Abstract
Dry eye disease (DED) is a common ocular surface condition causing symptoms of significant discomfort, visual disturbance, and pain. With recent advancements, DED has become recognized as a chronic self-perpetuating inflammatory condition triggered by various internal and environmental factors. DED has been shown to arise from the activation of both the innate and adaptive immune systems, leading to corneal epithelium and lacrimal gland dysfunction. While the cornea is normally avascular and thus imbued with angiogenic and lymphangiogenic privilege, various DED models have revealed activated corneal antigen-presenting cells in regional lymph nodes, suggesting the formation of new corneal lymphatic vessels in DED. The recent availability of reliable lymphatic cell surface markers such as LYVE-1 has made it possible to study lymphangiogenesis. Accordingly, numerous studies have been published within the last decade discussing the role of lymphangiogenesis in DED pathology. We systematically review the literature to identify and evaluate studies presenting data on corneal lymphangiogenesis in DED. There is considerable evidence supporting corneal lymphangiogenesis as a central mediator of DED pathogenesis. These findings suggest that anti-lymphangiogenic therapeutic strategies may be a viable option for the treatment of DED, a conclusion supported by the limited number of reported clinical trials examining anti-lymphangiogenic modalities in DED.
Keywords: dry eye disease, corneal lymphangiogenesis, anti-lymphangiogenic strategies, dry eye, angiogenesis
1. Introduction
The cornea consists of the epithelium, Bowman layer, stroma, Descemet membrane, and endothelium – from most exterior to most interior. The corneal epithelium contains superficial epithelial cells, central super basal epithelial cells, and an inner single layer of basal epithelial cells, which all together act as a barrier to chemicals, water, and microbes and provide a smooth optical surface for the internal part of the tear film-cornea interface that contributes to the refractive power of the eye. 1 Similarly, the Bowman layer helps the cornea maintain its shape. The organization of stromal fibers and extracellular matrix (ECM) forms the bulk of the structural framework of the cornea, and Descemet membrane serves as the basement membrane of the corneal endothelium. 1, 2 Dysfunctions of the cornea, along with the surrounding conjunctiva, eyelids, and lacrimal glands, can manifest as a variety of ocular surface disorders including blepharitis and meibomian gland dysfunction, allergic eye diseases, and, of interest in the present review, dry eye disease (DED). 3
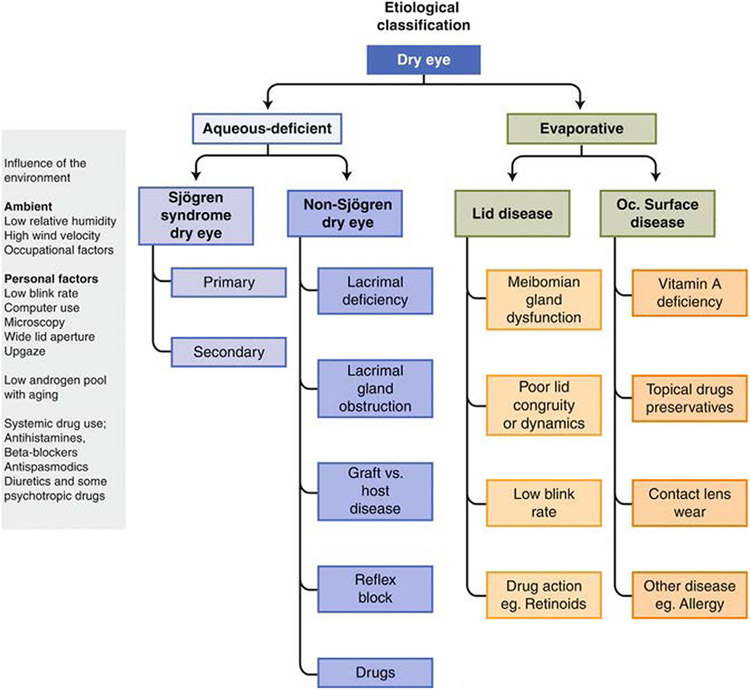
DED is a multifactorial disease of the ocular surface caused by tear film hyperosmolarity/instability and ocular surface damage/inflammation, which result in symptoms of discomfort and visual disturbance. 4 The tear film preserves the integrity of the ocular surface by maintaining a moist environment, lubricating the surface of the eye, decreasing the incidence of ocular surface infections, and washing away foreign particles that may damage the cornea.5 In DED, these essential functions of the tear film are impaired (Fig. 1). 6 There are two major types of DED: aqueous-deficient (from a lack of tear film) and evaporative (from variations in tear film composition). Aqueous-deficient DED is subdivided into Sjögren and non-Sjögren syndrome types, and the main cause of evaporative DED is Meibomian gland deficiency. The causes of the major subtypes of DED are summarized in Fig. 2. 7
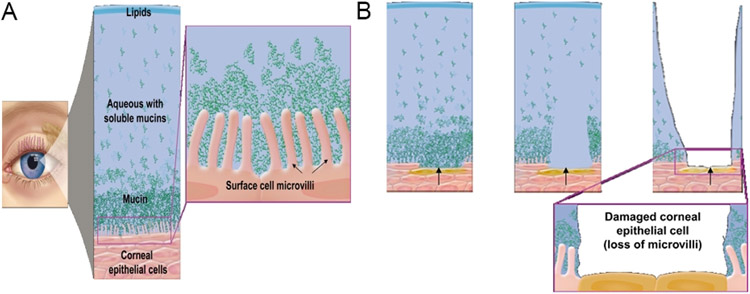
Figure 1 –
(A) Healthy tear film with lipid, aqueous, and mucin layers and healthy ocular surface with intact microvilli. (B) Progressive damage of corneal surface cells (lost microvilli) due to unhealthy tear film. 112 Reproduced with permission.
Figure 2 –
Etiological classification of dry eye. 113 Reproduced with permission.
Between 5 and34% of the world’s population suffers from DED. 8 Between 2003 and 2015, one study estimated the overall prevalence of DED in the United States to be 5.28%, with a higher prevalence among females at 7.33% and an increasing prevalence with age at 11.12% for individuals older than 50 years. Further, the annual prevalence of DED tripled from 2005 to 2012, was attributed to increasing awareness of DED as a treatable condition. 9 Clinically, DED is characterized by redness, burning, stinging, foreign body sensation, and photophobia, leading to visual impairment, especially during reading and driving. The following main risk factors for DED have been identified: age, female sex, post-menopausal estrogen therapy, antihistamines, collagen vascular disease, corneal refractive surgery, irradiation, hematopoietic stem cell transplantation, vitamin A deficiency, hepatitis C, and androgen insufficiency. 8 DED may develop secondarily to inflammatory diseases, hormonal imbalances, diabetes mellitus, thyroid disease, rheumatoid arthritis, systemic lupus erythematosus, long-term contact lens wear, previous eye surgery, systemic medication usage, and a host of environmental conditions (Fig. 2).10 The four most common clinical conditionals known to contribute to DED development are unspecified tear film insufficiency, rheumatoid arthritis, keratoconjunctivitis sicca, and Sjögren sicca syndrome. 9
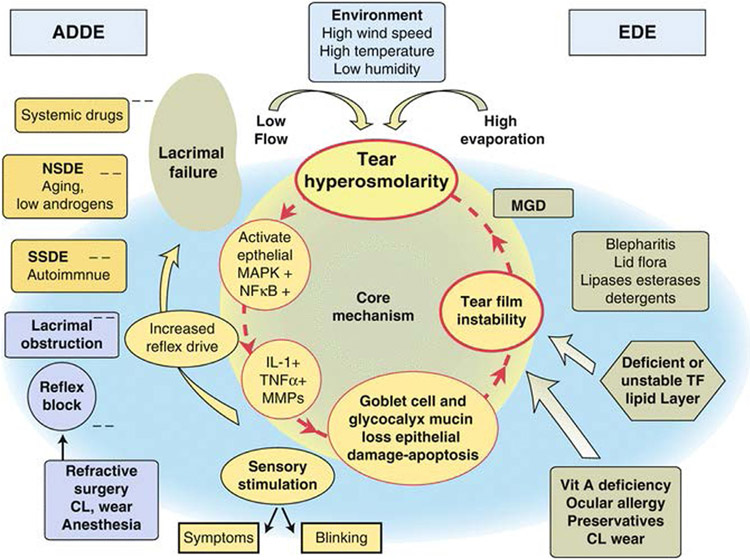
Previously, DED was thought to be merely a disease of tear film insufficiency; however, over the last two decades a growing body of research has redefined DED as a chronic, multifactorial condition involving the immune system. 11 Several mechanisms in the cornea, conjunctiva, and lacrimal glands work in a coordinated fashion to maintain the ocular surface and glandular homeostasis; dysfunction in any of these mechanisms may result in DED. Currently, it is thought that external factors (such as a desiccating environment and contact lens wearing) and/or intrinsic factors (such as aging or autoimmune conditions) act to initially disrupt the tear film. Upon tear film disruption, stress signaling pathways may be activated at the corneal epithelium, triggering activation of the innate immune system. Activation of the innate immune system may, in turn, activate the adaptive immune system, triggering further disruption of the tear film and resulting in a vicious, self-perpetuating cycle (Fig. 3). 12 In reality, it is difficult to separate the individual contributions of internal and external stressors to DED pathology; in many cases, both internal and external factors are involved in the induction of DED pathology. As DED has been increasingly understood as an immune-related condition, anti-inflammatory therapeutics have demonstrated significant promise as treatment options; however, these treatments are not effective in all DED patients and often do not address the root cause of immune system activation.
Figure 3 –
Mechanisms of dry eye. 113 Reproduced with permission.
