Abstract
Objective
This study aimed to provide, through an umbrella review, an overview of the effect of single exercise interventions during pregnancy on gestational diabetes mellitus (GDM) and hypertensive disorders of pregnancy (HDP). Also, to update the current evidence through an updated meta‐analysis.
Design
Umbrella review.
Setting
PubMed, EMBASE, Web of Science, Cochrane database of systematic reviews, Epistemonikos, SPORTDiscus, Clinicaltrials.gov, and PROSPERO register were searched from the database inception until August 2021.
Population
Peer‐reviewed systematic reviews and meta‐analyses of randomised controlled trials (RCTs) and RCTs samples.
Methods
Random‐effects model was used to calculate relative risk with 95% confidence interval in the updated meta‐analysis. The reference category was the groups that received usual prenatal care. AMSTAR 2 and the Cochrane Collaboration tool were used to assess the quality and GRADE approach was used to assess the overall certainly of evidence.
Main outcome measures
GDM and HDP relative risk.
Results
Twenty‐three systematic reviews and meta‐analyses; and 63 RCTs were included. Single exercise interventions reduced the incidence of GDM and HDP in most systematic reviews and meta‐analyses. Moreover, exercise interventions during pregnancy decrease the incidence of developing GDM and GH, particularly when they are supervised, have a low to moderate intensity level, and are initiated during the first trimester of pregnancy.
Conclusion
Based on the findings, obstetric and physical exercise professionals could recommend exercise interventions during pregnancy as an effective strategy to improve maternal outcomes.
Keywords: aerobic, diabetes, gestation, glucose, hypertension, maternal, motor activity, physical activity, pre‐eclampsia, pregnancy
Abbreviations
- CG
control group
- ES
effect size
- GDM
gestational diabetes mellitus
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HDP
hypertensive disorders of pregnancy
- IG
intervention group
- OR
odds ratio
- PA
physical activity
- RCT
randomised controlled trials
1. INTRODUCTION
Gestational diabetes mellitus (GDM), defined as ‘glucose intolerance and insulin resistance first detected during pregnancy’, 1 affects approximately 7% of pregnant women. In more than 50% of cases, GDM may progress to type 2 diabetes mellitus, hypertension and cardiovascular diseases within 5–15 years after pregnancy. 2 , 3 , 4 , 5 , 6 Additionally, GDM is associated with several maternal and perinatal complications, 7 such as hypertensive disorders of pregnancy (HDP), which include gestational hypertension (GH), pre‐eclampsia and eclampsia, 8 affecting 10% of pregnancies. 9 Likewise, HDP are associated with higher life‐long cardiovascular risk. 10 Otherwise, fetal overgrowth or macrosomia is one of the fetal complications that occur in up to 45% of GDM pregnancies. These babies have an increased risk of overweight, obesity and type 2 diabetes later in life. 11
Several studies have suggested that exercise is an effective strategy for preventing and treating diabetes and hypertension in the general population by reducing some of the mechanisms involved in inflammation, oxidative stress and endothelial dysfunction, all of which are pathophysiological mechanisms involved in the genesis of HDP and GDM. 9 , 12 , 13 That, in turn, is associated with obesity and physical inactivity. 14 However, the evidence about the effectiveness of exercise in avoiding the development of both disorders during pregnancy is still not consistent. Some reviews reported that physical activity (PA) programmes produced reductions in the prevalence of GDM. 15 , 16 Regarding the effect of exercise on pre‐eclampsia or HDP incidence, some studies did not find protective effect of exercise in the incidence of pre‐eclampsia or HDP. 17 , 18 , 19 , 20
Comprehensive evidence synthesis is needed to explore further reasons for these conflicting findings. 21 , 22 , 23 , 24 , 25 , 26 Hence, this umbrella review and updated meta‐analysis aimed to provide a comprehensive overview of the effect of exercise interventions during pregnancy on GDM and HDP.
2. METHODS
This umbrella review of systematic reviews and meta‐analyses was registered in PROSPERO (Registration number: CRD42019123410) and was developed according to PRISMA and the Cochrane Collaboration Handbook. 27 , 28 The review protocol has been published elsewhere. 29
2.1. Search methodology
The PubMed, EMBASE, Web of Science, Cochrane database of systematic reviews, Epistemonikos, SPORTDiscus, Clinicaltrials.gov and PROSPERO databases were systematically searched from inception to August 2021 for systematic reviews, meta‐analyses and randomised controlled trials (RCTs) aimed at assessing the effect of exercise interventions during pregnancy on GDM and HDP.
The search strategy combined the following relevant terms: (1) patients (pregnant OR pregnancy OR gravid OR gestation* OR maternal); (2) intervention (aerobic OR sport OR exercise OR fitness OR “physical exercise” OR “physical activity” OR “motor activity”); (3) outcome (diabetes OR “diabetes mellitus” OR DM OR “gestational diabetes” OR “glucose intolerance” OR glucose OR insulin OR hyperglycemia OR toxaemia OR preeclampsia OR pre‐eclampsia OR eclampsia OR “hypertensive disorders” OR “blood pressure” OR hypertension) (in more detail in Table S1). Additional reviews and RCTs were also identified by screening the references of the included reviews and meta‐analyses (Table S2).
The search strategy was conducted independently by two researchers (GSM and JAMH) to avoid any selection bias. Disagreements were solved by consensus, but if discrepancies persisted, a third researcher was consulted (VMV).
2.2. Study inclusion and exclusion criteria
Systematic reviews and meta‐analyses of RCTs written in any language evaluating the effect of exercise interventions during pregnancy on GDM and HDP were selected. Reviews had to contain a quantitative assessment of the exercise effect on GDM and HDP. Diagnostic criteria for GDM varied among studies and were established in each individual trial. The health conditions under HDP were defined by the American College of Obstetricians and Gynecologists (ACOG): 30 pre‐eclampsia–eclampsia, chronic hypertension, chronic hypertension with superimposed pre‐eclampsia and GH. Participants should be pregnant women with no absolute or relative contraindications to be included in exercise programmes according to the 2020 ACOG recommendations on PA and exercise during pregnancy and postpartum. 31 Moreover, we excluded articles that only included women with a specific disease or with a high incidence of GDM or HDP. Finally, exercise programmes of all exercise types at any level of intensity were included, but when the meta‐analysis included studies with an additional intervention with a nutritional or behavioural component, only information from RCTs with exclusive exercise intervention was extracted. Reviews were excluded if women in the control groups (CGs) were not receiving usual prenatal care.
Two researchers (GSM and JAMH) independently assessed for eligibility in duplicate all abstracts and full texts, as recommended by the Cochrane Handbook. 28 Disagreements on study eligibility were discussed with the study team and resolved by consensus.
2.3. Data extraction
Two researchers (GSM and JAMH) independently extracted data in ad hoc designed forms included in the umbrella review protocol. 29 Potential conflicts were discussed until consensus and a third researcher (VMV) was consulted when agreement was not reached. We collected first author, publication year, years for inclusion, number of RCTs included, intervention group (IG) and CG sample size, sample characteristics, outcomes of interest (GDM, GH, pre‐eclampsia), number of cases in each group, pooled risk measure (relative risk or odds ratio [OR]), effect size and its 95% CI of the largest study and overall effect size, the heterogeneity (I 2) and any reported measures of publication bias. For each study, we extracted the first author, publication year, country, recruitment years, IG and CG sample size, intervention characteristics (type of exercise, when exercise intervention started and ended, length, session duration, frequency, intensity and supervision), setting, the outcome of interest, and IG and CG cases.
