Abstract
Simple Summary
Despite clinical success, only a limited percentage of cancer patients are responsive to immunotherapy. Recently, gut microbiota modulation has been suggested as a tool to enhance immunotherapy efficacy, and mechanisms for these effects may be linked to microbial contributions—such as microbial-derived vitamins—to immune responses. While humans can acquire their vitamins from dietary sources, gut microbial-derived vitamins are crucial to the immune system’s function. The production of these vitamins can be altered by the bidirectional crosstalk between the immune system and the gut microbiome; however, their exact mechanism of action in bacterial communities and immune responses remains elusive. Further studies and clinical trials are needed to understand the role of microbial-derived vitamins in anti-tumor immune responses and their role in the efficacy of immunotherapies. This review will discuss the in-depth mechanisms of selective vitamins and their role in modulating immune responses, as well as their potential as immunotherapy enhancers.
Abstract
Not all cancer patients who receive immunotherapy respond positively and emerging evidence suggests that the gut microbiota may be linked to treatment efficacy. Though mechanisms of microbial contributions to the immune response have been postulated, one likely function is the supply of basic co-factors to the host including selected vitamins. Bacteria, fungi, and plants can produce their own vitamins, whereas humans primarily obtain vitamins from exogenous sources, yet despite the significance of microbial-derived vitamins as crucial immune system modulators, the microbiota is an overlooked source of these nutrients in humans. Microbial-derived vitamins are often shared by gut bacteria, stabilizing bioenergetic pathways amongst microbial communities. Compositional changes in gut microbiota can affect metabolic pathways that alter immune function. Similarly, the immune system plays a pivotal role in maintaining the gut microbiota, which parenthetically affects vitamin biosynthesis. Here we elucidate the immune-interactive mechanisms underlying the effects of these microbially derived vitamins and how they can potentially enhance the activity of immunotherapies in cancer.
Keywords: vitamins, gut microbiome, immunotherapy, immune checkpoint inhibitors, fecal microbiota transplant
1. Introduction
Although microbes can be localized in different niches of the body, the human gastrointestinal (GI) tract is home to the largest communities of microorganisms, their products, and genomes—collectively known as the microbiome [1,2,3]. The gut microbiota comprises protozoa, viruses, and fungi, but bacteria encompass the largest biomass [1,4,5,6,7]. The homeostasis between the gut microbiome and immune system is important to overall human health [6,8]. The microbiota of the gut also has a wider systemic influence on the host as microbial metabolites and other components pass across intestinal barriers which influence distal organs and the immune system. Hence, the host’s immune system and the gut microbiota interact in a symbiotic relationship and collectively form a “superorganism” [9,10].
While microbial-derived vitamins have been covered less in the literature, extensive reviews have previously detailed the vast array of roles of the other gut-derived metabolites on immune function and immunotherapy response [11,12,13,14,15]. Vitamins are essential micronutrients that act as precursors for essential enzymes for vital biochemical reactions and cellular functions in the body [16,17,18]. Vitamins can modulate the host’s immune response in the gut, highlighting their role as critical mediators in the interplay between the gut microbiome and the immune response [19,20]. Additionally, some vitamins, such as vitamin C, can act as an effective adjuvant to immunotherapy, further supporting the immunomodulatory functions of vitamins [21,22,23,24]. Vitamin E has been shown to enhance the efficacy of immune checkpoint inhibitors (ICIs) in mouse models of breast cancer and melanoma [25]. Studies displayed significantly better objective response rates in patients with melanoma, breast, prostate, and kidney cancers who took vitamin E supplements with anti-programmed death-1 (PD-1) or anti-PD-1 ligand (PD-L1) inhibitors than those who did not [25]. These results hint at a potential role for using vitamins in promoting the efficacy of immunotherapy in various malignancies.
Interestingly, gut bacteria produce specific vitamins alongside those obtained from dietary sources [26]. This review aims to elucidate the mechanisms underlying the effects of microbial-derived vitamins on immune response, the effect of deficiencies on immune-related diseases, and their potential effects on the response to immunotherapy against cancer. This review will discuss the roles of vitamins B6, B9, and B12 as an interconnected network of immunomodulators due to their similarities and overlapping functions in immune homeostasis. Furthermore, vitamin K2 will be analyzed alongside its interplay with vitamin D as a key inflammatory and immune response regulator. The many immunoregulatory roles of these vitamins have been simplified to provide an overall big-picture representation of their importance to the immune system and their potential promise in immunotherapeutic outcomes.
2. Gut Microbiota and Immune Homeostasis
The bidirectional crosstalk between the gut microbiota and the immune system is essential in developing and maintaining innate and adaptive immune responses. The host immune system impacts gut microbiota composition by properly balancing antigenic and infectious factors. Correspondingly, gut microbiota balance is critical to maintaining host immunity [7,27,28,29]. Specifically, the gut microbiota impacts the development of lymphoid tissues, sustains lymphocyte subpopulations in secondary immune organs, and helps local mucosal immunity regulation through direct contact with many immune cells [5,6,30]. Seventy percent of the body’s immune system can be found in the gut-associated lymphoid tissue (GALT)—making it the body’s largest immune organ [31,32]. The GALT is composed of mesenteric lymph nodes (MLNs), Peyer’s patches, and isolated lymphoid follicles (ILFs) [33,34]. Gut-associated immune cells differ in frequency and location along the cross-section of the intestines [32,35,36]. gut lamina propria contains cells from adaptive and innate immune cells, including T-cells and B-cells, natural killer (NK) cells, innate lymphoid cells (ILCs), and dendritic cells (DCs). The intraepithelial lymphocytes (IELs) mainly consist of CD8+ T-cells and are found in the intestinal epithelium and are more abundant in the small intestine than the large intestine [29,34,37,38,39]. T helper (Th) 17 and T regulatory cells (Tregs), the most copious T-cells of the gut mucosa, have an inversely related abundance along the intestinal tract. Th17 cells decrease in number from the proximal small intestine to the distal colon, while Tregs are more abundant in the colon [40]. Intestinal T-lymphocytes are the most abundant immune cells in the small and large intestines, distributed thoroughly in the GALT [33].
Gut immune cells respond to environmental cues, such as those delivered by the microbiota, and can modulate their response and effector mechanisms accordingly [41,42]. Germ-free (GF) mice show defects in intestinal immune structures, such as diminished amounts and sizes of MLNs and Peyer’s patches [43]. Additionally, GF mice lack fully developed GALTs compared to specific pathogen-free mice [43,44,45]. Gut bacteria also influence the development of naïve CD4+ T- cells and can mediate their differentiation into Th1, Th2, Th17, or Tregs, both within—the MLNs—and outside the intestines [46]. Decreased abundance of CD4+ T-cells and their lineages are displayed in GF mice compared to isotype-controlled mice [47,48].
While it is not completely understood how microbes influence the gut immune system, the most discernible is through the expression of pathogen-associated molecular patterns (PAMPs) that are recognized by pattern recognition receptors (PRRs) on immune cells [49]. PAMP-mediated activation of signaling cascades in PRRs induces the maturation of antigen-presenting cells (APCs), such as DCs and macrophages, and boosts T- and B-cell-related responses. APCs can stimulate the differentiation of Treg and T helper cells, in which the latter stimulates CD8+ cytotoxic T lymphocytes—found mainly in the intraepithelial compartment of the gut [28,30,49,50].GF mice display a reduced number and reduced cytotoxicity of intestinal CD8+ T-cells, highlighting the importance of gut microbial signals in the proper functioning of cytotoxic cells [51,52]. Furthermore, gut microbiota impacts the ability of gut-associated B-cells, consisting mainly of immunoglobulin (Ig) A-secreting plasma cells, to produce IgA and IgM antibodies [30,53,54]. The abundance and cellular composition of the Peyer’s patches in GF animals are significantly reduced, which in turn diminishes gut IgA levels, emphasizing the critical role of the gut microbiota in maintaining the affinity and functionality of B-cells [51,55,56,57]. Besides the lack of antibody production, GF mice have compromised innate and adaptive immune responses shown by their weaker responses to intracellular and extracellular infections [58,59]. Therefore, the gut microbiota and the immune system are interconnected as proper colonization of the gut ensures that all these immune cells develop and maintain immune balance.
3. Vitamin Absorption and Synthesis by Gut Bacteria
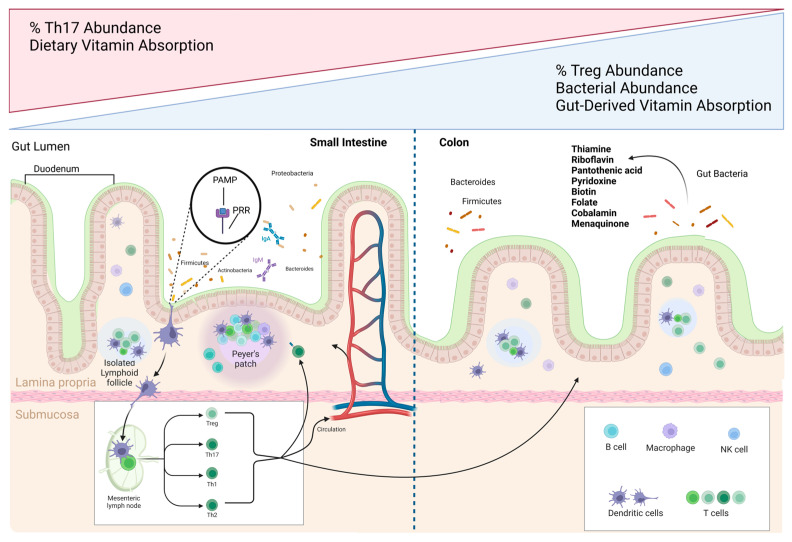
The site of absorption is a critical distinction between vitamins acquired from the diet versus gut bacteria. Most dietary vitamins are absorbed in the small intestine, whereas gut microbial-derived vitamins are predominantly generated and absorbed in the large intestine [39,60]. Some animals, such as rodents, are in fact coprophagic to take advantage of this microbial vitamin production and restriction of this leads to vitamin K and B deficiencies [61,62,63]. Similar to the regional distribution of immune cells, recent reports support an increased bacterial species diversity from the proximal to distal portions of the colon [64,65]. While studies have not yet identified links between regional variation in bacterial diversity and the abundance of immune cell communities, the microbiota is known to play a fundamental role in the induction, development, and function of the innate and adaptive immune system [36,66,67]. As the frequency and distribution of immune cells and bacterial populations vary along the length of the GI tract, the distinct absorption patterns of vitamins may have different implications for the host immune response (Figure 1) [68]. The duodenum contains the lowest bacterial abundance due to the high levels of oxygen, antimicrobial compounds, and lower pH levels from the stomach [68,69,70]. The GI tract includes functionally distinct regions whereby the proximal colon functions primarily in gut fermentation, and the distal colon focuses on extracting fluids and electrolytes [65]. Evidence directly alluding to the effects of these differences on site-specific immune responses in the host remains undetermined.
Figure 1.
The gastrointestinal (GI) tract contains varying frequencies and distributions of immune cells and vitamin-producing bacteria. The gut-associated lymphoid tissue (GALT) is essential to host immune defense and is comprised of mesenteric lymph nodes (MLNs), isolated lymphoid follicles (ILFs), and Peyer’s patches—found mainly in the small intestine. Gut microbes can express pathogen-associated molecular patterns (PAMPs) and immune cells recognize them through their pattern recognition receptors (PRRs). Antigen-presenting cells (APCs) undergo maturation upon PAMP-PRR interactions on their cell surfaces, which can then lead to the differentiation of naïve CD4+ T cells in the MLNs and outside the intestines and boost T- and B-cell-related responses. Th17 and Treg cells are the most abundant T cells in the gut and their concentration gradients along the tract share an inverse relationship. Bacterial abundances also vary along the GI tract, with the duodenum containing the lowest bacterial abundance which increases towards the colon, dominated by Firmicutes and Bacteroides in healthy individuals. Gut-derived vitamins are mainly generated and absorbed in the large intestine, while dietary vitamins are absorbed in the small intestine. Vitamin K2 and most members of the water-soluble B vitamins—thiamine, riboflavin, pantothenic acid, pyridoxine, biotin, folate, and cobalamin—are produced by gut bacteria and play many roles in immune function.
Bacterial-synthesized vitamins support the growth of the bacteria themselves, and any excess can be released and absorbed to benefit host health. While bacteria require vitamins for their essential cellular functions, not all bacteria are prototrophic and can produce vitamins [71,72]. Vitamin K2 menaquinone and most members of the water-soluble B vitamins—thiamine, riboflavin, pantothenic acid, pyridoxine, biotin, folate, and cobalamin—have been identified as gut microbial-derived vitamins [73,74]. A recent metagenomic study identified that most vitamin-producing microbiota could only synthesize one B or K2 vitamin [75]. In contrast, only 2.7% of those bacteria could produce five or more vitamins [76]. This implies that many prototrophs produce vitamins to support the growth and function of neighboring auxotrophs that cannot produce vitamins. Auxotrophs for vitamins B1, B2, B3, B5, and B9 represent between 20-50% of bacterial communities, whereas auxotrophs for B7 and B12 vitamins are more prevalent, representing up to over 50% of bacterial communities [77]. Out of the eight B vitamins, pyridoxine (B6), folate (B9), and cobalamin (B12) contain the highest estimated percentage of the daily reference intake (DRI) that the gut microbiota could provide at 86, 37, and 31%, respectively. Although bacterial utilization was not considered, the gut microbiota was estimated to produce over a quarter of the dietary recommended intakes for vitamins B6, B9, and B12 [78,79]. While absorption of dietary and gut-derived vitamins is predicted to vary between the small and large intestines, uncertainty remains on how these varied absorption patterns alter the body’s ability to utilize those vitamins and impact their downstream functions [80]. Regardless of these anomalies, these gut-microbial-derived vitamins are critical regulators of intestinal immune homeostasis. Furthermore, gut microbial communities are shown to have an interconnected synthesis and sharing of bioenergetic machinery [81,82].
