Abstract
Cells of Actinobacillus actinomycetemcomitans, a gram-negative pathogen responsible for an aggressive form of juvenile periodontitis, form tenaciously adherent biofilms on solid surfaces. The bacteria produce long fibrils of bundled pili, which are required for adherence. Mutations in flp-1, which encodes the major subunit of the pili, or any of seven downstream tad genes (tadABCDEFG) cause defects in fibril production, autoaggregation, and tenacious adherence. We proposed that the tad genes specify part of a novel secretion system for the assembly and transport of Flp pili. The predicted amino acid sequence of TadA (426 amino acids, 47,140 Da) contains motifs for nucleotide binding and hydrolysis common among secretion NTP hydrolase (NTPase) proteins. In addition, the tadA gene is the first representative of a distinct subfamily of potential type IV secretion NTPase genes. Here we report studies on the function of TadA. The tadA gene was altered to express a modified version of TadA that has the 11-residue epitope (T7-TAG) fused to its C terminus. The TadA-T7 protein was indistinguishable from the wild type in its ability to complement the fibril and adherence defects of A. actinomycetemcomitans tadA mutants. Although TadA is not predicted to have a transmembrane domain, the protein was localized to the inner membrane and cytoplasmic fractions of A. actinomycetemcomitans cells, indicating a possible peripheral association with the inner membrane. TadA-T7 was purified and found to hydrolyze ATP in vitro. The ATPase activity is stimulated by Triton X-100, with maximal stimulation at the critical micellar concentration. TadA-T7 forms multimers that are stable during sodium dodecyl sulfate-polyacrylamide gel electrophoresis in nonreducing conditions, and electron microscopy revealed that TadA-T7 can form structures closely resembling the hexameric rings of other type IV secretion NTPases. Site-directed mutagenesis was used to substitute Ala and Gln residues for the conserved Lys residue of the Walker A box for nucleotide binding. Both mutants were found to be defective in their ability to complement tadA mutants. We suggest that the ATPase activity of TadA is required to energize the assembly or secretion of Flp pili for tight adherence of A. actinomycetemcomitans.
Bacteria use sophisticated molecular machines to move macromolecules across their membranes (7, 43, 62). Secretion of proteins, such as proteases, pili, and toxins, can provide distinct selective advantages to bacteria in various environmental niches, and many of the well-studied secreted proteins are important colonization and virulence factors released by pathogenic bacteria. In addition, the rapid evolution that characterizes bacterial genomes is known to be driven in large part by mechanisms of gene exchange that transport DNA across membranes from one cell to another.
Despite the wide variety of substrates, macromolecular transport in bacteria is currently known to be carried out by five secretion mechanisms (types I to V), whose classification is broadly based on sequence, structure, and function (4, 22, 62). In type I and III secretion systems, proteins are transported across the inner and outer membranes in one step in a signal sequence-independent manner (1, 27, 45). Type I systems release proteins, such as hemolysin, through a transmembrane channel (24, 45). In type III secretion, proteins are secreted directly from the bacterial cytoplasm into the animal or plant host cell through a contact-mediated process (27). Type I, type III, and most of the type IV secretion systems are sec-independent pathways (4, 39, 40, 43). In contrast, proteins secreted by types II and V and at least one type IV system are first transported across the inner membrane to the periplasm in a sec-dependent manner (4, 22, 51). These proteins must have an N-terminal signal sequence, which is cleaved during translocation across the inner membrane (3, 14, 51). In type II systems, such as the well-studied pullulanase secretion system of Klebsiella spp., proteins are next transported from the periplasm across the outer membrane by a multiprotein apparatus (12, 51). Type IV secretion systems are known to transport proteins or protein-DNA complexes (4, 6, 7, 42). The type IV secretion systems that transport protein-DNA complexes include the conjugative-transfer systems of IncN plasmid pKM101 (67), IncP plasmid RP4 (39, 42), IncW plasmid R388 (39, 53), and the tumor-inducing T-DNA transfer system of Agrobacterium tumefaciens Ti plasmids (6, 7). Type IV systems that appear to be dedicated exclusively to protein transfer include the cag pathogenicity island of Helicobacter pylori (5), which secretes the high-molecular-weight CagA protein into gastric epithelial cells, and the secretion system for pertussis toxin of Bordetella pertussis (66). Recently, Legionella pneumophila was found to encode a type IV system that appears capable of secreting protein virulence factors and transporting DNA by conjugative transfer (58, 64). The list of organisms harboring type IV secretion systems has grown rapidly (for recent reviews see references 8 and 40). The recently classified type V secretion systems are autotransporters (22, 23). A well-studied example is the immunoglobulin A protease of Neisseria gonorrhoeae (34, 46). In this system, the C-terminal domain of the secreted protease mediates transport of the protein across the outer membrane.
Actinobacillus actinomycetemcomitans is a gram-negative facultative anaerobe responsible for an aggressive form of periodontal disease known as localized juvenile periodontitis, as well as other human diseases including endocarditis (17, 60, 69). A. actinomycetemcomitans produces several putative virulence factors, including a well-characterized RTX leukotoxin (17, 30, 36, 41). Colonies of fresh clinical isolates display characteristic wrinkled surfaces, referred to as rough-colony morphology (15, 25, 29, 31). When grown in broth, rough clinical isolates of A. actinomycetemcomitans adhere tightly to solid surfaces such as hydroxyapatite, glass, and plastic (16, 33, 54). The nonspecific adherence is remarkably tenacious, such that cells are resistant to vigorous shaking or vortex mixing of the culture tube. The cells also autoaggregate and produce long fibrils, which are required for tight adherence (15, 29, 31, 32, 54). The tight, nonspecific adherence of A. actinomycetemcomitans is likely to be important for colonization of the tooth surface (15, 31).
We have recently identified a cluster of seven genes (tadABCDEFG) required for tight adherence of A. actinomycetemcomitans (31). Loss of function of any of the tad genes in a rough clinical isolate results in severely diminished adherence and a smooth-colony morphology. The mutant cells no longer produce bundles of pili (fibrils) and fail to autoaggregate (31). We have also determined that mutations in the flp-1 gene, which encodes the major pilus subunit (28), cause a similar phenotype (32). The flp-1 gene, located approximately 5 kb upstream of tadA, is the first gene of an apparent operon that includes the tadABCDEFG cluster (32). Thus, the entire 12-kb region includes genes involved in the production of Flp fibrils. The TadB to TadG proteins are unrelated to proteins of known function, although they all contain at least one possible membrane-spanning domain and are thus predicted to be integral membrane proteins (31). We have proposed that Tad proteins form part of a membrane complex for the secretion and assembly of Flp fibrils required for adherence. Similar flp-tad gene clusters are widespread in Bacteria and Archaea, and the properties of this region in A. actinomycetemcomitans are suggestive of its acquisition by a horizontal-transfer event (31, 48). Little is known about the functions of the tad loci in other organisms. A related locus in Caulobacter crescentus with genes corresponding to those of the flp-1–tadC region was found to be responsible for the production of novel pili of unknown function (59), and a Pasteurella multocida tadD mutant is severely attenuated for virulence in mice (18).
