Abstract
Background:
Evaluation and interpretation of the literature on obstructive sleep apnea (OSA) allows for consolidation and determination of the key factors important for clinical management of the adult OSA patient. Toward this goal, an international collaborative of multidisciplinary experts in sleep apnea evaluation and treatment have produced the International Consensus statement on Obstructive Sleep Apnea (ICS:OSA).
Methods:
Using previously defined methodology, focal topics in OSA were assigned as literature review (LR), evidence-based review (EBR), or evidence-based review with recommendations (EBR-R) formats. Each topic incorporated the available and relevant evidence which was summarized and graded on study quality. Each topic and section underwent iterative review and the ICS:OSA was created and reviewed by all authors for consensus.
Results:
The ICS:OSA addresses OSA syndrome definitions, pathophysiology, epidemiology, risk factors for disease, screening methods, diagnostic testing types, multiple treatment modalities, and effects of OSA treatment on multiple OSA-associated comorbidities. Specific focus on outcomes with positive airway pressure (PAP) and surgical treatments were evaluated.
Conclusion:
This review of the literature consolidates the available knowledge and identifies the limitations of the current evidence on OSA. This effort aims to create a resource for OSA evidence-based practice and identify future research needs. Knowledge gaps and research opportunities include improving the metrics of OSA disease, determining the optimal OSA screening paradigms, developing strategies for PAP adherence and longitudinal care, enhancing selection of PAP alternatives and surgery, understanding health risk outcomes, and translating evidence into individualized approaches to therapy.
Keywords: atrial fibrillation, cardiovascular event, cerebrovascular disease, consensus, dementia, evidence-based medicine, home sleep apnea testing, hypertension, hypoglossal nerve stimulation, mortality, motor vehicle accidents, neurocognitive function, obstructive sleep apnea, outcomes, PAP adherence, perioperative management, polysomnography, positive airway pressure, screening, sleep, sleep disordered breathing, sleepiness, sleep surgery, surgical outcomes, systematic review, treatment outcomes, uvulopalatopharyngoplasty
I |. INTRODUCTION
Obstructive sleep apnea (OSA) is a complex and multifaceted disease with multiple associated symptoms and comorbidities. Work performed within the last decade has contributed to expanding knowledge of disease incidence, new approaches to diagnosis, and novel improvements in therapeutic options. OSA was first defined in 1965.1,2 For many years, the only therapy was tracheotomy tube placement to bypass upper airway (UA) obstruction. Continuous positive airway pressure (CPAP) therapy was introduced in 19813 and marked a pivotal discovery in OSA treatment. Since that time, growth in the literature and understanding of OSA as a heterogeneous and complex chronic disease has been exponential. Our abilities to diagnose OSA and to determine its far-reaching consequences have advanced significantly. OSA is currently recognized as a common and important major health issue, imposing a large cost on health systems around the world.
This International Consensus Statement on Obstructive Sleep Apnea (ICS:OSA) was created to summarize the best available evidence into a format that allows clinicians to examine diagnosis and management options for adult OSA, to understand the quality of evidence, and to translate the findings and recommendations into evidence-based care. Contributions came from more than 130 international authors from various OSA specialties including neurology, pulmonology, sleep medicine, otolaryngology, oral-maxillofacial surgery, dentistry, anesthesiology, psychiatry, cardiology, and sleep physiology. The specialists contributing to this statement represent a diverse set of expertise that encompass the multidisciplinary approach necessary to understand and treat OSA. Topics on OSA were assigned to experts who utilized a structured review process to evaluate and interpret the evidence. The ICS:OSA recommendations for diagnosis and management of OSA rely directly on the reviewed evidence with delineations of the benefits, harms, and costs that were considered for each recommendation.
This document highlights the current understanding and impact of OSA in adult patients. The ICS:OSA utilizes an evidence-based format defined by the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis in 2016 (ICAR-RS-2016),4 which was adapted from a framework5 that uses a blinded iterative review process. This method has been used for other subjects including allergic rhinitis (AR), skull base surgery, and olfaction. For ICS:OSA, experts in the fields of sleep medicine and sleep surgery contributed to its creation as both section authors and blinded reviewers of other sections. Each section attempts to emphasize published, peer-reviewed evidence where available and identify gaps in knowledge.
The ICS:OSA is not a clinical practice guideline (CPG) and does not employ the steps of a CPG creation. The ICS:OSA includes meta-analyses and other systematic reviews (SRs) when available for specific OSA topic areas but does not perform separate or new meta-analyses on the data summarized. This document aims to summarize and consolidate the best available knowledge on the diagnosis and treatment of OSA, to provide a standardized format to display the evidence, and to allow for alternative interpretations.
Recommendations exhibited in the ICS:OSA are based upon the best available evidence, but for many topics the level and quality of evidence are variable or weak. Summary recommendations should be assessed in the context of the evidence on which they are based and the populations of the studies themselves especially when attempting to translate the data for individualized recommendations. Recommendations in the ICS:OSA do not define standard of care or medical necessity and cannot dictate care of an individual patient. Variability in the presentation, symptoms, treatment responses, and tolerance levels of therapy is appreciated for all complex diseases and OSA is no exception.
The best evidence-based practice utilizes shareddecision making approaches that incorporate evidence with individual patient factors, values, expectations, and goals in creating individualized clinical decisions and recommendations. New and future research in OSA should aim to fill the knowledge gaps and strengthen the evidence that moves us toward optimal care of the OSA patient. As new and stronger evidence is examined, summary recommendations will require reevaluation and updates.
II |. METHODS
II.A |. Topic Development
The ICS:OSA document focused on incorporation and summarization of the published literature. The methodology for ICS:OSA followed that of prior International Consensus in Allergy and Rhinology documents,4,6,7 which involved a process adapted by Rudmik and Smith.5 The approach aims to maximize impact of published evidence by systematically evaluating the literature, grading the evidence, and creating evidence-based recommendations.
The ICS:OSA was divided into over 150 topics, each topic was assigned to a senior author who is a recognized expert in care of OSA patients. Topic generation spanned definitions of respiratory events in polysomnogram testing, controversies in different scoring definitions, epidemiology of disease, economic burden, risk factors, contributory factors for pathogenesis of OSA, diagnosis and screening tools, diagnostic testing modalities, medical comorbidities, medical management, and surgical management for OSA. A focus of the ICS:OSA included the many cardiovascular (CV), cognitive, and metabolic comorbidities associated with OSA which impact OSA screening and management. Separate sections were created to examine the evidence on the effects of PAP and surgical therapy for improving OSA-related symptoms and comorbidity risks.
A few topics based on disease definition or background information were assigned as literature reviews (LRs). Certain topics were not appropriate or lacked sufficient evidence and were assigned as evidence-based reviews (EBRs). Other topics had evidence to inform clinical recommendations were assigned as evidence-based reviews with recommendations (EBR-R).
For each topic, authors were asked to perform an SR of the literature using Ovid MEDLINE (1947-December 2019), EMBASE (1974-December 2019), and Cochrane Review databases. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standardized guidelines8 were used to inform the SRs. Randomized controlled trials (RCTs), meta-analyses, SRs, and published guidelines were first identified to provide the strongest level of evidence (LOE) if available. When these were not available, observational studies were used. Identified studies were included to ensure relevant studies were captured. The majority of the studies included were written in English. If the authors felt a non-English study should be included, the paper was translated and summarized. Important evolving topics did include papers after December 2019 if the paper significantly contributed new understanding or influenced the recommendations.
For EBR and EBR-R sections, relevant studies were displayed in a standardized format with quality of each study graded using the Oxford LOE (LOE Level 1a–5).9 Next an aggregate grade of evidence (A–D) was determined based on guidelines from the American Academy of Pediatrics Steering Committee on Quality Improvement and Management (AAP SCQIM)10 (see Table II.A.1). When appropriate, a recommendation was written using the AAP SCQIM (Table II.A.2). Each recommendation was based on the aggregate LOE along with an assessment of benefit, harm, and costs related to the specific topic.
TABLE II. A. 1.
Aggregate grade of evidence
| Grade | Research quality |
|---|---|
| A | Well-designed RCTs |
| B | RCTs with minor limitations Overwhelming consistent evidence from observational studies |
| C | Observational studies (case–control and cohort design) |
| D | Expert opinion Case reports Reasoning from first principles |
TABLE II.A.2.
AAP defined strategy for recommendation development10
| Evidence quality | Preponderance of benefijit over harm | Balance of benefijit and harm | Preponderance of harm over benefijit |
|---|---|---|---|
| A. Well-designed RCT’s | Strong recommendation | Option | Strong recommendation against |
| B. RCT’s with minor limitations; Overwhelmingly consistent evidence from observational studies | Recommendation | ||
| C. Observational studies (case–control and cohort design) | Recommendation against | ||
| D. Expert opinion, case reports, Reasoning from fijirst principles | Option | No recommendation |
II.B |. Iterative Review
Following the development of each ICS:OSA section in LR, EBR, or EBR-R formats, the section underwent a two-stage iterative review process using at least two independent reviewers. The purpose of the reviews was to evaluate the completeness of literature identification, determine accuracy of the grade of evidence, and ensure the recommendations were appropriate. Most sections were reviewed across disciplines. Following the review process, changes were agreed upon by both reviewers and initial author(s).
II.C |. ICS:OSA Statement Development
After review and edits were completed, the principal editors (J.L.C., A.N.G., and I.R.) synthesized all sections into the ICS:OSA statement. The document draft was then reviewed by all contributing authors. Once consensus was achieved on literature completeness and final recommendations, the final ICS:OSA statement was produced.
As each topic was authored by individuals, search results and level of evidence grading may vary and this document does not present every study published on every topic. Most sections do not include an exhaustive list of all studies ever performed and authors were given the ability to select the most relevant evidence. For certain topics, the literature is extensive and the section focused mainly on high-quality studies or SRs. The editors also made an effort to ensure recommendations aligned with published guidelines.
II.D |. Possible Adverse Effects of OSA Interventions
Throughout ICS:OSA, possible side effects or risks of testing or interventions were considered. In order to standardize a collection of these possible side effects and adverse effects, Table II.D defines typical adverse effects for a variety of OSA interventions for both the immediate and the long term. Risks for these effects should be considered when determining policy level recommendations. Each intervention has the additional risk of insufficient improvement of symptoms and continued OSA disease. Table II.D may not include all possible risks.
TABLE II.D.
Risks, side effects and adverse effects of common obstructive sleep apnea interventionsa
| Intervention | Possible side effects and adverse effects |
|---|---|
| Home sleep study testing | False negative result, diagnosis delay, sensor discomfort or failure, skin irritation (from adhesives). |
| In-laboratory sleep study testing | Discomfort, skin irritation (from adhesives), sensor discomfort, or failure. |
| Continuous positive airway pressure therapy or automatic positive airway pressure therapy | Discomfort, nasal complaints, oral dryness, skin irritation, allergy to mask materials, poor sleep quality, aerophagia, claustrophobia, mask leak, epistaxis, dizziness. |
| Bilevel positive airway pressure therapy | Discomfort, nasal dryness, oral dryness, skin irritation, poor sleep quality, aerophagia, claustrophobia, mask leak, epistaxis, dizziness. |
| Oral appliance | Discomfort, occlusal changes, jaw or temporomandibular joint pain, tongue irritation, excessive salivation, mouth dryness, damage to teeth. |
| Drug-induced sleep endoscopy | Excessive sedation, desaturation, laryngospasm, risk of anesthesia,b loss of airway, need for intubation, epistaxis. |
| Tonsillectomy | Acute postoperative pain and swelling, severe pain, temporary dysphagia, postoperative bleeding, infection, risk of anesthesia.b |
| Soft palate surgery (i.e., UPPP, ESP,LP) | Acute postoperative pain and swelling, dysphagia, taste change, postoperative bleeding, infection, temporary or permanent velopharyngeal insufficiency, long term globus sensation, pharyngeal dryness, oronasal fistula, risk of anesthesiab, death. UPPP surgery associated with 1.6% major complication rate and 0.09% mortality rate. |
| Genioglossus advancement | Acute postoperative pain and swelling, mandibular fracture, neurosensory changes, mandibular fracture, dental injury or dental pulp necrosis, postoperative bleeding or floor of mouth hematoma, infection, risk of anesthesia.b |
| Hyoid suspension | Acute postoperative pain and swelling, temporary dysphagia, infection, postoperative bleeding, infection, risk of anesthesia.b |
| Base of tongue surgery or lingual tonsillectomy | Acute postoperative pain, postoperative bleeding, infection, dysphagia, globus sensation, taste loss or change, tongue numbness, pharyngeal scarring, risk of anesthesia.b |
| Epiglottis surgery | Acute postoperative pain, postoperative bleeding, infection, dysphagia, changes in speech, taste change aspiration, globus, risk of anesthesia.b |
| Nasal and sinus surgery | Acute postoperative pain, postoperative bleeding, infection, orbital injury, septal perforation, cosmetic changes, lacrimal system injury, hyposmia/anosmia, vision changes or blindness, intracranial injury, cerebrospinal fluid leak, risk of anesthesia.b |
| Jaw surgery | Occlusal changes, facial neurosensory deficits, cosmetic changes in facial profile and structure, infection, bleeding, subcutaneous emphysema, malunion or nonhealing of the mandible, temporomandibular related pain, risk of anesthesia.b |
| Hypoglossal nerve stimulation surgery | Acute postoperative pain, postoperative bleeding, infection, tongue stiffness, tongue abrasion, transient tongue hemiparesis, lip weakness, device malfunction requiring revision surgery, device migration, pneumothorax, discomfort with use, risk of anesthesia.b |
| Tracheostomy | Acute postoperative pain, postoperative bleeding, infection, tube obstruction, tube displacement, tracheoesophageal fistula, pneumothorax, tracheal stenosis, trachea-innominate fistula, thyroid injury, voice changes, risk of anesthesia.b |
| Bariatric surgery | Acute postoperative pain, postoperative bleeding, infection, nausea, vomiting, gastroesophageal reflux, mucosal ulcers, gallstones, anastomotic leak, chronic malnutrition, dumping syndrome, hernia, need for revision surgery, risk of anesthesia.b |
May not include all possible risks of listed interventions. Surgical risks include common expected postoperative symptoms and rare long-term risks. All interventions have the risk for insufficient improvement in snoring and OSA.
Includes risk of sore throat, nausea, vomiting, damage to teeth, laryngospasm, aspiration, anaphylaxis, hypoxia, respiratory failure, cardiovascular collapse, or death.
III |. DEFINITIONS AND CLASSIFICATIONS RELATED TO OSA
III.A |. Sleep-Disordered Breathing
Sleep-disordered breathing (SDB) refers to a range of sleep-related breathing disorders that includes primary snoring, OSA, central sleep apnea (CSA), Cheyne-Stokes respiration, and sleep-related hypoventilation. The risk factors and underlying pathophysiological mechanisms for these disorders have overlapping as well as unique features. Each is associated with impaired ventilation during sleep and sleep disruption, although they differ in the underlying severity of gas exchange abnormalities, anatomic obstruction, and degree of underlying ventilatory control abnormality.
OSA is the most common sleep disorder in adults that is evaluated at sleep centers. It is defined on the basis of nighttime and daytime symptoms as well as objective data from a sleep study. Objective sleep testing, with the use of a home sleep apnea test (HSAT) or full polysomnography (PSG), records multiple channels of physiological data that allows identification of the key respiratory-related events that are used to assess SDB: apneas, hypopneas, and respiratory-event related arousals (RERAs), patterns of oxyhemoglobin saturation, sleep-state related parameters (in PSG only), and body position.
The American Academy of Sleep Medicine (AASM) publishes and regularly updates the guidelines that specify the technical requirements for sleep study data acquisition and scoring.11 Since 1999, these have been updated to address advances in technology (e.g., use of nasal pressure signals for airflow detection) as well as changes in hypopnea definitions (reflecting both updates to the evidence and pragmatic issues in operationalizing alternative definitions).
III.A.1 |. Respiratory event definitions
In 2012, the AASM updated the 2007 respiratory event scoring rules,12 which continue to be clarified.11 Note that the scoring of some events (e.g., hypopneas, RERAs) remains controversial, resulting in Recommended and Acceptable definitions and Optional recommendations. Definitions of relevant respiratory event subtypes are largely based on expert consensus and are summarized.
III.A.1.a |. Apnea
A drop in peak signal excursion by ≥90% of pre-event baseline for ≥10 s using an oronasal thermal signal (recommended sensor), positive airway pressure (PAP) device flow, or an alternative apnea sensor. No requirement for a desaturation or an arousal.
III.A.1.b |. Hypopnea
Recommended definition (AASM definition): A drop in peak signal excursion by ≥30% of pre-event baseline for ≥10 s using nasal pressure (recommended sensor), PAP device flow, or an alternative hypopnea sensor, AND a ≥3% oxygen desaturation from the pre-event baseline OR the event is associated with an electroencephalogram (EEG, cortical) arousal.
Alternative definition: A drop in peak signal excursion by ≥30% of pre-event baseline for ≥10 s using nasal pressure (recommended sensor), PAP device flow, or an alternative hypopnea sensor, AND a ≥4% oxygen desaturation from the pre-event baseline. This is the current definition used by Centers for Medicaid and Medicare Services (CMS).
III.A.1.c |. Respiratory Effort Related Arousal (RERA)
Sequence of breaths lasting ≥10 s characterized by increasing respiratory effort or by flattening of the inspiratory portion of the flow signal leading to an arousal from sleep when the sequence of breaths does not meet criteria for an apnea or hypopnea. Scoring of this event type is considered optional. Of note, the revised recommended hypopnea rules allow hypopneas to be recognized if associated with an arousal, thus identifying many events that previously would have been missed without the RERA classification.
Note: Apneas and hypopneas require comparison of breathing amplitude relative to a “baseline.” The AASM 2007 and 2012 manuals operationalize baseline as: “mean amplitude of stable breathing and oxygenation in the 2 min preceding the onset of the event (in individuals who have a stable breathing pattern during sleep) or the mean amplitude of the three largest breaths in the 2 min preceding onset of the event (in individuals without a stable breathing pattern).”
III.A.2 |. Summary metrics
Summary metrics of OSA severity tabulate the frequency of breathing disturbances to (1) use as thresholds for defining disease; and (2) assess disease severity, with higher indices considered to reflect more severe disease.
III.A.2.a |. Apnea hypopnea index (AHI)
The AHI is calculated as the number of respiratory events (apneas, hypopneas) divided by the number of hours of sleep documented during a PSG study. It is important to distinguish if all respiratory events are included (central and obstructive) or only obstructive events. Ideally, a total AHI inclusive of central and obstructive events would be reported along with a separate total for central apnea index.
III.A.2.b |. Respiratory event index (REI)
The REI is calculated as the number of respiratory events divided by the number of hours of estimated sleep using a HSAT. The recommended approach for estimating sleep time is by editing from the total recording time the periods when the participant is likely awake, as evidenced by artifact, movement, and characteristic changes in heart rate and breathing.
III.A.2.c |. Respiratory disturbance index (RDI)
The RDI is calculated as the number of respiratory events (apneas, hypopneas, and RERAs) divided by the number of hours of sleep documented during a PSG study.
III.A.2.d |. Oxygen desaturation index (ODI)
This metric refers to the number of dips (3% or 4%; ODI3%, ODI4%) in oxygen saturation (SaO2) relative toa local baseline per hour of sleep from an overnight sleep study. Unlike the AHI, the ODI is almost always automatically derived using analysis software. Software programs utilize various algorithms for averaging SaO2 signals, defining local baseline SaO2, requiring minimal durations of oxygen fall, and identifying and excluding artifacts.
III.A.3 |. Considerations in sleep study scoring and data interpretation
Event features:
There are three key features of event definitions that influence prevalence estimates of OSA: (1) Degree of associated oxygen desaturation, (2) Use of event-associated EEG arousal, and (3) Amplitude of breathing reduction (magnitude and duration). These dimensions relate to the prognostic importance of the features under analysis: oxygen desaturation captures the effect of reduced ventilation and increased work of breathing on blood oxygenation and resultant intermittent hypoxemia. Events with associated arousals provide information on whether the breathing disruption was sufficient to trigger central responses and fragment sleep. Magnitude of breathing reduction provides measures of airflow limitation amplitudes and airway collapsibility. Varying hypopnea definitions have mostly focused on the level of associated desaturation and presence/absence of an arousal and have not rigorously compared differences in approaches for quantifying flow limitation or ventilation.
Alternative hypopnea definitions impact AHI values:
All of the summary metrics are highly correlated with one another but can vary tremendously in the absolute numbers of events detected. In one of the first and largest studies, a 10-fold difference in OSA prevalence estimates was reported to result from use of different definitions of hypopnea and application of various disease-defining AHI thresholds.13 More recently, studies have estimated the reclassification of OSA severity that results from use of a “3% desaturation or arousal” hypopnea definition compared to a “4% desaturation” criterion. A series of calibration equations were published to allow imputation of AHI levels across definitions.14 This analysis highlighted that the largest differences in mean AHI occurred at lower OSA disease severity, with convergence at higher levels of disease severity, underscoring how different hypopnea definitions may reclassify individuals with mild to moderate OSA. A meta-analysis (MA) of 11 studies comparing these definitions calculated a sensitivity of 82.7% (95% confidence interval [CI] 0.72–0.90) and specificity of 93.2% (0.82–0.98) for the two definitions.15 The analysis estimated that an additional 20% of individuals would be classified with OSA using the “3% desaturation or arousal” hypopnea definition.
Comparisons of the prognostic utility of the various hypopnea definitions have not identified clear advantages to any single definition but have highlighted the need to adjust the thresholds used to define SDB disease severity (none, mild, moderate, and severe) according to the AHI (Table III.A.1). In a cross-sectional analysis of the community-based general population, HypnoLaus cohort, adjusted thresholds for defining moderate and severe OSA were shown to associate with both hypertension and diabetes. Specifically, this study suggested that the thresholds for defining disease severity need to be reduced by approximately one half for hypopnea definitions using a 4% desaturation criteria compared to the AASM hypopnea definition.16 In the Sleep Heart Health Study (SHHS), the application of the current AASM recommended definition (3% desaturation or arousal) resulted in an approximately doubling of the number of individuals classified with moderate OSA (AHI > 15). The group solely identified with use of the AASM definition had a high prevalence of hypertension as compared to the group classified using the more conservative 4% definition, supporting the importance of using more inclusive definitions for identifying individuals at risk for hypertension.17 It is important to recognize that OSA is associated with other comorbidities, in addition to hypertension and diabetes. The prognostic utility of different definitions may vary for sleepiness symptoms, other diseases, and mortality.
TABLE III.A.1.
Adult OSA severity classification
| OSA severity classification | AHI (events/h) |
|---|---|
| None | <5 |
| Mild | ≥5 to <15 |
| Moderate | ≥15 to <30 |
| Severe | ≥30 |
Population characteristics, such as age, gender, and obesity, also may influence the accuracy and prognostic value of alternative event definitions, requiring care in selecting definitions most appropriate for given populations. For example, women tend to have shorter apneas and experience less desaturation than men,18 but with aging, may experience increased rates of death and incident heart failure (HF).19 A study that utilized estimates of AHI based on different hypopnea definitions (from calibration equations) showed greater variation in effect estimates for CV disease when arousals were used in the hypopnea definition in the overall population but not in women, where an arousal-based definition appeared appropriate.20 Hypopnea definition and subsequent AHI scoring also have implications for insurance coverage for various OSA therapies.21
Scoring reliability:
Although there is active research developing automated tools for respiratory scoring, event identification is largely done by manual annotation by trained scorers. Accredited sleep laboratories need to document acceptable inter-scorer reliability using well-defined protocols. Consistency in scoring will vary according to the technician skill, quality of the underlying signals, and severity of the disorder. Apneas are generally considered easier to consistently score as their recognition requires identification of absent airflow. However, distinguishing event subtypes (obstructive, central, and mixed) is much more difficult, resulting in worse scorer reliability.22 Identifying subtle changes in ventilation can be difficult as is needed for hypopnea detection. One argument for requiring correlative data (desaturation, arousal) to identify a hypopnea is to provide additional signs of physiological disturbances over and beyond those identified through a non-calibrated estimate of breathing amplitude change, thus improving scoring reliability. In those cases, care is needed to ensure reliable arousal scoring, which is additionally dependent on the quality of the underlying EEG and electromyography (EMG) channels. For example, in the unattended PSGs in the SHHS, events were more reliably scored when desaturation criteria alone were used in comparison to inclusion of the arousal criteria.23
ODI versus AHI:
Given that hypopneas utilize oxygen desaturation criteria for event identification, it is not surprising that the AHI and ODI often are highly correlated, but dependent on the specific oximeter and AHI scoring definition used.24 The ODI is automatically derived using only a single sensor, providing objectivity, simplicity, and scalability. High diagnostic accuracy has been reported.25 A number of epidemiological studies and clinical trials have utilized the ODI for defining SDB eligibility criteria (e.g., SAVE26), or for characterizing risk of incident disease.27 However, the ODI may be less appropriate as a screening or prognostic index in individuals less likely to desaturate with respiratory events, such as younger patients, non-obese individuals, and women. Conversely, the ODI may be particularly useful to identify hypoxia-related stresses, which have been related to metabolic disease.16
Time spent with oxygen saturation <90% (T90):
Measures of hypoxemia, such as SaO2 nadir and time spent with arterial SaO2 less than 90% (T90), may be important in assessments of OSA severity and health risk determinations.28 It has been shown that more hypoxemia as measured by T90 and lower SaO2 nadir in those with similar AHI is associated with more inflammation (measured by C-reactive protein [CRP], platelet count, and endothelial stiffness).28–30 Recently a retrospective study found that having moderate to severe OSA and T90>20% of sleep time can be associated with a higher risk of hypertension, type 2 diabetes, and 5-year mortality compared to those with T90 < 20%.28 In addition, an SaO2 nadir of <75% correlated with increased risk of hypertension in this group.
III.B |. OSA and Subtype Definitions
OSA is a chronic disorder caused by repetitive collapse of the UA during sleep. Episodes of complete (apneas) and partial (hypopneas) cessation of airflow can lead to two main consequences: arousals from sleep and oxyhemoglobin desaturations. Apneas and hypopneas occur in all phases of sleep, but are more common in N1, N2 and rapid eye movement (REM) sleep stages than in N3 sleep.
III.B.1 |. Obstructive sleep apnea syndrome (OSAS)
OSAS diagnosis requires the patient to have31 symptoms of sleep-related breathing disturbances (snoring, snorting, gasping, or breathing pauses), excessive daytime sleepiness, or fatigue that occurs despite sufficient opportunity to sleep and is unexplained by other medical problems; and12 five or more episodes of predominantly obstructive respiratory events (obstructive or mixed apneas, hypopneas, or RERAs) per hour of sleep (AHI or RDI ≥ 5).31 OSA also may be diagnosed in the absence of symptoms if the AHI is ≥15 episodes/h.
If presenting daytime and nighttime symptoms or cardiometabolic comorbidities are caused by OSA, the term OSAS is used. However, the terms OSA and OSAS are often used interchangeably in the medical literature.
OSAS is recognized to be a heterogeneous syndrome.32 The classification that is most commonly used in clinical practice is the one based on frequency of obstructive events based on AHI (Table III.A.1). Frequency of respiratory events influences several important clinical consequences (hypertension, stroke) in a dose dependent fashion.33 Frequency-based OSA classification using AHI is utilized as an indication for therapy in current national insurance coverage guidelines in the United States (US).34
However, the classification based on AHI alone poorly addresses various phenotypes of the disease. An alternative classification based on presenting symptoms has been proposed which identifies three clusters of patients: a group with sleep disturbance, a group with excessive daytime sleepiness, and a group with minimal symptoms.35 Several other classifications, based on pathophysiology,36 comorbid conditions,37 and clinical outcomes,38 have also been described. Current guidelines for the treatment of OSA typically take into account several factors including the AHI, presence or absence of symptoms, and associated comorbidities.39
III.B.2 |. Positional OSA
Episodes of airway obstruction in OSA are more frequent and more severe in the supine compared to the nonsupine body position in nearly all patients.40 OSA patients who have an increase in breathing abnormalities while in the supine versus lateral position exhibit positional OSA (POSA). In non-positional patients, respiratory events appear in all positions of sleep. With POSA, changes in sleep position effect the overall AHI on a sleep study and account for night-to-night variability in sleep study results. The predominant sleep position and time in each position on the night of the study can be considered when evaluating OSA severity.41 Patients with POSA have lower body mass index (BMI), smaller neck circumference, longer posterior airway space measurements, and smaller lateral pharyngeal wall tissue volumes.41,42
Various definitions have been used to diagnose POSA. Cartwright’s definition is commonly used, which describes POSA when AHI in the supine position is greater than two times the AHI in non-supine sleep position. For a subset of patients with POSA, the airway only reaches critical collapsibility in the supine, but not in the nonsupine position.43 These patients exhibit supine-isolated OSA, who have respiratory disturbances exclusively in the supine position without abnormalities when nonsupine. Supine-isolated positional patients represent 27% of patients with POSA.44 POSA is associated with lower BMI and lower total AHI in males, lower AHI and higher sleepiness in premenopausal females, and lower AHI and lower Mallampati score in postmenopausal females.45
Treatment of POSA takes into account differences in the critical closing pressure between supine and non-supine positions of sleep. These are usually reflected by differences in pressures that need to be generated by PAP devices in different positions of sleep. Although not universally accepted, (auto-titrating positive airway pressure [APAP] devices may provide a better treatment option for patients with POSA than constant-pressure PAP, as they may produce higher pressures in supine, and lower pressures in non-supine body positions.
Treatment of patients with POSA may comprise of enforcement of non-supine sleep. Supine sleep can be avoided by employing the older tennis-ball technique (TBT), which involves placement of a bulky object on the patient’s back, or newer generation sleep position trainers (SPT) that include small, battery-powered devices attached to the neck or chest that provide vibrotactile feedback when in the supine position46 (see Section VIII.D.1).
TABLE IX.C.2.c.2.
Evidence for the association of DISE with improved outcomes
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Golbin et al.1650 | 2016 | 4 | Cohort | 1) Palate surgery ± tonsillectomy without DISE (n = 40) 2) Palate surgery ± tonsillectomy ± partial glossectomy ± partial epiglottectomy with DISE (n = 64) |
1) AHI 2) Epworth Sleepiness Scale score |
Both groups showed a significant decrease in AHI and no change in the Epworth score. There were no differences between the groups in either endpoint. |
| Pang et al.1651 | 2020 | 2b | Cohort | 1) Surgery without DISE (n = 156) 2) Surgery with DISE (n = 170) |
1) AHI 2) Systolic and diastolic blood pressure |
Surgical success rate was greater in group without DISE, as were the decreases in systolic and diastolic blood pressure. |
III.B.3 |. REM-related OSA
A number of physiological effects uniquely predispose airways to collapse in REM versus non-rapid eye movement (NREM) sleep. In REM, the genioglossus muscle activity is lower,47 the respiratory drive is lower,48 and the autonomic drive is higher than in NREM.49 These factors result in a longer duration of obstructive events and deeper hypoxemia in REM than in NREM.
The term “REM-related OSA” describes a condition where SDB is predominantly present in REM sleep. While definitions vary, most authors define REM-related OSA in terms of the frequency of SDB events (AHI) in REM and NREM sleep, with AHI in REM being at least double the AHI in NREM. Some groups add a qualifier of requiring an NREM AHI of <15 events/h.50–53 Depending on the definition used and the population examined, the prevalence of REM-related OSA varies from 13.5% to 36.7%.50,51,53
Clinically, REM-related OSA is more common in younger patients, in patients with mild to moderate OSA, and in women and African American patients.50,54,55 Excessive daytime sleepiness is usually less prominent in REM-related OSA than in non-REM-related OSA.56,57 Depression has been reported more commonly in patients with REM-related OSA than in non-REM-related OSA with combined significant impact on daytime sleepiness and quality of life (QOL).58,59
The clinical significance of the “REM-related OSA” entity is unclear, and some treat it merely as a mild form of OSA. Similarly, large studies failed to detect significant differences in symptoms or clinical outcomes between patients with REM-related (or REM-predominant) versus non-REM-related OSA.50,60–62 As a result, the current classification of sleep disorders does not list REM-related OSA as a separate entity.
III.B.4 |. Primary snoring
Isolated snoring, also known as primary snoring, describes a pattern of vibrational sounds related to narrowing of the UA during sleep without significant apnea or hypopnea events (AHI < 5) and without sleep-related daytime symptoms. Snoring is a reflection of partial collapse of the UA and increased airway resistance. The soft tissue that forms the UA is prone to collapse during sleep which is associated with turbulent airflow and pharyngeal tissue vibrations that create the sounds of snoring.
Airway collapsibility is higher in patients with sleep apnea than in primary snorers and healthy controls.63 Isolated snoring, upper airway resistance syndrome (UARS), and OSA can thus be viewed as three manifestations of progressive intermittent UA obstruction that occur during sleep. With progressive degrees and frequency of airway collapse as well as other modifying factors present in a given individual (arousal threshold, preexisting hypoxemia), intermittent obstruction of the airway is clinically reflected as a spectrum of SDB that presents as: (1) isolated snoring, then (2) obstruction-related arousals or desaturations (upper airway resistance syndrome or UARS), and finally (3) partial or complete cessation of airflow through the UA associated with desaturations and/or arousals (OSA). What determines a given individual’s propensity to move along the path of progression from isolated snoring to UARS to OSA is complex, but is likely affected by several factors including weight gain, sex, race, genetic factors, and advancement of age.64,65
Occasional snoring (snoring on three or less nights per week) is almost universal in the population with a prevalence of 38%–76% in different populations.66–69 Habitual snoring, defined as snoring on most nights of the week, is present in as many as 12%–25% of the population.66–69 Both occasional and habitual snoring are more frequent in men than in women.66–68
Since snoring is the primary nocturnal symptom of OSA, its presence should prompt direct questioning about other symptoms of OSA including pauses in breathing at night, unrefreshing sleep, and daytime sleepiness, especially when the presence of snoring is identified in a setting of OSA-related comorbidities.
III.B.5 |. Upper airway resistance syndrome (UARS)
Some patients have significant fragmentation of sleep due to obstructive events that do not meet formal criteria of apneas or hypopneas. In a PSG tracing, these patients usually display respiratory effort-related arousals (RERAs). RERAs are defined as >10 s sequences of breaths with increasing respiratory effort or flattening of the inspiratory portion of the flow signal, leading to an arousal.70 Guilleminault et al. have coined the term UARS71 to describe the clinical entity of nocturnal airway collapse leading to respiratory effort-related arousals that are associated with daytime symptoms, usually excessive daytime sleepiness. These events do not meet the criteria of OSA.
Most of the clinical and polysomnographic characteristics of UARS patients are based on a limited number of retrospective studies.72–74 The prevalence of UARS among members of a military academy was found to be 8.6%.75 Similar to OSA, the majority of patients with UARS are men, with M:F ratio of about 3:1.74 Patients with UARS tend to have lower BMI than those with OSA and the amount of weight gain in the years prior to diagnosis is typically less in UARS than in OSA patients.74 Daytime impairment in functioning tends to be worse in patients with UARS compared to those with mild OSA.74,76 On PSG, patients with UARS may have less N1 and N2 sleep than patients with OSA, but more N3 sleep.74 Several groups have reported an increased frequency of non-specific or functional somatic complaints in patients with UARS, compared to OSA patients including irritable bowel, insomnia, difficulty concentrating, cognitive problems, depression, and poor psychomotor performance.77–79 These features may be apparent on the initial presentation or may appear over time. It has been postulated that some of these associated symptoms may result from lack of specific therapy for UARS, as many insurers do not include UARS definitions in coverage policies for PAP therapy.80
The UARS is no longer recognized as a nosological entity by the current edition of International Classification of Sleep Disorders,81 as its pathophysiology is the same as OSA. As much as the term UARS has fallen out of favor, the value of this designation lies in underscoring the importance of respiratory-related sleep fragmentation in causing symptoms of sleepiness and impaired daytime functioning that may not be reflected by the AHI.
III.B.6 |. Obesity hypoventilation syndrome
Obesity hypoventilation syndrome (OHS) is defined by awake hypercapnia (PaCO2 ≥ 45 mmHg) in obese individuals (BMI ≥30 kg/m2) that is not otherwise explained by restrictive lung disorders or neuromuscular disease. This disorder represents the extreme end of the SDB spectrum. The prevalence of OHS in the general population is unknown, but conservative estimates range between 0.15% and 0.4% amongst adults in the US.82,83 However, in obese individuals referred to sleep clinics, prevalence rate increases to 10%–20%.84,85 In approximately 70% of those with OHS, severe OSA (i.e., AHI ≥30/h) is present while in 10% the abnormality is non-apneic sleep-dependent hypoventilation only, particularly in REM sleep.86 The exact mechanisms underlying why only some obese individuals with SDB hypoventilate are not fully understood, but appear to be the end result of a complex interplay between obesity, lung mechanics, respiratory drive, and neurohumoral factors.87
IV |. EPIDEMIOLOGY AND CONTRIBUTING FACTORS IN OSA
OSA is a common disorder in adults; its prevalence has increased with rising rates of obesity. OSA is strongly associated with CV and metabolic comorbidities; it is highly prevalent in populations with diabetes, hypertension, heart disease, and stroke. Environmental factors also contribute to OSA risk. The epidemiology of OSA depends on the criteria used to define the disease including how respiratory events are defined, AHI cut offs, and the manner of testing. The 4% desaturation criteria for hypopneas (≥4% oxygen desaturation) may be associated with a more cohesive group epidemiologically – typically older, male, obese, and with underlying cardiac disease (e.g., HF, coronary artery disease [CAD], and atrial fibrillation [AF]) or diabetes. The AASM definition of hypopneas for OSA classification (≥3% desaturation or arousal) is more inclusive and captures more variable sleep disruption phenotypes of OSA, seen in younger, nonobese, and women patients who tend not to desaturate as substantially.36
IV.A |. Prevalence
General population-based studies of OSA show a high prevalence of undiagnosed sleep apnea.88 An SR of OSA prevalence studies worldwide found a wide range (13%–33% in men and 6%–19% in women), likely due to methodological heterogeneity such as differences in sampling of the population, AHI cut-off applied, sleep-study scoring criteria used, and test type performed.89 In the US, the Wisconsin Sleep Cohort study90 and SHHS91 sampled large non-clinical cohorts in the 1990s. These studies found a general prevalence of 24% in men, 9% in women using the alternative definition of hypopnea (≥4% oxygen desaturation). A smaller subset, 3%–7% in men and 2%–5% in women, had OSAS based on AHI ≥ 5 with excessive daytime sleepiness.88,90,92 Population-based studies in Asia show similar prevalence to these estimates.93,94 An updated estimate based on the Wisconsin data in 2013 indicated higher rates of OSA in 14% of men and 5% of women ages 30–70 in the US over time, attributed to changes in population levels of obesity.95 Prevalence of OSAS was found to be even higher in a population study of Sao Paulo.96 A more recent population-based study in Switzerland found a very high prevalence: 23% in women and 50% in men.97 Using rates of obesity, race/ethnicity data, age, and regional prevalence data, it has been estimated that nearly one billion people have OSA worldwide, of which the vast majority is undiagnosed.98
IV.A.1 |. OSA prevalence and risk factors
Consistent across all epidemiological studies, the prevalence of OSA is associated with sex, obesity, and age. Prevalence is higher in men by 2:1, but rates increase in women after menopause and become nearly equal.99 As BMI increases, the risk of OSA rises with an increase in prevalence of AHI ≥ 15 from 3.6% in normal weight to 56% in those with BMI ≥40 among men aged 50–70 years.95 OSA incidence increases with age through age 60, although there also may be subsequent increased risk associated with aging.100 Other risk factors of OSA include a family history of OSA,101 and certain structural abnormalities of the viscerocranium, including reduced size of the mandible.102
Minority populations have lower rates of clinical diagnosis of OSA despite a higher prevalence than white US populations and greater burden of obesity, diabetes, and CV disease.103,104 Prevalence of OSA in relation to BMI differs by race/ethnicity in US studies, with Hispanic and Chinese American subjects having higher odds of OSA after adjustment for obesity.105 Differences in prevalence by race/ethnicity may be related to a number of factors, many of which are not well characterized (e.g., environmental exposures). In addition, variation of craniofacial features by ancestry background may be associated with OSA risk; for example, some studies indicate that some Asian populations may be at increased risk of OSA despite low BMI levels due to shorter midface and shorter cranial base measurements.106 Additionally, there are likely genetic factors that determine the amount of soft tissue in the UA that are responsible for family clustering of OSA.107
IV.A.2 |. OSA and cardiovascular disease
The prevalence of OSA is higher in those with CV disease. A recent MA estimated 49% of subjects hospitalized with cardiac disease (HF, acute coronary syndrome, and arrhythmias) had previously undiagnosed OSA.108 There is a very high prevalence of OSA in those with CAD, hypertension, HF, arrhythmias, and especially AF.109–112 Among subjects with resistant hypertension, OSA is highly prevalent (up to 85% in one series) and the most common secondary cause of hypertension.113
OSA is associated with a multitude of CV outcomes in observational studies, especially in studies utilizing the CMS definition of OSA, as most CV outcomes are thought to be associated with oxygen desaturation.111 Cohort studies have found severe OSA to be associated with higher odds of incident hypertension,114,115 stroke,116,117 and CV death.118 This is thought to be due to the increased nocturnal sympathetic activation, proinflammatory state due to cyclic hypoxemia, arousals, and negative intrathoracic pressures with occluded UA.110,119 Stroke has been consistently identified as a devastating outcome of untreated severe OSA, especially in middle aged adults.120
IV.A.3 |. OSA and metabolic disease
OSA is strongly correlated with diabetes, both of which are highly linked to obesity and increased waist/hip ratio. Obesity is the most consistent risk factor for both disorders, especially central adiposity.121 Trials enrolling obese diabetics have found that 86% have some degree of OSA.122 Weight loss trials show improvement in both diabetes outcomes and reduced sleep apnea severity.123 Additional metabolic risk factors include excess testosterone, growth hormone, and hypothyroidism.
IV.A.4 |. Environmental factors and OSA
Recent studies examining residential factors demonstrate an association between neighborhood features and sleep apnea. High density of fast-food outlets and lower mixed land use may foster OSA through promoting obesity. Neighborhoods with more parks, higher street connectivity, and aesthetic features promote walking. Residing in less favorable walking environments has been associated with more severe sleep apnea especially in male and obese individuals.124 Neighborhood disadvantage, living in poor residential areas, has been associated with childhood sleep apnea in several epidemiological studies.125,126 A recent MA also identifies that the social gradient is associated with OSA.127 This association may be related to obesogenic environments with limited options for recreation and healthy food128 and greater pollution in disadvantaged neighborhoods.129 Ambient air quality has also been associated with sleep apnea.130,131 The odds of sleep apnea increased by 39% as nitrous dioxide levels increased by 10 parts per billion (ppb) in US study.130 These environmental features may contribute to disparities in obesity and sleep health.
IV.B |. Economic Burden of OSA
OSA not only impacts health and functioning but also economic outcomes. Several economic topics related to OSA include: the costs of OSA due to its impact on health, attention, and productivity; the costs incurred in management; the cost effectiveness of diagnosis and treatment; and whether medical cost savings occur with treatment.
A 2016 AASM commissioned report estimated the economic impact of undiagnosed OSA in the US to be $149.6 billion in 2015 based on the interview of opinion leaders, the scientific literature, survey of patients, and CMS fee schedules.132 Direct economic costs included OSA-related comorbidities such as high blood pressure (BP), motor vehicle or workplace accidents, and compensating behaviors such as substance abuse. Indirect economic costs included decreased productivity at work, reduced QOL, and stress on interpersonal relationships. The largest economic impact was assigned to lost productivity ($86.9 billion) followed by medical comorbidities and reduced mental health ($30 billion) and motor vehicle accidents ($26.2 billion).
Patients with OSA have about twice the medical costs of controls prior to diagnosis.133,134 For example, a study of health maintenance organization members found average total medical costs in OSA patients prior to diagnosis was $2720 versus $1384 in age and gender matched controls (in 1996 dollars).134 In the SHHS, 256 subjects with OSA (AHI > 11 and sleepiness) had about 17% higher predicted healthcare utilization compared to subjects not meeting these OSA criteria after adjustment for age, gender, BMI, and study site.135 Similarly, in the Outcomes of Sleep Disorders in Older Men (MrOS) study, among 1316 elderly men, mean annualized total healthcare costs were 11.6% higher in untreated subjects with moderate to severe SDB than in those without SDB.136
With regards to OSA and productivity, data from the Danish National Patient Registry found lower employment (60% in OSA patients vs. 64% for age, sex, and region matched controls) and higher rate of public transfer payments (18% vs. 13% for controls). As a result, labor market income was 9.3% lower and social transfer payments were 18% higher in patients over the 8 years prior to and after diagnosis.133
Data from the National Safety Council and an MA of studies investigating collisions and OSA indicated that 800,000 drivers were involved in OSA-related motor vehicle collisions at a cost of $15.9 billion and 1400 lives in the year 2000 in the US ($23.9 billion adjusted to 2020 dollars).137
IV.B.1 |. Costs of treating OSA
The AASM commissioned-report estimated that in 2015, $12.4 billion was spent diagnosing and treating the 5.9 million US adults with diagnosed OSA.132 Costs related to OSA management vary based on whether laboratory or home-based diagnostic and management strategies are used. Based on data from a multicenter clinical trial using the payer perspective, the mean cost per patient over 3 months using home-based management was estimated at $1807 versus $2424 using lab-based management. Some of the cost advantage with use of home sleep testing ($167 vs. $782 for lab study) was offset by the need for additional testing in some patients who have failed or had non-diagnostic home studies.138 Management costs are highest at the time of diagnosis and tend to diminish over time. In a recent Australian cost effectiveness analysis (CEA), the average annual cost over 5 years for diagnosis and treatment of OSA using lab-based study was estimated at $579 Australian dollars (AUD; $495 US dollars).139
Cost effectiveness
CEA compares interventions based on the differences in costs needed to provide an additional quality-adjusted life year (QALY) gained. A number of CEA have evaluated the value of managing moderate to severe OSA from a payer perspective: the estimated cost per QALY over 5 years has varied considerably but generally support its value when compared to other accepted interventions (<$50,000 per QALY).140 Some CEA have considered downstream medical cost (due to strokes, heart attacks, and motor vehicle collisions) that could be prevented from OSA therapy. For example, Guest et al. estimated that cost savings exceeded the costs of treating OSA after 13 years.141 More recently, Streatfeild et al. estimated that from a healthcare system perspective, the estimated cost to treat OSA was $12,495 AUD (US $8746) per disability adjusted life year (Equivalent to QALY gained).139 They included the impact of estimated healthcare cost savings from reduced medical morbidity ($76 AUD/year). From a societal perspective, there was net cost saving of $326/year because of a projected $783/year cost saving due to the reduction of financial costs (due to gains in productivity, fewer accidents, and other savings).
Cost savings
Observational studies are equivocal regarding whether medical cost savings occur with OSA treatment and their magnitude. Concern has been expressed regarding the adequacy of methodology in some of these studies, because they do not include an appropriate control group, use a longitudinal cohort design, compare change in costs between groups (rather than relying only on costs in the posttreatment period), measure treatment adherence, adjust for potential confounders, and exclude costs of diagnosis and treatment (which naturally decline over time).142 A recent high-quality study in an older population found costs during the 2 years following diagnosis increased relative to pre-diagnosis levels in individuals regardless of OSA diagnosis or PAP adherence status.143 Medical costs increased about 8% less for the group fully adherent to CPAP relative to sleep apnea patients who did not receive therapy. It is likely that the impact of therapy on medical costs depends on factors such as the population characteristics (OSA severity and comorbidity), health system, effectiveness of therapy, and duration of observation.
In summary, untreated OSA is associated with substantial economic cost related to reduced productivity, medical comorbidity, and motor vehicle and work accidents. Medical costs in clinically identified OSA patients are about twice that for age-sex matched controls prior to diagnosis. In contrast, when OSA is identified in community-based research cohorts, cost differences are more modest (10%–20% more than similar patient without OSA). Costs for managing OSA are substantial though the cost effectiveness for the treatment of moderate to severe OSA with CPAP is well established. It is reasonable to expect some cost savings long-term with CPAP therapy due to downstream benefits, though there is a need for additional high-quality studies to clarify this issue with regards to the magnitude of savings and variation with patient characteristics, models of OSA care, and treatment adherence.
IV.C |. Risk Factors for OSA
IV.C.1 |. Sex
Most population-based studies show a two- to threefold greater prevalence of OSA among men compared to women.18,45,90,93,96,99,104,144–156 These findings span across a range of diagnostic modalities, hypopnea definitions, and AHI cutoffs. These findings are also consistent across ethnicities.45,93,96,146,148,149,153,157 Even when matched for BMI and age, OSA occurs more commonly in men, and with greater severity.155,156 Age and BMI are risk factors for OSA in both men and women, though the degrees of their impact vary between the sexes.147 Weight loss or gain impacts AHI greater in men than in women.155 The effect of BMI on OSA severity decreases with age for both sexes, with lesser impact for individuals older than age 60 years than in younger individuals.156 In a prospective cohort population-based study, Tishler et al.156 demonstrated that the AHI increases by 140% per 10-years in women (OR 2.41) but by only 15% in men (OR 1.15). This results in a narrowing of the sex difference in OSA risk with increasing age. Sex hormones are implicated in the differential risk between men and women. Post-menopausal women, and particularly those not on hormone-replacement therapy, are at increased risk for OSA compared to pre-menopausal women, even when correcting for age.99,144,147,154
Clinic-based prevalence studies also show men to have greater AHI compared to BMI- and age-matched women.155,158–160 The risk of mild OSA (AHI ≥ 5/h) is two-fold greater in men than women, while the risk of severe OSA (AHI ≥ 30/h) is almost eight-fold greater. In a clinic population of 26,425 adults, 21–80 years old, age was less of a factor in AHI severity in obese men than in obese women.161
Polysomnographic features of OSA also differ between men and women. Men with OSA tend to have more frequent apneas (vs. hypopneas), longer duration of apneas, and more severe oxygen desaturations.158,162 The distribution of apneas and hypopneas also differs between men and women. Women are more likely to have events during REM sleep.18,55,158,163–167 Several studies show that women tend to have less NREM events, but similar or greater REM events compared to age- and BMI-matched men. The difference in the prevalence and severity of OSA between men and women are affected by AHI definitions. For example, Won et al.18 showed defining respiratory events using lesser oxygen desaturation threshold levels and including arousals increased the relative proportion of women classified with OSA.
The reason for sex differences in the risk for OSA is not well understood but is thought to relate to several factors: (1) Facial anatomical distinctions, such as mandible position, impart different degrees of risk in men and women.168,169 (2) UA anatomy and function differ between sexes, with men demonstrating longer and more collapsible UAs.170–173 (3) Obesity174 and fat distribution175,176 differentially affect men and women’s propensity for OSA. Lim et al.176 found that while waist-to-hip ratio predicted OSA in both men and women, neck circumference was only predictive of men with OSA. (4) Loop gain and other measures of ventilatory control have been measured in men and women, and in post- and pre-menopausal women, with some evidence to suggest sex differences in respiratory control.177–182
In summary, there are significant sex differences in the prevalence, PSG features, and risk factors for OSA. Men are in general at greater risk for OSA during NREM sleep, while men and women share similar risk during REM sleep. More studies are needed to understand the sex and sleep stage-dependence of OSA. (Table IV.C.1a and IV.C.1b)
TABLE IV.C.1a.
Population studies on prevalence or incidence of OSA in men and women
| Study | LOE | Study design | Cohort | Sample population | OSA diagnostics/criteria | Conclusion |
|---|---|---|---|---|---|---|
| Young90 | 2c | Cross-sectional | Wisconsin Sleep Cohort 30–60 years N = 602 | Population-based | PSG AHI ≥ 5 |
Higher prevalence in men for all age groups Men 2–3.7 times greater prevalence |
| Bixler144 | 2c | Cross-sectional | 20–100 years N = 1741 |
Population-based | PSG AHI > 15 |
Prevalence greater in post-menopausal women, and in those not taking hormone replacement therapy |
| Shahar154 | 2c | Cross-sectional | Sleep Heart Health Study Women >50 years N = 2852 |
Population-based | HST IV AHI > 15 |
Hormone use associated with less OSA, particularly among women 50–59 years old (adjusted OR 0.36) |
| Young99 | 2c | Cross-sectional | Wisconsin Sleep Cohort Study Women 30–60 years N = 539 |
Population-based | PSG AHI ≥ 5 |
Menopausal transition associated with OSA after controlling for age, body habitus, and several lifestyle factors Postmenopausal women 2.6 times more likely to have AHI ≥ 5, and 3.5 times more likely to have AHI ≥ 15, compared to premenopausal women |
| Quintana-Gallego183 | 3b | Case–control | Spain sleep clinic Women mean age 58 ± 10 years; men mean age 53 ± 11 years N = 1745 |
Clinic-based | PSG AHI ≥ 10 or HST III RDI ≥ 10 |
Prevalence: 4.9:1 men to women ratio |
| Gabbay161 | 3b | Case–control | Israel multiple sleep centers 21–80 years N = 26,425 |
Clinic-based | PSG AHI ≥ 10 |
AHI increased with increasing age for both non-obese men and women Obesity affected AHI in men aged 20–40 years, but AHI did not change after age 40 years in obese men |
| Huang147 | 2b | Prospective cohort | Nurses’ Health Study (NHS) Post-menopausal women who were free of known OSA N = 50,473 NHS N = 53,827 NHSII |
Population-based | Medical record NHS: 12-year incidence NHSII: 20-year incidence |
Surgical menopause had 26% higher risk of incident OSA compared to naturally post-menopause, adjusted for age at menopause and other OSA risk factors |
| Won18 | 2c | Cross-sectional | MESA cohort Mean 69 ± 9 years N = 2057 |
Population-based | PSG AHI ≥ 15 |
Prevalence: 41% men, 22% women |
TABLE IV.C.1b.
Reasons for sex differences in OSA
| Study | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|
| Anatomy and anthropometric traits | |||||
| Polesel184 | 3b | Cross-sectional | Brazil N = 552 women N = 450 men |
Anthropometrics | Waist circumference and waist-to-height best predicted OSA in women Waist-to-height ratio and neck circumferences best predicted mild OSA in men, while BMI was associated with severe OSA |
| Cho168 | 3b | Cross-sectional | Korea Suspected OSA N = 2016 |
Anthropometrics Cephalometry | Men with OSA had increased soft palate length compared to controls Women with OSA had increased soft palate thickness and nasion angle than controls |
| Sutherland169 | 3b | Cross-sectional | Chinese and Australian sleep clinic N = 363 200 Chinese 163 Caucasian |
Craniofacial features by face photography ethnicity | Women with OSA had different facial features compared to controls, such as greater face width to eye width ratio, and reduced mandibular plane Men with OSA had increased mandibular plane angle relative to controls |
| Perri185 | 3b | Cross-sectional | Caucasian of European origin Sleep clinic N = 104 OSA N = 85 healthy |
Surface cephalometry | OSA patients had anthropometric and surface cephalometric phenotypes that differed in comparison with healthy subjects, however, sexual dimorphism levels (i.e., male–female ratios) were broadly similar in the two groups |
| Lim176 | 3b | Cross-sectional | Korean Clinic N = 151 |
Anthropometrics | Neck circumference predicted OSA in men only, while waist-to-hip ratio predicted OSA in both men and women |
| Dancey186 | 3b | Cross-sectional | Canada sleep clinic N = 3942 |
Neck circumference | Neck-to-height ratio predicted AHI, accounting for 19% of the variability, more significantly in men |
| Upper airway anatomy and function | |||||
| Brooks173 | 3b | Cross-sectional | Healthy volunteers N = 77 men N = 98 women |
Pharyngeal cross-sectional area during quiet breathing using the acoustic pulse technique | The men had a larger change in pharyngeal area with changing lung volume than the women |
| Segal172 | 2b | Cross-sectional | Chart review for those who have had PSG and CT neck N = 24 (15 men, nine women) |
Upper airway length | Men with OSA were found to have longer upper airway length even when normalized to body height compared with OSA women Correlation between upper airway length and AHI |
| Mohsenin171 | 3b | Cross-sectional | Sleep Clinic AHI ≥ 5 N = 71 |
Upper airway dimensions using acoustic reflectance while lower jaw was in the resting and retrusive posture | Men tend to have a larger but more collapsible airway during mandibular movement than women |
| Eckert187 | 3b | Cross-sectional | 12 healthy, eight OSA | Genioglossus electromyogram (GGEMG) | Reductions in GGEMG during REM sleep were not different between OSA patients and control subjects or between sexes |
| Malhotra170 | 3b | Case–control | Healthy volunteers age and BMI matched 19 men, 20 women |
MRI | Pharyngeal airway length and cross-sectional area, soft palate area, and airway volume were greater in men compared with women |
| Obesity and fat distribution | |||||
| Simpson188 | 3b | Cross-sectional | Western Australian Sleep Health Study Sleep clinic OSA patients (60 men, 36 women) |
Obesity measures using dual-energy absorptiometry | In women, percentage of fat in the neck region and body mass index together explained 33% of the variance in AHI In men, percentage of fat in the abdominal region and neck-to-waist ratio together accounted for 37% of the variance in AHI |
| Huang174 | 3b | Cross-sectional | Taiwanese Sleep center >18 years N = 2345 patients (339 females) |
BMI | AHI was associated with BMI more strongly in men |
| Harada175 | 3b | Cross-sectional | Japanese Sleep Clinic 271 men, 100 women with OSA |
CT scan measured visceral fat area | AHI was independently associated with visceral fat area only in men |
| Ventilatory control | |||||
| Sin177 | 3b | Cross-sectional | Canadian sleep center N = 219 (43 women, 176 men; 104 OSA, 115 no-OSA) |
Hypercapnic ventilatory response test (HCVR) | Elevated carbon dioxide and older age were significantly correlated with low HCVR in men, while BMI was associated with HCVR in women |
| Jordan179 | 3b | Cross-sectional | Healthy volunteers12 men, 11 women at both their luteal and follicular phases | Post-stimulus ventilatory decline (PSVD) | There were no significant differences in PSVD between men and women in either luteal or follicular phases |
| Syed178 | 2b | RCT | 10 men and seven women with OSA, with control 10 healthy men and 10 healthy women | Hypoxic ventilator response augmentation (HVRA) Ventilatory long-term facilitation (vLTF) |
HVRA and vLTF were enhanced in the OSA group compared with control Sex did not impact any measures Exposure to intermittent hypoxia during sleep led to a corresponding increase in respiratory events compared with sham exposure, irrespective of sex |
| Pillar181 | 3b | Cross-sectional | 8 normal women and eight age and BMI-matched men, during stable NREM | Upper airway mechanics Ventilation Activation of two dilator muscles, genioglossus and tensor palatini were monitored during loading |
Men developed more severe hypopnea in response to identical applied external loads than did women Men and women had near identical minute ventilation responses to total load, implying no differences in central drive or load response There were no significant increases in genioglossus or tensor palatini activation in response to loading in either sex |
| Zhou182 | 3b | Cross-sectional | 8 men (25–35 years) and 8 women in the midluteal phase of the menstrual cycle (21–43 years) Repeated studies in 6 women during the midfollicular phase |
Apneic threshold | In women, induction of a central apnea required an increase in tidal volume by 155% ± 29%, compared to men with an increase in tidal volume by 142% ± 13% Similarly, women required greater reduction in PETCO2 compared to men, to general a central sleep apnea There was no difference in the apneic threshold between the follicular and the luteal phase in women |
| Jordan180 | 3b | Cross-sectional | 11 men and 11 women were matched for severe OSA severity Second group of 12 men and 12 women were matched for body mass index |
Loop gain (LG) measured using proportional assist ventilator Critical closing pressure of upper airway (Pcrit) by progressive CPAP drop technique |
In the BMI-matched subgroup, women had less severe OSA during NREM sleep and lower Pcrit, but similar LG compared to men |
IV.C.2 |. Obesity
Obesity is one of the most significant risk factors for SDB.95,156,189,190 It is estimated that approximately 58% of adult OSA cases are attributable to obesity.191 OSA is prevalent in 44.6% of men and 13.5% of women aged 30–49 years with BMI of 30–39.9 kg/m2, compared to 7% of men and 1.4% of women whose BMI is less than 25 kg/m2.95 It is estimated that the prevalence of OSA in bariatric surgery patients is as much as 60%–83%,192,193 with weight loss after surgery resulting in significant reduction in AHI.192–195 Furthermore, it has been shown that 10% increase in body weight is associated with an increase in AHI of approximately 30%.189 Another study showed an increase in BMI by 1 standard deviation (SD) was associated with a threefold increase in risk of OSA.90 However, this association with BMI decreases with age, and after age 60 years may be less significant.95
According to the US National Health and Nutrition Examination Survey (NHANES), the prevalence of ageadjusted obesity (BMI > 30 kg/m2) has increased from 30.5% to 42.4%, and of severe obesity (>40 kg/m2) from 4.7% to 9.2% since 1999 to 2017 (the prevalence of both obesity and severe obesity was highest in non-Hispanic black adults).196 BMI correlates with body fat percentage, more so in women, but neither represents adipose tissue distribution nor differentiates between fat and lean tissue.197 Fat distribution, particularly in the upper body rather than total body, is the most important factor contributing to OSA.198
The mechanism by which obesity causes and progresses OSA is still unclear. Obesity, specifically central adiposity, increases pharyngeal collapsibility that causes recurrent episodes of UA obstruction in sleep apnea through both mechanical and neuromuscular effects.199–203 Central adiposity increases the mechanical load on the UA and decreases the compensatory neuromuscular response. This is thought to be mediated by adipokines that directly impact central nervous system activity.199 A study by Sands et al. showed that obese patients with OSA compared to those without OSA have worse pharyngeal collapsibility with less active pharyngeal muscle response.204 Despite the evidence of a link between obesity and central adiposity with OSA, there is variability in the prevalence and severity of OSA even in markedly obese patients suggesting there are likely other neuro-hormonal, anatomic, and genetic components involved.199
Although it is unclear whether a predominant mechanism of OSA in obesity is increased para-pharyngeal tissue, most studies suggest obese patients with OSA have a smaller UA cross-sectional area. Some studies suggest this is due to retropalatal fat deposition, while others suggest it is parapharyngeal fat or increased pharyngeal wall thickness that determines the development of OSA in obese individuals.200,202,205–208 Recent studies evaluating dynamic changes have shown that parapharyngeal fat is associated with concentric obstruction in the retropalatal area. They have also shown that parapharyngeal fat deposition in the subglosso-supraglottic area is associated with increased OSA severity independent of BMI or neck circumference.209,210
Investigators have also shown that caudal traction by the mediastinal and ribcage muscle attachments to the UA improves airway patency, suggesting UA patency is proportional to lung volumes.211,212 Caudal traction improves airway caliber by reducing transmural pressure and reducing compliance (i.e., increasing stiffness) of the pharyngeal muscles. In obese individuals, lung volumes are often reduced, which results in reduction in caudal traction, thereby contributing to UA collapse.211
It is not understood why some obese patients also have hypoventilation. Shimura et al. showed higher levels of leptin in hypercapnic OSA patients compared to eucapnic OSA patients, even after correcting for BMI, fat distribution, AHI, and mean SaO2. This suggests that leptin, an adipocyte derived hormone which is elevated in obesity, does not prevent hypoventilation in hypercapnic patients, despite it being a respiratory stimulant. It is possible that these obese individuals prone to hypercapnia have leptin resistance contributing to both obesity and hypoventilation.213
Further evidence that obesity is pathogenic in OSA for some individuals, is that weight loss results in marked improvement of OSA.122,214 Since obesity confers additional CV risk to OSA patients, weight loss directly benefits CV health.215 (Table IV.C.2)
TABLE IV. C. 2.
Obesity as a contributing factor for OSA
| Study | Year | LOE | Study design | Study groups/age/N | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Hoffstein212 | 1984 | 3b | Case–Control | Obese subjects; OSA versus non-OSA N = 19 Age=28–68 |
1. Cross-sectional area of pharynx 2. TLC (total lung capacity) to RV (residual volume) |
In obese patients with OSA, pharyngeal cross-sectional area is small and varies considerably with change in lung volume (and this change in size with change in lung volume was significantly different in the two groups) |
| Katz198 | 1990 | 3b | Cross-sectional | Canada Sleep clinic N = 123 |
PSG AHI ≥ 5 | External, internal neck circumference and degree of obesity are important predictors of OSA |
| Mezzanotte201 | 1992 | 3b | Case–control | Denver Veterans Affairs Hospital OSA and normal controls N = 25 Age = 40–46 years |
1. Supraglottic resistance 2. Genioglossal EMG 3. Minute ventilation 4. End tidal CO2 |
Neuromuscular compensation present during wakefulness from genioglossus may be lost during sleep in apneic patients |
| Shelton205 | 1993 | 3b | Case–control | Univ of Virginia Sleep clinic and control from community N = 30 Age = 23–65 |
MRI for adipose tissue volume | Adipose tissue is deposited in pharyngeal area in OSA patients, and the volume of this tissue is related to presence and degree of OSA |
| Young90 | 1993 | 2c | Cross-sectional | Wisconsin Sleep Cohort study N = 602 |
PSG AHI ≥ 5 | 1. Prevalence of OSA is 9% in women and 24% in men 2. Male sex and obesity strong risk factors 3. Increase in BMI by 1 SD is associated with three-fold increase in risk of OSA |
| Schwab200 | 1995 | 3b | Cross-sectional | Sleep Clinic at University of Pennsylvania N = 68 |
1. MRI 2. PSG RDI > 15 |
1. Lateral pharyngeal wall is larger in apneic patients 2. Wall thickness explains the largest part of variance in airway caliber |
| Peppard189 | 2000 | 2b | Prospective cohort | Population based N = 690 |
1. Percent change in AHI on PSG 2. Odds of developing moderate to severe SDB, with respect to change in weight |
1. 10% weight gain predicted an approximate 32% increase in AHI, with six-fold increase in odds of developing moderate to severe SDB 2. 10% weight loss predicted a 26% decrease in AHI |
| Stanchina203 | 2002 | 3b | Cross-sectional | Healthy individuals in Boston N = 15 Age = 24–32 |
During NREM sleep: 1. Genioglossus EMG 2. Epiglottic pressure 3. Airflow under different conditions |
Genioglossus muscle responds well during NREM sleep when hypercapnia is combined with resistive load, but is less responsive to either chemical stimuli (hypoxia, hypercapnia) or inspiratory resistive load alone |
| Young190 | 2002 | 2c | Cross-sectional | Sleep Heart Health Study N = 5615 |
In home PSG AHI ≥ 15 | Male sex, age, BMI, neck girth, snoring, and repeated breathing pause frequency were independent, significant correlates of AHI ≥ 15 |
| Schwab207 | 2003 | 3b | Case–Control | Penn Center for sleep disorder and control from community in same neighborhood N = 96 Age = 24–66 |
MRI of upper airway | Volume of lateral pharyngeal wall, total soft tissues and tongue larger in OSA than normal subjects |
| Tishler156 | 2003 | 2b | Prospective study | Cleveland family study N = 286 |
HST AHI ≥ 10 | Five-year incidence of OSA – 7.5% for moderate SDB and 16% for mild to moderate |
| Shimura213 | 2005 | 3b | Cross-sectional | Japanese sleep clinic patients N = 185 Age = 22–72 |
1. PSG AHI ≥ 5 2. CT scan for visceral and subcutaneous fat accumulation 3. Lung function 4. Leptin levels |
1. Location of body fat does not contribute to hypoventilation 2. Circulating leptin levels does not maintain alveolar hypoventilation in hypercapnic obese patients with OSA |
| Kairaitis 211 | 2007 | 5 | Animal study | Male NZ white rabbits N = 20 |
Upper airway extraluminal tissue pressure in lateral and anterior pharyngeal walls | Decrease in upper airway collapsibility due to lung volume related caudal traction |
| Foster122 | 2009 | 1b | RCT | 16 US centers (Overweight/obese with DM and OSA) N = 264 |
1. BMI 2. Waist and neck circumference 3. HbA1c |
1. The intensive lifestyle intervention (ILI) group lost more weight at 1 year than Diabetes support and education 2. ILI was associated with an adjusted decrease in AHI of 9.7 events/h |
| Flegal197 | 2010 | 2c | Cross-sectional | NHANES (National Health and Nutrition Examination Survey) population N = 5555 Age > 20 years |
BMI | In 2007–2008, the prevalence of obesity was 32.2% in men and 35.5 women |
| Ashrafian 192 | 2012 | 2a | SR(with heterogeneity) of cohort studies | Studies with metabolic interventions-33 Studies with lifestyle intervention-24 |
1. BMI 2. AHI |
Metabolic surgeries offer significant reduction in symptoms and measures of OSA by both weight dependent and independent mechanisms |
| Dixon194 | 2012 | 1b | Individual RCT (Bariatric surgery vs. conventional weight loss) | Australian Hospital-Obese patients (BMI of 35–55) and <6 months diagnosis of OSA (AHI > 20) N = 60 |
1. PSG (baseline to 2 year change in AHI) 2.Weight 3. CPAP adherence 4. Functional status |
In obese patients with OSA, bariatric surgery compared with conventional weight loss therapy did not result in a statistically greater reduction in AHI despite major differences in weight loss |
| Li202 | 2012 | 3b | Case-control | Chinese Han population (Otolaryngology Head & Neck Surgery Dept.) N = 28 |
1. MRI 2. Pharyngoscopy under general anesthesia |
OSA patients have more fat tissue adjacent to pharyngeal cavity, and the fat-deposition correlated to collapsibility |
| Peppard95 | 2013 | 2c | Cross-sectional | Wisconsin Sleep Cohort Study N = 1520 |
PSG AHI ≥ 5 and ESS>10 | Prevalence of SDB is increasing in the population (relative increases of between 14% and 55% depending on the group) |
| Chirinos 215 | 2014 | 1b | Individual RCT (Randomized to CPAP, weight loss and combined CPAP with weight loss) | Obese, moderate to severe OSA and CRP > 1 mg/L N = 181 |
1. CRP 2. Insulin sensitivity 3. Lipid levels 4. Blood pressure |
1. No difference in CRP level reduction 2. Weight loss provided incremental reduction in insulin resistance and TG level when combined with CPAP |
| Jang209 | 2014 | 3b | Cross-sectional | OSA patients from Korean Sleep Center N = 33 Age = 31–54 |
1. Facial CT 2. DISE (drug-induced sleep endoscopy) |
Parapharyngeal fat pad is associated with concentric narrowing of the retropalatal pharynx |
| Kim208 | 2014 | 3b | Case–control | University of Pennsylvania Center for Sleep and Circadian Neurobiology N = 121 |
MRI upper airway | Increased tongue volume and fat deposition at tongue base in OSA compared to controls |
| Pahkala206 | 2014 | 1b | RCT | Kuopio University Hospital, Finland N = 60 |
1. PSG 2. CT scan of upper airway |
1. Pharyngeal fat pad area was significantly larger and hyoid bone to cervical spine area longer in OSA than habitual snorers 2. Weight loss by lifestyle intervention-based program led to improvement in OSA by reducing both central obesity and pharyngeal fat pad |
| Sands204 | 2014 | 3b | Individual case–control studies | Overweight/obese without apnea (AHI < 15/h), overweight/obese with OSA (AHI ≥ 15/h) and normal weight/nonapneic N = 54 |
1. Pcrit (Pharyngeal critical closing pressure) 2. Pharyngeal muscle (greater genioglossus) EMG |
Overweight/obese without mod/severe OSA have increased (three times greater) pharyngeal muscle EMG activity during sleep responsiveness |
| Ashrafian195 | 2015 | 2a- | SR (with heterogeneity) of cohort studies (?but also has some RCT) | 19 surgical (n = 525) and 20 non-surgical (n = 825) studies | BMI and AHI before and after intervention | Surgical patients achieved a significant 14 kg/m2 weighted decrease in BMI with a 29/h weighted decrease in AHI. Non-surgical patients achieved a significant weighted decrease in BMI of 3.1 kg/m2 with a weighted decrease in AHI of 11/h |
| Ng214 | 2015 | 1b | RCT | Prince of Wales Hospital, Hongkong OSA patients with AH ≥ 15/h N = 104 |
1. HST AHI 2. ESS 3. SF-36 (Short Form Health) survey 4. BMI |
Lifestyle modification program (LMP) was more effective in reducing AHI from baseline (16.9% fewer events in LMP vs. 0.6% more events in control group with usual care) |
| Peroma193 | 2017 | 2b | Prospective cohort | Bariatric OSA patients who underwent bariatric surgery N = 132 | 1. PSG (12 months after surgery) 2. BMI 3. Neck and waist circumference |
Prevalence of OSA decreased from 71% at baseline to 44% at 12 months after surgery (p < 0.001) |
| Chen210 | 2019 | 3b | Cross-sectional | Taiwan Hospital (Otorhinolaryn- gology Dept.) N = 41 Age = 34–48 |
1. PSG AHI ≥ 5 2. Drug induced Sleep CT 3. BMI 4. Neck circumference |
Subglosso-supraglottic parapharyngeal fat pad area, independent of BMI, and neck circumference influenced severity of OSA |
| Hales196 | 2020 | 2c | Cross-sectional | US census from 2000 | BMI | 1. In 2017–2018, the age adjusted prevalence of obesity in adults was 42.4% 2. No significant differences between men and women 3. Severe obesity prevalence higher in women at 9.2% |
Abbreviations: AHI, apnea hypopnea index; BMI, body mass index; CT, computed tomography; DM, diabetes mellitus; EMG, electromyography; MRI, magnetic resonance imaging; OSA, obstructive sleep apnea; Pcrit, Pharyngeal critical pressure; PSG, polysomnography; SD, standard deviation.
IV.C.3 |. Craniofacial anatomy
There are significant craniofacial differences among OSA subjects when compared to healthy controls. Craniofacial analysis within the literature has primarily been based on cephalometric measurements obtained through lateral plain film cephalograms. Jamieson et al. first brought craniofacial morphology into OSA pathophysiology by examining cephalometry on sagittal plain films among 155 OSA patients and 17 non-OSA controls showing that OSA patients had a more acute cranial base angle and greater retroposition of the mandible.216 Numerous studies have since been published examining a variety of cephalometric measures among OSA patients obtained through lateral cephalograms as well as craniofacial measurements obtained through other modalities including computed tomography (CT) and magnetic resonance imaging (MRI).217–228 An SR and MA of 25 studies comparing lateral cephalometric measurements in OSA229 showed the strongest cephalometric measurements associated with OSA with the least variability and heterogeneity among studies were (see Figure IV.C.3):
Increased anterior facial height
Inferiorly and posteriorly positioned hyoid
FIGURE IV.C.3.
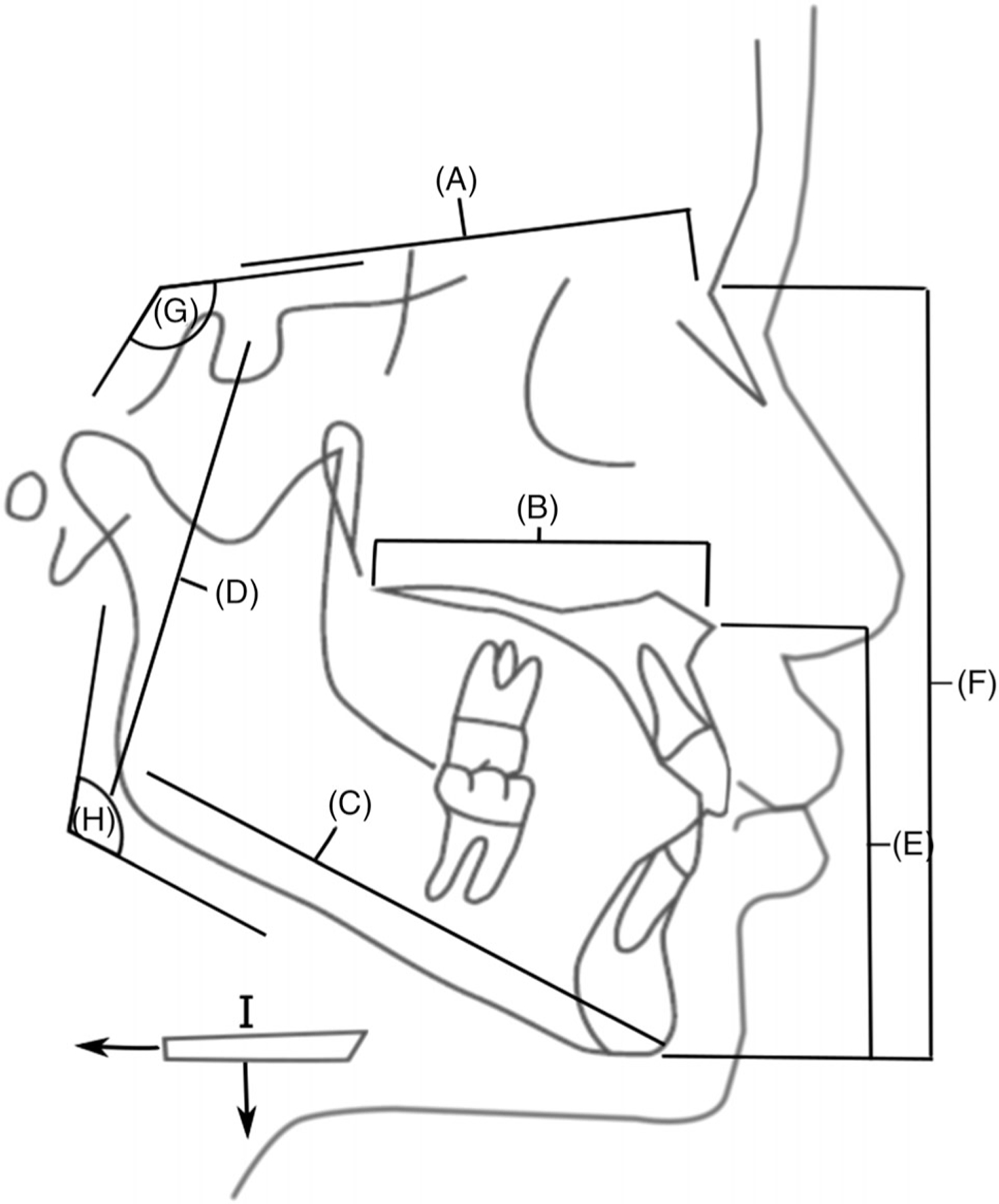
Common sagittal cephalometric findings associated with obstructive sleep apnea (OSA): (A) decreased cranial base length, (B) decreased maxillary length, (C) decreased mandibular length, (D) decreased posterior facial height, (E, F) increased anterior lower facial height and total facial height, (G) decreased cranial base angle, (H) decreased gonial angle (posterior rotation of mandible), (I) inferior and posterior displacement of the hyoid bone.
Additionally, the following cephalometric measurements are suggested to be associated with OSA:
Short anterior cranial base angle
Decreased cranial base length
Shorter maxillary length
Shorter mandibular length
Increased mandible rotation
Retroposition of the mandible
Decreased posterior facial height
However, there was greater variability and heterogeneity among studies for these measurements with some noting non-significant differences, while others did not measure all values in their cephalometry.229
In addition to lateral cephalometry, studies have also looked at transverse craniofacial features. Chi et al. compared both sagittal and coronal three-dimensional (3D) cephalometry obtained from MRI among OSA patients showing that both an inferiorly positioned hyoid as well as a smaller and shallower mandible was associated with OSA risk.224 Seto et al. measured interdental widths and showed that OSA patients had decreased inter-molar widths as well as higher palatal indexes which suggests a higher, deeper arched palate.230 Johal and Conaghan measured maxillary widths and found that OSA patients did not have shorter interdental widths but did show an association of OSA with increased palatal heights.231 Kuzucu et al. reported that OSA patients have decreased distances between the pterygoid hamuli along the posterior maxilla.232
Common craniofacial physical exam findings that are associated with OSA risk include: retrognathia, micrognathia, high arched palate, larger thyromental angles, and shorter thyromental distances.219,230,233–236 Dental findings may also be suggestive of underlying craniofacial deficiency and include: open bite, overbite, overjet, and proclination of the mandibular incisors.219,234,237
BMI is a confounding factor for craniofacial influences on OSA. OSA patients, when compared to BMI matched controls, showed craniofacial differences including a lower positioned hyoid, shorter mandibular body length, retroposition of the mandible, decreased mandibular ramus height, increased lower anterior facial height, and shorter cranial base length.238–241 Furthermore, comparing OSA among different ethnicities, studies have shown that Asian subjects have shorter cranial base angles, shorter maxillary lengths, shorter mandibular body lengths, greater mandibular ramus lengths, greater maxillary widths, and shallower maxillary depths while Caucasian subjects tend to have higher BMI and larger tongue volumes.151,242 In contrast, African Americans tend to have larger tongues than Caucasians and were less likely to be brachycephalic, a craniofacial form that is associated with reduced anterior–posterior facial dimensions.243,244 These studies suggest that craniofacial dimensions are a risk factor for OSA even when controlling for BMI and that different ethnicities may carry different OSA risks based on the contributions of BMI and craniofacial form.
In summary, there is a large body of evidence suggesting there are associated craniofacial factors in OSA. However, there is heterogeneity among studies regarding which specific craniofacial measurements are associated with OSA. All studies were also skewed heavily toward male patients and none focused primarily on female patients, making gender-based differences less clear. Increased anterior facial height and lower hyoid position were the most strongly associated with OSA while there is more mixed evidence regarding cranial base, maxillary, and mandibular measurements. These measurements appear more pronounced on non-obese patients suggesting that craniofacial factors play a greater role in OSA among thin patients. (Table IV.C.3)
TABLE IV. C. 3.
Evidence for craniofacial anatomy as a contributor to OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|
| Jamieson et al.216 | 1986 | 3b | Case–control study | Adult OSA patients (n = 155) and non-OSA controls (n = 17) | Lateral cephalometry | OSA patients had greater retroposition of the mandible and a more acute cranial base angle. |
| Hochban and Brandenburg217 | 1994 | 3b | Case–control study | Adult OSA patients (n = 403) and non-OSA controls (n = 88) | Lateral cephalometry | OSA patients had longer facial type, retroposition of the mandible, more dorsocaudally positioned hyoid bone, and an anteroposterior narrowing of the posterior airway space. |
| Frohberg et al.218 | 1995 | 3b | Case–control study | Adult OSA subjects (n = 30) and adult chronic snorers (n = 20) | Lateral cephalometry | OSA group had retroposition of maxilla and lower positions hyoid. |
| Lowe et al.219 | 1995 | 3b | Case–control study | Adult OSA subjects (n = 80) and non-OSA controls (n = 25) | Cephalometric measurements obtained from CT | OSA subjects have higher upper and lower facial heights, elongated maxillary and mandibular teeth, and proclined lower incisors. |
| Battagel et al.220 | 1996 | 3b | Case–control study | Adult OSA patients (n = 35) and healthy non-OSA controls (n = 24) | Lateral cephalometry | OSA patients had shorter cranial base length, shorter mandiblular body length, enlarged soft palate. |
| Tsai et al.221 | 2007 | 3b | Case–control study | Asian adult non-obese severe OSA patients (n = 46) and non-obese, snoring controls (n = 36) | Lateral cephalometry | Severe OSA patients had increased soft palate length and lower positioned hyoid bone compared to snorers. |
| Riha et al.222 | 2005 | 3b | Case–control study | Adult patients with OSA (n = 22) and non-OSA sibling controls (n = 22) | Lateral cephalometry | OSA patients had shorter mandibular body length and lower positioned hyoid compared to non-OSA siblings. |
| Johal et al.231 | 2007 | 3b | Case–control study | Adult OSA patients referred for oral appliance (n = 78) and gender matched healthy non-OSA controls (n = 78) | Lateral cephalometry | OSA patients the following differences compared to non-OSA controls: low set hyoid, retroposition of the mandible (SNB angle), increased soft palate length, thickness, and area. |
| Gungor et al.225 | 2013 | 3b | Case–control study | 16 Turkish adults with OSA and 16 Turkish non-OSA controls | Lateral cephalometry | OSA subjects had shorter midface length, protrusion of upper incisors, and lower positioned hyoid bone. |
| Banhiran et al.226 | 2013 | 3b | Case–control study | Adult OSA patients divided into mild (n = 43) and moderate/severe (n = 98) categories compared to non-OSA controls (n = 47) | Lateral cephalometry | Moderate/severe apneics had inferiorly positioned hyoid, shorter anterior cranial base angle, and longer soft palate compared to controls. There were no statistically significant differences between mild OSA patients and non-OSA controls. |
| Costa et al.227 | 2013 | 4 | Case series | Portuguese Adult OSA patients (n = 171) | Cephalometric measurements stratified by BMI | Caudally positioned hyoid, rotated mandible, and decreased hypopharyngeal airway caliber were associated with increasing AHI severity. |
| Sakat et al.228 | 2016 | 3b | Case–control study | Japanese adult severe OSA patients (n = 30) and BMI matched non-OSA controls (n = 10) | Craniofacial measurements on sagittal CT versus OSA | Retroposition of maxilla (SNA angle), retroposition of mandible (SNB angle), inferiorly positioned hyoid, shorter mandibular length were associated with OSA. |
| Neelapu et al.229 | 2017 | 3a | Systematic review and meta-analysis | Adult OSA patients and healthy non-OSA controls (n = 26 articles) | Lateral cephalometry | Significant weighted mean difference with insignificant heterogeneity was found for the following parameters between OSA patients and non-OSA controls: greater anterior lower facial height, lower position of hyoid bone, and decreased pharyngeal airway space. Significant weighted mean difference with significant heterogeneity was found for the following parameters between OSA patients and non-OSA controls: shorter cranial base and angle, decreased mandible, increased mandible rotation, decreased maxillary length, increased tongue area, increased soft palate area, and increased upper airway length. |
| Chi et al.224 | 2011 | 3b | Case–control study | Adult OSA patients(n = 55) and healthy non-OSA controls matched for age, sex, and race (n = 55) | Cephalometry based on MRI | Significant mean differences between OSA patients and non-OSA controls: Inferiorly and posteriorly displaced hyoid is associated with OSA. |
| Seto et al.230 | 2001 | 3b | Case–control study | Adult OSA patients (n = 29) and non-OSA controls (n = 21) | Lateral cephalometry, maxillary width, and height on dental exam | OSA patients had shorter mandibular body length and lower positioned hyoid compared to non-OSA patients. On maxillary width, OSA patients showed decreased inter-molar widths and increased palatal index. |
| Johal et al.223 | 2007 | 3b | Case–control study | Adult OSA patients referred for oral appliance (n = 78) and gender matched healthy non-OSA controls (n = 78) | Lateral cephalometry | OSA patients had the following differences compared to non-OSA controls: low set hyoid, retroposition of the mandible (SNB angle), increased soft palate length, thickness, and area. |
| Kuzucu et al.232 | 2020 | 4 | Case series | 89 Adult OSA subjects | Craniofacial measurements on CT | Narrower interpterygoid distance and smaller interpetrygoid to velopharyngeal length ratio was associated with increasing AHI. |
| Lam et al.236 | 2005 | 3b | Case–control study | Asian adults (n = 164) and White adults (n = 75) | Craniofacial measurements on physical exam obtained with patient sitting upright with head in neutral position | OSA subjects had; larger thyromental angle, neck circumference, BMI, and Mallampati scores. |
| Ferguson et al.238 | 1995 | 4 | Case series | Adult OSA patients divided into three groups based on neck circumference (n = 161) | Lateral cephalometry | Larger neck circumference apneics had lower hyoid bone positions, smaller neck circumference patients had smaller maxillary and mandibular lengths. |
| Tangugsorn et al.239 | 2000 | 3b | Case–control study | Obese OSA patients (n = 57), non-obese OSA patients (n = 48), and healthy non-OSA controls (n = 36) | Lateral cephalometry | Significant differences observed between OSA patients and non-OSA controls for the following parameters: inferiorly positioned hyoid, shorter cranial base, increased gonial angle, increased lower anterior facial height, decreased posterior facial height, large tongue, and large soft palate associated with OSA compared to non-OSA controls. Significant differences observed between non-obese and obese OSA patients for the following parameters: shorter mandibles, greater posteriorly displaced and rotated mandibles, greater anterior facial height, decreased posterior facial height. |
| Paoli et al.240 | 2001 | 3b | Case–control study | Obese adult OSA patients (n = 46) and non-obese adult OSA patients (n = 39) | Lateral cephalometry versus obesity | Retroposition of mandible (SNB angle), shorter cranial base, shorter mandibular ramus in non-obese OSA patients versus obese OSA patients. |
| Yu et al.241 | 2002 | 3b | Case–control study | Asian adult obese n = (33) and non-obese OSA patients (n = 29) and non-obese, non-OSA snoring patients (n = 13) | Lateral cephalometry | OSA patients have longer soft palates and lower hyoid position compared to snorers. |
| Lee et al.106 | 2010 | 3b | Case–control study | Caucasian adult OSA patients (n = 74) and Chinese adult OSA patients (n = 76) | Lateral cephalometry | Chinese OSA patients had shorter cranial base angle, shorter maxillary length, and shorter mandibular bodies compared to Caucasians. Chinese OSA patients had greater severity of OSA and lower BMI compared to Caucasians. |
| Xu et al.242 | 2020 | 3b | Case–control study | Icelandic adults (n = 108) and Chinese adults (n = 57) with OSA defined by ODI > 10 matched for age, gender, and ODI | Craniofacial dimensions as measured on MRI | Chinese subjects had bigger ANB angle, shorter mandibular corpus length, longer mandibular ramus length, and a wider and shallower maxilla. |
| Cakirer et al.244 | 2000 | 3b | Case–control study | Caucasian adults (n = 364) and African American adults (n = 165) and BMI <32 | Cranial and facial indices measured using calipers | Caucasians with AHI > 5 had lower cranial and facial indices compared to those with AHI <5 while African Americans had similar cranial and facial indices between the same groups. |
IV.C.4 |. Genetics
It has been known for over two decades that OSA aggregates in families. This is shown in the Cleveland Family Study in the US245 and also in studies in Scotland,246,247 Iceland,248 and Israel.249 The family aggregation is not explained by obesity.245 Individuals with a first-degree family member with OSA have approximately a two-fold increased risk of having the disorder.245 Structural risk factors for OSA – both soft tissues107 and craniofacial250,251 – also aggregate in families. Moreover, characteristics of the sleep study in OSA such as AHI, event duration, and measures of oxygen desaturation during sleep are heritable.252 Heart rate response to arousal is also heritable253 as is cardiopulmonary coupling,254 a measure of sleep depth.255 Extreme phenotypes have been demonstrated.256 Individuals with high phenotype risk scores but not OSA are extreme controls, while individuals with low phenotype risk scores but severe OSA are extreme cases. This suggests that there are likely to be rare genetic variants leading to these extreme phenotypes.
While family aggregation has been shown, progress on identifying relevant gene variants has been slow. There are different pathways to disease, such that OSA is a heterogeneous disorder. Obesity plays a major role.90 There are both soft tissue107 and craniofacial250,251 risk factors. There are also multiple physiological traits that determine risk for the disorder.36,257,258 The initial approach to identifying genes was linkage analysis,259,260 and most recently linkage has been used in conjunction with sequencing data to narrow windows of analysis to improve power,261 and applied to quantitative traits related to overnight SaO2. However, candidate gene studies have been underpowered and largely have not been replicated.262 More recently, progress has been made using genome-wide association studies263–265 or use of a special SNP panel.266
Genome-wide studies263–265 have largely focused on quantitative variables obtained from the sleep study to characterize SDB that were shown to be heritable.252 Based on primarily the Hispanic Community Health Study, a variant in GPR83 was found to be associated with AHI.263 However, the variant in GPR83 was specific to Caribbean background and data were unavailable for replication.
A larger genome-wide association study using data from multiple cohorts in both men and women looked for association with AHI in NREM sleep and REM sleep independently.265 While there were several suggestive associations, only one reached genome-wide significance, that is, a variant in RAI1 with NREM AHI in males (but not females) – a gene that has also been shown to show sexual dimorphism for cardiac disease and for adiposity traits in model organisms. Haploinsufficiency of the gene is implicated in the Smith–Magenis syndrome.267 Individuals with this syndrome have craniofacial abnormalities and circadian rhythm disturbances.268 Notably, significant sex by gene variants for sleep apnea was detected for multiple genes, supporting the importance of considering sex-specific genetic effects.
Studies have also focused on association with oxygen desaturation measures. Variants in the interleukin 18 receptor and hexokinase genes were associated with oxygen desaturation measures during sleep.269 This is based on a large multi-ethnic sample with replication. A later linkage-sequencing study also implicated multiple rare variants in the GTPase-activating protein DLC1261 with oxygen desaturation measures, with findings replicated in independent samples and supported by multiple sources of information, including genetic variants, gene expression, and methylation.
Admixture mapping has also been applied.270 This is an analytical tool that is applied to recently admixed populations where ancestors came from isolated continents.271 Novel variants were associated with AHI as well as oxygen desaturation measures. Notably, local African ancestry was shown to have a protective effect on the AHI and associated with shorter duration events. A region on chromosome 18q21 that included 20 variants on NARS/FECH was associated with both AHI and percentage time SaO2 < 90%, with 12 associations that replicated in independent cohorts. Evidence for a causal role in OSA was supported by finding that FECH expression was increased in association with lower nocturnal SaO2. These findings suggested a novel for iron metabolism in pathway influencing OSA phenotypes.
A genome-wide study that specifically examined both common and rare variants by whole genome sequencing264 identified novel rare variants associations with measures of desaturation, that is, ARMCX3, MRPS33, and C16orf90.
A Phenome-wide association study (PheWAS) study sought to determine support for previously identified variants.272 A PheWAS seeks association between gene variants, the diagnosis of OSA in the electronic health record273 or association with quantitative traits from sleep studies done clinically.272 For most of the previously described gene variants, there was no evidence of association with OSA diagnosis or with quantitative traits from the sleep study with findings from community-based cohorts which underwent standardized sleep apnea phenotyping.
Thus, studies of genetic variants in OSA are evolving. Recently, large biobanks have begun analyzing genetic associations with snoring and ICD-based OSA diagnosis.274 Future advances are expected as data from large clinical cohorts are combined with data from more deeply phenotyped research studies that also include data on gene function (expression, methylation).
V |. PATHOGENESIS OF OSA
V.A |. Contributory Factors for OSA: Anatomy
V.A.1 |. Nasal airway
The nasal cavity accounts for half of UA resistance.275 Several recent reviews indicate that nasal obstruction from septal deviation, inferior turbinate hypertrophy, nasal valve collapse, polyposis, and/or inflammation are common in patients with SDB, but the role of nasal obstruction as a contributing factor in the pathogenesis of OSA and SDB is controversial.276–278
Pathophysiology
Nasal breathing serves important physiological functions, including humidification, heating, and filtration of inhaled air.275 Nasal breathing is often decreased during sleep due to increased nasal mucosal congestion when recumbent279 and circadian variation in nasal resistance.280,281 Several physiologic mechanisms have been reported to explain the relationship between nasal airflow and breathing during sleep.
The first of these mechanisms is the Starling resistor model, which views the UA as a hollow tube282,283 with a collapsible pharyngeal segment bounded by the nasal and tracheal airways. The flow of air through the collapsible pharynx is influenced by upstream nasal, downstream tracheal, and transluminal pressure gradients. For example, when the nasal airway is obstructed, nasal resistance increases. In order to maintain stable maximum airflow from the nasal passages through the pharynx, the pharyngeal pressure drops, leading to increased collapsibility at the pharynx. In addition, by Pouseille’s law, airway resistance is proportional to the length of the airway and inversely proportional to the fourth power of the radius.284 Thus, even a small change in nasal airway patency can significantly increase airway resistance.285
The second reported mechanism is high nasal resistance promoting mouth breathing, which can predispose patients to SDB. During sleep, airway resistance is higher when breathing through the mouth compared to through the nose.286 Mouth breathing during sleep has been associated with up to 2.5 times higher airway resistance.287 This increased airway resistance can narrow the pharyngeal lumen leading to more frequent obstructive events, as demonstrated by Fitzpatrick et al. who found worsening of OSA severity when mouth breathing compared to nose breathing.287 Similarly, another study demonstrated that jaw opening during sleep, associated with posterior tongue displacement, is greater in patients with OSA than those without OSA.288
A third proposed factor is the nasal ventilatory reflex, which states that decreased nasal airflow can result in decreased activation of nasal receptors leading to decreased pharyngeal muscle tone and central respiratory drive and worsening of apneic events.289,290 This is supported by studies that have applied local anesthetic to nasal mucosa in healthy subjects resulting in significant increase in central and obstructive apnea events.291,292
Finally, it is postulated that nitrous oxide (NO) may play a role in maintaining pharyngeal muscle tone, spontaneous breathing, and sleep regulation.293 NO is produced in the nasal cavity and sinuses, and the total inspired quantity of nitrous oxide varies with nasal airflow.294 Despite these studies, its role in OSA development and regulation is still not completely understood.295
Clinical relationship between nasal obstruction and SDB
Several studies demonstrate that patients with SDB have greater rates of nasal obstruction. A recent study by Magliulo et al.276 demonstrated that 70% of patients with OSA had nasal obstruction confirmed by clinical examination and rhinomanometry. Another study of 49 OSA patients found that 45% reported subjective nasal obstruction.296
Data are conflicting regarding patients with nasal obstruction and risk of SDB. A study of 541 snorers who underwent posterior rhinomanometry concluded that nasal obstruction is an independent risk factor for OSA.297 The Wisconsin Sleep Study showed that those with self-reported nocturnal congestion had a three-fold increased incidence of habitual snoring.69 Conversely, a study by Miljeteig et al.298 divided 683 patients referred for PSG into three groups based on severity of nasal resistance and found no difference in apnea or snoring severity between groups.
Allergic rhinitis and SDB
A recent consensus statement on AR summarized the literature regarding the relationship between AR and sleep. The authors concluded that AR is associated with snoring, sleep fragmentation, and decreased sleep QOL, and successful treatment of AR can improve sleep quality. Many small studies with polysomnogram (PSG) data indicate that AR is associated with worsening PSG parameters, in terms of sleep efficiency, oxygen desaturation, and sleep architecture metrics, although results are mixed between studies.6
Nasal packing and SDB
Clinical studies and studies of normal subjects with artificially induced nasal obstruction have consistently demonstrated a greater predisposition to SDB.299–304 These studies obstructed the nasal cavity via the following measures: inflated balloons,303 petroleum jelly soaked gauze,300–302,304 or tape around the nares.299 Likewise, many studies have shown that the application of nasal packs during the management of epistaxis and post-nasal surgery can result in worsening sleep quality and increased apneic episodes.305–310
In summary, the literature suggests that nasal obstruction, either artificially or disease-induced, is associated with OSA-related symptoms such as snoring and poor quality sleep. Among those with SDB, nasal obstruction is highly prevalent. There does not appear to be a direct correlation between degree of nasal obstruction and SDB severity by AHI or oximetry.
V.A.2 |. Pharyngeal airway
Anatomic sites of airway obstruction during sleep have been assessed with a variety of techniques including pharyngeal pressure catheters placed at various sites in the UA, cine fluoroscopy, sleep endoscopy, CT, and MRI.311 Airway pressure monitoring studies have demonstrated that airway collapse is confined to or initiated in the oropharynx (OP) in the majority of patients.312
Pharyngeal shape
Several studies have identified differences in the pharyngeal lumen shape between patients with SDB and those without. Compared with normal subjects, habitual snorers have a generalized narrowing of the pharyngeal region.313 A normal pharyngeal lumen is elliptical in shape, with the long axis in the lateral dimension.314 In contrast, the lumen of snorers and OSA patients is circular or elliptical, with the long axis in the anterior–posterior dimension.314 This change in shape is likely a result of obstruction from the lateral pharyngeal walls. Studies have shown that the pharyngeal shape changes are most prominent at the retropalatal level200 and during sleep.315
In addition to shape, the length of the pharynx may also play an important role in OSA. Cephalometric studies demonstrate that lengthening of the pharynx is related to the severity of OSA.316 Similarly, increased rate of OSA in men may be primarily a result of a longer pharyngeal airway compared to female counterparts.317
Soft tissue factors
Several soft tissue factors can lead to pharyngeal narrowing or pharyngeal shape changes in adults with OSA including hypertrophy, inflammation, and/or edema.311 Thickened and collapsible lateral pharyngeal walls are a well-recognized factor in the pathophysiology of adult OSA.200,207,318 MRI studies confirm soft tissue enlargement in the UA leading to reduced size of the retropalatal airway, specifically, in OSA patients compared to control subjects.200,207,319 Lateral pharyngeal wall edema and enlarged lateral pharyngeal fat pads have been identified via tissue specimens and imaging in OSA patients.200,205,320–322 Lateral pharyngeal wall edema has been hypothesized to result from multiple factors including vascular congestion, inflammation secondary to trauma from snoring vibration, or pulmonary hypertension (PH) from recurrent hypoxic pulmonary artery vasoconstriction.323 An MRI study demonstrated that thickness of the lateral pharyngeal wall musculature was the predominant anatomic factor causing lateral airway narrowing in OSA patients as opposed to enlarged pharyngeal fat pads.200
Soft palate changes have also been noted in OSA patients including thickening of the soft palate324 and enlargement of the uvula.325 Soft palate position may also be related to OSA severity311,316,318,326
Many studies have assessed the relationship of tonsil size (both grade and volume) and OSA. The most recent study by Jara and Weaver studied an adult heterogeneous OSA population in the US and found that increasing tonsil grade was significantly associated with increasing OSA severity with adjusted data showing that every increase in tonsil grade as measured by the Brodsky classification system correlated to an increase in AHI of approximately 14 events/h.327 They also found that tonsil grade was more predictive of OSA than tonsil volume indicating that the actual tonsil volume may be less important than the proportion of space occupied within the OP.327 Other groups have studied the relationship between subjective (grade) or objective (volume/weight) tonsil evaluation and AHI and reported a trend toward significance328 or a significant association.318,329–334 To further support the role of enlarged tonsils in OSA, Camacho et al. performed an SR and MA of 17 studies evaluating tonsillectomy alone as treatment for adults with enlarged tonsils and OSA. They found a significant improvement in OSAS with a mean AHI decrease from 40.5 to 14.1 and a mean Epworth sleepiness scale (ESS) decrease from 11.6 to 6.1.335
Other groups have assessed a relationship between narrow fauces (posterior pillars of the tonsillar fossae) and OSA. Anatomically, the width of the fauces is generally defined as the diameter of the palatopharyngeal arch from oral view using a pharyngeal grading system.235 The grading system is based on the location of the palatopharyngeal arch intersection with the tongue. The presence of narrowing at the fauces has been previously defined as impingement of greater than 25% of the pharyngeal space by the peritonsillar tissues, excluding the tonsils.318 Studies report that the presence of narrow fauces is predictive of the presence of OSA.235,332,334 Woodson and Naganuma found that smaller endoscopically measured oropharyngeal spaces were correlated with elevated AHI.336
In summary, the OP is a common location of airway obstruction in subjects with SDB, and many anatomic factors have been identified as potential contributors to SDB including pharyngeal lumen size/shape, thickened lateral pharyngeal walls, elongated soft palate, enlarged uvula and/or tonsils, and narrow fauces.
V.A.3 |. Craniofacial structure
Craniofacial differences are present among OSA patients when compared to non-apneic controls.229 There are few studies examining how these anatomic factors contribute to the mechanism of UA collapse. A comprehensive review of the literature revealed five studies evaluating craniofacial measurements and physiologic factors that contribute to adult OSA. Sforza et al. measured critical closing pressures (upper airway critical closing pressure [Pcrit]) among 54 OSA patients and compared this to cephalometry. They found an association between greater Pcrit values (indicative of greater airway collapse) and longer soft palate length as well as with an inferiorly positioned hyoid.337 Genta et al. studied 34 Japanese-Brazilian men with OSA and also found an association between greater Pcrit values with an inferiorly and posteriorly positioned hyoid.338 Verin et al. compared UA resistance and cephalometry among OSA patients, snorers, and controls and found that an inferiorly and posteriorly positioned hyoid was associated with greater UA resistance among OSA patients.339 Watanabe et al. examined pharyngeal closing pressures at the velopharynx (VP) and OP among 54 Japanese OSA patients.340 Patients were stratified to either VP or VP + OP collapse which were the only two types of collapse patterns seen in their study. They found that patients with multilevel collapse (VP + OP) had a lower BMI, smaller maxillary and mandibular lengths, and a lower positioned hyoid compared to non-apneic controls while patients with only VP collapse had a larger BMI, and a lower positioned hyoid compared to non-apneic controls. No other cephalometric differences were seen among the groups. Comparing UA collapse among different ancestry background groups, Schorr et al. compared cephalometric measurements and Pcrit among Japanese-Brazilian OSA (n = 39) patients and Caucasian-Brazilians (n = 39).341 They found both groups had similar Pcrit values but that Japanese-Brazilian group had smaller cranial base angles, mandibular and maxillary lengths while the Caucasians-Brazilian group had greater BMI, tongue volume, and tongue length.
The studies to date suggest an association between several cephalometric values including: low hyoid position, short maxillary and mandibular length, short anterior cranial base length, and acute cranial base angles with higher Pcrit values, increased UA collapsibility, and greater UA resistance. Craniofacial influences may also be associated with increased risk for multilevel UA collapse. Furthermore, comparing OSA patients of different ethnic backgrounds have shown similar Pcrit values despite differences in BMI, tongue size, and craniofacial metrics suggesting that all these factors play a role in UA collapse. However, most studies had relatively small sample sizes. Therefore, larger studies controlling for BMI, soft tissue size, are needed to better elucidate the contributions of craniofacial metrics to OSA pathophysiology.
V.A.4 |. Upper airway fat
Tongue fat appears to play an important role in linking obesity and OSA risk. The pattern of fat deposition rather than BMI may be more indicative of OSA risk. There are only a handful of studies examining this relationship. Brennick et al. showed that among obese rats, there was greater deposition of fat within the tongue compared to non-obese rats.342 Kim et al. compared MRIs of the tongue between 90 obese OSA patients and compared them to 31 obese subjects without OSA. They showed that tongue fat volume calculated from the MRI was greater among the obese OSA patients versus obese subjects without apnea.208 Wang et al. compared tongue fat volumes among 67 obese OSA patients before and after a medical or surgical weight loss regimen.343 A reduction of tongue fat through weight loss correlated strongly with AHI reduction and was the primary UA mediator of the relationship between reductions in weight and AHI.
Although there is evidence that tongue fat is increased in obesity and that greater tongue fat is associated with OSA, how tongue fat influences the pathophysiology of OSA has not been well studied. There are no studies examining how tongue fat influences UA resistance, tongue muscle properties, and UA collapsibility. Future studies are needed to better understand the role of tongue fat in the pathogenesis of OSA.
V.B |. Contributory Factors for OSA: Physiology
V.B.1 |. Ventilatory stability/(loop gain)
“Loop gain” is an engineering term used to define the stability (“low” loop gain) or instability (“high” loop gain) of a negative feedback control system.180,344–347 Control of breathing is a negative feedback system in which chemoreceptors (e.g., in the carotid body; “controller”) and the lung (“plant”) try to maintain a PaCO2 at roughly 40 mmHg. In this setting, a high loop gain leads to large fluctuations in carbon dioxide (CO2). A useful analogy comes from consideration of room temperature in which a negative feedback control system tries to maintain a relatively constant temperature, for example, at 20 °C. If the thermostat (controller) were too sensitive, then any minor drop in room temperature would start the furnace (plant) and thus cause frequent fluctuations in room temperature. By analogy the CO2 levels are expected to fluctuate markedly if the chemoreceptors are too sensitive to minor CO2 perturbations. The temperature analogy is also helpful when one considers a plant that is too powerful. For example, if the room temperature were to fall to 19°C and in response the thermostat were to blast the temperature to 40°C, then the result would be major oscillations in room temperature. By analogy, if an individual were to respond to an increase in PaCO2 from 40 to 45 mmHg with hyperventilation lowering the PaCO2 down to 10 mmHg, then major fluctuations in CO2 would occur.348–350 Mathematically, loop gain is defined as the response (i.e., the increase in PaCO2 or room temperature) divided by the disturbance (i.e., initial drop in PaCO2 or room temperature): a major response to a minor disturbance (i.e., high loop gain) is considered destabilizing.
