Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is defined by the American Association of Oral and Maxillofacial Surgeons (AAOMS) as the presence of an exposed bone area in the maxillofacial region, present for more than eight weeks in patients treated with the use of antiresorptive or antiangiogenic agents, with no history of radiation or metastatic disease. Bisphosphonates (BF) and denosumab (DS) are widely used in adults for the management of patients with cancer and osteoporosis, and recently there has been an increase in their use in child and young patients for the management of disorders such as osteogenesis imperfecta (OI), glucocorticoid-induced osteoporosis, McCune-Albright syndrome (MAS), malignant hypercalcemia, and others. There are differences between case reports in adults compared to child and young patients related to the use of antiresorptive/antiangiogenic drugs and the development of MRONJ. The aim was to analyze the presence of MRONJ in children and young patients, and the relation with oral surgery. A systematic review, following the PRISMA search matrix based on the PICO question, was conducted in PubMed, Embase, ScienceDirect, Cochrane, Google Scholar, and manual search in high-impact journals between 1960 and 2022, publications in English or Spanish, including randomized and non-randomized clinical trials, prospective and retrospective cohort studies, cases and controls studies, and series and case reports. A total of 2792 articles were identified and 29 were included; all of them published between 2007 and 2022, identifying 1192 patients, 39.68% male and 36.24% female, aged 11.56 years old on average, using these drugs mainly for OI (60.15%); 4.21 years on average was the therapy time and 10.18 drug doses administered on average; oral surgery was observed in 216 subjects, reporting 14 cases of MRONJ. We concluded that there is a low presence of MRONJ in the child and youth population treated with antiresorptive drugs. Data collection is weak, and details of therapy are not clear in some cases. Deficiencies in protocols and pharmacological characterization were observed in most of the included articles.
Keywords: osteonecrosis, antiresorptive, MRONJ
1. Introduction
Bisphosphonates (BFs) are a group of antiresorptive drugs widely used in adults with pathological conditions, such as Paget’s disease, multiple myeloma, and conditions associated with cancer such as bone metastasis and hypercalcemia of malignancy [1,2,3,4,5,6,7,8,9,10,11,12,13].
The potential of BF therapy to improve survival rates remains controversial; however, its positive effect on the quality of life of patients with advanced bone cancer has been demonstrated [14]. Oral medications are also used for the management of osteoporosis and osteopenia [15,16].
BFs, especially those belonging to the nitrogen-containing subset, decrease bone resorption through inhibition of the enzyme farnesyl diphosphate synthase in the mevalonate pathway [17]. The mechanism is thought to involve interruption of the osteoclast cytoskeleton, impaired intracellular vesicular function, increased apoptosis, and decreased osteoclastic function [18,19].
In recent years, BFs have been reported to be associated with an increased risk of developing osteonecrosis of the jaws. The first description was reported in 2003 [20]; the most relevant report was presented by Marx [21]. Despite the fact that the first cases of osteonecrosis of the jaws were reported more than 15 years ago [21], the pathophysiology and mechanism is not completely clarified [22,23,24,25]. Hypotheses have been proposed that attempt to explain the unique location of this entity, exclusive to the jaws, including change in bone remodeling, over-suppression of bone resorption, inhibition of angiogenesis, frequent micro-trauma, suppression of innate or acquired immunity, vitamin D deficiency, soft tissue toxicity, inflammation, or infection [14].
On the other hand, in addition to BFs, denosumab (DS), which is a highly specific human monoclonal antibody (IgG2) against the human receptor activator of nuclear factor kappa-Β ligand (RANK Ligand), is also associated with the appearance of osteonecrosis of the jaws in a similar way to bisphosphonates [26,27,28].
In addition to antiresorptive drugs (BF and DS), there are agents from the group of antiangiogenic drugs related to osteonecrosis of the jaws, such as bevacizumab, sirolimus, sunitib, and others [29].
MRONJ is defined by the AAOMS as the presence of an area of exposed bone or the possibility of probing bone tissue through an intraoral or extraoral fistula in the maxillofacial region, present for more than eight weeks, in a patient with current or earlier treatment with antiresorptive or antiangiogenic agents, with no history of radiation therapy or metastatic disease in the jaws [14].
BF and DS are widely used in adults, and recently there has been an increase in their use in children and adolescents for the management of OI, glucocorticoid-induced osteoporosis, MAS, malignant hypercalcemia, or others [30,31].
There is an important difference between reports of MRONJ in adults compared to pediatric and young adolescent patients, even though these drugs are widely used in this population.
Currently, there are few reports in children and adolescents showing the relationship between OI, malignant pathology, osteoporosis, among others, and treatment with antiresorptive/antiangiogenic agents. The risk of developing MRONJ in such patients is possible; additionally, the influence of dental surgical treatments must be estimated.
The aim of this systematic review is to analyze the incidence of MRONJ in children and adolescents, and the relations with a history of invasive dental treatment.
2. Materials and Methods
In this systematic review, the PICO [32] question was established: Does MRONJ represent a risk in pediatric and adolescent patients treated with antiresorptive/antiangiogenic drugs in various pathologies? The systematic review performed using the PRISMA Statement (Preferred Reports for Systematic Reviews and Meta-analyses) as a guide [33,34].
The following inclusion criteria were considered: articles in English and Spanish, with child–youth population, undergoing treatment with antiresorptive and/or antiangiogenic drugs using intravenous route (IV) or oral route (po), with reports of cases of MRONJ. Case reports and case series, prospective and retrospective associated studies, case-control studies, randomized and non-randomized clinical trials were included. The exclusion criteria were literature review, treatment in subjects aged more than 18 years old, and the inability to access the full text.
In the first round of the search, abstracts were reviewed and all articles containing keywords were retained. Full versions were obtained for all the articles that met the inclusion criteria. In the second round of the search, a manual analysis of the references in each article was realized. A search for unpublished literature or data was not performed. Case reports and case series were also evaluated, in order to identify cases. The electronic search was complemented by a manual search as previously mentioned. In the third search round, each article was critically reviewed for validity and bias assessment and the following data were extracted: title, authors, journal, year of publication, type of study, total of pediatric and adolescent patients, total of pediatric and adolescent patients under antiresorptive/antiangiogenic treatment, sex, average age in years (range), indication for antiresorptive/antiangiogenic therapy, medication used, average time of use of the drug in years (range), number of average doses (range), data on dosage, route of administration, cumulative dose, adjunct medication therapy, MRONJ reports, comorbidities, MRONJ detection method, use of antimicrobial therapy.
The literature search strategy was carried out independently by two established evaluators (R.H and G.H) following the same search pattern. In case of disagreement during any stage of the review process, a third evaluator was included [32,33].
The following databases were incorporated into the systematic search: PubMed, Embase, ScienceDirect, Cochrane, Google Scholar, considering the literature from 1990 to April 2022. Manual search was included in this protocol, including articles from the area published in journals of high impact in selected languages (Journal of Oral and Maxillofacial Surgery, British Journal of Oral and Maxillofacial Surgery, Journal Of Neurosurgery, Asian Journal of Oral and Maxillofacial Surgery, Revista Española de Cirugía Oral y Maxilofacial, Revista Medicina Oral Patología Oral Cirugía Bucal). The following search terms were used: “Osteonecrosis jaw” or “ONJ” or “BRONJ” or “MRONJ” + “children” or “young” or “pediatric” or “paediatric” + “bisphosphonates” or “denosumab” or “antiangiogenics” + “osteogenesis imperfecta” or “Paget disease” or “fibrous dysplasia” or “cancer” in English, and “Osteonecrosis maxilar” o “ONM” o “OMAB” o “OMAM” o “ONMAM” + “niños” o “jóvenes” o “pediátrico” + “bifosfonatos” o “denosumab” o “antiangiogénicos” + “osteogenesis imperfecta” o “enfermedad de Paget” o “displasia fibrosa” o “cáncer” in Spanish.
3. Results
3.1. Selection
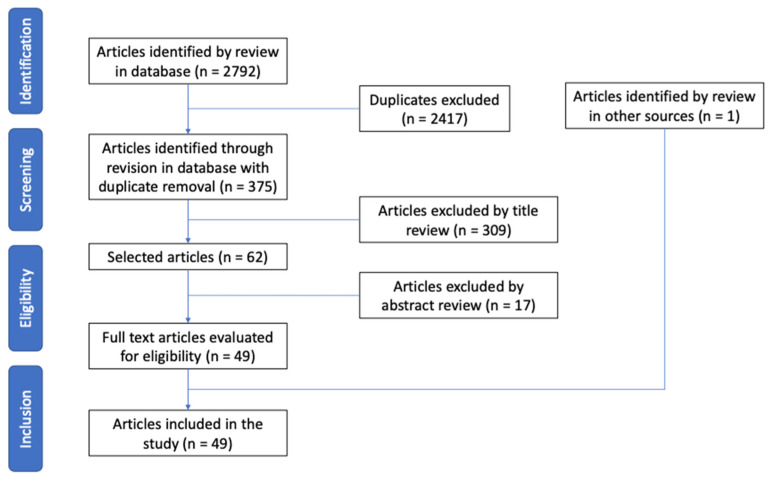
A total of 2792 articles were obtained in the five databases. After analysis of duplicate articles, 2417 were excluded. Another 309 articles were excluded in the title evaluation, and an additional 17 in the abstract review, making a total of 48 articles eligible for full-text evaluation; 1 article was added from the manual search. After the full-text review, 29 articles were included in the study that met the objectives of the systematic review [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] (Figure 1) (Table 1 and Table 2).
Figure 1.
Flow chart for the selection of articles based on the PRISMA matrix.
Table 1.
