Abstract
Many intracellular pathogens, including the protozoan parasite Toxoplasma gondii, live inside a vacuole that resides in the host cytosol. Vacuolar residence provides these pathogens with a defined niche for replication and protection from detection by host cytosolic pattern recognition receptors. However, the limiting membrane of the vacuole, which constitutes the host-pathogen interface, is also a barrier for pathogen effectors to reach the host cytosol and for the acquisition of host-derived nutrients. This review provides an update on the specialized secretion and trafficking systems used by Toxoplasma to overcome the barrier of the parasitophorous vacuole membrane thereby allowing the delivery of proteins into the host cell and the acquisition of host-derived nutrients.
Keywords: Toxoplasma gondii, translocon, secreted effectors, nutrient acquisition, parasitophorous vacuole, trafficking
Introduction
Toxoplasma gondii is an obligate intracellular parasite that can invade any nucleated cell from probably all warm-blooded animals. Even in immunocompetent hosts, it can cause chronic infections by forming semi-dormant tissue cysts in brain and muscle tissues that can persist for the life of the host. In healthy individuals it can cause mild symptoms and sometimes ocular complications, but it can become life-threatening for infants born to infected mothers and people with weakened immune systems (68). Key to Toxoplasma’s success is the coordinated secretion of an arsenal of proteins from three specialized secretory organelles called micronemes, rhoptries and dense granules. Micronemal proteins (MICs) are secreted in a calcium-dependent manner from the parasite’s apical end and mediate the attachment to the host cell (15, 40), which provides a signal for the rhoptries to secrete their contents. Proteins from the apical rhoptry neck (RONs) are secreted first and form a receptor that is inserted into the host cell plasma membrane thereby providing the handle to which MICs on the parasite plasma membrane (PPM) bind to and which it uses to pull itself inside (10). While it pulls on this handle it makes a transient break in the host cell plasma membrane through which it secretes rhoptry bulb proteins (ROPs) while a screw-like forward motion wraps the host cell plasma membrane around the parasite leading to the formation of the parasitophorous vacuole (PV) in the host cell cytosol (98). The PV is the parasite’s replication niche and is surrounded by the PV membrane (PVM), which is the interface between Toxoplasma and the host cell. Toxoplasma overcomes the barrier of the PVM by secreting dense granule proteins (GRAs) into the PV lumen after which some GRAs traffic to the PVM where they mediate the acquisition of host-derived nutrients while other GRAs form a translocon that delivers GRA effectors into the host cytosol (106). The mechanisms for the delivery of Toxoplasma’s secreted proteins to their correct location and the acquisition of host nutrients across the barrier of the PVM are slowly being unraveled and will be reviewed here.
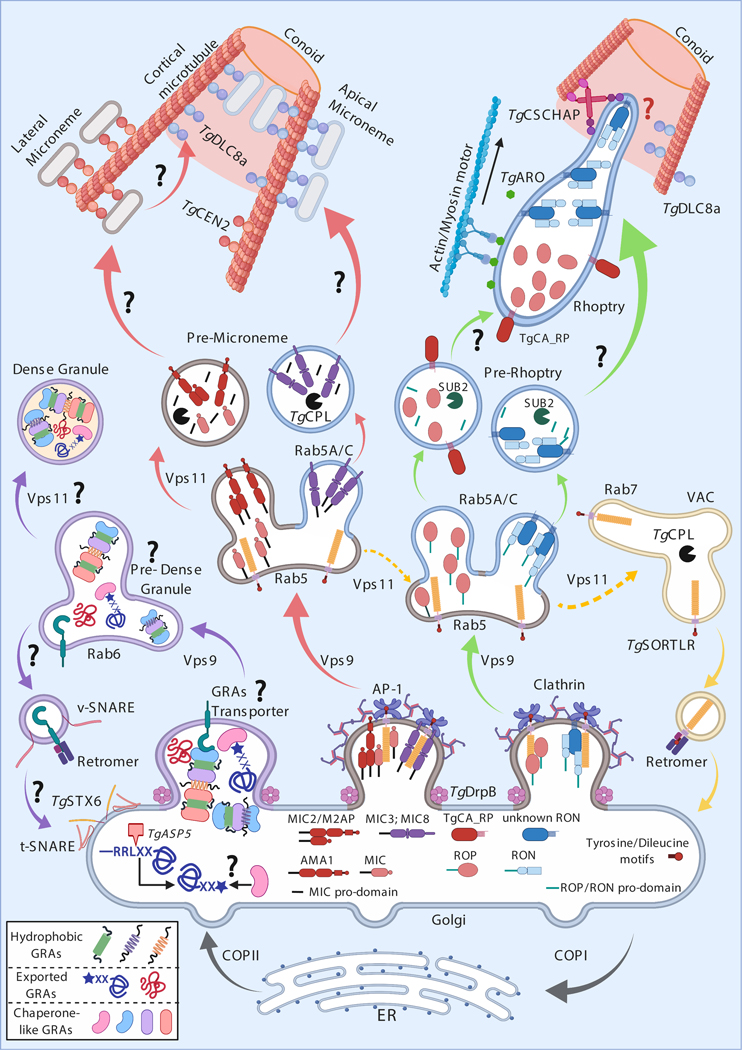
Trafficking pathways to deliver proteins to Toxoplasma’s specialized secretory organelles and the positioning of these organelles at secretion sites.
Toxoplasma secretory proteins need to be correctly trafficked to their respective secretory organelle. ROPs and MICs contain motifs that determine their trafficking to the rhoptries or micronemes while trafficking to dense granules is the default pathway of secretory proteins. The trafficking systems and sorting motifs in Toxoplasma were recently reviewed (127) and are summarized in Figure 1. The parasite attaches to the host cell with its apical end, which consists of the conoid (a cone-shaped structure of microtubules), two intra-conoid microtubules, and two polar rings (62). Therefore, micronemes and the neck of some rhoptry organelles must be closely appositioned to the apical end to allow fusion with the PPM and the secretion of MICs and RONs that mediate the attachment and invasion of the host cell. The positioning of rhoptries at the apical end of the parasite is mediated by the Armadillo Repeats-Only (TgARO) protein, which is anchored to the cytosolic face of the rhoptry membrane by the addition of a palmitoyl group by the palmitoyl acyl transferase TgDHHC7 (3, 88). TgARO interacts with myosin F (TgMyoF) (89), a myosin motor resembling the cargo transporter myosin Va (reviewed in (56)). By interacting with TgARO, TgMyoF could position rhoptries at the parasite’s apical end by walking on the filamentous actin cytoskeleton. At the apical end, the rhoptries are likely crosslinked to the intraconoid microtubules (86) by TgCSCHAP, a Coccidian Specific CORVET (class C core vacuole/endosome tethering)/HOPS (homotypic fusion and protein sorting) Associated Protein, in cooperation with microtubule associated protein Dynein Light Chain 8a (TgDLC8a) (74). This crosslinking to intraconoid microtubules would allow the membrane of the rhoptry neck to become closely appositioned to the PPM upon extrusion of the conoid. Microneme trafficking to exocytosis sites also depends on TgDLC8a (74).
Figure 1. Trafficking of proteins to Toxoplasma’s specialized secretory organelles.
Vesicles containing GRAs, MICs and RONs/ROPs are trafficked from the ER to the Golgi via the COPII complex (2) while the COPI complex assures retrograde transport (69). At the Golgi, each type of secretory protein localizes to a distinct Golgi region where MICs and RONs/ROPs could bind to the N-terminal domain of the transmembrane Sortilin-Like Receptor (TgSORTLR), which binds to the Adaptor Protein (AP)-1 complex with its C-terminal di-leucine motif (LL) (123). Some MICs (e.g., MIC2, AMA1) possess a transmembrane domain and a tyrosine motif (YxxΦ) (127) that could directly interact with AP1. The hydrophobic domains present in many GRAs are likely kept soluble by a chaperone-like protein, which could interact with an unknown GRA transporter protein. Exported GRAs are cleaved at the TEXEL motif (RRL) by the Golgi-resident ASP5 protease and the resultant N-terminal domain could be acetylated and associate with a chaperone-like protein. The scission of vesicles at the Golgi depends on the dynamin-related protein DrpB (14). MIC- and RON/ROP-containing vesicles are sorted from the Golgi (red and green arrows, respectively) and delivered to Rab5-/Vacuolar Protein Sorting (Vps)9-positive endosomal compartments (114) in a Clathrin/AP1 complex dependent manner (103). It is still unclear how MICs and RONs/ROPs dissociate from TgSORTLR. Subsequently, TgSORTLR could be transported from a Rab5- to a Rab7/VAC-positive endosomal compartment using the Vps11/HOPs complex (87), and finally recycled back to the Golgi by the retromer complex (115). Apical MICs (e.g., MIC3/8) and RONs/ROPs are delivered to their respective pre-organelles via Rab5A/C-positive vesicles (71) while delivery of lateral MICs (e.g., AMA1, MIC2/M2AP) is dependent on the Vps11/HOPs complex (87). In the pre-micronemes, the pro-domain of MICs could be cleaved by the protease TgCPL (37) while in the pro-rhoptries the SUB2 protease could cleave RON/ROP pro-domains (84). It is still unclear how pre-micronemes and pre-rhoptries mature into micronemes and rhoptries. After maturation, lateral and apical micronemes are attached to cortical microtubules using TgCEN2 and TgDLC8a, respectively. The transfer of lateral micronemes to the conoid to get secreted seems to be also dependent on TgDLC8a (74). An unknown protein could traffic RON proteins together from the Golgi, thereby promoting the localization of these proteins at the neck of the rhoptry. Subsequently, TgCA_RP could mediate the fusion of pre-rhoptries containing ROPs with the neck of the rhoptry (22). The mature rhoptry is then transported to the conoid using actin/myosin motors and TgARO. The final attachment of rhoptries to the conoid microtubule depends on TgCSCHAP (86) and TgDLC8a. The sorting of GRAs from the Golgi compartment (purple arrows) is dependent on Vps9 (114) but independent of Rab5-positive vesicles. Rab6 between the Golgi and endosome could be necessary for the formation of a putative pre-dense granule compartment (124). The retromer complex could also recycle the putative GRA transporter from the pre-dense granules to the Golgi, where syntaxin TgSTX6 together with t/v-SNARE assures vesicle fusion to the Golgi (63). It is unclear how GRAs are packed into mature dense granules.
