Abstract
The rising prevalence of obesity in Singapore is a harbinger for a corresponding increase in obesity-related complications such as type 2 diabetes mellitus (T2DM) and coronary heart disease. Obesity is a complex disease driven by multiple factors, and hence, treatment cannot follow a ’one-size-fits-all’ approach. Lifestyle modifications involving dietary interventions, physical activity and behavioural changes remain the cornerstone of obesity management. However, similar to other chronic diseases such as T2DM and hypertension, lifestyle modifications are often insufficient on their own, hence the importance of other treatment modalities including pharmacotherapy, endoscopic bariatric therapy and metabolic–bariatric surgery. Weight loss medications currently approved in Singapore include phentermine, orlistat, liraglutide and naltrexone–bupropion. In recent years, endoscopic bariatric therapies have evolved as an effective, minimally invasive and durable therapeutic option for obesity. Metabolic–bariatric surgery remains the most effective and durable treatment for patients with severe obesity, with an average weight loss of 25%–30% after one year.
Keywords: Endoscopic bariatric therapy, lifestyle modification, metabolic–bariatric surgery, treatment of obesity, weight loss pharmacotherapy
INTRODUCTION
Obesity is defined as a state of abnormal or excessive fat accumulation that may impair health. It is a burgeoning problem not just worldwide, but also locally. Based on the National Population Health Survey 2020, the prevalence of obesity in 2019–2020, denoted by a body mass index (BMI) of 30 kg/m2, had increased from 8.6% in 2013 to 10.5% in 2017.[1] As the risk for cardiovascular diseases and type 2 diabetes mellitus (T2DM) starts at a lower BMI for Asians, BMI ≥27.5 kg/m2 denotes a high-risk category for public health action in our local population. Also, 20.7% of our residents were in this high-risk category in 2019–2020,[1] which is an alarming statistic. In fact, it is likely that the prevalence of obesity will continue to increase due to the impact of the coronavirus disease 2019 (COVID-19) pandemic measures imposed to reduce viral transmission, culminating in an increasingly sedentary lifestyle.[2]
The greatest concern with these statistics is the impact on obesity-related complications including T2DM, coronary heart disease, non-alcoholic fatty liver disease and obstructive sleep apnoea.[3] Thus, it is not surprising that the prevalence of T2DM, hypertension and hyperlipidaemia in Singapore has increased from 2017 to 2020, in parallel with the increase in the prevalence of obesity.[1] Obesity also leads to increased mortality and reduced life expectancy.[4] This highlights the urgent need for us to take action to identify and treat obesity. While managing the various physical ailments, we should not forget the adverse effects of obesity on mental health — obesity is associated with an increased prevalence of depression, anxiety and eating disorders.[5] It is important to identify and address these mental health issues for the long-term treatment of obesity to be successful.
The treatment landscape of obesity has evolved over the last few years. The current treatment options for obesity (in order of increasing magnitude of weight loss) include lifestyle modification, anti-obesity pharmacotherapy, endoscopic bariatric therapies (EBTs) and metabolic–bariatric surgery (MBS). In this review, we provide updates on recent evidence in these modalities, with a focus on local data and experience, where available.
LIFESTYLE MODIFICATION
Lifestyle modifications remain the cornerstone of successful weight management. The importance of this can be reinforced through the obesity treatment pyramid, in which lifestyle modifications are at the base, without which the rest of the pyramid (other treatment modalities) would figuratively crumble down. Lifestyle modifications include three main components: dietary interventions, physical activity and behavioural changes.
Dietary interventions
The essential component of all dietary interventions for weight loss is reduction in caloric intake. A diet that contributes to a daily caloric deficit of at least 500 kcal below the estimated daily energy requirements should be recommended.[6,7] Many different diets that claim specific benefits with weight loss have emerged, such as the ketogenic diet, Atkins diet, Mediterranean diet and paleo diet. In general, these diets lead to a significant alteration in the dietary macronutrient composition. For example, the ketogenic diet provides the majority (65%–80%) of calories from fat, 20%–25% of calories from proteins and just 5%–10% of calories from carbohydrates.[8] The major concern is the risk of nutritional imbalance due to omission or excess of certain food groups; for instance, ketogenic diets have been reported to lead to severe hyperlipidaemia.[8] These diets are also highly challenging to maintain for a long term, particularly in our local context in which food choices tend to be predominantly carbohydrate based. Studies have shown that overall caloric restriction, rather than any specific dietary macronutrient composition, is the key factor in determining weight loss.[9] Intermittent fasting is a method of time-restricted energy restriction. However, compared to conventional energy restriction, the weight loss is similar while adherence may be poorer.[10,11] In short, it is important that doctors advise patients to focus on achieving caloric restriction, rather than any specific diet or method.