Distribution of variables of 29 articles related to MRONJ included in this systematic review.
| Authors (Year) | Pts. under ART/AAT | Sex | Avg. Age in yr. (Range) | Indication for ART/AAT | Medication | Medication Use Time (Range) | Avg. Doses Number (Range) | Dosage Data | Cumulative Dose | Concomitant Medication |
|---|---|---|---|---|---|---|---|---|---|---|
| August et al. (2011) [35] | 19 | 12 (M) 7 (F) |
12.50 (1.10 to 23.10) | Malignant pathology | ZD | NDA | 7.47 (1 to 21) | <10 yr.: 4 mg q28 days approx. >10 yr.: 0.08–0.16 mg/kg q28 days approx. |
NDA | Vincristine, cyclophosphamide, doxorubicin, ifosfamide, etoposide, methotrexate, topotecan, temozolamide, gemcitabine, docetaxel, irinotecan, vinorelbine, busulfan, melfalan. |
| Bredell et al. (2017) [36] | 5 | 2 (M) 3 (F) |
18.00 (3.90 to 26.00) | CGCL | DS | NDA | 14.20 (12 to 15) | Case 1: 70–100 mg SC once a wk. for the first mo. and then once a mo. Rest of cases: 120 mg SC initial dose, 120 mg on the 8th day and 15th day. Then 120 mg q4 wk. |
NDA | Calcitonin, IFN alpha, vitamin D and calcium supplements, intralesional corticosteroids. |
| Brown et al. (2008) [37] | 42 | NDA | 8.25 | OI, MAS, osteoporosis, transverse myelitis | PD (n = 1) ZD (n = 4) PD + ZD (n = 37) |
6.50 | 29.60 | PD: 1 mg/kg/dose q2 mo. approx. ZD: 0.04–0.05 mg/kg/dose q4 mo. approx. |
PD: 19.8 mg/kg corresponds to an equivalent dose in adults of 1190 mg. ZD: 0.49 mg/kg corresponds to an equivalent adult dose of 29.4 mg. |
Corticosteroids in a single case. |
| Carpenter et al. (2007) [38] | 18 | 11 (M) 7 (F) |
11.30 (5.80 to 17.10) | Low BMD | PD. AL or ZD as alternatives |
0.96 | NDA | PD: 1 mg/kg q mo. | NDA | Growth hormone, sex hormones, prednisone, calcium and/or vitamin D supplements. |
| Chahine et al. (2008) [39] | 278 | 136 (M) 142 (F) |
14.70 (0.70 to 32.00) | OI, osteoporosis, fibrous/bone dysplasia, neuromuscular disorders, rheumatic disorders, Crohn’s disease | PD | 4.60 (0.00 to 112) |
NDA | <2 yr.: 0.5 mg/kg q day for 3 consecutive days q2 mo. 2–3 yr.: 0.75 mg/kg q day for 3 consecutive days q3 mo. >3 yr.: 1 mg/kg q day 3 consecutive days q4 mo. Maximum dose: 60 mg q day. Maximum infusion concentration: 0.1 mg/mL. Infusion administration duration: 3–4 h. |
9 mg/kg (total annual dose). Avg. cumulative dose before tooth extraction: 40 mg/kg (2.5 to 81 mg/kg) in pts. who underwent tooth extraction and had records of PD Tx. (n = 45) |
NDA |
| Feehan et al. (2018) [40] | 33 | 18 (M) 15 (F) |
9.00 | OI | PD or ZD | 7.00 (4 to 11.5) |
NDA | NDA | NDA | Antidepressants (n = 6), proton pump inhibitor (n = 2), pain relievers (n = 1). |
| Goldsby et al. (2013) [41] | 24 | 8 (M) 16 (F) |
13.50 (7.00 to 22.00). | Malignant pathology | ZD | 0.64 | 8.00 | Induction dose (wk. 1 to 12): 1.2 mg/m2 (max. 2 mg) (n = 6); 2.3 mg/m2 (max. 4 mg) (n = 6); 3.5 mg/m2 (max. 6 mg) (n = 6). According to tolerance, the dose levels were scaled. A fourth group (n = 6) was added at a dose of 2.3 mg/m2 (max. 4 mg) after determining the maximum tolerated dose, in order to help assess the post-induction feasibility of ZD. |
NDA | Cisplatin, adriamycin, methotrexate, ifosfamide, etoposide, calcium and vitamin D supplements. |
| Idolazzi et al. (2017) [42] | 55 | 30 (M) 25 (F) |
12.60 (5.00 to 19.00) | OI | ND | 3.00 | 11.10 (3 to 13) | 2 mg/kg (max. 100 mg) q3 mo. for 3 yr. | NDA | Calcium and vitamin D supplements. |
| Ierardo et al. (2017) [43] | 20 | 12 (M) 8 (F) |
NDA (8.00 to 14.00) | OI | ND | NDA | NDA | Dose q3 mo. | NDA | NDA |
| Johannesen et al. (2009) [44] | 37 | 28 (M) 9 (F) |
10.80 (6.01 a 1550) | AFN | ZD | 1.18 | 6.70 | 1st dose: 0.0125 mg/kg. 2nd dose (at 6 wk.): 0.025 mg/kg. 3rd dose (12 wk. after the 1st dose): 0.025 mg/kg. Following dose: at 0.025 mg/kg 12 wk. apart. |
NDA | Calcium and vitamin D supplements. |
| Kumar et al. (2016) [45] | 26 | NDA | 7.00 (3.75 to 10.00) | OI | ZD | 3.00 (0.91 to 5.08) | 12,00 (3.66 to 20.3) | <1 yr.: 2 mg. >1 yr.: 4 mg q3 mo. |
48 mg/kg on avg. (16 to 80 mg/kg). | NDA |
| Li et al. (2011) [46] | 20 | 11 (M) 9 (F) |
10.60 | OI | IB | 2.00 | 8.00 | 2 mg q3 mo. | NDA | Calcium and vitamin D supplements. |
| Lindahl et al. (2016) [47] | 79 | 43 (M) 36 (F) |
6.75 (0.10 to 17.10). | OI | PD | 7.60 | NDA | Monthly infusion of 10 mg/m2 (1st 3 mo.), 20 mg/m2 (2nd 3 mo.), then 30 mg/m2. After 2 yr., the dose was adjusted in relation to the response (evaluated by bone densitometry, bone turnover markers in blood and urine, and regression of vertebral compression fractures). | NDA | Calcium and vitamin D supplements. |
| Maines et al. (2012) [48] | 102 | 47 (M) 55 (F) |
12.26 (3.10 to 23.40) | OI | ND | 6.81 (1.00 to 12.90) | NDA | Pts. who started treatment with less than 1 yr. (n = 15): Infusion 1 mg/kg/day for 2 consecutive days q3–4 mo. Rest of patients (n = 87): 2 mg/kg/dose in a single session q3–6 mo. |
Cumulative avg. dose: 1679 mg (144 to 5307 mg). Cumulative avg. dose (per kg): 50 mg/kg (10 to 100 mg/kg). | NDA |
| Malmgren et al. (2008) [49] | 64 | NDA | 8.10 (0.20 to 20.90). | OI | PD | 4.50 (0.50 to 12.50) | NDA | 1st 3 mo.: 10 mg/m2. 2nd 3 mo.: 20 mg/m2. Then: 30–40 mg/m2 in q mo. infusion. |
1623 mg/m2 on avg. (140–4020 mg/m2). | Steroids or cytostatics were never administered. |
| Milano et al. (2011) [50] | 1 | 1 (M) | 4.66 | OI | PD | NDA | 8.00 | Last dose before surgery: 60 mg. | NDA | Calcium and vitamin D supplements. |
| Moeini et al. (2013) [51] | 12 | 3 (M) 9 (F) |
13.00 (700 to 21.00) | Prostate thalassemia major | NDA | NDA | NDA | NDA | NDA | NDA |
| Naidu et al. (2014) [52] | 1 | 1 (F) | 9.00 | CGCL | DS | 1.50 | NDA | 120 mg q day for 28 days. Additionally, 2 loading doses were administered in 1mo. (8th and 15th day of Tx). | NDA | Calcium and vitamin D supplements. |
| Nasomyont et al. (2019) [53] | 123 | 69 (M) 54 (F) |
10.21 (0.01 to 20.60) | Osteoporosis | PD or ZD | NDA | 5.27 (1 to 48) | Individualized doses for each case. On avg. PD was administered: 9 mg/kg/yr. q2-4 mo. (Primary osteoporosis group). 4 mg/kg/yr. q3 mo. (secondary and glucocorticoid-induced osteoporosis groups). ZD: 0.1 mg/kg/year every 6 months. |
NDA | NDA |
| Ngan et al. (2013) [54] | 1 | 1 (F) | 12.00 | OI | PD | NDA | NDA | 1 mg/kg/dose q3 mo. | NDA | Calcium and vitamin D supplements. |
| Okawa et al. (2017) [55] | 31 | NDA | NDA | OI | NDA | NDA | NDA | NDA | NDA | NDA |
| Piperno-Neumann et al. (2018) [56] | 110 | NDA | NDA | Malignant pathology | ZD | 0.83 | 10.00 | >25 yr.: 10 monthly doses of 4 mg. 18–25 yr.: 10 monthly doses of 0.05 mg/kg for the first two cycles and 4mg for the remaining 8 cycles. Children <18 yr.: 10 monthly doses of 0.05 mg/kg in all cycles without exceeding 4 mg. |
NDA | Calcium and vitamin D supplements. Chemotherapy regimens with: Methotrexate, etoposide, ifosfamide, cisplatin, doxorubicin. |
| Putman et al. (2018) [57] | 14 | NDA | 14.70 (4.00 to 20.00) | Low BMD | AL (n = 12) y PD (n = 2) | 1.90 (AL group) | NDA | AL: 35 mg po once a wk. (n = 12). PD: (single dose IV) (n = 2). |
NDA | Vitamin D supplements, glucocorticoids. |
| Schwartz et al. (2008) [58] | 13 | 9 (M) 4 (F) |
9.10 (2 to 17.41) | OI | NDA | 4.56 | NDA | NDA | NDA | NDA |
| Simm et al. (2011) [59] | 20 | 9 (M) 11 (F) |
9.60 (3.30 to 16.50) | Osteoporosis | ZD | 1.70 (0.50 to 2.00) | NDA | 1st dose: 0.0125 mg/kg. 2nd dose: (at 12 wk.): 0.025 mg/kg. Following dose: (12 wk. after the previous dose): 0.025 mg/kg. |
0.1 mg/kg per year on avg. | Calcium and vitamin D supplements. |
| Tessaris et al. (2016) [60] | 13 | 6 (M) 7 (F) |
20.30 (7.00 to 27.00) | Fibrous dysplasia, MAS. | PD | 2.50 | NDA | 1 mg/kg/day for 3 consecutive days (1 daily infusion) repeating at intervals of 4–6 mo. | NDA | NDA |
| Uday et al. (2018) [61] | 2 | 1 (M) 1 (F) |
14.85 (14.00 to 15.70) | CGCL | DS | 2.45 | 32.00 (18.00 to 46.00) | 120 mg on days 1, 8, 15, and 28. Then q4 wk. (2.1 and 2.6 mg/kg/dose, respectively). | 5520 and 2160 mg, respectively. | Corticosteroids, chemotherapy, and bisphosphonates were not administered to patients. |
| Vuorimies et al. (2011) [62] | 17 | 8 (M) 9 (F) | 10.10 (1.50 to 16.80) | OI | ZD | 1.90 (1.00 to 3.20) | NDA | 1 infusion q6 mo. at a dose of 0.05 mg/kg (maximum 4.0 mg daily). | NDA | Calcium and vitamin D supplements. |
| Wagner et al. (2011) [63] | 12 | 9 (M) 3 (F) |
10.84 (2.20 to 14.50) | Osteoporosis | PD | 1.00 | 4.16 (1.00 to 21.00) | Protocol of 1 or 3 days depending on the healthcare center. 1st day: 1 mg/kg (max. 30mg). 2nd day: (according to serum calcium) >2.2 mmol/L: 1 mg/kg (maximum 60 mg). Between 2 and 2.2 mmol/L: 0.5 mg/kg (maximum 30 mg). <2 mmol/L: no more infusions. It is repeated q3 mo. |
NDA | Calcium and Vitamin D supplements, acetaminophen, codeine and morphine. |
AAT: antiangiogenic; AFN: avascular femoral necrosis; AL: alendronate; approx.: approximate; ART: antiresorptive; Avg.: average; BMD: bone mineral density; CGCL: central giant-cell lesion; DS: denosumab; F: female; IB: ibandronate; IV: intravenous; M: male; MAS: McCune-Albright syndrome; max.: maximum; mo.: month(s); ND: neridronate; NDA: No data available; OI: osteogenesis imperfecta; PD: pamidronate; po: per os (by mouth); pts.: patient(s); q: every; SC: subcutaneous; tx.: treatment; wk.: week(s); yr.: year(s); ZD: zoledronate.
