Abstract
Genetic polymorphisms may be involved with mercury levels and signs and symptoms of intoxication from this exposure. Therefore, the aims were to describe the frequency of the GSTP1 polymorphism and to evaluate its effects on mercury levels and neurological signs in three Munduruku indigenous villages in the Brazilian Amazon. One-hundred-and-seven indigenous (over 12 years old) were included and genotyped (rs1695) using a TaqMan validated assay. Then, associations were evaluated by binary logistic regression, using odds ratios (OR) and 95% confidence intervals (CI). Mean age was 27.4 ± 13.9 years old, 52.3% were male, mean hair mercury concentration was 8.5 ± 4.3, exceeding the reference limit (≥6.0 µg/g), and were different among the three villages: 13.5 ± 4.6 µg/g in Sawré Aboy, 7.4 ± 2.3 µg/g in Poxo Muybu and 6.9 ± 3.5 µg/g in Sawré Muybu. The minor allele frequency of GSTP1 G was significantly different among the villages: 57% Sawré Muybu, 21% Poxo Muybu and 15% Sawré Aboy. Finally, after adjustment, GSTP1 GG and GA genotypes were associated with lower levels of Hg (OR = 0.13; CI95% = 0.03–0.49) and abnormal somatosensory signs (OR = 3.7; 95%IC = 1.5–9.3), respectively. In conclusion, monitoring this population is imperative to identify individuals at higher risk of developing signs of chronic mercury exposure based on the genetic profile.
Keywords: mercury exposure, GSTP1, genetic polymorphism, neurotoxicity, environmental health, indigenous people, Brazilian Amazon
1. Introduction
Mercury is the third most toxic element on the planet and can be found in different forms in the environment, of which organic mercury (methylmercury-MeHg) is the most dangerous chemical form and a global public health concern [1]. Artisanal small-scale gold mining (ASGM) is one of the main sources of human exposure to elemental mercury (Hg(0)), and approximately 3000 tons of Hg were released into the environment between 1975 and 2002, mostly in South America [2]. In the last years, it is estimated that ASGM (also called garimpo) increased over 90% in the Brazilian Amazon [3]. The Amazonian indigenous peoples need natural resources to live and the ASGM aftereffects threaten their livelihoods and cause health risks [4].
In water bodies, the elemental mercury undergoes methylation, mediated by aquatic micro-organisms, and forms MeHg, which bioaccumulates in fish. MeHg is able to overcome the blood-brain barrier and reach the central and peripheral nervous systems, causing changes in gait, coordination, cognition, visual dysfunctions and loss of skin sensitivity [5,6]. The indigenous peoples are one of the largest consumers of fish in the world [7] and consequently, they are at risk of higher concentrations of MeHg in their bodies [4,8].
Several studies showed an association between levels of mercury in the body and genetic polymorphisms, either conferring higher body levels of Hg or signs and symptoms of intoxication from this exposure [9,10,11,12] or providing protection against these effects [9,11,13]. Recently, the delta aminolevulinic acid dehydratase (ALAD) 177 C>G (rs1800435) polymorphism was identified in two indigenous children from the Brazilian Amazon, who showed elevated Hg levels (>9 µg/g), exceeding the reference limit (≥6.0 µg/g), and several neurological symptoms such as visual field alterations, memory deficit, distal neuropathy and toe amyotrophy [14]. Therefore, the study of polymorphisms in genes involved in MeHg metabolism is of great importance [4,14].
The MeHg metabolism involves its conjugation to small tripeptide glutathione (GSH), favoring the elimination via the ABC-transporter system in the bile [15]. The γ-glutamyl-cysteine ligase (GCL) enzyme synthesizes the GSH and then, the glutathione S-transferases, particularly the pi 1 isoform (GSTP1) catalyzes the conjugation of GSH to MeHg [16,17]. The GSTP1 enzyme is the most widely expressed glutathione-s-transferase (GST) in different parts of the human body, such as the liver, brain, lung, placenta, erythrocytes, muscle and others [18,19]. The GSTP1 enzyme is encoded by the GSTP1 gene, a polymorphic member of the GST family, located on chromosome 11 (11q13.2) [20].
Single nucleotide polymorphisms (SNPs) in the GSTP1 gene have been associated with MeHg retention and its symptoms of intoxication [13,20,21], with the rs1695 SNP standing out due to its elevated frequency in different populations and its ability to directly alter enzymatic activity due to the amino-acid substitution at position 105 from isoleucine to valine (Ile105Val), altering the geometry of the substrate binding site and reducing (approximately 3-fold) the substrate affinity in vitro [18,19,21,22].
Thus, the aims of this study were (1) to describe the frequency of the GSTP1 rs1695 polymorphism in three Munduruku indigenous communities of the Brazilian Amazon and (2) to evaluate the effects of this SNP on the mercury levels and on the prevalence of neurological signs.
2. Materials and Methods
2.1. Study Design and Population
This study is part of a major project, described by Basta et al., 2021 [4] and approved by the National Ethics Committee of Human Research (protocol number 65671517.1.0000.5240). Here, a cross-sectional study was carried out with the Munduruku people of the middle Tapajós River, in Pará state, Brazilian Amazon, between October and November 2019, involving 107 indigenous adults from 3 villages (Sawré Muybu, Poxo Muybu and Sawré Aboy).
All residents over 12 years old were invited to participate in the study and there was no refusal. Therefore, a convenience sample was selected, without any probabilistic sampling methods. Then, all participants provided written informed consent and answered a questionnaire, which was applied by the research team during home visits and interviews with participating families [4].
2.2. Clinical and Neurological Evaluation
After the initial interview, participants were referred for clinical evaluation, where clinical and laboratory analysis were performed, and samples of hair and oral mucosa were collected for mercury exposure level determination and DNA extraction, respectively. The body mass index (BMI) was calculated as the weight (kg) divided by the square of height (m2). The Hemocue device was used to assess the hemoglobin (Hb) levels and determine the prevalence of anemia (Hb < 11.5 g/dL).
Participants underwent a systematized neurological examination protocol, specially developed for this research, carried out by three of the authors (RAAO, BDP and BHR). For somatosensory signs diagnosis, distal pinprick perception, distal thermal sensitivity, hallux or thumb vibration sensitivity and feet mechanical detection threshold were evaluated. Classical diagnostic research criteria for the diagnosis of peripheral polineuropathy, or neuropathy burden, were used [23] and included the presence of abnormalities in at least one somatic sensory domain and/or alterations in the ankle jerk reflex. Toe amyotrophy and ankle jerk reflex have been observed for the motor function diagnosis, while the brief cognitive screening battery (BCSB), verbal fluency test and stick design test were performed to evaluate the cognitive functions. The neurological assessment of the studied population is described in more detail elsewhere [6].
