Abstract
Mobile-health applications can be used to deliver timely and personalized health information to family and friends of chronically ill adults living in the community. This scoping review aims to investigate the nature and extent of native smartphone applications for informal caregivers. Six databases were searched for articles on applications across ten chronic conditions, namely heart disease, stroke, cancer, chronic obstructive respiratory disease, asthma, diabetes, Alzheimer’s disease or other dementia, rheumatoid arthritis, hypertension, and mood or anxiety disorders. In total, 36 articles were included, encompassing 26 applications. Of these, smartphone applications were designed for use only by caregivers (n = 15), with a few applications also intended to be used with patients (n = 5), healthcare providers (n = 4), or all three roles (n = 2). Most applications targeted a single chronic condition (n = 25), with Alzheimer’s and other dementia being the most common (n = 18). Only one application was designed for management of multiple chronic conditions. Long-term evaluation methods are needed to continually assess the impact of applications on a range of process and health outcomes, such as usability, caregiver burden, and quality of life. Additional directions to advance native smartphone applications for caregivers are discussed, including personalization and expansion of eligibility criteria.
Subject terms: Patient education, Quality of life
Introduction
In Canada, individuals with chronic conditions represent approximately 44.2% of the adult population aged 20 years or older, and at least one in every five adults is estimated to have two or more chronic illnesses1. Informal caregivers, such as family or friends, may be able to offer assistance with basic activities of daily living (ADL), such as feeding and walking, as well as instrumental activities of daily living (IADL), such as preparing meals, managing medications, and transportation2. Caregiving has been associated with varied outcomes, which could be positive such as personal growth, or negative outcomes such as emotional exhaustion3. Previous research has found that individuals in a caregiver role were at greater risk of injuries and illnesses, such as anxiety and depression4. The progressive and complex nature of some chronic conditions can lead some individuals to experience caregiver burden5. In particular, the transition to a high-intensity caregiving role (i.e., providing support for ADL) was found to be associated with the functional decline of family caregivers6. People may experience burden differently based on the type and frequency of the caregiving, as well as their own perceptions toward care-related tasks or problems5. With the growing prevalence of chronic conditions in an aging population, more attention is needed to prepare and support individuals in a caregiving role7,8.
In the past decade, smartphones have become sophisticated and affordable computing devices, playing a key role in digital inclusion9. Smartphones can have multiple applications (referred to as native apps), some of which may enable users to access online resources, including web-based applications. One of the key advantages of native apps over web-based applications is that the former is installed on the user’s mobile device10. Thereby, some native apps can be used offline by individuals who live in underserved or rural locations with limited or no access to broadband Internet. Moreover, native apps can also gain access to built-in components (e.g., camera, global positioning system, and biometric sensors) and other external devices connected through Bluetooth or Wi-Fi. System designers can leverage these technologies to design context-aware native apps that are tailored to user preferences and/or environment.
Digital health technologies (DHT), including native and web-based applications, have been explored as a means to deliver health interventions for caregivers11,12. For example, a systematic review in 2018 investigated web-based applications targeting chronic conditions in general and found a low-to-moderate positive impact on caregivers’ self-esteem, self-efficacy, mastery, and strain12. Considering that different chronic conditions may present unique challenges for informal caregivers, some reviews have focused on single chronic illnesses, such as dementia13,14 or cancer15. Even though these previous reviews investigated a broad range of technologies, the literature on native apps dedicated to specific chronic conditions is scarce. A study of two international application stores identified several native apps targeting chronic conditions in general, but found no supporting evidence for these apps in the literature16. Ultimately, a siloed approach for the management of chronic conditions may lead to the concomitant adoption of multiple native apps and create repetitive or redundant tasks for caregivers17. With multiple chronic conditions (MCC) becoming more prevalent in an aging population, informal caregivers will need tailored apps to support individuals with two or more chronic conditions living in the community18.
The objective of this scoping review is to summarize emergent research on native apps exclusively designed to support informal caregivers across various common types of chronic conditions. The research question is: what is known in the literature about native apps for informal caregivers of chronically ill patients living in the community?
Results
Article characteristics
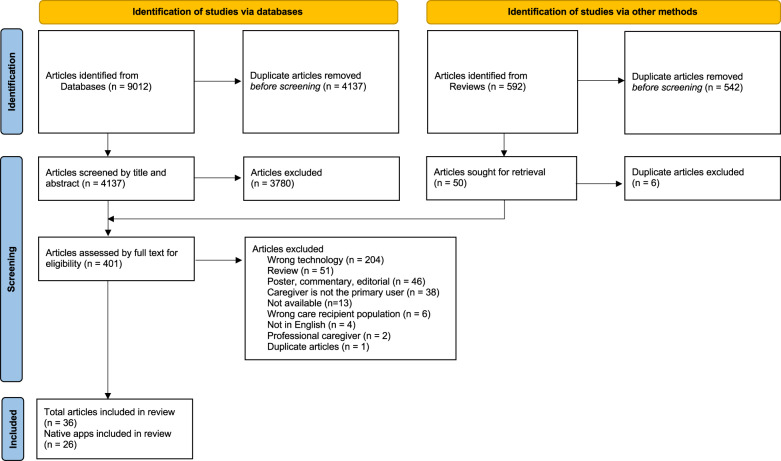
Searches of six databases yielded a total of 9012 articles. After removing duplicates, there were 4137 articles that were screened by title and abstract, resulting in 357 articles that were eligible for full text screening. In addition, another 44 articles were identified through hand-searching the citations of retrieved reviews that were eligible for full-text screening, resulting in a total of 401 articles that were screened by full text. After full-text screening, four conference proceedings and 32 journal articles were included in the review, totaling 36 articles, encompassing 26 native apps. Most apps were associated with only one study (n = 18). The results of the article-selection process are shown in the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA)19 flow diagram in Fig. 1. Table 1 describes the main characteristics of included articles. Most studies originated from research groups in the United States (n = 12) and the Netherlands (n = 5) and were published within the past two years, in 2019–2020. Most articles were intended for caregivers of a person with Alzheimer’s or other dementia (n = 27) and only one study addressed two or more chronic illnesses (i.e., MCC). Included studies presented varied research designs, with case studies being the most common design (n = 14). Findings of effectiveness studies were available for 5 apps, out of which 3 were publicly accessible.
Fig. 1. Flow diagram.
Preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram of the literature search19.
Table 1.
