Abstract
Traumatic brain injury (TBI) results when external physical forces impact the head with sufficient intensity to cause damage to the brain. TBI can be mild, moderate, or severe and may have long-term consequences including visual difficulties, cognitive deficits, headache, pain, sleep disturbances, and post-traumatic epilepsy. Disruption of the normal functioning of the brain leads to a cascade of effects with molecular and anatomical changes, persistent neuronal hyperexcitation, neuroinflammation, and neuronal loss. Destructive processes that occur at the cellular and molecular level lead to inflammation, oxidative stress, calcium dysregulation, and apoptosis. Vascular damage, ischemia and loss of blood brain barrier integrity contribute to destruction of brain tissue. This review focuses on the cellular damage incited during TBI and the frequently life-altering lasting effects of this destruction on vision, cognition, balance, and sleep. The wide range of visual complaints associated with TBI are addressed and repair processes where there is potential for intervention and neuronal preservation are highlighted.
Keywords: traumatic brain injury, light sensitivity, contrast sensitivity, neuroinflammation, visual acuity, headache, oxidative stress
1. Introduction
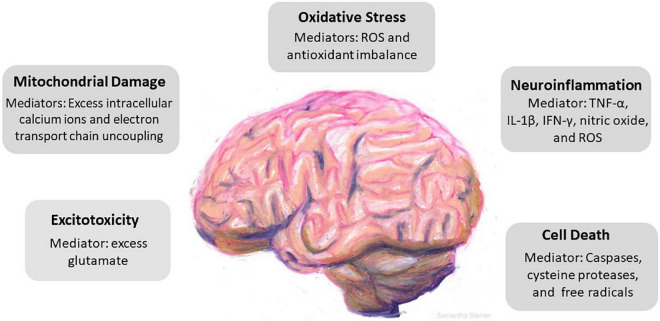
Traumatic brain injury (TBI) continues to be a major health concern in the United States and worldwide; often carrying long-term consequences that diminish quality of life and cause persistent cognitive impairment (Stocchetti and Zanier, 2016; Dewan et al., 2018). TBI can be mild, moderate or severe with about 80% of cases categorized as mild (Temkin et al., 2022). However, even mild TBI (mTBI) can lead to debilitating symptoms (McMahon et al., 2014; Ganti et al., 2019). It is misleading to assume that when the physical force applied to the head is weak, the consequences will be less. Some patients suffer substantially and for prolonged periods following mTBI, often with complaints of headache, dizziness, and memory issues (Prince and Bruhns, 2017; Hoffer and Balaban, 2019; Lu et al., 2020). Manifestations of TBI come on in phases, with a cascade of neurometabolic changes that affect the brain in complex and heterogeneous ways over a timespan ranging from days to years. Many TBI patients live with cognitive loss, behavioral problems, headaches and visual disturbances that interfere with their ability to work, socialize and fully participate in everyday life. This review will discuss our current knowledge of the functional and molecular deficits that result from TBI with an emphasis on the resulting impairments that affect the ability to carry out activities of daily living. Issues that link deficits in performance of key activities to visual sequelae involving focus, eyestrain, fatigue, and blurring will be highlighted. Beyond visual issues, cellular and molecular signaling pathways incited by TBI with resulting oxidative stress, inflammation, apoptosis, and autophagy are summarized in Figure 1 and discussed in detail below. Potential interventions to prevent or repair damage to the brain by limiting inflammation and facilitating neural regeneration are described.
FIGURE 1.
Cascade of cellular events driven by traumatic brain injury (TBI). TBI incites a series of responses that include excitotoxicity, mitochondrial damage, oxidative stress, neuroinflammation, and cell death. Key mediators in each pathological event is identified. Excess glutamate causes overactivation of N-Methyl-d-aspartate receptors (NMDAr) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAr) which induces neuronal overexcitation and swelling. Mitochondrial damage follows as a result of excess influx of intracellular calcium and uncoupling of the electron transport chain. The precipitous rise of reactive oxygen species and the brain parenchyma’s relatively low antioxidant capacity promotes oxidative stress. Neuroinflammation induces secondary damage via the release of proinflammatory cytokines, chemokines, and inflammatory mediators and ultimately leads to cell death.
2. Cellular response to traumatic brain injury
2.1. Excitotoxicity
A crucial feature of TBI is the acute phase, in which there is an immediate intractable excessive release of excitatory neurotransmitters that results from the stretching and tearing of brain tissue (Prins et al., 2013). This physical disruption leads to a cascade of pathological events called excitotoxicity (Hoffe and Holahan, 2022). The primary excitatory neurotransmitter released from presynaptic nerve terminals after an impact to cerebral tissues is glutamate (Chamoun et al., 2010; Thapa et al., 2021). In humans, up to a 50-fold increase in glutamate levels have been found, especially in patients with focal parenchymal contusions (Bullock et al., 1995; Bullock et al., 1998). The accumulation of glutamate in the synaptic cleft leads to repeated stimulation and overactivation of N-Methyl-d-aspartate receptors (NMDAr) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAr) on the post-synaptic membrane (Lozano et al., 2015). With NMDAr activation, sodium channels also open and neurons swell (Wang et al., 2016). Glutamate shifts AMPAr toward a subtype that is more permeable to calcium ions (Spaethling et al., 2008). The opening of calcium channels and excess entry of intracellular calcium ions incites a destructive cascade. A multitude of catabolic enzymes are released, including phospholipases that destroy cell and mitochondrial membranes, proteases that interfere with cytoskeletal organization, and endonucleases that precipitate DNA fragmentation. This culminates in apoptosis and necrosis and cell death (Wang et al., 2016).
The presence of excessive glutamate post-TBI is primarily due to failure of glutamate re-uptake (Tasker, 2012; Baracaldo-Santamaría et al., 2022). Synaptic glutamate is removed by excitatory amino acid transporters (EAATs) that are expressed predominantly in astrocytes (Rothstein et al., 1996). Evidence has demonstrated a 40% decline in the expression of astrocytic sodium-dependent glutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2) within 24 h following TBI, leading to a significant decrease in the resorption of glutamate (Rao et al., 1998; van Landeghem et al., 2006). Modulating effects of glutamate excitotoxicity has been explored as a mechanism for minimizing damage in TBI. However, pharmacologic antagonism of NMDA receptors has serious side effects including hallucinations, agitation, nausea (Morris et al., 1999; Ikonomidou and Turski, 2002).
Sowers et al. demonstrated excess glutamate oxidation in defined areas of injured hemispheres of rodents using matrix-assisted laser desorption ionization (MALDI)-imaging studies and metabolomics (Sowers et al., 2021). Glutamate is largely metabolized to glutamine in astrocytes. However, if oxidized, glutamate generates α-ketoglutarate, which yields ATP via the tricarboxylic acid (TCA) cycle. Sowers proposed that under conditions of great excess, glutamate oxidation may serve as a defense mechanism against glutamate excitotoxicity post- head injury and postulates that this mechanism may be leveraged to obtain better outcomes in TBI patients.
2.2. Mitochondrial damage
The massive accumulation of intracellular free calcium incited by depolarization promotes the activation of kinases and downstream enzymes capable of degrading phospholipids of the mitochondrial membrane (Jastroch et al., 2010). Although the mitochondria have protective calcium-buffering mechanisms in place, eventually the influx of calcium exceeds the buffering capacity and excess calcium ion accumulation in the mitochondria causes uncoupling of the electron transport chain located in the inner mitochondrial membrane (Duchen, 2012). This eliminates the concentration gradient between the mitochondrial matrix and the mitochondrial intermembrane space and reduces the capacity of the organelle to produce ATP (Bayir et al., 2005; McGovern and Barreto, 2021). The reduction of membrane potential via chemical uncoupling may serve as a defense against calcium overload by inhibiting uptake of calcium ions and serve a neuroprotective function in TBI, but loss of the gradient leads to permeabilization of the internal mitochondrial membrane as mitochondrial permeability transition pores open and ions and fluid flood the mitochondria. Eventually damage to the membrane becomes irreversible and bioenergetic collapse leads to cell death (Pandya et al., 2007).
The uncoupling of the electron transport chain also places oxidative stress on cells via free electrons that are easily trapped by oxygen. The subsequent buildup of reactive oxygen species (ROS) further intensifies the degradation of mitochondrial membrane phospholipids such as cardiolipin that are essential to maintain the selectivity and permeability of the inner mitochondrial membrane (Bayir et al., 2007; McGovern and Barreto, 2021). Furthermore, membrane degradation stimulates the cytosolic translocation of Cytochrome C, one of the mitochondria-dependent mechanisms for the activation of apoptosis or programmed cell death. Once in the cytosol, cytochrome C activates caspase proteins and other apoptotic proteins, eventually leading to apoptotic cell death (Kagan et al., 2005; Kumar, 2007; Chao et al., 2019).
2.3. Oxidative stress
Oxidative stress, defined as the imbalance of ROS and antioxidants, plays a crucial role in causing secondary damage post TBI (Birben et al., 2012). ROS levels in the brain rise precipitously following injury and may remain elevated for days afterward (Hall et al., 1993). Oxygen-derived free radicals known to place oxidative stress on the brain include hydrogen peroxide, superoxide anions, hydroxyl, and peroxyl radicals (Lewén et al., 2000; Hakiminia et al., 2022). These radicals can irreversibly oxidize macromolecules and injure cells. The brain parenchyma is especially vulnerable to oxidative damage due to its high oxidative metabolic activity, relatively low antioxidant capacity, and inadequate repair mechanisms (Shohami et al., 1997; Cernak et al., 2000; Ismail et al., 2020). In TBI, ROS are generated via mitochondrial leakage, the arachidonic acid cascade, and catecholamine oxidation (Hall et al., 1992; Marklund et al., 2001; Ismail et al., 2020). Neutrophils, which infiltrate the brain after TBI, are an important source of ROS and produce these molecules primarily by enzyme activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX)2 (Liu et al., 2018; Kim et al., 2022). NOX are a family of multi-subunit transmembrane enzymes that reduce molecular oxygen into ROS and catalyze formation of the superoxide anion (Bedard and Krause, 2007). NOX2 is the primary producer of ROS in brain tissue and thus plays a crucial role in the development of secondary injury after TBI (Choi et al., 2012). Zhang et al. (2012) demonstrated NOX activation in microglia significantly contributed to neuronal damage within 24–48 h post TBI and further raised ROS production. Oxidative damage can manifest as lipid peroxidation of neuronal, glial, and vascular cell membranes as well as myelin (Zhang et al., 2012; Daradkeh et al., 2021). Consequently, NOX inhibition has become a therapeutic target of investigation as a means to reduce oxidative damage in TBI and has met with some success in murine models (Angeloni et al., 2015; Chandran et al., 2018; Wang et al., 2022). The severity of injury in TBI can be correlated with the degree of ROS-related tissue damage and mitochondrial dysfunction (Tavazzi et al., 2005; Lee et al., 2021).
Arachidonic acid, normally stored in phospholipid membranes within cells, is released from membranes under hypoxic and inflammatory conditions and can then form neurotoxic metabolites. When arachidonic acid in the brain undergoes peroxidation and further reactions, it can generate a lipid hydroperoxide and an alkyl radical and ultimately neurotoxic aldehydes (carbonyls) (Yang et al., 2019).
Catecholamine are vulnerable to oxidation because they have a highly reactive quinonoid nucleus. Aberrant oxidation converts catecholamine molecules into quinones that may generate superoxide-free radicals that act as oxidizing agents, damaging neurons (Wang et al., 2021).
2.4. Neuroinflammation
Neuroinflammation inducing secondary damage to the brain is heralded by the release of proinflammatory cytokines, chemokines and inflammatory mediators (Ziebell and Morganti-Kossmann, 2010). The resulting inflammatory environment stimulates resident microglia and astrocytes while activated brain endothelial cells cause blood brain barrier (BBB) permeabilization which brings about infiltration of peripheral leukocytes (Acosta et al., 2013; Hernandez-Ontiveros et al., 2013; Liu et al., 2022). Inflammatory mediators are released by these leukocytes, specifically macrophages, neutrophils and lymphocytes, and play a crucial role in neuronal death (Mele et al., 2021). Activated resident microglia further attract immune cells into the injured areas of the brain. Microglial processes represent a first-line defense barrier between healthy and injured areas of the brain (Davalos et al., 2005; Haynes et al., 2006). When activated, microglia release oxidative metabolites such as nitric oxide, ROS, and pro-inflammatory cytokines such as TNF-α, interleukin (IL)-1β, and interferon (IFN)-γ (Lozano et al., 2015). ROS and cytokines then perpetuate the cycle by contributing to brain tissue damage (Kalra et al., 2022). Additionally, chronic complement dysregulation post-injury also plays a critical role in promoting neuroinflammation and neuronal cell death (Dong et al., 2023). Parry and colleagues found that formation of a specific component of the complement system, soluble membrane attack complex C5b-9 (sC5b-9), was elevated in TBI and may be involved in secondary injury and death of neurons (Parry et al., 2020). Toutonji et al. (2021) characterized the expression of 59 complement genes at different time points post-TBI and demonstrated an upregulated gene expression across most complement activation and effector pathways. The study found continued upregulation of C2, C3, and C4 expression up to 2 years following injury. Treatment using the targeted complement inhibitor, CR2-Crry, was also shown to significantly ameliorate TBI-induced transcriptomic changes at all time points after injury.
2.5. Cell death
Secondary damage after head trauma ultimately leads to neuronal cell death. Dying neural cells can exhibit either an apoptotic or a necrotic morphology (Raghupathi, 2004; Akamatsu and Hanafy, 2020). Caspases, cysteine proteases activated by proteolytic cleavage, are principal mediators of apoptotic cell death. Excitotoxicity, increased influx of intracellular calcium, as well as the production of free radicals brings about opening of mitochondrial permeability transition pores which leads to cytochrome C release into the cytosol (Bredesen, 2008; Akamatsu and Hanafy, 2020). The cytochrome C forms a complex with apoptotic protease activating factor-1, which then recruits and cleaves inactive procaspase-9 to activate caspase-9, which initiates the apoptotic cascade and activates caspase-3. Caspase-9 and caspase-3 are the key effector enzymes in neuronal apoptosis (Van Opdenbosch and Lamkanfi, 2019). The process that precedes cytochrome C release is thought to involve Bcl-2 family proteins that regulate the permeability of the outer mitochondrial membrane. TBI disturbs the balance between pro-apoptotic BAX, which forms pores in the outer mitochondrial membrane, and anti-apoptotic B cell lymphoma 2 (BCL-2), a pro-survival protein that binds to BAX (Wennersten et al., 2003; Stoica and Faden, 2010; Deng et al., 2020). Numerous natural and artificial caspase inhibitors have been identified and developed with the intention for therapeutic use in reducing neuronal cell death post TBI (Dhani et al., 2021; Feng et al., 2022; Unnisa et al., 2022).
3. The pupil and TBI
Pupils respond to three types of stimuli: they constrict in response to light falling on the retinal photoreceptors via the pupillary light reflex (PLR), constrict to bring near objects into focus via the accommodation reflex, and dilate in response to increased arousal triggered by strong emotions (Bradley et al., 2008; Mathôt, 2018; Bouffard, 2019; Figure 2). When one eye is exposed to illumination, the pupil of the other eye will constrict in a consensual response. The PLR is involved in both convergence and accommodation, with the parasympathetic system driving constriction through the sphincter pupillae muscles of the iris and the sympathetic system driving dilation through the dilator pupillae muscles of the iris. Dilation is mediated by activity in the locus coeruleus, hypothalamus, and superior colliculus (Mathôt, 2018). It should be noted that both constriction and dilation occur depending on the stimuli and there is a maximum amount the pupil can change. For example, dimming the light falling on the retina causes dilation of the pupils until the pupil diameter reaches maximum opening.
FIGURE 2.
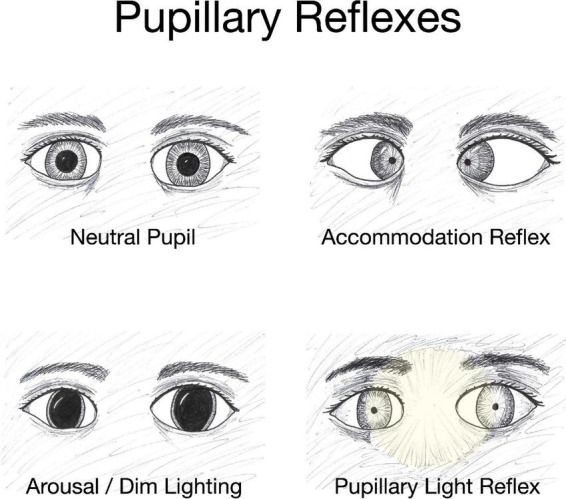
Pupillary reflexes. The diameter of the pupil changes in response to specific conditions such as variations in object distance and level of illumination. The accommodation reflex, an adjustment for near vision, results in pupillary constriction, and inward rotation of the eyes as an object draws nearer. Dim lighting elicits pupillary dilation as does emotional arousal. Increased brightness of lighting causes pupillary constriction and the effect is both direct (affecting the eye exposed to the light) and consensual with constriction of the pupil in the eye opposite to the light-stimulated eye.