Loop gain has several components which are sometimes referred to as controller and plant gain. Controller gain can be thought of as chemoresponsiveness, which is the amount that ventilation changes for a given change in CO2. High loop gain due to a high controller gain (i.e., overly sensitive thermostat in the analogy above) can be seen in clinical situations such as high altitude or heart failure.351,352 Plant gain on the other hand is a measure of the efficiency of CO2 excretion (i.e., too powerful furnace, or a very small room in our analogy), and is the amount that CO2 changes for a given change in ventilation.353,354 High loop gain due to high plant gain is clinically less common, but situations in which the lung volume decreases (e.g., supine sleep)355 can increase overall loop gain. Another component which is less often discussed is the so-called mixing gain which is a function of circulatory time and other factors.356 The overall loop gain is the product of the various gains, principally determined by the controller and the plant.
Clinically a high loop gain can express itself as periodic breathing, for example, at high altitude,357 or in congestive heart failure (CHF).358,359 In OSA, the contribution of loop gain to OSA pathogenesis is less clear, but many believe it plays an important role.345–347,360,361 Several studies have shown an elevated loop gain in OSA compared to controls, although some debate is ongoing as to whether the observed abnormalities are a cause or effect of the disease.362 In theory, a high loop gain could manifest as fluctuations in output from the central pattern generator in the brainstem. This output would affect the diaphragm as well as the UA muscles. When output from the central pattern generator is at its nadir, the UA would be vulnerable to collapse in those who are anatomically susceptible based on the low output to the UA dilator musculature. Of note, because loop gain is considered a response over a disturbance, a spontaneous respiratory event, for example, a hypopnea may be destabilizing if a patient experiences a marked response to such a respiratory disturbance. Some have coined the expression “apnea begets apnea” based on the self-perpetuating nature of the control system abnormalities.
From a surgical perspective, elevated loop gain may be important since it has been found to predict failure of sleep apnea surgeries, at least in some cases.363–365 Given the variability in OSA pathogenesis, the strategy to improve pharyngeal anatomy/collapsibility surgically may be prone to failure in the subset of patients in whom OSA is primarily caused by abnormal ventilatory control.36 In theory, interventions lowering loop gain (e.g., acetazolamide or oxygen)366,367 may be considered rescue strategies for such patients who have residual OSA despite UA surgical intervention.368
It may be possible to quantify loop gain directly from inlab or home sleep studies.369–371 More research is needed to test on reproducibility and if prospective measurements of loop gain allows identification of surgical responders, and if loop gain lowering interventions may be useful for (a subset) of patients with an incomplete response to sleep apnea surgeries.
V.B.2 |. Neuromuscular control
Patients with OSA typically have an anatomical compromise of the UA predisposing them to pharyngeal collapse.207,372 Through protective UA dilator muscle reflexes, the activity of the muscles is increased during wakefulness in OSA compared to matched controls.201 However, with sleep-onset there is loss of UA motor output leading to collapse of the vulnerable airway.373 Brainstem control of UA dilator muscles has been the subject of intense investigation.374,375
The UA in humans includes 23 pairs of muscles which support its patency. The genioglossus is an important, major UA dilator muscle. The genioglossus is a large muscle comprising the substance of the tongue and maintains patency of the retroglossal airway.350,354,376 Notably, the hypoglossal motor nucleus in the medulla of the brainstem provides the input for the genioglossus muscle. Additionally, it is a complex muscle which has both tonic activity (i.e., present throughout the respiratory cycle) as well as phasic activity (i.e., bursts with each inspiration).377,378 The phasic activity of the genioglossus is thought to be representative of other phasic UA dilator muscles; thus, the study of genioglossus motor control may be reflective of other phasic muscles (e.g., palatoglossus and hyoglossus).379 The genioglossus has been shown to have state dependence, that is, has activity during wakefulness which is attenuated at sleep onset. Indeed, there is a marked fall in genioglossus activity at the alpha–theta transition which may be important in compromising pharyngeal mechanics.373,380,381 Lastly, hypoglossal nerve stimulation (HNS) has been shown to be effective for treatment of OSA.382–384 HNS likely acts via tongue protrusors from the medial branch of the hypoglossal nerve, that is, largely through genioglossal stimulation.385–387
The genioglossus’ behavior is influenced by a number of important factors:
A negative pressure reflex (NPR) exists whereby a sub-atmospheric (negative inspiratory or suction) pressure leads to a robust activation of the genioglossus muscle.388–390 This reflex is thought to be important in modulating UA patency since it serves to restore pharyngeal patency in the face of a collapsing perturbation. The NPR has been mapped using neurochemical techniques and is thought to be regulated by cholinergic systems in the brainstem, for example, the peri-obex region which is heavily cholinergic.391 Pharmacological studies indicate manipulation of the cholinergic system may influence this reflex, recognizing the complex role of acetylcholine throughout the brain and systemically.392 A recent pilot study showed potential benefits of the combination of oxybutynin and atomoxetine on the AHI.393 A larger multicenter trial recently completed enrollment, but the results are not yet reported (NCT03919955).
Chemoreflexes are also thought to be important since hypoxia and CO2 may serve to activate the genioglossus muscle. CO2 stimulation may have differential effects on the diaphragm versus the UA dilator muscles. The combination of negative pressure plus CO2 may serve synergistically to activate the UA dilator muscles.203,317,394–396
The arousal response also has a major impact on genioglossus activity. When an individual awakens from sleep, there is a robust activation of the genioglossus muscle which is thought to restore pharyngeal patency.290,397,398 On the other hand, if sleep is maintained following UA collapse, then the accumulation of respiratory (CO2, negative pressure) stimuli may activate pharyngeal dilator muscles and thus restore pharyngeal patency without the need for repetitive arousal from sleep. Thus, the arousal threshold has become an important therapeutic target, but its manipulation is a double-edged sword: drugs (hypnotics, sedatives) which delay arousal may allow some stabilization of breathing but may also lead to severe hypoxemia prior to arousal.399–401 Consequently, patient selection becomes a critical factor in designing appropriate studies.377,402 At present, no RCTs have shown improvements in hard clinical outcomes, despite some potential physiological benefits.
A number of neurochemical influences can affect hypoglossal motor control. Monosynaptic projections from various brain structures have been shown in animal models including locus coeruleus (adrenergic), lateral dorsal/pedunculopontine tegmentum (cholinergic), and hypothalamic (orexinergic) and raphe neurons (serotonergic) among others. These neurochemical targets may allow augmentation of hypoglossal motor output and effect genioglossus activity.375,403–405
Other muscles are likely important, for example, the tensor palatini which receives its output from the mandibular branch of the trigeminal nerve.406–409 Of note, the neurobiology and control of different motor nuclei differ substantially from the standpoint of premotor inputs and neuropharmacology. The tensor palatini has primarily tonic activity (i.e., constant activity throughout the respiratory cycle) and thus has less in the way of respiratory modulation than the genioglossus or other phasic muscles.
Neuromuscular control is an important factor in UA patency. It can potentially be manipulated either pharmacologically or via electrical stimulation. Further study regarding underlying mechanisms and clinical trials focused on hard outcomes would be encouraged.
V.B.3 |. Arousal threshold
The arousal threshold refers to the propensity to wake up from sleep. Some people have a low arousal threshold meaning they wake up easily – or with minimal stimulus – whereas other people have a high arousal threshold – meaning they require considerable stimulus to arouse.397,410 The arousal threshold is thought to be important in OSA pathogenesis since roughly 1/3 of OSA patients are found to have a low arousal threshold and may wake up prematurely.398,411 The accumulation of respiratory stimuli during stable sleep has been shown to activate pharyngeal dilator muscles, which in many patients is both necessary and sufficient to stabilize breathing.394,396 Stanchina et al.203 showed a combination of CO2 and negative intrapharyngeal pressure could lead to robust activation of the UA dilator muscles during stable sleep. Thus, patients with a low arousal threshold may not experience sufficient accumulation of respiratory stimuli to activate the dilator muscles and thus repetitive airflow limitation is predicted. In contrast, patients with a high arousal threshold could get sufficient magnitude of respiratory stimuli for adequate duration to activate pharyngeal dilator muscles and thus stabilize breathing. The observation that even severe OSA patients have some periods of spontaneously occurring stable breathing has yielded discussion regarding potential therapies to manipulate the arousal threshold.398,412 Some view the arousal threshold as a double-edged sword. That is, therapies to increase the arousal threshold may be beneficial if this intervention allows dilator muscle activation and stabilization of breathing.413 On the other hand, an agent which raises the arousal threshold may yield substantial hypoxemia and hypercapnia which could impact end organ function.414 Thus, agents such as sedatives or hypnotics which can raise the arousal threshold may be beneficial at least in theory for select patients. However, the existing data suggest that any improvements in apnea which occur with these agents are relatively modest.400,401 Consequently, combinations of therapy may well be required to eliminate apnea using this approach.415 Another consideration is that these pharmacological agents have risks and benefits like all interventions and thus carefully performed outcome-based studies will be needed before any clinical recommendations can be made.
One strategy which has been discussed in the context of combination therapies is that of surgical rescue. Some data suggest that a low arousal threshold may be a risk factor for failure of UA surgery to achieve a surgical cure.363,364,416 In theory, the elevation of the arousal threshold may be a therapeutic target whereby an agent (e.g., trazodone or eszopiclone) could be used to elevate the arousal threshold in patients who have residual apnea, for example, following uvulopalatopharyngoplasty (UPPP).399 Such strategies would need to be studied carefully in the context of patient reported outcomes and hard endpoints.
Regarding the assessment of the arousal threshold, several techniques have been employed. The “gold standard” measurement was considered either esophageal manometry or intrapharyngeal pressure catheter measurements.410 However, Edwards et al. reported a regression formula which has considerable value in estimating the arousal threshold using clinically accessible data, such as the degree of hypoxemia, the arousal index, the AHI, and the occurrence of apneas versus hypopneas.411 Using this approach more than 60% of the variance in the arousal threshold can be predicted. In addition, Sands et al. developed a technique using signal processing of the polysomnographic recordings which can also estimate the arousal threshold using clinically available data.370
One important consideration is the fact that the arousal threshold is not a fixed trait but rather a dynamic phenomenon which changes with treatment.417,418 For example, many patients with a high arousal threshold with sleep apnea will experience a lowering of arousal threshold over time on therapy. This observation leads to speculation that the elevated arousal threshold seen in some OSA patients may be an adaptive phenomenon whereby elevation in the arousal threshold may allow the accumulation of respiratory stimuli, which could ultimately help to improve sleep to some extent.418 Another extension of this logic is that CPAP treatment in some patients may lead to insomnia since the lowering of arousal threshold may be associated with worsening of sleep quality.419 The same argument could be made for non-CPAP therapies as well. Further study is clearly required to determine the importance of arousal threshold in OSA pathogenesis and its importance in treatment of OSA both adjunctively (e.g., with CPAP)420 and as a rescue strategy (e.g., following failed UPPP).
VI |. DIAGNOSING OSA
VI.A |. Questionnaires for OSA
In-laboratory PSG is the gold standard for diagnosis of OSA, but can be expensive, inconvenient, and difficult to access. This is particularly true when considering screening in the general population or perioperatively. Additionally, PSG may not be readily available to all clinicians. Validated questionnaires are easily administered in all clinical settings and offer a rapid point-of-care tool to risk-stratify patients. Equally important is the assessment of the QOL impairment in patients with OSA as physiologic sleep measures are poor descriptors of QOL.421 Patient-reported outcome assessment is especially important when evaluating changes in QOL after treatment or over time. This review is based on more than 20 studies with reports ranging from Level 1a to 2b (overall grade C evidence) (Table VI.A.1).
TABLE VI.A.1.
Evidence for the role of validated screening, functional outcomes, and health-related QOL questionnaires for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Nagappa423 | 2015 | 1a | Systematic review | 1. Sleep, 2. Surgical, 3. General | Meta-analysis of STOP-BANG | STOP-BANG is adequate screening tool in sleep and surgical patients. |
| Abrishami429 | 2010 | 1a | Systematic review | 1. Sleep, 2. Surgical, 3. General | Meta-analysis of OSA screening questionnaires | STOP-BANG and Berlin had similar sensitivities. Studies were heterogenous. |
| Amra430 | 2017 | 1a | Systematic review | 1. Sleep, 2. Surgical, 3. General | Comparison of OSA screening questionnaires. | STOP-BANG had best sensitivity and specificity for moderate OSA. |
| Chiu431 | 2016 | 1a | Systematic review | 1. Sleep, 2. Surgical, 3. General | Meta-analysis of STOP-BANG, Berlin, STOP, and ESS | STOP-BANG most accurate at detecting OSA at all severity cutoffs. |
| Senaratna422 | 2017 | 1a | Systematic review | 1. Sleep, 2. Surgical, 3. General | Meta-analysis of Berlin questionnaire | Berlin is adequate screening tool in sleep and surgical patients. Studies were heterogenous. |
| Billings437 | 2014 | 1b | Cohort | Sleep clinic | Comparing psychometric properties of SAQLI and FOSQ | Both demonstrate responsiveness to CPAP treatment. Comparable reliability and validity. |
| Coutinho424 | 2019 | 1b | Cohort | Sleep clinic | Validate NoSAS as screening tool for OSA | NoSAS is an adequate screening tool for OSA. |
| Marti-Soler426 | 2016 | 1b | Cohort | General | NoSAS derivation and validation. Comparison to other screening questionnaires. | NoSAS adequate screening tool compared with Berlin and STOP-BANG. |
| Silva441 | 2016 | 1b | Cohort | 1. OSA, 2. non-OSA | Comparison of SAQLI, FOSQ, SF-36 scores with OSA severity | Scores correlate w/OSA severity in females, but not males. All demonstrated convergent validity. |
| Abma444 | 2016 | 2a | Systematic review | 1. Sleep, 2. Non-sleep | Review of quality of PROMs for OSA | Most PROMs not adequately assessed due to low quality studies. |
| Mollayeva 443 | 2016 | 2a | Systematic review | 1. Sleep, 2. Non-sleep | Meta-analysis of psychometric properties of PSQI | Adequate reliability and validity as QOL tool. |
| Kendzerska436 | 2013 | 2a | Systematic review | 1. Sleep, 2. Non-sleep | Overview of psychometric properties of ESS | Convergent validity lower than expected. Few high quality studies. |
| Rong428 | 2019 | 2b | Cohort | 1. OSA, 2. Primary snorers | Comparison of NoSAS to STOP-BANG | NoSAS and STOP-BANG had comparable sensitivities and ROC. |
| Flemons439 | 2002 | 2b | Cohort | OSA | Evaluate psychometric properties of SAQLI | Adequate properties as a QOL instrument. |
| Flemons438 | 1998 | 2b | Cohort | 1. OSA, 2. Primary snorers | Derivation of SAQLI | Excellent internal consistency and responsiveness is present. |
| Hong425 | 2018 | 2b | Cohort | Sleep clinic | Comparison of NoSAS to STOP-BANG, ESS, and Berlin | NoSAS is an adequate screening tool for OSA. |
| Lacasse440 | 2002 | 2b | Cohort | Sleep clinic | Validation of SAQLI | Adequate validity and responsiveness to treatment. |
| Peng427 | 2018 | 2b | Cohort | 1. OSA, 2. Primary snorers | Comparison of screening questionnaires | NoSAS, Berlin, and STOP-BANG have comparable sensitivities. |
| Weaver442 | 1997 | 2b | Cohort | 1. Sleep, 2. Non-sleep | Derivation of FOSQ | FOSQ had excellent reliability and demonstrated known-groups validity. |
Abbreviations: ESS, Epworth Sleepiness Scale; FOSQ, functional outcomes sleep questionnaire; PROMs, patient reported outcomes measure; PSQI, Pittsburgh sleep quality index; SAQLI, sleep apnea quality of life index.
V1.A.1 |. Screening questionnaires
Clinical questionnaires validated to assess the risk of OSA include the Berlin,422 STOP-BANG,423 and NoSAS424–428 screening tools.429–431 The Berlin questionnaire contains a total of 10 items divided into three categories: (1) daytime sleepiness, (2) BMI and hypertension, and (3) height and weight, where a positive score in two categories indicates high risk for OSA.422 The STOP-BANG is an 8-question survey including four subjective and four objective items with each positive item contributing 1 point for a maximum score of 8 points.423 The NoSAS is a newer tool developed in 2016 in sleep clinic patients and includes five items assessing mostly objective data such as BMI and neck circumference with scores ranging from 0 to 17 and a score ≧8 denoting high risk for OSA.426
A clinically useful questionnaire that assesses OSA risk should be able to identify patients with clinically relevant OSA (AHI ≥ 15), where an increased risk of cardiopulmonary disease and mortality has been established (true positive)432–434 and minimize the incorrect labeling of individuals without clinically relevant OSA as having the condition (false positive). The Berlin and STOP-BANG questionnaires have sensitivities of 82% and 94%, respectively,422,423 while the NoSAS has a sensitivity of 65%–90% in identifying sleep clinic patients with clinically relevant OSA.424–431 Additionally, the Berlin and STOP-BANG questionnaires have reported sensitivities of 79%–82% and 91% respectively among surgical patients and 89% and 88% respectively among general clinic patients.422,423 The ROC curves for the STOP-BANG at different OSA severity classes showed a STOP-BANG cutoff score of 5 had the best combination of sensitivity and specificity for moderate-severe OSA (AHI ≥ 15) in sleep medicine (60%, 72%) and surgical populations (45%, 56%).423 At this cutoff score, the STOP-BANG also has the best combination of sensitivity and specificity for severe OSA (AHI ≥ 30) in sleep clinic patients (66%, 66%) and surgical patients (56%, 74%).423
V1.A.2 |. Functional status measures and health-related quality of life questionnaires
It is well documented that, in general, patients with sleep apnea have limitations in daily activities and poorer QOL than normal healthy subjects.435 Thus, assessment of functional status and QOL is highly important to evaluate in addition to physiologic sleep measures when assessing OSA patients. Validated functional status and QOL measures most used in the clinical and research settings include the ESS,436 Sleep Apnea Quality of Life Index (SAQLI),437–441 Functional Outcomes of Sleep Questionnaire (FOSQ),437,441,442 and the Pittsburgh Sleep Quality Index (PSQI).443
Questionnaires can be divided into functional status questionnaires, which measure the ability to perform daily activities, and health-related QOL questionnaires, which capture the unique personal response to not being able to perform routine activities. Functional status questionnaires include the PSQI, ESS, and FOSQ. The PSQI was developed to measure multiple components of sleep quality and includes questions on sleep propensity (likelihood of falling asleep) over the past 1 month.443 The ESS assesses likelihood of falling asleep across eight scenarios on a scale of 0–3 with a higher score indicating more sleepiness.436 The psychometric properties of the FOSQ suggest that it offers a unique self-report measure of functional status as it relates to the impact on daily activities.442 The FOSQ has 30 questions in five domains: activity, vigilance, intimacy and sex, general productivity, and social outcome.437,441 The SAQLI is a health-related QOL questionnaire that is diseasespecific and includes questions focused on evaluating functioning across daily aspects of life affected by poor sleep.437,439–441 The SAQLI includes 35 questions across four domains: daily functioning, social interactions, emotional functioning, and symptoms and can be combined with a fifth domain that assesses negative impacts of treatment.437,439–441
When evaluating functional status and health-related QOL surveys, it is important to consider the reliability, validity, and responsiveness of these measures. Reliability of these questionnaires were reported in terms of internal consistency, the degree to which individual items on a survey are related as measured by Cronbach’s α, and test–retest reliability, the consistency of scores for the same patient over time measured by the intraclass correlation coefficient (ICC). All four of the QOL questionnaires demonstrated acceptable internal consistency (α ≥ 0.7)436,443 while the FOSQ and SAQLI demonstrated excellent internal consistency (α ≥ 0.9).437,438,440,442 The FOSQ and SAQLI demonstrated excellent test–retest reliability (ICC ≥ 0.9)439,440,442 while this test characteristic was not clearly assessed for the ESS and PSQI. Four studies assessed this factor in the ESS, but results of the test and retest populations are not comparable due to a long time interval between administrations or due to a change in test conditions.436 The data on test–retest reliability in the PSQI is limited to three studies that either did not include patients with a sleep disorder or had a long time interval between test administration.443 Measurement error, the error in a measurement that is not due to true differences in the construct being measured, was not reported for any questionnaire.
The validity of QOL and functional status instruments were measured in terms of convergent, divergent, and discriminative validity. Convergent validity measures the degree to which scores of a questionnaire correlate with other questionnaires with related constructs. The PSQI had strong correlations (r ≥ 0.7) with other questionnaires measuring sleep quality.443 The SAQLI showed moderate correlations (0.3 ≤ r < 0.7) with related domains of general health-status questionnaires such as the 36-item Short Form (SF-36) and the Global QOL Scale.437–441 The FOSQ showed moderate correlations with related domains of the SF-36 and the Sickness Impact Profile.437,441,442 The ESS showed a moderate correlation to the Maintenance Wakefulness Test and poor correlation (r ≤ 0.3) with the multiple sleep latency test (MSLT), both of which are objective daytime sleepiness measures.436 PSG measures (AHI, minimum O2 saturation) were either poorly or not significantly correlated with any of the questionnaires.436,437,441,443,444
Divergent validity assesses whether instruments with unrelated constructs in fact have low correlations with one another. Divergent validity was reported for the PSQI, which had non-significant correlations with unrelated instrument measures of bladder dysfunction and psychopathology.443 No other questionnaires reported on this measure. Discriminant validity refers to the ability of an instrument to distinguish between patients with sleep disorders and normal subjects. Discriminant validity was present for the ESS, PSQI, and FOSQ, but was not reported for the SAQLI.436,442,443
The ability of an assessment to measure change over time is important especially if the instrument is used in treatment effectiveness studies. The SAQLI and FOSQ both demonstrated the ability to detect change over time after treatment initiation.437–440 Both showed large effect sizes (d ≥ 0.8) after CPAP treatment in patients with OSA.437,439 In one study, the FOSQ showed a larger improvement in scores in patients who used >4h of CPAP nightly while there was no difference in scores for the SAQLI.437 (Table VI.A.1) An important limitation of this review is the heterogeneity of studies reporting on both screening and QOL questionnaires. Definitions of hypopnea and method used to detect OSA varied between studies resulting in wider ranges of reported sensitivity and specificity. Additionally, several studies used translated versions of the questionnaires that were not previously validated in those languages. Another limitation is the lack of highquality validation studies for all QOL measures. Future research should focus on using uniform definitions of hypopnea/apnea and utilize the gold standard PSG in validating OSA questionnaires and to further validate QOL surveys to estimate their adequacy as an outcome measure.
Overall, validated clinical questionnaires may be used as a tool to identify patients at high risk for OSA, monitor response to treatment, or evaluate the functional status and health-related QOL of patients. To screen patients suspected of sleep apnea in the clinic setting or preoperatively before planned surgery, the STOP-BANG questionnaire with a cutoff score of 5 is recommended. To measure functional status related to OSA, the FOSQ is recommended as it is disease-specific, assesses the effect of sleepiness comprehensively across domains, and has the strongest evidence for all measurement properties. (Table VI.A.2)
TABLE VI.A.2.
Functional status and health-related QOL questionnaires
| Internal consistencya | Test–retest reliabilityb | Measurement error | Convergent validityc | Divergent validity | Discriminative validity | Responsiveness | |
|---|---|---|---|---|---|---|---|
| ESS | Acceptable | ** | – | Poor to moderate | – | Present | – |
| PSQI | Acceptable | ** | – | Strong | Presentd | Present | – |
| SAQLI | Excellent | Excellent | – | Moderate | – | – | Present |
| FOSQ | Excellent | Excellent | – | Moderate | – | Present | Present |
Abbreviations: – Not reported;
not clearly assessed. ESS, Epworth Sleepiness Scale; FOSQ, functional outcomes sleep questionnaire; PSQI, Pittsburgh sleep quality index; SAQLI, sleep apnea quality of life index.
Based on Cronbach’s α values: unacceptable (α < 0.5), poor (0.5 ≤ α < 0.6), questionable (0.6≤ α <0.7), acceptable (0.7 ≤ α < 0.8), good (0.8 ≤ α < 0.9), excellent (α ≥ 0.9).
Based on intraclass correlation values: poor (ICC < 0.5), moderate (0.5 ≤ ICC < 0.75), good (0.75 ≤ ICC < 0.9), excellent (ICC ≥ 0.9).
Reported as degree of correlation with questionnaires measuring related constructs: poor (r < 0.3), moderate (0.3 ≤ r < 0.7), strong (r ≥ 0.7).
Indicates presence of poor or non-significant correlations with unrelated questionnaires.
VI.B |. Screening for OSA
VI.B.1 |. Primary care setting
Although a myriad of screening instruments exist for OSA, the majority of the literature centers around four main questionnaires: (1) Berlin, (2) STOP, (3) STOP-BANG, and (4) ESS. The Berlin questionnaire is composed of 10 items grouped into three domains to assess snoring severity, excessive daytime sleepiness, and history of hypertension or obesity.445 The STOP questionnaire is constructed in an even simpler format, with four yes or no questions corresponding to snoring, tiredness, observation (of apneas), and BP.446 The STOP-BANG questionnaire consists of the STOP items with an additional four questions for BMI, age, neck size, and gender.447 Lastly, the ESS is an 8-item questionnaire asking participants to rate their likelihood of falling asleep during various daytime activities.448 The convenience and low cost of questionnaires are well suited for the primary care setting.449–452 The four questionnaires mentioned above are the most widely discussed with respect to OSA screening in primary care and have each been validated on multiple occasions across a wide range of demographics.
Several studies have evaluated the differences in sensitivity and specificity between screening questionnaires.422,453,454 A recent multicenter prospective study enrolled 812 patients diagnosed with type 2 diabetes, obesity, HF, or resistant hypertension who completed multiple OSA screening questionnaires. Following a diagnostic sleep study, the STOP-BANG questionnaire (with “high-risk” set to 3 points or above) had a greater sensitivity than either the Berlin questionnaire or the OSA50 questionnaire (95%, 75%, and 88% sensitivity, respectively). After increasing the “high-risk” threshold to 5 or above on the STOP-BANG, it also had a greater specificity (69% compared to 38% for the Berlin and 21% for the OSA 50).455
The largest comparative study to date was a retrospective review by Silva et al. that included 4770 patients from the SHHS. For this cohort, the STOP-BANG had the highest sensitivity for moderate-to-severe OSA (87%). The 4-Variable screening tool (sex, BMI, BP, and snoring) had the highest specificity.456 A separate cohort study of 212 patients in Beijing similarly found the STOP-BANG questionnaire to be of superior predictive value than the ESS, Berlin, or STOP questionnaires.457 Additionally, a recent MA confirmed the STOP-BANG possessed the greatest pooled sensitivity. The ESS questionnaire, however, was shown to have markedly higher pooled specificity compared to the STOP, STOP-BANG, and Berlin.431 Although the STOP-BANG is the most sensitive screening questionnaire, the evidence is unclear as to which questionnaire is the most specific. In the primary care setting, a screening test with higher sensitivity may be preferred to ensure adequate diagnostic investigation and mitigate sequelae of untreated OSA.
There is very little evidence on the benefits of widespread screening for OSA in asymptomatic adults without comorbidities.429 A 2017 SR supervised by the US Preventive Services Task Force analyzed 110 studies with over 46,000 patients and found no information relating OSA screening to health outcomes.458 To adequately assess the impacts of screening, a randomized trial assigning patients to screening and control groups – with adequate sample size and follow-up – would be required. With respect to costs, the general sentiment behind the creation of the STOP and STOP-BANG questionnaires was to simplify previous instruments and reduce the burden of measurement on survey participants.447,457 Although there is no direct evidence describing the financial burden of implementing OSA screening, reports of other screening instruments have suggested minimal burden and disruption of the clinical workflow.446,459–461
In summary, the literature with respect to OSA screening in the primary care setting remains ambiguous. Support exists for the superior sensitivity and performance of the STOP-BANG as a screening tool, but no evidence has shown the long-term health impact of screening compared with no screening. Although the harms associated with screening appear limited, the precise cost-utility tradeoff of screening in the primary care setting has yet to be assessed. (Table VI.B.1)
TABLE VI.B. 1.
Evidence for screening for OSA in the primary care setting
| Study (authors) | Year | LOE (1a–5) | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Jonas et al.458 | 2017 | 2a | SR | 110 Studies (1994–2016) | Association of OSA screening with health outcomes | Insufficient evidence for OSA screening among asymptomatic adults; no studies were found comparing screening to no screening. |
| Abrishami et al.429 | 2012 | 2a | SR | 10 studies (1988–2008): one retrospective review, nine prospective cohorts | Sensitivity and specificity values of the Berlin, STOP, STOP-BANG, Wisconsin, and SASDQ questionnaires | Reviewed OSA screening questionnaires show inconsistent predictive values. |
| Kee et al.455 | 2018 | 2b | Prospective multicenter cohort | Patients with diagnosed comorbidities (diabetes, obesity, resistant hypertension, and heart failure) and a baseline home sleep study who completed the STOP-BANG, Berlin, and OSA50 questionnaires | Sensitivity and specificity values | STOP-BANG had the best sensitivity for screening purposes in patients with diabetes, obesity, resistant hypertension, and heart failure. |
| Silva et al.456 | 2011 | 2b | Prospective multicenter cohort | Sleep Heart Health Study (SHHS) Participants who completed baseline PSG, 4-Variable Screening Tool, STOP, STOP-BANG, and ESS questionnaires | Sensitivity and specificity values | STOP-BANG had the highest sensitivity while the 4-Variable had the highest specificity for moderate-to-severe OSA. |
| Luo et al.457 | 2014 | 2b | Prospective single center cohort | Patients both with and without OSA who completed the STOP, STOP-BANG, Berlin, and ESS questionnaires | Sensitivity and specificity values | STOP-BANG had the highest sensitivity among the reviewed questionnaires. |
| Tan et al.454 | 2017 | 2b | Prospective multicenter cohort | Singapore Health Study 2012 (SH2012) participants who completed home sleep study and Berlin questionnaire | Sensitivity and specificity values | Berlin questionnaire possesses a high sensitivity and NPV for severe OSA. |
| Senaratna et al.422 | 2017 | 2a | SR and meta-analysis | 35 Studies (2008–2016): 26 prospective cohorts, five retrospective reviews, four cross-sectional analyses | Pooled sensitivity and specificity values of the Berlin questionnaire | Limited evidence to suggest utility of Berlin questionnaire in general population. |
| Chiu et al.431 | 2017 | 2a | SR and meta-analysis | 108 Studies (1999–2016) | Pooled sensitivity and specificity values of Berlin, STOP, STOP-BANG, and ESS questionnaires | The STOP-BANG questionnaire is the most accurate tool for detecting mild, moderate, and severe OSA. |
| Tan et al.453 | 2016 | 2b | Prospective multicenter cohort | Singapore Health Study 2012 (SH2012) participants who completed home-based sleep study and STOP-BANG questionnaire | Sensitivity and specificity values | STOP-BANG has moderate sensitivity and high NPV for moderate-to-severe OSA. |
VI.B.2 |. Perioperative setting
The prevalence of OSA among surgical patients is 7%–10% and approximately 70% in patients undergoing bariatric surgery.462–464 The diagnosis of OSA is associated with increased risk for postoperative complications in general and increases cardiac adverse events,465–468 respiratory failure, oxygen desaturation, and intensive care admission.467,469–477 Moreover, these complications have increasingly resulted in malpractice lawsuits.478,479 Data about postoperative mortality is mixed but generally shows no increased 30-day mortality, which is thought to be due to increased monitoring in this group of patients.468,472,480–485 Identifying at risk patients is critical to perioperative planning given the increased risk of postoperative cardiopulmonary complications with OSA.486
As most surgical patients with OSA are undiagnosed, the Society of Anesthesia and Sleep Medicine Guidelines on preoperative screening and preparation of patients with OSA strongly recommends screening for OSA in the preoperative period.486
Screening will prompt practitioners to create a safer postoperative management plan, such as extended monitoring, the use of CPAP therapy, using less opioids and sedatives, and more regional anesthesia techniques whenever applicable. It also helps in deciding on the eligibility for ambulatory procedures.487
Sleep study testing is the standard for OSA diagnosis. However, it is not cost-effective or practical to screen patients using PSG in the perioperative setting due to highcost and inconvenience. There are often logistical issues of coordinating sleep study testing and surgery scheduling timelines. However, the preoperative assessment is a cost-effective opportunity to screen for and select high risk patients who can then be offered sleep study and treatment for OSA.488 In order to facilitate OSA detection, several predictive screening tools have been proposed. Using the questionnaires with clinical criteria is easy, inexpensive, and has an acceptable sensitivity. However, they should not be used as a diagnostic tool without confirmatory sleep study testing given the low to moderate specificity and the potential for misdiagnosis.446,464,486,489
Screening components
It is best if the patient is seen a few weeks before surgery, so a proper diagnosis and treatment are initiated. Patients can be screened by their primary care physician, by their surgeon, or by the anesthesia care provider in the preanesthesia clinic. Screening may take place in the hospital, or on the day of the procedure.
Preoperative screening
Preoperative evaluation of a patient for potential identification of OSA should include: medical history, screening questions, and physical exam. A focused review of history includes prior airway difficulty, hypertension, CV problems, craniofacial abnormalities, and any previous sleep studies. A physical examination should include evaluation of the airway, neck circumference, tonsil size, and tongue volume.490
Screening methods in the perioperative setting
Several pre-operative screening questionnaires are available. Only STOP-BANG, Berlin, American Society of Anesthesiologists (ASA) checklist, and perioperative sleep apnea prediction have been evaluated and validated in surgical populations.491
STOP-BANG (SB) questionnaire
The questionnaire builds on the STOP questionnaire (snoring, tiredness, observed apnea, and high BP) and adds the BMI, age, neck circumference, and gender criteria (BANG). Each item in the questionnaire is score as (yes/no) and is scored as (0–1) for a total score of 8. The STOP-BANG questionnaire has a high sensitivity where a score of ≥3 score is associated with an increased risk of moderate-to-severe OSA,470,492,493 and a higher rate of perioperative complications.469,471,473,475–477,485,486,494–496 On the other hand, the specificity ranges from 30% to 43%, which may yield high false positive rates and may increase unnecessary testing.446 The addition of serum HCO3− of at least 28 mmol/L to the STOP-BANG score improves the specificity but decreases its sensitivity.447
Perioperative Sleep Apnea Prediction Score (P-SAP)
The P-SAP score includes the following: snoring, thyromental distance <6 cm, type 2 diabetes mellitus (DM), high BP, Mallampati class III or IV, BMI > 30 kg/m2, age > 43 years, neck circumstance >40 cm, and male gender. A diagnostic threshold P-SAP score of 2 or higher showed excellent sensitivity (0.939) but low specificity (0.323), whereas a P-SAP score of 6 or higher had low sensitivity (0.239) but excellent specificity (0.911).464
American Society of Anesthesiologists (ASA) checklist
ASA OSA scoring checklist combines physical characteristics, history of apparent airway obstruction, and somnolence.497 Physical characteristics include signs and symptoms suggestive of the diagnosis of OSA (BMI > 35, craniofacial abnormalities, and neck circumference). Symptoms of apparent airway obstruction include: loud snoring, frequent snoring, observed pauses in breathing during sleep, and awakening from sleep with choking sensation. Somnolence is present if the patient reports fatigue despite adequate “sleep” or falling asleep easily in a non-stimulating environment. The ASA checklist was validated in surgical population with a moderate sensitivity (72%–87%) which was comparable to both the STOP and Berlin questionnaires.489
The Berlin questionnaire445
The Berlin questionnaire focuses on some risk factors for OSA. It includes questions about snoring, daytime sleepiness, and fatigue. Patients are also asked to provide information on history of hypertension, age, weight, height, sex, neck circumference, and ethnicity. Its sensitivity is 69–87% in surgical patients.489
DES-OSA score
The DES-OSA is a morphologic OSA prediction score; it takes into account the Mallampati score (MP), thyroid chin distance (DTC), BMI, neck circumference, and sex.498 Patients with MP class II scores 2 points, and 3 points if they are class III or IV. They are awarded points based on their DTC, neck circumference, and BMI. Patients who score 5 or above are likely to have OSA diagnosis.
Oxygen Desaturation Index (ODI)
Using overnight oximetry has been found to have a very good correlation with AHI on PSG in surgical patients.499 ODI is defined as the number of (3% or 4%) desaturation episodes per hour. The ODI > 10 demonstrated a sensitivity of 93% and a specificity of 75% to detect moderate and severe OSA. (Table VI.B.2)
TABLE VI.B. 2.
Association of OSA with worse postoperative outcomes
| Study | Year | LOE | Objective | Study design | Study groups | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|---|
| Khiani500 | 2009 | 3b | If the diagnosis of OSA using Berlin questionnaire will result in postoperative oxygen desaturation that needs supplemental oxygen following sedation for gastrointestinal procedures. | Cross sectional study | Berlin Questionnaire in patients undergoing sedation for GI procedures. | Rates of transient hypoxia, defined as a pulse oximetry measurement less than 92% requiring an increase in supplemental oxygen. | No significant difference in rate of transient hypoxia between the high- and low-risk groups (odds ratio 1.48; 95% CI, 0.58–3.80). Suggests that the majority of patients with no OSA can undergo conscious sedation for routine endoscopic procedures with standard monitoring practices. |
| Vasu et al.477 | 2010 | 2b | To determine whether high risk -OSA (HR-OSA) ≥ 3 on preoperative STOP-BANG (SB) questionnaire correlated with a higher rate of postoperative complications. | Retrospective Cohort study | Adults undergoing elective surgery at a tertiary care center who were administered the SB questionnaire. 135 patients were identified, 56 (41.5%) had high risk scores for OSA. | Pulmonary complications: Hypoxemia, atelectasis, pulmonary embolism, or pneumonia. Cardiac complications: New-onset atrial fibrillation, systemic hypotension, or myocardial infarction. |
Patients at high risk of OSA had a higher rate of postoperative complications compared with patients at low risk (19.6% vs. 1.3%; p < 0.001). The SB questionnaire is useful for preoperative identification of patients at higher risk for complications. |
| Coté et al.471 | 2010 | 2b | To de termine: The preoperative prevalence of OSA using SB questionnaire related to the need for airway maneuvers and sedation related complications. |
Prospective Cohort study | 231 consecutive patients undergoing advanced gastrointestinal procedures under sedation were identified by SB and were classified as high risk for OSA (score, ≥ 3 of 8; SB+) or low risk (SB<3). | -Airway Maneuvers (AM): - defined as a chin lift, modified mask ventilation, nasal airway, bag-mask ventilation, and endotracheal intubation. -Sedation-related complications (SRCs) were defined as any duration of pulse oximetry less than 90%, systolic blood pressure less than 90 mm Hg, apnea, or early procedure termination. |
The prevalence of SB+ was 43.3%. The frequency of hypoxemia was significantly higher among patients with SB+ than SB− (12.0% vs. 5.2%; relative risk [RR], 1.83; 95% confidence interval [CI], 1.32–2.54). The rate of AMs was also significantly higher among SB+ (20.0%) compared with SB− (6.1%) patients (RR, 1.8; 95% CI, 1.3–2.4). |
| Pereira et al.475 | 2013 | 2b | SB score can predict the risk of a patient having OSA and to evaluate the incidence high risk OSA (HR-OSA) in surgical patients admitted to the Post-Anesthesia Care Unit (PACU). | Prospective Cohort study | 340 adult patients after noncardiac and non-neurological surgery were admitted to PACU. 179 (52%) were considered HR-OSA. HR-OSA if SB score≥3 and Low-risk of OSA (LR-OSA) if SB score<3 (LR-OSA). |
Postoperative respiratory complications; residual neuromuscular blockade (NMB); hospital length of stay. | HR-OSA had: 1. More frequent hypoxia in PACU (9% vs. 3%, p = 0.012). 2. Higher incidence of residual neuromuscular blockade (NMB) (20% vs. 16%, p = 0.035). 3. Longer hospital stays. |
| Chia et al.495 | 2013 | 2b | To evaluate if the HR-OSA by SB can predict difficult intubation | Prospective cohort study | 200 patients undergoing surgery under general anesthesia. 83 with HR- OSA based on the SB score ≥ 3. Mallampati score and tonsil size, as well as demographic data, were recorded preoperatively for all patients. | Difficulty of intubation defined by Cormack & Lehane grade III or IV or the need of an intubation aid, or the need of three or more intubation attempts. 7% had difficulty of intubation. | Rate of difficult intubation was higher in HR- OSA patients than in LR-OSA patients. (13.3% vs. 2.6%. (p < 0.001). A SB ≥ 3 was seen more frequently in the difficult intubation patients (78.6% vs. 38.7%) (p = 0.009). |
| Acar494 | 2014 | 2b | To evaluate if the HR-OSA by SB can predict difficult intubation | Prospective cohort study | 200 patients undergoing surgery under general anesthesia. 83 with HR- OSA based on the SB score ≥ 3. Mallampati score and tonsil size, as well as demographic data, were recorded preoperatively for all patients. | Difficulty of intubation defined by Cormack & Lehane grade III or IV or the need of an intubation aid, or the need of three or more intubation attempts. 7% had difficulty of intubation. | Rate of difficult intubation was higher in HR- OSA patients than in LR-OSA patients. (13.3% vs. 2.6%. (p < 0.001). A SB ≥ 3 was seen more frequently in the difficult intubation patients (78.6% vs. 38.7%) (p = 0.009). |
| Mehta et al.501 | 2014 | 2b | To identify the prevalence of OSA by using the SB questionnaire and subsequent risk factors for airway interventions (AI) and sedation related adverse events (SRAE) | Prospective cohort study | 243 patients undergoing routine gastrointestinal procedures under sedation. HR-OSA = SB ≥ 3 score, low risk −OSA = SB score < 3 | Airway interventions (AI): Chin lift, mask ventilation, placement of nasopharyngeal airway, bag mask ventilation, unplanned endotracheal intubation, hypoxia, hypotension, or early procedure termination. |
48% with HR- OSA. An SB score ≥3 was found not to be associated with occurrence of AI (relative risk [RR] 1.07), or SRAE (RR 0.81, 95% CI, 0.53–1.2) after adjustment for propofol dose, BMI, smoking, and age. HR-OSA patients are not at higher risk for airway intervention or sedation-related adverse events SRAE. |
| Proczko et al.485 | 2014 | 2b | To determine if morbidly obese patients using CPAP have fewer and less severe perioperative complications and a shorter hospital stay than patients with at least three SB criteria and are not using CPAP therapy. | Retrospective Cohort Study | 3 groups of morbidly obese patients undergoing bariatric surgery. Group A: 99 patients who were diagnosed with PSG and used CPAP therapy before and after surgery. Group B: 182 patients who met at least three SB criteria and did not use CPAP. Group C: 412 patients who scored 1–2 items on the SB. |
Postoperative hospital stays and pulmonary complications. | Group B patients had a significantly higher rate of pulmonary complications, worse oxygen saturation, respiratory rates, and increased length of stay in hospital. There were also two cases of sudden death in this group. |
| Seet et al.476 | 2015 | 2b | Predict independent risk factors for intraoperative and early postoperative adverse events. | Retrospective cohort study | 5432 patients who underwent elective surgery were analyzed. | Adverse events: hypoxia, failed intubation or multiple attempts, dental injury, laryngospasm, bronchospasm, arrhythmia, hypertension and hypotension, unanticipated surgical bleeding, hypothermia, nerve injury, drug adverse reaction, equipment failure, unplanned ICU admission, post-anaesthesia care unit (PACU) time >2 h. | 7.4% had unexpected intraoperative and early postoperative adverse events. These events were greater in patients with SB scores ≥ 3 Patients with SB scores ≥ 5 had a fivefold increased risk of unexpected adverse events. |
| Chung | 2016 | 2a | Does the diagnosis of OSA changes the postoperative outcome? | Systematic Review | Patients undergoing surgery either under general or neuraxial anesthesia or sedation | Pulmonary, desaturation, difficult intubation, atrial fibrillation, cardiac complications, resource utilization, mortality. | The presence of OSA negatively influences perioperative outcomes. |
| Gokay et al.473 | 2016 | 4 | SB vs. Berlin OSA questionnaires for evaluating potential respiratory complications. | Prospective Cohort Study | 126 patients who underwent laparoscopic cholecystectomy. | Perioperative respiratory complications. | Both questionnaires found statistically significant differences between the low- and high-risk groups. |
| Chudeau et al.469 | 2016 | 2b | To evaluate whether the SB is predictive of perioperative respiratory complications in urgent surgery | Prospective cohort study | The SB questionnaire was used. 104 patients were HR-OSA and 85 LR-OSA. | Perioperative complications: respiratory complications, cardiac complications, neurologic complications, hospital length of stay and mortality. | HR-OSA vs. LR-OSA had: Higher respiratory complications (21% vs. 6%, a prolonged length of hospital stay (6 [3–12] vs. 4 [2–7] days. SB score was independently associated with respiratory complications (OR 1.44 [1.03–2.03]. |
| Setaro et al.502 | 2018 | 4 | To determine if longer monitoring of patients with OSA in the PACU improves patient outcomes after general anesthesia | Retrospec- tive cohort study | 602 patients were evaluated. 68 patients (11%) had a confirmed and a presumptive diagnosis of OSA on chart review and screening STOP > 1. | Oxygen desaturation <95%, PACU length of stay | Most patients (96.5%) did not experience oxygen desaturation regardless of OSA diagnosis or a positive STOP score. Patients with OSA did not experience a higher incidence of respiratory symptoms while in the PACU. |
| Diagnosis of OSA and postoperative morbidity | |||||||
| Memtsoudis et al.474 | 2011 | 2b | To analyze perioperative demographics and pulmonary outcomes of patients with OSA after orthopedic and general surgical procedures | Case–control study | 2,610,441 entries for orthopedic and 3,441,262 for general surgical procedures performed between 1998 and 2007. Of those, 2.52% and 1.40%, respectively, carried a diagnosis of OSA. | Aspiration pneumonia, adult respiratory distress syndrome (ARDS), pulmonary embolism (PE), and the need for intubation and mechanical ventilation. | OSA was associated with a significantly higher adjusted OR of developing pulmonary complications with the exception of PE. |
| Kaw et al.467 | 2012 | 1b | OSA is often undiagnosed before elective surgery and may predispose patients to perioperative complications. | Systematic review | 13 studies (n = 3942). Studies without controls, involving upper airway surgery, and with OSA diagnosed by ICD-9 codes alone were excluded. |
The incidence of postoperative desaturation, acute respiratory failure (ARF), postoperative cardiac events, and ICU transfers. | OSA was associated with significantly higher risk of postoperative cardiac events odds ratio (OR) 2.07; ARF OR 2.43; desaturation OR 2.27, and ICU transfer OR 2.81. |
| Opperer et al.468 | 2016 | 2a | The diagnosis of OSA has an impact on postoperative outcomes. | Systematic review | 413,304 OSA and 8,556,279 control patients. | Combined complications of Cardiac, pulmonary, airway, mortality complications and resource utilization. Length of hospital stay and ICU admissions. |
OSA patients had worse outcomes for pulmonary and combined complications, in-hospital mortality varied among studies. |
| Chan et al.465 | 2019 | 2b | To determine the association between OSA and 30-day risk of cardiovascular complications after major noncardiac surgery. | Prospective cohort study | 1364 patients recruited without prior diagnosis of OSA and undergoing major noncardiac surgery. Monitored with nocturnal pulse oximetry and measurement of cardiac troponin concentrations. | Primary outcome was a composite of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, and stroke within 30 days of surgery. | Rates for composite complications: 30.1% for severe OSA, 22.1% for moderate OSA, 19.0% for mild OSA, and 14.2% for no OSA. OSA and risk for complications was significant only among patients with severe OSA (adjusted HR, 2.23 [95% CI, 1.49–3.34]; and not among those with moderate or mild OSA. |
| Diagnosis of OSA and postoperative mortality | |||||||
| Lockhart et al,481 | 2013 | 2b | To determine whether a prior diagnosis of OSA, or a positive screen for OSA is associated with increased risk for 30 days and one year mortality. | Prospective cohort study | 14,962 patients, of whom 1939 (12.9%) reported a history of OSA. All patients completed preoperative OSA screening combination of the Berlin and Flemons STOP, and SB scores. | 30 day postoperative mortality and 1-year mortality. | Screening tools identified a high prevalence of undiagnosed patients at risk for OSA (9.5%–41.6%). Neither a prior diagnosis of OSA nor a positive screen for OSA risk was associated with increased 30-day or one-year postoperative mortality. |
| D’Apuzzo et al.480 | 2012 | 2b | To determine if patients with OSA havea higher likelihood of postoperative in-hospital complications or increased costs after revision arthroplasty. | Retrospective cohort study | Nationwide sample of 258, 455 patients who underwent revision total hip or knee arthroplasty. Of these patients, 16,608 (6.4%) had been diagnosed with OSA. |
In-hospital mortality, pulmonary embolism, and wound complications | OSA was associated with increased in-hospital mortality (odds ratio, 1.9; Pulmonary embolism (odds ratio, 2.1) Wound hematomas or seromas (odds ratio, 1.36) Increased postoperative charges ($61,044 vs. $58,813). |
| Mokhlesi et al,484 | 2013 | 2b | To determine if sleep disordered Breathing (SDB) is associated with higher hospital mortality, longer hospital stay, higher cost, respiratory and cardiac complications in bariatric surgeries | Retrospective Cohort study | Nationwide Inpatient Sample database of 91,028 adult patients undergoing bariatric surgeries | In-hospital death, total charges and length of stay, respiratory and cardiac complications | SDB was independently associated with decreased mortality (OR = 0.34), total charges (−$869), and length of stay. SDB associated with significantly increased emergent endotracheal intubation, noninvasive ventilation, and atrial fibrillation. |
| Lyons PG,503 | 2016 | 2b | To investigate the association between OSA and in-hospital mortality rapid response team (RRT) activation, ICU admission, intubation, and cardiac arrest on the wards in a large cohort of surgical and nonsurgical ward patients. | ∖Retrospective cohort | 93,676 ward admissions from 53,150 adult patients’ records were screened for the end points. OSA was identified in 5625 (10.6%) patients. | Primary outcome is in-hospital mortality. Secondary outcomes included length of stay (LOS), RRT activation, transfer to the ICU, endotracheal intubation, cardiac arrest on the wards, and a composite outcome of RRT activation, ICU transfer, and death. | OSA patients had more frequent RRT activations (1.5% vs. 1.1%) and ICU admission (8% vs. 7%) than controls but a lower inpatient mortality rate (1.1% vs. 1.4%). OSA was not associated with clinical deterioration and was associated with decreased in-hospital mortality. |
Abbreviations: HR-OSA, high risk-OSA; ICU, intensive care unit; LR-OSA, low risk-OSA; SB, STOP-BANG.
VI.C |. Symptoms Associated with OSA
VI.C.1 |. Snoring, gasping, excessive daytime sleepiness
OSA is characterized by repetitive episodes of partial and complete pharyngeal collapse. Accordingly, in patients with OSA, increased UA resistance from partial pharyngeal collapse may manifest as loud snoring. Complete pharyngeal collapse with cessation of airflow may result in witnessed apneas. Individuals with OSA may report nocturnal episodes of choking or gasping, and/or symptoms of daytime fatigue, sleepiness, tiredness, or even insomnia. Bedpartners may be affected.
Large community studies have demonstrated habitual snoring (occurring most nights of the week) in 34%–44% of the population, witnessed breathing pauses in 6%–29%, and daytime hypersomnolence in 18%–28% of individuals.88,145,456 Recent data from a population sample in the United Kingdom has shown a significant increase in the rate of reported witnessed apneas in the community over the last 20 years concurrently with a rise in obesity.504 The frequency of these symptoms is higher in those with OSA. Among individuals diagnosed with OSA, 38%–80% report snoring, 10%–67% report witnessed apneas, and 32%–40% report excessive daytime sleepiness (ESS ≥ 11). In general, increasing frequency of each symptom occurs with increasing AHI and OSA severity.88,456,505
Findings from several population-based and retrospective studies have demonstrated that habitual snoring and witnessed apneas are predictors for OSA.88,145,505,506 However, the most predictive symptom for identifying individuals with OSA may be nocturnal choking or gasping episodes.507 A large Canadian population study in 2009 found that 4.3% of the population reported awakening suddenly with gasping or choking rarely or sometimes, and 1.5% reported this symptom occurring once a week or more508; of individuals diagnosed with OSA, 14.4% reported nocturnal choking or gasping episodes rarely or sometimes (OR 3.52, 95% CI 1.92–6.46), and 11.2% reported this symptom once a week or more (OR 7.92, 95% CI 3.74–16.74). Furthermore, an SR by Myers et al. in 2013507 demonstrated that nocturnal choking or gasping episodes had a likelihood ratio (LR) of 3.3 (95% CI 2.1–4.6) for the diagnosis of OSA (AHI ≥ 10), while snoring was found to be less predictive (LR 1.1, 95% CI 1.0–1.1).
Despite being common in the general population, snoring or apneas may not be routinely screened for or reported in the primary care setting.509 Many validated questionnaires445,446,510 include some or all of these symptoms and may help identify patients at risk for OSA.509 Sex differences in reported sleep symptoms have been reported, with men being more likely to report snoring (34% vs. 19%) and women being more likely to report hypersomnolence (22.6% vs. 15.5%).90 Shepertycky et al.511 also demonstrated that women with OSA were more likely to report insomnia (OR 4.20, 95% CI 1.54–14.26) and less likely to report witnessed apneas (OR 0.66, 95% CI 0.38–1.12). These variations in OSA symptomatology are important to recognize not only for appropriate screening, but also may have broader implications for OSA treatment.420 (Table VI.C.1)
TABLE VI.C.1.
Evidence on the relationship between snoring, gasping, daytime sleepiness, and OSA
| Study | Year | LOE (1a–5) | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Myers et al.507 | 2013 | 1a | Systematic review | Community-screened patients and patients referred for sleep evaluation | In-laboratory PSG | Nocturnal choking or gasping episodes most predictive symptom (LR 3.3, 95% CI 2.1–4.6) for the diagnosis of OSA (AHI ≥ 10). Snoring, reported apneas, and excessive daytime sleepiness were less predictive of the diagnosis (LR 1.1 [95% CI 1.0–1.1], 1.4 [1.2–1.5], and 1.3 [1.1–1.4], respectively). |
| Young et al.90 | 1993 | 1b | Prospective cohort | Wisconsin Sleep Cohort Study population (n = 602) | In-laboratory PSG | In individuals with OSA (AHI ≥ 5), more men than women reported habitual snoring ± breathing pauses (34% vs. 19%), and more women than men reported hypersomnolence (22.6% vs. 15.5%). |
| Johns et al.510 | 1993 | 1b | Cross sectional survey | Adult patients suspected of having OSAS by history of snoring (n = 273) | Questionnaire (ESS) and in-laboratory PSG | Patients found to have OSAS (RDI > 5) had significantly higher levels of daytime sleepiness (measured by ESS) than primary snorers; ESS scores and thus daytime sleepiness increased significantly with increasing OSAS severity. |
| Netzer et al.445 | 1999 | 1b | Cross sectional survey | Adult primary care patients (n = 100) | Questionnaire (Berlin), in-home PSG | High-risk patients (in ≥2 categories) were more likely to meet criteria for OSAS (RDI > 5). High-risk qualification by one symptom category did not predict RDI as well as composite score. |
| Young et al.88 | 2002 | 1b | Prospective cohort | Sleep heart health study population (n = 5615) | Questionnaire, clinical examination, in-home PSG | Habitual snoring (OR 2.87, 95% CI 2.10–3.91), and breathing pauses occurring <3 nights/week (OR 1.78, 95% CI 1.34–2.37), or 3–7 nights/week (OR 4.03, 95% CI 2.87–5.67) were associated with moderate to severe OSA (AHI ≥ 15). |
| Chung et al.446 | 2008 | 1b | Diagnostic accuracy | Adult pre-operative patients (n = 177) | Questionnaire (STOP), in-laboratory PSG | Patients identified as high risk for OSA (≥2 “yes” answers) demonstrated a sensitivity of 65.6%, specificity of 60%, positive predictive value (PPV) 78.4%, and NPV 44.0% for OSA (AHI > 5). The STOP questionnaire was more sensitive in detecting patients with moderate (AHI > 15) to severe (AHI > 30) OSA. |
| Silva et al.456 | 2011 | 1b | Prospective cohort | Sleep heart health study population (n = 4770) | In-home PSG | Among individuals with moderate-to-severe SBD (RDI ≥ 15, <30) and severe SBD (RDI ≥ 30): 55.9% and 69.6%, respectively snore often/every night; 38.9% and 66.9% have witnessed apneas, and 32% and 40% have excessive daytime sleepiness (ESS≥11). |
| Johnson et al.506 | 2020 | 1b | Prospective cohort | Jackson Heart Sleep Study (n = 719) | Questionnaire (Including, STOP-bang, ESS), in-home PSG | Among an adult African American cohort with moderate to severe OSA (REI ≥ 15), 49.3% snore ≥3 nights/week (vs. 31.1% with mild or no OSA), 6.6% had witnessed apneas ≥3 nights/week (vs. 3.1%), and 22.4% had sleepiness (ESS > 10) (vs. 19.5%). |
| Durán et al.145 | 2001 | 2b | Cross-sectional | Community sample (n = 555) | Structured interview, in-laboratory PSG | Habitual snoring (OR 5.45) and breathing pauses during sleep (OR 13.4) are risk factors for moderate-severe OSA (AHI ≥ 15). Habitual snoring (OR 3.36) is also risk factor for mild OSA (AHI ≥ 5–14.9). Breathing pauses not significantly associated with mild OSA (OR 4.63). Daytime hypersomnolence is not associated with mild OSA (OR 1.37) or moderate-severe OSA (OR 1.05). |
| Shepertycky et al.511 | 2005 | 2b | Retrospective cohort | Population of patients diagnosed with OSAS (n = 260) | Questionnaire, in-lab PSG | At the time of OSAS diagnosis, women were more likely to present with insomnia (OR 4.20, 95% CI 1.54–14.26) and less likely to report witnessed apneas (OR 0.66, 95% CI 0.38–1.12). |
| Ustun et al.505 | 2016 | 2b | Retrospective cohort | Clinical Sleep Laboratory Database (n = 1992) | In-lab PSG | Patients found to have OSA (AHI > 5 or RDI > 10) were significantly more likely to self-report snoring (79.7%) and witnessed apneas (43.8%) than individuals without OSA. A clinical prediction model for diagnosing OSA using medical history features was superior to model performance using symptoms alone. |
| Li et al.512 | 2019 | 2b | Cross-sectional observa-tional study | Adult patients with snoring and suspected OSAS (n = 134) | Questionnaire (ESS and Montreal Cognitive Assessment), in-lab PSG | Daytime sleepiness, as measured by ESS, worsens with increasing OSAS severity. |
| Epstein et al.513 | 2009 | 5 | Guideline | Recommendations on the evaluation and management of adult patients with OSA | A comprehensive sleep history in a patient with suspected OSA should include evaluation for snoring, witnessed apneas, gasping/choking episodes, excessive sleepiness not explained by other factors (including assessment of sleepiness severity by ESS), total sleep amount, nocturia, morning headaches, sleep fragmentation/sleep maintenance insomnia, and decreased concentration and memory. |
VI.C.2 |. Nocturia
Nocturia, the need to urinate more than once per night,514 affects greater than 60% of adults aged 70 and older and is more prevalent in women.515,516 The prevalence of high risk for OSA (based on results from the Berlin questionnaire) studied in a sample of female patients, in an urogynecology clinic was greater in patients with nocturia (61.7%) compared to those without (24.1%) (OR 2.9, 95% CI 1.29–6.52).517 A recent MA demonstrated an association between OSA and risk of nocturia 1.41 (95% CI 1.26–1.59). Furthermore the authors’ report that patients with severe OSA had a higher incidence of nocturnal urination in comparison to patients with mild or moderate disease (relative risk [RR] 0.82; 95% CI 0.72–0.94). In men there was a statistically significant association between OSA and risk of nocturia (RR 1.487, 95% CI 1.087–2.034), which was not the case in women.518 To support the link between severity of OSA and nocturia, in a study comparing patients with and without nocturia, an increased ODI was associated with an increased probability of nocturia, in the multivariable model (OR = 1.03; 95% CI = 1.01–1.06).519 The higher the ODI score, the greater the probability of nocturia.
A chain of physiologic effects leads to diuresis and nocturia in the setting of OSA. Increased intra-thoracic negative pressures (needed to overcome a closed UA) lead to atrial stretch which triggers a signal of excessive fluid volume and the subsequent release of atrial natriuretic peptide induces diuresis.520,521
Nocturia has significant effects on QOL and in particular sleep quality, impacting total sleep time (TST) and sleep efficacy. In comparison to patients with OSA without nocturia, mean SaO2, time spent in desaturation below 90%, and ODI were worse in OSA patients with nocturia.516 Treatment with CPAP has been shown to improve nocturia in patients with OSA.522
VI.C.3 |. Caffeine intake
Excessive daytime sleepiness is a common symptom of OSA. One of the reasons to consume caffeinated products is to counteract sleepiness. Caffeine is a natural psychoactive substance which blocks adenosine receptors in the brain 30–60 min after consumption. As adenosine builds up during the day, the sleepier we become. Caffeine blocks this process, and as a consequence we remain alert and vigilant.
Bearing this is mind, assessing caffeine use as a marker of sleepiness is recommended when taking patient history in patients suspected of OSA. Studies on patients with untreated OSA have demonstrated that increased caffeine intake is associated with less cognitive impairment523 thereby indicating a potential therapeutic effect of caffeine and evidence for self-treatment of OSA. On the other hand, caffeine can delay onset of sleep and can interfere with our circadian rhythm.
Despite its common use, the prevalence of caffeine use in patients at risk for OSA is difficult to estimate. The SHHS provides the most comprehensive assessment of caffeine use in OSA patients.524 Women with SDB have the strongest correlations between caffeinated soda and OSA. There was only an association between caffeinated soda and severe SDB in men.524 Coffee and tea consumption were not associated with SDB.
Several trials were created to evaluate interactions among caffeine use, OSA, and CV risks. Bardwell et al. demonstrated that OSA patients consumed significantly greater caffeine than normal controls (295 vs. 103 mg/day of caffeine).525 Robinson et al. measured caffeine consumption before and after 4 weeks of CPAP. Although sleepiness improved after CPAP, caffeine intake did not change.526 Such research has contributed to the concept that OSA has independent effects on hypertension and other CV markers which are separate from the impact of caffeine use.
VI.D |. Physical Exam Findings Related to OSA
Evaluation of patients with OSA includes standard physical exam that evaluates for age, BMI, neck circumference, assessment of craniofacial structures, and standard UA examination of the nasal cavity, oral cavity, and OP. The ability of an individual physical exam finding to predict OSA alone is limited, but the entire physical exam can be considered in the context of the patient’s history to provide a better assessment of OSA risk.
Some goals of the physical exam for OSA aim to: (1) incorporate exam findings for use in OSA screening tools in conjunction with patient symptoms; (2) determine potential causes of airway obstruction, (3) identify potential anatomic concerns that may limit PAP therapy, and (4) aid in determining potential therapeutic targets for sleep surgery (see Section IX.C.1).
This section evaluates the components of the physical exam relevant to OSA evaluation and summarizes the available evidence that associates particular exam findings with OSA. Evidence tables are provided for specific exam findings that have adequate data.
Not all components of the physical exam are routinely performed, and the examination should be tailored to each individual patient after completing a thorough history and symptom review.
VI.D.1 |. BMI
Obesity is a well-established risk factor for the development and progression of OSA. A BMI greater than 30 increases risk for OSA350 and weight reduction is a treatment option that can improve OSA severity.189 BMI level also has implications for surgical outcomes with higher BMI associated with persistent OSA after surgery.527 BMI level should be included in the evaluation of OSA patients.
VI.D.2 |. Neck circumference
Neck circumference (NC) or collar size is associated with OSA in adults.184,528–534 It is measured as the circumference of the neck at the superior border of the cricothyroid membrane. Larger neck circumference is related to elevated BMI. Fat deposition in the tissues of the neck results in a smaller and more collapsible UA, thus increasing the likelihood of OSA.
An SR and MA of facial phenotype in adult OSA found that adults with OSA had significantly larger NC compared to controls.535 Neck circumference is associated with OSA severity.138,528 Specifically, NC > 40 cm (16 inch) is associated with snoring and OSA.528,529,536–538 Although it is positively correlated with OSA, NC alone is a poor predictor of OSA.539 The sensitivity of NC alone is insufficient to identify patients with sleep apnea, but increases when combined with other clinical, anthropometric, or cephalometric measures.537,540–542 One screening tool that incorporates NC is the STOP-BANG which is used to identify patients at high risk for OSA446 and uses NC or shirt collar size estimates with thresholds of 43 cm (17 inch) for males and 41 cm (16 inch) for females. (Table VI.D)
TABLE VI.D.
Neck circumference (NC) measurement in the diagnosis of OSA
| Study | Year | Study design | Study groups | Clinical endpoint | Conclusion | |
|---|---|---|---|---|---|---|
| Agha et al.535 | 2017 | 3a | Systematic review and meta-analysis of predominantly case–control studies | Not applicable | Not applicable. | Adults with OSA had an increased weighted mean difference in NC compared with controls, in five case–control studies. The pooled mean change was 1.26 mm (0.64–1.88), with large heterogeneity found between studies. |
| Kim et al.138 | 2015 | 3b | Cross-sectional retrospective study | Cohort of snoring Asian patients | Neck circumference in Asian patients with OSA compared to individuals with simple snoring. | NC predicted OSA presence and severity. |
| Kushida et al.537 | 1997 | 4 | Prospective cohort study | Consecutive patients referred for evaluation of sleep disorders | Evaluated measurements of the oral cavity with body mass index and NC, in predicting OSA. | A NC ≥ 40 cm is associated with OSA sensitivity of 61% and specificity 93%. |
| Stradling et al.533 | 1990 | 4 | Prospective study | Men 35–65 years old GP registry | Registry study. | NC was an independent predictor of nighttime hypoxia events. |
| Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine513 | 2009 | 5 | Guideline | Not applicable | Not applicable. | Features that may suggest the presence of OSA include increased NC (> 17 inch in men and > 16 inch in women). |
| Ahbab et al.528 | 2013 | 4 | Cohort study | OSA adults | Evaluate NC and metabolic syndrome parameters in OSA patients. | NC in severe OSA was significantly higher than in mild–moderate OSA. NC was an independent risk factor for severe OSA (odds ratio [OR]: 1.55. 95% confidence intervals [CI]: 1.09–2.21, p = 0.01). |
| Chang et al.529 | 2013 | 4 | Prospective cohort | Cohort of Chinese patients with snoring complaints in sitting position | Evaluate if snoring in a sitting position is a predictor of OSA in patients. | NC ≥40 cm as a predictor for OSA had sensitivity 48.6%, specificity 87.8%, and likelihood ratio 3.98. |
| Park et al.532 | 2014 | 4 | Retrospective cohort | Non-obese Asian patients who underwent PSG | Evaluate association between body weight and obstructive sleep apnea even in patients who are not obese. | AHI found to be positively correlated with the NC. NC is independently associated with OSA. |
| Davies et al.530 | 1990 | 4 | Prospective study | Cohort of patients referred for investigation of sleep disorders | Importance of NC, obesity, and radiographic pharyngeal dimensions for OSA. | NC of 42.5 cm (16.75 inch) for a 1.78 m (5 feet 10 inch) man) is 77% sensitive and 82% specific for significant OSA with positive predictive value (PPV) of 75%. |
| Yildirim et al.534 | 2015 | 4 | Prospective | Cohort of patients with a BMI ≥ 30 and OSA symptoms | Evaluate anthropometric measurements with OSA. | Significant positive correlation between AHI and neck circumference. |
| Mirrakhimov et al.531 | 2013 | 3a | Systematic review | Not applicable | Not applicable. | Neck circumference is associated with OSA. |
| Santos et al.542 | 2019 | 4 | Cohort study | Cohort civil servants 35–74 years | Compare BMI, several surrogate markers of body fat (in isolation or combined) and validated questionnaires for screening OSA. | Age and gender-adjusted NC (AUC = 0.733 [0.711–0.755]) had moderate accuracy as predictor for OSA. |
| Yusoff et al.538 | 2010 | 4 | Prospective | Cohort randomly selected express bus drivers | Identifying factors or conditions related with OSA. | NC (OR = 1.31, 95% CI 1.18–1.46) was significantly associated with OSA status. |
VI.D.3 |. Nasal exam
Nasal obstruction is associated with sleep apnea and treatment of obstruction improves sleep quality and CPAP tolerance296 (see Section V.A.1). A thorough nasal examination includes evaluation of external nasal structures and visualization of the nasal cavity using a nasal speculum and/or nasal endoscope. The examiner should identify any external nasal deformity, internal septal deviation, inferior turbinate hypertrophy, nasal valve collapse, nasal cavity inflammation, and adenoid hypertrophy. The Cottle and Modified Cottle Maneuvers may be beneficial in assessing for internal nasal valve collapse.543 The nasal exam is focused and based on patient symptoms of nasal obstruction. Nasal obstruction has implications in PAP tolerance544 and OSA-related symptoms.545
VI.D.4 |. Oral cavity and oropharynx exam
Examination of the oral cavity and OP is an important component of the OSA physical exam. Thickened and collapsible lateral pharyngeal walls and an enlarged uvula are well-recognized physical exam finding in adults with OSA.324,325 Several studies also demonstrate that a narrow palatopharyngeal arch, or fauces, is predictive of the presence and severity of OSA.235,332,334,336
Tonsil size is commonly reported using the Brodsky tonsil grading scale: 0 = surgically absent; 1+= 0%–25% OP obstruction; 2+= 25%–50% OP obstruction; 3+= 50%–75% OP obstruction; and 4+ = 75%–100% OP obstruction.546 Tonsil size may predict severity of OSA, and a study of adults with OSA demonstrated that each increase in tonsillar size correlates to an increase in AHI of approximately 14 events/h.327 Identification of enlarged tonsils in patients with OSA also has implications in treatment decisions.335
VI.D.5 |. Mallampati classification and Friedman tongue position
Common classification schema used for grading the oropharyngeal exam include the Mallampati classification (MC) and Friedman tongue position (FTP).
The MC was developed as a clinical indicator for difficult intubation and is performed by examining the seated patient with the mouth open and tongue protruded. Based on the visualized oropharyngeal structures (palate edge, uvula, and tonsils), a classification from I to IV is assigned. Class I represents complete visualization of the soft palate and uvula, class II includes partial visualization of the uvula and complete view of the soft palate, class III involves view of the base of the uvula, and class IV views of the hard palate only.
The MC has since been modified several times most notably as the FTP, in which the airway is assessed with the tongue remaining inside the oral cavity instead of protruded with the original MC.547,548 FTP was designed to grade the relative size of the tongue base and was used as a predictor for surgical success after UPPP. The modified Mallampati (MMP) also examines the oral cavity with the tongue at rest and is a variation of FTP.
Both the MC and FTP have been examined as predictors for the presence and severity of OSA, but conflicting evidence lends to uncertainty of their predictive values.547,549 MC is associated with AHI but not among women.550 Mallampati class IV is associated significantly with OSA.551 FTP and AHI are positively correlated with higher FTP grade associated with higher AHI547,552,553; however, low inter-examiner agreement in FTP scoring has been suggested.554
A recent review article concludes that MC and FTP may have limited predictive values for OSA when used independently, but they may play a role when incorporated into the overall patient assessment.548 (Table VI.D.5)
TABLE VI.D.5.
Evidence for the association between Mallampati classification, Friedman tongue position, and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Friedman et al.547 | 2013 | 3a | Systematic review | Adults with OSA | Systematic review and meta-analysis to assess the association between Mallampati and Friedman tongue position for OSA severity | Friedman tongue position and Mallampati classification were significantly associated with obstructive sleep apnea severity |
| Bins et al.549 | 2011 | 3a | Systematic review | Adults with OSA | Systematic review to evaluate the diagnostic value of the Mallampati score in patients suspected of OSAS | No evidence for diagnostic value of Mallampati score in patients suspected of having obstructive sleep apnea syndrome |
| Ruangsri et al.551 | 2016 | 3b | Case–control study | Group 1: OSA (n = 78); Group 2: no-OSA (n = 78) | Multivariate logistic regression analysis was used to identify the factors associated with OSA | Mallampati class IV was found to be significantly associated with OSA (adjusted odds ratio 5.040 (1.655, 15.358)) |
| Amra et al.550 | 2019 | 3b | Cross sectional study | Adults with OSA | Evaluated anthropometric data and the Mallampati classification for predicting the severity of OSA | Mallampati classification was found to be associated with AHI indices, but not among women patients |
| Friedman et al.547 | 2013 | 3a | Systematic review | Adults with OSA | Systematic review and meta-analysis to assess the association between Mallampati and Friedman tongue position for OSA severity | Friedman tongue position and Mallampati classification were significantly associated with obstructive sleep apnea severity |
| Banhiran et al.555 | 2014 | 3b | Cross sectional study | Group1 no OSA (n = 66); Group 2: OSA (AHI > 15/h) (n = 217) | Identify physical findings that may predict the presence of moderate to severe OSA in snoring patients | No significant difference was found in FTP between moderate to severe OSA and control patients (AHI < 5/h) |
| Lin et al.552 | 2019 | 4 | Retrospective cohort | Adult habitual snorers and adults with OSA | Identify standard clinical parameters that may predict AHI and OSA severity | FTP was found to bea reliable predictor of OSA (r = 0.504, 95% CI: 0.417–0.580) |
| Subramanian et al.553 | 2011 | 4 | Prospective cohort | Adult patients being screened for OSA | Develop a novel screening tool for the detection of OSA, combining self-reported historical factors with physical exam findings | FTP was found to bea reliable predictor of OSA (r = 0.14, p = 0.0019) |
VI.D.6 |. Laryngoscopy
Laryngoscopy may be included in the OSA physical exam if indicated based on patient history. Laryngoscopy can be used to identify lingual tonsillar hypertrophy, to diagnose masses or lesions within the UA, and to determine the shape, size and position of the epiglottis. While lingual tonsil grade as defined by Friedman et al. does not have a direct correlation with OSA, its presence may play a role in surgical planning.556,557 A recent study demonstrated that presence of a mega-epiglottis on laryngoscopy is an independent predictor of moderate and severe OSA. This study also demonstrated that a modified Cormack-Lehane score of 2 or more as measured on laryngoscopy may predict OSA.558
Physical exam summary
Specific physical exam findings may correlate to OSA risk and severity of disease however physical exam findings alone are insufficient for OSA diagnosis and severity estimations. Sleep study testing is required to achieve a diagnosis of OSA. The physical exam has a role in the evaluation for OSA specifically to: (1) incorporate exam findings into OSA screening tools for risk stratification; (2) identify potential anatomic concerns that may limit PAP therapy, and (3) aid in determining potential therapeutic targets for sleep surgery.
VI.E |. Imaging Findings Associated with OSA
VI.E.1 |. Lateral cephalometry
X-ray cephalometry is a widely available and inexpensive imaging modality that assesses the craniofacial skeleton and its association with soft tissue of the UA. It was introduced in the 1980s as part of routine sleep surgical evaluation where cephalometric measurement of the length of the soft palate and the position of the hyoid bone were associated with severe OSA.559 In an SR and MA, the following parameters significantly correlated with OSA: (1) a mandibular body length as measured from gonion to gnathion of less than 80 mm, (2) a sella, nasion, A point angle (SNA) of less than 75°, (3) an increased anterior lower facial height measured from the anterior nasal spine to the gnathion greater than 85 mm, and (4) hyoid bone more than 18 mm below the mandibular plane correlate strongly with OSA.229 While this study provides the highest LOE, caution must be exercised in generalizing its conclusions. There are methodological differences in standardization of image acquisition, magnification of cephalograms, matching of controls, article selection bias, and quality of the constituent studies. Studies generally did not account for ethnic differences in craniofacial structures. Additional limitations to cephalograms include lack of 3D assessment and low soft tissue detail. And despite the anatomic correlates, cephalograms can neither confirm nor exclude OSA. An MA linking adult OSA with cephalometric parameters failed to show a statistically significant difference when subjects with the disorder are compared to healthy controls.560 Moreover, because they are done awake and in the upright position, they do not account for changes in the airway during sleep.
Correlation of cephalometric parameters with the AHI on overnight PSG has been inconsistent in the literature. Analysis of preoperative clinical screening tests for OSA revealed that cephalometry alone may miss a significant proportion of patients with OSA in low-risk populations.561 Therefore, it cannot be relied upon for OSA screening and should be considered as only an investigative modality for OSA evaluation. The main benefits of cephalogram are its low cost and low-risk nature in providing anatomic assessment for OSA. It is most useful in treatment planning for orthognathic surgery.
VI.E.2 |. Cone-beam CT
Cone beam CT (CBCT) has the benefits of 3D volumetric assessment, which is more expensive than cephalogram, but increasingly available. High quality evidence supporting its use for OSA airway assessment is lacking. An SR of observational studies by Guijarro-Martínez and Swennen noted inconsistencies and discrepancies in the technique of imaging acquisition.562 Most studies did not control respiratory phase, mandibular position, and/or tongue position, which influence airway dimensions. Also, the most widely available CBCT scanners acquire images in upright position, which impacts airway analysis for OSA patients. Conclusions from upright CBCT airway dimensions may not apply to supine cross-sectional imaging as the gravitational effect in response to the postural change leads to a smaller cross-sectional area of UA due to movement of the hyoid bone, the mandible, the tongue, and UA muscle. Hsu and Wu showed that in the upright position, there is a significantly greater distance between the mandibular plane and the hyoid bone when compared to CBCT derived cephalometric images in the supine position among normal test subjects.563 Chen et al. performed an SR of the most relevant UA anatomical parameters related to OSA by CBCT, conventional CT, and MRI.564 On sagittal cross-sections, a soft palate measuring more than 38 mm in length and 10 mm in width, an oropharyngeal length measuring more than 70 mm, and tongue length more than 65 mm are correlated with OSA. That corresponds to a retropalatal cross-sectional area of less than 100 mm2 and a retroglossal cross-sectional area of less than 150 mm2 on axial views. Similar to cephalogram, CBCT can be of value as an adjunct in the anatomic evaluation of OSA and for surgical planning in orthognathic surgery.
VI.E.3 |. Other imaging types
Cine CT (ultra-fast CT) provides dynamic airway examination, an advantage over CBCT and conventional CT. It does not reliably distinguish between patients with OSA and primary snorers. Somnofluroscopy can distinguish snoring from apneas, but high radiation exposure and poor anatomical detail limit its utility. Sleep MRI provides superior soft tissue anatomical detail. Using sleep MRI, Liu et al. showed in a multivariate analysis for subjects matched for age and BMI that severity of OSA can be predicted by the presence of lateral pharyngeal wall collapse and low hyoid bone position.565 Limitations for the use of sleep MRI include lack of widespread availability, expense, and challenges involved in promoting sleep in an MRI scanner discomfort. Awake ultrasonography is a promising imaging modality in evaluating OSA. A recent MA by Singh et al. showed a number of parameters with moderate to good correlation with OSA.566 However, these parameters remain to be validated.
In the literature, there are only a few publications dealing with the evaluation of soft tissue and skeletal anatomy using MRI and lateral cephalometry with both control and OSA subjects. These studies utilized dynamic or ultrafast MRI sequences and cephalometric measures, but did not discriminate OSA from control subjects by sleep study or validated questionnaires.
The soft tissue landmarks in cephalometry are influenced by the superposition of all the structures present in the same plane, which makes some of the landmarks difficult to accurately and reliably identify. MRI provides unparalleled definition of UA soft tissue structures, without exposing patients to the ionizing radiation. However, MRI offers a less precise bony contour definition. Because the enhanced soft tissue resolution of MRI affords greater measurement accuracy and allows for the determination of additional airway measurements, which cannot be achieved through cephalometry (e.g., tongue volume, PAS area), MRI may be superior to radiocephalometry for the assessment of anatomic measures in OSA patients.567 (Table VI.E)
TABLE VI.E.
Evidence on imaging and OSA diagnosis
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Riley et al.559 | 1983 | 2b | Retrospective study | 15 adult OSA patients, 10 controls | Cephalogram | Length of the soft palate and the position of the hyoid bone serve as important cephalometric landmarks in OSA patients. |
| Neelapu et al.229 | 2017 | 2a | Systematic review, meta- analysis | 25 Studies | Cephalogram | Significant variables in OSA patients include increase in total anterior facial height, inferior hyoid bone position, and reduced pharyngeal airway space. |
| Armalaite et al.560 | 2015 | 2a | Systematic review, meta- analysis | 11 Studies | Cephalogram | The most reliable parameters in OSA subjects are MP-H and upper posterior pharyngeal space. However, the diagnosis of OSA cannot be made using cephalograms as the sole investigative modality. |
| Ramachandran et al.561 | 2009 | 2a | Systematic review, meta- analysis | 26 Studies | Multiple screening questionnaires, multiple history and physical examination elements, oximetry, cephalometry, and morphometry | No ideal preoperative screening tool exists for OSA in the surgical population. Preoperative screening tools including cephalometry may not identify a significant proportion of patients with OSA. |
| Guijarro- Martinez and Swennen562 | 2011 | 2a | Systematic review | 5 studies (relationship between upper airway and OSA) | CBCT | Statistically significant difference between OSA and non-OSA patients in the ratio of the airway cross-section area/square area. |
| Hsu and Wu563 | 2019 | 2b | Retrospective study | 21 normal subjects (no OSA) | Cephalogram, CBCT | Cephalograms in the upright position had a significantly larger MP-H when compared to CBCT derived cephalometric images in the supine position. |
| Chen et al.564 | 2016 | 2a | Systematic review | 8 studies | CBCT | The most significant anatomical characteristic related to the pathogenesis of OSA is the small cross-sectional area of the airway (CSAmin). It is unclear how soft tissue structures interact to control upper airway dimensions. |
| Liu et al.565 | 2015 | 2b | Case–control study | 15 mild adult OSA patients, 15 adult severe OSA patients | Sleep MRI | Based on sleep MRI, lateral pharyngeal wall collapse and low hyoid position are significantly associated with severe OSA. |
| Singh et al.566 | 2019 | 2a | Systematic review, meta-analysis | 21 Studies (3339 patients) | Ultrasound | Airway parameters correlated with OSA: neck circumference and retropalatal diameter shortening during MM, tongue base thickness during MM, resting tongue base thickness, tongue base width, and LPW thickening. Non-airway: carotid plaque presence and carotid intimal thickening. |
VI.F |. Diagnostic Testing for OSA
VI.F.1 |. Types of sleep studies
Traditionally, the standard way to diagnose OSA has been with PSG which is an overnight test conducted when one is asleep in a sleep lab with a sleep technologist in attendance. PSG uses ≥7 channels to monitor sleep, respiration, and muscle activity. Monitoring of brain activity started in 1875 and evolved to the Sleep Research Society developing a standardization for the scoring of sleep in 1968 using the Rechtschaffen and Kales (R and K) manual.568 R and K was the standard to score PSG until 2007 when the AASM published a scoring manual569 for scoring sleep studies that is followed by all accredited sleep centers and updated on an approximately annual basis. It describes types of sensors to be used, their placement, and how to score sleep, respiratory events, cardiac events, and limb movements. A standard PSG includes electroencephalography (EEG) with frontal, central, and occipital leads placed using the 10–20 international system, electro-oculography (EOG), chin electromyogram (EMG), lead II electrocardiogram (ECG), airflow monitoring via oral thermistor and a nasal pressure transducer, respiratory effort with inductance plethysmography, SaO2 via a pulse oximetry, and limb EMG.568 Other monitoring that is typically done is snoring via a snore microphone, body position sensor, and video monitoring. A PSG report will contain information about the patient’s sleep, limb movements, and the presence of SDB based on the AHI calculated during the PSG.
In general there are four types of sleep monitoring devices (I–IV) (Table VI.F.1). Type I is a PSG described above and types II–IV are portable sleep monitoring (PM) or HSAT.568 A type II study is portable monitoring done using the same channels as a type I device only it is unattended – meaning there is not a sleep technologist or sleep trained personnel with the patient while they are having the study done. Type III HSAT uses four to seven channels and type IV uses one to two channels with at least one being a pulse oximeter.568,570 Most HSATs do not directly record sleep. Therefore, as opposed to an AHI, HSATs report an REI which is the number of apneas and hypopneas per hours of recording time.571 The AASM recommends that HSATs record airflow, respiratory effort, and blood oxygenation.570,572 Most LRs and recommendations focus on type III devices. Type II devices are not typically conducted in the clinical setting and not much data exists on their efficacy. Of note, the naming of sleep monitoring tests (type I–IV) is non-descriptive and reflects a descending order of complexity/required channels. A separate mode of classifying sleep tests, the SCOPER criteria, have been proposed, but are not yet formally adopted. The Scoper criteria classify sleep tests by the parameters being assessed within a given individual test and is a reasonable alternative to the type I–IV classification.573
TABLE VI.F.1.
Overview of the types of sleep testing, strengths, and associated limitations
| Type I | Type II | Type III | Type IV | |
|---|---|---|---|---|
| Specifications | Full-attended polysomnography (≥7 channels) EEG, EOG, EMG, ECG, airflow, effort, oximetry |
Full-unattended polysomnography (≥7 channels) EEG, EOG, EMG, ECG, airflow, effort, oximetry |
Limited channel devices (four to seven channels) ECG, airflow, effort, oximetry |
One or two channels with one typically being oximetry Oximetry and other |
| Common examples | Routine polysomnography | NA | Alice PDX Apnealink PAT | Pulse oximetry |
| Strengths | Gold-standard sleep staging and event scoring | Done at home Diagnostic accuracy to rule in/out OSA | Done at home Sufficient to rule in OSA |
Done at home Simple set up |
| Limitations | Requires subject to stay overnight in the sleep lab and has greater associated costs | Requires technical expertise to set up and administer appropriately Not appropriate for complex patients |
High level of pre-test probability required in order to accurately diagnose OSA | Very limited data collected Additional testing often warranted |
A type III device that is FDA approved uses peripheral arterial tone (PAT) in combination with actigraphy, and pulse oximetry to diagnose OSA as opposed to the traditional combination of airflow, respiratory effort, and pulse oximetry used in other type III devices.574 The algorithm correlates low SaO2 with sympathetic tone (determined by the PAT) to determine if low oxygen is due to an obstructive event (event with decreased SaO2 associated with high sympathetic tone) or central event (events with decreased SaO2 associated with low sympathetic tone).574–576 It also uses an actigraph to identify which potential events occurred in wakefulness or sleep. The device is worn around the wrist and has two finger probes. The actigraph is in the body of the device and detects movement of the arm to approximate sleep and wake. Obstructive events cause increased sympathetic activity which will lead to vasoconstriction of digital blood vessels resulting in attenuation of the PAT signal.574,577 The device will report estimates of the AHI, sleep time, lowest SaO2, REM percentage, and oxygen percentage.574 Studies comparing PAT technology to PSG found a strong correlation of AHI and lowest SaO2 between the two study devices as well as reliable reproducibility with the PAT device results.574–576
VI.F.2 |. Home sleep studies
An HSAT is recommended for the evaluation of patients with high clinical probability of OSA along with a comprehensive sleep evaluation.578 SRs and meta-analyses of well-designed RCTs demonstrate that treatment outcomes and adherence are not statistically different between subjects diagnosed with HSAT or PSG.573,579 Current guidelines recommend using PSG or home sleep apnea testing with a technically adequate device for OSA diagnosis.578 These devices include type I–IV monitors (see section Types of Sleep Studies). There is increasing evidence supporting the use of some home-based type III and IV type sleep studies to “rule-in” but not “rule-out”580 moderate to severe OSA. Such devices may therefore prove useful in populations where there is high prevalence of OSA or when combined with validated sleep questionnaire(s) that enhance the pre-test probability of moderate to severe OSA.581 An 18% false negative rate for HSAT in high-risk patients has been observed572; therefore, if a single HSAT is negative, inconclusive, or technically inadequate, an in-laboratory attended 573,579 PSG should be performed. In addition, an MA of laboratory versus HSAT concluded that they both provide similar diagnostic information, but HSAT may underestimate severity of AHI by around 10%.582
Meta-analyses have been conducted comparing type III portable studies and type I (PSG) studies in uncomplicated patients at risk for OSA.583 The type III portable studies measured airflow, thoracoabdominal effort, SaO2, and body position. The results showed that type III devices are both sensitive and specific for the diagnosis of OSA. In addition, as disease severity increases, there is increased specificity and decreased sensitivity. The technical failure rate is also higher for portable monitoring done at home as opposed to in the lab.583 Therefore, evidence supports that type III studies are useful to diagnose OSA in those with a high pre-test probability of having moderate to severe OSA.138,573,579,582 MA comparing one channel, two channel, and four channel HSATs showed that the sensitivity is increased for devices using four channels compared to one or two channels.584,585 Also sensitivity decreases and specificity increases when the AHI cut off was moved from 5 to 15 events/h. It has been concluded that using type IV (one to two channels) devices may lead to a higher number of false positives and negatives compared to type III devices but may have a role in screening for OSA in areas where access to type I and III studies are limited.
Subjects with significant cardiorespiratory disease, potential respiratory muscle weakness due to neuromuscular conditions, awake hypoventilation or suspicion of sleep-related hypoventilation, chronic opioid medication use, history of stroke, or severe insomnia were excluded from most studies, and therefore a PSG is recommended over HSAT in these cases.579 However, there is emerging data from small RCTs that HSAT can be feasibly implemented after stroke or transient ischemic attack (TIA) or even patients in a stroke rehabilitation unit.586,587 Therefore, this population could be considered for testing with HSAT.
On a practical level, the patient’s insurance coverage may be the major determinant for which test is utilized. We recommend that if a single HSAT is negative, inconclusive, or technically inadequate, in-lab PSG be performed for the diagnosis of OSA.138 More robust evidence-based economic evaluation is needed to guide decision-makers about the cost and effectiveness of home-based testing compared to PSG. The gold standard diagnostic tool is PSG, yet the test is expensive, labor intensive, and timeconsuming. Home-based testing can broaden access to diagnostic services, in hopes to reduce the substantial economic burden related to OSA and provide consistent results signifying similar effectiveness.138
We recommend against home sleep testing for the routine assessment of isolated insomnia, restless legs syndrome (RLSs), or uncomplicated parasomnias if one of these conditions is considered the likely primary abnormality. HSAT may be considered if there is a high suspicion of overlapping OSA, as insomnia and OSA may co-exist in up to 30% of sleep clinic populations.588 We recommend that if an HSAT is used to rule in OSA, there are clearly defined pathways for assessing the pre-test probability and co-morbidities. We recommend that if HSAT confirms diagnosis of OSA, there is no further need to confirm with in-lab PSG.458,579 (Table VI.F.2) the recent AASM CPG. Namely, it addresses new literature assessing the utility of PSG in patients with significant cardiorespiratory disease, potential respiratory muscle weakness due to neuromuscular condition, awake hypoventilation, suspicion of sleep related hypoventilation, chronic opioid medication use, history of stroke, or severe insomnia.572
TABLE VI.F. 2.
Home sleep study testing compared to PSG
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Kushida et al.578 | 2005 | 1a | Guideline | 3464 studies | Standards of practice: AASM indications for PSG for sleep disorders. | For patients with high-pretest-probability, type III sleep study may be an acceptable alternative to full-night PSG. In the unattended setting, or in patients without high pretest probability stratification, the data does not support the use of these devices. |
| Collop et al.579 | 2007 | 1a | Systematic review | 70 studies | Clinical Guidelines for the use of unattended portable monitors in diagnosing OSA. | Portable monitoring for the diagnosis of OSA should be performed only in conjunction with a comprehensive sleep evaluation. In the absence of a comprehensive sleep evaluation, there is no indication for portable monitoring. |
| Collop et al.573 | 2011 | 1a | Systematic review | 27 RCTs | OOC testing with devices that measure Sleep, Cardiovascular, Oximetry, Position, Effort, Respiratory (SCOPER) parameters. | The literature is currently inadequate to state that a thermistor alone without any effort sensor is adequate to diagnose OSA. |
| Masa et al.580 | 2011 | 1b | Multicenter, randomized, blinded crossover study | 366 patients suspected of OSA | Diagnostic and cost effectiveness of home testing compared with in-hospital PSG. | Home respiratory polygraphy is a cost-effective alternative to polysomnography for sleep apnea/hypopnea diagnosis. Telematic procedures may help patients with limited mobility and those who live a long way from the sleep center. |
| Masa et al.589 | 2013 | 1b | Randomized control blinded trial | 348 patients with suspected OSA | Diagnostic cost-effectiveness of a sequential HRP scoring protocol compared with manual HRP scoring, and with in-hospital PSG. | Manual HRP scoring had better agreement than automatic HRP scoring; The sequential HRP protocol is a cost-effective alternative to PSG; and the cost savings of the sequential HRP protocol is low in comparison to the manual HRP protocol. |
| Kim et al.138 | 2015 | 1a | Economic analysis of RCT-home PAP | 373 at risk for moderate to severe OSA | Cost-minimization analysis of home vs. lab sleep study. | Per subject costs for the in-lab testing were $1840 compared to $1575 for home testing for the payer. Costs for the laboratory arm were $1697 compared to $1736 in the home arm for the provider. |
| Jonas et al.458 | 2017 | 1a | Randomeffects meta-analyses | 110 studies | Review primary care–relevant evidence on screening adults for OSA, test accuracy, and treatment of OSA. | There is uncertainty about the clinical utility of all potential screening tools. Although screening with multivariable apnea prediction, followed by home PM testing may have promise for distinguishing persons in the general population who are more or less likely to have OSA, current evidence is limited. |
| Abrahamyan et al.585 | 2018 | 1a | Systematic review and meta-analysis | 24 full-text articles for final review | Systematically review the evidence on diagnostic ability of type IV PMs compared to PSG for OSA diagnosis. | Use of Level 4 PMs in clinical practice can potentially widen access to diagnosis and treatment of OSA, but evidence is not strong. |
| Corral et al.590 | 2017 | 1b | Multicentric noninferiority RCT with two open parallel arms and a cost-effectiveness analysis | 430 screened patients with sleep apnea suspicion | Long-term effectiveness of home versus lab PSG in patients with intermediate-to-high sleep apnea suspicion. | The home testing protocol was noninferior to the PSG protocol based on the Epworth scale. Home testing was the Most cost-effective protocol, with a lower per-patient cost of 416.7€. |
| Douglas et al.591 | 2017 | 1a | Guideline/position paper utilizing well designed RCTs |
Consensus statement on the indications and performance of sleep studies in adults. | There is increasing evidence supporting the use of some home-based type III and IV type sleep studies to “rule-in” moderate to severe obstructive sleep apnea in high prevalence obstructive sleep apnea, and should be used under the supervision of an accredited sleep physician. | |
| Kapur et al.572 | 2017 | 1a | Systematic review and meta-analysis | 98 studies included in evidence-based recommendations and 86 included in meta-analysis Clinical practice guideline (AASM). |
Guidelines on appropriate and effective diagnosis of OSA. | Polysomnography, or home sleep apnea testing with a technically adequate device, is used for the diagnosis of OSA in uncomplicated adult patients presenting with signs and symptoms that indicate an increased risk of moderate to severe OSA. If a single home sleep apnea test is negative, PSG be performed for the diagnosis of OSA. |
| El Shayeb et al.583 | 2014 | 1a | Systematic review and meta-analysis | From 59 studies, 19 studies were included in the meta-analysis | Assess the diagnostic accuracy of Level 3 testing compared with Level 1 testing and to identify the appropriate patient population for each test. | Level 3 sleep studies are safe and convenient for diagnosing OSA in patients with a high pretest probability of moderate to severe forms of the condition without substantial comorbidities. |
| Rosen et al.592 | 2012 | 1b | Randomized, open-label, parallel group, unblinded, multicenter clinical trial | 7 AASM accredited centers recruited adults with high probability of OSA and ESS > 12 Home PAP study | To test the utility of an integrated clinical pathway for OSA diagnosis and CPAP treatment using portable monitoring devices. | A home-based strategy for diagnosis and treatment compared with in-laboratory PSG was not inferior in terms of acceptance, adherence, time to treatment, and functional improvements. |
| Gabriela et al.593. | 2019 | 2b | Randomized, prospective, cross-over and single blind clinical trial | 251 patients | Automatic validation of a new HRP system. | The automatic analysis of the HRP BTI-APNiA software presents a high validity in comparison to the AHI results measured by PSG. HRP BTI-APNiA is a valid alternative to PSG. |
| Flemons et al.594 | 2003 | 1a | Systematic review | 51 studies | To assess the utility of portable monitors in diagnosing sleep apnea in adults. | High-quality studies of type III monitors in the sleep laboratory attended setting had low false-positive rates; most studies found a threshold that distinguished patients with sleep apnea from those without. |
| Chesson et al.595 | 1997 | 2a | Systematic review | MEDLINE search; January 1966–April 1996 | Indications for PSG. | 22% failure rate of home-based study to diagnose OSA. |
| Garcia-Diaz et al.596 | 2007 | 1b | Prospective randomized study with blinded analysis | 62 patients with suspected OSA included | Utility and reliability of a respiratory polygraphy (RP) device with actigraphy in the diagnosis of sleep apnea-hypopnea syndrome. | HPR is an effective and reliable technique for the diagnosis of SAHS, although it is less sensitive than LRP. Wrist actigraphy improves the results of HRP only slightly. |
| Ayappa et al.597 | 2008 | 2b | Prospective study with blinded analysis | 102 subjects recruited. 96 returned to lab | Validity of the Apnea Risk Evaluation System (ARES) Unicorder, for the evaluation of sleep disordered breathing. | ARES Unicorder provides acceptably accurate estimates of SDB indices compared to conventional laboratory NPSG for both the simultaneous and in-home ARES data. |
| Garg et al.581 | 2014 | 1b | RCT crossover | 75 urban African Americans with high pre-test probability of OSA, identified with the Berlin questionnaire | Feasibility of home PM in an urban population at risk for OSA compared to in-laboratory polysomnography (PSG) and patient preference with respect to home PM versus PSG. | Home PM for diagnosis of OSA in a high-risk urban population is feasible, accurate, and preferred by patients. As home PM may improve access to care, the cost-effectiveness of this diagnostic strategy for OSA should be examined in underserved urban and rural populations. |
| Skomro et al.598 | 2010 | 1b | Randomized trial |
102 subjects | Compared subjective sleepiness, sleep quality, quality of life, BP, and CPAP adherence after 4 weeks of CPAP therapy in subjects diagnosed via lab versus home testing. | Compared with the home-based protocol, diagnosis and treatment of OSA in the sleep laboratory does not lead to superior 4-week outcomes in sleepiness scores, sleep quality, quality of life, BP, and CPAP adherence. |
| Abraham et al.584 | 2006 | 2b | Prospective multi-center study | 50 patients with NYHAIII CHF (SEARCH study) | Validity and clinical utility of home testing for SDB and arrhythmias in HF. | With CPS compared in in-lab PSG, the diagnostic accuracy was between 83% and 87% for OSA based on AHI of 5, 10, and 15. |
| Bravata et al.599 | 2017 | 1a | Randomized controlled intervention trial | 225 randomized patients | Evaluate whether the intervention strategy improved sleep apnea detection and treatment, and hypertension control among patients with chronic cerebrovascular disease and hypertension. | The use of portable polysomnography, and auto-titrating CPAP in the patients’ homes, improved both the diagnosis and the treatment for sleep apnea compared with usual care but did not lower blood pressure. |
| Kotzian et al.586 | 2018 | 1b | Single-blind, single center, randomized controlled trial | 55 patients (HOPES study) | Determine whether PAP adherence in patients who had a stroke with OSA can be improved by a PAP training strategy during in hospital rehabilitation combined with a telemedicine monitoring system after discharge. | Pre-results to our clinical experience, a severe SA in the screening PG remains a severe SA also in PSG. The diagnosis will not Change. We think that patients who had severely affected stroke need a quick access to therapy. |
| Fitzpatrick et al.600 | 2020 | 1b | Randomized, parallel, multicenter, single-blind, pragmatic controlled trial | 233 patients (SIESTA Trial) | To evaluate the accuracy of the clinical diagnosis of OSA informed by the home sleep study with a type IV portable monitor versus type I polysomnography | Home testing with portable devices plays a valuable role for diagnosing of OSA in a variety of settings. |
| Hui et al.601 | 2017 | 1b | Prospective, randomized controlled CPAP parallel study | 316 patients | Comparisons of home-based versus hospital-based approach in managing patients with suspected obstructive sleep apnea. | Home-based approach is non-inferior to hospital-based approach in managing patients with suspected OSAS, with shorter waiting time, and substantial cost savings. |
| Mulgrew et al.602 | 2007 | 1b | Randomized, controlled, open-label trial | 68 patients | To test the utility of a diagnostic algorithm in conjunction with ambulatory CPAP titration in initial management of obstructive sleep apnea. | In patients with a high probability of obstructive sleep apnea, PSG confers no advantage over the ambulatory approach in terms of diagnosis and CPAP titration. The ambulatory approach may improve adherence to treatment. When access to PSG is inadequate, the ambulatory approach can expedite management of patients in need of treatment. |
| Guerrero et al.603 | 2014 | 1b | Randomized, blinded, crossover study | 56 patients | Evaluate three night portable monitoring for OSA diagnosis. | Three consecutive nights of portable monitoring at home evaluated by a qualified sleep specialist is useful for the management of patients without high pretest probability of obstructive sleep apnea or with comorbidities. |
| Ferber et al.604 | 1994 | 2a | Systematic review | Literature review; MEDLINE (1966–1994) | Usefulness of portable Recording in the assessment of OSA. | No clear guidance for who is appropriate for home testing. |
| Morales et al.605 | 2012 | 2a | Prospective cohort study | 452 participants | Assess utility of home testing for the elderly with the complaint of daytime sleepiness. | Unattended, self-assembled, in-home sleep studies recording airflow and respiratory effort are most useful along with a comprehensive sleep history, is accurate in identifying severe OSAS in older adults. |
| Pietzsch et al.606 | 2011 | 1b | Decision- analytic Markov model using Tree-Age Pro 2009 Suite | Comparison of clinical health guidelines and health- economic studies | Benefits and cost-effectiveness of diagnostic tests. | For payers, a home-based diagnostic pathway for obstructive sleep apnea with robust patient support incurs fewer costs than a laboratory-based pathway. For providers, costs are comparable if not higher, resulting in a negative operating margin. |
VI.F.3 |. In-lab studies
Traditionally, in-lab PSG has been the testing modality of choice to diagnose and assess the severity of OSA.578 The 2017 AASM CPG recommended that PSG remains the diagnostic testing modality for patients suspected of having OSA who had significant comorbidities or who failed an initial HSAT, but that HSAT or PSG could be used to diagnose OSA for uncomplicated patients considered to be at risk of moderate to severe OSA.572 This diagnostic testing section reviewed the literature and updated the indications for PSG in the context of the recent AASM CPG. Namely, it addresses new literature assessing the utility of PSG in patients with significant cardiorespiratory disease, potential respiratory muscle weakness due to neuromuscular condition, awake hypoventilation, suspicion of sleep related hypoventilation, chronic opioid medication use, history of stroke, or severe insomnia.572
Heart failure with reduced ejection fraction (hFrEF or congestive heart failure [CHF]):
hFrEF patients are at high risk for SDB, including both OSA and CSA. HSATs have been evaluated for the diagnosis of SDB in multiple studies of patients with hFrEF, including three Level 1b studies, one Level 2b study, and one Level 3b study.584,607–610 In a study of 100 hFrEF patients undergoing simultaneous home PSG and a two channel version of the ApneaLink, accuracy for an AHI > 5 and >15 events/hour was reported at 94%, though the PSG required a 4% desaturation to qualify as a respiratory event and the HSAT could not distinguish between obstructive and central events.609 Aurora et al. reported that adding an effort belt to the IT (ApneaLink Plus) yielded similar accuracy and could distinguish obstructive versus central events (obstructive AHI r = 0.91, central AHI r = 0.998), though the study included only 57 inpatients with decompensated hFrEF.608 In a prospective multicenter cross-sectional study, stable hFrEF patients (N = 47 in the validation group) underwent simultaneous SleepMinder (a novel two channel touchless HSAT) and PSG.610 The SleepMinder required a 50% reduction in airflow only for a respiratory event, while the PSG required 30% drop in flow and 4% desaturations. The SleepMinder had an area under the curve (AUC) of 0.85 but misclassified 19% of patients using an AHI cutoff of 15 to define SDB and was unable to distinguish obstructive versus central events. The non-Level 1 studies in this group were downgraded due to small numbers and/or antiquated criteria used to define respiratory events.584,607
Chronic obstructive pulmonary disease (COPD):
Patients with COPD are at risk for OSA, but also hypoventilation and nocturnal hypoxemia. Limited data is available to assess the utility of HSATs in the diagnosis of SDB in patients with COPD (one Level 1b study and one Level 3b study).611,612 In prospective single center cross-sectional study of 90 outpatients with stable COPD and symptoms of OSA, Chang et al. evaluated one night with an HSAT device (Nox-T3) and then an in lab PSG with simultaneous Nox-T3 within 1 week.611 The best agreement was found when the studies were done on the same night, used a 4% desaturation criteria for respiratory events, and used an AHI cutoff of 15 to define SDB. Kappa values ranged from as low as 0.48 (different night, 3% desaturation criteria, AHI cutoff of 5) to 0.93 (same night, 4% desaturation criteria, AHI cutoff of 15). A small (N = 33) prospective single center cross-sectional study of patients with stable Gold II and III COPD and symptoms of OSA compared an HSAT (WatchPAT) to in lab PSG and found good sensitivity (92%–96%) with moderate specificities (55%–65%), and a concordance of 79% between the tests for an AHI > 15.612 However, of note, this later study required only a 50% drop in airflow (no associated desaturation) to define a respiratory event on the PSG.
Post-stroke:
Patients who have had a stroke have a roughly 60%–70% risk of having OSA.613 A single Level 2b study compared an HSAT device (Embletta) to simultaneous PSG within 3 days of an ischemic stroke.614 In a prospective single center cross-sectional study, Chernyshev et al. found HSAT was accurate for diagnosing OSA (accuracy 1.0 and 0.95 for AHI cutoff’s of 5 and 15). Both HSAT and PSG events were scored according to the 4% desaturation criteria. The main limitation to the study was that it included only 21 participants.
Technically inadequate or normal initial HSAT:
A Level 2b study performed as a retrospective single center review of patients with a high pretest probability of OSA who had a technically inadequate (N = 111) or normal HSAT (N = 127) but then a subsequent PSG, found OSA in 71% of the patients with technically inadequate studies and 24% of the patients with normal HSATs.615 Older age and lower ESS were associated with OSA on the subsequent PSG. (Table VI.F.3)
TABLE VI.F. 3.
