Abstract
Alpha-tocopherol is a member of the vitamin E family that functions as the principal fat-soluble antioxidant in vertebrates. Body-wide distribution of tocopherol is regulated by the hepatic alpha-tocopherol transfer protein (αTTP), which stimulates secretion of the vitamin from hepatocytes to circulating lipoproteins. This biological activity of αTTP is thought to stem from its ability to facilitate the transfer of vitamin E between membranes, but the mechanism by which the protein exerts this activity remains poorly understood. Using a fluorescence energy transfer methodology, we found that the rate of tocopherol transfer from lipid vesicles to αTTP increases with increasing αTTP concentration. This concentration dependence indicates that ligand transfer by αTTP involves direct protein-membrane interaction. In support of this notion, equilibrium analyses employing filtration, dual polarization interferometry, and tryptophan fluorescence demonstrated the presence of a stable αTTP·bilayer complex. The physical association of αTTP with membranes is markedly sensitive to the presence of vitamin E in the bilayer. Some naturally-occurring mutations in αTTP that cause the hereditary disorder Ataxia with vitamin E deficiency (AVED) diminish the effect of tocopherol on the protein-membrane association, suggesting a possible mechanism for the accompanying pathology.
Vitamin E is the major lipid-soluble antioxidant in numerous species. By virtue of its radical-trapping activity, vitamin E is thought to alleviate oxidative damage in cells, and thus to prevent various pathologies related to oxidative stress. Vertebrates selectively accumulate only one form of vitamin E from dietary mixtures, namely RRR-α-tocopherol (1,2). This preferential retention is achieved through degradation of other forms of vitamin E (e.g. 3,4), and the selective, high affinity binding of RRR-α-tocopherol (herein abbreviated tocopherol) by the hepatic alpha-tocopherol transfer protein (αTTP) (5,6). In vitro, αTTP binds tocopherol with high selectivity and affinity, and catalyzes transfer of the vitamin between membrane vesicles (7–9). In cultured hepatocytes, expression of αTTP enhances secretion of vitamin E to the culture media (10,11). It is generally believed that in vivo αTTP is critical for the incorporation of dietary RRR-α-tocopherol into circulating lipoproteins, which deliver the vitamin to target cells.
The role of αTTP in regulating whole-body levels of tocopherol is underscored by the fact that mutations in the ttpA gene cause hereditary vitamin E deficiency (ataxia with vitamin E deficiency, AVED; 12). AVED patients present progressive neurodegeneration and low plasma tocopherol levels. Multiple mutations in the ttpA gene were identified in AVED patients, which are thought to impair the protein’s cellular activities. Substitution mutations such as R59W, E141K, and R221W cause an early onset, severe form of the AVED syndrome, while the H101Q, A120T, and R192H substitutions are associated with a later-onset, milder form of the disorder (13–19). As these mutations have different effects on the protein’s inter-membrane transfer and secretion activities (20,21), they represent useful tools with which to study the molecular mechanisms underlying αTTP function.
The molecular mechanisms that underlie inter-membrane tocopherol transfer by αTTP are not known. Sec14p, a related protein from the CRAL-TRIO family, was proposed to facilitate lipid transfer by interacting directly with membranes and actively ‘extracting’ the ligand from the bilayer (22). However, no experimental evidence was put forward in support of this model. Other lipid transfer proteins are thought to employ multiple mechanisms to facilitate ligand transfer, such as bringing donor and acceptor particles to close proximity in the case of phospholipid transfer protein (PLTP, 23,24), physical association with the bilayer in the case of adipocyte- and heart- fatty acid binding proteins (25), or by binding lipids that have diffused from membranes into the aqueous milieu, such as in the case of the liver fatty acid binding protein (26). We report on our investigations into the mechanism by which αTTP facilitates the inter-membrane transfer of tocopherol. Specifically, we address the role of physical association between the protein and the membrane during the ligand transfer reaction.
Experimental Procedures
Details of the synthesis and fluorescent characteristics of NBD-tocopherol were previously reported (11,27).
Wild-type and mutant αTTP proteins were expressed and purified as previously described (20,27). Protein expression was induced with 0.25 mM IPTG overnight at 25°C. All proteins were stored in 20 mM Tris, pH 8.0, 150 mM NaCl, 50% glycerol at –20°C.
The affinity of αTTP for NBD-tocopherol was determined by fluorescence titrations as previously described (27). Briefly, 0.1–1 μM αTTP was titrated with increasing concentrations of the fluorescent ligand NBD-tocopherol, and NBD fluorescence was monitored at 526 nm (excitation = 466 nm) with a QuantaMaster 4 fluorimeter (Photon Technologies International).
