Introduction:
This systematic review and meta-analysis aimed to assess the efficacy and safety of cupping therapy in patients with metabolic syndrome (MetS).
Methods:
This systematic review focused on patients with MetS and included randomized controlled trials (RCTs) that compared the effects of cupping therapy with control groups. A total of 12 electronic databases were searched from inception until February 03, 2023. The main outcome after the meta-analysis was waist circumference; the others included anthropometric variables, blood pressure, lipid profile, fasting blood glucose level, and high-sensitivity C-reactive protein level. The incidence of adverse events and the follow-up courses were also evaluated. Risk of bias (ROB) was evaluated using ROB 2.0 from the Cochrane Handbook.
Results:
This systematic review included five studies involving 489 patients. Some risks of bias were also identified. The meta-analysis revealed a statistically significance in waist circumference (MD = −6.07, 95% CI: −8.44 to −3.71, P < .001, I2 = 61%, τ2 = 3.4), body weight (MD = −2.46, 95% CI: −4.25 to −0.68, P = .007, I2 = 0%, τ2 = 0) and body mass index (MD = −1.26, 95% CI: −2.11 to −0.40, P = .004, I2 = 0%, τ2 = 0) between the cupping therapy and control groups. However, there were no significant results in total fat percentage and blood pressure values. Regarding biochemical markers, cupping significantly lowered the concentration of low-density lipoprotein cholesterol (MD = −3.98, 95% CI: −6.99 to −0.96, P = .010, I2 = 0%, τ2 = 0) but had no significant effect on total cholesterol, triglyceride, high-density lipoprotein cholesterol, fasting blood glucose, and high-sensitivity C-reactive protein. 3 RCTs reported no adverse events.
Conclusions:
Despite some ROB and low to substantial heterogeneity of the included studies, cupping therapy can be considered a safe and effective complementary intervention for reducing waist circumference, body weight, body mass index, and low-density lipoprotein cholesterol in patients with MetS. In the future, well-designed, high-quality, rigorous methodology, and long-term RCTs in this population are required to assess the efficacy and safety of cupping therapy.
Keywords: adverse events, cup, cupping, dysmetabolic syndrome, Hijama, insulin resistance, metabolic syndrome, obesity, safety, syndrome X
1. Introduction
Metabolic syndrome (MetS), also known as dysmetabolic syndrome, or syndrome X, is defined by the World Health Organization (WHO) as a pathological disorder characterized by abdominal obesity, insulin resistance, hypertension, hyperglycemia, and atherogenic dyslipidemia.[1,2] In recent years, the prevalence of MetS has been reported to be 34.7% in the United States and 24.5% in mainland China.[3,4] MetS is associated with an increased risk of diabetes, cardiovascular disease, cerebrovascular diseases, and all-cause mortality, making it a serious public health concern.[3] Although there is some variation in the definition by different authoritative academic institutions, the diagnosis is based on waist circumference, body mass index (BMI), insulin resistance, blood glucose, blood pressure, and lipid profile.[5] Although its pathophysiology is not completely understood, obesity is believed to be at the core of MetS progression.[6] The development of MetS seems to be largely attributable to insulin resistance, excessive fatty acid flux, and proinflammatory states.[7] Insulin resistance is a pathogenic link between different metabolic abnormalities in MetS. It can be induced by different environmental factors such as diet and habits.[8] Lipotoxicity is caused by exposure to toxic levels of fatty acids, which leads to cell damage, and is involved in the pathogenesis of MetS. Adipokines are inflammatory cytokines secreted by adipose tissue. Adipokines are associated with low-grade state of inflammation that may contribute to the development of MetS and obesity-associated diseases.[2,9]
However, the 5 weight loss drugs approved by the Food and Drug Administration of the United States are highly limited in clinical use for MetS because of moderate to severe adverse reactions.[10] As there are insufficient treatments for obesity, complementary and alternative medicines are urgently required.
Cupping therapy is a technique that uses cups placed over the skin to create negative pressure through suction and has been widely used in clinical situations in Asian countries.[11] Cupping therapy was proved to have significant effect on low back pain, ankylosing spondylitis, knee osteoarthritis, neck pain, herpes zoster, migraine, plaque psoriasis, and chronic urticaria by the evidence of systematic review.[12] However, the efficacy of cupping therapy for metabolic syndrome still lacks sufficient evidence. A clinical trial showed cupping combined with diet control had significant effect on lowering BMI, body weight, waist circumference, hip circumference, total cholesterol (TC), triglycerides (TG), and high-sensitivity C-reactive protein (hs-CRP) in obese adult.[13] A randomized controlled trial from China also reported cupping could reduce waist circumference and BMI and lower the level of TC and TG in obese patient.[14] A network meta-analysis from Korea showed cupping plus acupressure was optimal for BMI reduction compared with non-treatment in childhood simple obesity.[15]
The mechanism how cupping can treat obesity is mainly by regulating the expression of pro-inflammatory factors such as hs-CRP, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) to improve insulin resistance and regulate metabolism of the body.[16] Cupping could increase the level of anti-inflammatory factors secreted by adipocytes, such as adiponectin, interleukin-10 (IL-10), and secreted frizzled related protein 5 (SFRP5).[16] Ahmed et al[17] investigated the effects of wet cupping therapy on inflammatory and immunological parameters in patients with rheumatoid arthritis. A significant reduction in the erythrocyte sedimentation rate and hs-CRP level was observed in the wet cupping group. Current research showed cupping intervention might benefit simple obesity by increasing blood circulation, regulating immune system, and reducing inflammation.[16] Recently, randomized controlled trials (RCTs) evaluated the efficacy of cupping for MetS. However, the results of different trials are controversial. Therefore, our study aimed to assess the efficacy of cupping for MetS using a systematic review and meta-analysis.
2. Materials and methods
This systematic review and meta-analysis was registered with PROSPERO (ID: CRD42022352216) on August 19, 2022, without amendments between the registration and the final article. The study was conducted in accordance with the PRISMA guidelines but without a published protocol. This study focused on RCTs examining the efficacy of cupping therapy in patients with MetS.
2.1. Search strategy
Nine English databases (PubMed, Embase, Cochrane, Alt HealthWatch, CINAHL, Medline, Health Source, Web of Science, Health and Psychosocial Instruments), 3 clinical trials repository (Clinical Trials.gov, World Health Organization International Clinical Trials Registry Platform, International Standard Randomised Controlled Trial Number) and 3 Chinese databases (CNKI, WanFang, and AiritiLibrary) were searched for RCTs published from database inception through February 03, 2023. The search strategy consisted of 2 components: Metabolic Syndrome (Metabolic Syndrome OR Plurimetabolic syndrome OR Dysmetabolic syndrome OR syndrome X OR insulin resistance) and Cupping (Cupping or Cups or Hijama or Al-Hijamah) (see Table S1, Supplemental Digital Content, http://links.lww.com/MD/I690, which illustrates the search strategy carried out in each database). To establish the eligibility of the studies, 2 reviewers (L.-K.W. and C.-Y.Y.) independently screened the records of the comprehensive searches by titles and abstracts or full text, as needed. If there is a disagreement, a third reviewer (Y.-C.C.) will help resolve this controversy.
