Abstract
Osteosarcoma, with poor survival after metastasis, is considered the most common primary bone cancer in adolescents. Notwithstanding the efforts of researchers, its five-year survival rate has only shown limited improvement, suggesting that existing therapeutic strategies are insufficient to meet clinical needs. Notably, immunotherapy has shown certain advantages over traditional tumor treatments in inhibiting metastasis. Therefore, managing the immune microenvironment in osteosarcoma can provide novel and valuable insight into the multifaceted mechanisms underlying the heterogeneity and progression of the disease. Additionally, given the advances in nanomedicine, there exist many advanced nanoplatforms for enhanced osteosarcoma immunotherapy with satisfactory physiochemical characteristics. Here, we review the classification, characteristics, and functions of the key components of the immune microenvironment in osteosarcoma. This review also emphasizes the application, progress, and prospects of osteosarcoma immunotherapy and discusses several nanomedicine-based options to enhance the efficiency of osteosarcoma treatment. Furthermore, we examine the disadvantages of standard treatments and present future perspectives for osteosarcoma immunotherapy.
Subject terms: Bone cancer, Cancer
Introduction
Osteosarcoma ranks first among malignant bone-related cancers in adolescents and has a complex heterogeneity and an abnormally produced immature osteoid matrix.1–3 Currently, the standard treatments for osteosarcoma are neoadjuvant chemotherapy (presurgery), surgical resection, and adjuvant chemotherapy (postsurgery).4,5 Despite the efforts of researchers, there has been no significant improvement in the 5-year survival rate of osteosarcoma patients over the past few decades, suggesting that existing therapeutic strategies are insufficient.6–8 Moreover, the above approaches cannot effectively eliminate all osteosarcoma cells due to nonspecific drug delivery, which is especially true for metastatic and circulating osteosarcoma cells, which might promote tumor recurrence and progression.9,10 Consequently, new therapeutic strategies against osteosarcoma urgently need to be explored.
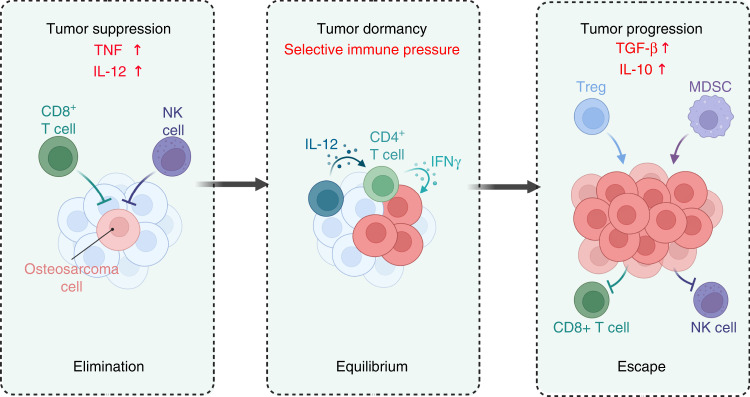
Recently, evidence has shown that the body’s immune system may be in a constant battle with osteosarcoma cells including three stages: immune clearance, balance, and escape11–13 (Fig. 1). Moreover, the immune system plays an important role in the execution and exertion of antitumor immunity. Therefore, utilizing the immunity of the organism for more efficient suppression and treatment of cancer has become a focus for researchers.11,14 The concept of harnessing the immune system for this purpose originated over 100 years ago when a physician named William Coley successfully treated several of his cancer patients with a combination of live and attenuated bacteria, later known as “Coley’s toxins”, after observing a subset of prior patients enter remission following their diagnosis with the common bacterial infection erysipelas.15,16 Notably, the immune microenvironment, the dictator of osteosarcoma treatment response, facilitates cancer cell escape of immune surveillance.17,18 Therefore, therapeutic agents that modulate the immune microenvironment and use existing immunity to eliminate osteosarcoma cells are gradually being recognized as new options with great application prospects.19,20 Unsurprisingly, immunotherapy shows benefits in terms of potent anti-osteosarcoma effects and suppression of metastasis and recurrence in comparison with conventional intervention strategies, including surgical resection and chemotherapy, which also show satisfactory efficacy in suppressing advanced osteosarcoma.21–23
Fig. 1.
The three stages of immune system attack of tumors: immune clearance, balance and escape
Along with rapid advances in immunology and biotechnology, nanoparticles have shown great promise for enhancing cancer immunotherapy.24–26 On the one hand, nanoparticles can effectively improve the pharmacokinetic parameters and reduce the side effects of therapeutic or imaging agents in cancer treatment by site-specific drug delivery.27,28 On the other hand, they can also target immune cells and organs to modulate the immune microenvironment to augment tumor immunotherapy.29,30 Therefore, versatile nanoplatforms, including biomimetic nanoparticles, inorganic nanomaterials, and organic nanomaterials, have been used to effectively modulate the immune microenvironment.31–33 Importantly, some immunomodulatory-based nanoplatforms have achieved satisfactory therapeutic effects in the preclinical study of osteosarcoma.34
For these reasons, managing the osteosarcoma immune microenvironment and using nanomedicines in enhanced immunotherapy are gaining widespread attention as personalized treatment regimens. This review, therefore, focuses on the current understanding of the characteristics and functions of the main immune components in the tumor microenvironment, including dendritic cells, T lymphocytes, tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), and natural killer (NK) cells. Moreover, we highlight that the benefits of nanomedicines in activating immune responses and reversing the immunosuppressive microenvironment hold great potential in osteosarcoma immunotherapy. Furthermore, the current challenges and future prospects of osteosarcoma immunotherapy are also discussed.
The immune microenvironment of osteosarcoma
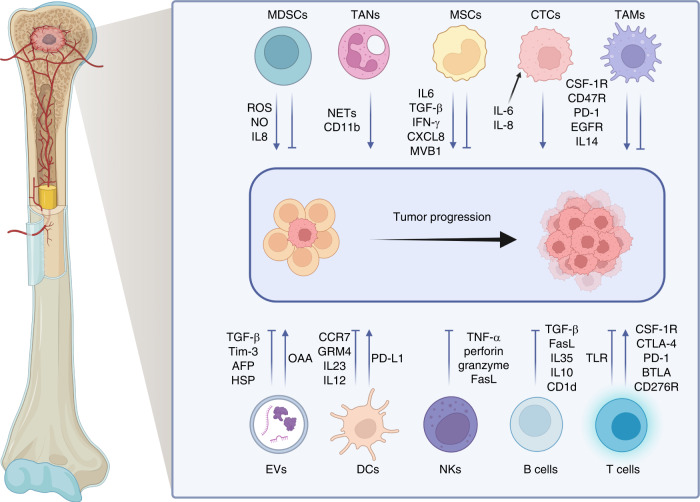
Osteosarcoma tissue is surrounded by massive immune cell infiltration, resulting in the creation of a complex immune microenvironment that allows osteosarcoma cells to grow within the bone by creating an immunosuppressive microenvironment to maintain their survival and proliferation.35–37 A robust immunosuppressive microenvironment is positively correlated with overactivation of molecules associated with immune suppression, such as indoleamine 2,3-dioxygenase (IDO), programmed cell death protein 1 (PD-1), interleukin-10 (IL-10), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and signal transducer and activator of transcription 3 (STAT3), due to their immunosuppressive effects mediated by myeloid-derived suppressor cells (MDSCs), TAMs, and regulatory T lymphocytes (Tregs)38–43 (Fig. 2). Consequently, there is an urgent need to gain an in depth understanding of and characterize the osteosarcoma immune microenvironment to develop advanced immunotherapies by utilizing these immunologic biomarkers.
Fig. 2.
The components in the immune microenvironment of osteosarcoma and the mechanisms of their protumor and antitumor effects
Dendritic cells (DCs) in the osteosarcoma immune microenvironment
DCs, the most common antigen-presenting cells (APCs) originating from the bone marrow, are mainly divided into DCs, DC1s, and DC2s.44–47 It should be noted that type 1 myeloid/conventional DCs (cDC1s) have an excellent profile of antigen presentation and cross-presentation and efficient T lymphocyte priming activity for initiating the immune response.48–50 There are significant differences in inflammatory infiltration among various types of osteosarcoma, but there is no difference in DCs. For instance, (DC-SIGN/CD11c+) DCs are more common in conventional high-grade osteosarcoma than in other sarcomas.51 Moreover, DC infiltration has been found to be associated with autophagy in osteosarcoma. For example, a recent study using a machine learning-based autophagy-related long noncoding RNA signature showed the association between infiltration of immune cells and the expression of autophagy-related genes, among which RUSC1-AS1 was adversely connected to the numbers of infiltrating immature DCs, mast cells, and macrophages.52 With osteosarcoma progression, osteosarcoma cells can develop DC- and phagocytosis-tolerant variants, which reduces DC activation and ultimately causes immune escape.53 It is well known that DCs can express glutamate metabotropic receptor 4 (GMR-4) and carcinogens to drive osteosarcoma pathogenesis. Agonists of GMR-4 or antibodies against IL-23 may be potential options for osteosarcoma immunotherapy.54,55 Additionally, DCs may also play a significant role in the pulmonary metastasis of advanced osteosarcoma.56 A comprehensive study showed that CCR7 contributed to the proliferation, deformation, and migration of DCs, thereby playing an important role in pulmonary metastasis of osteosarcoma. This work also suggested that the number of CD1c+ DCs was higher in pulmonary metastases than in primary and recurrent lesions.57
Several lines of evidence support the potential therapeutic value of DCs in osteosarcoma, but there are also some contradictory views. For example, PD-L1 levels were strongly related to the quantity of DCs and T lymphocytes in osteosarcoma.58 Additionally, DCs and TAMs were also found to be closely associated with survival time. Moreover, some studies have also suggested links between DCs and osteosarcoma prognosis based on immune classification, with fewer DCs than cytotoxic T lymphocytes and NK cells found in living patients.59
T lymphocytes in the immune microenvironment of osteosarcoma
T lymphocytes, a type of thymus-derived lymphocyte, mature and reside in peripheral immune organs.60,61 They contribute to cellular and humoral immunity and can be classified according to different criteria. For example, they are usually classified into effector, naive, and memory T lymphocytes during the activation stage.62,63 Based on the features of cell receptors, such as major histocompatibility complex (MHC) restriction and biodistribution, these cells are divided into αβT and γδT lymphocytes. In addition, they can also be divided into cytotoxic T lymphocytes (CTLs), helper T lymphocytes (Th lymphocytes), and Tregs according to their function.64–66 Overall, T lymphocyte classification is highly heterogeneous, and T lymphocyte infiltration plays a significant role in osteosarcoma immunotherapy.
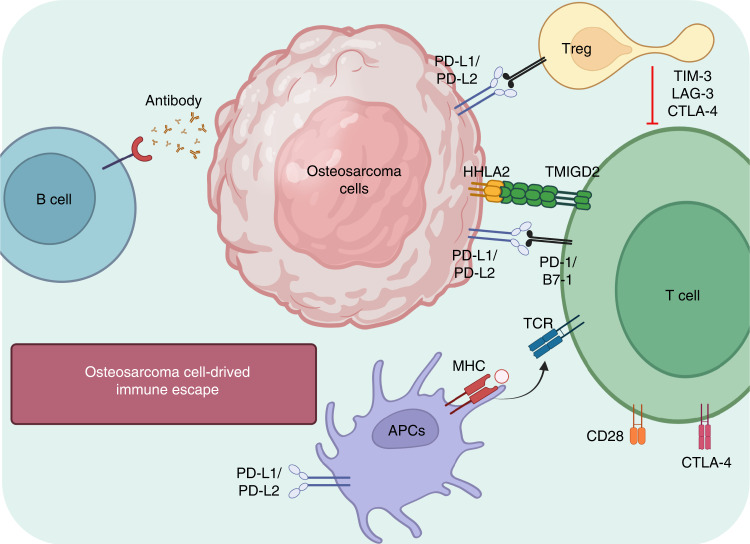
The majority of tumor-infiltrating lymphocytes are clustered at regions overexpressing human leukocyte antigen (HLA) class I in osteosarcomas, while effector T lymphocytes are mostly distributed at the border between healthy tissues and pulmonary metastases.67,68 Moreover, there are more T lymphocytes in metastases than in primary or recurrent lesions in situ.69 However, the levels of immune checkpoint and immunomodulatory molecules in metastatic lesions, such as PD-1, IDO, IFN-γ, and T lymphocyte immunoglobulin and mucin domain-containing protein-3 (TIM-3), have been found to be higher than those in primary tumors68,70–72 (Fig. 3). A recent report investigated infiltrating T lymphocytes in biopsy tumor tissue and peripheral blood samples from sixteen primary osteosarcoma patients,73 showing that there were more TIM-3+ PD-1-negative or -positive T lymphocytes in tumor tissue than in blood circulation, which indicated that the osteosarcoma immune microenvironment was suppressive. This study also showed that these immune infiltrating cells could promote the formation of an immunosuppressive microenvironment via crosstalk with each other in osteosarcoma, which suggested that the immune function of T lymphocytes could be suppressed by M2-type TAMs and that the consumption of CD163+ M2-type macrophages could activate the function and proliferation of T lymphocytes and the secretion of proinflammatory factors by M1-type macrophages.74 In conclusion, complex T-lymphocyte infiltration occurs in different regions and subgroups of osteosarcoma, involves various molecules, and plays different roles in antitumor immune responses.
Fig. 3.
Schematic illustration of immune checkpoints and the interplay between immune cells and osteosarcoma cells
Natural killer cells (NKs) in the immune microenvironment of osteosarcoma
NKs, considered nonspecific cytotoxic immune cells, are able to nonspecifically destroy infected abnormal cells (such as cancer cells) without prior activation or sensitization.75–77 They generally express suppressive surface receptors, such as killer-cell immunoglobulin-like receptors (KIRs), that can identify specific HLA class I molecules, including CD94/NK group 2 member A (NKG2A) and HLA-A, B and C.78–80 Moreover, they can be trained to lyse cancer cells with low expression of MHC class I produced from host cells.81,82 Their activated surface receptors generally include NKG2D and natural cytotoxic receptors (NCRs), which can recognize stress proteins on the surface of cancer cells, such as MHC class I peptide A/B (MICA/B), UL16-binding proteins (ULBPs), and the Fcg receptor CD16, which induces ADCC by recognizing the Fc portion of antibodies on opsonized cells.83–86 Coreceptors of NKG2D and NCRs, such as DNAM-1, can enhance the activation of NK cells for efficient immune responses.87 The balance between positive and negative signals received by NK cells determines their antitumor effects, which are mainly modulated by the secretion of cytotoxic granules (such as perforin and granzyme), the generation of cytokines (such as IFN-γ and TNF-α) activating antitumor immunity, and the expression of death receptor ligands on their surface.88
Osteosarcoma cells have been shown to be readily eliminated by NK cells in some preclinical studies.89,90 For example, cultivation of NKs from normal donors with osteosarcoma feeder cells for one week resulted in a median killing effect of approximately 46.1%.91,92 Notably, this cytotoxicity was not associated with the expression levels of NK receptor ligands but was significantly suppressed through the exposure of NKs to anti-DNAM-1 and anti-NKG2D antibodies.93 Similarly, blockade of the NKG2D receptor, but not that of DNAM-1, could greatly reverse the cytotoxicity of NK cells against osteosarcoma cells in in vitro assays.94,95 KIRs also play a significant role in osteosarcoma treatment: KIR receptor-ligand mismatched NK cells have an excellent in vitro anti-osteosarcoma effect, and this effect is further augmented when the HLA class I molecule is blocked in osteosarcoma cells.94,96,97 Furthermore, intraperitoneal administration of IL-2 in combination with intratumoral administration of activated and expanded NK cells can effectively mitigate bone impairment, suppress osteosarcoma volume, inhibit pulmonary metastasis, and clearly prolong mouse survival time.
The tumor-associated macrophage (TAM)-mediated immunosuppressive microenvironment in osteosarcoma
TAMs are generally considered to be derived from the myelomonocytic lineage and to develop from hematopoietic stem cells (HSCs).98–100 Moreover, they are generally recruited from the blood circulation to the site of the lesion to eliminate infection, inflammation or tumor cells, such as osteosarcoma cells.101–103 However, emerging evidence has recently indicated that TAMs can develop in embryos before the emergence of HSCs and maintain self-renewal and proliferation.104,105 Macrophages can be divided based on their origin into tissue-resident macrophages, mainly derived from the yolk sac, and blood monocytes, derived from the fetal liver and bone marrow.106
The quantity of TAMs may vary markedly in different solid tumors, including osteosarcoma, but they are the most abundant immune cell type in the tumor microenvironment, accounting for nearly 50% of the total tumor cells.38,107 TAMs play a significant role in matrix remodeling, inflammation and vascularization in antitumor immunity and modulation. In general, TAMs are characterized by protumor or antitumor effects based on the degree of malignancy of the tumor and their interactions with the tumor microenvironment because of their plasticity and heterogeneity.108,109 For instance, type-1 TAMs have the capability to phagocytose cancer cells and promote the secretion of inflammatory factors to improve antitumor immune responses.110,111 However, TAMs usually show an immunosuppressive type-2 phenotype in the tumor microenvironment and are prone to facilitate angiogenesis and extravascular invasion, which promote evasion of immune surveillance, eventually resulting in tumor progression, metastasis, and relapse.112,113 Moreover, CD14 and CD68 double-positive TAMs are the main immune infiltrating TAM subtype in bone-associated cancers, including osteosarcoma.51 An analysis of osteosarcoma patient RNA expression profiles, clinical features, and immune cell proportions showed that type 2 TAMs are the main immune cell type and are closely related to survival time.114 Another study using CD209 staining and gene expression analysis supported that there is accumulation of type-2-like TAMs in human osteosarcoma tissues and found that retinoic acid could modulate M2-like TAMs to suppress osteosarcoma initiation and stemness.115
Recently, emerging evidence has also confirmed that the quantity of TAMs in various tumor tissues is closely associated with the quantity of tumor blood vessels, suggesting that TAMs promote tumor angiogenesis.116–118 For example, various proangiogenic substances, such as fibroblast growth factor (FGF), matrix metallopeptidase 9 (MMP-9), and vascular endothelial growth factor (VEGF), are produced by TAMs to promote tumor progression and metastasis in various cancers, including osteosarcoma.119–121 Moreover, TAMs can also interact with various immune effector cells to induce an immunosuppressive tumor microenvironment. They can suppress the activity of T lymphocytes to facilitate tumor immune escape by overexpressing PD-1 and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) receptors.122 Furthermore, M2-like TAMs can also increase vascular extravasation, promote the survival and growth of metastases, suppress cytotoxic T lymphocytes, and maintain immunosuppressive Tregs to enhance tumor invasion and metastasis.123 As a result, the premetastatic niche is formed at distant lesions in specific metastatic sites, including bone, lung, and liver, with the assistance of TAMs.124
In addition to the above findings, recent reports have also shown that TAMs participate in local inflammatory regulation and drug resistance in osteosarcoma by interacting with other immune cells in the tumor microenvironment.125 However, distinct TAM subtypes may respond differently in osteosarcoma, causing different degrees of malignancy. Specific targeting of TAMs (such as CD163-positive TAMs) rather than pandepletion of TAMs has been shown to enhance the cytotoxic activity of T lymphocytes functioning in tumor suppression.73,126,127 Such information might prompt researchers to define specific TAM features and subtypes in human biopsies for enhanced TAM-specific targeting. Indeed, specific TAM subgroups, characteristics and signals continuously evolve over immunological progression, modulating either protumor or antitumor activity (summarized in Table 1). We have also highlighted the differences between human and murine TAMs (summarized in Table 2). Overall, with the deepening understanding of TAMs, investigators will have the option to manipulate TAMs using various approaches to enhance osteosarcoma immunotherapy.
Table 1.
Therapeutic TAM-modulating agents for osteosarcoma
| Therapeutic agent | Targeted cell or molecule | Mechanism | Reference |
|---|---|---|---|
| Mifamurtide (MTP-PE) | Monocytes and macrophages | Switches TAM polarization toward an intermediate M1-M2 phenotype | 474 |
|
pSTAT3, pAKT, IL-17R TNF-a, IL-1, IL-6, IL-8, NO, PGE2, and PGD2 LFA-1, ICAM-1 and, HLA-DR |
Switches TAM polarization toward an intermediate M1-M2 phenotype | 475 | |
| ATRA | CD117+ Stro-1+ cells, cancer stem cells, and macrophages | Decreases M2-like TAMs | 125 |
|
IL-1b, IL-4, IL-6, IL-13, and CXCL8 |
Decreases M2 phenotype polarization-mediated stemness | 476 | |
| Esculetin |
LM8 cells and macrophages Cyclin D1, CDK 4, MMP-2, TGF-b1, VEGF, IL-10, MCP-1, and pSTAT3 |
Downregulates essential cytokines (TGFb1, IL-10, and MCP-1) and proteins (pSTAT3) Involved in the differentiation of M2 macrophages | 299 |
| Zoledronic acid | Monocytes, DCs, and macrophages | Upregulates M1-like cytokines | 73 |
|
IL-1β, TNF-α, VEGF, IL-10, IDO, IL-12, and poly I:C TGF-β, Arg-1, and Fizz-1 |
Downregulates M2-like cytokines | 477 | |
|
Porous hollow iron nanoparticles |
Macrophages PI3K g and NF-kB p65 |
Upregulates NF-kB p65 and downregulates PI3K g in TAMs | 478 |
|
Chimeric antigen receptor macrophages |
T cells, dendritic cells, and macrophages ERK and NF-kB (P65) |
Upregulates pro-inflammatory pathways (interferon signaling, the TH1 pathway, and iNOS signaling) in M2 macrophages | 479 |
Table 2.
Differences between mouse and human macrophages
| Parameters | Murine TAMs | Human TAMs | Therapeutic implications |
|---|---|---|---|
|
M1-type versus M2- type polarization factors |
M1: IFNγ+LPS M2: M-CSF+IL-4 |
M1-type: GM-CSF, GMCSF+IFNγ, CSF+IFNγ M2: M-CSF+IL-4 (or IL-1 or IL-10) |
The M1/M2 model is not relevant for human TAM characterization |
| Specific markers |
M1: iNOS, CD80, MHC-II, IFNγ M2: Arg1, Ym1, VEGF |
M1: IL-1β, IL-6, TNFα, IL-12 M2: TGF-β, VEGF, EGF, PDGF, IL-10 |
Prototypic mouse M1 and M2 markers cannot be used to characterize human TAMs |
| Type II IFN expression | High expression | Low expression | Type II IFN production by murine TAMs may favor cytotoxic T lymphocyte responses and their reprogramming into antitumor Mφ |
| Polarization versus activation | Resting (M2) and LPS-stimulated (M1) cells are usually compared | Human Mφ and TAMs require stimulation (such as stimulation via CD40 or TLRs) to reveal their phenotypes | Human Mφ polarization and activation are two independent processes |
The tumor-associated neutrophil (TAN)-mediated immunosuppressive microenvironment in osteosarcoma
Neutrophils are important immune cells that are sensitive to pathogens and tissue impairment, accounting for nearly 50%-70% of total white blood cells in humans.128–130 Most clinical studies on neutrophils in osteosarcoma patients have focused on the ratio of neutrophils to lymphocytes or circulating neutrophils, and an increased ratio of neutrophils to lymphocytes pretreatment or presurgery might be closely associated with poor outcomes, indicating that this ratio can be applied as a prognostic marker for osteosarcoma.131–135 Neutrophils in the tumor microenvironment, also named TANs, show functional versatility and phenotypic heterogeneity similar to those of TAMs.136–138 However, there are limited reports about neutrophil infiltration in the osteosarcoma immune microenvironment.
