Abstract
Study Objective:
Adults with obstructive sleep apnea (OSA) show significant autonomic and neuropsychologic deficits, which may derive from damage to insular regions that serve those functions. The aim was to assess glial and neuronal status from anterior insular metabolites in OSA versus controls, using proton magnetic resonance spectroscopy (PMRS), and thus to provide insights for neuroprotection against tissue changes, and to reduce injury consequences.
Design:
Cross-sectional study.
Setting:
University-based medical center.
Participants:
Thirty-six patients with OSA, 53 controls.
Interventions:
None.
Measurements and Results:
We performed PMRS in bilateral anterior insulae using a 3.0-Tesla magnetic resonance imaging scanner, calculated N-acetylaspartate/creatine (NAA/Cr), choline/creatine (Cho/Cr), myo-inositol/creatine (MI/Cr), and MI/NAA metabolite ratios, and examined daytime sleepiness (Epworth Sleepiness Scale, ESS), sleep quality (Pittsburgh Sleep Quality Index, PSQI), and neuropsychologic status (Beck Depression Inventory II [BDI-II] and Beck Anxiety Inventory [BAI]). Body mass index, BAI, BDI-II, PSQI, and ESS significantly differed between groups. NAA/ Cr ratios were significantly reduced bilaterally, and left-sided MI/Cr and MI/NAA ratios were increased in OSA over controls. Significant positive correlations emerged between left insular MI/Cr ratios and apnea-hypopnea index values, right insular Cho/Cr ratios and BDI-II and BAI scores, and negative correlations appeared between left insular NAA/Cr ratios and PSQI scores and between right-side MI/Cr ratios and baseline and nadir change in O2 saturation.
Conclusions:
Adults with obstructive sleep apnea showed bilaterally reduced N-acetylaspartate and left-side increased myo-inositol anterior insular metabolites, indicating neuronal damage and increased glial activation, respectively, which may contribute to abnormal autonomic and neuropsychologic functions in the condition. The activated glial status likely indicates increased inflammatory action that may induce more neuronal injury, and suggests separate approaches for glial and neuronal protection.
Citation:
Yadav SK, Kumar R, Macey PM, Woo MA, Yan-Go FL, Harper RM. Insular cortex metabolite changes in obstructive sleep apnea. SLEEP 2014;37(5):951-958.
Keywords: anxiety, autonomic, magnetic resonance spectroscopy, choline, creatine, depression, myo-inositol, N-acetylaspartate
INTRODUCTION
Obstructive sleep apnea (OSA) subjects show autonomic and multiple neuropsychologic abnormalities, together with other physiological issues. Both structural injury and functional deficits appear in multiple brain sites in OSA subjects,1–4 and are especially prominent in the insular cortices, which assist regulation of autonomic and neuropsychologic functions.5–7 The insular injury in OSA appears as altered free water content,3,8 and impaired water diffusion within tissue.2,4 Determining the nature of metabolic deficits in insular areas, i.e., whether the deficit occurs preferentially to altered glia or neurons, may suggest interventions against central damage in the syndrome by providing insights into targeting support for glial or neuronal cells.
Although multiple brain sites are involved in autonomic and neuropsychologic regulation, including the amygdala, ventral medial prefrontal cortex, anterior thalamus, hippocampus, and cingulate cortex,9–11 the insular cortices are key structures to regulate such functions. These functions include an array of sympathetic and parasympathetic roles,5,12,13 pain modulation,14 and integration of somatosensory input with interoceptive autonomic action.5,6,12,15–17
The insular cortices consist of five primary gyri in humans, including anterior, middle, and posterior short gyri situated within the anterior insula, and the anterior and posterior long gyri, which lie within the posterior insula.18 All of these areas show differential response patterns to autonomic challenges,13 but the anterior insula especially is involved in sympathetic action,13,19 mood, attention, and anxiety modulation.3,8,20 The anterior, middle, and posterior short gyri (anterior insula) have reciprocal projections to the hypothalamus, which plays a significant role in autonomic and affective regulation via projections to the brainstem, limbic (including the amygdala), and cortical regions that regulate neuropsychologic functions.15,21–23 Since the functional and structural alterations that appear in the anterior insula in OSA have a unique potential to exert significant effects on autonomic and multiple neuropsychologic symptoms,1,2 we examined metabolite levels to provide indications of the nature of tissue changes in early stages of the syndrome. Such changes need to be identified to determine potential mechanisms for neuroprotection in the condition.
Proton magnetic resonance spectroscopy (PMRS) procedures are widely used to evaluate noninvasively regional metabolic changes in various clinical and research environments, and to suggest remedial interventions. Multiple brain metabolites, including N-acetylaspartate (NAA), a marker of neuronal density/ function, choline (Cho), an indicator of cell membrane turnover/ density, creatine (Cr), an index of energy metabolism, and myoinositol (MI), a marker of glial status, can be examined with PMRS procedures.24 Various metabolite ratios can be calculated with respect to Cr, which is considered a stable metabolite. The concentration of Cr does not change in most brain disease conditions,25–27 and changes in metabolite ratios are considered as alterations in numerator metabolites. The procedures allowed identification of pathology in various disease conditions, including brain tumors,28 epilepsy,29 encephalopathy,30 and hypoxic conditions,31 have been used to examine metabolites in hippocampal and frontal cortex sites in pediatric and adult OSA,32,33 and may help detect altered insular metabolites in adult OSA subjects.