2.3. Hair Mercury Analysis
Mercury exposure levels were determined by the measurements of mercury in the hair of participants. Hair samples were removed with the aid of stainless-steel dissection scissors, close to the scalp in the occipital region and stored in paper envelopes, individually identified. Then, the samples were sent to the Toxicology Laboratory, in the Environment Section of the Evandro Chagas Institute (IEC), in Belém-Pará, Brazil, for an analysis of total mercury levels (THg). The complete applied methodology was described by Basta et al., 2021 [4].
Mercury exposure levels <6.0 µg/g in hair were considered as a safe health limit, following the WHO recommendations [24]. Therefore, levels of hair Hg were categorized in ≤6.0 µg/g or >6.0 µg/g, according to the safety dose recognized by WHO and the median observed in the studied population.
2.4. DNA Extraction and GSTP1 Genotyping
Samples from oral mucosa epithelial cells were collected using sterile swabs, stored in a buffered solution, individually identified, and transported to the Laboratory of Pharmaceutical Science-LAPESF of the State University of Rio de Janeiro, West Zone Campus, in Rio de Janeiro-RJ. The access to the genomic DNA was performed using an extraction kit (Qiagen, Hilden, Germany), following the procedures recommended by the manufacturer. Briefly, samples are incubated at 56 °C with 20 µL of proteinase and 400 µL of lysis buffer to release intracellular material. The mixture is then centrifuged and 400 µL of ethanol is added, allowing DNA precipitation. Then, 700 µL of the mixture is transferred to a silica column, which, after centrifugation, retains the DNA. After that, two washing steps are carried out to remove PCR inhibitors, such as divalent cations and proteins, followed by a full-speed spin to remove all traces of wash buffers from the silica column. Finally, we use a low-salt buffer to elute the purified, ready-to-use DNA.
The genotyping analysis of the GSTP1 (chr11:67585218) A>G (rs1695) missense variant was performed using a TaqMan allelic discrimination assay (C_3237198_20) by a 7500 Real-Time System (Applied Biosystems, Foster City, CA, USA), as previously described [14] and the GSTP1 A>G allele frequency and genotype distribution were derived by gene counting.
2.5. Data Analysis
Continuous variables were presented as the mean, median, and their respective ranges. Linearity was tested with the Shapiro–Wilk normality test and the differences between groups for variables that did not present a normal distribution were evaluated by the Kruskal–Wallis (KW) nonparametric test. Categorical data were presented as number (n) and frequency (%) and analyzed using the chi-square test or Fisher’s exact test, if necessary. Deviations from Hardy–Weinberg equilibrium (HWE) in the GSTP1 A>G polymorphism frequency were assessed by the goodness-of-fit Chi-squared (χ2) test.
The associations between categorical variables and levels of mercury exposure were evaluated by determining the odds ratios (OR) and their respective 95% confidence intervals (95% CI), with adjustment for possible confounding factors, using binary logistic regression models. Age was considered a confounder for the association between the GSTP1 A>G SNP and Hg levels, while age and Hg levels were considered confounders for the association between the GSTP1 A>G SNP and the prevalence of neurological signs. All analyses were performed using IBM SPSS 20.0 Statistics for Windows (SPSS Inc., Chicago, IL, USA) and a significance level of 0.05 was adopted.
3. Results
The present study was conducted with 107 individuals older than 12 years old, residents from Poxo Muybu (31.8%), Sawré Aboy (21.5%) and Sawré Muybu (46.7%) villages. The majority of the individuals were male in Poxo Muybu and Sawré Aboy villages. The mean age in the overall population was 27.4 ± 13.9 years old (median 24.0, ranging from 12.0 to 72.0), with older individuals living in Sawré Muybu (58%) and younger individuals living in Sawré Aboy (60.8%). Approximately 5% of individuals showed anemia, and it was more common in residents of Sawré Aboy.
The mean level of mercury exposure in the studied population was 8.5 ± 4.3 (median 7.4, ranging from 2.0 to 22.8) and was not normally distributed (Shapiro–Wilk test p-value < 0.001). The Sawré Aboy village presented higher levels of mercury exposure when considering either the WHO cutoff point (6.0 µg/g) and the cutoff determined by the overall median of the studied population (7.4 µg/g). The Sawré Aboy residents also presented higher prevalence of abnormal somatosensory signs and cognitive functions (Table 1). When considering the prevalence of at least one abnormal neurological sign, 57.9% of individuals were affected in the overall population and a higher prevalence (82.6%) was found in Sawré Aboy residents (p-value = 0.004), comparing the three villages (data not shown).
Table 1.
Sociodemographic and clinical characteristics of the study population, according to the village of residence in Sawré Muybu indigenous territory, Pará State, Brazilian Amazon, 2019.
| Characteristics | Overall (n = 107) |
Village | |||
|---|---|---|---|---|---|
| Poxo Muybu | Sawré Aboy | Sawré Muybu | p-Value 1 | ||
| (n = 34) | (n = 23) | (n = 50) | |||
| Sex | |||||
| Female | 51 (47.7) | 16 (47.1) | 10 (43.5) | 25 (50.0) | 0.87 |
| Male | 56 (52.3) | 18 (52.9) | 13 (56.5) | 25 (50.0) | |
| Age (years) | |||||
| 12–19 | 36 (33.6) | 15 (44.1) | 11 (47.8) | 10 (20.0) | 0.05 |
| 20–24 | 19 (17.8) | 5 (14.7) | 3 (13.0) | 11 (22.0) | |
| 25–29 | 17 (15.9) | 2 (5.9) | 2 (8.7) | 13 (26.0) | |
| ≥30 | 35 (32.7) | 12 (35.3) | 7 (30.4) | 16 (32.0) | |
| BMI (kg/m2) | |||||
| <18.5 | 3 (2.8) | 2 (5.9) | 1 (4.3) | 0 (0.0) | 0.54 |
| 18.5–24.9 | 66 (61.7) | 19 (55.9) | 16 (69.6) | 31 (62.0) | |
| 25.0–29.9 | 34 (31.8) | 11 (32.4) | 5 (21.7) | 19 (36.0) | |
| ≥30.0 | 4 (3.7) | 2 (5.9) | 1 (4.3) | 1 (2.0) | |
| Hemoglobin 2 | |||||
| ≤11.5 | 5 (4.7) | 1 (3.0) | 3 (13.0) | 1 (2.0) | 0.1 |
| >11.5 | 101 (95.3) | 32 (97.0) | 20 (87.0) | 49 (98.0) | |
| Hg levels ( µ g/g) 3 | |||||
| ≤ 6.0 | 32 (30.5) | 10 (29.4) | 1 (4.3) | 21 (43.8) | 0.003 |
| > 6.0 | 73 (68.2) | 24 (70.6) | 22 (95.7) | 27 (56.2) | |
| ≤7.40 | 53 (50.5) | 19 (55.9) | 3 (13.0) | 31 (64.6) | <0.001 |
| >7.40 | 52 (49.5) | 15 (44.1) | 20 (87.0) | 17 (35.4) | |
| Somatosensory signs | |||||
| Normal | 66 (61.7) | 30 (88.2) | 10 (43.5) | 26 (52.0) | <0.001 |
| Abnormal | 41 (38.3) | 4 (11.8) | 13 (56.5) | 24 (48.0) | |
| Motor functions | |||||
| Normal | 85 (79.4) | 30 (88.2) | 17 (73.9) | 38 (76.0) | 0.3 |
| Abnormal | 22 (20.6) | 4 (11.8) | 6 (26.1) | 12 (20.4) | |
| Cognitive evaluations | |||||
| Normal | 68 (63.6) | 23 (67.6) | 9 (39.1) | 36 (72.0) | 0.02 |
| Abnormal | 39 (36.4) | 11 (32.4) | 14 (60.9) | 14 (28.0) | |
1 p-value obtained from the χ2 test (Pearson p-value) or Fisher’s exact test, when needed. 2 Missing information from 1 individual. 3 Missing information from 2 individuals.