Table 2.
Presence of MRONJ and comorbidities in the articles included in this systematic review.
| Authors (Year) | MRONJ Reported Cases | Comorbidities | MRONJ Detection Method | Use of Antimicrobial Therapy |
|---|---|---|---|---|
| August et al. (2011) [35] | No cases were reported. | NDA | Not specific. | N/A |
| Bredell et al. (2017) [36] | 1 case (case 2). MRONJ stage 2 (poor healing after exploratory surgery). | NDA | Not specific. | N/A |
| Brown et al. (2008) [37] | No cases were reported. | NDA | Clinical and radiographic evaluation. | NDA |
| Carpenter et al. (2007) [38] | No cases were reported. | NDA | Not specific. | N/A |
| Chahine et al. (2008) [39] | No cases were reported. | NDA | Search in medical record and follow up control notes from dentist that treats patient. | Prophylactic antibiotic therapy was used in 12 patients. |
| Feehan et al. (2018) [40] | No cases were reported. | Psychiatric (n = 4), cardiovascular (n = 3), respiratory (n = 1), endocrinology (n = 1) ENT (n = 3), others (n = 4). | Not specific. | N/A |
| Goldsby et al. (2013) [41] | No cases were reported. | NDA | Not specific. | N/A |
| Idolazzi et al. (2017) [42] | No cases were reported. | NDA | Not specific. | N/A |
| Ierardo et al. (2017) [43] | No cases were reported. | NDA | Radiographic and clinical evaluation. | Amoxicillin 50 mg/kg 30–60 min before surgery. Clindamycin 20 mg/kg was used in allergy sufferers 30–60 min before surgery. |
| Johannesen et al. (2009) [44] | No cases were reported. | NDA | Not specific. | N/A |
| Kumar et al. (2016) [45] | No cases were reported. | NDA | Not specific. | N/A |
| Li et al. (2011) [46] | No cases were reported. | They do not present rickets, hyperparathyroidism, other hereditary or metabolic bone pathologies, history of treatment with bisphosphonates, abnormal renal function (exclusion criteria). | Not specific. | N/A |
| Lindahl et al. (2016) [47] | No cases were reported. | NDA | Not specific. | N/A |
| Maines et al. (2012) [48] | No cases were reported. | NDA | Clinical examination by dental surgeon. | NDA |
| Malmgren et al. (2008) [49] | No cases were reported. | Its absence is presumed due to the non-administration of steroids or cytostatics. | Clinical and radiographic reviews every 6 months by a dentist or doctor. Radiographic evaluation in two children younger than 3 years was omitted. | Penicillin for 7 days in one case to treat a postoperative infection, which healed without complications. |
| Milano et al. (2011) [50] | No cases were reported. | NDA | Clinical evaluation. | Ampicillin 50 mg/kg single dose prior to surgical procedure. |
| Moeini et al. (2013) [51] | 12 cases. | NDA | Clinical evaluation. | N/A |
| Naidu et al. (2014) [52] | No cases were reported. | NDA | Not specific. | N/A |
| Nasomyont et al. (2019) [53] | No cases were reported. | NDA | Not specific. | N/A |
| Ngan et al. (2013) [54] | No cases were reported. | NDA | Clinical evaluation 1st month and 3rd month. There were no signs of infection, exposed bone, or osteonecrosis. | Amoxicillin for 5 days. |
| Okawa et al. (2017) [55] | No cases were reported. | NDA | Not specific. | NDA |
| Piperno-Neumann et al. (2018) [56] | No cases were reported. | NDA | Not specific. | N/A |
| Putman et al. (2018) [57] | No cases were reported. | NDA | Not specific. | N/A |
| Schwartz et al. (2008) [58] | No cases were reported. | NDA | Clinical evaluation in some cases. | 13–15 patients (not specify more details). |
| Simm et al. (2011) [59] | No cases were reported. | NDA | Dental evaluation. | N/A |
| Tessaris et al. (2016) [60] | No cases were reported. | NDA | Clinical and imaging evaluation (orthopantomography and CTCB). | NDA |
| Uday et al. (2018) [61] | 1 case. MRONJ Stage 2 after extraction of a fractured molar. | NDA | Clinical evaluation. | Amoxicillin and metronidazole as a treatment for MRONJ. |
| Vuorimies et al. (2011) [62] | No cases were reported. | NDA | Not specific. | N/A |
| Wagner et al. (2011) [63] | No cases were reported. | NDA | Not specific. | N/A |
ENT: ear, nose, throat; NDA: no data available.
3.2. Years of Publication and Types of Study
Articles published between 2007 and 2022 were included, these being 16 cohort studies (55.17%), 9 case series (31.03%), 2 case reports (6.89%), 1 randomized clinical trial (3.44%), and 1 non-randomized clinical trial (3.44%).
3.3. Patients by Sex
The total number of children and adolescents among the 29 articles was 1595; of these, 1192 received antiresorptive/antiangiogenic medication and were evaluated in search of MRONJ, of which 473 (39.68%) were male, 432 (36.24%) were female, and 287 (24.07%) gave no data about sex.
3.4. Patients by Age
In total, 89.65% (n = 26) of the articles presented data to calculate the average age, resulting in 11.56 years for 1031 patients (range 0.01 to 32.00 years). It is important to show that in 31.03% of the articles there are patient older than 20 years. In all, 10.34% (n = 3) of the articles did not provide data for the analysis of age [43,55,56].
3.5. Antiresorptive/Antiangiogenic Medication Used
Reported was the use of pamidronate (PD) (n = 451; 37.83%), zoledronate (ZD) (n = 257; 21.56%), neridronate (ND) (n = 177; 14.84%), unspecified between PD and ZD (n = 156; 13.08%), PD plus ZD (n = 37; 3.10%), ibandronate (IB) (n = 20; 1.67%), unspecified between PD, alendronate (AL), and ZD (n = 18; 1.51%), AL (n = 12; 1.00%), DS (n = 8; 0.67%). In 56 patients (4.69%), the involved drug was not specified [51,55,58]. Antiangiogenic drugs were not used in any patient.
3.6. Conditions for Antiresorptive Therapy
Osteogenesis imperfecta (OI) (n = 717; 60.15%) was the main reason for the use of these drugs. Other conditions were related to: osteoporosis/low bone mineral density (n = 203; 17.03%), malignant pathology (n = 153; 12.83%), fibrous dysplasias/bone dysplasias/MAS (n = 46; 3.85%), avascular femoral necrosis (n = 37; 3.10%), major prostatic thalassemia (n = 12; 1.00%), neuromuscular disorders (n = 11; 0.92%), giant-cell bone tumor (n = 8; 0.67%), rheumatic disorders (n = 3; 0.25%), Crohn’s disease (n = 1; 0.08%), and transverse myelitis (n = 1; 0.08%).
3.7. Drug Usage Time
A total of 27.58% (n = 8) of the articles did not show data to calculate the time involved in the use of antiresorptive therapy [35,36,43,50,51,53,54,55]. On the other hand, 68.96% (n = 20) of the articles showed data for the time analysis, resulting in 4.21 years on average for 948 patients [37,38,39,40,41,42,44,45,46,47,48,49,52,56,58,59,60,61,62,63]. The lowest average of treatment time was 0.64 years [41] and the longest was 7.60 years [47].
3.8. Dosage and Administration
A total of 55.17% (n = 16) of the articles did not present any data to calculate the drug dosage or administration [38,39,40,43,47,48,49,51,52,54,55,57,58,59,60,62]. On other hand, 44.82% (n = 13) of the articles showed data, resulting in 10.18 drug dosage on average used in 476 patients [35,36,37,41,42,44,45,46,50,53,56,61,63]. The lowest average dosage was 4.16 [63] and the highest was 32.00 [61].
In total, 92.11%. of the patients (n = 1098) received the drug through intravenous route (IV), 1.51% (n = 18) using multiple routes of administration, 1.00% (n = 12) using the oral route (po), and 0.67% (n = 8) through subcutaneous route (SC). Three articles did not include data about the route of administration (n = 56; 4.69%) [51,55,58].
3.9. Posology
In total, 41.37% of the articles (n = 12) used PD. In five of these articles (17.24%), doses of 1 mg/kg were used, with different strategies, as follows: 1 mg/kg/dose every 2 months approximately [37], 1 mg/kg every month [38], 1 mg/kg/dose every 3 months [54], 1 mg/kg daily for 3 consecutive days repeating at between 4 and 6 months [60], and 1 mg/kg on the first day (maximum 30 mg), according to serum calcium on the second day: >2.2 mmol/L: 1 mg/kg (maximum 60 mg); between 2 and 2.2 mmol/L: 0.5 mg/kg (maximum 30 mg); <2 mmol/L: no more infusions, repeating every 3 months [63].
In all, 6.89% of the articles (n = 2) established schemes based on body surface area and quarterly dose increases as follows: monthly infusions of 10 mg/m2 the first 3 months, 20 mg/m2 the second 3 months to continue 30 mg/m2, adjusting the dose after 2 years in relation to response (assessed by bone densitometry, blood and urine bone turnover markers, and regression of vertebral fractures by compression) [47], and 10mg/m2 the first 3 months, 20 mg/m2 the second 3 months to continue 30–40 mg/m2 in monthly infusions [49]. In all, 3.44% of the articles (n = 1) established schemes by age groups: 0.5 mg/kg daily for 3 consecutive days every 2 months in children under 2 years old, 0.75 mg/kg daily for 3 consecutive days every 3 months in patients aged 2 to 3 years, and 1 mg/kg daily for 3 consecutive days every 4 months in patients older than 3 years with a maximum dose of 60 mg per day, maximum infusion concentration rate of 0.1 mg/mL, and duration of infusion administration of 3–4 h [39]. On the other hand, in 3.44% of the articles (n = 1), the following scheme was established according to the pathology: 9 mg/kg/year every 2–4 months for patients with primary osteoporosis and 4 mg/kg/year every 3 months for patients with secondary and glucocorticoid-induced osteoporosis [53]. In 6.89% of the articles (n = 2), the data are limited, only exposing that the last dose before dental surgery was 60 mg [50] and a single dose [57]. In 3.44% (n = 1) of the articles, data on dosage or administration schedules of PD were not provided [40].