Study characteristics.
| Article characteristics | Number of articles out of N = 36 | References |
|---|---|---|
| Country of origina | ||
| Australia | 3 | 39,43,62 |
| Austria | 1 | 33 |
| Brazil | 1 | 36 |
| Denmark | 1 | 49 |
| Germany | 1 | 56 |
| Greece | 2 | 40,60 |
| India | 1 | 41 |
| Ireland | 1 | 40 |
| Mexico | 1 | 42 |
| Norway | 1 | 38 |
| Pakistan | 2 | 57,63 |
| Poland | 1 | 49 |
| South Korea | 1 | 51 |
| Spain | 2 | 28,49 |
| Sweden | 1 | 58 |
| The Netherlands | 5 | 31,37,40,44,47 |
| Turkey | 1 | 45 |
| United Kingdom | 2 | 26,40 |
| United States | 12 | 27,32,34,35,46,48,50,52–55,59 |
| Not specified EU Country | 1 | 61 |
| Publication year | ||
| 2011 | 1 | 42 |
| 2014 | 1 | 54 |
| 2015 | 1 | 55 |
| 2016 | 4 | 40,46,49,57 |
| 2017 | 4 | 31,47,48,56 |
| 2018 | 3 | 38,60,61 |
| 2019 | 10 | 27,32,34,36,37,39,41,43,44,50 |
| 2020 | 10 | 26,28,33,35,45,51,52,58,59,63 |
| 2021 | 2 | 53,62 |
| Chronic illnessa | ||
| Alzheimer’s or dementia | 27 | 26,27,31,33–38,42–44,46–56,58,60–62 |
| Anxiety or depression | 2 | 28,40 |
| Asthma | 0 | – |
| Cancer | 4 | 32,39,45,59 |
| Cerebrovascular disorders | 3 | 41,57,63 |
| COPDb | 1 | 40 |
| Diabetes | 1 | 40 |
| Heart failure | 1 | 40 |
| Hypertension | 0 | – |
| Rheumatoid arthritis | 0 | – |
| Research design | ||
| Case study | 14 | 26,31–34,36,38,39,42,43,46,48,55,62 |
| Descriptive | 4 | 54,56,60,61 |
| Observational, cross-sectional | 1 | 41 |
| Phenomenology | 1 | 40 |
| Pretest/posttest | 5 | 28,45,50,51,58 |
| Randomized controlled trial | 11 | 27,35,37,44,47,49,52,53,57,59,63 |
aMulti-site and multiple chronic conditions studies are counted in more than one category.
bCOPD Chronic obstructive pulmonary diseases.
Smartphone-application characteristics
In total, 26 native apps were identified in this review. Table 2 summarizes the main characteristics of the apps in terms of frequency of use, targeted platform(s), distribution, study type and year, categories of intended users, methods employed to build the app, and theoretical frameworks that informed app development. Additional information about the functionalities and/or system components of included apps can be found in Supplementary Table 2. The apps have been grouped by chronic condition, including Alzheimer’s and other dementia (n = 18), cancer (n = 4), stroke (n = 2), depression (n = 1), and MCC (n = 1). Most common components and/or system functionalities offered by native apps for caregivers include education about the chronic condition related to the patient’s care (n = 13), communication with family, friends, peers, or healthcare providers (n = 9), screening for risk factors (n = 7), self-care for the caregiver (n = 4), social networks (n = 3), personalized feedback (n = 3), and storing and/or sharing health information of the patient (n = 3), among others. The frequency of use refers to a recommended ‘dose’ of an intervention, which in the context of digital health interventions, is often related to how often participants are instructed to log in or perform specific tasks within the app. Owing to the diversity of approaches to measure the frequency of use, the usage was considered as either fixed (i.e., regular use of the app was suggested/requested, such as daily or weekly) or as needed (i.e., no frequency was defined for usage). Android was the most common platform (n = 18), and several native apps supported two or more platforms (n = 15), such as iOS and web platforms (i.e., the user can access the app from a browser, such as Safari or Google Chrome). Before 2016, most articles described private apps (i.e., apps that were only available to study participants or to external researchers upon request). After this date, many articles reported on public apps, which could be downloaded by the public from an app store such as Google Play (Google) or App Store (Apple).
Table 2.
Characteristics of native smartphone applications for caregivers grouped by chronic condition (italicized).
| Application name (Distribution) | Use frequency | Platform | Study type (Ref) | User | Method | Theoretical framework |
|---|---|---|---|---|---|---|
| Alzheimer’s or dementia | ||||||
| C-MMDa(Private) | As needed | Not specified, web |
Usability26 |
Caregiver, patient, provider | UCD | NRb |
| CareHeroes (Public) | As needed | Android, web |
Feasibility46, Pilot34 |
Caregiver, provider | UCD | Family-centered theory22 |
| CASTc (Private) | Fixed | Android | Feasibility48 | Caregiver | UCD | NR |
| Cubes (Public) | As needed | Android, iOS | Usability31 | Caregiver, provider | UCD | NR |
| Dea (Private) | As needed | Android | Usability33 | Caregiver | UCD | Meaningful Activity79 |
| Dementia Support for Carers (Private) | As needed | Android, iOS |
Protocol43, Development62 |
Caregiver | UCD | Family-centered theory22, empowerment model80, and adult learning theory81 |
| FamTechCare (Public) | Fixed | iOS, web |
Feasibility35, Cost-effectiveness53 |
Caregiver | NR | Reasons and management of behavioral and psychological symptoms of dementia24 |
| Inlife (Public) | As needed | Android, iOS, web |
Protocol47, Implementation37 |
Caregiver | UCD | NR |
| MemoryBoard (Private) | Fixed | Android, web | Usability42 | Caregiver, patient | UCD | NR |
| MITd(Public) | Fixed | iOS | Feasibility50 | Caregiver | NR | Mentalization theory22 |
| mYouTime (Private) | As needed | Android, iOS | Usability38 | Caregiver, provider | UCD | NR |
| PsyMate (Public) | Fixed | Android, iOS | Effectiveness44 | Caregiver | NR | Experience sampling method82 |
| SMAIe(Private) | Fixed | Android, web | Usability36 | Caregiver, provider | UCD | NR |
| Story-call (Private) | As needed | Android |
Development54, Pilot55 |
Caregiver | NR | Resilience model of family stress, adjustment, and adaptation23 |
| UnderstandAID (Private) | As needed | Android | Pilot49 | Caregiver | NR | NR |
| Unnamed (Private) | As needed | Android | Usability56 | Caregiver, patient | NR | NR |
| Unnamed (Private) | Fixed | Not specified | Effectiveness51 | Caregiver | MADL | Reasons and Management of Behavioral and Psychological Symptoms of Dementia24 |
| Unnamed (Private) | As needed | Not specified | Protocol58 | Caregiver | UCD | NR |
| Anxiety or depression | ||||||
| Happy (Public) | Fixed | Android, iOS | Pilot28 | Caregiver | NR | NR |
| Cancer | ||||||
| Caregiver Communication about Cancer (Public) | As needed | iOS | Acceptability32 | Caregiver | NR | Family caregiver communication typology83 |
| Carer Guide App (Public) | As needed | Android, iOS, web | Usability39 | Caregiver | UCD | TPBf 25 and UTAUTg 84 |
| Roadmap 2.0 (Public) | Fixed | Android, iOS | Protocol59 | Caregiver, patient | UCD | NR |
| Unnamed (Public) | As needed | Android, iOS | Effectiveness45 | Caregiver | NR | NR |
| Cerebrovascular disorder | ||||||
| Movies4Stroke (Private) | Fixed | Android |
Protocol57, Effectiveness63 |
Caregiver, patient | NR | Rogers’ diffusion of innovation theory85 |
| Unnamed (Private) | NR | Not specified | Acceptability41 | Caregiver, patient | NR | NR |
| Multiple chronic conditions | ||||||
| WELCOMEh(Private) | As needed | Not specified, web | Development40 | Caregiver, patient, provider | UCD | NR |
aC-MMD: CaregiversPro-MMD.
