Abstract
Tyrosine hydroxylase (TH) catalyzes the rate-limiting step in the catecholamine (CA) biosynthesis pathway, making TH a molecular target for controlling CA production, specifically dopamine. Dysregulation of dopamine is correlated with neurological diseases such as Parkinson’s disease (PD) and post-traumatic stress disorder (PTSD), among others. Previously, we showed that a 49-nucleotide guanine (G)-rich sequence within the human TH promoter adopts two different sets of G-quadruplex (GQ) structures (5ʹGQ and 3ʹGQ), where the 5ʹGQ uses G-stretches I, II, IV, and VI in TH49, which enhances TH transcription, while the 3ʹGQ utilizes G-stretches II, IV, VI, and VII, which represses transcription. Herein, we demonstrated targeted switching of these GQs to their active state using rationally designed DNA GQ Clips (5ʹGQ and 3ʹGQ Clips) to modulate endogenous TH gene expression and dopamine production. As a translational approach, we synthesized a targeted nanoparticle delivery system to effectively deliver the 5ʹGQ Clip in vivo. We believe this strategy could potentially be an improved approach for controlling dopamine production in a multitude of neurological disorders, including PD.
Keywords: tyrosine hydroxylase, G-quadruplex, dopamine, Parkinson’s disease, nucleic acids therapeutics, nanoparticles, targeted drug delivery
Graphical abstract
Tyrosine hydroxylase (TH) is key to dopamine synthesis. Reduced dopamine level is linked to Parkinson’s disease. We developed an oligonucleotide-based strategy that enhances dopamine synthesis by targeting TH promotor in human neurons. Using a nanotechnology-based targeted delivery approach, we showed this strategy also works in an animal model, suggesting its therapeutic potential.
Introduction
Tyrosine hydroxylase (TH) is a member of pterin-dependent monooxygenases that catalyzes the rate-determining step of catecholamine (CA) biosynthesis.1 Specifically, TH catalyzes the hydroxylation of L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) and is highly conserved.2 The downstream products of the CA biosynthesis pathway, namely dopamine, epinephrine, and norepinephrine, are vital as hormones and neurotransmitters within the central and peripheral nervous systems. Catecholamines play important roles in brain functions, such as attention,3 memory,4 cognition,5 and emotion,6,7 making regulation of these biological molecules important in modulating neurological functions. For example, dopaminergic dysfunction in humans is associated with a variety of health conditions, including Parkinson’s disease (PD), post-traumatic stress disorder (PTSD), schizophrenia, depression, drug addiction, and attention deficient disorder (ADD).8,9 Within PD, several pathological events have been linked to dopamine dysfunction, such as elevated reactive oxygen species (ROS), through metal dysregulation, Lewy body formation, and transcriptional regulation of TH.10, 11, 12 The critical role of TH in dopamine biosynthesis makes transcriptional regulation of TH expression an important molecular target for controlling dopamine synthesis to assist in the treatment of several neurological disorders, including PD.
We and others previously demonstrated that a guanine-rich (G-rich) region within the TH promoter plays an instrumental role in controlling the expression of the downstream gene.9,13 G-rich nucleic acid sequences possessing four G-stretches of two or more consecutive Gs can rearrange themselves into square planar G-quartets. In the presence of monovalent cations such as potassium and sodium, G-quartets can stack on top of each other to form a non-canonical secondary structure called G-quadruplex (GQ).14 In many instances, potential GQ-forming sequences are located within important biological regions of the genome, such as the promoter regions and recombination sites.15,16 GQs within the promoter regions of many genes, such as PDGF-A,16,17 VEGF,18,19 c-MYC,20 KRAS,21 and BCL2,22,23 act as a transcriptional repressor. Thus, the GQs in the gene promoter have been the obvious targets for small-molecule ligands that can control the stability of GQs in order to modulate transcription.24,25 However, small-molecule targeting has yielded mixed results because of its limited capability to alter the stability of GQs and also due to its poor selectivity in the presence of other GQ targets.25,26 To overcome the paucity of specific GQ-recognizing ligands, we intend to rationally develop nucleic-acid-based therapeutics to precisely target the desired GQ regions of TH promoter to modulate its expression and hence control the dopamine synthesis in neurons.
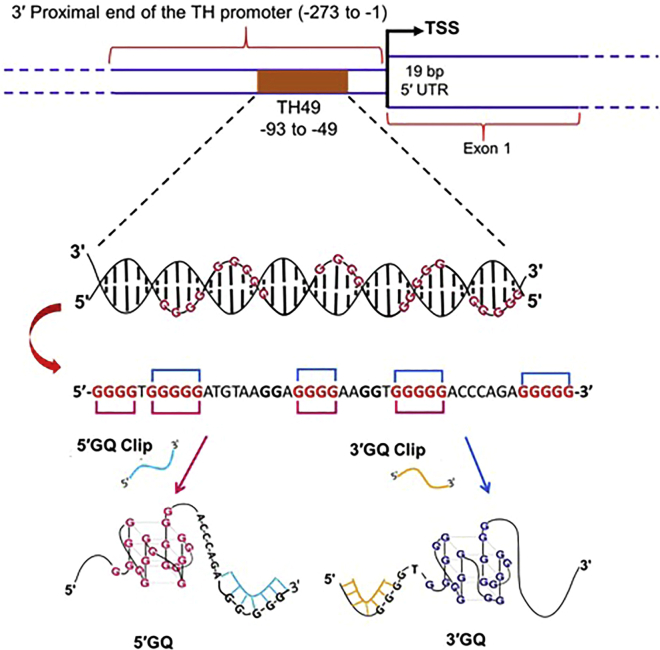
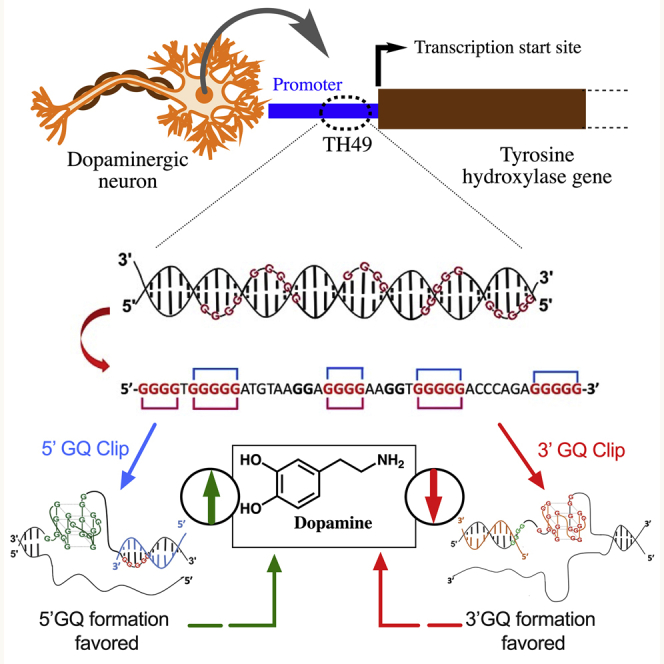
Previously, we reported that a 45-nucleotide (nt) G-rich sequence (TH49) within the 3ʹ end of the human TH promoter adopts two different sets of GQ structures (5ʹGQ and 3ʹGQ; Figure 1), where the 5ʹGQ structure enhances TH transcription, while the 3ʹGQ structure represses it.13 To exploit this unique feature of the GQ-forming region to modulate dopamine level, we created a novel DNA-molecule-based strategy that can regulate target GQ formation. Our designed GQ-targeting DNA Clips (GQ Clips) are complementary to a segment of the TH49 sequence in the TH promoter, as illustrated in Figure 1. Thus, binding of the engineered GQ Clip to the targeted G-stretches within TH49 blocks the formation of a particular GQ in the TH promoter and, in turn, favors the formation of the other GQ.
Figure 1.
Schematic representation of the position of TH49 within the tyrosine hydroxylase (TH) promoter and our strategy of blocking of a specific GQ formation in the TH promoter using GQ Clips
While the delivery of the DNA molecules to neuronal cells is difficult, targeted delivery of such sequences to the specific cells within the animal brain is undoubtedly more challenging. Nanoparticle drug delivery has been used as a way to overcome the limitations individual therapeutics may possess to improve upon drug retention, protection, and circulation.27 Specifically, gold nanoparticle and DNA nanoconjugates, referred to as spherical nucleic acids (SNAs), have displayed success in protection against DNA degradation and prolonged bioavailability for the treatment of various cancers.28 The addition of targeting moieties allows for specific cellular uptake, which is highly important for disease treatment, and the adjacent cellular entry pathway that can circumvent poor uptake of large, highly negatively charged DNA molecules.29 To harness the therapeutic advantage in terms of both targeting and efficient delivery, a nanoparticle delivery system was synthesized to deliver the 5′GQ Clip to dopaminergic neurons via targeting with a TrkB peptide aptamer, which exhibited a significantly improved response in cellulo compared to 5ʹGQ Clip only. Additionally, using a transgenic rat model in which the human TH promoter drives the expression of the reporter gene GFP, we were able to enhance GFP production in the rat brain. By being able to modulate the TH promoter activity, we believe this nucleic acid drug delivery system can potentially be an effective mechanism for controlling dopamine production, which eventually can help to treat various neurological disorders, especially PD, while potentially reducing the side effects commonly seen with current treatments.
Results
GQ Clip sequences bind and influence GQ formation within the human TH promoter
The TH49 sequence within the human TH promoter contains seven G-stretches and adopts two major GQ structures, named 5′GQ and 3′GQ, which play distinct roles in controlling TH transcription. However, G-stretches III and V are not involved for the formation of either 5′GQ or 3′GQ (Figure 1).13 While the 5′GQ structure enhances the cellular TH transcription, 3′GQ structure acts as a repressive element. Here, we have rationally designed two different DNA molecules, named GQ Clips, to specifically block the formation of either the 5′ or the 3′GQs within TH49 to favor the formation of one or the other GQ. For example, the 5′GQ Clip can bind with 3′ end of the sequence where G-stretch VII is located. Given that G-stretch VII is essential for the formation of 3′GQ, binding of 5′GQ Clip blocks the 3′GQ structure formation and in turn favors the formation of the 5′GQ structure. Meanwhile, the 3′GQ Clip can bind with 5′ end of the sequence where G-stretch I is located. Thus, 3′GQ Clip blocks the 5′GQ structure formation in the TH promoter, since 5′GQ structure cannot be formed without G-stretch I (Figure 1). The 3′ and 5′ GQ Clips are 20- and 21-nucleotide-long oligos, respectively, with phosphorothioate modifications at each end to increase stability against nucleases.