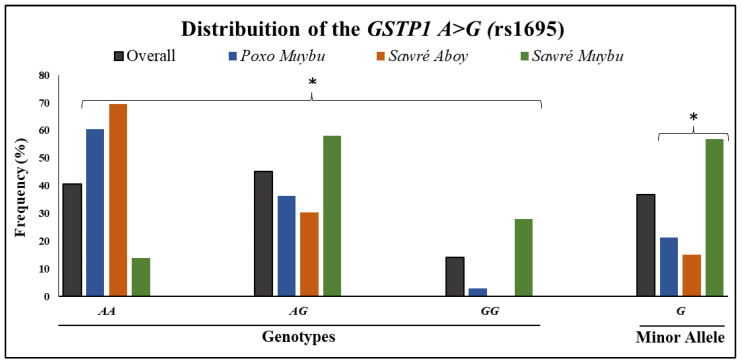
The rate of successful genotyping of the GSTP1 A>G SNP was 99.1%, since only a sample from one individual did not amplify during the PCR experiment. The distribution of the polymorphism was in Hardy–Weinberg equilibrium for the overall population (p-value = 0.79) and for each of the villages Poxo Muybu, Sawré Aboy and Sawré Muybu (p-values = 0.61, 0.39 and 0.19, respectively). The frequency distribution of the GSTP1 A>G polymorphism is presented in Figure 1. The distributions of the genotypes and minor allele frequency (MAF) among the three villages were statistically different (p-value < 0.0001), with Sawré Muybu residents presenting the highest frequency of the GSTP1 GG variant genotype and no individual from Sawré Aboy having that genotype. In addition, the Sawré Aboy village presented the highest frequency of the GSTP1 AA wild-type genotype.
Figure 1.
Distribution of the GSTP1 A>G (rs1695) genotypes (AA, AG, GG) and minor allele frequency (G) in the overall study population and in the 3 villages. * p-value < 0.0001 obtained from the χ2 test (Pearson p-value) when comparing the three villages, not including the overall group.
To investigate the associations between the GSTP1 A>G SNP and Hg exposure (Table 2), we first considered the Hg levels categorized according to the WHO guidelines (≤6.0 µg/g or >6.0 µg/g). It was observed that the GSTP1 GG genotype was associated with lower levels of Hg exposure in both crude and adjusted analyses. Furthermore, this association is slightly stronger in the recessive model (AA + AG versus GG). Interestingly, the Sawré Aboy residents showed significant (p-value < 0.0001) higher levels of Hg (13.5 ± 4.6 µg/g; median = 12.5, range = 4.8–22.8) compared with Poxo Muybu (7.4 ± 2.3 µg/g; median = 7.20, range = 2.8–12.9; p-value < 0.0001) and Sawré Muybu (6.9 ± 3.5 µg/g; median = 6.38, range = 2.0–16.0, p-value < 0.0001), and no individual from Sawré Aboy presented the GSTP1 GG variant genotype (Figure 1), which was associated with protection (lower levels of Hg).
Table 2.
Distribution of mercury exposure levels in the overall study population according to the GSTP1 A>G (rs1695) genotypes and alleles (Sawré Muybu indigenous territory, Pará State, Brazilian Amazon, 2019).
| GSTP1 (rs1695) A>G | Hg Levels (µg/g) | ||||
|---|---|---|---|---|---|
| ≤6.0 | >6.0 | p-Value 1 | Crude OR (95% IC) |
Adjusted OR 2 (95% IC) |
|
| (n = 32) | (n = 73) | ||||
| Genotypes | |||||
| AA | 11 (34.4) | 32 (44.4) | 0.001 | 1 | 1 |
| AG | 10 (31.2) | 36 (50.0) | 1.24 (0.47–3.30) | 1.23 (0.46–3.30) | |
| GG | 11 (34.4) | 4 (5.6) | 0.13 (0.03–0.48) | 0.13 (0.03–0.49) | |
| AA + GG | 21 (65.6) | 68 (94.4) | <0.001 | 1 | 1 |
| GG | 11 (34.4) | 4 (5.6) | 0.11 (0.03–0.39) | 0.12 (0.03–0.41) | |
| Alleles | |||||
| A | 32 (50.0) | 100 (69.4) | 0.007 | 1 | 1 |
| G | 32 (50.0) | 44 (30.6) | 0.44 (0.24–0.81) | 0.44 (0.24–0.81) | |
| GSTP1 (rs1695) A>G | Hg Levels (µg/g) | ||||
| ≤7.40 | >7.40 | p-value 1 |
Crude OR
(95% IC) |
Adjusted OR 2 (95% IC) | |
| (n = 53) | (n = 52) | ||||
| Genotypes | |||||
| AA | 21 (39.6) | 22 (43.1) | 0.05 | 1 | 1 |
| AG | 20 (37.7) | 26 (51.0) | 1.24 (0.54–2.86) | 1.24 (0.54–2.86) | |
| GG | 12 (22.6) | 3 (5.9) | 0.24 (0.06–0.97) | 0.24 (0.06–0.99) | |
| AA + GG | 41 (77.4) | 48 (94.1) | 0.02 | 1 | 1 |
| GG | 12 (22.6) | 3 (5.9) | 0.21 (0.06–0.81) | 0.22 (0.06–0.83) | |
| Alleles | |||||
| A | 62 (58.5) | 70 (68.6) | 0.13 | 1 | 1 |
| G | 44 (41.5) | 32 (31.4) | 0.64 (0.37–1.14) | 0.65 (0.37–1.14) | |
n = 105, missing information genotype for 1 individual and Hg levels from 2 individuals. 1 p-value obtained from the χ2 test (Pearson p-value) or Fisher’s exact test, when needed. 2 Odds ratio adjusted for age.
In addition, we investigated this association by considering the Hg levels categorized by the overall median (7.4 µg/g) and the results remained the same. Therefore, for the subsequent analysis, we considered only the Hg ≤ 6.0 (µg/g)2 versus > 6.0 (µg/g)2 classification.