A symbiotic relationship exists between prototrophs and auxotrophs in the gut, where vitamins are cross fed between microbes to support the function and stabilization of gut microbial communities [82]. Bacterial demands for vitamins can differ based on factors that affect the gut microbiome composition, such as age, lifestyle, diet, disease, and alcohol consumption [83,84]. Similarly, host factors can regulate vitamin demands in cells. Increased immune system activation requires enhanced immune cell functions, which may upregulate the need for vitamins to boost this activity [85]. GF mice not only have an underdeveloped immune system, but the absence of vitamin K and vitamin B12-producing bacteria places them at a higher risk of enduring vitamin deficiencies [41,42]. Whether gut bacterial-derived vitamins are sufficient to sustain the host’s immune responses or require additional dietary supplementation remains unclear. However, evidence in the literature emphasizes the importance of vitamins in the gut microbiome in promoting immune cell function and response.
4. Vitamin B6 as a Mediator of Lymphocyte Migration and Responses
Pyridoxine affects the maintenance of the gut barrier by mediating lymphocyte proliferation, activation, and maturation, as well as cytokine and antibody production [86,87]. Specifically, pyridoxine regulates T-cell homeostasis in modulating the Th1/Th2 cell balance, which drives cellular and humoral immunity, respectively [88,89]. Adequate amounts of pyridoxine maintain the Th1 immune response and inhibit Th2-mediated cytokine overactivity. Pyridoxine deficiency negatively impacts both cell-mediated and humoral immunity [89,90]. For example, pyridoxine deficiency decreases CD8+ lymphocyte proliferation and activation by suppressing the Th1 response and promoting the Th2 response [89,90]. Downregulation of Th1 responses results in reduced IFN-γ, interference with the induction of delayed-type hypersensitivity (DTH) reactions and lower ability of T-cells to modulate immunosurveillance of invading pathogens [91,92]. Overstimulated Th2 cells excessively produce type 2 cytokines, such as IL-4, IL-5, and IL-13, leading to the overactivity of pro-inflammatory responses [93,94]. This response further decreases antibody response and the formation of the pro-inflammatory cytokines IL1β, IL-2, and IL-2 receptors, which can cause antibody-mediated autoimmune diseases [93,95]. In vivo and in vitro studies have shown that vitamin B6 deficiency leads to defects in thymic epithelial cell function and lowers T lymphocyte abundance, leading to excessive Th2-driven inflammation [89]. Pyridoxine also regulates intestinal immune homeostasis and prevention of excess inflammation by managing IEL migration through sphingosine-1 phosphate (S1P) [96,97]. S1P is a bioactive sphingolipid metabolite and immunoregulator that regulates signaling pathways, such as the NF-κB pathway, in many cell types including macrophages and DCs. As an immunoregulator, S1P generates a gradient through its synthesis and degradation mediated by S1P lyase (SPL). It requires pyridoxal phosphate (PLP), or the biologically active form of vitamin B6, as a cofactor [96,98]. The S1P gradient is crucial in attenuating IEL traffic into the large intestines; however, heightened levels of S1P can lead to augmented inflammation by dysregulation of its maintenance of vascular integrity [99]. Vitamin B6 supplementation was shown to promote anti-inflammatory properties by promoting SPL, thus reducing S1P expression [98,100]. Therefore, vitamin B6 is essential in intestinal immunosurveillance by ensuring proper lymphocyte balance and IEL migration and activation.
5. Vitamin B9 in Treg Immunosurveillance
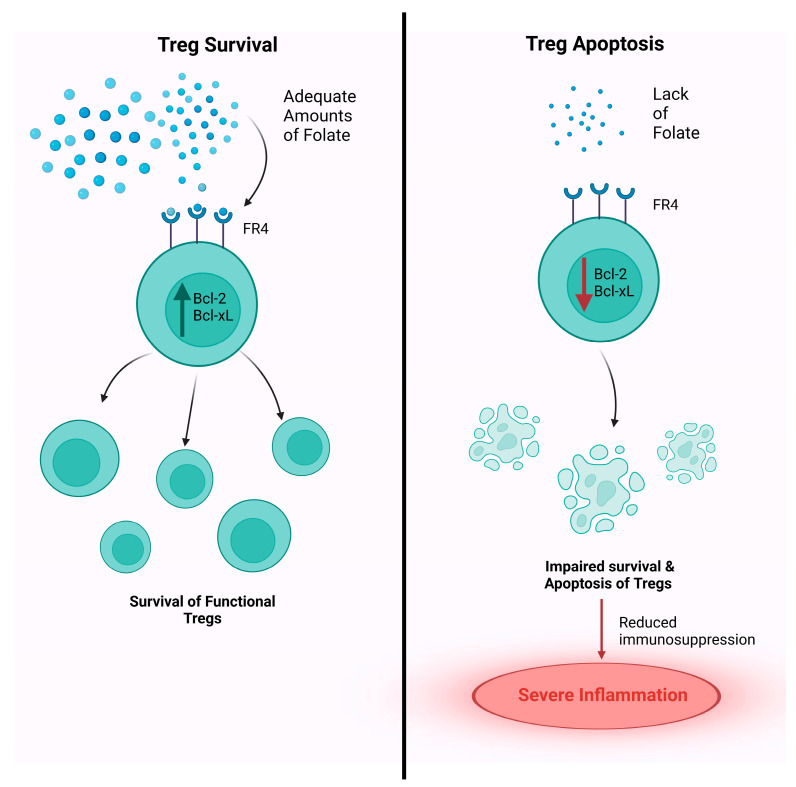
Folate’s main contribution to immune homeostasis derives from its requirement in Treg function [101]. Folate is required for immunosurveillance by stabilizing Tregs, specifically peripheral Tregs (pTregs), and aiding T-cells traffic from the colon to distant sites to assert their immune response [102,103]. Folate is required for the survival of mature Tregs but not the differentiation of immature T-cells into mature Tregs [103]. In vitro culture of Tregs under low vitamin B9 conditions leads to impaired cell survival, with decreased expression of anti-apoptotic Bcl2 molecules [104]. However, naïve T-cells retain their ability to differentiate into Tregs; which suggests that vitamin B9 is a survival factor for Tregs [103]. Once naïve T-cells differentiate into Tregs, they highly express folate receptor 4 (FR4), which regulates the development and function of Tregs [101,105]. High expression of FR4 on the surfaces of Tregs marks the important role of folate in the survival of these cells (Figure 2) [106,107]. Studies emphasized folate’s effect on the survival of Tregs by blocking FR4, which led to a significant reduction in the anti-apoptotic molecules Bcl-2 and Bcl-xL and hence, an increase in Treg apoptosis [107,108,109]. Mice fed with a vitamin B9-deficient diet exhibit increased susceptibility to intestinal inflammation [108]. Consistent with these findings, a deficiency of dietary vitamin B9 results in the impaired survival of the Tregs population in the small intestine [103]. Moreover, certain conventional T-cells that become autoreactive undergo apoptosis during development; however, a subset of cells undergoes a state of anergy known as Tan [110]. Gut microbial-derived folate can induce the conversion of conventional T-cells into Tan [103,106]. Tan also possesses FR4, indicating an overlapping relationship between Tan and Tregs [111]. Tan contains a greater number of methylation sites in the periphery compared to the thymus, which promotes transcriptional silencing and gene inactivation. The instability promoted by these methylation sites in Tan can promote the interconversion between Tan and Tregs [112,113,114]. Therefore, gut microbial-derived folate can drive epigenetic modifications through the loss of methylation sites in Tan to induce Treg replenishment. Decreased microbial-derived folate has been related to dysbiosis and the proliferation of autoreactive T lymphocytes that can lead to autoimmunity [112,115,116,117]. Reduced folate can lead to reduced SCFA production and cause an increased abundance of autoreactive immunogenic T effector cells (Teff) while reducing the abundance of Tregs [112,118,119,120]. Decreased Tregs trafficking from the colon to distant sites shifts the opportunity for such movement to Teffs [117,118]. Teffs target autoimmune target sites and skew the Teff to Treg ratio to override immune privilege and trigger autoimmune diseases such as colitis [117,121,122,123]. The effects of folate-deficiency on Treg immune profiles and gut dysbiosis can be consequential in immunotherapies such as ICIs, specifically in patients suffering from autoimmune diseases. For instance, patients with colitis may be characterized with dysbiosis in the gut ecosystem that potentiates the malabsorption of folate. Furthermore, ICIs can trigger inflammation throughout the body, particularly in organs that are targeted in autoimmunity, such as the intestines, and cause severe inflammation in those patients by dysregulating the Treg and Teff ratio. These adverse effects (AEs) may be detrimental to cancer patients suffering from such autoimmune diseases and folate deficiency, as the ICIs can enhance the AEs and may cause severe complications in patients [115,123,124]. Therefore, adequate folate is required in the surveillance and the immunological network of Tregs, which are linked to the control of autoimmunity and gut dysbiotic changes and should be taken into consideration in future immunotherapy studies.
Figure 2.
Folate is required for the survival of mature Tregs. Once naïve T cells differentiate into Tregs, they highly express folate receptor 4 (FR4), which regulates the development and function of Tregs. The binding of folate to FR4 stimulates the production of the anti-apoptotic molecules Bcl-2 and bcl-xL, allowing for the survival of functional Tregs. Folate deficiency affects the survival of Tregs, leading to a reduction Bcl-2 and Bcl-xL and hence, an increase in Treg apoptosis. Impaired Treg function may impair immunosuppression and lead to increased susceptibility to intestinal inflammation.
6. Vitamin B9 and B12 in Their Role as Co-Dependent Immunomodulators
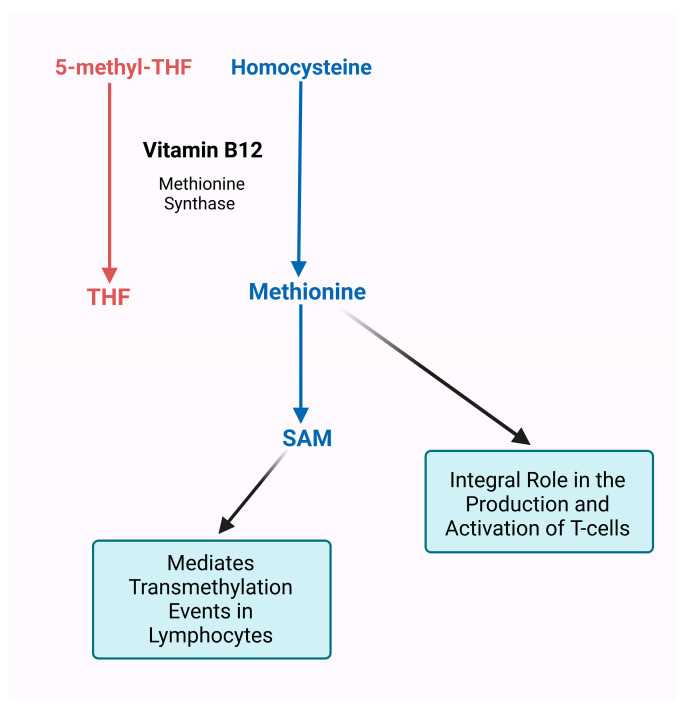
Interestingly, dietary and microbial-derived folate requires cobalamin (vitamin B12) to be absorbed in the intestinal wall, as without it, folate remains trapped in the colon [125]. Vitamin B12 is a cofactor for methionine synthase—important for catalyzing the formation of methionine from homocysteine—which is required to demethylate the inactive 5-methyl tetrahydrofolate to its active form tetrahydrofolate (THF) [126]. In cobalamin deficiency conditions, 5-methyl THF augmentation evokes a secondary folate deficiency affecting protein and DNA production as well as altering immune cell regulation and defense [85,127]. An inadequate amount of cobalamin can negatively affect folate functions, thereby inducing cobalamin deficiency that consequently promotes folate deficiency [128,129]. Upon absorption, cobalamin requires 5-methyl THF to produce methionine from homocysteine [130,131]. T-cells import methionine for protein synthesis and provide methyl groups to methylate the RNA and DNA required to drive the proliferation and differentiation of T-cells. Upregulation of the methionine transport Slc7a5 requires T-cell activation and is the rate-limiting factor for the generation of methyl groups [131,132]. In the absence of methionine, T-cells fail to expand and develop, leading to a weakened T-cell-mediated immune response [131,133]. Therefore, methionine is an integral portion of the production and activation of T-cells. Methionine is essential in supporting biochemical reactions by working alongside cobalamin and folate to synthesize S-adenosylmethionine (SAM) [130,134]. SAM is a major methyl donor in transmethylation reactions and is important for modulating biochemical reactions that support immune function [135,136,137,138,139,140,141]. T-cell activation upregulates methyltransferases, which depend upon SAM to methylate DNA, RNA, or proteins to allow for the differentiation and proliferation of those T-cells. The amount of SAM consumed is 4-10 times higher in activated lymphocytes compared to resting lymphocytes, and even in resting lymphocytes, the rates are 3–5 times greater than other cell types [142,143]. These findings indicate that lymphocytes are more sensitive to transmethylation events mediated by SAM for their function and activation than other cell types. Therefore, the interconnection of vitamin B12 with vitamin B9 through methionine and SAM metabolism demonstrates the necessity of not only ensuring that vitamin B12 can perform its essential functions but also ensuring that folate can be absorbed and perform its downstream immunomodulatory operations (Figure 3). Furthermore, folate affects cell-mediated immunity and immune cell proliferation [144,145]. Folate deficiency leads to reduced T-cell blastogenic response to certain mitogens [103,146]. Studies have also shown that antibody response is affected in folate-deficient hosts [147,148,149]. These alterations to humoral and cell-mediated immunity can reduce infection resistance [150,151].
Figure 3.