TgMyoF is also the molecular motor that binds to the surface of dense granules, likely via its WD40 domains, and delivers them to the parasite periphery (58) (Figure 2B). GRA46 has structural homology to myosin motors and was recently shown to localize to the periphery of dense granules and, unlike most other GRAs, GRA46 is not secreted (24). Thus, it is tempting to speculate that GRA46 also plays a role in the movement of dense granules. Once at the parasite periphery, dense granules are escorted along with Rab11A vesicles and transported along actin filaments to their secretion sites (65). However, contact of dense granules with the PPM is restrained by the inner membrane complex (IMC), a network of stitched alveoli underneath the PPM connected with the cytoskeleton. The diffusive-like motion of dense granules likely enhances their movement in between small gaps of the IMC (41) after which they can start fusion with the PPM (Figure 2B).
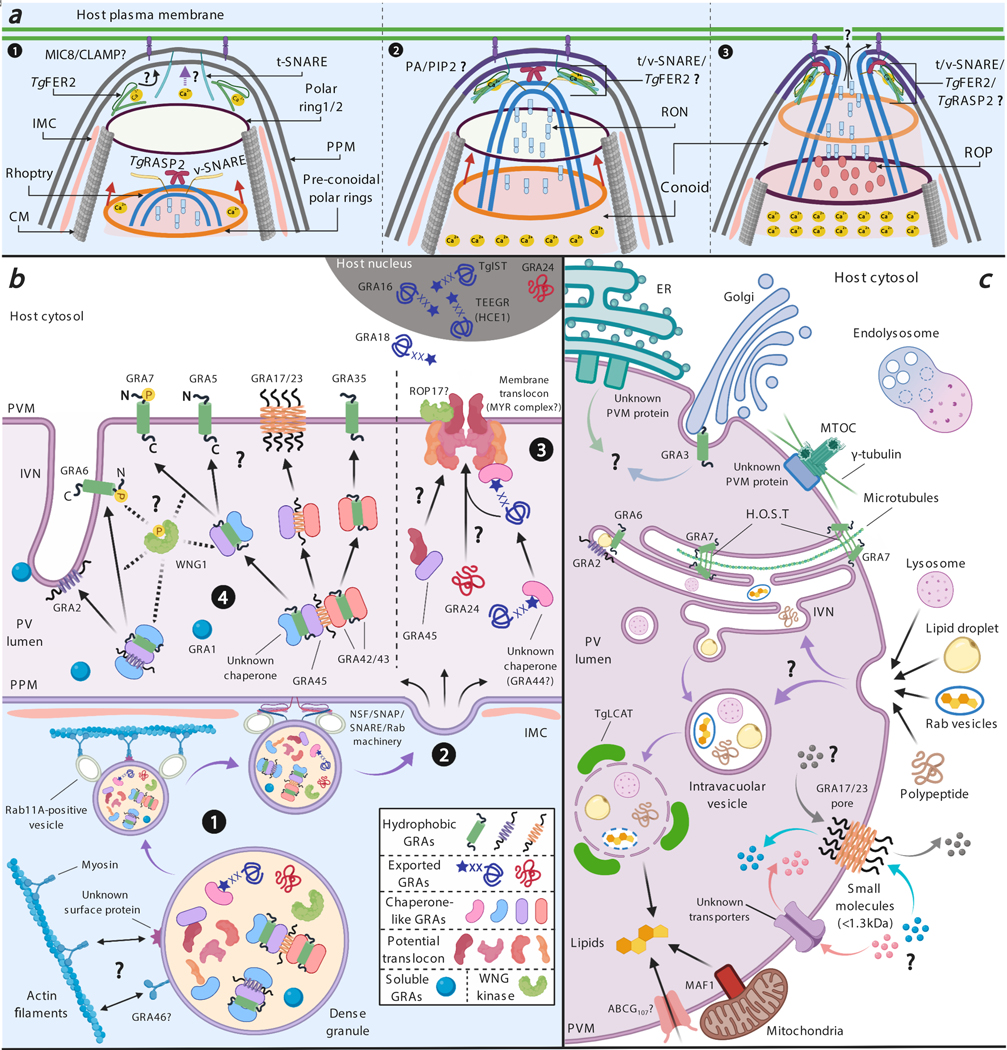
Figure 2. (A) Model for rhoptry secretion.
1) The process of host cell invasion raises the parasite internal [Ca2+] and the upper polar ring and conoid are extended until the base of the conoid protrudes beyond the lower polar ring (62). MICs are secreted, and MIC8/CLAMP could bind to the parasite and host plasma membrane (67, 120). The increase in [Ca2+] or the presence of MICs on the PPM could trigger a signaling cascade that changes the parasite phospholipid composition. The retracted conoid with rhoptry organelle moves through the cortical microtubules towards the apical polar ring. TgRASP and a putative v-SNARE protein are present at the membrane of the apical end of the rhoptry. A putative t-SNARE and TgFER2 at the PPM could bind to the membrane upon sensing a calcium signal. 2) When the conoid and the apical end of the rhoptry (containing RON proteins) are close to the apical polar ring, TgFER2 could mediate the interaction of the t/v-SNARE and bring the rhoptry membrane closer to the PPM, which could allow TgRASP2 to interact with PA/PIP2 (Phosphatidic Acid/Phosphatidylinositol (4,5)-bisphosphate) on the PPM. 3) The conoid ultimately passes through the apical polar ring, and the t/v-SNARE/TgFER2/TgRASP2 complex could mediate the fusion of the rhoptry membrane and the PPM allowing RONs/ROPs to be secreted. Possibly MIC/ROP/RON secretion changes the permeability of the host plasma membrane at the attachment site allowing RONs and ROPs to pass through and reach the cytosol. (B) Model for trafficking of GRAs from dense granules to their destinations. (1) Inside the dense granules, hydrophobic GRAs are packed as soluble oligomers via shielding of their hydrophobic domains by putative chaperone-like GRAs to avoid fusion with the dense granule membrane. Mature dense granules bind to myosin and/or Rab11A-positive vesicles, which mediate the transport of dense granules via actin tracks to the parasite’s periphery where they dock at IMC gaps. (2) Docking of dense granules at an IMC gap allows their fusion with the PPM via the NSF/SNAP/SNARE/Rab machinery resulting in exocytosis of GRAs into the PV lumen. (3) Once secreted into the PV lumen, ASP5-processed TEXEL-containing exported GRAs with potential N-terminal acylation are escorted by unknown chaperone GRAs to the PVM translocon, of which MYR1/2/3/4 and GRA44 may be components. GRA45 likely functions as a chaperone helping the translocon components traffic to and insert into the PVM. GRA24 is a TEXEL-negative exported protein and traffics to the membrane translocon via an unknown mechanism followed by translocation into the host cell. (4) Hydrophobic GRAs (transmembrane, amphipathic helices or α-helices) are escorted by chaperone GRAs to traffic inside the PV lumen and reach their destinations through an unknown mechanism. WNG kinase (WNG1) is involved in the eventual insertion of GRAs into the PVM or IVN via either directly phosphorylating the hydrophobic GRAs or phosphorylating the chaperone GRAs leading to the dissociation of hydrophobic GRA cargo from the chaperone. (C) Nutrient and small molecule import across the PVM. Host organelles, such as endoplasmic reticulum (ER), Golgi apparatus and the microtubule organizing center (MTOC), are recruited to the PVM. The relocation of the MTOC leads to the conversion of the microtubules network around the PVM bringing vesicular organelles, such as endolysosomal vesicles, to its vicinity where they can be used as nutrient resources for Toxoplasma. Host Golgi is retained in PVM invaginations by GRA3 whereas ER and MTOC are recruited via unknown PVM proteins. Host microtubules are recruited to invaginations of the PVM, mediated by GRA7, where they form a structure named H.O.S.T. responsible for cholesterol uptake derived from host lysosomes. Small nutrients are likely acquired through a membrane pore formed by GRA17/23 and/or other unknown PVM transporters. Host lysosomes, lipid droplets, Rab-positive vesicles derived from the endolysosomal system as well as polypeptides are invaginated into the PV and transiently stored at the IVN and intravacuolar vesicles of which the outer membrane can be digested by TgLCAT resulting in the release of nutrients into the PV lumen. GRA2 and GRA6 are present in the IVN and mediate the uptake of host cytosolic proteins. Host mitochondria are anchored to the PVM by MAF1. ABCG107 is a potential PVM transporter for scavenging host lipids and maintaining lipid homeostasis inside the PV lumen.
