Abstract
After recovering from the acute phase of coronavirus disease 2019 (COVID-19), many patients struggle with additional symptoms of long COVID during the chronic phase. Among them, the neuropsychiatric manifestations characterized by a short-term memory loss and inability to concentrate are called “brain fog”. Recent studies have revealed the involvement of “chronic neuro-inflammation” in the pathogenesis of brain fog following COVID-19 infection. In the COVID-related brain fog, similarly to neurodegenerative disorders caused by neuro-inflammation, brain leukocytes, such as microglia and lymphocytes, are hyperactivated, suggesting the overexpression of delayed rectifier K+-channels (Kv1.3) within the cells. In our previous patch-clamp studies, drugs, such as antihistamines, statins, nonsteroidal anti-inflammatory drugs, antibiotics and anti-hypertensive drugs, suppressed the Kv1.3-channel activity and reduced the production of pro-inflammatory cytokines. Additionally, newer generation antihistamines, antibiotics and corticosteroids strongly stabilize mast cells that directly activate microglia in the brain. Taking such pharmacological properties of these commonly used drugs into account, they may be useful in the treatment of COVID-related brain fog, in which the enhanced innate and adaptive immune responses are responsible for the pathogenesis.
Keywords: Brain fog, Long COVID (coronavirus disease), Chronic neuro-inflammation, Lymphocyte Kv1.3-channels, Mast cell stabilizers
Regardless of the severity of Coronavirus disease 2019 (COVID-19), a high proportion of patients struggle with “post-COVID-19 syndrome” or “long COVID”, a condition characterized by long-term health problems that persist after recovering from COVID-19 [1]. Long COVID potentially affects nearly every organ system, causing respiratory, cardiovascular, neurological symptoms and systemic manifestations including generalized fatigue, muscular weakness and sleep disorders [1]. Table 1 summarizes the symptoms, known mechanisms and rationalized treatment targets of acute and chronic phases of COVID-19 infection [1–4]. In long COVID, in addition to common neurological symptoms, such as headache, dizziness and numbness, some patients experience neuropsychiatric manifestations characterized by a short-term memory loss, inability to concentrate, depression and anxiety [5, 6]. These symptoms are called “brain fog”, indicating a cognitive impairment caused by neural circuit dysfunctions [5, 6]. Concerning the pathogenesis of the COVID-related brain fog, recent studies suggested the involvement of autoimmunity, viral neuro-invasion, oxidative stress, hypoxic neuronal injury or microvascular coagulopathies [7]. However, despite such findings, supportive management, such as cognitive behavioral therapy and the use of anti-depressants or herbal medications, is currently the mainstay of treatment for brain fog [6, 8, 9].
Table 1.
Summary of symptoms, mechanisms and treatment targets of acute and chronic phases of COVID-19
| Acute phase (first 4 weeks from infection) | Chronic phase (after 4 weeks) | |
|---|---|---|
| Symptoms |
• Nasopharyngeal (sore throat, runny nose) • Respiratory (cough, dyspnea) • Systemic (fever, headache, fatigue) |
• Respiratory (persistent cough, dyspnea, hypoxia) • Cardiovascular (palpitation, chest pain) • Neurological (headache, dizziness, numbness) • Neuropsychiatric (short-term memory loss, inability to concentrate, depression, anxiety) • Systemic (fatigue, muscular weakness, insomnia) |
| Known mechanisms |
• Viral attack • Inflammatory response • Cytokine storm |
• Immune response • Chronic inflammation • Pulmonary fibrosis • Coagulopathy |
| Treatment targets |
• Anti-viral medications • Immunomodulatory agents (cytokine inhibitors, immune globulin) |
• Supportive management • Rehabilitation • Vaccination • Anti-coagulation |
Recently, several studies have additionally revealed the involvement of “chronic neuro-inflammation” in the pathogenesis of COVID-related brain fog [5, 6, 10]. In patients with neuropsychiatric symptoms following COVID-19 infection, in addition to the elevation of serum C-reactive protein (CRP) levels [11], pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and interferon-α (IFN-α), were actually increased in both the peripheral blood and cerebrospinal fluid [12, 13]. Additionally, in the brain of these patients, microglia, the brain-resident macrophages that are stimulated by these cytokines [5, 6], were highly activated with the formation of microglial nodules [14, 15]. These nodules are the product of microglial phagocytosis of degenerating neurons that were attacked and killed by cytotoxic T-lymphocytes [16]. In patients with COVID-related brain fog, in addition to microglia, lymphocytes were also activated and actually increased in the brain [14, 15]. Therefore, the enhanced immune responses by these leukocytes were likely to be responsible for the pathogenesis of neuro-inflammation in COVID-related brain fog.
