Abstract
INTRODUCTION
Rehabilitation focuses on impairments, activity limitations and participation restrictions being informed by the underlying health condition. In the current absence of direct “evidence on” rehabilitation interventions for people with post-COVID-19 condition (PCC), we can search and synthesize the indirect “evidence relevant to” coming from interventions effective for the symptoms of PCC in other health conditions. The World Health Organization (WHO) required this information to inform expert teams and provide specific recommendations in their Guidelines. With this overview of reviews with mapping, we aimed to synthesize in a map the Cochrane evidence relevant to rehabilitation for fatigue, post-exertional malaise and orthostatic intolerance due to PCC.
EVIDENCE ACQUISITION
We searched the last five years’ Cochrane Systematic Review (CSRs) using the terms “fatigue,” “orthostatic intolerance,” “rehabilitation” and their synonyms in the Cochrane Library. We extracted and summarized the available evidence using a map. We grouped the included CSRs for health conditions and interventions, indicating the effect and the quality of evidence.
EVIDENCE SYNTHESIS
Out of 1397 CSRs published between 2016 and 2021, we included 32 for fatigue and 4 for exercise intolerance. They provided data from 13 health conditions, with cancer (11 studies), chronic obstructive pulmonary disease (7 studies), fibromyalgia (4 studies), and cystic fibrosis (3 studies) being the most studied. Effective interventions for fatigue included exercise training and physical activities, telerehabilitation and multicomponent and educational interventions. Effective interventions for exercise intolerance included combined aerobic/anaerobic training and integrated disease rehabilitation management. The overall quality of evidence was low to very low and moderate in very few cases. We did not identify CSRs that specifically addressed post-exertional malaise or orthostatic intolerance.
CONCLUSIONS
These results are the first step of indirect evidence able to generate helpful hypotheses for clinical practice and future research. They served as the basis for the three recommendations on treatments for these PCC symptoms published in the current WHO Guidelines for clinical practice.
Key words: COVID-19, Rehabilitation, Fatigue, Orthostatic intolerance, Post-acute COVID-19 syndrome
Introduction
This short paper is one of a series reporting the studies developed by Cochrane Rehabilitation for the World Health Organization Rehabilitation Program (WHO-RP) to synthesize the Cochrane evidence relevant to symptoms due to the post COVID-19 condition (PCC).1 Further details on this work have been reported elsewhere.2 We focus here on fatigue, post-exertional malaise and orthostatic intolerance.
Among a sample of 1.2 million individuals that experienced symptomatic SARS-CoV-2 infection between 2020 and 2021, 6.2% showed persistent fatigue with bodily pain, cognitive problems, or respiratory problems after three months after the initial infection.3 Observational studies showed that in Italian COVID-19 inpatients, 53% reported fatigue, and 22% were experiencing chest pain after two months;4 in a UK cohort of 100 COVID-19-survivors, ongoing fatigue is present after 4-8 weeks in more than two-thirds of them.5 In addition, Dani et al. described a series of patients characterized by debilitating symptoms following the viral infection attributable to orthostatic intolerance.6 Similarly to chronic fatigue syndrome, it has been proposed that in PCC, too, pro-inflammatory components like cytokines such as IFN gamma and IL-7 are supposed to compromise the regular functioning of the central nervous system,7, 8 resulting in a wide variety of symptoms mentioned above.
Currently, no evidence exists on specific treatments for such COVID-19 sequelae. However, rehabilitation interventions that proved effective for similar symptoms in other health conditions could be applied to people with PCC as a first meaningful clinical hypothesis (evidence relevant to).2 With the present review, we aimed to map the current Cochrane evidence on the efficacy of rehabilitation treatments proposed for fatigue, post-exertional malaise and orthostatic intolerance in other health conditions. This information can partially fill the knowledge gap in rehabilitation for people with PCC and help clinicians find the appropriate treatments for individual patients. This information can also generate research hypotheses for further studies.
