Abstract
Eyes absent (Eya), a protein conserved from plants to humans and best characterized as a transcriptional coactivator, is also the prototype for a novel class of eukaryotic aspartyl protein tyrosine phosphatases. This minireview discusses recent breakthroughs in elucidating the substrates and cellular events regulated by Eya's tyrosine phosphatase function and highlights some of the complexities, new questions, and surprises that have emerged from efforts to understand how Eya's unusual multifunctionality influences developmental regulation and signaling.
INTRODUCTION
Drosophila Eyes absent (Eya), the founding member of the Eya family, was molecularly defined in 1993 as a novel nuclear protein with essential roles in retinal cell survival and differentiation (1). Four years later, three key findings heightened interest in Eya family proteins. First, three mammalian Eya homologues, Eya1 Eya2, and Eya3, were identified and found to be expressed in the lens and nasal placodes, raising the possibility that the developmental program of eye specification is conserved between invertebrates and mammals (2, 3); the fourth vertebrate Eya gene, Eya4, was identified 2 years later (4). Functional conservation of Eya proteins was further emphasized by the demonstration that expression of murine Eya2 partially restores eye development in Drosophila eya mutants (5). Second, the discovery that loss-of-function mutations in Eya1 produced the ear, kidney and craniofacial defects associated with the autosomal-dominant disease branchio-oto-renal (BOR) syndrome (6–8) highlighted Eya's critical and pleiotropic roles in human development and disease. Third, parallel studies in Drosophila and in mammalian cultured cells revealed a molecular function for Eya as a transcriptional coactivator (5, 9–12). Eya is recruited to transcriptional complexes via a direct interaction between its highly conserved ∼270-amino-acid (aa) C-terminal motif, the Eya domain (ED) (Fig. 1), and Six family homeodomain DNA binding proteins. Together, Eya and Six operate as a composite transcription factor, with Six providing DNA binding specificity and the N-terminal half of Eya conferring transactivation.
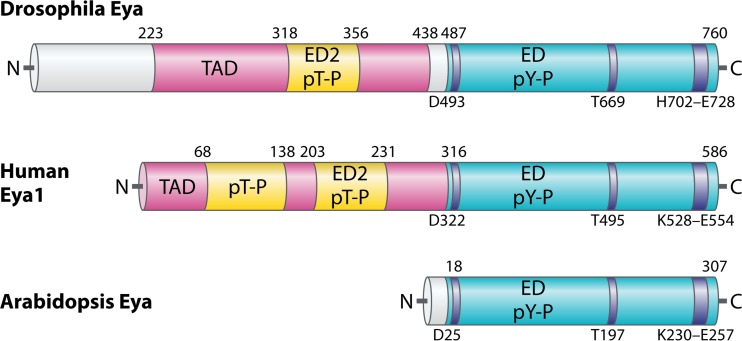
FIG 1.
Functional domains of Eya family proteins. Shown is a diagram, not drawn precisely to scale, comparing the Drosophila Eya, human Eya1, and Arabidopsis Eya proteins. The conserved Eya domain (ED) is depicted in blue, with dark bars marking motifs 1, 2, and 3 that define the HAD phosphotyrosine phosphatase (pY-P) domain; key catalytic residues are noted below. The aspartic acid in motif 1 is the most commonly mutated to generate “phosphatase-dead” Eya. The transactivation domain (TAD) is denoted by the pink N-terminal region in the fly and human proteins, with the embedded sequences implicated in phosphothreonine phosphatase (pT-P) activity in yellow. The pT-P motif overlaps a tyrosine-rich loosely conserved motif called Eya domain 2 (ED2). Gray areas denote stretches of protein sequence of unknown function with no conservation across species.
In addition to the many similarities, mammalian and Drosophila Eya proteins show a striking difference in subcellular localization. In contrast to Drosophila Eya, which appears constitutively nuclear (13), mammalian Eya proteins show significant cytoplasmic accumulation and rely on binding to Six for nuclear recruitment and retention (11, 13–20). Although initially dismissed as reflecting two slightly different mechanisms for regulating Eya-mediated transcriptional events, in retrospect, as discussed later in this minireview, this observation seems to have foretold a much more profound functional divergence between mammalian and fly Eya proteins.
Subsequent molecular genetic studies positioned Eya and Six as central players within a conserved network of transcription factors that is referred to as the retinal determination (RD) network (reviewed in references 21–24). Although Eya and most other RD genes were originally named for and defined by their pivotal roles in Drosophila eye specification (1, 25–27), subsequent studies showed that the RD network, either as a whole or in parts, operates across metazoans and interacts with a broad spectrum of signaling pathways to regulate the development and homeostasis of a variety of organs and tissues, including eye, ear, kidney, muscle, and nervous system. Study of Eya and the RD network has thus provided numerous insights into the modularity, conservation, and context specificity of regulatory networks across evolution. These topics have been reviewed extensively (23, 28–37) and will not be discussed here.
Even broader interest in Eya was piqued in 2003 when three groups reported that the ED carries intrinsic protein tyrosine phosphatase activity (38–40) (Fig. 1 and 2). This rather unique merger of transcriptional and catalytic activities is even more remarkable in that Eya is not a traditional thiol-based tyrosine phosphatase but rather belongs to the phosphatase subgroup of the haloacid dehalogenase (HAD) superfamily, a diverse collection of hydrolases that has been best studied in prokaryotes (reviewed in references 29, 41, and 42). Eya is thus the prototype of a novel class of eukaryotic protein phosphatases. Several recent reviews compare the distinguishing features of aspartyl-based versus thiol-based “classic” eukaryotic protein tyrosine phosphatases (43–46).
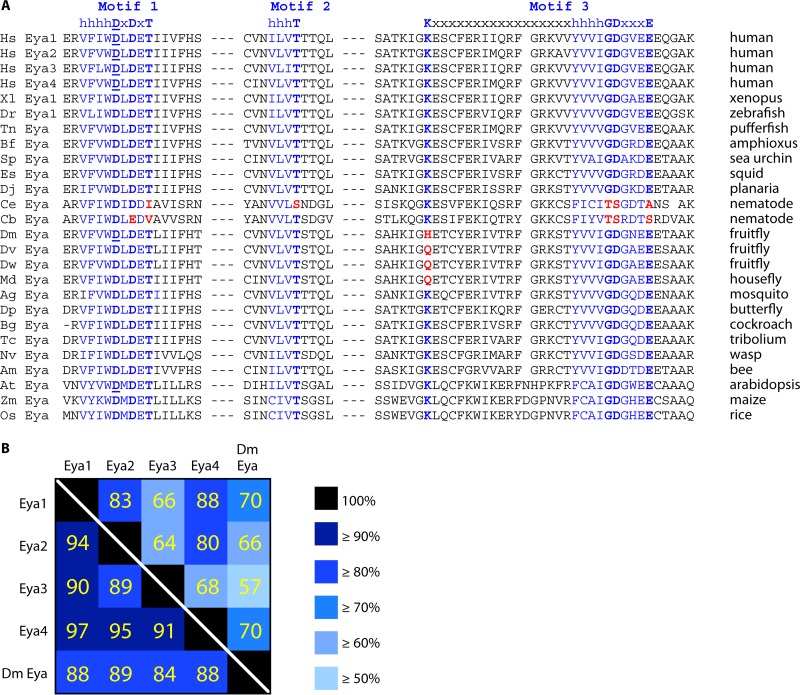
FIG 2.
