Abstract
INTRODUCTION
The aim of the study was to investigate the efficacy of rehabilitation programs for bladder disorders in patients with multiple sclerosis (MS) and to guide physicians in delineating therapeutic tools and programs for physiatrists, using the best current strategies.
EVIDENCE ACQUISITION
A search was conducted on PubMed, EMBASE, the Cochrane Library and Web of Science. Studies were eligible if they included adults with bladder disorders related to MS and described specific treatments of rehabilitation interest. The search identified 190,283 articles using the key words “multiple sclerosis” AND “rehabilitation” AND “urinary” OR “bladder,” of which the reviewers analyzed 81 full-texts; 21 publications met the criteria and were included in the systematic review.
EVIDENCE SYNTHESIS
The systematic review identified the specific rehabilitation treatments reported in the current literature. The meta-analysis compared the scores and scales used to quantify bladder disorders due to MS, both before and after rehabilitation or in a comparison with a control group.
CONCLUSIONS
The present study suggests the need of a specific therapeutic protocol, based on the degree of disability and symptom complexity in patients with MS-related neurogenic lower urinary tract dysfunction (NLUTD). Particularly, the meta-analysis shows the effectiveness of peripheral tibial nerve stimulation (PTNS) and pelvic floor muscle training (PFMT) for neurogenic detrusor overactivity (NDO). However, the goal of physiotherapy is to treat incontinence without making urinary retention worse and vice-versa, reducing the loss of urine urgency, while ensuring the emptying of the bladder.
Key words: Multiple sclerosis, Rehabilitation, Urologic diseases
Introduction
Neurogenic bladder or neurogenic lower urinary tract dysfunctions (NLUTD) occurs in up to 80% of patients with multiple sclerosis (MS) at some time during the course of their disease.1
NLUTD may cause failure to store urine (incontinence) and/or failure to empty the bladder (retention), representing a pervasive threat to their overall well-being, and having an impact on the quality of life (QoL) of patients with MS.
Lesions above the pontine micturition center are usually associated with neurogenic detrusor overactivity (NDO), characterized by involuntary detrusor contractions (urgency, frequency, urge incontinence and no or low postvoid residual urine). Urgency is the sudden desire and intense sensation of needing to pass urine; frequency is the need to urinate many times during the day or at night (nocturia) or both, but in normal or less-than-normal volumes; urge incontinence is an involuntary urine leak while having symptoms of urgency.
Medullary lesions (below the pons and above the sacral micturition center) are characterized by NDO with detrusor and external sphincter dyssynergia or DESD (urgency and/or urge urinary incontinence with variable postvoid residual urine), often associated with pelvic floor spasticity.
Infrasacral lesions involving the conus may lead to an absent or diminished detrusor activity (urinary retention), sometimes associated with urinary incontinence due to urethral incompetence and/or pelvic floor muscle weakness.
The most frequent urinary dysfunctions are related to NDO (urgency and urge incontinence)2 in up to 60% of MS patients;3 detrusor sphincter dyssynergia occurs in 35%,3 and detrusor underactivity in 25%.4 Untreated bladder disorders may lead to lower urinary tract (LUT) infections, kidney damage, emotional distress, sleep disturbances, social isolation, and a subsequent loss of QoL.1, 5
Appropriate management of NLUTD requires a multimodal and interdisciplinary approach and could improve the symptoms.6 Rehabilitation or conservative treatment plays a very important role in this field: it includes lifestyle interventions, complementary therapies, anti-incontinence devices, and pads, but in particular behavioral modification, pharmacological modulation, intermittent catheterization, electrical therapies and pelvic floor muscle training (PFMT), as well as peripheral tibial nerve stimulation (PTNS).4, 7
The goal of rehabilitation treatment is to reduce the loss of urgency or urinary incontinence, while ensuring the emptying of the bladder. To do this, it is necessary to establish the correct balance in the treatment of symptoms related to NDO and bladder retention.
The purpose of the present study was to investigate the efficacy of some rehabilitation protocols for the treatment of NLUTD (particularly of NDO) in MS patients, and to define therapeutic strategies by adopting the best tools and rehabilitation programs described in the current literature.
Evidence acquisition
Search strategy
The systematic review and meta-analysis used the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement,8 the Meta-Analyses of Observational Studies (MOOSE) Checklist9 and the population, intervention, comparison, outcome, and study design (PICOS) criteria.10 The search was conducted on PubMed, EMBASE, the Cochrane Library, and Web of Science with the following key words “multiple sclerosis” AND “rehabilitation” AND “urinary” OR “bladder.” In addition, the reference lists of related articles were manually examined for other suitable papers. Studies were eligible if they included adults with bladder disorders related to MS and described specific treatments of rehabilitation interest. In particular, according to the PICOS framework, studies were included for review if they met the following criteria: 1) population of interest (adults with bladder dysfunction); 2) intervention (rehabilitation program and physiatrist directed therapy); 3) comparison (no intervention or alternative therapeutic options); 4) outcomes (referred symptoms, self-reported questionnaires, urodynamic studies); and 5) design (published prospective and retrospective studies, and randomized controlled trials [RCTs]). Moreover, the choose of original English language text was another inclusion criterion.