Exocytosis of Toxoplasma’s secretory organelles.
The exocytosis of rhoptries is dependent on the secretion of micronemes (recently reviewed in (40) and not further discussed here). A specialized structure called the moving junction forms the interface between the invading parasite and the host cell. The moving junction consists of a complex between the micronemal adhesin AMA1 present on the parasite surface and RON2, which is secreted into the host cell plasma membrane where its cytosolic part forms a complex with host cytosolic RON4/5/8 (1, 72). Without AMA1 the moving junction is not formed but RONs are still secreted (85). In contrast, parasites that do not express MIC8 do not secrete RONs suggesting that MIC8 might influence rhoptry fusion with the PPM possibly in cooperation with the recently identified claudin-like apicomplexan microneme protein (CLAMP) (67, 120). Two other proteins involved in rhoptry exocytosis are TgFER2, which belongs to the Ferlin family of double C2-domain proteins (26), and TgRASP2, a member of the recently identified Rhoptry Apical Surface Proteins (TgRASP1/2/3) that contain C2 and PH (Pleckstrin Homology) domains (125). C2 and PH domains can mediate interaction with lipids, consistent with TgRASP2’s ability to bind phosphoinositides (PIPs) and phosphatidic acid (PA). Depletion of TgRASP2 or absence of its lipid-binding domain completely abolishes rhoptry exocytosis and invasion. TgRASP2 and TgFER2 possibly function as part of the machinery that allows the fusion between the rhoptry membrane and the PPM (Figure 2a).
Once Toxoplasma resides in the PV, dense granules fuse with the PPM in a process resembling classical exocytosis leading to the secretion of GRAs into the PV lumen, the space between the PPM and the PVM (41, 75). Even though a large GRA secretion event is observed shortly after invasion (17, 28, 41, 75), dense granule exocytosis was considered to be a constitutive calcium-independent process that involved the conserved eukaryotic N-ethylmaleimide-sensitive factor (NSF)/soluble NSF attachment protein (SNAP) receptor (SNARE)/Rab system (23, 65). GRAs can also be spontaneously released from extracellular parasites (73, 119) and this release can be induced by heat-inactivated serum (28) and negatively regulated by calcium (66). Thus, dense granule exocytosis appears to be driven by both constitutive and regulated processes.
Host cell modulation by ROPs.
At the time of invasion, an unknown Toxoplasma protein likely induces a transient break in the host cell membrane (126) allowing the secretion of ROPs into the host cell cytosol. Once ROPs are injected into the host cell cytosol, they reside there or are translocated to the host cell nucleus or to the PVM, based on the presence of specific signals. For example, the ROP16 kinase, which can directly phosphorylate host STAT3/6, and the rhoptry protein phosphatase 2 C (PP2C-hn) possess a nuclear localization signal (NLS), that mediates their translocation to the host nucleus (51, 113). Other ROP kinases or pseudokinases such as ROP17, ROP18 and ROP5 traffic back to the parasite’s nascent PVM, which is mediated by an arginine-rich amphipathic helix (RAH) domain (107). ROP5/17/18 form a complex on the PVM that inhibits the immunity-related guanosine triphosphatases (GTPases) (IRGs), which are induced by interferon-gamma (IFNγ) and can vesiculate and destroy the PVM (43).
To aid its migration from the site of infection to distant organs, Toxoplasma co-opts host immune cells as Trojan Horses (131). ROP17 enhances the tissue transmigration of infected monocytes which is dependent on the Rho-Rho-associated coiled-coil containing protein kinase (ROCK) pathway, which is generally involved in modulation of the cytoskeleton (39). However, because ROP17’s catalytic activity on the PVM also determines translocation of GRA effectors into the host cytosol (95) (discussed below), it is also possible that such a GRA effector modulates the Rho–ROCK pathway. The ROP Toxofilin is injected into the host cytosol upon invasion (76) where it disassembles the host cell cortical actin meshwork at the point of entry thereby facilitating invasion (35). Another cytosolic ROP called TgWIP (Wave complex Interacting Protein) was recently identified by an in vivo loss-of-function screen to be important for Toxoplasma dissemination. TgWIP mediates the disappearance of podosomes, which are actin-rich structures important for the adhesion of the cell to extracellular matrix, in infected dendritic cells and induces a hypermigratory phenotype in these cells (116). Migratory properties of host leukocytes are determined by the dynamic remodeling of the actin cytoskeleton, which can be influenced by the WAVE regulatory complex (WRC) and SHP2 phosphatase both of which are interacting with TgWIP (116). The function of most other ROP effectors is unknown.
Host cell modulation by exported GRAs.
A decade ago, GRAs were not considered to be secreted effectors that modulate the host cell. This changed when GRA15, which is secreted onto the PVM, was shown to activate the host nuclear factor-κB (NFκB) pathway (111). Since then, multiple PVM GRAs, including GRA6 (79), GRA7 (29), GRA17/GRA23 (52), GRA25 (118), GRA35 (129), and Mitochondrial Association Factor (MAF)1 (100), have been identified as parasite effectors that play an important role in the modulation of host cell signaling pathways, acquisition of host nutrients, or counteracting host innate defense mechanisms. Only few Toxoplasma effectors have been identified as exported beyond the PVM. GRA16 was the first GRA identified as exported beyond the PVM after which it localizes to the host cell nucleus, where it binds to host deubiquitinase HAUSP and protein phosphatase (PP)2A and regulates the host cell cycle (9). Subsequently, other exported GRAs that localize to the host nucleus were discovered: GRA24, which triggers and sustains phosphorylation of p38α Mitogen-activated protein (MAP) kinase and its translocation into host nuclei resulting in enhanced production of proinflammatory cytokines (12); TgIST, which blocks STAT1-dependent transcription and thereby counteracts IFNγ-induced parasite clearance (48, 94); TEEGR (Toxoplasma E2F4-associated EZH2-inducing gene regulator) (synonymous with inducer of Host Cyclin E (HCE)1), which binds host transcription factors E2F3 and E2F4 resulting in inhibition of host NFκB signaling and modulation of the host cell cycle (11, 96); and GRA28 with unknown function (90). GRA18 is so far the only GRA exported beyond the PVM that remains in the host cytosol where it interacts with Glycogen Synthase Kinase (GSK)3/PP2A-B56 leading to β-catenin upregulation and selective modulation of host immune gene expression (59).
Toxoplasma exported GRAs are processed in the Golgi.