Vitamin B12 and 5-methyl-tetrahydrofolate (THF) interact to produce methionine for T cell activation. Methionine synthase is a vitamin B12-dependent enzyme that produces methionine via homocysteine and 5-methyl-THF. The uptake of methionine is required in T-cells to provide methyl groups to RNA and DNA, needed for the cells to proliferate and differentiate. The absence of methionine leads to the failure of T-cell expansion and a weakened cell-mediated immune response. Methionine is essential to synthesize S-adenosylmethionine (SAM), which plays a major role in transmethylation reactions that support immune function. Lymphocytes depend upon SAM in activated states for transmethylation events involving DNA, RNA, or protein molecules, which are required for maintaining cell function.
7. Vitamin B6, B9, and B12 in the Regulation of Cytotoxic Immunity
Pyridoxine, folate, and cobalamin are involved in maintaining and enhancing cytotoxic and killer cells [152]. In vitro and in vivo studies show that the metabolism of these vitamins is required for CD8+ T-cell proliferation and their effector functions [118,127,146,153,154,155,156]. In contrast, a deficiency of these vitamins promotes DNA damage by arresting cell division at the S-phase and reduces cytotoxic cell expansion [153]. Furthermore, this deficiency inhibits cytotoxic T and NK cell activity, increasing dysregulation of the gut barrier and thus heightening the onset of infections [77]. Vitamin B9 deficiency has been suggested to inhibit CD8+ T-cell regulation as the ratio of CD4+ to CD8+ lymphocytes increases [146,157,158]. Pyridoxine deficiency leads to decreased NK cell activity and the increased onset of infection [154,159]. Similarly, cobalamin and folate regulate CD8+ T- and NK cell proliferation and antibody and Ig production in B-cells [13,158]. Reductions in Ig production such as reduced IgG and IgM levels, result in host immunodeficiency, putting the host at a higher risk of immune-prone diseases [55,160]. Similarly, reduced CD8+ T-cell and NK cell cytotoxicity can increase the host’s risk of infection and chronic inflammation by altering their immune risk phenotype [161,162,163]. Cytotoxic CD8+ T-cells constitute an important player in ICIs as they are associated with enhanced ICI-efficacy in cancer patients and hence, prolonged survival [164]. A lack of T-cells, specifically within the tumor, can lead to primary resistance to immunotherapy and poor disease prognosis in patients. Hence, vitamins B6, B9, and B12 are crucial in deciphering the efficacy of immunotherapy through their ability to regulate cytotoxic immunity and immune cell infiltration in tumors [165].
8. Vitamin K2 and D: More Than Calcium Metabolism and Bone Health
Vitamin D and vitamin K are fat-soluble vitamins that play an essential role in calcium metabolism and bone health. Vitamin D, or calcitriol, promotes the production of vitamin K-dependent proteins for calcium absorption, while vitamin K works to activate those proteins [166,167]. Evidence supports these vitamins work together not only in bone health but also in immune health [166,168]. Vitamin K can be separated into two distinct types, vitamin K1 and vitamin K2, each playing varying but significant roles. Vitamin K1 (phylloquinone) is derived from green plants and is acquired through the diet [169,170]. Vitamin K2, or menaquinone (MK), presents in several forms, predominating from obligate and facultative anaerobes [171]. Like vitamin B12, MKs can be remodeled by gut bacteria, hinting at possible modulation of the gut microbiome composition by the vitamin [172,173]. Gut microbiota with abundant levels of diverse bacteria can provide adequate levels of MK to significantly impact the host, pointing to an association between gut microbiota and sufficient vitamin K levels [172]. Similarly, vitamin D can be sourced from the diet as vitamin D2 or D3, or from the skin—through a photolytic process—as vitamin D3. Vitamin D2 or D3 is then converted into the inactive prohormone 25-hydroxycholecalciferol (25D) in the liver and then hydroxylated by the CYP27B1 enzyme into its active form 1,25-dihydroxyvitamin D (1,25D) [174]. While it is noted that vitamin D is not made in large quantities by gut bacteria; both 1,25D and 25D may possess bi-directional relationships with the gut microbiome and the immune system [175]. Vitamin D supplementation has been suggested to elevate the abundance of probiotics and to specifically increase bacteria that produce the SCFA butyrate, such as Akkermansia and Bifidobacterium [176]. Butyrate has been extensively reviewed for its ability to enhance the immune system-gut connection by modulating immune homeostasis and promoting healthy inflammatory responses in the colon [13,177,178,179,180]. Interestingly, evidence suggests that individuals with higher vitamin D levels possess a greater abundance of butyrate-producing bacteria shown to be associated with gut microbial diversity and better overall gut-immune health [181]. Furthermore, circulating levels of vitamin D may be involved in regulating immune homeostasis, which is in part tied to its interactions and alterations to gut microbiota composition [182]. Therefore, both MKs and Vitamin D levels demonstrate an intertwined relationship between gut microbiota composition and immune response regulation.
8.1. Vitamin K2 and D: Players in Effector and Tolerogenic Immunity
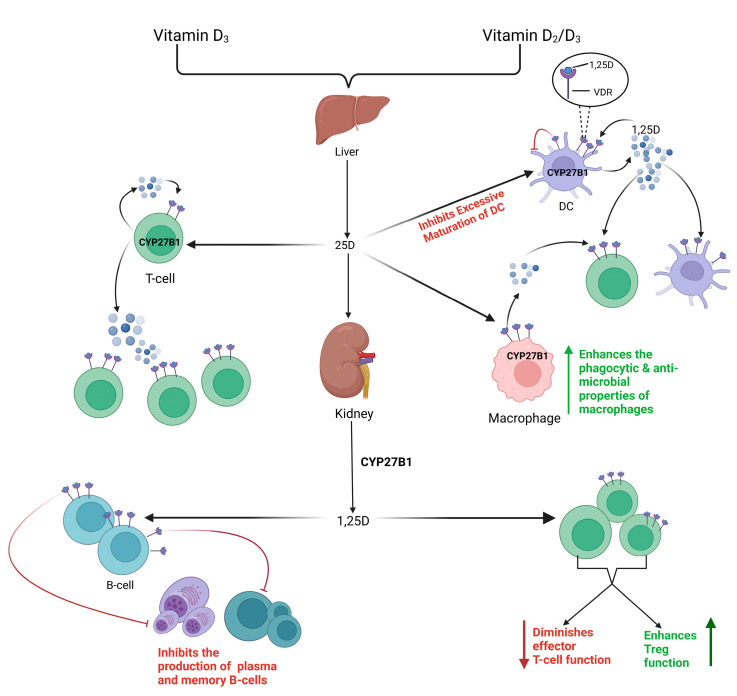
The combined effect of vitamin D and vitamin K2 in anti-tumor responses may be due to synergistic improvement in cellular differentiation outcomes and reduced hypercalcemic complications of standalone supplementation [183]. Potential reasoning for these effects may stem from calcitriol and MK’s comparable anti-inflammatory and immune-regulatory properties [184,185]. Interestingly, vitamin D receptors (VDR) and CYP27B1 are expressed in many cells, including immune cells such as APCs, T-cells, B-cells, and monocytes (Figure 4) [186,187,188]. The discovery of the extra-renal synthesis of 1,25D by immune cells suggests a more widespread influence of vitamin D on the immune system than ever before. While vitamin D is known to boost the phagocytic capabilities of monocytes and macrophages, it can promote their antimicrobial activity through VDR acting as a gene transcription regulator. Furthermore, 1,25D is important for tolerogenic modulation. The presence of CYP27B1 in DCs allows for the generation of 1,25D concentrations and modulation of immune responses [189,190]. Furthermore, the 1,25D-VDR complex in DCs inhibits the excessive maturation of these cells [191,192]. In vivo studies of VDR-KO mice found high levels of mature DCs and abnormal DC chemotaxis, highlighting the importance of vitamin D-VDR in self-tolerance development and autoimmunity [193,194]. Furthermore, vitamin D may possess immunological properties in the adaptive immune system via the expression of VDR on both T- and B-cells [195,196]. Direct effects of 1,25D on B-cell homeostasis are highlighted through its ability to inhibit memory and plasma cell generation, and due to its pro-apoptotic properties in autoreactive antibodies, which provide clinical significance in autoimmune diseases [174,195,197]. Furthermore, the different forms of vitamin D influence the proliferation and differentiation of T-cells via direct and indirect mechanisms. Direct impacts include the endocrine effects of systemic calcitriol on T-cells, the intracrine conversion of 25D to 1,25D by T-cells, and the paracrine effect of 1,25D stemming from DCs. T-cells may also be affected indirectly through the important role of 1,25D in APCs [198]. Vitamin D has been shown to attenuate Tregs phenotypes by elevating the levels of circulating Tregs and diminishing the effector functions of cytotoxic and helper T-cells to promote anti-inflammatory responses and support tolerance [19,197,199,200,201]. Therefore, the relationship between 1,25D and VDR is crucial for regulation of the innate immune system in promoting antimicrobial and tolerogenic immune responses, as well as in regulating adaptive immunity [202,203].
Figure 4.
Vitamin D receptors (VDR) and CYP27B1 are expressed in immune cells and regulate immune responses. The presence of CYP27B1 in antigen-presenting cells (APCs), T cells, B cells, and monocytes allows these immune cells to produce 1,25D, which can bind to its receptor VDR. The 1,25D-VDR complex inhibits the excessive maturation of dendritic cells (DCs). In monocytes and macrophages, 1,25D boosts their phagocytic capabilities and antimicrobial effects. 1,25D contribute to B-cell homeostasis by directly inhibiting memory and plasma cell generation. Furthermore, different forms of vitamin D can affect the proliferation and differentiation of T cells, such as the intracrine conversion of 25D to 1,25D by T cells. T cells may also be affected downstream of the production of 1,25D in APCs and macrophages. 1,25D can elevate the levels of circulating Tregs and reduce the effector functions of cytotoxic and helper T cells, which supports tolerance and highlights its anti-inflammatory properties.
8.2. Vitamin D and K Deficiencies as Preventable Risk Factors
Vitamin D’s role in immune responses highlights how vitamin D deficiency may skew these immune responses and become a risk factor for autoimmune diseases such as ulcerative colitis, rheumatoid arthritis, and thyroid diseases [204]. These risk factors should be considered in the context of vitamin D levels and ICI administration in cancer patients. ICIs have several AEs that correspond with the depicted autoimmune diseases related to vitamin D deficiency, targeting organs such as the thyroid, the bones, and the intestines. As vitamin D is a protective factor for several autoimmune diseases, it may be able to potentiate these ICI-induced AE by regulating immune responses [204,205].
The crucial role of vitamin D in the balance between effector and tolerogenic immunity can be demonstrated by vitamin K2 in relation to bone health. At in vitro doses related to bone cell function, inhibition of T-cell proliferation was suggested as not vitamin K1-induced but rather vitamin K2-specific, demonstrating vitamin K2’s immunomodulatory properties in T lymphocytes [206]. Further research is needed to better understand the immunoregulatory function of vitamin K2 on T-cells, specifically in immune cells.
Moreover, vitamin D is a modifiable risk factor for haematological diseases, as lower levels of vitamin D in patients with these diseases correlate with worse disease outcomes [207]. Vitamin D levels are low in patients with acute leukemia and these levels are shown to decline further after remission induction therapy [208]. Conversely, in Hodgkin’s lymphoma, supplemental vitamin D reduces the rate of tumor growth, demonstrated by the improved chemosensitivity of the tumors compared to vitamin D or chemotherapy treatments in standalone [207]. Similarly, higher levels of vitamin K are associated with anti-cancer effects, as individuals with a higher intake of vitamin K from their diet had a reduced risk of developing non-Hodgkin’s lymphoma [209]. Furthermore, vitamin K2 has been shown to prevent lymphoma in Drosophila [210]. Therefore, the serum levels of vitamins may not only be of use in differentiating the risks of developing various types of haematological disorders, but their role in immune responses may also provide downstream applications in cancer therapies.
8.3. Vitamin K2 and D in the Expression of Pro-Inflammatory Markers
Vitamin K2 and Vitamin D have anti-inflammatory effects marked by their ability to affect the expression of inflammatory markers [185,211,212,213]. Vitamin K2 has been shown to prevent cytokine storm—a phenomenon in which stimuli lead to extreme inflammatory response through rapid secretion of inflammatory cytokines [214,215]. Specifically, MK4 reduced the expression of NF-κB, a transcription factor for IL-6, which can reduce this cytokine levels [216,217]. MK4 is suggested as a more potent anti-inflammatory compound than vitamin K1 in primary fibroblastic and monocytic cell lines [213]. COVID-19 patients supplemented with vitamin K2 had reductions in IL-6 and tumor necrosis factor-alpha (TNFα) expression [215]. Reports to date have shown that lower levels of MK are associated with a more severe COVID-19 disease prognosis, which may correspond to the increased prevalence of IL-6 secretion and cytokine storms in those patients [218,219]. Similarly, vitamin K deficiencies are marked in many chronic GI inflammatory disorders and MK4 supplementation in a murine model of colitis was shown to provide immunosuppressive benefits by reducing pro-inflammatory cytokine production such as IL-6 [220]. Additionally, an in vitro atopic dermatitis study found that vitamin K2 significantly inhibited levels of the pro-inflammatory cytokines IL-17 and TNF-α [221]. Similarly, vitamin D’s importance to tolerogenic response extends to its ability to decrease the expression levels of T-cell cytokines, through VDR’s ability to suppress transcription of cytokine genes in activated T-cells [222,223,224]. Furthermore, evidence suggests that vitamin D can alter the Th1/Th17 pro-inflammatory phenotype to a more anti-inflammatory Th2/Treg phenotype [202,225]. Treatment with calcitriol has been credited to the inhibition of pro-inflammatory Th1 cytokines, specifically IL-1, IL-6, IL-8, IL-12, and TNF-α, as well as the induction of anti-inflammatory Th2 cytokines such as IL-10 and IL-4 [226,227,228,229,230,231,232,233,234,235,236]. A study found significant suppression of the pro-inflammatory IL-6 in healthy adults supplemented with a high dose of calcitriol [237]. Similar to MK, low vitamin D levels are associated with increased inflammatory markers, such as IL-6, in COVID-19 patients at risk of developing severe inflammatory conditions and higher mortality rates [238,239]. Furthermore, Vitamin D inhibits Th17 activity by suppressing IL-17 production at the transcriptional level in human T-cells, revealing a shift towards a tolerogenic phenotype [40,174]. Therefore, both vitamin K2 and D possess anti-inflammatory properties credited to their ability to regulate pro-inflammatory markers.
