Abstract
Background
This overview was originally published in 2017, and is being updated in 2022.
Chronic pain is typically described as pain on most days for at least three months. Chronic non‐cancer pain (CNCP) is any chronic pain that is not due to a malignancy. Chronic non‐cancer pain in adults is a common and complex clinical issue, for which opioids are prescribed by some physicians for pain management. There are concerns that the use of high doses of opioids for CNCP lacks evidence of effectiveness, and may increase the risk of adverse events.
Objectives
To describe the evidence from Cochrane Reviews and overviews regarding the efficacy and safety of high‐dose opioids (defined as 200 mg morphine equivalent or more per day) for CNCP.
Methods
We identified Cochrane Reviews and overviews by searching the Cochrane Database of Systematic Reviews in The Cochrane Library. The date of the last search was 21 July 2022. Two overview authors independently assessed the search results. We planned to analyse data on any opioid agent used at a high dose for two weeks or more for the treatment of CNCP in adults.
Main results
We did not identify any reviews or overviews that met the inclusion criteria. The excluded reviews largely reflected low doses or titrated doses, where all doses were analysed as a single group; we were unable to extract any data for high‐dose use only.
Authors' conclusions
There is a critical lack of high‐quality evidence, in the form of Cochrane Reviews, about how well high‐dose opioids work for the management of CNCP in adults, and regarding the presence and severity of adverse events.
No evidence‐based argument can be made on the use of high‐dose opioids, i.e. 200 mg morphine equivalent or more daily, in clinical practice. Considering that high‐dose opioids have been, and are still being used in clinical practice to treat CNCP, knowing about the efficacy and safety of these higher doses is imperative.
Keywords: Adult; Humans; Analgesics, Opioid; Analgesics, Opioid/adverse effects; Chronic Pain; Chronic Pain/drug therapy; Morphine; Morphine/adverse effects; Pain Management; Systematic Reviews as Topic
Plain language summary
High doses of opioid medicines for the management of chronic non‐cancer pain
Bottom line
There is no high‐quality evidence to show how well high doses of opioids work, or what side effects they cause, when they are used for adults with chronic pain that is not due to cancer.
Studies usually used doses below our cutoff. We need to know how well high‐dose opioid medicine works for chronic non‐cancer pain, and what side effects there may be.
Background
Opioids are a type of pain medicine related to morphine. Opioids have been used for many years as painkillers for moderate or severe chronic pain, which is pain that lasts for a long time (usually longer than three months).
Study characteristics
In this updated overview, we aimed to summarise the knowledge from Cochrane Reviews and overviews about opioid medicine for pain relief in adults who had chronic pain not due to cancer. We wanted to know how well opioid medicines worked when they were used at high doses (equal to 200 mg of morphine per day or more), and what side effects there might be.
Key results
Despite systematic searches, most recently conducted in July 2022, we did not find any information about this. Studies on opioids rarely reported on high‐dose use. When they included them as part of a study of people using all levels of doses, they did not report separate information for people who used high‐dose opioids.
Background
Description of the condition
In 2020, the International Association for the Study of Pain updated the definition of pain to reflect “[a]n unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (Raja 2020). The definition was contextually expanded, emphasising that pain is always a personal experience. Pain is influenced in varying degrees by biopsychosocial factors, and can become maladaptive, substantially and adversely impacting the sufferer’s functional and psychosocial well‐being (Raja 2020). About 49% of chronic pain sufferers in the United States reported that their pain resulted in work limitations (Yong 2022). In a Spanish study, 13.5% of chronic pain sufferers reported having lost or left their jobs because of their pain (De Sola 2016).
Chronic non‐cancer pain (CNCP) is very common in adults, estimated to impact about 20% (Breivik 2006; Dahlhamer 2018; Goldberg 2011; Moore 2014). Although there are several self‐report instruments to measure pain and its impact on the physical, social, emotional, and spiritual aspects of life, the personal and subjective nature of pain makes objective measurement impossible. Any assessment of pain is subjective and based on an individual's report (Breivik 2008). Notwithstanding, a person’s report of an experience as pain should be respected (Raja 2020).
Chronic pain is typically described as pain that persists past normal healing time, or pain lasting or recurring for more than three to six months (Bonica 1953).
Chronic non‐cancer pain is any chronic pain that is not due to a malignancy; CNCP is frequently divided into neuropathic pain, i.e. pain that appears to originate in the nerves; and non‐neuropathic or nociceptive pain, which appears to often have a musculoskeletal origin and to arise in muscles, bones, or ligaments.
Description of the interventions
The treatment of pain may encompass a variety of approaches, including pharmacological management. Effective pain therapy has been described as a reduction in pain intensity of at least 50% over baseline, or sometimes as a reduction of at least 30% over baseline, along with improvement in work‐ and function‐related outcomes. Effective pain therapy results in consistent improvements in fatigue, sleep, depression, quality of life, and work (Moore 2014).
Opioid therapy is used for the treatment of both acute and chronic painful conditions. There are numerous policies and country‐specific clinical guidelines to assist with the use of opioids for the management of chronic pain. The World Health Organization's (WHO) analgesic ladder guides the use of pain medications (including opioids and non‐opioids, such as non‐steroidal anti‐inflammatory drugs (NSAIDs)) in the management of pain (WHO 1996). Although originally formulated for cancer pain, this tool is now used for a broad range of chronic pain conditions. The American College of Occupational and Environmental Medicine (ACOEM) concludes that quality evidence does not support a superiority of opioids over NSAIDs or other medications for the treatment of CNCP (Hegmann 2014b). For acute pain, a recent systematic review and meta‐analysis of randomised trials, including findings from 47 trials (N = 6607), supported the notion that opioid prescribing at surgical discharge increases the risk of adverse events but does not reduce pain intensity (Fiore 2022).
This overview addressed the use of high‐dose opioids. As discussed below, we took this to mean opioids at doses of 200 mg morphine equivalent (MEQ) per day or more.
How the intervention might work
Opium is a plant‐derived substance; the primary ingredients are morphine and codeine. The term 'opioids' refers to either naturally occurring compounds (opiates) or synthetic compounds. As centrally acting analgesics, opioids bring about their action in the human body by binding to opioid receptors. The mu, kappa, and delta opioid receptors are widely distributed throughout the nervous system (Rachinger‐Adam 2011). Key among the opioid effects is analgesia; the focus of this overview is the use of opioids for their analgesic effect. Opioids bring about complex changes at the cellular and molecular level, decreasing pain perception and increasing tolerance to painful stimuli (Rass 2014).