Finally, the association between the GSTP1 A>G polymorphism and the prevalence of neurological signs was investigated. When considering the presence of any abnormal somatosensory sign, the GSTP1 AG genotype showed a positive association in both crude and adjusted analyses for age and Hg exposure levels (OR = 3.70 and 95% IC = 1.47–9.29; OR = 3.70 and 95% IC = 1.47–9.29, respectively), while the presence of abnormal motor or cognitive signs was not statistically significant (data not shown). In addition, each neurological sign individually and GSTP1 A>G polymorphism also investigated (Table 3 and Table 4). For the somatosensory signs, individuals carrying the GSTP1 AG genotype had an approximately 4-fold higher chance of having clinical signs of polyneuropathy, while the other signs were not significant among different GSTP1 genotypes (Table 3). There was a low prevalence of abnormal motor functions in the studied population (4.7% for toe amyotrophy and 16.8% for abnormal ankle jerk reflex) and all individuals who presented toe amyotrophy were carriers of the GSTP1 AG genotype (p-value = 0.04), however, it was not possible to estimate the OR and the respective 95% IC (Table 4). Regarding the cognitive evaluation, no association was observed with GSTP1 genotypes, however, the BCSB was significantly different among the groups, and approximately 42% of the altered results in this battery were from individuals carrying the GSTP1 AA genotype (Table 4), which was positively associated with higher levels of Hg in Table 2.
Table 3.
Distribution of the GSTP1 A>G (rs1695) genotypes and alleles in the overall study population according to the prevalence of somatosensory signs (Sawré Muybu indigenous territory, Pará State, Brazilian Amazon, 2019).
| GSTP1 (rs1695) A>G 1 | ||||
|---|---|---|---|---|
| Somatosensory Signs | AA | AG | GG | p-Value 2 |
| Distal pinprick perception | ||||
| Normal | 37 (45.7) | 33 (40.7) | 11 (13.6) | 0.15 |
| Abnormal | 6 (24.0) | 15 (60.0) | 4 (16.0) | |
| ORc (95% IC) | 1 | 2.80 (0.97–8.06) | 2.24 (0.54–9.40) | |
| ORa 3 (95% IC) | 1 | 3.16 (1.07–9.31) | 1.81 (0.39–8.43) | |
| Distal thermal sensitivity | ||||
| Normal | 37 (42.0) | 38 (43.2) | 13 (14.8) | 0.63 |
| Abnormal | 6 (33.3) | 10 (55.6) | 2 (11.1) | |
| ORc (95% IC) | 1 | 1.62 (0.54–4.92) | 0.95 (0.17–5.30) | |
| ORa 3 (95% IC) | 1 | 1.79 (0.58–5.53) | 0.67 (0.11–4.12) | |
| Hallux or thumb vibration sensitivity | ||||
| Normal | 39 (41.1) | 44 (46.3) | 12 (12.6) | 0.41 |
| Abnormal | 4 (36.4) | 4 (36.4) | 3 (27.3) | |
| ORc (95% IC) | 1 | 0.89 (0.21–3.78) | 2.44 (0.48–12.45) | |
| ORa3 (95% IC) | 1 | 0.98 (0.22–4.37) | 1.84 (0.29–11.84) | |
| Feet mechanical detection threshold | ||||
| Normal | 39 (42.9) | 39 (42.9) | 13 (14.3) | 0.43 |
| Abnormal | 4 (26.7) | 9 (60.0) | 2 (13.3) | |
| ORc (95% IC) | 1 | 2.25 (0.64–7.92) | 1.50 (0.25–9.16) | |
| ORa 3 (95% IC) | 1 | 2.50 (0.70–9.00) | 1.05 (0.15–7.18) | |
| Clinical signs of polyneuropathy 4 | ||||
| No | 34 (48.6) | 26 (37.1) | 10 (14.3) | 0.04 |
| Yes | 9 (25.0) | 22 (61.1) | 5 (13.9) | |
| ORc (95% IC) | 1 | 3.20 (1.26–8.09) | 1.89 (0.51–6.94) | |
| ORa 3 (95% IC) | 1 | 3.67 (1.42–9.53) | 1.41 (0.35–5.68) | |
ORc = crude odds ratio. 1 Missing information from 1 individual. 2 p-value obtained from the χ2 test (Pearson p-value) or Fisher’s exact test, when needed. 3 Odds ratio adjusted (ORa) for age and Hg exposure levels. 4 Neuropathy burden.
Table 4.
Distribution of the GSTP1 A>G (rs1695) genotypes and alleles in the overall study population according to the prevalence of motor and cognitive signs (Sawré Muybu indigenous territory, Pará State, Brazilian Amazon, 2019).
| GSTP1 (rs1695) A>G 1 | ||||
|---|---|---|---|---|
| Functions | AA | AG | GG | p-Value 2 |
| Motor | ||||
| Toe amyotrophy | ||||
| Normal | 43 (42.6) | 43 (42.6) | 15 (14.9) | 0.04 |
| Abnormal | 0 (0.0) | 5 (100.0) | 0 (0.0) | |
| ORc (95% IC) | 1 | - | - | |
| ORa 3 (95% IC) | 1 | - | - | |
| Ankle jerk reflex | ||||
| Normal | 36 (40.9) | 40 (45.5) | 12 (13.6) | 0.94 |
| Abnormal | 7 (38.9) | 8 (44.4) | 3 (16.7) | |
| ORc (95% IC) | 1 | 1.03 (0.34–3.12) | 1.29 (0.29–5.77) | |
| ORa 3 (95% IC) | 1 | 1.13 (0.36–3.52) | 0.97 (0.19–4.99) | |
| Cognitive | ||||
| BCSB 4 | ||||
| Normal | 33 (40.2) | 41 (50.0) | 8 (9.8) | 0.04 |
| Abnormal | 10 (41.7) | 7 (29.2) | 7 (29.2) | |
| ORc (95% IC) | 1 | 0.56 (0.19–1.64) | 2.89 (0.84–9.95) | |
| ORa 3 (95% IC) | 1 | 0.58 (0.20–1.73) | 3.51 (0.86–14.31) | |
| Verbal fluency test | ||||
| Normal | 29 (38.7) | 35 (46.7) | 11 (14.7) | 0.83 |
| Abnormal | 14 (45.2) | 13 (41.9) | 4 (12.9) | |
| ORc (95% IC) | 1 | 0.77 (0.31–1.89) | 0.75 (0.20–2.79) | |
| ORa 3 (95% IC) | 1 | 0.79 (0.32–1.98) | 1.05 (0.25–4.34) | |
| Stick design test | ||||
| Normal | 42 (40.4) | 47 (45.2) | 15 (14.4) | 0.84 |
| Abnormal | 1 (50.0) | 1 (50.0) | 0 (0.0) | |
| ORc (95% IC) | 1 | 0.89 (0.05–14.74) | - | |
| ORa 3 (95% IC) | 1 | 0.89 (0.05–14.87) | - | |
ORc = crude odds ratio. 1 Missing information from 1 individual. 2 p-value obtained from the χ2 test (Pearson p-value) or Fisher’s exact test, when needed. 3 Odds ratio adjusted (ORa) for age and Hg exposure levels. 4 Brief cognitive screening battery.
