Abstract
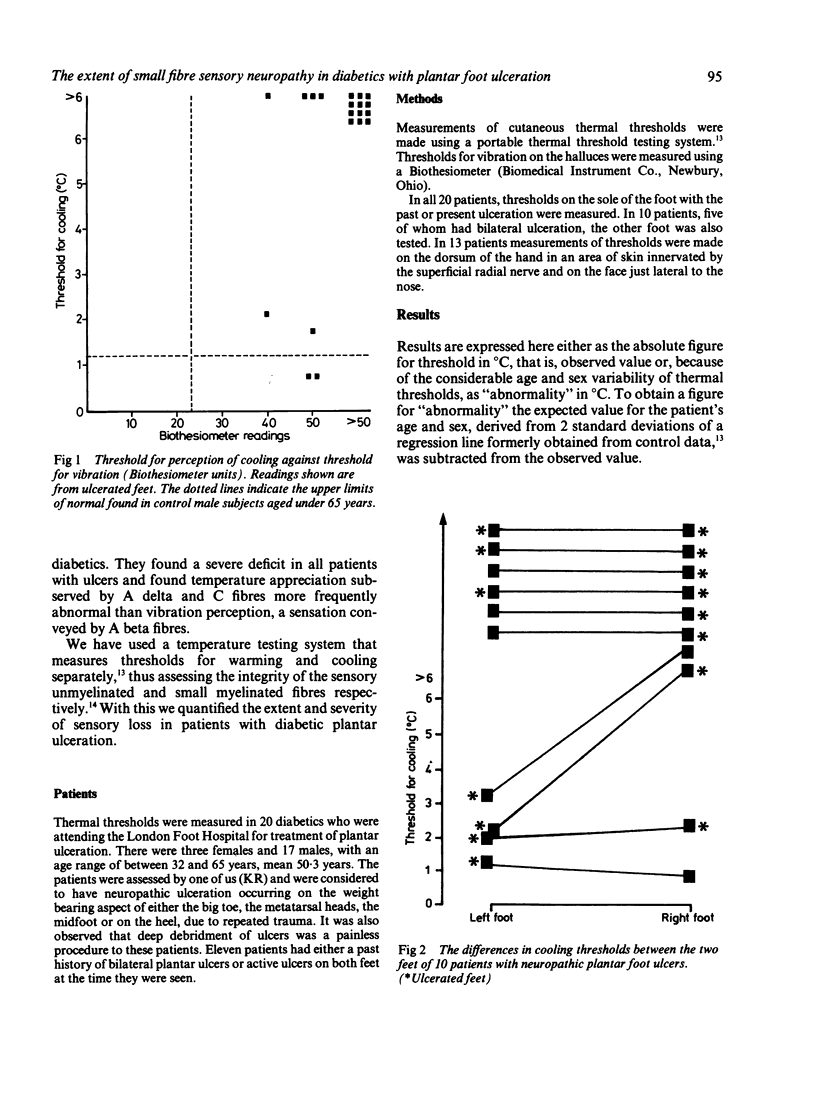
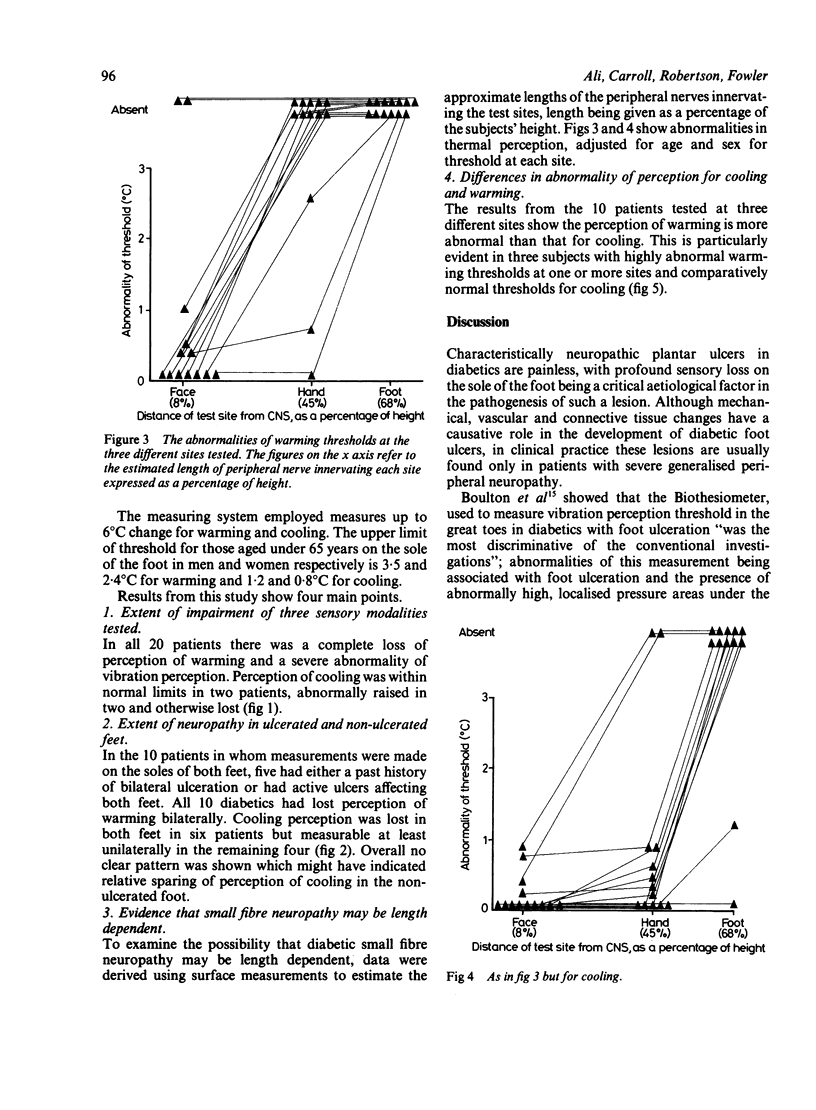
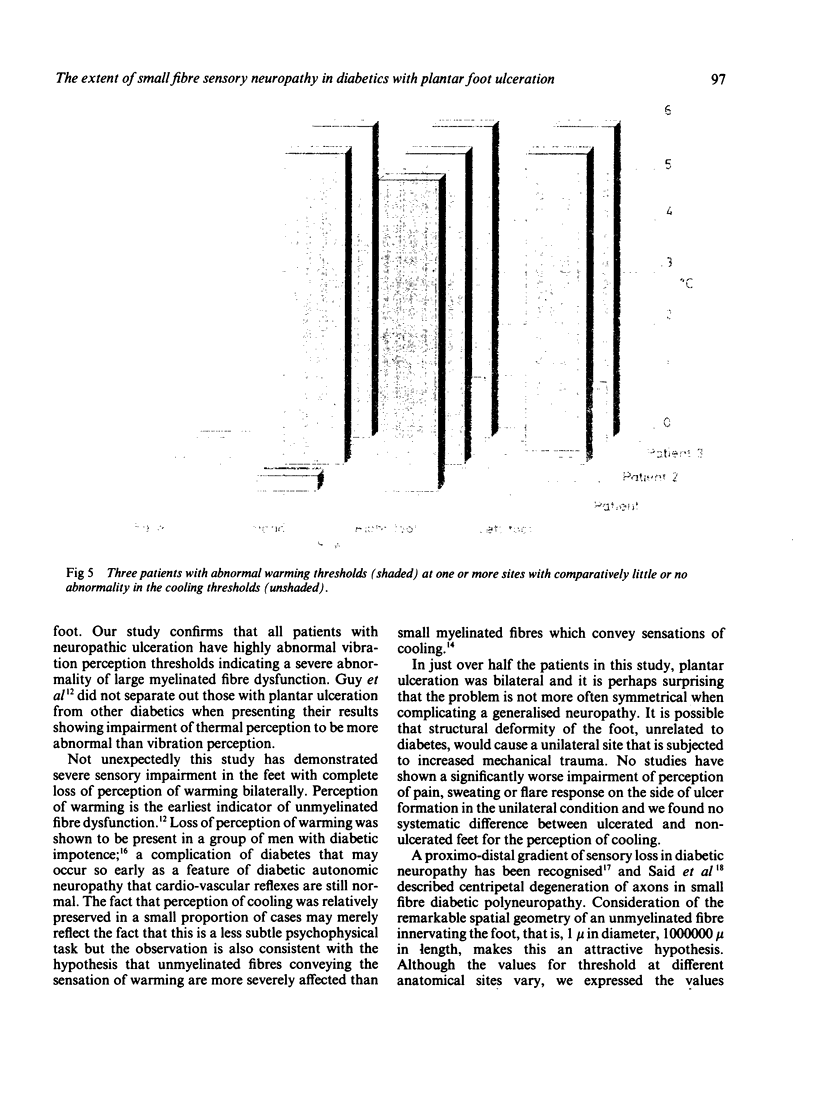
Thresholds for cutaneous warming and cooling stimuli were measured in 20 diabetics with neuropathic foot ulcers. All patients had a profound disturbance of sensory perception in the ulcerated foot with complete loss of perception of warming; thresholds for vibration and cooling were highly abnormal in all but two patients. Measurements of thermal threshold were made on both feet in 10 patients: warming was lost bilaterally in all, and cooling was bilaterally absent in six. There was no clear pattern of sensory loss in those diabetics with unilateral foot ulceration to suggest that sensory impairment was the determining factor for the development of a plantar ulcer. Measurements of thermal thresholds were made at additional sites in 13 patients and although the most marked abnormalities of sensation were always found in the feet, in some severe neuropaths, abnormal thresholds on the hand and even the face were demonstrated. Thresholds for warming were invariably more abnormal than thresholds for cooling. The diabetics with neuropathic ulceration in this study all had severe generalised peripheral nerve disease involving large myelinated as well as both small myelinated and unmyelinated sensory fibres. The quantitative evidence on the distribution of sensory loss for thermal sensations supports the hypothesis that the neuropathic process affecting the small myelinated and unmyelinated fibres is length dependent.