(A) Conservation of the HAD tyrosine phosphatase in Eya proteins from plants to humans. Shown is an alignment of three noncontiguous stretches of ED sequence, with motifs 1 to 3 that define the HAD family in blue and key catalytic residues in boldface. Intervening sequences have been removed, as indicated by dashed lines. The nucleophilic aspartic acid residue in motif 1 is underlined in those Eya proteins where in vitro phosphatase has been measured and shown to be abrogated by mutation of that residue. Residues in red highlight the sequence divergence of Drosophila and Caenorhabditis relative to all other species. Multisequence alignments were performed with the Cobalt Constraint-based multiple protein alignment tool using the following protein sequences: NP_001275503.1, CAA71310.1, CAA71311.1, CAA76636.1, NP_001083888.1, AAI54188.1, CAG09098.1, EEN65685.1, XP_011666140.1, AHA91757.1, CAD89531.1, CAB05707.2, CAP36181.1, AAN10587.1, EDW63329.1, EDW76186.1, XP_011290277.1, XP_314837.3, EHJ75518.1, CCX34986.1, EFA07446.1, XP_008208098.1, XP_006562598.1, AEC09093.1, ACG36155.1, and NP_001056580.1. Hs, Homo sapiens; Xl, Xenopus laevis; Dr, Danio rerio; Tn, Tetraodon nigroviridis; Bf, Branchiostoma floridae; Sp, Strongylocentrotus purpuratus; Es, Euprymna scolopes; Dj, Dugesia japonica; Ce, Caenorhabditis elegans; Cb, Caenorhabditis briggsae; Dm, Drosophila melanogaster; Dv, Drosophila virilis; Dw, Drosophila willisoni; Md, Musca domestica; Ag, Anopheles gambiae; Dp, Danaus plexippus; Bg, Blatella germanica; Tc, Tribolium castaneum; Nv, Nasonia vitripennis; Am, Apis mellifera; At, Arabidopsis thaliana; Zm, Zea mays; Os, Oryza sativa. (B) Pairwise comparisons of ED sequences of human Eya1 to -4 and Drosophila Eya highlight the high degree of conservation between paralogs and across species. Percentages of amino acid identities and similarities were calculated using LALIGN and are shown above and below the diagonal white line, respectively. Percentile bins are colored from dark to light in descending order. Focusing on percentage of identity, the ED of Eya3 appears to be the most divergent of the four paralogs, although the high degree of similarity across all four paralogs suggests the amino acid differences may have limited impact on function. A more extensive discussion of similarities and differences among the Eya paralogs can be found in the article by Tadjuidje and Hegde (34).
The discovery that Eya proteins have intrinsic protein tyrosine phosphatase activity raised many questions about how dephosphorylation of specific substrates might contribute to Eya's transcriptional functions and/or reveal completely new roles for Eya in other cellular processes. Below I consider the extent to which these questions have been answered and the new challenges that have emerged. Throughout the discussion, the term “phosphatase-dead Eya” refers to a version of the full-length protein in which one or more of the conserved residues that define the catalytic core have been mutated. The most commonly targeted is the nucleophilic aspartic acid (for example, D493 in Drosophila Eya, D327 in mouse Eya1, and D322 in human Eya1). For simplicity, although the specific Eya paralogs associated with the experiments highlighted in this minireview are noted, the generic term “Eya” is used quite liberally throughout the discussions.
THE GREAT DEBATE: IS Eya'S TYROSINE PHOSPHATASE ACTIVITY DEDICATED TO OR INDEPENDENT OF TRANSCRIPTION?
Although the bulk of phosphotyrosine (pY)-based signaling occurs cytoplasmically, many nuclear factors are tyrosine phosphorylated (reviewed in reference 47). While these modifications may all result from signaling events initiated in the cytoplasm, the idea of a parallel nuclear universe in which tyrosine kinases and phosphatases directly regulate nuclear events has appeal. Thus, the field eagerly proposed that Eya's tyrosine phosphatase activity might provide nucleus-autonomous regulation of the assembly, activity, or turnover of transcriptional complexes (38–40, 48).
The papers that originally documented Eya's tyrosine phosphatase activity provide evidence both for and against this hypothesis. Supporting the model, Rosenfeld and colleagues used transcriptional reporter assays in mammalian cultured cells to show that recruitment of Eya's phosphatase activity was needed to switch Six1-Dach1 transcriptional complexes from repressive to activating (38). Mechanistically, phosphatase-dead Eya failed to recruit polymerase II (Pol II) and the coactivator CBP, suggesting a direct contribution of phosphatase activity to the assembly and function of active transcriptional complexes (Fig. 3). Arguing against the model, phosphatase-dead Eya exhibited wild-type transactivation ability in reporter assays performed in Drosophila cultured cells (40).
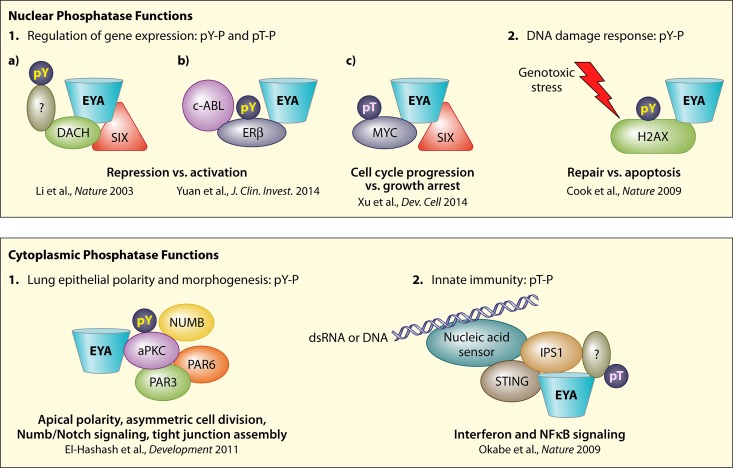
FIG 3.
Summary of Eya's phosphotyrosine and phosphothreonine phosphatase (pY-P and pT-P) functions in both nucleus and cytoplasm. Both phosphatase activities operate in in both compartments, although major gaps remain in substrate identification, as indicated by question marks. Phosphorylated residues are shown only on Eya substrates; other phospho-based regulation is not depicted. See the text and references for details (17, 20, 38, 64, 66, 92).
