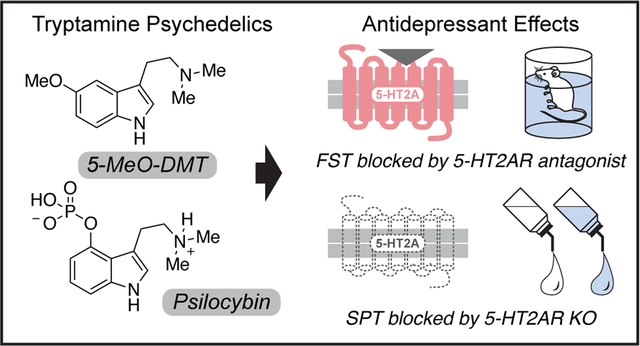
Abstract
Psychedelic compounds have displayed antidepressant potential in both humans and rodents. Despite their promise, psychedelics can induce undesired effects that pose safety concerns and limit their clinical scalability. The rational development of optimized psychedelic-related medicines will require a full mechanistic understanding of how these molecules produce therapeutic effects. While the hallucinogenic properties of psychedelics are generally attributed to activation of serotonin 2A receptors (5-HT2ARs), it is currently unclear if these receptors also mediate their antidepressant effects as several non-hallucinogenic analogs of psychedelics with antidepressant-like properties have been developed. Moreover, many psychedelics exhibit promiscuous pharmacology, making it challenging to identify their primary therapeutic target(s). Here, we use a combination of pharmacological and genetic tools to demonstrate that activation of 5-HT2A receptors is essential for tryptamine-based psychedelics to produce antidepressant-like effects in rodents. Our results suggest that psychedelic tryptamines can induce hallucinogenic and therapeutic effects through activation of the same receptor.
Keywords: psychedelic; psychoplastogen; neuroplasticity; structural plasticity; 5-HT2A receptor; psilocybin; 5-methoxy-N,N-dimethyltryptamine; TBG; depression
Graphical Abstract
Cortical atrophy and dysfunction are hallmarks of many neuropsychiatric diseases including depression, post-traumatic stress disorder (PTSD), and addiction.1,2,3 Psychoplastogens are small molecules with the potential to rapidly rescue these structural and functional deficits, leading to sustained therapeutic effects after a single administration.4 Classic psychedelics such as psilocybin, lysergic acid diethylamide (LSD), and 5-methoxy-N,N-dimethyltryptamine (5-MeO), are among the most potent and efficacious psychoplastogens known,5 and are increasingly being recognized for their potential to treat depression, anxiety disorders, and addiction.6,7 Recent data has indicated that their clinical8,9,10,11 and preclinical12,13,14 effects might be more robust than those of ketamine.
In addition to promoting the growth of neurons in the medial prefrontal cortex (mPFC),5,13,15 psychedelics produce profound subjective experiences.16 While the role of psychedelic-induced subjective effects in therapeutic outcomes is still highly debated,17,18 the importance of structural plasticity has been appreciated as a hallmark of antidepressant efficacy for many years.1,7,19,20 The majority, if not all, antidepressant interventions promote the growth of key neurons in the mPFC.1,7,19,20 A single dose of ketamine has been shown to induce spinogenesis in the mPFC,21 and Liston and co-workers demonstrated that this is required for the long-lasting antidepressant-like behavioral effects in rodents.22 Both ketamine and serotonergic psychedelics promote neuroplasticity by activating the mammalian target of rapamycin (mTOR), though their pharmacological targets are distinct.23,24,25,26,27 Recent evidence suggests that the psychoplastogenic effects of psychedelics are more enduring than those of ketamine,13 emphasizing the need to better understand their mechanisms of action.
There is strong consensus that serotonin 2A receptors (5-HT2ARs) mediate the hallucinogenic effects of psychedelics,28 as the 5-HT2R antagonist ketanserin (KETSN) blocks the subjective effects of both psilocybin and LSD in humans,29,30,31 and the head-twitch response (HTR)—a behavioral proxy of hallucinations in mice32—is absent when psychedelics are administered to 5-HT2AR knockout (KO) mice.33 However, it is currently unclear what role 5-HT2ARs play in the mechanisms by which serotonergic psychoplastogens produce long-lasting changes in neuronal structure and behavior—information necessary to engineer optimized neurotherapeutics related to psychedelics.
Several pieces of evidence suggest that 5-HT2ARs might be important for the therapeutic effects of psychedelics, but conflicting reports have prevented researchers from drawing any firm conclusions. While ketanserin and other 5-HT2R antagonists have been shown to antagonize the psychoplastogenic effects of psychedelics in vitro5 and eliminate their therapeutic effects in vivo,27,34,35 the selectivity of these agents is suboptimal.36 Moreover, two recent in vivo studies could not completely block the psychoplastogenic and antidepressant-like effects of psychedelics with ketanserin,13,37 though it is unclear if those studies employed doses sufficient to fully occupy the receptor. Challenges to the in vivo relevance of 5-HT2ARs are bolstered by the fact that psychedelics exhibit rich polypharmacology and activate several receptors hypothesized to promote plasticity and/or produce antidepressant effects (e.g., 5-HT1A, 5-HT1B, 5-HT2C, 5-HT6, 5-HT7, etc.). Additionally, our group and others have recently identified non-hallucinogenic analogues of psychedelics with therapeutic properties,34,38,39,40,41 calling into question the importance of 5-HT2AR activation in the therapeutic effects of serotonergic psychoplastogens. Here, we use a combination of pharmacological and genetic tools to demonstrate that activation of 5-HT2ARs is essential for the sustained therapeutic behavioral effects of psychedelics from the tryptamine family.