The exclusion criteria were animal studies, studies involving neurological diseases other than MS, and those not involving urinary symptoms. Any duplicate studies were also excluded. No publication data restrictions were applied,
In our meta-analysis we considered studies in which the authors compared numeric parameters that would be useful for quantifying urinary disorder severity before and after different rehabilitation treatments.
The present review was registered on PROSPERO: CRD42020220072.
Study selection and data collection process
A systematic search of the current literature was performed by two independent reviewers who identify studies that potentially met the inclusion criteria between September 2020 and October 2021. The titles and abstracts from the initial search were screened to identify relevant records and eligible studies. Any full texts selected were then reviewed and included in both the systematic review and the meta-analysis, Potentially, relevant references were screened subsequently. Two authors independently extracted data relating to the formal causation assessment and critically reviewed each included study in detail. A senior author, an expert in bladder and pelvic floor rehabilitation, provided the design, and supervised the drafting of the text.
Data extraction included the following information: names of authors, year of publication, sample characteristics, symptoms, diagnostic processes, rehabilitation program, comparisons and control groups, periodic assessments after treatment and follow-up, and outcomes identified after interventions (Supplementary Digital Material 1: Supplementary Table I). Descriptive features of publication characteristics and patient demographic variables were estimated. In Supplementary Digital Material 2: Supplementary Table II, study data were grouped on the basis of therapeutic strategies and rehabilitation techniques.
Risk of bias
The risk of bias assessment was performed by two independent review authors using the Cochrane risk of bias tool.11 The points for risk of bias included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Their adequacy, categorized as low, unclear, or high risk for each study, was analyzed by all the authors (Supplementary Digital Material 3: Supplementary Table III).
Quality of outcomes
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Guidelines for systematic reviews were used to evaluate the quality of the results.12-16 The rating of the quality of each study’s outcome was determined to indicate the degree of certainty (high, moderate, low, or very low) for the total effect estimates (Supplementary Table III).
Meta-analysis calculations
All statistical analyses were performed using MedCalc (MedCalc Software, Ostend, Belgium) version 18. The inconsistency test (I2) was used to verify the impact of study heterogeneity on the results of the meta-analysis. An I2 value of 50% was considered statistically significant for the included studies.17 We used a random-effect model to estimate the combined effect sizes.18 The Cochrane Collaboration methods were used to determine the quality of the identified studies,19 and publication bias was examined using funnel plots.
Continuous measures of mean values were extracted. The mean differences were estimated, in fact, the included studies used the same rating instruments to measure their outcome; thus, the units of measurement for the outcome of interest were the same across studies. The effect size was based on the summary information collected from the included multiple independent studies, the data were analyzed in two independent groups before and at the end of treatment, without considering the count of events over time, due to lack of specific data.
Evidence synthesis
Study selection
The database search identified 190,283 articles. After the titles and abstracts were screened, the reviewers analyzed 81 full texts. The eligibility of the studies was then assessed independently. A total of 21 publications met all the inclusion criteria and were therefore included in the present systematic review. The systematic review included the 6 articles that were also used for the meta-analysis.
According to grade quality of evidence most of the studies included in our systematic review had a low risk of bias, making the quality of evidence high (Supplementary Table III).
Study characteristics
All 21 prospective and retrospective studies and clinical trials included in the present review involved adults with bladder dysfunctions related to MS. A summary description of the included studies can be found in Supplementary Table I.
All study groups were not homogeneous for relevant general clinical features, such as clinical presentation, localization of demyelinating lesions, disease duration, severity of disability, diagnostic measures used for the assessment of bladder dysfunction, rehabilitation therapy, time of starting therapy, duration of treatment, and follow-up (Supplementary Table I). For sample inhomogeneity, we included only a few studies in the meta-analysis, comparing specific bladder symptoms after three months of the same rehabilitation therapy.
Study selection
Eligibility for inclusion in the present study was assessed independently. A total of 21 publications met the inclusion criteria and were included in the systematic review, of which 6 were used in the meta-analysis. Sixty studies were excluded for the following reasons: 21 did not present any therapeutic procedures, 9 included individuals with diagnoses other than MS, and 23 did not evaluate bladder dysfunctions related to MS. Additionally, case reports (N.=3) and studies not originally written in English (N.=4) were excluded. The study selection flowchart is presented in Figure 1.
Figure 1.
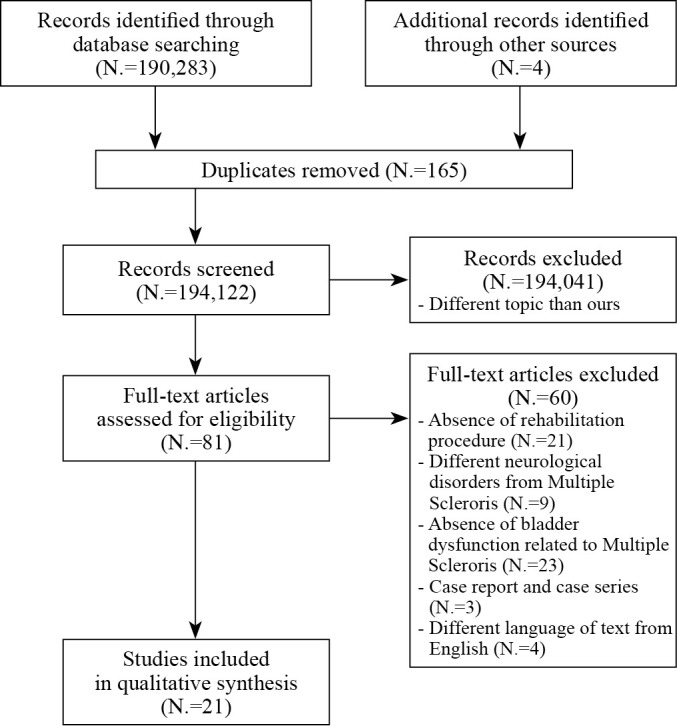
—Flowchart of the process for the literature search and extraction of studies meeting the inclusion criteria.