Subsequent studies bolstered the idea that Drosophila Eya's transcriptional function is less dependent on phosphatase activity than that of mammalian Eya. Lending support to the argument that phosphatase activity may not regulate Eya-Six transcriptional output in flies, examination of the genome-wide transcriptional response to overexpression of either wild-type or phosphatase-dead Drosophila Eya found that ∼80% of candidate target genes were normally regulated independent of Eya's catalytic activity (49). In contrast, three later studies concluded that phosphatase activity is central to the transcriptional response downstream of mammalian Eya-Six. First, Ahmed et al. identified a Six1-bound enhancer in the Atoh1 gene and showed that coexpression of Six1 with wild-type, but not phosphatase-dead, Eya1 was sufficient to activate Atoh1 expression and to induce hair cell fate in mouse cochlear explants (50). Although Eya substrates were not identified, assuming the developmental program that is initiated in these misexpression experiments accurately reflects the events of normal inner ear hair cell differentiation, the logical conclusion is that Eya1's tyrosine phosphatase activity is required for the transcriptional program that drives cell fate specification in the mammalian ear. Whether the mechanism involves attenuation of Dach-mediated repression as reported by Li et al. (38) remains to be tested. Second, Wu et al. compared the effects of expressing wild-type or phosphatase-dead Eya1 in cultured breast cancer cell lines and concluded that recruitment of tyrosine phosphatase activity was required to activate transcription at the cyclin D1 promoter to promote proliferation (51). Curiously, Eya1 was recruited to an AP-1 binding site in the promoter, rather than to the Six1-responsive element, implying a Six1-independent transcriptional function for Eya that is mechanistically distinct from the model proposed by Li et al. (38). Third, Eisner et al. identified Eya1 and Six1 as positive regulators of Sonic Hedgehog signaling during hindbrain development and showed that phosphatase-dead Eya failed to activate transcription of Gli1 and Ptch1 in transfected mouse embryonic fibroblasts derived from Eya1−/− animals (52). Putting aside the various caveats associated with the overexpression and cultured cell models used in all of these studies, the combined results at face value reveal fundamental differences in Eya function between flies and mammals. Only mammalian Eya seems to require tyrosine phosphatase activity to influence the assembly and activity of transcriptional complexes. This is an unexpected conclusion, given the high degree of evolutionary and functional conservation of Eya and of the genetic networks in which it operates.
Structural analyses have also contributed to the debate about how closely Eya's transcriptional and tyrosine phosphatase functions are intertwined. The first crystal structure published, of human Eya2, concluded that the Six binding surface and catalytic core are on opposite faces of the ED, with the Dach binding region sandwiched between (53). The authors postulated that this configuration might allow Dach-Eya interactions to bridge and coordinate transcriptional and phosphatase outputs, providing a physical model for the idea originally proposed by Rosenfeld and colleagues (38). A different picture emerged from analysis of a human Six1-Eya2 complex (54). This structure showed that Six1 interacts directly with the catalytic phosphatase domain of Eya2, rather than with an opposite side helix bundle as previously suggested (53). Although this study did not explore Dach interaction with the complex nor measure its catalytic activity, the binding configuration raises the possibility that Eya-Six transcriptional complexes block substrate access, physically separating Eya's two functions (54). However, because Six1 does not bind close to the active site, it may not interfere with substrate binding or dephosphorylation and could potentially even enhance it. The latter scenario has particularly interesting implications for contexts in which the assembly and output of Eya-Six transcriptional complexes are regulated by Eya's phosphatase activity.
A new twist emerged in 2013 when the Drosophila Eya field was turned upside down by the demonstration that Eya's tyrosine phosphatase activity is dispensable for normal development, fertility, and survival (55). Whereas earlier studies had relied on overexpression- and misexpression-based genetic assays to show compromised output from phosphatase-dead Eya (39, 40, 49, 56, 57), Mardon and colleagues used functional genomic transgenes to express Eya at endogenous levels, times, and places (55). Shockingly, three different phosphatase-dead Eya mutants fully complemented eya null alleles and exhibited completely wild-type function across a range of in vivo assays. Although redundancy with another phosphatase could mask an essential function, the simplest interpretation is that Eya's tyrosine phosphatase activity is dispensable for all essential developmental processes in the fly, at least under normal laboratory conditions.
Two indisputable conclusions from this study are, first, that tyrosine phosphatase function is not essential to Eya's role as a transcriptional coactivator in Drosophila, and, second, that the question of how Eya's transcriptional and phosphatase functions are integrated in vivo can only be answered using mammalian (or other vertebrate) models. Furthermore, in vivo validation of the “phosphatase regulates transcription” model must include identification of physiologically relevant substrates and target genes and cannot rely exclusively on overexpression- or misexpression-based approaches to demonstrate developmental regulation. Although generation of conditional phosphatase-dead alleles of endogenous mouse Eya genes will be labor intensive and costly, this strategy may well prove the fastest and most effective path toward identifying the in vivo requirements for Eya's tyrosine phosphatase activity.
What might be responsible for the genetic dispensability of Eya's tyrosine phosphatase function in Drosophila? As mentioned above, one possibility is a redundant phosphatase. Another is that Drosophila Eya simply lacks catalytic activity. Indeed, Drosophila Eya exhibits the weakest in vitro phosphatase activity of all Eya family proteins tested (39, 40, 58). However, the high degree of conservation of the ED across species (Fig. 2) makes it difficult to come up with a plausible molecular explanation for the loss-of-phosphatase-function hypothesis—for example, the EDs of Drosophila and human Eya1 share ∼70% amino acid identity and ∼88% similarity. Closer examination of the three noncontinuous sequence blocks that together form the catalytic core reveals divergence at one key residue. Specifically, the conserved lysine (K) that defines the start of motif 3 has been changed to either histidine (H) or glutamine (Q) in Drosophila and closely related flies such as the housefly. More distantly related insects, including cockroaches, butterflies, ants, wasps, bees, and Tribolium, all retain the K residue, as do all other invertebrates. If this K-H substitution is responsible for Drosophila Eya's lack of phosphatase activity, then flies are true outsiders among Eya-containing invertebrates, and if so, then mutation of H702 back to K should restore catalytic activity. Alternatively, it is possible that K699, a residue three amino acids N terminal to H702, marks the start of motif 3 in flies—indeed, our initial study showed that mutation of K699 compromised in vitro phosphatase activity and dampened output in overexpression-based assays in the fly (59). If this interpretation is correct, then further in-depth structural comparisons of Drosophila Eya will be required to understand the basis for its lost phosphatase activity. Finally, an additional prediction that emerges from the ED alignment is that Caenorhabditis elegans and closely related nematode Eya proteins likely lack tyrosine phosphatase activity, as they carry multiple substitutions at key catalytic residues. Assuming this is correct, then C. elegans offers another example in which tyrosine phosphatase activity is not essential for Eya-Six transcriptional output (60, 61).
With the two most tractable invertebrate models seemingly out of commission, and in light of the many unanswered questions that remain regarding the function and regulation of Eya's tyrosine phosphatase activity in mammals, it is worth mentioning the unique perspective that plant Eya proteins bring to the table. Plant Eya homologues have the conserved ED that defines the Eya superfamily (for example, Arabidopsis and human Eya1s share 32% amino acid identity and ∼60% similarity across the ED) but lack the N-terminal sequences that confer transactivation (58, 62) (Fig. 1 and 2). Plants also lack Six family representatives. Thus, although it needs to be confirmed experimentally, the assumption is that plant Eya is exclusively a tyrosine phosphatase. If so, then plants provide an opportunity to study the in vivo function and regulation of Eya tyrosine phosphatase activity without the complications associated with its other role as a transcription factor. The plant ED also stands out as having the strongest in vitro catalytic activity of all Eya family members tested (58). Considering how well Drosophila Eya's poor performance in a test tube foretold the fly's lack or limited use of tyrosine phosphatase potential in vivo (40, 55, 58), perhaps plant Eya offers the best path toward unraveling the mysteries of this novel eukaryotic phosphatase. From a structural biology perspective, determination of whether the plant ED fold has intrinsic Six binding potential might provide insight into the evolutionary trajectory that separates plant from animal Eya proteins, and by extension, the physical mechanisms by which animal Eya proteins integrate their dual functions as transcription factors and protein tyrosine phosphatases.