Our focus was to evaluate metabolites in an area critical for autonomic action and neuropsychologic deficits in OSA, and to determine the nature of changes using PMRS procedures. Based on earlier-demonstrated structural and functional alterations in the insular cortices, we hypothesized that bilateral anterior insular metabolites would be modified in OSA over control subjects.
METHODS
Participants
We included 36 newly-diagnosed, treatment-naïve OSA subjects and 53 control subjects. OSA subjects were recruited from the sleep disorders laboratory at the University of California at Los Angeles (UCLA) Medical Center. No subjects included in this study were taking any cardiovascular-altering medications (such as β-blockers, α-agonists, angiotensin-converting enzyme inhibitors, or vasodilators) or any mood-changing drugs (selective serotonin reuptake inhibitors, hemodynamic-altering or metabolic-altering drugs). OSA and control subjects with any previous history of heart failure, stroke, diagnosed cerebral conditions, metallic implants, or body weight more than 125 kg (scanner limitation) were excluded from the study. We interviewed control subjects, as well as their sleep partners, when available, to determine the potential for sleep disordered breathing, and subjects suspected of having such disturbed patterns underwent an overnight sleep study. Control subjects with a positive OSA diagnosis were excluded from this study (n = 3). Control subjects were healthy, without any medications that might alter brain tissue or any contraindications to the magnetic resonance imaging (MRI) scanner, and were recruited from the UCLA and West Los Angeles area. All procedures were approved by the Institutional Review Board at UCLA, and subjects provided written informed consent before participating in this study.
Overnight Polysomnography (PSG)
We performed overnight sleep studies in OSA subjects at the UCLA Sleep Disorders Center and Laboratory, which included 7 to 10 h (21:00 to 06:00) monitoring of electroencephalogram (EEG, central and occipital), digastric electromyogram (EMG), electrocardiogram (EKG, lead II), right and left extraocular eye movement (EOG), thoracic and abdominal wall movement, air flow, oxygen saturation, end-tidal carbon dioxide levels, snore volume, both-side leg movement, and sleep position. These data were digitized and stored on a computer for examination of sleep and oxygen saturation variables.
Examination of Sleep Quality and Daytime Sleepiness
Both OSA and control participants were evaluated for sleep quality with the Pittsburgh Sleep Quality Index (PSQI), and daytime sleepiness with the Epworth Sleepiness Scale (ESS). Both tests are self-administered questionnaires, and are commonly used indices of sleep quality and daytime sleepiness.34 Control subjects with a high score (either ESS or PSQI) were excluded from the study.
Neuropsychologic Assessment
We assessed both anxiety and depressive symptoms in OSA and control subjects using the Beck Anxiety Inventory (BAI) and Beck Depression Inventory II (BDI-II), respectively. Both inventories are self-administered questionnaires (21 questions; each score ranged from 0 to 3), with total scores ranging from 0 to 63 based on symptom severity.35,36 These symptoms were assessed since they are common in OSA, and the insula shows enhanced structural changes in depressed and anxious OSA over nondepressed and nonanxious OSA subjects, respectively.3,8
MRI and PMRS Procedures
All brain structural MRI and single-voxel PMRS procedures were performed on a 3.0-Tesla MRI scanner (Siemens, Magnetom, Tim-Trio, Erlangen, Germany) at the Department of Radiological Sciences MRI Research Center, using a receive-only eight-channel phased-array head coil. All subjects lay supine during structural MRI and PMRS procedures. To minimize head movement, we applied foam pads on both sides of the head. We acquired simultaneous proton-density and T2-weighted images [repetition time (TR) = 10,000 ms; echo time (TE1, 2) = 17, 134 ms; flip angle (FA) = 130°; matrix size = 256 × 256; field of view (FOV) = 230 × 230 mm2; slice thickness = 4.0 mm], covering the entire brain in the axial plane using a dual-echo turbo spin-echo pulse sequence. The magnetization prepared rapid acquisition gradient-echo pulse sequence (TR = 2,200 ms; TE = 2.2 ms; inversion time = 900 ms; FA = 9°; matrix size = 256 × 256; FOV = 230 × 230 mm2; slice thickness = 1.0 mm) was used to acquire the high-resolution T1-weighted images in the sagittal plane.
Voxel localizations were guided by high-resolution T1-weighted and T2-weighted images to avoid contamination from cerebrospinal fluid and white matter, and PMRS spectra were acquired from the bilateral anterior insulae from all subjects. Single-voxel PMRS was performed using point-resolved spectroscopy pulse sequence (TR = 3,000 ms, TE = 30 ms, spectral points = 2,048, spectral bandwidth = 1,500 Hz, averages = 144, voxel size = 10 × 10 × 10 mm3). The voxel size was chosen to fit well within the anterior insular cortices in all OSA and control subjects. Global and voxel shimming was performed manually by the operator before spectral data acquisition, and a full width at half maximum of < 18 Hz was achieved in all cases for voxel shimming.
