Abstract
Myofibroblasts are the construction workers of wound healing and repair damaged tissues by producing and organizing collagen/extracellular matrix (ECM) into scar tissue. Scar tissue effectively and quickly restores the mechanical integrity of lost tissue architecture but comes at the price of lost tissue functionality. Fibrotic diseases caused by excessive or persistent myofibroblast activity can lead to organ failure. This review defines myofibroblast terminology, phenotypic characteristics, and functions. We will focus on the central role of the cell, ECM, and tissue mechanics in regulating tissue repair by controlling myofibroblast action. Additionally, we will discuss how therapies based on mechanical intervention potentially ameliorate wound healing outcomes. Although myofibroblast physiology and pathology affect all organs, we will emphasize cutaneous wound healing and hypertrophic scarring as paradigms for normal tissue repair versus fibrosis. A central message of this review is that myofibroblasts can be activated from multiple cell sources, varying with local environment and type of injury, to either restore tissue integrity and organ function or create an inappropriate mechanical environment.
Myofibroblast activities are most prominent in the proliferative, remodeling, and maturation phases of wound healing after organ injury. If platelets are the pioneer troops that construct a provisional “pontoon bridge” made of fibrin for neutrophils and macrophages to perform their janitorial services and vascular cells to lay new plumbing, myofibroblast are both construction workers and engineers tasked to reestablish the architectural integrity of the damaged tissue. Myofibroblasts efficiently repair relatively minor and acute injuries by restoring tissue mechanical properties via secretion and remodeling of resistant collagenous extracellular matrix (ECM) that replaces the mechanically weaker fibrin clot (Fig. 1A). The resulting scar may not be esthetically perfect and not fully regenerate tissue architecture and function, but quickly (i.e., within days) provides mechanical strength—much like using superglue on a broken plastic piece. However, scar tissue (and superglue) leaves a mark on the connected surfaces and tends to break under high strain because it is lacking compliance. Myofibroblast activities can become excessive and cause pathological deformation with loss of tissue function instead of physiological repair—a process called fibrosis. Driving the superglue analogy further, myofibroblast in fibrosis behaves like a continuously dripping source of superglue that can damage the structure and create unwanted adhesions. Conversely, with missing myofibroblasts and their ECM, wounds never heal and become chronic. Both, excessively scarring and nonhealing wounds represent a significant health burden on patients and health care systems (Hinz 2016b; desJardins-Park et al. 2021).
Figure 1.
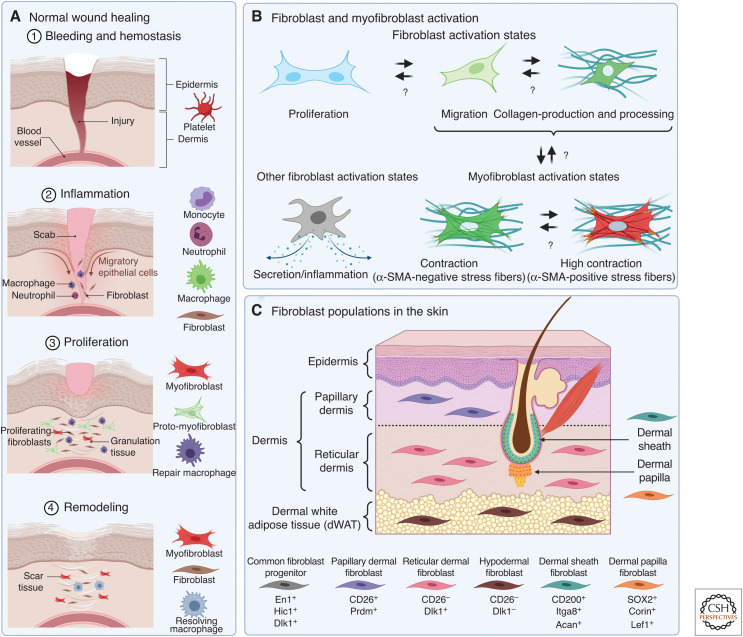
Fibroblasts and myofibroblasts in the skin. (A) Positioning of different myofibroblast activation states in the time line and cellular context of normal skin wound healing. (B) Fibroblast activation is a loose term that can entail enhanced proliferation, migration, collagen production, or acquisition of a secretory and/or inflammatory phenotype. Such pre-activated fibroblasts can proceed to undergo myofibroblast activation, which is characterized by the formation of contractile stress fibers that can contain α-smooth muscle actin (α-SMA). There is documented reversibility and plasticity between the different activation states and phenotypes in vitro and in animal models of fibrosis and healing, indicated by bidirectional arrows. Note that not all dermal fibroblast precursors will be able to acquire all activation states. (C) Schematic representation different fibroblast populations that reside in dermal and subcutaneous tissues and their molecular markers. Created with BioRender.com.
A striking example of excessive myofibroblast activity in the skin is the severe hypertrophic scarring following large area burns or trauma (Nabai and Ghahary 2017; Barnes et al. 2018; Yue et al. 2018; desJardins-Park et al. 2021; Sawant et al. 2021). Functional cutaneous tissues are replaced by dysfunctional scars in systemic sclerosis (SSc) (Denton and Khanna 2017; Henderson et al. 2019; Leask 2021) and fibrotic palmar fascia in Dupuytren's disease (Layton and Nanchahal 2019; Gonga-Cave et al. 2021). Fibrotic encapsulation by myofibroblasts of subcutaneous implants such as breast implants or glucose sensors is part of a foreign body response, which leads to disfigurement and implant failure (Noskovicova et al. 2021a). It is not entirely clear whether keloids represent a “myofibroblast disease” as these skin lesions are characterized by excessive accumulation of ECM rather than contractures and contain few myofibroblasts (Ehrlich et al. 1994; Sharma et al. 2019; Bell and Shaw 2021; Deng et al. 2021; Macarak et al. 2021). In other organs, loss of function due to the accumulation of myofibroblast-derived scar ECM can have lethal consequences, as in the lung (Denton and Khanna 2017; Reyfman et al. 2019; Volkmann and Varga 2019; Tsukui et al. 2020), liver (Schuppan et al. 2018), kidney (Falke et al. 2015), intestine (Wang et al. 2021), and the heart (Tallquist and Molkentin 2017; de Oliveira Camargo et al. 2021). Scarring of the eye severely impairs life quality (Hinz 2016a; Wilson 2020; O'Regan et al. 2021), and the scar tissue (stroma) forming around tumors can promote cancer progression and impede the delivery of drugs (Sahai et al. 2020; Davidson et al. 2021). The mechanisms that govern myofibroblast activities in normal healing but fail to do so in fibrosis and chronic wounds are not fully understood.
This review will define myofibroblast terminology, phenotypic characteristics, and functions for both newcomers and veterans of the wound healing field. For those unsatisfied with the entry statement attributing mere cleaning roles to inflammatory cells, we will conclude on the importance and hierarchy of myofibroblast–macrophage cross talk during the healing process. We will principally focus on the central role of cell and tissue mechanics in regulating tissue repair by controlling myofibroblast action and discuss how therapies based on targeting mechanical processes potentially can ameliorate wound healing outcomes. While myofibroblast physiology and pathology affect nearly all organs, we will emphasize skin wound healing and hypertrophic scarring as paradigms for normal tissue repair and fibrosis. One central message of this review is that myofibroblasts can be derived from multiple cell sources to perform repair functions that depend upon the local environment and type of injury. In contrast to the original view of myofibroblasts being terminally “differentiated” cells, we will develop that the myofibroblast should be considered an activation state that can be acquired by multiple precursor cells and—in some cases—is reversible (Soliman et al. 2021).
MYOFIBROBLASTS 101
Definition of the Myofibroblast
Since its discovery in the early 1970s in rat wound granulation and fibrotic tissue by Gabbiani et al. (1971), the myofibroblast has attracted increasing attention as additional roles have been revealed in the tumor stroma as cancer-associated fibroblasts (CAFs) (MacCarthy-Morrogh and Martin 2020; Sahai et al. 2020; Van Hove and Hoste 2022), as fibroblastic reticular cells in lymphoid organs and structures (Rodda et al. 2018; Perez-Shibayama et al. 2019; Fletcher et al. 2020), and as immunomodulating fibroblast-like synoviocytes (FLS) lining articular joints (Maglaviceanu et al. 2021; Schuster et al. 2021). The goal of selectively identifying and targeting pathological myofibroblasts while sparing beneficial populations has generated many different myofibroblast definitions and characteristics, not always adding clarity to a complex matter. When in doubt, history often provides an answer. The first published use of the term myofibroblast claims “…that fibroblasts, under certain conditions, are capable of modulating toward a cell type that is structurally and functionally close to smooth muscle; for these cells, the name ‘myofibroblast’ may be appropriate” (Majno et al. 1971). The smooth muscle cell (SMC) features referred to in these seminal studies included formation of actin–myosin bundles (stress fibers being the in vitro homologs) that generate contractile force (“myo”), whereas the “fibroblast” part of the name refers to cells with spindle-like morphology that are surrounded by and produce fibrous collagen-rich ECM (Virchow 1858), early on evidenced by abundant endoplasmic reticulum (Gabbiani et al. 1971). It is generally held that cell phenotypes missing either one of these most basic criteria should not be called myofibroblasts (Fig. 1B).
Enhanced expression of collagen type I, collagen α1 chain (ColIA1) promoter activity, and abundant ColIA1 mRNA are reliable indicators for the activation of fibroblastic cells from a state of low, homeostatic collagen turnover in healthy adult tissues (Kisseleva et al. 2012). However, contractile force is required to organize collagen fibers into scar tissue and to designate collagen-producing cells as myofibroblasts. Contractile stress fibers can assemble within minutes during myofibroblast activation, when globular (G-) actin monomers polymerize into filamentous (F-) actin that then aggregate into larger bundles together with myosin (Burridge and Guilluy 2016; Hinz et al. 2019). The transition of existing actin pools into stress fibers can occur without any transcriptional or translational changes and therefore is independent of gene expression. At the protein level, the fungal toxin phalloidin binds specifically to F-actin and reliably detects stress fibers in aldehyde-fixed cultured cells; however, this probe is incompatible with organic solvents and unsuitable for myofibroblast identification in paraffin-embedded tissue (Smith-Clerc and Hinz 2010).
Consistent with their SMC characteristics, myofibroblasts were shown to express the smooth muscle actin isoform α-smooth muscle actin (α-SMA) in stress fibers, and the corresponding antibody has become a gold-standard reagent to identify myofibroblasts in fibroproliferative tissues and in cell culture (Darby et al. 1990). We want to emphasize that expression of α-SMA is a hallmark for highly contractile myofibroblasts but not a myofibroblast prerequisite; formation of α-SMA-negative contractile stress fibers (e.g., composed of β-actin) already fulfils the myofibroblast criterion (Fig. 1B; Tomasek et al. 2002; Hinz et al. 2019; Younesi et al. 2021). Given that α-SMA is also expressed by other cells in normal and diseased tissues, including SMCs, pericytes, and dermal sheath cells in hair follicles (Heitman et al. 2020), additional histological inclusion and exclusion criteria should be used to define myofibroblasts (Pakshir et al. 2020). For instance, myofibroblasts do not typically express the SMC markers smooth muscle myosin, smoothelin, and desmin, which are also expressed in pericytes and SMCs, and are negative for keratins expressed in α-SMA-positive myoepithelium (Arnoldi et al. 2012; Tomasek et al. 2013). In contrast to myofibroblasts, pericytes and SMCs are negative or low for collagen expression in their normal state. Other frequently used, non-unique inclusion criteria for myofibroblasts are ECM proteins that are up-regulated in fibroproliferative conditions, such as the extradomain-A (ED-A) splice variant of fibronectin (FN) (Serini et al. 1998), periostin (Nikoloudaki et al. 2020), latent TGF-β-binding protein-1 (LTBP-1) (Rifkin et al. 2018), and tenascin-C (Yeo et al. 2018). Depending on tissues and conditions, activated myofibroblasts also express high levels of certain cell membrane proteins, including platelet-derived growth factor (PDGF) receptor β (PDGFRβ) (Henderson et al. 2013), α11β1 integrin (binds fibrillar collagen) (Zeltz et al. 2020), αv integrins (bind vitronectin, FN, and latent TGF-β) (Conroy et al. 2016), and cadherin-11 (binds cadherin-11 on other myofibroblasts or activated macrophages) (Lodyga et al. 2019; To and Agarwal 2019). More myofibroblast markers are to be expected to be discovered by single-cell RNA sequencing (scRNA-seq) studies performed with cells extracted from tissues under repair and in fibrosis. For instance, LRRC15 (leucine-rich repeat containing 15) has first been found to be expressed by CAFs activated into a contractile phenotype (“myCAF”) (Purcell et al. 2018) and now emerges as a pan-tissue discriminator and potential target of activated myofibroblasts. In contrast, interleukin (IL)-1-positive fibroblasts seem to be activated to perform immune-regulatory functions and lack myofibroblast features (“iCAF”) (Elyada et al. 2019; Dominguez et al. 2020; Buechler et al. 2021b).
As confounding as it may be for their detection and classification, plasticity is a vital feature of fibroblasts, and their activation can entail an array of features, including increased proliferation, migration, ECM secretion and cross-linking, degradation of collagen and other ECM components, phagocytosis, and even basic immune functions (Fig. 1B). None of these activation states alone qualify for the myofibroblast designation if stress fibers are absent, but these markers often precede and/or overlap the transition into contractile myofibroblasts. In the context of wound healing, it is intuitive that fibroblasts first must migrate from adjacent, intact tissue into the fibrin clot wound ECM that forms immediately after injury. Migration is accompanied and followed by proliferation and secretion of provisional ECM of fibrous collagen that is initially enriched in fine Col3 fibers (Volk et al. 2011). These early fibroblast activity states can then further activate into myofibroblasts that predominantly assemble Col1 fibers to generate an increasingly stiff environment augmented by the high contractile force produced by α-SMA stress fibers that are linked to collagens by integrins (Fig. 1B; Hinz et al. 2001a,b). Also intuitively, and for sheer energetic reasons, fibroblasts must specialize for the task in demand, which can explain why high collagen-expressing fibroblasts can lack α-SMA expression as an indicator for the highly contractile phenotype in screens (Tsukui et al. 2020) and vice versa. Whether producing concrete (collagen) with a cement mixer or shaping it with a trowel turns the (myofibroblast) construction worker into a different individuum is debatable and related to the question of where these industrious cells come from. It would also appear that different construction workers can take up a trowel and then look alike, as discussed in the next section.
Myofibroblast Origins
Despite carrying “fibroblast” in their name, myofibroblasts can be generated from different cell sources, including but not restricted to bona fide fibroblasts, pericytes, SMCs, and mesenchymal stromal cells (MSCs). In the past, efforts to establish specific fibroblast markers were limited, and fibroblasts were traditionally treated as the leftovers once all other cells of a tissue had been classified. More recent investigations in fetal and adult skin have identified distinct classes of fibroblasts with specific molecular markers, locations, and destinies (Fig. 1C). In skin, myofibroblast precursors with fibroblast character typically express collagen I, vimentin, PDGFRα (Iwayama et al. 2015; Li et al. 2018), β1 integrin (CD29) hypermethylated in cancer (Hic1) (Abbasi et al. 2020), Thy-1 (cluster of differentiation, CD90) (Jiang and Rinkevich 2018; Worthen et al. 2020), and fibroblast-specific protein 1 (Li et al. 2020). Fibroblasts do not typically express PDGFRβ (pericytes), desmin (SMCs and pericytes), or CD31 and VE-cadherin (endothelial cells), CD68 and CD45 (macrophages), and adiponectin and fatty acid-binding protein 4 (adipocytes) (Pakshir et al. 2020). These specific cell features help to discriminate fibroblasts from all the above-mentioned wound cells that also express the intermediate filament protein vimentin, an often used “fibroblast” marker in histology (Younesi et al. 2021). While vascular endothelial cells (Zhao et al. 2021) and circulating CD45-positive cells (fibrocytes, macrophages) (Sinha et al. 2018; Chong et al. 2019) seem to be possible—but more exotic—myofibroblast sources in healing and fibrotic skin, different fibroblast types (Chang et al. 2002; Jiang and Rinkevich 2018; Rognoni et al. 2018; Mascharak et al. 2020; Correa-Gallegos and Rinkevich 2021; Walker et al. 2021b), adipocytes (Festa et al. 2011; Marangoni et al. 2015; Shook et al. 2018), pericytes, and MSC are among the main contributors to the myofibroblast pool in the skin and other organs (Jiang and Scharffetter-Kochanek 2020; Soliman et al. 2021; Yokota et al. 2021).
The skin has been studied extensively to identify different fibroblast lineages and delineate their contribution to wound healing and scar formation by activating into myofibroblasts. The dermis is traditionally divided into two main layers: papillary dermis directly underlying the epidermis with lower fibroblast density, and deeper reticular dermis with higher fibroblast and ECM content. Other fibroblast-rich sources are the dermal white adipose tissue and fascia (Fig. 1C). Elegant lineage-tracing studies during murine development have demonstrated that early mesenchymal precursors give rise to skin fibroblast progenitors that share the same lineage, marked by the expression of engrailed 1 (En1), delta like noncanonical notch ligand 1 (Dlk1) and Hic1 (Driskell et al. 2013; Rinkevich et al. 2015). Flow cytometry and immunohistochemistry have distinguished two skin fibroblast populations that populate the papillary and reticular dermis. Early-stage papillary precursors in neonatal mouse skin express CD26, fibroblast activation protein (FAP), leucine-rich repeats, and immunoglobulin-like domains 1 (Lrig1), α8 integrin, and B-lymphocyte-induced maturation protein 1 (Blimp1), whereas precursors of reticular fibroblasts are enriched in CD90, peroxisome proliferator-activated receptor (PPAR)-γ, Ebf2, podoplanin, and Sca1 (Driskell et al. 2013; Korosec et al. 2019; Worthen et al. 2020). Advanced scRNAseq approaches reveal even higher complexities in skin fibroblast heterogeneity that have only begun to be appreciated (Philippeos et al. 2018; Tabib et al. 2018; He et al. 2020; Sole-Boldo et al. 2020; Vorstandlechner et al. 2020). A comprehensive meta-analysis of several scRNA-seq data sets has classified over 90% of all human dermal fibroblasts into three distinct categories based on the expression of apolipoprotein E and secreted frizzled-related proteins SFRP1 and SFRP2 (Ascension et al. 2021). SFRP2-positive fibroblasts are suggested to play roles in dermal homeostasis, apolipoprotein E-positive fibroblasts are implicated in diverse immune functions, and SFRP1-positive fibroblasts include specialized fibroblast subsets such as the dermal papilla cells and cells that populate the dermo–hypodermal junction. Hypodermal white fat tissue and fascia underlying the dermis are further repositories for cells that can undergo myofibroblast activation following cutaneous injury (Fig. 1C; Hepler et al. 2018; Correa-Gallegos and Rinkevich 2021; Plikus et al. 2021). Meta-analysis of fibroblast states and subtypes further revealed Pi16-positive and Col15a1-positive fibroblasts existing in almost all intact adult mouse tissues and therefore labeled “universal.” While Col15a1-positive universal fibroblasts are characterized by basement membrane ECM secretion profiles, the Pi16-positive universal fibroblasts appear to give rise to specialized organ fibroblasts (Buechler et al. 2021b).
As there seems to be no shortage of myofibroblast precursors in the skin, it is reasonable to ask whether all subtypes have the same potential to become activated, whether some populations are more prone to produce scar beyond normal healing, or whether some putative precursors are actually myofibroblast-impotent. Numerous scRNA-seq studies are currently being published, investigating mouse and human skin wound healing and fibrosis at single time points or longitudinally, from subjects with different ages, with and without consideration of spatial cell relationships (Salzer et al. 2018; Guerrero-Juarez et al. 2019; Phan et al. 2020, 2021; Foster et al. 2021). The fact that spatial relationships are typically lost in scRNA-seq is beginning to be solved by spatial transcriptomics and multiplexing technologies (Bingham et al. 2020). Collectively, the resulting data sets are “big” indeed, and meta-analyses will be required to analyze results across different studies as it has been done for normal adult skin (Ascension et al. 2021; Buechler et al. 2021b). Difficulties to consolidate data from multiple studies arise from varying tissue collection procedures, loci and time points, analysis designs (e.g., cluster annotation strategies), and at last “fibroblast” plasticity and phenotype switching during tissue repair (Rognoni et al. 2018; Goss et al. 2021). Those who place hope in meta-analyses of fibroblast contributions across different injured and fibrotic organs are referred to one recent scRNA-seq study performed on adult healthy mouse organs that showed as little as 20% overlap in gene expression among the populations clustered as fibroblasts (Muhl et al. 2020). Other limitations of scRNA-seq, when used alone, are possible discrepancies between transcript and protein expression and localization. mRNA profiles tell us what a cell desires to be, while protein expression reveals what it is. There is nevertheless tremendous potential in scRNA-seq profiling to devise therapeutic strategies that help to activate, inhibit, or introduce specific fibroblastic cell populations at different stages of wound healing to achieve significant wound healing outcomes. For instance, recent studies targeting proteins such as En1 and CD36 that are expressed in specific fibroblast populations show reduced myofibroblast activation in mouse models of hypertrophic scarring (Griffin et al. 2021; Mascharak et al. 2021a). It should further work in our favor that heterogenous mesenchymal cell populations can converge on a repair phenotype with one targetable function: remodeling wound tissue by contraction.
