Abstract
A single-blind double-dummy randomized study was conducted in diagnosed patients (n = 66) to compare the efficacy of Linseeds (Linum usitatissimum L.), Psyllium (Plantago ovata Forssk.), and honey in uncomplicated pelvic inflammatory disease (uPID) with standard drugs using experimental and computational analysis. The pessary group received placebo capsules orally twice daily plus a per vaginum cotton pessary of powder from linseeds and psyllium seeds, each weighing 3 gm, with honey (5 mL) at bedtime. The standard group received 100 mg of doxycycline twice daily and 400 mg of metronidazole TID orally plus a placebo cotton pessary per vaginum at bedtime for 14 days. The primary outcomes were clinical features of uPID (vaginal discharge, lower abdominal pain (LAP), low backache (LBA), and pelvic tenderness. The secondary outcomes included leucocytes (WBCs) in vaginal discharge on saline microscopy and the SF-12 health questionnaire. In addition, we also classified both (pessary and standard) groups using machine learning models such as Decision Tree (DT), Random Forest (RF), Logistic Regression (LR), and AdaBoost (AB). The pessary group showed a higher percentage reduction than the standard group in abnormal vaginal discharge (87.05% vs. 77.94%), Visual Analogue Scale (VAS)-LAP (80.57% vs. 77.09%), VAS-LBA (74.19% vs. 68.54%), McCormack pain scale (McPS) score for pelvic tenderness (75.39% vs. 67.81%), WBC count of vaginal discharge (87.09% vs. 83.41%) and improvement in SF-12 HRQoL score (94.25% vs. 86.81%). Additionally, our DT 5-fold model achieved the maximum accuracy (61.80%) in the classification. We propose that the pessary group is cost-effective, safer, and more effective as standard drugs for treating uPID and improving the HRQoL of women. Aucubin, Plantamajoside, Herbacetin, secoisolariciresinol diglucoside, Secoisolariciresinol Monoglucoside, and other various natural bioactive molecules of psyllium and linseeds have beneficial effects as they possess anti-inflammatory, antioxidant, antimicrobial, and immunomodulatory properties. The anticipated research work is be a better alternative treatment for genital infections.
Keywords: inflammatory disease, honey, L. usitatissimum L., P. ovata Forssk., herbal medicine, AI for medicine, Unani system of medicine, bioactive molecule, oxidative stress, drug, machine learning
1. Introduction
Pelvic inflammatory disease (PID) is an important gynaecological problem that is common in women of reproductive age living in developed or developing countries, with serious repercussions on their health and well-being [1,2]. It is an infectious and inflammatory disorder of the endometrium, fallopian tubes, ovaries, parametrium and peritoneum due to ascending infection of the endocervix, which presents varied patterns of polymicrobial infections and interrelated risks [3]. It is a common health issue in reproductive-age women in developed and developing countries [2]. Subclinical PID or uncomplicated pelvic inflammatory disease (uPID) can be defined as upper reproductive tract inflammation without any signs and symptoms of acute PID [4]. Uncomplicated PID must not be underestimated as it does not show clinical signs or symptoms, and it might destroy fallopian tubes as acute asymptomatic PID. Thus, asymptomatic sexually transmitted disease screening and early treatment are critical [4]. The CDC estimates that more than a million women experience an episode of PID every year [1]. Laparoscopic studies reveal that in 30–40% of cases, it is polymicrobial in aetiology. It leads to complications such as chronic pelvic pain, ectopic pregnancy, and infertility, and it increases health care costs [5]. The CDC has specified that for the clinical diagnosis of PID, at least one of the three minimum diagnostic criteria (viz., cervical motion tenderness or uterine tenderness/adnexal tenderness or lower abdominal pain) is a prerequisite. To increase the specificity of the minimum criteria, one or more of the following added criteria of PID are used to support the diagnosis: presence of a high number of WBCs in the vaginal fluid on saline microscopy, abnormal cervical or vaginal mucopurulent discharge, raised erythrocyte sedimentation rate, oral temperature >101 °F (>38.3 °C), raised C-reactive protein, and confirmation of N. gonorrhoea or C. trachomatis infection through laboratory diagnosis [1,2,6]. Uncomplicated PID is diagnosed on the basis of a clinical evaluation even though it has a lesser predictive value in comparison to laparoscopy [6]. The local signs and symptoms of uPID are copious, white or yellowish, and thin/thick discharge from the vagina with/without vulval itching, lower abdominal pain, and low backache during menses, menstrual disorders, dyspareunia, and infertility. Upon vaginal examination, eruptions are visualized very easily, there is tenderness on examination, and the uterine cervix is hypertrophied and can be palpated easily [7,8].
PID has developed as a silent killer that extensively disturbs women’s lives. The disease, if not treated, could result in acute morbidity and serious sequelae among women of reproductive age. The use of broad-spectrum antibiotics is the current centralized treatment according to guidelines [3]. Compliance is poor with antibiotic therapy for PID [4]. The most common and major side effects of antibiotics, analgesics, and anti-inflammatory drugs are headaches, gastrointestinal upsets, drowsiness, dizziness [9], renal impairment, and interactions with other medications [3]. Furthermore, an upsurge in multidrug resistant (MDR) pathogenic bacteria has been observed with a sharp reduction in conventional antibiotic therapy efficacy, which has increased the burden of disease in the population [10].
According to the World Health Organization (WHO), identified plants and their bioactive molecules would be the best alternative source to treat diseases. Unani classical texts and pharmacopoeia are enriched with medicines useful in uPID, such as the combination of Plantago ovata and Linum usitatissimum and honey [11,12], as they possess Muhallil al-Waram (anti-inflammatory), Musakkin (analgesic), and Dafi’-i-Ta’affun (antiseptic) properties [11]. Plantago ovata Forssk. belongs to the Family Plantaginaceae. The herb is found in northwestern India, cultivated to a small extent in West Bengal, Karnataka, and Coromandel Coast [13]. It is a soft, hairy, and small stemless annual herb. In India, ten species have been recorded, of which P. ovata is an important plant for its seeds [14,15]. The common name of P. ovata is Isapghol, which is derived from two Persian words—‘Isap,’ meaning a horse ear, and ‘Ghol,’ referring to the characteristic shape of its seed. The procedure and time of collection for the seeds include the spikes harvested in March–April, when they turn red. Usually, harvesting is completed in the early morning when a little dew is present, which prevents seed shedding. The material collected is threshed and winnowed and then sieved repeatedly until the seed is clear. For their preservation and storage, including the seeds, they are further cleaned and allowed to dry and then stored in airtight containers in cool and dry places [13]. The seeds are smooth, rosy-white, boat-shaped, ovoid-oblong, and convex on one side and concave on the other side. The outer layer of the ovule of the seed fuses with the inner epidermis on the concave side and forms a thin white membrane—the seed coat [15]. The microscopic longitudinal section is an oblong, elliptical and transverse section of seed that is oval in outline. The transverse section, cut through on one end of the seed, shows a central core of the radicle surrounded by the endosperm, whereas the other end shows two fleshy cotyledons. The seed coat structure is simple. The epidermis of the testa is composed of polyhedral cells, the walls of which are thickened by secondary deposits—the source of mucilage. In between the epidermis and the albumin, a thin brownish layer is found. Albumin is formed from thick-walled cells that are rich in matters such as fixed oil and proteins. The cells of the embryo are parenchymatous and packed with aleurone grains. The physicochemical parameters show that the organic chemical constituents present in psyllium seeds are protein, tannin, glycosides, fixed oils, and carbohydrates. The inorganic constituents are iron, zinc, potassium, and sodium [13]. It consists of various phytoconstituents such as pentosans, aldobionic acid, arabinose, galacturonic acid, rhamnose, fixed oil, proteins, xylose, galactose, uronic acid, glycoside-aucubin, tannin, and an active principle resembling acetylcholine. It also contains amino acids including alanine, glutamic acid, glycine, leucine, valine, lysine, and tyrosine, and fatty acids such as oleic, linoleic, and palmitic acids [16]. Its seeds are useful in hypercholesterolemia, gastrointestinal mucous-membrane inflammatory conditions and the genitourinary tract [14]. Pharmacologically, psyllium has been proven to have antibacterial, analgesic, antipyretic, and anti-inflammatory properties, and nephroprotective and hepatoprotective properties [17] in mice. Other studies on P. ovata have also proven it to have antimicrobial [18,19], anti-inflammatory, antipyretic [20,21] and antioxidant activities [22]. The aucubin in the seeds is responsible for its antibacterial properties. Plantamajoside, a bioactive molecule present in P. ovata, has been proven to have antibacterial activities [23].
Linum usitatissimum L. belongs to the Linaceae family. It is cultivated throughout the plains of India and up to altitudes of 2000 m above sea level [24]. Its seeds are commonly known as linseeds. The seeds contain two embryos: a thin endosperm and an embryo axis [25]. The testa is the seed coat or true hull. The seeds are about 4–6 mm in length and 2–2.5 mm in width. They are brown, elongated, ovate, flattened, rounded at one end, and obliquely pointed at the other end [26]. The procedure and time of collection is at the end of June and before the capsules or bolls ripen; the plants are heaved by hand, made up into stocks and left to dry in the field. The seeds are separated after the rippling process by threshing and winnowing the fruits. The seeds that are obtained are preserved and stored after drying and stored in a cool and dry place in air-tight containers. The organic chemical constituents are glucoside, oil, mucilage, protein, and tannin, and the inorganic constituents include potassium, magnesium, calcium, and sodium [24]. The taxonomy of the physicochemical parameters is summarized in Table 1 and Table 2, respectively. It also contains lignans, secoisolariciresinol diglucoside, alpha-linolenic acid, omega-3 fat [27], 30–40% fixed oil, 6% mucilage, and 25% protein. The two major fractions of the seed polysaccharides are aneutralarabinoxylan (75%) and acidic rhamnogalacturonan (25%). Linseed also contains amino acids such as threonine, lysine, and tyrosine [28]. In addition, it also contains tocopherols α, β, and γ forms as well as niacin [29]. It also contains 30.41% insoluble fibres and 10.22% soluble fibres [30]. The seeds also contain cyanogenic glycosides (prussic acid) [31]. Secoisolariciresinol diglucoside (SDG) is a phytoestrogen known to possess many favourable actions on human health and also contains antiviral, antibacterial, and antifungal properties [32]. Its seeds have a cancer-preventative effect and are effective for atherosclerosis, hypercholesterolemia, and chronic renal diseases because of the presence of an active principle-lignin secoisolariciresinol diglycoside [14,31]. Linseeds have been proven to have antimicrobial [30,33,34,35], anti-inflammatory, analgesic, antipyretic [36], and antioxidant activities [30]. Similarly, the antimicrobial activity of L. usitatissimum is mostly attributed to flavonoids, phenolic acid, and lignans [34].
Table 1.
Taxonomy of psyllium and linseeds.
| Taxonomy | Psyllium | Linseeds | Reference |
|---|---|---|---|
| Kingdom | Plantae | Plantae | [39,40] |
| Subkingdom | Viridiplantae | Viridiplantae | |
| Infrakingdom | Streptophyta | Streptophyta | |
| Superdivision | Embryophyta | Embryophyta | |
| Division | Tracheophyta | Tracheophyta | |
| Subdivision | Spermatophytina | Spermatophytina | |
| Class | Magnoliopsida | Magnoliopsida | |
| Superorder | Asteranae | Rosanae | |
| Order | Lamiales | Malpighiales | |
| Family | Plantaginaceae | Linaceae | |
| Genus | Plantago | Linum | |
| Species | Plantago ovata Forssk. | Linum usitatissimum L. |
Table 2.
Physicochemical parameters of whole seeds of psyllium and linseeds.
| Parameters | Psyllium | Linseeds | Reference |
|---|---|---|---|
| Ash value (%) | |||
| Total ash | 3.47 | 6.84 | [13,24] |
| Acid insoluble ash | 0.90 | 3.76 | |
| Water soluble ash | 1.10 | 0.41 | |
| Successive extractive value (%) | |||
| Petroleum ether | 4.22 | 43.0 | |
| Chloroform | 1.40 | 1.1 | |
| Ethanol | 2.45 | 4.91 | |
| Water | - | 2.36 | |
| Moisture content (%) | 4.45 | 5.65 | |
| Mucilage | 22% | 4.46% | [41,42] |
| Total phenolics mgGAE/g | 286.8 ± 6.05 | 7.24 ± 0.14 | [21,42] |
| Total flavonoids | 101.83 ± 12.9 mgRE/g | 1.74 ± 0.57 mg QE/g | [21,42] |
| Total tannins | 39.33 ± 1.52 mgCE/g | 1.35 ± 0.03 | [21,42] |
Through repeated digestion and regurgitation, honey is formed by foraging bees that gather flower nectar and process it. The enzymatic activities of the honey bee and its stomach’s acidic pH, together with diastase, invertase, and amylase, make a supersaturated aqueous solution composed of 80% sugars—mainly fructose and glucose, with minor amounts of maltose, sucrose, and other complex sugars. The most abundant peptides are defensin-1 and royal jelly protein isoforms, while the major enzymes include diastase (amylase), glucose oxidase, catalase, α-glucosidase, and acid phosphatase [37,38]. The attributable numerous bioactive properties of honey are due to the miscellaneous composition of honey [38]. The aforementioned enzymes as well as phenolic acids and flavonoids are related to its antioxidant activity [38].