The pupils can be an accessible and reliable indicator of the neural integrity of the visual system (Joshi and Gold, 2020). However, impaired pupillary responses can occur without damage to the neural integrity of the visual system as exemplified by arousal, which can trigger a pupillary response independent of the visual system (Bradley et al., 2008; Tapper et al., 2021). The PLR is more than just a reflex as it is influenced significantly by central brain function (Carrick et al., 2021). Patients who present with TBI commonly have associated pupillary abnormalities (Helmy et al., 2012). Patients with mTBI may present with pupillary responsivity that is significantly delayed, slowed, and reduced compared to the normal population (Ciuffreda et al., 2017; Thiagarajan and Ciuffreda, 2022). The value of quantitative pupillometry as a non-invasive method for assessing TBI is still being explored, but is not established (Master et al., 2020; Traylor et al., 2021).
In cases of severe head trauma, acute pupillary dilation is a neurological emergency. Pupillary dilation is hypothesized to be the consequence of uncal herniation causing mechanical compression of the oculomotor nerve and resulting in brain stem compromise (Ritter et al., 1999; Manley and Larson, 2002). Non-reacting pupils are associated with a poor prognosis (Marmarou et al., 2007). An isolated third cranial nerve palsy can cause anisocoria (uneven pupil size) and can be an ominous sign in the TBI patient. Third nerve palsy can be associated with expanding mass lesions such as extradural and subdural hematomas (Uberti et al., 2021). Anisocoria, especially with exposure to bright light, was shown to correspond to TBI severity in an analysis of prospectively collected registry data on 118 patients with blunt head trauma (Nyancho et al., 2021). An isolated third nerve palsy can also be seen in minor head trauma (Kim and Chang, 2013). The third nerve, also known as the oculomotor nerve, plays an essential role in oculomotor function (Condos et al., 2022). Damage to the third nerve causes efferent impairment of pupillary constriction and thus the pupil will become dilated and poorly responsive to light. As the third nerve has an important role in extraocular movements, in patients with third nerve palsy the eye appears down and turned outward on the ipsilateral side. The third nerve is also important in lid elevation via the levator muscle. Significant lid droopiness or ptosis can be a clear sign of third nerve damage (Nagendran et al., 2019).
Many believe that the afferent pupillary defect must be assessed in the TBI patient (Broadway, 2012). In head trauma there is often ocular trauma which can damage the optic nerve of the ipsilateral eye (Qiu et al., 2022). Trauma is not always evident and the decrease in vision may not be profound. The afferent pupillary defect generally does not cause anisocoria and can be a subtle but important finding. Testing for an afferent pupillary deficit involves the swinging flashlight test (Broadway, 2012). The pupil normally constricts to bright light and then redilates when light stimulus is removed. This happens to both pupils as this is a consensual or bilateral phenomenon. In the presence of an afferent pupillary defect, the pupil will paradoxically dilate on the involved side because of a decrease in afferent light input to the optic nerve on that side (Kennedy et al., 2013). Patients with an afferent pupillary defect need a thorough neuro-ophthalmologic evaluation as well as neuroimaging.
In addition to optic nerve damage, local trauma to the eye can damage the efferent loop to pupillary movement and can compromise the sphincter muscles responsible for pupillary constriction. The pupil may no longer be round if damage is asymmetric. Muscle damage can lead to traumatic pupillary dilation (traumatic mydriasis on the ipsilateral side) (Thuma et al., 2022).
Abnormal pupils are often observed in TBI patients with elevated intracranial pressure (Jahns et al., 2019). An early examination is crucial in assessing the TBI with its associated signs and decompressive craniectomy can control the refractory intracranial hypertension. In certain cases of TBI, lasting bilateral mydriasis and an absence of the PLR may be seen. Absence of PLR is generally associated with brainstem damage and a poor prognosis (Chaudhuri et al., 2009; Helmy et al., 2012). There is currently no effective treatment, but surgical craniectomy may be attempted (Athanasiou et al., 2017).
4. TBI and contrast sensitivity
Contrast sensitivity, an important characteristic of the visual system, refers to the ability to discriminate between shapes and objects when they do not differ much in brightness or color in comparison to the background and their border may be indistinct (Owsley, 2003; Richman et al., 2013). Eye care professionals commonly use the Snellen eye chart to assess monocular and binocular visual acuity by having the patient identify letters in dark black on a white background on a well-lit screen (Sloan, 1951; Ricci et al., 1998; Tsui and Patel, 2020). However, because these eye charts only measure high-contrast sensitivity, many patients may have excellent visual acuity but present with issues in vision on a day-to-day basis. Contrast sensitivity can be applied to detect aspects of visual quality change beyond acuity and perimetry that relate to driving, facial recognition and various of tasks of daily living (West et al., 2002; Ginsburg, 2003; Swan et al., 2019). Although these tasks involve multiple complex processes, measuring medium and low-contrast sensitivity can be useful, as it may uncover visual defects not seen on a regular eye exam (Boutet et al., 2015). The gold standard for measuring contrast sensitivity involves assessing contrast sensitivity as a function of spatial frequency (contrast sensitivity function), which is time-consuming and complex. Practical clinical measurement may employ pre-printed grating charts, contrast sensitivity letter charts such as the Pelli-Robson or Rabin chart, and computer-based determination of contrast sensitivity thresholds (Elliott et al., 1990; Rabin, 1994; Richman et al., 2015). Paper charts are wall-mounted and require external illumination. They are still widely used, inexpensive and show good reproducibility (Patel et al., 2009).
Traumatic brain injury has been shown to reduce contrast sensitivity (Alnawmasi et al., 2019; Honig et al., 2021). However, it is important to characterize the type of contrast sensitivity impacted; TBI patients have increased sensitivity to first-order motion stimuli, but have decreased sensitivity to contrast-defined and orientation-defined second-order stimuli (Spiegel et al., 2016). First-order stimuli are simply delineated from their backgrounds according to how much the object is illuminated, while second-order stimuli are recognized by the differences in their contrast or texture (Baker and Mareschal, 2001; Lu and Sperling, 2001). By utilizing a motion direction discrimination task, Piponnier et al. (2016) demonstrated that reaction times for second-order stimuli were slower than first-order stimuli in patients who had suffered TBI. Evaluation of contrast sensitivity in TBI patients in addition to testing their visual acuities can lead to more accurate assessment of functional vision. Through more complete visual examination, decreased contrast sensitivity in TBI patients can be detected and managed (Barnett and Singman, 2015). In treating contrast sensitivity deficits, some have advocated utilizing tinted lenses to increase contrast sensitivity, specifically under glare conditions (Lee et al., 2002; Lacherez et al., 2013).
5. Flashing lights after TBI
Flashing lights, or photopsia, is a common visual disturbance symptom characterized by seeing brief, flash-like stars in front of the eyes (Virdee and Mollan, 2020). The causes can be numerous and the etiology needs to be investigated. Patients who present with mTBI commonly have associated photopsia (Ciuffreda et al., 2021). In cases of head trauma, seeing stars is generally a transient event resulting from spontaneous firing of neurons in the occipital lobe upon impact and the interpretation of these random electrical impulses as stars by the brain (Moser et al., 2005; Chen et al., 2019).
The crucial clinical question is to ascertain whether the reported phenomena is unilateral or bilateral. Unilateral photopsia (flashing white lights in one eye only) usually originates within the eyeball. Photopsia experienced as flashing lights in both eyes simultaneously is usually central in etiology and represents activity in the brain (Virdee and Mollan, 2020). This is a key clinical distinction and may not be obvious to the patient. Colored lights are almost always of central etiology.
Unilateral photopsia requires a thorough ophthalmologic history and examination. Flashing lights can be accompanied by symptoms of floaters (dark spots in field of vision). This is most commonly caused by a posterior vitreous detachment (Byer, 1994; Verhoekx et al., 2021). The vitreous has attachments to the retina and retraction of these attachments can come about due to forces generated during TBI. This stimulates the retinal cells and is perceived as flashing lights. Tiny particles of debris and sometimes blood can be distributed as a result of this activity and can be perceived as floaters. Usually this vitreous separation is an innocuous event and does not damage the retina. Floaters often become asymptomatic after a few months. However, if there is sufficient traction upon the retina during this separation, a retinal tear can occur. This is a more serious event and can subsequently develop into a retinal detachment where the retina is torn loose from the choroid.
Retinal tears associated with head trauma may present as photopsia (Sornalingam et al., 2018). It is particularly important to assess for retinal tears and retinal detachment when evaluating head trauma because these are emergencies that require prompt intervention (Amos, 1999; Ibrar et al., 2021). Symptoms may be overlooked and attributed to the concussed state (Bedgood et al., 2015). The retina, an extension of the central nervous system (CNS), converts light signals into nerve impulses (Behar-Cohen et al., 2020). Retinal detachment can result in permanent visual loss and thus early examination is critical as tears can be treated to prevent detachment.
Visual snow syndrome (VSS), a rare condition characterized by the appearance of pixelated flickering dots in one plane in front of and throughout a visual field, may be incited by mTBI (Ciuffreda et al., 2021; Hang et al., 2021; Mehta et al., 2021; Werner and Gustafson, 2022). The pathophysiology of VSS is not well-understood, there is no effective treatment and it tends to persist over time (Fraser, 2022).
Bilateral flashing lights is a more complex phenomenon. Visual information is bifurcated at the optic chiasm as it enters the brain, so information is no longer segregated by origin from the left or right eye. This phenomenon going on in the left side of the brain impacts the right visual field and phenomenon going on in the right side of the brain impact the left visual field. Migraine auras are the most common cause of bilateral flashing lights or patterns of lights confined to one hemifield, but, as mentioned earlier, the impact from a TBI event can lead to brief, transient “seeing stars” due to pressure on the occipital lobe.
6. Visual and mental fatigue after TBI
6.1. Visual fatigue and light sensitivity
Traumatic brain injury can result in visual disturbances and discomfort of which the most common are blurred vision, double vision, light sensitivity, and visual fatigue (Greenwald et al., 2012; Armstrong, 2018). Approximately 50–70% of TBI patients have visual difficulties (Ciuffreda et al., 2007; Greenwald et al., 2012; Berthold-Lindstedt et al., 2017). Photosensitivity is seen in about 50% of mTBI patients and some relief may be given with tinted lenses (Truong et al., 2014). Light sensitivity can be assessed clinically under varying light sources and intensities or by patient self-reporting and validated questionnaires, but there is no established objective test for this somewhat subjective symptom (Truong and Ciuffreda, 2016). The etiology of light sensitivity after TBI is complex and has been postulated to involve damage to intrinsically photosensitive retinal ganglion cells (ipRGC) (Mostafa et al., 2021). These specialized cells are involved in circadian rhythm and other non-image associated functions and can be activated intrinsically via the photopigment melanopsin without input from rods or cones. In a mouse model, increases in melanopsin after TBI accompanied light aversion (Honig et al., 2019).
Oculomotor dysfunction is very common after TBI, with estimates of frequency of occurrence between 60 and 85% (Ciuffreda et al., 2007). Oculomotor dysfunction, attributed to damage to efferent pathways, is due to effects on cranial nerves, nerves controlling eye movements and vestibular pathology (Thiagarajan et al., 2014). When control of the position and movement of the eyes is impaired, there can be a delay in shifting focus between close and far fields, trouble with keeping focus on a close object, vergence-related abnormalities, and difficulties with tracking moving objects (Poltavski and Biberdorf, 2014; Kontos et al., 2017; Reneker et al., 2018). The consequences of oculomotor dysfunction may cause a person to skip lines when reading, perceive movement of print when reading, and experience eye strain (Capó-Aponte et al., 2017; Kapoor and Ciuffreda, 2002). Individuals with mTBI show complications in functional task abilities when engaging in reading comprehension. In reading tasks, those with mTBI exhibit more fixation time when reading words with lower frequencies. Overall, mTBI patients may display weaker functioning in reading in terms of accuracy, time of fixation, and a lower number of blinks (Ratiu et al., 2022). Sustained reading becomes very difficult and eyestrain is common. Convergence insufficiency with hampered ability to focus on a near target makes reading an arduous task. The need for ocular muscles to compensate for injury-induced diminished responsiveness leads to eyestrain and eye muscle fatigue. Headache may ensue (Ciuffreda et al., 2007). Patients with vergence problems may attain some relief with specially designed eyeglasses containing prism lenses and may be prescribed specific eye exercises (Aletaha et al., 2018).
6.2. Mental fatigue
In addition to visual fatigue, mental fatigue and feeling tired with low mental energy are common after TBI and can interfere with everyday living and fulfilling job responsibilities (Cantor et al., 2008; Johansson et al., 2009). It has been postulated that mental fatigue occurs because the injured brain is not working efficiently and has to expend extra energy to perform tasks that previously did not deplete reserves (Kohl et al., 2009). The brain becomes overloaded at low threshold and recovery is slowed compared to pre-trauma. When performing working memory tasks, the traumatized brain exhibits increased activity progressively over time in bilateral dorsolateral prefrontal cortex and the inferior parietal brain regions as a compensatory mechanism to meet demand and that this results in fatigue (Dettwiler et al., 2014; Sun et al., 2014). Trauma-induced changes in regional cerebral blood flow (rCBF) may help to explain fatigue symptoms. Magnetic resonance imaging studies show that self-assessment of perceived fatigue during a psychomotor vigilance task correlates to changes in rCBF (Möller et al., 2017). The rCBF differed in mTBI patients compared to healthy controls during the task in multiple brain regions including the left thalamus and superior frontal gyri, right precuneus and insula, and left/right medial frontal gyri and anterior cingulate cortex. During the task, the mTBI patients exhibited fatigue as slowing of performance over time. A correlation was found between self-perceived fatigue after a psychomotor vigilance task and high rCBF in the left medial frontal and anterior cingulate gyri. In a study by Ramage et al. (2019), pattern of functional connectivity, reflecting communication between brain regions, differed between control and mTBI subjects performing a behavioral task with heightened connectivity across all effort levels for the TBI subjects. This may reflect inefficiency of the activity of the neural network that leads to fatigue.
6.3. The intersection of mental fatigue and visual fatigue
Although the causes of the experience of mental exhaustion are not known, a correlation has been found between number of visual symptoms and self-reported feelings of mental fatigue (Berthold Lindstedt et al., 2019). The connection between visual processing and mental fatigue after TBI crystallizes in the work of Prak et al. (2021) in which a correlation was seen between self-reported fatigue and increased activation of the bilateral visual cortices in areas associated with motion perception, attention, and oculomotor pursuit.
Insomnia is a common symptom after TBI that may contribute to both eye fatigue and mental fatigue (Jain et al., 2014; Ouellet et al., 2015). Not surprisingly, there is a positive association between sleep disruption and fatigue in TBI patients (Englander et al., 2010; Cantor et al., 2012). The pattern of sleep disruption in TBI is heterogeneous and difficult to characterize (Raikes et al., 2019). In order to explore sleep characteristics of mTBI patients, Arbour et al. (2015) evaluated 34 mTBI subjects and 29 age-matched controls using polysomnographic recording at a sleep laboratory and found little difference in non-rapid eye movement (NREM) sleep patterns. They did find the mTBI group had increased beta power in the occipital derivation compared to the control group in all sleep cycles, which may result from injury to the brain as well as anxiety and pain. The eyes may be affected by insufficient sleep, leading to ocular discomfort, dry eyes, and itching (Kawashima et al., 2016; Li et al., 2018). Sleep disorders may make reading, driving, and viewing computer screens more difficult and uncomfortable (Engle-Friedman et al., 2018; Gibbings et al., 2022).