2.2. Inclusion criteria
Only randomized controlled trials (RCTs) reporting clinical efficacy comparing cupping intervention to non-cupping control group in Mets were included.
The included population was diagnosed with metabolic syndrome by a physician clinically, or met the diagnostic criteria for metabolic syndrome by international authoritative academic institutions, including WHO, National Cholesterol Education Program, American Heart Association, International Diabetes Federation, European Group for the Study of Insulin Resistance, American Association of Clinical Endocrinology, Chinese Society of Cardiology, and Chinese Diabetes Society.
Participants for inclusion should be primary MetS rather than comorbidity.
Eligible interventions comprise various cupping therapy (cupping, dry cupping, wet cupping, bleeding cupping, fire cupping, herbal cupping, moving cupping, needling cupping, Hijama or Al-Hijamah) with or without standard treatment for MetS.
Eligible control groups were sham cupping, blank control group, waiting group or standard treatment of MetS.
For outcome measurement, studies had to assess the resolution of metabolic syndrome. Primary outcome was the anthropometric parameter after the cupping intervention such as waist circumference, body weight, body mass index, or total fat percentage. Secondary outcomes could be metabolic parameters such as blood pressure values, lipid profile values, or biochemical markers.
2.3. Exclusion criteria
MetS belong to secondary metabolic diseases, such as hypothalamic lesions, hypothyroidism, polycystic ovary syndrome and Cushing syndrome.
Intervention group comprising more than Cupping therapy or standard care of MetS.
2.4. Data extraction and quality assessment
Two reviewers (L.-K.W. and C.-Y.Y) independently extracted the data, and a third reviewer (Y.-C.C.) was consulted if there was any disagreement. We recorded the first author, year of publication, type of study, location, sample size, age, sex, course of disease, diagnosis for inclusion, details of the intervention and control groups, treatment duration, outcome measures, adverse events (AEs), and follow-up (Table 1). Where data were missing or unclear, the corresponding authors were contacted via email to request for missing data. Extracted data were converted to the same unit, for example, from mmol/L to mg/dL, when measuring triglycerides. We assumed that the units of total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) should be mg/dL rather than mmol/L in the study by Farahmand et al[18] because of the normal range of laboratory values.
Table 1.
Main characteristics of the included studies.
| 1st author, year | Sample size (I/C) | Age ranges (I/C) | Mean ages (I/C) | M:F | Course of disease (I/C) | Diagnosis for inclusion | Intervention | Control | Outcome measures | Adverse event (I/C) | Follow up | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farahmand (2012)[18] | 126 (63/63) | 18–65 | 45.1/47.1 | NM | NM | Physician diagnosed | Wet Cupping + dietary advice | Dietary advice | 1) Anthropometric: weight, BMI, WC, HC, W: Hip, SBP, DBP 2) Laboratory: FBG, TC, TG, HDL-C, LDL-C, Fat %, total body fat mass |
No Adverse event | 6 wk | Iran |
| Farahmand (2014)[19] | 126 (63/63) | 18–65 | NM | NM | NM | Physician diagnosed | Wet Cupping + dietary advice | Dietary advice | hs-CRP, Hsp 27 antibody | No Adverse event | 6 wk | Iran |
| Wang (2015)[20] | 60 (30/30) | 19–46 | 31.5/30.6 | 52:8 | 1–10 yr | CSC Criteria 2007 | Fire Cupping + needle cupping + manipulation + herb | Herb | 1) Anthropometric: weight, BMI, WC, W:H, Fat %, SBP, DBP 2) Laboratory: FBG, TC, TG, HDL-C, LDL-C, hs-CRP, HOMA-IR, Chemerin, LP, Ob-R, TNF-α, ER |
No Adverse event | 4 wk | China |
| Liang (2019)[21] | 75 (25/25/25) | 25–70 | 47.9/51.8/51.2 | 16:59 | NM | CDS Criteria 2013 | 1. Fire Cupping + moving cupping with ointment 2. Acup |
Waiting group | WC, SBP, DBP, FBG, 2hBG, TG, HDL-C, Subcutaneous fat thickness, MetS prevalence | NM | Nil | China |
| Zhou (2021)[22] | 102 (51/51) | Nil | 45.6/44.9 | 60:42 | 3.4/3.4 yr | IDF Criteria | Moving Cupping + Acup | Acup | Weight, BMI, WC, W: Hip, AFT, VAI | NM | Nil | China |
2hBG = 2 hours postprandial blood glucose, Acup = acupuncture, AFT = abdominal fat thickness, BMI = body mass index, CDS = Chinese Diabetes Society, CSC = Chinese Society of Cardiology, DBP = diastolic blood pressure, ER = effective rate, Fat% = Body Total Fat percentage, FBG = fasting blood glucose, HDL-C = high density lipoprotein cholesterol, herb = Traditional Chinese herbal medicine, HOMA-IR = Homeostasis Model Assessment-Insulin Resistance index, hs-CRP = high sensitivity C-reactive protein, IDF = International Diabetes Federation, LDL-C = low density lipoprotein cholesterol, LP = leptin, MetS = metabolic syndrome, NM = not mentioned, Ob-R = obese gene receptor, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride, TNF-α = tumor necrosis factor-alpha, VAI = visceral adiposity index, W:H = waist:height, W:Hip = waist:hip circumference ratio, WC = waist circumference.
For critical appraisal, the methodological quality of the included studies was evaluated by 2 reviewers (L.-K.W. and C-Y. Y) independently using the assessment tool for Risk of Bias 2.0, from the Cochrane Handbook for Systematic Reviews of Interventions.[23,24] In addition, the level of evidence was analyzed using the GRADEpro Guideline Development tool (developed by Evidence Prime Inc.).[25] Any assessment discrepancies between reviewers were resolved via discussion or by a third reviewer (Y.-C. C).