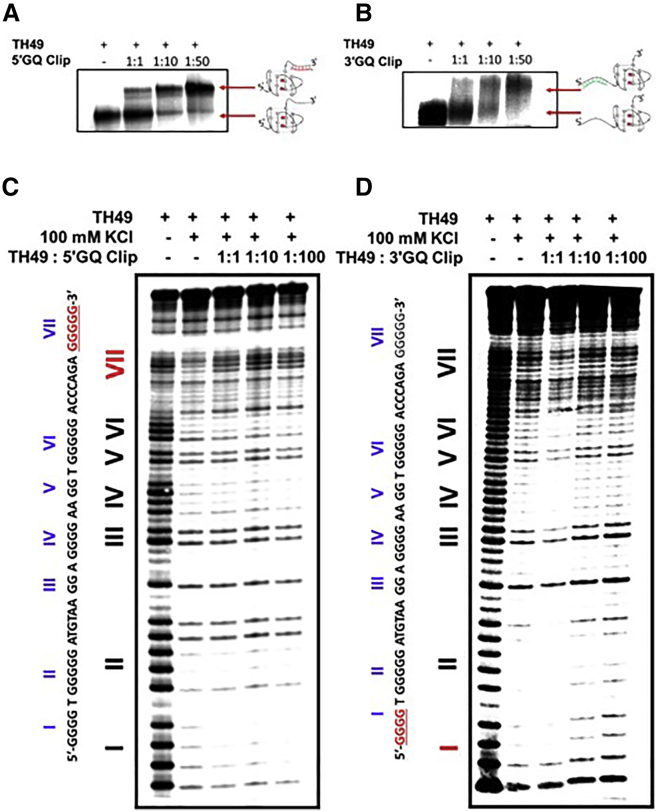
We performed a non-denaturing electrophoretic gel mobility shift assay (EMSA) to determine whether the GQ Clips can bind to the TH49 sequence and interfere with the GQ folding, which should result in changes in the migration patterns (Figures 2A and 2B). The ability of both GQ Clips (5′GQ Clip and 3′GQ Clip) to interact with TH49 was tested with increasing amounts of GQ Clip in the presence of 100 mM K+. As shown in Figure 2A, the leftmost lane shows the mobility of the native GQ adopted by the TH49 sequence in the absence of GQ Clips. As expected, in the presence of an increasing amount of 5′GQ Clip, another DNA species emerges, which has a slower mobility than the native TH49 GQs representing complexation of the 5′GQ Clip with TH49 GQ. The binding is more prominent as the concentration of the 5′GQ Clip increases, moving the equilibrium toward the slower-moving band (Figure 2A, lanes 2–4). Binding of the 3′GQ Clip to wtTH49 was also observed in Figure 2B, displaying a similar affinity but for the 5′ end of the TH49 sequence.
Figure 2.
GQ Clips bind to TH49 to elicit structural changes
(A and B) DNA Clips bind to TH49 in a dose-dependent fashion. Native PAGE of TH49 GQ (1 μM) in the absence and presence of increasing concentrations of 5′GQ Clip or 3′GQ Clip (0, 1, 10, and 50 μM). (C) The 5′GQ Clip blocks the formation of 3′GQ structure. DMS structural mapping of wtTH49 in the presence of systematically increasing the concentration of 5′GQ Clip was performed. Lane designations are as follows: lane 1, DMS probing at 0 mM K+; lane 2, DMS probing at 100 mM K+; lanes 3–5, DMS structural mapping in the presence of increasing concentrations of 3′GQ Clip at 100 mM K+. The TH49 sequence is shown on the left side and the G-stretches in the sequence and corresponding bands in the gel are labeled in red letters. (D) The 3′GQ Clip blocks the formation of 5′GQ structure. DMS structural mapping was repeated with the 3′GQ DNA Clip. The TH49 sequence is shown on the left side and the G-stretches in the sequence and corresponding bands in the gel are labeled in red letters.
Once initial binding of the GQ Clips with the TH49 sequence was confirmed, we performed dimethyl sulfate (DMS) structural mapping to determine the capability of the GQ Clips to block the targeted GQ within the TH49 and, in turn, promote the formation of the other GQ. The N7 of guanine in a B-form duplex DNA is not involved in Watson-Crick hydrogen bonding in GC base pairs. Therefore, the N7 position of guanine in the duplex and single-stranded DNA can be methylated by DMS, leading to subsequent DNA strand cleavage upon treatment with piperidine.30 However, in the context of a GQ, N7 of each guanine is hydrogen-bonded to N2 of the neighboring guanine to form a tetrad. Consequently, the N7 of such guanines are protected from the DMS methylation and the subsequent piperidine cleavage. The DMS footprinting of TH49 DNA in the presence of increasing 5′GQ Clip concentration is shown in Figure 2C. The leftmost lane, a no-KCl control, displays an unstructured sequence where all guanines in the sequence appear to be susceptible to DMS modification. This is expected, since the formation of GQ requires K+ as a monovalent cation for stabilization.31 This is the opposite of lane 2, as the structured TH49 is observed due to the presence of 100 mM K+. All five G-stretches (I, II, IV, VI, and VII) that are involved in the formation of the GQ structures are highly protected. However, the gradual increase of the intensity of bands corresponding to G-stretch VII was witnessed in the presence of 5′GQ Clip (Figures 2C, lanes 3–5, and S1), implying increasing deprotection of this particular G-stretch. Given that the G-stretch VII is only involved in the formation of 3′GQ structure, this observation strongly suggested that the binding of 5′GQ Clip to TH49 blocks the 3′GQ from adopting its conformation, and, as a result, the guanines in the G-stretch VII are increasingly modified by DMS. On the other hand, the intensity of bands corresponding to G-stretch I gradually increased with increasing 3′GQ Clip concentration in Figures 2D and S2. Since G-stretch I is only involved in the formation of 5′GQ structure, this observation provides clear evidence for the induction of 3′GQ structure by the 3′GQ Clip. Overall, the DMS assay provides strong evidence that the 5′GQ Clip favors the formation of the 5′GQ structure by blocking the adoption of 3′GQ in the G-rich region of the TH promoter and, on the other hand, the 3′GQ Clip binds with 5′ end of the TH49 sequence and blocks the 5′GQ formation.
To further assess the ability of GQ Clips to modulate target GQ formation, we designed mutated TH49 sequences (Table 1). The mutated TH49 sequences are such that, if the targeted GQ modulation is working as proposed, TH49 5′GQ mutant should not be able to form a GQ when treated with 5′GQ Clip, and TH49 3′GQ mutant should not be able to form a GQ upon the treatment with 3′GQ Clip (Figure S3). Indeed, that was exactly what we observed (Figure S4A). When TH49 5′mut is treated with 5′GQ Clip sequence, it loses its GQ feature (CD peak shifts from 263 nm to 270 nm), but there was no shift with the 3′GQ Clip treatment. On the other hand, the treatment of TH49 3′GQ mutant with 3′GQ Clip caused the reduction in the peak intensity of the GQ signal, but that was not the case with the treatment of TH49 3′GQ mutant with 5′GQ Clip (Figure S4B). To further test whether the reduction in the GQ peak intensity (263 nm) of TH49 3′GQ mutant upon treatment with 3′GQ Clip is truly due to their binding, we analyzed their CD behavior in two different Clip concentrations. As demonstrated in Figure S4C, we observed a concentration-dependent reduction in peak intensity of TH49 3′GQ mutant indicating the ability of 3′GQ Clip to bind to the target region of TH49 and modulate its GQ formation.
Table 1.
Oligodeoxynucleotide sequences used in the study
| Oligo name | Sequence |
|---|---|
| 3′GQ Clip | 5′-C∗C∗TGCCTGCTGTGCCTGAT∗G∗G-3′ |
| 5′GQ Clip | 5′-G∗C∗TGACGTCAAAGCCCCC∗T∗C-3′ |
| wtTH49 | 5′-CCATCAGGCACAGCAGGCAGG GGTGGGGGATGTAAGGAG GGGAAGGTGGGGGACCCA GAGGGGGCTTTGACGTCAGC-3′ |
| TH49 5′GQ mut | 5′-CCATCAGGCACAGCAGGCACATC TGGGGGATGTAAGGAGGGGA AGGTGGGGGACCCAGAGGGGGC TTTGACGTCAGC-3′ |
| TH49 3′GQ mut | 5′-CCATCAGGCACAGCAGGCAGGG GTGGGGGATGTAAGGAGGGGA AGGTGGGGGACCCAGAGCATC CTTTGACGTCAGC-3′ |
| Promotor control | 5′-G∗C∗ACGAGCCCCTGGTCCC∗C∗G-3′ |
| Scramble control | 5′-N∗N∗NNNNNNNNNNNNNNNN∗N∗N-3′ |
An asterisk (∗) denotes phosphorothioate modification, which renders the oligonucleotide exonuclease resistant.
The data presented herein, namely, the electromobility gel shift assay, the DMS footprints, and CD data, demonstrated the ability of engineered Clip sequences to modulate target GQ formation in TH49 sequence.
Cellular TH expression is controlled with the treatment of GQ Clip sequences
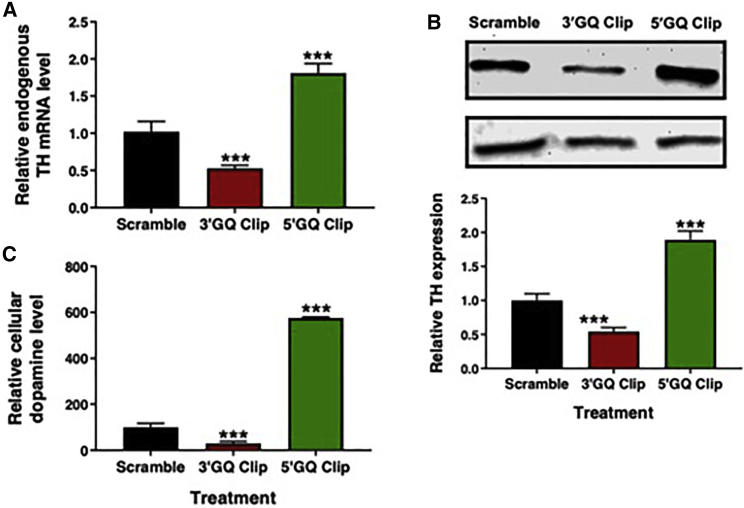
We wanted to test whether the in vitro modulation of TH49 GQs by DNA Clips can be replicated in the cells. Targeting the GQ region located proximal to the transcription initiation site (TIS) within the TH promoter should modulate the endogenous TH mRNA level. To investigate this, we transfected the GQ Clips (Table 1) in the human neuroblastoma (SH-SH5Y) cells. As shown in Figure 3A, the 3′GQ Clip reduced the endogenous TH mRNA level by 2-fold as compared to cells transfected with a scrambled control. This is presumably accomplished by blocking the 5′GQ structure, which in turn favors the formation of 3′GQ. Importantly, an increase of endogenous TH mRNA level (2-fold) was detected in cells that were treated with the 5′GQ Clip (Figure 3A). Blocking of 3′GQ structure favors the formation of the 5′GQ structure, which enhances TH transcription, resulting in the synthesis of more mRNA.