2.4. Assessment of risk of bias
The methodological quality of the included reviews and meta‐analyses was independently rated using the AMSTAR 2 tool by two researchers (GSM and JAMH). The AMSTAR2 is an instrument to critically assess the risk of bias of systematic reviews that consists of 16 different domains referring to relevant methodological aspects, which are answered with ‘yes’, ‘no’, ‘cannot answer’ and ‘partial yes’. The overall quality of the studies was categorised as follows: high, moderate, low or critically low. 32
Moreover, we assessed the risk of bias (quality) of the RCTs included in the updated meta‐analysis using the Cochrane Collaboration risk of bias tool (Table S13), which assesses eight potential sources of bias: random sequence generation, allocation concealment, blinding of participants, evaluator and outcome assessments, incomplete outcome data, missing data, and other. 33
The certainty of evidence and strength of recommendations from meta‐analyses were assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method. 34 This tool provides a system rate with four categories: ‘high’, when the systematic review or meta‐analysis includes at least two high‐quality primary studies; ‘moderate’, when it includes at least one high‐quality or two moderate‐quality primary studies; ‘low’ when it includes only moderate‐quality and/or inconsistent results studies; and ‘very low’, when no medium‐ to high‐quality studies were identified on this topic. Our starting point was ‘high’, and this grading decreased when we detected risk of bias, inconsistency of results (i.e. I 2 statistics >50%), indirectness of evidence (i.e. differences in intervention), imprecision (i.e. 95% CI includes 1.0) or publication bias (asymmetry in funnel plot). Additionally, the rating was increased if there was a large intervention effect, in case of a dose–response relation or if all plausible biases would decrease the magnitude of the intervention effect. 34 The GRADE assessment was performed independently by two researchers (GSM and JAMH) with discussion and agreement for any discrepancies.
2.5. Statistical analysis
2.5.1. Primary analysis
The primary analysis in the umbrella review was focused on one measure per individual study and each outcome. Forest plots were designed to synthesise all pooled estimates regarding the effect of exercise on the outcomes (GDM and HDP).
2.5.2. Assessment of summary effects and heterogeneity
The summary meta‐analytic odds ratio estimates and their corresponding 95% CI for each meta‐analysis were displayed graphically using a random‐effects model that was calculated by the original studies. 35 , 36 Additionally, a pooled analysis was performed with the RCTs included in the systematic reviews and meta‐analyses. The RCTs identified in the update with the estimates and corresponding 95% CI using the fixed‐effects or random‐effects model. 35 In our study, the significance level of the p values for Egger's test was set at 0.10. For continuous data, we calculated the mean difference, the incidence difference or the Glass Δ. 37 , 38 Trial sequence analysis (TSA) was conducted (Tables S20–S22). 39 The heterogeneity between study associations was assessed using the I 2 statistic. 40
Sensitivity analyses were conducted to assess the robustness of summary estimates and to detect whether any individual study accounted for a large proportion of heterogeneity.
2.5.3. Subgroup analyses and meta‐regression
Subgroup and meta‐regression analyses were performed with original study data to examine the influence of some potential mediators on outcomes. For this purpose, the following subgroups were established: (1) body mass index (BMI) categories (>25 kg/m2 or <25 kg/m2); (2) pregnancy trimester in which the intervention was initiated (first or second); (3) session duration (>45 min or <45 min) and (4) supervision during the exercise intervention or not.
2.5.4. Small studies effect assessment and excess significance biases
Egger's regression asymmetry test was used to calculate the evidence of small‐study effects. 41 Therefore, a p value less than 0.10 was considered to show evidence of small‐study effects. 41 Also, we assessed ‘p‐hacking’ 42 and publication bias with PET‐PEESE 43 , 44 and Rucker's analysis. 45
All statistical analyses and power calculations were performed using STATA V.15.1 software (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Study selection and study characteristics
We retrieved 696 systematic reviews and meta‐analyses after removing duplicates. The reasons to exclude studies are provided in Tables S18–S19. Finally, 23 systematic reviews and meta‐analyses were included after full‐text assessment (Tables 1 and 2), including 63 RCTs (10 485 and 11 192 women in IG and CG, respectively) (Figure S2). Among them, three RCTs published between 2016 and 2019 were not included in any systematic reviews and meta‐analyses, which were selected for the meta‐analysis update (Figure S3). 46 , 47 , 48 A characteristics summary of the included meta‐analyses and RCTs can be found in Tables 1 and 2, and Tables S3 and S4.
TABLE 1.
Meta‐analysis of randomised control trials of gestational diabetes mellitus
| Reference | Number of RCT | Sample IG/CG | Number of cases IG/CG | Type of measure (RR or OR) | RR/OR and 95% CI of the largest study | RR/OR and 95% CI | I 2 (%) |
|---|---|---|---|---|---|---|---|
| Aune et al. 24 | 12 | 2015/2030 | 129/191 | RR | 0.78 (0.47–1.28) | 0.69 (0.50–0.96) | 30.2 |
| Bennett et al. 49 | 9 | 1736/1260 | 111/135 | RR | 0.70 (0.49–0.99) | 0.65 (0.50–0.78) | 0 |
| Da Silva et al. 93 | 11 | 1673/1689 | NR | RR | 0.70 (0.49–0.99) | 0.67 (0.49–0.92) | 33 |
| Davenport et al. 21 | 27 | 3505/3429 | 271/380 | OR | 0.62 (0.40–0.98) | 0.62 (0.52–0.75) | 0 |
| Di Mascio et al. 56 | 4 | 836/850 | 20/50 | RR | NR | 0.41 (0.24–0.68) | NR |
| Díaz‐Burrueco et al. 50 | 10 | 1159/1516 | 123/204 | OR | NR | 0.65 (0.43–0.98) | 48 |
| Guo et al. 51 | 19 | Total:5883 | NR | RR | 0.54 (0.37–0.79) | 0.70 (0.59–0.84) | 14.9 |
| Han et al. 18 | 3 | 437/389 | 30/24 | RR | 1.21 (0.67–2.18) | 1.10 (0.66–1.84) | 0 |
| Magro‐Malosso et al. 57 | 7 | 748/602 | 40/53 | RR | NR | 0.61 (0.41–0.90) | NR |
| Ming et al. 53 | 8 | 1472/1509 | 88/144 | RR | 0.70 (0.49–0.99) | 0.58 (0.37–0.90) | 46 |
| Muhammad et al. 54 | 6 | 314/318 | 73/101 | RR | 0.54 (0.37–0.79) | 0.78 (0.51–1.19) | 49 |
| Nasiri‐Amiri et al. 58 | 8 | 727/714 | 143/196 | RR | 0.67 (0.47–0.96) | 0.76 (0.56–1.03) | 50 |
| Rogozinska et al. 26 | 27 | 3153/3015 | 240/347 | OR | NR | 0.66 (0.53–0.83) | 0 |
| Russo et al. 59 | 10 | 1715/1686 | 116/159 | RR | 0.70 (0.49–0.99) | 0.74 (0.57–0.97) | 12 |
| Sanabria et al. 15 | 8 | 1434/1439 | NR | RR | NR | 0.69 (0.52–0.91) | 0 |
| Doi et al. 55 | 11 | 722/745 | 100/148 | RR | 0.54 (0.37–0.79) | 0.69 (0.51–0.94) | 23.2 |
| Song et al. 60 | 10 | Total: 4161 | NR | RR | 0.94 (0.59–1.50) | 0.77 (0.54–1.09) | 38 |
| Xing et al. 61 | 10 | 794/786 | NR | RR | 1.30 (0.93. 1.82) | 0.71 (0.48–1.04) | 63.1 |
| Yin et al. 17 | 5 | 497/450 | 34/34 | RR | 1.21 (0.67–2.18) | 0.91 (0.57–1.44) | 26 |
| Yu et al. 62 | 6 | 651/719 | 117/181 | OR | 0.62 (0.40–0.98) | 0.59 (0.39–0.88) | 46 |
| Zheng et al. 63 | 5 | 550/583 | 116/168 | OR | 0.62 (0.40–0.98) | 0.62 (0.43–0.89) | 37 |
Abbreviations: CG, control group; IG, intervention group; OR, odds ratio; RCT, randomised controlled trial; RR, relative risk.