Evidence acquisition
The design of this study is an overview of reviews with mapping. We reported the methods used in a previous publication.2 In this short paper, we included Cochrane Systematic Reviews (CSRs) relevant to PCC that considered fatigue, post-exertional fatigue, weakness, post-exertional symptom exacerbation, post-exertional malaise, orthostatic intolerance, postural orthostatic tachycardia syndrome and autonomic nervous system dysfunction, as defined by the WHO. We divided the symptoms into three categories: fatigue, post-exertional malaise and orthostatic intolerance. We summarize the search string in Table I.
Table I. —List of impairments by WHO Rehabilitation Program relevant to post COVID-19 condition and outcomes included in the study.
| Impairment | Synonyms/variations | Outcomes |
|---|---|---|
| Fatigue | Fatigue, post-exertional fatigue, exhaustion, weaknessa,b,c | Any subjective or objective assessment of fatigue (e.g., Chronic Respiratory Questionnaire [CRQ] - fatigue domain; Borg fatigue score; Fatigue Severity Scale) |
| Post-exertional malaise | Post-exertional symptom exacerbation, post-exertional malaisec | Any scale of dyspnea/fatigue/pain assessed after any type of encoded physical effort (e.g., Borg RPE after 6-minutes walking test) |
| Orthostatic intolerance | Orthostatic intolerance, postural orthostatic tachycardia syndrome, autonomic nervous system dysfunctiond |
Not available Cochrane reviews corresponding to the search criteria |
Sources used for the selection of symptoms: a. Systematic Reviews results; b. Global Burden of Disease data; c. WHO clinical case definition development; d. Emerging evidence on underlying pathophysiology of autonomic nervous system dysfunction.
Evidence synthesis
Fatigue
We screened 1397 CSRs and excluded 1266 at the title and abstract stage. We screened 131 full texts, with 32 CSR meeting the inclusion criteria (Supplementary Digital Material 1: Supplementary Table I). Participants were adults with cancer (11 CSRs), chronic obstructive pulmonary disease (COPD) (four CSRs), fibromyalgia (four CSRs), chronic kidney disease (two CSRs), cystic fibrosis (two CSRs), multiple sclerosis (two CSRs), bronchiectasis (one CSR), chronic fatigue syndrome (one CSR), chronic respiratory disease (one CSR), facioscapulohumeral muscular dystrophy (one CSR), inflammatory bowel disease (one CSR), interstitial lung disease (one CSR), and traumatic brain injury (one CSR). The methodological quality assessed with AMSTAR 2 (A MeaSurement Tool to Assess systematic Reviews) was high in all CSRs (Supplementary Digital Material 2: Supplementary Table II). The quality of evidence has been evaluated in 23 reviews9-31 using the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) while it was not reported in the others.32-40
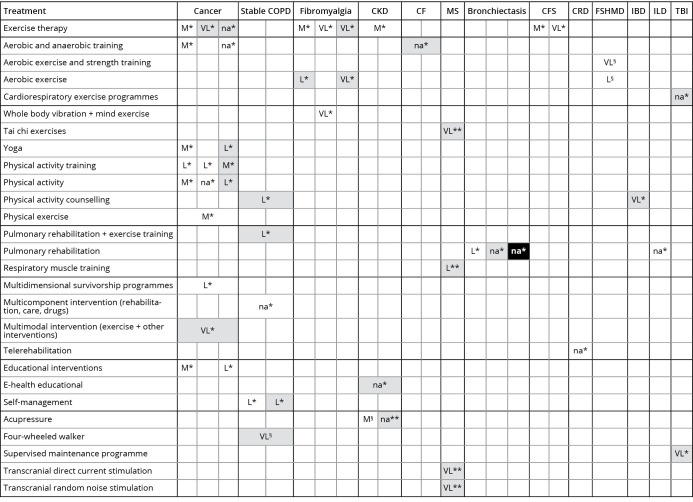
There is very low- to moderate-quality evidence for fatigue management with rehabilitation comprising exercise training and physical and aerobic activities applied with different frequencies, intensities, duration and types of interventions (Figure 1).
Figure 1.
