Abstract
Tirzepatide is a novel glucose‐dependent insulinotropic polypeptide/glucagon‐like peptide 1 (GLP‐1) receptor agonist approved in the United States as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes and under investigation for use in chronic weight management, major adverse cardiovascular events and the management of other conditions, including heart failure with preserved ejection fraction and obesity and non‐cirrhotic non‐alcoholic steatohepatitis. The Phase 3 SURPASS 1‐5 clinical trial programme was designed to assess efficacy and safety of once‐weekly subcutaneously injected tirzepatide (5, 10 and 15 mg), as monotherapy or combination therapy, across a broad spectrum of people with type 2 diabetes. Use of tirzepatide in clinical studies was associated with marked reductions of glycated haemoglobin (−1.87 to −2.59%, −20 to −28 mmol/mol) and body weight (−6.2 to −12.9 kg), as well as reductions in parameters commonly associated with heightened cardiometabolic risk such as blood pressure, visceral adiposity and circulating triglycerides. In SUPRASS‐2, these reductions were greater than with the GLP‐1 receptor agonist semaglutide 1 mg. Tirzepatide was well tolerated, with a low risk of hypoglycaemia when used without insulin or insulin secretagogues and showed a generally similar safety profile to the GLP‐1 receptor agonist class. Accordingly, evidence from these clinical trials suggests that tirzepatide offers a new opportunity for the effective lowering of glycated haemoglobin and body weight in adults with type 2 diabetes.
1. INTRODUCTION
Type 2 diabetes is characterized by a multiplicity of pathophysiological components, which includes insulin resistance, defective insulin secretion, adiposity, decreased incretin effect, increased glucagon secretion and dyslipidaemia. 1 , 2 , 3 , 4 , 5 Consequently, type 2 diabetes is more than a challenge of hyperglycaemia. For example, a retrospective study of more than one million adults with type 2 diabetes in the United States [median age of 65 years, diabetes duration of 4 years and glycated haemoglobin (HbA1c 6.8%)] reported that 82% had hypertension, 78% had obesity or overweight, 24% had chronic kidney disease and 22% cardiovascular disease. 6 Furthermore, a meta‐analysis of observational studies from 20 countries reported that the global prevalence of non‐alcoholic fatty liver disease among people with type 2 diabetes was 56% and the prevalence of non‐alcoholic steatohepatitis was 37%. 7
Obesity is the strongest risk factor for type 2 diabetes, 8 , 9 a major contributor to insulin resistance, 2 , 10 and is involved in the pathophysiology of hypertension, dyslipidaemia and non‐alcoholic fatty liver disease. 11 , 12 Given their intertwined pathophysiology, body weight loss can have beneficial effects on glycaemic control, insulin sensitivity and comorbidities. 10 , 13 The American Diabetes Association (ADA) recommends weight loss of at least 5% through diet, physical activity and behavioural therapy for most people with type 2 diabetes who have overweight or obesity. 14 Greater weight loss may offer the possibility of reversing the metabolic abnormalities of type 2 diabetes resulting in improvement of glycaemia up to the achievement of diabetes remission. 15 , 16 , 17 , 18 , 19 The possibility of achieving remission of type 2 diabetes associated with weight loss was reported in several studies evaluating different types of intervention (lifestyle changes, medication, bariatric surgery, or a combination of those), having different designs and conducted in different settings. The exact definitions of remission, based generally on the ability to maintain non‐diabetes glycaemia without glucose‐lowering treatment, differed between these studies. Nonetheless, the beneficial effects of greater weight loss on glycaemia reinforce the importance of weight management for people with type 2 diabetes. 19 Modest weight loss of at least 5% can improve cardiovascular risk factors such as blood pressure and lipids, 14 , 20 , 21 and weight losses of ≥7% may improve non‐alcoholic fatty liver disease. 22 , 23
Although several classes of glucose‐lowering agents are available as treatments for type 2 diabetes, half to three‐quarters of people may not meet individualized glycaemic targets. 24 , 25 , 26 , 27 , 28 With lifestyle intervention in the trial setting, as few as two‐fifths of participants achieve modest weight loss of ≥5% or ≥7% in the first year. 29 , 30 , 31 There is currently a need for more effective therapies to enable people to achieve glycaemic control, address the metabolic disorders associated with type 2 diabetes, and meet more ambitious weight loss targets, as part of individualized treatment plans.
Two gut‐derived incretin hormones, glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide 1 (GLP‐1) are secreted in response to nutrients, mediate the incretin effect and facilitate glycaemic control (Figure 1). 32 This effect is diminished in people with type 2 diabetes but can be partially restored with glucose‐lowering interventions. 33 , 34 , 35 , 36 , 37 GLP‐1 receptor agonists can improve glycaemic control and reduce body weight in people with type 2 diabetes through enhancement of glucose‐stimulated insulin secretion, reduced food intake, inhibition of glucagon secretion in hyperglycaemic or euglycaemic states, and delayed gastric emptying. 33 , 38 , 39 , 40 , 41 , 42 , 43 , 44 GIP also enhances glucose‐stimulated insulin secretion in people without type 2 diabetes whereas this effect is diminished in people with type 2 diabetes, but unlike GLP‐1, it stimulates glucagon secretion in hypoglycaemic states. 45 , 46 , 47 , 48 , 49 , 50 , 51 In normal physiology, these two incretin hormones exert relatively short‐lived effects at their respective receptors because of their rapid degradation by the dipeptidyl peptidase‐4 enzyme and a resulting half‐life of minutes. 52 Tirzepatide is a single modified peptide with GIP and GLP‐1 receptor agonism approved for treatment of people with type 2 diabetes in the United States and under investigation for its effects on chronic weight management, heart failure with preserved ejection fraction and obesity, major adverse cardiovascular events (MACE) and non‐cirrhotic non‐alcoholic steatohepatitis (NASH).
FIGURE 1.
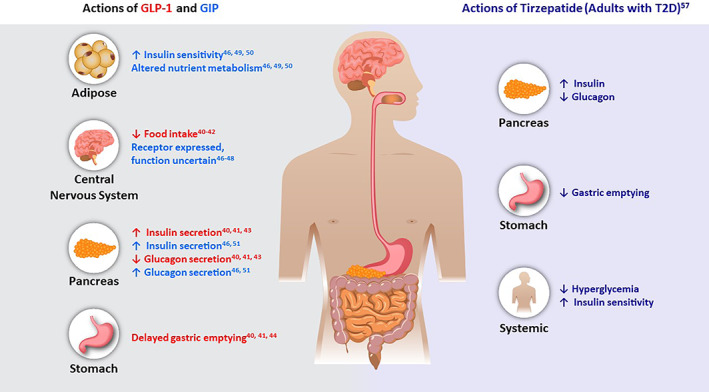
Gluco‐regulatory actions of GIP and GLP‐1 proposed based on preclinical and clinical studies, and actions of tirzepatide in adults with type 2 diabetes. GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1; T2D, type 2 diabetes
1.1. Tirzepatide molecule overview
Tirzepatide is a single modified GIP/GLP‐1 receptor agonist engineered from the GIP sequence to also have GLP‐1 activity. Tirzepatide was designed to achieve GIP receptor affinity comparable with that of native GIP and to bind the GLP‐1 receptor with approximately 5‐fold weaker affinity than native GLP‐1 (Panel). 53 The molecule is a 39 amino acid linear peptide that includes a C20 fatty diacid moiety and has a half‐life of approximately 5 days to enable once‐weekly subcutaneous injection. 54 In preclinical models, tirzepatide engages the GLP‐1 receptor as a biased agonist signalling towards the generation of cyclic adenosine monophosphate (cAMP) with reduced recruitment of β‐arrestin potentially leading to diminished GLP‐1 receptor internalization. 53 These experiments suggest this mechanism may enable sustained signalling of tirzepatide at the GLP‐1 receptor potentially contributing to a greater insulinotropic effect at pancreatic beta cells. 53 However, the clinical implications of biased agonism remain unclear.