TABLE 2.
Meta‐analysis of randomised control trials of hypertensive disorders of pregnancy
| Reference | Number of RCT | Sample IG/CG | Main outcome | Number of cases IG/CG | Type of measure (RR or OR) | RR/OR and 95% CI and of the largest study | RR/OR and 95% CI | I 2 (%) |
|---|---|---|---|---|---|---|---|---|
| Davenport et al. 21 | 23 | 2627/2689 | GH | 61/105 | OR | 1 (0.46–2.19) | 0.61 (0.43–0.85) | 0 |
| Magro‐Malosso et al. 57 | 16 | 2452/2189 | GH | 61/100 | RR | NR | 0.54 (0.40–0.74) | 10 |
| Xing et al. 61 | 5 | 428/424 | GH | NR | RR | 0.74 (0.35–1.55) | 0.53 (0.32–0.88)] | 3.7 |
| Davenport et al. 21 | 16 | 1719/1603 | PE | 34/49 | OR | 0.80 (0.27–2.40) | 0.59 (0.37–0.94) | 0 |
| Da Silva et al. 93 | 4 | 709/708 | PE | NR | RR | 0.99 (0.50, 1.96) | 0.93 (0.55–1.57) | 0 |
| Kramer et al. 64 | 1 | 43/39 | PE | 7/6/ | RR | 1.17 (0.44–3.08) | 1.17 (0.44–3.08) | NR |
| Magro‐Malosso et al., 57 | 6 | 1164/1066 | PE | 26/30 | RR | NR | 0.79 (0.45–1.38) | 0 |
| Xing et al. 61 | 5 | 499/505 | PE | NR | RR | NR | 1.00 (0.66–1.52) | 0 |
| Muhammad et al. 54 | 5 | 324/326 | GH and PE | 23/30 | RR | 0.75 (0.36–1.55) | 0.77 (0.46–1.30) | 0 |
| Rogozinska et al. 26 | 20 | 2513/2359 | HDP | 106/147 | OR | Not reported | 0.68 (0.49–0.93) | 0 |
Abbreviations: CG, control group; GH, gestational hypertension; HDP, hypertensive disorders of pregnancy; IG, intervention group; OR, odds ratio; PH, pre‐eclampsia; RCT, randomised controlled trial; RR, relative risk.
3.2. Risk of bias of included studies
The methodological quality scores of the 23 systematic reviews and meta‐analyses assessed by AMSTAR 2 are shown in Table S5. Seven studies scored moderate quality (33%), 18 , 21 , 22 , 49 , 50 , 51 , 52 five scored low quality (21%), 26 , 53 , 54 , 55 , 56 and 11 scored critically low methodological quality (53%). 15 , 17 , 24 , 25 , 57 , 58 , 59 , 60 , 61 , 62 , 63 Only six studies provided an a priori protocol register, 18 , 21 , 22 , 26 , 49 , 54 and seven reported a list of excluded studies. 18 , 21 , 22 , 24 , 49 , 53 , 64
According to the GRADE assessment, reviews included were considered low certainty. For the risk of bias, the scores were very serious because of the lack of blinding of participants (Table S6).
3.3. Synthesis of results
3.3.1. Gestational diabetes mellitus
Thirteen systematic reviews and meta‐analyses found a reduction of GDM incidence in the exercise group 15 , 21 , 24 , 26 , 27 , 49 , 50 , 51 , 53 , 55 , 56 , 59 , 62 , 65 and seven did not find effect of exercise on the incidence of GDM 18 , 54 , 58 , 60 (Figure 1 and Table S3). Among systematic review and meta‐analyses that included women with overweight or obesity, 21 , 26 , 49 , 54 , 58 , 61 only one found that exercise reduced GDM incidence (Figure S3 and Table S7). 63
FIGURE 1.
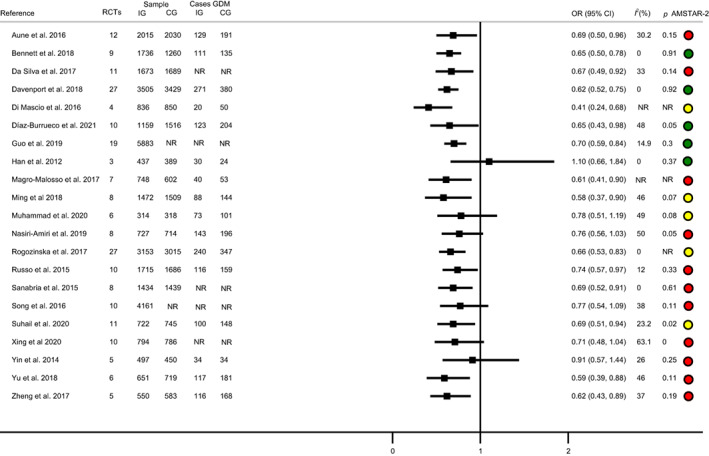
Exercise and gestational diabetes mellitus. Systematic review and meta‐analyses.
Regarding the updated meta‐analysis, 35 RCTs assessed the effect of exercise on GDM incidence, 46 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 whose pooled OR showed a decreased GDM incidence (OR 0.61; 95% CI 0.51–0.74; I 2 = 17.79; IG: n = 4935, CG: n = 5354) (Figure S4). The subgroup analyses (Table S8) showed that GDM incidence was reduced when the exercise intervention was: (1) initiated in the first trimester(OR 0.55; 95% CI 0.44–0.68; I 2 = 23.40; IG: n = 2775, CG: n = 2899); (2) supervised (OR 0.60; 95% CI 0.50–0.72; I 2 = 6.4%; IG: n = 3679, CG: n = 4670) and (3) light to moderate or moderate intensity (OR 0.58; 95% CI 0.39–0.87; I 2 = 0.00%; IG: n = 838, CG: n = 839; and OR 0.56; 95% CI 0.46–0.68; I 2 = 22.56%; IG: n = 2722, CG: n = 2887) (Table S8). Finally, longer exercise intervention did not reduce the GDM incidence more (OR −0.02, 95% CI −0.05 to 0.00; p = 0.06; IG: n = 4539, CG: n = 4936).
3.3.2. Hypertensive disorders of pregnancy
Eight systematic reviews and meta‐analyses assessed the effect of exercise on HDP incidence. 21 , 22 , 25 , 26 , 50 , 54 , 60 , 64 Three systematic reviews and meta‐analyses assessed the effect of exercise interventions on GH incidence, 21 , 22 , 61 five on pre‐eclampsia incidence, 21 , 22 , 25 , 61 , 64 three on HDP 22 , 26 , 50 and one on GH and pre‐eclampsia incidence together (Table S4). 54
All systematic reviews and meta‐analyses reported a GH 21 , 22 , 61 and HDP 22 , 26 , 50 incidence reduction in the exercise group compared with the CG. The studies that reported the effect of exercise on pre‐eclampsia incidence had mixed results: one found a decrease on the pre‐eclampsia incidence in the exercise group 21 and four did not find effect. 22 , 25 , 61 , 64 Finally, the only study that reported GH and pre‐eclampsia incidence together 54 did not find a decrease in the exercise group (Figure 2).