CONTRACTION IS THE KEY MYOFIBROBLAST FUNCTION
Granulation Tissue Is Contractile
The discovery of myofibroblasts as nonmuscle cells forming actin-myosin bundles in connective tissue ended a long-lasting dispute about whether wound size reduction is due to active cellular contraction (which it is) or cell-independent rearrangement of the collagen network ECM (“shrinkage”) (which it is not) (Gabbiani et al. 1971; Gabbiani 2021). Early observations with explanted wound granulation tissue revealed that agonists of SMC contraction stimulated contractile force (Majno et al. 1971). The ability to reorganize fibrous collagen networks from granulation tissue by cell contractile force is essential to form a scar ECM that can mechanically resist substantial external forces, particularly in mechanically active organs such as skin, heart, and lung (Walraven and Hinz 2018). Because wound tissue stiffness increases with increasing remodeling (Goffin et al. 2006), myofibroblasts must work against higher mechanical resistance as the scar matures. Not unlike humans working out in the gym, fibroblastic cells build up strength by enhancing stress fiber formation in their muscular apparatus with ongoing exercise and load. One outcome of fibroblast “bodybuilding” is neo-expression of α-SMA that hallmarks the transition from contractile α-SMA-negative (proto–) myofibroblasts into highly contractile myofibroblasts required for contraction of tissues under high stress (Tomasek et al. 2002). Consistently, expression of α-SMA in fibroblastic cells is controlled by the mechanical environment in vivo and in vitro, as discussed further below, and incorporation of α-SMA into stress fibers results in higher myofibroblast contractile force (Arora and McCulloch 1994; Hinz et al. 2001a,b). Expression of α-SMA is a slow instrument to control myofibroblast strength with an approximately linear relationship between force and α-SMA stress fiber levels in vitro (Hinz et al. 2001a). At least two additional mechanisms allow myofibroblasts to adapt their contractile activities to the changing wound environment more acutely: intracellular Ca2+ concentration fluctuations and/or the activity of the small GTPase RhoA/Rho-associated coiled-coil-forming protein kinase (ROCK). Both signaling pathways eventually converge to regulate stress fiber contraction through phosphorylation of the myosin light chain (MLC) (Fig. 2; Burridge et al. 2019; Hinz et al. 2019).
Figure 2.
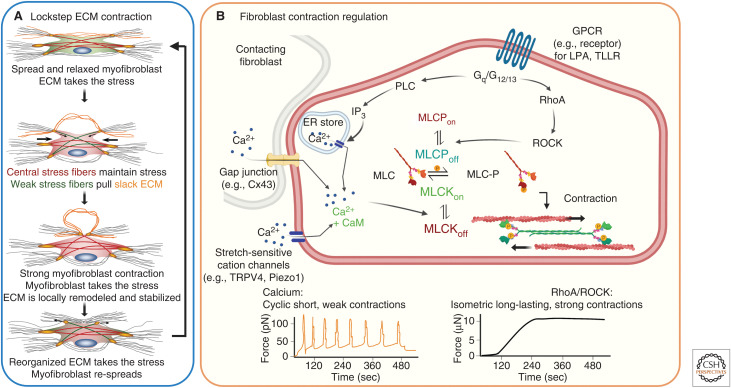
Modes and regulation of fibroblast contraction. (A) In vitro studies support a biphasic cyclic lockstep mechanism of myofibroblast contraction that produces in collagen contractures over several days during normal wound healing to years in scar tissue. In stressed extracellular matrix (ECM) environments, µN-strong and long-lasting (hours) isometric contractions create slack in collagen fibrils, which thereby become accessible to local remodeling by fast (minutes), short-ranged, and pN-weak contractile events. Proteolytic processing of relaxed collagen fibers allows formation of shorter neo-ECM that takes over the mechanical load and allows myofibroblast re-spreading. (B) Strong isometric contraction is produced through RhoA/Rho-associated coiled-coil protein kinase (ROCK) activation, following binding of G-protein-coupled receptor (GPCR) ligands like lysophosphatic acid (LPA) to the LPA1 receptor or the thrombin cleavage product TLLR to the plasminogen-activated receptor (PAR)-1. RhoA-activated ROCK phosphorylates and thereby inhibits myosin light chain (MLC) phosphatase (MLCP), which mediates maintained actomyosin stress fiber contraction. Alternatively, short periodic contractions are regulated by transient increases in cytosolic Ca2+ through binding of calmodulin (CaM), which activates the MLC kinase (MLCK). High levels of cytosolic Ca2+ are achieved by calcium release from the endoplasmic reticulum following inositol trisphosphate (IP3) generation by phospholipase C (PLC) downstream of GPCR signaling. Other Ca2+ sources are adjacent cells connecting through gap junctions or Ca2+ entry from the extracellular milieu through stress-sensitive plasma membrane channels like transient receptor potential cation channel subfamily V member 4 (TRPV4) or piezo1. Created with BioRender.com.
The Lockstep Model of Myofibroblast Contraction: Roles for Ca2+ and RhoA
Corresponding to their phenotypic similarity, myofibroblasts also share functional features with SMCs, such as the ability to maintain a contractile tone and slow rhythmic contractile behavior (Majno et al. 1971). Studies using three-dimensional collagen gel and two-dimensional compliant substrate cultures demonstrated that single myofibroblasts can develop persistent µN forces that create “slack” in stressed ECM (Hinz et al. 2001a; Follonier Castella et al. 2010a). Such locally stress-released ECM is then amenable to gradual shortening in ∼400 nm small and ∼100 pN weak contractile steps that occur with a frequency of ∼1/min. Although not experimentally shown at the single-cell level, acutely stress-released collagen fibers are prone to proteolytic processing (Flynn et al. 2010; Saini et al. 2020). Locally shortened and remodeled collagen fibers are proposed to take over the load from the isometrically contracting myofibroblast, allowing a new long-lasting lockstep contraction cycle after cell re-spreading (Tomasek et al. 2002). While low-frequency, high-force, and large-strain isotonic contraction are predominantly regulated by Rho/ROCK signaling, oscillation in cytosolic Ca2+ control the high-frequency, low-force, and low-strain isotonic contractions that locally reorganize collagen fibers (Fig. 2A; Follonier Castella et al. 2010b).
Extracellular and intracellular Ca2+ signaling is involved in almost all aspects of wound healing (Subramaniam et al. 2021). We have restricted our discussion to mechanisms that handle intracellular Ca2+ in the context of myofibroblast contraction to preface a later section on therapeutic strategies that target excessive myofibroblast activities in hypertrophic scarring and fibrosis. Ca2+ concentrations are as low as 10–100 nM in the cytosol of “resting” fibroblasts but can increase 10- to 100-fold within seconds following entry of extracellular Ca2+ through plasma membrane ion channels and/or influx of Ca2+ from endoplasmic reticulum (ER) stores (Janssen et al. 2015). The transmission of cytosolic Ca2+ through gap junctions has been shown to coordinate communication and contraction of physically contacting fibroblasts (Follonier et al. 2008). Abundance of the gap junction protein Cx43, nonexclusively expressed in fibroblasts, increases in (myo)fibroblasts after injury and in fibrosis, in correlation with increased contractility and inter-fibroblast communication. This pathway enhances wound closure, cell proliferation, accelerates wound reepithelialization, and regulates wound healing gene expression signatures in skin fibroblasts (Fig. 2B; Tarzemany et al. 2018; Costa et al. 2021; Montgomery et al. 2021).
The mechanical tugging of contractile myofibroblasts on each other or through ECM fibers is another mechanism that synchronizes the activity of individual myofibroblasts in a multicellular context (Hinz et al. 2004; Follonier et al. 2008; Liu et al. 2020). Mechanical events that generate high cytosolic Ca2+ levels in the myofibroblast include the opening of stretch-activated plasma membrane channels by acute mechanical stimulation (Murata et al. 2014) or by ECM stiffness (Godbout et al. 2013). Stretch-activated Ca2+ membrane channels that regulate fibroblast activation and activity in tissue repair and fibrosis include Piezo1, Piezo2, and members of the transient receptor potential vanilloid (TRPV) channel family, most notably TRPV4 (Fig. 2B). While most earlier studies investigated these receptors in cardiac and pulmonary (myo)fibroblasts (Rahaman et al. 2014; Arora et al. 2017; Bhattacharya and Hough 2019; Blythe et al. 2019; Grove et al. 2019; Dutta et al. 2020; Adapala et al. 2021; Stewart and Turner 2021), they have recently also attracted attention in the context of cutaneous wound healing (Anderson et al. 2017; He et al. 2021; Holt et al. 2021). For instance, Piezo1 plays an important role in detecting ECM elasticity and regulating cell behavior related to hypertrophic scarring (He et al. 2021). The binding of extracellular ATP to P2Y purinergic receptors in the cell membrane is another extracellular signaling event regulating cytosolic Ca2+ increases and fibroblast contractions through inositol trisphosphate (IP3)-mediated Ca2+ release from the ER (Ehrlich et al. 1986; Janssen et al. 2009).
G-protein-coupled receptor (GPCR) signaling following extracellular binding of ATP, lysophosphatidic acid (LPA), or TGF-β1 is critical in mediating Rho/ROCK regulation of persistent myofibroblast contraction in healing wounds and scar tissue (Tomasek et al. 2006; Yu and Brown 2015; Knipe et al. 2018). The binding of GPCR ligands results in the conversion of inactive Rho-GDP to Rho-GTP by guanine nucleotide exchange factors (GEFs). All Rho family members, RhoA, RhoB, and RhoC, regulate stress fiber contractility by regulating the activities of ROCK1 and ROCK2, which in turn enhance MLC phosphorylation either by inhibiting MLC phosphatase or activating MLC kinase (Loirand 2015; Pandya et al. 2017; Koenderink and Paluch 2018). The outcomes are strong and long-lasting isometric contractions, thereby enhancing overall cell stress that contributes to activation of myofibroblast contraction and persistence in a force feedback loop (Fig. 2B; Goffin et al. 2006; Vardouli et al. 2008; Ge et al. 2018; Oh et al. 2018). Conversely, ROCK inhibition with the specific antagonist Y27632 reduces granulation tissue contraction in mice (Tomasek et al. 2006). To add complexity, Ca2+ and Rho/ROCK signaling pathways intersect at various levels. For instance, Piezo1 stimulation during ventilator-induced rat lung injury activates the RhoA/ROCK1 pathway (Zhang et al. 2021). Activation of GPCRs, in addition to controlling RhoA activity, also leads to the production of IP3 and ER Ca2+ release; however, GPCR-related Ca2+ signaling appears to regulate fibroblast activation programs rather than acute contractile events (Wang et al. 2019; Eguchi et al. 2021; Seo et al. 2021). While multiple regulatory mechanisms control myofibroblast contraction over different time and length scales with different force levels, the collective outcomes are connective tissue remodeling and increased ECM tensile strength.
MECHANICS REGULATE MYOFIBROBLAST PHENOTYPE AND FUNCTION
Why Wounds Get Stiffer and Why It Matters
The ECM architecture of normal dermis protects embedded cells from mechanical stress, while wounding of the connective tissue initially exposes fibroblasts and other resident cells to a drastic change in the physical environment. The provisional matrix generated from plasma fibrin and fibronectin provides an initial strain-bearing scaffold for invasion of wound cells and release of growth factors, but fibrin matrices cannot resist large external forces (Rybarczyk et al. 2003; Feller et al. 2021). By using micro- and nano-indentation methods at the cellular level, the elastic modulus (“stiffness”) ranges from <1 kPa to a few tens of kPa in murine (Quesnel et al. 2019) and human intact dermis (Achterberg et al. 2014). Conversely, the modulus of the provisional matrix only ranges over a few hundred Pascals at fibrin concentrations equivalent to that of early wound tissue (Leung et al. 2007; Janmey et al. 2009; Achterberg et al. 2014). A recent study reports that murine in vivo wound strain ranges from ∼4% 3 d after wounding to ∼8% 21 d after wounding (Wietecha et al. 2020). Gradual replacement of fibrin with collagen I and III by infiltrating fibroblasts, fiber-rich ECM reestablishes mechanical tissue integrity associated with progressive stiffening of the initially soft granulation tissue (tens of kPa) (Goffin et al. 2006) into the stiff scar, which can reach moduli higher by an order of magnitude. Notably, the structure, alignment, and degree of collagen cross-linking in normal dermis and granulation tissue under maturation—even at stages with similar elastic moduli—are vastly different (Tschumperlin et al. 2018; Zhou et al. 2018; Wietecha et al. 2020). In addition to the replacement of fibrin with mature collagen fibers with different intrinsic mechanical properties (quality), an increase in protein amounts (quantity), and progressive alignment/remodeling of ECM fibers (architecture), wound stiffness is further enhanced by cross-linking enzymes. In addition to transglutamination, wound and fibrotic ECM resistance are further enhanced by the action of fibroblast-derived lysyl oxidases (LOXs) that catalyze the conversion of lysine molecules into highly reactive aldehydes that form covalent cross-links in ECM proteins, including collagen, LOX-like enzymes, and lysyl hydroxylases (Lucero and Kagan 2006; Walraven and Hinz 2018; Theocharis et al. 2019).
The importance of wound mechanics in the control of healing outcomes is well illustrated by surgical interventions. In human skin, the risk and severity of scarring are enhanced when the trauma or incision crosses anatomical lines of tension known as Langer's lines, for instance at joints or across the sternum but also occur widespread over other body locations. Surgeons (if they are good) are aware of these lines of stress and place their incisions in parallel with stress to reduce scarring (Mascharak et al. 2021b). Unlike humans who have tight skin, loose-skinned rodents typically heal without forming hypertrophic scars (Zomer and Trentin 2018); however, hypertrophy can be induced by suturing either passive splints or actuated expansion devices to the wound edges (Abercrombie et al. 1960; Aarabi et al. 2007; Son and Hinz 2021). In our own studies, we have frequently used this approach to demonstrate the enhanced generation of myofibroblast features in granulation tissue by mechanical stress (Hinz et al. 2001b; Klingberg et al. 2014; Coelho et al. 2017; Li et al. 2017). Mechanical strategies have also been used to induce myofibroblasts and fibrosis in other organs, by inducing pressure overloads in the liver, kidney, and heart tissue (Herrera et al. 2018; Santos and Lagares 2018; Tschumperlin et al. 2018; Lagares and Hinz 2021). Mechanical stress not only stimulates wound myofibroblast activation, but also prevents scar myofibroblasts from entering apoptosis, which normally clears these cells after healing (Parker et al. 2014; Hinz and Lagares 2020; Merkt et al. 2021).
Integration of Mechanosignaling by the Myofibroblast Cytoskeleton
Because restoration of mechanical ECM integrity is a critical aspect of tissue repair, myofibroblast activation and activities have evolved to tightly control wound healing mechanics by using various cell mechanosensing mechanisms (Hinz et al. 2019; Kuehlmann et al. 2020). We discussed above how stretch-mediated membrane channels of the Piezo and the TRPV families can regulate acute myofibroblast contractile dynamics (intracellular stress). This section summarizes how ECM-mediated mechanosignaling mechanisms control myofibroblast phenotype and function at the gene and protein expression level (Fig. 3). Fibroblasts typically perceive and transduce mechanical forces from the ECM via transmembrane integrins that are linked to the contractile actin cytoskeleton through adaptor proteins. Fibroblasts express the collagen-binding integrins α1β1, α2β1, and α11β1 (Zeltz and Gullberg 2016; Sawant et al. 2021). Deficiency of α11β1 but not of α1β1 and α2β1 integrins results in reduced myofibroblast activation and impaired wound healing (Schulz et al. 2015); concomitantly, integrin α11β1 has gained some traction as a myofibroblast marker (Zeltz et al. 2019; Alam et al. 2020). Although integrin α6β1 does not bind to fibrillar collagens, it has been implicated in fibroblast mechanosensing and myofibroblast activation, and it appears to regulate fibroblast-mediated proteolysis of basement membranes in the alveoli of patients with pulmonary fibrosis (Chen et al. 2016). Another interesting class of transmembrane collagen receptors comprises the discoidin domain receptors (DDRs) DDR1 and DDR2, which are receptor tyrosine kinases that specifically bind to fibrillar collagen (Marquez and Olaso 2014; Cario 2018), possibly with a preference for “wound” collagen types, such as collagen type III (Di Martino et al. 2022). The mechanisms of DDR-mediated mechanosensing are only beginning to be understood (Marquez and Olaso 2014; Coelho et al. 2017; Cario 2018; Coelho and McCulloch 2018). In contrast, it is well understood that integrin-mediated mechanotransduction occurs first by integrin conformational changes upon exertion of force, ultimately resulting in the formation of integrin clusters and their maturation into complex cell–ECM adhesions (Schnittert et al. 2018; Bachmann et al. 2019; Deville and Cordes 2019). Like integrins, some intracellular structural components of cell adhesions (e.g., talins, kindlins, and vinculin) are mechanoresponsive, and they are subject to strain-induced conformational changes that either allow adhesion reinforcements through binding of structural and actin-binding proteins (e.g., paxillin, filamin) or result in the recruitment and regulation of signaling molecules (e.g., focal adhesion kinase [FAK]) (Jansen et al. 2017; Sun et al. 2019).
Figure 3.
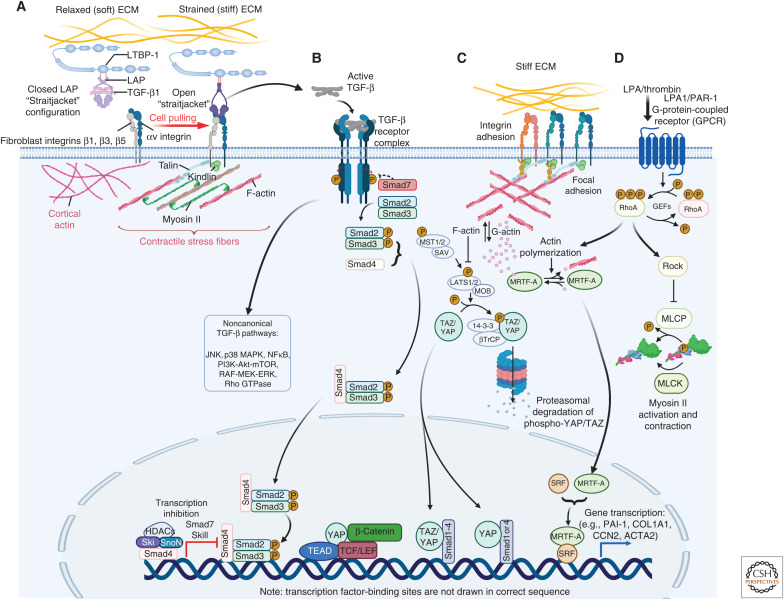
Mechanical regulation of myofibroblast activation. (A) Association of TGF-β1 with its latency-associated peptide (LAP) creates a small latent complex (SLC) that is stored in the extracellular matrix (ECM) by binding to the latent TGF-β1-binding protein 1 (LTBP-1). A contractile cytoskeleton and mechanical resistance in the ECM shifts integrins αvβ1, and possibly αvβ3 and αvβ5 into the active conformation that enables pulling on RGD-binding sites present in LAP. If stiff and/or strained ECM resists the SLC straitjacket pully open to release active TGF-β1. (B) Active TGF-β1 binds to the TGF-β receptor complex to promote canonical Smad signaling or other signaling pathways, for example c-Jun amino-terminal kinase (JNK), p38, extracellular signal-regulated kinases (ERKs), mitogen-activated protein kinase (MAPK), and Rho-RhoA/Rho-associated coiled-coil protein kinase (ROCK). Biochemical and biomechanical myofibroblast signaling pathways converge at multiple intersection points. (C) RhoA-mediated ROCK activation contributes to the polymerization of G- into F-actin following integrin-mediated mechanosignaling, or (D) G-protein-coupled receptor (GPCR) ligand binding. Actin polymerization liberates myocardin-related transcription factor A (MRTF-A) from G-actin to translocate into the nucleus. Together with serum response factor (SRF), MRTF-A drives the transcription of profibrotic gene products, such as CCN2 and ACTA2 (α-SMA). ROCK further regulates gene expression through the transcriptional coactivators Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). Translocation of YAP and TAZ and association with transcription factors such TEA domain family member (TEAD) results in the transcription of genes that promote cell proliferation and fibrogenesis like CCN2 and miR-21. Created with BioRender.com.