Meal replacements (MRs) are another useful weight loss modality. They come in various forms including shakes and bars. MRs are hypocaloric (200–250 kcal per serving), yet nutritionally complete. They can be used to replace one or two main meals a day and are particularly convenient when healthy food options are not readily available. MRs can also be used as part of a very-low-calorie diet (VLCD) of <800 kcal/day to replace all meals. The DiRECT study underscored the feasibility of using VLCD in the primary care setting.[12] In this trial, patients with T2DM and obesity took a low-energy formula diet (825–853 kcal/day) for 3 months, followed by structured food reintroduction for 2–8 weeks and then a weight maintenance phase.[12] After 1 year, their mean body weight fell by 10.0 kg. More importantly, 46% of subjects achieved remission of T2DM, defined as glycated haemoglobin (HbA1c) <6.5% after ≥2 months off all anti-diabetic medications.[12] At the end of 2 years, although there was some weight regain with a total weight loss of 7.6 kg, 36% of subjects continued to have remission of T2DM.[13]
Physical activity
Physical activity is an essential component of weight loss and has numerous other weight-independent health benefits, including reducing the risk of cardiovascular disease. We recommend at least 150–300 min of moderate-intensity aerobic exercise or 75–150 min of vigorous aerobic exercise a week, together with strength training exercises for 2 days or more in a week.[6,7] Individuals who are inactive should first start off with light-intensity exercise before progressing to higher-intensity exercise.[7] Instead of a continuous single session, physical activity can also be accumulated in bouts of at least 10 min, which may be useful for our patients with busy schedules and may improve their compliance.[7] In addition, just 10 min of daily exercise has been shown to lead to decreased mortality.[14]
Another meta-analysis has shown that 30–40 min of daily exercise can mitigate the mortality risk associated with a sedentary lifestyle.[15] Physical activity is essential for long-term weight maintenance, as evidenced by the finding that participants who maintained weight loss 6 years after ’The Biggest Loser’ competition had increased physical activity compared to their counterparts who regained weight.[16] It should, however, be emphasised that physical activity in the absence of dietary interventions is unlikely to lead to significant weight loss, thus underscoring the importance of combined lifestyle modifications.[17]
Behavioural changes
The third component of lifestyle modifications, behavioural changes, is the key ingredient for weight loss to occur. Research has shown it takes 18–254 days to develop a new habit,[18] underscoring the importance of sustained effort to establish new behavioural patterns. Thus, a multidisciplinary team (MDT) comprising dietitians, physiotherapists, psychologists and nurses is pivotal to the success of any weight management programme. Relevant aspects include education, goal setting, self-monitoring (weight, food intake and exercise), stimulus control and stress reduction.[6] Of note, cognitive behavioural therapy (CBT) was effective in increasing adherence to recommended weight loss behaviour, and weight loss programmes that included CBT strategies were found to achieve greater weight loss.[19]
Studies have shown that patients who attended a higher percentage of treatment sessions and lost more weight early on during a programme had greater long-term weight loss.[6] This is relevant when designing the structure of a weight management programme; it may be beneficial to have sessions closer together during the early ’intensive’ phase. An excellent illustration is the Look AHEAD study.[20] In this trial, the main objective was to achieve and maintain weight loss of ≥7% through comprehensive lifestyle intervention. In the first year, individual or group meetings were arranged every 1–2 weeks, after which the frequency of contact was reduced to once per month.[20] After 8 years, subjects maintained a weight loss of 4.7%, which is more than half the weight loss achieved at the end of the first year (8.5%).[21]
PHARMACOTHERAPY
Pharmacotherapy is an important adjunct to lifestyle modification in the management of obesity, similar to other chronic diseases. Medications may be considered in patients with a BMI of ≥30 kg/m2, or a BMI of ≥27 kg/m2 with obesity-related complications.[22] Apart from orlistat, all other weight loss medications work by suppressing appetite and hunger. Pharmacotherapy should be used together with lifestyle intervention as they work synergistically with additive weight loss benefits. Weight loss medications currently approved for use in Singapore include phentermine, orlistat, liraglutide and naltrexone–bupropion combination [Table 1].
Table 1.
Pharmacotherapy used for obesity management in Singapore.
| Class of drug | Placebo-subtracted weight loss | Potential side effects | Cardiovascular effects | Glucose-lowering effect | Route | |
|---|---|---|---|---|---|---|
| Licenced for short-term use | ||||||
|
| ||||||
| Phentermine | Sympathomimetic agent | 3.6–4.5 kg | Dry mouth, insomnia, agitation, palpitation | Not suitable in CV disease | Neutral | Tablet (once daily) |
|
| ||||||
| Licenced for long-term use | ||||||
|
| ||||||
| Orlistat | Lipase inhibitor | 2.9%–3.4% | Oily stool, diarrhoea, faecal incontinence | Safe | Neutral | Tablet (up to three times a day) |
|
| ||||||
| Liraglutide | GLP-1 receptor agonist | 5.4% | Nausea, vomiting, diarrhoea, constipation | Safe, may be of benefit | Benefit | Daily SC injection |
|
| ||||||
| Naltrexone–bupropion | Opioid antagonist (naltrexone)/dopamine and noradrenaline reuptake inhibitor (bupropion) | 4.2% | Nausea, vomiting, constipation, headache | Safe | Neutral | Tablet (twice daily) |
|
| ||||||
| Semaglutidea | GLP-1 receptor agonist | 14.5% | Nausea, vomiting, diarrhoea, constipation | Safe, may be of benefit | Benefit | Weekly SC injection |
aSubcutaneous semaglutide is currently approved only for treatment of T2DM in Singapore. CV: cardiovascular, GLP-1: glucagon-like peptide 1, SC: subcutaneous, T2DM: type 2 diabetes mellitus
Phentermine
Phentermine is a sympathomimetic amine approved for short-term use for treatment of obesity. Due to its low cost and easy administration, it is the most widely prescribed anti-obesity drug in the USA.[23] It leads to placebo-subtracted weight loss of about 3.6–4.5 kg after 6 months.[24] The side effects of phentermine are due to its effects on the sympathetic nervous system and can include insomnia, palpitations and constipation.[25] These side effects are usually mild and can be mitigated by starting at a low dose of 15 mg daily and using the lowest effective dose, uptitrating only as needed.
Phentermine may also variably increase the heart rate and blood pressure, and hence is contraindicated in those with cardiovascular disease or uncontrolled hypertension.[25] Regular blood pressure monitoring should be performed for those on phentermine. Despite the lack of randomised controlled trial (RCT) data, a large cohort study showed that long-term phentermine use is associated with greater weight loss without increased cardiovascular risk.[26] Hence, long-term phentermine use may be considered in low-risk individuals if it is well tolerated and effective.
Orlistat
Orlistat is a lipase inhibitor which reduces the absorption of dietary fat by up to 30%. It is taken at a dose of 120 mg three times a day with meals. Mean placebo-subtracted weight loss of 2.9%–3.4% is observed after 1 year of treatment with orlistat.[27,28] Gastrointestinal side effects, such as steatorrhoea, diarrhoea, oily spotting and faecal incontinence, are common and limit the use of orlistat. Adopting a low-fat and high-fibre diet can reduce some of these side effects.
Liraglutide
Liraglutide is a glucagon-like peptide 1 receptor agonist (GLP-1 RA) approved in Singapore for chronic weight management in adults and adolescents ≥12 years old. It is administered via daily subcutaneous (SC) injections. A large RCT has demonstrated weight loss of 5.6 kg (5.4%) over placebo with 3.0 mg of liraglutide over 1 year.[29] Liraglutide is also favoured to treat T2DM as it does not cause hypoglycaemia or weight gain. The side effects are primarily gastrointestinal and include nausea, vomiting, diarrhoea and constipation; these are usually transient and can be mitigated with gradual dose escalation. Tolerability can be improved by slower dose escalation (e.g. dose increase every 2–3 weeks instead of weekly), as this has only minor impact on the weight loss trajectory.[30] Cardiovascular safety is well established, with demonstrated reduction in cardiovascular events in T2DM patients at high cardiovascular risk.[31]
Naltrexone–bupropion combination
A fixed-dose combination of naltrexone (an opioid antagonist) and bupropion (a dopamine and noradrenaline reuptake inhibitor) recently approved in Singapore has shown an average placebo-subtracted weight loss of 4.8% after 1 year.[32] Common side effects include nausea, headache, insomnia and dry mouth. These side effects can be mitigated with gradual dose escalation, with a maximum total daily dose of 32 mg naltrexone–360 mg bupropion. It is contraindicated in patients with uncontrolled hypertension, seizure disorders, use of monoamine oxidase inhibitors, eating disorders, and drug or alcohol withdrawal.