Recently, there has been a growing recognition of the importance of corneal lymphatic vessels in mediating the activation and movement of immune cells at the corneal surface in DED. Initial reports identified activation of a T-helper cell type 1 (Th-1) type immune response in the regional draining lymph nodes of a mouse model of DED. 13 Further, activation of autoimmunity in local draining lymph nodes was observed in DED in vivo, suggesting the trafficking of ocular surface antigens to regional draining lymph nodes, through which pathologic activation of the adaptive immune system in DED occurs. 14 Lymphatic vessels play an important role in the afferent arm of the immune system by enabling movement of antigen-presenting cells (APCs) to regional lymph nodes; 15 however, under normal settings, the cornea is avascular and is privy to both immune and angiogenic privilege, raising the possibility that new lymphatic vessels form in the cornea in DED.16 In recent years with the development of reliable lymphatic markers, our understanding of the process of corneal lymphangiogenesis has grown significantly. Concomitantly, in the last decade, the role of corneal lymphangiogenesis as a central mediator of DED pathogenesis has also been increasingly recognized, making corneal lymphangiogenesis a potential therapeutic target in the treatment of DED. We briefly describe the mechanisms of corneal lymphangiogenesis and the pathophysiology of DED. Next, we systematically review the involvement of corneal lymphangiogenesis in DED. Then, we identify and discuss potential therapeutic strategies targeting corneal lymphangiogenesis in DED. Finally, weevaluate the potential role, or lack thereof, of hemangiogenesis in DED pathology and how we can learn from anti-hemangiogenesis therapeutics in our quest for DED anti-lymphangiogenesis therapeutics.
2. Immunopathogenesis of dry eye disease
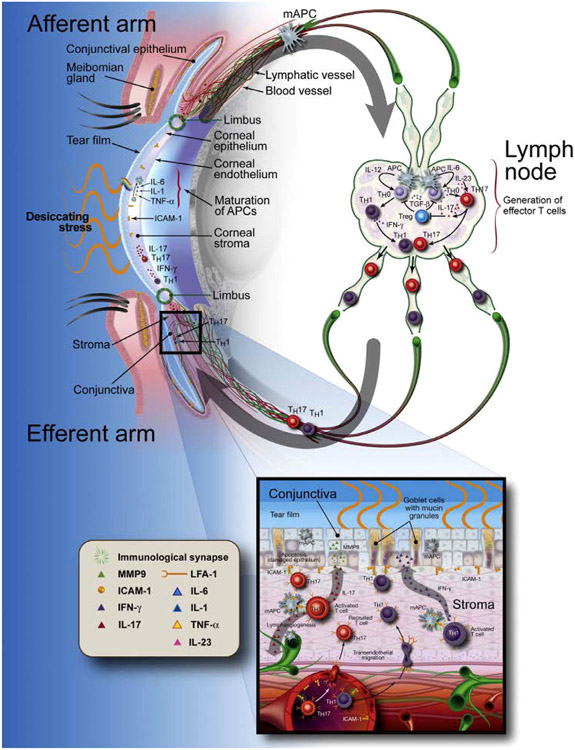
DED pathogenesis can involve activation of both the innate and adaptive immune systems (Fig. 4). 17, 18 Initially, the ocular surface faces an insult that results in localized acute inflammation. For instance, DED induced by environmental stress can result in a hyperosmolar tear film, which can initiate the innate system through activation of immature resident ocular surface APCs. Hyperosmolar stress induces the mitogen-activated protein kinase (MAPK) and pattern recognition receptor (PRR) pathways and prompts the corneal epithelium to secrete proinflammatory molecules/factors, such as interleukin-1beta (IL-1β), tumor necrosis factor alpha (TNF-α), matrix metalloproteinases (MMPs) such as MMP-3 and MMP-9, and apoptotic signals. These inflammatory cytokines amplify each other, resulting in a self-propagating cascade of inflammation.12, 17 Activation of the innate immune system may result in corneal epithelial disruption through loss of tight junctions, increased epithelial apoptosis, and gland disruption.
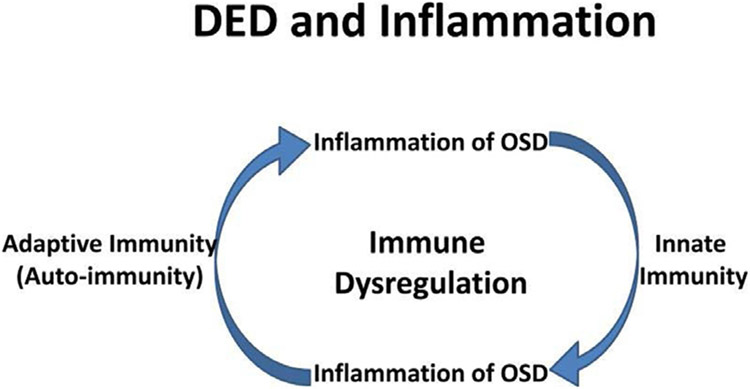
Figure 4 –
The current hypothesis of relationship between DED and inflammation. DED inflammation comprises both innate and adaptive immunity. It starts with an acute innate immune in response to environmental and/or microbial stresses, leading to activation and maturation of antigen-presenting cells (APCs). The matured APCs migrate into regional lymph nodes to generate and maintain dry eye- and ocular-specific autoreactive CD4+-T cells, autoantibody secreting B cells, leading to the adaptive immunity. Th17 cells back to the ocular surface through efferent blood vessels to promote production of inflammatory mediators, lymphangiogenesis and pathogenic immunocytic infiltration, leading to further damage of the ocular surface and progression of a chronic cycle of inflammation. Thus, the key mechanism in DED-related inflammation is not innate or adaptive immunity per se, but a abnormal inflammatory cycle sustained by dysregulation of immune response 17. Reproduced with permission.
DED pathogenesis may also involve activation of the adaptive immune system through several mechanisms. For example, conjunctival goblet cells, that are crucial to immune tolerance in the ocular surface condition the tolerogenic properties of APCs, which allow for the suppression of IL-12 production and Th-1 polarization. 19 This tolerogenic phenotype is thought to be due to goblet cell-derived soluble factors that downregulate dendritic cell expression of major histocompatibility complex (MHC) class II and its costimulatory molecules. 20 In addition, goblet cells are implicated in crosstalk with resident ocular surface dendritic cells, imbuing a tolerogenic phenotype. 21 Thus, loss of goblet cells may result in loss of the tolerogenic properties of APCs. Most notably, aqueous-deficient DED has been associated with goblet cell loss and increased interferon gamma (IFN-γ) expression in the conjunctiva. 19
Goblet cell loss and the release of cytokines IL-1 and TNF-α both work to induce migration of APCs to initiative adaptive immune responses. To aid the migration of APCs, lymphatic endothelial cells (LECs) upregulate expression of intercellular adhesion molecule-1 (ICAM-1). 22 Migrating APCs travel in a CCR-7 dependent manner through the afferent arm to regional lymph nodes where they interact with Th-0 cells through an immune synapse to promote T-cell differentiation. 23 Specifically, matured dendritic cells prime naive Th-0 cells into Th-1 and Th-17 antigen-specific effector T cells, which produce IFN-γ and IL-17, respectively, and are the primary lymphocytic cells involved in ocular surface inflammation related to DED. 13 IFN-γ has been implicated in goblet cell loss, further perpetuating the inflammation cascade. 24 IL-17 has been implicated in disruption of corneal epithelial barrier through expression of MMP-3 and MMP-9. 25
Regulatory T cell (Treg) control of pathogenic autoreactive T cells is crucial to maintaining self-tolerance. Niederkorn demonstrated that depletion of Tregs with anti-CD25 mAb exacerbated symptoms of DED in an in vivo murine model, suggesting that Tregs play an integral role in restraining the excessive immune activation that underlies DED pathology.26 Chauhan and coworkers demonstrated that the Th-17 effector cell proliferation remained unsuppressed in the presence of Tregs isolated from DED model mice.14 Additionally, IL-17 receptors are constitutively expressed on the ocular surface epithelium, suggesting the ocular surface may be especially susceptible to IL-17–induced inflammation. Chauhan and coworkers demonstrated that blocking IL-17 suppresses Th-17 cell proliferation, preserves Treg function, and decreases DED severity and progression in vivo. 14 These findings suggest that Th-17 cells are crucial effector cells that allow maintenance of the self-perpetuating cycle of inflammation. Dysfunction of Tregs due to IL-17 production allows for increased proliferation of Th-17 and Th-1 cells, along with their respective inflammatory cytokines.
Beyond the corneal epithelium, certain forms of DED may involve conjunctival dysfunction, resulting in squamous metaplasia, loss of goblet cells, and activation of inflammatory processes with CD4+ T-cell infiltration. DED may also result from lacrimal gland dysfunction. The lacrimal glands play several roles in maintaining a healthy ocular surface: they protect the ocular surface from invading pathogens, produce aqueous through acinar cells that add volume to the tear film, and secret growth factors for the maintenance of host tissue. 27 Lacrimal gland dysfunction can result in atrophy and loss of the secretory acini within the glands themselves due to fibrosis or apoptosis, once again leading to inflammation with CD4+ T-cell infiltration. 12 In ocular graft versus host disease, both cell-mediated and humoral immunity can result in infiltration, inflammation, and scarring of the lacrimal gland, conjunctiva, and ocular surface, eventually resulting in a decreased density of conjunctival goblet cells. 28 In summary, intrinsic/extrinsic factors induce the initial disruption of ocular surface homeostasis, resulting in activation of the innate and adaptive immune systems, further propagating DED pathology (Fig. 5).