Not surprisingly, DDRs, integrins, and cell–ECM adhesion complex proteins all play essential roles in normal and dysregulated wound healing. For instance, FAK is up-regulated during skin wound healing, and fibroblast-specific knockout of FAK in a mouse model of hypertrophic scarring generally reduces fibrotic wound features (Wong et al. 2012). Likewise, therapeutic delivery of a FAK inhibitor to large area wounds has been reported to accelerate wound closure and skin mechanical resistance while reducing scar formation (Ma et al. 2018; Chen et al. 2021). In addition to playing conventional outside-in signal transduction roles through specific ligand binding and mechanotransduction, specific fibroblastic αv integrins are further involved in a peculiar mechanism of myofibroblast mechanoregulation (Henderson et al. 2013; Hinz 2013). Integrin αvβ1 in all studied fibroblasts (Reed et al. 2015) and possibly αvβ3 and αvβ5 integrin in skin and heart fibroblasts (Asano et al. 2006; Sarrazy et al. 2014) bind to an RGD motif in the latency-associated peptide (LAP) of the myofibroblast-inducing growth factor TGF-β1 (Fig. 3A; Kim et al. 2018). LAP remains noncovalently associated with TGF-β1 upon secretion, often in complex with latent TGF-β1-binding protein-1 (LTBP-1), a large protein of the fibrillin family that sequesters latent TGF-β1 in the ECM for subsequent mechanical activation (Rifkin et al. 2018; Lodyga and Hinz 2020; Derynck et al. 2021). With the exception of αvβ8 integrin (Campbell et al. 2020), the transmission of contractile force of ∼100 pN via fibroblast αv integrins induces a conformational change in the latent TGF-β1 complex (Buscemi et al. 2011; Shi et al. 2011) that promotes the release of active TGF-β1 from the ECM (Wipff et al. 2007). Rather than being controlled by absolute cell force, the efficiency of TGF-β1 activation by fibroblast contraction is dependent on pre-strain in the ECM. Thus, availability of TGF-β1 during wound healing is directly regulated by the remodeling state of the ECM (i.e., the progress of healing) (Klingberg et al. 2014; Froese et al. 2016). We have recently reported that mechanical control of TGF-β1 and myofibroblast activation also gain importance in what starts as wound healing following surgical insertion of implant materials but eventually results in encapsulation of the foreign body (Noskovicova et al. 2021a). Fibroblast contact with stiff implant surfaces enhances the activation state and affinity of β1 integrins, the efficiency of TGF-β1 activation, and subsequent myofibroblast accumulation, whereas providing implants with a soft (2 kPa) coat suppresses implant fibrosis at all these steps (Noskovicova et al. 2021b).
Extracellular activation of TGF-β1 for subsequent binding to the TGF-β1 receptor complex and respective downstream signaling is only one of several mechanisms that mechanically regulate myofibroblast activation at the transcription level (Fig. 3B). Another principal strategy of fibroblasts to transduce extracellular strain into gene transcription is directly related to the status of intracellular actin polymerization (stress fiber formation) and the nuclear shuttling of cotranscription factors like myocardin-related transcription factor A (MRTF-A) (or megakaryocytic acute leukemia-1 [MKL-1]), Yes-associated protein (YAP), and transcriptional coactivator with PDZ-binding motif (TAZ). Given that TGF-β1 signaling, RhoA/ROCK, and other contractile stress-regulating pathways all control actin polymerization, their close, yet complex, entanglement with YAP/TAZ and MRTF-A signaling is not surprising (Fig. 3C; Miranda et al. 2017; Tschumperlin et al. 2018; Link et al. 2022).
MRTF-A is sequestered in the fibroblast cytosol by G-actin monomers and released upon G- to F-actin polymerization to translocate into the nucleus, where it drives the transcription of “muscle” proteins—including α-SMA—and collagen expression in conjunction with serum response factor (SRF) (Small et al. 2010; Luchsinger et al. 2011). Consequently, MRTF-A signaling is central in promoting wound healing (Crider et al. 2011; Varney et al. 2016) and various fibrotic disease states (Miranda et al. 2021). The profibrotic potency of MRTF-A is probably best shown by the fact that even bona fide epithelial cells can be transformed to a myofibroblast phenotype by nuclear MRTF-A in vitro (Charbonney et al. 2011). Like MRTF-A, the transcription coactivators YAP and TAZ shuttle to the nucleus of fibroblasts under stress to promote myofibroblast activation in wound repair and fibrosis (Fig. 3D; Liu et al. 2015; Panciera et al. 2017; Rognoni and Walko 2019). Counterintuitively, inhibition of nuclear YAP with verteporfin prolongs myofibroblast activation and induces scarring of dermal wounds generated in the spiny mouse (Brewer et al. 2021). Under normal conditions, spiny mice show true skin regeneration without any scarring after wounding, partly because their fibroblasts are not mechanically activated into myofibroblasts (Stewart et al. 2018). Although the stress-dependence of YAP and TAZ nuclear localization has been reported by numerous studies since the seminal discovery (Dupont et al. 2011), it is still not entirely clear how nuclear shuttling and mechanics are linked at the molecular level; even the nuclear translocation sequences and import regulation mechanisms are just beginning to be understood (Kofler et al. 2018).
It is also unclear whether forces transmitted to the nuclear envelope control nuclear pore permeability for YAP/TAZ translocation. However, a direct connection between ECM, integrins, filamentous cytoskeletal elements, the nuclear envelope, and even nuclear content can promote mechanotransduction in multiple cell types, including fibroblasts. Physical forces induce changes in the conformation and post-translational modifications of nuclear envelope proteins and alter chromatin condensation and epigenetic modification, which lead to changes in transcriptional activity (Mammoto et al. 2012; Discher et al. 2017; Maurer and Lammerding 2019; Dai et al. 2020). In seminal studies (Maniotis et al. 1997), pulling on integrins with ECM protein-coated magnetic beads or micropipettes not only induced nuclear deformation but extracted DNA and chromosomes from the nucleus of cultured cells. Upon exposure to physical cues, cytoskeletal conformation is sensed directly in the nucleus via protein complexes that connect the nuclear envelope with cytoskeletal proteins (Fig. 4A,B; Kirby and Lammerding 2018). In fibroblasts, extracellular force is transmitted to the nucleus through vimentin intermediate and actin microfilaments that connect at the outer nuclear membrane with the linker of the nucleoskeleton and cytoskeleton (LINC) complex. The LINC complex spans the nuclear envelope and is composed of nesprins (nuclear envelope spectrin repeat proteins), SUN (Sad1p and UNC-84 homology), and KASH (Klarsicht, ANC-1, and Syne homology) domain proteins (Bouzid et al. 2019; Janota et al. 2020; Pennacchio et al. 2020; Wong et al. 2021). On the inner nuclear membrane, lamins A, B, and C are the main components of a meshwork of fibrous proteins that form the nuclear lamina. Lamins attach to heterochromatin regions located in the nuclear periphery through different partners, such as LEM (LAP2, emerin, and MAN1), which modulate gene transcription (Dobrzynska et al. 2016). The expression ratio and spatial distribution of the three lamin isoforms (A, B, and C) change during cell differentiation and in response to physical cues. In the context of wound healing and fibrosis, an increased lamin A:C ratio stimulates persistent myofibroblast activation on a stiff environment through alteration of nuclear mechanical properties and gene expression (Walker et al. 2021a). In an animal model, mutated lamin A leads to defects in nuclear mechanotransduction, impairs the function of mechanosensitive transcription factors, and affects the expression of profibrotic genes that promote cardiac fibrosis (Osmanagic-Myers and Foisner 2019). Furthermore, lamin A content in the nuclear envelope linearly scale with collagen content in the ECM across different organs and stages of tissue repair and fibrosis (Swift et al. 2013). Thus, nuclear mechanics and related signaling appear to respond to the physical conditions of extracellular mechanical environment (Discher et al. 2017). The ECM–nuclear connection is a suitable mechanism to target myofibroblast activation, which is best studied in MSCs. The following section discusses how substrate physical properties can be adapted to render MSCs resistant to subsequent mechanical activation by generating “mechanical memory.”
Figure 4.
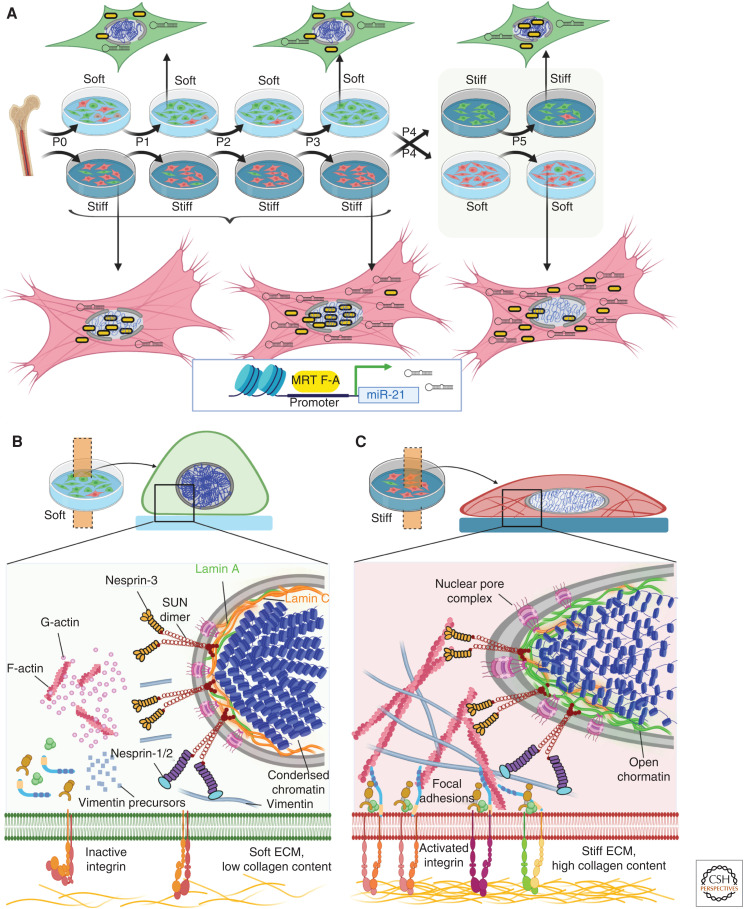
Nuclear mechanics and myofibroblast mechanical memory. (A) Integrins in the cell membrane act as mechanosensors and are those in their inactive forms if not bound to extracellular ligands and in low affinity confirmations under low intracellular stress or soft extracellular matrix (ECM). In such low stress conditions, both actin microfilament and vimentin intermediate filament polymerization is low and connections with the nuclear lamina are weak. Low lamin A:C ratios in the inner nuclear membrane and condensed chromatin characterize fibroblasts grown in soft environments. With high intracellular stress and binding to stiff ECM with high collagen content, integrins shift to their active confirmation and cluster into multicomponent focal adhesion structures. (B) Mechanotransduction pathways and stress originating at cell–ECM adhesions drive polymerization of G- to F-actin and formation of vimentin intermediate filaments that establish a direct connection between ECM adhesions and the linker of the nucleoskeleton and cytoskeleton (LINC) complex in the nuclear outer membrane. The LINC complex contains nuclear envelope spectrin repeat proteins (nesprins), and Sad1p and UNC-84 homology (SUN) proteins that span the nuclear envelope. Nesprin-3 connect SUN proteins to F-actin, whereas nesprins-1 and -2 connect to intermediate filaments. On the inner nuclear membrane, SUN dimers interact with lamin A attached to chromatin, which is thus forced to open in high stress conditions. (C) Imprinting of in vitro mechanical memory has been demonstrated for mesenchymal stromal cells (MSCs) directly isolated from bone marrow onto soft (5 kPa elastic modulus) and stiff (100 kPa) culture substrates. By continuously passaging on stiff or soft substrates (P = one passage of 7 d), MSCs are mechanically primed. On stiff substrates, the mechanosensitive myocardin-related transcription factor A (MRTF-A) localizes to the nucleus and drives continued transcription of the profibrotic micro-RNA miR-21; MRTF-A remains cytosolic on soft substrates. After the switch from stiff to soft substrates, MRTF-A acutely shifts to the cytosol, whereas miR-21 levels remain high due to low turnover rates and continue to promote myofibroblast activation for at least 2 more weeks as one keeper of mechanical memory. Created with BioRender.com.
Myofibroblast Mechanical Memory
In addition to transducing acute mechanical events into gene transcriptional activities, nuclear mechanics also regulate epigenetic processes that have a longer-lasting impact on fibroblastic cell fates. For instance, decoupling of the nucleus and cytoskeleton by disrupting the LINC complex was shown to change the fate of MSCs cultured on stiff substrates by regulating epigenetic modifiers. Such LINC-defective MSCs were unable to transduce extracellular physical cues to the nucleus and transcriptionally and permanently behave like inactivated MSCs grown on soft substrates (Killaars et al. 2019). We have coined the term “mechanical memory” to describe the observation that lung fibroblasts cultured (“primed”) on soft surfaces (5 kPa) for several passages are resistant to activation into myofibroblasts by subsequent exposure to physiologically relevant stiff substrates (100 kPa). Conversely, priming on stiff substrates propagates the myofibroblast phenotype even after several passages on a soft substrate (Fig. 4C). It is conceivable that mechanical memory contributes to the persistence of myofibroblasts in conditions of fibrosis, whereas restoration of normal tissue architecture and reduced mechanical load are suicide signals for myofibroblasts following normal wound healing (Lagares et al. 2017; Hinz and Lagares 2020). However, it should be emphasized that substrate-induced myofibroblast mechanical memory has so far only been demonstrated in vitro. At least in our own studies, mechanical priming is restricted to physiologically relevant soft and stiff substrates (<100 kPa) with primary fibroblastic cells that have never been exposed to rigid tissue culture plastic (GPa). While seeking proof for the generation of mechanical memory in vivo, we will restrict the following discussion of mechanical memory to studies performed with MSC that are typically expanded on rigid polystyrene for subsequent therapeutic grafting applications.
Administration of MSCs appears to be a promising therapy to accelerate and improve the healing of severe injuries such as large burn areas and to attenuate fibrosis (Gurtner et al. 2008; Amini-Nik et al. 2018; Lemos and Duffield 2018; Jiang and Scharffetter-Kochanek 2020). MSCs can be isolated from various adult tissues, including bone marrow and adipose tissue from extraembryonic sources such as amniotic fluid and umbilical cord (Pittenger et al. 2019). One feature of MSCs distinct from induced pluripotent stem cells and embryonic stem cells is MSC immuno-privilege due to lack of surface proteins that are recognized by natural killer cells (Faiella and Atoui 2016). Flying under the immune surveillance radar of the host reduces MSC clearance after xenografting in animals and has encouraged the production of allogeneic MSCs for therapeutic applications. However, MSCs are also prone to myofibroblast activation in profibrotic environments—a pathway that reduces their regenerative capacity (Hinz 2010). Furthermore, conventional (stiff surface) culture expansion of MSC to achieve the billions of cells required for transplants is selective for a fibro-osteogenic MSC fate (Engler et al. 2006; Talele et al. 2015). In contrast, “soft-primed” MSCs (5 kPa, 4 wk) preserved beneficial healing characteristics after transplant into a rat model of hypertrophic scarring, whereas conventionally cultured MSCs amplified scarring features (Li et al. 2017). Similarly, adipose stem cells primed for 2 wk on soft polymer substrates (1 kPa) preserved their phenotype and delayed development of profibrotic phenotypes after switching to a stiff substrate (120 kPa) and exhibited improved regeneration potential in a rat model of post-traumatic elbow contracture (Dunham et al. 2020). The molecular mechanisms through which mechanical environment creates lasting myofibroblast memory is a field of active investigation.
Memory appears to develop at different molecular levels with different molecular memory keepers having different lasting effects on memory (Kanoldt et al. 2019). Retention of mechanical responses was shown by inducing YAP/TAZ nuclear translocation in MSCs cultured on stiff substrates (36 kPa) for up to 10 d, followed by reduction of substrate modulus to 5.5 kPa with sustained nuclear retention of YAP/TAZ (Yang et al. 2014). Likewise, epithelial cells cultured on 50 kPa substrates for 3 d maintained multiple features for 2 d after substrate softening to 1 kPa substrates, including higher migration speed, larger focal adhesions, higher actin alignment, and high MLC phosphorylation levels (Nasrollahi et al. 2017). Using an experimental system of photoinduced polymer substrate softening, YAP/TAZ-mediated myofibroblast memory of MSCs was influenced both by duration and substrate stiffness after the switch to soft substrates (Yang et al. 2014). Myofibroblast memory in these studies generally refers to the preservation of α-SMA stress fiber formation even on soft substrates. Our own studies introduced the profibrotic microRNA miR-21 as another keeper of longer myofibroblast mechanical memory in rat bone marrow–derived MSCs (Li et al. 2017). MiR-21 drives a profibrotic program by binding and thus inhibiting transcription of genes that have antifibrotic actions, such as Smad, TGF-β-receptor type III, sprouty homologue 1 (SPRY1), and programmed cell death 4 (PDCD4) (summarized in Li et al. 2017). Transcription of miR-21 is promoted by nuclear translocation of the acutely mechanosensitive MRTF-A and miR-21 accumulates for the time that MSCs are exposed to stiff substrates. Mechanical memory is then retained, at least in part, by the low rates of miR-21 turnover for several days following substrate switching (Fig. 4C). In contrast to MSCs continuously grown on photo-modulated hydrogel substrates (Yang et al. 2014), MSCs in our studies were grown for three passages (4 wk) on stiff (100 kPa) silicone elastomer substrates and transferred onto 5 kPa soft substrates for another 2 wk. Thus, MSC are mechanically “reset” with every trypsin detachment at the beginning of a new passage. Concomitantly, acute mechanotransducers, like MRTF-A or YAP/TAZ, contribute to memory formation but cannot preserve memory in this system, because MSC detachment and soft substrate culture result in their nuclear export within minutes (Li et al. 2017). Furthermore, MSC-derived ECM cannot contribute to memory preservation after substrate switch because cells are detached with every transfer to a new passage.
The view that memory duration is determined by both, the exposure time to physical cues and the production and degradation kinetics of memory regulating factors—in this case, miR-21—is supported by recent mathematical models (Peng et al. 2017; Price et al. 2021). These computational models further predict that different cell types should reach a threshold for memory generation, depending on stiffness dosing and timing. More recent studies indeed suggest the existence of a “dosing threshold,” defining a point of no return for mechanically primed fibroblastic cells. Two populations of myofibroblasts were defined after priming heart valve interstitial cells for different durations on stiff culture substrates. “Transient myofibroblasts” that had been generated by 3 d of “priming” on a 4 kPa stiff hydrogel were inactivated after switching to 2 kPa soft gels for 2 d as evidenced by lost α-SMA expression. Conversely, “persistent myofibroblasts” remained activated for 2 d after soft substrate switch (2 kPa) following longer prior exposure of >7 d to moderately stiffer (4 kPa) environment (Walker et al. 2021a). It is yet unclear which molecular mechanisms would drive persistent myofibroblast activation following stiff priming. Epigenetic modifications such as regulating the level of DNA accessibility for transcription are ideally suited to preserve true long-term memory (Killaars et al. 2019; Walker et al. 2021a). It is beyond the scope of this review to discuss the role of epigenetics in persistent activation of myofibroblasts in scarring and fibrosis, and the reader is referred to recent reviews on the topic (Moran-Salvador and Mann 2017; Zhu et al. 2020; Tsou et al. 2021). Targeting epigenetic mechanisms may be a strategy to control myofibroblast persistence and activities in wound healing and fibrosis (Claveria-Cabello et al. 2020; Ruiz-Ortega et al. 2020; Skibba et al. 2020).
TARGETING MYOFIBROBLAST MECHANICS AND MECHANISMS TO IMPROVE WOUND HEALING
Inhibition of Myofibroblast Mechanotransduction and Contractile Pathways Reduces Scarring
Evidence that myofibroblast contractile force is key in driving normal and pathological healing and regulated by mechanical stress opens several opportunities to therapeutically target the myofibroblast force sensing and transmission mechanisms (Hinz et al. 2019; Tschumperlin and Lagares 2020; Fu et al. 2021a; Mascharak et al. 2021b). We will here briefly discuss the principles of existing strategies aiming at improving wound healing and reducing fibrosis by targeting mechanisms of ECM stiffening, myofibroblast contraction, stress perception, and mechanotransduction. At the macroscopic level, silicone membranes have been used to temporarily compensate for the loss of dermal structure after surgical interventions, protect resident cells from increased load, and thereby reduce the formation of skin hypertrophic scars (Wong et al. 2013; Barnes et al. 2018). At the tissue structure level, reducing the amount of ECM, its composition, and cross-linking that accompanies tissue repair is another approach to control myofibroblast activation (Lampi and Reinhart-King 2018; Walraven and Hinz 2018). Injection of collagenase into contracted nodules of palmar facia has been used as clinical therapy to reduce fibrosis in Dupuytren's disease (Hurst et al. 2009). However, degradation of collagen is difficult to imagine as a general approach to treat organ fibrosis (e.g., of lungs and heart) because crucial mechanical functions of connective tissue would be lost, we would fall apart. More specific targets in the ECM stiffening process are LOX and LOXL2 enzymes that facilitate collagen cross-linking and are expressed in activated fibroblasts (Vallet and Ricard-Blum 2019). Although the LOXL2 specific blocking antibody Simtuzumab (GS-6624) successfully prevents fibrosis in experimental and preclinical animal models (Barry-Hamilton et al. 2010), it failed in clinical phase 2 trials for lack of efficacy in treating lung fibrosis (NCT01769196); results of a phase 2 pilot trial treating liver fibrosis are pending (NCT01452308). It remains to be shown whether combination therapies using inhibitors of the ECM cross-linking LOX (e.g., β-aminopropionitrile) together with inhibitors of myofibroblast contractility (e.g., relaxin) are clinically more effective, as suggested by significantly decreased collagen deposition and reduced expression of α-SMA in a lung fibrosis model (Lin et al. 2017).