Diagnostic testing: indications for in-lab polysomnography
| Study | Year | LOE | Study design | Study groups | Clinical end point | Conclusion |
|---|---|---|---|---|---|---|
| CHF | ||||||
| Abraham et al.584 | 2006 | 2b | Prospective multicenter study comparing diagnostic accuracy of ClearPath System (CPS) vs. PSG. Stable CHF with EF ≤35% |
Night 1 did both in the lab and Night 2 (<3 nights apart) was home CPS. N = 50 |
RDI as determined by 3% desaturations for respiratory events and “flow change.” | 1) Same night: RDI>5 sens 92%, spec 52%, acc 73%, AUC NR RDI>15 sens 67%, spec 78%, acc 75%, AUC NR 2) Different night: RDI>5 acc 73% RDI>15 acc 77% |
| de Vries et al.609 | 2015 | 1b | Prospective single center cross-sectional study in patients with chronic heart failure (stable CHF by clinical judgment). | Subjects underwent the ApneaLink (2 channel – airflow + oximetry) and simultaneous PSG at home. N = 100 |
AHI as determined by respiratory events required 30% drop in airflow and 4% desaturations. | AHI > 5 sens 98%, spec 60%, acc NR, AUC 0.94 AHI>15 sens 92.9%, spec 91.9%, acc NR, AUC 0.94 ICC 0.85 for categories 3) 29% CSA, 19% OSA, 13% mixed 4) Best accuracy at AHI 15. 5) Cannot differentiate CSA vs. OSA |
| Araújo et al.607 | 2018 | 3b | Prospective single center cross-sectional study in patients with chronic heart failure (stable CHF by clinical judgment). | Subjects were studied by the ApneaLink (2 channel – airflow + oximetry) and PSG simultaneously during in the sleep laboratory. N = 35 |
AHI as determined by respiratory events required 50% drop in airflow and 3% desaturations. | AHI > 5 sens 81.8%, spec 61.5%, acc 74.2, AUC 0.85 AHI>15 sens 83.3%, spec 91.3%, acc 88.6, AUC 0.93 AHI correlation r = 0.79 Highest accuracy at AHI 15. Cannot differentiate CSA vs. OSA Downgraded due to small number and EF50%. |
| Aurora et al.608 | 2018 | 1b | Prospective single center cross sectional study in hospitalized patients with decompensated heart failure (stabilized at the time of testing). | Subjects underwent concurrent ApneaLink Plus (3 channel – airflow, oximetry and effort belt) and PSG. Recordings blindly scored for OSA and CSA. N = 57 |
AHI as determined by respiratory events that required a 50% drop in airflow and 3% desaturations (PSG allowed arousals). | AHI > 5 sens 95.8%, spec 80.0%, acc NR, AUC NR Central AHI>5 sens 90.9%, spec 100%, acc NR, AUC NR 3) ICC 0.89 for categories, Obs AHI r = 0.91, Central AHI r = 0.99 58.5% central AHI > 5 4) Higher accuracy for central vs. obstructive. 5) Cannot differentiate CSA vs. OSA. |
| Savage et al.610 | 2016 | 1b | Prospective multicenter cross-sectional study. Stable CHF patients with an EF<45%. | Subjects underwent simultaneous Sleep Minder (airflow + movement via electromagnetic signals) and PSG. Development (D) (n = 28) and validation (V) (n = 47) groups were studied. N = 75 |
AHI as determined by Sleep Minder (SM) required 50% reduction in airflow only. PSG required 30% drop in flow and 4% desaturations. | AHI > 5 sens NR, spec NR, acc NR, AUC NR AHI>15 sens 70%, spec 89%, acc NR, AUC 0.85. AUC 0.85 for all, 19% misclassified (> <AHI 15) by SM. 25%/34% (D/V) with OSA, 11%/9% with CSA. Best accuracy at AHI >30. Cannot differentiate CSA vs. OSA. |
| COPD | ||||||
| Chang et al.611 | 2019 | 1b | Prospective single center cross-sectional study in outpatients with stable COPD and symptoms of OSA. | Subjects did 1 night with Nox-T3 and then an in-lab PSG with simultaneous Nox-T3 within 1 week. N = 90 |
AHI as determined by 2 different definitions for respiratory events: 1) Nox-T3 and PSG respiratory events required 30% drop in airflow and 4% desaturation, and 2) Nox-T3 and PSG respiratory events required 30% drop in airflow and 3% desaturation or, for PSG, an arousal. | Same night 4%: AHI>5 sens 96%, spec 84%, kappa 0.82 AHI>15 sens 95%, spec 98%, kappa 0.93 Different night 4%: AHI>5 sens 95%, spec 78%, kappa 0.75 AHI>15 sens 74%, spec 98%, kappa 0.74 Same night 3% kappa AHI>5 0.58, kappa AHI>15 0.88 Different night 3% kappa AHI>5 0.48, kappa AHI>15 0.70 Nox-T3 more often found hypoxemia (15 vs. 5 with AHI < 5 and > 5 min with sats ≤88%.) |
| Jen et al.612 | 2020 | 3b | Prospective single center cross-sectional study in outpatients > 40 years old with stable Gold II and III COPD and OSA symptoms. | In random order, subjects underwent 1 night PSG and WatchPAT device in the lab and another night with the WatchPAT at home. N = 33 |
AHI as determined by WatchPAT and PSG. WatchPAT uses PAT with either a 3% with “arousal” or 4% desaturation. PSG required 50% drop in airflow without desaturation or arousals |
AHI>5 sens 96%, spec 55.6% AHI>15 sens 92.3%, spec 65.0% 3)Intraindividual AHI difference 78.8% concordance (AHI>15 and difference <10) |
| CVA | ||||||
| Chernyshev et al.614 | 2015 | 2b | Prospective single center cross-sectional study of inpatients admitted for acute ischemic stroke. Studied within 72 h of the CVA. | Subjects underwent a simultaneous PSG and HSAT within 3 days of their CVA. N = 21 BMI 33.1 (OSA) vs. 23.8 (no OSA), 66.6% had an AHI > 5, 48% had OSA, 19% had CSA |
AHI as determined by HSAT and PSG. Respiratory events required 30% drop and 4% desaturation. | For OSA only: AHI>5 sens 100%, spec 85.7%, acc NR, AUC 1.0 AHI>15 sens 100%, spec 83.9%, acc NR, AUC 0.95 Intraindividual AHI difference 1.5 Downgraded due to small number. |
| Failed HSAT | ||||||
| Zeidler et al.615 | 2015 | 2b | Retrospective single center review of patients referred for OSA evaluation who had a technically inadequate (N = 111) or normal HSAT (N = 127) but then had a subsequent PSG. | All had a high pretest probability of OSA. N = 238 |
Respiratory events scoring criteria not defined for HSAT or PSG. OSA defined by an AHI > 5. | 1) Technically inadequate HSAT: 71% with OSA: 38.7% mild, 32.4% moderate/severe 2) Normal HSATs: 24% OSA: 18.1% mild, 5.5% moderate/severe Older age and lower ESS were associated with OSA on PSG. |
VI.F.4 |. Oximetry
General population:
In the general population, two Level 1b studies,499,616 three Level 2b studies,617–619 and one Level 3b620 found reasonably good correlations between oximetry ODI and PSG AHIs, though primarily when the 4% desaturation criteria was used to score respiratory events. For example, in a study by Pataka et al., utilizing a 4% desaturation definition, there was good correlation between the ODI4% and the PSG ODI (r = 0.95), and for AHI > 15, ODI4% had a sensitivity, a specificity and an AUC of 82%, 94%, and 90%.616 However, a number of these studies were downgraded for using the oximetry off the PSG as the comparison to the PSG derived AHI,617,618 or for using highly selective populations.619
Two studies used acoustic devices to diagnosis OSA, one Level 2b study621 and one Level 4 study.622 In an exploratory study by Erdenebayar et al., there was moderate accuracy between in-lab PSG AHI and the piezoelectric sensor snoring index when worn at the same time as the PSG.621
Heart failure with reduced ejection fraction (hFrEF or CHF):
The accuracy of oximetry in patients with hFrEF was assessed by two Level 1b studies (Sharma et al., 2017 and Ward et al.)619,623 and one Level 3b study (Sharma et al., 2015).624 The study by Sharma et al., 2017, was a prospective single center, controlled trial of 105 inpatients with acute CHF, where simultaneous HSAT with ApneaLink and a high-resolution pulse oximetry (HRPO) was performed for a single night.619 The HSAT-derived REI was compared to the HRPO-derived ODI using 30% drop in flow with 4% oxygen desaturations. The receiver operating curve (ROC) analysis showed an area under the ROC curve (AUC) of 0.89 for REI > 5 events/h with 88% of the REI in the moderate–severe category being correctly classified. However, HRPO was unable to reliably differentiate between central and obstructive respiratory events. The second Level 1b study by Ward et al. was also a prospective, single center study of 173 CHF patients with simultaneous recording of unattended PSG, ambulatory electrocardiography and overnight pulse oximetry performed at the home or hospital per patient preference.623 The results demonstrated that at the cutoff of >7.5 desaturations/h, the ODI3% had a sensitivity 97%, a specificity 32%, a negative likelihood ratio 0.08, and a positive likelihood ratio 1.42. The diagnostic accuracy increased using a cutoff of 12.5 desaturations/h, with a sensitivity 93% and a specificity 73%. The ODI3% had an AUC of 0.92 (95% C.I. 0.88–0.96) for the detection of SDB in CHF, at the cutoff of >7.5 desaturations/h. The third study, by Sharma et al. (2015), a Level 3b study, was a prospective, single center study, in patients admitted with acute decompensated HF with a high clinical suspicion of SDB and an ODI4% ≥5 on overnight photoplethysmographic signal recording who underwent PSG.624 Among 86 patients who had oximetry, 68 underwent outpatient PSG within 4 weeks of discharge. Utilizing an AHI ≥ 5 to define OSA, the AUC was 0.82.
COPD:
One each of Level 1b, 2b, and 3b studies met criteria for inclusion.625–627 The study by Andrés-Blanco et al., a level 1b study, was a prospective single center in 193 COPD patients, where supervised portable oximetry was compared simultaneously to in-hospital PSG as well as to unsupervised portable oximetry at home.625 A control group of 100 non-COPD patients was also included. An ODI4% cutoff was used for the oximeter. There were no significant differences between COPD and non-COPD groups in both settings, particularly for severe OSAS. In a Level 2b study, Scott et al. compared overnight oximetry results to PSG in 50 COPD patients on long-term oxygen therapy.627 Pulse oximetry tracing interpretation had a modest diagnostic value in identifying OSA in patients with moderate to severe COPD; the AUC was 0.57–0.64.
Atrial fibrillation (AF):
One Level 2b study by Linz et al. met criteria for inclusion.25 It was a large prospective single center study in patients with documented AF (62% paroxysmal AF) who underwent PSG. Overnight oximetry from the PSG was used to determine the ODI and this was validated against the PSG AHI. In 439 patients with AF, the median AHI was 9.5 [3.6–21.0]/h and the prevalence of moderate (AHI 15–29/h) and severe SDB (AHI ≥ 30/h) was 17.3% and 16.6%, respectively. The ODI4% was able to detect moderate-to-severe SDB with an AUC of 0.95 and only severe SDB with AUC 0.93. An ODI4% of 7.6/h yielded a sensitivity and specificity for AHI ≥ 30/h of 89% and 83%, respectively. However, oximetry was unable to distinguish between central or obstructive respiratory events.
Post-stroke:
There were only two Level 2b oximetry studies and one Level 3b sonography study that were included in this analysis.628–630 Lin et al. performed a retrospective study in 254 acute stroke patients who underwent HSAT.629 The ODI3% from the pulse oximetry channel was compared to REI obtained from HSAT devices. Nocturnal pulse oximetry had a high diagnostic accuracy in predicting moderate to severe SDB in patients with acute stroke and the ODI3% was accurate in predicting SDB at different REI thresholds (REI ≥ 5, REI ≥ 15, and REI ≥ 30 events/h) with AUCs of 0.965, 0.974, and 0.951, respectively. Aaronson et al. also performed retrospective analysis of data obtained from 56 stroke patients who underwent nocturnal polygraphy and oximetry.628 Sensitivity, specificity, and positive and negative predictive values (NPVs) for the ODI4% ≥15 were, 77%, 100%, 100%, and 83%, respectively. Ryan et al. reported on 23 patients who were post-stroke and had SDB.630 Using a cutoff AHI of ≥15 by PSG to diagnose OSA, in-lab use of the BresoDx (single channel acoustic device) had a sensitivity of 90.0%, a specificity of 84.6%, and an overall accuracy of 87.0%. Bland–Altman plot showed close agreement, although a tendency for the BresoDx device to slightly overestimate the AHI. The AUCs for PSG diagnostic AHI thresholds of ≥5, ≥10, and ≥15 were 0.90, 0.91, and 1.00, respectively. Comparing the home use of BresoDx versus in-lab PSG, using an AHI threshold of ≥15, the BresoDx had a sensitivity of 100%, a specificity of 85.7%, and an overall accuracy was 91.3% (Table VI.F.4).
TABLE VI.F. 4.
Evidence for oximetry for diagnosis of OSA
| Study | Year | LOE | Study design | Study groups | Clinical end point | Conclusion |
|---|---|---|---|---|---|---|
| CHF | ||||||
| Sharma et al.619 | 2017 | 1b | Prospective single center, controlled trial of patients admitted with CHF. | Simultaneous measurement of apnea link and high-resolution pulse oximetry (HRPO) for a single night. N = 105 61 (58%) M |
HRPO-derived ODI (oxygen desaturation index) was compared with PM-derived respiratory event index (REI) using receiver operator characteristic (ROC) curve analysis and a Bland–Altman plot. | 1) ROC area under curve (AUC) was 0.89 for REI > 5 events/h. AUC ranged from 0.84 (REI ≤ 10 events/h) to 0.89 (REI ≤ 5 events/h and REI ≤ 20 events/h). 2) The Bland–Altman plot had good agreement. 3) 88% of the REI in moderate–severe category were correctly classified. 4) Cannot differentiate CSA vs. OSA. |
| Sharma et al.624 | 2015 | 3b | Prospective, single center cohort of consecutively admitted acute decompensated heart failure patients with high clinical suspicion of SDB. | Overnight (ON) inpatient oximetry (photoplethysmography) compared with outpatient PSG apnea hypopnea index (AHI). N = 105 subjects had ON oximetry and 68 underwent outpatient PSG within 4 weeks of discharge. |
PSG defined hypopneas as some drop in flow with 4% oxygen desaturation compared with ON oximetryODI of 4% desaturation using ROC analysis and Bland–Altman plot. | 1) ODI correlated with AHI with AUC of 0.82 on ROC for AHI ≥5. 2) The Bland–Altman plot had no major bias. |
| Ward et al.623 | 2012 | 1b | Prospective, single center cohort of CHF patients from cardiology clinics. | Simultaneous unattended PSG, ambulatory electrocardiography, and ON pulse oximetry at home or hospital N = 173 86% M. | Compared oximetry % ODI cutoff of >7.5 desaturations/h to PSG AHI >15/h. ODI used 3% desaturation. PSG defined hypopneas as 50% drop in flow with 3% desaturation or arousal. | 1) At a cutoff of >7.5 desaturations/h, the ODI3% had sensitivity 97%, specificity 32%, negative likelihood ratio (LR) 0.08, and positive LR 1.42. 2) At a cut-off of 12.5 desaturations/h, ODI3% sensitivity was 93% and specificity was 73%. 3) The 3% ODI had an AUC under ROC curve of 0.92 for detection of SDB in CHF, at the cutoff of >7.5 desaturations/h. |
| COPD | ||||||
| Andrés-Blanco et al.625 | 2017 | 1b | Prospective single center cohorts. | Simultaneous portable ON oximetry at home and in-hospital PSG; and unsupervised portable ON oximetry at home. Two independent validation datasets were analyzed: COPD versusnon-COPD. N = 110 non-COPD test set (69% M) and 68 COPD test group (88% M). |
A regression-based multilayer perceptron (MLP) artificial neural network (ANN) was trained to estimate AHI from portable oximetry recordings. Two independent validation datasets were analyzed: COPD vs. non-COPD. | 1. Portable ON oximetry-based ANN reached similar ICC values between the estimated and actual AHI for the non-COPD and the COPD groups either in the hospital (non-COPD: 0.937, COPD: 0.936) and at home (non-COPD: 0.731, COPD: 0.788) setting. 2. No significant differences in ROC between COPD and non-COPD groups in both settings. |
| Lajoie et al.626 | 2020 | 3b | Prospective cohort recruited from an ongoing multicenter trial. | Compared home ON oximetry and laboratory-based PSG in patients with moderate-to-severe COPD. N = 90 45 had OSA, 71% M 45 did not have OSA, 87% M |
ODI3% used for oximetry. AHI hypopnea definition not stated. |
1. Oxygen desaturation indices obtained with nocturnal oximetry and during PSG were not correlated (r = –0.27; p = 0.1). 2. Diagnosis of OSA in COPD should not be based solely on oximetry. OSA was confirmed in only 50% of subjects with oximetry tracings suggestive of OSA. |
| Scott et al.627 | 2014 | 2b | Consecutive chart review of the inpatient pulmonary rehabilitation service. | Subjects with moderate–severe COPD who were clinically prescribed oximetry and PSG. N = 59 46% M |
Criteria consisted of visually identified desaturation “events” (sustained desaturation ≥4%, 1 h time scale), “patterns” (≥3 similar desaturation/saturation cycles, 15 min time scale) and the automated oxygen desaturation index. Compared using AUC. | 1) Thirty-five were correctly identified as having OSA/no OSA with accuracy of 59%, a sensitivity and specificity of 59% and 60%, respectively; AUC 0.57. 2) Using software-computed desaturation events (hypoxemia ≥4% for ≥10 s) indexed at ≥15 events/h of sleep as diagnostic criteria, sensitivity was 60%, specificity was 63%, and the AUC 0.64. |
| Atrial Fibrillation | ||||||
| Linz et al.25 | 2018 | 2b | Prospectively single center cohort in patients with atrial fibrillation (AF) who underwent PSG. | Subjects with documented AF. N = 439 69% M |
ON oximetry from the PSG was used to determine the ODI. ODI was validated against PSG AHI. ODI4% used for oximetry, which came off PSG. PSG hypopnea definition was a 30% drop in flow for 10 s with either a 3% desaturation or an arousal. |
1) ODI was able to detect moderate-to-severe SDB (AHI ≥ 15/h) AUC: 0.951; severe SDB (AHI ≥ 30/h) AUC 0.932. 2) An ODI cut-off of 4.1/h had 91% sensitivity and 83% specificity in patients with and without AHI ≥ 15/h. 3) An ODI of 7.6/h yielded a sensitivity and specificity for AHI ≥ 30/h of 89% and 83%, respectively. 4) Cannot differentiate CSA vs. OSA. |
| CVA | ||||||
| Lin et al.629 | 2018 | 2b | Retrospective chart analysis. | Subjects with acute stroke or TIA underwent ON oximetry and HSAT. N = 254 50.7% M 232 (91.3%) were ischemic or TIA. |
ODI from pulse oximetry channel was compared to respiratory event index (REI) obtained from HSAT devices. ODI3% used for oximetry. REI3% used for HSAT. |
1) ODI3% had correlation (r = 0.902) and agreement with REI3%. 2) ODI3% was accurate in predicting SDB at different REI thresholds (REI ≥ 5, REI ≥ 15, and REI ≥ 30 events/h) with AUC of 0.965, 0.974, and 0.951, respectively. 3) An ODI3% ≥ 5 events/h rules in the presence of SDB (specificity 91.7%, PPV 96.3%). 4) An ODI3% ≥ 15 events/h rules in moderate to severe SDB (specificity 96.4%, PPV 95%) and an ODI3% < 5 events/h rules out moderate to severe SDB (sensitivity 100%, NPV 100%). |
| Aaronson et al.628 | 2012 | 2b | Retrospective study of stroke patients. | Compared polygraphy and oximetry from HSAT in stroke subjects. N = 56 62% male, 46% of the stroke patients had OSA. 69% with OSA were ischemic strokes. |
Compared REI to ODI. REI hypopneas defined as 50% drop in flow with a 4% desaturation. ODI used 4% desaturation. |
1) Sensitivity, specificity, and PPV and NPV for the ODI4% ≥15 were, respectively, 77%, 100%, 100%, and 83%. 2) ODI4% predicted 87% of the variance in the REI. 3) Given a 46% prevalence of OSA in stroke, the PPV of oximetry was 100% with an NPV of 83% |
| Ryan et al.630 | 2017 | 3b | Prospective cohort of patients with acute stroke in a stroke rehabilitation unit (SRU). | Compared testing with BresoDx – a portable single-channel acoustic device – both simultaneously during attended PSG in lab and unattended on the SRU. N = 23 48% M 78% had OSA (defined by AHI ≥15) on PSG. 74% of subjects were ischemic strokes. |
Compared PSG AHI to BresoDx AHI. PSG hypopneas defined by a 30% drop in flow with 3% desaturation or arousal. Determined AUC and Bland–Altman plot. |
1) Using cutoff AHI of ≥15 by PSG to diagnose OSA in-lab BresoDx had sensitivity of 90.0%, specificity of 84.6%, and accuracy of 87.0%. 2) Bland–Altman plot: good agreement, but BresoDx overestimated AHI by 4.4. 3) The AUCs for AHI in lab Breos vs. in-lab PSG at thresholds of ≥5, ≥10, and ≥15 were 0.90, 0.91, and 1.00, respectively. 4) For home BresoDx vs. in-lab PSG, at an AHI threshold of ≥15 had a sensitivity of 100%, specificity of 85.7%, and accuracy of 91.3%. |
| General | ||||||
| Pataka et al.616 | 2019 | 1b | Prospective study in a sleep clinic. | Compared sleep questionnaires STOP-BANG (SB), Berlin (BQ), Epworth Sleepiness Scale (ESS) completed by subjects with home oximetry and in laboratory PSG, to determine predictive value of test for CPAP initiation. N = 204 77.5% M |
Determine correlations and accuracy. Compared PSG and oximetry values as well. PSG hypopneas defined by a 30% drop in flow with 4% desaturation or arousal. ODI used 4% desaturations. |
1. Good correlation between oximetry ODI (ODIox) and PSG ODI (r = 0.95, p < 0.0001) and between ODIox and AHI (r = 0.811, p < 0.0001). ODIox ≥ 15 had sensitivity 89.3%, specificity 83.5%, PPV 87%, and NPV 86.4% for CPAP initiation. 2) Among questionnaires, ESS had highest specificity (68.6%) and PPV (68.6%) and SB had the highest sensitivity (98%) and NPV (80%) but the lowest specificity (11%) for CPAP initiation. Oximetry was superior to questionnaires for predicting CPAP treatment initiation. |
| Christensson et al.617 | 2018 | 2b | Prospective, observational multicenter trial of sleep clinic patients. | Subjects underwent HSAT (Nox-T3), ON oximetry, and STOP-BANG (SB) questionnaires. N = 449 subjects with suspected OSA. 61.5% M |
Compared REI to ODI. Compared REI to SB questionnaire scores. HSAT hypopneas defined by 30% drop in flow and 30% desaturation. ODI used 3% for oximetry. |
1) Strong correlation between REI and ODI3%, Spearman 0.96. 2) Positive correlation between SB score and ODI3%, Spearman ρ 0.50; An SB score of <2 almost excludes moderate to severe OSA, whereas nearly all OSA patients with an SB score ≥6 had OSA. |
| Sharma et al.619 | 2017 | 3b | Retrospective review of a large database of hospitalized inpatients. Only those high ODI on ON oximetry were offered PSG. |
Compared in-hospital ON HRPO to PSG post-discharge. N = 1410 underwent in-hospital HRPO with 1092 having and ODI4% ≥ 5. Of these, 680 underwent PSG post-discharge. 54% M (of HRPO group). |
Determined accuracy, AUC, and Bland–Altman plot of HRPO-determined ODI vs. AHI. ODI used 4% for oximetry. PSG hypopneas defined by a 30% drop in flow with 4% desaturation. |
1) ODI4% ≥5 had sensitivity 0.89 and pecificity 0.48. 2) ODI4% ≥ 15 had a sensitivity 0.65 and specificity 0.90. 3) ODI4% ≥5 had an AUC of 0.83 for an AHI ≥5 and 0.76 for an AHI ≥15. 4) Bland–Altman plot showed no significant bias when using ODI vs. AHI to define SDB. |
| Hang et al.618 | 2015 | 2b | Prospective study of sleep clinic patients undergoing PSG for suspected OSA. | Oximeter from PSG was used for ODI calculation without considering other PSG information. N = 699 (though only analyzed 544 with adequate TST and acceptable PSG signals) 77.1% M. PSG results: 20.6% had an AHI 5–15, 21.4% had an AHI >15–30, 46.3% had an AHI >30. |
Compared accuracy and AUC of ODI from PSG oximetry to AHI from same PSG. ODI used 3% and 4% desaturations. PSG defined hypopneas as 30% drop in flow with a 4% desaturation. |
1) For AHI ≥ 15, ODI3% had sensitivity, specificity, and accuracy of 86.1%, 92.4%, and 89.5%. 2) For AHI ≥15, ODI4% had sensitivity, specificity, and accuracy of 85.7%, 89.7%, and 87.8%. 3) AUC for severe OSA: 0.953–0.957; AUC of 0.921–0.924 for moderate to severe OSA patients. 4) Limitation due to removal of those with low TST on PSG. |
| Chung et al.499 | 2012 | 1b | Prospective study of patients presenting to presurgical clinic for elective surgery. | Subjects underwent unattended PSG and ON oximetry on the same night. N = 475 45.7% M |
Compared PSG AHI and ON oximetry ODI. Hypopnea definition was 30% drop in flow and 4% desaturation. ODI used 4% desaturation. |
1) ODI4% > 5 had a sensitivity, specificity, accuracy of 0.96, 0.67, 87% for an AHI >5; and 0.99, 0.39, 61.7% for AHI >15. 2) ODI4%>15 had a sensitivity, specificity, accuracy of 0.45, 0.98, 62.1% for an AHI >5, and 0.70, 0.93, 84% for AHI >15. 3) The AUC for ODI to predict AHI >5, AHI >15, and AHI >30 was 0.908, 0.931, and 0.958, respectively. |
| del Campo et al.620 | 2006 | 2b | Prospective study of cohort of patients undergoing PSG for suspected OSA. | Oximetry and PSG done at the same time. Approximate entropy (ApEn) (a mathematical tool) was calculated off oximetry and compared with PSG data. N = 187 (22.5% had COPD) 79% M |
Determined accuracy between PSG and ApEN. PSG hypopneas defined by 30% drop in flow with a 3% desaturation. ODI used 3% and 4% desaturations. |
1) AHI correlated with ApEn (r = 0.607; p < 0.001). 2) For AHI > 10, ApEn at 0.679 had sensitivity, specificity, PPV, and NPV of 88.3%, 82.9%, 88.3%, and 82.9%, respectively. |
| Erdenebayar et al.621 | 2017 | 2b | Prospective cross-sectional study of patients referred to a sleep clinic. | Subjects underwent an in-lab PSG and piezo-electric sensor at the same time. The piezo-electric sensor detected snoring and heartbeat information, and snoring index (SI) and features based on pulse rate variability (PRV) analysis. A support vector machine (SVM) was used as a classifier to detect OSA events. N = 45 70% M |
Compared accuracy of piezo-electric sensor with PSG. PSG scored per “AASM standards” but not defined further. |
1) Mild OSA detection: sensitivity, specificity, and accuracy of 72.5%, 74.2%, and 71.5%; moderate OSA detection: 85.8%, 80.5%, and 80.0%; and severe OSA: 70.3%, 77.1%, and 71.9%. 2) Automatic snoring detection had sensitivity, specificity, and accuracy of 88.5%, 96.1%, and 95.6%. 3) Heartbeat detection had sensitivity and PPV of 94.3% and 87.1%, all respectively. |
| Alakuijala et al.622 | 2016 | 4 | Prospective cross-sectional study of patients referred to a sleep clinic. | Subjects underwent a HSAT (Nox T3) at home. Periodic snoring data was collected from the same HSAT. N = 211 61% M There was no separate validation group. |
Analyzed the percentage of periodic snoring during HSAT and compared to the AHI from the HSAT. Correlations and Bland–Altman plot were analyzed. The HSAT defined hypopneas by 3% desaturations. |
1) AHI ranged from 0.1 to 116 events/h, and % of periodic snoring from 1% to 97%. 2) Positive correlation (r = 0.727, p < 0.001) between periodic snoring and AHI. 3) Sensitivity was 93.3%, specificity 35.1%, and NPV 75.0%. 4) Bland–Altman plot showed that periodic snoring percentage, and AHI agreed within range of various grades of OSA. |
| Neuromuscular disease | ||||||
| 2018 | 2b | Prospective cross-sectional study of patients followed for chronic respiratory failure due to neuromuscular disease, treated with chronic noninvasive ventilation (NIV). | All patients underwent the screening test panel (clinical evaluation, daytime arterial blood gas [ABG], nocturnal pulse oximetry [SpO2], and data from ventilator software), HSAT (Embletta Gold) and nocturnal transcutaneous CO2 (while on their NIV). N = 67 |
Compared accuracy among the tests. HSAT used 4% desaturation criteria. ODI3% used for oximetry. |
1. Nocturnal SpO2 and daytime ABG all failed to accurately detect nocturnal hypoventilation (NH). 2. ODI3% had a high sensitivity but low specificity for identifying obstructive events on NIV. |
|
Summary
This evidence-based review updated the review of the literature in the areas of diagnostic testing for patients with comorbid conditions and a moderate to high risk for OSA. While new data was found evaluating alternative diagnostic approaches, the weight of the evidence for each comorbidity did not support changing the 2017 CPG recommendations that in-lab PSG remains the diagnostic testing approach of choice for these patients.
Future research should account for several considerations. First and foremost, consideration should be given to the different definitions used to determine hypopneas as these differences will significantly impact the accuracy of testing that does not measure sleep and arousals, and as a result may affect long-term health outcomes in patients with OSA not associated with significant hypoxemia. Future work should also focus on the ability to predict patients likely to have false-negative HSAT or overnight oximetry testing results that may warrant follow-up testing, and what the impact of missed diagnosis may have on outcomes. Certain patient populations are at risk for complicated breathing disorders, including CSA and hypoventilation, and research is needed to advance technology for alternative testing devices that should take these factors into consideration. And finally, there is a lack of knowledge regarding the financial analysis of different approaches to diagnosing OSA in these specific high-risk patient populations.
VII |. COMORBIDITIES ASSOCIATED WITH OSA
VII.A |. Comorbidities Associated with OSA: Cardiovascular Disease
OSA is highly prevalent in the general population and in individuals with CV disease. OSA is characterized by repeated episodes of UA collapse during sleep, resulting in intermittent hypoxemia and arousals.632–635 The accompanying increase in sympathetic activity, inflammation, endothelial dysfunction, and elevated BP is associated with increased risk for CV morbidity and mortality.634,636–638
Many observational studies have demonstrated an association between OSA and incident CV disease, such as hypertension, AF, CAD, CHF, myocardial infarction (MI), stroke, and all-cause and CV mortality.
VII.A.1 |. Cardiovascular and all-cause mortality
A recent MA by Fu et al. examined the relationship between OSA and all-cause and CV mortality in 27 cohort studies. OSA increased risk for both all-cause mortality (HR 1.86, 95% CI = 1.81–1.91) and CV mortality (HR 2.36, 95% CI = 1.22–4.57). However, when OSA was stratified by severity, there was no significant association between mild OSA or moderate OSA and all-cause and CV mortality. Only severe OSA was an independent risk factor for both all-cause and CV mortality.639 On cluster analysis of the SantOSA cohort with moderate or severe OSA, the excessive sleepiness subtype was associated with an increased risk of incident CV mortality.640
VII.A.2 |. Cardiovascular disease
Compared to the general population, OSA is highly prevalent (38%–65%) in patients with CAD.635 Historically, evidence supports a significant association between OSA and CAD.
A large, population-based study was conducted to examine the cross-sectional relationship between OSA and cardiovascular disease (CVD). A cohort of 6424 subjects aged 40 and older from the SHHS underwent an unattended polysomnogram and categorized into quartiles of AHI. The first, second, third, and fourth AHI quartile ranges were 0–1.3, 1.4–4.4, 4.5–11.0, >11.0, respectively. Of the 6424 subjects, a total of 1023 subjects reported at least one CAD outcome, as defined as MI, angina, coronary revascularization procedure, HF, or stroke. Compared to the first and lowest quartile of AHI, the OR of prevalent CVD for the second, third and fourth highest quartiles were 0.98 (95% CI = 0.77–1.24), 1.28 (95% CI = 1.02–1.61), and 1.42 (95% CI = 1.13–1.78), respectively, indicating that severe OSA is independently associated with CAD events with a dose response relationship after adjusting for demographic variables, tobacco use, cholesterol, and hypertension variables.638
A secondary analysis of the SHHS cohort examined the prospective association of OSA and CAD. A subset from the SHHS cohort without CAD and HF at enrollment, and adequate data for analysis (4422 subjects, 56.4% women), were followed for a median of 8.7 years for incident CV disease. After adjusting for other risk factors, OSA was significantly associated and predicted incident CAD events, defined as MI, revascularization procedure, or coronary heart disease related death, however this was only observed in men aged 70 or younger (HR 1.10, 95% CI = 1.00–1.21 per 10-unit increase in AHI). Severe OSA (as defined by AHI ≥ 30 events/h) conferred a stronger increased risk of developing symptomatic CAD (HR 1.68, 95% CI = 1.02–2.76) again seen only in men aged 70 or younger.641 However, another analysis of the full SHHS cohort has shown severe OSA to be an independent predictor of death, and in particular death related to CAD. While this relationship was again strongest in men under 70 years of age (adjusted HR 2.09; 95% CI: 1.31–3.33), it was nonetheless seen across all patients with severe OSA in the study population (adjusted HR 1.46; 95% CI: 1.14–1.86).642
In addition to evidence supporting an independent link between OSA and incident CAD, there is evidence that OSA may be associated with recurrence of CAD events including restenosis after percutaneous coronary dilation and death among individuals with CAD and OSA.643 Nakashima et al. conducted a prospective cohort study to determine whether moderate to severe OSA was associated with an increased risk of adverse CV events in patients who underwent primary percutaneous coronary intervention (PCI). The cohort was comprised of 272 patients who were admitted to the Nagasaki Citizens Hospital with acute MI. Patients with moderate to severe OSA had independently and significantly increased acute coronary syndrome recurrence and major adverse cardiac events compared to patients with mild or no OSA.644
Participants in the other sentinel community-based prospective observational study of OSA, the Wisconsin sleep cohort, were significantly younger than those in the SHHS. This may provide at least a partial explanation for the much stronger association seen between OSA and CAD in the former, wherein an AHI of ≥30 conferred a greater than two-fold risk of incident CAD and HF events (adjusted HR 2.63; 95% CI: 1.13–6.10) over a period of approximately 18,000 person years.645 The fully adjusted model for incident CAD only was not statistically significant (HR 2.4, CI 0.99–6.0).
The inevitable meta-analyses suggest that OSA confers an increased risk of incident clinically overt CAD in men, with an apparent weaker relationship between OSA and CAD in women.633,635,639 An SR of untreated OSA and long-term adverse outcomes suggested that any negative effect of significant OSA on CV events was attenuated by female gender, age, a lack of daytime sleepiness, and obesity.637 Overall, there is relatively strong, but not uniform, evidence from clinical and population studies to support an important role for OSA in promoting the evolution of CAD, particularly in younger male patients. (Table VII.A.2)
TABLE VII.A.2.
Evidence on coronary artery disease and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endp oints | Conclusion |
|---|---|---|---|---|---|---|
| Bauters et al.632 | 2015 | 2b | Review | Review to determine the association between OSA and cardiovascular disease and impact of CPAP treatment on cardiovascular risk factors and outcomes. | Hypertension, stroke, ischemic heart disease, heart failure, atrial fibrillation, and cardiovascular mortality. | Moderate to severe OSA is independently associated with various forms of CV disease. |
| Bouzerda643 | 2018 | 2a | Systematic review | Review of pathophysiological mechanisms between OSA and cardiovascular disease and to determine prevalence of OSA in general population. | Systemic and pulmonary arterial hypertension, heart rhythm disorders, coronary heart disease, heart failure, and stroke. | Recommend screening for clinical symptoms of OSA in patients with cardiovascular disease. |
| Campos-Rodriguez et al.646 | 2012 | 2b | Prospective cohort study | Cohort of 116 women consecutively referred to two sleep clinics in Spain for suspected OSA between 1998 and 2007 categorized into five groups: 1) control without OSA 2) CPAP treated group with mild-moderate OSA 3) CPAP treated group with severe OSA 4) untreated group with mild-moderate OSA 5) untreated group with severe OSA |
Cardiovascular mortality, including death from stroke, myocardial infarction, heart failure, or arrhythmia. | Severe OSA is associated with cardiovascular mortality in women. Treatment with CPAP may reduce this risk. |
| Catalan-Serra et al.647 | 2019 | 2b | Prospective cohort study | Cohort of 1005 consecutive patients referred to sleep units at two Spanish University hospitals who were ≥65 years. They were categorized into four groups based on AHI values from sleep studies and CPAP adherence: 1. control group 2. untreated mild-moderate OSA 3. untreated severe OSA 4. CPAP-treated OSA |
Incidence of coronary heart disease and incidence of stroke after adjusting for OSA group, age, BMI, HTN, sex, smoking, ESS, and afib. | The incidence of stroke, but not coronary heart disease, is increased in elderly patients with untreated severe OSA. |
| Drager et al.636 | 2015 | 2b | Review | Review of pathogenesis and association between OSA and cardiovascular disease. | Cardiovascular disease including hypertension, autonomic dysfunction, insulin resistance, vascular dysfunction, lipid metabolism impairment, atherosclerosis. | OSA activates multiple intermediate pathways that lead to cardiovascular disease. |
| Fu et al.639 | 2016 | 2b | Meta-analysis | 27 cohort studies with 3,162,083 participants who were diagnosed with OSA by PSG. | All-cause or cardiovascular mortality. | Severe OSA is an independent risk factor for all-cause and cardiovascular mortality. CPAP use significantly reduced both all-cause and cardiovascular mortality in patients with OSA. |
| Ge et al.633 | 2013 | 2b | Meta-analysis | 6 cohort studies with 9165 adults who had been diagnosed with OSA, of any severity, confirmed by using a standardized polysomnography. | Cardiovascular mortality (defined as death from stroke, heart failure, myocardial infarction, or arrhythmia), and all-cause mortality. | Severe OSA is a strong independent predictor for future cardiovascular and all-cause mortality. CPAP treatment was associated with reduced risk of cardiovascular mortality. |
| Gonzanga et al.648 | 2015 | 2b | Review | Impact of OSA on cardiovascular disease and cardiovascular benefits of CPAP treatment. | Prevalence of OSA in patients with hypertension, coronary artery disease, atrial fibrillation, stroke, and heart failure. | OSA is highly prevalent in the general population and those with cardiovascular disease. Screening and treatment are needed to decrease cardiovascular risk. |
| Gottlieb et al.641 | 2010 | 2b | Prospective cohort study | Cohort of 4422 men and women who are ≥40 years old and free of coronary heart disease and heart failure followed for incident CHD and HF from the Sleep Heart Health Study. | Incident CHD (myocardial infarction, CHD death, or coronary revascularization procedure) and incident heart failure. | OSA is associated with increased risk of CV outcomes in community-dwelling middle-aged and older men. |
| Hla et al.645 | 2015 | 2b | Prospective cohort study | 1280 men and women from the Wisconsin Sleep Cohort who were free of CHD or HF at baseline and followed for 24 years. | Incident CHD or heart failure. | Untreated severe OSA was associated with incident coronary heart disease or heart failure. |
| Javaheri et al.634 | 2016 | 2b | Prospective cohort study | Cohort of 2865 older men from Osteoporotic Fractures in Men Study. | Incidence of heart failure. | Older men with elevated central sleep apnea index or Cheyne-Stokes breathing had an increased risk for incident HF. However, OSA was not significantly associated with incident HF. |
| Kasai et al.649 | 2010 | 2b | Review | Review of association between OSA and heart failure. | Cardiovascular function and autonomic function. | OSA has adverse cardiovascular effects and is associated with reduced survival in patients with HF. |
| Loke et al.650 | 2012 | 2a | Systematic Review | Association between OSA and incident cardiovascular events. | Stroke incidence and cardiovascular mortality. | OSA is an independent risk factor for stroke and cardiovascular mortality. |
| Martinez-Garcia et al.651 | 2012 | 2b | Prospective Cohort Study | Cohort of 943 elderly patients (≥65 years) with mild/mod OSA or severe OSA between 1998 and 2007 categorized into four groups: 1) control group (AHI<15) 2) untreated mild/mod OSA without CPAP 3) untreated severe OSA 4) OSA treated with CPAP |
Cardiovascular mortality defined as death from stroke, heart failure, or myocardial infarction. | Untreated severe OSA is significantly associated with cardiovascular mortality in the elderly. |
| Nakashima et al.644 | 2015 | 2b | Prospective cohort study | Cohort of 272 patients with acute myocardial infarction who underwent primary percutaneous coronary intervention within 12 h of onset. | MACE (cardiovascular mortality, acute coronary syndrome recurrence, and readmission for heart failure). | Pt with untreated moderate–severe OSA had an increased the risk of acute coronary syndrome recurrence. Moderate–severe OSA also increased risk for MI related percutaneous coronary intervention progressive lesions. |
| Punjabi et al.642 | 2009 | 2b | Prospective cohort study | Cohort of 6441 men and women from the Sleep Heart Health Study. | All-cause and cardiovascular mortality. | OSA is independently and significantly associated with all-cause and cardiovascular mortality with a stronger association in men 40–70 years old with severe OSA. |
| Rosen et al.637 | 2014 | 2b | Review | Review of the pathophysiology of OSA and heart failure and review of treatment options for OSA in patients with heart failure. | Incident heart failure and mortality. | Untreated OSA is an independent risk factor for increased mortality in heart failure patients. |
| Shah et al.652 | 2010 | 2b | Prospective cohort study | Cohort of 1436 patients ≥50 years of age who were referred during 1997–2001 to the Yale Center for Sleep Medicine for suspected sleep disordered breathing. | Cardiovascular outcomes myocardial infarction, coronary artery revascularization procedures, or cardiovascular mortality. | OSA increases the risk of coronary events or cardiovascular mortality. |
| Shahar et al.638 | 2001 | 2c | Cross-sectional study | Cohort of 6424 participants from the Sleep Heart Health Study. | Self-reported cardiovascular disease outcomes defined as MI, angina, coronary revascularization procedure, heart failure, or stroke. | OSA is associated CVD outcomes, but more strongly associated with self-reported heart failure and stroke than coronary heart disease. |
| Wang et al.635 | 2018 | 2a | Systematic review and meta-analysis | Review of nine studies (two RCTs and seven observational studies) for 1430 patients with CAD and CVD. | Adverse CVD event (MACE) -all-cause or cardiovascular death, myocardial infarction, stroke, repeat revascularization, or hospitalization for heart failure. | CPAP may prevent incident cardiovascular events in patients with CAD and OSA. However, this was only shown in observational studies, not in RCTs. |
| Yeboah et al.653 | 2011 | 3b | Nested case–control study | 5338 men and women from MESA study cohort. | Incident cardiovascular events as defined by myocardial infarction, angina, resuscitated cardiac arrest, stroke, stroke mortality, CHD mortality, or other CVD death as defined by the MESA protocol. | OSA, but not habitual snoring, was associated with incident CV events and all-cause mortality in adults without CVD. |
| Yu et al.654 | 2017 | 1b | Systematic review and meta-analysis | Review of 10 RCTs with 7266 patients to determine association of CPAP compared with standard care or sham PAP among adults with OSA or central sleep apnea. | Acute coronary syndrome events, stroke, or vascular events or death, major adverse cardiovascular events. | CPAP use was not significantly associated with reduced risk of cardiovascular outcomes or mortality for OSA patients. |
VII.A.3 |. Myocardial ischemia
OSA has been associated with numerous CV conditions, including CAD and myocardial ischemia. A number of possible pathophysiologic mechanisms have been implicated including sympathetic nervous system hyperactivity, hypertension, endothelial dysfunction, metabolic dysregulation, insulin resistance, and hypercoagulable state.641,655–663 In addition, OSA results in repetitive hypoxia and re-oxygenation and this has been associated with increased oxidative stress and systemic inflammation. These mechanisms may contribute to the increased risk of atherosclerosis and myocardial ischemia in patients with OSA.
OSA has been associated with coronary artery calcification,664 plaque instability,665 and vulnerability.644 During obstructive apneas, increased adrenergic tone and hypoxemia may increase the risk of myocardial ischemia.666,667 Interestingly, a temporal relationship between hypoxia and the development of ST changes and chest pain has been reported by Franklin et al.668 The severity of hypoxemia also appears to be a determinant of ST depression during sleep.669
Shah et al.652 have reported that in an observational cohort of over 1400 patients, OSA was associated with a two-fold increased risk of CV events or death even after adjustment for traditional risk factors. This suggests that OSA might independently increase the risk of coronary events. In patients with ST-segment elevation myocardial infarction (STEMI), the prevalence of undiagnosed OSA is almost 40%.670 In addition, the onset of MI is more likely to be during the nighttime.666 Patients who have had prior STEMI are more likely than the general population to have OSA, and have worse event free survival compared to STEMI patients without OSA.666,670
Major adverse CV events (defined as a composite of CV mortality, non-fatal MI, non-fatal stroke, and unplanned revascularization) after PCI also appear to be worse in patients with OSA.671,672 It is controversial however whether treatment of OSA with CPAP may reduce the risk of repeat revascularization after PCI.673,674 It has been reported that CPAP use of >4 versus <4 h per night may be associated with a significant reduction of CV risk.673 (Table VII.A.3)
TABLE VII.A.3.
Association between myocardial ischemia, coronary artery disease, and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Sorajia et al.664 | 2008 | 2b | Cross-sectional | 202 consecutive patients with no history of coronary disease who underwent electron-beam CT within 3 years of polysomnography between March 1991 and December 2003 were included. OSA was defined by an apnea hypopnea index (AHI)>or =5 events/h, and patients were grouped by quartiles of AHI severity. | Evaluated the association between OSA and the presence of subclinical coronary disease assessed by coronary artery calcification (CAC). | In patients without clinical coronary disease, the presence and severity of OSA is independently associated with the presence and extent of CAC. |
| Franklin et al.668 | 1995 | 2b | Prospective cohort | 10 consecutive men with severely disabling angina pectoris and nocturnal angina on at least two nights per week for at least 1 month. | Overnight recordings of nasal and oral airflow, abdominal and chest movements, oxygen saturation, body position, electrocardiogram, electro-oculograms, electroencephalograms, and submental electromyogram were registered. | Results show a relation between nocturnal angina pectoris and sleep apnea. |
| Mooe et al.669 | 2000 | 3b | Observational study | 132 men and 94 women | Overnight sleep study and Holter recording to examine disordered breathing (oxyhemoglobin desaturations > or = 4% and apnea-hypopneas), heart rates, and ST-segment depressions (> or = 1 mm, > or = 1 min). | Episodes of nocturnal myocardial ischemia are common in patients with angina pectoris. However, a temporal relationship between sleep-disordered breathing and myocardial ischemia is present only in a minority of the patients, but occurs more frequently in men and in more severely disordered breathing. |
| Kuniyoshi et al.666 | 2008 | 2b | Prospective cohort | 92 patients with MI for which the time of onset of chest pain was clearly identified. The presence of OSA was determined by overnight polysomnography. | Evaluate the day-night variation of acute myocardial infarction in patients with obstructive sleep. | The diurnal variation in the onset of MI in OSA patients is strikingly different from the diurnal variation in non-OSA patients. Patients with nocturnal onset of MI have a high likelihood of having OSA. These findings suggest that OSA may be a trigger for MI. |
| Xie et al.675 | 2016 | 2b | Prospective study | 112 patients without a prior diagnosis of sleep apnea underwent comprehensive polysomnography within a median of 7 days after MI. Patients were followed up at 6-monthly intervals (±2 weeks) for 48 months. | Investigate the effects of OSA on prognosis after MI, and to determine which specific measures of OSA severity best predicted outcomes. | Nocturnal hypoxemia in OSA is an important predictor of poor prognosis for patients after MI. |
| Nakashima et al.644 | 2015 | 2b | Prospective cohort | Patients with acute MI and followed over time. | The main study outcome measures were cardiac death, recurrence of ACS, and re-admission for heart failure. Major adverse cardiac events (MACEs) were defined as composite end points of individual clinical outcomes. | Moderate-to-severe OSA increased the risk of ACS and the incidence of PCI for progressive lesions. Increased plaque vulnerability might be related to these clinical manifestations. |
| Shah et al.652 | 2010 | 3b | Observational cohort study | 1436 enrolled patients, 1024 (71%) had an apnea hypopnea index > or = 5. | Study aimed to determine whether obstructive sleep apnea independently increases the risk of coronary events, including death from cardiovascular causes. | Obstructive sleep apnea increases the risk of coronary events or death from cardiovascular causes. |
| Lee et al.670 | 2011 | 2b | Prospective cohort | 120 patients underwent an overnight sleep study during index admission for STEMI. | Determine the effect of severe obstructive sleep apnea (OSA) on long-term outcomes after myocardial infarction. | 42% of the patients admitted with STEMI have undiagnosed severe OSA. Severe OSA carries a negative prognostic impact for this group of patients. It is associated with a lower event-free survival rate at 18-month follow-up. |
| Lee et al.671 | 2016 | 2b | Prospective study | Between December 2011 and April 2014, 1748 eligible patients were prospectively enrolled. The 1311 patients who completed a sleep study within 7 days of percutaneous coronary intervention formed the cohort for this analysis. Median follow-up of 1.9 years. | Hypothesized that OSA predicts subsequent major adverse cardiac and cerebrovascular events (MACCEs) in patients undergoing percutaneous coronary intervention. | OSA is independently associated with subsequent MACCEs in patients undergoing percutaneous coronary intervention. |
| Qu et al.672 | 2018 | 1a | Meta-analysis of prospective cohort studies | 7 studies including 2465 patients. | Followed up with patients after PCI, and evaluated their overnight sleep patterns within 1 month for major adverse cardiac events (MACEs) as primary outcomes including cardiac death, non-fatal myocardial infarction (MI), and coronary revascularization and secondary outcomes including re-admission for heart failure and stroke. | In patients after PCI, OSA appears to increase the risk of cardiac death, non-fatal MI, and coronary revascularization. |
| Xie et al.676 | 2018 | 2b | Prospective study | 112 post-myocardial infarction patients. | Investigate whether excessive daytime sleepiness (EDS) would be an independent prognostic factor after myocardial infarction. | EDS may be an independent prognostic factor of adverse outcome in post-myocardial infarction patients with moderate to severe sleep-disordered breathing. |
| Gottlieb et al.641 | 2010 | 1b | Prospective longitudinal epidemiological study | 1927 men and 2495 women > or = 40 years of age and free of coronary heart disease and heart failure at the time of baseline polysomnography were followed up for a median of 8.7 years. | Objective of this study was to assess the relation of obstructive sleep apnea to incident coronary heart disease and heart failure in a general community sample of adult men and women. | Obstructive sleep apnea is associated with an increased risk of incident heart failure in community-dwelling middle-aged and older men; its association with incident coronary heart disease in this sample is equivocal. |
| Schwarz et al.663 | 2015 | 1b | Systematic review and meta-analysis | Systematic review and meta-analysis evaluating RCTs on the effect of CPAP on endothelial function in OSA, assessed by flow-mediated dilatation (FMD) and other validated techniques. Eight RCTs comparing the effects of therapeutic CPAP versus subtherapeutic CPAP (or no intervention) on endothelial function involving 245 OSA patients were included in the systematic review. | Assess the effect CPAP therapy on endothelial function in patients with OSA. | In patients with OSA, CPAP therapy improves endothelial function significantly and to a clinically important extent. |
| Phillips et al.661 | 2012 | 2b | Randomized crossover trial | 28 patients received therapeutic or placebo CPAP, each for 2 months with a 1 month washout between treatments. After each treatment period, a 24 h coagulation study was conducted. | Plasminogen activator inhibitor-1 (PAI-1), D-dimer, fibrinogen, von Willebrand Factor (vWF), factor VIII (FVIII), factor VII (FVII), and factor V (FV) were determined at seven time points over the day and night. | CPAP may reduce cardiovascular in OSA, in part through reducing risk of thrombosis. |
| Milleron et al.658 | 2004 | 1a | Long-term prospective study | Studied 54 patients (mean age 57.3 ± 10.1 years) with both CAD (> or = 70% coronary artery stenosis) and OSA (apnea-hypopnoea index > or = 15). In 25 patients, OSA was treated with continuous positive airway pressure (n = 21) or upper airway surgery (n = 4); the remaining 29 patients declined treatment for their OSA. | A composite of cardiovascular death, acute coronary syndrome, hospitalization for heart failure, or need for coronary revascularization. | Treatment of OSA in CAD patients is associated with a decrease in the occurrence of new cardiovascular events, and an increase in the time to such events. |
| Wu et al.674 | 2015 | 2b | Prospective study | 390 patients with OSA who had undergone PCI. | Impact of OSA treatment with CPAP on percutaneous coronary intervention (PCI) outcomes. | Untreated moderate–severe OSA was independently associated with a significant increased risk of repeat revascularization after PCI. CPAP treatment reduced this risk. |
| Peker et al.673 | 2016 | 1b | Randomized controlled trial | Consecutive patients with newly revascularized CAD and OSA (apnea hypopnea index ≥15/h) without daytime sleepiness (Epworth Sleepiness Scale score <10) were randomized to auto-titrating CPAP (n = 122) or no positive airway pressure (n = 122). | Determine the effects of CPAP on long-term adverse cardiovascular outcome risk in patients with CAD with nonsleepy OSA. | Routine prescription of CPAP to patients with CAD with nonsleepy OSA did not significantly reduce long-term adverse cardiovascular outcomes in the intention-to-treat population. There was a significant reduction after adjustment for baseline comorbidities and compliance with the treatment. |
VII.A.4 |. Hypertension
OSA and hypertension (HTN) are highly prevalent conditions in the general population.95,97,677 Both conditions are highly comorbid, with 50% of subjects with OSA noted to have HTN678 and 50% of those with HTN found to have OSA.679 While OSA has been associated with several adverse CV consequences, the evidence linking OSA and HTN is the most robust.680,681 OSA and HTN have shared risk factors such as obesity,682 but the presence of OSA has been found to be an independent risk factor for prevalent as well as incident pre-HTN and HTN.115,660,683,684 More recent evidence suggests that untreated rapid eye movement (REM)-related OSA, a subtype of OSA where SDB events are predominantly confined to REM sleep, is significantly associated with the development of HTN.685
There is a dose–-response relationship between OSA and HTN, in that greater severity of OSA appears to confer a higher risk of HTN.115,660,684 Furthermore, the effects of OSA on BP seem to be more pronounced in subjects with subjective and objective daytime sleepiness686,687; these individuals may have a greater degree of desaturation and higher diastolic BP following SDB events.688 Finally, the temporal distribution, night-to-night variability, and degree of desaturation and/or autonomic response associated with SDB events may ultimately affect an individual’s risk of developing HTN.681
The prevalence of nocturnal non-dipping BP at night, an adverse CV prognostic risk factor, is high in subjects with OSA, in the range of 50%–80%.689,690 While the severity of respiratory abnormalities appears to be associated with a nocturnal non-dipping BP pattern in younger individuals, in older subjects, the severity of sleep disruption seems to correlate with nocturnal non-dipping status.691
Resistant HTN is defined by the use of three or more antihypertensive medications; refractory HTN refers to treatment with five or more medications.692,693 OSA is a secondary cause of HTN,694 and is particularly common in cases of resistant and refractory HTN.695–697 Over 50% of subjects with resistant HTN have underlying OSA.698–700 Furthermore, those with severe untreated OSA appear to have a significantly higher risk of having resistant HTN when compared to subjects with moderate OSA.701 A recent study demonstrated a two-fold increase in risk of resistant HTN in African-Americans with severe OSA, and it is notable that the burden of undiagnosed OSA is high in this population.702 (Table VII.A.4)
TABLE VII.A.4.
Association between OSA and refractory hypertension
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Pratt-Ubunama et al.699 | 2007 | 3b | Prospective, single-center, case–control study; all subjects underwent PSG (n = 46). | 1) RHTN (n = 17). 2) Controls with suspected OSA (n = 29). |
1. AHI. 2. PAC, renin concentration. |
OSA was common in subjects with RHTN (85%) and was more common and severe in men vs. women. PAC (but not renin concentration) correlated with AHI. |
| Pedrosa et al.679 | 2011 | 3b | Prospective cohort, study conducted at two outpatient HTN units (n = 125). | Consecutive patients with RHTN. | Evaluation for secondary caiuses of HTN. | Moderate–severe OSA was the most common condition associated with RHTN, seen in 64% of subjects. |
| Walia et al.703 | 2014 | 3b | Secondary analysis of baseline data from the Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT) RCT (n = 284). | Subjects with HTN; 64% had OSA, 10% had RHTN. | Association between RHTN and OSA. | RHTN was more prevalent in subjects with severe vs. moderate OSA (58.3% vs. 28.6%, p = 0.01) who were prescribed antihypertensives; those with severe OSA had four-fold higher adjusted odds of RHTN (OR 4.1, 95% CI: 1.7–10.2). |
| Johnson et al.702 | 2018 | 3b | Analysis of data from the Jackson Heart Study, community cohort (n = 913) | Subjects with HTN (n = 664); 25.7% with OSA (untreated in the vast majority), 14% with RHTN; majority female. | Association between OSA and RHTN in blacks. | Subjects with moderate–severe OSA had a two-fold higher odds of RHTN (OR 2.0; 95% CI: 1.14–3.67) after accounting for confounders. OSA and oxyhemoglobin saturation <90% were not associated with uncontrolled BP. |
| Martinez-Garcia et al.696 | 2018 | 3b | Cross-sectional study (n = 229) | Consecutive subjects with RHTN, 18% met criteria for refractory HTN. | Association between refractory HTN and presence/severity of OSA. | Subjects with refractory HTN had a two-fold higher risk of OSA (prevalence of moderate OSA was 95% and severe OSA was 64%) and greater OSA severity (AHI 41.8 vs. 33.8; p = 0.026) compared to those with RHTN. |
| Sapina-Beltran et al.700 | 2019 | 3b | Multicenter cohort study (n = 284) | Consecutive subjects with RHTN. | Prevalence of OSA in subjects with RHTN and association of OSA with BP control. | 83.5% had OSA, 25.7% moderate, and 26.1% severe OSA. Those with severe OSA had higher BP than those with mild/no OSA, with a greater effect noted on nighttime BP vs. those with no OSA. The prevalence of severe OSA was higher in those with uncontrolled BP (not statistically significant). |
Abbreviations: AHI, apnea hypopnea index, events/hour; BP, blood pressure; CI, confidence intervals; DBP, diastolic blood pressure; OSA, obstructive sleep apnea; PAC, plasma aldosterone concentration; PSG, polysomnography; RCT, randomized controlled trial; RHTN, resistant hypertension; SBP, systolic blood pressure.
VII.A.5 |. Atrial fibrillation
Patients with AF have a high prevalence of OSA. Direct comparison of studies regarding the concurrence of OSA and AF is complicated by varying respiratory event definitions and by inclusion in some studies of both OSA and CSA. Despite these limitations, studies that systematically evaluated AF patients using home or laboratory sleep testing report a prevalence of OSA (AHI ≥ 5) of 43%–85%, with prevalence >75% in most studies.704–710 Moderate or severe OSA (AHI ≥ 15) is reported in 20%–62%.330,704,706,710–715 When a comparison group of patients without AF was included, the prevalence of OSA was higher in those with AF than in controls in most707,715,716 but not all717 studies. Community-based cohort studies support an independent association of OSA with the development of AF. In the Multi-Ethnic Study of Atherosclerosis, the prevalence of AF increased from 4.0% in those without OSA to 7.5% in those with severe OSA.718 In two cohorts, the presence of AF was identified from the polysomnographic recording. In the SHHS, compared to those with AHI <5, participants with AHI ≥ 30 had an age-, sex-, BMI-, and prevalent CV disease-adjusted odds ratio (aOR) of 4.0 (95% CI 1.0–15.7) for the presence of AF,719 while in the Outcomes of Sleep Disorders in Older Men Study there was a dose-dependent association of OSA with prevalent AF.720
Longitudinal studies have yielded equivocal results regarding the association of OSA with incident AF. While studies using administrative claims data721 or patientreported diagnosis of OSA722 suggest an association of OSA with incident AF, these studies have a high risk of bias. Three large retrospective studies of incident AF in cohorts of patients referred for diagnostic PSG have been reported. In one, patients with AHI ≥ 5 were twice as likely to develop AF as those with AHI < 5, an effect that was limited to those under age 65; however, adjusting for age, sex, BMI, and prevalent CV disease, mean nocturnal SaO2 but not AHI was an independent predictor of incident AF.414 In another, the incidence of hospitalized AF increased progressively with increasing severity of OSA, although after multivariate adjustment that included BMI, neither AHI nor time at saturation <90% was a significant predictor of incident AF, except in those with >30% of sleep time at saturation <90%.723 It is unclear whether these measures of SaO2 reflect OSA or other conditions, such as reduced pulmonary or cardiac function, that might cause AF. In the third, AHI ≥ 5 was associated with an aOR of 1.55 (95% CI 1.21–2.00) for incident hospitalized AF after extensive covariate adjustment, with AF incidence increasing with greater severity of OSA.724 In contrast, two community-based cohort studies, each of which had shown a cross-sectional association of OSA with prevalent AF, found that CSA but not OSA was independently associated with increased incidence of AF.725,726
Data are more consistent regarding the association of OSA with recurrent AF. In six studies that systematically assessed the presence of OSA prior to treatment of AF, OSA was associated with an approximately two- to three-fold increased risk of recurrent AF following electrical cardioversion714 or pulmonary vein isolation (catheter ablation) procedures.708,709,711,713,727 A similar increased risk of AF recurrence was reported in several studies that did not systematically screen for OSA, but compared the risk of AF recurrence following ablation procedures in patients with a documented prior diagnosis of OSA to those without known OSA.728–730 The presence of severe OSA also predicts failure of antiarrhythmic drugs to suppress AF.731 These studies generally included a mix of patients with paroxysmal and persistent AF, and the finding of increased risk of recurrence in those with OSA does not appear to be limited to either group.
Putative pathophysiological mechanisms linking OSA to AF include both chronic cardiac structural changes and acute arrhythmogenic effects of obstructive events, and has been recently reviewed.732 Chronic changes include increased atrial dimension and slowed atrial conduction, while acute triggers to AF likely include atrial distension due to intrathoracic pressure swings along with hypoxemia, hypercapnia, and the associated acute elevation of sympathetic nervous system activity. In the SHHS, paroxysmal AF events were markedly more likely to occur during the 90 s following an obstructive apnea or hypopnea than during periods without obstructive events (OR 17.9, 95% CI 2.2–144.2).733 Similarly, in patients with paroxysmal AF, night-to-night variation in OSA severity predicts changes in AF burden.734 (Table VII.A.5)
TABLE VII.A.5.
Association between atrial fibrillation and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Gami et al.716 | 2004 | 3b | Case–control study | 1) 151 consecutive patients undergoing electrocardioversion for AF 2) 312 consecutive patients without AF referred to a general cardiology practice |
Presence of OSA identified based on a Berlin Questionnaire score of 2–3 | Adjusted OR of 2.19 (95% CI 1.40–3.42) for the association of AF and OSA |
| Porthan et al.717 | 2004 | 4 | Case–control study | 1) 59 patients with AF identified from hospital records, free of known causes of AF 2) 56 age- and sex-matched controls from general population registry, free of AF or known causes of AF |
Presence of “sleep apnea syndrome” based on AHI from in-lab cardiorespiratory polygraphy and sleep apnea symptoms | No significant difference between groups in prevalence of sleep apnea syndrome (32% in AF versus 29% in control) |
| Mehra et al.719 | 2006 | 3b | Nested case–control study within a community-based cohort study | Participants in the Sleep Heart Health Study 1) 228 subjects with AHI ≥30 on home polysomnography 2) 338 subjects with AHI <5, frequency-matched on age, sex, race/ethnicity, BMI |
Presence of arrhythmias on bipolar lead I ECG recorded on home polysomnography | Atrial fibrillation was present in 4.8% of severe OSA group and 0.9% of controls, with adjusted OR 4.02 (95% CI 1.03–15.74) for the association of AF and severe OSA |
| Stevenson et al.715 | 2008 | 3b | Case–control study | 1) 90 patients with paroxysmal or persistent AF 2) 45 patients referred to the same tertiary care center without AF, frequency match for age, and sex |
AHI >15 on home polysomnography | Mean AHI was higher in the AF group than the control group (23.2 [SD 19.3 vs. 14.7 [SD 12.4]), with adjusted OR 3.04 (95% CI1.24–7.46) for the association of AF and OSA |
| Braga et al.707 | 2009 | 3b | Case–control study | 1) 57 consecutive patients with chronic persistent AF in Sao Paulo, Brazil 2) 32 age-, sex-, and BMI-similar subjects from the Sao Paolo general population participating in the Epidemiologic Sleep Study (EPISONO) |
From in-lab polysomnography: 1) AHI 2) Sleep time with SaO2 <90% |
No significant difference between groups in mean AHI (24.3 [SD 16.5] vs. 19.1 [SD 15.3]), but higher prevalence of AHI ≥10 (81.6% vs. 60%, p = 0.03) and longer time with SaO2 <90% in the AF group |
| Bitter et al.706 | 2009 | 4 | Case series | 150 patients with persistent AF and normal left ventricular ejection fraction | AHI from cardiorespiratory polygraphy, classified as central or obstructive based on the predominant event type | OSA was present in 42.7% (18% mild, 12% moderate, 12.7% severe); an additional 31.3% had central sleep apnea |
| Mehra et al.720 | 2009 | 3b | Community-based cross-sectional cohort study | 2911 participants in the Outcomes of Sleep Disorders in Older Men Study | Association of nocturnal arrhythmias present on home polysomnography with total AHI, obstructive AHI (obstructive apneas plus all hypopneas), and central apnea index | There was a progressive increase in prevalence of AF with increasing quartile of total AHI, from 3.2% to 7.3%. The adjusted OR in the highest AHI quartile was 2.15 (95% CI 1.19–3.89). This effect was stronger for the central apnea index, and was non-significant for the obstructive AHI in adjusted analyses |
| Pathak et al.330 | 2014 | 4 | Case series | 149 patients referred for pulmonary vein isolation procedure for AF | Presence of severe OSA (AHI ≥30) on in-lab polysomnography | 86 of 149 patients (57.7%) had severe OSA |
| Kwon et al.718 | 2015 | 3b | Community-based cross-sectional cohort study | 2048 participants in the Multi-Ethnic Study of Atherosclerosis Sleep Study | Association of AHI from in-home polysomnography at MESA exam 5 with AF based on International Classification of Disease codes or ECG recordings over the preceding approximately 10 years (n = 92) or present on the polysomnogram ECG (an additional n = 8) | There was an increase in AF prevalence from 4.0% in those with none to mild OSA, 6.0% in those with moderate OSA, and 7.5% in those with severe OSA. The adjusted OR was 1.22 (95% CI 0.99–1.49) for each 1 SD increase in AHI. |
| Abuammar et al.704 | 2018 | 4 | Case series | 100 consecutive patients with AF without prior diagnosis of OSA recruited from arrhythmia clinics | Presence and severity of OSA from mean of 2 nights of home polysomnography | OSA was present in 85% of subjects (38% mild, 23% moderate, and 24% severe) |
| Traaen et al.710 | 2019 | 4 | Case series | 579 patients with paroxysmal AF admitted for pulmonary vein isolation procedure, without known OSA | Presence and severity of sleep apnea from mean of 2 nights of cardiorespiratory polygraphy | Sleep apnea was present in 83% of subjects (41% mild, 30% moderate, and 12% severe); this was OSA in 97.5% of those with sleep apnea, central sleep apnea in 2.5% |
| Gami et al.414 | 2007 | 2b | Retrospective cohort study | 3542 adults without history of AF referred for diagnostic polysomnography 1) OSA defined as AHI ≥5 (n = 2626), with group mean AHI 36 (SD 32) 2) No OSA | Incidence of AF based on electronic medical record review, with mean follow-up of 4.7 years | AF occurred in 2.1% of those without OSA, 4.3% of those with OSA, with unadjusted HR 2.18 (95% CI 1.34–3.54). This effect was restricted to those under age 65. In multivariate analysis, the difference between awake and asleep oxygen saturation was a strong predictor of incident AF. It is not clear in this study whether measures of OSA per se are independently associated with incident AF |
| Cadby et al.724 | 2015 | 2b | Retrospective cohort study | 6841 adults without history of AF referred for diagnostic polysomnography 1) OSA defined as AHI 0≥5 (n = 4352) 2) No OSA |
Incidence of hospitalization for AF based on review of Western Australia Hospital Morbidity and Mortality Data, with median follow-up of 11.9 years | AF occurred in 8.6% of those with OSA and 3.3% of those without OSA, with adjusted HR 1.55 (95% CI 1.21–2.00) for the association of OSA with incident AF. There was a modest dose–response relationship, with adjusted HR increasing from 1.48 in those with mild OSA to 1.73 in those with severe OSA. Both AHI and time at SaO2 <90% were associated with incident AF |
| May et al.725 | 2016 | 2b | Community-based cohort study | Of 2316 participants in the Outcomes of Sleep Disorders in Older Men Study who were free of AF at baseline, 852 had a follow-up sleep study after approximately 6 years and 843 of these had outcome data and were analyzed | Association of AHI from baseline in-home polysomnography with incident adjudicated or self-reported AF (the proportion of adjudicated versus self-report AF is not stated) | AF occurred in 10.0% of those with AHI <15 and 14.2% of those with AHI ≥15 (adjusted OR 1.15 [95% CI 0.72–1.84]). There was no association of AF with obstructive AHI; however, a central apnea index ≥5 was present in 48 participants at baseline and the incidence of AF in this group was 22.9% (adjusted OR 2.34 [95% CI 1.14–4.77]) |
| Tung et al.726 | 2017 | 2b | Community-based cohort study | Of 6441 participants in the Sleep Heart Health Study, 3346 had repeat polysomnography after approximately 5 years. Of these 388 were excluded due to restrictions on data use and 46 due to prevalent AF at baseline, yielding an analytic sample of 2912 participants. | Association of OSA and central sleep apnea with incident AF based on parent cohort adjudication or 12-lead ECG at the time of the second polysomnogram; median follow-up was 5.3 years; OSA was classified based on the obstructive AHI (OAHI, obstructive apneas plus all hypopneas per hour of sleep) | AF incidence increased with OSA severity from 9.6% of those with OAHI <5% to 16.4% with AHI ≥30; however, adjusting for age, sex, race and BMI there was no positive association of OSA with AF. Central apnea index ≥5 was present in 74 participants at baseline, and the incidence of AF in this group was 25.7% (adjusted OR 1.71, 95% CI 0.89–3.30, increasing to 3.00, 95% CI 1.40–6.44, with further adjustment for hypertension, diabetes, and cardiovascular disease) |
| Kendzerska et al.723 | 2018 | 2b | Retrospective cohort study | 8256 patients without prior history of arrhythmia referred for diagnostic polysomnography 1) 2263 with AHI < 5 2) 2260 with 5 ≤ AHI < 15 3) 1823 with 15 ≤ AHI ≤ 30 4) 2263 with AHI > 30 |
Time to first hospitalization with a diagnosis of AF or atrial flutter, using Ontario Provincial Health Administrative Administrative Data, with median follow-up of 10 years | In unadjusted analyses, incident hospitalized AF increased with increasing severity of OSA based on either AHI or percent time at SaO2 < 90%; however, in multivariate models that included BMI these associations were not significant, with hazard ratios < 1.0 for each OSA severity compared to AHI <5. Only when comparing those with more than 30% of sleep time at SaO2 < 90% (n = 463) to those with less severe hypoxemia, was the adjusted HR significant (1.77, 95% CI 1.15–2.74). |
| Mazza et al.714 | 2009 | 1b | Clinic-based cohort study | 158 consecutive patients admitted for electrocardioversion for AF; all had polysomnography the night prior to cardioversion 1) AHI ≥15 (n = 49) 2) AHI <15 (n = 109) |
Recurrence of AF over 1-year follow-up | AF recurred in 69% of patients with AHI ≥15 and in 43% of those with AHI <15 (adjusted OR 3.04, 95% CI 1.45–6.36) |
| Matiello et al.727 | 2010 | 1b | Clinic-based cohort study | 174 consecutive patients undergoing pulmonary vein isolation procedure for AF; all screened with Berlin Questionnaire, and the 51 with score of 2–3 underwent home cardiorespiratory polygraphy 1) Low risk Berlin Questionnaire or AHI <10 on polygraphy (n = 132) 2) AHI 10–<30 (n = 17) 3) AHI ≥30 (n = 25) |
Recurrence of AF over mean follow-up of 17 (SD 11.5) months | Estimated 1-year recurrence free survival was 48.5% in the low-risk group, 30.4% in those with AHI 10–<30, and 14.3% in those with AHI ≥30. Adjusted OR for recurrence was 1.57 (95% CI 0.83–3.00) for AHI 10–<30 and 1.87 (95% CI 1.11–3.16) for AHI ≥30 |
|
Bitter et al.711 |
2012 | 1b | Clinic-based cohort study | 82 consecutive patients undergoing pulmonary vein isolation procedure for AF, 75 evaluable; all underwent in-hospital cardiorespiratory polygraphy 1) AHI <15 (n = 53) 2) AHI ≥15 (n = 22, of which 15 were predominantly obstructive and seven were predominantly central) |
Recurrence of AF over a median follow-up of 12 months | AF recurred in 45.5% of those with AHI ≥15 and 24.5% of those with AHI <15. The adjusted HR for AF recurrence in those with AHI ≥15 was 3.20 (95% CI 1.14–8.95) compared to those with AHI <15. Not analyzed separately by obstructive versus central pattern |
| Szymanski et al.709 | 2015 | 1b | Clinic-based cohort study | 290 consecutive patients admitted for pulmonary vein isolation procedure for AF; in-hospital cardiorespiratory polygraphy the night prior to the procedure; polygraphy was inadequate in 22 patients and ablation was not performed in 14; 3 patients with central sleep apnea excluded 1) AHI <5 (n = 136) 2) AHI ≥5 (n = 115) |
Recurrence of AF over a mean follow-up of 30 months | AF recurred in 65.2% of those with AHI ≥5 and 45.6% of those with AHI <5. AF recurrence increased progressively with more severe OSA, to 81.8% in those with AHI ≥30. The adjusted OR for AF recurrence in those with AHI ≥5 was 2.58 (95% CI 1.91–4.10) |
| Kawakami et al.713 | 2016 | 1b | Clinic-based cohort study | 133 consecutive patients admitted for pulmonary vein isolation procedure for AF; in-hospital cardiorespiratory polygraphy the night prior to the procedure 1) AHI <15 (n = 69) 2) AHI 15–<30 (n = 39) 3) AHI ≥30 (n = 16) |
Recurrence of AF over a mean follow-up of 13 (SD 7) months | AF recurred in 25% of those with AHI <15, 49% of those with AHI 15–<30, and 69% of those with AHI ≥30. However, in multivariate analysis, the association of AHI with recurrent AF was significant only in the subgroup with paroxysmal AF (adjusted HR 1.04, 95% CI 1.002–1.065) |
VII.A.6 |. Congestive heart failure
HF is highly prevalent in the US with 6.5 million adults affected.735 HF is associated with significant mortality and is identified as the cause of one in every eight deaths in the US.736 Failure of the left ventricle is the most common subtype and can manifest as impairment of either systolic (hFrEF) or diastolic (HF with preserved ejection fraction [hFpEF]) function.737 OSA is more common in the hFrEF population, with prevalence estimates varying between 12% and 53%.738 A study of 700 patients with chronic stable hFrEF with ejection fraction (EF) ≤ 40% found a 36% prevalence of OSA, including 19% with severe OSA.739
In addition to shared risk factors like age and obesity, another proposed mechanism to explain the high prevalence of OSA in HF relates to nocturnal fluid shifting throughout the body. Excess interstitial fluid from the lower extremities redistributes during sleep when in the supine position. This rostral fluid shift correlates with an increase in neck circumference, decrease in diameter of the lumen of the pharynx, and increased propensity for obstruction as demonstrated by increase in AHI.740
Identification of OSA may be especially important in the setting of hFrEF, where it is an independent risk factor for mortality.741,742 Although large confirmatory studies are lacking, there is interest in how OSA may adversely affect outcomes in patients with HF and a number of mechanisms have been proposed. HF is a state of sympathetic over-activity, so the autonomic imbalances associated with OSA in response to hypoxemia and repetitive arousals from sleep may generate concomitant physiologic stress. Additionally, large swings in intrathoracic pressure due to inspiratory effort against a closed UA exert transmural pressures across the heart and great vessels, leading to increasing afterload, reduced stroke volume and increased myocardial oxygen consumption.680
Considering these underlying pathophysiologic mechanisms, PAP therapy would be expected to have beneficial clinical outcomes in patients with HF. In addition to relieving obstruction of the UA in OSA and reducing work of breathing, PAP decreases venous return (preload), and may blunt sympathetic activity. These physiologic benefits, however, have not yet translated to improvements in clinical outcomes in patients with OSA and HF, which are limited to small, short-term RCTs. Some have shown improvements in physiologic parameters that are surrogates for CV outcomes, but results have not been consistent and it is uncertain if these improvements translate into a meaningful clinical benefit.
In small and select groups of patients with OSA and hFrEF, CPAP has been associated with reduced systolic BP743,744 and heart rate,743 small but inconsistent changes in left ventricular EF (LVEF),743–747 decreased overnight urinary norepinephrine excretion,746 improved QOL,743,746 improved mean sleep-related SaO2,745 and decreased pulmonary artery systolic pressure.747 There are a number of studies that have reported echocardiographic measurements of LVEF before and after CPAP. However, LVEF is but one assessment in the clinical diagnosis of hFrEF, and some would argue a minor determinant. Patil et al. performed an MA of five RCTs measuring LVEF by echocardiography or radionuclide ventriculography to compare the efficacy of CPAP against control conditions in patients with hFrEF. While some studies showed modest improvements in the absolute value of LVEF associated with CPAP, most reported changes (typically less than 5%) that would not be considered clinically significant.39
Although most available studies focus on hFrEF, OSA appears to also be prevalent in hFpEF. Presence of OSA in hFpEF has been associated with increased brain natriuretic peptide (BNP) levels, which may serve as a marker for reduced cardiac function.748 Severity of OSA has also been associated with increasing severity of diastolic dysfunction.749 One small RCT reported improvement in diastolic function in hFpEF with nasal CPAP but there was no difference in heart rate, systolic function, ventricular structure, BP, or urinary catecholamines after 12 weeks of CPAP use.750
In terms of other treatment modalities, a recent randomized study of patients with hFrEF and OSA demonstrated benefits of exercise (three months of aerobic and strength training) as a stand-alone intervention and as an adjunctive therapy to CPAP. AHI decreased significantly in the exercise alone group (28 ± 17 to 18±12, p < 0.007) and the exercise + CPAP group (25 ± 15 to 10 ± 16, p < 0.007) compared to controls. Both exercise and CPAP improved NYHA functional class and daytime sleepiness as measured by the ESS. QOL improvements were maximal in the exercise groups.751
Prospective data analyzing the association between OSA and HF and high-quality data regarding benefits of treatment in this population are lacking (Table VII.A.6a).654,752 Several societies have made recommendations regarding the testing and treatment of those with hFrEF and OSA but these recommendations are largely based on low-quality evidence (Table VII.A.6b). Current evidence does not support the routine treatment of non-symptomatic OSA with CPAP in patients with hFrEF as a means to improve CV outcomes. Those with symptoms of OSA, such as excessive daytime sleepiness, should be offered treatment, in accordance with joint guidelines from national HF societies and the AASM.753 (Table VII.A.6a and VII.A.6b)
TABLE VII.A.6a.
Association between OSA and heart failure
| Study | Year | LOE | Study design | Study group | Relevant clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Gottlieb et al.641 |
2010 | 2b | Prospective cohort 4422 subjects Followed for a median of 8.7 years |
Participants had OSA on PSG and no coronary heart disease or heart failure at baseline | Incident CHF CHF-free survival | Incidence of CHF increased with increasing severity of OSA After adjustment for age, race, smoking, and BMI, AHI was associated with CHF in men but not women |
| Hla et al.645 | 2015 | 2b | Prospective cohort of people in South-Central Wisconsin 1546 participants |
Participants had OSA on PSG and no documented CHD or CHF at baseline CHD and CHF was self-reported |
Composite outcome of incident CHD or heart failure | After adjustment for age, sex, smoking, and BMI, OSA was associated with increased incidence of CHD or heart failure Association of OSA with incident CHD or CHF was attenuated when participants who reported using CPAP were included in the analysis |
| Kasai et al.754 | 2008 | 2b | Prospective cohort in Tokyo, Japan 88 patients followed for mean of 25.3 ± 15.3 months |
Moderate to severe OSA with AHI≥15 LVEF<50% and NYHA class II or greater symptoms 65 were treated with CPAP and 23 were untreated |
Event-free survival | Cumulative event-free survival was higher in patients w/OSA on CPAP vs. patients w/OSA not on CPAP Cumulative event-free survival was significantly lower in patients with poor compliance than in those with good compliance |
| Wang et al.742 | 2007 | 2b | Prospective, single-center, observational cohort Patients referred to Mount Sinai Hospital in Toronto 218 subjects were followed for a mean of 2.9 ± 2.2 years 164 had complete data |
Heart failure for at least 6 months LVEF ≤ 45% at rest NYHA class II-IV dyspnea All underwent PSG and were divided into categories: AHI < 15 and AHI ≥ 15 Those with central sleep apnea were excluded Patients with OSA were divided into “treated” and “untreated” groups |
Cumulative rate of death | Mortality was higher in those with untreated OSA compared to treated OSA after adjusting for LVEF, NYHA class, and age |
| Kaneko et al.743 | 2003 | 1b | RCT 24 patients | Heart failure for at least 6 months LVEF≤45% at rest NYHA class II-IV dyspnea No exacerbations within 3 months Optimal medical therapy OSA with AHI of ≥20 with >50% obstructive events Half of the patients were treated with CPAP, the other half were treated only with medical therapy |
Cardiovascular physiologic parameters as measured by trans-thoracic echocardiography: LVEF, LVEDV, LVESV | Nocturnal CPAP improves daytime left ventricular systolic function in patients with heart failure and OSA |
Abbreviations: CHD, coronary heart disease; CHF, congestive heart failure; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; OSA, obstructive sleep apnea; PSG, polysomnogram.
TABLE VII.A.6b.
Guidelines for testing and treatment of OSA in hFrEF
| Author | Question | Statement | Class | Level of evidence |
|---|---|---|---|---|
| AHA/ACC/HFSA | Should people with | A formal sleep assessment | IIa (moderate | C-LD (Limited data, |
| Guidelines on Management of Heart Failure753 | hFrEF be tested for OSA? | is reasonable for people with suspicion of SDB or EDS | recommendation) | randomized or non-randomized observational, or registry studies with limitations of design or execution, meta-analyses of such studies, physiological, or mechanistic studies in human subjects) |
| Should Patients with hFrEF and OSA be treated with CPAP? | In patients with CVD and OSA, CPAP may be reasonable to improve sleep quality and daytime sleepiness | IIb (weak recommendation) | B-R (moderate quality evidence from one or more RCTs/meta-analysis of moderate quality RCTs) | |
| AASM Guidelines for Evaluation and management of OSA513; AASM Clinical Practice Guideline for OSA572; AASM guidelines for treatment of adult OSA with PAP39 |
Who should be evaluated for OSA? | Patients with comorbidities considered “high risk” for OSA including HF | N/A | N/A |
| Where should patients with suspected OSA and significant comorbidities (including HF) be tested? | Consider initiating PAP using an in-lab strategy | Strong Recommendation | N/A | |
| Should PAP be recommended or withheld in non-sleepy OSA patients to reduce cardiovascular events or mortality? | Insufficient evidence | No recommendation | N/A | |
| Initiation of PAP therapy | In patients with significant comorbidities, PAP initiation using an in-lab strategy should be considered | N/A | N/A |
Abbreviations: AASM, American Academy of Sleep Medicine; ACC, American College of Cardiology; AHA, American Heart Association; ASV, adaptive servoventilation; CSA, central sleep apnea; CVD, cardiovascular disease; EDS, excessive daytime sleepiness; HFSA, Heart Failure Society of America; NYHA, New York Heart Association; PAP, positive airway pressure; SDB, sleep-disordered breathing.
VII.A.7 |. Cerebrovascular disease
Cerebrovascular accident (CVA) or stroke is one of the leading causes of death and disability globally.755,756 OSA is highly prevalent (55%) among the stroke population and significantly increases the risk for incident ischemic stroke.756
Johnson and Johnson conducted an MA of 29 studies consisting of 2343 patients with ischemic or hemorrhagic stroke and TIA patients. They found OSA was present in 65% of ischemic/hemorrhagic stroke and TIA patients, with greater prevalence in male patients, patients with recurrent strokes, and patients with strokes of unknown etiology.613
Most strokes result from a reduction in cerebral blood flow to a specific region of the brain. The subsequent brain injury from blood–brain barrier dysfunction starts a series of inflammation, oxidative stress, excitotoxicity, and apoptosis.755
OSA increases the risk for stroke through a variety of factors leading to vascular damage in the brain. The repeated hypoxia can lead to endothelium damage and release of pro-inflammatory factors, such as plasma cytokines, tumor necrosis factor-alpha, and interleukin-6. This may ultimately cause vascular dysfunction by increasing endothelin, neurovascular oxidative stress, and increasing susceptibility to injury. Moreover, the associated large negative intrathoracic pressure swings could result in mechanical stress on the heart and valves. This can result in nocturnal apneic-related right to left shunting through patent foramen ovale and consequently increase risk of embolism and stroke.33
A cohort of 5422 participants without a history of stroke and untreated OSA were followed for a median of 8.7 years for incident stroke. Men with moderately severe OSA were at increased risk for incident ischemic stroke (hazard ratio [HR] 2.86; 95% CI 1.10–7.39) after adjusting for demographic variables (age, race) and CV risk factors (BMI, smoking, systolic BP, use of antihypertensive medications, and diabetes). Furthermore, there seemed to be a dose response relationship in which the risk of stroke increased 6% with every one-unit increase in AHI, suggesting as severity of OSA increased, risk of stroke also increased. In women, stroke risk was not significantly associated with obstructive apnea-hypopnea index (OAHI) quartiles or desaturation levels, but increased risk was seen at OAHI levels greater than 25. After a minimum threshold of 25 obstructive events/h is met, with unit increase, there is a 2% increase in stroke HRs. Interestingly, arousal index was a significant negative predictor of incident stroke in women. Women with arousal index >12 was associated with decreased hazard rate of ischemic stroke compared to women with lower arousal index.33 Future studies should further explore the protective role arousal index may play in incident stroke in women.
An MA was conducted of 12 prospective cohort studies that followed a total of 25,760 participants for major CV events, fatal and nonfatal stroke, coronary heart disease, and all-cause mortality. It was found that severe OSA is independently associated with an increased risk of stroke, CV disease, and all-cause mortality. Three of the 12 studies examined the relationship between severe OSA and risk of stroke. Patients with severe OSA had an increased risk for stroke (combined RR = 2.15, 95% CI: 1.42, 3.24).757
Another MA assessed the risk of cerebrovascular events among OSA patients in 15 prospective studies and 43 nonprospective cohort studies (includes cross-sectional studies, case–control studies, and prospective observational studies) to ascertain the prevalence of OSA among patients with cerebrovascular (CV) disease. OSA was found to significantly increase the risk of fatal and non-fatal cerebrovascular disease (pooled HR = 1.94, 95% CI: 1.31–2.89) after adjusting for confounders. Furthermore, OSA was shown to be highly prevalent (58.8%) among patients with cerebrovascular disease and there was greater prevalence with increasing age.758
It has been reported that CVA patients with OSA experience longer hospitalization and rehabilitation admissions and higher mortality rates compared to CVA patients without OSA. A prospective cohort study examined the relationship and prevalence of OSA in patients with acute ischemic stroke. Of the 174 patients with acute ischemic stroke, only seven had a past medical history of OSA. Those patients diagnosed with OSA prior to acute ischemic stroke experienced significantly worse functional outcome as measured by lower modified Rankin scale (mRS) scores at hospital discharge after adjusting for age and stroke severity.759 Untreated OSA can cause impaired cognitive function, decreased concentration, and excessive daytime sleepiness, which ultimately prolongs the hospitalization stay and compromises rehabilitation participation.760,761
Therefore, it is recommended to screen for OSA in all patients presenting with TIA or ischemic or hemorrhagic stroke regardless of whether they are symptomatic or asymptomatic. Earlier diagnosis of OSA and early treatment could improve overall health and cognitive status and reduce the risk of recurrent stroke and stroke mortality. (Table VII.A.7)
TABLE VII.A.7.
Association between OSA and cerebrovascular disease
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Birkbak et al.762 | 2014 | 2a | Systematic review | 10 studies with 1203 stroke and TIA patients were reviewed to examine relationship between sleep disordered breathing and recurrent stroke and mortality. | All-cause mortality, recurrent vascular events, and case fatality in stroke/TIA patients. | Obstructive sleep disordered breathing is a risk factor for recurrent vascular events and all-cause mortality in stroke/TIA patients. |
| Culebras and Anwar763 | 2018 | 2a | Systematic review | Review of cerebrovascular complications of OSA. | Factors contributing to CVD. | OSA is highly prevalent in the stroke population. OSA increases the risk of hypertension, stroke, myocardial infarction, and afib and is closely linked to vascular dementia. OSA may have worse neurological outcomes in acute stroke patients and have worse recovery in stroke rehab. |
| Dong et al.764 | 2018 | 2a | Systematic review | 37 studies with 3242 patients were reviewed to determine prevalence of OSA. | Prevalence of OSA in patients with CVD. | OSA is highly prevalent in patients with CVD. Prevalence of AHI > 5 was 70.4% and prevalence of AHI > 10 was 61.9%. |
| Johnson and Johnson613 | 2010 | 2a | Meta-analysis | 29 studies with 2343 ischemic or hemorrhagic stroke and TIA patients were reviewed. | Prevalence of sleep disordered breathing (SDB) in ischemic and hemorrhagic stroke and TIA patients. | SDB is very common in stroke patients regardless of type of stroke or timing after stroke and is usually obstructive in nature. |
| Li et al.765 | 2014 | 2a | Meta-analysis | 10 cohort studies investigating the effect of OSA on incident ischemic and hemorrhagic stroke. | Incident ischemic and hemorrhagic stroke. | There was a significant association between OSA and the risk of fatal or non-fatal stroke after adjustment of established cardiovascular risk factors. |
| Mansu-khani et al.759 | 2011 | 2b | Prospective cohort study | 174 consecutive patients presenting with acute ischemic stroke in Saint Mary’s Hospital ED between June 2007 and March 2008. | Functional outcomes measured using modified Rankin scale at discharge. | Previous diagnosis of OSA was an independent predictor of worse functional outcome. Patients with definitive diagnosis of OSA before stroke are at increased risk of death within the first month after an acute ischemic stroke. |
| McDermott and Brown766 | 2020 | 2a | Systematic review | Assessing the directional relationship between the association between OSA and stroke. | Prevalence of OSA in the poststroke patient population. | Sleep apnea is an independent risk factor for stroke. OSA is highly prevalent poststroke and is associated with worse outcomes after stroke. |
| Mohammad et al.756 | 2019 | 3a | Case–control study | 107 patients admitted with acute ischemic stroke who were categorized into two groups: those who woke up with stroke symptoms (WUS) and those whose stroke occurred while awake (NWUS). | Risk for OSA assessed using the Berlin questionnaire. | Risk for OSA is high among the stroke population, with a greater prevalence among the WUS group. OSA is an important risk factor for ischemic stroke during sleep. |
| Redline et al.33 | 2010 | 2a | Prospective cohort study | Community-based sample of 5422 male and female participants in the Sleep Heart Health study without a history of stroke and untreated for OSA were followed for a median of 8.7 years. | Incidence of ischemic stroke. | There is a strong association between ischemic stroke and obstructive AHI in community-dwelling men with mild to moderate OSA. In women, stroke was not significantly associated with obstructive apnea-hypopnea index (OAHI) quartiles or desaturation levels, but an increased risk of stroke was observed in women with OAHI levels greater than 25. |
| Seiler et al.767 | 2019 | 2a | Systematic review and meta-analysis | 29 studies with 7096 patients with ischemic/hemorrhagic stroke or TIA were reviewed to determine prevalence of sleep disordered breathing. | Severity and prevalence of SDB. | Sleep disordered breathing is highly prevalent in patients after stroke and TIA. 70% of patients have an AHI > 5/h and 33% of patients have AHI > 30/h. |
| Wang et al.757 | 2013 | 2a | Meta-analysis | 12 prospective cohort studies with 25,760 participants were reviewed. | Incident fatal and non-fatal coronary heart disease, incident fatal and non-fatal stroke, and all-cause mortality. | Severe OSA significantly increases CVD risk, stroke, and all-cause mortality. A positive association with CVD was observed for moderate OSA but not for mild OSA. |
| Wu et al.758 | 2017 | 2a | Meta-analysis | 58 studies to determine prevalence of OSA among CV patients and risk for cerebrovascular events. | Prevalence of OSA | OSA is prevalent (58%) in patients with CV disease. As age increases, the prevalence of OSA increases. OSA is significantly increases risk for fatal or non-fatal CV disease. |
VII.B |. Comorbidities Associated with OSA: Pulmonary Disorders
VII.B.1 |. Primary pulmonary hypertension
PH, defined as a mean pulmonary artery pressure (mPAP) >20 mmHg,768 is a pathophysiological disorder769 that may be of primary origin or a sequalae of clinical conditions most notably CV and respiratory diseases.
The relationship between OSA and PH was first noted in 1976 by Tilkian et al.770 Few studies have detailed the link between World Health Organization (WHO) group 1 pulmonary arterial hypertension (PAH) and OSA, or the effects of treatment of OSA on hemodynamic variables in adult patients747,771–777 (Table VII.B.1). Although the mechanism behind PH associated with OSA is not entirely understood, it is postulated to be due to a combination of factors including pulmonary arteriolar remodeling, susceptibility to hypoxia, and underlying left heart disease.773 A study of WHO group I PAH patients showed there was no significant difference in mortality in patients with and without OSA; however, mortality was significantly higher in patients with nocturnal hypoxemia, defined as an average SpO2 < 90%, suggesting that duration and severity of nocturnal oxygen desaturation, well known to occur in OSA patients, is an important risk factor for development of PAH.776
TABLE VII.B.1.
Association between OSA and pulmonary hypertension
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Minic et al.775 | 2014 | 2b | Retrospective cross-sectional | Patients with group 1 PAH (n = 52) | Prevalence and clinical predictors of SDB and impact on outcomes | SDB present in 71% of PAH patients; 60% had significant hypoxemia (>10% of total sleep time at <90% oxyhemoglobin saturation). |
| Minai et al.774 | 2009 | 2b | Prospective cohort | OSA patients undergoing right heart catheterization for suspected PH | 1) Predictors of PAH in OSA patients 2) Survival in patients with OSA and PAH |
Female gender, younger age, obesity, and nocturnal desaturation were associated with development of PH and OSA patients. PH increases mortality in patients with OSA. |
| Imran et al.772 | 2016 | 2a | Systematic review | Patients with PH and OSA on CPAP (n = 222) | Mean pulmonary artery pressure | CPAP therapy is associated with a significantly lower PA pressure in patients with isolated OSA and PH (13.3 mmHg; 95% CI 12.7–14.0). |
| Sun et al.777 | 2016 | 2a | Systematic review | Patients with OSA on CPAP (n = 181) | Mean pulmonary artery pressure | CPAP therapy was associated with a statistically significant reduction in pulmonary artery pressure in patients with OSA |
| Arias et al.771 | 2006 | 2b | RCT (cross over) | 1) Severe OSA (n = 23) 2) Healthy controls (n = 10) |
Pulmonary artery systolic pressure | CPAP therapy was associated with a decrease in PASP in patients with PAH and OSA (28.9 + 8.6 to 24.0+5.8 mmHg; p < 0.0001). |
| Sharma et al.747 | 2019 | 2b | RCT | 1) Patients with OSA and heart failure (n = 11) 2) Patients with OSA and heart failure on CPAP (n = 10) |
Pulmonary artery systolic pressure | CPAP therapy was associated with a decrease in PASP in patients with PAH and OSA (58.6 ± 2.5 to 42.8 ± 2.7 mmHg; p = 0.025). |
Abbreviations: AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension; PSG, polysomnography; RCT, randomized controlled trial; RHC, right heart catheterization; SDB, sleep disordered breathing.
The prevalence of OSA in PAH and vice versa is not well elucidated. A study by Minic et al. showed that SDB (OSA, CSA, hypoventilation) was present in the majority of patients with PAH, with more than half of patients having significant nocturnal hypoxemia.775 Other reported risk factors including female gender, younger age, obesity, and nocturnal desaturations are thought to increase the risk of developing PAH in OSA patients.774
Although data on the effects of CPAP, the preferred treatment of OSA, on hemodynamic variables have been inconsistent, two SRs reported that CPAP therapy is associated with a reduction in mPAP in patients with OSA and PH.772,777 Another study showed that CPAP induced significant improvements in echocardiographic parameters, most notably pulmonary artery systolic pressure (PASP), further highlighting CPAP use and its positive effects on hemodynamic variables.771 Lastly, it was found that in patients with PH admitted for acute decompensated HF, the addition of 48 h of CPAP to standard care improved LVEF and significantly reduced PASP.747
In summary, there is some indication that survival for OSA patients with PH may be lower than that for OSA patients without PH.774 Based on limited studies, CPAP appears to improve hemodynamic variables including mPAP and PASP in patients with combined OSA and PAH; however, how these positive effects translate to meaningful clinical outcomes are still unclear. (Table VII.B.1)
VII.B.2 |. Chronic obstructive pulmonary disease (COPD)
The concurrent diagnosis of OSA and COPD in an individual is known as overlap syndrome (OS).778 OS is associated with a more severe clinical course compared to either disease alone.779–782 Shawon et al. conducted an SR to determine the prevalence and clinical outcomes of OS and reported a significantly higher prevalence of OS in patients with either OSA or COPD compared to the general population.783 OS patients experienced greater degree of nocturnal oxygen desaturation (SpO2 < 90%) and lower sleep efficiency compared to OSA patients. OS was also associated with increased CV complications (PH, AF, right ventricular dysfunction), COPD exacerbations, hospitalizations, and poorer QOL compared to either disease alone. (Table VII.B.2a)
TABLE VII.B.2a.
Prevalence and outcomes in OSA with coexisting COPD (overlap syndrome)
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Shawon et al.783 | 2016 | 2a | Systematic review | 1) General and hospital populations 1. COPD 2. OSA (n = 29,341; age 40–90) |
1) Prevalence of overlap syndrome 2) Clinical endpoints of overlap: cardiovascular disease, pulmonary hypertension, resistant hypertension, exacerbation of COPD, mortality, quality of life, hospitalization rate |
Overlap syndrome: High prevalence of overlap in either OSA (7.6%–55.7%) or COPD (2.9%–65.9%) population compared to general population (1.0%–3.6%). Overlap syndrome has greater nocturnal oxygen desaturations, reduced mean peripheral capillary oxygen saturation (SpO2) and increased sleep time spent with SpO2 < 90% (T90) and worse sleep quality than patients with only OSA. Overlap syndrome is associated with more frequent cardiovascular morbidity, poorer quality of life, more frequent COPD exacerbations and increased medical costs. |
| Donovan et al.779 | 2019 | 2b | Prospective cohort study | Participants from LOTT study (Long-term Oxygen Treatment Trial) with COPD assessed for OSA risk by STOP-BANG questionnaire Intermediate-to-high risk (score > 3) Low risk (score < 3) |
Composite of death First hospitalization Overall incidence of COPD exacerbations | High percentage of COPD patients were at intermediate to high risk of undiagnosed OSA based on modified STOP-BANG criteria and were associated with greater risk of poor outcomes. |
| Sanders et al.781 | 2003 | 2b | Prospective cohort study | Participants from Sleep Heart Health Study (community based), using polysomnography and spirometry results | 1. Determine association between OAD and SAH 2. Identify predictors of nocturnal oxyhemoglobin desaturation in OAD with and without SAH |
1. No association between mild OAD and SAH. 2. Independent of SAH, FEV1/FVC < 65% is associated with more severe nocturnal oxygen desaturation. 3. Nocturnal oxygen desaturation is greater in combined OAD and SAH compared with either disease alone. 4. Mild OAD patients without OSA have minimally disturbed sleep. |
| Du et al.780 | 2018 | 2b | Cross-sectional study | NHANES database (2005–2008) No COPD or OSA OSA COPD Overlap Syndrome |
Evaluate factors associated with all-cause mortality | Overlap syndrome and COPD were associated with higher all-cause mortality compared to neither disease and OSA alone. |
| Starr et al.782 | 2019 | 4 | Retrospective cohort study | Enrollment and claims data 2004–2013 from a 5% national sample of Medicare beneficiaries with COPD and Overlap Syndrome | Examine the diagnosed prevalence, trend, and patient characteristics of overlap syndrome | Prevalence: 11.0% of the COPD cohort had coexisting OSA compared with patients with COPD alone. Trend: younger age and male gender have a higher number of comorbid conditions and have more complex COPD. Over the 10-year study period, overlap syndrome increased from 4.04% in 2004 to 17.80% in 2013 based on visit for OSA. |
Abbreviations: COPD, chronic obstructive pulmonary disease; OAD, obstructive airway disease; OSA, obstructive sleep apnea; SAH, sleep apnea hypopnea.
VII.C |. Comorbidities Associated with OSA: Obesity
Obesity is the most common risk factor for OSA, diagnosed in more than 70% of OSA patients.784 Weight gain has been associated with greater SDB severity in both observational and intervention studies.189,191,785 The literature linking obesity with OSA is challenged by the sheer number of confounders among patients with metabolic disease, including behavioral variables (activity level, dietary indiscretion, and medication compliance), access to care, and socioeconomic status.
The relationship between excess weight and OSA is likely reciprocal. OSA fragments sleep, leading to chronic insufficient sleep and excessive daytime sleepiness. These factors lead to reduced activity levels as well as increased appetite, with resultant weight gain.786 Patients with OSA appear to be more susceptible to weight gain compared to similarly matched obese counterparts with significant weight gain reported in the year leading up to a diagnosis of OSA.787,788 Dysregulation of leptin, with adverse implications for weight loss, body fat storage, and distribution, is well documented in obese patients with OSA.788,789 Among a cohort of subjects with morbid obesity and OSA who underwent bariatric surgery, long-term weight loss outcomes were worse among those that did not continue to use CPAP postoperatively.790
Although untreated OSA is associated with weight gain, CPAP adherence has not been associated with weight loss and may even lead to weight gain.791–794 A number of theories have been put forth to explain the phenomena of weight gain following initiation of CPAP. Tachikawa et al. proposed that CPAP leads to a small reduction in basal metabolic rate, likely by eliminating additional energy expenditure due to breathing against an obstructive airway.795 Stenlof et al. have also found a reduction in energy expenditure with CPAP therapy.796 It is unclear if, and how, these reductions in energy expenditure translate to weight gain or inability to lose weight. Some studies have shown that those who gained weight with CPAP failed to record an increase in daytime activity and had more disordered eating behaviors.797,798 Others postulate that a resurgence of slow wave sleep following treatment of OSA may lead to increases in growth hormone and subsequent weight gain.795,799,800 These explanations are all speculative, and the best use of the information at present is tempering messages to patients that treatment of OSA will lead to a reduction in weight. While there are a number of meaningful outcomes from CPAP, including improvements in mood and QOL, a linear relationship with weight is unclear.
Impact of obesity on the management of OSA
Obesity exhibits both a mechanical and neurophysiologic effect on UA patency. Adipose deposition in the UA leads to a smaller lumen and increased collapsibility, both of which predispose to apnea.801 In addition, fat deposits around the thorax and abdomen reduce chest wall compliance and diminish functional residual capacity, which both increases oxygen demand and contributes to greater nocturnal hypoxia, especially among apneic patients.802
The critical closing pressure, Pcrit, is determined by mechanical and neural factors that regulate pharyngeal collapsibility. In obese patients an elevated Pcrit is due to UA adipose deposition.803 Obesity can also lead to reduced lung volumes, further compromised during supine sleep. These factors make OSA more common in obese individuals, contributing to increased severity of disease, longer apneic episodes, and more hypoxemia. These factors also reduce the efficacy of OSA-specific therapies.
PAP therapy remains the most commonly prescribed treatment for OSA. While efficacious, the treatment response may be reduced in obese patients, who tend to have greater residual AHIs compared with their non-obese counterparts. In addition, therapeutic pressures required to maintain UA patency are generally higher in obese patients.804 Not surprisingly, weight loss can lead to a reduction in required PAP pressures.805
Intuitively, the increased pressure requirements and diminished treatment response seen in obese patients may be expected to contribute to poor adherence. Interestingly, adherence rates are not lower among those with obesity, with some studies even demonstrating superior adherence in this population.793,806
Oral appliances (OAs) can provide both efficacious and effective treatment for patients with OSA, including those who are obese.807–810 However, similar to PAP, the therapeutic effect and likelihood of achieving successful therapy are decreased in obesity. The efficacy of OAs, as measured by the reduction in AHI, decreases with increasing weight in a non-linear relationship. Evidence shows that OAs are more efficacious and have a greater responder rate among those with lower BMIs.808,810,811 Likewise, the odds of having a high residual AHI are greater in those with increased weight, particularly among those with morbid obesity. As such, many consider morbid obesity to be a relative contraindication for OA therapy, although the use of OA as adjunct therapy requires further evaluation.
Surgical procedures specifically intended to reduce or eliminate SDB can present a treatment option for patients with OSA, particularly those unresponsive or intolerant to PAP therapy. The increased deposition of adipose tissue and greater collapsibility of the UAs commonly seen in obese patients may decrease the probability of a successful surgical outcome. Both the treatment effect and probability of successful therapy with UPPP decrease with increasing BMI.812 In addition, higher BMI is associated with greater risk for postoperative complications.813 Increased BMI may be accompanied by increased adiposity in the tongue base, leading to multilevel collapse of the UA necessitating multiple or staged surgical interventions. While this decreased probability for successful treatment does not necessarily negate surgery as a treatment option for obese patients, it does need to be weighed against the increased associated perioperative risks and higher chance for eventual recurrence of OSA. UA staging systems have been developed to help develop individualized approaches.814 Like other UA surgical procedures for OSA, HNS can provide a treatment option for those intolerant of PAP therapy. However, this therapy is only approved for a limited BMI range.815
A determination of whether untreated OSA leads to obesity is complicated by factors which are difficult to study in a controlled fashion, notably patient behaviors and adherence to interventions. The current LOE precludes a determination of whether OSA causes obesity and whether OSA therapies improve obesity.
VII.D |. Comorbidities Associated with OSA: Insulin Resistance
Understanding the relationship between OSA and metabolic disorders is of paramount importance for global health. OSA and metabolic disease are common bedfellows with obesity and associated comorbidities. The global impact is striking as 400–700 million individuals have diabetes or metabolic risk factors for the future development of diabetes, with healthcare costs in the tens of billions of dollars. These rates are arguably on track to double in the next 20 years.816,817
SDB is highly prevalent among individuals with impaired insulin sensitivity and diabetes, although underlying mechanisms are nebulous. Many studies control for variables such as age, gender, BMI, waist circumference, and race.657,818,819 Unfortunately, other relevant factors are generally ignored (activity level, exercise, diet, socioeconomic status, access to healthcare, and adherence with medical treatment). Potential pathophysiological mechanisms for a relationship between OSA and metabolic disease tend to be based on animal studies or uncontrolled cohorts. As a result, although a role for OSA in diabetes is compelling, the rigor of available evidence is not available for developing evidence-based conclusions or recommendations.
Among patients with OSA the available literature has shown a broad prevalence of prediabetes (20%–67%), based on impaired fasting glucose and impaired glucose tolerance testing.820 A linear correlation between OSA severity and insulin resistance has been documented with greater severity at diagnosis predicting the risk of incident diabetes.821,822 In a historical cohort of over 8000 patients, Kendzerska et al. found that those with an AHI of greater than or equal to 30 events/h had a 30% higher hazard of developing diabetes compared to those with AHI < 5 events/h.822 Aside from overall severity, severity in REM and time with an SaO2 less than 90% also increased risk of diabetes.
The mechanisms by which OSA may increase insulin resistance and impair glucose tolerance remain unclear. Both animal and human studies have linked intermittent hypoxia to insulin resistance.823,824 An MA by Iftikhar et al. evaluated 16 case–controlled studies to evaluate the association between OSA and insulin resistance. This study found a significant relationship for increasing HOMA-IR with baseline BMI, but not with age, AHI, or gender.656 Other studies have shown independent correlations between hypoxemia (increased ODI, increased time spent with SpO2 < 90%) and sleep fragmentation to higher glucose and insulin concentrations and insulin sensitivity.816,825
Ongoing research continues to evaluate the role of hypoxia and a pro-inflammatory state, focusing on intermittent hypoxia and increased adipose tissue lipolysis. Murphy et al.826 provided evidence in mice that intermittent hypoxia led to a pro-inflammatory phenotype of adipose tissue. In humans, OSA was associated with increased spontaneous lipolysis that correlated with the severity of OSA as well as insulin resistance and impaired insulin secretion in patients with type 2 DM.817 In a large-scale cross-sectional study, a significant positive interaction was observed between the severity of OSA and decreased lipoprotein(a) concentration.827 Several authors have outlined a role for episodic hypoxia during apneic events promoting increased adipose lipolysis and levels of free fatty acids systemically which have a pro-inflammatory effects leading to dysregulated glucose homeostasis and reduced insulin sensitivity.817 Building a link between underlying pathophysiologic mechanisms between OSA and metabolic disease may be confounded by heterogeneity in how metabolic disorders present clinically and deciphering the impact of OSA as opposed to the role of sleepiness, age, gender, and obesity.828 At this time, potential mechanisms for a causative relationship between OSA and metabolic disease are only hypothesis generating. There is not published evidence to date that firmly establishes a role for untreated OSA as a contributor to diabetes.
