Abstract
Objective:
Systematically review the existing evidence about couples-based interventions and postpartum contraceptive uptake and generate recommendations for future research.
Data sources:
PubMed, Web of Science, PsycINFO, Embase, and CINAHL through June 7, 2021.
Study selection and data extraction:
Studies with a couples-based intervention assessing postpartum contraceptive uptake. Two independent reviewers screened studies, extracted data, and assessed risk of bias with RoB-2 (Cochrane Risk of Bias 2) for randomized and ROBINS-I (Risk of Bias in Non-Randomized Studies – Interventions) for observational studies. Data were synthesized in tables, figures, and a narrative review.
Results:
925 papers were identified, 66 underwent full text review, and 17 articles, which included 18 studies – 16 randomized, 2 observational – were included. The lack of intervention and outcome homogeneity precluded meta-analysis and isolating the effect of partner involvement. Four studies were partner-required, where partner involvement was a required component of the intervention, and 14 were partner-optional. Unadjusted risk differences ranged from 0.01 to 0.51 in favor of couples-based interventions increasing postpartum contraceptive uptake versus standard of care. Bias assessment of the 16 randomized studies classified 8, 3, and 5 studies as at a high, some concern, and low risk of bias. Common sources of bias included intervention non-adherence and missing outcome data. One observational study was at a high and the other at a low risk of bias.
Conclusions:
Future studies that assess couples-based interventions must clearly define and measure how partners are involved in the intervention and assess how intervention adherence impacts postpartum contraceptive uptake.
Registration:
PROSPERO (CRD42021250358).
Keywords: Contraception, Sexual Partners, Spouses, Postpartum Period, Systematic Review
1. Introduction
Efforts to increase postpartum contraceptive uptake are essential to preventing negative maternal and fetal outcomes associated with short interpregnancy intervals [1–3]. Short interpregnancy intervals are associated with maternal and neonatal morbidity and mortality, including uterine rupture, hemorrhage, premature rupturing of membranes, preeclampsia, unsafe induced abortion, stillbirths, low birthweight, small for gestational age, and pre-term birth [4,5]. The World Health Organization (WHO) therefore recommends interpregnancy intervals of 24 months to minimize the risk of adverse maternal and child outcomes [4]. Other advantages to intentional birth spacing include a family’s ability to clothe, feed, shelter, provide emotional support, and educate offspring and maternal nutritional replacement after delivery [4,6,7].
Postpartum contraceptive uptake helps ensure adequate interpregnancy intervals, but its use is tied to social, cultural, economic, and health system norms [3]. Previous research suggests that interventions that integrate non-pregnant partners into existing maternal health systems may improve postpartum contraceptive uptake and reduce rapid repeat pregnancies, thus serving as a promising avenue for future work [8–22]. Given that pregnant people and their partners may interact with the health system frequently during antenatal, peripartum, and postpartum care, these periods present an opportunity to promote healthy timing and spacing of pregnancies. A 2016 review examined a broad range of facility- and community-based interventions to improve family planning uptake during the 12 months postpartum in lower and middle income countries [18,23–27]. Findings from the review suggested that couples-based interventions (6 of the 26 studies included) were likely to increase knowledge or use of postpartum contraceptive use but that the evidence base was too weak to make concrete policy reccomendations [18,23–27]. Additionally, the review only included studies published through 2015 and explored a variety of outcomes related to reproductive health and postpartum family planning, including knowledge of postpartum family planning services, use of postpartum family planning, and occurrence of pregnancy.
Given these data and their limitations, and extensive data showing that couples-based interventions can decrease sexual and substance-risk behavior [28,29], increase physical activity in pregnant people [30], increase access to HIV testing [31,32], and generally prompt to positive health behavioral change [33], we conducted an updated and targeted systematic review to identify, describe, compare, and synthesize research studies that specifically assessed the impact of couples-based interventions on postpartum contraceptive uptake. We evaluated how each study operationalized couples-based interventions and postpartum contraceptive methods. Finally, we assessed the risk of bias across each study using validated tools for randomized and observational studies to evaluate the evidence behind implementing couples-based interventions to improve postpartum contraceptive uptake. This review provides an important and timely synthesis of current literature to guide future interventions that seek to improve postpartum contraceptive uptake.
2. Methods
We followed the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) checklist [34].
2.1. Eligibility Criteria
We included primary studies with a partners-based intervention that evaluated postpartum contraceptive uptake. Eligible studies had to include people who were in their postpartum period, defined as up to six weeks from delivery. Interventions could occur at any time during the antenatal or postpartum period before outcome assessment. We excluded studies that specifically looked at contraceptive use after stillbirth or miscarriage because there are some data that shorter interpregnancy intervals may be appropriate in these instances [35]. We included articles, dissertations, and conference abstracts published in English, Spanish, or Portuguese in our initial search, based on the study team’s language capabilities, to capture all available evidence on the impact of couples-based interventions on postpartum contraceptive uptake. Upon full text review, we excluded articles in which non-pregnant partners were not included in the intervention, there was no intervention, or postpartum contraceptive uptake was not a measured outcome. We also excluded review articles and studies that contained insufficient information to assess the intervention and outcome, such as conference abstracts or studies without a control group.
2.2. Search Strategy
We searched PubMed, Web of Science, PsycINFO, Embase, and CINAHL without any search limits. We checked reference lists in reviewed articles and relevant reviews for additional eligible studies. Supplemental Table 1 shows the full search strategy.
2.3. Study Selection
The article selection process proceeded through three stages, with discordance resolved by author CMA, a third party. First, author DES used Rayyan [36,37], a free online tool with favorable user acceptability and feature functionality [38], to screen for duplicates and remove titles that were clearly irrelevant. In Stage 2, two reviewers (authors DES and LSP) screened abstracts of the remaining articles in Rayyan. In Stage 3, the full text of articles that passed abstract review were screened by the same two reviewers and the final set of articles were selected for data extraction. Article eligibility and screening processes detailing the number of studies included in each step of the review are reported according to the PRISMA checklist (Figure 1) [34].
Figure 1. Flow diagram.
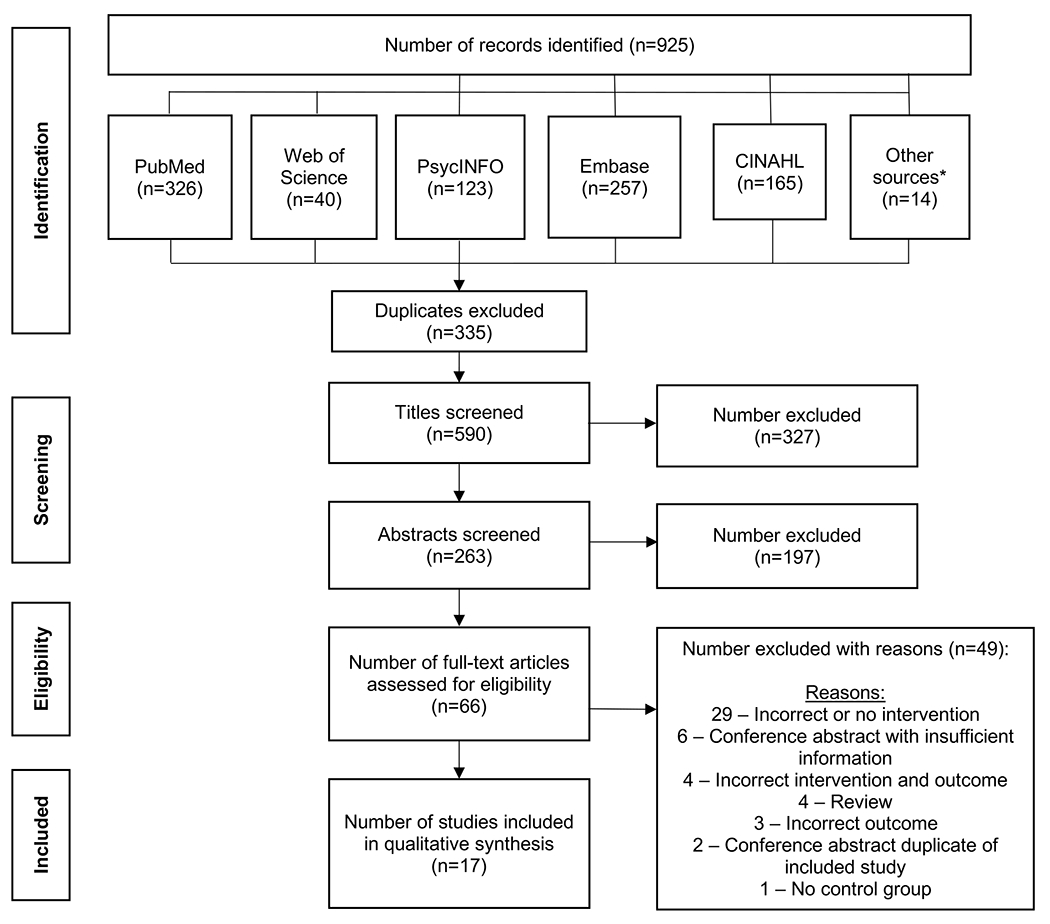
Papers identified in databases on June 7,2021. We screened out titles and abstracts that did not make reference to couples-based interventions or postpartum contraceptive use. In the full text reviews, incorrect interventions and outcomes included interventions and outcomes outside of our study inclusion criteria. For example, studies with interventions that only included the pregnant partner would be categorized as an incorrect intervention and excluded and studies with outcomes that only included knowledge about contraceptives, not contraceptive use, would be categorized as an incorrect outcome and excluded.