Clinical studies showed that the effects of tirzepatide on glycaemic control are underpinned by concurrent improvements in β‐cell function, insulin sensitivity and α‐cell function. 55 , 56 , 57 Tirzepatide (15 mg) significantly improved first phase, second phase, and total insulin secretion and insulin sensitivity. 57 In meal tolerance testing, tirzepatide also reduced fasting and meal‐stimulated glucagon secretion. 57 Evidence from studies in mice suggest that the improvements in insulin resistance with tirzepatide are both weight dependent and independent. 58 Initial studies in humans indicate that weight loss may only partly account for improvement in insulin sensitivity and that tirzepatide may provide greater improvement in insulin sensitivity than a selective GLP‐1 receptor agonist per unit weight loss, with this effect being most evident in those with greater weight loss. 55 , 59
Additional results from mechanism of action studies indicate that tirzepatide reduced energy intake and may reduce appetite, as assessed via a visual analogue scale. 60 , 61 However, these reductions did not differ from the GLP‐1 receptor agonist semaglutide. 61
PANEL. PRECLINICAL CHARACTERIZATION, PHARMACOKINETICS AND PHARMACODYNAMICS OF TIRZEPATIDE
Tirzepatide binds with high affinity to human GLP‐1 and GIP receptors expressed on transfected HEK293 cells. 54
Binding affinity [Ki +/−SEM (nM)]
GIP receptors: 0.135 +/−0.020
GLP‐1 receptors: 4.23 +/−0.23
Tirzepatide potently stimulates cAMP accumulation by human GLP‐1 and GIP receptors expressed on transfected HEK293 cells. 54
Intracellular cAMP accumulation [EC50 +/−SEM (nM)]
GIP receptors: 0.0224 +/−0.0053
GLP‐1 receptors: 0.934 +/−0.068
Tirzepatide stimulated cAMP accumulation in differentiated human adipocytes that express GIP receptors but not GLP‐1 receptors. The effect was comparable with that of GIP alone. 54
Pharmacokinetics below are average values from healthy single ascending dose cohorts administered 0.25‐8.0 mg doses subcutaneously. Pharmacokinetics in healthy participants are comparable with those with type 2 diabetes 54
- Geometric mean maximum observed drug concentration (C max) for 5.0 mg: 397 ng/ml
- Intersubject variability for C max ≤30% across doses
t 1/2: ~5 days
CL/F: 0.056 L/h
Vz/F: 9.5 L
Pharmacokinetics appear dose proportional, C max reached within 24‐48 h post‐dose.
Average accumulation following four weekly doses: 1.58.
Tirzepatide delays gastric emptying; e greatest after 1 dose and undergoes tachyphylaxis with repeated once‐weekly dosing. 64
Intrinsic factors
no clinically meaningful effect of renal or hepatic impairment 62 , 63 ;
dose adjustment may not be required in patients with renal impairment;
dose adjustment may not be required in patients with hepatic impairment.
Binding affinity and cAMP potency data are mean +/−SEM, C max, Cl/F and Vz/F are geometric mean (% CV). Where, CL/F, apparent total body clearance of drug following subcutaneous administration; T1/2, half‐life associated with the terminal rate constant in non‐compartmental analysis; Vz/F, apparent volume of distribution of drug during terminal phase following subcutaneous administration.
1.2. Dosing and administration
The preclinical characterization, clinical pharmacokinetics and pharmacodynamics of tirzepatide are presented in the Panel. Tirzepatide pharmacokinetics were similar in participants with renal impairment (lowest estimated glomerular filtration rate category: end stage renal disease (ESRD)) or hepatic [up to severe (class C) on Child‐Pugh score] impairment compared with healthy subjects, indicating that dose adjustment may not be required for these groups. 62 , 63 Tirzepatide also delays gastric emptying with this effect diminishing over time. 64 An approximate 20% reduction in the overall exposure of oral contraceptives was observed following the administration of a single 5 mg dose of tirzepatide. 65 These results were observed in a study conducted at a point where the effect on gastric emptying was maximal, namely following a single 5 mg dose. 65 This study reflects the effect of a single 5 mg dose of tirzepatide on oral contraceptive absorption and does not take into account tachyphylaxis observed with repeated dosing. 65 A gastric emptying delay is also observed in patients taking other GLP‐1 receptor agonists and the effect diminishes over time. 66 , 67 , 68 , 69 , 70 , 71 , 72 The dosing recommendation for tirzepatide is to initiate treatment at 2.5 mg and escalate in 2.5 mg doses at 4‐week intervals. 65
In the Phase 3 studies, as in the approved prescribing information, the tirzepatide initiating dose was 2.5 mg once weekly and tirzepatide was increased by 2.5 mg every 4 weeks. In the Phase 3 studies, this was done until the target dose (5, 10 or 15 mg) was reached (Figure 2). The prescribing information indicates that the 2.5 mg dose is for treatment initiation and is not intended for glycaemic control. 65 This gradual dose escalation scheme was informed by earlier studies that indicated a low starting dose with small dose increments improved tolerability and was associated with a more favourable side‐effect profile. 77 , 78
FIGURE 2.
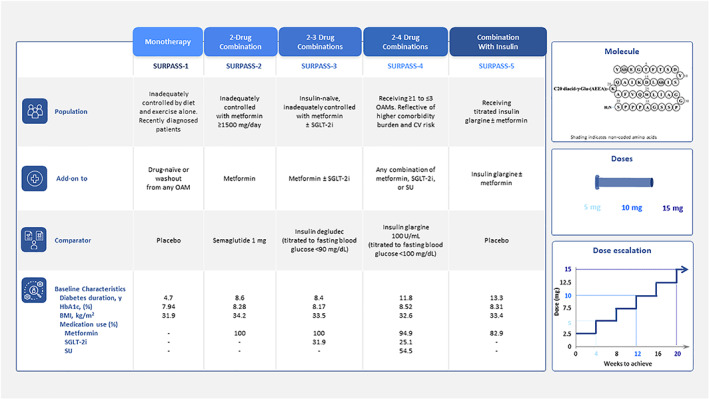
Overview of the Phase 3 SURPASS 1‐5 clinical programme 56 , 73 , 74 , 75 , 76 presenting population, baseline therapeutics, comparators and key baseline demographics for SURPASS 1‐5 reflecting the progression of disease; molecule structure (tirzepatide is a 39 amino acid synthetic peptide, GIP/GLP‐1 receptor agonist conjugated to a C20 fatty diacid moiety); and dose esclation sheme (doses initiated at 2.5 mg once weekly and increased by 2.5 mg every 4 weeks until assigned dose was reached and maintained for duration of trial). BMI, body mass index; CV, cardiovascular; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1; HbA1c, glycosylated haemoglobin A1c; OAM, oral antihyperglycaemic medication; SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea
1.3. Design of the SURPASS 1‐5 clinical programme
The Phase 3 SURPASS 1‐5 clinical trial programme was designed to capture a spectrum of patient populations reflective of the disease continuum and differing treatment options seen within clinical practice for type 2 diabetes. 56 , 73 , 74 , 75 , 76
Figure 2 provides an overview of the SURPASS 1‐5 studies. In addition to HbA1c measurement, SURPASS‐3 included a continuous glucose monitoring substudy to evaluate 24‐hour glucose profiles, as well as a magnetic resonance imaging (MRI) substudy to examine the effect of treatment on liver fat content and other measures, such as visceral fat. 79 , 80 The criteria for increased cardiovascular risk in SURPASS‐4 were known coronary, peripheral arterial or cerebrovascular disease, or aged ≥50 years with either history of chronic kidney disease and an estimated glomerular filtration rate of <60 ml/min per 1.73 m2 or history of congestive heart failure (New York Heart Association Class II or III). 75
As different populations were recruited to reflect the type 2 diabetes disease continuum, baseline characteristics (such as diabetes duration and mean HbA1c) varied across the studies (Figure 2).
Data presented here are from the efficacy estimand of each study, which represents on‐treatment efficacy without the influence of rescue therapy. Results from the efficacy estimand are generally aligned with those from the treatment‐regimen estimand, which represents efficacy irrespective of adherence to study drug or initiation of rescue therapy. 56 , 73 , 74 , 75 , 76
1.3.1. Study durations
Study durations of 40 weeks (SURPASS‐1, ‐2 and ‐5) and 52 weeks (SURPASS‐3) allowed for the gradual dose escalation scheme of up to 20 weeks to reach the 15 mg dose and a maintenance period to assess therapeutic efficacy of the 15 mg dose for periods up to 32 weeks. In SURPASS‐4, the primary endpoint was at 52 weeks with treatment continued to a maximum of 104 weeks for some participants.
1.3.2. Study comparators
Comparators included placebo (SURPASS‐1 and ‐5), the GLP‐1 receptor agonist semaglutide 1 mg, which was the highest available dose at trial initiation (SURPASS‐2), and titrated basal insulins, insulin degludec 100 U/ml (SURPASS‐3) and insulin glargine 100 U/ml (SURPASS‐4).
1.3.3. Insulin use in SURPASS‐3, ‐4 and ‐5
In SURPASS‐3 and ‐4, the basal insulin comparators were titrated using well‐established treat‐to‐target algorithms to reach a prespecified fasting blood glucose value. 74 , 75 The HbA1c change from baseline results in the insulin arms using this treat‐to‐target algorithm were comparable with those disclosed in Phase 3 trials. 81 , 82 , 83 The mean daily use at Week 52 was 48.8 U for insulin degludec and 43.5 U for insulin glargine 100 U/ml. In SURPASS‐5, background daily insulin glargine 100 U/ml (mean baseline: 0.4 U/kg/day) could be adjusted to maintain a target fasting blood glucose using self‐monitored blood glucose of <100 mg/dl. Whereas participants taking insulin glargine alone had an increase of 25.1 U (75%) from baseline at Week 40, participants taking tirzepatide required significantly less insulin [4.4 to −3.8 U (13% to −11%)]. 76 Of note, participants could lower insulin use but, per protocol, could not discontinue insulin therapy.
