Abstract
Objective
Acute respiratory distress syndrome (ARDS) is characterized by an acute inflammatory response in the lung parenchyma leading to severe hypoxemia. Because of its anti-inflammatory and immunomodulatory properties, omega-3 polyunsaturated fatty acids (ω-3 PUFA) have been administered to ARDS patients, mostly by the enteral route, as immune-enhancing diets with eicosapentaenoic acid, γ-linolenic acid, and antioxidants. However, clinical benefits of ω-3 PUFAs in ARDS patients remain unclear because clinical trials have found conflicting results. Considering the most recent randomized controlled trials (RCTs) and recent change in administration strategies, the aim of this updated systematic review and meta-analysis was to evaluate clinical benefits of ω-3 PUFA administration on gas exchange and clinical outcomes in ARDS patients.
Methods
We searched for RCTs conducted in intensive care unit (ICU) patients with ARDS comparing the administration of ω-3 PUFAs to placebo. The outcomes assessed were PaO2-to-FiO2 ratio evaluated early (3–4 d) and later (7–8 d), mortality, ICU and hospital length of stay (LOS), length of mechanical ventilation (MV), and infectious complications. Two independent reviewers assessed eligibility, risk of bias, and abstracted data. Data were pooled using a random effect model to estimate the relative risk or weighted mean difference (WMD).
Results
Twelve RCTs (n = 1280 patients) met our inclusion criteria. Omega-3 PUFAs administration was associated with a significant improvement in early PaO2-to-FiO2 ratio (WMD = 49.33; 95% confidence interval [CI] 20.88–77.78; P = 0.0007; I2 = 69%), which persisted at days 7 to 8 (WMD = 27.87; 95% CI 0.75–54.99; P = 0.04; I2 = 57%). There was a trend in those receiving ω-3 PUFA toward reduced ICU LOS (P = 0.08) and duration of MV (P = 0.06), whereas mortality, hospital LOS, and infectious complications remained unchanged. Continuous enteral infusion was associated with reduced mortality (P = 0.02), whereas analysis restricted to enteral administration either with or without bolus found improved early PaO2 and FiO2 (P = 0.001) and MV duration (P = 0.03). Trials at higher risk of bias had a significant reduction in mortality (P = 0.04), and improvement in late PaO2-to-FiO2 ratio (P = 0.003).
Conclusions
In critically ill patients with ARDS, ω-3 PUFAs in enteral immunomodulatory diets may be associated with an improvement in early and late PaO2-to-FiO2 ratio, and statistical trends exist for an improved ICU LOS and MV duration. Considering these results, administering ω-3 PUFAs appears a reasonable strategy in ARDS.
Keywords: Omega-3, Fish oil, Acute respiratory distress syndrome, Gas exchange
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by excessive lung parenchyma inflammation, which may lead to life-threatening hypoxemia. As described by the 2014 Berlin criteria [1], ARDS is developed in the first week after an initial insult, often from a non-pulmonary sepsis or trauma. The subsequent non-cardiogenic lung edema creates significant shunt and deteriorates the oxygenation of the organism. Therefore the PaO2-to-FiO2 ratio, which corresponds to the arterial pressure of oxygen divided by the inspired fraction of oxygen, is the preferred tool to quantify the severity of the ARDS.
Omega-3 PUFAs are now well known for their anti-inflammatory and immunomodulatory properties [2], [3]. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), ω-3 PUFAs mostly found in fish oil (FO), have been provided enterally or parenterally as constituents of immune-enhancing diets to critically ill patients with ARDS. γ-Linolenic acid (GLA), an ω-6 PUFA present in borage oil, has been co-administered enterally [4]. Several randomized controlled trials (RCTs) have been conducted over the past 20 years with the aim to evaluate the potential benefits of fish oil on clinical outcomes in ARDS patients. In 2008, after aggregating 3 RCTs, Pontes Arruda et al. [4] found a significant reduction of mortality and improvement of clinical outcomes related to mechanical ventilation, including improved gas exchange, after continuous infusion of an immune-enhancing diets with EPA, DHA and GLA. Nevertheless, more recent trials using an enteral bolus of ω-3 PUFAs as the administration strategy have shown conflicting results [5], [6]. According to current evidence, the 2015 Canadian Clinical Practice Guidelines [7] recommended that enteral formulas containing fish oils with borage oil and antioxidants should be considered in ARDS. With regard to enteral fish oil administered alone as a supplement, the Canadian guidelines concluded that there was insufficient data to make a recommendation. In 2016 the American Society for Parenteral and Enteral Nutrition and the Society of Critical Care Medicine [8] did not make a recommendation about the use of inflammation-modulating diets enriched with EPA and GLA in ARDS. More recently the ESPEN Expert Group [9] have supported the administration of fish oil in critically ill patients, although no specific recommendation in ARDS patients was made.
Thus, ω-3 PUFAs supplementation in ARDS patients remains controversial, current guidelines are divergent, and no meta-analyses reached a conclusion on the potential benefits on gas exchange. We conducted an updated systematic review and meta-analysis of ω-3 PUFAs use in critically ill patients with ARDS. The aim of the review was to elucidate whether ω-3 PUFAs administration by enteral or parenteral route could improve gas exchange as primary outcome, as well as other clinically relevant outcomes in ARDS.
Methods
The manuscript was written according to the PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-analysis) guidelines [10].
Data sources and searches
We conducted a literature search through Medline, CINAHL, EMBASE and Cochrane databases to find all RCTs published from inception to December 2017. Broad MeSH headings and keywords containing “fish oil,” “omega-3 polyunsaturated fatty acids,” “docosahexaenoic,” “eicosapentaenoic,” and “lipid emulsion” in combination with “critically ill,” “acute respiratory distress syndrome,” “acute lung injury,” or “intensive care unit” were used with no language restrictions. All terms were exploded when possible. Personal files and relevant articles’ reference lists were reviewed to optimize study identification. If a trial was only available as an abstract, if the publication was in inaccessible language for the authors, or if required data were missing during abstraction, the authors were contacted for additional details.