*Other sources include references from reviewed papers, clinical trials registries, and papers from relevant reviews identified through the literature search.
2.4. Data Extraction
Building from a previous review from LSP [39], we developed a data extraction and bias assessment form in Research Electronic Data Capture (REDCap), which allowed for duplicate extraction and scoring, secure data storage, and easy data export for analysis [40]. The REDCap data extraction form (Supplemental File 2) was modeled on the Cochrane data collection form for intervention reviews: RCTs and non-RCTs (Supplemental File 3) [41]. In brief, it included study country, year, design, number of participants, inclusion of people living with HIV, description and coding of intervention type, counts of participants by exposure status/intervention group, setting, definition of postpartum contraceptive use (e.g., “modern” versus “traditional”) or count of contraceptive use by type, timing of postpartum contraceptive use assessment, and crude and adjusted effect estimates (and type of effect estimate) and confidence intervals. Finally, because there is some disagreement as to how to operationalize contraceptive methods (e.g., “modern” versus “traditional” methods) [42], we recorded and interpreted how each included study did so and its implications for future research. DES and LSP independently used the REDCap data extraction form for each study and then came to a consensus for information from and risk of bias assessments for each included study. CMA resolved any disagreements.
2.5. Bias Assessment
In addition to the above defined data extraction, two reviewers independently assessed bias via the Cochrane Risk of Bias 2 (RoB 2) tool for randomized trials and Risk Of Bias In Non-Randomized Studies - of Interventions (ROBINS-I) tool for observational interventions (included in Supplemental File 3) [43,44]. Both bias assessments required that studies be classified as “low”, “some concerns”, or “high” risk of bias in the below-described domains and across all domains on each assessment tool [43,44]. RoB 2 assesses several domains of randomized trials that focus on trial design, conduct, and reporting with signaling questions that allow reviewers to systematically assess the risk of bias in any particular trial [43]. There are RoB 2 versions for parallel, cluster-randomized, and crossover trials [43]. ROBINS-I encourages reviewers to conceptualize the target trial that an observational trial is trying to emulate [44]. It then guides reviewers through signaling questions in various domains – confounding, selection, classification, missing data – which allow reviewers to come to a final determination of the risk of bias in any particular study [44].
For each randomized study, we evaluated bias relevant to the main analysis type presented in each study. Analysis types included intention-to-treat analyses, which measure the effect of the assigned treatment without consideration of protocol non-adherence, and per-protocol analyses, which measure the effect of the received treatment via consideration of adherence and loss-to-follow. Intention-to-treat analyses preserve the benefits of randomization and are less prone to confounding bias than per-protocol analyses, but per-protocol analyses often provide a better estimate of real-world treatment effectiveness. The trade-off between preserving the benefits of randomization and simplicity of analysis for intention-to-treat analysis versus the real-world effectiveness interpretation of per-protocol analysis has long been debated [45–47]. All risk of bias assessments are available at https://github.com/dannysack/postpartum_fp_review.
2.6. Data Analysis and Synthesis
The reviewed studies are summarized in a narrative review with detailed tables and descriptive statistics. We summarized key study characteristics from the data extraction and the effect estimates derived from each study. We then calculated unadjusted risk differences and 95% confidence intervals based on the reported number of outcome events in each study group (intervention and control) [48]. Studies were defined as “partner-required” if partner involvement was a required component of the intervention or “partner-optional” if the intervention included partners in at least one aspect of the intervention, but participation was not required. We planned to conduct a meta-analysis and create a conceptual framework relating partner engagement to postpartum contraceptive uptake, however, this was not possible due to excessive qualitative heterogeneity in the intervention and outcome definitions. After assessing bias in each individual study, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to present an overall summary of the quality of evidence for employing couples-based interventions to increase postpartum contraceptive uptake. We also comment on whether the extant literature may be able to inform practice or policy recommendations based on risk of bias, precision, consistency, directness, and likelihood of publication bias [49].
3. Results
3.1. Study Selection
Our initial search, on June 7, 2021, captured 925 papers across the five included databases and screened study references (Figure 1 and Supplementary Table 1). After manually confirming studies identified via Rayaan’s duplicate feature and examining the available title and author lists, we removed 335 duplicates prior to screening 590 titles and reviewing 263 abstracts. After screening out 327 titles and 197 abstracts that did not include references to couples-based interventions or postpartum contraception, we evaluated 66 full texts for final study eligibility (Figure 1). After excluding 49 studies that met the exclusion criteria, the remaining 17 articles were reviewed [15,19,24–27,50–60]. One included article, a doctoral dissertation [57], evaluated the same intervention in Kenya and Nigeria, which were carried out by two separate study teams; results from these are presented separately. No identified conference abstracts were included because they lacked sufficient detail to assess the intervention or the outcome. The lack of homogeneity in the interventions and outcomes precluded a meta-analysis.
3.2. Study Characteristics
Study characteristics are detailed in Tables 1 and 2. Of the 17 included papers, and 18 individual studies, seven were parallel randomized controlled trials, nine were clustered randomized controlled trials, and two were nonrandomized comparative trials – both observational studies with interventions implemented at the clinic, rather than the individual, level [15,19,24–27,50–60]. Studies were conducted between the late 1990s (two did not include data collection dates [50,51]) and 2018 and published between 2001 and 2021. Most of the included studies (n=10) were conducted in WHO’s African region [15,19,52,54–60], three were in the Eastern Mediterranean region [24,27,50], three were in the South-East Asian region [25,26,53], and one was in the European region [51]. Two studies specifically included people living with HIV [54,60].
Table 1.