Chronic post-traumatic headaches are a reoccurring issue for TBI patients that can augment both visual and mental fatigue (Faux and Sheedy, 2008; Voormolen et al., 2019; Ashina et al., 2020). The headaches may resemble a tension headache or have migraine-like qualities and may be accompanied by nausea, vomiting, and photophobia (Ashina et al., 2019). Nearly every type of headache listed by the International Headache Society can be linked to vision disorders after TBI (Dwyer, 2018; Quaid and Singman, 2022). Again, reading or looking at a computer screen becomes more challenging when headache is present. Eye fatigue and mental fatigue overlap in their ability to compromise quality of life after TBI, and both are exacerbated by disrupted sleep and headaches.
7. TBI and falls in older persons
For older persons, the rate of fall-induced TBIs is almost twice that of younger persons (Haring et al., 2015). Ground-level falls are the most common cause of injury in the geriatric population and the prevalence of fall-related TBI is steadily increasing (Harvey and Close, 2012; Korhonen et al., 2013; McGuire et al., 2017; Miyoshi et al., 2020). In the United States in 2013, TBI accounted for over 434,000 (2,232.2 per 100,000 population) emergency department visits, hospitalizations, and deaths in persons aged 75 and older (Thompson et al., 2006). TBI in older adults is a major cause of morbidity and mortality and a significant health and socioeconomic problem (Bailey et al., 2022). Over 60% of TBI cases in older individuals are due to unintentional falls, which become more likely as people age due to muscle weakness, balance issues, deteriorating vision, and effects of some widely used medications (Gale et al., 2016).
Balance problems are another major issue faced by our aging population. In fact, roughly one in five elderly persons experience problems related to dizziness or balance annually (Lin and Bhattacharyya, 2012). Certain medications may increase the likelihood of falls among the elderly (Gillespie et al., 2012; Hopewell et al., 2018). Hart et al. (2022) found that the dose of CNS-active medications, which include antidepressants, opioids, and benzodiazepines, was not reduced appreciably following a fall-related injury in older adults. This suggests that adjustment in CNS-active medications is not being utilized adequately to modify fall risk. Furthermore, Evans et al. (2011) conducted a study to gain a better understanding of the impact of multiple medication utilization by trauma patients 45 years and older. The study demonstrated that over 40% of the trauma patients were receiving five medications or more at the time of injury. As such, the patients were at a greater risk for complications, lower functional outcomes, and longer hospitalizations. Consequently, decreasing concurrent medication use may be beneficial for trauma patients and should be investigated further (Evans et al., 2011).
Traumatic brain injury in geriatric patients can have a compounding effect on vision in persons who may already have diabetic retinopathy, glaucoma, macular degeneration, cataracts, and other conditions (Reed-Jones et al., 2013; Ehrlich et al., 2019; Teo et al., 2021). Depending on location and severity, TBI can lead to damage to the optic nerve, optic tract, and occipital lobe. These injuries can manifest as blurred vision, double vision, and/or decreased peripheral vision (Sen, 2017). In order to avoid obstacles, older persons need to safely navigate their environment and, when visual input is insufficient, may require behavioral or assistive technology (Patla, 1997; Marigold and Patla, 2008; Slade et al., 2021). Visual impairments in the elderly are a major contributing factor in falls (Coleman et al., 2007; Freeman et al., 2007).
Falls may, of course, result in hospitalization without impacting the head or causing TBI, but falls are the most common cause of TBI in older adults (Fu et al., 2017; Hofmann et al., 2021). Intracranial hemorrhage can occur as a consequence of TBI and is one of the more serious potential complications associated with falls (de Wit et al., 2020a). Falling on level-ground is the most common cause of intracranial bleeding. de Wit et al. (2020b) found that 1 in 20 seniors who presented to the emergency department after a ground-level fall are diagnosed with intracranial bleeding. As such, patients taking anticoagulants are at an increased risk of intracranial hemorrhage and should be monitored appropriately. The major increase in TBI-related hospitalizations is driven by falls in the elderly and these falls are leading to a significant increase in diagnosed intracranial hemorrhages (Harvey and Close, 2012; Nederpelt et al., 2022).
Traumatic brain injury can lead to the development of cerebral microbleeds (CMB), small hemosiderin deposits that come from bleeding of injured small arteries, cerebral arterioles, or capillaries (Lee et al., 2018). CMB are associated with gait and balance issues as well as progressive cognitive decline (Altmann-Schneider et al., 2011; Yates et al., 2014; Yakushiji, 2015; Ungvari et al., 2017; Irimia et al., 2018). CMB are associated with slower processing speed, defective attention and executive function, and they have been linked to promoting major depressive episodes (Niogi et al., 2008; Wang et al., 2014; Akoudad et al., 2016). Aging has been shown to exacerbate microvascular fragility and promote the formation of CMB (Toth et al., 2021). The prevalence of CMB increases with advancing age and approaches 20% by age 65 (Vernooij et al., 2008; Altmann-Schneider et al., 2011; Ungvari et al., 2017; Irimia et al., 2018). As TBI can affect the elderly population disproportionately, it is important to monitor older persons in order to prevent any adverse effects resulting from CMB.
Traumatic brain injury in older persons is associated with poorer functional outcomes, higher risk of mobility restriction, permanent disability, and loss of independence (LeBlanc et al., 2006; Gardner et al., 2018; Gavrila Laic et al., 2021; Tyler et al., 2022). While the reasons for poorer outcomes are not fully known, the high incidence of comorbidities that accompany aging may contribute (Winter et al., 2022). Another factor may be the higher proportion of older persons using warfarin or other anticoagulants that increase risk of brain bleed with head trauma, leading to poorer outcomes (Zeeshan et al., 2018; Giner et al., 2022). Mortality is also extremely common following a TBI amongst this aging population (Susman et al., 2002; Sylliaas et al., 2009). Prevention of falls in older persons may involve multiple initiatives designed to minimize risk and improve stability, including balance and strength training, securing appropriate footwear, minimizing polypharmacy, appropriate vision aids, and environmental modifications (Moncada and Mire, 2017).
8. TBI and seizures
Patients who present with moderate to severe TBI often have associated seizures (Fordington and Manford, 2020). Seizures that occur immediately (within 24 h of trauma) are not considered “epileptic” and are attributed to the impact itself (Agrawal et al., 2006). Epilepsy developing after an acute brain insult is referred to as post-traumatic epilepsy (PTE) (Verellen and Cavazos, 2010; Anwer et al., 2021). In evaluating any patient presenting with a seizure of unclear etiology, history of head injury is crucial information. Seizure activity can worsen the consequences of TBI by depriving the brain of oxygen and causing release of inflammatory mediators. The seizure itself can act like a second brain injury (Mazzeo and Gupta, 2018). In mTBI, seizures are less common, but if a seizure occurs in the emergency room, or shortly after discharge, this can be a warning sign that important findings of significant intracranial injury may have been missed (Vaniyapong et al., 2020).
Traumatic brain injury is a key cause of PTE, a condition in which recurrent, unprovoked chronic seizures occur 2 weeks or more after the TBI event (Campbell et al., 2014). PTE is a common form of acquired epilepsy representing 5% of all cases of epilepsy (Fordington and Manford, 2020). It is estimated that between 3 and 5% of moderate TBI cases and between 25 and 50% of severe TBI cases go on to have PTE (Agrawal et al., 2006).
Epileptogenesis, the process through which changes occurring in the brain lead to seizures, is hypothesized to begin at the moment of the trauma itself, even though the latent period before epilepsy develops can last weeks, months or even years after the inciting injury (Benardo, 2003). Seizures tend to persist over time (Annegers et al., 1998; Steinmetz et al., 2013). The seizures originate in perilesional cortical and mesiotemporal regions (Englander et al., 2014; Perucca et al., 2019; Tubi et al., 2019). Although the pathways that lead from brain injury to seizure are not completely understood at the molecular level, PTE is thought to result from neuroinflammation, oxidative stress and neuronal loss (Li L. et al., 2021; Reddy et al., 2022). Predictors for likelihood of developing PTE include the presence of intracranial bleeding and more severe TBI (Englander et al., 2003; Pease et al., 2022).
There is currently no treatment to prevent onset of PTE, but it is imperative to evaluate patients for seizure activity immediately post-TBI as antiepileptic drugs can be given to manage associated symptoms (Englander et al., 2014; Reddy et al., 2022). An electroencephalogram (EEG) should be used when evaluating TBI patients because up to 25% of these patients may have sub-clinical seizure activity on EEG (Ronne-Engstrom and Winkler, 2006; Chen and Koubeissi, 2019). While drug treatment is generally effective in seizure control, some PTE patients are medication-resistant and difficult to manage (Gupta et al., 2014; Gugger et al., 2022).
Traumatic brain injury can also lead to psychogenic non-epileptic seizures (PNES) (Barry et al., 1998; Hingray et al., 2016). PNES resemble epileptic seizures in their symptoms, but are not caused by abnormal electrical discharges in the brain (Auxéméry et al., 2011). Rather, they are a type of non-epileptic seizure linked to underlying psychosocial stressors (LaFrance and Devinsky, 2002; Popkirov et al., 2018). PNES can co-occur in patients with epilepsy and can be a diagnostic challenge (El-Naggar et al., 2017). The misdiagnosis of PNES leads to ineffective treatments and poor patient outcomes (Barry et al., 1998; Gorenflo et al., 2022). Carefully working through the differential diagnosis with consideration of PNES after TBI will lead to appropriate treatment, which, for PNES generally consists of psychotherapy and psychopharmacological approaches (Carlson and Nicholson, 2017; Lopez and LaFrance, 2022).
9. Brain repair after TBI
As we described in Section “2. Cellular response to traumatic brain injury,” following TBI there is cell death and inflammation and the brain reacts in numerous ways that may be harmful or helpful. Many cell types participate in the process of healing and resolution. Support cells such as astroglia and microglia play an important role in determining recovery outcomes. This section discusses the post-TBI repair process at the cellular level.
9.1. Reactive astrogliosis
Astrocytes, the most abundant glial cell type within the CNS, are essential in maintaining CNS physiological homeostasis. This cell type maintains the integrity of the BBB, supports neuronal function, scavenges free radicals, and regulates extracellular glutamate (Cheng et al., 2019). In response to TBI and other CNS insults, astrocytes undergo a series of morphological and functional adaptations referred to as reactive astrogliosis. Astrogliosis is often referred to as a scar-forming process that occurs around a lesion marked by cell hypertrophy and proliferation and changes in gene expression (Cheng et al., 2019; Sofroniew, 2020; Li J. et al., 2021; Ayazi et al., 2022). Reactive astrocytes proliferate rapidly, densely packing and enclosing the injured area. They secrete inflammatory mediators and neurotrophic factors, and increase expression of intermediate filaments such as glial fibrillary acidic protein (GFAP) and vimentin (Herrmann et al., 2008; Karve et al., 2016; Gill et al., 2018; Hemati-Gourabi et al., 2022). Although this process can perpetuate detrimental CNS injuries through neuroinflammation and ROS generation, reactive astrogliosis can limit damage by forming a physical barrier that protects surrounding healthy tissue while also eliciting reparative effects through promotion of neurogenesis and synaptogenesis (Sheng et al., 2013; Liddelow and Barres, 2017; Adams and Gallo, 2018; Moulson et al., 2021). Thus, depending on the surrounding environment, the state of astrogliosis can manifest as either a neuroprotective or neurotoxic phenotype (Liddelow et al., 2017).
Some potential mechanisms of astrocyte-induced neuroprotection in TBI have been proposed (Song et al., 2002; Bylicky et al., 2018). Astrocytes produce the neurotrophic and mitogenic calcium binding protein S100β, which enhances neurogenesis within the hippocampus. Serum level of S100β is a well-established biomarker and predictor of CT abnormalities for early mTBI (Kahouadji et al., 2020). In male rats, hippocampal infusion of S100β following TBI improved cognitive functional recovery (Hayakata et al., 2004; Kleindienst et al., 2005). These effects are mediated by the facilitation of neuronal differentiation, proliferation, and survival of hippocampal progenitor cells (Hinkle et al., 1997; Kleindienst et al., 2013; Baecker et al., 2020; Zhou et al., 2020). Heme oxygenase induced by astrocytes after TBI catalyzes heme to carbon monoxide (CO), ferrous iron, and biliverdin. Choi (2018) demonstrated that low concentrations of CO promote neurogenesis, synaptic plasticity, and angiogenesis (Jung et al., 2020).
Studies are being done to find ways to manipulate astrocytes to control their reactivity and shift emphasis toward healing. In a mouse model of TBI, Wu et al. (2023) found that brain derived neurotrophic factor (BDNF) secretion by an activated astrocyte network could form a gradient to guide neuroblasts to the site of injury where they could potentially differentiate and facilitate repair.
Mature astrocytes can regress to an immature phenotype and show stem cell characteristics which could indicate a possible role in neuronal regeneration (Seri et al., 2001; Matsubara et al., 2021). Further, exosomes derived from healthy cultured primary rat astrocytes can protect against cognitive dysfunction, oxidative stress, and apoptosis in a rat model, pointing toward a possible therapeutic approach to attenuating TBI injury (Zhang et al., 2021).
9.2. Microglial activation
Microglial cells are innate immune cells of the CNS that promote repair through diverse mechanisms (Lyu et al., 2021). A dichotomous classification has been adopted to characterize microglia polarization into either a classically activated (M1) or an alternatively activated (M2) phenotype (Orihuela et al., 2016). M1 microglia lead to neuronal damage and brain dysfunction through pro-inflammatory factors, such as IL-1β, IL-6, and TNF-α. In contrast, M2 microglia are reparative in nature and reduce toxic cellular debris through phagocytosis, release neurotrophic factors, and resolve cerebral inflammation (Hu et al., 2015; Chai M. et al., 2022). Anti-inflammatory M2 microglia secrete neuroprotective cytokines, chemokines, and neurotrophic factors that contribute to BBB protection, remyelination, neurogenesis, angiogenesis, and axon regeneration (Yang et al., 2015; Ronaldson and Davis, 2020; Lyu et al., 2021). Microglia play a role in rapid closure of the BBB via chemotaxis of microglial processes after brain injury, a process mediated by the purinergic receptor P2YG protein–coupled 12 (P2RY12) (Lou et al., 2016; Császár et al., 2022). Choi et al. (2017) demonstrated M2 microglia-conditioned media induced by IL-4 increased the proliferation and differentiation of neural stem progenitor cells (NSPCs) in the ipsilateral subventricular zone of ex vivo ischemic brain sections. M2 microglia promote axonal regeneration through the secretion of protective molecules, such as arginase 1 (an enzyme that contributes to extracellular matrix deposition in wound healing) and BDNF (Louveau et al., 2015). Similarly, the anti- inflammatory phenotype may improve angiogenesis after brain injury through production of neuroprotective vascular endothelial growth factor (VEGF) and IL-8 (Medina et al., 2011). Unfortunately, anti-inflammatory treatments, including steroids, have not shown efficacy in improving TBI outcomes (Begemann et al., 2020).
9.3. Neuroinflammation
Neuroinflammation can often result in the uncontrolled release of toxic cytokines, proteases, glutamate, and free radicals. These effects are deleterious in nature to the injured CNS (Wee Yong, 2010; Simon et al., 2017). However neuroinflammation is not always synonymous with poor CNS outcomes (Bollaerts et al., 2017). T lymphocytes are a subset of inflammatory cells that have been found to facilitate axonal regeneration (Hauben et al., 2000). Ishii et al. (2012) demonstrated that the adoptive transfer of CD4+ T helper Th1, but not Th2 or Th17 cells, 4 days after traumatic spinal cord injury was associated with regrowth of the corticospinal tract and serotonergic fibers promoting locomotor and tactile recovery. Leukocytes and microglia are notable producers of neurotrophic factors, including oncomodulin, osteopontin, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor-2 (FGF-2), ciliary neurotrophic factor (CNTF), activin-A, glial-derived growth factor (GDNF), endothelin-2, insulin-like growth factor-1 (IGF-1), BDNF and neurotrophin-3 (Sousa-Victor et al., 2018). Many of these neurotrophic factors have been shown to be beneficial for the proliferation and differentiation of oligodendrocyte progenitor cells and for neurogenesis (Woodruff et al., 2004; Yuen et al., 2013; Yong et al., 2019).
While care of TBI patients is largely supportive, there is a growing understanding of the reparative process initiated within the CNS after injury. The ability of the brain to recover from injury is limited, but as new experimental data is acquired, targets for intervention to facilitate repair and regeneration may be identified and explored (Jin et al., 2022; Prabhakar et al., 2022).