bNR: not reported.
cCAST: Caregiver Assessment Using Serious Gaming Technology.
dMIT: Mentalizing Imagery Therapy.
eSMAI: Mobile System for Elderly Monitoring.
fTPB: Theory of planned behavior.
gUTAUT: Unified theory of acceptance and use of technology.
hWELCOME: Wearable Sensing and Smart Cloud Computing for Integrated Care to COPD Patients with Comorbidities.
Software design and development
The user-centered design (UCD) method (also referred to as human-centered design) was commonly cited as being used to develop native apps (n = 14), which meant that caregivers were engaged at different stages of software development to generate ideas or validate the function of the system20. Among the apps developed with UCD, several studies focused on improving usability (n = 6), as shown in Table 2. Usability studies were conducted on different versions of the app, ranging from paper-based prototypes in early formative research to functional apps in more advanced stages. Besides UCD, one study mentioned the mobile-application development lifecycle (MADL)21, derived from the waterfall method10. Most native apps were designed to be used only by caregivers (n = 15), with a few apps also targeting care recipients (n = 5), healthcare providers (n = 4), or all three roles (n = 2). Some of the articles referenced a theoretical framework when detailing the main features and/or components supported by the technology (n = 11). Frameworks used were specific to caregiving, such as the family-centered theory22 (n = 2) and the resiliency model of family stress, adjustment, and adaptation23 (n = 1), to the chronic condition, such as reasons and management of behavioral and psychological symptoms of dementia24 (n = 1), or to the intervention type, such as the theory of planned behavior25 (n = 1).
Study participants
The criteria for selecting participants in research with native apps varied greatly among the included studies. Table 3 presents a nonexhaustive list of the most frequent criteria used for selecting caregiver study participants. The most common inclusion criterion was self-identification as a primary caregiver (n = 21), which was less restrictive than the requirement to have a relationship with the care recipient, such as a partner, sibling, or child (n = 9). Moreover, several articles had inclusion criteria based on language skills (written or spoken) (n = 11), age (n = 9), living arrangements (e.g., sharing the same household or living within a short-distance drive of the care recipient) (n = 8), access to the Internet (n = 8), and access to a computer or smartphone (n = 6). Articles reported exclusion criteria less frequently (n = 24). These criteria included health issues (n = 7), cognitive impairment (n = 4), and caregiver burden (n = 3). The criteria to exclude caregivers based on burden was generally not defined in absolute terms and, instead, was individually assessed by the research team. Some articles reported challenges in recruiting caregivers who were available and interested to participate in the evaluation of the native app26–28 and consequently relaxed the selection criteria to address these challenges. As characteristics of caregivers participating in research, several studies reported the gender, education, caregiving experience, caregiving intensity, working situation, ethnicity, and/or frequency of technology use by participants.
Table 3.
Eligibility criteria of caregiver study participants.
| Characteristic | Used as inclusion criteria | Used as exclusion criteria |
|---|---|---|
| Access to computer/smartphone | 28,32,33,50,51,58 | 41,45 |
| Access to the Internet | 37,43,46,47,51,54,55,58 | |
| Age | 26,32,34,39,43,45,50,58,59 | 45,58 |
| Caregiver burden | 49 | 37,44,47 |
| Caregiving experience | 49,51,58 | |
| Cognitive impairment | 44,49,57,63 | |
| Familiarity with technology | 31,37,45,47,63 | 45 |
| Health issues | 28 | 28,37,44,47,49,51,58 |
| Language skills | 26,32,34,39,41,43,45,46,50,58,59 | |
| Literacy/education level | 45 | 45,49 |
| Living arrangements | 27,35,44,46,52,53,57,63 | |
| Relationship with care recipient | 27,28,31,35,36,38,44,49,56 | |
| Self-identify as caregiver | 26,32,33,37,39–41,43,45–48,50–55,57,59,63 | |
| Not reported/none | 42,60–62 | 26,27,31–36,38–40,42,43,46,48,52–56,59–62 |
Outcomes measured
According to the CONSORT-EHEALTH29 reporting guidelines, studies on DHT should describe use outcomes in addition to primary/secondary health outcomes. Use outcomes (e.g., engagement, frequency of use, or adherence) and nonuse outcomes (e.g., attrition) are examples of process outcomes required for the interpretation of results. Quality characteristics of the system, such as usability, effectiveness (i.e., accuracy and completeness with which users achieve specified goals), and efficiency (i.e., resources consumed to achieve goals)30, can facilitate process outcomes, such as adoption and acceptability. Overall, 14 articles investigated only process outcomes26,31–43; 11 articles investigated only health outcomes27,44–53; 7 articles investigated both28,54–59; and four articles did not investigate primary or secondary health outcomes for caregivers60–63.
The CONSORT-EHEALTH guidelines also recommend the use of qualitative and quantitative methods for a comprehensive evaluation of DHT; whereby qualitative methods help explore subjective perceptions of participants regarding the system, and quantitative methods (e.g., surveys and scales) provide an objective measure of an attribute and/or concept29. The majority of articles included in this review employed quantitative methods (n = 13)26–28,41,44,45,49,51–53,56,57,63 or a combination of quantitative and qualitative methods (n = 10)32,36,37,39,42,46,47,50,58,59. Table 4 lists the most common outcomes and associated quantitative instrument measures utilized in research, such as caregiver burden (n = 9), depression (n = 8), and quality of life (n = 4).
Table 4.
