Abstract
As we age, structural changes contribute to progressive decline in organ function, which in the heart act through poorly characterized mechanisms. Taking advantage of the short lifespan and conserved cardiac proteome of the fruit fly, we found that cardiomyocytes exhibit progressive loss of Lamin C (mammalian Lamin A/C homologue) with age, coincident with decreasing nuclear size and increasing nuclear stiffness. Premature genetic reduction of Lamin C phenocopies aging’s effects on the nucleus, and subsequently decreases heart contractility and sarcomere organization. Surprisingly, Lamin C reduction downregulates myogenic transcription factors and cytoskeletal regulators, possibly via reduced chromatin accessibility. Subsequently, we find a role for cardiac transcription factors in regulating adult heart contractility and show that maintenance of Lamin C, and cardiac transcription factor expression, prevents age-dependent cardiac decline. Our findings are conserved in aged non-human primates and mice, demonstrating that age-dependent nuclear remodeling is a major mechanism contributing to cardiac dysfunction.
Editor summary:
Kirkland et al. identify conserved age-dependent nuclear remodeling in Drosophila cardiomyocytes, dependent on declining Lamin C. They show that Lamin C loss induces reversible heart dysfunction by repressing myogenic transcription factors.
Introduction
With aging comes a progressive decline in organ function1,2, but age-related decline in heart performance is especially critical as cardiovascular disease is the leading cause of mortality worldwide3. Aging results in the progressive loss of structural organization4,5, which can limit contractility1,6 and result in heart failure7. High prevalence of age-related cardiac dysfunction may in part be because cardiomyocyte renewal is limited8 and therefore, maintenance of cardiac function over time must rely on compensatory mechanisms; these are multifaceted but are tightly linked to the integrity of key structural elements, e.g., intercalated discs, sarcomeres, and the extracellular matrix. Reducing force on cardiomyocytes (CMs) or compensating with transgenic overexpression of intercalated disc proteins can partially reverse heart dysfunction by restoring structural organization and gene expression6,7. Since physical forces transduced to the nucleus can impact chromatin organization and induce changes in gene expression9-11, nuclear changes may similarly be a mechanism contributing to age-associated cardiac dysfunction.
Structural changes in the nucleus are primarily governed by the nuclear lamina, an intermediate filament meshwork composed of A- and B-type Lamins. The lamina is tethered to the cytoskeleton12,13 via the linker of the cytoskeleton (LINC) complex9-11 as well as to chromatin14,15 via lamina associated domains (LADs)16. Along with the perinuclear cytoskeleton12,13 and chromatin14,15, the nuclear lamina regulates nuclear properties, including stiffness, size and shape17-26. In mechanically active tissues, Lamin mutations can give rise to muscular dystrophy27,28 and cardiomyopathies29, which also manifest in premature aging syndromes, e.g., Hutchinson Gilford Progeria (HGPS)30. Lamin mutations cause dysmorphic nuclei, epigenetic dysregulation and DNA damage31-34. However, changes in nuclear shape, which are conserved from invertebrates35,36 to humans37, have been observed upon aging in the absence of Lamin mutations. Age-related changes in nuclear shape also accompany loss of heterochromatin37,38 and accumulation of DNA damage37. In some cases, Progerin (truncated Lamin A) has been identified in aging skin39 and dilated cardiomyopathy40 in the absence of gene mutation. Furthermore, Lamin expression decreases with age in some tissues41-43, with loss of Lamin B being a well-known aging marker42 and which may decrease cardiomyocyte regenerative capacity and increase ploidy44. Lamin A and C (Lamin A/C, two splice variants of the lmna gene) are the dominant adult cardiac Lamins, and age-associated reduction has been observed in mouse cardiomyocytes41, but how lowered expression influences heart function and cardiac aging is unknown. Insights from Lamin A haploinsufficient mutant mice suggest Lamin reduction is as detrimental to heart function as progerin mutants; mice develop dilated cardiomyopathy via loss of sarcomere-nuclear coupling, show defective nuclear transport and fail to activate compensatory hypertrophic pathways45. Thus, age-associated changes to nuclear shape and Lamin composition, herein referred to as nuclear remodeling, could be a major mechanism contributing to organ dysfunction, yet mechanisms contributing to age-dependent nuclear changes and how they affect tissue function remain elusive.
To investigate a role for age-dependent nuclear remodeling in regulating heart function, we primarily employ the invertebrate Drosophila melanogaster. Drosophila have a short lifespan, possess a simple tubular heart, which has a conserved cardiac proteome to humans46, and importantly, undergo age-dependent cardiac decline5,47. We identified age-dependent remodeling unique to CM nuclei that is strongly influenced by an age-dependent reduction of Lamin C (LamC), the fly homologue to mammalian Lamin A/C. Genetic reduction of LamC in young flies phenocopies age-associated nuclear stiffening, decreases heart contractility and sarcomere disorganization, and ultimately shortens lifespan. We show that LamC loss decreases expression of cardiomyocyte transcription factors, as well as cytoskeletal regulators, possibly by reducing their chromatin accessibility. Genetic reduction of CM transcription factors phenocopies age-dependent loss of heart function, while preserving their expression by expressing LamC or Hand exogenously delays cardiac decline. CM age-associated nuclear shrinkage is conserved from flies to non-human primates and therefore presents nuclear remodeling as a major mechanism contributing to age-related organ dysfunction.
Results
Cardiomyocyte nuclei remodel during aging
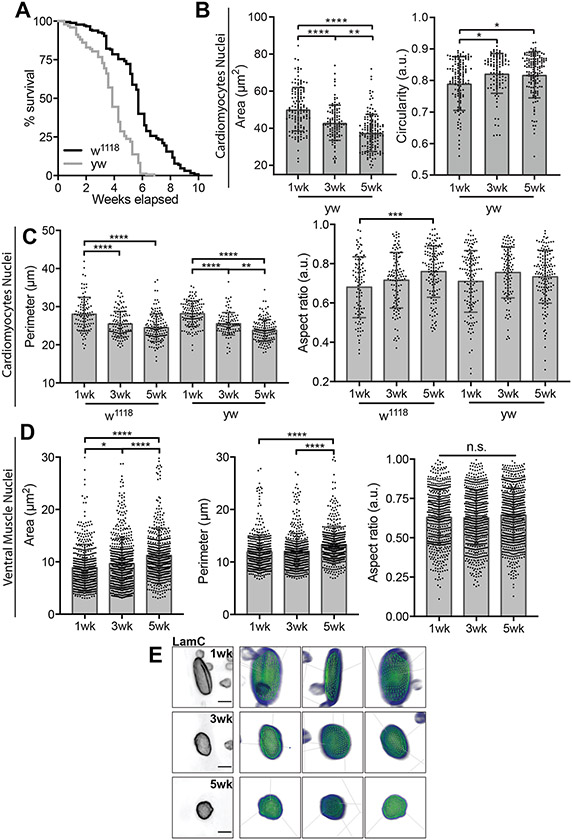
To understand whether age-associated nuclear remodeling influences heart function, we first sought to characterize how nuclear properties change upon aging in the Drosophila heart. Using two wildtype strains (w1118 and yw), we measured nuclear size and shape at 1, 3 and 5 weeks post-eclosure for surgically exposed hearts, and specifically the A2-A3 region (Fig. 1A). Our high-throughput two-dimensional segmentation approach showed that common to both strains, CM nuclei decrease in cross-sectional area and become more circular upon aging (Fig. 1B and Extended Data Fig. 1A-C), which is contrary to observations in other cell types, e.g., skeletal muscle nuclei36 and fibroblasts37. To exclude that our observations were an artifact of our protocol, we segmented nuclei from the syncytial ventral muscle that overlays the CM pairs within the same confocal images (Fig. 1A). Here, we found that ventral muscle nuclei increase in size upon aging, suggesting the reduction in nuclear size is CM specific (Extended Data Fig. 1D). Nuclear atrophy is conserved in three-dimensions, as we found that CM nuclear volume also decreases with age in w1118 flies (Fig. 1C and Extended Data Fig. 1E). Since morphology and mechanics are often linked, we measured nuclear stiffness at 1 and 5 weeks of age using atomic force microscopy (AFM). CM nuclei, selected based on Hand-promoter specific nuclear GFP expression and size (smaller than the pericardial nuclei), were more than 2-fold stiffer in aged flies (Fig. 1D). Together, our results show that CM nuclei become smaller, more circular, and stiffer with age.
Figure 1:
Age-Associated Changes in Cardiac Nuclear Morphology and Mechanics. (A) Schematic of ventral Drosophila body plan with the heart tube in the abdomen highlighted in blue. Expanded view of the heart tube shows a coronal (XY) confocal section through the heart tube (center) as well as a transverse (XZ) confocal section and schematic to highlight nuclear position in the luminal space (right). Asterisks indicate cardiomyocyte nuclei. Scale bar is 20 μm. (B) Images of w1118 fly nuclei (left) and plot of their corresponding 2D projection data (right). Scale bar is 5 μm. n = 96, 116, and 141 nuclei for 1- , 3-, and 5-week adults, respectively. The brightness of the LaminB staining was autoscaled to highlight nuclear edge and not to represent local protein concentration. (C) 3D renderings of cardiac nuclei (left) and their corresponding for volume and surface area. n = 27, 37, and 34 nuclei for 1- , 3-, and 5-week adult w1118 flies respectively. (D) Atomic force microscopy (AFM) nuclear indentation schematic (top) and plot of stiffness values, i.e., Young’s modulus, for nuclei of w1118 flies (bottom). n = 35 and 28 nuclei for 1- and 5-week adults, respectively. **p<10−2 and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test in (B-C) and two-sided unpaired t-test with Welch’s correction in (D). Error bars in (B-C) refer to mean +/− SD, and in (D) show min to max, with median and 25th and 75th interquartile range.
Cardiomyocyte Lamin RNA and protein decrease during aging
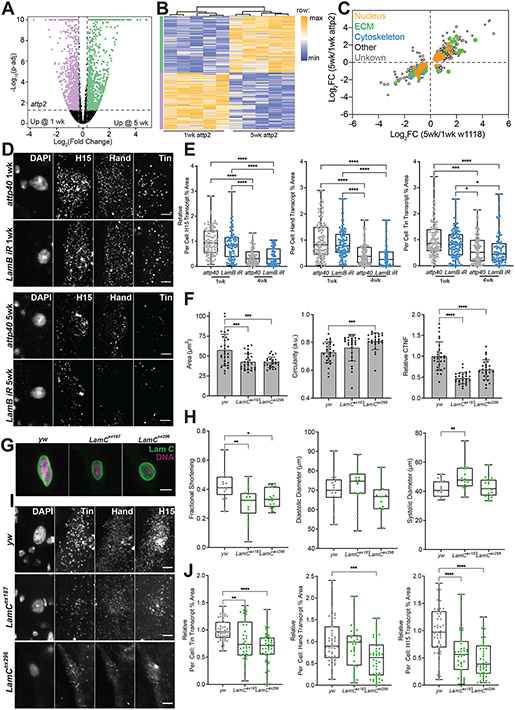
To identify candidate genes that may regulate age-associated nuclear structural changes, we performed bulk RNA sequencing on isolated Drosophila heart tubes (Fig. 2A; Table S1). Approximately 1,494 differentially expressed genes (DEGs; −1.25 > FC > 1.25, p-adj < 0.05) were identified, and based on gene ontology (GO) analysis, represented terms primarily related to the cytoskeleton and sarcomere, ECM and adhesion, and chromatin regulation and nuclear envelope (Fig. 2B; Table S2). Many DEGs in this latter ontology (Extended Data Fig. 2A) are common to age-related terms, e.g., DNA damage, repair, and histone regulation (Extended Data Fig. 2B). Interestingly, several nuclear envelope genes were downregulated including Lamin C (LamC), two homologues of Nesprin, LINC complex proteins, Klarischt (Klar) and Msp300 (Fig. 2C). Utilizing RNA in situ with Hybridization Chain Reaction (HCR)48 to visualize mRNA transcripts and confirm transcriptome analyses specifically in CMs, we found that LamC mRNA expression indeed decreases upon aging as did Lamin B (LamB) transcripts (Extended Data Fig. 2C-D) consistent with other aging systems49. Other cell types present in the heart may explain the absence of differential expression for LamB in bulk RNA sequencing (Fig. 1A). Single nuclear-RNA sequencing performed as part of the fly cell atlas50 indicates cardiomyocytes may represent only 3.5% of the heart nuclei and thus is a caveat to our Bulk-RNA sequencing approach. Subsequently, we verified via corrected total nuclear fluorescence (CTNF) that size-normalized expression of LamB and LamC decreased upon aging (Fig. 2D and Extended Data Fig. 2E). However, unlike in Progeria and aged donor fibroblasts where Lamin A/C relocates from nucleoplasm to nuclear envelope37, Lamins did not redistribute within aged nuclei (Extended Data Fig. 2E).