Figure 3.
GQ Clips can modulate TH expression and dopamine production in dopaminergic neurons
(A) The rationally designed GQ Clips modulate the endogenous TH transcription in human SH-SY5Y cells. Histograms representing the ratio of endogenous TH to GAPDH mRNA levels in SH-SY5Y cells. The mRNA level in the cells treated with scramble Clip was set to 1, and mRNA from GQ Clip-treated cells were normalized accordingly (data represent mean values ± the standard error of the mean (SEM); n = 9; ∗∗∗p < 0.001 from a Student’s t test). (B) GQ Clips modulate the endogenous TH protein expression. TH protein levels in SH-SY5Y cells were treated with 5′GQ Clip, 3′GQ Clip, and scrambled Clip as the control oligonucleotide. Western blot of the TH protein expression levels when treated with various oligonucleotides. Histogram representing densitometry analysis of the western blot image was performed using ImageJ software. The TH bands were normalized with corresponding GAPDH bands to correct for experimental loading errors. (C) Targeted control of endogenous dopamine production in SH-SY5Y cells by GQ Clips. Cellular dopamine levels in SH-SY5Y cells treated with 5′GQ Clip, 3′GQ Clip, and scrambled Clip sequences. The dopamine level of 2 × 106 cells was measured using HPLC equipped with ECD. The histogram represents a quantitative analysis of the cellular dopamine level. Data represented as mean ± SEM, and the significance of the data was determined by t test analysis (∗∗∗p < 0.001).
Next, western blot was used to measure TH protein expression in SH-SY5Y cells that were treated with the GQ Clips (Figure 3B). A 30% reduction in the TH protein expression was detected in the cells treated with the 3′GQ Clip, and the 5′GQ Clip increased TH expression by 60% as compared to the scrambled control. Thus, the quantitative RT-PCR (qRT-PCR) and western blot results confirmed the modulation of TH transcription by GQ Clips presumably by ensuring the formation of specific GQ structures in the TH promoter, which affects the TH protein production in the SH-SY5Y cells. In addition to the scramble control, we used a second control, which we termed as promoter control sequence, designed to bind within the TH promoter upstream of the targeted TH GQ region while not hindering other important stretches that are known to involve in the regulation of transcription (Figure S5).
Dopamine synthesis in the neuronal cells can be modulated with GQ Clips
Since the TH enzyme catalyzes the rate-limiting step in the catecholamine biosynthesis pathway, it was important to investigate whether the modulation of TH expression by the GQ Clips affects the synthesis of dopamine, a neurotransmitter that is produced in the subsequent step. For this, cellular analytes were isolated from the Clip-treated cells, and the relative presence of dopamine was measured using high-performance liquid chromatography (HPLC) separation followed by electrochemical detection. Significant changes in the cellular dopamine level were observed in the GQ Clip-treated cells (Figures 3C and S6). Treatment with the 3′GQ Clip displayed a 3-fold reduction in the dopamine level, while the 5′GQ Clip-treated cells showed a 5-fold increase. This suggests a novel therapeutic DNA sequence, which is capable of altering not only cellular TH mRNA and protein but also cellular dopamine levels by controlling the rate-limiting enzyme in the catecholamine biogenesis pathway using rationally designed exogenous agents. Several neurological disorders are associated with abnormal dopamine levels, making this a viable approach for correcting the dopamine level to elicit a therapeutic response.
Synthesis and characterization of a nanoparticle delivery system to improve the therapeutic potential of 5ʹGQ Clip
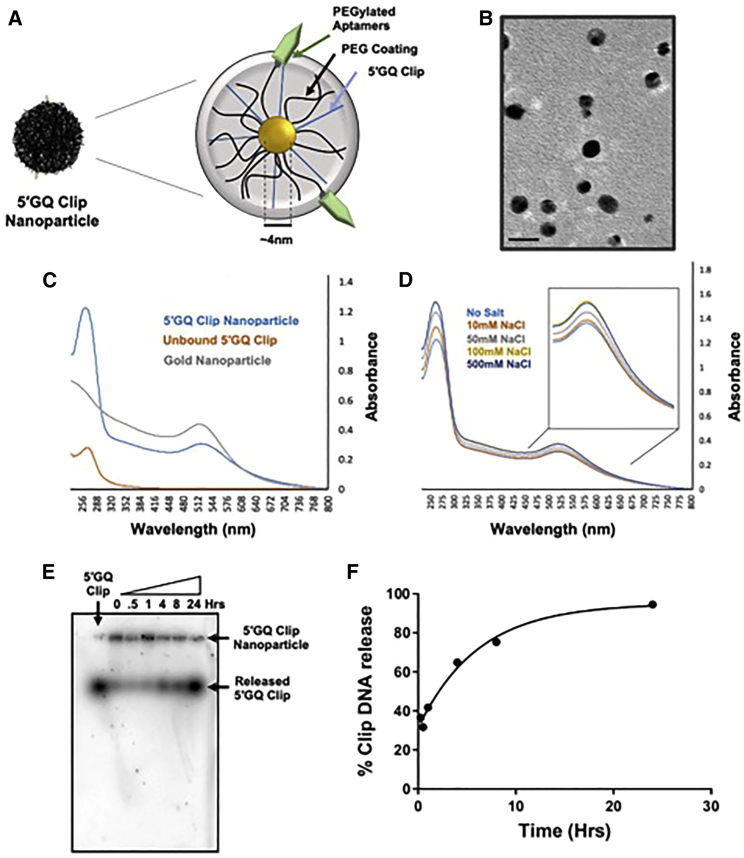
Based on our in vitro and in cellulo success of modulating TH expression using the GQ Clips, we wanted to advance this approach into an in vivo model for the potential treatment of neurological diseases, such as PD, where the 5′GQ Clip could be used to increase dopamine synthesis. Transfecting DNA molecules for therapeutic purposes is not a viable option for delivering nucleic acid agents into the neurons of an animal brain. Thus, to take full therapeutic advantage of the GQ Clips, we designed a nanoparticle complex to create a translation method for targeted neuronal uptake. To accomplish this, a nanoparticle complex (5ʹGQ Clip nanoparticle) was synthesized, comprising polyethylene glycol (PEG)-coated gold nanoparticle (AuNP) as a central tethering agent, PEGylated tyrosine receptor kinase B (TrkB), and transferrin receptor 1 (Tfr1) aptamers, and the 5′GQ Clip molecules (Figure 4A, S7–S13; Tables S1 and S2). Targeting aptamers have been paired with nanotechnology to particularly bypass negative pharmacokinetic characteristics previously associated with nucleic acid delivery.32 Utilizing this strategy, we believe the synthesized dual aptamer nanoparticle conjugate can be used for systemic injection of the 5′GQ Clip nanoparticle, which will cross the blood-brain barrier and be selectively taken up by dopaminergic neurons. For this purpose, we used a previously reported nucleic acid aptamer for binding to Tfr1 for brain uptake, and to target dopaminergic neurons we used a previously reported 18 amino acid sequence targeted to the TrkB receptor that is highly expressed on the cell surface.33, 34, 35 Transmission electron microscopy (TEM) images of 5ʹGQ Clip nanoparticle displayed the AuNPs were 4.5 ± 1 nm in size, which are advantageous for the passive excretion from the brain36 and the body37 based on selective sizing (Figure 4B). UV spectroscopy and gel electrophoresis were used to quantify DNA loading and stability of the 5′GQ Clip nanoparticle, monitored at 260 nm and 515 nm, respectively (Figures 4C, 4D, and S14). The characteristic AuNP 515 nm peak was determined to remain the same with increasing concentrations of NaCl indicating the enhanced stability of the nanoparticle complex, whereas a redshift would have indicated nanoparticle aggregation.
Figure 4.
Synthesis of a 5′GQ Clip nanoparticle system for the targeted delivery of 5ʹGQ Clip
(A) Schematic of targeted clip nanoparticle for treatment of dopaminergic neurons. (B) TEM of AuNP. The black bar represents 10 nm. (C and D) UV-vis spectroscopy was used to determine the loading and stability of the 5ʹGQ Clip nanoparticle. The absorbance of DNA at 260 nm was measured to quantitate conjugation onto the nanoparticle complex, as after conjugation and purification the increase in intensity was used to measure loading efficiency (~85%). The 5ʹGQ Clip nanoparticle was incubated in increasing concentrations of NaCl to assess the stability of the complex in salt. Through the addition of 500 mM NaCl, no redshift or broadening of the 515 nm AuNP-specific peak was observed, which both resemble nanoparticle aggregation. (E and F) In vitro release of Clip DNA from 5ʹGQ Clip nanoparticle via increased glutathione level. Glutathione has an affinity for the gold surface (ligand-metal interactions between thiol and gold), replacing the thiolated DNA. The DNA was radiolabeled, and the higher band resembled bound DNA to the nanoparticle complex, while the lower band was released DNA after being incubated in 10 mM glutathione for various time points. The bands were quantified using ImageJ software and the data plotted.
By using thiolated nucleic acids for AuNP conjugation, an inherent intracellular release mechanism is created where elevated intracellular levels of glutathione (GSH) can compete and replace ligands at the AuNP surface. We found that the 5′GQ Clip released in a time-dependent manner in the presence of 10 mM GSH, which is the average intracellular concentration (Figures 4E and 4F). The bands of the gel in Figure 4E were quantitated and plotted to generate a release curve (Figure 4F). The use of radiolabeled DNA allowed the detection of two distinct 5′GQ Clip bands. The upper slower-migrating band correlated with the DNA still attached to the nanoparticle, while the faster-migrating band corresponded to the released 5′GQ Clip. Within the first hour, 50% of the 5′GQ Clip was released from the nanoparticle complex, and after 24 h nearly all of the DNA was released.