Figure 5 –
The dry eye immunoinflammatory pathway. APC = antigen-presenting cell; ICAM-1 = intercellular adhesion molecule 1; IFN = interferon; IL = interleukin; LFA-1 = lymphocyte function-associated antigen 1; mAPC = mature antigen-presenting cell; MMP = matrix metalloproteinase; TH = T helper cell; TNF = tumor necrosis factor; Treg = regulatory T cell. 85 Reproduced with permission.
3. Mechanisms of corneal Lymphangiogenesis
As noted above, recent studies have demonstrated that corneal lymphatic vessels play an important role in mediating the activation and movement of immune cells at the corneal surface in DED. In physiological states, the cornea is avascular and lacks lymphatic vessels due to an active process that favors the production of anti-lymphangiogenic factors over pro-lymphangiogenic factors.29,30,31,32 This corneal clarity is essential to the proper optical performance of the cornea; however, pathological states like DED, inflammatory disorders, corneal graft rejection, infectious keratitis, contact-lens related hypoxia, alkali burns, neurotrophic ulceration, aniridia, and limbal stem cell deficiency 29 can all lead to lymphangiogenesis in the cornea. Ultimately, this corneal lymphangiogenesis causes reduced vision and may increase the risk of unwanted immune reactions. Understanding the mechanisms of corneal lymphangiogenesis will help contextualize future discussions of the role these lymphatic vessels play in DED pathogenesis.
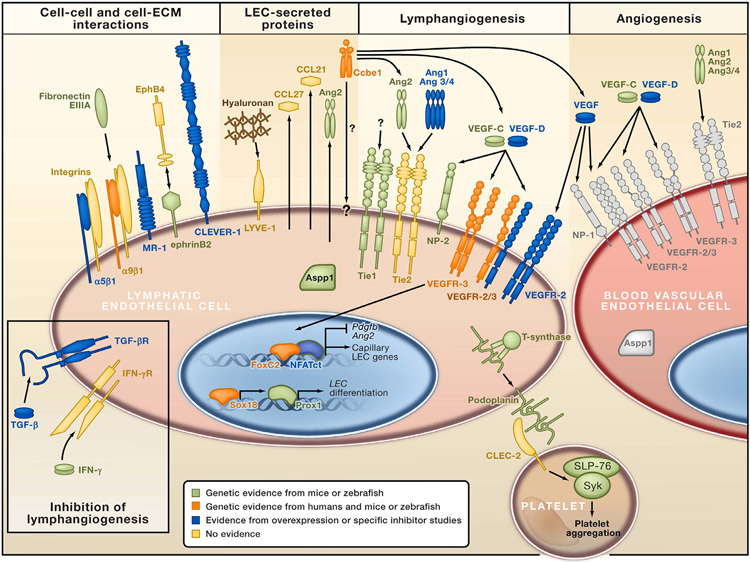
A variety of factors contribute to the growth of new lymphatic vessels in the cornea including, most importantly, the family of vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs). Other crucial pro-lymphangiogenic factors include fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), 33 angiopoietin, 34 and hepatocyte growth factor (HGF) 35 (Fig. 6). Anti-lymphangiogenic factors that are responsible for maintaining the physiologic avascularity of the cornea include soluble (s)VEGFR1, (s) VEGFR2, non-vascular VEGFR3, pigment epithelium-derived factor, and programmed death ligand 1 (PD-L1). More recently, new findings regarding the ways that transforming growth factor-beta induced protein
Figure 6 –
The transcription factor Prox1 drives expression of lymphatic endothelial-specific genes, whereas FOXC2 and NFAT1c regulate differentiation of the lymphatic collecting vessel phenotype. Podoplanin activates the CLEC-2 receptor in platelets, leading to the activation of the tyrosine kinase Syk and platelet aggregation. This mechanism is important for the separation of blood and lymphatic vascular systems. TGF-β and IFN-γ act as endogenous inhibitors of lymphangiogenesis. Note VEGFR and Tie expression in both lymphatic and blood vascular endothelial cells. 114 Adapted and reproduced with permission.
(TGFβIP) 36 and glycosaminoglycan hyaluronan 37 mediate corneal lymphangiogenesis have been revealed. Specifically, TGFβIp is highly upregulated in inflamed mouse corneas in correlation with macrophage infiltration, enhancing the effect of VEGF-C in promoting migration, tube formation, and adhesion of human LECs. 36 Hyaluronan regulates developmental and pathological corneal lymphangiogenesis by increasing VEGF-C expression in macrophages, promoting VEGF-C–induced LEC proliferation, and supporting growth of lymphatic vessels in the limbal corneal epithelium. 37
VEGFRs are a family of tyrosine kinase receptors. VEGFR3 expression is largely associated with LECs, VEGFR2 expression can be seen in both LECs and blood vascular endothelial cells, and VEGFR1 expression is very scarce in LECs. 38 Generally, the VEGFR ligands VEGF-A and VEGF-B are hemangiogenic, whereas VEGF-C and VEGF-D are mostly lymphangiogenic. Thus, corneal lymphangiogenesis is primarily caused by binding of VEGF-C and VEGF-D to VEGFR2 and VEGFR3. 39,40,41 In the VEGF-C/VEGFR3 pathway, binding of the VEGF-C ligand to VEGFR3 causes formation of homodimers, or heterodimers with VEGFR2, with downstream activation of extracellular signaling-related kinase (ERK)1/2 and AKT. 42 This eventually results in promotion of LEC proliferation, migration, and stabilization in the cornea. 43 Next, VEGF-D is known to activate both VEGFR2 and VEGFR3, to promote proliferation of blood vascular endothelial cells and LECs, and to be related to VEGF-C in structure. 44 Interestingly, VEGF-A has also been shown to have the ability to induce corneal lymphangiogenesis through recruitment of VEGFR1-positive macrophages, which secrete VEGF-C and -D, or by stimulating proliferation of LECs. 45, 46, 47 sVEGFR1 expressed in the cornea has been shown to act as an endogenous VEGF-A trap, thus acting as an anti-hemangiogenic/lymphangiogenic molecule. 16 Furthermore, VEGFR3 is constitutively expressed by the corneal epithelium and is also a key mediator of the physiologic avascularity of the cornea. 48 Finally, sVEGFR2 appears to play a key anti-lymphangiogenic role in the cornea; loss of sVEGFR2 in mice led to spontaneous lymphangiogenesis of the normally avascular cornea. 49
Next, FGF-2, also known as basic FGF (bFGF), has been shown to promote corneal lymphangiogenesis. Particularly, in a study of the mouse cornea, FGF-2 was shown to induce lymphangiogenesis via both direct and indirect mechanisms such as binding to LECs to stimulate their proliferation and migration. 50 51 Similarly, in a tumor microenvironment, FGF-2 and VEGF-C were shown to work collaboratively to promote lymphangiogenesis; specifically, FGF receptor-1 binding to FGF-2 and VEGFR3-mediated signaling was required for lymphatic tip cell formation. 52 In a murine cornea model, the Notch/Dll4 pathway was shown to mediate bFGF-induced corneal lymphangiogenesis; specifically, VEGF-A, Dll4, and Notch1 protein expression levels were higher in bFGF-induced animal models. 53 Conversely, neostatin-7 and collagen XVIII were shown to possess anti-lymphangiogenic activities by reducing bFGF-induced corneal lymphangiogenesis. 54
PD-L1 has been shown to act as an anti-angiogenic molecule in the cornea. In a suture-induced corneal angiogenesis model, PD-L1 knockout mice had significantly higher levels of angiogenesis compared to PD-L1 wild-type mice. 55 Additionally, pigment epithelium-derived factor has been shown to play a key anti-angiogenic role in the cornea through inhibition of endothelial cell growth, thus maintaining the physiologic avascularity of the cornea. 56
Finally, cell surface markers such as CD31, LYVE-1 and podoplanin can help isolate blood and lymphatic vessels in corneal buttons. Specifically, the CD31+++/LYVE-1-/podoplanin- profile indicates blood vessels, whereas the CD31+/LYVE1+++/podoplanin + profile suggests lymphatic vessels. 57, 58 The lymphatic marker LYVE-1 is the receptor for hyaluronan and has been shown to function in cell adhesion/transmigration in lymphangiogenesis, 59 and podoplanin has been implicated in promoting LEC growth in corneal wound healing. 60 The lymphatic marker prospero homeobox 1 (Prox1), a transcription factor, is also vital for lymphatic vessel development and maintenance. 61 62
4. Corneal Lymphangiogenesis in dry eye disease
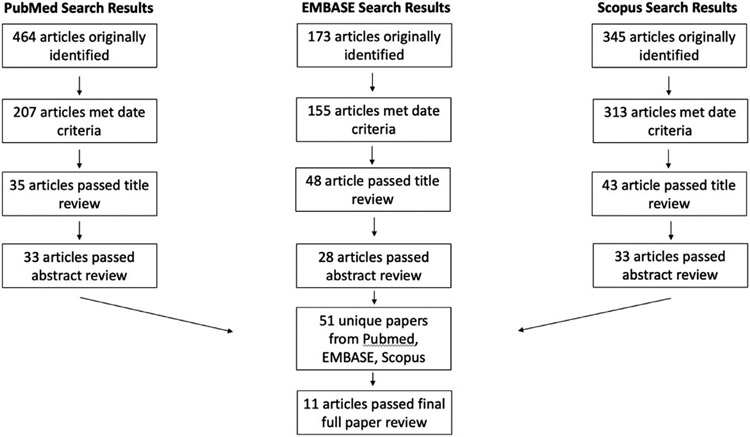
Our initial literature search criteria yielded 464 studies from MEDLINE, 173 studies from EMBASE, and 345 studies from SCOPUS. After passing through rounds of screening for date, title, abstract, and full paper review, 11 studies remained eligible for inclusion in our systematic review. A full summary of our literature search process is presented in Fig. 7, and an overview of the studies finally included in our systematic review is presented in the included TABLE.