Study selection
Studies were eligible if they corresponded to the following characteristics:
-
1.
Design: Randomized controlled design with parallel groups.
-
2.
Population: Patients 18 y and older hospitalized in any kind of intensive care unit (ICU) and presenting an ARDS as defined by the authors. When ambiguous, we considered the population to be from an ICU if the mortality in the control group was >5%.
-
3.
Intervention: Oral, enteral or parenteral ω-3 PUFA administration for at least 3 consecutive days.
-
4.
Comparator: Either placebo or a non-fish oil nutritional therapy administered in context of standard nutrition.
-
5.
Outcomes: The trials had to report either the primary outcome, PaO2-to-FiO2 ratio assessing patient's oxygenation, or any of the following secondary clinical outcomes: 30-d or hospital mortality; ICU length of stay (LOS); hospital LOS; duration of ventilation, including both invasive and non-invasive; or incidence of infectious complications.
Data extraction and quality assessment
Databases were searched and screened for relevant titles and abstracts. Using piloted, pretested forms, two independent reviewers (P.L.L. and W.M.) confirmed eligibility and abstracted data from the included citations, notably population, intervention strategy with dose, mean and length of administration, control strategy, primary and secondary outcome data, results, funding sources, and information on methodological quality for each study. If the contacted authors did not respond and the data sought was available in a previous publication for which authors had been contacted, we included the data in our analysis.
To be used, the authors had to report the mean value of the outcome and the standard deviation. When only interquartile range were reported, the authors were contacted. When exact data were unavailable but presented in a graph, authors were contacted and data were estimated visually from graphs. For the primary outcome of PaO2-to-FiO2 ratio, early assessment was subjectively defined as after 3 to 4 d of ω-3 administration and late was defined as 7 to 8 d, as are reported in most RCTs. When mean PaO2 and FiO2 were independently presented but the ratio was absent, authors were contacted and no ratio was calculated because of the imprecision and relation that we deemed existent between both variables. ICU and hospital LOS, and mechanical ventilation (MV) duration, were reported in days. If 28-d mortality was reported, it was used preferably for the aggregation. When 28-d mortality was not reported, we used hospital mortality. Data reported later than at day 28 were not aggregated. Finally, infections were defined accordingly to the authors’ definition.
Risk of bias
The risk of bias was assessed in duplicate by two independent reviewers simultaneously to data abstraction. A scoring system from 0 to 14 (see Supplementary Material) evaluated risk of bias accordingly to the following criteria:
-
1.
Randomization concealment
-
2.
Blinding
-
3.
Comparability of groups at baseline
-
4.
Intention-to-treat analysis
-
5.
Extent of follow-up
-
6.
Description of the intervention protocol
-
7.
Cointerventions similarities between groups
-
8.
Prespecified outcomes
For further analysis, a trial was designated as a level 1 study with low risk of bias if all the criteria were fulfilled: concealed randomization, blinded outcome adjudication, and an intention-to-treat analysis. A study was considered as level 2 study with higher risk of bias if any one of the above characteristics was unfulfilled.
Statistical Analysis
Review Manager (RevMan) 5.3 (Cochrane IMS, Oxford, UK) was used for all analyses, except for the test for asymmetry, Comprehensive Meta-Analysis v3 software (Biostat, Englewood, New Jersey). After aggregatin the available data from the trials reporting the analyzed outcome, the pooled risk ratio (RR) with 95% confidence intervals (CIs) was calculated using the Mantel-Haenszel test for each categorical variable. For continuous data, the inverse variance approach was used to estimate the overall weighted mean difference (WMD) and their confidence intervals. To estimate variances for Mantel-Haenszel and inverse variance estimators, the random effects model of DerSimonian and Laird [11] was used. To evaluate heterogeneity in the results, Mantel-Haenszel χ2 and Cochrane I2 value were respectively for its evaluation and quantification. Heterogeneity was quantified by the I2 measure (low, 25–49%; moderate, 50–74%; high, >75%) [12]. Differences between subgroups were analyzed using the test of subgroup differences described by Deeks et al. [13] and the results expressed using the P values [13]. To evaluate the risk of publication bias, we generated a funnel plot and tested for the asymmetry of the outcomes, as proposed by Egger et al. [14]. Throughout the statistical analysis, we considered a P value to be statistically significant if <0.05 and to be an indicator of trends if <0.10.
Subgroup and sensitivity analysis
Predefined subgroup analyses according to the risk of bias (level 1 versus level 2) were conducted. In a similar objective, subgroup analyses were conducted to compare single-center to multicenter studies. Considering the apparent differences in the results obtained in the last decade, probably because of new administration strategies, subgroup analysis comparing older (before 2011) and newer (after 2011) RCTs were conducted. Finally, sensitivity analyses restricted to oral or enteral administration and restricted to RCTs using continuous enteral administration of ω-3 PUFA were both conducted.