In osteosarcoma, TANs may have a longer lifespan under the stimulation of proinflammatory factors (such as IFN-γ) than circulating neutrophils.139,140 The neutrophil extracellular trap (NET)-mediated immunosuppressive microenvironment plays a significant role in immune escape during cancer immunotherapy and consists of a reticular chromatin structure composed of chromatin and granule proteins produced from neutrophils;141 this microenvironment facilitates tumor metastasis instead of conventional phagocytosis and killing factor secretion.142 For example, PAD4, which is overexpressed in osteosarcoma, plays an important role in forming NETs via extensive chromatin decondensation.143 Moreover, TANs can also be polarized to anticancer N1-like or procancer N2-like neutrophils, which are similar to M1- and M2-like macrophages.144–146 In this process, TGF-β produced from tumor and tumor microenvironment-associated cells can effectively promote the aggregation of type-2 neutrophils in tumor regions, resulting in functional and phenotypic neutrophil changes.144,147 In addition, the stimulation of TANs with IFN-γ and TNF-α can polarize type-2 neutrophils into type-1 neutrophils to efficiently suppress tumor growth.148 This phenotypic switch is closely related to changes in protein secretome profiles, including changes in the levels of secreted granule-associated proteins, adhesion molecules, chemokines, and cytokines.149–152 Compared with patients with metastasis, patients with nonmetastatic osteosarcoma present significantly higher expression levels of the neutrophil-specific marker CD11b.153 Infiltrating neutrophils further exert positive antitumor effects by coordinating the recruitment of immune cells to effectively mediate specific immunity while activating antibody-dependent cellular cytotoxicity (ADCC).154,155 Furthermore, the infiltration of neutrophils has also been reported to be associated with the expression of hypoxia-related genes.156,157 Emerging studies have indicated that hypoxia in the tumor microenvironment promotes tumor progression, and thus, hypoxia could be regarded as a prognostic factor for metastasis.32,158,159 There are significantly fewer N1-like TANs in groups with high hypoxia than in the groups without hypoxia, suggesting that hypoxia might contribute to evasion of immune surveillance and promotion of tumor metastasis by reducing antitumor immune cells.157 However, both studies described above only considered the total TAN number without taking functional differences between subtypes into account, which may be due to the difficulty in recognizing specific biomarkers. Therefore, more comprehensive studies are required to reveal the complex functions of TANs in osteosarcoma; furthermore, advanced treatments related to TANs are still being developed, and novel ideas derived from basic scientific research are required.
The myeloid-derived suppressor cell (MDSC)-mediated immunosuppressive microenvironment in osteosarcoma
MDSCs, as a group of immunosuppressive immature myeloid cells, are able to create an immunosuppressive tumor microenvironment by differentiating into tumor-associated DCs, TAMs, and TANs.160,161 They interact not only with the immune microenvironment but also with osteoblasts, osteoclasts, chondrocytes, and other stromal cells in the bone and joint microenvironment to facilitate the pathogenesis and metastasis of various tumors, including osteosarcoma.162,163 Generally, murine MDSCs are characterized by the coexpression of CD11b and Gr-1, and these cells are now further divided into granulocytic (G-MDSCs) and monocytic (M-MDSCs) based on their phenotypes and morphological characteristics. The former express have the cell surface marker phenotype CD11b+Ly6ClowLy6G+, while the latter are characterized by a CD11b+Ly6ChighLy6G- phenotype.164,165 Human MDSCs are different from murine MDSCs, which are characterized by no or low expression of HLA-DR and high expression of CD33. Unsurprisingly, the common myeloid biomarker CD11b is also a marker of human MDSCs, and the CD11b and CD33 double-positive HLA-DR-low population can be further classified as M or G MDSCs according to their CD14 or CD15 expression. It should be noted that CD15-positive cells usually exhibit a granulocytic morphology, while CD14-positive cells exhibit monocytic features.166,167
Recent reports have confirmed that early bone marrow mesenchymal stem cells (e-BMSCs) can serve as precursors of PMN-MDSCs and M-MDSCs.162,168,169 MDSCs interact most closely with T lymphocytes of all immune cells, and these interactions can lead to the production of reactive oxygen species (ROS) and ablate L-arginine in the tumor microenvironment to suppress the proliferation and boost the apoptosis of T lymphocytes and weaken T lymphocyte-mediated immunity.170,171 Different MDSC subtypes can also suppress the activity of T lymphocytes in different ways. For example, PMN-MDSCs can upregulate nicotinamide adenine dinucleotide phosphate (NADP) oxidase and activate STAT-3 to generate ROS, while M-MDSCs can modulate inducible nitric oxide synthase and activate STAT1 to release NO to suppress the function of T lymphocytes.172,173 Notably, MDSCs can inhibit not only the acquired antitumor immune response but also the innate antitumor immune response.174,175 For instance, MDSCs can impair the antigen presentation of DCs and phagocytosis of NK cells to promote tumor immune escape.176–178 In addition to suppressing immunity in the tumor microenvironment, MDSCs can also actively participate in the management of the immune microenvironment and tumor metastasis.179 Interestingly, under the stimulation of the hypoxic tumor microenvironment, MDSCs usually produce high levels of basic FGF, VEGF, VEGF analog Bv8, and MMP-9 to promote angiogenesis and the creation of a premetastatic niche, indicating a close association with pulmonary metastasis of osteosarcoma.180
Extracellular vesicles (EVs) in the immune microenvironment of osteosarcoma
Cancer cell-derived EVs are a group of heterogeneous nanovesicles secreted into the tumor microenvironment or circulation, encapsulating intact organelles, proteins, nucleic acids, and lipids (such as eicosanes, cholesterol, and fatty acids).181–183 Exosomes, a subclass of EVs, are produced by direct outward budding of the cell membrane.184,185 They induce the proliferation, metastasis, and chemotherapy resistance of osteosarcoma cells.185–187 These functions may be derived in part from interactions between tumor-derived exosomes and bone cells that form a microenvironment conducive to cancer cell homing.188 Notably, tumor cell- and immune cell-derived exosomes have been shown to carry TAAs and activate antitumor immune responses, resulting in the elimination of established tumors by CD8+ and CD4+ T lymphocytes, as well as directly inhibiting tumor cell proliferation and malignant tumor progression.189 In addition, emerging evidence supports that tumor-derived EVs can typically form an immunosuppressive microenvironment. As an example, the release of soluble MHC-I chain-related proteins and NKG2D soluble receptors from osteosarcoma-derived exosomes can suppress NK cell or CTL activity, thereby creating a conducive environment for osteosarcoma immune escape.190,191 Another study suggested that the level of exosomal PD-L1 in osteosarcoma patients with pulmonary metastasis was much higher than that in patients without pulmonary metastasis.191 In the study, a mouse model was used to evaluate the roles of exosomes expressing PD-L1, and the study showed that pulmonary metastasis significantly increased after exposure to such exosomes. Based on these studies, it appears that osteosarcoma cells can secrete exosomal PD-L1 to promote lung metastasis; therefore, detecting the level of exosomal PD-L1 in serum might be an important means to identify pulmonary metastasis for clinical treatment. A potential mechanism behind the utility of this strategy is related to PD-L1-induced immunosuppression, which subsequently enables expression of epithelial-mesenchymal transition (EMT)-related proteins, including N-cadherin, vimentin, fibronectin, and laminin 5. Another study reported that in 36% of cancer patients, exosomes containing IDO, which can modulate antitumor immune responses by regulating tryptophan (Trp) consumption and is involved in the formation of an inflammatory microenvironment to facilitate tumor angiogenesis, were identified in the majority of bodily fluids.192,193 These molecules in osteosarcoma-derived exosomes plays an indirect role in immune responses and may influence DC-mediated neoantigen presentation.190 Therefore, reversing the immunosuppressive microenvironment by regulating exosome release may be a new strategy to enhance osteosarcoma immunotherapy.
Mesenchymal stem cells (MSCs) in the immune microenvironment of osteosarcoma
MSCs, considered competitive clones that promote tumorigenesis, are another reason for the substantial heterogeneity found in osteosarcoma, which causes chemotherapy resistance, recurrence, and metastasis.194 Based on the expression of tumor stem cell-related genes, osteosarcoma patients can be divided into two clusters (Cluster 1 and Cluster 2).195 The immune microenvironment of Cluster 1 patients has fewer follicular helper T lymphocytes and macrophages and more cytotoxic T lymphocytes, resulting in an immune infiltrating phenotype and superior therapeutic effects compared to those of Cluster 2 patients. This suggests that MSCs affect the immune microenvironment of osteosarcoma, and different types of MSCs might lead to diverse immune infiltration phenotypes and outcomes. Moreover, a variety of studies have indicated that osteosarcoma might be derived from bone marrow-derived MSCs.196–199 MSCs are mature pluripotent stem cells found in different tissues, particularly in the dental pulp, bone, and adipose tissue, and play a significant role in modulating immunity, cell fusion and differentiation in osteosarcoma tumorigenesis.200 The histogenesis of osteosarcoma shows that naive MSCs and MSCs from tumors might play adverse roles in osteosarcoma progression. Naive MSCs generally have suppressive or supportive effects in cancer, while tumor-derived MSCs have the ability to facilitate epithelial-mesenchymal transition (EMT), thus exhibiting a robust immunosuppressive effect and promoting tumor cell proliferation.201 This MSC-mediated promotion of the proliferation and metastasis of osteosarcoma cells can be attributed to the following two points. On the one hand, the interaction between MSCs and osteosarcoma cells involves aquaporin 1 and IL-8. On the other hand, abnormal gene expression, including abnormal expression of TP53, Rb, C-MYC, IHH, and KRAS, can facilitate the reprogramming of MSCs into osteosarcoma cells.199,202–205 Notably, MSCs can also transform into tumor-associated fibroblasts (CAFs) after exposure to osteosarcoma cells, which can obviously promote osteosarcoma cell proliferation and metastasis.200,206 This process usually involves multiple substances, including monocyte chemotactic protein 1, growth-related oncogene-α, TGF-β, and intercellular adhesion factor. Moreover, CAFs can release extracellular matrix components to maintain cell proliferation and intercellular adhesion and communication to maintain malignant phenotypes and increase tumor heterogeneity. To some extent, osteosarcoma cells and MSCs have similar functions. For example, some studies have reported that osteosarcoma cells can also trigger the migration and invasion of endothelial cells and facilitate angiogenesis after exposure to MSCs.198,206–208 In regard to the immune response, MSCs can effectively release anti-inflammatory factors and suppress proinflammatory factors to assist osteosarcoma cells in immune escape, which is induced by autocrine or paracrine EVs, especially exosomes.209 An interesting study reported that MSCs can secrete EVs containing miRNAs/RNAs and proteins to suppress the proliferation and immune response of T lymphocytes.210 Additionally, TGF-β and IFN-γ secreted by MSC-derived EVs can induce the switch of mononuclear cells into Tregs.211 In addition to inhibiting T lymphocyte-related antitumor immunity, MSCs can also suppress the immune function of B lymphocytes. For example, MSC-derived exosomes can significantly increase the expression of MVB1 RNA and C-X-C motif chemokine ligand (CXCL) 8, which decreases the quantity of immunoglobulin M (IgM) in B lymphocytes.212 It should be noted that MSCs can also secrete IL-6 to promote the M2-like polarization of TAMs.213,214 Additionally, some cytokines, including IL10, hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF), CXCL2, CXCL20, and VEGF-C, also play a significant role in the increased accumulation of MSC-EVs at tumor sites and inflammation suppression.215
Circulating tumor cells (CTCs) in the immune microenvironment of osteosarcoma
Cancer cells, including osteosarcoma cells, can exist either in tumor tissues or in blood circulation, and such cells are named circulating tumor cells (CTCs).10 They have the ability to escape local therapy to survive at low levels with systemic interventions, such as radiotherapy, phototherapy and surgical resection, eventually leading to the metastasis and recurrence of osteosarcoma.216,217 Emerging evidence has also shown the important role of CTCs in the osteosarcoma immune microenvironment.218 For instance, previous studies have shown that suppression of IL-6 can decrease the number of CTCs to improve osteosarcoma treatment effects.219 This phenomenon was also found in another in vitro study that suggested that human IL-6 could activate the Janus-activated kinase/STAT3 and mitogen-activated protein kinase/extracellular signal modulated kinase 1/2 (MEK-1/ERK) pathways. These pathways have been shown to promote osteosarcoma cell proliferation, but only the Janus-activated kinase/STAT3 pathway can drive the migration of osteosarcoma cells. Therefore, one can speculate that the STAT3 pathway might facilitate the spread of CTCs for the formation of an immunosuppressive osteosarcoma microenvironment. In addition to IL-6, IL-8 also participates in CTC-mediated osteosarcoma progression by recruiting and activating T or B lymphocytes, neutrophils, basophils, and eosinophils. IL-8 released from self-seeded CTCs can effectively induce osteosarcoma cell proliferation and pulmonary metastasis in ex vivo assays.220 Consequently, suppressing the activity and secretion of IL-8 might be a potential antitumor strategy that can possibly be used in combination with various agonists or antagonists of other cytokines. Although the mechanism underlying the relationship between immune responses and CTCs is not clear, CTCs have been identified as potential predictive biomarkers and drug intervention targets for enhanced osteosarcoma immunotherapy. Furthermore, elimination of CTCs is being recognized as a more thorough and effective antitumor strategy.
Conventional immunotherapy for osteosarcoma
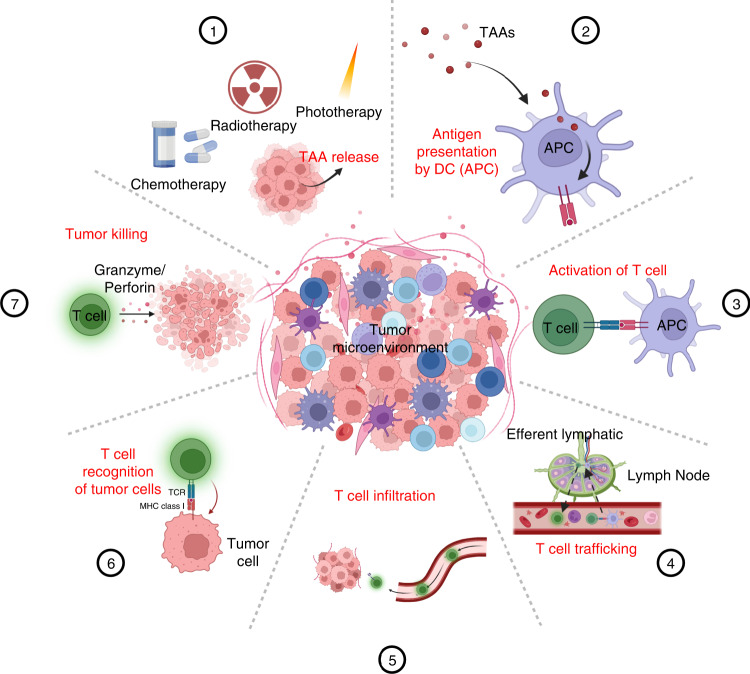
To better understand the immune response in osteosarcoma, the concept of the “tumor immunity cycle” has been used to illustrate approaches to enhance the effects of immunotherapy221 (Fig. 4). The crucial process of this cycle occurs in the tumor and regional lymph nodes, with immune cells traveling between these distinct regions.222–224 This immunity cycle starts with the release of neoantigens or TAAs from dying cancer cells, and these TAAs are subsequently captured and processed by APCs. After that, APCs present the processed TAAs on MHC-I and MHC-II molecules to naive T lymphocytes in draining lymph nodes, leading to the activation and production of CTLs to eliminate cells with specific TAAs. Activated CTLs multiply through clonal expansion, enter the blood circulation, and migrate from local lymph nodes to the tumor microenvironment. Once activated T lymphocytes arrive at tumor tissues, they can release cytotoxic substances such as perforin and granzyme B to eliminate tumor cells. These dying tumor cells in turn release more TAAs and costimulatory signals (such as DAMPs) to induce the antitumor immunity cascade.225,226 However, osteosarcoma can disrupt the essential elements of the tumor immunity cycle via an extensive negative feedback immunoregulatory mechanism, which is becoming increasingly recognized as a promising target for osteosarcoma immunotherapy.227
Fig. 4.
Schematic illustration of the cancer immunity cycle, which can be divided into seven main steps: (1) release of TAA; (2) presentation of TAAs; (3) activation and proliferation of T lymphocytes; (4−5) trafficking and infiltration of T lymphocytes; (6) T lymphocyte recognition of cancer cells; and (7) killing of cancer cells by T lymphocytes
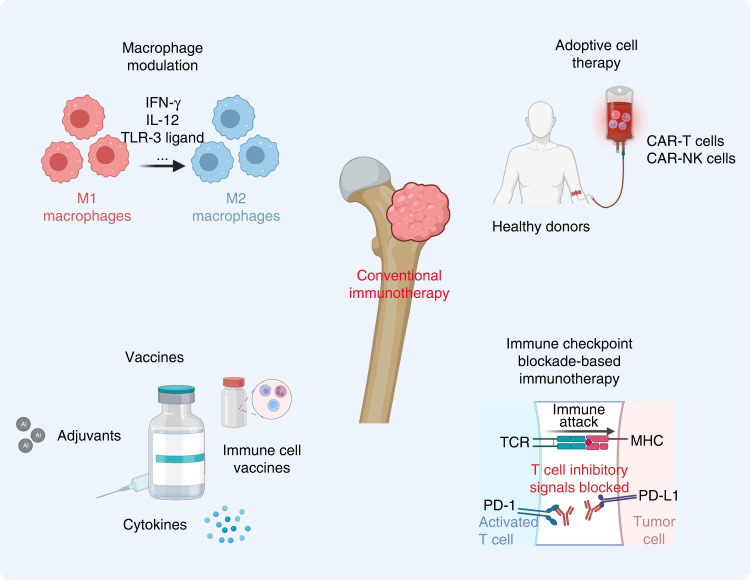
Macrophage modulation strategies for osteosarcoma immunotherapy
Macrophages are capable of responding to multiple stimuli in the tumor immune microenvironment via broad activation phenotypes due to their plasticity. As mentioned above, the transition from M1- to M2-like polarization of TAMs plays a significant role in the pulmonary metastasis of osteosarcoma. Thus, modulating macrophage polarization has been considered a promising approach for osteosarcoma immunotherapy (Fig. 5). Various agents have been used to modulate the polarization of macrophages to enhance antitumor immune responses, including Toll-like receptor (TLR) agonists, cytokines, and monoclonal antibodies.228 Moreover, several cytokines (including IFN-γ and IL-12) have been found to reprogram macrophages toward type 1-like phenotypes by activating the STAT signaling pathway.229 It should be noted that TLRs are important pathogen recognition receptors expressed by APCs, such as macrophages; therefore related agonists can mediate the switch of M2- to M1-like phenotypes to elicit an antitumor immune response.230 For example, Vidyarthi et al. found that murine colonic tumors polarized M2-like TAMs toward M1-like TAMs and inhibited tumor cell proliferation in an IFN-αβ signaling pathway-dependent manner by administering the TLR-3 ligand [poly (I: C)].231 In addition to cytokines and TLR agonists, antibodies, such as anti-CSF1 and anti-CD40 antibodies, are also used to facilitate the polarization of TAMs.232,233 Moreover, several drugs have also been shown to reprogram TAMs and have showing promising results in osteosarcoma treatment. Lipopolysaccharide (LPS)-activated M1-like TAMs in combination with IFN-γ presented significant inhibitory effects on osteosarcoma cell proliferation, and these effects could be regulated by soluble substances released by TAMs in an IL-1/TNF-α-independent manner.234 For example, all-trans retinoic acid (ATRA) can effectively suppress M2-like polarization and the secretion of MMP-12 to inhibit the invasion and pulmonary metastasis of osteosarcoma.235 Metformin (Met), which repolarizes TAMs to elicit antiangiogenic and antitumor effects, also plays a significant role in suppressing osteosarcoma cell proliferation by reprogramming the metabolic polarization of TAMs.236,237 Notably, gefitinib (Gef), an efficient EGFR inhibitor, can also repolarize the pulmonary macrophage phenotype by disturbing macrophage receptor-interacting protein kinase 2 (RIPK2) expression to suppress the invasion and metastasis of osteosarcoma.238–240 Additionally, Gef can also relieve postoperation-accelerated osteosarcoma metastasis and prolong overall survival time in a mouse model of osteosarcoma.241 Various compounds have also been derived from natural products, including epimedokoreanin B (derived from Epimedii Herba),242 onion A1 (derived from allium sulfides),243 and oleanolic acid (OA)/corosolic acid (CA).244,245 In an osteosarcoma mouse model, these compounds significant suppressed STAT3 activation to modulate type 2 TAM polarization, protecting against osteosarcoma progression and metastasis. Another study explored M2-like TAM antagonists or modulators, such as esculetin, wogonin (derived from the roots of Scutellaria baicalensis), resveratrol and synthetic hydroxystilbenes, xanthoangelol and 4-hydroxyderricin, for enhanced osteosarcoma immunotherapy.246–250 These compounds all effectively inhibited the activation and differentiation of type 2 macrophages to suppress osteosarcoma cell proliferation and metastasis.
Fig. 5.
Schematic illustration of current immunotherapy strategies for osteosarcoma
Osteosarcoma-associated vaccines
Osteosarcoma-associated vaccines are considered a novel immunotherapy method that exerts antitumor effects by activating a patient’s own endogenous immunity (Fig. 6). Studies on tumor antigens utilizing tumor-relevant substances composed of tumor proteins or peptides, autologous DCs, gangliosides, and autologous or allogeneic cancer cells to activate systemic immunity are ongoing.251–253 Adjuvants, cytokines, and other immunomodulators have also been applied in vaccine preparation to improve antitumor immunity.82 For instance, autologous tumor lysates were first used as tumor-associated vaccines and significantly prolonged the overall survival time of patients with osteosarcoma.254 Immune responses to peptides derived from the TAA papillomavirus binding factor (PBF) were found in 9 of 11 patients with refractory osteosarcoma.83 Moreover, T lymphocyte responses were detected in 20 of 28 patients with osteosarcoma and were related to long DFS (more than 5 years) in 2 patients with an anti-idiotypic antibody who received vaccination.84 The use of tumor vaccines is gaining momentum. Tumor vaccines are mainly divided into autologous cancer cell- and immune cell-based vaccines and noncell-based vaccines. Of these types, immune cell-based vaccines take full advantage of the activation of effector T lymphocytes by innate immunocytes, such as macrophages, DCs, and γδT lymphocytes. At the same time, however, the feasibility of modulating migration and activation is a major concern, as these processes are regulated by immunoinhibitory substances in the tumor microenvironment and the quantity and quality of compromised immune effector cells in patients.255 On the other hand, autologous cancer cell- and noncell-based vaccines have shown potential clinical value, as they can circumvent these barriers. It should be noted that the mechanisms of action of autologous cancer cell-based vaccines are independent of HLA-I, and the function of the patients’ immune system is to specifically select the most immunogenic antigen.256 Noncell-based vaccines generally have better antitumor effects and biocompatibility since they do not induce off-target effects.257
Fig. 6.