2. EFFICACY
2.1. Glycaemic efficacy
The primary endpoint for all five studies was change from baseline in HbA1c at either 40 or 52 weeks, with a baseline HbA1c of 7.94% to 8.52% (63‐70 mmol/mmol). 56 , 73 , 74 , 75 , 76 In all five studies, tirzepatide was associated with mean reductions from baseline in HbA1c ranging from −1.87% to −2.59% (−20 to −28 mmol/mol) (Figure 3A). These reductions were dose dependent and significantly greater with tirzepatide 5, 10 and 15 mg than placebo (0.04%, SURPASS‐1), semaglutide 1 mg (−1.86%, SURPASS‐2), insulin degludec (−1.34%, SURPASS‐3), insulin glargine 100 U/ml (−1.44%, SURPASS‐4) and placebo with background insulin (−0.93%, SURPASS‐5). Data from SURPASS‐4 indicate that HbA1c reductions were maintained at 78 and 104 weeks, although participant numbers were smaller at the latter time point, providing evidence for sustained glycaemic control with tirzepatide treatment beyond 1 year. 75 Across the five studies, the magnitude of HbA1c reductions achieved were greatest in SURPASS‐4 and ‐5, probably reflecting higher baseline HbA1c in these studies. In SURPASS‐1, the less pronounced dose‐response could reflect the relatively early course of type 2 diabetes and potentially more β‐cell function in this population, as all three tirzepatide doses led to near‐normoglycaemia at 40 weeks and presumably reaching a floor effect (mean HbA1c of 5.9%‐6.1%; 41‐43 mmol/mol).
FIGURE 3.
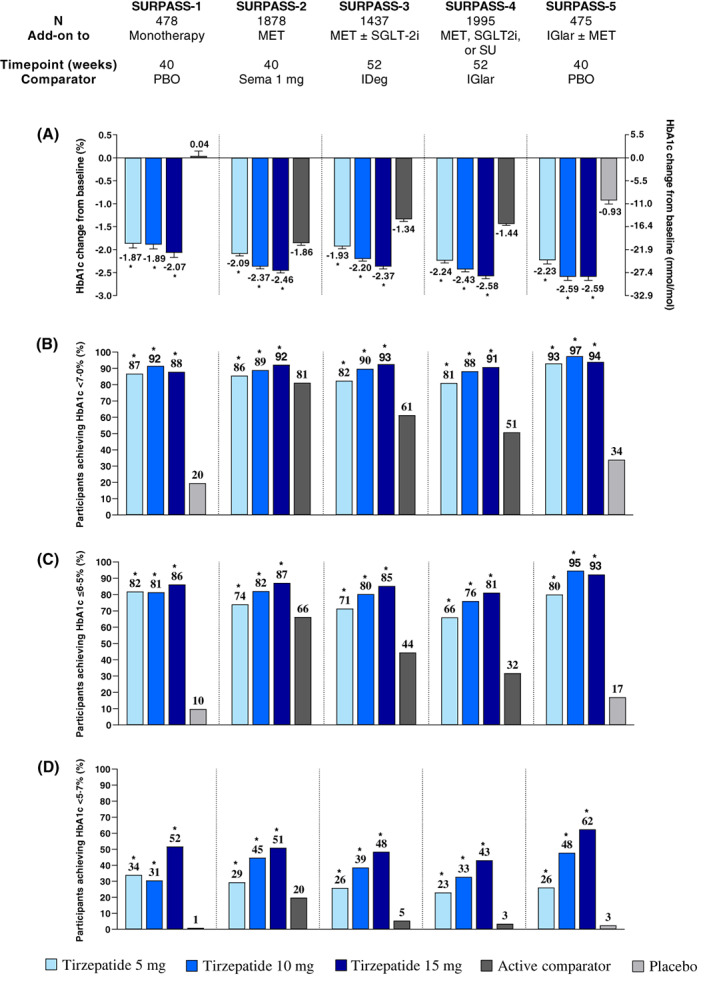
Glycaemic efficacy of tirzepatide in SURPASS 1‐5. 56 , 75 , 76 , 77 , 78 , 87 Data are estimated mean (SE) or percentage and from the modified intention‐to‐treat population (efficacy analysis set) of each study. (A) HbA1c change from baseline to the primary study endpoint; proportion of participants achieving HbA1c targets (B) <7.0%, (C) ≤6.5% and (D) <5.7%. *p < .05 versus placebo or active comparator. HbA1c, glycosylated haemoglobin A1c; IDeg, insulin degludec; IGlar, insulin glargine 100 U/ml; MET, metformin; N, number of patients who were randomly assigned and received at least one dose of study drug; PBO, placebo; Sema, semaglutide; SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea
Corresponding with the marked HbA1c reductions from baseline, substantial proportions of tirzepatide‐treated participants in each study achieved an HbA1c of <7.0% (53 mmol/mol) and ≤6.5% (48 nmol/mol), corresponding to treatment guideline recommendations for most people by the ADA and European Association for the Study of Diabetes (EASD). 84 , 85 Across the five trials, 81%‐97% of participants receiving tirzepatide achieved HbA1c <7% (53 mmol/mol) and 66%‐95% HbA1c ≤6.5% (48 mmol/mol) (Figure 3B,C). 56 , 73 , 74 , 75 , 76 For all doses and both treatment goals, these results were significantly greater compared with either placebo, semaglutide 1 mg, insulin degludec, insulin glargine 100 U/ml, or placebo with background insulin. More participants who received tirzepatide also achieved HbA1c <5.7% (39 mmol/mol) compared with all comparators (Figure 3D). 56 , 73 , 74 , 75 , 76
In SURPASS‐2, a composite endpoint assessing the proportion of participants who achieved HbA1c ≤6.5% (48 mmol/mol) without clinically significant (<54 mg/dl) or severe hypoglycaemia and with ≥10% weight loss was met by 32%‐60% of participants who received tirzepatide compared with 22% who received semaglutide 1 mg. 56
Fasting serum glucose (FSG) was significantly reduced by all tirzepatide doses in SURPASS‐1, ‐2 and ‐5 at either 40 or 52 weeks compared with placebo, semaglutide 1 mg and placebo with background insulin, respectively. 56 , 73 , 76 FSG levels at endpoint did not differ from insulin degludec in the 10‐ and 15‐mg groups in SURPASS‐3 and in SURPASS‐4 did not differ from insulin glargine 100 U/ml in the 5‐ and 10‐mg groups but were significantly lower in the 15‐mg group (Figure S1). In SURPASS‐3 and ‐4, significant reductions in FSG compared with baseline were apparent as early as 2 weeks (the earliest measurement) after treatment initiation when all participants received 2.5 mg. 74 , 75 Across the two studies and three dose groups, the magnitude of this change at 2 weeks was −30.3 to −34.0 mg/dl (−18% to −20%). In a small Phase 1 study, 24 h following treatment with a 2.5 mg initiating dose of tirzepatide, there was a non‐statistically significant (vs. placebo) decrease from baseline of –19 mg/dl in FSG. However, on Day 8 (pre‐dose) a statistically significant (vs. placebo) reduction of −39 mg/dl occurred, indicating early glucose‐lowering potential following tirzepatide initiation. 86
Overall daily mean, premeal daily mean and 2‐h postmeal daily mean measured during 7‐point self‐monitored blood glucose profiles indicate that tirzepatide enabled participants to maintain a significantly lower blood glucose level throughout the day in all five studies at 40 or 52 weeks. 56 , 73 , 74 , 75 , 76
In addition to the significant reductions in HbA1c and FSG, tirzepatide treatment significantly increased (to 73%) the proportion of a 24‐h period spent within a tight target glucose range (71‐140 mg/dl, 3.9‐7.8 mmol/L) compared with insulin degludec treatment (48%) at 52 weeks. 79 This time spent in tight target glucose range was also accompanied with a smaller within‐day glucose coefficient of variation (CV %) and significant reduction in CV% compared with insulin degludec, indicating low glycaemic variability with tirzepatide.
2.2. Weight loss
Across the five SURPASS studies and the spectrum of people with type 2 diabetes represented in them, the mean baseline body weight ranged from 86 to 95 kg. 56 , 73 , 74 , 75 , 76 Tirzepatide showed clinically meaningful reductions in body weight and a significantly higher proportion of participants achieving 5%, 10% and 15% body weight loss targets versus placebo and active comparators. 56 , 73 , 74 , 75 , 76
The average weight loss ranged from 6.2 to 12.9 kg across the three dose groups for all five studies. Weight loss followed an approximately dose proportional pattern where tirzepatide 5 mg was associated with 6.2 to 7.8 kg loss, 10 mg with 7.8 to 10.7 kg loss and for 15 mg 9.5 to 12.9 kg loss (Figure 4A). 56 , 73 , 74 , 75 , 76 All tirzepatide doses significantly reduced body weight compared with semaglutide 1 mg, and weight loss with tirzepatide 15 mg (−12.4 kg) was double that with semaglutide 1 mg (−6.2 kg). 56 Body weight reductions were also evident when tirzepatide was combined with therapies associated with weight gain, such as insulin or sulphonylureas. 39 , 75 , 76 , 85
FIGURE 4.