9.4. Stem cells
The use of stem cells for neural regeneration and restoration after TBI is an exciting cutting-edge area of exploration. The neurogenic regenerative capacity of endogenous neural progenitor cells has been reported in brain injury models in animal and in human studies (Macas et al., 2006; Sun, 2016). In the adult brain, endogenous neural stem cells are primarily localized to the subventricular zone of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus (Gage et al., 1998; Adugna et al., 2022). Recent studies targeting approaches to enhance the proliferation of these endogenous neural stem cells after TBI have demonstrated promising results. Chai Y. et al. (2022) showed in a rat brain injury model that transplanting an aligned fibrin hydrogel scaffold into the injury site promoted effective migration, differentiation, and maturation of endogenous neural stem cells, resulting in neurological functional recovery. This highly biomimetic scaffold mimics the parallel oriented structure of radial glia in the embryonic brain, a cell type that guides the directed migration of neurons in response to brain injury.
Transplantation of exogenous stem cells is also being investigated as a way to overcome the limited regenerative capabilities of the brain after TBI (Dekmak et al., 2018). Exogenous stem cell transplantation has been shown to accelerate immature neuronal development and increase endogenous cellular proliferation in damaged brain regions (Tajiri et al., 2013; Ngwenya et al., 2018; Adugna et al., 2022). Mesenchymal stromal cells (MSC) are multipotent stem cells with self-renewal and multi-differentiation abilities. Recent studies have demonstrated that exogenous MSC have the potential to treat TBI via their anti-inflammatory and antiapoptotic properties (Ferreira et al., 2018). In a rat model, MSC can also form a biobridge facilitating migration of endogenous neurogenic cells to the injured site (Tajiri et al., 2013). In the context of TBI, the secretome of MSC has the capacity to enhance endogenous neurogenesis (Liu et al., 2020; Badner and Cummings, 2022). Porcine and rodent models show that extracellular vesicles released by MSC can promote endogenous angiogenesis and neurogenesis, reduce inflammation, and facilitate cognitive and sensorimotor recovery after TBI (Abedi et al., 2022; Bambakidis et al., 2022). This highlights the significance of the trophic support these cells provide during exogenous application (Spees et al., 2016; Liu et al., 2020). Animal models have demonstrated reduced pro-inflammatory cytokine expression levels of IL-6, IL-1α, and IFN- γ after MSC transplantation via intraventricular infusion (Huang et al., 2019). Administration of autologous bone marrow MSCs (BM–MSCs) to patients during the subacute phase of TBI also resulted in improved neurological function in 40% of patients (Tian et al., 2013).
The therapeutic application of neural stem cell treatment, whether via manipulation of endogenous neural stem cells or implantation of exogenous neural stem cells, has notable potential to foster functional recovery in those manifesting TBI-related disability. However, further studies are needed to evaluate the safety and efficacy of stem cell transplantation in TBI in humans (Schepici et al., 2020).
10. Conclusion
Traumatic brain injury affects multiple aspects of neurologic and cognitive function, while also causing substantial pain and discomfort. The effects may be long-lasting and poorly responsive to treatment attempts. The intimate link between brain and eye leads to many manifestations of TBI that disturb vision, perception, and ability to perform essential everyday tasks such as reading, typing, driving, and navigating within the environment. Headaches, fatigue and eyestrain compound these problems. Although healing after TBI is difficult, intensive rehabilitation and interdisciplinary treatment including cognitive-behavioral therapy can improve overall functional outcomes (Vanderploeg et al., 2019; Alashram et al., 2022). Rescue or reprogramming of brain cells may be possible in the future, but the leap from animal models to humans is a large one and, at this time, there is no intervention with proven effectiveness to offer persons with TBI (Xu et al., 2022). Enhancing neural regeneration using stem cells is promising and is an area of active investigation.
Author contributions
AR and SR conceptualized the manuscript. AR, AZ, BJ, and DR wrote the initial manuscript and figures and further developed the manuscript. DP and AP provided meaningful edits in the draft phases of the review and contributed meaningful feedback in the writing process. All authors contributed to the article and approved the submitted version.
Acknowledgments
A special thanks to Dr. Amy D. Glass for formatting references. Original artwork in figures by Samantha M. Steiner. With special thanks to Mr. Edmonds Bafford.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abedi M., Hajinejad M., Atabi F., Sahab-Negah S. (2022). Exosome derived from human neural stem cells improves motor activity and neurogenesis in a traumatic brain injury model. Biomed. Res. Int. 2022:6409346. 10.1155/2022/6409346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta S. A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D. M., et al. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One 8:e53376. 10.1371/journal.pone.0053376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams K. L., Gallo V. (2018). The diversity and disparity of the glial scar. Nat. Neurosci. 21 9–15. 10.1038/s41593-017-0033-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adugna D. G., Aragie H., Kibret A. A., Belay D. G. (2022). Therapeutic application of stem cells in the repair of traumatic brain injury. Stem Cells Cloning 15 53–61. 10.2147/SCCAA.S369577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A., Timothy J., Pandit L., Manju M. (2006). Post-traumatic epilepsy: An overview. Clin. Neurol. Neurosurg. 108, 433–439. 10.1016/j.clineuro.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Akamatsu Y., Hanafy K. A. (2020). Cell death and recovery in traumatic brain injury. Neurotherapeutics 17 446–456. 10.1007/s13311-020-00840-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoudad S., Wolters F. J., Viswanathan A., de Bruijn R. F., van der Lugt A., Hofman A., et al. (2016). Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 73 934–943. 10.1001/jamaneurol.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alashram A. R., Padua E., Annino G. (2022). Virtual reality for balance and mobility rehabilitation following traumatic brain injury: A systematic review of randomized controlled trials. J. Clin. Neurosci. 105 115–121. 10.1016/j.jocn.2022.09.012 [DOI] [PubMed] [Google Scholar]
- Aletaha M., Daneshvar F., Mosallaei M., Bagheri A., Khalili M. R. (2018). Comparison of three vision therapy approaches for convergence insufficiency. J. Ophthalmic Vis. Res. 13 307–314. 10.4103/jovr.jovr_99_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnawmasi M. M., Chakraborty A., Dalton K., Quaid P., Dunkley B. T., Thompson B. (2019). The effect of mild traumatic brain injury on the visual processing of global form and motion. Brain Inj. 33 1354–1363. 10.1080/02699052.2019.1641842 [DOI] [PubMed] [Google Scholar]
- Altmann-Schneider I., Trompet S., de Craen A. J., van Es A. C., Jukema J. W., Stott D. J., et al. (2011). Cerebral microbleeds are predictive of mortality in the elderly. Stroke 42 638–644. 10.1161/STROKEAHA.110.595611 [DOI] [PubMed] [Google Scholar]
- Amos J. F. (1999). Differential diagnosis of common etiologies of photopsia. J. Am. Optom. Assoc. 70 485–504. [PubMed] [Google Scholar]
- Angeloni C., Prata C., Dalla Sega F. V., Piperno R., Hrelia S. (2015). Traumatic brain injury and NADPH oxidase: A deep relationship. Oxid. Med. Cell. Longev. 2015:370312. 10.1155/2015/370312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers J. F., Hauser W. A., Coan S. P., Rocca W. A. (1998). A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 338 20–24. 10.1056/NEJM199801013380104 [DOI] [PubMed] [Google Scholar]
- Anwer F., Oliveri F., Kakargias F., Panday P., Arcia Franchini A. P., Iskander B., et al. (2021). Post-traumatic seizures: A deep-dive into pathogenesis. Cureus 13:e14395. 10.7759/cureus.14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour C., Khoury S., Lavigne G. J., Gagnon K., Poirier G., Montplaisir J. Y., et al. (2015). Are NREM sleep characteristics associated to subjective sleep complaints after mild traumatic brain injury? Sleep Med. 16 534–539. 10.1016/j.sleep.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Armstrong R. A. (2018). Visual problems associated with traumatic brain injury. Clin. Exp. Optom. 101, 716–726. 10.1111/cxo.12670 [DOI] [PubMed] [Google Scholar]
- Ashina H., Iljazi A., Al-Khazali H. M., Ashina S., Jensen R. H., Amin F. M., et al. (2020). Persistent post-traumatic headache attributed to mild traumatic brain injury: Deep phenotyping and treatment patterns. Cephalalgia 40 554–564. 10.1177/0333102420909865 [DOI] [PubMed] [Google Scholar]
- Ashina H., Porreca F., Anderson T., Amin F. M., Ashina M., Schytz H. W., et al. (2019). Post-traumatic headache: Epidemiology and pathophysiological insights. Nat. Rev. Neurol. 15 607–617. 10.1038/s41582-019-0243-8 [DOI] [PubMed] [Google Scholar]
- Athanasiou A., Balogiannis I., Magras I. (2017). Lasting bilateral mydriasis after traumatic brain injury may not always be a lost case. Surg. Neurol. Int. 8:229. 10.4103/sni.sni_299_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auxéméry Y., Hubsch C., Fidelle G. (2011). Psychogenic non-epileptic seizures: A review. Encephale 37 153–158. 10.1016/j.encep.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Ayazi M., Zivkovic S., Hammel G., Stefanovic B., Ren Y. (2022). Fibrotic scar in CNS injuries: From the cellular origins of fibroblasts to the molecular processes of fibrotic scar formation. Cells 11:2371. 10.3390/cells11152371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badner A., Cummings B. J. (2022). The endogenous progenitor response following traumatic brain injury: A target for cell therapy paradigms. Neural Regen. Res. 17 2351–2354. 10.4103/1673-5374.335833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecker J., Wartchow K., Sehm T., Ghoochani A., Buchfelder M., Kleindienst A. (2020). Treatment with the neurotrophic protein S100B increases synaptogenesis after traumatic brain injury. J. Neurotrauma 37 1097–1107. 10.1089/neu.2019.6475 [DOI] [PubMed] [Google Scholar]
- Bailey M. D., Gambert S., Gruber-Baldini A., Guralnik J., Kozar R., Qato D., et al. (2022). Traumatic brain injury and risk of long-term nursing home entry among older adults: An analysis of medicare administrative claims data. J. Neurotrauma 40 86–93. 10.1089/neu.2022.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. L., Jr., Mareschal I. (2001). Processing of second-order stimuli in the visual cortex. Prog. Brain Res. 134 171–191. 10.1016/s0079-6123(01)34013-x [DOI] [PubMed] [Google Scholar]
- Bambakidis T., Dekker S. E., Williams A. M., Biesterveld B. E., Bhatti U. F., Liu B., et al. (2022). Early treatment with a single dose of mesenchymal stem cell derived extracellular vesicles modulates the brain transcriptome to create neuroprotective changes in a porcine model of traumatic brain injury and hemorrhagic shock. Shock 57 281–290. 10.1097/SHK.0000000000001889 [DOI] [PubMed] [Google Scholar]
- Baracaldo-Santamaría D., Ariza-Salamanca D. F., Corrales-Hernández M. G., Pachón-Londoño M. J., Hernandez-Duarte I., Calderon-Ospina C. A. (2022). Revisiting excitotoxicity in traumatic brain injury: From bench to bedside. Pharmaceutics 14:152. 10.3390/pharmaceutics14010152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B. P., Singman E. L. (2015). Vision concerns after mild traumatic brain injury. Curr. Treat. Options Neurol. 17:329. 10.1007/s11940-014-0329-y [DOI] [PubMed] [Google Scholar]
- Barry E., Krumholz A., Bergey G. K., Chatha H., Alemayehu S., Grattan L. (1998). Nonepileptic posttraumatic seizures. Epilepsia 39 427–431. 10.1111/j.1528-1157.1998.tb01395.x [DOI] [PubMed] [Google Scholar]
- Bayir H., Kagan V. E., Borisenko G. G., Tyurina Y. Y., Janesko K. L., Vagni V. A., et al. (2005). Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: Support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 225 673–684. 10.1038/sj.jcbfm.9600068 [DOI] [PubMed] [Google Scholar]
- Bayir H., Tyurin V. A., Tyurina Y. Y., Viner R., Ritov V., Amoscato A. A., et al. (2007). Selective early cardiolipin peroxidation after traumatic brain injury: An oxidative lipidomics analysis. Ann. Neurol. 62 154–169. 10.1002/ana.21168 [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 87 245–313. 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- Bedgood A., Rand S. E., Major J., Jr. (2015). Occult retinal detachment after mild traumatic brain injury: Case report and literature review. Clin. J. Sport Med. 25 e26–e27. 10.1097/JSM.0000000000000114 [DOI] [PubMed] [Google Scholar]
- Begemann M., Leon M., van der Horn H. J., van der Naalt J., Sommer I. (2020). Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: A meta-analysis. Sci. Rep. 10:16179. 10.1038/s41598-020-73227-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar-Cohen F., Gelizé E., Jonet L., Lassiaz P. (2020). Anatomy of the retina. Med. Sci. (Paris) 36 594–599. 10.1051/medsci/2020094 [DOI] [PubMed] [Google Scholar]
- Benardo L. S. (2003). Prevention of epilepsy after head trauma: Do we need new drugs or a new approach? Epilepsia 44 27–33. 10.1046/j.1528-1157.44.s10.2.x [DOI] [PubMed] [Google Scholar]
- Berthold Lindstedt M., Johansson J., Ygge J., Borg K. (2019). Vision-related symptoms after acquired brain injury and the association with mental fatigue, anxiety and depression. J. Rehabil. Med. 51 499–505. 10.2340/16501977-2570 [DOI] [PubMed] [Google Scholar]
- Berthold-Lindstedt M., Ygge J., Borg K. (2017). Visual dysfunction is underestimated in patients with acquired brain injury. J. Rehabil. Med. 49 327–332. 10.2340/16501977-2218 [DOI] [PubMed] [Google Scholar]
- Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. (2012). Oxidative stress and antioxidant defense. World Allergy Organ. J. 5 9–19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollaerts I., Van Houcke J., Andries L., De Groef L., Moons L. (2017). Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediat. Inflamm. 2017:9478542. 10.1155/2017/9478542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard M. A. (2019). The pupil. Continuum (Minneapolis, Minn.) 25 1194–1214. 10.1212/CON.0000000000000771 [DOI] [PubMed] [Google Scholar]
- Boutet I., Taler V., Collin C. A. (2015). On the particular vulnerability of face recognition to aging: A review of three hypotheses. Front. Psychol. 6:1139. 10.3389/fpsyg.2015.01139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. M., Miccoli L., Escrig M. A., Lang P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45 602–607. 10.1111/j.1469-8986.2008.00654.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen D. E. (2008). Programmed cell death mechanisms in neurological disease. Curr. Mol. Med. 8 173–186. 10.2174/156652408784221315 [DOI] [PubMed] [Google Scholar]
- Broadway D. C. (2012). How to test for a relative afferent pupillary defect (RAPD). Community Eye Health 25 58–59. [PMC free article] [PubMed] [Google Scholar]
- Bullock R., Zauner A., Myseros J. S., Marmarou A., Woodward J. J., Young H. F. (1995). Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann. N. Y. Acad. Sci. 765 290–297. 10.1111/j.1749-6632.1995.tb16586.x [DOI] [PubMed] [Google Scholar]
- Bullock R., Zauner A., Woodward J. J., Myseros J., Choi S. C., Ward J. D., et al. (1998). Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 89 507–518. 10.3171/jns.1998.89.4.0507 [DOI] [PubMed] [Google Scholar]
- Byer N. E. (1994). Natural history of posterior vitreous detachment with early management as the premier line of defense against retinal detachment. Ophthalmology 101 1503–1514. 10.1016/s0161-6420(94)31141-9 [DOI] [PubMed] [Google Scholar]