THE QUEST FOR Eya TYROSINE PHOSPHATASE SUBSTRATES
The surest way to define Eya's function as a tyrosine phosphatase is to identify substrates and then validate the physiological relevance of the regulation. Unfortunately, unlike kinases, where versatile in vitro assays combined with strong sequence specificity facilitate substrate identification, phosphatases tend to recognize three-dimensional motifs rather than linear sequences, making peptide-based screens and bioinformatic predictions unreliable. For this reason, knowledge of substrates remains limited even for the most extensively studied thiol-based tyrosine phosphatases. Adding a further challenge, the distinct aspartyl-based reaction chemistry used by Eya proteins precludes immediate implementation of approaches such as substrate trapping that have been developed for thiol-based phosphatases (63). Thus, although in vitro dephosphorylation of specific peptides has demonstrated specificity for phosphotyrosine and permitted comparison of enzymatic efficiency of different Eya proteins (39, 40, 58), it has not led to identification of actual substrates.
Returning briefly to the context of Eya as a transcription factor, no substrates linking Eya's tyrosine phosphatase activity to Eya-Six-mediated transcriptional regulation have been identified. Although Li et al.'s 2003 finding that Eya's tyrosine phosphatase activity can switch Six1-Dach1 complexes from repressive to activating (38) predicted that Dach1, a core component of the RD network, and Six1 itself might be Eya substrates, to my knowledge, there have been no reports of phosphotyrosine-based regulation of Six or Dach factors (Fig. 3). My lab's own efforts to explore phosphatase-substrate relationships between Eya and other RD proteins have yielded similarly negative results (I. Rebay, unpublished data). The one exception was our observation that phosphatase-dead Eya immunoprecipitated from Drosophila cultured cells showed greater levels of tyrosine phosphorylation than wild-type Eya and could be dephosphorylated in vitro by recombinant ED phosphatase (40). Efforts to identify the relevant kinase revealed that the Abelson (Abl) nonreceptor tyrosine kinase can directly phosphorylate Drosophila Eya and that this can promote its cytoplasmic accumulation (56). Genetic rescue assays using overexpressed phosphatase-dead Eya transgenes suggested a requirement for cytoplasmic tyrosine phosphatase activity, leading to a model in which Abl-mediated tyrosine phosphorylation recruits Eya phosphatase activity to cytoplasmic signaling centers, and autocatalytic dephosphorylation returns Eya to the nucleus to regulate gene expression (56). Somewhat ironically, although the relevance of these phenomena to Drosophila physiology has since been called into question (55), as discussed below, the prediction of cytoplasmic Eya tyrosine phosphatase activity has been validated in mammals (17, 18).
To date, three protein substrates of the mammalian Eya phosphotyrosine phosphatase have been identified: the histone H2A variant H2AX, the atypical protein kinase C zeta (aPKCζ), and the estrogen receptor beta (ERβ) (17, 18, 64–66) (Fig. 3). None of the three implicates Eya phosphatase activity in regulating Eya-Six-mediated transcriptional events, and all reveal new roles for Eya: H2AX in the repair versus apoptosis response to DNA damage, aPKCζ in epithelial polarity and asymmetric cell division, and ERβ in Six-independent modes of transcriptional regulation. A discussion of the three discoveries follows below.
An unconventional tyrosine kinase and phosphatase pair converges on H2AX.
In a mechanism conserved from plants to humans, DNA damage induces phosphorylation of the histone variant H2AX on S139 (67; reviewed in references 68 and 69). Phospho-specific antibodies against H2AX-pS139 mark the DNA damage sites, or foci, to which checkpoint and repair complexes are recruited. In 2009, mammalian H2AX was shown to be constitutively phosphorylated on Y142 by WSTF (Williams syndrome transcription factor), an unconventional tyrosine kinase better characterized as a core component of the WSTF-SNF2H chromatin remodeling complex (70, 71). Y142 phosphorylation decreased in response to DNA damage, revealing the need for a tyrosine phosphatase. Two groups showed that Eya provides this activity (64, 65).
In the first study, Cook et al. used a combination of in vitro and cultured human embryonic kidney cell assays to demonstrate that Eya dephosphorylation of H2AX-pY142 mediates the repair versus apoptosis response to DNA damage (64). To summarize the highlights, upon DNA damage, the checkpoint kinases ATM/ATR phosphorylate Eya on S219 (such phosphorylation had been previously noted in mass spectrometry studies [72–74]). This recruits Eya (Eya1 and Eya3) to DNA damage foci, where it dephosphorylates H2AX-pY142, which in turn enhances recruitment of the DNA damage checkpoint protein MDC1 and associated repair factors. When Eya was depleted, cells exposed to genotoxic stress had elevated H2AX-pY142 levels, failed to recruit MDC1-mediated repair complexes, and instead assembled proapoptotic complexes. These phenotypes could all be suppressed by expression of wild-type, but not phosphatase-dead, Eya, demonstrating a requirement for Eya's tyrosine phosphatase activity. In the second study, Krishnan et al. further confirmed Eya's ability to dephosphorylate H2AX-pY142 in vitro and showed that knockdown of endogenous Eya3 both enhanced the basal phosphorylation of Y142 and inhibited DNA damage-induced dephosphorylation in a cultured osteosarcoma cell line (65).
A number of interesting questions have arisen from identification of H2AX as an Eya substrate. First, is the WSTF-H2AX-Eya kinase-substrate-phosphatase relationship only relevant to a cell's response to genotoxic stress, or does the same network mediate cell survival versus death decisions that arise during normal development? Cook et al. noted an increase in H2AX-pS139 foci in the developing kidney of Eya1−/− null mutant mouse embryos and speculated that the renal apoptosis associated with Eya1 loss might result from compromised DNA damage response pathways (64). Whether increased H2AX-pS139 foci are observed in other developing tissues where Eya loss is associated with increased apoptosis (1, 75, 76) has not yet been reported. Second, might Six1 also be recruited to H2AX-pS139 foci? Although this possibility was not considered in the initial studies (64, 65), if the answer is “yes,” it would raise the possibility that the Eya-Six complex, and not just Eya itself, has dual nuclear functions—one dedicated to regulation of gene expression and the other as a phosphatase dedicated to the repair versus death response to genotoxic stress. Third, and returning to the question of the relevance of Eya's tyrosine phosphatase activity in Drosophila, might challenge of phosphatase-dead rescued flies (55) with genotoxic stress finally reveal a requirement for catalytic activity? Drosophila, like mammals, has a DNA damage-responsive H2A variant with the C-terminal Y142 (77), making this a formal possibility. However, plants, where Eya is postulated to operate exclusively as a tyrosine phosphatase, do not (78), suggesting that the enzyme-substrate relationship between Eya and H2AX cannot be a conserved commonality across the full spectrum of Eya-containing organisms. Fourth, might WSTF and Eya share other substrates? WSTF plays important roles in chromatin assembly, remodeling, and transcriptional regulation, and its loss-of-function phenotypes suggest broad developmental requirements in heart, thymus, neural crest, kidney, tooth, and brain (79–81). Whether its tyrosine kinase is relevant in those contexts and what substrates might be targeted are open questions, but it is tempting to speculate that this unconventional kinase might pair up with the unconventional Eya phosphatase to regulate a variety of substrates. Analogously, histone variants such as H2AX are emerging as important developmental factors with broader roles in chromatin modification and epigenetic regulation, although it is not yet known whether phosphorylation/dephosphorylation of Y142 is relevant to these contexts (82–84).
Eya is a bona fide cytoplasmic tyrosine phosphatase.