Next-generation GLP-1 RA
In recent years, newer GLP-1 RA compounds that allow for easier administration and show greater weight loss have been developed. One example is semaglutide, a GLP-1 RA currently available in Singapore in two forms for treatment of T2DM: a daily oral tablet and a weekly SC injection. SC semaglutide 2.4 mg weekly has also been approved in the USA to treat obesity, with demonstrated placebo-subtracted weight loss of 12.4%.[33] A head-to-head study comparing weekly semaglutide 2.4 mg with daily liraglutide 3.0 mg showed that weight loss with semaglutide (15.8%) was more than double that of liraglutide (6.4%).[34] The side effects are commonly gastrointestinal (nausea, diarrhoea, constipation) and typically mild and transient. Semaglutide has also been demonstrated to reduce cardiovascular events in T2DM patients.[35]
Tirzepatide is a dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) administered via weekly SC injection, recently approved for treatment of T2DM in the USA. Various Phase 3 RCTs have shown consistent robust improvements in glycaemic control and body weight without increased risk of hypoglycaemia.[36,37,38] A recent 72-week RCT in participants with obesity showed impressive weight loss of 20.9% with tirzepatide 15 mg versus 3.1% with placebo.[39] Also, 36.2% of participants achieved weight loss of ≥25%, which approaches the weight loss observed in bariatric surgery. Like other incretin-based therapies, adverse events observed are mostly mild to moderate transient gastrointestinal symptoms.
The ’efficacy–safety’ stopping rule
Patients should be reviewed within 3 months of commencement of anti-obesity pharmacotherapy for its safety, tolerability and efficacy.[24] Safety or significant tolerability concerns should prompt immediate cessation of the medication. As with all weight loss interventions, the amount of weight loss induced by pharmacotherapy varies between individuals and follows a largely normal distribution. In patients unable to maintain ≥5% weight loss after 3–4 months of maximum tolerated doses, the medication should be stopped.[6,40] On the other hand, if the medication is well tolerated and effective, it is reasonable to continue it as a long-term management for obesity, similar to treatment of other chronic diseases such as T2DM.
How is the most appropriate pharmacotherapy option selected?
The choice of anti-obesity medication for a given patient should be individualised based on several factors such as the desired weight loss, cost, mode of administration, contraindications, side effects and cardiovascular safety. For example, GLP-1 RA-based therapies provide superior weight loss and have excellent cardiovascular safety and metabolic benefits, but they are more expensive and require SC injections (albeit only once a week for semaglutide and tirzepatide). The ’efficacy–safety’ rule may be used to guide the decision to continue or stop the medication in the course of the weight loss journey.
ENDOSCOPIC BARIATRIC THERAPIES
EBTs have evolved as an effective, minimally invasive and durable therapeutic option for obesity and can be classified into gastric and small bowel interventions.[41] They were adapted from bariatric surgical procedures and attempt to mimic the mechanisms of the procedures. The gastric EBTs that are widely performed include intragastric balloons (IGBs) and endoscopic gastroplasties (EGs). In Singapore, both options are available for patients with obesity who show a suboptimal response to lifestyle modifications.
The key determinants of energy intake and eating behaviour are hunger, satiation (volume of food needed to reach fullness) and satiety (duration of fullness), regulated by the gut–brain axis.[42] Most obesity therapies, including IGBs and EGs, have focused on altering the gastric function and/or volume to reduce food intake, induce satiety and promote weight loss in obesity.
EBTs could be considered for a select group of patients after careful assessment by an MDT. The indications for EBT include: (1) patients with BMI ≥27 kg/m2 with or without medical comorbidities; (2) patients who are unsuitable for or who decline bariatric surgery; and (3) patients with significant weight regain post-bariatric surgery.[43]
Intragastric balloons
IGBs are the most well-established EBTs with substantial supporting evidence. They work by delaying gastric emptying and inducing early satiety.[44] IGBs differ in their design, filling volume, filling content (air vs. fluid), mode of deployment (endoscopic vs. fluoroscopy) and indwelling time (4–12 months).[45] The Orbera, Spatz and Elipse IGBs are currently available in Singapore.
In the short term (12 months), IGBs effectively induce weight loss ranging between 6% and 15%, compared to a lower weight loss (1%–5%) achieved with lifestyle intervention alone.[46] A meta-analysis demonstrated weight loss of 13.2% at 6 months with Orbera IGB.[47] Likewise, two large real-world studies of IGB demonstrated a favourable safety profile with no mortality, further confirming that IGB is a safe treatment option for obesity.[48,49] However, intolerance symptoms, including nausea and abdominal discomfort, are common in the early phase following IGB implantation and range in incidence between 2.8% and 16.6%.[48,49] A possible solution to minimise these symptoms includes early initiation of analgesia and antiemetics before IGB implantation.
Most of the weight loss occurs in the first few months after treatment with IGB, which is followed by a plateau, likely related to compensatory gastric dilatation, behaviour fatigue and change in resting energy expenditure.[50] Similarly, weight recidivism could occur following IGB removal. Continued follow-up with the MDT, concomitant use of adjuvant pharmacotherapy and personalisation of treatment would assist in weight loss maintenance.
Endoscopic gastroplasty
EGs are non-surgical procedures that involve suturing the stomach lumen and reducing its volume by 75%–80%, similar to surgical sleeve gastrectomy. This technique preserves the gastric anatomy and neurovascular supply. There are currently two EG platforms — endoscopic sleeve gastroplasty (ESG; Apollo Endosurgery, Austin, TX, USA) and primary obesity surgery endoluminal 2.0 (POSE-2.0; USGI Medical, San Clemente, CA, USA). Both interfere with gastric motility and accommodation, resulting in satiation.[51,52,53] We have pioneered the ESG procedure in Singapore, while POSE-2.0 will be introduced by 2023. The differences between the two procedures are the suture pattern (continuous vs. intermittent) and the suture material. ESG is generally associated with a significantly lower adverse event rate compared to laparoscopic sleeve gastrectomy (LSG). The pooled rate of all adverse events with ESG was 2.9% compared to 11.8% with LSG.[54] The reported adverse events with ESG include pain or nausea (1.08%), gastrointestinal bleeding (0.56%), perigastric collection (0.48%), pulmonary embolism (0.06%) and pneumoperitoneum (0.06%).[55]
In our extensive experience of performing ESG [Figure 1], we found weight loss of 16.2% and 20% at 6 and 12 months, respectively. Additionally, the patients showed significant improvement in T2DM, hypertension and fatty liver disease. The average period of stay was 24 h, and all patients recovered immediately without requiring prolonged bed rest or medications. None developed serious complications.[56] Our experience is aligned with the MERIT RCT and other published studies describing the short- and medium-term efficacy (5 years) of ESG together with an excellent safety profile (complication rate <2%).[57,58,59] The reversible nature of ESG and its positive impact on quality of life have extended its application to the adolescent population. Nonetheless, adherence to lifestyle modifications and multidisciplinary follow-up is essential for weight loss maintenance.[60]
Figure 1.