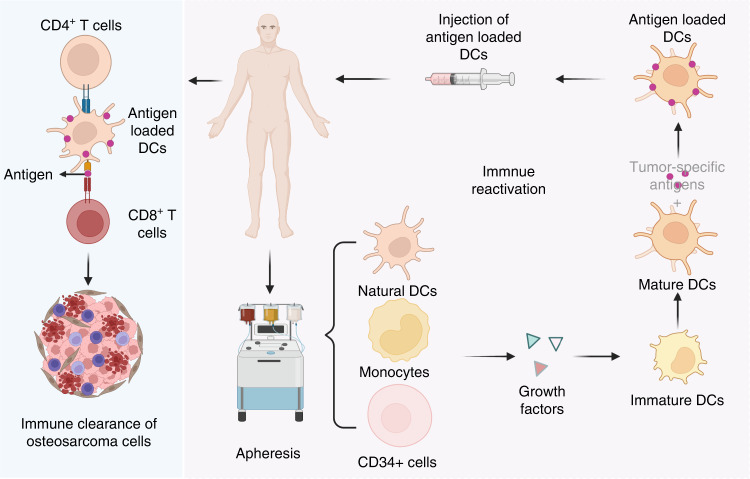
The main process used to generate ex vivo DC vaccines. Progenitor cells are extracted from patients via dialysis, induced to transition into mature DCs, and then loaded with tumor antigens for administration to patients. Tumor antigen-specific T lymphocytes are activated by injected antigen-loaded DCs to exert an antitumor effect
For example, melanoma-associated antigen 1 (MAGE-A1) was isolated and the first HLA-restricted anti-MAGE-A1 T lymphocytes and epitopes were identified using autologous CTL identification.258 Canine osteosarcomas are the only other spontaneous osteosarcomas in large animals, and their immune infiltrates resemble those of human osteosarcomas.90 It should be noted that mifamurtide (MTP-PE), the most effective systemic agent in canine osteosarcoma, has been approved in Europe and Japan for clinical use.90 Another example, DXS31-164, a Listeria monocytogenes vaccine (recombinant Listeria monocytogenes expressing a chimeric human HER2/neu construct), effectively triggers a HER2/neu-specific immune response to suppress pulmonary metastasis and prolong overall survival in a canine osteosarcoma model.259 The study also suggested the excellent effects of DXS31-164 in the treatment of HER2/neu-positive osteosarcoma patients. A recent study showed that the human anti-idiotypic vaccine 105AD7 was effective in most young osteosarcoma patients in clinical trials, without notable side effects.260 Another study also demonstrated that this vaccine could effectively elicit T-lymphocyte-mediated immunity in osteosarcoma patients and target a natural antigen (CD55) with amino acid and structural homology with 105AD7.261 Furthermore, various trials have also suggested that protein-based tumor vaccines, including those composed of tumor-rejection antigens and papillomavirus binding factors, can be used for specific immunotherapy in osteosarcoma and other malignant cancers due to shared overexpressed TAAs.262–267
DCs, considered professional APCs, make up approximately 0.3% of the total cell population in the blood and can promote the proliferation and differentiation of CTLs.268,269 Moreover, the quantity of DCs is strongly associated with the survival time of patients.270,271 Therefore, DC-based vaccines are the most common vaccination approach for pediatric sarcomas, and patients who received a DC vaccine had a prolonged survival time compared with control patients.272,273 In some encouraging cases, patients with recurrent or metastatic Ewing’s sarcoma, even in the presence of chemotherapy-induced immune cell loss, have shown significant improvement and extended survival time after receiving immunotherapy such as the DC vaccines, influenza vaccines, and autologous T lymphocytes.274 Cancer-associated vaccines have effectively enhanced T lymphocyte-mediated immunity even after treatment with chemotherapy or radiotherapy, but they have not yet achieved satisfactory effects in the treatment of most solid tumors, including osteosarcoma.275 Only one in ten patients showed a complete immune response while most patients showed progression in a phase I trial of a combined decitabine/DC vaccine for neuroblastoma and sarcoma.276 Considering the complex tumor microenvironment, this low level of response might be due to the interactions between various immune cells, including infiltrating type 2 macrophages, and the imbalance of Treg and Th17 cells.277 Consequently, further research in this area is still needed, with a focus on addressing the limitations of tumor vaccines in the immune microenvironment of osteosarcoma.
Osteosarcoma-associated cytokines
Recently, a growing number of reports have demonstrated that cytokines can activate cells in response to immunotherapy to improve the immune response, and some proinflammatory cytokines (such as IL-8 and TNF-α) and immunosuppressants are closely associated with osteosarcoma progression.278–280 Thus, unsurprisingly, cytokine-based treatments have been commonly used in tumor immunotherapy for over three decades due to their wide range of effects, such as activating T lymphocytes and regulating antigen presentation.281–283 Moreover, cytokines secreted by TAMs and mast cells can manage immune cells and activate the inflammatory response to foreign antigens during invasion.284 Among these cytokines, ILs play a significant role in the expression of cellular adhesion molecules (CAMs), which are involved in the binding of NK cells to targets.77,285
Activating immune-modulating cytokines, such as IL-12, can facilitate T or B lymphocyte maturation, proliferation, differentiation, and antibody production as well as activate CILs, NK cells, and other immune effector cells and have shown significant curative effects in melanoma and kidney cancer.286–288 For example, IL-12 plays a significant role in decreasing side effects, such as gastrointestinal bleeding caused by chemotherapeutic or radiotherapeutic agents, and increasing patient tolerance.289 Moreover, IL-15-activated NK cells can effectively lyse tumor cells in high-grade osteosarcoma patients, particularly when the NK cells are activated by other cytokines.290 In addition, a prospective study of patients with primary metastatic osteosarcoma showed a survival of more than 40% for patients at 3 years after treatment with chemotherapeutic agents and IL-2. This result is encouraging and higher than that reported elsewhere.291 However, there remain limitations to the clinical application of cytokines due to the adverse effects resulting from the overactivation of immune responses caused by administering high concentrations of cytokines.292,293 In Table 3, we have summarized recent preclinical studies on cytokines, vaccines, and other immunotherapies for osteosarcoma.
Table 3.
Preclinical immunotherapies in osteosarcoma
| Approach | Models | Key elements | Reference |
|---|---|---|---|
| Cytokines | Osteosarcoma cell lines, mice | IL-34, IL18, IL12 | 480–482 |
| CAR-T cells | Osteosarcoma cell lines, mice | CD166.BBζ, CAR-T cells, Sleeping Beauty-based components, CD8+ T cells, HER2-specific T cells | 48,353,483 |
| Vaccines | Osteosarcoma cell lines, DCs, mice | CD103+ dendritic cells, novel oncolytic vaccinia viruses, virus B1 kinase, tumor vaccines containing B7-1-transfected cells | 48,361 |
| Others | Osteosarcoma cell lines, mice, macrophages | Macrophage polarization | 476,484 |
Immune checkpoint blockade-based immunotherapy for osteosarcoma
Recently, some monoclonal antibodies, such as those targeting CTLA-4, B7-H3, PD-1, and the PD-1 ligand PD-L1, have been designed to block immune checkpoints and have attracted much interest because of their satisfactory antitumor efficicacy.294–298 However, clinical trial results to date suggest that most checkpoint blockers are less effective in treating solid tumors, including osteosarcoma.275 The reason for this is not completely understood, and it is possible that T lymphocytes are not the main effector cells inhibiting osteosarcoma in humans. A study detecting PD-1, PD-L1, and PD-L2 expression in 234 clinical samples from patients with musculoskeletal tumors reported that PD-1 and PD-L1 were negatively associated with prognosis and overall survival in osteosarcoma patients.19 This study also suggested that nivolumab, a PD-1 inhibitor, increased the proportions of CD4+ and CD8+ T lymphocytes and improved the cytotoxicity of CD8+ T lymphocytes to effectively suppress pulmonary metastasis of osteosarcoma in in vivo assays. Moreover, M2-like TAMs were reprogrammed and pulmonary metastasis of osteosarcoma was inhibited by decreasing the expression of PD-1.299 The current understanding of the mechanism of immune checkpoint blockers and the interaction between immune and osteosarcoma cells are summarized in Fig. 3.
In addition, the proportion of Tregs is significant increased in some tumor patients, and Tregs are considered important contributors to escape of immunological surveillance and poor immunotherapy outcomes.300–302 Hence, decreasing the quantity of Tregs is one of the main goals for Treg-based antitumor immunotherapy.303 For instance, anti-CD25 monoclonal antibodies have been used to reduce the number of CD4+ and CD25+ Tregs to promote specific CD8+ T lymphocyte-mediated cytotoxicity in breast cancer.304,305 In addition, blocking Tregs to modulate their immunosuppressive functions is also considered another effective strategy. Generally, ligands associated with immunosuppression are highly expressed on the Treg surface, including PD-L1, CTLA-4, and glucocorticoid-induced TNF receptor (GITR), which can be effectively blocked by specific inhibitors.174,306–308 Furthermore, enhancing the function of effector T lymphocytes to reverse the suppressive effect of Tregs has also been used in tumor immunotherapy.309 These ligands, which act as a brake for T lymphocytes, mainly induce TILs involved in antitumor immunity that fail to eliminate osteosarcoma cells, while related inhibitors can enhance T lymphocyte-induced antitumor immunity by MHC presentation to overcome obstacles and reverse this process.310,311
Notably, strategies aimed at the novel targets PD-1 and CTLA-4 represent a new era of antitumor immunotherapy and improve the potential for osteosarcoma therapy. PD-1 is a type 1 transmembrane protein that is usually found on the surface of activating effector T lymphocytes, B lymphocytes, and NK cells. It can interact with PD-L1 existing on the surface of tumor-infiltrating lymphocytes (TILs) and tumor cells. Strategies that suppress this interaction have shown significant therapeutic effects in cancer patients, including osteosarcoma patients.312,313 The expression levels of PD-L1 vary widely among osteosarcoma cell lines but are higher than those in parental cell lines.314 Moreover, the expression of PD-L1 shows a positive association with chemotherapy resistance and the quantity of TILs as well as with osteosarcoma cell proliferation.315,316 Consequently, high expression levels of PD-L1 are associated with worse survival time than low PD-L1 expression in osteosarcoma patients. Blocking the interaction of PD-1/PD-L1 may be a potential strategy to enhance the T lymphocyte-mediated immune response to improve osteosarcoma immunotherapy.
B7-H3 (also named CD276) is a member of the B7 family of molecules that can interact with CTLA-4 or PD-1.317,318 It is also generated in healthy tissues but is overexpressed in various tumor types, including osteosarcoma, and high CD276 expression is closely related to increased quantities of Tregs across tumor tissues, suggesting that CD276 has a positive effect on Treg-mediated suppression of T lymphocyte function.319–322 In a lymphoma mouse model, Lee et al. found that blocking the immune checkpoint CD276 effectively inhibited tumor progression, and combination of this strategy with an anti-PD-1 antibody led to further improved therapeutic efficacy in advanced tumors.318 Additionally, several preclinical reports have also shown the potential of using CAR-T lymphocytes or microRNAs (such as miR-124) to target B7-H3 to improve osteosarcoma immunotherapy.323–327 Recently, an orthotopic, spontaneously metastasizing osteosarcoma model was designed to predict the biocompatibility and efficacy of a new generation CAR-T-cell treatment and other therapeutic approaches for osteosarcoma metastasis.320 This model suggested that B7-H3 was highly and homogenously expressed in pediatric solid tumors. The CAR-T-cell strategy showed robust activity against various xenograft tumor models, and its efficacy was highly dependent on the density of TAAs. Notably, a phase I clinical trial using enoblituzumab (MGA271), considered a first-line monoclonal antibody, is in progress (NCT02982941) in adolescents with B7-H3-expressing osteosarcoma.328,329
CTLA-4 (also named CD152), a type 1 transmembrane glycoprotein receptor, is also expressed on Tregs and memory T lymphocytes and can bind to and compete with CD80 and CD86 on DCs due to their shared B7 ligands.330–333 Mechanistically, CTLA-4 can induce IDO to suppress T lymphocyte proliferation and cytokine secretion, leading to immunosuppression.334,335 It should be noted that ipilimumab, a human IgG4 monoclonal antibody, has been approved by the U.S. Food and Drug Administration (FDA) as a new generation immune checkpoint inhibitor against melanoma.336 Emerging evidence suggests that the risk of osteosarcoma is positively correlated with the expression of CTLA-4.337–341 Thus, CTLA-4 blocking agents and antagonists can restore antitumor immunity by activating B7 and CD28 signaling and depleting Tregs.342 In a phase I clinical trial in pediatric osteosarcoma, 25% of osteosarcoma patients who received ipilimumab developed stable disease with acceptable immune-associated side effects.343 However, the major issue with ipilimumab in pediatric patients is gastrointestinal side effects; therefore, safer and more effective immune checkpoint inhibitors and immunotherapies are urgently needed in the future. Table 4 summarizes preclinical studies of osteosarcoma immunotherapy.
Table 4.
Current immune checkpoint blockade therapies
| Therapeutic agent | Target site | Models | Clinical trial stage | Reference |
|---|---|---|---|---|
| Pembrolizumab | PD-1 inhibitor |
Recurrent or progressed osteosarcoma |
Single-arm, open-label, phase 2 trial | 485 |
| Nivolumab | PD-1 inhibitor | Recurrent or refractory osteosarcoma | Multicenter, open-label, single arm, phase 1–2 trial | 486 |
| Atezolizumab | PD-L1 inhibitor | Progressive osteosarcoma | Multicenter phase 1–2 study | 487 |
|
Apatinib+ camrelizumab |
PD-1 inhibitor+ tyrosine kinase inhibitor |
Patients (≥11 y) with metastatic or locally advanced osteosarcoma |
Single-arm, open-label, phase 2 trial | 488 |
|
Anti-PD-1/CTLA-4 antibodies+ Bempegaldesleukin |
PD-1/CTLA-4 inhibitor+ CD122- preferential IL-2 pathway agonist |
Disseminated K7M2-WT metastatic osteosarcoma mouse model, K7M3 primary tibial osteosarcoma mouse model, and DLM8 subcutaneous osteosarcoma mouse model |
Bempegaldesleukin (BEMPEG; NKTR-214) efficacy as a single agent and in combination with checkpoint inhibitor therapy in mouse models of osteosarcoma |
489 |
|
Nivolumab+ ipilimumab |
PD-1 inhibitor+ CTLA-4 inhibitor |
Locally advanced, unresectable, or metastatic osteosarcoma |
Two open-label, noncomparative, randomized, phase 2 trials | 490 |
Adoptive cell therapy for osteosarcoma
Active and adoptive immunotherapies are considered two immunotherapy formats for tumor treatment.344,345 The former, including DCs, pulsed vaccines, and cytokines, can effectively activate immune responses against cancer cells.346,347 Adoptive immunotherapy, considered passive immunotherapy, refers to the injection of in vitro-expanded cancer-specific cytotoxic immune cells, particularly T lymphocytes.63,348,349 Adoptive cell therapy (ACT) involves the collection of self immune cells, including NK cells and TILs, which are expanded in ex vivo culture and induced to express chimeric antigen receptors (CARs) or T lymphocyte receptors (TCRs) for ACT.350 The three main ACT types are CAR-modified T lymphocytes, TCR-modified T lymphocytes, and TILs. These approaches circumvent the shortcomings of both immune checkpoint blockade-targeted T lymphocyte activation and vaccine approaches due to their high affinity for specific TAAs and lack of a requirement for peptide recognition in the context of HLA. They have fewer side effects than chemotherapy.351
HER-2 receptors are expressed in 40% to 60% of primary osteosarcoma patients but at a low level. The outcome and biocompatibility of HER2-based CAR-T therapy have been investigated in trials in HER2-positive sarcoma, and dose-limiting toxicity has not been observed.352–354 Although it is expressed at a lower level, HER2 can also be efficiently recognized by CAR-T lymphocytes, suggesting that CAR-T cells have great potential to target cancer cells. By using an osteosarcoma mouse model, researchers found that metastatic cancer cells that were insensitive to chemotherapeutic agents could be efficiently eliminated by the IL-11 receptor α-chain (IL-11Rα) and CAR-T cells modified to target HER2.355 In a recent report, a cell membrane-modified and site-specific IL-12 (attIL12) was used to engineer peripheral blood mononuclear cells (PBMCs) rather than T lymphocytes to omit the expansion phase of the desired CAR-T cells.356 These IL12-targeted attIL12-PBMCs induced an observable antitumor effect in both heterogeneous osteosarcoma patient-derived xenograft tumors and metastatic osteosarcoma models with no significant side effects. Satisfactory outcomes of adoptive T lymphocyte transfer and ACT in osteosarcoma and other cancers have been reported in previous studies.357–360 As an example, CD166-specific T cells were obtained by virus-based transfer of the corresponding DNA plasmids. These cells were selectively expanded using IL-2 and IL-15, and the ability of CD166.BBζ CAR-T cells to kill CD166+ osteosarcoma cells was evaluated in vitro and in vivo. The CD166.BBζ CAR-T cells killed osteosarcoma cell lines in vitro, and the cytotoxicity degree was correlated with the levels of CD166 expression on the tumor cells. Intravenous injection of CD166.BBζ CAR-T cells into mice caused tumor regression with no obvious toxicity.361 These successful in vivo studies support further exploration, especially with regard to improving the outcomes of CD166-related therapies. However, safety-related modifications to avoid potential adverse effects of CAR-T treatment must be considered. The CD166-targeted T lymphocyte treatment discussed above represents a clinically appealing strategy for osteosarcoma patients with positive CD166 expression, offering a starting point for additional investigations of clinical osteosarcoma immunotherapy.
Unlike CAR-T-cell therapy, TCR T lymphocytes are generated from high-affinity and highly acidic TAA-specific T-lymphocyte clones, increasing the specificity and sensitivity of TCR T cells for targeting cell surface HLA.362 TCR T cells show excellent targeting efficacy compared to CARs or antibodies, penetrating tumors and binding to HLA-presented tumor intracellular and surface antigenic peptides.363 TILs are generally collected from resected tumors, followed by in vitro proliferation, and are then administered to subjects in considerable quantities.364 However, the strategies for isolation of TILs from osteosarcoma tissues and induction of their proliferation are not yet completely optimized, as the number of TILs acquired is frequently not adequate for immunotherapy.365 Another challenge in the treatment of osteosarcoma is the few immunoregulatory factors and excess inhibitory substances of osteosarcoma cells, which might suppress the activation and expansion of TILs.364 A new generation of TILs has shown good long-term persistence, which is consistent with the memory phenotypes of most T lymphocytes. For example, a novel therapy based on lifileucel (LN-144), an autologous, tumor-infiltrating lymphocyte product, was reported to induce a durable response and have excellent disease control in melanoma patients who had previously failed ICB therapy.366 In addition, ICB combined with TIL-based therapy may also be an effective therapeutic strategy for osteosarcoma patients who have progressed on monotherapy. Antagonistic anti-CTLA-4 antibodies have been verified to improve the HLA binding ability of TILs in melanoma as well as facilitate CD8+ TIL proliferation in Lewis lung carcinoma.256 A recent study also revealed that TIL-based strategies in combination with anti-PD1 antibody therapy showed excellent therapeutic efficacy in patients with pulmonary metastasis of osteosarcoma.367
Immunotherapy combinations for osteosarcoma
Recently, emerging evidence has shown that the expression level of PD-1 on CD8-positive TILs is greatly reduced after treatment with an anti-PD-L1 antibody, while the expression of CTLA-4 is increased in a metastatic osteosarcoma mouse model, indicating that PD-L1 and CTLA-4 may have complementary roles in inhibiting CD8+ CTL-induced antitumor immunity.368 These results provide enthusiasm for immunotherapy studies and the use of combination approaches. For example, the combination of α-PD-L1 and α-CTLA-4 antibody blockade leads to enhanced suppression of osteosarcoma metastasis and maintains osteosarcoma immunological surveillance in mouse models.368 In a clinical trial, approximately 30% of cancer patients were alive with a median of 13.6 months of follow-up in the nivolumab monotherapy arm, and the survival of half of the patients was significantly extended with the combination of ipilimumab and nivolumab therapy.369 Administration of anti-CTLA-4 antibody therapy combined with other immune stimulants, such as cryotreated tumor lysate-pulsed DCs, achieved increased antitumor immune responses in a murine osteosarcoma model.56 ICB antagonists are also able to improve the cytotoxicity of CAR-T lymphocytes and bispecific T-lymphocyte engager (BiTE) antibodies, resulting in reversal of the immunosuppression induced by released immunosuppressive factors and the lack of specific TAA presentation; these effects reactivate inhibitory and exhausted T lymphocytes.370–372 Encouraging outcomes from combination therapy with checkpoint antagonists and genetically engineered T lymphocytes or cancer vaccines have also been shown in phase I trials.373–375 As an example, the combination of CTLA-4 blockade and IL-21-activated polyclonal antigen-specific CTLs effectively inhibited melanoma metastasis in refractory patients, and this approach was shown to be biocompatible and achieved durable immunity in another patient, giving new hope for osteosarcoma immunotherapy.376,377
Combinational treatments with PD-L1/PD-1 blockade, CTLA-4 inhibitors, and small molecule IDO inhibitors have also resulted in strongly enhanced tumor suppression in multiple tumor models (including osteosarcoma models) because they expand infiltrating T lymphocytes and decrease Treg and MDSC numbers.378–380 For example, the combination of pembrolizumab and epacadostat has been generally well tolerated and presented satisfactory anticancer effects in various advanced cancers (such as endometrial cancer, kidney cancer, and melanoma) in multicenter clinical trials.381 However, pembrolizumab in combination with epacadostat did not extend progression-free survival or overall survival compared to placebo in combination with pembrolizumab in patients with unresectable or metastatic melanoma, indicating that IDO1 suppression may not be an effective strategy to improve anti-PD-1 therapy.382 However, adoptive ex vivo-expanded γδT lymphocyte transfer, especially combined with immunostimulants such as amino bisphosphonates, could be a promising approach for osteosarcoma immunotherapy.
Monoclonal antibodies, such as anti-GD2 antibodies, have also been found to improve antitumor immunity in combination with colony-stimulating factor 2 (CSF2) or IL-2 in solid tumors383,384 For example, some studies have reported that anti-ganglioside GD2 monoclonal antibodies can synergize with chemotherapeutic agents to cause endoplasmic reticulum (ER) stress-related cell death.385,386 Trials exploring checkpoint blockers in combination with adjuvant and neoadjuvant chemotherapy or site-specific treatment are also ongoing, such as studies assessing camrelizumab in combination with apatinib (NCT03359018) or pembrolizumab in combination with axitinib.312,387,388 These combination treatments may play a beneficial role during chemotherapy by decreasing tumor burden, exposing neoantigens by inducing tumor necrosis, and directly attacking tumor stromal cells.389 For instance, the preoperative chemotherapeutic agent ifosfamide in combination with the immunotherapeutic agent IL-18 can efficiently suppress the progression of pulmonary metastases in a murine osteosarcoma model.390 Another study also reported that the combination of α-PD-L1 antibody and L-arginine can effectively enhance anti-osteosarcoma immunity and greatly extend survival time.391 Some immune cells, such as DCs, have been shown to be efficacious in osteosarcoma therapy in combination with low-concentration chemotherapeutic agents, such as doxorubicin (DOX), which can effectively induce ICD to further activate immunity against osteosarcoma.392,393 CAR-T lymphocytes combined with antibodies and other T lymphocyte-based treatments, such as adoptive T-lymphocyte transfer, are also becoming increasingly common.394 Overall, combination immunotherapy can boost the duration of the immune response because the double-agent treatment activates antitumor immune memory effects in osteosarcoma patients who do not respond to monotherapy. Advances in both the understanding of immunotherapy and relevant technologies and further exploration of combination treatments in clinical trials will further boost therapeutic efficiency for patients with osteosarcoma.
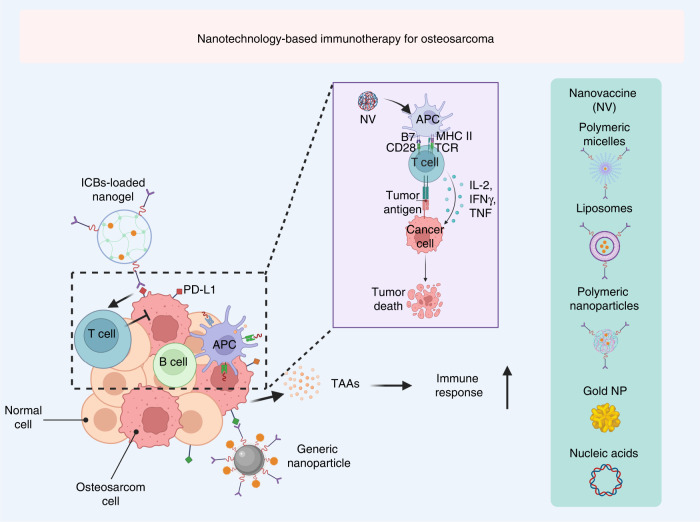
Nanotechnology-based immunotherapy for osteosarcoma
Some synthetic and natural polymer materials have shown immunomodulatory activity in the absence of external factors. For example, these materials can repolarize immunosuppressive type 1 TAMs into immune-supportive type 2 TAMs and reverse the immunosuppressive microenvironment of osteosarcoma. These materials generally have special physical properties, such as properties related to energy radiation and absorption, which can be harnessed to eliminate cancer cells, as in imaging-guided nanomedicine. Moreover, these nanoparticles have good optical and magnetic profiles and can be applied for photodynamic therapy (PDT), photothermal therapy (PTT), radiotherapy, and magnetothermal therapy in response to exogenous activation to induce immunogenic cell death (ICD) and augment antitumor immunity (Fig. 7). Furthermore, versatile nanoplatforms have been developed that can be rationally applied in living animals to deliver agents to specific sites or perform precise functions, which may be applied in the treatment of osteosarcomas that are resistant to immunotherapy, chemotherapy, and radiotherapy (Table 5). Consequently, nanoparticles can greatly facilitate the delivery of various anti-osteosarcoma agents, and relevant studies will provide new insights and strategies to destroy osteosarcoma cells with immunotherapy.