Quantitative instruments used to explore caregiving, health/wellbeing, and process outcomes.
| Outcome Group | Outcome(s) | Assessment instrument(s) | Ref |
|---|---|---|---|
| Caregiving | Burden, stress | Zarit Burden Interview (ZBI) (Custom*, 3-item)48 | 48 |
| Zarit Burden Interview (ZBI) Screening (4-item)86 | 46,54,55 | ||
| Zarit Burden Interview (ZBI) Short (12-item)86 | 27,56,58 | ||
| Zarit Burden Interview (ZBI) (22-item)87 | 49,51 | ||
| Caregiving | Caregiver competence, sense of competence | Caregiver Competence (CCS) (Custom, 4-item)88 | 49 |
| Short Sense of Competence Questionnaire (SSCQ) (7-item)89 | 27,44,47 | ||
| Caregiving | Quality of life, Care-related quality of life | CarerQoL (7-item)90 | 47,58 |
| PROMIS Global Health-10 scale91 | 59 | ||
| Quality of Life—Family Version (QoL-FV)92 | 45 | ||
| Health/wellbeing | Anxiety, depression, depressive symptoms | Center for Epidemiological Studies Depression Scale (CES-D) (20-item)93 | 27,28,44,49 |
| Hospital Anxiety and Depression Scale (HADS) (7-item)94 | 44,47 | ||
| Patient Health Questionnaire-2 (PHQ-2) (2-items)95 | 46 | ||
| Patient Health Questionnaire-9 (PHQ-9) (9-items)96 | 58 | ||
| Quick Inventory of Depressive Symptoms-Self-Rated (QIDS) (16-item)97 | 50 | ||
| Health/wellbeing | Social support, social support interactions, social support relations | Gain Through Group Involvement Scale (GAINSCL) (15-item)98 | 54,55 |
| Social Support List 12-Interactions (SSL12-I) (12-item)99 | 47 | ||
| Multidimensional Scale of Perceived Support (MSPSS) (12-item)100 | 47 | ||
| Health/wellbeing | Stress | Perceived Stress Scale (PSS) (10-item)101 | 44,47 |
| Process | Tool satisfaction, mobile application rating | Program Participation Questionnaire (PPQ) (Custom, 34-item)102 | 37,47 |
| User Version of Mobile App Rating Scale (uMARS) (20-item)103 | 43 | ||
| Process | Usability | ISONORM 9241/10 (7-item)104 | 56 |
| System Usability Scale (10-item)105 | 33,43 |
*An instrument labeled as “custom” indicates that it was adapted and/or shortened to fit research purposes.
Discussion
Principal findings
Smartphones offer a new avenue to support informal caregivers of chronically ill individuals. Recent reviews of international application stores identified several native apps targeting caregivers16, yet the scholarly literature about the design and evaluation of native apps remains scarce11. Literature reviews on technologies developed for caregivers have mainly focused on single chronic conditions, such as dementia, and include general-purpose apps, such as social media and videoconferencing64. In contrast to previous reviews, we investigated native apps within a broader set of chronic conditions, including combinations thereof. Also, this scoping review incorporates literature from different disciplines, such as psychology, medicine, and computer science. Despite the broader focus of this review, most included articles were published in the last 2 years, indicating the nascency of this growing research area. The following discussion focuses on supports for various chronic conditions, design and evaluation of DHT for informal caregivers, and research considerations.
Supports for chronic conditions
Much of the literature covered in this review consists of native apps for Alzheimer’s and other dementia (n = 18), many of which were exclusively designed for caregivers (n = 11) and presented components and/or features for the education of caregivers about dementia, socialization (e.g., support groups, social networks), and/or self-care (e.g., mindfulness, journaling). However, this review identified a lack of research on native apps among other prevalent chronic conditions for an adult population, such as COPD, arthritis, or diabetes, and only one app targeting MCC. It can be challenging to find appropriate guidance for two or more chronic conditions as caregivers may need to be in close communication with a wide variety of health care providers to understand the specific needs of care recipients18, which may include pain management, palliative care, and/or multiple medications (polypharmacy). Considering the high prevalence of MCC in the adult population, more research is needed to understand the supports required by caregivers for managing various comorbidities and, possibly, the extent to which existing apps for single chronic conditions may be appropriate for MCC.
Design and evaluation considerations
Smartphone applications could play an important role for the democratization of healthcare, and many of the apps found in this review offer new opportunities for caregivers to access health information. In addition to providing health information, some apps aim to fulfill specific caregiving needs, such as planning activities with the care recipient (e.g., social visits, appointments), making medical decisions (e.g., as a surrogate or shared-decision maker), or building caregiving skills (e.g., coping strategies, communication, competence). Caregivers may need support in different areas, and the personalization of apps has been suggested to support specific caregiving needs34. In particular, the personalization of apps could help mitigate issues concerning the quantity and quality of general health information, such as information overload18,65 and poor readability66. Several features may be explored to personalize apps based on common, foreseeable needs of caregivers, such as filters that direct users to specific resources, algorithms that make recommendations based on usage data and/or preferences (e.g., age, language, or location), or adaptive technologies that improve accessibility for individuals with a range of characteristics and capabilities67, such as closed captioning, text-to-speech, and coloring9. To meet caregiving needs that may be specific and/or time-sensitive, some DHT may introduce components that are individually tailored 27,42,44,52.
Several apps found in this review aimed to reduce caregiver burden. A previous meta-analysis found no effect of web-based interventions on caregiver burden, possibly due to heterogeneous technologies and caregiver characteristics observed among studies12. In this review, different versions of the Zarit Burden Scale were selected to measure burden, resulting in variability that could also make it difficult to compare research findings68. More recently, an understanding of informal caregiver burnout has emerged in which subjective burden is considered a measure of appraisal of the caregiving experience rather than as an outcome3. The use of theoretical frameworks for the design and evaluation of DHT has been recommended as a means to clarify relationships between intervention components and primary and/or secondary health outcomes69,70. However, only a few native apps in this review refer to a theoretical framework that is specific to caregiving, such as the family-centered theory22. Considering both positive and negative outcomes as part of a caregiver’s lived experience is important for a more balanced view3,71. Therefore, a comprehensive theory about caregiving could be particularly beneficial because it could help explain variations in health outcomes.
Research considerations
Considerations about the eligibility criteria and recruitment strategies of research participants are usually made early in the investigation of novel technologies and have implications for the interpretation and generalizability of research findings. Nonrecruitment and self-selection have been observed to limit the participation of vulnerable populations in research involving DHT72, and more research is needed to reach out to vulnerable caregivers who could be at greater risk of experiencing caregiving burden, such as those who dedicate several hours per day to caregiving, have low education, or face financial stress73. Some articles included in this scoping review have reported challenges to recruiting caregivers26–28 and the issue may have been compounded by strict selection criteria targeting individuals with specific abilities and/or material means, such as ownership of a smartphone of a particular brand, fluency in one language, or access to the Internet at home. Thereby, studies investigating DHT could have inadvertently excluded research participants within particularly important demographics74, such as caregivers who live in underserved or remote areas, who do not own smartphones, or who are cognitively impaired.