All known secretion systems have at least one protein with the highly conserved Walker A and B motifs for nucleotide binding and hydrolysis. These proteins are thought to use NTP hydrolysis to provide the energy for secretion complex assembly or the movement of macromolecules across membranes (6–8, 13, 49, 65). Among the large number of type IV secretion systems, NTP hydrolase (NTPase) activity has been reported for only a few, including VirB11 encoded by Ti plasmids (9), TrbB encoded by IncP plasmid RP4 (39), TrwD encoded by IncW plasmid R388 (39, 53), and HP0525 encoded by the cag pathogenicity island of H. pylori (39). The amino acid sequence of TadA displays similarity with those of predicted or known type IV secretion NTPases (31, 48). Recent extensive sequence and phylogenetic analyses of predicted secretion NTPase genes revealed the existence of a distinct TadA subfamily of predicted type IV NTPase genes that is widespread among gram-positive and gram-negative Bacteria as well as Archaea (48). Here we report that the TadA protein of A. actinomycetemcomitans is an ATPase required for fibril production and tenacious adherence.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A. actinomycetemcomitans strains (Table 1) were grown in AAGM (A. actinomycetemcomitans growth medium) broth (16). The glucose and NaHCO3 were added to the medium after autoclaving. AAGM agar for plates was made similarly, except that 40 g of Trypticase agar was substituted for the Trypticase soy broth. When appropriate, media for A. actinomycetemcomitans were supplemented with 4 μg of chloramphenicol/ml and 20 μg of nalidixic acid/ml. Broth cultures of A. actinomycetemcomitans were grown in screw-cap plastic tubes at 37°C for approximately 24 h. Plates were incubated at 37°C in a CO2-enriched environment in a sealed GasPak container (BBL) for 72 h. Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) broth with aeration or on LB agar. When appropriate, media for E. coli were supplemented with 50 μg of chloramphenicol/ml and 50 μg of kanamycin/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| A. actinomycetemcomitans | ||
| CU1000N | Spontaneous nalidixic acid-resistant mutant of rough clinical isolate CU1000 | 31 |
| AA1360 | CU1000N tadA mutant; tadA2::IS903φkan; Kmr | 31 |
| E. coli | ||
| Top10 | F−mcrA Δ(mrr hsdRMS mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pEK2 | pJAK16 with tadA from CU1000N expressed from the tac promoter | 31 |
| pJAK16 | Broad-host-range, mobilizable IncQ vector derived from pMMB67; lacIq; tacp | 63 |
| pRK21761 | RK2 oriT mutant used to mobilize plasmids from E. coli to A. actinomycetemcomitans; tetA::lacZYA | 63 |
| pSK174 | pJAK16 with tacp-tadA-T7 | This study |
| pSK194 | pJAK16 with tacp-tadA-T7(K210Q) | This study |
| pSK199 | pJAK16 with tacp-tadA-T7(K210A) | This study |
DNA procedures.
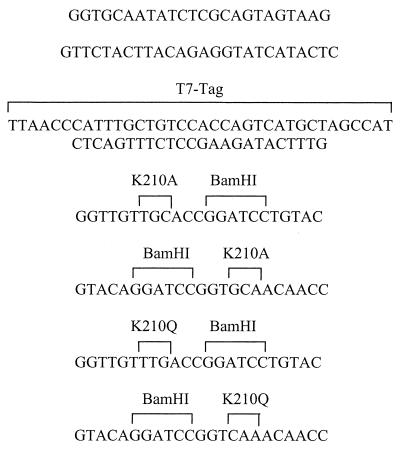
Plasmid DNA was isolated from E. coli using plasmid Mini-prep kits (Qiagen, Valencia, Calif.). Agarose gel electrophoresis has been described previously (57). DNA manipulations with restriction endonucleases and T4 DNA ligase were done according to the manufacturers' recommendations. Amplification of DNA by PCR was done with Taq DNA polymerase (56). The PCR primers used for the synthesis of tadA derivatives are presented in Table 2. To construct TadA-T7 proteins with substitutions at amino acid residue 210, we used the appropriate primers (Table 2) to amplify, as separate products, the 5′ and 3′ fragments of the gene. The products were then digested with the appropriate enzymes (Table 2) and ligated. The ligated products were reamplified using the flanking primer pair that amplified the whole gene. PCR products were cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.). Transformation of E. coli was done by the method of Cohen et al. (10) or by using Top10 OneShot cells according to the manufacturer's recommendations (Invitrogen). Nucleotide sequences were determined by the Columbia University DNA Sequencing Facility using Perkin-Elmer Applied Biosystems automated DNA sequencer 373A. For expression of genes in A. actinomycetemcomitans, the appropriate fragments were subcloned from pCR2.1 into pJAK16, a broad-host-range, mobilizable IncQ vector (Table 1), using standard techniques. Mobilization of pJAK16 derivatives into A. actinomycetemcomitans by conjugative transfer was described previously (63).
TABLE 2.
Properties of primers used in this study
TadA-T7 expression and localization in A. actinomycetemcomitans.
Single colonies of A. actinomycetemcomitans containing tacp-tadA-T7 expression plasmid pSK174 were used to inoculate each of four tubes containing 10 ml of AAGM with chloramphenicol and IPTG (isopropyl-β-d-thiogalactopyranoside; 0.1 mM). The cultures were incubated for 24 h, pooled, and then centrifuged at 12,000 × g for 10 min to pellet the cells. Fractionation was performed essentially as described by Haase et al. (21).
Transmission electron microscopy.
Colonies were resuspended in 0.01 M Tris-HCl (pH 7.5), and a drop of the suspension was placed on each Formvar- and carbon-coated copper grid (EM Sciences, Fort Washington, Pa.) and left for approximately 10 min. Grids were immersed in a drop of 1% uranyl acetate and removed immediately; the excess stain was wicked away. Grids were viewed in a JEOL 1200EX transmission electron microscope at 80.0 kV. For visualization of the TadA-T7 protein, a solution of protein (∼50 ng/μl) was added to the grid and then stained and examined.
Expression and purification of TadA-T7 in E. coli
Strains SK0378 and SK0108 were grown overnight in LB broth with chloramphenicol. One milliliter was removed and added to 100 ml of fresh LB broth plus chloramphenicol. This culture was grown for 30 min before IPTG was added to a final concentration of 1.0 mM. After 4 h of additional incubation, the cells were harvested by centrifugation at 12,000 × g for 10 min. The pellet was resuspended in 10 ml of sonication buffer (10 mM Tris-HCl [pH 8.0], 5.0 mM MgCl2, 100 mM NaCl). The cells were sonicated with four repeats of 10 2-s pulses on ice, followed by a 30-s pause. The sonicate was centrifuged at 6,000 × g for 10 min to remove any unbroken cells and large cell debris. The supernatant, which contained soluble protein, was passed through a 0.45-μm-pore-size filter (Pall Corp., Ann Arbor, Mich.). TadA-T7 was purified by using the T7-TAG affinity purification kit (Novagen, Madison, Wis.). One milliliter of the antibody-agarose slurry was used for every 100 ml of starting culture. Purification was carried out in gravity columns supplied by Novagen.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
Protein samples were boiled for 5 min and separated by electrophoresis through a 12 or 8% polyacrylamide gel with a stacking gel of 5% (2). The gel was run at 70 to 100 V until the dye ran to the end of the gel. Gels were either prepared for Western blot analysis or stained overnight with 0.5% Coomassie blue and destained in 50% methanol–10% acetic acid for 1 to 2 h. Detection of TadA-T7 was carried out using an anti-T7 monoclonal antibody (Novagen). Western blot analysis was performed as described by Rosche et al. (55).