FIGURE 2.
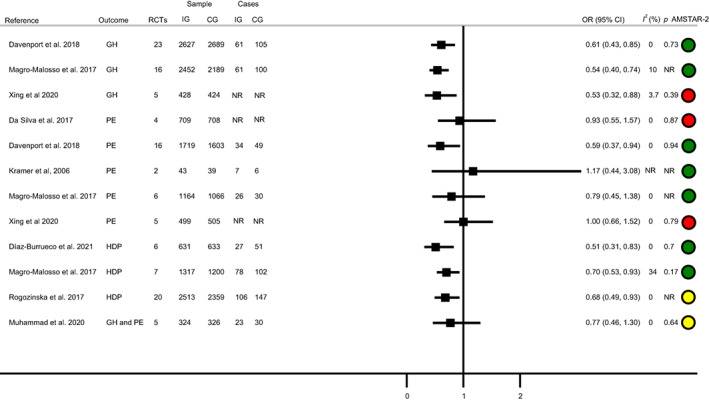
Exercise and hypertensive disorders of pregnancy. Systematic reviews and meta‐analyses.
Among the systematic review and meta‐analyses that included women with overweight or obesity, 21 , 26 , 54 , 61 one found a reduction in GH incidence, 61 while the others did not find an effect on GH 21 and HDP 21 , 26 , 54 , 61 (Figure S5 and Table S9).
Regarding the updated meta‐analysis of RCTs, 28 of them assessed the effect of exercise on HDP incidence. 47 , 48 , 68 , 70 , 71 , 74 , 77 , 80 , 82 , 83 , 85 , 86 , 87 , 89 , 90 , 91 , 92 , 94 , 97 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 Among them, 22 studies assessed the effect of exercise on GH incidence, 67 , 69 , 70 , 72 , 74 , 76 , 87 , 88 , 89 , 90 , 93 , 94 , 96 , 97 , 100 , 101 , 102 , 103 , 104 , 108 , 109 , 110 20 on pre‐eclampsia incidence, 47 , 72 , 74 , 77 , 79 , 82 , 87 , 90 , 92 , 93 , 94 , 95 , 100 , 101 , 105 , 106 , 107 , 111 , 112 , 113 one on HDP incidence 114 and the other RCT reported data on eclampsia (Table S3). 98
The pooled estimates show that exercise reduced the GH incidence (OR 0.53; 95% CI 0.40–0.71; I 2 = 0.00; IG: n = 2977, CG: n = 3067) (Figure S6). However, exercise did not reduce the incidence of preeclampsia (OR 0.81; 95% CI 0.61–1.07; I 2 = 0.00; IG: n = 2573, CG: n = 2771) (Figure S7). Subgroup analysis (Table S10) showed lower GH incidence in relation to their counterparts in the CGs in those studies that: (1) included women with all BMI categories (OR 0.50; 95% CI 0.37–0.68; I 2 = 0.00; IG: n = 2145, CG: n = 2332); (2) each session was longer than 45 minutes (OR 0.44; 95% CI 0.31–0.64; I 2 = 0.00; IG: n = 2166, CG: n = 2241); (3) started exercise interventions in the first and second trimester of pregnancy (OR 0.46; 95% CI 0.30–0.69; I 2 = 2.49; IG: n = 1451, CG: n = 1452 versus OR 0.40; 95% CI 0.20–0.79; I 2 = 0.00; IG: n = 1242, CG: n = 1255); (4) exercise was supervised (OR 0.55; 95% CI 0.41–0.73 I 2 = 0.00; IG: n = 2856, CG: n = 2957); and (5) the intensity of the exercise intervention was light to moderate or moderate, (OR 0.44; 95% CI 0.24–0.80; I 2 = 0.00%; IG: n = 657, CG: n = 658 and OR 0.58; 95% CI 0.41–0.81; I 2 = 22.56%; IG: n = 1874, CG: n = 1965). The pre‐eclampsia incidence was only reduced when initiated in the first trimester (OR 0.34; 95% CI 0.14–0.87; I 2 = 0.00%; IG: n = 1451, CG: n = 1452) (Table S11). Finally, longer exercise intervention did not reduce more HDP incidence (OR −0.02; 95% CI −0.05 to 0.00; p = 0.17; IG: n = 3266, CG: n = 3544).
Trial sequence analysis
The results of trial sequence analysis are presented in Tables S20–S22 and Figures [Link], [Link]. The required sample size was reached in the main analysis, except for the analysis that assessed the effect of PA intervention on pre‐eclampsia.
Additionally, the meta‐analysis of blood pressure value showed a reduction in the mean systolic blood pressure among women in the IG (−9.61 mmHg; 95% CI = −11.34 to −7.87 mmHg; I 2 = 93.83%; IG: n = 322, CG: n = 323) (Table S12).
Finally, there was no evidence of ‘p‐hacking’ (Tables S30 and S35, Figures S23–S25) or publication bias according to the analyses that assessed the effect of PA intervention on GDM, or of GH on GDM, GH and pre‐eclampsia (Figures S11–S22 and Tables S22–S28).
Sensitivity analyses showed that removing studies one‐by‐one did not modify the pooled odds ratio estimations or heterogeneity.
4. DISCUSSION
4.1. Main findings
Our umbrella review is the first that includes a meta‐analysis update to summarise the evidence of the effect of single exercise interventions during pregnancy on GDM and HDP incidence. Based on 21 systematic reviews and meta‐analyses, and 54 RCTs, we found that exercise interventions were more effective than standard prenatal care in reducing the incidence of GDM and GH by 39% and 47%, respectively. In contrast, our subgroup analyses showed no effect of exercise on the incidence of HDP in overweight and obese pregnant women or when sessions lasted less than 45 minutes. Meanwhile, our data suggest that exercise is more effective in reducing the GDM and HDP incidence when initiated in the first trimester of pregnancy, under supervision and with light to moderate intensity, whereas exercise only reduced the incidence of pre‐eclampsia when pregnant women started exercise in the first trimester of pregnancy. Trial sequence analysis indicates that additional studies are needed to elucidate the effect of PA interventions on pre‐eclampsia incidence.
4.2. Strengths and limitation
Among the strengths and what the study adds, we provide concrete recommendations about duration, intensity and the best moment to start PA intervention to reduce the incidence of GDM, GH and pre‐eclampsia, as well as the need for evidence about the effect of PA on pre‐eclampsia incidence.
Although this umbrella provides important insights, some limitations should be noted. First, most RCTs and meta‐analyses have methodological shortcomings mainly because blinding is not possible in this type of intervention. Second, many of the reviews included in our umbrella review included the same primary studies, revealing a large overlap between published systematic reviews and meta‐analyses (Tables S14–S17). We have conducted the pooled meta‐analysis with original studies where each study counted once to avoid multiple contributions of the same studies. Third, we excluded studies that only included women with specific diseases, GDM or HDP. Fourth, an important limitation is that most primary studies did not provide data on the pre‐pregnancy PA of participants; consequently, this important confounder was not controlled. Fifth, the included meta‐analyses had a broad variety in their primary studies, mainly because of their different included and excluded criteria or the dates for developing, between others. Finally, as a result of the large amount of data, we decided to include only outcomes regarding GDM and HDP of the protocol published.