Cellular uptake of 5′GQ Clip nanoparticle is dependent on TrkB aptamer
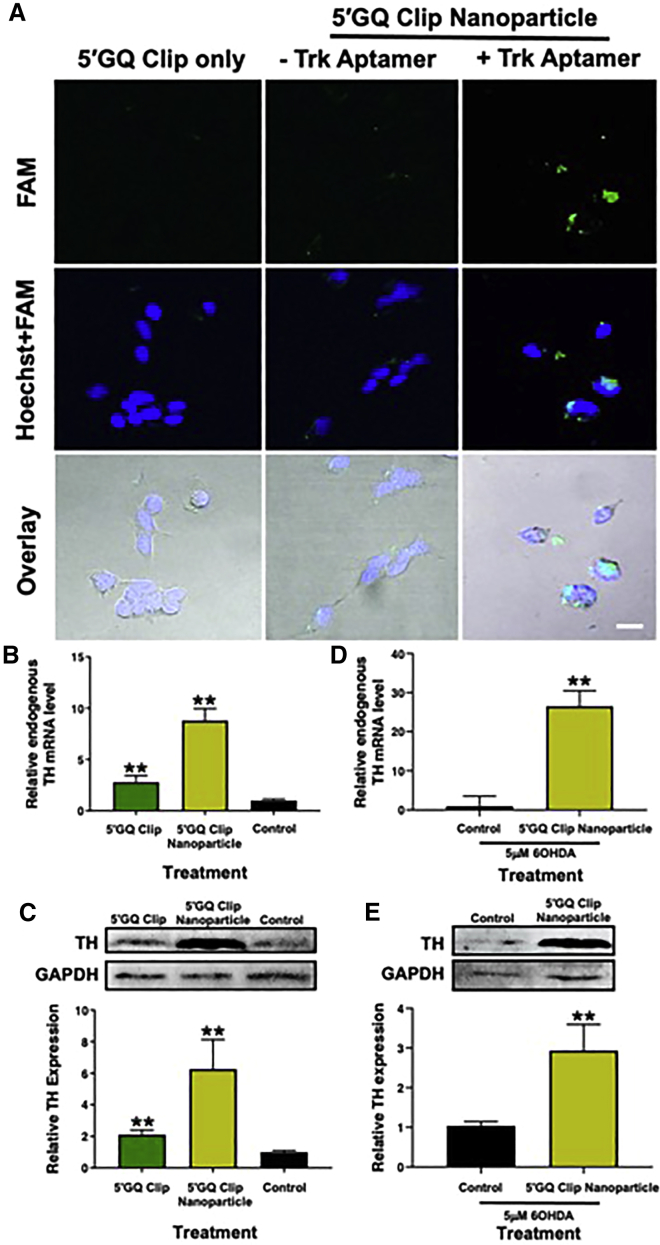
To visualize the improved uptake of the 5′GQ Clip sequence while attached to the nanoparticle complex, a fluorescently labeled (FAM) 5′GQ Clip sequence was used. Confocal microscopy images illustrate the uptake of the 5′GQ Clip sequence (no transfection), a no-aptamer 5′GQ Clip nanoparticle complex, and the 5′GQ Clip nanoparticle complex containing the TrkB aptamer in SH-SY5Y cells (Figure 5A). The 5′GQ Clip nanoparticle complex was observed to have dramatically higher cellular uptake when compared to the other two treatments. Specific binding to the receptor via the TrkB aptamer is expected to initiate receptor-mediated endocytosis, which helps the internalization of these larger complexes.38 These images highlight not only the ability of the 5′GQ Clip nanoparticle to enter the cells but also the incredibly limited uptake exhibited by both the free DNA and the no-aptamer nanoparticle. Both DNA and the no-aptamer 5′GQ Clip nanoparticle complex are large and highly charged, presumably making it challenging to enter the cell through non-receptor-mediated endocytosis mechanisms.39
Figure 5.
5ʹGQ Clip nanoparticle improves cellular efficacy
(A) Neuronal uptake of 5ʹGQ Clip nanoparticle is dependent on the Trk-B aptamer for successful cellular recognition. SH-SY5Y cells were incubated with 270 nM free fluorescently labeled 5′GQ Clip, no-aptamer 5′GQ Clip nanoparticle, or 5ʹGQ Clip nanoparticles (with TrkB). After 24 h, the TrkB aptamer was shown to be essential for cellular uptake presumably through receptor-mediated endocytosis and eventually reaching the nucleus. Scale bar: 10 μM. (B and C) 5ʹGQ Clip nanoparticle dramatically increases the expression of TH. SH-SH5Y cells were treated with 270 nM 5′GQ Clip nanoparticle and 5′GQ Clip for 24 h. Cellular RNA and protein were extracted, and the TH expression was normalized to GAPDH showing the nanoparticle complex increased expression by 9-fold and 6-fold, respectively. Data represented as mean ± SEM (∗∗p < 0.01). (D and E) 5ʹGQ Clip nanoparticle treatment can improve TH expression in disease-state cells. SH-SH5Y cells were treated with 270 nM 5ʹGQ Clip nanoparticle in the presence of 5 μM 6OHDA for 24 h. Similar to (A) and (B), qRT-PCR and western blot were used to verify the nanoparticle complex can increase mRNA and protein expression of TH 3-fold in the presence of high oxidative stress mimicking PD disease state. Data represented as mean ± SEM (∗∗p < 0.01).
Clip nanoparticle improved the functional efficiency of 5′GQ Clip in human neuronal cells
A large obstacle in nucleic acid therapeutics and drug delivery is cellular specificity and uptake. Oligonucleotide drugs, such as small interfering RNA (siRNA), anti-sense DNA, and micro-RNA, are handicapped by poor cellular uptake due to high overall net negative charge and large size, making pinocytosis less effective.32 To determine the possible therapeutic advantage of the 5′GQ Clip nanoparticle, initially the change in endogenous TH mRNA and protein levels were investigated. As seen before, the 5′GQ Clip increased TH expression by 2-fold, but the 5′GQ Clip nanoparticle caused a 9-fold increase when both were treated at the same concentration of 270 nM (Figure 5B). Western blot analysis of the cell lysates from 5′GQ Clip only and 5′GQ Clip nanoparticle-treated cells revealed similar results, where a 2-fold increase in TH protein expression occurred with the treatment of the 5′GQ Clip only, while the 5′GQ Clip nanoparticle contributed to an average of a 6.5-fold increase in TH protein expression (Figure 5C). Two other nanoparticle complexes were also synthesized as controls to test the necessity for the simultaneous presence of the 5′GQ Clip and TrkB aptamer on the 5′GQ Clip nanoparticle for it to be fully functional. The first nanoparticle complex replaced the 5′GQ Clip with a scrambled DNA sequence (scrambled Clip nanoparticle) but still possessed the TrkB aptamer. The second complex was a no-aptamer 5′GQ Clip nanoparticle that had the 5′GQ Clip therapeutic, PEG, and AuNP but no TrkB aptamer. Western blot analysis of the cell lysate after the treatment displayed the scramble Clip nanoparticle had no relative difference in protein expression compared to the untreated control, while the no-aptamer complex had a slight increase (1.5-fold) (Figure S15).
5′GQ Clip nanoparticle can increase TH expression in a toxin-induced PD cell model
PD is one of the most common and life-altering neurodegenerative diseases, involving the dysregulation of dopamine due to neuronal death.40 6-hydroxydopamine (6OHDA) is a neurotoxin that when supplemented in cellular growth medium promotes intracellular ROS and cell death mimicking PD.41 To examine the effect of how the 5′GQ Clip nanoparticle can modulate TH in a neurotoxin-mediated stressed environment, SH-SY5Y cells were treated with 5 μM 6OHDA with and without the 5′GQ Clip nanoparticle. In the presence of the 5′GQ Clip nanoparticle, the TH mRNA level was 25-fold higher in relation to the neurotoxin-only-treated cells (Figure 5D). Similarly, TH protein expression of the 5′GQ Clip nanoparticle-treated cells with 6OHDA was 3-fold higher than the neurotoxin treatment control (Figure 5E). Analyses of qRT-PCR and western blot were further performed with increasing 5′GQ Clip nanoparticle concentrations (270 nM and 540 nM) to observe if there was a dose-dependent response to the treatment. We observed that a 2-fold increase in 5′GQ Clip nanoparticle concentration indeed enhanced TH mRNA expression by ~2-fold. In relation to the no-treatment control and 6OHDA treatment, the 540 nM concentration saw a 5-fold and 20-fold increase in TH mRNA, respectively (Figure S16). In terms of protein expression, a ~2-fold increase was observed at 540 nM compared to the 270 nM treatment (Figure S17). This highlights that the system offers potential controllability in increasing TH expression as needed. Subsequently, SH-SY5Y cells were incubated with increasing concentrations of 6OHDA with and without 540 nM 5′GQ Clip nanoparticle to ensure that the nanoparticle did not perpetuate the cytotoxic effect of the disease state. The cellular viability assay displayed the opposite effect where the nanoparticle complex decreased 6OHDA toxicity by 1.5-fold (25 μM to 40 μM) (Figure S18). Thus, it is clear that the 5′GQ Clip nanoparticle provides a level of protection against the 6OHDA-mediated toxicity.
