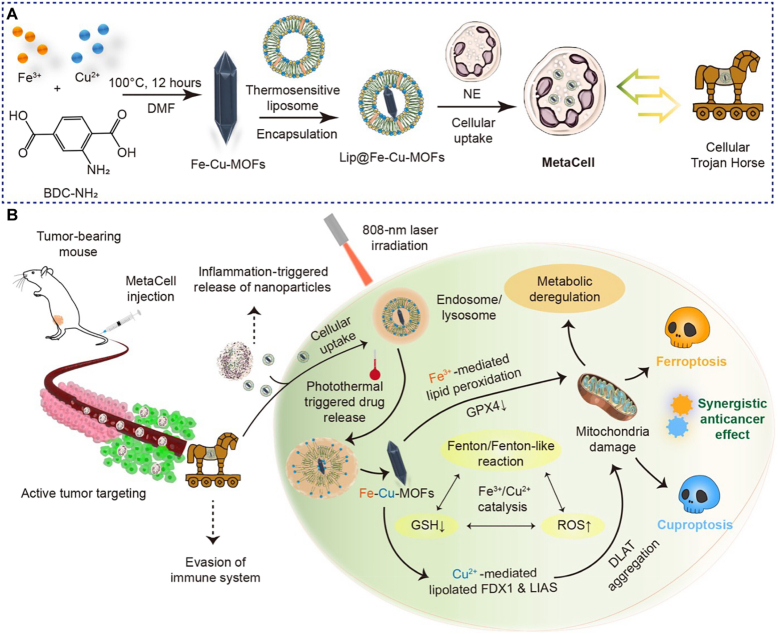
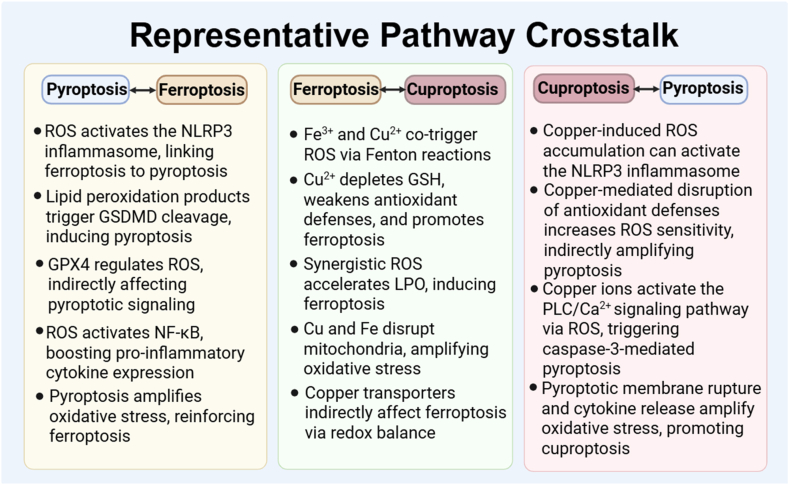
Abstract
Programmed cell death (PCD) plays a crucial role in preventing cancer initiation and progression. Among the diverse PCD pathways, cuproptosis, pyroptosis, and ferroptosis have garnered attention for their unique mechanisms, which not only directly eliminate tumor cells but also enhance anti-tumor immunity. However, the therapeutic efficacy of PCD inducers is often compromised by rapid compensatory pathways in tumor cells, accelerated drug metabolism, and a lack of specificity, which can result in severe side effects. Engineered nanomedicines offer distinct advantages by leveraging nanoscale physicochemical properties to optimize pharmacokinetics, efficacy, and safety in cancer therapy. These nanomedicines enable precise targeting of tumor cells while enhancing drug stability. Moreover, they can simultaneously activate multiple PCD pathways and integrate with conventional therapies to further amplify anti-tumor effects. This review systematically examines the pathophysiological roles, mechanisms, and therapeutic implications of cuproptosis, pyroptosis, and ferroptosis in cancer treatment, with an emphasis on their modulation by nanomedicines. It also explores the potential interactions among these PCD pathways and highlights recent advancements in nanomedicine-based combination therapies targeting multiple PCD mechanisms. Finally, the challenges, limitations, and prospects for the clinical translation and application of PCD-targeting nanomedicines are discussed.
Keywords: Programmed cell death, Biomaterial, Nanomedicine, Cuproptosis, Pyroptosis, Ferroptosis
Graphical abstract
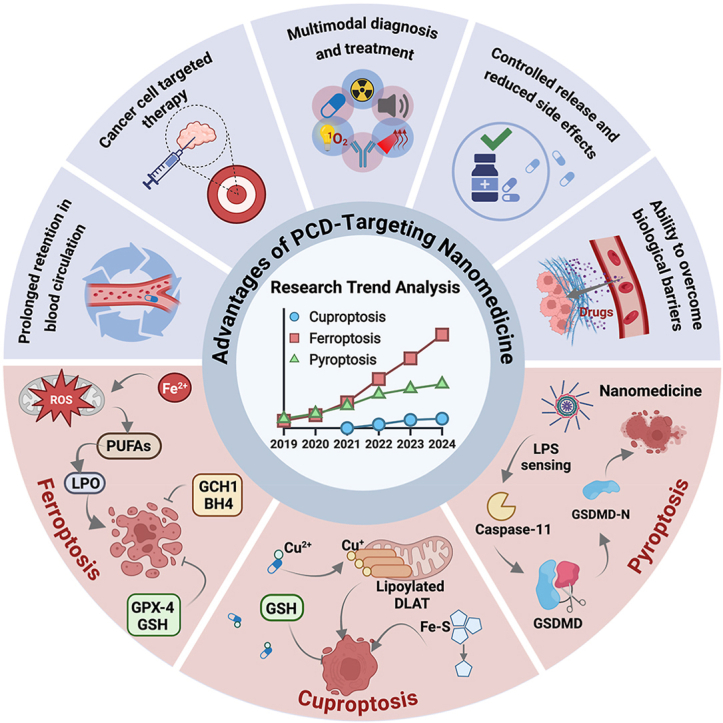
Highlights
-
•
Systematic analysis of pyroptosis, ferroptosis, and cuproptosis and their relevance in cancer therapy.
-
•
Highlights synergistic regulation among PCD pathways to enhance efficacy of multi-targeted cancer therapies.
-
•
Explores nanomedicine strategies to modulate PCD pathways and improve therapeutic precision and outcomes.
1. Introduction
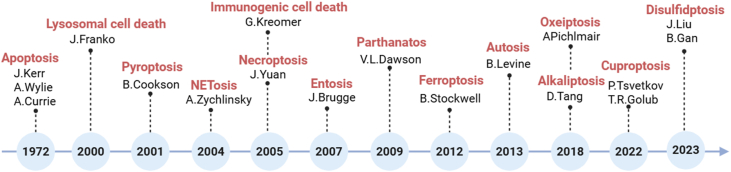
Programmed cell death (PCD) refers to a tightly regulated biological process through which cells undergo self-destruction in response to specific internal or external signals, thereby ensuring tissue homeostasis and organismal integrity [1]. Since the identification of apoptosis in 1972 as the first and most well-characterized form of PCD, research in this field has significantly expanded. A growing number of PCD subtypes have been discovered, including autophagy, pyroptosis, necroptosis, ferroptosis, and more recently, cuproptosis and alkaliptosis. Each type is distinguished by unique molecular mechanisms and signaling pathways, and they collectively contribute to a wide range of physiological processes and disease contexts, particularly cancer [2,3]. PCD processes are deeply implicated in cancer biology, where they influence tumor initiation, progression, immune escape, and resistance to therapy [4,5]. Importantly, the historical progression of PCD discovery also reflects the evolving understanding of cell death mechanisms [6,7]. A chronological summary of each PCD subtype's identification and major milestones are presented (Fig. 1), alongside a comparative overview of their biological functions and antitumor roles (Table 1). Certain PCD pathways can also activate the immune system [4]. When tumor cells die via specific PCD mechanisms, antigens and inflammatory mediators are released and recognized by the immune system, triggering an anti-tumor immune response [8]. Moreover, PCD influences the immune response in the tumor microenvironment (TME) to inhibit metastasis, which remains one of the most challenging aspects of cancer treatment [9,10]. By impairing the migratory capacity of tumor cells, PCD can significantly suppress the invasive growth of tumor [9]. For instance, pyroptosis, with its strong immunogenicity, is particularly effective at stimulating the immune system to clear tumor cells [11]. However, tumor cells frequently develop resistance to treatment by altering these death pathways or activating immune escape mechanisms, leading to diminished therapeutic efficacy [12]. Importantly, there is significant crosstalk among different PCD pathways. For instance, cuproptosis and ferroptosis have strong regulatory links that can jointly influence the tumor microenvironment and help predict patient prognosis [13]. Moreover, ferroptosis-related elements such as Fe2+ and reactive oxygen species (ROS)-inducing agents can also trigger pyroptosis through specific signaling pathways [14]. Therefore, a deeper understanding of the complex roles of PCD in cancer and the underlying mechanisms involved is essential for optimizing treatment strategies.
Fig. 1.
Chronology of the discovery of various PCD types. Created in BioRender. Zhang, Z. (2025) https://BioRender.com/a1uy4vz.
Table 1.
Comparative overview of classical and emerging PCD pathways.
| PCD Type | Causative Agents | Molecular Markers | Representative Inhibitors | Morphological Features |
|---|---|---|---|---|
| Apoptosis | Radiation, chemicals, viruses, growth factor deprivation | Caspase-3, Caspase-9, Annexin V, cleaved PARP | Z-VAD-FMK (pan-caspase inhibitor), Emricasan, Q-VD-OPh | Cell shrinkage Chromatin condensation Plasma membrane blebbing without rupture Formation of apoptotic bodies, cytoskeletal disintegration |
| Necroptosis | Toxins, hypoxia, physical damage, complement activation | RIPK1, RIPK3, pMLKL | Necrostatin-1 (RIPK1 inhibitor), GSK'872, NSA | Cytoplasm and organelles swelling Formation of necrosome Plasma membrane rupture Release of cell contents |
| Autophagy | Nutrient starvation, hypoxia, mTOR inhibition | ATG genes, Beclin-1, LC3-II, p62 | 3-MA, Wortmannin, Bafilomycin A1 | Formation of double-membraned autophagosomes Engulfment of cytoplasmic contents and organelles Fusion with lysosomes Intact nuclear morphology Preserved plasma membrane |
| Ferroptosis | Iron overload, ROS, Erastin, RSL3, GPX4 inhibition | GPX4, SLC7A11, ACSL4 | Ferrostatin-1, Liproxstatin-1, Deferoxamine (DFO) | Mitochondria shrinkage Increased mitochondria membrane density Crista destruction Outer membrane rupture |
| Pyroptosis | Bacterial infection, LPS, inflammasome activation | GSDMD, caspase-1, IL-1β | VX-765 (caspase-1 inhibitor), disulfiram, MCC950 | Cytoplasm swelling Formation of pyroptotic bodies Plasma membrane rupture Release of cell contents |
| Cuproptosis | Excess intracellular copper, elesclomol, FDX1 activation | FDX1, DLAT, lipoylated proteins | Tetrathiomolybdate (Cu chelator), BCS | Mitochondrial swelling Cristae loss Formation of electron-dense mitochondrial aggregates Nuclear morphology largely unchanged |
| Disulfidptosis | Cystine starvation, glucose starvation | SLC7A11, NADPH | NAC (antioxidant), disulfide scavengers, DTT, β-mercaptoethanol | Collapse of cytoskeletal structure F-actin contraction and detachment from the plasma membrane Leading to loss of cell shape Mitochondria and nuclei remain intact |
| Entosis | Cell detachment, stress | RhoA-ROCK, cadherins | Y-27632 (ROCK inhibitor), Fasudil | Formation of “cell-in-cell” structures Inner cell is engulfed by a large vacuole in the host cell Host cell nucleus adopts a crescent shape Inner cell undergoes degradation within the vacuole |
| NETosis | Bacterial/viral infection | ROS, PAD4 | Cl-amidine (PAD4 inhibitor), GSK484 | Chromatin decondensation and nuclear swelling Nuclear membrane rupture Plasma membrane rupture Release of decondensed chromatin and granular proteins Formation of web-like neutrophil extracellular traps (NETs) |
| Parthanatos | DNA damage, oxidative stress | PARP-1, PAR, AIF, MIF | Olaparib, PJ34 (PARP inhibitors) | Nuclear condensation Nuclear fragmentation Mitochondrial damage Chromatin marginalization |
| Lysosome-dependent Cell Death | Lysosomal membrane permeabilization (LMP) | Cathepsins | CA-074 Me (Cathepsin B inhibitor) | Lysosomal membrane permeabilization Cytoplasmic vacuolization Release of cathepsins Mitochondrial damage |
| Alkaliptosis | pH stress, JTC801 | NF-κB, JTC801, CA9 | Bafilomycin A1 (pH regulator) | Cytoplasmic alkalinization Plasma membrane destabilization Cell swelling |
Recent progress in understanding PCD has drawn significant attention to ferroptosis, cuproptosis, and pyroptosis, which have quickly become prominent areas of research. Interest in these pathways continues to grow, driven by their potential roles in cancer development and treatment. Emerging evidence suggests that these distinct forms of cell death may offer valuable therapeutic strategies by influencing tumor initiation, progression, and response to therapy. Although traditional PCD inducers have shown promise in targeting cancer cells and improving treatment efficacy, they come with limitations, including significant side effects, the development of drug resistance, and poor targeting efficiency [15]. The emergence of nanomedicines presents an innovative approach to overcoming the challenges [[16], [17], [18]]. Nanomedicines are multifunctional and can enhance drug targeting, improve solubility, and reduce systemic side effects [[19], [20], [21]]. They can also work synergistically with different PCD pathways, offering precise control over drug release and ensuring targeted delivery to tumor cells [22]. Furthermore, nanomedicines can be combined with other therapeutic modalities such as photothermal therapy (PTT), photodynamic therapy (PDT), and radiotherapy to further boost efficacy [[23], [24], [25], [26]]. The combination approach provides exciting possibilities for the development of novel cancer therapies.
While previous reviews have often examined ferroptosis, cuproptosis, or pyroptosis in isolation, this review builds on that foundation by offering an integrated analysis of these three emerging forms of PCD and their relevance in cancer therapy. We highlight not only their individual mechanisms but also their potential interplay, emphasizing how these pathways can be strategically targeted using nanomedicine. To the best of our knowledge, this is among the most comprehensive and up-to-date summaries of the mechanistic foundations and translational applications of ferroptosis, cuproptosis, and pyroptosis in the context of cancer nanotherapy. This review begins by outlining the core molecular events and signaling pathways that drive each PCD type, with particular attention to the cascade-like amplification processes that characterize them. By identifying critical regulatory nodes within these pathways, we aim to uncover therapeutic targets for the rational design of nanomedicine-based interventions. We then examine the interplay among these PCD mechanisms and how their interactions influence tumor progression. Following this, we discuss how nanomedicine can be engineered to modulate these PCD pathways, thereby enhancing anticancer efficacy through targeted delivery and controlled activation. Finally, we provide an overview of the current clinical progress in nanomedicine-based cancer treatments, along with key challenges and future directions. Compared to previous literature, this review offers a more systematic and integrated perspective, providing a theoretical foundation for the development of next-generation cancer nanotherapies that exploit the synergistic potential of multiple PCD pathways (Fig. 2).
Fig. 2.
This review aims to thoroughly explore the mechanisms of ferroptosis, cuproptosis, and pyroptosis (three prominent forms of PCD) in tumor therapy, providing a theoretical foundation for identifying potential therapeutic targets and offering guidance for the design and development of PCD-targeting nanomedicines. Created in BioRender. Zhang, Z. (2025) https://BioRender.com/ldqrajb.
2. Introduction of cuproptosis
Copper is an important trace nutrient that supports various physiological processes, including hematopoiesis, connective tissue formation, and nervous system function [27,28]. It also acts as a catalytic co-factor for enzymes involved in energy metabolism, mitochondrial respiration, and antioxidant defense [29]. Therefore, maintaining copper homeostasis is crucial for sustaining normal physiological functions. Imbalance in copper homeostasis is associated with several diseases, such as cancer, Wilson's disease, Menke's disease, Alzheimer's disease, and Parkinson's disease [[30], [31], [32], [33], [34]]. Imbalance in copper homeostasis can lead to elevated intracellular copper levels, resulting in cellular damage and cuproptosis. Cuproptosis is a recently discovered form of PCD, first introduced by Peter Tsvetkov in 2022 [35]. Researchers initially discovered that copper ionophores induce cell death by causing abnormal intracellular copper accumulation [35]. This led to the identification of a novel form of regulated cell death, distinct from known pathways, now termed cuproptosis. Cuproptosis is associated with several distinct morphological changes, including mitochondrial shrinkage, cell membrane rupture, ER damage, and chromatin fragmentation. The mechanism of cuproptosis has been observed across multiple genetic models characterized by disrupted copper homeostasis, suggesting that cuproptosis is a broadly relevant cellular process [35]. Importantly, the regulatory framework of cuproptosis extends beyond copper metabolism alone. It also involves the lipoylation pathway, iron-sulfur (Fe-S) cluster biogenesis and regulation, and cellular stress response and proteostasis [[36], [37], [38], [39]]. Together, these modules form a complex regulatory network that orchestrates the initiation and execution of cuproptosis. In the following sections, we provide a detailed overview of these pathways and their mechanistic roles in cuproptosis.
2.1. The role of copper ions in cells
Copper is prevalent in biological tissues and serves as an indispensable element for maintaining various physiological functions [29]. Copper was primarily found in organic complexes, many of which are metalloproteins that act as enzymes [40]. A small fraction of copper exists in its free forms, mainly as cuprous ions (Cu+) and cupric ions (Cu2+) [41]. The representative biological functions of copper are as follows.
-
1.
Enzyme catalysis: copper serves as a vital cofactor for many important enzymes, including tyrosinase, cytochrome c oxidase (COX), and SOD [42]. The enzymes are integral to various metabolic activities, such as energy production, antioxidant defense, pigment synthesis, and neurotransmitter production [29].
-
2.
Energy metabolism: in the mitochondrial respiratory chain, copper is involved in electron transport, particularly within COX [32]. It facilitates the transfer of electrons to oxygen, promoting adenosine triphosphate (ATP) synthesis and providing energy for cellular functions [43].
-
3.
Antioxidant defense: copper plays an important role in the body's antioxidant system, particularly through SOD, which helps convert harmful superoxide radicals into oxygen and hydrogen peroxide [44]. The process reduces oxidative stress and protects cells from damage [45].
-
4.
Iron metabolism: copper, as a key component of plasma copper-binding proteins, is involved in iron metabolism [46]. Ferroxidase I (ceruloplasmin) and Ferroxidase II play a crucial role in oxidizing ferrous ions (Fe2+) to ferric ions (Fe3+) [47]. The oxidation process facilitates the binding of iron to transferrin, which aids in the release of iron from storage sites, such as the liver and bone marrow, into the bloodstream, promoting red blood cell production [48]. A deficiency in copper can disrupt iron metabolism, leading to anemia [49].
-
5.
Connective tissue health: copper plays a key role in synthesizing collagen and elastin to maintain the structural integrity of skin, blood vessels, and bones [50]. It enhances the stability and elasticity of the tissues by promoting the hydroxylation of lysine and proline [51].
-
6.
Nervous system function: copper is involved in the formation of myelin and plays a vital role in the synthesis of dopamine [52]. Additionally, it is crucial in catalyzing the conversion of dopamine to the neurotransmitter norepinephrine and is closely related to the biosynthesis of catecholamines [53]. A deficiency in copper can lead to neurological issues, such as cognitive impairments and motor coordination problems [54].
-
7.
Immune function: copper is vital for the proper functioning of the immune system [55]. It supports the production and activity of white blood cells, enhancing the body's resistance to infections [56].
2.2. Mechanisms of copper accumulation in cells
Copper, an essential trace element, is vital for maintaining various physiological functions in the body [29]. Under normal conditions, cells regulate copper ion levels to ensure a stable, low concentration [57]. However, when the balance is disrupted by excessive copper accumulation, a specific form of cell death known as cuproptosis can be triggered. The process depends on the buildup of copper ions within the cell, highlighting the importance of controlling copper levels to either prevent or induce the response. The following section discusses the key mechanisms by which copper ion regulation functions as a “switch” for cuproptosis.
-
1.
Increased copper transporters: elevated levels of copper transporters on cell membrane, such as copper transporter 1 (CTR1), also known as solute carrier family 31 member 1 (SLC31A1), copper transporter 2 (CTR2), and divalent metal transporter 1 (DMT1), enhance the uptake of copper ions [58,59]. For instance, tumor tissues in breast invasive carcinoma (BRCA), esophageal carcinoma (ESCA), glioblastoma multiforme (GBM), and stomach adenocarcinoma (STAD) exhibit higher SLC31A1 gene expression, which encodes more CTR1 and promotes the uptake of Cu+ into cells [60].
-
2.
Reduction of copper exporters: a decrease in copper exporters, such as ATPase copper transporting alpha polypeptide (ATP7A) and ATPase copper transporting beta polypeptide (ATP7B), impairs the excretion of Cu+, resulting in higher intracellular Cu+ levels [61]. For instance, a hydrogen sulfide (H2S)-responsive copper hydroxyphosphate nanoparticles (Cu2 (PO4) (OH) NPs) can downregulate the expressions of ATP7A, which leads to copper accumulation [62].
-
3.
Copper carriers: copper carriers are lipid-soluble compounds capable of transporting copper ions directly into cells, thereby promoting intracellular copper accumulation. Common examples include elesclomol (ES) and disulfiram (DSF), which facilitate the uptake of Cu2+ [32,63]. In addition to these well-established agents, several novel copper carriers have been identified in recent years, such as 8-hydroxyquinoline and its derivatives, hydroxylated flavonoid-based copper carriers (HQFs), pyrithione, and the natural product-derived Pochonin D (PoD) [64].
-
4.
Inhibition of copper chelators: copper-binding molecules such as glutathione (GSH) and metallothionein 1/2 (MT1/2) regulate intracellular copper levels. Inhibiting their function increases free copper [65]. For instance, Buthionine-sulfoximine (BSO) inhibits glutathione synthase, reducing Cu+-GSH binding and promoting copper accumulation [66]. Other important proteins involved in copper chaperoning and coordination include copper chaperone for superoxide dismutase (CCS), antioxidant protein-1 (ATOX1), and cytochrome c oxidase copper chaperone (COX17), which participate in intracellular copper delivery and mitochondrial copper homeostasis [67].
2.3. Mechanisms of cuproptosis
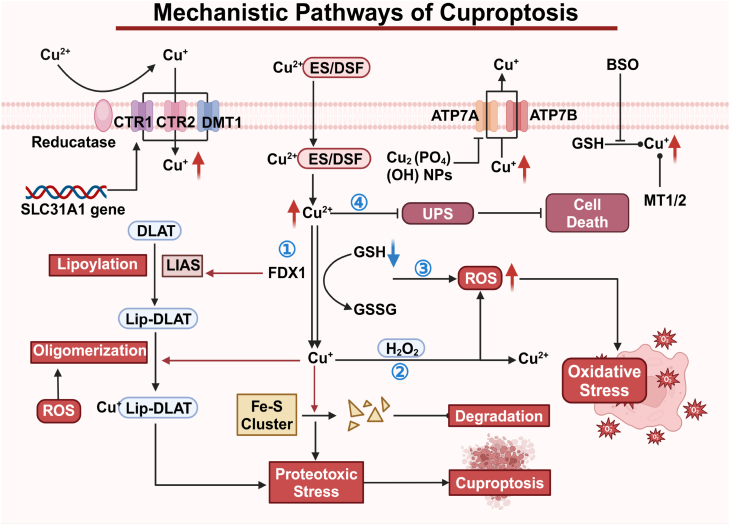
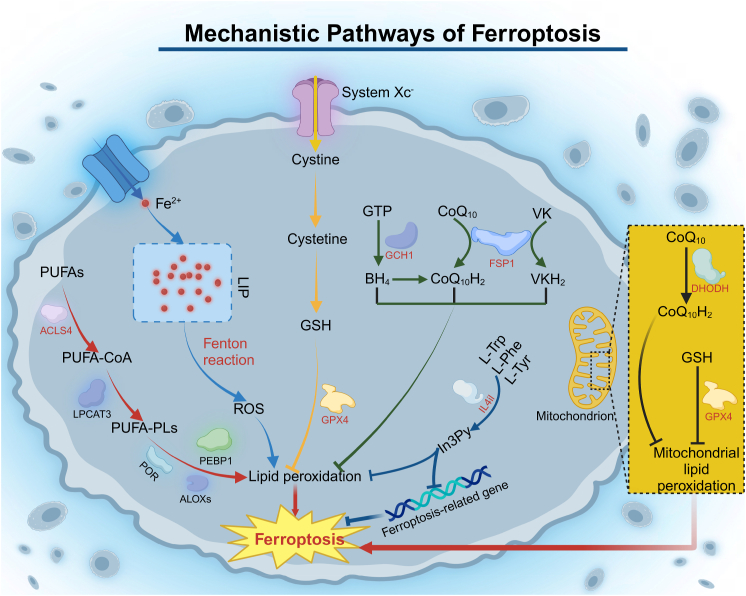
Excessive copper accumulation triggers a series of changes in cellular pathways and functions, such as oxidative stress and disruption of mitochondrial energy metabolism [29]. The alterations ultimately lead to a distinct form of PCD known as cuproptosis [35]. Below, we summarize the key mechanisms underlying cuproptosis Fig. 3).
-
1.
Copper-dependent cell death. Excess Cu2+ is transported into the mitochondria, where it is reduced to Cu+ by ferredoxin-1 (FDX1), which also plays a role upstream in the lipoic acid (LA) pathway [36]. Under its regulation, lipoic acid synthase (LIAS), a gene associated with the LA pathway, facilitates the attachment of the lipoyl group to tricarboxylic acid (TCA) cycle-associated enzymes, particularly dihydrolipoamide S-acetyltransferase (DLAT) [37]. Cu+ binds directly to lipoylated proteins, causing their oligomerization, and inhibits the formation of Fe-S cluster proteins [38]. The effects lead to proteotoxic stress and mitochondrial dysfunction, culminating in cell death.
-
2.
Oxidative stress activation. Excess Cu + reacts with hydrogen peroxide (H2O2) via the Fenton reaction, producing large amounts of ROS. The increase in ROS causes intracellular oxidative stress and contributes to cell death. Additionally, ROS can promote the aggregation of lipoylated DLAT in the mitochondria, further triggering cuproptosis [68].
-
3.
Reduction of GSH. Cu2+ can oxidize the reduced GSH to oxidized glutathione (GSSG), depleting the critical antioxidant. The process impairs the glutathione-based defense system, which normally neutralizes reactive hydroxyl groups, leading to the burst of ROS and eventually cell death [69].
-
4.
Disruption of the ubiquitin-proteasome system (UPS). The UPS is crucial for protein degradation. Cu2+ inhibits activity of proteasome, which is essential components of the UPS mechanism, and enhances interactions between ubiquitin and proteins, causing accumulation of misfolded proteins, inducing cell death [39].
Fig. 3.
Schematic of cuproptosis mechanism. The copper ionophores ES/DSF sends Cu2+ into the cells and then into the mitochondria. Cu2+ is reduced to Cu+, inducing proteotoxic stress via protein lipoylation and subsequent DLAT aggregation. Moreover, Cu+ inhibits Fe-S cluster proteins formation, inducing cuproptosis. Created in BioRender. Zhang, Z. (2025) https://BioRender.com/e66j274.
2.4. Relevance of cuproptosis in human cancers
Cuproptosis is a newly identified and mechanistically distinct form of PCD, driven by copper-induced mitochondrial stress. Unlike other PCD forms, it is tightly linked to mitochondrial metabolism and copper homeostasis, two pathways frequently altered in cancer. Emerging evidence suggests that cuproptosis plays a critical role in tumor biology, offering new opportunities for biomarker discovery and therapeutic intervention. Below, we summarize current insights into its relevance in human cancers.
-
1.
Dysregulated copper metabolism in tumors. Multiple cancer types, including hepatocellular carcinoma, breast cancer, glioblastoma, and lung adenocarcinoma, exhibit aberrant copper accumulation, largely driven by the overexpression of copper transporter SLC31A1 (CTR1) [60,70]. This not only reflects a metabolic reprogramming toward copper dependence but also renders these tumors particularly susceptible to cuproptosis-inducing agents. Notably, copper overload can disrupt mitochondrial function and trigger proteotoxic stress via lipoylated protein aggregation, the hallmark of cuproptosis [35].
-
2.
Prognostic and predictive biomarkers. Core components of the cuproptosis pathway, such as FDX1, LIAS, and LIPT1, have emerged as potential biomarkers for patient prognosis and therapeutic response [71]. Elevated FDX1 expression is associated with improved survival in cancers like hepatocellular and renal cell carcinoma, possibly due to increased vulnerability to copper-induced stress [71]. These molecular markers may help identify tumor subsets that are inherently more responsive to cuproptosis-based strategies.
-
3.
Therapeutic potential and selectivity. Pharmacological agents that perturb copper homeostasis—such as ES and DSF—have shown promising anti-tumor activity in preclinical models [72]. By exploiting the metabolic reliance of certain tumors on mitochondrial respiration, these compounds selectively trigger cuproptosis in cancer cells while sparing normal tissues [32,63]. Importantly, this mode of action is orthogonal to apoptosis or ferroptosis, making cuproptosis an attractive avenue for overcoming resistance to conventional therapies.
-
4.
Association with immune modulation. Emerging studies have revealed correlations between cuproptosis-related gene expression profiles and patterns of immune infiltration across multiple tumor types [73]. In clear cell renal cell carcinoma and melanoma, for instance, cuproptosis gene signatures are linked to T cell and macrophage enrichment, suggesting potential synergy with immune checkpoint blockade [74]. This highlights the possibility of integrating cuproptosis-targeted therapies with immunotherapeutic regimens.
-
5.
Cancer subtype vulnerability and therapeutic stratification. The sensitivity to cuproptosis varies not only among tumor types but also across molecular subtypes [59]. Tumors characterized by high oxidative phosphorylation activity, such as subsets of pancreatic ductal adenocarcinoma or kidney cancer, appear to be more dependent on lipoylated mitochondrial proteins, and thus more vulnerable to cuproptosis induction [75]. This subtype-specific susceptibility offers a rationale for precision targeting in selected patient populations.
Taken together, these findings underscore the growing recognition of cuproptosis as a clinically relevant vulnerability in cancer. Its tight connection to mitochondrial metabolism, tumor subtype specificity, and immune modulation distinguishes it from other PCD pathways and supports its development as a novel therapeutic axis.
2.5. Nanomedicine for tumor cuproptosis
Nanomedicine strategies targeting cuproptosis have advanced by focusing on key aspects of copper metabolism, including the uptake, transport, and excretion [73,76,77]. The approaches leverage a detailed understanding of copper regulation to enable precise interventions at different stages of copper homeostasis [58]. Compared to conventional treatments, nanomedicines provide significant benefits, such as customizable features, multi-target capabilities, and fewer side effects [78]. The advantages position nanomedicines as a promising avenue for developing targeted therapies to address tumor-related cuproptosis.