4.3. Interpretation
Our estimates of a 39% reduction in the incidence of developing GDM agree with those reported by most systematic reviews and meta‐analyses 15 , 21 , 24 , 26 , 49 , 53 , 55 , 58 , 61 , 62 , 65 and reveal that the reduction in GDM incidence still tends to be significant in most subgroup analyses (Table S8). However, six systematic reviews and meta‐analyses included in our umbrella review did not find a reduction in GDM. 17 , 18 , 54 , 57 , 59 , 60 Most of these were conducted in pregnant women with obesity and overweight. It is known that exercise interventions are generally less effective in women with a high pre‐pregnancy BMI. 49 This could be because the largest RCTs included initiated the intervention at 20 weeks of gestation, when insulin resistance is likely to have already developed. 75
The updated meta‐analysis shows that the incidence of GDM in the IGs is 39% lower than in the CGs. Furthermore, according to a previous systematic reviews and meta‐analyses, 21 the incidence of GDM was lower when the intervention started during the first trimester of pregnancy, was supervised, or had a light to moderate level of intensity. In addition, supervision during exercise performance has been highlighted as an important determinant in adherence to exercise interventions, and so it would increase the effectiveness in preventing GDM. 115
ACOG hypothesised that exercise could stimulate placental angiogenesis and improve maternal endothelial function. 116 Subsequent evidence supports that mild and moderate intensity exercise increases angiogenesis without increasing placental oxidative or endoplasmic stress. 117 However, the evidence about the effect of exercise on HDP incidence remains inconsistent. 21 , 54 Our analyses support, according to previous reports, 22 , 26 that exercise decreases GH but does not reduce the pre‐eclampsia incidence. 22 This could be attributed to differences in the sample characteristics, 54 heterogeneity in the exercise intervention design, or that the number of studies included in the meta‐analyses, or their statistical powers, was not sufficient 22 because pre‐eclampsia is not a common disorder, occurring in 1.4–4% of all pregnancies. 118
5. CONCLUSIONS
In summary, the current evidence supports that exercise has a beneficial effect on the incidence of GDM and GH in non–overweight or obese pregnant women. Furthermore, these benefits are greater when exercise interventions are supervised, have a low to moderate intensity level, and are initiated during the first trimester of pregnancy. Nevertheless, more high‐quality intervention studies are needed to accurately evaluate the safety and benefits of exercise programmes for specific pregnant populations, such as women with overweight and obesity, and whether higher intensity exercise interventions result in greater benefits in these groups. In addition, according to our data, to achieve greater benefits, the core of our recommendation for clinicians is that exercise should be supervised, initiated in the first trimester of pregnancy, and lasting more than 45 minutes per session. However, few studies have reported a reduction in HDP among women with overweight and obesity. More studies are needed to test the effectiveness of exercise interventions to reduce the incidence of pre‐eclampsia in pregnant women with excess weight.
AUTHOR CONTRIBUTIONS
All authors conceived and designed the study. GSM, JAMH and VMV acquired and collected the data. GSM, JAMH, RFR, CPM, CAB, ICR and VMV analysed the data. All authors drafted and critically revised the manuscript for important intellectual content and gave final approval of the version to be published.
FUNDING INFORMATION
This study was funded by the Consejería de Educación, Cultura y Deportes—Junta de Comunidades de Castilla‐La Mancha and FEDER funds (SBPLY/17/180501/000533). CP‐M is supported by a grant from the Universidad de Castilla‐La Mancha (2018‐CPUCLM‐7939).
CONFLICT OF INTEREST
None declared. Completed disclosure of interests form available to view online as supporting information.
Supporting information
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
ACKNOWLEDGEMENTS
None.
Martínez‐Vizcaíno V, Sanabria‐Martínez G, Fernández‐Rodríguez R, Cavero‐Redondo I, Pascual‐Morena C, Álvarez‐Bueno C, et al. Exercise during pregnancy for preventing gestational diabetes mellitus and hypertensive disorders: An umbrella review of randomised controlled trials and an updated meta‐analysis. BJOG. 2023;130(3):264–275. 10.1111/1471-0528.17304
DATA AVAILABILITY STATEMENT
The protocol can be accessed on PROSPERO (Registration number: CRD42019123410). The review protocol has also been published. 29 In addition, data can be accessed upon request by e‐mailing the corresponding author (gema.sanabria@uclm.es). Reuse only with permission and with citation. The data that support the findings of this study are available in the Supplementary material of this article.
REFERENCES
- 1. Committee on Practice Bulletins—Obstetric . ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–64. [DOI] [PubMed] [Google Scholar]
- 2. Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent type 2 diabetes among U.S. women. Diabetes Res Clin Pract. 2018;141:200–8. [DOI] [PubMed] [Google Scholar]
- 3. Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012‐2016. MMWR Morb Mortal Wkly Rep. 2016;67:1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eades CE, Cameron DM, Evans JMM. Prevalence of gestational diabetes mellitus in Europe: a meta‐analysis. Diabetes Res Clin Pract. 2017;129:173–81. 10.1016/j.diabres.2017.03.030 [DOI] [PubMed] [Google Scholar]
- 5. Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta‐analysis. Diabetes Care. 2011;34(1):223–9. 10.2337/dc10-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lind JM, Hennessy A, McLean M. Cardiovascular disease in women: the significance of hypertension and gestational diabetes during pregnancy. Curr Opin Cardiol. 2014;29:447–53. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43:514–31. [DOI] [PubMed] [Google Scholar]
- 8. Kampmann U, Madsen LSG. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, SOGC Hypertension Guideline Committee . Evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:575–6. [DOI] [PubMed] [Google Scholar]
- 10. Lane‐Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long‐term cardiovascular risks associated with adverse pregnancy outcomes. J Am Coll Cardiol. 2019;73:2106–16. [DOI] [PubMed] [Google Scholar]
- 11. Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(2):14–20. 10.1159/000371628 [DOI] [PubMed] [Google Scholar]
- 12. Sibai BM, Ross MG. Hypertension in gestational diabetes mellitus: pathophysiology and long‐term consequences. J Matern Neonatal Med. 2010;23:229–33. [DOI] [PubMed] [Google Scholar]
- 13. Thompson D, Berger H, Feig D, Gagnon R, Kader T, Keely E, et al. Diabetes and pregnancy. Can J Diabetes. 2013;37:S168–83. 10.1016/j.jcjd.2013.01.044 [DOI] [PubMed] [Google Scholar]
- 14. Padayachee C, Coombes JS. Exercise guidelines for gestational diabetes mellitus. World J Diabetes. 2015;6(8):1033–44. 10.4239/wjd.v6.i8.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanabria‐Martínez G, García‐Hermoso A, Poyatos‐León R, Álvarez‐Bueno C, Sánchez‐López M, Martínez‐Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta‐analysis. BJOG. 2015;122(9):1167–74. 10.1111/1471-0528.13429 [DOI] [PubMed] [Google Scholar]
- 16. Du MC, Ouyang YQ, Nie XF, Huang YRS. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: a meta‐analysis. Birth. 2018;46:211–21. [DOI] [PubMed] [Google Scholar]