Honey bioactive molecules include methylglyoxal, phenolics, royal jelly proteins (MRJPs), and oligosaccharides. In royal jelly, there are royalizing peptides, antimicrobial jellein MRJPs, and hydroxy-decenoic acid derivatives, notably 10-hydroxy-2-decenoic acid (10-HDA) [37]. Honey has anti-inflammatory, antimicrobial, and antioxidant properties, and therefore has a possible therapeutic role in the treatment of various diseases. The physicochemical parameters of honey are summarized in Table 3.
Table 3.
Physicochemical parameters of honey.
| Parameters | Values | Reference |
|---|---|---|
| Refractive index at 20 °C | 1.485 | [43] |
| Ash (%) | 0.28 | |
| Moisture (%) | 20.83 | |
| Total Soluble Solids (°Brix) | 77.65 | |
| P fund (mm) | 103 | |
| Colour | Light Amber | |
| Specific gravity | 1.40 | |
| pH | 4.06 | |
| Electrical conductivity (dS/m) | 0.77 | |
| Total reducing sugar (%) | 69.36 | |
| Sucrose (%) | 2.94 | |
| Glucose | 33.77 | |
| Fructose | 35.59 | |
| Hydroxy Methyl Furfural | 27.30 |
The main two bioactive molecules [44], polyphenols and flavonoids, act as antioxidants and are present in honey [38,45,46]. The taxonomy and physicochemical parameters of psyllium, linseeds, and honey are summarized in Table 1, Table 2 and Table 3. However, to date, these plant materials with honey have not been studied clinically to treat uPID, even though few studies related to complementary and alternative medicines have been conducted [9,47,48]. Hence, we evaluated and compared the efficacy of the vaginal application of a formulation prepared with L. usitatissimum, P. ovata, and honey [12] on uPID with standard drugs, doxycycline, and metronidazole. In addition, we also used the same machine learning models [49,50,51,52,53,54] including Decision Tree (DT) [55,56,57], Random Forest (RF) [58,59], Logistic Regression (LR), and AdaBoost (AB) with three cross-validation models including 2-fold, 5-fold, and 10-fold to classify the standard and pessary groups. The main contributions of this study are given below:
The pessary of psyllium and linseed with honey is beneficial for PID but has not been validated to date.
The pessary group was beneficial, safe, and cost-effective in curing uPID as they possess anti-inflammatory, antimicrobial, analgesic, and antioxidant activities as proven in in vitro and in vivo studies.
The design of an automatic classification model based on the experimental analysis of the pessary and standard groups on uPID occurring in reproductive-age women.
The design of a detection system based on medicine using a machine-learning classifier.
This paper is organized based on the CONSORT guidelines for randomized controlled trials, as are the introduction, materials and methods, results, discussion and conclusion of the study.
2. Materials and Methods
2.1. Study Design and Population
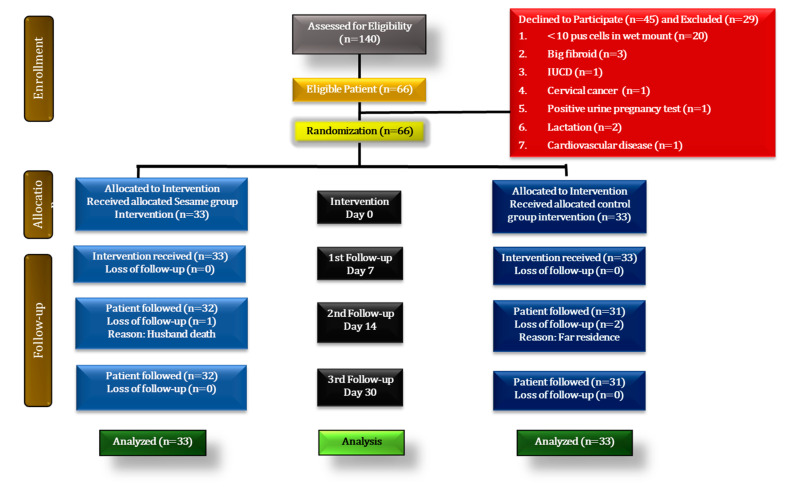
This study was a prospective, double-dummy, single-centre, parallel, and single-blind randomized controlled trial (RCT) conducted at our hospital. A total of 140 participants were examined for uPID. Out of 140 women, 74 were excluded from the study after preliminary screening and investigations (Figure 1). Then, 66 participants were randomly allocated to the pessary (n = 33) and standard (n = 33) groups with a 10% dropout after fulfilling the inclusion criteria. The first participant was recruited on February 18, 2017, and the last follow-up was completed on 8 January 2018.
Figure 1.
Consort statement for enrolment of participants.
Married women aged 18–45 years who were diagnosed with any of the clinical features of uPID as per classical Unani texts [60] (abnormal vaginal discharge, adnexal and cervical motion tenderness, low backache, pelvic discomfort, dysuria, lower abdominal pain, dyspareunia) were included [7]. In addition, participants were also included based on presence of the one of the minimum criteria (lower abdominal pain, bilateral adnexal tenderness, and cervical motion tenderness) for diagnosis of PID as per Centre for Disease Control (CDC) guidelines. Participants with uPID were defined as having a vaginal mucopurulent yellow or green discharge, abnormal cervical discharge or observed on the cervix, and the presence of leucorrhea defined as WBC > 10/HPF in the saline microscopy of vaginal fluid as additional criteria [6,61].
Those excluded were pregnant or lactating women; women with pelvic or tubo-ovarian abscess and/or any problem expected to require surgical intervention within 24 h of the start of treatment; the use of systemic antibacterial therapy less than 7 days before enrolment; a history of cardiovascular abnormalities, epilepsy, or impaired liver and renal function; any history of pelvic, uterine or abdominal surgery ≤30 days before treatment; an inability or intolerance to an oral antibiotic regimen, and a delivery or abortion within the last three months.
2.2. Data Collection and Assessment Tools
2.2.1. Data Collection
At the pre-study screening, we diagnosed women with uPID as shown by clinical features and one of the three minimum criteria from the CDC, abnormal vaginal discharge or cervicitis, and >10 WBC/HPF in the saline microscopy of vaginal fluid. At the baseline visit, relevant sociodemographic data, physical examination, and gynaecological examination were performed.
At baseline, the Visual Analogue Scale (VAS) for pain intensity to assess lower abdomen pain and lower back ache was used [61]. The VAS score is the most commonly used validated tool [48]. Participants were requested to mark on a colour-coding scale the VAS score that best described the actual status of their symptoms before the pelvic examination. The modified McCormack Pain Scale (McPS) was used to assess vaginal motion tenderness [47]. The amount of white discharge was estimated as absent (0), scanty (1) (normal moistness of the vagina and labia minora without staining or moistening the underclothes), moderate (2) (underclothes are soiled and require changing and washing frequently), or profuse (3) (requires the wearing of an extra pad, diaper or tampon) [62]. Clinical features such as dysuria, dyspareunia, and pruritus vulvae were considered present and relieved at baseline and follow-up. The SF-12 questionnaire was used to evaluate improvement in quality of life (QoL). The total Physical Composite Score (PCS) and Mental health Composite Score (MCS) of SF-12 HRQoL were calculated by utilizing the scores of twelve questions that ranged from 0 to 100; a score of 0 designated the lowest level of health, whereas 100 indicated the highest level of health.
Each patient was subjected to haematological biomarkers such as complete blood pressure, random blood sugar, erythrocyte sedimentation rate, and serological biomarkers, viz., HIV, VDRL, C-reactive protein, and complete urine analysis. The safety biochemical markers included blood urea, serum creatinine, phosphatase, aspartate transaminase, and alanine transaminase. Specific investigations such as pelvic ultrasonography, wet mount test (saline microscopy of vaginal discharge for WBC/HPF), and Pap smear were also performed to diagnose PID and its complications such as pelvic or tubo-ovarian abscess and cervical malignancy, respectively. A wet mount test was considered positive if WBC ≥ 10/HPF in vaginal discharge [48]. In known cases of controlled hypertension and hypothyroid participants, concomitant medication was continued as usual; however, the use of other concomitant medications that may have affected the outcome during the research period was not allowed.
2.2.2. Assessment of Safety of Pessary and Standard Groups
The safety assessment included clinical features, examination and safety biomarkers of kidney and liver function tests to detect hepatotoxicity and nephrotoxicity at post-intervention (day 14). During follow-up, each participant was questioned regarding side effects such as local irritation, itching, and burning sensation after inserting the medicated tampon.
2.2.3. Assessment of Haematological and Biochemical Markers
Blood from each participant was collected at the pre-screening visit. At room temperature, the collected blood was allowed to clot for 15–30 min, centrifuging the clotted blood at 3000–3500 rpm for 5–10 min separated the serum from it, and a valuation of biochemical markers was performed. Venous blood was collected in an EDTA tube for hemogram testing and was done by a COUNCELL V3 plus Automated haematology analyser. For the estimation of biochemical markers, a Chem 200 Technology Automated Biochemistry Analyzer was used. Glucose was estimated by the Glucose oxidase/peroxidase method [63]. ALT and AST levels were estimated by the UV-IFCC method [63]. The alkaline phosphatase level was estimated by 2-Amino-2-Methyl-1-propanol Buffer (IFCC) [64]. Serum urea level was valued by the Urease/Glutamate Dehydrogenase method [63]. Serum Creatinine level was estimated by the Picratte Method [65].
2.2.4. Follow-Up Assessment and Withdrawal Criteria
Participants came for a follow up on day 7 and day 14 during the treatment period and one follow up on day 30 without treatment. They were also questioned in a phone call on day 3 and day 5 of treatment. At each visit, the change in clinical features, the McCormack pain scale, the VAS pain scale, and the HRQoL of the women were recorded. The participants who failed to follow the trial protocol and showed signs of any drug adverse reactions were withdrawn.
2.3. Intervention Used in Both Groups
2.3.1. Selection and Identification
The Unani formulation for validation of uPID was carefully chosen from the Unani Pharmacopoeia [12]. Our institute’s pharmacy provided all trial-related medicines. The plant materials were directly procured from the local market by the institute’s pharmacy one week before the initiation of the trial. Then, these plant materials were sent for identification and authentication. We had procured certified honey from the Apiculture department, Gandhi Krishi Vigyana Kendra (GKVK), University of Agricultural Sciences, Government Institute, Bengaluru. The plant materials were identified as Linum usitatissimum L. whole seed and Plantago ovata Forsk whole seed at the Centre for Repository of Medicinal Resources, Trans-Disciplinary University, Bengaluru. For further reference, the specimens after identification were submitted to the institute with the voucher specimens (51/UQ/Res/2017).
2.3.2. Method of Preparation, Dispensing, and Training of the Participants
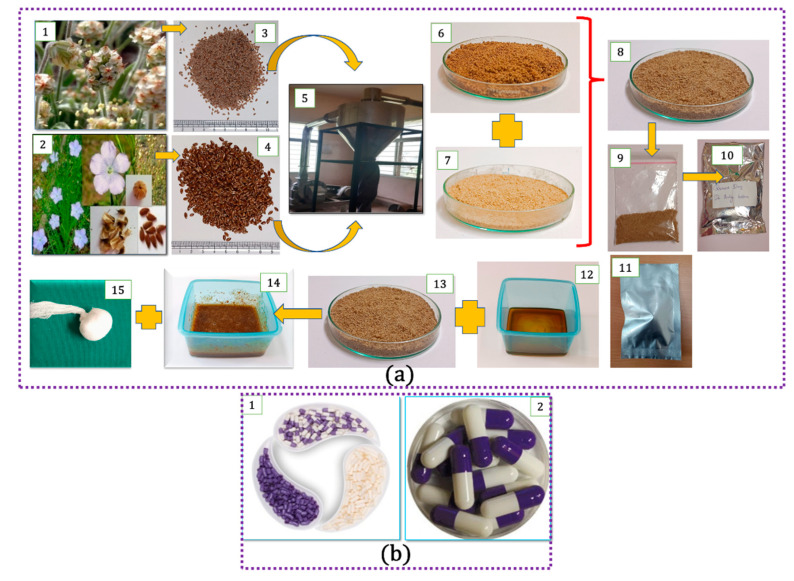
Seeds were cleaned and powdered in a hammer mill separately and passed through Mesh No. 100. The powder was filled into small airtight pouches (Figure 2).
Figure 2.
Depiction of pessary preparation: (a) (1) Plantago ovata plant; (2) Linum usitatissimum L. plant, seeds; (3) Plantago ovata plant, seeds; (4) Linum usitatissimum L plant, seeds; (5) powder-making machine; (6) powder of Linseeds; (7) powder of P. ovata; (8) mixed powder; (9) dispensing single dose in sachet; (10) sachets packed in the aluminium pouch; (11) dispensing of tampon; (12) honey; (13) mixed powder; (14) powder mixed with honey; (15) impregnation on tampon unfilled; and (b) (1) Unfilled and (2) filled placebo capsules.
Each participant received 14 sachets in an aluminium pouch, each of which contained 6 gm of a mixed powder of both medicines and 70 mL of honey in a glass bottle. Each participant also received 14 sterilized cotton tampons individually packed in an aluminium pouch. A measuring container was also supplied to the participant. Each participant was trained on how to pour 5 mL of honey into a measuring container and add one sachet of powder in the container and mix it well. Then, she was advised to impregnate the tampon and insert it deeply into the vagina at bedtime and was advised to remove it in the morning. The capsules (500 mg) were filled with microcrystalline edible cellulose powder. Each participant received an individual pack containing 28 capsules dispensed in non-transparent aluminium pouches.