2.5.1. Copper carrier-based nanomedicine for cuproptosis
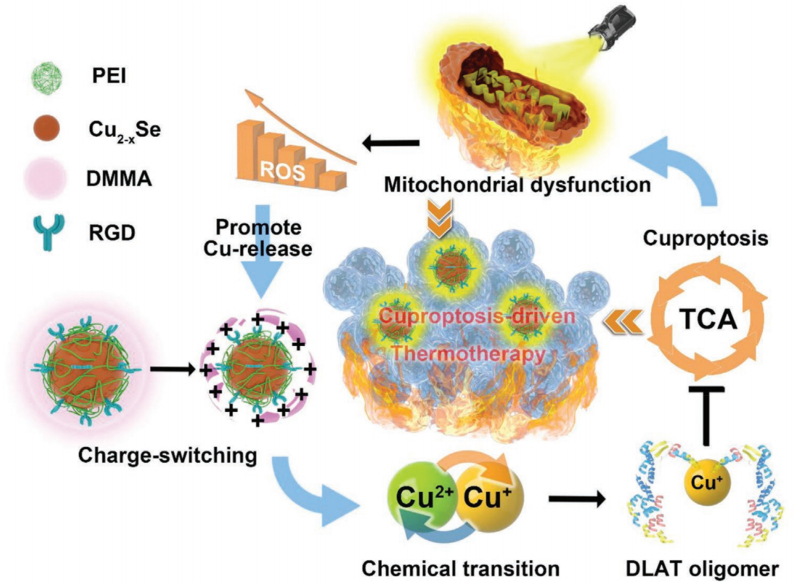
Copper carriers are complexes that load and transport copper ions into cells, potentially leading to excessive copper accumulation and enhanced cuproptosis. Various nanomedicines capable of carrying metal ions have been developed, including metal-organic frameworks (MOFs), bioresponsive polymers, nano-selenium alloy materials, and enzyme-engineered nonporous coordination polymers [76,[79], [80], [81]]. Chan et al. developed a nanosystem by encapsulating photothermal Cu2-xSe nanoparticles within bioresponsive dimethyl maleic anhydride (DMMA), which named DMMA@Cu2-xSe. Cu2-xSe acted as a copper carrier to address the deficiency of copper concentration by precisely targeting and accumulating copper in tumor tissues [76]. The reaction between DMMA and amino groups produced an amide that hydrolyzed under weakly acidic conditions, causing DMMA to separate from the nanosystem. The released Cu2-xSe was oxidized by high concentration of H2O2 to release Cu2+, which triggered the oligomerization of DLAT, an enzyme related to TCA cycle, and down-regulated the expression of key proteins in the TCA cycle and aerobic respiration, inhibiting mitochondrial aerobic respiration (Fig. 4). Upon entering the TME, the strongly positively charged poly(ethylene imine) (PEI), initially blocked by DMMA, was exposed, resulting in a positive surface charge on Cu2-xSe. The charge reversal enhanced the cellular uptake of Cu2-xSe. The aerobic respiratory depression induced by cuproptosis exacerbated mitochondrial damage during tumor hyperthermia. After laser irradiation, PTT further induced mitochondrial dysfunction, followed by the excessive production of intracellular ROS. DMMA@Cu2-xSe was oxidized by ROS and Cu2+ was released into tumor cells, which damaged the tumor cells, causing changes in the cell cycle. The synergistic photothermal-cuproptotic properties demonstrate enhanced efficacy of PTT, offering a promising new approach for cancer treatment.
Fig. 4.
Schematic illustration of the DMMA@Cu2-xSe nanosystem for cuproptosis-driven enhancement of thermotherapy. Reprinted with permission [76]. Copyright 2023, John Wiley and Sons.
In addition, Xu et al. developed a nanomedicine, GOx@[Cu(tz)], which successfully carried copper ions into cells. The nanomedicine was created by encapsulating glucose oxidase (GOx) within nonporous copper(I) 1,2,4-triazolate ([Cu(tz)]) [82]. After entering the cells, the nonporous structure was decomposed by the stimulation of overexpressed GSH. Cu+ from decomposed GOx@[Cu(tz)] can cause the accumulation of lipoylated DLAT, associated with the mitochondrial TCA cycle, leading to cuproptosis. In Gox@[Cu(tz)], the [Cu(tz)] component limited the interaction of GOx with circulating H2O2 by impeding the diffusion of blood glucose and convert oxygen (O2), ensuring that the catalytic activity of GOx was masked under physiological conditions. Under the stimulation of the overexpressed GSH, the restricted GOx catalytic activity was released to consume glucose, thereby enabling cancer starvation therapy. In addition, under near-infrared (NIR) light, a substantial amount of ·OH was produced by the Fenton-like redox reaction between H2O2 generated by glucose oxidation and Cu+. The reaction was further promoted by the restored activity of GOx@[Cu(tz)]. Furthermore, Cu2+ oxidized by H2O2 from surface Cu + also promoted Fenton-like reaction, producing more ·OH. In a similar approach aimed at enhancing copper ion delivery and amplifying oxidative stress, Yu et al. developed a semiconductor polymer nanoreactor, termed SPNLCu, which functioned as an efficient copper carrier. The system successfully delivered Cu2+ into tumor cells, inducing cuproptosis [81]. The innovative nanoreactor combined a sonodynamic semiconducting polymer nanoparticle (SPN) with lactate oxidase (LOx) using ROS-cleavable linkers and was further chelated with Cu2+. When exposed to ultrasound (US) irradiation, SPNLCu produced singlet oxygen (1O2), which broke the ROS-cleavable linkers and led to the release of LOx. The process depleted lactate and generated H2O2. The conversion of Cu2+ to Cu + facilitated the production of ·OH through a reaction with H2O2. Additionally, Cu+ reduced the expression levels of FDX1 and DLAT, culminating in cell death.
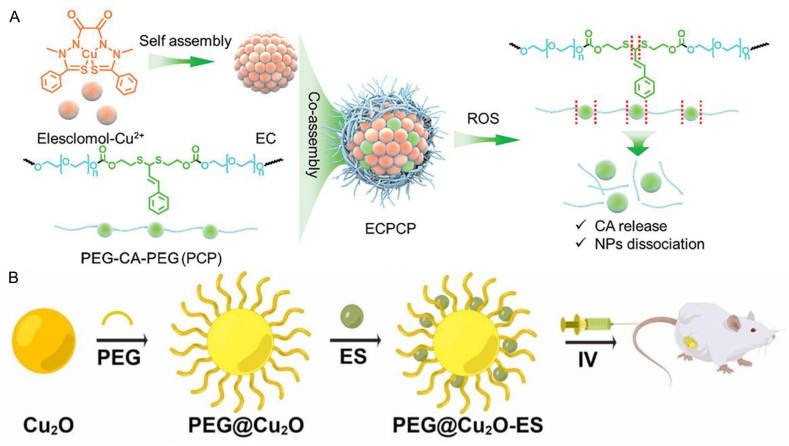
Additionally, single-molecule ES, a highly lipophilic copper-binding molecule, has been reported for metal ion loading, facilitating transport by forming complexes with copper ions [72]. Researchers have utilized the copper-binding properties of the compound to develop a variety of nanodrugs aiming at inducing cuproptosis. Guo et al. developed an innovative nanoparticle (NP@ESCu) by encapsulating ES and Cu2+ within a ROS-sensitive polymer (PHPM) [80]. When NP@ESCu was taken up by cancer cells, the ROS-sensitive thioketal bonds in PHPM were broken upon exposure to high ROS levels, then ES-Cu were released. ES could selectively transport Cu2+ to the cell mitochondria, and the released Cu2+ could bind to FDX1, a mitochondrial reductase involved in the formation of Fe-S clusters, which led to the reduction of Cu2+ to Cu+, inhibiting the generation of Fe-S clusters, causing Cu+ to bind directly to the lipoylated protein and ultimately inducing cell death. Meanwhile, ES was effluxed, which transported additional extracellular Cu2+ into cancer cells, leading to continuous Cu2+ accumulation and subsequent cuproptosis. To further optimize the therapeutic efficacy of ES-based copper delivery, Wu et al. engineered a refined system with a positive feedback loop to amplify oxidative stress and cuproptosis. The formulation integrated the ES-Cu compound (EC) with a novel ROS-responsive polymer (PCP), which was composed of cinnamaldehyde (CA) and polyethylene glycol (PEG), forming a nanoplatform, denoted as ECPCP (Fig. 5A) [56]. After cellular uptake, the PCP degraded and released CA and EC upon exposure to high levels of ROS. EC further broke down into ES and Cu2+. Cu2+ bound to FDX1 and was reduced to Cu+, which caused the lipoylation of DLAT and cell death. Meanwhile, ES transported extracellular Cu2+ into tumor cells, leading to the continuous accumulation of Cu2+ and cuproptosis. Cu2+ reacted with GSH, generating more toxic Cu+ and GSSG. The Fenton reaction, triggered by Cu+, and oxidative stress, induced by CA, promoted the production of ROS and the liberation of CA, forming a positive feedback mechanism that accelerated the dissociation of the ECPCP and further enhanced the anti-tumor effect.
Fig. 5.
A) The preparation process of ECPCP. B) Schematic diagram of PEG@Cu2O-ES inducing cuproptosis in breast cancer. Reprinted with permission [23,56]. Copyright 2024, John Wiley and Sons. Copyright 2024, Elsevier.
2.5.2. Nanomedicine for the regulation of copper exporters
Copper exporters are instrumental in the efflux of copper ions, with ATP7A and ATP7B serving as key representatives. Both ATP7A and ATP7B are P-type ATPase family members that facilitate copper ion transport within cells through multiple functional regions, including transport domains and ATP-hydrolyzing domains [61]. The domains work together to transport copper ions across cell membranes. The process initiates with copper ions binding to the copper-binding domains of either ATP7A or ATP7B, which then stimulates ATP hydrolysis and drives copper ions out of the cell [83]. In recent years, researchers have developed various nanomedicines aimed at inhibiting copper exporters, leading to elevated intracellular copper concentrations and triggering cuproptosis. For example, Jin et al. developed a nanoreactor, CCJD-FA, to increase intracellular copper levels by inhibiting ATP7B [77]. CCJD-FA encapsulated calcium peroxide nanoparticles (CaO2NPs) in a copper-based MOF shell, carrying JQ-1, a bromodomain inhibitor. In high-GSH environments, CCJD-FA dissociated to release Cu2+ and CaO2. Under acidic conditions, CaO2 produced H2O2, which reacted with Cu2+ to form Cu+ and O2. Notably, the released JQ-1 and O2 inhibited glycolysis in cancer cells, reducing ATP levels. The decrease in ATP disrupted ATP7B-mediated copper efflux, as ATP7B relies on ATP for energy, leading to increased intracellular copper ions and heightened sensitivity to cuproptosis. Similarly, Yan et al. developed an inhalable nanodevice named CLDCu to impact ATP7B function [84]. CLDCu featured a shell containing Cu2+ and a core loaded with DSF. Upon entering tumor cells, CLDCu disassembled in mild acidity, releasing DSF and Cu2+. DSF bound to Cu2+ to form bis(diethyldithiocarbamate)-copper (CuET), which substantially enhanced ROS generation. The increase in ROS damaged the MMP protein and inhibited ATP production. Consequently, ATP7B expression decreased, leading to reduced Cu+ export and elevated intracellular Cu+ levels, further inducing cuproptosis. The CLDCu-treated group showed significantly lower ATP levels and higher Cu+ content than other groups. While previous efforts focused on ATP7B, Liu et al. shifted attention to ATP7A, another key copper exporter, employing a distinct ROS-generating mechanism. Liu et al. designed a nanozyme called Cu-DBCO/CL to inhibit another copper exporter, ATP7A [85]. The nanozyme integrated cholesterol oxidase (CHO) into a copper-based framework. CHO effectively catalyzed the conversion of cholesterol to cholestenone and H2O2, generating ·OH, which elevated ROS levels. The increased ROS disrupted intracellular homeostasis, impaired mitochondrial function, and reduced ATP production, resulting in the inhibition of ATP7A and Cu+ accumulation. Li's group further reported a nanomedicine to inhibit Cu-ATPase, achieving notable results in enhancing cuproptosis (Fig. 5B) [23]. The formulation included Cu2O along with Cu2O-loaded ES. Cu2O generated ROS through a Fenton-like reaction, impairing Cu-ATPase function on cancer cell membranes and reducing Cu2+ efflux. In experiments, Cu-ATPase activity in the treated group decreased by about 80 % compared to the control, alongside a marked increase in intracellular copper ion concentration. The results demonstrated that the nanomedicine effectively induced a targeted rise in copper ions within treated cells.
2.5.3. Nanomedicine for the inhibition of copper chelators
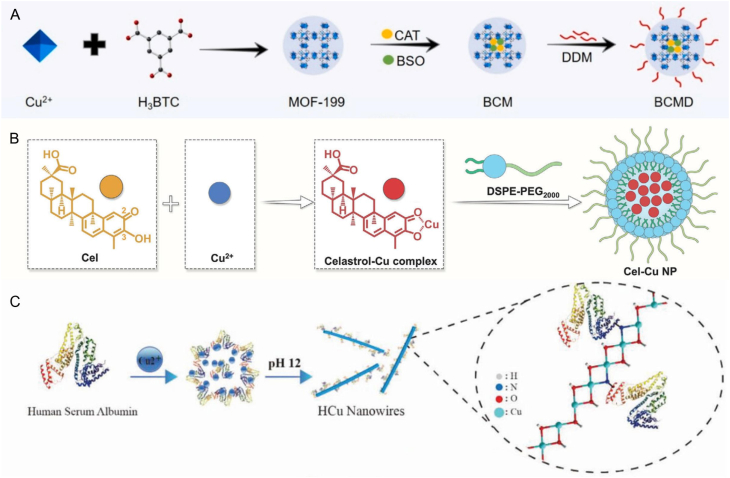
Copper chelators can bind to copper ions, resulting in a decrease in the concentration of free copper ions within cells. Two notable examples of such chelators are GSH and MT1/2. GSH is a thiol-containing copper chelator that can form GSH-Cu complexes [65]. The proteins MT1 and MT2, which are rich in thiols, bind copper ions in a pH-dependent manner through their cysteine residues [86]. Given the properties of the proteins in binding and storing copper, researchers have developed various nanomedicines aimed at inhibiting copper chelators. The inhibition can lead to an increase in the concentration of free copper ions in cells, thereby enhancing cuproptosis. Huang et al. developed a copper-based nanoplatform, named BSO-CAT@MOF-199@DDM (BCMD), designed to incorporate the inhibitor BSO within MOF-199 to modulate GSH levels effectively (Fig. 6A) [87]. Once internalized by tumor cells, BCMD dissociated in the mildly acidic tumor environment, releasing Cu2+ and BSO. The released BSO then inhibited glutamate-cysteine ligase (GCL), the rate-limiting enzyme in GSH synthesis, thereby effectively blocking GSH production. Additionally, BCMD triggered ICD, activating cytotoxic T cells to release IFN-γ. The process resulted in decreased expression of cystine/glutamate transporter proteins (SLC7A11/SLC3A2), essential for GSH synthesis as the transporters supply cysteine, a key precursor. As a result, GSH synthesis declined due to insufficient precursor availability. In GSH content assays, the BCMD treatment group showed the lowest GSH levels, confirming the effective action of BSO. While BCMD achieved GSH depletion by directly inhibiting its biosynthesis via enzymatic blockade, Lu et al. employed a distinct mechanism, utilizing celastrol to suppress the expression of key regulatory proteins involved in GSH production through NF-κB pathway inhibition. Cel and Cu2+ were used to synthesize a self-amplified cuproptosis nanoparticle (Cel-Cu NP) aimed at inhibiting GSH synthesis (Fig. 6B) [88]. After internalization by cells, Cel-Cu NP released Cu2+ and Cel. Cel inhibited the nuclear factor-kappa B (NF-κB) pathway, reducing the production of glutamate cysteine ligase modifier subunit (GCLM). The inhibition suppressed the expression of GCL, ultimately resulting in decreased GSH levels. Reduced GSH, which chelated copper ions, led to an increase in free intracellular copper, thereby amplifying cuproptosis. Gene set enrichment analysis revealed a decline in NF-κB signaling pathway activity and an increase in GSH metabolic pathway activity. The experimental results indicated that cells treated with Cel exhibited lower NF-κB and GCLM protein levels compared to those treated with PBS. Additionally, GSH levels in cells treated with Cel-Cu NPs decreased by 53 %. The findings suggested that Cel enhanced GSH depletion by inhibiting the NF-κB pathway, thereby promoting cuproptosis. In contrast to these biosynthesis-targeting strategies, Man et al. devised a direct GSH depletion platform using copper hydroxide nanowires (HCu nanowires), synthesized through an in situ biomineralization process involving Cu2+ and human serum albumin (HSA) at pH 12 (Fig. 6C) [89]. The innovative process involved combining Cu2+ with HSA, which imparted oxygen vacancies (OVs) to the HCu nanowires, enhancing their affinity for GSH. Once internalized by cells, HCu nanowires effectively depleted GSH and reduced Cu2+ to the more toxic Cu+. Additionally, HCu nanowires could directly oxidize GSH, effectively bypassing the conversion of Cu2+ and demonstrating a superior capacity for GSH consumption.
Fig. 6.
A) Schematic illustration of the fabrication process of BCMD. B) Schematic illustration of the preparation process of Cel-Cu NP. C) Schematic illustration of HCu nanowires. Reprinted with permission [[87], [88], [89]]. Copyright 2023, Elsevier. Copyright 2024, John Wiley and Sons. Copyright 2024, John Wiley and Sons.
In summary, researchers have developed various nanomedicines targeting key mechanisms to induce cuproptosis and suppress tumor growth and metastasis (Table 2). The strategies include enhancing copper transporters, reducing copper exporters, utilizing copper carriers, and inhibiting copper chelators. Future studies could explore additional proteins, ribonucleic acid (RNA), or DNA molecules within the pathways or investigate combination therapies that integrate cuproptosis with other mechanisms to achieve synergistic effects and improve therapeutic outcomes. However, these strategies still face several challenges in practical applications. Excessive administration of copper-containing agents may lead to systemic toxicity due to copper accumulation in non-target tissues. In addition, cuproptosis primarily relies on active mitochondrial metabolism, which may limit its efficacy against tumor subpopulations with low dependence on oxidative phosphorylation.
Table 2.
Applications of representative nanomedicines for cuproptosis.
| Nanomedicine structure | Mechanisms of action | Loaded drugs | Combined therapy | Ref. |
|---|---|---|---|---|
| DMMA@Cu2-xSe | Nanomedicine serves as copper carriers | DMMA | Photothermal therapy | [76] |
| GOx@[Cu(tz)] | GOx | Photothermal therapy | [82] | |
| SPNLCu | – | Immunotherapy | [81] | |
| Cu(I) NP | – | Immunotherapy | [90] | |
| CuD@PM | 5-azacytidine | Photodynamic therapy | [91] | |
| Cu/APH-M | – | Radiotherapy, Immunotherapy | [92] | |
| Na10[(PW9O34)2Cu4(H2O)2]·18H2O | – | Radiotherapy | [36] | |
| NP@ESCu | Utilization of copper carriers ES | PHPM | Immunotherapy | [80] |
| ECPCP | Cinnamaldehyde | Immunotherapy | [56] | |
| DSF/CuS-C | Utilization of copper carriers DSF | CpG | Photothermal therapy, Immunotherapy | [93] |
| M@HMnO2-DP | 5-azidobenzene-1, 3-diol 4-ethynylphenol |
– | [38] | |
| BCO-VCu | Reduction of copper exporters ATP7A and ATP7B |
– | Immunotherapy | [94] |
| HFn-Cu-REGO NPs | Regorafenib | Chemotherapy | [95] | |
| PEG@Cu2O-ES | – | Photothermal therapy, Immunotherapy | [23] | |
| Cu-DBCO/CL | Reduction of copper exporters ATP7A | Lysyl oxidase inhibitor | Immunotherapy | [85] |
| TPZ@Cu2Cl(OH)3-HA (TCuH) NPs | Tirapazamine | Photothermal therapy | [96] | |
| CAPSH | Hyaluronic acid | Thermodynamic therapy | [97] | |
| RNP@Cu2O@SPF | FA-PEG-COOH | Chemical dynamic therapy | [98] | |
| CCJD-FA | Reduction of copper exporters ATP7B | Bromodomain-containing protein 4 inhibitor | Immunotherapy | [77] |
| CLDCu | Chitosan | Immunotherapy | [84] | |
| BSO-CAT@MOF-199@DDM | Inhibition of copper chelators GSH | Buthionine-sulfoximine | Immunotherapy | [87] |
| Cel-Cu NP | Celastrol | Immunotherapy | [88] | |
| HCu nanowires | – | Chemodynamic therapy | [89] | |
| CuP/Er | Erastin | Immunotherapy | [73] | |
| CJS-Cu NPs | – | Photodynamic therapy | [99] | |
| AuBiCu-PEG NPs | Bi element | Radiotherapy, Immunotherapy | [100] | |
| HA-CuH-PVP | Hyaluronic acid | Hydrogen therapy | [101] |
3. Introduction of pyroptosis
Pyroptosis, a form of PCD, characterized by cell swelling and bubbling, culminating in membrane rupture, is primarily regulated by the caspase and gasdermin protein families. Pyroptosis is accompanied by the formation of transmembrane channels and the release of pro-inflammatory cellular components such as interleukin-1 beta (IL-1β) and interleukin-18 (IL-18) [102]. Pyroptosis is distinguished by its unique ability to trigger inflammation, setting it apart from apoptosis and necrosis [103]. Interestingly, in 1992, researchers utilized an electron microscope to observe Shigella flexneri-infected mouse macrophages and found that the cells exhibited morphology changes, such as bubbling, and pykoses. However, owing to the limited understanding of pyroptosis at that time, these macrophages were not deemed as pyroptosis but were instead categorized as apoptosis [104]. As the investigation of pyroptosis intensifies, the term “pyroptosis” was initially coined in 2001, combining the Greek roots “pyro” and “ptosis,” which respectively signify “fever” and “fall,” to characterize the particular form of cell death [105]. Pyroptosis is commonly initiated through the activation of caspase protein. Activation of caspase occurs in response to the infection of bacteria, viruses, or other pathogens, and stimulus such as the elevated levels of intracellular calcium ion (Ca2+) and ROS [106,107]. The activated caspase can subsequently target downstream gasdermin proteins, leading to the generation of its N-terminal domains. The N-terminal domains underwent conformational changes, exposing the membrane binding sites and forming four chain structures that could be inserted into the cell membrane, and were oligogenous to form pores, ultimately inducing pyroptosis [108].
3.1. Classification of caspase
In 1996, scientists proposed the term “caspase”, short for cysteine aspartic acid-specific protease, to delineate the enzymatic properties of this protein family. The term “caspase” is derived from “cysteine protease” due to its capacity to cleave peptide bonds at specific protein aspartate residues [109]. Caspases are a highly conserved intracellular protein series with a structure similar to IL-1β converting enzyme or Human Caenorhabditis elegans death protease. Activation of specific caspases can lead to apoptosis, inflammasome formation, and pyroptosis by utilizing the cysteine active center to recognize and cleave peptide bonds in target proteins (such as gasdermin, pro-IL-1β, and pro-IL-18) [109,110].
Caspases can be broadly categorized into apoptotic caspases and inflammatory caspases. The apoptotic caspases, namely caspase-2, -3, -6, -7, -8, -9, and -10, can be classified as initiator caspases and effector caspases based on their distinct roles in initiating apoptosis. Caspase-2, -8, -9, and -10 serve as initiator caspases, amplifying signals and activating corresponding effector caspases. As effector caspases, caspase-3, -6, and -7 execute the apoptosis process.
Inflammatory caspases encompass caspase-1, -4, -5, -11, and −12 [110]. Apart from caspase-12, the remaining inflammatory caspases can initiate inflammasome formation (e.g., IL-1β and IL-18) and trigger pyroptosis by cleaving downstream members of the gasdermin family [110]. Caspase-12, distinct from other inflammatory caspases, localizes in the endoplasmic reticulum (ER) and can be activated by ER stress, leading to ER-related apoptosis [111]. Caspase-14 is a unique caspase associated with epidermal differentiation, standing independent of the apoptotic and inflammatory categories [112]. It's worth noting that there is no strict delineation between apoptotic and inflammatory caspases, as apoptosis-related caspases, such as caspase-3 and -8, are also implicated in pyroptosis pathways [113,114]. In such instances, these inflammatory and apoptotic caspases participate in pyroptosis by cleaving specific gasdermin proteins.
3.2. Classification of gasdermin
In the early 21st century, the gasdermin gene was identified through the cloning of the hair-shedding fragment of chromosome 11 in recombination-induced mutation 3 mice [115,116]. Subsequent studies revealed that this gene family exhibited high expression in both the gastrointestinal tract (gastro) and skin tissue (dermato), leading to the terminology “gasdermin.” Furthermore, the heterogeneous expression of this gene in tissue cells was noted. For a long time, pyroptosis was understood as a caspase-1-dependent form of cell death, but the physiological role of gasdermin proteins and their involvement in the pyroptosis pathway remained unclear [105,117,118]. It wasn't until 2015 that researchers discerned the biological effects of the gasdermin family and their pivotal role in pyroptosis [119,120]. When the body's tissue cells are under assault from infections, tumors, asthma, systemic sclerosis, inflammatory bowel disease (IBD), and other factors, members of the gasdermin family exhibit varying degrees of expression in these tissues to address abnormal cells and preserve the body's equilibrium [[121], [122], [123], [124]]. The gasdermin protein family consists of six members encoded by six homologous genes, namely gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), gasdermin E (GSDME), and gasdermin F (GSDMF) [116] (Table 3).
Table 3.
Classification of gasdermins.
| Human | Mouse | Biodistribution | Pyroptosis-inducing ability | Related Disease | Ref. |
|---|---|---|---|---|---|
| GSDMA | GSDMA1-3 | Skin, esophagus, stomach, airway bladder epithelium | Yes | Ashama, systemic sclerosis, atopic dermatitis, mice alopecia | [129] |
| GSDMB | None | Airway, bowel, epithelium, stomach, esophagus, liver | Yes | Ashama, IBD, breast, gastric and lung cancer | [125,132] |
| GSDMC | GSDMC1-4 | Esophagus, skin, spleen, and vagina | Yes | atopic dermatitis, food allergy | [117,118] |
| GSDMD | GSDMD | Immune cells, placenta, gastrointestinal and esophageal epithelium, skin, airway | Yes | Parkinson's disease, sepsis, macular degeneration, IBD, acute kidney injury | [117,125] |
| GSDME | GSDME | Brain, endometrium, placenta, intestines | Yes | Autosomal dominant nonsyndromic hearing loss | [125,156] |
| GSDMF | GSDMF | Inner ear | No | Recessive nonsyndromic hearing impairment | [125] |
The gasdermin protein family shares highly conserved N and C domains in structure, with a protein bond linking these domains to form intact gasdermin [125]. The N domain can interact with membrane phospholipids, such as cardiolipin and phosphatidylserine, and induce pyroptosis [126,127]. However, due to the presence of the linking-protein bond and its coverage on the lipid-binding site on the N-terminal domain, the intact gasdermin proteins are self-inhibited, making GSDMs unable to exert their biological effects. Notably, during inflammation and tumorigenesis, immune cell-mediated immune inflammatory responses produce corresponding inflammasomes, which can eliminate self-inhibition through the activation of the caspase [125]. This process releases free N domains, which then assemble on the cell membrane, forming a transmembrane channel that causes membrane damage and pyroptosis. These channels allow the release of intracellular contents (e.g., lactate dehydrogenase (LDH), ATP) and inflammasomes (e.g., IL-1β, IL-18). Simultaneously, due to differences in internal and external ion concentrations, a significant influx of extracellular fluid into the cell leads to swelling and blistering, ultimately forming the typical morphology of pyroptosis [125,128].
3.2.1. GSDMA
The GSDMA (or FKSG9) protein is present in the epithelial tissues of the skin, esophagus, stomach, airway, and bladder in both humans and mice [129]. Notably, the expression pattern differs between the two species. In humans, a single GSDMA gene subtype is expressed, whereas in mice, three distinct GSDMA gene subtypes, namely GSDMA1, GSDMA2, and GSDMA3, are expressed in tissues. The human GSDMA and mouse GSDMA3 proteins feature GSDME-N capable of creating membrane pores and initiating pyroptosis. The N-terminal domain of GSDMA3 facilitates its insertion into the cell membrane by forming extended transmembrane β chains during the pyroptosis process [108]. GSDMA is implicated in the development of numerous diseases due to its differential expression in various tissues. In the respiratory system, the GSDMA is expressed in the airway epithelium, and the inflammatory response triggered by cell pyroptosis mediated by GSDMA is one of the mechanisms of the onset of asthma [117,122,130]. In the nervous system, monocyte-derived macrophage pyroptosis induced by the overexpression of GSDMA may be associated with relevance to the pathogenesis and modulation of systematic sclerosis [123]. In keratinocytes, the cysteine protease streptococcalpyrogenic exotoxin B (SpeB), produced by group A hemolytic streptococcus (GAS), can trigger the pyroptosis of keratinocytes by cleaving GSDMA, contributing to the dissemination of localized skin infections caused by GAS, ultimately leading to severe systemic infection [131].
3.2.2. GSDMB
GSDMB (or GSDML) is primarily expressed in the airway epithelium, intestinal epithelium, stomach, esophagus, liver, and various other tissues [125,132]. This gene is exclusive to humans and comprises four subtypes: GSDMB1, GSDMB2, GSDMB3, and GSDMB4. GSDMB shares structural similarities with other members of the gasdermin protein family [133]. However, the absence of exon 6—crucial to cell pyroptosis—results in significant differences in gene sequences between GSDMB1/2 and GSDMB3/4. This divergence subsequently leads to variations in the amino acid sequences of GSDMB1-N and GSDMB2-N compared to GSDMB3-N and GSDMB4-N. Notably, only the GSDME-N of GSDMB3 and GSDMB4 exhibit pore-forming activity [134,135].
GSDMB is implicated in the pathogenesis of numerous diseases. Within the respiratory system, it serves as a susceptibility gene for asthma [117]. In instances where asthma patients experience respiratory viral infections, GSDMB may enhance the expression of interferon-stimulating genes by promoting the mitochondrial antiviral signaling protein TANK-binding kinase 1, thereby exacerbating the inflammatory response within airway epithelia [136]. Furthermore, in the context of IBD, GSDMB expression is elevated in intestinal epithelial cells (IEC), where it plays a pivotal role in regulating IEC proliferation, migration, adhesion, and epithelial repair [137]. In tumor environments, toxic lymphocytes activate GSDMB in tumor cells, inducing pyroptosis, which represents an effective mechanism for tumor suppression [138].
3.2.3. GSDMC
GSDMC (or MLZE) is expressed in various tissues, including the esophagus, skin, spleen, and vagina [117,118]. In humans, this gene expresses a single subtype of GSDMC, whereas in mice, it is responsible for the expression of four subtypes: GSDMC1, GSDMC2, GSDMC3, and GSDMC4. The GSDMC gene plays a significant role in the proliferation, dissemination, and other malignant biological behaviors associated with tumors, such as kidney cancer, breast cancer, pancreatic ductal adenocarcinoma, and colorectal cancer [[139], [140], [141], [142], [143]]. This is achieved through the induction of genes associated with tumor stemness, epithelial-mesenchymal transition, immune evasion, and the inflammatory response that follows cell pyroptosis mediated by GSDMC protein. Nevertheless, it is noteworthy that GSDMC also exhibits anti-tumor effects. As a member of the gasdermin family, GSDMC possesses dual functional domains, with its anti-tumor efficacy manifested through the induction of pyroptosis in tumor cells by its N-terminal domain and the enhancement of cellular immunity [144,145].