- 17. Yin Y, Li X, Tao T, Luo BR, Liao SJ. Physical activity during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta‐analysis of randomised controlled trials. Br J Sports Med. 2014;48(4):290–5. 10.1136/bjsports-2013-092596 [DOI] [PubMed] [Google Scholar]
- 18. Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;(7):CD009021. 10.1002/14651858.CD009021.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rudra CB, Sorensen TK, Luthy DA, Williams MA. A prospective analysis of recreational physical activity and preeclampsia risk. Med Sci Sports Exerc. 2008;40:1581–8. [DOI] [PubMed] [Google Scholar]
- 20. Vollebregt KC, Wolf H, Boer K, van der Wal MF, Vrijkotte TG, Bonsel GJ. Does physical activity in leisure time early in pregnancy reduce the incidence of preeclampsia or gestational hypertension? Acta Obstet Gynecol Scand. 2010;89:261–7. [DOI] [PubMed] [Google Scholar]
- 21. Davenport MH, Ruchat S‐M, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta‐analysis. Br J Sports Med. 2018;52(21):1367–75. 10.1136/bjsports-2018-099355 [DOI] [PubMed] [Google Scholar]
- 22. Magro‐Malosso ER, Saccone G, Di Tommaso M, Roman A, Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2017;96(8):921–31. 10.1111/aogs.13151 [DOI] [PubMed] [Google Scholar]
- 23. Poyatos‐Leon R, Garcia‐Hermoso A, Sanabria‐Martinez G, Alvarez‐Bueno C, Cavero‐Redondo I, Martinez‐Vizcaino V. Effects of exercise‐based interventions on postpartum depression: a meta‐analysis of randomized controlled trials. Birth. 2017;44(3):200–8. 10.1111/birt.12294 [DOI] [PubMed] [Google Scholar]
- 24. Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose‐response meta‐analysis of epidemiological studies. Eur J Epidemiol. 2016;31(10):967–97. 10.1007/s10654-016-0176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva S, Ricardo LI, Evenson KR, Hallal PC. Leisure‐time physical activity in pregnancy and maternal‐child health: a systematic review and meta‐analysis of randomized controlled trials and cohort studies. Sports Med. 2017;47(2):295–317. 10.1007/s40279-016-0565-2 [DOI] [PubMed] [Google Scholar]
- 26. Rogozinska E, Marlin N, Betran AP, Astrup A, Barakat R, Bogaerts A, et al. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta‐analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119. 10.1136/bmj.j3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 28. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2, 2021. Part 1, Chapter V: Overviews of Reviews. Handbook . 2011;2:649. [Google Scholar]
- 29. Sanabria‐Martínez G, Poyatos‐León R, Notario‐Pacheco B, Álvarez‐Bueno C, Cavero‐Redondo I, Martinez‐Vizcaino V. Effects of physical exercise during pregnancy on mothers' and neonates' health: a protocol for an umbrella review of systematic reviews and meta‐analysis of randomised controlled trials. BMJ Open. 2019;9(9):e030162. 10.1136/bmjopen-2019-030162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American College of Obstetricians and Gynecologists . Task force on hypertension in pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–31. 10.1097/01.AOG.0000437382.03963.88 [DOI] [PubMed] [Google Scholar]
- 31. ACOG Committee opinion N 804. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2020;135(4):e178–88. 10.1097/AOG.0000000000003772 [DOI] [PubMed] [Google Scholar]
- 32. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guyatt GH. GrADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 36. Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–6. 10.7326/0003-4819-127-9-199711010-00008 [DOI] [PubMed] [Google Scholar]
- 37. Fusar‐Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100. 10.1136/ebmental-2018-300014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Stat Med. 2000;19(22):3127–31. [DOI] [PubMed] [Google Scholar]
- 39. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. J Clin Epidemiol. 2008;61(8):763–9. [DOI] [PubMed] [Google Scholar]
- 40. Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, Ltd; 2008. p. 243–96. 10.1002/9780470712184.ch9 [DOI] [Google Scholar]
- 41. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. 10.1136/BMJ.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simonsohn U, Nelson LD, Simmons JP. P‐curve: a key to the file‐drawer. J Exp Psychol Gen. 2014;143(2):534. [DOI] [PubMed] [Google Scholar]
- 43. Stanley TD. Meta‐regression methods for detecting and estimating empirical effects in the presence of publication selection. Oxf Bull Econ Stat. 2008;70(1):103–27. [Google Scholar]
- 44. Stanley TD, Doucouliagos H. Meta‐regression approximations to reduce publication selection bias. Res Synth Methods. 2014;5(1):60–78. [DOI] [PubMed] [Google Scholar]
- 45. Rücker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics. 2011;12(1):122–42. [DOI] [PubMed] [Google Scholar]
- 46. Pelaez M, Gonzalez‐Cerron S, Montejo R, Barakat R. Protective effect of exercise in pregnant women including those who exceed weight gain recommendations: a randomized controlled trial. Mayo Clin Proc. 2019;94(10):1951–9. 10.1016/j.mayocp.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 47. Taniguchi C, Sato C. Home‐based walking during pregnancy affects mood and birth outcomes among sedentary women: a randomized controlled trial. Int J Nurs Pract. 2016;22(5):420–6. 10.1111/ijn.12453 [DOI] [PubMed] [Google Scholar]
- 48. Khoram S, Loripoor M, Pirhadi M, Beigi M. The effect of walking on pregnancy blood pressure disorders in women susceptible to pregnancy hypertension: a randomized clinical trial. J Educ Health Promot. 2019;8(1):95. 10.4103/jehp.jehp_378_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bennett CJ, Walker RE, Blumfield ML, Gwini SM, Ma J, Wang F, et al. Interventions designed to reduce excessive gestational weight gain can reduce the incidence of gestational diabetes mellitus: a systematic review and meta‐analysis of randomised controlled trials. Diabetes Res Clin Pract. 2018;141:69–79. 10.1016/j.diabres.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 50. Díaz‐Burrueco JR, Cano‐Ibáñez N, Martín‐Peláez S, Khan KS, Amezcua‐Prieto C. Effects on the maternal‐fetal health outcomes of various physical activity types in healthy pregnant women. A systematic review and meta‐analysis. Eur J Obstet Gynecol Reprod Biol. 2021;262:203–15. 10.1016/j.ejogrb.2021.05.030 [DOI] [PubMed] [Google Scholar]
- 51. Guo X‐Y, Shu J, Fu X‐H, Chen XP, Zhang L, Ji MX, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta‐analysis and meta‐regression. BJOG. 2019;126(3):311–20. 10.1111/1471-0528.15467 [DOI] [PubMed] [Google Scholar]
- 52. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. 10.1002/14651858.CD000180 [DOI] [PubMed] [Google Scholar]
- 53. Ming WK, Ding W, Zhang CJP, Zhong L, Long Y, Li Z, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal‐weight women: a systematic review and meta‐analysis. BMC Pregnancy Childbirth. 2018;18(1):440. 10.1186/s12884-018-2068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muhammad HFL, Pramono A, Rahman MN. The safety and efficacy of supervised exercise on pregnant women with overweight/obesity: a systematic review and meta‐analysis of randomized controlled trials. Clin Obes. 2020;11:e12428. 10.1111/cob.12428 [DOI] [PubMed] [Google Scholar]
- 55. Doi SAR, Furuya‐Kanamori L, Toft E, Musa OAH, Mohamed AM, Clark J, et al. Physical activity in pregnancy prevents gestational diabetes: a meta‐analysis. Diabetes Res Clin Pract. 2020;168:108371. 10.1016/j.diabres.2020.108371 [DOI] [PubMed] [Google Scholar]