2.5. Data synthesis and analysis
The population size, mean, and standard deviation values at the end of treatment for cupping-induced changes in anthropometric parameters and biochemical markers were subjected to meta-analyses. The primary outcome was the mean difference (MD) in waist circumference with a 95% confidence interval (CI). Other outcome measures included anthropometric variables (weight, body mass index, and total fat percentage), blood pressure (systolic and diastolic blood pressures), lipid profile (total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein), fasting blood glucose, and high-sensitivity C-reactive protein (hs-CRP). The incidence of AEs and follow-up course were also investigated. A random-effects model was employed to pool the MDs using the R software (version 4.2.1; 2022, Vienna, Austria).[26] Heterogeneity between trials was determined using the tau square (τ2) and I square (I2) test, I2 values > 50% indicating high heterogeneity. If considerable heterogeneity was observed, meta-regression and subgroup analyses were performed. Funnel plots and Egger’s tests were used to examine potential publication bias. Statistical significance was set at P < .05. Sensitivity analysis was performed by reanalyzing with omitting any of the included studies to determine the robustness of the observed outcomes.
3. Results
3.1. Study search and characteristics
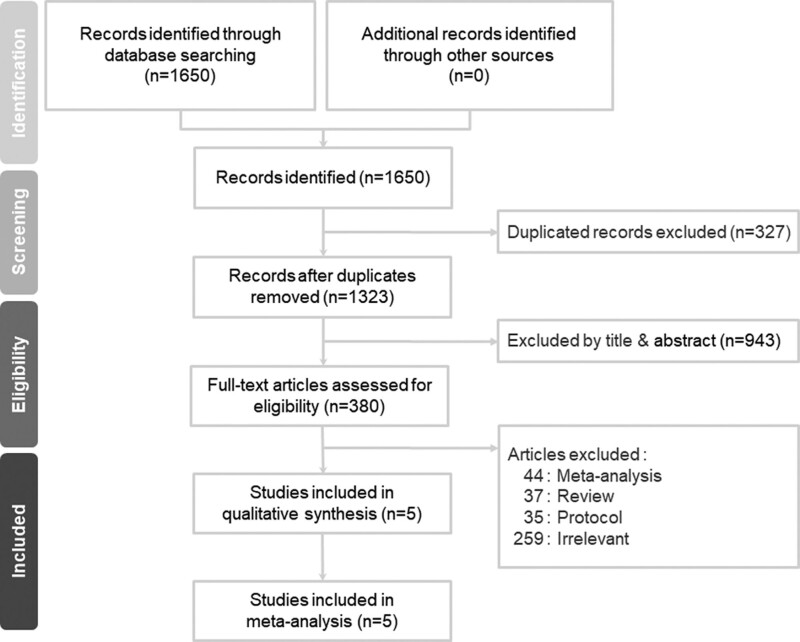
We retrieved 1323 non-duplicate citations and excluded 943 records based on their titles and abstracts. We included five studies after eliminating the remaining 375 articles that did not meet the inclusion criteria (Fig. 1). In total, 116 studies that did not include RCTs were excluded.[27] A total of 259 articles were excluded due to an irrelevant study design.[28]
Figure 1.
PRISMA flow diagram for the searching and identification of included studies.
Three of the included articles were published in journals with a science citation index,[18,19,21] while another article was a doctoral dissertation.[20] 2 trials were conducted in Iran, and 3 in China.[18–22] Table 1 summarizes the study characteristics and details of the methodology used in the five included studies.
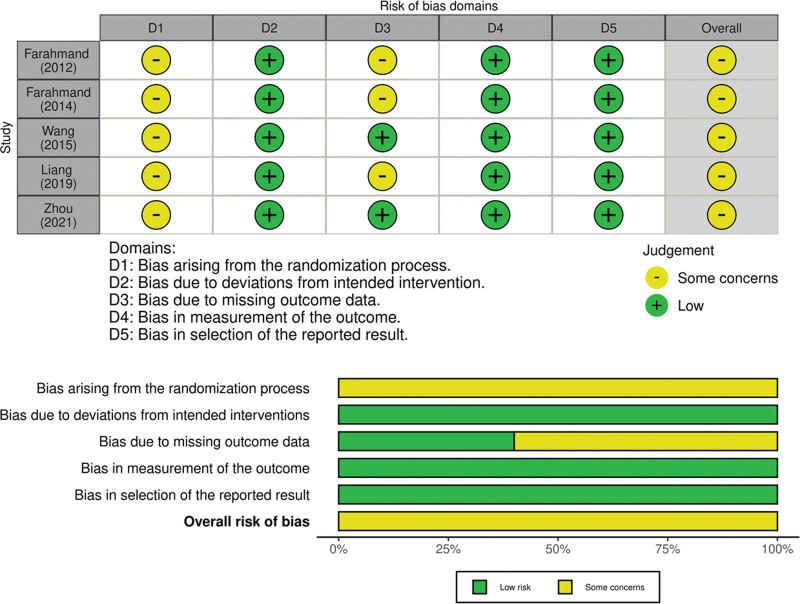
3.2. Risk of bias in the included studies
The risk of bias (ROB) assessment is shown in Figure 2. Five of the included RCTs were associated with some concerns. The main reason was the lack of reporting allocation sequence concealment during the randomization process. 3 RCTs assessed some concerns regarding bias due to missing outcome data domains. These 3 RCTs had several participants lost to follow-up and failed to complete the intention-to-treat analysis.
Figure 2.
Cochran Risk of Bias 2.0 assessment for included studies.
3.3. Outcome of interventions
3.3.1. Anthropometrics.
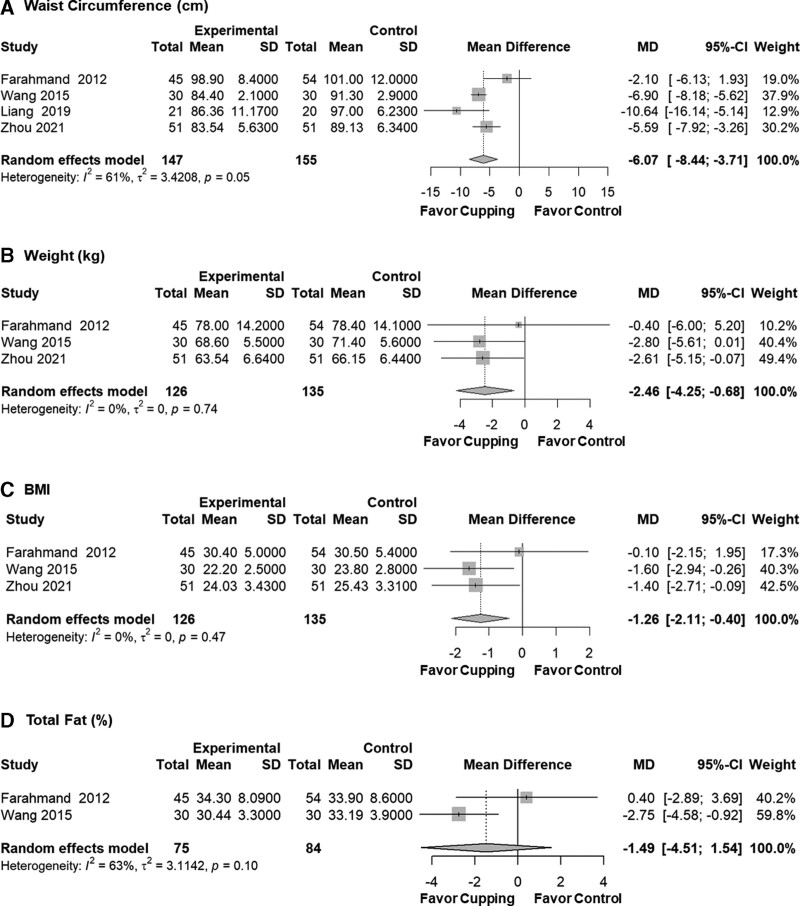
Our primary outcome was the MD and 95% CI of waist circumference after the cupping intervention. Compared to the control group, the cupping therapy group displayed a significantly lower waist circumference after treatment (MD = −6.07, 95% CI: −8.44 to −3.71, P < .001, I2 = 61%, τ2 = 3.4). Four studies addressing the meta-analysis of primary outcomes were all with some concerns risk of bias. Secondary outcomes such as body weight (kg) (MD = −2.46, 95% CI: −4.25 to −0.68, P = .007, I2 = 0%, τ2 = 0) and Body Mass Index (MD = −1.26, 95% CI: −2.11 to −0.40, P = .004, I2 = 0%, τ2 = 0) also showed significant results between cupping therapy group and control group. After meta-analysis, total fat percentage showed non-significant results (MD = −1.49, 95% CI: −4.51 to 1.54, P = .336, I2 = 63%, τ2 = 3.1), but tended to favor cupping (Fig. 3).