Results
Study selection and characteristics
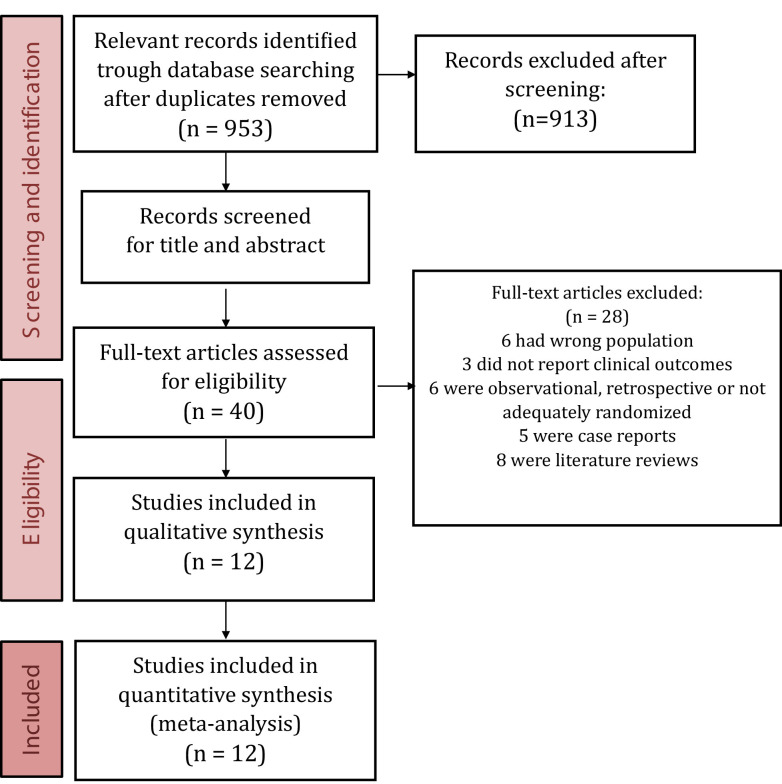
Of the 953 citations identified in our search, we reviewed the full text of 40. Ultimately, 12 RCTs and 1280 patients were included (Fig. 1 ).
Fig. 1.
PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-analysis) flow diagram.
A detailed description of all included trials is presented in Table 1 . The results of each trial are found in Table 2 . Five trials were multicenter, whereas the remaining recruited from a single center, and three trials were conducted before 2011. Three RCTs recruited sepsis-induced ARDS patients [4], [15], [16], one enrolled trauma patients [17], and the remainder recruited a heterogeneous ICU patient population with ARDS [5], [6], [18], [19], [20], [21], [22], [23].
Table 1.
Reported outcomes of included randomized controlled trials evaluating fish oil in ARDS patients
| Study | Population | Methods (score) | Intervention |
|---|---|---|---|
| Gadek et al. 1999[19] | ARDS patients from 5 ICUs n = 146 (5 centers) |
C. random: yes ITT: yes Blinding: yes (13) |
Fish oil, borage oil + antioxidants (Oxepa) vs. standard high-fat, low-CHO nutrition (Pulmocare) Received 9.8 g/d fish oils (EPA + DHA) |
| Singer 2006[23] | ARDS and ALI patients n = 100 (1 center) |
C. random: yes ITT: yes Blinding: no (11) |
Fish oil, borage oil + antioxidants (Oxepa) vs. standard high-fat, low-CHO nutrition (Pulmocare) |
| Pontes-Arruda 2006[16] | Severe sepsis or septic shock patients with ALI from 3 ICUs n = 165 (1 center) |
C. random: not sure ITT: yes Blinding: double (7) |
Fish oil, borage oil, + antioxidants (Oxepa) vs. standard high-fat, low-CHO nutrition (Pulmocare) Received 7.1 g/d of fish oils (EPA + DHA) |
| Rice et al. 2011[6] | ALI patients, mechanically ventilated from 44 ICUs n = 272 (44 centers) |
C. random: yes ITT: yes Blinding: yes (13) |
Fish oil supplement (6.84 g EPA, 3.4 g DHA, 5.92 g GLA) with 5.8 g protein, vitamins C and E, beta-carotene, selenium 120 cc boluses twice daily vs. isovolemic control solution (no EPA/DHA) with 52 g protein Both groups received EN feeding |
| Grau-Carmona et al. 2011[15] | Septic patients with ALI or ARDS n = 160 (11 centers) |
C. random: no ITT: no Blinding: yes (5) |
Fish oil, borage oil, + antioxidants (Oxepa) 52.5g pro/L vs. isocaloric, isonitrogenous, high-protein formula (Ensure Plus) 66.6 g pro/L isocaloric |
| Theilla et al. 2011[23] | ICU patients with pressure ulcers n = 40 (1 center) |
C. random: no ITT: yes Blinding: no (5) |
Fish oil, borage oil, antioxidants 66.1 g pro/day (Oxepa) vs. isocaloric/isonitrogenous polymeric formula (Jevity) 65.1 g pro/day |
| Stapleton et al. 2011[5] | ALI patients (trauma, sepsis, shock) from 5 ICUs n = 90 (5 centers) |
C. random: Yes ITT: Yes Blinding: Yes (12) |
Fish oil (9.75 g EPA, 6.75 g DHA/day × 14 d as bolus q6h) vs. 0.9% saline isonitrogenous diet |
| Gupta et al. 2011[20] | ICU patients with suspected ARDS (criteria not given) n = 61 (1 center) |
C. random: yes ITT: yes Blinding: double (9) |
Omegaven 10% in combination with standard enteral diet for 14 d; ω-3/ω-6 ratio of 1:2 |
| Elamin et al. 2012[18] | ARDS patients from 2 ICUs n = 22 (2 centers) |
C. random: yes ITT: no Blinding: double (7) |
EN formula containing fish oil, borage oil, and antioxidants (Oxepa) vs. EN formula of standard high-fat, low-CHO formula (Pulmocare) |
| Parish et al. 2014[21] | ARDS patients from 2 ICUs n = 58 (1 center) |
C. random: yes ITT: yes Blinding: double (7) |
EN formula (not specified) + 6 ω-3 soft gels/day (2 capsules q8h; 360 mg EPA and 240 mg DHA per two capsules) vs. EN formula (not specified) and placebo (not specified) |
| Kagan et al. 2015[17] | Multiple trauma or head injury patients from a single ICU n = 120 (1 center) |
C. random: yes ITT: yes Blinding: double (10) |
EN formula containing fish oil, borage oil, and antioxidants (Oxepa) vs. EN formula of standard high-fat, low-CHO nutrition (Pulmocare) |
| Shirai et al. 2015[16] | Sepsis-induced ARDS mechanically ventilated patients n = 46 (1 center) |
C. random: no ITT: yes Blinding: no (10) |
Enteral diet enriched with EPA, GLA, and antioxidants (Oxepa) vs. standard isocaloric enteral diet (Ensure Liquid) |
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; C. random, concealed randomization; CHO, carbohydrate; DHA, docosahexaenoic acid; EN, enteral nutrition; EPA, eicosapentaenoic acid; GLA, γ-linoleic acid; ICU, intensive care unit; ITT, intention to treat; MODS, multiple organ dysfunction syndrome; NR, non-reported; VAP, ventilator-associated pneumonia.