Rigorous research designs, such as randomized controlled trials, are an essential step in determining whether a DHT is able to achieve desired health outcomes70,75,76. This review identified a lack of studies aimed to provide evidence on the effectiveness of native apps for informal caregivers, with only five native apps investigated in clinical trials. For highly adaptable DHT in which research participants may be assigned different components and/or features (e.g., system notifications, frequency of use, or educational resources), complex research designs can be used to compare intervention variations75. Furthermore, considering that caregiving is an activity that may require substantial commitment over an extended period of time, long-term effects of using apps require further investigation77, including apps with custom-evaluative features to screen and/or follow-up on research participants.
Study limitations
Limitations to this study include that the search strategy may have missed potentially relevant articles not indexed by the databases selected in this review. To help mitigate this issue, an expert librarian revised the search strategy, and the reference lists of retrieved reviews were hand-searched to identify additional articles. It is also possible that tablet applications were excluded if compatibility with smartphones was not explicitly stated in the article. This scoping review aims to provide a preliminary map of the literature on native apps for informal caregivers, including apps at very early stages of research and development. As a common limitation of scoping reviews19, this review does not include a critical appraisal of the methodological quality and risk of bias of the included articles.
Conclusions
This scoping review explores emergent native apps aimed to support informal caregivers across a diverse set of chronic conditions. Most studies included in the scoping review target caregivers of individuals with Alzheimer’s and other dementia, but key application features and/or components (e.g., education, screening, and social support) could be useful for other caregiver groups as well. By investigating the design and development of apps across various common types of chronic illnesses, this review aims to support the development of DHT for those caring for individuals with MCC. Due to heterogeneous designs and methods employed in the evaluation of apps, as well as the scarce number of trials, limited evidence is currently available for meta-analysis of clinical effectiveness. Further research is needed to understand how DHT could benefit caregivers and care recipients and to personalize apps based on specific caregiving needs.
Methods
Design
This scoping review followed Arksey and O’Malley’s framework for performing scoping reviews78. The framework comprises of (i) identifying the research question; (ii) searching for relevant studies; (iii) selecting relevant studies; (iv) charting the data; and (v) collating, summarizing, and reporting the results. The search terms combined subject headings and text words related to the three concepts of caregivers, DHT, and chronic diseases. For the review, the ten major chronic diseases prevalent in Canada as identified by the Public Health Agency of Canada were used, which were namely heart disease, stroke, cancer, chronic obstructive respiratory disease (COPD), asthma, diabetes, Alzheimer’s disease or other dementia, rheumatoid arthritis, hypertension, and mood or anxiety disorders1. Search strategies were reviewed by an experienced biomedical librarian from the Gerstein Science Information Center, University of Toronto. All search strategies can be found in the the Supplementary Table 1. Relevant articles were searched in Medline, Embase, CINAHL, ProQuest, PsycINFO, and ACM Digital Library. References of retrieved reviews were hand-searched to identify additional relevant articles. The initial searches were conducted in December 2019 and subsequently repeated to capture additional articles indexed until January 2021.
Selection criteria
Articles were included if they met all of the following inclusion criteria: (i) care recipients are adults aged 18 years or older who have been diagnosed with one or more of the 10 chronic diseases stated above; (ii) the article describes or evaluates a native app that can be installed in the caregiver’s smartphone; and (iii) the app was purposively developed with a primary goal to support informal caregivers (e.g., family or friends of the care recipient). Articles could encompass full-length journal articles and conference proceedings. Conversely, articles were excluded if they met any of the following exclusion criteria: (i) the app targets institutionalized care recipients (e.g., long-term care, nursing homes, and hospitalized) and/or professional caregivers (e.g., clinicians, nurses, or paid support workers); (ii) the study investigates a general-purpose technology (e.g., social media, videoconferencing, and messenger) that is not tailor-made for caregiving; (iii) the article is not in English; or (iv) the article is a review, perspective, opinion, fast abstract, or commentary.
Selection of studies
Two researchers (MG and AS) independently applied the selection criteria to all articles retrieved through the search strategy in a two-phased process. First, the title and abstract of all entries in the dataset were screened in duplicate. Second, both reviewers screened the full text of the remaining entries to confirm their relevance to the research question. Disagreements on the selection of articles were resolved among the two reviewers in a consensus meeting. At this stage, one author (MG) hand-searched the reference list of retrieved reviews to identify additional articles not indexed by any of the databases searched in this review. The entire process was iterated once more when the database searches were repeated in January 2021. Mendeley (Elsevier) was used for managing references, Covidence (Veritas Health Innovation Ltd.) was used to support independent screening and data extraction, and Excel (Microsoft) was used for data analysis.
Data extraction and data analysis
Two authors (MG and AS) independently extracted data from included articles. Based on the CONSORT-EHEALTH guidelines29, the following information from the included articles was collected: (i) article characteristics (i.e., author(s), publication year(s), and country of origin); (ii) caregiver characteristics (e.g., age, gender, and education); (iii) study characteristics (e.g., theoretical framework, research design, purpose, selection criteria, and outcomes); and (iv) application details (e.g., targeted chronic conditions, platforms, distribution, usage mode, and development stage). Once the information from all included articles was extracted, descriptive quantitative analysis was used to summarize the frequency and distribution of native apps among platforms, chronic conditions, study design, caregiver participant-selection criteria, and investigated outcomes. Finally, all authors met to collectively discuss the areas in which the design and development of native apps for caregivers could be improved based on the information from the included articles.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Funding for this research was provided by the São Paulo Research Foundation (FAPESP), grants 2017/22107-2, 2018/24173-5, and the Canadian Institutes of Health Research Personalized Health Catalyst Grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author contributions
This work was first conceived by M.G. and E.S. Study selection, screening, and data extraction were completed by M.G. and A.S. M.G. led paper writing with significant contribution from all authors in editing and revisions. The final version of the paper was approved by all authors.
Data availability
The aggregate data extracted and analyzed for this scoping review are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-022-00567-z.
References
- 1.Branchard B, et al. At-a-glance - How healthy are Canadians? A brief update. Health Promot. Chronic Dis. Prev. Can. 2018;38:385–390. doi: 10.24095/hpcdp.38.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 3.Gérain, P. & Zech, E. Informal Caregiver Burnout? Development of a Theoretical Framework to Understand the Impact of Caregiving. Front. Psychol. 10, 1748 (2019). [DOI] [PMC free article] [PubMed]
- 4.Szabo S, Lakzadeh P, Cline S, Palma dos Reis R, Petrella R. The clinical and economic burden among caregivers of patients with Alzheimer’s disease in Canada. Int. J. Geriatr. Psychiatry. 2019;34:1677–1688. doi: 10.1002/gps.5182. [DOI] [PubMed] [Google Scholar]
- 5.Zarit, S. H. & Zarit, J. M. Family Caregiving. in Psychology and Geriatrics 21–43 (Elsevier, 2015). 10.1016/B978-0-12-420123-1.00002-2.