Protein export is well studied in Plasmodium spp, in which most exported proteins have a signal peptide (SP) followed by a downstream RxLxE/Q/D motif termed the Plasmodium export element (PEXEL) (82). PEXEL-containing proteins are post-translationally imported into the ER by a complex consisting of the Sec61 protein translocase, signal peptidase complex (SPC)25 and sometimes Sec62 (80), whereby the PEXEL is recognized and cleaved by the ER-resident aspartyl protease plasmepsin V (PMV), another member of the complex (7, 80, 112). PMV cleaves after the leucine of the PEXEL motif, which reveals a neo N-terminus that is acetylated (Ac-xE/Q/D) in the ER by an unknown N-acetyltransferase permitting a potential cargo selection mechanism by cargo receptors or chaperones for trafficking to the PVM translocon (8, 20). The Toxoplasma ortholog of Plasmodium PMV is the Golgi-resident Aspartyl Protease 5 (ASP5) (Figure 1) which cleaves the Toxoplasma export element (TEXEL) RRL. ASP5-mediated TEXEL cleavage is important for export of GRAs (25, 31, 57) and mutating the TEXEL sequence of exported GRAs impairs their export (25, 31). Unlike the Plasmodium PEXEL, the TEXEL cleaved by ASP5 is not always at the N-terminus close to the SP. This suggests that Toxoplasma applies another protein export mechanism where the SP directs GRA entry into the ER followed by transport to the Golgi where recognition and cleavage of TEXEL-containing GRAs by ASP5 takes place. Since there is no common feature within the neo N-terminus of ASP5 substrates, it is unclear how ASP5 cleavage would target GRAs for export. One potential model is that one or more chaperone proteins immediately take ASP5-cleaved substrates with them throughout the secretory pathway. Unexpectedly, ASP5 is also required for export of some TEXEL-negative proteins (TNEPs) such as GRA24 and TEEGR (HCE1) (11, 25, 31, 57, 96). The export of TNEPs either relies on TEXEL-containing translocon components for which ASP5 cleavage is needed for their correct function or on ASP5-dependent cleavage of TEXEL-containing chaperones that are needed for the correct trafficking of TNEPs to the translocon (discussed below). In Toxoplasma only a subset of ASP5 substrates are exported in contrast to Plasmodium where all reported PEXEL proteins are exported. For example, the TEXEL-containing proteins, GRA19, GRA20, and MYR1, are processed by ASP5 but they are not exported and instead localized to the PVM (25, 44, 57, 61). ASP5 also plays a critical role in the correct PV/PVM localization of TEXEL-negative proteins, such as GRA2, GRA3, GRA7 (57), and GRA35 (129). In addition, ASP5 abolishment results in a defective intravacuolar network (IVN), a network of membranous tubules in the PV lumen which are stabilized by the tubulogenic GRA2 and GRA6 proteins (83), and impairment of host mitochondrial recruitment at the PVM (25, 57). Given that many PV-localized GRAs are TEXEL-negative, ASP5 is likely indirectly influencing the localization of PVM-associated GRAs by affecting the function of proteins that help GRA trafficking to the PVM in a post-secretion event. In contrast to Plasmodium PMV, deletion of ASP5 is not lethal to Toxoplasma but leads to reduced in vitro parasite growth, impaired host cell modulation and enhanced susceptibility to immune responses (25, 57), probably due to the cumulative result of defects in protein export and correct localization of proteins at the PV/PVM (57).
Post-secretory trafficking of GRAs to their final destination.
After secretion into the PV lumen, GRAs stay there, associate with membranes of the PVM or the IVN, or are exported beyond the PVM into the host cytosol. Several Toxoplasma proteins mediating GRA trafficking and export have been discovered (25, 31, 44, 57, 81, 95) but the trafficking and export mechanisms at a molecular level are still unclear. Except for the exported GRAs, GRA1 (19), and Nucleoside Triphosphate Hydrolase (NTPases) (5, 119), all GRAs described so far are predicted to encode either a single-pass transmembrane domain (TMD) or amphipathic α-helices and associate with membranes of the IVN or PVM. Inside the dense granules, GRAs with a hydrophobic domain do not behave as type I transmembrane proteins and are not inserted in the dense granule membrane but instead are mixed between partially soluble and aggregated states (50). This sorting of GRAs with a single-pass TMD to dense granules only occurs in Toxoplasma itself, since when these proteins are expressed in host cells, they sort to the host cell plasma membrane (50). GRAs in the dense granules are partially packed in oligomeric complexes (13), likely their hydrophobic domains are either sequestered directly within the complex or shielded by chaperone-like proteins (Figure 2b), thereby explaining their export as soluble proteins. Once secreted into the PV lumen, a change in the microenvironment might lead to a subsequent conformational change exposing their hydrophobic domain. However, the precise mechanism that subsequently leads to their insertion into the PVM vs. IVN vs. PPM is still unclear. It was shown that the N-terminal domain between the SP and TMD of GRA5 and GRA6 contains an unknown signal that determines correct trafficking to the PVM and exchange of this domain leads to accumulation of these GRAs in the PV lumen (49, 50). How the N-terminal domain of these GRAs promotes a conformational change from a soluble stage to a transmembrane conformation remains to be determined. Possibly specific cofactors, such as protein chaperones delivered to the PV at the same time, recognize sorting motifs within this N-terminal domain and contribute to the post-secretory membrane insertion. Such a model is illustrated for protein trafficking in the cell envelope of gram-negative bacteria. Similar to the spatial structure of the Toxoplasma plasma membrane and PVM, the cell envelope of these bacteria consists of the plasma membrane, inner membrane, and outer membrane flanking the periplasm (121). For example in Escherichia coli, most non-folded outer membrane proteins after crossing the plasma membrane are assisted by chaperones to stay soluble followed by inserting into the outer membrane (34). A group of periplasmic chaperones called pilotins have been proposed to bind the C-terminus of outer membrane-localized lipoproteins followed by their co-transport to the outer membrane by the Lol system (70, 93).
Little is known about the Toxoplasma proteins involved in GRA trafficking after exocytosis into the PV lumen. GRA42 and GRA43, which are localized inside the PV lumen after secretion, mediate the correct trafficking of the PVM-associated GRA35 and GRA17/23 (129), suggesting GRA42/43 function as protein chaperones although they have no homology with any known protein chaperones. ASP5 also influences the PVM localization of GRAs, but GRA42/43 do not contain TEXEL motifs. It is therefore likely that other TEXEL-containing proteins function as regulators of GRA42 and GRA43 or function in the same pathway mediating the correct localization of PVM-associated GRAs. One candidate for such a protein is the kinase WNG1, a novel ASP5 substrate that localizes to the PV lumen (4, 24) and that is involved in the phosphorylation of several membrane-associated GRAs including GRA35 (4). Parasites without catalytically active WNG1 have reduced membrane association of GRA4, GRA6 and GRA7 as well as impaired IVN morphology, suggesting that WNG1 could directly phosphorylate membrane-associated GRAs as well as provide the energy for correct trafficking of these GRAs to their destination. An alternative hypothesis is that instead of directly phosphorylating the membrane-associated GRAs, WNG phosphorylates a chaperone-like GRA resulting in its disassociation from and release of its cargo leading to membrane insertion of a subset of GRAs where they are phosphorylated by host cytosolic kinases (4). Selective targeting of GRAs to PVM or IVN could further rely on the interactions between the N-terminal domain of GRAs and specific components of these membranes. For example, the lipid composition of the PVM or IVN could affect the localization of GRAs with a hydrophobic domain. Initially, the PVM is almost entirely derived from the host cell plasma membrane and rich in cholesterol and the ganglioside GM1 (21, 30). However, the PVM is depleted of cholesterol over time and parasite proteins are integrated into the PVM. Similarly, the IVN is initially derived from secretion of vesicular material from the parasite’s basal end, however late after infection IVN lipids are predominantly host cell derived (16). The lipid environment (thickness, composition, curvature) influences the affinity of proteins for certain membranes (102), which could contribute to GRA targeting. In agreement with this hypothesis, some PVM-localized GRAs, for example GRA7, could have specific affinities for certain lipids (e.g. phosphoinositides) that may be present on the PVM and IVN but not PPM (29). Furthermore, the PVM bilayer (13 nm) is thicker than the IVN bilayer (8 nm) which might affect preferential insertion into PVM vs. IVN (78).
GRA effector translocation across the PVM.
GRA export across the PVM into the host cytosol is only observed once the parasite has invaded, which differs from rhoptry effectors that are discharged into the host cell cytosol upon invasion. A forward genetic screen set up to discover Toxoplasma effectors responsible for host c-Myc induction identified MYR1 as the first Toxoplasma protein contributing to GRA export beyond the PVM (44). The export of GRA16, GRA18, GRA24, TgIST and TEEGR was absent in the parasites lacking MYR1 (11, 44, 48, 59, 96) whereas the functions of PVM-localized MAF1b and GRA15 were not affected (44). This suggests that MYR1 only affects the export of GRAs that translocate across the PVM but has no effect on the correct localization of GRAs to the PVM. Due to impairment in delivery of GRA effectors into the host cell, Δmyr1 parasite-infected cells have a dramatically reduced host transcriptional response to Toxoplasma infection (91). Subsequently, MYR2 and MYR3, which were identified from the same c-Myc induction mutagenesis screen, were also shown to be involved in export of GRAs across the PVM (81). Notably, both MYR1 and MYR3 localize at the PVM and form a stable interaction whereas MYR2 localizes at the periphery of the PV and did not associate with the other two MYR proteins (81). MYR4 was recently identified as MYR1-interacting protein and involved in GRA16 nuclear translocation (32). Surprisingly, ROP17, discovered from the same screen, was also shown to determine GRA translocation across the PVM (95). Unlike the MYR proteins, the action of ROP17 on GRA translocation takes place on the host cytosolic side of the PVM and depends on its catalytic residues (95). ROP17 is recruited to the PVM after secretion into the host cell and might phosphorylate and activate a component of the PVM translocation machinery. It should be noted that MYR1/2/3/4 have not been formally shown to form a translocation pore for protein export. It is also possible that they serve a role as chaperoning and/or docking proteins that help exported GRAs reach the protein-conducting pore at the PVM. Recent studies have discovered that the ASP5 substrates GRA44 and GRA45 are necessary for GRA16 and GRA24 export to the host nucleus (6, 32, 130). GRA45 possesses a small heat shock protein (HSP) domain and prevents aggregation of hydrophobic GRAs within the dense granules and influences the correct trafficking of GRAs to the PVM (130). Unlike the Plasmodium falciparum PTEX translocon, which contains the unfoldase HSP101 (60), there is no evidence that the Toxoplasma translocon contains an unfoldase and all known exported GRAs are intrinsically disordered proteins (55, 99). Fusion of the highly folded murine dihydrofolate reductase (DHFR) domain to highly disordered GRA16 blocks translocation of GRA16 as well as GRA24 to the host cell likely by clogging the translocation pore (31, 81). Taken together, Toxoplasma exported GRAs do not seem to require active unfolding for their export. Thus, GRA45 likely functions as a protein chaperone which helps to maintain the proper folding of GRAs destined for the PVM during their trafficking through the secretory system (Figure 2b). GRA45’s effect on GRA translocation across the PVM is therefore most likely due to a putative effect on the correct trafficking of translocon components to the PVM. GRA44 contains a putative acid phosphatase domain (6) but it is still unclear how it influences the protein export in Toxoplasma.