Fig. 7.
Schematic illustration of nanotechnology-based immunotherapy for osteosarcoma
Table 5.
Examples of different nanoparticles for osteosarcoma therapy
| Nanoplatform | Name | Therapeutic agent | Therapeutic application | Reference |
|---|---|---|---|---|
| Liposomes | Chol-SS-mPEG/HA-L | DOX | Chemotherapy | 491 |
| Hydrogels | SP@MX-TOB/GelMA | Tobramycin (TOB) | Chemotherapy, photothermal therapy | 492 |
| Chitosan |
ZSM-5/CS/DOX nanodisks |
DOX | Chemotherapy | 493 |
| Nano-oxides | PEG-GOFA/ICG | DOX and TH287 | Chemotherapy, photodynamic therapy | 494 |
| Nanocomposite oxides | β-TCP-Fe-GO | Fe3O4 magnetic particles | Hyperthermal therapy | 495 |
| Nanometals and nanoalloys | Au@AgNRs@BSA |
Au and Ag nanoparticles |
Photothermal therapy | 496 |
|
Carbon nanotubes |
SWCNTs | Single-walled carbon nanotube | Chemotherapy | 497 |
| Micelles | NP-PTX-DOX | PTX and DOX | Chemotherapy | 498 |
|
Dendritic macromolecules |
AG/G5-Dox NGs | DOX | Chemotherapy | 499 |
| Nanocapsules | IFS-LNC | Ifosfamide | Chemotherapy | 500 |
| Exosomes | EXO-DOX | DOX | Chemotherapy | 501 |
Targeting cancer cells for immunogenic cell death
Recently, a growing number of studies have reported that dying cancer cells can release immunoregulatory damage-associated molecular patterns (DAMPs) to effectively counteract specific or nonspecific antitumor immunity in immunotherapy.395,396 This promotion of cancer cell death by activating immunity is also referred to as immunogenic cell death (ICD).397,398 It is closely related to DAMPs, such as adenylate triphosphate (ATP), high mobility group protein B1 (HMGB1), surface calreticulin (CAT), and heat shock protein 70 (HSP70), which can mediate DC or TAM changes to improve antigen presentation and immune infiltration for effective immunotherapy.399,400 Moreover, the dying tumor cells can also release TAAs to recruit immune cells, and these TAAs serve as promising therapeutic targets for immunotherapy.401 Notably, versatile nanotechnology-based nanodelivery platforms are considered effective tools for inducing ICD because they can deliver useful concentrations of cytotoxic agents to cancer cells, resulting in improvements in the rate of ICD induced by different therapies, such as radiotherapy, chemotherapy, and phototherapy.31,402
Chemotherapy/radiotherapy
A number of reports have shown that dying osteosarcoma cells are immunogenic and can enhance immunity against osteosarcoma after treatment with certain chemotherapies or radiotherapy.393,403 Anthracyclines, including doxorubicin (DOX), mitoxantrone (MTO), oxaliplatin, bortezomib, and cyclophosphamide, are the most classic ICD inducers.404 For instance, a mitochondria-targeted nanomicelle (named OPDEA-PDCA) capable of initiating mitochondrial oxidative stress was designed to induce pyroptosis of osteosarcoma cells. In this nanoplatform, poly[2-(N-oxide-N,N-diethylamino)ethyl methacrylate] (OPDEA) is employed to target mitochondria, and modified dichloroacetate (DCA) is employed to suppress pyruvate dehydrogenase kinase 1 (PDHK1) to induce mitochondrial oxidative stress, which can lead to pyroptosis and further mediate ICD in osteosarcoma cell lines.405 However, OPDEA-PDCA can also promote the release of soluble PD-L1. Thus, OPDEA-PDCA in combination with an anti-PD-L1 monoclonal antibody substantially inhibits osteosarcoma cell proliferation and enhances T lymphocyte activation. In addition, a pH-responsive autophagy-regulated nanoplatform (named CBZP), which encapsulates both the natural product curcumin (CUR) to enhance the antitumor immunity of PD-1/PD-L1 blockade by mediating autophagic cell death-mediated ICD and the immune checkpoint inhibitor BMS1166 to simultaneously suppress the PD-1/PD-L1 interaction to improve tumor immunogenicity and improve T lymphocyte immunity, has also been developed for osteosarcoma immunotherapy.406 After being taken up by osteosarcoma cells, the pH-responsive nanoplatform triggers autophagy and increases the intracellular acidic environment, which in turn further promotes the release of CUR to augment autophagic activity. Importantly, administration of CBZP to orthotopic osteosarcoma-bearing mice presented excellent antitumor efficacy and achieved a long-term immune response to inhibit tumor recurrence, which was also accompanied by improved DC maturation and tumor infiltration of CD8+ T lymphocytes. In summary, this nanoplatform has shown beneficial anti-osteosarcoma effects by integrating an agent to induce ICD activation and immune checkpoint inhibitors, thus shedding light on the use of autophagy modulation as a promising modality for osteosarcoma treatment.
Phototherapy
Near-infrared (NIR) laser-activated phototherapy has been widely used in basic research of osteosarcoma.34,407–409 PTT mainly relies on a high local temperature to inhibit tumor cell proliferation, which can effectively eliminate cancer cells and enhance antitumor immune responses.410–412 Moreover, PTT driven by nanoparticles has also been shown to induce effective treatment outcomes and is able to facilitate local heating of tumor tissues without impairing the surrounding normal tissue.413 Additionally, PDT, as another noninvasive therapeutic approach, can also be activated by a laser of a specific wavelength, resulting in cytotoxic ROS accumulation in the presence of endogenous O2 to eventually induce the death of tumor cells.414–416 Notably, studies have reported that cancer cells can also release TAAs after laser exposure, promoting the secretion of cytokines and the maturation of DCs, which helps them activate T lymphocytes to induce robust antitumor immunity.417,418 These results suggest that dying cancer cells releasing TAAs after phototherapy can induce adjuvant effects by stimulating an immune response, and thus, this strategy can be considered an “automatic vaccine”.419,420 For instance, a tumor cell membrane-modified Au nanoplatform (named C-RAuNC) was encapsulated with the ferroptosis agonist RSL3 and administered to prevent osteosarcoma drug resistance.421 In this nanoplatform, RSL3 can inhibit glutathione peroxidase (GPX4) to mediate ferroptosis. Moreover, Au nanoparticles encapsulated in tumor cell membranes can achieve site-specific delivery, controlled drug release, effective phototherapy, and amplified ICD to enhance the immune response and thus overcome osteosarcoma drug resistance. In another example, encouraging results of therapy for osteosarcoma drug resistance were achieved by C-R-AuNCs, which are believed to be a promising modality for the future clinical treatment of drug-resistant osteosarcoma. In PDT research on osteosarcoma, increased expression of HSP70 has been shown in the MG-63 cell line, which is in line with enhancement of immunity and indicates the promise of ICD induced by PDT in osteosarcoma therapy.422
Chemodynamic therapy
In addition to the above approaches, chemodynamic therapy (CDT) can also improve immunogenicity for osteosarcoma immunotherapy.423,424 CDT, a noninvasive treatment with a high tissue penetration depth compared to phototherapy strategies, can generate ROS via Fenton or Fenton-like reactions or sonosensitizers to induce tumor ablation, enhance immunogenicity, and achieve better antitumor immunity.424–426 For instance, alendronate (ALD)/K7M2 cell membrane-encapsulated hollow manganese dioxide (HMnO2) nanoplatforms were used as nanocarriers to encapsulate ginsenoside Rh2 (Rh2) for magnetic resonance imaging (MRI)-guided chemodynamic-immunotherapy combination treatment in osteosarcoma.427 ALD and K7M2 cell membranes were successively modified on the surface of the nanoparticles and encapsulated with Rh2. This tumor microenvironment-responsive nanoplatform showed good osteosarcoma-targeting and homing abilities, excellent GSH-responsive drug release and an excellent MRI profile, and useful chemodynamic-immunotherapy combination treatment effects. These designed nanoplatforms can efficiently induce ICD, activate CD4+/CD8+ T lymphocytes in vivo, and promote the expression of BAX, BCL-2 and Caspase-3 at the cellular level to improve antitumor immunity. Further results have suggested that these nanoparticles improve the release of TNF-α, IFN-γ, and IL-6 into the serum and suppress the production of FOXP3+ T lymphocytes in tumors. Furthermore, these nanoplatforms greatly inhibit tumor cell proliferation in situ in tumor-bearing mice. Consequently, the combination of such efficient and biocompatible nanoplatforms with immunotherapy and chemodynamic therapy will likely produce excellent strategies for osteosarcoma treatment.
Targeting DCs for enhanced osteosarcoma immunotherapy
Capsaicin, a chemotherapeutic agent, can effectively induce ICD to improve the DC-mediated phagocytosis of MG-63 cells and the presentation of relevant antigens to enhance the immune response.428 Additionally, the liposomal-muramyl tripeptide phosphatidylethanolamine, not only as monotherapy but also in combination with other therapeutic agents, can also activate DCs and stimulate T lymphocyte proliferation to extend overall survival without inducing metastasis.429 Moreover, efficient site-specific delivery of immunomodulators or immunostimulatory factors to innate immune cells (i.e., DCs) or tumor-draining lymph nodes is useful for enhancing the immune response for nanovaccines.430 Generally, almost all types of delivery platforms, such as nucleic acids, polymeric micelles, polymer nanoparticles, liposomes, and inorganic nanoparticles, can be rationally used for therapeutic nanovaccines.431 It should be noted that antigens can be in the form of recombinant proteins, synthetic long or short peptides, DNA, or RNA because nanocarriers can prevent cargo degradation.432 In addition, the most commonly studied nanoadjuvants in tumor vaccines, including oligodeoxynucleotides (ODNs), monophosphoryl lipid A (MPLA), 5′-C-phosphate-G-3′ (CpG), LPS, TLR agonists, polyinosinic: polycytidylic acid (poly I:C), and agonists of stimulator of IFN genes (STING), can efficiently augment antitumor immunity and greatly improve the therapeutic effect of these cancer vaccines.433–435 Similarly, this flexibility makes nanoscale vaccines suitable for boosting antitumor immunity, as they may mediate a robust cellular and humoral immune response in vivo. Moreover, these nanovaccines can not only be efficiently transported from the site of administration to the tumor-draining lymph nodes, triggering robust antitumor immunity and immune memory, but also boost DC maturation and accelerate the efficacy of targeting DCs, leading to stronger T lymphocyte-mediated antitumor immunity.436–438 Tuohy et al. developed a robust hyperthermia-based nanotherapy platform in a murine osteosarcoma model. The researchers found that osteosarcoma-bearing mice with osteomyelitis showed a higher proportion of “nonclassical” monocytes (Ly6Clo) than all other groups.439 There were significant changes in monocyte expression of various chemokine receptors, such as CXCR2, CCR2, and CXCR4, among the experimental groups. Monocytes from osteosarcoma-bearing mice treated with hyperthermia therapy showed greater chemotaxis than monocytes from osteosarcoma-bearing mice with osteomyelitis. In addition, the tetrafunctional amphiphilic blocker copolymer 704 was used to deliver a fractalkine (FKN)-encoding plasmid to evaluate its antimetastatic effects.440 FKN was described as an excellent candidate to induce robust antitumor immunity in various tumors.
Targeting the immunosuppressive microenvironment of osteosarcoma
Immunosuppressive factors in the tumor microenvironment act as the main substances that promote tumorigenesis and malignant progression.441,442 Strategies that modulate the immunosuppressive microenvironment of tumors can polarize immune cells to a phenotype suitable for antitumor immunity. Nanoparticles can be used for temporal and spatial modulation of Tregs, TAMs, TANs, and MDSCs as well as immunosuppressive soluble substances, presenting a potential strategy for transitioning cold tumors to hot tumors.443–445 Consequently, the site-specific, targeted and precise modulation of these tumor-relevant immunosuppressive cells should provide rationally designed nanoplatforms to effectively deliver immunotherapeutic agents to the tumor microenvironment in vivo.446–448
MDSCs mainly accumulate in peripheral lymphoid organs or tumor tissues and induce tumorigenesis, malignant progression, and metastasis by secreting immunosuppressive factors (such as IL-10, IDO, and arginase-1 (Arg-1)), activating Tregs and suppressing the activity of T lymphocytes and NK cells.449–451 Therefore, blocking, reprogramming, or depleting MDSCs has been considered a promising strategy for restoring the antitumor immune response.452 For instance, a recent study reported robust nanoparticles (HA/ZIF-8@Gem/D-1-MT NPs) that could efficiently encapsulate the chemotherapeutic agent gemcitabine and the IDO inhibitor 1-methyl-DL-tryptophan (1-MT) to deplete MDSCs and suppress IDO.453 Release of gemcitabine effectively induced encouraging chemotherapeutic effects on osteosarcoma cells with subsequent MDSC depletion, and IDO in osteosarcoma cells was suppressed by 1-MT, leading to synergistic antitumor immune effects. Efforts are underway to develop treatments that reduce tumor-related inflammation and the numbers of immunosuppressive cells. Another study suggested that a previously reported deep-tissue imaging strategy that uses indocyanine green-encapsulated calcium phosphosilicate nanoparticles (ICG-CPSNPs) could be used as an immunomodulatory agent to enhance antitumor immune responses. The theranostic application of ICG-CPSNPs as photosensitizers for PDT effectively inhibited tumor cell proliferation in a murine model of metastatic osteosarcoma by reducing inflammation-expanded immature myeloid cells. Consequently, this therapeutic approach was also named PhotoImmunoNanoTherapy.454
Immune checkpoint blockade for osteosarcoma
Nanotechnology has provided a potential approach to overcome the side effects of ICB agents. For example, a recent study reported on a locally deliverable ICB nanogel that was injectable and NIR-sensitive and could be used for postsurgical osteosarcoma immunotherapy.455 This injectable nanogel with an adjustable solution-to-gel transition temperature was generated by copolymerization of thermosensitive and zwitterionic monomers. Subsequently, combined with multifunctional mesoporous nanomaterials, this nanocomposite could absorb NIR laser irradiation, leading to hyperthermia; this effect triggered a retro Diels-Alder (D-A) reaction to degrade the coating layer on nanoparticles, resulting in NIR-triggered drug release. Moreover, in an osteosarcoma postsurgical recurrence model, researchers found that this versatile delivery platform with favorable biocompatibility could effectively avoid early leakage of the drug and greatly enhance drug accumulation in tumors. In addition, the long-term controlled drug release of this nanoplatform greatly increased the quantity of active T lymphocytes, leading to a satisfactory antirecurrence effect. In summary, this study suggests that the locally injectable nanogel is a promising tool for postoperative treatment of patients with osteosarcoma. Moreover, another study also investigated the ability of PD-L1 downregulation with PDT combined with the autophagy inhibitor 3-methyladenine (3-MA) to prevent the long-distance metastasis of osteosarcoma.456 A significant tumor inhibition effect mediated by PDT was found in a partial resection model of osteosarcoma, revealing the potential clinical value of PDT during tumor surgery. At the same time, this study also found that the expression of PD-L1 was downregulated in tumor tissues in response to treatment with nanoparticles, which markedly enhanced antitumor immune responses to suppress osteosarcoma cell proliferation and metastasis. Notably, this immunological response triggered by the combination of the autophagy inhibitor and PDT inhibited osteosarcoma growth in in vitro and in vivo assays, suggesting the potential clinical utility of this strategy. Therefore, the combination of PDT with multimodal treatments may be promising for osteosarcoma immunotherapy.
CAR-T cells for osteosarcoma immunotherapy
CAR-T cells have greatly influenced the current treatment landscape of hematological malignancies and shown significant clinical therapeutic benefits, and these positive results have generated enthusiasm for research in solid tumors, including osteosarcoma.23,457–459 However, the treatment of solid tumors faces a unique set of challenges in comparison with hematological tumors.460,461 For instance, the lack of selectively and uniformly generated TAAs and the immunosuppressive tumor microenvironment are considered the biggest obstacles to successful CAR-T-cell therapy.462–464 A combination of costimulatory factors and cytokines has been developed to improve CAR-T-cell activity and augment antitumor immunity. The expression of transgenic cytokines on CAR-T cells also enhances proliferative activity.465 At the same time, killing of T lymphocytes induced by redirected universal cytokines was achieved using a nuclear factor of activated T lymphocyte (NFAT)-responsive promoter that could promote the release of cytokines when TAAs were recognized by CARs, resulting in minimal side effects and increased cytokine concentrations in the tumor tissue.466 To promote migration to tumor sites, CAR-T cells have been engineered to coexpress CCL5 and CXCL9, which forms a feedback loop to amplify lymphocyte implantation via efficient CD8+ cell recruitment.467 Nanobiotechnology has been used to address these significant challenges to enhance the efficacy of CAR-T-cell therapy.468 For instance, a recent report showed that iron oxide nanoparticles can be used to label CAR-T cells for a clinically translatable approach, enabling noninvasive monitoring of the labeled CAR-T cells with magnetic particle imaging (MPI), photoacoustic imaging (PAT), and MRI for detection of their distribution in the body.469 The study showed enhanced nanoparticle internalization in CAR-T cells that did not affect their proliferation, viability, or function using a custom-made microfluidics device for T-cell labeling by mechanoporation. Multimodal imaging showed that T lymphocytes labeled with the Fe2O3 nanoparticles homed to osteosarcoma and off-target sites in mice, whereas no T lymphocytes were observed in groups treated with unlabeled cells. This manuscript details the successful labeling of CAR-T cells with ferumoxytol, opening up the possibility of detecting CAR-T cells in solid tumors. Moreover, a clinical trial (NCT04433221) that combined CAR-T cells with low-dose chemotherapeutic agents that are capable of regulating surface PD-L1 expression is in progress. Notably, CD28-based and CD28-CD3z-OX40 CAR-T-cell therapy have been used In clinical trials (NCT00902044 and NCT01953900) in sarcoma patients to enhance the costimulatory response. Importantly, multitarget CAR-T cells have also been developed to enhance TAA recognition and suppress the cancer recurrence induced by the overgrowth of certain TAA-negative cells or cells with low TAA expression.
Conclusions and perspectives
Osteosarcoma is a primary malignant bone tumor, mainly develops in adolescence and has a poor prognosis. The immune microenvironment in osteosarcoma is complex, has high plasticity, and is closely associated with immune escape, uncontrolled proliferation, and metastasis of osteosarcoma cells. Therefore, remodeling the immunosuppressive microenvironment of osteosarcoma should be a strategy to remove small lesions and CTCs for efficacious osteosarcoma treatment. In this review, we systematically discussed the roles of various immune-associated cells in the immune microenvironment in osteosarcoma and the progress of relevant immunotherapy regimens and clinical applications. In particular, we highlighted the use of nanotechnology to modulate the immunosuppressive osteosarcoma microenvironment for enhanced osteosarcoma immunotherapy, thus providing guidance for the study, diagnosis, and treatment of osteosarcoma in the future.
Current immunotherapies for osteosarcoma, including tumor antigen vaccines, ICB strategies, and CAR-T therapy, have shown satisfactory therapeutic efficacy, but the promotion of metastasis by and immunosuppressive nature of the tumor microenvironment are still two major barriers to successful therapeutic outcomes. Overall, modulating the immune microenvironment of tumors to overcome immunosuppression and favor an antitumor immune response may be an important strategy for overcoming the current challenges of tumor therapy. A growing amount of evidence supports a comprehensive approach that can mediate antitumor immunity while overcoming the immunosuppressive tumor microenvironment, which should significantly boost clinical therapeutic outcomes.
Although such immunotherapy approaches have many advantages, there are also some disadvantages, including the extremely high cost, significant individual differences in immune responses, poor in vivo pharmacokinetic characteristics, and substantial adverse effects of systemic delivery, all of which need to be overcome. Thus, the research described above also has limitations. First, the immune microenvironment of osteosarcoma is complicated and dynamic and varies by disease type and duration and in different individuals. The current knowledge of the role of immune microenvironment components in osteosarcoma has been formed based on similar studies on other cancers, though the results may be both ambiguous and disease-specific. Moreover, most of the research is still in the preclinical stage, with insufficient evidence for translational medicine and nanotechnology-based drug delivery systems. Furthermore, antitumor immunity affects the whole body, and immune-associated treatments need to be both biocompatible and efficient. Such treatments may also result in new and serious side effects if not used properly; therefore, the side effects of these therapeutic agents must be seriously considered. Owing to strong genomic heterogeneity, targeted immunotherapy has not effectively improved overall survival for decades.470 Novel prognostic markers assessable at diagnosis are vital to identifying subsets of osteosarcoma. The focus of clinical trials has now shifted to serial phase II studies to evaluate the activity of novel agents in recurrent and refractory disease. In-depth analyses have revealed profound genomic instability and heterogeneity across patients, with nearly universal TP53 aberration. The complexity of the genome may support the role for immunotherapy.471 Characteristic gene expression classifiers can be applied to distinguish different categories of patients at initial diagnosis or during the postoperation phase and can be helpful in guiding the formulation and modification of immunotherapy treatment strategies.472
There has been continuous progress of nanomaterials and production technology, and nanotechnology provides great opportunities for effective control of specific immune responses and enhancement of osteosarcoma immunotherapy. However, osteosarcoma immunotherapy strategies based on nanotechnology are still in development but clearly have great potential. Therefore, multivariate diagnostic models and grading systems for osteosarcoma that consider immune components to promote accurate treatment and diagnosis of osteosarcoma should be explored. Novel methods that are more efficient than current treatment options and are also safe need to be developed, immunotherapy should be combined with other treatment modalities, and less expensive and more efficient production processes need to be pursued to achieve effective osteosarcoma treatment. Furthermore, more specific biomarkers are required for recognizing immune and nonimmune cells, identifying the interactions between the components of the immune microenvironment, exploring more suitable therapeutic targets, and integrating multidisciplinary knowledge and multitechnological support. Additionally, rational modification of the physicochemical properties of nanoparticles may be able to overcome immune escape, inhibit tumor cell proliferation and tumor progression, enhance cell targeting and drug accumulation in the tumor, and control drug release. In particular, the particle size and specific surface features of nanoparticles are special elements to be considered in the specific delivery of immunoregulators for effective targeting of the tumor microenvironment. Nanoplatforms with a size of 100 nm can be exuded from blood vessels and target tumors, spreading over a limited range in the extracellular space. However, a large number of xenograft models have confirmed that 10-100 nm nanoplatforms can readily reach and accumulate in tumor tissues after entering the blood circulatory system.473 Notably, nanoparticles with suitable particle size and slightly positive or negative charges have repulsive effects, which can decrease phagocytosis and clearance by the reticuloendothelial system. Consequently, the control of surface charge and steric stabilization can minimize the nonspecific interactions of nanoplatforms and prevent the loss of nanoplatforms at nontargeted sites.
Acknowledgments
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation (No. 2019B030302012), National Key Research and Development Project (No. 2020YFA0509400), National Natural Science Foundation of China (No. 81821002, 82130082), Excellent Young Scientists Fund of Natural Science Foundation of Henan Province (222300420072), Distinguished Young Scientists Fund of Henan Provincial Health Commission (YXKC2020025), and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21004 and ZYGD22007).