A number of opioid actions besides analgesia have been described. These include euphoria (Schulteis 1996), sedation, drowsiness, and endocrine dysregulation (Vuong 2010). Opioids also alter sleep regulation, and are associated with poor sleep quality, insomnia, respiratory depression, sleep apnoea and sleep‐disordered breathing (Zutler 2011). Physiological dependence on opioids may develop rapidly after the initiation of opioid use, and opioid abuse and dependence (i.e. opioid use disorders (OUD)) are a major concern with this group of medications. Opioid addiction is also a significant problem, and its neurobiology suggests that increasing doses of opioids are desired over time (Kosten 2002).
Besides other symptoms, the use of opioids is associated with the development of somnolence and sedation (Els 2017). Although each case is individually evaluated, the use of opioids is generally not recommended for people engaged in safety‐sensitive work (Hegmann 2014a).
Although a number of adverse effects have been identified with the acute administration of opioids in novice users, chronic opioid use has been suggested to result in fewer medical problems (Rass 2014). However, some of these adverse effects are serious and potentially lethal, and may not decrease with long‐term use. In addition, long‐term opioid therapy at higher doses has been found to be associated with lower self‐efficacy for pain management, and increased fear‐avoidance beliefs, which are robust predictors of treatment outcome (Morasco 2017).
Why it is important to do this overview
Opioids are commonly used, and used at high doses, for the treatment of pain, including CNCP (Zutler 2011). The opioid doses used for CNCP vary between practitioners, payers, and countries. The use of opioids for CNCP has increased, despite safety concerns (Bohnert 2012; Chapman 2010; Kidner 2009), and despite a lack of clear evidence of the benefit of higher doses (Morasco 2017).
There are several studies examining the prescribing trends for opioids in recent decades for the treatment of CNCP. For example, an analysis of Workers' Compensation Board (WCB) data (where most claimants with pain would have non‐malignant pain) from Manitoba, Canada demonstrated a dramatic increase in the average opioid dose prescribed over time, from less than 500 mg MEQ per year in 1998 to over 6000 mg MEQ per year in 2010 (Kraut 2015). People using opioids at high doses may account for a significant portion of total opioid consumption. Use by the top 3% of WCB claimants on opioids in Alberta, Canada accounted for 80% of the total MEQ used (Boyko 2016). In England, there was a 34% increase in the number of opioid items prescribed between 1998 and 2016, representing a 127% increase when accounting for the oral morphine equivalency (Curtis 2019). Only 12% of strong opioids in England were prescribed for cancer pain in 2014 (Zin 2014); from 2010 to 2014, buprenorphine and codeine had the greatest increase in prescription rates (Mordecai 2018).
A recent study of 41 general practices in England found that 19.3% of the people prescribed opioids were given a dose equal to, or above 120 mg morphine a day; the most commonly cited reasons were musculoskeletal pain of the back, neck, joints, or limbs (Ponton 2018).
The use of high‐dose opioids for CNCP has come under review because of questions about the effectiveness of opioids for CNCP, and the potential for adverse events, abuse, and addiction (Franklin 2014; Häuser 2014; Katz 2015; NOUGG 2010; Nuckols 2014). Three and a half decades ago, Portenoy and Foley described an addiction risk of lower than 1% (Portenoy 1986). However, evidence suggests that opioid abuse and addiction are well documented, and not uncommon among people with chronic pain, with rates of addiction averaging between 8% and 12% (Vowles 2015). Opioid addiction can potentially develop even if these drugs are used for the management of severe pain (Kosten 2002; Huffman 2015; Vowles 2015). This is further supported by a 2020 study, which found that 26.5% of people using opioids to treat CNCP met the criteria for OUD, with 9.0% meeting the criteria for moderate or severe OUD (Boscarino 2020).
With increasing opioid doses, the risk for addiction increases. Huffman and colleagues reported that a 50 mg increases in oral morphine dose almost doubled the risk of addiction; a 100 mg increase in dose was associated with a three‐fold increase in risk (Huffman 2015). Furthermore, there is the potential for serious adverse events, such as sleep apnoea, sleep‐disordered breathing, and respiratory depression, which may lead to opioid‐associated deaths; such adverse outcomes of opioid use demonstrate a clear dose dependency (Jungquist 2012; Walker 2007).
Hegmann and colleagues summarised the substantial increase in the use of opioids, along with the increase in deaths associated with opioid use (Hegmann 2014b). Opioid‐related deaths are common, and can occur even when the prescription is in accordance with guidelines. Sixty percent of opioid‐related deaths in the USA occurred in people who were given prescriptions according to prescribing guidelines of medical boards (with 20% of deaths with doses up to 100 mg MEQ per day, and 40% in people prescribed dosages above that threshold). The remaining 40% of deaths occurred in people abusing the drugs (Manchikanti 2012a). Abuse of opioids includes multiple prescriptions, 'double‐doctoring', and drug diversion.
A consensus has emerged that while long‐term opioid therapy for non‐cancer pain may be appropriate for selected individuals, it should not be the rule (Manchikanti 2012b). Further, a strong and reproducible dose‐response relationship for efficacy led to the recommendation for a MEQ dose limit of 50 mg per day; higher doses are only recommended with documented functional improvement, risk‐benefit consideration, and monitoring of adverse events (Hegmann 2014b).
For people with chronic pain, at short‐term follow‐up, opioids are associated with small beneficial effects versus placebo, but are also associated with increased risk of short‐term harms, and do not appear to be superior to non‐opioid therapy. Evidence of opioid use on intermediate‐term and long‐term benefits remains very limited, and additional evidence confirms an association between opioids and increased risk of serious harms that appears to be dose‐dependent (Chou 2020). In a recent study, opioid users reported no improvement for pain symptoms, physical function, emotional function, and social/family disability (Veiga 2019). Opioid users reported higher satisfaction with care and outcomes at one‐year follow‐up, but at two years, they only reported improvement in satisfaction with outcomes (Veiga 2019). Opioids showed limited effectiveness in long‐term CNCP management, as opioid users presented no improvements in functional outcomes or quality of life (Veiga 2019).
Guidelines and research reports vary in what they consider to be a high opioid dose. A previous Canadian guideline designated a dose of opioids in excess of 200 mg MEQ per day as a 'watchful dose' (NOUGG 2010); this guideline has been updated, and the current Canadian guideline recommends dose limits of 90 mg and 50 mg MEQ per day (Busse 2017). This is more aligned with the dose recommendations in the ACOEM guideline (Hegmann 2014b), and the CDC guideline (Dowell 2016). Other recent guidelines recommend maximum doses in the order of 100 mg or 120 mg MEQ per day. For example, a German guideline, based on a systematic review, recommended that the morphine equivalent dose should not exceed 120 mg per day (Häuser 2014). We adopted a dose of 200 mg MEQ per day as the 'high‐dose' threshold for this overview, as use at this level would generally be considered as clearly 'high dose'.
Another Cochrane overview complements this present overview, Adverse events associated with medium‐ and long‐term use of opioids for chronic non‐cancer pain: an overview of Cochrane Reviews (Els 2017).