Overnight PSG Data Evaluation
All overnight PSG recordings were evaluated by physicians at UCLA. Various sleep states, sleep disordered breathing, and physiology were first examined for total sleep time, and number and type of breathing conditions (central, obstructive, or mixed episodes). A registered PSG technician evaluated all sleep records, and then each PSG was reviewed by the sleep physician. Each one minute data epoch was scored as awake (W), rapid eye movement sleep (R), or sleep stages N1-N3, using the PSG data based on revised scoring criteria.37 We defined an OSA event as cessation of airflow lasting longer than 10 sec with continued diaphragmatic effort, and a central sleep apnea as cessation of airflow and respiratory effort lasting longer than 10 sec.37 The apnea-hypopnea index (AHI) is defined as a severity index of sleep disordered breathing, which comprises both apneas and hypopneas, and is derived by dividing the number of apnea and hypopnea episodes by the total sleep time. An OSA subject was categorized as follows: mild OSA—AHI was 5 or more, but fewer than 15 events/h, moderate OSA—15 or more, but fewer than 30 events/h, and severe OSA—more than 30 events/h.37 We calculated the total number and duration of apnea, AHI, number of arousals, oxygen saturation variables, total sleep time, time in each state, and sleep efficiency variables from all PSG data.
Post Processing of PMRS Data
Insular metabolites of interest were NAA, Cr, Cho, and MI. Signal quantification of these metabolites was performed using curve fitting with standard software available in the MRI scanner, provided by the manufacturer. After baseline correction, the NAA peak was assigned at 2.02 ppm, Cr at 3.02 ppm, Cho at 3.2 ppm, and MI at 3.56 ppm, and automatic curve-fitting procedures were used to obtain signal integrals. Using NAA, Cr, Cho, and MI metabolite amplitudes, metabolite ratios, including NAA/Cr, Cho/Cr, MI/Cr, and MI/NAA were calculated. All spectra were visually assessed for artifacts according to criteria described elsewhere,38 and only spectra with adequate quality were included in the analysis.
Statistical Analysis
The IBM statistical package for the social sciences (IBM SPSS, version 20, Armonk, New York) was used for data analyses. Demographic and biophysical factors, metabolite ratios, sleep variables, and neuropsychologic scores were assessed between groups by independent samples t-tests and Chi-square tests. Pearson's correlation procedures were used to determine association between metabolite ratios and biophysical, sleep, oxygen saturation variables, and neuropsychologic scores in OSA subjects. A value of P < 0.05 was chosen to establish statistical significance.
RESULTS
Demographics, Biophysical, Sleep, and Oxygen Saturation Indices, and Neuropsychologic Variables
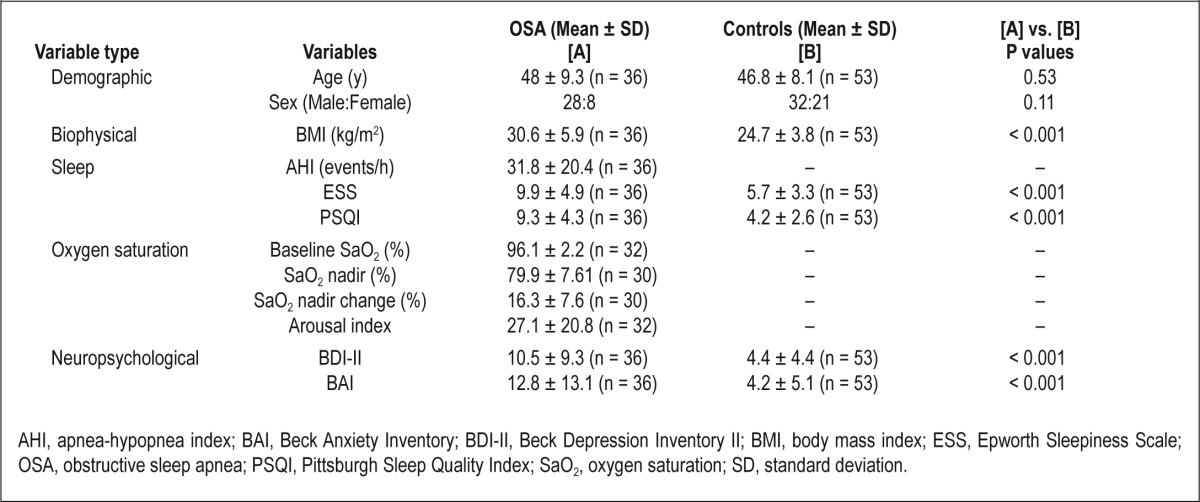
Demographic, biophysical, sleep, oxygen saturation variables, and neuropsychologic scores of OSA and control subjects are summarized in Table 1. No significant differences in age (P = 0.53) or sex (P = 0.11) appeared between groups. Body mass index (P < 0.001), BAI (P < 0.001), BDI-II (P < 0.001), PSQI (P < 0.001), and ESS (P < 0.001) significantly differed between groups.
Table 1.
Demographic, biophysical, sleep, neuropsychologic, and oxygen saturation data of OSA and control subjects
Insular Metabolite Ratios of OSA and Controls
Metabolite ratios, derived from both left and right anterior insulae of OSA and control subjects, are shown in Table 2, and representative spectra, derived from the left insular cortex from one OSA and one control subject, are shown in Figure 1. NAA/Cr ratios were significantly decreased bilaterally (left insula, P = 0.026; right insula, P = 0.037) in OSA subjects over controls. Significantly increased MI/Cr (P = 0.03) and MI/NAA (P = 0.001) ratios appeared only on the left side in OSA subjects over control subjects, while the right side showed no significant difference (MI/Cr, P = 0.7; MI/NAA, P = 0.12). No significant differences in Cho/Cr ratios appeared in OSA subjects over control subjects (left, P = 0.20; right, P = 0.96).