In IEC, the N6 adenosine methylation of the polyadenosine RNA transcript of the GSDMC gene is crucial for maintaining the activity of intestinal stem cells, which is essential for the proper morphological development of normal IEC [146]. Furthermore, following intestinal worm infection, the expression of O-linked N-acetylglucosamine protein (O-GlcNAc) transferase is induced, resulting in the O-GlcNAc modification of signal transducer and activator of transcription 6. This modification promotes the release of GSDMC-mediated IL-33 in IEC, which participates in the immune response to worm infection [147]. Additionally, other studies indicate that the anti-helminthic response in the intestinal epithelium is also associated with a type 2 immune response, which is induced by mast cell protease 1 secreted by mast cells that are bound to the surface of IEC, following the cleavage of intestinal epithelial GSDMC [148].
3.2.4. GSDMD
GSDMD is widely expressed in various tissues, including skin, airway epithelium, placenta, esophagus, and gastrointestinal epithelium [117,125]. This gene, located on chromosome 8 adjacent to the GSDMC gene, is expressed in both murine and human models and plays a critical role in pyroptosis and NETosis (a form of inflammatory cell death in neutrophils) [149]. The palmitoylation of the GSDMD protein at Cys191/192 facilitates the formation of pores in the cell membrane by both GSDMD N-terminal (GSDMD-NT) and full-length GSDMD proteins [150]. These transmembrane channels enable the release of intracellular pathogenic substances into the extracellular space, allowing immune cells to capture them, thereby fulfilling an immune function in conjunction with cell pyroptosis [151]. Moreover, during muscle repair, macrophages secrete metabolites through the pores formed by GSDMD, contributing to the activation of muscle stem cells and facilitating the repair and regeneration of muscle tissue [152]. In the central nervous system, GSDMD is implicated in the activation of microglia and mediates the pyroptosis of dopaminergic neurons, exacerbating Parkinson's disease in patients [153]. Additionally, in the liver, GSDMD-mediated pyroptosis is recognized as a significant mechanism contributing to the development of nonalcoholic cirrhosis [154]. In autoimmune diseases, GSDMD has been shown to play a role in the pathogenesis of systemic lupus erythematosus through the mediation of neutrophil pyroptosis [155].
3.2.5. GSDME
GSDME (or DFNA5) is expressed in various tissues, including cochlea, brain, endometrium, placenta, and bowel [125,156]. Initially identified as a gene mutation associated with hearing loss in humans, GSDME has recently gained attention for its role in triggering pyroptosis [113]. The GSDME protein possesses a structure analogous to other proteins within the gasdermin family, which includes the pyroptotic N-domain. Beyond its ability to induce pyroptosis via the N-domain, ultraviolet irradiation-induced DNA damage in cervical cancer cells can also stimulate pyroptosis mediated by the full-length GSDME protein [157]. Within the human body, the activated GSDME protein is implicated in the onset and progression of numerous diseases. In particular, it contributes to neuronal damage in brain and spinal neurons by compromising mitochondrial integrity, which is associated with frontotemporal dementia and various neurodegenerative diseases, such as amyotrophic lateral sclerosis [158]. Furthermore, during the development of atherosclerosis, GSDME can induce macrophage pyroptosis, thereby facilitating disease progression [159]. In patients with rheumatoid arthritis, GSDME-induced pyroptosis in synovial macrophages and monocytes plays a significant role in disease development [160]. Additionally, enhancing the expression levels of GSDME through pharmacological interventions has emerged as a promising strategy in cancer treatment [161,162].
3.2.6. GSDMF
GSDMF (or PJVK, DFNB59, Pejvakin) is expressed in various tissues, including brain, internal ear, heart, and lung tissues [125]. Despite its affiliation with the gasdermin family, the capacity of GSDMF to initiate pyroptosis remains undefined [[163], [164], [165]]. The GSDMF gene is recognized as a crucial component in hearing preservation. Within the inner ear, the expression of GSDMF may enhance peroxisome activity in hair cells in response to noise stimuli, thereby conferring a protective effect on the auditory system. Mutations within this gene can lead to hearing impairment in humans [166].
3.3. Formation of pyroptotic channel
It is evident that the ultimate manifestation of pyroptosis is facilitated by the transmembrane channels of the GSDMs protein family. In recent years, research into the assembly of GSDMA3, GSDMB, and GSDMD-based channels has progressively elucidated the structure of pyroptotic pores and the distinct conformational alterations of GSDMs-N during pore formation, thereby enhancing the understanding of the pyroptosis process [108,133,167].
The GSDMs-N subunit is comprised of a globular domain and a β-barrel domain. The globular domain forms the edge of the pore, encompassing α and β elements. The portion of the globular domain near the lipid membrane consists of positively charged α1, α3, and β1-β2 loop structures, which are masked in the self-inhibited state of the GSDMs protein [108]. The β-barrel structure constitutes the main transmembrane region, with 2 β-hairpin structures (β3-β5, β7-β8), each consisting of 2 inverse parallel β-chains. The β7-β8 hairpin structure and the α1 helix and β1-β2 loop structure in the globular domain serve as lipid binding sites in the membrane pore binding of GSDMs-N.The structure of GSDMs-N inserted into the cell membrane is often likened to a left hand, with the globular domain as the palm, the four inverted parallel beta chains as the fingers, the membrane binding site α1 as the thumb structure attached to the palm, and the β1-β2 loop as the wrist [108,167]. In the self-inhibited state, GSDMs-N resembles a clenched fist, with four β-chains helically hiding the membrane binding site α1, and β1-β2 loops hidden by connecting chains [108,133,167].
When the self-inhibition state of the GSDMs protein family is relieved, GSDMS-N does not directly change its conformation but uses the conformation in the self-inhibition state to oligomerize through hydrogen bonding, hydrophobic force, and the interaction between charges to form a structure called pre-pore. The pre-pore is structured like a yo-yo, divided into two rings, the bottom ring not inserted into the membrane, thus unable to form holes [167]. Subsequently, the GSDMs-N subunit undergoes a significant conformational change resembling the expansion of a clenched fist. This change involved the α1 helix serving as the neutral axis, the spherical domain rotating away from the membrane, and the formation and rotation of two membrane-inserting β-hairpin structures toward the central axis of the pore. Consequently, a transmembrane β-barrel structure with a diameter slightly smaller than the pre-pore was created. During this process, the membrane lipid binding site α1 helix became exposed, and the β7-β8 hairpin structure formed, facilitating the further localization of the membrane pore on the cell membrane. This transition completes the transformation from the pre-pore to the pore, enabling GSDMs-N to position and perforate the membrane [108,167].
The pores formed by GSDMs-N are structurally similar but have different numbers of monomers and parameters. For instance, the GSDMA3-N hole is composed of 26–28 monomers, with a 27-monomer hole having the highest three-dimensional structure confidence. The GSDMB-N hole is composed of 24–26 monomers, with the 24-monomer hole having the most reliable three-dimensional structure. The GSDMD-N hole is composed of 31–34 monomers, with the 33-monomer hole having the highest reliability in the three-dimensional structure [108,133,167]. Although the inner diameter of these cavities varies, it is sufficient to allow the passage of cellular contents and inflammatory mediators produced during pyroptosis into the extracellular matrix [125].
In summary, the GSDMs protein family can exert their important biological effects in pyroptosis by undergoing a process of self-inhibition disinhibition, pre-pore transformation, and pre-pore-to-pore transition.
3.4. Mechanisms of pyroptosis
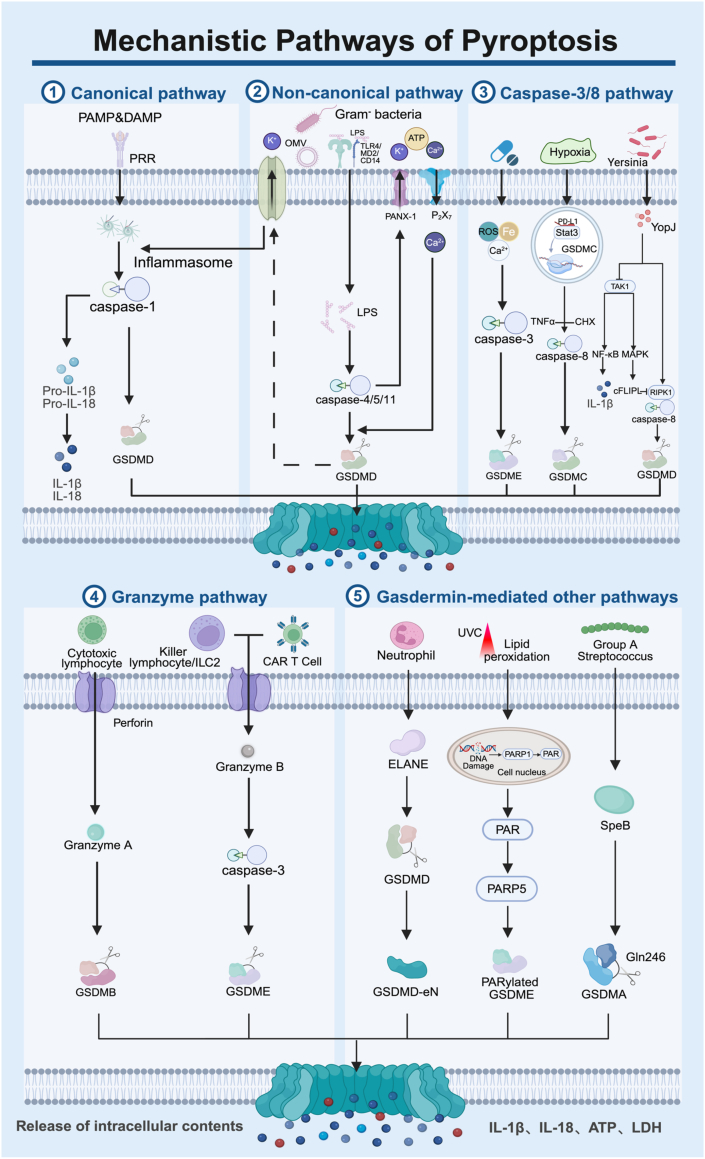
Two pivotal protein families, caspases and gasdermins, form the cornerstone of understanding the pyroptotic pathway. These proteins are intricately linked during the pathogenesis of pyroptosis. Traditionally, pyroptosis is thought to be initiated by activating caspases through associated inflammatory factors. Activated caspases then cleave GSDMD, ultimately triggering pyroptosis. According to the above theory, the pathways of pyroptosis are divided into caspase-1 mediated canonical pathway and caspase-4/5/11 mediated noncanonical pathway [107]. With the deepening research on pyroptosis, the pathway has extended to include the caspase-3/8-mediated and granzyme-mediated pathways [168,169]. In these pathways, pyroptosis is primarily initiated by caspase-mediated cleavage of gasdermin, producing N-terminal domains that oligomerize to form transmembrane pores in the cytomembrane. However, as research progresses, alternative pathways beyond these relatively conserved mechanisms have been identified. Examples include group A GAS virulence factor SpeB and neutrophil elastase proteinase cleaving GSDMD to induce pyroptosis, as well as the intact GSDME protein directly triggering pyroptosis [131,158,170]. The following paragraphs present a comprehensive introduction of the pyroptosis pathways (Fig. 7).
Fig. 7.
Illustration of the mechanisms of the canonical pathway, non-canonical pathway, caspase-3/8 pathway, granzyme pathway, and gasdermin-mediated other pathways. Created in BioRender. Zhang, Z. (2025) https://BioRender.com/rdy3rde.
3.4.1. Canonical pathway
The canonical pathway involves the activation of caspase-1 by inflammatory factors, which initiates the cleavage of the GSDMD protein, leading to the process of pyroptosis. Both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are recognized by pattern recognition receptors (PRRs) situated on the cell membrane. This recognition leads to the formation of specific inflammasomes, such as Nod-like receptor family pyrin domain-containing 3 (NLRP3). Activated inflammasomes subsequently convert the inactive pro-caspase-1 into its active form, bioactive caspase-1. This enzyme cleaves GSDMD to produce GSDMD-N, resulting in the formation of pores within the cellular membrane that culminate in pyroptosis. Additionally, caspase-1 cleaves pro-IL-1β and pro-IL-18, generating the active forms of IL-1β and IL-18. These cytokines are then released through pyroptotic channels, serving as pro-inflammatory mediators to enhance the immune response [107,169].
3.4.2. Non-canonical pathway
The non-canonical pathway pertains to the action of lipopolysaccharide (LPS) produced by gram-negative bacteria, which activates the cleavage of GSDMD via caspase-4, -5, and -11, subsequently initiating pyroptosis. LPS can infiltrate the cytoplasm through two primary mechanisms: one involves the mediation of TLR4/MD2/CD14 during the process of endocytosis by host cells. At the same time, the other relies on the endocytosis of LPS-rich outer membrane vesicles (OMVs) produced by the bacteria [171,172]. Once inside the cytoplasm, LPS can directly activate caspase-4, -5, and -11, which cleaves GSDMD, yielding the pyroptotic GSDMD-N fragment, responsible for forming transmembrane channels.
The cleavage of GSDMD instigates the efflux of K+, subsequently stimulating the activation of the intracellular NLRP3 inflammasome [119,[171], [172], [173], [174], [175]]. Although caspase-4, -5, and -11 are unable to directly convert pro-IL-1β and pro-IL-18 into their active forms (IL-1β and IL-18), these cytokines can still be produced and secreted through the NLRP3/caspase-1 pathway in certain cell types [163]. Furthermore, caspase-11 can induce pyroptosis through additional mechanisms. The pannexin-1 (PANX-1) ion channel, widely present on the cell membrane, can be cleaved by caspase-11, facilitating the passage of ions such as K+ and bioactive molecules like ATP. Purinergic receptor P2X ligand-gated ion channel 7 (P2X7) receptors, ligand-gated ion channels located on the cell membrane, can be activated by extracellular ATP, resulting in Ca2+ influx—an essential event in GSDMD-N-mediated pyroptosis [176,177]. The activity of caspase-11, triggered by cytoplasmic LPS, leads to the cleavage of PANX-1, allowing the efflux of ATP and K+. Extracellular ATP and K+ subsequently activate PANX-1, causing an influx of Ca2+. This rise in intracellular calcium activates caspase-11, which further cleaves GSDMD to generate the membrane-insertive GSDMD-N fragment, ultimately culminating in pyroptosis induced by the interaction of caspase-11-activated PANX-1 and P2X7 [175].
3.4.3. Caspase-3/8 mediated pathway
Caspase-3/8, traditionally recognized as an apoptotic caspase, is increasingly understood to extend its functions beyond apoptosis, particularly in the context of pyroptosis. Numerous studies have documented the capacity of caspase-3 to facilitate this pathway. Many chemotherapeutic agents utilized in cancer treatment target caspase-3, thereby inducing pyroptosis in tumor cells via the cleavage of GSDME. This process results in the generation of GSDME-N, which subsequently forms pyroptotic pores and exerts anti-tumor effects [113]. Notable examples of such agents include the topoisomerase inhibitors topotecan, etoposide, cisplatin, and irinotecan. Furthermore, advancements in novel technologies and materials, including PDT, acoustic dynamics, and nanomaterials, have enabled the activation of caspase-3 through modifications to the TME. This can be achieved by elevating the levels of ROS within tumor cells or through the overload of calcium and iron, thereby promoting the pyroptosis of tumor cells and contributing to therapeutic anti-tumor strategies [[178], [179], [180], [181], [182]]. Furthermore, it has the potential to enhance the caspase-3/GSDME-mediated pyroptosis pathway by increasing the expression of GSDME in tumor cells, thereby facilitating the objective of tumor treatment [183].
Caspase-8 serves as a pivotal switch in cell death patterns, possessing the ability to initiate pyroptosis [184]. Under hypoxic conditions, pyroptosis can be induced via the nPD-L1-GSDMC-caspase-8 signaling pathway. Programmed death ligand 1 (PD-L1) promotes its nuclear translocation through physical interaction with phosphorylated Stat3 in the nucleus, thereby enhancing the transcriptional activity of GSDMC. In conjunction with tumor necrosis factor-alpha (TNF-α) and cycloheximide (CHX), caspase-8 cleaves GSDMC to form GSDMC-N, ultimately triggering pyroptosis [114]. Additionally, the virulence protein Yersinia outer protein J (YopJ), secreted by Yersinia, can inhibit transforming growth factor-β-activated kinase 1 (TAK1) and activate the receptor interacting protein kinase 1 (RIPK1)-caspase-8 pathway to induce pyroptosis. TAK1 is involved in the modulation of NF-κB and mitogen-activated protein kinase (MAPK) pathways, increasing the expression of IL-1β and cellular FLICE-inhibitory protein (cFLIPL), both of which are linked to the regulation of cell death. A heightened expression of cFLIPL serves to inhibit the RIPK1-caspase-8 pathway. Conversely, reduced levels of cFLIPL can stimulate caspase-8 activity, facilitating GSDMD cleavage and the induction of pyroptosis [185,186].
3.4.4. Granzyme-mediated pathway
Granzyme A, produced by cytotoxic lymphocytes, enters the target cell via perforin and cleaves GSDMB, leading to the induction of pyroptosis [138]. Additionally, chimeric antigen receptor (CAR) T cells exhibit a strong affinity for target cells, which enhances their capacity to release perforin and granzyme B. Granzyme B enters the cell through the perforin pore, activating the intracellular pyroptotic caspase-3/GSDME pathway. Moreover, granzyme B secreted by cytotoxic lymphocytes, as well as a second group of innate lymphoid cells (ILC2), can directly cleave GSDME, resulting in the formation of the pyroptotic N-terminal domain and subsequently triggering pyroptosis [162,187].
3.4.5. Gasdermin mediated other pathways
-
(1)
The cysteine protease SpeB, a virulence factor produced by GAS, cleaves GSDMA at the amino acid residue Gln246. This cleavage results in the polymerization of GSDMA-N, leading to the formation of channels within the cell membrane, which subsequently induces pyroptosis [131].
-
(2)
The formation of GSDMD-eNT, mediated by the neutrophil protease ELANE, which is slightly smaller than the GSDMD-NT generated by caspase-4/5/11, can oligomerize on the membrane to create transmembrane pores, ultimately leading to the process of pyroptosis [170].
-
(3)
The DNA damage induced by UVC irradiation stimulates the nuclear enzyme PARP1 to synthesize poly (ADP-ribose) (PAR) polymers. These PAR polymers, once released into the cytoplasm, can activate PARP5, which enhances GSDME polymerization and reduces its autoinhibition. Concurrently, UVC exposure promotes cytochrome C-mediated phospholipid peroxidation, which can be detected by PARylated GSDME, resulting in oxidative oligomerization of the plasma membrane and ultimately leading to pyroptosis [157].
The diverse pathways associated with pyroptosis underscore the involvement of multiple mechanisms, suggesting the existence of potential yet-to-be-discovered pathways beyond the canonical, non-canonical, caspase-3/8-mediated, and granzyme-mediated pathways.
3.5. Nanomedicine for tumor pyroptosis
Pyroptosis is a specialized form of cell death characterized by a robust immune response. When it occurs in tumor cells, pyroptosis promotes the infiltration of immune cells into the TME and enhances the body's innate immune response through cell lysis and the release of inflammatory mediators. This process facilitates the clearance of tumors and decreases the likelihood of adverse events, such as cancer recurrence and metastasis, thereby achieving a more effective antitumor response [188]. Numerous chemotherapeutic agents, including metformin and doxorubicin, as well as acoustic and photodynamic therapies, can induce pyroptosis in cells. However, their therapeutic efficacy is significantly limited by the challenges associated with effective delivery into tumor cells [188]. In this regard, nanomedicine presents a promising solution, as it can overcome these limitations by enabling targeted delivery to tumors, achieving high intracellular drug concentrations, and efficiently initiating pyroptosis. Upon cellular entry, nanomaterials disassemble and release their active components. This process directly induces pyroptosis in the targeted cells and also indirectly activates the caspase and/or gasdermin protein families by modifying the TME, such as by elevating ROS levels and triggering Ca2+ influx [189,190].
In the pathways described in the preceding sections, nanodrugs are capable of inducing pyroptosis through the activation of various pyroptotic proteins. The caspase-3/GSDME pathway, which is commonly employed, represents a significant mechanism in the caspase-3/caspase-8 mediated pathway [191,192]. For example, Zheng et al. employed a one-pot gas diffusion method to combine CaCO3 and curcumin (CUR) to create biodegradable Ca2+ nano modulators (CaNMs), designed to induce pyroptosis by causing mitochondrial Ca2+ overload [190]. CaCO3 served both as a carrier for CUR and a Ca2+ source within tumor cells. Once internalized, CaNMs could degrade rapidly and increase the intracellular Ca2+ concentration within the acidic intracellular environment. Meanwhile, the CUR facilitated the release of Ca2+ from the ER to the cytoplasm while impeding the efflux of intracellular Ca2+ ions. The elevated intracellular Ca2+ level caused mitochondrial Ca2+ overload, leading to increased cytochrome C and reactive oxygen species production. Subsequently, cytochrome C activated caspase-3, resulting in GSDME cleavage. The resultant GSDME-N of GSDME were associated with cell membrane phospholipids, progressing to pyroptosis via transmembrane channel formation. Microscopic and bio-TEM observations unveiled notable swelling and bubbling in 4T1 cells treated with CaNMs, accompanied by severe damage to the cell membranes and organelles. These observations matched the typical morphological alterations associated with pyroptosis, thus emphasizing the significance of pyroptosis in mediating tumor cell death induced by mitochondrial Ca2+ overload. The study offers novel insights and strategies for leveraging pyroptosis in cancer treatment.
In contrast to calcium nanomaterials (CaNMs), which promote the activation of caspase-3/GSDME through calcium overload, the subsequent nanomaterials activate caspase-3/GSDME by integrating chemotherapy with PDT. This approach increases the concentration of intracellular ROS, leading to the activation of caspase-3/GSDME and the induction of pyroptosis. A nano-targeting chimera (L@NBMZ) was developed to enhance photodynamic pyroptosis by encapsulating the photosensitizer sulfur-substituted Nile Blue (NBSEt) and MZ1, a BRD4 protein-degrading proteolysis targeting chimera (PROTAC), within liposomes [189]. Upon internalization, the L@NBMZ could release NBSEt and MZ1 when exposed to laser irradiation. MZ1 could induce pyroptosis via the caspase-3 pathway and inhibit breast cancer cell proliferation by suppressing gene transcription. The NBSEt generated ROS, specifically light-activated superoxide radicals (O2−•), to enhance the function of MZ1, which synergistically led to the production of cleaved caspase-3. The activated caspase-3 cleaved GSDME to generate its GSDME-N. These domains combined with cell membrane phospholipids to form a transmembrane channel, leading to pyroptosis. Cells in the light-exposed L@NBMZ group exhibited typical morphological changes indicative of pyroptosis, such as loss of filopodial tips, cell swelling, and bubbling. The extracellular fluid showed increased cell membrane permeability, a typical feature of pyroptosis. Additionally, cells in the L@NBMZ group exhibited green DCF labels in both normoxia and hypoxia environments, demonstrating the potential efficacy of nanopharmaceuticals in light-driven PROTAC therapy by effectively combating tumor cells with O2−•. In summary, this proposed PROTAC enhancer assembly strategy advances anti-cancer treatment, offering a valuable reference for expanding PROTAC application and developing light-driven PROTAC therapy.
In contrast to the direct activation of caspase-3/GSDME, the subsequent pharmacological agents that target caspase-9 and cytochrome C facilitate the indirect activation of caspase-3/GSDME. Chen et al. developed an acid-activated nanosensitizer (ANPS) library by covalently linking the photosensitizer chlorin e6 (Ce6) and the fluorescence quencher QSY21 to mPEG-b-P(R1-r-R2). This library comprised 9 nanoparticles, each containing Ce6 and QSY21 in equal proportions [193]. The ANPS nanoparticles demonstrated distinct pH sensitivity, aligning with the pH range of endosomal maturation. They remained inactive during systemic circulation and extracellular distribution but were triggered to O2 to 1O2 upon exposure to laser and acidic environments. Upon internalization, particularly within early endosome, the ANPS library induced singlet oxygen generation (SOG), which led to lipid peroxidation (LPO) and subsequently activated phospholipase C (PLC) and increased inositol triphosphate levels. This elevation in cytoplasmic Ca2+ concentrations initiated mitochondrial apoptosis mediated by cytochrome C and caspase-9, culminating in activated caspase-3. Caspase-3 then cleaved GSDME, generating GSDME-N that interacted with cell membrane phospholipids, forming transmembrane channels that increased cell membrane permeability, ultimately inducing pyroptosis. The ANPS library effectively targeted oxidative stress within specific acidic endocytic compartments, demonstrating a practical strategy for location-dependent, organelle-specific tunability of pyroptosis while minimizing systemic toxicity. This approach lays the groundwork for designing nanomaterials with tunable pyroptosis activity for targeted treatment of tumors through early endosomal signaling.
Nanomedicine can be administered through various modalities, including the use of metal ions. Furthermore, delivery platforms for nanomedicine that incorporate biological substances, such as bacteria, demonstrate significant potential for application in this field. Wang et al. developed a novel adaptive pyroptosis inducer named LPZ by combining polyamine oxidase (PAO) with imidazole skeleton-67 zeolite (ZIF-67) to create the PAO@ZIF-67 (PZ) nanostructure, which was then electrostatically adsorbed onto the surface of Lactobacillus rhamnosus (LGG) [194]. Mediated by LGG, LPZ could selectively bind to tumor cells due to the higher expression of mucins in cancer cells than normal cells while minimizing systemic cytotoxicity. Once adhered to the tumor membranes, LPZ could facilitate the initiation of the pyroptosis pathway via ROS-mediated damage to cancer cell membranes. Meanwhile, the LGG metabolized carbohydrates via anaerobic respiration to generate lactic acid, lowering the intracellular pH and promoting the dissociation of PZ from LPZ. This process facilitated ROS induction by PZ, followed by endocytosis of the isolated PZ of tumor cells. In the acidic intracellular environment, PAO and superoxide dismutase (SOD)-like ZIF-67 increased H2O2 production, while peroxide (POD)-like ZIF-67 promoted the conversion of H2O2 to ·OH. Subsequent bursts of intracellular ROS activated the nucleotide-binding oligomer domain, leading to the activation of caspase-1, which cleaved GSDMD to generate the GSDMD-N. These domains formed transmembrane channels, increasing membrane permeability, releasing cellular contents, and ultimately triggering pyroptosis. Simultaneously, combining LPZ with 2,3-dioxygenase (IDO) inhibitors could stimulate immune activation, restraining the growth and lung metastasis of bilateral transplanted 4T1 tumors. This integration of probiotics and enzyme-like metal-organic framework has enabled the construction of self-adaptive pyroptosis inducers, resulting in potent pyroptosis and effective immunotherapy.
The integration of immunotherapy enhances the anti-tumor efficacy associated with cell pyroptosis. Su et al. constructed a ROS/pH dual-response carrier, mPEG-b-P(MTE-co-PDA), using the reversible addition-fragmentation chain transfer polymerization method. Through self-assembly, the signal transducer and transcriptional activator 3 (STAT3) inhibitor niclosamide (NI) were loaded onto the carrier to create the MPNPs (Fig. 8A). [195]. A two-step red-mediated recombination was utilized to generate the ICP34.5 and ICP47 deletion herpes simplex virus (HSV-1 Δ34.5/Δ47, named oHSV). The oHSV induced the ER stress, leading to the generation of ROS. Meanwhile, MPNPs were internalized into lysosomes and released NI within the acidic and high ROS environment. The dissociative NI, on the one hand, could reduce the proliferation capacity and stemness of tumor cells by inhibiting STAT3. On the other hand, the nanoparticle could mediate the loss of mitochondrial membrane potential (MMP), uncoupling of oxidative phosphorylation, and production of mitochondrial superoxide, which activated caspase-3 with replicated oHSV. Cleaved by activated caspase-3, GSDME generated the GSDME-N, which interacted with cell membranes to create pores, resulting in pyroptosis. Moreover, the combination of MPNPs and oHSV increased the expression of PD-L1 on the surface of tumor cells. When used with anti-PD-1 therapy, the anti-tumor ability, tumor recurrence, and metastasis inhibition were significantly improved. The inflammatory response induced by pyroptosis increased the proportion of mature dendritic cells, which could elicit T cell activation, including CD8+ T cells, CD44+ CD62L− effector memory T cells, and CD44+ CD62L+ central memory T cells, alongside a decrease in immunosuppressive Treg cells (Fig. 8B). This nanomedical strategy overcomes the limitations of immune checkpoint blockade and provides a new approach to individualized tumor treatment.
Fig. 8.
A) The schematic illustration of the MPNPs preparation. B) Illustration showing the mechanisms that MPZPs induced pyroptosis and anti-tumor immunotherapy. Reprinted with permission [195]. Copyright 2023, John Wiley and Sons.
Researchers have made significant advancements in developing various nanomedicines that target key mechanisms or molecular proteins within pyroptotic pathways to inhibit the biological behaviors of tumor cells, including metastasis, proliferation, and recurrence (Table 4). These approaches involve the targeting of caspase3/GSDME, caspase1/GSDMD, the granzyme pathway, and the upregulation of GSDMB to enhance the efficiency of pyroptosis induction in tumor cells, thereby achieving effective tumor suppression. While existing studies have leveraged the biological implications of pyroptosis for creating and applying nanochemical therapeutics, future research could benefit from exploring the activation of additional pyroptotic pathways. This may include investigating other pyroptotic proteins such as GSDMA and GSDMC and the synergistic potential of combining pyroptosis with alternative cell death mechanisms through specialized pharmacological agents to enhance anti-tumor efficacy. While pyroptosis can stimulate strong immune responses against tumors, its long-term effectiveness is often limited by the suppressive nature of the tumor microenvironment. For example, studies have shown that pyroptosis can promote dendritic cell maturation and T cell activation, but the presence of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment can suppress this immune activation [196,197]. Therefore, combination strategies involving immune checkpoint inhibitors and other immunomodulatory approaches require further optimization to enhance therapeutic outcomes while minimizing adverse effects.
Table 4.
Representative nanomedicines for tumor pyroptosis.
| Structure of nanomedicine | Structural diagram | Loaded drugs | Combined therapy | Ref. |
|---|---|---|---|---|
| L@NBMZ |  |
NBSEt, MZ1 and PROTAC | Photodynamic therapy | [189] |
| MPNPs |  |
Mpeg-b-P(MTE-co-PDA) | Immunotherapy | [195] |
| PCL@GSK-diABZI/aPD-1 |  |
diABZI-C2-NH2, GSK | Immunotherapy | [198] |
| Oxidation-sensitive nanoparticles (NP1) and decitabine nanomicelles (NP2) |
 |
Decitabine | Photodynamic therapy | [183] |
| NCSNPs |  |
NLG and SNAP | – | [199] |
| CS-1@PB[HM] NPs |  |
CS-1 | Photodynamic therapy | [200] |
| GSDMBNT mRNA@LNPs |  |
AA3-Dlin LNP | Immunotherapy | [201] |
| PMC |  |
Mn, Ce6 | Photodynamic therapy | [202] |
| TPL@TFBF |  |
TPL, Fe3+, TA | – | [203] |
| TFOS LP |  |
Na2S2O8 | – | [204] |
| CS-HAP@ATO NPs |  |
CS, ATO | – | [205] |
| FeMn@R@H |  |
Fe, Mn, R848 | – | [8] |
| PSNAs |  |
PEI | – | [206] |
| VTPA |  |
Mn, IONPs | – | [207] |
| HSA@BCN and HSA@Tz-Ru1 |  |
Tz-Ru1, Ac4ManN-BCN | Sonodynamic therapy | [208] |
| YBS‐BMS NPs‐RKC |  |
YBS and BMS | Photodynamic therapy | [209] |
Copyright 2021, 2023, 2024, John Wiley and Sons. Copyright 2023, Springer Nature. Copyright 2023, Elsevier. Copyright 2024, American Chemical Society.