In view of the ongoing prescribing of high‐dose opioids for CNCP by some physicians, with its potential for serious harm, we thought it was important to search for new evidence concerning the practice; this motivated the present overview update.
Objectives
To describe the evidence from Cochrane Reviews and overviews regarding the efficacy and safety of high‐dose opioids (defined as 200 mg morphine equivalent or more per day) for chronic non‐cancer pain.
Methods
We aimed to provide an overview of the evidence from Cochrane Reviews and overviews for the efficacy and safety of any opioid agent used at a high dose, administered by any route and frequency of administration, for the treatment of chronic non‐cancer pain (CNCP) in adults. Typically, we expected trial durations of at least two weeks to be relevant for a chronic painful condition. Reviews including shorter trials would have been critically assessed. If a review included a majority of data from inappropriately short trials, we might have excluded the review from the overview, depending on the condition studied.
Criteria for considering reviews for inclusion
We had planned to include all Cochrane Reviews and overviews of randomised controlled trials that assessed the efficacy and safety of opioids in adults with CNCP.
We expected the following of a Cochrane Review or overview in order to be included in our overview.
There was a clearly defined research question relevant to the use of opioids in CNCP.
The Cochrane Review or overview performed a systematic search for relevant evidence, providing details of databases searched and the search strategies.
The Cochrane Review or overview detailed criteria for the inclusion of studies. Included studies in Cochrane Reviews are typically randomised controlled trials (RCT), as this study design is associated with a lower risk of bias than non‐randomised study designs.
Outcomes of interest and data on the high‐dose range (200 mg morphine equivalent (MEQ) or more per day) were reported.
Outcomes of interest
When choosing outcome measures to assess the efficacy and safety of interventions in our overview, we preferred outcome measures that were of known utility. Our preferred outcome measures were based on the guidance for systematic reviews in the pain field (Moore 2010). We planned to consider group average values if no, or insufficient data for responder analyses were available. Our outcomes of interest are listed below; we planned to extract and analyse data for opioid doses of 200 mg morphine equivalent or more per day.
Pain‐related outcomes
Proportion of participants with at least 50% pain reduction over study baseline
Proportion of participants with at least 30% pain reduction over study baseline
Proportion of participants below 30/100 mm on the visual analogue pain scale (i.e. no worse than mild pain)
Treatment group average scores for pain intensity or pain relief
Work‐ and function‐related outcomes
Work time missed
Interference with work (subjective rating scales)
Function (SF‐36, SF‐12 scales); proportion of participants with at least 30% improvement in functional abilities
Quality of life (any scale)
Adverse event outcomes
Proportion of participants with any adverse event
Proportion of participants with any serious adverse event
Proportion of participants withdrawing due to adverse events
We planned to note the imputation methods used in the trials, and discuss the limitations resulting from them. We would have preferred data with 'baseline observation carried forward' methodology over data with 'last observation carried forward', if there was a choice.
Search methods for identification of reviews
Electronic searches
Originally, we searched the Cochrane Database of Systematic Reviews (CDSR; in the Cochrane Library, Issue 4, 2017) across all years. The date of the last search for the original review was 18 April 2017, using the search strategy presented in Appendix 1. For this update, we searched the CDSR in the Cochrane Library, Issue 7, 2022, from April 2017 to 21 July 2022, using the search strategy presented in Appendix 2 (modified only to accommodate the updates to the CDSR interface).
Data collection and analysis
Selection of reviews
Two overview authors independently screened the results of the electronic search by title and abstract. We obtained the full‐text versions of potentially relevant reviews and overviews, and two overview authors subsequently applied the selection criteria to determine eligibility for inclusion. Disagreements were resolved by consensus or by discussion with a third overview author.
Data extraction and management
Two overview authors planned to independently extract data, using a standardised form. We planned to resolve discrepancies by consensus, and to consult a third overview author and make a majority decision should we not have achieved resolution.
Using standardised and piloted data extraction forms, we planned to extract data on the following.
Objectives of the Cochrane Review or overview
Number of studies and participants included in the Cochrane Review or overview
Study and participant baseline characteristics
Inclusion and exclusion criteria applied in the Cochrane Review or overview
Chronic pain conditions studied
Opioid medication, dose and frequency of administration
Our outcomes of interest
Route(s) of administration covered in the Cochrane Review or overview
Declarations of competing interest and sponsorship of the Cochrane Review or overview authors
Assessment of methodological quality of included reviews
For the original overview, two overview authors intended to independently undertake a formal quality assessment of the included Cochrane Reviews and overviews using criteria modified from the AMSTAR guidance (Assessing the Methodological Quality of Systematic Reviews (Moore 2010; Shea 2007)). For this update, we planned to use criteria modified from the AMSTAR‐2 guidance (A critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both (Shea 2017)). The overview authors planned to reconcile differences through discussion, or if needed, by consulting a third overview author.
Data synthesis
Our primary aim, given the expected methodological and clinical heterogeneity between the Cochrane Reviews and overviews that we would include in our overview, was to perform a qualitative evidence synthesis. However, if feasible and appropriate, we would have conducted quantitative meta‐analyses. For meta‐analysis, we intended to use either a fixed‐effect model or a random‐effects model, as determined by between‐study heterogeneity (I² statistic). In addition to assessing statistical heterogeneity, we planned to also consider clinical heterogeneity between the studies.
We planned to use a fixed‐effect model when there was no evidence of significant between‐study heterogeneity. We planned to calculate relative benefit or risk with 95% confidence intervals. We planned to calculate numbers needed to treat for an additional beneficial outcome or an additional harmful outcome from the pooled number of events, using the method of Cook and Sackett (Cook 1995). We planned to follow the guidance detailed in the Cochrane Handbook for Systematic Reviews of Interventions for our overview and meta‐analyses methodology (Higgins 2022). We planned to undertake our analyses across opioid agents (by conversion to mg morphine equivalent (MEQ)), and also for the individual agents. We planned to undertake separate analyses by trial duration. We defined short‐term treatment as treatment lasing less than two weeks; medium‐term lasted at least two weeks but less than two months; long‐term was two months or longer.
We planned to assess the quality of the body of evidence with the GRADE approach as applied in Cochrane Reviews (Higgins 2022). See Appendix 3 for a further description of the GRADE system.
Results
For the original version of this overview, we searched the Cochrane Library using a combination of controlled vocabulary (MeSH) and text terms (Appendix 1). The search resulted in 735 records, including 723 Cochrane Reviews and 12 Cochrane overviews. Three hundred and twenty‐one of the Cochrane Reviews were duplicates, resulting in 414 records (402 reviews and 12 overviews). We excluded 362 reviews and five overviews based on the title or abstract, leaving 40 reviews and seven overviews for detailed examination.