—Evidence map of fatigue symptom. Lines represent the interventions. Columns represents the health conditions where the searched outcome has been considered. Colors into each cell reported the type of effect (effect against the intervention – black; effect in favor of the intervention – white; no definite results – grey). Quality of evidence was reported into each cell with the following acronyms: VL: very low-quality; L: low-quality; M: moderate-quality; H: high-quality; na: not available. Comparisons: *Control group; **sham group; §no intervention. CFS: chronic fatigue syndrome; CKD: chronic kidney disease; IBD: inflammatory bowel disease; CRD: chronic respiratory disease; CF: cystic fibrosis; ILD: interstitial lung disease; COPD: chronic obstructive respiratory disease; FSHMD: facio-scapulo-humeral muscular dystrophy; MS: multiple sclerosis; TBI: traumatic brain injury.
Such interventions have been used with benefits (measured through structured questionnaires and self-perception scales) in patients with cancer, fibromyalgia, chronic kidney disease, cystic fibrosis, chronic fatigue syndrome, inflammatory bowel disease and neurological conditions. Studies on fatigue in chronic respiratory diseases (COPD and bronchiectasis, in particular) support (low-quality evidence) the use of pulmonary rehabilitation, multicomponent interventions and telerehabilitation, as well as self- and educational interventions. In addition, educational programs also seem to be effective in cancer patients (low- to moderate-quality of evidence). Finally, acupressure effectively reduced fatigue perception (measured by the Piper Fatigue Scale) of people with chronic kidney disease, compared to no treatment (moderate-quality of evidence).
Exercise intolerance
We included 4 CSRs (Supplementary Digital Material 3: Supplementary Table III). Participants were adults with stable COPD (3) and cystic fibrosis (1). The results of the AMSTAR 2 assessment indicated the high methodological quality of the CSRs (Supplementary Digital Material 4: Supplementary Table IV). The quality of evidence has been evaluated in two studies39, 41 using the GRADE approach, while it was not in the other two.36, 42
The effective interventions described were combined aerobic/anaerobic training compared to no physical training, integrated disease rehabilitation management compared to control, neuromuscular electrical stimulation (NMES) plus exercise compared to exercise only or NMES compared to usual care. However, only the latter has been demonstrated to promote beneficial effects in terms of endurance time, with very low-quality evidence, in stable COPD (Figure 2).39
Figure 2.
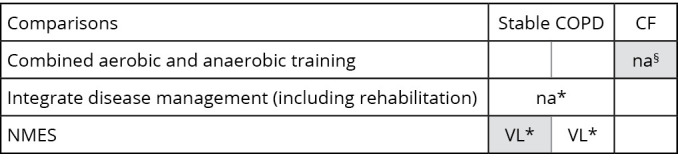
—Evidence map of post-exertional malaise symptom. Lines represent the interventions. Columns represents the health conditions where the searched outcome has been considered. Colors into each cell reported the type of effect (effect against the intervention – black; effect in favor of the intervention – white; no definite results – grey). Quality of evidence was reported into each cell the with the following acronyms: VL: very low-quality; L: low-quality; M: moderate-quality; H: high-quality; na: not available. Comparisons: *Control group; §no intervention. NMES: neuromuscular electrical stimulation; COPD: chronic obstructive respiratory disease; CF: cystic fibrosis.
Orthostatic intolerance and post-exertional malaise
We did not identify CSRs that specifically addressed post-exertional malaise or orthostatic intolerance.
Discussion
This paper maps the current Cochrane evidence on the efficacy of rehabilitation interventions for managing fatigue, post-exertional malaise and orthostatic intolerance in health conditions different from PCC. CSRs focusing on various health conditions showed that fatigue can be effectively managed mainly through various exercise and physical activity modalities. Studies in people affected by chronic respiratory disease or cancer support telerehabilitation, multicomponent and educational interventions. While we did not find CSRs specifically focused on post-exertional malaise and orthostatic intolerance, evidence on the treatment of COPD endorses using NMES as an add-on to exercise training to improve exercise tolerance.
When implementing the “evidence relevant to”, we need to check 1) if there are specific pathophysiological mechanisms of PCC suggesting avoiding any of the identified treatments; 2) if there are treatments specific for the reported health conditions that would not be appropriate for PCC. Obviously, in the implementation phase, the need to check individual contraindications in single patients remains. The WHO identified one red flag for PCC rehabilitation: post-exertional symptom exacerbation.43 This can represent an individual contra-indication for all the treatments considered below.