A total of 10.34% of the articles (n = 3) used ND with the following schemes: 2 mg/kg (maximum 100 mg) every 3 months for 3 years, [42] one dose every 3 months without other data [43], and 1 mg/kg/dose day for 2 consecutive days every 3–4 months in patients younger than 1 year, and 2 mg/kg/dose in a single session every 3–6 months in the rest of the patients [48].
In total, 6.89% of the articles (n = 2) used AL. In one of them, the following schedule was applied: 35 mg once a week [57]. The other article lacks data about dosage and administration schedules [38]. In all, 3.44% of the articles (n = 1) used IB, at a dose of 2 mg every 3 months [46].
In total, 37.93% of the articles (n = 11) used ZD. In all, 6.89% (n = 2) did not provide data about drug dosage [38,40]. On the other hand, 6.89% (n = 2) of the articles [44,59] used doses of 0.0125 mg/kg (first dose) and 0.025 mg/kg (second dose), administered 6 weeks after the first dose, with a third dose of 0.025 mg/kg being added 12 weeks after the first dose in one of the articles [44]. The schedules for both articles included next doses of 0.025 mg/kg with a 12-week interval. Additionally, 10.34% (n = 3) of the articles applied schemes according to age. The schemes included the administration of 4 mg approximately every 28 days for those over 10 years of age and 0.08 to 0.16 mg/kg approximately every 28 days for those under 10 years of age, [35] 2 mg for those under 1 year of age and 4 mg for those over 1 year of age every 3 months [45], and 10 monthly doses of 4 mg for those over 25 years of age; 10 monthly doses of 0.05 mg/kg the first two cycles and 4 mg the remaining eight cycles for patients between 18 and 25 years old; 10 monthly doses of 0.05 mg/kg in all cycles without exceeding 4 mg for patients under 18 years [56]. The rest of the articles where ZD was used applied the following schemes: 0.05 mg/kg every 6 months (maximum 4 mg per dose), [62] 0.04–0.05 mg/kg/dose every 4 months approximately, [37] scheme of induction doses between weeks 1 and 12 of treatment by cohorts: 1.2 mg/m2 (maximum 2 mg); 2.3 mg/m2 (maximum 4 mg); 3.5 mg/m2 (maximum 6 mg); according to tolerance, dose levels were scaled, adding a fourth cohort at a dose of 2.3 mg/m2 (maximum 4 mg) after determining the maximum tolerated dose [41] and 0.1 mg/kg/year every 6 months [53].
In total, 10.34% of the articles (n = 3) used DS. The doses used in the three articles were 120 mg via subcutaneous with loading doses at 8 and 15 days, except for one article [61] that reports loading doses at 28 days, to then continue with doses every 4 weeks [36,61] or every 28 days [52]. In one article, a patient was treated with a dose of 70–100 mg (initial dose) and loads at 8, 15, 21, and 28 days, and then continued once a month [36]. Conversely, 10.34% (n = 3) of the articles did not present data on dosage and posology [51,55,58].
3.10. Cumulative Dose
In total, 24.13% (n = 7) of the articles presented data about cumulative doses, in the following way: 19.8 mg/kg of PD corresponding to an equivalent dose in adults of 1190 mg and 0.49 mg/kg of ZD corresponding to adult equivalent dose of 29.4 mg [37], cumulative average dose of 40 mg/kg of PD (range 2.5 to 81 mg/kg) in patients who underwent tooth extraction, [39] 48 mg/kg of ZD on average (range 16 to 80 mg/kg) [45], 1679 mg average cumulative dose of ND (range 144 to 5307 mg); 50 mg/kg of ND on average (range from 10 to 100 mg/kg), [48] 1623 mg/m2 of PD on average (range of 140 to 4020 mg/m2), [49] 0.1 mg/kg of ZD per year on average [59], and 5520 and 2160 mg of DS for both patients study subjects [61].
Conversely, 75.86% of the articles (n = 22) did not present data on cumulative doses [35,36,38,40,41,42,43,44,46,47,50,51,52,53,54,55,56,57,58,60,62,63].
3.11. Concomitant Medication
In total, 51.72% of the articles (n = 15) reported the administration of vitamin D and calcium supplements [36,38,41,42,44,46,47,50,52,54,56,57,59,62,63]. In all, 13.79% (n = 4) of the articles reported the use of corticosteroids [36,37,38,57], 13.79% (n = 4) of the articles reported the use of antineoplastic agents [35,36,41,56], 6.89% (n = 2) of the articles reported the use of hormonal therapy [36,38], 6.89% (n = 2) of the articles reported the use of non-steroidal analgesics [40,63], 3.44% (n = 1) of the articles reported the use of antidepressants [40], 3.44% (n = 1) of the articles reported the use of proton pump inhibitors [40], and 3.44% (n = 1) reported the use of opiates (codeine and morphine) [63]. On the other hand, 6.89% of the articles (n = 2) express the non-use of cytostatic and steroids [49] and corticosteroids, chemotherapy, or bisphosphonates [61]. In all, 31.03% of the articles (n = 9) did not report data on other medications used [39,43,45,48,51,53,55,58,60].
3.12. Osteonecrosis of the Jaws
In total, 89.65% of the articles (n = 26) did not report cases of MRONJ or healing impairment in studies where dental procedures were included [35,37,38,39,40,41,42,43,44,45,46,47,48,49,50,52,53,54,55,56,57,58,59,60,62,63]. In 10.34% (n = 3) of the articles was reported: 1 case of impaired healing after exploratory maxillary surgery in a patient diagnosed with central giant-cell granuloma, which the authors correlated with stage 2 of MRONJ [36], 12 cases of MRONJ including thickening of the lamina dura in seven patients, full-thickness sclerosis in six patients, sclerotic changes in the mandibular canal in three patients, poorly healed or non-healed post-extraction socket and periapical radiolucency in five patients, widening of the periodontal ligament and osteolysis in four patients, bone sequestration in three patients, oroantral fistulas in two patients, widening of soft tissue and mild periosteal reaction in one patient. The mandible was involved in nine patients and the maxilla in three patients [51]; one case, after 44 doses of DS, showed a fracture of a lower molar by a sports accident, requiring tooth extraction. The risk of developing MRONJ was discussed. The risks of relapses of giant-cell bone tumor vs MRONJ were evaluated, and it was decided to allow the continuity of DS treatment after complete healing of the mucosa without bone exposure. The patient returned 2 months later, with acute pain in the post-extraction tooth socket and the presence of exposed bone tissue (stage 2 MRONJ according to the American Association of Oral and Maxillofacial Surgeons classification). DS treatment was stopped after a total of 46 doses (cumulative dose 98 mg/kg). Cultures of the area showed Streptococcus milleri and alpha-hemolytic Streptococcus. Non-surgical treatment of MRONJ was performed at the beginning (amoxicillin, metronidazole, and mouth washes) without success. Debridement and sequestrectomy were subsequently performed showing a moderate amount of necrotic bone tissue around the post-extraction socket with complete recovery after the surgery [61].
3.13. MRONJ Detection Method
In total, 55.17% of the articles (n = 16) do not show data on the MRONJ detection method [35,36,38,40,41,42,44,45,46,47,50,55,56,57,62,63]. In 37.93% of the studies (n = 11) were reported the use of clinical evaluation for the confirmation of MRONJ [37,39,43,48,49,50,51,54,58,59,60,61], and additionally, imaging exploration was used in 17.24% of articles (n = 5) [37,43,49,58,60]. In one article (3.44%), the search in notes of follow-up by the physician in the medical records was used [39].
3.14. Invasive Procedures
Invasive dental procedures were carried out in 37.93% of the articles (n = 11) [36,37,39,43,48,49,50,54,55,58,61] including 216 patients (18.12% of all patients) with 13.17 years old in average [36,37,39,49,50,54,61]; 30.09% (n = 65) were male, 21.75% (n = 47) were female, and 48.14% (n = 104) gave no information about sex. PD was used in 63.42% of patients (n = 137), ND in 10.18% (n = 22), unspecified between PD and ZD in 5.09% (n = 11), DS in 0.92% (n = 2). The drug is not specified in 20.37% of patients (n = 44) (Table 3).
Table 3.
Distribution of data from patients undergoing invasive dental procedures and MRONJ report.