Table 2.
Insular metabolite ratios of OSA and control subjects

Figure 1.

Left insular spectra from one obstructive sleep apnea (age 44.7 y; male) and one age- and sex-matched control subject (age 45.7 y; male). Peaks of NAA at 2.02 ppm, Cr at 3.02 ppm, Cho at 3.2 ppm, and MI at 3.56 ppm are shown; spectra are processed with Java-based magnetic resonance spectroscopy software (jMRUI, V 3.0) for display. OSA, obstructive sleep apnea; Cr, creatine; Cho, choline; MI, myoinositol; NAA, N-acetylaspartate.
Correlations Between Metabolite Ratios and AHI, Sleep, Oxygen Saturation Variables, BAI, and BDI-II Scores
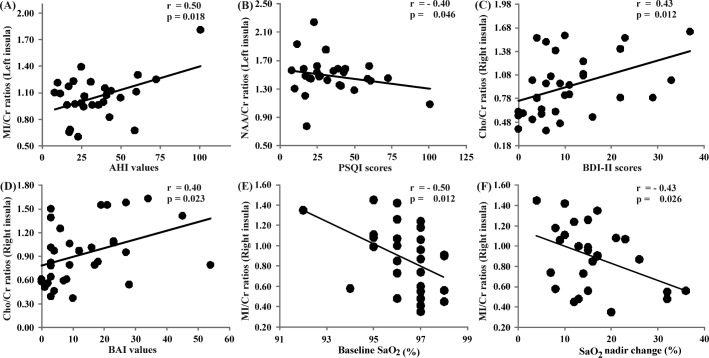
In OSA subjects, significant positive correlations emerged between the left insular MI/Cr ratios and AHI values (r = 0.45, P = 0.02), right insular Cho/Cr ratios and BDI-II scores (r = 0.43, P = 0.01), and right insular Cho/Cr ratios and BAI values (r = 0.4, P = 0.02; Figure 2). Negative correlations emerged between left insular NAA/Cr ratios and PSQI scores (r = -0.4, P = 0.046; Figure 2), and between right-side MI/Cr ratios and baseline oxygen saturation (r = -0.46, P = 0.01; Figure 2) and oxygen saturation nadir change (r = -0.43, P = 0.03; Figure 2).
Figure 2.
Correlations between PMRS-derived metabolite ratios with OSA severity, sleep quality, oxygen saturation variables, and neuropsychologic scores in OSA subjects. Significant positive correlations appeared between the left insular MI/Cr ratios and AHI values (A). A significant negative correlation emerged between the left insular NAA/Cr ratios and PSQI scores (B). A significant positive correlation appeared between right insular Cho/Cr ratios and BDI-II scores (C), and right insular Cho/Cr ratios and BAI values (D). A significant negative correlation appeared between right insular MI/Cr ratios and baseline oxygen saturation (E) and oxygen saturation nadir change indices (F). AHI, apnea-hypopnea index; BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory II; Cho, choline; Cr, creatine; MI, myo-inositol; NAA, N-acetylaspartate; OSA, obstructive sleep apnea; PMRS, proton magnetic resonance spectroscopy; PSQI, Pittsburgh Sleep Quality Index.
DISCUSSION
Overview
OSA subjects showed significant bilaterally reduced insular cortex NAA/Cr ratios, a finding normally associated with neuronal damage,25 and increased MI/Cr and MI/NAA ratios on the left side, suggesting excessive glial activation in that area with the presence of significant neurodegenerative processes.39 The presence of abnormal anterior insular metabolite levels strengthens other evidence indicating damage to that subregion, and the finding of glial activation indicates that the injurious processes are ongoing, with the potential for glial changes to induce even further neuronal damage. Since the anterior insulae play such critical roles in autonomic and neuropsychologic functions, many of which are abnormal in OSA, the implications for intervention in the syndrome are substantial. The outcomes suggest that determination of processes involved in aberrant glial activation and targeting for protection against such exaggerated action may be useful as neuroprotection in the syndrome.
Reduced NAA and Neuronal Damage/Loss of Function in Insular Cortices
The significantly reduced bilateral NAA/Cr ratios suggest that insular neurons have been affected. All brain conditions with tissue pathology, except Canavan disease, show reduced NAA, and that reduction is traditionally considered to reflect loss of neuronal structure in chronic disease states, and neuronal functional loss in acute conditions.40,41
Other PMRS studies in OSA subjects, performed in frontal, parietal, and occipital cortices, as well as in cingulate and hippo-campal sites, have shown decreased NAA/Cr ratios in the condition.32,33,42–45 These studies, together with the findings here, suggest that widespread NAA declines appear in OSA, and that the accompanying neuronal damage/loss of function to such metabolic signs may be extensive. However, a recent study showed increased NAA/Cr ratios in hippocampal sites in subjects with severe OSA, compared to mild OSA subjects, and the authors concluded that increased NAA/Cr ratios in the hippocampus, an opposite finding to that here and findings of others,32,33,42–45 result from decreased Cr levels from chronic hypoxemia.46 This discrepancy could arise from differences in magnetic field strength (higher magnetic field used here would improve signal-to noise), use of head coil, echo time differences on the PMRS pulse sequence, and shimming issues.47 We used a higher magnetic field, an eight-channel coil, and lower echo times for data acquisition, all factors contributing to more-accurate assessment of spectral signals. Of substantial importance, inadequate shimming can result in unclear separation of Cho and Cr peaks,46 which affects Cho and Cr metabolite levels and interpretation of findings.