The first cytoplasmic Eya tyrosine phosphatase substrate, aPKCζ, emerged from exploration of the requirement for Eya1 in lung epithelial morphogenesis (17, 18). Briefly, apically localized cytoplasmic Eya1 protein was found to overlap both the aPKCζ-Par polarity complex and the tight junction proteins ZO-1, occludin, and claudin in mouse embryonic lung epithelia. In either the embryonic lung or in MLE-15 lung epithelial cells, Eya1 loss disrupted both complexes, leading to defects in spindle orientation, plane of cell division, Numb localization, Notch activity, barrier formation, and cell fate. Expression of wild-type, but not phosphatase-dead, Eya1 rescued all phenotypes. Probing deeper into biochemical mechanism, El-Hashash et al. showed that loss of Eya1 led to hyperphosphorylation and prolonged activation of aPKCζ, which in turn disrupted phosphorylation of apical and junctional substrates. Again, wild-type, but not phosphatase-dead, Eya1 restored the normal phosphorylation balance. Both coimmunoprecipitation studies and in vitro phosphatase assays using immunopurified proteins suggested that direct interactions with aPKCζ recruit Eya1 to the apical-junctional domain, where its tyrosine phosphatase activity helps set the precise levels of aPKCζ activity needed for proper localization and function of apical-junctional complexes. Identification of the specific pY residue(s) targeted by Eya will be needed to define the regulatory mechanism more precisely.
As discussed above for H2AX, the work by El-Hashash and colleagues (17–19) has opened up new directions for exploring Eya function and regulation. Of particular significance to the field, this is the first demonstration of a cytoplasmic function for Eya during animal development. Previously, although cytoplasmic Eya localization had been noted, it was generally assumed to reflect a sequestration mechanism that regulated Eya's transcriptional functions rather than a site of important activity (11, 15, 20, 85). As the bulk of cellular phosphotyrosine-based signaling occurs cytoplasmically, cytoplasmic Eya could influence a broad range of processes via dephosphorylation of a variety of substrates.
Eya tyrosine phosphatase activity and cancer: insight from ERβ.
Elevated Eya expression has been noted in a variety of human cancers and correlates with tumor aggressiveness and poor prognosis (reviewed in references 34, 86, and 87). Co-overexpression of Eya's transcriptional partner Six1 and altered regulation of Eya-Six target genes relevant to proliferation, survival, and metastasis suggest Eya's function as a transcription factor is central to the oncogenic mechanism (reviewed in reference 86). The contributions of Eya's tyrosine phosphatase activity to tumor initiation and progression are less well understood, and consideration of possible mechanisms parallels the broader debate on the relationship between tyrosine phosphatase activity and Eya-Six transcription discussed earlier in this minireview. One area of current controversy is whether Eya's catalytic activity is solely required for tumor invasiveness or whether it also drives proliferation. As discussed below, recent identification of ERβ as a direct Eya2 substrate offers partial resolution.
To set the stage, Pandey et al. (16) argue compellingly that cytoplasmic Eya tyrosine phosphatase activity drives the altered cell motility that accompanies metastasis and invasion but does not regulate tumor cell proliferation. Specifically they showed that overexpression of either Eya1, Eya2, or Eya3 increased the proliferation, motility, invasiveness, and transformation behaviors of cultured breast cancer cells. In contrast, overexpression of phosphatase-dead versions increased proliferation but did not alter motility, invasion, or transformation. Using an in vivo metastasis assay, small interfering RNA (siRNA) knockdown of Eya3 reduced the frequency of lung metastases when breast cancer cells were injected into mice; metastatic efficiency was restored by reexpressing wild-type, but not phosphatase-dead, Eya3. Screens for chemical inhibitors of Eya's tyrosine phosphatase activity have identified compounds that inhibit the increased motility and invasiveness of Eya-expressing cultured cells, further highlighting the relevance of its catalytic activity to metastasis (88, 89). A major limitation to these studies is that direct substrates were not identified, leaving the molecular mechanisms responsible for the phosphatase-driven cellular behaviors uncertain. Given that disruptions to apical polarity and junctional complexes are well-established contributors to epithelial-to-mesenchymal transition (EMT) and metastatic disease (reviewed in reference 90), perhaps the Eya-aPKCζ connection discovered in the lung (17, 18) might also be relevant to cancer progression.
A study by Wu et al. (51) reached the opposite conclusion to the study by Pandey et al. (16), namely, that Eya's tyrosine phosphatase activity is essential for promotion of proliferation of cultured breast cancer cells via modulation of the transcriptional complexes that activate cyclin D expression (51). As mentioned earlier, although Six1 also directly activates cyclin D expression, Eya1 phosphatase activity appears targeted toward complexes assembled at an AP-1 binding site rather than to those at the Six1 binding site. Putting aside the caveat of lack of substrate identification, the results suggest that Eya's tyrosine phosphatase activity promotes proproliferation transcriptional programs, but via a Six-independent mechanism.
Identification of the estrogen receptor ERβ as a direct Eya substrate strengthens this argument (66). ERβ has well-documented antiproliferative effects in a variety of cancer cell types, including breast cancer cells, and low ERβ expression generally correlates with poor prognosis in human patients (reviewed in reference 91). Yuan et al. (66) provide new insight into how ERβ antitumorigenic activity may be regulated. Briefly, they identify Y36 of ERβ as a phosphorylatable switch that is toggled by the opposing action of the c-Abl tyrosine kinase and the Eya2 tyrosine phosphatase. Using a combination of cultured cell assays and xenografts, the authors show that elevated Eya2 can drive dephosphorylation of ERβ-Y36, which in turn attenuates activation of the gene expression programs associated with ERβ's antitumorigenic effects. Mechanistically, pY36 provides a docking site for the coactivator p300, and so Eya2 dephosphorylation of this residue prevents assembly of an active transcriptional complex. Suggesting the phospho-switch may be relevant in vivo, immunohistochemical examination of human breast tumor samples revealed a negative correlation between Eya2 and pY36 levels, and low pY36 levels in turn correlated with aggressive disease progression and poor patient survival.
In conclusion, although in some contexts Eya's tyrosine phosphatase activity may only be relevant to tumor cell motility and invasiveness, in others it seems likely to promote the transcriptional programs that drive proliferation. Whether chemical inhibition of catalytic activity will prove therapeutically beneficial in any context remains to be tested.
DÉJÀ VU ALL OVER AGAIN—Eya IS A NONCANONICAL THREONINE PHOSPHATASE
While the focus of this minireview is to summarize progress in understanding Eya's tyrosine phosphatase function, the discussion would be incomplete without brief mention of the 2009 discovery by Nagata and colleagues that the weakly conserved tyrosine-rich motif in the N-terminal half of Eya, originally identified as Eya domain 2 (ED2) (3), carries a novel phosphothreonine (pT)-specific phosphatase activity (92) (Fig. 1). Okabe et al. first identified a role for Eya4 in regulating innate immunity, and in the course of asking whether Eya's tyrosine phosphatase function was relevant, a “control” experiment uncovered unexpected catalytic activity in the N-terminal half of the protein(92). This new phosphatase activity was mapped to the ED2 region, defined as pT specific, and although no substrates were identified, the activity was assumed to be cytoplasmic based on Eya4's subcellular localization and interaction partners (Fig. 3). Although the catalytic mechanism and protein fold remain unknown, two follow-up studies identified mouse Eya3 as having the strongest in vitro threonine phosphatase activity of the four Eya paralogs, defined more precisely the sequence boundaries of the domain (93), and showed that both Eya's threonine phosphatase activity and its role in regulating innate immunity are likely to be conserved in Drosophila (57). The latter conclusion was based on heat shock-driven rescue transgenes, and so given the hard lesson with the tyrosine phosphatase activity (55), it will need to be confirmed with genomic constructs.