4. Introduction of ferroptosis
A type of erastin-induced cell death that can be inhibited by iron chelator deferoxamine was discovered by Dr. Brent R. Stockwell and named as ferroptosis in 2012 [210]. Then, ferroptosis was defined as a novel PCD characterized by the iron-dependent accumulation of lipid peroxides to fatal levels [211]. Compared to other types of morphological changes in PCD, such as cell swelling, chromatin condensation, and nuclear fragmentation, cells undergoing ferroptosis show signs of mitochondrial membrane shrinkage and rupture, along with an increase in mitochondrial membrane density [212]. In recent years, it has been reported that ferroptosis is one of the important mechanisms causing various diseases, such as cancer, neurodegeneration, cardiovascular diseases, autoimmune diseases, endocrine metabolic diseases, and ischemic injuries [[213], [214], [215], [216], [217], [218]]. In order to seek treatment for the diseases above, the mechanism behind ferroptosis has been extensively studied, which can provide potential therapeutic targets and treatment strategies for improved outcomes.
4.1. The existence of iron ions in human body
Iron is one of the most abundant transition metals in the human body. The absorption of iron mainly depends on the intestinal endothelial cells of the duodenum and the upper part of the jejunum, and the iron content in human body of an adult is about 4–5 g [219]. Humans have evolved efficient mechanisms for storing iron but lack a dedicated system for its excretion [220]. The only way to maintain iron homeostasis is regulating iron absorption, specifically, which relies on regulating the levels and function of iron transport proteins. Since IEC turnover approximately every three days, iron stored in these cells is lost in feces [221]. Additionally, a very small amount of iron is excreted into bile, which is the only natural mechanism for excreting iron, totaling a daily loss of 1–2 mg of iron [220]. The distribution of iron in the human body is as follows.
-
●
The iron in hemoglobin: accounts for 60-70 % of the total iron in the human body. Mainly present in red blood cells, responsible for transporting oxygen. Each hemoglobin molecule contains four iron ions [222].
-
●
The iron in hepcidin: accounts for 20-30 % of total iron in the human body. Mainly present in hepatocytes, playing a role in iron storage [223].
-
●
The iron in transferrin: accounts for 5-10 % of the total iron in the human body. Iron ions are transported in the plasma in the form of transferrin-bound [224].
-
●
The iron in myoglobin: accounts for about 5 % of the total iron in the human body. Mainly present in skeletal muscles, responsible for oxygen transport in muscles [225].
-
●
The iron exists in other forms: a small amount of iron ions exists in free form inside and outside the cells. Some iron proteins, such as iron-sulfur proteins, also contain small amounts of iron ions [226].
4.2. The physiological functions of iron ions
Iron, as an essential trace element, performs numerous vital physiological functions in the human body. The specific roles are as follows.
-
1.
Synthesis of hemoglobin and myoglobin: iron ions are one of the important components of hemoglobin and myoglobin, and maintaining normal levels of iron ions ensures an adequate supply of oxygen to organs and muscles [227].
-
2.
Participating in energy metabolism: iron ions participate in energy metabolism mainly through their crucial role in the respiratory chain. For example, COX is an iron-containing enzyme. In the mitochondria of cells, the electron transport chain is a critical step in the generation of ATP [228]. During this process, the iron ions in COX can undergo valence state changes (conversion between Fe2+ and Fe3+), enabling them to accept electrons from other electron carriers and then transfer them to oxygen molecules. This process drives the transport of protons (H+) across the membrane, creating an electrochemical gradient.
-
3.
Immune function: in terms of immune function, iron ions mainly participate in physiological processes related to ferritin and lactoferrin. Ferritin can prevent microorganisms from obtaining excessive iron ions for their own growth and reproduction by regulating the concentration of iron ions, meanwhile, the iron in ferritin can act as an essential cofactor to maintain the stability of the Regulatory T cell lineage and is involved in immune responses [229]. Lactoferrin is a glycoprotein that can bind and transport iron ions. Lactoferrin has antimicrobial activity and plays a role in inflammatory responses and immune regulation [230].
-
4.
Maintaining normal neurological function: in terms of neurotransmitter synthesis, iron ions are involved in the synthesis of neurotransmitters such as dopamine. Iron ions are cofactors for the key enzyme (tyrosine hydroxylase) involved in dopamine synthesis, which converts tyrosine into levodopa (L-DOPA), and subsequently synthesizes dopamine [231]. Iron ions are also crucial in myelination. A deficiency of iron ions leads to poor myelination, which slows the conduction speed of nerve impulses, affecting the transmission of neural signals and resulting in cognitive and motor dysfunction [232].
-
5.
Ensuring normal cell proliferation: in the DNA synthesis process, iron ions participate in the activity regulation of ribonucleotide reductase (RNR). RNR is a key enzyme in DNA synthesis, which converts ribonucleotides into deoxyribonucleotides, the raw materials for DNA synthesis [233]. Iron ions are essential cofactors for RNR, necessary for the enzyme to function. When iron ions are sufficiently supplied, RNR can operate normally, ensuring that cells have enough DNA raw materials during division and proliferation, allowing for smooth replication.
4.3. The balance of iron ions in human body
Both excessive and insufficient iron ions can lead to changes in cellular homeostasis, causing a series of diseases. Only when maintained in a relatively balanced state can iron ions exert normal physiological functions.
-
1.Iron deficiency can lead to the following representative diseases:
-
●Anemia: iron is a critical component of hemoglobin, which is necessary for oxygen transport in the blood. Iron deficiency can result in anemia, which is marked by a reduction in red blood cell count or a deficiency of hemoglobin in the blood [234]. This can result in fatigue, weakness, and shortness of breath.
- ●
-
●Immune system dysfunction: iron deficiency can impair the function of immune system, increasing the risk of infections [236].
-
●
-
2.Iron excess can lead to the following representative diseases:
-
●Liver disease: excess iron can accumulate in the liver, leading to cirrhosis, liver cancer, and liver failure [237]. This is often seen in hereditary hemochromatosis, which is a genetic condition that leads to excessive iron absorption from the diet.
-
●Heart problems: iron overload can cause heart problems, including heart failure and arrhythmias, due to the deposition of iron in the heart muscle [237].
-
●Diabetes: excess iron can impair pancreatic function, which may trigger insulin resistance and diabetes [237].
- ●
-
●Cancer: excess body iron stores or elevated iron intake has been associated with an increased risk of certain cancers, including liver and colon cancer [236].
-
●
4.4. Mechanisms of ferroptosis
Iron-dependent lipid peroxides accumulation is the occurrence basis of ferroptosis. In brief, cellular Fe catalyzes the production of ROS through the Fenton reaction. These ROS then attack polyunsaturated fatty acids (PUFAs) in the lipid bilayers, initiating a deadly cascade of LPO. Simultaneously, LPO accumulation also occurs in mitochondria, which also promotes the ferroptosis to some extent. Additionally, cells also possess a defense system against ferroptosis, such as glutathione peroxidase 4 (GPX4), an enzyme that helps protect cells from oxidative damage. When this defense system collapses, it further exacerbates ferroptosis. There are a total of 6 representative pathways that regulate the accumulation of LPO to influence the occurrence of ferroptosis (Fig. 9).
-
1.
Lipid metabolism: acylcoenzyme A (CoA) synthetase long-chain family member 4 (ACSL4) and lysophospholipid CoA acyltransferase 3 (LPCAT3) facilitate incorporation of PUFAs into membrane lipids to attain PUFA-PLs, then PUFA-PLs are oxidated to LPO by PE-binding protein 1 (PEBP1), P450 oxidoreductase (POR) and arachidonate lipoxygenases (ALOXs), thereby leading to ferroptosis.
-
2.
Iron metabolism: Fe2+ is stored in labile iron pool (LIP) inside the cell. When there is a disturbance in iron metabolism, excessive intracellular Fe2+ leads to the production of a large amount of ROS via the Fenton reaction. The ROS stimulates the generation and accumulation of LPO, ultimately causing ferroptosis.
-
3.
GPX4 and GSH suppress LPO accumulation: inhibiting the transport function of the system Xc− (the cystine-glutamate antiporter consisting of subunits SLC7A11 and SLC3A2) reduces the intake of cystine to reduce the synthesis of GSH, which leads to the inactivation of GPX4 to reduce the clearance of LPO.
-
4.GPX4-independent control of LPO accumulation:
-
●Ferroptosis suppressor protein 1 (FSP1)/ubiquinone-10 (CoQ10) pathway: the downregulation of FSP1 directly inhibits the clearance of LPO by preventing the conversion of CoQ10 to ubiquinol (CoQ10H2).
-
●GTP cyclohydrolase-1 (GCH1)/tetrahydrobiopterin (BH4) pathway: under the catalytic action of GCH1, GTP is converted to BH4, which not only directly reduces LPO, but also generates CoQ10H2 to further decrease LPO.
-
●Dihydroorotate dehydrogenase (DHODH) pathway: inactivation of mitochondrial DHODH hinders the generation of CoQ10H2, which further leads to a decrease in mitochondrial LPO clearance.
-
●
-
5.
The amino acid oxidase interleukin-4-induced-1 (IL4i1)- mediated pathway: the indole-3-pyruvate (In3Py) generated by IL4il inhibits ferroptosis by scavenging free radicals and modulating the expression of genes that mitigate ferroptosis.
-
6.
Mitochondrial lipid peroxidation: the accumulation of LPO can also occur in mitochondria, and this process can be inhibited by GPX4 in mitochondria and CoQ10H2 generated through DHODH catalysis.
Fig. 9.
Illustration shows that the disorder of lipid metabolism and iron metabolism in cells induces the accumulation of lipid peroxide and ferroptosis, and the cells prevent ferroptosis by producing reducing substances. Created in BioRender. Zhang, Z. (2025) https://BioRender.com/q45f149.
4.5. Nanomedicine for tumor ferroptosis
In many kinds of tumors such as breast cancer, liver cancer, clear cell renal cell carcinoma, melanoma, and ovarian cancer cells, the induction of ferroptosis has been shown to inhibit tumor growth, development, and metastasis [[239], [240], [241], [242], [243]]. A growing number of ferroptosis inducers have been discovered for tumor chemotherapy, however, direct use of ferroptosis inducer is not an ideal approach, primarily due to their shortcomings: rapid excretion, significant side effects, low tumor accumulation, and more crucially, their dependence on a single mechanism to trigger ferroptosis, which renders them vulnerable to cellular defense mechanisms, thus reducing the efficacy of treatmen [244,245]. Nanomedicines release ferroptosis inducers during their biodegradation process, activating multiple ferroptosis pathways, which can generate synergistic effects to enhance the therapeutic efficacy of nanomedicine. Nanomedicine modification can enhance specificity towards tumor tissue, prolonging drug circulation and action [191,192,246]. Furthermore, these nanomedicines can activate other PCD pathways simultaneously (such as autophagy and apoptosis) to exert their therapeutic effects, or enhance the therapeutic effect of classical cancer treatment strategies such as chemotherapy, radiotherapy, immunotherapy, and PTT, which render nanomedicine superior to conventional ferroptosis inducers [10,[247], [248], [249], [250], [251]].
Among the above-mentioned mechanisms, iron metabolism is considered the most fundamental mechanism of ferroptosis, and the GPX4-dependent mechanism is first discovered and studied, so researchers have focused on these two mechanisms to develop nanomedicines. For example, A novel nanomedicine, Fe3O4@PCBMA-SIM, was designed to treat triple-negative breast cancer (TNBC) by inducing ferroptosis [251,252]. Firstly, Fe3O4@PCBMA was synthesized from Fe3O4 and carboxymethyl methacrylate (CBMA) by modified reflux precipitation polymerization method, then simvastatin (SIM) was loaded into Fe3O4@PCBMA (Fig. 10A). With the increasing GSH concentration and the decreasing pH value, Fe3O4@PCBMA-SIM represented powerful biodegradability, releasing substantial SIM and Fe2+. The generation of GPX4 is related to the mevalonate (MVA) pathway under the action of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR). Therefore, SIM was used to inhabit HMGCR to adjust the MVA pathway and reduce the production of GPX4. As a key factor in ferroptosis, the lack of GPX4 induced the accumulation of LPO. In addition, the Fe2+ catalyzed the formation of highly ROS through the Fenton reaction, exacerbating the accumulation of LPO to enhance ferroptosis (Fig. 10B). As is well known, detecting the accumulation of LPO is reasonable evidence for detecting ferroptosis, which exhibited that the Fe3O4@PCBMA-SIM group engendered the most ROS and LPO.
Fig. 10.
A) Synthesis and biodegradation process of Fe3O4@PCBMA-SIM nanomedicine. B) Schematic diagram of ferroptosis induced by Fe3O4@PCBMA-SIM. Reprinted with permission [252]. Copyright 2021, Springer Nature.
In addition to using Fe2+ to induce ferroptosis through the Fenton reaction to generate ROS, Mn2+ can also generate ROS through a process called a Fenton-like reaction, leading to ferroptosis though two pathways. A nanocleaner designed to eliminate GSH for eradicating tumors through ferroptosis has been developed [253]. Manganese-doped silica nanoparticles (MnMSN) are part of this nanomedicine, with the unique structure of Mn-O bonds being easily reduced by GSH. By loading sorafenib onto MnMSN to obtain MnMSN@SFB and further modifying the surface with folate-grafted PEG (FaPEG) through silica hydrolysis reaction, the final nanomedicine named FaPEGMnMSN@SFB is obtained. In the TME, GSH can reduce the Mn-O bond in MnMSN, while being oxidized to GSSG. The decomposition of Mn-O bond consumes GSH, thereby reducing the intracellular GSH level. Sorafenib can block the transport system, inhibit GSH biosynthesis, further reduce intracellular GSH content, leading to the inactivation of GPX4 and accumulation of LPO. In the presence of Mn2+ catalysis, more ·OH is generated through Fenton-like reaction, further exacerbating oxidative stress and promoting ferroptosis.
Unlike the example above, there are also nanomedicines that focus on the accumulation of LPO inside mitochondria, ultimately triggering ferroptosis via three mechanisms (DHODH pathway, iron metabolism, and GPX4-dependent mechanisms). An albumin-based nanomedicine co-loaded with sorafenib and atovaquone (ATO, used in the clinic that targets mitochondria) was designed to induce the augmented ferroptosis in TNBC (Fig. 11A), which can target tumor tissue due to the binding affinity with secreted protein acidic and rich in cysteine (SPARC) and glycoprotein 60 (gp60) [254]. The sorafenib decreased the synthesis of GSH by inhibiting the transport activity of the System Xc−. As a substrate of GPX4, the diminished GSH limited the GPX4 to revert reactive lipid peroxides produced by Fe2+-mediated Fenton reaction, leading to the occurrence of ferroptosis (Fig. 11B). In addition, ATO was used to inhibit the activity of DHODH in mitochondria, which could enhance ferroptosis by both preventing the conversion of CoQ10 to reductive DHODH-CoQ10H2 to decrease the removal of peroxidized lipids and damaging the repair system of tumor cells. Simultaneously, ATO competitively bound to mitochondrial complex III and cytochrome C to inhibit the oxidative phosphorylation process, and increased production of ROS, further enhancing the accumulation of peroxidized lipids. Compared to other groups, the ATO/SRF@BSA-treated group exhibited the hallmark mitochondrial morphology of ferroptosis, such as atrophic mitochondria and vague membrane edges after treatment, which verified that ATO/SRF@BSA induced ferroptosis (Fig. 11C).
Fig. 11.
A) Synthesis process of ATO/SRF@BSA nanomedicine. B) A schematic diagram illustrates the mechanisms of ferroptosis induced by ATO/SRF@BSA. C) TEM images of mitochondria of 4T1 cells after receiving different administrations (scale bar = 500 nm). Reprinted with permission [254]. Copyright 2024, Elsevier.
Similarly, a hyaluronic acid (HA)-based iron nanomedicine called FHA NPS has also been developed for inducing ferroptosis through three pathways [255]. HA was a naturally occurring polysaccharide with excellent biocompatibility and a ligand for the CD44 receptor overexpressed on tumor cells, allowing the nanomedicine to target and kill tumor cells. When this drug bound to CD44 on the surface of tumor cells and was taken up by the cells, it releases iron ions to generate a large amount of ROS through the Fenton reaction, leading to the accumulation of LPO in the cells. Additionally, it depletes GSH to inactivate GPX4 and reduces intracellular levels of vitamin E and ferrostatin-1, a ferroptosis inhibitor, resulting in the decreased clearance of lipid peroxides in the cells and ferroptosis. Furthermore, a nanomedicine that induce ferroptosis through four mechanisms was developed by Wang and his colleges [256]. Firstly, PEGylated SP94 (an amino acid sequence which can specifically bind to receptors on the surface of liver cancer cells, exhibiting targeting ability) is combined with Graphdiyneoxide (GDYO) to generate GDYO@SP94. Then, DOX-Fe2+, sorafenib, and SLC7A11-i are loaded onto GDYO@SP94 to obtain the nanomedicine GDYO@SP94/DOX-Fe2+/sorafenib/SLC7A11-i (MNMG). This nanomedicine enters cells through endocytosis and undergoes biodegradation in acidic lysosomes to release Fe2+, DOX, sorafenib, and SLC7A11-i. The large amount of ROS generated through the Fenton reaction by Fe2+, and the ferroptosis-induced effect of Fe2+ was further enhanced in the presence of abundant H2O2 produced by DOX. Sorafenib directly inhibits System XC− reducing the production of intracellular GSH, decreasing the activity of GPX4, disrupting the stability of the cellular redox system, leading to the accumulation of LPO and the occurrence of iron-induced cell death. On the other hand, SLC7A11-i affects the translation of System XC− related RNA, leading to reduced expression of System XC−, thereby controlling the activity of GPX4 to induce ferroptosis. The treatment effect is greatly enhanced by synergistically inducing iron-dependent cell death through four distinct mechanisms.
In summary, nanomedicines have emerged as a highly promising approach for inducing ferroptosis (Table 5). Their unique physicochemical properties enable precise regulation of the intricate network of ferroptosis-related signaling pathways, offering significantly greater efficacy compared to conventional therapies. Through various modifications, nanomedicines can achieve highly specific and targeted delivery to pathological cells or tissues, maximizing therapeutic effects while minimizing potential damage to surrounding healthy tissues. Additionally, nanomedicines function as exceptional drug carriers, particularly for delivering ferroptosis inducers, allowing for the co-delivery of multiple therapeutic agents. Their superior physical and chemical stability, compared to traditional chemotherapeutic drugs, reduces toxicity and adverse side effects. Furthermore, their sustained-release properties enable the controlled, gradual release of loaded drugs, extending their duration of action and facilitating continuous ferroptosis induction. These advantages collectively contribute to improved and more durable therapeutic outcomes.
Table 5.
Nanomedicines for inducing cancer ferroptosis.
| Structure of nanomedicines | Tumor targeting methods | Ferroptosis inducing mechanisms | Loaded drugs | Ref. |
|---|---|---|---|---|
| FaPEGMnMSN@SFB | FaPEG modification |
|
|
[253] |
| ATO/SRF@BSA | SPARC and gp60 modification |
|
|
[254] |
| FHA NPS | HA modification |
|
[255] | |
| GDYO@SP94/DOX-Fe2+/sorafenib/SLC7A11-i | PEGylated SP94 modification |
|
|
[256] |
| CM-DHA@GSSF | A549 cell membrane modification |
|
|
[257] |
| iRGD - PEG/AuNCs@FePt | Targeting peptide iRGD modification |
|
[258] | |
| FeS2@CP | PEG-bPEI modification |
|
[250] | |
| Fe3O4@PCBMA-SIM | EPR |
|
|
[252] |
| BC - LNPs@PTX | EPR |
|
|
[259] |
| PEG - PDA@Mn | EPR |
|
[260] | |
| SQ890@Fe | EPR |
|
|
[261] |
| PFC/TRIM37@Fe - TA@HA | HA modification |
|
|
[262] |
| Fe3O4@Chl/Fe | Carboxyphenoxyboronic acid modification |
|
[263] | |
| IONP@PTX | EPR |
|
|
[264] |
| Fe3O4-PEG-PLGA | EPR |
|
[265] | |
| PION@E6 | EPR |
|
[266] | |
| DOX@HFON | Supermagneticness |
|
|
[267] |
| BSO&OXA@MOF-LR | Tumor homing peptide RGD modification |
|
|
[268] |
| HSN | EPR |
|
|
[269] |
| mSiO2@MnOx-mPEG | EPR |
|
|
[270] |
Despite the promising potential of ferroptosis-based nanomedicine, several limitations remain. First, the effectiveness of ferroptosis is highly dependent on intracellular iron levels, ROS generation, and the suppression of antioxidant systems. However, tumor cells often develop compensatory mechanisms, such as upregulation of GPX4 or enhanced GSH synthesis, to resist ferroptosis. Additionally, the heterogeneous tumor microenvironment—such as hypoxia or acidic conditions—can interfere with drug release and ferroptosis induction efficiency. Therefore, improving the stability and targeting ability of nanocarriers, and enhancing their synergy with ferroptosis pathways or other therapies, is crucial for achieving better therapeutic outcomes.
5. Nanomedicine for combined PCD
Combining distinct PCD pathways targets complementary vulnerability nodes in cancer cells, thereby enhancing antitumor efficacy by activating diverse cell death mechanisms, such as cuproptosis, pyroptosis, and ferroptosis. Since these pathways operate through distinct mechanisms, relying on a single pathway may fail to eliminate tumor cells entirely. Tumor cells can adapt and develop resistance to single-mode therapies, diminishing treatment effectiveness [271]. By integrating multiple PCD pathways, this approach addresses tumor resistance and reduces the risk of therapeutic failure due to drug resistance. Nanomedicine plays a pivotal role in this strategy, as it enables the simultaneous delivery of multiple drugs or integrates external stimuli, such as PTT or radiotherapy [21,24]. Additionally, nanomedicine offers superior targeting accuracy and controlled release capabilities, maximizing therapeutic efficacy. To achieve these advantages, various shared nanomedicine design strategies have been developed to promote the activation of different PCD pathways (Table 6). The combined PCD pathway strategy in nanomedicine represents a promising direction for cancer therapy. Its capacity to enhance treatment efficacy, reduce side effects, and enable personalized and precise interventions positions it as a transformative approach in oncology.
Table 6.
Shared nanomedicine design strategies for PCD pathways.
| Shared strategies | PCD | Structure of nanomedicine | Ref. |
|---|---|---|---|
| pH-responsive release | Cuproptosis | DMMA@Cu2-xSe, CLDCu, BSO-CAT@MOF-199@DDM | [76,84,87] |
| Pyroptosis | CaNMs, ANPS, PAO@ZIF-67 | [190,272,273] | |
| Ferroptosis | GDYO@SP94/DOX-Fe2+/sorafenib/SLC7A11-i | [256] | |
| GSH-responsive release | Cuproptosis | GOx@[Cu(tz)], CCJD-FA | [77,82] |
| Pyroptosis | SAS/DOX@OHS-PEG | [274] | |
| Ferroptosis | FaPEGMnMSN@SFB | [253] | |
| ROS-triggered release | Cuproptosis | SPNLCu, NP@ESCu, ECPCP | [56,80,81] |
| Pyroptosis | L@NBMZ | [189] | |
| Ferroptosis | Fe3O4@GOx@Tuftsin | [275] | |
| ROS/pH dual-responsive release | Pyroptosis | MPNPs | [195] |
| GSH/pH dual-responsive release | Ferroptosis | Fe3O4@PCBMA-SIM | [252] |
| Metal ion delivery | Cuproptosis | Cel-Cu NP, HCu nanowires, PEG@Cu2O-ES | [23,88,89] |
| Pyroptosis | FeMn@R@H | [8] | |
| Ferroptosis | ZCPO@HA | [276] |
5.1. Nanomedicines for synergistic induction of cuproptosis and pyroptosis
The synergistic induction of cuproptosis and pyroptosis through nanomedicine represents a promising strategy to enhance anti-tumor immunity. Both cell death mechanisms amplify oxidative stress, disrupt mitochondrial metabolism, and remodel the tumor immune TME, collectively promoting robust anti-tumor responses. Leveraging nanocarriers for combined therapies offers advantages such as improved drug delivery efficiency, minimized side effects, and reduced risk of resistance compared to monotherapies. Several innovative approaches have been developed to exploit these synergistic effects. Liu et al. reported a MOF-based nanovaccine, Cu-THBQ/AX, which transformed immunologically “cold” tumors into “hot” tumors by promoting oxidative stress and mitochondrial dysfunction [277]. The nanovaccine triggered ROS production, activated PLC, and increased intracellular calcium levels, leading to mitochondrial apoptosis, ATP depletion, and suppression of the copper efflux protein ATP7A. These effects caused copper ion accumulation and cuproptosis, while PLC-mediated signaling induced inflammatory pyroptosis. Similarly, Zhu et al. developed degradable copper-doped layered double hydroxide (Cu-LDH) nanoparticles that initiated cuproptosis and pyroptosis through lysosomal rupture [278]. Copper ion overload disrupted mitochondrial function, while inflammasome activation and caspase-1 cleavage of GSDMD initiated pyroptosis, releasing DAMPs and enhancing immune activation. Wang et al. designed a multifunctional platform, hCZAG, using GOx to drive oxidative stress and synergistically induce these cell death mechanisms [279]. ROS accumulation triggered DLAT protein aggregation for cuproptosis and activated caspase-1 for pyroptosis, further releasing DAMPs and enhancing adaptive immune responses.
Other nanoplatforms demonstrated unique strategies to enhance the synergy between cuproptosis and pyroptosis. Zhao et al. introduced a tumor-targeted copper-based therapy utilizing Cu2(PO4)(OH) nanoparticles activated by H2S, which transformed into ultrasmall Cu9S8 nanoparticles in the H2S-rich TME [62]. The transformation enhanced ROS production, disrupted mitochondrial function, and downregulated ATP7A, inducing cuproptosis. Concurrently, ROS-mediated inflammasome activation triggered pyroptosis, remodeling the TME and amplifying systemic anti-tumor immunity. Qiao et al. designed copper-quinone-GOx nanoparticles (CQG NPs), inspired by “Taiji philosophy,” to integrate these mechanisms into a self-reinforcing therapeutic approach (Fig. 12A) [280]. CQG NPs depleted GSH, alleviated hypoxia-induced suppression of the NLRP3 inflammasome, and disrupted antioxidant defenses. Copper ions released by CQG NPs induced cuproptosis, while H2O2 generated by GOx activated caspase-1 to trigger pyroptosis, effectively reversing the immunosuppressive TME (Fig. 12B). Finally, Zhu et al. developed Cu-Pic/HA nanoparticles to deplete intracellular polyamines and synergistically induce cuproptosis and pyroptosis [22]. Polyamine depletion disrupted mitochondrial function, causing copper ion accumulation and toxic protein aggregation for cuproptosis. Concurrently, NLRP3 inflammasome activation and caspase-1 activity promoted pyroptosis, with enhanced palmitoylation efficiency of GSDMD amplifying the process. The combined pathways remodeled the TME, activating robust systemic immune responses. Together, these innovative strategies demonstrate the transformative potential of integrating cuproptosis and pyroptosis into nanomedicine to overcome tumor resistance and improve immunotherapy outcomes, offering a robust dual-strike approach for durable anti-tumor immunity.
Fig. 12.
A) The preparation process of CQG NPs. B) A schematic illustration depicting the synergistic anticancer effects of cuproptosis and pyroptosis triggered by CQG NPs. Reprinted with permission [280]. Copyright 2023, John Wiley and Sons.
The core of the synergistic mechanism between cuproptosis and pyroptosis lies in the fact that oxidative stress and mitochondrial dysfunction induced by cuproptosis provide key activating signals for pyroptosis [277]. In cuproptosis, copper ion accumulation leads to DLAT protein aggregation, impairing mitochondrial respiration and inducing proteotoxic stress. This process generates high levels of ROS, which serve as key activators of inflammasomes and create a permissive environment for pyroptosis [279]. Several signaling pathways, including PLC/Ca2+ influx and H2O2-mediated oxidative signals, act as bridges between the two mechanisms [277]. Additionally, copper ions exacerbate cellular stress by depleting antioxidant defenses such as GSH and downregulating the copper export protein ATP7A. These changes not only reinforce cuproptosis through a positive feedback loop but also intensify ROS signaling, further promoting inflammasome activation [280]. The coordinated activation of cuproptosis and pyroptosis enhances tumor cell killing while promoting the release of Immunomodulatory factors that reshape the immunosuppressive tumor microenvironment and elicit a more robust systemic anti-tumor immune response [22].
5.2. Nanomedicines for synergistic induction of ferroptosis and cuproptosis
Ferroptosis and cuproptosis are two distinct forms of PCD that share certain mechanistic similarities. Both pathways can promote the accumulation of ROS through Fenton or Fenton-like reactions, exacerbating cellular damage. The mechanisms also deplete the cellular antioxidant system, leading to GSH depletion, and induce mitochondrial dysfunction. Combining these pathways can significantly enhance the cytotoxic effects on tumor cells, providing a novel and promising strategy for cancer treatment. Some nanomedicines that synergize ferroptosis and cuprotosis have been proposed to enhance their therapeutic efficacy. Chen et al. proposed a novel Cellular Trojan Horsecell termed MetaCell for targeted cancer intervention, leveraging combined cuproptosis and ferroptosis [79]. By cleverly encapsulating the thermosensitive liposomal bimetallic Fe-Cu metal-organic frameworks (Lip@Fe-Cu-MOFs) in living neutrophils, immune cells are transformed into specialized carriers to target tumor cells (Fig. 13A). Upon cellular uptake, Lip@Fe-Cu-MOFs released Fe-Cu-MoF, which had the property of producing local heat under NIR light, when locally heated to a specific temperature. The property promoted photothermal effects and induced cuproptosis and ferroptosis simultaneously (Fig. 13B). Fe3+ and Cu2+ within Lip@Fe-Cu-MOFs catalyzed Fenton/Fenton-like reactions, generating ROS, depleting GSH, and disrupting cellular antioxidant defenses, leading to ferroptosis. Additionally, Cu2+ induced the oligomerization of lipoylated DLAT, a specific component of the TCA cycle, affecting mitochondrial function, thereby causing cuproptosis. Studies have demonstrated that Lip@Fe-Cu-MOFs had efficacy in targeting solid tumors, significantly inhibiting tumor growth. The integrated approach, combining the disruption of metal ion homeostasis with PTT, shows great promise for next-generation cancer treatments. Simultaneously, Zhu et al. developed a carrier-free nanoparticle, Ce6@Cu NPs, by combining Cu2+ with the sonosensitizer Ce6 to promote cuproptosis and ferroptosis [281]. Ce6@Cu NPs exhibited excellent blood-brain barrier (BBB) penetration, allowing for substantial accumulation at tumor sites. Once internalized by cancer cells, Ce6@Cu NPs was activated by US, promoting the elevation of 1O2 levels that induced the oxidation of PUFAs, subsequently causing lethal LPO. The generated 1O2 also depleted GSH, inhibiting GPX4 activity and further accelerating LPO, thereby inducing irreversible ferroptosis. Additionally, the increase of Cu+ was caused by the reaction of Cu2+ and reductive GSH, reducing the expression of FDX-1 and LIAS. The process caused the oligomerization of DLAT, leading to proteotoxic stress and cuproptosis. In vivo experiments demonstrated that, when irradiated with US, Ce6@Cu NPs possessed a higher cytotoxicity and had a significant inhibitory effect on the growth of tumor.