Participants, interventions, and comparators
The studies included in the present systematic review adhered to the PICOS criteria. This systematic review included original studies regarding the treatment of bladder dysfunction due to MS. The participant selection was consistent for all included studies, as all participants were adults with bladder disorders related to MS. The included studies described rehabilitation interventions, comparing patients with MS-related bladder disorders to healthy controls, or to different rehabilitation protocols or follow-up periods.
The outcomes of the studies in the present review included clinical assessments, diagnostic scales, and cystoscopy results. All of the studies used validated measurement tools and clearly showed their results. The present study was designed as a retrospective and prospective, RCT study, following the recommendations of the Oxford Center for Evidence-Based Medicine (Supplementary Table I). The meta-analysis focused on the quantitative results of rehabilitation treatments that were reported in more than one article.
Outcomes
The main outcome was to define the best therapeutic strategies, based on the rehabilitation techniques described in the available literature (Supplementary Table II). The secondary outcome was to guide physicians in delineating therapeutic tools and programs for physiatrists, using the best current strategies.
Timeframes
Most of the studies assessed the participants at 3 months post-treatment,5, 20-27 single study immediately after therapy,28 and several at other time points post-treatment: 2 months,29 9 weeks,30-32 6 months.20, 29-31, 33 Only 4 studies followed participants for a longer period, with the last assessment after one year,20 and two years post-treatment.34, 35
Comparing studies: subjective and objective bladder outcome measures
Most studies evaluated the validity of peripheral tibial nerve stimulation (PTNS) and pelvic floor muscle training (PFMT) in patients with bladder dysfunctions, after a subjective assessment based on the symptoms, these studies reported significant results in daytime frequency,5, 20-25, 36 nocturia,5, 20-26, 36 urge incontinence,5, 20, 22, 24, 25 voided volume.20-26, 28, 29, 36 Additional symptoms were reported as having improved after specific treatments: urge incontinence after intravaginal electrostimulation33 and voided volume with sacral neuromodulation,34 and neuromuscular electrical stimulation.30
Other evaluation tools are assessment scales that showed subjective improvements in results after specific treatments. The international Consultation on Incontinence Questionnaire Short Form (ICIQ-SF) and the Over-Active Bladder Awareness Tool – 8-item (OAB-V8) showed significant improvements after both PTNS20 and PFMT.24, 27, 37 The Expanded Disability Status Scale (EDSS) showed improvements5, 25, 26, 28, 30, 31, 32 after PFMT and PTNS.21, 36
To obtain objective bladder outcome measures, urodynamic studies and the Power, Endurance, Repetitions, Fast and Every Contraction Timed (PERFECT) scheme are the most used tools. According to urodynamic studies, the maximum cystometric capacity increased after PTNS22, 26, 36 and PFMT,24, 25 cystometric capacity and the reflex volume increased by >30% after PTNS without correlation with PFMT clinical efficiency,26 and no significant differences were recorded in maximum detrusor pressure after PFMT and PTNS.24, 28 PERFECT scheme showed that both PFMT5, 24, 25, 37 and intravaginal electrostimulation33 gave an improvement in power, endurance, repetitions, and contraction time.
Comparing studies: techniques for bladder dysfunction management
PFMT5, 24, 25, 27, 29, 31, 32, 38, 39 and PTNS20-26, 28, 29, 36 were the most used therapies, and for this reason, we analyzed their validity in our meta-analysis. Further studies are needed for other techniques less described in the current literature, such as intravaginal electrical stimulation,31, 33 electromyography (EMG) biofeedback,25, 31, 32, 39 neuromuscular electrical stimulation,25, 30, 31, 39 and sacral neuromodulation.34
In addition to PFMT and PTNS, among the techniques for the treatment of bladder dysfunctions in MS, several rehabilitation programs involved specific exercises to rehabilitate the bladder and pelvic floor muscles.5, 24, 25, 27, 30, 31, 37, 39 Significant results were recorded in voided volume, ICIQ-SF,5, 28 EDSS,5, 25, 28, 35 and PERFECT5, 25 results after rehabilitation training5, 25, 28, 35 and self-guided rehabilitation,5, 25, 28 although the statistical comparison of these outcomes was not analyzed because their samples were not homogeneous (different follow-up period, and/or different treatment).
Meta-analysis
Of the 21 studies included in the present systematic review, six studies were included in the meta-analysis (Supplementary Digital Material 4: Supplementary Table IV, Supplementary Table V, Supplementary Table VI).
Supplementary Table III summarizes the GRADE of the studies included in the meta-analysis, the absence of significative limitations, inconsistency, and incomplete data make the quality of evidence high.