ATPase assay.
ATP hydrolysis was assayed by measuring the release of 32P-labeled inorganic phosphate from [γ-32P]ATP (New England Nuclear; 6,000 Ci/mmol). The reaction mixture (4 μl) containing 30 ng of protein, 10 μM [γ-32P]ATP (100 Ci/mmol) in buffer A (25 mM potassium salt of HEPES [pH 7.0], 12.5 mM MgCl2, 5 mM β-mercaptoethanol, 10% [vol/vol] glycerol) was incubated at 30°C for 30 min. The reaction was stopped by adding 0.5 μl of 50% trichloroacetic acid. The reaction mixture was spotted on a thin-layer chromatography (TLC) plate (polyethyleneimine-cellulose with fluorescent indicator; Fisher Scientific). The TLC plate was developed in 1 M formic acid–0.5 M LiCl. ATPase activity was measured by excising the spots containing radioactivity and counting the amount of radioactivity using a scintillation counter. Both the product and unreacted substrate were counted and used for ATPase activity calculations.
In situ ATPase activity.
The method of Koronakis et al. (37) was modified for the detection of ATPase activity on SDS-polyacrylamide gels. Protein electrophoresis was carried out in SDS–8% polyacrylamide gels by following standard protocols with the following modifications. The SDS concentration in the sample loading buffer, the gel, and the gel running buffer was 0.05% instead of 0.1%. Sample buffer with 5% β-mercaptoethanol was used. Protein samples were not heated before being loaded on the gel. The electrophoresis apparatus, including running buffers, was cooled in ice before use, and electrophoresis was done at low voltage (65 V) in ice to prevent protein denaturation. Following electrophoresis, the gel was soaked in buffer A (see above) containing 0.1% Triton X-100 with gentle shaking for 1 h during which time the buffer was changed twice. After this the gel was incubated in buffer A containing 2 mM ATP for 60 min at 37°C and then stained with detection solution (0.034% malachite green, 0.1% Triton X-100, and 10.5 g of ammonium molybdate per liter of 1 M HCl). Another gel was run in parallel and stained with Coomassie blue or blotted with an anti-T7-TAG antibody to determine the position of migration of TadA-T7.
RESULTS
Construction of functional, epitope-tagged TadA.
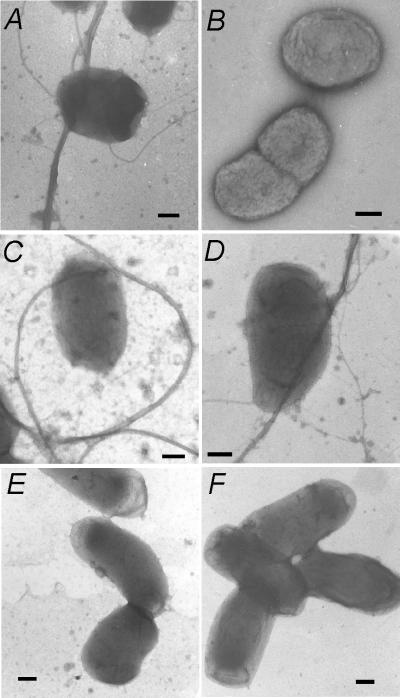
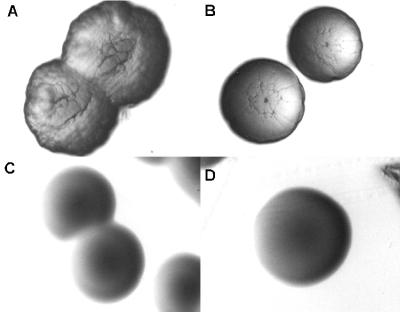
To facilitate the study of the TadA protein of A. actinomycetemcomitans, we constructed a gene fusion to express the TadA polypeptide with the 11-amino-acid T7-TAG epitope added to its C terminus. For regulated expression, the gene fusion (tadA-T7) was placed downstream of the IPTG-inducible tac promoter on a broad-host-range IncQ vector. Induction in E. coli (data not shown) and A. actinomycetemcomitans (Fig. 1) resulted in expression of a polypeptide of the expected molecular mass, as determined by Western blot analysis with a monoclonal anti-T7-TAG antibody. To determine if the TadA-T7 polypeptide is functional in vivo, we tested its ability to complement a tadA mutation in A. actinomycetemcomitans. The tadA mutant is defective in nonspecific tight adherence to surfaces, fails to express long, bundled fibrils, and forms smooth colonies in contrast to the rough colonies of wild-type strains (31). Earlier results with an isogenic plasmid containing the wild-type tadA gene showed that leaky expression from the tac promoter was sufficient to achieve complementation (31) (Fig. 2). We found that tadA-T7 was likewise able to complement the tadA mutation in the absence of induction. The low-level expression of tadA-T7 restored tight adherence (data not shown) and fibril production (Fig. 2C) and conferred a rough phenotype on colonies (Fig. 3B). These results show that the TadA-T7 fusion protein is functional in vivo.
FIG. 1.
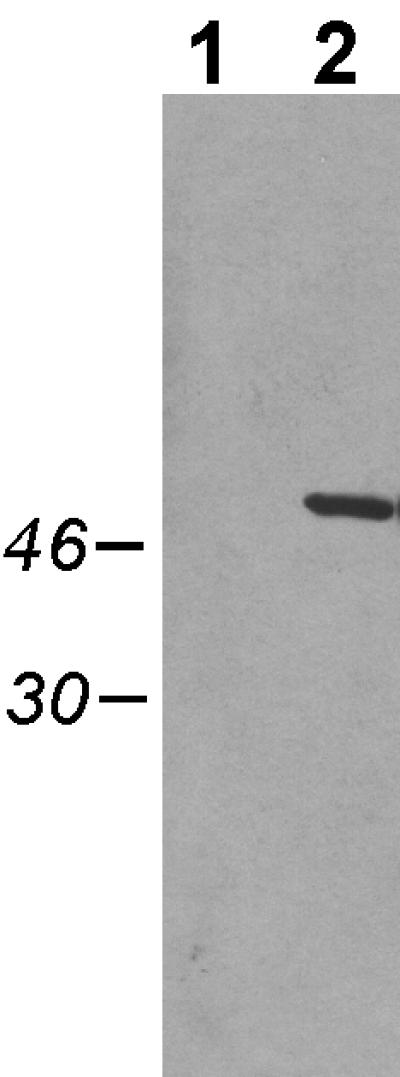
Expression of TadA-T7 in A. actinomycetemcomitans visualized by Western blot analysis. Cells were grown overnight in the presence of 0.1 mM IPTG, pelleted, and then resuspended in SDS loading dye, boiled, and separated by SDS-PAGE through a 12% polyacrylamide gel, as described in Materials and Methods. Lane 1, strain CU1000N carrying pJAK16 (vector); lane 2, strain CU1000N carrying pSK174 (tadA-T7). Numbers (left), molecular masses of relevant markers in kilodaltons.