Competing interests
The authors declare no competing interests.
Footnotes
Consent for publication All authors have read and approved the final manuscript.
These authors contributed equally: Hailong Tian, Jiangjun Cao
Contributor Information
Haijiao Mao, Email: maohaijiao@nbu.edu.cn.
Yi Zhang, Email: zhangyi@zzu.edu.cn.
Canhua Huang, Email: hcanhua@scu.edu.cn.
References
- 1.Liu W, et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022;53:102344. doi: 10.1016/j.redox.2022.102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li HB, et al. METTL14-mediated epitranscriptome modification of MN1 mRNA promote tumorigenicity and all-trans-retinoic acid resistance in osteosarcoma. EBioMedicine. 2022;82:104142. doi: 10.1016/j.ebiom.2022.104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Wang L, Guo H, Zhang W, Shao Z. Single-cell transcriptomics reveals the regulative roles of cancer associated fibroblasts in tumor immune microenvironment of recurrent osteosarcoma. Theranostics. 2022;12:5877–5887. doi: 10.7150/thno.73714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu L, et al. Circular RNA circFIRRE drives osteosarcoma progression and metastasis through tumorigenic-angiogenic coupling. Mol. Cancer. 2022;21:167. doi: 10.1186/s12943-022-01624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukamoto S, et al. Effect of adjuvant chemotherapy on periosteal osteosarcoma: a systematic review. Jpn. J. Clin. Oncol. 2022;52:888–896. doi: 10.1093/jjco/hyac059. [DOI] [PubMed] [Google Scholar]
- 6.Horkoff MJ, et al. A population-based analysis of the presentation and outcomes of pediatric patients with osteosarcoma in Canada: a report from CYP-C. Can. J. Surg. 2022;65:E527–e533. doi: 10.1503/cjs.008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu T, et al. Immune microenvironment in osteosarcoma: components, therapeutic strategies and clinical applications. Front. Immunol. 2022;13:907550. doi: 10.3389/fimmu.2022.907550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piperno-Neumann S, et al. Results of API-AI based regimen in osteosarcoma adult patients included in the French OS2006/Sarcome-09 study. Int. J. Cancer. 2020;146:413–423. doi: 10.1002/ijc.32526. [DOI] [PubMed] [Google Scholar]
- 9.Lamhamedi-Cherradi SE, et al. Transcriptional activators YAP/TAZ and AXL orchestrate dedifferentiation, cell fate, and metastasis in human osteosarcoma. Cancer Gene Ther. 2021;28:1325–1338. doi: 10.1038/s41417-020-00281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Wu W, Deng S, Shao Z, Jin X. TRAIP modulates the IGFBP3/AKT pathway to enhance the invasion and proliferation of osteosarcoma by promoting KANK1 degradation. Cell Death Dis. 2021;12:767. doi: 10.1038/s41419-021-04057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R, Hu Y, Liu T, Wang Y. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of osteosarcoma cancer. BMC Cancer. 2021;21:1345. doi: 10.1186/s12885-021-09042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan B, et al. Analysis of immune gene expression subtypes reveals osteosarcoma immune heterogeneity. J. Oncol. 2021;2021:6649412. doi: 10.1155/2021/6649412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bain, J. M. et al. Immune cells fold and damage fungal hyphae. Proc. Natl. Acad. Sci. USA118, e2020484118 (2021). [DOI] [PMC free article] [PubMed]
- 14.Matsiko A. Cancer immunotherapy making headway. Nat. Mater. 2018;17:472. doi: 10.1038/s41563-018-0091-8. [DOI] [PubMed] [Google Scholar]
- 15.DeLucia DC, Lee JK. Development of cancer immunotherapies. Cancer Treat. Res. 2022;183:1–48. doi: 10.1007/978-3-030-96376-7_1. [DOI] [PubMed] [Google Scholar]
- 16.Starnes CO. Coley’s toxins. Nature. 1992;360:23. doi: 10.1038/360023b0. [DOI] [PubMed] [Google Scholar]
- 17.Zaaboub R, et al. Nurselike cells sequester B cells in disorganized lymph nodes in chronic lymphocytic leukemia via alternative production of CCL21. Blood Adv. 2022;6:4691–4704. doi: 10.1182/bloodadvances.2021006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu B, et al. UBR5 promotes tumor immune evasion through enhancing IFN-γ-induced PDL1 transcription in triple-negative breast cancer. Theranostics. 2022;12:5086–5102. doi: 10.7150/thno.74989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng B, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J. Hematol. Oncol. 2018;11:16. doi: 10.1186/s13045-018-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, et al. Imaging-guided/improved diseases management for immune-strategies and beyond. Adv. Drug Deliv. Rev. 2022;188:114446. doi: 10.1016/j.addr.2022.114446. [DOI] [PubMed] [Google Scholar]
- 21.Ali S, et al. The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic Leukemia and diffuse Large B-Cell lymphoma. Oncologist. 2020;25:e321–e327. doi: 10.1634/theoncologist.2019-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Y, et al. Immune checkpoints in osteosarcoma: recent advances and therapeutic potential. Cancer Lett. 2022;547:215887. doi: 10.1016/j.canlet.2022.215887. [DOI] [PubMed] [Google Scholar]
- 23.Brohl AS, et al. Immuno-transcriptomic profiling of extracranial pediatric solid malignancies. Cell Rep. 2021;37:110047. doi: 10.1016/j.celrep.2021.110047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian LR, et al. Nanodrug regulates lactic acid metabolism to reprogram the immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Biomater. Sci. 2022;10:3892–3900. doi: 10.1039/D2BM00650B. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Li L, Zhang B, Xu ZP. MnO(2)-shelled Doxorubicin/Curcumin nanoformulation for enhanced colorectal cancer chemo-immunotherapy. J. Colloid Interface Sci. 2022;617:315–325. doi: 10.1016/j.jcis.2022.02.132. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Wen P, Li J, Kataoka K. Targeted nanomedicine in cisplatin-based cancer therapeutics. J. Control Rel. 2022;345:709–720. doi: 10.1016/j.jconrel.2022.03.049. [DOI] [PubMed] [Google Scholar]
- 27.Rehman S, et al. Unraveling enhanced brain delivery of paliperidone-loaded lipid nanoconstructs: pharmacokinetic, behavioral, biochemical, and histological aspects. Drug Deliv. 2022;29:1409–1422. doi: 10.1080/10717544.2022.2069880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian, H. et al. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol.15, 132 (2022). [DOI] [PMC free article] [PubMed]
- 29.Chen J, et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl. Acad. Sci. USA. 2022;119:e2207841119. doi: 10.1073/pnas.2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh VK, et al. CD44 receptor-targeted nanoparticles augment immunity against tuberculosis in mice. J. Control Rel. 2022;349:796–811. doi: 10.1016/j.jconrel.2022.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T, et al. Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy. Bioact. Mater. 2022;16:418–432. doi: 10.1016/j.bioactmat.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, et al. Advances of bacteria-based delivery systems for modulating tumor microenvironment. Adv. Drug Deliv. Rev. 2022;188:114444. doi: 10.1016/j.addr.2022.114444. [DOI] [PubMed] [Google Scholar]
- 33.Wu, F. et al. Modulation of the tumor immune microenvironment by Bi(2) Te(3) -Au/Pd-based theranostic nanocatalysts enables efficient cancer therapy. Adv. Healthc. Mater. 11, e2200809 (2022). [DOI] [PubMed]
- 34.Liu K, et al. Photothermal-triggered immunogenic nanotherapeutics for optimizing osteosarcoma therapy by synergizing innate and adaptive immunity. Biomaterials. 2022;282:121383. doi: 10.1016/j.biomaterials.2022.121383. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, et al. Subtype classification and prognosis signature construction of osteosarcoma based on cellular senescence-related genes. J. Oncol. 2022;2022:4421952. doi: 10.1155/2022/4421952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierrevelcin, M. et al. Engineering Novel 3D models to recreate high-grade osteosarcoma and its immune and extracellular matrix microenvironment. Adv. Healthc. Mater.11, e2200195 (2022). [DOI] [PubMed]
- 37.Somaiah N, et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-centre phase 2 trial. Lancet Oncol. 2022;23:1156–1166. doi: 10.1016/S1470-2045(22)00392-8. [DOI] [PubMed] [Google Scholar]
- 38.Xie X, et al. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022;16:107–119. doi: 10.1016/j.bioactmat.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L. et al. Self-splittable transcytosis Nanoraspberry for NIR-II Photo-immunometabolic cancer therapy in deep tumor tissue. Adv Sci (Weinh)9, e2204067 (2022). [DOI] [PMC free article] [PubMed]
- 40.Huang X, et al. Dual-responsive nanosystem based on TGF-β blockade and immunogenic chemotherapy for effective chemoimmunotherapy. Drug Deliv. 2022;29:1358–1369. doi: 10.1080/10717544.2022.2069877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mi, H. et al. Quantitative spatial profiling of immune populations in pancreatic ductal adenocarcinoma reveals tumor microenvironment heterogeneity and prognostic biomarkers. Cancer82, 4359–4372 (2022). [DOI] [PMC free article] [PubMed]
- 42.Li, M. et al. Tumor-derived exosomes deliver the tumor suppressor miR-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene41, 4618–4632 (2022). [DOI] [PMC free article] [PubMed]
- 43.Kuo, C. L. et al. A Fc-VEGF chimeric fusion enhances PD-L1 immunotherapy via inducing immune reprogramming and infiltration in the immunosuppressive tumor microenvironment. Cancer Immunol. Immunother. 72, 351–369 (2022). [DOI] [PMC free article] [PubMed]
- 44.Poulin LF, Lasseaux C, Chamaillard M. Understanding the cellular origin of the mononuclear phagocyte system sheds light on the myeloid postulate of immune paralysis in sepsis. Front. Immunol. 2018;9:823. doi: 10.3389/fimmu.2018.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin X, et al. Human Blood CD1c+ Dendritic cells encompass CD5high and CD5 low subsets that differ significantly in phenotype, gene expression, and functions. J. Immunol. 2017;198:1553–1564. doi: 10.4049/jimmunol.1600193. [DOI] [PubMed] [Google Scholar]
- 46.Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154:3–20. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talker SC, et al. Precise delineation and transcriptional characterization of bovine blood dendritic-cell and monocyte subsets. Front. Immunol. 2018;9:2505. doi: 10.3389/fimmu.2018.02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou, Y. et al. Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103(+) conventional dendritic cells. J. Immunother. Cancer8, e000474 (2020). [DOI] [PMC free article] [PubMed]
- 49.Meyer MA, et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 2018;9:1250. doi: 10.1038/s41467-018-03600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makino, K. et al. Generation of cDC-like cells from human induced pluripotent stem cells via Notch signaling. J. Immunother. Cancer10, e003827 (2022). [DOI] [PMC free article] [PubMed]
- 51.Inagaki Y, et al. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin. Sarcoma Res. 2016;6:13. doi: 10.1186/s13569-016-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang GZ, et al. Development of a machine learning-based autophagy-related lncrna signature to improve prognosis prediction in osteosarcoma patients. Front Mol. Biosci. 2021;8:615084. doi: 10.3389/fmolb.2021.615084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le, T., Su, S., Kirshtein, A. & Shahriyari, L. Data-driven mathematical model of osteosarcoma. Cancers 13, 2367 (2021). [DOI] [PMC free article] [PubMed]
- 54.Kansara M, et al. Infiltrating myeloid cells drive osteosarcoma progression via GRM4 regulation of IL23. Cancer Discov. 2019;9:1511–1519. doi: 10.1158/2159-8290.CD-19-0154. [DOI] [PubMed] [Google Scholar]
- 55.Jones KB. Dendritic cells drive Osteosarcomagenesis through newly identified oncogene and tumor suppressor. Cancer Discov. 2019;9:1484–1486. doi: 10.1158/2159-8290.CD-19-0994. [DOI] [PubMed] [Google Scholar]
- 56.Kawano M, Itonaga I, Iwasaki T, Tsumura H. Enhancement of antitumor immunity by combining anti-cytotoxic T lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed dendritic cells in murine osteosarcoma. Oncol. Rep. 2013;29:1001–1006. doi: 10.3892/or.2013.2224. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020;11:6322. doi: 10.1038/s41467-020-20059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koirala P, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016;6:30093. doi: 10.1038/srep30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le T, Su S, Shahriyari L. Immune classification of osteosarcoma. Math. Biosci. Eng. 2021;18:1879–1897. doi: 10.3934/mbe.2021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawir DF, Sikora K, O’Meara CP, Schorpp M, Boehm T. Pervasive changes of mRNA splicing in upf1-deficient zebrafish identify rpl10a as a regulator of T cell development. Proc. Natl. Acad. Sci. USA. 2020;117:15799–15808. doi: 10.1073/pnas.1917812117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel A, Kerndl M, Schabbauer G, Sharif O. Protocol to assess the tolerogenic properties of adoptively transferred dendritic cells during murine experimental autoimmune encephalomyelitis. STAR Protoc. 2022;3:101653. doi: 10.1016/j.xpro.2022.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gan X, et al. An anti-CTLA-4 heavy chain-only antibody with enhanced T(reg) depletion shows excellent preclinical efficacy and safety profile. Proc. Natl. Acad. Sci. USA. 2022;119:e2200879119. doi: 10.1073/pnas.2200879119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia, S. et al. miR-150 promotes progressive T cell differentiation via inhibiting FOXP1 and RC3H1. Hum. Immunol. 83, 778–788 (2022). [DOI] [PubMed]
- 64.Itahashi K, Irie T, Nishikawa H. Regulatory T-cell development in the tumor microenvironment. Eur. J. Immunol. 2022;52:1216–1227. doi: 10.1002/eji.202149358. [DOI] [PubMed] [Google Scholar]
- 65.Liang J, et al. Tumor-associated regulatory T cells in non-small-cell lung cancer: current advances and future perspectives. J. Immunol. Res. 2022;2022:4355386. doi: 10.1155/2022/4355386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schroeter CB, et al. Crosstalk of microorganisms and immune responses in autoimmune neuroinflammation: a focus on regulatory t cells. Front. Immunol. 2021;12:747143. doi: 10.3389/fimmu.2021.747143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yabe H, et al. Prognostic significance of HLA class I expression in Ewing’s sarcoma family of tumors. J. Surg. Oncol. 2011;103:380–385. doi: 10.1002/jso.21829. [DOI] [PubMed] [Google Scholar]
- 68.Ligon, J. A. et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J. Immunother. Cancer9, e001772 (2021). [DOI] [PMC free article] [PubMed]
- 69.Sundara YT, et al. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol. Immunother. 2017;66:119–128. doi: 10.1007/s00262-016-1925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tobita S, et al. Successful continuous nivolumab therapy for metastatic non-small cell lung cancer after local treatment of oligometastatic lesions. Thorac. Cancer. 2020;11:2357–2360. doi: 10.1111/1759-7714.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavon I, Heli C, Brill L, Charbit H, Vaknin-Dembinsky A. Blood levels of co-inhibitory-receptors: a biomarker of disease prognosis in multiple sclerosis. Front. Immunol. 2019;10:835. doi: 10.3389/fimmu.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su M, Huang CX, Dai AP. Immune checkpoint inhibitors: therapeutic tools for breast cancer. Asian Pac. J. Cancer Prev. 2016;17:905–910. doi: 10.7314/APJCP.2016.17.3.905. [DOI] [PubMed] [Google Scholar]
- 73.Han Q, Shi H, Liu F. CD163(+) M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int Immunopharmacol. 2016;34:101–106. doi: 10.1016/j.intimp.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 74.Matsuo T, et al. Extraskeletal osteosarcoma with partial spontaneous regression. Anticancer Res. 2009;29:5197–5201. [PubMed] [Google Scholar]
- 75.Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat. Rev. Drug Disco. 2022;21:559–577. doi: 10.1038/s41573-022-00413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Croft CA, et al. Notch, RORC and IL-23 signals cooperate to promote multi-lineage human innate lymphoid cell differentiation. Nat. Commun. 2022;13:4344. doi: 10.1038/s41467-022-32089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le, H., Spearman, P., Waggoner, S. N. & Singh, K. Ebola virus protein VP40 stimulates IL-12- and IL-18-dependent activation of human natural killer cells. JCI Insight7, e158902 (2022). [DOI] [PMC free article] [PubMed]
- 78.Pende D, et al. Killer Ig-like Receptors (KIRs): Their role in NK cell modulation and developments leading to their clinical exploitation. Front. Immunol. 2019;10:1179. doi: 10.3389/fimmu.2019.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng G, Jia L, Yang AG. Roles of HLA-G/KIR2DL4 in breast cancer immune microenvironment. Front. Immunol. 2022;13:791975. doi: 10.3389/fimmu.2022.791975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.D’Amico S, et al. ERAP1 controls the interaction of the inhibitory receptor KIR3DL1 with HLA-B51:01 by affecting natural killer cell function. Front. Immunol. 2021;12:778103. doi: 10.3389/fimmu.2021.778103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boudreau JE, Hsu KC. Natural killer cell education and the response to infection and cancer therapy: stay tuned. Trends Immunol. 2018;39:222–239. doi: 10.1016/j.it.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borrego F, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol. Immunol. 2002;38:637–660. doi: 10.1016/S0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 83.Barrow AD, Martin CJ, Colonna M. The natural cytotoxicity receptors in health and disease. Front. Immunol. 2019;10:909. doi: 10.3389/fimmu.2019.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee GH, et al. Clinical impact of natural killer Group 2D receptor expression and that of its ligand in ovarian carcinomas: a retrospective study. Yonsei Med. J. 2021;62:288–297. doi: 10.3349/ymj.2021.62.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kegasawa T, et al. Soluble UL16-binding protein 2 is associated with a poor prognosis in pancreatic cancer patients. Biochem. Biophys. Res. Commun. 2019;517:84–88. doi: 10.1016/j.bbrc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 86.Tsertsvadze, T., Mitskevich, N., Bilanishvili, A., Girdaladze, D. & Porakishvili, N. Phagocytosis and expression of FCg-receptors and CD180 on monocytes in chronic lymphocytic leukemia. Georgian Med. News 88–93 (2017). [PubMed]
- 87.Sivori S, et al. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 2019;16:430–441. doi: 10.1038/s41423-019-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol. 2019;40:142–158. doi: 10.1016/j.it.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Ogiwara Y, et al. Blocking FSTL1 boosts NK immunity in treatment of osteosarcoma. Cancer Lett. 2022;537:215690. doi: 10.1016/j.canlet.2022.215690. [DOI] [PubMed] [Google Scholar]
- 90.Razmara AM, et al. Natural killer and T cell infiltration in canine osteosarcoma: clinical implications and translational relevance. Front. Vet. Sci. 2021;8:771737. doi: 10.3389/fvets.2021.771737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim KS, Mimura K, Kua LF, Shiraishi K, Kono K. Implication of highly cytotoxic natural killer cells for esophageal squamous cell carcinoma treatment. J. Immunother. 2018;41:261–273. doi: 10.1097/CJI.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 92.Baek HJ, et al. Ex vivo expansion of natural killer cells using cryopreserved irradiated feeder cells. Anticancer Res. 2013;33:2011–2019. [PubMed] [Google Scholar]
- 93.Cho D, et al. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin. Cancer Res. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernández L, et al. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. 2015;368:54–63. doi: 10.1016/j.canlet.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 95.Zhu S, et al. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm. Res. 2015;32:779–792. doi: 10.1007/s11095-013-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Otegbeye F, et al. Natural killer cell alloreactivity predicted by killer cell immunoglobulin-like receptor ligand mismatch does not impact engraftment in umbilical cord blood and haploidentical stem cell transplantation. Transpl. Cell Ther. 2022;28:483.e481–483.e487. doi: 10.1016/j.jtct.2022.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, et al. Mesenchymal stem cells enhance the impact of KIR receptor-ligand mismatching on acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia but not in those with acute lymphocytic leukemia. Hematol. Oncol. 2021;39:380–389. doi: 10.1002/hon.2867. [DOI] [PubMed] [Google Scholar]
- 98.Arvanitakis, K., Koletsa, T., Mitroulis, I. & Germanidis, G. Tumor-associated macrophages in hepatocellular carcinoma pathogenesis, prognosis and therapy. Cancers14, 226 (2022). [DOI] [PMC free article] [PubMed]
- 99.Izumi Y, et al. An antibody-drug conjugate that selectively targets human monocyte progenitors for anti-cancer therapy. Front. Immunol. 2021;12:618081. doi: 10.3389/fimmu.2021.618081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu XQ, et al. Increased expression of tribbles homolog 3 predicts poor prognosis and correlates with tumor immunity in clear cell renal cell carcinoma: a bioinformatics study. Bioengineered. 2022;13:14000–14012. doi: 10.1080/21655979.2022.2086380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelleher FC, O’Sullivan H. Monocytes, macrophages, and osteoclasts in osteosarcoma. J. Adolesc. Young-. Adult Oncol. 2017;6:396–405. doi: 10.1089/jayao.2016.0078. [DOI] [PubMed] [Google Scholar]
- 102.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brifault C, Gilder AS, Laudati E, Banki M, Gonias SL. Shedding of membrane-associated LDL receptor-related protein-1 from microglia amplifies and sustains neuroinflammation. J. Biol. Chem. 2017;292:18699–18712. doi: 10.1074/jbc.M117.798413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shapouri-Moghaddam A, et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 106.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016;99:180–185. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Huang Q, et al. The role of tumor-associated macrophages in osteosarcoma progression - therapeutic implications. Cell Oncol. 2021;44:525–539. doi: 10.1007/s13402-021-00598-w. [DOI] [PubMed] [Google Scholar]
- 108.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kielbassa K, Vegna S, Ramirez C, Akkari L. Understanding the origin and diversity of macrophages to tailor their targeting in solid cancers. Front. Immunol. 2019;10:2215. doi: 10.3389/fimmu.2019.02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dudzinski SO, et al. Leptin augments antitumor immunity in obesity by repolarizing tumor-associated macrophages. J. Immunol. 2021;207:3122–3130. doi: 10.4049/jimmunol.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahirwar DK, et al. Slit2 inhibits breast cancer metastasis by activating M1-like phagocytic and antifibrotic macrophages. Cancer Res. 2021;81:5255–5267. doi: 10.1158/0008-5472.CAN-20-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szulc-Kielbik I, Kielbik M. Tumor-associated macrophages: reasons to be cheerful, reasons to be fearful. Exp. Suppl. 2022;113:107–140. doi: 10.1007/978-3-030-91311-3_4. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, et al. HMGA2 facilitates colorectal cancer progression via STAT3-mediated tumor-associated macrophage recruitment. Theranostics. 2022;12:963–975. doi: 10.7150/thno.65411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deng C, et al. Reprograming the tumor immunologic microenvironment using neoadjuvant chemotherapy in osteosarcoma. Cancer Sci. 2020;111:1899–1909. doi: 10.1111/cas.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shao XJ, et al. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharm. Sin. 2019;40:1343–1350. doi: 10.1038/s41401-019-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 117.Jakab M, Rostalski T, Lee KH, Mogler C, Augustin HG. Tie2 receptor in tumor-infiltrating macrophages is dispensable for tumor angiogenesis and tumor relapse after chemotherapy. Cancer Res. 2022;82:1353–1364. doi: 10.1158/0008-5472.CAN-21-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J, Zhou X, Hao H. Macrophage phenotype-switching in cancer. Eur. J. Pharm. 2022;931:175229. doi: 10.1016/j.ejphar.2022.175229. [DOI] [PubMed] [Google Scholar]
- 119.Sun Q, et al. Lenvatinib for effectively treating antiangiogenic drug-resistant nasopharyngeal carcinoma. Cell Death Dis. 2022;13:724. doi: 10.1038/s41419-022-05171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hang X, et al. BCL-2 isoform β promotes angiogenesis by TRiC-mediated upregulation of VEGF-A in lymphoma. Oncogene. 2022;41:3655–3663. doi: 10.1038/s41388-022-02372-0. [DOI] [PubMed] [Google Scholar]