- 6.Liu H, Lou VWQ. Transitioning into spousal caregiving: contribution of caregiving intensity and caregivers’ multiple chronic conditions to functional health. Age Ageing. 2019;48:108–114. doi: 10.1093/ageing/afy098. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard, S. C., Given, B., Petlick, N. H. & Bemis, A. Supporting Family Caregivers in Providing Care. in Patient Safety and Quality: An Evidence-Based Handbook for Nurses (ed. Hughes, R. G.) 1–341–1–404 (Agency for Healthcare Research and Quality (US), 2008). [PubMed]
- 8.Schulz R, Czaja SJ. Family caregiving: a vision for the future. Am. J. Geriatr. Psychiatry. 2018;26:358–363. doi: 10.1016/j.jagp.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker, R. Digital Inclusion. In Developing Inclusive Mobile Apps 17–49 (Apress, 2020). 10.1007/978-1-4842-5814-9_2.
- 10.Jabangwe R, Edison H, Duc AN. Software engineering process models for mobile app development: A systematic literature review. J. Syst. Softw. 2018;145:98–111. [Google Scholar]
- 11.Lorca-Cabrera J, et al. Effectiveness of health web-based and mobile app-based interventions designed to improve informal caregiver’s well-being and quality of life: a systematic review. Int. J. Med. Inform. 2020;134:104003. doi: 10.1016/j.ijmedinf.2019.104003. [DOI] [PubMed] [Google Scholar]
- 12.Ploeg J, et al. Caregiver-focused, web-based interventions: systematic review and meta-analysis (part 2) J. Med. Internet Res. 2018;20:e11247. doi: 10.2196/11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter G, Monaghan C, Santin O. What is known from the existing literature about peer support interventions for carers of individuals living with dementia: a scoping review. Health Soc. Care Community. 2020;28:1134–1151. doi: 10.1111/hsc.12944. [DOI] [PubMed] [Google Scholar]
- 14.Faieta, J. Development and evaluation of an Alzheimer’s Disease mHealth Device Evaluation Tool. in Dissertation Abstracts International. B, The Sciences and Engineering82, The Ohio State University (2021).
- 15.Shin JY, Choi SW. Online interventions geared toward increasing resilience and reducing distress in family caregivers. Curr. Opin. Support. Palliat. Care. 2020;14:60–66. doi: 10.1097/SPC.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorca-Cabrera J, et al. Mobile applications for caregivers of individuals with chronic conditions and/or diseases: quantitative content analysis. Int. J. Med. Inform. 2021;145:104310. doi: 10.1016/j.ijmedinf.2020.104310. [DOI] [PubMed] [Google Scholar]
- 17.van Velsen L, Beaujean DJ, van Gemert-Pijnen JE. Why mobile health app overload drives us crazy, and how to restore the sanity. BMC Med. Inform. Decis. Mak. 2013;13:23. doi: 10.1186/1472-6947-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price ML, Surr CA, Gough B, Ashley L. Experiences and support needs of informal caregivers of people with multimorbidity: a scoping literature review. Psychol. Health. 2019;35:36–69. doi: 10.1080/08870446.2019.1626125. [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ISO 9124-210. Ergonomics of human-system interaction - Part 210: Human-centred design for interactive systems (2019).
- 21.Vithani, T. & Kumar, A. Modeling the mobile application development lifecycle. in International MultiConference of Engineers and Computer Scientists (IMECS) 1–5 (2014).
- 22.Bamm EL, Rosenbaum P. Family-centered theory: origins, development, barriers, and supports to implementation in rehabilitation medicine. Arch. Phys. Med. Rehabil. 2008;89:1618–1624. doi: 10.1016/j.apmr.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Yeh P-M, Bull M. Use of the resiliency model of family stress, adjustment and adaptation in the analysis of family caregiver reaction among families of older people with congestive heart failure. Int. J. Older People Nurs. 2012;7:117–126. doi: 10.1111/j.1748-3743.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- 24.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369–h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajzen, I. & Fishbein, M. Understanding Attitudes Predicting Social Behavior (Prentice-Hall, 1980).
- 26.Howe D, et al. The CAREGIVERSPRO-MMD platform as an online informational and social support tool for people living with memory problems and their carers: an evaluation of user engagement, usability and usefulness. J. Appl. Gerontol. 2020;39:1303–1312. doi: 10.1177/0733464819885326. [DOI] [PubMed] [Google Scholar]
- 27.Williams KN, et al. Supporting family caregivers with technology for dementia home care: a randomized controlled trial. Innov. aging. 2019;3:igz037. doi: 10.1093/geroni/igz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otero P, Hita I, Torres ÁJ, Vázquez FL. Brief psychological intervention through Mobile App and conference calls for the prevention of depression in non-professional caregivers: a pilot study. Int. J. Environ. Res. Public Health. 2020;17:4578. doi: 10.3390/ijerph17124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eysenbach G. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J. Med. Internet Res. 2011;13:e126. doi: 10.2196/jmir.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ISO/IEC 25010. Systems and software engineering — Systems and software Quality Requirements and Evaluation (SQuaRE) — System and software quality models (2011).
- 31.Boessen ABCG, Verwey R, Duymelinck S, van Rossum E. An online platform to support the network of caregivers of people with dementia. J. Aging Res. 2017;2017:3076859. doi: 10.1155/2017/3076859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittenberg E, Xu J, Goldsmith J, Mendoza Y. Caregiver communication about cancer: development of a mhealth resource to support family caregiver communication burden. Psychooncology. 2019;28:365–371. doi: 10.1002/pon.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rettinger, L. et al. A mixed-methods evaluation of a supporting app for informal caregivers of people with dementia. in Proceedings of the 13th ACM International Conference on PErvasive Technologies Related to Assistive Environments 1–8 (ACM, 2020). 10.1145/3389189.3397981.