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. E., Delbridge L., Le Quesne L. P. The role of autonomic neuropathy in diabetic foot ulceration. J Neurol Neurosurg Psychiatry. 1986 Sep;49(9):1002–1006. doi: 10.1136/jnnp.49.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M. E., Le Quesne P. M. Quantitative sweat test in diabetics with neuropathic foot lesions. J Neurol Neurosurg Psychiatry. 1986 Sep;49(9):1059–1062. doi: 10.1136/jnnp.49.9.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton A. J., Hardisty C. A., Betts R. P., Franks C. I., Worth R. C., Ward J. D., Duckworth T. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care. 1983 Jan-Feb;6(1):26–33. doi: 10.2337/diacare.6.1.26. [DOI] [PubMed] [Google Scholar]
- Ctercteko G. C., Dhanendran M., Hutton W. C., Le Quesne L. P. Vertical forces acting on the feet of diabetic patients with neuropathic ulceration. Br J Surg. 1981 Sep;68(9):608–614. doi: 10.1002/bjs.1800680904. [DOI] [PubMed] [Google Scholar]
- Deanfield J. E., Daggett P. R., Harrison M. J. The role of autonomic neuropathy in diabetic foot ulceration. J Neurol Sci. 1980 Aug;47(2):203–210. doi: 10.1016/0022-510x(80)90004-0. [DOI] [PubMed] [Google Scholar]
- Delbridge L., Ctercteko G., Fowler C., Reeve T. S., Le Quesne L. P. The aetiology of diabetic neuropathic ulceration of the foot. Br J Surg. 1985 Jan;72(1):1–6. doi: 10.1002/bjs.1800720102. [DOI] [PubMed] [Google Scholar]
- Fowler C. J., Carroll M. B., Burns D., Howe N., Robinson K. A portable system for measuring cutaneous thresholds for warming and cooling. J Neurol Neurosurg Psychiatry. 1987 Sep;50(9):1211–1215. doi: 10.1136/jnnp.50.