The data included in the meta-analysis showed significant results and validity of PTNS after three months of treatment for the following symptoms: daytime frequency, nocturia, urgency incontinence, and voided volume (P<0.001). No significant results were observed after PFMT in voided volume. After three months of PFMT, endurance and fast contraction portions of the PERFECT scheme showed significant results (P=0.002), which differed from power and repetition (P>0.05). A significant improvement in maximum cystometric capacity (P<0.001) was recorded after three months of PTNS.
Heterogeneity and risk of bias
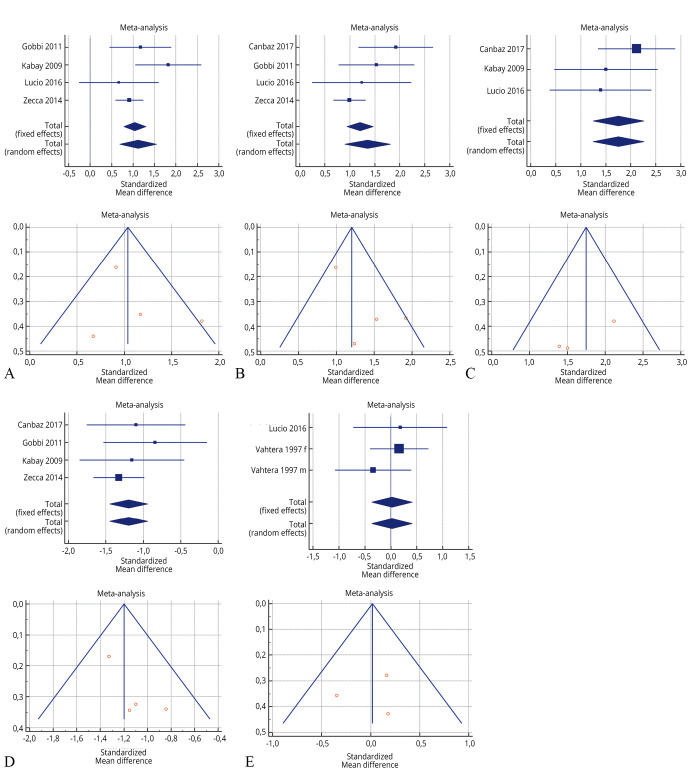
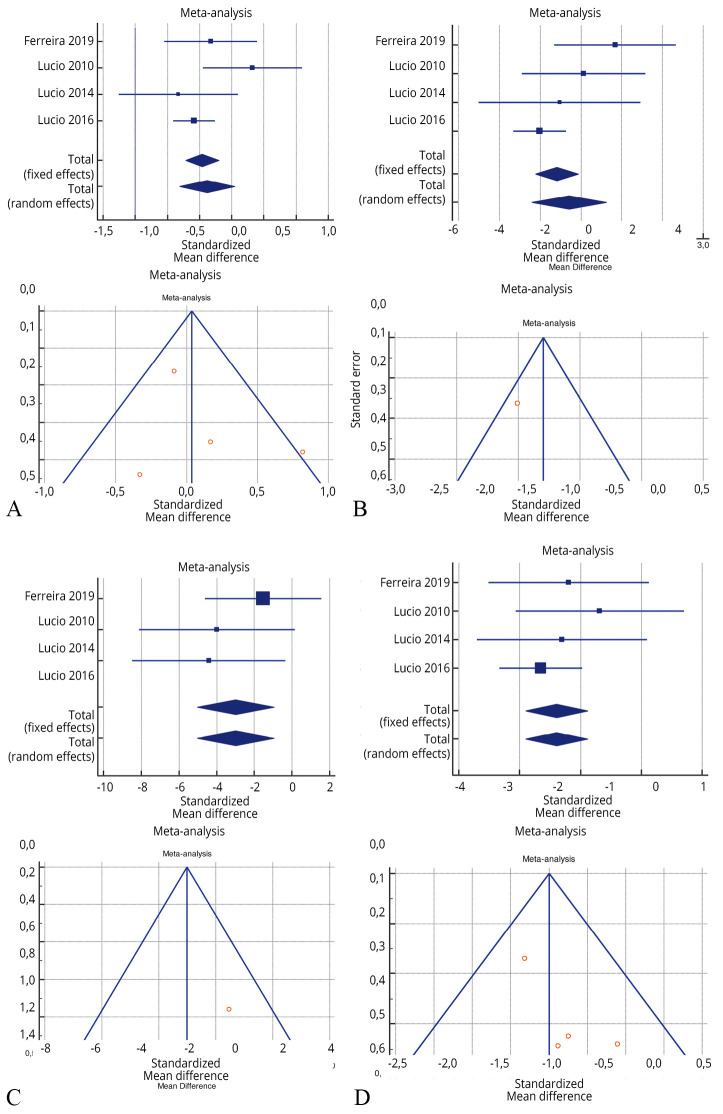
The risk of bias assessment for the individual studies is presented in Supplementary Table III and also in the funnel plot (Figure 2,20, 21, 23, 26, 30, 33 Figure 3,25, 26, 33, 39 Figure 422, 23, 26, 27).
Figure 2.
—Forest plot of Supplementary Table V: A) effectiveness of 3 months of peripheral tibial nerve stimulation in daytime frequency before and after treatment; B) effectiveness of 3 months of peripheral tibial nerve stimulation in nocturia before and after treatment; C) effectiveness of 3 months of peripheral tibial nerve stimulation in urge incontinence before and after treatment; D) effectiveness of 3 months of peripheral tibial nerve stimulation in voided volume before and after treatment; and E) effectiveness of 3 months of pelvic floor muscle training in voided volume before and after treatment.20, 21, 23, 26, 30, 33
Figure 3.