2.3.3. Route of Administration and Dosage
Placebo: two capsules orally BID plus per vaginum tampon (cotton pessary/hamul) of fine powder of L. usitatissimum seeds (3 gm) and Plantago ovata seeds (3 gm) with honey (5 mL) at bedtime for 14 days was advised as per the traditional method in Unani classical text.
2.3.4. Standard Controlled Group
For 14 days, each participant received doxycycline 100 mg twice daily and metronidazole 400 mg three times daily [66], as well as a placebo (palm sugar) vaginal tampon at bedtime.
2.4. Outcomes
For primary outcome measures, changes in clinical features (abnormal vaginal discharge and other symptoms), VAS pain scale for LAP and LBA and McCormack pain scale were assessed. The primary endpoint included a change in clinical response, defined as a 60% or greater reduction in vaginal discharge, VAS total score for pain intensity, and McPS score at day 14 when compared to baseline [61]. The percentage reduction (%R) score was calculated in Equation (1) below:
| (1) |
For secondary outcomes, changes in WBC cells in saline microscopy of vaginal discharge from the wet mount test [61] and SF-12 health survey HRQoL questionnaire [67] were assessed. The secondary endpoint included clinical cure defined as WBC < 10/HPF in the saline microscopy of the vaginal discharge from a wet mount test and a 60% or more improvement in HRQoL.
2.5. Sample Size Estimation
The sample size estimation was carried out after a discussion with a biostatistician. The present study was of two groups, so the sample size was calculated around the mean difference of the pelvic tenderness score. The mean and SD were taken from the previous study mean (µ1 = 1.05 and µ2 = 0.50) and standard deviation (σ1 = 1.05 and σ2 = 0.82) [48]. The level of significance was p < 0.05. For a confidence interval of 95% and a power of the study of 90%, the following formula was used to calculate the sample size. As a result, it is calculated in Equations (2) and (3) below:
| (2) |
| (3) |
The sample size required in each group was 24. We included 33 participants in each group considering 10% dropout.
2.6. Randomization, Allocation, Blinding, and Treatment Adherence
Participants were randomly assigned into the pessary (n = 33) and standard (n = 33) groups after fulfilling the inclusion criteria. A computer-generated open list of a random number in a single block with a sequence of random assignments was used for simple randomization. However, the order of randomization was masked from the first researcher until the interventions were allocated to each participant. The double-dummy technique was used for masking and blinding. The medicine was dispensed in non-transparent aluminium pouches for masking. The participants were blinded. The participants were asked to bring back the empty pouches, and the number of unused sachets returned was counted and recorded on day 14 to check for compliance with the treatment. The data collection method for the sachet adherence measurement was similar to the pill count. If a participant failed to return dispensed sachets as a dropout, then it was assumed that sachets were not used and it was treated in the same way as returns. This variable was coded as 0% adherence if the participant dropped out of the treatment in the first post-randomization of the study and did not once return any sachets [68,69]. The formula used to calculate the compliance is in Equation (4) below:
| (4) |
2.7. Statistical Methods
The statistical software SPSS 18.0 version 3.2.2 was used for data analysis. The results were presented in terms of mean ± SD for continuous variables and number (%) of categorical measurements. The alpha was 5% with a 95% confidence interval with a two-sided p-value. The Fisher’s or Chi-square test was applied for the comparison of the proportions wherever necessary. An intragroup comparison for normally distributed data, a paired Student’s t-test, and for skewed data, a Wilcoxon matched paired test was performed. The analysis included the student’s t-test (normally distributed data) and the Mann–Whitney U test (skewed data) for intergroup comparison. The intention-to-treat principle uses data from all randomized subjects with at least two post-randomization outcome measures for all efficacy variables. The last observation carried forward method was performed to impute missing data. The significance level was described as p > 0.05 (not significant) and p < 0.001 (extremely significant).
3. Results
3.1. Sociodemographic Characteristics
The study groups were homogenous, with no statistically significant differences between the pessary and standard groups (p > 0.05) in terms of age (31.36 ± 5.65 vs. 30.79 ± 5.40 years), age of menarche (12.63 ± 6.25 vs. 12.47 ± 5.78 years), menstrual cycle (29.72 ± 3.99 vs. 28.54 ± 3.77 days), duration of menstrual flow (4.63 ± 1.13 vs. 4.75 ± 0.96 days), duration of marriage (12.63 ± 6.25 vs. 12.46 ± 5.39 years) and body mass index (27.14 ± 4.73 vs. 26.16 ± 5.39 Kg/m2). In the pessary and standard groups, 18 (54.5%) and 17 (51.5%) participants were from the upper or lower class, respectively. In pessary and standard groups, 12 (36.4%) vs. 8 (24.42%) and 3 (9.1%) vs. 5 (15.2%) participants were from the lower and upper middle classes, respectively.
3.2. Primary Outcomes
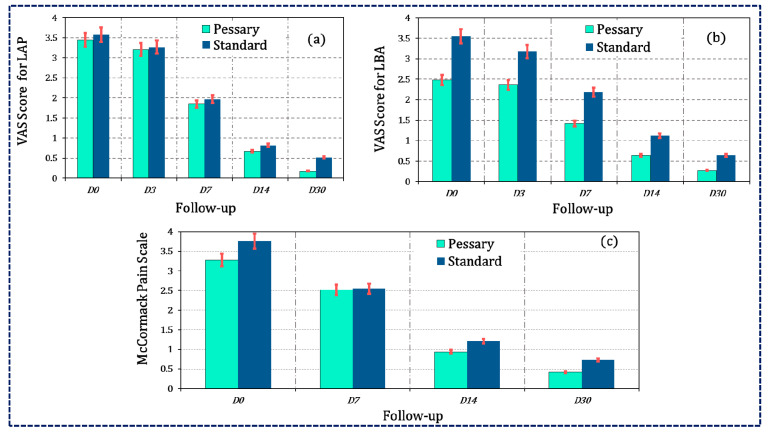
Between the groups, visual analogue scale score, modified McCormack pain scale (Table 4 and Figure 3), and the comparison for clinical features (Table 5) were statistically insignificant, (p > 0.05) on day 0 and each follow up, whereas the intragroup comparison at day 14 compared to day 0 was statistically extremely significant (p < 0.001) in both the groups. The clinical response was more than 70% for all primary outcomes.
Table 4.
The primary outcome measure based on VAS score for lower abdominal pain, lower backache (LBA), and Modified McCormack (McPS) for pelvic tenderness.
| VAS for Lower Abdominal Pain | Pessary Group (n = 33) | Standard Group (n = 33) | p-Value |
|---|---|---|---|
| D0 | 3.45 ± 2.21 | 3.58 ± 2.65 | 0.841 |
| D7 | 1.85 ± 1.46 | 1.97 ± 1.78 | 0.763 |
| D14 | 0.67 ± 0.89 * | 0.82 ± 1.33 ** | 0.589 |
| D30 | 0.18 ± 0.46 * | 0.52 ± 1.09 ** | 0.112 |
| %Pain Reduction (PR) | 80.57% | 77.09% | |
| VAS for lower back ache | |||
| D0 | 2.48 ± 2.29 | 3.55 ± 2.69 | 0.090 |
| D7 | 1.42 ± 1.44 | 2.18 ± 1.98 | 0.080 |
| D14 | 0.64 ± 0.93 * | 1.12 ± 1.47 ** | 0.115 |
| D30 | 0.27 ± 0.63 * | 0.64 ± 1.08 ** | 0.100 |
| % Pain Reduction PR | 74.19% | 68.54% | |
| Modified McCormack Pain Scale (McPS) for pelvic tenderness | |||
| D0 | 3.82 ± 1.59 | 3.76 ± 1.50 | 0.874 |
| D7 | 2.52 ± 1.20 | 2.55 ± 1.00 | 0.912 |
| D14 | 0.94 ± 1.00 * | 1.21 ± 1.11 ** | 0.298 |
| D30 | 0.42 ± 0.66 * | 0.73 ± 1.01 ** | 0.154 |
| % Pain Reduction (PR) | 75.39% | 67.81% | |
* p < 0.001 and ** p < 0.001 on day 14 and day 30 from day 0 in the pessary group and standard group respectively; unpaired ‘t’ test and paired ‘t’ test; % PR: % pain reduction at day 14 compared to day 0.
Figure 3.
Graphical depiction of (a) VAS score for LAP, (b) VAS score for LBA, and (c) McCormack pain scale for pelvic tenderness.
Table 5.
The primary outcome measures are based on abnormal vaginal discharge and associated symptoms of uPID.
| Abnormal Vaginal Discharge (AVD) | Pessary Group (n = 33) | Standard Group (n = 33) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | 2.78 ± 0.41 | 2.72 ± 0.51 | 0.79 | ||||||
| Day 14 | 0.36 ± 0.54 | 0.60 ± 0.86 | 0.39 | ||||||
| p value | <0.0001 | <0.0001 | |||||||
| % AVD reduction | 87.05% | 77.94% | |||||||
| Associated symptoms | Present | Relieved | Present | Relieved | |||||
| Dyspareunia | |||||||||
| Day 0 | 21 (100) | 0 | 18 (100) | 0 | |||||
| Day 14 | 3 (14.28) | 18 (85.71) | 4 (22.22) | 14 (77.77) | 0.41 | ||||
| p value | <0.0001 | <0.0001 | |||||||
| Dysuria | |||||||||
| Day 0 | 10 (100) | 0 | 10 (100) | 0 (0) | |||||
| Day 14 | 0 (0) | 10 (100) | 2 (20) | 8 (80) | 0.23 | ||||
| Pruritus vulvae | 0 | ||||||||
| Day 0 | 23 (100) | 0 | 19 (100) | 0 | |||||
| Day 14 | 0 | 23 (100) | 3 (15.78) | 16 (84.21) | 0.08 | ||||
| AVD as per severity grading | |||||||||
| AVD Grading | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | -- |
| Day 0 | 0 | 0 | 7 (21.2) | 26 (78.8) | 0 | 1 (3) | 7 (21.2) | 25 (75.8) | - |
| Day 14 | 22 (66.7) | 10 (30.3) | 1 (3) | 0 * | 19 (57.6) | 10 (30.3) | 2 (6.1) | 2 (6.1) ** | - |
| Day 30 | 28 (84.8) | 5 (15.2) | 0 | 0 * | 25 (75.8) | 5 (15.2) | 2 (6.1) | 1 (3) ** | - |
* p < 0.001 and ** p < 0.001 on day 14 and day 30 from day 0 in the pessary group and standard group respectively; unpaired ‘t’ test and paired ‘t’ test; % PR: % pain reduction at day 14 compared to day 0. Chi-square test and Fisher Exact test; AVD as per severity grading: 0—absent; 1—mild; 2—moderate and 3—profuse.
3.3. Secondary Outcomes
3.3.1. WBC Count on Saline Microscopy of Vaginal Discharge
The WBC count in the wet mount in the pessary and standard groups on day 0 was 31 ± 11.14 and 36.36 ± 13.00/HPF on saline microscopy, respectively, and was statistically insignificant (p > 0.05), whereas on day 14, it was 4.69 ± 8.21 and 6.03 ± 8.97, respectively, and was also statistically insignificant (p > 0.05). The intragroup comparison was statistically significant with p < 0.0001 in both groups. The percent reduction in the pessary and standard groups on day 14 was 87.09% and 83.41%, respectively.
In the pessary group on day 0, 33.3% (n = 11/33), 30.3% (n = 10/33), 24.2% (n = 8/33), and 12.1% (n = 4/33) showed 11–20, 21–30, 31–40, and >40 WBC/HPF in the saline microscopy, respectively. On day 14, 45.5% (n = 15/33) had no WBC cells and 45.5% (n = 15/33) had 1–10 WBC/HPF, respectively. Three percent (n = 1/33) each showed 11–20, 21–30, and 31–40 WBC/HPF in the saline microscopy, with p < 0.0001 on day 14 from day 0. In the standard group on day 0, 15.2% (n = 5/33), 30.3% (n = 10/33), 30.3% (n = 10/33), and 24.2% (n = 8/33) had WBC/HPF values of 11–20, 21–30, 31–40, and > 40, respectively. On day 14, 0% (n = 0/33), 84.8% (n = 28/33), 6.1% (n = 2/33), 6.1% (n = 2/33), and 3% (n = 1/33) showed no WBC, 1–10, 11–20, 21–30, and 31–40 WBC/HPF, respectively, with p < 0.0001 from day 0.
3.3.2. Heath-Related QoL Assessed by SF-12 Questionnaire
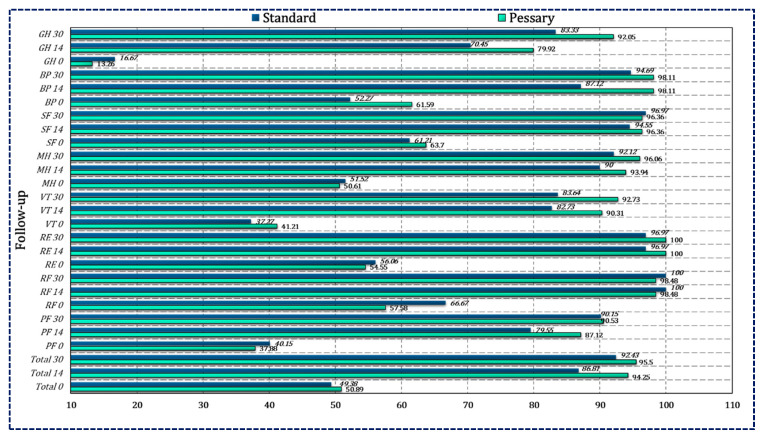
The intragroup and intergroup comparison for the total score, eight domains of the SF-12, and the PCS and MCS scores were compared on days 0, 14 and 30. Table 6 summarizes the results of the SF-12 questionnaire. Between the groups, the comparison for vitality was statistically significant at day 30 (p < 0.037). The improvement of the SF-12 score was more than 70% in both groups (Figure 4).