The earlier-demonstrated metabolic alterations in hippocampal and other cortical areas in OSA are significant32,33,42,44,48,49 in that injury to the hippocampus typically affects short-term memory and cognitive processing. Demonstration of insular metabolite changes here supplements those earlier hippocampal and cortical findings; the cardiovascular roles of the insula are expressed through projections to hippocampal and hypothalamic sites, as well as through the cingulate and frontal cortices, among other areas, and hippocampal, cingulate, and ventral frontal cortex roles in cardiovascular regulation are now well described.50–52
Increased MI and Glial Activation OSA
Increased MI/Cr and MI/NAA ratios appeared in the left insula in OSA. MI represents spectroscopically detected free intracellular organic osmolytes, is a component of cell membrane and myelin sheath structures,53 is considered a glial cell marker, and is involved in signal transduction pathways. Increased MI levels accompany many disease conditions, including human immunodeficiency virus, Alzheimer's disease, and multiple sclerosis, and are interpreted as glial activation due to immune-reactive responses.54–56 The findings of increased MI, reflected as increased MI/Cr and MI/NAA ratios in OSA subjects here, also suggest the presence of glial activation, possibly resulting from intermittent hypoxic or ischemic conditions inducing inflammatory responses and neurodegenerative processes, as reported in Alzheimer's disease and other brain conditions,57–60 and indicate increased glial cell membrane turnover or myelin sheath damage.39 If the process is of an inflammatory nature, the unilateral changes in MI, with the right side showing an increasing, albeit nonsignificant trend, suggests more severe changes on the left insula over the right insula.
The asymmetrical outcome may stem from the larger cerebral blood flow on the right side,61 with the consequences of hypoxemic periods during apnea having a relatively reduced effect on the right over the left side. However, this possibility is speculative.
Implications of Glial Activation on the Left Insula
Enhanced unilateral inflammatory actions in the insular cortex, which may contribute to more neuronal injury in that area, have clinical implications for autonomic regulation. Since the left insula receives fibers from, and projects predominantly to the anterior cingulate cortex over the right insula,23 is preferentially involved in mediating parasympathetic influences,5 and presumably counteracts sympathetic output from the right side, the reduced respiratory-related arrhythmia in OSA during nonapneic periods may derive from those alterations,62 i.e., deficient left-sided insula action would be less effective in modulating high sympathetic tone with parasympathetic action, resulting in reduced respiratory-related arrhythmia. Lowered respiratory-related arrhythmia has long been associated with a number of cardiovascular concerns, including sudden death,63 and OSA subjects are at risk for sudden, unexplained cardiac death.64 The particular mechanisms underlying reduced respiratory-related arrhythmia in OSA are still being explored, but may involve disturbed integration of afferent thoracic or aortic pressure sensors with nucleus ambiguous vagal influences to offset sympathetic outflow, likely modulated by insular projections.65
Correlations: Metabolite Ratios, Sleep and Oxygen Saturation Characteristics, and Neuropsychologic Scores
Negative correlations between NAA/Cr ratios and PSQI scores within the OSA group appeared, suggesting that the insular changes may affect sleep characteristics. The neuropsychologic consequences are not confined to reduced sleep quality; the positive correlations between Cho/Cr ratios and BDI-II and BAI scores suggest that the metabolically abnormal sites may contribute to the elevated depression and anxiety symptoms, which are found in a substantial portion of OSA patients (more than 50%),66 with both BDI-II and BAI scores significantly differing here from healthy control subjects. Both PSQI and ESS scores varied between OSA and control subjects; however, neither score showed significant correlations with any insular metabolites, suggesting that the insular cortices played less of a role in sleep characteristics. Since MI/Cr ratios and AHI values were positively correlated, OSA severity may directly contribute to the extent of anterior insula tissue changes. We observed significant negative correlations between right insula MI/NAA ratios and baseline oxygen saturation and oxygen saturation nadir change indices, indicating that the degree of hypoxia affects insular injury in the condition.
Autonomic Irregularities in OSA
Alterations in insular systems, such as those resulting from stroke, lesions, or electrical stimulation, have been associated with altered sympathetic outflow,67 hypertension,68 cardiac arrhythmia,67 and myocardial infarction.69 The enhanced sympathetic outflow likely contributes to many of the deleterious cardiovascular consequences and to profuse sweating,70 impaired cerebral perfusion,71 and, by virtue of sympathetic influences on the pancreas, glucagon and insulin regulation,72 potentially leading to the close association of poor glucose regulation found in OSA.73
The initial descriptions of insular cortex injury emerged from both structural changes indicated by diffusion tensor imaging and T2-relaxometry procedures, and functional deficits from functional MRI responses to autonomic and ventilatory challenges.1–4 The structural aberrations were reflected as altered water diffusion, abnormal organization, and increased free water content within the tissue, and functional deficits appeared as muted, delayed, or inverse signal responses to autonomic challenges in a different OSA group,1 as well as in the same OSA subject cohort used here.74 The functional deficits were complex, and involved both parasympathetic and sympathetic changes; the relationships of those deficits to metabolite changes will be detailed in a subsequent publication.