Table 2.
Summary of randomized clinical trials evaluating ω-3 PUFAs in ARDS
| Study | Mortality (%) |
Infections (%) |
Length of stay (days) |
Duration of ventilation (days) |
Other |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fish oils | Standard | Fish oils | Standard | Fish oils | Standard | Fish oils | Standard | Fish oils | Standard | |
| Gadek et al. 1999[19] | 28-d 11/70 (16) |
28-d 19/76 (25) |
NR | NR | ICU 11.0 ± 7.5 Hospital 27.9 ± 17.6 |
ICU 14.8 ± 11.0 Hospital 31.1 ± 20.4 |
9.6 ± 7.5 | 13.2 ± 11.9 | New organ failures | |
| 7/70 (10) | 19/76 (25) | |||||||||
| Singer et al. 2006[22] | 28-d 14/46 (30) |
28-d 26/49 (53) |
NR | NR | ICU 13.5 ± 11.8 |
ICU 15.6 ± 11.8 |
12.1 ± 11.3 | 14.7 ± 12 | NR | |
| Pontes-Arruda et al. 2006[4] | 28-d 26/83 (31) |
28-d 38/82 (46) |
NR | NR | ICU 17.2 ± 4.9 |
ICU 23.4 ± 3.5 |
14.64 ± 4.3 (55) | 22.19 ± 5.1 | New organ dysfunction | |
| 38% | 81% | |||||||||
| Rice et al. 2011[6] | 60-d 38/143 (27) |
60-d 21/129 (16) |
VAP 10/143 (7) Bacteremia 16/143 (11) |
VAP 10/129 (8) Bacteremia 14/129 (11) |
ICU-free days 14.0 ± 10.5 |
ICU-free days 16.7 ± 9.5 |
Ventilator-free days 14.0 ± 11.1 |
Ventilator-free days 17.2 ± 10.2 |
Non-pulmonary organ failure–free days | |
| 12.3 ± 11.1 | 15.5 ± 11.4 | |||||||||
| Grau-Carmona et al. 2011[15] | 28-d 11/61 (18) |
28-d 11/71 (16) |
VAP 32/61 (53) |
VAP 34/71 (48) |
ICU 16 (11–25) |
ICU 18 (10–30) |
10 (6–14) | 9 (6–18) | Nutritional intake 1 (kcal/day) | |
| 718 (1189–1965) | 1599 (1351–1976) | |||||||||
| Theilla et al. 2011[23] | NR | NR | NR | NR | ICU 26.1 ± 14.2 |
ICU 21.2 ± 9.1 |
NR | NR | Change in pressure ulcers Scale | |
| 1.5 | 0.3 | |||||||||
| Stapleton et al. 2011[5] | Hospital 10/41 (22) 60-d 9/41 (23) |
Hospital 10/49 (20) 60-d 12/49 (24) |
NR | NR) | ICU 11.9 ± 10.6 Hospital 23.0 ± 18.3 ICU-free days 12 ± 11 Hospital-free days 23 ± 19 |
ICU 17.4 ± 14.8 Hospital 27.6 ± 20.6 ICU-free days 11 ± 10 Hospital- free days 27.5 ± 22 |
8.6 ± 9.0 (38) Ventilator-free days 14.8 ± 10 |
12.9 ± 12.2 Ventilator-free days 14.0 ± 10 |
Nutritional intake in first wk | |
| 7362 ± 3800 kcal | 7495 ± 3831 kcal | |||||||||
| Gupta et al. 2011[20] | ICU 7/31 (23) Hospital 9/31 (29) |
ICU 13/30 (43) Hospital 14/30 (47) |
NR | NR | ICU 15.96 ± 7.6 Hospital 21.5 ± 13.5 |
ICU 15.88 ± 6.5 Hospital 26.6 ± 18.2 |
11.8 ± 10.6 | 10.7 ± 14.6 | NR | |
| Elamin et al. 2012[18] | 28-d 0/9 (0) |
28-d 1/8 (12.5) |
NR | NR | ICU 12.8 |
ICU 17.5 |
6.7 | 8.2 | MODS score at 7 d Lower in fish oil group (P < 0.06) MODS score at 28 d Lower in fish oil group (P < 0.05) |
|
| Parish et al. 2014[21] | 28-d 7/29 (26) |
28-d 9/29 (32) |
NR | NR | ICU 15 ± 3.5 |
ICU 15.6 ± 4.3 |
Ventilator-free days 6.6 ± 2 |
Ventilator-free days 6 ± 2.5 |
NR | |
| Kagan et al. 2015[17] | 28-d 8/62 (13) |
28-d 5/58 (8) |
VAP 25/62 (40) Wound infection 12/62 Bacteremia14/62 |
VAP 22/58 (38) Wound infection 10/58 Bacteremia 3/58 |
ICU 19.5 ± 15.3 Hospital 33.1 + 25.7 |
ICU 16.4 ± 11.3 Hospital 27.1 + 17.3 |
17 ± 15.1 | 13.6 ± 10.7 | New organ failure | |
| 31/62 | 23/58 | |||||||||
| Shirai et al. 2015[16] | 60-d 3/23 (13) |
60-d 3/23 (13) |
10/23 (44) | 12/23 (52) | ICU 17.6 ± 8.2 |
ICU 25.87 ± 12.5 |
13.6 ± 4.8 | 17.8 ± 8.6 | NR | |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; MODS, multiorgan dysfunction syndrome; NR, not reported; PUFA, polyunsaturated fatty acid; VAP, ventilator-associated pneumonia.