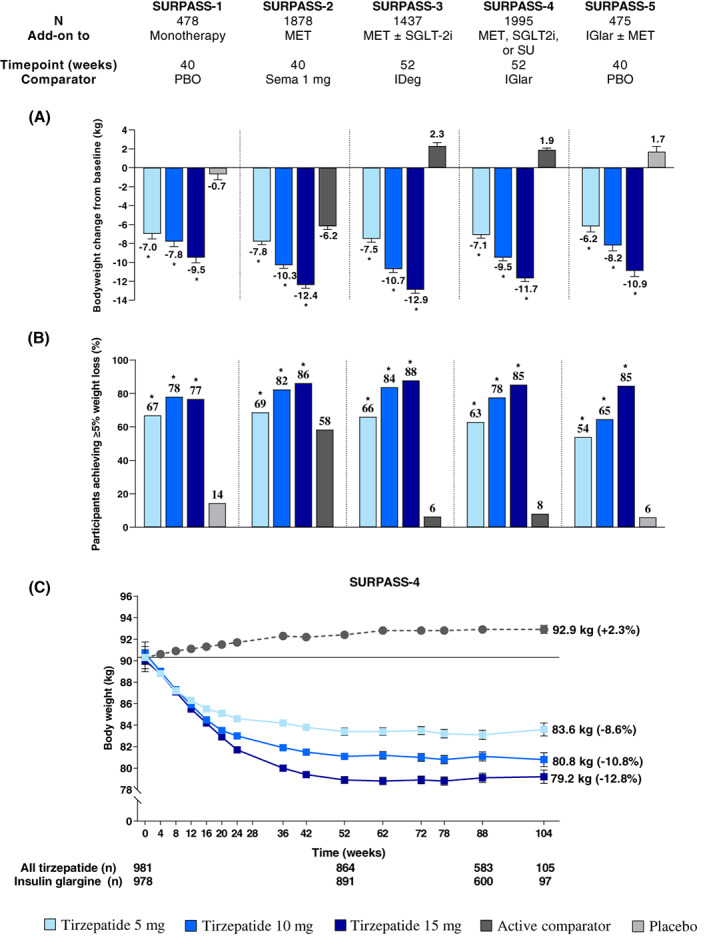
Body weight loss with tirzepatide in SURPASS 1‐5. 56 , 73 , 74 , 75 , 76 , 87 Data are estimated mean (SE) or percentage and from the modified intention‐to‐treat population (efficacy analysis set) of each study. (A) Body weight change from baseline to the primary study endpoint; (B) proportion of participants achieving ≥5% weight loss at the primary study endpoint; (C) body weight change from baseline over time in SURPASS‐4. Solid line indicates baseline values. *p < .05 versus placebo or active comparator. IDeg, insulin degludec; IGlar, insulin glargine 100 U/ml; MET, metformin; N, number of patients who were randomly assigned and received at least one dose of study drug; PBO, placebo; Sema, semaglutide; SGLT‐2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea
Up to 88% of participants who were assigned tirzepatide 15 mg reached at least 5% weight loss from baseline at Week 52, where the average starting weight was 94.3 kg and 32% were being treated with metformin plus an sodium‐glucose cotransporter‐2 (SGLT2) inhibitor (range 77%‐88% for SURPASS 1‐5) (Figure 4B). 56 , 73 , 74 , 75 , 76 Among participants who received tirzepatide 15 mg, at least 10% weight loss was achieved by up to 69% (range 47%‐69%), and at least 15% weight loss was achieved by up to 43% (range 27%‐43%) (Figure S2A,B). 56 , 73 , 74 , 75 , 76 The proportion of participants assigned to tirzepatide who reached these weight loss targets was consistently higher compared with semaglutide 1 mg (Figures 4B and S2A,B). 56 , 87 In tirzepatide‐treated participants who used background insulin, 7% to 85% reached the weight loss targets of at least 5%, 10% or 15% (Figures 4B and S2A,B). 76
Weight reduction with tirzepatide began during the dose‐escalation phase as early as 4 weeks after treatment initation and continued without appearing to plateau by the primary study endpoints at 40 or 52 weeks. 56 , 73 , 74 , 75 , 76 In SURPASS‐4, with a variable treatment period up to 104 weeks, weight loss reached a plateau at about 1 year and was sustained for up to 18 months to 2 years (Figure 4C). 75
2.3. Cardiometabolic measures
The substantial reductions in body weight achieved with tirzepatide were accompanied by significant reductions in waist circumference compared with placebo or active comparator in each study. 56 , 73 , 74 , 75 , 76 For example, in SURPASS‐2 waist circumference decreased by 6.9 to 9.9 cm with tirzepatide and 5.6 cm with semaglutide 1 mg at Week 40 from a mean baseline waist circumference of 109.3 cm. 56 Similarly in SURPASS‐4, a reduction in waist circumference was observed to 52 weeks in the tirzepatide arms and this was sustained through to 104 weeks, although participant numbers gradually decreased beyond 52 weeks. 75
Regarding lipid profile changes, at 40 weeks in SURPASS‐2, triglyceride levels were lower in those who received tirzepatide (−19.0% to −24.8%) than semaglutide 1 mg (−11.5%) and high‐density lipoprotein cholesterol levels were higher (6.8%‐7.9% vs. 4.4%). 56 Changes in low‐density lipoprotein cholesterol (−5.2% to −7.7% vs. −6.4%) and total cholesterol (−5.5% to −6.3% vs −4.8%) levels were similar between tirzepatide and semaglutide 1 mg. In the high cardiovascular risk population of SURPASS‐4, all tirzepatide arms reduced triglycerides (−16.3% to −22.5% vs. −6.4%), low‐density lipoprotein cholesterol (−6.8% to −8.3% vs 1.4%) and total cholesterol (−5.1% to −5.6% vs. 0%) and increased high‐density lipoprotein cholesterol (6.7%‐10.8% vs. 2.9%) compared with baseline and insulin glargine 100 U/ml. 75 These improvements occurred on top of background lipid‐lowering therapy use, which 82% used at baseline with little change throughout the study. 75
Normal liver fat content is generally considered to be <5%, although this varies depending on the measurement method used. 88 A ≥30% relative reduction in liver fat assessed by MRI‐proton density fat fraction (PDFF) has been associated with improvement in NASH. 89 In a meta‐analysis, people with a ≥30% relative decline in MRI‐PDFF were more likely to have a histological response (51% vs. 14%, p < .001) and NASH resolution (41% vs. 7%; p < .001) than those with a smaller decline. 90 In people with type 2 diabetes, elevated liver fat content and increased visceral fat have been associated with an increased risk of cardiometabolic complications. 91 , 92 In the MRI substudy of SURPASS‐3, tirzepatide reduced liver fat content (measured by MRI‐PDFF) to a significantly greater extent than insulin degludec (−8.09% vs. −3.38%) at Week 52. 80 More tirzepatide‐treated participants achieved a liver fat content of ≤10% (60%‐78% vs. 35%) and a relative decrease in liver fat content from baseline of ≥30% (67%‐81% vs. 32%). In addition, up to 48% of participants who received tirzepatide reached a liver fat content <6%. Tirzepatide was also associated with clinically meaningful reductions in abdominal visceral adipose tissue (−1.10 to −1.65 L) and abdominal subcutaneous adipose tissue (−1.40 to −2.25 L) volumes, both of which increased in the insulin degludec arm (0.38 and 0.63 L, respectively).