- 34.Ruggiano N, et al. The potential of information technology to navigate caregiving systems: perspectives from dementia caregivers. J. Gerontol. Soc. Work. 2019;62:432–450. doi: 10.1080/01634372.2018.1546786. [DOI] [PubMed] [Google Scholar]
- 35.Williams, K. N., Shaw, C. A., Perkhounkova, Y., Hein, M. & Coleman, C. K. Satisfaction, utilization, and feasibility of a telehealth intervention for in-home dementia care support: A mixed methods study. Dementia. 10.1177/1471301220957905 (2020). [DOI] [PMC free article] [PubMed]
- 36.Costa Stutzel M, et al. Multi-part quality evaluation of a customized mobile application for monitoring elderly patients with functional loss and helping caregivers. BMC Med. Inform. Decis. Mak. 2019;19:140. doi: 10.1186/s12911-019-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dam AEH, et al. Process evaluation of a social support platform “Inlife” for caregivers of people with dementia. Internet Inter. 2019;15:18–27. doi: 10.1016/j.invent.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halbach T, Solheim I, Ytrehus S, Schulz T. A mobile application for supporting dementia relatives: a case study. Stud. Health Technol. Inform. 2018;256:839–846. [PubMed] [Google Scholar]
- 39.Heynsbergh N, Heckel L, Botti M, O SC, Livingston PM. Development of a Smartphone App for Informal carers of people with cancer: processes and learnings. JMIR Form. Res. 2019;3:e10990. doi: 10.2196/10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kayyali R, et al. Qualitative investigation into a wearable system for chronic obstructive pulmonary disease: the stakeholders’ perspective. BMJ Open. 2016;6:e011657. doi: 10.1136/bmjopen-2016-011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmood A, et al. Acceptability and attitude towards a mobile-based home exercise program among stroke survivors and caregivers: a cross-sectional study. Int. J. Telemed. Appl. 2019;2019:5903106. doi: 10.1155/2019/5903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarro, R. F. & Favela, J. Usability assessment of a pervasive system to assist caregivers in dealing with repetitive behaviors of patients with dementia. in Proceedings of the 4th International Conference on PErvasive Technologies Related to Assistive Environments 28:1–28:8 (ACM, 2011). 10.1145/2141622.2141656.
- 43.Rathnayake S, Moyle W, Jones CJ, Calleja P. Development of an mHealth application for family carers of people with dementia: a study protocol. Collegian. 2019;26:295–301. [Google Scholar]
- 44.Bartels, S. L. et al. The necessity for sustainable intervention effects: lessons-learned from an experience sampling intervention for spousal carers of people with dementia. Aging Ment. Health 1–11. 10.1080/13607863.2019.1647130 (2019). [DOI] [PubMed]
- 45.Uysal N, et al. Empowering caregivers in the radiotherapy process: the results of a randomized controlled trial. Support. Care Cancer. 2020 doi: 10.1007/s00520-020-05743-z. [DOI] [PubMed] [Google Scholar]
- 46.Brown EL, et al. CareHeroes Web and AndroidTM apps for dementia caregivers: a feasibility study. Res. Gerontol. Nurs. 2016;9:193–203. doi: 10.3928/19404921-20160229-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dam AEH, de Vugt ME, van Boxtel MPJ, Verhey FRJ. Effectiveness of an online social support intervention for caregivers of people with dementia: the study protocol of a randomised controlled trial. Trials. 2017;18:395. doi: 10.1186/s13063-017-2097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes JC, Banerjee T, Goodman G, Lawhorne L. A preliminary qualitative analysis on the feasibility of using gaming technology in caregiver assessment. J. Technol. Hum. Serv. 2017;35:183–198. [Google Scholar]
- 49.Núñez-Naveira L, et al. UnderstAID, an ICT platform to help informal caregivers of people with dementia: a pilot randomized controlled study. Biomed. Res. Int. 2016;2016:5726465. doi: 10.1155/2016/5726465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sikder AT, et al. Mentalizing imagery therapy mobile app to enhance the mood of family dementia caregivers: feasibility and limited efficacy testing. JMIR Aging. 2019;2:e12850. doi: 10.2196/12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park E, Park H, Kim EK. The effect of a comprehensive mobile application program (CMAP) for family caregivers of home‐dwelling patients with dementia: a preliminary research. Japan J. Nurs. Sci. 2020;17:e12343. doi: 10.1111/jjns.12343. [DOI] [PubMed] [Google Scholar]
- 52.Shaw CA, Williams KN, Perkhounkova Y, Hein M, Coleman CK. Effects of a video-based intervention on caregiver confidence for managing dementia care challenges: findings from the FamTechCare clinical trial. Clin. Gerontol. 2020;43:508–517. doi: 10.1080/07317115.2020.1729917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw CA, Williams KN, Lee RH, Coleman CK. Cost‐effectiveness of a telehealth intervention for in‐home dementia care support: Findings from the FamTechCare clinical trial. Res. Nurs. Health. 2021;44:60–70. doi: 10.1002/nur.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis BH, Nies MA, Shehab M, Shenk D. Developing a pilot e-mobile app for dementia caregiver support: lessons learned. Online J. Nurs. Inform. 2014;18:21–28. [Google Scholar]
- 55.Davis BH, Shehab M, Shenk D, Nies M. E-mobile pilot for community-based dementia caregivers identifies desire for security. Gerontechnology. 2014;13:332–336. [Google Scholar]
- 56.Megges H, Freiesleben SD, Jankowski N, Haas B, Peters O. Technology for home dementia care: a prototype locating system put to the test. Alzheimer’s Dement. Transl. Res. Clin. Inter. 2017;3:332–338. doi: 10.1016/j.trci.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamal AK, et al. Translating knowledge for action against stroke-using 5-minute videos for stroke survivors and caregivers to improve post-stroke outcomes: study protocol for a randomized controlled trial (Movies4Stroke) Trials. 2016;17:52. doi: 10.1186/s13063-016-1175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabir ZN, et al. Care of family caregivers of persons with dementia (CaFCa) through a tailor-made mobile app: study protocol of a complex intervention study. BMC Geriatr. 2020;20:305. doi: 10.1186/s12877-020-01712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rozwadowski M, et al. Promoting health and well-being through mobile health technology (Roadmap 2.0) in family caregivers and patients undergoing hematopoietic stem cell transplantation: protocol for the development of a mobile randomized controlled trial. JMIR Res. Protoc. 2020;9:e19288. doi: 10.2196/19288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzallas, A. T. et al. Designing a gamified social platform for people living with dementia and their live-in family caregivers. in Proceedings of the 11th PErvasive Technologies Related to Assistive Environments Conference 476–481 (ACM, 2018). 10.1145/3197768.3201560.
- 61.Solachidis, V. et al. Two examples of online eHealth platforms for supporting people living with cognitive impairments and their caregivers. in Proceedings of the 11th PErvasive Technologies Related to Assistive Environments Conference 449–454 (ACM, 2018). 10.1145/3197768.3201556.