Figure 3.
Anthropometric results after meta-analysis by mean difference. BMI = body mass index, CI = confidence interval, MD = mean difference.
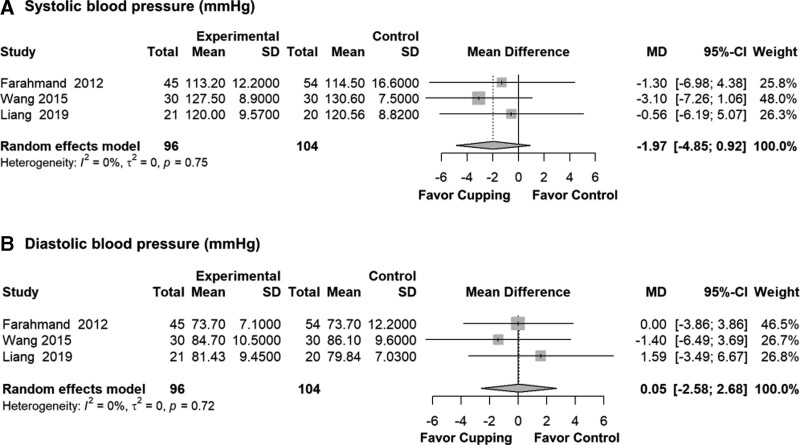
3.3.2. Blood pressure.
Meta-analyses revealed no significant evidence of the effects of cupping compared with the control group on systolic blood pressure (SBP) (MD = −1.97, 95% CI: −4.85 to 0.92, P = .181, I2 = 0%, τ2 = 0) and diastolic blood pressure (DBP) (MD = 0.05, 95% CI: −2.58 to 2.68, P = .969, I2 = 0%, τ2 = 0) (Fig. 4).
Figure 4.
Blood pressure results after meta-analysis by mean difference. CI = confidence interval, MD = mean difference.
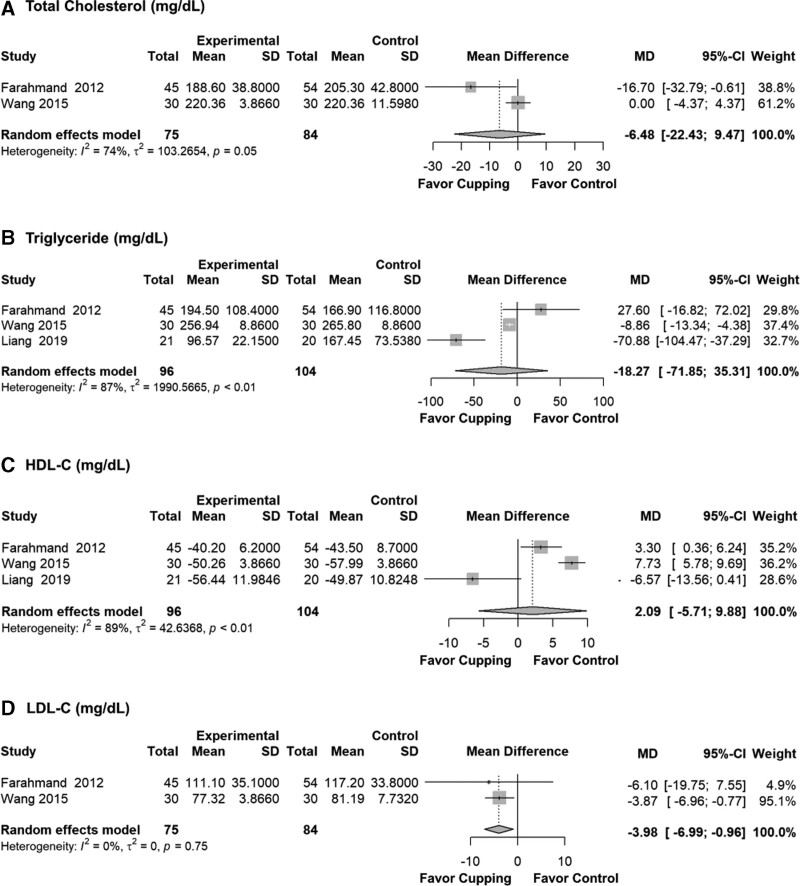
3.3.3. Lipid profile.
For LDL-C, cupping therapy had a significant effect on lowering the LDL-C blood concentration (MD = −3.98, 95% CI: −6.99 to −0.96, P = .010, I2 = 0%, τ2 = 0). It showed non-significant results about the effect of cupping on TC, TG, and HDL-C (Fig. 5).
Figure 5.
Lipid profile results after meta-analysis by mean difference. CI = confidence interval, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, MD = mean difference.