- 56. Di Mascio D, Magro‐Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal‐weight women and risk of preterm birth: a systematic review and meta‐analysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(5):561–71. 10.1016/j.ajog.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 57. Magro‐Malosso ER, Saccone G, Di Mascio D, Di Tommaso M, Berghella V. Exercise during pregnancy and risk of preterm birth in overweight and obese women: a systematic review and meta‐analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2017;96(3):263–73. 10.1111/aogs.13087 [DOI] [PubMed] [Google Scholar]
- 58. Nasiri‐Amiri F, Sepidarkish M, Shirvani MA, Habibipour P, Tabari NSM. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: a systematic review and meta‐analysis. Diabetol Metab Syndr. 2019;11:72. 10.1186/s13098-019-0470-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Russo LM, Nobles C, Ertel KA, Chasan‐Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta‐analysis. Obstet Gynecol. 2015;125(3):576–82. 10.1097/AOG.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 60. Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta‐analysis of randomized controlled trials. Obes Rev. 2016;17(10):960–9. 10.1111/obr.12442 [DOI] [PubMed] [Google Scholar]
- 61. Xing Y, Wang X, Zhang W, Jiang H. The effect of exercise on maternal complications and birth outcomes in overweight or obese pregnant women: a meta‐analysis. Ann Palliat Med. 2020;9(6):4103–12. 10.21037/apm-20-2097 [DOI] [PubMed] [Google Scholar]
- 62. Yu Y, Xie R, Shen C, Shu L. Effect of exercise during pregnancy to prevent gestational diabetes mellitus: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med. 2018;31(12):1632–7. 10.1080/14767058.2017.1319929 [DOI] [PubMed] [Google Scholar]
- 63. Zheng J, Wang H, Ren M. Influence of exercise intervention on gestational diabetes mellitus: a systematic review and meta‐analysis. J Endocrinol Invest. 2017;40(10):1027–33. 10.1007/s40618-017-0673-3 [DOI] [PubMed] [Google Scholar]
- 64. Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;2006(3):CD000180. 10.1002/14651858.CD000180.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. da Silva SG, da Silva BGC, Coll CVN, Domingues MR, Evenson KR, Hallal PC. Effects of an exercise intervention on gestational weight gain. Med Sci Sports Exerc. 2016;48:644. 10.1249/01.mss.0000486931.59434.ce [DOI] [Google Scholar]
- 66. Barakat R, Lucia A, Ruiz JR. Resistance exercise training during pregnancy and newborn's birth size: a randomised controlled trial. Int J Obes (Lond). 2009;33(9):1048–57. 10.1038/ijo.2009.150 [DOI] [PubMed] [Google Scholar]
- 67. Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33(7):1457–9. 10.2337/dc09-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cordero Y, Mottola MF, Vargas J, Blanco M, Barakat R. Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc. 2015;47(7):1328–33. 10.1249/MSS.0000000000000547 [DOI] [PubMed] [Google Scholar]
- 69. Daly N, Farren M, McKeating A, O'Kelly R, Stapleton M, Turner MJ. A medically supervised pregnancy exercise intervention in obese women: a randomized controlled trial. Obstet Gynecol. 2017;130(5):1001–10. 10.1097/AOG.0000000000002267 [DOI] [PubMed] [Google Scholar]
- 70. Garnæs KK, Mørkved S, Salvesen Ø, Moholdt T. Exercise training in pregnancy for women with Bmi ≥ 28. A randomized controlled trial. Med Sci Sports Exerc. 2016;48:931–2. 10.1249/01.mss.0000487788.31806.bb [DOI] [Google Scholar]
- 71. Guelfi KJ, Ong MJ, Crisp NA, Fournier PA, Wallman KE, Grove JR, et al. Regular exercise to prevent the recurrence of gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2016;128:819–27. 10.1097/AOG.0000000000001632 [DOI] [PubMed] [Google Scholar]
- 72. Hui AL, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean HJ, et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre‐pregnancy body mass index in a randomized control trial. BMC Pregnancy Childbirth. 2014;14:331. 10.1186/1471-2393-14-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ko CW, Napolitano PG, Lee SP, Schulte SD, Ciol MA, Beresford SAA. Physical activity, maternal metabolic measures, and the incidence of gallbladder sludge or stones during pregnancy: a randomized trial. Am J Perinatol. 2014;31(1):39–48. 10.1055/s-0033-1334455 [DOI] [PubMed] [Google Scholar]
- 74. Kong KL, Campbell CG, Foster RC, Peterson AD, Lanningham‐Foster L. A pilot walking program promotes moderate‐intensity physical activity during pregnancy. Med Sci Sports Exerc. 2014;46(3):462–71. 10.1249/MSS.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 75. Nobles C, Marcus BH, Stanek EJ 3rd, Braun B, Whitcomb BW, Manson JE, et al. The effect of an exercise intervention on gestational weight gain: the behaviors affecting baby and you (B.A.B.Y.) study: a randomized controlled trial. Am J Health Promot. 2018;32(3):736–44. 10.1177/0890117117732409 [DOI] [PubMed] [Google Scholar]
- 76. Okido MM, Valeri FL, Martins WP, Ferreira CHJ, Duarte G, Cavalli RC. Assessment of foetal wellbeing in pregnant women subjected to pelvic floor muscle training: a controlled randomised study. Int Urogynecol J. 2015;26(10):1475–81. 10.1007/s00192-015-2719-4 [DOI] [PubMed] [Google Scholar]
- 77. Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24‐28 weeks: a randomised controlled trial. Br J Sports Med. 2012;46(9):656–61. 10.1136/bjsports-2011-090009 [DOI] [PubMed] [Google Scholar]
- 78. Oostdam N, van Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. 2012;119(9):1098–107. 10.1111/j.1471-0528.2012.03366.x [DOI] [PubMed] [Google Scholar]
- 79. Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Neonatal Med. 2014;27(13):1348–52. 10.3109/14767058.2013.858318 [DOI] [PubMed] [Google Scholar]
- 80. Renault KM, Nørgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The treatment of obese pregnant women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210(2):134.e1–9. 10.1016/j.ajog.2013.09.029 [DOI] [PubMed] [Google Scholar]
- 81. Cordero Rodríguez Y, Puente MP, De M, Abad M, Santaella MP, Carballo RB. Can moderate physical exercise during pregnancy act as a factor in preventing gestational diabetes. Rev Int Cienc Deporte. 2012;8(27):3–19. 10.5232/ricyde2012.02701 [DOI] [Google Scholar]
- 82. Ruiz JR, Perales M, Pelaez M, Lopez C, Lucia A, Barakat R. Supervised exercise‐based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc. 2013;88(12):1388–97. 10.1016/j.mayocp.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 83. Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. 2016;123(4):588–97. 10.1111/1471-0528.13738 [DOI] [PubMed] [Google Scholar]
- 84. Simmons D, Devlieger R, van Assche A, Jans G, Galjaard S, Corcoy R, et al. Effect of physical activity and/or healthy eating ongdm risk: the Dali lifestyle study. J Clin Endocrinol Metab. 2017;102(3):903–13. 10.1210/jc.2016-3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stafne SN, Salvesen KÅ, Romundstad PR, Eggebø TM, Carlsen SM, Mørkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol. 2012;119(1):29–36. 10.1097/AOG.0b013e3182393f86 [DOI] [PubMed] [Google Scholar]
- 86. Tomić V, Sporiš G, Tomić J, Milanović Z, Zigmundovac‐Klaić D, Pantelić S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat Med J. 2013;54(4):362–8. 10.3325/cmj.2013.54.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ussher M, Lewis S, Aveyard P, Manyonda I, West R, Lewis B, et al. The London exercise and pregnant smokers (LEAP) trial: a randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technol Assess. 2015;19(84):1–135. 10.3310/hta19840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barakat R, Pelaez M, Lopez C, Montejo R, Coteron J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: results of a randomized controlled trial. J Matern Neonatal Med. 2012;25(11):2372–6. 10.3109/14767058.2012.696165 [DOI] [PubMed] [Google Scholar]