| Authors | Number of pts. | Avg. Age (Range) | Sex | Medication | Invasive Procedures | Time of Invasive Procedure (during tx./After tx./Unknown) | Duration of ART tx. Prior to the inVasive Procedure (Avg. in yr.) | Cumulative Dose Prior to the Invasive Procedure | Use of Antibiotic Therapy | Post-Operatory Follow-up Time (Range) in yr. | MRONJ Report |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bredell et al. [36] | 1 | 18.00 | 1(F) | DS | Maxillary exploratory surgery. | 1/0/0 | 0.66 | NDA | NDA | NDA | |
| Brown et al. [37] | 11 | 6.61 (1.65 to 12.47) | NDA | PD and ZD | 20 primary teeth extractions, 4 permanent teeth surgical extractions included, 2 simple permanent teeth extractions, 1 canine surgical exposure included, 1 odontoma excision. | 28/0/0 | PD (n = 11): 3.50 ZD (n = 6): 1.20 |
PD: 1250 mg on avg. ZD: 11.1 mg on avg. |
NDA | NDA | 1 case (stage 2). |
| Chahine et al. [39] | 113 | 14.00 (2.00 to 30.00). (Data from only 66 patients). | 41 (M) 25 (F) (Data from only 66 patients). |
PD | 178 primary tooth extractions, 72 permanent teeth extractions (32 simple and 40 surgical). | 163/87/0 | 4.60 (Data from only 66 patients) |
40 mg/kg on avg. (Data from only 66 patients). |
Applied in 12 patients. (Data from only 66 patients). |
0.23 (0.00 to 3.75). Telephone follow-up: 2.48 (0.12 to 10.38) | None |
| Ierardo et al. [43] | 20 | NDA (8.00 to 14.00) |
12 (M) 8 (F) |
ND | 35 scaling (tart removal), 20 germenectomies, 15 primary tooth extractions, 5 canine extractions included, 8 frenilectomies, 3 ankylosed molar extractions. | 86 / 0 / 0 | NDA | NDA | Used with 2 patients. Amoxicillin 50 mg/kg 30–60 min before surgery. Clindamycin 20 mg/kg was used in allergy sufferers 30–60 min before surgery. | NDA (2 to 5) | None |
| Maines et al. [48] | 2 | NDA | NDA | ND | 2 pulpectomies. | 2/0/0 | NDA | NDA | NDA | NDA | None |
| Malmgren et al. [49] | 22 | 12.20 (3.40 to 31.90) | 10 (M) 12 (F) |
PD | 30 tooth extractions, 6 surgical extractions, 1 orthognathic surgery, 1 implant surgery, 1 endodontic treatment. | 33/6/0 | 3.60 | NDA | NDA | 3.10 (0.90 to 8.00) | None |
| Milano et al. [50] | 1 | 4.66 | 1 (M) | PD | 5 primary tooth extractions. | 5/0/0 | NDA | NDA | Not applied. | 2.75 | None |
| Ngan et al. [54] | 1 | 12.00 | 1 (F) | PD | 6 primary tooth extractions. | 6 / 0 / 0 | NDA | NDA | Amoxicillin for 5 days. | 0.25 | None |
| Okawa et al. [55] | 31 | NDA | NDA | NDA | 67 primary tooth extractions. | 67 / 0 / 0 | NDA | NDA | NDA | NDA | None |
| Schwartz et al. [58] | 13 | NDA (2.00 to 16.00) |
NDA | NDA | 50 extractions of primary teeth and 10 of permanent teeth. | 39 / 11 / 10 | NDA | NDA | Used in 12 patients, not used with 2 and there is no data from 1 patient. | NDA | None |
| Uday et al. [61] | 1 | 19.00 | 1 (M) | DS | 1 permanent tooth extraction. | 1/0/0 | NDA | 98 mg/kg | NDA | NDA | 1 case (stage 2). |
ART: antiresorptive; avg.: average; DS: denosumab; ND: neridronate; PD: pamidronate; pts.: patient(s); tx.: treatment; yr.: year(s); ZD: zoledronate.
A total of 545 invasive procedures were performed: primary tooth extractions (n = 371; 68.07%), permanent tooth surgical extractions (n = 123; 22.56%), periodontal treatment (n = 35; 6.42%), frenectomy (n = 8; 1.46%), endodontic treatments (n = 3; 0.55%), orthognathic surgery (n = 1; 0.18%), odontoma excision (n = 1; 0.18%), implant surgery (n = 1; 0.18%), exploratory maxillary surgery (n = 1; 0,18%), and surgical exposure of canine included (n = 1; 0.18%). In total, 79.08% (n = 431) of the procedures were performed during antiresorptive therapy, 19.08% (n = 104) were performed after the end of therapy, and in 1.83% (n = 10), the time for dental treatment is not clear.
In total, 36.36% of the articles (n = 4) showed data on the time using the antiresorptive treatments prior to invasive procedures: 0.66 years on average [36], 3.50 years on average for patients under treatment with PD, and 1.20 years on average for patients under treatment with ZD [37], 4.60 years on average [39], and 3.60 years on average [49]. In all, 27.27% of the articles (n = 3) presented data on cumulative doses, these being the following: 1250 mg on average for patients under treatment with PD and 11.10 mg on average for patients under treatment with ZD [37], 40 mg/kg on average [39] and 98 mg/kg [61].
In total, 45.45% of the articles (n = 5) presented data about use of antibiotic therapy, with two articles (18.18%) showing a treatment related to: amoxicillin 50 mg/kg administered 30–60 min before surgery, clindamycin 20 mg/kg 30–60 min before surgery in allergic patients, used in two patients [39] and without the use of antibiotic therapy [50].
In total, 36.36% of the articles [39,50,54] presented data on the postoperative follow-up time, these being 1.58 years of average postoperative follow-up. In one article (11.11%) with 2.48 years follow-up, the postoperative control was performed calling all the subjects or parents [39].
In 81.81% (n = 9) of the articles, no cases of MRONJ were reported [37,39,43,48,49,50,54,55,58]. In 18.18% of the articles, two cases of MRONJ were reported [36,61].
3.15. Risk of Bias Assessment
Due to the presence of different types of study, it was necessary to apply different checklists [64,65,66,67], in order to check the quality of the articles (Table 4).
Table 4.
Assessment of methodological quality and risk of bias in the 29 articles included in this systematic review.
| Cohort Study | |||
|---|---|---|---|
| Authors | Verification List | Assessment | Interpretation |
| Brown et al. [37] | Checklist SIGN Methodology 3 (cohort studies) | Acceptable | Medium risk |
| Carpenter et al. [38] | High quality | Low risk | |
| Chahine et al. [39] | Low quality | High risk | |
| Feehan et al. [40] | High quality | Low risk | |
| Idolazzi et al. [42] | High quality | Low risk | |
| Johannesen et al. [44] | High quality | Low risk | |
| Kumar et al. [45] | Acceptable | Medium risk | |
| Li et al. [46] | Low quality | High risk | |
| Lindahl et al. [47] | High quality | Low risk | |
| Maines et al. [48] | High quality | Low risk | |
| Malmgren et al. [49] | High quality | Low risk | |
| Nasomyont et al. [53] | High quality | Low risk | |
| Okawa et al. [55] | High quality | Low risk | |
| Putman et al. [57] | High quality | Low risk | |
| Voumiries et al. [62] | High quality | Low risk | |
| Wagner et al. [63] | High quality | Low risk | |
| Reports and case series | |||
| August et al. [35] | Murad et al. Methodological quality and synthesis of case series and case reports | 2/5 | High risk |
| Bredell et al. [36] | 5/5 | Low risk | |
| Ierardo et al. [43] Milano et al. [50] |
1/5 | High risk | |
| 4/5 | Medium risk | ||
| Moeini et al. [51] | 0/5 | High risk | |
| Naidu et al. [52] | 5/5 | Low risk | |
| Ngan et al. [54] | 4/5 | Medium risk | |
| Schwartz et al. [58] | 4/5 | Medium risk | |
| Simm et al. [59] | 5/5 | Low risk | |
| Tessaris et al. [60] | 5/5 | Low risk | |
| Uday et al. [61] | 5/5 | Low risk | |
| Non-randomized clinical trials | |||
| Goldsby et al. [41] | Sterne et al. ROBINS-I tool for assessing risk of bias in non-randomized studies of interventions | Low risk | Low risk |
| Randomized clinical trials | |||
| Piperno-Neumann et al. [56] | Checklist SIGN Methodology 2 (controlled trials) | High quality | Low risk |
4. Discussion
The time involved in the antiresorptive therapy is a risk factor for development of MRONJ [14]. Among patients with malignant pathology exposed to denosumab and zoledronate, the incidence of MRONJ increases directly proportional to the years of treatment, reaching a plateau between 2 and 3 years of treatment. The incidence of MRONJ is established at 1.3% for denosumab and 1.88% for zoledronate after 3 years of therapy [68]. For orally administered medication, patients without MRONJ have an average duration of treatment of 3.5 years, while patients who developed MRONJ have an average of 4.4 years [69,70]. Within the results of this systematic review, 27.58% of the articles failed to show data to calculate the duration of treatment [35,36,43,50,51,53,54,55]. In contrast, 68.96% of the articles present data that allow us to calculate the average duration of antiresorptive therapy as 4.21 years [37,38,39,40,41,42,44,45,46,47,48,49,52,56,58,59,60,61,62,63]. We can think that this group of patients show higher risk of developing MRONJ.
Another important factor in estimating the risk of MRONJ is related to the pathology under treatment. For example, the prevalence of MRONJ in patients with malignant disease exposed to zoledronate is 1% (100 cases per 1000 patients); in contrast, the same population exposed to placebo have a risk of developing MRONJ 50 to 100 times higher [71,72,73,74], this risk being comparable to patients with cancer exposed to denosumab [21,68,75]. In patients with osteoporosis, the prevalence is 0.1% with an increase to 0.21% when the use of the drug is for more than 4 years [69,70]. If compared with cancer patients, the risk is 100 times lower [14]. Even though, within the population of this systematic review, there are patients with malignant pathology (12.83%) and osteoporosis (17.03%), the main indication of antiresorptive therapy in the child–youth population was osteogenesis imperfecta (60.15%). The differences in indications for antiresorptive therapy between the pediatric/adolescence population and adults represent a limitation for the comparison of risks.
Some drugs, when administered simultaneously with antiresorptives, represent an increased risk of developing MRONJ, such as corticosteroids [76,77] and antiangiogenics [78,79]. Within the results of this study, 31.03% of the articles did not report data on the use of concomitant medication [39,43,45,48,51,53,55,58,60]. In all, 13.79% of the articles reported the use of corticosteroids [36,37,38,57]. Of the 68.96% (n = 20) of the articles that reported the use of concomitant medication, only two articles reported cases of MRONJ (two patients), and for that reason, the use of concomitant medication does not appear to be a risk factor in the development of MRONJ.
In total, 55.17% of the articles (n = 16) in this systematic review do not present data on the MRONJ detection method [35,36,38,40,41,42,44,45,46,47,50,55,56,57,62,63]. In 37.93% of the articles (n = 11), the use of clinical evaluation for the detection of MRONJ was reported [37,39,43,48,49,50,51,54,58,59,60,61], and, additionally, an imaging was described in 24% of the articles (n = 5) [37,43,49,58,60]. However, they do not report data about the oral health status of patients prior to initiation and during antiresorptive therapy, and few studies describe the criteria for the diagnosis of MRONJ.
As previously stated, the risks of developing MRONJ are related to the pathology and drug. In this systematic review, some articles presented dosing schedules that were applied to treat different pathologies in the same way [35,37,39], and other articles observed the use of different antiresorptive drugs to treat the same pathology [37,38,40]. The data provided by these articles make it impossible to analyze the risk of MRONJ in children and adolescents, especially because not all antiresorptive drugs have the same relative potency. In the case of bisphosphonates, which constitute the largest group of antiresorptive drugs, these differences appear; for example, zoledronate is at least ten times more potent than IB, which in turn is a thousand times more powerful than etidronate [80]. This increase in potency and, in turn, in toxicity is due to the presence of nitrogen within the molecular chain of bisphosphonate [81,82,83]. This fact makes it essential that studies using bisphosphonates include information about the drug and the cumulative doses, in order to contribute to establishing risk groups in these populations. In this systematic review, 3 studies lack data on the drugs used [51,55,58], 6 studies do not present specific data on dosage [40,43,50,51,55,58], and 22 studies do not present data on cumulative doses [35,36,38,40,41,42,43,44,46,47,50,51,52,53,54,55,56,57,58,60,62,63].