- 121.Huang, C. Y. et al. Fluoroquinolones suppress TGF-β and PMA-induced MMP-9 production in cancer cells: implications in repurposing quinolone antibiotics for cancer treatment. Int. J. Mol. Sci. 22, 11602 (2021). [DOI] [PMC free article] [PubMed]
- 122.Patel SS, et al. The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4-positive T cells that are PD-1-negative. Blood. 2019;134:2059–2069. doi: 10.1182/blood.2019002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang P, et al. Macrophages promote coal tar pitch extract-induced tumorigenesis of BEAS-2B cells and tumor metastasis in nude mice mediated by AP-1. Asian Pac. J. Cancer Prev. 2014;15:4871–4876. doi: 10.7314/APJCP.2014.15.12.4871. [DOI] [PubMed] [Google Scholar]
- 125.Han Y, et al. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019;440-441:116–125. doi: 10.1016/j.canlet.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 126.Etzerodt A, et al. Specific targeting of CD163(+) TAMs mobilizes inflammatory monocytes and promotes T cell-mediated tumor regression. J. Exp. Med. 2019;216:2394–2411. doi: 10.1084/jem.20182124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamaguchi, Y. et al. PD-L1 blockade restores CAR T cell activity through IFN-γ-regulation of CD163+ M2 macrophages. J. Immunother. Cancer10, e004400 (2022). [DOI] [PMC free article] [PubMed]
- 128.Eruslanov EB, Singhal S, Albelda SM. Mouse versus human neutrophils in cancer: a major knowledge gap. Trends Cancer. 2017;3:149–160. doi: 10.1016/j.trecan.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 130.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 131.Yang S, Wu C, Wang L, Shan D, Chen B. Pretreatment inflammatory indexes as prognostic predictors for survival in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2020;13:515–524. [PMC free article] [PubMed] [Google Scholar]
- 132.Liu B, et al. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci. Rep. 2016;6:39862. doi: 10.1038/srep39862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xia WK, et al. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016;14:127. doi: 10.1186/s12957-016-0889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasquez L, et al. Pretreatment Neutrophil-to-Lymphocyte ratio and lymphocyte recovery: independent prognostic factors for survival in pediatric sarcomas. J. Pediatr. Hematol. Oncol. 2017;39:538–546. doi: 10.1097/MPH.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 135.Yapar A, et al. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt Dis. Relat. Surg. 2021;32:489–496. doi: 10.52312/jdrs.2021.79775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu, L., Saxena, S., Awaji, M. & Singh, R. K. Tumor-associated neutrophils in cancer: going pro. Cancers11, 564 (2019). [DOI] [PMC free article] [PubMed]
- 137.Filep JG. Targeting neutrophils for promoting the resolution of inflammation. Front. Immunol. 2022;13:866747. doi: 10.3389/fimmu.2022.866747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 139.Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 140.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–322. doi: 10.1016/S0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 141.Carestia A, Kaufman T, Schattner M. Platelets: New bricks in the building of neutrophil extracellular traps. Front. Immunol. 2016;7:271. doi: 10.3389/fimmu.2016.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 143.Leshner M, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bronte V, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mishalian I, et al. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun R, et al. Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget. 2014;5:12621–12634. doi: 10.18632/oncotarget.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 150.Jaillon S, et al. Neutrophil diversity, and plasticity in tumour progression and therapy. Nat. Rev. Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 151.Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019;16:601–620. doi: 10.1038/s41571-019-0222-4. [DOI] [PubMed] [Google Scholar]
- 152.Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang B, et al. Identification of prognostic biomarkers associated with metastasis and immune infiltration in osteosarcoma. Oncol. Lett. 2021;21:180. doi: 10.3892/ol.2021.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Furumaya C, Martinez-Sanz P, Bouti P, Kuijpers TW, Matlung HL. Plasticity in Pro- and anti-tumor activity of neutrophils: shifting the balance. Front. Immunol. 2020;11:2100. doi: 10.3389/fimmu.2020.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang X, et al. Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review) Int. J. Oncol. 2016;49:857–867. doi: 10.3892/ijo.2016.3616. [DOI] [PubMed] [Google Scholar]
- 156.Zhang C, et al. Neutrophils correlate with hypoxia microenvironment and promote progression of non-small-cell lung cancer. Bioengineered. 2021;12:8872–8884. doi: 10.1080/21655979.2021.1987820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fu Y, et al. Development and validation of a hypoxia-associated prognostic signature related to osteosarcoma metastasis and immune infiltration. Front. Cell Dev. Biol. 2021;9:633607. doi: 10.3389/fcell.2021.633607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y, et al. CXCR4-guided liposomes regulating hypoxic and immunosuppressive microenvironment for sorafenib-resistant tumor treatment. Bioact. Mater. 2022;17:147–161. doi: 10.1016/j.bioactmat.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang W, et al. Engineering micro oxygen factories to slow tumour progression via hyperoxic microenvironments. Nat. Commun. 2022;13:4495. doi: 10.1038/s41467-022-32066-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dou, A. & Fang, J. Heterogeneous myeloid cells in tumors. Cancers13, 3772 (2021). [DOI] [PMC free article] [PubMed]
- 161.De Vlaeminck Y, González-Rascón A, Goyvaerts C, Breckpot K. Cancer-associated myeloid regulatory cells. Front Immunol. 2016;7:113. doi: 10.3389/fimmu.2016.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ling Z, Yang C, Tan J, Dou C, Chen Y. Beyond immunosuppressive effects: dual roles of myeloid-derived suppressor cells in bone-related diseases. Cell Mol. Life Sci. 2021;78:7161–7183. doi: 10.1007/s00018-021-03966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Horlad H, et al. Corosolic acid impairs tumor development and lung metastasis by inhibiting the immunosuppressive activity of myeloid-derived suppressor cells. Mol. Nutr. Food Res. 2013;57:1046–1054. doi: 10.1002/mnfr.201200610. [DOI] [PubMed] [Google Scholar]
- 164.Wu, S. Y. & Chiang, C. S. Distinct role of CD11b(+)Ly6G(-)Ly6C(-) Myeloid-derived cells on the progression of the primary tumor and therapy-associated recurrent brain tumor. Cells9, 51 (2019). [DOI] [PMC free article] [PubMed]
- 165.Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med. Microbiol Immunol. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- 166.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bottomley MJ, Harden PN, Wood KJ, Hester J, Issa F. Dampened Inflammatory signalling and myeloid-derived suppressor-like cell accumulation reduces circulating monocytic HLA-DR density and may associate with malignancy risk in long-term renal transplant recipients. Front. Immunol. 2022;13:901273. doi: 10.3389/fimmu.2022.901273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mukherjee S, et al. IL-6 dependent expansion of inflammatory MDSCs (CD11b+ Gr-1+) promote Th-17 mediated immune response during experimental cerebral malaria. Cytokine. 2022;155:155910. doi: 10.1016/j.cyto.2022.155910. [DOI] [PubMed] [Google Scholar]
- 169.Scirocchi F, et al. Immune effects of CDK4/6 inhibitors in patients with HR(+)/HER2(-) metastatic breast cancer: Relief from immunosuppression is associated with clinical response. EBioMedicine. 2022;79:104010. doi: 10.1016/j.ebiom.2022.104010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Joshi S, Sharabi A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharm. Ther. 2022;235:108114. doi: 10.1016/j.pharmthera.2022.108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fionda C, Abruzzese MP, Santoni A, Cippitelli M. Immunoregulatory and effector activities of nitric oxide and reactive nitrogen species in cancer. Curr. Med. Chem. 2016;23:2618–2636. doi: 10.2174/0929867323666160727105101. [DOI] [PubMed] [Google Scholar]
- 172.Sasidharan Nair V, Saleh R, Toor SM, Alajez NM, Elkord E. Transcriptomic analyses of myeloid-derived suppressor cell subsets in the circulation of colorectal cancer patients. Front. Oncol. 2020;10:1530. doi: 10.3389/fonc.2020.01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Porta C, et al. Tumor-derived Prostaglandin E2 promotes p50 NF-κB-dependent differentiation of monocytic MDSCs. Cancer Res. 2020;80:2874–2888. doi: 10.1158/0008-5472.CAN-19-2843. [DOI] [PubMed] [Google Scholar]
- 174.Xin B, et al. Enhancing the therapeutic efficacy of programmed death ligand 1 antibody for metastasized liver cancer by overcoming hepatic immunotolerance in mice. Hepatology. 2022;76:630–645. doi: 10.1002/hep.32266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang X, et al. CD147 mediates epidermal malignant transformation through the RSK2/AP-1 pathway. J. Exp. Clin. Cancer Res. 2022;41:246. doi: 10.1186/s13046-022-02427-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang Y, Li C, Liu T, Dai X, Bazhin AV. Myeloid-derived suppressor cells in tumors: from mechanisms to antigen specificity and microenvironmental regulation. Front. Immunol. 2020;11:1371. doi: 10.3389/fimmu.2020.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J. Cancer. 2013;4:3–11. doi: 10.7150/jca.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang N, et al. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFβRII-SMADS pathway. J. Nanobiotechnology. 2021;19:437. doi: 10.1186/s12951-021-01138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu H, Sun X, Gong X, Wang G. Human umbilical cord mesenchymal stem cells derived exosomes exert antiapoptosis effect via activating PI3K/Akt/mTOR pathway on H9C2 cells. J. Cell Biochem. 2019;120:14455–14464. doi: 10.1002/jcb.28705. [DOI] [PubMed] [Google Scholar]
- 183.Jiao Y, et al. Tumor cell-derived extracellular vesicles for breast cancer specific delivery of therapeutic P53. J. Control Release. 2022;349:606–616. doi: 10.1016/j.jconrel.2022.07.020. [DOI] [PubMed] [Google Scholar]
- 184.De Martino, V. et al. Extracellular vesicles in osteosarcoma: antagonists or therapeutic agents? Int. J. Mol. Sci.22, 12586 (2021). [DOI] [PMC free article] [PubMed]
- 185.Hareendran S, Yang X, Sharma VK, Loh YP. Carboxypeptidase E and its splice variants: Key regulators of growth and metastasis in multiple cancer types. Cancer Lett. 2022;548:215882. doi: 10.1016/j.canlet.2022.215882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Y, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11:1473–1492. doi: 10.7150/thno.51245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Du M, et al. Genome-wide CRISPR screen identified Rad18 as a determinant of doxorubicin sensitivity in osteosarcoma. J. Exp. Clin. Cancer Res. 2022;41:154. doi: 10.1186/s13046-022-02344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cappariello, A. & Rucci, N. Tumour-derived Extracellular Vesicles (EVs): A dangerous “Message in A Bottle” for Bone. Int. J. Mol. Sci.20, 4805 (2019). [DOI] [PMC free article] [PubMed]
- 189.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev. Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 190.Troyer RM, et al. Exosomes from Osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp. Cell Res. 2017;358:369–376. doi: 10.1016/j.yexcr.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 191.Wang J, et al. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J. Nanobiotechnology. 2020;18:151. doi: 10.1186/s12951-020-00710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Isla Larrain MT, et al. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumour Biol. 2014;35:6511–6519. doi: 10.1007/s13277-014-1859-3. [DOI] [PubMed] [Google Scholar]
- 193.Wang S, Ma F, Feng Y, Liu T, He S. Role of exosomal miR‑21 in the tumor microenvironment and osteosarcoma tumorigenesis and progression (Review) Int J. Oncol. 2020;56:1055–1063. doi: 10.3892/ijo.2020.4992. [DOI] [PubMed] [Google Scholar]
- 194.Schiavone K, Garnier D, Heymann MF, Heymann D. The heterogeneity of Osteosarcoma: The role played by cancer stem cells. Adv. Exp. Med. Biol. 2019;1139:187–200. doi: 10.1007/978-3-030-14366-4_11. [DOI] [PubMed] [Google Scholar]
- 195.Xu A, et al. Cell Differentiation trajectory-associated molecular classification of Osteosarcoma. Genes. 2021;12:1685. doi: 10.3390/genes12111685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sarhadi, V. K., Daddali, R. & Seppänen-Kaijansinkko, R. Mesenchymal stem cells and extracellular vesicles in osteosarcoma pathogenesis and therapy. Int. J. Mol. Sci.22, 11035 (2021). [DOI] [PMC free article] [PubMed]
- 197.Sole A, et al. Unraveling Ewing Sarcoma Tumorigenesis originating from patient-derived mesenchymal stem cells. Cancer Res. 2021;81:4994–5006. doi: 10.1158/0008-5472.CAN-20-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mannerström B, et al. Epigenetic alterations in mesenchymal stem cells by osteosarcoma-derived extracellular vesicles. Epigenetics. 2019;14:352–364. doi: 10.1080/15592294.2019.1585177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang JY, et al. Generation of Osteosarcomas from a combination of Rb silencing and c-Myc overexpression in human mesenchymal stem cells. Stem Cells Transl. Med. 2017;6:512–526. doi: 10.5966/sctm.2015-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chang X, Ma Z, Zhu G, Lu Y, Yang J. New perspective into mesenchymal stem cells: Molecular mechanisms regulating osteosarcoma. J. Bone Oncol. 2021;29:100372. doi: 10.1016/j.jbo.2021.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kawano M, Tanaka K, Itonaga I, Iwasaki T, Tsumura H. Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment. Cell Commun. Signal. 2018;16:13. doi: 10.1186/s12964-018-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Du L, et al. CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis. Cell Death Dis. 2018;9:714. doi: 10.1038/s41419-018-0745-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pelagalli, A., Nardelli, A., Fontanella, R. & Zannetti, A. Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci.17, 1102 (2016). [DOI] [PMC free article] [PubMed]
- 205.Deng, Q. et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. Elife8, e50208 (2019). [DOI] [PMC free article] [PubMed]
- 206.Pietrovito L, et al. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018;12:659–676. doi: 10.1002/1878-0261.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Baglio SR, et al. Blocking tumor-educated MSC Paracrine activity halts Osteosarcoma progression. Clin. Cancer Res. 2017;23:3721–3733. doi: 10.1158/1078-0432.CCR-16-2726. [DOI] [PubMed] [Google Scholar]
- 208.Lin L, et al. Conditioned medium of the osteosarcoma cell line U2OS induces hBMSCs to exhibit characteristics of carcinoma-associated fibroblasts via activation of IL-6/STAT3 signalling. J. Biochem. 2020;168:265–271. doi: 10.1093/jb/mvaa044. [DOI] [PubMed] [Google Scholar]
- 209.Chang AI, Schwertschkow AH, Nolta JA, Wu J. Involvement of mesenchymal stem cells in cancer progression and metastases. Curr. Cancer Drug Targets. 2015;15:88–98. doi: 10.2174/1568009615666150126154151. [DOI] [PubMed] [Google Scholar]
- 210.Lagerweij T, Pérez-Lanzón M, Baglio SR. A preclinical mouse model of osteosarcoma to define the extracellular vesicle-mediated communication between tumor and mesenchymal stem cells. J. Vis. Exp. 2018;135:56932. doi: 10.3791/56932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Q, et al. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J. Cell Physiol. 2018;233:6832–6840. doi: 10.1002/jcp.26436. [DOI] [PubMed] [Google Scholar]
- 212.Khare D, et al. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-Lymphocytes. Front. Immunol. 2018;9:3053. doi: 10.3389/fimmu.2018.03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li W, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10:918. doi: 10.1038/s41419-019-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jia XH, et al. Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects. Oncotarget. 2016;7:20934–20944. doi: 10.18632/oncotarget.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mardpour S, et al. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J. Cell Physiol. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 216.Chang L, Asatrian G, Dry SM, James AW. Circulating tumor cells in sarcomas: a brief review. Med. Oncol. 2015;32:430. doi: 10.1007/s12032-014-0430-9. [DOI] [PubMed] [Google Scholar]
- 217.Wu ZJ, Tan JC, Qin X, Liu B, Yuan ZC. Significance of circulating tumor cells in osteosarcoma patients treated by neoadjuvant chemotherapy and surgery. Cancer Manag Res. 2018;10:3333–3339. doi: 10.2147/CMAR.S176515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cortini, M. et al. Exploring metabolic adaptations to the acidic microenvironment of Osteosarcoma cells unveils Sphingosine 1-Phosphate as a valuable therapeutic target. Cancers 13, 311 (2021). [DOI] [PMC free article] [PubMed]
- 219.Zhang Y, et al. Interleukin-6 suppression reduces tumour self-seeding by circulating tumour cells in a human osteosarcoma nude mouse model. Oncotarget. 2016;7:446–458. doi: 10.18632/oncotarget.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu T, et al. Self-seeding circulating tumor cells promote the proliferation and metastasis of human osteosarcoma by upregulating interleukin-8. Cell Death Dis. 2019;10:575. doi: 10.1038/s41419-019-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sun Q, et al. Nanomedicine and macroscale materials in immuno-oncology. Chem. Soc. Rev. 2019;48:351–381. doi: 10.1039/C8CS00473K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Preusser M, Berghoff AS, Thallinger C, Zielinski CC. Cancer immune cycle: a video introduction to the interaction between cancer and the immune system. ESMO Open. 2016;1:e000056. doi: 10.1136/esmoopen-2016-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim KS, Youn YS, Bae YH. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. J. Control Release. 2019;311-312:85–95. doi: 10.1016/j.jconrel.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 224.Meng Z, et al. Tumor immunotherapy boosted by R837 nanocrystals through combining chemotherapy and mild hyperthermia. J. Control Release. 2022;350:841–856. doi: 10.1016/j.jconrel.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 225.Yang J, Zhang C. Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:e1612. doi: 10.1002/wnan.1612. [DOI] [PubMed] [Google Scholar]
- 226.Galiana I, et al. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanoSenolytic. J. Control Rel. 2020;323:624–634. doi: 10.1016/j.jconrel.2020.04.045. [DOI] [PubMed] [Google Scholar]
- 227.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 228.Anfray, C., Ummarino, A., Andón, F. T. & Allavena, P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells9, 46 (2019). [DOI] [PMC free article] [PubMed]
- 229.van Dalen, F. J., van Stevendaal, M., Fennemann, F. L., Verdoes, M. & Ilina, O. Molecular repolarisation of tumour-associated macrophages. Molecules24, 9 (2018). [DOI] [PMC free article] [PubMed]
- 230.Shime H, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc. Natl. Acad. Sci. USA. 2012;109:2066–2071. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vidyarthi A, et al. TLR-3 stimulation Skews M2 macrophages to M1 through IFN-αβ signaling and restricts tumor progression. Front. Immunol. 2018;9:1650. doi: 10.3389/fimmu.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Helleberg Madsen N, Schnack Nielsen B, Larsen J, Gad M. In vitro 2D and 3D cancer models to evaluate compounds that modulate macrophage polarization. Cell Immunol. 2022;378:104574. doi: 10.1016/j.cellimm.2022.104574. [DOI] [PubMed] [Google Scholar]
- 233.Ubil E, et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Invest. 2018;128:2356–2369. doi: 10.1172/JCI97354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pahl JH, et al. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-γ. J. Exp. Clin. Cancer Res. 2014;33:27. doi: 10.1186/1756-9966-33-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Correction: All-Trans retinoic acid prevents osteosarcoma metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol. Res.8, 280, (2020). [DOI] [PubMed]
- 236.Wang JC, et al. Metformin’s antitumour and anti-angiogenic activities are mediated by skewing macrophage polarization. J. Cell Mol. Med. 2018;22:3825–3836. doi: 10.1111/jcmm.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Uehara T, et al. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int. Immunol. 2019;31:187–198. doi: 10.1093/intimm/dxy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Maloney C, et al. Gefitinib inhibits invasion and metastasis of Osteosarcoma via inhibition of macrophage receptor interacting Serine-Threonine Kinase 2. Mol. Cancer Ther. 2020;19:1340–1350. doi: 10.1158/1535-7163.MCT-19-0903. [DOI] [PubMed] [Google Scholar]
- 239.Yang M, et al. MYLK4 promotes tumor progression through the activation of epidermal growth factor receptor signaling in osteosarcoma. J. Exp. Clin. Cancer Res. 2021;40:166. doi: 10.1186/s13046-021-01965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang S, et al. Stattic sensitizes osteosarcoma cells to epidermal growth factor receptor inhibitors via blocking the interleukin 6-induced STAT3 pathway. Acta Biochim. Biophys. Sin. 2021;53:1670–1680. doi: 10.1093/abbs/gmab146. [DOI] [PubMed] [Google Scholar]
- 241.Kallis MP, et al. Pharmacological prevention of surgery-accelerated metastasis in an animal model of osteosarcoma. J. Transl. Med. 2020;18:183. doi: 10.1186/s12967-020-02348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nohara T, et al. Antitumor allium sulfides. Chem. Pharm. Bull. 2017;65:209–217. doi: 10.1248/cpb.c16-00844. [DOI] [PubMed] [Google Scholar]
- 243.Nohara T, et al. Thiolane-type sulfides from garlic, onion, and Welsh onion. J. Nat. Med. 2021;75:741–751. doi: 10.1007/s11418-021-01533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fujiwara Y, Takeya M, Komohara Y. A novel strategy for inducing the antitumor effects of triterpenoid compounds: blocking the protumoral functions of tumor-associated macrophages via STAT3 inhibition. Biomed. Res. Int. 2014;2014:348539. doi: 10.1155/2014/348539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chen X, et al. Oleanolic acid inhibits osteosarcoma cell proliferation and invasion by suppressing the SOX9/Wnt1 signaling pathway. Exp. Ther. Med. 2021;21:443. doi: 10.3892/etm.2021.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kimura Y, Sumiyoshi M. Anti-tumor and anti-metastatic actions of wogonin isolated from Scutellaria baicalensis roots through anti-lymphangiogenesis. Phytomedicine. 2013;20:328–336. doi: 10.1016/j.phymed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 247.Kimura Y, Sumiyoshi M. Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur. J. Pharm. 2015;746:115–125. doi: 10.1016/j.ejphar.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 248.Sumiyoshi M, Taniguchi M, Baba K, Kimura Y. Antitumor and antimetastatic actions of xanthoangelol and 4-hydroxyderricin isolated from Angelica Keiskei roots through the inhibited activation and differentiation of M2 macrophages. Phytomedicine. 2015;22:759–767. doi: 10.1016/j.phymed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 249.Kimura Y, Sumiyoshi M. Resveratrol prevents tumor growth and metastasis by inhibiting Lymphangiogenesis and M2 macrophage activation and differentiation in tumor-associated macrophages. Nutr. Cancer. 2016;68:667–678. doi: 10.1080/01635581.2016.1158295. [DOI] [PubMed] [Google Scholar]
- 250.Kimura Y, Sumiyoshi M, Baba K. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res. 2016;36:137–148. [PubMed] [Google Scholar]
- 251.Caldeira, J. C., Perrine, M., Pericle, F. & Cavallo, F. Virus-like particles as an immunogenic platform for cancer vaccines. Viruses 12, 488 (2020). [DOI] [PMC free article] [PubMed]
- 252.Ying K, et al. Macrophage membrane-biomimetic adhesive polycaprolactone nanocamptothecin for improving cancer-targeting efficiency and impairing metastasis. Bioact. Mater. 2023;20:449–462. doi: 10.1016/j.bioactmat.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sun L, et al. Long-term effect of mobile phone-based education and influencing factors of willingness to receive HPV vaccination among female freshmen in Shanxi Province, China. Hum. Vaccin Immunother. 2022;18:2051990. doi: 10.1080/21645515.2022.2051990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Marcove RC, Miké V, Huvos AG, Southam CM, Levin AG. Vaccine trials for osteogenic sarcoma. A preliminary report. CA Cancer J. Clin. 1973;23:74–80. doi: 10.3322/canjclin.23.2.74. [DOI] [PubMed] [Google Scholar]