We retrieved full texts of all 40 reviews and the seven overviews. We decided to also retrieve component studies from reviews that might have described high‐dose opioid use. Therefore, we also obtained full‐text copies of 27 potentially relevant component studies from five of the reviews (Chaparro 2012, Chaparro 2013, McNicol 2013, Santos 2015, Wiffen 2015a). Only one of these reviews had separate data on high‐dose use (Wiffen 2015a). However, this was still ineligible, as people with and without cancer were included, and the data for those with non‐cancer pain were not analysed separately (Bohme 2003).
No review or overview met our inclusion criteria. This was primarily due to three reasons. Some reviews focused on cancer or acute pain. Others did not include opioids as an intervention. Reviews that did include opioids for CNCP studied a range of opioid doses, with all doses (including those below and above our cut‐off of 200 mg morphine equivalent per day) analysed as a single group, without providing separate data for high‐dose use. The reasons for exclusion are detailed in Table 1 for reviews and Table 2 for overviews.
1. Characteristics of excluded reviews.
| Study | Reason for exclusion |
| Alviar 2011 | Opioids studied but not at high doses specifically |
| Bao 2016 | Cancer pain |
| Bell 2012 | Cancer pain |
| Brettschneider 2013 | No opioids studied |
| Cepeda 2006 | Opioids studied but not at high doses specifically |
| Chaparro 2012 | Opioids studied but not at high doses specifically |
| Chaparro 2013 | Opioids studied but not at high doses specifically |
| Cheong 2014 | No opioids studied |
| de Oliveira 2016 | Opioids studied but not at high doses specifically |
| Duehmke 2006 | Opioids studied but not at high doses specifically |
| Enthoven 2016 | Opioids studied but not at high doses specifically |
| Fraquelli 2016 | Acute pain |
| Gaskell 2014 | Opioids studied but not at high doses specifically |
| Gaskell 2016 | Review with no included studies |
| Hadley 2013 | Cancer pain |
| Hróbjartsson 2010 | No opioids studied |
| Marks 2011 | No opioids studied |
| McNicol 2013 | Opioids studied but not at high doses specifically |
| McNicol 2015 | Withdrawn from publication |
| Moore 2015a | No opioids studied |
| Mujakperuo 2010 | No opioids studied |
| Nicholson 2017 | Cancer pain |
| Quigley 2013 | Withdrawn from publication |
| Radner 2012 | No opioids studied |
| Ramiro 2011 | Opioids studied but not at high doses specifically |
| Richards 2011 | Opioids studied but not at high doses specifically |
| Santos 2015 | Opioids studied but not at high doses specifically |
| Schmidt‐Hansen 2015a | Cancer pain |
| Schmidt‐Hansen 2015b | Cancer pain |
| Seidel 2013 | Opioids studied but not at high doses specifically |
| Singh 2015 | No opioids studied |
| Staal 2008 | Opioids studied but not at high doses specifically |
| Stannard 2016 | Opioids studied but not at high doses specifically |
| Stanton 2013 | No opioids studied |
| Straube 2014 | Cancer pain |
| Whittle 2011 | Opioids studied but not at high doses specifically |
| Wiffen 2015a | Opioids studied but with mixed cancer and non‐cancer group |
| Wiffen 2015b | Cancer pain |
| Wiffen 2016 | Cancer pain |
| Zeppetella 2015 | Cancer pain |
2. Characteristics of excluded overviews.
| Overview | Reason for exclusion |
| da Costa 2014 | Opioids studied but not at high doses specifically |
| Derry 2016 | Opioids studied but not at high doses specifically |
| Haroutiunian 2012 | Opioids studied but not at high doses specifically |
| Moore 2015 | No opioids studied |
| Noble 2010 | Opioids studied but not at high doses specifically |
| O'Connell 2013 | No opioids studied |
| Rubinstein 2011 | Opioids studied but not at high doses specifically |
For this update, we searched the Cochrane Library using a combination of MeSH and text terms, with the syntax updated to accommodate the new Cochrane Library interface (Appendix 2). We identified 78 records, including 75 Cochrane Reviews and three Cochrane overviews. We excluded 71 reviews and one overview based on the title and abstract, leaving four reviews and two overviews to be reviewed as full‐text reports. None of these six records met the inclusion criteria for our overview. Table 3 details the reasons for exclusion.
3. Characteristics of excluded reviews and overviews, 2022 update.
| Review or Overview | Reason for exclusion |
| Cooper 2017 | Opioids studied but not at high doses specifically |
| Eccleston 2017 | Opioids studied but not at high doses specifically |
| Els 2017a | Opioids studied but not at high doses specifically |
| Els 2017b | Previous version of this overview |
| McNicol 2017 | Opioids studied but not at high doses specifically |
| Thorpe 2018 | Opioids studied but not at high doses specifically |
Our study selection is illustrated by the flow diagram in Figure 1, which combines data from the original overview and this update.
1.
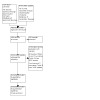
PRISMA study selection flow diagram
Description of included reviews
No Cochrane Reviews or overviews met our inclusion criteria.
Methodological quality of included reviews
No Cochrane Reviews or overviews were included.
Effect of interventions
No Cochrane Reviews or overviews were identified that met our inclusion criteria.
Discussion
Summary of main results
No Cochrane Reviews or overviews were included in this overview.
Overall completeness and applicability of evidence
There is a lack of high‐quality evidence, in the form of Cochrane Reviews, on the efficacy and safety of high‐dose opioids for the management of chronic non‐cancer pain (CNCP) in adults. No evidence‐based statements can be made on the use of high‐dose opioids in clinical practice.
Quality of the evidence
Our overview aimed to assess all the available evidence from Cochrane Reviews and overviews on the use of high‐dose opioids in the management of CNCP in adults. We found no such evidence.
Potential biases in the overview process
We know of no potential biases in our overview process. It is unlikely that there is a substantial body of high‐quality evidence that examines how well high‐dose opioids work for CNCP, or what adverse effects they may have.
Agreements and disagreements with other studies or reviews
This overview is consistent with other reviews (Dowell 2016; Hegmann 2014b). There does not appear to be a body of dependable, high‐quality clinical evidence to either support or refute the use of high‐dose opioids for CNCP.
Authors' conclusions
Implications for practice.
There is an absence of high‐quality evidence to support or refute the use of high‐dose opioids for CNCP. Opioids are associated with increased risks for adverse events, including addiction, overdose, and death (Dowell 2016).
For persons suffering from chronic non‐cancer pain
When selecting a treatment modality and dosage, risks and benefits must be considered. People should be made aware of the potential risks, and the absence of evidence for benefit of high‐dose opioids for CNCP. Physicians should have frank and open conversations with people in this regard, supplemented with a discussion on other, evidence‐based, strategies.