Brain leukocytes, such as microglia and T-lymphocytes, mainly express delayed rectifier K+-channels (Kv1.3) on their cell membranes [17, 18]. The channels play a pivotal role in the activation and the proliferation of the leukocytes themselves, which thus trigger the innate and adaptive immune responses [17, 18]. Previously, in our rat models with advanced-stage chronic kidney disease (CKD), we demonstrated that both macrophages and T-lymphocytes had distinctly proliferated, and pro-inflammatory cytokines, such as IL-2 and TNF-α, were significantly increased within the inflamed kidneys [17, 19]. In these macrophages and T-lymphocytes, the expression of Kv1.3-channels was up-regulated and the pharmacological inhibition of the channels significantly slowed the progression of renal fibrosis. From these results, the Kv1.3-channels were considered primarily to over-activate the immune responses, which subsequently facilitated the progression of CKD [17, 19]. Recently, besides CKD and other chronic diseases, such as chronic obstructive pulmonary disease and inflammatory bowel disease [17, 20], neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’ s disease, multiple sclerosis and schizophrenia, are now also regarded as chronic inflammatory diseases [21, 22]. In these neuro-inflammatory diseases, macrophages and lymphocytes were actually over-activated or had proliferated within the brain, and the expression of Kv1.3-channels was up-regulated within the cells [21, 22].
In the management of COVID-related brain fog, first of all, it is important to rule out other causes of brain fog, such as strokes and seizures, which may warrant additional evaluation and medications. Additionally, in the treatment of COVID-related brain fog, a multi-disciplinary and individual approach should be required for each patient [8]. This includes the evaluation of (1) cognition, (2) neuroinflammation markers, (3) psychological factors and (4) sleep disorders. Recent clinical studies have revealed the therapeutic efficacies of antihistamines and anti-cholesterol drugs (statins) [6, 23, 24]. Despite the lack of pharmacological evidence, these agents actually ameliorated the neuropsychiatric symptoms together with a reduction in peripheral inflammatory markers [6, 24]. On the other hand, an in vitro study additionally demonstrated the efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) for neuro-inflammation, since these drugs directly reduced the activity of microglia [25]. In our patch-clamp studies using murine thymocytes, antihistamines (cetirizine, fexofenadine, azelastine, terfenadine), statins (pravastatin, lovastatin, simvastatin) and NSAIDs (indomethacin, diclofenac, salicylate) suppressed the activity of lymphocyte Kv1.3-channels and thus reduced the pro-inflammatory cytokine production [26–28]. These findings would further clarify the additional pharmacological mechanisms by which antihistamines, statins and NSAIDs are effective for COVID-related brain fog, where the enhanced immune responses are responsible for the pathogenesis (Fig. 1). In our following patch-clamp studies, we additionally demonstrated the inhibitory properties of antibiotics (clarithromycin, chloroquine) and anti-hypertensive drugs (nifedipine, benidipine, diltiazem, verapamil) on lymphocytes Kv1.3-channels [17, 29–31]. Taking such pharmacological properties of these commonly used drugs into account, they would also be beneficial in the treatment of COVID-related brain fog, since the channel blockade could suppress the activity of brain macrophages (microglia) and lymphocytes (Fig. 1).
Fig. 1.