The prevalence for the subjects included in this review is close to 1.1%. If we consider the results of Moeini et al. [51], the prevalence decreases to 0.16%. The analysis of bias is presented in Table 4 [64,65,66,67] and because we included articles with different methodological designs, the use of different tools was necessary.
According to AAOMS [14], dentoalveolar surgery is considered a major risk factor for the development of MRONJ. Several studies reported that among patients with MRONJ, dental extraction is a very common variable (52% to 61% of patients) [79,84,85]. In this review, 216 patients received 545 invasive treatments performed mostly during antiresorptive therapy, including dental extraction in primary and permanent dentition, surgery for odontoma, dental implant surgery, and orthognathic surgery. Only two cases of MRONJ (0.92% of patients) were reported after exploratory maxillary surgery in a patient with central giant-cell granuloma [36] and tooth extraction from a fractured molar resulting from a sport accident [61]. These data allow us to clarify that invasive treatments in this population do not appear to be a risk factor for MRONJ.
The pediatric skeleton presents a thicker overlying periosteum, a greater osteogenic potential, and a greater remodeling potential than adult bone [86]. This creates evident physiological differences between the pediatric and adult skeleton, preventing their direct comparison [37]. Growing bone is more porous than adult bone because Haversian canals occupy much more space within bone mass [87]. Additionally, there is more vascularization [88], which may the most important protection against MRONJ. These physiological differences make the calculation of cumulative doses more complex in the child–youth population [37]. Is important to note that recent studies showed that chronic exposure to zoledronic acid induced significant reduction in osteogenic differentiation in in vitro models and this fact can be included in the conditions for the treatment [89]. In this line, exosomes can help to reduce the risk of side effects in the treatment under antiresorptive drugs [90].
Growth rates are higher in children and adolescence compared to adults, demonstrated by levels of biochemical markers of bone resorption and apposition such as serum alkaline phosphatase, serum osteocalcin, pyridoline and deoxypyridoline, NTX and CTX of mature collagen type I, serum calcium, among others [91]. Maybe, the high bone turnover may serve as a compensatory factor in the face of complications related to the effects of antiresorptive therapy, even long-standing ones, reducing the half-life of antiresorptive drugs.
It is important to note that in some stages of the bone growth process there is a delay in the apposition of minerals, as well as an increase in cortical porosity, showing an increased risk of fractures in early adolescence [92,93]. On the other hand, osteoclastic cells have not decreased in child and adolescent subjects, in contrast to adults or the elderly [94]. In the same line, the presence of vitamin D and calcium is important in subjects under antiresorptive drug therapy and in children this fact can be easier to control than in the adult population [95].
Anatomical factors could be another protective factor, since the primary teeth present shorter and narrower roots with some root resorption at the time of the extraction, and sockets are smaller with less requirements for bone resorption; in addition, the alveolar process being in growth with active bone apposition [54].
It was described that the development of MRONJ in patients with osteoporosis is related to suppuration, use of bisphosphonate, tooth extraction, and anemia [96]. Others showed that dental extractions, dental implants, and apical or periodontal surgery are the main factors involved in MRONJ [97]; additional risk factors such as dental prostheses with poor adaptation, excessive chewing force, poor oral hygiene, periodontal disease, and morphological bone irregularities have been related to MRONJ [98]. These conditions are absent in children and adolescents, and the masticatory force is lower in this group, showing another protective condition against MRONJ [99]. In the same line, Actinomyces spp. show a role in the pathogenesis of MRONJ [100,101] and this type of biofilm show differences between the child and adult populations.
As reported in this review and in others previously published [102], the main problem to compare results between studies was the lack in standardization, process and route in drugs, posology, timing, administration, and dosage, making it complex to compare results. The lack in data, low prevalence, and differences in methodology make it difficult to perform a metanalysis. The latest position paper of the AAOMS [103] states that there are very limited data describing the occurrence of MRONJ in the pediatric population for osteogenesis imperfecta and other conditions. No cases of MRONJ with sufficient evidence have been reported so far.
Additionally, the role of surgical management in improving MRONJ at early stages has also become a topic of discussion in the literature; it has even been included in the latest position paper of the American Association of Oral and Maxillofacial Surgery [103].
Surgical intervention should be explored and presented as a treatment option in an attempt to slow disease progression with the recognition that early surgical intervention may predict beneficial outcomes for patients [104].
Active clinical and radiographic surveillance is critical in the nonsurgical management of patients with Stage 1, 2, and 3 diseases to monitor signs of disease progression. In patients who demonstrate failure of nonsurgical therapy, early surgical intervention is recommended. In patients with a progressive clinical or radiographic picture in the disease or more advanced disease at presentation, the use of surgery is recommended [103,105]. MRONJ resection should be performed without first instituting prolonged nonoperative measures. MRONJ represents a complex wound for which surgical therapy can be performed in a timely manner [10]. Although controversy exists between operative and non-operative therapies, surgical treatment of patients has shown maintenance of mucosal coverage, improvement in quality of life, and timely resumption of antiresorptive therapy for all stages of MRONJ [106]. Nonetheless, the lack of evidence in pediatric and young patients makes it impossible at the moment to have any recommendations of early treatment in this populations treated with the drugs discussed in this research.
5. Conclusions
There is a low presence of MRONJ in the child and youth population. Data collection is weak, and details of therapy are not clear in some cases. Deficiencies in protocols and pharmacological characterization were observed in most of the included articles. The biological and physiological conditions involved in bone growth and development, as well as the proper dental and oral conditions, could be the most important protective factor against MRONJ. For future articles, it is recommended that a proper collection of the data is performed, where the drugs, time of treatment, possible trigger procedures of MRONJ, treatment, and follow-up must be recorded so further studies can be developed properly.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available by request: henryagg@gmail.com.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.McLeod N., Brennan P., Ruggiero S. Bisphosphonate osteonecrosis of the jaw: A historical and contemporary review. Surgeon. 2012;10:36–42. doi: 10.1016/j.surge.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Yang G., Singh S., Chen Y., Hamadeh I.S., Langaee T., McDonough C.W., Holliday L.S., Lamba J.K., Moreb J.S., Katz J., et al. Pharmacogenomics of osteonecrosis of the jaw. Bone. 2019;124:75–82. doi: 10.1016/j.bone.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Major P., Lortholary A., Hon J., Abdi E., Mills G., Menssen H.D., Yunus F., Bell R., Body J., Quebe-Fehling E., et al. Zoledronic Acid Is Superior to Pamidronate in the Treatment of Hypercalcemia of Malignancy: A Pooled Analysis of Two Randomized, Controlled Clinical Trials. J. Clin. Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 4.Shibahara T. Antiresorptive Agent-Related Osteonecrosis of the Jaw (ARONJ): A Twist of Fate in the Bone. Tohoku J. Exp. Med. 2019;247:75–86. doi: 10.1620/tjem.247.75. [DOI] [PubMed] [Google Scholar]
- 5.Eguia A., Bagán-Debón L., Cardona F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal. 2020;25:e71–e83. doi: 10.4317/medoral.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillner B.E., Ingle J.N., Chlebowski R.T., Gralow J., Yee G.S., Janjan N.A., Cauley J.A., Blumenstein B.A., Albain K.S., Lipton A., et al. American Society of Clinical Oncology 2003 Update on the Role of Bisphosphonates and Bone Health Issues in Women With Breast Cancer. J. Clin. Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Nicolatou-Galitis O., Schiødt M., Mendes R.A., Ripamonti C., Hope S., Drudge-Coates L., Niepel D., Van den Wyngaert T. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019;127:117–135. doi: 10.1016/j.oooo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Saad F. RESPONSE: Re: Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients With Metastatic Hormone-Refractory Prostate Cancer. JNCI J. Natl. Cancer Inst. 2004;96:1480–1481. doi: 10.1093/jnci/djh295. [DOI] [PubMed] [Google Scholar]
- 9.Rosen L., Gordon D., Tchekmedyian N., Yanagihara R., Hirsh V., Krzakowski M., Pawlicki M., De Souza P., Zheng M., Urbanowitz G., et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 10.Giudice A., Barone S., Diodati F., Antonelli A., Nocini R., Cristofaro M.G. Can Surgical Management Improve Resolution of Medication-Related Osteonecrosis of the Jaw at Early Stages? A Prospective Cohort Study. J. Oral Maxillofac. Surg. 2020;78:1986–1999. doi: 10.1016/j.joms.2020.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Matys T. Medication-related Osteonecrosis of the Jaw. Radiology. 2021;301:548. doi: 10.1148/radiol.2021211142. [DOI] [PubMed] [Google Scholar]
- 12.Rosen Lee S. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 13.Berenson J.R., Hillner B.E., Kyle R.A., Anderson K., Lipton A., Yee G.C., Biermann J.S. American Society of Clinical Oncology clinical practice guidelines: The role of bisphosphonates in multiple myeloma. J. Clin. Oncol. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Ruggiero S.L., Dodson T., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O’Ryan F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Imam B., Aziz K., Khan M., Zubair T., Iqbal A. Role of Bisphosphonates in Postmenopausal Women with Osteoporosis to Prevent Future Fractures: A Literature Review. Cureus. 2019;11:e5328. doi: 10.7759/cureus.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal S.M., Qamar I., Zhi C., Nida A., Aslam H.M. Role of Bisphosphonate Therapy in Patients with Osteopenia: A Systemic Review. Cureus. 2019;11:e4146. doi: 10.7759/cureus.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allegra A., Alonci A., Penna G., Granata A., Nastro Siniscalchi E., Oteri G., Loddo S., Teti D., Cicciù D., Saverio De Ponte F., et al. Bisphosphonates Induce Apoptosis of Circulating Endothelial Cells in Multiple Myeloma Patients and in Subjects with Bisphosphonate-Induced Osteonecrosis of the Jaws. Acta Haematol. 2010;124:79–85. doi: 10.1159/000313787. [DOI] [PubMed] [Google Scholar]
- 18.Giudice A., Antonelli A., Muraca D., Fortunato L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020;31:813–818. doi: 10.4103/ijdr.IJDR_689_19. [DOI] [PubMed] [Google Scholar]