9. Vitamins and Immunotherapy: Enhancement of Therapy and Prevention of Side Effects
Gut microbial-derived vitamins have immunomodulatory properties and may enhance ICI response rates [84,176]. The capability of the host’s immune system to fight against tumor cells is key to successful outcomes in immunotherapy. Unfortunately, ICI immunotherapies are shown to be only clinically effective in 10-40% of cancer patients and can trigger autoreactive adverse reactions [240,241]. Novel treatment strategies that target gut microbiota composition and diversity are currently in the works to overcome such limitations [242,243,244,245,246].
Since 2015, several preclinical and clinical studies have been hailed as milestones in the link between gut microbiota and ICI responses. Notably, certain bacterial species (i.e., Bifidobacterium, Akkermansia) were marked as elevated in immunotherapy responders versus non-responders throughout multiple studies [247,248]. Oral administration of Bifidobacterium spp. in tumor-bearing mice promoted DC maturation leading to enhanced CD8+ T-cell priming and improved anti-PD1 efficacy [247,249,250]. Furthermore, supplementation with Bacteroides fragilis in antibiotic-treated mice augmented the efficacy of anti-cytotoxic -T-lymphocyte- associated protein 4 (CTLA-4) therapy by stimulating DC maturation and thus, Th1 immune-mediated responses [248]. Similarly, enhanced anti-tumor immunosurveillance to immunotherapy and elevations in intestinal metabolites were found with oral administration of Akkermansia muciniphila—in which several strains are proposed to be vitamin B12 producers—in animal studies [251,252]. In human studies, fecal microbiota transplantation (FMT) from ICI responders to antibiotic-treated mice ameliorated anti-tumor responses, which was not seen in FMTs from non-responders [253,254]. Oral supplementation with A. muciniphila following FMT in non-responders improved anti-PD1 response in lung, renal cell, and urothelial carcinoma patients by recruiting CCR9+ CXCR3+ CD4+ T-cells into tumor beds [254,255]. In melanoma patients, ICI responders with higher abundances of Faecalibacterium and Ruminococcaceae—auxotrophic for most B vitamins—showed increased CD4+ T- and CD8+ T-cells in their periphery [72,253,256,257]. In 2021, two seminal clinical trials displayed that FMT from ICI responders with anti-PD1 therapy reprogrammed the tumor microenvironment to overcome resistance to PD1-blockade in a subset of melanoma patients [258,259]. Similarly, an emphasis on the relationship between gut microbiota and immunotherapy response efficacy has been implicated in studies looking at the link between antibiotic-induced dysbiosis and attenuations to ICI efficacy [15,260,261,262,263,264]. Antibiotic-treated patients with advanced solid tumors displayed reduced survival rates and poor ICI responses, which were associated with reduced gut microbiota diversity and abundance [56,263,265]. Furthermore, dysbiotic gut microbiomes may be unable to perform vital functions which may impact vitamin biosynthesis and ultimately deplete vitamin-producing bacteria [266,267,268]. Ampicillin, Chloramphenicol, Ciprofloxacin, and Vancomycin have been shown to reduce vitamin biosynthesis by affecting the gut transcriptome [266]. Therefore, the interconnection between the gut microbiota and the immune system has the potential to shape the response to immunotherapy.
A symbiotic gut microbiota provides adequate endogenous vitamin production to support immune function (Figure 5), and inversely, vitamins can beneficially modulate the ecology of the gut microbiota. Disruptions to the bidirectional relationship between vitamins and the gut microbiome can potentially cause the loss of beneficial bacteria and vital micronutrients [84]. It is currently unfeasible to determine the exact proportion of microbially-derived vitamin deficiency compared to dietary vitamin deficiency in individuals. However, vitamin deficiency has been postulated as a link to gut dysbiosis—marked by a detrimental loss in probiotics and commensals—causing detrimental effects on the immune system and potentiating inflammatory diseases [176,269,270]. B vitamins, vitamin K, and many dietary vitamins have established immunoregulatory properties that coincide with positive immunotherapy response rates. The mechanistic role of vitamin B6 in inducing T-cell subtype determination and inducing PD-1/PD-L1 blockage renders it a natural immunoregulator and a potential option for combination immunotherapy [271]. Comparably, PD-L1 expression is significantly reduced in the presence of vitamins B6 and B9 and thus, corresponded to reduced proliferation of pro-monocytic lymphoma cells in vitro [127]. Furthermore, Vitamin D supplementation can suppress tumor angiogenesis and induce anti-inflammatory effects within the tumor microenvironment, promoting apoptosis and autophagic death of tumor cells [272]. In a clinical trial of tuberculosis patients, supplementation with high doses of vitamin D and adjunctive therapy reduced inflammatory responses more quickly than in patients who received no supplementation [273]. These results support vitamin D’s tolerogenic immunologic properties that may provide potential therapeutic promises as an adjuvant with cancer therapies [190]. Vitamin D has also shown potent anti-cancer benefits alongside treatment in patients with melanoma, prostate, breast, and colorectal cancers [24]. Similarly, vitamins may modulate the human gut microbiome and immune barrier function in terms of metabolic activity and bacterial composition. Evidence suggests that vitamin D and B vitamins increase the abundance of butyrate-producing bacteria and the gut-mucosal integrity bacteria A. muciniphila [74]. The effect of vitamins in sustaining and promoting the growth of such immunomodulatory bacteria emphasizes the bidirectional symbiosis between vitamins and gut bacteria and its potential for immunotherapy enhancement. Prospective studies are needed to analyze alterations in metabolite production from gut microbial modulation production and the indirect impacts of their vitamins on immunotherapy response.
Figure 5.
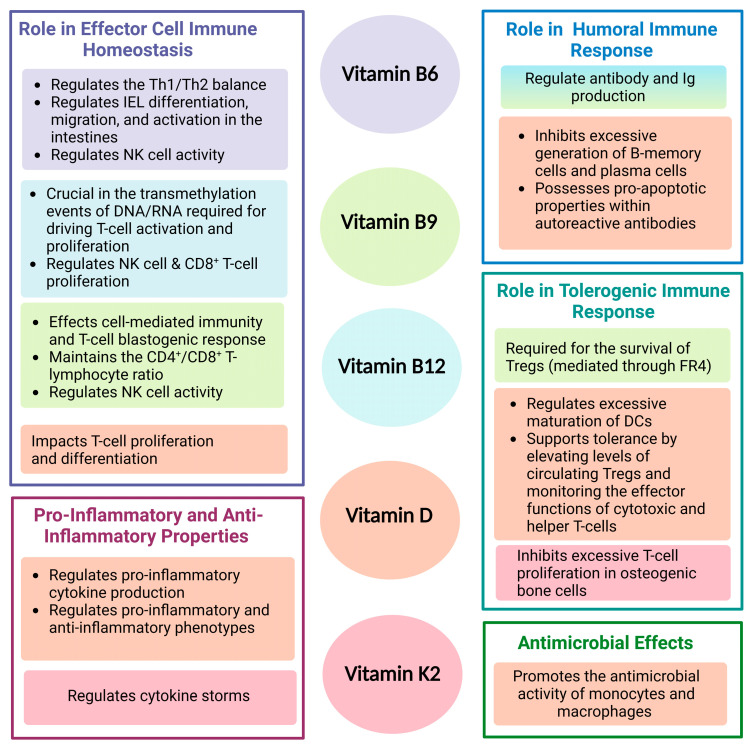
Summary of the Immune Functions of Vitamins B6, B9, B12, D, and K2.
There are many immune-related adverse events (AEs) that occur in ICI treatments, such as colitis and peripheral neuropathy [274,275,276,277,278]. Several bacterial species, including Bacteroidaceae, Bifidobacterium animalis, Bifidobacteria infantis, Faecalibacterium prausnitzii, and Lactobacillaceae are associated with the maintenance of tolerogenic responses in the gut [51,264,279,280,281,282,283,284,285,286,287,288,289]. The bacteria are deemed protective against ICI-induced colitis through nitric oxide production, shifts in the Th1/Th2 balance, and induced Treg differentiation. These protective effects may be potentiated by the synthesis of group B vitamins by these bacterial species [24,28,30,261,290,291,292,293,294]. Therefore, vitamins, or specifically gut-derived vitamins, may be critical in the interplay between the gut microbiota and the prevention of ICI-induced AEs. Clinical evidence suggests that vitamin B12 can efficiently reduce the toxicity of cancer treatments due to its necessity in the nervous system and red blood cell synthesis [130,295]. Vitamin B12 is used alongside several chemotherapies to hinder the severity of drug-induced peripheral neuropathy [127,296]. Similarly, patients treated with ICIs while being administered vitamin D reported a reduced risk of ICI-induced colitis [205]. It remains unclear the extent to which these immune responses vary between microbial-derived or dietary vitamins and if these differences could drive variations in immunotherapy sensitivity in patients. Nevertheless, vitamins have the potential to enhance immunotherapy responses while simultaneously preventing AEs, however, clinical trials are needed to determine the specific roles of vitamins and how their levels correlate to responses in the immune-gut axis of cancer patients.
10. Conclusions
The gut microbiome has gained immense attention over the past decade, due to the interconnection of its microbes and products with the immune system and its potential as an immunomodulatory target in anti-cancer therapy. The extent of the fundamental mechanisms and pathways of gut-microbial vitamins in immune-mediated cancers remains elusive; however, the bidirectional relationship between vitamins and the gut-immune axis warrants potential consideration for immunotherapy. Gut-derived vitamins are essential in ensuring cellular and humoral immunity’s proper development and function, playing an integral defensive role in anti-tumor responses. These micronutrients, in turn, drive a healthy and diverse gut microbial composition that supports the immune system. Such effects shed light on the potential to improve clinical outcomes in immunotherapy patients and minimize the risk of adverse-therapeutic effects. As many questions about gut-derived vitamins remain unanswered, many more studies, including randomized clinical trials, are required to elucidate their role in the efficacy of immunotherapies and their specific effects on immune cells and anti-tumor immune responses.
Author Contributions
H.G. conceptualized, wrote the initial draft of the manuscript, conducted the literature review, revised, edited, and created the figures; figures were created with BioRender.com (accessed on 16 January 2023); J.A.C., J.P.B. and S.M.V. contributed to the conceptualization, revised, edited, finalized, and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
S.M.V. is a member of the board of directors of IMV Inc. The rest of the authors declare no conflict of interest.