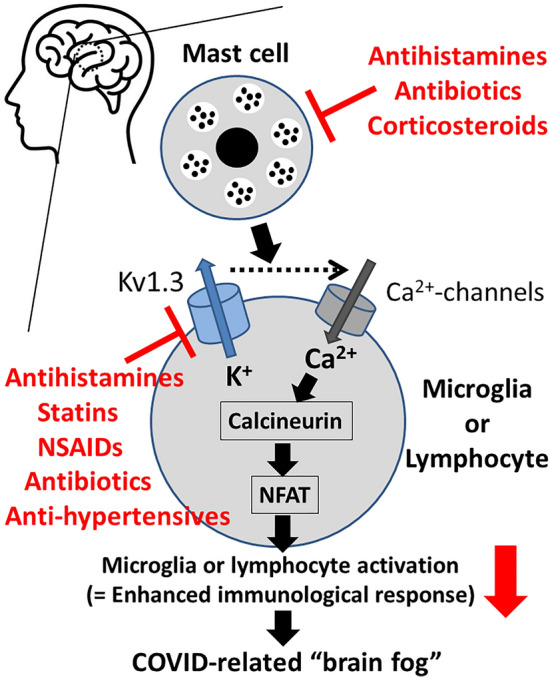
Roles of mast cells and Kv1.3-channels in the activation pathway of brain leukocytes (microglia or lymphocytes) and as the targets of commonly used drugs for COVID-related brain fog. Kv1.3-channels promote calcium influx and trigger the proliferation and activation of brain macrophages (microglia) or lymphocytes. The increased cytosolic calcium concentration stimulates the phosphatase calcineurin, which de-phosphorylates the nuclear factor of activated T cells (NFAT), causing its accumulation in the nucleus and binding to the promoter region of cytokine-encoding genes. Antihistamines, statins, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics and anti-hypertensives, which inhibit Kv1.3-channels, or antihistamines, antibiotics and corticosteroids, which stabilize mast cells, directly or indirectly suppress the activity of brain leukocytes and the subsequent immunological response
Additionally, recent studies also revealed the contribution of mast cells to the pathogenesis of neuro-inflammation in COVID-related brain fog [32]. According to these studies, brain mast cells that produce pro-inflammatory cytokines directly increased the activity of microglia (Fig. 1). These findings strongly indicated the additional pharmacological efficacy of suppressing mast cells in the treatment of COVID-related brain fog. In our separate patch-clamp studies, by monitoring the changes in the whole-cell membrane capacitance in rat peritoneal mast cells, we provided in vitro evidence that newer generation antihistamines (olopatadine, ketotifen, cetirizine, levocetirizine), antibiotics (clarithromycin) and corticosteroids (hydrocortisone, dexamethasone) strongly inhibit the process of exocytosis [33–37]. In morphological analyses as well, these drugs actually suppressed the degranulation from mast cells, suggesting their pharmacological efficacy as potent mast cell stabilizers. By stabilizing brain mast cells and thus repressing the activity of microglia, they could also be used in the treatment of COVID-related brain fog (Fig. 1). However, in when antibiotics and corticosteroids, we have to be very careful since the overuse of antibiotics has caused a worldwide problem with antibiotic resistance [38], while the use of corticosteroids has been associated with a worse clinical outcome of COVID-19 [39].
Multisystem Inflammatory Syndrome in Adults (MIS-A) is a condition recently recognized by the US Center for Disease Control (CDC) [40]. It is characterized by diffuse multiorgan symptoms, including malaise, myalgia, chest tightness, brain fog and other neuropsychiatric symptoms, which persisted for months after COVID-19 infection. Since these symptoms are very similar to those associated with Mast Cell Activation Syndrome (MCAS), its possibility should also be evaluated in any patients who present symptoms of COVID-related brain fog [41]. MCAS could be treated with a liposomal formulation of flavone luteolin together with rupatadine, an antihistamine [41]. They inhibit the release of pro-inflammatory mediators from mast cells, such as platelet activating factor (PAF) and other chemokines, that are responsible for the pathogenesis of cytokine storms in COVID-19.
Conclusion
Drugs, such as antihistamines, statins, NSAIDs, antibiotics and anti-hypertensive drugs, suppressed the Kv1.3-channel activity and pro-inflammatory cytokine production from leukocytes. Additionally, newer generation antihistamines, antibiotics and corticosteroids strongly stabilized mast cells, which directly activate microglia in the brain. Given the pharmacological properties of these commonly used drugs, they may be useful in the treatment of COVID-related brain fog since the enhanced innate and adaptive immune responses are responsible for the pathogenesis. Nevertheless, we must be very careful in implementing these medications in humans.
Author contributions
I.K. wrote the main manuscript text and prepared the figure and table. The author reviewed the manuscript.
Funding
Funding was provided by Salt Science Research Foundation (Grant No.: 2218).