VII.E |. Comorbidities Associated with OSA: Cognitive Impairment and Dementia
VII.E.1 |. OSA and cognitive performance
Several quantitative reviews evaluating clinical studies have found that OSA is associated with deficits in cognitive performance. Two meta-reviews have examined the quantitative reviews, and both identified associations between OSA and cognitive deficits across many domains.829,830 Cognitive domains commonly found to be negatively impacted by OSA include attention, vigilance, executive function, processing speed, and subdomains of memory. Although the majority of meta-analyses have demonstrated cognitive deficits in patients with OSA compared to healthy controls, these results have been variable and somewhat inconsistent (Table VII.E).
TABLE VII.E.
Association between OSA and cognitive impairment
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Olaithe et al.830 | 2018 | 2a | Meta-review | 7 OSA meta-analyses (2003–2016): Individuals with OSA and healthy controls. Also included were 5 COPD studies, 2 insomnia studies, 4 sleep deprivation studies. |
Cognitive deficits in OSA, COPD, insomnia, or sleep deprivation. | Cognitive deficits in OSA include attention, memory, executive function, psychomotor function, visuospatial, and language abilities. Small to large effects were found in most studies across most domains. |
| Bucks et al.829 | 2013 | 2a | Meta-review | 5 quantitative reviews (2001–2012): older adults (≥50 years) with OSA vs. controls/norms. | Domains of cognitive function in OSA. | OSA is associated with deficits in attention/vigilance, delayed verbal and visual long-term memory, visuospatial/constructional abilities, and executive function, but does not appear to affect language ability or psychomotor function. The data are equivocal for working memory, short-term memory, and global cognitive functioning. Treatment with CPAP appears to improve executive dysfunction, delayed long-term verbal and visual memory, attention/vigilance, and global cognitive functioning. |
| Bubu et al.832 | 2020 | 2a | Systematic review | 68 studies (cross-sectional, longitudinal, and RCTs, 1985–2019). Studies were stratified based on age: young/middle-aged (30–60 years) or older (>60 years) adults. |
1. OSA and cognitive performance/impairment. 2. OSA and subsequent development of mild cognitive impairment/dementia. 3. OSA and biomarkers of Alzheimer disease. |
1. OSA and cognition: In young and middle-aged adults, OSA is often associated with cognitive impairment, although unclear whether OSA precedes cognitive decline. CPAP treatment improves certain cognitive domains. In older adults, the OSA-cognition associations are variable and depend on study type and setting. 2. OSA and MCI/dementia: lack of longitudinal studies for young/middle-aged adults and more research is needed. In older adults, OSA is often associated with the development of MCI/AD. CPAP treatment improved sleep quality and cognitive function in AD patients with OSA. 3. OSA and biomarkers of AD: in middle-aged and older adults, there is an association between OSA and biomarkers of AD pathology in cross-sectional studies. Limited prospective studies show contrasting associations. |
| Wang et al.841 | 2020 | 2a | Systematic review/review | 40 studies (cohort, case study, RCT). | 1. Cognitive impairments associated with OSA. 2. OSA and Alzheimer disease pathological markers. 3. Cognitive performance with CPAP treatment. |
1. Individuals with OSA had worse cognitive performance than controls in domains of attention, executive function, intelligence, memory, psychomotor speed, and alertness. 2. AD pathological markers appear to be increased in OSA. 3. CPAP therapy could improve cognitive impairments in OSA, particularly if duration of therapy is >4 weeks. |
| Mubashir et al.834 | 2019 | 3a | Systematic review | 5 studies (4 cross-sectional, 1 retrospective cohort, 2011–2015) reporting the prevalence of OSA among adult patients (>18 years) with mild cognitive impairment (MCI). | 1. Prevalence of OSA in MCI. 2. Risk of OSA in MCI. |
1. There is a highly variable prevalence of OSA in MCI population (11%–71%), compared to 4%–70% in included control groups. 2. One study recruiting from memory clinics showed >3-fold risk of OSA in patients with MCI compared to control group. There were no differences in risk of OSA between MCI and control groups in the other included studies with control populations. |
| Zhu and Zhao831 | 2018 | 2a | Meta-analysis | 6 cohort studies (2011–2016): 19,940 adults ≥18 years, with and without sleep-disordered breathing. | Association between sleep-disordered breathing and the incidence of cognitive decline. | Baseline SDB is independently associated with risk of cognitive decline (higher risk of mild cognitive impairment compared with dementia). Stratified analyses suggest gender difference (higher risk for incidence of cognitive decline in females, not males). |
| Cross et al.842 | 2017 | 3a | Systematic review and meta-analysis | 13 studies (cross-sectional, case–control, 1985–2016): 5104 adults, ≥50 years, with or without OSA. |
Effect of OSA on neuropsychological performance. | There was a small negative association between OSA and all combined neuropsychological outcomes, but this association may be due to publication bias (calculations accounting for bias resulted in a null association). Association between OSA and cognition in older age is highly variable and depends on type/setting of study. |
| Stranks et al.843 | 2016 | 3a | Meta-analysis | 19 studies (1987–2012) of adult OSA patients vs. healthy controls. | Cognitive function of OSA patients. | For OSA patients, statistically significant negative effect sizes were found in the domains of non-verbal memory, concept formation, psychomotor speed, construction, executive functioning, perception, motor control and performance, attention, speed of processing, working and verbal memory, verbal functioning, and verbal reasoning. The domain of perception was not impaired. Non-verbal memory, concept formation, and psychomotor speed were most impaired. |
| Emamian et al.833 | 2016 | 3a | Meta-analysis | 5 cross-sectional studies (1983–1989) of Alzheimer disease patients vs. healthy controls. | Odds ratio for OSA in Alzheimer disease vs. healthy control. | Patients with AD had a 5× higher chance of presenting with OSA than healthy age-matched individuals (aggregate odds ratio was 5.05 and homogeneous). |
| Vaessen et al.844 | 2015 | 3a | Systematic review | 12 studies (9 case–control, 1 longitudinal uncontrolled, 1 descriptive uncontrolled, 1 cross-sectional) with adult patients with OSA vs. controls or non-OSA population. | Cognitive complaints in untreated OSA. | Concentration complaints were increased in untreated OSA patients compared to primary snorers and healthy controls. Memory and executive function may be similarly increased, however insufficient data precluded firm conclusions. Cognitive complaints may be related to higher levels of subjective sleepiness. The authors stress the importance of the difference between subjective cognitive complaints and objective cognitive impairment. |
| Kilpinen et al.845 | 2014 | 3a | Systematic review | 44 studies: comparison of OSA patients to healthy controls. | Information processing speed. | Information processing speed was reduced in OSA patients in half of the studies. Reduced information processing speed was seen 75% of the time when compared to norm-referenced data. CPAP treatment improved processing speed marginally when compared to placebo/conservative treatment. |
| Wallace and Bucks846 | 2013 | 2a | Meta-analysis | 42 studies: 2294 adults with untreated OSA, and 1364 healthy controls. | Tasks associated with episodic memory (immediate recall, delayed recall, learning, and/or recognition memory). | Compared to healthy controls, adults with OSA had significant impairment in verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuo-spatial episodic memory (immediate and delayed recall), but not visual immediate recall or visuo-spatial learning. Compared to norms, adults with OSA had significant impairment only in verbal immediate and delayed recall. |
| Saunamäki and Jehkonen847 | 2007 | 2a | Systematic review | 40 studies assessing executive function in OSA. | Domains of executive function. | Executive function domains that were most adversely affected in OSA include working memory, phonological fluency, cognitive flexibility, and planning. CPAP improved performance times, cognitive flexibility, and planning, but did not significantly improve deficits in working memory and phonological fluency. |
| Aloia et al.848 | 2004 | 2a | Systematic review | 37 studies (1985–2002) | Pattern of cognitive deficits in OSA, cognitive domains improved by treatment, possible mechanisms of cognitive dysfunction. | Findings were equivocal for most cognitive domains. Attention/vigilance, executive function, and memory were impaired in the majority of reviewed studies (6/8, 6/9, and 7/11 studies, respectively). Treatment improved attention/vigilance in most studies and did not improve constructional abilities or psychomotor function. |
| Beebe et al.849 | 2003 | 2a | Meta-analysis | 25 studies: 1092 patients with OSA and 899 healthy controls. | Neuropsychological outcome domains in untreated OSA. | Untreated OSA had significant impact on vigilance and executive functioning, and negligible impact on intellectual and verbal functioning. Data were mixed for visual and motor functioning, and memory functioning. |
| Fulda and Schulz850 | 2003 | 3a | Meta-analysis | 54 studies (1985–2000): 1635 SRBD patients compared with 1737 controls. | Cognitive dysfunction in SRBD. | Compared with controls, SRBD patients demonstrated: Moderate to large reductions in mental flexibility, visual delayed-memory retrieval, and driving simulation performance. Small to moderate reductions in focused and sustained attention, verbal delayed-memory retrieval, verbal fluency, and composite measures of general intellectual functioning. No difference in divided attention, concept formation and reasoning, and verbal or visual immediate-memory performance. Other domains not assessed due to insufficient data or between-study heterogeneity. |
VII.E.2 |. OSA and mild cognitive impairment (MCI)/dementia
The association between OSA and MCI/dementia is a growing area of research. A large MA of 19,940 patients has shown that SDB at baseline is associated with risk of cognitive decline.831 A recent SR of 68 studies found that OSA is often associated with the development of MCI and Alzheimer’s disease (AD) in older adults,832 and that OSA is often associated with cognitive impairment in young and middle-aged adults. An MA showed that patients with AD had a 5× higher chance of presenting with OSA than healthy age-matched individuals.833 In MCI patients, the association is less clear, with a highly variable prevalence of OSA (11%–71%) based on OSA diagnostic methods and patient recruitment locations834 (Table VII.E).
Several cross-sectional, cohort, and case–control studies examined the relationship between OSA and dementia. Cross-sectional studies showed high rates of OSA among dementia patients.835,836 One recent study found a very high prevalence of OSA in patients with mild to moderate AD (116 of 128 patients, or 90.6%).835 In a retrospective cohort study, patients with SDB were 1.58 times more likely to develop AD than those without SDB.837 Large cohort studies focusing on OSA and dementia appear inconsistent. A prospective cohort study from the SHHS found that elderly cognitively-normal (mean age = 76.9 years, n = 208) individuals with OSA were found to have increased risk of dementia compared to those without OSA, independent of age, gender, and ApoE4 allele status.838 In contrast, the Atherosclerosis Risk in Communities Study, a 15-year prospective cohort study, found that sleep apnea severity and nocturnal hypoxemia were not associated with cognitive decline.839 In another analysis of the same cohort, OSA was not associated with risk of incident dementia; however, when adjudicated outcomes were used, severe OSA was associated with risk of dementia in later life.840
Overall, most evidence suggests that OSA may represent a modifiable risk factor for dementia, but conflicting and inconsistent data exist. (Table VII.E)
VII.F |. Comorbidities Associated with OSA: Cancer
The epidemiological association between OSA and cancer was first published in 2012, and since then, a total of 21 studies have been published [PubMed]. The consensus is that higher OSA severity (e.g., more hypoxic burden) is associated with an increase in cancer risk.
Two population-based epidemiological studies with long-term (>20 years) follow-up reported a significant association between OSA severity and all cancers.851,852 Five epidemiologic studies with a shorter (<20 years) follow-up also reported a significant correlation between OSA severity and all cancers. These include increased cancer incidence853 and increased cancer mortality in patients <65 years old with severe OSA854; an increased cancer incidence in patients <45 years old with severe OSA855; an increased cancer prevalence in women with OSA, but not in men856; and a higher overall incidence of cancer in a veteran population with OSA.857 Two shorter follow-up studies reported no association between OSA and all cancers.858,859 However, when epidemiological studies assessed an association of OSA and specific cancers, results were heterogeneous but support a higher incidence of breast cancer,858 primary central nervous system cancers,860 nasal and prostate cancer,861 colorectal cancer,862 and melanoma, kidney, breast, and uterine cancer.863
VII.F.1 |. Risk factors
VII.F.1.a |. Changes in upper airway anatomy
There is a strong increased incidence of OSA in patients with head and neck cancer – both on presentation864 and after radiation therapy,865 likely related to changes in craniofacial anatomy.
VII.F.1.b |. Increased hypoxic burden
Increased hypoxic burden, specifically, cyclical intermittent hypoxia (CIH) is hypothesized as the primary mechanism on OSA and increased cancer risk. Higher OSA severity as measured by PSG, specifically those related to an increased hypoxic burden (e.g., T90), has been associated with melanoma aggressiveness,866 pancreatic cancer progression,867 lung cancer prevalence,868 and cancer mortality.869 In addition, potential mechanisms that associate hypoxic burden with increased cancer risk include: significantly reduced levels of circulating invariant natural killer T cells and function (in OSA patients without cancer),870 alterations of molecules related to HIF1 (in OSA patients with lung cancer),868 increased microRNAs involved with some cancer types (miR-1254 and miR-320e in OSA patients without cancer),871 and upregulation of circulating TGF-β1, VEGF, and Foxp3 + Tregs (in OSA patients with lung cancer).872 To date, there have not been studies specifically assessing the effect of CPAP modifying cancer risk.
VII.F.2 |. Potential mechanisms
In vitro models have provided two main findings: (1) CIH results in changes that are distinct from sustained hypoxia; (2) CIH results in molecular changes that promote cancer progression or metastases. Most CIH mechanisms that have been explored center around stabilizing HIF1873 and measuring downstream effects. Examples of these downstream effects are conversion to cancer stem cell properties,874 increased NOX1 subunit of NADPH oxidase,875 increased monocyte polarization toward a tumor-promoting phenotype,876 glycolysis,873 and extracellular matrix remodeling.877 Other studies focused on why cancer cells exposed to CIH may be more resistant to therapy. These mechanisms include a decrease in topo alpha mRNA and protein,878 changes in proteasome function,879 and small interfering RNA.880 Lastly, CIH may increase expression of genes associated with metastases,881 and cancer cell migration.882
VII.F.2.a |. Animal models
Animal models have demonstrated that CIH increases cancer progression.883 Although most mouse models placed the cancer in the flank (considered to be inherently more hypoxic884 than typical primary sites such as the lung) important findings have emerged: CIH accelerates tumor growth,885 increases tumor angiogenesis,886 increases metastases,887 reduces immunosurveillance,888 increases spontaneous tumorigenesis,889 and increases molecular markers of tumor aggression including PD-L1.890
These exciting areas of clinical, in vitro, and animal studies demonstrate an association of CIH, one of the common features of OSA, to cancer risk. This association could lead to an OSA subtype that would benefit from aggressive screening and treatment, and may identify novel cancer targets.
VII.G |. Comorbidities Associated with OSA: Nasal Disorders
Nasal obstruction or congestion can be caused by both inflammatory and structural factors. Common inflammatory diseases causing nasal obstruction include rhinitis, sinusitis, and nasal polyps. Structurally, obstruction may occur at the level of the external or internal nasal valves, septum, and inferior turbinates. Nasal obstruction, AR, and chronic sinusitis have repeatedly been demonstrated as significant risk factors for habitual snoring, chronic excessive daytime sleepiness, and SDB.69,891–893
VII.G.1 |. Allergic rhinitis
Several studies have specifically investigated the relationship between AR and SDB and have demonstrated that AR negatively impacts sleep quality and successful treatment with nasal corticosteroids and/or montelukast improves sleep disturbance. In a population-based study of individuals enrolled in the Wisconsin Sleep Cohort Study, chronic severe nasal congestion was identified as an independent risk factor for habitual snoring and participants with nasal congestion due to allergy were 1.8 times more likely to suffer from moderate to severe SDB.69,893 In a cohort study of AR and non-AR patients, seasonal AR was associated with increased daytime sleepiness and QOL impairment.892 While data on the effects of AR on polysomnogram (PSG) parameters remains mixed, medical management of AR has been demonstrated to improve PSG parameters such as improved O2 nadir, and supine AHI levels.894 Intranasal corticosteroids have been shown to improve congestion and daytime somnolence in patients with perennial rhinitis. Pooled data from three double-blind crossover RCTs of budesonide, flunisolide, and fluticasone demonstrated significantly decreased nasal congestion and sleepiness in treated patients.891 Kiely et al. performed a double-blind crossover RCT on the effect of intranasal steroid on SDB, assessed with PSG and nasal resistance at baseline and after 4-week treatment, and found a significant but small reduction in average AHI in patients with OSA using fluticasone.895 (Table VII.G.1)
TABLE VII.G. 1.
Allergic rhinitis and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusions |
|---|---|---|---|---|---|---|
| Young et al.893 | 1997 | 1b | Prospective population-based cohort study | Population-based sample of individuals (n = 911, response rate 50%) enrolled in Wisconsin Sleep Cohort Study | Relationship between nasal obstruction and sleep-disordered breathing assessed via polysomnography, rhinometry, and questionnaires | Participants with nighttime symptoms of rhinitis (5+ nights/month) were significantly more likely to report habitual snoring (3–7 nights/week), chronic excessive daytime sleepiness, or nonrestorative sleep than those who rarely had symptoms (p < 0.0001). Those with nasal congestion due to allergy were 1.8× more likely to have moderate to severe sleep-disordered breathing. |
| Craig et al.891 | 2005 | 1a | Pooled data from three double-blind crossover RCT | 69 patients with allergic rhinitis without OSA | Effect of intranasal corticosteroids in patients with perennial rhinitis on reduction of congestion and daytime somnolence | Pooled data of budesonide, flunisolide, and fluticasone demonstrated significantly decreased nasal congestion and sleepiness in treated patients. A correlation between reduction in nasal congestion and an improvement in sleep (p < 0.01) and daytime somnolence (p = 0.01) was demonstrated. |
| Stuck et al.892 | 2004 | 1b | Prospective, cohort study | 25 patients with seasonal allergic rhinitis and 25 healthy volunteers assessed with questionnaires (ESS, SF-36) and polysomnography performed before and during pollen season | Effect of seasonal allergic rhinitis on subjective and objective sleep patterns | Seasonal allergic rhinitis leads to increased daytime sleepiness (p = 0.006) and quality of life impairment (p < 0.0001). |
| Santos et al.896 | 2008 | 1b | Double blind crossover RCT | 31 patients with persistent allergic rhinitis and sleep disturbances treated with montelukast or placebo | Effect of rhinitis therapy on patient-reported sleep quality and symptoms of daytime sleepiness | Montelukast treatment demonstrated significant improvement in reported daytime somnolence (p = 0.0089) and daytime fatigue (p = 0.0087). |
| Golden et al.897 | 2010 | 1b | Double blind crossover RCT | 24 patients with perennial AR received azelastine vs. saline | Effect of topical nasal antihistamines on symptoms of rhinitis, sleep, and daytime somnolence | Azelastine reduced rhinorrhea (p = 0.03) and improved subjective sleep quality (p = 0.04) but did not reduce daytime somnolence. |
| Lavigne et al.894 | 2013 | 2b | Prospective cohort study | 21 patients with OSA and allergic rhinitis and 34 patients with OSA without allergic rhinitis | Effect of corticosteroid treatment on disease severity with sleep studies and biopsies obtained from the inferior turbinate, nasopharynx, and uvula | Improved O2 nadir, supine AHI, and daytime somnolence in AR group (p = 0.05). |
| Kiely et al.895 | 2004 | 1b | RCT | OSA with AHI > 10 (n = 13) vs. patients without OSA. Intervention: fluticasone spray for 4 weeks | AHI, ESS, snoring | Reduction in AHI in patients with OSA and AR compared to placebo (23.3 vs. 30.3). Most patients continued to have significant OSA. |
VII.G.2 |. Nasal obstruction
Nasal obstruction is a significant risk factor for SDB. While data remains limited regarding objective measures of SDB following treatment for nasal obstruction, literature has shown that sleep quality is compromised in patients with chronic nasal obstruction, with significant improvement in subjective sleep quality after medical and surgical treatment. Anatomic nasal obstruction, including septal deviation, internal/external valve collapse, and inferior turbinate hypertrophy, has been repeatedly identified as common findings in patients with SDB.6,898
The effect of medical management of nasal obstruction on SDB has been studied extensively. Topical nasal decongestion and/or external dilator strip for chronic nasal obstruction has been shown to decrease snoring, but has not demonstrated a significant, persistent change in AHI or improvement in sleep quality.895–897,899–904 In a double-blind crossover RCT investigating the effects of topical nasal decongestant on nasal conductance, symptom scores, and PSG findings, there was a significant decrease in AHI at time of maximal decongestion but no significant change in overall AHI or sleep quality.900 Djupesland et al. conducted a double-blind crossover RCT on the effects of external nasal dilation on SDB, assessed by PSG, acoustic rhinometry, and questionnaire. While nasal dimensions increased significantly with external dilator compared to placebo, there was no significant decrease in AHI. Kerr et al. studied the effect of nasal resistance reduction by application of topical vasoconstrictor and insertion of vestibular stents in patients with OSA. On posterior rhinomanometry and polysomnogram, it was found that while reduction of nasal resistance resulted in no significant change in AHI, significant improvement in subjective sleep quality was reported.902 Sinonasal surgery continues to play a key role in the management of OSA given demonstrated improvement in CPAP tolerance. Further study of the effect of treatment of nasal obstruction on objective polysomnographic parameters is reviewed elsewhere. (Table VII.G.2)
TABLE VII.G. 2.
Association between nasal obstruction and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusions |
|---|---|---|---|---|---|---|
| Young et al.69 | 2001 | 1b | Prospective population-based cohort study | Population-based sample of individuals enrolled in ongoing Wisconsin Sleep Cohort Study. In-laboratory polysomnography was performed on subset (n = 1032). | Relationship between nasal congestion and snoring. | Chronic severe nasal congestion is an independent risk factor for habitual snoring at baseline and 5-year follow-up (OR 4.9; 95% confidence interval, 2.8–8.8). |
| An et al.899 | 2019 | 1b | Double blind crossover RCT | 15 patients with OSA and chronic nasal obstruction without obvious pharyngeal narrowing completed two overnight polysomnograms (randomly applying oxymetazoline or placebo). | Effects of nasal patency on sleep architecture in nasal obstruction- predominant obstructive sleep apnea patients by applying nasal decongestant. | Oxymetazoline resulted in significant increase in REM sleep (p = 0.027) and reduction of stage 1 sleep (p = 0.004). AHI in supine position was significantly reduced (p = 0.001). |
| Kerr et al.902 | 1992 | 1b | Single-blind crossover study | 10 patients with OSA receiving oxymetazoline and nasal dilator vs. placebo. | Effect of nasal resistance reduction on OSA and nasal airflow assessed via posterior rhinomanometry and PSG. | Reduction of nasal resistance resulted in no change in AHI but improved subjective sleep quality (p < 0.001). |
| Stradling et al.533 | 1991 | 2b | Cross sectional study | 1001 men surveyed regarding sleep quality and underwent polysomnography. | Independent predictors of snoring and obstructive sleep apnea. | Multiple linear regression identified nasal stuffiness as a significant independent predictor of snoring but not OSA. |
| Vidigal et al.905 | 2012 | 2c | Case–control study | 47 with moderate/severe OSAS and 20 matched controls. | To evaluate nasal obstruction in patients with OSA compared to controls via questionnaires, physical exam, rhinoscopy, nasal inspiratory peak flow (NIPF), and acoustic rhinometry (AR). | OSA group had a significantly higher score on the nasal symptoms scale (p < 0.01) and higher frequency of nasal alterations including presence of septal deviation (p = 0.01) and inferior nasal turbinate hypertrophy (p < 0.01). NIPF and AR parameters were not significantly different. |
| McLean et al.903 | 2005 | 1b | Single blind crossover RCT | 10 patients with nasal obstruction and OSA. | Effect of topical decongestant and external dilator strip on nasal resistance, mouth breathing during sleep, and OSA severity. | While AHI reduced by mean 12 (range 3–22) with treatment, no correlation identified between nasal resistance change and AHI change (r2 = 0.001; p = 0.92). |
| Clarenbach et al.900 | 2008 | 1b | Double blind crossover RCT | 12 patients with chronic nasal congestion and OSA treated with nasal xylometazoline or placebo for 1 week. | Effects of topical nasal decongestant on nasal conductance, symptom scores, polysomnography. | While there was a significant decrease in AHI at time of maximal decongestion (p < 0.05), there was no significant change in overall AHI or sleep quality. |
| Djupesland et al.901 | 2001 | 1b | Double blind crossover RCT | 18 heavy snorers without severe OSA (mean AHI 9.3) reporting nocturnal nasal obstruction. | Effects of external nasal dilation (Breathe Right) assessed by polysomnography, acoustic rhinometry, and questionnaire. | Nasal dimensions increased significantly (p < 0.001) with active dilator compared to placebo. In habitual snorers (AHI < 10) with severe morning obstruction, no significant reduction in AHI (p = 0.06). |
| Ishii et al.545 | 2015 | 2a | Meta-analysis | 10 studies meeting criteria with 320 patients: two RCTs, seven prospective studies, and one retrospective study. | Effect of isolated nasal surgery on OSA. | Isolated nasal surgery for patients with nasal obstruction and OSA improved ESS by 3.53 (95% CI [0.64, 6.23]) and RDI by 11.06 (95% CI [5.92, 16.19]), but had no significant AHI improvement (95% CI [–1.6, 11.62]). |
| Li et al.906 | 2011 | 2a | Meta-analysis | 11 prospective noncontrolled clinical trials on outcomes of nasal surgery for OSA. | Effect of nasal surgery on daytime sleepiness, snoring, and polysomnogram. | Mean AHI decreased from 35.2 ± 22.6 to 33.5 ± 23.8 after nasal surgery (p = 0.69). ESS scores decreased from 10.6 ± 3.9 to 7.1 ± 3.7 (p < 0.001). Significant improvement in snoring assessed by questionnaires and visual analog scale (p < 0.05). |
| Yamasaki et al.907 | 2020 | 2b | Prospective cohort study | Patients undergoing nasal surgery surveyed through 24 months postoperatively. | Longitudinal snoring symptoms and nasal obstruction after functional nasal surgery for patients with SDB. | OSA patients achieved clinically significant improvement on Snoring Outcome Survey through 24 months (p > 0.05). |
| Bosco et al.908 | 2020 | 2b | Prospective cohort study | 34 patients with AHI > 15 and septal deviation treated with nasal surgery. | Effects of nasal surgery on upper airway collapse, assessed using drug-induced sleep endoscopy. | Before nasal surgery, 74% of the patients demonstrated multilevel obstruction. After nasal surgery, 50% patients showed multilevel collapse (p < 0.05) with significant improvement shown in hypopharyngeal collapse. |
| Li et al.909 | 2009 | 2b | Prospective, controlled nonrandomized study | 66 patients with OSA (AHI > 5, BMI < 33) and chronic nasal obstruction (surgical, n = 44; control, n = 22). | Effect of septoplasty with inferior turbinate reduction versus medical therapy (steroid or decongestant spray, saline lavage, or oral antihistamine) on snoring, daytime sleepiness, and respiratory adverse events assessed via questionnaire, rhinomanometry, and polysomnogram at baseline and 3 months. | Significantly improved nasal resistance found in the surgical group (p < 0.001). Both groups revealed insignificant changes in polysomnographic parameters. In subgroup analysis, a significant effect of nasal surgery on AHI was found when patients were divided by Friedman tongue position (FTP) into “low” (FTP 1 or 2) and “high” (FTP 3 or 4) (p = 0.007). |
| Nakata et al.910 | 2008 | 2b | Prospective cohort study | 49 OSA patients with symptomatic nasal obstruction. | Effect of nasal surgery on nasal resistance, sleep apnea, and sleep quality in patients with OSA assessed via polysomnography before and after surgery. | While there was no significant change in AHI, nasal surgery decreased nasal resistance (p < 0.001), ameliorated sleep-disordered breathing (increased nadir oxygen saturation, p < 0.01; shortened apnea–hypopnea duration, p < 0.05), and improving sleep quality and daytime sleepiness in OSAS (ESS scores, p < 0.001). |
| Shuaib et al.911 | 2015 | 2c | Retrospective cohort study | 26 patients with chief complaint of nasal obstruction found to have septal and nasal valve obstruction on examination, who subsequently underwent functional rhinoplasty. | Effect of nasal surgery on nasal resistance, sleep apnea, and sleep quality in patients with OSA assessed via polysomnography before and after functional septorhinoplasty. | Mean AHI preoperatively was 24.7, which dropped to mean postoperative AHI 16, a reduction of 35% (p = 0.013). Among patients with BMI < 30 resulted in 57% mean AHI reduction, from 22.5 to 9.6 (p < 0.01). |
| Hisamatsu et al.912 | 2015 | 2c | Retrospective cohort study | 45 patients with moderate or severe OSA and high nasal resistance assessed by rhinomanometry underwent compound nasal surgery (septoplasty, turbinate reduction, and submucosal resection of the posterior nasal nerve). | Effects of nasal surgery on OSA assessed using polysomnography at 3 months, daytime sleepiness, nasal allergy symptoms, and health-related QOL. | Postoperative improvement was demonstrated in at least one polysomnography parameter in 57% and 75% patients with moderate or severe OSA, respectively. Quality of life measures were also significantly improved. |
| Silvoniemi et al.898 | 1997 | 2c | Cross-sectional study | 46 patients with severe nasal obstruction due to septal deviation. | Sleep-disordered breathing as assessed by rhinomanometry and whole night sleep recording. | Thirty-one patients (67%) had also heavy disturbing snoring, and apnea periods during sleep were reported by 10 cases. |
| Lenders et al.913 | 1991 | 2c | Case–control study | 45 habitual snorers and 22 patients with OSA examined by PSG, rhinomanometry, and acoustic rhinometry. | Association between anatomic nasal obstruction and sleep-disordered breathing. | In 97% of these patients, inferior turbinate hypertrophy was found by acoustic rhinometry, while increased nasal resistance of various degrees was measured in 93% of all patients by active anterior rhinomanometry. |
VII.G.3 |. Chronic rhinosinusitis
Sleep quality is compromised in patients with chronic sinusitis (chronic rhinosinusitis CRS), with significant improvement in subjective sleep quality after medical and surgical treatment.914–916 Among World Trade Center responders, CRS was an independent risk factor for OSA.917 Data is limited regarding objective measures of sleep following treatment for CRS. In a prospective cohort study of 405 patients undergoing endoscopic sinus surgery for medically refractory CRS 15% of CRS patients were found to have a history of comorbid OSA based on chart review prior to treatment.914 PSG-confirmed OSA was identified in approximately 65% of CRS patients undergoing surgery in another prospective cohort study.918 Preliminary research has suggested that immune mediators associated with CRS may contribute to SDB.918 (Table VII.G.3)
TABLE VII.G. 3.
Association of chronic rhinosinusitis and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|
| Quinn et al.916 | 2017 | 2a | Meta-analysis | 6 prospective cohort studies; two retrospective case series | Relationship between sleep-disordered breathing and CRS. | Sleep quality is compromised in CRS patients with significant improvement in subjective sleep quality after medical and surgical treatment. Data is limited regarding objective measures of sleep following treatment for CRS. |
| Alt et al.915 | 2019 | 2b | Case–control study | 52 patients with CRS and 56 controls | Objective sleep measures in CRS patients assessed by home polysomnogram. | Patients with CRS were found to have an increased number of awakenings (p = 0.004), lower average overnight oxygen saturation (p = 0.042), increased REM latency (p = 0.016), and increased snoring at >40 dB (p = 0.034). |
| Alt et al.914 | 2015 | 2b | Prospective cohort study | 405 patients undergoing endoscopic sinus surgery for medically refractory chronic rhinosinusitis without nasal polyposis | Impact of comorbid OSA on sleep dysfunction in patients with CRS following functional endoscopic sinus surgery as assessed by Pittsburgh Sleep Quality Index (PSQI). | 15% of CRS patients had comorbid OSA and had substantial disease-specific QOL improvements following endoscopic sinus surgery (p < 0.05). |
| Rotenberg et al.919 | 2015 | 2b | Prospective cohort study | 53 patients undergoing endoscopic sinus surgery for medically refractory chronic rhinosinusitis without nasal polyposis | Sleep quality following sinus surgery recorded at baseline and 6 months after surgery assessed with Epworth Sleepiness Scale (ESS) and the Pittsburgh Sleep Quality Index (PSQI). | Sinus surgery for CRS is associated with improved patient-reported sleep quality (p < 0.01). |
| Tosun et al.920 | 2009 | 2b | Prospective cohort study | 27 patients with nasal polyposis underwent endoscopic sinus surgery with polypectomy | Effect of endoscopic sinus surgery on sleep quality in patients with chronic nasal obstruction resulting from nasal polyposis evaluated by questionnaire and polysomnography before and 3 months after the surgery. | While endoscopic sinus surgery with polypectomy significantly improved snoring and daytime sleepiness in patients with chronic nasal obstruction due to nasal polyposis (p < 0.01), it did not demonstrate a significant change in AHI (p = 0.55). |
| Yalaman-chali et al.921 | 2014 | 2c | Retrospective cohort study | 56 patients with OSA and chronic rhinosinusitis who underwent septoplasty with bilateral submucosal inferior turbinate reduction and concurrent endoscopic sinus surgery | Impact of combined nasal surgery and endoscopic sinus surgery on OSA assessed by polysomnography before and 3 months after the surgery. | AHI improved significantly after combined nasal surgery and endoscopic sinus surgery in patients with moderate OSA (p = 0.023) and severe OSA (p = 0.034), while patients with mild OSA did not have significant changes in AHI. |
| Sunderram et al.917 | 2019 | 2b | Cross sectional study | 601 subjects enrolled in the WTC Health Program underwent two nights of home sleep testing | Association between CRS and OSA. | CRS is an independent risk factor for OSA (odds ratio of 1.80; p 5.006), even after adjusting for age, BMI, sex, GERD, and EtOH use. |
| Jiang et al.918 | 2016 | 2b | Cross sectional study | 139 CRS patients who underwent functional endoscopic sinus surgery | Impact of CRS on sleep-disordered breathing as assessed by PSG and ESS. | OSA was diagnosed in 64.7% of patients with CRS, but there was no correlation with the severity of rhinosinusitis. The study did not assess for change in PSG or ESS outcomes after treatment. |
| Alt et al.922 | 2014 | 2b | Cross sectional study | 20 CRS subjects completed disease-specific QOL and olfactory instruments | Association between immune mediators that have been associated with CRS and sleep-disordered breathing as assessed by Pittsburgh Sleep Quality Index (PSQI). | Increased expression of TGF-β (r = −0.443; p = 0.050) and IL-4 (r = −0.548; p = 0.012) correlated with sleep dysfunction, whereas IL-13 expression was linearly associated with worse sleep quality (PSQI scores r = −0.417; p = 0.075). |
VII.H |. Comorbidities Associated with OSA: Gastroesophageal Reflux Disease
The relationship between OSA and gastroesophageal reflux disease (GERD) is complex and our understanding of a causative relationship and effects of treatment continues to evolve. Numerous studies have established a high incidence of GERD among patients with SDB.923–936 Population-based studies have demonstrated that greater than 75% of patients with OSA report nocturnal reflux symptoms, which have been associated with sleep fragmentation and overall poor sleep quality.927,930,936 Laryngopharyngeal reflux (LPR), which occurs when gastric contents breach both the lower esophageal sphincter (LES) and upper esophageal sphincter (UES), presents with symptoms such as chronic cough, hoarseness, and throat clearing, and has also been associated with SDB.923,924,926–930,932,933,936,937 While the causal relationship between obstructive events and nocturnal reflux has yet to be established, a cycle of increases in respiratory effort with negative intrathoracic pressure leading to reflux of gastric contents at the level of the LES during sleep has been suggested. However, in a prospective cohort study of 27 adults with reflux confirmed by two validated instruments (Reflex Symptom Index (RSI) ≥ 13 and Reflux Finding Score (RFS) ≥ 7) and OSA, no temporal association between reflux and obstructive respiratory events was identified on PSG with concomitant multichannel intraluminal impedance (MCII)-pH esophageal monitoring.936
While the high incidence of nocturnal reflux among patients with OSA has been repeatedly demonstrated, the correlation between severity of OSA and GERD remains controversial with studies demonstrating variable findings. Though the gold standard for identifying reflux events consists of pH-monitoring studies and outpatient MCII, these tests are costly and most retrospective cohort studies rely on clinical diagnoses based on endoscopic findings and validated patient questionnaires such as the RSI and the RFS. Caparroz et al. found that patients with LPR based on endoscopic findings and elevated RSI were more likely to have a higher mean AHI and higher percentage of sleep time with oxyhemoglobin saturation below 90%.924 Elhennawi et al. studied the relationship between OSA and LPR using 24-h pH monitoring and found that while the number of nocturnal reflux episodes and total duration of reflux during sleep were significantly correlated to the degree of OSA, daytime reflux was not related to the degree of OSA.926 Ultimately, the severity of OSA has not been shown to consistently correlate with gastroesophageal reflux.926,932,933
While patients with OSA and gastroesophageal reflux have been shown to have a higher BMI than patients with OSA alone, the exact relationship between OSA, obesity, and gastroesophageal reflux has yet to be elucidated.924,933,938–941 Obesity is a known risk factor for both OSA and GERD and weight loss has been shown to improve both OSA and nocturnal reflux symptoms in obese patients.930,937,939,940 In a recent prospective cohort study on the influence of OSA on GERD in obesity, obese patients without OSA, nonobese patients with OSA, and obese patients with OSA underwent simultaneous PSG and esophageal manometry and pH monitoring. There was no significant difference in the total number of objective reflux events among obese patients with and without OSA. However, obese patients with OSA had a significantly greater number of reflux events compared to the non-obese group with OSA. In multivariate analysis, BMI was a significant predictor of the number of objective reflux events, but AHI showed no significant association with GERD severity.941
Treatment of GERD has demonstrated significant reduction in snoring and patient-reported daytime sleepiness and improved sleep quality.927,940 However, treatment of gastroesophageal reflux has not been shown to improve OSA severity based on objective polysomnographic parameters.940 Treatment for OSA with CPAP therapy and/or multilevel surgery (MLS) has been shown to significantly improve subjective parameters of reflux.928,929 In a study of 73 patients who underwent MLS for OSA, mean RSI score decreased significantly after surgery.928 In a study of 44 patients with symptoms of SDB and reflux, subjects underwent 24 h pH monitoring simultaneously with PSG at 0 and 3 months after CPAP treatment and showed significant improvement of subjective parameters of reflux, such as RSI and RFS.927 Improvement in objective parameters of gastroesophageal reflux with treatment for OSA has yet to be demonstrated.
Ultimately, the relationship between OSA and GERD is multifactorial and closely tied to obesity. Treatment for both OSA and reflux results in subjective improvement in reflux and sleep quality, but objective improvement demonstrated through polysomnogram and/or pH monitoring is lacking. Further study of the causal relationship between reflux and SDB using objective parameters is needed. (Table VII.H)
TABLE VII.H.
Association between OSA and GERD
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Teklu et al.935 | 2020 | 2c | Cross-sectional study | 101 adult patients who underwent polysomnogram | Relationship between reported reflux symptoms and OSA | Patients with OSA have worse symptoms of laryngopharyngeal reflux based on RSI (p = 0.003). |
| Morse et al.931 | 2004 | 2b | Cross sectional study | 136 patients referred for polysomnogram | Relationship between OSA and GERD in a large group of patients with OSA | Subjective reports of sleep quality were affected by GERD severity, but no significant correlation between OSA and GERD. |
| Sundar et al.934 | 2010 | 2b | Cross sectional study | 75 patients with isolated chronic cough | Rates of OSA among patients with chronic cough | 37% of patients with chronic cough had a diagnosis of GERD. 44% were found to have OSA. 93% of the patients that had interventions to optimize their sleep-disordered breathing reported improvement in cough. |
| Chan et al.925 | 2010 | 2b | Cross sectional study | 108 consecutive patients referred for evaluation of SDB | Prevalence and severity of chronic cough in patients with sleep-disordered breathing | 33% of patients with SDB reported a chronic cough. Patients with SDB and chronic cough were predominantly females (p < 0.001), reported nocturnal heartburn (p = 0.03), and rhinitis (p = 0.02) compared to those without SDB. |
| Caparroz et al.937 | 2019 | 2b | Cross sectional study | 56 patients with moderate or severe OSA | Prevalence of laryngopharyngeal reflux in patients with moderate and severe OSA by reflux symptom index questionnaire and indirect videolaryngoscopy | 64.3% of patients with moderate or severe OSA had laryngopharyngeal reflux (positive RSI and/or positive endolaryngeal reflux finding score). BMI was a predictor of reflux presence. In patients with positive score for endoscopic findings and reflux symptom index, there was a trend toward significance for a higher mean AHI and a percentage of sleep time with oxyhemoglobin saturation below 90% (p = 0.05). |
| Lee et al.929 | 2018 | 3b | Case–control study | 19 OSA patients with unilevel complete obstruction and 69 OSA patients with multilevel complete obstruction on drug-induced sleep endoscopy (DISE) | Relationship between level of obstruction determined by DISE, and laryngopharyngeal reflux (LPR)-related clinical parameters | No significant correlation between OSA severity and LPR-related symptoms. Multilevel complete obstruction on DISE did not affect the LPR-related clinical parameters (p > 0.05). |
| Kim et al.928 | 2017 | 2b | Cross sectional study | 73 patients who underwent multilevel surgery for OSA | Effect of multilevel surgery for OSA on symptoms of laryngopharyngeal reflux (LPR) and polysomnogram | Treatment for OSA using multilevel surgery reduced symptoms of LPR; mean RSI score decreased from 11.48 ± 7.95 to 4.95 ± 6.19 after surgery (p < 0.001). |
| Altintas et al.923 | 2017 | 2b | Cross sectional study | 62 patients with AHI > 5 | Relationship between presence of LPR and level of depression and anxiety in patients with OSA assessed via questionnaire and laryngeal examination | There were significantly higher levels of depression and anxiety in patients with LPR and OSA (p =0.016). A positive correlation was found between RSI and AHI scores (r = 0.338; p = 0.007). |
| Elhennawi et al.926 | 2016 | 2b | Cross sectional study | 62 patients with OSA | Relationship between OSA and LPR assessed with ambulatory 24-h pH monitoring | LPR is common in patients with OSA (66%). Patients with severe OSA have significantly higher nocturnal LPR (p < 0.05). Number of reflux episodes and total duration of reflux during sleep are significantly correlated to degree of OSA (p < 0.05). Daytime reflux was not related to degree of OSA (p > 0.05). |
| Qu et al.932 | 2015 | 2b | Case–control study | 36 OSA patients and 10 healthy controls underwent 24-h double-probed combined esophageal multichannel intraluminal impedance and pH monitoring simultaneously with polysomnography | Esophageal functional changes observed in OSA | 63.9% of patients had both OSA and LPR by pH monitoring and polysomnogram. Significant differences were found in the onset velocity of liquid swallows (p = 0.029) and percent relaxation of the lower esophageal sphincter (LES) during viscous swallows (p = 0.049) between patients with OSA versus healthy controls. |
| Rodrigues et al.933 | 2014 | 2b | Cross sectional study | A total of OSA patients divided into obese group (n = 39) and non-obese patients (n = 66) | Relationship between obesity on LPR and OSA | In the obese group, mean RSI was 6.7 in patients with mild OSA and 11.53 in patients with moderate to severe OSA (p < 0.05). No correlation between OSA severity and RSI in non-obese group. |
| Xavier et al.936 | 2019 | 1b | Cross sectional study | 27 adults with LPR confirmed by two validated instruments (RSI ≥ 13 and reflux finding score ≥ 7) and OSA underwent full polysomnography with concomitant multichannel intraluminal impedance-pH esophageal monitoring | Temporal correlation between reflux episodes and respiratory events in patients with LPR and OSA | Among patients with well-established laryngopharyngeal reflux and OSA, there is no temporal association between reflux and obstructive respiratory events. |
| Eryılmaz et al.927 | 2012 | 1b | Prospective cohort study | 44 patients underwent double probed 24 h pH monitoring simultaneously with polysomnography due to the complaints of SDB and reflux, at 0 and 3 months | Effect of OSA therapy on LPR parameters | OSA and LPR coexist frequently. LPR treatment did not improve the polysomnographic parameters, but significantly reduced ESS (p = 0.02) and snoring (p = 0.007). Although CPAP treatment significantly improved subjective parameters of reflux, such as RSI and RFS (p = 0.016 for both), there was no significant improvement in objective parameters of 24-h pH monitoring. |
| Magliulo et al.930 | 2018 | 2a | Meta-analysis | 10 papers studying LPR in OSA were included with 870 identified OSA patients | Incidence of LPR in OSA patients | There is a high incidence of LPR (45.2%) among OSA patients. AHI severity did not correlate with presence of laryngopharyngeal reflux (p = 0.3). OSA patients with LPR had higher BMI compared with LPR patients (p = 0.001). |
| Caparroz et al.924 | 2019 | 2b | Cross sectional study | 70 patients with moderate or severe OSA underwent validated questionnaires, laryngoscopy to calculate the Reflux Finding Score (RFS), and fiber-optic endoscopic evaluation of swallowing (FEES) | Association between presence of dysphagia with signs and symptoms suggestive of LPR in patients with moderate and severe OSA | Although 17.9% of patients presented with findings suggestive of concomitant LPR and dysphagia, there was no statistically significant association between these two conditions. |
| Kim et al.939 | 2018 | 1b | Cross sectional study | 216 patients underwent both PSG and EGD | Relationship between OSA and GERD | Endoscopically proven GERD was associated with more severe OSA (p = 0.01). GERD symptoms were also associated with worse sleep quality (p = 0.03). |
| Rassamee- hiran et al.940 | 2016 | 2a | Meta-analysis | 2 randomized trials and 4 prospective cohort studies on the effect of treatment for GERD on OSA | Association between PPI treatment for GERD and improvement in OSA | No differences in AHI before and after treatment with PPIs (SMD 0.21; 95% CI [−0.11, 0.54]). |
| Gilani et al.938 | 2016 | 2b | Retrospective study | Adults with OSA and GERD and potentially confounding conditions were identified in the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey | Association between GERD and OSA controlling for concurrent confounders in a national database | Significant positive association between GERD and OSA was observed, which persisted after adjustment for age, sex, race/ethnicity, sino-nasal obstruction, inflammatory disorders, obesity, asthma, and lung disorders (OR 1.94, 95% CI [1.07–3.54], p = 0.030). |
| Shepherd et al.941 | 2016 | 1b | Prospective cohort study | 20 obese individuals (BMI > 30), nine non-obese individuals (BMI < 30) with moderate-to-severe OSA, and 17 obese control subjects (BMI > 30) underwent high-resolution esophageal manometry, 24-h esophageal pH-impedance monitoring, and in-laboratory polysomnography | Effect of OSA on GERD independent of obesity | The two obese groups did not show any significant differences in the total number of acidic reflux events (41 ± 20 vs. 28 ± 16); however, the obese OSA group had a greater number of acidic reflux events compared to the non-obese OSA group (22 ± 12 events, p < 0.05). In multivariate analysis, BMI significantly predicted number of acidic reflux events (r(2) = 0.16, p = 0.01). However, AHI showed no significant association with GERD severity. |
| Xavier et al.942 | 2013 | 2c | Cross-sectional study | 74 patients with OSA | Prevalence of symptoms of reflux in OSA patients assessed with questionnaire | 98% of the subjects with suspected OSA had symptoms suggestive of LPR; prevalence was significantly higher among obese individuals (p = 0.002). |
VII.I |. Comorbidities Associated with OSA: Other Sleep Disorders
VII.I.1 |. Insomnia
The available literature addressing the prevalence of insomnia in patients with OSA (Table VII.I.1)588,943–956 suffers from several significant shortcomings. Variable definitions are used for OSA and insomnia and different studies focus on “insomnia symptoms” versus “insomnia disorder.” The former is likely to be more common in OSA patients as it requires the presence of less significant symptoms. Additionally, very few studies examined cohorts that are representative of the general population. Only three studies were available with representative population samples946,948,951 while 10 studies were convenience samples. Thus, the estimates of prevalence may not generalize to the broader population.
TABLE VII.I.1.
Prevalence of insomnia in patients with OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Zhang et al.956 | 2019 | 4 | Systematic review and meta-analysis | 31 studies assessing the prevalence of insomnia symptoms in OSA; four studies assessing the prevalence of OSA in patients with insomnia; two studies assessing prevalence of both insomnia in OSA and OSA in insomnia | Presence of insomnia symptoms or diagnosis, PSG | 38% of patients with OSA had comorbid insomnia. In patients with insomnia, the pooled prevalence rates of OSA were 35% for AHI ≥ 5 events/h and 29% for AHI ≥ 15 events/h. |
| Luyster et al.588 | 2010 | 4 | Systematic review | 4 studies assessing the prevalence of insomnia symptoms in OSA; three studies assessing the prevalence of OSA in patients with insomnia | Presence of insomnia symptoms or diagnosis, PSG | 39%–58% of patients with OSA had comorbid insomnia symptoms. 29%–67% of patients with insomnia had an AHI of >5 events/h. |
| Gupta and Knapp946 | 2014 | 2c | Retrospective, cross-sectional, case–control study of epidemiologic databases | 62,253,910 ± 5,274,747 (unweighted count = 7234) patient visits with diagnosis of OSA from 1995–2010 | ICD-9 coded diagnosis of sleep apnea or insomnia | 6.4% ± 0.9% of patient visits for OSA also contained a diagnosis of insomnia. Visits with both OSA and insomnia were significantly more likely to also list essential hypertension (OR = 1.83, 95% CI 1.27–2.65). |
| Sivertsen et al.951 | 2013 | 2c | Historical cohort case–control design | 6892 participants aged40–45 years | Karolinska Sleep Questionnaire, Norwegian official registry data, Health information from the Hordaland Health Study in western Norway (1997–1999) | 8.4% of the population had OSA and 0.6% had comorbid insomnia. |
| Lang et al.948 | 2017 | 2b | Prospective, general population, cohort study | 837 randomly selected men without prior diagnosis of OSA | At-home unattended PSG; 36-item Short Form (SF-36) vitality scale, Beck Depression Inventory | 46% of the population had OSA; of these, 14.5% had comorbid insomnia. |
| Stelzer et al.953 | 2020 | 4 | Prospective, cross-sectional case series | 238 patients with OSA | ICSD-3, PSG-3, Beck Depression, and Anxiety Inventories | 29% of patients diagnosed with OSA had comorbid insomnia. Depression and anxiety were higher in the group with insomnia. |
| Wallace and Wohlgemuth955 | 2019 | 4 | Prospective, cross-sectional, case series | 630 veterans with a new diagnosis of OSA | Insomnia Severity Index (ISI), PSG | 74% of patients with OSA had moderate or severe insomnia. |
| Cho et al.945 | 2018 | 4 | Prospective, cross-sectional case series | 476 patients with OSA seen at two tertiary hospital sleep centers | ISI, PSG, SF-36, PSQI, Beck Depression Inventory | 29.2% of patients with OSA had significant insomnia. Patients with both OSA and insomnia had higher rates of heart disease, lower quality of life, and worse depression than those with OSA only. No significant differences in CPAP adherence between those with vs. without insomnia. |
| Taskaban et al.954 | 2018 | 4 | Prospective, cross-sectional case series | 197 consecutive patients with OSA seen in a sleep laboratory | PSG, WHO Quality of Life form | 18.8% of patients with OSA had comorbid insomnia. Patients with both OSA and insomnia had lower physical and social scores than those with OSA only. |
| Bjorvatn et al.944 | 2014 | 4 | Prospective, cross-sectional, general population sample case series | 1502 randomly selected participants from the general population | Bergen Insomnia Scale. Self- or spouse-reported OSA symptoms | 6.2% of participants were diagnosed with OSA; of these, 57.6% reported comorbid insomnia. |
| Mysliwiec et al.949 | 2013 | 4 | Prospective cross-sectional case series | 110 military personnel who returned from combat with sleep disturbances | ICSD-2, PSG | 24.5% diagnosed with OSA alone and 38.2% with comorbid OSA and insomnia. Patients with both OSA and insomnia were significantly more likely to meet criteria for depression. |
| Bjornsdottir et al.943 | 2013 | 4 | Prospective cross-sectional case series | 705 adults with OSA | Basic Nordic Sleep Questionnaire, HSAT | 68.3% had comorbid insomnia, 15.5% exhibited symptoms of initial insomnia, 59.3% had symptoms of middle insomnia, and 27.7% exhibited symptoms of late insomnia, with overlap between groups. |
| Nguyen et al.950 | 2010 | 4 | Prospective, cross-sectional, case series | 148 consecutive patients with OSA treated in a sleep clinic | ISI, PSG | 50% of patients had moderate to severe comorbid insomnia. Presence of insomnia did not significantly affect CPAP adherence. |
| Smith et al.952 | 2004 | 4 | Prospective, cross-sectional, case series | 105 patients with OSA | ISI, PSG, sleep diary, reported daytime consequences of insomnia, Depression Anxiety Scale-21 | 35% of patients had comorbid insomnia. Patients with both OSA and insomnia had increased levels of depression, anxiety, and stress. |
| Krakow et al.947 | 2001 | 4 | Retrospective cross-sectional case series | 231 patients with OSA | Reported insomnia symptoms, sleep diary; reported psychiatric disorders; reported use of sedative and psychotropic medications | 50% reported insomnia complaints. OSA + insomina had significantly more psychiatric disorders and physical/mental symptoms that disrupted sleep. OSA + insomnia also reported greater use of sedative and psychotropic medications. |
Insomnia symptoms and insomnia disorder are quite common in OSA patients; however, the above-noted limitations are reflected in the wide-range of prevalence estimates found. The reported rates of an insomnia disorder diagnosis in patients with OSA ranged from 6.4% to 74%. Notably, the majority of studies found rates that were clustered in the range of 29%–50%. For insomnia symptoms, the prevalence varied from 7% to 68% with most estimates being in the range of 36%–58%. Notably, the studies with the lowest prevalence rates tended to be the general population studies.
Despite significant limitations in the literature, it is clear that insomnia symptoms and insomnia disorder are seen commonly in OSA patients. Co-morbid insomnia and sleep apnea (COMISA) is associated with greater impairment of sleep quality, reduced QOL, complex diagnostic decisions, and reduced acceptance and response rates to treatments that require adherence (i.e., PAP, mandibular advancement device [MAD], and HNS therapy).955,957 Effective management of patients with both insomnia and OSA requires targeted therapies for both disorders.958 Treatment reviewed in Sections VIII.A.5.i and VIII.B.9. (Table VII.I.1)
VII.I.2 |. Sleep movement disorders
In patients with OSA, specific considerations are warranted in the presence of comorbid RLSs, periodic limb movements (PLMs), REM sleep behavior disorder (RBD), and sleep-related movement disorders. While the evidence supporting specific recommendations is limited, the available data provide indications regarding clinicallyimportant aspects of OSA management in these patient populations.
RBD is an REM-related parasomnia marked by dreamenactment behavior and absence of atonia during REM sleep.81 RBD may be idiopathic, that is, occurring in the absence of clear etiology, or may be secondary to underlying neurodegenerative synuclein-mediated disorders,959 structural lesions, or narcolepsy.960 Screening for OSA is indicated in patients with history of dream-enactment behavior, as untreated SDB can mimic RBD.961 The presence of OSA may be associated with reduced response to RBD treatments such as clonazepam.962 On the other hand, treatment of OSA in patients with RBD may improve RBD manifestations.963 Individuals with a neurodegenerative disorder who are diagnosed with RBD may be particularly at risk for OSA.964
RLS is a marked by sensory symptoms, often in the limbs, combined with urge to move the limbs that temporarily improves with limb movement and exhibits diurnal variation.81 OSA may be comorbid with RLS, and treatment of OSA is indicated in patients with RLS and can improve RLS severity.965,966
PLMs may emerge after initiation of CPAP for the treatment of OSA, with risk factors including older age967 and female sex.968 PLMs cormorbid with OSA may be associated with increased REM latency and reduced stage 3 sleep.969 There may not be a significant impact of treatment-emergent PLMs on daytime sleepiness.970,971 Nevertheless, presence of PLMs has important clinical implications as they may indicate greater risk of AF and other CV diseases,972–975 perhaps related to increased sympathetic activation.974 Patients with OSA and treatmentemergent PLMs thus warrant age- and comorbidityappropriate monitoring for cardiac disease.972–975
As for other sleep-related movement disorders, screening for OSA may be indicated in select patients with bruxism though a clear relationship between sleep bruxism and OSA has not been established.976,977 Finally, limited data indicate that in select adults with rhythmic movement disorder it may be appropriate to perform PSG to identify OSA.978 (Table VII.I.2)
TABLE VII.I.2.
Association between sleep movement disorders and OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| REM behavior disorder | ||||||
| Li et al.962 | 2016 | 4 | Case series | 39 iRBD patients assessed pre- and post-treatment with clonazepam | Modified RBD questionnaire, ESS, PSG measures | Clonazepam reduced subjective measures of sleep related injury, nightmares, limb movements, and objective measures of REM sleep tonic mentalis surface EMG activity. Residual symptoms were common. Presence of OSA was associated with reduced response to clonazepam. |
| Iranzo et al.961 | 2005 | 3b | Case–control study | 16 patients with dream enactment behavior and witnessed apneas were compared, via VPSG, with 20 healthy controls | Describe VPSG features of patients with OSA that mimic behaviors of RBD | Severe OSA may mimic the symptoms of RBD. A clinical history of dream enactment behavior but normal atonia during REM sleep should suggest this RBD mimic VPSG is mandatory to establish the diagnosis of RBD, and identify or exclude other causes of dream-enacting behaviors. |
| Gabryelska et al.963 | 2017 | 4 | Cross-sectional survey study | 72 out of 120 patients previously diagnosed with RBD and OSA responded to a mailed questionnaire: 27 patients reported use of CPAP. 45 patients reported noncompliance with CPAP | Determine the prevalence of obstructive sleep apnea (OSA) in RBD patients and determine whether continuous positive airway pressure (CPAP) therapy improved RBD symptoms | OSA is a common comorbidity of RBD, being self-reported in 89% of patients with diagnosed RBD. CPAP therapy might improve self-reported RBD symptoms. |
| Bugalho et al.979 | 2017 | 3b | Case–control study | 10 patients with RBD and AHI > 14 were compared to 22 with RBD with AHI ≤14 | To understand the influence of OSA on symptoms in RBD | RBD patients with OSA had less O2 desaturation compared to those without OSA. |
| Koo et al.964 | 2018 | 3b | Case–control study | 35 patients with idiopathic RBD compared to 42 patients with RBD plus a neurodegenerative Parkinsonian syndrome (25 with PD and 17 with MSA) | Compare sleep parameters in RBD patients with vs. without neurodegenerative Parkinsonian syndromes | RBD patients with diagnosed neurodegenerative Parkinsonian syndromes may be more prone to OSA than patients with idiopathic RBD. |
| Restless leg syndrome | ||||||
| Rodrigues et al.965 | 2006 | 4 | Case series | 17 patients with OSA and RLS or PLMs underwent CPAP titration and follow-up assessment 3 months later | Determine if CPAP therapy improves RLS, as measured by the IRLS, in patients with OSA | IRLS severity decreased significantly post-CPAP compared to pre-CPAP levels. |
| Silva et al.966 | 2017 | 4 | Case series | 28 patients with RLS and OSA were reviewed for evolution of RLS symptoms following CPAP therapy | Determine if CPAP therapy for OSA is associated with improved RLS symptoms | RLS symptoms improved following CPAP initiation and allowed for reductions in medications for RLS. |
| Lakshmanan et al.980 | 2019 | 3b | Case–control study | IRLS scores were compared in 325 individuals with OSA who received PAP therapy vs. 109 controls, and participants were screened for RLS using a single question | Determine if CPAP therapy for OSA is associated with improved RLS symptoms, as measured with IRLS | OSA patients with RLS who reported adherence to PAP had significant improvements in RLS symptoms compared to those who reported non-adherence. |
| PLMS/PLMD | ||||||
| Wu et al.974 | 2018 | 3b | Case–control | 30 patients with OSA and treatment-emergent PLMs vs. 30 patients with OSA without treatment-emergent PLMs | HRV spectral analysis (FFT) applied to investigate basal autonomic regulation reflecting cardiovascular risk in patients with OSA with treatment-emergent PLMs. Parameters examined included RMSSD, LF, HF, and LF/HF ratio | PLMS emerging after CPAP therapy was associated with a decreased HRV during leg movement-free intervals and a shift toward sympathetic predominance in basal autonomic regulation. |
| Ren et al.968 | 2016 | 3b | Case–control | (1) 182 females with OSA matched for severity and age (2) 182 males with OSA matched for severity and age |
Differences in PLMS in males vs. females with OSA | In age groups of 55 or less, females with OSA are significantly more likely to have PLMS (PLMI ≥ 15) than males with OSA of similar severity (OR 2.48; 95% CI 1.06–5.79). |
| Haba-Rubio et al.971 | 2005 | 4 | Case series | In 57 patients diagnosed with OSA on CPAP: (1) 22 patients with PLMS (PLM index ≥ 5/h) (2) 35 patients without PLMS |
Examine the role of PLMS on objective and subjective parameters of sleepiness before and after CPAP use in OSA patients | The presence of PLMS showed no correlation with increased sleepiness as measured by MSLT or by ESS after a 1 year follow-up on CPAP. |
| Al-Alawi et al.970 | 2006 | 3b | Case series | In data of 795 patients who underwent polysomnography: (3) 351 patients had PLMS and OSA (4) 26 patients had PLMS without OSA |
Examine the prevalence of PLM arousals in OSA patients Examine relationship of PLM arousal index with risk factors |
There was a higher PLMI in OSA subjects (44% had both). Patient with PLM arousal index ≥ 5/h had higher relationships with predisposing conditions (depression, Fibomyalgia, and DM), older age, more predisposing medications, obesity, and more likely to have OSA. There was no difference in the relationship with ESS or hypertension between patient with OSA only or OSA and PLM with arousals. |
| Xie et al.975 | 2017 | 2b | Cross-sectional study | In 15,414 patients who attended a PSG, 50.8% had PLMI>15/h, 36.1% had PLM>30/h, and 13.1% had PLMI between 15 and 30/h. The prevalence of atrial fibrillation was 15.3% | Examine whether PLMS is associated with the prevalence of atrial fibrillation in a group of patients with Sleep Disordered Breathing (SDB) | In a multivariate adjustment model, only mild SDB patients with PLMI > 30/h had 1.21 times higher odds for atrial fibrillation compared with patients with PLMI < 15/h (OR 1.21, 95% CI 1.00–1.47, p-value = 0.048). Similarly, PLMAI > 5/h had higher odds compared to PLMAI < 1 for atrial fibrillation in mild SDB patients (OR 1.27, 95% CI 1.03–1.56, p-value = 0.024). |
| Iriarte et al.969 | 2009 | 4 | Cross-sectional study | In a referral sleep center for sleep symptoms, patients were recruited and grouped: (1) 35 patients with no diagnosis for OSA and PLMS (2) 94 patients with OSA only (3) 37 patients with PLMS only (4) 64 patients with PLMS and OSA |
Examine the importance of PLMS on sleep quality in patient referred for sleep symptoms in patients with or without OSA | In a multivariate analysis, adjusting for age, sex, and AHI, PLMS was associated significantly with an increase in REM latency of 38 min (CI 95% CI 4.4–71.6; p-value = 0.03) and a decrease stage NREM 3 and 4 of 3.7% (CI 95% CI 0.2–7.2; p-value = 0.04), when comparing all four groups. The effects of PLMS on sleep architecture parameters were worse in the PLMS only group as compared to OSA only or combined OSA and PLMS group. |
| Loewen et al.981 | 2009 | 3b | Case–control | (1) 12 patients with OSA and end-stage renal disease (2) 18 patients with OSA and normal renal function |
Differences in effect of PLMS on subjective and objective measures of sleep quality | |
| Aritake-Okada et al.967 | 2012 | 3b | Case–control | 997 patients diagnosed with OSA: 67 in persistent group (PLMI ≥ 15/h in both diagnositic and CPAP titration study), 80 in CPAP-emergent group (PLMI < 15/h in diagnostic, PLMI ≥ 15/h in CPAP titration study), 40 CPAP-disappeared group (PLMI ≥ 15/h in diagnostic study, PLMI < 15/h in CPAP titration study), non-PLMS group (PLMI < 15/h in diagnostic and CPAP titration study). | Examine the change in prevalence of PLMS in diagnostic and CPAP titration study in OSA patients (AHI > 20 events/h). Examine the associated factors on PLMS groups | PLMS were significantly increased from diagnostic to CPAP titration study in the persistent group, CPAP-emergent group, and non-PLMS group and decreased in the CPAP-disappeared group (all p-value < 0.001). In a multivariate regression analysis, CPAP-emergent PLMS group was associated with older age (age > 47 years: OR: 1.69, 95% CI 1.05–2.71, p-value = 0.03)) and higher AHI (diagnostic AHI > 30/h: OR: 2.19, CI: 1.16–4.11, p-value: 0.015). |
| Drakatos et al.972 | 2016 | 3b | Case–control | 49 males without comorbid cardiovascular disease: eight controls, 13 with PLMs (defined as PLMI > 15/h), 17 OSA (defined as AHI > 10/h) and 11 OSA + PLMs | Compare stiffness index derived from the digital volume pulse (SI-DVP) among the groups | Patients with PLMS had higher arterial stiffness measures compared to controls. The OSA/PLMS group had the highest SI-DVP. |
| Xie et al.982 | 2019 | 3b | Case–control | 14,444 PSGs conducted over a 4-year period were examined. 314 patients with CSA completed the study with CPAP titration and in cases of persistent CSA, ASV titration | To examine changes in PLMS in response to ASV for CSA | In the age group >68, presence of heart failure was associated with increases in PLMI and PLMAI, even after adjustment for age and severity of HF. |
| Murase et al.973 | 2014 | 3b | Case–control study | 46 patients with OSA and PLMs were compared to 208 patients with OSA without PLMS | Compare inflammatory markers in OSA patients with vs. without PLMs | The OSa and PLMS group had higher CRP and fibrinogen levels compared to the OSA group without PLMS. |
| SRRMD; Aggregate level of evidence: D; (Level 4: one study) | ||||||
| Chiaro et al.978 | 2017 | 4 | Case Series | 5 patients with diagnosed SRRMD with RMEs seen following OSA events | Investigate the role of sleep apnea as a trigger for rhythmic motor events as a respiratory related arousal mechanism | SRRMD in adult patients may imply sleep-disordered breathing, possibly associated with longer respiratory events. |
| Bruxism; Aggregate level of evidence: B; (Level 2a: two studies) | ||||||
| Lopes977 | 2019 | 2a | Systematic review | Systematic review; 200 articles identified after initial search and seven included in the qualitative synthesis | Examine the association between sleep bruxism and OSA | Well-designed studies are lacking but based on available evidence, OSA patients do not experience sleep bruxism significantly more than controls. A subtype of patients with OSA may have sleep bruxism. Occurrence of sleep bruxism has been proposed as a protective mechanism for respiratory events. |
| Jokubauskas976 | 2017 | 2a | Systematic review | Systematic review; 691 articles identified after initial search and three included in the synthesis | Examine the association between sleep bruxism and OSA | There are insufficient data to establish an association between OSA and sleep bruxism. Sleep bruxism events occur during microarousal events resulting from apneas/hypopneas and most often are temporally related to the termination of an apnea/hypopnea. |
VII.I.3 |. Narcolepsy
Narcolepsy is a chronic neurologic disorder characterized by excessive daytime sleepiness and abnormal regulation of REM sleep. Narcolepsy type 1 (NT1) refers to narcolepsy with cataplexy and NT2 to narcolepsy without cataplexy. NT1 occurs with a prevalence of 25–50 per 100,000 people.983 It is associated with a deficiency of hypocretin, also called orexin, which is a neuropeptide hormone secreted by a small group of cells located in the lateral hypothalamus. NT1 is thought to be autoimmune in nature; though no antibody has been detected, there is a strong HLA association to HLA-DQB1*06:02.984 The primary diagnostic test for NT1 and NT2 is the MSLT, consisting of overnight PSG followed by a series of five daytime naps at 2-h intervals and assessing average sleep latency across the naps and the number of sleep-onset REM periods (SOREMPs), that is, REM during the naps.
Patients with narcolepsy have an increased risk of multiple comorbidities compared to age matched controls including obesity, diabetes, depression, thyroid disease, and hypertension; however, OSA is the most common comorbidity, both at time of diagnosis and follow-up.985 The incidence of OSA is approximately 25% in those with narcolepsy and nearly half of these patients have moderate to severe OSA.986,987 At the time of the initial diagnosis of narcolepsy, the odds of having a diagnosis of OSA compared to matched controls is markedly increased, ranging from 18 to 69.985,988,989
There is on average a 6–10-year delay to diagnosis for those with narcolepsy,986,990 which underscores the need to be alert to the frequency with which OSA and narcolepsy can co-occur. If treatment of OSA results in no or minimal improvement in excessive daytime sleepiness, the provider should consider the possibility of another sleep disorder, such as narcolepsy or other central disorder of hypersomnolence.987 The presence of cataplexy, unique to NT1, can also assist in this differentiation. If pursuing PSG and MSLT to quantify sleepiness in a patient with treated OSA and persistent excessive daytime sleepiness, the test result validity is maximized when OSA treatment is used during the polysomnogram and during MSLT naps.
It is also possible for a patient to be misdiagnosed with narcolepsy when the etiology of sleepiness is OSA alone, as polysomnographic findings similar to narcolepsy can result from untreated or undertreated OSA.991 Five percent of those with sleep related breathing disorders can have two or more SOREMPs on an MSLT, suggesting REM periods during naps are less specific to narcolepsy than initially believed.991 Male sex, sleepiness, short REM latency on nocturnal polysomnogram, and minimal SaO2 are risk factors for two or more SOREMPs in a patient with OSA.
Among the treatments for narcolepsy are daytime stimulant medications and sodium oxybate. While sodium oxybate can improve excessive daytime sleepiness and cataplexy, it is also known to be a respiratory depressant at higher doses, raising concern that it could worsen sleep apnea. Though current data is mixed regarding this possibility,992–995 the use of sodium oxybate in a patient with narcolepsy could potentially contribute to the development or worsening of OSA and repeat screening for this may pursued in certain clinical contexts.
In summary, OSA is a common comorbidity in patients with narcolepsy. OSA is a more common explanation for excessive daytime sleepiness than narcolepsy based on population prevalence. However, in patients with excessive daytime sleepiness, it is imperative to confirm efficacy of OSA treatment before ruling out narcolepsy. (Table VII.I.3)
TABLE VII.I.3.
Narcolepsy and prevalence of comorbid sleep apnea
| Study | Year | LOE | Study design | N/age/cohort | Sample population | Conclusion |
|---|---|---|---|---|---|---|
| Sansa et al., 2010987 | 2010 | 2c | Cross-sectional | N = 133; ages 11–80 | Single university hospital sleep clinic | AHI > 10 events/h in 24.8% of those with narcolepsy, 30% of these led to a delay in diagnosis of 6.1 ± 7.8 years. Excessive daytime sleepiness improved in 21% of those treated with CPAP. |
| Frauscher et al., 2013986 | 2013 | 2c | Cross-sectional | N = 100, ages 16–78; Innsbruck narcolepsy cohort | Single center, academic facility, tertiary referral center | 24% of those with narcolepsy had sleep-related breathing disorders: 14 had mild sleep apnea, eight moderate sleep apnea, and two had severe sleep apnea. The majority had obstructive sleep apnea syndrome (21/24); 2/24 had mixed sleep apnea syndrome; and 1/24 central sleep apnea syndrome. |
| Jennum et al., 2013996 | 2013 | 3b | Case–control | N = 757; age <20 to >80; Danish National patient registry | Patients diagnosed with narcolepsy in Denmark | Prior to narcolepsy diagnosis OR for sleep apnea diagnosis 44.5, 95% CI 13.1–151.3; after the narcolepsy diagnosis OR for sleep apnea 19.2, 95% CI 7.7–48.3. |
| Pizza et al., 2013997 | 2013 | 2c | Cross–sectional | N = 35; mean age 40 ± 16 | Narcolepsy with cataplexy patients seen at a university hospital | 31% of patient had sleep-disordered breathing; Mild SDB was diagnosed in 4 (11%) subjects, moderate SDB in 4 (11%), and severe SDB in 3 (9%). In five out of 11 (46%) patients with SDB, apneas were mostly obstructive, in one it was central. The remaining five patients (46%) showed mixed, obstructive and central apneas. |
| Black et al., 2017988 | 2017 | 3b | Case–control | N = 9321; mean age 46.1 ± 13.3 | US medical claims data (private insurance and Medicare) | Sleep apnea was present in 51.4% of those with narcolepsy and 5.8% of matched controls, 45.6% excess prevalence, p < 0.0001, OR 18.7, 95% CI (17.5–20.0). |
| Jennum et al., 2017989 | 2017 | 3b | Case–control | N = 339; 20–50 years old; Danish National Patient Registry | Patients diagnosed with narcolepsy in Denmark | Prior to narcolepsy diagnosis OR for sleep apnea 34.6, 95% CI 18–66.5; after narcolepsy diagnosis OR 35.2 for sleep apnea, 95% CI 19.4–63.9. |
| Cohen et al., 2018985 | 2018 | 2b | Prospective cohort | N = 68; ages 5–74; Rochester Epidemiology Project | Population-based in Olmsted Count, MN | At time of narcolepsy diagnosis OR of having OSA 69.25, 95% CI 9.26–517.99; OR of having OSA after observation period 13.55, 95% CI 5.08–36.14. |
| Filardi et al., 2020998 | 2020 | 3b | Case–control | N = 38; ages 2–18 | Children and adolescents with narcolepsy with cataplexy at academic medical center | No difference in prevalence of sleep-disordered breathing between narcolepsy and controls. |
VII.J |. Conditions Associated with OSA: Pregnancy
Due to a number of physiologic and hormonal changes, pregnancy is a time of unique vulnerability among women for SDB. High estrogen levels can lead to nasal congestion (i.e., rhinitis of pregnancy)999 and may contribute to UA narrowing.1000 While high progesterone levels increase ventilatory drive, whether this effect promotes or protects against respiratory instability and SDB during sleep is uncertain.1001 Diaphragmatic elevation leads to decreased functional residual capacity, which in turn contributes to reduced oxygen reserve; these changes become more pronounced in the supine position and during late pregnancy.1001
Both subjective and objective measures of SDB increase with advancing pregnancy.1002–1004 By the end of the second trimester, women report increases in the frequency of snoring, gasping or snorting, and witnessed apneas compared to the first trimester1002; these symptoms generally further increase until delivery. Recently, when more than 3300 women underwent home sleep testing in the prospective, multicenter SDB substudy of the Nulliparous Pregnancy Outcomes Study (nuMoM2b), the prevalence of SDB (AHI ≥ 5 events/h) increased from 3.6% in early pregnancy to 8.3% in mid-pregnancy.1003 The vast majority of SDB cases in the nuMOM2b cohort were mild,1003 a similar finding to other studies.1001 Older age, higher BMI, larger neck circumference, non-Hispanic black race, smoking, and chronic hypertension were all associated with increased AHI.1003 Other studies have identified similar risk factors for gestational OSA.1005,1006
An accumulating body of data demonstrates an increased risk of cardiometabolic complications among women with gestational SDB.1001 Women in the nuMOM2b study with SDB in early or mid-pregnancy were nearly twice as likely to develop preeclampsia compared to women without SDB. The odds for hypertensive disorders of pregnancy (gestational hypertension or preeclampsia) were increased only among women with mid-pregnancy SDB, and not among women with early pregnancy SDB. The nuMoM2b study also observed an approximately three-fold increase in the odds for gestational DM among women with early and mid-pregnancy SDB, compared to women without SDB.
A recent metanalysis examined associations between subjectively and objectively measured SDB and multiple maternal and fetal outcomes, with preeclampsia, gestational hypertension, and gestational diabetes mellitus (GDM) having the largest numbers of studies available for inclusion (i.e., 15–20 individual studies).1007 In these analyses, the risks for gestational hypertension, preeclampsia, and GDM were all increased among women reporting SDB symptoms.1007 Among women with objectively diagnosed OSA, the risk for preeclampsia more than doubled, whereas risks for gestational hypertension and GDM nearly doubled.1007 The risk for cesarean section also increased significantly among women with either subjective or objective SDB.1007 Other studies have found increased risks for severe maternal morbidity including eclampsia, cardiomyopathy, pulmonary embolism, and in-hospital mortality.1008,1009
Studies examining fetal outcomes have largely focused on preterm birth and growth-related outcomes, especially small for gestational age (SGA) births.1007 A recent metanalysis found that the risk for preterm birth increased approximately 50% among women who reported subjective SDB symptoms or had been diagnosed with OSA.1007 While some individual studies have observed increased risks for SGA infants among women with SDB, two meta-analyses did not find an increased risk for SGA births among women with SDB symptoms or objectively documented OSA.1007,1010
Case reports and case series suggest that treatment of SDB during pregnancy improves maternal-fetal outcomes.1011,1012 However, there is a paucity of controlled trial data. Several trials examining the effect of CPAP therapy on outcomes including BP, glycemic control, and CV risk are in progress.1001,1013 The current approach to treatment is extrapolated from recommendations and data in the general population, as there are no pregnancy-specific guidelines. CPAP therapy is generally considered first-line treatment for gestational OSA, as it is widely effective and can be initiated quickly. Furthermore, auto-adjusting CPAP can accommodate changes in SDB severity during and after pregnancy. Women with preexisting OSA may also benefit from switching at least temporarily from fixed CPAP or OAs to auto-adjusting CPAP therapy during pregnancy to accommodate fluctuations in OSA severity. For women with positional (i.e., supine) SDB, the left lateral sleep position may be sufficient to avoid apneic events and maximize venous return via the inferior vena cava.1014
While pregnancies in women with OSA are widely considered high risk,1015 clinical practice guidelines specifically for management of pregnant women with OSA have not been offered. The use of continuous pulse oximetry during labor and the postpartum period can identify hypoxic episodes and guide management.1015 Early consultation with the anesthesia service can help to avoid the use of general anesthesia, which poses increased risks to individuals with OSA,1016 should delivery by cesarean section be indicated.
Postpartum, women receiving treatment for OSA should resume therapy as soon as is feasible. The use of opioids should be carefully considered, given increased risks for morbidity and mortality among OSA patients taking opioids.1015,1017 In a group of women in the immediate postpartum period (within 48 h after delivery), elevating the upper body to 45° during sleep reduced the AHI significantly compared to sleeping in a non-elevated position.1018 This low technology intervention was well tolerated and can be easily recommended to patients.
While the severity of gestational OSA generally improves after delivery,1019–1021 women diagnosed with or suspected of having OSA during pregnancy should be followed postpartum to determine whether OSA has resolved or requires further treatment.1015,1017 Little is known about how frequently SDB during pregnancy persists or recurs afterwards, or about the effect of gestational SDB on risk later in life for adverse cardiometabolic outcomes such as hypertension and DM.
VIII |. MEDICAL TREATMENT FOR OSA
VIII.A |. Medical Management of OSA: Positive Airway Pressure (PAP) Therapy
VIII.A.1 |. Types of PAP for OSA
VIII.A.1.a |. Types of PAP: Continuous positive airway pressure (CPAP)
CPAP, the gold standard OSA treatment, acts as a pneumatic splint to maintain UA patency. Typically, after OSA diagnosis, CPAP is initiated with in-lab titration polysomnogram. An SR by Patil et al.39 demonstrated a clinically significant reduction in disease severity as evidenced by complete resolution or near resolution of the AHI; improvement in subjective sleepiness (reduction in ESS score of 2.4 points based on metanalysis of 38 RCTs, and ability to maintain wakefulness based on the Maintenance of Wakefulness Test [MWT]); sleep related QOL; and BP. Two additional SRs1022,1023 that studied the effect CPAP therapy had on QOL showed similar results. The randomized clinical trial by Ponce et al.1022 compared the use of CPAP to no CPAP in 154 patients and demonstrated that the use of CPAP decreased subjective sleepiness based on a reduction in the ESS by 2.6 points. A positive correlation between duration of CPAP use and reduction in ESS score was also seen. The Zhao study1023 was an SR and demonstrated that the use of CPAP therapy in non-sleepy individuals with OSA improved health-related quality of life (HRQOL) by using the SF-36 questionnaire. Improvements were specifically seen in vitality, general health, bodily pain, and physical health. Given the extent of conflicting data about effect of CPAP use on CV outcomes, the SR by Patil et al.39 noted that there is “insufficient and inconclusive evidence to either recommend or withhold PAP to treat non-sleepy adults with OSA as a means to reduce CV events or mortality.”
An SR of empirical studies with observational or experimental designs with monetized health economic outcomes of OSA treatments based on comparisons showed that, compared with no treatment, PAP was associated with favorable economic outcomes; these outcomes were greater with adherence to PAP therapy.1024 In 2015, the average cost of CPAP inclusive of testing, appointments, treatment devices, and surgery, if necessary, was estimated at $2105 per patient per year.132 (Table VIII.A.1.a)
TABLE VIII.A.1.a.
Evidence for the use of CPAP to treat OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion | ||
|---|---|---|---|---|---|---|---|---|
| Wang et al.841 | 2019 | 2a | SR | 1. CPAP 2. no therapy |
Cognitive decline | CPAP likely improves memory impairment in patients with OSA and cognitive decline. | ||
| Ponce et al.1022 | 2019 | 1b | RCT | 145 patients 1. CPAP 2. no CPAP |
ESS, QSQ, sleep related symptoms, anxiety, and depression | CPAP therapy improved HRQOL measures in patients over the age of 70. | ||
| Patil et al.39 | 2019 | 1a | SR | 336 studies that met inclusion criteria; 184 studies provided data suitable for meta-analyses | Multiple outcomes of sleep apnea | CPAP reduces disease severity, sleepiness, blood pressure, and motor vehicle accidents. | ||
| Zheng et al.1025 | 2019 | 1a | SR | 17 studies comparing 1. CPAP, 2. control/placebo/sham CPAP in treatment of anxiety and depression in patients with OSA | Various measurements of anxiety and depression | CPAP reduced symptoms of depression. | ||
| Ning et al.1026 | 2019 | 1a | SR | 14 RCTs evaluating effect of CPAP therapy on various cardiac biomarkers | Cardiac biomarkers | CPAP therapy improves levels of inflammatory biomarkers. | ||
| Khan et al.1027 | 2018 | 1a | SR | 7 RCTs studying the use of CPAP in reducing MACE in patients with moderate to severe sleep apnea | MACE: MI, CV mortality | CPAP therapy of greater than 4 h a night significantly reduced MACE. | ||
| Wang et al.635 | 2018 | 2a | SR | 9 studies (seven observational and two RCTs) studying cardiovascular outcomes in patients with coronary artery disease and OSA | MACE: cardiovascular death, stroke, hospitalization for heart failure | CPAP may decrease cardiovascular events in patients with CAD and OSA. | ||
| Labarca et al.1028 | 2018 | 1a | SR | 6 RCTs studying effects of CPAP therapy in OSA and type II diabetes | HbA1c, fasting glucose | CPAP therapy does not significantly reduce HbA1c or fasting glucose levels. | ||
| Gupta et al.1029 | 2019 | 1b | RCT | 70 patients with OSA and recent stroke randomized to 1. CPAP, 2. non-CPAP | New vascular events | CPAP therapy reduces the presence of new vascular events in patients with a stroke. | ||
| Congrete et al.1030 | 2018 | 2a | SR | 7 studies reviewing the risk of recurrent atrial fibrillation after catheter ablation in patients with obstructive sleep apnea | Recurrent episodes of atrial fibrillation | Treatment of OSA with CPAP decreased the incidence of recurrent atrial fibrillation. | ||
| Joyerux-Faure et al.1031 | 2018 | 1b | RCT | 37 patients with obstructive sleep apnea and resistant hypertension randomized to 1. CPAP 2. Sham CPAP |
Blood pressure | Treatment with CPAP lowered nocturnal systolic blood pressure. | ||
| Zhao et al.1023 | 2017 | 1b | RCT | 169 Patients with moderate to severe OSA and CVD randomized to 1. Medical therapy 2. Medical therapy + sham CPAP 3. Medical therapy + CPAP 4. Medical therapy + CPAP + motivational enhancement. |
Health-related quality of life | CPAP improves health related QOL metrics. | ||
| Abuzaid et al.1032 | 2017 | 1a | SR | 4 RCTs studying cardiovascular outcomes in patients with moderate to severe OSA treated with CPAP | MACE | CPAP therapy did not reduce the number of cardiovascular events. | ||
| Campos-Rodriguez et al.1033 | 2017 | 1b | RCT | 307 women with moderate to severe OSA randomized to 1. CPAP 2. Conservative therapy |
Blood pressure | CPAP therapy significantly decreases diastolic blood pressure. | ||
| Wickwire et al.1024 | 2019 | 1a | SR | Empirical studies with observational or experimental designs with monetized health economic outcomes of OSA treatments based on comparisons | Health economic outcomes | Relative to no treatment, PAP was associated with favorable economic outcomes. PAP adherence was positively associated with greater economic outcomes. | ||
| Hoyos et al.1034 | 2015 | 1a | SR | 7 RCTs studying the effect of CPAP therapy on blood pressure | Blood pressure | CPAP therapy reduces nocturnal systolic and diastolic blood pressures. | ||
Abbreviations: APAP, auto-titrating CPAP; BPAP, bilevel PAP; CAD, coronary artery disease; CPAP, continuous PAP; CV, cardiovascular; ESS, Epworth Sleepiness Scale; HbA1c, glycated hemoglobin; HRQOL, health-related quality of life; MACE, major adverse cardiovascular events; MI, myocardial infarction; OSA, obstructive sleep apnea; PAP, positive airway pressure; QOL, quality of life; QSQ, Quebec Sleep Questionnaire; RCT, randomized control trial.
VIII.A.1.b |. Types of PAP: Auto-titrating positive airway pressure (APAP)
Two SRs39,1035 evaluated the differences between APAP and CPAP devices. Both modalities resulted in a clinically significant reduction of the AHI, sleepiness as measured by the ESS, and QOL. There was no significant difference between the two treatment modalities. The randomized equivalence trial of 208 sleepy patients (mean ESS 13.1) by Bloch et al.1036 comparing APAP to CPAP demonstrated reductions of ESS scores of 6.3 and 6.2, respectively. The systemic review by Ip et al.1035 demonstrated that APAP reduced ESS score by 0.5 points when compared to CPAP, though this increased benefit is likely not clinically relevant. One of the presumed benefits of APAP therapy is that it will lead to increased compliance due to its ability to automatically adjust pressures based on patient need. The SR by Patil et al.39 did not demonstrate any difference in PAP adherence as measured by hours used, nights used, or nights used with greater than 4 h of usage per night. While the SR by Ip et al.1035 did show an 11-min increase in PAP usage per day with APAP therapy, this is likely to be clinically insignificant. These studies did not evaluate peak or mean pressures achieved during APAP use. In addition, an RCT demonstrated that APAP was not found to lower mask leak rates when compared to CPAP.1037 Additional RCTs1036,1038 demonstrated similar findings in the reduction of sleepiness1036 and improvement in QOL measures.1039
APAP was shown to have no added benefit in BP reduction when compared to CPAP. The large meta review performed by Ip et al.1035 included three RCTs that evaluated the effect of APAP and CPAP on BP and found that there was no significant difference between the two modalities. The RCT by Pepin et al.1038 was a double-blind RCT, which randomized 322 patients with OSA to receive either APAP or CPAP, found reductions in diastolic BP with CPAP but not APAP in the intention to treat analysis.
Patil et al.39 examined the comparison between in-lab titration of CPAP and home APAP (without lab titration) for initiation of PAP therapy. Analysis showed similar effects on OSA severity, sleepiness, and adherence with APAP initiated at home. Patients value preferences and resource utilization are considerations when choosing in-home APAP initiation versus CPAP titration. CPAP or APAP for the treatment of OSA is recommended. (Table VIII.A.1.b)
TABLE VIII.A.1.b.
Evidence for APAP for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Wimms et al.1038 | 2019 | 1b | RCT | 301 patients with mild OSA randomized to 1. APAP + standard care, 2. standard care | QOL from SF-36 | APAP usage improved QOL in patients with mild obstructive sleep apnea. |
| Patil et al.39 | 2019 | 1a | SR | 26 RCTs comparing APAP to CPAP | OSA severity, adherence, sleepiness, QOL, neurocognitive function | No difference between APAP and CPAP. |
| Lebret et al.1037 | 2019 | 1b | RCT | 276 patients with OSA randomized to 1. CPAP, 2. APAP | Mask leak | PAP modality did not affect level of mask leak. |
| Bloch et al.1036 | 2018 | 1b | RCT | 208 patients randomized to 1. CPAP, 2. APAP(5–15 cmH2O) | Subjective and objective sleepiness | APAP is effective in treating EDS. |
| Pepin et al.1039 | 2016 | 1b | RCT | 322 patients with OSA randomized to 1. CPAP, 2. APAP | Difference in office based systolic blood pressure after 4 months of therapy | APAP can lower office based systolic blood pressure. |
| Ip et al.1035 | 2012 | 1a | SR | 24 RCTs comparing CPAP to APAP | Adherence | Eleven-minute increase in PAP adherence with APAP. |
Abbreviations: APAP, auto-titrating CPAP; BPAP, bilevel PAP; CPAP, continuous PAP; OSA, obstructive sleep apnea; PAP, positive airway pressure; QOL, quality of life; RCT, randomized control trial.
VIII.A.1.c |. Types of PAP: Bilevel PAP
Few studies included in the SR studied bilevel PAP (BPAP). An SR of PAP treatments for OSA found no benefit for BPAP over CPAP.39 Review of studies showed no significant difference between BPAP, CPAP, and APAP for the improvement in OSA severity, sleepiness, and QOL. BPAP also confers no significant advantage over CPAP or APAP in improved adherence, except as a potential therapy option for patients nonadherent to CPAP.39 Patients who require high PAP levels greater than what CPAP devices can deliver may benefit from the use of BPAP. For patients with routine OSA, BPAP devices may cost more and may not provide sufficient reduction in the AHI if the expiratory pressure setting is too low, thus BPAP should not be considered in these patients.
A single center RCT that studied an enhanced education support program for patients with OSA reported no difference in PAP adherence, nightly duration of PAP use or reduction in subjective daytime sleepiness between use of BPAP, CPAP, or APAP.1040
BPAP may be indicated when CPAP is not tolerated. Ballard et al.1041 examined 100 patients who were persistently noncompliant with CPAP after mask optimization, humidification, and education measures and performed a double-blind randomized trial of standard CPAP versus BPAP use and showed more participants in the BPAP group achieved compliance of PAP use more than 4 h per night. (Table VIII.A.1.c)
TABLE VIII.A.1.c.
Evidence for use of BPAP for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Patil et al.39 | 2019 | 1a | SR | 5 RCTs comparing BPAP or auto-BPAP to CPAP. | OSA severity, treatment adherence, sleepiness, QOL | No difference between BPAP or auto-BPAP and CPAP for OSA severity, sleepiness, QOL. |
| Sarac et al.1040 | 2016 | 1b | RCT | 115 patients with OSA randomized to standard care or enhanced educational support. | PAP adherence rates, PAP hours/night, reduction in Epworth Sleepiness Scale Score | No stated difference between BPAP, CPAP, and APAP. |
| Ballard et al.1041 | 2007 | 1b | RCT | 104 OSA patients persistently noncompliant with CPAP: double blind randomized CPAP vs. flexible BPAP. | Compliance with PAP | More on BPAP 25/51 (49%) achieved compliance vs. 15/53 (28%) randomized to CPAP. |
Abbreviations: APAP, auto-titrating CPAP; BPAP, bilevel PAP; CPAP, continuous PAP; OSA, obstructive sleep apnea; PAP, positive airway pressure; RCT, randomized control trial; QOL, quality of life.
VIII.A.1.d |. Types of PAP: Adaptive servo-ventilation
ASV is a form of auto-adjustable bilevel positive pressure support with a backup rate which is used to treat central sleep apnea syndromes (CSAS). ASV devices provide expiratory positive airway pressure (EPAP) to achieve and maintain UA patency during sleep as well as variable inspiratory pressure support (IPS) and auto-backup rate (BUR) in order to stabilize the respiratory control circuit and alleviate cyclical episodes of hyperand hypoventilation. The EPAP supplied by ASV devices is either titrated to the critical airway opening pressure needed to achieve UA patency during an attended PSG or auto-adjusted by the device (if equipped with auto-EPAP capabilities).1042,1043 IPS and the BUR are dynamically auto-adjusted, in relation to the patient’s native respiratory efforts and device-specific minute-ventilation-associated targets, with the goal of preventing episodes of respiratory instability.1044,1045 Importantly, the proprietary algorithms used for dynamic IPS and BUR adjustment have been shown to vary by device,1045,1046 which could potentially lead to difficulties achieving therapeutic targets.1045
ASV therapy is not recommended for uncomplicated OSA.39 However, there is a limited body of literature to support its use in cases of persistent treatment-emergent central sleep apnea (TECSA).1047–1055 TECSA is defined by the initial presence of OSA (obstructive AHI ≥ 5 events/h in at least 2 h of recorded sleep) followed by resolution of obstructive events with CPAP titration, and the subsequent appearance of central respiratory events with ongoing CPAP therapy (residual central AHI [CAHI] ≥ 5/h in at least 2 h of recorded sleep time).81,1042 TECSA does not include patients with CHF-associated CSAS. ASV can be used in CSAS with concurrent HF and opioid-associated CSAS.1056 Updated guidelines examined the increased risk for cardiac mortality in patients with an LVEF less than 45% and moderate to severe CSA predominant SDB. Guidelines recommend against the use of ASV in patients with LVEF ≤ 45%.1057
VIII.A.2 |. PAP use: mask interfaces
CPAP therapy was originally designed for use with a nasal interface that would deliver positive pressure through the nares and act as a pneumatic splint, pushing the soft palate and tongue forward and away from the posterior oropharyngeal wall to prevent UA occlusion.3 Over time, numerous nasal masks, oronasal masks, nasal pillow interfaces, and even oral masks have been developed for use with CPAP, with the goal of maximizing mask tolerability, a major determinant of CPAP adherence.1058 Nevertheless, questions about which types of CPAP interfaces are associated with the best adherence and about the effectiveness of oronasal and oral interfaces persist.
A 2006 Cochrane review of CPAP delivery interfaces for OSA identified only four randomized, controlled studies that met the stringent criteria for inclusion and concluded that the optimal form of CPAP delivery interface remained unclear.1059 The authors indicated that nasal pillows or the Oracle oral mask could be useful alternatives for patients unable to tolerate conventional nasal masks. Similarly, they suggested that oronasal masks should not be recommended for initial use but could be considered among individuals whose nasal symptoms limited nasal mask use. Subsequently, an MA that included five randomized and eight non-randomized trials found that oronasal masks were associated with a significantly higher CPAP level, higher residual AHI, and poorer adherence compared to nasal masks.1060 While data from a total of 4563 individuals with OSA was included, the majority of the data came from a single study.1061 Limitations including moderate to high heterogeneity between studies with regards to CPAP level and adherence were noted.
Most recently, as part of an SR focused on the use of PAP for treatment of OSA in adults, the AASM published a series of meta-analyses evaluating the effects of different PAP interfaces on outcomes including reducing AHI, improving adherence to PAP therapy and sleepiness.39 These analyses utilized data from eight RCTs and three observational studies. Residual AHI was higher using oronasal masks compared to nasal interfaces, though the difference was not clinically significant. Adherence to CPAP was similar when intranasal (nasal pillows) and nasal interfaces were compared; however, adherence was significantly higher with nasal interfaces compared to oronasal interfaces. No significant differences in sleepiness were observed in comparisons of oronasal and nasal masks, nor in comparisons between nasal pillows and nasal masks. Data were insufficient to perform analyses examining differences in QOL or interface-associated side effects.
The current review identified eleven small (n = 14–85) randomized trials of different mask interfaces that examined outcomes including optimal CPAP pressure, residual AHI on CPAP, CPAP adherence (duration or frequency of use), ESS score, side effects, and patient preference.1062–1072 Most were crossover trials in which participants were assigned to use CPAP with nasal masks and oronasal masks in random order1063,1065,1068,1069,1071; two trials included a third arm using nasal pillows.1064,1065
In one trial, oronasal mask use required significantly higher final CPAP pressures to treat OSA compared to use of nasal masks or nasal pillows.1064 Another found no difference between oronasal and nasal masks in optimal CPAP pressures.1071 Four trials found significantly higher residual AHIs using oronasal masks compared to nasal masks, despite delivery of the same or similar CPAP pressures during both study arms,1063,1065,1069,1071 but differences were not clinically meaningful.
While some studies have observed significantly longer durations of CPAP use with nasal masks compared to oronasal masks,1065,1068 others have found no significant differences.1063,1069 Comparisons of objective and subjective sleep quality and daytime sleepiness have demonstrated more slow wave and TST during CPAP titration using nasal masks compared to oronasal masks,1071 better sleep quality using CPAP with nasal masks compared to oronasal masks,1069 and less sleepiness (i.e., lower ESS scores) using nasal masks compared to oronasal masks.1068 Patients also expressed an overall preference for nasal masks compared to oronasal masks.1068,1069,1071
When nasal masks and nasal pillows were compared, no significant differences were observed in 95th percentile auto-titrating pressures.1072 Three randomized crossover trials observed no significant differences in mean nightly duration of CPAP use or residual AHI when comparing nasal masks to nasal pillows.1067,1070,1072 Patients reported similar or fewer adverse effects with nasal pillow use compared to nasal mask use.1067,1070 Sleep quality,1072 ESS scores, and QOL1067,1070 generally did not differ during nasal pillow use compared to nasal mask use. Patients did not express a clear preference for nasal pillows or nasal masks in these studies.1070,1072
Oral masks have been compared to nasal masks in two randomized crossover studies.1062,1066 Significant differences between the interfaces were not observed when effective CPAP pressure,1062 average duration of CPAP use1062,1066 residual AHI during PAP titration,1062 and sleepiness1062 were compared. The side effect profiles differed: while oral mask use was associated with more dry mouth and throat, gum discomfort, and excessive salivation, nasal mask use was associated with more complaints of nasal congestion and mask leak.1062,1066
Four observational studies comparing CPAP mask interfaces were identified.1061,1073–1075 The largest was a prospective cohort study of 2311 newly diagnosed OSA patients who were given a choice of nasal masks, oronasal masks, or nasal pillows.1061 Users of oronasal masks and nasal pillows were more likely to be non-adherent to CPAP (mean use <4 h/night) at follow-up compared to nasal mask users. Oronasal mask users were more likely than the other groups to report side effects and to find CPAP inconvenient. Two other studies have observed that effective CPAP pressure and residual AHI were higher with oronasal mask use compared to nasal masks1074 and/or nasal pillows.1075
In general, the data suggest that nasal interfaces should be utilized initially in patients starting CPAP therapy. The use of oronasal masks is associated with the need for higher levels of CPAP pressure, and with higher residual AHI. Compared to nasal masks, adherence in several studies was lower with oronasal mask use. Mostly, outcomes including CPAP adherence and residual AHI were similar when nasal pillows and nasal masks were compared. Data on use of oral masks was particularly sparse. Oronasal and oral masks may be appropriate for a select group of patients who are predominantly mouth breathers, or who have large air leaks during sleep due to mouth opening, but these studies have not been performed. (Table VIII.A.2)
TABLE VIII.A.2.
Summary of the evidence for PAP mask interfaces
| Study | Year | LoE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Patil et al.39 | 2019 | 1a | Meta-analyses | 8 RCTs, three observational studies | Residual AHI, CPAP adherence, sleepiness, QOL, side effects | No significant differences between NP and NM in residual AHI or adherence. Higher residual AHI and lower CPAP adherence using ONM compared to NM. No differences in sleepiness between NM and NP, or between NM and ONM. |
| Goh et al.1065 | 2019 | 2b | Randomized unblinded crossover | Newly diagnosed moderate-severe OSA (n = 85) 1. Nasal masks 2. Oronasal masks 3. Nasal pillows |
CPAP adherence, residual AHI | Significantly better adherence with NM compared to ONM and NP. Significantly higher residual AHI with ONM compared to NM and NP. |
| Rowland et al.1069 | 2018 | 2b | Randomized unblinded crossover | Moderate-severe OSA (n = 48) 1. Nasal masks 2. Nasal masks plus chinstrap 3. Oronasal masks |
CPAP adherence, residual AHI, sleepiness, patient satisfaction, sleep quality, side effects, patient preference | No significant different in CPAP adherence. Significantly higher residual AHI with ONM compared to NM or NM plus chin strap. No significant differences in sleepiness. Greater comfort, better sleep quality and overall preference for NM compared to ONM. |
| Andrade et al.1060 | 2018 | 1b | Meta-analysis | 5 randomized, eight nonrandomized trials (n = 4563) | CPAP level, residual AHI, CPAP adherence | ONM associated with higher CPAP level, higher residual AHI, lower adherence than nasal masks. |
| Deshpande et al.1075 | 2016 | 4 | Retrospective observational | OSA patients having CPAP titration studies (n = 358) 1. Nasal masks (34.6%) 2. Oronasal masks (46.1%) 3. Nasal pillows (19.3%) |
CPAP therapeutic level, residual AHI | CPAP level higher for ONM compared with NP and NM. Residual AHI higher for ONM than NM and NP. |
| Bettinzoli et al.1074 | 2014 | 4 | Retrospective observational | OSA patients with AHI ≥ 15 (n = 109) 1. Nasal masks (61.5%) 2. Oronasal masks (38.5%) |
CPAP therapeutic level, residual AHI, mask preference | Patients using ONM had significantly higher therapeutic level and higher residual AHI. |
| Ebben et al.1063 | 2014 | 2b | Randomized unblinded crossover | Moderate-severe OSA naïve to CPAP (n = 14) 1. Nasal masks 2. Oronasal mask |
Residual AHI, CPAP adherence | Residual AHI was significantly higher using ONM compared to NM. No significant difference in CPAP adherence. |
| Borel et al.1061 | 2013 | 2b | Prospective observational cohort | OSA newly prescribed CPAP (n = 2311) 1. Nasal masks (62.4%) 2. Oronasal masks (26.2%) 3. Nasal pillows (11.4%) |
CPAP adherence, CPAP level, CPAP-related side effects | Both ONM and NP associated with higher risk of non-adherence than NM. ONM associated with higher pressures than NM or NP. Proportion reporting side effects significantly higher with ONM than NM. |
| Zhu et al.1072 | 2013 | 2b | Randomized unblinded crossover | OSA using CPAP with NM for ≥6 months (n = 20) 1. Nasal masks 2. Nasal pillows |
CPAP daily usage, residual AHI, CPAP level, mask leak, mask performance/preference | No significant differences in average daily usage, pressure levels, mask leak, or residual AHI. Minor differences in side effects, no differences in patient preference. |
| Ebben et al.1064 | 2012 | 2b | Randomized unblinded trial | OSA with AHI >5/h (n = 55) 1. Nasal masks 2. Oronasal masks 3. Nasal pillows |
Final pressure levels, AHI on CPAP, final mask leak from CPAP titration study | Patients titrated using ONM required significantly higher final pressure levels compared to NM or NP. Final pressures not significantly different between NM and NP. Final AHIs not significantly different. Mask leak greater using ONM compared to NP but not NM. |
| Ryan at al.1070 | 2011 | 2b | Randomized unblinded crossover | OSA (AHI ≥ 10/h) naïve to CPAP (n = 21) 1. Nasal masks 2. Nasal pillows |
CPAP adherence, residual AHI, sleepiness, side effects, QOL, preference | No significant differences between NM and NP in adherence, residual AHI, sleepiness, side effects, QOL, or interface preference. |
| Teo et al.1071 | 2011 | 2b | Randomized single-blind crossover | OSA (RDI > 15/h) naïve to CPAP (n = 24) 1. Nasal masks 2. Oronasal mask |
PSG titration study CPAP level, residual RDI, mask leak, SWS, TST | CPAP levels not significantly different between NM and ONM. Residual AHI, arousals, mask leak all significantly greater with ONM than NM. Significantly less SWS and TST with ONM. Greater satisfaction with NM than ONM. |
| Chai-Coetzer et al.1059 | 2006 | 1a | Systematic review | 4 RCTs 1. 2 studies comparing NM vs. OM 2. 1 study comparing NM vs. NP 3. 1 study comparing NM vs. ONM |
CPAP compliance, sleep physiological parameters, ESS, OSA symptoms, adverse effects, interface satisfaction | Optimum CPAP delivery interface remains unclear given the limited number of available studies. |
| Anderson et al.1062 | 2003 | 2b | Randomized single-blind crossover, intention to treat analysis | OSA (AHI > 20/h) naïve to CPAP (n = 21) 1. Nasal masks 2. Oral masks |
PSG variables, questionnaires, compliance | No significant differences in PSG variables during CPAP titration between OM and NM. No differences in residual AHI, CPAP compliance, CPAP pressure, sleepiness, overall side effects, or mask preference. |
| Beecroft et al.1073 | 2003 | 4 | Prospective observational | OSA (AHI > 5/h) naïve to CPAP (n = 98) 1. Nasal masks (66%) 2. Oronasal mask (7%) 3. Oral masks (23%) |
Optimal CPAP level, residual AHI, self-reported adherence, mask comfort, side effects | No significant difference in optimal CPAP levels, residual AHI, self-reported usage, or satisfaction between NM, ONM, and OM users. |
| Khanna et al.1066 | 2003 | 2b | Randomized unblinded trial | Patients with OSA (RDI > 15/h, n = 38) 1. Nasal masks 2. Oral masks |
CPAP compliance, overall satisfaction, side effects | No significant differences in CPAP compliance between NM and OM users. Overall satisfaction similar between groups, types of side effects differed by mask type. Dropout rates similar between groups. |
| Massie et al.1067 | 2003 | 2b | Randomized unblinded crossover | Patients with OSA (n = 39) 1. Nasal masks 2. Nasal pillows |
CPAP compliance, residual AHI, sleepiness, QOL, side effects, overall satisfaction | Higher percentage of days using CPAP for NP compared to NM but no differences in overall adherence. No significant differences in residual AHI or QOL. Better sleep quality and overall satisfaction with nasal pillows. |
| Mortimore et al.1068 | 1998 | 2b | Randomized unblinded crossover | New OSA patients naïve to CPAP (n = 20) 1. Nasal masks 2. Oronasal mask |
Compliance, symptoms on CPAP, side effects | Higher CPAP compliance and less sleepiness using NM compared to ONM. Significantly fewer side effects with NM compared to ONM. |
Abbreviations: AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; NM, nasal masks; NP, nasal pillows; ONM, oronasal masks; OM, oral masks; OSA, obstructive sleep apnea; PSG, polysomnogram; QOL, quality of life; RCT, randomized controlled trial; RDI, respiratory disturbance index; SWS, slow wave sleep; TST, total sleep time.
VIII.A.3 |. PAP titration paradigms
The immediate goal of an efficacious PAP titration is to determine the pressure required to eliminate apneas, hypopneas, and inspiratory flow limitation (IFL)39,1077,1078 whilst preventing arousal and central apnea events. The midterm and longer-term goals are for patient acceptance of PAP therapy, continued effectiveness in eliminating respiratory events during sleep, ongoing patient adherence to therapy, and improvement in symptoms and outcomes related to SDB. PAP titration paradigms can be viewed along three dimensions: method of pressure changes (manual versus automatic), PAP titration setting (in-lab versus at-home), and time spent titrating pressure (partial or full night, or multiple nights).