Sonicated unilamellar vesicles were prepared from egg-yolk phosphatidylcholine, NBD-tocopherol, TRITC-DHPE (Molecular Probes), and butylated hydroxytoluene (molar ratio 98.2: 0.8: 0.5: 0.5) by sonication in SET buffer (0.25 M sucrose, 1 mM EDTA, 50 mM Tris, pH 8.0) and used at 25 μM in a stopped-flow apparatus (TGK Scientific, UK). Tocopherol-containing lipid vesicles were prepared from egg yolk phosphatidylcholine, RRR-α-tocopherol, and butylated hydroxytoluene (molar ratio 93.5:6:0.5) by sonication in SET buffer. Sonicated vesicles were centrifuged at 100,000 × g at 4°C for 1 hour, and the resultant supernatant was purged with argon and stored at 4°C in the dark for up to two weeks. Large unilamellar vesicles (LUVs) for dual polarization interferometry were prepared by thirteen repeated extrusion steps through a 100 nm polycarbonate filter (LipoFast, Avestin, Ottawa) in 10 mM potassium phosphate, 137 mM sodium chloride, pH 7.4.
Movement of NBD-tocopherol from membranes into TTP’s binding pocket was examined by monitoring the fluorescence resonance energy transfer (FRET) between NBD-tocopherol and the fluorescent lipid TRITC-DHPE as described earlier (27). Briefly, small unilamellar vesicles containing NBD-tocopherol and TRITC-DHPE were prepared as described above, and mixed with purified recombinant αTTP. Release of FRET between NBD-tocopherol and the fluorescent lipid was measured by monitoring the time-dependent change in TRITC-DHPE fluorescence (excitation = 466 nm, emission = 575 nm). Raw fluorescence data were fitted to the sum of a single exponential process and a linear term (representing chromophore bleaching), and the obtained pseudo first-order half-life values are reported. FRET experiments were done at protein concentrations > 5 μM, where more than 95% of the ligand was extracted, thus minimizing contribution from back-reactions (re-association of NBD-tocopherol with the bilayer).
To evaluate the association of αTTP with membranes, the protein was incubated with sonicated vesicles for 30 minutes at room temperature, and filtered through a centrifugal concentrator (Micro-con YM-100, Millipore, 11,000 × g for 30 minutes). Lipid vesicles and associated protein were retained above the filter, while buffer and free protein flow through the filter to the lower chamber (28). Vesicle-associated protein was recovered with SET buffer supplemented with 150 μM Triton X-100. Samples were resolved on SDS-PAGE, visualized by Coomassie staining, and quantitated by densitometry.
Protein-membrane interactions were also measured using dual polarization interferometry (DPI) on an Analight Bio 200 with an unmodified sensor chip (Farfield Scientific Ltd., UK). Extruded vesicles were deposited onto the surface of the sensor chip at a flow rate of 25 μl/min for 8 minutes. The deposited phospholipids were allowed to equilibrate under flow conditions until a stable layer formed with a final thickness 3–10 nm before protein was injected. The association of αTTP to adsorbed phospholipids in the first 450 seconds was fit to a one-site exponential equation, which yielded the maximum specific mass of bound protein adsorbed at a given concentration of αTTP. After each injection of protein the sensor chip was regenerated with 80% ethanol.
Results
The defining biochemical activity of αTTP is catalysis of tocopherol transfer between lipid bilayers (7,8,29). To understand the molecular mechanisms by which the protein exerts this activity, we examined the first step of the transfer reaction, namely, transfer of tocopherol from lipid bilayers into TTP’s binding pocket. Two possible mechanisms could be envisioned to underlie ligand transfer process. One involves physical association of αTTP with the lipid bilayer, followed by ‘extraction’ of tocopherol into the protein’s binding pocket (reaction i):
| (i) |
Alternatively, the protein may facilitate transfer by binding the vitamin following its passive diffusion from membranes, thereby shifting the equilibrium partitioning of tocopherol toward the aqueous milieu (reaction ii):
| (ii) |
To distinguish between these two mechanisms, we examined the rate of ligand sequestration from its FRET partner in the presence of different αTTP concentrations. If αTTP directly interacts with the bilayer (reaction i), the rate of ligand sequestration is expected to depend on protein concentration. On the other hand, if the protein binds tocopherol in the aqueous buffer (reaction ii), the reaction rate will be limited by the rate of spontaneous ligand dissociation from the membrane. In this case, the rate of tocopherol extraction will be independent of protein concentration. We utilized a fluorescence resonance energy transfer (FRET) assay that we recently developed to monitor ligand transfer. In this system, FRET occurs between NBD-tocopherol and a fluorescently-labeled lipid, (TRITC-DHPE), when both are present in the same bilayer (27). Sequestration of NBD-tocopherol by αTTP results in loss of FRET between the lipid and NBD-tocopherol, which is readily detected fluorimetrically (see Experimental Procedures).