Table 6.
The secondary outcome measure based on SF-12 HRQoL.
| Domains SF-12 HRQoL | Period | Pessary Group (n = 33) | Standard Group (n = 33) | p-Value |
|---|---|---|---|---|
| Total score | Day 0 | 50.89 ± 23.38 | 49.38 ± 26.85 | 0.808 |
| Day 14 | 94.25 ± 5.80 a | 86.81 ± 13.14 b | 0.004 | |
| Day 30 | 95.50 ± 6.86 a | 92.43 ± 11.88 b | 0.203 | |
| Physical function (PF) | Day 0 | 37.88 ± 35.42 | 40.15 ± 33.62 | 0.79 |
| Day 14 | 87.12 ± 19.64 a | 79.55 ± 19.22 b | 0.118 | |
| Day 30 | 90.53 ± 18.50 a | 90.15 ± 18.69 b | 0.934 | |
| Role limitation due to Physical Problems (RF) | Day 0 | 57.58 ± 25.38 | 66.67 ± 27 | 0.164 |
| Day 14 | 98.48 ± 8.70 a | 100.00 ± 0.00 b | 0.321 | |
| Day 30 | 98.48 ± 8.70 a | 100.00 ± 0.00 b | 0.321 | |
| Role limitation due to Emotional Problems (RE) | Day 0 | 54.55 ± 38.25 | 56.06 ± 39.05 | 0.874 |
| Day 14 | 100.00 ± 0.00 a | 96.97 ± 17.41 b | 0.321 | |
| Day 30 | 100.00 ± 0.00 a | 96.97 ± 17.41 b | 0.321 | |
| Energy/Fatigue/Vitality (VT) | Day 0 | 41.21 ± 29.97 | 37.27 ± 33.66 | 0.617 |
| Day 14 | 90.30 ± 11.32 a | 82.73 ± 18.59 b | 0.05 | |
| Day 30 | 92.73 ± 10.98 a | 83.64 ± 21.91 b | 0.037 | |
| Emotional well-being Mental Health (MH) | Day 0 | 50.61 ± 30.1 | 51.52 ± 32.32 | 0.906 |
| Day 14 | 93.94 ± 9.33 a | 90.00 ± 12.25 b | 0.147 | |
| Day30 | 96.06 ± 8.99 a | 92.12 ± 12.19 b | 0.14 | |
| Social Functioning (SF) | Day 0 | 63.70 ± 33.69 | 61.21 ± 38.39 | 0.781 |
| Day 14 | 96.36 ± 12.45 a | 94.55 ± 9.05 b | 0.5 | |
| Day 30 | 96.36 ± 12.45 a | 96.97 ± 7.28 b | 0.81 | |
| Bodily Pain (BP) | Day 0 | 61.59 ± 26.07 | 52.27 ± 28.89 | 0.174 |
| Day 14 | 98.11 ± 10.88 a | 87.12 ± 17.81 b | 0.004 | |
| Day 30 | 98.11 ± 10.88 a | 94.69 ± 12.1 b | 0.39 | |
| General Health (GH) | Day 0 | 13.26 ± 16.52 | 16.67 ± 20.41 | 0.459 |
| Day 14 | 79.92 ± 18.73 a | 70.45 ± 19.22 b | 0.047 | |
| Day30 | 92.05 ± 18.70 a | 83.33 ± 20.41 b | 0.075 |
Paired and Unpaired ‘t’ Test; a p < 0.001 and b p < 0.001 on day 14 and day 30 from day 0 in the pessary and standard groups, respectively.
Figure 4.
Comparative analysis between both standard and pessary groups based on SF-12 HRQoL at each follow-up.
3.4. Haematological and Biochemical Markers for Safety Parameters
None of the participants complained of any local irritation, itching, or burning sensation. The haematological and biochemical markers for the safety of the liver and kidneys and other investigations are summarized in Table 7. The statistical difference was found to be insignificant for all investigations before and after treatment. Further, none of the participants in either group reported any serious adverse events. In addition, Section 4 explains in detail the hepatoprotective and nephroprotective in vitro and in vivo research conducted for the safety of linseeds and psyllium.
Table 7.
Haematological and biochemical markers in the pessary and standard group.
| Variables | Period | Pessary Group (n = 33) | Standard Group (n = 33) | p-Value |
|---|---|---|---|---|
| Random Blood Sugar (mg %) | Day 0 | 99.76 ± 12.97 | 99.88 ± 14.74 | 0.972 |
| Erythrocyte Sedimentation Rate (mm/1 h) | Day 0 | 35.85 ± 13.54 | 33.70 ± 14.59 | 0.537 |
| Day 14 | 30.91 ± 10.09 a | 35.36 ± 14.37 b | 0.15 | |
| Safety biomarkers | ||||
| Haemoglobin (Hb%) (gm%) | Day 0 | 11.35 ± 1.48 | 11.21 ± 1.15 | 0.665 |
| Day 14 | 11.17 ± 1.33 a | 11.26 ± 1.09 b | 0.779 | |
| Total Leucocyte Counts (cells/cumm) | Day 0 | 6515.15 ± 1439.21 | 6112.12 ± 1437.83 | 0.259 |
| Day 14 | 6309.09 ± 1673.20 a | 5975.75 ± 1267.4 b | 0.365 | |
| Aspartate aminotransferase (IU/L) | Day 0 | 22.28 ± 7.32 | 22.31 ± 9.52 | 0.988 |
| Day 14 | 21.33 ± 6.76 a | 22.24 ± 9.10 b | 0.647 | |
| Alanine transaminase (IU/L) | Day 0 | 24.62 ± 11.47 | 23.79 ± 11.3 | 0.769 |
| Day 14 | 23.76 ± 9.22 a | 24.97 ± 12.61 b | 0.657 | |
| Alkaline phosphatase (IU/L) | Day 0 | 199.60 ± 55.35 | 207.18 ± 42.04 | 0.533 |
| Day 14 | 204.52 ± 39.59 a | 202.27 ± 41.91 b | 0.824 | |
| Blood Urea (mg %) | Day 0 | 22.64 ± 5.63 | 23.12 ± 3.85 | 0.686 |
| Day 14 | 24.45 ± 3.59 a | 23.70 ± 4.21 b | 0.434 | |
| Serum Creatinine (mg %) | Day 0 | 0.76 ± 0.09 | 0.78 ± 0.10 | 0.52 |
| Day 14 | 0.76 ± 0.09 a | 0.75 ± 0.08 b | 0.579 | |
| Other investigations | ||||
| C-Reactive Protein | ||||
| Positive | Day 0 | 10 (100) | 8 (100) | 0.78 |
| Negative | Day 14 | 5 (50) | 5 (62.5) | 1.00 |
| Ultrasonography of abdomen | ||||
| Normal | - | 16 (48.5) | 21 (63.6) | 0.32 |
| PID | 17 (48.5) | 12 (36.4) | ||
| PH of vaginal discharge | Day 0 | 5.21 ± 1.05 | 4.70 ± 1.69 | 0.145 |
| Day 14 | 4.42 ± 0.71 | 3.48 ± 1.44 | 0.016 | |
| p value | 0.007 | 0.03 | ||
| Pap smear | ||||
| Normal | 6 (18.2) | 6 (18.2) | ||
| Inflammatory | Day 0 | 21 (63.6) | 24 (72.7) | 0.548 |
| Bacterial Vaginosis | 6 (18.2) | 3 (9.1) | ||
| Normal | 22 (66.7) | 10 (30.3) | ||
| Inflammatory | Day 14 | 11 (33.33) | 22 (66.7) | 0.003 |
| Bacterial Vaginosis | (0) | 1 (3) | ||
| p value | p < 0.001 | 0.365 | ||
Fisher’s exact test; a p > 0.05 and b p > 0.05 on day 14 from day 0 in the pessary and standard group respectively.
3.5. Classification of the Pessary and Standard Groups using Machine Learning Techniques
For the classification, we used four machine learning techniques such as DT, RF, LR, and AB with three cross-validation models including 2-fold, 5-fold, and 10-fold to classify the pessary and standard groups (Table 8). The standard mathematical expression of the performance evaluation methods such as sensitivity (SENS), specificity (SPEC), accuracy (ACC), and precision (PREC) [70,71,72,73,74] are mentioned in Equations (5)–(8):
Table 8.
Classification of the pessary and standard groups based on different machine learning models.
| Cross Validation-Fold | Classifier | SENS | SPEC | ACC | PREC |
|---|---|---|---|---|---|
| 2 | DT | 57.40 | 59.80 | 57.40 | 55.70 |
| RF | 55.90 | 58.40 | 55.90 | 54.20 | |
| LR | 57.50 | 54.20 | 51.50 | 50.00 | |
| AB | 48.50 | 56.80 | 48.50 | 48.50 | |
| 5 | DT | 61.80 | 63.90 | 61.80 | 60.00 |
| RF | 52.90 | 55.60 | 52.90 | 51.40 | |
| LR | 48.50 | 51.50 | 48.50 | 46.90 | |
| AB | 50.00 | 54.20 | 50.00 | 50.20 | |
| 10 | DT | 51.50 | 54.20 | 51.50 | 50.30 |
| RF | 58.80 | 61.20 | 58.80 | 57.30 | |
| LR | 52.90 | 55.60 | 52.90 | 51.20 | |
| AB | 50.00 | 54.20 | 50.00 | 49.70 | |
| mean | 53.30 | 56.63 | 53.30 | 52.11 | |
| ±SD | 4.27 | 3.55 | 4.27 | 3.87 | |
Bold number means highest classification performances.
| (5) |
| (6) |
| (7) |
| (8) |
In the 2-fold model, DT achieved maximum performance in terms of sensitivity (57.40%), specificity (59.80%), accuracy (57.40%), and precision (55.70%). Additionally, LR achieved minimum performance in terms of sensitivity (57.50%), specificity (54.20%), accuracy (51.50%), and precision (50.00%). In the 5-fold, DT achieved maximum performance in terms of sensitivity (61.80%), specificity (63.90%), accuracy (61.80%), and precision (60.00%). Additionally, LR achieved minimum performance in terms of sensitivity (48.50%), specificity (51.50%), accuracy (48.50%), and precision (46.90%). In 10-fold, RF achieved maximum performance in terms of sensitivity (58.80%), specificity (61.20%), accuracy (58.80%), and precision (57.30%). Additionally, AB achieved minimum performance in terms of sensitivity (50.00%), specificity (54.20%), accuracy (50.00%), and precision (49.70%). The average performance was also found in terms of sensitivity (53.30%), specificity (56.63%), accuracy (53.30%), and precision (52.11%). However, our DT 5-fold model was better for classification in this study.
4. Discussion
4.1. Major Findings
The present study confirmed that a formulation prepared with linseeds, psyllium seeds, and honey was a safe and effective alternative herbal treatment to control uPID clinically. The clinical response for the primary and secondary outcome measures of the pessary group was above 70%. The observed effects of the formulation may be attributed to the synergistic effect of all individual ingredients. Overall, the compliance of the participant in the pessary and the standard groups were 98.46% and 96.92%, respectively.
4.2. Outcome Parameters
Concerning the primary outcome parameters, abnormal vaginal discharge is reported in thirty per cent of the population of India. Reproductive tract infections (RTIs) are a silent epidemic that distresses women’s lives [75,76] in developing nations. Intergroup comparison in the VAS score for LAP and LBA was not significant (p > 0.05) on day 14. However, the pessary group showed a higher percentage reduction than the standard group in abnormal vaginal discharge (87.05% vs. 77.94%), VAS-LAP (80.57% vs. 77.09%), LBA (74.19% vs. 68.54%), and McPS score (75.39% vs. 67.81%). In this study, the pessary group showed a reduction in vaginal discharge of 87.05%, a VAS score for LAP of 80.57% and 74.19% for LBA, and a McPS score similar to the findings reported in the previous studies [47,48,61]. Saeedi et al. [69] observed a noteworthy decrease in the symptoms. Similarly, another study carried out to evaluate the role of Shivagutika for 60 days in PID concluded that Shivagutika efficiently decreases the symptoms and clinically controlled the infection [9]. Another study also reported amelioration of abnormal vaginal discharge, backache, and other clinical features related to endo cervicitis [77].