Neuropsychologic Deficits in OSA
Multiple neuropsychologic abnormalities, including high depression and anxiety symptoms, are common in OSA subjects.75 OSA subjects with depression show neural injury in various brain sites, including anterior insular areas, and that injury appears beyond the alterations found in OSA without depression,8 and a similar pattern of structural changes appear in anxious versus nonanxious OSA subjects.3 The anterior insula projects to the anterior cingulate cortex, an area especially involved in mood and anxiety functions, as shown by structural and functional MRI studies,76,77 and failed interactions between the anterior insula and anterior cingulate may well underlie a portion of the depression and anxiety symptoms in OSA.
Effects of Intermittent Hypoxia/Ischemia on Neurons and Glia
Intermittent hypoxia is a major characteristic of OSA. Animal models of sleep apnea and in vitro studies show that intermittent hypoxia can damage neurons, glia, and peripheral ganglia, and alter axonal and neuronal chemistry by production of oxygen free radicals and mitochondrial dysfunction.78–81 Reactive oxygen species activate signaling pathways and transcription factors, accompanied by lipid peroxidation, as well as protein and nucleic acid oxidation. These signaling pathways and transcription factors trigger inflammatory pathways, and inflammation activates endothelial, glial, and other cells, eliciting neuronal and axonal changes and glial cell activation in acute conditions.82
Abnormal blood flow changes occur during apnea in OSA,83 and apnea-induced hypoxemia, combined with altered cerebral perfusion, may lead to ischemia and vascular changes. Ischemic conditions can trigger inflammatory responses, and vascular alterations can reduce blood flow, further enhancing ischemia; thus, both ventilatory-induced hypoxia and accompanying vascular changes may compound the regional injury.
Potential Glial Interventions for OSA
The indications that insular glia are affected in relatively early stages of the syndrome (OSA subjects were recently diagnosed), and that OSA severity directly links to MI metabolite levels have implications for intervention for the syndrome. A focus on means to protect glia before neuronal injury occurs, using interventions such as enhancing adenosine triphosphate function by micronutrients or other means, and treatment for the breathing condition in addition to conventional positive airway pressure techniques may be useful. Anti-inflammatory pharmacologic agents may be helpful for glial support, as well as targeted interventions to address the vasoconstriction and hindered vascular reactivity that accompanies sleep deprivation of OSA.84
CONCLUSION
OSA subjects show bilaterally reduced insular NAA metabolite ratios, indicating neuronal damage/loss of function, and left-sided increased MI, suggesting increased glial activation in those sites. The abnormal insular metabolites may contribute to altered insular function which results in autonomic and neuropsychologic deficits in the condition, including the enhanced sympathetic discharge, reduced respiratory-related arrhythmia, and depression and anxiety symptoms. These findings of abnormal metabolites in OSA may result from intermittent hypoxia, failure of adequate perfusion, or impaired micronutrient support accompanying the condition. The activated glial status suggests increased inflammatory action, which may lead to more neuronal injury, and suggests that glial support may require additional or separate means for protection different from those required for neurons alone in the syndrome.
DISCLOSURE STATEMENT
This was not an industry supported study. This research work was supported by National Institutes of Health R01 HL113251. The authors have indicated no financial conflicts of interest. The work for this study was performed at the University of California at Los Angeles, Los Angeles, CA.
ACKNOWLEDGMENTS
The authors thank Ms. Rebecca Harper, Mr. Edwin Valladares, and Drs. Rebecca Cross and Stacy Serber for their help with data collection.
ABBREVIATIONS
- OSA
obstructive sleep apnea
- PMRS
proton magnetic resonance spectroscopy
- NAA
N-acetylaspartate
- Cho
choline
- Cr
creatine
- MI
myo-inositol
- MRI
magnetic resonance imaging
- PSQI
Pittsburgh Sleep Quality Index
- ESS
Epworth Sleepiness Scale
- BAI
Beck Anxiety Inventory
- BDI-II
Beck Depression Inventory II
- TR
repetition time
- TE
echo time
- FA
flip angle
- FOV
field of view
- AHI
apnea-hypopnea index
- SaO2
oxygen saturation
Footnotes
A commentary on this article appears in this issue on page 835.