We chose to use 5-methoxy-N,N-dimethyltryptamine (5-MeO) for our initial studies due to its pharmacokinetic properties. When administered via intraperitoneal injection, 5-MeO is rapidly cleared from the body with a half-life of approximately 12 mins,42 making pharmacological blocking studies quite feasible. While we had previously reported that 5-MeO can increase dendritic arbor complexity in immature cortical cultures, the effects of 5-MeO on more mature neurons was not known.38 Therefore, we first confirmed that 5-MeO can promote spinogenesis like the related tryptamines N,N-dimethyltryptamine (DMT) and psilocin.5,13 When compared head-to-head with ketamine, 5-MeO produced comparable effects on dendritic spine density in mature (DIV21) cultures of cortical neurons (Figure 1A–C). Pretreatment with the 5-HT2 antagonist ketanserin prevented 5-MeO from promoting dendritic spine growth (Figure 1D).
Figure. 1. 5-MeO promotes cortical spine growth.

(A) Schematic depicting the experimental design for spinogenesis studies. (B) Rat embryonic cortical cultures were treated on DIV21 with compounds (10 μM), and dendritic spine density was assessed 24 h later. Representative images are shown. (C) Quantification of dendritic spine density demonstrated that both 5-MeO and KET increased spine density compared to the VEH control. (D) The effect of 5-MeO (1 μM) on spinogenesis was blocked by ketanserin (10 μM, 15 min pretreatment). VEH = vehicle; KET = ketamine; 5-MeO = 5-methoxy-N,N-dimethyltryptamine, KETSN = ketanserin; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to VEH controls, one-way ANOVA with Dunnett’s post hoc test.
For our in vivo studies, we chose to use a 10 mg/kg dose of 5-MeO, as this has been shown to be the lowest dose that reliably produces a HTR.38,43 To assess the antidepressant effects of 5-MeO, we utilized a version of the forced swim test (FST) in mice that has proven particularly effective for assessing the rapid and sustained effects of psychoplastogens (Figure 2A).39,40 On Day 1, male mice were subjected to a FST to provide some stress and induce a depressive phenotype. On Day 2, mice were first pretreated with either ketanserin (4 mg/kg, IP) or vehicle (saline, IP). After 10 mins, they were administered a dose of 5-MeO (10 mg/kg, IP) or vehicle and head-twitch behavior (Figure 2B) and locomotor activity (Figure 2C) were immediately assessed.
Figure. 2. 5-MeO produces an antidepressant-like effect through activation of 5-HT2 receptors.
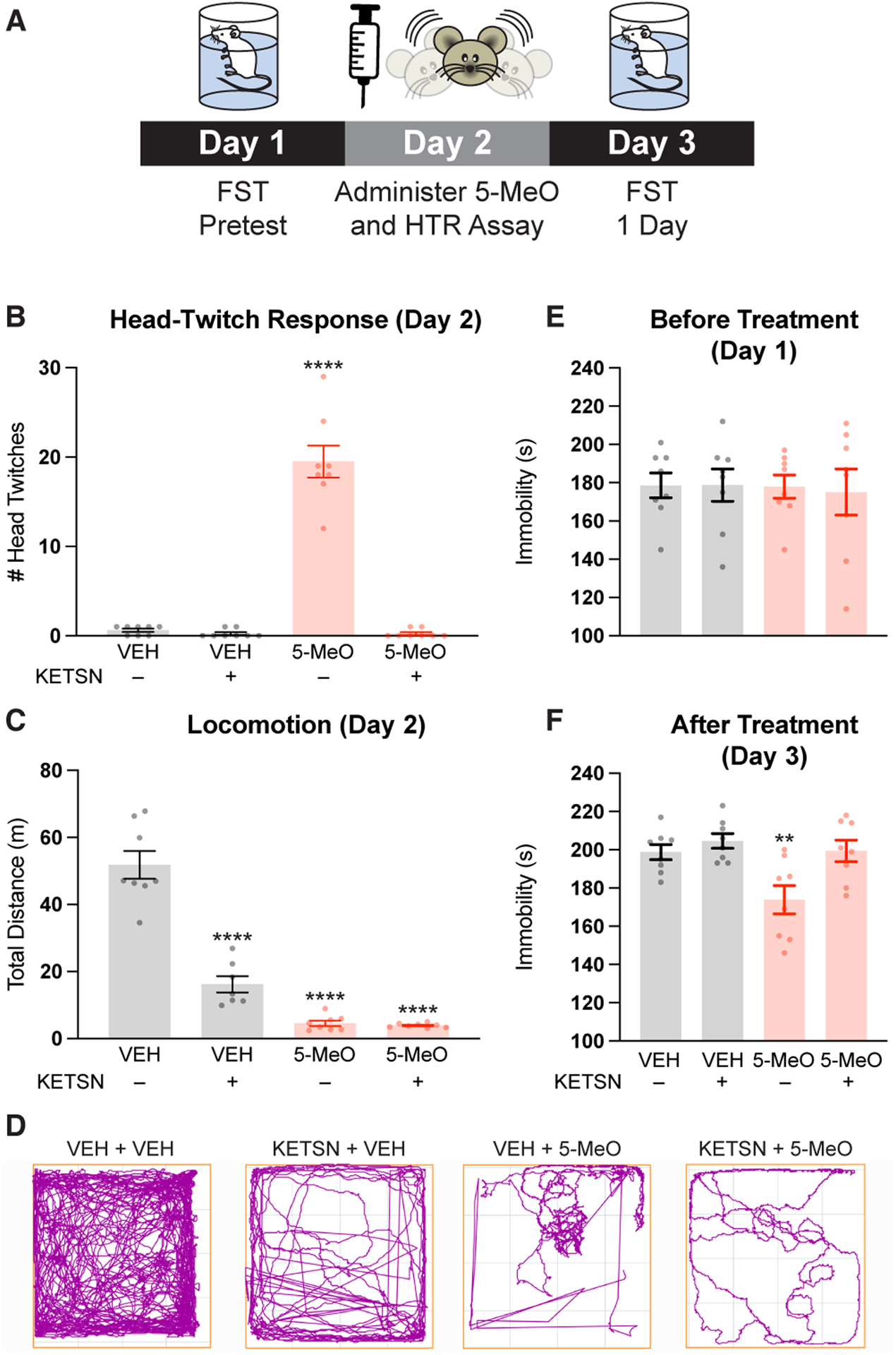
(A) Schematic of the FST design. (B–D) Male mice were administered VEH or KETSN (4 mg/kg, IP) 10 min prior to administration of 5-MeO (10 mg/kg, IP). Ketanserin completely blocked the HTR induced by 5-MeO (B), but did not block 5-MeO-induced hypolocomotion (C and D). (E) A pretest demonstrated that immobility in the FST was the same for all groups prior to compound administration. (F) Pretreatment with KETSN (4 mg/kg, IP) blocks the antidepressant-like effect of 5-MeO-DMT (10 mg/kg, IP) in the FST. 5-MeO = 5-methoxy-N,N-dimethyltryptamine; VEH = vehicle, KETSN = ketanserin. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to VEH – KETSN controls, one-way ANOVA with Dunnett’s post hoc test.