- Bylicky M. A., Mueller G. P., Day R. M. (2018). Mechanisms of endogenous neuroprotective effects of astrocytes in brain injury. Oxid. Med. Cell. Longev. 2018:6501031. 10.1155/2018/6501031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. N., Gandhi A., Singh B., Churn S. B. (2014). Traumatic brain injury causes a tacrolimus-sensitive increase in non-convulsive seizures in a rat model of post-traumatic epilepsy. Int. J. Neurol. Brain Disord. 1 1–11. 10.15436/2377-1348.14.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J. B., Ashman T., Gordon W., Ginsberg A., Engmann C., Egan M., et al. (2008). Fatigue after traumatic brain injury and its impact on participation and quality of life. J. Head Trauma Rehabil. 23 41–51. 10.1097/01.HTR.0000308720.70288.af [DOI] [PubMed] [Google Scholar]
- Cantor J. B., Bushnik T., Cicerone K., Dijkers M. P., Gordon W., Hammond F. M., et al. (2012). Insomnia, fatigue, and sleepiness in the first 2 years after traumatic brain injury: An NIDRR TBI model system module study. J. Head Trauma Rehabil. 27 E1–E14. 10.1097/HTR.0b013e318270f91e [DOI] [PubMed] [Google Scholar]
- Capó-Aponte J. E., Jorgensen-Wagers K. L., Sosa J. A., Walsh D. V., Gregory G. L., Temme L. A., et al. (2017). Visual dysfunctions at different stages after blast and non-blast mild traumatic brain injury. Optom. Vis. Sci. 94 7–15. 10.1097/OPX.0000000000000825 [DOI] [PubMed] [Google Scholar]
- Carlson P., Nicholson P. K. (2017). Psychological interventions for psychogenic non-epileptic seizures: A meta-analysis. Seizure 45 142–150. 10.1016/j.seizure.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Carrick F. R., Azzolino S. F., Hunfalvay M., Pagnacco G., Oggero E., D’Arcy R. C. N., et al. (2021). The pupillary light reflex as a biomarker of concussion. Life (Basel) 11:1104. 10.3390/life11101104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I., Savic V. J., Kotur J., Prokic V., Veljovic M., Grbovic D. (2000). Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J. Neurotrauma 17 53–68. 10.1089/neu.2000.17.53 [DOI] [PubMed] [Google Scholar]
- Chai M., Su G., Gao J., Chen W., Wu Q., Dong Y., et al. (2022). Molecular mechanism of the protective effects of M2 microglia on neurons: A review focused on exosomes and secretory proteins. Neurochem. Res. 47 3556–3564. 10.1007/s11064-022-03760-4 [DOI] [PubMed] [Google Scholar]
- Chai Y., Zhao H., Yang S., Gao X., Cao Z., Lu J., et al. (2022). Structural alignment guides oriented migration and differentiation of endogenous neural stem cells for neurogenesis in brain injury treatment. Biomaterials 280:121310. 10.1016/j.biomaterials.2021.121310 [DOI] [PubMed] [Google Scholar]
- Chamoun R., Suki D., Gopinath S. P., Goodman J. C., Robertson C. (2010). Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 113 564–570. 10.3171/2009.12.jns09689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran R., Kim T., Mehta S. L., Udho E., Chanana V., Cengiz P., et al. (2018). A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J. Cereb. Blood Flow Metab. 38 1818–1827. 10.1177/0271678X17738701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H., Lin C., Zuo Q., Liu Y., Xaio M., Xu X., et al. (2019). Cardiolipin-dependent mitophagy guides outcome after traumatic brain injury. J. Neurosci. 39 1930–1943. 10.1253/NEUROSCI.3415-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri K., Malham G. M., Rosenfeld J. V. (2009). Survival of trauma patients with coma and bilateral fixed dilated pupils. Injury 40 28–32. 10.1016/j.injury.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Chen B. S., Lance S., Lallu B., Anderson N. E. (2019). Visual snow: Not so benign. J. Clin. Neurosci. 64 37–39. 10.1016/j.jocn.2019.03.023 [DOI] [PubMed] [Google Scholar]
- Chen H., Koubeissi M. Z. (2019). Electroencephalography in epilepsy evaluation. Continuum (Minneapolis, Minn.) 25 431–453. 10.1212/CON.0000000000000705 [DOI] [PubMed] [Google Scholar]
- Cheng X., Wang J., Sun X., Shao L., Guo Z., Li Y. (2019). Morphological and functional alterations of astrocytes responding to traumatic brain injury. J. Integr. Neurosci. 18 203–215. 10.31083/j.jin.2019.02.110 [DOI] [PubMed] [Google Scholar]
- Choi B. Y., Jang B. G., Kim J. H., Lee B. E., Sohn M., Song H. K., et al. (2012). Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 1481 49–58. 10.1016/j.brainres.2012.08.032 [DOI] [PubMed] [Google Scholar]
- Choi J. Y., Kim J. Y., Kim J. Y., Park J., Lee W. T., Lee J. E. (2017). M2 phenotype microglia-derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp. Neurobiol. 26 33–41. 10.5607/en.2017.26.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. K. (2018). Role of carbon monoxide in neurovascular repair processing. Biomol. Ther. (Seoul) 26 93–100. 10.4062/biomolther.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K. J., Han M. E., Tannen B., Ruttner D. (2021). Visual snow syndrome: Evolving neuro-optometric considerations in concussion/mild traumatic brain injury. Concussion 6:CNC89. 10.2217/cnc-2021-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K. J., Joshi N. R., Truong J. Q. (2017). Understanding the effects of mild traumatic brain injury on the pupillary light reflex. Concussion 2:CNC36. 10.2217/cnc-2016-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K. J., Kapoor N., Rutner D., Suchoff I. B., Han M. E., Craig S. (2007). Occurrence of oculomotor dysfunctions in acquired brain injury: A retrospective analysis. Optometry 78 155–161. 10.1016/j.optm.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Coleman A. L., Cummings S. R., Yu F., Kodjebacheva G., Ensrud K. E., Gutierrez P., et al. (2007). Binocular visual-field loss increases the risk of future falls in older white women. J. Am. Geriatr. Soc. 55 357–364. 10.1111/j.1532-5415.2007.01094.x [DOI] [PubMed] [Google Scholar]
- Condos A., Sullivan M. A., Hawley D., Cho A., Cathey M. (2022). Not just down and out: Oculomotor nerve pathologic spectrum. Curr. Probl. Diagn. Radiol. 51 217–224. 10.1067/j.cpradiol.2020.12.006 [DOI] [PubMed] [Google Scholar]
- Császár E., Lénárt N., Cserép C., Környei Z., Fekete R., Pósfai B., et al. (2022). Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 219:e20211071. 10.1084/jem.20211071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daradkeh G., Al-Maashani A., Al-Abrawi S., Amiri R., Al-Barashdi J. (2021). Adequacy and importance of dietary antioxidants in traumatic brain injury patients. J. Nutr. Food Sci. 11:792. [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J. V., Zuo Y., Jung S., et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8 752–758. 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- de Wit K., Parpia S., Varner C., Worster A., McLeod S., Clayton N., et al. (2020a). Clinical predictors of intracranial bleeding in older adults who have fallen: A cohort study. J. Am. Geriatr. Soc. 68 970–976. 10.1111/jgs.16338 [DOI] [PubMed] [Google Scholar]
- de Wit K., Merali Z., Kagoma Y., Mercier É. (2020b). Incidence of intracranial bleeding in seniors presenting to the emergency department after a fall: A systematic review. Injury 51 157–163. 10.1016/j.injury.2019.12.036 [DOI] [PubMed] [Google Scholar]
- Dekmak A., Mantash S., Shaito A., Toutonji A., Ramadan N., Ghazale H., et al. (2018). Stem cells and combination therapy for the treatment of traumatic brain injury. Behav. Brain Res. 340 49–62. 10.1016/j.bbr.2016.12.039 [DOI] [PubMed] [Google Scholar]
- Deng H., Yue J. K., Zusman B. E., Nwachuku E. L., Abou-Al-Shaar H., Upadhyayula P. S., et al. (2020). B-cell lymphoma 2 (bcl-2) and regulation of apoptosis after traumatic brain injury: A clinical perspective. Medicina 56:300. 10.3390/medicina56060300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettwiler A., Murugavel M., Putukian M., Cubon V., Furtado J., Osherson D. (2014). Persistent differences in patterns of brain activation after sports-related concussion: A longitudinal functional magnetic resonance imaging study. J. Neurotrauma 31 180–188. 10.1089/neu.2013.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M. C., Rattani A., Gupta S., Baticulon R. E., Hung Y. C., Punchak M., et al. (2018). Estimating the global incidence of traumatic brain injury. J. Neurosurg. 1 1–18. 10.3171/2017.10.JNS17352 [DOI] [PubMed] [Google Scholar]
- Dhani S., Zhao Y., Zhivotovsky B. (2021). A long way to go: Caspase inhibitors in clinical use. Cell Death Dis. 12 1–13. 10.1038/s41419-021-04240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. Q., Ge Q. Q., Lu S. H., Yang M. S., Zhuang Y., Zhang B., et al. (2023). Tandem Mass Tag-based proteomics analysis reveals the vital role of inflammation in traumatic brain injury in a mouse model. Neural Regen. Res. 18 155–161. 10.4103/1673-5374.343886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. (2012). Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 464 111–121. 10.1007/s00424-012-1112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer B. (2018). Posttraumatic headache. Semin. Neurol. 38 619–626. 10.1055/s-0038-1673692 [DOI] [PubMed] [Google Scholar]
- Ehrlich J. R., Hassan S. E., Stagg B. C. (2019). Prevalence of falls and fall-related outcomes in older adults with self-reported vision impairment. J. Am. Geriatr. Soc. 67 239–245. 10.1111/jgs.15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. B., Sanderson K., Conkey A. (1990). The reliability of the Pelli-Robson contrast sensitivity chart. Ophthalmic Physiol. Opt. 10 21–24. [PubMed] [Google Scholar]
- El-Naggar H., Moloney P., Widdess-Walsh P., Kilbride R., Delanty N., Mullins G. (2017). Simultaneous occurrence of nonepileptic and epileptic seizures during a single period of in-patient video-electroencephalographic monitoring. Epilepsia Open 2 467–471. 10.1002/epi4.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J., Bushnik T., Duong T. T., Cifu D. X., Zafonte R., Wright J., et al. (2003). Analyzing risk factors for late posttraumatic seizures: A prospective, multicenter investigation. Arch. Phys. Med. Rehabil. 84:365373. 10.1053/apmr.2003.50022 [DOI] [PubMed] [Google Scholar]
- Englander J., Bushnik T., Oggins J., Katznelson L. (2010). Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj. 24 1379–1388. 10.3109/02699052.2010.523041 [DOI] [PubMed] [Google Scholar]
- Englander J., Cifu D. X., Diaz-Arrastia R. (2014). Information/education page. Seizures and traumatic brain injury. Arch. Phys. Med. Rehabil. 95 1223–1224. 10.1016/j.apmr.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle-Friedman M., Mathew G. M., Martinova A., Armstrong F., Konstantinov V. (2018). The role of sleep deprivation and fatigue in the perception of task difficulty and use of heuristics. Sleep Sci. 11 74–84. 10.5935/1984-0063.20180016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. C., Gerlach A. T., Christy J. M., Jarvis A. M., Lindsey D. E., Whitmill M. L., et al. (2011). Pre-injury polypharmacy as a predictor of outcomes in trauma patients. Int. J. Crit. Illn. Inj. Sci. 1 104–109. 10.4103/2229-5151.84793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux S., Sheedy J. (2008). A prospective controlled study in the prevalence of posttraumatic headache following mild traumatic brain injury. Pain Med. 9 1001–1011. 10.1111/j.1526-4637.2007.00404.x [DOI] [PubMed] [Google Scholar]
- Feng X., Ma W., Chen J., Jiao W., Wang Y. (2022). Ulinastatin alleviates early brain injury after traumatic brain injury by inhibiting oxidative stress and apoptosis. Acta Cir. Bras. 37:e370108. 10.1590/acb370108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J. R., Teixeira G. Q., Santos S. G., Barbosa M. A., Almeida-Porada G., Gonçalves R. M. (2018). Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 9:2837. 10.3389/fimmu.2018.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordington S., Manford M. (2020). A review of seizures and epilepsy following traumatic brain injury. J. Neurol. 267 3105–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C. L. (2022). Visual snow: Updates on pathology. Curr. Neurol. Neurosci. Rep. 22 209–217. 10.1007/s11910-022-01182-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E. E., Munoz B., Rubin G., West S. K. (2007). Visual field loss increases the risk of falls in older adults: The Salisbury eye evaluation. Invest. Ophthalmol. Vis. Sci. 48 4445–4450. 10.1167/iovs.07-0326 [DOI] [PubMed] [Google Scholar]
- Fu W. W., Fu T. S., Jing R., McFaull S. R., Cusimano M. D. (2017). Predictors of falls and mortality among elderly adults with traumatic brain injury: A nationwide, population-based study. PLoS One 12:e0175868. 10.1371/journal.pone.0175868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Kempermann G., Palmer T. D., Peterson D. A., Ray J. (1998). Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 36 249–266. [DOI] [PubMed] [Google Scholar]
- Gale C. R., Cooper C., Sayer A. A. (2016). Prevalence and risk factors for falls in older men and women: The English longitudinal study of ageing. Age Ageing 45 789–794. 10.1093/ageing/afw129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganti L., Stead T., Daneshvar Y., Bodhit A. N., Pulvino C., Ayala S. W., et al. (2019). GCS 15: When mild TBI isn’t so mild. Neurol. Res. Pract. 1:6. 10.1186/s42466-018-0001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. C., Dams-O’Connor K., Morrissey M. R., Manley G. T. (2018). Geriatric traumatic brain injury: Epidemiology, outcomes, knowledge gaps, and future directions. J. Neurotrauma 35 889–906. 10.1089/neu.2017.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrila Laic R. A., Bogaert L., Vander Sloten J., Depreitere B. (2021). Functional outcome, dependency and well-being after traumatic brain injury in the elderly population: A systematic review and meta-analysis. Brain Spine 1:100849. 10.1016/j.bas.2021.100849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbings A., Ray L. B., Gagnon S., Collin C. A., Robillard R., Fogel S. M. (2022). The EEG correlates and dangerous behavioral consequences of drowsy driving after a single night of mild sleep deprivation. Physiol. Behav. 252:113822. 10.1016/j.physbeh.2022.113822 [DOI] [PubMed] [Google Scholar]
- Gill J., Latour L., Diaz-Arrastia R., Motamedi V., Turtzo C., Shahim P., et al. (2018). Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 91 e1385–e1389. 10.1212/WNL.0000000000006321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie L. D., Robertson M. C., Gillespie W. J., Sherrington C., Gates S., Clemson L. M., et al. (2012). Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 9:CD007146. 10.1002/14651858.CD007146.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner J., Mesa Galán L., Yus Teruel S., Guallar Espallargas M. C., Pérez López C., Isla Guerrero A., et al. (2022). Traumatic brain injury in the new millennium: New population and new management. Neurologia (Engl. Ed.) 37 383–389. 10.1016/j.nrleng.2019.03.024 [DOI] [PubMed] [Google Scholar]
- Ginsburg A. P. (2003). Contrast sensitivity and functional vision. Int. Ophthalmol. Clin. 43 5–15. 10.1097/00004397-200343020-00004 [DOI] [PubMed] [Google Scholar]
- Gorenflo R., Ho R., Carrazana E., Mitchell C., Viereck J., Liow K. K., et al. (2022). Identification of risk factors and distinguishing psychogenic nonepileptic seizures from epilepsy: A retrospective case-control study. Clin. Neurol. Neurosurg. 217:107221. 10.1016/j.clineuro.2022.107221 [DOI] [PubMed] [Google Scholar]
- Greenwald B. D., Kapoor N., Singh A. D. (2012). Visual impairments in the first year after traumatic brain injury. Brain Inj. 26 1338–1359. 10.3109/02699052.2012.706356 [DOI] [PubMed] [Google Scholar]
- Gugger J. J., Kennedy E., Panahi S., Tate D. F., Roghani A., Van Cott A. C., et al. (2022). Multimodal quality of life assessment in post-9/11 veterans with epilepsy: Impact of drug resistance, traumatic brain injury, and comorbidity. Neurology 98 e1761–e1770. 10.1212/WNL.0000000000200146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K., Sayed N., Ding K., Agostini M. A., Van Ness P. C., Yablon S., et al. (2014). Subtypes of post-traumatic epilepsy: Clinical, electrophysiological, and imaging features. J. Neurotrauma 31 1439–1443. 10.1089/neu.2013.3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakiminia B., Alikiaii B., Khorvash F., Mousavi S. (2022). Oxidative stress and mitochondrial dysfunction following traumatic brain injury: From mechanistic view to targeted therapeutic opportunities. Fundam. Clin. Pharmacol. 36 612–662. 10.1111/fcp.12767 [DOI] [PubMed] [Google Scholar]
- Hall E. D., Andrus P. K., Yonkers P. A. (1993). Brain hydroxyl radical generation in acute experimental head injury. J. Neurochem. 60 588–594. 10.1111/j.1471-4159.1993.tb03189.x [DOI] [PubMed] [Google Scholar]
- Hall E. D., Yonkers P. A., Andrus P. K., Cox J. W., Anderson D. K. (1992). Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J. Neurotrauma 9(Suppl. 2) S425–S442. [PubMed] [Google Scholar]