Transport of small molecules across the PVM.
Toxoplasma’s growth is dependent on nutrient scavenging from the host cell as it is auxotrophic for nutrients such as purines, tryptophan, arginine and cholesterol. Additionally the parasite has salvage pathways for amino acids, sphingolipids and pyrimidines (27). The PVM-host cytoplasm is the interface through which parasite proteins mediate the uptake and transport of nutrients into the PV lumen. The subsequent absorbance of these nutrients is via membrane transporters located in the PPM (97, 105). The PVM is selectively permeable allowing the charge-independent diffusion of molecules up to 1.3 kDa (117). The PVM-localized GRA17 and GRA23 play a role in the permeability of the PVM to small molecules (52) (Figure 2c). Parasites without GRA17 form “bubble” vacuoles that have decreased permeability to small molecules. The expression of Plasmodium translocon protein EXP2, a GRA17/23 orthologue, in Δgra17 parasites rescued this phenotype, which suggested that EXP2 might have a dual function as a nutrient pore and translocon (52), which was recently confirmed (47). Parasites without GRA23 display no obvious phenotype, however, when GRA23 is overexpressed in Δgra17 parasites it rescues the defect in the PVM permeability to small molecules suggesting it can also form a permeability pore. The simultaneous deletion of GRA17 and GRA23 is synthetically lethal, indicating that these proteins function in similar pathways for nutrient transport across the PVM. Because the deletion of GRA17 generates swollen vacuoles it is possible that the GRA17 pore is also involved in the elimination of metabolic waste products and the absence of the pore leads to an osmotic imbalance in the PV lumen, which could explain why 60% of Δgra17 bubble vacuoles eventually disrupt, killing the parasites inside. GRA17, GRA23 and EXP2 lack TMDs and their sizes and multiple α-helices suggest a functional analogy to α-pore forming toxins (αPFTs) (52). αPFTs exist as soluble, inactive monomers that, in the context of their targets, subsequently assemble and multimerize into active complexes that can form large pores in phospholipid membranes (54). A recent Cryo-EM structure of the PTEX complex showed that seven EXP2 monomers form a protein-conducting channel spanning the membrane (60). Super resolution Scanning EM of the Toxoplasma PVM revealed pores ranging from 10 to 200 nm and the number of pores in the PVM increased with the length of the intracellular cycle (36), which is consistent with GRAs forming these pores. Whether these pores constitute GRA17/23 nutrient pores and/or pores formed by the translocon mediating GRA export is currently unknown. The presence of pores can explain the traffic of small molecules, however it is unclear how macromolecules larger than 1.3 KDa reach the PV lumen, the only classical transporter identified so far in the PVM is the Toxoplasma ADP binding cassette sub-family G (ABCG107) likely involved in lipid acquisition (42). The presence of additional transporters in the PVM remains to be determined.
Uptake of host-derived vesicles and host cytosolic proteins through the PVM.
Many intracellular pathogens obtain host nutrients by recruiting host organelles and scavenging essential molecules from them. After Toxoplasma invasion, the PV is located to a perinuclear area and recruits the microtubule organizing center (MTOC) by interaction of the PVM with γ-tubulin through an unknown mechanism (128). Recruitment of the MTOC to the PVM likely contributes to the subsequent recruitment of the Golgi complex, ER, and endolysosomal vesicles (108). The conversion of vesicular trafficking towards the PV facilitates membrane fusion events for delivery of host cell nutrients into the PV. Rapidly after invasion ER profiles and Golgi complex stacks are located around the PV (122), possibly to obtain N-glycans and sphingolipids (46, 110). Sec22b is required for recruitment of ER to the PVM. The intricate connections between the ER and PVM also allow Toxoplasma antigens to be translocated to the ER and subsequently retrotranslocated via the ERAD system to the host cytosol where they can be loaded onto MHC I for antigen presentation to CD8 T cells (18, 53, 77).
Host mitochondria are recruited to the vicinity of the PV of type I and III, but not type II, strains, which is mediated by the PVM-localized GRA protein MAF1b (100). There is some evidence that Toxoplasma recruits mitochondria to scavenge specific lipids: deletion of the Acyl coenzyme A (CoA)-binding protein (ACBP2) in type II parasites results in a defect in cardiolipin metabolism, which can be rescued by expressing type I MAF1b in the type II Δacbp2 strain (45). On the other hand, mitochondria recruited to the PV can also limit parasite growth by siphoning of fatty acids and degrading them thereby limiting Toxoplasma’s access to these fatty acids (101). Toxoplasma intercepts and ‘ingest’ host Rab-derived vesicles loaded with sphingolipids, lipid droplets containing neutral lipids (109), and endolysosomes filled with cholesterol (29), through a phagocytic-like process whereby the PVM surrounds these vesicles after which they pinch off and localize inside the PV lumen (92). The lecithin:cholesterol acyltransferase (TgLCAT), a GRA secreted into the PV lumen, likely degrades the limiting membrane of these vesicles promoting the release of their cargo into the PV lumen (104). Toxoplasma can also ingest host cytoplasmic proteins through an unknown mechanism. Because ingested host proteins are rapidly digested inside the VAC, an acidic lysosome-like organelle, the ingestion of host proteins can only be observed in parasites where the VAC’s major digestive enzyme Cathepsin L (CPL) is knocked out (38) or inhibited. Toxoplasma might ingest host proteins to acquire amino acids, similar to how Plasmodium acquires amino acids from ingested hemoglobin (64). The Toxoplasma proteins that mediate the ‘ingestion’ of host proteins or Rab-derived vesicles at the PVM or mediate the transport of Rab-derived vesicle cargo from the PV lumen across the PPM are currently unknown. In Δgra2 parasites, which do not have an IVN, the ingestion of host cytosolic proteins, Rab-vesicles, and lipid droplets is significantly reduced, suggesting that the IVN functions as a conduit to provide nutrients to Toxoplasma (29, 109, 110). GRA7 is responsible for the membrane constriction that leads to the formation of an intravacuolar membrane and host microtubule bridge named H.O.S.T (Host-Organelle Sequestering Tubulo-structures) that is important for uptake of lysosome-derived vesicles (29). GRA3 is present in the PVM, where it binds specifically to lipids and promotes structural modifications in the PVM that lead to the interaction and retention of the Golgi complex. GRA3 is also involved in the disruption of anterograde traffic to retain host nutrients from the secretory pathway (33). Likely other yet to be identified GRAs are involved in the nutrient uptake mechanisms displayed by Toxoplasma.
Future Issues list:
What proteins form the membrane pore or channel on the PVM and do GRA44/GRA45, MYR1/2/3/4, and ROP17 play a role in the formation and/or regulation of the translocon or the transport of effectors to the translocon?
What are the proteins that prevent aggregation of GRAs inside the dense granules, mediate the conformational change of GRAs in the PV lumen, and help GRAs insert into or associate with different membrane fractions in the PV?
What is the Toxoplasma protein(s) recognizing the neo N-terminus of ASP5-cleaved GRAs and how are they and TNEPs subsequently transported to the PVM translocon?
What is the molecular mechanism of dense granule exocytosis?
What is the molecular mechanism involved in the host cytosolic protein uptake?
What is the molecular mechanism of ROP secretion into the host cell cytosol?
Acknowledgements:
JPJS is supported by the National Institutes of Health (R01-AI080621, R21AI139387).