Funding Statement
Funding was provided by a Canadian Institutes of Health Research grant MOP#389137 to S.M.V. and a Catalyst Grant from the Western University IDI program.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ursell L.K., Metcalf J.L., Parfrey L.W., Knight R. Defining the human microbiome. Nutr. Rev. 2012;70((Suppl. S1)):S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-Bacterial Mutualism in the Human Intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 5.Ley R.E., Peterson D.A., Gordon J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Wu H.-J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi N., Li N., Duan X., Niu H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017;4:14. doi: 10.1186/s40779-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill S.R., Pop M., DeBoy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sender R., Fuchs S., Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar M., Chen Y., Ma Z., Zhang X., Shi D., Khan J.A., Liu H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022;8:350–360. doi: 10.1016/j.aninu.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godlewska U., Bulanda E., Wypych T.P. Bile acids in immunity: Bidirectional mediators between the host and the microbiota. Front. Immunol. 2022;13:949033. doi: 10.3389/fimmu.2022.949033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021;18:1161–1171. doi: 10.1038/s41423-020-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorgdrager F.J.H., Naudé P.J.W., Kema I.P., Nollen E.A., de Deyn P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019;10:2565. doi: 10.3389/fimmu.2019.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W., Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021;18:866–877. doi: 10.1038/s41423-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna M., Jaqua E., Nguyen V., Clay J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022;26:89–97. doi: 10.7812/TPP/21.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonardi R., Jackowski S. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus. 2007;2:2–17. doi: 10.1128/ecosalplus.3.6.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rucker R.B., Stites T. New perspectives on function of vitamins. Nutrition. 1994;10:507–513. [PubMed] [Google Scholar]
- 19.Mora J.R., Iwata M., von Andrian U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019;9:3160. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedhiafi T., Inchakalody V.P., Fernandes Q., Mestiri S., Billa N., Uddin S., Merhi M., Dermime S. The potential role of vitamin C in empowering cancer immunotherapy. Biomed. Pharmacother. 2022;146:112553. doi: 10.1016/j.biopha.2021.112553. [DOI] [PubMed] [Google Scholar]
- 22.Magrì A., Germano G., Lorenzato A., Lamba S., Chilà R., Montone M., Amodio V., Ceruti T., Sassi F., Arena S., et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020;12:1–13. doi: 10.1126/scitranslmed.aay8707. [DOI] [PubMed] [Google Scholar]
- 23.Sasidharan Nair V., Song M.H., Oh K.I. Vitamin C Facilitates Demethylation of the Foxp3 Enhancer in a Tet-Dependent Manner. J. Immunol. 2016;196:2119–2131. doi: 10.4049/jimmunol.1502352. [DOI] [PubMed] [Google Scholar]
- 24.Yuen R.C.-F., Tsao S.-Y. Embracing cancer immunotherapy with vital micronutrients. World J. Clin. Oncol. 2021;12:712–724. doi: 10.5306/wjco.v12.i9.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan X., Duan Y., Xiao Y., Sun K., Qi Y., Zhang Y., Ahmed Z., Moiani D., Yao J., Li H., et al. Vitamin E Enhances Cancer Immunotherapy by Reinvigorating Dendritic Cells via Targeting Checkpoint SHP1. Cancer Discov. 2022;12:1742–1759. doi: 10.1158/2159-8290.CD-21-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uebanso T., Shimohata T., Mawatari K., Takahashi A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020;64:2000426. doi: 10.1002/mnfr.202000426. [DOI] [PubMed] [Google Scholar]
- 27.Ganal-Vonarburg S.C., Duerr C.U. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology. 2020;159:39–51. doi: 10.1111/imm.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W., Deng Y., Chu Q., Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Szczyrek M., Bitkowska P., Chunowski P., Czuchryta P., Krawczyk P., Milanowski J. Diet, Microbiome, and Cancer Immunotherapy-A Comprehensive Review. Nutrients. 2021;13:2217. doi: 10.3390/nu13072217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vighi G., Marcucci F., Sensi L., di Cara G., Frati F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008;153:3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiertsema S.P., van Bergenhenegouwen J., Garssen J., Knippels L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients. 2021;13:886. doi: 10.3390/nu13030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coker M.O., Madan J.C. The microbiome and immune system development. Dev. Microbiome Lessons Early Life. 2020;1:43–66. doi: 10.1016/B978-0-12-820602-7.00003-9. [DOI] [Google Scholar]
- 34.Mörbe U.M., Jørgensen P.B., Fenton T.M., von Burg N., Riis L.B., Spencer J., Agace W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021;14:793–802. doi: 10.1038/s41385-021-00389-4. [DOI] [PubMed] [Google Scholar]
- 35.Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 36.Vijay-Kumar M., Chassaing B., Kumar M., Baker M., Singh V. Mammalian gut immunity. Biomed. J. 2014;37:246. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aron-Wisnewsky J., Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 2016;12:169–181. doi: 10.1038/nrneph.2015.191. [DOI] [PubMed] [Google Scholar]
- 38.De Vos W.M., Tilg H., van Hul M., Cani P.D. Gut microbiome and health: Mechanistic insights. Gut. 2022;71:1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham V.T., Dold S., Rehman A., Bird J.K., Steinert R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021;95:35–53. doi: 10.1016/j.nutres.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Denning T.L., Norris B.A., Medina-Contreras O., Manicassamy S., Geem D., Madan R., Karp C.L., Pulendran B. Functional Specializations of Intestinal Dendritic Cell and Macrophage Subsets That Control Th17 and Regulatory T Cell Responses Are Dependent on the T Cell/APC Ratio, Source of Mouse Strain, and Regional Localization. J. Immunol. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiebiger U., Bereswill S., Heimesaat M.M. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: Lessons learned from germfree and gnotobiotic animal models. Eur. J. Microbiol. Immunol. 2016;6:253–271. doi: 10.1556/1886.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen A.K., Hansen C.H.F., Krych L., Nielsen D.S. Impact of the gut microbiota on rodent models of human disease. World J. Gastroenterol. 2014;20:17727–17736. doi: 10.3748/wjg.v20.i47.17727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macpherson A.J., Harris N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 44.Dianda L., Hanby A.M., Wright N.A., Sebesteny A., Hayday A.C., Owen M.J. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am. J. Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 45.Manca C., Boubertakh B., Leblanc N., Deschênes T., Lacroix S., Martin C., Houde A., Veilleux A., Flamand N., Muccioli G.G., et al. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J. Lipid Res. 2020;61:70–85. doi: 10.1194/jlr.RA119000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiechers C., Zou M., Galvez E., Beckstette M., Ebel M., Strowig T., Huehn J., Pezoldt J. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell. Mol. Immunol. 2021;18:1211–1221. doi: 10.1038/s41423-021-00647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niess J.H., Leithäuser F., Adler G., Reimann J. Commensal Gut Flora Drives the Expansion of Proinflammatory CD4 T Cells in the Colonic Lamina Propria under Normal and Inflammatory Conditions. J. Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 48.Strauch U.G., Obermeier F., Grunwald N., Gürster S., Dunger N., Schultz M., Griese P.D., Mälher M., Schölmerich J., Rath H.C. Influence of intestinal bacteria on induction of regulatory T cells: Lessons from a transfer model of colitis. Gut. 2005;54:1546–1552. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negi S., Das D.K., Pahari S., Nadeem S., Agrewala J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019;10:2441. doi: 10.3389/fimmu.2019.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marinelli L., Tenore G.C., Novellino E. Probiotic species in the modulation of the anticancer immune response. Semin. Cancer Biol. 2017;46:182–190. doi: 10.1016/j.semcancer.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas H. Bacterial strains modulate CD8+ T cell function and cancer immunity. Nat. Rev. Gastroenterol. Hepatol. 2019;16:141. doi: 10.1038/s41575-019-0120-3. [DOI] [PubMed] [Google Scholar]
- 53.Keppler S.J., Goess M.C., Heinze J.M. The Wanderings of Gut-Derived IgA Plasma Cells: Impact on Systemic Immune Responses. Front. Immunol. 2021;12:670290. doi: 10.3389/fimmu.2021.670290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pabst O., Slack E. IgA and the intestinal microbiota: The importance of being specific. Mucosal Immunol. 2020;13:12–21. doi: 10.1038/s41385-019-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demirdag Y.Y., Gupta S. Update on Infections in Primary Antibody Deficiencies. Front. Immunol. 2021;12:634181. doi: 10.3389/fimmu.2021.634181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim M., Qie Y., Park J., Kim C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lécuyer E., Rakotobe S., Lengliné-Garnier H., Lebreton C., Picard M., Juste C., Fritzen R., Eberl G., McCoy K.D., Macpherson A.J., et al. Segmented Filamentous Bacterium Uses Secondary and Tertiary Lymphoid Tissues to Induce Gut IgA and Specific T Helper 17 Cell Responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez B., Prioult G., Bibiloni R., Nicolis I., Mercenier A., Butel M.-J., Waligora-Dupriet A.-J. Germ-free status and altered caecal subdominant microbiota are associated with a high susceptibility to cow’s milk allergy in mice. FEMS Microbiol. Ecol. 2011;76:133–144. doi: 10.1111/j.1574-6941.2010.01035.x. [DOI] [PubMed] [Google Scholar]
- 59.Taurog J.D., Richardson J.A., Croft J.T., Simmons W.A., Zhou M., Fernández-Sueiro J.L., Balish E., Hammer R.E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Said H.M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–372. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sukemori S., Ikeda S., Kurihara Y., Ito S. Amino acid, mineral and vitamin levels in hydrous faeces obtained from coprophagy-prevented rats. J. Anim. Physiol. Anim. Nutr. 2003;87:213–220. doi: 10.1046/j.1439-0396.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 62.Sukemori S., Kurosawa A., Ikeda S., Kurihara Y. Investigation on the growth of coprophagy-prevented rats with supplemented vitamin B12. J. Anim. Physiol. Anim. Nutr. 2006;90:402–406. doi: 10.1111/j.1439-0396.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 63.Bogatyrev S.R., Rolando J.C., Ismagilov R.F. Self-reinoculation with fecal flora changes microbiota density and composition leading to an altered bile-acid profile in the mouse small intestine. Microbiome. 2020;8:19. doi: 10.1186/s40168-020-0785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Guryn K., Leone V., Chang E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe. 2019;26:314–324. doi: 10.1016/j.chom.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belkaid Y., Hand T.W. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hacquard S., Garrido-Oter R., González A., Spaepen S., Ackermann G., Lebeis S., McHardy A.C., Dangl J.L., Knight R., Ley R., et al. Microbiota and Host Nutrition across Plant and Animal Kingdoms. Cell Host Microbe. 2015;17:603–616. doi: 10.1016/j.chom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dieterich W., Schink M., Zopf Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018;6:116. doi: 10.3390/medsci6040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed S., Macfarlane G.T., Fite A., McBain A.J., Gilbert P., Macfarlane S. Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl. Environ. Microbiol. 2007;73:7435–7442. doi: 10.1128/AEM.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodionov D.A., Arzamasov A.A., Khoroshkin M.S., Iablokov S.N., Leyn S.A., Peterson S.N., Novichkov P.S., Osterman A.L. Micronutrient Requirements and Sharing Capabilities of the Human Gut Microbiome. Front. Microbiol. 2019;10:1316. doi: 10.3389/fmicb.2019.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soto-Martin E.C., Warnke I., Farquharson F.M., Christodoulou M., Horgan G., Derrien M., Faurie J.-M., Flint H.J., Duncan S.H., Louis P. Vitamin Biosynthesis by Human Gut Butyrate-Producing Bacteria and Cross-Feeding in Synthetic Microbial Communities. MBio. 2020;11:e00886-20. doi: 10.1128/mBio.00886-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu Q., Li P. Probiotics and Prebiotics in Human Nutrition and Health. InTech; London, UK: 2016. Biosynthesis of Vitamins by Probiotic Bacteria. [DOI] [Google Scholar]
- 74.Pham V.T., Fehlbaum S., Seifert N., Richard N., Bruins M.J., Sybesma W., Rehman A., Steinert R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome—A pilot study. Gut Microbes. 2021;13:1875774. doi: 10.1080/19490976.2021.1875774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daisley B.A., Koenig D., Engelbrecht K., Doney L., Hards K., Al K.F., Reid G., Burton J.P. Emerging connections between gut microbiome bioenergetics and chronic metabolic diseases. Cell Rep. 2021;37:110087. doi: 10.1016/j.celrep.2021.110087. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Q., Lin L., Xie F., Jin W., Zhu W., Wang M., Qiu Q., Li Z., Liu J., Mao S. Metagenomic insights into the microbe-mediated B and K2 vitamin biosynthesis in the gastrointestinal microbiome of ruminants. Microbiome. 2022;10:109. doi: 10.1186/s40168-022-01298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peterson C.T., Rodionov D.A., Osterman A.L., Peterson S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients. 2020;12:3380. doi: 10.3390/nu12113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magnúsdóttir S., Ravcheev D., de Crécy-Lagard V., Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshii K., Hosomi K., Sawane K., Kunisawa J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma V., Rodionov D.A., Leyn S.A., Tran D., Iablokov S.N., Ding H., Peterson D.A., Osterman A.L., Peterson S.N. B-Vitamin Sharing Promotes Stability of Gut Microbial Communities. Front. Microbiol. 2019;10:1485. doi: 10.3389/fmicb.2019.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zengler K., Zaramela L.S. The social network of microorganisms—How auxotrophies shape complex communities. Nat. Rev. Microbiol. 2018;16:383–390. doi: 10.1038/s41579-018-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson L.N., Koech P.K., Plymale A.E., Landorf E.V., Konopka A., Collart F.R., Lipton M.S., Romine M.F., Wright A.T. Live Cell Discovery of Microbial Vitamin Transport and Enzyme-Cofactor Interactions. ACS Chem. Biol. 2016;11:345–354. doi: 10.1021/acschembio.5b00918. [DOI] [PubMed] [Google Scholar]
- 84.Morowitz M.J., Carlisle E.M., Alverdy J.C. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg. Clin. N. Am. 2011;91:771–785. doi: 10.1016/j.suc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maggini S., Pierre A., Calder P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chandra R.K., Sudhakaran L. Regulation of immune responses by vitamin B6. Ann. N. Y. Acad. Sci. 1990;585:404–423. doi: 10.1111/j.1749-6632.1990.tb28073.x. [DOI] [PubMed] [Google Scholar]
- 87.Trakatellis A., Dimitriadou A., Trakatelli M. Pyridoxine deficiency: New approaches in immunosuppression and chemotherapy. Postgrad Med. J. 1997;73:617–622. doi: 10.1136/pgmj.73.864.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elmadfa I., Meyer A.L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets. 2019;19:1100–1115. doi: 10.2174/1871530319666190529101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qian B., Shen S., Zhang J., Jing P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017;2017:1–12. doi: 10.1155/2017/2197975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stach K., Stach W., Augoff K. Vitamin B6 in Health and Disease. Nutrients. 2021;13:3229. doi: 10.3390/nu13093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mak T.W., Saunders M.E. Allergy and Hypersensitivity. Immune Response. 2006;1:923–962. doi: 10.1016/B978-012088451-3.50030-2. [DOI] [Google Scholar]