Figure 2:
Natural Aging Downregulates Nuclear Envelope Proteins. (A) Volcano plot and heat map of bulk RNA-seq from surgically dissected heart tubes. Fold change (FC) represents 5-week w1118 fly hearts normalized to 1-week old hearts and p-adjusted was computed from quadruplicate repeats. 1,494 differentially expressed genes (DEGs) were assessed from cutoffs of −0.32 > log2(FC) > 0.32 (or −1.25 > FC > 1.25) and p-adj < 0.05 (dashed lines) from comparisons of 15 fly hearts in quadruplicate biological replicates; DEGs increasing and decreasing with age are shown in green and purple, respectively. Heat maps are normalized within each gene/row. (B) The top 100 molecular function and cellular component gene ontological terms are plotted based on elimination pruning p-value. Terms related to cytoskeleton & sarcomere, ECM & adhesion, and chromatin remodeling & nuclear envelope are annotated by color. (C) Given as absolute fold change, the expression of selected genes associated with nuclear envelope terms are plotted, with Lamin C (LamC) expression noted with italics. (D) Confocal projection images of cardiomyocyte nuclei showing Lamin B (LamB) and C expression with age (left). Scale bar is 5 μm. (right) Corrected total nuclear fluorescence (CTNF), which adjusts for nucleus size, is plotted for LamB and LamC as a function of adult fly age. n = 141, 124, 116, 114, 107, and 69 nuclei/condition from left to right for LamC and LamB aged 1-, 3-, or 5-weeks of adulthood, respectively. ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test. Error bars refer to mean +/− SD.
Lamin C reduction phenocopies age-related nuclear and cytoskeletal remodeling, and shortens lifespan
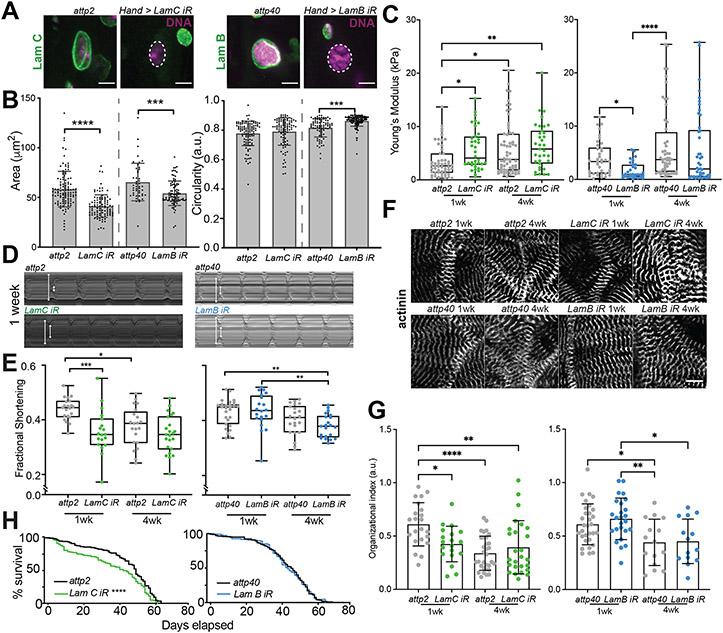
Strong evidence from patients with laminopathies23,37 suggest that Lamins regulate cell function and contribute to heart dysfunction51. However, evidence does not suggest what effects, if any, Lamins might have in aged hearts. Therefore, we sought to determine the effect of Lamin downregulation on CM function. We utilized Hand4.2-Gal4, a heart (CM and pericardial cells) specific driver, to drive the expression of interfering RNA (RNAi) and verified cardiac-specific knock down (KD) for LamB and LamC, relative to their background controls (attp40 for LamB RNAi and attp2 with Luciferase control RNAi for LamC RNAi; Fig. 3A and Extended Data Fig. 3A-B). To mark nuclei, we stained the Lamin isoform not targeted by RNAi and found that LamC and LamB reduction decreased nuclear area and perimeter compared to age-matched controls. Only LamB RNAi nuclei were more circular at 1 week than the attp40 control (Fig. 3B and Extended Data Fig. 3C). By 4 weeks of adulthood, controls undergoing age-associated remodeling more closely mirrored RNAi effects observed at 1 week (Extended Data Fig. 3D). These distinct effects on nuclear morphology indicate that LamC and LamB may influence nuclear properties differently. Indeed, nuclei extracted from LamC RNAi hearts at 1 week were stiffer than age-matched controls and mimicked 4 week-aged control nuclei, while LamB RNAi heart nuclei were softer than controls and did not phenocopy aging (Fig. 3C).
Figure 3:
LamC, but not LamB, impacts Cardiomyocyte Aging, Heart Function, and Lifespan. (A) Confocal cross-section images are shown for transgenic flies knocking down LamB and LamC by RNAi (right) and their background fly line (left). Dashed lines indicate nuclear position based on DNA. Scale bar is 5 μm. (B) Plots quantifying nuclear area (left) and circularity (right) based on confocal images of LamB and LamC RNAi lines and their genetic control background at 1-week of adulthood. n = 111, 95, 46, and 90 (hearts/condition; left to right). (C) Plot of Young’s modulus values is shown for nuclei of the indicated adult ages for LamC (green), LamB (blue) RNAi, and their control strains (grey). n = 53, 36, 63, 35, 38, 31, 52, and 46 (hearts/condition; left to right). (D) Representative kymographs of surgically exposed heart tubes for LamC (green), LamB (blue) RNAi, and their control strains. White arrows mark the diastolic and systolic diameters. (E) Fractional shortening at 1- and 4-weeks of adulthood is plotted for LamC (green) and LamB (blue) RNAi, and their control strains. n = 21,18, 21, 23, 27, 20, 22, and 20 (hearts/condition; left to right). (F) Representative images of α-actinin staining for the indicated transgenic flies and their control backgrounds (paired by row) used to calculate Organizational index in (G) . Scale bar is 10 μm. (G) Organizational index is plotted for each heart tube. n = 22,19, 32, 26, 31, 25, 15, and 14 (hearts/condition; left to right). (H) Kaplan-Meier survival curve for LamC (green) and LamB (blue) RNAi, and their control strains. 102, 148, 95, and 200 flies for attp2, LamC RNAi, attp40, and LamB RNAi, respectively, were used in the plot. *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test for (B-G). ****p<10−4 based on Log-Rank (Mantel-Cox) test in (H). Error bars in (B, G) refer to mean +/− SD, and in (C,E) min to max, with median and 25th and 75th interquartile range.
Along with nuclear stiffness, we found differential effects on heart function upon LamB or LamC reduction. Surgically exposed hearts from 1 week and 4 week-old adults were subjected to live, high-speed imaging52. We observed that 1 week LamC RNAi hearts decreased in fractional shortening (Fig. 3D-E), i.e., the difference of systolic and diastolic heart diameters divided by the diastolic diameter, relative to 1 week old controls (Extended Data Fig. 3E-F) and were therefore less contractile. Conversely, LamB RNAi hearts only exhibited age-associated decreases in fractional shortening comparable to the attp40 background control (Fig. 3D-E). Since hearts with reduced LamC were less contractile, we examined how organized the sarcomeres, i.e., the contractile unit of CMs, were to assess whether organization might account for reduced fractional shortening. Using an automated, unbiased Fourier transform analytic53, we found a significant decrease in cardiomyocyte sarcomere organization for 1 week old LamC RNAi hearts relative to age-matched controls, which phenocopies the less organized 4-week control adult heart. Consistent with functional data, LamB RNAi hearts exhibited only age-associated reduction in sarcomere organization (Fig. 3F-G). As heart function is tightly linked to survival in Drosophila, we observed that only LamC RNAi flies had shortened lifespan (Fig. 3H), especially for adult flies of median age, compared to the background control. These results suggest that LamC loss during aging may contribute to heart dysfunction via sarcomere disorganization.
Aging and Lamin C reduction alter chromatin accessibility at mesoderm transcription factor loci
While heart dysfunction may occur via sarcomere disorganization, the common upstream mechanism for LamC RNAi and natural aging are unknown. Given Lamins’ role in anchoring chromatin and their link to cardiomyopathies54, we hypothesized that cardiac dysfunction induced by Lamin deficits could be mediated by changes in age-associated chromatin organization. Assay for Transposase-Accessible Chromatin sequencing55 (ATAC-Seq) was performed on isolated heart nuclei, and we verified detection of accessibility peaks mapping to Drosophila heart-specific and enriched genes Hand and tinman (tin) and sarcomere genes Tropomyosin and Mhc (Fig. 4A). Subsequently, we compared differentially accessible regions (DARs) for 1- and 5-week wildtype (w1118), 1-week LamC RNAi (verses attp2 background control) and 1-week LamB RNAi (verses attp40 background control) hearts (−1.25 > FC > 1.25, p-adj < 0.1). There were more DARs with aging compared to RNAi hearts (Fig. 4B; Table S3), likely because aging impacts all cardiac-related cells whereas the RNAi was expressed only in CMs, which are a subset of all cells present in the heart tube (Fig. 1A). Surprisingly with less LamC, hearts had more DARs that were less accessible vs. more accessible, an imbalance also observed in aging hearts (67.5% less accessible and 55.9% more accessible DARs). Conversely, LamB RNAi had fewer DARs overall and fewer that were less accessible (38.0%). Thus, while nuclei get smaller and stiffer in aged and LamC RNAi hearts, there are also changes in chromatin accessibility.
Figure 4:
Chromatin Accessibility Decreases with Age and LamC RNAi at Sites of Myogenic Control. (A) Map of accessibility peaks for Drosophila cardiac transcription factor genes Hand (top) and tinman (tin; upper middle) and sarcomere genes Tropomyosin (Tm1 and Tm2; lower middle) and Mhc (bottom). Data is shown in triplicate sequencing runs using 1-week adult w1118 flies, i.e., 3 lines plotted in the panel for 50 pooled hearts per replicate. (B) Volcano plots of the indicated aging or transgenic comparisons of differentially accessible regions (DARs) from ATAC-seq, assessed from −0.32 > log2(FC) > 0.32 (or −1.25 > FC > 1.25) and p-adj < 0.05. The number of peaks is annotated at the bottom for each comparison indicating if the region is more (black) or less (red) accessible relative to the comparator line. (C) Scatter plot is shown for ATAC-seq data comparing the Log2(FC) in accessibility for genes based on effects from aging and LamC (green) or LamB (blue) RNAi. Percentage of data in each quadrant are shown. (D) Top gene ontological terms are plotted for co-downregulated peaks (closest associated gene) in aged and LamC RNAi fly comparisons and ordered based on the false discovery rate p-value. (E) Genes within the anatomical structure term were plotted for their fold change for aging (gray) and LamC RNAi (green). Genes names in red represent myogenic transcription factors or muscle-specific structure proteins. (F) Map of accessibility peaks for the myogenic transcription factor Hand. Arrows indicate the location of a common DAR in Hand that is present and reduces in aged and LamC RNAi flies but not in LamB RNAi flies. Multiple lines per map indicate multiple sequencing runs of biological replicates. DAR fold change is annotated for each comparison.
These data could suggest that LamC reduction might have effects on specific chromatin domains during aging, thus we asked to what extent the same genes were affected in the same direction (more or less accessible) for both aging and 1-week LamC RNAi. Analysis of DARs common to both datasets indicated that 68% of DARs were co-regulated, with more than half of the common DARs being less accessible (Fig. 4C; Table S4). Conversely, more than half of the DARs shared between LamB RNAi and aging were mutually more accessible. These results indicate that LamC and LamB may differentially contribute to age-associated changes, with LamC reduction conferring a decrease in accessibility associated with a decline in heart performance.
To better understand how mutual DARs might contribute to loss of function, we identified the ontological terms associated with the genes most proximal to the less accessible regions. Mutually less accessible genes revealed terms for contractile fibers and cell cortex in addition to differentiation, development, and morphogenesis (Fig. 4D; Table S5). Interestingly, the most highly significant terms’ contributed genes included the Snail-type transcription factor Escargot and the heart-specific transcription factor Hand (Fig. 4E), which do not change in accessibility for LamB RNAi (Fig. 4F). Hand is required for invertebrate and vertebrate CM specification56, and therefore may function beyond development to maintain cardiac programs. If reduced chromatin accessibility leads to protein loss upon aging, it is possible that downstream cardiac expression could be dysregulated.
Lamin C loss induces aging expression profile and represses cardiomyocyte transcription factors
To assess whether altered chromatin accessibility might lead to transcriptional dysregulation, and if other cardiac specific transcription factors were affected, we performed bulk-RNA sequencing for aged attp2 background control and LamC RNAi hearts. We observed 344 differentially expressed genes (DEGs) resulting from heart-specific LamC loss at 1-week of age, and 1,998 DEGs between 1- and 5-week attp2 background flies (−1.25 > FC > 1.25, padj < 0.05; Fig. 5A, Table S6-7). When comparing the attp2 and w1118 backgrounds, we found 688 common DEGs as a function of age that either increased or decreased mutually (Extended Data Fig. 4A-C; Table S8). We then identified mutually significant DEGs from LamC RNAi and aged hearts and observed that 110 DEGs of known function were present in both conditions (lower left and upper right quadrants, Fig. 5B; Table S9). The common DEGs yield biological process GO terms related to aging (red, Fig. 5C; Table S9), suggesting that LamC loss creates differential gene expression similar to natural aging. We also observed terms previously identified from ATAC-Seq, including anatomical structure development and morphogenesis (blue, Fig. 5C), in which CM transcription factors tin and H15 were downregulated (Fig. 5D). The imperfect overlap of the RNA and ATAC-sequencing may be accounted for by differences in processing of heart tissue prior to isolation, e.g. RNA extraction was performed from intact hearts immersed in Quizol, while nuclei were first isolated by mechanical disruption and detergent-based lysis for ATAC-sequencing. HCR validated CM specificity of tin, H15, and Hand (as found to be less accessible in ATAC-Seq) and showed that all three were reduced upon aging and LamC RNAi (Fig. 5E-F). Conversely for LamB RNAi, hearts showed only an aging phenotype and no effect from the loss of LamB (Extended Data Fig. 4D-E). Since the CM transcriptional network is highly conserved with mammals, and the potential significance of cardiac transcriptional changes on heart function, we focus our attention on Hand, Tin and H15 herein.