Findings on exercise and physical interventions align with guidelines that support the integration of exercise training in managing cardiorespiratory, oncological and neurological disorders. There is consensus that such training can benefit the overall quality of life, including fatigue reduction.44-47 In particular, the 2021 European Society of Cardiology guidelines emphasizes the importance for individuals with chronic conditions to be as active as possible even if they cannot achieve the weekly target of 150 minutes of moderate-intensity physical activity.44
In addition to physical activity, we found evidence for the use of telerehabilitation in managing fatigue. Even if it has been studied as a specific intervention, telemedicine is a means to propose different therapies, not a treatment per se. Telemedicine has been available for several years,48 but the COVID-19 pandemic has strongly promoted its development and implementation. A recent systematic review confirms that, with the currently available evidence, telerehabilitation can be considered a feasible and safe option to deliver rehabilitation remotely, providing good continuity of care even in pandemics.49
Evidence on the use of self-management and education programs supports the literature promoting patient empowerment to reduce disability and health services utilization (i.e. fewer outpatient visits and hospital admissions).50 These treatments could be appropriate for people with PCC as well.
All the other interventions that showed efficacy for fatigue (acupressure, pulmonary rehabilitation, survivorship programs, transcranial stimulation, walker) or exercise intolerance (NMES as an add-on to exercises) seem highly correlated to the specific diseases studied in each CSR. For now, they could be regarded as a hypothesis for research but not as clinical solutions in PCC patients.
Looking at the indirect evidence provided with this work and at the current direct evidence coming from the rapid living systematic review produced by Cochrane Rehabilitation,51, 52 the experts conveyed by the WHO provided the following conditional recommendations for the clinical rehabilitation management of adults with PCC:43
Post-exertional symptom exacerbation: “education and skills training on energy conservation techniques such as pacing approaches. The provision and training in using assistive products and environmental modifications may be useful for people experiencing moderate to severe PESE.”43
Fatigue: “a combination of education, skills training on energy conservation techniques such as pacing approaches and, in the absence of post-exertional symptom exacerbation, a cautious return to symptom titrated physical exercise training. The provision and training in the use of assistive products and environmental modifications may be considered for people experiencing levels of fatigue that limit instrumental activities of daily living. Psychological support may be offered to support coping with the symptom”.43
Orthostatic intolerance: “a combination of education and skills training on self-management strategies and, in the absence of post-exertional symptom exacerbation, physical exercise training. Environmental modifications may be useful to support activities of daily living for people experiencing difficulties with upright positions or standing”.43
Also, the WHO proposed a strong recommendation related to the red-flag post-exertional symptom exacerbation: “In adults with post COVID-19 condition exertional desaturation and cardiac impairment following COVID-19 should be ruled out and managed before consideration of physical exercise training. While orthostatic intolerance and post-exertional symptom exacerbation (PESE) are amenable to rehabilitation, their presence will require interventions to be modified in view of these diagnoses for rehabilitation to be safe.”43
Strengths and limitations of the study
Our map of CSRs focuses on the best current evidence relevant to rehabilitation for people with PCC. However, other high-quality systematic reviews could not be considered in the selection process because they were not included in the Cochrane Library.
We must interpret the findings carefully because very few CSRs showed moderate-quality evidence, while all the others were very low- or low-quality. In addition, the current results did not provide specific evidence on PCC management. Still, they can partially fill the knowledge gap in rehabilitation and help clinicians find the appropriate treatments for individual patients. These findings can also highlight new research priorities for further studies.
Conclusions
Specific rehabilitation interventions successfully used in different health conditions may improve fatigue and exercise intolerance due to PCC. Future research priorities should include producing new and particular evidence on PCC and improving the methodological quality of primary studies in people with chronic diseases. Further, there is a need for CSRs to consider the symptoms of post-exertional malaise and orthostatic intolerance.
Supplementary Digital Material 1
Supplementary Table I
Characteristics of the studies included on fatigue.