—Forest plot of Supplementary Table VI: effectiveness of 3 months of pelvic floor muscle training in PERFECT scheme (P power, E endurance, R repetitions, F fast contraction, ECT every timed contraction) versus no treatment.25, 26, 33, 39
Figure 4.
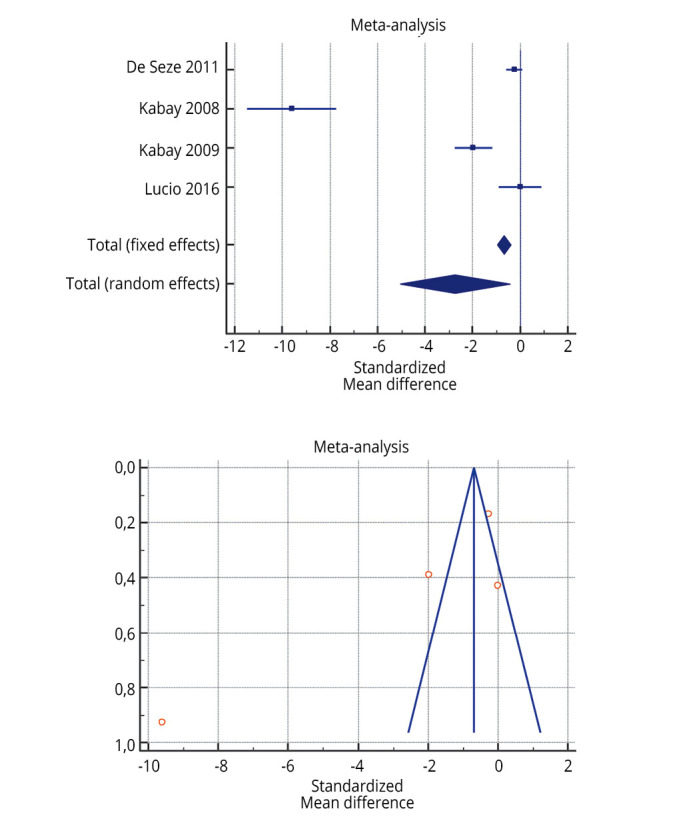
—Forest plot of Supplementary Table VII: effectiveness of 3 months of peripheral tibial nerve stimulation in maximum cystometric capacity before and after treatment.22, 23, 26, 27
As shown in Supplementary Table IV, Supplementary Table V, Supplementary Table VI, the Inconsistency test (I2) verified the impact of study heterogeneity on the results of the meta-analysis. Heterogeneity between studies was very low (I2=0.00%) for urge incontinence and voided volume, low-moderate (I2=46.80-51.49%) for daytime frequency and nocturia, and high for maximum cystometric capacity (I2=97.33%). The I2 was very heterogeneous for the PERFERCT scheme (0.00-95.65%).
Discussion
This systematic review identified the specific rehabilitation therapies used to treat urinary dysfunction in patients with MS, as described in the available literature. The meta-analysis compared the scores and the scales used to quantify MS-related bladder disorders after treatment with various rehabilitation strategies to evaluate the validity of these studies. The data from the meta-analysis highlighted an improvement in symptoms after PTNS, but only partial improvement after PFMT. Some articles reported significant improvements with intravaginal electrical stimulation, EMG biofeedback, neuromuscular electrical stimulation, and sacral neuromodulation, however, because of the excessively heterogeneous samples, a statistical comparison with meta-analysis of these techniques was not performed.
The present systematic review showed that any management of bladder dysfunctions in patients with MS can improve symptoms and QoL. Many studies pointed out that specific counselling and keeping a bladder diary may help patients to better understand and quantify their problems. Additionally, there were several recommendations that may be useful for incontinence, such as drinking water regularly throughout the day (1.5-2 L/day) but not late at night, and not delaying micturition in case of urgency when using aids such as insets, condom urinals, or other devices.