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. J., Sitzoglou K., Ali Z., Halonen P. The conduction velocities of peripheral nerve fibres conveying sensations of warming and cooling. J Neurol Neurosurg Psychiatry. 1988 Sep;51(9):1164–1170. doi: 10.1136/jnnp.51.9.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R. J., Clark C. A., Malcolm P. N., Watkins P. J. Evaluation of thermal and vibration sensation in diabetic neuropathy. Diabetologia. 1985 Mar;28(3):131–137. doi: 10.1007/BF00273859. [DOI] [PubMed] [Google Scholar]
- Le Quesne P. M., Fowler C. J. A study of pain threshold in diabetics with neuropathic foot lesions. J Neurol Neurosurg Psychiatry. 1986 Oct;49(10):1191–1194. doi: 10.1136/jnnp.49.10.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. A., Caskey P. E., Tuck R. R., Fealey R. D., Dyck P. J. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983 Nov;14(5):573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- Parkhouse N., Le Quesne P. M. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. 1988 May 19;318(20):1306–1309. doi: 10.1056/NEJM198805193182005. [DOI] [PubMed] [Google Scholar]
- Parkhouse N., Le Quesne P. M. Quantitative objective assessment of peripheral nociceptive C fibre function. J Neurol Neurosurg Psychiatry. 1988 Jan;51(1):28–34. doi: 10.1136/jnnp.51.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. P., Le Quesne L. P. Method of healing diabetic forefoot ulcers. Br Med J (Clin Res Ed) 1983 Feb 5;286(6363):436–437. doi: 10.1136/bmj.286.6363.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said G., Slama G., Selva J. Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy. Brain. 1983 Dec;106(Pt 4):791–807. doi: 10.1093/brain/106.4.791. [DOI] [PubMed] [Google Scholar]