The AASM has published several guidelines detailing practice parameters and guidelines for PAP titration39,1079,1080 with the goal being to identify a pressure that reduces AHI to <5 events/h in all sleep positions and sleep stages. The gold standard PAP titration is an in-laboratory technician-guided, manual titration with continuous monitoring of airflow, leak, sleep, and SaO2.1079 This approach assumes a single PAP can be determined that maintains airway patency despite changes in sleep stage, sleep position, and other factors that change UA collapsibility such as weight change, seasonal allergies, use of medication, and alcohol. With the introduction of APAP devices this provided an alternative approach to identifying a therapeutic pressure where 90% or 95% of all titration pressures fall.1080 The effective pressure values identified by manual titration versus auto-algorithms have not demonstrated systematic differences. Titration of BPAP is not addressed here as BPAP is not recommended over CPAP or APAP for routine treatment of OSA in the absence of sleep-related breathing disorders associated with hypercapnia.39
An SR and MA of 10 RCTs comparing APAP at-home and in-lab PAP titration for initiation of PAP demonstrates high grade evidence that both approaches are effective in reducing OSA severity (AHI), daytime sleepiness, and for PAP adherence and QOL measures.39 However, study participants were predominately those with moderate to high OSA severity and excluded patients with significant comorbidities. Of importance, these comparisons assume equivalent levels of patient CPAP education and mask fitting prior to titration plus equal opportunity for therapy support post-initiation.
Split-night titration, where the first couple of hours of the PSG are for diagnostic purposes and the remainder of the study involves PAP titration, compared to a fullnight titration, is not significantly different in terms of outcomes such as subjective sleepiness or adherence over time.1081–1083 However, certain criteria must be met to initiate a split-night PSG, that is, moderate–severe OSA and least 3 h available for CPAP titration.572 This results in the exclusion of those with mild OSA from the research comparing full night and split titration paradigms and contributes to the weak recommendation regarding the appropriateness of split night PSGs for all patients.572 Additionally, there is recent evidence that the prevalence of TECSA tends to be higher for split-night compared to full night titration studies.1042,1084 Nevertheless, there is no evidence that treatment-emergence central sleep apnea poses any harm to the patient.39
Determination of effective pressures using equations that combine anthropomorphic and OSA severity variables have been suggested as alternatives to manual titration.1085 Although these equations may provide a starting pressure for titration, their use as the sole determinant of a patient’s therapeutic pressure has not been accepted as they differ from manually determined pressures by up to 5 cmH2O.1075,1086,1087
From the perspective of the treating physician, avoidance of delays to therapy initiation is a pertinent driver for the chosen PAP titration paradigm. The only RCT assessing patient preference demonstrated a preference for home management602 compared to an in-lab titration. As the necessity for increased telehealth technology grows, we will likely see a concomitant increase in telemonitoring approaches to PAP titration, perhaps including patientdirected titration paradigms.1088–1090 Based on the above considerations that all show no clinically significant differences in outcomes between titration paradigms, the choice of strategy should be based on patient preferences and abilities, judgment of the sleep clinician, and availability of resources.
VIII.A.4 |. PAP adherence
VIII.A.4.a |. Measurement of PAP adherence
The use of PAP is objectively measured by the device and defined as the number of hours PAP is used at the prescribed pressure. This is most often reported as a single summary measure of the amount of time PAP was used across the 24-h day. While the term “use” is the most concise descriptor, treatment compliance and adherence are terms that are often utilized for prescribed medical interventions. Treatment adherence, implying a partnership between provider and patient, is the more commonly used term and is more consistent with the broader chronic illness medical literature.
CPAP adherence is in large part a behavioral measurement because the act of putting on a mask at night prior to sleep requires voluntary action. PAP adherence is therefore a measure of the extent to which an individual uses their prescribed medical device and can be categorized into 0 h of use, >0 h of use, or missing data. Missing data represents problems with data measurement (e.g., internal CPAP device problems) or transmission (e.g., poor cellular coverage). In these cases, the only way to know if PAP was used is to ask the patient directly. Some studies include a CPAP use questionnaire to determine if the patient had a medical reason to not use CPAP for some amount of time. In this case, a true 0 may be reclassified as missing data.
The quantity of PAP use as a marker of adherence requires some clarification. Measurement of the amount of CPAP use does not take into account the amount of sleep during a defined 24-h period. Because the CPAP device does not measure sleep, it is not known whether CPAP is used during wake or sleep periods. CPAP is often used during times of attempted sleep. To increase accuracy of measurement, one should consider combining CPAP with a reliable and valid measure of sleep duration to determine the percentage of the actual sleep period that CPAP is used to account for individual differences in sleep and wake. CPAP devices also do not measure respiratory effort, and unless equipped with an oxygen probe, do not monitor oxygenation.
The medical prescription for PAP includes a pressure mode and pressure level(s). Pressure modes include fixed and auto-adjusting continuous single and bilevel pressure modes. The amount of PAP use and the efficacy of PAP in controlling SDB can be affected by pressure mode, pressure level, and/or mask type and fit. In addition, it should be stated that the CPAP device provides measures of treatment efficacy, including residual AHI and mask leak.
Given the single measure of time a PAP device is used in a given 24 h period is the foundation of defining adherence, it is important to understand additional limitations that exist when objective device-reported measurements are not available. In this case, estimated CPAP use provided by patients (or their bedpartners) is the next best surrogate. The mean difference between subjectively and objectively measured CPAP adherence is 0.96 h (see Table VIII.A.4a), meaning that patients tend to overestimate their PAP use by approximately 1 h per night.
TABLE VIII.A.4a.
Subjective versus Objective measures of CPAP adherence
| Study | Year | n | LOE | Study design | Clinical end-pointa | Conclusion |
|---|---|---|---|---|---|---|
| Kribbs et al.1091 | 1993 | 35 | 2b | Prospective cohort | SR: 6.3 ± 1.47; Obj: 5.1 ± 1.87 | Subjective was 1.2 h more than objective |
| Rauscher et al.1099 | 1993 | 63 | 2b | Prospective cohort | SR: 6.3 ± 0.2 SEMa; Obj: 4.9 ± 0.3 SEMa | Subjective was 1.4 h more than objective |
| Meurice et al.1100 | 1994 | 44 | 2b | Prospective cohort | SR: 7.0 ± 1.65; Obj: 6.02 ± 2.48 | Subjective was 1.0 h more than objective |
| Pepin et al.1101 | 1995 | 193 | 2b | Prospective cohort | SR: 7.4 ± 2.0; Obj: 6.5 ± 3.0 | Subjective was 0.9 h more than objective |
| Engleman et al.1102 | 1996 | 204 | 2b | Prospective cohort | SR: 6.0 ± 1.9; Obj: 5.1 ± 2.5 | Subjective was 0.9 h more than objective |
| Drake et al.1103 | 2003 | 71 | 2b | Prospective cohort | SR: 5.86 ± 2.02; Obj: 5.04 ± 2.59 | Subjective was 0.8 h more than objective |
| Means et al.1104 | 2003 | 39 | 2b | Prospective cohort | SR: 4.82 ± 2.49; Obj: 4.31 ± 2.31 | Subjective was 0.5 h more than objective |
n, sample size.
Abbreviations: LOE, level of evidence; SD, standard deviation; SEM, standard error of measurement, SR, subjective report, Obj, objective measure.
Data all in mean ± SD except for row with mean ± SEM.
While PAP adherence is measured as a continuous variable (i.e., number of hours per night), it is often reported as a categorical variable (adherent/not adherent) to foster understanding and discussion. The threshold most often used for “adherent” is used >4 h per night on >70% of nights over a rolling 30-day period over a defined period of time. This threshold was based on a very early study of 35 CPAP users that showed a bimodal distribution,1091 which was then subsequently adopted as policy for coverage determinations.1092–1094 Since that time, very few studies have examined this relationship, though increasing data suggests disparities exist with PAP adherence across socioeconomic and racial groups1095–1097 and use of the 4-h, 70% threshold may be contributing to reduced access to treatment by rendering users below this threshold ineligible for coverage. One study found that more CPAP use was associated with better outcomes, finding a linear dose–response relationship up through 7 h of use.1098 This suggests current threshold definitions should be reexamined and that more research is needed to identify clinically meaningful adherence thresholds. (Table VIII.A.4a)
VIII.A.4.b |. Predictors of PAP adherence
Predictors of PAP adherence are factors that have been found to be independently associated with treatment use.
While the term “predictor” implies causation, most if not all studies in this literature are based on finding an association between a given factor and PAP adherence. PAP adherence predictors are most often categorized into the following groups: sociodemographic (e.g., age, sex), disease-related (e.g., AHI, treatment-related (e.g., pressure mode, pressure level), and psychological (e.g., mood) factors. For the purposes of this review, we are separating out behavioral predictors into their own category (e.g., selfefficacy, readiness to change). Predictors are most often measured before starting CPAP therapy but are sometimes measured after some CPAP usage. In addition, predictors may be measured as change variables, such that a difference score is calculated between a defined follow-up period and baseline.
Table VIII.A.4b provides the list of primary PAP adherence studies and predictor categories studied. Although some studies included early adherence as a predictor for comparison purposes, this analysis does not include early adherence level as a predictor since the goal is to identify independent factors associated with future PAP adherence, and it is well known that past behavior predicts future behavior.1105 For example, Aloia et al. found that adherence measured at 1 week and 3 months accounted for 52% and 79% of the variance in 6-month adherence, respectively, and further did not find other measured predictors that could independently account for any additional variance.1106 Understanding factors that influence PAP adherence behavior will inform the creation of effective interventions to promote PAP use.1107
TABLE VIII.A.4.b.
Predictor of PAP adherence
| Study | Year | LOE | n | Predictor categories | Effect size/outcomes | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| McArdle et al.1114 | 1999 | 4 | 1211 | Dem, OSA | Snoring history, AHI ≥ 15 and ESS > 10 were independent predictors of CPAP adherence. Early adherence was the strongest predictor of subsequent adherence. | This was one of the first studies to systematically examine predictors of CPAP adherence, finding the higher AHI and sleepiness levels were associated with CPAP adherence. | ||||
| Pelletier-Fleury et al.1115 | 2001 | 2b | 163 | Dem, OSA, other | Female sex, a BMI of >30 kg/m2, an ESS score of >15, an AHI of >30/h, and a level of nCPAP of <12 cmH2O were independent predictors of poor CPAP adherence. Age was not associated. | This study largely replicated the findings of McArdle et al. by finding that higher AHI and sleepiness levels were independently associated with CPAP adherence, as were BMI, female sex, and CPAP pressure. | ||||
| Sin et al.1116 | 2002 | 2b | 296 | Dem, OSA, CPAP | Higher age, greater reduction in ESS score, and female sex were all associated with higher CPAP adherence. | This was first study to show the change in ESS score was associated with CPAP adherence, such that the larger the decrease in sleepiness level, the higher the CPAP adherence. | ||||
| Stepnowsky et al.1117 | 2002 | 2b | 23 | Dem, OSA, CPAP, Psych, Beh | Ways of coping independently accounted for 20% of the variance, beyond that by AHI and ESS (total model = 35%). Active (but not passive) coping was the primary driver (15% variance). | Two components of active coping (confrontive coping and planful problem solving) were identified through post hoc analyses as being the most important predictors of CPAP adherence. | ||||
| Stepnowsky et al.1118 | 2002 | 2b | 51 | Dem, OSA, CPAP, Beh | Social Cognitive Theory (SCT) variables accounted for an adjusted 40% and Transtheoretical Model variables 33% of the variance in CPAP adherence. | Self-efficacy was the driving factor of the SCT variables and decisional-balance index was the driving factor for the TM variables. | ||||
| Lewis et al.1113 | 2004 | 2b | 80 | Dem, OSA, CPAP, Psych, other | Stepwise regression identified two (out of 21) predictors: Recent Life Events and Initial Problems on the first night of CPAP. Though not included in the regression, change scores (for ESS, anxiety and depression) were all significant. | Having a major recent life event over the last 6 months and reporting problems with CPAP on the first night of use were both associated with lower CPAP adherence. The study also identified change scores as being potentially important predictors. | ||||
| Lloberes et al.1119 | 2004 | 2b | 133 | Dem, OSA, CPAP | No predictors were identified for 3-month CPAP use at 3 months, higher AHI and impaired QOL before treatment were found to be independent predictors of 1-year CPAP adherence. | Higher AHI and impaired QOL at baseline were predictive of CPAP adherence. | ||||
| Wild et al.1120 | 2004 | 2b | 119 | Dem, OSA, CPAP, Psych, Beh | 24% of the variance in CPAP adherence was explained by ESS, AHI, BMI, pressure, and psych variables (health value, internal locus of control, and powerful others). | The three psychological variables explained 6% of the variance in CPAP adherence, which is relatively low compared to some measured behavioral variables. | ||||
| Aloia et al.1106 | 2005 | 2b | 98 | Dem, OSA, CPAP, Beh | Dem, OSA, and CPAP predictors non-significant; Beh predictors measured at 1 week and 3 months accounted for 23% and 42% of variance, respectively. | Self-efficacy, readiness to change, and decisional balance were all significant predictors of 6-month CPAP adherence. | ||||
| Stepnowsky et al.1121 | 2006 | 4 | 58 | Dem, OSA, CPAP, Beh | Social Cognitive Theory (SCT) variables accounted for 12% and Transtheoretical Model variables 16% of the variance in CPAP adherence. Note that CPAP pressure and side effects accounted for 31% of the variance. | Self-efficacy was the driving factor of the SCT variables and decisional-balance index was the driving factor for the TM variables. Of note, CPAP pressure and side effects were important predictors in this study of experienced CPAP users. | ||||
| Kohler et al.1122 | 2010 | 4 | 639 | Dem, OSA, CPAP | Multivariate analysis (including gender, age, neck circumference, ESS, ODI, and participation in a research study) indicated that ODI event rate was significantly associated with long-term compliance with CPAP (ODI: HR 0.97, SE 0.01, p < 0.001, 95% CI 0.96–0.98). | Higher baseline Oxygen Desaturation Index values were associated with higher CPAP adherence. | ||||
| Wallace et al.1123 | 2013 | 4 | 248 | Dem, OSA, CPAP, Beh, other | The following predictors were found to be independently associated with CPAP adherence: fewer insomnia symptoms and higher self-efficacy. Being Black was associated with lower adherence. | Key feature of this study was the study of three races (Black, White, and Hispanic). White and Hispanics had higher adherence rates. Fewer insomnia symptoms and higher self-efficacy were the only predictors identified. | ||||
| Wallace et al.1124 | 2013 | 2b | 124 | Dem, OSA, CPAP, Psych, Beh, other | 7-day CPAP adherence: Lower ISI scores were associated with higher 7-day adherence (6% variance). 30-day CPAP adherence: The model with baseline ISI, ESS < 10, outcome expectations and self-efficacy accounted for 50% of the variance in CPAP adherence; a second model that added week 1 adherence then accounted for an additional 30% of the variance. | While ISI was an important predictor for 7-day adherence, it was not significant for 30-day adherence. Lower ESS and higher self-efficacy and outcome expectations were associated with higher 30-day adherence in this sample of Hispanic Veterans. Early adherence again was a predictor of subsequent adherence. | ||||
| Schoch et al.1125 | 2014 | 4 | 1756 | Dem, OSA | Cox regression found that only ESS, ODI, and AHI were significantly associated with long-term adherence (while age, gender, BMI, and ESS were not significantly associated). | Higher baseline sleepiness, oxygen desaturation, and AHI scores were associated with higher 3-year CPAP adherence rates. | ||||
| Budhirja et al.1126 | 2016 | 2b | 1105 | Dem, OSA | 2-month CPAP adherence: age, presence of CVD and decrease in ESS score. 6-month CPAP adherence: age, AHI, lower anxiety, decrease in ESS score. | Consistent predictors across both time points include age and decrease in ESS score; other potentially important predictors include AHI, presence of CVD, and lower anxiety. One additional interesting predictor found in this RCT was belief that one is in the active treatment group. | ||||
| Dzierzewski et al.1127 | 2016 | 4 | 191 | Dem, OSA, CPAP, Psych, Beh | Age, self-efficacy, and self-efficacy by pressure interaction accounted for 29% variance in CPAP adherence. | First study to find that self-efficacy has a moderating effect on adherence such that higher self-efficacy scores related to adherence only for those with higher pressure levels. | ||||
| Jacobsen et al.1128 | 2017 | 4 | 695 | Dem, OSA | Using Cox regression, higher AHI and ESS scores and lower smoking levels were related to higher adherence. | This was one of the larger studies of CPAP users with a median duration of use of 3 years. | ||||
| Hoshino et al.1129 | 2018 | 4 | 161 | Dem, OSA | REM-related OSA was the strongest independent predictor to distinguish good adherence (OR = 64; p < 0.001) and poor adherence (OR = 3.2; p = 0.014) from those who stopped using CPAP over a 6-month time period. 0/43 patients in the good adherence group had REM-related OSA. | Based on this study, further research is warranted on the predictive value of the of REM-related OSA on CPAP adherence. | ||||
| Liou et al.1130 | 2018 | 4 | 119 | Dem, OSA, CPAP | After adjusting for demographics, comorbidities, and sleep apnea severity, changing sleeping location = 1×/month was associated with 77% lower odds of reaching CMS adherence compared to rarely changed sleeping location. | This was the first known study to rigorously evaluation “changing sleeping location = 1×/month” asa predictor of CPAP adherence and emphasizes some of the logistical challenges patients face in using a complex medical device on a nightly basis. | ||||
| Philip et al.1131 | 2018 | 4 | 404 | Dem, OSA, CPAP, Beh | The model containing six variables (age, BMI, AHI, number of years with CPAP, ISI total score, and self-efficacy) explained 19% of the variance in CPAP adherence. | Individual independent predictors in this study included age, BMI, ISI total score, and self-efficacy. | ||||
| Wallace et al.1124 | 2018 | 2b | 53 | Dem, OSA, CPAP, Psych, Beh | In adjusted analyses, pre-treatment insomnia symptoms (early, late, and aggregated nocturnal symptoms) and sleep dissatisfaction were predictive of lower CPAP use at 6 months. | Insomnia symptoms, measured at multiple time points, were negatively associated with CPAP adherence. | ||||
| Baron et al.1132 | 2020 | 2b | 92 | Dem, OSA, Psych, Beh, other | Perceived partner autonomy was significantly associated with CPAP adherence, beyond the covariates (age, gender, ODI, sleep-related impairment, OSA risk perception, and self-efficacy), which were all significant. | Perceived partner autonomy (defined as a sense of choice or volition regarding behavior) was found to be significantly related to 2-month CPAP adherence. | ||||
Predictor categories: Beh, behavioral; CPAP, CPAP-related; Dem, demographic; OSA, OSA-related; Psych, psychological; n, sample size. LOE, levels of evidence.
Studies were selected for inclusion based on these three criteria: (1) primary objective was to study the predictors of CPAP adherence, (2) examination of groups of predictors from two or more categories; and (3) utilization of multivariate analysis.
There are several important findings by predictor category:
Sociodemographic:
While sociodemographic variables were the most studied predictor category, only increasing age (n = 2 studies), increasing BMI (n = 2), and female sex (n = 1) were found to be significantly associated with higher CPAP adherence across 22 studies. CPAP adherence is also influenced by race, with reduced adherence reported in Black, Hispanic, and Asian Pacific Islander users compared to white users.1108–1112 Variables such as overall sleep duration, presence of comorbid insomnia, latency to sleep onset, socioeconomic status, and education levels contribute in part to reduced use of PAP.
CPAP-related.
One study found the presence of reporting problems on the first night of CPAP use was associated with reduced adherence.1113
OSA-related.
The most common OSA-related predictors were baseline disease severity (e.g., AHI and ODI) and symptom severity (e.g., daytime sleepiness as measured by the ESS), with worse disease and symptoms at baseline being related to higher subsequent CPAP adherence. Three other notable findings from this category were: (1) change in ESS (i.e., reduction in sleepiness) was predictive of higher CPAP use, which represented the only change score across the included studies; (2) higher baseline insomnia symptoms were associated with higher subsequent CPAP use; and (3) REM-related OSA was found in one study.
Psychological.
A wide variety of predictors were found in this category, but each only once: ways of coping, health locus of control, health value, anxiety, and perceived partner autonomy.
Behavioral.
This class of predictors was primarily driven by self-efficacy (in 7/22 studies). The decisional-balance index, which is a relative weighting of the pros and cons of sleep apnea, was identified in two studies. This class of predictors has the advantage of being modifiable (i.e., amenable to change), and therefore can provide the basis for an intervention.
Other.
The other category included a variety of predictors including presence of CV disease, smoking history, and change in sleeping location.
Attempting to identify predictors of PAP adherence is an important area of investigation. Much progress has been made in recent years, with multivariate models explaining ∼40%–50% of the variance in PAP adherence scores. Predictors of adherence have been identified. It seems clear that patients with more severe disease and sleepiness symptoms at baseline and those who experience a relief of those symptoms (i.e., perceive a benefit) tend to use CPAP more. Behavioral predictors, including self-efficacy and readiness for change, represent factors that significantly increase PAP adherence and have the additional benefit of being modifiable and a basis for interventions. The primary goal of identifying PAP adherence predictors is to help design interventional protocols and to inform the sleep care team about important factors to help provide care. While predictors could potentially identify patients who would not benefit from PAP, none of the PAP adherence studies specifically addressed this issue.
Despite PAP being considered a complex treatment regimen, there are very few studies of predictors derived from a systems perspective, for example, factors related to the provision of health services (quality of care provided, type of care provided, organization that provides the care [DME vs. Clinic]); cost (whether to patient or reimbursement levels to care team that likely affects amount and/or quality of care); or disparities (socioeconomic status or race/ethnicity). (Table VIII.A.4.b)
VIII.A.5 |. Optimization of PAP therapy
VIII.A.5.a |. Educational interventions for PAP adherence
Educational interventions focus on improving the understanding of OSA, its consequences, and benefits of treatment. The underlying premise is that imparting knowledge can affect personal beliefs and preconceptions that may ultimately lead to changes in behavior.
The type and method of educational therapies varied among different studies, which included printed documents, video clips, web-based education, telephone calls, face-to-face individual counseling sessions, personalized feedback, and review of individual PSGs. These interventions were largely passive and did not require patients’ active participation. While some studies included additional study arms, every study included a separate educational arm. The study by Richards et al. was included in this section despite having an intervention arm called “Cognitive Behavioral Therapy (CBT)” group. This was due to the fact that despite the name of the study arm, the intervention only entailed reading or observing prepared materials and did not involve active engagement from the participants.1133
The effects of educational interventions on CPAP adherence are mixed, with three studies showing no significant effect on adherence and the other four showing increased compliance with therapy to various degrees. The 2014 Cochrane review showed moderate-quality evidence that short-term educational interventions lead to a small increase in average PAP use of about 35 min per night.1134 Additionally, the SR by Patil et al. showed moderate quality evidence that PAP adherence increased by 0.6 h/night with an educational intervention and is the basis for the AASM guidelines that strongly recommends the use of education with PAP therapy.39 (Table VIII.A.5.a)
TABLE VIII.A.5.a.
Educational interventions and PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Patil et al.39 | 2019 | 1a | Systematic review seven RCTs | RCTs examining the impact of a pure educational intervention as an adjunct to PAP therapy to improve adherence with PAP | PAP usage | Moderate quality evidence that showed significant improvement in PAP adherence by 0.6 h/night in adults with OSA with an educational intervention compared to usual care. Based on this systematic review, AASM guidelines strongly recommends that educational interventions be given with initiation of PAP therapy in adults with OSA. |
| Wozniak et al.1134 | 2014 | 1a | Systematic review 30 RCTs | RCTs examining the effectiveness of educational, supportive, or behavioral strategies in encouraging CPAP usage | CPAP usage | Low- to moderate-quality evidence that all three types of interventions led to increasing machine use. Supportive interventions increased machine usage by 50 min per night, educational interventions increased machine usage by 35 min per night, behavior therapy increased machine usage by 1.44 h per night. |
| Chervin et al.1135 | 1997 | 1b | RCT N = 33 Duration:8 weeks |
1. Intervention 1: telephone call each week during trial; N = 12 2. Intervention, 2: two printed documents; N = 14 3. Control: no additional support; N = 7 |
Machine usage at 1–2 months; dropouts/lost-to follow-up | No significant differences in CPAP adherence between intervention groups and control. |
| Falcone et al.1136 | 2014 | 1b | RCT N = 206 Duration: 12 months |
1. Standard support: sleep medicine physician gave a full explanation of the need for and benefits of CPAP 2. Educational support: standard support and viewing of the rapid-eye-movement phase of diagnostic and CPAP-titration PSG, with explanations and attention to flow and oxyhemoglobin saturation curve |
CPAP usage at 1, 3, 12 months; sleepiness; retention rate | The educational support group had higher retention rates at 1, 3, and 12 months. CPAP use at 1, 3, and 12 months was higher in the educational support group but did not reach significance. |
| Hwang et al.1137 | 2017 | 1b | Four-arm, randomized, factorialdesign clinical trial N = 1455 Duration: 90 days |
1:1:1:1 to one of four arms: 1. Web-based OSA education (Tel-ed); N = 380 2. Telemonitoring and automated feedback (Tel-TM); N = 375 3. Tel-ed + tel-TM (Tel-both); N = 346 4. Usual care; N = 354 |
CPAP usage at 90 days | Tel-TM and Tel-both groups showed significant increase in average usage at 90 days. Tel-ed had no significant effect on CPAP usage at 90 days. |
| Hwang et al.1137 | 2017 | 1b | Four-arm, randomized, factorialdesign clinical trial N = 1455 Duration: 90 days |
1:1:1:1 to one of four arms: 1. Web-based OSA education (Tel-ed); N = 380 2. Telemonitoring and automated feedback (Tel-TM); N = 375 3. Tel-ed + tel-TM (Tel-both); N = 346 4. Usual care; N = 354 |
CPAP usage at 90 days | Tel-TM and Tel-both groups showed significant increase in average usage at 90 days. Tel-ed had no significant effect on CPAP usage at 90 days. |
| Pengo et al.1138 | 2018 | 1b | RCT N = 112 Duration: 6 weeks |
1. Negative: negatively framed messages in additional to CPAP read out to patients during CPAP collection appointment and during weekly phone calls; N = 36 2. Positive: positively framed messages in addition to CPAP read out to patients during CPAP collection appointment and during weekly phone calls; N = 37 3. Standard care: explanation of importance of treating OSA, APAP introduction and instruction for use, compliance assessment at 2 weeks; N = 39 |
APAP usage, % days used >4 h, sleepiness, withdrawals at 2 and 6 weeks | Patients in the positive group had higher APAP use compared to the negative and control groups at 2 weeks. There was no difference in compliance between groups at 6 weeks, 2 months, and 6 months. The positive group had a reduction in the dropout rate at 6 weeks. |
| Richards et al.1133 | 2007 | 1b | RCT N = 100 Duration: 28 days |
1. Treatment as usual: standardized group education session, explanation of CPAP titration and equipment, explanation of side effects; N = 50 2. Cognitive behavioral therapy (CBT): two 1-h CBT interventions that included a standardized educational slide presentation, 15-min video of real-life CPAP users, an additional booklet on sleep, OSA/CPAP, and general health |
Machine usage, withdrawal | The CBT group had increased mean nightly usage at 28 days. |
| Roecklein et al.1139 | 2010 | 1b | RCT N = 30 Duration: 3 months |
1. Standard education: written information from American Academy of Sleep Medicine on OSA, snoring and PAP therapy for OSA; N = 16 2. Personalized feedback: written personalized feedback report with severity of disease, self-reported daytime sleepiness, individually estimated risk of adverse health outcome, and risk of MVA; feedback addressed barriers using CPAP, ambivalence about treatment and difficulties of behavior changed and promoted self-efficacy and personal responsibility; N = 14 |
Objective CPAP usage, self-reported CPAP usage | There was no significant difference in CPAP usage. |
| Sarac et al.1040 | 2017 | 1b | RCT N = 115 Duration: 6 months |
1. Standard support: general explanation of OSA and CPAP; N = 63 2. Educational support: additional 20 min education by sleep MD including viewing own PSG, comparing PSG from diagnostic and CPAP titration studies that emphasized obstructive events and O2 desaturations; N = 52 |
CPAP usage, N of adherent participants, sleepiness, withdrawal | Average PAP usage was increased in the educational support group compared to the standard support group at long-term follow-up. |
VIII.A.5.b |. Supportive interventions for PAP adherence
Supportive interventions include telemonitoring under various formats, including telephone calls, texting, personalized support platforms, and peer buddy systems. These interventions engage patients, increase support, and reinforce strategies to increase CPAP adherence.
One RCT by Fox et al. had a telemedicine intervention arm which provided a modem enabled PAP device, which sent information regarding adherence, interface leak, and residual AHI directly to a web-based database. A coordinator reviewed the transmitted information and contacted the patient if there was a significant reduction in PAP usage or increase in pressure or residual AHI. At 3 months, PAP adherence was significantly greater in the modem PAP group compared to the standard group. The telemedicine group utilized an additional 65 min of technician time.1140 A different type of supportive intervention was used by Hoy et al. in which the intensive support arm received standard support in addition to education at home with the partner, two additional nights in the hospital, and home visits by sleep nurses. This group had higher CPAP usage than those in the usual care group at 6 months.1141 Parthasarathy et al. used a peer buddy system, in which trained peers with OSA and good CPAP adherence were paired with newly diagnosed participants and engaged in face-to-face sessions as well as telephone conversations. CPAP adherence was greater in the peer buddy system group after 3 months.1142
Out of the 13 RCTs, seven showed that supportive interventions had a positive impact on CPAP adherence, while six showed no difference between the groups. The 2014 Cochrane review showed low-quality evidence that supportive interventions increased CPAP usage by 50 min per night compared to control.1134 (Table VIII.A.5.b)
TABLE VIII.A.5.b.
Supportive interventions for PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Wozniak et al.1134 | 2014 | 1a | Systematic review 30 RCTs |
RCTs examining the effectiveness of educational, supportive, or behavioral strategies in encouraging CPAP usage | CPAP usage | Low- to moderate-quality evidence that all three types of interventions led to increasing machine use. Supportive interventions increased machine usage aby 50 min per night. Educational interventions increased machine usage by 35 min per night. Behavior therapy increased machine usage by 1.44 h per night. |
| Chervin et al.1135 | 1997 | 1b | RCT N = 33 Duration: 8 weeks |
1. Intervention 1: telephone call each week during trial; N = 12 2. Intervention 2: two printed documents; N = 14 3. Control: no additional support; N = 7 |
Machine usage at 1–2 months; dropouts/lost-to-follow-up | There were no significant differences in CPAP adherence between intervention groups and control. |
| DeMolles et al.1143 | 2004 | 1b | RCT N = 30 Duration: 8 weeks |
1. Usual care (UC): usual medical care, patient education, and demonstration of equipment use; N = 15 2. Telephone-linked communication technology (TLC): UC + computerized digitized human speech program. TLC asks questions designed to elicit information from participant regarding adherence, education, and reinforcement; N = 15 |
CPAP usage at 2 months; sleep-related symptoms | There was no difference in average nightly CPAP use between the groups, but the TLC group had fewer sleep-related symptoms. |
| Fox et al.1140 | 2012 | 1b | RCT N = 75 Duration: 12 weeks |
1. Telemedicine intervention: physiologic information (adherence, air leak, residual AHI) was transmitted daily to a website that could be reviewed. If problems were identified, the patient was advised over the phone or visited the PAP coordinator; N = 39 2. Standard care |
Machine usage, adherence after 3 months, subjective sleep quality, any side effects | PAP adherence was significantly greater in the telemedicine group compared with the standard group. An additional 65 min of technician time was spent on patients in the telemedicine group. |
| Hoet et al.1144 | 2017 | 1b | RCT N = 46 |
1. Usual care: group education session 1 month after CPAP initiation, and medical visit at 1.5 and 3 months; N = 23 2. Telemonitoring: telemonitoring device attached to CPAP, through which participant data was analyzed and patients were contacted in the case of air leak, residual AHI>10/h, or CPAP use less than 3 h in 3 consecutive days); N = 23 |
CPAP usage at 3 months; time to delay to first technical intervention after CPAP initiation | Compliance at 3 months was significantly better in the telemonitoring group. Telemedicine reduces delay to first technical intervention in CPAP-treated patients. |
| Hoy et al.1141 | 1999 | 1b | RCT N = 80 Duration: 6 months |
1. Standard support: video education, titration of CPAP pressure overnight, nurses telephoned on days 2, 21, reviewed in hospital at 1, 3, 6 months; N = 40 2. Intensive support: standard support + initial education at home with partner, 2 extra nights in hospital, sleep nurses’ home visits to participant and partner at 7, 14, 28 days, and 4 months after starting CPAP; N = 40 |
Machine usage at 6 months | Intensive support group had higher CPAP usage than those in the usual care group. |
| Hwang et al.1137 | 2017 | 1b | Four-arm, randomized, factorialdesign clinical trial N = 1455 Duration: 90 days |
1:1:1:1 to one of four arms: 1. Web-based OSA education (Tel-Ed); N = 380 2. Telemonitoring and automated feedback (Tel-TM); N = 375 3. Tel-ed + tel-TM (Tel-both); N = 346 4. Usual care; N = 354 |
CPAP usage at 90 days | Tel-TM and Tel-both groups showed significant increase in average usage at 90 days. Tel-ed had no significant effect on CPAP usage at 90 days. |
| Mendelson et al.1145 | 2014 | 1b | RCT N = 107 |
1. Standard care: baseline evaluation, patients were contacted at day 2 to ask about adherence and to troubleshoot, met with sleep specialist at 4 weeks and at 4 months; N = 53 2. Telemedicine: standard care + smart phone for uploading BP measurements, CPAP adherence, sleepiness, and QOL data; participants received daily pictograms containing health-related messages; N = 54 |
Home self-measured BP, CPAP usage, cardiovascular risk evolution, sleepiness, QOL, fatigue, dyspnea, withdrawals | CPAP adherence did not differ between the groups. Self-measured BP did not improve in either group. |
| Munafo et al.1146 | 2016 | 1b | RCT N = 122 Duration: 3 months |
1. Standard of care 2. SOC + telehealth messaging program: patients and providers were messaged based on CPAP device data |
CPAP usage, sleepiness, residual AHI, resource use | Adherence rates were similar in both groups. There was a significant reduction in the mean aggregate time required to coach a patient in the telehealth group versus standard of care group. |
| Parthasarathy et al.1142 | 2013 | 1b | RCT N = 39 Duration: 90 days |
1. Usual care: educational brochures regarding OSA and CPAP therapy; N = 17 2. Peer buddy system: trained peers with OSA and good CPAP adherence record were paired with newly diagnosed participants and participated in two face to face sessions and 8 telephone conversations; N = 22 |
Participant ratings of acceptability of peer-buddy system; CPAP adherence | Weekly CPAP adherence was greater in the intervention group. |
| Pepin et al.1147 | 2019 | 1b | RCT N = 306 Duration: 6 months |
1. Usual care; N = 149 2. Multimodal telemonitoring: CPAP related factors, BP, physical activity recorded by connected devices. Symptoms and QOL recorded via electronic questionnaires. Patients received demonstration home telemonitoring use and explanation of why monitoring these physiological variables was relevant; N = 157 |
SBP, CPAP usage at 6 months, sleepiness, QOL, physical activity | Self-measured BP did not differ significantly between groups. There was a significant increase in CPAP adherence and an improvement in daytime sleepiness and QOL in favor of the multimodal telemonitoring. |
| Stepnowsky et al.1148 | 2007 | 1b | RCT N = 45 Duration: 2 months |
1. Usual care: telephone call from staff at 1 week, office visit at 1 month; N = 21 2. Telemonitoring: Compliance and efficacy data garnered as objective compliance data and subjective reports of usage. Follow up tailored to how CPAP used by participants; N = 24 |
CPAP usage, % nights with CPAP use >4h | There were no statistically significant differences in CPAP compliance. |
| Stepnowsky et al.1149 | 2013 | 1b | RCT N = 241 Duration: 4 months |
1. Telemonitoring: allow both the patient and provider access to telemonitored adherence and efficacy data on a daily basis and act on the data collaboratively to guide CPAP management and troubleshoot problems, emphasize ways for patient to express their preferences and needs; N = 126 2. Usual care: CPAP instruction and setup by a healthcare provider, follow up at 1 week and 1 month; N = 115 |
CPAP usage at 2 and 4 months | There was higher CPAP adherence at 2- and 4-months in the telemonitoring group compared to control. |
| Turino et al.1150 | 2017 | 1b | RCT N = 100 |
1. Standard management: a short instruction session on CPAP device use and 1-month was less visit; N = 48 2. Telemonitoring program: daily information on CPAP adherence, pressures, mask leak, and respiratory events were sent to the database; automatic alarms for the provider were generated if mask leak >30 L/min for >30% of the night or usage <4 h/night on 2 consecutive nights. Provider contacted the patient, providing case by case problem solving; N = 52 |
Machine usage at 1, 3 months; cost-effectiveness | No significant difference in CPAP compliance between the groups. Telemedicine was less expensive than standard management and was cost-effective. |
VIII.A.5.c |. Behavioral interventions and PAP adherence
Behavioral interventions focus on modifying behavioral constructs to improve adherence to CPAP. These interventions require participant engagement and aim to enhance behavioral action, motivation for change, self-efficacy, and outcome expectations.
All six RCTs show increased compliance with behavioral interventions, which include motivational enhancement therapy, CBT, and progressive muscle relaxation training. The 2014 Cochrane review showed low-quality evidence that behavioral interventions increased CPAP usage by 1.44 h per night compared to control.1134
Most of these interventions are not time intensive. Aloia et al. study included two 45-min cognitive behavioral sessions with a therapist that increased compliance at 12 weeks.1151 A study by Bakker et al. utilized motivational enhancement via two 1-h in-person sessions, in addition to phone calls and an educational video. This intervention resulted in a significantly improved average nightly adherence (99 min/night) within the treatment group at 12 months.1152 An additional study by Lai et al. utilized motivational enhancement via a 20-min patientcentered interview, a 25-min video, and 10-min telephone follow-up. Within this study the intervention group had higher CPAP use, a four-fold increase in the number using CPAP for ≥70% of days with ≥4 h per night, and greater improvements in daytime sleepiness as well as treatment self-efficacy at 3 months.1153
In summary, behavioral therapies are effective, timelimited interventions that can positively influence CPAP adherence. More studies are needed to determine whether these interventions have long-term effects and which group of patients will benefit most. (Table VIII.A.5.c)
TABLE VIII.A.5.c.
Behavioral interventions and PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Patil et al.39 | 2019 | 1a | Systematic review Six RCTs |
RCTs examining the efficacy of a behavioral intervention as an adjunct to PAP therapy to improve adherence with PAP | PAP usage | There was moderate to high quality level evidence that significant improvement in PAP adherence by 1.2 h/night in adults with OSA with an educational intervention compared to usual care. Based on this systematic review, AASM guidelines conditionally recommend that behavioral interventions be given during the initial period of PAP therapy in adults with OSA. |
| Wozniak et al.1134 | 2014 | 1a | Systematic review Thirty RCTs |
RCTs examining the effectiveness of educational, supportive, or behavioral strategies in encouraging CPAP usage | CPAP usage | There was low- to moderate-quality evidence that all three types of interventions led to increasing machine use. Supportive interventions increased machine usage by 50 min per night. Educational interventions increased machine usage by 35 min per night. Behavior therapy increased machine usage by 1.44 h per night. |
| Aloia et al.1151 | 2001 | 1b | RCT N = 12 Duration: 12 weeks |
1. Cognitive behavior intervention (two 45-min sessions with a therapist designed to educate subjects on the consequences of OSA and efficacy of CPAP); N = 6 2. Control (same therapist contact, but no information on OSA or CPAP); N = 6 |
Machine usage at 1, 4, 12 weeks; N of adherent participants (≥6h per night); vigilance testing | The intervention increased compliance at 12 weeks. A modest cognitive-behavioral intervention may substantially increase CPAP use and vigilance in older adults. |
| Bakker et al.1152 | 2016 | 1b | RCT N = 83 Duration: 12 months |
1. Active CPAP; N = 42 2. Active CPAP + ME (motivational enhancement; 1-h in-person sessions at baseline and week 1, educational video, phone calls with psychologist); N = 41 |
CPAP usage at 6, 12 months | Average nightly adherence for 6 months was significantly higher (99 min/night) with CPAP + ME compared with CPAP alone. |
| Dantas et al.1154 | 2015 | 1b | RCT N = 41 Duration: 2 months |
1. Intervention group (motivation strategies applied according to patient’s motivation, assessed by the degree of confidence and conviction); N = 20 2. Control 1 (only standard information about APAP) 3. Control 2 (routine procedures) |
APAP usage at 1 and 2 months; % of days of APAP use (>4 h/night on 60% of the nights); sleepiness; AHI | There was higher adherence to APAP and lower AHI at 2 months in the intervention group compared to control groups. |
| Lai et al.1153 | 2014 | 1b | RCT N = 100 Duration: 3 months |
1. Control group (usual advice on importance of CPAP therapy and its care); N = 51 2. Usual care + brief motivation enhancements program directed at enhancing the subjects’ knowledge, motivation, and self-efficacy to use CPAP through a 25-min video, a 20-min patient-centered interview, and a 10-min telephone follow-up; N = 49 |
CPAP usage at 1 and 3 months; N of adherent participants; intention to use; sleepiness; self-efficacy; QOL | The intervention group had better CPAP use, a four-fold increase in the number using CPAP for ≥70% of days with ≥4 h/day, greater improvements in daytime sleepiness, and treatment self-efficacy compared with the control group. |
| Olsen et al.1155 | 2012 | 1b | RCT N = 106 Duration: 52 weeks |
1. Motivational interviewing intervention (three sessions of CPAP-specific nurse led motivational interview therapy one month apart); N = 53 2. Control (standard 1 on one 45-min education session, follow-up at two to 4 weeks by MD and 2 months by RN); N = 53 |
CPAP acceptance and adherence, FOSQ, self-efficacy measure for sleep apnea, ESS | The intervention group had improved CPAP acceptance and overall adherence rates compared to standard care alone. |
| Wang et al.1156 | 2012 | 1b | RCT N = 152 Duration: 12 weeks |
1. PMR (progressive muscle relaxation training): one night of CPAP titration in the hospital, 12 × 40 min group PMR practice sessions over 12 weeks, 1 per week. Self-practice of PMR before each CPAP treatment; N = 38 2. EDU (education): 3 nights of CPAP titration in, 4 h group education session on OSA and CPAP, brochure on benefits of CPAP and 20 min video on how to optimize CPAP treatment, 24-h consultation telephone line to sleep nurse; N = 38 3. EDU + PMR: 3 nights of CPAP titration in the hospital; N = 38 4. Control: 1 night of CPAP titration in the hospital; N = 38 |
CPAP usage at 4, 8, 12 weeks; N of adherent participants (≥4 h/night, 9/14 nights); sleepiness, sleep quality, anxiety, depression | EDU + PMR showed significant improvement in CPAP adherence, sleepiness, and sleep quality compared to control at 4, 8, and 12 weeks of intervention. EDU only showed significant improvement in CPAP adherence, sleepiness, and sleep quality at 4 weeks of intervention. The PMR group showed no significant improvement over time. |
VIII.A.5.d |. Aerophagia management and PAP adherence
Aerophagia – excessive and repetitive air swallowing that can result in abdominal pain and bloating – is a reported side effect of PAP. Aerophagia is reported in 16% of CPAP users1101 and is associated with GERD.1157 The interventions that help reduce aerophagia are largely anecdotal and are targeted towards reducing therapeutic pressure. While many studies report decreased average pressures with the use of APAP versus CPAP in addition to increased compliance, there has only been one trial that prospectively studied whether APAP reduces aerophagia symptoms. In this double-blinded, randomized crossover study by Shirlaw et al., APAP reduced symptoms of aerophagia.1158 (Table VIII.A.5.d)
TABLE VIII.A.5.d.
Evidence for aerophagia management and PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Shirlaw et al.1158 | 2017 | 1b | Double-blinded randomized crossover trial (n = 56) | 2 weeks on each therapy mode in random order 1. APAP 6–20 cm H2O 2. CPAP |
Usage hours and visual analog scale to measure symptoms of aerophagia | APAP reduces the symptoms of aerophagia while not affecting compliance compared with CPAP. |
VIII.A.5.e |. Claustrophobia management and PAP adherence
Claustrophobia can negatively affect adherence in PAP users. One review shows that almost half the participants stated that they would not use CPAP if it made them feel claustrophobic, caused nasal symptoms, or disturbed their bed partner.1159 In a secondary analysis of a prospective, longitudinal study of 97 OSA patients, 63% had claustrophobic tendencies whichwere associated with non-adherence.1160 To date, only a few studies have looked at behavioral interventions that target claustrophobia to increase compliance. Two case reports have shown success with in vivo desensitization or exposure therapy to treat claustrophobia to improve CPAP usage.1161,1162 One retrospective case series of 13 patients showed that after exposure therapy, patients used CPAP for significantly longer compared to pre-treatment.1163 Larger-scale, randomized control studies are required to validate the effects of behavioral therapy to treat claustrophobic reactions to optimize CPAP adherence. (Table VIII.A.5.e)
TABLE VIII.A.5.e.
Evidence for claustrophobia management and PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Means and Edinger1163 | 2007 | 4 | Case series | One or more sessions of exposure therapy with a behavioral sleep psychologist | CPAP adherence data | Patients used CPAP significantly longer after exposure therapy compared to pre-treatment. |
| Edinger et al.1161 | 1993 | 4 | Case report | Male apnea patient, who initially failed CPAP due to claustrophobia, underwent in vivo desensitization | CPAP tolerance | The patient had increased tolerance to CPAP and continued to use CPAP after desensitization. |
| McCrae et al.1162 | 2006 | 4 | Case report | Patient with trauma-related claustrophobic response to CPAP underwent graduated in vivo exposure over a 3-month period | CPAP compliance | The patient reported increased CPAP compliance without anxiety and decreased symptoms of OSA. |
VIII.A.5.f |. Humidification and PAP adherence
UA symptoms-nasal congestion, dry nose and mouth, and sore throat are common undesirable effects of PAP and are associated with decreased adherence to therapy. Koutsourelakis et al.1164 showed that CPAP with heated humidification reduced pre-existing nasal airway inflammation and decreased the level of pro-inflammatory cytokines. By reducing nasal symptomatology, heated humidification is an intervention aimed to improve comfort and compliance.
Humidification systems are built into current PAP devices and are widely available for use.However, the clinical significance of reduced nasal inflammation on PAP adherence, subjective daytime sleepiness, and PAP-related side effects remains unclear.
There have been several SRs evaluating the effect of heated humidification on compliance. The first, published in 2018, reviewed nineRCTs, and found no improvement in compliance or ESS scores with heated humidification.1165 Even in the subgroup analysis of subjects with nasal symptoms prior to PAP therapy, a group expected to benefit most from humidification, there was no statistical difference in PAP usage time. Another SR by Patil et al. in 2019 reviewed nine RCTs, also showed no clinically significant difference in adherence with or without humidification. However, it did show a significant reduction in incidence of side effects based on three RCTs, two of which were included in the 2018 review.39 In a recent Cochrane review, humidification was found to increase average night use by 0.37 h per person per night based on six RCTs, but it was low-certainty evidence.1166 (Table VIII.A.5.f)
TABLE VIII.A.5.f.
Humidification and PAP use
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Zhu et al.1165 | 2018 | 1a | Systematic review and meta-analysis | 1. Heated humidification 2. No heated humidification |
PAP compliance and subjective daytime sleepiness | There was no improvement in compliance or ESS scores. |
| Patil et al.39 | 2019 | 1a | Systematic review | 1. Heated humidification 2. No heated humidification |
Adherence, sleepiness, QOL, PAP-related side effects | There were no clinically significant differences in adherence, sleepiness, or QOL. The heated humidification group had significant reduction in PAP-related side effects. |
| Kennedy et al. Cochrane Review1166 | 2019 | 1a | Systematic review | 1. Heated humidification + fixed CPAP 2. Fixed CPAP alone |
1. Primary outcome: machine usage 2. Secondary outcomes: symptom scores (ESS), quality of life scores (SF-36), sleep disruption (AHI), adverse events, patient preference |
There was low-certainty evidence that humidification increased average night usage and improved ESS. There was insufficient evidence on QOL, sleep disruption, and adverse events. There was no significant difference in patient preference. |
| Palm et al.1167 | 2018 | 2b | Cohort study | Patients with OSA on CPAP | Adherence | The use of humidifier is associated with greater adherence to CPAP. |
| Jacobsen et al.1128 | 2017 | 2b | Retrospective cohort study | Patients with OSA on CPAP | Adherence | The frequency of patients offered humidification was higher in persistent CPAP users. |
| Wiest et al.1168 | 2002 | 3b | Case–control study | 1. Heated humidification 2. No heated humidification |
Patient comfort and acceptance | The use of heated humidification was not associated with improvement in comfort or treatment acceptance. |
VIII.A.5.g |. Nasal obstruction and PAP adherence
Patients initiating PAP therapy may have pre-existing nasal obstruction due to a variety of inflammatory or structural etiologies. These may limit PAP adherence because nasal PAP interfaces require nasal airway patency.
Multiple observational studies found that increased objective nasal resistance on anterior rhinomanometry was predictive of nasal PAP intolerability.544,1169 Additional observational studies have found that smaller nasal cavities on acoustic rhinometry at the time of PAP initiation led to lower PAP adherence at 3 months1170 and 2 years.1171 Subjective baseline nasal obstruction alone did not affect PAP adherence at 2 years follow-up.1171 Objective nasal function testing is not standard clinical practice and not routinely used to identify patients at the highest risk of non-adherence.
New subjective nasal symptoms can arise as a result of PAP use and can cause PAP non-adherence. As many as 65% of patients using PAP report nasal congestion, dry nose or throat, and discomfort likely due to mucosal swelling and the drying effects of positive pressure.1172 In an international RCT, adults who developed PAP-related nasal side effects were associated with lower PAP adherence at 12 months post-initiation but not at 24 months.1173 The long-term adherence in this study may have improved due to symptom management with intranasal corticosteroid and heated humidified air during the study. Advances in PAP technology to limit nasal congestion may contribute to more promising recent evidence. Värendh et al.’s recent prospective cohort study found decreased subjective and objective nasal obstruction after 2 years of PAP therapy.1171
No studies have directly examined how the presence of rhinitis affects CPAP adherence.
There is mixed evidence on CPAP use in patients with OSA and AR alters nasal symptomatology. One cohort study found that CPAP users with baseline AR have less improvement in nasal obstruction than those without AR.1174 A smaller cohort study found increased cytological inflammation in both AR and non-AR patients, but worse nasal dryness in non-AR patients.1175
There is insufficient evidence on the effect of chronic sinusitis alone on CPAP adherence. One small experimental study applied 2 h of CPAP on 20 cmH2O to patients with CRS with polyps but without OSA and found a reduction in nasal polyp size but no change in subjective nasal obstructive symptoms or objective acoustic rhinometry.1176 (Table VIII.A.5.g)
TABLE VIII.A.5.g.
Effects of nasal obstruction on PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Värendh et al.1171 | 2019 | 2b | Prospective cohort | 728 CPAP users evaluated with acoustic rhinometry | CPAP adherence, subjective, and objective nasal obstruction | Small nasal volumes at baseline associated with lower adherence, subjective and objective nasal obstruction decreased after 2 years of PAP use. |
| Van Ryswyk et al.1173 | 2019 | 2b | Cohort study (secondary analysis of SAVE RCT) | CPAP users in SAVE trial. | PAP adherence at 12 and 24 months | Nasal symptoms found to be predictive at 12-months but NOT found to be predictive of adherence at 24-months. |
| Sugiura et al.544 | 2006 | 2b | Prospective cohort | 77 OSA patients evaluated with anterior rhinomanometry | Initial CPAP acceptance vs. non-acceptance | Higher objective nasal resistance was a predictor of initial CPAP non-acceptance via nasal interface. |
| Li et al.1170 | 2005 | 2b | Prospective cohort | 60 OSA patients with small and large minimal nasal cross-sectional area based on acoustic rhinometry | CPAP use hours/night after 3 months | Smaller nasal cavities associated with lower adherence but subjective nasal stuffiness did not correlate with objective nasal dimensions. |
Management of nasal obstruction for PAP adherence.
Nasal obstruction management strategies that may affect PAP adherence include conservative, medical, and surgical options. When patients cannot tolerate a conventional nasal mask, they can trial nasal pillows or oronasal/full face masks as second-line interfaces, though adherence with nasal masks is known to be superior to oronasal masks.1059 For nasal obstruction and dryness, the addition of sodium hyaluronate nasal spray or saline nasal spray is associated with significant improvement in PAP adherence in a randomized trial.1177 The effect of heated humidification on nasal symptoms and PAP adherence has mixed evidence.1166,1178
Intranasal corticosteroid sprays are widely used and effective for patients with established allergic and non-AR, and are commonly prescribed for OSA patients with presumed nasal congestion to increase CPAP adherence. An SR and MA of two RCTs studying unselected OSA patients found only a small 24-min increase in CPAP use in favor of nasal steroid use at 4 weeks.1179 Notably, the efficacy of the treatment may be diluted as both studies included unselected OSA patients with no subgroup analyses for the presence of clinical rhinitis or severity of nasal obstruction. Although clinically logical, there is insufficient published evidence on the efficacy of intranasal corticosteroids for improving CPAP adherence specifically in patients with AR.
Nasal surgery has shown benefit for improving PAP adherence. An SR and MA performed by Camacho et al. demonstrated that isolated nasal surgery converted 89% (57 of 64 patients) of non-PAP users to PAP-tolerant users, and improved objective CPAP use hours from 3.0 ± 3.1 h per night to 5.5 ± 2.0 h per night in short-term follow-up for 33 patients in whom objective data was available.1180 The studies included had high heterogeneity in patient population and selection. Clinicians should use this evidence in combination with best judgment for appropriate patient selection to ensure that nasal obstruction is the primary contributor to CPAP non-adherence, and whether the degree of obstruction on examination is significant enough to warrant surgical correction. The use of surgery for decreasing PAP pressures will be discussed in another section, though improvement in adherence as a result of lower pressures should be acknowledged. Kempfle et al. used financial modeling to determine cost-effective ways to improve CPAP adherence. Compared to medical management of nasal obstruction, inferior turbinate reduction was found to be more cost-effective in the short-term while septoplasty was more cost-effective in the long-term.1181 (Table VIII.A.5.g.1)
TABLE VIII.A.5.g.1.
Evidence for the nasal obstruction treatment to improve PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Charakorn et al.1179 | 2017 | 1a | Systematic review and meta-analysis of two RCT (144 patients) | 1. CPAP with nasal steroids 2. CPAP only |
PAP adherence, subjective nasal symptoms. | Nasal steroids provided small but non-significant increase in average nightly PAP use (0.4 h); subjective nasal symptoms were not significantly affected. |
| Camacho et al.1180 | 2015 | 3a | Systematic review and meta-analysis; 11 studies looked at adherence (153 patients) | CPAP users who underwent isolated nasal surgery | Pressure reduction, self-reported adherence, objective adherence. | Isolated nasal surgery reduced CPAP pressures and may increase CPAP use (self-reported and objective measures). |
| La Mantla et al.1177 | 2017 | 1b | RCT (102 patients) | 1. Sodium hyaluronate + CPAP 2. Saline + CPAP 3. CPAP only |
Mini-Rhinoconj QOL score, CPAP adherence, sleepiness, nasal resistance. | Sodium hyaluronate and saline groups showed improved CPAP adherence at 4 weeks. |
| Ryan et al.1178 | 2009 | 1b | RCT (125 patients) | 1. Dry CPAP 2. Humidified CPAP 3. CPAP with INCS |
Objective adherence, self-reported surveys. | Humidifier decreased nasal symptoms; no difference in adherence or QOL between groups at 4 weeks. |
| Kempfle et al.1181 | 2016 | 2c | Economic analysis | 1. IT reduction 2. Septoplasty 3. Medical treatment |
Total cost of untreated OSA, surgery, complications, post-op compliance, QOL. | Surgical options are cost-effective over multiple model assumptions. |
VIII.A.5.h |. Treatment emergent central sleep apnea (TECSA)
TECSA, previously known as complex sleep apnea before its definition in the International Classification of Sleep Disorders, 3rd edition (ICSD-3), is the emergence or persistence of central apneas with the treatment of OSA in the absence of another cause of CSA.
The prevalence of TECSA ranges from 3.5% to 19.8% at the time of titration, but can also emerge later with 0.7% to 4.2% having a new finding of TECSA after a month or more of CPAP therapy).1182
The most common treatment is continued use of CPAP at the lowest pressure setting which limits the obstructive AI to less than 5 events/h. An SR of the natural history of TECSA1182 found that majority of cases resolved with CPAP use when reassessed 4–28 weeks after starting CPAP therapy. In this group, persistence of TECSA was found in 31.1% (range 14.3%–46.2%) of TECSA patients across five studies.1182
In an EBRR of therapies for the treatment of TECSA, three randomized controlled studies (RCT) involved ASV. Morgenthaler et al. showed that ASV decreased AHI compared to CPAP both at the time of titration and at 90-day follow-up.1052 Dellweg et al. showed that in patients with TECSA after 6 weeks of CPAP use, both ASV with auto-adjusting EPAP and BPAP with backup rate similarly reduce AHI at the time of titration but the ASV arm maintained a lower AHI after 6 weeks of use.1049 Javaheri et al. compared two ASV devices available in 2011 (BiPAP-AutoSV advanced and BiPAP-AutoSV) in a mixed population of TECSA patients and found greater improvement in the AHI with the BiPAP-Auto-advanced device.1183 The remaining lower quality studies all supported the use of ASV in various ways. ASV improved sleep efficiency in those with TECSA and heart disease with preserved EF > 50%,1184 ASV improved adherence when switching from CPAP in patients with presumed TECSA,1053 and ASV improves AHI compared to CPAP, BPAP-S, or BPAP-ST. Kuzniar et al. compared two ASV devices available in 2011 (VPAP-AdaptSV and BiPAP-AutoSV) and found them to be comparable; however, the populations varied as the study was not randomized.1046 Javaheri et al. found that default ASV settings reduced the overall AHI, central AI, obstructive AI, and hypopnea index AHI, CAI, OAI, and HI over 90 days with sustained adherence and improvement in ESS and subjective sleep quality.1185
Insufficient evidence was found for treating TECSA with modalities used for other forms of CSA including phrenic nerve stimulation, supplemental O2 or CO2, and medications such as acetazolamide, theophylline, or hypnotic agents.
At the time of diagnosis both CPAP and ASV are treatment options as most TECSA resolves with CPAP use; however, when TECSA is severe, ASV can be used. The evidence suggests that ASV is more effective than CPAP for persistent TECSA (Recommendation, grade B). However, more studies are needed to predict those who will develop persistent TECSA due to the excess cost of ASV. (Table VIII.A.5.h)
TABLE VIII.A.5.h.
Evidence for treatment of TECSA
| Study | Year | LOE | Study design | Study group | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Morgenthaler et al.1052 | 2014 | 1b | RCT | 1) ASV; 2) CPAP | Primary endpoint: AHI < 10 at 90 days; Secondary endpoints: compliance, ESS, and SAQLI |
1) ASV reduces AHI more than CPAP. |
| Dellweg et al.1049 | 2013 | 1c | RCT | 1) ASV; 2) NPPV (bilevel PAP with backup rate) | Primary endpoint: AHI after 6 weeks. Secondary endpoint: AHI on ASV titration |
1) ASV showing sustained significant improvement at 6 weeks; 2) ASV and NPPV similarly treated TECSA on the titration. |
| Javaheri et al.1183 | 2011 | 1c | RCT | 1) ASV (BiPAP autoSV Advanced); 2) ASV (BiPAP autoSV) | Primary endpoint: AHI on ASV titration | 1) BiPAP autoSV Advanced lowered AHI more than BiPAP autoSV. |
| Pépin et al.1053 | 2018 | 3b | Case–control | 1) CPAP switched to ASV; 2) ASV; 3) CPAP | Primary endpoint: Adherence data; Secondary endpoint: Average AHI |
1) Increased adherence in patients switching from CPAP to ASV. 2) Lower AHI in patients switching from CPAP to ASV. |
| Heider et al.1184 | 2018 | 3b | Case–control | 1) HFpEF with TECSA; 2) HFpEF with CSA | Primary endpoint: Sleep fragmentation and quality at ASV titration and after 27 months of ASV use | 1) AHI and arousal index improve with ASV. 2) Increased sleep efficiency only in TESCA group with ASV. 3) ESS improves with ASV. |
| Neu et al.1186 | 2017 | 3b | Case–control | 1) AutoCPAP with TECSA; 2) AutoCPAP without TECSA | Primary endpoint: AHI; Secondary endpoints: Quality of life, sleep fragmentation |
1) Auto-titrating CPAP has relative treatment failure for TECSA compared to those without TECSA. |
| Kuzniar et al.1046 | 2011 | 3b | Retrospective cohort | 1) ASV (VPAP AdaptSV); 2) ASV (BiPAP autoSV) | Primary endpoint: AHI; Secondary endpoints: adherence data, sleepiness using ESS |
1) Both ASV devices were comparable. 2) Baseline populations were significantly different. |
| Allam et al.1047 | 2007 | 3bCase–control |
1) ASV; 2) CPAP; 3) BPAP-S; 4) BPAP-ST; CPAP + O2 | Primary endpoint: AHI on titration | 1) ASV reduced AHI more than other modalities. | |
| Javaheri et al.1185 | 2015 | 4 | Case series | 1) ASV using default settings | Primary endpoint: AHI; Secondary endpoints: subjective sleep quality using Likert scale and adherence and therapy data at 90 days | 1) Default ASV settings improve AHI. 2) Adherence, sleep disordered breathing, ESS, and subjective sleep quality are improved at 90 days. |
| Brown et al.1048 | 2011 | 4 | Case series | 1) ASV | Primary endpoint: AHI on titration | ASV can be effective in reducing AHI. |
VIII.A.5.i |. Insomnia treatment and PAP adherence
Patients with insomnia and OSA show reduced acceptance and lower average nightly use of CPAP therapy compared to patients with OSA alone.958 Published studies on the effects of insomnia therapies for patients with OSA and comorbid insomnia are limited to studies evaluating nonmedication therapies (see Table 10.c.2.b).957,1187–1191 The SR concluded that these trials demonstrate the beneficial effects of therapist-administered cognitive behavioral therapy for insomnia (CBTi) on improving insomnia symptoms and increasing CPAP therapy usage.1189 Two of these RCTs have demonstrated an improvement in CPAP adherence by an average of 48 and 61 min per night 6 months after CBTi administration. Two RCTs found no between-group differences in average CPAP use.1188,1192 There is preliminary evidence that CBTi delivered by trained therapists may be more effective than self-administered CBTi using a self-guided resource.
Studies have also examined the effect of sedativehypnotic medications on OSA severity; however, these medicines are not currently recommended as first-line or long-term treatment for insomnia due to risks for side-effects, dependence, and withdrawal.1193 Further evaluation of the therapeutic potential of medications that do not alter OSA severity is required. (Table VIII.A.5.i)
TABLE VIII.A.5.i.
Insomnia treatment and PAP adherence
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Sweetman et al.1189 | 2019 | 1b | Systematic review | 4 RCTs considered | CPAP adherence, insomnia outcomes: Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), Bergen Insomnia Scale (BSI) | RCTs provide tentative support for the effect of therapist-administered CBTi in improving insomnia symptoms and increasing CPAP use in OSA patients with comorbid insomnia; CBTi delivered by trained therapists may be more effective than self-administered CBTi. |
| Ong et al.1188 | 2020 | 1b | RCT | 121 adults with OSA and comorbid insomnia randomized to: 1) CBTi followed by PAP, 2) CBTi concurrent with PAP, or 3) PAP only | Apnea diagnosed with PSG; Primary Outcome: 90-day PAP adherence; Secondary outcomes: PSQI, ISI |
No significant group differences in PAP adherence between groups; compared to PAP alone, the concomitant treatment arms led to greater improvement in ISI. |
| Alessia et al.1187 | 2020 | 1b | RCT | 125 with comorbid insomnia and newly-diagnosed OSA by PSG (AHI≥15 events/h) randomized to: 1) 5-weekly sessions integrating CBTi with a PAP adherence program provided by a “sleep coach” or 2) 5-weekly sleep education control sessions | Apnea diagnosed with PSG (AHI ≥ 15 events/h); PSQI, 7-day sleep diary, 7-day actigraphy; objective PAP use, ISI, Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire-10 (FOSQ-10). | Six months after CBTi intervention, 90-day PAP use was increased by 0.9 h/night. CBTi led to greater improvement in PSQI, ISI, ESS, FOSQ-10, diary sleep onset latency, and sleep efficiency; and actigraphic sleep efficiency. |
| Sweetman et al.957,1190–1191 | 2019, 2020 | 1b | RCT | 145 patients with insomnia and untreated OSA randomized to: 1) a four-session CBTi programme followed by CPAP or 2) no treatment followed by CPAP | Insomnia diagnosed with ICSD-3; apnea diagnosed with PSG (AHI ≥ 15); Sleepiness evaluated with ESS, ISI, Dysfunctional Beliefs and Attitudes about Sleep Questionnaire Primary outcome: AHI |
The CBTi group showed a 7.5 event/h greater AHI difference compared to control (p = 0.012), a greater reduction in total number of nocturnal awakenings (p = 0.031), and higher initial CPAP treatment acceptance (99% vs. 89%; p = 0.034). By 6 months after intervention, the CBTi group had 61 min greater average nightly adherence to subsequent CPAP (p = 0.023) and greater improvement of global insomnia severity, and dysfunctional sleep-related cognitions (p < 0.001) |
| Bjorvatn et al.1192 | 2018 | 1b | RCT | 164 patients with HSAT-based OSA diagnosis randomized along with CPAP to: 1) a self-help book for insomnia or 2) sleep hygiene advice | BIS, ISI Apnea: AHI ≥ 5 events/h |
No differences were found between groups; BIS and ISI improved significantly in both groups. |
VIII.A.5.j |. Long-term PAP adherence assessment
PAP therapy is the mainstay of treatment for OSA and can include auto-adjusting PAP, CPAP, and BiPAP, among others. Predictors of PAP adherence include severe OSA (AHI ≥ 30), symptoms of excessive daytime sleepiness (Epworth score >10 or ≥12)1114,1194 and early PAP adherence in the first week or month of treatment.1141,1195 As OSA is a chronic condition, PAP should be continued as long-term treatment to maintain symptomatic improvements.
The goals of PAP therapy are to improve day and nighttime symptoms and reduce risks of CV and neurocognitive morbidities. For AHI, the recommended metrics during PAP titration are: optimal AHI < 5, good AHI < 10, and adequate >75% AHI reduction1079; these metrics can be reasonably applied to analysis of AHI data from PAP machines with small adjustments as described in the American Thoracic Society (ATS) Statement from 2013.1196 The ATS statement describes the current standards and issues with CPAP tracking systems for monitoring PAP adherence and outlined a clinical algorithm for PAP adherence management. Currently, with the availability of PAP data, long-term therapy should optimally include both patient assessment and PAP data analysis, which can be performed on routine basis when patients are doing well and also when new clinical issues arise.
Several trials have assessed PAP adherence and treatment responses for follow-up durations from 3 months up to 10 years. Patient education has been shown to improve adherence.1134 A large database analysis showed an outstanding 87% adherence at 90 days in patients who enrolled in internet-based active patient engagement software.1197 Applications that allow OSA patients to access and track their own CPAP adherence data on computers and smartphones are also available (e.g., SleepMapper from Respironics) and have been shown to increase hours of usage 1 h or more over the initial 3 months of CPAP use.1198 Additionally, automatic feedback messages via text messaging, emails, or phone calls, directly to patients based on predetermined thresholds for CPAP usage have also shown improved 90-day CPAP adherence.1137 These studies demonstrate the value of methods to improve and maintain adherence.
Despite the importance of long-term PAP therapy for chronic OSA, the current emphasis is on the initial 3 months of PAP,39 and there are limited data on long-term PAP after 3 months; therefore, long-term guidelines are based on expert opinion. After the initial 3 months of treatment, CMS provides ongoing coverage for patients who are successfully adherent, showing PAP use for ≥4h on ≥70% of nights; coverage includes mask, headgear, and tubing every 3 months for patients receiving clinical benefit.
For protocol guidelines, CMS mandates ongoing annual clinical follow-up, either with PCP or specialist, to assess PAP therapy, or sooner if new clinical issues arise. Assessment should include subjective symptoms, weight changes, new medical issues, changes in medication, and mask fit/comfort. A review of PAP objective data including PAP hours, residual AHI, and leak is highly valuable to confirm subjective reports. If a full face mask shows high leak value, which can occur due to a larger contact area between mask and face, consider switching to a nasal mask or pillows. If nasal masks or pillows show high leak value, consider a chin strap or a full face mask. Patient education regarding the rationale for therapy and also maintaining clean and effective equipment should consistently be emphasized, since it has been shown to improve adherence.
When patients are on APAP, PAP pressure can vary based on weight and sleep position; if AHI data from APAP is in optimal or good ranges (<5 or <10, respectively), then continue APAP therapy and reassure the patient. For higher APAP AHI values, consider interventions to optimize mask or nasal pillows, or higher AHI values persist, consider polysomnogram for all-night titration. When patients using fixed-pressure CPAP experience significant weight change of >10%, adjusting PAP pressure may be clinically necessary; therefore, consider polysomnogram for all-night titration to optimize PAP pressure. For adherent patients who are benefitting from PAP, annual follow-up is appropriate. In non-adherent patients, closer follow-up within 3–6 months is warranted. For patients who either cannot or would not use PAP, alternative therapies should be pursued.1196
Since OSA is a chronic medical condition, long-term adherence to PAP is important for long-term symptom management and reduction of CV risks. Long-term PAP use is supported by both evidence and expert opinion to maintain symptomatic improvements. Since PAP adherence improves with a range of educational and patient engagement approaches, regular use of one or more of these approaches should also be emphasized. Annual follow-up should include subjective symptom assessment, objective PAP data analysis, and patient education.
VIII.B |. Efficacy of PAP for Symptoms and Comorbidities
VIII.B.1 |. PAP and daytime sleepiness
OSA commonly presents with daytime sleepiness. Sleepiness can be measured both subjectively and objectively. Additionally, there are methods to measure objective wakefulness.
An SR1199 from 2006 evaluated the effect of CPAP compared to placebo or conservative management on objective/subjective sleepiness and objective wakefulness in subjects with mild to moderate OSA. Meta-analyses showed a reduction in the ESS score of 1.2 points (95% CI: 0.5, 1.9), an increase in the MWT sleep latency of 2.1 min (95% CI: 0.5, 3.7), and no significant change in the MSLT values. An additional SR1200 that compared subjects receiving CPAP to sham CPAP, oral placebo, usual care, or a dental device demonstrated a reduction in ESS score of 2.7 points (95% CI: −3.5, −2.0) for all levels of symptom severity, with greatest improvement in the subjects that were sleepiest (mean difference −5.0 points, 95% CI: −6.5, −3.5). MWT values improved in CPAP versus control (mean difference 3.3 min, 95% CI: 1.3, 5.3); however, MWT values were not significantly different in CPAP versus dental devices (0.7 min, 95% CI: 1.6, 2.9). MSLT values were not significantly different in CPAP versus control for all participants (mean difference 0.6 min, 95% CI: −0.7, 1.9); however, there was a statistically significant increase in mean sleep latency of 2.3 min (95% CI: 9, 3.7, I2 0%) for subjects with severe disease, as measured by AHI. A recent SR39 evaluated subjective and objective sleepiness and objective wakefulness in subjects treated with PAP compared to control groups. The control groups primarily used sham PAP or no PAP, with one study using nasal dilator strips, one study using sleep hygiene and counselling, and one study utilizing usual care. The MA of 38 RCTs demonstrated a clinically significant decrease in ESS score by 2.4 points in subjects using PAP compared with controls (95% CI: −2.8, −1.9). An MA of seven RCTs showed a reduction in MWT or Oxford Sleep Resistance Test (OSLER) sleep latency by 0.5 min in subjects treated with PAP (95% CI: 0.2, 0.8) compared to control subjects. An MA of seven RCTs found no significant difference in mean sleep latency in subjects treated with PAP compared to controls (mean difference 0.25 min, 95% CI: −0.89, 1.38). (Table VIII.B.1)
TABLE VIII.B. 1.
Evidence for improvement in sleepiness with PAP
| Year | LOE | Study design | Study groups | Clinical end-point | Conclusion | |
|---|---|---|---|---|---|---|
| Marshal et al.1199 | 2006 | 1a | SR | 7 RCTs comparing CPAP to placebo or conservative management in subjects with mild to moderate OSA | Objective and subjective daytime sleepiness and objective wakefulness | Meta-analyses demonstrated a significant reduction in subjective sleepiness (ESS) by 1.2 points (95% CI: 0.5, 1.9), improved objective daytime wakefulness (MWT) by 2.1 min (95% CI 0.5, 3.7), and no significant change in MSLT. |
| McDaid et al.1200 | 2009 | 1a | SR | 48 studies comparing CPAP vs. alternatives (oral placebo, sham CPAP, dental device, and usual care) | Objective and subjective daytime sleepiness and sleepiness and wakefulness | CPAP significantly reduced ESS score compared to control by 2.7 points (95% CI: −3.5, −2.0). CPAP improved daytime wakefulness (MWT) by 3.3 min (95% CI: 1.3, 5.3). CPAP did not significantly change MSLT values for all participants, but there was a benefit (mean difference 2.3 min, 95% CI: 9, 3.7, I2 0%) for those with severe disease (as measured by AHI). |
| Patil et al.39 | 2019 | 1a | SR | Up to 38 RCTs comparing PAP vs. alternatives (predominantly sham PAP or no PAP, but also nasal dilator strips, sleep hygiene and counselling, and usual care) | Objective and subjective daytime sleepiness and objective wakefulness | Meta-analyses of 38 RCTs demonstrated a clinically significant decrease in ESS score by 2.4 points with the use of PAP compared with controls (95% CI: −2.8, −1.9). A meta-analysis of seven RCTs showed a reduction in MWT or OSLER sleep latency by 0.5 min in subjects treated with PAP (95% CI: 0.2, 0.8). A meta-analysis of seven RCTs found no significant difference in mean sleep latency in subjects treated with PAP compared to controls. |
VIII.B.2 |. PAP and OSA severity
CPAP therapy has been utilized as a non-invasive treatment for OSA since the 1980s. Multiple SRs have consistently demonstrated that CPAP is effective at reducing the AHI.39,1201,1202 CPAP was shown to be superior to placebo, conservative management, or positional therapy (PT) during follow-up PSG over weeks to months.1201 An SR1202 of eight RCTs demonstrated a mean decrease in AHI of 17 events/h compared to control groups in seven RCTs and a decrease in AHI of 9 events/h compared to a control group in one RCT crossover study. A recent SR39 using two different meta-analyses evaluated 11 RCTs that compared CPAP to control interventions (sham CPAP, conservative measures, no intervention, sham surgery, a placebo tablet, or nasal dilator strips). One MA demonstrated a mean decrease in AHI of 23 events/h with CPAP therapy compared to control interventions. The other MA showed a mean decrease in AHI of 29 events/h comparing subjects pre- and post-treatment. The mean difference in AHI was most pronounced in study groups where the mean pre-treatment AHI was greater than 30 events/h. (Table VIII.B.2)
TABLE VIII.B. 2.
Evidence for PAP to improve OSA severity
| Year | LOE | Study design | Study groups | Clinical end-points | Conclusion | |
|---|---|---|---|---|---|---|
| Gay et al.1201 | 2006 | 1a | SR | 11 RCTs comparing CPAP vs. placebo, conservative management, or positional therapy | AHI | CPAP was superior to placebo, conservative management, and positional therapy as demonstrated on PSG several weeks or months after treatment. |
| Giles et al.1202 | 2006 | 1a | SR | 8 RCTs comparing CPAP to control interventions (sham CPAP, conservative measures, no intervention, or placebo tablet) | AHI | Seven RCTs (parallel and partial crossover studies) showed a significant mean difference in AHI of −17 events/h with CPAP therapy. One RCT (crossover study) demonstrated a difference in AHI of −9 events/h with CPAP therapy. |
| Patil et al.39 | 2019 | 1a | SR | 11 RCTs comparing CPAP to a control intervention (sham CPAP, conservative measures, no intervention, sham surgery, placebo tablet, or nasal dilator strips) | AHI | Meta-analysis of RCTs showed a significant mean difference in AHI of −23 events/h with CPAP therapy. Another meta-analysis of these RCTs demonstrated a significant mean difference in AHI of −29 events/h before and after CPAP therapy. |
VIII.B.3 |. PAP and mortality risk
OSA is a common disorder that is known to be associated with a variety of poor health outcomes, including increased mortality.95 PAP therapy is an effective form of treatment for OSA and is considered to be standard first-line therapy.
A recent multi-center RCT from Spain1203 did not demonstrate significant benefit with CPAP therapy compared to usual care for either all-cause mortality or CV mortality in a group of non-sleepy subjects with acute coronary syndrome. It is important to note that the mean adherence to CPAP therapy in this trial was only 2.78 h/night. An earlier metanalysis39 evaluated both RCTs and non-randomized trials comparing PAP therapy to no PAP therapy in middle-aged and older subjects with at least moderate OSA. This SR performed two meta-analyses and had conflicting conclusions regarding all-cause mortality. The MA of four RCTs demonstrated that CPAP therapy did not reduce all-cause mortality; however, there was variability in CPAP adherence among the four trials. An MA of nine non-RCTs did demonstrate a clinically significant reduction in all-cause mortality with CPAP with a risk ratio of 0.4 (95% CI 0.24–0.69). An additional RCT from China1204 evaluated subjects with moderate to severe OSA who had uncontrolled hypertension and coronary heart disease. This study demonstrated a non-significant trend toward lower severe CV and cerebrovascular events with one death in the control group.
In the face of cohort data showing a significant effect of CPAP therapy on reduction in all-cause mortality and negative findings from multiple RCTs, it is difficult to interpret the overall benefit of PAP. Pack et al. summarized the biases and limitations associated with the negative RCT studies on the effects of PAP therapy and CV events. Specifically, RCT studies were secondary prevention studies and all trials excluded OSA subjects with excessive sleepiness who are increased risk for CV events and most likely to benefit from treatment.1205 RCTs also had inadequate adherence to PAP therapy and on secondary analysis of data in adherent patients, CPAP benefit was found to be consistent with prior epidemiological studies.1206 (Table VIII.B.3)
TABLE VIII.B. 3.
Evidence for PAP use and mortality risk reduction
| Year | LOE | Study design | Study groups | Clinical end-point | Conclusion | |
|---|---|---|---|---|---|---|
| Sanchez-de-la-Torre et al.1203 | 2020 | 1b | RCT | 1264 patients hospitalized for acute coronary syndrome and AHI > 15 randomized to CPAP treatment plus usual care vs. usual care alone ISAACC study: Impact of sleep apnea syndrome in the evolution of acute coronary syndrome – effect of intervention with CPAP |
Cardiovascular mortality and all-cause mortality | No significant benefit with CPAP therapy for cardiovascular mortality (hazard ratio 0.83) or all-cause mortality (hazard ratio 0.82), however, the mean adherence to CPAP therapy was only 2.78 h/night. |
| Patil et al.39 | 2019 | 1a, 2a | SR | 4 RCTs comparing CPAP to no CPAP 9 non-randomized trials comparing PAP vs. control conditions |
All-cause mortality | Meta-analysis of RCTs showed that CPAP therapy did not reduce all-cause mortality (risk ratio 0.96); however, there was variability in CPAP adherence. Meta-analysis of non-randomized trials demonstrated a clinically significant reduction in all-cause mortality (risk ratio 0.4). |
| Huang et al.1204 | 2015 | 1b | RCT | 83 patients randomized to CPAP vs. no therapy | Severe cardiovascular and cerebrovascular events (including death) | There was a non-significant trend toward lower severe cardiovascular and cerebrovascular events with one death in the control group. |
VIII.B.4 |. PAP and cardiovascular outcomes
VIII.B.4.a |. PAP and control of hypertension
There are many published RCTs measuring BP before and after initiation of CPAP therapy.39 Although several SRs and meta-analyses have noted reductions in BP with CPAP treatment of OSA, particularly moderate to severe OSA, caution is warranted because of heterogeneity in the pooled studies.39,654,1207–1211 Examples include variability in severity of OSA, presence of daytime sleepiness, control conditions, blinding, adherence to CPAP treatment, measurement of BP, HTN status at baseline, lack of controlled antihypertensive treatment, and duration of follow-up. A majority of the subjects included in the studies were middle-aged, obese, and male. Meta-analyses with overall low to moderate homogeneity of included studies are shown in Table 2. The recent MA by Yu et al., comprising patients with both OSA and CSA, was largely driven by the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial and did not show a reduction in major adverse CV events.654,1212 However, a borderline statistically significant (p = 0.05) reduction of systolic BP with PAP was noted. It is notable that adherence to CPAP in the SAVE trial was suboptimal at a mean of 3.3 h per night and REM-related SDB events, most of which tend to occur in the latter part of the night, were likely left untreated.
The magnitude of reduction of 24-h mean BP with CPAP treatment of OSA is modest, in the range of 2–4 mmHg, may be related to the severity of OSA1213 and to the number of hours of nightly usage of the device.39,1207–1209 Based on a metanalysis of antihypertensive medication RCTs, clinically significant 24-h systolic BP reduction as low as 1–2 mmHg is associated with decreased risk of stroke and major CV events; larger BP reductions are associated with larger reductions in risk for stroke, major CV events, and total mortality.1214 The effect on BP appears to be sustained over time with continued use of CPAP and some evidence suggests that fixed CPAP devices may be better at decreasing BP than auto-titrating devices (APAP), although better tolerated APAP may be used for longer durations.39 Additionally, some studies have evaluated the synergistic effects of antihypertensive medication with CPAP in decreasing BP in those with HTN,39 but the evidence on the optimal timing of antihypertensive medication to lower nocturnal BP and eliminate the nocturnal non-dipping pattern of BP is conflicting. While CPAP treatment has been shown to reduce BP in those with existing pre-HTN and HTN, the evidence for the impact of treatment of OSA on incident HTN is mixed.39
Meta-analyses have indicated a greater magnitude of BP reduction with CPAP therapy, in the range of 5–7 mmHg in systolic BP and 3–5 mmHg in diastolic BP, in those with resistant HTN.1210,1211 However, not all reports are consistent,39 likely relating to differences in baseline severity of OSA and adherence to CPAP. These meta-analyses have shown a significant decrease in nighttime BP as well as a long-term (12 weeks) reduction in mean and diastolic BP with CPAP device usage of 4 or more hours a night.39,1210,1211
Individuals with OSA who report daytime sleepiness appear to demonstrate greater decline in BP compared to those who are not sleepy.39,1211 Precision medicine may help identify subgroups of patients most likely to benefit in terms of BP reduction from optimal CPAP treatment. These factors, amongst others, may help individualize treatment of OSA in the future to help decrease the risk of HTN and other adverse long-term CV consequences. (Table VIII.B.4.a)
TABLE VIII.B. 4.a.
Evidence for PAP therapy and blood pressure control
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Bazzano et al.1207 | 2007 | 1a | Meta-analysis of 16 RCTs (n = 818) – low heterogeneity | 1) OSA, treated with CPAP for at least 2 weeks (and up to 24 weeks, with good adherence in most studies). 2) OSA controls (sham-CPAP, pill, or usual care). OSA was mostly moderate-severe. All but 5 studies used ambulatory BP monitoring. |
1) SBP 2) DBP 3) MAP |
Mean SBP decreased by 2.46 mmHg (95% CI: −4.31 to −0.62), mean DBP by 1.83 mmHg (95% CI: −3.05 to −0.61) and MAP by 2.22 mmHg (95% CI: −4.38 to −0.05) with CPAP. Decreases in nocturnal and daytime BP were not statistically different. Dose–response relationship between mean hourly usage of CPAP and reduction in SBP was noted. |
| Fava et al.1209 | 2014 | 1a | Meta-analysis of 29 RCTs (n = 1820) – moderate heterogeneity | 1) OSA, treated with CPAP (good adherence in most studies) for 2–52 weeks. 2) OSA controls (sham-CPAP, pill, or usual care). OSA was mostly moderate–severe. Most studies used ambulatory BP monitoring, some included subjects with HTN. |
1) SBP 2) DBP |
Mean SBP decreased by 2.6 ± 0.6 mmHg and DBP by 2.0 ± 0.4 mmHg; p < 0.001) with CPAP. Daytime SBP and DBP fell by 2.2 ± 0.7 and 1.9 ± 0.6 mmHg respectively and nighttime SBP and DBP by 3.8 ± 0.8 and 1.8 ± 0.6 mmHg, respectively. Higher severity of OSA was associated with a greater decline in mean SBP. |
| Yu et al.654 | 2017 | 1a | Meta-analysis of 10 RCTs evaluating the primary outcome; two of these and two others, i.e., a total of four studies (n = 3017) measured BP before and after CPAP) – low to moderate heterogeneity | 1) OSA, treated with CPAP (most with suboptimal adherence). 2) OSA, receiving sham-CPAP or usual care. |
Major adverse cardiovascular events. BP (SBP, DBP) was an “inter-mediate” outcome | Pooled mean difference in SBP was 0.20 mmHg (95%CI: −2.29 to −1.89, p = 0.05 and DBP was 0.21 mmHg (95%CI: −1.06 to −0.65, p = 0.80) in CPAP vs. control groups. |
| Patil et al.39 | 2019 | 1a | Meta-analysis of 26 RCTs (n = 2444 had 24-h BP measured before and after) – moderate heterogeneity | 1) OSA, treated with CPAP (many with suboptimal adherence). 2) OSA, various control conditions (sham-CPAP, pill, or usual care). OSA was mostly moderate-severe. Five studies included subjects with HTN, five with RHTN. |
1) Multiple outcomes assessed, one of which was BP (SBP, DBP, and mean BP) | Mean nighttime SBP and DBP fell by 4.2 mmHg (95%CI: −6.0 to −2.5) and 2.3 mmHg (95%CI: −3.7 to −0.9) respectively with CPAP. Mean daytime SBP and DBP fell by −2.8 mmHg (95%CI: −4.3 to −1.2) and −2.0 mmHg (95%CI: −3.0 to −0.9), respectively; 24-h SBP, DBP, and mean BP all decreased significantly. In those with HTN, nighttime SBP and DBP, daytime SBP and DBP, and 24-h SBP and mean BP all decreased. In those with RHTN, significant reductions in nighttime SBP and DBP, daytime DBP, 24-h SBP and DBP, but no clinically significant reduction in daytime SBP was noted. |
| Bratton et al.1208 | 2015 | 1a | Meta-analysis of 51 studies (n = 4888) – moderate heterogeneity | 1) OSA, on CPAP. 2) OSA, on OA. 3) OSA, inactive control. follow-up ranged between 4 and 157 weeks, with variable adherence to CPAP. Many studies had subjects with HTN, including RHTN. Forty-four studies compared CPAP to inactive control, three compared OA to inactive control, one compared CPAP to OA, and three compared those on CPAP, OA, and inactive control. |
1) SBP 2) DBP |
CPAP reduced SBP by 2.5 mmHg (95%CI: −1.5 to −3.5; p < 0.001) and DBP by 2.0 mmHg (95%CI: −1.3 to −2.7; p < 0.001) vs. inactive control; a dose–response relationship between hours of usage of CPAP and reduction in SBP/DBP was noted. OA reduced SBP by 2.1 mmHg (95%CI: −0.8 to −3.4; p = 0.002) and DBP by 1.9 mmHg (95%CI: −0.5 to −3.2; p = 0.008). There were no significant differences in reduction in SBP/DBP between CPAP and OA in the network meta-analysis. |
| Iftikhar et al.1210 | 2014 | 1a | Meta-analysis (two observational studies, n = 44; four RCTs, n = 320) – moderate heterogeneity | 1) OSA and RHTN, treated with CPAP for 2–6 months. 2) OSA and RHTN, not treated. OSA was mostly severe. CPAP adherence was good. All but one study used ambulatory BP monitoring. |
1) 24-h SBP 2) 24-h DBP |
24-h SBP decreased by 7.21 mmHg (95%CI: −9.04 to −5.38; p < 0.001) and DBP by 4.99 mmHg (95%CI: −6.01 to −3.96; p < 0.001) with CPAP. Ambulatory SBP and DBP from four RCTs showed a decrease of 6.74 mmHg (95% CI: −9.98 to −3.49; p < 0.001) and 5.94 mmHg (95% CI: −9.40 to −2.47; p = 0.001), respectively. |
| Liu et al.1211 | 2016 | 1a | Meta-analysis of five RCTs (n = 216), moderate heterogeneity | 1) OSA and RHTN, treated with CPAP for 8 weeks to 6 months. 2) OSA and RHTN, not treated or on sham CPAP. OSA was moderate-severe. CPAP adherence was good and subjects reported no or mild sleepiness at baseline. |
1) 24-h SBP and DBP 2) Daytime SBP and DBP 3) Nocturnal SBP and DBP |
24-h SBP fell by 4.78 mmHg (95%CI: −7.95 to −1.61) and 24-h DBP by 2.95 mmHg (95%CI: −5.37 to −0.53) with CPAP. Nocturnal DBP decreased by 1.53 mmHg (95%CI: −3.07–0) with CPAP. |
Abbreviations: BP, blood pressure; CI, confidence intervals; CPAP, continuous positive airway pressure; CSA, central sleep apnea; CV, cardiovascular; DBP, diastolic blood pressure; MAP, mean arterial pressure; OA, oral appliance; OSA, obstructive sleep apnea; RCT, randomized controlled trial; RHTN, resistant hypertension; SBP, systolic blood pressure.
VIII.B.4.b |. PAP and control of atrial fibrillation
Several observational studies have evaluated the association of CPAP treatment for OSA with risk of recurrent AF following electrical cardioversion or ablation procedures. These studies have generally found that patients with OSA who are not treated with CPAP are significantly more likely to have recurrent AF than CPAP-treated patients, with two- to six-fold higher adjusted risk of recurrence.708,729,730,1215,1216 No significant difference in rate of recurrence was seen, however, in two very small studies712,728 and one large, retrospective cohort study.1217 A single randomized clinical trial has been published comparing CPAP to usual care for prevention of recurrence following electrical cardioversion of AF. In this study, only 25 of a planned 180 patients were randomized, with no significant difference in outcome between groups.1218 In a randomized trial evaluating the impact of CPAP versus supportive care in older adults with OSA, CV outcomes were a secondary endpoint. Incident AF was not separately analyzed but was reported in a supplementary table and occurred in 16.5% of supportive care patients over 12 months of follow-up versus 7.6% in the CPAP group, a difference that is not statistically significant.1219
A recent randomized clinical trial tested the effect of 5 months of treatment with CPAP plus usual care to usual care alone on AF burden (percentage of time in AF) in 109 patients with paroxysmal AF who were found on screening to have moderate or severe OSA (defined as AHI ≥ 15). Despite a mean nightly CPAP use of 4.4 h, the adjusted difference in AF burden between CPAP and usual care was 0.6% (95% CI: −2.6–1.3; p = 0.52), nor was there a significant difference in the percentage of patients achieving a 25% reduction in AF burden or in the frequency or duration of AF episodes.710 Limiting the analysis to those using CPAP ≥4 h per night did not alter the findings. (Table VIII.B.4.b)
TABLE VIII.B. 4.b.
Evidence for use of PAP and atrial fibrillation control
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Kanagala et al.1216 | 2003 | 4 | Clinic-based cohort study | Patients referred for DC cardioversion; OSA diagnosis based on prior documented polysomnography; CPAP use based on self-report 1. 12 patients with treated OSA 2. 27 patients with untreated (or inadequately treated) OSA 3. 79 patients without known OSA |
Recurrence of AF at 12 months | AF recurred in 82% of untreated OSA patients, which was significantly high than the 42% of treated OSA patients (p = 0.01) and 53% in the patients without known OSA (p = 0.009). |
| Jongnarangsin et al.728 | 2008 | 4 | Clinic-based cohort study | 324 patients referred for radiofrequency catheter ablation of AF (pulmonary vein isolation, other atrial regions if needed; OSA diagnosis based on prior documented polysomnography 1. 18 patients with treated OSA 2. 14 patients with untreated OSA 3. 292 patients without known OSA |
Recurrence of AF over a mean follow-up of 7 (SD 4) months | AF recurred in 37% of patients without known OSA and 59% of patients with OSA (p = 0.02). The recurrence rate was 50% in the CPAP-treated OSA patients and 71% in the untreated OSA patients (p = 0.29). In multivariate analysis, the adjusted OR for AF recurrence was 3.04 (95% CI 1.11–8.32) in those with OSA compared to those without known OSA. |
| Patel et al.730 | 2010 | 2b | Clinic-based, multi-center cohort study | 3000 consecutive patients undergoing pulmonary vein and posterior wall isolation procedure for AF; OSA diagnosis based on prior documented polysomnography with AHI >15 and >80% of events obstructive; CPAP use based on self-report 1. 315 OSA patients with regular CPAP use 2. 325 OSA patients with no or erratic CPAP use 3. 2360 patients without known OSA |
Recurrence of AF over a mean follow-up of 32 (SD 14) months | AF recurred in 22% of patients without known OSA and 27% of patients with OSA. The adjusted OR for AF recurrence was 1.59 (95% CI 1.25–2.08) for OSA versus no known OSA. Among those with OSA, AF recurred in 21% of those treated with CPAP and 32% of those not using CPAP (p = 0.003). The adjusted OR for AF recurrence was 0.16 (95% CI 0.07–0.35) for CPAP use versus no or erratic CPAP use. |
| Fein et al.1215 | 2013 | 4 | Clinic-based cohort study | 92 patients undergoing pulmonary vein isolation for the treatment of AF; OSA diagnosis based on prior documented polysomnography with AHI >15 and ≥80% of events obstructive; method of CPAP use determination not stated 1. 32 patients with treated OSA 2. 30 patients with untreated OSA 3. 30 patients without known OSA |
AF-free survival | The 1-year AF-free survival was 71.9% in those with CPAP-treated OSA, significantly higher than the 33.3% in those with untreated OSA (p = 0.02) but not significantly different from the 66.7% in those without OSA. Compared to those without OSA, the adjusted HR for AF recurrence was 2.15 (95% CI 1.10–5.44) in those with OSA not using CPAP and 0.7 (95% CI 0.3–1.59) in those with OSA using CPAP. |
| Naruse et al.708 | 2013 | 2b | Clinic-based cohort study | 153 patients undergoing pulmonary vein isolation for the treatment of AF; all patients underwent polysomnography approximately 1 week after the procedure, with OSA defined as AHI ≥ 5 and ≥50% of events obstructive; objective CPAP data 1. 82 patients with CPAP-treated OSA (60 with use ≥4 h/night, 22 with use <4 h/night) 2. 34 patients with untreated OSA 3. 37 patients without OSA |
AF recurrence over mean follow-up of 19 (SD 10) months | AF recurred in 53% of those with untreated OSA, 30% in those treated with CPAP, and 22% of those without OSA. The multivariate adjusted HR for AF recurrence was 2.61 (95% CI 1.12–6.09) for presence of OSA and 0.41 (95% CI 0.22–0.76) for CPAP use. |
| Neilan et al.729 | 2013 | 2b | Clinic-based cohort study | 720 consecutive patients referred for pulmonary vein isolation for AF; OSA diagnosis based on prior documented polysomnography; CPAP usage by self-report 1. 71 patients with treated OSA (CPAP use >4 h/night) 2. 71 patients with untreated OSA (or CPAP use <4 h/night) 3. 578 patients without known OSA |
Late AF recurrence (AF occurring >3 months after PVI) over median follow-up of 42 months | The cumulative incidence of late AF was 68% in patients with untreated OSA, 35% in treated OSA, and 30% in those without OSA. Compared to those without OSA, the multivariate adjusted HR for AF recurrence was 2.79 (95% CI 1.97–3.94) in untreated OSA and 1.14 (95% CI 0.741.76) in treated OSA. |
| McMillan et al.1219 | 2014 | 2b | Randomized multi-center clinical trial | 278 patients with 4% oxygen desaturation index >7.5 events/h plus Epworth Sleepiness Scale score of ≥9 1. CPAP plus “best supportive care” 2. “Best supportive care” alone |
Incident AF was reported as part of a secondary composite endpoint and was not itself a pre-specified endpoint | Supplementary table S13b includes baseline prevalence and 12-month incidence of AF. In the Best Supportive Care group, incident AF occurred in 14 of 85 patients free of AF at baseline, versus seven of 92 in the CPAP group (OR 0.42 for CPAP group, p = 0.07). |
| Caples et al.1218 | 2019 | 2b | Randomized clinical trial | 25 patients with recent successful electrocardioversion for AF screened for enrollment; of 1757 patients screened, 34 were enrolled and underwent in-lab polysomnography; 25 with AHI ≥ 5 and predominantly obstructive events were randomized to: 1. CPAP plus usual |
Recurrence of AF | 36% of participants withdrew or were lost to follow-up prior to 1-year follow-up; three patients in each group had recurrence of AF (p = 0.98). This study was markedly underpowered to observe a treatment effect. |
| Hojo et al.712 | 2019 | 4 | Clinic-based cohort study | 100 patients undergoing pulmonary vein isolation for AF; all had cardiorespiratory polygraphy at baseline, with OSA defined as AHI ≥ 15 and ≥50% of events obstructive; all patients had a second PVI procedure at 6 months, after which CPAP was recommended based on local guidelines 1. 11 patients with treated OSA 2. 23 patients with untreated OSA 3. 66 patients without OSA |
Recurrence of AF or other atrial tachyarrhythmia following the second PVI procedure | No significant difference among groups was noted in rate of AF recurrence (12.1% in no OSA group, 9.1% un treated OSA group, 8.7% in untreated OSA group). |
| Srivali et al.1217 | 2019 | 2b | Retrospective clinic-based cohort study | 429 patients with a diagnosis of sleep apnea by in-lab PSG (351 OSA, 21 CSA, 57 mixed sleep apnea), with a subsequent diagnosis of AF, treated with PVI or with electrical or chemical cardioversion 1. 269 PAP-adherent patients, defined as ≥4 h/night at least 70% of nights 2. 160 PAP non-adherent patients |
Recurrence of AF over a median follow-up of 4.6 months; analyzed for all sleep apnea and separately by type of sleep apnea | Time to recurrence of AF did not differ between PAP-adherent and non-adherent patients overall or for any sleep apnea type. In OSA patients, median recurrence-free survival was 9.4 months in the adherent and 9.5 months in the non-adherent group. PVI (vs. cardioversion) was the strongest predictor of recurrence-free survival, and was more common in PAP non-adherent (33%) than PAP adherent (20%) patients; however, the authors state that in multivariate models adjusting for type of intervention, there was no significant difference between PAP adherent and non-adherent patients in hazard of AF recurrence. |
| Traaen et al.710 | 2021 | 1b | Randomized clinical trial | Patients with paroxysmal atrial fibrillation, screened for sleep apnea with two nights of home respiratory polygraphy with a mean AHI ≥ 15, and use of CPAP ≥4 h on each night of a 7-night run-in period 1. Usual care alone (n = 54, no dropouts), baseline AF burden 5.0% 2. Usual care plus CPAP (n = 55, one dropout), baseline AF burden 5.6%; mean CPAP use 4.4 (SD 1.9) h/night |
Primary outcome: between-group difference in change in AF burden (percent of time in AF) from the 1-month period prior to treatment to the final 3 months of the 5-month treatment period Secondary outcome: percent of patients with a ≥25% reduction in AF burden Post-hoc analyses: (1) frequency of AF episodes; (2) duration of AF episodes |
AF burden decreased from 5.0% to 4.3% in the control group and from 5.6% to 4.1% in the CPAP group (adjusted mean difference −0.6%, 95% CI −2.6 to +1.3%, p = 0.52). No significant difference in “per protocol” analysis including only those using CPAP ≥4 h/night. The percent of patients with a ≥25% reduction in AF burden was 41% in the CPAP group and 31% in the usual care group (adjusted mean difference −9.3%, 95% CI −26.4 to +8.7%, p = 0.33). No significant difference in frequency or duration of AF events. Serious adverse events occurred in 13% of CPAP and 4% of control patients. |
VIII.B.4.c |. PAP and CVA
Despite the high prevalence of OSA in the stroke population and the well-established literature stating OSA is an independent risk factor for cerebrovascular events, it is not common practice to evaluate and treat OSA as a part of post-stroke workup.
A recent small RCT of stroke patients who were admitted for neurorehabilitation was conducted to determine if OSA treatment with CPAP would improve cognitive and functional outcomes post-stroke. Twenty patients were randomly assigned to receive 4 weeks of CPAP treatment and 16 patients were assigned to receive treatment as usual (control). Patients who received and were compliant with 4 weeks of CPAP showed greater improvement in cognitive status (attention and executive functioning) compared to the control group. However, there was no significant difference in functional status (neurological status and ADL) between the treatment and control groups.1220
There have been mixed results about the efficacy of CPAP treatment on stroke outcomes, but currently there is promising evidence suggesting CPAP improves cognitive functioning of stroke patients, which is consistent with the benefits of CPAP in the general OSA population. In an RCT by Gupta et al.,1029 patients with moderate sleep apnea (AHI > 15 events/h) and recent stroke were randomized to receive either standard of care for stroke or standard of care + CPAP. The patients in the CPAP + standard of care group were found to have a non-statistically significant decrease in new vascular events and a statistically significant improvement in post-stroke function based on the Barthel Index.
Therefore, it is recommended to screen for OSA in all patients presenting with TIA or ischemic or hemorrhagic stroke regardless of whether they are symptomatic or asymptomatic. Earlier diagnosis of OSA and early treatment could improve overall health and cognitive status and reduce the risk of recurrent stroke and stroke mortality. The standard of treatment of OSA is CPAP. However, CPAP is poorly tolerated in both the general population and even less so in the stroke patient population. Apart from the general obstacles, such as mask claustrophobia, nasal congestion, and mask leak, there are unique difficulties stroke patients face, including aphasia, facial or oropharyngeal weakness, and limb weakness. Thus, alternative options for treating OSA should also be considered. The effects of alternative OSA treatments on risk of recurrent stroke have not been reported. (Table VIII.B.4.c)
TABLE VIII.B. 4.c.
PAP after CVA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Aaronson et al. 1220 | 2016 | 1b | Randomized control trial | 36 stroke patients who were admitted to a neurorehabilitation unit with OSA were randomly assigned to receive 4 weeks of CPAP therapy (20) or standard of care (16) | Cognitive status measured by neuropsychological examination, and functional status measured by two neurological scales and a measure of activities of daily living (ADL). Recovery rate in the two groups of OSA patients |
CPAP treatment significantly improves attention and executive functioning of stroke patients with OSA. CPAP treatment did not significantly improve functional status (neurological and ADL). |
| Bravata et al.1221 | 2011 | 1b | Randomized control trial | 70 acute TIA patients were randomized to control (25) or treatment group (45) who received auto-CPAP for two nights and those in treatment group with OSA received auto-CPAP for remaining 90 days | Prevalence of OSA CPAP adherence rate among TIA patients Recurrent vascular events such as recurrent TIA, stroke, hospitalization for CHF, MI, death |
OSA is highly prevalent among patients with acute TIA. Increasing auto-CPAP use was not significantly associated with reduced rate of recurrent vascular events. |
| Bravata et al.599 | 2017 | 1b | Randomized control trial | 225 points with cerebrovascular disease and HTN from the GoToSleep study at two Veteran Health Affairs centers were randomized to control group or treatment group of auto-CPAP for 1 year | OSA prevalence, OSA treatment as measured by CPAP adherence, and HTN control | There is a high prevalence of OSA among patients with chronic cerebrovascular disease and hypertension. Portable polysomnography and auto-CPAP use improved the diagnosis and the treatment for OSA compared with standard care, but did not lower blood pressure. |
| Catalan-Serra et al.647 | 2019 | 2b | Prospective cohort study | Cohort of 1005 consecutive patients referred to Sleep Units at two Spanish University hospitals who were ≥65 years. They were categorized into four groups based on AHI values from sleep studies and CPAP adherence: 1) control group 2) untreated mild-moderate OSA 3) untreated severe OSA 4) CPAP-treated OSA |
Incidence of coronary heart disease and incidence of stroke after adjusting for OSA group, age, BMI, HTN, sex, smoking, ESS, and AF | The incidence of stroke, but not coronary heart disease, is increased in elderly patients with untreated severe OSA. |
| Gupta et al.1029 | 2018 | 1b | RCT | 70 patients with OSA and recent stroke randomized to 1. CPAP, 2. non-CPAP | New vascular events. Secondary: clinical stroke outcomes and neuropsycholog- ical parameters | Nonsignificant lower rate of vascular event in CPAP group (3%) vs. non-CPAP group (15%). Significantly improved clinical stroke outcomes (modified Rankin scale) at 12-months in CPAP group. |
| Khot et al.761 | 2016 | 1b | Randomized control trial | Pilot study: 40 patients in stroke rehabilitation who were randomized to APAP or sham CPAP without testing for OSA | Change in Functional Independence Measure (FIM) was assessed between rehabilitation admission and discharge | There were positive trends toward better FIM score improvement with APAP compared to sham CPAP. |
| Kim et al.1222 | 2016 | 2a | Systematic review and meta-analysis | 8 studies (one RCT and five cohort and two administrative data) to evaluate effects of CPAP on risk of stroke | Stroke incidence and mortality | Treating with CPAP in patients with OSA might decrease the risk of stroke as seen only in the cohort studies. |
| McKee et al.1224 | 2020 | 2b | Retrospective observational study | 527 post-stroke patients at inpatient rehab who underwent overnight oximetry and those at high risk for OSA (3%ODI > 10) were on trial of Auto-CPAP | Recovery measured by changes in functional and cognitive outcomes calculated by functional independence measure (FIM) scores | APAP in high-risk patients was poorly tolerated and did not improve FIM. |
| Parra et al.1225 | 2015 | 1b | Randomized control trial 235 patients <75 years old admitted with ischemic stroke for the first time and with moderate-severe OSA with an AHI ≥20 events randomized to standard care or nasal CPAP during the acute stroke phase | Cardiovascular events: cardiac ischemic events, recurrent stroke, and cardiovascular mortality | Ischemic stroke patients treated with early nasal CPAP improves long-term survival with better neurological recovery compared to the control group. | |
| Parra et al.1226 | 2011 | 1b | Randomized control trial | 140 patients admitted with first time ischemic stroke and AHI ≥ 20 events/h randomized to early nasal CPAP or standard care during acute stroke phase | Cardiovascular events: cardiac ischemic events, stroke recurrence, and cardiovascular mortality Neurological parameters measured by Barthel index, Canadian scale, Rankin scale, and SF-36 |
Patients who received nasal CPAP treatment earlier had significantly greater and faster neurological recovery compared to the control group. Stroke patients treated with CPAP experienced less Incident cardiovascular events in the first 24 months compared to control group. |
| Ryan et al.1227 | 2011 | 1b | Randomized control trial | Patients with ischemic or hemorrhagic stroke patients with moderate to severe OSA were assigned to standard rehabilitation alone (22) or to CPAP (22) | Motor, functional, and neurocognitive recovery as measured by Canadian Neurological scale, the 6-min walk test distance, sustained attention response test, and the digit or spatial span-backward | CPAP in stroke patients undergoing rehabilitation improved functional and motor, but not neurocognitive outcomes. |
| Sánchez-de-la-Torre et al.1203 | 2020 | 1b | Randomized control trial | 2551 patients admitted with acute coronary syndrome (ACS) symptoms who had a respiratory polygraphy within 24–72 h of admission. Patients had a sleep study and those with OSA were randomized to either CPAP with standard care or standard care alone. Those ACS patients without OSA were the control group | Prevalence of cardiovascular events: cardiovascular death or non-fatal events [acute myocardial infarction, non-fatal stroke, hospital admission for heart failure, and new hospitalizations for unstable angina or transient ischemic attack] | The prevalence of cardiovascular events was not significantly associated with CPAP use in patients with ACS and OSA compared to standard care treatment. Moreover, OSA diagnosis was not associated with worse prognosis in ACS patients. |
VIII.B.4.d |. PAP for heart failure
The prevalence of OSA is significantly higher than the general population, ranging from 11% to 37%, in patients with HF.1228,1229 In the SHHS, a cross-sectional analysis in 6424 individuals demonstrated that OSA was associated with increased relative OR of 2.38 (1.22–4.62) of having HF.638 Treatment of OSA in individuals with HF has been shown to directly improve cardiac mechanical function in addition to various factors that perpetuate the pathologic abnormalities in HF.