- Hang C., Leishangthem L., Yan Y. (2021). Not all cases of visual snows are benign: Mimics of visual snow syndrome. Neuropsychiatr. Dis. Treat. 17 3293–3300. 10.2147/NDT.S338111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring R. S., Narang K., Canner J. K., Asemota A. O., George B. P., Selvarajah S., et al. (2015). Traumatic brain injury in the elderly: Morbidity and mortality trends and risk factors. J. Surg. Res. 195 1–9. 10.1016/j.jss.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Hart L. A., Walker R., Phelan E. A., Marcum Z. A., Schwartz N. R. M., Crane P. K., et al. (2022). Change in central nervous system-active medication use following fall-related injury in older adults. J. Am. Geriatr. Soc. 70 168–177. 10.1111/jgs.17508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey L. A., Close J. C. (2012). Traumatic brain injury in older adults: Characteristics, causes and consequences. Injury 43 1821–1826. 10.1016/j.injury.2012.07.188 [DOI] [PubMed] [Google Scholar]
- Hauben E., Butovsky O., Nevo U., Yoles E., Moalem G., Agranov E., et al. (2000). Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J. Neurosci. 20 6421–6430. 10.1523/JNEUROSCI.20-17-06421.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakata T., Shiozaki T., Tasaki O., Ikegawa H., Inoue Y., Toshiyuki F., et al. (2004). Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock 22 102–107. 10.1097/01.shk.0000131193.80038.f1 [DOI] [PubMed] [Google Scholar]
- Haynes S., Hollopeter G., Yang G., Kurpius D., Dailey M., Gan W., et al. (2006). The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9 1512–1519. 10.1038/nn1805 [DOI] [PubMed] [Google Scholar]
- Helmy A., Kirkpatrick P. J., Seeley H. M., Corteen E., Menon D. K., Hutchinson P. J. (2012). Fixed, dilated pupils following traumatic brain injury: Historical perspectives, causes and ophthalmological sequelae. Acta Neurochir. Suppl. 114 295–299. 10.1007/978-3-7091-0956-4_57 [DOI] [PubMed] [Google Scholar]
- Hemati-Gourabi M., Cao T., Romprey M. K., Chen M. (2022). Capacity of astrocytes to promote axon growth in the injured mammalian central nervous system. Front. Neurosci. 16:955598. 10.3389/fnins.2022.955598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ontiveros D. G., Tajiri N., Acosta S., Giunta B., Tan J., Borlongan C. V. (2013). Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 4:30. 10.3389/fneur.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Imura T., Song B., Qi J., Ao Y., Nguyen T. K., et al. (2008). STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 28 7231–7243. 10.1523/JNEUROSCI.1709-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingray C., Biberon J., El-Hage W., de Toffol B. (2016). Psychogenic non-epileptic seizures (PNES). Rev. Neurol. (Paris) 172 263–269. 10.1016/j.neurol.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Hinkle D. A., Baldwin S. A., Scheff S. W., Wise P. M. (1997). GFAP and S100beta expression in the cortex and hippocampus in response to mild cortical contusion. J. Neurotrauma 14 729–738. 10.1089/neu.1997.14.729 [DOI] [PubMed] [Google Scholar]
- Hoffe B., Holahan M. R. (2022). Hyperacute excitotoxic mechanisms and synaptic dysfunction involved in traumatic brain injury. Front. Mol. Neurosci. 15:831825. 10.3389/fnmol.2022.831825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer M., Balaban C. (2019). Neurosensory disorders in mild traumatic brain injury, 1st Edn. London: Academic Press, 452. [Google Scholar]
- Hofmann V., Deininger C., Döbele S., Konrads C., Wichlas F. (2021). Mild traumatic brain injury in older adults: Are routine second cCT scans necessary? J. Clin. Med. 10:3794. 10.3390/jcm10173794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig M. G., del Mar N. A., Henderson D. L., O’Neal D., Doty J. B., Cox R., et al. (2021). Raloxifene modulates microglia and rescues visual deficits and pathology after impact traumatic brain injury. Front. Neurosci. 15:701317. 10.3389/fnins.2021.701317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig M. G., Del Mar N. A., Henderson D. L., Ragsdale T. D., Doty J. B., Driver J. H., et al. (2019). Amelioration of visual deficits and visual system pathology after mild TBI via the cannabinoid Type-2 receptor inverse agonism of raloxifene. Exp. Neurol. 322:113063. 10.1016/j.expneurol.2019.113063 [DOI] [PubMed] [Google Scholar]
- Hopewell S., Adedire O., Copsey B. J., Boniface G. J., Sherrington C., Clemson L., et al. (2018). Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 7:CD012221. 10.1002/14651858.CD012221.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Leak R. K., Shi Y., Suenaga J., Gao Y., Zheng P., et al. (2015). Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 11 56–64. 10.1038/nrneurol.2014.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Freeman W. D., Edenfield B. H., Brott T. G., Meschia J. F., Zubair A. C. (2019). Safety and efficacy of intraventricular delivery of bone marrow-derived Mesenchymal stem cells in hemorrhagic stroke model. Sci. Rep. 9:5674. 10.1038/s41598-019-42182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrar A., Panayiotis M., Mohamed E. (2021). Recognising and managing retinal detachments. Brit. J. Hosp. Med. (Lond.) 82 1–11. 10.12968/hmed.2021.0145 [DOI] [PubMed] [Google Scholar]
- Ikonomidou C., Turski L. (2002). Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 1 383–386. 10.1016/s1474-4422(02)00164-3 [DOI] [PubMed] [Google Scholar]
- Irimia A., Van Horn J. D., Vespa P. M. (2018). Cerebral microhemorrhages due to traumatic brain injury and their effects on the aging human brain. Neurobiol. Aging 66 158–164. 10.1016/j.neurobiolaging.2018.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Jin X., Ueno M., Tanabe S., Kubo T., Serada S., et al. (2012). Adoptive transfer of Th1-conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 3:e363. 10.1038/cddis.2012.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail H., Shakkour Z., Tabet M., Abdelhady S., Kobaisi A., Abedi R., et al. (2020). Traumatic brain injury: Oxidative stress and novel anti-oxidants such as mitoquinone and edaravone. Antioxidants (Basel) 9:943. 10.3390/antiox9100943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns F. P., Miroz J. P., Messerer M., Daniel R. T., Taccone F. S., Eckert P., et al. (2019). Quantitative pupillometry for the monitoring of intracranial hypertension in patients with severe traumatic brain injury. Crit. Care 23:155. 10.1186/s13054-019-2436-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Mittal R. S., Sharma A., Sharma A., Gupta I. D. (2014). Study of insomnia and associated factors in traumatic brain injury. Asian J. Psychiatr. 8 99–103. 10.1016/j.ajp.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Jastroch M., Divakaruni A. S., Mookerjee S., Treberg J. R., Brand M. D. (2010). Mitochondrial proton and electron leaks. Essays Biochem. 47 53–67. 10.1042/bse0470053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G., Ho J. W., Keeney-Bonthrone T. P., Ober R. A., Liu B., Chtraklin K., et al. (2022). Recombinant human MG53 protein attenuates brain lesion size in a large animal model of traumatic brain injury. J. Trauma Acute Care Surg. 93 613–619. 10.1097/TA.0000000000003746 [DOI] [PubMed] [Google Scholar]
- Johansson B., Berglund P., Ronnback L. (2009). Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 23 1027–1040. 10.3109/02699050903421099 [DOI] [PubMed] [Google Scholar]
- Joshi S., Gold J. I. (2020). Pupil size as a window on neural substrates of cognition. Trends Cogn. Sci. 24 466–480. 10.1016/j.tics.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E., Koh S.-H., Yoo M., Choi Y. K. (2020). Regenerative potential of carbon monoxide in adult neural circuits of the central nervous system. Int. J. Mol. Sci. 21:2273. 10.3390/ijms21072273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V. E., Tyurin V. A., Jiang J., Yurina Y. Y., Ritov V. B., Amoscato A. A., et al. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1 223–232. 10.1038/nchembio727 [DOI] [PubMed] [Google Scholar]
- Kahouadji S., Salamin P., Praz L., Coiffier J., Frochaux V., Durif J., et al. (2020). S100B blood level determination for early management of ski-related mild traumatic brain injury: A pilot study. Front. Neurol. 11:856. 10.3389/fneur.2020.00856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S., Malik R., Singh G., Bhatia S., Al-Harrasi A., Mohan S., et al. (2022). Pathogenesis and management of traumatic brain injury (TBI): Role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology 30 1153–1166. 10.1007/s10787-022-01017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor N., Ciuffreda K. J. (2002). Vision disturbances following traumatic brain injury. Curr. Treat. Options Neurol. 4 271–280. 10.1007/s11940-002-0027-z [DOI] [PubMed] [Google Scholar]
- Karve I. P., Taylor J. M., Crack P. J. (2016). The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 173 692–702. 10.1111/bph.13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M., Uchino M., Yokoi N., Uchino Y., Dogru M., Komuro A., et al. (2016). The association of sleep quality with dry eye disease: The Osaka study. Clin. Ophthalmol. 1 1015–1021. 10.2147/OPTH.S99620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. A., Noble J., Wong A. M. (2013). Examining the pupils. CMAJ 185:E424. 10.1503/cmaj.120306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Chang H. (2013). Isolated oculomotor nerve palsy following minor head trauma: Case illustration and literature review. J. Korean Neurosurg. Soc. 54 434–436. 10.3340/jkns.2013.54.5.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Cho A. Y., Kim H. C., Ryu D., Jo S. A., Jung Y. S. (2022). Effects of natural polyphenols on oxidative stress-mediated blood-brain barrier dysfunction. Antioxidants (Basel) 20:197. 10.3390/antiox11020197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A., Grunbeck F., Buslei R., Emtmann I., Buchfelder M. (2013). Intraperitoneal treatment with S100B enhances hippocampal neurogenesis in juvenile mice and after experimental brain injury. Acta Neurochir. (Wien) 155 1351–1360. 10.1007/s00701-013-1720-2 [DOI] [PubMed] [Google Scholar]
- Kleindienst A., McGinn M. J., Harvey H. B., Colello R. J., Hamm R. J., Bullock M. R. (2005). Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J. Neurotrauma 22 645–655. 10.1089/neu.2005.22.645 [DOI] [PubMed] [Google Scholar]
- Kohl A. D., Wylie G. R., Genova H. M., Hillary F. G., Deluca J. (2009). The neural correlates of cognitive fatigue in traumatic brain, injury using functional MRI. Brain Inj. 23 420–432. 10.1080/02699050902788519 [DOI] [PubMed] [Google Scholar]
- Kontos A. P., Deitrick J. M., Collins M. W., Mucha A. (2017). Review of vestibular and oculomotor screening and concussion rehabilitation. J. Athl. Train. 52, 256–261. 10.4085/1062-6050-51.11.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen N., Niemi S., Parkkari J., Sievänen H., Kannus P. (2013). Incidence of fall-related traumatic brain injuries among older Finnish adults between 1970 and 2011. JAMA 309 1891–1892. 10.1001/jama.2013.3356 [DOI] [PubMed] [Google Scholar]
- Kumar S. (2007). Caspase function in programmed cell death. Cell Death Differ. 14 32–43. 10.1038/sj.cdd.4402060 [DOI] [PubMed] [Google Scholar]
- Lacherez P., Saeri A. K., Wood J. M., Atchison D. A., Horswill M. S. (2013). A yellow filter improves response times to low-contrast targets and traffic hazards. Optom. Vis. Sci. 90 242–248. 10.1097/OPX.0b013e3182815783 [DOI] [PubMed] [Google Scholar]
- LaFrance W. C., Jr., Devinsky O. (2002). Treatment of nonepileptic seizures. Epilepsy Behav. 3(Suppl. 5) 19–23. 10.1016/s1525-5069(02)00505-4 [DOI] [PubMed] [Google Scholar]
- LeBlanc J., de Guise E., Gosselin N., Feyz M. (2006). Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Inj. 20 779–790. 10.1080/02699050600831835 [DOI] [PubMed] [Google Scholar]
- Lee J. E., Stein J. J., Prevor M. B., Seiple W. H., Holopigian K., Greenstein V. C., et al. (2002). Effect of variable tinted spectacle lenses on visual performance in control subjects. CLAO J. 28 80–82. [PubMed] [Google Scholar]
- Lee J., Sohn E. H., Oh E., Lee A. Y. (2018). Characteristics of cerebral microbleeds. Dement. Neurocogn. Disord. 17 73–82. 10.12779/dnd.2018.17.3.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Lee M., Ko D. G., Choi B. Y., Suh S. W. (2021). The role of NADPH oxidase in neuronal death and neurogenesis after acute neurological disorders. Antioxidants (Basel) 10:739. 10.3390/antiox10050739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewén A., Matz P., Chan P. H. (2000). Free radical pathways in CNS injury. J. Neurotrauma 17 871–890. 10.1089/neu.2000.17.871 [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Qin S. (2021). Molecular mechanisms and signaling pathways of reactive astrocytes responding to traumatic brain injury. Histol. Histopathol. 36 921–929. 10.14670/HH-18-338 [DOI] [PubMed] [Google Scholar]
- Li L., Kumar U., You J., Zhou Y., Weiss S. A., Engel J., et al. (2021). Spatial and temporal profile of high-frequency oscillations in posttraumatic epileptogenesis. Neurobiol. Dis. 161:105544. 10.1016/j.nbd.2021.105544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ning K., Zhou J., Guo Y., Zhang H., Zhu Y., et al. (2018). Sleep deprivation disrupts the lacrimal system and induces dry eye disease. Exp. Mol. Med. 50:e451. 10.1038/emm.2017.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Barres B. A. (2017). Reactive astrocytes: Production, function, and therapeutic potential. Immunity 46 957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. W., Bhattacharyya N. (2012). Balance disorders in the elderly: Epidemiology and functional impact. Laryngoscope 122 1858–1861. [DOI] [PubMed] [Google Scholar]
- Liu X. Y., Wei M. G., Liang J., Xu H. H., Wang J. J., Wang J., et al. (2020). Injury-preconditioning secretome of umbilical cord mesenchymal stem cells amplified the neurogenesis and cognitive recovery after severe traumatic brain injury in rats. J. Neurochem. 153 230–251. 10.1111/jnc.14859 [DOI] [PubMed] [Google Scholar]
- Liu Y. W., Zhang J., Bi W., Zhou M., Li J., Xiong T., et al. (2022). Histones of neutrophil extracellular traps induce CD11b expression in brain pericytes via dectin-1 after traumatic brain injury. Neurosci. Bull. 38 1199–1214. 10.1007/s12264-022-00902-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-W., Li S., Dai S.-S. (2018). Neutrophils in traumatic brain injury (TBI): Friend or foe? J. Neuroinflammation 15:146. 10.1186/s12974-018-1173-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M. R., LaFrance W. C. (2022). Treatment of psychogenic nonepileptic seizures. Curr. Neurol. Neurosci. Rep. 8 467–474. 10.1007/s11910-022-01209-3 [DOI] [PubMed] [Google Scholar]
- Lou N., Takano T., Pei Y., Xavier A. L., Goldman S. A., Nedergaard M. (2016). Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. U.S.A. 113 1074–1079. 10.1073/pnas.1520398113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A., Nerriere-Daguin V., Vanhove B., Naveilhan P., Neunlist M., Nicot A., et al. (2015). Targeting the CD80/CD86 costimulatory pathway with CTLA4-Ig directs microglia toward a repair phenotype and promotes axonal outgrowth. Glia 63 2298–2312. 10.1002/glia.22894 [DOI] [PubMed] [Google Scholar]
- Lozano D., Gonzales-Portillo G. S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., et al. (2015). Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 8 97–106. 10.2147/NDT.S65815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Li F., Wang P., Chen H., Chen Y. C., Yin X. (2020). Altered hypothalamic functional connectivity in post-traumatic headache after mild traumatic brain injury. J. Headache Pain 21:93. 10.1186/s10194-020-01164-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. L., Sperling G. (2001). Three-systems theory of human visual motion perception: Review and update. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 18 2331–2370. 10.1364/josaa.18.002331 [DOI] [PubMed] [Google Scholar]
- Lyu J., Jiang X., Leak R. K., Shi Y., Hu X., Chen J. (2021). Microglial responses to brain injury and disease: Functional diversity and new opportunities. Transl. Stroke Res. 12 474–495. 10.1007/s12975-020-00857-2 [DOI] [PubMed] [Google Scholar]