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, et al. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 77(6):895–907 [DOI] [PubMed] [Google Scholar]
- 3.Beck JR, Fung C, Straub KW, Coppens I, Vashisht AA, et al. 2013. A Toxoplasma Palmitoyl Acyl Transferase and the Palmitoylated Armadillo Repeat Protein TgARO Govern Apical Rhoptry Tethering and Reveal a Critical Role for the Rhoptries in Host Cell Invasion but Not Egress [DOI] [PMC free article] [PubMed]
- 4.Beraki T, Hu X, Broncel M, Young JC, O’Shaughnessy WJ, et al. 2019. Divergent kinase regulates membrane ultrastructure of the Toxoplasma parasitophorous vacuole. Proc. Natl. Acad. Sci. U. S. A 116(13):6361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudes D, Peck KR, Afifi MA, Beckers CJ, Joiner KA. 1994. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem 269(46):29252–60 [PubMed] [Google Scholar]
- 6.Blakely WJ, Holmes MJ, Arrizabalaga G. 2019. The secreted acid phosphatase domain-containing GRA44 from Toxoplasma gondii is required for C-myc induction in infected cells. Work. Pap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boddey JA, Hodder AN, Gunther S, Gilson PR, Patsiouras H, et al. 2010. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 463(7281):627–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boddey JA, Moritz RL, Simpson RJ, Cowman AF. 2009. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic. 10(3):285–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, et al. 2013. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe. 13(4):489–500 [DOI] [PubMed] [Google Scholar]
- 10.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, et al. 2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem 280(40):34245–58 [DOI] [PubMed] [Google Scholar]
- 11.Braun L, Brenier-Pinchart MP, Hammoudi PM, Cannella D, Kieffer-Jaquinod S, et al. 2019. The Toxoplasma effector TEEGR promotes parasite persistence by modulating NF-kappaB signalling via EZH2. Nat Microbiol. 4(7):1208–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, et al. 2013. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med 210(10):2071–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun L, Travier L, Kieffer S, Musset K, Garin J, et al. 2008. Purification of Toxoplasma dense granule proteins reveals that they are in complexes throughout the secretory pathway. Mol. Biochem. Parasitol 157(1):13–21 [DOI] [PubMed] [Google Scholar]
- 14.Breinich MS, Ferguson DJP, Foth BJ, van Dooren GG, Lebrun M, et al. 2009. A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Curr. Biol 19(4):277–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullen HE, Bisio H, Soldati-Favre D. 2019. The triumvirate of signaling molecules controlling Toxoplasma microneme exocytosis: Cyclic GMP, calcium, and phosphatidic acid. PLoS Pathog. 15(5):e1007670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caffaro CE, Boothroyd JC. 2011. Evidence for host cells as the major contributor of lipids in the intravacuolar network of Toxoplasma-infected cells. Eukaryot. Cell 10(8):1095–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carruthers VB, Sibley LD. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol 73(2):114–23 [PubMed] [Google Scholar]
- 18.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, et al. 2011. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 147(6):1355–68 [DOI] [PubMed] [Google Scholar]
- 19.Cesbron-Delauw MF, Guy B, Torpier G, Pierce RJ, Lenzen G, et al. 1989. Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A 86(19):7537–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang HH, Falick AM, Carlton PM, Sedat JW, DeRisi JL, Marletta MA. 2008. N-terminal processing of proteins exported by malaria parasites. Mol. Biochem. Parasitol 160(2):107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charron AJ, Sibley LD. 2004. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic. 5(11):855–67 [DOI] [PubMed] [Google Scholar]
- 22.Chasen NM, Asady B, Lemgruber L, Vommaro RC, Kissinger JC, et al. 2017. A Glycosylphosphatidylinositol-Anchored Carbonic Anhydrase-Related Protein of Toxoplasma gondii Is Important for Rhoptry Biogenesis and Virulence. mSphere. 2(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaturvedi S, Qi H, Coleman D, Rodriguez A, Hanson PI, et al. 1999. Constitutive calcium-independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. J. Biol. Chem 274(4):2424–31 [DOI] [PubMed] [Google Scholar]
- 24.Coffey MJ, Dagley LF, Seizova S, Kapp EA, Infusini G, et al. 2018. Aspartyl Protease 5 Matures Dense Granule Proteins That Reside at the Host-Parasite Interface in Toxoplasma gondii. MBio. 9(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coffey MJ, Sleebs BE, Uboldi AD, Garnham A, Franco M, et al. 2015. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife. 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman BI, Saha S, Sato S, Engelberg K, Ferguson DJP, et al. 2018. A Member of the Ferlin Calcium Sensor Family Is Essential for Toxoplasma gondii Rhoptry Secretion. MBio. 9(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppens I. 2014. Exploitation of auxotrophies and metabolic defects in Toxoplasma as therapeutic approaches. Int. J. Parasitol 44(2):109–20 [DOI] [PubMed] [Google Scholar]
- 28.Coppens I, Andries M, Liu JL, Cesbron-Delauw MF. 1999. Intracellular trafficking of dense granule proteins in Toxoplasma gondii and experimental evidences for a regulated exocytosis. Eur. J. Cell Biol 78(7):463–72 [DOI] [PubMed] [Google Scholar]
- 29.Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, et al. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 125(2):261–74 [DOI] [PubMed] [Google Scholar]
- 30.Coppens I, Joiner KA. 2003. Host but Not Parasite Cholesterol Controls Toxoplasma Cell Entry by Modulating Organelle Discharge [DOI] [PMC free article] [PubMed]
- 31.Curt-Varesano A, Braun L, Ranquet C, Hakimi MA, Bougdour A. 2016. The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell. Microbiol 18(2):151–67 [DOI] [PubMed] [Google Scholar]
- 32.Cygan AM, Theisen TC, Mendoza AG, Marino ND, Panas MW, Boothroyd JC. 2019. Co-immunoprecipitation with MYR1 identifies three additional proteins within the Toxoplasma parasitophorous vacuole required for translocation of dense granule effectors into host cells. bioRxiv, p. 867788. [DOI] [PMC free article] [PubMed]
- 33.Deffieu MS, Alayi TD, Slomianny C, Tomavo S. 2019. The Toxoplasma gondii dense granule protein TgGRA3 interacts with host Golgi and dysregulates anterograde transport. Biol. Open 8(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Geyter J, Tsirigotaki A, Orfanoudaki G, Zorzini V, Economou A, Karamanou S. 2016. Protein folding in the cell envelope of Escherichia coli. Nat Microbiol. 1(8):16107. [DOI] [PubMed] [Google Scholar]
- 35.Delorme-Walker V, Abrivard M, Lagal V, Anderson K, Perazzi A, et al. 2012. Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J. Cell Sci [DOI] [PMC free article] [PubMed]
- 36.de Souza W, Attias M. 2015. New views of the Toxoplasma gondii parasitophorous vacuole as revealed by Helium Ion Microscopy (HIM). J. Struct. Biol 191(1):76–85 [DOI] [PubMed] [Google Scholar]
- 37.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. 2006. Vacuolar H -ATPase Activity Is Required for Endocytic and Secretory Trafficking in Arabidopsis [DOI] [PMC free article] [PubMed]
- 38.Dou Z, McGovern OL, Di Cristina M, Carruthers VB. 2014. Toxoplasma gondii Ingests and Digests Host Cytosolic Proteins. MBio. 5(4):e01188–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, Sibley LD. 2019. The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nature Microbiology [DOI] [PMC free article] [PubMed]
- 40.Dubois DJ, Soldati-Favre D. 2019. Biogenesis and secretion of micronemes in Toxoplasma gondii. Cell. Microbiol 21(5):e13018 [DOI] [PubMed] [Google Scholar]
- 41.Dubremetz JF, Achbarou A, Bermudes D, Joiner KA. 1993. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res 79(5):402–8 [DOI] [PubMed] [Google Scholar]
- 42.Ehrenman K, Sehgal A, Lige B, Stedman TT, Joiner KA, Coppens I. 2010. Novel roles for ATP-binding cassette G transporters in lipid redistribution in Toxoplasma. Mol. Microbiol 76(5):1232–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. 2014. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe. 15(5):537–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franco M, Panas MW, Marino ND, Lee M-CW, Buchholz KR, et al. 2016. A Novel Secreted Protein, MYR1, Is Central to Toxoplasma’s Manipulation of Host Cells. MBio. 7(1):e02231–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Y, Cui X, Fan S, Liu J, Zhang X, et al. 2018. Comprehensive Characterization of Toxoplasma Acyl Coenzyme A-Binding Protein TgACBP2 and Its Critical Role in Parasite Cardiolipin Metabolism. MBio. 9(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garénaux E, Shams-Eldin H, Chirat F, Bieker U, Schmidt J, et al. 2008. The dual origin of Toxoplasma gondii N-glycans. Biochemistry. 47(47):12270–76 [DOI] [PubMed] [Google Scholar]