REFERENCES
- 1.Harper RM, Macey PM, Henderson LA, et al. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol. 2003;94:1583–95. doi: 10.1152/japplphysiol.00881.2002. [DOI] [PubMed] [Google Scholar]
- 2.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26:480–91. doi: 10.1002/da.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res. 1996;6:131–40. doi: 10.1007/BF02281899. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009;364:1933–42. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 8.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. 2013;37:340–8. doi: 10.1016/j.neubiorev.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Neckel H, Quagliotto E, Casali KR, Montano N, Dal Lago P, Rasia-Filho AA. Glutamate and GABA in the medial amygdala induce selective central sympathetic/parasympathetic cardiovascular responses. Can J Physiol Pharmacol. 2012;90:525–36. doi: 10.1139/y2012-024. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Res. 1990;533:66–72. doi: 10.1016/0006-8993(90)91796-j. [DOI] [PubMed] [Google Scholar]
- 13.Macey PM, Wu P, Kumar R, et al. Differential responses of the insular cortex gyri to autonomic challenges. Auton Neurosci. 2012;168:72–81. doi: 10.1016/j.autneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu X, Gao Z, Wang X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–35. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–74. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZH, Dougherty PM, Oppenheimer SM. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience. 1999;94:351–60. doi: 10.1016/s0306-4522(99)00339-5. [DOI] [PubMed] [Google Scholar]
- 17.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–90. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 18.Ture U, Yasargil DC, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–33. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 20.Hatton SN, Lagopoulos J, Hermens DF, Naismith SL, Bennett MR, Hickie IB. Correlating anterior insula gray matter volume changes in young people with clinical and neurocognitive outcomes: an MRI study. BMC Psychiatry. 2012;12:45. doi: 10.1186/1471-244X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–20. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- 22.Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 23.Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999;56:919–26. doi: 10.1001/archneur.56.8.919. [DOI] [PubMed] [Google Scholar]
- 25.Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton MR spectroscopy of metabolite concentrations in temporal lobe epilepsy and effect of temporal lobe resection. Epilepsy Res. 2009;83:168–76. doi: 10.1016/j.eplepsyres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Yadav SK, Saksena S, Srivastava A, et al. Brain MR imaging and 1HMR spectroscopy changes in patients with extrahepatic portal vein obstruction from early childhood to adulthood. AJNR Am J Neuroradiol. 2010;31:1337–42. doi: 10.3174/ajnr.A2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazaro L, Bargallo N, Andres S, et al. Proton magnetic resonance spectroscopy in pediatric obsessive-compulsive disorder: longitudinal study before and after treatment. Psychiatry Res. 2012;201:17–24. doi: 10.1016/j.pscychresns.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita Y, Kajiwara H, Yokota A, Koga Y. Proton magnetic resonance spectroscopy of brain tumors: an in vitro study. Neurosurgery. 1994;35:606–13. doi: 10.1227/00006123-199410000-00005. discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 29.Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994;44:1411–7. doi: 10.1212/wnl.44.8.1411. [DOI] [PubMed] [Google Scholar]
- 30.Yadav SK, Srivastava A, Srivastava A, et al. Encephalopathy assessment in children with extra-hepatic portal vein obstruction with MR, psychometry and critical flicker frequency. J Hepatol. 2010;52:348–54. doi: 10.1016/j.jhep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Kucharczyk J, Moseley M, Kurhanewicz J, Norman D. MRS of ischemic/ hypoxic brain disease. Invest Radiol. 1989;24:951–4. doi: 10.1097/00004424-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Halbower AC, Degaonkar M, Barker PB, et al. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Med. 2006;3:e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Donoghue FJ, Wellard RM, Rochford PD, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. 2012;35:41–8. doi: 10.5665/sleep.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: the CARDIA study. Sleep. 2006;29:1503–6. doi: 10.1093/sleep/29.11.1503. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 37.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 38.Kreis R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 2004;17:361–81. doi: 10.1002/nbm.891. [DOI] [PubMed] [Google Scholar]
- 39.Hattingen E, Raab P, Franz K, Zanella FE, Lanfermann H, Pilatus U. Myo-inositol: a marker of reactive astrogliosis in glial tumors? NMR Biomed. 2008;21:233–41. doi: 10.1002/nbm.1186. [DOI] [PubMed] [Google Scholar]
- 40.Leary SM, Davie CA, Parker GJ, et al. 1H magnetic resonance spectroscopy of normal appearing white matter in primary progressive multiple sclerosis. J Neurol. 1999;246:1023–6. doi: 10.1007/s004150050507. [DOI] [PubMed] [Google Scholar]
- 41.De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–7. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- 42.Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, Chen W. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001;71:334–9. doi: 10.1136/jnnp.71.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alchanatis M, Deligiorgis N, Zias N, et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J. 2004;24:980–6. doi: 10.1183/09031936.04.00127603. [DOI] [PubMed] [Google Scholar]
- 44.Tonon C, Vetrugno R, Lodi R, et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30:305–11. doi: 10.1093/sleep/30.3.305. [DOI] [PubMed] [Google Scholar]
- 45.Sarchielli P, Presciutti O, Alberti A, et al. A 1H magnetic resonance spectroscopy study in patients with obstructive sleep apnea. Eur J Neurol. 2008;15:1058–64. doi: 10.1111/j.1468-1331.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 46.Alkan A, Sharifov R, Akkoyunlu ME, et al. MR spectroscopy features of brain in patients with mild and severe obstructive sleep apnea syndrome. Clin Imaging. 2013;37:989–92. doi: 10.1016/j.clinimag.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Inglese M, Spindler M, Babb JS, Sunenshine P, Law M, Gonen O. Field, coil, and echo-time influence on sensitivity and reproducibility of brain proton MR spectroscopy. AJNR Am J Neuroradiol. 2006;27:684–8. [PMC free article] [PubMed] [Google Scholar]