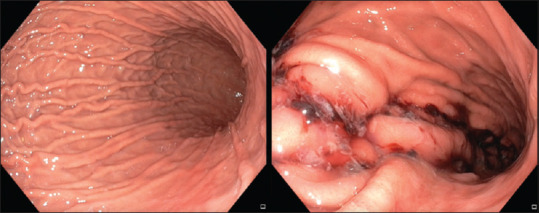
Endoscopic images of the stomach before and after endoscopic sleeve gastroplasty for a patient with obesity.
METABOLIC–BARIATRIC SURGERY
From 2004 to 2020, the number of MBS procedures performed in Singapore increased significantly and the procedure had evolved during that period [Table 2]. Earlier procedures such as the laparoscopic adjustable gastric banding (LAGB) have been replaced by procedures with better efficacy. The most popular procedure in Singapore and worldwide is the LSG, followed by the Roux-en-Y gastric bypass (RYGB), while one-anastomosis gastric bypass (OAGB) has regained popularity due to recent endorsement by the American Society of Metabolic and Bariatric Surgery.
Table 2.
Metabolic–bariatric surgery procedures from 2004 to 2020 in Singapore.a
| Type of procedure | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAGB | 102 | 131 | 52 | 12 | 15 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||||||
| LSG | 0 | 0 | 3 | 3 | 5 | 27 | 189 | 193 | 252 | 277 | 292 | 363 | 373 | 256 |
|
| ||||||||||||||
| RYGB | 0 | 0 | 0 | 1 | 2 | 8 | 70 | 103 | 93 | 108 | 110 | 80 | 84 | 61 |
|
| ||||||||||||||
| OAGB | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 1 | 19 |
|
| ||||||||||||||
| BPD | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||||||
| Gastric plication | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
|
| ||||||||||||||
| Revisional surgery | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 17 | 21 | 17 | 15 |
|
| ||||||||||||||
| Total | 102 | 131 | 55 | 16 | 22 | 54 | 275 | 296 | 347 | 401 | 419 | 464 | 475 | 351 |
aData from the Obesity & Metabolic Surgery Society of Singapore. BPD: biliopancreatic diversion with duodenal switch, LAGB: laparoscopic adjustable gastric banding, LSG: laparoscopic sleeve gastrectomy, OAGB: one_anastomosis gastric bypass, RYGB: Roux-en-Y gastric bypass
MBS remains the most effective and durable treatment for patients with severe obesity, with the average weight loss documented being 25% and 30% for LSG and RYGB, respectively,[22] achieving significant improvement of obesity-related complications such as T2DM.[61] The weight loss of patients is also sustained, with an average weight regain of 5%–10% documented from their lowest weight after 10 years.[62,63] Data from our cohort has shown that 55.9% of patients with T2DM could achieve diabetes remission (HbA1c ≤6% without DM medications) 1 year after bariatric surgery.[64] The significant glycaemic benefit of MBS has led to its endorsement as a recommended treatment option for patients with T2DM and obesity.[65]
The large, sustained weight loss and metabolic improvements observed with MBS are not just due to restriction or malabsorption, but also due to changes in gut hormones, bile acids and gut microbiome, which lead to reduction in hunger and increased satiety.[66] The safety of MBS has improved significantly with a ten-fold decrease in mortality — from 0.5% with RYGB in a meta-analysis published in 2004[67] to 0.05% for LSG in a more recent meta-analysis.[68]
Indications for MBS
Indications for MBS are primarily based on BMI as follows: >40 kg/m2, or >35 kg/m2 with obesity-related complications. Locally, these BMI cut-offs are lowered by 2.5 kg/m2 due to higher propensity for metabolic complications at lower BMI in Asians.[69] Due to better benefit–risk profile of MBS over the years, there have been recent recommendations to further lower the BMI threshold to >35 kg/m2, or >30 kg/m2 with comorbidities.[70]
Types of bariatric surgery
Laparoscopic sleeve gastrectomy
In LSG, 60%–80% of the stomach is removed along the greater curvature to create a 'sleeve’ of stomach along the lesser curve[71] [Figure 2]. LSG is popular owing to its efficacy and low surgical risk, which is comparable to cholecystectomy.[68] Because of its quick postoperative recovery, LSG can be done as a short-stay procedure, with most patients requiring just an overnight stay in the hospital.
Figure 2.
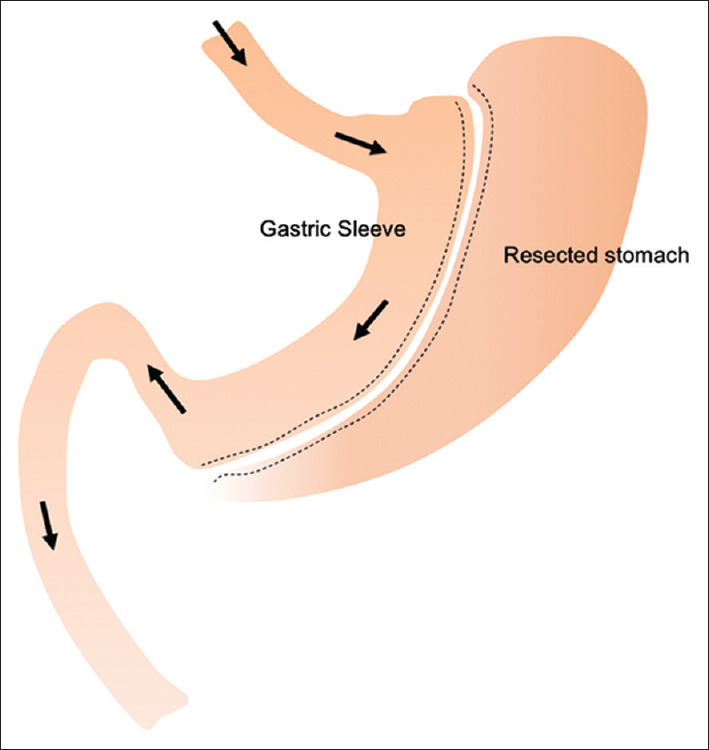
Diagram shows sleeve gastrectomy anatomy.