TH-GFP can be modulated by 5′GQ Clip nanoparticle in vivo
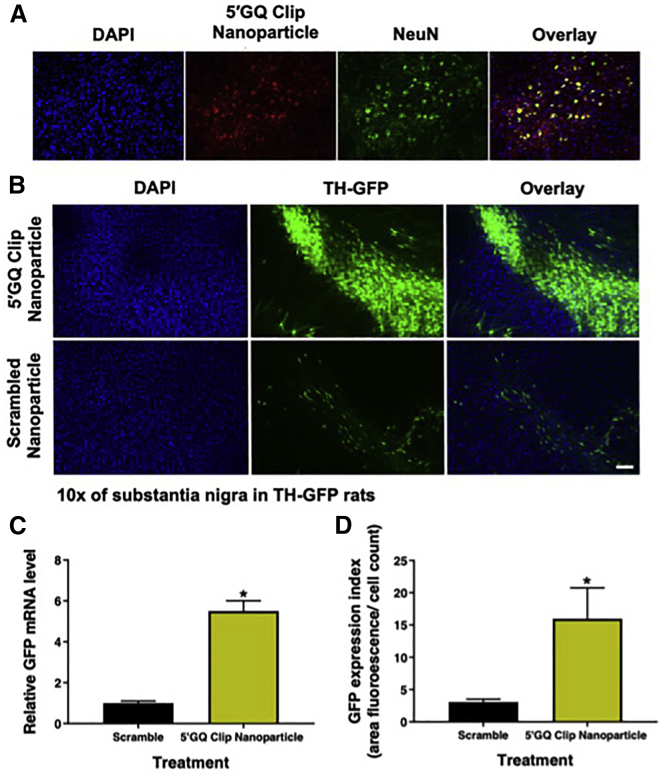
To achieve the desired effect on neurological disorders, targeted neuronal uptake is necessary for therapeutic efficacy, specifically being able to localize into neurons of the substantia nigra. To examine this, the 5′GQ Clip nanoparticle was subjected to in vivo experimentation to test if the design of the drug delivery complex could indeed reach the dopaminergic regions of an animal brain. Rats were intravenously injected via the tail vein with 10 μg of hexachlorofluorescein (HEX)-labeled 5′GQ Clip nanoparticle and sacrificed after 24 h. Rat brains were harvested, fixed, and sliced into 20 μm sections that contained the substantia nigra region containing the targeted dopaminergic neurons. Using confocal microscopy, HEX staining was observed across the slices, and higher magnification images showed HEX staining colocalized with neurons (NeuN-positive cells) (Figure 6A). This demonstrates the ability of the 5′GQ Clip nanoparticle to not only enter the brain but also target the dopaminergic neurons (as seen in Figure 5A). This was a highly encouraging result, given that many neurological drugs encounter the common lacunae of the inability to cross the BBB.
Figure 6.
5′GQ Clip nanoparticle is taken up by neurons and can increase the expression of GFP in TH-GFP transgenic rat model
(A) Confocal fluorescent images display successful cellular recognition and uptake of the 5′GQ Clip nanoparticle complex in rat brains. Rats were treated with 10 μg of total 5′GQ Clip DNA (conjugated to the 5ʹGQ Clip nanoparticle). After 24 h, cellular uptake in the substantia nigra of treated rats. (B) Confocal fluorescent images show successful upregulation of GFP through targeting of the TH promoter by the 5′GQ Clip nanoparticle complex in rat brains. Transgenic TH-GFP rats were treated with 10 μg 5′GQ Clip or scrambled nanoparticles. After 24 h, GFP expression was assessed in the substantia nigra of treated rats. The activity of the TH promoter is shown to greatly increase in the presence of the 5′GQ Clip compared to the scramble sequence (or endogenous GFP expression). Scale bar: 100 μM. (C) 5′GQ Clip nanoparticle treatment in TH-GFP rats shows a 5-fold increase in GFP mRNA expression. Cellular RNA was extracted, and qRT-PCR was used to quantify GFP mRNA concentrations compared to GAPDH. (D) GFP protein expression is increased with 5′GQ Clip nanoparticle treatment in TH-GFP rats. Quantification of GFP within rats (n = 3) of the substantia nigra shows an average of a 5-fold increase in fluorescence per cell. Data represented as mean ± SEM (∗p < 0.05).
With successful uptake into neurons, the therapeutic relevance of the 5′GQ Clip nanoparticle was next assessed in a transgenic TH-GFP rat model. The human TH promoter sequence is not conserved in the rat or mouse genome, so we used a commercially available model developed by the Lacovitti lab.9,42 This model utilized GFP expression driven by the human TH gene promotor that encompasses the 49-nucleotide region targeted by the 5′GQ Clip. It has been previously reported that GFP expression is exhibited with high specificity to dopaminergic regions, including the substantia nigra and striatum.42 This model allows us to modulate reporter GFP expression using our 5′GQ Clip nanoparticle. The TH-GFP rats were treated intravenously with 10 μg of 5′GQ Clip nanoparticle with 5′GQ Clip or scrambled DNA and sacrificed after 24 h. Specific brain areas were studied using immunofluorescence staining and imaging of the respective tissue sections. The 5′GQ Clip nanoparticle caused a dramatic increase in GFP expression, suggesting the 5′GQ Clip’s ability to target the human TH promoter, thereby increasing the expression of the linked transgene (Figures 6B, 6D, and S19). Neurons within the entirety of substantia nigra displayed higher fluorescence, aligning with the data in Figure 6A, where 5′GQ Clip nanoparticle uptake was observed in the majority of the neurons. ImageJ software was used to further assess the changes in fluorescence between the two treatments. The total area of fluorescence was normalized against the number of nuclei (>1,500 nuclei per image), displaying a robust 5-fold increase in GFP fluorescence.
Further analysis was performed to examine the mRNA levels in the presence of each treatment from brain slices containing the substantia nigra. qRT-PCR showed the 5′GQ Clip nanoparticle-treated rats had over a 5-fold increase in mRNA GFP expression when compared with scrambled Clip nanoparticle-treated rat tissue (Figure 6C). Successful increase in GFP mRNA and protein expression in vivo establishes that the 5′GQ Clip nanoparticle complex can not only reach neurons within the substantia nigra but also be potentially used as a viable system to modulate TH and therefore dopamine in an in vivo system for the treatment of PD and other dopaminergic diseases.
Discussion
The human TH promoter harbors a unique biochemical region that allows precise control of endogenous TH expression and therefore dopamine production, providing an opportunity to improve upon current therapeutic approaches. This precise control is inherent to the ability of the GQ Clips to bind and regulate the specific GQ formation within the TH promoter. Nucleic acid therapeutics provide a high level of specificity, but major challenges exist in the realm of cellular targeting and uptake. Here, we have introduced a novel DNA-based approach, with a translational nanoparticle delivery system, to control specific GQ formation within the human TH promoter and, in turn, the TH expression culminating in up- or downregulation of dopamine production. Our in vitro and in vivo studies described in this report have laid the groundwork for the advancement of GQ Clip-centric neurological disease therapy, such as PD, where the 5′GQ Clip could be used to enhance dopamine level.
The GQ structure has attracted interest as a therapeutic target due to the diverse roles it plays at different stages in gene regulation,20,43, 44, 45 with profound implications in many human diseases, such as cancer,25,46 diabetes,47,48 neurodegeneration,13,49,50 and cardiovascular disease.51 The most common approach for targeting GQs has been the use of small-molecule ligands, with the intention to differentiate GQs from other secondary structures, including the non-targeted GQs, and alter their stability.52,53 Nevertheless, the main obstacles for using small molecules to target GQs in the cell are their lack of specificity toward the targeted GQ and bioavailability.25 The induction of selective GQ formation in the TH promoter (for example the 5′GQ) by small molecules is even more challenging due to the low level of structural variation between the 5′GQ and 3′GQ structures, making it difficult to target one over the other to modulate the increase or decrease of TH expression, respectively. This creates a need to develop rational therapeutic design approaches for targeting specific GQs, which in our case are a specific set of GQs within the endogenous human TH promoter. Other strategies have been developed to target specific quadruplexes that take advantage of using antisense nucleic acid sequences to target nearby regions to achieve single GQ targeting precision. Tassinari et al.54 recently developed a naphthalene diimide-peptide nucleic acid (PNA) complex that was able to distinguish specific GQs while also using the aromatic naphthalene to improve the stability of the target GQ. The small-molecule-PNA conjugate was able to recognize specific GQs of choice within the HIV-long terminal repeat (LTR) based on the sequence of the antisense PNA as a new GQ-targeting therapeutic approach. Our lab designed a locked nucleic acid therapeutic that was able to modulate the equilibrium in the pre-miRNA 92b structure between a folded GQ and duplex products leading to an anticancer effect.55 In a separate approach, our lab induced synthetic RNA-DNA GQs within the 5′ UTR of eIF-4E mRNA as designed roadblocks to prevent protein production.56 However, these strategies, along with other nucleic acid therapeutic approaches, possess pharmacokinetic disadvantages, making a delivery approach essential for in vivo efficacy.57
While the possibility of nucleic acid therapeutics has been a promising idea for the treatment of brain disorders, the underlying pitfalls make it challenging to accomplish a therapeutic success. Some of the obstacles include off-target toxicities, limited localization at diseased sites due to poor serum stability, passage through BBB, and lack of cellular recognition. So far, several nanoparticle compositions have established the value of material complexes for the treatment of neurodegenerative diseases.58 Two small-molecule nanoparticle delivery approaches for brain disorder treatment consisted of a poly(n-butylcyanoacrylate) and polysorbate 80 nanoparticle for treating Alzheimer’s disease resulting in a 4-fold increase in rivastigmine uptake, while the other used a peptide-targeted PLGA-PEG nanoparticle for the improved delivery of odorranalectin for the treatment of PD.59,60 Additionally, a PEI nanoparticle was complexed with α-synuclein siRNA through charge-charge interactions to create longer circulation times and increased stability of the siRNA cargo.61 The nanoparticles were able to localize in dopaminergic neurons and reduce protein expression in a dose-dependent manner. Our study looked to further the ability to use nanomaterials for effective treatment of neurodegeneration by utilizing targeting ligands to actively home in on the dopaminergic neurons while using a polymer coating for stability and elongated circulation time. The addition of the aptamers has allowed the 5′GQ Clip nanoparticle to elicit better outcomes when compared to the 5′GQ Clip treatments, as was evident from the qRT-PCR and WB data. There are two major explanations that could potentially be attributed to these results. (1) The nanoparticle has inherent protection against DNA degradation through the PEG shell that presumably engulfs the DNA, allowing for limited accessibility to nucleases.62 The increased stability could potentially provide the 5′GQ Clip longer availability in cell studies. (2) Targeting aptamers are known to enter the cell via receptor-mediated endocytosis, making cellular uptake more efficient as compared to DNA only.63 DNA molecules do not efficiently pass through the cell membrane due to high net-negative charges and size, which is displayed by the minimal uptake of DNA alone observed in the confocal microscopy experiments (Figure 5A). Both of these factors are thought to contribute to a 4.5-fold increase in mRNA and a 3-fold increase in protein production in cellulo compared to the DNA treatment, further displaying the ability to increase in vivo GFP mRNA and protein production both by 5-fold in the TH-GFP transgenic rat model. There are also a number of strategies that can be implemented to improve upon current nucleic acid drug delivery obstacles, such as endosomal escape, within the nanoparticle complex. Small molecules (chloroquine), pore-forming peptides, and fusogenic biomolecules have shown promise to induce endosomal escape as improvements to nanomaterial design, which may further improve the efficacy of the DNA GQ Clips.64, 65, 66 Together, these highlight the beneficial pairing of nucleic acid therapeutics and nanotechnology that can be extended for a range of drug delivery applications in brain disorder treatment.