Study Characteristics
| First Author, Year, Ref Study Years Study Location |
Study Design Study Size: total enrolled (included in the final analysis), clusters |
Study Population | Intervention Description | Intervention Type | Outcome Definition | Main Findings Effect Estimates (95% CI) |
Other Analysis/Bias Considerations Risk of Bias Tool: Overall Bias and Direction |
| Soliman, 1999 [50] Unclear Mansoura, Egypt |
RCT 200 (200) |
Pregnant women in their third trimester attending a university-affiliated outpatient antenatal clinic and half of their partners | Three 1-hour counseling sessions for woman and partner using GATHER technique, timing not specified, likely between second trimester and delivery | Partner-Optional | Injection, minipill, IUD, LAM, sterilization, barrier/condom use, or abstinence at 3 months postpartum | Increased contraceptive uptake among women who received intervention with their partner involved versus standard of care RD = 0.48 (0.37, 0.59) |
No adjustment for baseline covariates; no information on missing data; small sample size RoB 2: High risk of bias likely favors intervention |
| Turan, 2001 [68] Unclear Istanbul, Turkey |
RCT 333 (279) |
Nulliparous women 4+ months pregnant and some of their partners attending medical school-affiliated antenatal clinic | Group educational sections, an educational booklet, and dedicated question phone line, timing not specified, likely from 4 months to delivery | Partner-Required | IUD, pills, sterilization, or condom use at 4 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.15 (–) OR = 1.49 (–) |
Poor intervention adherence and unclear results reporting RoB 2: high risk of bias, direction dependent on most prominent source of bias |
| Kunene, 2004 [52] 2001-2002 KwaZulu Natal, South Africa |
CRT 2082 (1423), 8 clusters |
Women 10-30 weeks pregnant, living with partner or in a relationship of > 1 year duration, attending urban or rural clinics in township near Durban | Improved existing antenatal services, new educational booklet, individual and group counseling, with non-pregnant partners invited to 3 sessions, 2 during pregnancy, 1 six weeks postpartum | Partner-Required | “Any method” or “modern method” (not well defined) at 6 months postpartum | No statistical difference in contraceptive uptake by group RD = 0 (−0.04, 0.04) |
Differential loss to follow up in control versus intervention group, did not account for clustering, no baseline covariate adjustment, lots of missing outcome data (36% in control, 27% in intervention) RoB 2: High risk of bias in likely unpredictable direction, but may favor standard of care |
| Caleb-Varkey, 2004 [53] 2000-2002 Delhi, India |
Non-equivalent control group 1067 (632), 6 clusters |
Women 10-26 weeks pregnant attending antenatal services at six dispensaries affiliated with national medical provider and their partners | Trained providers to conduct behavior change communication, new educational materials, implemented updates clinical practices including syphilis screening and a postpartum mother-baby clinic | Partner-Optional | Sterilization, pills, copper IUD, condoms, injection, jelly, or natural or traditional methods at 6-9 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.13 (0.05, 0.21) |
Lots of missing data (40.7%), no adjustment for covariates, some discrepancies in results reporting ROBINS-I: High risk of bias, unpredictable direction given extent of missingness |
| Abdel-Tawab, 2008 [27] 2005-2007 Assiut and Sohag, Egypt |
CRT 1416 (1409), 30 clusters |
Pregnant women and their partners attending antenatal care at 20 clinics participating in the study | Health services model: birth spacing messages in ante- and post-partum care Community awareness model: awareness-raising activities for men, educational activities for women, men, and health providers |
Partner-Optional | Contraceptive use at 10-12 months postpartum | Communities that just increased women’s education improved more than those with just the community awareness activities, both were better than standard of care RD = 0.07 (0.01, 0.13) |
Poorly described randomization and outcome, analysis did not account for clustering or consider baseline covariates RoB 2: High risk of bias, unpredictable direction |
| Saeed, 2008 [24] 2006-2007 Islamabad, Pakistan |
RCT 648 (600) |
All women admitted to labor and delivery at hospital regardless of duration of pregnancy or delivery outcome and some of their partners | 20-minute informal contraceptive counseling session in presence of husband or close relative with a take home educational leaflet | Partner-Optional | Pills, injection, copper IUD, condoms, or sterilization at 8-12 weeks postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.51 (0.44, 0.57) |
Do not clearly describe how many non-pregnant partners were involved or adherence RoB 2: Low risk of bias, if present, likely favors intervention |
| Sebastian, 2012 [25] 2006-2007 Uttar Pradesh, India |
CRT 1197 (959), 4 clusters |
Women 4-7 months pregnant, aged <25, in the study villages, and with one or no children | Community health worker led educational campaign on healthy timing and spacing of pregnancy, postpartum care, and postpartum contraception for women, her oldest female family member, and a separate campaign with similar messages directed at men in the community | Partner-Optional | Pills, condoms, IUD, or sterilization at 9 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.27 (0.21, 0.33) aOR = 3.66 (2.72, 4.91) |
Lots of missing outcome data (19.9%), did not account for clustering in power calculation or analysis, intervention poorly described RoB 2: Some concerns for bias, unpredictable direction |
| Villar-Loubet, 2012 [54] 2010-2011 Mpumalanga, South Africa |
CRT 478 (453), 12 clusters |
Women 24-30 weeks pregnant, aged 18+, who completed HIV testing and counseling, and their partners attending participating clinics | 4 weekly, 90-120 minute gender-concordant group educational sessions emphasizing cognitive-behavioral skills and information about HIV prevention, medication adherence, condom use, among other things, with “homework” encouraged for partners | Partner-Optional | Couples classified as consistent condom users if both members reported condom use 100% of the time during sex | Increased consistent condom use among intervention couples versus active control (non-contraception-related sessions - see text for details) RD = 0.10 (−0.07, 0.28) |
Potential for social desirability bias, selective reporting of outcome, and lack of adjustment for baseline covariate imbalances RoB 2: Some concerns for bias, favors intervention |
| Ahmed, 2015 [26] 2007-2011 Sylhet, Bangladesh |
Clustered quasi-experimental 4321 (4083), 4 clusters |
Pregnant women attending antenatal care within one of the four included communities who did not experience stillbirth or neonatal death | Postpartum family planning messaging added to general antenatal care in clinics and in community health worker standard of care (both at individual and group level). Revisited at postpartum visits every 2 months and participants provided with take home educational materials | Partner-Optional | LAM, pill, condoms, IUD, implant, sterilization, withdrawal, or periodic abstinence at 24 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.11 (0.08, 0.14) aHR = 2.57 (–) |
Robust analytic methods including control for potential confounders and accounting for clustering ROBINS-I: Low risk of bias |
| Daniele, 2018 [19] 2015-2016 Bobo-Dioulasso, Burkina Faso |
RCT 1144 (1115) |
Women 20-36 weeks pregnant, aged 15-45, cohabiting with a man, and expected to give birth in a primary health center (instead of at a hospital) | Interactive group discussion for non-pregnant partners, individual couples counseling during pregnancy, and postnatal couple counseling session before discharge | Partner-Required | Implants, IUD, injectable, pills, and sterilization at 8 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.07 (0.01, 0.12) aRD = 0.06 (0.01, 0.12) |
Potential cross-over between intervention and control groups RoB 2: Low risk of bias, if any bias, direction would depend on whether cross-over/adherence, or unblinded data collectors/social desirability is stronger |
| Harrington, 2019 [69] 2016-2017 Kisumu and Siaya, Kenya |
RCT 260 (254) |
Women 28+ weeks pregnant, aged 14+, able to read and response to text messages themselves or with reliable assistance, daily access to a mobile phone, HIV negative | Once weekly tailored text messages with content centered around family planning once weekly tailored to gestational age or postpartum week. After contraceptive initiation, messages designed to support continuation and manage side effects. Non-pregnant partner could opt-in for messages | Partner-Optional | Sterilization, implant, copper IUD, injectable, and pills at 6 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.13 (0.01, 0.24) aRR = 1.26 (1.04, 1.52) |
Only minor concern is social desirability bias of outcome reporting RoB 2: Low risk of bias, if any bias, direction would depend on whether cross-over, or social desirability is stronger |
| Tran, 2019 [55] 2016-2017 Passoré, Burkina Faso |
CRT 614 (523), 8 clusters |