Eight studies administered an enteral formula (Oxepa, Abbott Nutrition, Chicago, IL) containing 4.6 g/L of DHA, 1.9 g/L EPA, 4.6 g/L GLA, and antioxidants [4], [15], [16], [17], [18], [19], [22], [23], whereas others used Ultimate Omega (Nordic Naturals, Watsonville, CA) [5], FO capsules [21], parenteral Omegaven (Fresenius Kabi, Bad Homburg vor der Hohe, Germany) [20], and a non-specified enteral formula [6]. Administration duration varied across different trials. A total of 11 trials administered ω-3 PUFAs enterally.
Individual study risk of bias is presented in Table 1. The mean methodological score was evaluated on a 14-point scale. The mean score of all RCTs was 9.1 and the median value was 10 (range 5–13). Randomization was concealed in 8 out of 12 (67%) trials, whereas intention-to-treat analysis was performed in 10 out of 12 (83%) trials and double blinding in 9 out of 12 (75%) of the studies.
Primary outcome
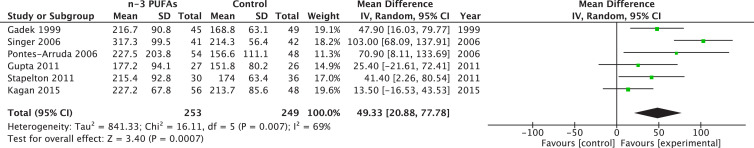
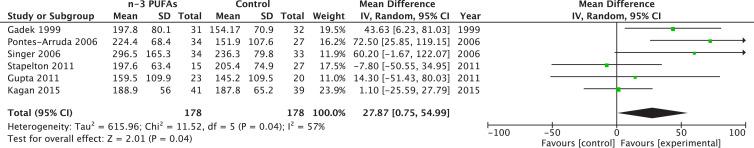
Six different trials reported a PaO2-to-FiO2 ratio at day 3 to 4 (early) totaling 502 patients, whereas six trials reported PaO2-to-FiO2 ratio at day 7 to 8 (late) for a total of 306 patients. The pooled estimate suggests that ω-3 PUFAs reduce early PaO2-to-FiO2 ratio (WMD 49.33; 95% CI 20.88–77.78; P = 0.0007; Fig. 2 ) and late (WMD 27.87; 95% CI 0.75–54.99; P = 0.04). Heterogeneity was moderate with I2 value of 69% and 57%, respectively (Fig. 3 ).
Fig. 2.
Effects of fish oil on early PaO2/FiO2 in acute respiratory distress syndrome. CI, confidence interval; df, degrees of freedom; IV, instrumental variables; PUFA, polyunsaturated fatty acids; SD, standard deviation.
Fig. 3.
Effects of fish oil on late PaO2/FiO2 in acute respiratory distress syndrome. CI, confidence interval; df, degrees of freedom; IV, instrumental variables; PUFA, polyunsaturated fatty acids; SD, standard deviation.
Secondary outcomes
Overall effect on mortality
When the 10 trials totaling 1165 patients were aggregated, there was no effect on mortality (RR = 0.84; 95% CI 0.57–1.24; P = 0.38; see Supplemental Fig. 1). No statistical heterogeneity was found in the data. Seven of these nine trials reported 28-d mortality and two reported hospital mortality. Two trials were not included in the aggregation because only 60-d mortality was reported.
ICU length of stay
A total of nine trials and 754 patients were aggregated to evaluate ω-3 PUFAs administration on ICU LOS. No statistical benefits existed, but a trend was found toward a reduction (WMD –2.28, 95% CI –4.82 to 0.25; P = 0.08; see Supplemental Fig. 2). High heterogeneity existed in the data, with I2 evaluated at 79%. Unfortunately, three trials [6], [15], [18] did not report data in units interpretable for this meta-analysis.
Hospital length of stay
When aggregating the four trials and 411 patients reporting hospital LOS as an outcome, no effect was found (see Supplemental Fig. 3).
Overall effect on ventilator days
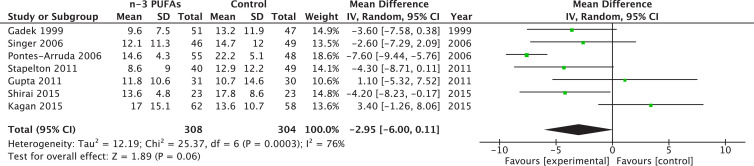
Seven trials reported data on MV duration, for a total of 612 patients. After aggregation, statistical significance was not reached, but a trend existed toward an improvement of MV duration (WMD –2.95; 95% CI –6.00 to 0.11; P = 0.06; see Fig. 4 ). However, heterogeneity was highly significant with a I2 at 76%. Three RCT reported data in non-usable variables for this aggregation [6], [15], [21].
Fig. 4.
Effects of fish oil on mechanical ventilation days in acute respiratory distress syndrome (ARDS). CI, confidence interval; df, degrees of freedom; IV, instrumental variables; PUFA, polyunsaturated fatty acids; SD, standard deviation.
Overall new infections
No effect was found when the four trials (n = 570) reporting infections after ω-3 administration were aggregated (see Supplemental Fig. 4). No heterogeneity existed in this analysis.
Subgroup analysis
Higher versus lower methodological quality
A trial was considered as having high methodological quality if it was a level 1 trial, as previously defined. A significant difference existed in both subgroups regarding early PaO2-to-FiO2 ratio (P = 0.0004) and late PaO2-to-FiO2 ratio (P = 0.01). The value was improved only in the level 2 trials, with lower methodological quality. No significant difference in subgroups existed regarding mortality (P = 0.08) and duration of MV (P = 0.0l9).
Single versus multicenter trials
When compared, no differences between subgroups existed regarding both early and late PaO2-to-FiO2 ratio. Notwithstanding, late PaO2-to-FiO2 ratio had a tendency toward a reduction only in single center trials (RR = 34.31, 95% CI –5.44 to 74.05, P = 0.09). No differences existed regarding other outcomes. Interestingly, ICU LOS and MV duration had a significant improvement in multicenter trials (P = 0.002 and P = 0.009, respectively).