Fig. 13.
A) The preparation process of MetaCell. B) A schematic illustration depicting the synergistic anticancer effects of cuproptosis and ferroptosis triggered by MetaCell. Reprinted with permission [79]. Copyright 2024, American Association for the Advancement of Science.
In summary, although copper and iron maintain distinct homeostatic regulatory pathways, emerging evidence highlights a growing synergy in their roles in mediating PCD. Copper can facilitate the generation of ROS through Fenton-like reactions, thereby exacerbating iron-dependent lipid peroxidation, a hallmark of ferroptosis [79]. In parallel, copper-induced disruption of antioxidant defense systems, such as glutathione depletion or inhibition of antioxidant enzymes, may further sensitize cells to ferroptotic damage [281]. Moreover, copper transporters, including CTR1/2 and ATP7A/B, not only regulate intracellular copper homeostasis but may also indirectly modulate ferroptotic sensitivity by influencing cellular redox dynamics [58,59]. These insights suggest that dual targeting of copper and iron metabolism could represent a promising therapeutic strategy to enhance specificity and efficacy while potentially overcoming resistance mechanisms in cancer treatment. Copper also interacts with other metal, such as zinc, a cofactor of copper-zinc superoxide dismutase (SOD1), works in concert with copper to regulate the antioxidant defense [67]. In addition, the zinc transporter ZnT1 can facilitate the entry of Cu2+ into cells, thereby contributing to intracellular copper distribution and metabolism [282]. Manganese, on the other hand, has been shown to bind tumor antigens released during copper-induced cell death, triggering a robust anti-tumor immune response and offering a novel strategy for cancer therapy [283].
5.3. Nanomedicines for synergistic induction of ferroptosis and pyroptosis
The combined application of ferroptosis and pyroptosis can leverage their complementary effects, significantly enhancing the elimination of tumor cells. Both pathways promote tumor suppression by generating ROS and disrupting cellular homeostasis. The processes create an inflammatory environment that activates anti-tumor immunity, further strengthening the immune response against cancer. Additionally, traditional medicines that induce pyroptosis have limitations such as poor targeting and low induction efficiency. Therefore, novel nanomedicines that can synergistically induce ferroptosis and pyroptosis have been proposed to improve these limitations and synergistically enhance therapeutic efficacy. Zhang et al. designed a dual inducer of ferroptosis and pyroptosis named COF-919, which was obtained by integrating aggregation-induced emission luminogens into a non-metallic covalent organic framework (COF) [284]. After entering the cells, the PDT and PTT induced by COF-919 promoted the occurrence of ferroptosis and pyroptosis. Firstly, COF-919 has a good ability to generate ROS, depleting intracellular GSH, leading to low expression of GPX4 and causing the accumulation of LPO in cells, ultimately resulting in ferroptosis. Secondly, COF-919, with its strong NIR light absorption and low energy band characteristics, triggers pyroptosis through photothermal conversion and ROS generation. Specifically, COF-919 activates caspase-3 and cleaves GSDME to generate GSDME-N, which aggregates to form membrane pores, releasing cellular contents and thereby exacerbating inflammation and pyroptosis. Western blot results indicate low expression of GPX4 and high expression of GSDME-N in tumor tissues, confirming the feasibility of COF-919 in inducing ferroptosis and pyroptosis. Li and her team also proposed a new nano micelle named NO/PTX, which delivers paclitaxel (PTX) loaded into a biodegradable polymer micelle composed of monomethoxypoly(ethylene glycol) and polylactic acid (mPEG-PLA-NO) [285]. After being taken up by tumor cells, NO/PTX significantly reduced the levels of intracellular GSH peroxidase and SOD, leading to the synthesis of a large amount of ROS within the cell. High levels of ROS, on one hand, deplete intracellular GSH, inhibit the function of GPX4, causing the accumulation of LPO and ultimately resulting in ferroptosis. On the other hand, it activates the inflammasome, upregulates the levels of caspase-1, thereby activating GSDMD and causing cell membrane rupture and exacerbation of inflammation, which in turn promotes the maturation and secretion of cytokine precursors pro-IL-1β and pro-IL-18, ultimately leading to pyroptosis.
Recent studies have revealed significant crosstalk between ferroptosis and pyroptosis, primarily mediated through oxidative stress and inflammatory signaling [285]. Lipid peroxidation products generated during ferroptosis can activate the NLRP3 inflammasome, leading to the cleavage of GSDMD and the execution of pyroptosis [285]. Moreover, GPX4, a key negative regulator of ferroptosis, also modulates ROS levels that influence inflammasome activation [286]. The NF-κB pathway, often triggered by ROS, further promotes the expression of pro-inflammatory cytokines such as IL-1β and IL-18, facilitating the convergence of ferroptotic and pyroptotic cell death [287]. This crosstalk highlights the potential for dual-targeting strategies in inflammatory tumors.
5.4. PCD-based nanomedicine in combination with clinically useable tumor therapies
Combining PCD-based nanomedicine with clinically established tumor therapies presents a promising strategy to enhance therapeutic efficacy and overcome treatment resistance. Traditional therapies such as chemotherapy, PDT, and radiotherapy can interact with PCD pathways, either by inducing or enhancing cell death, thus creating opportunities for synergistic therapeutic designs [76,92,95]. For instance, PDT generates ROS, which can trigger ferroptosis by inducing lipid peroxidation [288]. Nanosystems co-delivering photosensitizers and ferroptosis inducers such as iron ions or GPX4 inhibitors have demonstrated enhanced tumor inhibition via dual ROS-mediated pathways [289]. Chemotherapy drugs like doxorubicin and cisplatin have been shown to activate pyroptosis by inducing GSDME cleavage through caspase-3 activation [290]. When delivered via nanocarriers along with pyroptosis sensitizers (e.g., LPS, inflammasome activators), these drugs can shift cancer cell death modes and enhance immunogenicity [291]. Radiotherapy has also been reported to sensitize cells to ferroptosis by disrupting redox balance and increasing intracellular iron levels, which can be further amplified by nanocarriers carrying ferroptosis activators [292]. Moreover, nanomedicines can improve the delivery and tumor selectivity of conventional therapies, reduce systemic toxicity, and enable controlled activation in response to tumor microenvironmental cues [293]. These combined strategies not only enhance therapeutic potency but also broaden the spectrum of responsive tumors, including those resistant to monotherapies [294]. In summary, integrating PCD-based nanomedicine with clinically used tumor therapies leverages multiple death pathways, improves therapeutic specificity, and holds great promise for translational oncology. Future research should further explore the mechanistic basis of PCD synergy with conventional treatments to develop rational combination regimens.
More importantly, beyond their direct cytotoxic effects, PCD-inducing nanomedicines can modulate immune responses by regulating the expression of immune checkpoint molecules such as PD-L1. Targeting PD-L1 has emerged as an effective strategy to block immune checkpoints and activate cytotoxic T cells [295]. Recent studies have underscored the potential of PCD-based nanomedicines in enhancing antitumor immunity through immune modulation [[295], [296], [297]]. For example, a nanoplatform combining cisplatin with metformin-modified chitosan was shown to disrupt mitochondrial function and effectively suppress PD-L1 overexpression in tumor cells [296]. Berberine (BBR) has also been reported to inhibit PD-L1 overexpression, helping to overcome resistance to photodynamic immunotherapy [297]. In addition, a variety of agents—including siRNA, CRISPR-based tools, microRNAs, and peptides—have been explored for their ability to directly or indirectly suppress PD-L1 expression [295]. These approaches highlight the potential of integrating PCD-inducing strategies with immune checkpoint modulation, and many more possibilities remain to be explored in future research. Compared with conventional treatment strategies that primarily rely on non-specific cytotoxicity or broad-spectrum immune activation, PCD-inducing nanomedicines offer a more targeted and multifaceted approach [298]. PCD-inducing nanomedicines serve as both a bridge and an amplifier in modulating immune checkpoints: they not only suppress immune evasion by downregulating PD-L1 through intracellular signaling pathways, but also enhance antitumor immunity by activating immune responses [195].
5.5. Synergistic nanotherapeutic strategies targeting multiple PCD pathways
Multifunctional nanoplatforms that simultaneously activate multiple PCD pathways can achieve multidimensional tumor eradication. Different PCD mechanisms are interlinked through oxidative stress amplification and redox imbalance, mitochondrial dysfunction and metabolic collapse with inflammatory signaling activation, forming positive feedback loops that amplify death signals (Fig. 14) [79,284]. For instance, oxidative stress and mitochondrial dysfunction can concurrently initiate several death pathways, and stimulate antitumor immune responses [280]. Rather than occurring in isolation, these PCD modalities often share upstream triggers—such as ROS accumulation, metal ion imbalance, and inflammasome activation—facilitating functional coupling and synergistic amplification [79,285]. This coordinated mechanism not only enhances cytotoxicity and raises the threshold for therapeutic resistance but also effectively remodels the tumor immune microenvironment [22]. Such synergy provides a theoretical foundation for the rational design of nanomedicines, steering them toward multi-target, multi-mechanism integrated strategies for precision cancer therapy.
Fig. 14.
Crosstalk among ferroptosis, pyroptosis, and cuproptosis pathways. Created in BioRender. Zhang, Z. (2025) https://BioRender.com/c5c7bnw.
6. Challenges and future perspectives
Nanomedicine, as an innovative drug delivery system, has garnered significant attention in recent years, particularly within the field of drug development. PCD-targeting nanomedicines, which are specifically designed to induce cancer cell death through targeted mechanisms, hold tremendous research potential. However, their progress is constrained by several limitations and challenges, primarily associated with the design, characterization, and performance of these nanomedicines under experimental conditions. Overcoming these obstacles is essential for driving meaningful advancements in this promising field. The following sections outline the representative drawbacks, limitations, and challenges in nanomedicine for PCD.
-
1.
Targeting specificity: Targeting specificity remains a significant challenge in nanomedicine, particularly in delivering therapeutics selectively to tumors while minimizing effects on healthy tissues. Although ligands such as RGD peptides and folic acid (FA) show promise for tumor targeting, their clinical use is often limited by factors including variable receptor expression across tumors, instability of ligands under physiological conditions, and unintended interactions that cause off-target toxicity. In particular, certain receptors targeted by these ligands may also be expressed in normal cells, reducing targeting specificity and increasing the risk of off-target toxicity. For instance, FA targets the folate receptor alpha (FR-α), which is frequently overexpressed in cancers such as ovarian and lung adenocarcinomas; however, FR-α is also expressed in healthy tissues like the kidneys, leading to unintended accumulation and off-target toxicity [299]. These challenges complicate the development of precise delivery systems capable of distinguishing cancerous from normal cells. To address this, it is crucial to refine current targeting strategies by exploring multifunctional or environment-responsive systems that can adapt to the complex and heterogeneous nature of the tumor microenvironment, thereby improving targeting accuracy and therapeutic efficacy.
-
2.
Cell Type Specificity: PCD pathways display distinct activation patterns depending on the cell type, which has important implications for the design and effectiveness of nanomedicine-based therapies. For example, GSDME-mediated pyroptosis is more common in breast and colorectal cancers due to higher GSDME expression [125]. Ferroptosis is frequently observed in hepatocellular and pancreatic cancers, where iron metabolism is often disrupted [300]. Likewise, cuproptosis tends to occur more often in lung adenocarcinoma and kidney cancer, which are characterized by elevated mitochondrial activity and copper accumulation [59]. These differences make it challenging to apply PCD-based nanomedicines universally. To improve selectivity and reduce unintended effects on healthy cells, nanomedicines should be tailored to the dominant PCD pathway in each tumor type. This can be achieved through the use of stimuli-responsive release systems and selective surface modifications based on cell-specific markers.
-
3.
Pharmacokinetics and biosafety: nanomedicines have emerged as a promising tool for targeted tumor therapy due to the unique size and properties [301]. The pharmacokinetics of nanomedicines, which involves the distribution, metabolism, and elimination of nanomedicines, is crucial for ensuring the desired results in treatment [302]. To achieve this, a comprehensive understanding of the interactions between nanomedicines and biological barriers is crucial, as these interactions directly influence the biodistribution of the nanomedicine [303,304]. Furthermore, the development of nanomedicines with adjustable elimination rates holds potential in minimizing the risk of off-target accumulation, which may cause undesirable side effects [305]. Degradable nanomedicines, along with those designed for controlled release, are being explored as strategies to enhance removal, thereby reducing long-term toxicity concerns and ensuring sustained therapeutic efficacy [306].
-
4.
Systemic toxicity: the systemic toxicity of nanomedicines is a critical issue in clinical applications, primarily due to the tendency to accumulate in healthy tissues and organs [307,308]. Liver and kidney toxicity are particularly concerning, as these organs are essential for detoxifying and filtrating of nanomedicines [309,310]. The liver is particularly vulnerable to toxicity due to its function in processing foreign substances like nanomedicines. Similarly, the kidneys, responsible for the elimination of waste products, are susceptible to nanomedicine accumulation, leading to potential damage and impaired organ function [311]. The challenge highlights the necessity for effective strategies to reduce nanomedicine buildup in unintended organs, thus improving the effectiveness and overall performance of nanomedicines [312]. Moreover, successful therapeutic delivery requires overcoming biological barriers such as the blood-brain barrier and cellular membranes, making the development of advanced carrier systems for efficient intracellular drug delivery essential [313].
-
5.
Bioavailability and clearance: the bioavailability and systemic circulation of nanomaterials, such as MOFs and liposomes, face significant challenges due to rapid clearance by the immune system, primarily through the mononuclear phagocyte system [314,315]. The rapid elimination significantly reduces the bioavailability of nanomaterials at the intended tumor sites, limiting the therapeutic potential [316,317]. Additionally, nanomedicines exhibit non-selective distribution, which can result in the particles accumulating in unintended areas. The improper accumulation reduces treatment effectiveness and disrupts the intended therapeutic outcome [318,319]. While the unique properties of nanomedicines offer significant potential for enhanced tumor targeting, controlling the accumulation of nanomedicines in non-target organs remains a major obstacle [320,321]. Addressing the challenge requires the development of strategies to manipulate nanomedicine clearance pathways, improving the targeting efficiency of nanomedicines and optimizing clinical application [322,323].
-
6.
Therapeutic resistance: the effectiveness of nanomedicines in cancer therapy is significantly hindered by the development of resistance mechanisms in tumor cells [324]. Upon exposure to nanomedicines that induce PCD, tumor cells can activate various survival pathways, allowing them to evade the therapeutic effects, thereby undermining the success of nanomedicine-based treatments [325]. Furthermore, tumor heterogeneity exacerbates the challenge of selectively targeting malignant cells [326]. The tumor microenvironment, which is characterized by variable pH, enzyme activity, and ROS levels, contributes to inconsistent drug release, diminishing the therapeutic outcomes [327]. Current advancements emphasize the development of nanomedicine systems that leverage microenvironmental factors, such as pH-responsive drug release, enzyme-triggered activation, and ROS-sensitive mechanisms [328]. The strategies aim to enhance the precision of nanomedicines, improving the selective targeting of cancer cells while minimizing off-target effects [329]. By optimizing the interplay between nanomedicines and the tumor microenvironment, the approaches seek to improve both therapeutic efficacy and safety, offering a promising avenue for advancing cancer treatment [330].
Nanomedicine has achieved significant progress in experimental research, demonstrating remarkable therapeutic potential. However, the transition from laboratory breakthroughs to clinical applications poses a series of complex challenges. Addressing these obstacles will require innovative and multidisciplinary solutions. With continued advancements in nanotechnology, the field holds the promise of revolutionizing cancer treatment. The following sections explore future perspectives on clinical translation and application.
-
1.
Clinical trail landscape: Since no targeted PCD nanomedicines have been found to be applied in clinical trials yet, existing classic therapeutic agents, such as antimalarial drugs and chemotherapy drugs, have been repurposed in clinical trials to modulate PCD pathways, like ferroptosis, cuproptosis, and pyroptosis. The table below presents representative approved drugs that have been reported to regulate PCD, highlighting the mechanisms of action in modulating PCD pathways, rather than focusing on clinical trial research involving nanomedicines (Table 7). The approach aims to enhance the understanding of clinical trial progress and improve the value of translational medicine, while providing insight into the design of future PCD-targeted nanomedicines, emphasizing the strategic direction for further research and clinical applications.
-
2.
Immune modulation: Nanomedicines hold great promise in cancer immunotherapy due to their unique interactions with both tumor and immune cells. Different PCD pathways activate the immune system through distinct mechanisms. Pyroptosis has the capacity to induce immune cell death, initiate the release of pro-inflammatory cytokines, and activate and recruit immune cells within the tumor microenvironment, thereby further promoting cell death, cytokine release, and immune response activation [331]. Ferroptosis not only induces tumor-specific immune responses but also regulates the antitumor interactions between tumor cells and macrophages, demonstrating broad application potential [332]. Cuproptosis can effectively trigger immunogenic cell death based on the release of tumor-associated antigens, helping to prevent tumor recurrence [97]. Combining PCD-inducing nanoplatforms with checkpoint inhibitors offers a synergistic therapeutic strategy. Designing nanomedicines that not only kill tumor cells but also enhance anti-tumor immunity may provide more comprehensive and effective cancer treatments.
-
3.
Integration with advanced technologies: emerging platforms such as 3D bioprinting, organ-on-a-chip systems, and advanced imaging techniques offer valuable opportunities to refine the design, preclinical evaluation, and real-time monitoring of nanomedicines. These technologies hold significant promise for improving therapeutic efficacy and enabling patient-specific treatment strategies. Given the limited predictive value of conventional 2D cell cultures and animal models, the adoption of standardized, physiologically relevant models, such as patient-derived organoids and humanized mouse models, is essential for accurately assessing the efficacy and safety of PCD-targeting nanomedicines.
-
4.
Optimization for tumor microenvironment delivery: umor microenvironments are highly heterogeneous and dynamic, often presenting barriers such as hypoxia, acidosis, and elevated ROS. Engineering nanoparticles to respond to these cues (e.g., stimuli-responsive or self-adaptive designs) may significantly improve their penetration and precision. For example, pH-sensitive polymeric nanoparticles that remain stable at physiological pH but release their payload in acidic tumor regions have demonstrated improved drug accumulation and antitumor efficacy.
-
5.
Identification of predictive biomarkers for PCD susceptibility: Identifying reliable biomarkers to predict tumor sensitivity to specific PCD pathways is essential for patient stratification and personalized nanomedicine design. Incorporating biomarker-based screening can enhance treatment efficacy and reduce off-target risks. For instance, elevated expression of FDX1 has been linked to increased susceptibility to cuproptosis, indicating its potential as a predictive biomarker for copper-based therapies.
-
6.
Regulatory and ethical challenges: The clinical translation of nanomedicines is often impeded by regulatory and ethical concerns. The absence of approved PCD inducers on the market further complicates this process. Regulatory frameworks must evolve to accommodate the unique physicochemical properties and potential risks associated with nanomedicines.
-
7.
Personalized nanomedicine: the growing demand for personalized treatment options in cancer therapy underscores the importance of tailoring treatments to individual patient characteristics. Advancements in genomics and bioinformatics can facilitate the development of nanomedicines designed specifically for personalized medicine, promoting more effective and targeted therapies.
-
8.
Integration of multi-modal therapies: enhancing the efficacy of cancer treatment hinges on the integration of multi-modal therapies. Future research should focus on designing nanomedicines capable of simultaneously activating multiple PCD pathways or combining different therapeutic approaches to improve treatment outcomes.
-
9.
Exploring Emerging PCD Pathways: new forms of PCD are being actively researched for their potential applications in cancer treatment. The emerging pathways provide opportunities for developing novel targeted therapeutic strategies, potentially leading to more effective cancer treatments.
Table 7.
Representative drugs that can modulate PCD pathways.
| Drugs | Current application | PCD types | Reported PCD-targeting mechanisms | Ref. |
|---|---|---|---|---|
| Mefloquine | Malaria infections | Ferroptosis |
|
|
| Dihydroartemisinin | Malaria infections | Ferroptosis |
|
|
| Sulfasalazine | Inflammatory bowel disease and rheumatic diseases | Ferroptosis |
|
|
| Acetaminophen | Antipyretic, analgesic and anti-inflammatory | Ferroptosis |
|
|
| Cisplatin | Cancer therapy | Ferroptosis |
|
|
| Lapatinib and siramesine | Breast cancer | Ferroptosis |
|
|
| Statins | Hypercholesterolemia and cardiovascular diseases | Ferroptosis |
|
|
| Dimethyl fumarate | Multiple sclerosi | Ferroptosis |
|
|
| Olaparib | Ovarian cancer | Ferroptosis |
|
|
| Orlistat | Obesity | Ferroptosis |
|
|
| Flubendazole | Nematode and cestode infections | Ferroptosis |
|
|
| Cetuximab | Colorectal cancer | Ferroptosis |
|
|
| Salinomycin | Anticoccidial and antiparasitic applications | Ferroptosis |
|
|
| Elesclomol | Solid tumors | Cuproptosis |
|
|
| Disulfiram | Psychiatric disorders | Cuproptosis |
|
|
| Tetrathiomolybdate | Hepatolenticular degeneration | Cuproptosis |
|
|
| Metformin | Type 2 diabetes | Pyroptosis |
|
|
| Mannose | Urinary tract infections | Pyroptosis |
|
|
7. Conclusions
This review highlights the tremendous potential of nanomedicines in regulating PCD pathways including pyroptosis, ferroptosis, and cuproptosis. The emergence of nanomedicines offers unprecedented precision and multifunctionality in controlling cell death mechanisms. Their potential applications suggest a transformative shift in therapeutic strategies. Nanomedicines are designed to release active components within specific cellular microenvironments, allowing for precise control over cell death pathways, thereby enhancing therapeutic efficacy and minimizing side effects. The characteristic demonstrates significant advantages, particularly in cancer treatment. However, the clinical translation of nanomedicines faces multiple challenges. The foremost concern is safety, which requires extensive toxicological studies to ensure their safety and effectiveness in humans. Additionally, the reproducibility and cost control of nanomedicine production are critical factors for successful clinical application. Future research must address these issues to fully exploit the unique advantages of nanomedicines in regulating PCD and treating diseases. With continuous advancements in nanotechnology and a deeper understanding of PCD mechanisms, nanomedicines are expected to play an even greater role in personalized and precision medicine. This will provide strong support for clinical treatments and has the potential to revolutionize existing approaches to cancer therapy.
CRediT authorship contribution statement
Zhan Zhang: Supervision, Writing – original draft, Methodology, Conceptualization, Writing – review & editing, Formal analysis, Investigation. Yuanzhen Wu: Writing – original draft, Writing – review & editing, Investigation. Yanchen Liu: Investigation, Writing – original draft, Writing – review & editing. Jingyu Zhang: Writing – review & editing, Investigation, Writing – original draft. Yan Zhang: Writing – original draft, Investigation, Writing – review & editing. Yunlu Dai: Investigation, Writing – review & editing, Writing – original draft, Supervision. Caigang Liu: Funding acquisition, Supervision, Resources.
Ethics approval and consent to participate
This review article does not require any ethical approval or allied consents for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Zhan Zhang, Yuanzhen Wu, Yanchen Liu, Jingyu Zhang, and Yan Zhang contributed equally to this work. This work was supported by the National Natural Science Foundation of China (32301112), the Natural Science Foundation of Liaoning Province (2023-BS-108), China Postdoctoral Science Foundation (20241171040, 2024MD754001), and Shenyang U35 Youth Top-notch Talent Project (RC231061).
Footnotes
Peer review under the responsibility of editorial board of Bioactive Materials.
Contributor Information
Zhan Zhang, Email: zhangzhan@cmu.edu.cn.
Yunlu Dai, Email: yldai@um.edu.mo.
Caigang Liu, Email: liucg@sj-hospital.org.