- 92.He D., Wu L., Kim H.K., Li H., Elmets C.A., Xu H. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 2009;183:1463–1470. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berger A. Th1 and Th2 responses: What are they? BMJ. 2000;321:424. doi: 10.1136/bmj.321.7258.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romagnani S. Th1/Th2 Cells. Inflamm. Bowel Dis. 1999;5:285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Bretscher P. On Analyzing How the Th1/Th2 Phenotype of an Immune Response Is Determined: Classical Observations Must Not Be Ignored. Front. Immunol. 2019;10:1234. doi: 10.3389/fimmu.2019.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abe A., Shayman J.A. Sphingolipid Catabolism. Encycl. Biol. Chem. 2004;1:82–87. doi: 10.1016/B0-12-443710-9/00726-2. [DOI] [Google Scholar]
- 97.Garattini E., Terao M. Xanthine Oxidoreductase and Aldehyde Oxidases. Compr. Toxicol. Third Ed. 2018;10–15:208–232. doi: 10.1016/B978-0-12-801238-3.99184-0. [DOI] [Google Scholar]
- 98.Du X., Yang Y., Zhan X., Huang Y., Fu Y., Zhang Z., Liu H., Zhang L., Li Y., Wen Q., et al. Vitamin B6 prevents excessive inflammation by reducing accumulation of sphingosine-1-phosphate in a sphingosine-1-phosphate lyase–dependent manner. J. Cell Mol. Med. 2020;24:13129–13138. doi: 10.1111/jcmm.15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Obinata H., Hla T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019;31:617–625. doi: 10.1093/intimm/dxz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao P., Liu I.D., Hodgin J.B., Benke P.I., Selva J., Torta F., Wenk M.R., Endrizzi J.A., West O., Ou W., et al. Responsiveness of sphingosine phosphate lyase insufficiency syndrome to vitamin B6 cofactor supplementation. J. Inherit. Metab. Dis. 2020;43:1131–1142. doi: 10.1002/jimd.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Workman C.J., Szymczak-Workman A.L., Collison L.W., Pillai M.R., Vignali D.A.A. The development and function of regulatory T cells. Cell Mol. Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kunisawa J., Hashimoto E., Ishikawa I., Kiyono H. A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS ONE. 2012;7:e32094. doi: 10.1371/journal.pone.0032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kunisawa J., Kiyono H. Vitamin-Mediated Regulation of Intestinal Immunity. Front. Immunol. 2013;4:189. doi: 10.3389/fimmu.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao D.-Z. Effects of folic acid on epithelial apoptosis and expression of Bcl-2 and p53 in premalignant gastric lesions. World J. Gastroenterol. 2005;11:1571. doi: 10.3748/wjg.v11.i11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walker L.S.K. Regulatory T cells: Folate receptor 4: A new handle on regulation and memory? Immunol. Cell Biol. 2007;85:506–507. doi: 10.1038/sj.icb.7100115. [DOI] [PubMed] [Google Scholar]
- 106.Tian Y., Wu G., Xing J.-C., Tang J., Zhang Y., Huang Z.-M., Jia Z.C., Zhao R., Tian Z.-Q., Wang S.-F., et al. A novel splice variant of folate receptor 4 predominantly expressed in regulatory T cells. BMC Immunol. 2012;13:30. doi: 10.1186/1471-2172-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamaguchi T., Hirota K., Nagahama K., Ohkawa K., Takahashi T., Nomura T., Sakaguchi S. Control of Immune Responses by Antigen-Specific Regulatory T Cells Expressing the Folate Receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 108.Kinoshita M., Kayama H., Kusu T., Yamaguchi T., Kunisawa J., Kiyono H., Sakaguchi S., Takeda Y. Dietary Folic Acid Promotes Survival of Foxp3+ Regulatory T Cells in the Colon. J. Immunol. 2012;189:2869–2878. doi: 10.4049/jimmunol.1200420. [DOI] [PubMed] [Google Scholar]
- 109.Zhang X., Zhao Q., Cao M., Li X., Chen X., He M., Liu Y., Zhao J. Folate Receptor 4-Expressing T cell Is Associated with Disease-Free Survival in Patients with Esophageal Squamous Cell Carcinoma. Dis. Markers. 2022;2022:4351949. doi: 10.1155/2022/4351949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crespo J., Sun H., Welling T.H., Tian Z., Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kalekar L.A., Mueller D.L. Relationship between CD4 Regulatory T Cells and Anergy In Vivo. J. Immunol. 2017;198:2527–2533. doi: 10.4049/jimmunol.1602031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mölzer C., Wilson H.M., Kuffova L., Forrester J.V. A Role for Folate in Microbiome-Linked Control of Autoimmunity. J. Immunol. Res. 2021;2021:9998200. doi: 10.1155/2021/9998200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schietinger A., Greenberg P.D. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valdor R., Macian F. Induction and stability of the anergic phenotype in T cells. Semin. Immunol. 2013;25:313–320. doi: 10.1016/j.smim.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dornmair K., Goebels N., Weltzien H.-U., Wekerle H., Hohlfeld R. T-cell-mediated autoimmunity: Novel techniques to characterize autoreactive T-cell receptors. Am. J. Pathol. 2003;163:1215–1226. doi: 10.1016/S0002-9440(10)63481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Engevik M.A., Morra C.N., Röth D., Engevik K., Spinler J.K., Devaraj S., Crawford S.E., Estes M.K., Kalkum M., Versalovic J. Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 2019;10:2305. doi: 10.3389/fmicb.2019.02305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jäger A., Kuchroo V.K. Effector and Regulatory T-cell Subsets in Autoimmunity and Tissue Inflammation. Scand. J. Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kurniawan H., Kobayashi T., Brenner D. The emerging role of one-carbon metabolism in T cells. Curr. Opin. Biotechnol. 2021;68:193–201. doi: 10.1016/j.copbio.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 119.Nogal A., Valdes A.M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1–24. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu C.-H., Huang T.-C., Lin B.-F. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J. Nutr. Biochem. 2017;41:65–72. doi: 10.1016/j.jnutbio.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Jones P., Lucock M., Scarlett C.J., Veysey M., Beckett E.L. Folate and Inflammation–links between folate and features of inflammatory conditions. J. Nutr. Intermed Metab. 2019;18:100104. doi: 10.1016/j.jnim.2019.100104. [DOI] [Google Scholar]
- 122.Okeke E.B., Uzonna J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019;10:680. doi: 10.3389/fimmu.2019.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Skapenko A., Leipe J., Lipsky P.E., Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 2005;7((Suppl. 2)):S7–S14. doi: 10.1186/ar1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang M., Zhou Q., Dorfman R.G., Huang X., Fan T., Zhang H., Fan T., Zhang H., Zhang J., Yu C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16:84. doi: 10.1186/s12876-016-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alpers D.H. Absorption and blood/cellular transport of folate and cobalamin: Pharmacokinetic and physiological considerations. Biochimie. 2016;126:52–56. doi: 10.1016/j.biochi.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Laudert D., Hohmann H.P. Application of Enzymes and Microbes for the Industrial Production of Vitamins and Vitamin-Like Compounds. Compr. Biotechnol. Second. Ed. 2011;3:583–602. doi: 10.1016/B978-0-08-088504-9.00214-2. [DOI] [Google Scholar]
- 127.Mikkelsen K., Apostolopoulos V. Nutrition and Immunity. Springer International Publishing; Cham, Switzerland: 2019. Vitamin B12, Folic Acid, and the Immune System; pp. 103–114. [DOI] [Google Scholar]
- 128.Hughes C.F., Ward M., Hoey L., McNulty H. Vitamin B 12 and ageing: Current issues and interaction with folate. Ann. Clin. Biochem. Int. J. Lab. Med. 2013;50:315–329. doi: 10.1177/0004563212473279. [DOI] [PubMed] [Google Scholar]
- 129.Selhub J., Morris M.S., Jacques P.F., Rosenberg I.H. Folate–vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am. J. Clin. Nutr. 2009;89:702S–706S. doi: 10.3945/ajcn.2008.26947C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allen L.H. Vitamin B-12. Adv. Nutr. 2012;3:54–55. doi: 10.3945/an.111.001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Klein Geltink R.I., Pearce E.L. The importance of methionine metabolism. eLife. 2019;8:e47221. doi: 10.7554/eLife.47221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sinclair L.V., Howden A.J., Brenes A., Spinelli L., Hukelmann J.L., Macintyre A.N., Liu X., Thomson S., Taylor P.M., Rathmell J.C., et al. Antigen receptor control of methionine metabolism in T cells. eLife. 2019;8:e44210. doi: 10.7554/eLife.44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roy D.G., Chen J., Mamane V., Ma E.H., Muhire B.M., Sheldon R.D., Shorstova T., Koning R., Johnson R.M., Esaulova E., et al. Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metab. 2020;31:250–266.e9. doi: 10.1016/j.cmet.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 134.Froese D.S., Fowler B., Baumgartner M.R. Vitamin B 12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019;42:673–685. doi: 10.1002/jimd.12009. [DOI] [PubMed] [Google Scholar]
- 135.Abbasi I.H.R., Abbasi F., Wang L., Abd El Hack M.E., Swelum A.A., Hao R., Yao J., Cao Y. Folate promotes S-adenosyl methionine reactions and the microbial methylation cycle and boosts ruminants production and reproduction. AMB Express. 2018;8:65. doi: 10.1186/s13568-018-0592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Annibal A., Tharyan R.G., Schonewolff M.F., Tam H., Latza C., Auler M.M.K., Grönke S., Partridge L., Antebi A. Regulation of the one carbon folate cycle as a shared metabolic signature of longevity. Nat. Commun. 2021;12:3486. doi: 10.1038/s41467-021-23856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ding W., Smulan L.J., Hou N.S., Taubert S., Watts J.L., Walker A.K. s-Adenosylmethionine Levels Govern Innate Immunity through Distinct Methylation-Dependent Pathways. Cell Metab. 2015;22:633–645. doi: 10.1016/j.cmet.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Djokic G., Korcok D., Djordjevic V., Agic A., Rankovic A., Djukic D. The effects of S-adenosyl-L-methionine-vitamin B complex on mild and moderate depressive symptoms. Hippokratia. 2017;21:140–143. [PMC free article] [PubMed] [Google Scholar]
- 139.EFSA NDA Panel (EFSA Panel on Dietetic Products N and A Scientific Opinion on Dietary Reference Values for folate. EFSA J. 2014;12:3893. doi: 10.2903/j.efsa.2014.3893. [DOI] [Google Scholar]
- 140.Ullah H., Khan A., Rengasamy K.R.R., di Minno A., Sacchi R., Daglia M. The Efficacy of S-Adenosyl Methionine and Probiotic Supplementation on Depression: A Synergistic Approach. Nutrients. 2022;14:2751. doi: 10.3390/nu14132751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mehdi A., Rabbani S.A. Role of Methylation in Pro- and Anti-Cancer Immunity. Cancers. 2021;13:545. doi: 10.3390/cancers13030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.German D.C., Bloch C.A., Kredich N.M. Measurements of S-adenosylmethionine and L-homocysteine metabolism in cultured human lymphoid cells. J. Biol. Chem. 1983;258:10997–11003. doi: 10.1016/S0021-9258(17)44376-6. [DOI] [PubMed] [Google Scholar]
- 143.Lawson B.R., Eleftheriadis T., Tardif V., Gonzalez-Quintial R., Baccala R., Kono D.H., Theofilopoulos A.N. Transmethylation in immunity and autoimmunity. Clin. Immunol. 2012;143:8–21. doi: 10.1016/j.clim.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dhur A., Galan P., Hercberg S. Folate status and the immune system. Prog. Food Nutr. Sci. 1991;15:43–60. [PubMed] [Google Scholar]
- 145.Stover P.J. Physiology of Folate and Vitamin B 12 in Health and Disease. Nutr. Rev. 2004;62:S3–S12. doi: 10.1111/j.1753-4887.2004.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 146.Courtemanche C., Elson-Schwab I., Mashiyama S.T., Kerry N., Ames B.N. Folate Deficiency Inhibits the Proliferation of Primary Human CD8+ T Lymphocytes In Vitro. J. Immunol. 2004;173:3186–3192. doi: 10.4049/jimmunol.173.5.3186. [DOI] [PubMed] [Google Scholar]
- 147.Ramaekers V.T., Rothenberg S.P., Sequeira J.M., Opladen T., Blau N., Quadros E.V., Selhub J. Autoantibodies to Folate Receptors in the Cerebral Folate Deficiency Syndrome. N. Engl. J. Med. 2005;352:1985–1991. doi: 10.1056/NEJMoa043160. [DOI] [PubMed] [Google Scholar]
- 148.Saeed F., Nadeem M., Ahmed R.S., Tahir Nadeem M., Arshad M.S., Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016;27:205–229. doi: 10.1080/09540105.2015.1079600. [DOI] [Google Scholar]
- 149.Watkins D., Rosenblatt D.S. Immunodeficiency and inborn disorders of vitamin B12 and folate metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2020;23:241–246. doi: 10.1097/MCO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 150.Farmer J.T., Dietert R.R. A Comprehensive Guide to Toxicology in Preclinical Drug Development. Academic Press; Cambridge, MA, USA: 2013. Immunotoxicology Assessment in Drug Development; pp. 365–381. [DOI] [Google Scholar]
- 151.Pecora F., Persico F., Argentiero A., Neglia C., Esposito S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients. 2020;12:3198. doi: 10.3390/nu12103198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gombart A.F., Pierre A., Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bargiela D., Cunha P.P., Veliça P., Foskolou I.P., Barbieri L., Rundqvist H., Johnson R.S. Vitamin B6 Metabolism Determines T Cell Anti-Tumor Responses. Front. Immunol. 2022;13:1–14. doi: 10.3389/fimmu.2022.837669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ha C., Miller L.T., Kerkvliet N.I. The effect of vitamin B6 deficiency on cytotoxic immune responses of T cells, antibodies, and natural killer cells, and phagocytosis by macrophages. Cell Immunol. 1984;85:318–329. doi: 10.1016/0008-8749(84)90246-6. [DOI] [PubMed] [Google Scholar]
- 155.Tina Suksmasari B.H. Multivitamin Supplementation Supports Immune Function and Ameliorates Conditions Triggered By Reduced Air Quality. Vitam. Miner. 2015;4:1–15. doi: 10.4172/2376-1318.1000128. [DOI] [Google Scholar]
- 156.Yasuda H., Tsutsui M., Ando J., Inano T., Noguchi M., Yahata Y., Tanaka M., Tsukune Y., Masuda A., Shirane S., et al. Vitamin B6 deficiency is prevalent in primary and secondary myelofibrosis patients. Int. J. Hematol. 2019;110:543–549. doi: 10.1007/s12185-019-02717-8. [DOI] [PubMed] [Google Scholar]
- 157.Funada U., Wada M., Kawata T., Mori K., Tamai H., Kawanishi T., Kunou A., Tanka N., Tadokoro T., Maekawa A. Changes in CD4+CD8−/CD4−CD8+ Ratio and Humoral Immune Functions in Vitamin B12-Deficient Rats. Int. J. Vitam. Nutr. Res. 2000;70:167–171. doi: 10.1024/0300-9831.70.4.167. [DOI] [PubMed] [Google Scholar]
- 158.Partearroyo T., Úbeda N., Montero A., Achón M., Varela-Moreiras G. Vitamin B(12) and folic acid imbalance modifies NK cytotoxicity, lymphocytes B and lymphoprolipheration in aged rats. Nutrients. 2013;5:4836–4848. doi: 10.3390/nu5124836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parra M., Stahl S., Hellmann H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells. 2018;7:84. doi: 10.3390/cells7070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Justiz Vaillant A.A., Qurie A. StatPearls. StatPearls Publishing; Tampa, FL, USA: 2022. Immunodeficiency. [Google Scholar]
- 161.Rosenberg J., Huang J. CD8+ T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr. Opin. Chem. Eng. 2018;19:9–20. doi: 10.1016/j.coche.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Uzhachenko R.V., Shanker A. CD8+ T Lymphocyte and NK Cell Network: Circuitry in the Cytotoxic Domain of Immunity. Front. Immunol. 2019;10:1906. doi: 10.3389/fimmu.2019.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weigelin B., den Boer ATh Wagena E., Broen K., Dolstra H., de Boer R.J., Figdor C.G., Textor J., Friedl P. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 2021;12:5217. doi: 10.1038/s41467-021-25282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bruni D., Angell H.K., Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer. 2020;20:662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 165.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Ballegooijen A.J., Pilz S., Tomaschitz A., Grübler M.R., Verheyen N. The Synergistic Interplay between Vitamins D and K for Bone and Cardiovascular Health: A Narrative Review. Int. J. Endocrinol. 2017;2017:7454376. doi: 10.1155/2017/7454376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wen L., Chen J., Duan L., Li S. Vitamin K-dependent proteins involved in bone and cardiovascular health (Review) Mol. Med. Rep. 2018;18:3–15. doi: 10.3892/mmr.2018.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goddek S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int. J. Infect. Dis. 2020;99:286–290. doi: 10.1016/j.ijid.2020.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Halder M., Petsophonsakul P., Akbulut A.C., Pavlic A., Bohan F., Anderson E., Maresz K., Kramaan R., Schurgers L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019;20:896. doi: 10.3390/ijms20040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schurgers L.J., Vermeer C. Determination of Phylloquinone and Menaquinones in Food. Pathophysiol. Haemost. Thromb. 2000;30:298–307. doi: 10.1159/000054147. [DOI] [PubMed] [Google Scholar]