Several studies showed the effectiveness of individualized PFMT,40, 41 bladder rehabilitation programs,5, 24, 38, 40, 41 as well as toilet training.42 For overactive bladder, PFMT may promote an increase in urethral pressure and detrusor relaxation, activating the pudendal-pelvic reflex with pelvic floor muscle contraction.43 Neuromodulation may be used to treat lower urinary tract symptoms, such as urinary incontinence and retention, refractory to medical therapy. Limited data are available for progressive neurologic diseases, such as MS and only a few studies demonstrated the efficacy of these treatments in patients with MS. Stimulation of the lower sensory motor nerves potentiates the somatic afferent branches of sacral spinal roots, inhibiting detrusor overactivity.44, 45 According to the meta-analysis, PTNS may improve daytime frequency, nocturia, urgency episodes, voided volume, and urge incontinence. Intravaginal electrostimulation inhibits the involuntary contraction of the detrusor muscle, resulting in better outcomes than PFMT alone.46 Sacral nerve modulation can be used for refractory symptoms, clinically isolated syndrome and stable relapsing-remitting disease.34 PTNS stimulates the peripheral tibial nerve, which inhibits bladder activity by depolarizing somatic sacral and lumbar afferent fibers.20 PTNS appeared to be effective in improving bothersome symptoms, such as urgency, in more than 80% of patients treated with this method, and also appeared to reduce frequency and urge urinary incontinence, therefore having a positive impact on QoL.26 The efficacy of PTNS appears in the first week of treatment and remains stable when done 20 min daily for 3 months.26 A study highlighted that the neuromodulating effects of electrical stimulation of the posterior tibial nerve had no beneficial acute effects,28 although prolonged percutaneous tibial nerve stimulation may lead to a persistent improvement of lower urinary tract symptoms.37 The high efficiency of PTNS associated with its excellent patient tolerance indicates the benefits of this treatment alone, or in combination with other rehabilitation programs, for neurogenic bladder dysfunction in patients with MS.21-23, 26, 28, 36, 47
The combination of intravaginal electrostimulation with PFMT could be an efficient protocol for improving strength, particularly with improvement in fast fibers that are responsible for vigorous and reflex contractions,46 reducing micturition frequency, urgency, and incontinence. Electrical stimulation of the pelvic floor combined with PFMT and EMG biofeedback constitutes an effective treatment for lower urinary tract dysfunction in males.29
Another important element, described in a few of the studies reviewed, is the role of home exercises in the treatment of bladder dysfunction.27, 32, 37, 46 In this case, the appropriate instruction of patients on how to perform home pelvic floor muscle training is the key for the success of self-guided rehabilitation.27
Limitations of the study
As concerns the limits of this study, the differences in the number of participants, as well as the types and duration of interventions, may have affected the results of the present review; however, PTMS seems to improve daytime frequency, nocturia, urgency episodes, voided volume, and urge incontinence episodes. Additionally, study heterogeneity did not permit a comparison with other results in the meta-analysis. Furthermore, a few studies indicated a risk of bias. Therefore, the included studies should be interpreted with caution until more studies are conducted to further evaluate this topic.
Conclusions
Multiple sclerosis is a complex and generally progressive disease requiring a multidisciplinary approach to patient care. The management of urinary disorders in MS patients is primarily a conservative one, knowing well that a urologic program in MS should be designed to promote continent, low-pressure bladder storage and controlled emptying in order to minimize the symptoms in a manner that promotes an improved QoL. Thus, the goal of rehabilitation treatment is to treat NDO without making bladder emptying worse. To do this, it is necessary to establish the correct balance in the treatment of symptoms related to NDO and bladder retention and the integration and use of more rehabilitation techniques may potentially have the best results. Pharmacologic treatment together with rehabilitation strategies, such as PFMT and PTNS, allow the specialist in physical and rehabilitative medicine to combine the different therapeutic programs and choose the best rehabilitation program, with full decisional and clinical practice autonomy and customizing the individual therapeutic project. This study shows particularly the effectiveness of PTNS and PFMT for NDO, however, with the need of a specific therapeutic protocol, based on the degree of disability and symptom complexity in patients with MS. Despite the range of available tools, robust trials are lacking, and therapeutic and rehabilitative approaches vary. Future studies may benefit from longitudinal follow-up with an explicit therapeutic program for care. Nevertheless, more research is needed to determine the best diagnostic methodologies, and to delineate a definitive therapeutic protocol. A therapeutic protocol should have objective, reproducible, repeatable outcomes, and guide the physiatrists to use a common language with the other members of a multidisciplinary team who treat patients with MS.
Supplementary Digital Material 1
Supplementary Table I
Characteristics and outcomes of studies included in the systematic review.
Supplementary Digital Material 2
Supplementary Table II
Therapeutic strategies and rehabilitation techniques of the current literature.
Supplementary Digital Material 3
Supplementary Table III
Risk of bias summary for each included study and GRADE quality of evidence.
Supplementary Digital Material 4
Supplementary Table IV
Bladder diary parameters before and after 3 months of treatment.
Supplementary Table V
PERFECT scheme (P power, E endurance, R repetitions, F fast contractions) after 3 months of treatment between a treated group and a control group.
Supplementary Table VI
Maximum cystometric capacity before and after 3 months of treatment with peripheral tibial nerve stimulation.