Figure 7 –
Overview of Systematic Literature Search. MEDLINE, EMBASE, and Scopus were utilized. After filtering for date, title, abstract, and full text, 11 articles discussing corneal lymphangiogenesis in dry eye disease were selected for inclusion.
Table 1 –
Overview of included studies
| Author | Ref | Year | Title | DED model | Findings on Lymphangiogenesis in DED |
|---|---|---|---|---|---|
| Goyal et al. | 63 | 2010 | Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? | Murine model, controlled-environment chamber | This was the first study to directly show induction of lymphangiogenesis in a mouse model of DED. In DED, lymphangiogenesis is induced without accompanying hemangiogenesis. Additionally, there is increased influx of CD11b+/LYVE-1+ cells in the cornea in DED. Lymphangiogenesis is associated with the homing of CD11b+ APCs to draining lymph nodes. |
| Lee et al. | 64 | 2011 | Therapeutic efficacy of topical epigallocatechin gallate (EGCG) in murine dry eye | Murine model, controlled-environment chamber | DED results in increased infiltration of CD11b+ cells into the cornea and increased expression of pro-inflammatory cytokines. Treatment with topical 0.1% epigallocatechin gallate resulted in improvement in clinical signs of DED, decreases in inflammatory cytokines, and only a modest, non-significant decrease in lymphangiogenesis. |
| Chauhan et al. | 72 | 2011 | A novel pro-lymphangiogenic function for Th17/IL-17 | Murine model, controlled-environment chamber | In a model of DED, blockade of IL-17 resulted in inhibition of lymphangiogenesis; Th-17–secreted IL-17 may play a key role in the promotion of lymphangiogenesis by inducing the expression of VEGF-D. |
| Okanobo et al. | 66 | 2012 | Efficacy of topical blockade of interleukin-1 in experimental dry eye disease | Murine model, controlled-environment chamber | DED was induced using a desiccating environment; DED induction resulted in increased CD11b+ infiltration, increased corneal lymphatic growth, and increased corneal IL-1B expression. Treatment with topical IL-1Ra (IL-1 receptor antagonist) resulted in decreased infiltration of CD11b+ cells, decreased corneal lymphatic growth, and decreased corneal IL-1B expression. |
| Goyal, Chauhan, Dana | 65 | 2012 | Blockade of prolymphangiogenic VEGF-C suppresses dry eye disease | Murine model, controlled-environment chamber | DED induced by a desiccating environment resulted in an in-growth of lymphatic vessels to the center of the cornea. Blockade of VEGF-C resulted in significant reductions in lymphatic caliber and lymphatic area, a significant reduction in CD11b+ infiltration, and improvement in clinical signs of DED. |
| Cho, Archer, and Ambati | 68 | 2014 | Dry eye predisposes to corneal neovascularization and lymphangiogenesis after corneal injury in a murine model | Murine model, scopolamine-induced DED | The effects of scopolamine-induced DED in corneal injury were investigated in a mouse model. In this model, DED corneas had more CD11b+ infiltration compared to normal corneas, but no differences in neovascularization and lymphangiogenesis were seen between the groups. Upon injury (incision), DED corneas had significantly greater levels of neovascularization, lymphangiogenesis and CD11b+ infiltration than normal eyes subjected to injury. |
| Seo et al. | 70 | 2014 | Activation of HIF-1α (hypoxia inducible factor-1α) prevents dry eye-induced acinar cell death in the lacrimal gland | Murine model, controlled-environment chamber | In a mouse model of DED, LYVE-1–expressing structures were upregulated in lacrimal glands. |
| Kwon et al. | 69 | 2016 | Comparison of postoperative corneal changes between dry eye and non-dry eye in a murine cataract surgery model | Murine model, scopolamine-induced DED | Greater levels of neovascularization, lymphangiogenesis, and CD11b+ cell infiltration were observed in mice with anticholinergic-induced DED subjected to cataract surgery than in normal mouse corneas after cataract surgery. Without cataract surgery, DED mice had greater CD11b+ infiltration compared to normal mice, but no differences in neovascularization were observed. DED can cause greater postoperative inflammation. |
| Min et al. | 71 | 2016 | Activation of Dll4/Notch Signaling and Hypoxia-Inducible Factor-1 Alpha Facilitates Lymphangiogenesis in Lacrimal Glands in Dry Eye | Murine model, controlled-environment chamber | In a mouse model of DED, dry eye significantly upregulated Dll4/Notch signaling and lymphangiogenesis in lacrimal grands. HIF-1a suppression led to lower levels of Dll4/Notch signaling as well as lower levels of lymphangiogenesis. Formation of lymphatic vessels reduced CD45+ cell infiltration in lacrimal glands. |
| Ji et al. | 67 | 2018 | Corneal lymphangiogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease | Murine model, controlled-environment chamber | In a LYVE-1Cre;VEGFR2flox mice model of DED, fewer lymphatic vessels were observed at the ocular surface. Further, there were lower levels of proinflammatory cytokines and fewer dendritic cells and effector T-cells in regional lymph nodes, directly demonstrating that lymphatics in DED are key in the afferent arm of the adaptive immune system. |
| Wang et al. | 75 | 2019 | The important role of the chemokine axis CCR7-CCL19 and CCR7-CCL21 in the pathophysiology of the immuno-inflammatory response in dry eye disease | Murine model, controlled-environment chamber | In a mouse model of DED, Wang et al. found dendritic cells localized inside and around lymphatic vessels of the cornea, and that dendritic cell migration to lymphatic vessels is mediated by the CCR7-CCL19 and CCR7-CCL21 chemokine axis |
Goyal et al. were the first to demonstrate the new formation of corneal lymphatic vessels in a mouse model of DED. 63 In their study, Goyal and coworkers observed an early increase in VEGF-D/VEGFR3 levels followed by increases in VEGF-C, VEGF-A and VEGFR2, which led to the induction of corneal lymphangiogenesis. 63 By 2 weeks after induction of DED, significant increases in lymphatic vessel area and lymphatic vessel caliber were observed in the center of the cornea, accompanied by increased recruitment of CD11b+/LYVE1+ monocytic cells to the cornea. Goyal and coworkers demonstrated that in DED induced by desiccating stress, corneal lymphangiogenesis occurs alone without accompanying hemangiogenesis, a finding replicated in several other studies. 64 65 66 Importantly, corneal lymphangiogenesis was associated with an increased presence of activated APCs (MHC-II+ CD11b+) in regional draining lymph nodes, providing initial evidence that newly formed corneal lymphatic vessels in DED serve as the link between the ocular surface and the activation of the adaptive immune response in regional lymph nodes.
Further work since the initial study by Goyal and coworkers in 2010 has provided more insight into the central role that corneal lymphangiogenesis plays in the pathology of DED. In 2012 in a mouse model of DED, They demonstrated that intraperitoneal injection of anti-VEGF-C antibody led to significant reductions in corneal lymphatic area and caliber. 65 Additionally, treatment with anti-VEGF-C antibody led to decreased activation and recruitment of CD11b+ cells, along with significant decreases in pro-inflammatory cytokines (i.e., IL1-α, IL1-β, IL-6, and IL-17), which have been implicated in DED pathogenesis. Finally, in vivo blockade of VEGF-C led to functional improvement, as evidenced by a significant decrease in corneal fluorescein staining scores. Through the suppression of VEGF-C expression, Goyal and coworkers provided strong direct evidence that corneal lymphangiogenesis plays a key role in DED pathogenesis.
Through the use of a conditional LYVE-1;VEGFR2 mice knockdown model, Ji and coworkers provided further evidence that newly formed corneal lymphatic vessels mediate DED pathology through the trafficking of immune cells. 67 In conditional knockdown mice it was shown that a reduction in corneal lymphatic vessel coverage was associated with a decreased level of corneal erosion in DED. Furthermore, similar to the results from the 2012 study of Goyal and coworkers, decreased levels of pro-inflammatory cytokines (TNF-α, IL-1β, IFN-γ, IL-8) were observed at the ocular surface in LYVE-1;VEGFR2 conditional knockdown mice (8). Interestingly, in LYVE-1;VEGFR2 conditional knockdown DED mice, regional draining lymph nodes were smaller compared to those in wild-type DED mice, and reductions were observed in the numbers of a variety of activated immune cells (CD207+, IL-17+CD4+, CD11b+, IFN-γ CD4+) present in the regional lymph nodes of conditional knockdown mice. Finally, prevention of lymphangiogenesis in knockdown mice preserved the integrity of corneal nerves. These results provide direct evidence that the newly formed corneal lymphatics in DED are central to the trafficking of immune cells from the ocular surface to regional lymph nodes for activation of an immune response. The results from the 2018 study by Ji and coworkers and the 2012 study by Goyal and coworkers complement one another, and together these studies provide strong evidence that corneal lymphangiogenesis is a central mediator of DED pathogenesis.
There is also evidence that the presence of DED predisposes the cornea to subsequent lymphangiogenesis and neovascularization upon injury. In a scopolamine (anti-cholinergic)-induced DED mouse model, Cho and coworkers observed that ocular surgical insults induced greater levels of neovascularization, lymphangiogenesis, and inflammation compared with the levels induced by ocular surgical insults in non-DED mice. 68 Similar results were observed in a model of cataract surgery conducted in DED and non-DED mice. 69 Interestingly, in scopolamine-induced DED, Cho and coworkers observed no increases in lymphangiogenesis or neovascularization, which is in contrast to the increased lymphangiogenesis observed in desiccating stress-induced DED. 63, 68
In addition to lymphangiogenesis at the ocular surface, the formation of new lymphatic vessels has been observed in the lacrimal glands in DED. Seo and coworkers observed that, upon induction of DED in vivo, there is an increase in LYVE-1+ structures in the lacrimal gland. 70 Lymphatic vessels appear to play different roles in lacrimal glands compared to the cornea. In the lacrimal glands, the formation of lymphatic vessels appears to be mediated by HIF1-α regulation of Dll4/Notch signaling, and the formation of lymphatic vessels reduces CD45+ cell infiltration in lacrimal glands in DED. 71 More studies are necessary to evaluate the role of lymphatics in lacrimal glands in DED.