FIG. 2.
Complementation of a tadA mutation for fibril formation. Cells were stained with 1% uranyl acetate and then viewed by electron microscopy, as described in Materials and Methods. (A) CU1000N tadA+; (B to F) AA1360 mutant with pJAK16 vector (B), wild-type tadA plasmid pEK2 (C), wild-type tadA-T7 plasmid pSK174 (D), tadA-T7(K210A) plasmid pSK199 (E), and tadA-T7(K210Q) plasmid pSK194 (F). Scale bars, 200 nm.
FIG. 3.
Complementation of the tadA mutation with tadA-T7 for rough-colony morphology. (A) CU1000N tadA+; (B to D) AA1360 tadA mutant with wild-type tadA-T7 plasmid pSK174 (B), tadA-T7(K210A) plasmid pSK199 (C), and tadA-T7(K210Q) plasmid pSK194 (D). The rough appearance of the colonies in panel B is typical for complemented tad mutations, as previously described (31).
Localization of TadA-T7.
Type IV secretion NTPases are thought to function at the inner membrane (9, 52, 53). To determine the localization pattern of TadA-T7, wild-type and tadA mutant strains of A. actinomycetemcomitans carrying the tadA-T7 plasmid were treated with 0.1 mM IPTG. Whole-cell extracts were prepared and separated into cytoplasmic, inner membrane, and outer membrane fractions, using the procedure of Haase et al. (21) as described in Materials and Methods. While enzymatic markers for assessing the purity of the fractions in A. actinomycetemcomitans have not yet been established, Coomassie staining of polypeptides separated by SDS-PAGE showed reproducibly distinct patterns for the three fractions (data not shown). Western blot analysis of these fractions with a monoclonal anti-T7-TAG antibody revealed that TadA-T7 could be found in both the cytoplasm and the inner membrane fractions (Fig. 4).
FIG. 4.
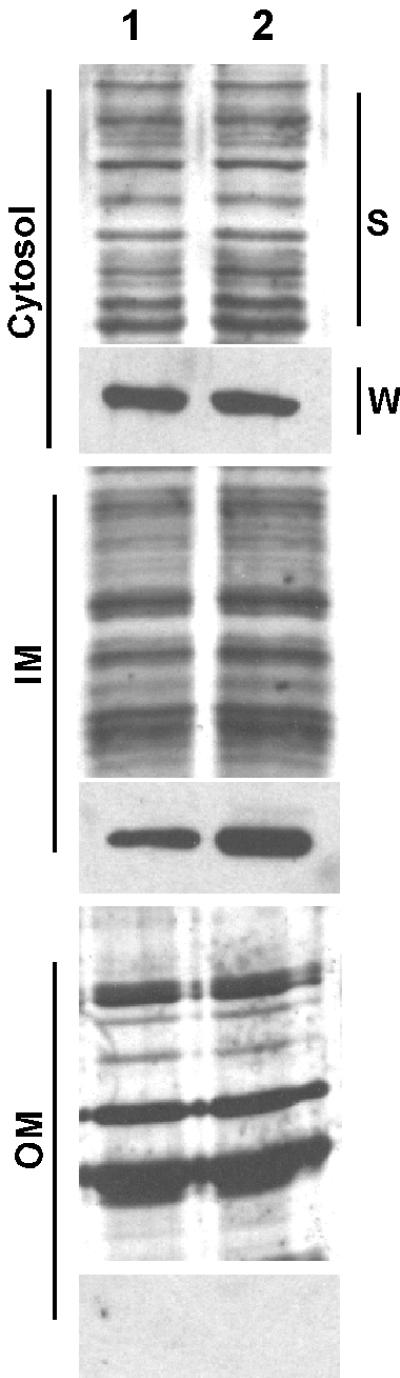
Cellular location of TadA-T7. Fractionation of cells into cytoplasmic (cytosol), inner membrane (IM), and outer membrane (OM) fractions was done by the method of Haase et al. (21), as described in Materials and Methods. All strains carry the TadA-T7-expressing plasmid. Samples were separated by SDS-PAGE through a 12% polyacrylamide gel. Lane 1, AA1360 tadA mutant; lane 2, CU1000N tadA+. S, silver-stained gel; W, Western blot with anti-T7-TAG monoclonal antibody.
Purification of TadA-T7.
The TadA-T7 protein was purified from E. coli by affinity chromatography using agarose beads containing the anti-T7-TAG monoclonal antibody, as described in Materials and Methods. After separation by SDS-PAGE, the peak protein fraction appeared to be >95% pure, as evidenced by the presence of only one band by Coomassie blue staining and Western blot analysis (Fig. 5). The yield was approximately 1 mg of purified protein/liter of starting culture. For a control, an extract from E. coli cells containing the vector plasmid (pJAK16) was mock purified by the same procedure as that used for the TadA-T7 fusion protein. The corresponding fractions from the mock purification did not show a protein band after SDS-PAGE and Coomassie staining or Western blot analysis. These fractions were used as a negative control in the ATPase assays (below).
FIG. 5.
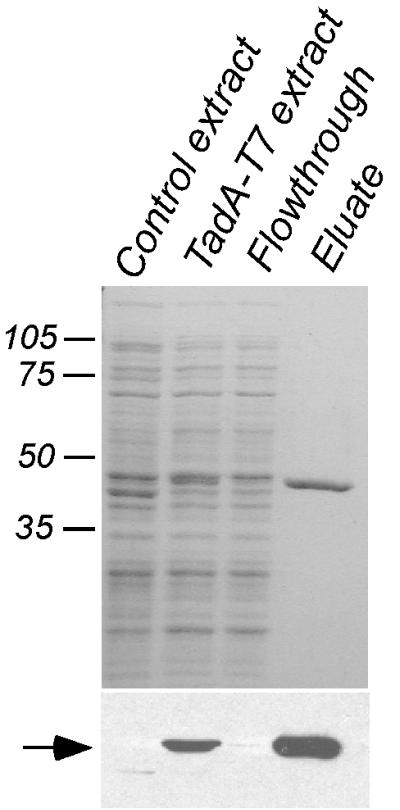
Purification of TadA-T7 from E. coli. TadA-T7 was purified by affinity chromatography, as described in Materials and Methods. (Top) 12% polyacrylamide gel stained with Coomassie blue; (bottom) Western blot analysis with anti-T7-TAG antibody. Numbers, molecular masses of relevant markers in kilodaltons. Arrow, TadA-T7. No additional bands were seen when threefold more eluate was loaded.
Multimer formation by TadA-T7.