Figure 1 shows the kinetics of FRET release at various protein concentrations. We observed a pronounced dependence of the reaction half-life on αTTP concentrations from 8.7 ± 0.4 s (5 μM αTTP; n = 10) to 3.9 ± 0.2 s (30 μM αTTP; n = 9, Figure 1B). The concentration-dependent nature of this process suggests that αTTP extracts tocopherol from membranes through a direct, collision-mediated mechanism.
Figure 1. Sequestration of membrane-bound tocopherol by αTTP.

A) Time-dependent changes in FRET between NBD-tocopherol and fluorescent liposomes (25 μM in SET buffer) in the presence of 5 μM or 30 μM αTTP. Fluorescence of TRITC-DHPE was monitored at 575 nm upon excitation at 466 nm. Data collection was initiated upon mixing of the two samples in the stopped-flow device. B) Dependence of the rate of tocopherol sequestration on αTTP concentrations. Rate constants were extracted from the time-dependent decrease in FRET as described in Experimental Procedures. Shown are averages and standard deviations from >5 independent measurements at each protein concentration.
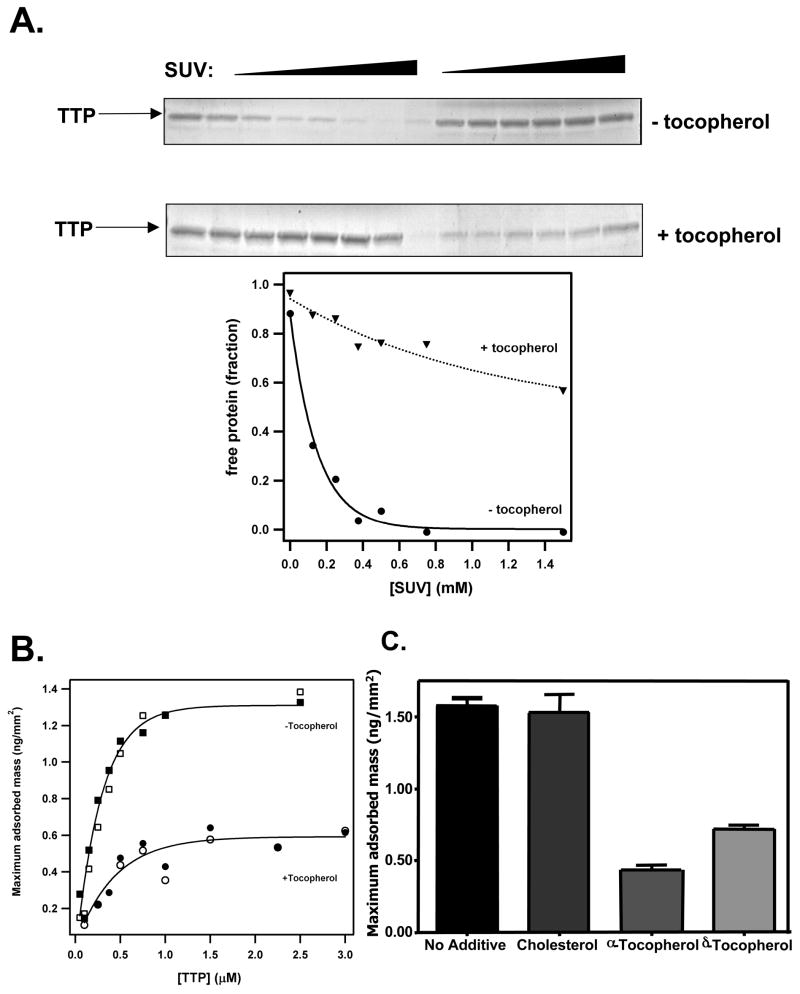
To further examine a direct αTTP –membrane interaction, we used a size-exclusion filtration assay that allows for the detection of stable protein-vesicle complexes (28). Purified αTTP was incubated with varying concentrations of sonicated unilamellar vesicles and the mixture was passed through a centrifugal filtration device (MWCO = 100 kDa). Vesicles and associated protein were retained by the filter, while free protein passed through the filter to the lower chamber. As shown in Figure 2A, the fraction of lipid-bound αTTP increased with increasing vesicle concentration. This effect was not due to non-specific ‘trapping’ of the protein by the vesicles, since another soluble protein (GST) did not associate with the vesicles under identical conditions (Figure 2A). A physical interaction between αTTP and lipid bilayers was also evident from the fluorescence properties of the protein. The presence of five tryptophans in αTTP’s primary structure gives rise to a characteristic intrinsic fluorescence spectrum when the protein is excited at 295 nm, (λmax emission = 328 nm; Figure 2B). In the presence of lipid vesicles, however, the emission spectrum is red-shifted by 4 nm (λmax emission = 332 nm). Such a spectral shift is often observed when the local environment of tryptophan residues is changed to one of a more polar nature (30). These observations indicate that αTTP undergoes a distinct conformational change upon association with lipid bilayers. We also evaluated the interaction between αTTP and bilayers using dual polarization interferometry (DPI) measurements. Specifically, we used this method to probe the interactions between soluble αTTP and a lipid bilayer adsorbed onto the surface of a sensor chip (see Experimental Procedures). Alpha-TTP formed a distinct layer on the immobilized phospholipids, the specific mass of which was dependent on protein concentration (Figure 3B).