Roux-en-Y gastric bypass
The RYGB procedure consists of creation of a gastric pouch, which is connected to the distal jejunum to form the Roux limb. The disconnected bilio-pancreatic limb is then anastomosed 75–150 cm along the Roux limb, forming a Y-configuration [Figure 3]. The distal stomach, duodenum and proximal jejunum are thus bypassed, triggering changes in gut hormones and bile acid metabolism. Meta-analyses showed greater weight loss, improved metabolic outcomes and lower incidence of postoperative gastro-oesophageal reflux disease (GERD) with RYGB compared to LSG.[22,72]
Figure 3.
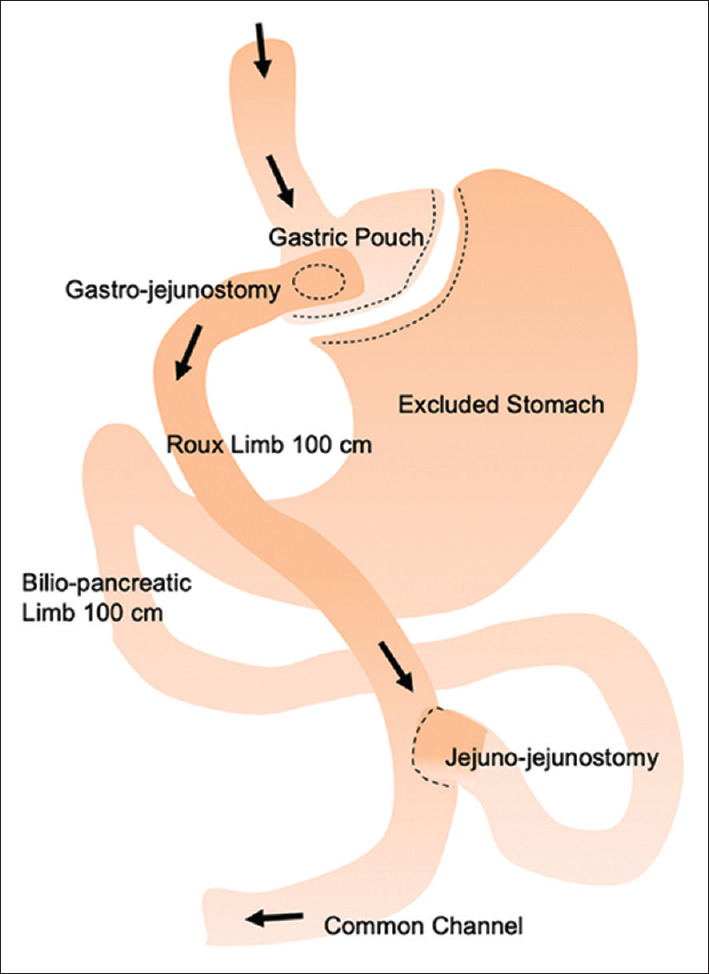
Diagram shows roux-en-Y gastric bypass anatomy.
One-anastomosis gastric bypass
The OAGB operation consists of two components. A narrow lesser-curvature gastric pouch is created, followed by a jejunal bypass with a gastro-jejunostomy anastomosis, bypassing the duodenum and proximal jejunum [Figure 4]. The OAGB is a simpler and safer operation compared to RYGB,[73] with comparable weight loss and metabolic outcomes.[74] Our long-term data showed OAGB achieved greater excess weight loss (EWL) (62.5% in 5 years) and T2DM resolution (71.9%) compared to RYGB and LSG.[75]
Figure 4.
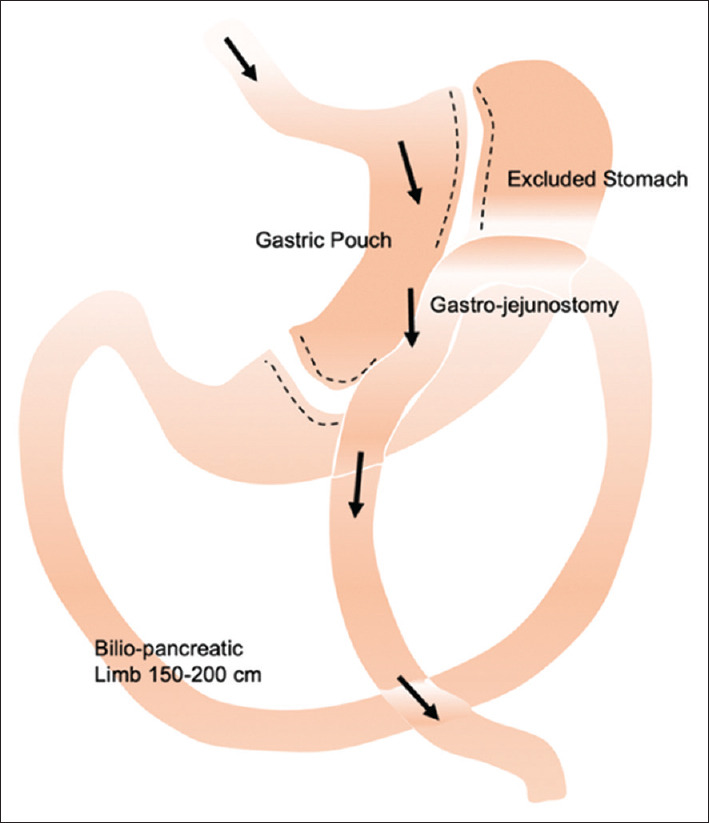
Diagram shows one-anastomosis gastric bypass anatomy.
Revisional surgery
Revisional surgery is the fastest growing category of MBS worldwide, and the number of revisional surgeries performed tripled from 6% in 2013 to 16.7% of all procedures in 2019. Common indications of revisional surgery are GERD and weight regain following primary surgery. Conversion from LSG to RYGB may engage additional neurohormonal mechanisms that alter energy homeostasis, leading to weight loss.[76] We found that revisional surgery was highly effective in treating GERD post-LSG and produced an additional weight loss of 24% in patients with insufficient weight loss.[77]
Procedure selection and preoperative workup
Once a decision has been made for MBS, systematic nutritional, psychosocial and anatomical assessments should be organised. Screening and optimisation of obesity-related complications should be performed, together with assessment of commitment to lifestyle change and expectations post-surgery via an MDT approach. The choice of procedure depends on factors such as initial BMI, presence of diabetes, GERD, perioperative risk, and patient and surgeon preferences. Although RYGB can result in greater weight reduction and T2DM resolution, mortality and morbidity are higher with the procedure. LSG has a lower rate of perioperative complications, but higher rates of weight regain, which may necessitate future revisional surgery. VLCD of <800 kcal/day is usually prescribed 2 weeks before surgery to reduce hepatic steatosis and improve the technical aspects of surgery.[78]
Complications
Surgical complications or nutritional deficiencies may arise following MBS. Serious surgical complications such as leaks or haemorrhage are rare (<2%). Erosive oesophagitis is common after LSG,[71] and it can be treated with proton pump inhibitors or revisional surgery to RYGB in refractory cases.[77] The pillars of managing surgical complications post-MBS are early diagnosis and a multidisciplinary 'step-up’ approach. First-line treatment consists of supportive measures including transfusion and sepsis control, followed by radiological or endoscopic therapy if feasible. Reoperations are reserved for life-threatening complications or if other measures fail.