- 47.Garten M, Nasamu AS, Niles JC, Zimmerberg J, Goldberg DE, Beck JR. 2018. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat Microbiol. 3(10):1090–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gay G, Braun L, Brenier-Pinchart MP, Vollaire J, Josserand V, et al. 2016. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-gamma-mediated host defenses. J. Exp. Med 213(9):1779–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gendrin C, Bittame A, Mercier C, Cesbron-Delauw MF. 2010. Post-translational membrane sorting of the Toxoplasma gondii GRA6 protein into the parasite-containing vacuole is driven by its N-terminal domain. Int. J. Parasitol 40(11):1325–34 [DOI] [PubMed] [Google Scholar]
- 50.Gendrin C, Mercier C, Braun L, Musset K, Dubremetz JF, Cesbron-Delauw MF. 2008. Toxoplasma gondii uses unusual sorting mechanisms to deliver transmembrane proteins into the host-cell vacuole. Traffic. 9(10):1665–80 [DOI] [PubMed] [Google Scholar]
- 51.Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 6(1):73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, et al. 2015. The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe. 17(5):642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. 2009. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J. Exp. Med 206(2):399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Frêche B. 2008. Bacterial pore-forming toxins: the (w)hole story? Cell. Mol. Life Sci 65(3):493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hakimi M-A, Bougdour A. 2015. Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Curr. Opin. Microbiol 26:24–31 [DOI] [PubMed] [Google Scholar]
- 56.Hammer JA 3rd, Sellers JR. 2011. Walking to work: roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol 13(1):13–26 [DOI] [PubMed] [Google Scholar]
- 57.Hammoudi PM, Jacot D, Mueller C, Di Cristina M, Dogga SK, et al. 2015. Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS Pathog. 11(10):e1005211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heaslip AT, Nelson SR, Warshaw DM. 2016. Dense granule trafficking in Toxoplasma gondii requires a unique class 27 myosin and actin filaments. Mol. Biol. Cell 27(13):2080–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He H, Brenier-Pinchart MP, Braun L, Kraut A, Touquet B, et al. 2018. Characterization of a Toxoplasma effector uncovers an alternative GSK3/beta-catenin-regulatory pathway of inflammation. Elife. 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho C-M, Beck JR, Lai M, Cui Y, Goldberg DE, et al. 2018. Malaria parasite translocon structure and mechanism of effector export. Nature. 561(7721):70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsiao CH, Luisa Hiller N, Haldar K, Knoll LJ. 2013. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic. 14(5):519–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu K, Roos DS, Murray JM. 2002. A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J. Cell Biol 156(6):1039–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson AJ, Clucas C, Mamczur NJ, Ferguson DJ, Meissner M. 2013. Toxoplasma gondii Syntaxin 6 is required for vesicular transport between endosomal-like compartments and the Golgi complex. Traffic. 14(11):1166–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juge N, Moriyama S, Miyaji T, Kawakami M, Iwai H, et al. 2015. Plasmodium falciparum chloroquine resistance transporter is a H+-coupled polyspecific nutrient and drug exporter. Proc. Natl. Acad. Sci. U. S. A 112(11):3356–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kannan V, Sylia C, Elisabeth W, Nicolas B, Javier P, et al. 2019. Rab11A regulates the constitutive secretory pathway during Toxoplasma gondii invasion of host cells and parasite replication. Work. Pap. [Google Scholar]
- 66.Katris NJ, Ke H, McFadden GI, van Dooren GG, Waller RF. 2019. Calcium negatively regulates secretion from dense granules in Toxoplasma gondii. Cell. Microbiol 21(6):e13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kessler H, Herm-Götz A, Hegge S, Rauch M, Soldati-Favre D, et al. 2008. Microneme protein 8--a new essential invasion factor in Toxoplasma gondii. J. Cell Sci 121(7):947–56 [DOI] [PubMed] [Google Scholar]
- 68.Kim K, Weiss LM. 2004. Toxoplasma gondii: the model apicomplexan. Int. J. Parasitol 34(3):423–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klumperman J. 2000. Transport between ER and Golgi. Curr. Opin. Cell Biol 12(4):445–49 [DOI] [PubMed] [Google Scholar]
- 70.Koo J, Burrows LL, Howell PL. 2012. Decoding the roles of pilotins and accessory proteins in secretin escort services. FEMS Microbiol. Lett 328(1):1–12 [DOI] [PubMed] [Google Scholar]
- 71.Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, et al. 2013. An Overexpression Screen of Toxoplasma gondii Rab-GTPases Reveals Distinct Transport Routes to the Micronemes [DOI] [PMC free article] [PubMed]
- 72.Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, et al. 2005. The rhoptry neck protein RON4 relocalizes at the moving junction during Toxoplasma gondii invasion. Cell. Microbiol 7(12):1823–33 [DOI] [PubMed] [Google Scholar]
- 73.Lecordier L, Mercier C, Sibley LD, Cesbron-Delauw MF. 1999. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol. Biol. Cell 10(4):1277–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lentini G, Dubois DJ, Maco B, Soldati-Favre D, Frénal K. 2019. The roles of CEN2 and DLC8a in apical secretory organelles discharge of Toxoplasma gondii. Traffic [DOI] [PubMed]
- 75.Leriche MA, Dubremetz JF. 1990. Exocytosis of Toxoplasma gondii dense granules into the parasitophorous vacuole after host cell invasion. Parasitol. Res 76(7):559–62 [DOI] [PubMed] [Google Scholar]
- 76.Lodoen MB, Gerke C, Boothroyd JC. 2010. A highly sensitive FRET-based approach reveals secretion of the actin-binding protein toxofilin during Toxoplasma gondii infection. Cell. Microbiol 12(1):55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez J, Bittame A, Massera C, Vasseur V, Effantin G, et al. 2015. Intravacuolar Membranes Regulate CD8 T Cell Recognition of Membrane-Bound Toxoplasma gondii Protective Antigen. Cell Rep. 13(10):2273–86 [DOI] [PubMed] [Google Scholar]
- 78.Magno RC, Lemgruber L, Vommaro RC, De Souza W, Attias M. 2005. Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc. Res. Tech 67(1):45–52 [DOI] [PubMed] [Google Scholar]
- 79.Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, et al. 2014. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J. Exp. Med 211(10):2013–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marapana DS, Dagley LF, Sandow JJ, Nebl T, Triglia T, et al. 2018. Plasmepsin V cleaves malaria effector proteins in a distinct endoplasmic reticulum translocation interactome for export to the erythrocyte. Nat Microbiol. 3(9):1010–22 [DOI] [PubMed] [Google Scholar]
- 81.Marino ND, Panas MW, Franco M, Theisen TC, Naor A, et al. 2018. Identification of a novel protein complex essential for effector translocation across the parasitophorous vacuole membrane of Toxoplasma gondii. PLoS Pathog. 14(1):e1006828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matthews KM, Pitman EL, de Koning-Ward TF. 2019. Illuminating how malaria parasites export proteins into host erythrocytes. Cell. Microbiol 21(4):e13009 [DOI] [PubMed] [Google Scholar]
- 83.Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF. 2002. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol. Biol. Cell 13(7):2397–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller SA, Thathy V, Ajioka JW, Blackman MJ, Kim K. 2003. TgSUB2 is a Toxoplasma gondii rhoptry organelle processing proteinase. Mol. Microbiol 49(4):883–94 [DOI] [PubMed] [Google Scholar]
- 85.Mital J, Meissner M, Soldati D, Ward GE. 2005. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol. Biol. Cell 16(9):4341–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morlon-Guyot J, Berry L, Sauquet I, Singh Pall G, El Hajj H, et al. 2018. Conditional knock-down of a novel coccidian protein leads to the formation of aberrant apical organelles and abrogates mature rhoptry positioning in Toxoplasma gondii. Mol. Biochem. Parasitol 223:19–30 [DOI] [PubMed] [Google Scholar]
- 87.Morlon-Guyot J, Pastore S, Berry L, Lebrun M, Daher W. 2015. Toxoplasma gondii Vps11, a subunit of HOPS and CORVET tethering complexes, is essential for the biogenesis of secretory organelles. Cell. Microbiol 17(8):1157–78 [DOI] [PubMed] [Google Scholar]
- 88.Mueller C, Klages N, Jacot D, Santos JM, Cabrera A, et al. 2013. The Toxoplasma Protein ARO Mediates the Apical Positioning of Rhoptry Organelles, a Prerequisite for Host Cell Invasion [DOI] [PubMed]
- 89.Mueller C, Samoo A, Hammoudi P-M, Klages N, Kallio JP, et al. 2016. Structural and functional dissection of Toxoplasma gondii armadillo repeats only protein. J. Cell Sci 129(5):1031–45 [DOI] [PubMed] [Google Scholar]