PD is one of the most common neurodegenerative diseases where treatment methods are centered around increase dopamine levels. But, as with most neurological disorders, available treatment options are non-curative and can become less effective with the progression of the disease. L-DOPA therapy is the main treatment used currently for dopamine deficiency, since replacement therapy with dopamine is not possible due to its inability to cross the brain capillary endothelial wall, which forms the BBB in vivo.67 Although L-DOPA replacement therapy has been the basis of dopamine deficiency for a long period, this treatment method has shown many side effects, including difficulties in performing voluntary movements (dyskinesia), gastroesophageal reflux, vomiting, and suppression of appetite.67 Most importantly, typically 4–6 years after starting treatment, patients will develop motor complications. These obstacles make finding new options to develop co-treatment or replacement of current L-DOPA therapy imperative. Monoamine oxidase B inhibitors (MAOB) have been studied as a possible pathway to further increase plasma dopamine levels in conjunction with L-DOPA treatment as a possibility to improve drug effectiveness, but they also possess side effects. Zhang et al.67,68 reported an alternative approach to biologically replacing dopamine production using TH transvascular gene therapy with transferrin antibody-conjugated immunoliposomes. The gene therapy conjugate was able to specifically increase TH production in vivo, displaying the ability to improve upon motor symptoms in a 6OHDA toxin model. Because the sequence varies at the all-important G-rich region between the primate and rodent TH promoter sequences, we had to use a transgenic TH-GFP rat model to verify the ability for the 5′GQ Clip nanoparticle in vivo by targeting the human TH promoter. We believe our approach of delivering 5′ GQ Clips provides the capability to create a much higher level of precision when it comes to dopamine production compared to the current approaches. It is also important to note that this strategy has the ability to not only increase dopamine production as observed in the case of the 5′GQ Clip but also reduce dopamine production as achieved by using the 3′GQ Clip, which can be instrumental in several other dopamine-related diseases, such as PTSD.
Our study verifies that the targeting of the TH promoter region can systematically control dopamine production, which can be beneficial for various neurological diseases, especially when we harness the dopamine-enhancing role of the 5′GQ Clip, which can directly improve upon many disease treatments where increase in dopamine levels is necessary. Based on previous concepts, we have developed a strategy to modulate GQ function using nucleic acid therapeutics, where we designed a strategy to modulate the formation of two separate GQ structures within the same 49-nucleotide stretch of the human TH promoter shown to play an instrumental role in controlling TH expression and dopamine production. By pairing the nucleic acid therapeutics with the nanoparticle platform, we were able to improve on the modulation of the human TH promoter activity in cellulo for both normal and 6OHDA-stressed human neuronal cells. Increased TH activity in the in vivo TH-GFP transgenic rat model further supports the viability of the 5′GQ Clip nanoparticle as a proof of concept for neurological diseases, such as PD, where specifically further analysis is needed to observe how the increase in TH level affects the disease condition. The development of a humanized TH model in a rodent system will be vital to evaluate the potential effects of the TH modulation on motor symptoms in PD.
Materials and methods
Oligonucleotides
All of the DNA oligonucleotides used in this study were purchased from Integrated DNA Technologies. Oligonucleotides were purified using 17% denaturing PAGE and were extracted via a crush-and-soak method by tumbling the gel slices at 4°C in a solution of 300 mM NaCl, 10 mM Tris-HCl (pH 7.5), and 0.1 mM EDTA. Samples were concentrated with 2-butanol and ethanol precipitated with 3 volumes of ice-cold 100% ethanol. The salt was removed by washing the DNA pellets with ice-cold 70% ethanol.
Radiolabeling of DNA oligonucleotides
The DNA sequences were 5′ end-radiolabeled by incubating with T4 polynucleotide kinase (NEB) and [γ-32P]ATP (PerkinElmer) for 45 min at 37°C. The radiolabeled DNA oligonucleotides were purified by 17% denaturing PAGE and extracted from the gel via the crush-and-soak method.
Native gel electrophoretic mobility shift assay
The blocking of specific GQ formation was achieved by mixing of the TH49 template with an increasing amount of the corresponding GQ Clip (1:1, 1:10, 1:50) in 150 mM KCl, 10 mM Tris-HCl, and 0.1 mM EDTA (pH 7.5). Then, the mixtures were heated to 95°C for 10 min followed by slow cooling to room temperature over a 90-min period. The complexes were resolved by 10% native polyacrylamide gel electrophoresis in Tris-borate-EDTA buffer supplemented with 150 mM KCl for 6 h in a 4°C cold room. The gel was exposed to a phosphorimager screen and then visualized by Typhoon Phosphorimager FLA 9500 (GE Healthcare, Life Sciences).
DMS structure mapping
Samples for DMS structure mapping were prepared by mixing the appropriate amount of GQ Clips with 1 μM unlabeled TH49 template, 10 mM Tris-HCl buffer (pH 7.4), 100,000 cpm 5′ end-radiolabeled wtTH49 template, and 150 mM KCl or no KCl in a final volume of 30 μL. DNA structures were folded as described above. Then samples were treated with 1% DMS for 2 min at room temperature before the methylation reactions were stopped by adding 300 μL of stop buffer (300 mM sodium acetate, 250 mg/mL sheared salmon sperm DNA, and 2 M β-mercaptoethanol). DNA samples were ethanol precipitated with 3 volumes of ice-cold 100% ethanol, and the DNA pellets were washed with 70% ethanol. The pellets were then dried in a vacuum centrifuge and treated with 70 μL of freshly prepared 10% piperidine for 30 min at 95°C. The cleaved products were resolved on a 12% denaturing polyacrylamide gel, and the dried gel was exposed to a phosphorimager screen and visualized on a Typhoon FLA 9500 Phosphorimager (GE Life Sciences).
Cell culture
The SH-SY5Y cells were cultured in Eagle’s minimum essential medium (EMEM)/F-12 (Corning) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics streptomycin, penicillin, and amphotericin B, at 37°C in 5% CO2 in a humidified incubator. The cells were grown in 6-well plates with ~500,000 cells per well and allowed to grow until they reached ~80% confluency. For transfection of the GQ DNA Clips, jetPRIME transfection reagents were used per the manufacturer’s protocol for 6 h in 6-well plates. Briefly, for each well (total volume of 2 mL), 2 μg of DNA GQ Clips were mixed with jetPRIME transfection reagents in a 1:2 ratio and incubated for 10 min. After transfection for 6 h, transfection medium was replaced with new medium.
RNA isolation and qRT-PCR
Cells were treated for 24 h with media containing Clip sequences, scrambled sequence, or nanoparticle complexes. The cells were then washed three times with full-growth medium, and total cellular RNA was extracted from treated SH-SY5Y cells using a TRIzol reagent as per manufacturer’s protocol. The cDNA was synthesized using qScript cDNA SuperMix (Quanta Biosciences). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and TH mRNAs were subjected to qRT-PCR using a Perfecta SYBR Green Super Mix (Quanta Biosciences) on an Eppendorf Mastercycler RealPlex2 in the presence of the appropriate set of primers. The relative mRNA levels were estimated by the comparative Ct method (Livak method).69,70
RNA was isolated and purified from the rat brain regions using TRIzol reagent per the manufacturer’s protocol and stored at −80°C until it was assayed. The cDNA was synthesized using qScript cDNA SuperMix (Quanta Biosciences). The GAPDH and GFP mRNAs were subjected to qRT-PCR as discribed above. For transfection of the GQ DNA Clips, jetPRIME transfection reagents were used as per manufacturer’s protocol for 6 h in 6-well plates. Briefly, for each well (total volume of 2 mL), 2 μg of DNA GQ Clips were mixed with jetPRIME transfection reagents in 1:2 ratio and incubated for 10 min. After transfection for 6 h, transfection medium was replaced with new medium.
Western blotting
Cells were treated for 24 h with media containing Clip sequence, scrambled sequence, or nanoparticle complexes. The cells were then washed three times with full growth medium, and total cellular protein was extracted from treated SH-SY5Y cells using a TRIzol reagent as per manufacturer’s protocol. Protein lysate was separated by 15% SDS-PAGE. The proteins were detected by mouse monoclonal TH antibody (Sigma, T1299) at 1:200 dilution and GAPDH (G-9, sc-365062) antibody at 1:10,000 dilution. Horseradish peroxidase-conjugated goat anti-mouse IgG (sc-2005) was used as secondary antibody at a dilution 1:1,000. Proteins were visualized by Western Blotting Luminol Reagent (sc-2048) in a GE imaginer.
Detection of dopamine
Cells were transfected with DNA oligos as described above. After transfection, the cells were trypsinized, and the cellular components were precipitated with 0.1 M perchloric acid solution. The level of dopamine present in the supernatant was analyzed via HPLC equipped with an electrochemical detector. Briefly, the supernatant was syringe filtered through a 0.22 μm nylon membrane filter and placed into the autosampler vials for HPLC-ECD analysis on a Thermo Scientific Ultimate 3000 system consisting of an ESA Model 582 pump set at 0.5 mL/min solvent flow. C-18 reversed-phase column (Waters Corporation) was used for the analysis. Dopamine was detected with an electrochemical detector (Coulochem III, ESA) with a PEEK filter-protected 5011A analytical cell (ESA, 5 nA; guard electrode, 205 mV; analytical electrode, 250 mV). Chromatograms were recorded using Chromeleon software, which also controlled the pump, autosampler, and detector. The HPLC solvent consisted of 15% v/v acetonitrile, 10% v/v methanol, 150 mM sodium phosphate buffer set to pH 5.3 with citric acid, 4.75 mM citric acid, and 50 μM EDTA. The HPLC solvents were vacuum degassed before use.
Preparation of gold nanoparticles
Gold nanoparticles were synthesized following a previously reported protocol.71 The solution was then filter sterilized using a 0.2 μm cellulose acetate filter (Corning). Ten kilodalton membrane cut-off 50 mL centrifuge tubes were used to concentrate the nanoparticle solution to 2 mL. Particle size was then analyzed by UV-vis spectroscopy, TEM, and dynamic light scattering (DLS). A molar extinction coefficient of the 5 nM AuNP was used to obtain nanoparticle concentration via UV-vis spectroscopy (Cary 5000, Agilent).