3. SAFETY
3.1. Overview and adverse events
The safety profile of tirzepatide was generally similar to that of the GLP‐1 receptor agonist class and tirzepatide‐related safety findings were consistent across SURPASS 1‐5. 56 , 73 , 74 , 75 , 76 Tirzepatide was generally well tolerated. However, discontinuation because of adverse events varied between tirzepatide and comparators in some studies (Table S1). 56 , 73 , 74 , 75 , 76 Overall, 3% to 11% of participants who received tirzepatide reported treatment discontinuation because of adverse events, in comparison with placebo (3%), semaglutide 1 mg (4%), basal insulins (1%‐5%) and placebo with background insulin (3%). Considering SURPASS‐2 specifically, adverse events were the most common reason for treatment discontinuation with tirzepatide and semaglutide. This was more common with tirzepatide 10 [40 participants (9%)] and 15 mg [40 participants (9%)] than with tirzepatide 5 mg [28 participants (6%)] and semaglutide 1 mg [19 participants (4%)]. 56 The proportion of participants experiencing ≥1 treatment‐emergent adverse event (TEAE) was similar between tirzepatide and comparator in each study (Table S1), and similar TEAEs emerged as common across the studies (Table S2). 56 , 73 , 74 , 75 , 76
Generally, the proportions of participants who reported serious adverse events (SAEs) were similarly distributed between tirzepatide and comparator treatment arms (Table S1), and the range of SAEs reported is consistent with that reported for semaglutide 1 mg in the SUSTAIN programme. 93 , 94 , 95 , 96 , 97 A higher number of SAEs was reported in tirzepatide‐treated participants than those who received semaglutide 1 mg in SURPASS‐2. The most frequent SAE reported was COVID‐19‐related pneumonia in both the tirzepatide (0.4%) and semaglutide 1 mg (0.9%) groups. 56 Overall, 79 deaths occurred in the SURPASS 1‐5 studies, 41 (1%) in the tirzepatide arms and 38 (2%) in the comparator arms (Table S1). In SURPASS‐2, there were five deaths related to COVID‐19, with the death of a sixth being adjudicated as from cardiovascular causes but with suspected COVID‐19. 56 These occurred in five (0.4%) participants who received tirzepatide and one who received semaglutide (0.2%). In the high‐risk population of SURPASS‐4, six (0.6%) COVID‐19‐related deaths were reported in the tirzepatide arms and eight (0.8%) in the insulin glargine 100 U/ml arm. 75 There was one COVID‐19‐related death in a tirzepatide‐treated participant in SURPASS‐3 (<0.1%) and none in SURPASS‐1 or ‐5. 73 , 74 , 76 No deaths were considered related to study medications.
3.2. Gastrointestinal adverse events
As expected from the GLP‐1 receptor agonist class, gastrointestinal adverse events (including nausea, vomiting and diarrhoea) occurred; their incidence was similar to those in the semaglutide 1‐mg group of the SUSTAIN programme. 93 , 94 , 95 , 96 , 97 In SURPASS‐2, with direct comparison with semaglutide 1 mg, gastrointestinal adverse events (40%‐46% vs. 41%), nausea (17%‐22% vs. 18%), vomiting (6%‐10% vs. 8%) and diarrhoea (13%‐16% vs. 12%) were reported for participants on tirzepatide and semaglutide 1 mg. 56 As shown in time‐course plots (Figure 5), most events were mild or moderate in severity and occurred more frequently in the dose‐escalation phase in all groups. In SURPASS‐4 (a population with a relatively long duration of diabetes, high background medication use, high number of comorbidities and the longest follow‐up period) nausea was reported in 12%‐23% on tirzepatide and 2% on insulin glargine 100 U/ml; vomiting in 5%‐9% and 2%; and diarrhoea in 13%‐22% and 4%, respectively. 75 Gastrointestinal side effects were also reported more frequently than insulin glargine 100 U/ml with GLP‐1 receptor agonists. 94 , 98 , 99 Consistent with the low rate of study treatment discontinuation, ≤3% of participants who received at least one dose of tirzepatide discontinued treatment because of either nausea, vomiting or diarrhoea (Table S1). 56 , 73 , 74 , 75 , 76
FIGURE 5.
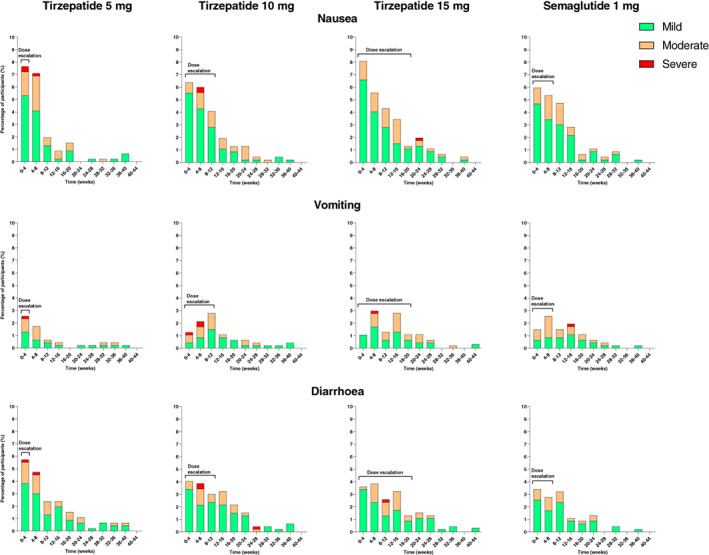
Incidence of nausea, vomiting and diarrhoea in SURPASS‐2. 56 Data are percentage of participants who reported a new event relative to participants at risk during a time interval (incidence) and from the modified intention‐to‐treat population (safety analysis set). Dose escalation indicates the time period before reaching the maintenance dose of tirzepatide 5 mg (4 weeks), 10 mg (12 weeks), and 15 mg (20 weeks) and semaglutide 1 mg (8 weeks)
3.3. Hypoglycaemia
Despite the magnitude of the observed effect of tirzepatide on glycaemia, the incidence of hypoglycaemia, defined as clinically significant [blood glucose <54 mg/dl (<3 mmol/L)] or severe, was low across the SURPASS studies. 56 , 73 , 74 , 75 , 76 The incidence of hypoglycaemia was lower for insulin‐naïve participants assigned to tirzepatide (1%‐2%) compared with insulin degludec (7%) and for tirzepatide (6%‐9%) compared with insulin glargine 100 U/ml (19%). 74 , 75 In the high cardiovascular risk population of SURPASS‐4, 145 hypoglycaemic episodes occurred in the tirzepatide arms and 492 in the insulin glargine 100 U/ml arm. 75 The incidence appeared to be dependent on concomitant medication use; hypoglycaemia occurred in 10%‐14% of tirzepatide‐treated participants using a sulphonylurea at baseline and 1%‐3% of those who did not use a sulphonylurea. In SURPASS‐5 (in which participants used background insulin) tirzepatide did not increase hypoglycaemia compared with placebo (14%‐19% vs. 13%, respectively). 76 These results suggest that the risk of hypoglycaemia attributable to tirzepatide is low when not combined with an insulin secretagogue or insulin.
3.4. Blood pressure and cardiovascular outcomes
A cardiovascular meta‐analysis, including people across the diabetes clinical spectrum, indicated that tirzepatide did not increase the risk of major cardiovascular events versus control (placebo or active comparator). 100 Overall, 142 people had at least one MACE‐4 (cardiovascular death, myocardial infarction, stroke and hospitalization because of unstable angina) event, and the hazard ratio for tirzepatide versus pooled control was 0.80 (95% CI: 0.57‐1.11). Per regulatory guidance, an upper bound of the 95% CI <1.8 for MACE‐4 indicates that a drug is not associated with an unacceptably high risk for cardiovascular events versus comparators. 101 , 102 , 103 , 104 There was also no increased risk in the secondary composite outcome MACE‐3 (cardiovascular death, myocardial infarction and stroke). The ongoing SURPASS‐CVOT, which is anticipated to be completed in 2024, will evaluate the cardiovascular efficacy of tirzepatide compared with dulaglutide 1.5 mg (NCT04255433); as such there is no cardiovascular indication reflected on the prescribing information for tirzepatide.