References
- 1.Yang F., Zhang G., An N., Dai Q., Cho W., Shang H., Xing Y. Interplay of ferroptosis, cuproptosis, and PANoptosis in cancer treatment-induced cardiotoxicity: mechanisms and therapeutic implications. Semin. Cancer Biol. 2024;106–107:106–122. doi: 10.1016/j.semcancer.2024.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Wang H., Shu L., Lv C., Liu N., Long Y., Peng X., Ling H., Tao T., Tang J., Cheng Y., Liu S., Xiao D., Tao Y. BRCC36 deubiquitinates HMGCR to regulate the interplay between ferroptosis and pyroptosis. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202304263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang X., Peng Q., Peng M., Oyang L., Wang H., Liu Q., Xu X., Wu N., Tan S., Yang W., Han Y., Lin J., Xia L., Tang Y., Luo X., Dai J., Zhou Y., Liao Q. Cellular metabolism: a key player in cancer ferroptosis. Cancer Commun. 2024;44:185–204. doi: 10.1002/cac2.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J., Tai Z., Wu J., Li L., Zhang T., Liu J., Zhu Q., Chen Z. Nanomedicine-induced programmed cell death enhances tumor immunotherapy. J. Adv. Res. 2024;62:199–217. doi: 10.1016/j.jare.2023.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Mo W., Hang Z., Huang Y., Yi H., Sun Z., Lei A. Cuproptosis: harnessing transition metal for cancer therapy. ACS Nano. 2023;17:19581–19599. doi: 10.1021/acsnano.3c07775. [DOI] [PubMed] [Google Scholar]
- 6.Qiu X., Jiang W., Guo X., Du C., Wang X., Tian Y., An H., Wang J., Luo Y., Guo Y., Luo P., Teng H., Li P., Sun Y., Cao Y., Zhang A., Zhou Z., Ren J. Laser boosting the influx of calcium ions to enhance gasdermin e-dependent pyroptosis driven by a dual-layer polydopamine nanoagonist. Chem. Eng. J. 2023;476 doi: 10.1016/j.cej.2023.146748. [DOI] [Google Scholar]
- 7.Zhou S., Liu J., Wan A., Zhang Y., Qi X. Epigenetic regulation of diverse cell death modalities in cancer: a focus on pyroptosis, ferroptosis, cuproptosis, and disulfidptosis. J. Hematol. Oncol. 2024;17:22. doi: 10.1186/s13045-024-01545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Z., Chen G., Zhong M., Lin L., Mai Z., Tang Y., Chen G., Ma W., Li G., Yang Y., Yu Z., Yu M. An acid-responsive MOF nanomedicine for augmented anti-tumor immunotherapy via a metal ion interference-mediated pyroptotic pathway. Biomaterials. 2023;302 doi: 10.1016/j.biomaterials.2023.122333. [DOI] [PubMed] [Google Scholar]
- 9.Yan C., Liu Y., Zhao G., Yang H., Lv H., Li G., Li Y., Fu Y., Sun F., Feng Y., Li Y., Zhao Z. Inhalable metal-organic framework-mediated cuproptosis combined with PD-L1 checkpoint blockade for lung metastasis synergistic immunotherapy. Acta Pharm. Sin. B. 2024;14:2281–2297. doi: 10.1016/j.apsb.2024.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G., Sun L., Gu X., Ai L., Yang J., Zhang Z., Hou P., Wang Y., Ou X., Jiang X., Qiao X., Ma Q., Niu N., Xue J., Zhang H., Yang Y., Liu C. FSIP1 enhances the therapeutic sensitivity to CDK4/6 inhibitors in triple-negative breast cancer patients by activating the Nanog pathway. Sci. China Life Sci. 2023;66:2805–2817. doi: 10.1007/s11427-023-2343-y. [DOI] [PubMed] [Google Scholar]
- 11.Yu B., Wang Y., Bing T., Tang Y., Huang J., Xiao H., Liu C., Yu Y. Platinum prodrug nanoparticles with COX-2 inhibition amplify pyroptosis for enhanced chemotherapy and immune activation of pancreatic cancer. Adv. Mater. 2024;36 doi: 10.1002/adma.202310456. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Wang T., Yan S., Tang J., Zhang Y., Wang L., Xu H., Tu C. Crosstalk of pyroptosis and cytokine in the tumor microenvironment: from mechanisms to clinical implication. Mol. Cancer. 2024;23:268. doi: 10.1186/s12943-024-02183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu N., Chen M. Crosstalk between ferroptosis and cuproptosis: from mechanism to potential clinical application. Biomed. Pharmacother. 2024;171 doi: 10.1016/j.biopha.2023.116115. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B., Zhang J.-y., Liu X.-s., Chen H.-z., Ai Y.-l., Cheng K., Sun R.-y., Zhou D., Han J., Wu Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–1185. doi: 10.1038/s41422-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luobin L., Wanxin H., Yingxin G., Qinzhou Z., Zefeng L., Danyang W., Huaqin L. Nanomedicine-induced programmed cell death in cancer therapy: mechanisms and perspectives. Cell Death Discov. 2024;10:386. doi: 10.1038/s41420-024-02121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z., Sang W., Xie L., Dai Y. Metal-organic frameworks for multimodal bioimaging and synergistic cancer chemotherapy. Coord. Chem. Rev. 2019;399 doi: 10.1016/j.ccr.2019.213022. [DOI] [Google Scholar]
- 17.Zhang Z., Xie L., Ju Y., Dai Y. Recent advances in metal-phenolic networks for cancer theranostics. Small. 2021;17 doi: 10.1002/smll.202100314. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z., Dalan R., Hu Z., Wang J.W., Chew N.W., Poh K.K., Tan R.S., Soong T.W., Dai Y., Ye L., Chen X. Reactive oxygen species scavenging nanomedicine for the treatment of ischemic heart disease. Adv. Mater. 2022;34 doi: 10.1002/adma.202202169. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Yu X., Shi X., Shen M. Cancer nanomedicine based on polyethylenimine-mediated multifunctional nanosystems. Prog. Mater. Sci. 2022;124 doi: 10.1016/j.pmatsci.2021.100871. [DOI] [Google Scholar]
- 20.Sang W., Zhang Z., Dai Y., Chen X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 2019;48:3771–3810. doi: 10.1039/c8cs00896e. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., Sang W., Xie L., Li W., Li B., Li J., Tian H., Yuan Z., Zhao Q., Dai Y. Polyphenol-Based Nanomedicine Evokes immune activation for combination cancer treatment. Angew Chem. Int. Ed. Engl. 2021;60:1967–1975. doi: 10.1002/anie.202013406. [DOI] [PubMed] [Google Scholar]
- 22.Zhu G., Xie Y., Wang J., Wang M., Qian Y., Sun Q., Dai Y., Li C. Multifunctional copper-phenolic nanopills achieve comprehensive polyamines depletion to provoke enhanced pyroptosis and cuproptosis for cancer immunotherapy. Adv. Mater. 2024;36 doi: 10.1002/adma.202409066. [DOI] [PubMed] [Google Scholar]
- 23.Li W., Xiao Y., Guo G., Peng J., Zhu N., Chen Z., Peng B., Jiang Z., Li B., Yu G., Guo Z., Liang M., Guo W. Cuprous oxide nanocomposites with photothermal (PTT) and chemical dynamics (CDT) effects induce cuproptosis in breast cancer using the strategy of increasing inflow and reducing outflow. Nano Today. 2024;56 doi: 10.1016/j.nantod.2024.102223. [DOI] [Google Scholar]
- 24.Zhang Z., Li B., Xie L., Sang W., Tian H., Li J., Wang G., Dai Y. Metal-Phenolic network-enabled lactic acid consumption reverses immunosuppressive tumor microenvironment for sonodynamic therapy. ACS Nano. 2021;15:16934–16945. doi: 10.1021/acsnano.1c08026. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z., Li X., Liu W., Chen G., Liu J., Ma Q., Hou P., Liang L., Liu C. Polyphenol nanocomplex modulates lactate metabolic reprogramming and elicits immune responses to enhance cancer therapeutic effect. Drug Resist. Updates. 2024;73 doi: 10.1016/j.drup.2024.101060. [DOI] [PubMed] [Google Scholar]
- 26.Sang W., Zhang Z., Wang G., Xie L., Li J., Li W., Tian H., Dai Y. A triple-kill strategy for tumor eradication reinforced by metal-phenolic network nanopumps. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202113168. [DOI] [Google Scholar]
- 27.Lee S., Chung C.Y., Liu P., Craciun L., Nishikawa Y., Bruemmer K.J., Hamachi I., Saijo K., Miller E.W., Chang C.J. Activity-Based sensing with a metal-directed acyl imidazole strategy reveals cell type-dependent pools of Labile Brain copper. J. Am. Chem. Soc. 2020;142:14993–15003. doi: 10.1021/jacs.0c05727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J., Huang Z., Tan J., Pan J., Chen S., Wan W. Copper ion/gallic acid MOFs-laden adhesive pomelo peel sponge effectively treats biofilm-infected skin wounds and improves healing quality. Bioact. Mater. 2024;32:260–276. doi: 10.1016/j.bioactmat.2023.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Min J., Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Targeted Ther. 2022;7:378. doi: 10.1038/s41392-022-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandmann O., Weiss K.H., Kaler S.G. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14:103–113. doi: 10.1016/S1474-4422(14)70190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deas E., Cremades N., Angelova P.R., Ludtmann M.H., Yao Z., Chen S., Horrocks M.H., Banushi B., Little D., Devine M.J., Gissen P., Klenerman D., Dobson C.M., Wood N.W., Gandhi S., Abramov A.Y. Alpha-Synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in parkinson's disease. Antioxidants Redox Signal. 2016;24:376–391. doi: 10.1089/ars.2015.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guthrie L.M., Soma S., Yuan S., Silva A., Zulkifli M., Snavely T.C., Greene H.F., Nunez E., Lynch B., De Ville C., Shanbhag V., Lopez F.R., Acharya A., Petris M.J., Kim B.E., Gohil V.M., Sacchettini J.C. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science. 2020;368:620–625. doi: 10.1126/science.aaz8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei P., Ayton S., Bush A.I. The essential elements of Alzheimer's disease. J. Biol. Chem. 2021;296 doi: 10.1074/jbc.REV120.008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo J., Cheng J., Zheng N., Zhang X., Dai X., Zhang L., Hu C., Wu X., Jiang Q., Wu D., Okada H., Pandolfi P.P., Wei W. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1 dependent manner. Adv. Sci. (Weinh.) 2021;8 doi: 10.1002/advs.202004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsvetkov P., Coy S., Petrova B., Dreishpoon M., Verma A., Abdusamad M., Rossen J., Joesch-Cohen L., Humeidi R., Spangler R.D., Eaton J.K., Frenkel E., Kocak M., Corsello S.M., Lutsenko S., Kanarek N., Santagata S., Golub T.R. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y., Wang D., Gu C., Wang X., Zhu S., Zheng Z., Zhang F., Yan J., Gu Z. A cuproptosis nanocapsule for cancer radiotherapy. Nat. Nanotechnol. 2024 doi: 10.1038/s41565-024-01784-1. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y., Pan R.J., An Z.H., Liu Q.M., Zhou S.H. Analysis of immune infiltration and cuproptosis-related genes in acute myocardial infarction. Eur. Heart J. 2023;44 doi: 10.1093/eurheartj/ehad655.1381. [DOI] [Google Scholar]
- 38.Zhu J., You Y., Zhang W., Wang W., Jiang M., Pu F., Ren J., Qu X. Sensitizing cuproptosis by endogenous copper-triggered bioorthogonal nanoremodeler. Nano Today. 2024;55 doi: 10.1016/j.nantod.2024.102196. [DOI] [Google Scholar]
- 39.Xu W., Suo A., Aldai A.J.M., Wang Y., Fan J., Xia Y., Xu J., Chen Z., Zhao H., Zhang M., Qian J. Hollow Calcium/Copper bimetallic amplifier for Cuproptosis/Paraptosis/Apoptosis cancer therapy via Cascade reinforcement of endoplasmic reticulum stress and mitochondrial dysfunction. ACS Nano. 2024;18:30053–30068. doi: 10.1021/acsnano.4c11455. [DOI] [PubMed] [Google Scholar]
- 40.Kim B.E., Nevitt T., Thiele D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y., Yang X., Jang W.J., Yan M., Yoon J. Binding- and activity-based small molecule fluorescent probes for the detection of Cu+, Cu2+, Fe2+ and Fe3+ in biological systems. Coord. Chem. Rev. 2025;522 doi: 10.1016/j.ccr.2024.216201. [DOI] [Google Scholar]
- 42.Tisato F., Marzano C., Porchia M., Pellei M., Santini C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010;30:708–749. doi: 10.1002/med.20174. [DOI] [PubMed] [Google Scholar]
- 43.Wikstrom M., Krab K., Sharma V. Oxygen activation and energy conservation by cytochrome c oxidase. Chem. Rev. 2018;118:2469–2490. doi: 10.1021/acs.chemrev.7b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tainer J.A., Getzoff E.D., Richardson J.S., Richardson D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983;306:284–287. doi: 10.1038/306284a0. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Chen W., Yin J., Xia S., Jiang Y., Ge Q., Liu J., Wang M., Hou Z., Bai Y., Shi P. Biomineralized ZIF-8 encapsulating SOD from hydrogenobacter thermophilus: maintaining activity in the intestine and alleviating intestinal oxidative stress. Small. 2024 doi: 10.1002/smll.202402812. [DOI] [PubMed] [Google Scholar]
- 46.Williams D.M., Loukopoulos D., Lee G.R., Cartwright G.E. Role of copper in mitochondrial iron metabolism. Blood. 1976;48:77–85. [PubMed] [Google Scholar]
- 47.Bradley J.M., Svistunenko D.A., Lawson T.L., Hemmings A.M., Moore G.R., Le Brun N.E. Three aromatic residues are required for electron transfer during iron mineralization in bacterioferritin. Angew Chem. Int. Ed. Engl. 2015;54:14763–14767. doi: 10.1002/anie.201507486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nai A., Lidonnici M.R., Rausa M., Mandelli G., Pagani A., Silvestri L., Ferrari G., Camaschella C. The second transferrin receptor regulates red blood cell production in mice. Blood. 2015;125:1170–1179. doi: 10.1182/blood-2014-08-596254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen E.L., Gonzalez-Ibanez A.M., Mendoza P., Ruiz L.M., Riedel C.A., Simon F., Schuringa J.J., Elorza A.A. Copper deficiency-induced anemia is caused by a mitochondrial metabolic reprograming in erythropoietic cells. Metallomics. 2019;11:282–290. doi: 10.1039/c8mt00224j. [DOI] [PubMed] [Google Scholar]
- 50.Rimar D., Rosner I., Slobodin G., Rozenbaum M., haj T., Jiries N., Kaly L., Boulman N., Vadas Z. AB0141 Lysyl oxidase is correlated with fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2014;72:A828.3–A82828. doi: 10.1136/annrheumdis-2013-eular.2464. [DOI] [PubMed] [Google Scholar]
- 51.Chen W., Yang A., Jia J., Popov Y.V., Schuppan D., You H. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology. 2020;72:729–741. doi: 10.1002/hep.31236. [DOI] [PubMed] [Google Scholar]
- 52.Ferreira J., Michiels J., Herregraven M., Korevaar P.A. Myelin surfactant assemblies as dynamic pathways guiding the growth of electrodeposited copper dendrites. J. Am. Chem. Soc. 2024;146:19205–19217. doi: 10.1021/jacs.4c04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monzani E., Nicolis S., Dell'Acqua S., Capucciati A., Bacchella C., Zucca F.A., Mosharov E.V., Sulzer D., Zecca L., Casella L. Dopamine, oxidative stress and protein-quinone modifications in parkinson's and other neurodegenerative diseases. Angew Chem. Int. Ed. Engl. 2019;58:6512–6527. doi: 10.1002/anie.201811122. [DOI] [PubMed] [Google Scholar]
- 54.Madsen E., Gitlin J.D. Copper and iron disorders of the brain. Annu. Rev. Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 55.Voli F., Lerra L., Kimpton K., Saletta F., Cirillo G., Kavallaris M., Vittorio O. Harnessing copper in cancer to enhance anti-tumor immune response. Ann. Oncol. 2018;29 doi: 10.1093/annonc/mdy487.030. [DOI] [Google Scholar]
- 56.Wu H., Zhang Z., Cao Y., Hu Y., Li Y., Zhang L., Cao X., Wen H., Zhang Y., Lv H., Jin X. A self-amplifying ROS-Responsive nanoplatform for simultaneous cuproptosis and cancer immunotherapy. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202401047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siotto M., Squitti R. Copper imbalance in Alzheimer's disease: overview of the exchangeable copper component in plasma and the intriguing role albumin plays. Coord. Chem. Rev. 2018;371:86–95. doi: 10.1016/j.ccr.2018.05.020. [DOI] [Google Scholar]
- 58.Tang D., Kroemer G., Kang R. Targeting cuproplasia and cuproptosis in cancer. Nat. Rev. Clin. Oncol. 2024;21:370–388. doi: 10.1038/s41571-024-00876-0. [DOI] [PubMed] [Google Scholar]
- 59.Xie J., Yang Y., Gao Y., He J. Cuproptosis: mechanisms and links with cancers. Mol. Cancer. 2023;22:46. doi: 10.1186/s12943-023-01732-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong F.S., Ren C.Y., Jia R., Zhou Y., Chen J.H., Ma Y. Systematic pan-cancer analysis identifies SLC31A1 as a biomarker in multiple tumor types. BMC Med. Genom. 2023;16:61. doi: 10.1186/s12920-023-01489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bitter R.M., Oh S., Deng Z., Rahman S., Hite R.K., Yuan P. Structure of the Wilson disease copper transporter ATP7B. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abl5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao F., Liang L., Wang H., Wang C., Su D., Ying Y., Li W., Li J., Zheng J., Qiao L., Mou X., Che S., Yu J. H2S‐Activated ion‐interference therapy: a novel tumor targeted therapy based on copper‐overload‐mediated cuproptosis and pyroptosis. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202300941. [DOI] [Google Scholar]
- 63.Zhou J., Yu Q., Song J., Li S., Li X.L., Kang B.K., Chen H.Y., Xu J.J. Photothermally triggered copper payload release for cuproptosis-promoted cancer synergistic therapy. Angew Chem. Int. Ed. Engl. 2023;62 doi: 10.1002/anie.202213922. [DOI] [PubMed] [Google Scholar]
- 64.Gao S., Ge H., Gao L., Gao Y., Tang S., Li Y., Yuan Z., Chen W. Silk fibroin nanoparticles for enhanced cuproptosis and immunotherapy in pancreatic cancer treatment. Adv. Sci. (Weinh.) 2025;12 doi: 10.1002/advs.202417676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santoro A., Calvo J.S., Peris-Diaz M.D., Krezel A., Meloni G., Faller P. The Glutathione/Metallothionein System challenges the design of efficient O(2) -Activating copper complexes. Angew Chem. Int. Ed. Engl. 2020;59:7830–7835. doi: 10.1002/anie.201916316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu S., Wang P., Qin J., Pei Y., Wang Y. GSH‐Depleted nanozymes with dual‐radicals enzyme activities for tumor synergic therapy. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202102160. [DOI] [Google Scholar]
- 67.Yang Z., Su W., Wei X., Pan Y., Xing M., Niu L., Feng B., Kong W., Ren X., Huang F., Zhou J., Zhao W., Qiu Y., Liao T., Chen Q., Qu S., Wang Y., Guan Q., Li D., Zen K., Chen Y., Qin C., Wang Y., Zhou X., Xiang J., Yao B. Hypoxia inducible factor-1alpha drives cancer resistance to cuproptosis. Cancer Cell. 2025;43:937–954 e9. doi: 10.1016/j.ccell.2025.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Dai Y., Zhu L., Li X., Zhang F., Chen K., Jiao G., Liu Y., Yang Z., Guo Z., Zhang B., Shen Q., Zhao Q. A biomimetic cuproptosis amplifier for targeted NIR-II fluorescence/photoacoustic imaging-guided synergistic NIR-II photothermal immunotherapy. Biomaterials. 2024;305 doi: 10.1016/j.biomaterials.2023.122455. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X., Zhang Z., Shu Q., Xu C., Zheng Q., Guo Z., Wang C., Hao Z., Liu X., Wang G., Yan W., Chen H., Lu C. Copper clusters: an effective antibacterial for eradicating multidrug‐resistant bacterial infection in vitro and in vivo. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202008720. [DOI] [Google Scholar]
- 70.Wu C., Tan J., Wang X., Qin C., Long W., Pan Y., Li Y., Liu Q. Pan-cancer analyses reveal molecular and clinical characteristics of cuproptosis regulators. Imeta. 2023;2:e68. doi: 10.1002/imt2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W., Lu K., Jiang X., Wei Q., Zhu L., Wang X., Jin H., Feng L. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J. Exp. Clin. Cancer Res. 2023;42:142. doi: 10.1186/s13046-023-02720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng P., Zhou C., Lu L., Liu B., Ding Y. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res. 2022;41:271. doi: 10.1186/s13046-022-02485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Liu J., Chen Y., Weichselbaum R.R., Lin W. Nanoparticles synergize ferroptosis and cuproptosis to potentiate cancer immunotherapy. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202310309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Zang L., Guan L., Ren X., Xia Z., Li Z., Meng Z., Lian H. Designed tumor microenvironment-remodeling bispolyphenol nanoparticles combined with αPD-L1 for enhanced melanoma immunotherapy. Chem. Eng. J. 2025;503 doi: 10.1016/j.cej.2024.158442. [DOI] [Google Scholar]
- 75.Vasan K., Werner M., Chandel N.S. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020;32:341–352. doi: 10.1016/j.cmet.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan L., Liu Y., Chen M., Su Y., Guo J., Zhu L., Zhan M., Chen T., Lu L. Cuproptosis‐driven enhancement of thermotherapy by sequentially response Cu2‐xSe via copper chemical transition. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202302054. [DOI] [Google Scholar]
- 77.Jin X.-K., Liang J.-L., Zhang S.-M., Huang Q.-X., Zhang S.-K., Liu C.-J., Zhang X.-Z. Orchestrated copper-based nanoreactor for remodeling tumor microenvironment to amplify cuproptosis-mediated anti-tumor immunity in colorectal cancer. Mater. Today. 2023;68:108–124. doi: 10.1016/j.mattod.2023.06.024. [DOI] [Google Scholar]
- 78.Chen Y. Nanotechnology for next-generation cancer immunotherapy: state of the art and future perspectives. J. Contr. Release. 2023;356:14–25. doi: 10.1016/j.jconrel.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Chen K., Zhou A., Zhou X., He J., Xu Y., Ning X. Cellular Trojan Horse initiates bimetallic Fe-Cu MOF-mediated synergistic cuproptosis and ferroptosis against malignancies. Sci. Adv. 2024;10 doi: 10.1126/sciadv.adk3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo B., Yang F., Zhang L., Zhao Q., Wang W., Yin L., Chen D., Wang M., Han S., Xiao H., Xing N. Cuproptosis induced by ROS responsive nanoparticles with Elesclomol and copper combined with αPD‐L1 for enhanced cancer immunotherapy. Adv. Mater. 2023;35 doi: 10.1002/adma.202212267. [DOI] [PubMed] [Google Scholar]
- 81.Yu N., Zhou J., Ding M., Li M., Peng S., Li J. Sono‐triggered Cascade lactate depletion by semiconducting polymer nanoreactors for cuproptosis‐immunotherapy of pancreatic cancer. Angew. Chem. Int. Ed. 2024;63 doi: 10.1002/anie.202405639. [DOI] [PubMed] [Google Scholar]
- 82.Xu Y., Liu S.Y., Zeng L., Ma H., Zhang Y., Yang H., Liu Y., Fang S., Zhao J., Xu Y., Ashby C.R., Jr., He Y., Dai Z., Pan Y. An enzyme-engineered nonporous Copper(I) coordination polymer nanoplatform for cuproptosis-based synergistic cancer therapy. Adv. Mater. 2022;34 doi: 10.1002/adma.202204733. [DOI] [PubMed] [Google Scholar]
- 83.Fatemi N., Sarkar B. Molecular mechanism of copper transport in Wilson disease. Environ. Health Perspect. 2002;110(Suppl 5):695–698. doi: 10.1289/ehp.02110s5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan C., Lv H., Feng Y., Li Y., Zhao Z. Inhalable nanoparticles with enhanced cuproptosis and cGAS-STING activation for synergistic lung metastasis immunotherapy. Acta Pharm. Sin. B. 2024;14:3697–3710. doi: 10.1016/j.apsb.2024.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y., Niu R., Zhao H., Wang Y., Song S., Zhang H., Zhao Y. Single-site nanozymes with a highly conjugated coordination structure for antitumor immunotherapy via cuproptosis and cascade-enhanced T lymphocyte activity. J. Am. Chem. Soc. 2024;146:3675–3688. doi: 10.1021/jacs.3c08622. [DOI] [PubMed] [Google Scholar]
- 86.Krezel A., Maret W. The bioinorganic chemistry of Mammalian metallothioneins. Chem. Rev. 2021;121:14594–14648. doi: 10.1021/acs.chemrev.1c00371. [DOI] [PubMed] [Google Scholar]
- 87.Huang Q.-X., Liang J.-L., Chen Q.-W., Jin X.-K., Niu M.-T., Dong C.-Y., Zhang X.-Z. Metal-organic framework nanoagent induces cuproptosis for effective immunotherapy of malignant glioblastoma. Nano Today. 2023;51 doi: 10.1016/j.nantod.2023.101911. [DOI] [Google Scholar]
- 88.Lu S., Li Y., Yu Y. Glutathione-Scavenging Celastrol-Cu nanoparticles induce self-amplified cuproptosis for augmented cancer immunotherapy. Adv. Mater. 2024;36 doi: 10.1002/adma.202404971. [DOI] [PubMed] [Google Scholar]
- 89.Man R., Xia M., Li H., Tian F., Zhang J., Yu Z., Tang B. Human serum albumin mediated controllable synthesis of defect-rich copper hydroxide nanowire for cuproptosis-based anti-tumor therapy. Adv. Healthcare Mater. 2024 doi: 10.1002/adhm.202401078. [DOI] [PubMed] [Google Scholar]
- 90.Hu F., Huang J., Bing T., Mou W., Li D., Zhang H., Chen Y., Jin Q., Yu Y., Yang Z. Stimulus-responsive copper complex nanoparticles induce cuproptosis for augmented cancer immunotherapy. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202309388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang S., Liu Y., Su M., Wang Y., Wang W., Wang W., Yang Q., Zhang Z., Hong X., Sun Z., Xiao Y. Near-infrared activatable copper nanoplatforms synergize with the 5-Azacytidine prodrug to potentiate cuproptosis. Angew Chem. Int. Ed. Engl. 2024 doi: 10.1002/anie.202411609. [DOI] [PubMed] [Google Scholar]
- 92.Pei P., Wang Y., Shen W., He Q., Han X., Zhang C., Xie Y., Zhou G., Zhao Y., Hu L., Yang K. Oxygen‐driven cuproptosis synergizes with radiotherapy to potentiate tumor immunotherapy. Aggregate. 2024;5 doi: 10.1002/agt2.484. [DOI] [Google Scholar]
- 93.Zhao C., Tang X., Chen X., Jiang Z. Multifaceted carbonized metal-organic frameworks synergize with immune checkpoint inhibitors for precision and augmented cuproptosis cancer therapy. ACS Nano. 2024;18:17852–17868. doi: 10.1021/acsnano.4c04022. [DOI] [PubMed] [Google Scholar]
- 94.Du Y., Zhao X., He F., Gong H., Yang J., Wu L., Cui X., Gai S., Yang P., Lin J. A vacancy-engineering ferroelectric nanomedicine for cuproptosis/apoptosis Co-Activated immunotherapy. Adv. Mater. 2024;36 doi: 10.1002/adma.202403253. [DOI] [PubMed] [Google Scholar]
- 95.Jia W., Tian H., Jiang J., Zhou L., Li L., Luo M., Ding N., Nice E.C., Huang C., Zhang H. Brain-targeted HFn-Cu-REGO nanoplatform for site-specific delivery and manipulation of autophagy and cuproptosis in glioblastoma. Small. 2023;19 doi: 10.1002/smll.202205354. [DOI] [PubMed] [Google Scholar]
- 96.Qiao L., Ou Y., Li L., Wu S., Guo Y., Liu M., Yu D., Chen Q., Yuan J., Wei C., Ou C., Li H., Cheng D., Yu Z., Li Z. H(2)S-driven chemotherapy and mild photothermal therapy induced mitochondrial reprogramming to promote cuproptosis. J Nanobiotechnology. 2024;22:205. doi: 10.1186/s12951-024-02480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guan M., Cheng K., Xie X.T., Li Y., Ma M.W., Zhang B., Chen S., Chen W., Liu B., Fan J.X., Zhao Y.D. Regulating copper homeostasis of tumor cells to promote cuproptosis for enhancing breast cancer immunotherapy. Nat. Commun. 2024;15 doi: 10.1038/s41467-024-54469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu X., Bai Z., Wang H., Wang H., Hou D., Xu Y., Wo G., Cheng H., Sun D., Tao W. CRISPR-Cas9 gene editing strengthens cuproptosis/chemodynamic/ferroptosis synergistic cancer therapy. Acta Pharm. Sin. B. 2024;14:4059–4072. doi: 10.1016/j.apsb.2024.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng J., Ge H., Guo M., Zhang T., Hu Q., Yao Q., Long S., Sun W., Fan J., Du J., Peng X. Photoinduced cuproptosis with tumor-specific for metastasis-inhibited cancer therapy. Small. 2024;20 doi: 10.1002/smll.202304407. [DOI] [PubMed] [Google Scholar]
- 100.He C., Zhu N., Chen Y., Zheng Y., Chen S., Wu Z., Zheng S., Hu H., Qi L., Hou M., Shen Z., Zhao B., Guo W., Yan C. Reshaping immunosuppressive tumor microenvironment using Ferroptosis/Cuproptosis nanosensitizers for enhanced radioimmunotherapy. Adv. Funct. Mater. 2024 doi: 10.1002/adfm.202409966. [DOI] [Google Scholar]
- 101.He G., Pan Y., Zeng F., Qin S., Luan X., Lu Q., Xie C., Hu P., Gao Y., Yang J., He B., Song Y. Microfluidic synthesis of CuH nanoparticles for antitumor therapy through hydrogen-enhanced apoptosis and cuproptosis. ACS Nano. 2024;18:9031–9042. doi: 10.1021/acsnano.3c12796. [DOI] [PubMed] [Google Scholar]
- 102.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang S., Guo S., Guo J., Du Q., Wu C., Wu Y., Zhang Y. Cell death pathways: molecular mechanisms and therapeutic targets for cancer. MedComm. 2024;5 doi: 10.1002/mco2.693. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zychlinsky A., Prevost M.C., Sansonetti P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 105.Cookson B.T., Brennan M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 106.Tweedell R.E., Malireddi R.K.S., Kanneganti T.D. A comprehensive guide to studying inflammasome activation and cell death. Nat. Protoc. 2020;15:3284–3333. doi: 10.1038/s41596-020-0374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei X., Xie F., Zhou X., Wu Y., Yan H., Liu T., Huang J., Wang F., Zhou F., Zhang L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022;19:971–992. doi: 10.1038/s41423-022-00905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruan J., Xia S., Liu X., Lieberman J., Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557:62–67. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 110.Man S.M., Kanneganti T.D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 112.Lippens S., Kockx M., Knaapen M., Mortier L., Polakowska R., Verheyen A., Garmyn M., Zwijsen A., Formstecher P., Huylebroeck D., Vandenabeele P., Declercq W. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y., Gao W., Shi X., Ding J., Liu W., He H., Wang K., Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 114.Hou J., Zhao R., Xia W., Chang C.W., You Y., Hsu J.M., Nie L., Chen Y., Wang Y.C., Liu C., Wang W.J., Wu Y., Ke B., Hsu J.L., Huang K., Ye Z., Yang Y., Xia X., Li Y., Li C.W., Shao B., Tainer J.A., Hung M.C. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sato H., Koide T., Masuya H., Wakana S., Sagai T., Umezawa A., Ishiguro S., Tamai M., Shiroishi T. A new mutation Rim3 resembling Re(den) is mapped close to retinoic acid receptor alpha (Rara) gene on mouse chromosome 11. Mamm. Genome. 1998;9:20–25. doi: 10.1007/s003359900673. [DOI] [PubMed] [Google Scholar]