Supplementary Digital Material 2
Supplementary Table II
AMSTAR 2 assessment for reviews on fatigue symptom.
Supplementary Digital Material 3
Supplementary Table III
Summary of included studies on post-exertional malaise symptom.
Supplementary Digital Material 4
Supplementary Table IV
AMSTAR 2 assessment for reviews on post-exertional malaise symptom.
References
- 1.World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. 2021 [Internet]; Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [cited 2022, Jul 1].
- 2.Negrini S, Kiekens C, Cordani C, Arienti C, De Groote W. Cochrane “evidence relevant to” rehabilitation of people with post COVID-19 condition. The concept and methodology of the Cochrane Rehabilitation project for the World Health Organization Rehabilitation Programme. Eur J Phys Rehabil Med 2022;58:853–6. https://doi.org/ 10.23736/S1973-9087.22.07793-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, Ballouz T, et al. ; Global Burden of Disease Long COVID Collaborators. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36215063&dopt=Abstract 10.1001/jama.2022.18931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan AT, Drew DA, Nguyen LH, Joshi AD, Ma W, Guo CG, et al. ; COPE Consortium. The COronavirus Pandemic Epidemiology (COPE) Consortium: A Call to Action. Cancer Epidemiol Biomarkers Prev 2020;29:1283–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32371551&dopt=Abstract 10.1158/1055-9965.EPI-20-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 2021;93:1013–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32729939&dopt=Abstract 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 6.Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond) 2021;21:e63–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33243837&dopt=Abstract 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Bharti S, Garg I. Post COVID fatigue: can we really ignore it? Indian J Tuberc 2022;69:238–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35379408&dopt=Abstract 10.1016/j.ijtb.2021.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol 2021;268:751–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32734353&dopt=Abstract 10.1007/s00415-020-10108-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AL, Burge AT, Holland AE. Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2017;9:CD011699. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28952156&dopt=Abstract 10.1002/14651858.CD011699.pub2 [DOI] [PMC free article] [PubMed]
- 10.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev 2018;1:CD011292. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29376559&dopt=Abstract 10.1002/14651858.CD011292.pub2 [DOI] [PMC free article] [PubMed]
- 11.Bennett S, Pigott A, Beller EM, Haines T, Meredith P, Delaney C. Educational interventions for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2016;11:CD008144. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27883365&dopt=Abstract 10.1002/14651858.CD008144.pub2 [DOI] [PMC free article] [PubMed]
- 12.Cheng KK, Lim YT, Koh ZM, Tam WW. Home-based multidimensional survivorship programmes for breast cancer survivors. Cochrane Database Syst Rev 2017;8:CD011152. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28836379&dopt=Abstract 10.1002/14651858.CD011152.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loughney LA, West MA, Kemp GJ, Grocott MP, Jack S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst Rev 2018;12:CD012280. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30536366&dopt=Abstract 10.1002/14651858.CD012280.pub2 [DOI] [PMC free article] [PubMed]
- 14.Knips L, Bergenthal N, Streckmann F, Monsef I, Elter T, Skoetz N. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev 2019;1:CD009075. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30702150&dopt=Abstract 10.1002/14651858.CD009075.pub3 [DOI] [PMC free article] [PubMed]
- 15.McGettigan M, Cardwell CR, Cantwell MM, Tully MA. Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst Rev 2020;5:CD012864. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32361988&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 16.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;9:CD005001. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27650122&dopt=Abstract 10.1002/14651858.CD005001.pub3 [DOI] [PMC free article] [PubMed]
- 17.Grande AJ, Silva V, Sawaris Neto L, Teixeira Basmage JP, Peccin MS, Maddocks M. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev 2021;3:CD010804. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33735441&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 18.Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev 2017;1:CD010802. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28045199&dopt=Abstract 10.1002/14651858.CD010802.pub2 [DOI] [PMC free article] [PubMed]