- 171.Bentley R., Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol. Rev. 1982;46:241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ellis J.L., Karl J.P., Oliverio A.M., Fu X., Soares J.W., Wolfe B.E., Hernandez C.J., Mason J.B., Booth S.L. Dietary vitamin K is remodeled by gut microbiota and influences community composition. Gut Microbes. 2021;13:1–16. doi: 10.1080/19490976.2021.1887721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lai Y., Masatoshi H., Ma Y., Guo Y., Zhang B. Role of Vitamin K in Intestinal Health. Front. Immunol. 2022;12:5491. doi: 10.3389/fimmu.2021.791565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Prietl B., Treiber G., Pieber T., Amrein K. Vitamin D and Immune Function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cantorna M.T., Lin Y.-D., Arora J., Bora S., Tian Y., Nichols R.G., Patterson A.D. Vitamin D Regulates the Microbiota to Control the Numbers of RORγt/FoxP3+ Regulatory T Cells in the Colon. Front. Immunol. 2019;10:1772. doi: 10.3389/fimmu.2019.01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Singh P., Rawat A., Alwakeel M., Sharif E., al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020;10:21641. doi: 10.1038/s41598-020-77806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Danne C., Sokol H. Butyrate, a new microbiota-dependent player in CD8+ T cells immunity and cancer therapy? Cell Rep. Med. 2021;2:100328. doi: 10.1016/j.xcrm.2021.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schulthess J., Pandey S., Capitani M., Rue-Albrecht K.C., Arnold I., Franchini F., Chomka A., Ilott N.E., Johnston D.G.W., Pires E., et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity. 2019;50:432–445.e7. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Siddiqui M.T., Cresci G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021;14:6025–6041. doi: 10.2147/JIR.S300989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thomas R.L., Jiang L., Adams J.S., Xu Z.Z., Shen J., Janssen S., Ackermann G., Vanderschueren D., Pauwels S., Knight R., et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020;11:5997. doi: 10.1038/s41467-020-19793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Luthold R.V., Fernandes G.R., Franco-de-Moraes A.C., Folchetti L.G.D., Ferreira S.R.G. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism. 2017;69:76–86. doi: 10.1016/j.metabol.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 183.Xv F., Chen J., Duan L., Li S. Research progress on the anticancer effects of vitamin K2 (Review) Oncol. Lett. 2018;15:8926–8934. doi: 10.3892/ol.2018.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Krajewska M., Witkowska-Sędek E., Rumińska M., Stelmaszczyk-Emmel A., Sobol M., Majcher A., Pyrzak B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022;13:1152. doi: 10.3389/fendo.2022.920340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simes D.C., Viegas C.S.B., Araújo N., Marreiros C. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019;20:4150. doi: 10.3390/ijms20174150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Adams J.S., Hewison M. Update in Vitamin, D. J. Clin. Endocrinol. Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Battault S., Whiting S.J., Peltier S.L., Sadrin S., Gerber G., Maixent J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013;52:429–441. doi: 10.1007/s00394-012-0430-5. [DOI] [PubMed] [Google Scholar]
- 188.Holick M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 189.Enioutina E.Y., Bareyan D., Daynes R.A. TLR-Induced Local Metabolism of Vitamin D3 Plays an Important Role in the Diversification of Adaptive Immune Responses. J. Immunol. 2009;182:4296–4305. doi: 10.4049/jimmunol.0804344. [DOI] [PubMed] [Google Scholar]
- 190.Lin R. Crosstalk between Vitamin D Metabolism, VDR Signalling, and Innate Immunity. Biomed. Res. Int. 2016;2016:1–5. doi: 10.1155/2016/1375858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barragan M., Good M., Kolls J.K. Regulation of Dendritic Cell Function by Vitamin D. Nutrients. 2015;7:8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Griffin M.D., Lutz W.H., Phan V.A., Bachman L.A., McKean D.J., Kumar R. Potent Inhibition of Dendritic Cell Differentiation and Maturation by Vitamin D Analogs. Biochem. Biophys. Res. Commun. 2000;270:701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 193.Bruce D., Yu S., Ooi J.H., Cantorna M.T. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int. Immunol. 2011;23:519–528. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Griffin M.D., Lutz W., Phan V.A., Bachman L.A., McKean D.J., Kumar R. Dendritic cell modulation by 1α,25 dihydroxyvitamin D 3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 196.Mahon B.D., Wittke A., Weaver V., Cantorna M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 197.Prietl B., Pilz S., Wolf M., Tomaschitz A., Obermayer-Pietsch B., Graninger W., Pieber T.R. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: Vitamin D treatment for autoimmune diseases? Isr. Med. Assoc. J. 2010;12:136–139. [PubMed] [Google Scholar]
- 198.Hewison M. An update on vitamin D and human immunity. Clin. Endocrinol. 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 199.Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F.J., O’Garra A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 200.Fisher S.A., Rahimzadeh M., Brierley C., Gration B., Doree C., Kimber C.E., Cajide A.P., Lamikanra A.A., Roberts D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE. 2019;14:e0222313. doi: 10.1371/journal.pone.0222313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hayes C.E., Hubler S.L., Moore J.R., Barta L.E., Praska C.E., Nashold F.E. Vitamin D Actions on CD4+ T Cells in Autoimmune Disease. Front. Immunol. 2015;6:100. doi: 10.3389/fimmu.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Aranow C. Vitamin D and the immune system. J. Investig. Med. 2011;59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carlberg C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019;10:2211. doi: 10.3389/fimmu.2019.02211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kostoglou-Athanassiou I., Athanassiou L., Athanassiou P. Vitamin D Deficiency. IntechOpen; London, UK: 2020. Vitamin D and Autoimmune Diseases. [DOI] [Google Scholar]
- 205.Grover S., Dougan M., Tyan K., Giobbie-Hurder A., Blum S.M., Ishizuka J., Qazi T., Elias R., Vora K.B., Ruan A.B., et al. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer. 2020;126:3758–3767. doi: 10.1002/cncr.32966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Myneni V., Mezey E. Immunomodulatory effect of vitamin K2: Implications for bone health. Oral Dis. 2018;24:67–71. doi: 10.1111/odi.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Borchmann S., Cirillo M., Goergen H., Meder L., Sasse S., Kreissl S., Bröckelmann P.J., Tresckow B.V., Fuchs M., Ullrich R.T., et al. Pretreatment Vitamin D Deficiency Is Associated with Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. J. Clin. Oncol. 2019;37:3528–3537. doi: 10.1200/JCO.19.00985. [DOI] [PubMed] [Google Scholar]
- 208.Naz A., Qureshi R.N., Shamsi T.S., Mahboob T. Vitamin D levels in patients of acute leukemia before and after remission-induction therapy. Pak. J. Med. Sci. 2012;29:1–14. doi: 10.12669/pjms.291.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cerhan J.R., O’Connor H.M., Fredericksen Z.S., Liebow M., Macon W.R., Wang A.H., Zent C.S., Ansell S.M., Slager S.L., Call T.G., et al. Abstract 2811: Vitamin K intake and risk of non-Hodgkin lymphoma (NHL) Cancer Res. 2010;70:2811. doi: 10.1158/1538-7445.AM10-2811. [DOI] [Google Scholar]
- 210.Dragh M.A., Xu Z., Al-Allak Z.S., Hong L. Vitamin K2 Prevents Lymphoma in Drosophila. Sci. Rep. 2017;7:17047. doi: 10.1038/s41598-017-17270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kołakowski A., Kurzyna P.F., Bzdęga W., Żywno H., Harasim-Symbor E., Chabowski A., Konstantynowicz-Nowicka K. Influence of vitamin K2 on lipid precursors of inflammation and fatty acids pathway activities in HepG2 cells. Eur. J. Cell Biol. 2021;100:151188. doi: 10.1016/j.ejcb.2021.151188. [DOI] [PubMed] [Google Scholar]
- 212.Ohsaki Y., Shirakawa H., Hiwatashi K., Furukawa Y., Mizutani T., Komai M. Vitamin K Suppresses Lipopolysaccharide-Induced Inflammation in the Rat. Biosci. Biotechnol. Biochem. 2006;70:926–932. doi: 10.1271/bbb.70.926. [DOI] [PubMed] [Google Scholar]
- 213.Reddi K., Henderson B., Meghji S., Wilson M., Poole S., Hopper C., Harris M., Hodges S.J. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by Naphthoquinone (vitamin K) compounds. Cytokine. 1995;7:287–290. doi: 10.1006/cyto.1995.0034. [DOI] [PubMed] [Google Scholar]
- 214.Kudelko M., Yip T.F., Hei Law G.C., Lee S.M.Y. Potential Beneficial Effects of Vitamin K in SARS-CoV-2 Induced Vascular Disease? Immuno. 2021;1:17–29. doi: 10.3390/immuno1010003. [DOI] [Google Scholar]
- 215.Visser M.P.J., Dofferhoff A.S.M., van den Ouweland J.M.W., van Daal H., Kramers C., Schurgers L.J., Jansen R., Walk J. Effects of Vitamin D and K on Interleukin-6 in COVID-19. Front. Nutr. 2022;8:1282. doi: 10.3389/fnut.2021.761191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Saputra W.D., Aoyama N., Komai M., Shirakawa H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019;20:2317. doi: 10.3390/ijms20092317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Berenjian A., Sarabadani Z. How menaquinone-7 deficiency influences mortality and morbidity among COVID-19 patients. Biocatal Agric. Biotechnol. 2020;29:101792. doi: 10.1016/j.bcab.2020.101792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mangge H., Prueller F., Dawczynski C., Curcic P., Sloup Z., Holter M., Herrmann M., Meinitzer A. Dramatic Decrease of Vitamin K2 Subtype Menaquinone-7 in COVID-19 Patients. Antioxidants. 2022;11:1235. doi: 10.3390/antiox11071235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shiraishi E., Iijima H., Shinzaki S., Nakajima S., Inoue T., Hiyama S., Kawai S., Araki M., Yamaguchi T., Hayashi Y., et al. Vitamin K deficiency leads to exacerbation of murine dextran sulfate sodium-induced colitis. J. Gastroenterol. 2016;51:346–356. doi: 10.1007/s00535-015-1112-x. [DOI] [PubMed] [Google Scholar]
- 221.Zhang M., Miura T., Suzuki S., Chiyotanda M., Tanaka S., Sugiyama K., Kawashima H., Hirano T. Vitamin K2 Suppresses Proliferation and Inflammatory Cytokine Production in Mitogen-Activated Lymphocytes of Atopic Dermatitis Patients through the Inhibition of Mitogen-Activated Protein Kinases. Biol. Pharm. Bull. 2021;44:7–17. doi: 10.1248/bpb.b20-00079. [DOI] [PubMed] [Google Scholar]
- 222.Edfeldt K., Liu P.T., Chun R., Fabri M., Schenk M., Wheelwright M., Keegan C., Krutzik S.R., Adams J.S., Hewison M., et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. USA. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C., et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 224.Matthews D., LaPorta E., Zinser G.M., Narvaez C.J., Welsh J. Genomic vitamin D signaling in breast cancer: Insights from animal models and human cells. J. Steroid Biochem. Mol. Biol. 2010;121:362–367. doi: 10.1016/j.jsbmb.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lemire J.M., Adams J.S., Sakai R., Jordan S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ahangar-Parvin R., Mohammadi-Kordkhayli M., Azizi S.V., Nemati M., Khorramdelazad H., Taghipour Z., Hassan Z., Moazzeni S.M., Jafarzadeh A. The Modulatory Effects of Vitamin D on the Expression of IL-12 and TGF-β in the Spinal Cord and Serum of Mice with Experimental Autoimmune Encephalomyelitis. Iran. J. Pathol. 2018;13:10–22. [PMC free article] [PubMed] [Google Scholar]
- 227.Azimi A., Ghajarzadeh M., Sahraian M.A., Mohammadifar M., Roostaei B., Samani S.M.V., Shabestari H.R.F., Hanaei S. Effects of Vitamin D Supplements on IL-10 and INFγ Levels in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Maedica. 2019;14:413–417. doi: 10.26574/maedica.2019.14.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Calton E.K., Keane K.N., Newsholme P., Soares M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE. 2015;10:e0141770. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dadaei T., Safapoor M.H., Asadzadeh Aghdaei H., Balaii H., Pourhoseingholi M.A., Naderi N., Zojaji H., Azimzadeh P., Mohammadi P., Zali M.R. Effect of vitamin D3 supplementation on TNF-α serum level and disease activity index in Iranian IBD patients. Gastroenterol. Hepatol. Bed Bench. 2015;8:49–55. [PMC free article] [PubMed] [Google Scholar]
- 230.Das P., Babaei P., Nielsen J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019;20:208. doi: 10.1186/s12864-019-5591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dauletbaev N., Herscovitch K., Das M., Chen H., Bernier J., Matouk E., Berube J., Rousseau S., Lands L.S. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br. J. Pharmacol. 2015;172:4757–4771. doi: 10.1111/bph.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ene C.-D., Anghel A.-E., Neagu M., Nicolae I. 25-OH Vitamin D and Interleukin-8: Emerging Biomarkers in Cutaneous Melanoma Development and Progression. Mediat. Inflamm. 2015;2015:1–8. doi: 10.1155/2015/904876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hashemi R., Hosseini-Asl S.S., Arefhosseini S.R., Morshedi M. The impact of vitamin D3 intake on inflammatory markers in multiple sclerosis patients and their first-degree relatives. PLoS ONE. 2020;15:e0231145. doi: 10.1371/journal.pone.0231145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kong J., Grando S.A., Li Y.C. Regulation of IL-1 Family Cytokines IL-1α, IL-1 Receptor Antagonist, and IL-18 by 1,25-Dihydroxyvitamin D3 in Primary Keratinocytes. J. Immunol. 2006;176:3780–3787. doi: 10.4049/jimmunol.176.6.3780. [DOI] [PubMed] [Google Scholar]
- 235.Silberstein M. COVID-19 and IL-6: Why vitamin D (probably) helps but tocilizumab might not. Eur. J. Pharmacol. 2021;899:174031. doi: 10.1016/j.ejphar.2021.174031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Song E.K., Moser D.K., Lennie T.A. Abstract 20661: Vitamin D Deficiency, Tumor Necrosis Factor-Alpha, and Event-Free Survival in Patients With Heart Failure. Circulation. 2014;130:A20661. doi: 10.1161/circ.130.suppl_2.20661. [DOI] [Google Scholar]
- 237.Müller K., Diamant M., Bendtzen K. Inhibition of production and function of interleukin-6 by 1,25-dihydroxyvitamin D3. Immunol. Lett. 1991;28:115–120. doi: 10.1016/0165-2478(91)90108-M. [DOI] [PubMed] [Google Scholar]
- 238.Bayraktar N., Turan H., Bayraktar M., Ozturk A., Erdoğdu H. Analysis of serum cytokine and protective vitamin D levels in severe cases of COVID-19. J. Med. Virol. 2022;94:154–160. doi: 10.1002/jmv.27294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020;32:2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Davis I.D. An overview of cancer immunotherapy. Immunol. Cell Biol. 2000;78:179–195. doi: 10.1046/j.1440-1711.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 241.Esfahani K., Roudaia L., Buhlaiga N., del Rincon S v Papneja N., Miller W.H. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020;27:S87–S97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fecal Microbial Transplantation Non-Small Cell Lung Cancer and Melanoma (FMT-LUMINATE) n.d. [(accessed on 14 January 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04951583?term=fmt&cond=immune+checkpoint+inhibitor&draw=2&rank=7.