In the secondary outcome parameters, the reduction of the WBC count in the saline microscopy was significant, with p < 0.001 in both groups from baseline, whereas between the groups, the comparison was statistically insignificant. However, the percent reduction of the WBC count was higher in the pessary than in the standard group (87.09% vs. 83.41%). In a previous study, the use of Arq Mako and Sharbat Kasni showed a 76.6% reduction in the WBC count [47], which was comparatively less effective than the present study. Health-related QoL improvement was seen in both groups. The Pap smear showed inflammatory cells in 21 (63.6%) participants before treatment; after treatment, the Pap smear was normal in 10 (48.5%) participants in the pessary group. Similar findings were reported in a previous study [78]. Studies have revealed that pelvic inflammatory disease is associated with bacterial vaginosis. The Pap smear finding showed that, in all participants, uPID associated with bacterial vaginosis was cured in the pessary group. Kahkashan et al. [79] also reported the beneficial effect of the Unani medicine Pistacia integerrima for bacterial vaginosis. On day 14, the SF-12 total score was 94.25 ± 5.80 in the pessary and 86.81 ± 13.14 in the standard group, with a significant difference (p < 0.004). The pessary group exhibited a meaningfully higher improvement in HRQoL than the standard group. Haggerty et al. [80], in their study, reported the women with chronic pelvic pain after PID had lower QoL. They concluded that chronic pelvic pain has a significant impact on physical and mental function, which worsens as pain intensity increases. They also discussed that women had lower scores for general health, bodily pain, vitality, physical functioning, social functioning, and mental health. This finding completely correlates with the present study. Previous human studies have revealed that inflammation plays a significant role in pelvic inflammatory disease progression [81]. Studies have reported that L. usitatissimum has analgesic and anti-inflammatory properties [82]. The improvement in HRQoL can be accredited to the anti-inflammatory, antioxidant, analgesic [14] and immunomodulatory [83] properties of P. ovata. L. usitatissimum has analgesic [26] and antioxidant properties [27]. L. usitatissimum is well known for the presence of omega-3 fatty acids and vitamins, and has the highest source of dietary lignan [84], which helps in improving quality of life. It also has antidepressant and antipyretic properties [36].
4.3. Overall Interpretation
The amelioration of abnormal vaginal discharge, LAP, LBA, McPS, and the absence of pus cells in the wet mount was probably attributed to the traditional ethnomedicinal properties such as the anti-inflammatory (Muhallil al-Waram), antiseptic (Dafi’-i-Ta’affun) and analgesic (Musakkin) properties of P. ovata, L. usitatissimum and honey [11]. Further, P. ovata has Qabid (astringent), Mudirr-i-Bawl (diuretic) and Mulayyin (laxative) properties and is useful in inflammatory conditions of the genitourinary tract [14], and gonorrhoea [15]. Honey has anti-inflammatory, antioxidant, antimicrobial, and immunomodulatory activities [37].
4.4. Antimicrobial Activity of Bioactive Molecules of Linseeds, Psyllium, and Honey
A previous study revealed that the methanolic extract of P. ovata takes more significant action against Gram-positive bacteria [18]. Another study showed that the silver nanoparticles of P. ovata seed shows good antibacterial activity against Streptococcus aureus [19]. Fons et al. [85] reported that the Plantago species’ secondary bioactive metabolites, i.e., iridoids, phenol, sterols, polysaccharides, alkaloids, and coumarins, can be supplemented as drugs to treat human diseases. Furthermore, a range of biological activities such as wound healing activity [83], antioxidant [20], analgesic, anti-inflammatory, immune-modulating, and anti-ulcerogenic activities of P. ovata have been proven. The plantamajoside is a caffeic acid derivative mainly credited for its antibacterial activity [23].
L. usitatissimum has anti-inflammatory, analgesic, diuretic, and laxative properties [11]. The mucilaginous infusion “Linseed tea” is useful for gonococcal infection [15]. Various pharmacological studies have proven its analgesic, antipyretic [36], Gram-negative and Gram-positive antibacterial properties [32,86]. One study screened its inhibitory effects against four types of Gram-positive and -negative bacteria, namely S. aureus, B. cereus, K. pneumoniae, and P. aeruginosa, for four different extracts of L. usitatissimum seeds. The petroleum ether extract established considerable inhibitory effects against all four bacteria. The ethanol extract also showed significant antibacterial activities [26,34,86]. Linseed protein extract showed antibacterial activity against the selected micro-organisms, especially Gram-negative bacteria [35]. Another study similarly stated the high antibacterial properties of linseed extract against E. coli and S. aureus [35].
In a previous study, Bodhankar et al. [10] assessed twelve medicinal plants for antimicrobial activity against selected pathogens as a substitute treatment for PID. They concluded that the active components exhibited antimicrobial activity against tested pathogens (Streptococci species, S. aureus, Klebsiella, Salmonella and Candida albicans), and the plants’ bioactive compounds included flavonoids, saponins, terpenoids, alkaloids, phenols and fixed oil. Similarly, the antimicrobial activity of L. usitatissimum is mostly attributed to flavonoids, phenolic acid, and lignans [34].
Honey can kill various bacterial strains as it contains a very high content of methylglyoxal (MGO), equal to about 1500 mg/kg. In addition, it acts against methicillin-resistant S. aureus. Nevertheless, honey also contains various other unidentified compounds that have potential action and antibacterial activity. A study has shown that MGO acts against S. aureus and B. subtilis. Other antibacterial factors isolated from honey are glycoproteins with high-mannose N-glycans [37]. Honey’s enzymatic glucose oxidation reaction and some of its physical aspects are the main sources of antimicrobial activity. In addition, the antimicrobial activity of honey’s other factors includes high osmotic pressure/low WA, low protein content, low pH/acidic environment, low redox potential due to the high level of reducing sugars, a viscosity that limits dissolved oxygen and other chemical agents/phytochemicals [45]. Glucose oxidase, hydrogen peroxide, low WA and water acidity are important properties of honey that do not help in the growth of yeast and bacteria [87]. Molan [88] reported that Escherichia coli and Staphylococcus aureus can be significantly prevented by manuka honey. It has been illustrated that the antibacterial activity of honey is effective on many bacterial pathogens and fungi [45].
4.5. Inflammatory Process in PID and Anti-Inflammatory and Antioxidant Activities of Linseeds, Psyllium, and Honey
Psyllium seeds and linseeds have analgesic, antipyretic, and anti-inflammatory properties [11,17]; hence, they were perhaps able to reduce pus cells in a vaginal wet mount. Pelvic discomfort depreciates the QoL and is a major and annoying issue for participants as well as physicians.
The inflammatory and idiopathic biological processes and the psychogenic, neuropathic, mixed and nociceptive processes are various causes of pelvic pain disorders. Tissue damage as the body’s reaction causes inflammatory pain, and the consequent inflammatory procedure activates “silent nociceptors” that usually don’t respond to thermal or mechanical stimuli. The essential causes of any type of pain are inflammation and their inflammatory response, which are mediated by inflammatory bio mediators (growth factors, neurotransmitters, neuropeptides, and cytokines). An injury to soft tissue or nerves may lead to the release of these inflammatory mediators and stimulates peripheral terminals of sensory nerve fibres, and released inflammatory neuropeptides are activated by the reverse firing of these sensory nerves. Inflammatory neuropeptides promote vascular permeability and vasodilation, and they recruit more immune cells (T helper cell) and triggers sensory nerve fibres in the surrounding area (neurogenic inflammation). Amplified input from peripheral pain receptors changes the central processing at the CNS level and modifies central processing mechanisms. In addition, local tissue inflammation also results in secondary hyperalgesia and central sensitization. Therefore, this can result in a syndrome that includes fever, joint pain, diffuse muscle pain, spasms, lethargy, and anorexia [89]. In response to the bacterial infection of N. gonorrhoea, an in vitro study has proven that lipid mediators important for inflammatory hyper nociception are produced from dendritic (PGE2) cells and neutrophils (leukotriene B4). In acute pelvic inflammatory diseases, elevated levels of both mediators have been noted in the peritoneal fluid [90]. Cyclooxygenase 1 and 2 are produced from arachidonic acid that produces prostaglandins (PGs), and PGs play an essential role in causing pain and inflammation. Currently, for pain relief and to reduce inflammation, common drugs such as steroids and nonsteroids are beneficial [91]. Therefore, targeting these responses may help to manage pain relief [90]. The source of any type of pain is inflammation and the inflammatory response. Hence, pelvic inflammatory disease can also cause pain.
Kopaei [82] confirmed the analgesic and anti-inflammatory effects of linseeds in mice. Linseeds show dose-dependent analgesic activities similar in part to morphine and might be used as an analgesic and anti-inflammatory agent [82]. The extract had flavonoid, phenolic, and flavonol compounds with antioxidant potential. Morsi et al. [92] estimated the total phenolic and flavonoid contents and also investigated the anti-inflammatory, antioxidant, and anticancer activities of various linseed extracts. The methanolic extract exhibited higher antioxidant activity. The GC-MS analysis displayed that the methanolic extract, ethyl acetate, and butanol fractions had 32, 40, and 36 compounds, respectively [92]. Mechchate et al. [93] investigated the anti-inflammatory and antidiabetic activity of a free polyphenol fraction of linseeds. Carrageenan is a chemical that helps the release of inflammatory mediators and was used in the experiment to induce acute inflammation. Carrageenan induced a biphasic response in the first hour after injection, inducing oedema formation and the release of histamine, serotonin and kinins. In the second phase, 2–3 h after the injection, it caused the release of PGs. PGs is a well-known cause of inflammation. The authors confirmed that the polyphenol fraction of linseeds had an anti-inflammatory effect in phase 1 and 2 of the carrageenan-induced inflammation [93]. The treatment with lignan extracts showed a significant reduction in malondialdehyde (MDA), whereas the levels of serum glutathione (GSH), glutathione peroxidase (GPx), catalase (CAT), superoxidase dismutase (SOD), zinc and manganese were significantly increased in paracetamol, with lignan-treated groups showing the antioxidant potential of lignans [94]. Khedher et al. determined the antioxidant and antiulcerogenic properties of psyllium seed ethanolic extract in rats and reported that during pre-treatment with psyllium, the oxidative stress status in the stomach tissues showed a significant increase in SOD, CAT, and GPx (antioxidant enzyme levels) with a significant decrease in lipid peroxidation. In conclusion, psyllium extract protects against gastric ulcers because of its antioxidant effect and the presence of bioactive molecules [21]. The phytochemical evaluation of the psyllium extract exhibited high numbers of flavonoids, polyphenols, and tannins possibly responsible for its anti-oxidative effect [95]. The above-mentioned compounds have crucial roles in quenching singlet and triplet oxygen or decomposing peroxides and absorbing and neutralizing free radicals [96]. Reddy et al. [83] confirmed the anti-inflammatory and antibacterial activities of psyllium.
The phenolic compound in honey has been an important contributor to its antioxidant capacity. The Italian multifloral honey extract contains major components, viz., the flavonoids, genistin, apigenin, luteolin, daidzein, kaempferol, quercetin, and chrysin. These compounds have inhibited the release of pro-inflammatory TNF-α and IL-1β. Immunomodulatory effects have also been reported for honey proteins [37]. Naturally, the level of enzymatic antioxidants such as SOD, CAT, GPx, and nonenzymatic antioxidants such as Vit. C and E decrease in the blood circulation and tissue as a result of an increase in inflammatory factors and damage. Hence, antioxidants have the potential to get rid of pain and inflammation [97]. Some plant compounds such as carotenoids, saponins, vitamin C, organic acids, alkaloids, tannins, anthocyanins, glycosides, vitamin E, and caffeic acid derivatives are effective in pain relief as they possess analgesic and anti-inflammatory effects [98]. Flavonoids have analgesic and anti-inflammatory activities [99] that are based on prostaglandin production inhibition in the central and peripheral system; flavonoids possibly prevent the metabolism of arachidonic acid.
Linseeds are traditionally used to ameliorate pain and inflammation. Linseeds include phenolic compounds, phenols, carotenoids, flavones, flavonoids, tannins, lignans, antocianins, vitamins E and C, and amino acids such as tyrosine, tryptophan, and proline. These plant bio compounds have diverse roles such as antioxidant activities [97]. Psyllium contains flavonoids and phenolics that have a reduced capacity and ROS scavenging activities. Psyllium seeds that had 78% polyunsaturated, 15% saturated, and 7% monounsaturated fatty acids were found [100] to contain flavonoids (natural polyphenol compounds) in their plants and have analgesic and anti-inflammatory effects [101]. These results are consistent with studies conducted on most medicinal compounds that have analgesic and anti-inflammatory properties. They prove their beneficial effects in reducing prostaglandins, their mediators, and enzymes that produce pain and inflammation as a significant peripheral factor in reducing the pain–inflammation process [102]. Further, lipoxygenase enzymes are related to various inflammatory-related diseases. Screening for the lipoxygenase (LOX)-inhibitory activities of linseed lignan extracts in various methods has been reported. Linseed extracts are also a potential source of novel therapeutics to treat many diseases related to the LOX enzyme [103].
TNF-α is a marker for inflammatory illness and it is a pro-inflammatory cytokine that has a critical role in modifying many physiological events as well as inflammation. P. psyllium extract successfully reduced CCl4-induced inflammation and hence TNF-α expression, indicating its healing effect on liver cells via the controlling of cytokine expression and the underlying inflammatory process [104]. Aucubin (a type of iridoid glycoside) and Lantamajoside exhibited anti-inflammatory properties through the inhibitory effect of TPA (12-o-tetradecanoylphorbol-13-acetate) [105] and arachidonic acid metabolism, respectively. Plantamajoside has an inhibitory effect on arachidonic acid metabolism, and therefore has anti-inflammatory [106] and also antioxidant properties [107]. Lantamajoside from psyllium has antioxidant and radical scavenging properties [83]. Tannins are recognized to ‘tan’ the mucosal outermost layer, interpreting it as less permeable and more resistant to injury or irritation [108]. The protective effects are associated with the antioxidant activities and antiradical power of psyllium extract [21]. The anti-inflammatory potential of honey is due to its phenolic content [109]. The pro-inflammatory activities of cyclooxygenase-2 and/or inducible nitric oxide synthase (iNOS) are suppressed by flavonoids and phenolic bioactive compounds [46]. Inducible nitric oxide synthase, tyrosine kinase, ornithine decarboxylase, and cyclooxygenase-2 are regulated by honey and its compounds. Numerous types of honey have been revealed to persuade tumour necrosis factor-alpha, IL-6 and interleukin-1 beta production. In addition, honey increases B and T lymphocytes, monocytes, neutrophils, eosinophils, antibodies, and natural killer cell generation in both primary and secondary immune responses in tissue culture [45].