PEGylation of TrkB aptamer
TrkB peptide aptamer (CENLYFQSGSMAHPYFAR) was purchased from Genscript (Piscataway, NJ, USA) and purified using HPLC (C-18 reversed-phase column, Waters Corporation). The peptide solution was syringe filtered through a 0.22 μm nylon membrane and placed into the autosampler vials for HPLC-UV analysis using an Agilent 1100 Series HPLC Value HPLC System. The mobile phase consisted of 1% trifluoroaceticacid in acetonitrile gradient (10% to 80% acetonitrile over 30 min, 80% acetonitrile for 5 min, and 10% acetonitrile for 5 min). A flow rate of 1.0 mL/min was used, and the flow was monitored with an Agilent 1100 series UV detector at 190 nm. Peak areas were acquired with Agilent Chemstation software. The HPLC solvents were vacuum degassed before use. The purified peptide was conjugated to bismalemide (BM)-(PEG) in excess using the manufacturer’s protocol (Pierce, Thermo Fisher). The BM(PEG)-peptide was purified using 1000 Da MWCO dialysis. Both the peptide and the BM(PEG) were examined for proper size using ESI-MS (Figures S11 and S12). A 1.5:1 molar ratio of BM(PEG)-peptide to 5 kDa SH-PEG-SH was mixed in water over 24 h at room temperature to create the thioether product. The PEG-conjugated aptamer was purified via 3 kDa MWCO centrifuge tubes.
Synthesis of the 5′GQ Clip nanocomplexes
First, AuNP and PEG aptamers were mixed at a 1:1.5 molar ratio and shaken for 1 min. Afterward, a 1:5:2 ratio by volume of AuNP-aptamer complex, 5 kDa SH-PEG-COOH 1 mM solution, and 1 mM 5′GQ Clip DNA were mixed and shaken for 1 h at 4°C. The nanocomplex was purified by dialysis with 100,000 kDa MWCO centrifuge tubes (Millipore). The resulting nanoparticle complex was added to increasing amounts of NaCl to test stability in salt. UV-Vis spectrophotometer was used to assess the AuNP peak at 515 nm for aggregation.
Detection of 5′GQ Clip release from nanoparticle complexes by agarose gel
The 5′ end-radiolabeled single-stranded oligonucleotides were done as described previously. The labeled Clip DNA was conjugated onto the nanoparticles with cold DNA to equal the normal nanoparticle loading. The nanoparticle samples were then incubated with 10 mM glutathione for various time points. The samples were analyzed on a 1% agarose gel. The gel was exposed to a phosphorimager screen and then visualized by Typhoon Phosphorimager FLA 9500 (GE Healthcare, Life Sciences).
Cellular uptake detection by confocal fluorescence microscopy
SH-SY5Y cells were seeded overnight at a density of 75,000 cells per well, respectively, in an 8-well chamber slide. Cells were then treated for 24 h with media containing nanoparticle complexes conjugated with fluorescently labeled HEX 5′GQ Clip. The cells were then washed three times with full growth medium and immediately analyzed for intracellular Dox distribution under an Olympus 1,000× confocal microscope.
Animals
Adult male TH-GFP transgenic rats (245–300 g: Taconic Biosciences, Germantown, NY, USA) and Fischer 344 rats (240–300 g: Charles River, Wilmington, MA, USA) were individually housed in Plexiglas cages (60 × 30 × 24 cm3). Rats were allowed approximately 7 days to acclimate to the colony after shipment before being handled for approximately 4 days before experiments were performed. Food and water were provided ad libitum. Studies were performed in accordance with the guidelines of the Public Health Service (PHS) Guide to the Care and Use of Laboratory Animals and approved by the Kent State University Institutional Animal Care and Use Committee.
In vivo neuronal uptake
5′GQ Clip nanoparticle was synthesized with fluorescently labeled HEX 5′GQ Clip. Fischer 344 rats were briefly anesthetized under isoflurane and intravenously injected via the tail vein with 10 μg of HEX-labeled 5′GQ Clip nanoparticles. Twenty-four hours later, animals were deeply anesthetized with pentobarbital and transcardially perfused with 250 mL saline followed by 400 mL 4% paraformaldehyde. Rat brains were harvested, post-fixed for 24 h in paraformaldehyde prior to placement in 30% sucrose, and sliced into 20 μm sections spanning the region of the substantia nigra containing the targeted dopaminergic neurons. Confocal microscopy was used where neurons were stained with NeuN to observe HEX-positive neurons.
Modulation of TH-GFP in a transgenic rat model
Two groups of TH-GFP rats (n = 3) were treated intravenously via the tail vein with 10 μg (in reference to loaded DNA) of 5′GQ Clip nanoparticle or scrambled nanoparticle and perfused 24 h later as described previously. Rat brains were harvested, fixed, and sliced into 20 μm sections spanning the region of the substantia nigra containing the targeted dopaminergic neurons. Confocal microscopy was used to observed GFP expression. ImageJ software was used to quantify the fluorescence between the two treatments. The total area of fluorescence was normalized against the number of nuclei (>1,500 nuclei per image).
RNA isolation and qPCR
RNA was isolated and purified from the brain regions collected using TRIzol reagent as per manufacturer’s protocol. Purified RNA was checked for quality and quantity on a Nanodrop. Only those samples that had 260/280 and 260/230 ratios greater than 1.7 were processed further. RNA was stored at −80°C until it was assayed. The cDNA was synthesized using qScript cDNA SuperMix (Quanta Biosciences). The GAPDH and GFP mRNAs were subjected to qRT-PCR using a Perfecta SYBR Green Super Mix (Quanta Biosciences) on an Eppendorf Mastercycler RealPlex2 in the presence of the appropriate set of primers. The relative mRNA levels were estimated by the comparative Ct method (Livak method).69,70
Statistical analyses
If not stated otherwise, results are mean values ± standard error of the mean (SEM) of at least three independent experiments, or results show one representative experiment of a minimum of three. Statistical analyses were performed on all available data. Unless otherwise mentioned, statistical significance was determined using the two-tailed Student’s t test, with p values ≤ 0.05 considered statistically significant.
Acknowledgments
The research was supported by Kent State University and the Division of Research and Sponsored Programs. We would like to acknowledge Dr. Naveen Kumar Singhal for the help with ex vivo experimentation, Dr. Dirk Friedrich for the help with mass spectrometric detection of dopamine, and Nicole West for technical support.
Author contributions
S.B., M.M.F., N.B., and P.K. conceived the project, and S.B. supervised the project. M.M.F., N.B., and P.K. designed and undertook the cellular, biochemical, and molecular biology experiments. N.B., J.J., and P.K. undertook the animal and postmortem tissue works. B.C. and T.M. performed CD experiments. N.B., M.M.F., P.K., and S.B. analyzed and interpreted the data and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.05.013.
Supplemental information
References
- 1.Ullrich R., Hofrichter M. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 2007;64:271–293. doi: 10.1007/s00018-007-6362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molinoff P.B., Axelrod J. Biochemistry of catecholamines. Annu. Rev. Biochem. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- 3.Tripp G., Wickens J.R. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Arnsten A.F. Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 5.Assadi S.M., Yücel M., Pantelis C. Dopamine modulates neural networks involved in effort-based decision-making. Neurosci. Biobehav. Rev. 2009;33:383–393. doi: 10.1016/j.neubiorev.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Anisman H., Zacharko R.M. Behavioral and neurochemical consequences associated with stressors. Ann. N Y Acad. Sci. 1986;467:205–225. doi: 10.1111/j.1749-6632.1986.tb14630.x. [DOI] [PubMed] [Google Scholar]
- 7.Brooks D.J., Piccini P. Imaging in Parkinson’s disease: the role of monoamines in behavior. Biol. Psychiatry. 2006;59:908–918. doi: 10.1016/j.biopsych.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Daubner S.C., Le T., Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee K., Wang M., Cai E., Fujiwara N., Baker H., Cave J.W. Regulation of tyrosine hydroxylase transcription by hnRNP K and DNA secondary structure. Nat. Commun. 2014;5:5769. doi: 10.1038/ncomms6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkkinen L., O’Sullivan S.S., Collins C., Petrie A., Holton J.L., Revesz T., Lees A.J. Disentangling the relationship between lewy bodies and nigral neuronal loss in Parkinson’s disease. J. Parkinsons Dis. 2011;1:277–286. doi: 10.3233/JPD-2011-11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrez S., Jabir N.R., Shakil S., Greig N.H., Alam Q., Abuzenadah A.M., Damanhouri G.A., Kamal M.A. A synopsis on the role of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 2012;11:395–409. doi: 10.2174/187152712800792785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhath M.M., Thompson M., Ray S., Sewell A., Balci H., Basu S. G-Quadruplex-Enabling Sequence within the Human Tyrosine Hydroxylase Promoter Differentially Regulates Transcription. Biochemistry. 2015;54:5533–5545. doi: 10.1021/acs.biochem.5b00209. [DOI] [PubMed] [Google Scholar]