Optimization of BP and heart rate are part of core strategies recommended in the clinical guidelines for HF management.753 Untreated OSA in patients with HFrEF increases systolic BP,1230 whereas CPAP treatment of OSA in this population improves systolic and diastolic BP,743,1230–1232 heart rate,743,1232 heart rate variability (HRV),1233 oxidative metabolism,1234 and sympathetic activity.1232,1235
CPAP treatment of OSA in patients with HFrEF is associated with a modest increase in LVEF,745,1231,1234,1236–1239 whereas this increase is not observed in OSA individuals without HFrEF.1237 The impact of CPAP on LVEF was more prominent in individuals with a baseline LVEF > 30% in a study by Egea et al.745 While another study did not show an improvement in LVEF with CPAP use, adherence to PAP therapy was low and efficacy of treatment on autoCPAP was not reported, limiting the analysis of PAP impact.1239 Kasai et al. showed that the use of CPAP in OSA patients with HFrEF improved event-free survival compared to no CPAP use, with even greater improvement in event-free survival in individuals with higher CPAP use.754
OSA is associated with diastolic dysfunction1240 and with left ventricular hypertrophy.1241 CPAP use among patients with HFpEF and OSA also improves diastolic function750,1242–1244 and reduces LV thickness.1241,1244 Adherence to therapy appears to determine impact of CPAP on diastolic function in OSA patients as well. In a study by Glantz et al., no improvement was seen in diastolic function with CPAP in non-sleepy patients with OSA; however, in post hoc analysis, it was noted that the use of CPAP for >4 h per night resulted in improved diastolic function.1245 (Table VIII.B.4.d1 and VIII.B.4.d2)
TABLE VIII.B. 4.d1.
Evidence for PAP and clinical outcomes in HFrEF
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Tkacova et al.1230 | 1998 | 3b | Prospective CS | Pharmacologically treated HF patients with OSA were studied during PSG and recordings were done before, during, and after CPAP application. n = 8 |
BP and esophageal pressures before onset of sleep and during stage 2 NREM sleep before, during, and after application of CPAP | 1. OSA was associated with increased systolic BP and systolic LVPtm from wakefulness to stage 2 NREM sleep. 2. CPAP alleviated OSA, improved SaO2, and reduced systolic BP in stage 2 NREM sleep, LVPtm, Pes amplitude, and RR.1230 |
| Usui et al.1232 | 2005 | 1b | RCT | Pharmacologically treated HF patients with LVEF < 45% and OSA (AHI ≥ 20/h) randomized to PAP and usual therapy vs. only usual therapy. n = 17 |
Recording at baseline and at 1 month the morning after PSG: ECG BP peroneal MSNA With subjects awake, resting quietly supine, and breathing without apnea |
1. In OSA patients treated with CPAP, there was significant reductions in daytime MSNA (from 58 ± 4 to 48 ± 5 bursts/min; 84 ± 4 bursts/100 heart beats to 72 ± 5 bursts/100 heart beats; p < 0.001 and p = 0.003, respectively), systolic BP (from 135 ± 5 to 120 ± 6 mmHg, p = 0.03), and HR (from 69 ± 2 min (−1) to 66 ± 2 min (−1); p = 0.013). 2. In control OSA patients, there were no changes in MSNA, systolic BP, or HR.1232 |
| Kaneko et al.743 | 2003 | 1b | RCT | Pharmacologically treated HF patients with LVEF ≤ 45% and OSA (AHI ≥ 20/h) randomized to PAP and usual therapy vs. only usual therapy. n = 24 |
Recording at baseline and at 1 month: AHI Daytime BP and HR LV end-diastolic dimensions LVEF |
1. In OSA patients treated with CPAP, there was significant reductions in AHI, daytime systolic BP, HR, LV end-diastolic dimension and improved LVEF (absolute improvement by 8.8%). 2. In control OSA patients, there were no changes in AHI, daytime systolic BP, HR, LV end-diastolic dimensions, and LVEF.743 |
| Gilman et al.1233 | 2008 | 1b | RCT | Pharmacologically treated HF patients with LVEF ≤ 45% and OSA (AHI ≥ 20/h) randomized to PAP and usual therapy vs. only usual therapy. n = 19 |
Morning HF-HRV at baseline and at 1 month | 1. In OSA patients treated with CPAP, there was HF-HRV increased significantly during wakefulness in the morning. 2. In control OSA patients, there were no changes in HF-HRV during wakefulness in the morning.1233 |
| Mansfield et al.746 | 2004 | 1b | RCT | Pharmacologically treated HF with LVEF < 55% and NYHA II, AHI ≥ 5/h, CSA ≤ 20%) randomized to PAP and usual therapy vs. only usual therapy. n = 55 |
Recording at baseline and at 3 months: LVEF Overnight urinary norepinephrine BP quality of life |
1. In OSA patients treated with CPAP, there was increased LVEF (absolute improvement by 5%), reduced urine norepinephrine excretion (42% decrease), ESS, and minimum oxygen saturation. 2. No changes in blood pressure or peak oxygen consumption were noted 3. In control OSA patients, there were no significant changes in LVEF, urine norepinephrine or ESS.746 |
| Smith et al.1239 | 2007 | 1b | RCT with crossover design | Pharmacologically treated symptomatic HF with LVEF <45% and NYHA class II–VI with OSA (AHI ≥ 15/h) randomized to APAP vs. sham CPAP for 6 weeks each in crossover design. n = 23 |
Recordings at baseline, 6 and 13 weeks (1 week washout before cross over): Clinical assessment TTE CPET 6 min walk distance (MWD) neurohumoral markers (NT-pro-BNP and NT-pro-ANP) Oxford Sleep Resistance Test Quality of life |
1. CPAP improved daytime sleepiness, but not LVEF, 6 MWD, Peak VO2, VE/VCO2, or quality of life. 2. Study limitations were poor adherence (∼3 h) and use of autoCPAP without report of treatment efficacy in AHI reduction.1239 |
| Malone et al.1238 | 1991 | 3b | Prospective CS | Pharmacologically treated, obese patients with idiopathic cardiomyopathy, LVEF < 55%, and severe OSA. n = 8 |
Recording at baseline, 4 weeks after nasal CPAP, and 1 week after withdrawal of nasal CPAP TTE |
1. CPAP results in mean LVEF increase of 37% to 49% from pre-treatment to 4 weeks after therapy. 2. Withdrawal of CPAP resulted in reduction of LVEF from 53% to 45%.1238 |
| Egea et al.745 | 2008 | 1b | RCT | Pharmacologically treated HF with LVEF < 45% and OSA with AHI > 10/h and randomized to PAP vs. sham CpAP. n = 60 |
Recording and baseline and 3 months of treatment with optimal CPAP or sham-CPAP LVEF 6 min walking test ESS SF-36 NYHA Dyspnea |
1. LVEF improved by an absolute 2.2% in the treatment group with even greater improvement in patients with LVEF > 30%. 2. ESS with significant improvement in both groups. 3. No change in NYHA, 6 min walk distance, SF-36, or dyspnea.745 |
| Johnson et al.1237 | 2008 | 2b | Prospective CS | HF patients with NYHA class II or III and LVEF < 40% and AHI > 15/h. Patients with OSA were treated with CPAP and those without were Not. n = 12 |
TTE at baseline (awake, before, and after acute CPAP administration) and after CPAP therapy (7 weeks) in OSA patients Recording was done at baseline and follow-up for non-OSA patients |
1. In OSA patients, acute CPAP resulted in decreased stroke volume and LVEF by ∼5% compared to baseline. 2. In OSA patients, chronic CPAP resulted in increased stroke volume and increased LVEF from 38.4% to 43.4%. 3. There was no change in LVEF, diastolic function, or filling pressures in patients without OSA.1237 |
| Ferrier et al.1236 | 2008 | 2b | Prospective CS | Outpatient patients with HF (LVEF < 45%) and OSA (AHI > 15/h) treated with CPAP and control group with HF (LVEF < 45%) and AHI < 10/h. n = 26 |
Recording at baseline and at 6 months Minnesota HF score ESS Shuttle walk distance BNP Urine catecholamines TTE |
1. In the study group, there was absolute improvement in LVEF by 4.7%. 2. In the study group, there was decrease in the LV end-diastolic volume, systolic blood pressure, and ESS. 3. Walk distance, catecholamines, BNP levels, and symptoms remained unchanged in both groups.1236 |
| Yoshinaga et al.1234 | 2007 | 2b | Prospective CS | Outpatients with HF (LVEF < 40%, NYHA II or III, unchanged > 4 weeks) with and without OSA (AHI > 15/h). n = 12 |
Oxidative metabolism using mono-exponential fit of the of the myocardial [11C] acetate positron emission tomography time-activity curve (correlate for myocardial oxygen consumption) Myocardial efficiency from work metabolic index and at baseline, short-term CPAP and after 6 ± 3 weeks of CPAP |
1. Short-term CPAP reduced oxidative metabolism and stroke volume index, but did not change work metabolic index. 2. Longer-term CPAP improved left ventricular ejection fraction by 5%, reduced oxidative metabolism and improved work metabolic index.1234 |
| Kasai et al.754 | 2008 | 2b | Prospective CS | Medically optimized heart failure with LVEF ≤ 50%, without hospitalizations in the preceding month and AHI ≥ 15/h, NYHA class II or above, CAI < 50% of overall AHI. Participants grouped into CPAP untreated, “more compliant” and “less compliant” groups. n = 88 |
Baseline recordings: BMI heart rate ESS LVEF by echo plasma norepinephrine NYHA class etiology of HF (ischemic or nonischemic) presence of AFIB administered medications Primary end point: event-free survival at 25.3 months |
1. Use of CPAP was associated with improved event-free survival compared to no CPAP use. 2. Greater adherence to CPAP therapy (∼6.0 h/night) was associated with greater improvement in event free-survival than less adherence (∼3.5 h/night).754 |
| Wang et al.742 | 2007 | 2b | Prospective CS | Medically optimized heart failure patients with LVEF ≤ 45% and NYHA class II to IV with OSA (AHI ≥ 15) with a CPAP treated and untreated group. n = 164 |
Primary end point: Cumulative rate of death from the date of the diagnostic sleep study until January 1, 2005 |
There was trend for reduced mortality in the treated OSA group.742 |
| Hall et al.1235 | 2014 | 1b | RCT | Medically optimized patients with HFrEF (EF < 45%, NYHA ≥ II and OSA (AHI > 10, OA > 80%) randomized to CPAP versus usual care. n = 45 |
Evaluated at baseline and 6–8 weeks after therapy initiation: ECG echocardiography c-acetate and c-hydroxyephedrine PET imaging |
1. Short-term CPAP increased hydroxyephedrine retention, indicating improved sympathetic nerve function in patients with OSA and HFrEF. 2. No changes in hemodynamic, LV, or energetics parameters in the whole study population.1235 |
| Aggarwal et al.1231 | 2014 | 1a | SR | Systemic review and meta-analysis with pooled data from 15 randomized controlled trials in which patients had sleep disordered breathing and heart failure and consisted of a group receiving PAP therapy and another group receiving no PAP therapy or sham PAP therapy. | End points analyzed are: Left ventricular ejection fraction Diastolic blood pressure Systolic blood pressure Heart rate Mortality |
1. A significant improvement in LVEF was noted with CPAP (mean difference of 5.05%; 95% CI 3.72–6.38), diastolic blood pressure (mean difference of –1.67; 95% CI of –3.09–0.25) and heart rate (mean difference of –5.92; 95% CI of –10.12–1.72). 2. No significant changes in mortality (OR 0.63; 95% CI 0.40–1.00) and systolic blood pressure (mean difference –6.35; 95% CI –16.11–2.41) were noted. |
TABLE VIII.B. 4.d2.
Evidence for PAP and clinical outcomes in HFpEF
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Arias et al.750 | 2005 | 1b | RCT | Patients with OSA (AHI ≥ 10/h and excessive daytime sleepiness with ESS ≥ 10) and healthy control subjects OSA patients were randomized to 12 weeks of sham CPAP vs. effective CPAP and crossed-over to alternate therapy n = 32 |
Recording at baseline, after effective nasal CPAP therapy for 12 weeks, and sham CPAP therapy for 12 weeks TTE Blood pressures Urine catecholamines |
1. Diastolic function improved in the OSA patients after 12 weeks ofeffective CPAP therapy. 2. There was also significant increase in ratio of peak early filling velocity (E) to peak late filling velocity (A) of diastolic transmitral flow (E/A) and reduction in mitral deceleration time and isovolumic relaxation. |
| Alchanatis et al.1243 | 2000 | 2b | Prospective CS | Patients with OSA (AHI > 15/h) n = 15 |
Blood pressure and TTE at baseline and after 12–14 weeks of nasal CPAP therapy | 1. At baseline, there was diastolic dysfunction present in OSA patients and was improved (increased E/A ratio, decreased IVRT) after 12 weeks of CPAP therapy. |
| Cloward et al.1241 | 2003 | 2b | Prospective CS | Patient with desaturation index >40/h and ≥20% cumulative time spent with arterial oxygen saturation (SaO2) < 90% n = 25 |
TTE at baseline, 1 and 6 months following initiation of PAP therapy | 1. Severe OSA was associated with left ventricular hypertrophy (LVH), left atrial enlargement (LAE), right atrial enlargement (RAE), and right ventricular hypertrophy (RVH). 2. Those compliant with CPAP therapy, there significant reductions in LVH after 6 months of CPAP therapy as measured by interventricular septal distance. 3. RAE and LAE were unchanged after CPAP therapy. |
| Dursunoglu et al.1244 | 2007 | 2b | Prospective CS | Patients with severe OSA (AHI > 33/h) n = 25 |
TTE and blood pressure in patients with severe OSA at baseline and 6 months of CPAP therapy | 1. There was significant reduction in LV wall thickness (interventricular septum and LV posterior wall) in the CPAP treated group. 2. There was significant improvement in LV global dysfunction after 6 months of PAP therapy. |
| Shivalkar et al.1246 | 2006 | 2b | Prospective CS | Patients with severe, symptomatic OSA, and age- and gender-matched controls n = 40 |
TTE and blood pressure at baseline and after 6 months of PAP | 1. PAP therapy significantly improved heart rate and blood pressure. 2. PAP resulted in improved pulmonary artery pressures, interventricular septum thickness, right ventricle dimensions, mitral annular systolic tissue doppler velocity (Sm), tricuspid annular Sm, and right ventricle free wall Sm. |
| Akar Bayram et al.1242 | 2009 | 2b | Prospective CS | Newly diagnosed OSA patients with AHI ≥ 15 and control patients without OSA n = 46 |
TTE at baseline and after 6 months of PAP therapy in the study group compared to control patients | 1. Patients who were on CPAP therapy had improved systolic and diastolic function after 6 months of CPAP therapy. |
| Glantz et al.1245 | 2017 | 1b | RCT | Revascularized CAD patients with non-sleepy OSA (AHI ≥ 15, ESS < 10) were assigned to CPAP or no-CPAP Group n = 171 |
Echocardiographic measurements were obtained at baseline, 3 and 12 months | 1. There was no improvement in diastolic dysfunction in non-sleepy CAD patients with OSA on CPAP therapy. 2. CPAP use of >4 h/night resulted in improved diastolic dysfunction in post hoc analysis. |
| Craig et al1247 | 2015 | 1b | RCT | 2 centers randomized 238 patients to 6 months of CPAP or standard care n = 238 |
Echocardiograms were done for 168 patients and cardiac magnetic resonance scans were done for 68 patients. Baseline ODI was 13.5/h and ESS was 8.4. Baseline LV ejection fraction was well preserved (60.4%) | 1. CPAP had no significant effect on ECHO-derived LA area or early to late left ventricular filling velocity (E/A). 2. Small change in echo-derived LV end diastolic volume (EDV) with CPAP was noted (−5.9 mL with 95% CI −10.6 to −1.2; p = 0.015). 3. No significant changes were detected by CMR or LV mass index or LVEF. |
VIII.B.4.e |. PAP and cardiovascular events
OSA may increase the risk of CAD three-fold relative to the general population (16.2% CAD in OSA patients versus 5.4% in patients without OSA).673 The Sleep Heart Health cohort reported a prevalence of self-reported CAD ranging from 9% to 19% in OSA patients depending on the severity of the SDB.638 The risk of CAD is highest in those with severe OSA.
Observational data have shown that CPAP therapy may attenuate this risk of CAD development from 16.2% to 3.9%, when there is adherence to CPAP and efficacy of CPAP in resolving OSA.1248 Multiple long-term studies evaluating CPAP use in OSA spanning five to 10 years have shown reduction in CAD-related mortality and CV events with CPAP.118,1249,1250 Doherty et al. showed that death from cardiac events was reduced from 14.8% to 1.9% with PAP therapy compared to untreated OSA, and total cardiac events were also reduced with PAP therapy relative to untreated OSA (18% vs. 31%, respectively) over an average of 7 years.1250 An additional retrospective study showed improved CAD mortality with PAP treatment in moderate to severe OSA but did not show reduction in CAD events.1251
Prospective RCTs investigating the impact of CPAP therapy for newly diagnosed OSA after index cardiac or vascular event did not show reduction in secondary cardiac or vascular events, including CV death.26,673,1203 These studies may have failed to demonstrate benefits of PAP therapy in CV risk reduction due to poor adherence (<4h per night).26,1203 Adherence may play a role in improving CV outcomes, as demonstrated by Peker et al. in which the secondary analysis of the RCT, individuals using CPAP for more than 4 h per night relative to those nonadherent to therapy had a significant reduction in cardiac events.673 (Table VIII.B.4.e)
TABLE VIII.B. 4.e.
Evidence for PAP and cardiovascular risk reduction
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Peker et al.1248 | 2006 | 2b | Prospective CS | Patients age 30–69 who underwent in-lab polysomnography and were not known to have heart disease were stratified by presence or absence of OSA (AHI ≥15), and treatment with PAP therapy if OSA diagnosis present n = 308 |
Cohort was observed over 7 years for development of CAD and CAD related fatality. | 1. 16.2% of patients with OSA and 5.4% of patients without OSA developed CAD. 2. Patients on effective therapy for OSA (PAP use >50% of sleep time, use of oral appliance, or effectively treated with surgery) had reduction in incidence of CAD to 3.9%. |
| Peker et al.673 | 2016 | 1b | RCT | Patients with newly revascularized CAD and OSA (AHI ≥ 15/h) without daytime sleepiness (ESS < 10) randomized to auto-titrating PAP or No PAP n = 122 |
Primary endpoint was first event of repeat revascularization, MI, or cardiovascular mortality. Median follow up was 57 months. | 1. Incidence of the primary endpoint did not differ significantly in patients who did vs. did not receive CPAP (18.1% vs. 22.1%; HR 0.80 with 95%CI 0.46–1.41, p = 0.449). 2. Significant cardiovascular risk reduction in those who used CPAP for ≥4 vs. <4h per night or did not receive treatment (HR 0.29 with 95% CI 0.10–0.86, p = 0.026). |
| McEvoy et al.26 | 2016 | 1b | RCT | Adults between 45 and 75 years of age with moderate to severe OSA and coronary or cerebrovascular disease were randomized to receive CPAP treatment plus usual care or usual care alone. n = 2717 |
The primary end-point was death from cardiovascular causes, MI, CVA, or hospitalization for unstable angina, heart failure, or TIA. | 1. After a mean follow-up of 3.7 years, there was no significant difference in the primary end point in the CPAP vs. usual care group (HR 1.10 with 95% CI 0.91–1.32, p = 0.34). 2. No significant effect on any individual or other composite cardiovascular end point was observed. 3. Mean duration of adherence to CPAP therapy was 3.3 h per night and mean AHI decreased from 20 to 3.7 events/h. |
| Sanchez-de-la-Torre et al.1203 | 2020 | 1b | RCT | Patients with ACS at 15 hospitals in Spain. Non-sleepy, adult patients, admitted to hospital for documented symptoms of ACS. Respiratory polygraphy completed in the first 24–72 h after admission. OSA patients were randomly assigned to CPAP plus usual care or usual care alone group n = 1264 |
Cohort was followed for minimum of 1 year. The primary endpoint was the prevalence of composite of cardiovascular event (cardiovascular death or non-fatal events such as MI, CVA, hospital admission for heart failure, and new hospitalizations for unstable angina or TIA). Cohort was followed for median of 3.35 years. |
1. The prevalence of cardiovascular events was similar between patients in the CPAP and control groups HR 0.89 with 95% CI of 0.68–1.17, p = 0.40) during follow-up. 2. Mean time of adherence to CPAP was 2.78 h/night. 3. The prevalence of cardiovascular events seem not to be related to CPAP compliance or OSA severity. |
| Marin et al.118 | 2005 | 2b | Prospective CS | Men with OSA (AHI ≥ 5/h) and age- and BMI-matched snorers and healthy adults without sleep apnea, stratified further by whether or not sleep apnea was treated n = 1651 |
Cohort was observed for up to 10.1 years for development of CAD and CAD-related fatality. | PAP treatment of OSA was associated with lower nonfatal (0.64 per 100 person-years) and fatal cardiovascular events (0.35 per 100 person-years), than untreated severe sleep apnea (1.06 and 2.13, respectively), untreated mild to moderate sleep apnea (0.55 and 0.89, respectively). |
| Doherty et al.1250 | 2005 | 2b | Prospective CS | Patients with OSA (AHI ≥ 5) receiving CPAP for at least 5 Years n = 168 |
Cohort was observed for average follow-up of 7.5 years. Prevalence of cardiovascular was evaluated on those intolerant of CPAP versus those actively using CPAP. | 1. Death from cardiac events was lower in the CPAP-treated patients than untreated patients (1.9% vs. 14.8%, p = 0.009). 2. Total cardiac events were also lower in CPAP-treated patients (18% vs. 31%, respectively, p < 0.05). |
| Buchner et al.1249 | 2007 | 2b | CS | Patients that underwent polysomnography and had OSA (AHI ≥ 5) n = 384 |
Fatal and nonfatal cardiac events and PAP adherence were evaluated over a median follow-up of 6 years. | 1. In treated patients, AHI reduced from an average of 30.8 to 5.6/h. 2. Cardiovascular events were less frequent in treated patients (14.3%) than untreated patients (28.3%). 3. Estimated event-free survival at 10 years was 79.7% in treated versus 51.8% in untreated. 4. Estimated event-free survival at 10 years was 80.3% in treated versus 51.8% in untreated. |
| da Silva Paulitsch et al.1252 | 2019 | 1a | SR | Randomized controlled trials that compared CPAP with no active treatment in adults with OSA and CVD n = 3314 |
Primary outcomes were: All-cause mortality Cardiovascular mortality Acute MI CVA Any major cardiovascular event |
The duration (median) of CPAP treatment varied from 1 to 56.9 months. 1. The pooled RR was 0.58 (95% CI 0.6–1.23) for cardiovascular death. 2. The pooled RR was 1.11 (95% CI 0.76–1.62) for MI. 3. The pooled RR was 0.93 (95% CI 0.70–1.24) for any major cardiovascular event. |
| Wang et al.635 | 2018 | 2a | SR | Observational studies and randomized controlled trials that included patients with OSA and CAD and reported impact of PAP therapy versus no PAP therapy. n = 1430 |
Primary outcomes were major adverse cardiovascular event (MACE) including: All-cause or cardiovascular death Myocardial infarction Stroke Repeat revascularization Hospitalization for heart failure |
Median duration of follow-up was 36–86.5 months. 1. Treatment with CPAP was associated with significantly lower risk of MACE in six observational studies (RR 0.61; 95% CI 0.39–0.49) but this was not reproduced in two RCTs (RR 0.57; 95% CI 0.32–1.02). 2. CPAP significantly reduced the risk of all-cause death (four observational studies) and cardiovascular death (three observational studies), which were not confirmed in RCTs. |
| Labarca et al.752 | 2020 | 2a | SR | Pooled data from eight RCTs including adults with OSA (AHI ≥ 5) with average follow-up 6–84 months. Three studies included patients in sleep clinics (primary prevention), whereas five studies included patients with known CAD (secondary prevention) n = 5817 |
Outcomes observed were relative risk of: Major adverse cardiovascular events (MACE) CV mortality Myocardial infarction Unstable angina AFIB Heart failure |
CPAP did not improve cardiovascular outcomes. Heterogeneity in studies including study population (primary versus secondary prevention), bias due to lack of blinding, and inclusion of sleepy and nonsleepy patients cited. |
| Abuzaid et al.1032 | 2017 | 1a | SR | Randomized controlled trials with adult patients with OSA who were assigned to either PAP therapy or non-PAP therapy (standard therapy) n = 3780 |
Primary outcome: Major cardiovascular event Other outcomes: Cardiac mortality Myocardial infarction Angina pectoris Stroke Transient ischemic attack |
1. Compared with medical therapy alone, CPAP use was not associated with reduced risk of major adverse cardiovascular events (RR 0.94; 95% CI 0.78–1.15) EXCEPT in the subgroup that wore CPAP > 4 h/night (RR 0.70; 95% CI 0.52–0.94). 2. No reduction in the risk of cardiac mortality (RR 1.14; 95% CI 0.66–1.97), myocardial infarction (RR 0.96; 95% CI 0.64–1.44), and angina pectoris (RR 1.16; 95% CI0.9–1.50) was noted. 3. Subgroup analysis of CPAP adherence demonstrated that CPAP use was not associated with decreased risk of heart failure (RR 0.91; 95% CI 0.50–1.66). |
| Barbé et al.38 | 2012 | 1b | RCT | Nonsleepy patients with moderate to severe OSA (AHI ≥20, ESS ≤ 10), randomized to CPAP versus no treatment n = 725 |
Incidence of hypertension or cardiovascular events assessed over an average of 4 years. | CPAP use was not associated with a statistically significant reduction in HTN or cardiovascular events. The hypertension or cardiovascular event incidence density rate was 9.20 per 100 person-years (95% CI, 7.36–11.04) in the CPAP group and 11.02 per 100 person-years (95% CI, 8.96–13.08) in the control group. |
| Cassar et al.1251 | 2007 | 2b | Retrospective CS | Patients with moderate to severe OSA (AHI ≥ 15) on polysomnography between 1992 and 2004 who underwent PCI, | Observed for: Cardiac death General mortality Major adverse cardiac events Major adverse cardiac or | 1. Treatment of OSA reduced number of cardiac deaths from 10% to 3% at 5 years. 2. No difference in number of major cardiac events or adverse cerebrovascular events. |
VIII.B.5 |. PAP and cognitive measures
Sleep apnea treatment could potentially influence the central nervous system (CNS) through amelioration of recurrent nocturnal hypoxia, sympathetic activation, sleep deprivation, and inflammation, amongst others, which may accelerate decline in cognitive function if left untreated.1253–1255 If the benefits of CPAP are largely due to reduced daytime sleepiness, the related cognitive benefits would cease shortly after stopping CPAP or may attenuate over time. However, it is also possible that CPAP has disease-modifying effects that provide a long-term, sustained benefit that fundamentally alters the trajectory of age-related cognitive decline by reducing neuronal cell death and maintaining neural plasticity.832,1253,1256,1257
Several mechanisms could underlie the potential for long-term cognitive benefits. From a vascular perspective, OSA episodes lead to sympathetic vasoconstriction, which can increase the risk of hypertension and subsequent end-organ damage, including neuronal cell death from cerebrovascular injury.1258 Imaging studies in sleep apnea patients have shown evidence of multiple changes, including in hippocampal subfields.1256,1259,1260 OSA may also act on several elements of the staged biomarker model of more severe cognitive impairment, such as in AD where Amyloid-β is the first detectable stage, followed by tau pathology, neuronal injury, memory dysfunction, and decreased cognitive function.1253,1256,1257 Specifically, nocturnal hypoxia and oxidative stress can increase the risk of diffuse neuritic plaques.1256,1261 Sleep fragmentation, can also have multiple negative consequences with long-term sequelae impacting this AD model: (1) reduced glymphatic clearance of Aβ1262; and (2) increased cerebrospinal fluid tau levels.1257 Apolipoprotein E may mediate these effects.1263 Complicating this process is the growing body of evidence that this relationship can be bi-directional.1257 For example, tauopathy can lead to neuronal loss in key sleep-wake centers, such as the locus coeruleus.1264 Cholinergic neurons are important in respiratory control, including UA motor neurons, and are often affected in AD.1265
A subsection of the 2019 AASM SR and MA evaluated RCTs for the neurocognitive improvements with CPAP therapy for OSA and concluded no significant difference between PAP and control groups.39 Of note, the studies in the MA include patients of a wide range of ages, with a wide range of severity of OSA, with variable symptoms (including a group of non-sleepy patients), and a variable treatment duration (1–12 months). No included studies selectively enrolled patients with dementia or MCI. Given the complex relationship between OSA and cognitive function, it is likely that certain subsets of patients (such as MCI/dementia patients, or elderly patients with severe OSA) may derive greater cognitive benefits from CPAP therapy than the general adult OSA population. Additionally, other quantitative reviews of RCTs (see Table 9b) indicate a slight benefit in cognitive function from CPAP use,1255,1266–1269 but three of these reviews have reported significant benefit in only one cognitive domain each – attention (Kylstra),1266 vigilance (Pan),1268 or memory (Labarca).1267 Of note, two of the reviews focused on elderly patients,1267,1269 and one focused on AD patients.1255 Many RCTs evaluating CPAP and neurocognitive function have found modest improvements in certain cognitive domains, while other studies have found no significant effect. Many of the published RCTs are limited by small sample sizes and/or short trial durations (as short as 1 week of treatment), with the majority not exceeding 3 months of CPAP treatment. Based on the available evidence, it is uncertain that treatment of OSA with CPAP therapy improves neurocognitive function. Quantitative reviews of the data demonstrate inconsistent benefits, but this may be due to the variable quality and study populations of the RCTs on which they are based.
Data suggests that treatment of OSA with CPAP therapy may offer benefit with minimal risk.832,1253,1255–1257,1259,1263,1264,1270,1271 PAP effects on cognitive function in cognitively normal adults may differ from outcomes in cognitively impaired adults.
In cognitively normal adults, several RCTs have been performed examining the effect of PAP on cognitive function. The most notable was the APPLES study, which showed an improvement in sleepiness (both objective and subjective), but no sustained impact on cognitive function aside from a transient improvement at two months in executive and frontal lobe function.1272 One possible explanation suggests that the study sample from this cohort had sufficient cognitive reserve that there was minimal room for improvement.1273 A 2015 MA of 13 studies found a significant but small effect size (d = 0.12) in improved vigilance measures only. A later 2020 MA of 14 studies noted that those with more sleepiness or more severe OSA are more likely to benefit, with a significant but small effect size on attention and speed of information processing only (standardized mean difference [SMD] = 0.17).1274 Most of the studies are limited by short trial durations ranging from 1 to 24 weeks, whereas prior neuroimaging studies suggest effects may require up to 12 months to be apparent.1274
In cognitively impaired populations, there are relatively few RCTs. One RCT in mild-moderate AD patients with OSA was performed using sham-CPAP versus CPAP for a 3-week period, then the sham-CPAP group crossed over to CPAP for a subsequent 3 weeks.1275 They noted statistically significant improvements in a composite endpoint as well as on two individual tests (Hopkins Verbal Learning Test-Revised and Trail Making) when including the 3 week + cross-over data.1275 An additional long-term follow-up (13.3 months) was done in a subset of five CPAP adherent versus five non-CPAP adherent patients from this cohort. While findings were not statistically significant due to the sample size, the effect size on the composite measure was small to moderate at 0.4.1276 In MCI patients, Richards et al. conducted a quasi-experimental study with 1-year follow-up that demonstrated moderate to large effect sizes for psychomotor/cognitive processing and other domains.1271 A secondary analysis published in 2020 from the same cohort showed that elderly MCI patients with mild OSA also derived cognitive benefit from CPAP adherence.1277 Only two studies with >1-year follow-up have been conducted on the effect of CPAP in older adults with MCI and sleep apnea. One retrospective study (MCI n = 62, follow-up 2–3 years) suggested a sustained benefit.1278 However, another recent retrospective study (MCI n = 96, mean follow-up n = 2.8 years) showed no cognitive benefit.1279 MCI patients are heterogeneous with only a subset having high Aβ burden, tauopathy or neurodegeneration associated with increased risk of progression to AD with dementia. The impact of OSA may vary depending upon MCI subtype.1261,1280
Research elucidating which patients might derive the most cognitive benefit from CPAP usage can help focus treatment efforts on certain patient populations (i.e., elderly, MCI/dementia patients). Certain subsets of patients may derive greater cognitive benefits from CPAP therapy. Given the ethical limitations related to long-term placebo treatment in at-risk populations, additional research using large cohort studies is warranted to better understand cognitive effects of PAP over time. (Table VIII.B.5)
TABLE VIII.B. 5.
PAP to improve cognitive measures
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Cognitively intact (i.e., inclusion criteria did not require mild cognitive impairment or dementia) | ||||||
| Kushida et al. (APPLES trial)1272 | 2012 | 1b | Randomized, double-blind, controlled trial comparing CPAP to sham-CPAP (placebo), multicenter trial | 1098 participants enrolled across five sites, with assessments at baseline, 2 and 6 months. | Neurocognitive domains: 1) Executive and frontal lobe function (Sustained Working Memory Test-Overall Mid-Day Score); 2) Attention and psychomotor function (Pathfinder Number Test-Total Time); 3) Learning and memory (Buschke Selective Reminding Test-Sum Recall). Sleepiness: 1) Epworth Sleepiness Scale; 2) Maintenance of Wakefulness Test (MWT) | Neurocognitive domains: transient improvement in executive and frontal lobe function only at 2 months, but no sustained benefit. Sleepiness: CPAP led to improvements in subjective and objective sleepiness, especially in those with an AHI>30 events/h. |
| Pan et al.1270 | 2015 | 1a | Meta-analysis of randomized controlled trials (duration 1–24 weeks) | PubMed, CINAHL, Medline, PsycInfo, EMBASE, Cochrane Library, CNKI, WanFang, VIP, and CBMdisc from June 1971 to July 2014. 13 studies were identified involving 1744 participants. 1098 were from APPLES trial. Did not include trials of cognitively impaired patients. | Cognitive domains: 1) attention; 2) vigilance; 3) processing speed; 4) working memory; 5) memory; 6) verbal fluency; 7) visuoconstructive skills | CPAP led to a small but statistically significant improvement in vigilance only. |
| Dalmases et al.1281 | 2015 | 2b | Open label randomized pilot study of CPAP vs. conservative care for 3 months | 33 cognitively intact participants age >65 years with severe OSA enrolled at a single site, parallel arm study. | Cognitive domains: 1) episodic memory; 2) short-term memory; 3) executive function-working memory, speed, visuomotor speed, mental flexibility, and verbal fluency. MRI scans at baseline and 3 months also | CPAP led to statistically significant improvements in episodic, short-term memory, speed of mental processing, and mental flexibility. MRI scan showed reduced cortical thinning and increased right middle frontal gyrus connectivity in the CPAP group. No change in Epworth Sleepiness Scale. |
| McMillan et al.1282 | 2015 | 1b | Open label randomized study of CPAP + best supportive care (BSC) vs. BSC alone | 278 participants age > 65 years with an oxygen desaturation index >7.5 events/h + Epworth Sleepiness Scale ≥ 9 for a 12-month period. | Cognitive measures included the Mini-Mental Status Exam, Trail Making Test Part B, Digit Symbol Substitution Test, and reaction time test | CPAP did not lead to any cognitive changes. Of note, CPAP objective adherence (defined as ≥4 h of use/night) was 35% and median usage was 2:22 (h:min)/night at 12 months. |
| Bubu et al.832 | 2020 | 2a | Systematic review | PubMed/Medline, Embase, Psych INFO, and Cochrane library for clinical trials published prior to May 1, 2019 related to OSA and cognitive function. | Included research in cognitively intact and cognitively impaired populations | CPAP treatment may offer cognitive benefits for middle-aged and older adults, including those with Alzheimer’s disease. |
| Martinez-Garcia et al.1283 | 2015 | 2b | Open label randomized study of CPAP vs. no CPAP for 3 months | 224 older adults age ≥70 years, with severe OSA (AHI ≥ 30 events/h) enrolled at 12 sites; excluded participants with severe impairment of cognitive performance and ESS ≥ 18. | Cognitive domains: executive function, visual attention, speed of processing/mental flexibility, and working memory. Other domains: subjective sleepiness, anxiety, depression, and sleep quality | CPAP treatment led to statistically significant improvements in depression, anxiety, and sleepiness (small, moderate, and large effect sizes, respectively). Statistically significant improvements for working memory (digit symbol test and Trail Making A) that were small and moderate, respectively. No effect on other domains. |
| Patil et al.39 | 2019 | 1a | AASM systematic review and meta-analysis | 9 RCTs of variable patient populations with mild-mod OSA; control groups were sham CPAP, oral placebo, or conservative treatment. Duration of RCTs was at least 1 month (1–12 months). | Neurocognitive function across domains of executive function, processing speed, attention and vigilance, memory, and intelligence | Meta-analyses showed no clinically significant difference between CPAP and control groups in any of the neurocognitive domains. |
| Wang et al.1274 | 2020 | 1a | Meta-analysis of randomized controlled trials (duration 1–24 weeks) | PubMed, EMBASE, and Cochrane Library were systematically searched for RCTs from database inception to October 24, 2019. 14 studies were identified, involving 1926 participants; 843 were from APPLES trial. Did not include trials of cognitively impaired patients except for one study of Parkinson’s disease patients and one of stroke patients. | Cognitive domains: 1) attention and speed of information processing; 2) executive function; and 3) memory. Also reviewed individual cognitive scales when available, such as Trail Making A/B and subjective sleepiness | CPAP led to a small but statistically significant improvement in attention and vigilance only in severe OSA. No difference in subjective sleepiness. Of note, did not include APPLES trial data and large heterogeneity existed for sleepiness data analyses. |
| Cognitively impaired (i.e., mild cognitive impairment or Alzheimer’s disease) | ||||||
| Ancoli-Israel et al.1275 | 2008 | 1b | Randomized, controlled trial comparing CPAP to sham-CPAP (placebo) | 52 participants with mild to moderate Alzheimer’s disease and OSA. Assessed at baseline and 3 weeks, then the placebo sham-CPAP arm crossed over to receive CPAP. | Fourteen cognitive measures assessing multiple domains including attention, vigilance, psychomotor speed, memory, and executive function. Also derived a composite neurocognitive score from these 14 items | No significant difference in cognitive measures for a priori analyses, possibly due to sample size. When an additional analysis was conducted including the cross-over data (pre-/post-paired comparison), statistically significant benefits noted on the composite score, Hopkins Verbal Learning Test-Revised (HVLT-R), and Trail Making test. |
| Richards et al.1271 | 2019 | 2b | Quasi-experimental pilot study comparing CPAP adherent and non-adherent participants | 54 participants with mild cognitive impairment. Assessed at one year follow-up. | Memory (Hopkins Verbal Learning Test-Revised), psy- chomotor/cognitive processing speed (Digit Symbol subtest from the Wechsler Adult Intelligence Scale Substitution Test). Progression measures were the Everyday Cognition, Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change Scale, and Clinical Dementia Rating Scale | At 1-year: statistically significant improvements in psychomotor/cognitive processing speed in the CPAP adherent patients; small to moderate but not statistically significant improvements noted in memory, attention, and everyday function. |
| Perez-Cabezas et al.1255 | 2020 | 2a | Systematic review of randomized controlled trials and cohort studies for CPAP treatment of OSA in Alzheimer’s disease (AD) | Review of publications in PubMed, PEDro, SCOPUS, SPORTDiscus, CINHAL and Web of Science up to July 2019 using PRISMA framework | Identified five studies involving CPAP treatment of patients with AD and OSA: three clinical trials and two pilot studies. Treatment duration ranged from 3 weeks to 3 months | Noted some evidence to support attenuation of cognitive decline in AD patients, but there were several methodological limitations and a paucity of studies. |
VIII.B.6 |. PAP and insulin resistance
OSA and obesity are independently linked with insulin resistance. PAP therapy has been associated with reductions in glycemic index and insulin sensitivity among obese patients with OSA and insulin resistance.1284–1286 Unfortunately, evidence has generally been low quality with unclear clinical significance. While several studies have demonstrated improvements in glycemic control associated with PAP use,1284,1285 others have failed to find benefit.1286 These observational studies may have been limited by patient heterogeneity and duration of follow-up. Relatively few clinical trials have evaluated the impact of PAP on glycemic control, most of which have failed to demonstrate a positive impact of PAP on insulin sensitivity.1287,1288 These trials had limited duration of follow-up (1–3 months). A more recent randomized clinical trial explored the benefits of PAP among patients with inadequately controlled type 2 DM and OSA.1289 Compared with no treatment, PAP therapy produced significant improvement in glycemic control, particularly for patients with more severe hypoxemia and elevated inflammatory markers (IL-1Β) at baseline. Subsequent SRs and meta-analyses have failed to show a clinically significant benefit of PAP in reducing glycemic index and insulin sensitivity in OSA.1028,1290 Evidence for PAP as an adjunctive therapy to improve glycemic control in patients with type 2 diabetes and OSA is evolving and may hold for insulin control and obesity for patients with SDB. Further research delineating the relationship of variables such as OSA severity, sleep quantity, sleep architecture, degree of daytime sleepiness, and PAP adherence metrics are necessary to better quantify benefits. Comprehensive weight loss strategies, including medically supervised very-low calorie diet and bariatric surgery when conservative measures fail, are worthy of consideration in patients with OSA and insulin resistance. (Table VIII.B.6)
TABLE VIII.B. 6.
PAP to improve insulin resistance
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Abud et al.1290 | 2019 | 1A | SR | 9 RCTs evaluating CPAP therapy vs. no therapy in non-diabetic adults with OSAHS | HOMA index, fasting glucose | CPAP improves HOMA IR index |
| Labarca et al.1028 | 2018 | 1A | SR | 6 RCTs evaluating effect of HbA1c or glycemic control using CPAP treatment versus no treatment for patients with T2DM | HbA1c, fasting glucose | CPAP does not improve HbA1c or fasting glucose |
| Martinez-Ceron, Fernandez-Navarro et al.1291 | 2016 | 2A | SR | 28 non-randomized studies and nine RCTs evaluating the effects of CPAP on glucose metabolism in OSA patients | Effect of CPAP on glucose metabolism | CPAP could improve the control of glucose metabolism |
| Martinez-Ceron, Barquiel et al.1289 | 2016 | 1B | RCT | 50 patients with T2DM and OSA 1. CPAP, 2. no CPAP | Multiple markers of glycemic control | CPAP resulted in improved glycemic control and insulin resistance |
| Myhill et al.1286 | 2012 | 2B | Randomized parallel group intervention | 59 patients with T2DM and new dx of OSA 1. early CPAP (<1 week), 2. late CPAP (1–2 months) | CVD risk factors | CPAP decreased blood pressure and pulse rate but did not influence metabolic control |
| Comondore et al.1287 | 2009 | 1B | RCT (randomized crossover trial) | 13 patients with OSA 1. CPAP or no therapy for 4 weeks followed by 4 week washout, 2. crossover to other intervention | Fasting morning blood and urine, 24-h BP measurements, enothelial function | CPAP showed potential improvements in a variety of cardiovascular biomarkers |
| West et al.1288 | 2007 | 1B | RCT | 42 men with T2DM and new dx of OSA 1. CpAP, 2. placebo CPAP | IR, HbA1c | CPAP does not improve HbA1c or IR |
| Hassaballa et al.1285 | 2005 | 2B | Cohort study | 38 patients with T2DM and OSA | HbA1c | CPAP leads to a significant drop in HbA1c |
| Babu et al.1284 | 2005 | 2B | Cohort study | 25 patients with T2DM before and after CPAP therapy for OSA | Interstitial glucose levels, HbA1c | CPAP improved glycemic control in obese subjects with T2DM |
Abbreviations: BP, blood pressure; CPAP, continuous PAP; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment of insulin resistance; IR, insulin resistance; OSA, obstructive sleep apnea; OSAHS, obstructive sleep apnea hypopnea syndrome; RCT, randomized control trial; T2DM, type 2 diabetes mellitus.
VIII.B.7 |. PAP and motor vehicle accidents
OSA has been associated with driving accidents in recent cohort studies1292 and meta-analyses.39,1293,1294 This association may be the result of a number of neurocognitive sequelae of OSA, including daytime sleepiness and impairments in sustained attention.
Meta-analyses that include data from RCTs which assessed performance in driving simulators have concluded that sufficient evidence supports the role of CPAP in reducing the risk of driving accidents in patients with OSA.1295–1298
In our SR of the English-language literature to review the evidence of the effects of CPAP on driving accidents, we found three meta-analyses39,1293,1294 and one observational study.1292 These data support the beneficial effects of CPAP in reducing driving accidents and improving performance on a driving simulator.
The most recent MA and SR conducted in 2019 by Patil et al. assessed the literature related to the treatment of OSA with CPAP therapy. Four of the included studies were randomized clinical trials1295–1298 that assessed performance in a driving simulator, and an additional ten were observational studies1299–1308 in which a motor vehicle crash rate risk ratio was available, comparing PAP pre-treatment versus post-treatment. Crashes were ascertained by self-report or objective reports.
Self-reported sleepiness was present among participants of three of the randomized trials.1296,1297 One trial recruited patients with mild OSA and self-reported sleepiness1296 and another1295 included those with mild to moderate OSA without sleepiness. Comparison groups included an oral placebo tablet,1296,1297,1309 sham CPAP,1295 or advice regarding weight loss and sleep hygiene counseling.1298,1310 Duration of the trials ranged 1–6 months. The metaanalyses of the four RCTs did not demonstrate a clinically significant reduction in the number of percent of obstacles hit when assessed by driving simulator; however, these results should be interpreted cautiously, since performance on-the-road may not correlate with performance in a simulated, controlled laboratory setting. The observational data, by contrast, demonstrated that PAP compared to no treatment results in a clinically significant reduction in motor vehicle accidents. This effect was independent of whether or not PAP therapy was initiated directly at home versus following an in-laboratory titration; and independent of the type of PAP administered (auto-adjusting, bilevel, or standard continuous). Patients receiving pressure-profile PAP had outcomes similar to those receiving fixed PAP. The overall quality of evidence was rated as low to moderate in this MA due to limitations in study design and ascertainment of outcomes. The data demonstrated that PAP compared to no treatment results in a clinically significant reduction in motor vehicle accidents.
In an earlier MA, Tregear et al.1294 included nine observational studies1102,1299,1300,1302–1304,1307,1308,1311 examining crash risk of drivers with OSA Preversus post-CPAP. All of these observational studies except two1102,1311 were also included in the MA by Patil et al.39 They concluded that PAP treatment results in motor vehicle crash risk reduction, with a risk ratio of 0.278, 95% CI: 0.22–0.35; p < 0.001. They also concluded that daytime sleepiness improves significantly after just one night of PAP therapy, and that performance on a driving simulator improves significantly within 2–7 days of using PAP therapy.
A third MA by Antonopoulus et al. included eight of the studies also included in the study by Tregear et al.,1294 as well as an abstract and several others.1312–1317 The outcomes assessed in response to CPAP included real accidents (10 studies, N = 1221 patients)1102,1299,1300,1302–1304,1307,1308,1316,1318; near-miss accidents (five studies, N = 769 patients)1102,1307,1308,1316,1318; and performance on a driving simulator one abstract1316 (five studies, N = 110 patients).1312–1314,1317 The authors concluded that CPAP was both highly effective and costeffective in preventing traffic accidents among patients with OSA, and that CPAP reduced not only real accidents, but also near-misses and performance on a driving simulator. They further advised that the number of patients needed to be treated with CPAP to avoid one real road traffic accident was five and to avoid a near-miss was only two. Therefore, they concluded that CPAP constitutes an efficient use of healthcare resources in the treatment of OSA.1293
In addition to the studies included in these metaanalyses, an additional retrospective study1292 evaluated data from a large-scale, employer-mandated program to screen, diagnose, and monitor OSA treatment adherence. Drivers identified as having OSA by PSG (n = 1613) were compared against those (n = 403) without OSA and a control group of drivers (n = 2016) who were matched based on experience-at-hire and length of job tenure. Auto-adjusting positive airway pressure (APAP) treatment was provided to all drivers with OSA. Treatment adherence was monitored objectively using data retrieved from the APAP devices. The analysis group included (n = 682) who adhered fully, n = 571 who adhered partially, and n = 360 with no adherence. Those with OSA and no adherence to APAP had a Department-of-Transportation reportable crash rate that was five-fold greater (incidence rate ratio = 4.97, 95% CI: 2.09–10.63) than matched controls (0.070 vs. 0.014 per 100,000 miles). Those who adhered fully to APAP had crash rates that were statistically similar to those of controls (incidence rate ratio = 1.02, 95% CI: 0.48–2.04; 0.014 per 100,000 miles). The program also resulted in the removal of non-treatment-adherent drivers through dismissal or voluntary exit from the company, and retention of adherent drivers. (Table VIII.B.7)
TABLE VIII.B. 7.
Summary of data evaluating the efficacy of PAP in lowering crash rates by subjective or objective reports and in driving simulator
| Study | Year | LOE (1a–5) | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Patil et al.39 | 2019 | 2A | Systematic review, meta-analysis | 10 observational studies of crash rates 2 randomized clinical trials in driving simulators |
Crash rate (Pre- vs. post-treatment) risk ratio Proficiency in driving simulator (difference in mean number and percent obstacles hit) |
PAP associated with reduction in Crash rates [risk ratio 0.28].No significant change in Mean number obstacles hit in simulator: PAP treated group; mean difference = −0.08 |
| Tregear et al.1294 | 2010 | 2A | Systematic review, meta-analysis | 9 observational studies | Crash risk ratio Pre- vs. post-CPAP | PAP reduced Crash risk: risk ratio 0.278; p < 0.001 |
| Antonopoulos et al.1293 | 2011 | 2A | Systematic review, meta-analysis | 15 studies included: Real accidents: 10 studies (1221 participants) Near-miss accidents: five studies (769 participants) Performance in driving simulator 6 studies (110 participants) Total: 1 RCT with low risk of bias 6 observational studies with low risk of bias 2 observational studies with high risk of bias 6 case reports or series |
Outcome measures: odds ratios (ORs), incident rate ratios (IRRs), risk differences (RDs), and mean differences Incident rate ratio = incidence rate of accidents after CPAP divided by the incidence rate before CPAP Number needed to treat (NNT) derived from risk differences |
PAP associated with significant reduction in real accidents and near-misses: Real accidents (10 studies): OR 0.21, IRR 0.45 NNT (95CI) = 5 Near-miss accidents (five studies): OR 0.09; IRR 0.23 NNT = 2 Driving performance (six studies): CPAP was associated with a significant reduction in accident-related events in the driving simulator |
| Burks et al.1292 | 2016 | 2B | Retrospective, controlled cohort | OSA positive n = 1613 cases, OSA negative n = 403 Controls n = 2016 matched on experience-at-hire and length of job tenure, matched on the date of each diagnosed driver’s polysomnogram Cases categorized by adherence to automaticallyadjusting PAP: “Full Adherence” (n = 682) “Partial Adherence” (n = 571) “No Adherence” (n = 360) |
Preventable Department-of-Transportation-reportable crashes/100,000 miles, based on subgroup | “No Adherence” cases had a five-fold higher preventable reportable crash rate than matched controls: IRR = 4.97; (0.070 vs. 0.014 per 100,000 miles) “Full Adherence” cases had crash rates statistically similar to controls: IRR = 1.02; 0.014 per 100,000 miles |
VIII.B.8 |. PAP and work productivity
OSA has been shown in observational studies to reduced work-productivity. However, the effect of CPAP on work productivity has only been studied in a very limited fashion, even though improvements in OSA-related symptoms such as daytime sleepiness, which affect productivity, are well appreciated. An SR of the English-language literature was performed to identify studies of CPAP effects on work productivity. Of the five studies identified, four of them were prospective, observational cohort studies, and one cross-sectional with retrospective recall of the outcomes assessed.
Outcome measures between studies varied and included both validated and non-validated work productivity surveys. Validated questionnaires included the Work Role Functioning Questionnaire (WRFQ; impact of health on work performance), Job Content Questionnaire (JCQ; job stress), Maslach Burnout Inventory–General Survey (MBI-GS; a measure of burnout), Shirom-Melamed Burnout Questionnaire (SMBQ; a measure of burnout), Indice de Impactode la Enfermedad en la Productividad Laboral (IMPALA; work productivity), and the Work Limitation Questionnaire (WLQ; work productivity).
Participants were middle-aged, predominantly male, and had moderate-severe OSA. In all studies, outcomes were assessed before and after a period of CPAP use with a follow-up period from 3 months to 2 years (three studies had 6-month follow-up). One study asked participants to recall symptoms experienced 6 months prior to the time of assessment.1311 Four of the five studies1311,1319,1321 indicated a high level of adherence to CPAP, which was an inclusion criterion in only three of the studies.1319–1321 There was no comparison group in any of the studies. The sample size of the studies included ranged from 33 to 254 participants.
The earliest published evidence,1311,1321 which used Likert-scale questions, reported improvements in job productivity, concentrating on new tasks, learning tasks, performing monotonous tasks, and work-related absenteeism. More recent studies1319,1320,1322 used standardized questionnaires, which differed between the studies, thus preventing direct comparisons. One study,1319 which only included participants adherent to CPAP, reported improvements in work productivity, mental and social demands, and schedule management compared to pre-treatment scores. Another study,1320 which also only assessed participants who adhered to CPAP, demonstrated improvements in job productivity, professional efficacy, and burnout but not job stress or job satisfaction. The final study,1322 which had followed up from only 7.7% of participants that had completed pre-treatment surveys, reported improvements in time management, mental–interpersonal relationships, and work output.
In summary, data regarding the efficacy of PAP in impacting workplace productivity is limited and confined to a few studies that consist of case series and low-quality cohort studies. They are limited by the lack of uniformity in the definition of OSA, lack of blinding,1311,1319–1322 relatively small numbers of participants,1320–1322 short follow-up periods,1311,1319–1321 use of non-validated1311,1321 and subjective assessment tools,1311,1319–1322 recall bias,1311 selection bias,1311,1319–1322 and failure to fully control for adherence to PAP therapy.1311,1321,1322 (Table VIII.B.8)
TABLE VIII.B. 8.
PAP and work productivity
| Study | Year | LOE (1a–5) | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Botokeky et al.1319 | 2019 | 4 | Observational cohort | Participants with OSA adherent to CPAP (N = 39) Follow-up assessment: 89 days (SD: 58) |
WRFQ | OSA participants on CPAP had improvements in schedule management, productivity, mental demands, and social demands compared to pre-treatment scores. |
| Jurádo-Gámez et al.1320 | 2015 | 4 | Observational cohort | Participants with OSA adherent to CPAP (N = 54) Follow-up assessment: 6 months |
JCQ MBI-GS SMBQ Job satisfaction index IMPALA | OSA participants on CPAP demonstrated improvements in job productivity, professional efficacy, and burnout compared to pre-treatment scores. No changes in job stress or satisfaction was observed with CPAP use. |
| Mulgrew et al.1322 | 2007 | 4 | Observational cohort | Participants with OSA (N = 33 of 428 initially surveyed) Follow-up assessment: 2 years |
WLQ | OSA participants on CPAP demonstrated improvements in time management, mental-interpersonal relationships, and work output. |
| Scharf et al.1311 | 1999 | 4 | Cross-sectional study with recall assessment | Participants with OSA (N = 254 of 316 initially surveyed) Follow-up assessment: 6 months |
“Rate your productivity at your job” (10-point Likert scale) “How many days of work have you missed because of illness, tiredness or sleepiness over the past 6 months” |
OSA participants treated with CPAP demonstrated improvements in work-related absenteeism and job productivity. |
| Ulfberg et al.1321 | 1999 | 4 | Observational cohort | Participants with OSA (N = 34) Follow-up assessment: 6 months |
5-Point Likert scale “Do you have difficulty doing your job due to sleepiness or tiredness” “How difficult is it for you to concentrate on new tasks?” “How difficult is it for you to learn new tasks?” “How difficult is it for you to perform monotonous tasks?” |
OSA participants treated with CPAP demonstrated improvements in job productivity and areas including concentrating on new tasks, learning tasks, and performing monotonous tasks. |
Abbreviations: IMPALA, índice de impacto de la enfermedad en la productividad laboral (job productivity questionnaire); JCQ, Job Content Questionnaire; MBI-GS, Maslach Burnout Inventory–General Survey; SMBQ, Shirom-Melamed burnout questionnaire; WLQ, Work Limitation Questionnaire; WRFQ, Work Role Functioning Questionnaire.
VIII.B.9 |. PAP and insomnia
Few studies addressed the question of whether CPAP improves insomnia in patients with OSA (see Table VIII.A.6.i).943,1323 Evidence shows approximately 20%–50% improvement in self-reported insomnia symptoms with use of PAP for OSA. Improvement in sleep maintenance difficulties occurred more commonly than improvement in difficulty with sleep onset or early morning awakening. There is high variability in study design, insomnia definitions, and duration of follow-up time between the studies. (Table VIII.A.6.i)
TABLE VIII.A.6.i.
PAP and improvement in insomnia
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Glidewell et al.1323 | 2014 | 4 | Retrospective cohort study | Archival data from 68 PAP-treated sleep apnea patients | Insomnia Severity Index (ISI), PSG | For the 53 patients with pretreatment insomnia symptoms, ISI scores improved in 45.3% and persisted in 54.7% following PAP treatment. |
| Bjornsdottir et al.943 | 2013 | 3b | Prospective longitudinal cohort study | 705 adults with OSA | Basic Nordic Sleep Questionnaire, HSAT | Symptoms of sleep maintenance insomnia improved significantly among patients using PAP (from 59.4% to 30.7%, p < 0.001). Symptoms of initial insomnia tended to persist regardless of PAP treatment, and symptoms of late insomnia were more likely to improve among patients not using PAP. |
| Sweetman et al.1190 | 2019 | 2 | Prospective trial | 73 adults treated with CPAP. Those who had CBTi were excluded | ISI (6-months) | CPAP therapy led to a 36% improvement in ISI. |
VIII.B.10 |. PAP and obesity hypoventilation syndrome
Distinguishing OHS from eucapnic OSA is important as there are significant differences in clinical outcomes seen between the two groups. In addition to hypercapnic respiratory failure, people with OHS are more likely to develop chronic HF and PH,1324,1325 present with more comorbidities,1326 utilize more health care resources,133,1327 report poorer HRQOL, experience more socioeconomic deprivation,1328 and have lower survival rates compared to those with eucapnic OSA.1326,1329 PAP therapy, in the form of CPAP or the various modes of bilevel ventilatory support (spontaneous [BVS-S], spontaneous-timed [BVS-ST], and volume-targeted pressure support [BVS-VTPS]), has formed the cornerstone of treatment for OHS. While the evidence supporting one mode of PAP over another has been limited, many patients have traditionally been managed with BVS despite its higher cost and need for greater expertise to set up and monitor. Studies evaluating the different modes of BVS, primarily BVS-ST against BVS-VTPS, have failed to show significant differences in clinical outcomes in the medium term (up to 3 months), so long as therapy is set up to deliver equivalent levels of tidal volume and/or CO2 control.1330–1332 Although CPAP does not directly address hypoventilation, it can relieve UA obstruction and reduce the work of breathing.1333 Three RCTs86,1334,1335 and two meta-analyses1336,1337 comparing CPAP to bilevel therapy over the medium term in ambulatory OHS patients with concomitant OSA (OHS + OSA) found similar improvements in awake CO2 and daytime symptoms between the two therapy modes. Nevertheless, as OHS is most commonly a life-long condition, it has been unclear if these early and equivalent improvements with CPAP would be maintained over a more extended period. Two recent long-term comparative studies have shed some light on this issue. A prospective cohort study following 252 newly diagnosed patients with OHS for at least 2 years1338 reported better therapy adherence and larger improvements in depression scores in those prescribed BVS compared to CPAP. However, all other measures including resolution of awake hypercapnia, improvement in HRQOL, and mortality were similar between therapies. In a long-term RCT with a median follow-up period of 5 years, Masa et al.1339 reported the outcomes of 204 stable OHS patients with severe OSA allocated to CPAP or BVS. No significant difference between groups was observed for any outcome measured including hospitalization days, healthcare utilization, mortality, CV events, awake blood gases, HRQOL, or symptoms. Hence, current data suggest that while improvements in some parameters such as awake PaCO2 and cardiac function may occur more rapidly with BVS,1334,1340 the longer-term efficacy of these PAP modalities is similar for stable OHS with OSA.1339
Given CPAP is more cost effective than BVS,1341 and both PAP modalities provide similar long-term clinical outcomes,1337,1339,1342 CPAP is now suggested as the initial therapy for stable patients with OHS and concomitant severe OSA.1337,1343 Nevertheless, CPAP will fail in a proportion of individuals. Patients at the greatest risk of a suboptimal response are those with higher baseline PaCO2 levels,1334 more restrictive spirometry, and lower AHI.1344 Consequently, close monitoring is needed to ensure an appropriate response to therapy is achieved.1343
Bilevel therapy remains the therapy of choice in OHS patients without severe OSA (i.e., AHI < 30/h). A randomized trial comparing BVS-VTPS to lifestyle changes alone in 98 stable patients with OHS without OSA showed that by 2 months only the BVS group showed significant improvements in CO2, sleepiness, and PSG parameters.1345 Extending follow-up to at least 3 years, the authors found benefits favoring BVS in terms of improved blood gases and QOL.1342 However, no differences between groups in hospitalization days per year, other health resource use, CV events, or mortality were observed. For those presenting with acute respiratory decompensation, bilevel therapy also remains the therapy of choice.1346,1347 However, even in this latter group a proportion can be stepped down to CPAP after a period of clinical stability without compromising awake blood gases, QOL, or sleep quality.1348,1349
Adherence to therapy is an important modifiable factor influencing therapy response, irrespective of the PAP mode used. In a post-hoc analysis of data from their long-term PAP trial, Masa et al.1339 found patients with OHS + OSA who were more adherent to PAP (>4 h/day) also experienced lower hospitalization days per patient-year, hospital and intensive care unit (ICU) admissions, emergency room visits, and mortality than those less adherent. The type of PAP used did not alter these outcomes. In a case–control study which included 206 OHS patients and 236 eucapnic OSA patients, Kreivi et al.1326 found that untreated or non-adherent OHS patients had an adjusted 5-year mortality rate of 27% compared to 2% in those adherent to PAP therapy and 4% in OSA patients who abandoned CPAP. Similarly, amongst OHS patients without severe OSA, higher adherence to bilevel therapy was associated with fewer emergency room visits and lower mortality.1342 Unlike OSA, there is a lack of literature around strategies for improving adherence to PAP in patients with OHS.
Although it appears from cohort studies1326,1329,1336,1350 and long-term RCTs,1339,1342 death from respiratory failure is significantly reduced with PAP therapy, the impact on CV risk and overall mortality is less marked. Even with PAP therapy, OHS is associated with poorer survival compared to OSA alone.1329 This highlights the need for early detection of this disorder before significant comorbidities develop, along with the use of multimodal therapy beyond PAP therapy including weight loss programs and pulmonary rehabilitation to improve outcomes. Although weight loss is an obvious therapeutic target, there is a lack of good quality data around how best this can be achieved and maintained, and what target weight loss is needed to achieve clinically relevant benefits.1351,1352
VIII.B.11 |. PAP in overlap syndrome (COPD)
Concurrent OSA and COPD is known as OS.778 Studies have shown that CPAP improves respiratory muscle function, work of breathing, gas exchange, and functional status in OS patients.1353,1354 Several mechanisms may explain these results, such as bronchodilation, reduction of respiratory load, improvement of ventilation/perfusion ratio, and changes in central chemoreceptor sensitivity. Two prospective cohort studies provided the best available evidence on CPAP and survival in patients with OS.1355,1356 (Table VIII.7c) Marin et al. evaluated CPAP on first-time hospitalization from COPD exacerbation and mortality in three groups; OS with CPAP, OS without CPAP, and COPD without OSA.1356 All three groups had similar markers of COPD severity and received similar COPD management. At baseline, patients with OS had a higher incidence of COPD exacerbations. The investigators reported a significant mortality increase and higher hospitalization rates in the OS group without CPAP compared to the OS group with CPAP and the COPD group. OS patients without CPAP carry a worse prognosis compared to OS with CPAP and COPD without OSA. Machado et al. evaluated CPAP on OS patients with moderate-to-severe OSA and hypoxemic/hypercapnic COPD on long-term oxygen supplementation.1355 The authors reported a 5-year survival benefit in the CPAP treated group versus the non-CPAP group.
Stanchina et al. analyzed the relationship between CPAP adherence and survival in 227 OS patients.1357 Multivariate analysis showed that more time on CPAP was associated with reduced risk of death in a dose-dependent manner. A recent retrospective cohort study analyzed the effect of CPAP therapy on healthcare utilization in the 1-year pre- and post-CPAP initiation in Medicare beneficiaries with OS.1358 CPAP was associated with significant reductions in COPD related hospitalizations, particularly in older adults (≥75 years), higher COPD complexity (defined by comorbid respiratory and procedures1359), and those with three or more medical co-morbidities.
BPAP therapy is widely accepted for acute COPD exacerbations and data also suggest benefit in stable hypercapnic COPD patients in the outpatient setting.1360–1362 None of the studies however reported concurrent OSA diagnosis, so it is unclear if BPAP would be as beneficial as CPAP in OS patients, particularly those with more severe gas exchange abnormalities including daytime hypercapnia. In a post hoc analysis of OS patients on CPAP versus BPAP, those who benefited from BPAP had a higher BMI, lower FEV1 and FEV1/FVC, and worse daytime gas exchange.1363 Randomized clinical trials are needed to determine the role of BPAP versus CPAP in OS.
In summary, there is overwhelming evidence that patients with comorbid OSA and COPD are at risk for increased morbidity and mortality compared with either disease alone. CPAP therapy seems to mitigate these risks; however, this has yet to be proven with randomized clinical trials. Also, further studies are needed to determine the role of BPAP on clinical outcomes in OS. (Table VIII.B.11)
TABLE VIII.B. 11.
Positive airway pressure (PAP) therapy and clinical outcomes in overlap syndrome
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Marin et al.1356 | 2010 | 2b | Prospective cohort | 228 patients with Overlap on CPAP 213 patients with Overlap not on CPAP 210 patients with COPD only |
1. Mortality and first-time hospitalization from COPD exacerbation in Overlap Syndrome 2. Effect of CPAP on mortality and first-time hospitalization |
Overlap syndrome patients had higher incidence of hospitalization from COPD exacerbations; (RR 1.70; 1.21–2.38). Overlap syndrome is associated with increased mortality compared with COPD alone; (RR 1.79; 1.16–2.77). CPAP improves mortality in overlap syndrome patients compared to overlap patients without CPAP. |
| Machado et al.1355 | 2010 | 2b | Prospective cohort | Overlap syndrome patients with moderate-severe OSAS and severe COPD on LTOT (N = 95) Treatment with CPAP No CPAP |
Effect of CPAP on survival | Significant 5-year survival advantage with CPAP (71%) treatment compared to no CPAP (26%); (p < 0.01). |
| Singh et al.1358 | 2019 | 2b | Retrospective cohort | 5% national sample of Medicare beneficiaries with COPD and on CPAP therapy Pre-CPAP Post-CPAP |
Effect of CPAP on emergency room visits and hospitalizations for all-cause and COPD related conditions in 1-year pre- and post-CPAP initiation | Hospitalization rates for COPD related conditions were lowered in 1-year post-CPAP treatment compared to 1-year pre-CPAP. Hazard ratio for death due to respiratory failure or cardiovascular disease between groups was 0.19 (95% CI 0.08–0.48). No change in ER visits for COPD related conditions or for any cause. CPAP was more beneficial in older adults, higher COPD complexity, and three or more medical comorbidities. |
| Stanchina et al.1357 | 2013 | 4 | Retrospective cohort (post hoc analysis) | Overlap syndrome patients identified from large outpatient database analyzed for CPAP adherence | Mortality | Greater hours on CPAP were associated with reduction in mortality; (HR 0.71, p < 0.001). |
| Kuklisova et al.1363 | 2018 | 2b | Retrospective cohort (post hoc analysis) | Post hoc analysis of large database with overlap patients using CPAP versus bilevel PAP | Determinants of CPAP failure | PCO2 awake and CT90% predicted failure, bilevel PAP was well tolerated and effective. |
COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; CT90%, sleep time with SpO2 < 90%; ER, emergency room; HR, hazard ratio; LTOT, long-term oxygen therapy; PCO2, partial pressure arterial carbon dioxide.
VIII.C |. Medical Management of OSA: Oral Appliances
VIII.C.1 |. Tongue retention devices
A tongue retaining device (TRD) uses suction force to anteriorly displace the tongue during sleep. Unlike other OAs, a TRD isolates the tongue and obviates the need to advance the mandible. However, combination devices do exist.1364 Such devices can be acquired over the counter or can be customized. The first report of such therapy dates back almost four decades. TRD offers potential advantages relative to other therapies for OSA due to their portability and low cost. There are currently no Cochrane Review of clinical guidelines on the role of TRD in the management of OSA.
The literature on TRD for OSA is small, with only 16 published studies and one unpublished study, involving only 278 patients in total.1365 The majority of reports are case series, with only six RCTs. The predominant outcome has been the AHI, but oxygen desaturation, arousal index, and snoring have also been assessed. A minority of studies explored patient reported outcomes, including daytime sleepiness and side-effects. All studies demonstrate a reduction in AHI, on average approximately 50% (range 13.5%–67.4%).1365 Most studies have included OSA in the moderate to severe range and SR of 16 studies shows an average Pre-TRD AHI of 34 reducing to a post-TRD AHI of 16.1365 One study did not detect a difference in the treatment outcome for mild, moderate, or severe OSA.1366 Several studies have suggested a greater reduction in supine compared to non-supine AHI,1367,1368 although OSA by body position has not been reported in most studies. A similar reduction in the ODI has been observed across studies that assessed this outcome. The small number of studies that assessed subjective sleepiness have demonstrated an improvement in the ESS. The treatment duration has generally only been for up to 6 months, and only a few case series in small numbers of patients have evaluated long-term efficacy. An RCT compared TRD to mandibular repositioning device (MRD) and showed a similar reduction in mean AHI (15 events/h, 55% reduction in AHI) with both therapies, but a higher proportion of patients achieving a complete or partial response with MRD treatment (68% vs. 45%). Furthermore, compliance was poorer with TRD and 91% of patients preferred MRD treatment.1366 Another study compared TRD to CPAP in a randomized cross-over study and revealed a significantly greater reduction in mean AHI as well as better improvement of sleep-related oxygen parameters with CPAP therapy than with TRD. However, there was no significant difference between interventions for other sleep parameters, daytime sleepiness, and patient’s QOL.1369 A majority of patients (48%) preferred CPAP to TRD due to their perceptions of greater efficacy and less side effects with CPAP therapy.
Side effects of TRD occur in approximately two thirds of patients and include tongue numbness, tongue pain, tooth or gum pain, and dry mouth,1370 and likely contribute to device intolerance.
There are current gaps in knowledge regarding long-term efficacy, adherence, and side effects, as well as patient characteristics associated with a beneficial outcome. (Table VIII.C.1)
TABLE VIII.C.1.
Summary of evidence for tongue retention devices for OSA
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Banhirian et al.1369 | 2019 | 2b | RCT (cross-over) | 1. TRD 2. CPAP 27 Subjects |
1. AHI (WatchPAT) 2. FOSQ 3. ESS 4. Side effects 5. Adherence |
TRD reduced AHI less than CPAP, no difference in ESS or FOSQ scores. |
| Chang et al.1365 | 2017 | 1a | Systematic review | TRD 242 Subjects | 1. AHI 2. LSAT 3. ESS 4. ODI |
Reduced AHI and ODI, increased LSAT, and decreased ESS. |
| Deane et al.1366 | 2009 | 1b | RCT (crossover) | 1. TRD 2. MRD |
1. AHI 2. ESS 3. Snoring (subjective) 4. Sleep quality 5. Side effects 6. Treatment preference |
Similar AHI reduction for TRD and MRD, but higher |
| Lazard et al.1370 | 2009 | 4 | Retrospective case series | TRD 55 Subjects | 1. AHI 2. Snoring 3. ESS |
AHI improvement at 5 years. |
Abbreviations: AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Score; FOSQ, Functional Outcomes of Sleep Questionnaire; LSAT, lowest oxygen saturation; MRD, mandibular repositioning device; ODI, oxygen desaturation index; RCT, randomized controlled trial; TRD, tongue retaining device.
VIII.C.2 |. Mandibular repositioning devices (MRD)
MRDs attach to the upper and lower dental arches and secures the mandible in a more anterior, or protruded, position relative to the maxilla. They are also commonly referred to as mandibular advancement splints, appliances, or devices (MAS/MAA/MAD), or MRD or OA. They are generally the preferred OA type for the treatment of OSA, owing to stronger evidence of effectiveness and great patient acceptance compared to TRD. The forward positioning of the mandible while wearing MRD increases the pharyngeal airway space and reduces collapsibility, representing key mechanisms for improvement in OSA. Current clinical guidelines of the American Academies of Sleep Medicine and Dental Sleep Medicine recommend that MRD should be prescribed, rather than no therapy, for adult OSA patients who are intolerant of CPAP or who prefer alternative therapy.809
MRD is manufactured from impressions (digital or physical) of an individual’s dentition and hence are customized devices. Prefabricated or “boil and bite” devices also exist. An MA of three crossover trials compared customized and prefabricated MRDs in a total of 129 participants.1371 Customized MRDs were superior in AHI reduction, as well as the proportion of treatment responders (AHI < 5/h or 50% reduction in AHI).1371 Additionally, in crossover trials patient preference and self-reported adherence was in favor of the customized MRD.1371 Therefore, customized devices offer clear advantages for clinical effectiveness, as reflected in the clinical guidelines.809 We provide a summary of the evidence for the effect MRD has on AHI, subjective and objective health outcomes, and therapy side effects.
MRD candidacy – dentition evaluation
Dental contraindications to MRD therapy have been found to present in up to 34% of 100 consecutive patients undergoing PSG.1372 The dentist conducts an odontologic examination, including patient history and examination to characterize oral diseases and occlusal conditions. To be suitable for MRD therapy, candidates generally require sufficient healthy teeth and alveolar ridge to retain the device, the absence of temporomandibular disorders (TMDs), and adequate protrusive jaw function.1373 The presence of 8–10 teeth in each arch and a minimum 5 mm protrusive capacity of the mandible are usually considered necessary for optimal results from MRD treatment. However, lack of sufficient teeth may not be an absolute contraindication as the use of a dental implant-retained MRD has been reported in edentulous and partially dentate patients.1374 Moreover, TRDs have also been proposed as a treatment alternative for edentulous patients. The periodontal status of OSA patients warrants consideration. Periodontal disease may pose a contraindication to MRD therapy due to substantial tooth mobility.1372,1373 Dental or periodontal care may be required before the use of MRD (estimated in some 16% of patients).1372
MRD candidacy – TMJ/associated disorders
A comprehensive temporomandibular joint (TMJ) assessment is an important prerequisite to treatment with MRD. The prevalence of TMD in OSA patients varies from 2% to 52%,1372,1375 with 50% of subjects complaining of myofascial pain associated with and without limited mouth opening and arthralgia.1375 Another study highlights the association between the two disorders, finding a 28.4% OSA prevalence rate in 53 TMD patients, highlighting the need to consider diagnostic sleep testing in TMD patients complaining of sleep disturbances.1376
There have been numerous studies that have collectively reported TMD as a result of MRD use. However, in an MRI study, translation of the mandibular condyle (movement out of the glenoid fossa of the temporal bone) with MRD therapy was equal to or less than that observed with maximum jaw opening in the absence of significant TMJ morphological alterations.1377 Nonetheless, many patients experience TMJ related pain in the initial stages of treatment which is usually transient and not a contraindication to MRD use. To date, two studies advocate the use of mandibular jaw exercises to manage TMJ side effects with MRD use.1375,1378
MRD outcomes – polysomnographic indices
Pre- to post-comparison of customized MRD therapy in 33 studies (n = 1259) show a mean reduction in AHI (or RDI) of 13.9 events/h,809 as 21 studies (n = 904) demonstrated a modest increase in minimum SaO2. Only six studies (n = 399) evaluated change in ODI and showed a mean reduction 12.8 events/h. Arousal index was measured in only 16 studies (n = 704) and showed a mean reduction of 10.8/h.809 Additionally, 16 studies (n = 675) evaluated REM sleep and noted a small but statistically significant increase in REM sleep; clinical significance of this increase is unknown and furthermore, no change in sleep efficiency between the two study nights with and without MRD was detected.809 An MA of cross-over trials of MRD versus control appliance (i.e., inactive device that does not produce mandibular advancement) showed improvements in AHI, minimum SaO2, and arousal index with MRD, indicating mandibular advancement is key to efficacy (four studies, n = 155).1379
MRD outcomes – daytime sleepiness (Epworth Sleepiness Score)
A 2015 network MA of the effects of OSA therapy on ESS identified five studies (n = 271) assessing MRD versus inactive control and three studies (n = 244) which compared CPAP, MRD, and an inactive control.1380 In both pairwise and network analysis, MRD reduced ESS versus inactive control (−1.7 points; p < 0.0001).1380
MRD outcomes –– blood pressure
BP is the most common objective health outcome reported in MRD studies. A 2015 network MA of the effects of OSA therapy on BP assessed MRD versus inactive control (six studies, n = 473).1208 Compared to inactive control, MRD were associated with a reduction in systolic blood pressure (SBP) of 2.1 mmHg (p = 0.002) and in diastolic blood pressure (DBP) of 1.9 mmHg (p = 0.008).1208
MRD outcomes – health-related quality of life
The SF-36 Health Survey is widely used to evaluate HRQOL. The SF-36 has multiple domains to evaluate different aspects of QOL. An SR and MA of RCTs incorporating the SF-36 identified two RCTs (one parallel; 25–29 participants, one cross-over; 83 participants) which compared SF-36 between MRD and inactive control device.1381 Pairwise MA demonstrated improvement in the mental component score for MRD versus control (2.7 points; p = 0.041).1381 The physical component score did not show a significant improvement versus control device (1.3 points; p = 0.18).1381
MRD side effects
Long-term use of MRD is associated with progressive but generally minor tooth movements, but not craniofacial changes. An SR and MA to evaluate dental and skeletal changes associated with MRD included 12 studies in MA (follow-up periods 2–7 years). In a single study (n = 489), tooth movements were associated with ongoing MRD usage, with decreases in overjet after MRD treatment (−0.99 mm; p < 0.00001) and overbite (−1.00 mm; p < 0.00001).1382 No significant changes in skeletal relationships (craniofacial angles between landmarks sellanasion line and point A on the maxilla [SNA], sella-nasion line and point B on the mandible [sella, nasion, B point angle SNB], point A-nasion line and point B [ANB]) or mandibular rotation were evident. Additional side effects of MRD use include pain and sensitivity of the teeth, mouth dryness, occlusion changes, and TMJ exacerbation or pain.
Factors related to MRD efficacy
Inter-individual variability in AHI reduction with MRD is common and several studies have sought to describe patient factors associated with AHI reduction using MRD. A recent MA suggests “responders” to MRD therapy tend to have the following characteristics: lower age (n = 998; −3.1 years; p < 0.0001), female gender, lower BMI (n = 970; −1.96 kg/m2; p < 0.00001), smaller neck circumference (n = 688; −1.04 cm; p < 0.0001), and lower baseline AHI (n = 914; −4.56 events/h; p < 0.00001), as well as some craniofacial characteristics (retracted maxilla and mandible, narrower airway, and shorter soft palate).1383 It is also suggestive that MRD are more effective in POSA than non-POSA patients (four out of five studies).1383 Although these average differences are statistically significant, the narrow mean differences do not make these factors useful as clinical selection tools. (Table VIII.C.2)
TABLE VIII.C.2.
Summary of evidence for mandibular repositioning devices
| Study | Year | LOE (1a–5) | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Araie et al.1382 | 2018 | 1a | Systematic review and meta-analysis | MRD 12 Studies (489 subjects) | Cephalometry, dental casts | Long-term use associated with dental changes |
| Bratton et al.1380 | 2015a | 1a | Network meta-analysis | 1. MRD 2. Control 3 studies (271 subjects) |
Epworth Sleepiness Score | MRD reduces daytime sleepiness (ESS) compared to inactive control |
| Bratton et al.1208 | 2015b | 1a | Network meta-analysis | 1) MRD 2)Control 3 studies (229 subjects) |
Blood pressure | MRD reduce blood pressure compared to inactive control device |
| Chen et al.1383 | 2020 | 1a | Systematic review and meta-analysis | MRD 41 Studies (1854 subjects) |
1. Age 2. Gender 3. BMI 4. Neck circumference 5. Cephalometry 6. AHI |
On average MRD “responders” are younger, female, less obese, with lower baseline AHI |
| Johal et al.1371 | 2017 | 1a | Systematic review and meta-analysis | 1. Customized MRD 2. Prefabricated MRD 3 studies (129 subjects) |
1. AHI 2. Proportion treatment responders |
Customized devices have definable advantages including greater efficacy and patient preference and adherence |
| Kuhn et al.1381 | 2017 | 1a | Systematic review and meta-analysis | 1. MRD 2. Inactive control 3 studies (177 subjects) |
SF-36 (mental and physical components) | MRD was associated with improvement in both physical and mental component scores, but only two studies |
| Lim et al.1379 | 2006 | 1a | Cochrane systematic review and meta-analysis | 1. MRD 2. Inactive control 13 Studies (553 subjects) |
1. AHI 2. LSAT 3. Arousal index |
MRD reduce polysomnographic indices of OSA versus inactive control devices |
| Ramar et al.809 | 2015 | 1a | Clinical practice guideline | 1. Pre- and post-MRD 2. MRD versus sham-MRD 3. MRD versus CPAP 42 Studies (1307 subjects) |
1. AHI 2. LSAT 3. ODI 4. Arousal index 5. REM% 6. Sleep efficiency |
It is recommended that MRD is prescribed for adult OSA patients, rather than no therapy, who are intolerant of CPAP or prefer alternate therapy |
Abbreviations: AHI, apnea hyponea index; BMI, body mass index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Score; LSAT, lowest oxygen saturation; MRD, mandibular repositioning device; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; %REM, percent of total sleep time spent in rapid eye movement sleep stage; SF-36, 36-Item Short Form.
VIII.C.2.a |. MRD efficacy versus CPAP
Many studies have compared the effects of MRD versus CPAP, reporting on efficacy of treatment and subjective and objective health outcomes.
Efficacy (AHI)
Efficacy of both MRD and CPAP depends on compliance to therapy. Most data on MRD compliance has been limited to self-report.1384 Patient acceptance and comfort levels have direct implications for successful therapy. CPAP is better at reducing AHI, as illustrated by an MA of 12 RCTs (n = 824) showing greater average AHI reduction (8.2 events/h; p < 0.001).1385
Treatment usage (hours per night)
Six RCTs comparing CPAP and MRD included self-reported MRD usage, as well as either objective or self-report CPAP usage.1385 There was evidence of high heterogeneity between studies. Overall CPAP compliance was found to be significantly lower than self-report MRD compliance by 1.1 h per night.1385 An additional assessment of MRD versus CPAP for adherence included 11 studies (n = 442), with adherence favoring MRD by 0.7 h per night, again with high study heterogeneity.809 Eight studies were included in an MA of dropouts for each treatment (CPAP, MRD) and did not find a difference.1385
Daytime sleepiness (ESS)
A 2015 network MA of 67 studies (n = 6873) compared CPAP, MRD, and inactive control devices for effect on ESS.1380 CPAP treatment produced greater reductions in ESS than MRD by 0.8 points (p = 0.015). An MA of 12 RCTs comparing CPAP and MRD did not find a statistically significant improvement in ESS score, with a difference in means of only 0.6 points (p = 0.203).1385 There was statistical heterogeneity detected between studies and a sensitivity analysis concluded more studies with balanced baseline ESS values are needed to understand if there truly is a statistical difference in ESS between treatments or not.1385
Blood pressure
Network MA (N = 4888 participants) confirms both CPAP and MRD reduce BP relative to control.1208 There was no significant difference between CPAP and MRD in their association with change in SBP (−0.5 mmHg; p = 0.55) or in DBP (−0.2 mmHg; p = 0.82].
Quality of life
In MA of the effect of CPAP versus control treatment (11 studies), CPAP improved both the mental (1.6 points; p = 0.047) and physical component (1.8 points; p = 0.005) scores of the SF-36 questionnaire.1381 There was no difference between CPAP and MRD in pairwise and network MA of the physical and mental component scores for HRQOL.1381 However, the authors acknowledge the limited data on MRD and wider CIs, and although this study is suggestive that MRD may be just as effective, further RCTs comparing CPAP and MRD are ultimately needed. The Functional Outcomes of Sleep Questionnaire (FOSQ) assesses disease specific QOL. Four RCTs (N = 559) of MRD and CPAP found no difference in FOSQ changes between CPAP and MRD (difference in means of 0.03; p = 0.788)1385; however, quality of evidence (GRADE) is moderate due to risk of bias.1385
Cognitive function
There have been two trials comparing CPAP and MRD that have used trail making B test (n = 244), which assesses executive functioning. Statistical heterogeneity between studies was not found. No difference in mean cognitive performance was detected using this test (mean difference of −3.5; p = 0.395)1385; however, quality of evidence (GRADE) is very low due to risk of bias, inconsistency, and imprecision.1385 (Table VIII.C.2.a)
TABLE VIII.C.2.a.
Summary of evidence for mandibular repositioning device efficacy versus CPAP
| Study | Year | LOE (1a–5) | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Bratton et al.1380 | 2015a | 1a | Network meta-analysis | 1. MRD 2. CPAP 3. Control 67 Studies6873 Subjects |
Epworth Sleepiness Score | CPAP more effective than MRD in reducing ESS (although possibility of publication bias in favor of CPAP that may contribute to difference) |
| Bratton et al.1208 | 2015b | 1a | Network meta-analysis | 1. MRD 2. CPAP 3. Control 51 Studies4888 Subjects |
Blood pressure | Both MRD and CPAP reduce BP relative to control. There was no difference in BP reduction between CPAP and MRD |
| Kuhn et al.1381 | 2017 | 1a | Systematic review and meta-analysis | 1. MRD 2. CPAP 23 Studies2342 Subjects |
SF-36 (mental and physical components) | Suggestive that MRD may be similarly effective to CPAP, but more RCTs needed |
| Ramar et al.809 | 2015 | 1a | Clinical practice guideline | 1. MRD 2. CPAP 15 Studies491 Subjects |
Polysomnographic indices, adherence, side effects, blood pressure, quality of life, sleepiness | It is recommended that MRD is prescribed for adult OSA patients, rather than no therapy, who are intolerant of CPAP or prefer alternative therapy |
| Schwarz et al.1385 | 2018 | 1a | Systematic review and meta-analysis | 1. CPAP 2. MAS 12 (824) [AHI]Six (525) [compliance]Four (559) [FOSQ]10 (950) [ESS]Two (244) [cognitive function] |
1. AHI 2. Compliance 3. FOSQ 4. ESS 5. Cognitive function |
CPAP better reducing AHI. No difference SF-36, FOSQ, cognitive performance. ESS potentially favors CPAP, adherence greater in MRD |
Abbreviations: AHI, apnea hyponea index; BP, blood pressure; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Score; FOSQ, Functional Outcomes of Sleep Questionnaire; MRD, mandibular repositioning.
VIII.D |. Medical Management of OSA: Behavioral and Adjunctive Therapies
VIII.D.1 |. Positional therapy for positional OSA
In patients with POSA, disease severity depends on the total sleeping time (TST) in the supine position PT aims at preventing patients from sleeping in the supine position in order to improve or resolve POSA. The majority of early studies on PT used the so-called TBT where a bulky mass is strapped to the patient’s back.1386 TBT is effective in reducing the overall AHI and the percentage of TST in supine position, but long-term compliance is poor. This is due to discomfort and no improvement, or even deterioration of sleep quality and/or daytime alertness.1387,1388 Compliance rates of TBT reported in the literature range from 40% to 70% in the short-term to only 10% in the long-term.1387–1390
A new generation of small, lightweight, battery-powered vibrotactile smart devices, which are either attached to the trunk (or neck), was introduced. Almost all research is with chest-worn devices (Table VIII.D.1). When the patient moves to the supine position, these sleep position trainers (SPT) provide a vibrating stimulus aiming to turn the patient to a non-supine sleeping position without awakening the user. In a recent MA, data from studies reporting on the effect of new-generation SPT devices were included. Data from six studies showed that mean AHI was significantly reduced from a mean 21.8–9.9 events/h (54% change) and AHI SMD was −1.94 (large effect). Pooled mean reduction in percentage of TST in the supine position also declined from 40.1% to 6.5% (84% reduction).1391 Short-term compliance (4 weeks to 3 months of therapy) is high, varying from 76% to 96%, when defined as at least 4 h of use per night during 7 days a week.1391–1400,1402,1403
TABLE VIII.D. 1.
Evidence for positional therapy for positional OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion | |||
|---|---|---|---|---|---|---|---|---|---|
| Eijsvogel et al.1396 | 2015 | 2b | RCT | 55 patients with positional OSA (defined as supine AHI ≥ 2 times nonsupine AHI and non-supine AHI < 10) with chest worn device (n = 29, SPT, NightBalance) vs. tennis ball technique (n = 26) | AHI, compliance (use ≥4 h/day and ≥5 days/week) | Equal reduction AHI (SPT: 11.4 vs. 3.9, TBT: 13.1 vs. 5.8), better compliance in SPT arm (SPT: 75.9% vs. TBT: 42.3%, p = 0.01). | |||
| Laub et al.1397 | 2017 | 1b | RCT | 101 patients with positional OSA (defined as supine AHI ≥2 times nonsupine AHI, supine AHI > 10 and non-supine AHI < 10) with chest worn device (n = 52, SPT, NightBalance) vs. no treatment (n = 49) | AHI, % supine sleep, sleepiness, compliance (SPT use >4 h/night all week) | At 2 months, AHI improved in the SPT group (from 18.1 to 10.4 on per-protocol analysis, p = 0.008) and remained unchanged in the control group (p = 0.2). Percentage of time supine improved in both groups and ESS did not change in either group. Compliance with SPT was 75.5%. | |||
| Benoist et al.1392 | 2017 | 2b | RCT | 99 patients with positional OSA (defined as supine AHI ≥ 2 times nonsupine AHI) with chest worn device (n = 48, SPT, NightBalance) vs. oral appliance therapy (n = 51) | AHI, compliance | SPT and oral appliance equally effective. At 3 months, AHI decreased in both groups (SPT: 13.9–8.7, oral appliance: 13.2–8.1, p < 0.001 for both). Only SPT decreased percentage of time in supine sleep (42.4–14.2, p < 0.001). | |||
| de Ruiter et al.1395 | 2017 | 1a | RCT | 58 patients with positional OSA (defined as supine AHI ≥ 2 times nonsupine AHI) with chest worn device (n = 29, SPT, NightBalance) vs. oral appliance therapy (n = 29) | AHI, compliance | SPT and oral appliance equally effective. At 12 months, AHI decreased in both groups (SPT: 13.2–7.1, oral appliance: 13.4–5.0, p < 0.001 for both). Only SPT decreased percentage of time in supine sleep (41.6–12.7, p < 0.001). | |||
| Berry et al.1393 | 2019 | 1b | Non-inferiority RCT, cross over | 117 patients with positional OSA (defined as AHI ≥15 or AHI 10–15 with ESS > 10 and supine AHI ≥ 2 times nonsupine AHI) with CPAP vs. chest worn device (SPT, NightBalance) followed by crossover | Adherence, AHI, PROMs | SPT non-inferior to CPAP with improved AHI in both groups (SPT: 21.5–7.3, CPAP: 21.5–3.7). SPT had greater nightly adherence than CPAP (345.3 vs. 286.98 min, p < 0.0001). | |||
| Mok et al.1403 | 2020 | 2b | Non-inferiority RCT, crossover | 40 patients with ESS 10–16, positional OSA (defined as supine AHI ≥ 2 times nonsupine AHI, supine AHI > 10 and non-supine AHI < 10) with CPAP vs. neck worn device (Night Shift) | AHI, ESS | With regard to decrease in AHI, PT inferior to CPAP (PT: 23.4–13.0, CPAP: 23.4–4.0, p < 0.001). With regard improvement in ESS, PT noninferior to CPAP. |
|||
| Srijithesh et al.1405 | 2019 | SR, Cochrane | 323 patients with OSA treated with positional therapy vs. CPAP (n = 72) or positional therapy vs. inactive control (n = 251) | AHI, ESS | Positional therapy was less effective than CPAP for reducing AHI (6.4 fewer events per hour with CPAP, 95% CI 3.00–9.79; low-certainty evidence) but compliance may be higher. Positional therapy was more effective than inactive control in reducing AHI and ESS. | ||||
| van Maanen et al.1401 | 2013 | 2b | Single arm | 36 patients with positional OSA (defined as supine AHI > 2 times nonsupine AHI) with chest worn device (Sleep Position Trainer [SPT]NightBalance) | AHI, sleep efficiency, ESS, FOSQ, compliance | At 1 month, the median percentage of supine sleeping time decreased from 49.9% to 0.0% (p < 0.001) and median AHI decreased from 16.4 to 5.2 (p < 0.001). | |||
| van Maanen et al.1402 | 2012 | 2b | Single arm | 36 patients with positional OSA (defined as supine AHI > 2 times nonsupine AHI) with simple neck worn device | AHI, sleep quality | Mean AHI decreased from 27.7 to 12.8 (p < 0.01) and mean percentage of supine sleeping time decreased from 40% to 19% with use of the device. | |||
| van Maanen et al.1400 | 2014 | 2b | Single arm | A total of patients with positional OSA (defined as supine AHI > 2 times nonsupine AHI) and data from chest worn device (SPT, NightBalance) | Sleep efficiency, ESS, FOSQ, compliance | Long-term reduction of percentage of supine sleep from 21% to 3% (p < 0.001) at 6 months and improvement of ESS and QOL. | |||
| Levendowsky et al.1398 | 2014 | 1b | Single arm | 30 patients with positional OSA (defined as supine AHI ≥ 1.5 times nonsupine AHI) with neck worn device (NightShift) | AHI, SO2, depression score, sleep architecture | Improvement of mean AHI from 24.7 to 7.5 (p < 0.00001), decrease in percentage of time O2 sat <90% (from 4.2 to 1.2, p < 0.01), and improved sleep architecture. | |||
| Beyers et al.1394 | 2019 | 1b | Single arm | 58 patients with positional OSA (defined as supine AHI ≥ 2 times nonsupine AHI) with chest worn device (SPT, NightBalance) | Long-term AHI, % supine sleep, sleep quality, compliance | At 1 year, 85% of patients were still using the device and 75% reported subjective improvement in sleep quality. Mean AHI improved from 16.4 to 6.2 (p < 0.001), percentage of time in supine sleep was reduced from 37.1% to 1.4% (p < 0.001). | |||
| Dieltjens et al.1404 | 2015 | 1b | RCT | 20 patients with residual positional OSA after mandibular advancement device use (defined as supine AHI ≥ 2 times nonsupine AHI, supine AHI>10 and non-supine AHI<10) with chest worn device (SPT, NightBalance) vs. combination of chest worn device and mandibular advancement device | AHI, compliance | SPT and MAD equally effective at reducing AHI and ODI, combination more effective with decrease in AHI from 20.9 to 5.5, p < 0.008). | |||
Long-term compliance of SPT varies between 64.4% and 75.5%.1391–1400,1402,1403 The impact of compliance on SPT effectiveness is illustrated in an RCT study where 55 patients were treated with either SPT or TBT. Both SPT and TBT were equally effective in reducing respiratory events in those with mild POSA with a normal ESS. However, compared to TBT, sleep quality, QOL, and compliance was significantly greater in the SPT group.1396
In a recent RCT, the effectiveness of MAD versus PT was compared.1392,1395 The effect after 3 and 12 months was the same but there were more treatment-related side effects in the MAD arm. Two RCTs compared CPAP and SPT in the treatment of mild POSA. In the first study, APAP was compared with PT in a randomized crossover trial format. SPT was found to be noninferior in treatment efficacy and greater adherence.1393 A total of 117 participants were randomized (58 SPT first, 59 APAP first). The AHI with PT was higher compared to APAP (mean ± SD, 7.29 ± 6.8 vs. 3.71 ± 5.1 events/h, p < 0.001). The mean AHI difference (SPT-APAP) was 3.58. The average nightly adherence (all nights) was greater with PT (345.3 ± 111.22 vs. 286.98 ± 128.9 min, p < 0.0001). Participants found the SPT to be more comfortable and easier to use, and 53% reported a preference for PT assuming both devices were equally effective. In the other, smaller (N = 40) RCT, a neck SPT was used and compared to CPAP. ESS difference with SPT did not meet the noninferiority margin.1403
Others have looked at combination therapy with PT and MAD therapy.1404 In one study of persistent POSA with MAD therapy, patients underwent consecutive randomized PSG tests with SPT and with SPT + MAD. The effects of MAD and PT were comparable with approximately a 50% reduction of the AHI, but then a further 25% reduction was reported when MAD and PT were combined. A recent Cochrane review was generalized to all categories of OSA and included both TBT and SPT devices.1405 The review described a greater improvement in AHI with CPAP compared to PT, but no difference in ESS or QOL between the groups. Studies that compared PT to an inactive control group showed improved ESS and AHI in the PT group. (Table VIII.D.1)
VIII.D.2 |. Weight management for OSA
The role of weight loss on severity of OSA has been studied. A total of eight papers were identified that reported results of RCTs of medical weight loss interventions against controls, of which seven were RCTs on adult patients and one was an MA (Table IX.1.b).122,1406–1411
Study durations ranged from 4 weeks to 1 year. All RCTs included a small number of participants that ranged from 11 to 264. Low calorie diet was compared to control in six RCTs,1407,1408,1410–1413 diet and exercise were compared to control in two RCTs122,1409 and inpatient rehabilitation program group (including individualized exercise training, education activities sessions, and dietary management) was compared to control in one RCT. One RCT demonstrated efficacy of the Mediterranean diet for reduction in CV endpoints, weight loss, and has been shown to reduce the REM-related AHI at 3 months.1414 The MA included is the most recent ATS clinical practice guideline for the role of weight management in the treatment of adult OSA.1415 Medical weight loss was superior to control in reducing AHI in all included studies.
The mean difference in weight change with weight loss in the six RCTs ranged from −5 to −18.7 kg. The resultant mean difference in AHI ranged from −4 to −25.122,1406–1409,1411 One RCT only reported mean difference in BMI (−1.8 kg/m2) and percent change in AHI (−16.9%).1410 In the ATS MA, there were only four RCTs that examined the impact of weight loss on OSA severity, and the mean difference (or reduction) in AHI was −8.5 events/h (95% CI −10.8 to −6.2).1415 Given the benefits of weight loss programs, and their limitations related to their role in the management of OSA, the ATS made a strong recommendation for comprehensive weight loss programs, but underlined that this recommendation is of very low certainty in the estimated effect.
Neither serious adverse effects nor deaths were reported in any of the included studies. Cost analysis was not performed in any of the studies.
Weight loss medications can be beneficial in some patients. The Endocrine Society recommends weight loss agents as adjunctive therapy for patients unsuccessful in losing weight despite lifestyle modifications, diet, and exercise.1416 The benefits of pharmacologic weight loss in patients with OSA have been explored, although only two RCTs have measures related to OSA specific outcomes.1417,1418 A new medication for weight loss includes glucagon-like peptide-1 receptor agonists (GLP-1 RA) such as semaglutide (15). GLP-1RA medications stimulate insulin secretion and reduce glucagon and may also delay gastric emptying.1419 A recent randomized, double-blind study looked at the effects of semaglutide combined with intensive behavioral therapy and low-calorie diet. When compared to placebo, once-weekly subcutaneous semaglutide resulted in a 10.3% greater decrease in body weight and more participants lost at least 5% of their body weight.1420 Daily liraglutide demonstrated reduction in AHI (−6.1 events/h) which was associated with the degree of weight loss in post hoc analysis.1417 Overall, there are limited data showing that weight loss medications improve measures of outcomes related to OSA. However, given the benefits of weight loss in general, these medications should be considered to promote weight loss in patients who cannot achieve weight loss goals with calorie restriction regimens. (Table VIII.D.2)
TABLE VIII.D. 2.
Evidence for the role of weight loss in the management of obstructive sleep apnea (OSA)
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| López-Padrós et al.1412 | 2020 | 2b | RCT | 1. Intensive weight loss program with low (600–800 kcal/day) calorie diet for 15 days followed by a 1200 kcal/day diet during the remainder of the initial 12 weeks, and a 1200–1800 kcal/day Mediterranean diet for the last 36 weeks (n = 22) 2. Control group (n = 20) |
AHI (PSG) | In obese patients with severe OSA (AHI > 30), an intensive weight loss program is superior to control in reducing weight and OSA severity. Diet group: MD in weight at 3 months: −10.5 kg 12 months: −8.2 kg MD in AHI at 3 months: −23.7 12 months: −19.4 Control group: MD in weight at 3 months: −2.3 kg at 12 months: −0.1 kg MD in AHI at 3 months: −9 at 12 months: −13.5 |
| Hudgel et al.1415 | 2018 | 2a | SR with meta-analysis | 4 RCTs that examined the impact of weight loss on OSA | AHI | MD in weight was −11.6 kg; MD in BMI was −4.1 kg/m2
AHI MD −8.5 ESS MD −2.4 points For patients with OSA who are overweight or obese, suggest a reduced-calorie diet (with or without exercise/increased physical activity) rather than no diet (conditional recommendation, very low certainty in the estimated effect). |
| Fernandes et al.1407 | 2015 | 2b | RCT | 800 Cal diet group (n = 11) Control group (n = 10) Age (20–55) |
pAHI (with WatchPAT) | Weight loss superior to control in improving OSA severity. Mean differences below.Diet group:Change in weight: −5.57 kgChange in pAHI: −7.22 Control group:Change in weight: 0.43 kgChange in pAHI: 0.13 (p = 0.04) |
| Make-Nunes et al.1409 | 2015 | 2b | RCT | Hypocaloric diet (decrease of 500 kcal/day) and exercise training group (n = 16) Control group (n = 8) | AHI (PSG) | Hypocaloric diet and exercise training superior to control in improving OSA severity. Mean differences below. Hypocaloric diet and exercise training group Change in weight: −5 kg Change in AHI: −16 Control group: Change in weight: 1 kg Change in AHI: 11 Change in body weight was associated with change in AHI. |
| Ng et al.1410 | 2015 | 2b | RCT | Dietician led lifestyle modification program (diet) group (n = 61) Control group: usual care (n = 43) |
AHI | In patients with moderate to severe OSA, lifestyle modification was superior to usual care in reducing the severity of OSA. Dietician led lifestyle modification program (diet) group: Change in BMI: −1.8 kg/m2 Percent change in AHI: −16.9% Control group: Change in BMI: −0.6 kg/m2 Percent change in AHI + 0.6% |
| Desplan et al.1406 | 2014 | 2b | RCT | Inpatient rehabilitation program group including individualized exercise training, education activities sessions, and dietary management (n = 11) Control group: one-month education activity sessions (n = 11) |
AHI, ODI (PSG) | In patients with moderate to severe OSA, intervention was superior to control in reducing AHI and ODI. Inpatient rehabilitation program: Change in BMI: −0.8kg/m2 Change in AHI: −12.6 Change in ODI: −5.5 Control group: Change in BMI: 0 kg/m2 Change in AHI: 5.6 Change in ODI: 5.2 Change in arousals: 6.7 |
| Papandreou et al.1414 | 2012 | 2b | RCT | Mediterranean diet, CPAP, and lifestyle intervention (n = 20) Prudent diet, CPAP, and lifestyle intervention (n = 20) |
AHI (PSG) | In patient with moderate to severe OSA (AHI > 15) and BMI ≥ 30 kg/m2, Mediterranean diet was only superior to control in reducing AHI during REM sleep. Otherwise, there were no significant differences in other sleep parameters between the two groups. Changes in weight and BMI did not reach statistical significance between the two groups. |
| Foster et al.122 | 2009 | 2a | RCT | Intervention group: diet, exercise, and training (n = 125) Control group: three group sessions for diabetes management (n = 139) |
AHI, ODI (unattended overnight PSG) | Intervention superior to control in reducing AHI reported as adjusted mean differences. Diet and exercise group: Change in weight: 10.8 kg Change in BMI: −3.8kg/m2 Change in AHI: −5.4 Change in ODI: −5.5 Control group: Change in weight: 0.6 kg Change in BMI: 0.2 kg/m2 Change in AHI: 4.2 Change in ODI: 1.2 |
| Johansson et al.1408 | 2009 | 2b | RCT | Intervention group: Very low calorie (2.3 MJ/day) liquid diet (n = 30) Control group: usual diet (n = 33) |
pAHI, ODI (WatchPAT) | Very low calorie diet superior to usual diet in reducing pAHI. Low energy diet improved OSA in obese men, with the greatest effect in patients with severe disease. Mean differences below. Intervention group: Change in weight: −18.7 kg Change in BMI: −5.7 kg/m2 Change in pAHI: −25 Change in ODI: −19 Control group: Change in weight: 1.1 kg Change in BMI: 0.3 kg/m2 Change in pAHI: −2 Change in ODI: −1 |
| Tuomilehto et al.1411 | 2009 | 2b | RCT | Intervention: very low calorie diet program 600–800 kcal/day (with supervised lifestyle modification (n = 35) Control: routine lifestyle counseling (n = 37) |
AHI, mean SaO2 | Intervention superior to control in reducing AHI in mild OSA, and improving mean oxygen saturation, together with weight loss. Mean differences below. Diet group: Change in weight: −10.7 kg Change in BMI: −3.5 kg/m2 Change in AHI: −4.0 Change in mean SaO2: 0.8 Control group: Change in weight: −2.4 kg Change in BMI −0.8 kg/m2 Change in AHI: 0.3 Change in mean SaO2: −0.3 |
| Nerfeldt et al.1413 | 2008 | 2b | RCT | Intervention: weight reduction program consisting of a low-calorie diet and group meetings (n = 6) Control: expectancy followed by crossover after 8 weeks (n = 5) |
AHI, ODI (Micro Digi trapper-type 3) | Intervention was superior to control in reducing ODI in males with BMI ≥30. Mean differences below. Diet group: Change in weight: −18.5 kg Change in BMI: −4.8 kg/m2 Change in ODI: −50 Control group: Change in weight: −13 kg Change in BMI: −3.7 kg/m2 Change in ODI: −1 |
| Pharmacotherapy | ||||||
| Blackman et al.1417 | 2016 | 1b | RCT | Liraglutide 3 mg with diet (500 kcal/day) and exercise (n = 180) Placebo with diet (500 kcal/day) and exercise (n = 179) |
AHI (PSG) | Intervention was superior to placebo in reducing AHI in patients with obesity (BMI ≥30 kg/m2) and mild–moderate OSA (AHI ≥ 15). Mean differences below. Liraglutide group: Change in weight: −6.7 kg ± 0.5 Change in BMI: −2.2 ± 0.2 kg/m2 Change in AHI: −12.2 ± 1.8 Placebo group: Change in weight: −1.9 kg ± 0.4 Change in BMI: −0.6 ± 0.1 kg/m2 Change in AHI: −6.0 ± 2.0 Significant treatment difference in AHI, weight, SBP, and HbA1c between groups. |
| Winslow et al.1418 | 2012 | 2b | RCT | Phentermine 15 mg plus extended-release topiramate 92 mg (n = 22) Placebo (n = 23) |
AHI (PSG) | Treatment was superior to placebo in reducing AHI. Mean differences below. Phentermine and topiramate group: Change in weight: −11.0 ± 1.24 kg Change in AHI: −31.5 ± 4.2 Placebo group: Change in weight: −4.5 ± 1.21 kg Change in AHI: −16.6 ± 4.5 |
Abbreviations: AHI, apnea hypopnea index; CWLP, comprehensive weight loss program; EDS, excessive daytime sleepiness; ESS, Epworth sleepiness score; MD, mean difference; N, number; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; pAHI, PAT AHI; PAT, peripheral arterial tonometry; RCT, randomized controlled trial; SaO2, oxygen saturation; SBP, systolic blood pressure; SR, systematic review.