For policymakers
In the absence of evidence to support or refute the efficacy of high‐dose opioids, and in view of the established risks, the use of high‐dose opioids for treating CNCP should not be recommended.
For funders
In the absence of evidence to support or refute the efficacy of high‐dose opioids, they should not be recommended.
Implications for research.
General
Although we know that opioids are commonly prescribed to people, including at high doses, for the treatment of CNCP, there is no evidence to refute or support the efficacy of this clinical practice. However, the risks associated with high‐dose opioid use are well established. We do not at present have dependable information about how well high‐dose opioids work, or what adverse effects may accompany their use when they are used on a long‐term basis for CNCP.
Design
On the one hand, the absence of high‐quality evidence for high‐dose opioid use would typically call for more randomised controlled trials to be conducted, specifically addressing this question. On the other hand, the potential for significant adverse events with high‐dose opioids provides an argument against conducting these studies. The ethics of conducting studies on high‐dose opioids needs to be considered carefully.
Where possible, data from people receiving high‐dose opioids in existing datasets should be re‐analysed separately.
Measurement
Many of the outcomes of interest for high‐dose opioids are the same as for opioids studied at a lower dose range. Dworkin 2005 outlined six domains that should be considered for chronic pain clinical trials, including pain, physical functioning, emotional functioning, treatment satisfaction, symptoms, and adverse events, and participant disposition.
What's new
| Date | Event | Description |
|---|---|---|
| 23 March 2023 | New search has been performed | Updated overview |
| 23 March 2023 | New citation required but conclusions have not changed | Updated overview |
History
Protocol first published: Issue 7, 2016 Review first published: Issue 10, 2017
| Date | Event | Description |
|---|---|---|
| 4 January 2018 | Amended | Minor amendment to Declarations of interest section. |
Acknowledgements
The authors would like to gratefully acknowledge the assistance of Liz Dennett, librarian at the University of Alberta, for her assistance with the search for the overview update.
Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS) supported the authors in the development of this updated overview.
Editorial contributions:
The following people conducted the editorial process for this update:
Sign‐off Editor (final editorial decision): Neil O'Connell, Brunel University London; Co‐ordinating Editor Cochrane Pain, Palliative and Supportive Care (PaPaS) Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Helen Wakeford, Executive Editor, Cochrane Central Editorial Service Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service Copy Editor (copy editing and production): Victoria Pennick, Cochrane Copy‐edit Group
Appendices
Appendix 1. Cochrane Database of Systematic Reviews (in the Cochrane Library) search strategy
#1 MeSH descriptor: [Pain] explode all trees
#2 pain*:ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Analgesics, Opioid] explode all trees
#5 opioid*:ti,ab,kw (Word variations have been searched)
#6 codeine or oxycodone or tramadol or hydromorphone or morphine or fentanyl:ti,ab,kw (Word variations have been searched)
#7 meperidine or pethidine or dextropropoxyphene or methadone or buprenorphine or pentazocine or hydrocodone or opium or butorphanol:ti,ab,kw (Word variations have been searched)
#8 tapentadolol or papaveretum or meptazinol or dipipanone or dihydrocodeine or diamorphine:ti,ab,kw (Word variations have been searched)
#9 #4 or #5 or #6 or #7 or #8
#10 #3 and #9
Appendix 2. Cochrane Database of Systematic Reviews (in the Cochrane Library) updated search strategy
#1 MeSH descriptor: [Pain] explode all trees
#2 (pain*):ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Analgesics, Opioid] explode all trees
#5 (opioid*):ti,ab,kw (Word variations have been searched)
#6 (codeine or oxycodone or tramadol or hydromorphone or morphine or fentanyl):ti,ab,kw (Word variations have been searched)
#7 (meperidine or pethidine or dextropropoxyphene or methadone or buprenorphine or pentazocine or hydrocodone or opium or butorphanol):ti,ab,kw (Word variations have been searched)
#8 (tapentadolol or papaveretum or meptazinol or dipipanone or dihydrocodeine or diamorphine):ti,ab,kw (Word variations have been searched)
#9 {OR #4‐#8}
#10 #3 and #9
#11 #10 in Cochrane Reviews
#12 #11 with Cochrane Library publication date Between Apr 2017 and Jul 2022
Appendix 3. GRADE Assessment
The GRADE system uses the following criteria for assigning grade of evidence.
High quality: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different
Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect
Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect
Grade of evidence is decreased further if the following are present.
Serious (‐1) or very serious (‐2) limitation to study quality
Important inconsistency (‐1)
Some (‐1) or major (‐2) uncertainty about directness
Imprecise or sparse data (‐1)
High probability of reporting bias (‐1)
Differences between protocol and review
TJ joined as an author after the protocol was published.
We considered both Cochrane overviews and Cochrane Reviews to be eligible for this overview. We did this be inclusive of both these forms of Cochrane evidence synthesis products.
Contributions of authors
SS conceived of the idea. CE, RH, DK, BS, VL, and SS contributed to the protocol for this overview. TJ joined the project after the protocol was published. TJ did the searching with the help of the PaPaS Review Group and the assistance of Liz Dennett. CE, TJ, BS, and VL screened the abstracts. CE, TJ, BS, RH, and DK assessed Cochrane Reviews and overviews for inclusion, with guidance from SS. CE, TJ, and SS drafted the full overview. CE, TJ, BS, and SS drafted the overview update. All overview authors approved the current version of the overview.
Sources of support
Internal sources
-
National Institute for Health and Care Research, UK
This project was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
External sources
-
WCB Alberta, Canada
We acknowledge funding from the Workers’ Compensation Board ‐ Alberta (Edmonton, Alberta) for the project 'Effectiveness and safety of high dose opioid therapy in WCB Alberta claimants', of which the original version of this overview was part. This overview update was supported by a program grant from the Workers’ Compensation Board ‐ Alberta (Edmonton, Alberta), 'Program of research and training in Preventive Medicine'.
-
Alberta School of Business, Canada
We acknowledge funding from the Alberta School of Business, University of Alberta, for the project “Effectiveness and safety of high dose opioid therapy in WCB Alberta claimants”, of which the original version of this overview was part.
Declarations of interest
Charl Els: none known; he is an addiction psychiatrist and occupational physician, employed in a regulatory setting
Tanya D Jackson: none known; she is a clinical psychologist whose practice includes people with chronic pain
Reidar Hagtvedt: none known
Diane Kunyk: none known
Barend Sonnenberg: none known; he is a family physician and a medical consultant at the Workers' Compensation Board, Alberta, primarily assessing claims where opioids are prescribed to workers
Vernon G Lappi: he is a retired specialist occupational medicine physician. He discloses that he is the former Director of Medical Services of the Workers' Compensation Board ‐ Alberta, and that he has personal equity holdings in Berkshire Hathaway and Procter and Gamble.