- 255.Wang Z, et al. Innate immune cells: a potential and promising cell population for treating Osteosarcoma. Front. Immunol. 2019;10:1114. doi: 10.3389/fimmu.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tsukahara T, et al. The future of immunotherapy for sarcoma. Expert Opin. Biol. Ther. 2016;16:1049–1057. doi: 10.1080/14712598.2016.1188075. [DOI] [PubMed] [Google Scholar]
- 257.Shemesh CS, et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol. Ther. 2021;29:555–570. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 259.Mason NJ, et al. Immunotherapy with a HER2-targeting Listeria Induces HER2-specific immunity and demonstrates potential therapeutic effects in a Phase I trial in Canine Osteosarcoma. Clin. Cancer Res. 2016;22:4380–4390. doi: 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
- 260.Pritchard-Jones K, et al. Immune responses to the 105AD7 human anti-idiotypic vaccine after intensive chemotherapy, for osteosarcoma. Br. J. Cancer. 2005;92:1358–1365. doi: 10.1038/sj.bjc.6602500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ullenhag GJ, et al. T-cell responses in osteosarcoma patients vaccinated with an anti-idiotypic antibody, 105AD7, mimicking CD55. Clin. Immunol. 2008;128:148–154. doi: 10.1016/j.clim.2008.03.512. [DOI] [PubMed] [Google Scholar]
- 262.Li D, Toji S, Watanabe K, Torigoe T, Tsukahara T. Identification of novel human leukocyte antigen-A*11:01-restricted cytotoxic T-lymphocyte epitopes derived from osteosarcoma antigen papillomavirus binding factor. Cancer Sci. 2019;110:1156–1168. doi: 10.1111/cas.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tsukahara T, et al. Specific targeting of a naturally presented osteosarcoma antigen, papillomavirus binding factor peptide, using an artificial monoclonal antibody. J. Biol. Chem. 2014;289:22035–22047. doi: 10.1074/jbc.M114.568725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Holland D, et al. Activation of the enhancer of Zeste homologue 2 gene by the human papillomavirus E7 oncoprotein. Cancer Res. 2008;68:9964–9972. doi: 10.1158/0008-5472.CAN-08-1134. [DOI] [PubMed] [Google Scholar]
- 265.Peng W, Huang X, Yang D. EWS/FLI-l peptide-pulsed dendritic cells induces the antitumor immunity in a murine Ewing’s sarcoma cell model. Int. Immunopharmacol. 2014;21:336–341. doi: 10.1016/j.intimp.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 266.Tsuda N, et al. Expression of a newly defined tumor-rejection antigen SART3 in musculoskeletal tumors and induction of HLA class I-restricted cytotoxic T lymphocytes by SART3-derived peptides. J. Orthop. Res. 2001;19:346–351. doi: 10.1016/S0736-0266(00)90031-7. [DOI] [PubMed] [Google Scholar]
- 267.Tsukahara T, et al. Identification of human autologous cytotoxic T-lymphocyte-defined osteosarcoma gene that encodes a transcriptional regulator, papillomavirus binding factor. Cancer Res. 2004;64:5442–5448. doi: 10.1158/0008-5472.CAN-04-0522. [DOI] [PubMed] [Google Scholar]
- 268.He F, et al. GATA3/long noncoding RNA MHC-R regulates the immune activity of dendritic cells in chronic obstructive pulmonary disease induced by air pollution particulate matter. J. Hazard Mater. 2022;438:129459. doi: 10.1016/j.jhazmat.2022.129459. [DOI] [PubMed] [Google Scholar]
- 269.Wang Q, et al. Lymph node-targeting nanovaccines for cancer immunotherapy. J. Control Rel. 2022;351:102–122. doi: 10.1016/j.jconrel.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 270.Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer. 2018;65:e27227. doi: 10.1002/pbc.27227. [DOI] [PubMed] [Google Scholar]
- 271.Dallal RM, et al. Paucity of dendritic cells in pancreatic cancer. Surgery. 2002;131:135–138. doi: 10.1067/msy.2002.119937. [DOI] [PubMed] [Google Scholar]
- 272.Morales E, Olson M, Iglesias F, Luetkens T, Atanackovic D. Targeting the tumor microenvironment of Ewing sarcoma. Immunotherapy. 2021;13:1439–1451. doi: 10.2217/imt-2020-0341. [DOI] [PubMed] [Google Scholar]
- 273.Reinhardt B, et al. Long-term outcomes after gene therapy for adenosine deaminase severe combined immune deficiency. Blood. 2021;138:1304–1316. doi: 10.1182/blood.2020010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mackall CL, et al. A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin. Cancer Res. 2008;14:4850–4858. doi: 10.1158/1078-0432.CCR-07-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ma L, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Krishnadas DK, et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol. Immunother. 2015;64:1251–1260. doi: 10.1007/s00262-015-1731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Letizia M, et al. Store-operated calcium entry controls innate and adaptive immune cell function in inflammatory bowel disease. EMBO Mol. Med. 2022;14:e15687. doi: 10.15252/emmm.202215687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Xiao H, et al. Effect of the cytokine levels in serum on osteosarcoma. Tumour Biol. 2014;35:1023–1028. doi: 10.1007/s13277-013-1136-x. [DOI] [PubMed] [Google Scholar]
- 279.Challagundla N, Shah D, Yadav S, Agrawal-Rajput R. Saga of monokines in shaping tumour-immune microenvironment: Origin to execution. Cytokine. 2022;157:155948. doi: 10.1016/j.cyto.2022.155948. [DOI] [PubMed] [Google Scholar]
- 280.Wang J, Mi S, Ding M, Li X, Yuan S. Metabolism and polarization regulation of macrophages in the tumor microenvironment. Cancer Lett. 2022;543:215766. doi: 10.1016/j.canlet.2022.215766. [DOI] [PubMed] [Google Scholar]
- 281.Burgess M, Tawbi H. Immunotherapeutic approaches to sarcoma. Curr. Treat. Options Oncol. 2015;16:26. doi: 10.1007/s11864-015-0345-5. [DOI] [PubMed] [Google Scholar]
- 282.Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022;19:237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 283.Tuzlak S, et al. Repositioning T(H) cell polarization from single cytokines to complex help. Nat. Immunol. 2021;22:1210–1217. doi: 10.1038/s41590-021-01009-w. [DOI] [PubMed] [Google Scholar]
- 284.Wang T, Secombes CJ. The cytokine networks of adaptive immunity in fish. Fish. Shellfish Immunol. 2013;35:1703–1718. doi: 10.1016/j.fsi.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 285.Kubo S, et al. Early B cell factor 4 modulates FAS-mediated apoptosis and promotes cytotoxic function in human immune cells. Proc. Natl Acad. Sci. USA. 2022;119:e2208522119. doi: 10.1073/pnas.2208522119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zebley CC, et al. Proinflammatory cytokines promote TET2-mediated DNA demethylation during CD8 T cell effector differentiation. Cell Rep. 2021;37:109796. doi: 10.1016/j.celrep.2021.109796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kitazawa T, Streilein JW. Studies on delayed systemic effects of ultraviolet B radiation on the induction of contact hypersensitivity, 3. Dendritic cells from secondary lymphoid organs are deficient in interleukin-12 production and capacity to promote activation and differentiation of T helper type 1 cells. Immunology. 2000;99:296–304. doi: 10.1046/j.1365-2567.2000.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rudman SM, et al. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin. Cancer Res. 2011;17:1998–2005. doi: 10.1158/1078-0432.CCR-10-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Srinoulprasert Y, et al. Differential cytokine profiles produced by anti-epileptic drug re-exposure of peripheral blood mononuclear cells derived from severe anti-epileptic drug patients and non-allergic controls. Cytokine. 2022;157:155951. doi: 10.1016/j.cyto.2022.155951. [DOI] [PubMed] [Google Scholar]
- 290.Buddingh EP, et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol. Immunother. 2011;60:575–586. doi: 10.1007/s00262-010-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Meazza C, et al. Primary metastatic osteosarcoma: results of a prospective study in children given chemotherapy and interleukin-2. Med. Oncol. 2017;34:191. doi: 10.1007/s12032-017-1052-9. [DOI] [PubMed] [Google Scholar]
- 292.Rivoltini L, et al. Phenotypic and functional analysis of lymphocytes infiltrating paediatric tumours, with a characterization of the tumour phenotype. Cancer Immunol. Immunother. 1992;34:241–251. doi: 10.1007/BF01741792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nasr S, et al. A phase I study of interleukin-2 in children with cancer and evaluation of clinical and immunologic status during therapy. A Pediatric Oncology Group Study. Cancer. 1989;64:783–788. doi: 10.1002/1097-0142(19890815)64:4<783::AID-CNCR2820640402>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 294.Rodig, S. J. et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med.10, eaar3342 (2018). [DOI] [PubMed]
- 295.Marin-Acevedo JA, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 2018;11:39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Melaiu O, Lucarini V, Giovannoni R, Fruci D, Gemignani F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. Semin Cancer Biol. 2022;79:18–43. doi: 10.1016/j.semcancer.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 297.Kubo T, et al. Immunopathological basis of immune-related adverse events induced by immune checkpoint blockade therapy. Immunol. Med. 2022;45:108–118. doi: 10.1080/25785826.2021.1976942. [DOI] [PubMed] [Google Scholar]
- 298.Kyu Shim M, Yang S, Sun IC, Kim K. Tumor-activated carrier-free prodrug nanoparticles for targeted cancer Immunotherapy: Preclinical evidence for safe and effective drug delivery. Adv. Drug Deliv. Rev. 2022;183:114177. doi: 10.1016/j.addr.2022.114177. [DOI] [PubMed] [Google Scholar]
- 299.Dhupkar P, Gordon N, Stewart J, Kleinerman ES. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018;7:2654–2664. doi: 10.1002/cam4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shi M, et al. Blockage of the IDO1 pathway by charge-switchable nanoparticles amplifies immunogenic cell death for enhanced cancer immunotherapy. Acta Biomater. 2022;150:353–366. doi: 10.1016/j.actbio.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 301.Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022;15:61. doi: 10.1186/s13045-022-01282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Liu N, Chang CW, Steer CJ, Wang XW, Song G. MicroRNA-15a/16-1 prevents hepatocellular carcinoma by disrupting the communication between Kupffer cells and regulatory T cells. Gastroenterology. 2022;162:575–589. doi: 10.1053/j.gastro.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 303.Serr I, Kral M, Scherm MG, Daniel C. Advances in human immune system mouse models for personalized treg-based immunotherapies. Front. Immunol. 2021;12:643544. doi: 10.3389/fimmu.2021.643544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Onda M, Kobayashi K, Pastan I. Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc. Natl. Acad. Sci. USA. 2019;116:4575–4582. doi: 10.1073/pnas.1820388116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hong H, et al. Depletion of CD4+CD25+ regulatory T cells enhances natural killer T cell-mediated anti-tumour immunity in a murine mammary breast cancer model. Clin. Exp. Immunol. 2010;159:93–99. doi: 10.1111/j.1365-2249.2009.04018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Atif, S. M., Mack, D. G., Martin, A. K. & Fontenot, A. P. Protective role of tissue-resident Tregs in a murine model of beryllium-induced disease. JCI Insight 7, e156098 (2022). [DOI] [PMC free article] [PubMed]
- 307.Mijnheer G, et al. Conserved human effector Treg cell transcriptomic and epigenetic signature in arthritic joint inflammation. Nat. Commun. 2021;12:2710. doi: 10.1038/s41467-021-22975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Amoozgar Z, et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat. Commun. 2021;12:2582. doi: 10.1038/s41467-021-22885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Liang Y, et al. Blockade of PD-1/PD-L1 increases effector T cells and aggravates murine chronic graft-versus-host disease. Int. Immunopharmacol. 2022;110:109051. doi: 10.1016/j.intimp.2022.109051. [DOI] [PubMed] [Google Scholar]
- 310.Wagner LM, Adams VR. Targeting the PD-1 pathway in pediatric solid tumors and brain tumors. Onco. Targets Ther. 2017;10:2097–2106. doi: 10.2147/OTT.S124008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yoshida K, et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer. 2020;20:25. doi: 10.1186/s12885-019-6499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Thanindratarn P, Dean DC, Nelson SD, Hornicek FJ, Duan Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019;15:100221. doi: 10.1016/j.jbo.2019.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018;18:39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 314.Cascio MJ, et al. Canine osteosarcoma checkpoint expression correlates with metastasis and T-cell infiltrate. Vet. Immunol. Immunopathol. 2021;232:110169. doi: 10.1016/j.vetimm.2020.110169. [DOI] [PubMed] [Google Scholar]
- 315.Shen JK, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol. Res. 2014;2:690–698. doi: 10.1158/2326-6066.CIR-13-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mochizuki Y, et al. Telomerase-specific oncolytic immunotherapy for promoting efficacy of PD-1 blockade in osteosarcoma. Cancer Immunol. Immunother. 2021;70:1405–1417. doi: 10.1007/s00262-020-02774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Flem-Karlsen K, Fodstad Ø, Nunes-Xavier CE. B7-H3 immune checkpoint protein in human cancer. Curr. Med. Chem. 2020;27:4062–4086. doi: 10.2174/0929867326666190517115515. [DOI] [PubMed] [Google Scholar]
- 318.Lee YH, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–1045. doi: 10.1038/cr.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wang Y, et al. Comprehensive surfaceome profiling to identify and validate novel cell-surface targets in Osteosarcoma. Mol. Cancer Ther. 2022;21:903–913. doi: 10.1158/1535-7163.MCT-21-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Talbot LJ, et al. A Novel Orthotopic implantation technique for osteosarcoma produces spontaneous metastases and illustrates dose-dependent efficacy of B7-H3-CAR T Cells. Front. Immunol. 2021;12:691741. doi: 10.3389/fimmu.2021.691741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yin SJ, Wang WJ, Zhang JY. Expression of B7-H3 in cancer tissue during osteosarcoma progression in nude mice. Genet. Mol. Res. 2015;14:14253–14261. doi: 10.4238/2015.November.13.9. [DOI] [PubMed] [Google Scholar]
- 322.Wang L, et al. Roles of coinhibitory molecules B7-H3 and B7-H4 in esophageal squamous cell carcinoma. Tumour Biol. 2016;37:2961–2971. doi: 10.1007/s13277-015-4132-5. [DOI] [PubMed] [Google Scholar]
- 323.Majzner RG, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wang L, et al. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol. 2016;37:14939–14947. doi: 10.1007/s13277-016-5386-2. [DOI] [PubMed] [Google Scholar]
- 325.Gill J, Gorlick R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021;18:609–624. doi: 10.1038/s41571-021-00519-8. [DOI] [PubMed] [Google Scholar]
- 326.Whittle SB, et al. Charting a path for prioritization of novel agents for clinical trials in osteosarcoma: A report from the Children’s Oncology Group New Agents for Osteosarcoma Task Force. Pediatr. Blood Cancer. 2021;68:e29188. doi: 10.1002/pbc.29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wang L, Zhang GC, Kang FB, Zhang L, Zhang YZ. hsa_circ0021347 as a potential target regulated by B7-H3 in Modulating the malignant characteristics of Osteosarcoma. Biomed. Res. Int. 2019;2019:9301989. doi: 10.1155/2019/9301989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Aggarwal, C. et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J. Immunother. Cancer10, e004424 (2022). [DOI] [PMC free article] [PubMed]
- 329.Hińcza-Nowak, K. et al. Immune profiling of medullary thyroid cancer-an opportunity for immunotherapy. Genes12, 1534 (2021). [DOI] [PMC free article] [PubMed]
- 330.Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front. Oncol. 2014;4:385. doi: 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Xiang J, Gu X, Qian S, Chen Z. Graded function of CD80 and CD86 in initiation of T-cell immune response and cardiac allograft survival. Transpl. Int. 2008;21:163–168. doi: 10.1111/j.1432-2277.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- 333.Kennedy A, et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat. Immunol. 2022;23:1365–1378. doi: 10.1038/s41590-022-01289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Heeren, A. M. et al. Immune landscape in vulvar cancer-draining lymph nodes indicates distinct immune escape mechanisms in support of metastatic spread and growth. J. Immunother. Cancer9, e003623 (2021). [DOI] [PMC free article] [PubMed]
- 335.Pinato, D. J. et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J. Immunother. Cancer9, e003311 (2021). [DOI] [PMC free article] [PubMed]
- 336.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 337.Markel JE, et al. Using the spleen as an in vivo systemic immune barometer alongside osteosarcoma disease progression and immunotherapy with α-PD-L1. Sarcoma. 2018;2018:8694397. doi: 10.1155/2018/8694397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wang SD, et al. The role of CTLA-4 and PD-1 in anti-tumor immune response and their potential efficacy against osteosarcoma. Int. Immunopharmacol. 2016;38:81–89. doi: 10.1016/j.intimp.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 339.Liu S, Geng P, Cai X, Wang J. Comprehensive evaluation of the cytotoxic T-lymphocyte antigen-4 gene polymorphisms in risk of bone sarcoma. Genet. Test. Mol. Biomark. 2014;18:574–579. doi: 10.1089/gtmb.2014.0023. [DOI] [PubMed] [Google Scholar]
- 340.He J, et al. Association between CTLA-4 genetic polymorphisms and susceptibility to osteosarcoma in Chinese Han population. Endocrine. 2014;45:325–330. doi: 10.1007/s12020-013-0050-8. [DOI] [PubMed] [Google Scholar]
- 341.Hingorani P, et al. Increased CTLA-4(+) T cells and an increased ratio of monocytes with loss of class II (CD14(+)HLA-DR(lo/neg)) found in aggressive pediatric sarcoma patients. J. Immunother. Cancer. 2015;3:35. doi: 10.1186/s40425-015-0082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Deppong CM, et al. CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-β. J. Immunol. 2013;191:3082–3089. doi: 10.4049/jimmunol.1300830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Merchant MS, et al. Phase I Clinical trial of Ipilimumab in pediatric patients with advanced solid tumors. Clin. Cancer Res. 2016;22:1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Carnevale J, et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature. 2022;609:174–182. doi: 10.1038/s41586-022-05126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Nguyen, A. et al. HDACi promotes inflammatory remodeling of the tumor microenvironment to enhance epitope spreading and antitumor immunity. J. Clin. Invest.132, e159283 (2022). [DOI] [PMC free article] [PubMed]
- 346.Huang R, et al. GP96 and SMP30 protein priming of dendritic cell vaccination induces a more potent CTL Response against Hepatoma. J. Health. Eng. 2022;2022:2518847. doi: 10.1155/2022/2518847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Bolhassani A, et al. Modified DCs and MSCs with HPV E7 antigen and small HSPS: Which one is the most potent strategy for eradication of tumors? Mol. Immunol. 2019;108:102–110. doi: 10.1016/j.molimm.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 348.Qi T, McGrath K, Ranganathan R, Dotti G, Cao Y. Cellular kinetics: A clinical and computational review of CAR-T cell pharmacology. Adv. Drug Deliv. Rev. 2022;188:114421. doi: 10.1016/j.addr.2022.114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Elnaggar M, et al. Triple MAPK inhibition salvaged a relapsed post-BCMA CAR-T cell therapy multiple myeloma patient with a BRAF V600E subclonal mutation. J. Hematol. Oncol. 2022;15:109. doi: 10.1186/s13045-022-01330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yee C, Lizee GA. Personalized therapy: tumor antigen discovery for adoptive cellular therapy. Cancer J. 2017;23:144–148. doi: 10.1097/PPO.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 351.Wang W, et al. Hepatobiliary Tumor Organoids reveal HLA class I neoantigen landscape and antitumoral activity of neoantigen peptide enhanced with immune checkpoint inhibitors. Adv. Sci. 2022;9:e2105810. doi: 10.1002/advs.202105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Salawu A, et al. A Phase 2 trial of Afatinib in patients with solid tumors that harbor genomic aberrations in the HER family: The MOBILITY3 Basket Study. Target Oncol. 2022;17:271–281. doi: 10.1007/s11523-022-00884-z. [DOI] [PubMed] [Google Scholar]
- 353.Ahmed N, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric antigen receptor-modified T cells for the immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Geary RL, Corrigan LR, Carney DN, Higgins MJ. Osteosarcoma and second malignant neoplasms: a case series. Ir. J. Med. Sci. 2019;188:1163–1167. doi: 10.1007/s11845-019-02027-2. [DOI] [PubMed] [Google Scholar]
- 355.Huang G, et al. Genetically modified T cells targeting interleukin-11 receptor α-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 2012;72:271–281. doi: 10.1158/0008-5472.CAN-11-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yang Q, et al. Membrane-anchored and tumor-targeted IL12 (attIL12)-PBMC therapy for osteosarcoma. Clin. Cancer Res. 2022;28:3862–3873. doi: 10.1158/1078-0432.CCR-22-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Davila ML, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Théoleyre S, et al. Phenotypic and functional analysis of lymphocytes infiltrating osteolytic tumors: use as a possible therapeutic approach of osteosarcoma. BMC Cancer. 2005;5:123. doi: 10.1186/1471-2407-5-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Wang Y, et al. Anti-CD166/4-1BB chimeric antigen receptor T cell therapy for the treatment of osteosarcoma. J. Exp. Clin. Cancer Res. 2019;38:168. doi: 10.1186/s13046-019-1147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhao Q, et al. Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons. Front. Immunol. 2021;12:658753. doi: 10.3389/fimmu.2021.658753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Brameshuber M, et al. Monomeric TCRs drive T cell antigen recognition. Nat. Immunol. 2018;19:487–496. doi: 10.1038/s41590-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Muthana M, et al. Macrophage delivery of an oncolytic virus abolishes tumor regrowth and metastasis after chemotherapy or irradiation. Cancer Res. 2013;73:490–495. doi: 10.1158/0008-5472.CAN-12-3056. [DOI] [PubMed] [Google Scholar]
- 365.Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sarnaik AA, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 2021;39:2656–2666. doi: 10.1200/JCO.21.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Wang C, Li M, Wei R, Wu J. Adoptive transfer of TILs plus anti-PD1 therapy: An alternative combination therapy for treating metastatic osteosarcoma. J. Bone Oncol. 2020;25:100332. doi: 10.1016/j.jbo.2020.100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J. Immunother. Cancer. 2015;3:21. doi: 10.1186/s40425-015-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.D’Angelo SP, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Krupka C, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30:484–491. doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
- 371.Sun C, Dotti G, Savoldo B. Utilizing cell-based therapeutics to overcome immune evasion in hematologic malignancies. Blood. 2016;127:3350–3359. doi: 10.1182/blood-2015-12-629089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Tabata, R., Chi, S., Yuda, J. & Minami, Y. Emerging immunotherapy for acute myeloid leukemia. Int. J. Mol. Sci.22, 1944 (2021). [DOI] [PMC free article] [PubMed]
- 373.Sierro SR, et al. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur. J. Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 374.Fu J, et al. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74:4042–4052. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Houser KV, et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nat. Med. 2022;28:383–391. doi: 10.1038/s41591-021-01660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Wang Z, Li B, Ren Y, Ye Z. T-cell-based immunotherapy for Osteosarcoma: challenges and opportunities. Front. Immunol. 2016;7:353. doi: 10.3389/fimmu.2016.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Chapuis AG, et al. T-cell Therapy using Interleukin-21-primed Cytotoxic T-Cell Lymphocytes combined with Cytotoxic T-Cell Lymphocyte Antigen-4 blockade results in long-term cell persistence and durable tumor regression. J. Clin. Oncol. 2016;34:3787–3795. doi: 10.1200/JCO.2015.65.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Fulgenzi CAM, et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: A network metanalysis of phase III trials. Eur. J. Cancer. 2022;174:57–67. doi: 10.1016/j.ejca.2022.06.058. [DOI] [PubMed] [Google Scholar]