4. Discussion
The present study described the association of a single nucleotide polymorphism from a gene involved in the Hg metabolism and the presence of neurological signs of chronic mercury exposure in 107 indigenous adults residing in a region of the Brazilian Amazon affected by illegal mining activities. The Hg levels and GSTP1 A>G polymorphism genotype frequencies were significantly different among the three villages (Poxo Muybu, Sawré Aboy and Sawré Muybu). The GSTP1 AA genotype was associated with higher levels of Hg and the heterozygotic genotype (GSTP1 GA) was associated with a higher chance of having an abnormal somatosensory sign and neuropathy burden. In addition, the Sawré Aboy residents showed: (i) higher levels of Hg and (ii) higher frequency of the GSTP1 AA genotype.
Our findings retake the discussion initiated by Basta et al., 2021, who observed in this same population that Hg exposure levels and impaired neurological functions were higher in the Sawré Aboy village, which is located downstream from the Jamanxin River and is the closest village to the mining activities in comparison to the Sawré Muybu and Poxo Muybu ones [4,6]. In addition, these findings can also be explained by the higher frequency of the GSTP1 AA genotype, associated with higher Hg levels, in Sawré Aboy residents compared to the other two villages (Sawré Muybu and Poxo Muybu).
The distribution profile of the GSTP1 A>G (rs1695) polymorphism varies according to the population, with the MAF (allele G) ranging from 24.7% to 43% in the Brazilian population [25,26,27,28,29,30,31]. As far as we know, there is no data on GSTP1 polymorphism frequency from other indigenous Brazilian populations focusing on mercury exposure. A recent study investigated the GSTP1 frequency in the nonindigenous Brazilian population and observed that the MAF (ranging from 30.7% to 31.5%) did not differ between the groups based on skin color: black, mulatto, nonwhite and white [32]. Here, the GSTP1 MAF in the overall indigenous population was 36.8%, similar (41.8%) to that described in the indigenous from the Amazon admitted to the Indian House of Health in Boa Vista, Roraima, Brazil (CASAI/RR), aiming to evaluate the genetic profile (TP53 and GSTP1 genes) and prostatic features [26]. However, a significant difference was found among the three villages: 21.2% Poxo Muybu, 15.2% Sawré Aboy and 57.0% Sawré Muybu. The Munduruku are an indigenous community from the Brazilian Amazon that lives isolated from other Amerindian populations and rarely display admixture with nonindigenous. Despite living near each other for centuries, these populations maintain their distinctiveness, according to the substantial differences found in their genetic profiles [4]. For this reason, it is not possible to extrapolate these results, not even for the same ethnic group. Therefore, the implementation of an individual genetic diagnosis is necessary.
The GST superfamily is comprised of 16 genes, including GSTP1, and encodes vital defense enzymes involved in the detoxification of reactive oxygen species and metal biotransformation [33,34]. GSTP1 is a key enzyme because it is the most widely expressed GST in the body. In addition to being frequent, the GSTP1 A>G non-synonymous polymorphism changes significantly the enzyme-substrate affinity [18,19,35,36,37]. The literature is not conclusive about the relationship between the GSTP1 A>G polymorphism and levels of Hg exposure. Our results contrast with studies where the minor allele GSTP1 G was associated with higher Hg levels from different exposure sources and biological matrices such as hair [9,38] and urine [13], and is agreement with other studies where the GSTP1 G showed a protective effect against elevated Hg levels dosed from hair [22] and blood samples [21,39]. One possible reason for the discrepancy among the studies is the use of different biological matrices to measure the Hg exposure, depending on the route of exposure, which may not present the same association with the polymorphism in the GSTP1 gene, which is mostly involved with the metabolism of MeHg ingested from the diet [9,15,39]. Furthermore, this raises the question of whether Hg dosed from hair samples reflects the circulating levels of the metal or, instead, represents how much is being excreted from the organism. Nevertheless, the measurement of Hg in hair samples is advantageous due to the simple collection and transportation, which are easier than for blood and urine samples [40,41].
There is evidence that the GSTP1 polymorphism is associated with several neurological conditions, such as multiple sclerosis [42] and autism spectrum disorders [43]. To date, however, no other clinical study has found an association between the GSTP1 A>G polymorphism and signs of neurotoxicity induced by MeHg. We believe that the enzymatic alteration caused by this SNP may alter the MeHg exposure levels in the peripheral and central nervous systems. This, in addition to the enhancement of oxidative stress, may play a role in the pathogenesis of the neurological abnormalities found in this series [44]. In the present study, despite the association found for the GSTP1 AG genotype, it is not possible to conclude that the SNP is involved with the onset of neurological signs and symptoms due to the small number of individuals enrolled and the low prevalence of these outcomes in the population. Furthermore, this might be due to the low prevalence of the neurological signs and symptoms of chronic mercury exposure and the low frequency of the GSTP1 AA genotype in Sawré Muybu and the GSTP1 GG genotype in Poxo Muybu and Sawré Aboy, ensuring a higher frequency of the GSTP1 AG genotype in the overall population. In addition, the individuals were young, and this kind of outcome is more prevalent in later ages, which could be better investigated in a longitudinal observation of this population, that also has an elevated frequency of polymorphism associated with mercury exposure levels.
Studies with indigenous communities have multiple ethical aspects that need to be considered, such as the cultural importance of the indigenous body and, therefore, all specimens that can be collected, such as tissue, blood, and hair [45]. In the present study, we were able to count on the engagement of community leaders, who made it possible to collect hair and oral mucosa samples and perform the needed analysis. This access was allowed thanks to the effort and competence of the multidisciplinary team that was assembled to evaluate this population, involving biologists, physicians, nurses, psychologists, and pharmaceutical geneticists. Efforts are being made to create a closer interaction between researchers and indigenous communities, to increase research recruitment and also to provide reports of individual results obtained with the studies [4]. This approach is especially important when genetic studies are carried out because individuals must be aware of the benefits of engaging in the research [4,14,45].
Although some limitations need to be discussed, such as nonmeasured variables that would be important to evaluate, such as occupation, alcohol consumption and smoking status, those can be sources of bias. Furthermore, the small sample size may lead to relatively weak power to detect the real association between the polymorphism and the neurological signs and symptoms. However, the potential benefits that can be provided to the communities must be considered. Therefore, in order to reduce some of these limitations and expand the clinical evaluation carried out among the Munduruku indigenous people, a longitudinal study is being designed to evaluate levels of mercury exposure in different matrices and the chronic effects of mercury exposure, in addition to identifying possible polymorphisms as susceptibility biomarkers of these events. Furthermore, the villages are in a high-risk area for ASGM since levels of Hg are extremely high, which reveals the extreme vulnerability of this population and the need for constant environmental and individual monitoring through a wide genetic evaluation that can easily be carried out with noninvasive biological matrices, such as the oral mucosa cells used in this work.