When the TadA-T7 preparations were separated by SDS-PAGE in the absence of β-mercaptoethanol, Western blot analysis revealed multiple higher-molecular-weight species, which were able to survive the strong denaturing conditions of 0.1% SDS and boiling for 5 min (Fig. 6). The predominant species were those migrating at the expected positions of TadA-T7 dimers, trimers, and higher multimers. That the high-molecular-weight species were true multimers of TadA-T7 was indicated by their conversion to monomers in the presence of 5% β-mercaptoethanol (Fig. 6). Furthermore, matrix-assisted laser desorption ionization mass spectrometry of an extracted trimer-size band confirmed that this species was composed entirely of TadA-T7 (data not shown). Additional evidence that TadA-T7 can exist as a multimer was obtained by electron microscopy of purified TadA-T7 protein preparations, which revealed the existence of ring-shaped structures with a central region, where the negative stain was able to penetrate (Fig. 7). The outer diameter of the ring is approximately 17 nm, and there is a central electron-dense region 3 nm in diameter. The shape and dimensions are suggestive of the hexameric ring structures seen for the secretion ATPase proteins TrbB (encoded by plasmid RP4) and cag HP0525 (of H. pylori) (38). No difference in the appearance of TadA-T7 was observed when ATP was present.
FIG. 6.

Multimer formation by TadA-T7 visualized by Western blot analysis. Proteins were boiled in loading buffer in the absence or presence of β-mercaptoethanol (β-me) and separated by SDS-PAGE through an 8% polyacrylamide gel. Arrow, monomeric TadA-T7. The right panel is an overexposed version of the left to show minor bands. Numbers, molecular masses of relevant markers in kilodaltons.
FIG. 7.

Electron microscopy of TadA-T7 protein. Purified TadA-T7 was stained in 1% uranyl acetate and viewed in the electron microscope, as described in Materials and Methods. (Top) Typical field of view showing many protein molecules. Scale bar, 50 nm. (Bottom) Selected images of individual ring-like molecules, with electron-dense centers.
TadA-T7 is an ATPase.
The predicted amino acid sequence of TadA contains the conserved motifs for nucleotide binding and hydrolysis commonly found in NTPases: Walker A and B boxes and an Asp box (Fig. 8A) (53, 65). We therefore tested purified TadA-T7 for ATPase activity. Incubation of TadA-T7 with [γ-32P]ATP resulted in the release of 32P-labeled inorganic phosphate, as detected by TLC (Fig. 8B, lane 2). The identity of the labeled phosphate band was confirmed by comparing its migration on TLC plates to that of unlabeled mono-, di-, and triphosphates and adenosine mono-, di-, and triphosphates (data not shown). Mock-purified protein from E. coli cells containing the plasmid vector lacking tadA-T7 did not exhibit ATPase activity (Fig. 8B, lane 3). To confirm that the observed ATPase activity resulted from the TadA-T7 protein, the activity of the purified protein was assayed in situ after separation in polyacrylamide gels, as described in Materials and Methods. We first separated TadA-T7 under denaturing conditions by SDS-PAGE. The protein was then renatured in situ with Triton X-100 and assayed. The ATPase activity band (Fig. 8C, lane 2) corresponded with the TadA-T7 band detected by Coomassie blue staining (Fig. 8C, lane 1) and Western blot analysis (data not shown). We conclude that ATPase activity is a property of TadA.
FIG. 8.
ATPase activity of TadA-T7. (A) Schematic of the TadA amino acid sequence with nucleotide-binding and hydrolysis motifs common to other secretion NTPases. (B) TLC showing [γ-32P]ATP hydrolysis by TadA-T7 (left) and its stimulation by Triton X-100 (right). Reactions and TLC were done as described in Materials and Methods. Lanes 1 and 4, no TadA-T7; lanes 2 and 5, TadA-T7; lane 3, mock-purified extract lacking TadA-T7; lane 6, TadA-T7 in 250 μM Triton X-100. The right panel was underexposed to allow quantitation. (C) In situ ATPase activity of TadA-T7. Purified TadA-T7 was subjected to SDS-PAGE, renatured in the gel by treatment with Triton X-100, and assayed for ATPase activity as described in Materials and Methods. Lane 1, Coomassie blue-stained gel showing the position of TadA-T7 (arrow); lane 2, malachite green staining of released inorganic phosphate.
Characterization of the ATPase activity.
The ATPase activity of TadA-T7 requires the presence of Mg2+ or Ca2+, and it is completely inhibited in the presence of 5 mM EDTA (data not shown). Using standard methods, the Km for ATP was determined to be 9 μM and the specific activity was determined to be 15 nmol/min/mg of protein. ATPase activity was inhibited by ribo- and deoxyribonucleotides, suggesting that they can compete with the substrate for binding to the active site. At 100 μM concentrations, the percentages of inhibition of ATPase activity were 92 (dATP), 70 (dGTP), 58 (dTTP), and 58% (dCTP).
Because TadA-T7 was found to be membrane associated, we tested the effect of nonionic detergent Triton X-100 on ATPase activity. We observed an increase in activity over a range of Triton X-100 concentrations, with a maximum enhancement of about 10-fold at the critical micellar concentration (250 μM) (Fig. 8B; compare lanes 5 and 6).
Mutants altered in the Walker A box.
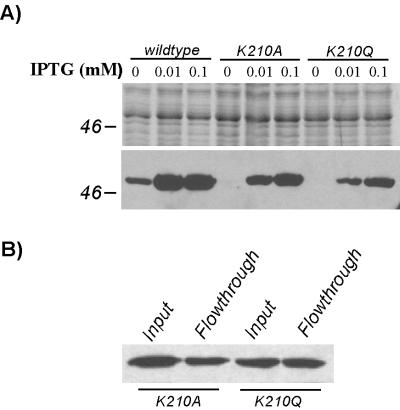
The highly conserved lysine residue of conserved Walker A box sequence GXXGXGKT/S is known to interact with bound nucleotides. Substitution of different amino acids for this lysine has been shown to decrease or abolish NTPase activity in several proteins (11, 35, 53, 61). We used site-directed mutagenesis to change the corresponding lysine residue (K210) of the TadA-T7 Walker A box to alanine (K210A) and glutamine (K210Q). Expression of the mutant genes was induced by IPTG, and the proteins were examined by SDS-PAGE. Western blot analysis revealed that the mutant proteins accumulated to significantly lower levels than the wild-type TadA-T7 (Fig. 9). To determine if the K210A and K210Q alleles could complement the tadA mutation in A. actinomycetemcomitans, we used appropriate concentrations of IPTG to express the mutant proteins to levels similar to that of uninduced TadA-T7, which allows complementation. Even at levels of expression comparable to or greater than that of wild-type TadA-T7, the mutations failed to complement the tadA mutation. The cells showed no evidence of adherence, fibril production (Fig. 2E and F), or ability to produce rough colonies (Fig. 3C and D). However, expression of the K210A and K210Q mutants in wild-type tadA+ A. actinomycetemcomitans resulted in a noticeable decrease of adherence, which could be indicative of a partially dominant-negative phenotype (data not shown). The mutant proteins were also localized by the cell fractionation methods described above, and the localization patterns of the mutant proteins were the same as that of the wild-type TadA-T7 protein (data not shown). These results suggest that the failure to complement the tadA mutation does not result from insufficient protein or improper localization of the mutant TadA-T7 polypeptides. We conclude that the conserved lysine at position 210, predicted to be important for ATP binding, is essential for TadA function in vivo.