Through alteration or shortening of the tract, MBS can cause nutritional deficiencies. Thorough nutritional assessment is advised as vitamin D, B12, folate and iron deficiencies are common in patients with severe obesity, and these should be corrected before surgery.[79] Routine supplementation of multivitamin, calcium and vitamin D is recommended after MBS.[80]
Adequate protein intake is essential to mitigate the loss of lean muscle mass, and protein intake of at least 60 g/day and up to 1.5 g/kg ideal body weight/day is recommended.[81] Liquid protein supplements and MR may be considered to achieve target protein intake, especially in the early postoperative period.
DISCUSSION
It is increasingly recognised that obesity is a disorder of the energy homeostasis system, rather than simply due to passive fat accumulation from energy excess.[82] Weight loss is accompanied by a reduction in energy expenditure and resting metabolic rate and changes in appetite-regulating hormones, causing increased hunger and reduced satiety.[83,84] In response to weight loss, these adaptive mechanisms create the perfect ’metabolic storm’ for subsequent weight regain.
There are three main considerations in obesity management: efficacy, risks and cost. Lifestyle modification is cheap and accessible and remains the cornerstone of obesity management. However, as lifestyle modifications alone typically achieve only modest long-term weight loss, other treatment modalities are essential. Recent advances in anti-obesity pharmacotherapy show promising results with weight loss, similar to bariatric surgery. However, in the long term, surgery is likely to be more cost-effective, given the high costs of these drugs.[85,86] EBTs can be considered in those who prefer less-invasive options; they can induce reasonable weight loss and improve the comorbid conditions.
Given that obesity is a complex disease driven by multiple factors, treatment cannot follow a ’one-size-fits-all’ approach. Most of the management options discussed may be employed synergistically — pharmacotherapy plays an important role as an adjunct to lifestyle intervention in the weight maintenance phase after initial weight loss with lifestyle intervention[87,88] or in settings of inadequate weight loss or weight regain after bariatric surgery.[89] Endoscopic revisions could be performed to augment weight loss in patients with insufficient weight loss after bariatric surgery.[90]
CONCLUSION
Obesity is a chronic disease associated with multiple systemic complications, and it is increasing in prevalence and starting at a younger age. Stigma is a common, yet under-recognised barrier to the timely treatment of obesity. Although lifestyle modifications remain the cornerstone of successful weight management, they are often insufficient on their own, hence the importance of other treatment modalities including pharmacotherapy, EBTs and bariatric surgery. In particular, the newer pharmacological agents and EBTs are promising modalities that can achieve more weight loss at a lower risk. Weight regain following initial weight loss is extremely common, and multiple treatment modalities may be synergistically adopted to mitigate this.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ministry of Health, Singapore. National Population Health Survey 2019/20. [Last accessed on 2022 Nov 30]. Available from: https://www.Moh.Gov.Sg/Resources-Statistics/ Reports/National-Survey-2019-20 .
- 2.Chua MWJ, Zheng S. Obesity and COVID-19: The clash of two pandemics. Obes Res Clin Pract. 2020;14:380–2. doi: 10.1016/j.orcp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinlen D, Cody D, O’Shea D. Complications of obesity. QJM. 2018;111:437–43. doi: 10.1093/qjmed/hcx152. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Cupples LA, Stokes A, Liu CT. Association of obesity with mortality over 24 years of weight history: Findings from the Framingham heart study. JAMA Netw Open. 2018;1:e184587. doi: 10.1001/jamanetworkopen.2018.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarwer DB, Polonsky HM. The psychosocial burden of obesity. Endocrinol Metab Clin North Am. 2016;45:677–88. doi: 10.1016/j.ecl.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 7.Lee YS, Biddle S, Chan MF, Cheng A, Cheong M, Chong YS, et al. Health promotion board-ministry of health clinical practice guidelines: Obesity. Singapore Med J. 2016;57:292–300. doi: 10.11622/smedj.2016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahreem A, Rakha A, Rabail R, Nazir A, Socol CT, Maerescu CM, et al. Fad diets: Facts and fiction. Front Nutr. 2022;9:960922. doi: 10.3389/fnut.2022.960922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA. 2014;312:923–33. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 10.Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11:2442. doi: 10.3390/nu11102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannen ST, Maldonado SG, Nonnenmacher T, Sowah SA, Gruner LF, Watzinger C, et al. Adherence and dietary composition during intermittent vs. continuous calorie restriction: Follow-up data from a randomized controlled trial in adults with overweight or obesity. Nutrients. 2021;13:1195. doi: 10.3390/nu13041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018;391:541–51. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 13.Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344–55. doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Maurice PF, Graubard BI, Troiano RP, Berrigan D, Galuska DA, Fulton JE, et al. Estimated number of deaths prevented through increased physical activity among US adults. JAMA Intern Med. 2022;182:349–52. doi: 10.1001/jamainternmed.2021.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekelund U, Tarp J, Fagerland MW, Johannessen JS, Hansen BH, Jefferis BJ, et al. Joint associations of accelero-meter measured physical activity and sedentary time with all-cause mortality: A harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med. 2020;54:1499–506. doi: 10.1136/bjsports-2020-103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerns JC, Guo J, Fothergill E, Howard L, Knuth ND, Brychta R, et al. Increased physical activity associated with less weight regain six years after “The Biggest Loser” competition. Obesity (Silver Spring) 2017;25:1838–43. doi: 10.1002/oby.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox CE. Role of physical activity for weight loss and weight maintenance. Diabetes Spectr. 2017;30:157–60. doi: 10.2337/ds17-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lally P, Jaarsveld Cv, Potts H, Wardle J. How are habits formed: Modelling habit formation in the real world. Euro J Soc Psychol. 2010;40:998–1009. [Google Scholar]
- 19.Toh A. Understanding matters of the mind in obesity. Singapore Fam Physician. 2021;47:22–6. [Google Scholar]