Figure 2. Physical association of αTTP with lipid vesicles.
A) Purified recombinant αTTP (or GST; 1.5 μM) was incubated with the indicated concentration of sonicated vesicles for 30 minutes. Free protein was separated from membrane-bound protein by centrifugal filtration as described in Experimental Procedures. Shown is a representative of three independent experiments. B) Fluorescence emission spectrum of αTTP (0.8 μM; excitation = 295 nm) was monitored in the presence or absence of 15 mM sonicated vesicles. Fluorescence intensities were normalized at the wavelength of maximal emission, and corrected for scattering. Representative of three experiments.
Figure 3. Effect of vitamin E on the interaction between αTTP and membranes.
A) Filtration experiments were performed as in Figure 2A, in the presence or absence of RRR-α-tocopherol (6 mol %). Shown is a representative of 3 independent experiments. B) DPI. Shown is the specific mass of αTTP adsorbed to immobilized phospholipid layers containing (or not) RRR-α-tocopherol (6 mol%) at different protein concentrations. C) Maximum specific adsorbed mass observed with 500 nM αTTP flowed over adsorbed phospholipids containing 6 mol% of either cholesterol, RRR-α-tocopherol, or RRR-δ-tocopherol.
The presence of vitamin E greatly affected the partitioning of αTTP between the aqueous and the lipid phases; in filtration experiments, the apparent affinity of αTTP to lipid vesicles was markedly reduced by the presence of RRR-α-tocopherol (Figure 3A). In DPI measurements, we found that the amount of lipid-adsorbed αTTP was reduced by > 2-fold in the presence of vitamin E (Figures 3B-C). Importantly, this effect was specific, since another neutral lipid, cholesterol, did not influence the αTTP-lipid interaction (Figure 3C). Further evidence for the specificity of the ligand effect is the observation that δ-tocopherol exhibited a significantly weaker effect on the affinity of αTTP for membranes as compared to the effect of α-tocopherol (Figure 3C). This difference likely reflects the reduced affinity of αTTP for δ-tocopherol (5,6). We conclude from these data that the sensitivity of the αTTP-lipid interaction to ligands is a specific attribute of vitamin E, that is most attenuated by the protein’s natural ligand.
To gain further insight into the molecular mechanisms of ligand-transfer, we examined the catalytic efficacy of αTTP mutants associated with the AVED disorder. Table 1 summarizes the effects of these mutations on the binding affinity of αTTP for NBD-tocopherol. Both the GST-fused and the ‘naked’ (untagged) forms of αTTP bind tocopherol with high affinity (Kd ~ 10 nM), demonstrating that the presence of the amino-terminal tag does not perturb the native conformation of the protein. While none of the AVED-affected residues are present in the ligand binding pocket of the protein (Figure 4A), the naturally occurring R59W and H101Q mutations cause a significant reduction in αTTP’s affinity for NBD-tocopherol (Kd = 39 nM and 71 nM, respectively, Table 1). The affinities of the remaining mutants for NBD-tocopherol are similar to that of the wild-type protein (Kd = 3–27 nM, Table 1).
Table 1.
Affinity of mutant αTTP proteins for NBD-tocopherol.
| TTP variant | Clinical phenotype | Kd (nM)a |
|---|---|---|
| Wild typeb | - | 8.5 ± 6.3 |
| Wild type | - | 9.8 ± 2.3 |
|
| ||
| R192Hb | AVED (mild) | 26.8 ± 7.7 |
| H101Qb | AVED (mild) | 38.9 ± 12.7 |
| A120Tb | AVED (mild) | 9.2 ± 5.4 |
|
| ||
| R59Wb | AVED (severe) | 70.8 ± 17.6 |
| E141Kb | AVED (severe) | 11.7 ± 2.7 |
| R221Wb | AVED (severe) | 21.7 ± 11.4 |
Averages and standard deviations from >3 titrations are shown.