Figure 5:
LamC Loss Transcriptionally Regulates Myogenic Transcription Factors. (A) Volcano plot and heat map of bulk RNA-seq from surgically dissected heart tubes from LamC RNAi flies compared to attp2 control background flies at 1-week of age. 344 DEGs were assessed from cutoffs of −0.32 > log2(FC) > 0.32 (or −1.25 > FC > 1.25) and p-adj < 0.05 (dashed lines) from comparisons of 15 fly hearts in quadruplicate biological replicates; DEGs increasing and decreasing with LamC RNAi are shown in green and purple, respectfully. Heat maps are normalized within each gene/row. (B) Scatter plot is shown comparing Log2 Fold Change from aging (1- and 5-weeks of age) and LamC RNAi compared to control background (attp2). Number of DEGs represent genes of known function. Data was categorized based on which cellular component ontology term it most closely matches. Distance from the red dashed line of unity was used identify co-regulated genes whose (C) biological process ontology terms were annotated and ordered based on their false discovery rate p-value. (D) Genes within the anatomical structure term were plotted for their fold change for aging (gray) and LamC RNAi (green). Genes names in red represent myogenic transcription factors or muscle-specific structure proteins. (E) Representative images of in situ hybridization chain reaction for transcription factors H15, Hand, and tin, co-stained with DAPI, for LamC RNAi and control attp2 flies at 1- and 4-weeks of adulthood with quantification (F) for each transcription factor is also shown (right) and quantifies the per cell percent area covered by each transcript. For H15, n = 39, 76, 64, and 45 cells for 1-week control, 1-week LamC RNAi, 4-week control, and 4-week LamC RNAi, respectively. For Hand, n = 71, 84, 52, and 43 cells for 1-week control, 1-week LamC RNAi, 4-week control, and 4-week LamC RNAi, respectively. For tin, n = 69, 101, 103, and 85 cells for 1-week control, 1-week LamC RNAi, 4-week control, and 4-week LamC RNAi, respectively. *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test. Bars in (F) represent min to max, with median and 25th and 75th interquartile range.
To confirm that LamC-dependent nuclear remodeling mediates the reduction of CM transcription factors rather than any off-target effect of the RNAi, we utilized heterozygous LamC null mutant flies57. We observed that LamC excision mutants, LamC187 (375 base pair deletion in the first exon) and LamC296 (560 base pair deletion in the first exon), reduce LamC expression and nuclear size compared to wildtype background controls (Extended Data Fig. 4F-G) and similarly to LamC RNAi (Fig. 3A-B). We identified a corresponding reduction in heart contractility compared to the background control, which for at least LamC187 induced systolic dysfunction alike heart-specific LamC RNAi (Extended Data Fig. 4H; Fig. 3D-E). Subsequently we confirmed that the CM transcription factors Tin, Hand and H15 were also downregulated in at least one heterozygous LamC mutant line (Extended Data Fig. 4I-J). Thus, we confirm a conserved LamC-dependent role in regulating CM transcription factor expression.
Our results thus far show that LamC loss occurs with age, makes nuclei smaller and stiffer, decreases CM transcription factor accessibility and expression and then disrupts sarcomeres to cause contractile dysfunction. However, our results do not yet establish if loss of a myogenic program is critical for adult myocyte function.
Adult-onset myogenic transcription factor loss induces heart dysfunction while maintain Lamin C preserves heart function
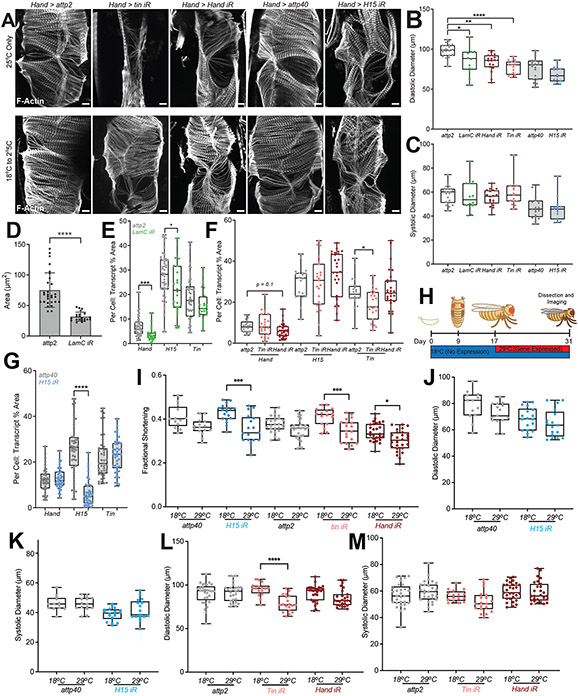
The importance of myogenic transcription factors is highlighted by significant sarcomere defects present when any one factor is silenced throughout development (Extended Data Fig. 5A; upper panel). We therefore next sought to assess whether CM transcription factor loss in adulthood, due to age associated LamC loss, could also influence heart function. To approach this, we reared Hand-Gal4 RNAi flies from the embryonic to pupal stages at 18°C to lower the efficiency of the Gal4-UAS expression system58, and then upon elcosure the adult fly was transferred to 25°C to induce strong expression while aging (Fig. 6A). In the case of the CM transcription factors KD, the lowered efficiency of the Gal4-UAS decreased the expression of the RNAi to allow for structurally normal heart development (Extended Data Fig. 5A; lower panel). We then assessed how RNAi in the adult fly influenced heart function; we observed a decrease in fractional shortening for Hand and Tin RNAi that proved similar to LamC RNAi by the same approach. While H15 RNAi did not yield a significant decrease, it indicates a trend towards decreased contractility as well (Fig. 6B, Extended Data Fig. 5B-G). In addition, we used a complementary method, employing the temperature sensitive suppressor of Gal4, Tub-Gal80ts, with the heart specific driver Hand-Gal4 to assess the role of CM transcriptions factors in the adult heart. To suppress Gal4-UAS driven expression during development, flies are reared at 18°C, then at eclosure, 50% of adult flies were maintained at the permissive (18°C) or shifted to the non-permissive temperature (29°C) at which denaturation of the Gal80 suppressor enables high efficiency Gal4-UAS expression (Extended Data Fig. 5H). Live heart imaging showed that loss of each transcription factor in adults, i.e., 2 weeks post-temperature shift, still caused a significant decrease in fractional shortening compared to control backgrounds, which exhibited a slight but insignificant reduction in fractional shortening possibly due to relative differences in aging between flies maintained each temperature since higher temperatures accelerate aging (Extended Data Fig. 5I-M).
Figure 6:
Myogenic Transcription Factors maintain heart contractility in aged flies
(A) Schematic of temperature sensitive transgenic expression where 18°C minimizes Gal4 mediated expression up to adult fly eclosure. At 25°C transgene expression is high enabling assessment of gene role in adult heart. (B) Quantification of fractional shortening for surgically exposed hearts with Hand-Gal4 driving expression of LamC RNAi (green), Hand RNAi (red), Tin RNAi (pink) and respective control attp2 (grey), and H15 RNAi (blue) with attp40 control (grey) with temperature schedule shown in (A), n = 10, 12, 18, 15, 19 and 14 (heart tubes left to right). (C) Fractional shortening plots for Hand-Gal4 driven expression of LamC OE by the regime shown in (A) with control, over expression of LacZ (A). n = 20, 20, 16, 19, 18, 16 (Heart tubes/age, left to right). (D) Representative images of RNA in situ with hybridization chain reaction for Hand, H15 and Tin transcripts, with corresponding quantification of the per cell, percentage area for each transcript, for LacZ control and LamC OE in (E) and Hand OE in (G) each at 1, 4 and 7 weeks at 25°C. (E) n = 36, 47, 27, 34, 29, 43 (CM nuclei/Genotype/age, left to right). Scale bar in (D) is 5 μm. (F) Fractional shortening plots for Hand-Gal4 driven expression of Hand OE by the regime shown in (A) with control, over expression of LacZ (A). n = 20, 20, 16, 21, 19, 18 (Heart tubes/age, left to right). (G) n = 36, 47, 27, 30, 35, 35 (Note same control; CM nuclei/age, left to right). *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Kruskal-Wallis test and Dunn’s comparisons test in (B), and two-way ANOVA with Sidaks multiple comparisons test. In all graphs, bars represent min to max, with median and 25th and 75th interquartile range.
Conversely, we asked if adult-onset LamC overexpression could preserve myogenic factor expression and function with age. Using the method described in Fig. 6A, we induced higher levels of exogenous LamC expression upon eclosure and aged flies up to 7 weeks at 25°C. We observed that nuclear area was preserved despite aging when compared to the LacZ over-expression (OE) control; we note that LamC OE induced nuclear divisions, and thus data represents total nuclear area per cardiomyocyte (Extended Data Fig. 6A-B). Subsequently, we found that sustained LamC expression up to 7 weeks, preserved fractional shortening and cardiac transcription factor expression compared to control (Fig. 6C-E). These observations were consistent with results using the alternative tub-Gal80ts temperature-sensitive induction system (Extended Data Fig. 6E-O). Finally, we asked whether overexpression of a single CM transcription factor was sufficient to preserve heart function upon aging and to mimic the influence of preserved LamC using the 18°C to 25°C method (Fig. 6A). We found that Hand OE generated viable adult flies, preserved heart contractility up to 7 weeks compared to controls, and lead to elevated expression of Hand, Tin, and H15 transcription factors compared to controls for at least 4 weeks of age, suggesting a degree of co-regulation (Fig. 6F-G, Extended Data Fig. 6C-D). Together, our results establish that adult loss of myogenic programs is mediated by age associated LamC loss, which may modulate their chromatin accessibility, and subsequently reduces adult cardiomyocyte function (Fig. 7A).
Figure 7:
Nuclear remodeling induces adult-onset transcription factor loss, a process conserved in Mice and Non-human Primates. (A) Schematic depicting how age-associated, cardiac-specific reduction of nuclear lamins reduces nuclear volume and chromatin accessibility, especially for myogenic transcription factors. With less muscle transcription from key cardiac loci, sarcomeres become disordered with age and heart function is reduced. Lamin overexpression can overcome age-associate reduction and preserve function. (B) Immunofluorescent staining of mouse heart sections at 9 and 29 months of age with anti-LamA/C (green) and DAPI (magenta). Scale bar = 30 μm. Below shows plots of projected nuclear area and circularity with respective ages. n = 3 biological replicates and 3 technical replicates. (C) Immunofluorescent staining of monkey left ventricle sections at 8.9 and 25.5 years of age with anti-LamA/C (green) and DAPI (magenta). Scale bar = 30 μm. Below shows plots of projected nuclear area and circularity with respective ages. n = 3 biological replicates and 3 technical replicates. qPCR results are plotted for (D) mouse and (E) rhesus macaque for the myogenic transcription factors Hand 1, Hand 2, Nkx2–5, and TBX20 as normalized by housekeepers Eef1e1, Rpl4, and ACTB for mouse and Rpl13a, Rpl20, and TUBB2 for macaque. Data were normalized to maximum and minimum expression within each gene and housekeeper for heatmap. Bar graph and regression indicate the average expression and standard error of the mean across all genes and housekeepers for each animal; r2 = 0.50 and 0.71 for mouse and macaque, respectively. *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by two-sided unpaired t-test (B and C, circularity) and with Welch’s correction in (B and C, nuclear area). Significance in (D) and (E) indicate deviation from 0 for simple linear regression. Error bars in (B, left and center, C) refer to mean +/− SD, and in (B, right) min to max, with median and 25th and 75th interquartile range.
Nuclear Remodeling and Myogenic Transcription Factor Loss is Conserved in Mice and Non-human Primates
Despite physiological differences between tubular and chambered hearts, there is surprising overlap between the Drosophila and human cardiac proteomes46. We therefore sought to assess whether similar structural and transcriptional changes are conserved from the fly heart to the mammalian heart41. We observed in both mouse and monkey heart sections that nuclear size decreased and circularity increased upon aging, as we found in the fly heart tube (Fig. 7B-C). Furthermore, immunofluorescence staining of the mouse heart sections confirmed reduction of Lamin A/C (Fig. 7B), consistent with Drosophila. Subsequently, we found mammalian homologues of fly transcription factors, Hand1, Nkx2.5 (homologue of tin), and Tbx20 (homologue of H15) significantly decreased expression in aging mice hearts (Fig. 7D and Extended Data Fig. 7A-B) and Hand1, Hand2 and Nkx2.5 significantly decreased in expression in aging non-human primate rhesus macaque hearts (Fig. 7E and Extended Data Fig. 7C-D), when normalized to at least one of three different, stable housekeeping genes. These data suggest that the functional decline attributed to cardiomyocyte transcription factor loss in flies could be a conserved mechanism, caused in part by physical remodeling of the nucleus.
Discussion
The role nuclear remodeling has on heart function during natural aging has thus far been largely unexplored. Here, we demonstrate that CM nuclear remodeling, i.e., age-related loss of nuclear lamins, is intimately linked with tissue-level dysfunction. Genetically inducing nuclear remodeling leads to reduction in heart contractility, sarcomere disorganization and shortens lifespan by mimicking transcriptional changes that occur in natural aging. Our findings suggest that transcriptional misregulation downstream of nuclear remodeling may occur due to altered chromatin accessibility and, strikingly, this represses CM fate transcription factors and sarcomeric structural components. Importantly, we show that preserving “youthful” nuclear properties, e.g., high Lamin expression and nuclear morphology, maintains CM transcription factor expression and heart function. These changes are conserved in both mice and non-human primates further demonstrating nuclear remodeling and myogenic transcriptional programs as potential therapeutic targets for preserving heart function during aging.