- 116.Tamura M., Tanaka S., Fujii T., Aoki A., Komiyama H., Ezawa K., Sumiyama K., Sagai T., Shiroishi T. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89:618–629. doi: 10.1016/j.ygeno.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 117.Panganiban R.A., Nadeau K.C., Lu Q. Pyroptosis, gasdermins and allergic diseases. Allergy. 2024 doi: 10.1111/all.16236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Schutter E., Roelandt R., Riquet F.B., Van Camp G., Wullaert A., Vandenabeele P. Punching holes in cellular membranes: biology and evolution of gasdermins. Trends Cell Biol. 2021;31:500–513. doi: 10.1016/j.tcb.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 119.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 120.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S., Cuellar T., Haley B., Roose-Girma M., Phung Q.T., Liu P.S., Lill J.R., Li H., Wu J., Kummerfeld S., Zhang J., Lee W.P., Snipas S.J., Salvesen G.S., Morris L.X., Fitzgerald L., Zhang Y., Bertram E.M., Goodnow C.C., Dixit V.M. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 121.Liu X., Lieberman J. Knocking 'em dead: Pore-Forming proteins in immune defense. Annu. Rev. Immunol. 2020;38:455–485. doi: 10.1146/annurev-immunol-111319-023800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Washington C., 3rd, Dapas M., Biddanda A., Magnaye K.M., Aneas I., Helling B.A., Szczesny B., Boorgula M.P., Taub M.A., Kenny E., Mathias R.A., Barnes K.C., Khurana Hershey G.K., Kercsmar C.M., Gereige J.D., Makhija M., Gruchalla R.S., Gill M.A., Liu A.H., Rastogi D., Busse W., Gergen P.J., Visness C.M., Gold D.R., Hartert T., Johnson C.C., Lemanske R.F., Jr., Martinez F.D., Miller R.L., Ownby D., Seroogy C.M., Wright A.L., Zoratti E.M., Bacharier L.B., Kattan M., O'Connor G.T., Wood R.A., Nobrega M.A., Altman M.C., Jackson D.J., Gern J.E., McKennan C.G., Ober C. African-specific alleles modify risk for asthma at the 17q12-q21 locus in African Americans. Genome Med. 2022;14:112. doi: 10.1186/s13073-022-01114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moreno-Moral A., Bagnati M., Koturan S., Ko J.H., Fonseca C., Harmston N., Game L., Martin J., Ong V., Abraham D.J., Denton C.P., Behmoaras J., Petretto E. Changes in macrophage transcriptome associate with systemic sclerosis and mediate GSDMA contribution to disease risk. Ann. Rheum. Dis. 2018;77:596–601. doi: 10.1136/annrheumdis-2017-212454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Christodoulou K., Wiskin A.E., Gibson J., Tapper W., Willis C., Afzal N.A., Upstill-Goddard R., Holloway J.W., Simpson M.A., Beattie R.M., Collins A., Ennis S. Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes. Gut. 2013;62:977–984. doi: 10.1136/gutjnl-2011-301833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu X., Xia S., Zhang Z., Wu H., Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021;20:384–405. doi: 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.C., Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 127.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen X., He W.T., Hu L., Li J., Fang Y., Wang X., Xu X., Wang Z., Huang K., Han J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Broz P., Pelegrin P., Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020;20:143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 130.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S., von Mutius E., Farrall M., Lathrop M., Cookson W. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deng W., Bai Y., Deng F., Pan Y., Mei S., Zheng Z., Min R., Wu Z., Li W., Miao R., Zhang Z., Kupper T.S., Lieberman J., Liu X. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature. 2022;602:496–502. doi: 10.1038/s41586-021-04384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katoh M., Katoh M. Evolutionary recombination hotspot around GSDML-GSDM locus is closely linked to the oncogenomic recombination hotspot around the PPP1R1B-ERBB2-GRB7 amplicon. Int. J. Oncol. 2004;24:757–763. [PubMed] [Google Scholar]
- 133.Wang C., Shivcharan S., Tian T., Wright S., Ma D., Chang J., Li K., Song K., Xu C., Rathinam V.A., Ruan J. Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Nature. 2023;616:590–597. doi: 10.1038/s41586-023-05832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhong X., Zeng H., Zhou Z., Su Y., Cheng H., Hou Y., She Y., Feng N., Wang J., Shao F., Ding J. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature. 2023;616:598–605. doi: 10.1038/s41586-023-05872-5. [DOI] [PubMed] [Google Scholar]
- 135.Oltra S.S., Colomo S., Sin L., Pérez-López M., Lázaro S., Molina-Crespo A., Choi K.H., Ros-Pardo D., Martínez L., Morales S., González-Paramos C., Orantes A., Soriano M., Hernández A., Lluch A., Rojo F., Albanell J., Gómez-Puertas P., Ko J.K., Sarrió D., Moreno-Bueno G. Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells. Cell Death Differ. 2023;30:1366–1381. doi: 10.1038/s41418-023-01143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu T., Liu S., Rui X., Cao Y., Hecker J., Guo F., Zhang Y., Gong L., Zhou Y., Yu Y., Krishnamoorthyni N., Bates S., Chun S., Boyer N., Xu S., Park J.A., Perrella M.A., Levy B.D., Weiss S.T., Mou H., Raby B.A., Zhou X., Gasdermin B. An asthma-susceptibility gene, promotes MAVS-TBK1 signalling and airway inflammation. Eur. Respir. J. 2024;63 doi: 10.1183/13993003.01232-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rana N., Privitera G., Kondolf H.C., Bulek K., Lechuga S., De Salvo C., Corridoni D., Antanaviciute A., Maywald R.L., Hurtado A.M., Zhao J., Huang E.H., Li X., Chan E.R., Simmons A., Bamias G., Abbott D.W., Heaney J.D., Ivanov A.I., Pizarro T.T. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell. 2024;187:1011–1015. doi: 10.1016/j.cell.2024.01.010. [DOI] [PubMed] [Google Scholar]
- 138.Zhou Z., He H., Wang K., Shi X., Wang Y., Su Y., Wang Y., Li D., Liu W., Zhang Y., Shen L., Han W., Shen L., Ding J., Shao F. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368 doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 139.Cui Y.Q., Meng F., Zhan W.L., Dai Z.T., Liao X. High expression of GSDMC is associated with poor survival in kidney clear cell cancer. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/5282894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun K., Chen R.X., Li J.Z., Luo Z.X. LINC00511/hsa-miR-573 axis-mediated high expression of Gasdermin C associates with dismal prognosis and tumor immune infiltration of breast cancer. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-19247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miguchi M., Hinoi T., Shimomura M., Adachi T., Saito Y., Niitsu H., Kochi M., Sada H., Sotomaru Y., Ikenoue T., Shigeyasu K., Tanakaya K., Kitadai Y., Sentani K., Oue N., Yasui W., Ohdan H. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated apc, promoting colorectal cancer proliferation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei J., Xu Z., Chen X., Wang X., Zeng S., Qian L., Yang X., Ou C., Lin W., Gong Z., Yan Y. Overexpression of GSDMC is a prognostic factor for predicting a poor outcome in lung adenocarcinoma. Mol. Med. Rep. 2020;21:360–370. doi: 10.3892/mmr.2019.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu R., Li J., Aicher A., Jiang K., Tondi S., Dong S., Zheng Q., Tang S., Chen M., Guo Z., Šabanović B., Ananthanarayanan P., Jiang L., Sapino A., Wen C., Fu D., Shen B., Heeschen C. Gasdermin C promotes stemness and immune evasion in pancreatic cancer via pyroptosis-independent mechanism. Adv. Sci. (Weinh.) 2024 doi: 10.1002/advs.202308990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang S., Chang C.W., Huang J., Zeng S., Zhang X., Hung M.C., Hou J. Gasdermin C sensitizes tumor cells to PARP inhibitor therapy in cancer models. J. Clin. Investig. 2024;134 doi: 10.1172/jci166841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu Y., Lu Y., Ning B., Su X., Yang B., Dong H., Yin B., Pang Z., Shen S. Intravenous delivery of living Listeria monocytogenes elicits Gasdmermin-Dependent tumor pyroptosis and motivates anti-tumor immune response. ACS Nano. 2022;16:4102–4115. doi: 10.1021/acsnano.1c09818. [DOI] [PubMed] [Google Scholar]
- 146.Du J., Sarkar R., Li Y., He L., Kang W., Liao W., Liu W., Nguyen T., Zhang L., Deng Z., Dougherty U., Kupfer S.S., Chen M., Pekow J., Bissonnette M., He C., Li Y.C. N(6)-adenomethylation of GsdmC is essential for Lgr5(+) stem cell survival to maintain normal colonic epithelial morphogenesis. Dev. Cell. 2022;57:1976–1994.e8. doi: 10.1016/j.devcel.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao M., Ren K., Xiong X., Xin Y., Zou Y., Maynard J.C., Kim A., Battist A.P., Koneripalli N., Wang Y., Chen Q., Xin R., Yang C., Huang R., Yu J., Huang Z., Zhang Z., Wang H., Wang D., Xiao Y., Salgado O.C., Jarjour N.N., Hogquist K.A., Revelo X.S., Burlingame A.L., Gao X., von Moltke J., Lin Z., Ruan H.B. Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and Gasdermin C. Immunity. 2022;55:623–638.e5. doi: 10.1016/j.immuni.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang L., He H., Guo X.K., Wang J., Wang W., Li D., Liang S., Shao F., Liu W., Hu X. Intraepithelial mast cells drive gasdermin C-mediated type 2 immunity. Immunity. 2024;57:1056–1070.e5. doi: 10.1016/j.immuni.2024.03.017. [DOI] [PubMed] [Google Scholar]
- 149.Katoh M., Katoh M. Identification and characterization of human DFNA5L, mouse Dfna5l, and rat Dfna5l genes in silico. Int. J. Oncol. 2004;25:765–770. [PubMed] [Google Scholar]
- 150.Du G., Healy L.B., David L., Walker C., El-Baba T.J., Lutomski C.A., Goh B., Gu B., Pi X., Devant P., Fontana P., Dong Y., Ma X., Miao R., Balasubramanian A., Puthenveetil R., Banerjee A., Luo H.R., Kagan J.C., Oh S.F., Robinson C.V., Lieberman J., Wu H. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature. 2024;630:437–446. doi: 10.1038/s41586-024-07373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feng S., Fox D., Man S.M. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J. Mol. Biol. 2018;430:3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 152.Chi Z., Chen S., Yang D., Cui W., Lu Y., Wang Z., Li M., Yu W., Zhang J., Jiang Y., Sun R., Yu Q., Hu T., Lu X., Deng Q., Yang Y., Zhao T., Chang M., Li Y., Zhang X., Shang M., Xiao Q., Ding K., Wang D. Gasdermin D-mediated metabolic crosstalk promotes tissue repair. Nature. 2024 doi: 10.1038/s41586-024-08022-7. [DOI] [PubMed] [Google Scholar]
- 153.Zhang X., Zhang Y., Wang B., Xie C., Wang J., Fang R., Dong H., Fan G., Wang M., He Y., Shen C., Duan Y., Zhao J., Liu Z., Li Q., Ma Y., Yu M., Wang J., Fei J., Xiao L., Huang F. Pyroptosis-mediator GSDMD promotes Parkinson's disease pathology via microglial activation and dopaminergic neuronal death. Brain Behav. Immun. 2024;119:129–145. doi: 10.1016/j.bbi.2024.03.038. [DOI] [PubMed] [Google Scholar]
- 154.Xu B., Jiang M., Chu Y., Wang W., Chen D., Li X., Zhang Z., Zhang D., Fan D., Nie Y., Shao F., Wu K., Liang J. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2018;68:773–782. doi: 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 155.Miao N., Wang Z., Wang Q., Xie H., Yang N., Wang Y., Wang J., Kang H., Bai W., Wang Y., He R., Yan K., Wang Y., Hu Q., Liu Z., Li F., Wang F., Ginhoux F., Zhang X., Yin J., Lu L., Wang J. Oxidized mitochondrial DNA induces gasdermin D oligomerization in systemic lupus erythematosus. Nat. Commun. 2023;14:872. doi: 10.1038/s41467-023-36522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Laer L., Huizing E.H., Verstreken M., van Zuijlen D., Wauters J.G., Bossuyt P.J., Van de Heyning P., McGuirt W.T., Smith R.J., Willems P.J., Legan P.K., Richardson G.P., Van Camp G. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat. Genet. 1998;20:194–197. doi: 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- 157.Zhou B., Jiang Z.H., Dai M.R., Ai Y.L., Xiao L., Zhong C.Q., Wu L.Z., Chen Q.T., Chen H.Z., Wu Q. Full-length GSDME mediates pyroptosis independent from cleavage. Nat. Cell Biol. 2024 doi: 10.1038/s41556-024-01463-2. [DOI] [PubMed] [Google Scholar]
- 158.Neel D.V., Basu H., Gunner G., Bergstresser M.D., Giadone R.M., Chung H., Miao R., Chou V., Brody E., Jiang X., Lee E., Watts M.E., Marques C., Held A., Wainger B., Lagier-Tourenne C., Zhang Y.J., Petrucelli L., Young-Pearse T.L., Chen-Plotkin A.S., Rubin L.L., Lieberman J., Chiu I.M. Gasdermin-E mediates mitochondrial damage in axons and neurodegeneration. Neuron. 2023;111:1222–1240.e9. doi: 10.1016/j.neuron.2023.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei Y., Lan B., Zheng T., Yang L., Zhang X., Cheng L., Tuerhongjiang G., Yuan Z., Wu Y. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 2023;14:929. doi: 10.1038/s41467-023-36614-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhai Z., Yang F., Xu W., Han J., Luo G., Li Y., Zhuang J., Jie H., Li X., Shi X., Han X., Luo X., Song R., Chen Y., Liang J., Wu S., He Y., Sun E. Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor-induced caspase 3/Gasdermin E-Mediated pyroptosis. Arthritis Rheumatol. 2022;74:427–440. doi: 10.1002/art.41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang X., Zhang P., An L., Sun N., Peng L., Tang W., Ma D., Chen J. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm. Sin. B. 2020;10:1397–1413. doi: 10.1016/j.apsb.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Z., Zhang Y., Xia S., Kong Q., Li S., Liu X., Junqueira C., Meza-Sosa K.F., Mok T.M.Y., Ansara J., Sengupta S., Yao Y., Wu H., Lieberman J. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi J., Gao W., Shao F. Pyroptosis: Gasdermin-Mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 164.Liang X., Qin Y., Wu D., Wang Q., Wu H. Pyroptosis: a double-edged sword in lung cancer and other respiratory diseases. Cell Commun. Signal. 2024;22:40. doi: 10.1186/s12964-023-01458-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang X., Tang Z. Role of gasdermin family proteins in cancers. Int. J. Oncol. 2023;63 doi: 10.3892/ijo.2023.5548. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Delmaghani S., Defourny J., Aghaie A., Beurg M., Dulon D., Thelen N., Perfettini I., Zelles T., Aller M., Meyer A., Emptoz A., Giraudet F., Leibovici M., Dartevelle S., Soubigou G., Thiry M., Vizi E.S., Safieddine S., Hardelin J.P., Avan P., Petit C. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell. 2015;163:894–906. doi: 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 167.Xia S., Zhang Z., Magupalli V.G., Pablo J.L., Dong Y., Vora S.M., Wang L., Fu T.M., Jacobson M.P., Greka A., Lieberman J., Ruan J., Wu H. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607–611. doi: 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu M., Deng F., Song X., Zhao H., Yan F. The crosstalk between immune cells and tumor pyroptosis: advancing cancer immunotherapy strategies. J. Exp. Clin. Cancer Res. 2024;43:190. doi: 10.1186/s13046-024-03115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Y., Pan R., Ouyang Y., Gu W., Xiao T., Yang H., Tang L., Wang H., Xiang B., Chen P. Pyroptosis in health and disease: mechanisms, regulation and clinical perspective. Signal Transduct. Targeted Ther. 2024;9:245. doi: 10.1038/s41392-024-01958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kambara H., Liu F., Zhang X., Liu P., Bajrami B., Teng Y., Zhao L., Zhou S., Yu H., Zhou W., Silberstein L.E., Cheng T., Han M., Xu Y., Luo H.R. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 2018;22:2924–2936. doi: 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ding J., Shao F. SnapShot: the noncanonical inflammasome. Cell. 2017;168:544–544 e1. doi: 10.1016/j.cell.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 172.Vanaja S.K., Russo A.J., Behl B., Banerjee I., Yankova M., Deshmukh S.D., Rathinam V.A.K. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and Caspase-11 activation. Cell. 2016;165:1106–1119. doi: 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yi Y.S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017;152:207–217. doi: 10.1111/imm.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Meunier E., Dick M.S., Dreier R.F., Schurmann N., Kenzelmann Broz D., Warming S., Roose-Girma M., Bumann D., Kayagaki N., Takeda K., Yamamoto M., Broz P. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 175.Yang D., He Y., Munoz-Planillo R., Liu Q., Nunez G. Caspase-11 requires the Pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bartlett R., Stokes L., Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- 177.Wang D., Zheng J., Hu Q., Zhao C., Chen Q., Shi P., Chen Q., Zou Y., Zou D., Liu Q., Pei J., Wu X., Gao X., Ren J., Lin Z. Magnesium protects against sepsis by blocking gasdermin D N-terminal-induced pyroptosis. Cell Death Differ. 2020;27:466–481. doi: 10.1038/s41418-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peng Y., Mo R., Yang M., Xie H., Ma F., Ding Z., Wu S., Lam J.W.Y., Du J., Zhang J., Zhao Z., Tang B.Z. Mitochondria-Targeting AIEgens as pyroptosis inducers for boosting Type-I photodynamic therapy of tongue squamous cell carcinoma. ACS Nano. 2024 doi: 10.1021/acsnano.4c06808. [DOI] [PubMed] [Google Scholar]
- 179.Wei W., Wang H., Ren C., Deng R., Qin Q., Ding L., Li P., Liu Y., Chang M., Chen Y., Zhou Y. Ultrasmall enzyodynamic PANoptosis nano-inducers for ultrasound-amplified hepatocellular carcinoma therapy and lung metastasis inhibition. Adv. Mater. 2024 doi: 10.1002/adma.202409618. [DOI] [PubMed] [Google Scholar]
- 180.Xu J., Qiu W., Liang M., Ye M., Hu J., Ma X., Shi X., Xue P., Kang Y., Xiao B., Xu Z. Dual-stimulus phototherapeutic nanogel for triggering pyroptosis to promote cancer immunotherapy. J. Contr. Release. 2023;358:219–231. doi: 10.1016/j.jconrel.2023.04.030. [DOI] [PubMed] [Google Scholar]
- 181.Chen Y., Li W., Kwon S., Wang Y., Li Z., Hu Q. Small-molecule ferritin degrader as a pyroptosis inducer. J. Am. Chem. Soc. 2023;145:9815–9824. doi: 10.1021/jacs.3c01852. [DOI] [PubMed] [Google Scholar]
- 182.Wang Y., Niu B., Tian Y., Lan H., Zhou Z., Li Y., Zhao S., Zhang Y., Yang C., Kong L., Zhang Z. Mitoxantrone combined with engineered TRAIL-nanovesicles for enhanced cancer immunotherapy via converting apoptosis into pyroptosis. Adv. Healthcare Mater. 2024 doi: 10.1002/adhm.202401723. [DOI] [PubMed] [Google Scholar]
- 183.Ding F., Liu J., Ai K., Xu C., Mao X., Liu Z., Xiao H. Simultaneous activation of pyroptosis and cGAS-STING pathway with Epigenetic/photodynamic nanotheranostic for enhanced tumor photoimmunotherapy. Adv. Mater. 2024;36 doi: 10.1002/adma.202306419. [DOI] [PubMed] [Google Scholar]
- 184.Fritsch M., Günther S.D., Schwarzer R., Albert M.C., Schorn F., Werthenbach J.P., Schiffmann L.M., Stair N., Stocks H., Seeger J.M., Lamkanfi M., Krönke M., Pasparakis M., Kashkar H. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 185.Muendlein H.I., Jetton D., Connolly W.M., Eidell K.P., Magri Z., Smirnova I., Poltorak A. cFLIP(L) protects macrophages from LPS-Induced pyroptosis via inhibition of complex II formation. Science. 2020;367:1379–1384. doi: 10.1126/science.aay3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sarhan J., Liu B.C., Muendlein H.I., Li P., Nilson R., Tang A.Y., Rongvaux A., Bunnell S.C., Shao F., Green D.R., Poltorak A. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li Z., Ma R., Tang H., Guo J., Shah Z., Zhang J., Liu N., Cao S., Marcucci G., Artis D., Caligiuri M.A., Yu J. Therapeutic application of human type 2 innate lymphoid cells via induction of granzyme B-mediated tumor cell death. Cell. 2024;187:624–641.e23. doi: 10.1016/j.cell.2023.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao L., Cheng H., Tong Z., Cai J. Nanoparticle-mediated cell pyroptosis: a new therapeutic strategy for inflammatory diseases and cancer. J Nanobiotechnology. 2024;22:504. doi: 10.1186/s12951-024-02763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang D., Zou Y., Huang H., Yin J., Long S., Sun W., Du J., Fan J., Chen X., Peng X. A PROTAC augmenter for photo-driven pyroptosis in breast cancer. Adv. Mater. 2024;36 doi: 10.1002/adma.202313460. [DOI] [PubMed] [Google Scholar]
- 190.Zheng P., Ding B., Zhu G., Li C., Lin J. Biodegradable Ca(2+) nanomodulators activate pyroptosis through mitochondrial Ca(2+) overload for cancer immunotherapy. Angew Chem. Int. Ed. Engl. 2022;61 doi: 10.1002/anie.202204904. [DOI] [PubMed] [Google Scholar]
- 191.Wang X., Hua P., He C., Chen M. Non-apoptotic cell death-based cancer therapy: molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B. 2022;12:3567–3593. doi: 10.1016/j.apsb.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li Y., Guo Y., Zhang K., Zhu R., Chen X., Zhang Z., Yang W. Cell death pathway regulation by functional nanomedicines for robust antitumor immunity. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202306580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen B.L., Yan Y., Yang Y., Cao G., Wang X., Wang Y.Q., Wan F.J., Yin Q.Q., Wang Z.H., Li Y.F., Wang L.T., Xu B., You F.P., Zhang Q., Wang Y.G. A pyroptosis nanotuner for cancer therapy. Nat. Nanotechnol. 2022;17:788–798. doi: 10.1038/s41565-022-01125-0. [DOI] [PubMed] [Google Scholar]
- 194.Wang W.J., Zhang L., Zhang Y.J., Liu X.M., Song A.J., Ren J.S., Qu X.G. A self-adaptive pyroptosis inducer: optimizing the catalytic microenvironment of nanozymes by membrane-adhered microbe enables potent cancer immunotherapy. Adv. Mater. 2024;36:11. doi: 10.1002/adma.202310063. [DOI] [PubMed] [Google Scholar]
- 195.Su W., Qiu W., Li S.J., Wang S., Xie J., Yang Q.C., Xu J., Zhang J., Xu Z., Sun Z.J. A dual-responsive STAT3 inhibitor nanoprodrug combined with oncolytic virus elicits synergistic antitumor immune responses by igniting pyroptosis. Adv. Mater. 2023;35 doi: 10.1002/adma.202209379. [DOI] [PubMed] [Google Scholar]
- 196.Zhong Y.T., Qiu Z.W., Zhang K.Y., Lu Z.M., Li Z.F., Cen Y., Li S.Y., Cheng H. Plasma membrane targeted photodynamic nanoagonist to potentiate immune checkpoint blockade therapy by initiating tumor cell pyroptosis and depleting infiltrating B cells. Adv. Mater. 2025;37 doi: 10.1002/adma.202415078. [DOI] [PubMed] [Google Scholar]
- 197.Imianowski C.J., Chen Q., Workman C.J., Vignali D.A.A. Regulatory T cells in the tumour microenvironment. Nat. Rev. Cancer. 2025 doi: 10.1038/s41568-025-00832-9. [DOI] [PubMed] [Google Scholar]
- 198.Xiao H., Li X., Liang S., Yang S., Han S., Huang J., Shuai X., Ren J. Dual-Responsive nanomedicine activates programmed antitumor immunity through targeting lymphatic system. ACS Nano. 2024;18:11070–11083. doi: 10.1021/acsnano.3c11464. [DOI] [PubMed] [Google Scholar]
- 199.Hu W., Ye B., Yu G., Yang H., Wu H., Ding Y., Huang F., Wang W., Mao Z. Dual-Responsive supramolecular polymeric nanomedicine for self-cascade amplified cancer immunotherapy. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202305382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Long Y., Fan J., Zhou N., Liang J., Xiao C., Tong C., Wang W., Liu B. Biomimetic Prussian blue nanocomplexes for chemo-photothermal treatment of triple-negative breast cancer by enhancing ICD. Biomaterials. 2023;303 doi: 10.1016/j.biomaterials.2023.122369. [DOI] [PubMed] [Google Scholar]
- 201.Li F., Zhang X.Q., Ho W., Tang M., Li Z., Bu L., Xu X. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat. Commun. 2023;14:4223. doi: 10.1038/s41467-023-39938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Feng Y., Wang G., Li W., Yan J., Yu X., Tian H., Li B., Dai Y. PhotoPyro-Induced cGAS-STING pathway activation enhanced anti-melanoma immunotherapy via a manganese-coordinated nanomedicine. Adv. Healthcare Mater. 2024;13 doi: 10.1002/adhm.202302811. [DOI] [PubMed] [Google Scholar]
- 203.Wang S., Guo Q., Xu R., Lin P., Deng G., Xia X. Combination of ferroptosis and pyroptosis dual induction by triptolide nano-MOFs for immunotherapy of Melanoma. J Nanobiotechnology. 2023;21:383. doi: 10.1186/s12951-023-02146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hu R., Chen X., Li Z., Zhao G., Ding L., Chen L., Dai C., Chen Y., Zhang B. Liquid nanoparticles for nanocatalytic cancer therapy. Adv. Mater. 2023;35 doi: 10.1002/adma.202306469. [DOI] [PubMed] [Google Scholar]
- 205.Yang Y., Yang J., Zhu N., Qiu H., Feng W., Chen Y., Chen X., Chen Y., Zheng W., Liang M., Lin T., Yu J., Guo Z. Tumor-targeting hydroxyapatite nanoparticles for remodeling tumor immune microenvironment (TIME) by activating mitoDNA-pyroptosis pathway in cancer. J Nanobiotechnology. 2023;21:470. doi: 10.1186/s12951-023-02231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.He T., Wen J., Wang W., Hu Z., Ling C., Zhao Z., Cheng Y., Chang Y.C., Xu M., Jin Z., Amer L., Sasi L., Fu L., Steinmetz N.F., Rana T.M., Wu P., Jokerst J.V. Peptide-driven proton sponge nano-assembly for imaging and triggering lysosome-regulated immunogenic cancer cell death. Adv. Mater. 2024;36 doi: 10.1002/adma.202307679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nadeem S., Yang C., Du Y., Li F., Chen Z., Zhou Y., Lee J.Y., Ding Q., Ling D. A virus-spike tumor-activatable pyroptotic agent. Small. 2021;17 doi: 10.1002/smll.202006599. [DOI] [PubMed] [Google Scholar]
- 208.Xu X., Zheng J., Liang N., Zhang X., Shabiti S., Wang Z., Yu S., Pan Z.Y., Li W., Cai L. Bioorthogonal/ultrasound activated oncolytic pyroptosis amplifies in situ tumor vaccination for boosting antitumor immunity. ACS Nano. 2024;18:9413–9430. doi: 10.1021/acsnano.3c11023. [DOI] [PubMed] [Google Scholar]
- 209.Wang H., He Z., Gao Y., Feng D., Wei X., Huang Y., Hou J., Li S., Zhang W. Dual-pronged attack: ph-driven membrane-anchored NIR dual-type nano-photosensitizer excites immunogenic pyroptosis and sequester immune checkpoint for enhanced prostate cancer photo-immunotherapy. Adv. Sci. (Weinh.) 2023;10 doi: 10.1002/advs.202302422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yagoda N., von Rechenberg M., Zaganjor E., Bauer A.J., Yang W.S., Fridman D.J., Wolpaw A.J., Smukste I., Peltier J.M., Boniface J.J., Smith R., Lessnick S.L., Sahasrabudhe S., Stockwell B.R. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang C., Liu X., Jin S., Chen Y., Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol. Cancer. 2022;21:47. doi: 10.1186/s12943-022-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Huang L., McClatchy D.B., Maher P., Liang Z., Diedrich J.K., Soriano-Castell D., Goldberg J., Shokhirev M., Yates J.R., 3rd, Schubert D., Currais A. Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death. Cell Death Dis. 2020;11:828. doi: 10.1038/s41419-020-03020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fang X., Ardehali H., Min J., Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023;20:7–23. doi: 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li P., Jiang M., Li K., Li H., Zhou Y., Xiao X., Xu Y., Krishfield S., Lipsky P.E., Tsokos G.C., Zhang X. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 2021;22:1107–1117. doi: 10.1038/s41590-021-00993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li D., Jiang C., Mei G., Zhao Y., Chen L., Liu J., Tang Y., Gao C., Yao P. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12 doi: 10.3390/nu12102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Meng Z., Liang H., Zhao J., Gao J., Liu C., Ma X., Liu J., Liang B., Jiao X., Cao J., Wang Y. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119935. [DOI] [PubMed] [Google Scholar]
- 219.Hurrell R., Egli I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010;91:1461s–1467s. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 220.Abbaspour N., Hurrell R., Kelishadi R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 221.Yiannikourides A., Latunde-Dada G.O. A short review of iron metabolism and pathophysiology of iron disorders. Medicines (Basel) 2019;6 doi: 10.3390/medicines6030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Salnikow K. Role of iron in cancer. Semin. Cancer Biol. 2021;76:189–194. doi: 10.1016/j.semcancer.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 223.Koonyosying P., Garbowski M., Schaeper U., Martinez A., Porter J.B. Iron chelation efficiency in human hepatocytes is enhanced by exogenous hepcidin. Blood. 2023;142 doi: 10.1182/blood-2023-182214. 2468-2468. [DOI] [Google Scholar]
- 224.Parrow N.L., Li Y., Feola M., Guerra A., Casu C., Prasad P., Mammen L., Ali F., Vaicikauskas E., Rivella S., Ginzburg Y.Z., Fleming R.E. Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood. 2019;134:1373–1384. doi: 10.1182/blood.2018893099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Robach P., Cairo G., Gelfi C., Bernuzzi F., Pilegaard H., Vigano A., Santambrogio P., Cerretelli P., Calbet J.A., Moutereau S., Lundby C. Strong iron demand during hypoxia-induced erythropoiesis is associated with down-regulation of iron-related proteins and myoglobin in human skeletal muscle. Blood. 2007;109:4724–4731. doi: 10.1182/blood-2006-08-040006. [DOI] [PubMed] [Google Scholar]
- 226.Lill R., Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 227.Coates T.D. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic. Biol. Med. 2014;72:23–40. doi: 10.1016/j.freeradbiomed.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim S.L., Shin S., Yang S.J. Iron homeostasis and energy metabolism in obesity. Clin Nutr Res. 2022;11:316–330. doi: 10.7762/cnr.2022.11.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu Q., Carlos A.R., Braza F., Bergman M.L., Kitoko J.Z., Bastos-Amador P., Cuadrado E., Martins R., Oliveira B.S., Martins V.C., Scicluna B.P., Landry J.J., Jung F.E., Ademolue T.W., Peitzsch M., Almeida-Santos J., Thompson J., Cardoso S., Ventura P., Slot M., Rontogianni S., Ribeiro V., Domingues V.D.S., Cabral I.A., Weis S., Groth M., Ameneiro C., Fidalgo M., Wang F., Demengeot J., Amsen D., Soares M.P. Ferritin heavy chain supports stability and function of the regulatory T cell lineage. Embo j. 2024;43:1445–1483. doi: 10.1038/s44318-024-00064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lu J., Francis J., Doster R.S., Haley K.P., Craft K.M., Moore R.E., Chambers S.A., Aronoff D.M., Osteen K., Damo S.M., Manning S., Townsend S.D., Gaddy J.A. Lactoferrin: a critical mediator of both host immune response and antimicrobial activity in response to streptococcal infections. ACS Infect. Dis. 2020;6:1615–1623. doi: 10.1021/acsinfecdis.0c00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Berthou C., Iliou J.P., Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2022;3:263–275. doi: 10.1002/jha2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cheli V.T., Correale J., Paez P.M., Pasquini J.M. Iron metabolism in oligodendrocytes and astrocytes, implications for myelination and remyelination. ASN Neuro. 2020;12 doi: 10.1177/1759091420962681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sanvisens N., Bañó M.C., Huang M., Puig S. Regulation of ribonucleotide reductase in response to iron deficiency. Mol. Cell. 2011;44:759–769. doi: 10.1016/j.molcel.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bonyadian M., Moeini E., Ebrahimnejad H., Askari N., Karimi I. The effect of iron sulfate nanoparticles and their fortified bread on wistar rats and human cell lines. J. Trace Elem. Med. Biol. 2022;73 doi: 10.1016/j.jtemb.2022.127005. [DOI] [PubMed] [Google Scholar]
- 235.Gerlach M., Ben-Shachar D., Riederer P., Youdim M.B. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J. Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 236.Kalinowski D.S., Stefani C., Toyokuni S., Ganz T., Anderson G.J., Subramaniam N.V., Trinder D., Olynyk J.K., Chua A., Jansson P.J., Sahni S., Lane D.J., Merlot A.M., Kovacevic Z., Huang M.L., Lee C.S., Richardson D.R. Redox cycling metals: pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochim. Biophys. Acta. 2016;1863:727–748. doi: 10.1016/j.bbamcr.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 237.Cézard C., Rabbind Singh A., Le Gac G., Gourlaouen I., Ferec C., Rochette J. Phenotypic expression of a novel C282Y/R226G compound heterozygous state in HFE hemochromatosis: molecular dynamics and biochemical studies. Blood Cells Mol. Dis. 2014;52:27–34. doi: 10.1016/j.bcmd.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 238.Liu Y., Nguyen M., Robert A., Meunier B. Metal ions in alzheimer's disease: a key role or not? Acc. Chem. Res. 2019;52:2026–2035. doi: 10.1021/acs.accounts.9b00248. [DOI] [PubMed] [Google Scholar]
- 239.Zheng C., Zhang B., Li Y., Liu K., Wei W., Liang S., Guo H., Ma K., Liu Y., Wang J., Liu L. Donafenib and GSK-J4 synergistically induce ferroptosis in liver cancer by upregulating HMOX1 expression. Adv. Sci. (Weinh.) 2023;10 doi: 10.1002/advs.202206798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chang K., Chen Y., Zhang X., Zhang W., Xu N., Zeng B., Wang Y., Feng T., Dai B., Xu F., Ye D., Wang C. DPP9 stabilizes NRF2 to suppress ferroptosis and induce sorafenib resistance in clear cell renal cell carcinoma. Cancer Res. 2023;83:3940–3955. doi: 10.1158/0008-5472.Can-22-4001. [DOI] [PubMed] [Google Scholar]