- 90.Nadipuram SM, Kim EW, Vashisht AA, Lin AH, Bell HN, et al. 2016. In Vivo Biotinylation of the Toxoplasma Parasitophorous Vacuole Reveals Novel Dense Granule Proteins Important for Parasite Growth and Pathogenesis. MBio. 7(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naor A, Panas MW, Marino N, Coffey MJ, Tonkin CJ, Boothroyd JC. 2018. MYR1-Dependent Effectors Are the Major Drivers of a Host Cell’s Early Response to Toxoplasma, Including Counteracting MYR1-Independent Effects. MBio. 9(2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nolan SJ, Romano JD, Coppens I. 2017. Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii. PLoS Pathog. 13(6):e1006362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okuda S, Tokuda H. 2011. Lipoprotein sorting in bacteria. Annu. Rev. Microbiol 65:239–59 [DOI] [PubMed] [Google Scholar]
- 94.Olias P, Etheridge RD, Zhang Y, Holtzman MJ, Sibley LD. 2016. Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-gamma-Dependent Gene Expression. Cell Host Microbe. 20(1):72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Panas MW, Ferrel A, Naor A, Tenborg E, Lorenzi HA, Boothroyd JC. 2019. Translocation of Dense Granule Effectors across the Parasitophorous Vacuole Membrane in Toxoplasma-Infected Cells Requires the Activity of ROP17, a Rhoptry Protein Kinase. mSphere. 4(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panas MW, Naor A, Cygan AM, Boothroyd JC. 2019. Toxoplasma Controls Host Cyclin E Expression through the Use of a Novel MYR1-Dependent Effector Protein, HCE1. MBio. 10(2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parker KER, Fairweather SJ, Rajendran E, Blume M, McConville MJ, et al. 2019. The tyrosine transporter of Toxoplasma gondii is a member of the newly defined apicomplexan amino acid transporter (ApiAT) family. PLoS Pathog. 15(2):e1007577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavlou G, Biesaga M, Touquet B, Lagal V, Balland M, et al. 2018. Toxoplasma Parasite Twisting Motion Mechanically Induces Host Cell Membrane Fission to Complete Invasion within a Protective Vacuole. Cell Host Microbe. 24(1):81–96.e5 [DOI] [PubMed] [Google Scholar]
- 99.Pellegrini E, Palencia A, Braun L, Kapp U, Bougdour A, et al. 2017. Structural Basis for the Subversion of MAP Kinase Signaling by an Intrinsically Disordered Parasite Secreted Agonist. Structure. 25(1):16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pernas L, Adomako-Ankomah Y, Shastri AJ, Ewald SE, Treeck M, et al. 2014. Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol. 12(4):e1001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pernas L, Bean C, Boothroyd JC, Scorrano L. 2018. Mitochondria Restrict Growth of the Intracellular Parasite Toxoplasma gondii by Limiting Its Uptake of Fatty Acids. Cell Metab. 27(4):886–97 [DOI] [PubMed] [Google Scholar]
- 102.Phillips R, Ursell T, Wiggins P, Sens P. 2009. Emerging roles for lipids in shaping membrane-protein function. Nature. 459(7245):379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pieperhoff MS, Schmitt M, Ferguson DJP, Meissner M. 2013. The role of clathrin in post-Golgi trafficking in Toxoplasma gondii. PLoS One. 8(10):e77620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pszenny V, Ehrenman K, Romano JD, Kennard A, Schultz A, et al. 2016. A Lipolytic Lecithin:Cholesterol Acyltransferase Secreted by Toxoplasma Facilitates Parasite Replication and Egress. J. Biol. Chem 291(8):3725–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajendran E, Hapuarachchi SV, Miller CM, Fairweather SJ, Cai Y, et al. 2017. Cationic amino acid transporters play key roles in the survival and transmission of apicomplexan parasites. Nat. Commun 8:14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rastogi S, Cygan AM, Boothroyd JC. 2019. Translocation of effector proteins into host cells by Toxoplasma gondii. Curr. Opin. Microbiol 52:130–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reese ML, Boothroyd JC. 2009. A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole. Traffic. 10(10):1458–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romano JD, Coppens I. 2013. Host Organelle Hijackers: a similar modus operandi for Toxoplasma gondii and Chlamydia trachomatis: co-infection model as a tool to investigate pathogenesis. Pathog. Dis 69(2):72–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romano JD, Nolan SJ, Porter C, Ehrenman K, Hartman EJ, et al. 2017. The parasite Toxoplasma sequesters diverse Rab host vesicles within an intravacuolar network. J. Cell Biol 216(12):4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romano JD, Sonda S, Bergbower E, Smith ME, Coppens I. 2013. Toxoplasma gondii salvages sphingolipids from the host Golgi through the rerouting of selected Rab vesicles to the parasitophorous vacuole. Mol. Biol. Cell 24(12):1974–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, et al. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med 208(1):195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE. 2010. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 463(7281):632–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saeij JPJ, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 445(7125):324–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakura T, Sindikubwabo F, Oesterlin LK, Bousquet H, Slomianny C, et al. 2016. A Critical Role for Toxoplasma gondii Vacuolar Protein Sorting VPS9 in Secretory Organelle Biogenesis and Host Infection. Sci. Rep 6:38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sangaré LO, Alayi TD, Westermann B, Hovasse A, Sindikubwabo F, et al. 2016. Unconventional endosome-like compartment and retromer complex in Toxoplasma gondii govern parasite integrity and host infection. Nat. Commun 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sangaré LO, Ólafsson EB, Wang Y, Yang N, Julien L, et al. 2019. In Vivo CRISPR Screen Identifies TgWIP as a Toxoplasma Modulator of Dendritic Cell Migration. Cell Host Microbe. 26(4):478–92.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwab JC, Beckers CJ, Joiner KA. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U. S. A 91(2):509–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shastri AJ, Marino ND, Franco M, Lodoen MB, Boothroyd JC. 2014. GRA25 is a novel virulence factor of Toxoplasma gondii and influences the host immune response. Infect. Immun 82(6):2595–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sibley LD, Niesman IR, Asai T, Takeuchi T. 1994. Toxoplasma gondii: secretion of a potent nucleoside triphosphate hydrolase into the parasitophorous vacuole. Exp. Parasitol 79(3):301–11 [DOI] [PubMed] [Google Scholar]
- 120.Sidik SM, Huet D, Ganesan SM, Huynh M-H, Wang T, et al. 2016. A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell. 166(6):1423–35.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol 2(5):a000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sinai A, Webster P, Joiner K. 1997. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci 110:2117–28 [DOI] [PubMed] [Google Scholar]
- 123.Sloves P-J, Delhaye S, Mouveaux T, Werkmeister E, Slomianny C, et al. 2012. Toxoplasma Sortilin-like Receptor Regulates Protein Transport and Is Essential for Apical Secretory Organelle Biogenesis and Host Infection [DOI] [PubMed]
- 124.Stedman TT, Sussmann AR, Joiner KA. 2003. Toxoplasma gondii Rab6 mediates a retrograde pathway for sorting of constitutively secreted proteins to the Golgi complex. J. Biol. Chem 278(7):5433–43 [DOI] [PubMed] [Google Scholar]
- 125.Suarez C, Lentini G, Ramaswamy R, Maynadier M, Aquilini E, et al. 2019. A lipid-binding protein mediates rhoptry discharge and invasion in Plasmodium falciparum and Toxoplasma gondii parasites. Nat. Commun 10(1):4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suss-Toby E, Zimmerberg J, Ward GE. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. U. S. A 93(16):8413–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Venugopal K, Marion S. 2018. Secretory organelle trafficking in Toxoplasma gondii: A long story for a short travel. Int. J. Med. Microbiol 308(7):751–60 [DOI] [PubMed] [Google Scholar]
- 128.Walker ME, Hjort EE, Smith SS, Tripathi A, Hornick JE, et al. 2008. Toxoplasma gondii actively remodels the microtubule network in host cells. Microbes Infect. 10(14–15):1440–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y, Cirelli KM, Barros PDC, Sangare LO, Butty V, et al. 2019. Three Toxoplasma gondii Dense Granule Proteins Are Required for Induction of Lewis Rat Macrophage Pyroptosis. MBio. 10(1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Sangaré LO, Paredes-Santos TC, Krishnamurthy S, Hassan MA, et al. 2019. A genome-wide loss-of-function screen identifies Toxoplasma gondii genes that determine fitness in interferon gamma-activated murine macrophages. Work. Pap. [Google Scholar]
- 131.Weidner JM, Barragan A. 2014. Tightly regulated migratory subversion of immune cells promotes the dissemination of Toxoplasma gondii. Int. J. Parasitol 44(2):85–90 [DOI] [PubMed] [Google Scholar]