At the cellular level, inhibition of myofibroblast contraction seems the most straightforward approach to prevent tissue contractures. Various strategies have been shown effective in different models of fibrosis by intercepting the contraction axis formed by GPCR ligands, GPCRs, RhoA/ROCK, and myosin activity. Inhibition of the GPCR receptor for lysophosphatidic acid (LPA) LPA1 reduces contraction-mediated activation of TGF-β1 and myofibroblast formation in lung fibrosis (Jenkins et al. 2006; Tager et al. 2008) and has long been proposed as a therapeutic modulator to control myofibroblasts in physiological wound healing (Parizi et al. 2000). Modified LPA1 ligands are being tested as imaging agents for positron emission tomography diagnostics in pulmonary fibrosis (NCT04069143). Downstream of GPCRs, the ROCK inhibitors relaxin and fasudil both reduce organ fibrosis and wound contraction (Bond et al. 2011; Huang et al. 2011; Zhou et al. 2013) and selective depletion of different ROCK isoforms protects mice from induced pulmonary fibrosis (Knipe et al. 2018). A limitation of myofibroblast contraction inhibition by blocking RhoA/ROCK signaling is lack of specificity. For example, RhoA/ROCK are critical regulators of vascular and airway SMC contraction and cardiomyocyte contraction in the heart, which likely excludes systemic therapies but leaves a window for safe local applications, such as topical treatment of skin wounds. So far, fasudil has not been tested in clinical trials to treat fibrosis, but it is being evaluated as a vascular tone modulator in Reynaud's disease of patients with SSc (NCT00498615, phase 3) and atherosclerosis (NCT00498615, phase 2). Relaxin is being tested for several clinical applications, and phase 3 trials have been completed for applications in diffuse SSc (NCT00704665). The ROCK pathway inhibitor, pravastatin, completed clinical phase 2 trials to treat patients with radiation-induced skin fibrosis (NCT01268202). More specific myofibroblast contraction targets are selected GPCRs, as recently demonstrated for the dopamine receptor D1 in lung fibrosis (Haak et al. 2019, 2020) and the GPCR for estrogen in pancreatic stellate cells (Dooling and Discher 2019). One advantage of considering GPCRs as anti-myofibroblast targets is their clinical use for other disease applications and the opportunity for drug repurposing. Other “specific” targets in the myofibroblast contractile apparatus are α-SMA, which has been pharmacologically tested (Hinz et al. 2002), specific tropomyosin isoforms that support organization of α-SMA into stress fibers (Prunotto et al. 2015), or—so far only hypothetically—actin isoform-specific nucleators of the mDia family (Chen et al. 2017).
Other prime targets are different elements of the mechanosensing and mechanotransduction cascades that control myofibroblast activity and activation (Chen et al. 2021). Other potential targets are the extracellular domains of the integrins, which are involved in myofibroblast attachment and activation of TGF-β1, fibrillar collagen-binding DDRs, and specific cadherins, most notably cadherin-11, which are up-regulated during myofibroblast activation (Hinz 2013; Kim et al. 2018; Moll et al. 2019; Riley and Merryman 2021). Specific antibodies and small molecule inhibitors against fibroblast integrins have been tested in experimental models (Reed et al. 2015) and are on the verge of entering clinical trials. Phase 1 and phase 2 clinical trials have been performed with antibodies directed against the TGF-β1-activating epithelial integrin αvβ6 (STX-100/BG00011) to treat and diagnose pulmonary fibrosis (NCT01371305). Abituzumab is a monoclonal antibody directed against αv integrin with promising safety and tolerability profiles in oncologic clinical trials (Wirth et al. 2014; Hussain et al. 2016). A phase 2 trial studying abituzumab in SSc was terminated early due to difficulties in identifying subjects who met eligibility criteria (NCT02745145). No clinical trials are listed for DDRs and/or cadherin-11. Inhibition of the cytoplasmic mediator FAK downstream of integrin-mediated mechanotransduction with antibodies and/or small molecules can reduce myofibroblast accumulation in various animal models of organ fibrosis (Horowitz et al. 2007; Lagares et al. 2012; Zhang et al. 2017; Zhao et al. 2017), including mouse hypertrophic scarring (Wong et al. 2012; Ma et al. 2018). FAK inhibitors have not been tested yet in clinical trials for the treatment of fibrosis. In addition, inhibition of mechanosensitive fibroblast membrane channels like Piezo1 and TRPV4 and gap junction components such as connexin 43 are being pursued to treat “channelopathies” like those causing cardiac dysfunction (Andelova et al. 2020; Jiang et al. 2021; Lawhorn et al. 2021) These compounds have not yet been tested in the clinical treatment of organ fibrosis (Rahaman et al. 2014; Inoue et al. 2019; Prakoura et al. 2019; Adapala et al. 2021; Fu et al. 2021b; Stewart and Turner 2021) and are just beginning to be considered for wound healing therapies (Becker et al. 2016; Montgomery et al. 2018; He et al. 2021). As inhibitors of these receptors, only the TRPV4 channel blocker GSK2798745 has advanced to clinical trials targeting pulmonary edema in congestive heart failure (NCT02497937) but not fibrosis.
Finally, mechanotransduction therapies can intercept regulation and activity of the transcription factors MRTF-A, YAP, and TAZ (Dey et al. 2020). Deletion of MRTF-A or inhibition with the small molecule compound CCG-1423 suppresses myofibroblast formation in culture and reduces skin and pulmonary fibrosis in mice (Shiwen et al. 2015; Sisson et al. 2015). Therapeutic targeting of TAZ and YAP using dimethyl fumarate suppresses fibrogenesis in cell culture and ameliorates skin fibrosis in mice (Toyama et al. 2018). Likewise, YAP inhibition using verteporfin prevents myofibroblast activation of Dupuytren fibroblasts (Piersma et al. 2015) and reduces experimental kidney fibrosis (Szeto et al. 2016). In a mouse model of hypertrophic scarring, inhibition of YAP signaling with verteporfin inhibits postnatal expression of En-1, a marker and driver of scar-promoting dermal fibroblasts and facilitates regenerative healing by En-1-negative dermal fibroblast populations (Mascharak et al. 2021a). MRTF-A inhibitors are not currently tested in clinical trials. Verteporfin is in clinical trials for various localized applications—mostly with a different mode of action (i.e., not targeting YAP) in photodynamic therapy. Systemic therapies targeting TAZ and YAP are difficult to imagine because of their key regulatory roles in many cellular processes. However, fibrogenic fibroblasts up-regulate GPCRs upstream of YAP signaling, which are being proposed as specific targets in pulmonary fibrosis and other connective tissue disorders (Haak et al. 2019, 2020).
Mechanical Stimulation of Myofibroblasts to Improve Healing of Chronic Wounds
So far, we have mainly discussed strategies that aim at eliminating or inhibiting myofibroblast functions in skin hypertrophic scarring and organ fibrosis. Here, we will briefly consider the consequences of myofibroblast depletion for the normal tissue repair process and how mechanical stimulation can be used in clinical practice to reestablish missing or dysfunctional granulation tissue in the context of nonhealing, chronic wounds (Hinz 2016b; Lebonvallet et al. 2018; desJardins-Park et al. 2021). The prevalence of nonhealing chronic wounds increases with ongoing age and in the presence of risk factors such as diabetes and vasculopathies (desJardins-Park et al. 2021). Infections, exacerbated inflammation, ischemia, hypoxia, and hyperglycemia characteristic for chronic wound beds all repress the formation of myofibroblasts and vascularization, resulting in an overall poorly developed granulation tissue (Hinz 2016b; Bonte et al. 2019; Wan et al. 2021; Yokota et al. 2021). Tissue hypoxia resulting from poor vascularization inhibits fibroblast proliferation and formation of reparative collagen ECM; several crucial events in collagen fiber assembly and cross-linking depend on adequate oxygen concentrations (Scheid et al. 2000). Even in the presence of collagen, ECM remodeling and wound closure is impaired due to the lack of activated myofibroblasts, granulation tissue contraction (Alizadeh et al. 2007; Modarressi et al. 2010), delayed reepithelialization, and failure of neovessels to sprout and mature in the wound bed (Tandara and Mustoe 2004). For instance, in vitro hypoxia suppresses myofibroblast activation by reducing the activities of RhoA and MRTF-A activity (Modarressi et al. 2010; Leinhos et al. 2019).
All these aspects of poor healing can be rescued—at least to some extent—by external mechanical stimulation, of which negative-pressure wound therapy (NPWT) is the most widely used approach. NPWT has been introduced more than 20 years ago and since then has seen many technological updates and clinical applications, including treatment of diabetic foot ulcers and incision healing after surgery (Hunter et al. 2007; Expert Working Group 2008). All NPWTs—not unlike vacuum cleaners—are based on the application of negative pressure to wound sites using an adjustable external unit to generate the negative pressure, a tube, a pad, and a foam dressing that is inserted into the wound bed. In addition to removing wound exudate and infectious microorganisms by suction, applied negative pressure generates micromechanical strains and fluid flow shear forces that are transmitted into the wound matrix at the level of the foam material (Orgill and Bayer 2011; Singh et al. 2020; Ozkan et al. 2021). Enhanced accumulation of myofibroblasts has been described for wound beds that underwent NPWT (Heit et al. 2012). It would exceed the scope of this review to delve into the thousands of published studies describing the different actions of various NPWT permutations and a few hundred works investigating the mechanisms of NPWT actions. NPWT and other mechanical therapies will be, in principle, beneficial for chronic wound healing therapies by stimulating the very same myofibroblast mechanotransduction and -transmission mechanisms that should be inhibited in hypertrophic/fibrotic conditions (Daigle et al. 2013).
Beyond Mechanics: Myofibroblast Therapies to Improve Wound Healing Outcomes
Myofibroblasts are mechanically active cells but not all myofibroblast activities are mechanically controlled, and most current anti-scarring therapies are targeting other effectors of myofibroblast activation, deactivation, and fate programs (Friedman et al. 2013; Allinovi et al. 2018; Schuppan et al. 2018; Sato et al. 2019; Volkmann and Varga 2019). Although TGF-β1 is arguably the most potent single factor driving myofibroblast activation, antifibrotic therapies directly inhibiting the TGF-β1 cytokine or the TGF-β1 receptor complex have been unsuccessful due to the pleiotropic character of the growth factor. In the context of dermal wound healing, TGF-β1 is promoting myofibroblast activation but also suppressing inflammation and controlling epidermal overgrowth (Werner and Grose 2003). Inhibition of latent TGF-β1 presentation and activation mechanisms have already been discussed above and are reviewed elsewhere in more detail (Lodyga and Hinz 2020). In addition to mechanical tension and TGF-β1, multiple growth factors and cytokines have been described as myofibroblast regulators. Prominent examples include other members of the TGF-β family, PDGF, and fibroblast growth factor (FGF) family members, which are being investigated for their potential antifibrotic activity (Morikawa et al. 2016; Lodyga and Hinz 2020). Activins and bone morphogenetic proteins (BMPs) are drawing increasing attention with regard to their role in regulating fibroblast activation and their value as therapeutic targets (Wietecha et al. 2020; Duffy et al. 2021; Krepinsky 2022). PDGF has long been studied as a key regulator of myofibroblast activation (Klinkhammer et al. 2018) and was more recently found to coordinate a complex vascular niche in Dupuytren's disease (Layton et al. 2022). FGF appears to play context-dependent roles in regulating fibrosis. For instance, adding recombinant FGF-2 resulted in decreased fibrosis in a model of pulmonary fibrosis (Dolivo et al. 2017b) and decreased myofibroblast differentiation of human dermal fibroblasts in vitro (Dolivo et al. 2017a). Conversely, in a lung cancer model, FGF-2 promoted cell proliferation, epithelial-to-mesenchymal transition, and metastasis through the FGF receptor 1-ERK1/2-SOX2 signaling axis (Wang et al. 2018). Two recent studies report that delivery of FGF-2 in preclinical mouse models improved angiogenesis, cell migration, and reduced fibrosis (Lu et al. 2020; Jin et al. 2021). The effects of other members of the FGF family has been extensively reviewed elsewhere (Ornitz and Itoh 2022). The pleiotropic character of TGF-β1, PDGF, and FGF and their context-dependent actions render these factors difficult to target in antifibrosis therapies.
Presently, only two drugs are licensed to treat (pulmonary) fibrosis, nintedanib and pirfenidone. Nintedanib has also been approved for the treatment of lung fibrosis in SSc as a positive outcome of the SENSCIS trial (Distler et al. 2019). The efficacy of pirfenidone is currently being investigated in clinical trials in patients with SSc (NCT01933334, NCT03068234, NCT03856853, NCT03221257). Their broad action on kinases involved in profibrotic signaling pathways is advantageous to tackle redundancies in tissue repair pathways but also causes substantial side effects (King et al. 2014; Richeldi et al. 2014; Sun et al. 2018). Other tyrosine kinase inhibitors are proposed to disrupt fibrotic signaling pathways and reduce fibroblast activation. For instance, imatinib, commercially known as Glivec, is a tyrosine kinase inhibitor currently used to treat various hematological malignancies. Several studies investigated imatinib in the context of fibrotic diseases but definitive conclusions on its efficacy cannot be drawn from the small negative trials (Pope et al. 2011; Fraticelli et al. 2014). Further studies have been hampered mainly by its poor tolerability. Nilotinib is an inhibitor of the c-ABL and PDGFR tyrosine kinases, which has shown antifibrotic effects in a mouse model of bleomycin-induced fibrosis and on dermal fibroblast from patients with SSc (Manley et al. 2010; Rhee et al. 2011).
The renin angiotensin aldosterone system (RAAS) is best known for its key role in regulating blood volume, blood pressure, and sodium homeostasis. However, angiotensin II (ang II), the central hormone produced in the RAAS, also acts in many tissues including the skin and immune system and contributes to tissue fibrosis (Gan et al. 2018; AlQudah et al. 2020). In normal skin, RAAS components are present at low expression levels, including the ang II receptors type 1 and 2 (AT1R, AT2R) in dermal fibroblasts, epidermal keratinocytes, and vasculature where it localizes with angiotensinogen, renin, and angiotensin-converting enzyme 2 (Steckelings et al. 2004). RAAS components are up-regulated and play roles in all phases of wound healing following skin injury, including hemostasis, recruitment and activation of keratinocytes and fibroblasts, wound contraction, and ECM remodeling (Bernasconi and Nystrom 2018). Notably, ang II was among the factors used in seminal studies to induce granulation contraction (Majno et al. 1971) and directly triggers myofibroblast contraction through Ca2+ influx (Follonier et al. 2008). Ang II further stimulates TGF-β synthesis and secretion and to directly activate a TGF-β signaling pathways, primarily through canonical SMAD signaling and noncanonical MAPK signaling (Tan et al. 2018). Deletion of the AT1R delays skin wound healing in mice, whereas AT2R deficiency has a mixed effect, disrupting early stages of skin repair while accelerating later stages. Part of this dual action appears to be related to differential actions of fibroblasts. While ang II induces collagen synthesis in skin fibroblasts via AT1R, it inhibits collagen production via AT2R. Likewise, ang II signaling through AT1R enhances expression of the tissue inhibitor of metalloproteinases-1 (TIMP-1) and thereby collagen degradation, whereas AT2R signaling inhibits TIMP-1 expression and thus promotes collagen degradation (Min et al. 2004). Finally, Ang II was also shown to up-regulate CCN2 and TGF-β in cultured fibroblasts through activation of p38-MAPK signaling pathways (Gu et al. 2012). Consequently, the clinically approved ATR antagonist losartan and resveratrol have antifibrotic effects in various organs (Ramirez and Rifkin 2012). A systematic review of the resveratrol literature on suggests a supporting action on wound healing by reducing hypertrophic scarring (Hecker et al. 2022). Losartan was shown to suppress fibrogenesis of cultured keloid fibroblasts (Ikeda et al. 2013) and skin wound myofibroblasts (Hedayatyanfard et al. 2018); however, at the same time, it reduced the success of skin grafting on porcine full-thickness wounds (Akershoek et al. 2017).
Among the more “unusual” approaches to fight fibrosis, blockade of the ubiquitin-proteasome pathway has been shown to prevent lung and skin fibrosis after injury in a murine model, in part by inhibiting TGF-β (Mutlu et al. 2012). A small, double-blind clinical trial is underway studying the effect of the proteasome inhibitor in combination with mycophenolate mofetil (NCT02370693). Cannabinoids exert anti-inflammatory effects by binding to their two receptors, CB1r and CB2r. Both cannabinoid receptors are overexpressed in dermal fibroblasts of patients with SSc, suggesting that modulating cannabinoid signals may ameliorate fibrotic responses (Akhmetshina et al. 2009). In vitro treatment of SSc fibroblasts with WIN55,212–2, a synthetic CB1r cannabinoid agonist, reduces collagen production, myofibroblast transformation, and TGF-β expression (Balistreri et al. 2011). Lenabasum is a synthetic CB2r agonist that showed greater improvement of the combined response index in diffuse cutaneous SSc compared to placebo in a recent phase 2 trial. Lenabasum was also associated with a significant benefit on skin involvement. In skin biopsies, lenabasum down-regulates gene transcripts associated with inflammation and fibrosis (Spiera et al. 2020).
IL-4 and IL-13 are two hallmark type 2 inflammatory cytokines, shown to induce TGF-β production by infiltrating macrophages and to promote a distinct fibrotic response (Lee et al. 2001; Gieseck et al. 2018). In addition to this conventional role, IL-13 is also suggested to directly stimulate collagen-producing myofibroblasts (Gieseck et al. 2016). Preclinical studies demonstrate that antibodies neutralizing the IL-13 or IL-4 receptors reduce fibrosis (Xue et al. 2015; Hart et al. 2017). IL-13 and IL-4 inhibitors alone or in combination have also been tested in the clinic in phase 2 trials to treat pulmonary fibrosis, SSc, and keloids (NCT01266135, NCT00581997, NCT01872689). A bi-specific antibody directed against IL-4 and IL-13 function reduces Rodnan skin scores of patients with diffuse cutaneous SSc (NCT02921971). IL-11 is a member of the IL-6 cytokine family that has been associated with a range of fibroinflammatory conditions. TGF-β1 can initiate an autocrine loop of IL-11 signaling in pulmonary fibroblasts, which, in a largely ERK-dependent manner, triggers the translation of profibrotic proteins. Therapeutic targeting of IL-11 was shown to ameliorate fibrotic outcomes in preclinical models of pulmonary fibrosis and was associated with a reduction in pathological ERK signaling (Ng et al. 2020; Widjaja et al. 2021).
It seems appropriate to finish this part on myofibroblast therapeutics with a few words about death. In their seminal studies, Gabbiani and colleagues demonstrated that most myofibroblasts undergo apoptosis upon completion of tissue repair (Darby et al. 1990; Desmouliere et al. 1995). Consequently, persistence of myofibroblasts in hypertrophic scarring has been suggested to result from an acquired resistance to cell death in diseased conditions (Thannickal and Horowitz 2006; Fattman 2008). Instead, myofibroblasts were later shown to be committed to suicide in fibrotic conditions by mitochondrial apoptotic priming, which is controlled by a complex balance of effector, activator, prosurvival, and sensitizer proteins belonging to the BCL-2 family (Merkt et al. 2020, 2021). Novel therapeutic strategies are emerging to capitalize on myofibroblasts addiction to survival factors and targeting apoptotic pathways to treat fibrosis (Kuehl and Lagares 2018; Tschumperlin and Lagares 2020). For instance, the inhibitor of several BCL proteins, ABT-263 (navitoclax) cell specifically induces myofibroblast apoptosis in mouse dermal and lung fibrosis (Lagares et al. 2017; Pan et al. 2017), and in liver and lung myofibroblasts (Rizvi et al. 2014; Yosef et al. 2016; Moncsek et al. 2018). Likewise, functional inhibition of BCL proteins that shift the balance to prosurvival, using the mimetic drug A-1331852 protects mice from induced liver fibrosis by driving liver myofibroblasts into suicide (Moncsek et al. 2017). Drugs targeting different components of mitochondrial apoptotic pathways are tested in clinical trials in patients with various tumors but not yet for applications in fibrosis and scarring. In addition to targeting apoptosis pathways, combinations of drugs specifically ablating senescent cells like death-primed myofibroblasts have entered phase 1 human clinical trials to treat patients with lung fibrosis (NCT02874989) with first promising results (Justice et al. 2019). Notably, there exists an intricate relation between extracellular and intracellular stress and the probability of myofibroblasts to enter programmed cell death. For instance, the relative expression of BCL-2 family proteins is regulated by YAP/TAZ and RhoA/ROCK signaling and tackling myofibroblast mechanotransduction pathways is likely to also interfere with life–death decisions and persistence of myofibroblasts (Hinz and Lagares 2020).