- Macas J., Nern C., Plate K. H., Momma S. (2006). Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J. Neurosci. 26 13114–13119. 10.1523/JNEUROSCI.4667-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley G. T., Larson M. D. (2002). Infrared pupillometry during uncal herniation. J. Neurosurg. Anesthesiol. 14 223–228. 10.1097/00008506-200207000-00009 [DOI] [PubMed] [Google Scholar]
- Marigold D. S., Patla A. E. (2008). Visual information from the lower visual field is important for walking across multi-surface terrain. Exp. Brain Res. 188 23–31. 10.1007/s00221-008.1335-7 [DOI] [PubMed] [Google Scholar]
- Marklund N., Clausen F., Lewander T., Hillered L. (2001). Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J. Neurotrauma 18 1217–1227. 10.1089/089771501317095250 [DOI] [PubMed] [Google Scholar]
- Marmarou A., Lu J., Butcher I., McHugh G. S., Murray G. D., Steyerberg E. W., et al. (2007). Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: An IMPACT analysis. J. Neurotrauma 24 270–280. 10.1089/neu.2006.0029 [DOI] [PubMed] [Google Scholar]
- Master C. L., Podolak O. E., Ciuffreda K. J., Metzger K. B., Joshi N. R., McDonald C. C., et al. (2020). Utility of pupillary light reflex metrics as a physiologic biomarker for adolescent sport-related concussion. JAMA Ophthalmol. 138 1135–1141. 10.1001/jamaophthalmol.2020.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt S. (2018). Pupillometry: Psychology, physiology, and function. J. Cogn. 1:16. 10.5334/joc.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S., Matsuda T., Nakashima K. (2021). Regulation of adult mammalian neural stem cells and neurogenesis by cell extrinsic and intrinsic factors. Cells 10:1145. 10.3390/cells10051145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo A. T., Gupta D. (2018). Monitoring the injured brain. J. Neurosurg. Sci. 62 549–562. 10.23736/S0390-5616.18.04465-X [DOI] [PubMed] [Google Scholar]
- McGovern A. J., Barreto G. E. (2021). Mitochondria dysfunction and inflammation in traumatic brain injury: Androgens to the battlefront. Androg. Clin. Res. Therap. 2 304–315. 10.1089/andro.2021.0017 [DOI] [Google Scholar]
- McGuire C., Kristman V. L., Martin L., Bédard M. (2017). Characteristics and incidence of traumatic brain injury in older adults using home care in Ontario from 2003–2013. Can. Geriatr. J. 20 2–9. 10.5770/cgj.20.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon P., Hricik A., Yue J. K., Puccio A. M., Inoue T., Lingsma H. F., et al. (2014). Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI study. J. Neurotrauma 31 26–33. 10.1089/neu.2013.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina R. J., O’Neill C. L., O’Doherty T. M., Knott H., Guduric-Fuchs J., Gardiner T. A., et al. (2011). Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol. Med. 17 1045–1055. 10.2119/molmed.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. G., Garza I., Robertson C. E. (2021). Two hundred and forty-eight cases of visual snow: A review of potential inciting events and contributing comorbidities. Cephalalgia 41 1015–1026. 10.1177/0333102421996355 [DOI] [PubMed] [Google Scholar]
- Mele C., Pingue V., Caputo M., Zavattaro M., Pagano L., Prodam F., et al. (2021). Neuroinflammation and hypothalamo-pituitary dysfunction: Focus of traumatic brain injury. Int. J. Mol. Sci. 22:2686. 10.3390/ijms22052686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Kondo Y., Hirano Y., Ishihara T., Sueyoshi K., Okamoto K., et al. (2020). Characteristics, injuries, and clinical outcomes of geriatric trauma patients in Japan analysis: An of the nationwide trauma registry database. Sci. Rep. 10:19148. 10.1038/s41598-020-76149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller M. C., Nordin L. E., Bartfai A., Julin P., Li T. Q. (2017). Fatigue and cognitive fatigability in mild traumatic brain injury are correlated with altered neural activity during vigilance test performance. Front. Neurol. 21:496. 10.3389/fneur.2017.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada L. V. V., Mire L. G. (2017). Preventing falls in older persons. Am. Fam. Physician 96 240–247. [PubMed] [Google Scholar]
- Morris G., Bullock R., Marshal S., Marmarou A., Maas A., Marshall L. (1999). Failure of competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: Results of two phase III clinical trials. J. Neurosurg. 91 737–743. 10.3171/jns1999.91.5.0737 [DOI] [PubMed] [Google Scholar]
- Moser R. S., Schatz P., Jordan B. D. (2005). Prolonged effects of concussion in high school athletes. Neurosurgery 57 300–306. 10.1227/01.neu.0000166663.98616.e4 [DOI] [PubMed] [Google Scholar]
- Mostafa J., Porter J., Queener H. M., Ostrin L. A. (2021). Intrinsically photosensitive retinal ganglion cell-driven pupil responses in patients with traumatic brain injury. Vision Res. 188 174–183. 10.1016/j.visres.2021.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulson A. J., Squair J. W., Franklin R. J. M., Tetzlaff W., Assinck P. (2021). Diversity of reactive astrogliosis in CNS pathology: Heterogeneity or plasticity? Front. Cell. Neurosci. 15:703810. 10.3389/fncel.2021.703810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagendran S. T., Lee V., Perry M. (2019). Traumatic orbital third nerve palsy. Br. J. Oral Maxillofac. Surg. 57 578–581. 10.1016/j.bjoms.2019.01.029 [DOI] [PubMed] [Google Scholar]
- Nederpelt C. J., Naar L., Meier K., van Wijck S. F. M., Krijnen P., Velmahos G. C., et al. (2022). Treatment and outcomes of anticoagulated geriatric trauma patients with traumatic intracranial hemorrhage after falls. Eur. J. Trauma Emerg. Surg. 48 4297–4304. 10.1007/s00068-022-01938-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwenya L. B., Mazumder S., Porter Z. R., Minnema A., Oswald D. J., Farhadi H. F. (2018). Implantation of neuronal stem cells enhances object recognition without increasing neurogenesis after lateral fluid percussion injury in mice. Stem Cells Int. 2018:4209821. 10.1155/2018/4209821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S. N., Mukherjee P., Ghajar J., Johnson C., Kolster R. A., Sarkar R., et al. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 29 967–973. 10.3174/ajnr.A0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyancho D., Atem F. D., Venkatachalam A. M., Barnes A., Hill M., Traylor J. I., et al. (2021). Anisocoria correlates with injury severity and outcomes after blunt traumatic brain injury. J. Neurosci. Nurs. 53 251–255. 10.1097/JNN.0000000000000613 [DOI] [PubMed] [Google Scholar]
- Orihuela R., McPherson C. A., Harry G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173 649–665. 10.1111/bph.13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet M. C., Beaulieu-Bonneau S., Morin C. M. (2015). Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 14 746–757. 10.1016/S1474-4422(15)00068-X [DOI] [PubMed] [Google Scholar]
- Owsley C. (2003). Contrast sensitivity. Ophthalmol. Clin. North Am. 16 171–177. 10.1016/s0896-1549(03)00003-8 [DOI] [PubMed] [Google Scholar]
- Pandya J. D., Pauly J. R., Nukala V. N., Sebastian A. H., Day K. M., Korde A. S., et al. (2007). Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma 24 798–811. 10.1089/neu.2006.3673 [DOI] [PubMed] [Google Scholar]
- Parry J., Hwang J., Stahel C. F., Henderson C., Nadeau J., Stacey S., et al. (2020). Soluble terminal complement activation fragment sC5b-9: A new serum biomarker for traumatic brain injury. Eur. J. Trauma Emerg. Surg. 47 1491–1497. 10.1007/s00068-020-01407-z [DOI] [PubMed] [Google Scholar]
- Patel P. J., Chen F. K., Rubin G. S., Tufail A. (2009). Intersession repeatability of contrast sensitivity scores in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 50 2621–2625. 10.1167/iovs.08-2407 [DOI] [PubMed] [Google Scholar]
- Patla A. E. (1997). Understanding the roles of vision in the control of human locomotion. Gait Posture 5 54–69. [Google Scholar]
- Pease M., Gonzalez-Martinez J., Puccio A., Nwachuku E., Castellano J. F., Okonkwo D. O., et al. (2022). Epilepsy after severe traumatic brain injury. Ann. Neurol. 92 663–669. 10.1002/ana.26443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca P., Smith G., Santana-Gomez C., Bragin A., Staba R. (2019). Electrophysiological biomarkers of epileptogenicity after traumatic brain injury. Neurobiol. Dis. 123 69–74. 10.1016/j.nbd.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piponnier J.-C., Forget R., Gagnon I., McKerral M., Jean-François J.-F., Faubert J. (2016). First- and second-order stimuli reaction time measures are highly sensitive to mild traumatic brain injuries. J. Neurotrauma 33 242–253. 10.1089/neu.2014.3832 [DOI] [PubMed] [Google Scholar]
- Poltavski D. V., Biberdorf D. (2014). Screening for lifetime concussion in athletes: Importance of oculomotor measures. Brain Inj. 28 475–485. 10.3109/02699052.2014.888771 [DOI] [PubMed] [Google Scholar]
- Popkirov S., Carson A. J., Stone J. (2018). Scared or scarred: Could ‘dissociogenic’ lesions predispose to nonepileptic seizures after head trauma? Seizure 58 127–132. 10.1016/j.seizure.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Prabhakar N. K., Khan H., Grewal A. K., Singh T. G. (2022). Intervention of neuroinflammation in the traumatic brain injury trajectory: In vivo and clinical approaches. Int. Immunopharmacol. 108:108902. 10.1016/j.intimp.2022.108902 [DOI] [PubMed] [Google Scholar]
- Prak R. F., Marsman J. C., Renken R., van der Naalt J., Zijdewind I. (2021). Fatigue following mild traumatic brain injury relates to visual processing and effort perception in the context of motor performance. Neuroimage Clin. 32:102783. 10.1016/j.nicl.2021.102783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C., Bruhns M. E. (2017). Evaluation and treatment of mild traumatic brain injury: The role of neuropsychology. Brain Sci. 7 105. 10.3390/brainsci7080105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M., Greco T., Alexander D., Giza C. C. (2013). The pathophysiology of traumatic brain injury at a glance. Dis. Mod. Mech. 6 1307–1315. 10.1242/dmm.011585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Boucher M., Conley G., Li Y., Zhang J., Morriss N., et al. (2022). Traumatic brain injury-related optic nerve damage. J. Neuropathol. Exp. Neurol. 81 344–355. 10.1093/jnen/nlac018 [DOI] [PubMed] [Google Scholar]
- Quaid P. T., Singman E. L. (2022). Post-traumatic headaches and vision: A review. NeuroRehabilitation 50 297–308. 10.3233/NRE-228013 [DOI] [PubMed] [Google Scholar]
- Rabin J. (1994). Optical defocus: Differential effects on size and contrast letter recognition thresholds. Invest. Ophthalmol. Vis. Sci. 35 646–648. [PubMed] [Google Scholar]
- Raghupathi R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathol. 14 215–222. 10.1111/j.1750-3639.2004.tb00056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikes A. C., Satterfield B. C., Killgore W. D. S. (2019). Evidence of actigraphic and subjective sleep disruption following mild traumatic brain injury. Sleep Med. 54 62–69. 10.1016/j.sleep.2018.09.018 [DOI] [PubMed] [Google Scholar]
- Ramage A. E., Tate D. F., New A. B., Lewis J. D., Robin D. A. (2019). Effort and fatigue-related functional connectivity in mild traumatic brain injury. Front. Neurol. 29:1165. 10.3389/fneur.2018.01165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. L., Başkaya M. K., Doğan A., Rothstein J. D., Dempsey R. J. (1998). Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J. Neurochem. 70 2020–2027. 10.1046/j.1471-4159.1998.70052020.x [DOI] [PubMed] [Google Scholar]
- Ratiu I., Fissel-Brannick S., Whiting M., Murnion L., Azuma T. (2022). The impact of mild traumatic brain injury on reading comprehension and eye movements: Preliminary results. J. Commun. Disord. 96:106197. 10.1016/j.jcomdis.2022.106197 [DOI] [PubMed] [Google Scholar]
- Reddy D. S., Golub V. M., Ramakrishnan S., Abeygunaratne H., Dowell S., Wu X. (2022). A comprehensive and advanced mouse model of post-traumatic epilepsy with robust spontaneous recurrent seizures. Curr. Protoc. 2:e447. 10.1002/cpz1.447 [DOI] [PubMed] [Google Scholar]
- Reed-Jones R. J., Solis G. R., Lawson K. A., Loya A. M., Cude-Islas D., Berger C. S. (2013). Vision and falls: A multidisciplinary review of the contributions of visual impairment to falls among older adults. Maturitas 75 22–28. 10.1016/j.maturitas.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Reneker J. C., Cheruvu V. K., Yang J., James M. A., Cook C. E. (2018). Physical examination of dizziness in athletes after a concussion: A descriptive study. Musculoskelet. Sci. Pract. 34 8–13. 10.1016/j.msksp.2017.11.012 [DOI] [PubMed] [Google Scholar]
- Ricci F., Cedrone C., Cerulli L. (1998). Standardized measurement of visual acuity. Ophthalmic Epidemiol. 5 41–53. 10.1076/opep.5.1.41.1499 [DOI] [PubMed] [Google Scholar]
- Richman J., Spaeth G. L., Wirostko B. (2013). Contrast sensitivity basics and a critique of currently available tests. J. Cataract Refract. Surg. 39 1100–1106. 10.1016/j.jcrs.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Richman J., Zangalli C., Lu L., Wizov S. S., Spaeth E., Spaeth G. L. (2015). The Spaeth/Richman contrast sensitivity test (SPARCS): Design, reproducibility and ability to identify patients with glaucoma. Br. J. Ophthalmol. 99 16–20. 10.1136/bjophthalmol-2014-305223 [DOI] [PubMed] [Google Scholar]
- Ritter A. M., Muizelaar J. P., Barnes T., Choi S., Fatouros P., Ward J., et al. (1999). Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery 44 941–948. 10.1097/00006123-199905000-00005 [DOI] [PubMed] [Google Scholar]
- Ronaldson P. T., Davis T. P. (2020). Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 40 S6–S24. 10.1177/0271678X20951995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronne-Engstrom E., Winkler T. (2006). Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurol. Scand. 114 47–43. 10.1111/j.1600-0404.2006.00652.x [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Dykes-Hoberg M., Pardo C. A., Bristol L. A., Jin L., Kuncl R. W., et al. (1996). Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16 675–686. 10.1016/s.0896-6273(00)80086-0 [DOI] [PubMed] [Google Scholar]
- Schepici G., Silvestro S., Bramanti P., Mazzon E. (2020). Traumatic brain injury and stem cells: An overview of clinical trials, the current treatments and future therapeutic approaches. Medicina (Kaunas) 56:137. 10.3390/medicina56030137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N. (2017). An insight into the vision impairment following traumatic brain injury. Neurochem. Int. 111 103–107. 10.1016/j.neuint.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J. M., McEwen B. S., Alvarez-Buylla A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21 7153–7160. 10.1523/JNEUROSCI.21-18-07153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W. S., Hu S., Feng A., Rock R. B. (2013). Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 38 2148–2159. 10.1007/s11064-013-1123-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E., Beit-Yannai E., Horowitz M., Kohen R. (1997). Oxidative stress in closed-head injury: Brain antioxidant capacity as an indicator of functional outcome. J. Cereb. Blood Flow Metab. 17 1007–1019. 10.1097/00004647-199710000-00002 [DOI] [PubMed] [Google Scholar]
- Simon D. W., McGeachy M. J., Bayır H., Clark R. S., Loane D. J., Kochanek P. M. (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13 171–191. 10.1038/nrneurol.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade P., Tambe A., Kochenderfer M. J. (2021). Multimodal sensing and intuitive steering assistance improve navigation and mobility for people with impaired vision. Sci. Robot. 6:eabg6594. 10.1126/scirobotics.abg6594 [DOI] [PubMed] [Google Scholar]
- Sloan L. L. (1951). Measurement of visual acuity; a critical review. AMA Arch. Ophthalmol. 45 704–725. 10.1001/archopht.1951.01700010719013 [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. (2020). Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends Immunol. 41 758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Stevens C. F., Gage F. H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature 417 39–44. 10.1038/417039a [DOI] [PubMed] [Google Scholar]