All pregnant women in third trimester attending and planning to deliver in an enrolled health center. Eligible health centers offered ante-, peri-, and post-partum care, had at least three contraceptive methods (barrier, short-term, and long-term) available, had no contraceptive stock-outs during the preceding 6 months, and had an average of 30 deliveries per month | 6 component intervention: 3 facility-based: refresher training for providers, supportive provider supervision, increased service availability to 7 days a week 3 individual-based: postpartum contraceptive counseling tool, appointment cards, and an invitation letter for partners |
Partner-Optional | Implant, IUD, injectable, pills, emergency contraceptive, condoms, sterilization, and LAM at 12 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.26 (0.18, 0.34) aPR = 1.79 (1.30, 2.47) |
Small number of clusters, did not track non-pregnant partner participation by design RoB 2: Low risk of bias, if any bias, would be likely favor intervention given social desirability given that analysis adjusted for baseline covariates and clustering |
| Abdulkadir, 2020 [56] 2015 Kano, Nigeria |
RCT 150 (137) |
Women 32-38 weeks pregnant, aged 15-45, receiving antenatal care at teaching hospital | Antenatal contraceptive counseling for woman at recruitment, second session 4 weeks later with non-pregnant partner present using GATHER method | Partner-Required | Contraceptive use at 4 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.17 (0.01, 0.34) |
Lack of blinding in exposure and outcome assessment, potential derivations from primary analysis plan RoB 2: High risk of bias, likely favors intervention, but is unpredictable because of missingness |
| Whiting-Collins, 2020 [57] 2017-2018 Kisumu and Machakos, Kenya |
CRT 1017 (631), 20 clusters |
Women 16-20 weeks pregnant, aged 15+, not planning to move in the next 18 months and their partners | 6 group antenatal visits and 4 group postnatal visits for women and (postnatally) their infants with postpartum contraceptives discussed at antenatal meetings 3, 4, and 5 and partners invited to meeting 3 | Partner-Optional | Time to first use of sterilization, IUD, injection, pill, emergency contraception, condom, or “other” method from 12-month postpartum survey | No statistical difference in time to or total contraceptive uptake by group RD = 0.07 (0.0, 0.14) aHR = 1.12 (0.93, 1.34) |
No adjustment for baseline covariates, missing outcome data (37.7%) RoB 2: High risk of bias, unpredictable direction due to missingness |
| Whiting-Collins, 2020 [57] 2017-2018 Nasarawa, Nigeria |
CRT 1075 (873), 20 clusters |
Women 16-20 weeks pregnant, aged 15+, not planning to move in the next 18 months and their partners | 6 group antenatal visits and 4 group postnatal visits for women and (postnatally) their infants with postpartum contraceptives discussed at antenatal meetings 3, 4, and 5 and partners invited to meeting 3 | Partner-Optional | Time to first use of sterilization, IUD, injection, pill, emergency contraception, condom, or “other” method from 12-month postpartum survey | No statistical difference in time to or total contraceptive uptake by group (RD = 0.01) RD = 0.27 (0.21, 0.34) aHR = 1.01 (0.72, 1.60) |
No adjustment for baseline covariates, missing outcome data (18.8%) RoB 2: High risk of bias, unpredictable direction due to missingness |
| Tran, 2020 [58] 2016-2018 Kinshasa, Democratic Republic of Congo |
CRT 690 (519), 8 clusters |
All pregnant women in third trimester attending and planning to deliver in an enrolled health center. Eligible health centers offered ante-, peri-, and post-partum care, had at least three contraceptive methods (barrier, short-term, and long-term) available, had no contraceptive stock-outs during the preceding 6 months, and had an average of 30 deliveries per month | 6 component intervention: 3 facility-based: refresher training for providers, supportive provider supervision, increased service availability to 7 days a week 3 individual-based: postpartum contraceptive counseling tool, appointment cards, and an invitation letter for partners |
Partner-Optional | Implant, IUD, injectable, pills, emergency contraceptive, condoms, sterilization, and LAM at 12 months postpartum | No statistical difference in contraceptive uptake by group, but similar effect estimate to Tran, 2019 RD = 0.10 (0.02, 0.19) aPR = 1.58 (0.74, 3.38) |
High-level standard of care in control group may have blunted effect, small number of clusters RoB 2: High risk of bias, unpredictable direction due to missingness, intervention contamination favors control |
| Coulibaly, 2021 [59] 2018 Centre-North Region, Burkina Faso |
CRT 571 (485), 8 clusters |
Follow up study of Tran, 2019, same study population | Same as Tran, 2019 | Partner-Optional | Implant, IUD, injectable, pills, emergency contraceptive, condoms, sterilization, and LAM at 24 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.11 (0.02, 0.19) aPR = 1.21 (0.19, 1.61) |
Moderate among of missing data (14.7% in control, 10.5% in intervention), small number of clusters, did not adjust for sufficient baseline covariates, small number of clusters RoB 2: Some concerns of bias, most likely favors comparator giving missingness was similar across groups |
| Atukunda, 2021 [60] 2016-2018 Mbarara, Uganda |
RCT 320 (317) |
Women living with HIV admitted to a postnatal ward in a public teaching hospital, aged 18+, who either had a male sexual partner or intended to have one within the next two years | Up to 40 minutes of a structured immediate postpartum counseling session with a primary sexual partner if available. Women using contraceptive pills at 6 months postpartum received daily reminders for 4 months, then weekly reminders for 2 months | Partner-Optional | Condom, injectable, contraceptive pills, cooper IUD, implant, and, if used with every sexual encounter, diaphragm or cervical cap use at 12 months postpartum | Increased contraceptive uptake with intervention versus standard of care RD = 0.11 (0.01, 0.20) aOR = 1.75 (1.24-2.95) |
Few non-pregnant partners participated, but their pariticipation was optional per the design RoB 2: Low risk of bias |
Abbreviations: RCT – randomized controlled trial; CRT – cluster randomized controlled trial; IUD – intrauterine device; LAM – lactational amenorrhea; GATHER method: Greeting clients, Ask them about themselves, Tell them about different FP methods, Helping choose a method, Explaining how to use method, Return for follow up; RD – risk difference; RR – risk ratio; OR – odds ratio; HR – hazard ratio; PR – prevalence ratio; 95% CI – 95% confidence interval; LTFU – loss to follow up; a – denotes adjustment for baseline covariates and/or cluster effect (e.g., aOR – adjusted odds ratio); vs. – versus; (–) – insufficient information available to calculate a confidence interval or not reported; RoB 2 – Cochrane Risk of Bias 2 tool for randomized trials; ROBINS-I – Risk of Bias In Non-Randomized Studies - of Interventions
The sample size of studies with individual interventions ranged from 137 to 1115 [15,19,24,50,51,56,60], whereas studies with clustered interventions range of sample sizes from 453 to 4083 participants and included between 4 and 30 clusters [25–27,52–55,57–59]. There was a broad range of contraceptive uptake in the control (26-90%) and intervention groups (33-91%). The control group in all included studies was the local standard of care during the study period [15,19,24–27,50–60]. Villar-Loubet et al. (2012) also provided participants in the control group with time-matched educational videos on pregnancy-related health topics (e.g., exercise, diabetes prevention, etc.) rather than intervention sessions (gender-concordant group educational sessions emphasizing cognitive-behavioral skills and information about condom use and HIV prevention and management) [54].
Most studies suggested that the intervention was associated with increased postpartum contraceptive uptake, with unadjusted risk differences ranging from 0.01 to 0.51 (Figure 2) [15,19,24–27,50–60]. Among the ten studies that presented adjusted effect estimates with 95% confidence intervals [15,19,25,26,55,57–60] – one risk difference, one risk ratio, two odds ratios, three prevalence ratios, and three hazard ratios – five were statistically significant [15,19,25,55,60], favoring the intervention over the control group (Table 1). The direction of the effects was somewhat similar, although there was too much heterogeneity in both the interventions and the outcomes to conduct a meta-analysis.
Figure 2. Unadjusted Risk Differences in Randomized Studies.

Forest plot of unadjusted risk differences across included randomized studies.
Abbreviations: CAM – Community Awareness Model; (–) – insufficient information available to calculate a confidence interval or not reported
3.3. Intervention and Adherence Heterogeneity
Fourteen studies were characterized as partner-optional [15,24–27,50,53–55,57–60] and four were characterized as partner-required (two conducted before 2003 and two in 2015) [19,51,52,56]. Examples of partner-optional interventions included a single counseling session (of many) where non-pregnant partners were invited [57], partner educational materials distributed at antenatal visits or via another opt-in educational method [15,50,53,54], and an invitation letter that pregnant partners could choose to give their non-pregnant partner to attend antenatal and postnatal visits that included contraceptive counseling [55,58,59]. Partner-optional studies increased postpartum contraceptive uptake from 7% to 51% across studies [15,24–27,50,53–55,57–60]. Partner-required interventions included contraceptive couples counseling or group sessions with both partners present and increased postpartum contraceptive uptake from 0% to 17% [19,51,52,56].