CONCLUSION AND OUTLOOK: WITH A LITTLE HELP FROM FRIENDS
This review focused on fibroblasts and myofibroblasts as key effector cells in normal and dysregulated wound healing—purposely ignoring other cellular actors in the repair process. Although ECM compositional and mechanical properties play a major role in (mis-) instructing myofibroblasts, another hallmark of hypertrophic scar and chronic wound environments is subacute, unresolved, and/or dysregulated inflammation. Macrophages have long been recognized as key immune cells required to both amplify and resolve inflammation in the healing wound to orchestrate tissue repair (Eming et al. 2021). Macrophage recruitment and activation from tissue-resident sources or from circulating bone marrow–derived monocytes is part of the normal inflammatory response against injury (Davies et al. 2013; Wynn and Vannella 2016; Jakubzick et al. 2017). Macrophages produce factors that aid in the activation and proliferation of other immune cells, stem cells, and fibroblasts. For instance, dermal macrophages have been shown to promote fibroblast proliferation in the skin by secreting TGF-α, basic fibroblast growth factor, and PDGF (Shook et al. 2018). During wound resolution, tissue-resident macrophages die or repopulate the site, while recruited monocyte-derived macrophages undergo apoptosis or persist and with the potential to gain self-renewing capabilities (Watanabe et al. 2019). Persistence of macrophages beyond physiological repair (Janssen et al. 2011; Gibbings et al. 2015), dysregulation of macrophage activation (polarization) (Lavin et al. 2014; Braga et al. 2015; Gibbings et al. 2015), and continued recruitment of “second-wave” recruited macrophages (Arnold et al. 2007; Kharraz et al. 2013) into the existing fibrotic environment have all been shown to contribute to fibrosis rather than resolution (Forbes and Rosenthal 2014; Misharin et al. 2017). Although the persistence of macrophages often results in pathological repair, completely abrogating inflammatory responses offers no benefit and equally disrupts physiological wound healing (Vannella and Wynn 2017).
Miscommunication between inflammatory macrophages and myofibroblast has been identified as one key factor in tipping the balance between physiological repair and pathological fibrosis (Buechler et al. 2021a; Schuster et al. 2021). Beyond traditional paracrine signaling, our own studies demonstrated that myofibroblast contractions are transmitted through fibrous collagen ECM and attract mechanosensitive and migratory macrophages (Pakshir et al. 2019). Macrophages in the proximity of myofibroblast establish cadherin-11-mediated adhered junctions that allow for prolonged contact of the two cell types in cocultures and in fibrotic mouse and human lung tissues (Lodyga et al. 2019). One characteristic of this macrophage–myofibroblast fibrotic niche, at least in vitro, are high levels of active TGF-β1 that eventually enhance myofibroblast phenotype and contractile function (Lodyga et al. 2019). While this study demonstrated that direct contact with macrophages is essential, the mode of macrophage-induced TGF-β1 activation remains elusive. Moreover, the role of macrophages in the wound healing process has been shown to be context dependent. For example, small wounds in young mice that lack macrophages heal in a scarless manner (Martin et al. 2003). In conclusion, in addition to eliminating or deactivating myofibroblasts by conventional biochemical and mechanical therapeutics, understanding how macrophages in their many phenotypic variants interact with fibroblastic cells provides unique opportunities to even reeducate myofibroblasts into scar-resolving cells.
ACKNOWLEDGMENTS
The research of B.H. is supported by a foundation grant from the Canadian Institutes of Health Research (#375597) and support from the John Evans Leadership funds (#36050, #38861, and 38430) and innovation funds (“Fibrosis Network, #36349”) from the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). R.S. received a stipend from the Mathematics of Information Technology and Complex Systems (Mitacs fund #IT13623). M.E. is supported by an Ontario Graduate Scholarship (OGS), and F.Y. is a recipient of a Mary H. Beatty Fellowship from the School of Graduate Studies, University of Toronto. Schemes in figures have been produced using Biorender.com.
Footnotes
Editors: Xing Dai, Sabine Werner, Cheng-Ming Chuong, and Maksim Plikus
Additional Perspectives on Wound Healing: From Bench to Bedside available at www.cshperspectives.org
REFERENCES
- Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. 2007. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 21: 3250–3261. 10.1096/fj.07-8218com [DOI] [PubMed] [Google Scholar]
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, Jaffer A, Sharma N, Hagner A, Shah P, et al. 2020. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27: 396–412.e396. 10.1016/j.stem.2020.07.008 [DOI] [PubMed] [Google Scholar]
- Abercrombie M, James DW, Newcombe JF. 1960. Wound contraction in rabbit skin, studied by splinting the wound margins. J Anat 94: 170–182. [PMC free article] [PubMed] [Google Scholar]
- Achterberg VF, Buscemi L, Diekmann H, Smith-Clerc J, Schwengler H, Meister JJ, Wenck H, Gallinat S, Hinz B. 2014. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J Invest Dermatol 134: 1862–1872. 10.1038/jid.2014.90 [DOI] [PubMed] [Google Scholar]
- Adapala RK, Katari V, Teegala LR, Thodeti S, Paruchuri S, Thodeti CK. 2021. TRPV4 mechanotransduction in fibrosis. Cells 10: 3053. 10.3390/cells10113053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akershoek JJ, Brouwer KM, Vlig M, Boekema B, Beelen RHJ, Middelkoop E, Ulrich MMW. 2017. Differential effects of Losartan and Atorvastatin in partial and full thickness burn wounds. PLoS ONE 12: e0179350. 10.1371/journal.pone.0179350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetshina A, Dees C, Busch N, Beer J, Sarter K, Zwerina J, Zimmer A, Distler O, Schett G, Distler JH. 2009. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum 60: 1129–1136. 10.1002/art.24395 [DOI] [PubMed] [Google Scholar]
- Alam J, Musiime M, Romaine A, Sawant M, Melleby AO, Lu N, Eckes B, Christensen G, Gullberg D. 2020. Generation of a novel mouse strain with fibroblast-specific expression of Cre recombinase. Matrix Biol Plus 8: 100045. 10.1016/j.mbplus.2020.100045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh N, Pepper MS, Modarressi A, Alfo K, Schlaudraff K, Montandon D, Gabbiani G, Bochaton-Piallat ML, Pittet B. 2007. Persistent ischemia impairs myofibroblast development in wound granulation tissue: a new model of delayed wound healing. Wound Repair Regen 15: 809–816. 10.1111/j.1524-475X.2007.00312.x [DOI] [PubMed] [Google Scholar]
- Allinovi M, De Chiara L, Angelotti ML, Becherucci F, Romagnani P. 2018. Anti-fibrotic treatments: a review of clinical evidence. Matrix Biol 68–69: 333–354. 10.1016/j.matbio.2018.02.017 [DOI] [PubMed] [Google Scholar]
- AlQudah M, Hale TM, Czubryt MP. 2020. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol 91–92: 92–108. 10.1016/j.matbio.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Nik S, Dolp R, Eylert G, Datu AK, Parousis A, Blakeley C, Jeschke MG. 2018. Stem cells derived from burned skin: the future of burn care. EBioMedicine 37: 509–520. 10.1016/j.ebiom.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelova K, Egan Benova T, Szeiffova Bacova B, Sykora M, Prado NJ, Diez ER, Hlivak P, Tribulova N. 2020. Cardiac connexin-43 hemichannels and Pannexin1 channels: provocative antiarrhythmic targets. Int J Mol Sci 22: 260. 10.3390/ijms22010260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EO, Schneider ER, Bagriantsev SN. 2017. Piezo2 in cutaneous and proprioceptive mechanotransduction in vertebrates. Curr Top Membr 79: 197–217. 10.1016/bs.ctm.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. 2007. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069. 10.1084/jem.20070075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldi R, Chaponnier C, Gabbiani G, Hinz B. 2012. Heterogeneity of smooth muscle. In Muscle: fundamental biology and mechanisms of disease (ed. Hill J), pp. 1183–1195. Elsevier, New York. [Google Scholar]
- Arora PD, McCulloch CA. 1994. Dependence of collagen remodelling on α-smooth muscle actin expression by fibroblasts. J Cell Physiol 159: 161–175. 10.1002/jcp.1041590120 [DOI] [PubMed] [Google Scholar]
- Arora PD, Di Gregorio M, He P, McCulloch CA. 2017. TRPV4 mediates the Ca2+ influx required for the interaction between flightless-1 and non-muscle myosin, and collagen remodeling. J Cell Sci 130: 2196–2208. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. 2006. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 168: 499–510. 10.2353/ajpath.2006.041306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascension AM, Fuertes-Alvarez S, Ibanez-Sole O, Izeta A, Arauzo-Bravo MJ. 2021. Human dermal fibroblast subpopulations are conserved across single-cell RNA sequencing studies. J Invest Dermatol 141: 1735–1744.e1735. 10.1016/j.jid.2020.11.028 [DOI] [PubMed] [Google Scholar]
- Bachmann M, Kukkurainen S, Hytonen VP, Wehrle-Haller B. 2019. Cell adhesion by integrins. Physiol Rev 99: 1655–1699. 10.1152/physrev.00036.2018 [DOI] [PubMed] [Google Scholar]
- Balistreri E, Garcia-Gonzalez E, Selvi E, Akhmetshina A, Palumbo K, Lorenzini S, Maggio R, Lucattelli M, Galeazzi M, Distler JW. 2011. The cannabinoid WIN55, 212-2 abrogates dermal fibrosis in scleroderma bleomycin model. Ann Rheum Dis 70: 695–699. 10.1136/ard.2010.137539 [DOI] [PubMed] [Google Scholar]
- Barnes LA, Marshall CD, Leavitt T, Hu MS, Moore AL, Gonzalez JG, Longaker MT, Gurtner GC. 2018. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care (New Rochelle) 7: 47–56. 10.1089/wound.2016.0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. 2010. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017. 10.1038/nm.2208 [DOI] [PubMed] [Google Scholar]
- Becker DL, Phillips AR, Duft BJ, Kim Y, Green CR. 2016. Translating connexin biology into therapeutics. Semin Cell Dev Biol 50: 49–58. 10.1016/j.semcdb.2015.12.009 [DOI] [PubMed] [Google Scholar]
- Bell RE, Shaw TJ. 2021. Keloid tissue analysis discredits a role for myofibroblasts in disease pathogenesis. Wound Repair Regen 29: 637–641. 10.1111/wrr.12923 [DOI] [PubMed] [Google Scholar]
- Bernasconi R, Nystrom A. 2018. Balance and circumstance: the renin angiotensin system in wound healing and fibrosis. Cell Signal 51: 34–46. 10.1016/j.cellsig.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Hough RF. 2019. Piezo1 in the lung: at last! Am J Respir Cell Mol Biol 60: 609–610. 10.1165/rcmb.2018-0418ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham GC, Lee F, Naba A, Barker TH. 2020. Spatial-omics: novel approaches to probe cell heterogeneity and extracellular matrix biology. Matrix Biol 91–92: 152–166. 10.1016/j.matbio.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe NM, Muraki K, Ludlow MJ, Stylianidis V, Gilbert HTJ, Evans EL, Cuthbertson K, Foster R, Swift J, Li J, et al. 2019. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J Biol Chem 294: 17395–17408. 10.1074/jbc.RA119.009167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JE, Kokosis G, Ren L, Selim MA, Bergeron A, Levinson H. 2011. Wound contraction is attenuated by fasudil inhibition of Rho-associated kinase. Plast Reconstr Surg 128: 438e–450e. 10.1097/PRS.0b013e31822b7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte F, Girard D, Archambault JC, Desmouliere A. 2019. Skin changes during ageing. Subcell Biochem 91: 249–280. 10.1007/978-981-13-3681-2_10 [DOI] [PubMed] [Google Scholar]
- Bouzid T, Kim E, Riehl BD, Esfahani AM, Rosenbohm J, Yang R, Duan B, Lim JY. 2019. The LINC complex, mechanotransduction, and mesenchymal stem cell function and fate. J Biol Eng 13: 68. 10.1186/s13036-019-0197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga TT, Agudelo JS, Camara NO. 2015. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol 6: 602. 10.3389/fimmu.2015.00602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CM, Nelson BR, Wakenight P, Collins SJ, Okamura DM, Dong XR, Mahoney WM, McKenna A, Shendure J, Timms A, et al. 2021. Adaptations in Hippo-Yap signaling and myofibroblast fate underlie scar-free ear appendage wound healing in spiny mice. Dev Cell 56: 2722–2740.e2726. 10.1016/j.devcel.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler MB, Fu W, Turley SJ. 2021a. Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 54: 903–915. 10.1016/j.immuni.2021.04.021 [DOI] [PubMed] [Google Scholar]
- Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien R, Roose-Girma M, et al. 2021b. Cross-tissue organization of the fibroblast lineage. Nature 593: 575–579. 10.1038/s41586-021-03549-5 [DOI] [PubMed] [Google Scholar]
- Burridge K, Guilluy C. 2016. Focal adhesions, stress fibers and mechanical tension. Exp Cell Res 343: 14–20. 10.1016/j.yexcr.2015.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Monaghan-Benson E, Graham DM. 2019. Mechanotransduction: from the cell surface to the nucleus via RhoA. Philos Trans R Soc Lond B Biol Sci 374: 20180229. 10.1098/rstb.2018.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. 2011. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 21: 2046–2054. 10.1016/j.cub.2011.11.037 [DOI] [PubMed] [Google Scholar]
- Campbell MG, Cormier A, Ito S, Seed RI, Bondesson AJ, Lou J, Marks JD, Baron JL, Cheng Y, Nishimura SL. 2020. Cryo-EM reveals integrin-mediated TGF-β activation without release from latent TGF-β. Cell 180: 490–501.e416. 10.1016/j.cell.2019.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario M. 2018. DDR1 and DDR2 in skin. Cell Adh Migr 12: 386–393. 10.1080/19336918.2018.1485618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. 2002. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci 99: 12877–12882. 10.1073/pnas.162488599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonney E, Speight P, Masszi A, Nakano H, Kapus A. 2011. β-Catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol Biol Cell 22: 4472–4485. 10.1091/mbc.e11-04-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Qu J, Huang X, Kurundkar A, Zhu L, Yang N, Venado A, Ding Q, Liu G, Antony VB, et al. 2016. Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat Commun 7: 12564. 10.1038/ncomms12564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Arora PD, McCulloch CA, Wilde A. 2017. Cytokinesis requires localized β-actin filament production by an actin isoform specific nucleator. Nat Commun 8: 1530. 10.1038/s41467-017-01231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kwon SH, Henn D, Kuehlmann BA, Tevlin R, Bonham CA, Griffin M, Trotsyuk AA, Borrelli MR, Noishiki C, et al. 2021. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat Commun 12: 5256. 10.1038/s41467-021-25410-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SG, Sato S, Kolb M, Gauldie J. 2019. Fibrocytes and fibroblasts—where are we now. Int J Biochem Cell Biol 116: 105595. 10.1016/j.biocel.2019.105595 [DOI] [PubMed] [Google Scholar]
- Claveria-Cabello A, Colyn L, Arechederra M, Urman JM, Berasain C, Avila MA, Fernandez-Barrena MG. 2020. Epigenetics in liver fibrosis: could HDACs be a therapeutic target? Cells 9: 2321. 10.3390/cells9102321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho NM, McCulloch CA. 2018. Mechanical signaling through the discoidin domain receptor 1 plays a central role in tissue fibrosis. Cell Adh Migr 12: 348–362. 10.1080/19336918.2018.1448353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho NM, Arora PD, van Putten S, Boo S, Petrovic P, Lin AX, Hinz B, McCulloch CA. 2017. Discoidin domain receptor 1 mediates myosin-dependent collagen contraction. Cell Rep 18: 1774–1790. 10.1016/j.celrep.2017.01.061 [DOI] [PubMed] [Google Scholar]
- Conroy KP, Kitto LJ, Henderson NC. 2016. αv integrins: key regulators of tissue fibrosis. Cell Tissue Res 365: 511–519. 10.1007/s00441-016-2407-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Gallegos D, Rinkevich Y. 2021. Cutting into wound repair. FEBS J 10.1111/febs.16078 [DOI] [PubMed] [Google Scholar]
- Costa E, Okesola BO, Thrasivoulou C, Becker DL, Deprest JA, David AL, Chowdhury TT. 2021. Cx43 mediates changes in myofibroblast contraction and collagen release in human amniotic membrane defects after trauma. Sci Rep 11: 16975. 10.1038/s41598-021-94767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider BJ, Risinger GM, Haaksma CJ Jr, Howard EW, Tomasek JJ. 2011. Myocardin-related transcription factors A and B are key regulators of TGF-β1-induced fibroblast to myofibroblast differentiation. J Invest Dermatol 131: 2378–2385. 10.1038/jid.2011.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai EN, Heo SJ, Mauck RL. 2020. “Looping in” mechanics: mechanobiologic regulation of the nucleus and the epigenome. Adv Healthc Mater 9: e2000030. 10.1002/adhm.202000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle P, Despatis MA, Grenier G. 2013. How mechanical deformations contribute to the effectiveness of negative-pressure wound therapy. Wound Repair Regen 21: 498–502. 10.1111/wrr.12052 [DOI] [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G. 1990. α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63: 21–29. [PubMed] [Google Scholar]
- Davidson S, Coles M, Thomas T, Kollias G, Ludewig B, Turley S, Brenner M, Buckley CD. 2021. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol 21: 704–717. 10.1038/s41577-021-00540-z [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. 2013. Tissue-resident macrophages. Nat Immunol 14: 986–995. 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CC, Hu YF, Zhu DH, Cheng Q, Gu JJ, Feng QL, Zhang LX, Xu YP, Wang D, Rong Z, et al. 2021. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat Commun 12: 3709. 10.1038/s41467-021-24110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton CP, Khanna D. 2017. Systemic sclerosis. Lancet 390: 1685–1699. 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- de Oliveira Camargo R, Abual'anaz B, Rattan SG, Filomeno KL, Dixon IMC. 2021. Novel factors that activate and deactivate cardiac fibroblasts: a new perspective for treatment of cardiac fibrosis. Wound Repair Regen 29: 667–677. 10.1111/wrr.12947 [DOI] [PubMed] [Google Scholar]
- Derynck R, Turley SJ, Akhurst RJ. 2021. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol 18: 9–34. 10.1038/s41571-020-0403-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- desJardins-Park HE, Gurtner GC, Wan DC, Longaker MT. 2021. From chronic wounds to scarring: the growing healthcare burden of under- and over-healing wounds. Adv Wound Care (New Rochelle) 10.1089/wound.2021.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. 1995. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146: 56–66. [PMC free article] [PubMed] [Google Scholar]
- Deville SS, Cordes N. 2019. The extracellular, cellular, and nuclear stiffness, a trinity in the cancer resistome—a review. Front Oncol 9: 1376. 10.3389/fonc.2019.01376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Varelas X, Guan KL. 2020. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov 19: 480–494. 10.1038/s41573-020-0070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, Naba A, Aguirre-Ghiso JA, Bravo-Cordero JJ. 2022. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer 3: 90–107. 10.1038/s43018-021-00291-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Smith L, Cho S, Colasurdo M, Garcia AJ, Safran S. 2017. Matrix mechanosensing: from scaling concepts in ‘omics data to mechanisms in the nucleus, regeneration, and cancer. Annu Rev Biophys 46: 295–315. 10.1146/annurev-biophys-062215-011206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, Raghu G, Sauter W, Girard M, Alves M, et al. 2019. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 380: 2518–2528. 10.1056/NEJMoa1903076 [DOI] [PubMed] [Google Scholar]
- Dobrzynska A, Gonzalo S, Shanahan C, Askjaer P. 2016. The nuclear lamina in health and disease. Nucleus 7: 233–248. 10.1080/19491034.2016.1183848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolivo DM, Larson SA, Dominko T. 2017a. FGF2-mediated attenuation of myofibroblast activation is modulated by distinct MAPK signaling pathways in human dermal fibroblasts. J Dermatol Sci 88: 339–348. 10.1016/j.jdermsci.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolivo DM, Larson SA, Dominko T. 2017b. Fibroblast growth factor 2 as an antifibrotic: antagonism of myofibroblast differentiation and suppression of pro-fibrotic gene expression. Cytokine Growth Factor Rev 38: 49–58. 10.1016/j.cytogfr.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW, Castiglioni A, et al. 2020. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 10: 232–253. 10.1158/2159-8290.CD-19-0644 [DOI] [PubMed] [Google Scholar]
- Dooling LJ, Discher DE. 2019. Inhibiting tumor fibrosis and actomyosin through GPCR activation. Trends Cancer 5: 197–199. 10.1016/j.trecan.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, et al. 2013. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–281. 10.1038/nature12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy L, Henderson J, Brown M, Pryzborski S, Fullard N, Summa L, Distler JHW, Stratton R, O'Reilly S. 2021. Bone morphogenetic protein antagonist gremlin-1 increases myofibroblast transition in dermal fibroblasts: implications for systemic sclerosis. Front Cell Dev Biol 9: 681061. 10.3389/fcell.2021.681061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham C, Havlioglu N, Chamberlain A, Lake S, Meyer G. 2020. Adipose stem cells exhibit mechanical memory and reduce fibrotic contracture in a rat elbow injury model. FASEB J 34: 12976–12990. 10.1096/fj.202001274R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Dutta B, Arya RK, Goswami R, Alharbi MO, Sharma S, Rahaman SO. 2020. Role of macrophage TRPV4 in inflammation. Lab Invest 100: 178–185. 10.1038/s41374-019-0334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi A, Coleman R, Gresham K, Gao E, Ibetti J, Chuprun JK, Koch WJ. 2021. GRK5 is a regulator of fibroblast activation and cardiac fibrosis. Proc Natl Acad Sci 118: e2012854118. 10.1073/pnas.2012854118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP, Rajaratnam JB, Griswold TR. 1986. ATP-induced cell contraction in dermal fibroblasts: effects of cAMP and myosin light-chain kinase. J Cell Physiol 128: 223–230. 10.1002/jcp.1041280213 [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G. 1994. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 145: 105–113. [PMC free article] [PubMed] [Google Scholar]
- Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, et al. 2019. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 9: 1102–1123. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Murray PJ, Pearce EJ. 2021. Metabolic orchestration of the wound healing response. Cell Metab 33: 1726–1743. 10.1016/j.cmet.2021.07.017 [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Expert Working Group. 2008. Vacuum assisted closure: recommendations for use. A consensus document. Int Wound J 5: iii–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiella W, Atoui R. 2016. Immunotolerant properties of mesenchymal stem cells: updated review. Stem Cells Int 2016: 1859567. 10.1155/2016/1859567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. 2015. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol 11: 233–244. 10.1038/nrneph.2014.246 [DOI] [PubMed] [Google Scholar]
- Fattman CL. 2008. Apoptosis in pulmonary fibrosis: too much or not enough? Antioxid Redox Signal 10: 379–385. 10.1089/ars.2007.1907 [DOI] [PubMed] [Google Scholar]
- Feller T, Connell SDA, Arismall io RRAS. 2021. Why fibrin biomechanical properties matter for hemostasis and thrombosis. J Thromb Haemost 20: 6–16. 10.1111/jth.15531 [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. 2011. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146: 761–771. 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Baker AT, Lukacs-Kornek V, Knoblich K. 2020. The fibroblastic T cell niche in lymphoid tissues. Curr Opin Immunol 64: 110–116. 10.1016/j.coi.2020.04.007 [DOI] [PubMed] [Google Scholar]
- Flynn BP, Bhole AP, Saeidi N, Liles M, Dimarzio CA, Ruberti JW. 2010. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS ONE 5: e12337. 10.1371/journal.pone.0012337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follonier L, Schaub S, Meister JJ, Hinz B. 2008. Myofibroblast communication is controlled by intercellular mechanical coupling. J Cell Sci 121: 3305–3316. 10.1242/jcs.024521 [DOI] [PubMed] [Google Scholar]
- Follonier Castella L, Buscemi L, Godbout C, Meister JJ, Hinz B. 2010a. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J Cell Sci 123: 1751–1760. 10.1242/jcs.066795 [DOI] [PubMed] [Google Scholar]
- Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. 2010b. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res 316: 2390–2401. 10.1016/j.yexcr.2010.04.033 [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Rosenthal N. 2014. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 20: 857–869. 10.1038/nm.3653 [DOI] [PubMed] [Google Scholar]
- Foster DS, Januszyk M, Yost KE, Chinta MS, Gulati GS, Nguyen AT, Burcham AR, Salhotra A, Ransom RC, Henn D, et al. 2021. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc Natl Acad Sci 118: e2110025118. 10.1073/pnas.2110025118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraticelli P, Gabrielli B, Pomponio G, Valentini G, Bosello S, Riboldi P, Gerosa M, Faggioli P, Giacomelli R, Del Papa N, et al. 2014. Low-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res Ther 16: R144. 10.1186/ar4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Sheppard D, Duffield JS, Violette S. 2013. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5: 167sr161. 10.1126/scitranslmed.3004700 [DOI] [PubMed] [Google Scholar]
- Froese AR, Shimbori C, Bellaye PS, Ask K, Inman M, Obex S, Fatima S, Jenkins G, Gauldie J, Kolb M. 2016. Stretch induced activation of TGF-β1 in pulmonary fibrosis. Am J Respir Crit Care Med 194: 84–96. 10.1164/rccm.201508-1638OC [DOI] [PubMed] [Google Scholar]
- Fu S, Panayi A, Fan J, Mayer HF, Daya M, Khouri RK, Gurtner GC, Ogawa R, Orgill DP. 2021a. Mechanotransduction in wound healing: from the cellular and molecular level to the clinic. Adv Skin Wound Care 34: 67–74. 10.1097/01.ASW.0000725220.92976.a7 [DOI] [PubMed] [Google Scholar]
- Fu Y, Wan P, Zhang J, Li X, Xing J, Zou Y, Wang K, Peng H, Zhu Q, Cao L, et al. 2021b. Targeting mechanosensitive piezo1 alleviated renal fibrosis through p38MAPK-YAP pathway. Front Cell Dev Biol 9: 741060. 10.3389/fcell.2021.741060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. 2021. 50 years of myofibroblasts: how the myofibroblast concept evolved. Methods Mol Biol 2299: 1–5. 10.1007/978-1-0716-1382-5_1 [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majno G. 1971. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27: 549–550. 10.1007/BF02147594 [DOI] [PubMed] [Google Scholar]
- Gan W, Ren J, Li T, Lv S, Li C, Liu Z, Yang M. 2018. The SGK1 inhibitor EMD638683, prevents Angiotensin II–induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis 1864: 1–10. 10.1016/j.bbadis.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Ge J, Burnier L, Adamopoulou M, Kwa MQ, Schaks M, Rottner K, Brakebusch C. 2018. Rhoa, Rac1, and Cdc42 differentially regulate αSMA and collagen I expression in mesenchymal stem cells. J Biol Chem 293: 9358–9369. 10.1074/jbc.RA117.001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM, Bratton DL, Janssen W, Jakubzick CV. 2015. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 126: 1357–1366. 10.1182/blood-2015-01-624809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseck RL III, Ramalingam TR, Hart KM, Vannella KM, Cantu DA, Lu WY, Ferreira-Gonzalez S, Forbes SJ, Vallier L, Wynn TA. 2016. Interleukin-13 activates distinct cellular pathways leading to ductular reaction, steatosis, and fibrosis. Immunity 45: 145–158. 10.1016/j.immuni.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseck RL III, Wilson MS, Wynn TA. 2018. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 18: 62–76. 10.1038/nri.2017.90 [DOI] [PubMed] [Google Scholar]
- Godbout C, Follonier Castella L, Smith EA, Talele N, Chow ML, Garonna A, Hinz B. 2013. The mechanical environment modulates intracellular calcium oscillation activities of myofibroblasts. PLoS ONE 8: e64560. 10.1371/journal.pone.0064560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. 2006. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J Cell Biol 172: 259–268. 10.1083/jcb.200506179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonga-Cave BC, Pena Diaz AM, O'Gorman DB. 2021. Biomimetic analyses of interactions between macrophages and palmar fascia myofibroblasts derived from Dupuytren's disease reveal distinct inflammatory cytokine responses. Wound Repair Regen 29: 627–636. 10.1111/wrr.12928 [DOI] [PubMed] [Google Scholar]
- Goss G, Rognoni E, Salameti V, Watt FM. 2021. Distinct fibroblast lineages give rise to NG2+ pericyte populations in mouse skin development and repair. Front Cell Dev Biol 9: 675080. 10.3389/fcell.2021.675080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MF, Borrelli MR, Garcia JT, Januszyk M, King M, Lerbs T, Cui L, Moore AL, Shen AH, Mascharak S, et al. 2021. JUN promotes hypertrophic skin scarring via CD36 in preclinical in vitro and in vivo models. Sci Transl Med 13: eabb3312. 10.1126/scitranslmed.abb3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove LM, Mohan ML, Abraham S, Scheraga RG, Southern BD, Crish JF, Naga Prasad SV, Olman MA. 2019. Translocation of TRPV4-PI3Kγ complexes to the plasma membrane drives myofibroblast transdifferentiation. Sci Signal 12: eaau1533. 10.1126/scisignal.aau1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Liu X, Wang QX, Tan HW, Guo M, Jiang WF, Zhou L. 2012. Angiotensin II increases CTGF expression via MAPKs/TGF-β1/TRAF6 pathway in atrial fibroblasts. Exp Cell Res 318: 2105–2115. 10.1016/j.yexcr.2012.06.015 [DOI] [PubMed] [Google Scholar]
- Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, Ma D, Liu Y, Yamaga K, Shestova O, Gay DL, Yang Z, et al. 2019. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun 10: 650. 10.1038/s41467-018-08247-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008. Wound repair and regeneration. Nature 453: 314–321. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- Haak AJ, Kostallari E, Sicard D, Ligresti G, Choi KM, Caporarello N, Jones DL, Tan Q, Meridew J, Diaz Espinosa AM, et al. 2019. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med 11: eaau6296. 10.1126/scitranslmed.aau6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak AJ, Ducharme MT, Diaz Espinosa AM, Tschumperlin DJ. 2020. Targeting GPCR signaling for idiopathic pulmonary fibrosis therapies. Trends Pharmacol Sci 41: 172–182. 10.1016/j.tips.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart KM, Fabre T, Sciurba JC, Gieseck RL III, Borthwick LA, Vannella KM, Acciani TH, de Queiroz Prado R, Thompson RW, White S, et al. 2017. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci Transl Med 9: eaal3694. 10.1126/scitranslmed.aal3694 [DOI] [PubMed] [Google Scholar]
- He H, Suryawanshi H, Morozov P, Gay-Mimbrera J, Del Duca E, Kim HJ, Kameyama N, Estrada Y, Der E, Krueger JG, et al. 2020. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol 145: 1615–1628. 10.1016/j.jaci.2020.01.042 [DOI] [PubMed] [Google Scholar]
- He J, Fang B, Shan S, Xie Y, Wang C, Zhang Y, Zhang X, Li Q. 2021. Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1. Cell Death Dis 12: 226. 10.1038/s41419-021-03481-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker A, Schellnegger M, Hofmann E, Luze H, Nischwitz SP, Kamolz LP, Kotzbeck P. 2022. The impact of resveratrol on skin wound healing, scarring, and aging. Int Wound J 19: 9–28. 10.1111/iwj.13601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayatyanfard K, Ziai SA, Niazi F, Habibi I, Habibi B, Moravvej H. 2018. Losartan ointment relieves hypertrophic scars and keloid: a pilot study. Wound Repair Regen 26: 340–343. 10.1111/wrr.12648 [DOI] [PubMed] [Google Scholar]
- Heit YI, Dastouri P, Helm DL, Pietramaggiori G, Younan G, Erba P, Munster S, Orgill DP, Scherer SS. 2012. Foam pore size is a critical interface parameter of suction-based wound healing devices. Plast Reconstr Surg 129: 589–597. 10.1097/PRS.0b013e3182402c89 [DOI] [PubMed] [Google Scholar]
- Heitman N, Sennett R, Mok KW, Saxena N, Srivastava D, Martino P, Grisanti L, Wang Z, Ma'ayan A, Rompolas P, et al. 2020. Dermal sheath contraction powers stem cell niche relocation during hair cycle regression. Science 367: 161–166. 10.1126/science.aax9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. 2013. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624. 10.1038/nm.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Distler J, O'Reilly S. 2019. The role of epigenetic modifications in systemic sclerosis: a druggable target. Trends Mol Med 25: 395–411. 10.1016/j.molmed.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC, et al. 2018. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7: e39636. 10.7554/eLife.39636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J, Henke CA, Bitterman PB. 2018. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 128: 45–53. 10.1172/JCI93557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. 2010. The myofibroblast in connective tissue repair and regeneration. In Regenerative medicine and biomaterials for the repair of connective tissues (ed. Ralphs CAJ), pp. 39–82. Woodhead, Cambridge. [Google Scholar]
- Hinz B. 2013. It has to be the αv: myofibroblast integrins activate latent TGF-β1. Nat Med 19: 1567–1568. 10.1038/nm.3421 [DOI] [PubMed] [Google Scholar]
- Hinz B. 2016a. Myofibroblasts. Exp Eye Res 142: 56–70. 10.1016/j.exer.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Hinz B. 2016b. Targeting the myofibroblast to improve wound healing. In Wound healing biomaterials, Volume I: therapies and regeneration (ed. Ågren MS), pp. 69–100. Woodhead, Cambridge. [Google Scholar]
- Hinz B, Lagares D. 2020. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol 16: 11–31. 10.1038/s41584-019-0324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. 2001a. α-Smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12: 2730–2741. 10.1091/mbc.12.9.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. 2001b. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159: 1009–1020. 10.1016/S0002-9440(10)61776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G, Chaponnier C. 2002. The NH2-terminal peptide of α-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol 157: 657–663. 10.1083/jcb.200201049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. 2004. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15: 4310–4320. 10.1091/mbc.e04-05-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, McCulloch CA, Coelho NM. 2019. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp Cell Res 379: 119–128. 10.1016/j.yexcr.2019.03.027 [DOI] [PubMed] [Google Scholar]
- Holt JR, Zeng WZ, Evans EL, Woo SH, Ma S, Abuwarda H, Loud M, Patapoutian A, Pathak MM. 2021. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. eLife 10: e65415. 10.7554/eLife.65415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. 2007. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19: 761–771. 10.1016/j.cellsig.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Gai Y, Yang N, Lu B, Samuel CS, Thannickal VJ, Zhou Y. 2011. Relaxin regulates myofibroblast contractility and protects against lung fibrosis. Am J Pathol 179: 2751–2765. 10.1016/j.ajpath.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Teot L, Horch R, Banwell PE. 2007. Evidence-based medicine: vacuum-assisted closure in wound care management. Int Wound J 4: 256–269. 10.1111/j.1742-481X.2007.00361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J. 2009. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med 361: 968–979. 10.1056/NEJMoa0810866 [DOI] [PubMed] [Google Scholar]
- Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K, Group PS. 2016. Differential effect on bone lesions of targeting integrins: randomized phase II trial of abituzumab in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res 22: 3192–3200. 10.1158/1078-0432.CCR-15-2512 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Torigoe T, Matsumoto Y, Fujita T, Sato N, Yotsuyanagi T. 2013. Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen 21: 616–623. 10.1111/wrr.12062 [DOI] [PubMed] [Google Scholar]
- Inoue R, Kurahara LH, Hiraishi K. 2019. TRP channels in cardiac and intestinal fibrosis. Semin Cell Dev Biol 94: 40–49. 10.1016/j.semcdb.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, Olson LE. 2015. PDGFRα signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29: 1106–1119. 10.1101/gad.260554.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick CV, Randolph GJ, Henson PM. 2017. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 17: 349–362. 10.1038/nri.2017.28 [DOI] [PubMed] [Google Scholar]
- Janmey PA, Winer JP, Weisel JW. 2009. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 6: 1–10. 10.1098/rsif.2008.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janota CS, Calero-Cuenca FJ, Gomes ER. 2020. The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol 63: 204–211. 10.1016/j.ceb.2020.03.001 [DOI] [PubMed] [Google Scholar]
- Jansen KA, Atherton P, Ballestrem C. 2017. Mechanotransduction at the cell–matrix interface. Semin Cell Dev Biol 71: 75–83. 10.1016/j.semcdb.2017.07.027 [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Farkas L, Rahman T, Kolb MR. 2009. ATP stimulates Ca2+-waves and gene expression in cultured human pulmonary fibroblasts. Int J Biochem Cell Biol 41: 2477–2484. 10.1016/j.biocel.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. 2011. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184: 547–560. 10.1164/rccm.201011-1891OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ, Mukherjee S, Ask K. 2015. Calcium homeostasis and ionic mechanisms in pulmonary fibroblasts. Am J Respir Cell Mol Biol 53: 135–148. 10.1165/rcmb.2014-0269TR [DOI] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. 2006. Ligation of protease-activated receptor 1 enhances αvβ6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest 116: 1606–1614. 10.1172/JCI27183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Rinkevich Y. 2018. Defining skin fibroblastic cell types beyond CD90. Front Cell Dev Biol 6: 133. 10.3389/fcell.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Scharffetter-Kochanek K. 2020. Mesenchymal stem cells adaptively respond to environmental cues thereby improving granulation tissue formation and wound healing. Front Cell Dev Biol 8: 697. 10.3389/fcell.2020.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Yin K, Wu K, Zhang M, Wang S, Cheng H, Zhou Z, Xiao B. 2021. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat Commun 12: 869. 10.1038/s41467-021-21178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Quesada C, Aliabouzar M, Kripfgans OD, Franceschi RT, Liu J, Putnam AJ, Fabiilli ML. 2021. Release of basic fibroblast growth factor from acoustically-responsive scaffolds promotes therapeutic angiogenesis in the hind limb ischemia model. J Control Release 338: 773–783. 10.1016/j.jconrel.2021.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, et al. 2019. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40: 554–563. 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoldt V, Fischer L, Grashoff C. 2019. Unforgettable force: crosstalk and memory of mechanosensitive structures. Biol Chem 400: 687–698. 10.1515/hsz-2018-0328 [DOI] [PubMed] [Google Scholar]
- Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz-Canoves P. 2013. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm 2013: 491497. 10.1155/2013/491497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killaars AR, Grim JC, Walker CJ, Hushka EA, Brown TE, Anseth KS. 2019. Extended exposure to stiff microenvironments leads to persistent chromatin remodeling in human mesenchymal stem cells. Adv Sci (Weinh) 6: 1801483. 10.1002/advs.201801483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Sheppard D, Chapman HA. 2018. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol 10: a022293. 10.1101/cshperspect.a022293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. 2014. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- Kirby TJ, Lammerding J. 2018. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol 20: 373–381. 10.1038/s41556-018-0038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, et al. 2012. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci 109: 9448–9453. 10.1073/pnas.1201840109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B. 2014. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol 207: 283–297. 10.1083/jcb.201402006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhammer BM, Floege J, Boor P. 2018. PDGF in organ fibrosis. Mol Aspects Med 62: 44–62. 10.1016/j.mam.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Knipe RS, Probst CK, Lagares D, Franklin A, Spinney JJ, Brazee PL, Grasberger P, Zhang L, Black KE, Sakai N, et al. 2018. The Rho kinase isoforms ROCK1 and ROCK2 each contribute to the development of experimental pulmonary fibrosis. Am J Respir Cell Mol Biol 58: 471–481. 10.1165/rcmb.2017-0075OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderink GH, Paluch EK. 2018. Architecture shapes contractility in actomyosin networks. Curr Opin Cell Biol 50: 79–85. 10.1016/j.ceb.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Kofler M, Speight P, Little D, Di Ciano-Oliveira C, Szaszi K, Kapus A. 2018. Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nat Commun 9: 4966. 10.1038/s41467-018-07450-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosec A, Frech S, Gesslbauer B, Vierhapper M, Radtke C, Petzelbauer P, Lichtenberger BM. 2019. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J Invest Dermatol 139: 342–351. 10.1016/j.jid.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Krepinsky JC. 2022. Activin B, a new player in kidney fibrosis? J Pathol 256: 363–365. 10.1002/path.5847 [DOI] [PubMed] [Google Scholar]
- Kuehl T, Lagares D. 2018. BH3 mimetics as anti-fibrotic therapy: unleashing the mitochondrial pathway of apoptosis in myofibroblasts. Matrix Biol 68–69: 94–105. 10.1016/j.matbio.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Kuehlmann B, Bonham CA, Zucal I, Prantl L, Gurtner GC. 2020. Mechanotransduction in wound healing and fibrosis. J Clin Med 9: 5. 10.3390/jcm9051423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagares D, Hinz B. 2021. Animal and human models of tissue repair and fibrosis: an introduction. Methods Mol Biol 2299: 277–290. 10.1007/978-1-0716-1382-5_20 [DOI] [PubMed] [Google Scholar]
- Lagares D, Busnadiego O, Garcia-Fernandez RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, et al. 2012. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum 64: 1653–1664. 10.1002/art.33482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagares D, Santos A, Grasberger PE, Liu F, Probst CK, Rahimi RA, Sakai N, Kuehl T, Ryan J, Bhola P, et al. 2017. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci Transl Med 9: eaal3765. 10.1126/scitranslmed.aal3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi MC, Reinhart-King CA. 2018. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci Transl Med 10: eaao0475. 10.1126/scitranslmed.aao0475 [DOI] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326. 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorn BG, Brnardic EJ, Behm DJ. 2021. TRPV4 antagonists: a patent review (2015–2020). Expert Opin Ther Pat 31: 773–784. 10.1080/13543776.2021.1903432 [DOI] [PubMed] [Google Scholar]