- 89. Vinter CA, Jensen DM, Ovesen P, Beck‐Nielsen H, Jørgensen JS. The LiP (lifestyle in pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34(12):2502–7. 10.2337/dc11-1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216(4):340–51. 10.1016/j.ajog.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 91. Price BB, Amini SB, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes–a randomized trial. Med Sci Sports Exerc. 2012;44(12):2263–9. 10.1249/MSS.0b013e318267ad67 [DOI] [PubMed] [Google Scholar]
- 92. Rakhshani A, Nagarathna R, Mhaskar R, Mhaskar A, Thomas A, Gunasheela S. The effects of yoga in prevention of pregnancy complications in high‐risk pregnancies: a randomized controlled trial. Prev Med. 2012;55(4):333–40. 10.1016/j.ypmed.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 93. da Silva SG, Hallal PC, Domingues MR, Bertoldi AD, Silveira MFD, Bassani D, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;14(1):175. 10.1186/s12966-017-0632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barakat R, Pelaez M, Lopez C, Lucia A, Ruiz JR. Exercise during pregnancy and gestational diabetes‐related adverse effects: a randomised controlled trial. Br J Sports Med. 2013;47(10):630–6. 10.1136/bjsports-2012-091788 [DOI] [PubMed] [Google Scholar]
- 95. Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot. 2014;29(1):2–8. 10.4278/ajhp.130131-QUAN-56 [DOI] [PubMed] [Google Scholar]
- 96. Barakat R, Pelaez M, Montejo R, Refoyo I, Coteron J. Exercise throughout pregnancy does not cause preterm delivery: a randomized, controlled trial. J Phys Act Health. 2014;11(5):1012–7. 10.1123/jpah.2012-0344 [DOI] [PubMed] [Google Scholar]
- 97. Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. 2016;214(5):649.e1–8. 10.1016/j.ajog.2015.11.039 [DOI] [PubMed] [Google Scholar]
- 98. Barakat R, Refoyo I, Coteron J, Franco E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Brazilian . J Phys Ther. 2019;23(2):148–55. 10.1016/j.bjpt.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bisson M, Alméras N, Dufresne SS, Robitaille J, Rhéaume C, Bujold E, et al. A 12‐week exercise program for pregnant women with obesity to improve physical activity levels: an open randomised preliminary study. PLoS One. 2015;10(9):e0137742. 10.1371/journal.pone.0137742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Haakstad LAH, Bø K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2011;16(2):116–25. 10.3109/13625187.2011.560307 [DOI] [PubMed] [Google Scholar]
- 101. Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab. 2010;95(5):2080–8. 10.1210/jc.2009-2255 [DOI] [PubMed] [Google Scholar]
- 102. Murtezani A, Paçarada M, Ibraimi Z, Nevzati A, Abazi N. The impact of exercise during pregnancy on neonatal outcomes: a randomized controlled trial. J Sports Med Phys Fitness. 2014;54(6):802–8. [PubMed] [Google Scholar]
- 103. Pelaez M, Gonzalez‐Cerron S, Montejo R, Barakat R. Pelvic floor muscle training included in a pregnancy exercise program is effective in primary prevention of urinary incontinence: a randomized controlled trial. NeurourolUrodyn. 2014;33(1):67–71. 10.1002/nau.22381 [DOI] [PubMed] [Google Scholar]
- 104. Perales M, Mateos S, Vargas M, Sanz I, Lucia A, Barakat R. Fetal and maternal heart rate responses to exercise in pregnant women. A randomized controlled trial. Arch Med Deport. 2015;32(6):361–7. [Google Scholar]
- 105. de Oliveria Melo AS, Silva JLP, Tavares JS, Barros VO, Leite DFB, Amorim MMR. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol. 2012;120(2):302–10. 10.1097/AOG.0b013e31825de592 [DOI] [PubMed] [Google Scholar]
- 106. Erkkola R. The influence of physical training during pregnancy on physical work capacity and circulatory parameters. Scand J Clin Lab Invest. 1976;36(8):747–54. 10.3109/00365517609081933 [DOI] [PubMed] [Google Scholar]
- 107. Kasawara KT, Burgos CSG, do Nascimento SL, Ferreira NO, Surita FG, Silva JLPE. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: a randomized controlled trial. ISRN Obstet Gynecol. 2013;2013:1–8. 10.1155/2013/857047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Barakat R, Ruiz JR, Stirling JR, Zakynthinaki M, Lucia A. Type of delivery is not affected by light resistance and toning exercise training during pregnancy: a randomized controlled trial. Am J Obstet Gynecol. 2009;201(6):590.e1–6. 10.1016/j.ajog.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 109. Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol. 2011;204(5):402.e1–7. 10.1016/j.ajog.2011.01.043 [DOI] [PubMed] [Google Scholar]
- 110. Garnæs KK, Mørkved S, Salvesen Ø, Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP trial). PLoS Med. 2016;13(7):e1002079. 10.1371/journal.pmed.1002079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Collings CA, Curet LB, Mullin JP. Maternal and fetal responses to a maternal aerobic exercise program. Am J Obstet Gynecol. 1983;145(6):702–7. 10.1016/0002-9378(83)90576-8 [DOI] [PubMed] [Google Scholar]
- 112. Elden H, Ostgaard HC, Fagevik‐Olsen M, Ladfors L, Hagberg H. Treatments of pelvic girdle pain in pregnant women: adverse effects of standard treatment, acupuncture and stabilising exercises on the pregnancy, mother, delivery and the fetus/neonate. BMC Complement Altern Med. 2008;8(1):1–13. 10.1186/1472-6882-8-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kordi R, Abolhasani M, Rostami M, Hantoushzadeh S, Mansournia MA, Vasheghani‐Farahani F. Comparison between the effect of lumbopelvic belt and home based pelvic stabilizing exercise on pregnant women with pelvic girdle pain; A randomized controlled trial. J Back Musculoskelet Rehabil. 2013;26(2):133–9. 10.3233/BMR-2012-00357 [DOI] [PubMed] [Google Scholar]
- 114. Bhartia N, Jain S, Shankar N, Rajaram S, Gupta M. Effects of antenatal yoga on maternal stress and clinical outcomes in north Indian women: a randomised controlled trial. J Indian Assoc Clin Med. 2019;1(20):10–4. [Google Scholar]
- 115. Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum: a systematic review and meta‐analysis of randomized controlled trials. Prev Med. 2013;56(6):351–64. 10.1016/j.ypmed.2013.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. ACOG . The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin No. 202. Obstet Gynecol. 2019;133(1):e1–e25. 10.1097/AOG.0000000000003018 [DOI] [Google Scholar]
- 117. Hardy DB, Mu X, Marchiori KS, Mottola MF. Exercise in pregnancy increases placental angiogenin without changes in oxidative or endoplasmic reticulum stress. Med Sci Sports Exerc. 2021;53:1846–54. 10.1249/MSS.0000000000002647 [DOI] [PubMed] [Google Scholar]
- 118. Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population‐based trends in pregnancy hypertension and pre‐eclampsia: an international comparative study. BMJ Open. 2011;1(1):e000101. 10.1136/bmjopen-2011-000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Data Availability Statement
The protocol can be accessed on PROSPERO (Registration number: CRD42019123410). The review protocol has also been published. 29 In addition, data can be accessed upon request by e‐mailing the corresponding author (gema.sanabria@uclm.es). Reuse only with permission and with citation. The data that support the findings of this study are available in the Supplementary material of this article.