Across all studies, the description and reporting of partner involvement was somewhat lacking, and in 17 out of 18 studies, participant and/or partner adherence to the intervention was low or unreported [15,19,24–27,50–60]. For example, Saeed et al. (2008), which assessed the impact of a 20-minute informal contraceptive counseling session in presence of husband or close relative immediately after birth, did not report how frequently non-pregnant partners attended the educational sessions [24]. Additionally, in three studies with the same design implemented in different locations with different follow up durations, which are referred to as the Yam Daabo studies [55,58,59], participants had the option to bring a letter home inviting partners into care, as one of six intervention components. The Yam Daabo studies generally aimed to increase contraceptive uptake at 12 months postpartum, but did not describe how many participants opted into the letter component of the intervention or how many partners engaged in care [55,58,59]. Furthermore, across both study sites, only six control group participants (n=592) and 39 intervention group participants (n=572) completed all recommended study visits [55,58]. Additionally, Daniele et al. (2018) reported that while 77% of non-pregnant partners in the intervention group attended the group session for men and 64% attended the first couples counseling session, only 56% attended the postnatal couple counseling session [19].
3.4. Postpartum Contraceptive Definitions
Postpartum contraceptive uptake was measured anywhere from 2 to 24 months after birth and contraceptive definitions varied across studies (Supplemental Table 2). Specifically, seven studies assessed contraceptive use 6 or fewer months postpartum [15,24,50–52,54,56], nine studies assessed contraceptive use from 6 to 12 months postpartum [19,22,25,27,53,55,57,58], and two studies measured postpartum contraceptive use at more than 12 months after delivery [26,59]. Additionally, 11 studies made specific distinctions between “modern” and “traditional” contraceptive methods and the other seven did not make any delineation between the two. Of the studies that differentiated between “modern” and “traditional” contraceptives, there was notable heterogeneity, particularly surrounding how to characterize lactational amenorrhea (LAM). Some studies considered LAM use prior to six months postpartum as “modern” [55,58,59], whereas others did not specify whether it was “modern” or “traditional” [26]. Daniele et al. (2018) and Harrington et al. (2019) defined “effective modern methods” as methods with a typical use failure rate of less than ten percent [15,19], with Harrington et al. (2019) further specifying that the “modern” methods had to be available in Kenya at their study sites (including sterilization, implant, copper IUD, injectable, and oral contraception). Finally, Villar-Loubet et al. (2012) and Atukunda et al. (2021) included barrier methods as “modern” (only condoms in Villar-Loubet et al.’s case) only if participants reported using them with every sexual encounter regardless of the method’s typical use failure rate [22,54].
3.5. Risk of Bias Assessment
Table 1 includes a column summarizing the risk of bias assessment for each study. Risk of bias assessments for included randomized studies are presented in Figure 3 and Supplemental Table 3 and risk of bias assessments for observational studies are presented in Supplemental Table 4.
Figure 3. Risk of Bias Assessment in Randomized Trials.

Heatmaps of the signaling questions (a) and final risk of bias assessment (b) for the included randomized controlled trials. “Rand” refers to randomization, “IntDeriv” refers to intervention derivations, “MissOut” refers to missing outcomes, “OutMeas” refers to outcome measurement, and “SelRes” refers to the selection of the reported results. In (a), “RoB” refers to Risk of Bias, whereas in (b), each number refers to the specific signaling question. Signalling questions for the risk of bias assessment are presented in Supplemental File 3. Additionally, specific details about how bias was assessed within each study are presented in Supplementary Table 3.
All randomized studies used intention-to-treat analyses [15,19,24,25,27,50–52,54–60], so bias assessments evaluate the validity of their intention-to-treat approach, which is not impacted by protocol non-adherence. Eight of 16 randomized studies had a high risk of bias [27,50–52,56–58], five studies had a low risk of bias [15,19,24,55,60], and the remaining three had some concerns about a risk of bias [25,54,59]. For the most part, randomization was unbiased, although several studies did not clearly define how participants or clusters were randomized to treatment or intervention arms [25,27,50]. In several of the interventions, particularly in clustered randomized trials, there was some evidence that crossover may have occurred [25,52,58,59], although it is unclear how much this may have biased findings toward the standard of care group. Since many of these studies employed partner-optional interventions [15,24–27,50,53–55,57–60], partner intervention non-adherence was not an intervention deviation and thus did not signal high risk of bias in our risk of bias assessments. Though not influential on the validity of the reported effect measure for the pre-specified analysis plan (i.e. intention-to-treat analysis), such study design decisions limit our ability to isolate the effect of partner involvement and limits our interpretation of how partner involvement impacted postpartum contraceptive uptake. Importantly, missing outcome data were particularly high in several of the studies, with levels that ranged between 19% and 38% in studies characterized as high risk of bias [27,50–52,56–58]. No studies reported any information about how they addressed missing baseline covariate data [15,19,24,25,27,50–52,54–60]. All included studies, with the exception of Atukunda et al. (2021) which combined self-report with medical record confirmation [22], used self-report to assess postpartum contraceptive uptake, which can overestimate the impact of the intervention due to social desirability bias [15,19,24,25,27,50–52,54–59]. Furthermore, several of the cluster-randomized studies did not account for clustering or baseline covariates in their analysis [27,50,52,56,57,59].
Of the eight studies published before 2015, and therefore described in previous reviews [18], only Saeed et al. (2008) was characterized as at a low risk of bias [24]. Of the ten studies published in 2015 or later, and therefore newly reviewed in this analysis, four were characterized as high risk of bias and one was characterized as at some concern of bias [56–59]. Of the five characterized as a low risk of bias [15,19,22,26,55], four out of five were characterized as partner-optional interventions and one was a partner-required intervention. Ahmed et al. (2015) provided pregnant people with educational materials to take home to their partners [26], Harrington et al. (2019) allowed partners to opt-in to text messages with education content [15], Tran et al. (2019) sent an invitation letter to partners as one part of six intervention components [55], and Atukunda et al. (2021) provided contraceptive counseling and a voucher after delivery to postpartum people and, if present, their partners [22]. Daniele et al. (2018), the only partner-required, low risk of bias study, included interactive group discussions for partners, couples counseling during pregnancy, and postnatal couples counseling [19]. They reported poor adherence among partners, likely limiting the impact of the intervention [19].
Of the two observational studies, one was classified as at a high risk of bias [53], while the other was classified as at a low risk of bias [26]. Caleb-Varkey et al. (2004) had a high risk of bias because it was unclear how confounding was handled, there was a large amount of missing outcome data (~41%), potential cross-over effects by providers working at both intervention and control clinics, and varying incorporation of partners across clinical sites [53]. Ahmed et al. (2015) had a low risk of bias because it considered and included confounding variables in the analysis and had low levels missing outcome data, but it was unclear how it handled missing covariates [26]. Both studies, like the randomized controlled trials, likely had some social desirability bias influencing outcome reporting, likely favoring intervention arms.
3.6. Summary of the Evidence via GRADE Criteria
Based on the above-described risk of bias assessments and other study characteristics, we concluded that, according to GRADE, there is low certainty evidence that couples-based interventions impact postpartum contraceptive uptake [49]. Despite consistent precision and direction in reported estimates of effect from the included studies, the lack of homogeneity in terms of study design, intervention description, and outcome definition inhibit our ability to provide any moderate or strong recommendations for use of specific couples-based interventions. Furthermore, since most of the interventions were “partner-optional” rather than “partner-required”, these findings cannot be directly applied to how couples-based interventions that require participation from both partners may impact postpartum contraceptive uptake.