Sebastian Straube: none known; he is a specialist occupational medicine physician and some of the people he assesses have chronic pain. He is a Cochrane editor but was not involved in the editorial process for this overview.
New search for studies and content updated (no change to conclusions)
References
References to excluded reviews
Alviar 2011
- Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD006380. [DOI: 10.1002/14651858.CD006380.pub2] [DOI] [PubMed] [Google Scholar]
Bao 2016
- Bao YJ, Hou W, Kong XY, Yang L, Xia J, Hua BJ, et al. Hydromorphone for cancer pain. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD011108. [DOI: 10.1002/14651858.CD011108.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2012
- Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD003351. [DOI: 10.1002/14651858.CD003351.pub2] [DOI] [PubMed] [Google Scholar]
Brettschneider 2013
- Brettschneider J, Kurent J, Ludolph A. Drug therapy for pain in amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD005226. [DOI: 10.1002/14651858.CD005226.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cepeda 2006
- Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD005522. [DOI: 10.1002/14651858.CD005522.pub2] [DOI] [PubMed] [Google Scholar]
Chaparro 2012
- Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD008943. [DOI: 10.1002/14651858.CD008943.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaparro 2013
- Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004959. [DOI: 10.1002/14651858.CD004959.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheong 2014
- Cheong YC, Smotra G, Williams ACC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD008797. [DOI: 10.1002/14651858.CD008797.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2017
- Cooper TE, Chen J, Wiffen PJ, Derry S, Carr DB, Aldington D, et al. Morphine for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011669. [DOI: 10.1002/14651858.CD011669.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
da Costa 2014
- da Costa BR, Nüesch E, Kasteler R, Husni E, Welch V, Rutjes AWS, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD003115. [DOI: 10.1002/14651858.CD003115.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Oliveira 2016
- Oliveira CO, Carvalho LB, Carlos K, Conti C, Oliveira MM, Prado LB, et al. Opioids for restless legs syndrome. Cochrane Database of Systematic Reviews 2016, Issue 6. Art. No: CD006941. [DOI: 10.1002/14651858.CD006941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2016
- Derry S, Stannard C, Cole P, Wiffen PJ, Knaggs R, Aldington D, et al. Fentanyl for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD011605. [DOI: 10.1002/14651858.CD011605.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duehmke 2006
- Duehmke RM, Hollingshead J, Cornblath DR. Tramadol for neuropathic pain. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD003726. [DOI: 10.1002/14651858.CD003726.pub3] [DOI] [PubMed] [Google Scholar]
Eccleston 2017
- Eccleston C, Fisher E, Thomas KH, Hearn L, Derry S, Stannard C, et al. Interventions for the reduction of prescribed opioid use in chronic non‐cancer pain. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD010323. [DOI: 10.1002/14651858.CD010323.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Els 2017a
- Els C, Jackson TD, Kunyk D, Lappi VG, Sonnenberg B, Hagtvedt R, et al. Adverse events associated with medium‐ and long‐term use of opioids for chronic non‐cancer pain: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD012509. [DOI: 10.1002/14651858.CD012509.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Els 2017b
- Els C, Jackson TD, Hagtvedt R, Kunyk D, Sonnenberg B, Lappi VG, et al. High‐dose opioids for chronic non‐cancer pain: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD012299. [DOI: 10.1002/14651858.CD012299.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Enthoven 2016
- Enthoven W, Roelofs PD, Deyo RA, Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD012087. [DOI: 10.1002/14651858.CD012087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraquelli 2016
- Fraquelli M, Casazza G, Conte D, Colli A. Non-steroid anti-inflammatory drugs for biliary colic. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD006390. [DOI: 10.1002/14651858.CD006390.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaskell 2014
- Gaskell H, Moore RA, Derry S, Stannard C. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD010692. [DOI: 10.1002/14651858.CD010692.pub2] [DOI] [PubMed] [Google Scholar]
Gaskell 2016
- Gaskell H, Moore RA, Derry S, Stannard C. Oxycodone for pain in fibromyalgia in adults. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD012329. [DOI: 10.1002/14651858.CD012329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadley 2013
- Hadley G, Derry S, Moore RA, Wiffen PJ. Transdermal fentanyl for cancer pain. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD010270. [DOI: 10.1002/14651858.CD010270.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haroutiunian 2012
- Haroutiunian S, McNicol ED, Lipman AG. Methadone for chronic non-cancer pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD008025. [DOI: 10.1002/14651858.CD008025.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2010
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD003974. [DOI: 10.1002/14651858.CD003974.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 2011
- Marks JL, Colebatch AN, Buchbinder R, Edwards CJ. Pain management for rheumatoid arthritis and cardiovascular or renal comorbidity. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD008952. [DOI: 10.1002/14651858.CD008952.pub2] [DOI] [PubMed] [Google Scholar]
McNicol 2013
- McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD006146. [DOI: 10.1002/14651858.CD006146.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicol 2015
- McNicol ED, Strassels S, Goudas L, Lau J, Carr DB. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD005180. [DOI: 10.1002/14651858.CD005180.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicol 2017
- McNicol ED, Ferguson MC, Schumann, R. Methadone for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012499. [DOI: 10.1002/14651858.CD012499.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Derry S, Aldington D, Wiffen PJ. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults - an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD011407. [DOI: 10.1002/14651858.CD011407.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for fibromyalgia in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD011824. [DOI: 10.1002/14651858.CD011824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mujakperuo 2010
- Mujakperuo HR, Watson M, Morrison R, Macfarlane TV. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD004715. [DOI: 10.1002/14651858.CD004715.pub2] [DOI] [PubMed] [Google Scholar]
Nicholson 2017
- Nicholson AB, Watson GR, Derry S, Wiffen PJ. Methadone for cancer pain. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003971. [DOI: 10.1002/14651858.CD003971.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noble 2010
- Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD006605. [DOI: 10.1002/14651858.CD006605.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connell 2013
- O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL. Interventions for treating pain and disability in adults with complex regional pain syndrome- an overview of systematic reviews. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD009416. [DOI: 10.1002/14651858.CD009416.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2013
- Quigley C. Hydromorphone for acute and chronic pain. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD003447. [DOI: 10.1002/14651858.CD003447.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radner 2012