Kd values were determined using GST fusion proteins; n = 3.
Figure 4. Sequestration of membrane-bound tocopherol by αTTP mutants.
A) Location of AVED-affected residues relative to the ligand-binding pocket of αTTP. Plotted after Meier et al. (42; PDB accession 1OIP). B) Time-dependent changes in FRET between NBD-tocopherol and fluorescent liposomes in the presence of wild-type, R192H, E141K, H101Q, or R59W variants of αTTP (30 μM active protein). Conditions are as in Figure 1.
The efficacy of wild-type αTTP and several AVED variants in catalyzing the sequestration of tocopherol from membranes is shown in Figure 4B. The rate at which the R192H or H101Q mutants facilitated ligand removal was essentially indistinguishable from the wild-type protein (t1/2 = 4.1 and 4.8 seconds for the R192H and H101Q mutants, respectively, at 30 μM protein). Loss of FRET induced by the E141K and R59W mutants, on the other hand, occurred at a significantly slower rate (t1/2 = 7.9 and 7.01 seconds, respectively). These observations indicate that the clinical phenotype associated with a particular mutation in αTTP (i.e. mutations associated with the severe versus the mild forms of AVED) is correlated with the competence of the protein in sequestering tocopherol from lipid bilayers.
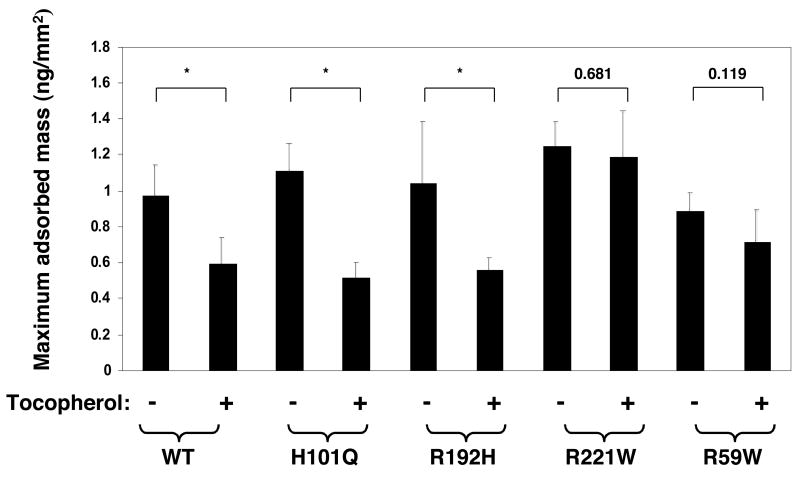
Another correlation between in vitro activity and clinical severity is evident when inspecting the concentration dependence of the ligand transfer rate for the different mutants (Figure 5). All αTTP variants tested facilitated the sequestration of tocopherol to a similar extent (ca. 20% change in emission at 575 nm). However, the kinetic characteristics of this change were markedly different. Alpha-TTP variants associated with the late-onset, mild phenotype of AVED (i.e. harboring the R192H, H101Q or A120T substitutions) exhibited tocopherol transfer rates that are highly dependent on protein concentration, similar to the wild-type protein (i.e. a 2-fold decrease in t1/2 between 5 μM and 30 μM protein; Figure 5). In contrast, the rate for protein-induced FRET loss decreased by only 20% for the R221W mutant, and did not exhibit any significant concentration dependence in the case of the E141K and R59W variants of αTTP, associated with the severe form of AVED (Figure 5). We previously reported similar kinetic distinctions between these αTTP mutant classes in their ability to catalyze the vesicle-to-vesicle transfer of radiolabeled tocopherol (20). These observations raise the possibility that naturally occurring mutations in αTTP alter the protein’s ability to interact with membranes. To directly address this issue, we quantitated the interaction of various AVED mutants with lipids using DPI. As shown in Figure 6, all proteins associated with phospholipids to a similar extent as the wild-type protein (ca. 1 ng protein adsorbed per mm2). However, the effect of vitamin E on the protein-lipid interaction was strikingly different amongst the different mutant classes. Alpha-TTP variants associated with the mild, late-onset form of AVED (i.e R192H, H101Q) exhibited pronounced sensitivity to the presence of vitamin E, similar to the wild-type protein: the mass of lipid-adsorbed αTTP was reduced by ca. 50% in the presence of tocopherol, as would be expected if αTTP·tocopherol has reduced affinity for the membrane. Mutant proteins associated with the severe, early-onset form of AVED (i.e. R221W, R59W), on the other hand, were not affected by the presence of tocopherol in the bilayer. That the severe AVED mutations diminish the ligand sensitivity of αTTP-membrane interaction was also observed in filtration experiments (data not shown). These data raise the possibility that a decline in tocopherol sensitivity, rather than differences in affinity for membranes per se, gives rise to the functional defects associated with the severe AVED phenotype.