Our observations of age-associated nuclear remodeling in Drosophila, mice and non-human primates cardiomyocytes are in contrast to existing observations in C. elegans intestinal cells35, Drosophila skeletal muscle36, aged human fibroblasts37 and what is currently understood for progeria-related laminopathies19,23,33,37. Rather than increasing in size and dysmorphia, we observe that aging CM nuclei atrophy and become rounder. Although more recent findings also indicate towards tissue-specific nuclear remodeling in C.elegans59,60. We also, for the first time to our knowledge, demonstrate that CM nuclei stiffen upon aging in situ, an observation only seen previously in cell culture for progeria cells and only after multiple rounds of passaging19. Further supported by our assessment of non-cardiomyocyte ventral muscle nuclei that hypertrophy with age within the heart tube, our findings suggest that cardiomyocytes have specific mechanisms mediating nuclear remodeling.
In the context of Drosophila CMs, we sought to understand how nuclear remodeling occurred upon aging and identified that nuclear lamins, LamC and LamB, in addition to nesprin-related proteins Klar and Msp300, were downregulated upon aging. Consistent with our data, Lamin B has been previously reported to be downregulated with age43,49,61 possibly due to its role in senesence42, while a functional role for age-associated Lamin A/C reduction has not previously been explored. We found that genetically reducing LamC prematurely was sufficient to induce aging-like nuclear atrophy, but conversely, overexpression was required to change nuclear size in Xenopus and HeLa25. While A and B-type Lamins differentially contribute to nuclear mechanics20, we observed that reduction of A-type LamC increased CM nuclear stiffness despite cultured cells’ nuclei soften with reduced Lamin A/C expression20,26. These differences could be accounted for by several hypotheses; First, Drosophila LamB and LamC could have differing functions compared to mammalian counterparts, although in other cell types, there is conservation between Drosophila and human Lamins62. Second, it is increasingly apparent that nuclei respond differently in 2D and 3D environments. In 2D cell culture, nuclear wrinkling indicates membrane laxity, whereas in 3D environments63,64, wrinkling is dependent on actin filaments intrusion into the perinuclear space and wrinkling infers high membrane tension63. Third, cell- or developmental-specific differences may result in alternative mechanics upon Lamin depletion. For example, Jevtic et al., show that in differentiated Xenopus cells, very high levels of Lamins can in fact decrease nuclear size25. Fourth, cell-specific LADs at the nuclear periphery show unique phenotypes upon Lamin A mutations in hiPSC-derived CMs versus adipocytes and hepatocytes65. Thus, differential Lamin-chromatin interactions could similarly contribute to altered mechanical regulation in aging cardiomyocytes verses other cell types.
Given the linkage of nuclear lamina to sarcomeres via the LINC complex and chromatin via LADs66 as well as the functional deficits we uncovered, our data provide confirmation of a role for A-type Lamins in age-dependent regulation of heart function. Removal of Lamins disrupts chromatin attachment to the nuclear periphery, higher-order chromatin organization, and can influence gene expression67-71. These studies focus predominantly on stem cell fate and maturation, yet our data now suggests differences in postmitotic tissues. We identified that in both aging and LamC reduction, differentially accessible peaks were skewed towards decreased accessibility, despite evidence that heterochromatin is lost in Lamin A/C mutants and with aging23,31. Correspondingly, studies specifically disrupting LADs yield conflicting results depending on cell origin; Chang et al., reported chromatin decompaction and redistribution in breast cancer cells71, while Ulianov et al., found that topological-associated domains decondensed but global chromatin density increased in embryonic-derived Drosophila S2 cells69. Similarly, maintaining lamina but disrupting chromatin attachment increased chromatin compaction in C. elegans embryos70. Disrupting LADs can also show localized alterations in accessibility, with recent work demonstrating Lamin B loss leads to repositioning of disease causing loci away from the nuclear periphery in post-mitotic neurones72 and alter repressive H3K9me3 marks in C. elegans73. Two available datasets analyzing B-type LADs in Drosophila74,75 identified 327 LAD’s in protein coding regions, 40% of which corresponded to peaks in our dataset. However, less than 4% of the LADs were differentially regulated with LamC RNAi and, and <2% changed with LamB RNAi, suggesting an LAD-independent aging mechanism. These conflicting examples, along with our data, suggest that accessibility both globally, and locally for specific loci, could be context specific, our data proposes that in the context of aging, reduced accessibility is coupled to dysfunction.
We show that ultimately, LamC-mediated nuclear remodeling appears to be a conserved process in vertebrates that reduces the expression of cardiomyocyte transcription factors, e.g., Hand/HAND1/2, Tin/NKX2-5 and H15/Tbx20. We observe that Hand specifically is less accessible with aging and LamC reduction. In Drosophila, the highly conserved Tin is an early initiator of cardiogenesis and binds between Hand exons 3 and 476, an intron we observe to have reduced accessibility upon LamC reduction (Fig. 4F). Thus, reduced gene accessibility could further downregulate Hand and downstream myogenic transcription. We predict reduced chromatin accessibility might also account for the reduction of Tin/NKX2-5 and H15/Tbx20 with age across flies, mice, and monkeys. Our findings provide a new Lamin-mediated interpretation for previous observations of reduced NKX2-5 in aged, isolated mouse cardiomyocytes77 and provides them with a role beyond development. We show in Drosophila that their adult-specific reduction gives rise to a marked reduction in heart function, supported by studies that find an adult-specific role for TBX20 when deleted in mice78-80. Consistent with these observations, CM transcription factors are misregulated in remodeling events leading to heart failure81, e.g., HAND is downregulated in rodent hypertrophy82 and in human cardiomyopathy83. Furthermore, senescent myoblasts in aged skeletal muscle, exhibit altered myogenic transcription factors and result in smaller myotubes84,85. Together, Lamin-mediated misregulation of myogenic transcriptional programs likely has a significant impact on mediating heart dysfunction during aging and may precede the development of heart failure. Since preserving LamC, maintained CM transcription factor expression and heart function despite aging in flies, and heart function was also preserved with adult Hand expression, our findings suggest nuclear lamina remodeling is upstream of myogenic transcription regulation and a unique mechanism in age-related organ dysfunction. Furthermore, our work presents several avenues for investigating therapeutic interventions to increase healthspan into advanced age.
Methods
Drosophila melanogaster
Fly stocks were raised in non-crowded conditions on standard fly food medium consisting of yeast, cornstarch and molasses (10% yeast, 12% sugar and 1.5% agar). Flies were raised at 25°C except for the temperature sensitive fly crosses (HandGal4, Fig. 6 and HandGal4, TubGal80ts; TubGal80ts, Extended Data Fig. 5 and 6) which were raised at 18°C until eclosure, then transferred to 25°C (Fig. 6), or 18°C and 29°C (Extended Data Fig. 5 and 6) respectively. Freshly eclosed flies were collected and aged such that day of collection was day 1. Flies were transferred to fresh food every 2-3 days. Female flies were used for subsequent heart analysis to ensure consistent heart morphology. The following fly lines were used from the Bloomington stock center: white-1118, w1118, yellow-white yw, attp2; UAS-Luciferase (#31603), UAS-LamC RNAi (#31621), attp40 (#36304), UAS-LamB RNAi (#57501), UAS-Stinger-GFP (#84277), UAS-tinman RNAi (#50663), UAS-H15 RNAi (#57415), UAS-Hand RNAi (#28977). Hand4.2Gal4 was acquired from Olsen Laboratory76 and modified by the Bodmer lab to make Hand4.2Gal4, TubGal80ts; TubGal80ts. UAS-LamC, Lamc187 and LamC296 were gifted by the Walrath laboratory.
Mouse
All mouse experiments were performed in according to the guidelines established by the Institutional Animal Care and Use Committee at the University of California San Diego. Use of aged C57BL/6 mice was approved by the University of California San Diego Institutional Animal Care and Use Committee under study #S08172. All animals were provided with food and water ad libitum until the specific age time point at which point animals were euthanized by asphyxiation followed by cervical dislocation. The lower section of the left ventricle was removed from five young (5-month), three juvenile (9-month), four adult (14-month), and three aged (24-month) old mice and snap-frozen in liquid nitrogen immediately after resection and stored at −80°C. The remainder of the heart was washed in PBS before embedding in OCT for cryosectioning. OCT boats containing hearts were frozen on dry ice with methyl butane before storage at −80°C.
Rhesus Macaque
Ten adult male rhesus monkeys (ages: 8.87, 9.7, 10.66, 12.88, 14.12, 18.81, 19.59, 23.39, 24.73, and 25.48 years of age) were maintained at the NIA in accordance with NIH Institutional Animal Care and Use Committee protocol AG000238-07 (Effects of Aging on Experimental Atherosclerosis in Nonhuman Primates). Left ventricular samples from macaque where flash-frozen for qPCR analysis or formalin fixed, paraffin embedded and subsequently sectioned for immunofluorescence analysis.
Fly heart dissection
Flies were anaesthetized with FlyNap® (Carolina Biological Supply Co.) and dissected in artificial hemolymph that was oxygenated using aerators as previously described52.
Immunofluorescence and imaging
Hearts dissected in oxygenated artificial hemolymph were relaxed using 10mM EGTA in oxygenated artificial hemolymph and immediately fixed with 4% formaldehyde in the same EGTA hemolymph solution for 20 minutes. The hearts were then rinsed 3 x with phosphate buffered saline (PBS) and washed 3 x 10 minutes with 0.5% Triton 100-X in PBS (PBST). The hearts were then blocked with 1% BSA in PBST (PBST-BSA) for 30 minutes. Primary antibodies were prepared as indicated below in PBST-BSA and incubated overnight at 4°C. PBST and PBST-BSA washes were repeated and secondary antibody with DAPI and Phalloidin were prepared in PBST-BSA and incubated for 1.5-2 hours at room temperature. Following secondary incubation, the hearts were washed 3 x 20 minute with PBST and then rinsed 3 x with PBS to remove detergent. Antibodies and Dyes: Mouse anti-LamC (DSHB, LC28.26), 1:500. Mouse anti-LamB, 1:100 (DSHB, ADL195). Mouse anti-actinin (DSHB, 2G3-3D7) 1:100. DAPI (Sigma), 1:500. Rhodamine-Phalloidin (ThermoFisher, R415), 1:250. Donkey anti-mouse Alexa Fluor 488 (ThermoFisher, A21202), 1:500.
For imaging, the cuticle around the hearts was subsequently trimmed down to a small rectangle to prevent obstruction of the heart, then hearts were transferred to Fluormount® G slide mounting medium for antibody-based imaging or ProLong™ Glass Mountant (Invitrogen) for HCR imaging. The A2-A3 region of the heart was imaged on a Zeiss LSM780 inverted confocal microscope with a 40X objective, 1x Zoom, 0.44μm depth resolution for nuclear imaging or 0.88μm for actinin and HCR imaging, and at a resolution of 2148 x 1076 XY pixels.
Mouse heart sections embedded in OCT were cryosectioned and stored at −80°C prior to fixation and staining. Slides were directly fixed with 4% PFA in PBS for 20 minutes at −20°C with regular agitation to prevent freezing. Slides were subsequently washed 3 × 5 minutes with PBS and permeabilized for 1 hour with 1% PBS-Triton 100-X. Primary antibody was prepared in 10% Fetal Bovine Serum (FBS) with PBS (anti-Lamin A/C 1:250, Cell Signalling 4C11) and incubated overnight at 4°C. Slides were subsequently washed 3 x 5 minutes with PBS before applying secondary antibody (Donkey anti-mouse Alexa Fluor 488, ThermoFisher, A21202, 1:500) and DAPI (Sigma). Slides were then washed 3 x 5 minutes with PBST and then PBS. Finally, samples were prepared for imaging using ProLong™ Glass Antifade Mountant (Invitrogen). Samples were imaged on a Keyence All-in-One BZ-X Series Fluorescence Microscope, with a 60X objective, 1x Zoom, 1μm depth resolution and 1920 x 1440 XY pixel resolution.
Macaque heart sections were received from the NIA. For staining and imaging, slides were first rehydrated using the following steps: 2 x 10 minutes with Xylene, 100% ethanol, 95% ethanol (in DI water), 70% ethanol and 50% ethanol before rinsing with DI water. Slides were subsequently immersed in PBS with 0.5% Triton X-100 for 30 minutes and incubated with DAPI (Sigma) for 30 minutes, prior to 3 x 5 minute washes with PBST and 3 x 5 minutes with PBS. Slides were prepared using ProLong™ Glass Antifade Mountant (Invitrogen) and imaged as described for mouse heart sections.
Fly nuclear morphology and intensity analysis
For two-dimensional analysis of nuclear morphology, 3D stack images were acquired of the A2-A3 region of the heart as described above in the (Methods: Immunofluorescence and Imaging). The A2-A3 heart region possesses 3-4 cardiomyocyte pairs and therefore 6-8 total CM nuclei. Using ImageJ, the CM nuclei were cropped from the larger heart image, within a 22.172μm / 2242pixel box with the minimal number of z slices to eliminate out-of-plane nuclei from the cuticle or ventral muscle overlaying the CM nucleus. The cropped nuclei were then segmented in FIJI by the macro included in the supplementary software file.
The results were then saved and analyzed in excel. To calculate aspect ratio (AR) the minor axis was divided by the major axis and to calculate the circularity the excel function “=(4*PI()*C2)/D2^2” was used. The data was presented, and the appropriate statistical tests performed in prism.