- 19.Aghaloo T.L., Felsenfeld A.L., Tetradis S. Osteonecrosis of the jaw in a patient on Denosumab. J. Oral Maxillofac. Surg. 2010;68:959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Goodger N.M., Pogrel M.A. Osteonecrosis of the jaws associated with cancer chemotherapy. J. Oral Maxillofac. Surg. 2003;61:1104–1107. doi: 10.1016/S0278-2391(03)00328-8. [DOI] [PubMed] [Google Scholar]
- 21.Marx R.E. Pamidronate (Aredia) and Zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003;61:1115–1117. doi: 10.1016/S0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 22.Reid Ian R., Bolland M.J., Grey A.B. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41:318–320. doi: 10.1016/j.bone.2007.04.196. [DOI] [PubMed] [Google Scholar]
- 23.Matthew R.A., Burr D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009;67((Suppl. 5)):61–70. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Landesberg R., Woo V., Creemers S., Cozin M., Marolt D., Vunjak-Novakovic G., Kousteni S., Raghavan S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 2011;1218:62–79. doi: 10.1111/j.1749-6632.2010.05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita J., McCauley L. Antiresorptives and osteonecrosis of the jaw. J. Evide. Based Dent. Pract. 2012;13:233–247. doi: 10.1016/S1532-3382(12)70046-5. [DOI] [PubMed] [Google Scholar]
- 26.Geusens P. Emerging treatments for postmenopausal osteoporosis–focus on denosumab. Clin. Interv. Aging. 2009;4:241–250. doi: 10.2147/CIA.S3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewiecki E.M. Denosumab update. Curr. Opin. Rheumatol. 2009;21:369–373. doi: 10.1097/BOR.0b013e32832ca41c. [DOI] [PubMed] [Google Scholar]
- 28.Egloff-Juras C., Gallois A., Salleron J., Massard V., Dolivet G., Guillet J., Phulpin B. Denosumab-related osteonecrosis of the jaw: A retrospective study. J. Oral Pathol. Med. 2018;47:66–70. doi: 10.1111/jop.12646. [DOI] [PubMed] [Google Scholar]
- 29.Rosella D., Papi P., Giardino R., Cicalini E., Piccoli L., Pompa G. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J. Int. Soc. Prev. Community Dent. 2016;6:97–104. doi: 10.4103/2231-0762.178742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baroncelli G., Bertelloni S. The use of bisphosphonates in pediatrics. Horm. Res. Paediatr. 2014;82:290–302. doi: 10.1159/000365889. [DOI] [PubMed] [Google Scholar]
- 31.Boyce A.M. Denosumab: An emerging therapy in pediatric bone disorders. Curr. Osteoporos. Rep. 2017;15:283–292. doi: 10.1007/s11914-017-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishmore M., Panat S.R., Aggarwal A., Updhyay N., Alok A. Evidence based dental care: Integrating clinical expertise with systematic research. J. Clin. Diagn. Res. 2014;8:259–262. doi: 10.7860/JCDR/2014/6595.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Urrútia G., Bonfill X. Declaración PRISMA: Una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med. Clin. 2010;135:507–511. doi: 10.1016/j.medcli.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 35.August K.J., Dalton A., Katzenstein H., George B., Olson T.A., Wasilewski-Masker K., Rapkin L.B. The use of zoledronic acid in pediatric cancer patients. Pediatr. Blood Cancer. 2011;56:610–614. doi: 10.1002/pbc.22681. [DOI] [PubMed] [Google Scholar]
- 36.Bredell M., Rordorf T., Kroiss S., Rücker M., Zweifel D.F., Rostetter C. Denosumab as a treatment alternative for central giant cell granuloma: A long-term retrospective cohort study. J. Oral Maxillofac. Surg. 2018;76:775–784. doi: 10.1016/j.joms.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Brown J.J., Ramalingam L., Zacharin M.R. Bisphosphonate-associated osteonecrosis of the jaw: Does it occur in children? Clin. Endocrinol. 2008;68:863–867. doi: 10.1111/j.1365-2265.2008.03189.x. [DOI] [PubMed] [Google Scholar]
- 38.Carpenter P.A., Hoffmeister P., Chesnut C.H., Storer B., Charuhas P.M., Woolfrey A.E., Sanders J.E. Bisphosphonate therapy for reduced bone mineral density in children with chronic graft-versus-host disease. Biol. Blood Marrow. Transplant. 2007;13:683–690. doi: 10.1016/j.bbmt.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Chahine C., Cheung M.S., Head T.W., Schwartz S., Glorieux F.H., Rauch F. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J. Pediatr. 2008;153:719–720. doi: 10.1016/j.jpeds.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Feehan A., Zacharin M.R., Lim A.S., Simm P.J. A comparative study of quality of life, functional and bone outcomes in osteogenesis imperfecta with bisphosphonate therapy initiated in childhood or adulthood. Bone. 2018;113:137–143. doi: 10.1016/j.bone.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Goldsby R., Fan T.M., Villaluna D., Wagner L.M., Isakoff M.S., Meyer J., Randall R.L., Lee S., Kim G., Bernstein M., et al. Feasibility and dose discovery analysis of zoledronic acid with concurrent chemotherapy in the treatment of newly diagnosed metastatic osteosarcoma: A report from the Children’s Oncology Group. Eur. J. Cancer. 2013;49:2384–2391. doi: 10.1016/j.ejca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Idolazzi L., Fassio A., Viapiana O., Rossini M., Adami G., Bertoldo F., Antoniazzi F., Gatti D. Treatment with neridronate in children and adolescents with osteogenesis imperfecta: Data from open-label, not controlled, three-year Italian study. Bone. 2017;103:144–149. doi: 10.1016/j.bone.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Ierardo G., Bossù M., D’Angeli G., Celli M., Sfasciotti G. Bisphosphonates therapy in children with Osteogenesis imperfecta: Clinical experience in oral surgery. Oral Implantol. 2017;30:311–316. doi: 10.11138/orl/2017.10.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johannesen J., Briody J., McQuade M., Little D.G., Cowell C.T., Munns C.F. Systemic effects of zoledronic acid in children with traumatic femoral head avascular necrosis and Legg–Calve–Perthes disease. Bone. 2009;45:898–902. doi: 10.1016/j.bone.2009.04.255. [DOI] [PubMed] [Google Scholar]
- 45.Kumar C., Panigrahi I., Aradhya A.S., Meena B.L., Khandelwal N. Zoledronate for Osteogenesis imperfecta: Evaluation of safety profile in children. J. Pediatr. Endocrinol. Metab. 2016;29:947–952. doi: 10.1515/jpem-2015-0351. [DOI] [PubMed] [Google Scholar]
- 46.Li M., Xia W.B., Xing X.P., Yu W., Hu Y.Y., Jiang Y., Wang O., Liu H.J., Han L.W., Meng X.W., et al. Benefit of infusions with ibandronate treatment in children with osteogenesis imperfecta. Chin. Med. J. (Engl.) 2011;124:3049–3053. [PubMed] [Google Scholar]
- 47.Lindahl K., Kindmark A., Rubin C.-J., Malmgren B., Grigelioniene G., Söderhäll S., Ljunggren Ö., Åström E. Decreased fracture rate, pharmacogenetics and BMD response in 79 Swedish children with osteogenesis imperfecta types I, III and IV treated with Pamidronate. Bone. 2016;87:11–18. doi: 10.1016/j.bone.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Maines E., Monti E., Doro F., Morandi G., Cavarzere P., Antoniazzi F. Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J. Bone Miner. Metab. 2012;30:434–438. doi: 10.1007/s00774-011-0331-3. [DOI] [PubMed] [Google Scholar]
- 49.Malmgren B., Aström E., Söderhäll S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J. Oral Pathol. Med. 2008;37:196–200. doi: 10.1111/j.1600-0714.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 50.Milano M., Wright T., Loechner K. Dental implications of osteogenesis imperfecta: Treatment with IV bisphosphonate: Report of a case. Pediatr. Dent. 2011;33:349–352. [PubMed] [Google Scholar]
- 51.Moeini M., Moeini M., Lotfizadeh N., Alavi M. Radiography finding in the jaws in children taking bisphosphonate. Iran J. Ped. Hematol. Oncol. 2013;3:114–118. [PMC free article] [PubMed] [Google Scholar]
- 52.Naidu A., Malmquist M.P., Denham C.A., Show S. Management of central giant cell granuloma with subcutaneous denosumab therapy. J. Oral Maxillofac. Surg. 2014;72:2469–2484. doi: 10.1016/j.joms.2014.06.456. [DOI] [PubMed] [Google Scholar]
- 53.Nasomyont N., Hornung L.N., Gordon C.M., Wasserman H. Outcomes following intravenous bisphosphonate infusion in pediatric patients: A 7-year retrospective chart review. Bone. 2019;121:60–67. doi: 10.1016/j.bone.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Ngan K.K., Bowe J., Goodger N. The risk of bisphosphonate-related osteonecrosis of the jaw in children. A case report and literature review. Dent. Update. 2013;40 doi: 10.12968/denu.2013.40.9.733. [DOI] [PubMed] [Google Scholar]
- 55.Okawa R., Kubota T., Kitaoka T., Kokomoto K., Ozono K., Nakano K. Oral manifestations of Japanese patients with osteogenesis imperfecta. Pediat. Dent. J. 2017;27:73–78. doi: 10.1016/j.pdj.2017.02.001. [DOI] [Google Scholar]
- 56.Piperno-Neumann S., Le Deley M.C., Rédini F., Pacquement H., Marec-Bérard P., Petit P., Brisse H., Lervat C., Gentet J.C., Entz-Werlé N., et al. Sarcoma Group of UNICANCER; French Society of Pediatric Oncology (SFCE); French Sarcoma Group (GSF-GETO). Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1070–1080. doi: 10.1016/S1470-2045(16)30096-1. [DOI] [PubMed] [Google Scholar]
- 57.Putman M.S., Simoneau T., Feldman H., Haagensen A., Boyer D. Low bone density and fractures before and after pediatric lung transplantation. Bone. 2018;111:129–134. doi: 10.1016/j.bone.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz S., Joseph C., Iera D., Vu D.D. Bisphosphonates, osteonecrosis, osteogenesis imperfecta and dental extractions: A case series. J. Can Dent. Assoc. 2008;74:537–542. [PubMed] [Google Scholar]
- 59.Simm P.J., Johannesen J., Briody J., McQuade M., Hsu B., Bridge C., Little D.G., Cowell C.T., Munns C.F. Zoledronic acid improves bone mineral density, reduces bone turnover and improves skeletal architecture over 2 years of treatment in children with secondary osteoporosis. Bone. 2011;49:939–943. doi: 10.1016/j.bone.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Tessaris D., Matarazzo P., Lala R., Defabianis P. Odontoiatric perspectives and osteonecrosis of the jaw as a possible adverse effect of bisphosphonates therapy in fibrous dysplasia and McCune-Albright syndrome. J. Pediatr. Endocrinol. Metab. 2016;29:333–336. doi: 10.1515/jpem-2015-0300. [DOI] [PubMed] [Google Scholar]
- 61.Uday S., Gaston C.L., Rogers L., Parry M., Joffe J., Pearson J., Sutton D., Grimer R., Högler W. Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J. Clin. Endocrinol. 2018;103:596–603. doi: 10.1210/jc.2017-02025. [DOI] [PubMed] [Google Scholar]
- 62.Vuorimies I., Toiviainen-Salo S., Hero M., Mäkitie O. Zoledronic acid treatment in children with osteogenesis imperfecta. Horm. Res. Paediatr. 2011;75:346–353. doi: 10.1159/000323368. [DOI] [PubMed] [Google Scholar]
- 63.Wagner S., Poirot I., Vuillerot C., Berard C. Tolerance and effectiveness on pain control of Pamidronate® intravenous infusions in children with neuromuscular disorders. Ann. Phys. Rehabil. Med. 2011;54:348–358. doi: 10.1016/j.rehab.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Checklist, SIGN Methodology. 3: Cohort Studies. Edinburgh, Scotland: Scottish Intercollegiate Guideline Network. 2012. [(accessed on 6 January 2022)]. Available online: https://www.sign.ac.uk/what-we-do/methodology/checklists/
- 66.Checklist, SIGN Methodology. “2: Controlled Trials”. 2014. [(accessed on 6 January 2022)]. Available online: https://www.sign.ac.uk/what-we-do/methodology/checklists/
- 67.Sterne J., Hernán M.A., Reeves B., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 69.US Food and Drug Administration Background Document for Meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. 9 September 2011. [(accessed on 6 January 2022)];2014 Available online: https://www.fda.gov/advisory-committees/drug-safety-and-risk-management-advisory-committee/drug-safety-and-risk-management-advisory-committee-roster.