Figure 5. Dependence of tocopherol sequestration rates on protein concentration.
Rates of αTTP-induced sequestration of NBD-tocopherol were measured as described in Figure 1, using various concentrations of the indicated αTTP variants. Shown are averages and standard deviations from at least 5 independent measurements at each protein concentration.
Figure 6. Association of AVED variants with membranes.
Maximum specific adsorbed mass observed with 500 nM of the indicated form of αTTP to phospholipid membranes lacking or containing 6 mol% RRR-α-tocopherol was measured using DPI as described in Experimental Procedures. Data are representative of at least 3 independent measurements. P-values were calculated with unpaired t-tests and highly significant differences (p < 0.03) are denoted by an asterisk.
Discussion
The critical role of αTTP in regulating whole-body status of vitamin E is evident from the observations that mutations in the human ttpA gene lead to vitamin E deficiency with ataxia. Similarly, mice in which expression of the αTTP gene is disrupted display reduced vitamin E levels and neurological disorders (31–33). Two biochemical activities are ascribed to αTTP: stimulation of tocopherol secretion from cultured hepatocytes (10,11,21,34), and catalysis of tocopherol transfer between membrane vesicles in vitro (6,20,29,35). Neither the molecular mechanism underlying tocopherol transfer, nor the relationship between this activity and the physiological function of αTTP are known at present.
We showed that the rate by which αTTP sequesters membrane-bound tocopherol increases with increasing protein:vesicles ratio, suggesting that the ligand transfer activity is mediated by direct protein-membrane interactions. In support of this notion, equilibrium measurements utilizing filtration, DPI, and intrinsic fluorescence spectroscopy demonstrated that a stable complex is formed between αTTP and membranes. These observations suggest that inter-membrane transfer of tocopherol by αTTP involves at least 3 principal steps: 1) association between αTTP and the lipid bilayer, 2) ‘extraction’ of tocopherol from the bilayer into TTP’s binding pocket, and 3) dissociation of the holo-protein from the membrane, similar to the proposed mechanism for ligand transfer by Sec14 (22). Ligand transfer is not likely to be limited by association between αTTP and membranes, since in solution this step is likely to be too fast (diffusion-controlled). Possibly, sequestration of tocopherol by αTTP (step 2) is the rate-determining step of the ligand transfer reaction, which is monitored in our kinetic FRET measurements.
An important feature of the interaction between αTTP and lipid vesicles is its marked sensitivity to the presence of vitamin E. It is possible that this ‘bias’ contributes to the directionality of tocopherol distribution within the hepatocyte, i.e. that preferential affinity results in net fluxes of tocopherol from vitamin E-containing to vitamin E-poor membranes. The molecular mechanism by which αTTP ‘senses’ the presence of tocopherol in lipid bilayers is not known. The protein may be sensitive to ligand-induced changes in the physical properties of the bilayer, such as fluidity and surface tension, as is the case for other lipid binding and transfer proteins (36–41). Our observations that cholesterol does not influence αTTP-lipid interactions argue against this mechanism. Alternatively, the presence of tocopherol in the binding pocket may stabilize αTTP in solution, as predicted from a structural comparison of the ‘apo’ and ‘holo’ conformations (42). The reduced sensitivity of the ‘severe’ αTTP mutants (e.g. R59W and R221W) mutants to the presence of tocopherol supports this explanation. Thus, we propose that ligand-induced protein conformation, rather than changes in physical properties of the bilayer, underlie the ligand sensitivity of the αTTP-membrane interaction.
We previously observed that αTTP variants that cause the severe form of AVED in humans (i.e. the R59W, E141K, or R221W substitutions) are impaired in catalyzing vesicle-to-vesicle transfer of radiolabeled tocopherol (20). In the present study, we observed that the rate at which these mutants sequester membrane-bound tocopherol is independent of protein concentration, unlike the wild-type αTTP or mutants associated with the mild form of AVED. It is possible that the insensitivity of the severe mutants to tocopherol is at the root of the functional defect, since it could lead to altered partitioning of αTTP between different membranes, and compromise the directionality of tocopherol transfer. This explanation could also account for the inability of such mutants to facilitate the transfer of NBD-tocopherol from lysosomes to the plasma membranes in intact cells (e.g. the R221W mutant; 21).