5. Conclusions
This is the first study to evaluate the association of the GSTP1 A>G polymorphism with Hg levels and the neurological signs that can be caused by chronic exposure to heavy metals in an extremely vulnerable population from the Brazilian Amazon. The Hg levels were significantly higher in Sawré Aboy residents, followed by those from Poxo Muybu and Sawré Muybu, while the GSTP1 G allele, which is associated with lower levels of Hg, was more frequent in Sawré Aboy residents, followed by Poxo Muybu residents, and finally Sawré Muybu residents. The GSTP1 AA and GA genotypes were associated with higher levels of Hg and a higher chance of having an abnormal somatosensory sign, respectively. Our findings highlight the importance of monitoring the indigenous communities in the Brazilian Amazon, who live in a vulnerable situation caused by the abandonment of the state. In conclusion, it is evident that further wide evaluation is imperative to this population, in order to identify individuals at higher risk of developing signs and symptoms of chronic mercury exposure. Thus, individual genetic diagnosis tests are crucial, since it is not possible to extrapolate data from other populations, even from other Amerindians.
Acknowledgments
Through Juarez Saw, Jairo Saw, Valdemar Poxo, and Alessandra Korap, we thank the Munduruku people for the trust placed in our team and the support in carrying out the research. We thank the team from the Rio Tapajós Indigenous Special Sanitary District, who spared no efforts to support us in all stages of the fieldwork. In particular, we thank nurses Alan Marcelo Simon and Lygia Catarina de Oliveira. We thank João Paulo Goes Pereira for the mercury analysis in the fish samples. The authors thank the technical assistance of Jessica Vilarinho Cardoso from Laboratório de Pesquisa de Ciências Farmacêuticas-LAPESF (https://lapesfuezo.wixsite.com/website, accessed on 4 January 2023)) at Universidade do Estado do Rio de Janeiro, Campus Zona Oeste (UERJ-ZO).
Author Contributions
Conceptualization, P.C.B. and J.A.P.; methodology, R.A.A.d.O., B.H.R., B.D.P., M.C.d.S., J.A.P., I.M.d.J. and M.d.O.L.; formal analysis, M.C.d.S., R.A.A.d.O., D.E.M., I.M.d.J. and M.d.O.L.; investigation, J.A.P., A.C.S.d.V., P.C.B. and M.C.d.S.; resources, P.C.B., J.A.P. and S.S.H.; data curation, A.C.S.d.V., P.C.B., M.C.d.S. and J.A.P.; writing—original draft preparation, M.C.d.S. and J.A.P.; writing—review and editing, A.C.S.d.V., P.C.B., R.A.A.d.O., D.E.M., and S.S.H.; visualization S.S.H., P.C.B. and J.A.P.; supervision, P.C.B. and J.A.P.; project administration, P.C.B.; funding acquisition, P.C.B., S.S.H., D.E.M. and J.A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the National School of Public Health at Fundação Oswaldo Cruz (REC/ENSP) and the Brazilian National Research Ethics Commission of the National Health Council (CONEP/CNS), CAAE: 65671517.1.0000.5240, with Opinion No. 2.262.686 favorable to its performance. In compliance with Convention No. 169 of the International Labor Organization (ILO), the study began with a pre-study consultation, carried out in August 2019, during a visit to the villages, in which two authors (SSH and PCB) and local indigenous leaders participated. At the time, the study objectives were presented and discussed (https://youtu.be/oFEYEGxNmns?t=4, accessed on 4 January 2023). After answering questions and receiving approval of the proposal from the communities, we received support from the coordination of the Special Indigenous Sanitary District of the Tapajós River through the multidisciplinary indigenous health team to carry out the study. In addition, the interviews and data collection started only after the participants had their questions answered and given formal consent in the Informed Consent Form (ICF) by the children’s guardians.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the vice presidency of environment, care and health promotion (VPAAS) of Fundação Oswaldo Cruz through the Decentralized Execution of Resources Document No. 175/2018, Process: 25000.209221/2018-18, signed between the Fundação Oswaldo Cruz and the Special Department for Indigenous Health, both under the Ministry of Health. This study was supported by the Brazilian agency Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). A funding body contributed to the acquisition of research inputs.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rice K.M., Walker E.M., Wu M., Gillette C., Blough E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Pub. Health. 2014;47:74–83. doi: 10.3961/jpmph.2014.47.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lacerda L. Updating Global Hg Emissions from Small-Scale Gold Mining and Assessing Its Environmental Impacts. Environ. Geol. 2003;43:308–314. doi: 10.1007/s00254-002-0627-7. [DOI] [Google Scholar]
- 3.Siqueira-Gay J., Sánchez L.E. The Outbreak of Illegal Gold Mining in the Brazilian Amazon Boosts Deforestation. Reg. Environ. Chang. 2021;21:28. doi: 10.1007/s10113-021-01761-7. [DOI] [Google Scholar]
- 4.Basta P.C., Viana P.V.D.S., Vasconcellos A.C.S.D., Périssé A.R.S., Hofer C.B., Paiva N.S., Kempton J.W., Ciampi de Andrade D., Oliveira R.A.A.D., Achatz R.W., et al. Mercury Exposure in Munduruku Indigenous Communities from Brazilian Amazon: Methodological Background and an Overview of the Principal Results. Int. J. Environ. Res. Public. Health. 2021;18:9222. doi: 10.3390/ijerph18179222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasconcellos A.C.S.D., Barrocas P.R.G., Ruiz C.M.V., Mourão D.D.S., Hacon S.D.S. Burden of Mild Mental Retardation Attributed to Prenatal Methylmercury Exposure in Amazon: Local and Regional Estimates. Ciênc. Saúde Coletiva. 2018;23:3535–3545. doi: 10.1590/1413-812320182311.15812016. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira R.A.A.D., Pinto B.D., Rebouças B.H., Ciampi de Andrade D., Vasconcellos A.C.S.D., Basta P.C. Neurological Impacts of Chronic Methylmercury Exposure in Munduruku Indigenous Adults: Somatosensory, Motor, and Cognitive Abnormalities. Int. J. Environ. Res. Public. Health. 2021;18:10270. doi: 10.3390/ijerph181910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos G.M.D., Santos A.C.M.D. Sustentabilidade da pesca na Amazônia. Estud. Av. 2005;19:165–182. doi: 10.1590/S0103-40142005000200010. [DOI] [Google Scholar]