FIG. 9.
Expression properties of TadA-T7 lysine (K210) mutants. (A) A. actinomycetemcomitans tadA mutant strains (AA1360) expressing wild-type TadA-T7, TadA-T7(K210A), or TadA-T7(K210Q) were grown for ∼24 h in the presence 0, 0.01, or 0.1 mM IPTG. Top, Coomassie blue-stained gel (12% polyacrylamide) to demonstrate total amount of protein loaded; bottom, Western blot analysis with anti-T7-TAG antibody to detect TadA-T7. (B) Failure of TadA-T7(K210A) and -K210Q mutant polypeptides to bind to the T7-TAG affinity column. Extracts from E. coli expressing either TadA-T7(K210A) or TadA-T7(K210Q) were passed over affinity columns as described in Materials and Methods. Approximately equal amounts of protein were detected in both the input and flowthrough fractions.
DISCUSSION
Recent extensive phylogenetic analysis of 148 genes for putative secretion NTPases has revealed that tadA of A. actinomycetemcomitans is representative of a large subfamily of predicted type IV secretion NTPase genes that are widespread among Bacteria and Archaea (48). In this work, we constructed an epitope-tagged version of TadA (TadA-T7) that is functional in vivo. The purified protein has ATPase activity, and mutations that alter the conserved lysine of the Walker A nucleotide-binding motif inactivate TadA-T7 function in vivo. As expected for a secretion NTPase (6, 40), the protein appeared to be associated with the inner membrane. The tad gene cluster of A. actinomycetemcomitans is required for expression of long fibrils that mediate tenacious, nonspecific adherence to surfaces (31). These results are consistent with our proposal that TadA is the energy module for secretion or assembly of the Flp pili.
The ATPase activity was strongly indicated by the predicted amino acid sequence of TadA. The most obvious features of the sequence are the Walker A (P loop), Walker B, and the Asp boxes (Fig. 8A) common among NTPases. A His box of unknown function and conserved in other type IV NTPases is also present (Fig. 8A) (53). Despite the presence of the conserved nucleotide-binding motifs, few predicted secretion NTPases have been shown to hydrolyze NTPs (9, 39, 50). The recently determined crystal structure of the type IV secretion NTPase HP0525 (68) has added functional significance to the conserved residues found in TadA. Walker A box residues, which coordinate the β- and γ-phosphate moieties of nucleotide di- and triphosphates, are identical in TadA and HP0525. The Glu residues predicted to be involved in ATP hydrolysis in HP0525 are present in TadA at positions 235 (in the Asp box) and 280 (in Walker box B). The Arg residue of Walker box B that coordinates the γ-phosphate of ATP in HP0525 is present at position 272 of TadA. Other residues outside these common motifs are also conserved. For example, the HP0525 Arg and Ser residues that coordinate the β-phosphate of ADP and the Arg residue predicted to be in contact with the γ-phosphate of ATP are found at the corresponding positions in TadA (at Arg 164 and Ser 167 and at Arg 146, respectively).
As a direct test of the role of nucleotide binding in TadA, we found that the highly conserved Lys 210 residue in the Walker A box is required for function in vivo. The Walker A box TadA-T7 mutants revealed an unexpected property of the C terminus. The mutant proteins were expressed, albeit to significantly lower levels than wild-type TadA-T7, and they could be detected by Western blot analysis. However, when we attempted to purify the mutant proteins to test for defects in ATP hydrolase activity, we found that neither mutant would bind to the affinity column (Fig. 9B). The crystal structure of the HP0525 secretion NTPase indicates that the conformation of the protein depends on interactions of the N-terminal and C-terminal domains with bound nucleotides (68). Thus, a point mutation of TadA predicted to affect the ability of nucleotides to bind may have resulted in an altered protein conformation in which the C-terminal tag is unavailable for binding to the affinity column.
The predicted amino acid sequence of TadA does not indicate an obvious transmembrane domain. Nevertheless, TadA-T7 is a soluble protein that could be found both in the cytoplasm and associated with the inner membrane, and its ATP hydrolase activity was stimulated by Triton X-100. These results suggest that TadA may associate peripherally with the cytoplasmic side of the inner membrane. Thus, it is possible that the TadA polypeptide interacts directly with the membrane; however, additional studies are required to confirm the localization pattern observed for TadA-T7. Cytoplasmic and inner membrane localization is characteristic of other secretion NTPases, such as the PulE protein of the type II system for pullulanase secretion, as well as type IV NTPase VirB11 encoded by Ti plasmids (52). Membrane association is held as a likely possibility for TrbB encoded by IncP plasmid RP4 (19), and it has also been predicted for the type IV ATPase HP0525 on the basis of its structure (68). One face of the hexameric ring structure of HP0525 is largely hydrophobic, and this face could mediate contact with the membrane.
Another possibility is exemplified by a number of type II and type IV secretion NTPases, such as PulE (49) and VirB11 (6), which have been shown to interact with other components of the secretion apparatus that are embedded in the inner membrane. Except for TadA, the proteins encoded by the tadABCDEFG cluster of A. actinomycetemcomitans are predicted to be integral membrane proteins (31), and it is reasonable to expect that TadA is localized to the membrane by interaction with one or more of these proteins. We examined the predicted amino acid sequence of TadA for clues to any such protein-protein interactions. The COILS program (44) strongly predicts a coiled coil in the N-terminal region (amino acids 17 to 50) (Fig. 8A). The predicted coiled coil would contain four heptad repeats that resemble a leucine zipper motif (70). Coiled-coil domains were also predicted for all members of the TadA subfamily of NTPases present in gram-negative bacteria and several of the TadA homologs in Archaea (data not shown). We also found possible coiled-coil domains in the N-terminal regions in several other type IV secretion NTPases, but their scores were significantly lower than that of TadA. The coiled-coil domain of TadA could be involved in homodimer formation, or it might mediate interactions with other proteins of the secretion system and may account for the peripheral association of TadA with the inner membrane (26). Promising partners for heterologous interaction with TadA are the TadB and TadD proteins, which are likely inner membrane proteins with six and one predicted transmembrane domains, respectively. TadB and TadD are each predicted to form a coiled-coil domain involving at least three heptad repeats. We are currently investigating whether TadA membrane association is dependent on the presence of TadB and/or TadD.
Type IV secretion NTPases TrbB, HP0525, and TrwD are able to form homomultimers, including dimers, tetramers, and hexameric rings (38). The recently determined crystal structure of the HP0525 NTPase revealed how six subunits can interact to form a cylindrical pore (68). The ATP hydrolase activity is proposed to modulate a conformational shift of the C-terminal domains of the subunits to open one end of the pore and allow the substrate to initiate its translocation across the membrane. Our results suggest that TadA, which is homologous with HP0525, TrbB, and TrwD, is able to form a similar structure. Electron microscopy of purified TadA-T7 revealed the presence of ring-like structures with dimensions similar to those of the hexameric rings observed for TrbB, TrwD, and HP0525. We have also detected stable lower oligomers of wild-type TadA-T7 (Fig. 6) and the Walker A box mutants (data not shown) in the absence of a reducing agent. TadA has a single cysteine, but intermolecular disulfide bond formation cannot readily account for all the observed species.