Pretreatment with KETSN completely blocked the HTR induced by 5-MeO (Figure 2B). Interestingly, both KETSN and 5-MeO reduced locomotion (Figure 2C and D) as compared to vehicle treated controls. Though no differences in FST behavior were observed between groups prior to treatment (Figure 2E), 5-MeO produced a robust antidepressant response 24 h following administration (Figure 2F). Moreover, this response was completely blocked by pretreatment with KETSN, while KETSN alone had no impact on FST behavior (Figure 2F). We reasoned that initial reports claiming that KETSN cannot completely block the antidepressant-like effects of psilocybin13,37 might be due to pharmacokinetic considerations related to the longer pretreatment interval (1 h vs 10 min) and/or partial receptor occupancy (low vs high dose KETSN). In fact, a 1 mg/kg dose of KETSN administered to rats only blocks ~30% of cortical 5-HT2ARs.44 As expected, pretreatment of mice with a higher dose of KETSN (4 mg/kg) just 10 min prior to administration of 5-MeO completely blocked both the acute HTR and the sustained antidepressant-like effects of 5-MeO in the FST measured 24 h later.
We previously demonstrated that a single dose of DMT (10 mg/kg, IP) produces antidepressant effects in the FST,14 increases dendritic spine density in vivo, and increases both the frequency and amplitude of spontaneous excitatory postsynaptic currents measured ex vivo.5 Unpublished results from our lab and others45 suggests that 5-MeO produces similar effects. Therefore, we were interested to determine if a 10 mg/kg dose of 5-MeO would alter the intrinsic excitability of cortical neurons. One day following administration of 5-MeO to male and female mice, we sacrificed the animals and measured the electrical properties of cortical neurons via ex vivo electrophysiology. The resting membrane potential of layer 5 neurons was slightly more depolarized 24 h after 5-MeO administration, but there were otherwise no significant changes in intrinsic excitability (Figure 3 and Table 1).
Figure 3. Intrinsic excitability is not impacted by a single administration of 5-MeO.
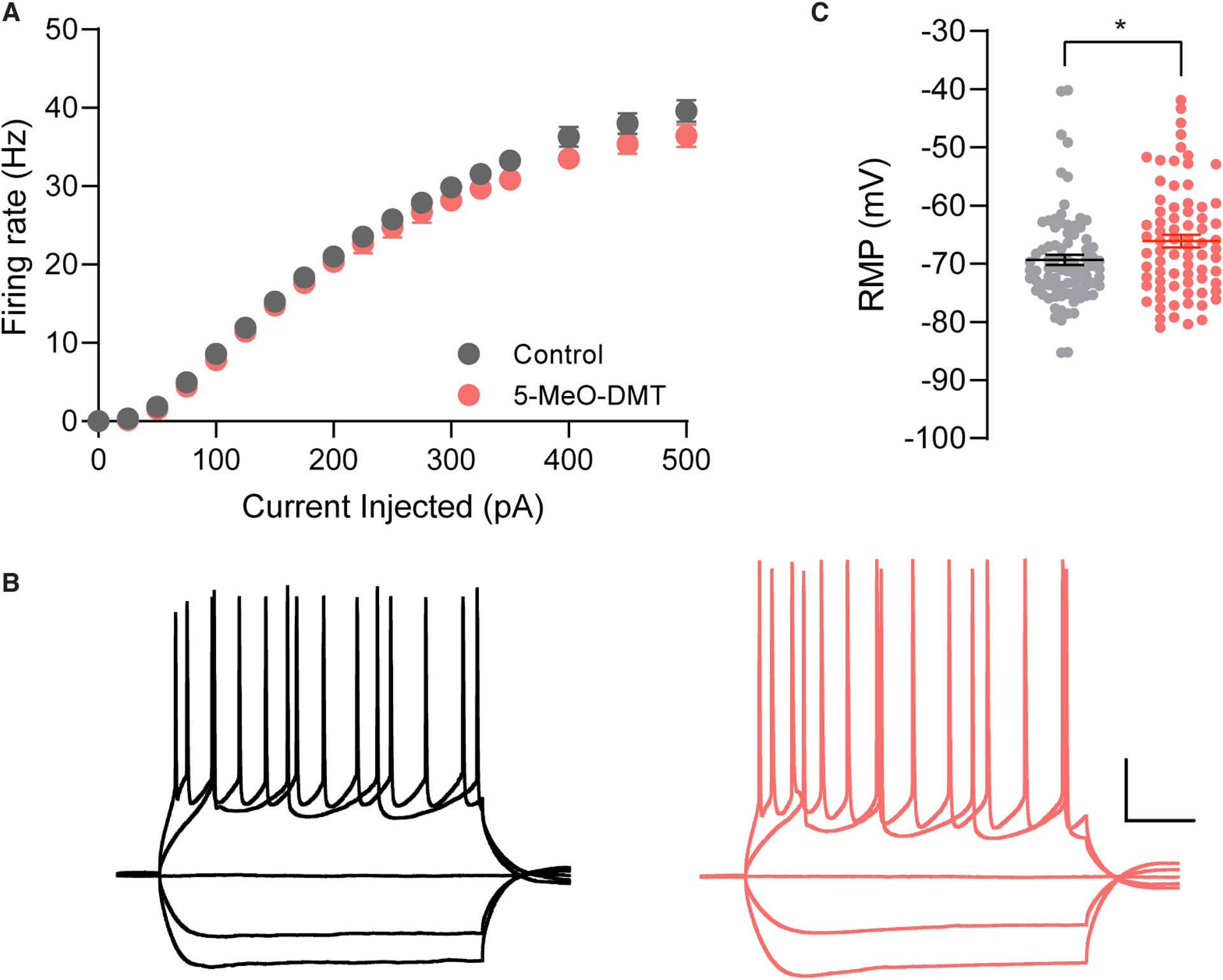
(A) Layer 5 mPFC pyramidal neurons from animals administered a single dose of 5-MeO (10 mg/kg, IP) 24 h prior to tissue collection showed no significant difference in action potential firing rates in response to depolarizing current injection (n = 75 neurons, 6 animals per group) compared to neurons from animals that were given saline (n = 87 neurons, 6 animals per group). (B) Sample traces of neurons from animals treated with vehicle (black) and 5-MeO (red) in response to +200, +100, 0, −100, and −200 pA current injections (scale bars: 200 ms, 20 mV). (C) Resting membrane potential (RMP) was significantly more depolarized in animals treated with 5-MeO (p = 0.020), but all other intrinsic cellular properties were largely unchanged by the drug treatment. Data represent mean ± SEM. *p < 0.05, Student’s t-test.