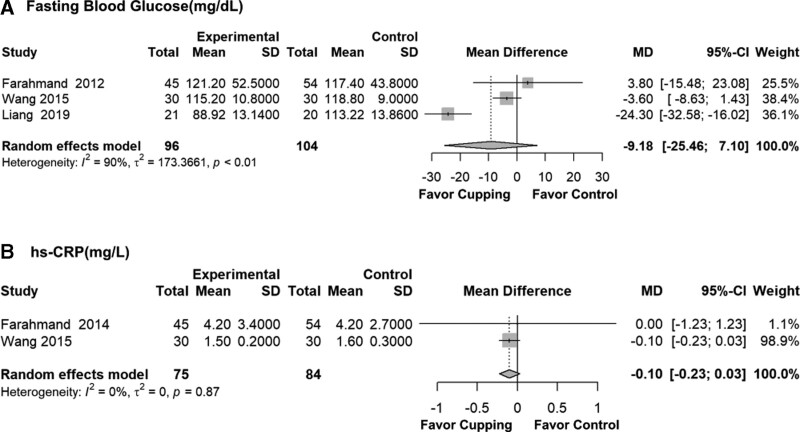
3.3.4. Blood sugar.
The MD and 95% CI of fasting blood glucose (FBG) after the meta-analysis showed non-significance with high heterogeneity (MD = −9.18, 95% CI: −25.46 to 7.10, P = .269, I2 = 90%, τ2 = 173.4) (Fig. 6).
Figure 6.
Biochemical marker results after meta-analysis by mean difference. CI = confidence interval, hs-CRP = high-sensitivity C-reactive protein, MD = mean difference.
3.3.5. hs-CRP.
There was a non-significant result, but with a tendency to favor the cupping group for hs-CRP (MD = −0.10, 95% CI: −0.23 to 0.03, P = .131, I2 = 0%, τ2 = 0) (Fig. 6).
3.4. Adverse events
Three of the 5 RCTs reported no AEs during the research period,[18–20] while Liang et al and Zhou did not mention safety-related data during trials.[21,22] Wang[20] reported that there were no abnormalities in the blood test, urine test, stool test, or function of the heart, liver, and kidney. No local skin injuries, infections, scalding, or allergies were observed.[20]
3.5. Follow-up
Wang[20] reported that the Traditional Chinese medicine symptom score of MetS still made a significant difference after 4 weeks of follow-up (cupping: control = 16.4 ± 2.1: 26.1 ± 3.4, P < .05). This indicates that cupping therapy has long-term effects on MetS.
3.6. Cupping parameters
The details of the cupping procedures, including the treatment period, total number of treatments, frequency of treatment, cupping retention time, number of cupping points, and cupping point selection, are summarized in Table 2. The most common selected point was the Zhongwan (Ren-12) in the conception vessel and the trapezius muscle between the 2 scapulae.
Table 2.
Details of the cupping method.
| 1st author year | Treatment period | Frequency of treatment | Total number of treatments | Cupping retention times | Number of cupping points | Cupping points selection |
|---|---|---|---|---|---|---|
| Farahmand (2012)[18] | 12 wk | Once per 6 wk | 2 | NM | Several | Between 2 scapulae (T1–T3) |
| Farahmand (2014)[19] | 12 wk | Once per 6 wk | 2 | NM | Several | Trapezius muscle near scapular spine |
| Wang (2015)[20] | 60 d | Everyday | 60 | 30 min | 2 | Zhongwan (Ren-12), Shuifen (Ren-9) |
| Liang (2019)[21] | 8 wk | Twice per week | 16 | 5 min | 6–8 | Zhongwan (Ren-12), Qihai (Ren-6) Tianshu (St-25, B), Daimai(GB-26, B), Liangmen (St-21, B), Shuidao (St-28, B) |
| Zhou (2021)[22] | 30 d | Once every 2 d | 15 | 5–10 min | Several | Abdomen around the umbilicus |
B = bilateral, NM = not mentioned.
3.7. Heterogeneity analysis
Substantial heterogeneity was found after meta-analysis of the waist circumference of the cupping therapy group compared with that of the control group (I2 = 61%, τ2 = 3.4). Heterogeneity was still severe in the results for total fat percentage and TC, TG, HDL-C, and FBG levels. Low to moderate heterogeneity was observed in the results of weight, BMI, SBP, DBP, LDL-C, and hs-CRP levels. After meta-regression, heterogeneity came from the variable “Location” (see Graph S2, Supplemental Digital Content, http://links.lww.com/MD/I691, which illustrates the result of meta-regression of the mean difference in waist circumference). Thus, subgroup analysis by “Location” was performed, and the residual heterogeneity of I2 decreased to 0.0% (see Graph S3, Supplemental Digital Content, http://links.lww.com/MD/I692, which illustrates subgroup analysis by location of waist circumference in the meta-analysis).
3.8. Sensitivity analysis
We performed sensitivity analyses for each of the parameters by omitting any of the included studies. For example, regarding the outcome of waist circumference, the meta-analysis results remained significant (MD = −6.33, 95% CI: −10.59 to −2.07, P < .01, I2 = 72%, τ2 = 10.6) after omitting the study by Zhou.[22] We confirmed that the results obtained from these sensitivity analyses were consistent with those without omitting studies (see Graph S4, Supplemental Digital Content, http://links.lww.com/MD/I693, which illustrates the sensitivity analyses for each of the parameters by omitting any of the included studies), which showed the robustness of our assessment.
3.9. GRADE certainty of evidence
The level of evidence, as assessed by GRADEpro,[25] is shown in Table 3. The quality of evidence was moderate for the waist circumference assessment because of the inconsistency caused by considerable heterogeneity (I2 = 61%, τ2 = 3.4). For weight, BMI, LDL-C, the quality of evidence was high because of the low heterogeneity without study limitations, inconsistency, indirectness, imprecision, or publication bias.
Table 3.
GRADE certainty of evidence of outcomes.