Older versus newer trials
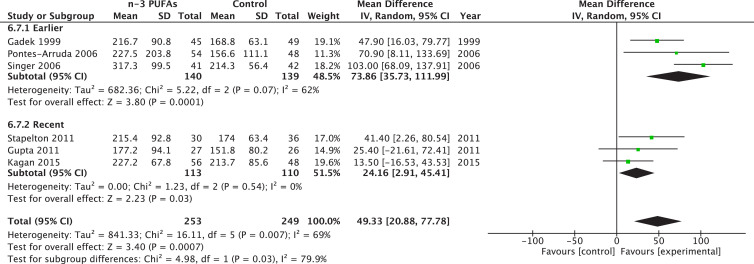
A significant subgroup effect was identified on PaO2-to-FiO2 ratio based on years of publication (P = 0.03), with a larger benefit for RCTs published before 2011 (WMD 73.86, 95% CI 35.73–111.99, P = 0.0001; I2 = 62%, P = 0.07; Fig. 5 ). Early PaO2-to-FiO2 ratio also had an improvement in more recent trials (RR = 24.16, 95% CI 2.91–77.78, P = 0.03; I2 = 0%). However, late PaO2-to-FiO2 ratio only had a significant improvement in those studies published before 2011 (RR = 55.89, 95% CI 29.49–82.28, P < 0.0001; I2 = 0%). The P value for subgroup differences between both group of trials was significant (P = 0.001). Regarding mortality, we found an effect only in those earlier trials (RR = 0.49, 95% CI 0.32–0.76, P = 0.001; I2 = 0%; test for subgroup differences, P = 0.001). Moreover, earlier studies found a significant effect on ICU LOS (WMD –4.73, 95% CI –7.05 to –2.41, P < 0.0001; I2 = 48%) and MV days (WMD –5.10, 95% CI –8.53 to –1.66, P = 0.004; I2 = 63%). No effect on hospital LOS was found in earlier or later studies.
Fig. 5.
Effect of fish oil on gas exchange in older and newer trials. CI, confidence interval; df, degrees of freedom; IV, instrumental variables; PUFA, polyunsaturated fatty acids; SD, standard deviation.
Sensitivity analysis restricted to oral/enteral
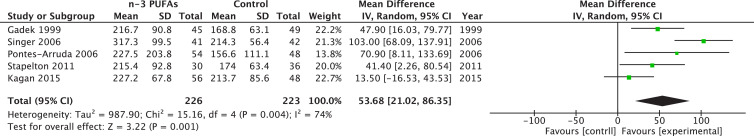
When we restricted the analysis to those trials administering ω-3 PUFAs by oral or enteral route, we found a significant effect on early PaO2-to-FiO2 ratio (RR = 53.68, 95% CI 21.02–83.65, P = 0.001; I2 = 74%%, P = 0.004; Fig. 6 ), with only a tendency to improve late PaO2-to-FiO2 ratio (RR = 30.12, 95% CI –0.86 to 61.09, P = 0.06; I2 = 65%, P = 0.02). Moreover, we found a tendency toward an improvement in ICU LOS (MWD –2.61, 95% CI –5.35 to 0.14, P = 0.06; I2 = 79%, P < 0.0001) and a significant effect on MV duration in those ARDS patients who received ω-3 PUFAs (MWD –3.44; 95% CI –6.59 to –0.28; P = 0.03; I2 = 77%; P = 0.0006). Finally, no effect on mortality and hospital LOS was found.
Fig. 6.
Effect of fish oil on gas exchange in acute respiratory distress syndrome: Sensitivity analysis restricted to oral/enteral administration. CI, confidence interval; df, degrees of freedom; IV, instrumental variables; PUFA, polyunsaturated fatty acids; SD, standard deviation.
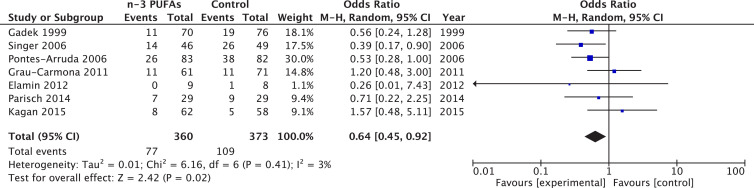
Sensitivity analysis restricted to continuous enteral
When six trials administering fish oils by enteral route were aggregated, we found a significant reduction in mortality (RR = 0.64, 95% CI 0.45–0.92, P = 0.02; I2 = 3%) (Fig. 7 ). No other clinical outcomes could be evaluated.
Fig. 7.
Effect of enteral fish oil on mortality in acute respiratory distress syndrome: Sensitivity analysis restricted to continuous infusion. CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel test; PUFA, polyunsaturated fatty acids.
Publication bias
There was no indication that potential publication bias influenced the reported results in the meta-analysis. However, funnel plots were created for each study outcome and the tests of asymmetry were significant for overall infections (odds ratio [OR] –2.14, 95% CI –3.23, –1.04, P = 0.01). Nonetheless, test for asymmetry was not significant for any other clinical outcome (early PaO2-to-FiO2 ratio, P = 0.56; late PaO2-to-FiO2 ratio, P = 0.80; mortality, P = 0.62; ICU LOS, P = 0.98; hospital LOS, P = 0.54, and MV days, P = 0.47).
Discussion
ARDS is characterized by neutrophilic inflammation, hyperpermeability, and alveolar and vascular fibrin deposition, which is mediated by series-2 prostanoids and thromboxanes, and series-4 leukotrienes derived from cyclooxygenase and 5-lypoxygenase enzymes, respectively [24], [25], [26], [27]. Kumar et al. [28] have determined that patients at risk of developing ARDS have low levels of anti-inflammatory ω-3 PUFAs (25% of normal), whereas those with a diagnosis of ARDS have levels as low as 6% of normal.