Table 1.
Intrinsic Excitability of Layer 5 Pyramidal Neurons in the mPFC
| Property | Vehicle (N = 85) | 5-MeO (N = 75) | Student’s t-test | P Value |
|---|---|---|---|---|
| RMP (mV) | −69.33 ± 0.87 [−85 to −40] |
−66.09 ± 1.09 [−81 to −42] |
t(158)=2.35 | 0.020* |
| Rinput (MΩ) | 206.4 ± 7.52 [80.1 to 384.6] |
205.6 ± 6.86 [102.0 to 391.7] |
t(158)=0.07 | 0.942 |
| Sag (mV)A | −0.059 ± 0.004 [−0.002 to −0.164] |
−0.055 ± 0.005 [−0.012 to −0.188] |
t(158)=0.63 | 0.531 |
| Rheobase (pA) | 94.1 ± 8.1 [3.3 to 380] |
75.6 ± 4.1 [18.7 to 212] |
t(158)=1.94 | 0.054 |
| AP Threshold (mV)A | −57.5 ± 0.9 [−76.9 to −26.5] |
−54.7 ± 1.1 [−69.6 to −31.9] |
t(158)=1.97 | 0.051 |
| AP Height (mV) | 133.1 ± 1.8 [91.2 to 182] |
120.9 ± 1.7 [86.0 to 171] |
t(158)=0.72 | 0.474 |
| AP half-width (ms) | 1.65 ± 0.06 [0.93 to 3.17] |
1.57 ± 0.06 [0.84 to 3.31] |
t(158)=0.88 | 0.381 |
| AHP Peak (mV) | −3.02 ± 0.18 [−6.73 to −0.188] |
−2.59 ± 0.17 [−6.57 to −0.001] |
t(158)=1.70 | 0.090 |
Mean ± SEM [range]
Significant at p < 0.05
junction potential not adjusted
Next, we were interested in determining if the antidepressant effects of 5-MeO were as rapid as those induced by ketamine. Previous work demonstrated that ketamine produces both rapid (<24 h) and sustained (>24 h) antidepressant effects in mice, though only the latter requires spinogenesis in the mPFC.22 To assess both rapid and sustained effects in the FST, we modified our experimental design slightly (Figure 4A) and tested 5-MeO and ketamine head-to-head in male and female mice. As before, we performed a pretest on Day 1, with all groups demonstrating comparable levels of immobility. In contrast to our previous FST experiment, we assessed immobility 30 mins and 7 days after compound administration. Unlike ketamine, 5-MeO did not induce a rapid antidepressant-like effect within 30 mins (Figure 4B). However, it did produce a sustained antidepressant effect that was evident 7 days after treatment (Figure 4B), which is consistent with the time course for psychoplastogen-induced spinogenesis.22,46 Effects in male and female mice were similar (Figure S1).
Figure 4. 5-MeO produces sustained, but not rapid, antidepressant-like effects in the FST.