Data availability
Enquiries about data availability should be directed to the authors.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin Y, Wu Y, Zhong P, Hou B, Liu J, Chen Y, Liu J. A clinical staging proposal of the disease course over time in non-severe patients with coronavirus disease 2019. Sci Rep. 2021;11:10681. doi: 10.1038/s41598-021-90111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, Gralinski LE, Rondina MT, van de Veerdonk FL, Hoffmeister KM, Griffin JH, Nugent D, Moon K, Morrissey JH. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22:639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arish M, Qian W, Narasimhan H, Sun J. COVID-19 immunopathology: from acute diseases to chronic sequelae. J Med Virol. 2023;95:e28122. doi: 10.1002/jmv.28122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanou MI, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas GP, Rizos E, Boutati E, Grigoriadis N, Krogias C, Giannopoulos S, Tsiodras S, Gaga M, Tsivgoulis G. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. 2022;13:20406223221076890. doi: 10.1177/20406223221076890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 7.Monje M, Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110:3484–3496. doi: 10.1016/j.neuron.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan K, Lin Y, Prewitt KM, Potter DA. Multidisciplinary approach to brain fog and related persisting symptoms post COVID-19. J Health Serv Psychol. 2022;48:31–38. doi: 10.1007/s42843-022-00056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jegal KH, Yoon J, Kim S, Jang S, Jin YH, Lee JH, Choi SM, Kim TH, Kwon S. Herbal medicines for post-acute sequelae (fatigue or cognitive dysfunction) of SARS-CoV-2 infection: a phase 2 pilot clinical study protocol. Healthcare (Basel). 2022;10:1839. doi: 10.3390/healthcare10101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkataramani V, Winkler F. Cognitive deficits in long Covid-19. N Engl J Med. 2022;387:1813–1815. doi: 10.1056/NEJMcibr2210069. [DOI] [PubMed] [Google Scholar]
- 11.Hugon J, Msika EF, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269:44–46. doi: 10.1007/s00415-021-10655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borsini A, Merrick B, Edgeworth J, Mandal G, Srivastava DP, Vernon AC, Nebbia G, Thuret S, Pariante CM. Neurogenesis is disrupted in human hippocampal progenitor cells upon exposure to serum samples from hospitalized COVID-19 patients with neurological symptoms. Mol Psychiatry. 2022;27:5049–5061. doi: 10.1038/s41380-022-01741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milan A, Salles P, Pelayo C, Uribe-San-Martin R. Acute to chronic electro-clinical manifestations of neuro-COVID and the long-haul consequences in people with epilepsy: a review. Cureus. 2022;14:e26020. doi: 10.7759/cureus.26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou JJ, Movassaghi M, Gordy D, Olson MG, Zhang T, Khurana MS, Chen Z, Perez-Rosendahl M, Thammachantha S, Singer EJ, Magaki SD, Vinters HV, Yong WH. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathol. 2021;2:2. doi: 10.17879/freeneuropathology-2021-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144:2696–2708. doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troscher AR, Wimmer I, Quemada-Garrido L, Kock U, Gessl D, Verberk SGS, Martin B, Lassmann H, Bien CG, Bauer J. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 2019;137:619–635. doi: 10.1007/s00401-019-01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazama I. Physiological significance of delayed rectifier K(+) channels (Kv1.3) expressed in T lymphocytes and their pathological significance in chronic kidney disease. J Physiol Sci. 2015;65:25–35. [DOI] [PMC free article] [PubMed]
- 18.Fordyce CB, Jagasia R, Zhu X, Schlichter LC. Microglia Kv1.3 channels contribute to their ability to kill neurons. J Neurosci. 2005;25:7139–7149. doi: 10.1523/JNEUROSCI.1251-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazama I, Baba A, Matsubara M, Endo Y, Toyama H, Ejima Y. Benidipine suppresses in situ proliferation of leukocytes and slows the progression of renal fibrosis in rat kidneys with advanced chronic renal failure. Nephron Exp Nephrol. 2014;128:67–79. doi: 10.1159/000368080. [DOI] [PubMed] [Google Scholar]
- 20.Kazama I. Roles of lymphocyte Kv1.3-channels in gut mucosal immune system: novel therapeutic implications for inflammatory bowel disease. Med Hypotheses. 2015;85:61–63. doi: 10.1016/j.mehy.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Li G, Guo J, Zhang Z, Zhang S, Zhu Y, Cheng J, Yu L, Ji Y, Tao J. Kv1.3 channel as a key therapeutic target for neuroinflammatory diseases: state of the art and beyond. Front Neurosci. 2019;13:1393. doi: 10.3389/fnins.2019.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato Y, Kuwana R, Kazama I. Suppressing leukocyte Kv1.3-channels by commonly used drugs: a novel therapeutic target for schizophrenia? Drug Discov Ther. 2022;16:93–95. doi: 10.5582/ddt.2022.01031. [DOI] [PubMed] [Google Scholar]