4. Discussion
We conducted an updated, comprehensive systematic review of couples-based interventions and their impact on postpartum contraceptive uptake. Our literature search and subsequent analysis identified 18 studies across 17 articles that evaluated the association between partner-based interventions and postpartum contraceptive uptake. We identified and reviewed ten additional studies compared to the last review that examined partner-based interventions and postpartum contraception from 2016 [18]. Additionally, our review included a GRADE recommendation based on updated risk of bias assessments and other study characteristics [18,49]. While all the included studies reported a positive or null association between interventions that included couples and postpartum contraceptive uptake – none suggested decreased postpartum contraceptive uptake after involving non-pregnant partners – none allowed us to isolate the effect of a partner’s involvement, or the type of partner involvement, on postpartum contraceptive uptake. For example, two studies at low risk of bias, a 20-minute couples counseling session in the immediate postpartum period that increased postpartum contraceptive uptake approximately 51% at 8-12 weeks postpartum [24] and a 40-minute postpartum session that increased contraceptive uptake 11% at 12 months postpartum [60], intended, but did not require non-pregnant partner inclusion. These single sessions are simple to study further and implement individually, or as part of some of the more complex interventions that also address other components of the pregnancy health system, but neither allow us to speculate on how the counseling sessions impacted postpartum contraceptive uptake through the pregnant partner, the non-pregnant partner, or the combination of both partners because of planned heterogeneity in how the intervention was implemented. Finally, there was significant heterogeneity in how partners were incorporated into the intervention and how postpartum contraceptive uptake was defined, limiting the generalizability of any one type of intervention to a population outside of each study. Our findings suggest that couples-based interventions remain an understudied component of postpartum contraceptive uptake.
Interestingly, and somewhat concerningly, of the nine cluster randomized studies, all had fewer than 40 clusters and seven either did not adjust for or insufficiently adjusted for baseline covariates, with some studies not accounting for clustering in the analysis [25,27,52,54,57,59]. Failing to account for clustering would not impact the point estimate, but it would make the confidence intervals artificially narrow, limiting the interpretability of those studies [61,62]. Not including baseline covariates in cluster randomized trials, on the other hand, may lead to residual confounding, which can bias point estimates [61,62]. Cluster randomized trials are an important study design when assessing interventions – such as partner-based interventions – where communities served by the same clinical site may communicate outside of where the intervention is implemented. Attempting a parallel randomized controlled trial in such a setting risks frequent information sharing among intervention and control participants in the same community, potentially blunting the effect of the intervention. Turner et al. (2017) provides an excellent summary of key considerations when planning and analyzing cluster randomized trials [61,61]. Bie et al. (2021) provides additional insight into cluster randomized trial analysis in the context of small cluster sizes [63].
Furthermore, given the poor intervention adherence among non-pregnant partners (in part because they were not required to attend interventions in most reviewed studies), variable information on intervention fidelity, and missing outcome data prevalence across the included studies, it is important to consider how non-adherence and loss to follow-up may have influenced study findings and interpretations. Since all included randomized trials used intention-to-treat analyses, their goal was to assess the effect of the assigned intervention, rather than the effect of intervention adherence on postpartum contraceptive uptake. Future analyses may want to consider alternative approaches that account for non-adherence or loss to follow up, such as “per protocol” or “as treated” analyses. Adherence-corrected analyses can account for time-varying confounding via inverse probability weighting, instrumental variable analyses, or g-estimation [45,65]. Interventions that prove highly effective among a particular sub-set of participants, or within a particular context, may be amenable to further development to make them more effective among a broader range of participants [64]. However, such analyses would require reliable data collection specific to which participants adhered to which components of the invervention and, if possible, why differential adherence exists. Since none of the reviewed studies published sufficiently detailed information, it is hard to speculate on why particular studies had lower adherence, however, the low risk of bias studies with the fewest intervention components, such as Saeed et al. (2008) and Atukunda et al. (2021) [24,60], tended to have the most complete outcome data. This suggests that increased intervention complexity may increase the likelihood of poor adherence and follow up.
Our systematic review has several limitations. Data extraction was subject to reporting in the primary studies, which was variable and occasionally incomplete. This made it difficult to fully assess the risk of bias in several studies and we tended to assign a higher risk of bias when we were not able to find all the necessary information. Furthermore, by virtue of our focus on couples-based interventions, we did not systematically explore interventions only targeted towards pregnant people, which likely provide additional key insight into the effort to improve healthy timing and spacing of pregnancies. We had hoped to conduct a meta-analysis of studies that included partners-based interventions and develop a conceptual model of how partners may influence postpartum contraceptive uptake among pregnant people. Unfortunately, most included studies (14/18) were characterized as partner-optional, meaning that partners were included in at least one aspect of the intervention, but there was little information about partner participation in the intervention, which made it difficult to isolate the effect of including partners in interventions aimed at increasing postpartum contraceptive uptake. In addition to preventing us from conducting a meta-analysis or creating a conceptual framework, the intervention heterogeneity limited our ability to comment specifically on the validity of the partner component of any intervention (since we could only assess the risk of bias in the intervention as a whole).
We suggest that future studies assessing the impact of couples-based interventions on postpartum contraceptive uptake take the following steps to advance our collective understanding of their potential impact on postpartum contraceptive uptake:
Clearly define and measure which components of the intervention include partners and measure each component’s impact on postpartum contraceptive uptake. Since the included studies did not make a strong case for partner-optional versus partner-required interventions, either may be appropriate for future research questions depending on the existing norms and needs in the relevant community.
Describe intervention adherence in the pregnant and non-pregnant partners separately and jointly. For example, only one of the reviewed studies at low risk of bias, Daniele et al. (2018) [19], explicitly stated intervention adherence among non-pregnant partners. Appropriate assessment of non-pregnant partner adherence to the intervention under investigation would allow for secondary analyses that evaluate the impact of pregnant and non-pregnant partner adherence on postpartum contraceptive uptake (such as “per protocol” analyses). These analyses would help with implementation and adaptation of interventions for interested communities.
Clearly define the types of contraceptives available in the study – use a standardized definition – and continue follow up for long enough (18-24 months) such that postpartum contraceptive uptake can help faciliate healthy timing and spacing of pregnancies [4]. This would require interventions to consider additional outcomes relevant to healthy timing and spacing of pregnancies, such as repeat pregnancies during the study period. These events are less frequent than contraceptive use (and hence more difficult to power a study towards) but may be a more relevant clinical and policy question.
Design appropriately powered studies based on the design (e.g., if using a cluster randomized trial, make sure to include an appropriate number of clusters and account for clustering in the analysis). This would avoid completing underpowered studies that do not directly contribute to the knowledge base, which is simultaneously an inappropriate use of financial and human resources and unethical for study participants. This is especially concerning if the trial designs are too heterogeneous to perform meta-analysis.
Finally, none of the reviewed articles mentioned any potentially negative outcomes due to including non-pregnant partners in pregnancy care. This is of concern given evidence from quantitative and qualitative studies assessing couples-based interventions for people with HIV where engaging some non-pregnant partners in care inadvertently led to decreased access to care for pregnant people [66,67]. It is not clear whether including a controlling or otherwise coercive non-pregnant partner in pregnancy care – such as in an attempt to increase postpartum contraceptive uptake – improves the relationship by educating non-pregnant partners on how to treat their pregnant partner or removes autonomy from pregnant partners. Interventions should therefore screen pregnant partners for potentially abusive non-pregnant partners, to avoid harm in the first place. They should also consider measuring relationship functioning at the start and end of interventions and measuring harms that might be secondary to including non-pregnant partners in care, such as interpersonal violence, depression, and anxiety.
In conclusion, while the current literature supports that partner-based interventions may increase the likelihood of postpartum contraceptive uptake, the best type of partner-based intervention and the level of adherence necessary to see improvements in postpartum contraceptive uptake remain unclear. Research that addresses these gaps in the literature can inform interventions that improve postpartum contraceptive uptake. Such analyses would benefit from collaboration that centers pregnant people in intervention planning, a coordinated set of intervention types, outcome measures, and follow up times to best guide clinical and policy recommendations.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Ms. Rachel Walden from the Annette and Irwin Eskind Family Biomedical Library at the Vanderbilt University School of Medicine for assistance shaping the search terms for each database.