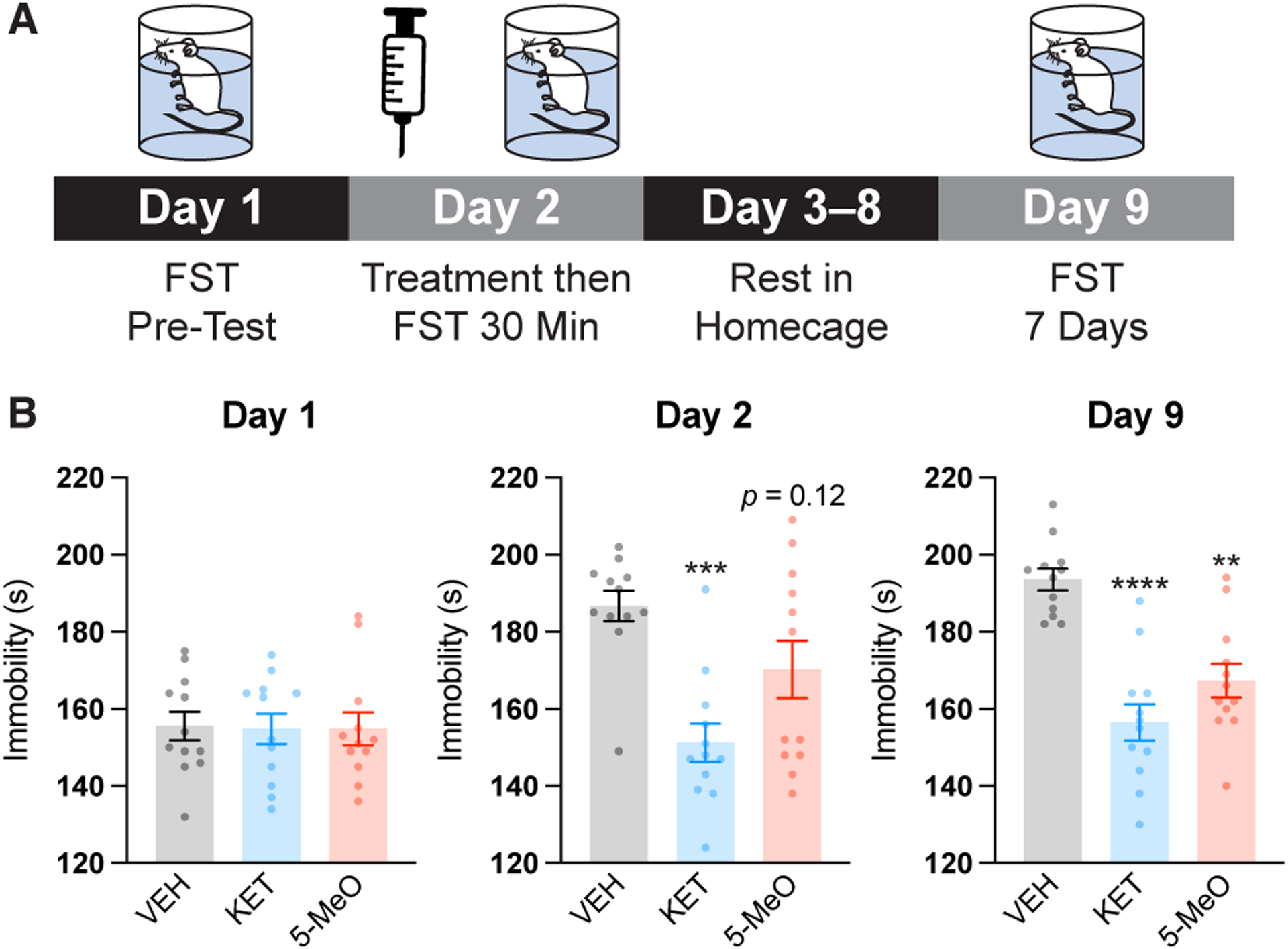
(A) Schematic depicting the experimental design for FST studies. A pre-test was performed on Day 1. On Day 2, compounds were administered to male and female mice (5-MeO, 10 mg/kg, IP; KET, 3 mg/kg, IP) and a FST was performed 30 mins later. The animals were returned to their home cages for 1 week before another FST was performed. (B) The treatment groups did not exhibit any differences during the pre-test. Ketamine produced a rapid antidepressant-like effect within 30 mins of administration, and both ketamine and 5-MeO decreased immobility 7 days after treatment. VEH = vehicle; KET = ketamine; 5-MeO = 5-methoxy-N,N-dimethyltryptamine. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to VEH controls, one-way ANOVA with Dunnett’s post hoc test.
While KETSN is a useful pharmacological tool, it is known to target several receptors in addition to 5-HT2ARs (e.g., VMAT2, 5-HT2C, H1, and α1 receptors).36 Unfortunately, confounding effects prevent the use of the more selective 5-HT2AR antagonist volinanserin (MDL 100907) from being used as a pharmacological tool to block antidepressant-like effects. Unlike ketanserin, volinanserin stimulates cortical neuron growth in culture (Olson Lab, unpublished results) and is known to impact behaviors relevant to depression.41 Therefore, genetic tools are essential to fully elucidate the role of 5-HT2ARs in the neurobiological effects of psychedelics. We were inspired by recent reports demonstrating that 2,5-dimethoxy-4-iodoamphetamine (DOI) cannot promote spine growth in the mPFC,47 increase the expression of plasticity-related genes,48 facilitate fear extinction learning, or reduce fear expression49 when administered to 5-HT2AR KO mice.47,48,49 As DOI has an amphetamine substructure, it was unclear if these results would extend to psychedelics of the tryptamine family given that this chemical scaffold exhibits more promiscuous pharmacology. Moreover, it was unclear if the long-lasting antidepressant-like effects of psychedelics also require 5-HT2AR activation. Thus, we were interested in assessing the antidepressant-like effects of tryptamine psychedelics using 5-HT2AR KO mice.
Though the FST has good predictive validity for antidepressant efficacy, there is debate over its use in antidepressant drug discovery.50 In fact, genetic background can have a dramatic impact on FST behavior, and we have found that wild type 129S6/SvEv mice do not respond to ketamine or 5-MeO in the FST (Figure S2). As this is the genetic background of our 5-HT2A KO line, we opted to subject these animals to an alternative test assessing anhedonia—a key aspect of depression that psilocybin has been shown to ameliorate.37 A previous report was unable to block the antidepressant effects of psilocybin in the sucrose preference test (SPT) or the female urine sniffing test using KETSN.37 Given the challenges associated with matching pharmacokinetics to achieve optimal receptor occupancy during blocking studies in vivo, we hypothesized that the antidepressant effects of psilocybin might be absent in 5-HT2A KO animals.