References
- 1.European Multiple Sclerosis Platform. Recommendations on rehabilitation services for persons with multiple sclerosis in Europe. Brussels: EMSP; 2004. [Google Scholar]
- 2.Amarenco G, de Sèze M, Ruffion A, Sheikh Ismael S. Clinical and urodynamic evaluations of urinary disorders in multiple sclerosis. Ann Phys Rehabil Med 2014;57:277–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24980885&dopt=Abstract 10.1016/j.rehab.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 3.Al Dandan HB, Coote S, McClurg D. Prevalence of Lower Urinary Tract Symptoms in People with Multiple Sclerosis: A Systematic Review and Meta-analysis. Int J MS Care 2020;22:91–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32410904&dopt=Abstract 10.7224/1537-2073.2019-030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Sèze M, Ruffion A, Denys P, Joseph PA, Perrouin-Verbe B; GENULF. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler 2007;13:915–28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17881401&dopt=Abstract 10.1177/1352458506075651 [DOI] [PubMed] [Google Scholar]
- 5.Lúcio AC, Perissinoto MC, Natalin RA, Prudente A, Damasceno BP, D’ancona CA. A comparative study of pelvic floor muscle training in women with multiple sclerosis: its impact on lower urinary tract symptoms and quality of life. Clinics (São Paulo) 2011;66:1563–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22179160&dopt=Abstract 10.1590/S1807-59322011000900010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallien P, Gich J, Sánchez-Dalmau BF, Feneberg W. Multidisciplinary management of multiple sclerosis symptoms. Eur Neurol 2014;72(Suppl 1):20–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25278120&dopt=Abstract 10.1159/000367620 [DOI] [PubMed] [Google Scholar]
- 7.DI Benedetto P , Finazzi-Agrò E. Conservative management of adult neurogenic lower urinary tract dysfunction. Eur J Phys Rehabil Med 2017;53:981–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29260540&dopt=Abstract 10.23736/S1973-9087.17.04980-2 [DOI] [PubMed] [Google Scholar]
- 8.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25555855&dopt=Abstract 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10789670&dopt=Abstract 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 10.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 2014;14:579. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25413154&dopt=Abstract 10.1186/s12913-014-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Oxford: Cochrane Collaboration; 2008. [Google Scholar]
- 12.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21195583&dopt=Abstract 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011;64:1283–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21839614&dopt=Abstract 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21247734&dopt=Abstract 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol 2011;64:1277–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21802904&dopt=Abstract 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE Working Group . GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 2011;64:1303–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21802903&dopt=Abstract 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 17.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16784338&dopt=Abstract 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16807131&dopt=Abstract 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22008217&dopt=Abstract 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canbaz Kabay S, Kabay S, Mestan E, Cetiner M, Ayas S, Sevim M, et al. Long term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months results. Neurourol Urodyn 2017;36:104–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26352904&dopt=Abstract 10.1002/nau.22868 [DOI] [PubMed] [Google Scholar]
- 21.Gobbi C, Digesu GA, Khullar V, El Neil S, Caccia G, Zecca C. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Mult Scler 2011;17:1514–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21757534&dopt=Abstract 10.1177/1352458511414040 [DOI] [PubMed] [Google Scholar]
- 22.Kabay SC, Yucel M, Kabay S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic study. Urology 2008;71:641–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18387393&dopt=Abstract 10.1016/j.urology.2007.11.135 [DOI] [PubMed] [Google Scholar]
- 23.Kabay S, Kabay SC, Yucel M, Ozden H, Yilmaz Z, Aras O, et al. The clinical and urodynamic results of a 3-month percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol Urodyn 2009;28:964–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19373898&dopt=Abstract 10.1002/nau.20733 [DOI] [PubMed] [Google Scholar]
- 24.Zecca C, Digesu GA, Robshaw P, Puccini F, Khullar V, Tubaro A, et al. Motor and sensory responses after percutaneous tibial nerve stimulation in multiple sclerosis patients with lower urinary tract symptoms treated in daily practice. Eur J Neurol 2014;21:506–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24387787&dopt=Abstract 10.1111/ene.12339 [DOI] [PubMed] [Google Scholar]
- 25.Lúcio AC, Campos RM, Perissinotto MC, Miyaoka R, Damasceno BP, D’ancona CA. Pelvic floor muscle training in the treatment of lower urinary tract dysfunction in women with multiple sclerosis. Neurourol Urodyn 2010;29:1410–3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20976816&dopt=Abstract 10.1002/nau.20941 [DOI] [PubMed] [Google Scholar]
- 26.Lúcio A, Dʼancona CA, Perissinotto MC, McLean L, Damasceno BP, de Moraes Lopes MH. Pelvic Floor Muscle Training With and Without Electrical Stimulation in the Treatment of Lower Urinary Tract Symptoms in Women With Multiple Sclerosis. J Wound Ostomy Continence Nurs 2016;43:414–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27014935&dopt=Abstract 10.1097/WON.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 27.de Sèze M, Raibaut P, Gallien P, Even-Schneider A, Denys P, Bonniaud V, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: results of a multicenter prospective study. Neurourol Urodyn 2011;30:306–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21305588&dopt=Abstract 10.1002/nau.20958 [DOI] [PubMed] [Google Scholar]
- 28.Rafii F, Sajjadi M, Shareinia H, Sarraf P. Pelvic Floor Muscle Training Instruction to Control Urinary Incontinence and its Resulting Stress, Anxiety and Depression in Patients with Multiple Sclerosis. Jundishapur J Chron Dis Care 2017;6:37333. [Google Scholar]