| Outcomes | Number of participants (studies) | Illustrative comparative risks* (95% CI) | Absolute effect (95% CI) | Certainty of evidence (GRADE) | |
|---|---|---|---|---|---|
| Control | Cupping | ||||
| Waist circumference (cm) | 302 (4 studies) | The mean WC loss in the control groups ranged from 3.9 lost to 0.33 lost | The mean WC loss in the intervention groups was 6.07 lower than control group | MD 6.07 lower (8.44–3.71 lower) | ⊕⊕⊕⊝ moderate |
| Weight (kg) | 261 (3 studies) | The mean Weight loss in the control groups ranged from 2.9 lost to 7.9 lost | The mean Weight loss in the intervention groups was 2.46 lower than control group | MD 2.46 lower (4.25–0.68 lower) | ⊕⊕⊕⊕ high |
| BMI | 261 (3 studies) | The mean BMI loss in the control groups ranged from 1.3 lost to 2.5 lost | The mean BMI loss in the intervention groups was 1.26 lower than control group | MD 1.26 lower (2.11–0.40 lower) | ⊕⊕⊕⊕ high |
| Total fat (%) | 159 (2 studies) | The mean total Fat loss in the control groups ranged from 0.2 lost to 1.29 lost | The mean total Fat loss in the intervention groups was 1.49 lower than control group | MD 1.49 lower (4.51 lower–1.54 higher) | ⊕⊕⊝⊝ low |
| SBP (mm Hg) | 200 (3 studies) | The mean SBP loss in the control groups ranged from 9.7 lost to 2.78 gain | The mean SBP loss in the intervention groups was 1.97 lower than control group | MD 1.97 lower (4.85 lower–0.92 higher) | ⊕⊕⊕⊝ moderate |
| DBP (mm Hg) | 200 (3 studies) | The mean DBP loss in the control groups ranged from 6 lost to 0.06 gain | The mean DBP loss in the intervention groups was 0.05 higher than control group | MD 0.05 higher (2.58 lower–2.68 higher) | ⊕⊕⊕⊝ moderate |
| TC (mg/dL) | 159 (2 studies) | The mean TC loss in the control groups ranged from 19.3 lost to 3.1 gain | The mean TC loss in the intervention groups was 6.48 lower than control group | MD 6.48 lower (22.43 lower–9.47 higher) | ⊕⊕⊝⊝ low |
| TG (mg/dL) | 200 (3 studies) | The mean TG loss in the control groups ranged from 71.9 lost to 18.6 lost | The mean TG loss in the intervention groups was 18.27 lower than control group | MD 18.27 lower (71.85 lower to 35.31 higher) |
⊕⊝⊝⊝ very low |
| HDL-C (mg/dL) | 200 (3 studies) | The mean HDL-C gain in the control groups ranged from 5.0 lost to 15.5 gain | The mean HDL-C gain in the intervention groups was 2.09 higher than control group | MD 2.09 higher (5.71 lower–9.88 higher) | ⊕⊝⊝⊝ very low |
| LDL-C (mg/dL) | 159 (2 studies) | The mean LDL-C loss in the control groups ranged from 15.4 lost to 0.9 lost | The mean LDL-C loss in the intervention groups was 3.98 lower than control group | MD 3.98 lower (6.99 lower–0.96 lower) | ⊕⊕⊕⊕ high |
| FBG (mg/dL) | 200 (3 studies) | The mean FBG loss in the control groups ranged from 21.6 lost to 8.3 gain | The mean FBG loss in the intervention groups was 9.18 lower than control group | MD 9.18 lower (25.46 lower–7.10 higher) | ⊕⊝⊝⊝ very low |
| hs-CRP (mg/L) | 159 (2 studies) | The mean hs-CRP loss in the control groups ranged from 0.6 lost to 0.2 lost | The mean hs-CRP loss in the intervention groups was 0.10 lower than control group | MD 0.10 lower (0.23 lower–0.03 higher) | ⊕⊕⊕⊝ moderate |
Population: patients with metabolic syndrome/Intervention: cupping therapy/Comparison: control group.
BMI = body mass index, DBP = diastolic blood pressure, FBG = fasting blood glucose, HDL-C = high density lipoprotein cholesterol, hs-CRP = high sensitivity C-Reactive Protein, LDL-C = low density lipoprotein cholesterol, MD = mean difference, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride, WC = waist circumference.
4. Discussion
4.1. Summary of evidence
To the best of our knowledge, this is the first systematic review and meta-analysis to evaluate the efficacy of cupping therapy for MetS. Five RCTs, involving 489 participants, were included in this study. The pooled metabolic parameter data showed that cupping had a significant effect on waist circumference, body weight, and BMI, but not significant on total fat percentage and blood pressure. As for biochemical markers, cupping significantly lowered the concentration of LDL-C, but had no effect on total cholesterol, triglyceride, HDL-C, fasting blood glucose, and hs-CRP. In addition, cupping therapy had a long-term effect on follow-up, without AEs. In evidence-based medicine, there is very little evidence of cupping therapy for metabolic indices. In previous study, wet cupping was shown to improve fatty liver severity and homeostatic model assessment for insulin resistance in patients with nonalcoholic fatty liver disease.[29] By reducing insulin resistance, wet cupping can potentially relieve MetS. A few small and poor methodological studies have suggested that wet cupping alone significantly reduces blood pressure compared with antihypertensive medication.[30] In a randomized controlled clinical trial, the cupping and acupoint catgut embedding group had significant differences compared with the acupoint catgut embedding group alone in the weight, waist circumference, hip circumference, leptin and leptin-adiponectin ratio of simple obesity patients.[31] Based on the results of our meta-analysis, cupping therapy can be considered a safe and effective complementary intervention for reducing waist circumference, body weight, BMI, and LDL-C levels in patients with MetS.
4.2. Pathophysiology and mechanism
Although the mechanism by which cupping influences MetS remains unclear, there are 3 deductions based on evidence-based medicine. First, a previous randomized clinical trial suggested that wet cupping might be an effective method for reducing LDL-C levels in healthy young men.[32] Farahmand et al and Wang revealed that cupping tended to lower LDL-C levels in patients with MetS,[18,20] but these results were not statistically significant. After our meta-analysis, cupping significantly reduced the LDL-C concentration in Mets. By lowering blood LDL-C levels, cupping may have a protective effect against atherosclerosis and the progression of MetS. Second, an increasing number of studies have demonstrated that obesity and MetS are related to chronic inflammation and oxidative status.[33] In a mice study, the anti-inflammatory lipids such as Prostaglandin E1 (PGE1) and 5,6 epoxyeicosatrienoic acid (5,6-EET) were significantly increased while pro-inflammatory lipids such as hydroxyeicosatetraenoic acid (12-HETE) and Thromboxane B2 (TXB2) were deceased after cupping treatment. A significant reduction in the erythrocyte sedimentation rate and hs-CRP level was observed in the wet cupping group compared to control group of Ahmed et al[17] study in patients with rheumatoid arthritis. By reducing inflammation, wet cupping may have the potential to treat chronic inflammatory diseases such as MetS. Third, adipokines play crucial roles in the regulation of metabolism. In humans, “chemerin” is a newly discovered adipokine that is involved in inflammation, adipogenesis, angiogenesis, and energy metabolism.[34] Wang[20] showed that fire cupping and needle cupping can significantly reduce the level of chemerin in patients with MetS compared with the control group. Cupping might regulate body metabolism by lowering chemerin concentration.[20]
Our systematic review and meta-analysis showed cupping therapy significantly decrease waist circumference, body weight, body mass index, and LDL-C level. The extract mechanisms underlying the beneficial effects of cupping therapy remain unclear. However, previous investigators have postulated theories to explain the clinical benefits of cupping therapy in this study. These theories include the “activation of immune system,” which lead to immunological effects and hormonal adjustments and “blood detoxification,” which results in releasing of toxins and removal of wastes.[35] The investigators also suggested the notion that these theories may overlap or work interchangeably to produce various therapeutic effects in specific diseases.[35]
Previous findings regarding the therapeutic effects of cupping therapy are mostly positive in population with metabolic syndrome, but are controversial in healthy population. A randomized controlled trial in obese patient reported cupping could reduce waist circumference, BMI, and the level of TC and TG.[14] However, the improvements of other anthropometric or metabolic parameters were not observed. In a randomized controlled trial of healthy young men population, it was found that a substantial decrease in LDL-C and in the LDL-C/HDL-C ratio in the wet cupping group compared to the control, whereas no statistically significant effects were noted in TG, TC, and HDL-C.[32] Thus, the differences in the population studied, sampling size, follow-up time, treatment time and heterogeneity of the cupping method may all contribute to the differences in the therapeutic effects of cupping therapy.