After performing a baseline SPT, we induced a depressive state by administering corticosterone (CORT) chronically over 10 days (Figure 5A). At the end of this 10-day period, both WT and 5-HT2AR KO mice exhibited reduced preferences for sucrose as compared to the baseline test conducted prior to CORT administration (Figure 5B). Next, we administered a single dose of psilocybin (10 mg/kg, IP), waited 24 h, and then assessed sucrose preference. A single dose of psilocybin rescued CORT-induced anhedonia in WT mice, but this antidepressant-like effect was absent in 5-HT2AR KO mice (Figure 5C).
Figure 5. Psilocybin rescues CORT-induced anhedonia through activation of 5-HT2ARs.

(A) Schematic depicting the experimental design. A SPT was performed on Day 1 followed by 10 days of chronic CORT administration. A second SPT was performed on Day 12 prior to administration of VEH or PSY (10 mg/kg, IP) on Day 13. On day 14, a third SPT was performed. (B) Chronic administration of CORT induced anhedonia in both WT and 5-HT2AR KO mice. (C) A single administration of PSY rescued CORT-induced anhedonia in WT, but not 5-HT2AR KO mice. Administration of VEH had no effect in either genotype. VEH = vehicle; PSY = psilocybin; WT = wild type. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Student’s t-test (paired).
Here, we used a combination of rapidly cleared psychedelics, a traditional 5-HT2 antagonist, and 5-HT2AR KO mice to clearly demonstrate that 5-HT2R activation is necessary for tryptamine psychedelics to produce some long-lasting antidepressant-like effects in assays such as the FST and SPT. However, other antidepressant-like effects may result from the activation of other receptors depending on the specific compound employed. Whether 5-HT2AR activation also mediates other aspects of their therapeutic properties (e.g., anti-addictive effects) remains an open question.
While psychedelics show promise for treating certain patient populations, their hallucinogenic effects drastically limit their clinical scalability.7,18 The advent of non-hallucinogenic analogues of psychedelics with therapeutic properties38,39,40,34,41 has opened new opportunities for developing scalable alternatives that solve this issue. However, to engineer optimized compounds, we need to fully understand their mechanistic underpinnings. Currently, it is unclear if non-hallucinogenic analogues of psychedelics produce their psychoplastogenic and antidepressant-like effects through the classic psychedelic receptor, or if they simply exhibit weak affinity/efficacy at 5-HT2ARs while targeting a different neuroreceptor. Understanding the molecular and circuit-level mechanisms by which psychedelics and their non-hallucinogenic congeners produce sustained therapeutic effects will be essential to develop scalable treatments tailored to specific disease indications.
METHODS
Data Analysis and Statistics.
Treatments were randomized, and data were analyzed by experimenters blinded to treatment conditions. Statistical analyses were performed using GraphPad Prism (version 9.1.2) unless noted otherwise. All comparisons were planned prior to performing each experiment. Data are represented as mean ± SEM, unless noted otherwise, with asterisks indicating *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Drugs.
Many of the drugs used in these studies were purchased from commercial sources including corticosterone (Spectrum, C1487), ketamine hydrochloride (KET, Spectrum, K1068), and ketanserin tartrate (KETSN, Alfa Aesar, J62798). The hemifumarate salt of 5-methoxy-N,N-dimethyltryptamine (5-MeO) was synthesized as described previously.38 Psilocybin (PSY) was synthesized according to the procedure outlined in the supporting information. KET, KETSN, 5-MeO and PSY all dissolved easily in saline prior to administration. All compounds synthesized in-house were judged to be analytically pure based on NMR and LC-MS data. For cell culture experiments, VEH = 0.1% biology grade dimethyl sulfoxide (Sigma-Aldrich). For in vivo experiments, VEH = USP grade saline (0.9%).
Animals.
All experimental procedures involving animals were approved by the University of California, Davis (UC Davis) Institutional Animal Care and Use Committee (IACUC) and adhered to principles described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were either obtained from Jackson Laboratory (Sacramento, CA) or bred in house unless noted otherwise. Power analyses were conducted to ensure appropriate sample size for all experiments involving animals. Animals were housed 2–5 animals of the same sex per cage and were given ad libitum access to food and water unless noted otherwise. Lights in the vivarium were turned on at 07:00 hours and turned off at 19:00 hours. UC Davis is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Spinogenesis Assays Using Cultured Rat Cortical Neurons.
Spinogenesis experiments were performed as previously described5 with the exception that cells were treated on DIV20 and fixed 24 h after treatment on DIV21. The images were taken on a Nikon HCA Confocal microscope a with a 100x/NA 1.45 oil objective. DMSO and ketamine (10 μM) were used as vehicle and positive controls, respectively.
Forced Swim Test (FST).
Mice (C57/BL6J, 9–10 weeks of age at time of experiment) were obtained from the Jackson Lab and housed in a UC Davis vivarium. After 1 week of habituation to the vivarium each mouse was handled for approximately 1 minute by an experimenter for 3 consecutive days leading up to the first FST. All experiments were carried out by the same experimenter who performed the handling. During the FST, mice underwent a 6 min swim session in a clear Plexiglas cylinder 40 cm tall, 20 cm in diameter, and filled with 30 cm of 24 ± 1°C water. Fresh water was used for every mouse. After habituation to the experimenter, drug-naïve mice first underwent a pre-test swim to induce a depressive phenotype. Immobility scores for all mice were determined after the pre-test and mice were randomly assigned to treatment groups to generate groups with similar average immobility scores to be used for the following FST sessions. The next day, mice received IP injections of drug and FST sessions were conducted at varying time points (i.e., 30 min, 24 h, 7 d) depending on the specific experiment. Mice were dried and returned to their home cages between FST sessions. All FST sessions were performed between the hours of 08:00 and 13:00. Experiments were video-recorded and manually scored offline by an experimenter blinded to treatment conditions. Immobility time was defined as passive floating or remaining motionless with no activity other than that needed to keep the mouse’s head above water and was scored for the last 4 min of each 6 min trial. For the experiment described in Figure 2, KETSN (4 mg/kg) or VEH was administered to male mice 10 min prior to receiving IP injections of 5-MeO (10 mg/kg) or VEH (saline). For the experiment described in Figure 4, 5-MeO (10 mg/kg), ketamine (3 mg/kg), or VEH (saline) was administered to both male and female mice 30 min prior to a FST. An additional FST was performed 7 d later. The experimenter who performed the tests was male.