Currently, our evidence indicates that the genes within the flp-1 through tadG region constitute a secretion system responsible for the export of Flp1. In this model, TadA might then act as the energizing protein. Alternatively, TadA could use the energy from ATP hydrolysis to serve as a chaperone during the secretion process. Following the transport of Flp1 through the bacterial inner membrane, the pilin subunit might contact RcpA, whose gene lies upstream of tadA and within the 12-kb region. RcpA has similarity to PulD (21), a protein that forms a channel in the bacterial outer membrane during type II secretion (20, 47). While TadA and RcpA are similar to proteins of known secretion systems, this region likely encodes a secretion system that is distinct from both type II and type IV systems. Phylogenetic analyses with tadA and flp-1 support this notion (32, 48), and an extensive examination and analysis of the entire region have strengthened this hypothesis (manuscript in preparation).
In summary, we report the first demonstration of ATPase activity by a member of the newly identified and widespread TadA subfamily of type IV secretion NTPases (48). The results support our hypothesis that the proteins encoded by the flp-tad region of A. actinomycetemcomitans constitute a novel secretion apparatus required for nonspecific adherence and that TadA provides energy for assembly or secretion. We have also suggested that related gene clusters in a wide variety of Bacteria and Archaea code for systems that help their host organisms colonize various niches. It seems likely that the highly conserved TadA homologs encoded by these loci also possess an ATP hydrolase activity required for function.
ACKNOWLEDGMENTS
We thank Paul Planet and Paul Freimuth for insightful discussions.
This work was supported in part by an NIH research grant to D. H. Figurski and an NIH traineeship to S.C.K.
REFERENCES
- 1.Aepfelbacher M, Zumbihl R, Ruckdeschel K, Jacobi C A, Barz C, Heesemann J. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol Chem. 1999;380:795–802. doi: 10.1515/BC.1999.099. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M. Short protocols in molecular biology: a compendium of methods from Current Protocols in Molecular Biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1989. [Google Scholar]
- 3.Binet R, Letoffe S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 4.Burns D L. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. doi: 10.1016/s1369-5274(99)80004-6. [DOI] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie P J. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol Microbiol. 2001;40:294–305. doi: 10.1046/j.1365-2958.2001.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie P J, Vogel J P. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 2000;8:354–360. doi: 10.1016/s0966-842x(00)01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie P J, Ward J E, Jr, Gordon M P, Nester E W. A gene required for transfer of T-DNA to plants encodes an ATPase with autophosphorylating activity. Proc Natl Acad Sci USA. 1989;86:9677–9681. doi: 10.1073/pnas.86.24.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S N, Chang A C, Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA. 1972;69:2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delepelaire P. PrtD, the integral membrane ATP-binding cassette component of the Erwinia chrysanthemi metalloprotease secretion system, exhibits a secretion signal-regulated ATPase activity. J Biol Chem. 1994;269:27952–27957. [PubMed] [Google Scholar]
- 12.d'Enfert C, Chapon C, Pugsley A P. Export and secretion of the lipoprotein pullulanase by Klebsiella pneumoniae. Mol Microbiol. 1987;1:107–116. doi: 10.1111/j.1365-2958.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 13.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 15.Fine D H, Furgang D, Kaplan J, Charlesworth J, Figurski D H. Tenacious adhesion of Actinobacillus actinomycetemcomitans strain CU1000 to salivary-coated hydroxyapatite. Arch Oral Biol. 1999;44:1063–1076. doi: 10.1016/s0003-9969(99)00089-8. [DOI] [PubMed] [Google Scholar]
- 16.Fine D H, Furgang D, Schreiner H C, Goncharoff P, Charlesworth J, Ghazwan G, Fitzgerald-Bocarsly P, Figurski D H. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology. 1999;145:1335–1347. doi: 10.1099/13500872-145-6-1335. [DOI] [PubMed] [Google Scholar]
- 17.Fives-Taylor P M, Meyer D H, Mintz K P, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology 2000. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Fuller T E, Kennedy M J, Lowery D E. Identification of Pasteurella multocida virulence genes in a septicemic mouse model using signature-tagged mutagenesis. Microb Pathog. 2000;29:25–38. doi: 10.1006/mpat.2000.0365. [DOI] [PubMed] [Google Scholar]
- 19.Grahn A M, Haase J, Bamford D H, Lanka E. Components of the RP4 conjugative transfer apparatus form an envelope structure bridging inner and outer membranes of donor cells: implications for related macromolecule transport systems. J Bacteriol. 2000;182:1564–1574. doi: 10.1128/jb.182.6.1564-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilvout I, Hardie K R, Sauvonnet N, Pugsley A P. Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J Bacteriol. 1999;181:7212–7220. doi: 10.1128/jb.181.23.7212-7220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase E M, Zmuda J L, Scannapieco F A. Identification and molecular analysis of rough-colony-specific outer membrane proteins of Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:2901–2908. doi: 10.1128/iai.67.6.2901-2908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson I R, Cappello R, Nataro J P. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 2000;8:529–532. doi: 10.1016/s0966-842x(00)01853-9. [DOI] [PubMed] [Google Scholar]
- 23.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 24.Holland I B, Kenny B, Blight M. Haemolysin secretion from E coli. Biochimie. 1990;72:131–141. doi: 10.1016/0300-9084(90)90138-7. [DOI] [PubMed] [Google Scholar]
- 25.Holt S C, Tanner A C, Socransky S S. Morphology and ultrastructure of oral strains of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. Infect Immun. 1980;30:588–600. doi: 10.1128/iai.30.2.588-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J C. A guided tour in protein interaction space: coiled coils from the yeast proteome. Proc Natl Acad Sci USA. 2000;97:12935–12936. doi: 10.1073/pnas.97.24.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T, Tanimoto I, Ohta H, Kato K, Murayama Y, Fukui K. Molecular characterization of low-molecular-weight component protein, Flp, in Actinobacillus actinomycetemcomitans fimbriae. Microbiol Immunol. 1998;42:253–258. doi: 10.1111/j.1348-0421.1998.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 29.Inouye T, Ohta H, Kokeguchi S, Fukui K, Kato K. Colonial variation and fimbriation of Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1990;57:13–17. doi: 10.1016/0378-1097(90)90405-f. [DOI] [PubMed] [Google Scholar]
- 30.Kachlany S C, Fine D H, Figurski D H. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect Immun. 2000;68:6094–6100. doi: 10.1128/iai.68.11.6094-6100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kachlany S C, Planet P J, Bhattacharjee M K, Kollia E, DeSalle R, Fine D H, Figurski D H. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in Bacteria and Archaea. J Bacteriol. 2000;182:6169–6176. doi: 10.1128/jb.182.21.6169-6176.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kachlany S C, Planet P J, DeSalle R, Fine D H, Figurski D H, Kaplan J B. flp-1, the first representative of a new pilin gene subfamily, is required for nonspecific adherence of Actinobacillus actinomycetemcomitans. Mol Microbiol. 2001;40:542–554. doi: 10.1046/j.1365-2958.2001.02422.x. [DOI] [PubMed] [Google Scholar]