In this work we have systematically reviewed 12 eligible clinical trials enrolling 1280 ARDS patients with ARDS to evaluate the effects of fish oils as monotherapy or in combination with other nutrients, provided as a continuous infusion or in daily boluses via enteral (EN) or parenteral nutrition. Based on the analysis of trials that met our selection criteria, we found that ω-3 PUFAs, particularly when they are provided in combination with GLA and antioxidants as continuous infusion, are associated with a significant improvement in gas exchange. Nonetheless, this immunomodulatory strategy did not influence any clinical outcome in the overall analysis. Fish oils provided by enteral route produced a significant reduction in mortality, but the mortality effect was mostly driven from trials published before 2011, with almost 50% of the effect derived from the earlier studies.
None of the trials were powered to detect an effect on mortality, and mortality from the Rice et al. [6] study was not included because this trial only reported 60-day mortality. The OMEGA study [6] enrolled 272 patients with acute lung injury, of whom 143 were randomly assigned to receive EPA, DHA plus GLA and antioxidants, or placebo, whereas all of them received standard ARDS network lung protective ventilation. However, the trial was terminated early after the first interim analysis found a trend toward higher mortality in those patients who received the immunomodulatory diet. Although high serum fatty acid levels were identified in supplemented patients, the primary outcome (ventilator-free days) and inflammation biomarkers did not improve [6]. The difference between results found in earlier trials (before 2011) might also be explained by change of practice regarding therapeutic strategies to the ARDS patient.
The sensitivity analysis (excluding the trials that provided pure fish oil) aggregated six studies and found a significant reduction in mortality (P = 0.02). At this point, we considered why early trials providing an immunomodulatory diet as continuous infusion reported beneficial results, whereas later trials did not find benefits. In an elegant editorial, Heyland and Cook [29] speculated that the bolus delivery provided in the OMEGA study could have blunted the immunomodulatory and anti-inflammatory effects of the enteral diet. An alternative explanation may involve the greater daily dose of protein received by patients in the control group. Controls received up to 20 g/d of protein more than patients in the intervention group, which may have positively influenced clinical outcomes in the control group. Nonetheless, the Grau-Carmona study [15] that used a standard care control formula also showed negative results. Moreover, the study by Stapleton et al. [5] published in 2011, which provided a daily bolus of pure fish oil, also failed to find any effect on clinical outcomes or biomarkers of systemic inflammation.
In 2008 the first meta-analysis evaluating an immune-enhancing diet (IED) with fish oils, borage oil, and antioxidants found a significant reduction in the risk of mortality as well as relevant improvement in oxygenation in mechanically ventilated patients with ARDS [4]. An in-depth review of this analysis led to the conclusion that, when aggregating the data from Gadek et al. [19], the standard errors were used instead of the standard deviation. This led to exacerbated and overemphasized benefits for all the continuous variables (ICU LOS, hospital LOS) that can be elicited by analyzing the small confidence interval of the trial in the forest plots. A recent meta-analysis by Chen et al. [30] evaluated how ω-3 fatty acids influenced mortality in patients with sepsis-induced ARDS. After aggregating nine RCTs using EN and 16 RCTs using parenteral nutrition, no improvement was found on mortality. Nonetheless, a signal existed in the EN subgroup (P = 0.04) and a correlation existed between ω-6 to ω-3 ratio for reduction of mortality (P = 0.02). These findings are concordant with our results, except for the effect of ratio of ω-6 to ω-3 ratio, which was not analyzed.
The strength of this work resides in the various strategies to limit bias, including comprehensive literature search, specific criteria for research, and inclusion and analysis, as well as duplicate data abstraction. We focused on clinically relevant outcomes for ICU patients. Nonetheless, several limitations limit the strength of the conclusions. First, the protocol of this systematic review and meta-analysis was not previously registered. A second limitation is the risk for publication bias regarding overall infections, and the limited number of trials included in the prespecified subgroup analyses, which cancelled many planned subgroup analyses.
Two main points led to a moderate risk of indirectness. First, FO was administered in 8 of the 12 included trials as a mixture with other nutrients (Oxepa) and the positive results in our analyses seem to be driven mostly by these mixed substrate trials. It is therefore hard to conclude any specific benefits of FO. The second point relates to the various ARDS definitions used over the years for inclusion of patients into studies. Nonetheless, most authors reported their diagnosis criteria, and we acknowledge that their definition corresponded to the most recent Berlin criteria. The risk of imprecision is moderate, considering that most of the outcomes crossed the no-effect line and presented a wide confidence interval. Inconsistency remained present throughout the analysis with I2 heterogeneity value up to 79%. Only mortality analysis found less heterogeneity. These findings come from the wide range of daily doses, as well as the variety in lengths of administration of fish oils among the different trials, which unfortunately weaken any possible recommendations for clinical practice.
Conclusions
In this meta-analysis we found that ω-3 PUFAs plus GLA and antioxidants may be able to significantly improve pulmonary gas exchange and are associated with a trend toward reduced ICU length of stay and MV duration in critically ill patients with ARDS. Nonetheless, a clinical and statistically significant heterogeneity was found. Moreover, the treatment effect seemed greatest in those trials published before 2011. The therapeutic effect could depend on the route and/or the method of administration. Continuous infusion of an immunomodulatory formula to enterally fed patients might optimize the therapeutic effect of fish oil. Further well-powered clinical trials are warranted and should aim at evaluating how ω-3 PUFAs can modulate the ARDS pathophysiology.
Footnotes
Conflict of interest: P.L.L. received speaking honorarium from Fresenius Kabi. G.H. and W.M. have received financial reimbursement from Baxter Healthcare for consultancy work, advisory boards, and speaking engagements. F.D.A. has no conflict of interest to declare.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.nut.2018.10.026.
Appendix. Supplementary materials
Supplemental Fig. 1. Effects of fish oil on mortality in acute respiratory distress syndrome (ARDS). CI, confidence interval.
Supplemental Fig. 2. Effects of fish oil on ICU length of stay in acute respiratory distress syndrome (ARDS). CI, confidence interval; ICU, intensive care unit.