Head-Twitch Response (HTR) and Locomotion.
The head-twitch response assay was performed as described previously.38 Male mice were obtained from Jackson Laboratory (Sacramento, CA) and were approximately 8 weeks old at the time of the experiments. Compounds were administered via IP injection (5 mL/kg) using 0.9% saline as the vehicle. Behavior was video recorded for 20 mins immediately after injection and locomotion was quantified using ANYmaze Video Tracking System, version 7.07 (Stoelting Co.). Head-twitch response behavior was scored by two blinded observers, and the results were averaged (Pearson correlation coefficient = 0.93). For blocking studies investigating the role of the 5HT2AR in the effects of 5-MeO, male mice were injected with KETSN (4 mg/kg) or VEH (saline) 10 min before 5-MeO (10 mg/kg) or VEH (saline). Video recording was performed for 20 mins after the second set of injections.
Electrophysiology (Intrinsic Excitability).
Male and female C57BL6J mice (~8 weeks old) were given an intraperitoneal injection of 5-MeO (10 mg/kg) or vehicle (saline). After 24 h, mice were anesthetized with isoflurane and transcardially perfused with ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1 NaH2PO4, 2.5 CaCl2 and 1.3 MgSO4 (Sigma-Aldrich). Brains were rapidly removed and 300 μm coronal mPFC slices were cut on a Leica VT1200 vibratome (Buffalo Grove, IL) with ice-cold ACSF solution. Slices were incubated in 32°C NMDG solution containing the following (in mM): 93 NMDG, 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, and 0.5 CaCl251 for 10 minutes, then transferred to room temperature ACSF, and held for at least 45 minutes before recording. All solutions were vigorously perfused with 95% O2/5% CO2. Whole-cell recordings were obtained from Layer 5 pyramidal mPFC neurons under visual guidance (DIC/infrared optics). For all whole-cell current clamp recordings, borosilicate glass pipettes were fabricated with resistances of 4–6 MΩ. Pipettes were filled with the following intracellular solution (in mM): 135 K+ gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 NaATP, 0.4 NaGTP, pH = 7.3, 290 mOsm. Series resistance was monitored throughout and cells in which series resistance varied by more than 20% during a recording were discarded. Recordings were collected with a MultiClamp 700B amplifier (Molecular Devices), filtered at 2 kHz, digitized at 10 kHz, and data analyzed using pClamp 10 software (Molecular Devices). Frequency-current relationships for evoked firing were determined by injecting 500 ms current steps with amplitudes increasing by 25 pA, from 0 to 500 pA from the resting potential. Current injections were recorded in increasing order three times per cell. Values obtained from the responses elicited by the same current injection were averaged. For input resistance, 500 ms current steps of 0 to −200 pA were injected in −20 pA increments. Steady-state responses were measured as the average change in voltage in the last 100 ms of the pulse. Sag currents were measured during the 100 pA hyperpolarizing steps and calculated as the initial voltage trough minus the steady-state voltage change. Firing frequency versus injected current was measured as the number of spikes per 500 ms step in 25 pA increments from 0 to 200 pA. Rheobase was determined by injecting 0.5 ms square pulses in 2 pA steps and recording the strength of the first pulse to elicit an action potential. AP threshold, AP height, AP half-width, and peak AHP were calculated by injecting a 2 ms square pulse of 1.8 nA. Liquid junction potentials were not corrected. Each treatment group had 3 male and 3 female animals; no significant difference between sexes was observed so the data were combined.
Sucrose Preference Test (SPT).
Male and female wild type and 5-HT2A KO mice (129S6/SvEv background), at ~13–20 weeks of age were given 1 week to acclimate to the new vivarium, then transferred to new cages (individually housed). Once individually housed, the mice were given one bottle of water and one bottle of 1% sucrose for 24 h to assess baseline sucrose preference. Animals that did not exhibit a baseline sucrose preference (i.e., animals that drank more water than sucrose solution) were removed from the experiment. After the baseline SPT, animals were group housed and given daily IP injections of 20 mg/kg of corticosterone (CORT) in DMSO (2 mL/kg) for 10 days. After 10 days of CORT treatment, animals were singly housed and given access to a bottle of 1% sucrose and a bottle of water for 24 h to assess sucrose preference (Post-CORT). The results from this SPT were used to randomly assign treatment groups with comparable Post-CORT sucrose preferences. Next, animals were administered VEH (saline) or psilocybin (10 mg/kg) via IP injection (5 mL/kg). A SPT was performed 24 h after treatment (Post-Drug). Sucrose preference was calculated as the amount of sucrose solution consumed minus the amount of water consumed, divided by the total amount of liquid consumed. Data points were omitted if bottles leaked during the test.
Supplementary Material
Acknowledgement
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997 to DEO), two NIH training grants (T32GM099608 to MV and EVB; T32MH112507 to LPC, and HNS), the Camille and Henry Dreyfus Foundation (DEO), a sponsored research agreement with Delix Therapeutics (DEO), and a UC Davis Provost’s Undergraduate Fellowship (SDP). The Olympus FV1000 confocal used in this study was purchased using NIH Shared Instrumentation Grant 1S10RR019266-01. We thank the MCB Light Microscopy Imaging Facility, which is a UC Davis Campus Core Research Facility, for the use of this microscope. We thank Javier Gonzalez-Maeso for providing 5-HT2AR KO animals and Calvin Ly for performing preliminary plasticity studies with volinanserin.