- 20.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Look ARG. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–66. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 23.Saxon DR, Iwamoto SJ, Mettenbrink CJ, McCormick E, Arterburn D, Daley MF, et al. Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009-2015. Obesity (Silver Spring) 2019;27:1975–81. doi: 10.1002/oby.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee PC, Dixon J. Pharmacotherapy for obesity. Aust Fam Physician. 2017;46:472–7. [PubMed] [Google Scholar]
- 25.Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann N Y Acad Sci. 2014;1311:1–13. doi: 10.1111/nyas.12328. [DOI] [PubMed] [Google Scholar]
- 26.Lewis KH, Fischer H, Ard J, Barton L, Bessesen DH, Daley MF, et al. Safety and effectiveness of longer-term phentermine use: Clinical outcomes from an electronic health record cohort. Obesity (Silver Spring) 2019;27:591–602. doi: 10.1002/oby.22430. [DOI] [PubMed] [Google Scholar]
- 27.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–62. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 28.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: A systematic review and meta-analysis. JAMA. 2016;315:2424–34. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 30.Papathanasiou T, Strathe A, Agerso H, Lund TM, Overgaard RV. Impact of dose-escalation schemes and drug discontinuation on weight loss outcomes with liraglutide 3.0 mg: A model-based approach. Diabetes Obes Metab. 2020;22:969–77. doi: 10.1111/dom.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 33.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 34.Rubino DM, Greenway FL, Khalid U, O’Neil PM, Rosenstock J, Sorrig R, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: The STEP 8 randomized clinical trial. JAMA. 2022;327:138–50. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 36.Ludvik B, Giorgino F, Jodar E, Frias JP, Fernandez Lando L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398:583–98. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstock J, Wysham C, Frias JP, Kaneko S, Lee CJ, Fernandez Lando L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–55. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 38.Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 39.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 40.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: A systematic and clinical review. JAMA. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jirapinyo P, Thompson CC. Endoscopic bariatric and metabolic therapies: Surgical analogues and mechanisms of action. Clin Gastroenterol Hepatol. 2017;15:619–30. doi: 10.1016/j.cgh.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acosta A, Camilleri M, Abu Dayyeh B, Calderon G, Gonzalez D, McRae A, et al. Selection of antiobesity medications based on phenotypes enhances weight loss: A pragmatic trial in an obesity clinic. Obesity (Silver Spring) 2021;29:662–71. doi: 10.1002/oby.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. A pathway to endoscopic bariatric therapies. Surg Obes Relat Dis. 2011;7:672–82. doi: 10.1016/j.soard.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Vargas EJ, Bazerbachi F, Calderon G, Prokop LJ, Gomez V, Murad MH, et al. Changes in time of gastric emptying after surgical and endoscopic bariatrics and weight loss: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:57–68.e5. doi: 10.1016/j.cgh.2019.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee PC, Dixon J. Medical devices for the treatment of obesity. Nat Rev Gastroenterol Hepatol. 2017;14:553–64. doi: 10.1038/nrgastro.2017.80. [DOI] [PubMed] [Google Scholar]
- 46.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic bariatric therapy: A guide to the intragastric balloon. Am J Gastroenterol. 2019;114:1421–31. doi: 10.14309/ajg.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 47.Force ABET, Committee AT, Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, et al. ASGE bariatric endoscopy task force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425–38.e5. doi: 10.1016/j.gie.2015.03.1964. [DOI] [PubMed] [Google Scholar]
- 48.Vargas EJ, Pesta CM, Bali A, Ibegbu E, Bazerbachi F, Moore RL, et al. Single fluid-filled intragastric balloon safe and effective for inducing weight loss in a real-world population. Clin Gastroenterol Hepatol. 2018;16:1073–80.e1. doi: 10.1016/j.cgh.2018.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agnihotri A, Xie A, Bartalos C, Kushnir V, Sullivan S, Islam S, et al. Real-world safety and efficacy of fluid-filled dual intragastric balloon for weight loss. Clin Gastroenterol Hepatol. 2018;16:1081–8.e1. doi: 10.1016/j.cgh.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Chan DL, Cruz JR, Mui WL, Wong SKH, Ng EKW. Outcomes with Intra-gastric balloon therapy in BMI<35 non-morbid obesity: 10-year follow-up study of an RCT. Obes Surg. 2021;31:781–6. doi: 10.1007/s11695-020-04986-3. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Nava G, Negi A, Bautista-Castano I, Rubio MA, Asokkumar R. Gut and metabolic hormones changes after endoscopic sleeve gastroplasty (ESG) vs. laparoscopic sleeve gastrectomy (LSG) Obes Surg. 2020;30:2642–51. doi: 10.1007/s11695-020-04541-0. [DOI] [PubMed] [Google Scholar]
- 52.Vargas EJ, Rizk M, Gomez-Villa J, Edwards PK, Jaruvongvanich V, Storm AC, et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: A comparative prospective study. Gut. 2022:gutjnl-2022-327816. doi: 10.1136/gutjnl-2022-327816. doi: 10.1136/gutjnl-2022-327816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez Nava G, Arau RT, Asokkumar R, Maselli DB, Rapaka B, Matar R, et al. Prospective multicenter study of the primary obesity surgery endoluminal (POSE 2.0) procedure for treatment of obesity. Clin Gastroenterol Hepatol. 2023;21:81–9. doi: 10.1016/j.cgh.2022.04.019. e4. [DOI] [PubMed] [Google Scholar]
- 54.Mohan BP, Asokkumar R, Khan SR, Kotagiri R, Sridharan GK, Chandan S, et al. Outcomes of endoscopic sleeve gastroplasty; how does it compare to laparoscopic sleeve gastrectomy. A systematic review and meta-analysis? Endosc Int Open. 2020;8:E558–65. doi: 10.1055/a-1120-8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, Adam A, Neto MG, Badurdeen D, et al. Efficacy and safety of endoscopic sleeve gastroplasty: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:1043–53.e4. doi: 10.1016/j.cgh.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Asokkumar R, Lim CH, Tan AS, Lee PC, Eng A, Tan J, et al. Safety and early efficacy of endoscopic sleeve gastroplasty (ESG) for obesity in a multi-ethnic Asian population in Singapore. JGH Open. 2021;5:1351–6. doi: 10.1002/jgh3.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abu Dayyeh BK, Bazerbachi F, Vargas EJ, Sharaiha RZ, Thompson CC, Thaemert BC, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. Lancet. 2022;400:441–51. doi: 10.1016/S0140-6736(22)01280-6. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Nava G, Asokkumar R, Bautista-Castano I, Laster J, Negi A, Fook-Chong S, et al. Endoscopic sleeve gastroplasty, laparoscopic sleeve gastrectomy, and laparoscopic greater curve plication: Do they differ at 2 years? Endoscopy. 2021;53:235–43. doi: 10.1055/a-1224-7231. [DOI] [PubMed] [Google Scholar]