- 62.Rathnayake S, Moyle W, Jones C, Calleja P. Co-design of an mHealth application for family caregivers of people with dementia to address functional disability care needs. Inform. Health Soc. Care. 2021;46:1–17. doi: 10.1080/17538157.2020.1793347. [DOI] [PubMed] [Google Scholar]
- 63.Kamal A, et al. Effect of 5-minute movies shown via a Mobile Phone App on risk factors and mortality after stroke in a low- to middle-income country: randomized controlled trial for the stroke caregiver dyad education intervention (Movies4Stroke) JMIR mHealth uHealth. 2020;8:e12113. doi: 10.2196/12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faieta, J., Sheehan, J. & DiGiovine, C. Mhealth interventions to improve health and quality of life related outcomes for informal dementia caregivers: A scoping review. Assist. Technol. 1–13 10.1080/10400435.2020.1829174 (2021). [DOI] [PubMed]
- 65.Khaleel I, et al. Health information overload among health consumers: a scoping review. Patient Educ. Couns. 2020;103:15–32. doi: 10.1016/j.pec.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Mcinnes N, Haglund BJA. Readability of online health information: implications for health literacy. Inform. Heal. Soc. Care. 2011;36:173–189. doi: 10.3109/17538157.2010.542529. [DOI] [PubMed] [Google Scholar]
- 67.Persson H, Åhman H, Yngling AA, Gulliksen J. Universal design, inclusive design, accessible design, design for all: different concepts—one goal? On the concept of accessibility—historical, methodological and philosophical aspects. Univers. Access Inf. Soc. 2015;14:505–526. [Google Scholar]
- 68.Yu J, Yap P, Liew TM. The optimal short version of the Zarit Burden Interview for dementia caregivers: diagnostic utility and externally validated cutoffs. Aging Ment. Health. 2019;23:706–710. doi: 10.1080/13607863.2018.1450841. [DOI] [PubMed] [Google Scholar]
- 69.Pingree S, et al. The value of theory for enhancing and understanding e-health interventions. Am. J. Prev. Med. 2010;38:103–109. doi: 10.1016/j.amepre.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Monitoring and Evaluating Digital Health Interventions (2016).
- 71.Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: a reappraisal from population-based studies. Gerontologist. 2015;55:309–319. doi: 10.1093/geront/gnu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poli A, Kelfve S, Motel-Klingebiel A. A research tool for measuring non-participation of older people in research on digital health. BMC Public Health. 2019;19:1487. doi: 10.1186/s12889-019-7830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden. JAMA. 2014;311:1052. doi: 10.1001/jama.2014.304. [DOI] [PubMed] [Google Scholar]
- 74.Veinot TC, Mitchell H, Ancker JS. Good intentions are not enough: how informatics interventions can worsen inequality. J. Am. Med. Inform. Assoc. 2018;25:1080–1088. doi: 10.1093/jamia/ocy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray E, et al. Evaluating Digital Health Interventions. Am. J. Prev. Med. 2016;51:843–851. doi: 10.1016/j.amepre.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Enam A, Torres-Bonilla J, Eriksson H. Evidence-based evaluation of ehealth interventions: systematic literature review. J. Med. Internet Res. 2018;20:e10971. doi: 10.2196/10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthew-Maich N, et al. Designing, implementing, and evaluating mobile health technologies for managing chronic conditions in older adults: a scoping review. JMIR mHealth uHealth. 2016;4:e29. doi: 10.2196/mhealth.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 2005;8:19–32. [Google Scholar]
- 79.Phinney A, Chaudhury H, O’connor DL. Doing as much as I can do: the meaning of activity for people with dementia. Aging Ment. Health. 2007;11:384–393. doi: 10.1080/13607860601086470. [DOI] [PubMed] [Google Scholar]
- 80.Cox EO. Empowerment-oriented practice applied to long-term care. J. Soc. Work Long. Term. Care. 2001;1:27–46. [Google Scholar]
- 81.Knowles, M. S. Andragogy in Action: Applying Modern Principles of Adult Learning (Jossey-Bass, 1984).
- 82.Larson, R. & Csikszentmihalyi, M. The experience sampling method. in Flow and the Foundations of Positive Psychology 21–34 (Springer Netherlands, 2014). 10.1007/978-94-017-9088-8_2.
- 83.Goldsmith J, Wittenberg E, Platt CS, Iannarino NT, Reno J. Family caregiver communication in oncology: advancing a typology. Psychooncology. 2016;25:463–470. doi: 10.1002/pon.3862. [DOI] [PubMed] [Google Scholar]
- 84.Venkatesh, V., Morris, M. G., Davis, G. B. & Davis, F. D. User acceptance of information technology: toward a unified view. MIS Q. 27, 425 (2003).
- 85.Rogers EM. A prospective and retrospective look at the diffusion model. J. Health Commun. 2004;9:13–19. doi: 10.1080/10810730490271449. [DOI] [PubMed] [Google Scholar]
- 86.Bédard M, et al. The Zarit Burden interview. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 87.Zarit, S. H., Orr, N. K. & Zarit, J. M. The hidden victims of Alzheimer’s disease: Families under stress. (New York University Press, 1985).
- 88.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 89.Vernooij-Dassen MJFJ, Persoon JMG, Felling AJA. Predictors of sense of competence in caregivers of demented persons. Soc. Sci. Med. 1996;43:41–49. doi: 10.1016/0277-9536(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 90.Brouwer WBF, van Exel NJA, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual. Life Res. 2006;15:1005–1021. doi: 10.1007/s11136-005-5994-6. [DOI] [PubMed] [Google Scholar]
- 91.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual. Life Res. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okcin F, Karadakovan A. Reliability and validity of the quality of life-family version (QOL-FV) in Turkish family caregivers of patients with cancer. Asian Pac. J. Cancer Prev. 2012;13:4235–4240. doi: 10.7314/apjcp.2012.13.9.4235. [DOI] [PubMed] [Google Scholar]
- 93.Radloff LS. The CES-D scale. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 94.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 95.Kroenke K, Spitzer RL, Williams JBW. The patient health questionnaire-2. Med. Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 96.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rush AJ, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 98.Kaye LW. Assessing the efficacy of a self-help support group program for older women. J. Women Aging. 1996;7:11–30. [Google Scholar]
- 99.Kempen GIJM, Van Eijk LM. The psychometric properties of the SSL12-I, a short scale for measuring social support in the elderly. Soc. Indic. Res. 1995;35:303–312. [Google Scholar]
- 100.Marziali E, Garcia LJ. Dementia caregivers’ responses to 2 internet-based intervention programs. Am. J. Alzheimer’s Dis. Other Demen. 2011;26:36–43. doi: 10.1177/1533317510387586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 102.Dam AEH, van Boxtel MPJ, Rozendaal N, Verhey FRJ, de Vugt ME. Development and feasibility of Inlife: A pilot study of an online social support intervention for informal caregivers of people with dementia. PLoS ONE. 2017;12:e0183386. doi: 10.1371/journal.pone.0183386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stoyanov SR, Hides L, Kavanagh DJ, Wilson H. Development and validation of the user version of the mobile application rating scale (uMARS) JMIR mHealth uHealth. 2016;4:e72. doi: 10.2196/mhealth.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neyer FJ, Felber J, Gebhardt C. Entwicklung und Validierung einer Kurzskala zur Erfassung von Technikbereitschaft. Diagnostica. 2012;58:87–99. [Google Scholar]
- 105.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int. J. Hum. Comput. Interact. 2008;24:574–594. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The aggregate data extracted and analyzed for this scoping review are available from the corresponding author on reasonable request.