As with the tyrosine phosphatase, identification of substrates, including those subject to dual pT- and pY Eya-mediated dephosphorylation, exploration of different contexts and subcellular compartments in which threonine phosphatase activity is relevant, and determination of how it interfaces with Eya's other two functions are all important priorities. Regarding the relationship between Eya's threonine phosphatase and transcriptional roles, although the two are subcellularly separated in the context of innate immunity, the ED2 is embedded within the transactivation motif, raising the possibility of regulation of transcriptional complex dynamics and output. A recent study exploring Eya1 function in regulating the self-renewal and proliferation of nephron progenitors in the embryonic kidney makes a strong argument for such a possibility by identifying Myc as a direct substrate (20) (Fig. 3). Briefly, the authors defined a nuclear Eya1-Six2-Myc complex that leads to Eya1 dephosphorylating T58 on Myc. Phosphorylation of T58 by Gsk3 had been previously implicated in targeting Myc for degradation (94), and so in the absence of Eya1-Six2, the failure to dephosphorylate can deplete the Myc pool and lead to cell cycle arrest. Myc's prominent role as an oncogene suggests the relationship could also be relevant in cancer cells. More broadly, given how frequently threonine phosphorylation regulates transcription factor complex assembly and output, opportunities for Eya-mediated regulation of other nuclear factors in both normal and oncogenic contexts seem vast.
Concluding remarks.
The unusual multifunctionality of Eya family proteins, combined with their pleiotropic functions in normal development and disease, will undoubtedly motivate further study and reveal novel modes of cellular regulation. With the advent of sensitive phospho-proteomic approaches to identify a broader spectrum of Eya substrates and clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 technology to manipulate endogenous genes in vivo, the next decade of research promises to provide clear resolution to some of the ongoing debates and perhaps also toss out a few more intriguing surprises.
ACKNOWLEDGMENTS
I thank all past and present Rebay lab members for many stimulating conversations that helped develop the ideas put forth in this minireview. Special thanks go to T. Davis, C. Hoi, and N. Sanchez-Luege for helpful suggestions on the manuscript.
My lab's work on Eya has been supported by NIH R01 EY012549.
REFERENCES
- 1.Bonini NM, Leiserson WM, Benzer S. 1993. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 2.Xu P-X, Woo I, Her H, Beier DR, Maas RL. 1997. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development 124:219–231. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman JE, Bui QT, Steingrimsson E, Nagle DL, Fu W, Genin A, Spinner NB, Copeland NG, Jenkins NA, Bucan M, Bonini NM. 1997. Cloning and characterization of two vertebrate homologs of the Drosophila eyes absent gene. Genome Res 7:128–141. doi: 10.1101/gr.7.2.128. [DOI] [PubMed] [Google Scholar]
- 4.Borsani G, DeGrandi A, Ballabio A, Bulfone A, Bernard L, Banfi S, Gattuso C, Mariani M, Dixon M, Donnai D, Metcalfe K, Winter R, Robertson M, Axton R, Brown A, van Heyningen V, Hanson I. 1999. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum Mol Genet 8:11–23. doi: 10.1093/hmg/8.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Bonini NM, Bui QT, Gray-Board GL, Warrick JM. 1997. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development 124:4819–4826. [DOI] [PubMed] [Google Scholar]
- 6.Vincent C, Kalatzis V, Abdelhak S, Chaib H, Compain S, Helias J, Vaneecloo FM, Petit C. 1997. BOR and BO syndromes are allelic defects of EYA1. Eur J Hum Genet 5:242–246. [PubMed] [Google Scholar]
- 7.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. 1997. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet 15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- 8.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, Konig R, Vigneron J, Weissenbach J, Petit C, Weil D. 1997. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet 6:2247–2255. doi: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- 9.Xu P-X, Cheng J, Epstein JA, Maas RL. 1997. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci U S A 94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. 1997. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91:881–891. doi: 10.1016/S0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. 1999. Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol 19:6815–6824. doi: 10.1128/MCB.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver SJ, Davies EL, Doyon L, Rebay I. 2003. Functional dissection of Eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol 23:5989–5999. doi: 10.1128/MCB.23.17.5989-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonini NM, Leiserson WM, Benzer S. 1998. Multiple roles of the eyes absent gene in Drosophila. Dev Biol 196:42–57. doi: 10.1006/dbio.1997.8845. [DOI] [PubMed] [Google Scholar]
- 14.Buller C, Xu X, Marquis V, Schwanke R, Xu P-X. 2001. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum Mol Genet 10:2775–2781. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- 15.Fougerousse F, Durand M, Lopez S, Suel L, Demignon J, Thornton C, Ozaki H, Kawakami K, Barbet P, Beckmann JS, Maire P. 2002. Six and Eya expression during human somitogenesis and MyoD gene family activation. J Muscle Res Cell Motil 23:255–264. doi: 10.1023/A:1020990825644. [DOI] [PubMed] [Google Scholar]
- 16.Pandey RN, Rani R, Yeo EJ, Spencer M, Hu S, Lang RA, Hegde RS. 2010. The Eyes Absent phosphatase-transactivator proteins promote proliferation, transformation, migration, and invasion of tumor cells. Oncogene 29:3715–3722. doi: 10.1038/onc.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Hashash AH, Turcatel G, Al Alam D, Buckley S, Tokumitsu H, Bellusci S, Warburton D. 2011. Eya1 controls cell polarity, spindle orientation, cell fate and Notch signaling in distal embryonic lung epithelium. Development 138:1395–1407. doi: 10.1242/dev.058479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.El-Hashash AH, Turcatel G, Varma S, Berika M, Al Alam D, Warburton D. 2012. Eya1 protein phosphatase regulates tight junction formation in lung distal epithelium. J Cell Sci 125:4036–4048. doi: 10.1242/jcs.102848. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.El-Hashash AH, Al Alam D, Turcatel G, Bellusci S, Warburton D. 2011. Eyes absent 1 (Eya1) is a critical coordinator of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Dev Biol 350:112–126. doi: 10.1016/j.ydbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Wong EY, Cheng C, Li J, Sharkar MT, Xu CY, Chen B, Sun J, Jing D, Xu P-X. 2014. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev Cell 31:434–447. doi: 10.1016/j.devcel.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wawersik S, Maas RL. 2000. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet 9:917–925. doi: 10.1093/hmg/9.6.917. [DOI] [PubMed] [Google Scholar]
- 22.Pappu KS, Mardon G. 2004. Genetic control of retinal specification and determination in Drosophila. Int J Dev Biol 48:913–924. doi: 10.1387/ijdb.041875kp. [DOI] [PubMed] [Google Scholar]
- 23.Silver S, Rebay I. 2005. Signaling circuitries in development: insights from the retinal determination gene network. Development 132:3–13. [DOI] [PubMed] [Google Scholar]