Across the SURPASS studies, tirzepatide generally decreased blood pressure over time with no return to baseline by the primary endpoint. 56 , 73 , 74 , 75 , 76 Taking SURPASS‐2 as an example, from a mean baseline systolic blood pressure of 130.6 mmHg and diastolic blood pressure of 79.2 mmHg, mean systolic and diastolic blood pressure decreased with tirzepatide (up to −6.5 and −2.9 mmHg, respectively), compared with decreases of −3.6 and −1.0 mmHg, respectively, with semaglutide 1 mg at 40 weeks. 56 In SURPASS‐4, mean systolic and diastolic blood pressures decreased with tirzepatide (up to −4.8 and −1.0 mmHg, respectively) and increased with insulin glargine 100 U/ml (1.3 and 0.7 mmHg, respectively) at 52 weeks. 75 A similar trend was observed over the remainder of the study. As expected from the GLP‐1 class, transient increases in pulse rate were seen in the SURPASS studies, with the magnitude of increase being similar to that observed with GLP‐1 receptor agonists in the literature and semaglutide 1 mg in SURPASS‐2. 56 , 73 , 74 , 75 , 76 , 105 , 106 , 107 Although the mechanistic pathway is not fully known, GLP‐1 receptor agonists have shown neutral or protective cardiovascular effects in cardiovascular outcomes trials. 108
3.5. Adverse events of special interest
The occurrence of adverse events of special interest are presented in Table S3. 56 , 73 , 74 , 75 , 76 Family or personal history of medullary thyroid carcinoma was an exclusion criterion of SURPASS 1‐5. No cases of medullary thyroid carcinoma were reported during any of the trials. 56 , 73 , 74 , 75 , 76 The prescribing information for tirzepatide includes a warning for the risk of thyroid C‐cell tumours. Hypersensitivity and injection site reactions were infrequent with tirzepatide treatment. 56 , 73 , 74 , 75 , 76 Few cases of adjudicated pancreatitis and cholelithiasis were reported. 56 , 73 , 74 , 75 , 76 Participants with a history of proliferative diabetic retinopathy, diabetic maculopathy, or non‐proliferative diabetic retinopathy requiring acute treatment were excluded from the Phase 3 studies. Few TEAEs of diabetic retinopathy were reported. 56 , 73 , 74 , 75 , 76
In SURPASS‐2 reductions in estimated glomerular filtration rate, urine albumin/creatinine ratio, alanine transaminase and aspartate aminotransferase at 40 weeks were comparable for tirzepatide and semaglutide 1 mg. 56 Although antidrug antibodies have been detected in 51.1% among antidrug antibody‐evaluable participants treated with tirzepatide across the Phase 3 studies there was no discernible impact on efficacy, safety, or pharmacokinetics. Antidrug antibodies have also been reported to varying extents with GLP‐1 receptor agonists, again with little apparent clinical relevance. 109 , 110
4. DISCUSSION
When considering treatment options for type 2 diabetes, in addition to glycaemic effects, factors such as comorbidities, hypoglycaemia risk, effects on body weight, side effects and patient preferences, including frequency of administration, should be considered. 39 , 85 , 111 In addition to improving glycaemia, the take away from the SURPASS 1‐5 studies showed efficacy of tirzepatide to reduce visceral adiposity, blood pressure, triglycerides, waist circumference, body weight and liver fat content. These improvements were apparent across the diabetes continuum when used as monotherapy in people newly diagnosed with type 2 diabetes and as combination therapy with metformin, SGLT2 inhibitors and/or sulphonylureas or insulin. The head‐to‐head SURPASS‐2, ‐3 and ‐4 trials showed that tirzepatide achieved greater glucose lowering and weight lowering than semaglutide 1 mg, titrated insulin glargine 100 U/ml and titrated insulin degludec.
The vast majority of participants treated with tirzepatide achieved HbA1c targets of <7.0% and ≤6.5% (53 and 48 mmol/mol) recommended by ADA/EASD and AACE/ACE for most people with type 2 diabetes. 84 , 85 , 111 A higher proportion of participants who received tirzepatide also reached an HbA1c <5.7% (39 mmol/mol; reflecting near‐normoglycaemia) than with comparators, including semaglutide 1 mg. 56 , 73 , 74 , 75 , 76 This may offer further benefit in reducing the risk of micro‐ and macrovascular complications, provided it can be achieved without significant hypoglycaemia. 84 , 112 The risk of hypoglycaemia with tirzepatide treatment remained low when not combined with an insulin secretagogue or insulin.
Treatment options for people with type 2 diabetes that provide clinically meaningful weight loss have been limited to selective GLP‐1 receptor agonists in high doses and to a lesser extent SGLT2 inhibitors. 39 , 113 Consistent with the current awareness that weight management is an important factor in overall diabetes care, 14 , 19 , 39 tirzepatide provided clinically meaningful weight reductions, even when used in conjunction with therapies associated with weight gain. These reductions were of a greater magnitude than with semaglutide 1 mg in SURPASS‐2. Ameliorative effects of 15% weight loss on type 2 diabetes progression to date have been shown with non‐pharmacotherapy means, but to date pharmacotherapies have not been able to achieve this. 19 However, the weight loss results in people with type 2 diabetes treated with tirzepatide suggest that the more ambitious targets of 10% or 15% weight loss may be feasible for some people taking tirzepatide, thus potentially conferring additional glycaemic benefit. Data from SURMOUNT‐1 also indicate the potential of tirzepatide as a therapeutic for people living with obesity without type 2 diabetes. After 72 weeks of tirzepatide treatment (up to 15 mg), mean body weight changes were up to −23.6 kg (−23%), and up to 96%, 90% and 78% achieved ≥5%, ≥10% and ≥15% body weight loss, respectively. 114
As semaglutide 2 mg has only been recently approved (2022) for use in Europe and the United States, the SURPASS Phase 3 clinical trial programme was limited to those doses available at the time (1.0 mg) and did not include the semaglutide 2 mg dose. However, an adjusted indirect treatment comparison using data from SURPASS‐2 and SUSTAIN FORTE, leveraged the semaglutide 1 mg comparator to assess the treatment effect of semaglutide 2 mg and tirzepatide 5, 10 and 15 mg on an aggregate data population. 115 In this adjusted indirect treatment comparison, tirzepatide 10 and 15 mg significantly reduced HbA1c and body weight versus semaglutide 2 mg whereas there were no differences between tirzepatide 5 mg and semaglutide 2 mg. The magnitude of these differences were also clinically meaningful, with an estimated treatment difference for HbA1c reduction of 0.4% and body weight reduction of 3‐5 kg for tirzepatide 10 and 15 mg compared with semaglutide 2 mg.
Use of a GLP‐1 receptor agonist is recommended by ADA/EASD in people with type 2 diabetes and established atherosclerotic cardiovascular disease and should be considered for those at high risk of cardiovascular disease to reduce MACE risk. 116 The ongoing SURPASS‐CVOT (NCT04255433) will provide definitive evidence on the impact of tirzepatide on cardiovascular risk relative to the GLP‐1 receptor agonist dulaglutide, which is also indicated for the reduction in cardiovascular risk for people with established cardiovascular disease or multiple risk factors. Cardiovascular outcome trials for some GLP‐1 receptor agonists, including semaglutide 1 mg, focused on populations with established cardiovascular disease. 117 , 118 , 119 In contrast, most participants in the REWIND cardiovascular outcome trial for dulaglutide 1.5 mg, which showed reduced cardiovascular outcomes with a similar effect size to other GLP‐1 receptor agonist trials, had cardiovascular risk factors without established cardiovascular disease. 120 , 121 This indicates that dulaglutide may be a robust comparator for tirzepatide in the SURPASS‐CVOT. Currently available data from SURPASS indicate beneficial effects on blood pressure and lipids. Data from the cardiovascular meta‐analysis, including the high cardiovascular risk population in SURPASS‐4, suggest no increased cardiovascular risk with tirzepatide. 100
SURPASS 1‐5 did not include all population groups. For example, women who were pregnant or breastfeeding were excluded from the studies. Although there was no upper age limit across SURPASS 1‐5 and the age profile varied across the studies, reflecting the different inclusion and exclusion criteria, few participants were ≥85 years of age. While SURPASS 1‐5 excluded people <18 years of age, study NCT05260021 will evaluate tirzepatide in paediatric and adolescent participants.
5. CONCLUSION
In the Phase 3 SURPASS‐1‐5 studies, which included >6000 people with type 2 diabetes, tirzepatide was associated with clinically meaningful reductions in both HbA1c and body weight when used across the diabetes treatment continuum, from monotherapy to combination with basal insulin. These data were generally representative of a broad spectrum of people with type 2 diabetes in terms of disease duration, comorbidities and complications, similar to clinical practice. In each study, the magnitude of glucose‐lowering efficacy and weight loss with tirzepatide was greater than with comparators, which included semaglutide 1 mg and titrated basal insulin glargine 100 U/ml and insulin degludec. Tirzepatide was well tolerated, and its safety profile appears generally similar to the GLP‐1 receptor agonist class. These findings suggest that tirzepatide may be a useful therapy for many people with type 2 diabetes as part of individualized patient‐centred care.
CONFLICT OF INTEREST
CDB reports consulting fees from Eli Lilly and Company, Novo Nordisk, AstraZeneca, Boehringer‐Ingelheim and Abbott Diagnostics; and payment/honoraria from Eli Lilly and Company and Novo Nordisk. CB reports consulting fees from Abbott, BI, Eli Lilly and Company, and Merck; and payment/honoraria from BI, Intas, Merck and Novo. CW reports research funding from Eli Lilly and Company, Abbott, Corcept, Regeneron, Mylan and Novo Nordisk; participation on a Data Safety Monitoring Board for the NIH Artificial Pancreas Project; and is president of the Endocrine Society. AH, SEA and JP are employees and shareholders of Eli Lilly and Company.
Supporting information
Figure S1 Fasting serum glucose in SURPASS‐3 and ‐41,2
Figure S2. Participants achieving ≥10% and ≥15% weight loss in SURPASS 1‐51‐5
Table S1. Overview of adverse events in SURPASS 1‐51‐5
Table S2. Treatment‐emergent adverse events in the SURPASS 1‐5 (occurring in ≥5% of participants in any treatment group)1‐5
Table S3. Adverse events of special interest in SURPASS 1‐51‐5
ACKNOWLEDGMENTS
We thank Shweta Urva, Tamer Coskun, Josh Levine and William Roell (Eli Lilly and Company) for their time and expertise in reviewing sections of this manuscript and Praveen Shetty (Eli Lilly and Company) for assistance with figure creation.