- 48.Bartlett DJ, Rae C, Thompson CH, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med. 2004;5:593–6. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Algin O, Gokalp G, Ocakoglu G, Ursavas A, Taskapilioglu O, Hakyemez B. Neurochemical-structural changes evaluation of brain in patients with obstructive sleep apnea syndrome. Eur J Radio. 2012;81:491–5. doi: 10.1016/j.ejrad.2010.12.092. [DOI] [PubMed] [Google Scholar]
- 50.Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. NeuroImage. 2007;35:698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 51.Kimmerly DS, O'Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol. 2005;569:331–45. doi: 10.1113/jphysiol.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523:259–70. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 54.Kapeller P, Ropele S, Enzinger C, et al. Discrimination of white matter lesions and multiple sclerosis plaques by short echo quantitative 1H-magnetic resonance spectroscopy. J Neurol. 2005;252:1229–34. doi: 10.1007/s00415-005-0847-3. [DOI] [PubMed] [Google Scholar]
- 55.Chang L, Lee PL, Yiannoutsos CT, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. NeuroImage. 2004;23:1336–47. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 56.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55:210–7. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2005;384:23–8. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 58.Niddam DM, Tsai SY, Lu CL, Ko CW, Hsieh JC. Reduced hippocampal glutamate-glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am J Gastroenterol. 2011;106:1503–11. doi: 10.1038/ajg.2011.120. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Zhao C, Yu L, Zhou W, Li K. Regional metabolic changes in the hippocampus and posterior cingulate area detected with 3-Tesla magnetic resonance spectroscopy in patients with mild cognitive impairment and Alzheimer disease. Acta Radiol. 2009;50:312–9. doi: 10.1080/02841850802709219. [DOI] [PubMed] [Google Scholar]
- 60.Franczak M, Prost RW, Antuono PG, Mark LP, Jones JL, Ulmer JL. Proton magnetic resonance spectroscopy of the hippocampus in patients with mild cognitive impairment: a pilot study. J Comput Assist Tomogr. 2007;31:666–70. doi: 10.1097/RCT.0b013e318031bc31. [DOI] [PubMed] [Google Scholar]
- 61.Chiron C, Jambaque I, Nabbout R, Lounes R, Syrota A, Dulac O. The right brain hemisphere is dominant in human infants. Brain. 1997;120:1057–65. doi: 10.1093/brain/120.6.1057. [DOI] [PubMed] [Google Scholar]
- 62.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldberger AL, Rigney DR, Mietus J, Antman EM, Greenwald S. Nonlinear dynamics in sudden cardiac death syndrome: heartrate oscillations and bifurcations. Experientia. 1988;44:983–7. doi: 10.1007/BF01939894. [DOI] [PubMed] [Google Scholar]
- 64.Gami AS, Somers VK. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovasc Electrophysiol. 2008;19:997–1003. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 65.Hersi AS. Obstructive sleep apnea and cardiac arrhythmias. Ann Thorac Med. 2010;5:10–7. doi: 10.4103/1817-1737.58954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asghari A, Mohammadi F, Kamrava SK, Tavakoli S, Farhadi M. Severity of depression and anxiety in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2012;269:2549–53. doi: 10.1007/s00405-012-1942-6. [DOI] [PubMed] [Google Scholar]
- 67.Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF. Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Res. 1991;550:115–21. doi: 10.1016/0006-8993(91)90412-o. [DOI] [PubMed] [Google Scholar]
- 68.Ben Salem D, Walker PM, Bejot Y, et al. N-acetylaspartate/creatine and choline/creatine ratios in the thalami, insular cortex and white matter as markers of hypertension and cognitive impairment in the elderly. Hypertens Res. 2008;31:1851–7. doi: 10.1291/hypres.31.1851. [DOI] [PubMed] [Google Scholar]
- 69.Cheshire WP, Jr, Saper CB. The insular cortex and cardiac response to stroke. Neurology. 2006;66:1296–7. doi: 10.1212/01.wnl.0000219563.87204.7d. [DOI] [PubMed] [Google Scholar]
- 70.Kahn A, Groswasser J, Rebuffat E, et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–92. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 71.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–48. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- 72.Gerich JE, Charles MA, Grodsky GM. Regulation of pancreatic insulin and glucagon secretion. Ann Rev Physiol. 1976;38:353–88. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- 73.Peltier AC, Consens FB, Sheikh K, Wang L, Song Y, Russell JW. Autonomic dysfunction in obstructive sleep apnea is associated with impaired glucose regulation. Sleep Med. 2007;8:149–55. doi: 10.1016/j.sleep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 74.Macey PM, Kumar R, Woo MA, Yan-Go FL, Harper RM. Heart rate responses to autonomic challenges in obstructive sleep apnea. PloS One. 2013;8:e76631. doi: 10.1371/journal.pone.0076631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kales A, Caldwell AB, Cadieux RJ, Vela-Bueno A, Ruch LG, Mayes SD. Severe obstructive sleep apnea--II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38:427–34. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 76.Longe O, Maratos FA, Gilbert P, et al. Having a word with yourself: neural correlates of self-criticism and self-reassurance. NeuroImage. 2010;49:1849–56. doi: 10.1016/j.neuroimage.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 77.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic--cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 78.Gozal E, Gozal D, Pierce WM, et al. Proteomic analysis of CA1 and CA3 regions of rat hippocampus and differential susceptibility to intermittent hypoxia. J Neurochem. 2002;83:331–45. doi: 10.1046/j.1471-4159.2002.01134.x. [DOI] [PubMed] [Google Scholar]
- 79.Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–8. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- 80.Aviles-Reyes RX, Angelo MF, Villarreal A, Rios H, Lazarowski A, Ramos AJ. Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: implications for sleep apnea. J Neurochem. 2010;112:854–69. doi: 10.1111/j.1471-4159.2009.06535.x. [DOI] [PubMed] [Google Scholar]
- 81.Douglas RM, Ryu J, Kanaan A, et al. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol. 2010;298:C1594–602. doi: 10.1152/ajpcell.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 83.Balfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med. 1994;150:1587–91. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- 84.Phillips DJ, Schei JL, Rector DM. Vascular compliance limits during sleep deprivation and recovery sleep. Sleep. 2013;36:1459–70. doi: 10.5665/sleep.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]