- 33.Kagermeier A S, London J. Actinobacillus actinomycetemcomitans strains Y4 and N27 adhere to hydroxyapatite by distinctive mechanisms. Infect Immun. 1985;47:654–658. doi: 10.1128/iai.47.3.654-658.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klauser T, Pohlner J, Meyer T F. The secretion pathway of IgA protease-type proteins in gram-negative bacteria. Bioessays. 1993;15:799–805. doi: 10.1002/bies.950151205. [DOI] [PubMed] [Google Scholar]
- 35.Klose M, Schimz K L, van der Wolk J, Driessen A J, Freudl R. Lysine 106 of the putative catalytic ATP-binding site of the Bacillus subtilis SecA protein is required for functional complementation of Escherichia coli secA mutants in vivo. J Biol Chem. 1993;268:4504–4510. [PubMed] [Google Scholar]
- 36.Kolodrubetz D, Dailey T, Ebersole J, Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989;57:1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koronakis V, Hughes C, Koronakis E. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol Microbiol. 1993;8:1163–1175. doi: 10.1111/j.1365-2958.1993.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 38.Krause S, Barcena M, Pansegrau W, Lurz R, Carazo J M, Lanka E. Sequence-related protein export NTPases encoded by the conjugative transfer region of RP4 and by the cag pathogenicity island of Helicobacter pylori share similar hexameric ring structures. Proc Natl Acad Sci USA. 2000;97:3067–3072. doi: 10.1073/pnas.050578697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause S, Pansegrau W, Lurz R, de la Cruz F, Lanka E. Enzymology of type IV macromolecule secretion systems: the conjugative transfer regions of plasmids RP4 and R388 and the cag pathogenicity island of Helicobacter pylori encode structurally and functionally related nucleoside triphosphate hydrolases. J Bacteriol. 2000;182:2761–2770. doi: 10.1128/jb.182.10.2761-2770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai E M, Kado C I. The T-pilus of Agrobacterium tumefaciens. Trends Microbiol. 2000;8:361–369. doi: 10.1016/s0966-842x(00)01802-3. [DOI] [PubMed] [Google Scholar]
- 41.Lally E T, Kieba I R, Demuth D R, Rosenbloom J, Golub E E, Taichman N S, Gibson C W. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem Biophys Res Commun. 1989;159:256–262. doi: 10.1016/0006-291x(89)92431-5. [DOI] [PubMed] [Google Scholar]
- 42.Lanka E, Wilkins B M. DNA processing reactions in bacterial conjugation. Annu Rev Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- 43.Lory S. Secretion of proteins and assembly of bacterial surface organelles: shared pathways of extracellular protein targeting. Curr Opin Microbiol. 1998;1:27–35. doi: 10.1016/s1369-5274(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 44.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 45.Menestrina G, Moser C, Pellet S, Welch R. Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology. 1994;87:249–267. doi: 10.1016/0300-483x(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 46.Meyer T F, Pohlner J, van Putten J P. Biology of the pathogenic Neisseriae. Curr Top Microbiol Immunol. 1994;192:283–317. doi: 10.1007/978-3-642-78624-2_13. [DOI] [PubMed] [Google Scholar]
- 47.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Planet P J, Kachlany S C, DeSalle R, Figurski D H. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc Natl Acad Sci USA. 2001;98:2503–2508. doi: 10.1073/pnas.051436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Possot O, d'Enfert C, Reyss I, Pugsley A P. Pullulanase secretion in Escherichia coli K-12 requires a cytoplasmic protein and a putative polytopic cytoplasmic membrane protein. Mol Microbiol. 1992;6:95–105. doi: 10.1111/j.1365-2958.1992.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 50.Possot O, Pugsley A P. Molecular characterization of PulE, a protein required for pullulanase secretion. Mol Microbiol. 1994;12:287–299. doi: 10.1111/j.1365-2958.1994.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 51.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rashkova S, Spudich G M, Christie P J. Characterization of membrane and protein interaction determinants of the Agrobacterium tumefaciens VirB11 ATPase. J Bacteriol. 1997;179:583–591. doi: 10.1128/jb.179.3.583-591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivas S, Bolland S, Cabezon E, Goni F M, de la Cruz F. TrwD, a protein encoded by the IncW plasmid R388, displays an ATP hydrolase activity essential for bacterial conjugation. J Biol Chem. 1997;272:25583–25590. doi: 10.1074/jbc.272.41.25583. [DOI] [PubMed] [Google Scholar]
- 54.Rosan B, Slots J, Lamont R J, Listgarten M A, Nelson G M. Actinobacillus actinomycetemcomitans fimbriae. Oral Microbiol Immunol. 1988;3:58–63. doi: 10.1111/j.1399-302x.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosche T M, Siddique A, Larsen M H, Figurski D H. Incompatibility protein IncC and global regulator KorB interact in active partition of promiscuous plasmid RK2. J Bacteriol. 2000;182:6014–6026. doi: 10.1128/jb.182.21.6014-6026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 58.Segal G, Russo J J, Shuman H A. Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol Microbiol. 1999;34:799–809. doi: 10.1046/j.1365-2958.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 59.Skerker J M, Shapiro L. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 2000;19:3223–3234. doi: 10.1093/emboj/19.13.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontology 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 61.Stephens K M, Roush C, Nester E. Agrobacterium tumefaciens VirB11 protein requires a consensus nucleotide-binding site for function in virulence. J Bacteriol. 1995;177:27–36. doi: 10.1128/jb.177.1.27-36.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thanassi D G, Hultgren S J. Multiple pathways allow protein secretion across the bacterial outer membrane. Curr Opin Cell Biol. 2000;12:420–430. doi: 10.1016/s0955-0674(00)00111-3. [DOI] [PubMed] [Google Scholar]
- 63.Thomson V J, Bhattacharjee M K, Fine D H, Derbyshire K M, Figurski D H. Direct selection of IS903 transposon insertions by use of a broad-host-range vector: isolation of catalase-deficient mutants of Actinobacillus actinomycetemcomitans. J Bacteriol. 1999;181:7298–7307. doi: 10.1128/jb.181.23.7298-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel J P, Isberg R R. Cell biology of Legionella pneumophila. Curr Opin Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- 65.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winans S C, Walker G C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985;161:402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeo H, Savvides S N, Herr A B, Lanka E, Waksman G. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol Cell. 2000;6:1461–1472. doi: 10.1016/s1097-2765(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 69.Zambon J J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhu H, Celinski S A, Scholtz J M, Hu J C. The contribution of buried polar groups to the conformational stability of the GCN4 coiled coil. J Mol Biol. 2000;300:1377–1387. doi: 10.1006/jmbi.2000.3936. [DOI] [PubMed] [Google Scholar]