- 241.Hong X., Roh W., Sullivan R.J., Wong K.H.K., Wittner B.S., Guo H., Dubash T.D., Sade-Feldman M., Wesley B., Horwitz E., Boland G.M., Marvin D.L., Bonesteel T., Lu C., Aguet F., Burr R., Freeman S.S., Parida L., Calhoun K., Jewett M.K., Nieman L.T., Hacohen N., Näär A.M., Ting D.T., Toner M., Stott S.L., Getz G., Maheswaran S., Haber D.A. The lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses ferroptosis. Cancer Discov. 2021;11:678–695. doi: 10.1158/2159-8290.Cd-19-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang C.K., Chen T.J., Tan G.Y.T., Chang F.P., Sridharan S., Yu C.A., Chang Y.H., Chen Y.J., Cheng L.T., Hwang-Verslues W.W. MEX3A mediates p53 degradation to suppress ferroptosis and facilitate ovarian cancer tumorigenesis. Cancer Res. 2023;83:251–263. doi: 10.1158/0008-5472.Can-22-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu C., Sun L., Niu N., Hou P., Chen G., Wang H., Zhang Z., Jiang X., Xu Q., Zhao Y., Wang Y., Shi Y., Liu M., Yang Y., Qian W., Wang J., Liu C. Molecular classification of hormone receptor-positive/HER2-positive breast cancer reveals potential neoadjuvant therapeutic strategies. Signal Transduct. Targeted Ther. 2025;10:97. doi: 10.1038/s41392-025-02181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Du Y., Guo Z. Recent progress in ferroptosis: inducers and inhibitors. Cell Death Discov. 2022;8:501. doi: 10.1038/s41420-022-01297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sun S., Shen J., Jiang J., Wang F., Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Targeted Ther. 2023;8:372. doi: 10.1038/s41392-023-01606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Luo L., Wang H., Tian W., Li X., Zhu Z., Huang R., Luo H. Targeting ferroptosis-based cancer therapy using nanomaterials: strategies and applications. Theranostics. 2021;11:9937–9952. doi: 10.7150/thno.65480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen Z., Li R., Fang M., Wang Y., Bi A., Yang L., Song T., Li Y., Li Q., Lin B., Jia Y., Fu S., Fu S., Xiong H. Integrated analysis of FKBP1A/SLC3A2 axis in everolimus inducing ferroptosis of breast cancer and anti-proliferation of T lymphocyte. Int. J. Med. Sci. 2023;20:1060–1078. doi: 10.7150/ijms.84872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lei G., Mao C., Yan Y., Zhuang L., Gan B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 2021;12:836–857. doi: 10.1007/s13238-021-00841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hao X., Zheng Z., Liu H., Zhang Y., Kang J., Kong X., Rong D., Sun G., Sun G., Liu L., Yu H., Tang W., Wang X. Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol. 2022;56 doi: 10.1016/j.redox.2022.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li M., Wang M., Huang J., Tang S., Yang J., Xu Z., Xu G., Chen X., Liu J., Yang C. High-performance pyrite nano-catalyst driven photothermal/chemodynamic synergistic therapy for osteosarcoma. J Nanobiotechnology. 2024;22:141. doi: 10.1186/s12951-024-02419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bu J., Zhang Y., Wu S., Li H., Sun L., Liu Y., Zhu X., Qiao X., Ma Q., Liu C., Niu N., Xue J., Chen G., Yang Y., Liu C. KK-LC-1 as a therapeutic target to eliminate ALDH+ stem cells in triple negative breast cancer. Nat. Commun. 2023;14 doi: 10.1038/s41467-023-38097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yao X., Xie R., Cao Y., Tang J., Men Y., Peng H., Yang W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnology. 2021;19:311. doi: 10.1186/s12951-021-01058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tang H., Li C., Zhang Y., Zheng H., Cheng Y., Zhu J., Chen X., Zhu Z., Piao J.G., Li F. Targeted manganese doped silica nano GSH-cleaner for treatment of liver cancer by destroying the intracellular redox homeostasis. Theranostics. 2020;10:9865–9887. doi: 10.7150/thno.46771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhou T.J., Zhang M.M., Liu D.M., Huang L.L., Yu H.Q., Wang Y., Xing L., Jiang H.L. Glutathione depletion and dihydroorotate dehydrogenase inhibition actuated ferroptosis-augment to surmount triple-negative breast cancer. Biomaterials. 2024;305 doi: 10.1016/j.biomaterials.2023.122447. [DOI] [PubMed] [Google Scholar]
- 255.Bae C., Kim H., Kook Y.M., Lee C., Kim C., Yang C., Park M.H., Piao Y., Koh W.G., Lee K. Induction of ferroptosis using functionalized iron-based nanoparticles for anti-cancer therapy. Mater. Today Bio. 2022;17 doi: 10.1016/j.mtbio.2022.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang L., Tong L., Xiong Z., Chen Y., Zhang P., Gao Y., Liu J., Yang L., Huang C., Ye G., Du J., Liu H., Yang W., Wang Y. Ferroptosis-inducing nanomedicine and targeted short peptide for synergistic treatment of hepatocellular carcinoma. J Nanobiotechnology. 2024;22:533. doi: 10.1186/s12951-024-02808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Huang Y., Lin Y., Li B., Zhang F., Zhan C., Xie X., Yao Z., Wu C., Ping Y., Shen J. Combination therapy to overcome ferroptosis resistance by biomimetic self-assembly nano-prodrug. Asian J. Pharm. Sci. 2023;18 doi: 10.1016/j.ajps.2023.100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhang Z., Lo H., Zhao X., Li W., Wu K., Zeng F., Li S., Sun H. Mild photothermal/radiation therapy potentiates ferroptosis effect for ablation of breast cancer via MRI/PA imaging guided all-in-one strategy. J Nanobiotechnology. 2023;21:150. doi: 10.1186/s12951-023-01910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Song G., Li M., Fan S., Qin M., Shao B., Dai W., Zhang H., Wang X., He B., Zhang Q. Boosting synergism of chemo- and immuno-therapies via switching paclitaxel-induced apoptosis to mevalonate metabolism-triggered ferroptosis by bisphosphonate coordination lipid nanogranules. Acta Pharm. Sin. B. 2024;14:836–853. doi: 10.1016/j.apsb.2023.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen Z., Li Z., Li C., Huang H., Ren Y., Li Z., Hu Y., Guo W. Manganese-containing polydopamine nanoparticles as theranostic agents for magnetic resonance imaging and photothermal/chemodynamic combined ferroptosis therapy treating gastric cancer. Drug Deliv. 2022;29:1201–1211. doi: 10.1080/10717544.2022.2059124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tang L., Ling M., Syeda M.Z., Sun R., He M., Mu Q., Zhu X., Huang C., Cui L. A smart nanoplatform for enhanced photo-ferrotherapy of hepatocellular carcinoma. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1022330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tian Y., He X., Yuan Y., Zhang S., Wang C., Dong J., Liu Z., Jing H. TME-responsive nanoplatform with glutathione depletion for enhanced tumor-specific mild photothermal/gene/ferroptosis synergistic therapy. Int. J. Nanomed. 2024;19:9145–9160. doi: 10.2147/ijn.S475698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chin Y.C., Yang L.X., Hsu F.T., Hsu C.W., Chang T.W., Chen H.Y., Chen L.Y., Chia Z.C., Hung C.H., Su W.C., Chiu Y.C., Huang C.C., Liao M.Y. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J Nanobiotechnology. 2022;20:373. doi: 10.1186/s12951-022-01575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chen H., Wen J. Iron oxide nanoparticles loaded with paclitaxel inhibits glioblastoma by enhancing autophagy-dependent ferroptosis pathway. Eur. J. Pharmacol. 2022;921 doi: 10.1016/j.ejphar.2022.174860. [DOI] [PubMed] [Google Scholar]
- 265.Lomphithak T., Helvacioglu S., Armenia I., Keshavan S., Ovejero J.G., Baldi G., Ravagli C., Grazú V., Fadeel B. High-dose exposure to polymer-coated iron oxide nanoparticles elicits autophagy-dependent ferroptosis in susceptible cancer cells. Nanomaterials. 2023;13 doi: 10.3390/nano13111719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wen J., Chen H., Ren Z., Zhang P., Chen J., Jiang S. Ultrasmall iron oxide nanoparticles induced ferroptosis via Beclin1/ATG5-dependent autophagy pathway. Nano Converg. 2021;8:10. doi: 10.1186/s40580-021-00260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhang M., Bao S., Qiu G., Liang J., Wang Q., Zhu X., Qin G., Liu J., Zhao C. An magnetic-targeting nano-diagnosis and treatment platform for TNBC. Breast Cancer. 2023;15:101–119. doi: 10.2147/bctt.S387793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rao Z., Xia Y., Jia Q., Zhu Y., Wang L., Liu G., Liu X., Yang P., Ning P., Zhang R., Zhang X., Qiao C., Wang Z. Iron-based metal-organic framework co-loaded with buthionine sulfoximine and oxaliplatin for enhanced cancer chemo-ferrotherapy via sustainable glutathione elimination. J Nanobiotechnology. 2023;21:265. doi: 10.1186/s12951-023-01998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jiang Y., Zhao X., Huang J., Li J., Upputuri P.K., Sun H., Han X., Pramanik M., Miao Y., Duan H., Pu K., Zhang R. Transformable hybrid semiconducting polymer nanozyme for second near-infrared photothermal ferrotherapy. Nat. Commun. 2020;11:1857. doi: 10.1038/s41467-020-15730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang J., Ding L., Yu L., Wang Y., Ge M., Jiang Q., Chen Y. Nanomedicine enables autophagy-enhanced cancer-cell ferroptosis. Sci Bull (Beijing). 2021;66:464–477. doi: 10.1016/j.scib.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 271.Qiao X., Zhang Y., Zhang Z., Niu N., Li H., Sun L., Ma Q., Bu J., Liu J., Chen G., Xue J., Yang Y., Liu C. KCNJ15 deficiency promotes drug resistance via affecting the function of lysosomes. Asian J. Pharm. Sci. 2023;18 doi: 10.1016/j.ajps.2023.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen B., Yan Y., Yang Y., Cao G., Wang X., Wang Y., Wan F., Yin Q., Wang Z., Li Y., Wang L., Xu B., You F., Zhang Q., Wang Y. A pyroptosis nanotuner for cancer therapy. Nat. Nanotechnol. 2022;17:788–798. doi: 10.1038/s41565-022-01125-0. [DOI] [PubMed] [Google Scholar]
- 273.Wang W., Zhang L., Zhang Y., Liu X., Song A., Ren J., Qu X. A self-adaptive pyroptosis inducer: optimizing the catalytic microenvironment of nanozymes by membrane-adhered microbe enables potent cancer immunotherapy. Adv. Mater. 2024;36 doi: 10.1002/adma.202310063. [DOI] [PubMed] [Google Scholar]
- 274.Jiang C., Li X., Pan F., Zhang L., Yu H., Zhang J., Zou J., Zhong T., Zhang D., Yang Y., Li Y., Zhang P. Ferroptosis and pyroptosis co‐activated nanomodulator for “cold” tumor immunotherapy and lung metastasis inhibition. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202211698. [DOI] [Google Scholar]
- 275.Zhang L., Qiu M., Wang R., Li S., Liu X., Xu Q., Xiao L., Jiang Z.X., Zhou X., Chen S. Monitoring ROS responsive Fe(3)O(4)-based nanoparticle mediated ferroptosis and immunotherapy via (129)Xe MRI. Angew Chem. Int. Ed. Engl. 2024;63 doi: 10.1002/anie.202403771. [DOI] [PubMed] [Google Scholar]
- 276.Liu B., Chen X., Zhu Y., Chen H., Tan J., Yang Z., Li J., Zheng P., Feng L., Wang Q., Gai S., Zhong L., Yang P., Cheng Z., Lin J. One-step symbiosis of bimetallic peroxides nanoparticles to induce ferroptosis/cuproptosis and activate cGAS-STING pathway for enhanced tumor immunotherapy. Adv. Mater. 2025;37 doi: 10.1002/adma.202500337. [DOI] [PubMed] [Google Scholar]
- 277.Liu Y., Niu R., Zhang X., Zhang B., Chen X., Guo J., Song S., Wang Y., Zhang H., Zhao Y. Metal-organic framework-based nanovaccine for relieving immunosuppressive tumors via hindering efferocytosis of macrophages and promoting pyroptosis and cuproptosis of cancer cells. ACS Nano. 2024;18:12386–12400. doi: 10.1021/acsnano.4c01518. [DOI] [PubMed] [Google Scholar]
- 278.Zhu G., Wang M., Qiao L., Xie Y., Wang J., Li L., Sun Q., Zheng P., Li C. Lysosomal rupture‐mediated “Broken Window Effect” to amplify cuproptosis and pyroptosis for high‐efficiency cancer immunotherapy. Adv. Funct. Mater. 2024;34 doi: 10.1002/adfm.202400496. [DOI] [Google Scholar]
- 279.Wang Y.Y., Li S.L., Zhang X.Y., Jiang F.L., Guo Q.L., Jiang P., Liu Y. "Multi-in-One" yolk-shell structured nanoplatform inducing pyroptosis and antitumor immune response through Cascade reactions. Small. 2024;20 doi: 10.1002/smll.202400254. [DOI] [PubMed] [Google Scholar]
- 280.Qiao L., Zhu G., Jiang T., Qian Y., Sun Q., Zhao G., Gao H., Li C. Self-destructive copper carriers induce pyroptosis and cuproptosis for efficient tumor immunotherapy against dormant and recurrent tumors. Adv. Mater. 2024;36 doi: 10.1002/adma.202308241. [DOI] [PubMed] [Google Scholar]
- 281.Zhu Y., Niu X., Ding C., Lin Y., Fang W., Yan L., Cheng J., Zou J., Tian Y., Huang W., Huang W., Pan Y., Wu T., Chen X., Kang D. Carrier-free self-assembly nano-sonosensitizers for sonodynamic-amplified cuproptosis-ferroptosis in glioblastoma therapy. Adv. Sci. (Weinh.) 2024;11 doi: 10.1002/advs.202402516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Li Y., Ma J., Wang R., Luo Y., Zheng S., Wang X. Zinc transporter 1 functions in copper uptake and cuproptosis. Cell Metab. 2024;36:2118–2129 e6. doi: 10.1016/j.cmet.2024.07.009. [DOI] [PubMed] [Google Scholar]
- 283.Xie L., Gong J., He Z., Zhang W., Wang H., Wu S., Wang X., Sun P., Cai L., Wu Z., Wang H. A copper-manganese based nanocomposite induces cuproptosis and potentiates anti-tumor immune responses. Small. 2025;21 doi: 10.1002/smll.202412174. [DOI] [PubMed] [Google Scholar]
- 284.Zhang L., Song A., Yang Q.C., Li S.J., Wang S., Wan S.C., Sun J., Kwok R.T.K., Lam J.W.Y., Deng H., Tang B.Z., Sun Z.J. Integration of AIEgens into covalent organic frameworks for pyroptosis and ferroptosis primed cancer immunotherapy. Nat. Commun. 2023;14:5355. doi: 10.1038/s41467-023-41121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Li H., Deng X., Zhang Z., Yang Z., Huang H., Ye X., Zhong L., Xu G., Liu R., Fang Y. Nitric oxide/paclitaxel micelles enhance anti-liver cancer effects and paclitaxel sensitivity by inducing ferroptosis, endoplasmic reticulum stress and pyroptosis. RSC Adv. 2023;13:31772–31784. doi: 10.1039/d3ra04861f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhu L., Hu J., Wu X., Zhang J., Xu X., Huang X., Tian B., Zhao C.X., Du Y., Wu L. Programmed enhancement of endogenous iron-mediated lysosomal membrane permeabilization for tumor ferroptosis/pyroptosis dual-induction. Nat. Commun. 2025;16:3017. doi: 10.1038/s41467-025-58124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhao P., Yin S., Qiu Y., Sun C., Yu H. Ferroptosis and pyroptosis are connected through autophagy: a new perspective of overcoming drug resistance. Mol. Cancer. 2025;24:23. doi: 10.1186/s12943-024-02217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang H., Qiao C., Guan Q., Wei M., Li Z. Nanoparticle-mediated synergistic anticancer effect of ferroptosis and photodynamic therapy: novel insights and perspectives. Asian J. Pharm. Sci. 2023;18 doi: 10.1016/j.ajps.2023.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang Q., Chen D., Liu X., Deng Z., Li J., Zhu S., Ma B., Liu R., Zhu H. High photocytotoxicity Iridium(III) complex photosensitizer for photodynamic therapy induces antitumor effect through GPX4-Dependent ferroptosis. Small. 2025;21 doi: 10.1002/smll.202403165. [DOI] [PubMed] [Google Scholar]
- 290.Bi J., Han Y., Han X., Wu Y., Liao S., Zhang Y., Han X., Wang Z., Li J., Zheng A., Bi W. Activation of pyroptosis and immunogenic cell death by targeted liposomal cisplatin for enhanced chemo-immunotherapy for osteosarcoma. Nano Today. 2025;62 doi: 10.1016/j.nantod.2025.102717. [DOI] [Google Scholar]
- 291.Mehrotra P., Maschalidi S., Boeckaerts L., Maueroder C., Tixeira R., Pinney J., Burgoa Cardas J., Sukhov V., Incik Y., Anderson C.J., Hu B., Keceli B.N., Goncalves A., Vande Walle L., Van Opdenbosch N., Sergushichev A., Hoste E., Jain U., Lamkanfi M., Ravichandran K.S. Oxylipins and metabolites from pyroptotic cells act as promoters of tissue repair. Nature. 2024;631:207–215. doi: 10.1038/s41586-024-07585-9. [DOI] [PubMed] [Google Scholar]
- 292.Zhu W., Cheng X., Xu P., Gu Y., Xu H., Xu J., Wang Y., Zhang L.W., Wang Y. Radiotherapy-driven nanoprobes targeting for visualizing tumor infiltration dynamics and inducing ferroptosis in myeloid-derived suppressor cells. J. Am. Chem. Soc. 2024;146:22455–22468. doi: 10.1021/jacs.4c05650. [DOI] [PubMed] [Google Scholar]
- 293.Eweje F., Ibrahim V., Shajii A., Walsh M.L., Ahmad K., Alrefai A., Miyasato D., Davis J.R., Ham H., Li K., Roehrl M., Haller C.A., Liu D.R., Chen J., Chaikof E.L. Self-assembling protein nanoparticles for cytosolic delivery of nucleic acids and proteins. Nat. Biotechnol. 2025 doi: 10.1038/s41587-025-02664-2. [DOI] [PubMed] [Google Scholar]
- 294.Zhang H., Lv J., Wu H., He Y., Li M., Wu C., Lv D., Liu Y., Yang H. Endogenous/Exogenous dual-responsive nanozyme for photothermally enhanced ferroptosis-immune reciprocal synergistic tumor therapy. Sci. Adv. 2025;11 doi: 10.1126/sciadv.adq3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhou Z., Wang H., Li J., Jiang X., Li Z., Shen J. Recent progress, perspectives, and issues of engineered PD-L1 regulation nano-system to better cure tumor: a review. Int. J. Biol. Macromol. 2024;254 doi: 10.1016/j.ijbiomac.2023.127911. [DOI] [PubMed] [Google Scholar]
- 296.Zhou Z., Liu Y., Jiang X., Zheng C., Luo W., Xiang X., Qi X., Shen J. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int. J. Biol. Macromol. 2023;224:797–809. doi: 10.1016/j.ijbiomac.2022.10.167. [DOI] [PubMed] [Google Scholar]
- 297.Zheng C., Luo W., Liu Y., Chen J., Deng H., Zhou Z., Shen J. Killing three birds with one stone: multi-stage metabolic regulation mediated by clinically useable berberine liposome to overcome photodynamic immunotherapy resistance. Chem. Eng. J. 2023;454 doi: 10.1016/j.cej.2022.140164. [DOI] [Google Scholar]
- 298.Cai S., Chen Z., Wang Y., Wang M., Wu J., Tong Y., Chen L., Lu C., Yang H. Reducing PD-L1 expression with a self-assembled nanodrug: an alternative to PD-L1 antibody for enhanced chemo-immunotherapy. Theranostics. 2021;11:1970–1981. doi: 10.7150/thno.45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Numasawa K., Hanaoka K., Saito N., Yamaguchi Y., Ikeno T., Echizen H., Yasunaga M., Komatsu T., Ueno T., Miura M., Nagano T., Urano Y. A fluorescent probe for rapid, high-contrast visualization of folate-receptor-expressing tumors in vivo. Angew Chem. Int. Ed. Engl. 2020;59:6015–6020. doi: 10.1002/anie.201914826. [DOI] [PubMed] [Google Scholar]
- 300.Ru Q., Li Y., Chen L., Wu Y., Min J., Wang F. Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects. Signal Transduct. Targeted Ther. 2024;9:271. doi: 10.1038/s41392-024-01969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kumar S., Shukla M.K., Sharma A.K., Jayaprakash G.K., Tonk R.K., Chellappan D.K., Singh S.K., Dua K., Ahmed F., Bhattacharyya S., Kumar D. Metal-based nanomaterials and nanocomposites as promising frontier in cancer chemotherapy. MedComm. 2023;4 doi: 10.1002/mco2.253. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ranjbar Bahadori S., Mulgaonkar A., Hart R., Wu C.Y., Zhang D., Pillai A., Hao Y., Sun X. Radiolabeling strategies and pharmacokinetic studies for metal based nanotheranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1671. [DOI] [PubMed] [Google Scholar]
- 303.Mohammadi M.R., Corbo C., Molinaro R., Lakey J.R.T. Biohybrid nanoparticles to negotiate with biological barriers. Small. 2019;15 doi: 10.1002/smll.201902333. [DOI] [PubMed] [Google Scholar]
- 304.Lenders V., Koutsoumpou X., Phan P., Soenen S.J., Allegaert K., de Vleeschouwer S., Toelen J., Zhao Z., Manshian B.B. Modulation of engineered nanomaterial interactions with organ barriers for enhanced drug transport. Chem. Soc. Rev. 2023;52:4672–4724. doi: 10.1039/d1cs00574j. [DOI] [PubMed] [Google Scholar]
- 305.Jiang S., Hao B., Song X., Jiang Y., Guo J., Wang Y., Wang Q., Wang X., Xu T., Wu X., Chan K.F., Chiu P.W.Y., Zhang L. Living microalgae-based magnetic microrobots for calcium overload and photodynamic synergetic cancer therapy. Adv. Healthcare Mater. 2025;14 doi: 10.1002/adhm.202403866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chauhan D.S., Reddy B.P.K., Mishra S.K., Prasad R., Dhanka M., Vats M., Ravichandran G., Poojari D., Mhatre O., De A., Srivastava R. Comprehensive evaluation of degradable and cost-effective plasmonic nanoshells for localized photothermolysis of cancer cells. Langmuir. 2019;35:7805–7815. doi: 10.1021/acs.langmuir.8b03460. [DOI] [PubMed] [Google Scholar]
- 307.Hitchcock J., White A.L., Hondow N., Hughes T.A., Dupont H., Biggs S., Cayre O.J. Metal-shell nanocapsules for the delivery of cancer drugs. J. Colloid Interface Sci. 2020;567:171–180. doi: 10.1016/j.jcis.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 308.Liyanage P.Y., Hettiarachchi S.D., Zhou Y., Ouhtit A., Seven E.S., Oztan C.Y., Celik E., Leblanc R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Canc. 2019;1871:419–433. doi: 10.1016/j.bbcan.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Asmatulu E., Andalib M.N., Subeshan B., Abedin F. Impact of nanomaterials on human health: a review. Environ. Chem. Lett. 2022;20:2509–2529. doi: 10.1007/s10311-022-01430-z. [DOI] [Google Scholar]
- 310.Barabadi H., Mobaraki K., Ashouri F., Noqani H., Jounaki K., Mostafavi E. Nanobiotechnological approaches in antinociceptive therapy: animal-based evidence for analgesic nanotherapeutics of bioengineered silver and gold nanomaterials. Adv. Colloid Interface Sci. 2023;316 doi: 10.1016/j.cis.2023.102917. [DOI] [PubMed] [Google Scholar]
- 311.Dubashynskaya N.V., Petrova V.A., Skorik Y.A. Biopolymer drug delivery systems for oromucosal application: recent trends in pharmaceutical R&D. Int. J. Mol. Sci. 2024;25 doi: 10.3390/ijms25105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kafle U., Agrawal S., Dash A.K. Injectable nano drug delivery systems for the treatment of breast cancer. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14122783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chen H., Zhou M., Zeng Y., Miao T., Luo H., Tong Y., Zhao M., Mu R., Gu J., Yang S., Han L. Biomimetic lipopolysaccharide-free bacterial outer membrane-functionalized nanoparticles for brain-targeted drug delivery. Adv. Sci. (Weinh.) 2022;9 doi: 10.1002/advs.202105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Haftek M., Abdayem R., Guyonnet-Debersac P. Skin minerals: key roles of inorganic elements in skin physiological functions. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23116267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mills J.A., Liu F., Jarrett T.R., Fletcher N.L., Thurecht K.J. Nanoparticle based medicines: approaches for evading and manipulating the mononuclear phagocyte system and potential for clinical translation. Biomater. Sci. 2022;10:3029–3053. doi: 10.1039/d2bm00181k. [DOI] [PubMed] [Google Scholar]
- 316.Ansari M.A., Shoaib S., Chauhan W., Gahtani R.M., Hani U., Alomary M.N., Alasiri G., Ahmed N., Jahan R., Yusuf N., Islam N. Nanozymes and carbon-dots based nanoplatforms for cancer imaging, diagnosis and therapeutics: current trends and challenges. Environ. Res. 2024;241 doi: 10.1016/j.envres.2023.117522. [DOI] [PubMed] [Google Scholar]
- 317.Zhang X., Zhao Q., Yang J., Wang T., Chen F., Zhang K. Tumor microenvironment-triggered intratumoral in-situ biosynthesis of inorganic nanomaterials for precise tumor diagnostics. Coord. Chem. Rev. 2023;484 doi: 10.1016/j.ccr.2023.215115. [DOI] [Google Scholar]
- 318.Liu Z., Cao T., Xue Y., Li M., Wu M., Engle J.W., He Q., Cai W., Lan M., Zhang W. Self‐amplified photodynamic therapy through the 1O2‐Mediated internalization of photosensitizers from a ppa‐bearing block copolymer. Angew. Chem. 2020;132:3740–3746. doi: 10.1002/ange.201914434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tran X., Mulac D., Langer K. Investigating the selectivity of the targeting properties of trastuzumab-bound nanoparticles to HER2/neu positive cells in co-culture. J. Drug Deliv. Sci. Technol. 2024;100 doi: 10.1016/j.jddst.2024.106040. [DOI] [Google Scholar]
- 320.Patra S., Pradhan B., Nayak R., Behera C., Rout L., Jena M., Efferth T., Bhutia S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021;73:310–320. doi: 10.1016/j.semcancer.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 321.Di Filippo L.D., de Carvalho S.G., Duarte J.L., Luiz M.T., Paes Dutra J.A., de Paula G.A., Chorilli M., Conde J. A receptor-mediated landscape of druggable and targeted nanomaterials for gliomas. Mater. Today Bio. 2023;20 doi: 10.1016/j.mtbio.2023.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hallan S.S., Amirian J., Brangule A., Bandere D. Lipid-based nano-sized cargos as a promising strategy in bone complications: a review. Nanomaterials. 2022;12 doi: 10.3390/nano12071146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lim A.W., Talley N.J., Walker M.M., Storm G., Hua S. Current status and advances in esophageal drug delivery technology: influence of physiological, pathophysiological and pharmaceutical factors. Drug Deliv. 2023;30 doi: 10.1080/10717544.2023.2219423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Xu Y., Hsu J.C., Xu L., Chen W., Cai W., Wang K. Nanomedicine-based adjuvant therapy: a promising solution for lung cancer. J Nanobiotechnology. 2023;21:211. doi: 10.1186/s12951-023-01958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wang Y., Cui Y., Zhao Y., Zhao Q., He B., Zhang Q., Wang S. Effects of surface modification and size on oral drug delivery of mesoporous silica formulation. J. Colloid Interface Sci. 2018;513:736–747. doi: 10.1016/j.jcis.2017.11.065. [DOI] [PubMed] [Google Scholar]
- 326.Cheng P.S.W., Zaccaria M., Biffi G. Functional heterogeneity of fibroblasts in primary tumors and metastases. Trends Cancer. 2025;11:135–153. doi: 10.1016/j.trecan.2024.11.005. [DOI] [PubMed] [Google Scholar]
- 327.Wang Y., Wang J., Jiao Y., Chen K., Chen T., Wu X., Jiang X., Bu W., Liu C., Qu X. Redox-active polyphenol nanoparticles deprive endogenous glutathione of electrons for ROS generation and tumor chemodynamic therapy. Acta Biomater. 2023;172:423–440. doi: 10.1016/j.actbio.2023.09.037. [DOI] [PubMed] [Google Scholar]
- 328.Zhang P., Chen D., Li L., Sun K. Charge reversal nano-systems for tumor therapy. J Nanobiotechnology. 2022;20:31. doi: 10.1186/s12951-021-01221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Higuchi T., Klimek K., Groener D., Chen X., Werner R.A. Norepinephrine transporter-targeted cancer theranostics-new Horizons. Clin. Nucl. Med. 2025;50:44–51. doi: 10.1097/RLU.0000000000005567. [DOI] [PubMed] [Google Scholar]
- 330.Huang X., Hou S., Li Y., Xu G., Xia N., Duan Z., Luo K., Tian B. Targeting lipid metabolism via nanomedicine: a prospective strategy for cancer therapy. Biomaterials. 2025;317 doi: 10.1016/j.biomaterials.2024.123022. [DOI] [PubMed] [Google Scholar]
- 331.Li F., Zhang X.-Q., Ho W., Tang M., Li Z., Bu L., Xu X. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat. Commun. 2023;14 doi: 10.1038/s41467-023-39938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Jiang Q., Wang K., Zhang X., Ouyang B., Liu H., Pang Z., Yang W. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020;16 doi: 10.1002/smll.202001704. [DOI] [PubMed] [Google Scholar]
- 333.Tao Q., Liu N., Wu J., Chen J., Chen X., Peng C. Mefloquine enhances the efficacy of anti-PD-1 immunotherapy via IFN-gamma-STAT1-IRF1-LPCAT3-induced ferroptosis in tumors. J. Immunother. Cancer. 2024;12 doi: 10.1136/jitc-2023-008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lin R., Zhang Z., Chen L., Zhou Y., Zou P., Feng C., Wang L., Liang G. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016;381:165–175. doi: 10.1016/j.canlet.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 335.Lim J., Lee J., Boo Y., Kim W.J. A polymeric iron oxide nanocomplex loaded with sulfasalazine: an approach for inducing ferritinophagy-assisted ferroptosis for anti-cancer therapy. Nanoscale. 2024;16:742–751. doi: 10.1039/d3nr04733d. [DOI] [PubMed] [Google Scholar]
- 336.Gai C., Yu M., Li Z., Wang Y., Ding D., Zheng J., Lv S., Zhang W., Li W. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. J. Cell. Physiol. 2020;235:3329–3339. doi: 10.1002/jcp.29221. [DOI] [PubMed] [Google Scholar]
- 337.Kapper C., Oppelt P., Arbeithuber B., Gyunesh A.A., Vilusic I., Stelzl P., Rezk-Fureder M. Targeting ferroptosis in ovarian cancer: novel strategies to overcome chemotherapy resistance. Life Sci. 2024;349 doi: 10.1016/j.lfs.2024.122720. [DOI] [PubMed] [Google Scholar]
- 338.Ma S., Henson E.S., Chen Y., Gibson S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.208. e2307-e2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sahebkar A., Foroutan Z., Katsiki N., Jamialahmadi T., Mantzoros C.S. Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: is there a role for statin therapy? Metabolism. 2023;146 doi: 10.1016/j.metabol.2023.155659. [DOI] [PubMed] [Google Scholar]
- 340.Singhal R., Mitta S.R., Das N.K., Kerk S.A., Sajjakulnukit P., Solanki S., Andren A., Kumar R., Olive K.P., Banerjee R., Lyssiotis C.A., Shah Y.M. HIF-2alpha activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J. Clin. Investig. 2021;131 doi: 10.1172/JCI143691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Teng K., Ma H., Gai P., Zhao X., Qi G. SPHK1 enhances olaparib resistance in ovarian cancer through the NFκB/NRF2/ferroptosis pathway. Cell Death Discov. 2025;11 doi: 10.1038/s41420-025-02309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wu J., Zhang X., Sun D., Shi X., Sun J., Luo C., He Z., Zhang S. Molecularly engineering a lipid nanoregulator destroying the last defense of ferroptosis in breast cancer therapy. Chem. Eng. J. 2024;495 doi: 10.1016/j.cej.2024.153366. [DOI] [Google Scholar]
- 343.Khachigian L.M. Emerging insights on functions of the anthelmintic flubendazole as a repurposed anticancer agent. Cancer Lett. 2021;522:57–62. doi: 10.1016/j.canlet.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 344.Yang J., Mo J., Dai J., Ye C., Cen W., Zheng X., Jiang L., Ye L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12:1079. doi: 10.1038/s41419-021-04367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Antoszczak M., Muller S., Caneque T., Colombeau L., Dusetti N., Santofimia-Castano P., Gaillet C., Puisieux A., Iovanna J.L., Rodriguez R. Iron-Sensitive prodrugs that trigger active ferroptosis in drug-tolerant pancreatic cancer cells. J. Am. Chem. Soc. 2022;144:11536–11545. doi: 10.1021/jacs.2c03973. [DOI] [PubMed] [Google Scholar]
- 346.Xiao Y., Yin J., Liu P., Zhang X., Lin Y., Guo J. Triptolide-induced cuproptosis is a novel antitumor strategy for the treatment of cervical cancer. Cell. Mol. Biol. Lett. 2024;29:113. doi: 10.1186/s11658-024-00623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wang L., Li K., Lin X., Yao Z., Wang S., Xiong X., Ning Z., Wang J., Xu X., Jiang Y., Liu D., Chen Y., Zhang D., Zhang H. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 2019;450:22–31. doi: 10.1016/j.canlet.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 348.Guo Y., Li Y., Zhang M., Ma R., Wang Y., Weng X., Zhang J., Zhang Z., Chen X., Yang W. Polymeric nanocarrier via metabolism regulation mediates immunogenic cell death with spatiotemporal orchestration for cancer immunotherapy. Nat. Commun. 2024;15:8586. doi: 10.1038/s41467-024-53010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]