- 8.Gimenes T.C., Penteado J.O., dos Santos M., da Silva Júnior F.M.R. Methylmercury in Fish from the Amazon Region—A Review Focused on Eating Habits. Water. Air. Soil Pollut. 2021;232:199. doi: 10.1007/s11270-021-05151-x. [DOI] [Google Scholar]
- 9.Chan P.H.Y., Chan K.Y.Y., Schooling C.M., Hui L.L., Chan M.H.M., Li A.M., Cheung R.C.K., Lam H.S. Association between Genetic Variations in GSH-Related and MT Genes and Low-Dose Methylmercury Exposure in Children and Women of Childbearing Age: A Pilot Study. Environ. Res. 2020;187:109703. doi: 10.1016/j.envres.2020.109703. [DOI] [PubMed] [Google Scholar]
- 10.Lozano M., Murcia M., Soler-Blasco R., González L., Iriarte G., Rebagliato M., Lopez-Espinosa M.-J., Esplugues A., Ballester F., Llop S. Exposure to Mercury among 9-Year-Old Children and Neurobehavioural Function. Environ. Int. 2021;146:106173. doi: 10.1016/j.envint.2020.106173. [DOI] [PubMed] [Google Scholar]
- 11.Parajuli R.P., Goodrich J.M., Chan H.M., Lemire M., Ayotte P., Hegele R.A., Basu N. Variation in Biomarker Levels of Metals, Persistent Organic Pollutants, and Omega-3 Fatty Acids in Association with Genetic Polymorphisms among Inuit in Nunavik, Canada. Environ. Res. 2021;200:111393. doi: 10.1016/j.envres.2021.111393. [DOI] [PubMed] [Google Scholar]
- 12.Sirivarasai J., Chaisungnern K., Panpunuan P., Chanprasertyothin S., Chansirikanjana S., Sritara P. Role of MT1A Polymorphism and Environmental Mercury Exposure on the Montreal Cognitive Assessment (MoCA) Neuropsychiatr. Dis. Treat. 2021;17:2429–2439. doi: 10.2147/NDT.S320374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina Pérez O.M., Flórez-Vargas O., Rincón Cruz G., Rondón González F., Rocha Muñoz L., Sánchez Rodríguez L.H. Glutathione-Related Genetic Polymorphisms Are Associated with Mercury Retention and Nephrotoxicity in Gold-Mining Settings of a Colombian Population. Sci. Rep. 2021;11:8716. doi: 10.1038/s41598-021-88137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perini J.A., Silva M.C., Vasconcellos A.C.S.D., Viana P.V.S., Lima M.O., Jesus I.M., Kempton J.W., Oliveira R.A.A., Hacon S.S., Basta P.C. Genetic Polymorphism of Delta Aminolevulinic Acid Dehydratase (ALAD) Gene and Symptoms of Chronic Mercury Exposure in Munduruku Indigenous Children within the Brazilian Amazon. Int. J. Environ. Res. Public. Health. 2021;18:8746. doi: 10.3390/ijerph18168746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballatori N., Clarkson W. Biliary Secretion of Glutathione and of Glutathione-Metal Complexes. Fundam Appl Toxicol. 1985;5:816–831. doi: 10.1016/0272-0590(85)90165-4. [DOI] [PubMed] [Google Scholar]
- 16.Custodio H.M., Broberg K., Wennberg M., Jansson J.-H., Vessby B., Hallmans G., Stegmayr B., Skerfving S. Polymorphisms in Glutathione-Related Genes Affect Methylmercury Retention. Arch. Environ. Health Int. J. 2004;59:588–595. doi: 10.1080/00039890409603438. [DOI] [PubMed] [Google Scholar]
- 17.Lu S.C. Glutathione Synthesis. Biochim. Biophys. Acta BBA—Gen. Subj. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strange R.C., Jones P.W., Fryer A.A. Glutathione S-Transferase: Genetics and Role in Toxicology. Toxicol. Lett. 2000;112–113:357–363. doi: 10.1016/S0378-4274(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T., Coggan M., Shaw D.C., Board P.G. Electrophoretic and Immunological Analysis of Human Glutathione S-Transferase Isozymes. Ann. Hum. Genet. 1987;51:95–106. doi: 10.1111/j.1469-1809.1987.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 20.Wahlberg K., Love T.M., Pineda D., Engström K., Watson G.E., Thurston S.W., Yeates A.J., Mulhern M.S., McSorley E.M., Strain J.J., et al. Maternal Polymorphisms in Glutathione-Related Genes Are Associated with Maternal Mercury Concentrations and Early Child Neurodevelopment in a Population with a Fish-Rich Diet. Environ. Int. 2018;115:142–149. doi: 10.1016/j.envint.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parajuli R.P., Goodrich J.M., Chou H.-N., Gruninger S.E., Dolinoy D.C., Franzblau A., Basu N. Genetic Polymorphisms Are Associated with Hair, Blood, and Urine Mercury Levels in the American Dental Association (ADA) Study Participants. Environ. Res. 2016;149:247–258. doi: 10.1016/j.envres.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodrich J.M., Basu N. Variants of Glutathione S-Transferase Pi 1 Exhibit Differential Enzymatic Activity and Inhibition by Heavy Metals. Toxicol. In Vitro. 2012;26:630–635. doi: 10.1016/j.tiv.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.England J.D., Gronseth G.S., Franklin G., Miller R.G., Asbury A.K., Carter G.T., Cohen J.A., Fisher M.A., Howard J.F., Kinsella L.J., et al. Distal Symmetric Polyneuropathy: A Definition for Clinical Research. Neurology. 2005;64:199. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- 24.Joint FAO/WHO Expert Committee on Food Additives (JECFA) Toxicological Evaluation of Certain Food Additives and Contaminants; Proceedings of the 33rd Meeting of the Joint FAO/WHO Expert Committee on Food Additives; Geneva, Switzerland. 21–30 March 1989; Geneva, Switzerland: World Health Organization; 1989. [Google Scholar]
- 25.Magno L.A.V., Talbot J., Talbot T., Borges Santos A.M., Souza R.P., Marin L.J., Moreli M.L., de Melo P.R.S., Corrêa R.X., Rios Santos F., et al. Glutathione S-Transferase Variants in a Brazilian Population. Pharmacology. 2009;83:231–236. doi: 10.1159/000205823. [DOI] [PubMed] [Google Scholar]