- 23.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70:61–67. doi: 10.1136/jim-2021-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer L. Lots of long COVID treatment leads, but few are proven. Proc Natl Acad Sci U S A. 2022;119:e2213524119. doi: 10.1073/pnas.2213524119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajmone-Cat MA, Bernardo A, Greco A, Minghetti L. Non-steroidal anti-inflammatory drugs and brain inflammation: effects on microglial functions. Pharmaceuticals (Basel) 2010;3:1949–1965. doi: 10.3390/ph3061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazama I, Maruyama Y, Murata Y. Suppressive effects of nonsteroidal anti-inflammatory drugs diclofenac sodium, salicylate and indomethacin on delayed rectifier K+-channel currents in murine thymocytes. Immunopharmacol Immunotoxicol. 2012;34:874–878. doi: 10.3109/08923973.2012.666249. [DOI] [PubMed] [Google Scholar]
- 27.Kazama I, Baba A, Maruyama Y. HMG-CoA reductase inhibitors pravastatin, lovastatin and simvastatin suppress delayed rectifier K(+)-channel currents in murine thymocytes. Pharmacol Rep. 2014;66:712–717. doi: 10.1016/j.pharep.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Saito K, Abe N, Toyama H, Ejima Y, Yamauchi M, Mushiake H, Kazama I. Second-generation histamine H1 receptor antagonists suppress delayed rectifier K(+)-channel currents in murine thymocytes. Biomed Res Int. 2019;2019:6261951. doi: 10.1155/2019/6261951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Baba A, Tachi M, Maruyama Y, Kazama I. Suppressive effects of diltiazem and verapamil on delayed rectifier K(+)-channel currents in murine thymocytes. Pharmacol Rep. 2015;67:959–964. doi: 10.1016/j.pharep.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Kazama I, Maruyama Y. Differential effects of clarithromycin and azithromycin on delayed rectifier K(+)-channel currents in murine thymocytes. Pharm Biol. 2013;51:760–765. doi: 10.3109/13880209.2013.764539. [DOI] [PubMed] [Google Scholar]
- 31.Kazama I, Tamada T, Tachi M. Usefulness of targeting lymphocyte Kv1.3-channels in the treatment of respiratory diseases. Inflamm Res. 2015;64:753–765. doi: 10.1007/s00011-015-0855-4. [DOI] [PubMed] [Google Scholar]
- 32.Theoharides TC, Cholevas C, Polyzoidis K, Politis A. Long-COVID syndrome-associated brain fog and chemofog: luteolin to the rescue. BioFactors. 2021;47:232–241. doi: 10.1002/biof.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baba A, Tachi M, Maruyama Y, Kazama I. Olopatadine inhibits exocytosis in rat peritoneal mast cells by counteracting membrane surface deformation. Cell Physiol Biochem. 2015;35:386–396. doi: 10.1159/000369704. [DOI] [PubMed] [Google Scholar]
- 34.Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, Matsubara M, Saito K, Yamauchi M, Miura C, Kazama I. Anti-allergic drugs tranilast and ketotifen dose-dependently exert mast cell-stabilizing properties. Cell Physiol Biochem. 2016;38:15–27. doi: 10.1159/000438605. [DOI] [PubMed] [Google Scholar]
- 35.Mori T, Abe N, Saito K, Toyama H, Endo Y, Ejima Y, Yamauchi M, Goto M, Mushiake H, Kazama I. Hydrocortisone and dexamethasone dose-dependently stabilize mast cells derived from rat peritoneum. Pharmacol Rep. 2016;68:1358–1365. doi: 10.1016/j.pharep.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Kazama I, Saito K, Baba A, Mori T, Abe N, Endo Y, Toyama H, Ejima Y, Matsubara M, Yamauchi M. Clarithromycin dose-dependently stabilizes rat peritoneal mast cells. Chemotherapy. 2016;61:295–303. doi: 10.1159/000445023. [DOI] [PubMed] [Google Scholar]
- 37.Fujimura R, Asada A, Aizawa M, Kazama I. Cetirizine more potently exerts mast cell-stabilizing property than diphenhydramine. Drug Discov Ther. 2022;16:245–250. doi: 10.5582/ddt.2022.01067. [DOI] [PubMed] [Google Scholar]
- 38.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 39.Cespedes MDS, Souza J. Coronavirus: a clinical update of Covid-19. Rev Assoc Med Bras. 1992;2020(66):116–123. doi: 10.1590/1806-9282.66.2.116. [DOI] [PubMed] [Google Scholar]
- 40.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theoharides TC, Conti P. COVID-19 and multisystem inflammatory syndrome, or is it mast cell activation syndrome? J Biol Regul Homeost Agents. 2020;34:1633–1636. doi: 10.23812/20-EDIT3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Enquiries about data availability should be directed to the authors.