4.3. Heterogeneity
Substantial heterogeneity was found among the included RCTs in the meta-analysis result of waist circumference. After meta-regression, heterogeneity came from the variable “Location.” Subgroup analysis by variable “Location” was performed, and the residual heterogeneity decreased to 0.0%. Low to moderate heterogeneity was observed in the results of weight, BMI, SBP, DBP, LDL-C, and hs-CRP levels. However, heterogeneity was still severe in the results for total fat percentage and TC, TG, HDL-C, and FBG levels. Heterogeneity between studies may have arisen from the clinical perspective or study design, such as differences in cupping methods, cupping point selection, treatment frequency, or treatment course.
4.4. Implications for clinical practice and future research
The results of this meta-analysis can be applied only to patients with MetS. Based on these results, no conclusive recommendations can be made regarding cupping therapy for the parameters of MetS. Despite some ROB and low to substantial heterogeneity of the included studies, cupping therapy can be considered a safe and effective complementary intervention for reducing waist circumference, body weight, BMI, and LDL-C in individuals with MetS. Cupping might be a complementary and alternative treatment for patients with AEs associated with weight loss drugs. By previous studies, acupuncture has been proven beneficial in the treatment of MetS and could serve as an alternative therapy for MetS-associated conditions.[36] However, fear of acupuncture needles limits the utilization of acupuncture. Thus, an increasing number of Traditional Chinese medicine doctors are currently treating MetS by cupping. In the future, well-designed, high-quality, rigorous methodology, and long-term RCTs are required to assess the efficacy and safety of cupping therapy in patients with MetS. Future RCTs should ensure adequate randomization, allocation sequence concealment, intention-to-treat analysis, and blinding of the outcome assessors.
4.5. Limitations
This systematic review and meta-analysis had 3 main limitations. First, only 5 RCTs met the inclusion criteria were included. More RCTs evaluating the efficacy of cupping for MetS are required in the future. Second, the original heterogeneity of the MD on waist circumference after meta-analysis was high (I2 = 61%). After subgroup analysis by variable “Location,” the residual heterogeneity by I-square decreased to 0.0%. Third, there are various cupping methods, including dry cupping, wet cupping, bleeding cupping, fire cupping, flash cupping, herbal cupping, moving cupping, retained cupping, needling cupping, Hijama, and Al-Hijamah. Therefore, the efficacy and function of cupping should be discussed in more detail.
5. Conclusions
After meta-analysis, the pooled data of metabolic indices showed that cupping had a significant effect on waist circumference, body weight, BMI, and LDL-C, with moderate to high certainty of evidence. Cupping had no significant effect on total fat percentage, SBP, DBP, total cholesterol, triglyceride, HDL-C, fasting blood glucose, and hs-CRP in Mets patients. Despite some ROB and low to substantial heterogeneity of the included studies, cupping therapy can be considered a safe and effective complementary intervention for reducing waist circumference, body weight, BMI, and LDL-C in individuals with MetS. In the future, well-designed, high-quality, rigorous methodology, and long-term RCTs in this population are required to assess the efficacy and safety of cupping therapy.
Acknowledgments
The authors are grateful to Yu Ru Kou for his valuable suggestions provided during the revision of this manuscript.
Author contributions
Conceptualization: Li-Kung Wu, Yi-Chen Chen, Chih-Yu Yen, Chen-Ying Chang Chien, Jian-Ruei Ciou, Hsiao-Hsiang Torng, Chun-Yu Lai, Tsung-Jung Ho.
Data curation: Li-Kung Wu, Yi-Chen Chen, Chung-Shan Hung.
Formal analysis: Li-Kung Wu, Yi-Chen Chen, Chung-Shan Hung, Chen-Ying Chang Chien.
Methodology: Li-Kung Wu, Yi-Chen Chen, Jian-Ruei Ciou, Hsiao-Hsiang Torng, Yuan-Yuan Liu, Yen-Lun Kung, Huei-Kai Huang, Tsung-Jung Ho.
Resources: Li-Kung Wu, Chih-Yu Yen, Jian-Ruei Ciou, Shiuan Hua, Peng-Nien Lu.
Software: Li-Kung Wu, Yi-Chen Chen, Tsung-Jung Ho.
Supervision: Li-Kung Wu, Chung-Shan Hung, Yen-Lun Kung, Huei-Kai Huang, Zhong-Kui Chen, Tsung-Jung Ho.
Validation: Li-Kung Wu, Huei-Kai Huang, Zhong-Kui Chen.
Visualization: Li-Kung Wu, Chung-Shan Hung, Yi-Chin Chang, Shiuan Hua, Peng-Nien Lu, Yuan-Yuan Liu, Chun-Yu Lai, Tsung-Jung Ho.
Writing – original draft: Li-Kung Wu, Yi-Chen Chen, Chen-Ying Chang Chien, Yi-Chin Chang, Chun-Yu Lai.
Writing – review & editing: Li-Kung Wu, Yi-Chen Chen, Tsung-Jung Ho.
Supplementary Material
Abbreviations:
- AEs
- adverse events
- BMI
- body mass index
- CI
- confidence interval
- DBP
- diastolic blood pressure
- FBG
- fasting blood glucose
- HDL-C
- high-density lipoprotein cholesterol
- hs-CRP
- high-sensitivity C-reactive protein
- LDL-C
- low-density lipoprotein cholesterol
- MD
- mean difference
- MetS
- metabolic syndrome
- RCTs
- randomized controlled trials
- ROB
- risk of bias
- SBP
- systolic blood pressure
- TC
- total cholesterol
- TG
- triglycerides
- WHO
- World Health Organization
L-KW and Y-CC contributed equally to this work.
Research was fully funded by Buddhist Tzu Chi Foundation Tzu Chi Research Department with funding Number TCRD110-16.
No ethical approval was required because data from previously published articles in which informed consent was obtained by the primary investigators were retrieved and analyzed.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Wu L-K, Chen Y-C, Hung C-S, Yen C-Y, Chang Chien C-Y, Ciou J-R, Torng H-H, Chang Y-C, Hua S, Lu P-N, Liu Y-Y, Lai C-Y, Kung Y-L, Huang H-K, Chen Z-K, Ho T-J. The efficacy and safety of cupping as complementary and alternative therapy for metabolic syndrome: A systematic review and meta-analysis. Medicine 2023;102:13(e33341).