Supplemental Fig. 3. Effects of fish oil on hospital length of stay in acute respiratory distress syndrome (ARDS). CI, confidence interval.
Supplemental Fig. 4. Effects of fish oil on infectious complications in acute respiratory distress syndrome (ARDS). CI, confidence interval.
References
- 1.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Calder P.C. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 3.Singer P., Shapiro H., Theilla M., Anbar R., Singer J., Cohen J. Anti-inflammatory properties of ω-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 4.Pontes-Arruda A., Aragao A.M., Albuquerque J.D. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton R.D., Martin T.R., Weiss N.S., Crowley J.J., Gundel S.J., Nathens A.B., et al. A phase II randomized placebo-controlled trial of ω-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice T.W., Wheeler A.P., Thompson B.T., deBoisblanc B.P., Steingrub J., Rock P., et al. Enteral ω-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines CCP. Section 4. Composition of enteral nutrition. Available at: http://www.criticalcarenutrition.com. Accessed: June 2018.
- 8.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 9.Calder P.C., Adolph M., Deutz N.E., Grau T., Innes J.K., Klek S., et al. Lipids in the intensive care unit: recommendations from the ESPEN Expert Group. Clin Nutr. 2018;37:1–18. doi: 10.1016/j.clnu.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 11.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks J. H.P. Statistical algorithms in review manager. Available at: http://ims.cochrane.org/revman/documentation/Statistical-methods-in-RevMan-5.pdf.
- 14.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau-Carmona T., Moran-Garcia V., Garcia-de-Lorenzo A., Heras-de-la-Calle G., Quesada-Bellver B., Lopez-Martinez J., et al. Effect of an enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid and anti-oxidants on the outcome of mechanically ventilated, critically ill, septic patients. Clin Nutr. 2011;30:578–584. doi: 10.1016/j.clnu.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Shirai K., Yoshida S., Matsumaru N., Toyoda I., Ogura S. Effect of enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with sepsis-induced acute respiratory distress syndrome. J Intensive Care. 2015;3:24. doi: 10.1186/s40560-015-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagan I., Cohen J., Stein M., Bendavid I., Pinsker D., Silva V., et al. Preemptive enteral nutrition enriched with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in severe multiple trauma: a prospective, randomized, double-blind study. Intensive Care Med. 2015;41:460–469. doi: 10.1007/s00134-015-3646-z. [DOI] [PubMed] [Google Scholar]
- 18.Elamin E.M., Miller A.C., Ziad S. Immune enteral nutrition can improve outcomes in medical-surgical patients with ARDS: a prospective randomized controlled trial. J Nutr Disord Ther. 2012;2:109. doi: 10.4172/2161-0509.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadek J.E., DeMichele S.J., Karlstad M.D., Pacht E.R., Donahoe M., Albertson T.E., et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A., Govil D., Bhatnagar S., Gupta S., Goyal J., Patel S., et al. Efficacy and safety of parenteral ω 3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med. 2011;15:108–113. doi: 10.4103/0972-5229.83019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parish M., Valiyi F., Hamishehkar H., Sanaie S., Asghari Jafarabadi M., Golzari S.E., et al. The effect of omega-3 fatty acids on ARDS: a randomized double-blind study. Adv Pharm Bull. 2014;4:555–561. doi: 10.5681/apb.2014.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer P., Theilla M., Fisher H., Gibstein L., Grozovski E., Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 23.Theilla M., Schwartz B., Zimra Y., Shapiro H., Anbar R., Rabizadeh E., et al. Enteral ω-3 fatty acids and micronutrients enhance percentage of positive neutrophil and lymphocyte adhesion molecules: a potential mediator of pressure ulcer healing in critically ill patients. Br J Nutr. 2012;107:1056–1061. doi: 10.1017/S0007114511004004. [DOI] [PubMed] [Google Scholar]
- 24.Palombo J.D., Lydon E.E., Chen P.L., Bistrian B.R., Forse R.A. Fatty acid composition of lung, macrophage and surfactant phospholipids after short-term enteral feeding with ω-3 lipids. Lipids. 1994;29:643–649. doi: 10.1007/BF02536099. [DOI] [PubMed] [Google Scholar]
- 25.Bulger E.M., Maier R.V. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000;28:N27–N36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 26.Lewis R.A., Austen K.F., Soberman R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 27.Barham J.B., Edens M.B., Fonteh A.N., Johnson M.M., Easter L., Chilton F.H. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000;130:1925–1931. doi: 10.1093/jn/130.8.1925. [DOI] [PubMed] [Google Scholar]
- 28.Kumar K.V., Rao S.M., Gayani R., Mohan I.K., Naidu M.U. Oxidant stress and essential fatty acids in patients with risk and established ARDS. Clin Chim Acta. 2000;298:111–120. doi: 10.1016/s0009-8981(00)00264-3. [DOI] [PubMed] [Google Scholar]
- 29.Cook D.J., Heyland D.K. Pharmaconutrition in acute lung injury. JAMA. 2011;306:1599–1600. doi: 10.1001/jama.2011.1470. [DOI] [PubMed] [Google Scholar]
- 30.Chen H., Wang S., Zhao Y., Luo Y., Tong H., Su L. Correlation analysis of ω-3 fatty acids and mortality of sepsis and sepsis-induced ARDS in adults: data from previous randomized controlled trials. Nutr J. 2018;17:57. doi: 10.1186/s12937-018-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Effects of fish oil on mortality in acute respiratory distress syndrome (ARDS). CI, confidence interval.
Supplemental Fig. 2. Effects of fish oil on ICU length of stay in acute respiratory distress syndrome (ARDS). CI, confidence interval; ICU, intensive care unit.
Supplemental Fig. 3. Effects of fish oil on hospital length of stay in acute respiratory distress syndrome (ARDS). CI, confidence interval.
Supplemental Fig. 4. Effects of fish oil on infectious complications in acute respiratory distress syndrome (ARDS). CI, confidence interval.