One of the most unique aspects of corneal lymphangiogenesis in desiccating stress-induced DED is the induction of lymphatic vessel growth without concomitant blood vessel growth; however, it is still largely unclear how this process occurs. Classically, innate immune signals have been shown to be important for the induction of lymphangiogenesis. 72 In a mouse model of DED, Okanobo and coworkers showed that blockade of IL-1 through ocular surface application of IL-1 receptor antagonist (IL-1Ra) ameliorated symptoms of DED and decreased corneal lymphangiogenesis, possibly through reduced infiltration of macrophages. 66 Thrombospondin-1 was also shown to play an important immunoregulatory role in the corneal epithelium in DED; its topical application decreased activation of dendritic cells, decreased levels of proinflammatory cytokines, and improved clinical markers of DED in a mouse model. 73 In other models, thrombospondin-1 has been shown to be an important antilymphangiogenic molecule in the cornea, suggesting that thrombospondin-1 may be an important regulator of corneal lymphangiogenesis in DED pathogenesis. 74 Further, it has been recently shown that the expression of chemokines and receptor such as CCR-7, CCL19, and CCL21 is increased in DED, and the expression of these chemokines and receptor is correlated with the activation of dendritic cell migration to lymphatic vessels. 75
While innate immune signals largely regulate the induction of lymphangiogenesis, there is evidence that Th-17 cell-secreted IL-17 plays a role in the progression of lymphangiogenesis in DED. 72 Chauhan and coworkers showed that IL-17 is directly involved in inducing the expression of VEGF-D, and IL-17 blockade results in a significant decrease in corneal lymphangiogenesis along with improvements in corneal disease scores. 72 While the work of Chauhan and colleagues and others provides some insight into lymphangiogenesis in DED as a VEGF-C/D/VEGFR3, IL-17 driven process, more studies are necessary to fully elucidate the mechanisms by which lymphangiogenesis is induced in DED.
5. Therapeutic implications of the involvement of Lymphangiogenesis in dry eye disease
5.1. Current therapeutic strategies
The treatment and management of DED depend on the cause, severity, and progression of the condition. Existing therapeutic strategies for DED target tear volume/quality, artificial tear compensation, and inflammation. Artificial tears are the most common treatment for DED and act to replace the tear film lost in DED. Additionally, artificial tears can dilute the concentration of pro-inflammatory cytokines at the ocular surface, thereby diminishing the immune-related symptoms accompanying DED. As DED has been increasingly understood as an immune-related condition, anti-inflammatory therapeutics have demonstrated significant promise as a treatment option. Thus far, the most popular immunomodulatory agent used to treat DED has been topical cyclosporine, which has been shown to inhibit the production of inflammatory mediators and reduce the symptoms of DED. 76, 77 Corticosteroids have successfully been used to treat DED-driven corneal epithelial disease, 78, 79 while tetracyclines and dietary supplementation of essential fatty acids have also shown some benefit in reducing corneal irregularity. 10 Of particular interest to this review are the therapeutic strategies focused on inhibiting the self-perpetuating inflammatory cascade involved in DED. Currently, there are only two Food and Drug Administration approved drugs for DED: a 0.05% ophthalmic cyclosporine A solution and a 5% lymphocyte function-associated antigen-1 (LFA-1) antagonist lifitegrast ophthalmic solution.
Cyclosporine A is an anti-inflammatory agent that specifically and reversibly inhibits the phosphatase activity of calcineurin that is required for the transcription of T-cell activating/proliferative cytokines such as IL-2. 8, 80 Cyclosporine A is administered topically due to the severe side effects associated with its systemic administration, which include nephrotoxicity, hypertension, hepatotoxicity and increased susceptibility to infection. 81 A regimen of 0.05% cyclosporine eye drops applied twice daily yielded significant improvements in tear production, goblet cell density, corneal epithelial/barrier functions and tear film break-up time in DED patients; 82 however, topical cyclosporine A was not effective in all patients and had side effects such as local ocular burning, redness, visual blurring, foreign body sensation and epiphora. 83, 84
Lifitegrast is a novel LFA-1 integrin antagonist that competitively inhibits LFA-1 binding with ICAM-1. LFA-1 is expressed on the leukocyte surface and as such is crucial for the afferent arm of dendritic cell migration to lymph nodes and the efferent arm of T-cell migration to the ocular surface 12. Topical administration of 5% lifitegrast exhibits strong inhibition of T-cell adhesion to ICAM-1 surfaces, rapid absorption into ocular tissues, and rapid clearance from systemic circulation. 85 Overall, DED symptoms were well managed with 5% lifitegrast, with patients experiencing improved eye dryness and improved corneal epithelial and barrier health (improved corneal fluorescein staining scores). 86 A 5% lifitegrast ophthalmic solution is well tolerated with minor side effects, including installation-site irritation, pruritus and reaction, reduced visual acuity, and dysgeusia. 87
Additional therapies include topical corticosteroids, topical non-steroidal anti-inflammatory drugs (NSAIDs), and antibiotics. Topical corticosteroids have been shown to reduce inflammation in DED, but long-term use of corticosteroids is associated with increased intraocular pressure, glaucoma, cataracts, and ocular infections. 88 Corticosteroids inhibit inflammation on a genomic level, inhibiting expression of pro-inflammatory genes and suppressing anti-inflammatory genes. 89 Short-term use of corticosteroids results in moderate relief of symptoms, improved corneal fluorescein staining, reduced tear osmolarity, and decreased expression of inflammatory cytokines. 78
NSAIDs used to control ocular inflammation generally are nonselective COX inhibitors and are usually delivered as a topical ophthalmic solution. 88 Various trials have demonstrated the effectiveness of topical NSAIDs in reducing DED-induced inflammation.90, 91, 92 However, topical NSAIDs are associated with conjunctival stinging, conjunctival hyperemia, corneal melt, corneal infiltrates, and corneal perforation. 93, 94
Other non-widely used therapies include tetracyclines, which are antibiotics known to inhibit activation of MAPK and nuclear factor-kappa beta (NF-κβ) pathways, thereby reducing MMP synthesis and activity, IL-1 expression, TNF-induced collagenase activity, and B cell activation. However, while the use of tetracyclines has been extensively studied in ocular rosacea, meibomian gland dysfunction, and corneal surface irregularity, their efficacy in treating DED remains underex plored. 8, 10 So far, it is apparent that doxycycline, by improving meibomian gland function and preserving corneal smoothness, may indirectly promote ocular surface healing in dry eye conditions .95 Doxycycline has also been shown to reduce the expression of MMP-9 and MAPK, which are generally increased in the desiccating stress model of DED. 96Tetracyclines also have a high likelihood of side effects, such as gastrointestinal and skin problems, and thus are recommended for only short-term use. 8 Additionally, macrolides, another class of antibiotics, also exhibit anti-inflammatory effects by suppressing inflammatory cytokines, MMPs and chemokines that are produced in DED. 97
5.2. Potential therapeutic strategies targeting Lymphangiogenesis in dry eye disease
Given the central role that corneal lymphangiogenesis plays in the pathology of DED, strategies that target corneal lymphangiogenesis are of interest. As our understanding of corneal lymphangiogenesis has advanced in recent years, several therapeutics targeting lymphangiogenesis offer promise in treating DED. Indeed, several peer-reviewed pre-clinical studies using murine models of DED demonstrated the inhibition of corneal LA in DED through various methods such as peritoneal anti-VEGF-C antibody injections and conditional knockdown (Lyve-1Cre;VEGFR2flox mice); these findings demonstrate the potential for targeting corneal lymphangiogenesis as a therapeutic strategy in DED. 65, 67
There have been several clinical studies evaluating the use of antilymphangiogenic therapies in DED. Among the most revolutionary ophthalmologic advancements in recent years, bevacizumab (anti-VEGF-A) has shown promise in treating a range of corneal and retinal conditions. While VEGF-A is perhaps best known for its role in the induction of hemangiogenesis, VEGF-A has also been shown to be involved in the process of lymphangiogenesis through the recruitment of VEGF-C/D–secreting macrophages as well as through VEGF-A/VEGFR2 signaling. 46, 98 Supporting this concept, bevacizumab has been shown to be a potent inhibitor of both corneal angiogenesis and lymphangiogenesis in both in vivo and in vitro models.45 Given the central involvement of corneal lymphangiogenesis in DED and the anti-lymphangiogenic and anti-inflammatory potential of bevacizumab, bevacizumab may be a viable therapeutic option for DED patients, particularly those with severe DED. To our knowledge, there have been two clinical trials evaluating the efficacy of bevacizumab in treating DED. 99, 100 In their 2015 study, Jiang and coworkers evaluated the safety and efficacy of subconjunctival bevacizumab injection in 32 patients (64 eyes) with DED using a self-controlled case series study design. 99 They found that subconjunctival injection of bevacizumab was well tolerated and produced a significant decrease in ocular surface disease index scores, a significant increase in tear break up time (TBUT), and a significant increase in goblet cell density relative to baseline at 1 week, 1 month, and 3 months after injection. Surprisingly, they found no improvement in corneal staining post bevacizumab, whereas the 2012 study by Goyal and coworkers found improvements in corneal staining in a murine model of DED after anti-VEGF-C treatment. 65, 99 This difference in corneal staining outcome may be explained by the use of VEGF-C blockade in the murine model and the use of VEGF-A blockade (bevacizumab) in the human clinical trial. 65, 99
In a prospective double-blind, randomized trial reported in 2020, Kasetsuwan and coworkers studied the efficacy of daily topical bevacizumab 0.05% eye drops in DED. 100 They observed significant improvement in tear break up time scores by 12 weeks and significant decreases in ocular surface disease index scores at 1, 4, and 12 weeks after the start of the study in the bevacizumab group relative to the placebo group. Importantly, they reported no significant side effects associated with topical bevacizumab treatment. Additionally, their study showed an improvement in corneal staining, which is in contrast to the results of Jiang and coworkers. 99 Several factors may explain this discrepancy, including a difference in bevacizumab delivery method and different study designs. On the basis of these two clinical trials and the basic science lit erature on VEGF-A, bevacizumab may be a viable therapeutic in DED due, at least in part, to its anti-lymphangiogenic potential.