4.6. Haematological and Biochemical Markers for Safety Parameters
The haematological and biochemical markers for the safety of blood, liver, and kidney showed no statistical difference before and after treatment and were within the normal range. This shows that both groups were safe on the liver, kidney, and blood, showing no toxicity of these medications in the body fluids and organs. It indicates that the research drugs were found to be safe. Further, clinically, the research drugs did not show any side effects during or after the completion of the trial. However, in the control group, the first three participants showed gastrointestinal and other symptoms such as pain in the epigastric region, vomiting, decreased appetite, headache, and giddiness after 2 days of ingestion of the standard drugs. Then onwards, all the participants were advised to take a tablet of pantoprazole 20 mg twice daily before eating food to avoid side effects. Khedher [21] investigated and tested animals that received different doses of Psyllium extract (100–1000 mg/kg). The data showed no acute toxicity or lethality up to 1000 mg/kg. Another study showed that animals who received psyllium extract at variable doses showed no signs of toxicity, morbidity or changes in animal behaviour. Analysis of serum renal and hepatic biomarkers showed that a dose of up to 1000 mg/kg of the extract was safe without any side effects [104].
4.7. Hepatoprotective and Nephroprotective Active of Psyllium and Linseeds
This section also explains in detail the hepatoprotective and nephroprotective in vitro and in vivo research of linseeds and psyllium. Previous studies have confirmed the hepatoprotective [17], immunomodulatory [83], and nephroprotective properties of P. ovata [15]. Further, the hepatoprotective and nephroprotective [110] properties of L. usitatissimum are also proven. Bagheri et al. [17] reported that an aqueous extract of psyllium seed showed hepatoprotective properties, and the tissue maintained normal architecture in the histological analysis of liver rats. They concluded that Psyllium seed extract was comparable to the standard hepatoprotective drug silymarin. They observed that indomethacin caused degenerative changes and cell necrosis in the liver’s cells and decreased hepatic microsomal cytochrome P-450 and prostaglandin [104]. Jumaily and Al Azawi [22] concluded that liver and renal damage from paracetamol-induced hepato-nephrotoxicity can be prevented, and pure lignan was better than partial lignan in rabbits. Another study [111] observed the hepatoprotective action of a linseeds chutney supplemented diet and was a probable source of antioxidants, and it might be used in protection against hepatotoxic damage induced by carbon tetrachloride (CCl4) in Wister rats.
4.8. Strength, Limitations and Future Recommendations
This was the first kind of study wherein a clinical intervention in which the ability of linseeds, psyllium and honey to reduce clinical features, VAS score, McCormack pain scale, and improve the HRQoL in uPID were investigated. Furthermore, it was a single-blind, prospective, parallel, randomized, controlled, and double-dummy study with intent-to-treat analysis and good patient compliance. Moreover, this study also included machine learning wherein an automatic classification model based on experimental analysis of the pessary and standard groups on uPID occurring in reproductive-age women was performed.
Due to the short duration of follow-up, specific diagnostic tests such as cervical swab culture, NAAT, and endometrial biopsy were not used to ascertain the microbiological and histological cure rates because of time restrictions and limited resources. Also, the data could not be generalized to all participants with acute PID, considering disease severity. Therefore, we suggest the aforementioned specific diagnostic tests for future studies. In addition, trial medicine might be researched in acute and complicated PID. This study was to validate the Unani pharmacopeial formula for its efficacy and safety. The authors also recommend carrying out the dosage form modification and standardization; the quantitative analysis of its bioactive molecules responsible for antimicrobial, anti-inflammatory and antioxidant activities; and a stability evaluation of the finished product. Additionally, the presence of active constituents in the bloodstream could be checked to assess their absorption and safety. Hence, elaborative pharmacokinetics and pharmacodynamics studies are recommended. Further, we would collect a large number of participants and design a hybrid model based on machine learning techniques to classify the drug and non-drug groups.
5. Conclusions
This data authenticates the claim of Unani scholars and is in consonance that the pessary group was as efficacious as the standard group for the treatment of uPID and thereby improved women’s HRQoL. Further, the treatment in the pessary group was a safe and economical alternative to antimicrobials to treat uPID. Healthcare providers can recommend the aforementioned Unani drugs to women to cure uPID. In addition, our DT machine learning model is more effective for the classification of both groups. Furthermore, double-blind RCTs and phase IV trials for a longer duration are recommended. In addition, well-designed studies with mechanisms need to be considered.
Acknowledgments
The authors would like to thank Wu, Singh, Siddiqui, Ansari, Gul, and Perween for the help, support and motivation of the study. We also acknowledge Qudsia Sultana, Dallas, USA for proof reading this article.
Author Contributions
S.Q., A.S. and M.B.B.H.: conceptualization, data curation, formal analysis, methodology, validation, software, supervision, writing, and resources; K.R., F.A. and A.u.H.: investigation, project administration, and supervision; B.A.A., M.A.A., and R.M.G.: resources, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
We received approval from the scientific review and Institutional Ethical Committee (NIUM/IEC/2015-16/014/ANQ/06 dated 17.03.2016). The trial has been registered in the clinical trial registry of India as CTRI/2018/03/012375. The authors performed the study as per the “Declaration of Helsinki” and “GCP” guidelines according to the Ministry of AYUSH, India.
Informed Consent Statement
All randomized participants gave written informed consent.
Data Availability Statement
The data is available through the request via Prof. Dr. Arshiya Sultana (drarshiya@yahoo.com).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This publication was supported by the National Natural Science Foundation of China (U2001207 and 61872248), Guangdong NSF (2017A030312008), Shenzhen Science and Technology Foundation (ZDSYS20190902092853047 and R2020A045), the Project of DEGP (2019KCXTD005), and the Guangdong “Pearl River Talent Recruitment Program” (2019ZT08X603). Additionally, this research was supported by Ministry of AYUSH, India.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Das B.B., Ronda J., Trent M. Pelvic Inflammatory Disease: Improving Awareness, Prevention, and Treatment. Infect. Drug Resist. 2016;9:191–197. doi: 10.2147/IDR.S91260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sultana A., Mehdi S., Rahman K., Fazmiya M.J.A., Heyat M.B.B., Akhtar F., Baig A.A. Computational Intelligence in Healthcare Applications. Elsevier; Amsterdam, The Netherlands: 2022. Recent Advancements of Pelvic Inflammatory Disease: A Review on Evidence-Based Medicine; pp. 101–120. [Google Scholar]
- 3.Dhasmana D., Hathorn E., McGrath R., Tariq A., Ross J.D.C. The Effectiveness of Nonsteroidal Anti-Inflammatory Agents in the Treatment of Pelvic Inflammatory Disease: A Systematic Review. Syst. Rev. 2014;3:79. doi: 10.1186/2046-4053-3-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judlin P., Liao Q., Liu Z., Reimnitz P., Hampel B., Arvis P. Efficacy and Safety of Moxifloxacin in Uncomplicated Pelvic Inflammatory Disease: The MONALISA Study. BJOG Int. J. Obstet. Gynaecol. 2010;117:1475–1484. doi: 10.1111/j.1471-0528.2010.02687.x. [DOI] [PubMed] [Google Scholar]
- 5.Kotecha P.V., Patel S.V., Baxi R.K., Shah S., Mehta K.G., Diwanji M. Treatment Seeking Pathway of Pid (Pelvic Inflammatory Disease) Patients Attending Government Hospital Vadodara, India. Natl. J. Community Med. 2011;2:186–190. [Google Scholar]
- 6.Dayal S., Singh A., Chaturvedi V., Krishna M., Gupta V. Pattern of Pelvic Inflammatory Disease in Women Who Attended the Tertiary Care Hospital among the Rural Population of North India. Muller J. Med. Sci. Res. 2016;7:100–104. doi: 10.4103/0975-9727.185005. [DOI] [Google Scholar]
- 7.Khan A. Iksir-i-A‘Zame. Idarae Kitabus Shifa; New Delhi, India: 2011. [Google Scholar]
- 8.Savaris R.F., Fuhrich D.G., Maissiat J., Duarte R.V., Ross J. Antibiotic Therapy for Pelvic Inflammatory Disease. Cochrane Database Syst. Rev. 2020;2020:CD010285. doi: 10.1002/14651858.CD010285.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vishwesh B.N., Bhat S.K. A Clinical Study to Evaluate the Role of Shivagutika in Pelvic Inflammatory Disease. J. Ayurveda Holist. Med. 2014;2:27–32. [Google Scholar]
- 10.Bodhankar M., Pachori R.R., Kulkarni N.S. Medicinal Plants as an Alternative for Treating Pelvic Inflammatory Disease. Int. J. Biochem. Mol. Biol. 2015;3:23–32. [Google Scholar]
- 11.Kabir al-Din M. Makhzan Al-Mufradat. Idarae Kitabus Shifa; New Delhi, India: 2007. [Google Scholar]
- 12.Arzani M.A. Qarabadeen Qadri. I’jaz Publishing House; New Delhi, India: 2009. [Google Scholar]
- 13.Standardisation of Single Drugs of Unani Medicine. Part-II. 1st ed. Central Council for Research in Unani Medicine (CCRUM); New Delhi, India: 1992. Psyllium. [Google Scholar]
- 14.Khare C.P. Indian Medicinal Plants: An Illustrated Dictionary (Google EBook) Springer; Berlin/Heidelberg, Germany: 2007. 900p [Google Scholar]
- 15.Prajapati N.D. Agro’s Dictionary of Medicinal Plants. Agrobios; Jodhpur, India: 2003. [Google Scholar]
- 16.Deokar G., Kshirsagar S., Deore P., Kakulte H. Pharmaceutical Benefits of Plantago ovate (Isabgol Seed): A Review. Pharm. Biol. Eval. 2016;3:32–41. [Google Scholar]
- 17.Bagheri S.M., Zare-Mohazabieh F., Momeni-Asl H., Yadegari M., Mirjalili A., Anvari M. Antiulcer and Hepatoprotective Effects of Aqueous Extract of Plantago ovata Seed on Indomethacin-Ulcerated Rats. Biomed. J. 2018;41:41–45. doi: 10.1016/j.bj.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motamedi H., Darabpour E., Gholipour M., Seyyed Nejad S.M. Antibacterial Effect of Ethanolic and Methanolic Extracts of Plantago ovata and Oliveria decumbens Endemic in Iran against Some Pathogenic Bacteria. Int. J. Pharmacol. 2010;6:117–122. doi: 10.3923/ijp.2010.117.122. [DOI] [Google Scholar]
- 19.Bokaeian M., Fakheri B.A., Mohasseli T., Saeidi S. Antibacterial Activity of Silver Nanoparticles Produced by Plantago ovata Seed Extract against Antibiotic Resistant Staphylococcus aureus. Int. J. Infect. 2015;2:176–178. doi: 10.17795/iji-22854. [DOI] [Google Scholar]
- 20.Sagar S., Goudar G., Sreedhar M., Panghal A., Sharma P. Characterization of Nutritional Content and in Vitro—Antioxidant Properties of Plantago ovata Seeds. Int. J. Food Nutr. Sci. 2020;9:27. doi: 10.4103/ijfns.ijfns_27_20. [DOI] [Google Scholar]
- 21.Khedher A., Dhibi S., Bouzenna H., Akermi S., El Feki A., Teles P.H.V., Almeida J.R.G.S., Hfaiedh N. Antiulcerogenic and Antioxidant Activities of Plantago ovata Ethanolic Extract in Rats. Brazilian J. Biol. 2022;84:e255120. doi: 10.1590/1519-6984.255120. [DOI] [PubMed] [Google Scholar]
- 22.Al-Jumaily E.F., AL-Azawi A.H. Antioxidant and Hepatoprotective Activity of Purelignan from Flaxseed (Linum usitatissimum L.) Onacetaminophen Induced Toxicity in Male Rabbit. Int. J. Res. Stud. Biosci. 2015;3:8–16. [Google Scholar]
- 23.Ravn H., Brimer L. Structure and Antibacterial Activity of Plantamajoside, a Caffeic Acid Sugar Ester from Plantago Major Subs Major. Phytochemistry. 1988;27:3433–3437. doi: 10.1016/0031-9422(88)80744-1. [DOI] [Google Scholar]
- 24.Standardisation of Single Drugs of Unani Medicine. Part-II. Central Council for Research in Unani Medicine; Delhi, India: 1992. Linseeds; pp. 276–281. [Google Scholar]
- 25.Bernacchia R., Preti R., Vinci G. Chemical Composition and Health Benefits of Flaxseed. Austin J. Nutr. Food Sci. 2014;2:1–9. [Google Scholar]
- 26.Heena Umer K., Zeenat F., Ahmad W., Ahmad Hakim Syed Ziaul Hasan Govt I., Vakil Khan A., Fahmeeda Zeenat C., Ahmad I. Therapeutics, Phytochemistry and Pharmacology of Alsi (Linum usitatissimum Linn.): An Important Unani Drug. J. Pharmacogn. Phytochem. 2017;6:377–383. [Google Scholar]
- 27.Mohamed El-Feky F., El-Sherbiny I.M., El-Fedawy M.G., Abdel-Mogib M. Phytochemical Studies on Linum usitatissimum Seeds and the Nanoformulation of the Bioactive Butanol Extract. Int. J. Sci. Eng. Appl. 2016;5:76–91. doi: 10.7753/IJSEA0502.1006. [DOI] [Google Scholar]
- 28.Azamat Kizi K.S. Antibacterial Activity of Linum usitatissimum L. Seeds and Active Compound Detection. Eur. Int. J. Multidiscip. Res. Manag. Stud. 2022;2:263–266. doi: 10.55640/eijmrms-02-04-50. [DOI] [Google Scholar]
- 29.Tavarini S., Castagna A., Conte G., Foschi L., Sanmartin C., Incrocci L., Ranieri A., Serra A., Angelini L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules. 2019;24:3729. doi: 10.3390/molecules24203729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim Y.Y., Gui B., Arnison P.G., Wang Y., Reaney M.J.T. Flaxseed (Linum usitatissimum L.) Bioactive Compounds and Peptide Nomenclature: Areview. Trends Food Sci. Technol. 2014;38:5–20. doi: 10.1016/j.tifs.2014.03.011. [DOI] [Google Scholar]
- 31.Goyal A., Sharma V., Upadhyay N., Gill S., Sihag M. Flax and Flaxseed Oil: An Ancient Medicine & Modern Functional Food. J. Food Sci. Technol. 2014;51:1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuk M., Dorotkiewicz-Jach A., Drulis-Kawa Z., Arendt M., Kulma A., Szopa J. Bactericidal Activities of GM Flax Seedcake Extract on Pathogenic Bacteria Clinical Strains. BMC Biotechnol. 2014;14:70. doi: 10.1186/1472-6750-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-bayati F.A. Antibacterial Activity of Linum usitatissimum L. Seeds and Active Compound Detection. Rafidain J. Sci. 2007;18:27–36. [Google Scholar]
- 34.Son H.J., Song K. Bin Antimicrobial Activity of Flaxseed Meal Extract against Escherichia coli O157:H7 and Staphylococcus aureus Inoculated on Red Mustard. J. Microbiol. Biotechnol. 2017;27:67–71. doi: 10.4014/jmb.1607.07036. [DOI] [PubMed] [Google Scholar]
- 35.Hassan M., Tehrani H., Batal R., Kamalinejad M., Mahbubi A. Extraction and Purification of Flaxseed Proteins and Studying Their Antibacterial Activities. J. Plant Sci. 2014;2:70. doi: 10.11648/j.jps.20140201.21. [DOI] [Google Scholar]
- 36.Kaithwas G., Mukherjee A., Chaurasia A.K., Majumdar D.K. Antiinflammatory, Analgesic and Antipyretic Activities of Linum usitatissimum L. (Flaxseed/Linseed) Fixed Oil. Indian J. Exp. Biol. 2011;49:932–938. [PubMed] [Google Scholar]
- 37.Cornara L., Biagi M., Xiao J., Burlando B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combarros-Fuertes P., Estevinho L.M., Dias L.G., Castro J.M., Tomás-Barberán F.A., Tornadijo M.E., Fresno-Baro J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019;67:688–698. doi: 10.1021/acs.jafc.8b05436. [DOI] [PubMed] [Google Scholar]
- 39.Linum usitatissimum L. Taxonomic Serial No.: 29226. Integrated Taxonomic Information System (ITIS)—Report. [(accessed on 15 January 2023)]; Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=29226#null.