- Sornalingam K., Borman A. D., Ashworth J. (2018). Nonaccidental injury presenting as unilateral retinal detachment in two infants. J. AAPOS 22 231–233. 10.1016/j.jaapos.2017.10.018 [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P., Jasper H., Neves J. (2018). Trophic factors in inflammation and regeneration: The role of MANF and CDNF. Front. Physiol. 9:1629. 10.3389/fphys.2018.01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers J. L., Sowers M. L., Shavkunov A. S., Hawkins B. E., Wu P., DeWitt D. S., et al. (2021). Traumatic brain injury induces region-specific glutamate metabolism changes as measured by multiple mass spectrometry methods. iScience 24:103108. 10.1016/j.isci.2021.103108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethling J. M., Klein D. M., Singh P., Meaney D. F. (2008). Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J. Neurotrauma 25 1207–1216. 10.1089/neu.2008.0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees J. L., Lee R. H., Gregory C. A. (2016). Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 7:125. 10.1186/s13287-016-0363-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D. P., Reynaud A., Ruiz T., Laguë-Beauvais M., Hess R., Farivar R. (2016). First- and second-order contrast sensitivity functions reveal disrupted visual processing following mild traumatic brain injury. Vision Res. 122 43–50. 10.1016/j.visres.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Steinmetz S., Tipold A., Löscher W. (2013). Epilepsy after head injury in dogs: A natural model of posttraumatic epilepsy. Epilepsia 54 580–588. 10.1111/epi.12071 [DOI] [PubMed] [Google Scholar]
- Stocchetti N., Zanier E. R. (2016). Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 20:148. 10.1186/s13054-016-1318-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica B. A., Faden A. I. (2010). Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics 7 3–12. 10.1016/j.nurt.2009.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. (2016). Endogenous neurogenic cell response in the mature mammalian brain following traumatic injury. Exp. Neurol. 275 405–410. 10.1016/j.expneurol.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Lim J., Meng J., Kwok K., Thakor N., Bezerianos A. (2014). Discriminative analysis of brain functional connectivity patterns for mental fatigue classification. Ann. Biomed. Eng. 42 2084–2094. 10.1007/s10439-014-1059-8 [DOI] [PubMed] [Google Scholar]
- Susman M., DiRusso S. M., Sullivan T., Risucci D., Nealon P., Cuff S., et al. (2002). Traumatic brain injury in the elderly: Increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma 53 219–224. 10.1097/00005373-200208000-00004 [DOI] [PubMed] [Google Scholar]
- Swan G., Shahin M., Albert J., Herrmann J., Bowers A. R. (2019). The effects of simulated acuity and contrast sensitivity impairments on detection of pedestrian hazards in a driving simulator. Transp. Res. Part F Traffic Psychol. Behav. 64 213–226. 10.1016/j.trf.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylliaas H., Idland G., Sandvik L., Forsen L., Bergland A. (2009). Does mortality of the aged increase with the number of falls? Results from a nine-year follow-up study. Eur. J. Epidemiol. 24 351–355. 10.1007/s10654-009-9348-5 [DOI] [PubMed] [Google Scholar]
- Tajiri N., Kaneko Y., Shinozuka K., Ishikawa H., Yankee E., McGrogan M., et al. (2013). Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One 8:e74857. 10.1371/journal.pone.0074857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper A., Gonzalez D., Nouredanesh M., Niechwiej-Szwedo E. (2021). Pupillometry provides a psychophysiological index of arousal level and cognitive effort during the performance of a visual-auditory dual-task in individuals with a history of concussion. Vision Res. 184 43–51. 10.1016/j.visres.2021.03.011 [DOI] [PubMed] [Google Scholar]
- Tasker R. C. (2012). Spreading depolarisations and traumatic brain injury: Time course and mechanisms. Lancet Neurol. 11:389. 10.1016/S1474-4422(12)70084-4 [DOI] [PubMed] [Google Scholar]
- Tavazzi B., Signoretti S., Lazzarino G., Amorini A. M., Delfini R., Cimatti M., et al. (2005). Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery 56 582–589. 10.1227/01.neu.0000156715.04900.e6 [DOI] [PubMed] [Google Scholar]
- Temkin N., Machamer J., Dikmen S., Nelson L. D., Barber J., Hwang P. H., et al. (2022). Risk factors for high symptom burden three months after traumatic brain injury and implications for clinical trial design: A transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma 39 1524–1532. 10.1089/neu.2022.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo Z. L., Tham Y. C., Yu M., Chee M. L., Rim T. H., Cheung N., et al. (2021). Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 128 1580–1591. 10.1016/j.ophtha.2021.04.027 [DOI] [PubMed] [Google Scholar]
- Thapa K., Khan H., Singh T. G., Kaur A. (2021). Traumatic brain injury: Mechanistic insight on pathophysiology and potential therapeutic targets. J. Mol. Neurosci. 71 1725–1742. 10.1007/s12031-021-01841-7 [DOI] [PubMed] [Google Scholar]
- Thiagarajan P., Ciuffreda K. J. (2022). Accommodative and pupillary dysfunctions in concussion/mild traumatic brain injury: A Review. NeuroRehabilitation 50 261–278. 10.3233/NRE-228011 [DOI] [PubMed] [Google Scholar]
- Thiagarajan P., Ciuffreda K. J., Capo-Aponte J. E., Ludlam D. P., Kapoor N. (2014). Oculomotor neurorehabilitation for reading in mild traumatic brain injury (mTBI): An integrative approach. Neurorehabilitation 34 129–146. 10.3233/NRE-131025 [DOI] [PubMed] [Google Scholar]
- Thompson H. J., McCormick W. C., Kagan S. H. (2006). Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 54 1590–1595. 10.1111/j.1532-5415.2006.00894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuma T. B. T., Bello N. R., Rapuano C. J., Wasserman B. N. (2022). Resolution of traumatic mydriasis and accommodative dysfunction eight years after sweetgum ball ocular injury. Am. J. Ophthalmol. Case Rep. 26:101552. 10.1016/j.ajoc.2022.101552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Wang X., Wang X., Wang L., Wang X., Wu S., et al. (2013). Autologous bone marrow mesenchymal stem cell therapy in the subacute stage of traumatic brain injury by lumbar puncture. Exp. Clin. Transplant. 11 176–181. 10.6002/ect.2012.0053 [DOI] [PubMed] [Google Scholar]
- Toth L., Czigler A., Horvath P., Kornyei B., Szarka N., Schwarcz A., et al. (2021). Traumatic brain injury-induced cerebral microbleeds in the elderly. Geroscience 43 125–136. 10.1007/s11357-020-00280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutonji A., Mandava M., Guglietta S., Tomlinson S. (2021). Chronic complement dysregulation drives neuroinflammation after traumatic brain injury: A transcriptomic study. Acta Neuropathol. Commun. 9:126. 10.1186/s40478-021-01226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor J. I., El Ahmadieh T. Y., Bedros N. M., Al Adli N., Stutzman S. E., Venkatachalam A. M., et al. (2021). Quantitative pupillometry in patients with traumatic brain injury and loss of consciousness: A prospective pilot study. J. Clin. Neurosci. 91 88–92. 10.1016/j.jocn.2021.06.044 [DOI] [PubMed] [Google Scholar]
- Truong J. Q., Ciuffreda K. J. (2016). Objective pupillary correlates of photosensitivity in the normal and mild traumatic brain injury populations. Mil. Med. 181 1382–1390. 10.7205/MILMED-D-15-00587 [DOI] [PubMed] [Google Scholar]
- Truong J. Q., Ciuffreda K. J., Han M. H., Suchoff I. B. (2014). Photosensitivity in mild traumatic brain injury (mTBI): A retrospective analysis. Brain Inj. 28 1283–1287. 10.3109/02699052.2014.915989 [DOI] [PubMed] [Google Scholar]
- Tsui E., Patel P. (2020). Calculated decisions: Visual acuity testing (Snellen chart). Emerg. Med. Pract. 22 CD1–CD2. [PubMed] [Google Scholar]
- Tubi M. A., Lutkenhoff E., Blanco M. B., McArthur D., Villablanca P., Ellingson B., et al. (2019). Early seizures and temporal lobe trauma predict post-traumatic epilepsy: A longitudinal study. Neurobiol. Dis. 123 115–121. 10.1016/j.nbd.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler C. M., Perrin P. B., Klyce D. W., Arango-Lasprilla C. J., Dautovich N. D., Rybarczyk B. D. (2022). Predictors of 10-year functional independence trajectories in older adults with traumatic brain injury: A model systems study. NeuroRehabilitation 10.3233/NRE-220165 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Uberti M., Hasan S., Holmes D., Ganau M., Uff C. (2021). Clinical significance of isolated third cranial nerve palsy in traumatic brain injury: A detailed description of four different mechanisms of injury through the analysis of our case series and review of the literature. Emerg Med. Int. 2021:5550371. 10.1155/2021/5550371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Tarantini S., Kirkpatrick A. C., Csiszar A., Prodan C. I. (2017). Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 312 H1128–H1143. 10.1152/ajpheart.00780.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnisa A., Greig N. H., Kamal M. A. (2022). Inhibition of Caspase 3 and Caspase 9 mediated apoptosis: A multimodal therapeutic target in traumatic brain injury. Curr. Neuropharmacol. 20:121898. 10.2174/1570159X20666220327222921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Landeghem F. K., Weiss T., Oehmichen M., Von Deimling A. (2006). Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J. Neurotrauma 23 1518–1528. 10.1089/neu.2006.23.1518 [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N., Lamkanfi M. (2019). Caspases in cell death, inflammation, and disease. Immunity 50 1352–1364. 10.1016/j.immuni.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg R. D., Belanger H. G., Curtiss G., Bowles A. O., Cooper D. B. (2019). Reconceptualizing rehabilitation of individuals with chronic symptoms following mild traumatic brain injury. Rehabil. Psychol. 64 1–12. 10.1037/rep0000255 [DOI] [PubMed] [Google Scholar]
- Vaniyapong T., Phinyo P., Patumanond J., Ratanalert S., Limpastan K. (2020). Development of clinical decision rules for traumatic intracranial injuries in patients with mild traumatic brain injury in a developing country. PLoS One 15:e0239082. 10.1371/J.pone.0239082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verellen R. M., Cavazos J. E. (2010). Post-traumatic epilepsy: An overview. Therapy 7 527–531. 10.2217/THY.10.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoekx J. S. N., van Overdam K. A., Gishti O., van Leeuwen O., Crama N. (2021). [Acute onset of floaters, even without flashes, is an urgent ophthalmic warning sign]. Ned. Tijdschr. Geneeskund. 165:D5850. Article in Dutch, [PubMed] [Google Scholar]
- Vernooij M. W., van der Lugt A., Ikram M. A., Wielopolski P. A., Niessen W. J., Hofman A., et al. (2008). Prevalence and risk factors of cerebral microbleeds: The Rotterdam scan study. Neurology 70 1208–1214. 10.1212/01.wnl.0000307750.41970.d9 [DOI] [PubMed] [Google Scholar]
- Virdee J., Mollan S. P. (2020). Photopsia. Pract. Neurol. 20 415–419. 10.1136/practneurol-2019-002460 [DOI] [PubMed] [Google Scholar]
- Voormolen D. C., Haagsma J. A., Polinder S., Maas A. I. R., Steyerberg A. W., Vuleković P., et al. (2019). Post-concussion symptoms in complicated vs. uncomplicated mild traumatic brain injury patients at three and six months post-injury: Results from the CENTER-TBI Study. J. Clin. Med. 8:1921. 10.3390/jcm8111921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Cui D., Gao L. (2016). Traumatic brain injury: A review of characteristics, molecular basis and management. Front. Biosci. 21:890–899. 10.2741/4426 [DOI] [PubMed] [Google Scholar]
- Wang W., Wu X., Yang C. S., Zhang J. (2021). An unrecognized fundamental relationship between neurotransmitters: Glutamate protects against catecholamine oxidation. Antioxidants (Basel) 10:1564. 10.3390/antiox10101564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhang X., Lin L., Ren J., He R., Sun K. (2022). Inhibition of NADPH oxidase 2 (NOX2) reverses cognitive deficits by modulating excitability and excitatory transmission in the hippocampus after traumatic brain injury. Biochem. Biophys. Res. Commun. 617(Pt 1) 1–7. 10.1016/j.bbrc.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Wang X., Wei X. E., Li M. H., Li W. B., Zhou Y. J., Zhang B., et al. (2014). Microbleeds on susceptibility-weighted MRI in depressive and non-depressive patients after mild traumatic brain injury. Neurol. Sci. 35 1533–1539. 10.1007/s10072-014-1788-3 [DOI] [PubMed] [Google Scholar]
- Wee Yong V. (2010). Inflammation in neurological disorders: A help or a hindrance? Neuroscientist 16 408–420. 10.1177/1073858410371379 [DOI] [PubMed] [Google Scholar]
- Wennersten A., Holmin S., Mathiesen T. (2003). Characterization of Bax and Bcl-2 in apoptosis after experimental traumatic brain injury in the rat. Acta Neuropathol. 105 281–288. 10.1007/s00401-002-0649-y [DOI] [PubMed] [Google Scholar]
- Werner R. N., Gustafson J. A. (2022). Case report: Visual snow syndrome after repetitive mild traumatic brain injury. Opt. Vis. Sci. 99 413–416. 10.1097/OPX.0000000000001862 [DOI] [PubMed] [Google Scholar]
- West S. K., Rubin G. S., Broman A. T., Muñoz B., Bandeen-Roche K., Turano K. (2002). How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch. Ophthalmol. 120 774–780. 10.1001/archopht.120.6.774 [DOI] [PubMed] [Google Scholar]
- Winter L., Mensinger J. L., Moriarty H. J., Robinson K. M., McKay M., Leiby B. E. (2022). Age moderates the effect of injury severity on functional trajectories in traumatic brain injury: A study using the NIDILRR traumatic brain injury model systems national dataset. J. Clin. Med. 11:2477. 10.3390/jcm11092477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. H., Fruttiger M., Richardson W. D., Franklin R. J. (2004). Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 25 252–262. 10.1016/j.mcn.2003.10.014 [DOI] [PubMed] [Google Scholar]
- Wu N., Sun X., Zhou C., Yan J., Cheng C. (2023). Neuroblasts migration under control of reactive astrocyte-derived BDNF: A promising therapy in late neurogenesis after traumatic brain injury. Stem Cell Res. Ther. 14:2. 10.1186/s13287-022-03232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zheng L. X., Chen X. S., Pang Q. Y., Yan Y. N., Liu R., et al. (2022). Brain-specific loss of Abcg1 disturbs cholesterol metabolism and aggravates pyroptosis and neurological deficits after traumatic brain injury. Brain Pathol. e13126. 10.1111/bpa.13126 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji Y. (2015). Cerebral microbleeds: Detection, associations and clinical implications. Front. Neurol. Neurosci. 37:78–92. 10.1159/000437115 [DOI] [PubMed] [Google Scholar]
- Yang B., Fritsche K. L., Beversdorf D. Q., Gu Z., Lee J. C., Folk W. R., et al. (2019). Yin-Yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Front. Neurol. 10:642. 10.3389/fneur.2019.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Salayandia V. M., Thompson J. F., Yang L. Y., Estrada E. Y., Yang Y. (2015). Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J. Neuroinflammation 12 26. 10.1186/s12974-015-0245-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates P. A., Villemagne V. L., Ellis K. A., Desmond P. M., Masters C. L., Rowe C. C. (2014). Cerebral microbleeds: A review of clinical, genetic, and neuroimaging associations. Front. Neurol. 4:205. 10.3389/fneur.2013.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong H., Rawji K. S., Ghorbani S., Xue M., Yong V. W. (2019). The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 16 540–546. 10.1038/s41423-019-0223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen T. J., Johnson K. R., Miron V. E., Zhao C., Quandt J., Harrisingh M. C., et al. (2013). Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain 136 1035–1047. 10.1093/brain/awt024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeshan M., Jehan F., O’Keeffe T., Khan M., Zakaria E. R., Hamidi M., et al. (2018). The novel oral anticoagulants (NOACs) have worse outcomes compared with warfarin in patients with intracranial hemorrhage after TBI. J. Trauma Acute Care Surg. 85 915–920. 10.1097/TA.0000000000001995 [DOI] [PubMed] [Google Scholar]
- Zhang Q. G., Laird M. D., Han D., Nguyen K., Scott E., Dong Y., et al. (2012). Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One 7:e34504. 10.1371/journal.pone.0034504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Hong J., Zhang H., Zheng W., Yang Y. (2021). Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY) 13 21642–21658. 10.18632/aging.203508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Shao A., Yao Y., Tu S., Deng Y., Zhang J. (2020). Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun. Signal. 18:62. 10.1186/s12964-020-00549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell J. M., Morganti-Kossmann M. C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7 22–30. 10.1016/j.nurt.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]