- 19.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2017;4:CD003200. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28444695&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 20.Natale P, Ruospo M, Saglimbene VM, Palmer SC, Strippoli GF. Interventions for improving sleep quality in people with chronic kidney disease. Cochrane Database Syst Rev 2019;5:CD012625. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31129916&dopt=Abstract 10.1002/14651858.CD012625.pub2 [DOI] [PMC free article] [PubMed]
- 21.Voet NB, van der Kooi EL, van Engelen BG, Geurts AC. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev 2019;12:CD003907. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31808555&dopt=Abstract 10.1002/14651858.CD003907.pub5 [DOI] [PMC free article] [PubMed]
- 22.Bidonde J, Busch AJ, Schachter CL, Webber SC, Musselman KE, Overend TJ, et al. Mixed exercise training for adults with fibromyalgia. Cochrane Database Syst Rev 2019;5:CD013340. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31124142&dopt=Abstract 10.1002/14651858.CD013340 [DOI] [PMC free article] [PubMed]
- 23.Kim SY, Busch AJ, Overend TJ, Schachter CL, van der Spuy I, Boden C, et al. Flexibility exercise training for adults with fibromyalgia. Cochrane Database Syst Rev 2019;9:CD013419. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31476271&dopt=Abstract 10.1002/14651858.CD013419 [DOI] [PMC free article] [PubMed]
- 24.Bidonde J, Busch AJ, van der Spuy I, Tupper S, Kim SY, Boden C. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst Rev 2017;9:CD011755. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28950401&dopt=Abstract 10.1002/14651858.CD011755.pub2 [DOI] [PMC free article] [PubMed]
- 25.Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Góes SM, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev 2017;6:CD012700. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28636204&dopt=Abstract 10.1002/14651858.CD012700 [DOI] [PMC free article] [PubMed]
- 26.Farrell D, Artom M, Czuber-Dochan W, Jelsness-Jørgensen LP, Norton C, Savage E. Interventions for fatigue in inflammatory bowel disease. Cochrane Database Syst Rev 2020;4:CD012005. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32297974&dopt=Abstract 10.1002/14651858.CD012005.pub2 [DOI] [PMC free article] [PubMed]
- 27.Rietberg MB, Veerbeek JM, Gosselink R, Kwakkel G, van Wegen EE. Respiratory muscle training for multiple sclerosis. Cochrane Database Syst Rev 2017;12:CD009424. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29267988&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 28.Amatya B, Young J, Khan F. Non-pharmacological interventions for chronic pain in multiple sclerosis. Cochrane Database Syst Rev 2018;12:CD012622. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30567012&dopt=Abstract 10.1002/14651858.CD012622.pub2 [DOI] [PMC free article] [PubMed]
- 29.Burge AT, Cox NS, Abramson MJ, Holland AE. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2020;4:CD012626. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32297320&dopt=Abstract 10.1002/14651858.CD012626.pub2 [DOI] [PMC free article] [PubMed]
- 30.Malaguti C, Dal Corso S, Janjua S, Holland AE. Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2021;8:CD013569. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34404111&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 31.Hassett L, Moseley AM, Harmer AR. Fitness training for cardiorespiratory conditioning after traumatic brain injury. Cochrane Database Syst Rev 2017;12:CD006123. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29286534&dopt=Abstract 10.1002/14651858.CD006123.pub3 [DOI] [PMC free article] [PubMed]
- 32.Cavalheri V, Burtin C, Formico VR, Nonoyama ML, Jenkins S, Spruit MA, et al. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev 2019;6:CD009955. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31204439&dopt=Abstract 10.1002/14651858.CD009955.pub3 [DOI] [PMC free article] [PubMed]
- 33.Peddle-McIntyre CJ, Singh F, Thomas R, Newton RU, Galvão DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev 2019;2:CD012685. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30741408&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 34.Stevenson JK, Campbell ZC, Webster AC, Chow CK, Tong A, Craig JC, et al. eHealth interventions for people with chronic kidney disease. Cochrane Database Syst Rev 2019;8:CD012379. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31425608&dopt=Abstract 10.1002/14651858.CD012379.pub2 [DOI] [PMC free article] [PubMed]