- 40.Plantago ovata Forssk. Taxonomic Serial No.: 504438. Integrated Taxonomic Information System (ITIS)—Report. [(accessed on 15 January 2023)]; Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=504438#null.
- 41.Greenberg D. A Quantitative and Qualitative Evaluation of Official and Unofficial Species of Psyllium Seeds and Their Mucilages. J. Am. Pharm. Assoc. 1948;37:139–145. doi: 10.1002/jps.3030370404. [DOI] [PubMed] [Google Scholar]
- 42.El Abdali Y., Allali A., Agour A., Mikou K., Lahkimi A., Eloutassi N., Bouia A. Phytochemical Screening, and in Vitro Antiradical and Immunostimulant Potential of Linum usitatissimum L. Pharmacologyonline. 2021;3:602–614. [Google Scholar]
- 43.Vijayakumar K.T., Neethu T., Bhat N.S., Nayimabanu T., Varsharani H. Physico-Chemical Property of Different Floral Honeys of Bangalore Region, Karnataka. J. Entomol. Zool. Stud. 2020;8:846–854. [Google Scholar]
- 44.Fazmiya M.J.A., Sultana A., Rahman K., Heyat M.D.B., Sumbul, Akhtar F., Khan S., Appiah S.C.Y. Current Insights on Bioactive Molecules, Antioxidant, Anti-Inflammatory, and Other Pharmacological Activities of Cinnamomum camphora Linn. Oxid. Med. Cell. Longev. 2022;2022:9354555. doi: 10.1155/2022/9354555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samarghandian S., Farkhondeh T., Samini F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017;9:121–127. doi: 10.4103/0974-8490.204647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viuda Martos M., Ruiz Navajas Y., Fernández López J., Pérez-Alvarez J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008;73:R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 47.Parween A., Tabassum K., Begum W. Effect of Arq Mako and Sharbat Kasni in Waram Al Rahim (Pelvic Inflammatory Disease): An Observational Clinical Trial. Int. J. Adv. Res. Dev. 2017;2:7–13. [Google Scholar]
- 48.Sayed A., Shameem I., Mubeen U., Qabalat I., Niswan A., Medicine U., Qabalat I., Niswan A., Medicine U., Qabalat I., et al. Effect of Arq brinjasif in Mild Pelvic Inflammatory Disease—A Randomised Controlled Trial. Int. J. Med. Res. 2016;1:91–96. [Google Scholar]
- 49.Teelhawod B.N., Akhtar F., Heyat M.B.B., Tripathi P., Mehrotra R., Asfaw A.B., Al Shorman O., Masadeh M. Machine Learning in E-Health: A Comprehensive Survey of Anxiety; Proceedings of the 2021 International Conference on Data Analytics for Business and Industry, ICDABI 2021; Sakheer, Bahrain. 25–26 October 2021; pp. 167–172. [Google Scholar]
- 50.Sultana A., Begum W., Saeedi R., Rahman K., Heyat M.B.B., Akhtar F., Son N.Y., Ullah H. Experimental and Computational Approaches for the Classification and Correlation of Temperament (Mizaj) and Uterine Dystemperament (Su’-I-Mizaj Al-Rahim) in Abnormal Vaginal Discharge (Sayalan Al-Rahim) Based on Clinical Analysis Using Support Vector Mach. Complexity. 2022;2022:1–16. doi: 10.1155/2022/5718501. [DOI] [Google Scholar]
- 51.Iqbal M.S., Abbasi R., Bin Heyat M.B., Akhtar F., Abdelgeliel A.S., Albogami S., Fayad E., Iqbal M.A. Recognition of MRNA N4 Acetylcytidine (Ac4C) by Using Non-Deep vs. Deep Learning. Appl. Sci. 2022;12:1344. doi: 10.3390/app12031344. [DOI] [Google Scholar]
- 52.Pal R., Adhikari D., Heyat M.B.B., Guragai B., Lipari V., Brito Ballester J., De la Torre Díez I., Abbas Z., Lai D. A Novel Smart Belt for Anxiety Detection, Classification, and Reduction Using IIoMT on Students’ Cardiac Signal and MSY. Bioengineering. 2022;9:793. doi: 10.3390/bioengineering9120793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bin Heyat M.B., Akhtar F., Sultana A., Tumrani S., Teelhawod B.N., Abbasi R., Kamal M.A., Muaad A.Y., Lai D., Wu K. Role of Oxidative Stress and Inflammation in Insomnia Sleep Disorder and Cardiovascular Diseases: Herbal Antioxidants and Anti-Inflammatory Coupled with Insomnia Detection Using Machine Learning. Curr. Pharm. Des. 2022;28:3618–3636. doi: 10.2174/1381612829666221201161636. [DOI] [PubMed] [Google Scholar]
- 54.Akhtar F., Bin Heyat M.B., Li J.P., Patel P.K., Rishipal, Guragai B. Role of Machine Learning in Human Stress: A Review; Proceedings of the 2020 17th International Computer Conference on Wavelet Active Media Technology and Information Processing, ICCWAMTIP 2020; Chengdu, China. 18–20 December 2020; pp. 170–174. [Google Scholar]
- 55.Bin Heyat M.B., Lai D., Khan F.I., Zhang Y. Sleep Bruxism Detection Using Decision Tree Method by the Combination of C4-P4 and C4-A1 Channels of Scalp EEG. IEEE Access. 2019;7:102542–102553. doi: 10.1109/ACCESS.2019.2928020. [DOI] [Google Scholar]
- 56.Tripathi P., Ansari M.A., Gandhi T.K., Mehrotra R., Bin Heyat M.B., Akhtar F., Ukwuoma C.C., Muaad A.Y., Kadah Y.M., Al-Antari M.A., et al. Ensemble Computational Intelligent for Insomnia Sleep Stage Detection via the Sleep ECG Signal. IEEE Access. 2022;10:108710–108721. doi: 10.1109/ACCESS.2022.3212120. [DOI] [Google Scholar]
- 57.Lai D., Bin Heyat M.B., Khan F.I., Zhang Y. Prognosis of Sleep Bruxism Using Power Spectral Density Approach Applied on EEG Signal of Both EMG1-EMG2 and ECG1-ECG2 Channels. IEEE Access. 2019;7:82553–82562. doi: 10.1109/ACCESS.2019.2924181. [DOI] [Google Scholar]
- 58.Bin Heyat M.B., Akhtar F., Abbas S.J., Al-Sarem M., Alqarafi A., Stalin A., Abbasi R., Muaad A.Y., Lai D., Wu K. Wearable Flexible Electronics Based Cardiac Electrode for Researcher Mental Stress Detection System Using Machine Learning Models on Single Lead Electrocardiogram Signal. Biosensors. 2022;12:427. doi: 10.3390/bios12060427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chola C., Bin Heyat M.B., Akhtar F., Al Shorman O., Bibal Benifa J.V., Muaad A.Y.M., Masadeh M., Alkahatni F. IoT Based Intelligent Computer-Aided Diagnosis and Decision Making System for Health Care; Proceedings of the 2021 International Conference on Information Technology, ICIT 2021—Proceedings; Amman, Jordan. 14–15 July 2021; pp. 184–189. [Google Scholar]
- 60.Sultana A., Heyat M.B.B., Rahman K., Kunnavil R., Fazmiya M.J.A., Akhtar F., Sumbul, Vidal Mazón J.L., Rodríguez C.L., De La Torre Díez I. A Systematic Review and Meta-Analysis of Premenstrual Syndrome with Special Emphasis on Herbal Medicine and Nutritional Supplements. Pharmaceuticals. 2022;15:1371. doi: 10.3390/ph15111371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moghtadaei P., Sardari F., Esmaeilian S. Comparison of Ceftriaxone Plus Weekly Azithromycin or Daily Ofloxacin for Outpatient Treatment of Pelvic Inflammatory Disease: A Randomized Clinical Trial. J. Fam. Reprod. Health. 2008;2:87–93. [Google Scholar]
- 62.Bhat T.A., Begum W. Efficacy of Tamarindus indicus, Melia azadirach and Santalum album in syndromic management of abnormal vaginal discharge: A single-blind randomised controlled trial. J. Complement. Integr. Med. 2017;15:19. doi: 10.1515/jcim-2015-0023. [DOI] [PubMed] [Google Scholar]
- 63.Thomas L. Clinical Laboratory Diagnostics, 1 st ed. TH-Books Sgesellschaft; Frankfurt, Germany: 1998. [Google Scholar]
- 64.Tietz N.W., Rinker A.D., Shaw L.M. IFCC Reference Procedure for Measurement of Catalytic Concentration of Enzymes at 37 °C. Part 9. Reference Procedure for Measurement of Catalytic Concentration of Alkaline Phosphatase. Clin. Chem. Lab. Med. 2011;49:1439–1446. doi: 10.1515/CCLM.2011.621. [DOI] [PubMed] [Google Scholar]
- 65.Burtis C.A., Ashwood E.R., Bruns D.E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. Saunder’s Co.; Bloomington, MN, USA: 2005. [Google Scholar]
- 66.Sharma J.B., Chanana C., Kumar S., Roy K., Malhotra N. Comparison of Ofloxacin & Ornidazole with Probiotic versus Doxycycline & Metronidazole for the Outpatient Treatment of Pelvic Inflammatory Disease. JK Sci. 2007;9:66–69. [Google Scholar]
- 67.Anjum A., Sultana A. A Randomized Comparative Study of Herbal Decoction of Cassia fistula Linn Pod’s Pericarp and Myristica fragrans Houtt Arils vs. Mefenamic Acid in Spasmodic Dysmenorrhoea. J. Complement. Integr. Med. 2019;16:1–12. doi: 10.1515/jcim-2018-0105. [DOI] [PubMed] [Google Scholar]
- 68.Lam W.Y., Fresco P. Medication Adherence Measures: An Overview. Biomed. Res. Int. 2015;2015:217047. doi: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saeedi R., Sultana A., Rahman K., Belal Bin Heyat M., Kamal M.A., Ishawu M. Efficacy of Acacia nilotica Linn. Pod’s Sitz Bath plus Vaginal Pessary in Syndromic Management of Abnormal Vaginal Discharge: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2022;2022:5769555. doi: 10.1155/2022/5769555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Said R.R., Heyat M.B.B., Song K., Tian C., Wu Z. A Systematic Review of Virtual Reality and Robot Therapy as Recent Rehabilitation Technologies Using EEG-Brain–Computer Interface Based on Movement-Related Cortical Potentials. Biosensors. 2022;12:1134. doi: 10.3390/bios12121134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nawabi A.K., Sheng J., Abbasi R., Iqbal M.S., Heyat M.B.B., Akhtar F., Wu K., Twumasi B.A. Segmentation of Drug-Treated Cell Image and Mitochondrial-Oxidative Stress Using Deep Convolutional Neural Network. Oxid. Med. Cell. Longev. 2022;2022:5641727. doi: 10.1155/2022/5641727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tripathi P., Ansari M., Akhtar F., Heyat M.B.B., Mehrotra R., Yatoo A.H., Teelhawod B.N., Asfaw A.B., Baig A.A. Automatic Epileptic Seizure Detection Based on the Discrete Wavelet Transform Approach Using an Artificial Neural Network Classifier on the Scalp Electroencephalogram Signal. Comput. Intell. Healthc. Appl. 2022;10:157–173. doi: 10.1016/b978-0-323-99031-8.00012-0. [DOI] [Google Scholar]