For three-dimensional analysis of nuclear morphology, the FIJI 3D Mesh plugin86 was used on the LamC channel of the cropped nuclei stacks. The parameters for seeding and expanding the mesh were as follows: gamma, 200.0; pressure, 0.06; image weight, 0.05; beta 0.0; alpha, 1.0; steric neighbors, 0.0; divisions, 3.0. Volume and surface area from exported results were copied to prism for graphing and statistical testing.
The corrected total nuclear fluorescence (CTNF) was calculated using ImageJ. Cropped nuclear stacks, a binary image of the Lamin channel and max projection image were generated as described above in Nuclear Morphology Analysis. An ROI is generated from the binary and overlaid on the max projection image and area and integrated density are measured for the Lamin channel. The relevant macro is included in a supplementary software file.
Subsequently, to account for changes in nuclear size, the background is subtracted relative the area. A clear region outside of the nucleus is selected from the Lamin channel and the mean intensity measured. Then, the following equation is used to calculate the approximate protein amount: Corrected Total Nuclear Fluorescence = Integrated Density – (Mean Intensity x Nuclear Area).
Lamin localization
A custom python code87 was modified to assess the intensity of Lamin at radially increasing distances from the center of the nucleus to the periphery for the max projected images also generated for nuclear morphology and intensity analysis (Figure Extended Data Fig. 2F). The average mean intensity measurement at periphery was then divided by the average mean intensity the center to obtain the fold enrichment of Lamin at the periphery.
Sarcomere organization
Using ImageJ and confocal stack images of actinin stained hearts, the dorsal region of the A2-A3 region was projected to isolate a planar region of sarcomeres and eliminate actinin-stained sarcomeres from the ventral side of the CMs and ventral muscle. ROIs with a single layer of sarcomeres uninterrupted by non-CM cells were then cropped and saved. The isolated actinin regions from A2-A3 region were then batch processed using a published matlab code53 which uses a scanning Fourier transform to calculate organizational index. The input parameters included a sarcomere length of 2.5-3.2μm, a sarcomere directionality of 90°, a scanning resolution of 16 and at the appropriate pixel to μm ratio.
Lifespan assay
To determine lifespan, virgin females were collected and up to 30 flies separated into each vial. The flies were maintained at 25°C and transferred to fresh food every 2-3 days, when dead flies were also counted.
Live heart imaging
Hearts were dissected as previously described52 and heart function was assessed using high speed digital imaging (142fps, 9300 EM-CCD cameras, Hamamatsu), a 10X water-immersion lens and HCImageLive software (Hamamatsu). Using semi-automatic optical heart beat analysis software (SOHA)88, fractional shortening was calculated from the end diastolic diameter (EDD) and end systolic diameters (ESD): (FS = EDD-ESD/EDD).
Nuclear extraction
30-60 dissected hearts were removed from their cuticle and transferred to 1ml of ice-cold Nuclei EZ lysis buffer (Sigma-Alrdich Nuclei EZ Prep isolation kit) in a 1ml glass douncer. 20 loose strokes followed by 10 minutes on ice and then 15 tight strokes aided dissociation of nuclei from the hearts. The solution was transferred to a low-bind Eppendorf and centrifuged at 500 x g for 5 minutes. The supernatant was removed, the nuclear pellet resuspended in fresh ice-cold Nuclei EZ lysis buffer and incubated on ice for 5 minutes. Spinning, removal of supernatant, resuspension and incubation on ice was repeated once more. The samples were then centrifuged once more at 500 x g before removing the supernatant and resuspending the pellet in PBS for nuclear AFM or Nuclei EZ storage buffer for ATAC Seq samples.
Atomic Force Microscopy
For atomic force microscopy, isolated nuclei in PBS were spun (500g, 3 minutes) on to 12mm coverslips coated with Poly-D-Lysine (1 μg/μl was used to coat coverslips for 5 minutes, then rinsed with purified water and left to dry overnight). Coverslips were transferred to a glass slide, secured with vacuum grease, and covered in a PBS droplet for AFM. Indentation experiments were performed on an MFP-3D Bio Atomic Force Microscope (Oxford Instruments) mounted in a Ti-U fluorescent inverted scope (Nikon Instruments, Melville, NY) and used Asylum Research 13, Igor Pro 6.34A software. Nanoworld PNP-TR tips were calibrated for their spring constant using the thermal noise method and used for probing isolated nuclei. A trigger force of 2nN, an approach Velocity constant of at 2 μm/s and a force distance of 6 μm were used to generate a force map with 12 points across 2μm2. Hand4.2-Gal4 was used to drive expression of GFP, and thus only GFP-positive nuclei were selected for indentation. The software was used to calculate the Young’s modulus using the Hertz equation89. Any poor fits to the indentation curve were excluded. Then, the average Young’s modulus was calculated from the force map.
Bulk RNA sequencing
For gene expression analysis, corresponding adult flies were dissected as previously described52 to expose the heart. Fat cells were carefully removed from either side of the length of the heart. A minimum of 15 hearts were then pulled from the cuticle using fine forceps and pooled together in Eppendorf tubes containing 300μl of Qiazol lysis reagent. Hearts were mechanically homogenized using a handheld tissue homogenizer and plastic pestles. Afterward, a further 400μl of Qiazol lysis reagent was added and the tube flash frozen in liquid nitrogen. Samples were stored for up to 2 weeks at −80°C until RNA extraction was performed. Total RNA was then extracted and purified using the Qiagen miRNeasy Mini kit (Cat. No. 217004) as per the protocol. The purified RNA was then processed by the Institute for Genomic Medicine at University California San Diego. RNA integrity was analyzed using an Agilent Tape station system and precise RNA concentration determined using a Qubit 2.0 Fluorometer. Libraries were built using the Illumina TruSeq Stranded RNA, High Throughput Library Prep Kit and sequenced on a NovaSeq 6000 for samples with RIN numbers at 9.0 and above.
RNA-Sequencing data was analyzed by ROSALIND® (https://rosalind.onramp.bio/), with a HyperScale architecture developed by ROSALIND, Inc. (San Diego, CA). Reads were trimmed using cutadapt90. Quality scores were assessed using FastQC91. Reads were aligned to the Drosophila melanogaster genome build dm6 using STAR92. Individual sample reads were quantified using HTseq93 and normalized via Relative Log Expression (RLE) using DESeq2 R library94. Read Distribution percentages, violin plots, identity heatmaps, and sample MDS plots were generated as part of the QC step using RSeQC95. DEseq2 was also used to calculate fold changes and p-values and perform optional covariate correction. Clustering of genes for the final heatmap of differentially expressed genes was done using the PAM (Partitioning Around Medoids) method using the fpc R library96. Hypergeometric distribution was used to analyze the enrichment of pathways, gene ontology, domain structure, and other ontologies. The topGO R library97, was used to determine local similarities and dependencies between GO terms in order to perform Elim pruning correction. Several database sources were referenced for enrichment analysis, including Interpro98, NCBI99 MSigDB100,101, REACTOME102, WikiPathways103. Enrichment was calculated relative to a set of background genes relevant for the experiment. Panther was used to assess GO terms for gene lists generated in Rosalind.
Hybridization Chain Reaction (HCR)
Hearts were dissected as previously described52 to expose the heart in a 2.5mm dish. The hearts were relaxed with 10mM EGTA in oxygenated hemolymph and fixed with 4% formaldehyde in 0.1% Tween 20, PBS for 20 minutes. Next, the hearts were washed 2 x 5 minutes with 0.1% Tween 20, PBS. Then on ice, hearts were incubated, each for 5 minutes, with 25%, 50%, 75%, 100%, 75%, 50% and finally 25% methanol in PBS. Hearts were then permeabilized for 2 hours at room temperature with 1% Triton 100-X in PBS. A second fixation was repeated at room temperature and samples were washed 2 x 5 minutes with 0.1% Tween, PBS on ice. Then, a 50% 0.1% Tween 20, PBS; 50% 5X SSCT (20XSSC, 10% Tween 20, Ultrapure water) solution was used to wash the samples for 5 minutes on ice and replaced by 5X SSCT for a further 5 minutes. The cuticle with the heart attached was then trimmed down to a small rectangle and carefully transferred to a 96 well plate well (each containing a maximum of 7 hearts). Within the well, the hearts were incubated with probe hybridization buffer (Molecular Instruments) on ice for 5 minutes, then the plate was transferred to 37°C for 30 minutes. 2μl of each probe designed by Molecular Instruments was prepared in 200μl of probe hybridization buffer and incubated overnight with the hearts at 37°C. The following day, the samples were washed 4 - 15 minutes with probe wash buffer (Molecular Instruments); 2 x 5X SSCT and 1 x 5 minutes with amplification buffer (Molecular Instruments). To prepare the hairpins for fluorescence amplification, 2μl of corresponding h1 and h2 were heated to 95°C for 90 seconds and cooled in the dark for 30 minutes. The cooled hairpins were then added to 100μl of amplification buffer and incubated with the hearts overnight at room temperature in the dark. On the next day, while maintained in the dark, the samples were washed 2 x 5 minutes with 5X SSCT; 2 x 30 minutes with 5X SSCT; 1 x 5 minutes with 5X SSCT and finally rinsed 3 x with PBS. DAPI (1:250) was added with the first 5X SSCT 30-minute wash or stained subsequently for 15 minutes in PBST, followed by 3 x 10-minute PBST washes and 3 x PBS rinses. Samples were prepared and imaged as described above (Methods section: Immunofluorescence and Imaging).
To quantify RNA expression levels, the processed hearts were imaged as described in the Immunofluorescence and Imaging section and then imported into ImageJ. For Hand, Tinman and H15 quantification, the A2-A3 heart region confocal stack was converted to a max projection, duplicated and then binarized. Using the max projected image as a guide, the cytoplasmic pockets surrounding the CM nuclei were then then traced, the ROI copied to the binary imaged for particle analysis. As the segmentation was imperfect for transcripts very close together and to account for differences in pocket size, the % area covered by the transcripts was used to assess statistical significance in Prism (Graphpad).
As Lamin C and B are expressed in cells other than the CM nuclei, i.e., the ventral muscle nuclei and the cuticle, the narrowest stacks were taken around the nuclear-cytoplasmic pocket to eliminate interfering non-CM transcripts, and then the same analysis was conducted as for H15, Tinman and H15. The macro is included in the supplementary software file.
Bulk ATAC Sequencing
ATAC-seq was performed on 2,000-5,000 nuclei per sample as outlined elsewhere. Samples were permeabilized in cold nuclear permeabilization buffer ((0.2% IGEPAL-CA630 (I8896, Sigma), 1 mM DTT (D9779, Sigma), Protease inhibitor (05056489001, Roche), and 5% BSA (A7906, Sigma) in PBS (10010-23, Thermo Fisher Scientific)) for 5 minutes on a rotator at 4°C followed by centrifugation for 5 min at 500g at 4°C. After decanting supernatant, the pellet was resuspended in cold tagmentation buffer ((33 mM Tris-acetate (pH = 7.8) (BP-152, Thermo Fisher Scientific), 66 mM K-acetate (P5708, Sigma), 11 mM Mg-acetate (M2545, Sigma), 16% DMF (DX1730, EMD Millipore) in molecular biology grade water (46000-CM, Corning)) followed by incubation with Tagmentation enzyme (FC-121-1030; Illumina) at 37°C with shaking for 30 min. Tagmented DNA was purified using MinElute PCR purification kit (28004, QIAGEN). The resulting libraries were amplified using NEBNext High-Fidelity 2X PCR Master Mix (M0541, NEB) with primer extension at 72°C for 5 minutes, denaturation at 98°C for 30 s, followed by 8 cycles of denaturation at 98°C for 10s, annealing at 63°C for 30s and extension at 72°C for 60s. After purification of amplified libraries using MinElute PCR purification kit (28004, QIAGEN), double sided size selection was performed using SPRIselect beads (B23317, Beckman Coulter) with 0.55X beads and 1.5X to sample volume.
Sample Processing from FASTQ - FASTQ files were submitted through the UCSD Epigenetics ATAC-seq pipeline (https://github.com/epigen-UCSD/atac_seq_pipeline), based on the ENCODE pipeline. Briefly, reads were aligned using bowtie2, converted to uncompressed BAM files, sorted and index using: bowtie2-X2000 --mm --local −1 $fastq1 −2 $fastq2 ∣ samtools view -Su /dev/stdin ∣ samtools sort & index > xxx.PE2SE.bam &.bai 2> align.log. Poorly mapped, (<30 mapping score), duplicate, multimapped, and mitochondrial reads were removed using samtools and picard. Tn5 adapters were removed by truncating + end reads by 4 base pairs and – end reads by 5 base pairs, and then written to final output BAMs.
Computational Analysis –
Analysis was conducted as in Whitehead et al.,104 and is described as follows: BAM files were downloaded from UCSD Center for Epigenomics, sorted and indexed with samtools. Peakcalling was performed using MACS2 using the following commands: callpeak -f BAMPE -g dm - -q 0.01 --nomodel --shift −100 --extsize 200 --keep-dup all. MACS .xls output files and sorted BAMs were used to construct a Diffbind3.0.9 sample sheet for each comparison: 1- week vs 5-week w1118 samples, wildtype vs LamB iR attp40 samples, and wildtype vs LamC iR attp2 samples. Samples were read into R Studio using dba(), count densities per peak were calculated using dba.count(), filtering out peaks with <1 read per sample and a summit width of 100 (as recommended by the Diffbind3 vignette). Differential accessibility was calculated using the EdgeR wrapper of dba.analyze(). BED files were generated for each comparison using dba.report() and annotated using HOMER annotatePeaks.pl. Regions were filtered based on a log2 fold change of 0.32 and FDR of ≤ 0.1. Common features between comparisons were isolated using dplyr’s inner_join function of the “Nearest.Refseq” column output of HOMER. Plots were generated using ggplot2 and ggrepel packages. Panther was used to assess GO terms for gene lists.