- 35.Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev 2021;1:CD013040. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33511633&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 36.Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev 2017;11:CD002768. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29090734&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 37.Moran F, Bradley JM, Piper AJ. Non-invasive ventilation for cystic fibrosis. Cochrane Database Syst Rev 2017;2:CD002769. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28218802&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 38.Dowman L, Hill CJ, May A, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021;2:CD006322. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34559419&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 39.Hill K, Cavalheri V, Mathur S, Roig M, Janaudis-Ferreira T, Robles P, et al. Neuromuscular electrostimulation for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018;5:CD010821. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29845600&dopt=Abstract 10.1002/14651858.CD010821.pub2 [DOI] [PMC free article] [PubMed]
- 40.Dennett EJ, Janjua S, Stovold E, Harrison SL, McDonnell MJ, Holland AE. Tailored or adapted interventions for adults with chronic obstructive pulmonary disease and at least one other long-term condition: a mixed methods review. Cochrane Database Syst Rev 2021;7:CD013384. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34309831&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 41.Gendron LM, Nyberg A, Saey D, Maltais F, Lacasse Y. Active mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018;10:CD012290. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30306545&dopt=Abstract 10.1002/14651858.CD012290.pub2 [DOI] [PMC free article] [PubMed]
- 42.Poot CC, Meijer E, Kruis AL, Smidt N, Chavannes NH, Honkoop PJ. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2021;9:CD009437. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34495549&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 43.World Health Organization. Clinical management of COVID-19: living guideline, 15 September 2022. Geneva: World Health Organization; 2022. [PubMed] [Google Scholar]
- 44.Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. ESC National Cardiac Societies ; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. [Erratum in: Eur Heart J 2022 Sep 09] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34458905&dopt=Abstract 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 45.Singh SJ, ZuWallack RL, Garvey C, Spruit MA, American Thoracic Society/European Respiratory Society Task Force on Pulmonary Rehabilitation . Learn from the past and create the future: the 2013 ATS/ERS statement on pulmonary rehabilitation. Eur Respir J 2013;42:1169–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24178930&dopt=Abstract 10.1183/09031936.00207912 [DOI] [PubMed] [Google Scholar]
- 46.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 2019;51:2375–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31626055&dopt=Abstract 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y, Lai B, Mehta T, Thirumalai M, Padalabalanarayanan S, Rimmer JH, et al. Exercise Training Guidelines for Multiple Sclerosis, Stroke, and Parkinson Disease: Rapid Review and Synthesis. Am J Phys Med Rehabil 2019;98:613–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30844920&dopt=Abstract https://doi.org/ 10.1097/PHM.0000000000001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil 2017;31:625–38. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27141087&dopt=Abstract 10.1177/0269215516645148 [DOI] [PubMed] [Google Scholar]
- 49.Brigo E, Rintala A, Kossi O, Verwaest F, Vanhoof O, Feys P, et al. Using Telehealth to Guarantee the Continuity of Rehabilitation during the COVID-19 Pandemic: A Systematic Review. Int J Environ Res Public Health 2022;19:10325. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36011959&dopt=Abstract 10.3390/ijerph191610325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rochfort A, Beirne S, Doran G, Patton P, Gensichen J, Kunnamo I, et al. Does patient self-management education of primary care professionals improve patient outcomes: a systematic review. BMC Fam Pract 2018;19:163. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30268092&dopt=Abstract 10.1186/s12875-018-0847-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceravolo MG, de Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid “living” review on rehabilitation needs due to COVID-19: update to March 31st, 2020. Eur J Phys Rehabil Med 2020;56:508–14. 10.23736/S1973-9087.20.06329-7 [DOI] [PubMed] [Google Scholar]
- 52.Ceravolo MG, Andrenelli E, Arienti C, Côté P, de Sire A, Iannicelli V, et al. Rehabilitation and COVID-19: rapid living systematic review by Cochrane Rehabilitation Field - third edition. Update as of June 30th, 2021. Eur J Phys Rehabil Med 2021;57:850–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34749491&dopt=Abstract 10.23736/S1973-9087.21.07301-9 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Characteristics of the studies included on fatigue.
Supplementary Table II
AMSTAR 2 assessment for reviews on fatigue symptom.
Supplementary Table III
Summary of included studies on post-exertional malaise symptom.
Supplementary Table IV
AMSTAR 2 assessment for reviews on post-exertional malaise symptom.