- 29.Fjorback MV, van Rey FS, van der Pal F, Rijkhoff NJ, Petersen T, Heesakkers JP. Acute urodynamic effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with MS. Eur Urol 2007;51:464–70, discussion 471–2. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16956713&dopt=Abstract 10.1016/j.eururo.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 30.Vahtera T, Haaranen M, Viramo-Koskela AL, Ruutiainen J. Pelvic floor rehabilitation is effective in patients with multiple sclerosis. Clin Rehabil 1997;11:211–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9360033&dopt=Abstract 10.1177/026921559701100304 [DOI] [PubMed] [Google Scholar]
- 31.McClurg D, Ashe RG, Lowe-Strong AS. Neuromuscular electrical stimulation and the treatment of lower urinary tract dysfunction in multiple sclerosis—a double blind, placebo controlled, randomised clinical trial. Neurourol Urodyn 2008;27:231–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17705160&dopt=Abstract 10.1002/nau.20486 [DOI] [PubMed] [Google Scholar]
- 32.McClurg D, Ashe RG, Marshall K, Lowe-Strong AS. Comparison of pelvic floor muscle training, electromyography biofeedback, and neuromuscular electrical stimulation for bladder dysfunction in people with multiple sclerosis: a randomized pilot study. Neurourol Urodyn 2006;25:337–48. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16637070&dopt=Abstract 10.1002/nau.20209 [DOI] [PubMed] [Google Scholar]
- 33.Ferreira AP, Pegorare AB, Salgado PR, Casafus FS, Christofoletti G. Impact of a pelvic floor training program among Women with Multiple Sclerosis: A Controlled Clinical Trial. Am J Phys Med Rehabil 2016;95:1–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25888662&dopt=Abstract 10.1097/PHM.0000000000000302 [DOI] [PubMed] [Google Scholar]
- 34.McClurg D, Lowe-Strong A, Ashe R. Pelvic floor training for lower urinary tract dysfunction in MS. Nurs Times 2009;105:45–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19326656&dopt=Abstract [PubMed] [Google Scholar]
- 35.Khan F, Pallant JF, Pallant JI, Brand C, Kilpatrick TJ. A randomised controlled trial: outcomes of bladder rehabilitation in persons with multiple sclerosis. J Neurol Neurosurg Psychiatry 2010;81:1033–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20542931&dopt=Abstract 10.1136/jnnp.2010.206623 [DOI] [PubMed] [Google Scholar]
- 36.Engeler DS, Meyer D, Abt D, Müller S, Schmid HP. Sacral neuromodulation for the treatment of neurogenic lower urinary tract dysfunction caused by multiple sclerosis: a single-centre prospective series. BMC Urol 2015;15:105. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26498275&dopt=Abstract 10.1186/s12894-015-0102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zecca C, Digesu GA, Robshaw P, Singh A, Elneil S, Gobbi C. Maintenance percutaneous posterior nerve stimulation for refractory lower urinary tract symptoms in patients with multiple sclerosis: an open label, multicenter, prospective study. J Urol 2014;191:697–702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24076308&dopt=Abstract 10.1016/j.juro.2013.09.036 [DOI] [PubMed] [Google Scholar]
- 38.Pereira CM, Castiglione M, Kasawara KT. Effects of physiotherapy treatment for urinary incontinence in patient with multiple sclerosis. J Phys Ther Sci 2017;29:1259–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28744060&dopt=Abstract 10.1589/jpts.28.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lúcio AC, D’Ancona CA, Lopes MH, Perissinotto MC, Damasceno BP. The effect of pelvic floor muscle training alone or in combination with electrostimulation in the treatment of sexual dysfunction in women with multiple sclerosis. Mult Scler 2014;20:1761–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24876156&dopt=Abstract 10.1177/1352458514531520 [DOI] [PubMed] [Google Scholar]
- 40.Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 2000;(2):CD001308. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10796768&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 41.Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002;100:72–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12100806&dopt=Abstract 10.1097/00006250-200207000-00012 [DOI] [PubMed] [Google Scholar]
- 42.Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults (Cochrane Review). Cochrane Database Syst Rev 2000;(2):2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafik A, Shafik IA. Overactive bladder inhibition in response to pelvic floor muscle exercises. World J Urol 2003;20:374–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12682771&dopt=Abstract 10.1007/s00345-002-0309-9 [DOI] [PubMed] [Google Scholar]
- 44.Yamanishi T, Kaga K, Fuse M, Shibata C, Uchiyama T. Neuromodulation for the Treatment of Lower Urinary Tract Symptoms. Low Urin Tract Symptoms 2015;7:121–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26663726&dopt=Abstract 10.1111/luts.12087 [DOI] [PubMed] [Google Scholar]
- 45.Tracey JM, Stoffel JT. Secondary and tertiary treatments for multiple sclerosis patients with urinary symptoms. Investig Clin Urol 2016;57:377–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27847911&dopt=Abstract 10.4111/icu.2016.57.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva Ferreira AP, de Souza Pegorare AB, Miotto A, Junior, Salgado PR, Medola FO, Christofoletti G. A Controlled Clinical Trial on the Effects of Exercise on Lower Urinary Tract Symptoms in Women With Multiple Sclerosis. Am J Phys Med Rehabil 2019;98:777–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30932917&dopt=Abstract 10.1097/PHM.0000000000001189 [DOI] [PubMed] [Google Scholar]
- 47.Andersson KE, Appel R, Cardozo L, Chapple C, Drutz H, Fourcroy J, et al. Pharmacological treatment of urinary incontinence. ICS; 2005 [Internet]. Available from: https://www.ics.org/Publications/ICI_3/v2.pdf/chap14.pdf [cited 2022, Jan 24].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Characteristics and outcomes of studies included in the systematic review.
Supplementary Table II
Therapeutic strategies and rehabilitation techniques of the current literature.
Supplementary Table III
Risk of bias summary for each included study and GRADE quality of evidence.
Supplementary Table IV
Bladder diary parameters before and after 3 months of treatment.
Supplementary Table V
PERFECT scheme (P power, E endurance, R repetitions, F fast contractions) after 3 months of treatment between a treated group and a control group.
Supplementary Table VI
Maximum cystometric capacity before and after 3 months of treatment with peripheral tibial nerve stimulation.