Footnotes
Supporting Information
methods for synthesizing psilocybin, 1H and 13C NMR spectra, LC-MS chromatogram for psilocybin
Disclosure
DEO is a co-founder of Delix Therapeutics, Inc., serves as the Chief Innovation Officer and Head of the Scientific Advisory Board, and has sponsored research agreements with Delix Therapeutics.
REFERENCES
- 1.Duman RS, Aghajanian GK, Sanacora G & Krystal JH Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22, 238–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnsten AF, Raskind MA, Taylor FB & Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol. Stress 1, 89–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein RZ & Volkow ND Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12, 652–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson DE Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci 12, 1–4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ly C et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 23, 3170–3182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inserra A, De Gregorio D & Gobbi G Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol Rev 73, 202–277 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Vargas MV, Meyer R, Avanes AA, Rus M & Olson DE Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry 12, 727117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis AK et al. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psych 78, 481–489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carhart-Harris R et al. Trial of Psilocybin versus Escitalopram for Depression. New Eng. J. Med 384, 1402–1411 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Carhart-Harris R et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharm 235, 399–408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin GM; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression, N. Eng. J. Med, 387, 1637–1648 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Hibicke M, Landry AN, Kramer HM, Talman ZK & Nichols CD Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci 11, 864–871 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Shao LX et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron LP, Benson CJ, Dunlap LE & Olson DE Effects of N,N-dimethyltryptamine (DMT) on rat behaviors relevant to anxiety and depression. ACS Chem. Neurosci 9, 1582–1590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleksandrova LR & Phillips AG Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci 42, 929–942 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Griffiths RR, Richards WA, Johnson MW, McCann UD & Jesse R Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol, 22, 621–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaden DB & Griffiths RR The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci 4, 568–572 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson DE The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci 4, 563–567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castrén E & Monteggia LM Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 90, 128–136 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Castrén E & Antila H Neuronal plasticity and neurotrophic factors in drug responses. Molecular Psychiatry 22, 1085–1095 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phoumthipphavong V, Barthas F, Hassett S, & Kwan AC. Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal cortex. eNeuro 3, e0133–15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moda-Sava RN et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364, eaat8078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ly C et al. Stimulation with Psychoplastogens is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci 4, 452–460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson DE Biochemical Mechanisms Underlying Psychedelic-Induced Neuroplasticity. Biochemistry 61, 127–136 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadriu B et al. Ketamine and Serotonergic Psychedelics: Common Mechanisms Underlying the Effects of Rapid-Acting Antidepressants. Int. J. Neuropsychopharmacol 24, 8–21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savalia NK, Shao LX & Kwan AC A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci 44, 260–275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Gregorio D et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc. Natl. Acad. Sci 118, e2020705118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwan AC; Olson DE; Preller KH; Rother BL The Neural Basis of Psychedelic Action. Nat. Neurosci, 25, 1407–1419 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H & Hell D Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Preller KH et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife 7, e35082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holze F et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacol 46, 537–544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halberstadt AL, Chatha M, Klein AK, Wallach J & Brandt SD Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Maeso J et al. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 53, 439–452 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Cao D et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375, 403–411 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Serra YA et al. Role of 5-HT2A receptors in the effects of ayahuasca on ethanol self-administration using a two-bottle choice paradigm in male mice. Psychopharmacol 239, 1679–1687 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casey AB, Cui M, Booth RG & Canal CE “Selective” serotonin 5-HT2A receptor antagonists. Biochem. Pharmacol 200, 115028 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hesselgrave N, Troppoli TA, Wulff AB, Cole AB & Thompson SM Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad. Sci 118, e2022489118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunlap LE et al. Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogs Through Structure-Activity Relationship Studies. J. Med. Chem, 63, 1142–1155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cameron LP et al. A Non-Hallucinogenic Psychedelic Analogue with Therapeutic Potential. Nature 589, 474–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong C et al. Psychedelic-Inspired Drug Discovery Using an Engineered Biosensor. Cell, 10, 2779–2792.e18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan AL; et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature, 610, 582–591 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen H-W, Jiang X-L, Winter JC & Yu A-M Psychedelic 5-Methoxy-N,N-dimethyltryptamine: Metabolism, Pharmacokinetics, Drug Interactions, and Pharmacological Actions. Curr. Drug Metab 11, 659–666 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halberstadt AL; Koedood L; Powell SB; Geyer MA Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol, 25, 1548–1561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrett RJ, & Sanders-Bush E Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor. J. Pharmacol. Exp. Ther 275, 1050–1057 (1995) [PubMed] [Google Scholar]
- 45.Jefferson SJ, Gregg I, Dibbs M, Liao C, Wu H, Davoudian PA, Sprouse JS, Sherwood AM, Kaye AP, Pittenger C, & Kwan AC 5-MeO-DMT modifies innate behaviors and promotes structural neural plasticity in mice. bioRxiv, (2022). 10.1101/2022.11.03.515044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ly C; Greb CA; Vargas MV; Duim WC; Grodzki ACG; Lein PJ; Olson DE Transient Stimulation with Psychoplastogens is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci, 4, 452–460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de la Fuente Revenga M et al. Prolonged epigenetic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep 37, 109836 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desouza LA et al. The Hallucinogenic Serotonin2A Receptor Agonist, 2,5-Dimethoxy-4-Iodoamphetamine, Promotes cAMP Response Element Binding Protein-Dependent Gene Expression of Specific Plasticity-Associated Genes in the Rodent Neocortex. Front. Mol. Neurosci 14, 790213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pędzich BD et al. Effects of a psychedelic 5-HT2A receptor agonist on anxiety-related behavior and fear processing in mice. Neuropsychopharm 47, 1304–1314 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hendrie C, Pickles A. The failure of the antidepressant drug discovery process is systemic. J. Psychopharmacol 27, 407–416 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Ting JT et al. Preparation of Acute Brain Slices Using an Optimized N-Methyl-D-glucamine Protective Recovery Method. J. Vis. Exp 53825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