VIII.D.3 |. Sedative medications and effect on OSA
A sedative is defined as “A drug taken for its calming or sleep-inducing effect” and is often used by people who are struggling to fall or stay asleep.1421 Early in the history of OSA, case reports suggested that some sedatives worsened breathing during sleep.1422,1423 Benzodiazepines, commonly used sedative medications, likely affect respiration through multiple mechanisms, including sedation, muscle relaxation, and increasing N2 sleep.1424 A 2015 Cochrane review reviewed 14 randomized controlled studies of patients with OSA and 10 opiate, hypnotic, and sedating medications including: remifentanil (infusion), eszopiclone, zolpidem, brotizolam, flurazepam, nitrazepam, temazepam, triazolam, ramelteon, and sodium oxybate. The findings demonstrated that no evidence that the pharmacological compounds assessed have a deleterious effect on the severity of OSA as measured by change in AHI or ODI, though some statistically significant decreases in oxygen level were observed with remifentanil, zolpidem, and triazolam.1425
Alcohol is often considered a sedative and has effects similar to that of benzodiazepines. Alcohol use in a 1982 case report dramatically worsened SDB in seven patients with known snoring and/or OSA.1426 However, in contrast to the benzodiazepine data above, alcohol increased in the severity of OSA in a statistically significant manner, both in terms of AHI and mean blood oxygen level in a 14-study MA.1427 Most of the studies in the MA used between 2 and 3 standard alcohol drinks as the tested dosage.
Sedating anti-depressants including trazodone, protriptyline, mirtazapine, paroxetine, and fluoxetine have been studied without evidence that they negatively impact OSA severity.1428 Suvorexant, a newer medication with an orexin antagonism mechanism, did not impact OSA severity in a study of 26 patients with mild–moderate OSA.1429 Antihistamines are often used as over-the-counter sedative medications; no evidence has suggested that these medications are respiratory depressants or that they impact the ventilatory response.1424
Sedative medications are frequently used in the perioperative setting, both before and after surgery. Scientific data suggest that patients with OSA are at higher risk for adverse events in the peri-operative setting.468 In specific, patients with OSA and high arousal thresholds may be particularly sensitive to sedatives and narcotic medications with an increased risk of respiratory arrest in the peri-operative period. Limiting sedative use as possible and close monitoring is suggested in patients with OSA undergoing surgery.1428
While the majority of this section has focused on the potential worsening of SDB with use of sedatives, there is some data suggestive that sedative medications may be helpful in some patients with OSA. For instance, one study of eszopiclone use in patients with OSA demonstrated a statistically significant reduction of AHI.400 One current theory suggests that in appropriate patients, sedativesmay increase the arousal threshold to facilitate the experience of sufficient magnitude of respiratory stimuli for an adequate duration which activates pharyngeal dilatormuscles and thus stabilizes breathing. With fewer arousals and stabilized breathing, deeper sleep may be obtained further reducing the occurrence of respiratory events. Thus, for the sedative to be an effective treatment, the sedative must increase the arousal threshold, and the patient must have a mild to moderately collapsible UA, responsive and effective UA dilator muscles, and a low to moderate arousal threshold.1428
VIII.D.4 |. Nasal obstruction and sinusitis management for OSA treatment
The evaluation and management of nasal obstruction plays an important role in OSA as the nose regulates nasal resistance and stimulates ventilation.1430 Nasal obstruction is thought to contribute to OSA and is known to interfere with treatment options including CPAP and OAs.1431 Addressing nasal obstruction can be done with surgical management (septoplasty, inferior turbinate reduction, and endoscopic sinus surgery), medical management (nasal corticosteroid sprays, nasal decongestants, oral antihistamines, leukotriene antagonists, and internal or external nasal dilators), or a combination of surgery and medical management. The medical management of nasal obstruction investigated is primarily limited to studies examining the use of intranasal corticosteroid sprays and/or mechanical stents to hold the nasal cavity open during nasal respiration. Although there are a number of RCTs evaluating the above medical therapies, data consist of short follow-up times, heterogenous patient populations, and the inherent challenge of establishing an objective measurement of nasal obstruction.
The conglomeration of literature overall suggests an improvement in both subjective and objective sleep in patients with rhinitis and OSA with the use of intranasal corticosteroids (Table 1). Craig et al. evaluated the effect of intranasal corticosteroid use on multiple patient-reported sleep symptoms using pooled data from three randomized placebo-controlled, cross-over trials and found that improved nasal congestion in patients with perennial AR correlated highly with improved sleep (p < 0.01) and daytime sleepiness (p = 0.01).891 In the only SR published on this topic, Liu et al. demonstrated that intranasal corticosteroid sprays were effective in decreasing AHI (SMD −0.73) in patients with OSA.1432 However, the overall effectiveness of intranasal corticosteroid therapy for decreasing OSA severity is still undetermined. Kiely et al. performed a randomized placebo-controlled, crossover study demonstrating improved sleep outcomes using intranasal corticosteroids in patients with AR and OSA. AHI was significantly reduced in 13 patients with OSA and rhinitis treated with fluticasone for 4 weeks as compared to placebo (23.3 vs. 30.3 p < 0.05). Despite improvements, most patients continued to have significant OSA.895 In divergence, Smith et al. in 2019 performed a randomized placebo controlled double blind investigation examining patients with an AHI less than 15, demonstrating fluticasone and montelukast did not significantly decrease AHI or ESS1433 while the TST and % of REM sleep improved. The role of intranasal corticosteroids in improving CPAP adherence in patients with OSA also remains undetermined. In an MA of two RCTs evaluating corticosteroid use and objective CPAP compliance, Charakorn et al. found that while CPAP use increased by an average of 0.4 h per night in treated patients, this benefit did not reach statistical significance and there was no change in percentage of nights with CPAP use.1179
Clarenbach et al. performed a randomized placebocontrolled, cross-over study examining the benefits of acutely improving nasal obstruction by using a decongestant (oxymetazoline). They demonstrated AHI significantly decreased at the time of maximal pharmacological effect (30–210 min) (27.3 vs. 33.2), but overall AHI did not change.900 Despite these short-term AHI reductions, routine nasal decongestant use is not recommended secondary to known rebound congestion and architectural changes in the sinonasal epithelium.
Chronic rhinosinusitis
There are limited data evaluating the impact of medical therapies (topical nasal corticosteroid sprays, saline irrigations, oral antibiotics, and oral corticosteroids) on patient sleep quality outcome measures in CRS. In a study by Alt et al.,1434 patients undergoing both medical and surgical therapy for CRS were followed longitudinally after each intervention. Patients in the surgical cohort were found to have greater improvement in PSQI as compared with their medical therapy counterparts. Additional study is required to confirm these findings.
Allergic rhinitis
Oral antihistamines and leukotriene inhibitors have also been investigated as medical therapies for comorbid AR and OSA, though data are limited. Acar et al. evaluated the impact of desloratadine treatment in both the presence and absence of concomitant mometasone nasal sprays in a 2013 RCT. No additional benefit was noted in objective sleep parameters when desloratadine was added.1435 Similarly, Smith et al. evaluated the combination of montelukast and fluticasone as compared to placebo on AHI in mild OSA patients in a double-blind placebo RCT. While there was no significant reduction in AHI, TST, and percentage of REM sleep improved.1433 The potential sedative effect of oral antihistamine treatment in OSA patients warrants consideration if antihistamine therapy is considered for the management of comorbid AR. Further study is needed to delineate the role of oral antihistamine and leukotriene inhibitors for OSA therapy. (Table VII.D.4)
TABLE VII.D. 4.
Evidence for medical therapies for nasal obstruction and obstructive sleep apnea
| Study | Year | LOE | Study design | Study groups/intervention | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Topical corticosteroids, oral antihistamines, leukotriene inhibitors | ||||||
| Kiely et al.895 | 2004 | 1b | RCT | 1. OSA: AHI > 10 fluticasone 2. No OSA Intervention:Fluticasone spray × 4 weeks |
1. AHI 2. NAR 3. EpSS 4. Snoring |
AHI was significantly reduced in 13 patients with OSA and rhinitis treated with fluticasone for 4 weeks as compared to placebo (23.3 vs. 30.3). Despite improvements, most patients continued to have significant OSA. |
| Stuck et al.892 | 2004 | 3b | Prospective controlled cohort | 1. Seasonal AR patients 2. Non-AR patients Intervention:None – study designed to evaluate the impact of AR on sleep |
PSG SF-36 Daytime sleepiness |
In patients with AR during allergy season, AHI significantly worsened as compared to non-AR patients. This difference was not clinically meaningful. |
| Lavigne et al.894 | 2013 | 3b | Prospective cohort | 1. OSA w AR 2. OSA w/o AR Intervention:Mometasone spray × 10–12 weeks |
PSG EpSS |
Decrease in oxygen nadir in AR points with OSA. Significant improvement in supine AHI in OSA patients with AR. Improvement in nasal symptom scores. |
| Tam et al.1436 | 2019 | 4 | Case series | OSA Intervention: Fluticasone spray × 4 weeks |
PSQI EpSS NOSE |
Decreases in PSQI, EpSS, and NOSE after 4 weeks of fluticasone. |
| Meltzer et al.1437 | 2010 | 1b | RCT Double blind placebo-controlled | Moderate to severe perennial AR Intervention: Mometasone spray |
PSG TNSS RQLQ EpSS |
No significant difference in AHI in perennial AR patients AHI with mometasone (increase in AHI from 2.57 to 3.53) TNSS, RQLQ, EpSS significantly improved. |
| Acar et al.1435 | 2013 | 1b | RCT Double blinded placebo controlled | Groups: 1. Mometasone spray 2. Mometasone spray + desloratadine 3. Desloratadine 4. Placebo Duration: 6 weeks |
PSG EpSS |
Nasal corticosteroid spray significantly reduced AHI (29–>24). There was no additional benefit of adding the oral antihistamine. EpSS improved. |
| Smith et al.1433 | 2019 | 1b | RCT Double blinded placebo control |
OSA with AHI greater than 5 and less than 15 1. Montelukast + fluticasone 2. Placebo |
PSG EpSS |
Fluticasone and Montelukast did not significantly decrease AHI or Epss. AHI in patients with chronic rhinitis (n = 4) significantly worsened. Total sleep time and %REM sleep improved. |
| Liu et al.1432 | 2016 | 1a | SR and Metanalysis of RCTs | Intranasal corticosteroids for OSA: five RCTs included – two adult studies (Acar and Kiely) | PSG | Intranasal corticosteroid was effective for OSA in decreasing AHI (SMD −0.73). Effectiveness of intranasal corticosteroid therapy for decreasing OSA severity is undetermined. |
| Craig et al.891 | 2005 | 1a | RCT Double blind placebo- controlled crossover |
Perrenial AR without OSA Intervention: 1. Budesonide, flunisolide, or fluticasone 2. Placebo |
PSG Sleepiness and nasal congestion severity based on symptom diaries |
Intranasal corticosteroid decreased nasal congestion and sleepiness. Changes in PSG (fluticasone group only) were not statistically significant. |
| Charakorn et al.1179 | 2017 | 1a | SR and meta-analysis of RCTs |
Intranasal corticosteroids for OSA treated with CPAP: two RCTs included |
CPAP compliance | Intranasal corticosteroid increased CPAP usage 0.4 h per night, but this was not statistically significant. Percentage of nights with CPAP use did not change. |
| Nasal decongestants | ||||||
| Clarenbach et al.900 | 2008 | 1b | RCT placebo- controlled crossover |
OSA with nasal congestion Intervention: Oxymetazoline |
PSG | In 12 patients with OSA, AHI significantly decreased at time of maximal pharmacological effect (30–210 min) (27.3 vs. 33.2), but overall AHI was not significantly different between oxymetazoline and placebo. |
| Mclean et al.903 | 2005 | 1b | RCT single blind placebo- controlled crossover |
OSA and nasal obstruction. Nasal obstruction defined by turbinate hypertrophy ± nasal valve collapse Intervention: Breathe right (external dilator Oxymetazoline × 2 |
Nasal resistance PSG | Nasal resistance was significantly decreased. AHI fell 12 points with oxymetazoline and external dilator. This was statistically significant but did not provide a clinically effective treatment. |
| Kerr et al.902 | 1992 | 4 | Case series | 10 OSA patients of varying severity Intervention: Vestibular dilator, oxymetazoline |
PSG Nasal resistance |
Improvement in arousals/h and sleep quality. No change in apnea. |
| Koutsourelakis et al.1438 | 2013 | 1b | RCT double blinded crossover |
OSA with AHI > 10 Intervention: Nasal tramazoline and dexamethasone spray BID |
PSG Nasal resistance EpSS |
Mean decrease in AHI of 21% (−6.1) after 1 week of therapy compared to placebo. |
VIII.D.5 |. Nasal dilators for OSA
Internal and external nasal dilators have been studied as a treatment option for snoring and OSA. While many studies have shown some benefit with snoring, the vast majority of studies have not found improvement in OSA.904,1431,1439–1441
An SR by Camacho et al. examined nasal dilators (both internal and external) and their effect on snoring and OSA.1442 Fourteen studies were analyzed, with five studies utilizing internal dilators and nine studies using external dilators; only two studies showed that nasal dilators reduced OSA parameters. An MA showed that both internal and external nasal dilators did not have an effect on the AHI or sleep architecture but internal nasal dilators did have a small improvement on the AI.1442
In the study by Hoijer et al., 10 patients underwent two sleep studies with and without an internal nasal dilator (Nozovent) with a reduction in the AI by 47%.1443 However, it is important to point out that three out of the 10 patients had a pre-treatment AI less than 5 and one patient worsened with the use of the nasal dilator. The study excluded other measures of OSA such as AHI and RDI. A study of external nasal dilators (Breathe right strips) on patients with OSA in 26 patients1444 showed that 19 out of 26 patients had a reduction in RDI, and sub-analysis of the patients that benefited showed a positive effect in patients with nasal obstruction, minor or no pharyngeal obstruction, and age under 55 years.1444
In a randomized crossover study comparing external nasal dilator strips to placebo strips, no reduction in AHI was noted. Within this study a subset of patients with severe nasal obstruction, based on rhinometry, did experience a significant but small reduction in AHI (7.4–5.4) and improved mean SpO2 (92.4–96.7).901 Other studies showed an increase in nasal cross-sectional area and reduction in nasal resistance with nasal dilators but without effects on objective OSA outcomes such as AHI.902,1439,1445
The evidence suggests that nasal dilators do not improve objective sleep parameters in patients with OSA, especially in patients in the moderate to severe OSA.1442 However, it does appear that a small subset of patients with severe nasal obstruction and OSA may potentially benefit from nasal dilators.901,1444 While nasal dilators (internal and external) are not recommended as monotherapy for OSA, they may still play a role as an adjunct treatment, as it has been shown that nasal dilators could reduce the amount of pressure required by CPAP devices which may improve adherence.1180,1446 (Table VIII.D.5)
TABLE VIII.D. 5.
Evidence for effects of nasal dilators on OSA
| Study | Year | LOE (1a–5) | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Yagihara et al.1441 | 2017 | 2b | Randomized crossover study | 26 patients with severe OSA randomized to either 1 month of nasal dilator strips or nasal CPAP and then crossover after 2 weeks. | Sleep architecture, AHI, arousal index, oxygen saturation, sleep questionnaires. | Nasal dilator strips in patients with severe OSA had no effect on objective sleep outcomes (sleep architecture and respiratory events). |
| Camacho et al.1442 | 2016 | 1a | SR | 14 studies (five internal dilators, nine external dilators). Most of the studies were individual case–control or prospective case-series. | Effect of nasal dilators on sleep stages, AHI, apnea index, lowest oxygen saturation, snoring, sleepiness, and sub-analysis comparing internal to external dilators. | Nasal dilators did not improve AHI or most PSG metrics except for internal nasal dilators which had a mild improvement on the apnea index. |
| Amaro et al.1447 | 2012 | 2b | Randomized crossover study | 12 patients with acromegaly and moderate to severe OSA underwent a randomized crossover study using CPAP and nasal dilator strips. | Sleep architecture, apnea index, hypopnea index, apnea hypopnea index, central apnea, oxygen saturation, sleep questionnaires. | Nasal dilator strips had no reduction on objective sleep parameters; subjective sleep improvement, but less than in the CPAP group. |
| Djupesland et al.901 | 2001 | 2b | Randomized crossover study | 18 patients with nocturnal nasal obstruction, severe snoring, and without severe OSA (mean AHI 9.3) underwent a randomized crossover study comparing external nasal dilator strips vs. placebo strips. | Nasal patency, AHI, oxygen saturation, sub-analysis based on nocturnal nasal dimensions. | Improvement in AHI and oxygen saturation was noted only by a subset of patients with severe nasal obstruction based on rhinometry. |
| Pevernagie et al.904 | 2000 | 2b | Double-blind crossover RCT | Effect of external nasal dilators on chronic rhinitis patient with snoring. Mean AHI for the group was 6. | Effect on snoring, sleep architecture, objective sleep parameters. | External nasal dilators improved snoring but had no effect on objective sleep apnea measures. |
| Schonhofer et al.1431 | 2000 | 4 | Individual case series | 21 patients with OSA who used the internal nasal dilator (Nozovent) for 1 month. | Effect on snoring, sleep architecture, objective sleep parameters, sleep questionnaires. | No effect on objective sleep parameters or snoring. |
| Gosepath et al.1444 | 1999 | 4 | Individual case series | 26 patients undergoing polysomnography with and without an external nasal dilator (Breathe right nasal strips). | Effect on RDI, correlation with other factors such as comorbidities, age, and rhinologic factors. | Nineteen out of the 26 patients experienced a reduction in RDI. |
| Bahammam et al.1439 | 1999 | 2b | Double-blind crossover RCT | 18 patients in a double blinded crossover RCT comparing external nasal dilators to placebo. | Effect on nasal airway, sleep architecture, AHI, oxygen saturation. | External nasal dilators had no effect on AHI. |
| Redline et al.1310 | 1998 | 3b | Prospective cohort | Effect of CPAP vs. nasal dilators and conservative management. | Snoring, RDI, oxygen saturation, sleep questionnaires. | CPAP was superior to conservative therapy (included nasal dilators). |
| Wenzel et al.1440 | 1997 | 4 | Individual case series | 1. 30 patients with OSA. 2. 20 patients with snoring without OSA. |
Effect on objective sleep parameters and snoring. | Neither the degree of OSA or snoring in patients without OSA was changed. |
| Hoffstein et al.1448 | 1993 | 4 | Individual case series | 15 patients underwent sleep study with and without an internal nasal dilator (Nozovent). | Snoring, apneas, hypopneas, oxygen saturation. | Internal nasal dilator had a weak effect on snoring and no effect on obstructive sleep apnea measures. |
| Hoijer et al.1443 | 1992 | 4 | Individual case series | 10 patients used an internal nasal dilator (Nozovent) for 10 nights, and then they had two sleep studies one with and one without the device at a random order. | Rhinomanometry, apnea index, oxygen saturation, snoring noise, questionnaires. | Internal nasal dilator reduced the apnea index, severity of oxygen desaturation, and snoring noise. |
| Metes et al.1445 | 1992 | 4 | Individual case series | Effect of internal nasal dilator (Nozovent) on nasal resistance and sleep outcomes in a subset of patients who are heavy snorers with OSA. | Nasal resistance, snoring, apnea index, hypopnea index, oxygen saturation. | Internal nasal dilators improved nasal resistance significantly but there was no effect on objective sleep outcomes in a subset of patients who heavy snorers and have OSA. |
| Kerr et al.902 | 1992 | 4 | Individual case series | 10 OSA patients treated with a nasal dilator and topical nasal vasoconstriction. | Rhinomanometry, sleep architecture, oxygen saturation, AHI, apnea index. | Despite a 73% mean drop in nasal resistance, there was no improvement in objective obstructive sleep apnea measures. |
VIII.D.6 |. Surfactants for OSA treatment
Multiple physiologic and anatomical factors contribute to the presence and severity of OSA. Surface tension of the UA lining is one potential target for the treatment of OSA. In awake patients, it has been shown that by applying surfactant to the UA and reducing surface tension, less intraluminal pressure is required to reopen a closed pharynx.1449 This led to studies of the effects of UA surface tension on OSA.
There are a limited number of studies looking at the role of surfactants in OSA treatment. Three studies have been found which demonstrated a modest improvement in OSA after surfactant administration.1450–1452 Jokic et al. studied the effect of surfactant on OSA in a double-blinded crossover RCT in 10 patients.1450 The patients underwent two sleep studies, one night with a placebo and the other night with surfactant. The mean AHI on the surfactant night was 14 compared to 24 on the placebo night (95% CI 6–13).1450 Of note, this was the only published RCT studying the effect of surfactant on OSA.
The two other studies identified were both prospective cohort studies.1451,1452 Kirkness et al. demonstrated that surfactant decreased the surface tension of the UA lining as well as the severity of the RDI, and the correlation between the two was statistically significant.1451 This study involved nine patients who underwent two separate sleep studies, one diagnostic and the other interventional. The RDI decreased from 51 on the diagnostic night to 35 (p < 0.03) on the test night when surfactant was administered.1451 Morrell et al. performed a prospective cohort study recruiting nine patients with OSA to study the effect of surfactant on the RDI by comparing it to saline. The protocol involved studying patients on two separate nights with one night collecting data on pre- and post-saline instillation while the second night involved pre- and post-surfactant administration. Seven patients were able to complete the study and the data collected showed that the RDI was reduced with surfactant administration (pre-surfactant RDI 79.7 vs. post-surfactant RDI 59.6 [p < 0.05]) whereas saline did not decrease RDI (pre-saline 75.3 vs. post-saline 79.9).1452
One concern with surfactant is the practicality of administration by patients given that the studies published report instillation via nasal catheters targeting the nasopharynx positioned above the level of the soft palate. Perhaps a more practical form of application that could be considered is delivery via a nasal spray or an inhaler. Additionally, while surfactant appeared to reduce OSA severity in the studies discussed, OSA was not cured in any of those studies, and the improvement was modest. Surfactant may potentially play a role as an adjunct treatment or be a suitable monotherapy option for individuals with mild OSA; however, the evidence is still lacking. Larger RCTs are needed to further elucidate the utilities of surfactant in OSA management and define appropriate roles in treatment. (Table VIII.D.6)
TABLE VIII.D. 6.
Evidence for the role of surfactant in obstructive sleep apnea treatment
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Kirkness et al.1451 | 2003 | 3b | Prospective cohort | 9 patients with OSA | Effect of surfactant on surface tension of the upper airway lining and severity of OSA | Surfactant reduced the surface tension of the upper airway lining and RDI. |
| Morrell et al.1452 | 2002 | 3b | Prospective Cohort | 1. 19 Asymptomatic adult snorers 2. 9 adults with OSA |
1. Effect of surfactant on upper airway mechanics in snorers 2. Effect of surfactant compared to saline on OSA |
Surfactant reduced airway resistance at peak inspiratory pressure. Surfactant reduced OSA severity while saline did not. |
| Jokic et al.1450 | 1998 | 2b | Double-blind crossover RCT | 1. 10 OSA patients receive surfactant 2. Same 10 patients crossover to receive placebo |
AHI scores, sleep architecture, arousal index | Surfactant reduced OSA severity compared to placebo but there was no effect on sleep architecture. |
VIII.D.7 |. Supplemental oxygen for OSA treatment
Supplemental oxygen has been investigated as a therapy for intermittent nocturnal hypoxia in OSA for over 30 years, particularly for those patients that are intolerant to CPAP. Approximately 25%–50% of patients with OSA have been shown to either refuse CPAP therapy or experience intolerance.1453 While administration of oxygen has been shown to consistently improve the mean oxyhemoglobin saturation to levels comparable to CPAP,1454 long-term oxygen therapy can prolong the duration of apnea hypopnea events due to suppression of hypoxic ventilatory drive, worsen CO2 retention, and lead to respiratory acidosis.1455 Oxygen therapy has not been shown to improve subjective sleep quality or daytime functioning,1456,1457 or reduce the frequency of apnea hypopnea events.1454 Short-term oxygen therapy was evaluated for postoperative patients for 3 days and was shown to reduce AHI, ODI, and improve SaO2.1458
The effect of oxygen therapy on BP in patients with OSA has been recently evaluated with well-designed randomized studies.1459–1461 Patients with OSA are at increased risk for nocturnal fluctuations in BPs and daytime hypertension. Recurrent nocturnal hypoxemia increased sympathetic stimulation and alteration of the renin angiotensin aldosterone system are the proposed mechanisms for elevation in BP and increased CV risk in these patients. Norman et al. studied 46 patients with moderate and severe OSA who were randomized to receive 2 weeks of placebo, CPAP, or supplemental oxygen. Patients were monitored with 24-h ambulatory BP monitoring at the end of treatment period. Patients in the oxygen group experienced an improvement in the mean saturation and nadir saturation but oxygen did not lower daytime or nighttime BP.1460 Gottlieb et al. conducted the Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT) study and randomized patients with moderate to severe sleep apnea to receive CPAP or nocturnal supplemental oxygen or education on sleep hygiene for 12 weeks.1459 At the end of the study, patients randomized to CPAP experienced a significant reduction in mean 24-h BP as compared to supplemental oxygen. The results of these two randomized studies suggest that nocturnal oxygen therapy does not significantly impact BP in patients with sleep apnea; however, both studies used low flow oxygen at 2–3 L/min and excluded patients with most severe sleep apnea. The recently published SOX (Supplemental Oxygen during CPAP Withdrawal) trial was a single center, double blind, and crossover trial with randomized treatment order. The results demonstrated that supplemental oxygen at 5 L/min eliminated the morning rise in BP experienced by patients with moderate to severe sleep apnea after CPAP withdrawal, as compared with room air. Patients were not excluded based on sleep apnea or hypoxia severity.1461 Larger, multi-center studies are needed before any treatment recommendations can be made in the use of supplemental oxygen in sleep apnea.
Oxygen therapy may have a role in OSA co-morbid with other conditions (e.g., HF, COPD) but rigorous studies are lacking.
In this era of precision and personalized approach to medicine, identifying patients most likely to benefit from oxygen therapy is prudent. Sands et al. demonstrated that elevated loop gain in combination with greater pharyngeal patency could be used to predict responsiveness to oxygen therapy in their cohort of patients with moderate to severe OSA that underwent diagnostic PSG.1462 In a proof of concept study, Wang et al. determined that a 10-min awake ventilatory chemoreflex test to assess response to hypercapnia and hypoxia could indicate response to low flow oxygen therapy in patients with OSA.1463 Future research on the role of oxygen therapy in OSA must focus on recognizing oxygen responders. Larger studies are needed to establish the duration and dose of oxygen therapy and long-term outcomes. Additionally, safety of long-term oxygen therapy in patients with OSA should be ascertained given concerns for CO2 retention and acidosis. (Table VIII.D.7)
TABLE VIII.D. 7.
Evidence on supplemental oxygen and OSA treatment
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Phillips et al.1457 | 1990 | 1b | Randomized crossover | 1. Nasal oxygen 2. Nasal air 3. Nasal CPAP |
SDB events and Oxygen saturation Sleep architecture and daytime sleepiness |
Oxygen did not improve daytime sleepiness or SDB events but improved oxygen saturation. |
| Loredo et al.1456 | 2006 | 1b | Randomized, double blind, placebo controlled parallel study | 1. CPAP 2. Placebo CPAP 3. Oxygen at 3 L/min |
Sleep architecture and arousals Daytime sleepiness | Oxygen did not improve sleep quality when compared to CPAP, it improved oxygen saturation. |
| Norman et al.1460 | 2006 | 1b | Randomized double blind, placebo controlled study | 1. CPAP 2. Placebo CPAP 3. Oxygen at 3 L/min |
Change in 24 h ambulatory BP monitoring AHI, ODI, mean oxygen saturation, average SpO2 nadir during desaturations, time with saturation <90% | Oxygen did not improve 24-h ambulatory BP parameters. Both the CPAP and oxygen groups had a higher mean nocturnal SpO2 compared with the placebo group. |
| Mehta et al.1454 | 2013 | 1a | Systematic review and meta-analysis | 14 studies that included O2 therapy and/or CPAP as a study intervention | Effects of oxygen on AHI, apnea duration, and nocturnal hypoxemia | Oxygen improved O2 saturation, but may increase duration of apnea hypopnea events. |
| Gottlieb et al.1459 | 2014 | 1b | Randomized controlled trial | 1. CPAP 2. Oxygen at 2 L/min 3. Education on sleep hygiene and healthy lifestyle |
24 h mean arterial blood pressure at 12 weeks | 24 h mean BP was lower in the CPAP group, no significant difference was noted in the oxygen group. |
| Liao et al.1458 | 2017 | 1b | Randomized controlled study | 1. Oxygen at 3 L/min for three postoperative nights 2. Control group (no oxygen) |
Oxygen saturation PSG data | Supplemental oxygen improved oxygenation, AHI without increasing duration of respiratory events. |
| Sands et al.1462 | 2018 | 1b | Single-blinded randomized-controlled crossover | 1. 40% supplemental oxygen 2. Room air |
PSG variables Blood pressure and self-reported sleep quality | Increased loop gain in combination with improved airway could be used to predict response to oxygen therapy in patients with OSA that were off CPAP treatment. |
| Wang et al.1463 | 2018 | 1b | Randomized parallel group double-blind placebo-controlled clinical trial |
1. Oxygen at 3 L/min 2. Air |
Ventilatory response threshold test PSG parameters | 10 min wakefulness test predicted response to oxygen therapy. |
| Turnbull et al.1461 | 2019 | 1b | Randomized double blind crossover trial after CPAP withdrawal | 1. Oxygen at 5 L/min 2. Air |
Change in home morning BP Home morning HR, office BP and HR, levels of urine sympathetic metabolites, AHI, ESS, OSLER test |
Oxygen improved the morning rise in BP and improved oxygen saturation. It did not improve objective or subjective sleepiness. |
VIII.D.8 |. Myofunctional therapy for OSA
UA dilator muscles are thought to be crucial in maintaining airway patency, and muscle training whilst awake via myofunctional therapy (MT) can reduce airway collapsibility in patients with OSA.1464 MT exercises involve the patient performing a series of prescribed isotonic and isometric oropharyngeal exercises to improve muscle tone and soft tissue position in the oropharyngeal airway. These exercises include vowel sound repetition, tongue movements along the hard palate and floor of mouth, exerting facial musculature, and swallow/breathing techniques.1464,1465
The majority of well-designed studies, where MT was analyzed against a control group, report a positive effect of MT in lowering polysomnographic (AHI, SaO2 parameters) measures, as well as improvements in secondary outcomes such as Epworth Sleepiness Score (ESS), snoring, CPAP compliance, and subjective QOL scores. An SR by Camacho et al. found MT provides an AHI reduction of approximately 50% in selected adults.1465
Within the literature, the prescription of MT exercise type and duration is non-standardized, leading to difficulty in ascertaining where the treatment effect lies. Most RCTs are not powered for multiple outcome measures, and might better be described as “exploratory” rather than “conclusive.” The most comprehensive description of MT exercises lies within the Guimaraes et al. (2009) paper – where soft palate vowel maneuvers, tongue exercise, facial muscle movements, breathing, and chewing exercises are all combined.1464 Patients were prescribed intensive and detailed sequences of exercises with some observed by speech pathologists which may not be practical in real life paradigms. Unfortunately, follow-up protocols between studies varied significantly, making generalizability a challenge.
The pathophysiological mechanism of improvement after treatment with MT is not well defined. MT may improve airway dilator tone, resisting collapse, as well as improve airway volume through tongue fat reduction and neck adiposity reduction. A significant neck circumference reduction was observed in a treatment group without significant AHI reduction.1464,1466 However, this finding was not reproduced in other studies.1467 The mechanism accounting for reported increased CPAP compliance also needs further investigation.1468
Based on the available evidence MT may have a role in the armamentarium of treatment options for mild/moderate OSA – perhaps as an adjunct to more definitive treatments. Further data is needed to isolate the mechanism of action of MT, as well as the optimal exercise regime and duration, which may be too involved and nuanced to be practical. A paucity of long-term studies with clinical significance exists, thus further research is warranted. (Table VIII.D.8)
TABLE VIII.D. 8.
Evidence on myofunctional therapy to treat OSA
| Study | Year | LOE | Study design | Study groups | Clinical end point | Conclusion |
|---|---|---|---|---|---|---|
| Guimaraes et al.1464 | 2009 | 1b | RCT, age 25–65 recent diagnosis moderate OSAS n = 31 | 1) 3 months Sham therapy (n = 15, control)2) 3 months oropharyngeal exercises – tongue, soft palate, oropharyngeal wall, facial muscles (n = 16, treatment group) | 1) PSG (AHI) 2) Snoring (Berlin questionnaire) 3) Subjective sleepiness (ESS) 4) Sleep quality (Pittsburgh questionnaire) **Note anthropometric measures similar pre- to post-treatment, except significant reduction in neck circumference (cm) in treatment group 39.6 ± 3.6 to 38.5±4 |
1. AHI significantly reduced in treatment group, 22.4 ± 4.8 to 13.7 ± 8.5. No significant change in control group. 2. Reduced snoring frequency and intensity in treatment group. 3. Reduced ESS in treatment group 14 ± 5 to8 ± 6. 4. Improved sleep quality in treatment group. |
| Camacho et al.1465 | 2015 | 1a | Systematic review | Included data from total nine studies: 2 RCTs 3 Prospective case series (no controls) 2 Retrospective case Series 1 Retrospective case report 1 Abstract *Combined, 120 adult patients studied |
1) Polysomnography 2) Snoring 3) Subjective sleepiness (ESS) |
MT provides a reduction in AHI of approximately 50% (24.5 ± 14.3 to 12.3± 11.8). Reduction in snoring scores (72%). Reduction in ESS in post-MT (14.8 ± 3.5 to 8.2 ± 4.1). |
| Diaferia et al.1469 | 2013 | 1b | RCT, 185 consecutive male patients with no previous OSAS treatment | 3 months of treatment: 1) Placebo (sham therapy)f 2) MT 3) CPAP without MT 4) Combination Group – treated with both MT and CPAP |
1) Polysomnography (AHI) 2) Subject Sleepiness (ESS) 3) Quality of life questionnaires (FOSQ, WHOQOL-Bref, SF-36) |
1. Significant reduction in AHI in MT group vs. placebo: 28.0 ± 22.7 to 13.9 ± 18.5. 2. CPAP compliance superior in combination group (hours per night): 5.1 ± 2.3 vs. CPAP alone 3.6 ± 1.8 (p = 0.02). 3. QOL scores improved in all questionnaires. |
| Ieto et al.1467 | 2015 | 1b | RCT, 39 patients in final analysis. Primary snoring, or mild–moderate OSA included | 3 months treatment: 1) Control group (sham). n = 20 2) Therapy group: Daily MT n = 19. |
1) Snoring primary measure a) Snore Index/h as measured in PSG b) Bed partner subjective score 2) Pre- and post-treatment AHI, SpO2 minimums 3) Sleepiness (ESS)/sleep quality (Pittsburgh index) |
1) Therapy group had significantly lower snore index. 2) Non-significant difference in pre- to post-therapy AHI in treatment group. 3) Pittsburgh sleep quality index significantly better in treatment group. 4) Non-significant change in ESS. |
| Diaferia et al.1468 | 2015 | 1b | RCT, 100 patients in final analysis | 3 months treatment 4 Treatment groups 1) Placebo (neck exercises). n = 24 2) MT (3× 20 min sessions. Each day). n = 27. 3) CPAP. n = 27 4) Combination CPAP + myofunctional therapy. n = 22 |
1) CPAP adherence 2) AHI/SpO2 min 3) Subjective sleepiness (ESS) |
1. CPAP adherence was 30% in CPAP only group vs. 65% in combined group. Non-significant difference in CPAP pressures. 2. Myofunctional therapy alone group significant reduction in AHI (28–13.9, p ≤ 0.001). 3. Significant reduction in ESS in myofunctional therapy alone, and combination groups. |
Abbreviations: ESS, Epworth Sleepiness Score; MT, myofunctional therapy; PSG, polysomnogram; RCT, randomized controlled trial; SpO2 min, minimum SaO2.
IX |. SURGICAL TREATMENT FOR OSA
IX.A |. Surgical Candidacy
Surgery for OSA is considered a second line treatment for patients who fail CPAP.1482,1483 With CPAP non-adherence rates of 30%–40%, alternative therapies to CPAP, including UA surgery may be considered in treating a significant portion of OSA patients.1470,1484 This section delves further into candidates for UA surgery based on BMI criteria, what constitutes CPAP failure, and which patients could be considered for primary surgery for OSA.
IX.A.1 |. Evaluation of PAP failure prior to surgery
As CPAP is considered the primary first-line treatment for OSA, defining CPAP failure is crucial to determining surgical candidacy. CPAP nonadherence is complex and multifactorial, influenced by many factors including patient knowledge, perception of treatment and disease state, bed partner involvement, and mask interface.1470 A widely accepted definition of adequate CPAP adherence is based on the US Centers for Medicare and Medicaid decision memo describes greater than 4 h of use per night for greater than 70% of nights.21,1471 Despite inherent problems with this arbitrary definition, it has remained the standard since early papers on CPAP adherence were published in the 1990s.1091,1472,1473
Evidence has emerged on patient adherence to CPAP and what constitutes adequate treatment. Treatment outcomes differ based on the outcome metric selected (i.e., self-reported clinical outcomes, polysomnogram generated data, and CV/disease-oriented outcomes). Weaver et al. observed a linear dose–response relationship in objective daytime sleepiness as well as patient self-reported sleepiness and QOL with increasing levels of CPAP use.1098 Stradling et al. showed a similar dose response relationship in an RCT of CPAP use.1474 Neither study identified a treatment threshold. Campos-Rodriguez et al. in a prospective cohort associated at least 1 h/night with a lower mortality.1475 Antic et al. showed improved neurocognitive and behavioral outcomes with increased CPAP use.1476 In summary, evidence varies on the effectiveness of CPAP with different levels of use, different patient populations, and different treatment outcome measures. No true definition of CPAP failure exists.1107,1196 Overall, despite extensive evidence that CPAP is the most definitive way to resolve OSA, there is minimal data to define CPAP failure based on adherence. Determining which patients require surgery to effectively treat their OSA may require more than fitting them into strict criteria of CPAP usage.
When counseling patients about surgical candidacy and surgical options, individualized discussion on experiences with CPAP and acceptance of CPAP as a long-term solution for OSA are important components of the shared-decision making process. (Table IX.A.1)
TABLE IX.A.1.
Evidence for defining CPAP non-adherence
| Study | Year | LOE | Study design | Study groups | Clinical end-points | Conclusion |
|---|---|---|---|---|---|---|
| Faccenda et al.1477 | 2001 | 1b | RCT crossover study | 1. Control placebo pill (n = 70) 2. CPAP use group (n = 71) | Change in BP, ESS, FOSQ | Patients on CPAP with use >3.5 h/night had a drop in BP 1.5 mmHg. |
| Redline et al.1310 | 1999 | 1b | RCT | RDI < 30 and nonsleepy patients randomized 1. Control conservative therapy (n = 52) 2. CPAP users (n = 59) |
Change in ESS, mood, MSLT, functional status, neuropsychologic testing | Average CPAP use 3.1 h/night, CPAP group had significant improvements in RDI, MSLT, O2 nadir, and ESS score. Improvement in O2 saturation and RDI was significantly grearter in CPAP than control group. |
| Barbé et al.38 | 2012 | 1b | RCT | Nonsleepy (ESS < 10) OSA patients with AHI > 20 1. No intervention (n = 366) 2. CPAP (n = 357) |
Change in BP and cardiovascular events | No significant difference found between groups, but in those with CPAP use >4 h/night, there was a significant decrease in the incidence density ratio of hypertension or cardiovascular events. |
| Stradling et al.1474 | 2000 | 1b | RCT | 1. Therapeutic CPAP 2. Subtherapeutic CPAP as control |
1. CPAP adherence 2. Post-treatment neurocognitive and behavioral tests |
Degree of improvement correlated significantly with amount of CPAP use in the therapeutic CPAP group, but did not in the subtherapeutic CPAP group. |
| Kribbs et al.1091 | 1993 | 3b | Cohort | CPAP users (n = 35) | CPAP adherence | Average use 4.88 h/night, and only 46% of the patients met criteria for regular use of >4 h/night. Very few patients, (2/35), used CPAP for >7 h/night. |
| Engleman et al.1472 | 1994 | 3b | Cohort | CPAP users (n = 54) | CPAP adherence | CPAP use <5 h/night, did not correlate with OSA severity. |
| Reeves-Hoche at al.1473 | 1994 | 3b | Cohort | CPAP users (n = 38) | CPAP adherence | Mean hours of CPAP use at effective pressure was for 4.3 h/night. |
| Weaver et al.1098 | 2007 | 3b | Cohort | Moderate to severe OSA | 1. CPAP adherence 2. Post-treatment neurocognitive and behavioral tests |
Increased CPAP usage is associated with increased objective and subjective sleepiness measures. In those with severe OSA, greatest propotion normalized ESS scores with 4 h/night of CPAP, analysis shows further improvement can be obtained with more hours of use. |
| Antic et al.1476 | 2011 | 3b | Cohort | Moderate to severe OSA | 1. CPAP adherence 2. Post-treatment neurocognitive and behavioral tests |
Increasing CPAP adherence improves behavioral and neurocognitive outcomes in a linear fashion, even highly compliant patients may not attain normal functional status. |
| Schwab et al.1196 | 2013 | 5 | Expert opinion | None | None | There is insufficient evidence to support 4 h/night for 70% of nights in a consecutive 30-day period as the definition of adherence. |
| Campos-Rodriguez et al.1475 | 2005 | 3b | Case–control | 1. Noncompliant CPAP use, <1 h/night 2. Mildly compliant, 1–6 h/night 3. Compliant, >6 h/night |
Mortality rate | Patients who use CPAP >1 h/night have a significantly lower mortality than those who use it <1 h/night. |
| Zimmerman et al.1478 | 2006 | 3b | Case–control | 1. CPAP poor users, <2 h/night (n = 14) 2. CPAP moderate users, 2–6 h/night (n = 25) 3. CPAP optimal users, >6 h/night (n = 19) |
Memory performance measured by HVLT-R | Patients with OSA and memory impairment have improvement in memory scores if they used CPAP >6 h/night. |
| Aloia et al.1479 | 2010 | 3b | Cohort | CPAP users with neuropsychologic testing prior to starting treatment and 3–6 months after (n = 150) | Changes in neuropsychologic tests before and after CPAP treatment | Based on the reimbursement criteria for CPAP of 4 h/night for 70% of nights during consecutive 30-day period during the first 3 months of use, 37% of patients would be considered nonadherent. However, both adherent and nonadherent patients experienced similar improvements in neuropsychologic testing, and these improvements accumulated at the 6-month point. |
| Krakow et al.1480 | 2016 | 3b | Case–control | 1. Noncompliant CPAP use, < 2h/night (n = 13) 2. Subcompliant CPAP use, 3–4 h/night (n = 21) 3. Compliant CPAP use, >4h/night (n = 59) | Changes in insomnia, sleepiness, and nocturia based on CPAP adherence | Both compliant and subcompliant CPAP users had improvements in insomnia, sleepiness, and nocturia. Patients exhibited a dose dependent response to CPAP use. |
| Sawyer et al.1107 | 2011 | 5 | Expert opinion | None | None | The historical level of CPAP use of 4 h/night is not necessarily relevant given the evidence of a dose response relationship between CPAP use and benefits. |
| Brown et al.21 | 2010 | 5 | Expert opinion | None | None | The CMS guidelines for reimbursement of CPAP, 4 h/night for >70% of nights during consecutive 30-day period during the first 3 months of use, is inappropriate considering the evidence of a dose response to CPAP use. |
| Ravesloot et al.1481 | 2011 | 5 | Expert opinion | None | None | Using mean AHI instead of arbitrary compliance rates for CPAP use is a more effective way of measuring CPAP adherence, as worsened OSA severity requires higher CPAP usage to be deemed effective. |
IX.A.2 |. BMI criterion for surgical candidacy
Elevated BMI plays a crucial role in the development and severity of OSA. Obesity increases airway collapsibility, contributes to mechanical changes, and decreases neuromuscular tone.803 The prevalence of OSA in morbidly obese (BMI > 40 kg/m2) patients is 40%–90%, with BMI being a major predictor of AHI severity.350 In addition, a 10% weight loss has been linked to a corresponding 30% decrease in AHI.189,803
BMI has also been shown to play a role in UA surgery success in management of OSA. Much of our knowledge on this subject is based on post-hoc analyses of cohorts to see if BMI differences affects surgical success. Studies suffer significant selection bias with morbidly obese patients (BMI > 36–40 kg/m2) often excluded due to a belief that UA surgery is inadequate.814
The effect of BMI on OSA surgery outcomes is not universally accepted. Friedman et al. in 2003 produced a 4-level staging system for determining candidates for palate and tongue surgery for OSA based on tongue position in the mouth, with stage 4 representing morbidly obese patients that were not appropriate for surgery. However, this study showed that BMI in patients without morbid obesity (BMI < 40 kg/m2) had no significant effect on surgical success.814,1485 Furthermore, in a group with BMI < 34 kg/m2 and anatomically favorable patients with Friedman staging 1–2, Browaldh et al. showed no effect of BMI on the success rate of UPPP. An SR by Choi et al. showed that BMI was not a significant negative predictor of success after UPPP.1486,1487 In contrast, a study by Shie et al. retrospectively analyzed the effect of obesity on a cohort of patients with severe OSA (AHI > 30) and found it to be a significant negative predictor of success.527 An SR of long-term outcomes of UPPP showed BMI was a significant factor in surgical failure.812
Hypoglossal nerve stimulation (HNS) is one surgical therapy that has focused on excluding patients with severely elevated BMI due to the belief it may have decreased effectiveness in more obese patients. One of the original exclusion criteria for the clinical trial for the device was a BMI < 32 kg/m2.383,1488 However, in a small population, Huntley et al. in 2018 found no difference in short-term surgical success for patients with BMI between 32 and 34 mg/kg2.1489 However, this continues to be a source of controversy.
For non-palatal surgery, an SR by Camacho et al. examined studies on maxillomandibular advancements (MMAs) and tracheostomies performed on patients with BMI ≥ 40 kg/m2. Despite a small number of patients, MMA showed success in this population. Tracheostomy is considered a last resort and often confined to this population, with evidence of improving OSA.1490
Conflicting published evidence exists to use BMI as criteria for surgical candidacy. Heterogeneity of outcomes by procedure is high and for many procedures the data for patients with higher BMI > 40 kg/m2 does not exist. Overall, BMI ≥ 40 kg/m2 is considered a poor predictor of surgical success. Inadequate data is available to make specific recommendations on lesser degrees of obesity, but it is suggested that other clinical and anatomic features should be considered before excluding surgical therapy. (Table IX.A.2)
TABLE IX.A.2.
Evidence for BMI as a criterion for surgical candidacy
| Study | Year | LOE | Study design | Study groups | Clinical end-points | Conclusion |
|---|---|---|---|---|---|---|
| Choi et al.1486 | 2016 | 2a | Systematic review | OSA patients who underwent single level UPPP (n = 15 studies) | Surgical success as determined by AHI < 20 and AHI decrease by 50% | BMI does not affect surgical success in the immediate period; only Friedman 3 and low hyoid were negative predictors |
| He et al.812 | 2019 | 2a | Systematic review | Studies of patients with OSA who had single level UPPP (n = 11 studies) | Surgical success as determined by AHI < 20 and AHI decrease by 50% | BMI negatively affects long term surgical success |
| Camacho et al.1490 | 2015 | 2a | Systematic review | Studies of morbidly obese (BMI > 40) patients with OSA who underwent MMA or tracheostomy 1. MMA (n = 34 patients) 2. Tracheostomy (n = 14 patients) |
Surgical success determined by AHI < 20 and decrease by 50% | AHI significantly improved after MMA or tracheostomy in morbidly obese patients. However, studies are limited, making it difficult to draw definitive conclusions |
| Browaldh et al.1487 | 2013 | 2b | RCT | Moderate to severe OSA patients with BMI < 36 kg/m2, Friedman stage 1–2. Separated by Friedman stage and BMI 30 kg/m2 1. Control group, no intervention (n = 33) 2. Intervention, UPPP (n = 32) |
Primary outcome change in AHI | Intervention resulted in mean 60% improvement in AHI compared to 11% in control, and this was independent of BMI, tonsil size, or Friedman stage |
| Huntley et al.1489 | 2018 | 3b | Case control | OSA patients undergoing hypoglossal nerve stimulation 1. BMI < 32 kg/m2 (n = 113) 2. BMI > 32 kg/m2 (n = 40) |
Surgical success, > 50% reduction in AHI and AHI < 20 postoperatively | There was no difference in surgical success between the two groups |
| Heiser et al.1488 | 2019 | 3b | Case control | OSA patients who underwent hypoglossal nerve stimulation in the ADHERE registry (n = 508, mean BMI 29.3) | Analysis of predictors of surgical success and failure | On posthoc analysis, for each 1 unit increase in BMI, there was a 9% decrease in odds of surgical success. Increasing age was a positive predictor for success |
| Shie et al.527 | 2013 | 3b | Case control | Patients with severe OSA, failed CPAP, and underwent UPPP 1. BMI < 27 kg/m2 (n = 56) 2. BMI > 27 kg/m2 (n = 61) |
Surgical success, > 50% reduction in AHI or AHI < 20 postoperatively | Obesity was a significant negative predictor of treatment success |
| Li et al.1491 | 2006 | 3b | Case control | OSA patients with BMI < 40kg/m2 who underwent UPPP (n = 110) | Surgical success as determined by AHI < 20 and AHI decrease by 50% | Friedman staging had a significant predictive value on surgical success, and BMI was not predictive (no patients with BMI 40) |
| Martino et al.1492 | 2006 | 4 | Case series | Obese, OSA patients with average BMI > 30 kg/m2 (mean 36 kg/m2) and tonsillar hypertrophy (n = 7) | Posttreatment reduction in AHI compared to baseline | Six of seven patients had reduction in AHI by at least 50% |
| Vicente et al.1493 | 2006 | 4 | Case series | Severe OSA patients who underwent tongue base suspension and UPPP (n = 54, BMI < 40) | Surgical success as determined by AHI < 20, AHI decrease by 50%, and ESS < 11 | 78% of patients experienced surgical success at 3 years follow-up, and BMI at baseline was the only predictor of surgical success |
| Friedman et al.1485 | 2002 | 4 | Case series | Patients with OSA undergoing UPPP (n = 151) 1. Friedman stage 1 (n = 31) 2. Friedman stage 2 (n = 79) 3. Friedman stage 3 (n = 74) |
Surgical success as determined by RDI < 20 and RDI decrease by 50% | Lower Friedman stages at any BMI <40 kg/m2 did not affect outcome |
| Chandrashekariah et al.1494 | 2016 | 4 | Case series | OSA patients who had undergone UPPP, obese (BMI ≥ 30 kg/m2, average BMI 41 kg/m2), and had persistent elevated AHI (n = 11) | Improvement in CPAP adherence | Eight of 11 patients had improvement in PAP adherence, with increased mean of 48.6 min per night |
IX.A.3 |. Surgery as primary treatment for OSA
In most patients CPAP continues to be considered the first line treatment of severe OSA. Primary surgery as first-line therapy remains a strategy with an absence of high-level data to support its use. Despite this, primary surgery is advocated for some patients with mild or moderate OSA (where comparable clinical outcomes are observed) or in selected patients with appropriate anatomic features (such as craniofacial abnormalities or marked tonsil hypertrophy).1495
An SR by Rotenberg et al. explored both studies evaluating CPAP and surgical therapies, commenting specifically on trials that compared the two. Two trials, one RCT by Woodson et al. and another non-RCT by Ceylan et al., compared temperature-controlled radiofrequency tissue ablation (TCRFTA) of the tongue and palate to CPAP, both finding minimal differences between the two on objective PSG parameters and subjective parameters such as the ESS.1495–1497 Another study by Weaver et al. compared a cohort of patients who had undergone UPPP versus prescribed CPAP treatment and found that the CPAP patients had a 31% higher mortality.1498 Despite limitations, these data suggest that there may be a phenotype of patient who may benefit from interventions other than CPAP as the first line.
Certain anatomic features predispose patients to surgical success. Hypertrophic tonsils and a low tongue position, with the combination encompassed in the Friedman staging system, portend a good result for palate surgery.547 A study by Rotenberg et al. explored palate surgery for Friedman stage 1 patients, which showed a success rate (based on Sher’s criteria) of 87.5%.1499 An SR of tonsillectomy for OSA by Camacho et al. revealed that isolated tonsillectomy is effective as a treatment for patients with hypertrophied tonsils and mild-moderate OSA.335 A study by Senchak et al. showed substantial improvement in the AHI of a population of young adults with large tonsils undergoing primary tonsillectomy for reasons other than OSA.1500 Although the number of patients with such favorable anatomy is small, it does raise the point that surgery can provide a simple and efficient way to treat OSA in the appropriate situation and patient population.
MMA is another technique where anatomic factors can make primary surgery favorable. The original Stanford protocol by Riley et al. in 1993 kept MMA as a phase 2 surgery to be performed after a more conservative approach such as palatoplasty failed.1501,1502 However, there is minimal data on using it as a primary surgical approach in OSA. An RCT by Vicini et al. compared primary MMA to CPAP on patients with severe OSA (AHI > 30). They found MMA to be non-inferior to CPAP as a treatment.1503 Liu et al. in a retrospective review showed that MMA was effective for stabilizing lateral pharyngeal wall collapse, an area that is difficult to treat with intrapharyngeal surgery.1504 Patients with dentofacial abnormalities, specifically class II occlusion, are also good candidates for primary MMA.1505
In general, for the majority of patients, CPAP represents the best primary option. Practice parameters for surgery for OSA published by Aurora et al. in 2010 and Kent et al. in 2021 continue to recommend CPAP as the primary option given the lack of good evidence showing surgery as a primary modality to treat OSA.1482,1506 Further research is necessary to explore primary surgery for OSA. (Table IX.A.3)
TABLE IX.A.3.
Evidence for surgery as a primary treatment for OSA
| Study | Year | LOE | Study design | Study groups | Clinical end-points | Conclusion |
|---|---|---|---|---|---|---|
| Sundaram et al.1483 | 2005 | 1a | Cochrane systematic review | Surgical RCTs for treatment of OSA (n = 709 patients combined) | Effectiveness of surgery for OSA | There is insufficient evidence to support surgery being consistently effective for OSA management. More RCTs are necessary to specify subgroups that would benefit most from surgery. |
| Woodson et al.1497 | 2003 | 1b | RCT | Patients with mild to moderate OSA randomized to 1. Sham-placebo (n = 30) 2. CPAP (n = 30) 3. Temperature controlled radiofrequency tissue ablation, TCRFTA (n = 30) |
AHI, quality of life measures | Both CPAP and TCRFTA had significant improvement over sham-placebo. There was no significant difference in outcomes between CPAP and TCRFTA. |
| Vicini et al.1503 | 2010 | 1b | RCT | Patients with severe OSA (AHI > 30) randomized to 1. APAP group (n = 25) 2. MMA group (n = 25) |
Reduction in AHI and ESS | MMA is a viable alternative surgical therapy to CPAP, with a noninferior success rate. |
| Rotenberg et al.1495 | 2016 | 2a | Systematic review | 1. Systematic review of CPAP trials (n = 82 trials) 2. Systematic review of surgery trials (n = 69 trials) 3. Systematic review of CPAP vs. surgery trials (n = 3 trials) |
Comparison of success between CPAP and surgery for OSA | Lack of long-term follow-up and low adherence in CPAP trials, along with evidence of surgical success, should change our thought that surgery cannot be a primary treatment in OSA. |
| Aurora et al.1482 | 2010 | 2a | systematic review | Review of effectiveness surgical trials (n = 79 trials) | Publishing of practice parameters for surgical management of OSA | Due to lack of strong evidence favoring surgical management of OSA, CPAP should always be considered as the initial treatment for OSA. |
| Walker-Engstrom et al.1507 | 2002 | 2b | RCT | Patients with mild to moderate OSA 1. Dental appliance group (n = 32) 2. UPPP group (n = 40) |
PSG parameters at 1 and 4 years follow-up, surgical success determined as AHI < 10 | Oral appliance had better success rate of 62% over UPPP, with success rate of 33%. Compliance with oral appliance was 62% at 4-year follow-up. |
| Lojander et al.1508 | 1996 | 2b | RCT | 1. Nasal CPAP vs. conservative treatment (n = 44) 2. UPPP with or without mandibular osteotomy vs. conservative treatment (n = 32) |
ESS and posttreatment ODI | CPAP was successful but compliance was an issue. Surgery also showed significant improvement compared to control. No comparison can be made in this study between CPAP and surgery. |
| Weaver et al.1498 | 2004 | 3b | Cohort | VA Patients with OSA 1. Using CPAP (no compliance data) (n = 18,754) 2. Underwent UPPP (n = 2072) |
Posttreatment overall survival | Patients on CPAP had a 31% higher probability of mortality at any time relative to patients who underwent UPPP. |
| Senchak et al.1500 | 2015 | 3b | Cohort | Cohort of military patients undergoing tonsillectomy for reasons other than OSA. Out of 156, n = 19 with OSA | Posttreatment AHI | Patients had 82% reduction in AHI. In population of young overweight men with large tonsils and moderate OSA, tonsillectomy alone can be effective treatment. |
| Ceylan et al.1496 | 2009 | 3b | Case–control | Mild to moderate OSA 1. nasal CPAP group (n = 21) 2. Temperature controlled radiofrequency tissue volume reduction group (TCRTVR) (n = 26) |
ESS and post treatment PSG | No significant difference between success in the multilevel minimally invasive surgery group (TCRTVR) and nasal CPAP group. |
| Rotenberg et al.1499 | 2014 | 3b | Case–control | Patients with moderate to severe OSA who failed CPAP and had favorable anatomy for palate surgery | 1. 1-year posttreatment AHI 2. 1-year posttreatment ESS 3. 1-year posttreatment sleep apnea quality of life index (SAQLI-E) 4. 1-year posttreatment BP |
By AHI, 85.7% of patients achieved surgical success. SAQLI-E scores improved significantly after surgery. A subset of patients can be managed more effectively with surgery than CPAP. |
| Kent et al.1506 | 2021 | 1a | Meta-analysis | Evaluation of two RCTs and 15 observational studies. For RCTs patients were randomized to surgery or no treatment | AHI/RDI, ESS, LSAT, sleep-related QOL, snoring, ODI, blood pressure, death, persistent side effects | Recommend PAP as initial therapy for adults with OSA and a major upper airway abnormality. Harm for initial trial of PAP therapy is low. Recommendation was conditional. |
IX.B |. Perioperative Management of OSA
IX.B.1 |. Anesthesia considerations in OSA and upper airway surgery
Patients with OSA are at increased risk for complications associated with anesthesia. Risks may be higher after UA surgery given anatomical changes to the airway and soft tissue edema postoperatively. Multiple large-scale studies have examined the associations between OSA and adverse perioperative outcomes (Table IX.B.1a).466–468,1509–1511 Although results are heterogeneous, studies focused on outcomes surrounding sleep surgery demonstrate that OSA is associated with multiple perioperative complications including difficult airway management, UA obstruction, and postoperative respiratory failure.
TABLE IX.B.1a.
Evidence for perioperative outcomes associated with OSA and upper airway surgery
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Opperer et al.468 | 2016 | 2a | SR | OSA (n = 413,304) and non-OSA (n = 8,556,279) patients undergoing non-sleep surgery with general/neuraxial anesthesia or sedation. | Varying postoperative outcomes including pulmonary complications, cardiac complications, oxygen desaturation, difficult airway management. | The presence of OSA is associated with an increased risk of postoperative complications. |
| Kaw et al.467 | 2012 | 2a | SR | Patients undergoing non-cardiac, non-sleep surgery (n = 3942). | Varying postoperative outcomes, primarily cardiac/respiratory complications. | The incidence of postoperative desaturation, respiratory failure, postoperative cardiac events, and ICU transfers was higher in patients with OSA. |
| Hai et al.466 | 2014 | 2a | SR | Patients undergoing non-sleep surgery (n = 7162). | Outcomes: acute respiratory failure, cardiac complications, postoperative ICU transfer. | OSA was associated with significant increase in risk of respiratory failure, cardiac events, and ICU transfer postoperatively. |
| Vasu et al.1511 | 2012 | 2a | SR | Patients undergoing surgery, excluding bariatric, and sleep-apnea procedures (n = 108,968). | Varying perioperative complications. | Patients with OSA are at increased risk for perioperative complications. |
| Corso et al.1509 | 2018 | 2a | SR | Patients with OSA undergoing surgery (46 studies). | All articles containing relevant evidence on epidemiology, pathophysiologic mechanisms, and perioperative interventions on OSA. | Preoperative screening of OSA patients is of relevance given the increased perioperative morbidity of these patients. |
| Riley et al.1517 | 1997 | 2b | Retrospective cohort | OSA patients undergoing various upper airway procedures (n = 182). | Fifty-four perioperative factors were analyzed. | Intraoperative airway risks can be reduced by use of fiberoptic intubation in patients with increased neck circumference and skeletal deficiency. Patients with OSA are at a significantly increased risk for hypertension. Nasal CPAP eliminated the postoperative risk of hypoxemia, which allowed the use of adequate analgesics. |
| Vest et al.1514 | 2013 | 3b | Retrospective case–control | Ninety adults with difficult tracheal intubation were compared with 81 gender-matched controls. | Predictors of difficult intubation. | In adult subjects, OSA history was not a predictor of difficult intubation. Other patient or anesthesiologist factors (lower BMI, higher Mallampati scores) should be assessed for their association with difficult intubation. |
| Iyer et al.1530 | 2011 | 2b | Retrospective cohort | Consecutive series of patients who had undergone laparoscopic gastric banding (n = 267). | The incidence of difficult intubation, early postoperative complications, and its attendant risk factors were studied. | Severe OSA and neck circumference greater than 44 cm were factors associated with difficult intubation in morbidly obese patients who presented for bariatric surgery. |
| Kheterpal et al.1531 | 2009 | 2b | Prospective cohort | Consecutive of patients undergoing general anesthesia over 4-year period (n = 50,000). | The primary outcome was impossible mask ventilation defined as the inability to exchange air during bag-mask ventilation attempts, despite multiple providers, airway adjuvants, or neuromuscular blockade. Secondary outcomes included the final, definitive airway management technique and direct laryngoscopy view. | A total of 77 cases of impossible mask ventilation (0.15%) were observed. Neck radiation changes, male sex, sleep apnea, Mallampati III or IV, and presence of beard were identified as independent predictors. |
| Kim et al.1516 | 2006 | 3b | Retrospective case–control | Patients who had undergone UPPP (n = 90). | OSA patients were compared with 90 age and sex-matched control patients with respect to the prevalence of difficult intubation. | The occurrence of difficult intubation can be predicted using AHI in patients who undergo UPPP surgery for OSAS. |
| Leong et al.1510 | 2017 | 3a | SR | Patients with (n = 4626) and without OSA (n = 64,684) who underwent airway management for surgery. | Difficulty of airway management (mask ventilation, tracheal intubation, failed supraglottic airway device). | OSA was found to bea risk factor associated with difficult tracheal intubation and difficult mask ventilation. There was no association between OSA and difficult supraglottic airway use. |
| Corso et al.1515 | 2011 | 3b | Retrospective case–control | OSA patients (n = 145) who underwent sleep surgery were compared with control patients (n = 1263) who underwent other otolaryngologic surgery. | Difficult intubation. | Difficult intubation occurred more frequently in patients with OSA. In OSA patients in whom difficult intubation was found, body mass index (BMI), AHI, and LSAT were not different from values obtained in OSA patients who underwent easy intubation. |
| Siyam et al.1518 | 2002 | 3b | Retrospective case–control | Anesthetic management of OSA patients (n = 36 were compared with non-OSA controls (n = 77). | Difficult intubation. | Difficult intubation occurred more often in sleep apnea patients. No relationship was found between severity of OSA and the occurrence of difficult intubation. |
| Ulnik et al.1532 | 2000 | 2b | Prospective Cohort | Patients who underwent sleep surgery (n = 38). | Postoperative course and occurrence of complications within 72 h. | Within the first 72 h after surgery, no complications were observed. Patients with BMIs greater than 35 were at increased risk for postoperative desaturations. The uncomplicated OSAS patient, one without significant comorbid factors, can be treated in a safe and prudent fashion outside of an intensive care unit. |
| Kim et al.1525 | 2005 | 2b | Retrospective cohort | OSA who underwent UPPP surgery with/without tonsillectomy under general anesthesia (n = 90). | Apnea-hypopnea indices (AHI), preoperative lowest arterial saturation (LSAT-PREOP) levels, percentages of obstruction at the upper level of the uvula during apnea (AL-U), need for an airway in the postanesthesia care unit (PACU) or during the first postoperative night in a ward (POPN1), LSAT-PACU, LSAT-POPN1, and the incidence of postoperative bleeding or other complications. | Immediate postoperative complications and oxygen saturation are associated with OSAS severity and the level of obstruction, inducing apnea in those who have undergone UPPP for OSAS. |
| Talei et al.1533 | 2013 | 2b | Retrospective cohort | Patients who underwent UPPP with or without septoplasty for OSA (n = 32). | Perioperative, clinical, and anesthetic records were reviewed for any complications and risks, defined as any adverse event delaying surgical progress or recovery along with any additional risk to patient safety. | Review of 32 patients failed to show any life-threatening risks or complications. |
Difficult airway management
In evaluating difficult airway management, a qualitative SR to determine the influence of OSA on perioperative outcomes included 61 studies with a total of 413,304 OSA patients and 8,556,279 control patients468; the majority of studies reported that difficult intubation was more common for OSA patients than for controls. A review of 492,239 cases performed at four institutions documented a 0.4% rate of difficult mask ventilation (grade 3 or 4) combined with difficult laryngoscopy (grade 3 or 4 view) in patients with OSA. OSA was an independent predictor of difficult mask ventilation and laryngoscopy, as were increased age, male sex, Mallampati III or IV, neck radiation, limited thyromental distance, and limited jaw protrusion.1512 Two smaller studies in patients undergoing non-sleep surgery did not report a relationship between OSA and difficult airway management.1513,1514 One limitation of the SRs and meta-analyses is that they typically excluded patients undergoing sleep surgery. When populations of patients undergoing sleep surgery were examined, the majority of studies showed an association between OSA and difficult airway management.1510,1515–1518 OSA patients who underwent UPPP had higher rates of difficult intubation when compared to age- and sex-matched controls.1516 Furthermore, the rate of difficult intubation positively correlated with OSA severity, especially for patients with an AHI greater than or equal to 40 events/h.1516 Based on strong evidence supporting an association between OSA and difficult airway management, the ASA practice guidelines recommend that patients with known or suspected OSA be managed according to the Practice Guidelines for Management of the Difficult Airway: An Updated Report.1519,1520 A recent consensus statement recommended modifications to management such as using a ramped position for induction and intubation, noninvasive positive pressure ventilation during induction, and weighing the advantages of rapid sequence induction against the risk of rapid oxygen desaturation and difficult airway management.1509,1521 (Table IX.B.1a)
Perioperative sedatives and opiates
Acute UA obstruction is another concern in the perioperative management of patients undergoing sleep surgery. Studies demonstrate an increased tendency for OSA patients to develop acute UA obstruction in the setting of minimal sedation when compared to otherwise healthy adults. In particular, hypoglossal nerve activity is sensitive to minimal levels of anesthesia and inappropriate sedation can lead to loss of genioglossus tone and consequently UA patency.1522–1524 In addition, postoperative complications and SaO2 may be associated with OSA severity. A retrospective cohort evaluation of patients who underwent UPPP with or without tonsillectomy demonstrated that 8.9% developed postoperative oxygen desaturation and the mean AHI of patients who developed complications was significantly higher than of those without complications (68.1 vs. 49.3 events/h, respectively).1525 It is recommended that no sedating medications be given to at-risk OSA patients preoperatively. Intraoperatively, acute obstruction following extubation is positively correlated with the amount of opioid administered for patients with OSA.1526 Thus, it is recommended that sedative pre-medications not be routinely used in OSA patients and, if necessary, be delivered judiciously with close monitoring.1521 Similarly, intraoperative opioid use should also be minimized to decrease the risk of difficult extubation and immediate postoperative respiratory failure. Proper care during extubation should be taken to ensure that the patient has completely recovered consciousness, neuromuscular blockade is fully reversed, and if possible, the patient placed into a semi-upright or lateral position (Table IX.B.1b).1521,1522
TABLE IX.B.1b.
Evidence for reduction of preoperative sedative and intraoperative opiate use
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Ravesloot et al.1521 | 2019 | 2a | SR and expert consensus | Studies on operative care of OSA patients (n = 164) | Consensus of experts (n = 12) on preoperative, intraoperative, and postoperative care topics | Strong recommendation to avoid use of sedatives (quality of evidence: very low) and opioids (quality of evidence: low). |
| Esclamado et al.1526 | 1989 | 2b | Retrospective Cohort | OSA patients who underwent sleep surgery (n = 135) | Various patient demographics and perioperative complications, including difficulty intubating and failed extubation | Acute obstruction with extubation was significantly and positively correlated with dose of narcotic analgesia intraoperatively. |
| Connolly et al.1522 | 1991 | 4 | Case report | One patient who underwent UPPP and review of literature | Complications after anesthesia | Recommendation for limitation to regional anesthesia, inhaled anesthetic, and/or short-acting opioids when possible, and to avoid sedatives and opioids due to high sensitivity in OSA patients. |
Airway edema management
With regards to postoperative respiratory failure, systemic steroids are commonly used to address postoperative airway edema in patients undergoing sleep surgery despite the fact that the immediate postoperative effect of UA surgery on OSA severity is unclear. The use of steroids is supported by a small study (n = 8) of postoperative edema following laser-assisted uvulopalatoplasty (LAUP) with pre- and postoperative PSG, MRI, and videoendoscopy. These authors demonstrated that 48–72 h after LAUP, mean RDI nearly doubled from 11.3 to 21.7 events/h while cross-sectional area of the airway decreased slightly from 49.8 to 47.9 mm2.1527 Similarly, a study of patients undergoing MMA noted pharyngeal edema on nasopharyngolaryngoscopy after surgery in all 70 patients and hypopharyngeal hematoma in 6% of patients, though ultimately none had airway difficulty.1528 In light of these findings, articles based on expert opinion recommend that systemic steroids should be considered to reduce airway edema with optimal dosing of one dose prior to surgery followed by several doses postoperatively.1521,1529 (Table IX.B.1c)
TABLE IX.B.1c.
Evidence for administration of perioperative systemic steroids after sleep surgery
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Ravesloot et al.1521 | 2019 | 2a | SR and expert consensus | Studies on operative care of OSA patients (n = 164) | Consensus of experts (n = 12) on preoperative, intraoperative, and postoperative care topics | Weak recommendation for postoperative methods to reduce edema, including steroids (level of evidence: very low). If used, agents should be administered pre- and several times postoperatively. |
| Mickelson et al.1529 | 2007 | 5 | Expert opinion | N/A | Postoperative airway edema | Recommendation for one dose of dexamethasone (5–10 mg) pre- and several times postoperatively. |
IX.B.2 |. Perioperative CPAP use and sleep surgery
Patients with OSA have been shown to have greater risks for perioperative adverse events, such as oxygen desaturation, difficult intubation, cardiac complications, and AF.468,1534 In the outpatient setting, PAP has been consistently demonstrated to be effective in controlling OSA symptoms and reducing consequent cardiopulmonary effects. In the perioperative setting, PAP reduces UA edema and increases lung functional residual capacity and volume.1509,1535 Therefore, it is important to consider the effect of PAP use in the perioperative management of OSA patients undergoing surgery.
The immediate effect of UA surgery on the severity of OSA in the postoperative period is unknown as increased airway volume and stability may be counteracted by factors such as local tissue edema and the use of sedating medications.1521 Presently, there are no studies specifically addressing the perioperative use of PAP in OSA patients undergoing UA surgery, likely because PAP failure is often a reason for surgery.1521 A retrospective cohort study from 2001 showed that OSA patients undergoing elective hip/knee replacement surgery had a significantly increased risk of postoperative cardiopulmonary complications and that the subset of these OSA patients who used continuous PAP (CPAP) preoperatively had a lower rate of such complications.1536 The current practice guideline recommendations regarding CPAP use for patients with OSA are based on expert opinion surveys of the ASA; they suggest that providers consider the preoperative initiation of CPAP, particularly if OSA is severe.1519
There is a robust body of literature evaluating the benefits of postoperative PAP on cardiopulmonary outcomes in patients with OSA undergoing non-sleep surgery.1509,1535–1540 In contrast, the literature on PAP use following sleep surgery specifically is limited.1541 One RCT evaluating the effect of CPAP use in 16 patients after UPPP and septoplasty showed that CPAP was effective in preventing BP elevation during sleep in the immediate postoperative period.1541 An MA which included pooled data from six studies (three observational studies, two RCTs, and one case series) concluded that there was no significant difference in postoperative adverse events between CPAP and non-CPAP groups.1535 However, these studies were largely heterogeneous, the majority of patients did not undergo surgeries to specifically address OSA, and the two RCTs included were not designed to address the perioperative effects of CPAP.
A recent review and consensus statement by a group of UA surgery experts produced several recommendations surrounding the perioperative use of PAP in patients with OSA undergoing UA surgery.1521 The following recommendations reached >90% expert consensus and are all based on “very low” quality of evidence based on the GRADE system1542:
Perioperative treatment with PAP should be considered to potentially reduce the risk of postoperative airway complications after UA surgery in patients with OSA without contraindications. A few relative contraindications include: un-cooperative patients, unstable cardiorespiratory status, facial trauma or burns, copious respiratory secretions, severe nausea and vomiting, and severe air-trapping diseases. Patients who have surgery that involves the skull base or the orbital wall should avoid PAP as it can be led to pneumocephalus and/or air in the orbit.
PAP therapy should be used preoperatively in OSA patients already receiving PAP.
Full-face mask PAP in OSA patients may be used after nasal surgery if PAP is indicated, tolerated, and not contraindicated.
Recommend against the use of PAP after maxillofacial surgery.1521
There is high variability in the resumption of PAP use after nasal, sinus, and skull base surgery in patients with OSA. This is principally related to the paucity of data on best practices including clinical outcomes and complications related to early or late resumption of PAP in patients with OSA. Generally, practitioners agree that for nasal surgery and sinus surgery without a skull base or orbital defect, resumption of CPAP within the first 2 weeks is appropriate. With a skull base or orbital defect, resumption of PAP is principally dependent on the size of the defect but can also be dependent on PAP pressure and individual patient dependence on PAP therapy. With increasing size of skull base defects with a CSF leak, the delay in initiation of PAP therapy varies from 14+ days from small CSF leaks to 21+ days for more significant leaks.1543
In summary, although the perioperative use of PAP is generally encouraged in cases of non-UA surgery, especially cardiothoracic and major abdominal surgeries, PAP use in the patients undergoing UA surgery for OSA has not been well-studied and limited evidence has shown equivocal results. (Table IX.B.2)
TABLE IX.B.2.
Evidence of the effects of perioperative CPAP use and sleep surgery
| Study | Year | LOE | Study design | Population size (n) and study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Ravesloot et al.1521 | 2019 | 1b | Review and consensus | 164 articles published on or before December 31, 2011 | Forty-seven questions covering preoperative, intraoperative, and postoperative care | The use of perioperative PAP may reduce airway complications after sleep surgery, if not otherwise contraindicated. |
| de Araújo et al.1541 | 2013 | 1b | RCT | OSA patients subjected to UPPP ± septoplasty (n = 16) 1. CPAP (n = 10) 2. Non-CPAP (n = 6) |
AHI Nocturnal blood pressure Nocturnal heart rate Nocturnal norepinephrine/epinephrine levels |
AHI increased in patients without CPAP on postoperative day 1. Nocturnal blood pressure and catecholamine levels were increased in non-CPAP group. |
IX.B.3 |. Postoperative disposition after sleep surgery
Postoperative monitoring is important in the OSA patient population and should involve metrics such as SaO2 levels, respiratory rate, and, ideally, end-tidal CO2 levels.1521 Methods to specifically address postoperative UA edema, such as corticosteroid administration (see “Anesthesia Considerations”), head-of-bed elevation, and avoidance of supine positioning when possible are also recommended in the postoperative period.1521
The spectrum of surgeries available for OSA management varies widely and consequently postoperative disposition can include a combination of: ICUs, stepdown units, inpatient ward, post-anesthesia recovery units (post-anesthesia care unit [PACU]), and same-day discharge home. Decisions regarding postoperative disposition should take into account the type of surgery performed, patient comorbidities, OSA severity, and if applicable, adherent use of PAP.1521,1544 A large-scale analysis of 3130 adults who underwent UPPP showed that increased AHI (OR 1.23, p = 0.05), elevated BMI (OR 1.34, p = 0.04), and the presence of medical comorbidities (OR 1.47, p = 0.002) were each associated with increased rates of serious postoperative complications.1544,1545
Depending on the type of sleep surgery, there are varying levels of evidence regarding optimal postoperative disposition to minimize adverse events while preventing the waste of resources. Procedures that do not directly involve the OP and hypopharynx, such as isolated nasal surgery and hypoglossal nerve stimulator implantation, are generally safe to perform in an ambulatory setting. Current consensus guidelines propose that prolonged postoperative monitoring is not required in isolated nasal surgery.1521 HNS surgery is unique compared to other surgical interventions for OSA in that the native airway anatomy is preserved and there is less postoperative pain compared to UA soft tissue surgeries. Therefore, HNS patients have been shown to be safely managed in an ambulatory setting and can typically be discharged home the same day following surgery provided the degree of OSA and severity of co-morbidities do not warrant overnight monitoring.815,1546–1548 For minimally-invasive, office-based procedures including radiofrequency (RF) to the palate or base of tongue and palate suspension or implant placement, the use of local anesthetic alone allows patients to safely go home the same day.1549–1554
In the case of palatoplasty surgery, postoperative outcomes of UPPP and its modifications (transpalatal advancement, expansion pharyngoplasty) have been examined. Several cohort analyses examined complication rates in the immediate postoperative period and determined that there is often no need for routine ICU or inpatient prolonged postoperative monitoring.813,1555–1563 Complication rates were generally low and serious events such as acute oxygen desaturations often occurred within a few hours after surgery and could be addressed in the recovery room.1559,1561,1563 Therefore, although postoperative monitoring, usually involving an overnight inpatient stay, has been recommended for patients with OSA undergoing UPPP, same-day discharge after a several-hour stay in the postoperative recovery room may be appropriate in select cases. Other factors to be considered should include the patients’ severity of OSA, SaO2 nadir, comorbidities, and BMI.813,1521,1545,1557
Few studies evaluate postoperative protocols for hypopharyngeal sleep apnea surgery, such as base of tongue reduction, hyoid suspension, and transoral robotic surgery (TORS). Current data suggests that airway-related complication rates may be higher in hypopharyngeal surgeries.1550,1564 However, existing literature on hypopharyngeal surgery lacks large-volume studies focused on specific procedures within the lower pharynx and results are inconclusive. One study of 22 patients who underwent hypopharyngeal surgery for OSA demonstrated no instances of postoperative desaturation, pulmonary edema, or airway compromise requiring re-intubation.1565 MLS may warrant augmented postoperative monitoring, though existing data on management do not provide a sufficient basis for management recommendations.
For MMA, which involves advancement of the nasopharyngeal, retropalatal, and hypopharyngeal airway, there are few studies that examine the optimal immediate postoperative management regimen. Review of existing literature shows that patients generally require postoperative monitoring in ICU or inpatient hospital units.1564,1566 Further research regarding these types of airway procedures is warranted and current expert consensus suggests patients undergoing hypopharyngeal airway surgery or MMA for OSA should at least have overnight postoperative monitoring in the hospital facility.1521
Overall, OSA patients are susceptible to multiple postoperative complications such as acute respiratory failure, oxygen desaturation, or inadequate pain control. The frequency and severity of postoperative issues are dependent on the type of surgery performed, patient comorbidities, and the severity of OSA. Postoperative disposition should be determined based on these same factors. (Table IX.B.3)
TABLE IX.B.3.
Evidence for postoperative disposition after sleep surgery
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Terris et al.1563 | 1998 | 2b | Retrospective cohort | OSA patients who underwent surgery (n = 109). | Postoperative and follow-up complications and adverse events. | Routine postoperative intensive care monitoring for all adult patients undergoing sleep surgery is unnecessary. Although high-risk patients cannot always be identified preoperatively, significant complications generally emerge within 2 h after surgery. A decision regarding the level of postoperative monitoring needed may be made with confidence during the period of time that the patient is in the recovery room. |
| Mickelson et al.1558 | 1998 | 2b | Retrospective cohort | OSA patients who underwent UPPP (n = 347). | Postoperative complications and escalation of care. | Except for one patient, all complications that occurred on the surgical ward were treated without transfer to the intensive care unit. These findings suggest that UPPP is a safe procedure and that postoperative monitoring in an intensive care setting is not necessary for most patients. |
| Rocke et al.1545 | 2013 | 2b | Retrospective cohort | OSA patients (n = 104) who underwent surgical treatment were triaged to ICU, routine ward bed, or discharge home based on preoperative patient factors and type of surgery. | Postoperative complications. | A postoperative disposition protocol can be effectively used to triage patients to less than intensive postoperative care. |
| Pang et al.1559 | 2012 | 2b | Retrospective cohort | OSA patients who underwent multilevel airway surgery (n = 487). | Surgical complications. | Routine postoperative admission to the intensive care unit for all patients with OSA is unnecessary. These patients should be closely monitored in the post-anesthesia care unit area after surgery, and based on the outcome of this period, they can be observed overnight in either the high-dependency unit or the general ward. |
| Rotenberg et al.1560 | 2010 | 1b | Prospective cohort | OSA patients who underwent surgery (n = 121). | 1) Incidence of respiratory complications requiring nursing intervention, 2) level of postoperative blood oxygen saturation. | The incidence of respiratory events requiring intervention in the early postoperative course of OSA patients was low (3.4%). Routine postoperative inpatient monitoring may not be required in many cases. |
| Cillo et al.1555 | 2015 | 2b | Retrospective cohort | OSA patients who underwent surgery (intra- and extra-pharyngeal procedures) (n = 267). | Surgical complications. | The incidence of respiratory events requiring intervention in the early postoperative course of OSA patients was low (2.2%). Intra-pharyngeal complications 1.2% and extrapharyngeal complications 3.8%. |
| Hathaway et al.1557 | 2006 | 2b | Retrospective cohort | OSA patients who underwent UPPP ± tonsillectomy, septoplasty, or supraglottoplasty (n = 110). | Admission rate and surgical complications. | Admission was required in 20 (18%) patients whereas 90 (82%) were discharged on the day of surgery. Admission due to desaturation was noted in 3 (3%) patients. Careful preoperative selection of patients should permit many patients to undergo UPPP as outpatient surgery. Factors requiring admission should be assessed in the early postoperative period. |
| Spiegel et al.1561 | 2005 | 2b | Retrospective cohort | OSA patients who underwent UPPP (n = 117). | Postoperative complications. | The majority of complications after UPPP with or without additional procedures occur within 1–2 h after surgery. Postoperative oxygen desaturation is usually no worse than that that was observed on preoperative polysomnography findings. A 2–3 h observation period followed by same day discharge may be suitable for patients after UPPP although numbered studied was limited. |
| Strocker et al.1562 | 2008 | 2b | Retrospective cohort | Moderate to severe OSA patients who underwent UPPP (n = 40). | Postoperative outcomes. | Patients with significant OSA safely underwent UPPP as an outpatient procedure in this small cohort. |
| Kandasmy et al.813 | 2013 | 2b | Retrospective cohort | OSA patients who underwent UPPP (n = 345). | Postoperative complications. | The incidence of post-UPPP complications is much lower than the literature has historically suggested. Selected patients should be able to safely undergo outpatient UPPP. Patients with higher AHI, higher BMI, or multiple comorbidities are at higher risk for postoperative complications and are most appropriate for overnight monitoring. |
| Gessler et al.1556 | 2003 | 2b | Retrospective cohort | OSA patients who underwent tonsillectomy and UPPP (n = 130). | Postoperative complications. | Patients who undergo tonsillectomy and UPPP do not require monitoring in an intensive care unit postoperatively. |
| Glazer et al.1567 | 2014 | 2b | Retrospective cohort | Moderate to severe OSA patients who underwent TORS for OSA (n = 166). | Major and minor complication rates. | All patients were admitted to ICU postoperatively with median days in the ICU and hospital 1 and 2, respectively. Complications are similar to those seen with other surgical treatments of OSA and were only predicted by ASA score and number of procedures performed. |
| Nelson et al.1565 | 2015 | 2b | Retrospective cohort | OSA patients who underwent hypopharyngeal surgery (n = 22). | Postoperative complications. | No patient experienced intra-operative complications, postoperative O2 desaturation <90%, prolonged admission for inadequate pain control, pulmonary edema, or airway compromise requiring re-intubation. |
IX.B.4 |. Opioid use for pain control after sleep surgery
Patients with OSA may be particularly susceptible to the respiratory depressant effects of common anesthetic agents and opioid pain medications.1519 Existing literature lacks high-quality large-volume studies examining the influence of differing pain management regimens on the postoperative outcomes of patients undergoing sleep surgery. One Cochrane review did not find any significant effect of sedative and hypnotic medications on the AHI in a non-surgical setting. However, significant decreases in SaO2 nadirs were observed with remifentanil, zolpidem, and triazolam.1425 The current recommendation, based on anecdotal evidence, first principles, and expert consensus, is to minimize or avoid perioperative opioid use in adult OSA patients when possible and instead, utilize alternative pain management options to augment patient comfort and reduce the need for opioids.1519,1521,1526,1529,1568 An assessment of 42 patients, who underwent UPPP and were treated with acetaminophen as the sole analgesic agent, reported no instances of significant oxygen desaturation in the immediate postoperative period, but pain scores were not recorded.1568
Although it is optimal to completely avoid opioids after sleep surgery, some UA procedures are associated with significant postoperative pain requiring multimodality therapy. If necessary, experts agree that opioids should be given in a setting where oximetry can be monitored, especially for intramuscular or intravenous routes of opioid administration.1519 Furthermore, if systemic opioids are administered through a patient-controlled device, background fixed-rate infusions should be avoided or strictly monitored.1519
Alternative pain medications are recommended to replace or reduce opioid requirement in the postoperative period after sleep surgery. Several RCTs have compared the efficacy and safety of intranasal butorphanol, a synthetic opioid agonist-antagonist that does not cause dose-related respiratory depression, with commonly used medications such as meperidine and fentanyl. In the immediate postoperative period following UPPP, intranasal butorphanol was shown to be equally efficacious to oral mefenamic acid and intramuscular meperidine in pain relief1549,1569 and additionally, reduced the incidence of postoperative cognitive dysfunction in patients undergoing sleep surgery.1569
Non-opioid analgesics, such as intravenous ketorolac, have also been shown to be safe and non-inferior to meperidine and mefenamic acid.1570 Additionally for patients undergoing UPPP, the intraoperative and postoperative administration of a selective COX2 inhibitor, parecoxib, as well as the preemptive submucosal infiltration of ropivacaine have both been shown to reduce postoperative pain compared to placebo.1571,1572 Local ropivacaine infiltration has also been shown to reduce postoperative patient-controlled consumption of morphine.1571
Multiple studies have demonstrated the safety and efficacy of dexmedetomidine in achieving analgesia in the ICU setting and during non-airway surgeries,1573–1575 but investigations in patients undergoing sleep surgery have not shown any reduction in opioid requirements with dexmedetomidine use.1576
In the tonsillectomy literature, two SRs from 2011 and 2019 evaluated RCTs on postoperative analgesic regimens with overall recommendations supporting a multimodal strategy including acetaminophen, non-steroidal antiinflammatory drugs, as well as dexamethasone.1577,1578. Finally, other suggested pain control alternatives mentioned in the 2019 expert consensus statement include intraoperative ketamine, intraoperative/postoperative intravenous lidocaine, ice application, magnesium, and alpha-2 agonists.1521,1579,1580,1582 (Table IX.B.4)
TABLE IX.B.4.
Pain management after sleep surgery
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Diakos et al.1577 | 2011 | 1a | SR | RCTs (n = 580) with adults undergoing tonsillectomy where peri-operative steroids were used | Pain level compared dexamethasone use with control or placebo | Dexamethasone significantly reduced pain and postoperative nausea and vomiting in adults undergoing tonsillectomy. |
| Mason et al.1425 | 2015 | 1a | SR | RCTs (n = 293) with adult OSA patients where participants were randomly assigned to use opiates or opioids, sedatives, hypnotics, or placebo | Effect on OSA severity | No evidence suggests that the pharmacological compounds assessed have a deleterious effect on the severity of OSA as measured by change in AHI or ODI. Significant clinical and statistical decreases in minimum overnight SpO2 were observed with remifentanil, zolpidem 20 mg and triazolam 0.25 mg. |
| Tolska et al.1583 | 2019 | 1a | SR | RCTs (n = 1816) reporting on analgesics and dexamethasone for post-tonsillectomy pain in adults and adolescents (>13 years) | Pain intensity | Paracetamol, gabapentinoids, and dexamethasone reduced pain on the day of surgery. Ketoprofen, ibuprofen, indomethacin, lornoxicam, parecoxib, rofecoxib, and dextromethorphan reduced pain intensity, need for rescue analgesics, or both on the day of surgery. Dexamethasone in multiple doses provided analgesia beyond the first postoperative day. |
| Titirungruang et al.1578 | 2019 | 1a | SR | RCTs (n = 6327) of steroids in adults and children who underwent tonsillectomy | Pain, nausea/vomiting, hemorrhage outcomes | IV steroids significantly decreased immediate postoperative pain severity, as well as nausea/vomiting. |
| Yang et al.1569 | 2015 | 1b | RCT | OSA patients (n = 260, 65 in each group) who underwent H-UPPP 1. Intranasal butorphanol 2. Intravenous butorphanol 3. Intranasal fentanyl 4. Intravenous saline (control) |
Postoperative day 1, 6, 12, 18, 24, 36, 48 – hour pain scores: VAS and Brureggemann comfort scale Cognitive dysfunction evaluated by Mini-Mental Status Examination assessed 1 day before, and 1, 3, and 7 days postoperatively |
Intranasal administration of butorphanol is safe and effective, reducing postoperative usage of analgesic and the incidence of postoperative cognitive dysfunction in patients undergoing H-UPPP. |
| Huang et al.1549 | 2009 | 1b | RCT | OSA patients (n = 12) who underwent UPPP 1. Transasal butorphanol (n = 7) 2. Oral mefenamic acid and intramuscular meperidine (n = 5) |
Pain scores at 12, 24, 72-h postoperatively VAS Clinical Global Impression in Severity and Improvement Postoperative pain-related morbitidies (PRM) and quality of life in bodily pain (QOL-BP) were evaluated 72-h postoperatively |
Transnasal butorphanol can safely alleviate wound pain after UPPP. No significant difference in degree of pain relief was found between the two groups. |
| Lee et al.1570 | 2007 | 1b | RCT | Patients who underwent surgical OSA treatments (n = 90, 45 in each group) 1. Intravenous ketorolac 2. Oral mefenamic acid and intramuscular meperidine |
Postoperative discomfort by self-assessment questionnaire on 1st and 3rd days after surgery Patient satisfaction with postoperative pain treatment evaluated at 1 month postoperatively |
Short-term administration of intravenous ketorolac is noninferior to the conventional regimen and represents a safe treatment for wound pain after OSA surgery. |
| Xie et al.1572 | 2013 | 1b | RCT | UPPP patients (n = 40, 20 in each group) randomized to two groups 1. local block with ropivacaine + saline 2. local block with ropivacaine + intra- and postoperative parecoxib infusion |
VAS pain score at 24 and 48 h after surgery; postoperative adverse effects | Intravenous parecoxib combined with incision-local ropivacaine provided effective postoperative analgesia. |
| Li et al.1571 | 2014 | 1b | RCT | UPPP patients (n = 50, 25 in each group) randomized to 2 groups 1. submucosal infiltration of ropivacaine + epinephrine 2. submucosal infiltration of saline + epinephrine |
Cumulative patient-controlled morphine consumption, VAS scores at 4, 8, 12, 24, 48 h postoperatively at rest and with swallowing, opioid-related adverse effects | Patients who received preemptive submucosal infiltration with 0.33% ropivacaine expressed significantly decreased pain scores and also demonstrated lower morphine consumption postoperatively. |
| Chawla et al.1576 | 2010 | 3b | Retrospective case–control | Patients who underwent OSA surgery (n = 268) 1. Dexmedetomidine used (n = 125) 2. Dexmedetomidine not used (n = 143) |
Mean arterial pressure Use of anti-hypertensives Use of opioids | Dexmedetomidine improves hemodynamic stability in patients undergoing sleep surgery. No difference was noted in opioid dosage between case and control groups. |
IX.C |. Surgical Planning: Level of Obstruction Assessment
IX.C.1 |. Physical examination for surgical planning
Physical examination has long been considered a critical component in the assessment of patients pursuing surgical intervention for OSA. It is vital in identifying any anatomic abnormality that may contribute to UA obstruction. However, to date, the physical exam has had limited utility in determining the site and mechanism of obstruction. Here, we identify the literature that describes physical examination findings for assessment of surgical treatment of OSA.
BMI
Obesity and an elevated BMI have long been known to be a factors in the pathophysiology of UA obstruction leading to OSA and surgical outcomes related to its treatment. In Friedman’s scoring system, a BMI above 40 is considered exclusion criteria for patients undergoing UPPP.814, 1485 Part of the rationale behind this association is related to potential fat deposition in the tongue and parapharyngeal space. Cadaveric studies indicate that as BMI increases, adipose tissue may accumulate in the tongue, increasing the potential for hypopharyngeal obstruction.208 Additionally, literature suggests that increasing BMI is associated with greater tendency for complete concentric collapse (CCC) of the velum on druginduced sleep endoscopy (DISE), which may predict an unfavorable phenotype in surgical outcomes.1584 Patients with obesity often have other comorbidities such that bariatric surgery and weight management may be more appropriate and result in a greater likelihood in resolution of OSA.
Facial exam
External examination allows for the identification of craniofacial abnormalities such as deformities of the external nose, midface hypoplasia, retrognathia, large neck circumference, and low position of the hyoid relative to the mandibular plane. Exam findings of retrognathia and midface defects contribute to considerations for skeletal surgery and can be used in combination with cephalometry or CBCT imaging craniofacial analyses.1585 While craniofacial abnormalities (such as a low hyoid to mandibular plane) have been studied in the context of alternative OSA treatments, there is conflicting data in their abilities to predict outcomes with MAD or with surgical interventions.1585,1586
Nasal exam
Nasal obstruction can play a significant role in the pathophysiology and management of OSA. While correction of nasal obstruction alone does not significantly reduce the overall AHI, it can improve sleep quality and tolerance of CPAP (Section IX.D.2).545,1587,1588
The nasal exam aims to identify anatomic abnormalities in patients with nasal obstruction. Examination of the external nasal structure as well as anterior rhinoscopy with a nasal speculum and headlight are essential parts of the nasal exam. Anterior rhinoscopy allows for identification of turbinate hypertrophy and evaluation of septal deviation, which can occur as a spur along the maxillary crest, a high septal deflection, or a caudal obstruction. The Cottle and modified Cottle maneuvers are useful in assessing for internal nasal valve collapse. Nasal examination can be further enhanced with use of nasal endoscopy, which may help identify nasal polyps, inflammation associated with chronic sinusitis, posterior nasal septal deviations, enlarged adenoid tissue, and in rare cases, tumors.
Oral cavity/oropharyngeal exam
Examination of the oral cavity allows identification of the shape of the hard palate, size of the tongue, and presence and size of palatine tonsils (PTs). Identification of enlarged PTs may identify the OP as the primary source of obstruction (both in children and adults) and suggest tonsillectomy as a reasonable initial surgical option. Additionally, oral cavity and oropharyngeal examination allows assessment of Mallampati and FTP, which in combination with tonsillar size, has been used in the Friedman scoring system (FSS). Lower FSS scores have been associated with improved outcomes in UPPP and by extension may identify the OP and palate as the principal sources of UA obstruction.327,814,1589,1590 Anthropometric measurement can be taken in the OP and may have predictive value in severity of OSA.1591 However, there remains no correlation with site and mechanism of collapse.
Laryngoscopic exam
The use of a fiberoptic endoscope can be used to examine the entire UA from the nose to the subglottis. It can be used at the level of the velum to identify maxillary constriction (narrowed distance between posterior edge of hard palate and posterior pharyngeal wall) as well as the oblique versus vertically oriented palate.1592 This technique can also be used to identify enlarged lingual tonsillar tissue557,1593,1594 and epiglottis anatomy such as an omega shaped epiglottis.1595 Other abnormalities of the UA, such as laryngeal stenosis, vocal fold paralysis, inflammatory supraglottic changes, and neoplastic masses, can be identified with fiberoptic endoscopy.1596 An adjunct to this exam is the Mueller maneuver which acts to simulate UA obstruction.1597 However, the Mueller maneuver does not readily reproduce the pattern of airway collapse associated with physiologic sleep and has not been shown to correlate with surgical outcomes related to site of obstruction.1598–1600
Physical examination remains an important adjunct in the evaluation of patients pursuing options for treatment of OSA. However, there is limited evidence that supports the use of awake physical examination for identifying the source or character of obstruction as a predictor for surgical intervention and OSA outcomes. (Table IX.C.1)
TABLE IX.C.1.
Evidence for physical exam for surgical planning
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Friedman et al.1485 | 2002 | 4 | Retrospective cohort | Retrospective analysis without control group | Surgical success | The use of clinical staging system identifies potential for success or failure with UPPP. |
| Friedman et al.814 | 2004 | 4 | Prospective cohort | Prospective analysis without control group | Surgical success | The use of clinical staging system identifies potential for success or failure with UPPP and those for whom tongue base intervention should be added to intervention. |
| Kim et al.208 | 2014 | 3a | Case–control | Obese non-apneic (control) and obese apneic patients (study) | Volume of tongue fat | Increase deposition of tongue fat in apneics compared to obese non-apneic controls. |
| Vroegop et al.1584 | 2014 | 4 | Observational study | Patients undergoing DISE | Patterns of collapse identified during DISE | Overview of patterns of collapse in a large cohort of SDB patients who underwent DISE. |
| Denolf et al.1585 | 2016 | 3a | Meta-analysis | Review of available literature on cephalometry | Using cephalometry to predict outcomes of MAD | Cephalometric patterns cannot reliably predict treatment outcomes from MAD. |
| Hong et al.1586 | 2016 | 3a | Systematic review | Review of available literature on upper airway evaluation in patients with OSA | Use of multiple modalities for evaluation of OSA | Physical examination cannot reliably predict site and mechanism of obstruction. |
| Ishii et al.545 | 2015 | 3a | Meta-analysis | Ten studies included | AHI, RDI, ESS | Isolated nasal surgery offered improvement in ESS and RDI. No improvement in AHI. |
| Kim et al.1587 | 2004 | 4 | Case series | Subjects undergoing nasal surgery Pre- and postoperative sleep study |
RDI Apnea index (AI) Oxygen desaturation index (ODI) |
Significant improvement in RDI, AI, and OSI with nasal surgery. |
| Osborn et al.1588 | 2013 | 5 | Expert opinion | Discussion of various medical and surgical treatment options for nasal obstruction. | ||
| Nuckton et al.1590 | 2006 | 2b | Prospective case series | Patients evaluated at Sleep Center | Likelihood of Mallampati score to predict OSA | Increased Mallampati score associated with risk of OSA diagnosis and increased severity. |
| Holmlund et al.1589 | 2016 | 2b | Multicenter prospective interventional study | Adult patients with tonsillar hypertrophy and OSA undergoing tonsillectomy | Resolution of OSA and reduction in AHI | Significant improvement in AHI. |
| Jara and Weaver327 | 2018 | 2c | Retrospective cohort study | Patients undergoing tonsillectomy for OSA | Association between palatine tonsil (PT) grade and volume and OSA | PT grade associated with PT volume and AHI. PT volume not associated with AHI. |
| Friedman et al.814 | 2004 | 2b | Prospective cohort study | One cohort of patients evaluated preoperatively using Friedman staging system and one cohort not | Surgical success rates | Cohort evaluated preoperatively with Friedman staging system and managed accordingly had improved surgical outcomes. |
| Olszewska et al.1592 | 2019 | 5 | Expert opinion | Review of palate anatomy. | ||
| Dündar et al.1593 | 1996 | 5 | Case report | Patient with OSA and lingual tonsillar hypertrophy | Surgical outcome | Improvement in disease severity. |
| Tang and Friedman557 | 2018 | 2c | Retrospective cohort study | Patients with lingual tonsillar hypertrophy and OSA compared to those without OSA | Association of lingual tonsil size and OSA diagnosis | No significant correlation between lingual tonsil size and diagnosis of OSA. |
| Friedman et al.1594 | 2017 | 5 | Expert opinion | |||
| Delakorda and Ovsenik1595 | 2019 | 2c | Retrospective cohort study | Evaluation of epiglottis shape in patients with OSA undergoing DISE | Epiglottic shape in those patients with tongue base and epiglottic obstruction seen on DISE | Many patients with tongue base and epiglottic obstruction on DISE had abnormal epiglottis shape (flat). |
| Hsu et al.1597 | 2004 | 2b | Prospective cohort study | Quantitative computer-assisted videoendoscopy performed in patients with OSA and compared to cohort without OSA | Retropalatal and retrolingual airway measurements and their association with OSA | Retropalatal and retrolingual measurements found predictive of OSA. |
| Fernández-Julián et al.1598 | 2014 | 2b | Prospective cohort study | Comparison of surgical recommendations based on clinical evaluation vs. DISE | Correlation of surgical recommendation and type of evaluation | DISE evaluation provided more information for surgical planning, particularly at the level of the hypopharynx and larynx. |
| Yegïn et al.1600 | 2017 | 2c | Retrospective cohort study | OSA patients undergoing evaluation with Muller’s maneuver and DISE | Difference in upper airway obstruction findings with Muller’s maneuver and DISE | DISE may have improved ability to diagnose site(s) and configuration of upper airway collapse when compared to Muller’s maneuver. |
| Soares et al.1599 | 2013 | 2c | Retrospective cohort study | Patients with OSA undergoing office evaluation with Muller’s maneuver and DISE | Location in airway obstruction | Significant difference in finding of severe retrolingual obstruction seen on DISE compared to Muller’s maneuver. |
IX.C.2 |. Drug-induced sleep endoscopy (DISE)
IX.C.2a |. DISE methods
Assessment of the site(s) of obstruction is critical to successful surgical treatment of OSA. Multiple evaluation techniques have been developed to examine an individual’s pattern of UA obstruction; each has important strengths and weaknesses. Traditional evaluation of the UA with physical examination techniques performed in the office are commonly performed during wakefulness and include largely static observations rather than dynamic assessments. As such, they may not be ideal methods to assess the UA during breathing and sleep.
Flexible fiberoptic endoscopy of the UA during natural sleep has been performed in research and limited clinical settings,1601–1604 but logistical challenges have prevented broad clinical adoption. In 1991 Croft and Pringle described the technique of “sleep nasendoscopy” as the fiberoptic examination of the UA under conditions of spontaneous ventilation and pharmacologic sedation.1605 This has been renamed DISE,1606 based on the use of sedation to achieve physiologic changes similar to natural sleep during the performance of endoscopy.
Consensus-based recommendations have been offered regarding many technical aspects of DISE, albeit mostly guided by expert opinion.1607 This section will focus on the choice of sedative agent, as DISE has been performed with a variety of agents, most often using propofol or a combination of midazolam and propofol but with more-recent interest in dexmedetomidine.
UA patency relies on pharyngeal dilator muscle tone and changes in lung volume that counteract collapsing forces, principally intraluminal negative pressure generated during inspiration and anatomical narrowing of the airway.1608 OSA patients maintain pharyngeal patency with greater dilator (genioglossus) muscle tone during wakefulness, but sleep onset results in marked decreases in muscle tone due to loss of the wakefulness stimulus and decreases in negative pressure reflex activity and lung volume.396,1609–1614 Because the purpose of DISE is to evaluate the pattern of obstruction in OSA, DISE’s accuracy depends on reproducing the changes in UA physiology seen in natural sleep.
Most anesthetic agents have a wide spectrum of action from sedation to general anesthesia, with dose-dependent effects on muscle tone, airway collapsibility, responses to chemosensory input, and airflow. A recent review of sedative agents commonly used in DISE focused on propofol, midazolam, and dexmedetomidine and included a discussion of physiologic changes and their comparison to natural sleep.1615 The interest in transition from wakefulness to unconscious sedation is based on the concept of a thalamocortical switch determining consciousness or unconsciousness (no response to verbal stimulation) that may be common to natural sleep and sedation.1616,1617
Hillman found that increasing levels of sedation with propofol are consistent with a switch, as changes in UA collapsibility, levels of EEG activity, and muscle tone occurred at this transition from consciousness to unconsciousness disproportionate to changes in propofol concentration.1618 Unconscious sedation with propofol is associated with decreases in genioglossus tone to 10% of maximum awake activity,1618,1619 which is one-half to one-third of tone seen during non-REM sleep in individuals with no sleep apnea,1610 but greater than during REM sleep in patients both with and without OSA.187 Rabelo showed that low doses of propofol have been associated with similar levels of SDB as seen in natural sleep,1620 and this study and another by Berry showed that propofol did not induce snoring or airway obstruction in those without SDB during natural sleep.1620,1621 Propofol does induce changes in EEG waveforms,1622 making a comparison to sleep staging difficult or impossible; it is unclear whether this is relevant to DISE as a surgical evaluation technique.
Midazolam also induces EEG waveform changes,1623 but moderate doses of midazolam in a small study of males with OSA produced similar levels of SDB (AHI) and UA collapsibility to those seen in natural sleep.1603,1624
Dexmedetomidine has been examined in DISE, based on its use in many procedures requiring sedation. Dexmedetomidine has demonstrated similar EEG waveforms compared to natural sleep1625 but likely does not produce the same changes in UA muscle tone that occur in natural sleep.1626
Two studies have examined potential associations between sedative agent and DISE findings based on DISE performed in the same study participants with different sedative agents.1627 Yoon et al. showed excellent agreement between findings with propofol versus dexmedetomidine,1628 whereas Viana found a greater degree of tongue-related obstruction with propofol and midazolam versus dexmedetomidine but otherwise found no differences.1629
Overall, propofol has been the most-studied sedative agent, with some similarities in UA physiology to those seen in natural sleep at the transition to unconsciousness. Studies performed with propofol, midazolam, or a combination have demonstrated validity1621,1630 and moderate to good test–retest and inter-rater reliability.1631–1633 However, more research with all sedative agents will help to define their roles in DISE.
DISE classification scheme
For many years, surgical evaluation techniques focused on categorizing patients according to the Fujita classification system that encompasses the two primary regions of pharyngeal UA obstruction: the palatal/velopharyngeal and hypopharyngeal/retroglossal/retrolingual regions.1634 However, there are two major limitations of region-based classifications. First, there is substantial anatomical overlap between these regions, including the extension of the lateral pharyngeal walls throughout the length of the pharynx and the physical overlap of the tongue and soft palate. Second, a region-based approach may not direct surgical treatment adequately. For example, in patients with hypopharyngeal/retroglossal/retrolingual obstruction, the oropharyngeal lateral walls, tongue, and epiglottis can each play a prominent role.
There has been a long-standing interest in describing the pattern of obstruction during DISE, and multiple groups have proposed grading systems with varying degrees of complexity.1605,1635–1637 Initially, these focused on the regions outlined by Fujita1605,1636 but some of these incorporated specific combinations of pharyngeal structures1635 or the entire UA (Nose OP Hypopharynx and Larynx classification, or NOHL).1637
Because DISE is an examination of the pharynx and because pharyngeal procedures may exert differential effects on pharyngeal structures, distinguishing between the structural contributions may play a critical role in procedure selection and improvement of outcomes. In 2011, Kezirian et al. developed the VOTE classification (Figure IX.C.2) to characterize the four most common structures that play a role in pharyngeal airway obstruction: the velum, OP lateral walls, tongue, and epiglottis.1627 The VOTE classification encompasses these structures in various combinations of the degree of airway narrowing (none, partial, or complete) and configuration (anteroposterior, lateral, or concentric [combination of anteroposterior and lateral]). It is important to note that epiglottis-related obstruction can be defined as limited to those cases in which the epiglottis obstructs the airway independently or as occurring additionally in cases where another structure (such as the tongue) mechanically displaces the epiglottis to obstruct the airway in combination; it is not clear which definition is better, but any study should identify the choice and maintain consistency.