Calcium dobesilate is a drug that has been shown to be clinically safe and to be a potent inhibitor of FGF, a pro-lymphangiogenic factor.101, 102 [n a small self-controlled case series study, the efficacy of calcium dobesilate eye drops was evaluated in the treatment of severe DED in 8 patients. 103 All patients had significantly improved Schirmer tear test scores, significantly decreased corneal epitheliopathy as assessed by fluorescein corneal staining, and improvement in DED symptoms (e.g., dryness, foreign body sensation, photophobia, eye pain, blurred vision). These results are promising but are limited by the study design and very small study size. Nevertheless, this study provided preliminary evidence in a human trial that calcium dobesilate, and more generally, targeting of FGF, offers promise in the treatment of severe DED.
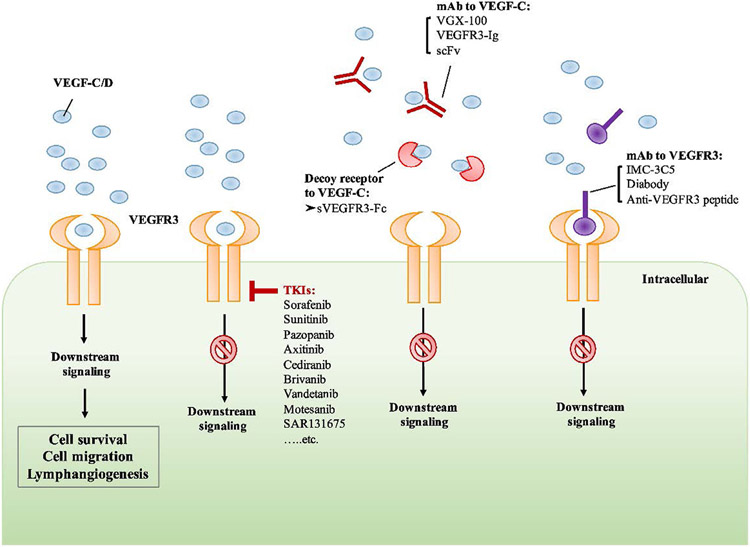
The clinical trials reported thus far related to strategies targeting lymphangiogenesis in DED provide limited, but promising, evidence that targeting corneal lymphangiogenesis represents a viable strategy in treating DED; however, there are several other strategies targeting corneal lymphangiogenesis that have yet to be evaluated in DED human trials. One potential anti-lymphangiogenic strategy is targeting VEGF-C/D binding to VEGFR2/3 (Fig. 8). As previously discussed, targeting VEGF-C through anti-VEGF-C antibodies represents a potent antilymphangiogenic strategy and has been shown to reduce DED-related symptoms in vivo. 65 Anti-VEGF-C has been shown to be a viable strategy targeting lymphangiogenesis in a variety of models including corneal transplantation and tumor metastasis. 104, 105 Furthermore, VEGF-D has been shown to act synergistically with VEGF-C and may thus represent another viable therapeutic target in the inhibition of corneal lymphangiogenesis 44. As VEGFR3 represents a binding partner of both VEGF-C and VEGF-D, inhibition of VEGFR3 signaling may also represent a viable anti-lymphangiogenic strategy. 106
Figure 8 –
Potential Anti-Lymphangiogenic Strategies targeting VEGF-C/D binding to VEGFR2/3. The signaling pathways of vascular endothelial growth factors and vascular endothelial growth factor receptors (VEGFs/VEGFRs) and their biological functions. The three tyrosine kinase (TK) receptors have specific binding capabilities. VEGF-A, VEGF-B, and PlGF can bind to VEGFR1 and mediate its biological functions. The binding of VEGF-A, VEGF-C, and VEGF-D can stimulate the activation of VEGFR2, resulting in cell proliferation and angiogenesis. VEGF-C and VEGF-D bind to VEGFR3 and induce downstream signaling which mediates cell survival and lymphangiogenesis. Neuropilin 1 (NRP1) and neuropilin 2 (NRP2) can function as co-receptors for VEGFR2 and VEGFR3. The binding of VEGF-A isoforms and NRP1 can form a complex with VEGFR2, leading to the induction of downstream signaling which regulates the proliferation and migration of endothelial cells. VEGF-C/D bind to NRP2 and forms a complex with VEGFR3, activating the VEGFR3 signaling which enhances the proliferation of lymphatic endothelial cells (LECs) and lymphangiogenesis. MKK4, Mitogen-activated protein kinase kinase-4; JNK1/2, c-Jun N-terminal kinase-1/2; PI3K, phosphoinositide-3 kinase; AKT/PKB, AKT/protein kinase B; PKC, protein kinase C; ERK, extracellular signal–related kinase; SHC-GRB2, Src homology domain containing growth factor receptor–bound protein 2 115. Reproduced with permission.
Targeting FGF2 represents a viable strategy in DED, and preliminarily, the results from the calcium dobesilate clinical trial suggest that FGF can be targeted successfully in humans. 103 Sunitinib, a multi-target tyrosine kinase inhibitor, has also been shown to be a potent inhibitor of corneal lymphangiogenesis and thus may be viable in DED. 107 Finally, Ang-Tie signaling represents a potent lymphangiogenic pathway and targeting of Ang-Tie has been shown to have oncologic and ophthalmologic utility through anti-lymphangiogenic mechanisms. 106, 108 In summary, there are a number of anti-lymphangiogenic strategies tested using in vivo corneal disease models that, if successfully translated to clinical studies, could bolster the treatment of DED.
6. Does hemangiogenesis play a role in dry eye disease pathogenesis?
A discussion of the role that corneal lymphangiogenesis plays in DED pathogenesis necessitates an acknowledgement of whether corneal hemangiogenesis plays a role in DED pathogenesis as well. Hemangiogenesis is a complex process with a series of steps, including interaction with an angiogenic stimulus followed by blood vessel sprouting, elongation and branching, lumen formation, anastomosis, and, finally, stabilization or regression.109 Similar to corneal lymphangiogenesis, growth factors and other biological cues play crucial roles in corneal hemangiogenesis, including VEGFs, FGF, PDGF, and TGF-β. 109 Unfortunately, the field of research investigating the role of corneal hemangiogenesis in DED is incredibly barren, like its lymphangiogenesis counterpart, with only 10 studies returned by a PubMed search for “Dry Eye Disease” AND “Angiogenesis.”
Goyal and coworkers recognized the emphasis being given to pathologic angiogenesis in various corneal diseases but reported that to their knowledge there are no data regarding corneal hemangiogenesis in DED. 63 In corneal diseases like keratitis, chemical burns, and neovascularization, clinically visible blood vessels arise in the cornea and coexist with lymphatic vessels to act as efferent and afferent arms of the immune response, respectively; however, some of the previously reviewed studies reported selective corneal lymphangiogenesis in DED pathogenesis. 63 65 Furthermore, Stevenson and coworkers confirmed the general consensus that DED produces lymphangiogenesis without associated hemangiogenesis in experimental and clinical settings. 110 Still, without a critical mass of literature investigating the role of corneal hemangiogenesis in DED pathogenesis, further studies must be conducted to verify this assertion.
Interestingly, a peer-reviewed study found that DED predisposes the cornea to injury-induced neovascularization and lymphangiogenesis in a murine model. This finding does not support a role for hemangiogenesis in DED pathogenesis and instead suggests a different sequence of events in which DED may precede pathological hemangiogenesis. 68 In tandem with this finding, one study demonstrated that DED corneas have greater amounts of inflammation and neovascularization after ocular surface surgery than non-DED corneas and trapping VEGF-A decreased both angiogenesis and inflammation in these DED corneas.111
Even with the lack of related studies, corneal hemangiogenesis is still relevant in the discussion of DED therapeutics. As one article pointed out, there are 26 clinically approved approaches to treat pathological angiogenesis while none exist for lymphangiogenesis. 106 Thus, antiangiogenesis therapeutics could serve as an inspiration for developing anti-ymphangiogenesis therapeutics for the treatment of DED, especially since many of the factors that promote hemangiogenesis also work to promote lymphangiogenesis. As previously explained, the well-known antiangiogenesis drugs bevacizumab (which targets VEGF-A) and calcium dobesilate (which targets FGF) have both been tested in patients with DED with promising results. 99, 100, 103 There is incredible promise for the potential application of VEGF pathway target sites for future antilymphangiogenesis drugs (Fig. 9). Further development of antilymphangiogenesis therapeutics for the treatment of DED is of paramount importance.