- Layton T, Nanchahal J. 2019. Recent advances in the understanding of Dupuytren's disease. F1000Res 8: F1000 Faculty Rev-231. 10.12688/f1000research.17779.1 [DOI] [Google Scholar]
- Layton TB, Williams L, Yang N, Zhang M, Lee C, Feldmann M, Trujillo G, Furniss D, Nanchahal J. 2022. A vasculature niche orchestrates stromal cell phenotype through PDGF signaling: importance in human fibrotic disease. Proc Natl Acad Sci 119: e2120336119. 10.1073/pnas.2120336119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. 2021. The hard problem: mechanotransduction perpetuates the myofibroblast phenotype in scleroderma fibrosis. Wound Repair Regen 29: 582–587. 10.1111/wrr.12889 [DOI] [PubMed] [Google Scholar]
- Lebonvallet N, Laverdet B, Misery L, Desmouliere A, Girard D. 2018. New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation. Exp Dermatol 27: 950–958. 10.1111/exd.13681 [DOI] [PubMed] [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. 2001. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med 194: 809–821. 10.1084/jem.194.6.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhos L, Peters J, Krull S, Helbig L, Vogler M, Levay M, van Belle GJ, Ridley AJ, Lutz S, Katschinski DM, et al. 2019. Hypoxia suppresses myofibroblast differentiation by changing RhoA activity. J Cell Sci 132: jcs223230. 10.1242/jcs.223230 [DOI] [PubMed] [Google Scholar]
- Lemos DR, Duffield JS. 2018. Tissue-resident mesenchymal stromal cells: implications for tissue-specific antifibrotic therapies. Sci Transl Med 10: eaan5174. 10.1126/scitranslmed.aan5174 [DOI] [PubMed] [Google Scholar]
- Leung LY, Tian D, Brangwynne CP, Weitz DA, Tschumperlin DJ. 2007. A new microrheometric approach reveals individual and cooperative roles for TGF-β1 and IL-1β in fibroblast-mediated stiffening of collagen gels. FASEB J 21: 2064–2073. 10.1096/fj.06-7510com [DOI] [PubMed] [Google Scholar]
- Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz B. 2017. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16: 379–389. 10.1038/nmat4780 [DOI] [PubMed] [Google Scholar]
- Li R, Bernau K, Sandbo N, Gu J, Preissl S, Sun X. 2018. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. eLife 7: e36865. 10.7554/eLife.36865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li Y, Liu S, Qin Z. 2020. Extracellular S100A4 as a key player in fibrotic diseases. J Cell Mol Med 24: 5973–5983. 10.1111/jcmm.15259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Sung YK, Jiang X, Peters-Golden M, Nicolls MR. 2017. Simultaneously targeting myofibroblast contractility and extracellular matrix cross-linking as a therapeutic concept in airway fibrosis. Am J Transplant 17: 1229–1241. 10.1111/ajt.14103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link PA, Choi KM, Diaz Espinosa AM, Jones DL, Gao AY, Haak AJ, Tschumperlin DJ. 2022. Combined control of the fibroblast contractile program by YAP and TAZ. Am J Physiol Lung Cell Mol Physiol 322: L23–L32. 10.1152/ajplung.00210.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, et al. 2015. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357. 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yu H, Zhao H, Wu Z, Long Y, Zhang J, Yan X, You Z, Zhou L, Xia T, et al. 2020. Matrix-transmitted paratensile signaling enables myofibroblast-fibroblast cross talk in fibrosis expansion. Proc Natl Acad Sci 117: 10832–10838. 10.1073/pnas.1910650117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodyga M, Hinz B. 2020. TGF-β1: a truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol 101: 123–139. 10.1016/j.semcdb.2019.12.010 [DOI] [PubMed] [Google Scholar]
- Lodyga M, Cambridge E, Karvonen HM, Pakshir P, Wu B, Boo S, Kiebalo M, Kaarteenaho R, Glogauer M, Kapoor M, et al. 2019. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-β. Sci Signal 12: eaao3469. 10.1126/scisignal.aao3469 [DOI] [PubMed] [Google Scholar]
- Loirand G. 2015. Rho kinases in health and disease: from basic science to translational research. Pharmacol Rev 67: 1074–1095. 10.1124/pr.115.010595 [DOI] [PubMed] [Google Scholar]
- Lu X, Jin H, Quesada C, Farrell EC, Huang L, Aliabouzar M, Kripfgans OD, Fowlkes JB, Franceschi RT, Putnam AJ, et al. 2020. Spatially-directed cell migration in acoustically-responsive scaffolds through the controlled delivery of basic fibroblast growth factor. Acta Biomater 113: 217–227. 10.1016/j.actbio.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. 2006. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63: 2304–2316. 10.1007/s00018-006-6149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger LL, Patenaude CA, Smith BD, Layne MD. 2011. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem 286: 44116–44125. 10.1074/jbc.M111.276931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Kwon SH, Padmanabhan J, Duscher D, Trotsyuk AA, Dong Y, Inayathullah M, Rajadas J, Gurtner GC. 2018. Controlled delivery of a focal adhesion kinase inhibitor results in accelerated wound closure with decreased scar formation. J Invest Dermatol 138: 2452–2460. 10.1016/j.jid.2018.04.034 [DOI] [PubMed] [Google Scholar]
- Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J. 2021. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol 30: 132–145. 10.1111/exd.14243 [DOI] [PubMed] [Google Scholar]
- MacCarthy-Morrogh L, Martin P. 2020. The hallmarks of cancer are also the hallmarks of wound healing. Sci Signal 13: eaay8690. 10.1126/scisignal.aay8690 [DOI] [PubMed] [Google Scholar]
- Maglaviceanu A, Wu B, Kapoor M. 2021. Fibroblast-like synoviocytes: role in synovial fibrosis associated with osteoarthritis. Wound Repair Regen 29: 642–649. 10.1111/wrr.12939 [DOI] [PubMed] [Google Scholar]
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. 1971. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science 173: 548–550. 10.1126/science.173.3996.548 [DOI] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, Ingber DE. 2012. Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 125: 3061–3073. 10.1242/jcs.093005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. 1997. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci 94: 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, Mestan J, Trappe J, Wartmann M, Fabbro D. 2010. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta 1804: 445–453. 10.1016/j.bbapap.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, Varga J. 2015. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67: 1062–1073. 10.1002/art.38990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez J, Olaso E. 2014. Role of discoidin domain receptor 2 in wound healing. Histol Histopathol 29: 1355–1364. [DOI] [PubMed] [Google Scholar]
- Martin P, D'Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. 2003. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol 13: 1122–1128. 10.1016/S0960-9822(03)00396-8 [DOI] [PubMed] [Google Scholar]
- Mascharak S, des Jardins-Park HE, Longaker MT. 2020. Fibroblast heterogeneity in wound healing: hurdles to clinical translation. Trends Mol Med 26: 1101–1106. 10.1016/j.molmed.2020.07.008 [DOI] [PubMed] [Google Scholar]
- Mascharak S, des Jardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, et al. 2021a. Preventing engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372: eaba2374. 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascharak S, des Jardins-Park HE, Davitt MF, Guardino NJ, Gurtner GC, Wan DC, Longaker MT. 2021b. Modulating cellular responses to mechanical forces to promote wound regeneration. Adv Wound Care (New Rochelle) 10.1089/wound.2021.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Lammerding J. 2019. The driving force: nuclear mechanotransduction in cellular function fate, and disease. Annu Rev Biomed Eng 21: 443–468. 10.1146/annurev-bioeng-060418-052139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkt W, Bueno M, Mora AL, Lagares D. 2020. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Semin Cell Dev Biol 101: 104–110. 10.1016/j.semcdb.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkt W, Zhou Y, Han H, Lagares D. 2021. Myofibroblast fate plasticity in tissue repair and fibrosis: deactivation, apoptosis, senescence and reprogramming. Wound Repair Regen 29: 678–691. 10.1111/wrr.12952 [DOI] [PubMed] [Google Scholar]
- Min LJ, Cui TX, Yahata Y, Yamasaki K, Shiuchi T, Liu HW, Chen R, Li JM, Okumura M, Jinno T, et al. 2004. Regulation of collagen synthesis in mouse skin fibroblasts by distinct angiotensin II receptor subtypes. Endocrinology 145: 253–260. 10.1210/en.2003-0673 [DOI] [PubMed] [Google Scholar]
- Miranda MZ, Bialik JF, Speight P, Dan Q, Yeung T, Szaszi K, Pedersen SF, Kapus A. 2017. TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism. J Biol Chem 292: 14902–14920. 10.1074/jbc.M117.780502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MZ, Lichner Z, Szaszi K, Kapus A. 2021. MRTF: basic biology and role in kidney disease. Int J Mol Sci 22: 6040. 10.3390/ijms22116040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, et al. 2017. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214: 2387–2404. 10.1084/jem.20162152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, Hinz B. 2010. Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol 130: 2818–2827. 10.1038/jid.2010.224 [DOI] [PubMed] [Google Scholar]
- Moll S, Desmouliere A, Moeller MJ, Pache JC, Badi L, Arcadu F, Richter H, Satz A, Uhles S, Cavalli A, et al. 2019. DDR1 role in fibrosis and its pharmacological targeting. Biochim Biophys Acta Mol Cell Res 1866: 118474. 10.1016/j.bbamcr.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Moncsek A, Al-Suraih MS, Trussoni CE, O'Hara SP, Splinter PL, Zuber C, Patsenker E, Valli PV, Fingas CD, Weber A, et al. 2017. Targeting senescent cholangiocytes and activated fibroblasts with Bcl-xL inhibitors ameliorates fibrosis in Mdr2−/− mice. Hepatology 67: 247–259. 10.1002/hep.29464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncsek A, Al-Suraih MS, Trussoni CE, O'Hara SP, Splinter PL, Zuber C, Patsenker E, Valli PV, Fingas CD, Weber A, et al. 2018. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma—extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout Mdr2−/− mice. Hepatology 67: 247–259. 10.1002/hep.29464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Ghatnekar GS, Grek CL, Moyer KE, Gourdie RG. 2018. Connexin 43-based therapeutics for dermal wound healing. Int J Mol Sci 19: 1778. 10.3390/ijms19061778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Richardson WJ, Marsh S, Rhett JM, Bustos F, Degen K, Ghatnekar GS, Grek CL, Jourdan LJ, Holmes JW, et al. 2021. The connexin 43 carboxyl terminal mimetic peptide αCT1 prompts differentiation of a collagen scar matrix in humans resembling unwounded skin. FASEB J 35: e21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Salvador E, Mann J. 2017. Epigenetics and liver fibrosis. Cell Mol Gastroenterol Hepatol 4: 125–134. 10.1016/j.jcmgh.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M, Derynck R, Miyazono K. 2016. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8: a021873. 10.1101/cshperspect.a021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhl L, Genove G, Leptidis S, Liu J, He L, Mocci G, Sun Y, Gustafsson S, Buyandelger B, Chivukula IV, et al. 2020. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun 11: 3953. 10.1038/s41467-020-17740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Ito S, Furuya K, Takahara N, Naruse K, Aso H, Kondo M, Sokabe M, Hasegawa Y. 2014. Ca2+ influx and ATP release mediated by mechanical stretch in human lung fibroblasts. Biochem Biophys Res Commun 453: 101–105. 10.1016/j.bbrc.2014.09.063 [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Budinger GR, Wu M, Lam AP, Zirk A, Rivera S, Urich D, Chiarella SE, Go LH, Ghosh AK, et al. 2012. Proteasomal inhibition after injury prevents fibrosis by modulating TGF-β1 signalling. Thorax 67: 139–146. 10.1136/thoraxjnl-2011-200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabai L, Ghahary A. 2017. Hypertrophic scarring in the rabbit ear: a practical model for studying dermal fibrosis. Methods Mol Biol 1627: 81–89. 10.1007/978-1-4939-7113-8_6 [DOI] [PubMed] [Google Scholar]
- Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, Pathak A. 2017. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146: 146–155. 10.1016/j.biomaterials.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B, Cook SA, Schafer S. 2020. Interleukin-11 signaling underlies fibrosis, parenchymal dysfunction, and chronic inflammation of the airway. Exp Mol Med 52: 1871–1878. 10.1038/s12276-020-00531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoloudaki G, Creber K, Hamilton DW. 2020. Wound healing and fibrosis: a contrasting role for periostin in skin and the oral mucosa. Am J Physiol Cell Physiol 318: C1065–C1077. 10.1152/ajpcell.00035.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskovicova N, Hinz B, Pakshir P. 2021a. Implant fibrosis and the underappreciated role of myofibroblasts in the foreign body reaction. Cells 10: 1794. 10.3390/cells10071794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskovicova N, Schuster R, van Putten S, Ezzo M, Koehler A, Boo S, Coelho NM, Griggs D, Ruminski P, McCulloch CA, et al. 2021b. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-β. Nat Biomed Eng 5: 1437–1456. 10.1038/s41551-021-00722-z [DOI] [PubMed] [Google Scholar]
- Oh RS, Haak AJ, Smith KMJ, Ligresti G, Choi KM, Xie T, Wang S, Walters PR, Thompson MA, Freeman MR, et al. 2018. RNAi screening identifies a mechanosensitive ROCK-JAK2-STAT3 network central to myofibroblast activation. J Cell Sci 131: jcs209932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan A, O'Brien CJ, Eivers SB. 2021. The lysophosphatidic acid axis in fibrosis: implications for glaucoma. Wound Repair Regen 29: 613–626. 10.1111/wrr.12929 [DOI] [PubMed] [Google Scholar]
- Orgill DP, Bayer LR. 2011. Update on negative-pressure wound therapy. Plast Reconstr Surg 127: 105S–115S. 10.1097/PRS.0b013e318200a427 [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. 2022. New developments in the biology of fibroblast growth factors. WIREs Mech Dis e1549. 10.1002/wsbm.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanagic-Myers S, Foisner R. 2019. The structural and gene expression hypotheses in laminopathic diseases—not so different after all. Mol Biol Cell 30: 1786–1790. 10.1091/mbc.E18-10-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan B, Uysal CA, Ertas NM. 2021. Practical things you should know about wound healing and vacuum-assisted closure management. Plast Reconstr Surg 147: 358e–359e. 10.1097/PRS.0000000000007569 [DOI] [PubMed] [Google Scholar]
- Pakshir P, Alizadehgiashi M, Wong B, Coelho NM, Chen X, Gong Z, Shenoy VB, McCulloch CA, Hinz B. 2019. Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat Commun 10: 1850. 10.1038/s41467-019-09709-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakshir P, Noskovicova N, Lodyga M, Son DO, Schuster R, Goodwin A, Karvonen H, Hinz B. 2020. The myofibroblast at a glance. J Cell Sci 133: jcs227900. 10.1242/jcs.227900 [DOI] [PubMed] [Google Scholar]
- Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen M, Lin S, Huang L, Chung EJ, Citrin DE, et al. 2017. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys 99: 353–361. 10.1016/j.ijrobp.2017.02.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciera T, Azzolin L, Cordenonsi M, Piccolo S. 2017. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18: 758–770. 10.1038/nrm.2017.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya P, Orgaz JL, Sanz-Moreno V. 2017. Actomyosin contractility and collective migration: may the force be with you. Curr Opin Cell Biol 48: 87–96. 10.1016/j.ceb.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizi M, Howard EW, Tomasek JJ. 2000. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res 254: 210–220. 10.1006/excr.1999.4754 [DOI] [PubMed] [Google Scholar]
- Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. 2014. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635. 10.1172/JCI71386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Liu L, MacLean AL, Wong CW, Zhao W, Nie Q. 2017. A mathematical model of mechanotransduction reveals how mechanical memory regulates mesenchymal stem cell fate decisions. BMC Syst Biol 11: 55. 10.1186/s12918-017-0429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio FA, Nastaly P, Poli A, Maiuri P. 2020. Tailoring cellular function: the contribution of the nucleus in mechanotransduction. Front Bioeng Biotechnol 8: 596746. 10.3389/fbioe.2020.596746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Shibayama C, Gil-Cruz C, Ludewig B. 2019. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol Rev 289: 31–41. 10.1111/imr.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QM, Fine GM, Salz L, Herrera GG, Wildman B, Driskell IM, Driskell RR. 2020. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. eLife 9: e60066. 10.7554/eLife.60066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QM, Sinha S, Biernaskie J, Driskell RR. 2021. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp Dermatol 30: 92–101. 10.1111/exd.14244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippeos C, Telerman SB, Oules B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, et al. 2018. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 138: 811–825. 10.1016/j.jid.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma B, de Rond S, Werker PM, Boo S, Hinz B, van Beuge MM, Bank RA. 2015. YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am J Pathol 185: 3326–3337. 10.1016/j.ajpath.2015.08.011 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. 2019. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 4: 22. 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, Driskell RR, Rosenthal N, Biernaskie J, Horsley V. 2021. Fibroblasts: origins, definitions, and functions in health and disease. Cell 184: 3852–3872. 10.1016/j.cell.2021.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J, McBain D, Petrlich L, Watson S, Vanderhoek L, de Leon F, Seney S, Summers K. 2011. Imatinib in active diffuse cutaneous systemic sclerosis: results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum 63: 3547–3551. 10.1002/art.30549 [DOI] [PubMed] [Google Scholar]
- Prakoura N, Hadchouel J, Chatziantoniou C. 2019. Novel targets for therapy of renal fibrosis. J Histochem Cytochem 67: 701–715. 10.1369/0022155419849386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CC, Mathur J, Boerckel JD, Pathak A, Shenoy VB. 2021. Dynamic self-reinforcement of gene expression determines acquisition of cellular mechanical memory. Biophys J 120: 5074–5089. 10.1016/j.bpj.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunotto M, Bruschi M, Gunning P, Gabbiani G, Weibel F, Ghiggeri GM, Petretto A, Scaloni A, Bonello T, Schevzov G, et al. 2015. Stable incorporation of α-smooth muscle actin into stress fibers is dependent on specific tropomyosin isoforms. Cytoskeleton (Hoboken) 72: 257–267. 10.1002/cm.21230 [DOI] [PubMed] [Google Scholar]
- Purcell JW, Tanlimco SG, Hickson J, Fox M, Sho M, Durkin L, Uziel T, Powers R, Foster K, McGonigal T, et al. 2018. LRRC15 is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Cancer Res 78: 4059–4072. 10.1158/0008-5472.CAN-18-0327 [DOI] [PubMed] [Google Scholar]
- Quesnel K, Shi-wen X, Hutchenreuther J, Xiao Y, Liu S, Peidl A, Naskar D, Siqueira WL, O'Gorman DB, Hinz B, et al. 2019. CCN1 expression by fibroblasts is required for bleomycin-induced skin fibrosis. Matrix Biol Plus 3: 100009. 10.1016/j.mbplus.2019.100009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, et al. 2014. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest 124: 5225–5238. 10.1172/JCI75331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. 2012. Is losartan the drug for all seasons? Curr Opin Pharmacol 12: 223–224. 10.1016/j.coph.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D. 2015. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med 7: 288ra279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, et al. 2019. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199: 1517–1536. 10.1164/rccm.201712-2410OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee CK, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim YK, Kim KH, Kim TJ, Kim JW. 2011. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration 82: 273–287. 10.1159/000327719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. 2014. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- Rifkin DB, Rifkin WJ, Zilberberg L. 2018. LTBPs in biology and medicine: LTBP diseases. Matrix Biol 71–72: 90–99. 10.1016/j.matbio.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley LA, Merryman WD. 2021. Cadherin-11 and cardiac fibrosis: a common target for a common pathology. Cell Signal 78: 109876. 10.1016/j.cellsig.2020.109876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, et al. 2015. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151. 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Mertens JC, Bronk SF, Hirsova P, Dai H, Roberts LR, Kaufmann SH, Gores GJ. 2014. Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J Biol Chem 289: 22835–22849. 10.1074/jbc.M114.563064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, Barres BA, Luster AD, Ye CJ, Cyster JG. 2018. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity 48: 1014–1028.e1016. 10.1016/j.immuni.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Walko G. 2019. The roles of YAP/TAZ and the hippo pathway in healthy and diseased skin. Cells 8: 411. 10.3390/cells8050411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E, Pisco AO, Hiratsuka T, Sipila KH, Belmonte JM, Mobasseri SA, Philippeos C, Dilao R, Watt FM. 2018. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol Syst Biol 14: e8174. 10.15252/msb.20178174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. 2020. Targeting the progression of chronic kidney disease. Nat Rev Nephrol 16: 269–288. 10.1038/s41581-019-0248-y [DOI] [PubMed] [Google Scholar]