- 59.Sharaiha RZ, Hajifathalian K, Kumar R, Saunders K, Mehta A, Ang B, et al. Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Clin Gastroenterol Hepatol. 2021;19:1051–7.e2. doi: 10.1016/j.cgh.2020.09.055. [DOI] [PubMed] [Google Scholar]
- 60.Negi A, Asokkumar R, Ravi R, Lopez-Nava G, Bautista-Castano I. Nutritional management and role of multidisciplinary follow-up after endoscopic bariatric treatment for obesity. Nutrients. 2022;14:3450. doi: 10.3390/nu14163450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. 2018;61:257–64. doi: 10.1007/s00125-017-4513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 63.Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: A randomized clinical trial. JAMA Surg. 2015;150:931–40. doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee PC, Tham KW, Ganguly S, Tan HC, Eng AKH, Dixon JB. Ethnicity does not influence glycemic outcomes or diabetes remission after sleeve gastrectomy or gastric bypass in a multiethnic Asian cohort. Obes Surg. 2018;28:1511–8. doi: 10.1007/s11695-017-3050-6. [DOI] [PubMed] [Google Scholar]
- 65.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by International Diabetes Organizations. Diabetes Care. 2016;39:861–77. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 66.Dixon JB, Lambert EA, Lambert GW. Neuroendocrine adaptations to bariatric surgery. Mol Cell Endocrinol. 2015;418:143–52. doi: 10.1016/j.mce.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 67.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 68.Robertson AGN, Wiggins T, Robertson FP, Huppler L, Doleman B, Harrison EM, et al. Perioperative mortality in bariatric surgery: Meta-analysis. Br J Surg. 2021;108:892–7. doi: 10.1093/bjs/znab245. [DOI] [PubMed] [Google Scholar]
- 69.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 70.Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2022;18:1345–56. doi: 10.1016/j.soard.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 71.Lim CH, Lee PC, Lim E, Tan J, Chan WH, Tan HC, et al. Correlation between symptomatic gastro-esophageal reflux disease (GERD) and erosive esophagitis (EE) post-vertical sleeve gastrectomy (VSG) Obes Surg. 2019;29:207–14. doi: 10.1007/s11695-018-3509-0. [DOI] [PubMed] [Google Scholar]
- 72.Sharples AJ, Mahawar K. Systematic review and meta-analysis of randomised controlled trials comparing long-term outcomes of Roux-En-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2020;30:664–72. doi: 10.1007/s11695-019-04235-2. [DOI] [PubMed] [Google Scholar]
- 73.Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Ann Surg. 2005;242:20–8. doi: 10.1097/01.sla.0000167762.46568.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robert M, Espalieu P, Pelascini E, Caiazzo R, Sterkers A, Khamphommala L, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): A multicentre, randomised, open-label, non-inferiority trial. Lancet. 2019;393:1299–309. doi: 10.1016/S0140-6736(19)30475-1. [DOI] [PubMed] [Google Scholar]
- 75.Toh BC, Chan WH, Eng AKH, Lim EKW, Lim CH, Tham KW, et al. Five-year long-term clinical outcome after bariatric metabolic surgery: A multi-ethnic Asian population in Singapore. Diabetes Obes Metab. 2018;20:1762–5. doi: 10.1111/dom.13263. [DOI] [PubMed] [Google Scholar]
- 76.Dharmaratnam VM, Lim E, Eng A, Chan WH, Tan HC, Ho E, et al. Revisional surgery or pharmacotherapy for insufficient weight loss and weight regain after primary bariatric procedure: A descriptive study. Obes Surg. 2022;32:3298–304. doi: 10.1007/s11695-022-06191-w. [DOI] [PubMed] [Google Scholar]
- 77.Lim CH, Lee PC, Lim E, Eng A, Chan WH, Tan HC, et al. Resolution of erosive esophagitis after conversion from vertical sleeve gastrectomy to Roux-en-Y gastric bypass. Obes Surg. 2020;30:4751–9. doi: 10.1007/s11695-020-04913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarnoff M, Kaplan LM, Shikora S. An evidenced-based assessment of preoperative weight loss in bariatric surgery. Obes Surg. 2008;18:1059–61. doi: 10.1007/s11695-008-9603-y. [DOI] [PubMed] [Google Scholar]
- 79.Lee PC, Ganguly S, Dixon JB, Tan HC, Lim CH, Tham KW. Nutritional deficiencies in severe obesity: A multiethnic Asian cohort. Obes Surg. 2019;29:166–71. doi: 10.1007/s11695-018-3494-3. [DOI] [PubMed] [Google Scholar]
- 80.Lee PC, Dixon J. Bariatric-metabolic surgery: A guide for the primary care physician. Aust Fam Physician. 2017;46:465–71. [PubMed] [Google Scholar]
- 81.Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the obesity management task force of the European Association for the study of obesity for the post-bariatric surgery medical management. Obes Facts. 2017;10:597–632. doi: 10.1159/000481825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. Obesity pathogenesis: An endocrine society scientific statement. Endocr Rev. 2017;38:267–96. doi: 10.1210/er.2017-00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 85.Boyers D, Retat L, Jacobsen E, Avenell A, Aveyard P, Corbould E, et al. Cost-effectiveness of bariatric surgery and non-surgical weight management programmes for adults with severe obesity: A decision analysis model. Int J Obes (Lond) 2021;45:2179–90. doi: 10.1038/s41366-021-00849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauren BN, Lim F, Krikhely A, Taveras EM, Woo Baidal JA, Bellows BK, et al. Estimated cost-effectiveness of medical therapy, sleeve gastrectomy, and gastric bypass in patients with severe obesity and type 2 diabetes. JAMA Netw Open. 2022;5:e2148317. doi: 10.1001/jamanetworkopen.2021.48317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384:1719–30. doi: 10.1056/NEJMoa2028198. [DOI] [PubMed] [Google Scholar]
- 88.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA. 2021;325:1414–25. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barenbaum SR, Zhao AS, Saunders KH, Aronne LJ, Shukla AP. Management of weight regain following bariatric surgery: Behavioral intervention and pharmacotherapy. Expert Rev Endocrinol Metab. 2022;17:405–14. doi: 10.1080/17446651.2022.2101993. [DOI] [PubMed] [Google Scholar]
- 90.Bulajic M, Vadala di Prampero SF, Boskoski I, Costamagna G. Endoscopic therapy of weight regain after bariatric surgery. World J Gastrointest Surg. 2021;13:1584–96. doi: 10.4240/wjgs.v13.i12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]