De Block C, Bailey C, Wysham C, Hemmingway A, Allen SE, Peleshok J. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes Metab. 2023;25(1):3‐17. doi: 10.1111/dom.14831
Funding information Eli Lilly and Company
DATA AVAILABILITY STATEMENT
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://www.vivli.org.
REFERENCES
- 1. DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(1):15019. [DOI] [PubMed] [Google Scholar]
- 2. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(9):1671‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taskinen M‐R, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239(2):483‐495. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co‐prevalence of comorbidities among patients with type 2 diabetes mellitus. Cur Med Res Opin. 2016;32(7):1243‐1252. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol. 2019;71(4):793‐801. [DOI] [PubMed] [Google Scholar]
- 8. Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non‐insulin‐dependent diabetes mellitus in women: the nurses' health study. Am J Epidemiol. 1997;145(7):614‐619. [DOI] [PubMed] [Google Scholar]
- 9. Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure‐wide umbrella review of meta‐analyses. PLoS One. 2018;13(3):e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840‐846. [DOI] [PubMed] [Google Scholar]
- 11. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875‐880. [DOI] [PubMed] [Google Scholar]
- 12. Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65(suppl_1):S57‐S63. [DOI] [PubMed] [Google Scholar]
- 13. Clamp LD, Hume DJ, Lambert EV, Kroff J. Enhanced insulin sensitivity in successful, long‐term weight loss maintainers compared with matched controls with no weight loss history. Nutr Diabetes. 2017;7(6):e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S100‐S110. [DOI] [PubMed] [Google Scholar]
- 15. Sjöholm K, Sjöström E, Carlsson LM, Peltonen M. Weight change‐adjusted effects of gastric bypass surgery on glucose metabolism: 2‐ and 10‐year results from the Swedish obese subjects (SOS) study. Diabetes Care. 2016;39(4):625‐631. [DOI] [PubMed] [Google Scholar]
- 16. Lean ME, Leslie WS, Barnes AC, et al. Primary care‐led weight management for remission of type 2 diabetes (DiRECT): an open‐label, cluster‐randomised trial. Lancet. 2018;391(10120):541‐551. [DOI] [PubMed] [Google Scholar]
- 17. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care‐led weight‐management intervention for remission of type 2 diabetes: 2‐year results of the DiRECT open‐label, cluster‐randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344‐355. [DOI] [PubMed] [Google Scholar]
- 18. Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308(23):2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394‐405. [DOI] [PubMed] [Google Scholar]
- 20. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33(8):1759‐1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 2019;1(6):468‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romero‐Gómez M, Zelber‐Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829‐846. [DOI] [PubMed] [Google Scholar]
- 24. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med. 2021;384(23):2219‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pantalone KM, Misra‐Hebert AD, Hobbs TM, et al. The probability of A1c goal attainment in patients with uncontrolled type 2 diabetes in a large integrated delivery system: a prediction model. Diabetes Care. 2020;43(8):1910‐1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: a meta‐analysis. Diabetes Res Clin Pract. 2018;137:137‐148. [DOI] [PubMed] [Google Scholar]
- 27. Stone MA, Charpentier G, Doggen K, et al. Quality of care of people with type 2 diabetes in eight European countries: findings from the guideline adherence to enhance care (GUIDANCE) study. Diabetes Care. 2013;36(9):2628‐2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blonde L, Aschner P, Bailey C, Ji L, Leiter LA, Matthaei S. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diab Vasc Dis Res. 2017;14(3):172‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Look Ahead Research Group . Eight‐year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22(1):5‐13. 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Penn L, White M, Lindström J, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European diabetes prevention study RCT. PloS One. 2013;8(2):e57143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gasbjerg LS, Helsted MM, Hartmann B, et al. Separate and combined glucometabolic effects of endogenous glucose‐dependent Insulinotropic polypeptide and glucagon‐like peptide 1 in healthy individuals. Diabetes. 2019;68(5):906‐917. [DOI] [PubMed] [Google Scholar]
- 33. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metab. 2018;27(4):740‐756. [DOI] [PubMed] [Google Scholar]
- 34. Gasbjerg LS, Bergmann NC, Stensen S, et al. Evaluation of the incretin effect in humans using GIP and GLP‐1 receptor antagonists. Peptides. 2020;125:170183. [DOI] [PubMed] [Google Scholar]
- 35. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon‐like peptide 1 [7‐36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. J Clin Invest. 1993;91(1):301‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45(8):1111‐1119. [DOI] [PubMed] [Google Scholar]
- 37. Højberg PV, Zander M, Vilsbøll T, et al. Near normalisation of blood glucose improves the potentiating effect of GLP‐1 on glucose‐induced insulin secretion in patients with type 2 diabetes. Diabetologia. 2008;51(4):632‐640. [DOI] [PubMed] [Google Scholar]
- 38. Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon‐like peptide‐1 (7‐36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326(20):1316‐1322. [DOI] [PubMed] [Google Scholar]
- 39. American Diabetes Association . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S111‐S124. [DOI] [PubMed] [Google Scholar]
- 40. Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 2004;17(3):183‐190. [Google Scholar]
- 41. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696‐1705. [DOI] [PubMed] [Google Scholar]
- 42. Szayna M, Doyle ME, Betkey JA, et al. Exendin‐4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141(6):1936‐1941. [DOI] [PubMed] [Google Scholar]
- 43. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon‐like peptide‐1 7‐36: a physiological incretin in man. Lancet. 1987;2(8571):1300‐1304. [DOI] [PubMed] [Google Scholar]
- 44. Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon‐like peptide‐1 (GLP‐1)‐(7‐36) amide in type 2 (noninsulin‐dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81(1):327‐332. [DOI] [PubMed] [Google Scholar]
- 45. Stensen S, Gasbjerg LS, Krogh LL, et al. Effects of endogenous GIP in patients with type 2 diabetes. Eur J Endocrinol. 2021;185(1):33‐45. [DOI] [PubMed] [Google Scholar]
- 46. Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of GLP‐1? Trends Endocrinol Metab. 2020;31(6):410‐421. [DOI] [PubMed] [Google Scholar]
- 47. Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin‐vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133(6):2861‐2870. [DOI] [PubMed] [Google Scholar]
- 48. Kaplan AM, Vigna SR. Gastric inhibitory polypeptide (GIP) binding sites in rat brain. Peptides. 1994;15(2):297‐302. [DOI] [PubMed] [Google Scholar]
- 49. Mohammad S, Ramos LS, Buck J, Levin LR, Rubino F, McGraw TE. Gastric inhibitory peptide controls adipose insulin sensitivity via activation of camp‐response element‐binding protein and p110β isoform of phosphatidylinositol 3‐kinase. J Biol Chem. 2011;286(50):43062‐43070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Starich GH, Bar RS, Mazzaferri EL. GIP increases insulin receptor affinity and cellular sensitivity in adipocytes. Am J Physiol. 1985;249(6 Pt 1):E603‐E607. [DOI] [PubMed] [Google Scholar]
- 51. Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37(5):826‐828. [DOI] [PubMed] [Google Scholar]
- 52. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1‐2):8‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP‐1 receptor agonist. JCI Insight. 2020;5(17):e140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP‐1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP‐1 receptor agonist tirzepatide improves beta‐cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(2):388‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503‐515. [DOI] [PubMed] [Google Scholar]
- 57. Heise T, Mari A, DeVries JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double‐blind, parallel‐arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10:418‐429. doi: 10.1016/S2213-8587(22)00085-7. [DOI] [PubMed] [Google Scholar]
- 58. Samms RJ, Christe ME, Collins KA, et al. GIPR agonism mediates weight‐independent insulin sensitization by tirzepatide in obese mice. J Clin Invest. 2021;131(12):e146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mather KJ, Mari A, Li J, et al. 714‐P: greater improvement in insulin sensitivity per unit weight loss with tirzepatide compared with selective GLP‐1 receptor agonism. Diabetes. 2022;71(Supplement_1):714. [Google Scholar]
- 60. Ohwaki K, Furihata K, Oura T, Imaoka T. 969‐P: effects of tirzepatide on meal intake and appetite in Japanese patients with type 2 diabetes. Diabetes. 2020;69(Supplement_1):969‐P. [Google Scholar]
- 61. Heise T, De Vries JH, Coskun T, et al. 338‐OR: tirzepatide reduces appetite, energy intake, and fat mass in people with T2D. Diabetes. 2022;71(Supplement_1):338‐OR. [Google Scholar]