Figure 9 –
Schematic of VEGF pathway target sites of FDA-approved anti-angiogenesis drugs and potential VEGF pathway target sites for future anti-lymphangiogenesis drugs. Axitinib, Bevacizumab, Cabozantinib, Nintedanib, Pazopanib, Pegaptanib, Ramuricumab, Ranibizumab, Regorafenib, Sorafenib, Sunitinib, and Vandetanib are approved anti-angiogenic therapies that target VEGF-mediated angiogenesis. Inhibition of a VEGF ligand, a VEGFR binding site, or VEGFR tyrosine kinase activity leads to a reduction in angiogenesis. This strategy can potentially be translated for lymphangiogenesis-targeted drug design. Development of anti-lymphangiogenic therapies may follow the design of anti-angiogenic therapies by interrupting the lymphangiogenic factor VEGF-C, its receptors VEGFR2 and VEGFR3, or the tyrosine kinase activity of VEGFR2 and VEGFR3. HA, hemangiogenic; LA, lymphangiogenic. 106 Reproduced with permission.
Conclusions
Despite recent advancements recognizing DED as a chronic inflammatory condition, a significant number of patients remain afflicted by DED that does not respond to currently available treatments. We conducted a systematic review of research highlighting corneal lymphangiogenesis as a key mediator of DED pathology. Induction of corneal lymphangiogenesis facilitates the movements of corneal APCs to regional lymph nodes for activation of an immune response. Thus, targeting lymphangiogenesis may represent a viable therapeutic strategy, as shown through various in vivo models and a limited number of human clinical trials. More studies are necessary to evaluate fully antilymphangiogenic therapies in DED, but the current research suggests that targeting corneal lymphangiogenesis represents a promising strategy.
Method of literature search.
To identify published studies investigating the involvement of corneal lymphangiogenesis in DED pathogenesis, a systematic review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement by two independent reviewers. Searches were conducted using MEDLINE, EMBASE, and Scopus during August 2020. Search terms included combinations of “corneal lymphangiogenesis,” “lymphangiogenesis”, “lymphatic”, “lymphatics”, “lymphatic vessels,” “neo-lymphangiogenesis”, “angiogenesis”, “dry eye”, and “dry eye disease.” Because the earliest report on the involvement of corneal lymphangiogenesis in dry eye disease was published in 2010 by Goyal and colleagues, we searched for studies published within the last 15 years (January 1, 2006 through August 1, 2020). 63 Studies identified after the initial search were screened independently by two reviewers to determine whether they presented data concerning lymphangiogenesis in DED. Studies were screened by title, abstract, and finally, full text to assess the discussion of corneal lymphangiogenesis and DED. For inclusion, studies must have presented original data regarding the involvement of corneal lymphangiogenesis in DED.
Acknowledgments
Publication of this article was supported by National Institutes of Health grants EY10101 (D.T.A.), EY01792 and EY027912 (MIR); I01 BX002386, I01 BX004234, and the Eversight, Midwest Eye Bank Award (J.H.C); and an unrestricted grant from Research to Prevent Blindness (RPB), New York, NY.
Abbreviations:
- AKT
protein kinase B (PKB)
- AKR
mouse thymoma protein
- APCs
antigen-presenting cells
- ANG
angiopoietin
- CAM
cell adhesion molecules
- CCL
C-C chemokine ligand
- CCR
C-C chemokine receptor
- CD
cluster of differentiation
- CD4+ T cells
cluster of differentiation 4+ T cells
- COX
cyclo-oxygenase
- DE
dry eye
- DED
dry eye disease
- Dll4/Notch1
delta-like ligand 4/Notch1
- EC
endothelial cells
- ECM
extracellular matrix
- EFNB2/EPHB4
ephrin B2/EphB4
- EMBASE
Excerpta Medica Database
- ERK
extracellular signaling-related kinase
- FDA
Food and Drug Administration
- FGF/bFGF
fibroblast growth factor/basic fibroblast growth factor
- HGF
hepatocyte growth factor
- HIF-α
hypoxia inducible factor-alpha
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon gamma
- IL
interleukin
- IL-1Ra
interleukin 1 receptor antagonist
- LFA-1
lymphocyte function-associated antigen-1
- LEC
lymphatic endothelial cell
- LN
lymph nodes
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- MAPK
mitogen-activated protein kinase
- MEDLINE
Medical Literature Analysis and Retrieval System Online, or MEDLARS Online
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- NF-κβ
nuclear factor-kappa beta
- NRP
neuropilin
- NSAIDs
non-steroidal anti-inflammatory drugs
- OSDI
ocular surface disease index
- PD-L1
programmed death ligand 1
- PDGF
platelet-derived growth factor
- PEDF
pigment epithelium-derived factor
- PlGF
placental growth factor
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROX1
prospero-related homeobox-1
- PRR
pattern recognition receptors
- TBUT
tear break-up time
- TGFβ
transforming growth factor-beta
- TGFβIP
transforming growth factor-beta induced protein
- Th0
cognate naïve T ells
- Th-1/Th17
T-helper cell type 1, 17
- TIE2
TEK receptor tyrosine kinase
- TNF
tumor necrosis factor
- Treg
regulatory T cells
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor.
Footnotes
Declaration of competing interests
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
REFERENCES
- 1.Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira RC. Wilson SE. Descemet’s membrane development, structure, function and regeneration. Exp Eye Res. 2020;197:108090–7. [DOI] [PubMed] [Google Scholar]
- 3.Khanna RC. Ocular surface disorders. Community Eye Health. 2017;30:S1–2. [PMC free article] [PubMed] [Google Scholar]
- 4.Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary.. Ocul Surf. 2017;15:802–12. [DOI] [PubMed] [Google Scholar]
- 5.Yokoi N, Bron AJ, Georgiev GA. The precorneal tear film as a fluid shell: the effect of blinking and saccades on tear film distribution and dynamics. Ocul Surf. 2014;12:252–66. [DOI] [PubMed] [Google Scholar]
- 6.Dartt DA, Willcox MD. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013;117:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Yiu SC. Dry eye disease: a review of diagnostic approaches and treatments. Saudi J Ophthalmol. 2014;28:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(quiz 82):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States health care system. Am J Ophthalmol. 2019;202:47–54. [DOI] [PubMed] [Google Scholar]
- 10.Javadi MA, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011;6:192–8. [PMC free article] [PubMed] [Google Scholar]
- 11.The definition and classification of dry eye disease: report of the definition and classification subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92. [DOI] [PubMed] [Google Scholar]
- 12.Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124:S4–s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Annan J, Chauhan SK, Ecoiffier T, et al. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50:3802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan SK, Dohlman TH, Dana R. Corneal lymphatics: role in ocular inflammation as inducer and responder of adaptive immunity. J Clin Cell Immunol. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens. 2014;40:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Periman LM, Perez VL, Saban DR, et al. The immunological basis of dry eye disease and current topical treatment options. J Ocul Pharmacol Ther. 2020;36:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko BY, Xiao Y, Barbosa FL, et al. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight. 2018;3(3):e98222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015;10:e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbosa FL, Xiao Y, Bian F, et al. Goblet cells contribute to ocular surface immune tolerance-implications for dry eye disease. Int J Mol Sci. 2017;18(5):978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saban DR. The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation. Ocul Surf. 2014;12:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–60. [DOI] [PubMed] [Google Scholar]
- 25.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–7. [DOI] [PubMed] [Google Scholar]
- 27.Conrady CD, Joos ZP, Patel BC. Review: the lacrimal gland and its role in dry eye. J Ophthalmol. 2016;2016:7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SK. Ocular graft vs. host disease. Ocul Surf. 2005;3:S177–9. [DOI] [PubMed] [Google Scholar]
- 29.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 30.Gao X, Guo K, Santosa SM, et al. Application of corneal injury models in dual fluorescent reporter transgenic mice to understand the roles of the cornea and limbus in angiogenic and lymphangiogenic privilege. Sci Rep. 2019;9:12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JH, Han KY, Azar DT. Wound healing fibroblasts modulate corneal angiogenic privilege: interplay of basic fibroblast growth factor and matrix metalloproteinases in corneal angiogenesis. Jpn J Ophthalmol. 2010;54:199–205. [DOI] [PubMed] [Google Scholar]
- 33.Jitariu AA, Cimpean AM, Kundnani NR, Raica M. Platelet-derived growth factors induced lymphangiogenesis: evidence, unanswered questions and upcoming challenges. Arch Med Sci. 2015;11:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen D, Grimaldo S, Sessa R, et al. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2014;55:3320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajiya K, Hirakawa S, Ma B, et al. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin T, Zhang X, Lu Y, Gong L. TGFBIp mediates lymphatic sprouting in corneal lymphangiogenesis. J Cell Mol Med. 2019;23:7602–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun M, Puri S, Mutoji KN, et al. Hyaluronan derived from the limbus is a key regulator of corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2019;60:1050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauniyar K, Jha SK, Jeltsch M. Biology of vascular endothelial growth factor C in the Morphogenesis of lymphatic vessels. Front Bioeng Biotechnol. 2018;6:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh SJ, Jeltsch MM, Birkenhager R, et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. [DOI] [PubMed] [Google Scholar]
- 40.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. 1998;95:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38:258–68. [DOI] [PubMed] [Google Scholar]
- 42.Deng Y, Zhang X, Simons M. Molecular controls of lymphatic VEGFR3 signaling. Arterioscler Thromb Vasc Biol. 2015;35:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clahsen T, Buttner C, Hatami N, et al. Role of endogenous regulators of hem- and Lymphangiogenesis in corneal transplantation. J Clin Med. 2020;9(2):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stacker SA, Achen MG. Emerging roles for VEGF-D in human disease. Biomolecules. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–52. [DOI] [PubMed] [Google Scholar]