Quantitative PCR for Monkey and Mouse left ventricle
Total RNA was isolated from mouse and monkey frozen left ventricle sections by first, grinding frozen tissue in a pestle and mortar with liquid nitrogen to ensure samples did not degrade. Ground tissue was transferred to an Eppendorf and resuspended in 600ul of RLT lysis buffer from the RNeasy mini RNA extraction kit (Qiagen). The suspension was then transferred to a QIAshredder column and centrifuged at <10,000 x g for 5 minutes for further homogenization. The supernatant was collected and total RNA was extracted using the RNAeasy mini RNA extraction kit (Qiagen) as per the protocol. RNA quality was assessed using an Agilent Tape station system. Poly(A)+ RNA was reverse transcribed using oligo(dT) reagent of the SuperScript IV First-Strand Synthesis kit (Thermofisher) and cDNA library generated using manufacturers protocol with a final RNase step. RT-qPCR was then performed in triplicate for each sample using SYBR Green PCR Master mix (Thermofisher) and the CFX96 hardware (Biorad). Each gene of interest was normalized to three housekeeping genes105,106 using the delta CT equation 2−(AvgCqGOI – AvgCqHK) . Primer sequences are shown in Table S10 and validated for specificity by melt temperature, and efficiency by DNA concentration titration are shown below.
Statistics and Reproducibility
Microsoft Excel 2011, Matlab 2020a, Python and Prism 9 Software were used to present data and conduct statistical analysis. The respective statistical tests and n numbers are described in the figure legends. For nuclear morphology and intensity analysis and HCR, 6-8 nuclei were cropped from the A2-A3 heart section and a minimum of 7 hearts were assessed. For RNA extraction, 15 hearts were collected per condition, and at least three biological replicated were acquired. For nuclear extraction 30-50 hearts were extracted per condition and 3-5 replicates were obtained. For SOHA live heart imaging, >13 hearts were imaged and analyzed. For actinin organization, >14 hearts were analyzed. For lifespan assays, more than 100 flies were recorded. The following statistical significance cut off was applied: n.s. p>0.05, * p<0.05, **p<0.01, ***p<0.01, ****p<0.0001. No tests were conducted to measure statistical power or normality of distributions. Data were only excluded if met criteria of the ROUT method for identifying one or more outliers with a Q of 1%, conducted in GraphPad Prism. Neither the experiments or analysis were randomized or blinded.
Extended Data
Extended Data Fig. 1. Nuclear Dynamics in Cardiac and Skeletal Muscle Cells.
(A) Kaplan-Meier survival curve for yw (gray) and w1118 (black) flies. n = 103 and flies, respectively, were used in the plot. p<10−3 based on Log-Rank (Mantel-Cox) test between the two strains. (B) Plots of 2D projected area (left) and circularity data (right) for yw flies. n = 129, 108, and 143 for yw flies at 1-, 3-, and 5-weeks, respectively. (C) Cardiomyocyte nuclear area (left) and aspect ratio (right) plotted for w1118 and yw flies as a function of adult age. n = 96, 116, and 141 nuclei for for w1118 flies and n = 129, 108, and 143 for yw flies at 1-, 3-, and 5-weeks of adulthood, respectively. (D) Ventral muscle nuclear area (left), perimeter (center), and aspect ratio (right) from w1118 flies at 1-, 3-, and 5-weeks of adulthood. n = 528, 604, 661 ventral muscle nuclei from w1118 flies at 1-, 3-, and 5-weeks of adulthood. (E) Representative images of the 3D wireframe mesh of cardiomyocyte nuclei from w1118 flies at 1- (top), 3- (middle), and 5-weeks (bottom) of adulthood. Scale bar is 5 μm. *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test. Error bars in all graphs refer to mean +/− SD.
Extended Data Fig. 2. Natural Aging Downregulates LamC and LamB but does not affect their localization.
(A) MA plot of all genes from 1- and 5-week adult w1118 fly hearts showing log2 fold change (FC) and mean normalized expression counts. Data is shown in black for genes −1.25 < FC < 1.25 (dashed lines) or p-adj > 0.05. Open circles represent genes that do not map to a nuclear ontological term. Green and purple data represent DEGs that are up- or down-regulated in 5-week adult flies, respectively. (B) Cellular component and molecular function ontological terms for genes associated with the nuclear envelope GO term, organized by their elimination pruning p-value. (C) Representative images of 1 and 5 week w1118 fly heart nuclei stained for DNA, and LamC (green) and LamB (purple) mRNA transcripts. Scale bar, 5 μm. (D) Left plot shows area percentage occupied by mRNA transcripts per cardiomyocyte in 1- and 5-week-old adult w1118 fly hearts. Right plot normalizes data to mean area at 1 week for each transcript. n = 49, 36, 49, and 36 nuclei from w1118 flies at 1 and 5 weeks for LamC and LamB, respectively. (E) Corrected total nuclear fluorescence (CTNF) of 1, 3, and 5 week yw flies for LamC (top) and LamB (bottom). n = 94, 106, and 133 nuclei for LamC and n = 173, 115, and 90 nuclei for LamB for 1, 3, and 5 week adults, respectively. (F) Image showing a representative nucleus with multiple lines radiating out from its centroid (left) to create line plots that are averaged into a single radial profile of the fluorescent intensity (right). Lower panel, the ratio of edge to center intensity is plotted. n = 72, 63, 73, and 33 nuclei from w1118 flies at 1 and 5 weeks for LamC and LamB, respectively. n = 51, 87, 77, and 42 nuclei from yw flies at 1 and 5 weeks for LamC and LamB, respectively. *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test. Bars in (D) refer to min to max, with median and 25th and 75th interquartile range and error bars in (E-F) refer to mean +/− SD.
Extended Data Fig. 3. Validation and Morphological and Functional Characterization of LamB and LamC RNAi lines.
Corrected total nuclear fluorescence (CTNF) for cardiomyocytes from (A) LamC RNAi and (B) LamB RNAi fly lines and their respective controls, i.e., attp2 and attp40, n = 47, 36, 79, and 69 nuclei from attp2 and LamC RNAi flies at 1 and 4 weeks (left to right). n = 53, 29, 65, and 61 nuclei from attp40 and LamB RNAi flies at 1 and 4 weeks (left to right). (C) Plots quantifying nuclear perimeter (left, n = 111, 96, 46, and 89 nuclei/condition) and aspect ratio (right, n = 113, 97, 46, and 92 nuclei/condition) for LamB, LamC RNAi and their genetic controls. (D) Plots quantifying nuclear area, perimeter, aspect ratio, and circularity (left to right) for LamB and LamC RNAi lines and their genetic control background at 4 weeks. For all plots, n = 136, 101, 86, and 95 nuclei/condition left to right. (E-F) Plots of diastolic and systolic diameters, and shortening velocity determined from SOHA imaging for attp2 control and LamC RNAi flies (E), and attp40 control and LamB RNAi flies (F) at 1 and 4 weeks. Each LamC RNAi plots, n = 21, 23, 25, and 27 nuclei/condition, left to right. Each LamB RNAi plots, n = 31, 21, 26, and 25 nuclei/condition, left to right. For panels A-D, *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 from a two-sided unpaired t-test at each time point and RNAi line. For E-F, *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test. Error bars in (A-D) refer to mean +/− SD, and bars in (E, F) refer to min to max, with median and 25th and 75th interquartile range.
Extended Data Fig. 4. Validation of transgenic fly background, effects of LamB on myogenic transcription factor expression, and LamC heterozygous flies.
(A) Volcano plot and (B) heat map of bulk RNA-seq from surgically dissected attp2 heart tubes. Fold change represents 5-week attp2 fly hearts normalized to 1-week old hearts and p-adj was computed from quintuplicate. 1,998 differentially expressed genes (DEGs) were assessed from cutoffs of −0.32 > log2(FC) > 0.32 (or −1.25 > FC > 1.25) (dashed lines) from comparisons of 15 fly hearts per replicate; DEGs increasing and decreasing with age are shown in green and purple, respectively. Heatmap columns were hierarchically clustered using Euclidean distance and linkage shown by the dendrogram. Heat maps are normalized within each gene/row. (C) 635 of 688 genes were co-regulated DEGs (92.3%) in the w1118 and attp2 control fly hearts, and plotted based on their fold change with age. DEGs were annotated based on their ontological categorization as nuclear (orange), extracellular matrix (ECM; green), or cytoskeletal (blue). A subset of DEGs either did not fit those categories (black/white) or lacked a known ontology (gray). Only 7.7% of all DEGs were dysregulated. (D) Representative images of attp40 control and LamB RNAi flies at 1 and 4 weeks showing H15, Hand and Tin transcription factor mRNA and DAPI. Scale bar is 5 μm. (E) Quantification of the per cell percentage area for each transcript in attp40 control and LamB RNAi flies at 1 and 4 weeks. For H15, n = 93, 87, 85, and 71 cells, left to right. For Hand, n = 111, 96, 105, and 76 cells, left to right. For Tin, n = 105, 118, 102, and 76 cells, left to right. (F) Quantification of cardiomyocyte nuclear area, circularity and LamC corrected total nuclear fluorescence for 1 week aged female control yw/w1118 and heterozygous LamC excision mutants yw/w1118;LamCex187/+ and yw/w1118;LamC296/+ fly hearts and their representative images in (G) showing staining for LamC (green) and DNA (Magenta), scale bare = 5μm. For (F), n = 29, 26, 25 (CM nuclei, left to right) (H) Quantification of heart parameters: fractional shortening, diastolic diameter and systolic diameter for background control and heterozygous LamC excision mutants. n = 17, 16, 16 (heart tubes, left to right) (I) Representative images of Hand, H15 and Tin mRNA in heterozygous LamC excision mutants, presented with quantification of per cell percentage area for each transcript in (J). n = 52, 39, 46 (CM nuclei, left to right). *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by one-way ANOVA with Tukey multiple comparisons test. Box plots in (E, H, J) refer to min to max, with median and 25th and 75th interquartile range and error bars in (F) refer to mean +/− SD.
Extended Data Fig. 5. Effects of Adult Myogenic Transcription Factor Loss on of Heart Tube Morphology and Function.
(A) Representative images of A2-A3 heart region visualized by phalloidin (F-Actin) with the indicated transgenes expressed under the control of the Hand-Gal4 promoter. Scale bar is 10 μm. At least three heart tubes were imaged for each condition. (B) Diastolic and (C) systolic diameters of heart tubes from control fly lines (attp40 and attp2) and their corresponding transgenic flies expressing the indicated RNAi. n = 10, 12, 18, 15, 19 and 14 (heart tubes, left to right). (D) CM nuclear area in attp2 control and LamC RNAi hearts subject to regime described in Fig. 6A. n = 29 and 22 (CM Nuclei, left to right). (E-G) Quantification of the per cell, percentage area for Hand, H15 and Tin transcripts upon KD of (E) LamC, (F) Hand (red),Tin (pink) and respective control attp2 (grey), and (G) H15 (blue) and attp40 control (grey), induced as in Fig. 6A. n = 32 and 26 (CM Nuclei/genotype) in (E), n = 20, 23, 28 (CM Nuclei/genotype, left to right) in (F) and n = 43 and 42 (CM Nuclei/genotype) in (G). (H) Schematic of temperature sensitive transgenic expression where 29°C enables transgenic expression due to the denaturation of Gal4 transcription factor suppressor, Gal80ts. (I) Fractional shortening of surgically exposed heart tubes at 18°C and 29°C for controls (black), and KD of tin (pink), Hand (brown), and H15 (blue) with corresponding diastolic and systolic diameters in (J-M). n = 13, 15, 18, 15, 26, 27, 16, 19, 29, 27, 29, and 21 (heart tubes/transgene/ temperature; left to right as shown in (I)). *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by independent t-test and one-way ANOVA with Kruskal-Wallis test and Dunn’s comparisons test. Box plots show to min to max, with median and 25th and 75th interquartile range and error bars in (D) refer to mean +/− SD.
Extended Data Fig. 6. Effects of LamC and Myogenic Transcription Factor Overexpression.
For panels A-D and as outlined in Figure 6A, flies were reared at 18°C and shifted to 25°C upon eclosure. Nuclear area (A) and CTNF (B) for hearts overexpressing LacZ (control) and LamC from 1 to 7 weeks. n = 31, 41, 23, 30, 26, and 31 cells (A, left to right) and n = 31, 31, 24, 29, 30, and 40 cells (B, left to right). (C) Diastolic and (D) systolic diameters of LacZ and Hand OE flies at 1, 4, and 7 weeks of adulthood. (E) Representative images (left) for nuclei from flies at 18°C and 29°C expressing GFPNLS transgene or LamC OE from HandGal,tubGal80ts;tubGal80ts based upon regime described in S5H. Scale bar is 5 μm. Plots of (F) projected nuclear area, (G) circularity, and (H) and corrected total nuclear fluorescence (CTNF) of LamC protein as a function of temperature and transgene expression. n = 39, 40, 46, and 41 nuclei (left to right). (I) For GFPNLS and LamC OE at 18°C and 29°C, representative images of LamC mRNA transcripts. Scale bar is 5 μm. (J) Quantification of LamC transcript area per CM in GFPNLS and LamC OE hearts, at 18°C and 29°C. n = 24, 36, 20 and 40 CMs, left to right. Diastolic (K) and systolic (L) diameters, and (M) fractional shortening for GFPNLS and LamC OE at 18°C and 29°C. n = 32, 19, 30 and 26 heart tubes, left to right. (N). A plot for each transcription factor is shown and quantifies the per cell, percentage area for each transcript. For all transcripts, n = 50, 56, 49 and 35 cells, left to right. (O) Representative images for tin (pink), Hand (brown), and H15 (blue) transcripts in GFPNLS and LamC OE at 18°C and 29°C. Scale bar is 5 μm. *p<0.05, **p<10−2, ***p<10−3, and ****p<10−4 by two-way ANOVA with Sidaks multiple comparisons test (A-D) and one-way ANOVA with Kruskal-Wallis test and Dunn’s comparisons test (F-N). Error bars in (A, B, F-H) refer to mean +/− SD and all box plots display min to max, with median and 25th and 75th interquartile range.