As shown by the observations presented here and in previous studies (20), αTTP mutations that are associated with the mild AVED pathology (R192H, H101Q, A120T) do not impede the ability of the protein to catalyze tocopherol transfer in vitro. These observations suggest that additional physiological factors may contribute to αTTP actions in vivo. The nature of these factors and their role in regulating tocopherol status remain to be clarified.
Acknowledgments
This work was supported in part by award DK067494 to D.M., training grant T32-DK715827 to S.M. from the National Institutes of Health, and by a grant from the Natural Sciences and Engineering Research Council of Canada to J.A. We are grateful to Valerie Cross for technical help in the initial stages of the study.
The abbreviations used
- αTTP
alpha-tocopherol transfer protein
- AVED
ataxia with vitamin E deficiency
- DOPC
1,2-Dioleoyl-sn-Glycero-3-Phosphatidylcholine
- DOPS
1,2-Dioleoyl-sn-Glycero-3-Phosphoserine
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- GST
glutathione-S-transferase
- IPTG
isopropyl-β-D-thiogalactopyranoside
- MWCO
molecular weight cut-off
- NBD-tocopherol
(R)-2,5,7,8-tetramethyl-chroman-2- oxadiazol-4-ylamino)-nonyl]-chroman-6-ol
- PC
phosphatidylcholine
- PMSF
phenylmethylsulfonyl fluoride
- PS
phosphatidylserine
- tocopherol
RRR-α-tocopherol
- TRIS
tris(hydroxymethyl)aminomethane
- TRITC-DHPE
N-(6-tetramethylrhodaminethiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt
References
- 1.Traber MG, Kayden HJ. Am J Clin Nutr. 1989;49(3):517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 2.Behrens WA, Madere R. J Am Coll Nutr. 1986;5:91–96. doi: 10.1080/07315724.1986.10720116. [DOI] [PubMed] [Google Scholar]
- 3.Sontag TJ, Parker RS. J Biol Chem. 2002;277(28):25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 4.Swanson JE, Ben RN, Burton GW, Parker RS. J Lipid Res. 1999;40(4):665–671. [PubMed] [Google Scholar]
- 5.Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, Manor D, Atkinson J. Biochemistry. 2003;42(21):6467–6474. doi: 10.1021/bi034086v. [DOI] [PubMed] [Google Scholar]
- 6.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. FEBS Lett. 1997;409(1):105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 7.Mowri H, Nakagawa Y, Inoue K, Nojima S. Eur J Biochem. 1981;117(3):537–542. doi: 10.1111/j.1432-1033.1981.tb06370.x. [DOI] [PubMed] [Google Scholar]
- 8.Verdon CP, Blumberg JB. Anal Biochem. 1988;169(1):109–120. doi: 10.1016/0003-2697(88)90261-8. [DOI] [PubMed] [Google Scholar]
- 9.Arita M, Sato Y, Miyata A, Tanabe T, Takahashi E, Kayden HJ, Arai H, Inoue K. Biochem J. 1995;306(Pt 2):437–443. doi: 10.1042/bj3060437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita M, Nomura K, Arai H, Inoue K. Proc Natl Acad Sci U S A. 1997;94(23):12437–12441. doi: 10.1073/pnas.94.23.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D. J Lipid Res. 2005;46 (10):2072–2082. doi: 10.1194/jlr.M500143-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M. Nat Genet. 1995;9(2):141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 13.Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Am J Hum Genet. 1998;62(2):301–310. doi: 10.1086/301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotoda T, Arita M, Arai H, Inoue K, Yokota T, Fukuo Y, Yazaki Y, Yamada N. N Engl J Med. 1995;333(20):1313–1318. doi: 10.1056/NEJM199511163332003. [DOI] [PubMed] [Google Scholar]
- 15.Yokota T, Shiojiri T, Gotoda T, Arai H. N Engl J Med. 1996;335(23):1770–1771. doi: 10.1056/NEJM199612053352315. [DOI] [PubMed] [Google Scholar]
- 16.Yokota T, Shiojiri T, Gotoda T, Arita M, Arai H, Ohga T, Kanda T, Suzuki J, Imai T, Matsumoto H, Harino S, Kiyosawa M, Mizusawa H, Inoue K. Ann Neurol. 1997;41(6):826–832. doi: 10.1002/ana.410410621. [DOI] [PubMed] [Google Scholar]