- 46.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang LK, Garcia-Cardena G, Farnebo F, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101:11658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin JW. Min M, Larrieu-Lahargue F, et al. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao R Ji H. Feng N, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A. 2012;109:15894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie F, Zhang X. Luo W, et al. Notch signaling pathway is involved in bFGF-induced corneal Lymphangiogenesis and Hemangiogenesis. J Ophthalmol. 2019;2019:9613923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kojima T, Azar DT. Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin Y, Chauhan SK, El Annan J, et al. A novel function for programmed death ligand-1 regulation of angiogenesis. Am J Pathol. 2011;178:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao C, Sima J, Zhang SX, et al. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci. 2004;45:1758–62. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Stockard CR, Harkins L, et al. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech Histochem. 2008;83:179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroedl F, Kaser-Eichberger A, Schlereth SL, et al. Consensus statement on the immunohistochemical detection of ocular lymphatic vessels. Invest Ophthalmol Vis Sci. 2014;55:6440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gale NW, Prevo R, Espinosa J, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maruyama Y, Maruyama K, Kato Y, et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Invest Ophthalmol Vis Sci. 2014;55:4813–22. [DOI] [PubMed] [Google Scholar]
- 61.Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–7. [DOI] [PubMed] [Google Scholar]
- 62.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goyal S, Chauhan SK, El Annan J, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128:819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HS, Chauhan SK, Okanobo A, et al. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea. 2011;30:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. 2012;130:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okanobo A, Chauhan SK, Dastjerdi MH, et al. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol. 2012;154:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji YW, Lee JL, Kang HG, et al. Corneal lymphangiogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease. Ocul Surf. 2018;16:306–13. [DOI] [PubMed] [Google Scholar]
- 68.Cho YK, Archer B, Ambati BK. Dry eye predisposes to corneal neovascularization and lymphangiogenesis after corneal injury in a murine model. Cornea. 2014;33:621–7. [DOI] [PubMed] [Google Scholar]
- 69.Kwon JW, Chung YW, Choi JA, et al. Comparison of postoperative corneal changes between dry eye and non-dry eye in a murine cataract surgery model. Int J Ophthalmol. 2016;9:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seo Y, Ji YW, Lee SM, et al. Activation of HIF-1α (hypoxia inducible factor-1α) prevents dry eye-induced acinar cell death in the lacrimal gland. Cell Death Dis. 2014;5:e1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Min JH, Lee CH, Ji YW, et al. Activation of Dll4/Notch signaling and hypoxia-inducible factor-1 alpha facilitates Lymphangiogenesis in lacrimal glands in dry eye. PLoS One. 2016;11:e0147846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chauhan SK, Jin Y, Goyal S, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan X, Chen Y. Foulsham W, et al. The immunoregulatory role of corneal epithelium-derived thrombospondin-1 in dry eye disease. Ocul Surf. 2018;16:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cursiefen C, Maruyama K, Bock F,et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang T, Li W, Cheng H, et al. The Important Role of the Chemokine Axis CCR7-CCL19 and CCR7-CCL21 in the Pathophysiology of the Immuno-inflammatory Response in Dry Eye Disease. Ocul Immunol Inflamm. 2019:1–12. [DOI] [PubMed] [Google Scholar]
- 76.Pisella PJ, Labetoulle M, Doan S, et al. Topical ocular 0.1% cyclosporine A cationic emulsion in dry eye disease patients with severe keratitis: experience through the French early-access program. Clin Ophthalmol. 2018;12:289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen D, Zhang S, Bian A, et al. Efficacy and safety of 0.05% cyclosporine ophthalmic emulsion in treatment of Chinese patients with moderate to severe dry eye disease: A 12-week, multicenter, randomized, double-masked, placebo-controlled phase III clinical study. Medicine (Baltimore). 2019;98:e16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cutolo CA, Barabino S, Bonzano C, Traverso CE. The use of topical corticosteroids for treatment of dry eye syndrome. Ocul Immunol Inflamm. 2019;27:266–75. [DOI] [PubMed] [Google Scholar]
- 79.Kallab M, Szegedi S, Hommer N, et al. topical low dose preservative-free hydrocortisone reduces signs and symptoms in patients with chronic dry eye: a randomized clinical trial. Adv Ther. 2020;37:329–41. [DOI] [PubMed] [Google Scholar]
- 80.Mandal A, Gote V, Pal D, et al. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36:36. [DOI] [PubMed] [Google Scholar]
- 81.Kashani S, Mearza AA. Uses and safety profile of ciclosporin in ophthalmology. Expert Opin Drug Saf. 2008;7:79–89. [DOI] [PubMed] [Google Scholar]
- 82.Wan KH, Chen LJ, Young AL. Efficacy and Safety of Topical 0.05% Cyclosporine Eye Drops in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-analysis. Ocul Surf. 2015;13:213–25. [DOI] [PubMed] [Google Scholar]
- 83.Heidari M, Noorizadeh F, Wu K, et al. Dry eye disease: emerging approaches to disease analysis and therapy. J Clin Med. 2019;8(9):1439–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Utine CA. Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18:352–61. [DOI] [PubMed] [Google Scholar]
- 85.Perez VL. Pflugfelder SC, Zhang S, et al. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14:207–15. [DOI] [PubMed] [Google Scholar]
- 86.Keating GM. Lifitegrast Ophthalmic Solution 5%: A review in dry eye disease. Drugs. 2017;77:201–8. [DOI] [PubMed] [Google Scholar]
- 87.Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of Lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: a 1-year, multicenter, randomized, placebo-controlled study. Cornea. 2016;35:741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X, VJ M, Qu Y, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. 2006;148:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Dong F, Chen W, et al. Clinical efficacy of 0.1% pranoprofen in treatment of dry eye patients: a multicenter, randomized, controlled clinical trial. Chin Med J (Engl). 2014;127:2407–12. [PubMed] [Google Scholar]
- 91.Fujishima H, Fuseya M, Ogata M, Murat D. Efficacy of bromfenac sodium ophthalmic solution for treatment of dry eye disease. Asia Pac J Ophthalmol (Phila). 2015;4:9–13. [DOI] [PubMed] [Google Scholar]
- 92.Sosne G, Ousler GW. Thymosin beta 4 ophthalmic solution for dry eye: a randomized, placebo-controlled, Phase II clinical trial conducted using the controlled adverse environment (CAE™) model. Clin Ophthalmol. 2015;9:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaynes BI, Fiscella R. Topical nonsteroidal anti-inflammatory drugs for ophthalmic use: a safety review. Drug Saf. 2002;25:233–50. [DOI] [PubMed] [Google Scholar]
- 94.Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: clinical importance, pathogenesis, and risk mitigation. Surv Ophthalmol. 2020;65:1–11. [DOI] [PubMed] [Google Scholar]
- 95.De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–56. [DOI] [PubMed] [Google Scholar]
- 96.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–35. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L, Su Z, Zhang Z, et al. Effects of azithromycin on gene expression profiles of proinflammatory and anti-inflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease. JAMA Ophthalmol. 2015;133:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang X, Lv H, Qiu W, et al. Efficiency and safety of subconjunctival injection of anti-VEGF agent - bevacizumab - in treating dry eye. Drug Des Devel Ther. 2015;9:3043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasetsuwan N, Chantaralawan K, Reinprayoon U, Uthaithammarat L. Efficacy of topical bevacizumab 0.05% eye drops in dry eye disease: A double-masked, randomized trial. PLoS One. 2020;15:e0234186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allain H, Ramelet AA, Polard E, Bentué-Ferrer D. Safety of calcium dobesilate in chronic venous disease, diabetic retinopathy and haemorrhoids. Drug Saf. 2004;27:649–60. [DOI] [PubMed] [Google Scholar]
- 102.Fernandez IS, Cuevas P, Angulo J, et al. Gentisic acid, a compound associated with plant defense and a metabolite of aspirin, heads a new class of in vivo fibroblast growth factor inhibitors. J Biol Chem. 2010;285:11714–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cuevas P, Outeiriño LA, Azanza C, et al. Improvement in the signs and symptoms of dry eye disease with dobesilate eye drops. Mil Med Res. 2015;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alitalo AK, Proulx ST, Karaman S, et al. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013;73:4212–21. [DOI] [PubMed] [Google Scholar]
- 105.Hajrasouliha AR, Funaki T, Sadrai Z, et al. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2012;53:1244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamakawa M, Doh SJ, Santosa SM, et al. Potential lymphangiogenesis therapies: learning from current antiangiogenesis therapies-A review. Med Res Rev. 2018;38:1769–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Detry B, Blacher S, Erpicum C, et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2013;54:3082–93. [DOI] [PubMed] [Google Scholar]
- 108.Zhang L, Li G, Sessa R, et al. Angiopoietin-2 blockade promotes survival of corneal transplants. Invest Ophthalmol Vis Sci. 2017;58:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Logsdon EA, Finley SD, Popel AS. Mac Gabhann F: a systems biology view of blood vessel growth and remodelling. J Cell Mol Med. 2014;18:1491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kwon JW, Choi JA, Shin EY, et al. Effect of trapping vascular endothelial growth factor-a in a murine model of dry eye with inflammatory neovascularization. Int J Ophthalmol. 2016;9:1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bron AJ. The definition and classification of dry eye disease.Chan C, editor. Dry Eye: A Practical Approach. Switzerland: Berlin, Heidelberg, Springer Berlin Heidelberg; 2015. p. 1–19.. [Google Scholar]
- 114.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–76. [DOI] [PubMed] [Google Scholar]
- 115.Hsu MC, Pan MR, Hung WC. Two birds, one stone: double hits on tumor growth and Lymphangiogenesis by targeting vascular endothelial growth factor receptor 3. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]