Contributor Information
Li-Kung Wu, Email: windcolor0606@gmail.com.
Yi-Chen Chen, Email: samtati@gmail.com.
Chung-Shan Hung, Email: melody1088105@gmail.com.
Chih-Yu Yen, Email: 104318127@gms.tcu.edu.tw.
Chen-Ying Chang Chien, Email: cccy0727@gmail.com.
Jian-Ruei Ciou, Email: jraeuy@gmail.com.
Hsiao-Hsiang Torng, Email: myps4815@gmail.com.
Yi-Chin Chang, Email: kosamo84@gmail.com.
Shiuan Hua, Email: a0988214883@gmail.com.
Peng-Nien Lu, Email: peterboy638@gmail.com.
Yuan-Yuan Liu, Email: ttisban2@gmail.com.
Chun-Yu Lai, Email: nick9016tv@gmail.com.
Yen-Lun Kung, Email: kung818@gmail.com.
Huei-Kai Huang, Email: drhkhuang@gmail.com.
Zhong-Kui Chen, Email: samtati@gmail.com.
References
- [1].Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36:14–20. [DOI] [PubMed] [Google Scholar]
- [3].Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323:2526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li R, Li W, Lun Z, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1–23. [DOI] [PubMed] [Google Scholar]
- [7].Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. [DOI] [PubMed] [Google Scholar]
- [8].Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–56. [DOI] [PubMed] [Google Scholar]
- [9].Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461–70. [DOI] [PubMed] [Google Scholar]
- [10].Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet. 2016;387:1947–56. [DOI] [PubMed] [Google Scholar]
- [11].Cao H, Li X, Liu J. An updated review of the efficacy of cupping therapy. PLoS One. 2012;7:e31793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Choi TY, Ang L, Ku B, et al. Evidence map of cupping therapy. J Clin Med. 2021;10:1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shi SM, Zuo BW. Observation on curative effect of cupping combined with diet control in treating simple obesity. J Hebei Med Univ. 2009. [Google Scholar]
- [14].Jiang DW, Chai K, Zhou JY. Efficacy evaluation of cupping treating overweight/obese patients with the phlegm-dampness constitution and effect on serum leptin. Zhejiang J Tradit Chin Med. 2020. [Google Scholar]
- [15].Lee B, Kwon CY. Comparative effectiveness of East Asian traditional medicine for childhood simple obesity: a systematic review and network meta-analysis. Int J Environ Res Public Health. 2022;19:12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen LZ, Wang YM, Liu T, et al. Cupping intervention on simple obesity based on inflammation correlation. Acta Chin Med. 2021. [Google Scholar]
- [17].Ahmed SM, Madbouly NH, Maklad SS, et al. Immunomodulatory effects of blood letting cupping therapy in patients with rheumatoid arthritis. Egypt J Immunol. 2005;12:39–51. [PubMed] [Google Scholar]
- [18].Farahmand SK, Gang LZ, Saghebi SA, et al. The effects of wet cupping on coronary risk factors in patients with metabolic syndrome: a randomized controlled trial. Am J Chin Med. 2012;40:269–77. [DOI] [PubMed] [Google Scholar]
- [19].Farahmand SK, Gang LZ, Saghebi SA, et al. The effects of wet cupping on serum high-sensitivity C-reactive protein and heat shock protein 27 antibody titers in patients with metabolic syndrome. Complement Ther Med. 2014;22:640–4. [DOI] [PubMed] [Google Scholar]
- [20].Wang L. The Effect of Evaluation Research on Treatment of Metabolic Syndrome through the Main and Collateral Channels Modulating Mechanism doctoral degree. Changchun University of Chinese Medicine; 2015. Available at: https://d-wanfangdata-com-cn.hlsw.tzuchi.com.tw:8443/thesis/ChJUaGVzaXNOZXdTMjAyMTA1MTkSCUQwMTYyODcxORoIaTRnYThhM2I%3D. [Google Scholar]
- [21].Liang C-M, Hu H, Wang X-M, et al. A clinical study on medical cupping for metabolic syndrome with abdominal obesity. Tradit Med Res. 2019;4:4–11. [Google Scholar]
- [22].Zhou M. 51 cases of abdominal obesity with metabolic syndrome treated by abdominal vacuum moving cupping combined with acupuncture. Tradit Chin Med Res. 2021;34:39–41. [Google Scholar]
- [23].Higgins JPT TJ, Chandler J, Cumpston M, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- [24].McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. [DOI] [PubMed] [Google Scholar]
- [25].Prime MUaE. GRADEpro GDT. GRADEpro Guideline Development Tool [Software]: gradepro.org.; 2022.
- [26].Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- [27].Al Bedah AMN, Khalil MKM, Sohaibani I, et al. Evaluation of wet cupping therapy: systematic review of randomized clinical trials. J Altern Complement Med. 2016;22:768–77. [DOI] [PubMed] [Google Scholar]
- [28].Irct2016080228664N. The effect of wet cupping on regulation of vaginal bleeding in Pcos. 2016. Available at: http://wwwwhoint/trialsearch/Trial2aspx?TrialID=IRCT2016080228664N2.
- [29].Bashiri H, Bozorgomid A, Shojaeimotlagh V. Efficacy of Hijamat (wet cupping therapy) in Iranian patients with nonalcoholic fatty liver disease: a controlled clinical trial. Turk J Med Sci. 2020;50:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lu S, Du S, Fish A, et al. Wet cupping for hypertension: a systematic review and meta-analysis. Clin Exp Hypertens. 2019;41:474–80. [DOI] [PubMed] [Google Scholar]
- [31].Yang QL, Li X. Influence of catgut embedding at shumu point combined with acupoint cupping on leptin-adiponectin ratio in simple obesity patients. Yunnan J Tradit Chin Med Mater Med. 2022;43:54–8. [Google Scholar]
- [32].Niasari M, Kosari F, Ahmadi A. The effect of wet cupping on serum lipid concentrations of clinically healthy young men: a randomized controlled trial. J Altern Complement Med. 2007;13:79–82. [DOI] [PubMed] [Google Scholar]
- [33].Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Helfer G, Wu QF. Chemerin: a multifaceted adipokine involved in metabolic disorders. J Endocrinol. 2018;238:R79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Al-Bedah AMN, Elsubai IS, Qureshi NA, et al. The medical perspective of cupping therapy: effects and mechanisms of action. J Tradit Complement Med. 2019;9:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li X, Jia HX, Yin DQ, et al. Acupuncture for metabolic syndrome: systematic review and meta-analysis. Acupunct Med. 2021;39:253–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.