- 26.Lima Junior M.M.D., Reis L.O., Ferreira U., Cardoso U.O., Barbieri R.B., Mendonça G.B.D., Ward L.S. Unraveling Brazilian Indian Population Prostate Good Health: Clinical, Anthropometric and Genetic Features. Int. Braz. J. Urol. 2015;41:344–352. doi: 10.1590/S1677-5538.IBJU.2015.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chagas B.S., Gurgel A.P.A.D., Júnior S.S.L.P., Lima R.C.P., Cordeiro M.N., Moura R.R., Coelho A.V.C., Nascimento K.C.G., Neto J.C.S., Crovella S., et al. Research Article Synergic Effect of Oral Contraceptives, GSTP1 Polymorphisms, and High-Risk HPV Infection in Development of Cervical Lesions. Genet. Mol. Res. 2017;16:1–9. doi: 10.4238/gmr16039742. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira de Araújo Melo C., Cidália Vieira T., Duarte Gigonzac M.A., Soares Fortes J., Moreira Duarte S.S., da Cruz A.D., Silva D.D.M.E. Evaluation of Polymorphisms in Repair and Detoxification Genes in Alcohol Drinkers and Non-drinkers Using Capillary Electrophoresis. Electrophoresis. 2020;41:254–258. doi: 10.1002/elps.201900193. [DOI] [PubMed] [Google Scholar]
- 29.Ferracini A.C., Lopes-Aguiar L., Lourenço G.J., Yoshida A., Lima C.S.P., Sarian L.O., Derchain S., Kroetz D.L., Mazzola P.G. GSTP1 and ABCB1 Polymorphisms Predicting Toxicities and Clinical Management on Carboplatin and Paclitaxel-Based Chemotherapy in Ovarian Cancer. Clin. Transl. Sci. 2021;14:720–728. doi: 10.1111/cts.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barros J.B.D.S., Santos K.D.F., Azevedo R.M., de Oliveira R.P.D., Leobas A.C.D., Bento D.D.C.P., Santos R.D.S., Reis A.A.D.S. No Association of GSTP1 Rs1695 Polymorphism with Amyotrophic Lateral Sclerosis: A Case-Control Study in the Brazilian Population. PLoS ONE. 2021;16:e0247024. doi: 10.1371/journal.pone.0247024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Sousa Barros J.B., de Faria Santos K., da Cruz Pereira Bento D., Prado Assunção L.D., da Silva Santos R., da Silva Reis A.A. Influence of GSTP1 Rs1695 Polymorphism on Survival in Male Patients’ Amyotrophic Lateral Sclerosis: A Genetic Association Study in Brazilian Population. Mol. Biol. Rep. 2022;49:1655–1659. doi: 10.1007/s11033-021-06724-z. [DOI] [PubMed] [Google Scholar]
- 32.Rossini A., Rapozo D.C.M., Amorim L.M.F., Macedo J.M.B., Medina R., Neto J.F.N., Gallo C.V.M., Pinto L.F.R. Frequencies of GSTM1, GSTT1, and GSTP1 Polymorphisms in a Brazilian Population. Genet. Mol. Res. 2002;1:233–240. [PubMed] [Google Scholar]
- 33.Nebert D.W., Vasiliou V. Analysis of the Glutathione S-Transferase (GST) Gene Family. Hum. Genom. 2004;1:460. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yohannes Y.B., Nakayama S.M.M., Yabe J., Toyomaki H., Kataba A., Nakata H., Muzandu K., Ikenaka Y., Choongo K., Ishizuka M. Glutathione S-Transferase Gene Polymorphisms in Association with Susceptibility to Lead Toxicity in Lead- and Cadmium-Exposed Children near an Abandoned Lead-Zinc Mining Area in Kabwe, Zambia. Environ. Sci. Pollut. Res. 2022;29:6622–6632. doi: 10.1007/s11356-021-16098-1. [DOI] [PubMed] [Google Scholar]
- 35.Johansson A.-S., Stenberg G., Widersten M., Mannervik B. Structure-Activity Relationships and Thermal Stability of Human Glutathione Transferase P1-1 Governed by the H-Site Residue 105. J. Mol. Biol. 1998;278:687–698. doi: 10.1006/jmbi.1998.1708. [DOI] [PubMed] [Google Scholar]
- 36.Ali-Osman F., Akande O., Antoun G., Mao J.-X., Buolamwini J. Molecular Cloning, Characterization, and Expression in Escherichia Coli of Full-Length CDNAs of Three Human Glutathione S-Transferase Pi Gene Variants. J. Biol. Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 37.Hu X., Xia H., Srivastava S.K., Herzog C., Awasthi Y.C., Ji X., Zimniak P., Singh S.V. Activity of Four Allelic Forms of Glutathione S-Transferase HGSTP1-1 for Diol Epoxides of Polycyclic Aromatic Hydrocarbons. Biochem. Biophys. Res. Commun. 1997;238:397–402. doi: 10.1006/bbrc.1997.7311. [DOI] [PubMed] [Google Scholar]
- 38.Gundacker C., Wittmann K.J., Kukuckova M., Komarnicki G., Hikkel I., Gencik M. Genetic Background of Lead and Mercury Metabolism in a Group of Medical Students in Austria. Environ. Res. 2009;109:786–796. doi: 10.1016/j.envres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Engström K.S., Strömberg U., Lundh T., Johansson I., Vessby B., Hallmans G., Skerfving S., Broberg K. Genetic Variation in Glutathione-Related Genes and Body Burden of Methylmercury. Environ. Health Perspect. 2008;116:734–739. doi: 10.1289/ehp.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Branco V., Caito S., Farina M., Teixeira da Rocha J., Aschner M., Carvalho C. Biomarkers of Mercury Toxicity: Past, Present, and Future Trends. J. Toxicol. Environ. Health Part B. 2017;20:119–154. doi: 10.1080/10937404.2017.1289834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crespo-Lopez M.E., Augusto-Oliveira M., Lopes-Araújo A., Santos-Sacramento L., Takeda P.Y., de Matos Macchi B., do Nascimento J.L.M., Maia C.S., Lima R.R., Arrifano G.P. Mercury: What Can We Learn from the Amazon? Environ. Int. 2021;146:106223. doi: 10.1016/j.envint.2020.106223. [DOI] [PubMed] [Google Scholar]
- 42.Mann C.L.A., Davies M.B., Boggild M.D., Alldersea J., Fryer A.A., Jones P.W., Ko C.K., Young C., Strange R.C., Hawkins C.P. Glutathione S-Transferase Polymorphisms in MS: Their Relationship to Disability. Neurology. 2000;54:552. doi: 10.1212/WNL.54.3.552. [DOI] [PubMed] [Google Scholar]
- 43.Williams T.A., Mars A.E., Buyske S.G., Stenroos E.S., Wang R., Factura-Santiago M.F., Lambert G.H., Johnson W.G. Risk of Autistic Disorder in Affected Offspring of Mothers With a Glutathione S-Transferase P1 Haplotype. Arch. Pediatr. Adolesc. Med. 2007;161:356–361. doi: 10.1001/archpedi.161.4.356. [DOI] [PubMed] [Google Scholar]
- 44.Fu Z., Xi S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods. 2020;30:167–176. doi: 10.1080/15376516.2019.1701594. [DOI] [PubMed] [Google Scholar]
- 45.Aramoana J., Koea J., on behalf of the CommNETS Collaboration An Integrative Review of the Barriers to Indigenous Peoples Participation in Biobanking and Genomic Research. JCO Glob. Oncol. 2020;6:83–91. doi: 10.1200/JGO.18.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.