Funding:
DES is supported by the National Institute of Mental Health of the National Institutes of Health [F30MH123219] and by NIGMS of the National Institutes of Health [T32GM007347]. LSP is supported by the National Institutes of Allergy and Infectious Diseases [F31AI15261401A1]. CMA is supported by the National Institute of Mental Health [K01MH107255; R01MH113478]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project used REDCap, which is supported by CTSA award number UL1TR002243 from the National Center for Advancing Translational Sciences.
Footnotes
Competing Interests: None declared.
Ethics and Consent: Not required.
Data availability:
Data extracted from the included studies and code for generating the figures is available at https://github.com/dannysack/postpartum_fp_review
References
- [1].Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth Spacing and Risk of Adverse Perinatal Outcomes: A Meta-analysis. JAMA 2006;295:1809–23. 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- [2].Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol 2007;196:297–308. 10.1016/j.ajog.2006.05.055. [DOI] [PubMed] [Google Scholar]
- [3].Cleland J, Conde-Agudelo A, Peterson H, Ross J, Tsui A. Contraception and health. Lancet Lond Engl 2012;380:149–56. 10.1016/S0140-6736(12)60609-6. [DOI] [PubMed] [Google Scholar]
- [4].World Health Organization. Report of a WHO technical consultation on birth spacing: Geneva, Switzerland: 13-15 June 2005 2007. [Google Scholar]
- [5].American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health, American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Louis JM, Bryant A, Ramos D, Stuebe A, et al. Interpregnancy Care. Am J Obstet Gynecol 2019;220:B2–18. 10.1016/j.ajog.2018.11.1098. [DOI] [Google Scholar]
- [6].RamaRao S, Townsend J, Askew I. Correlates of inter-birth intervals: Implications of optimal birth spacing strategies in Mozambique. Reprod Health 2006. 10.31899/rh4.1191. [DOI] [Google Scholar]
- [7].van Eijsden M, Smits LJM, van der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr 2008;88:147–53. 10.1093/ajcn/88.1.147. [DOI] [PubMed] [Google Scholar]
- [8].Feyissa TR, Harris ML, Melka AS, Loxton D. Unintended Pregnancy in Women Living with HIV in Sub-Saharan Africa: A Systematic Review and Meta-analysis. AIDS Behav 2019;23:1431–51. 10.1007/s10461-018-2346-4. [DOI] [PubMed] [Google Scholar]
- [9].Audet CM, Chire YM, Vaz LM, Bechtel R, Carlson-Bremer D, Wester CW, et al. Barriers to Male Involvement in Antenatal Care in Rural Mozambique. Qual Health Res 2016;26:1721–31. 10.1177/1049732315580302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kraft JM, Wilkins KG, Morales GJ, Widyono M, Middlestadt SE. An evidence review of gender-integrated interventions in reproductive and maternal-child health. J Health Commun 2014;19 Suppl 1:122–41. 10.1080/10810730.2014.918216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eliason S, Baiden F, Quansah-Asare G, Graham-Hayfron Y, Bonsu D, Phillips J, et al. Factors influencing the intention of women in rural Ghana to adopt postpartum family planning. Reprod Health 2013;10:34. 10.1186/1742-4755-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hartmann M, Gilles K, Shattuck D, Kerner B, Guest G. Changes in couples’ communication as a result of a male-involvement family planning intervention. J Health Commun 2012;17:802–19. 10.1080/10810730.2011.650825. [DOI] [PubMed] [Google Scholar]
- [13].Mboane R, Bhatta MP. Influence of a husband’s healthcare decision making role on a woman’s intention to use contraceptives among Mozambican women. Reprod Health 2015;12:36. 10.1186/s12978-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olakunde BO, Sam-Agudu NA, Patel TY, Hunt AT, Buffington AM, Phebus TD, et al. Uptake of permanent contraception among women in Sub-Saharan Africa: a literature review of barriers and facilitators. Contraception 2019. 10.1016/j.contraception.2018.12.007. [DOI] [PubMed] [Google Scholar]
- [15].Harrington EK, McCoy EE, Drake AL, Matemo D, John-Stewart G, Kinuthia J, et al. Engaging men in an mHealth approach to support postpartum family planning among couples in Kenya: a qualitative study. Reprod Health 2019;16:17. 10.1186/s12978-019-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Akaba G, Ketare N, Tile W. A community-based, mixed-methods study of the attitudes and behaviors of men regarding modern family planning in Nigeria. Int J Gynecol Obstet 2016;135:86–90. 10.1016/j.ijgo.2016.04.009. [DOI] [PubMed] [Google Scholar]
- [17].Blackstone SR, Nwaozuru U, Iwelunmor J. Factors Influencing Contraceptive Use in Sub-Saharan Africa: A Systematic Review. Int Q Community Health Educ 2017;37:79–91. 10.1177/0272684X16685254. [DOI] [PubMed] [Google Scholar]
- [18].Blazer C, Prata N. Postpartum family planning: current evidence on successful interventions. Open Access J Contracept 2016;7:53–67. 10.2147/OAJC.S98817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Daniele MA, Ganaba R, Sarrassat S, Cousens S, Rossier C, Drabo S, et al. Involving male partners in maternity care in Burkina Faso: a randomized controlled trial. Bull World Health Organ 2018;96:450–61. 10.2471/BLT.17.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chinaeke EE, Fan-Osuala C, Bathnna M, Ozigbu CE, Olakunde B, Ramadhani HO, et al. Correlates of reported modern contraceptive use among postpartum HIV-positive women in rural Nigeria: an analysis from the MoMent prospective cohort study. Reprod Health 2019;16:2. 10.1186/s12978-018-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yargawa J, Leonardi-Bee J. Male involvement and maternal health outcomes: systematic review and meta-analysis. J Epidemiol Community Health 2015;69:604–12. 10.1136/jech-2014-204784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Atukunda EC, Mugyenyi GR, Atuhumuza EB, Kaida A, Boatin A, Agaba AG, et al. Factors Associated with Pregnancy Intentions Amongst Postpartum Women Living with HIV in Rural Southwestern Uganda. AIDS Behav 2019;23:1552–60. 10.1007/s10461-018-2317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang Y, Merkatz R, Zhu H, Roberts K, Sitruk-Ware R, Cheng L, et al. The free perinatal/postpartum contraceptive services project for migrant women in Shanghai: effects on the incidence of unintended pregnancy. Contraception 2014;89:521–7. 10.1016/j.contraception.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [24].Saeed GA, Fakhar S, Rahim F, Tabassum S. Change in trend of contraceptive uptake--effect of educational leaflets and counseling. Contraception 2008;77:377–81. 10.1016/j.contraception.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [25].Sebastian MP, Khan ME, Kumari K, Idnani R. Increasing postpartum contraception in rural India: evaluation of a community-based behavior change communication intervention. Int Perspect Sex Reprod Health 2012;38:68–77. 10.1363/3806812. [DOI] [PubMed] [Google Scholar]
- [26].Ahmed S, Ahmed S, McKaig C, Begum N, Mungia J, Norton M, et al. The Effect of Integrating Family Planning with a Maternal and Newborn Health Program on Postpartum Contraceptive Use and Optimal Birth Spacing in Rural Bangladesh. Stud Fam Plann 2015;46:297–312. 10.1111/j.1728-4465.2015.00031.x. [DOI] [PubMed] [Google Scholar]
- [27].Abdel-Tawab N, Loza S, Zaki A. Helping Egyptian women achieve optimal birth spacing intervals through fostering linkages between family planning and maternal/child health services. Reprod Health 2008. 10.31899/rh4.1136. [DOI] [Google Scholar]