- 73.Ullah H., Heyat M.B.B., Akhtar F., Muaad A.Y., Ukwuoma C.C., Bilal M., Miraz M.H., Bhuiyan M.A.S., Wu K., Damaševičius R., et al. An Automatic Premature Ventricular Contraction Recognition System Based on Imbalanced Dataset and Pre-Trained Residual Network Using Transfer Learning on ECG Signal. Diagnostics. 2022;13:87. doi: 10.3390/diagnostics13010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ullah H., Bin Heyat M.B., Akhtar F., Sumbul, Muaad A.Y., Islam M.S., Abbas Z., Pan T., Gao M., Lai D. An End-to-End Cardiac Arrhythmia Recognition Method with an Effective DenseNet Model on Imbalanced Datasets Using ECG Signal. Comput. Intell. Neurosci. 2022;2022:9475162. doi: 10.1155/2022/9475162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sultana A., Baig K., Rahman K., Mehdi S., Heyat M.B.B., Akhtar F., Baig A.A. Computational Intelligence in Healthcare Applications. Elsevier; Amsterdam, The Netherlands: 2022. Contemporary Overview of Bacterial Vaginosis in Conventional and Complementary and Alternative Medicine; pp. 33–53. [Google Scholar]
- 76.Saeedi R., Deoband J.T., Khan M.A., Farzana M.U.Z.N. A Conceptual Literary Exploration of Sayalan Al-Rahim (Abnormal Vaginal Discharge) in Unani Medicine. SLJIM. 2020;5:414–423. [Google Scholar]
- 77.Qhuddsia Q.N., Sultana A., Jahan I. Efficacy of Unani Formulations in Chronic Endocervicitis: A Randomized Comparative Study. Int. J. Pharm. Pharm. Sci. 2015;7:101–104. [Google Scholar]
- 78.Moylan S., Berk M., Dean O.M., Samuni Y., Williams L.J., O’Neil A., Hayley A.C., Pasco J.A., Anderson G., Jacka F.N., et al. Oxidative & Nitrosative Stress in Depression: Why so Much Stress? Neurosci. Biobehav. Rev. 2014;45:46–62. doi: 10.1016/j.neubiorev.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Baig K., Sultana A., Rahman K. A Randomized Comparative Study of Kakrasingi (Pistacia integerrima J. L. Stewart Ex Brandis) and Metronidazole in Bacterial Vaginosis. J. Herb. Med. 2022;36:100609. doi: 10.1016/j.hermed.2022.100609. [DOI] [Google Scholar]
- 80.Haggerty C. Lower Quality of Life among Women with Chronic Pelvic Pain after Pelvic Inflammatory Disease. Obstet. Gynecol. 2003;102:934–939. doi: 10.1016/s0029-7844(03)00695-1. [DOI] [PubMed] [Google Scholar]
- 81.Li X.H., Liu Y.R., Jiang D.H., Tang Z.S., Qian D.W., Song Z.X., Chen L., Shi X.B., Yang N.J., Yan Y.F., et al. Research on the Mechanism of Chinese Herbal Medicine Radix Paeoniae Rubra in Improving Chronic Pelvic Inflammation Disease by Regulating PTGS2 in the Arachidonic Acid Pathway. Biomed. Pharmacother. 2020;129:110052. doi: 10.1016/j.biopha.2020.110052. [DOI] [PubMed] [Google Scholar]
- 82.Rafieian-kopaei M., Shakiba A., Sedighi M., Bahmani M. The Analgesic and Anti-Inflammatory Activity of Linum usitatissimum in Balb/c Mice. J. Evid.-Based Complement. Altern. Med. 2017;22:892–896. doi: 10.1177/2156587217717416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reddy P.R.T., Vandana K., Prakash S. Antibacterial and Anti-Inflammatory Properties of Plantago ovata Forssk. Leaves and Seeds against Periodontal Pathogens: An in Vitro Study. AYU Int. Q. J. Res. Ayurveda. 2018;39:226–229. doi: 10.4103/ayu.AYU_176_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nowak D.A., Snyder D.C., Brown A.J., Demark-Wahnefried W. The Effect of Flaxseed Supplementation on Hormonal Levels Associated with Polycystic Ovarian Syndrome: A Case Study. Curr. Top. Nutraceutical Res. 2007;5:177–181. [PMC free article] [PubMed] [Google Scholar]
- 85.Fons F., Gargadennec A., Rapior S. Culture of Plantago Species as Bioactive Components Resources: A 20-Year Review and Recent Applications. Acta Bot. Gall. 2008;155:277–300. doi: 10.1080/12538078.2008.10516109. [DOI] [Google Scholar]
- 86.Haroon M., Iqbal M.J., Hassan W., Ali S., Ahmed H., Hassan S.U. Evaluation of Methanolic Crude Extract of Linum usitatissimum for the Removal of Biofilm in Diabetic Foot Isolates. Brazilian J. Biol. 2023;83:3–4. doi: 10.1590/1519-6984.245807. [DOI] [PubMed] [Google Scholar]
- 87.Patton T., Barrett J., Brennan J., Moran N. Use of a Spectrophotometric Bioassay for Determination of Microbial Sensitivity to Manuka Honey. J. Microbiol. Methods. 2006;64:84–95. doi: 10.1016/j.mimet.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Molan P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001;2:13–19. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 89.Omoigui S. The biochemical origin of pain: The origin of all pain is inflammation and the inflammatory response. Part 2 of 3—Inflammatory profile of pain syndromes. Med. Hypotheses. 2009;69:1169–1178. doi: 10.1016/j.mehy.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu S.X., Gray-Owen S.D. Gonococcal Pelvic Inflammatory Disease: Placing Mechanistic Insights into the Context of Clinical and Epidemiological Observations. J. Infect. Dis. 2021;224:S56–S63. doi: 10.1093/infdis/jiab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheraghi J., Valadi A. Effect of Antinociceptive and Anti-Inflammatory Component of Limonene. Iran J. Med. Aromaterapic Plants. 2010;6:415–422. [Google Scholar]
- 92.Morsi E.A., Ahmed H.O., Abdel-Hady H., El-Sayed M., Shemis M.A. GC-Analysis, and Antioxidant, Anti-Inflammatory, and Anticancer Activities of Some Extracts and Fractions of Linum usitatissimum. Curr. Bioact. Compd. 2020;16:1306–1318. doi: 10.2174/1573407216666200206095954. [DOI] [Google Scholar]
- 93.Mechchate H., Es-Safi I., Conte R., Hano C., Amaghnouje A., Jawhari F.Z., Radouane N., Bencheikh N., Grafov A., Bousta D. In Vivo and in Vitro Antidiabetic and Anti-Inflammatory Properties of Flax (Linum usitatissimum L.) Seed Polyphenols. Nutrients. 2021;13:2759. doi: 10.3390/nu13082759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-jumaily E.F., Al-azawi A.H. Hepatoprotective Activity Of Lignan Compound From Flaxseed (Linum usitatissimum L.) against Acetaminophen-Induced Hepatotoxicity In Rabbits. World J. Pharm. Pharm. Sci. 2013;3:56–72. [Google Scholar]
- 95.Chaouche T.M., Haddouchi F., Ksouri R., Atik-Bekkara F. Evaluation of Antioxidant Activity of Hydromethanolic Extracts of Some Medicinal Species from South Algeria. J. Chin. Med. Assoc. 2014;77:302–307. doi: 10.1016/j.jcma.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 96.Pareek S., Paliwal R., Mukherjee S. Effect of Juice Extraction Methods and Processing Temperature-Time on Juice Quality of Nagpur Mandarin (Citrus reticulata Blanco) during Storage. J. Food Sci. Technol. 2011;48:197–203. doi: 10.1007/s13197-010-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakhlawy F.S. Inheritance of Oil Content, Unsaturated Fatty Acid Composition and Iodine Number of Oil in Flax. Menofiya J. Agric. Res. Egypt. 1996;20:483–492. [Google Scholar]
- 98.Ghannadi A.R., Ghassemi-Dehkordi N. Pharmacognostical Investigations on Sambucus ebulus L. and Sambucus nigra L. DARU. 1997;7:55–65. [Google Scholar]
- 99.Samani B.H., Khoshtaghaza M.H., Lorigooini Z., Minaei S., Zareiforoush H. Analysis of the Combinative Effect of Ultrasound and Microwave Power on Saccharomyces Cerevisiae in Orange Juice Processing. Innov. Food Sci. Emerg. Technol. 2015;32:110–115. doi: 10.1016/j.ifset.2015.09.015. [DOI] [Google Scholar]
- 100.Patel M.K., Mishra A., Jha B. Non-Targeted Metabolite Profiling and Scavenging Activity Unveil the Nutraceutical Potential of Psyllium (Plantago ovata Forsk) Front. Plant Sci. 2016;7:431. doi: 10.3389/fpls.2016.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koo K.L.K., Ammit A.J., Tran V.H., Duke C.C., Roufogalis B.D. Gingerols and Related Analogues Inhibit Arachidonic Acid-Induced Human Platelet Serotonin Release and Aggregation. Thromb. Res. 2001;103:387–397. doi: 10.1016/S0049-3848(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 102.Lantz R.C., Chen G.J., Sarihan M., Sólyom A.M., Jolad S.D., Timmermann B.N. The Effect of Extracts from Ginger Rhizome on Inflammatory Mediator Production. Phytomedicine. 2007;14:123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Jandali R., Agha M.I.H. Lipoxygenase Inhibitory Activity of Some Extracts Prepared from Flaxseed (Linum usitatissimumv L.) Curr. Bioact. Compd. 2021;18:e091221197172. doi: 10.2174/1574893616666211012091140. [DOI] [Google Scholar]
- 104.Abouzied M.M., Mahmoud S.M., Wahid A., Ahmed A.E., Okasha A.M., Soliman H.A., Thagfan S.S.A., Attia E.Z. A Study of the Hepatoprotective Effect of Plantago psyllium L. Seed Extract against Carbon Tetrachloride Induced Hepatic Injury in Rats. J. Appl. Biomed. 2020;18:80–86. doi: 10.32725/jab.2020.006. [DOI] [PubMed] [Google Scholar]
- 105.Del Carmen Recio M., Giner R.M., Manez S., Rios J.L. Structural Considerations on the Iridoids as Anti-Inflammatory Agents. Planta Med. 1994;60:232–234. doi: 10.1055/s-2006-959465. [DOI] [PubMed] [Google Scholar]
- 106.Murai M., Tamayama Y., Nishibe S. Phenylethanoids in the Herb of Plantago Lanceolata and Inhibitory Effect on Arachidonic Acid-Induced Mouse Ear Edema. Planta Med. 1995;61:479–480. doi: 10.1055/s-2006-958143. [DOI] [PubMed] [Google Scholar]
- 107.Nishibe S., Sasahara M., Jiao Y., Yuan C.L., Tanaka T. Phenylethanoid Glycosides from Plantago Depressa. Phytochemistry. 1993;32:975–977. doi: 10.1016/0031-9422(93)85238-M. [DOI] [Google Scholar]
- 108.De Jesus N.Z.T., de Souza Falcão H., Gomes I.F., de Almeida Leite T.J., de Morais Lima G.R., Barbosa-Filho J.M., Tavares J.F., da Silva M.S., de Athayde-Filho P.F., Batista L.M. Tannins, Peptic Ulcers and Related Mechanisms. Int. J. Mol. Sci. 2012;13:3203–3228. doi: 10.3390/ijms13033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al Waili N.S., Boni N. Natural Honey Lowers Plasma Prostaglandin Concentrations in Normal Individuals. J. Med. Food. 2003;6:129–133. doi: 10.1089/109662003322233530. [DOI] [PubMed] [Google Scholar]
- 110.Ghule A.E., Jadhav S.S., Bodhankar S.L. Renoprotective Effect of Linum Usitatissimum Seeds through Haemodynamic Changes and Conservation of Antioxidant Enzymes in Renal Ischaemia-Reperfusion Injury in Rats. Arab J. Urol. 2011;9:215–221. doi: 10.1016/j.aju.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shakir K.A.F., Madhusudhan B. Hypocholesterolemic and Hepatoprotective Effects of Flaxseed Chutney: Evidence from Animal Studies. Indian J. Clin. Biochem. 2007;22:117–121. doi: 10.1007/BF02912893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available through the request via Prof. Dr. Arshiya Sultana (drarshiya@yahoo.com).