- 17.Yokota T, Uchihara T, Kumagai J, Shiojiri T, Pang JJ, Arita M, Arai H, Hayashi M, Kiyosawa M, Okeda R, Mizusawa H. J Neurol Neurosurg Psychiatry. 2000;68(4):521–525. doi: 10.1136/jnnp.68.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang J, Kiyosawa M, Seko Y, Yokota T, Harino S, Suzuki J. Jpn J Ophthalmol. 2001;45(6):672–676. doi: 10.1016/s0021-5155(01)00425-7. [DOI] [PubMed] [Google Scholar]
- 19.Hentati A, Deng HX, Hung WY, Nayer M, Ahmed MS, He X, Tim R, Stumpf DA, Siddique T, Ahmed Ann Neurol. 1996;39(3):295–300. doi: 10.1002/ana.410390305. [DOI] [PubMed] [Google Scholar]
- 20.Morley S, Panagabko C, Shineman D, Mani B, Stocker A, Atkinson J, Manor D. Biochemistry. 2004;43(14):4143–4149. doi: 10.1021/bi0363073. [DOI] [PubMed] [Google Scholar]
- 21.Qian J, Atkinson J, Manor D. Biochemistry. 2006;45(27):8236–8242. doi: 10.1021/bi060522c. [DOI] [PubMed] [Google Scholar]
- 22.Sha B, Phillips SE, Bankaitis VA, Luo M. Nature. 1998;391(6666):506–510. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- 23.Ponsin G, Qu SJ, Fan HZ, Pownall HJ. Biochemistry. 2003;42(15):4444–4451. doi: 10.1021/bi027006g. [DOI] [PubMed] [Google Scholar]
- 24.Desrumaux C, Labeur C, Verhee A, Tavernier J, Vandekerckhove J, Rosseneu M, Peelman F. J Biol Chem. 2001;276(8):5908–5915. doi: 10.1074/jbc.M008420200. [DOI] [PubMed] [Google Scholar]
- 25.Wootan MG, Bernlohr DA, Storch J. Biochemistry. 1993;32(33):8622–8627. doi: 10.1021/bi00084a033. [DOI] [PubMed] [Google Scholar]
- 26.Hsu KT, Storch J. J Biol Chem. 1996;271(23):13317–13323. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 27.Morley S, Cross V, Cecchini M, Nava P, Atkinson J, Manor D. Biochemistry. 2006;45(4):1075–1081. doi: 10.1021/bi052271y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Ball JM, Billheimer JT, Schroeder F. Biochem J. 1999;344(Pt 2):593–603. [PMC free article] [PubMed] [Google Scholar]
- 29.Sato Y, Hagiwara K, Arai H, Inoue K. FEBS Lett. 1991;288(1–2):41–45. doi: 10.1016/0014-5793(91)80999-j. [DOI] [PubMed] [Google Scholar]
- 30.Lackowicz JR. Principles of Fluorescence Spectroscopy. Plenum Press; New York: 1983. [Google Scholar]
- 31.Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, Packer L, Traber MG, Farese RV., Jr Proc Natl Acad Sci U S A. 2000;97(25):13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokota T, Igarashi K, Uchihara T, Jishage K, Tomita H, Inaba A, Li Y, Arita M, Suzuki H, Mizusawa H, Arai H. Proc Natl Acad Sci U S A. 2001;98(26):15185–15190. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard SW, Terasawa Y, Farese RV, Jr, Traber MG. Am J Clin Nutr. 2002;75(3):555–560. doi: 10.1093/ajcn/75.3.555. [DOI] [PubMed] [Google Scholar]
- 34.Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H. Genes Cells. 2003;8(10):789–800. doi: 10.1046/j.1365-2443.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- 35.Murphy DJ, Mavis RD. J Biol Chem. 1981;256(20):10464–10468. [PubMed] [Google Scholar]
- 36.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Mol Cell. 2004;15(2):259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Rao CS, Chung T, Pike HM, Brown RE. Biophys J. 2005;89(6):4017–4028. doi: 10.1529/biophysj.105.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg RB, Cook VR, Jones JB, Kussie P, Tall AR. J Biol Chem. 1994;269(47):29588–29591. [PubMed] [Google Scholar]
- 39.Gargouri Y, Moreau H, Verger R. Biochim Biophys Acta. 1989;1006(3):255–271. doi: 10.1016/0005-2760(89)90012-x. [DOI] [PubMed] [Google Scholar]
- 40.Lowe ME. J Lipid Res. 2002;43(12):2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 41.Wu F, Corsico B, Flach CR, Cistola DP, Storch J, Mendelsohn R. Biochemistry. 2001;40(7):1976–1983. doi: 10.1021/bi002252i. [DOI] [PubMed] [Google Scholar]
- 42.Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. J Mol Biol. 2003;331(3):725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]