Extended Data Fig. 7. Myogenic transcription factor expression in the left ventricles of aged non-human primates and mice.
(A) Expression of three housekeeping genes for mice are plotted as a function of age with a linear and associated p-value shown. Data is plotted for raw Cq values. (B) Expression of four transcription factors in mice is shown, normalized to each housekeeper gene. Data normalized to Eef1e1 (black), Rpl4 (medium gray), and ACTB (light gray) are plotted using the left and right y-axes, depending on the axis label colors. P-values for each fit are shown in the upper right corner. (C) Expression of three housekeeping genes for rhesus macaques are plotted as a function of age with a linear and associated p-value shown. Data is plotted for raw Cq values. (D) Expression of four transcription factors in rhesus macaques is shown, normalized to each housekeeper gene. Data normalized to Rpl13a (black) and TUBB2 (light gray) use the left y-axis whereas Rpl32 uses the right y-axis (medium gray). P-values for each fit are shown in the upper right corner.
Supplementary Material
Supplementary Table 5 Gene Ontologies for co-downregulated DAR in Aging and LamC iR. This table annotates the gene ontologies for biological process (BP) and cellular component (CC) for co-downregulated genes based on the closest assigned gene for the peak and associated DAR. Data is annotated (left to right) for: GO complete name, Drosophila melanogaster peak, number of genes in dataset, expected gene number, gene over/under, fold Enrichment, raw P-value, FDR, and -Log10 (FDR p-val).
Supplementary Table 4 Analysis of DARs common to Aging vs LamC RNAi and Aging vs LamB RNAi. This table annotates co-regulated DAR from ATAC-sequencing data for LamB RNAi vs. attp40 flies and for LamC RNAi vs. attp2 flies. In tabs one (Age_and_LamC_EdgeR) and three (Age_and_LamB_EdgeR), each comparison is denoted with “x” and “y” corresponding to RNAi vs. control and 5wk vs. 1wk aging flies, respectively, and annotated for (left to right): peak number, chromosome, peak start location, peak end location, peak annotation, detailed peak annotation, distance to transcription start site (TSS), nearest promoter ID, Entrez ID of nearest gene, Unigene ID for nearest gene, nearest Refseq, gene name, gene alias, gene description, gene type, gene concentration, conc_group1, conc_group2, fold change, p-value, and false discovery rate (FDR). In tabs two (Age and LamC Genes Only) and four (Age and LamB Genes Only), data is annotated just for the fold change for each gene associated with the DAR.
Supplementary Table 6 LamC RNAi-mediated Transcriptome Changes in attp2 fly background. Table showing changes in the transcriptome of LamC RNAi flies at 1-week of adulthood versus their control attp2 background flies. Data plotted is annotated for common gene name followed by (left to right): gene description, fold change for the comparison of LamC/attp2, log2 Fold Change value, p-Value, p-Adj, Average Log2 Expression, raw Fragments Per Kilobase of transcript per Million mapped reads (FPKM), gene ID, and any gene aliases, if available.
Supplementary Table 8 Co-regulated Transcriptome Changes in attp2 vs w1118 fly background. Table showing differentially expressed genes as a function of age between the attp2 and w1118 fly genotypes. Only genes differentially expressed with age in both systems are shown. Data is annotated for common gene symbol followed by (left to right): gene ID, fly base alias, gene description, and then in the attp2 fly first and w1118 second, we show the log2 fold change value with age, p-value for the age fold change, and p-adj value for the age fold change.
Supplementary Table 9 Co-regulated Transcriptome Changes in LamC RNAi vs attp2 and Biological Process ontology terms. Mutually significant DEGs from LamC RNAi and aged fly hearts are listed in this table. In the first tab listing co-regulated genes, data is annotated (left to right) by: symbol, gene ID, gene alias, gene description, base mean gene expression with age, log2 fold change with age, p-adjusted for age fold change, base mean gene expression for LamC RNAi vs. attp2, log2 fold change for LamC RNAi vs. attp2, p-adjusted for LamC RNAi vs. attp2 fold change, protein type, additional protein description, protein classification, and the type of cellular component ontological terms associated with the gene, e.g., cytoskeleton, nucleus, extracelluar matrix, other, or unknown. In the second tab, PANTHER biological process ontological terms are annotated for co-regulated terms.
Supplementary Table 7 Age-Associated Transcriptome Changes of attp2 flies. Table showing changes in the transcriptome of attp2 flies. Data plotted is annotated for common gene name followed by (left to right): gene description, fold change for the comparison of 5-week/1-week adults, log2 Fold Change value, p-Value, p-Adj, Average Log2 Expression, raw Fragments Per Kilobase of transcript per Million mapped reads (FPKM), gene ID, and any gene aliases, if available.
Supplementary Table 10 Primer Sequences. All forward and reverse primer sequences for mouse and rhesus macaque are shown here.
Supplementary Table 2 Gene Ontologies of Age-Associated Transcriptome Changes of w1118 flies. Table showing gene ontologies associated with transcriptome changes in w1118 flies. Data listed is annotated for (left to right): gene ontology identifier, common name, p-value, false discovery rate (FDR)-adjusted p-Value, Elim pruning p-Value, Number of Genes in Term, Number of Genes that are also in this Filter or Cluster, Number of Up-regulated genes, Number of Down-regulated genes, and the negative Log10 Elim pruning p-Value.
Supplementary Table 1 Age-Associated Transcriptome Changes of w1118 flies. Table showing changes in the transcriptome of w1118 flies. Data listed is annotated for common gene name followed by (left to right): gene description, fold change for the comparison of 5-week/1-week adults, log2 Fold Change value, p-Value, p-Adj, Average Log2 Expression, raw Fragments Per Kilobase of transcript per Million mapped reads (FPKM), gene ID, and any gene aliases, if available.
Supplementary Table 3 Differentially Accessible Chromatin Regions for Age, Lam C RNAi and LamB RNAi. This table annotates ATAC-sequencing data for age comparisons in the w1118 background, in the LamB RNAi vs. attp40 flies, and in the LamC RNAi vs. attp2 flies. In each tab, data annotates (left to right): peak number, chromosome, peak start location, peak end location, peak annotation, detailed peak annotation, distance to transcription start site (TSS), nearest promoter ID, Entrez ID of nearest gene, Unigene ID for nearest gene, nearest Refseq, gene name, gene alias, gene description, gene type, gene concentration, conc_group1, conc_group2, fold change, p-value, and false discovery rate (FDR).
Acknowledgements
The authors would like to thank Dr. Kristen Jepsen of the Institute for Genomic Medicine (UCSD) for technical assistance with sequencing; Elsa Molina (Sanford Consortium Stem Cell Core) for access and maintenance of the LSM 780 microscope; Dr. Karen Christman, Dr. Ryan Middleton, Jerry Tuler and Dr. Katie Birmingham (UCSD) for use and dissection of hearts from the aged mouse model; Dr. Nissi Varki and Swati Choudhury of the Mouse Phenotyping Core (UCSD) for assistance with histological preparations; and UCSD undergraduate students Scott Skalak, Mustafa Naguib, Ishta Madan, Jiyoung Yun and Sanjana Sharma for assistance with fly husbandry and immunostaining experiments. We would like to thank the Bloomington Drosophila Stock Center (P40OD018537), Dr. Rolf Bodmer, Dr. Karen Ocorr, Dr. Joerg Grosshans and Dr. Lori Walrath for providing fly strains. The authors acknowledge funding and equipment support from the National Institutes of Health (R01AG045428 to A.J.E. and S10OD026929 to UCSD IGM Genomics Center) as well as intramural support from the National Institute on Aging (to E.G.L. and M.W.). Fellowship support was provided by the American Heart Association (20POST35180048 to N.J.K.), the National Science Foundation (to A.J.W. and P.B.), the ARCS Foundation (to A.J.W.), and the National Institutes of Health (T32GM008666 to J.D.H.). Work at the Center for Epigenomics was supported in part by the UC San Diego School of Medicine.
Footnotes
Competing Interests
The authors declare no competing interests.
Data availability
RNA-Seq and ATAC-Seq data is deposited at Gene Omnibus Express (GEO) Accession GSE185967 and GSE185923, respectively. Source data files are provided and all supporting data from this study are available from the corresponding author upon reasonable request.
Code availability
Software to image fly hearts, analyze their contraction, and create kymographs, i.e., Semi-automatic Optical Heartbeat Analysis, SOHA, is available at http://sohasoftware.com/index.html.
Python code to assess Lamin distribution is available at http://englea52.github.io/Englerlab/. Any ImageJ macros have been included in the supplementary software file.
References
- 1.Phillip JM, Aifuwa I, Walston J & Wirtz D The Mechanobiology of Aging. Annu Rev Biomed Eng 17, 113–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert HTJ & Swift J The consequences of ageing, progeroid syndromes and cellular senescence on mechanotransduction and the nucleus. Exp Cell Res 378, 98–103 (2019). [DOI] [PubMed] [Google Scholar]
- 3.CDC, N. Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative . (2015). [Google Scholar]
- 4.Sessions AO et al. Extracellular matrix downregulation in the Drosophila heart preserves contractile function and improves lifespan. Matrix Biol 62, 15–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushik G et al. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci Transl Med 7, 292ra99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sessions AO & Engler AJ Mechanical Regulation of Cardiac Aging in Model Systems. Circ Res 118, 1553–1562 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birks EJ Molecular changes after left ventricular assist device support for heart failure. Circ. Res 113, 777–791 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Van Berlo JH et al. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S, Irianto J & Discher DE Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol 216, 305–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janota CS, Calero-Cuenca FJ & Gomes ER The role of the cell nucleus in mechanotransduction. Current Opinion in Cell Biology vol. 63 204–211 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Saucerman JJ, Tan PM, Buchholz KS, McCulloch AD & Omens JH Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nature Reviews Cardiology vol. 16 361–378 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatau SB et al. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. U. S. A 106, 19017–19022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramdas NM & Shivashankar GV Cytoskeletal Control of Nuclear Morphology and Chromatin Organization. J. Mol. Biol 427, 695–706 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Stephens AD, Banigan EJ, Adam SA, Goldman RD & Marko JF Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol Biol Cell 28, 1984–1996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens AD et al. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol Biol Cell 29, 220–233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Steensel B & Belmont AS Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 169, 780–791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl KN, Ribeiro AJ & Lammerding J Nuclear shape, mechanics, and mechanotransduction. Circ Res 102, 1307–1318 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatzifrangkeskou M, Kah D, Lange JR, Goldmann WH & Muchir A Mutated lamin A modulates stiffness in muscle cells. Biochem. Biophys. Res. Commun (2020) doi: 10.1016/j.bbrc.2020.05.102. [DOI] [PubMed] [Google Scholar]
- 19.Verstraeten VLRM, Ji JY, Cummings KS, Lee RT & Lammerding J Increased mechanosensitivity and nuclear stiffness in Hutchinson–Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell 7, 383–393 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammerding J et al. Lamins a and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem 281, 25768–25780 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Srivastava LK, Ju Z, Ghagre A & Ehrlicher AJ Spatial distribution of lamin A determines nuclear stiffness and stress-mediated deformation. bioRxiv 1–15 (2019) doi: 10.1101/765263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buxboim A et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol 24, 1909–1917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl KN et al. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A 103, 10271–10276 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt A et al. Developmental control of nuclear size and shape by kugelkern and kurzkern. Curr. Biol 16, 543–552 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Jevtić P et al. Concentration-dependent effects of nuclear lamins on nuclear size in xenopus and mammalian cells. J. Biol. Chem 290, 27557–27571 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ & Discher DE Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. U. S. A 104, 15619–15624 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonne G et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet 21, 285–288 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Di Barletta MR et al. Different mutations in the LMNA gene cause autosomal dominant autosomal recessive Emery-Dreifuss muscular dystrophy. Am. J. Hum. Genet 66, 1407–1412 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.T. MRG et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J. Am. Coll. Cardiol 41, 771–780 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Capell BC, Collins FS & Nabel EG Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res 101, 13–26 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Scaffidi P & Misteli T Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat. Med 11, 440–445 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B et al. Genomic instability in laminopathy-based premature aging. Nat. Med 11, 780–785 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Goldman RD et al. Accumulation of mutant lamin A progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A 101, 8963–8968 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shumaker DK et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci 103, 8703 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haithcock E et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl. Acad. Sci 102, 16690–16695 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandt A, Krohne G & Großhans J The farnesylated nuclear proteins KUGELKERN and LAMIN B promote aging-like phenotypes in Drosophila flies. Aging Cell 7, 541–551 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Scaffidi P & Misteli T Lamin A-dependent nuclear defects in human aging. Science (80-. ). 312, 1059–1063 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson K et al. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 8, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClintock D et al. The Mutant Form of Lamin A that Causes Hutchinson-Gilford Progeria Is a Biomarker of Cellular Aging in Human Skin. PLoS One 2, e1269 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messner M et al. Upregulation of the aging related LMNA splice variant progerin in dilated cardiomyopathy. PLoS One 13, e0196739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afilalo J et al. Age-related changes in lamin A/C expression in cardiomyocytes. Am. J. Physiol. Circ. Physiol 293, H1451–H1456 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Freund A, Laberge RM, Demaria M & Campisi J Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 23, 2066–2075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Zheng X & Zheng Y Age-associated loss of lamin-b leads to systemic inflammation and gut hyperplasia. Cell 159, 829–843 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han L et al. Lamin B2 Levels Regulate Polyploidization of Cardiomyocyte Nuclei and Myocardial Regeneration. Dev Cell (2020) doi: 10.1016/j.devcel.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikolova V et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C–deficient mice. J. Clin. Invest 113, 357–369 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cammarato A et al. A mighty small heart: the cardiac proteome of adult Drosophila melanogaster. PLoS One 6, e18497 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura M et al. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell 13, 431–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi HMT et al. Third-generation in situ hybridization chain reaction: Multiplexed, quantitative, sensitive, versatile, robust. Dev. 145, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.bin Imtiaz MK et al. Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity. Cell Stem Cell 28, 967–977.e8 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Li H et al. Fly Cell Atlas: A single-nucleus transcriptomic atlas of the adult fruit fly. 2432, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prakash A et al. Cardiac abnormalities in patients with hutchinson-gilford progeria syndrome. JAMA Cardiol. 3, 326–334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogler G & Ocorr K Visualizing the beating heart in Drosophila. J. Vis. Exp 1425 (2009) doi: 10.3791/1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salick MR et al. The scanning gradient Fourier transform (SGFT) method for assessing sarcomere organization and alignment. J. Appl. Phys 127, 194701 (2020). [Google Scholar]