- [28].Koenig LJ, Whitaker DJ, Royce RA, Wilson TE, Ethier K, Fernandez MI. Physical and Sexual Violence During Pregnancy and After Delivery: A Prospective Multistate Study of Women With or at Risk for HIV Infection. Am J Public Health 2006;96:1052–9. 10.2105/AJPH.2005.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jiwatram-Negrón T, El-Bassel N. Systematic review of couple-based HIV intervention and prevention studies: advantages, gaps, and future directions. AIDS Behav 2014;18:1864–87. 10.1007/s10461-014-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Choi J, Fukuoka Y. Spousal influence on physical activity in physically inactive pregnant women: A cross-sectional study. Health Care Women Int 2018;39:263–74. 10.1080/07399332.2017.1402333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Audet CM, Blevins M, Chire YM, Aliyu MH, Vaz LM, Antonio E, et al. Engagement of Men in Antenatal Care Services: Increased HIV Testing and Treatment Uptake in a Community Participatory Action Program in Mozambique. AIDS Behav 2016;20:2090–100. 10.1007/s10461-016-1341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crepaz N, Tungol-Ashmon MV, Vosburgh HW, Baack BN, Mullins MM. Are couple-based interventions more effective than interventions delivered to individuals in promoting HIV protective behaviors? A meta-analysis. AIDS Care 2015;27:1361–6. 10.1080/09540121.2015.1112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arden-Close E, McGrath N. Health behaviour change interventions for couples: A systematic review. Br J Health Psychol 2017;22:215–37. 10.1111/bjhp.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sundermann AC, Hartmann KE, Jones SH, Torstenson ES, Velez Edwards DR. Interpregnancy Interval After Pregnancy Loss and Risk of Repeat Miscarriage. Obstet Gynecol 2017;130:1312–8. 10.1097/AOG.0000000000002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khabsa M, Elmagarmid A, Ilyas I, Hammady H, Ouzzani M. Learning to identify relevant studies for systematic reviews using random forest and external information. Mach Learn 2015:1–18. 10.1007/s10994-015-5535-7. [DOI] [Google Scholar]
- [38].Harrison H, Griffin SJ, Kuhn I, Usher-Smith JA. Software tools to support title and abstract screening for systematic reviews in healthcare: an evaluation. BMC Med Res Methodol 2020;20:7. 10.1186/s12874-020-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Peetluk LS, Ridolfi FM, Rebeiro PF, Liu D, Rolla VC, Sterling TR. Systematic review of prediction models for pulmonary tuberculosis treatment outcomes in adults. BMJ Open 2021;11:e044687. 10.1136/bmjopen-2020-044687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. , editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane: 2021. [Google Scholar]
- [42].Hubacher D, Trussell J. A definition of modern contraceptive methods. Contraception 2015;92:420–1. 10.1016/j.contraception.2015.08.008. [DOI] [PubMed] [Google Scholar]
- [43].Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- [44].Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials 2012;9:48–55. 10.1177/1740774511420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Stampfer MJ, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiol Camb Mass 2008;19:766–79. 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hernán MA, Robins JM. Per-Protocol Analyses of Pragmatic Trials. N Engl J Med 2017;377:1391–8. 10.1056/NEJMsm1605385. [DOI] [PubMed] [Google Scholar]
- [48].Confidence Interval for Two Independent Samples, Dichotomous Outcome n.d. https://sphweb.bumc.bu.edu/otlt/mph-modules/bs/bs704_confidence_intervals/bs704_confidence_intervals7.html (accessed September 10, 2021).
- [49].Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Soliman MH . Impact of antenatal counselling on couples’ knowledge and practice of contraception in Mansoura, Egypt. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit 1999;5:1002–13. [PubMed] [Google Scholar]
- [51].Turan JM, Nalbant H, Bulut A, Sahip Y. Including expectant fathers in antenatal education programmes in Istanbul, Turkey. Reprod Health Matters 2001;9:114–25. [DOI] [PubMed] [Google Scholar]
- [52].Kunene B, Beksinska M, Zondi S, Mthembu N, Mullick S, Ottolenghi E, et al. Involving men in maternity care: South Africa. Reprod Health 2004. 10.31899/rh4.1204. [DOI] [Google Scholar]
- [53].Caleb-Varkey L, Mishra A, Das A, Ottolenghi E, Huntington D, Adamchak S, et al. Involving men in maternity care in India. Reprod Health 2004. 10.31899/rh4.1167. [DOI] [Google Scholar]
- [54].Villar-Loubet OM, Cook R, Chakhtoura N, Peltzer K, Weiss SM, Shikwane ME, et al. HIV knowledge and sexual risk behavior among pregnant couples in South Africa: the PartnerPlus project. AIDS Behav 2013;17:479–87. 10.1007/s10461-012-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tran NT, Seuc A, Coulibaly A, Landoulsi S, Millogo T, Sissoko F, et al. Post-partum family planning in Burkina Faso (Yam Daabo): a two group, multi-intervention, single-blinded, cluster-randomised controlled trial. Lancet Glob Health 2019;7:e1109–17. [DOI] [PubMed] [Google Scholar]
- [56].Abdulkadir Z, Grema BA, Michael GC, Omeiza SY. Effect of Antenatal Couple Counselling on Postpartum Uptake of Contraception among Antenatal Clients and their Spouses attending Antenatal Clinic of a Northern Nigeria Tertiary Hospital: A Randomized Controlled Trial. West Afr J Med 2020;37:695–702. [PubMed] [Google Scholar]
- [57].Whiting-Collins LJ. Postpartum family planning among women attending group-based antenatal and postnatal care in Kenya and Nigeria: A cluster randomized control trial. The Johns Hopkins University, 2020. [Google Scholar]
- [58].Tran NT, Seuc A, Tshikaya B, Mutuale M, Landoulsi S, Kini B, et al. Effectiveness of post-partum family planning interventions on contraceptive use and method mix at 1 year after childbirth in Kinshasa, DR Congo (Yam Daabo): a single-blind, cluster-randomised controlled trial. Lancet Glob Health 2020;8:e399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Coulibaly A, Baguiya A, Garanet F, Tran NT, Millogo T, Yaméogo WME, et al. Yam Daabo interventions’ effects on postpartum family planning use in Burkina Faso at 24 months after childbirth. BMC Public Health 2021;21:946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Atukunda EC, Mugyenyi GR, Musiimenta A, Kaida A, Atuhumuza EB, Lukyamuzi EJ, et al. Structured and sustained family planning support facilitates effective use of postpartum contraception amongst women living with HIV in South Western Uganda: A randomized controlled trial. J Glob Health 2021;11:04034. 10.7189/jogh.11.04034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Turner EL, Li F, Gallis JA, Prague M, Murray DM. Review of Recent Methodological Developments in Group-Randomized Trials: Part 1-Design. Am J Public Health 2017;107:907–15. 10.2105/AJPH.2017.303706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Turner EL, Prague M, Gallis JA, Li F, Murray DM. Review of Recent Methodological Developments in Group-Randomized Trials: Part 2-Analysis. Am J Public Health 2017;107:1078–86. 10.2105/AJPH.2017.303707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bie R, Haneuse S, Huey N, Schildcrout J, McGee G. Fitting marginal models in small samples: A simulation study of marginalized multilevel models and generalized estimating equations. Stat Med 2021;n/a. 10.1002/sim.9126. [DOI] [PubMed] [Google Scholar]
- [64].Toh S, Hernán MA. Causal Inference from Longitudinal Studies with Baseline Randomization. Int J Biostat 2008;4:22. 10.2202/1557-4679.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9:e029954. 10.1136/bmjopen-2019-029954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Aliyu MH, Sam-Agudu NA, Shenoi S, Goga AE, Ramraj T, Vermund SH, et al. Increasing male engagement in the prevention of vertical transmission of HIV: what works in sub-Saharan Africa? BMJ 2019;365:l1965. 10.1136/bmj.l1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Peneza AK, Maluka SO. “Unless you come with your partner you will be sent back home”: strategies used to promote male involvement in antenatal care in Southern Tanzania. Glob Health Action 2018;11:1449724. 10.1080/16549716.2018.1449724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. Am J Public Health 2017;107:863–9. 10.2105/AJPH.2017.303744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Harrington EK, Drake AL, Matemo D, Ronen K, Osoti AO, John-Stewart G, et al. An mHealth SMS intervention on Postpartum Contraceptive Use Among Women and Couples in Kenya: A Randomized Controlled Trial. Am J Public Health 2019;109:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data extracted from the included studies and code for generating the figures is available at https://github.com/dannysack/postpartum_fp_review