- 24.Kumar JP. 2010. Retinal determination the beginning of eye development. Curr Top Dev Biol 93:1–28. doi: 10.1016/B978-0-12-385044-7.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. 1994. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 26.Quiring R, Walldorf U, Kloter U, Gehring WJ. 1994. Homology of the Eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 27.Czerny T, Halder G, Kloter U, Souabni A, Gehring WJ, Busslinger M. 1999. twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol Cell 3:297–307. doi: 10.1016/S1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- 28.Brodbeck S, Englert C. 2004. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol 19:249–255. doi: 10.1007/s00467-003-1374-z. [DOI] [PubMed] [Google Scholar]
- 29.Jemc J, Rebay I. 2007. The Eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu Rev Biochem 76:513–538. doi: 10.1146/annurev.biochem.76.052705.164916. [DOI] [PubMed] [Google Scholar]
- 30.Kumar JP. 2009. The molecular circuitry governing retinal determination. Biochim Biophys Acta 1789:306–314. doi: 10.1016/j.bbagrm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grocott T, Tambalo M, Streit A. 2012. The peripheral sensory nervous system in the vertebrate head: a gene regulatory perspective. Dev Biol 370:3–23. doi: 10.1016/j.ydbio.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Zaffran S, Kelly RG. 2012. New developments in the second heart field. Differentiation 84:17–24. doi: 10.1016/j.diff.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Treisman JE. 2013. Retinal differentiation in Drosophila. Wiley Interdiscip Rev Dev Biol 2:545–557. doi: 10.1002/wdev.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadjuidje E, Hegde RS. 2013. The Eyes Absent proteins in development and disease. Cell Mol Life Sci 70:1897–1913. doi: 10.1007/s00018-012-1144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu P-X. 2013. The EYA-SO/SIX complex in development and disease. Pediatr Nephrol 28:843–854. doi: 10.1007/s00467-012-2246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong EYM, Ahmed M, Xu P-X. 2013. EYA1-SIX1 complex in neurosensory cell fate induction in the mammalian inner ear. Hear Res 297:13–19. doi: 10.1016/j.heares.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moody SA, LaMantia A-S. 2015. Transcriptional regulation of cranial sensory placode development. Curr Top Dev Biol 111:301–350. doi: 10.1016/bs.ctdb.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. 2003. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 39.Rayapureddi JP, Kattamuri C, Steinmetz BD, Frankfort BJ, Ostrin EJ, Mardon G, Hegde RS. 2003. Eyes absent represents a class of protein tyrosine phosphatases. Nature 426:295–298. doi: 10.1038/nature02093. [DOI] [PubMed] [Google Scholar]
- 40.Tootle TL, Silver SJ, Davies EL, Newman V, Latek RR, Mills IA, Selengut JD, Parlikar BEW, Rebay I. 2003. The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature 426:299–302. doi: 10.1038/nature02097. [DOI] [PubMed] [Google Scholar]
- 41.Aravind L, Koonin EV. 1998. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci 23:469–472. doi: 10.1016/S0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 42.Seifried A, Schultz J, Gohla A. 2013. Human HAD phosphatases: structure, mechanism, and roles in health and disease. FEBS J 280:549–571. doi: 10.1111/j.1742-4658.2012.08633.x. [DOI] [PubMed] [Google Scholar]
- 43.Tautz L, Critton DA, Grotegut S. 2013. Protein tyrosine phosphatases: structure, function, and implication in human disease. Methods Mol Biol 1053:179–221. doi: 10.1007/978-1-62703-562-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonks NK. 2013. Protein tyrosine phosphatases—from housekeeping enzymes to master regulators of signal transduction. FEBS J 280:346–378. doi: 10.1111/febs.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatzihristidis T, Desai N, Hutchins AP, Meng T-C, Tremblay ML, Miranda-Saavedra D. 2015. A Drosophila-centric view of protein tyrosine phosphatases. FEBS Lett 589:951–966. doi: 10.1016/j.febslet.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Zhao S, Sedwick D, Wang Z. 2015. Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene 34:3885–3894. doi: 10.1038/onc.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cans C, Mangano R, Barila D, Neubauer G, Superti-Furga G. 2000. Nuclear tyrosine phosphorylation: the beginning of a map. Biochem Pharmacol 60:1203–1215. doi: 10.1016/S0006-2952(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 48.Rebay I, Silver SJ, Tootle TL. 2005. New vision from Eyes absent: transcription factors as enzymes. Trends Genet 21:163–171. doi: 10.1016/j.tig.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Jemc J, Rebay I. 2007. Identification of transcriptional targets of the dual-function transcription factor/phosphatase eyes absent. Dev Biol 310:416–429. doi: 10.1016/j.ydbio.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed M, Wong EYM, Sun J, Xu J, Wang F, Xu P-X. 2012. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell 22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu K, Li Z, Cai S, Tian L, Chen K, Wang J, Hu J, Sun Y, Li X, Ertel A, Pestell RG. 2013. EYA1 phosphatase function is essential to drive breast cancer cell proliferation through cyclin D1. Cancer Res 73:4488–4499. doi: 10.1158/0008-5472.CAN-12-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisner A, Pazyra-Murphy MF, Durresi E, Zhou P, Zhao X, Chadwick EC, Xu P-X, Hillman RT, Scott MP, Greenberg ME, Segal RA. 2015. The Eya1 phosphatase promotes Shh signaling during hindbrain development and oncogenesis. Dev Cell 33:22–35. doi: 10.1016/j.devcel.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung S-K, Jeong DG, Chung SJ, Kim JH, Park BC, Tonks NK, Ryu SE, Kim SJ. 2010. Crystal structure of ED-Eya2: insight into dual roles as a protein tyrosine phosphatase and a transcription factor. FASEB J 24:560–569. doi: 10.1096/fj.09-143891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrick AN, Cabrera JH, Smith AL, Chen XS, Ford HL, Zhao R. 2013. Structure-function analyses of the human SIX1-EYA2 complex reveal insights into metastasis and BOR syndrome. Nat Struct Mol Biol 20:447–453. doi: 10.1038/nsmb.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin M, Jusiak B, Bai Z, Mardon G. 2013. Eyes absent tyrosine phosphatase activity is not required for Drosophila development or survival. PLoS One 8:e58818. doi: 10.1371/journal.pone.0058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong W, Dabbouseh NM, Rebay I. 2009. Interactions with the Abelson tyrosine kinase reveal compartmentalization of eyes absent function between nucleus and cytoplasm. Dev Cell 16:271–279. doi: 10.1016/j.devcel.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Sano T, Guan Y, Nagata S, Hoffmann JA, Fukuyama H. 2012. Drosophila EYA regulates the immune response against DNA through an evolutionarily conserved threonine phosphatase motif. PLoS One 7:e42725. doi: 10.1371/journal.pone.0042725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rayapureddi JP, Kattamuri C, Chan FH, Hegde RS. 2005. Characterization of a plant, tyrosine-specific phosphatase of the aspartyl class. Biochemistry 44:751–758. doi: 10.1021/bi0481794. [DOI] [PubMed] [Google Scholar]
- 59.Tootle TL, Lee PS, Rebay I. 2003. CRM1-mediated nuclear export and regulated activity of the receptor tyrosine kinase antagonist YAN require specific interactions with MAE. Development 130:845–857. doi: 10.1242/dev.00312. [DOI] [PubMed] [Google Scholar]