- Rybarczyk BJ, Lawrence SO, Simpson-Haidaris PJ. 2003. Matrix-fibrinogen enhances wound closure by increasing both cell proliferation and migration. Blood 102: 4035–4043. 10.1182/blood-2003-03-0822 [DOI] [PubMed] [Google Scholar]
- Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. 2020. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20: 174–186. 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini K, Cho S, Dooling LJ, Discher DE. 2020. Tension in fibrils suppresses their enzymatic degradation—a molecular mechanism for “use it or lose it.” Matrix Biol 85–86: 34–46. 10.1016/j.matbio.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer MC, Lafzi A, Berenguer-Llergo A, Youssif C, Castellanos A, Solanas G, Peixoto FO, Stephan-Otto Attolini C, Prats N, Aguilera M, et al. 2018. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175: 1575–1590.e1522. 10.1016/j.cell.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Santos A, Lagares D. 2018. Matrix stiffness: the conductor of organ fibrosis. Curr Rheumatol Rep 20: 2. 10.1007/s11926-018-0710-z [DOI] [PubMed] [Google Scholar]
- Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B. 2014. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res 102: 407–417. 10.1093/cvr/cvu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Yanagihara T, Kolb MRJ. 2019. Therapeutic targets and early stage clinical trials for pulmonary fibrosis. Expert Opin Investig Drugs 28: 19–28. 10.1080/13543784.2019.1554054 [DOI] [PubMed] [Google Scholar]
- Sawant M, Hinz B, Schonborn K, Zeinert I, Eckes B, Krieg T, Schuster R. 2021. A story of fibers and stress: matrix-embedded signals for fibroblast activation in the skin. Wound Repair Regen 29: 515–530. 10.1111/wrr.12950 [DOI] [PubMed] [Google Scholar]
- Scheid A, Wenger RH, Christina H, Camenisch I, Ferenc A, Stauffer UG, Gassmann M, Meuli M. 2000. Hypoxia-regulated gene expression in fetal wound regeneration and adult wound repair. Pediatr Surg Int 16: 232–236. 10.1007/s003830050735 [DOI] [PubMed] [Google Scholar]
- Schnittert J, Bansal R, Storm G, Prakash J. 2018. Integrins in wound healing fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev 129: 37–53. 10.1016/j.addr.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Schulz JN, Zeltz C, Sorensen IW, Barczyk M, Carracedo S, Hallinger R, Niehoff A, Eckes B, Gullberg D. 2015. Reduced granulation tissue and wound strength in the absence of α11β1 integrin. J Invest Dermatol 135: 1435–1444. 10.1038/jid.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. 2018. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol 68–69: 435–451. 10.1016/j.matbio.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Schuster R, Rockel JS, Kapoor M, Hinz B. 2021. The inflammatory speech of fibroblasts. Immunol Rev 302: 126–146. 10.1111/imr.12971 [DOI] [PubMed] [Google Scholar]
- Seo Y, Heo Y, Jo S, Park SH, Lee C, Chang J, Jeon DK, Kim TG, Han G, Namkung W. 2021. Novel positive allosteric modulator of protease-activated receptor 1 promotes skin wound healing in hairless mice. Br J Pharmacol 178: 3414–3427. 10.1111/bph.15489 [DOI] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. 1998. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol 142: 873–881. 10.1083/jcb.142.3.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JR, Lebeko M, Kidzeru EB, Khumalo NP, Bayat A. 2019. In vitro and ex vivo models for functional testing of therapeutic anti-scarring drug targets in keloids. Adv Wound Care (New Rochelle) 8: 655–670. 10.1089/wound.2019.1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. 2011. Latent TGF-β structure and activation. Nature 474: 343–349. 10.1038/nature10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwen X, Stratton R, Nikitorowicz-Buniak J, Ahmed-Abdi B, Ponticos M, Denton C, Abraham D, Takahashi A, Suki B, Layne MD, et al. 2015. A role of myocardin related transcription factor-A (MRTF-A) in scleroderma related fibrosis. PLoS ONE 10: e0126015. 10.1371/journal.pone.0126015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, Lopez-Giraldez F, Dash BC, Munoz-Rojas AR, Aultman KD, Zwick RK, Lei V, et al. 2018. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362: eaar2971. 10.1126/science.aar2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Chopra K, Sabino J, Brown E. 2020. Practical things you should know about wound healing and vacuum-assisted closure management. Plast Reconstr Surg 145: 839e–854e. 10.1097/PRS.0000000000006652 [DOI] [PubMed] [Google Scholar]
- Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S. 2018. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun 9: 936. 10.1038/s41467-018-03208-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, Kim KK, Keshamouni VG, White ES, Zhou Y, et al. 2015. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol 185: 969–986. 10.1016/j.ajpath.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibba M, Drelich A, Poellmann M, Hong S, Brasier AR. 2020. Nanoapproaches to modifying epigenetics of epithelial mesenchymal transition for treatment of pulmonary fibrosis. Front Pharmacol 11: 607689. 10.3389/fphar.2020.607689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN. 2010. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 107: 294–304. 10.1161/CIRCRESAHA.110.223172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Clerc J, Hinz B. 2010. Immunofluorescence detection of the cytoskeleton and extracellular matrix in tissue and cultured cells. Methods Mol Biol 611: 43–57. 10.1007/978-1-60327-345-9_4 [DOI] [PubMed] [Google Scholar]
- Sole-Boldo L, Raddatz G, Schutz S, Mallm JP, Rippe K, Lonsdorf AS, Rodriguez-Paredes M, Lyko F. 2020. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun Biol 3: 188. 10.1038/s42003-020-0922-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman H, Theret M, Scott W, Hill L, Underhill TM, Hinz B, Rossi FMV. 2021. Multipotent stromal cells: one name, multiple identities. Cell Stem Cell 28: 1690–1707. 10.1016/j.stem.2021.09.001 [DOI] [PubMed] [Google Scholar]
- Son DO, Hinz B. 2021. A rodent model of hypertrophic scarring: splinting of rat wounds. Methods Mol Biol 2299: 405–417. 10.1007/978-1-0716-1382-5_27 [DOI] [PubMed] [Google Scholar]
- Spiera R, Hummers L, Chung L, Frech TM, Domsic R, Hsu V, Furst DE, Gordon J, Mayes M, Simms R, et al. 2020. Safety and efficacy of lenabasum in a phase II, randomized, placebo-controlled trial in adults with systemic sclerosis. Arthritis Rheumatol 72: 1350–1360. 10.1002/art.41294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. 2004. Human skin: source of and target organ for angiotensin II. Exp Dermatol 13: 148–154. 10.1111/j.0906-6705.2004.0139.x [DOI] [PubMed] [Google Scholar]
- Stewart L, Turner NA. 2021. Channelling the force to reprogram the matrix: mechanosensitive ion channels in cardiac fibroblasts. Cells 10: 990. 10.3390/cells10050990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DC, Serrano PN, Rubiano A, Yokosawa R, Sandler J, Mukhtar M, Brant JO, Maden M, Simmons CS. 2018. Unique behavior of dermal cells from regenerative mammal, the African Spiny Mouse, in response to substrate stiffness. J Biomech 81: 149–154. 10.1016/j.jbiomech.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Subramaniam T, Fauzi MB, Lokanathan Y, Law JX. 2021. The role of calcium in wound healing. Int J Mol Sci 22: 6486. 10.3390/ijms22126486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YW, Zhang YY, Ke XJ, Wu XJ, Chen ZF, Chi P. 2018. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-β1/Smad/CTGF signaling pathway. Eur J Pharmacol 822: 199–206. 10.1016/j.ejphar.2018.01.027 [DOI] [PubMed] [Google Scholar]
- Sun Z, Costell M, Fassler R. 2019. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol 21: 25–31. 10.1038/s41556-018-0234-9 [DOI] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341: 1240104. 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, et al. 2016. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol 27: 3117–3128. 10.1681/ASN.2015050499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabib T, Morse C, Wang T, Chen W, Lafyatis R. 2018. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J Invest Dermatol 138: 802–810. 10.1016/j.jid.2017.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. 2008. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14: 45–54. 10.1038/nm1685 [DOI] [PubMed] [Google Scholar]
- Talele NP, Fradette J, Davies JE, Kapus A, Hinz B. 2015. Expression of α-smooth muscle actin determines the fate of mesenchymal stromal cells. Stem Cell Rep 4: 1016–1030. 10.1016/j.stemcr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Molkentin JD. 2017. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol 14: 484–491. 10.1038/nrcardio.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WQ, Fang QQ, Shen XZ, Giani JF, Zhao TV, Shi P, Zhang LY, Khan Z, Li Y, Li L, et al. 2018. Angiotensin-converting enzyme inhibitor works as a scar formation inhibitor by down-regulating Smad and TGF-β-activated kinase 1 (TAK1) pathways in mice. Br J Pharmacol 175: 4239–4252. 10.1111/bph.14489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandara AA, Mustoe TA. 2004. Oxygen in wound healing—more than a nutrient. World J Surg 28: 294–300. 10.1007/s00268-003-7400-2 [DOI] [PubMed] [Google Scholar]
- Tarzemany R, Jiang G, Jiang JX, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, Hakkinen L. 2018. Connexin 43 regulates the expression of wound healing-related genes in human gingival and skin fibroblasts. Exp Cell Res 367: 150–161. 10.1016/j.yexcr.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Horowitz JC. 2006. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 3: 350–356. 10.1513/pats.200601-001TK [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis AD, Manou D, Karamanos NK. 2019. The extracellular matrix as a multitasking player in disease. FEBS J 286: 2830–2869. 10.1111/febs.14818 [DOI] [PubMed] [Google Scholar]
- To S, Agarwal SK. 2019. Macrophages and cadherins in fibrosis and systemic sclerosis. Curr Opin Rheumatol 31: 582–588. 10.1097/BOR.0000000000000657 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. 2002. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani G, Martin MD, Haaksma CJ, Hinz B. 2006. Contraction of myofibroblasts in granulation tissue is dependent on Rho/Rho kinase/myosin light chain phosphatase activity. Wound Repair Regen 14: 313–320. 10.1111/j.1743-6109.2006.00126.x [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ, Schwartz RJ, Howard EW. 2013. Whole animal knockout of smooth muscle α-actin does not alter excisional wound healing or the fibroblast-to-myofibroblast transition. Wound Repair Regen 21: 166–176. 10.1111/wrr.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama T, Looney AP, Baker BM, Stawski L, Haines P, Simms R, Szymaniak AD, Varelas X, Trojanowska M. 2018. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J Invest Dermatol 138: 78–88. 10.1016/j.jid.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumperlin DJ, Lagares D. 2020. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacol Ther 212: 107575. 10.1016/j.pharmthera.2020.107575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumperlin DJ, Ligresti G, Hilscher MB, Shah VH. 2018. Mechanosensing and fibrosis. J Clin Invest 128: 74–84. 10.1172/JCI93561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou PS, Varga J, O'Reilly S. 2021. Advances in epigenetics in systemic sclerosis: molecular mechanisms and therapeutic potential. Nat Rev Rheumatol 17: 596–607. 10.1038/s41584-021-00683-2 [DOI] [PubMed] [Google Scholar]
- Tsukui T, Sun KH, Wetter JB, Wilson-Kanamori JR, Hazelwood LA, Henderson NC, Adams TS, Schupp JC, Poli SD, Rosas IO, et al. 2020. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun 11: 1920. 10.1038/s41467-020-15647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet SD, Ricard-Blum S. 2019. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem 63: 349–364. 10.1042/EBC20180050 [DOI] [PubMed] [Google Scholar]
- Van Hove L, Hoste E. 2022. Activation of fibroblasts in skin cancer. J Invest Dermatol 142: 1026–1031. 10.1016/j.jid.2021.09.010 [DOI] [PubMed] [Google Scholar]
- Vannella KM, Wynn TA. 2017. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol 79: 593–617. 10.1146/annurev-physiol-022516-034356 [DOI] [PubMed] [Google Scholar]
- Vardouli L, Vasilaki E, Papadimitriou E, Kardassis D, Stournaras C. 2008. A novel mechanism of TGFβ-induced actin reorganization mediated by Smad proteins and Rho GTPases. FEBS J 275: 4074–4087. 10.1111/j.1742-4658.2008.06549.x [DOI] [PubMed] [Google Scholar]
- Varney SD, Betts CB, Zheng R, Wu L, Hinz B, Zhou J, Van De Water L. 2016. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J Cell Sci 129: 774–787. 10.1242/jcs.170589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. 1858. Die Cellularpathologie in ihrer Begründung: auf physiologische und pathologische Gewebelehre. A. Hirschwald, Berlin. [PubMed] [Google Scholar]
- Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. 2011. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 194: 25–37. 10.1159/000322399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann ER, Varga J. 2019. Emerging targets of disease-modifying therapy for systemic sclerosis. Nat Rev Rheumatol 15: 208–224. 10.1038/s41584-019-0184-z [DOI] [PubMed] [Google Scholar]
- Vorstandlechner V, Laggner M, Kalinina P, Haslik W, Radtke C, Shaw L, Lichtenberger BM, Tschachler E, Ankersmit HJ, Mildner M. 2020. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J 34: 3677–3692. 10.1096/fj.201902001RR [DOI] [PubMed] [Google Scholar]
- Walker CJ, Crocini C, Ramirez D, Killaars AR, Grim JC, Aguado BA, Clark K, Allen MA, Dowell RD, Leinwand LA, et al. 2021a. Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nat Biomed Eng 5: 1485–1499. 10.1038/s41551-021-00709-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JT, Flynn LE, Hamilton DW. 2021b. Lineage tracing of Foxd1-expressing embryonic progenitors to assess the role of divergent embryonic lineages on adult dermal fibroblast function. FASEB Bioadv 3: 541–557. 10.1096/fba.2020-00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walraven M, Hinz B. 2018. Therapeutic approaches to control tissue repair and fibrosis: extracellular matrix as a game changer. Matrix Biol 71–72: 205–224. [DOI] [PubMed] [Google Scholar]
- Wan R, Weissman JP, Grundman K, Lang L, Grybowski DJ, Galiano RD. 2021. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair Regen 29: 573–581. 10.1111/wrr.12954 [DOI] [PubMed] [Google Scholar]
- Wang K, Ji W, Yu Y, Li Z, Niu X, Xia W, Lu S. 2018. FGFR1-ERK1/2-SOX2 axis promotes cell proliferation, epithelial-mesenchymal transition, and metastasis in FGFR1-amplified lung cancer. Oncogene 37: 5340–5354. 10.1038/s41388-018-0311-3 [DOI] [PubMed] [Google Scholar]
- Wang Y, Sherrard A, Zhao B, Melak M, Trautwein J, Kleinschnitz EM, Tsopoulidis N, Fackler OT, Schwan C, Grosse R. 2019. GPCR-induced calcium transients trigger nuclear actin assembly for chromatin dynamics. Nat Commun 10: 5271. 10.1038/s41467-019-13322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F. 2021. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev 302: 211–227. 10.1111/imr.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Alexander M, Misharin AV, Budinger GRS. 2019. The role of macrophages in the resolution of inflammation. J Clin Invest 130: 2619–2628. 10.1172/JCI124615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Grose R. 2003. Regulation of wound healing by growth factors and cytokines. Physiol Rev 83: 835–870. 10.1152/physrev.2003.83.3.835 [DOI] [PubMed] [Google Scholar]
- Widjaja AA, Viswanathan S, Jinrui D, Singh BK, Tan J, Wei Ting JG, Lamb D, Shekeran SG, George BL, Schafer S, et al. 2021. Molecular dissection of pro-fibrotic IL11 signaling in cardiac and pulmonary fibroblasts. Front Mol Biosci 8: 740650. 10.3389/fmolb.2021.740650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietecha MS, Pensalfini M, Cangkrama M, Muller B, Jin J, Brinckmann J, Mazza E, Werner S. 2020. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat Commun 11: 2604. 10.1038/s41467-020-16409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE. 2020. Corneal myofibroblasts and fibrosis. Exp Eye Res 201: 108272. 10.1016/j.exer.2020.108272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol 179: 1311–1323. 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Heidenreich A, Gschwend JE, Gil T, Zastrow S, Laniado M, Gerloff J, Zuhlsdorf M, Mordenti G, Uhl W, et al. 2014. A multicenter phase 1 study of EMD 525797 (DI17E6), a novel humanized monoclonal antibody targeting αv integrins, in progressive castration-resistant prostate cancer with bone metastases after chemotherapy. Eur Urol 65: 897–904. 10.1016/j.eururo.2013.05.051 [DOI] [PubMed] [Google Scholar]
- Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, et al. 2012. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18: 148–152. 10.1038/nm.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Beasley B, Zepeda J, Dauskardt RH, Yock PG, Longaker MT, Gurtner GC. 2013. A mechanomodulatory device to minimize incisional scar formation. Adv Wound Care (New Rochelle) 2: 185–194. 10.1089/wound.2012.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong X, Loo TH, Stewart CL. 2021. LINC complex regulation of genome organization and function. Curr Opin Genet Dev 67: 130–141. 10.1016/j.gde.2020.12.007 [DOI] [PubMed] [Google Scholar]
- Worthen CA, Cui Y, Orringer JS, Johnson TM, Voorhees JJ, Fisher GJ. 2020. CD26 identifies a subpopulation of fibroblasts that produce the majority of collagen during wound healing in human skin. J Invest Dermatol 140: 2515–2524.e2513. 10.1016/j.jid.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462. 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. 2015. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 6: 7158. 10.1038/ncomms8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Tibbitt MW, Basta L, Anseth KS. 2014. Mechanical memory and dosing influence stem cell fate. Nat Mater 13: 645–652. 10.1038/nmat3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SY, Lee KW, Shin D, An S, Cho KH, Kim SH. 2018. A positive feedback loop bi-stably activates fibroblasts. Nat Commun 9: 3016. 10.1038/s41467-018-05274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota M, Haffner N, Kassier M, Brunner M, Shambat SM, Brennecke F, Schniering J, Marques Maggio E, Distler O, Zinkernagel AS, et al. 2021. Staphylococcus aureus impairs dermal fibroblast functions with deleterious effects on wound healing. FASEB J 35: e21695. 10.1096/fj.201902836R [DOI] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, et al. 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7: 11190. 10.1038/ncomms11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younesi FS, Son DO, Firmino J, Hinz B. 2021. Myofibroblast markers and microscopy detection methods in cell culture and histology. Methods Mol Biol 2299: 17–47. 10.1007/978-1-0716-1382-5_3 [DOI] [PubMed] [Google Scholar]
- Yu OM, Brown JH. 2015. G protein-coupled receptor and RhoA-stimulated transcriptional responses: links to inflammation, differentiation, and cell proliferation. Mol Pharmacol 88: 171–180. 10.1124/mol.115.097857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Yu X, Petersen F, Riemekasten G. 2018. Recent advances in mouse models for systemic sclerosis. Autoimmun Rev 17: 1225–1234. 10.1016/j.autrev.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Zeltz C, Gullberg D. 2016. The integrin-collagen connection—a glue for tissue repair? J Cell Sci 129: 1284. 10.1242/jcs.188672 [DOI] [PubMed] [Google Scholar]
- Zeltz C, Alam J, Liu H, Erusappan PM, Hoschuetzky H, Molven A, Parajuli H, Cukierman E, Costea DE, Lu N, et al. 2019. α11β1 integrin is induced in a subset of cancer-associated fibroblasts in desmoplastic tumor stroma and mediates in vitro cell migration. Cancers (Basel) 11: 765. 10.3390/cancers11060765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D. 2020. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin Cancer Biol 62: 166–181. 10.1016/j.semcancer.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Zhang J, Fan G, Zhao H, Wang Z, Li F, Zhang P, Zhang J, Wang X, Wang W. 2017. Targeted inhibition of focal adhesion kinase attenuates cardiac fibrosis and preserves heart function in adverse cardiac remodeling. Sci Rep 7: 43146. 10.1038/srep43146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang L, Huang T, Lu D, Song Y, Wang L, Gao J. 2021. Mechanosensitive cation channel Piezo1 contributes to ventilator-induced lung injury by activating RhoA/ROCK1 in rats. Respir Res 22: 250. 10.1186/s12931-021-01844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XK, Yu L, Cheng ML, Che P, Lu YY, Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, et al. 2017. Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci Rep 7: 4032. 10.1038/s41598-017-04317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Patel J, Kaur S, Sim SL, Wong HY, Styke C, Hogan I, Kahler S, Hamilton H, Wadlow R, et al. 2021. Sox9 and Rbpj differentially regulate endothelial to mesenchymal transition and wound scarring in murine endovascular progenitors. Nat Commun 12: 2564. 10.1038/s41467-021-22717-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. 2013. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108. 10.1172/JCI66700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, Bitterman PB, Corley RA, Ding BS, Engler AJ, et al. 2018. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol 73: 77–104. 10.1016/j.matbio.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Hou Q, Li M, Fu X. 2020. Molecular mechanism of myofibroblast formation and strategies for clinical drugs treatments in hypertrophic scars. J Cell Physiol 235: 4109–4119. 10.1002/jcp.29302 [DOI] [PubMed] [Google Scholar]
- Zomer HD, Trentin AG. 2018. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 90: 3–12. 10.1016/j.jdermsci.2017.12.009 [DOI] [PubMed] [Google Scholar]