- Radner H, Ramiro S, Buchbinder R, Landewé R, Heijde D, Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD008951. [DOI: 10.1002/14651858.CD008951.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramiro 2011
- Ramiro S, Radner H, Heijde D, Tubergen A, Buchbinder R, Aletaha D, et al. Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis). Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD008886. [DOI: 10.1002/14651858.CD008886.pub2] [DOI] [PubMed] [Google Scholar]
Richards 2011
- Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD008920. [DOI: 10.1002/14651858.CD008920.pub2] [DOI] [PubMed] [Google Scholar]
Rubinstein 2011
- Rubinstein SM, Middelkoop M, Assendelft WJJ, Boer MR, Tulder MW. Spinal manipulative therapy for chronic low-back pain. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD008112. [DOI: 10.1002/14651858.CD008112.pub2] [DOI] [PubMed] [Google Scholar]
Santos 2015
- Santos J, Alarcão J, Fareleira F, Vaz-Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD009923. [DOI: 10.1002/14651858.CD009923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt‐Hansen 2015a
- Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Oxycodone for cancer-related pain. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003870. [DOI: 10.1002/14651858.CD003870.pub5] [DOI] [PubMed] [Google Scholar]
Schmidt‐Hansen 2015b
- Schmidt-Hansen M, Bromham N, Taubert M, Arnold S, Hilgart JS. Buprenorphine for treating cancer pain. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD009596. [DOI: 10.1002/14651858.CD009596.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidel 2013
- Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004844. [DOI: 10.1002/14651858.CD004844.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2015
- Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD005614. [DOI: 10.1002/14651858.CD005614.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Staal 2008
- Staal JB, Bie R, Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low-back pain. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD001824. [DOI: 10.1002/14651858.CD001824.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stannard 2016
- Stannard C, Gaskell H, Derry S, Aldington D, Cole P, Cooper TE, et al. Hydromorphone for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011604. [DOI: 10.1002/14651858.CD011604.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2013
- Stanton TR, Wand BM, Carr DB, Birklein F, Wasner GL, O'Connell NE. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004598. [DOI: 10.1002/14651858.CD004598.pub3] [DOI] [PubMed] [Google Scholar]
Straube 2014
- Straube C, Derry S, Jackson KC, Wiffen PJ, Bell RF, Strassels S, et al. Codeine, alone and with paracetamol (acetaminophen), for cancer pain. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD006601. [DOI: 10.1002/14651858.CD006601.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorpe 2018
- Thorpe J, Shum B, Moore RA, Wiffen PJ, Gilron I. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD010585. [DOI: 10.1002/14651858.CD010585.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittle 2011
- Whittle SL, Richards BL, Husni E, Buchbinder R. Opioid therapy for treating rheumatoid arthritis pain. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD003113. [DOI: 10.1002/14651858.CD003113.pub3] [DOI] [PubMed] [Google Scholar]
Wiffen 2015a
- Wiffen PJ, Derry S, Moore RA, Stannard C, Aldington D, Cole P, et al. Buprenorphine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011603. [DOI: 10.1002/14651858.CD011603.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2015b
- Wiffen PJ, Derry S, Naessens K, Bell RF. Oral tapentadol for cancer pain. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011460. [DOI: 10.1002/14651858.CD011460.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2016
- Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD003868. [DOI: 10.1002/14651858.CD003868.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeppetella 2015
- Zeppetella G, Davies AN. Opioids for the management of breakthrough pain in cancer patients. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD004311. [DOI: 10.1002/14651858.CD004311.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bohme 2003
- Bohme K, Likar R. Efficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS), in the treatment of patients with chronic pain. A randomised, double-blind, placebo-controlled study. Pain Clinic 2003;15(2):193-202. [DOI: 10.1163/156856903321579334] [DOI] [Google Scholar]
Bohnert 2012
- Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. American Journal of Psychiatry 2012;169(1):64-70. [DOI: 10.1176/appi.ajp.2011.10101476] [DOI] [PubMed] [Google Scholar]
Bonica 1953
- Bonica JJ. The Management of Pain. Philadelphia: Lea & Febiger, 1953. [Google Scholar]
Boscarino 2020
- Boscarino JA, Withey CA, Dugan RJ, Hu Y, Auciello J, Alfieri T. Opioid medication use among chronic non-cancer pain patients assessed with a modified drug effects questionnaire and the association with opioid use disorder. Journal of Pain Research 2020;13:2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyko 2016
- Boyko R, Els C, Lappi V, Sonnenberg B, Kunyk D, Hagtvedt R, et al. Opioid prescription analysis for Workers’ Compensation Board of Alberta claimants. Poster presented at the 34th Annual Conference of the Occupational and Environmental Medical Association of Canada; 2016 Sept 25; Whistler, British Columbia, Canada.
Breivik 2006
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain 2006;10(4):287-333. [DOI] [PubMed] [Google Scholar]
Breivik 2008
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EB, et al. Assessment of pain. British Journal of Anaesthesia 2008;101(1):17-24. [DOI: 10.1093/bja/aen103] [DOI] [PubMed] [Google Scholar]
Busse 2017
- Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189(18):E659-66. [DOI: 10.1503/cmaj.170363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2010
- Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. Journal of Pain 2010;11(9):807-29. [DOI: 10.1016/j.jpain.2010.02.019] [DOI] [PubMed] [Google Scholar]
Chou 2020
- Chou R, Hartung D, Turner J, Blazina I, Chan B, Levander X, et al. Opioid Treatments for Chronic Pain. Comparative Effectiveness Review No. 229. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I.) AHRQ Publication No. 20-EHC011. Rockville, MD: Agency for Healthcare Research and Quality; April 2020. Available at effectivehealthcare.ahrq.gov/products/opioids-chronic-pain/research. [DOI: 10.23970/AHRQEPCCER229] [DOI] [PubMed]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 2019
- Curtis HJ, Croker R, Walker AJ, Richards GC, Quinlan J, Goldacre B. Opioid prescribing trends and geographical variation in England, 1998–2018: a retrospective database study. The Lancet Psychiatry 2019;6(2):140-50. [DOI] [PubMed] [Google Scholar]
Dahlhamer 2018
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR. Morbidity and Mortality Weekly Report 2018;67(36):1001-6. [DOI: 10.15585/mmwr.mm6736a2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Sola 2016
- De Sola H, Salazar A, Dueñas M, Ojeda B, Failde I. Nationwide cross-sectional study of the impact of chronic pain on an individual’s employment: relationship with the family and the social support. BMJ Open 2016;6(12):e012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowell 2016
- Dowell D, Haegerich T, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. JAMA 2016;315(15):1624-45. [DOI: 10.1001/jama.2016.1464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2005
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. [DOI: 10.1016/j.pain.2004.10.019] [DOI] [PubMed] [Google Scholar]
Fiore 2022