- 70.Lo J.C., O’Ryan F.S., Gordon N.P., Yang J., Hui R.L., Martin D., Hutchinson M., Lathon P.V., Sanchez G., Silver P., et al. Predicting Risk of Osteonecrosis of the Jaw with Oral Bisphosphonate Exposure (PROBE) Investigators. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J. Oral Maxillofac. Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qi W.X., Tang L.N., He A.N., Yao Y., Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: A meta-analysis of seven randomized controlled trials. Int. J. Clin. Oncol. 2014;19:403–410. doi: 10.1007/s10147-013-0561-6. [DOI] [PubMed] [Google Scholar]
- 72.Coleman R., Woodward E., Brown J., Cameron D., Bell R., Dodwell D., Keane M., Gil M., Davies C., Burkinshaw R., et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01–04) for women with stage II/III breast cancer. Breast Cancer Res. Treat. 2011;127:429–438. doi: 10.1007/s10549-011-1429-y. [DOI] [PubMed] [Google Scholar]
- 73.Mauri D., Valachis A., Polyzos I.P., Polyzos N.P., Kamposioras K., Pesce L.L. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: A meta-analysis. Breast Cancer Res. Treat. 2009;116:433–439. doi: 10.1007/s10549-009-0432-z. [DOI] [PubMed] [Google Scholar]
- 74.Scagliotti G.V., Hirsh V., Siena S., Henry D.H., Woll P.J., Manegold C., Solal-Celigny P., Rodriguez G., Krzakowski M., Mehta N.D., et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: Subgroup analysis from a randomized phase 3 study. J. Thorac. Oncol. 2012;7:1823–1829. doi: 10.1097/JTO.0b013e31826aec2b. [DOI] [PubMed] [Google Scholar]
- 75.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., De Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 76.Grbic J.T., Black D.M., Lyles K.W., Reid D.M., Orwoll E., McClung M., Bucci-Rechtweg C., Su G. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: Data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J. Am. Dent. Assoc. 2010;141:1365–1370. doi: 10.14219/jada.archive.2010.0082. [DOI] [PubMed] [Google Scholar]
- 77.Tsao C., Darby I., Ebeling P.R., Walsh K., O’Brien-Simpson N., Reynolds E., Borromeo G. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J. Oral Maxillofac. Surg. 2013;71:1360–1366. doi: 10.1016/j.joms.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Guarneri V., Miles D., Robert N., Diéras V., Glaspy J., Smith I., Thomssen C., Biganzoli L., Taran T., Conte P. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 2010;122:181–188. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 79.Saad F., Brown J.E., Poznak C.V., Ibrahim T., Stemmer S.M., Stopeck A.T., Diel I.J., Takahashi S., Shore N., Henry D.H., et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 80.Marx R.E. Oral and Intravenous Bisphosphonate Induced Osteonecrosis of the Jaws: History, Etiology, Prevention, and Treatment. Quintessence Publishing; Hanover Park, IL, USA: 2007. Modes of action and pharmacokinetics of the bisphosphonate family; pp. 11–15. [Google Scholar]
- 81.Glowacki J. Bisphosphonates and bone. Ortho. J. Harvard. Med. Sch. 2005;25:64–67. [Google Scholar]
- 82.Lasseter K., Porras A.G., Denker A., Santhanagopal A., Daifotis A. Pharmacokinetic considerations in determining the terminal elimination half-lives of bisphosphonates. Clin. Drug Investig. 2005;25:107–114. doi: 10.2165/00044011-200525020-00003. [DOI] [PubMed] [Google Scholar]
- 83.Russell R.G., Croucher P.I., Rogers M.J. Bisphosphonates: Pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9((Suppl. 2)):S66–S80. doi: 10.1007/PL00004164. [DOI] [PubMed] [Google Scholar]
- 84.Vahtsevanos K., Kyrgidis A., Verrou E., Katodritou E., Triaridis S., Andreadis C.G., Boukovinas I., Koloutsos G.E., Teleioudis Z., Kitikidou K., et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 85.Fehm T., Beck V., Banys M., Lipp H.P., Hairass M., Reinert S., Solomayer E.F., Wallwiener D., Krimmel M. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol. Oncol. 2009;112:605–609. doi: 10.1016/j.ygyno.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 86.Flynn J.M., Schwend R.M. Management of pediatric femoral shaft fractures. J. Am. Acad. Orthop. Surg. 2004;12:347–359. doi: 10.5435/00124635-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 87.Ogden J.A., Ganey T.M., Ogden D.A. Fractures in Children. 4th ed. Lippincott Raven; Philadelphia, PA, USA: 1996. The biological aspects of children’s fractures; pp. 19–52. [Google Scholar]
- 88.Maes C., Kronenberg H. Pediatric Bone. Academic Press; Cambridge, MA, USA: 2012. Postnatal bone growth: Growth plate biology, bone formation, and remodeling; pp. 55–82. [Google Scholar]
- 89.Di Vito A., Chiarella E., Baudi F., Scardamaglia P., Antonelli A., Giudice D., Barni T., Fortunato L., Giudice A. Dose-dependent effects of zoledronic acid on human periodontal ligament stem celss: An In vitro pilot study. Cell Transplant. 2020;29:0963689720948497. doi: 10.1177/0963689720948497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giudice A., Antonelli A., Chiarella E., Baudi F., Barni T., Di Vito A. The case of medication-related osteonecrosis of the jaw addressed from a pathogenic point of view. Innovative therapeutic strategies: Focus on the most recent discoveries on oral mesenchymal stem cell-derived exosomes. Pharmaceuticals. 2020;13:423. doi: 10.3390/ph13120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stagi S., Cavalli L., Iurato C., Seminara S., Brandi M.L., De Martino M. Bone metabolism in children and adolescents: Main characteristics of the determinants of peak bone mass. Clin. Cases Miner. Bone Metab. 2013;10:172–179. [PMC free article] [PubMed] [Google Scholar]
- 92.Kirmani S., Christen D., van Lenthe G.H., Fischer P.R., Bouxsein M.L., McCready L.K., Melton III L.J., Riggs B.L., Amin S., Müller R., et al. Bone structure at the distal radius during adolescent growth. J. Bone Miner. Res. 2009;24:1033–1042. doi: 10.1359/jbmr.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rauch F., Neu C., Manz F., Schoenau E. The development of metaphyseal cortex—Implications for distal radius fractures during growth. J. Bone Miner. Res. 2001;16:1547–1555. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 94.On S.W., Cho S.W., Byun S.H., Yang B.E. Various therapeutic methods for the treatment of medication-related osteonecrosis of the jaw (MRONJ) and their limitations: A narrative review on new molecular and cellular therapeutic approaches. Antioxidants. 2021;10:680. doi: 10.3390/antiox10050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drake M., Clarke B.L., Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo. Clin. Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Felsenberg D., McCauley L.K., O’Ryan F., Reid I.R., Ruggiero S.L., Taguchi A., Tetradis S., Watts N.B., Brandi M.L., Peters E., et al. International Task Force on Osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 97.Japanese Allied Committee on Osteonecrosis of the Jaw. Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S., Soen S., Taguchi A., Nagata T., Urade M., et al. Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2017;35:6–19. doi: 10.1007/s00774-016-0810-7. [DOI] [PubMed] [Google Scholar]
- 98.Taguchi A., Shiraki M., Morrison A. Antiresorptive agent-related osteonecrosis of the jaw in osteoporosis patients from Asian countries. Osteoporos Sarcopenia. 2017;3:64–74. doi: 10.1016/j.afos.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Julia-Sánchez S., Álvarez-Herms J., Cirer-Sastre R. The influence of dental occlusion on dynamic balance and muscular tone. Front. Physiol. 2020;31:1626. doi: 10.3389/fphys.2019.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sampaio-Maia B., Monteiro-Silva F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent. Res. J. 2014;11:291–301. [PMC free article] [PubMed] [Google Scholar]
- 101.Russmueller G., Seemann R., Weiss K., Stadler V., Speiss M., Perisanidis C., Fuereder T., Willinger B., Sulzbacher I., Steininger C. The association of medication-related osteonecrosis of the jaw with Actinomyces spp. infection. Sci. Rep. 2016;17:31604. doi: 10.1038/srep31604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duarte N.T., Rech B.O., Martins I.G., Franco J., Ortega K. Can children be affected by bisphosphonate-related osteonecrosis of the jaw? A systematic review. Int. J. Oral Maxillofac. Surg. 2020;49:183–191. doi: 10.1016/j.ijom.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Ruggiero S.L., Dodson T.B., Aghaloo T., Carlson E.R., Ward B.B., Kademani D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022;80:920–943. doi: 10.1016/j.joms.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Ristow O., Ruckschloss T., Muller M., Berger M., Kargus S., Pautke C., Engel M., Hoffmann J., Freudlsperger C. Is the conservative nonsurgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Craniomaxillofac. Surg. 2019;47:491–499. doi: 10.1016/j.jcms.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 105.Carlson E.R., Schlott B.J. Anti-resorptive osteonecrosis of the jaws. Facts forgotten, questions answered, lessons learned. Oral Maxillofac. Surg. Clin. N. Am. 2014;26:171–191. doi: 10.1016/j.coms.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 106.Park H., Copeland C., Henry S., Barbul A. Complex wounds and their management. Surg. Clin. N. Am. 2010;90:1181–1194. doi: 10.1016/j.suc.2010.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available by request: henryagg@gmail.com.