- 54.Cheedipudi Sirisha M et al. Genomic Reorganization of Lamin-Associated Domains in Cardiac Myocytes Is Associated With Differential Gene Expression and DNA Methylation in Human Dilated Cardiomyopathy. Circ Res 124, 1198–1213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buenrostro JD, Giresi PG, Zaba LC, Chang HY & Greenleaf WJ Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidson EH & Erwin DH Gene Regulatory Networks and the Evolution of Animal Body Plans. Science (80-. ). 311, 796 LP – 800 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Schulze SR et al. Molecular genetic analysis of the nested Drosophila melanogaster lamin C gene. Genetics 171, 185–196 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brand AH & Perrimon N Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993). [DOI] [PubMed] [Google Scholar]
- 59.McGee MD et al. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell 10, 699–710 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao M et al. Segmentation and classification of two-channel C. elegans nucleus-labeled fluorescence images. BMC Bioinformatics 18, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dreesen O et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol 200, 605–617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulze SR et al. A comparative study of Drosophila and human A-type lamins. PLoS One 4, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cosgrove BD et al. Nuclear envelope wrinkling predicts mesenchymal progenitor cell mechano-response in 2D and 3D microenvironments. Biomaterials 270, 120662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nava MM et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 181, 800–817.e22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhoades JH, Prosser BL & Musunuru K Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes Article Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Stem Cell 1–17 (2021) doi: 10.1016/j.stem.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Steensel B & Belmont AS Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 169, 780–791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng X et al. Lamins Organize the Global Three-Dimensional Genome from the Nuclear Periphery. Mol. Cell 71, 802–815.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu B et al. Plant lamin-like proteins mediate chromatin tethering at the nuclear periphery. Genome Biol. 20, 1–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ulianov SV et al. Nuclear lamina integrity is required for proper spatial organization of chromatin in Drosophila. Nat Commun 10, 1176 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawh AN et al. Lamina-Dependent Stretching and Unconventional Chromosome Compartments in Early C. elegans Embryos. Mol. Cell 78, 96–111.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang L et al. Nuclear peripheral chromatin-lamin B1 interaction is required for global integrity of chromatin architecture and dynamics in human cells. Protein Cell (2020) doi: 10.1007/s13238-020-00794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noguchi A et al. Decreased Lamin B1 Levels Affect Gene Positioning and Expression in Postmitotic Neurons. Neurosci. Res (2021) doi: 10.1016/j.neures.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 73.Li C-L et al. Region-specific H3K9me3 gain in aged somatic tissues in Caenorhabditis elegans. PLOS Genet. 17, e1009432 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pickersgill H et al. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat. Genet 38, 1005–1014 (2006). [DOI] [PubMed] [Google Scholar]
- 75.van Bemmel JG et al. The Insulator Protein SU(HW) Fine-tunes nuclear lamina interactions of the drosophila genome. PLoS One 5, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han Z & Olson EN Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132, 3525–3536 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Bodyak N Gene expression profiling of the aging mouse cardiac myocytes. Nucleic Acids Res. 30, 3788–3794 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen T et al. Tbx20 regulates a genetic program essential to adult mouse cardiomyocyte function. J. Clin. Invest 121, 4640–4654 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakabe NJ et al. Dual transcriptional activator and repressor roles of TBX20 regulate adult cardiac structure and function. Hum. Mol. Genet 21, 2194–2204 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stennard FA et al. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development 132, 2451–2462 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Akazawa H & Komuro I Roles of cardiac transcription factors in cardiac hypertrophy. Circ. Res 92, 1079–1088 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Thattaliyath BD, Livi CB, Steinhelper ME, Toney GM & Firulli AB HAND1 and HAND2 are expressed in the adult-rodent heart and are modulated during cardiac hypertrophy. Biochem. Biophys. Res. Commun 297, 870–875 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Natarajan A et al. Human eHAND, but not dHAND, is down-regulated in cardiomyopathies. J. Mol. Cell. Cardiol 33, 1607–1614 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Bigot A et al. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol. Cell 100, 189–199 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Musarò A et al. Enhanced Expression of Myogenic Regulatory Genes in Aging Skeletal Muscle. Exp. Cell Res 221, 241–248 (1995). [DOI] [PubMed] [Google Scholar]
- 86.Smith MB, Chaigne A & Paluch EK An active contour ImageJ plugin to monitor daughter cell size in 3D during cytokinesis. Methods Cell Biol 137, 323–340 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Beri P et al. Cell adhesiveness serves as a biophysical marker for metastatic potential. Cancer Res. Canres.1794.2019 (2019) doi: 10.1158/0008-5472.CAN-19-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ocorr K, Fink M, Cammarato A, Bernstein S & Bodmer R Semi-automated Optical Heartbeat Analysis of small hearts. J. Vis. Exp 1435 (2009) doi: 10.3791/1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hertz H Ueber den kontakt elastischer koerper. J. fuer die Reine Angew. Math 92, 156 (1881). [Google Scholar]
- 90.Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10–12 (2011). [Google Scholar]
- 91.Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 92.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.A. S, P. PT & H. W HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014 1512 15, 1–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.W. L, W. S & L. W RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 (2012). [DOI] [PubMed] [Google Scholar]
- 96.CRAN - Package fpc. https://cran.r-project.org/web/packages/fpc/index.html.
- 97.Bioconductor - topGO. https://bioconductor.org/packages/release/bioc/html/topGO.html.
- 98.Mitchell AL et al. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 47, D351–D360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LY G et al. The NCBI BioSystems database. Nucleic Acids Res. 38, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Subramanian A et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liberzon A et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.F. A et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 46, D649–D655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DN S et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 46, D661–D667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whitehead AJ, Hocker JD, Ren B & Engler AJ Improved epicardial cardiac fibroblast generation from iPSCs. J. Mol. Cell. Cardiol 164, 58–68 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahn K et al. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol. Biol 9, 1–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruiz-Villalba A et al. Reference genes for gene expression studies in the mouse heart. Sci. Rep 7, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 5 Gene Ontologies for co-downregulated DAR in Aging and LamC iR. This table annotates the gene ontologies for biological process (BP) and cellular component (CC) for co-downregulated genes based on the closest assigned gene for the peak and associated DAR. Data is annotated (left to right) for: GO complete name, Drosophila melanogaster peak, number of genes in dataset, expected gene number, gene over/under, fold Enrichment, raw P-value, FDR, and -Log10 (FDR p-val).
Supplementary Table 4 Analysis of DARs common to Aging vs LamC RNAi and Aging vs LamB RNAi. This table annotates co-regulated DAR from ATAC-sequencing data for LamB RNAi vs. attp40 flies and for LamC RNAi vs. attp2 flies. In tabs one (Age_and_LamC_EdgeR) and three (Age_and_LamB_EdgeR), each comparison is denoted with “x” and “y” corresponding to RNAi vs. control and 5wk vs. 1wk aging flies, respectively, and annotated for (left to right): peak number, chromosome, peak start location, peak end location, peak annotation, detailed peak annotation, distance to transcription start site (TSS), nearest promoter ID, Entrez ID of nearest gene, Unigene ID for nearest gene, nearest Refseq, gene name, gene alias, gene description, gene type, gene concentration, conc_group1, conc_group2, fold change, p-value, and false discovery rate (FDR). In tabs two (Age and LamC Genes Only) and four (Age and LamB Genes Only), data is annotated just for the fold change for each gene associated with the DAR.
Supplementary Table 6 LamC RNAi-mediated Transcriptome Changes in attp2 fly background. Table showing changes in the transcriptome of LamC RNAi flies at 1-week of adulthood versus their control attp2 background flies. Data plotted is annotated for common gene name followed by (left to right): gene description, fold change for the comparison of LamC/attp2, log2 Fold Change value, p-Value, p-Adj, Average Log2 Expression, raw Fragments Per Kilobase of transcript per Million mapped reads (FPKM), gene ID, and any gene aliases, if available.
Supplementary Table 8 Co-regulated Transcriptome Changes in attp2 vs w1118 fly background. Table showing differentially expressed genes as a function of age between the attp2 and w1118 fly genotypes. Only genes differentially expressed with age in both systems are shown. Data is annotated for common gene symbol followed by (left to right): gene ID, fly base alias, gene description, and then in the attp2 fly first and w1118 second, we show the log2 fold change value with age, p-value for the age fold change, and p-adj value for the age fold change.
Supplementary Table 9 Co-regulated Transcriptome Changes in LamC RNAi vs attp2 and Biological Process ontology terms. Mutually significant DEGs from LamC RNAi and aged fly hearts are listed in this table. In the first tab listing co-regulated genes, data is annotated (left to right) by: symbol, gene ID, gene alias, gene description, base mean gene expression with age, log2 fold change with age, p-adjusted for age fold change, base mean gene expression for LamC RNAi vs. attp2, log2 fold change for LamC RNAi vs. attp2, p-adjusted for LamC RNAi vs. attp2 fold change, protein type, additional protein description, protein classification, and the type of cellular component ontological terms associated with the gene, e.g., cytoskeleton, nucleus, extracelluar matrix, other, or unknown. In the second tab, PANTHER biological process ontological terms are annotated for co-regulated terms.
Supplementary Table 7 Age-Associated Transcriptome Changes of attp2 flies. Table showing changes in the transcriptome of attp2 flies. Data plotted is annotated for common gene name followed by (left to right): gene description, fold change for the comparison of 5-week/1-week adults, log2 Fold Change value, p-Value, p-Adj, Average Log2 Expression, raw Fragments Per Kilobase of transcript per Million mapped reads (FPKM), gene ID, and any gene aliases, if available.
Supplementary Table 10 Primer Sequences. All forward and reverse primer sequences for mouse and rhesus macaque are shown here.
Supplementary Table 2 Gene Ontologies of Age-Associated Transcriptome Changes of w1118 flies. Table showing gene ontologies associated with transcriptome changes in w1118 flies. Data listed is annotated for (left to right): gene ontology identifier, common name, p-value, false discovery rate (FDR)-adjusted p-Value, Elim pruning p-Value, Number of Genes in Term, Number of Genes that are also in this Filter or Cluster, Number of Up-regulated genes, Number of Down-regulated genes, and the negative Log10 Elim pruning p-Value.
Supplementary Table 1 Age-Associated Transcriptome Changes of w1118 flies. Table showing changes in the transcriptome of w1118 flies. Data listed is annotated for common gene name followed by (left to right): gene description, fold change for the comparison of 5-week/1-week adults, log2 Fold Change value, p-Value, p-Adj, Average Log2 Expression, raw Fragments Per Kilobase of transcript per Million mapped reads (FPKM), gene ID, and any gene aliases, if available.
Supplementary Table 3 Differentially Accessible Chromatin Regions for Age, Lam C RNAi and LamB RNAi. This table annotates ATAC-sequencing data for age comparisons in the w1118 background, in the LamB RNAi vs. attp40 flies, and in the LamC RNAi vs. attp2 flies. In each tab, data annotates (left to right): peak number, chromosome, peak start location, peak end location, peak annotation, detailed peak annotation, distance to transcription start site (TSS), nearest promoter ID, Entrez ID of nearest gene, Unigene ID for nearest gene, nearest Refseq, gene name, gene alias, gene description, gene type, gene concentration, conc_group1, conc_group2, fold change, p-value, and false discovery rate (FDR).
Data Availability Statement
RNA-Seq and ATAC-Seq data is deposited at Gene Omnibus Express (GEO) Accession GSE185967 and GSE185923, respectively. Source data files are provided and all supporting data from this study are available from the corresponding author upon reasonable request.
Software to image fly hearts, analyze their contraction, and create kymographs, i.e., Semi-automatic Optical Heartbeat Analysis, SOHA, is available at http://sohasoftware.com/index.html.
Python code to assess Lamin distribution is available at http://englea52.github.io/Englerlab/. Any ImageJ macros have been included in the supplementary software file.