- 62. Urva S, Quinlan T, Landry J, Martin J, Loghin C. Effects of renal impairment on the pharmacokinetics of the dual GIP and GLP‐1 receptor agonist tirzepatide. Clin Pharmacokinet. 2021;60(8):1049‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Urva S, Quinlan T, Landry J, Ma X, Martin JA, Benson CT. Effects of hepatic impairment on the pharmacokinetics of the dual GIP and GLP‐1 receptor agonist tirzepatide. Clin Pharmacokinet. 2022;61:1057‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Urva S, Coskun T, Loghin C, et al. The novel dual glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1 (GLP‐1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long‐acting GLP‐1 receptor agonists. Diabetes Obes Metab. 2020;22(10):1886‐1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mounjaro [package insert] . Indianapolis, IN: Eli Lilly and Company; 2022. https://pi.lilly.com/us/mounjaro-uspi.pdf?s=pi
- 66. Adlyxin [package insert] . Bridgewater, NJ: Sanofi US; 2021. https://products.sanofi.us/adlyxin/adlyxin.pdf
- 67. Bydureon [package insert] . Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2022. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/6b02db6d-7a95-4a90-88ae-5f5ac7397755/6b02db6d-7a95-4a90-88ae-5f5ac7397755_viewable_rendition__v.pdf
- 68. Byetta [package insert] . Wilmington, DE. AstraZeneca Pharmaceuticals LP; 2022. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/ce8afab9-2b45-436d-957c-a73978d09e93/ce8afab9-2b45-436d-957c-a73978d09e93_viewable_rendition__v.pdf
- 69. Ozempic [package insert] . Bagsvaerd, Denmark: Novo Nordisk A/S; 2022. https://www.novo-pi.com/ozempic.pdf
- 70. Rybelsus [package insert] . Bagsvaerd, Denmark: Novo Nordisk A/S; 2022. https://www.novo-pi.com/rybelsus.pdf
- 71. Victoza [package insert] . Plainsboro, NJ: Novo Nordisk Inc.; 2021. https://www.novo-pi.com/victoza.pdf
- 72. Trulicity [package insert] . Indianapolis, IN: Eli Lilly and Company; 2022. https://pi.lilly.com/us/trulicity-uspi.pdf
- 73. Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP‐1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS‐1): a double‐blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143‐155. [DOI] [PubMed] [Google Scholar]
- 74. Ludvik B, Giorgino F, Jódar E, et al. Once‐weekly tirzepatide versus once‐daily insulin degludec as add‐on to metformin with or without sodium‐glucose co‐transporter‐2 inhibitors in patients with type 2 diabetes (SURPASS‐3): a randomised, open‐label, parallel‐group, phase 3 trial. Lancet. 2021;398(10300):583‐598. [DOI] [PubMed] [Google Scholar]
- 75. Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS‐4): a randomised, open‐label, parallel‐group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811‐1824. [DOI] [PubMed] [Google Scholar]
- 76. Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS‐5 randomized clinical trial. JAMA. 2022;327(6):534‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frias JP, Nauck MA, Van J, et al. Efficacy and tolerability of tirzepatide, a dual glucose‐dependent insulinotropic peptide and glucagon‐like peptide‐1 receptor agonist in patients with type 2 diabetes: a 12‐week, randomized, double‐blind, placebo‐controlled study to evaluate different dose‐escalation regimens. Diabetes Obes Metab. 2020;22(6):938‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP‐1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo‐controlled and active comparator‐controlled phase 2 trial. Lancet. 2018;392(10160):2180‐2193. [DOI] [PubMed] [Google Scholar]
- 79. Battelino T, Bergenstal RM, Rodriguez A, et al. Efficacy of once‐weekly tirzepatide versus once‐daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS‐3 CGM): a substudy of the randomised, open‐label, parallel‐group, phase 3 SURPASS‐3 trial. Lancet Diabetes Endocrinol. 2022;10(6):407‐417. [DOI] [PubMed] [Google Scholar]
- 80. Gastaldelli A, Cusi K, Fernandez Lando L, Bray R, Brouwers B, Rodriguez A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS‐3 MRI): a substudy of the randomised, open‐label, parallel‐group, phase 3 SURPASS‐3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393‐406. [DOI] [PubMed] [Google Scholar]
- 81. Pan C, Gross JL, Yang W, et al. A multinational, randomized, open‐label, treat‐to‐target trial comparing insulin degludec and insulin glargine in insulin‐naïve patients with type 2 diabetes mellitus. Drugs R D. 2016;16(2):239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zinman B, Philis‐Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN once long). Diabetes Care. 2012;35(12):2464‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080‐3086. [DOI] [PubMed] [Google Scholar]
- 84. American Diabetes Association . Glycemic targets: standards of medical care in diabetes — 2021. Diabetes Care. 2021;44(Supplement 1):S73‐S84. [DOI] [PubMed] [Google Scholar]
- 85. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract. 2020;26(1):107‐139. [DOI] [PubMed] [Google Scholar]
- 86. Furihata K, Mimura H, Urva S, Oura T, Ohwaki K, Imaoka T. A phase 1 multiple‐ascending dose study of tirzepatide in Japanese participants with type 2 diabetes. Diabetes Obes Metab. 2022;24(2):239‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Davies MJ, Frias JP, Rosenstock J, et al. Efficacy and safety of tirzepatide versus semaglutide once weekly as add‐on therapy to metformin in people with type 2 diabetes (SURPASS‐2). Presention at the: the American Diabetes Associaton ‐ 81st Annual Scientific Sessions; June 25‐29 2021; Virtual Meeting.
- 88. Petäjä EM, Yki‐Järvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD‐A systematic review. Int J Mol Sci. 2016;17(5):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Loomba R. MRI‐proton density fat fraction treatment response criteria in nonalcoholic steatohepatitis. Hepatology. 2021;73(3):881‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stine JG, Munaganuru N, Barnard A, et al. Change in MRI‐PDFF and histologic response in patients with nonalcoholic steatohepatitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2021;19(11):2274‐2283.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brouwers B, Schrauwen‐Hinderling VB, Jelenik T, et al. Metabolic disturbances of non‐alcoholic fatty liver resemble the alterations typical for type 2 diabetes. Clin Sci (Lond). 2017;131(15):1905‐1917. [DOI] [PubMed] [Google Scholar]
- 92. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616‐627. [DOI] [PubMed] [Google Scholar]
- 93. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 94. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 95. Sorli C, Harashima S‐i, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 96. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly Semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care. 2018;41(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 97. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38(12):2241‐2249. [DOI] [PubMed] [Google Scholar]
- 99. Russell‐Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre‐specified meta‐analysis. Nat Med. 2022;28(3):591‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. US Food and Drug Association . Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.fda.gov/media/71297/download
- 102. European Medicines Agency . Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus_en.pdf
- 103. US Food and Drug Administration . Guidance for industry. Type 2 diabetes mellitus: evaluating the safety of new drugs for improving glycemic control. 2020.
- 104. Gore MO, McGuire DK. Cardiovascular disease and type 2 diabetes mellitus: regulating glucose and regulating drugs. Curr Cardiol Rep. 2009;11(4):258‐263. [DOI] [PubMed] [Google Scholar]
- 105. Zaccardi F, Htike ZZ, Webb DR, Khunti K, Davies MJ. Benefits and harms of once‐weekly glucagon‐like peptide‐1 receptor agonist treatments: a systematic review and network meta‐analysis. Ann Intern Med. 2016;164(2):102‐113. [DOI] [PubMed] [Google Scholar]
- 106. Lorenz M, Lawson F, Owens D, et al. Differential effects of glucagon‐like peptide‐1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta‐analysis. BMJ Open. 2013;3(1):e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP‐1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta‐analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019;21(11):2576‐2580. doi: 10.1111/dom.13847. [DOI] [PubMed] [Google Scholar]
- 109. Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon‐like peptide‐1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35(1):e3070. doi: 10.1002/dmrr.3070. [DOI] [PubMed] [Google Scholar]
- 110. Hinnen D. Glucagon‐like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectrum. 2017;30(3):202‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. De Block CEM, Dirinck E, Verhaegen A, Van Gaal LF. Efficacy and safety of high‐dose glucagon‐like peptide‐1, glucagon‐like peptide‐1/glucose‐dependent insulinotropic peptide, and glucagon‐like peptide‐1/glucagon receptor agonists in type 2 diabetes. Diabetes Obes Metab. 2022;24(5):788‐805. [DOI] [PubMed] [Google Scholar]
- 114. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205‐216. [DOI] [PubMed] [Google Scholar]
- 115. Vadher K, Patel H, Mody R, et al. Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: an adjusted indirect treatment comparison. Diabetes Obes Metab. 2022;24(9):1861‐1868. doi: 10.1111/dom.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221‐228. [DOI] [PubMed] [Google Scholar]
- 117. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 119. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the researching cardiovascular events with a weekly INcretin in diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 121. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Fasting serum glucose in SURPASS‐3 and ‐41,2
Figure S2. Participants achieving ≥10% and ≥15% weight loss in SURPASS 1‐51‐5
Table S1. Overview of adverse events in SURPASS 1‐51‐5
Table S2. Treatment‐emergent adverse events in the SURPASS 1‐5 (occurring in ≥5% of participants in any treatment group)1‐5
Table S3. Adverse events of special interest in SURPASS 1‐51‐5
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://www.vivli.org.