- 243.Fecal Microbiota Transplantation to Improve Efficacy of Immune Checkpoint Inhibitors in Renal Cell Carcinoma (TACITO) n.d. [(accessed on 14 January 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04758507?term=fmt&cond=immune+checkpoint+inhibitor&draw=2&rank=4.
- 244.Fecal Microbiota Transplantation with Immune Checkpoint Inhibitors in Lung Cancer n.d. [(accessed on 14 January 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05502913?term=fmt&cond=immune+checkpoint+inhibitor&draw=2&rank=5.
- 245.FMT Combined with Immune Checkpoint Inhibitor and TKI in the Treatment of CRC Patients with Advanced Stage n.d. [(accessed on 14 January 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05279677?term=fmt&cond=immune+checkpoint+inhibitor&draw=2&rank=3.
- 246.Fecal Microbiota Transplantation in Patients with Malignancies Not Responding to Immune Checkpoint Inhibitor Therapy n.d. [(accessed on 14 January 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05273255?term=fmt&cond=immune+checkpoint+inhibitor&draw=2&rank=1.
- 247.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.-L., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duoing C.P.M., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2022;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li L., McAllister F. Too much water drowned the miller: Akkermansia determines immunotherapy responses. Cell Rep. Med. 2022;3:100642. doi: 10.1016/j.xcrm.2022.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kirmiz N., Galindo K., Cross K.L., Luna E., Rhoades N., Podar M., Flores G.E. Comparative Genomics Guides Elucidation of Vitamin B 12 Biosynthesis in Novel Human-Associated Akkermansia Strains. Appl. Environ. Microbiol. 2020;86:e02117-19. doi: 10.1128/AEM.02117-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Grajeda-Iglesias C., Durand S., Daillère R., Iribarren K., Lemaitre F., Derosa L., Aprahamian F., Bossut N., Nirtmalathasan N., Madeo F., et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging. 2021;13:6375–6405. doi: 10.18632/aging.202739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.-L., Luke J.J., Gaweski T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Routy B., le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 255.Wojas-Krawczyk K., Kalinka E., Grenda A., Krawczyk P., Milanowski J. Beyond PD-L1 Markers for Lung Cancer Immunotherapy. Int. J. Mol. Sci. 2019;20:1915. doi: 10.3390/ijms20081915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mao J., Wang D., Long J., Yang X., Lin J., Song Y., Xie F., Xun Z., Wang Y., Wang Y., et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer. 2021;9:e003334. doi: 10.1136/jitc-2021-003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 259.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.-M., Morrison R.M., Debalsio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Duan H., Yu L., Tian F., Zhai Q., Fan L., Chen W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 2022;62:1427–1452. doi: 10.1080/10408398.2020.1843396. [DOI] [PubMed] [Google Scholar]
- 261.Elkrief A., Derosa L., Zitvogel L., Kroemer G., Routy B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes. 2019;10:424–428. doi: 10.1080/19490976.2018.1527167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jing Y., Chen X., Li K., Liu Y., Zhang Z., Chen Y., Liu Y., Wang Y., Lin S.H., Diao L., et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J. Immunother. Cancer. 2022;10:e003779. doi: 10.1136/jitc-2021-003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Reed J.P., Devkota S., Figlin R.A. Gut microbiome, antibiotic use, and immunotherapy responsiveness in cancer. Ann. Transl. Med. 2019;7:S309. doi: 10.21037/atm.2019.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zitvogel L., Ma Y., Raoult D., Kroemer G., Gajewski T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science. 2018;359:1366–1370. doi: 10.1126/science.aar6918. [DOI] [PubMed] [Google Scholar]
- 265.Derosa L., Hellmann M.D., Spaziano M., Halpenny D., Fidelle M., Rizvi H., Long N., Plodkowski A.J., Arbour K.C., Chaft J.E., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shevchuk Y.M., Pharm D., Conly J.M. Antibiotic-associated hypoprothrombinemia. Infect. Dis. Newsl. 1992;11:43–46. doi: 10.1016/0278-2316(92)90002-U. [DOI] [PubMed] [Google Scholar]
- 268.Guarner F., Malagelada J.-R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 269.Lurz E., Horne R.G., Määttänen P., Wu R.Y., Botts S.R., Li B., Rossi L., Johnson-Henry K.C., Pierre A., Surette M.G., et al. Vitamin B12 Deficiency Alters the Gut Microbiota in a Murine Model of Colitis. Front. Nutr. 2020;7:83. doi: 10.3389/fnut.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tabatabaeizadeh S.-A., Tafazoli N., Ferns G.A., Avan A., Ghayour-Mobarhan M. Vitamin D, the gut microbiome and inflammatory bowel disease. J. Res. Med. Sci. 2018;23:75. doi: 10.4103/jrms.JRMS_606_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yuan J., Li J., Shang M., Fu Y., Wang T. Identification of vitamin B6 as a PD-L1 suppressor and an adjuvant for cancer immunotherapy. Biochem. Biophys. Res. Commun. 2021;561:187–194. doi: 10.1016/j.bbrc.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 272.Leyssens C., Verlinden L., Verstuyf A. The future of vitamin D analogs. Front. Physiol. 2014;5:122. doi: 10.3389/fphys.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Coussens A.K., Wilkinson R.J., Hanifa Y., Nikolayevskyy V., Elkington P.T., Islam K., Timms P.M., Venton T.R., Bothamley G.H., Packe G.E., et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. USA. 2012;109:15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Albarrán V., Chamorro J., Rosero D.I., Saavedra C., Soria A., Carrato A., Gajate P. Neurologic Toxicity of Immune Checkpoint Inhibitors: A Review of Literature. Front. Pharmacol. 2022;13:1–18. doi: 10.3389/fphar.2022.774170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Martins F., Sofiya L., Sykiotis G.P., Lamine F., Maillard M., Fraga M., Shabafrouz K., Ribi C., Cairoli A., Guex-Crosier Y., et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 276.Portenkirchner C., Kienle P., Horisberger K. Checkpoint Inhibitor-Induced Colitis-A Clinical Overview of Incidence, Prognostic Implications and Extension of Current Treatment Options. Pharmaceuticals. 2021;14:367. doi: 10.3390/ph14040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shi J., Niu J., Shen D., Liu M., Tan Y., Li Y., Huang Y., Cui L., Guan Y., Zhang L. Clinical diagnosis and treatment recommendations for immune checkpoint inhibitor-related adverse reactions in the nervous system. Thorac. Cancer. 2020;11:481–487. doi: 10.1111/1759-7714.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Som A., Mandaliya R., Alsaadi D., Farshidpour M., Charabaty A., Malhotra N., Mattar M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases. 2019;7:405–418. doi: 10.12998/wjcc.v7.i4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Alameddine J., Godefroy E., Papargyris L., Sarrabayrouse G., Tabiasco J., Bridonneau C., Yazdanbakhsh K., Sokol H., Altare F., Jotereau F. Faecalibacterium prausnitzii Skews Human DC to Prime IL10-Producing T Cells Through TLR2/6/JNK Signaling and IL-10, IL-27, CD39, and IDO-1 Induction. Front. Immunol. 2019;10:143. doi: 10.3389/fimmu.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cheng J., Laitila A., Ouwehand A.C. Bifidobacterium animalis subsp. lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021;8:1094. doi: 10.3389/fnut.2021.790561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ding Y.-H., Qian L.-Y., Pang J., Lin J.-Y., Xu Q., Wang L.-H., Huang D.-S., Zou H. The regulation of immune cells by Lactobacilli: A potential therapeutic target for anti-atherosclerosis therapy. Oncotarget. 2017;8:59915–59928. doi: 10.18632/oncotarget.18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fox J.G., Ge Z., Whary M.T., Erdman S.E., Horwitz B.H. Helicobacter hepaticus infection in mice: Models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Galdeano C.M., Perdigón G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim J.Y., Bang S.-J., Kim J.-Y., Choi E.J., Heo K., Shim J.-J., Lee J.-L. The Probiotic Strain Bifidobacterium animalis ssp. lactis HY8002 Potentially Improves the Mucosal Integrity of an Altered Intestinal Microbial Environment. Front. Microbiol. 2022;13:817591. doi: 10.3389/fmicb.2022.817591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Maier E., Anderson R.C., Altermann E., Roy N.C. Live Faecalibacterium prausnitzii induces greater TLR2 and TLR2/6 activation than the dead bacterium in an apical anaerobic co-culture system. Cell Microbiol. 2018;20:e12805. doi: 10.1111/cmi.12805. [DOI] [PubMed] [Google Scholar]
- 286.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 287.Miller L., Lehtoranta L., Lehtinen M. The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis. Nutrients. 2017;9:191. doi: 10.3390/nu9030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.O’Mahony C., Scully P., O’Mahony D., Murphy S., O’Brien F., Lyons A., Sherlock G., MacSharry L., Kiely B., Shanahan F., et al. Commensal-Induced Regulatory T Cells Mediate Protection against Pathogen-Stimulated NF-κB Activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wexler H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bertrand A., Kostine M., Barnetche T., Truchetet M.-E., Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bhatt A.P., Redinbo M.R., Bultman S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017;67:326–344. doi: 10.3322/caac.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Dubin K., Callahan M.K., Ren B., Khanin R., Viale A., Ling L., No D., Gobourne A., Littman E., Huttenhower C., et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Morgado M., Plácido A., Morgado S., Roque F. Management of the Adverse Effects of Immune Checkpoint Inhibitors. Vaccines. 2020;8:575. doi: 10.3390/vaccines8040575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Villéger R., Lopès A., Carrier G., Veziant J., Billard E., Barnich N., Gagniere J., Vazeille E., Bonnet M. Intestinal Microbiota: A Novel Target to Improve Anti-Tumor Treatment? Int. J. Mol. Sci. 2019;20:4584. doi: 10.3390/ijms20184584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kennedy D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients. 2016;8:68. doi: 10.3390/nu8020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Todorova T.T., Ermenlieva N., Tsankova G. Immunotherapy-Myths, Reality, Ideas, Future. InTech; London, UK: 2017. Vitamin B12: Could It Be a Promising Immunotherapy? [DOI] [Google Scholar]