- 380.Ju, F. et al. Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade. J. Immunother. Cancer10, e004762 (2022). [DOI] [PMC free article] [PubMed]
- 381.Mitchell TC, et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037) J. Clin. Oncol. 2018;36:3223–3230. doi: 10.1200/JCO.2018.78.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Long GV, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–1097. doi: 10.1016/S1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 383.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kushner BH, et al. Humanized 3F8 anti-GD2 Monoclonal antibody dosing with granulocyte-macrophage colony-stimulating factor in patients with resistant neuroblastoma: A Phase 1 Clinical trial. JAMA Oncol. 2018;4:1729–1735. doi: 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhu W, et al. Anti-ganglioside GD2 monoclonal antibody synergizes with cisplatin to induce endoplasmic reticulum-associated apoptosis in osteosarcoma cells. Pharmazie. 2018;73:80–86. doi: 10.1691/ph.2018.7836. [DOI] [PubMed] [Google Scholar]
- 386.Buondonno I, et al. Endoplasmic reticulum-targeting doxorubicin: a new tool effective against doxorubicin-resistant osteosarcoma. Cell Mol. Life Sci. 2019;76:609–625. doi: 10.1007/s00018-018-2967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Xie, L. et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial. J. Immunother. Cancer8, e000798 (2020). [DOI] [PMC free article] [PubMed]
- 388.Regan DP, et al. Losartan Blocks Osteosarcoma-elicited monocyte recruitment, and combined with the kinase inhibitor toceranib, exerts significant clinical benefit in canine metastatic Osteosarcoma. Clin. Cancer Res. 2022;28:662–676. doi: 10.1158/1078-0432.CCR-21-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 390.Yamada N, et al. Immunotherapy with interleukin-18 in combination with preoperative chemotherapy with ifosfamide effectively inhibits postoperative progression of pulmonary metastases in a mouse osteosarcoma model. Tumour Biol. 2009;30:176–184. doi: 10.1159/000236410. [DOI] [PubMed] [Google Scholar]
- 391.He X, Lin H, Yuan L, Li B. Combination therapy with L-arginine and α-PD-L1 antibody boosts immune response against osteosarcoma in immunocompetent mice. Cancer Biol. Ther. 2017;18:94–100. doi: 10.1080/15384047.2016.1276136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kawano M, et al. Dendritic cells combined with doxorubicin induces immunogenic cell death and exhibits antitumor effects for osteosarcoma. Oncol. Lett. 2016;11:2169–2175. doi: 10.3892/ol.2016.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Zhang Y, et al. Hyaluronate-based self-stabilized nanoparticles for immunosuppression reversion and immunochemotherapy in osteosarcoma treatment. ACS Biomater. Sci. Eng. 2021;7:1515–1525. doi: 10.1021/acsbiomaterials.1c00081. [DOI] [PubMed] [Google Scholar]
- 394.Ramos-Cardona XE, Luo W, Mohammed SI. Advances and challenges of CAR T therapy and suitability of animal models (Review) Mol. Clin. Oncol. 2022;17:134. doi: 10.3892/mco.2022.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wu L, et al. Biomimetic nanocarriers guide extracellular ATP Homeostasis to remodel energy metabolism for activating innate and adaptive immunity system. Adv. Sci. 2022;9:e2105376. doi: 10.1002/advs.202105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Gao, S., Yang, X., Xu, J., Qiu, N. & Zhai, G. Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: the horizons in cancer treatment. ACS Nano15, 12567–12603 (2021). [DOI] [PubMed]
- 397.Li Q, et al. A Three-in-One Immunotherapy Nanoweapon via Cascade-amplifying cancer-immunity cycle against tumor metastasis, relapse, and postsurgical regrowth. Nano Lett. 2019;19:6647–6657. doi: 10.1021/acs.nanolett.9b02923. [DOI] [PubMed] [Google Scholar]
- 398.Zhang D, et al. Cold to Hot: Rational design of a minimalist multifunctional photo-immunotherapy nanoplatform toward boosting immunotherapy capability. ACS Appl. Mater. Interfaces. 2019;11:32633–32646. doi: 10.1021/acsami.9b09568. [DOI] [PubMed] [Google Scholar]
- 399.Zhou S, et al. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: Four birds with one stone. J. Control Rel. 2020;328:617–630. doi: 10.1016/j.jconrel.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 400.Ren X, et al. An injectable hydrogel using an immunomodulating gelator for amplified tumor immunotherapy by blocking the arginase pathway. Acta Biomater. 2021;124:179–190. doi: 10.1016/j.actbio.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 401.Vitale M, et al. Oncolytic adenoviral vector-mediated expression of an Anti-PD-L1-scFv improves anti-tumoral efficacy in a Melanoma Mouse Model. Front. Oncol. 2022;12:902190. doi: 10.3389/fonc.2022.902190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem. Int. Ed. Engl. 2019;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Buondonno I, et al. Mitochondria-targeted Doxorubicin: A new therapeutic strategy against Doxorubicin-Resistant Osteosarcoma. Mol. Cancer Ther. 2016;15:2640–2652. doi: 10.1158/1535-7163.MCT-16-0048. [DOI] [PubMed] [Google Scholar]
- 404.Kepp O, Senovilla L, Kroemer G. Immunogenic cell death inducers as anticancer agents. Oncotarget. 2014;5:5190–5191. doi: 10.18632/oncotarget.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Jin, J. et al. Mitochondria-targeting polymer Micelle of Dichloroacetate induced pyroptosis to enhance osteosarcoma immunotherapy. ACS Nano, 16, 10327–10340 (2022). [DOI] [PubMed]
- 406.Ge YX, et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials. 2022;282:121407. doi: 10.1016/j.biomaterials.2022.121407. [DOI] [PubMed] [Google Scholar]
- 407.Liao J, Han R, Wu Y, Qian Z. Review of a new bone tumor therapy strategy based on bifunctional biomaterials. Bone Res. 2021;9:18. doi: 10.1038/s41413-021-00139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Yu W, et al. A review and outlook in the treatment of osteosarcoma and other deep tumors with photodynamic therapy: from basic to deep. Oncotarget. 2017;8:39833–39848. doi: 10.18632/oncotarget.16243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Huang X, et al. Rationally designed Heptamethine Cyanine photosensitizers that amplify tumor-specific endoplasmic reticulum stress and boost antitumor immunity. Small. 2022;18:e2202728. doi: 10.1002/smll.202202728. [DOI] [PubMed] [Google Scholar]
- 410.Wang C, et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014;26:8154–8162. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 411.Lou H, et al. A small-molecule based organic nanoparticle for photothermal therapy and near-infrared-iib imaging. ACS Appl. Mater. Interfaces. 2022;14:35454–35465. doi: 10.1021/acsami.2c11706. [DOI] [PubMed] [Google Scholar]
- 412.Huang X, et al. Black phosphorus-synergic nitric oxide nanogasholder spatiotemporally regulates tumor microenvironments for self-amplifying immunotherapy. ACS Appl Mater. Interfaces. 2022;14:37466–37477. doi: 10.1021/acsami.2c10098. [DOI] [PubMed] [Google Scholar]
- 413.Guevara ML, Persano F, Persano S. Nano-immunotherapy: Overcoming tumour immune evasion. Semin Cancer Biol. 2021;69:238–248. doi: 10.1016/j.semcancer.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 414.Tian, H. et al. A targeted nanomodulator capable of manipulating tumor microenvironment against metastasis. J. Control. Release348, 590-600 (2022). [DOI] [PubMed]
- 415.Zhu M, et al. Bioinspired design of seco-chlorin photosensitizers to overcome phototoxic effects in photodynamic therapy. Angew. Chem. Int. Ed. Engl. 2022;61:e202204330. doi: 10.1002/anie.202204330. [DOI] [PubMed] [Google Scholar]
- 416.Ren Q, Yi C, Pan J, Sun X, Huang X. Smart Fe(3)O(4)@ZnO core-shell nanophotosensitizers potential for combined chemo and photodynamic skin cancer therapy controlled by UVA radiation. Int. J. Nanomed. 2022;17:3385–3400. doi: 10.2147/IJN.S372377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Li Z, et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv. Sci. 2022;9:e2201734. doi: 10.1002/advs.202201734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Alves, C. G. et al. Heptamethine cyanine-loaded nanomaterials for cancer immuno-photothermal/photodynamic therapy: a review. Pharmaceutics14, 1015 (2022). [DOI] [PMC free article] [PubMed]
- 419.Chen Q, Chen M, Liu Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019;48:5506–5526. doi: 10.1039/C9CS00271E. [DOI] [PubMed] [Google Scholar]
- 420.Liu Y, et al. In situ tumor vaccination with calcium-linked degradable coacervate nanocomplex co-delivering photosensitizer and TLR7/8 agonist to trigger effective anti-tumor immune responses. Adv. Mater. 2022;11:e2102781. doi: 10.1002/adhm.202102781. [DOI] [PubMed] [Google Scholar]
- 421.Zhu L, Meng D, Wang X, Chen X. Ferroptosis-driven nanotherapeutics to reverse drug resistance in tumor microenvironment. ACS Appl. Bio Mater. 2022;5:2481–2506. doi: 10.1021/acsabm.2c00199. [DOI] [PubMed] [Google Scholar]
- 422.Yanase S, et al. Enhancement of the effect of 5-aminolevulinic acid-based photodynamic therapy by simultaneous hyperthermia. Int. J. Oncol. 2005;27:193–201. [PubMed] [Google Scholar]
- 423.Ding M, et al. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022;149:334–346. doi: 10.1016/j.actbio.2022.06.041. [DOI] [PubMed] [Google Scholar]
- 424.Li, Z. et al. Immunogenic cell death augmented by manganese zinc sulfide nanoparticles for metastatic Melanoma Immunotherapy. ACS Nano16, 15471–15483 (2022). [DOI] [PubMed]
- 425.Li, X. et al. Protein-delivering nanocomplexes with Fenton reaction-triggered cargo release to boost cancer immunotherapy. ACS Nano16, 14982–14999 (2022). [DOI] [PubMed]
- 426.Xiong G, et al. Near-Infrared-II light-induced mild Hyperthermia activate Cisplatin-Artemisinin nanoparticle for enhanced chemo/chemodynamic therapy and immunotherapy. Small Methods. 2022;6:e2200379. doi: 10.1002/smtd.202200379. [DOI] [PubMed] [Google Scholar]
- 427.Fu L, Zhang W, Zhou X, Fu J, He C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact. Mater. 2022;17:221–233. doi: 10.1016/j.bioactmat.2022.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Jin T, Wu H, Wang Y, Peng H. Capsaicin induces immunogenic cell death in human osteosarcoma cells. Exp. Ther. Med. 2016;12:765–770. doi: 10.3892/etm.2016.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Mori K, Rédini F, Gouin F, Cherrier B, Heymann D. Osteosarcoma: current status of immunotherapy and future trends (Review) Oncol. Rep. 2006;15:693–700. [PubMed] [Google Scholar]
- 430.Leleux J, Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv. Health. Mater. 2013;2:72–94. doi: 10.1002/adhm.201200268. [DOI] [PubMed] [Google Scholar]
- 431.Musetti S, Huang L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy. ACS Nano. 2018;12:11740–11755. doi: 10.1021/acsnano.8b05893. [DOI] [PubMed] [Google Scholar]
- 432.Lybaert L, Vermaelen K, De Geest BG, Nuhn L. Immunoengineering through cancer vaccines - A personalized and multi-step vaccine approach towards precise cancer immunity. J. Control Rel. 2018;289:125–145. doi: 10.1016/j.jconrel.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 433.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Maiti, G. et al. Matrix lumican endocytosed by immune cells controls receptor ligand trafficking to promote TLR4 and restrict TLR9 in sepsis. Proc. Natl Acad. Sci. USA118, e2100999118 (2021). [DOI] [PMC free article] [PubMed]
- 435.Shen CF, et al. Innate immune responses of vaccinees determine early neutralizing antibody production after ChAdOx1nCoV-19 vaccination. Front. Immunol. 2022;13:807454. doi: 10.3389/fimmu.2022.807454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Min Y, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell Activity: A case for synergistic therapies. Cancer Cell. 2017;31:311–325. doi: 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Li Q, et al. Elastic nanovaccine enhances dendritic cell-mediated tumor immunotherapy. Small. 2022;18:e2201108. doi: 10.1002/smll.202201108. [DOI] [PubMed] [Google Scholar]
- 439.Tuohy JL, et al. Assessment of a novel nanoparticle hyperthermia therapy in a murine model of osteosarcoma. Vet. Surg. 2018;47:1021–1030. doi: 10.1111/vsu.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Richard-Fiardo P, et al. Effect of fractalkine-Fc delivery in experimental lung metastasis using DNA/704 nanospheres. Cancer Gene Ther. 2011;18:761–772. doi: 10.1038/cgt.2011.42. [DOI] [PubMed] [Google Scholar]
- 441.Wang G, et al. The Anti-fibrosis drug Pirfenidone modifies the immunosuppressive tumor microenvironment and prevents the progression of renal cell carcinoma by inhibiting tumor autocrine TGF-β. Cancer Biol. Ther. 2022;23:150–162. doi: 10.1080/15384047.2022.2035629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zhang, J. et al. Arginine supplementation targeting tumor-killing immune cells reconstructs the tumor microenvironment and enhances the antitumor immune response. ACS Nano, 16, 12964–12978 (2022). [DOI] [PubMed]
- 443.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Disco. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 444.Wang, M. et al. Pyroptosis remodeling tumor microenvironment to enhance pancreatic cancer immunotherapy driven by membrane anchoring photosensitizer. Adv. Sci.9, e2202914 (2022). [DOI] [PMC free article] [PubMed]
- 445.Wu P, et al. Manipulating offense and defense signaling to fight cold tumors with carrier-free nanoassembly of fluorinated Prodrug and siRNA. Adv. Mater. 2022;34:e2203019. doi: 10.1002/adma.202203019. [DOI] [PubMed] [Google Scholar]
- 446.Björnmalm M, Thurecht KJ, Michael M, Scott AM, Caruso F. Bridging bio-nano science and cancer nanomedicine. ACS Nano. 2017;11:9594–9613. doi: 10.1021/acsnano.7b04855. [DOI] [PubMed] [Google Scholar]
- 447.Li T, et al. Spatially targeting and regulating tumor-associated macrophages using a raspberry-like micellar system sensitizes pancreatic cancer chemoimmunotherapy. Nanoscale. 2022;14:13098–13112. doi: 10.1039/D2NR03053E. [DOI] [PubMed] [Google Scholar]
- 448.Xia T, et al. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses. J. Hematol. Oncol. 2022;15:37. doi: 10.1186/s13045-022-01253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017;46:3830–3852. doi: 10.1039/C6CS00592F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Bhatia SN, Chen X, Dobrovolskaia MA, Lammers T. Cancer nanomedicine. Nat. Rev. Cancer. 2022;22:550–556. doi: 10.1038/s41568-022-00496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Sanseviero E, Kim R, Gabrilovich DI. Isolation and phenotyping of splenic myeloid-derived suppressor cells in murine cancer Models. Methods Mol. Biol. 2021;2236:19–28. doi: 10.1007/978-1-0716-1060-2_3. [DOI] [PubMed] [Google Scholar]
- 452.Chen H, et al. METTL3 inhibits antitumor immunity by targeting m(6)A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology. 2022;163:891–907. doi: 10.1053/j.gastro.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 453.Fan Q, et al. Nanoengineering a metal-organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2,3-dioxygenase and myeloid-derived suppressor cells. J. Exp. Clin. Cancer Res. 2022;41:162. doi: 10.1186/s13046-022-02372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Barth BM, et al. PhotoImmunoNanoTherapy reveals an anticancer role for sphingosine kinase 2 and dihydrosphingosine-1-phosphate. ACS Nano. 2013;7:2132–2144. doi: 10.1021/nn304862b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Guo R, et al. NIR responsive injectable nanocomposite thermogel system against osteosarcoma recurrence. Macromol. Rapid Commun. 2022;43:e2200255. doi: 10.1002/marc.202200255. [DOI] [PubMed] [Google Scholar]
- 456.Yu W, et al. Autophagy inhibitor enhance ZnPc/BSA nanoparticle induced photodynamic therapy by suppressing PD-L1 expression in osteosarcoma immunotherapy. Biomaterials. 2019;192:128–139. doi: 10.1016/j.biomaterials.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 457.Raglow Z, et al. Targeting glycans for CAR therapy: The advent of sweet CARs. Mol. Ther. 2022;30:2881–2890. doi: 10.1016/j.ymthe.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Klatt MG, et al. A TCR mimic CAR T cell specific for NDC80 is broadly reactive with solid tumors and hematologic malignancies. Blood. 2022;140:861–874. doi: 10.1182/blood.2021012882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Pant, A. & Jackson, C. M. Supercharged chimeric antigen receptor T cells in solid tumors. J. Clin. Invest.132, e162322 (2022). [DOI] [PMC free article] [PubMed]
- 460.Li H, et al. Scattered seeding of CAR T cells in solid tumors augments anticancer efficacy. Natl. Sci. Rev. 2022;9:nwab172. doi: 10.1093/nsr/nwab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Luo M, Zhang H, Zhu L, Xu Q, Gao Q. CAR-T cell therapy: challenges and optimization. Crit. Rev. Immunol. 2021;41:77–87. doi: 10.1615/CritRevImmunol.2021037253. [DOI] [PubMed] [Google Scholar]
- 462.Zuo, Y. H., Zhao, X. P. & Fan, X. X. Nanotechnology-based chimeric antigen receptor T-cell therapy in treating solid tumor. Pharmacol. Res.184, 106454 (2022). [DOI] [PubMed]
- 463.Wang G, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol. Ther. 2023;31:134–153. doi: 10.1016/j.ymthe.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Young RM, Engel NW, Uslu U, Wellhausen N, June CH. Next-generation CAR T-cell therapies. Cancer Discov. 2022;12:1625–1633. doi: 10.1158/2159-8290.CD-21-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Ma X, et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Tian Y, Li Y, Shao Y, Zhang Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J. Hematol. Oncol. 2020;13:54. doi: 10.1186/s13045-020-00890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Dangaj D, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35:885–900.e810. doi: 10.1016/j.ccell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Chen X, et al. Enhancing adoptive T cell therapy for solid tumor with cell-surface anchored immune checkpoint inhibitor nanogels. Nanomedicine. 2022;45:102591. doi: 10.1016/j.nano.2022.102591. [DOI] [PubMed] [Google Scholar]
- 469.Kiru, L. et al. In vivo imaging of nanoparticle-labeled CAR T cells. Proc. Natl. Acad. Sci. USA119, e2102363119 (2022). [DOI] [PMC free article] [PubMed]
- 470.Song YJ, et al. Immune landscape of the tumor microenvironment identifies prognostic gene signature CD4/CD68/CSF1R in Osteosarcoma. Front. Oncol. 2020;10:1198. doi: 10.3389/fonc.2020.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr. Opin. Pediatr. 2016;28:26–33. doi: 10.1097/MOP.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Song YJ, et al. Gene expression classifier reveals prognostic osteosarcoma microenvironment molecular subtypes. Front. Immunol. 2021;12:623762. doi: 10.3389/fimmu.2021.623762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Yu W, Liu R, Zhou Y, Gao H. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent. Sci. 2020;6:100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Şimşek M, Ataş E, Bağrıaçık E, Günal A, Ünay B. Type 4 hypersensitivity development in a case due to mifamurtide. Turk. J. Pediatr. 2020;62:694–699. doi: 10.24953/turkjped.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 475.van Dongen M, et al. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. Int J. Cancer. 2010;127:899–909. doi: 10.1002/ijc.25113. [DOI] [PubMed] [Google Scholar]
- 476.Wolf-Dennen K, Gordon N, Kleinerman ES. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology. 2020;9:1747677. doi: 10.1080/2162402X.2020.1747677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Tsagozis P, Eriksson F, Pisa P. Zoledronic acid modulates antitumoral responses of prostate cancer-tumor associated macrophages. Cancer Immunol. Immunother. 2008;57:1451–1459. doi: 10.1007/s00262-008-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Punzo F, et al. Mifamurtide and TAM-like macrophages: effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget. 2020;11:687–698. doi: 10.18632/oncotarget.27479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Ségaliny AI, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int. J. Cancer. 2015;137:73–85. doi: 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]
- 481.Guan Y, et al. Inhibition of IL-18-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy against osteosarcoma cancer. J. Bone Oncol. 2017;9:59–64. doi: 10.1016/j.jbo.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Hu, J. et al. Cell membrane-anchored and tumor-targeted IL-12 (attIL12)-T cell therapy for eliminating large and heterogeneous solid tumors. J. Immunother. Cancer10, e003633 (2022). [DOI] [PMC free article] [PubMed]
- 483.Jeong, S. N. & Yoo, S. Y. Novel oncolytic virus armed with cancer suicide gene and normal vasculogenic gene for improved anti-tumor activity. Cancers12, 1070 (2020). [DOI] [PMC free article] [PubMed]
- 484.Yahiro K, et al. Activation of TLR4 signaling inhibits progression of osteosarcoma by stimulating CD8-positive cytotoxic lymphocytes. Cancer Immunol. Immunother. 2020;69:745–758. doi: 10.1007/s00262-020-02508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Xin Yu J, et al. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat. Rev. Drug Discov. 2020;19:163–164. doi: 10.1038/d41573-019-00182-w. [DOI] [PubMed] [Google Scholar]
- 486.Gao W, Zhou J, Ji B. Evidence of Interleukin 21 reduction in Osteosarcoma patients due to PD-1/PD-L1-mediated suppression of follicular helper T cell functionality. DNA Cell Biol. 2017;36:794–800. doi: 10.1089/dna.2017.3669. [DOI] [PubMed] [Google Scholar]
- 487.Alsaab HO, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharm. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022;19:37–50. doi: 10.1038/s41571-021-00552-7. [DOI] [PubMed] [Google Scholar]
- 490.Huang W, Ran R, Shao B, Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res. Treat. 2019;178:17–33. doi: 10.1007/s10549-019-05371-0. [DOI] [PubMed] [Google Scholar]
- 491.Chi Y, et al. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control Rel. 2017;261:113–125. doi: 10.1016/j.jconrel.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 492.Yin J, et al. MXene-based hydrogels endow polyetheretherketone with effective osteogenicity and combined treatment of osteosarcoma and bacterial infection. ACS Appl Mater. Interfaces. 2020;12:45891–45903. doi: 10.1021/acsami.0c14752. [DOI] [PubMed] [Google Scholar]
- 493.Yang F, Wen X, Ke QF, Xie XT, Guo YP. pH-responsive mesoporous ZSM-5 zeolites/chitosan core-shell nanodisks loaded with doxorubicin against osteosarcoma. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018;85:142–153. doi: 10.1016/j.msec.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 494.Huang X, et al. Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma. Acta Biomater. 2020;109:229–243. doi: 10.1016/j.actbio.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 495.Zhang Y, et al. 3D-printed bioceramic scaffolds with a Fe(3)O(4)/graphene oxide nanocomposite interface for hyperthermia therapy of bone tumor cells. J. Mater. Chem. B. 2016;4:2874–2886. doi: 10.1039/C6TB00390G. [DOI] [PubMed] [Google Scholar]
- 496.Yue J, et al. Bull serum albumin coated Au@Agnanorods as SERS probes for ultrasensitive osteosarcoma cell detection. Talanta. 2016;150:503–509. doi: 10.1016/j.talanta.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 497.Miao Y, et al. Single-walled carbon nanotube: One specific inhibitor of cancer stem cells in osteosarcoma upon downregulation of the TGFβ1 signaling. Biomaterials. 2017;149:29–40. doi: 10.1016/j.biomaterials.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 498.Li Y, Hou H, Zhang P, Zhang Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020;27:1044–1053. doi: 10.1080/10717544.2020.1785049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Gonçalves M, et al. Dendrimer-assisted formation of fluorescent nanogels for drug delivery and intracellular imaging. Biomacromolecules. 2014;15:492–499. doi: 10.1021/bm401400r. [DOI] [PubMed] [Google Scholar]
- 500.Wang SQ, Zhang Q, Sun C, Liu GY. Ifosfamide-loaded lipid-core-nanocapsules to increase the anticancer efficacy in MG63 osteosarcoma cells. Saudi J. Biol. Sci. 2018;25:1140–1145. doi: 10.1016/j.sjbs.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Wei H, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int. J. Nanomed. 2019;14:8603–8610. doi: 10.2147/IJN.S218988. [DOI] [PMC free article] [PubMed] [Google Scholar]