- 60.Amin NM, Lim S-E, Shi H, Chan TL, Liu J. 2009. A conserved Six-Eya cassette acts downstream of Wnt signaling to direct non-myogenic versus myogenic fates in the C. elegans postembryonic mesoderm. Dev Biol 331:350–360. doi: 10.1016/j.ydbio.2009.05.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirose T, Galvin BD, Horvitz HR. 2010. Six and Eya promote apoptosis through direct transcriptional activation of the proapoptotic BH3-only gene egl-1 in Caenorhabditis elegans. Proc Natl Acad Sci U S A 107:15479–15484. doi: 10.1073/pnas.1010023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda Y, Hatano S, Sentoku N, Matsuoka M. 1999. Homologs of animal eyes absent (eya) genes are found in higher plants. Mol Gen Genet 262:131–138. doi: 10.1007/s004380051067. [DOI] [PubMed] [Google Scholar]
- 63.Flint AJ, Tiganis T, Barford D, Tonks NK. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A 94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. 2009. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krishnan N, Jeong DG, Jung SK, Ryu SE, Xiao A, Allis CD, Kim SJ, Tonks NK. 2009. Dephosphorylation of the C-terminal tyrosyl residue of the DNA damage-related histone H2A.X is mediated by the protein phosphatase Eyes absent. J Biol Chem 284:16066–16070. doi: 10.1074/jbc.C900032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan B, Cheng L, Chiang H-C, Xu X, Han Y, Su H, Wang L, Zhang B, Lin J, Li X, Xie X, Wang T, Tekmal RR, Curiel TJ, Yuan Z-M, Elledge R, Hu Y, Ye Q, Li R. 2014. A phosphotyrosine switch determines the antitumor activity of ERβ. J Clin Invest 124:3378–3390. doi: 10.1172/JCI74085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 68.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. 2002. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev 12:162–169. doi: 10.1016/S0959-437X(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 69.Harper JW, Elledge SJ. 2007. The DNA damage response: ten years after. Mol Cell 28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, Ishibe-Murakami S, Wang B, Tempst P, Hofmann K, Patel DJ, Elledge SJ, Allis CD. 2009. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 457:57–62. doi: 10.1038/nature07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aydin ÖZ, Vermeulen W, Lans H. 2014. ISWI chromatin remodeling complexes in the DNA damage response. Cell Cycle 13:3016–3025. doi: 10.4161/15384101.2014.956551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. 2007. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 73.Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA, Gygi SP, Livingstone M, Zhang H, Polakiewicz RD, Comb MJ. 2007. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci U S A 104:19855–19860. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lavin MF, Kozlov S. 2007. ATM activation and DNA damage response. Cell Cycle 6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 75.Xu P-X, Adams J, Peters H, Brown MC, Heaney S, Maas R. 1999. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 76.Xu P-X, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. 2002. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development 129:3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baldi S, Becker PB. 2013. The variant histone H2A.V of Drosophila—three roles, two guises. Chromosoma 122:245–258. doi: 10.1007/s00412-013-0409-x. [DOI] [PubMed] [Google Scholar]
- 78.Kawashima T, Lorković ZJ, Nishihama R, Ishizaki K, Axelsson E, Yelagandula R, Kohchi T, Berger F. 2015. Diversification of histone H2A variants during plant evolution. Trends Plant Sci 20:419–425. doi: 10.1016/j.tplants.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Barnett C, Krebs JE. 2011. WSTF does it all: a multifunctional protein in transcription, repair, and replication. Biochem Cell Biol 89:12–23. doi: 10.1139/O10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnett C, Yazgan O, Kuo H-C, Malakar S, Thomas T, Fitzgerald A, Harbour W, Henry JJ, Krebs JE. 2012. Williams Syndrome Transcription Factor is critical for neural crest cell function in Xenopus laevis. Mech Dev 129:324–338. doi: 10.1016/j.mod.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitagawa H, Fujiki R, Yoshimura K, Oya H, Kato S. 2011. Williams syndrome is an epigenome-regulator disease. Endocr J 58:77–85. doi: 10.1507/endocrj.K10E-393. [DOI] [PubMed] [Google Scholar]
- 82.Lang J, Smetana O, Sanchez-Calderon L, Lincker F, Genestier J, Schmit A-C, Houlné G, Chabouté M-E. 2012. Plant γH2AX foci are required for proper DNA DSB repair responses and colocalize with E2F factors. New Phytol 194:353–363. doi: 10.1111/j.1469-8137.2012.04062.x. [DOI] [PubMed] [Google Scholar]
- 83.Singh I, Ozturk N, Cordero J, Mehta A, Hasan D, Cosentino C, Sebastian C, Krüger M, Looso M, Carraro G, Bellusci S, Seeger W, Braun T, Mostoslavsky R, Barreto G. 2015. High mobility group protein-mediated transcription requires DNA damage marker γ-H2AX. Cell Res 25:837–850. doi: 10.1038/cr.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santoro SW, Dulac C. 2015. Histone variants and cellular plasticity. Trends Genet 31:516–527. doi: 10.1016/j.tig.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan X, Brass LF, Poncz M, Spitz F, Maire P, Manning DR. 2000. The alpha subunits of Gz and Gi interact with the eyes absent transcription cofactor Eya2, preventing its interaction with the Six class of homeodomain-containing proteins. J Biol Chem 275:32129–32134. doi: 10.1074/jbc.M004577200. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Han N, Zhou S, Zhou R, Yuan X, Xu H, Zhang C, Yin T, Wu K. 9 April 2015. The DACH/EYA/SIX gene network and its role in tumor initiation and progression. Int J Cancer doi: 10.1002/ijc.29560. [DOI] [PubMed] [Google Scholar]
- 87.Blevins MA, Towers CG, Patrick AN, Zhao R, Ford HL. 2015. The SIX1-EYA transcriptional complex as a therapeutic target in cancer. Expert Opin Ther Targets 19:213–225. doi: 10.1517/14728222.2014.978860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tadjuidje E, Wang TS, Pandey RN, Sumanas S, Lang RA, Hegde RS. 2012. The EYA tyrosine phosphatase activity is pro-angiogenic and is inhibited by benzbromarone. PLoS One 7:e34806. doi: 10.1371/journal.pone.0034806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krueger AB, Drasin DJ, Lea WA, Patrick AN, Patnaik S, Backos DS, Matheson CJ, Hu X, Barnaeva E, Holliday MJ, Blevins MA, Robin TP, Eisenmesser EZ, Ferrer M, Simeonov A, Southall N, Reigan P, Marugan J, Ford HL, Zhao R. 2014. Allosteric inhibitors of the Eya2 phosphatase are selective and inhibit Eya2-mediated cell migration. J Biol Chem 289:16349–16361. doi: 10.1074/jbc.M114.566729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ye X, Weinberg RA. 2015. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haldosén L-A, Zhao C, Dahlman-Wright K. 2014. Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 382:665–672. doi: 10.1016/j.mce.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Okabe Y, Sano T, Nagata S. 2009. Regulation of the innate immune response by threonine-phosphatase of Eyes absent. Nature 460:520–524. doi: 10.1038/nature08138. [DOI] [PubMed] [Google Scholar]
- 93.Sano T, Nagata S. 2011. Characterization of the threonine-phosphatase of mouse eyes absent 3. FEBS Lett 585:2714–2719. doi: 10.1016/j.febslet.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 94.Welcker M, Orian A, Jin J, Grim JE, Grim JA, Harper JW, Eisenman RN, Clurman BE. 2004. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A 101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]