- Fiore JF, El-Kefraoui C, Chay MA, Nguyen-Powanda P, Do U, Olleik G, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet 2022;399:2280-93. [DOI] [PubMed] [Google Scholar]
Franklin 2014
- Franklin GM, American Academy of Neurology. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2014;83(14):1277-84. [DOI: 10.1212/WNL.0000000000000839] [DOI] [PubMed] [Google Scholar]
Goldberg 2011
- Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hegmann 2014a
- Hegmann KT, Weiss MS, Bowden K, Branco F, DuBrueler K, Els C, et al. ACOEM practice guidelines: opioids and safety-sensitive work. Journal of Occupational and Environmental Medicine 2014;56(7):e46-53. [DOI: 10.1097/JOM.0000000000000237] [DOI] [PubMed] [Google Scholar]
Hegmann 2014b
- Hegmann KT, Weiss MS, Bowden K, Branco F, DuBrueler K, Els C, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. Journal of Occupational and Environmental Medicine 2014;56(12):e143-59. [DOI: 10.1097/JOM.0000000000000352] [DOI] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Huffman 2015
- Huffman KL, Shella ER, Sweis G, Griffith SD, Scheman J, Covington EC. Nonopioid substance use disorders and opioid dose predict therapeutic opioid addiction. Journal of Pain 2015;16(2):126-34. [DOI: 10.1016/j.jpain.2014.10.011] [DOI] [PubMed] [Google Scholar]
Häuser 2014
- Häuser W, Bock F, Engeser P, Tölle T, Willweber-Strumpfe A, Petzke F. Clinical practice guideline: long-term opioid use in non-cancer pain. Deutsches Ärzteblatt International 2014;111(43):732-40. [DOI: 10.3238/arztebl.2014.0732] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jungquist 2012
- Jungquist CR, Flannery M, Perlis ML, Grace JT. Relationship of chronic pain and opioid use with respiratory disturbance during sleep. Pain Management Nursing 2012;13(2):70-9. [DOI: 10.1016/j.pmn.2010.04.003] [DOI] [PubMed] [Google Scholar]
Katz 2015
- Katz JA, Swerdloff MA, Brass SD, Argoff CE, Markman J, Backonja M, et al. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2015;84(14):1503-4; Author Response 1504-5. [DOI: 10.1212/WNL.0000000000001485] [DOI] [PubMed] [Google Scholar]
Kidner 2009
- Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. Journal of Bone and Joint Surgery 2009;91(4):919-27. [DOI: 10.2106/JBJS.H.00286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosten 2002
- Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Science & Practice Perspectives 2002;1(1):13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraut 2015
- Kraut A, Shafer LA, Raymond CB. Proportion of opioid use due to compensated workers' compensation claims in Manitoba, Canada. American Journal of Industrial Medicine 2015;58(1):33-9. [DOI: 10.1002/ajim.22374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manchikanti 2012a
- Manchikanti L, Helm S 2nd, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain Physician 2012;15(3 Suppl):ES9-38. [PubMed] [Google Scholar]
Manchikanti 2012b
- Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, et al, American Society of Interventional Pain Physicians. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain. Part 2 – guidance. Pain Physician 2012;15(3 Suppl):S67-116. [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al, ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386-9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore AR, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79-94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Morasco 2017
- Morasco BJ, Yarborough BJ, Smith NX, Dobscha SK, Deyo RA, Perrin NA, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. The Journal of Pain 2017;18(4):437-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mordecai 2018
- Mordecai L, Reynolds C, Donaldson LJ, C Williams AC. Patterns of regional variation of opioid prescribing in primary care in England: a retrospective observational study. British Journal of General Practice 2018-03-01;68(668):e225-33. [DOI: 10.3399/bjgp18x695057] [DOI] [PMC free article] [PubMed] [Google Scholar]
NOUGG 2010
- National Opioid Use Guideline Group (NOUGG). Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. nationalpaincentre.mcmaster.ca/opioid/documents.html (accessed 12 May 2017).
Nuckols 2014
- Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Annals of Internal Medicine 2014;160(1):38-47. [DOI: 10.7326/0003-4819-160-1-201401070-00732] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ponton 2018
- Ponton R, Sawyer R. Opioid prescribing in general practice: use of a two-stage review tool to identify and assess high-dose prescribing. British Journal of Pain 2018;12(3):171-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Portenoy 1986
- Portenoy R, Foley K. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain 1986;25(2):171-86. [DOI] [PubMed] [Google Scholar]
Rachinger‐Adam 2011
- Rachinger-Adam B, Conzen P, Azad SC. Pharmacology of peripheral opioid receptors. Current Opinion in Anaesthesiology 2011;24(4):408-13. [DOI: 10.1097/ACO.0b013e32834873e5] [DOI] [PubMed] [Google Scholar]
Raja 2020
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161(9):1976-82. [DOI: 10.1097/j.pain.0000000000001939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rass 2014
- Rass O, Schacht RL, Marvel CL, Mintzer MZ. Chapter 11. Opioids. In: Allen DN, Woods SP, editors(s). Neuropsychological aspects of substance use disorders: Evidence-based perspectives. New York: Oxford University Press, 2014:231-53. [Google Scholar]
Schulteis 1996
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochemical Research 1996;21(11):1437-54. [DOI] [PubMed] [Google Scholar]
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:1-10. [DOI: 10.1186/1471-2288-7-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shea 2017
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI: 10.1136/bmj.j4008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veiga 2019
- Veiga DR, Monteiro-Soares M, Mendonça L, Sampaio R, Castro-Lopes JM, Azevedo LF. Effectiveness of opioids for chronic noncancer pain: a two-year multicenter, prospective cohort study with propensity score matching. The Journal of Pain 2019;20(6):706-15. [DOI] [PubMed] [Google Scholar]
Vowles 2015
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015;156(4):569-76. [DOI: 10.1097/01.j.pain.0000460357.01998.f1] [DOI] [PubMed] [Google Scholar]
Vuong 2010
- Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocrine Reviews 2010;31(1):98–132. [DOI: 10.1210/er.2009-0009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2007
- Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. Journal of Clinical Sleep Medicine 2007;3(5):455-61. [PMC free article] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization. Cancer pain relief: with a guide to opioid availability. 2nd edition. Geneva: World Health Organization, 1996. [Google Scholar]
Yong 2022
- Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain 2022;163(2):e328-32. [DOI] [PubMed] [Google Scholar]
Zin 2014
- Zin CS, Chen LC, Knaggs RD. Changes in trends and pattern of strong opioid prescribing in primary care. European Journal of Pain 2014;18(9):1343-51. [DOI: 10.1002/j.1532-2149.2014.496.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zutler 2011
- Zutler M, Holty JE. Opioids, sleep, and sleep-disordered breathing. Current Pharmaceutical Design 2011;17(15):1443-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Els 2017
- Els C, Jackson TD, Kunyk D, Lappi VG, Sonnenberg B, Hagtvedt R, et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD012509. [DOI: 10.1002/14651858.CD012509] [DOI] [PMC free article] [PubMed] [Google Scholar]
Els 2017c
- Els C, Jackson TD, Hagtvedt R, Kunyk D, Sonnenberg B, Lappi VG, et al. High‐dose opioids for chronic non‐cancer pain: an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD012299. [DOI: 10.1002/14651858.CD012299.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