- 14.Williamson J.R., Raghuraman M.K., Cech T.R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989;59:871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 15.Yang D., Okamoto K. Structural insights into G-quadruplexes: towards new anticancer drugs. Future Med. Chem. 2010;2:619–646. doi: 10.4155/fmc.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Y., Hurley L.H. Structures, folding patterns, and functions of intramolecular DNA G-quadruplexes found in eukaryotic promoter regions. Biochimie. 2008;90:1149–1171. doi: 10.1016/j.biochi.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Y., Rezler E.M., Gokhale V., Sun D., Hurley L.H. Characterization of the G-quadruplexes in the duplex nuclease hypersensitive element of the PDGF-A promoter and modulation of PDGF-A promoter activity by TMPyP4. Nucleic Acids Res. 2007;35:7698–7713. doi: 10.1093/nar/gkm538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun D., Guo K., Rusche J.J., Hurley L.H. Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents. Nucleic Acids Res. 2005;33:6070–6080. doi: 10.1093/nar/gki917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo K., Gokhale V., Hurley L.H., Sun D. Intramolecularly folded G-quadruplex and i-motif structures in the proximal promoter of the vascular endothelial growth factor gene. Nucleic Acids Res. 2008;36:4598–4608. doi: 10.1093/nar/gkn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui-Jain A., Grand C.L., Bearss D.J., Hurley L.H. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. USA. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogoi S., Paramasivam M., Spolaore B., Xodo L.E. Structural polymorphism within a regulatory element of the human KRAS promoter: formation of G4-DNA recognized by nuclear proteins. Nucleic Acids Res. 2008;36:3765–3780. doi: 10.1093/nar/gkn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dexheimer T.S., Sun D., Hurley L.H. Deconvoluting the structural and drug-recognition complexity of the G-quadruplex-forming region upstream of the bcl-2 P1 promoter. J. Am. Chem. Soc. 2006;128:5404–5415. doi: 10.1021/ja0563861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai J., Chen D., Jones R.A., Hurley L.H., Yang D. NMR solution structure of the major G-quadruplex structure formed in the human BCL2 promoter region. Nucleic Acids Res. 2006;34:5133–5144. doi: 10.1093/nar/gkl610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel D.J., Phan A.T., Kuryavyi V. Human telomere, oncogenic promoter and 5′-UTR G-quadruplexes: diverse higher order DNA and RNA targets for cancer therapeutics. Nucleic Acids Res. 2007;35:7429–7455. doi: 10.1093/nar/gkm711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balasubramanian S., Hurley L.H., Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat. Rev. Drug Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidle S. Quadruplex Nucleic Acids as Novel Therapeutic Targets. J. Med. Chem. 2016;59:5987–6011. doi: 10.1021/acs.jmedchem.5b01835. [DOI] [PubMed] [Google Scholar]
- 27.Davis M.E., Chen Z.G., Shin D.M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 28.Rosi N.L., Giljohann D.A., Thaxton C.S., Lytton-Jean A.K.R., Han M.S., Mirkin C.A. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tijerina P., Mohr S., Russell R. DMS footprinting of structured RNAs and RNA-protein complexes. Nat. Protoc. 2007;2:2608–2623. doi: 10.1038/nprot.2007.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharyya D., Mirihana Arachchilage G., Basu S. Metal Cations in G-Quadruplex Folding and Stability. Front Chem. 2016;4:38. doi: 10.3389/fchem.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaczmarek J.C., Kowalski P.S., Anderson D.G. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. doi: 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranjan S., Sood R., Dudas J., Glueckert R., Schrott-Fischer A., Roy S., Pyykkö I., Kinnunen P.K. Peptide-mediated targeting of liposomes to TrkB receptor-expressing cells. Int. J. Nanomedicine. 2012;7:3475–3485. doi: 10.2147/IJN.S32367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bien-Ly N., Yu Y.J., Bumbaca D., Elstrott J., Boswell C.A., Zhang Y., Luk W., Lu Y., Dennis M.S., Weimer R.M., et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J. Exp. Med. 2014;211:233–244. doi: 10.1084/jem.20131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porciani D., Signore G., Marchetti L., Mereghetti P., Nifosì R., Beltram F. Two interconvertible folds modulate the activity of a DNA aptamer against transferrin receptor. Mol. Ther. Nucleic Acids. 2014;3:e144. doi: 10.1038/mtna.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Y., Meyers J.D., Agnes R.S., Doane T.L., Kenney M.E., Broome A.M., Burda C., Basilion J.P. Addressing brain tumors with targeted gold nanoparticles: a new gold standard for hydrophobic drug delivery? Small. 2011;7:2301–2306. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond.) 2016;11:673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porciani D., Cardwell L.N., Tawiah K.D., Alam K.K., Lange M.J., Daniels M.A., Burke D.H. Modular cell-internalizing aptamer nanostructure enables targeted delivery of large functional RNAs in cancer cell lines. Nat. Commun. 2018;9:2283. doi: 10.1038/s41467-018-04691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foroozandeh P., Aziz A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018;13:339. doi: 10.1186/s11671-018-2728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 41.Simola N., Morelli M., Carta A.R. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox. Res. 2007;11:151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 42.Iacovitti L., Wei X., Cai J., Kostuk E.W., Lin R., Gorodinsky A., Roman P., Kusek G., Das S.S., Dufour A., et al. The hTH-GFP reporter rat model for the study of Parkinson’s disease. PLoS ONE. 2014;9:e113151. doi: 10.1371/journal.pone.0113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun D., Thompson B., Cathers B.E., Salazar M., Kerwin S.M., Trent J.O., Jenkins T.C., Neidle S., Hurley L.H. Inhibition of human telomerase by a G-quadruplex-interactive compound. J. Med. Chem. 1997;40:2113–2116. doi: 10.1021/jm970199z. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y. Chemistry in human telomere biology: structure, function and targeting of telomere DNA/RNA. Chem. Soc. Rev. 2011;40:2719–2740. doi: 10.1039/c0cs00134a. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez R., Miller K.M., Forment J.V., Bradshaw C.R., Nikan M., Britton S., Oelschlaegel T., Xhemalce B., Balasubramanian S., Jackson S.P. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat. Chem. Biol. 2012;8:301–310. doi: 10.1038/nchembio.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Cian A., Lacroix L., Douarre C., Temime-Smaali N., Trentesaux C., Riou J.F., Mergny J.L. Targeting telomeres and telomerase. Biochimie. 2008;90:131–155. doi: 10.1016/j.biochi.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Schonhoft J.D., Bajracharya R., Dhakal S., Yu Z., Mao H., Basu S. Direct experimental evidence for quadruplex-quadruplex interaction within the human ILPR. Nucleic Acids Res. 2009;37:3310–3320. doi: 10.1093/nar/gkp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connor A.C., Frederick K.A., Morgan E.J., McGown L.B. Insulin capture by an insulin-linked polymorphic region G-quadruplex DNA oligonucleotide. J. Am. Chem. Soc. 2006;128:4986–4991. doi: 10.1021/ja056097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koukouraki P., Doxakis E. Constitutive translation of human α-synuclein is mediated by the 5′-untranslated region. Open Biol. 2016;6:160022. doi: 10.1098/rsob.160022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simone R., Balendra R., Moens T.G., Preza E., Wilson K.M., Heslegrave A., Woodling N.S., Niccoli T., Gilbert-Jaramillo J., Abdelkarim S., et al. G-quadruplex-binding small molecules ameliorate C9orf72 FTD/ALS pathology in vitro and in vivo. EMBO Mol. Med. 2018;10:22–31. doi: 10.15252/emmm.201707850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou W., Brand N.J., Ying L. G-quadruplexes-novel mediators of gene function. J. Cardiovasc. Transl. Res. 2011;4:256–270. doi: 10.1007/s12265-011-9258-2. [DOI] [PubMed] [Google Scholar]
- 52.Folini M., Venturini L., Cimino-Reale G., Zaffaroni N. Telomeres as targets for anticancer therapies. Expert Opin. Ther. Targets. 2011;15:579–593. doi: 10.1517/14728222.2011.556621. [DOI] [PubMed] [Google Scholar]
- 53.Collie G.W., Parkinson G.N. The application of DNA and RNA G-quadruplexes to therapeutic medicines. Chem. Soc. Rev. 2011;40:5867–5892. doi: 10.1039/c1cs15067g. [DOI] [PubMed] [Google Scholar]
- 54.Tassinari M., Zuffo M., Nadai M., Pirota V., Sevilla Montalvo A.C., Doria F., Freccero M., Richter S.N. Selective targeting of mutually exclusive DNA G-quadruplexes: HIV-1 LTR as paradigmatic model. Nucleic Acids Res. 2020;48:4627–4642. doi: 10.1093/nar/gkaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirihana Arachchilage G., Kharel P., Reid J., Basu S. Targeting of G-Quadruplex Harboring Pre-miRNA 92b by LNA Rescues PTEN Expression in NSCL Cancer Cells. ACS Chem. Biol. 2018;13:909–914. doi: 10.1021/acschembio.7b00749. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya D., Nguyen K., Basu S. Rationally induced RNA:DNA G-quadruplex structures elicit an anticancer effect by inhibiting endogenous eIF-4E expression. Biochemistry. 2014;53:5461–5470. doi: 10.1021/bi5008904. [DOI] [PubMed] [Google Scholar]
- 57.Xu L., Anchordoquy T. Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J. Pharm. Sci. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldsmith M., Abramovitz L., Peer D. Precision nanomedicine in neurodegenerative diseases. ACS Nano. 2014;8:1958–1965. doi: 10.1021/nn501292z. [DOI] [PubMed] [Google Scholar]
- 59.Wilson B., Samanta M.K., Santhi K., Kumar K.P., Paramakrishnan N., Suresh B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008;1200:159–168. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 60.Wen Z., Yan Z., Hu K., Pang Z., Cheng X., Guo L., Zhang Q., Jiang X., Fang L., Lai R. Odorranalectin-conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control. Release. 2011;151:131–138. doi: 10.1016/j.jconrel.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Helmschrodt C., Höbel S., Schöniger S., Bauer A., Bonicelli J., Gringmuth M., Fietz S.A., Aigner A., Richter A., Richter F. Polyethylenimine Nanoparticle-Mediated siRNA Delivery to Reduce α-Synuclein Expression in a Model of Parkinson’s Disease. Mol. Ther. Nucleic Acids. 2017;9:57–68. doi: 10.1016/j.omtn.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh P., Han G., De M., Kim C.K., Rotello V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 63.Zhou J., Rossi J.J. Cell-specific aptamer-mediated targeted drug delivery. Oligonucleotides. 2011;21:1–10. doi: 10.1089/oli.2010.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dowdy S.F., SF Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 65.Maxfield F.R., FR Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J. Cell Biol. 1982;95:676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou K.K., Pan H., Schlesinger P.H., Wickline S.A., KK A role for peptides in overcoming endosomal entrapment in siRNA delivery - A focus on melittin. Biotechnol. Adv. 2015;33:931–940. doi: 10.1016/j.biotechadv.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pardridge W.M. Tyrosine hydroxylase replacement in experimental Parkinson’s disease with transvascular gene therapy. NeuroRx. 2005;2:129–138. doi: 10.1602/neurorx.2.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Schlachetzki F., Zhang Y.F., Boado R.J., Pardridge W.M. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum. Gene Ther. 2004;15:339–350. doi: 10.1089/104303404322959498. [DOI] [PubMed] [Google Scholar]
- 69.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 70.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 71.Beals N., Thiagarajan P.S., Soehnlen E., Das A., Reizes O., Lathia J.D., Basu S. Five-Part Pentameric Nanocomplex Shows Improved Efficacy of Doxorubicin in CD44+ Cancer Cells. ACS Omega. 2017;2:7702–7713. doi: 10.1021/acsomega.7b01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.