FIGURE IX.C.2.
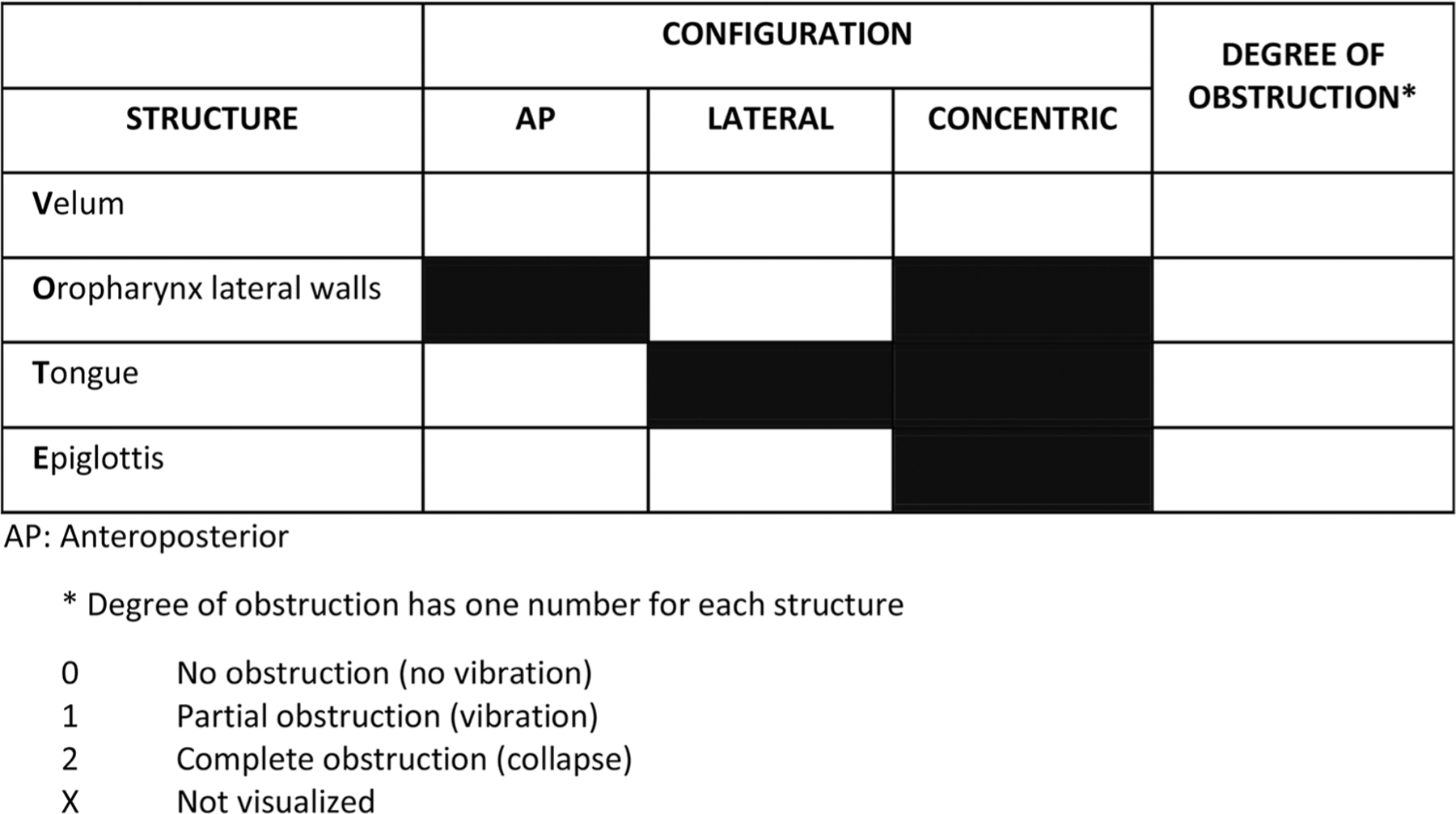
The VOTE classification1627
Although each of these structures represents a combination of numerous muscles and other tissue types, the VOTE classification is based on the way these structures may be visualized during DISE. The VOTE classification is used most commonly around the world, but ultimately the choice of a system or any improvements must be based on research identifying the specific DISE findings associated with treatment outcomes.
DISE findings and associations
DISE findings differ from one individual to another, likely reflecting differences in the anatomical contribution to OSA across individuals. However, one might expect there to be associations between DISE findings and clinical characteristics, principally AHI and BMI. Vroegop has provided the only study of sufficient size to explore these associations among 1249 study participants.1584 Increasing AHI was associated with greater odds of: obstruction related to multiple VOTE structures; complete versus partial degree of obstruction related to the velum, oropharyngeal lateral walls, and tongue (especially complete concentric velum-related obstruction); and oropharyngeal lateral wall-related obstruction. In the same study, they also showed that increasing BMI was associated with: greater odds of complete concentric velum-related obstruction; greater odds of oropharyngeal lateral wall-related obstruction; and lesser odds of complete tongue-related obstruction.
Cadaveric1638 and live208,343 human studies have demonstrated that fat deposition can occur in tissues surrounding the UA, especially within the tongue itself. While the tongue has been studied most thoroughly, it is likely that the same fat deposition occurs in other structures of the head and neck. Fat deposition would be expected to increase the volume of tissues, increasing OSA severity (AHI) and the contributions of the enlarged structures to airway obstruction. In this sense, it is somewhat surprising that increasing BMI was associated with lesser odds of complete tongue-related obstruction, but the apparent lesser odds may reflect an even greater odds of obstruction from other structures (such as the oropharyngeal lateral walls).
IX.C.2.b |. DISE findings inform surgical outcomes
The key to any surgical evaluation technique is the extent to which findings are associated with outcomes of surgery. Despite almost three decades of experience with DISE, the literature on the association with surgical outcomes is limited (Table IX.C.2.b). Single-center cohort studies have examined AHI outcomes for palate surgery (almost all study participants) with or without additional procedures.1636,1639–1644 These studies have generally (but not universally) shown that palate surgery outcomes are improved in the presence of velum-related obstruction only during DISE, with the exception of complete concentric velum-related obstruction that has been associated with poorer outcomes.
TABLE IX.C.2.b.
Association between DISE findings and surgical outcomes
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Iwanaga et al.1636 | 2003 | 4 | Cohort | Region(s) of obstruction during DISE (n = 60) | Postoperative reduction in AHI | Anteroposterior velum- and tonsil-related obstruction associated with better outcomes after palate surgery than complete concentric velum-related obstruction or multilevel obstruction. |
| Hessel and Vries1640 | 2004 | 4 | Cohort | Region(s) of obstruction during DISE (n = 55) | Postoperative AHI < 20 | Hypopharyngeal obstruction associated with poorer outcomes after palate ± other procedures. |
| Koutsourelakis et al.1642 | 2012 | 4 | Cohort | DISE findings based on VOTE classification (n = 49) | Postoperative AHI <10 and AHI reduction >50% | Complete circumferential velum-related obstruction and complete tongue-related obstruction associated with poorer outcomes after palate ± other procedures. |
| Soares et al.1643 | 2012 | 4 | Cohort | DISE findings based on VOTE classification (n = 34) | Postoperative AHI <20 and AHI reduction >50% | Complete oropharyngeal lateral wall-related obstruction and complete epiglottis-related obstruction associated with poorer outcomes after various procedures. |
| Zhang et al.1644 | 2015 | 4 | Cohort | DISE findings based on VOTE classification (n = 43) | Postoperative AHI <20 and AHI reduction >50% | DISE findings not associated with outcomes of palate procedures. |
| Blumen et al.1639 | 2015 | 4 | Cohort | DISE findings based on VOTE classification (n = 24) | Postoperative AHI <10 and AHI reduction >50% | Complete circumferential velum-related obstruction and complete tongue-related obstruction associated with poorer outcomes after tonsillectomy ± palate surgery. |
| Hsu and Jacobowitz1641 | 2017 | 4 | Cohort | DISE findings based on VOTE classification (n = 38) | Postoperative AHI <20 and AHI reduction >50% | Complete circumferential velum-related obstruction associated with smaller, but still significant, AHI reduction following palate surgery compared to noncircumferential collapse. |
| Green et al.1646 | 2019 | 2b | Cohort | DISE findings based on VOTE classification (n = 275) | Postoperative AHI <15 and AHI reduction >50% | Oropharyngeal lateral wall-related obstruction (partial or complete) and complete tongue-related obstruction were associated with poorer outcomes, with and without adjustment for type of surgery. |
| Huyett et al.1647 | 2020 | 2b | Cohort | DISE findings based on VOTE classification (n = 343) | Postoperative AHI <15 and AHI reduction >50% | Complete (vs. partial/none) oropharyngeal lateral wall-related obstruction was associated with poorer outcomes, and complete (vs. partial/none) tongue-related obstruction was associated with better outcomes of hypoglossal nerve stimulation. |
Unfortunately, these studies were limited by multiple concerns: single-center studies relying on the DISE scoring of the unblinded operating surgeon, small sample size and limited statistical power, and the incorporation of multiple combinations of procedures without the ability to perform statistical adjustment for the type of procedure. To address these limitations, two recent larger, multicenter cohort studies incorporated DISE video reviews by multiple surgeons blinded to the type of procedure and surgical outcome. Of note, both studies excluded adults with marked tonsillar hypertrophy (3+ or 4+ on the Brodsky1645 or Friedman1485 scales), where DISE may not be able to visualize other structures contributing to airway obstruction, aside from the tonsils themselves due to their large size. Both studies included adjustment for tonsil size, the factor most clearly associated with surgery outcomes.
The first study examined pharyngeal surgery and showed that complete or partial (vs. none) oropharyngeal lateral wall-related obstruction was associated with poorer surgical outcomes (adjusted OR 0.51; 95% CI 0.27, 0.93).1646 Complete tongue-related obstruction was associated with a lower odds of surgical response in moderate to severe OSA (adjusted OR 0.52; 95% CI 0.28, 0.98), with findings that were similar but not statistically significant in other analyses. Among numerous statistical analyses was the suggestion that tongue resection procedures may be associated with better outcomes in the presence of complete tongue-related obstruction. Surgical outcomes were not clearly associated with the degree and configuration of velum-related obstruction or the degree of epiglottisrelated obstruction. Limitations of the study include an inability to differentiate specific palate surgery techniques to identify whether these techniques had differential associations with DISE findings (e.g., whether newer palate surgery techniques can address oropharyngeal lateral-wall related obstruction).
The second study examined HNS and AHI outcomes on the post-implantation titration polysomnogram. Complete (vs. partial/none) tongue-related obstruction was associated with increased odds of treatment response (78% vs. 68%, p = 0.043).1647 Complete (vs. partial/none) oropharyngeal lateral wall-related obstruction was also associated with lower odds of surgical response (58% vs. 74%, p = 0.042). Limitations of this study include the fact that the analysis was based on titration sleep study outcomes rather than full-night efficacy studies.
Both of these studies have limitations, and there will be benefits to additional research, including studies with larger sample sizes that may increase statistical power to analyze DISE findings for velum- and epiglottis-related obstruction. However, these two cohort studies provide higher-level evidence indicating that certain DISE findings are associated with surgical outcomes. (Table IX.C.2.b)
IX.C.2.c |. DISE for treatment planning and to improve outcomes
Three studies have examined whether DISE changes the treatment plan for adults considering alternatives to PAP therapy, finding that DISE changed the treatment plan in a substantial proportion of study participants (Table IX.C.2.c.1).1598,1648,1649 A more-important question is whether DISE improves surgical outcomes, a question addressed by two other studies (Table IX.C.2.c.2). One single-center study showed that DISE was potentially associated with more-aggressive surgery without a difference in outcomes.1650 A multi-center study showed better AHI and BP outcomes in a group undergoing surgery without DISE, compared to those undergoing DISE, in spite of the latter undergoing more tongue-directed procedures (but fewer nasal procedures).1651 Importantly, there was statistical adjustment for age, gender, and BMI in the latter study, but not for tonsil size.
TABLE IX.C.2.c.1.
Studies examining whether DISE changes the treatment plan
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Gillespie et al.1649 | 2012 | 4 | Case series | DISE findings based on multiple classifications. (n = 37) | Change in surgical treatment plan | Change in 62% (23/37) overall, including all with epiglottis-related obstruction during DISE (n = 14). |
| Eichler et al.1648 | 2013 | 4 | Case series | DISE findings based on VOTE classification. (n = 97) | Change in treatment plan (surgery and/or oral appliance) | Change in 78% (76/97) overall. |
| Fernández-Julián et al.1598 | 2014 | 4 | Cohort | DISE findings based on own classification. (n = 162) | Change in surgical treatment plan | Change in >40% overall. |
Because DISE is an evaluation tool for treatment selection, sleep endoscopy is primarily indicated for those patients who do not tolerate PAP therapy who are considering multiple options, such as various procedures (and possibly the use of MRDs). If BMI or other factors (e.g., large tonsils) would limit the choice of options to a single primary approach, DISE is not generally recommended because it may not inform treatment selection.
Ultimately, understanding when and how to use DISE is dependent on studies of the association between DISE findings and surgical outcomes. The recent multicenter studies may have more clearly delineated the role of DISE in procedure selection and, more importantly, counseling patients regarding surgical outcomes (e.g., recommending specific approaches or outcome expectations in the presence of complete oropharyngeal lateral-wall related obstruction). If so, systematic protocols and rigorous studies are needed to determine when and how to use DISE in improving treatment outcomes. (Table IX.C.2.c.1 and IX.C.2.c.2)
IX.D |. Surgical Treatment for OSA
IX.D.1 |. Sinus surgery
CRS and OSA have been demonstrated to negatively impact patient reported sleep quality.1434 Endoscopic sinus surgery, in turn, has been shown to improve patient reported sleep quality for CRS patients through an assortment of patient reported outcome measures (PROMs) such as the ESS, the PSQI, and the Sinonasal Outcomes Test-22.915 Despite patient reported improvements in validated PROMs, data supporting the use of endoscopic sinus surgery as a surgical therapy to improve objective OSA measures is lacking. Studies examining the impact of endoscopic sinus surgery on PSG measures are limited by small sample sizes, heterogenous patient populations (CRS without nasal polyposis, CRS with nasal polyposis), and variable surgical approaches (i.e., polypectomy vs. complete/full endoscopic sinus surgery, concurrent septoplasty).1652 Of the studies that evaluate objective parameters such as the AHI, very few include endoscopic sinus surgery alone.920,1653–1655 In the Tosun et al. case series, the mean AHI score did not significantly change following surgery. AHI scores decreased in 11 patients, increased in 11 patients, and did not change in five patients in the postoperative period. Jiang et al. found a statistically significant reduction in AHI in CRS patients with moderate OSA. Yalamanchali et al. investigated concurrent septoplasty and inferior turbinate reduction and endoscopic sinus surgery in 57 patients.921 Patients with moderate and severe OSA were found to have statistically significant reductions in AHI, however no patient in this study achieved a reduction in their OSA severity category following surgery. In an SR of three articles evaluating AHI outcomes after endoscopic sinus surgery, the effect size, as measured by the SMD for sinus surgery was −0.20, indicating a trivial to small reduction in AHI for CRS patients with nasal polyposis undergoing sinus surgery.1652 There is a paucity of high-quality data to support the use of endoscopic sinus surgery to improve objective measures of OSA severity.
In contrast to the nominal impact of endoscopic sinus surgery on AHI outcomes, data examining the impact of surgery on validated outcomes measures including the ESS and PSQI are promising. Several prospective cohort studies have evaluated sleep outcomes with the ESS.919–921 Each of these studies demonstrated significant improvement in subjective sleep as measured by the ESS. These results are best summarized in the MA from Sukato et al. in which the SMD was −0.94 indicating a large and statistically significant improvement following sinus surgery. Similarly, Alt et al.,915,1434,1656 Little et al.,1657 and Rotenberg and Pang919 demonstrated statistically significant improvements in the PSQI. The SMD of −0.80 in the Sukato MA confirmed a large and statistically significant improvement in the PSQI. Together, these outcomes are consistent with a robust improvement in sleep quality for CRS patients undergoing endoscopic sinus surgery. (Table IX.D.1)
TABLE IX.D.1.
Evidence for endoscopic sinus surgery for the treatment of obstructive sleep apnea
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Series et al.1658 | 1993 | 4 | Case series | Patients undergoing nasal airway surgery or polypectomy | RDI Oxygen saturation REM Number of arousals Total apnea index Cephalometric measurements | Increase in REM. No differences in other measures. |
| Tosun et al.920 | 2009 | 4 | Case series | CRSwNP with body mass index less than 28 kg/m2 | VAS (snoring) EpSS AHI Oxygen saturation Number of arousals % of sleep stages Rapid eye movement stage | Snoring VAS and EpSS significantly improved. No difference noted in AHI after polypectomy and sinus surgery. No difference in saturation, arousals, sleep stages or eye movement. |
| Gunhan et al.1653 | 2011 | 4 | Case series | CRSwNP. OSA patients excluded | AHI Erectile dysfunction | AHI Significantly reduced from 2 to 1.5. |
| Yalamanchali et al.921 | 2014 | 4 | Case series | Mild OSA Moderate OSA Severe OSA | AHI Oxygen saturation Sleep efficiency Sleep parameters |
AHI was statistically significantly reduced in severe and moderate AHI categories. “Successful surgery”, defined as a reduction in AHI <20 or a 50% reduction in AHI was only achieved in 3.6% of patients. No patients achieved a change in OSA severity. |
| Uz et al.1655 | 2017 | 4 | Case series | CRSwNP undergoing sinus surgery | 1. PSQI 2. AHI |
AHI decreased significantly from 13.3 to 11.2 at 6 months postoperatively |
| Sukato et al.1652 | 2018 | 3a | Systematic review of case series | 1. AHI 2. PSQI 3. ESS |
Random effects meta-analysis suggests small improvement after surgery. | |
| Jiang and Liang1654 | 2019 | 4 | Case series | Non-OSA vs. mild, moderate, and severe OSA | 1. Sinonasal outcomes test -−20 2. AHI |
Increase in AHI in non-OSA patients. Statistically significant decrease in moderate OSA patients |
| Alt et al.1656 | 2014 | 4 | Case–contol | CRSwNP and CRSsNP | PSQI | Significant improvement in PSQI |
| Alt et al.1434 | 2017 | 4 | Case–control | CRSwNP and CRSsNP Medical vs. surgical management |
PSQI | Significant improvement in PSQI in surgically managed group but not medically managed group |
| Rotenberg and Pang919 | 2015 | 4 | Case series | CRSsNP | PSQI ESS |
Significant improvement in both ESS and PSQI |
| Little et al.1657 | 2020 | 4 | Case series | CRS | PSQI SNOT-22 |
Significant improvement in PSQI, SNOT-22 |
Abbreviations: AHI, apnea/hypopnea index; CRSwNP, chronic rhinosinusitis with nasal polyposis; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; PSQI, Pittsburgh Sleep Quality Index; RDI, respiratory disturbance index; REM, rapid eye movement; VAS, visual analog scale.
IX.D.2 |. Nasal surgery
Nasal obstruction is a known contributor to OSA.275 Studies have shown that airflow resistance in the nasal cavity is significantly higher in patients with OSA, suggesting that surgical correction of nasal obstruction may be an important treatment modality.1659 The goal of nasal surgery is to correct anomalies in the nasal passage and to relieve clinical symptoms of nasal obstruction that have implications in OSA management.906
Impact on OSA severity and symptoms
Three meta-analyses have examined the role of nasal surgery as a treatment for OSA. These studies do not provide consensus on whether nasal surgery is effective at reducing AHI, but all showed significant improvement in ESS. Most studies were limited by small sample sizes and combined a variety of different nasal surgery procedures.
In a 2011 MA, Li et al.906 found no significant change in AHI after nasal surgery. This study suggested that variance in pharyngeal anatomy may be a factor in surgical outcomes. The study did find a significant decrease in ESS. A 2015 MA of 10 studies by Ishii et al.545 also found no significant improvement in AHI but did show significant improvement in RDI and ESS after nasal surgery. The studies analyzed included a variety of nasal surgery techniques and approaches including septoplasty alone, septoplasty with turbinate reduction, and septoplasty with turbinate reduction plus nasal valve reconstruction. The variability of nasal surgeries, along with variable methods for evaluating the response to nasal surgery among the studies, limited the analysis. The most recent 2017 MA by Wu et al.1660 found a significant, but small, decrease in AHI following nasal surgery. This MA included 17 studies with pre- and postoperative quantitative data. There was a statistically significant improvement in AHI of −4.15 events/hour (95% CI −6.48 to −1.83). ESS also improved significantly by −4.08 points (95% CI −5.27 to −2.88). This analysis utilized an analysis that sub-grouped the collected data according to SD of change.
It is important to note that all three meta-analyses found significant improvement in ESS, an important measure of daytime sleepiness related to OSA. Nasal surgery has been shown to improve subjective sleep quality, daytime sleepiness, snoring, and sleep-related QOL measures in patients with nasal obstruction and SDB. Most of the included studies were prospective or retrospective studies without controls with considerable heterogeneity and inconsistent patient selection criteria, increasing the potential for bias. Small sample sizes and short follow-up periods limit generalizability of the data.
The role of nasal surgery in improving PAP tolerance is addressed in Section VIII.A.5.g.
Nasal surgery compared to medical therapy and controls
One non-randomized study compared surgery with septoplasty and inferior turbinate reduction versus medical therapy with sprays, saline lavage, and antihistamines. No significant changes were found in PSG metrics, but subgroup analysis showed patients with lower BMI and lower FTP (1 or 2) were associated with better subjective outcomes for snoring and somnolence after nasal surgery.909
In the only RCT comparing results for patients who underwent septoplasty versus sham surgery, AHI remained unchanged but ESS scores decreased in the group that underwent septoplasty.1661 There have not been any RCTs assessing the effect of turbinate reduction alone on AHI.
Overall, existing pooled analyses have shown mixed results in AHI change after nasal surgery in OSA patients, but certain patients may have a higher chance of surgical benefit. Non-obese BMI, lower FTP, positional dependence of OSA, and baseline mild OSA may be potential predictors for significant improvements in OSA severity after nasal surgery.899,1662 (Table IX.D.2)
TABLE IX.D.2.
Evidence for nasal surgery to treat OSA
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Wu et al.1660 | 2017 | 2a | Meta-analysis | 17 studies (2002–2016): 1 RCT, 2 nonrandomized control, 11 prospective, 3 retrospective | Association of isolated nasal surgery with improvement in apnea hypopnea index (AHI), ESS. | Both AHI and ESS improved significantly after nasal surgery. AHI change 4.15 events/h (−6.5 to −1.8). |
| Ishii et al.545 | 2015 | 2a | Meta-analysis | 10 studies: two RCTs, seven prospective, one retrospective | AHI, RDI, ESS. | No significant improvement in AHI; significant improvement in RDI and ESS. RDI declined by 11 events/h (−16 to −6). |
| Li et al.906 | 2011 | 2a | Meta-analysis | 13 studies (1999–2009): one RCT, one nonrandomized control, nine prospective, two cross-sectional | Changes in nasal resistance, AHI, ESS, snoring. | Changes in AHI after nasal surgery were not statistically significant; variable results in reduction of snoring; statistically significant reduction in ESS. |
| Koutsourelakis et al.1661 | 2008 | 1b | RCT | 49 OSA patients with fixed nasal obstruction due to deviated septum | Association of septoplasty with increased nasal breathing epochs and reduction in AHI. | No significant decrease in AHI; change in AHI after surgery inversely related to change in nasal breathing epochs. |
| Shuaib et al.911 | 2015 | 4 | Case series | 26 patients with septal and nasal valve obstruction | Association between functional septorhinoplasty and AHI. | Significant decrease of 35% in mean postoperative AHI. |
| Yalamanchali et al.921 | 2014 | 4 | Case series | 56 patients with mild, moderate, or severe OSA | Association between combined nasal surgery and endoscopic sinus surgery on measurements of OSA and sleep architecture. | Small, statistically significant reduction in AHI in patients with moderate to severe OSA. |
| Moxness et al.1663 | 2014 | 2b | Prospective cohort | 59 patients with OSA and clinically significant nasal obstruction | Association of either 1) septoplasty alone, or 2) septoplasty with turbinate reduction with OSA parameters. Evaluated 3 months postop. | Significant reduction in AHI in the group that had septoplasty with turbinate reduction (17.4–11.7 postop) Sleepiness improved in both groups. |
| Park et al.1659 | 2014 | 4 | Case series | 25 patients with reduced nasal cavity patency and narrowing of retroglossal or retropalatal airways with OSA | Association of septoplasty and turbinoplasty with improvement in OSA parameters (AHI, RDI, ESS). | Significant decrease in AHI and RDI; reduction in subjective symptoms. ESS improved even in those categorized as AHI non-responders. |
| Li et al.909 | 2009 | 2b | Cohort | 66 patients with OSA and chronic nasal obstruction | Association of nasal surgery with ESS, PSG parameters, AHI, snoring (Snoring Outcome Survey). | Significantly improved SOS and ESS; insignificant changes in polysomnographic parameters. |
| Hu et al.1664 | 2013 | 2b | Cohort | 79 OSA/hypopnea syndrome patients with nasal obstruction | Association of nasal surgery with polysomnographic parameters (AHI), and nasal resistance. | Inconsistent decrease in AHI depending on severity of OSAHS; significant decrease in nasal resistance. |
IX.D.3 |. Tonsillectomy
Historically reserved as a treatment for recurrent infections or tonsillar malignancies, only a few studies have discussed the use of isolated tonsillectomy as a treatment for OSA syndrome. There is, however, a more substantial body of evidence supporting palatine tonsillectomy as part of UPPP,1665,1667–1670,2001 a common surgical treatment for adult OSA. The efficacy of UPPP is augmented when combined with tonsillectomy, resulting in additional improvement in the AHI, daytime sleepiness, and snoring.1665,1667–1670,2001
Several publications have shown an association between the objective tonsil volume, as well as the subjective tonsil grade, and AHI. A PT grading scale has been used to predict OSA severity and the efficacy of tonsillectomy in treating OSA. A higher grade, which corresponds with larger and more exophytic tonsils, corresponds with higher preoperative AHI values and a more significant reduction in AHI after removal.1669,1671 A small series noted improvement in AHI in selected patients with small tonsils after tonsillectomy.1672 Jara and Weaver327 found a strong correlation between the subjective and objective tonsil measures that were consistent with the findings of previous studies, and they suggested that PT grade, rather than volume, may be more predictive of the severity of OSA. Cahali et al. found a correlation between tonsillar hypertrophy and OSA but did not find a correlation between tonsillar size and OSA severity.1673
Tan et al. evaluated the efficacy of tonsillectomy as an isolated surgical treatment for the reduction of the RDI and other sleep study parameters in patients with OSA with significant tonsil hypertrophy (grade 3–4).1674 This study showed that in the group with RDI > 60, an average reduction of RDI by 57.6 events/hour was achieved. They concluded that tonsillectomy alone might be considered as an effective first-line surgical procedure in the treatment of OSA in patients with grade 3 or 4 tonsils, reserving other UA procedures for a later stage if necessary.
An MA by Camacho et al.335 reviewed 17 manuscripts analyzing isolated tonsillectomy for OSA in the adult population, but there was no manuscript with evidence level 3a or above. In their analysis, tonsillectomy for hypertrophied tonsils (or grade 2–4) resulted in AHI decrease of 65.2%. Surgical success was achieved in 85.2% of patients, and 57.4% of the patients were cured. All patients with AHI < 30 were successfully treated with tonsillectomy, while 84% were cured. They concluded that tonsillectomy can be a viable option as the sole treatment for patients with mild-moderate OSA and tonsil hypertrophy.
Holmlund et al.1589 studied 28 patients (ages 18–59 years) with OSA who had an AHI of >10 and large tonsils (grade 3–4) who underwent tonsillectomy. They found a significant decrease in the AHI as well as an improved ESS score. Senchak et al.1500 chose an endpoint of reduction of at least 50% in AHI as well as absolute AHI of <15 events/h and found a beneficial effect for adult tonsillectomy. Nakata et al. examined the efficacy of isolated tonsillectomy in 30 patients with severe OSA and showed a success rate of 40%. In patients with BMI < 25, the success rate was 100%, but tonsillectomy did not affect snoring values.1675 A subsequent study by Nakata et al.1676 examined the role of tonsillectomy on nasal resistance and OSA and found that it was beneficial in reducing both nasal resistance and AHI. Tan et al.1674 who examined the role of tonsillectomy as the sole treatment in 34 patients, as well as Verse et al. who treated 11 patients1677 suffering from OSA, also reported positive results.
Tonsillectomy appears to be an effective treatment for OSA in adults with tonsil hypertrophy. It was shown that tonsillectomy alone can significantly improve the QOL and reduce the AHI in a select population of adults with OSA and tonsillar hypertrophy.1678 Tonsillectomy can be successful as a treatment for adult OSA, especially among patients with grade 3 or 4 tonsils and mild to moderate OSA.335 (Table IX.D.3)
TABLE IX.D.3.
Evidence on tonsillectomy for treatment of OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusions |
|---|---|---|---|---|---|---|
| Smith et al.1678 | 2017 | 4 | CS | 29 OSA patients | Sher’s criteria | Tonsillectomy appears to be an effective treatment for OSA in a select population of adults with tonsillar hypertrophy. |
| Holmlund et al.1589 | 2016 | 3b | PCC | 28 OSA patients with AHI > 10, Friedman grade III and IV tonsils | Change in AHI, ESS 6 months after surgery | Tonsillectomy may be an effective treatment for adult patients with OSA and large tonsils. |
| Camacho et al.335 | 2016 | 3a | SR/MA | NA | NA | Isolated tonsillectomy can be successful as a treatment option for adult OSA, especially among patients with large tonsils, and mild to moderate OSA (AHI < 30/h). |
| Senchak et al.1500 | 2015 | 3b | PCC | 19 OSA patients | Reduction of AHI by at least 50% to an absolute index of <15 | Adult tonsillectomy alone has a beneficial effect in the treatment of OSA. |
| Tan et al.1674 | 2014 | 3b | PCS | 34 OSA patients | Sher’s criteria | Tonsillectomy alone may be considered as an effective, first-line surgical procedure in the treatment of OSA. |
| Stow et al.1679 | 2012 | 4 | RCS | 13 OSA patients with tonsillar hypertrophy | Sher’s criteria | In selected adult subjects, tonsillectomy with intercurrent nasal surgery should be considered an effective treatment for OSA. |
| Nakata et al.1676 | 2007 | 3b | PCS | 20 OSA patients | AHI decrease | The reduction in nasal resistance induced by simple tonsillectomy could play an important role in improving OSAS, similar to nasal surgery and adenoidectomy. |
| Martinho et al.1492 | 2006 | 4 | PCS | 7 OSA patients with moderate obesity and tonsil hypertrophy | AHI decrease | Tonsillectomy for OSAHS in obese patients with obstructive palatine tonsil hypertrophy caused a significant reduction in AHI. |
| Nakata et al.1675 | 2006 | 3b | PCS | 30 OSA patients with tonsillar hypertrophy | Sher’s criteria | Simple tonsillectomy is a beneficial modality to treat OSA in selected patients (larger tonsils, low body mass index). |
| Verse et al.1677 | 2000 | 3b | PCS | 11 OSA patients with tonsillar hypertrophy | Sher’s criteria | In carefully selected patients, tonsillectomy should be considered an effective and safe surgical option for the treatment of OSA. |
| Miyazaki et al.1680 | 1998 | 4 | RCS | 10 OSA patients | PSG, AHI, 3 months postoperatively | Certain subjects can be effectively treated through tonsillectomy solely. |
| Houghton et al.1681 | 1997 | 4 | RCS | 7 OSA patients | PSG, 3 months postoperatively | Adult patients with tonsillomegaly may represent a subgroup of patients with OSA who would benefit from surgery aimed at the oropharynx. |
| Cheong et al.1682 | 1990 | 4 | RCS | 14 OSA patients | Postoperative PSG | Tonsillectomy offered a simple and effective therapy for patients with tonsillar hypertrophy. |
| Aubert-Tulkens et al.1683 | 1989 | 4 | RCS | 7 severe OSA patients | Postoperative AI | In adults with long-standing sleep apnea syndrome, surgical correction of nasal or pharyngeal abnormalities should not be expected to normalize sleep and breathing. |
| Moser et al.1684 | 1987 | 4 | RCS | 6 OSA patients with adenotonsillar hypertrophy | >2 months postoperative AI | Many adults with OSA can clearly benefit from the removal of hypertrophied tonsils. |
| Rubin et al.1685 | 1983 | 4 | RCS | 23 moderate-severe OSA patients (seven underwent tonsillectomy, 16 treated by SMR) | Postoperative PSG, AI | Tonsillectomy improved the AI. |
| Orr et al.1672 | 1981 | 4 | RCS | 6 OSA patients with tonsillar hypertrophy | ESS, PSG in four patients | Even relatively mild tonsillar enlargement can play an important role in the pathogenesis of obstructive sleep apnea. |
Abbreviations: CS, case series; PCC, prospective case–control study; PCS, prospective cohort study; RCS, retrospective cohort study; SR, systematic review.
IX.D.4 |. Soft palate surgery
IX.D.4.a |. Uvulopalatopharyngoplasty (UPPP)
A standard or traditional UPPP was first described in the literature by Fujita in 1981. A standard UPPP is based on palate and uvula tissue resection. UPPP has typically included tonsillectomy and trimming of the free-edge of the soft palate and uvula with suture closure. UPPP is the most commonly performed surgery for the management of OSA. Studies examining the efficacy of surgical procedures for OSA have been case series that have been evaluated in several SR and MA studies.812,1486,1634,1686–1688 Sher et al. was one of the first papers looking at the efficacy of UPPP for OSA. They found that the overall success rate based on greater than 50% reduction in the severity of AHI and an AHI of less than 20 (Sher criteria) was approximately 40%.1634 When examining the differences between responders and non-responders, they found that patients with only retropalatal collapse (52% success rate) had higher success rates than those with retrolingual or mixed collapse (5% success rate). It should be noted that the Sher criteria for success is limiting and modern studies examine the effect of surgery on AHI and ESS change.
Several studies show standard UPPP decreases overall AHI by 33%–50%.812,1686,1688 The effect has mainly measured with short-term analyses, with mixed data on long-term deterioration of respiratory events.812,1688 Aside from AHI, other studies have also looked at impact of UPPP on daytime sleepiness, CV events, cerebrovascular events, and overall mortality.
A metanalysis on isolated UPPP outcomes examined AHI change in RCTs and cohort studies, the majority of studies demonstrated a reduction in respiratory events and daytime sleepiness after UPPP.1688 In two RCTs, UPPP groups had significant AHI and ESS reductions compared to controls with no treatment.1666,2001 Data before and after UPPP in three RCTs and three cohort studies (196 patients) showed significant 49.5% reduction in AHI from mean 35.4 to 17.2 events/h (mean difference of −19.14)1487,1666,1795,2020. Similar significant AHI improvements were seen in prospective cohort studies. Predictors for treatment success included BMI, age, OSA severity, and clinical and anatomic staging systems.1688 Choi et al. found that the greatest pre-operative predicator of surgical success for UPPP was location of oropharyngeal narrowing.1486 Friedman stage I was a strong predicator of success while Friedman stage III and low hyoid position were negative predictors after UPPP. Halle et al. published evidence that UPPP has beneficial effect on mortality, cerebrovascular disease, but not on cardiac arrhythmias.1687 Further evidence on the effect of UPPP on mortality and cardiovascular outcomes are presented in Section IX.E.
Major complications related to UPPP surgery include UA edema, postoperative bleeding with or without the need for surgical intervention, velopharyngeal insufficiency (VPI), or nasal regurgitation. In comparison to modern variations of the UPPP (see Section IX.D.4.b), a standard UPPP appears to have lower surgical efficacy with potentially higher complication rates. Sher et al. found that the incidence of mild VPI 2 years after UPPP was as high 39.4%. More recent studies have found the VPI rates to be much lower due to changes in surgical techniques.1688 Postoperative bleeding requiring return to the operating room ranged from 2% to 8%.1634,1688 Velopharyngeal stenosis complication rates were found to be around 1.8%.1688 Overall complication rate reported for UPPP is approximately 1.3%.1544 (Table IX.D.4.a)
TABLE IX.D.4.a.
Evidence for uvulopalatopharyngoplasty for the treatment for obstructive sleep apnea
| Study | Year | LOE | Study design | Study group(s) | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|
| He et al.812 | 2019 | 2a | Systematic review and meta-analysis of 11 studies (eight standard UPPP, one submucosal UPPP, one extended UPPP, and one uvulopalatal flap) | 1) UPPP or modified UPPP with short-term (<1 year, n = 435 patients) and/or long-term (>34 months, n = 368) follow-up | 1. AHI 2. RDI 3. ODI 4. Lowest arterial oxygen sat (LO2Sat) 5. CT90 6. ESS 7. Snoring scores |
Long-term outcomes showed an overall 46.1% (mean difference −15.4 events/h) decrease of AHI. Efficacy decreased between short and long-term follow-up with AHI increase by 12.3 and surgical response declined from 67% to 44% in long term group. BMI, LO2Sat, and CT90 were significantly correlated with long-term surgical response. |
| Stuck et al.1688 | 2018 | 1a | SR of 53 studies (seven RCTs, six non-randomized controlled trials, and 40 prospective cohort studies) with meta-analysis Focus on isolated, standard UPPP. Modifications to UPPP and concomitant other surgeries were excluded |
1. UPPP with or without tonsillectomy 2. Control (n = 49) 3 RCTs compared UPPP with control group |
1. AHI 2. ESS 3. Cardiovascular disease |
Pooled analysis: after UPPP, AHI reduced from mean pre-surgery 35.3 to 17.9 (49.5%) and ESS score reduced from mean 11.7 to 7.3. Success rate ranged 35–95%. Compared to non-treated controls in two RCTs, UPPP improved both AHI (mean difference of −18.6, sig mean difference −1.03 = large effect) and ESS (significant mean difference of −5.37). Before and after UPPP in three RCTs showed 49.5% AHI reduction, mean difference −20.4. Mixed results from long-term studies. Complications of UPPP include upper airway edema, postoperative bleeding (0%–7.8%), VPI (2%–8%, all temporary), and velopharyngeal stenosis (1.8%). |
| Halle et al.1687 | 2017 | 3a | Systematic review | 1. UPPP (n = 6704) 2. Tracheotomy (n = 157) 3. Skeletal surgery (n = 125) 4. HNS (n = 46) 5. Controls (n = 6394) |
1. AHI 2. Cardiovascular event 3. Cerebrovascular disease 4. Mortality |
UPPP group had significant variability in success of reducing AHI. Lower-level evidence suggests that UPPP has beneficial effect on mortality but not on cardiac arrhythmias compared to those not undergoing surgery. Several retrospective studies found no difference in survival between CPAP users and UPPP. Lower-level evidence suggests that patients undergoing UPPP had a relative risk of 0.45 of incident cerebrovascular disease compared to those not undergoing surgery. Effect on blood pressure is undetermined. |
| Choi et al.1486 | 2016 | 3a | Meta-analysis of 15 retrospective case series | UPPP | Predictors of success – age, BMI, preoperative AHI, Friedman stage, cephalometric measurements | Friedman stage I is strong predicator of >50% postoperative reduction in AHI after UPPP (OR [95% CI] 4.4 [2.3–8.5], p < 0.001) while stage III and low hyoid position are negative predictors. Age, BMI, and preoperative AHI did not differ between patients with and without AHI reduction. |
| Caples et al.1686 | 2010 | 2a | SR and meta-analysis of 15 observational studies | UPPP (n = 950) | Percent reduction in AHI (95% CI) | Percent reduction in AHI was 33% (95% CI: 23%–42%). Postoperative residual AHI remained elevated, averaging 29.8/h. Side effects of surgery include difficulty swallowing/nasal regurgitation, taste disturbances, and voice changes. 1%–2% risk of a life-threatening adverse events and 0.2% risk of death following UPPP. |
| Sher et al.1634 | 1996 | 3a | SR | UPPP (n = 992) | 1. AHI 2. Surgical success rate (>50% reduction in AHI or RDI) |
Overall surgical success rate was 40%. Patients with only retropalatal collapse had higher success rates (mean AHI change −74.6%, mean RDI change −32.7%) than those with retrolingual or mixed collapse (mean AHI change −22.8%, mean RDI change −6.5%) Mild VPI at 2 years was 39.4%. Postoperative bleeding requiring return to the OR was between 2% and 5%. |
IX.D.4.b |. Expansion sphincter pharyngoplasty
Expansion sphincter pharyngoplasty (ESP) was first palate edge. The palatopharyngeus muscle is isolated and left with its posterior surface partially attached to described by Pang and Woodson in 2007.1689 The technique creates tension in the lateral pharyngeal walls and palatopharyngeus muscles in order to treat velopharyngeal collapse and attempt to prevent lateral oropharyngeal wall collapse as seen on DISE. The procedure, and its subsequent modifications, consists of a tonsillectomy and palatopharyngeus muscle rotation flap that is anterosupero-laterally rotated and secured to the hamulus of the hard palate or near the junction of the lateral hard the posterior horizontal superior pharyngeal constrictor muscles. A partial uvulectomy (optional) and closure of the anterior and posterior tonsillar pillars complete the procedure.1689
When compared to a standard or traditional UPPP techniques, ESP involves less tissue resection and has been shown to have significantly better surgical success rates based on the Sher criteria.1690,1691 The vast majority of studies are case series in which surgical success rate of ESP ranges from 57% to 86.6%.1690–1699 In an SR of five papers, the overall pooled success rate of ESP was 86.3%.1690 The authors acknowledge that one of the limitations of their SR was the heterogeneity of the patient population, patient selection criteria, small sample sizes, and inclusion of ESP with MLS in some studies.
When looking at other clinical outcomes, ESP is associated with improved ESS.1699–1701 One study examining ESP and CV events showed patients with successful outcomes after ESP also had a decrease in cardiac sympathetic activity. Another study suggested that ESP can improve CRP but only in patients achieving AHI < 5 events/h after surgery.1693
Several studies have looked at the efficacy of ESP in comparison to other UPPP variations, such as barbed pharyngoplasty, relocation pharyngoplasty, uvulopalatal flap, z-pharyngoplasty, and suspension pharyngoplasty.1691,1697,1702 Surgical success rates are similar between ESP and these other UPPP variations. ESP appears to have the lowest complication rates amongst palatoplasty procedures in a retrospective case series.1702 ESP has also been shown to be effective in treating lateral oropharyngeal collapse and circumferential palate collapse patterns seen on DISE.1695,1703
ESP is a well-tolerated surgical procedure that is effective in AHI and ESS reduction. Individual outcomes will vary depending on BMI, age, and anatomic clinical features. (Table IX.D.4.b)
TABLE IX.D.4.b.
Evidence for expansion sphincter pharyngoplasty for OSA
| Study | Year | LOE | Study design | Study groups | Clinical end points | Conclusion |
|---|---|---|---|---|---|---|
| Pang et al.1690 | 2016 | 2a | SR and meta-analysis of five studies (one cohort and four comparative cohort) | 1. Expansion sphincter pharyngoplasty (n = 155) 2. UPPP (n = 63) 3. Adeno-tonsillectomy (n = 25) |
1. AHI 2. Surgical success rate (reduction of postoperative AHI >50%) |
ESP had significantly lower postoperative AHI than UPPP (standardized mean difference −7.32, 95% CI (−11.11, −3.52), p = 0.0002). Overall pooled success rate of ESP was 86.3%. |
| Hong et al.1695 | 2019 | 4 | Prospective case series | Expansion sphincter pharyngoplasty patients with moderate or severe OSA (n = 63) | AHI Cross-sectional pharyngeal area |
Mean AHI decreased from 35.5 to 17.3 (mean difference, 18.1; 95% CI, 16.3–20.0). Change in lateral pharyngeal collapse in patients with moderate or severe OSA (based on cross-sectional pharyngeal retropalatal areas with CT scan). Postoperative DISE was not employed. |
| Plaza et al.1701 | 2019 | 4 | Prospective case series | Expansion sphincter pharyngoplasty (n = 75) | AHI ESS Surgical success rate (Sher criteria, AHI reduction >50% and to <20 events/h). |
Surgical success was 90% and 33% of patients were cured (AHI < 5). Mean AHI decreased from 22.1 to 8.6 (p < 0.001). Mean ESS decreased from 11.5 to 4.6 (p < 0.001). Well-tolerated; no significant complications. |
| Lorusso et al.1698 | 2018 | 4 | Prospective case series | Expansion sphincter pharyngoplasty in patients with moderate or severe OSA (n = 20), with or without nasal surgery, tonsillectomy, and/or hyoid bone suspension | 1. AHI 2. Surgical success rate (Sher criteria, AHI reduction >50% and to <20 events/h) |
Surgical success rate was 65%. (100% in the ESP + tonsillectomy group [n = 4]). Mean AHI decreased from 41.7 to 17.4 overall and 24.2 to 8 in the ESP + tonsillectomy group. |
| Bosco et al.1704 | 2019 | 4 | Prospective case series | Expansion sphincter pharyngoplasty | Surgical success rate | Surgical success rate was 82.4%. Well-tolerated. |
| Karakoc et al.1697 | 2018 | 4 | Prospective case series | 1. Expansion sphincter pharyngoplasty (n = 31) 2. Cahali lateral phayngoplasty (n = 28) 3. Anterior palatoplasty (n = 20) |
AHI ESS Surgical success rate (AHI reduction >50% and to <15 events/h) |
Surgical success rate was 74% in expansion group. Mean AHI decreased in both the ESP group, from 26.8 to 9.1 (p < 0.001), and in the CLP group. Mean ESS decreased in all groups and from 11.1 to 4.3 (p < 0.001) in the expansion group. Snoring also decreased significantly in all groups. |
| Despeghel et al.1700 | 2017 | 4 | Prospective case series | Expansion sphincter pharyngoplasty in patients with 1. AHI < 5 (n = 16) 2. AHI ≥ 5 (n = 19) |
AHI ESS |
53% had reduction in AHI > 50%. AHI reduction of 22% (mild OSA), 31% (moderate OSA), and 58% (severe OSA). ESS improved from 10.4 to 5.8 at 3 months postoperatively and 5.9 1 year. Minimal postoperative complications. |
| Pang et al.1699 | 2016 | 4 | Prospective case series | Expansion sphincter pharyngoplasty with (n = 61) or without (n = 12) nasal surgery | AHI ESS Surgical success rate (Sher criteria, AHI reduction >50% and to <20 events/h) |
Success rate was 86% (88.5% in combined nose/palate surgery and 75% in palate surgery alone, p = 0.081). AHI improved in all patients and was statistically significant (26.3–12.6, p < 0.001). ESS had statistically significant improvement (11.5–2.9, p < 0.001). |
| Binar et al.1693 | 2017 | 3 | Prospective case series | 1. Expansion sphincter pharyngoplasty (n = 23) 2. CPAP (n = 28) |
AHI C-reactive protein Surgical success rate (AHI reduction >50% and to <20 events/h) |
Success rate of ESP was 69.6% and 26% of patients were cured (AHI <5). Mean AHI decreased from 32.3 to 11.8 (p < 0.001). Only patients with AHI < 5 after surgery had improvement in CRP. CPAP group did not have significant change in CRP. |
| Liu et al.1703 | 2020 | 2b | Prospective cohort study | Expansion sphincter palatoplasty in patients with complete circumferential collapse (n = 12) | AHI Collapse pattern |
Mean AHI decreased from 54.0 to 33.1. All patients converted to either no collapse at the level of the velum (n = 3), complete anterior–posterior collapse (n = 7), or partial anterior–posterior collapse (n = 2). |
| Babademez et al.1692 | 2019 | 4 | Retrospective case series | 1. Expansion sphincter pharyngoplasty with anterior palatoplasty (n = 53) 2. Barbed palatoplasty (n = 45) |
AHI ESS Surgical success rate (AHI reduction >50% and to <20 events/h) |
Success rate was 86.6% in BP and 84.9% in ESP with anterior palatoplasty. Both surgeries significantly improved AHI (25.9–7.4 in BP, 28.5–9.1 in ESP with anterior palatoplasty, both p < 0.001). No significant difference in success rate or AHI reduction ratios between the surgeries. Both surgeries significantly improved ESS (11.2–3.4 in BP, 12.6–4.1 in ESP with anterior palatoplasty, both p < 0.001). Selecting a threshold of a 50% reduction in AHI and AHI less than 20 events/h, success rates were 86.6% in BP group and 84.9% in ESPwAP group. |
| Pang et al.1702 | 2019 | 4 | Retrospective case series | 1. Expansion sphincter pharyngoplasty (n = 84) 2. Barbed reposition phayngoplasty (n = 40) 3. Relocation pharyngoplasty (n = 8) 4. Uvulopalatoflap (n = 11) 5. Suspension pharyngoplasty (n = 9) 6. Modified UPPP(n = 64) 7. Z-pharyngoplasty (n = 1) |
Postoperative complications | Complication rates: dry throat (overall: 7.8%, ESP: 0%, mUPPP: 15.6%), globus sensation (overall: 11.5%, ESP: 0%, mUPPP: 15.6%). Throat phlegm (overall: 10.1%, ESP: 0%, mUPPP: 15.6%). Scar sensation (overall: 3.7%, ESP: 0%, mUPPP: 3.1%). Dysphagia (overall 0.5%, ESP: 0%, mUPPP: 0%). mUPPP, suspension pharyngoplasty, and relocation pharyngoplasty had highest symptom complaints. ESP had no symptom complaints. |
| Guler et al.1694 | 2018 | 4 | Retrospective case series | Expansion sphincter pharyngoplasty (n = 67) | AHI ESS Minimum O2 saturation Surgical success rate (AHI reduction >50% and to <20 events/h) |
Surgical success rate was 67.2%. Significant improvement in AHI from 18.3 to 8.0 (p = 0.001). ESS improved from 8.3 to 5.6 (p = 0.001) and minimum O2 saturation improved from 83.9% to 88.7% (p = 0.02). |
| Huntley et al.1696 | 2018 | 4 | Retrospective case series | 1. Expansion sphincter pharyngoplasty (n = 33) 2. Upper airway stimulation (n = 75) |
AHI ESS O2 nadir Surgical success rate (AHI reduction >50% and to <20 events/h) |
Surgical success of ESP was 63.6% vs. 86.7% for UAS. |
| Rashwan et al.1691 | 2018 | 4 | Retrospective case series | 1. Expansion sphincter pharyngoplasty (n = 25) 2. Barbed reposition pharyngoplasty (n = 25) 3. UPPP (n = 25) |
AHI ODI LOS |
Both BRP and ESP are more effective than UPPP in improving AHI and ODI and lowest oxygen saturation. No difference in mean change in LOS between the three groups. |
| Suslu et al.1705 | 2017 | 4 | Retropsective case series | Expansion sphincter pharyngoplasty (n = 28) | AHI Heart rate variability Surgical success rate (AHI reduction >50% and to <20 events/h) | Surgical success rate of ESP was 57.1%. Patient with successful outcomes after ESP had a decrease in cardiac sympathetic activity. |
IX.D.4.c |. Lateral pharyngoplasty
Lateral pharyngoplasty (LP) involves dissection and repositioning of palatopharyngeus and superior pharyngeal constrictor muscles in order to splint lateral pharyngeal walls (LPW) and thus decrease UA collapsibility at the VP and OP.1706 The repositioning of the muscle flaps promotes structural changes to the retropalatal space.1707 In OSA patients, the LPW narrow the UA during wakefulness,200,319 they further enlarge in volume in obstructed respiration during sleep,1708,1709 and are particularly collapsible in severe OSA during natural sleep.1710
In the first description of LP for OSA treatment in 2003, Cahali1706 found improvement in the AHI, snoring complaints, amount of deep sleep, and ESS score in a median follow-up of 8 months. Patients included in this study had retropalatal collapse on awake examination. There have been three randomized parallel group trials comparing LP to UPPP, and all included patients examined while awake.1707,1711,1712 Two of these trials included cases with only retropalatal collapse,1707,1712 and the outcome favored LP in one,1707 whereas the other study reported 100% success in both groups.1712 The third randomized trial included patients with retropalatal and retroglossal collapse and the outcome favored LP in patients with moderate OSA, without significant differences in the remaining groups.1711
By selecting surgeries according to the pattern of retropalatal collapse found on awake examination, Karacoc et al.1697 reported that only LP and ESP reduced AHI, whereas anterior palatoplasty did not. All interventions decreased ESS score and snoring. In a retrospective case series including only patients with retropalatal collapse on DISE, Carrasco-Llatas et al.1713 reported a 70% success rate (Sher’s criteria1634) with LP.
Some modifications in LP technique were included, focusing on reducing the stretch of UA tissues. In one of them, De Paula Soares et al.1714 reported decrease in BP during sleep and over a 24-h period 6 months after LP, along with decrease in AHI. The reported success rate with LP varies widely from 50% to 100% with consistent decrease in AHI across the studies. By performing the most recent version of LP, Elzayat et al.1715 reported a 70% success rate in a series of non-selected patients. These authors did DISE preoperatively (not for the purpose of selecting the procedure) and repeated DISE in surgical failures and found that, by excluding patients with complete hypopharyngeal collapse on DISE, LP improves 75% of patients with OSA with a success rate of 90%.1715
Bleeding and swallowing function are important considerations when performing any palatopharyngoplasty. Only one study reported two cases of postoperative bleeding after LP, which did not require return to the operating room.1697 The rate of bleeding after LP seems similar to other palatopharyngoplasties.1697 A few studies reported cases with transient dysphagia, which resolved after 1–6 months after LP.1697,1706,1707 (Table IX.D.4.c)
TABLE IX.D.4.c.
Evidence on lateral pharyngoplasty for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Cahali1706 | 2003 | 2b | Prospective cohort | LP (n = 10) Oral findings and Müller maneuver: only retropalatal collapse, bulky lateral pharyngeal tissues |
PSG ESS |
LP significantly reduced median AHI (from 41.2 to 9.5, p = 0.009), snoring, ESS (from 13 to 5, p = 0.011) and increased slow wave sleep (from 5.3% to 16.3% of time, p = 0.037). Successa of LP was 60%. |
| Cahali et al.1707 | 2004 | 1b | Randomized parallel group trial | 1 LP (n = 15) 2 UPPP (n = 12) Oral findings and Müller maneuver: only retropalatal collapse, bulky lateral pharyngeal tissues |
PSG ESS CT scan |
Only LP significantly reduced mean AHI (from 41.6 to 15.5, p = 0.002) and increased slow wave sleep (from 9.8% to 16.3% of time, p = 0.03). Significant decrease in AHI in LP vs. UPPP (p = 0.05). Both interventions significantly reduced ESS. No differences in postoperative upper airway CT measurements. Successa of LP was 53.3%. |
| Tunçel et al.1716 | 2012 | 4 | Retrospective case series | 1 LP (n = 9) 2 ZP (n = 13) 3 LP or ZP plus TBS (n = 13) Oral findings and Müller maneuver: only retropalatal collapse for LP |
PSG | AHI significantly reduced in all groups (LP: 9–4, p = 0.007; ZP: 15–6, p < 0.001; combined surgery: 18–8, p = 0.002). Greater improvement with multilevel intervention. Success ratesa are not available. |
| De Paula Soares et al.1714 | 2014 | 2b | Prospective cohort | LP (n = 18) Oral findings: clearly identifiable posterior tonsillar pillars | PSG ABPM |
LP significantly reduced AHI (from 33.5 to 20.9, p = 0.02), arousal index (31.6–16.7, p = 0.005) and T90% (10.6%–0.9%, p = 0.008). LP significantly reduced systolic (7.4 mmHg decrease, p = 0.006) and diastolic blood (4.2 mmHg decrease, p = 0.03) pressure during sleep and over a 24-h period. Successa of LP was 50%. |
| Chi et al.1711 | 2015 | 1b | Randomized parallel group trial | 1 LP plus UPPP (n = 25) 2 UPPP (n = 29) Oral findings and Müller maneuver: retropalatal and retroglossal collapse |
PSG | Significant decrease in AHI in LP with UPPP (30.7%) vs. UPPP (10.8%, p = 0.02) in moderate OSA. Similar but nonsignificant reduction in AHI in mild and severe OSA. Success ratesa non available. |
| Carrasco-Llatas et al.1713 | 2015 | 3b | Retrospective case series | 1 LP (n = 10) 2 PPR (n = 22) 3 UPPP (n = 7) 4 ZP (n = 4) 5 ESP (n = 10) Oral findings and DISE: only retropalatal collapse |
PSG | All interventions significantly reduced AHI. AHI decreased in LP group from 48.0 to 15.2 (p < 0.05) and successa of LP was 70%. |
| Dizdar et al.1712 | 2015 | 2b | Randomized parallel group trial | 1 LP (n = 14) 2 UPPP (n = 9) Oral findings and Müller maneuver: only retropalatal collapse, bulky lateral pharyngeal tissues |
PSG ESS |
Both interventions significantly reduced AHI (LP: 23.4–11.3, p < 0.05) and ESS (LP: 15.3–6.8). Successa of both interventions were 100%. |
| Karacoc et al.1697 | 2018 | 2b | Prospective cohort | 1 LP (n = 28) 2 ESP (n = 31) 3 AP (n = 20) Oral findings and Muller maneuver: only retropalatal collapse. For LP: bulky lateral pharyngeal tissues and primarily lateral wall collapse |
PSG ESS Snoring |
Only LP and ESP significantly reduced AHI, (LP: 17.69–12.05, p = 0.004). All interventions significantly decreased ESS (LP: 13.2–8.3) and snoring. Successa,b of LP was 64%. |
| Elzayat et al.1715 | 2020 | 2b | Prospective cohort | LP (n = 40) Non-selected (excluded previous palatal surgery) |
PSG DISE |
LP significantly reduced AHI (from 34.7 to 16.6, p < 0.001) and increased baseline and min O2 saturation during sleep. Successa was 70%. Complete postoperative hypopharyngeal collapse occurred in 83.3% of non-responders. By excluding cases with preoperative complete hypopharyngeal collapse (25% of cases), successa would be 90%. |
Abbreviations: ABPM, ambulatory blood pressure monitoring; AP, anterior palatoplasty; CT, computed tomography; DISE, drug-induced sleep endoscopy; ESP, expansion sphincter pharyngoplasty; ESS, Epworth sleepiness scale; LP, lateral pharyngoplasty; PPR, partial palate resection; PSG, polysomnography; T90%, percentage of TST with oxyhemoglobin saturation <90%; TBS, tongue base suspension; UPPP, uvulopalatopharyngoplasty; ZP, Z-palatoplasty.
Success based on Sher’s criteria1634 = reduction in AHI ≥ 50% with final AHI < 20/h (or
<15/h).
IX.D.4.d |. Transpalatal advancement pharyngoplasty
First described by Woodson and Toohill (1993), transpalatal advancement pharyngoplasty (TPA) was originally described for OSA patients who had failed the traditional UPPP surgery1717 and were found to have continued retropalatal collapse. These patients typically have a long bony palate antero-posteriorly and a narrow retropalatal region. The TPA involves targeted excision of a bony window near the posterior edge of the bony hard palate, leaving a small strip of bone adherent to the soft palate aponeurosis. The soft palate aponeurosis is then pulled and sutured forward anteriorly, thereby widening the retropalatal space. Woodson and Toohill1717 demonstrated in 11 patients the success rate was 67%. In the successful group the RDI decreased from 52.8 ± 12.2 to 12.3 ± 2.8. When examining the whole cohort, RDI decreased from 73.3 ± 29.4 to 25.1 ± 28.2. The main complications included a transient oronasal fistula (ONF), transient dysphagia, and serous otitis media. Woodson1718 also showed a significant increase in the retropalatal area in seven patients after the TPA with area increase by 220% from 61.5 to 135.0 mm2, and retropalatal closing pressure decreased 9.2 cmH2O from 5 to −4.2 cmH2O. Woodson et al.1719 then studied success rates of TPA against traditional UPPP surgery. Both were associated with improvement in RDI; however, TPA was associated with a larger RDI reduction (TPA: 48.3 ± 24.6 to 19.8 ± 16.8, UPPP: 47.9 ± 30.0 to 30.9 ± 24.2, p < 0.0001). In the TPA group, the postoperative AHI change was greater (30.9 ± 24.2 points vs. 19.8 ± 16.8, p < 0.02). For patients with Friedman stage 3 tongue position, the OR of 50% or greater AHI reduction to level <20 events/h with TPA compared with UPPP was 3.80.
As the most difficult complication to treat is the ONF, Shine and Lewis1720 demonstrated in 89 patients who underwent TPA, the type of incision used made a significant difference in incidence of postoperative ONF after adjusting for age, sex, previous tonsillar and UPPP surgery, smoking histories, and pre-operative disease severity. Another study1721 on 59 TPA patients (single surgeon data) showed overall complication rate was 25.4% (15/59), the most common of which was transient VPI (8/59, 13.6%). ONF was seen in 4/59 (6.8%) of patients. The only significant contributing factor for the development of ONF was the presence of a high-arched palate.
An MA by Volner et al.1722 analyzed 199 OSA patients (five studies) who underwent the TPA. The mean preoperative and postoperative AHI (199 patients) an overall relative reduction of 64.8% although the studies demonstrated significant heterogeneity. The SMD for TPA demonstrated a large magnitude of effect on AHI −1.76 [95% CI −2.4, −1.1]. Lowest oxygen saturation (LSAT, 70 patients) also improved with after TPA with a mean difference of 3.55 percentage points. (Table IX.D.4.d)
TABLE IX.D.4.d.
Evidence for transpalatal advancement surgery for OSA
| Study | Year | LOE | Study design | Study groups | Clinical end point | Conclusion |
|---|---|---|---|---|---|---|
| Woodson and Toohill1717 | 1993 | 4 | Retrospective | Previous UPPP failures (n = 11) | RDI | TPA led to RDI reduction from 73.3 ± 29.4 to 25.1 ± 28.2 (p < 0.001). Main complications included transient oronasal fistula (ONF), transient dysphagia, and serous otitis media. |
| Woodson1718 | 1996 | 4 | Retrospective cohort study | TPA UPPP |
Quantitative endoscopic area assessment | TPA results in an increase in velopharyngeal area from 61.5 to 135.0 mm2 (p < 0.001). |
| Woodson et al.1719 | 2005 | 3 | Retrospective cohort study | TPA (n = 30) UPPP (n = 44) |
AHI | AHI reduction was greater with TPA vs. UPPP. Postop AHI was lower in TPA group (17.1 ± 30.1) vs. the UPPP group (28.5 ± 25.6, p < 0.04). TPA group had larger AHI change (30.9 ± 24.2 points vs. 19.8 ± 16.8, p < 0.02). |
| Volner et al.1722 | 2017 | 3a | Systemic review with meta-analysis (five studies) | TPA (n = 199) | AHI LSAT |
1. AHI reduction from 54.6 ± 23.0 to 19.2 ± 16.8; mean difference −36.3. 2. LSAT improved from 81.9 ± 8.1 to 85.4 ± 6.9. |
IX.D.4.e |. Radiofrequency palatoplasty
RF surgery of the soft palate was first introduced by Powell et al. in 1998 as a treatment in habitual snoring and/or mild OSA.1723 The RF technique utilizes heating of the tissue via an RF electrode to create a precisely controlled submucosal scar, stiffening of the targeted tissue, and volume reduction in the applied area. Literature has shown volumetric tissue reduction of the soft palate and further amelioration of the symptoms following treatment.1724–1726 Due to the simple technique requiring only local anesthesia, this procedure can be done in an office outpatient setting.1727
The efficacy of RF surgery has been demonstrated in numerous prospective clinical trials. The short-term efficacy of this treatment includes reduction in snoring, excessive daytime sleepiness, and OSA severity.1724,1728–1735 An SR by Bäck et al. demonstrated that RF of the soft palate could reduce symptoms of snoring, at least in the short term.1736 On the contrary, the latest RCT conducted by Holmlund’s group reported no significant clinical benefit on daytime sleepiness, snoring, or apnea severity one year after RF palatoplasty.1737 Modifying factors that impact the success of RF have been reported. Ferguson et al. reported that multi-lesion RF with higher energy levels per treatment increased the efficacy compared to single-lesion therapy.1738 A study by Bäck et al. revealed that RF surgery of the soft palate is not recommended as a single-stage approach in mild OSA.1739 RF may be suitable as a first-step treatment particularly due to its minimally invasive nature, but additional treatment sessions may be needed to increase long-term efficacy.1740–1742 Adverse events reported were minimal and included mucosal sloughing, pain, localized edema of the tissues, and oronasal fistulization.1743 There was no significant impact on vowels and voice quality after RF surgery of the soft palate.1744,1745 (Table IX.D.4.e)
TABLE IX.D.4.e.
Radiofrequency palatoplasty for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Bäck et al.1724 | 2001 | 3a | Prospective, non-randomized | 21 healthy men with habitual snoring for at least 1 year | Epworth Sleepiness Scale (ESS), visual analogue scale (VAS) scores of symptoms, cephalometric analysis, inflammatory laboratory parameters | RF thermal ablation of the soft palate seems to be effective for snoring and excessive daytime sleepiness. |
| Bäck et al.1725 | 2002 | 3a | Prospective, non-randomized | 20 non-obese habitual snorers | Snoring, daytime sleepiness, soft palate dimensions on magnetic resonance imaging (MRI) | Significant changes in the soft palate dimensions and in the T1-signal intensity were seen on MRI, along with decreased snoring and daytime sleepiness, after RF palatoplasty. |
| Bäck et el.1739 | 2009 | 1b | Randomized single-blinded placebo-controlled | 32 patients with mild OSA | Primary measures: apnea/hypopnea index (AHI), ESS, and a 36-item short-form quality-of-life (QOL) questionnaire Secondary measures: soft tissue airway parameters in cephalometric radiographs, snoring scores, and rates of adverse events |
RF surgery of the soft palate is not recommended as a single-stage treatment in mild OSA. AHI did not differ between patients after RF surgery (11.0 preoperatively vs. 13.0 postoperatively) vs. placebo (12.0 preoperatively vs. 11.0 postoperatively, p = 0.63) |
| Bäck et al.1736 | 2009 | 1b | Systemic review | 30 controlled or prospective studies of RF palatoplasty in snoring with at least 10 adults without moderate/severe OSA | ESS, AHI snoring severity, postoperative discomfort | RF surgery is a safe operative procedure, which may reduce symptoms of snoring in short-term follow-up. Of six studies reporting AHI and/or RDI, none reported significant change following RF. |
| Balsevičius et al.1743 | 2015 | 3a | Prospective, non-randomized | 28 patients with mild/moderate OSA who underwent two sessions of RF within the interval from 6 to 8 weeks | VAS scores of symptoms, AHI, Sleep Apnea Quality of Life Index, Beck Depression Inventory – second edition (BDI-II), ESS | RF with nine lesions to the soft palate seems to be an effective and safe treatment modality associated with low morbidity in selected mild/moderate OSA. Mean AHI decreased from 13.7 ± 5.9 to 8.3 ± 4.9 (p < 0.01) and 60.7% of patients experienced an AHI reduction >50%. |
| Tatla et al.1735 | 2003 | 3b | Prospective, nonrandomized study | Ten patients with chronic habitual snoring or mild OSA | BMI, PSG, snoring VAS, subjective assessment of pain | RF palatoplasty has minimal morbidity, high patient acceptability, minimal analgesia requirements, and little inconvenience to patients. Decrease in AHI was not significant. |
| Powell et al.1723 | 1998 | 2b | Prospective nonrandomized | 22 patients with sleep-disordered breathing | PSG, cephalometric radiographs, VAS scores of symptoms (pain, speech, snoring, and swallowing), and infrared thermal imaging | There was documented tissue reduction and improvement in symptoms in all patients. Apnea and hypopnea indices initially worsened in the 48–72 h immediately following treatment but decreased to pre-treatment levels by 10–12 weeks; long-term decrease was not significant. |
| Sher et al.1732 | 2001 | 2b | Prospective, nonrandomized multicenter study | 113 patients with habitual disruptive snoring who had a respiratory disturbance index (RDI) < 15 and minimum oxygen saturation not less than 85% | VAS scores of symptoms (pain, snoring and sleep quality) and ESS | The multiple lesion RF protocol was the most successful. Temperature-controlled RF was found to be a minimally invasive, well-tolerated procedure. Mean RDI increased from 6.4 to 8.7 for the overall cohort (p < 0.0031). |
| Boudewyns et al.1729 | 2000 | 2b | Prospective, nonrandomized multi-center clinical study | 45 non-apneic snorers | ESS, snoring index | RF ablation applied at the midline of the soft palate, with a maximum of three treatment sessions, improves snoring and ESS in the majority of patients without serious adverse events. |
| Hofmann et al.1741 | 2006 | 2b | Prospective clinical trial | 79 patients with primary snoring or mild OSA (47: uvulopalatopharyngoplasty [UPPP]; 32: RF palatoplasty) | Subjective snoring scores and AHI | The success rate of RF surgery of the soft palate is clearly lower compared to UPPP. Snoring scores improved in both groups, while AHI and HI only improved in the UPPP group. |
| Holmlund et al.1737 | 2014 | 1b | Randomized controlled trial | 35 men with snoring and mild/no OSA | ESS, Basic Nordic Sleep Questionnaire, AHI with home sleep study | RF soft palate has no significant effect on daytime sleepiness, snoring, or AHI 1 year after surgery. |
| Hukins et al.1730 | 2000 | 3b | Prospective, non- randomized | 20 adults with loud habitual snoring without clinically significant OSA | VAS scores of symptoms (snoring intensity, pain, difficulty in swallowing), and changes in speech, polysomnography and lateral cephalometry | RF palatoplasty is well tolerated with very low morbidity. It is associated with subjective improvement in snoring in most patients. Change in AHI is not significant. |
| Birkent et al.1744 | 2008 | 3a | Prospective, non-randomized | 26 habitual snorers and mild OSA patients (AHI < 10) | Acoustic evaluation made by the multidimensional Voice program | RF of the soft palate as a treatment for snoring and mild OSA does not havea significant impact on voice quality. |
| Hultcrantz et al.1731 | 2009 | 3b | Prospective, non-randomized | 29 patients with habitual snoring; 10 of 29 patients underwent electromyography (EMG) of palatoglossal muscles | Snoring VAS, bedpartner estimated hours of sleep and EMG changes of palatoglossal muscles | RF treatment for snoring may lead to long-term improvement in one out of four cases. Pre-evaluation with EMG may predict the outcome. |
| Ferguson et al.1738 | 2001 | 2b | Prospective, non-randomized | 47 habitual snorers without symptoms suggestive of OSA (16: single-lesion treatments, 31: multilesion treatments) | VAS scores for snoring and pain | Multilesion RF palatoplasty using higher energy levels per treatment is safe and has increased efficacy without increased complications relative to single-lesion therapy. |
| Blumen et al.1728 | 2013 | 4 | Retrospective review | 105 subjects with simple snoring or mild OSA | To determine whether snoring intensity measured after the first session of soft palate RF predicts the final treatment outcome | Snoring intensity after the first RF session helps predict the final outcome of RF-assisted stiffening of the soft palate for simple snoring. |
| Haraldsson et al.1745 | 2002 | 3a | Prospective, non-randomized | 16 habitual snorers (all patients had an oxygen desaturation index <6) | Objective (nasal-oral ratio meter) and subjective (speech-language pathologist) speech evaluation | RF soft palate for snoring did not show any significant adverse effect on velopharyngeal function and voice quality. |
| Kermadec et al.1740 | 2014 | 4 | Observational retrospective study | 77 patients underwent soft palate RF treatment for snoring with AHI <20 (at least 3 years’ follow-up) | A postal questionnaire including snoring VAS, demographics, cardiovascular risk factors (body weight, hypertension and diabetes) | Relapse of snoring was observed in nearly all patients. Most patients did not comply with the follow-up instructions and did not seek other forms of treatment when recurrence occurred. |
| Stuck et al.1733 | 2004 | 2a | Systemic review | Habitual snorers and mild OSA (22 original articles published in peer-reviewed journals) | Snoring VAS and snoring index | RF surgery of the soft palate leads to a significant reduction of subjective snoring, and snoring is reduced to a tolerable level. |
| Stuck et al.1734 | 2005 | 1b | Randomized, placebo-controlled trial | 26 patients with primary snoring (AHI < 15, BMI < 35) divided into treatment and placebo arms | Bed partner with snoring VAS | Snoring scores for the RF arm were significantly better than the placebo arm, although the reduction in snoring was only moderate. |
| Stuck et al.1742 | 2009 | 3a | Prospective clinical trial | 19 patients with primary snoring (AHI < 15, BMI < 32) 18 months post-procedure | Questionnaire on snoring VAS, and the overall satisfaction with the procedure | A relapse in snoring can be expected in some patients after RF-assisted uvulopalatoplasty (RF-UPP). |
| Emery et al.1727 | 2000 | 3b | Prospective, non-randomized | 43 snoring and/or mild OSA patients whose snoring intensity was bothersome to their bed partner | Safety, character of pain, and effects on speech, swallowing, and snoring severity | RF palatoplasty is well-tolerated and not painful. |
| Blumen et al.1726 | 2002 | 3a | Prospective, non-randomized | 30 patients with simple snoring or mild OSA having soft palate obstruction | Efficacy and tolerance of RF and laser-assisted uvulopalatopharyngoplasty (LAUP) | RF was as effective as LAUP in reducing snoring in the short-term, and was better tolerated. |
IX.D.4.f |. Pillar implants
Pillar implants were initially introduced in 2003 for the treatment of snoring and were later approved for mild OSA. Three implants consisting of non-resorbable woven polyethylene terephthalate (each 18 mm long and 2 mm in diameter) are inserted into the soft palate, one in the midline of the junction between the hard to soft palate, and one lateral to either side with a space of 3 mm in-between. The procedure is performed under local anesthesia in an office setting.
The intention is to extend the hard palate by stiffening the soft palate through chronic inflammatory, fibrous capsule formation around the implant and thereby to prevent a retropalatal collapse. This process should also reduce tissue vibrations during respiration.
In the majority of studies a significant positive effect on AHI, snoring intensity, LSAT, and daytime sleepiness (ESS) was observed selecting patients with the following criteria: mild to moderate OSA, BMI ≤32 kg/m2, no sign of airway collapse other than palate, and a length of ≥20 mm of the soft palate.1746–1753 In two of the three RCTs, a significant postoperative improvement of the AHI and the superiority of the implant over placebo was shown,1748,1750,1752 one RCT showed elevation in AHI in both treatment and placebo groups.1752
An initial significant improvement in AHI after the first 90 days showed consistency for the follow-up after 12–15 months,1754,1755 whereas patients without improvement did not show any improvement either after 15 months.1755 A long-term follow-up with up to 36 months also showed a permanent significant improvement in AHI and ESS but also a significant change in BMI which was not described further.1756 Other studies, however, showed no significant improvement in AHI in mild to moderate OSA after implantation.1757,1758
When implanted in patients with moderate to severe OSA, the implant showed nearly no effect or a significant but clinically meaningless improvement regarding RDI and ESS.1759,1760 AHI was unchanged, but the implantation significantly reduced the needed CPAP-pressure and increased CPAP-adherence: 11.2 versus 9.2 cmH2O (p < 0.001), and 5.7 vs. 6.4 h (p < 0.001). Body weight was not measured postoperatively.1759 However, these results could not be confirmed in in a subsequent RCT at constant body weight.1761 There is no objective data on other sleep parameters.
The above stated effects have to be measured against the morbidity of the surgery: in the vast majority the occurring mild pain, speech and swallowing difficulties were selflimiting within the first 24–72 h up to 3–6 months1757,1762 or did not occur at all.1747 Dysphagia in 14.8% and foreign body sensation in 25.9% persisted in one study1758 and was selflimiting in most other cases.1757,1760–1762 Infections occurred in 0%1750 to 2%1752 and irritations or ulcerations in 7.5%,1753 and only one needed antibiotics. There is only one isolated report of an abscess.1756 Extrusion rates were published between 0%,1750,1760,1761 4%,1752 and 9.9%1753 to 14.3%1754 of implanted patients. Complete resolution was reached after 2 weeks.1753
In summary, pillar implants can be recommended as sole therapy if the above selection criteria are fulfilled, or as part of a multimodality and multilevel treatment plan, since the potential benefits outweigh the possible harm. It cannot be recommended in patients with severe OSA or obesity. The costs may be significant and should be measured against other therapeutic options and the possible need to treat a persisting OSA. (Table IX.D.4.f)
TABLE IX.D.4.f.
Evidence on pillar implants for sleep apnea
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Maurer et al.1750 | 2012 | 1b | Randomized, double-blind, placebo-controlled trial | Mild to moderate OSA (study group n = 11, placebo group n = 11) • AHI 10–40/h • BMI ≤32 kg/m2 • Soft palate length >25 mm • Tonsil size <50% of the airway • No signs of retrolingual obstruction in clinical exam • No previous history of upper airway surgery other than nasal, adenoid, or tonsil |
Ninety days follow-up of AHI, snoring, LSAT, and ESS | AHI, snoring and LSAT with significant improvement. Placebo group: no significant difference in AHI and ESS. |
| Steward et al.1752 | 2008 | 1b | Randomized, double-blind, sham-placebo-controlled, multi-institutional study | Mild to moderate OSA(study group n = 50, placebo group n = 50) • BMI <32 kg/m2 • AHI 5–40/h • Tonsil size <III |
Three months follow-up of AHI and ESS, LSAT and PVT, snoring | Increase of AHI in both groups, pre- and post-interventional: study vs. placebo. ESS and LSAT improved compared to placebo. No significant change in PVT or snoring. |
| Hu et al.1760 | 2018 | 4 | Retrospective, single-institutional | Severe OSA with retropalatal collapse (n = 10) • RDI > 30/h • No nasal obstruction • Tonsil size <III • Soft palate length 30–40 mm |
Thirty-three months follow-up, ESS, snoring, RDI, LSAT, sleep efficiency. RDI reduction of <20/h and a reduction of ≥50% | Improvement in snoring, ESS, RDI, and LSAT. |
| Bertoletti et al.1759 | 2009 | 4 | Non-randomized, single-institutional pilot-study | Moderate to severe OSA with CPAP-therapy and palatal implant (n = 21) • Titrated CPAP pressure >10 cm H2O • AHI > 20/h • BMI > 30 kg/m2 |
Ninety days follow-up, CPAP pressure | No significant difference of pre- and postoperative AHI and SpO2. Significant difference between average CPAP pressure levels (mean reduced from 11.2 to 9.3 cmH2O). |
| Server et al.1758 | 2016 | 4 | Retrospective, non-randomized study | Mild OSA and simple snoring (n = 27) • BMI < 30 kg/m2 • AHI < 15/h • Tonsils size <50% of the airway • The same bed-partner for 6 years • Tonsil size <50% of the airway • Extrusion excluded patients |
Six years follow-up considering snoring, ESS, mouth dryness, foreign body sensation in the throat | Snoring decreased significantly. No significant change of AHI or ESS. |
| Friedman et al.1748 | 2008 | 1b | Randomized, double-blinded, placebo-controlled, clinical trial | Mild to moderate OSA(study group n = 31, placebo group n = 31) • AHI 5–39/h • BMI < 32 kg/m2 • soft palate length ≥20 mm but <35 mm • tonsil size <III |
Three months follow-up of AHI, QOL (SF-36), snoring, ESS | Significant improvement of AHI (from 23.8 to 15.9 in treatment group), QOL, snoring, and ESS. |
| Nordgård et al.1751 | 2006 | 4 | Prospective, non-randomized study | Mild to moderate OSA (n = 25) • AHI 10–30/h • BMI ≤30 kg/m2 • Soft palate length >25 mm • Tonsil size <50% of the airway • No significant nasal stenosis • Bed partner present |
Three months follow-up of AHI, ESS, and snoring | Significant reduction of AHI, ESS, and snoring. |
| Walker et al.1753 | 2006 | 4 | Prospective, non-randomized study conducted at five clinical sites | Mild to moderate OSA (n = 53) • Palatal contribution to OSA • AHI 10–30/h • BMI ≤32 kg/m2 • No nasal obstruction or palatal surgery • Bed partner |
Three months follow-up of AHI, ESS and snoring intensity (VAS), LSAT | Significant improvement of AHI, ESS, and snoring. |
| Choi et al.1757 | 2015 | 4 | Non-randomized study conducted at five clinical sites | Simple snoring to mild OSA (n = 29) • AHI <15/h • BMI <25 kg/m2 • Tonsil size grade 1 or 2 • Palate-tongue position 1 or 2 • Suspected retropalatal obstruction • Soft palate length >25 mm • Tonsil size <III |
Three to six months follow-up of snoring (VAS), ESS | Significant improvement of snoring and ESS. No significant change in AHI, RDI, LSAT or snore loudness. Improvement in AHI for patients with mild OSA. |
| Friedman et al.1762 | 2006b | 4 | Non-randomized, retrospective review | Snoring and OSA (n = 125), groups: PIT only (n = 29), adjunctive nasal procedure (n = 37), adjustive oropharyngeal procedure (n = 55), failed previous UPPP (n = 4) • BMI 19.5–39 kg/m2 |
Six months follow-up of Snoring, ESS, AHI, and AHI | No significant improvement in AHI. Significant improvement of AHI in combination with nasal airway procedures in mild OSA |
| Goessler et al.1749 | 2007 | 4 | Prospective, non-randomized study | OSA (n = 16) • AHI 10–30/h • BMI ≤30 kg/m2 • Soft palate length >25 mm • Tonsils size <50% of the airway • No previous pharyngeal surgery, nasal or retrolingual obstruction |
Three months follow-up on AHI, snoring (VAS), ESS | Significant improvement of AHI, snoring, and ESS. |
| Choi et al.1746 | 2013 | 1a | Meta-analysis of seven studies including two RCTs | OSA (total n = 363) | Three to 29 months of follow-up of AHI, ESS, snore sound loudness, and extrusion rate | Significant improvement of snoring sound loudness, ESS, and AHI. Extrusion rate was 9.3%. AHI change was significant in most studies, pre-treatment AHI for all studies was <25, |
| Nordgård et al.1751 | 2006 | 4 | Prospective, non-randomized study | Mild to moderate OSA (n = 25) • AHI 10–30/h • BMI ≤30 kg/m2 • Soft palate length >25 mm • Tonsil size <50% of the airway • No significant nasal stenosis • Bed partner present |
Three months follow-up of AHI, ESS, and snoring | Significant reduction of AHI, ESS, and snoring. |
| Walker et al.1753 | 2006 | 4 | Prospective, non-randomized study conducted at five clinical sites | Mild to moderate OSA (n = 53) • Palatal contribution to OSA • AHI 10–30/h • BMI ≤32 kg/m2 • No nasal obstruction or palatal surgery • Bed partner |
Three months follow-up of AHI, ESS and snoring intensity (VAS), LSAT | Significant improvement of AHI, ESS, and snoring. |
| Choi et al.1757 | 2015 | 4 | Non-randomized study conducted at five clinical sites | Simple snoring to mild OSA (n = 29) • AHI <15/h • BMI <25 kg/m2 • Tonsil size grade 1 or 2 • Palate-tongue position 1 or 2 • Suspected retropalatal obstruction • Soft palate length >25 mm • Tonsil size <III |
Three to six months follow-up of snoring (VAS), ESS | Significant improvement of snoring and ESS. No significant change in AHI, RDI, LSAT or snore loudness. Improvement in AHI for patients with mild OSA. |
| Friedman et al.1762 | 2006b | 4 | Non-randomized, retrospectivereview | Snoring and OSA (n = 125), groups: PIT only (n = 29), adjunctive nasal procedure (n = 37), adjustive oropharyngeal procedure (n = 55), failed previous UPPP (n = 4) • BMI 19.5–39 kg/m2 |
Six months follow-up of Snoring, ESS, AHI, and AHI | No significant improvement in AHI. Significant improvement of AHI in combination with nasal airway procedures in mild OSA |
| Goessler et al.1749 | 2007 | 4 | Prospective, non-randomized study | OSA (n = 16) • AHI 10–30/h • BMI ≤30 kg/m2 • Soft palate length >25 mm • Tonsils size <50% of the airway • No previous pharyngeal surgery, nasal or retrolingual obstruction |
Three months follow-up on AHI, snoring (VAS), ESS | Significant improvement of AHI, snoring, and ESS. |
| Choi et al.1746 | 2013 | 1a | Meta-analysis of seven studies including two RCTs | OSA (total n = 363) | Three to 29 months of follow-up of AHI, ESS, snore sound loudness, and extrusion rate | Significant improvement of snoring sound loudness, ESS, and AHI. Extrusion rate was 9.3%. AHI change was significant in most studies, pre-treatment AHI for all studies was <25, posttreatment mean AHI was elevated >10 in most studies (significant mean difference −0.378, % change from 12% to 50%). |
| Walker et al.1755 | 2007 | 4 | Prospective case series conducted at four clinical sites | Continuation (Walker et al. 2006) of follow up (n = 22/52) • Palatal contribution to OSA • AHI 10–30/h • BMI ≤32 kg/m2 • Soft palate length can accommodate 18 mm implant with no previous surgery • No nasal obstruction or palatal surgery • Bed partner available |
Fifteen months follow-up of AHI, ESS, and snoring intensity | Patients with a significant decrease in AHI after 90 days (13/22) maintained this decrease, patients without a decrease in AHI after 90 days did not show any improvement. Snoring intensity remained improved in all patients. ESS in patients without AHI decrease returned to baseline level (after 15 months) from initial significant improvement after 90 days. Those with AHI decrease remained improved. |
| Nordgård et al.1754 | 2007 | 4 | Prospective case series | Mild to moderate OSA (n = 26) • AHI 10–30/h • BMI ≤30 kg/m2 • Soft palate length >25 mm • Tonsils size <50% of the airway • No significant nasal stenosis and available bed partner |
One year follow-up on AHI, ESS, snoring intensity, and pain | Significant improvement in AHI ESS and snoring after 3 and 12 months compared to baseline. |
| Neruntarat1756 | 2011 | 4 | Prospective study | Mild to moderate OSA (n = 92) • AHI ≤30/h • BMI ≤30 kg/m2 • Soft palate length >20 mm |
Twenty-six to 32 months follow-up on AHI, ESS, snoring intensity, and LSAT | Significant decrease in AHI, ESS, snoring intensity, and LSAT. |
| Gillespie et al.1761 | 2011 | 1b | Randomized, double-blind, placebo-controlled multicenter study in four different referral centers | Moderate to severe OSA with pressure related CPAP-complaints (study group n = 26, placebo group n = 25) • AHI >15/h prior to CPAP-use • Prescribed CPAP pressure ≥7 cmH2O • Intolerance due to high pressure, mouth leak or mouth dryness • Soft palate length ≥25 mm • No nasal obstruction • CPAP-titration at baseline to identify lowest optimal pressure • Tonsil size <III |
Ninety days follow-up of CPAP pressure after PI, adherence, and CPAP-satisfaction | At constant weight no significant reduction of CPAP pressure and no increase in CPAP compliance was detected. Only CPAP-satisfaction was significantly better in study group vs. placebo. |
| Friedmann et al.1747 | 2006 | 4 | Prospective, nonrandomized study | Persistence of mild to moderate OSA and snoring after UPPP (n = 23) • History of UPPP-surgery • AHI 5–40/h • Residual palate ≥20 mm Persistent retropalatal obstruction |
Six months follow-up on AHI, snoring levels, ESS, QOL, and LSAT | Significant decrease in snoring level and ESS. Improvement of AHI and LSAT. |
IX.D.4.g |. Palate suture suspension
The use of sutures to achieve an antero-apical suspension of the soft palate and lateral stabilization of the pharyngeal walls presents a fairly new surgical technique in treating sleep apnea, which has been developed with the intention of reduced morbidity and increased efficacy. Predecessors such as ESP or UPPP have shown to be effective, nonetheless they present more invasive techniques compared to those using suture suspension.1763
There are several similar techniques using suspending sutures: barbed reposition pharyngoplasty,1764 barbed suspension pharyngoplasty,1765 the barbed roman blinds technique,1766 the Alianza technique,1767 barbed anterior pharyngoplasty,1768 barbed ESP,1769 expansion pharyngoplasty by simple suture suspension,1770 and modified barbed soft palatal webbing flap palatopharyngoplasty using barbed sutures.1771
The aim behind a suture suspension technique is an anterior-lateral soft palate displacement and stabilization of lateral pharyngeal wall by anchoring the sutures to fibrous and bony holds (i.e., pterygomandibular raphe or hamulus pterygoideus) without needing any fibromuscular resection or relocation. Most techniques use a self-locking bidirectional barbed suture that is inserted in a certain manner into the soft palate and both pillars including the tonsillar fossa.
The target group presents with retropalatal and lateral pharyngeal wall collapse as documented in DISE. Studies included patients with BMI < 35 or <30 kg/m2 and sleep apnea severity ranging from mild to severe. All studies suggest an effective treatment of OSA patients ranging from mild to severe cases, a statistically significant reduction in endpoints such as AHI, ODI, LSAT, ESS, and reduction of snoring. Some studies suggest the barbed reposition pharyngoplasty to be equally effective as ESP and more effective than UPPP.1763 In 2019, Vicini et al. published results of an RCT comparing the BRP to a control group (observation) where the surgical BRP group had nasal surgery, tonsillectomy, and BRP. The 6-months follow-up showed a significant reduction in AHI and other relevant endpoints in patients that underwent surgery.1691 Most studies and techniques include simultaneous or prior tonsillectomy and some studies included surgery at other levels.
The postoperative morbidity of patients who underwent suture suspension surgery is reported as low. Common short-term complications were postoperative pain, dysphagia, and in some cases suture extrusion. All papers stated an overall well-performable intervention with minor sideeffects.1702,1772–1774 Further data is needed to evaluate the long-term outcomes.
Unfortunately, the LOE of most existing studies is low as, except for the RCT by Vicini et al., most studies were either non-randomized single-arm studies or retrospective studies. Furthermore, the often-performed MLS in addition to suture suspension prohibits a conclusion about the specific effectiveness of suture suspension techniques.
In summary, suture suspension surgery appears to be an effective approach, when performed on selected patients. Compared to other surgical techniques, such as UPPP or ESP, suture suspension seems less invasive. (Table IX.D.4.g)
TABLE IX.D.4.g.
Evidence on palate suture suspension for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Askar et al.1775 | 2018 | 4 | Single-center, prospective, uncontrolled case series | 29 Patients -OSA (AHI > 15/h) -Surgical technique: double suspension suture + modified anterior palatoplasty -Tonsillectomy in patients with tonsil grade 2–4 (n = 17) -History of tonsillectomy (n = 8) -Mean BMI 24.1 kg/m2 -Pre-op DISE: retropalatal collapse |
-10–18 months follow-up -AHI -LSAT -ESS, VAS for snoring -Increase in retropalatal space measured during surgery |
-Mean AHI dropped from a mean of 28.6 ± 5.5 to 8.3 ± 2.96 -AHI < 10/h was achieved in 98.65% (n = 26) -The mean LSAT increased significantly from 79.4 ± 3.5 to 93.1 ± 3.1 -ESS showed significant improvements (14.5 ± 2.4 to 3.4 ± 1) -VAS for snoring significantly decreased from 7.9 ± 1.3 to 1.9 ± 1.2) -Retropalatal space widened significantly at the end of surgery |
| Askar et al.1776 | 2018 | 4 | Single-center, prospective single-arm study | 22 Patients -OSA (AHI > 5/h) -Surgical technique: double palatal suspension sutures technique -BMI < 30 kg/m2 -Pre-op DISE: retropalatal obstruction |
-6-months follow-up -AHI, LSAT -ESS -VAS for snoring |
-Significant improvement of AHI (29.7 ±5.5 to 7.9 ±3.7 (p < 0.0001), LSAT (80.34 ± 4.42 to 90.3 ±4.75), ESS (12.3 ± 3.7 to 4.6 ± 1.9) -Snoring disappeared in 86.4% of patients and was reduced in 13.6% -Mean inter-pillar distance and the retropalatal distance increased significantly after completion of the procedure |
| Babademez et al.1692 | 2019 | 4 | Single-center retrospective comparative chart review | -Mild-moderate OSA -Surgical technique: BRP (n = 45), ESP with AP (n = 53) -Simultaneous tonsillectomy performed |
-Mean follow-up 18.8 months -Surgical success ratea -AHI -ESS |
-Overall success ratea was 85.7%, with no significant statistical difference between BP and ESPw/AP -AHI reduction in ESPw/AP from 28.5/h to 9.1/h and in BRP from 25.9/h to 7.4/h -BP group required less postoperative analgesic treatment |
| Babademez et al.1777 | 2019 | 4 | Prospective, non-randomized two-armed study | 34 Patients -Mild–moderate OSA -Surgical technique: BRP (n = 17) vs. mBRP involving barbed uvula lift (n = 17) -All patients received tonsillectomy -Pre-op DISE: retro-palatal obstruction of any type, no hypopharyngeal obstruction -BMI < 35 kg/m2 -No nasal obstruction, tonsillar hypertrophy < grade 2 |
-Mean follow-up 6–9 months -AHI, ESS, Snoring |
-82% and 95% success ratea for BRP and mBRP, respectively, with no significant difference between the two procedures (p > 0.05) |
| Cammaroto et al.1763 | 2017 | 4 | Retrospective, comparison study | 30 Patients -OSA (mean AHI > 30/h) -BRP (n = 10) or UPPP (n = 10) or ESP (n = 10) + TORS with supraglottoplasty (SGP) and temporary tracheostomy, tonsillectomy and septo-turbinoplasty in all patients -BMI < 30 kg/m2 |
-At least 6-months follow-up -AHI -ESS |
-All patients received the same multilevel surgical procedure except for the palatal surgery, which was different -Surgical success rate in BRP (and ESP) was 90% (50% in UPPP) -AHI reduction in BRP and ESP, ESS reduction in all patients |
| El-Ahl et al.1770 | 2016 | 4 | Single-center prospective uncontrolled case series | 24 Patients -OSA (AHI < 15/h) -Surgical technique: expansion pharyngoplasty by simple suture suspension -No tonsillectomy was performed -Tonsil size grade 0–2 -BMI < 30 kg/m2 -Pre-op DISE: retropalatal obstruction of any type, lateral pharyngeal wall collapse, no retroglossal collapse |
-6-months follow-up -AHI, LSAT, ESS, VAS for snoring |
-Mean AHI dropped significantly from 28.6 ± 4.2 preoperatively to 8.9 ± 4.9 postoperatively -Mean LSAT level increased significantly from 79.25 ± 4.12 to 89.29 ± 5.28 -Mean ESS decreased from 11.7 ± 2.9 to 5.1 ± 2.2 -VAS for snoring reduced significantly. Snoring completely disappeared in 75% |
| Elbassiouny et al.1771 | 2016 | 4 | Single-center prospective uncontrolled case series | 21 Patients -OSA -Surgical technique: modified barbed soft palatal webbing flap palatopharyngoplasty using barbed sutures -Included tonsillectomy where tonsillar collapse was documented in DISE (n = 4, 19%) -VAS for snoring |
-6-months follow-up -AHI, LSAT |
-95% Surgical success ratea -Significant reduction in AHI (45.7 ± 2.6 to 12.3 ± 3.9), increase in LSAT (74 ± 4 to 89 ± 2%) -Only three patients had residual lateral wall collapse in postoperative DISE |
| Mantovani et al.1766 | 2015 | 4 | Pilot longitudinal single-arm study | 32 Patients -Severe OSA (AHI > 30/h) -Surgical technique: barbed roman blinds technique -Pre-op DISE: retropalatal lateral obstruction -All patients had formerly undergone tonsillectomy -BMI < 30 kg/m2, BMI-variation < 0.2 within follow-up |
-Mean follow-up 12 months -Success ratea -AHI -t<90%spO2 -ESS |
-Surgical success ratea of 84.4% -Significant reduction in AHI (36.9 ± 4.5/h to 13.7 ± 4.5/h (p < 0.001)), t < 90% and ESS) |
| Mantovani et al.1767 | 2017 | 4 | Single-arm, non-randomized, pilot study | 19 Patients -OSA (AHI 5–30/h) -Surgical technique: Alianza technique -DISE: concentric pharyngeal collapse at velum -All patients post-tonsillectomy -BMI < 30 kg/m2 |
-6-months follow-up -AHI, ESS -VAS for snoring |
-Significant reduction in AHI (22.4±5 vs. 7.4±9.4, p = 0.002), (ESS 11.3 ± 5 vs. ESS 3.9 ± 4, p < 0.001) -Reduction in snoring (9.5 ± 0.7 vs. 2.1 ± 1.7; p < 0.01) |
| Rashwan et al.1691 | 2018 | 3b | Interventional comparative study | 75 Patients -Moderate–severe OSA -Pre-op DISE: palatal obstruction with lateral pharyngeal wall collapse -Surgical techniques: BRP (n = 25), UPPP (n = 25), ESP (n = 25) -BMI < 35 kg/m2 -All patients received tonsillectomy and nasal surgery (turbinoplasty and/or septoplasty) |
-6-months follow-up with PSG -Delta(Δ)-AHI, -ESS, -ODI, -LSAT |
-Significant reduction of all end-points in all groups (p < 0.05) -Mean AHI reduction highest in BRP group (: Δ-AHI: BRP −15.76 ± 14.5/h vs. ESP: −10.1 ± 5.3/h vs. UPPP: −6.1 ± 5.5) -Mean ESS reduction highest in BRP group (Δ-ESS: BRP: −5.52±4.1 vs. ESP: −4.84 ± 3.3 vs. UPPP: −1.36 ± 1.9; p < 0.005) -No significant difference in reduction of LSAT between the three groups |
| Vicini et al.1764 | 2019 | 1b | Single-center prospective randomized two-armed controlled trial | 50 Patients (group A: 25 BRP, group B: 25 observation) -Diagnosis: moderate–severe OSA (AHI >15/h) -Surgical technique: BRP -All patients received tonsillectomy and nasal surgery -Age 18–65 years -BMI < 35 kg/m2 -Pre-op DISE: severe circular palatal collapse and severe transversal pharyngeal collapse with none or mild tongue collapse |
-6-months follow-up -AHI, ODI, LSAT -ESS |
-Significant reduction of AHI, ODI, LSAT, and ESS (p < 0.05) in BRP group -AHI and ODI in BRP group superior to observation (Δ-AHI BRP: −15.75±14.7 vs. control: −5 ± 13.75, p = 0.01; Δ-ODI BRP: −15.1 ± 17.9 vs. control: −2.84 ± 14.55, p = 0.01) -LSAT improvement not significant -ESS reduction not significantly different -No significant changes in control group observed -Higher baseline AHI predicts more significant postoperative absolute AHI reduction -Both groups showed no significant change in BMI |
Abbreviations: AP, anterior palatoplasty; BRP, barbed reposition pharyngoplasty; DISE, drug-induced sleep endoscopy; ESP, expansion sphincter pharyngoplasty; LSAT, lowest oxygen saturation; mBRP, modified barbed reposition pharyngoplasty; TORS, transoral robotic tongue base reduction; UPPP, uvulo palato pharyngoplasty.
Surgical success defined as 50% reduction in AHI and AHI < 20 (if not indicated otherwise).
IX.D.5 |. Tongue and hypopharyngeal surgery
IX.D.5.a |. Tongue base radiofrequency ablation
Tongue base radiofrequency (TBRF) was first described by Powell et al.1778 Energy delivered through an RF or coblation device causes submucosal coagulation and ablation which leads to soft tissue fibrosis. While it was initially proposed that its effect would occur primarily through volumetric tissue reduction, Stuck et al.1779 and Blumen et al.1780 could not find any tongue base volume changes in MRI scans after TBRF, and these authors suggested that the benefits of TBRF were the result of the scar formation process.1781
An SR of TBRF outcomes is challenging, due to significant variability of devices, techniques used, presence or absence of procedures performed simultaneously, patient selection criteria, outcomes measured, and paucity of data on long-term results.
As the available evidence is most commonly focused on MLS, the assessment of a sole effect of each intervention is challenging. In this review, we only selected studies in which individual analysis for TBRF was performed. We excluded papers where other procedures were performed but no individual role of TBRF was assessed, and those with duplicate results. This review included 12 articles, including one SR and MA.1782
Three techniques regarding TBRF have been described: transoral through the dorsal tongue, transoral through the ventral aspect of the tongue, and the transcervical approach. The first is the most commonly used technique. With the exception of den Herder et al.,1781 all papers found a positive role for TBRF, despite significant methodological heterogeneity. Two articles suggested a decrease in efficacy over time.1783,1784
A metanalysis by Baba et al. included three non-randomized, parallel group comparative trials (CPAP, submucosal minimally invasive lingual excision (SMILE), and lingual suspension), and seven prospective case series.1782 They found 40% reduction on RDI (p < 0.0001), statistically significant improvement in LSAT (p = 0.002), and improved sleepiness score (p < 0.00001) with short term (<2 months) follow-up.
It must be noted that none of the included studies had a placebo or non-treated control group though Woodson et al.1785 had a cohort of non-surgical patients under CPAP treatment. Two articles compared UPPP alone and UPPP plus TBRF,1786,1787 and both found a higher AHI reduction with the addition of TBRF. Friedman et al. found that specifically stage III patients (tongue base obstruction) were not significantly improved by UPPP alone (control group) but were significantly improved by UPPP plus TBRF (experimental group).
Two studies compared TBRF with other techniques.1783,1788 Friedman et al. found a statistically significant difference favoring SMILE, in patients with stage III anatomy (tongue base obstruction) and patients with severe OSA,1788 however SMILE presented a higher complication rate. Fibbi et al. compared TBRF with tongue base suspension and found no difference between the groups.1783
There is significant heterogeneity on inclusion criteria among studies, not allowing a pooled analysis. While some articles studied subjects who failed prior surgeries or severe cases,1780,1785,1789 others include primarily mild or moderate cases.1790 Some authors used DISE to indicate surgery,1787 while others only relied only on physical examination. Obesity, another variable assessed differently among selected studies, was a predictor of failure as shown by several authors.1789,1791
Woodson et al. found that TBRF was useful in mild OSA patients.1785 This finding was also highlighted by Fibbi et al., who observed 75% success rate after 6 months, and 33% after 2 years, concluding that their high ratio of success was attributed to the sample selection (mild OSA with AHI < 20 and tongue base obstruction Fujita III).1783 Riley et al. found that a lower preoperative AHI and higher LSAT are statistically related to better response.1789
Available evidence suggests low complication rates. Major complications include severe tongue edema, lingual artery lesion, hypoglossal nerve injury, and tongue abscess. While Babae et al. analyzed complications in their metanalysis, no cumulative data for complications was provided.1782 It is worth mentioning that Blumen et al. performed a transcervical approach with ultrasonography guidance to prevent lingual artery and hypoglossal nerve lesions.1780
In summary, the available literature suggests that TBRF has the ability to improve OSA as an adjuvant treatment in well selected patients with tongue base obstruction and without severe OSA. However, there remain questions about the optimal patient selection and the long-term effects. (Table IX.D.5.a)
TABLE IX.D.5.a.
Evidence on tongue base radiofrequency for OSA treatment
| Study | Year | LOE | Study design | Study group(s) | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|
| Babae et al.1782 | 2015 | 3a | Systematic review and metanalysis | TBRF alone (n: not reported) | RDI, AHI, LSAT, ESS, VAS | RDI 40% reduction (p < 0.0001). LSAT RoM = 1.05 (p = 0.02). ESS reduction RoM = 0.59 (p < 0.00001) and VAS. Snoring RoM = 0.48 (p < 0.00001). |
| Fibbi et al.1783 | 2009 | 4 | Cohort | TBRF (n = 13) Lingual suspension (n = 13) |
Surgical success (AHI >50% decrease, final < 20); AHI, AI, LSAT, Change in ESS and VAS | Success 75% with TBRF at 6 months; 33% at 24 months. Reduction in AHI after 6 months (14.8–4.7) and 24 months (8.7). ESS and VAS improved at 6 and 24 months. No sig difference between techniques. |
| VandenBroek et al.1787 | 2008 | 4 | Retrospective cohort | UPPP alone (n = 38) UPPP + TBRF (n = 37) | Surgical success (>50% decrease and AHI < 20) | 42% success in UPPP alone vs. 49% in combined group, no sig difference. |
| Den Herder et al.1781 | 2006 | 4 | Quasi-experimental | TBRF pre- (n = 22) and posttreatment (n = 10) | Surgical success (>50% decrease AHI < 20 or AI < 10), AHI, ESS, VAS | 33% success. No significant change in AHI, ESS postprocedure. Improved snoring. |
| Blumen et al. 1780 | 2006 | 4 | Quasi-experimental | TBRF before (n = 10) and after treatment (n = 10) | RDI, AI, HI, LSAT, snoring volume, ESS, tongue volume on MRI | RDI improved (52 to 33.6, p = 0.016); AI LSAT, ESS, Snoring VAS improved. No change in tongue volume on MRI. |
| Riley et al.1789 | 2003 | 4 | Quasi-experimental | TBRF before (n = 20) and after treatment (n = 19) | AHI, LSAT, ESS, VAS (speech and swallow) | Significant AHI improvement (35.1 to 15.1); LSAT (82% to 86.3%); ESS (12.4 to 7.3); No difference in VAS for speech and swallow. |
| Friedman et al.1786 | 2003 | 4 | Retrospective cohort | UPPP alone (n = 134) UPPP + TBRF (n = 143) | Surgical success (>50% decline to RDI < 15) | UPPP alone success rates by Friedman Stage I: 80.6%; Stage II: 37.9%; Stage III: 8.1%. UPPP + TBRF Stage II: 55.1% Stage III: 33% Significant difference. |
| Stuck et al.1790 | 2002 | 4 | Quasi-experimental | TBRF before (n = 20) and after treatment (n = 18) | Success >50% decline to RDI < 15; RDI, mean O2 sat, ESS, VAS for snoring | Success in 33%; Responders 55%. Improved ESS and VAS for snoring (significant). No difference in RDI, mean O2 sat. |
| Li et al.1784 | 2002 | 4 | Quasi-experimental | TBRF before (n = 18) and after treatment (n = 16) | RDI, AI, HI, LSAT, ESS, SF-36 in short and long term | Short term: sig improvements in RDI, AI, LSAT. Long term: Improved RDI, AI, LSAT, ESS, VAS snoring, and SF-36 scores. |
| Woodson et al.1785 | 2001 | 4 | Quasi-experimental | TBRF before (n = 73) and after treatment (n = 56) | Success >50% decline AHI <20, AHI, AI, LSAT, SF36, SNORE-25, ESS, FOSQ | Success: 20%. Sig improvements in AHI, AI, ESS, FOSQ, VAS snoring. |
| Powell et al.1778 | 1999 | 4 | Quasi-experimental | TBRF before (n = 18) and after treatment (n = 18) | RDI, AI, HI, LSAT; Tongue volume on MRI, SF-36, ESS | Significant improvement in RDI, AI, LSAT, ESS; 40% cure rate; Reduced tongue volume. |
IX.D.5.b |. Genioglossus advancement
In 1986, Riley et al. first described the inferior sagittal osteotomy of the mandible with hyoid myotomy and genioglossus muscle advancement (GA) for the treatment of OSA.1792 The objective for this procedure was to stabilize the hypopharyngeal airway by moving the genioglossus muscle forward, reducing its collapse and subsequently increasing the pharyngeal airway during sleep. Since the initial description, the procedure has undergone several modifications.
Genioglossus advancement with or without hyoid surgery (HS) was designed to increase the retrolingual space.1634 Because GA is rarely performed in isolation and more commonly performed in conjunction with other surgeries to address different levels of airway collapse, there is limited evidence evaluating the sole effect of GA. There can be significant heterogeneity in surgical techniques, thereby limiting a systematic analysis. Several osteotomy designs have been used to advance the genioglossus musculature, including standard genioplasty, inferior sagittal osteotomy, circular genioplasty, mortised genioplasty, or genial bone advance trephine system. Variations in osteotomies may impact nearby muscles including the geniohyoid, digastric, and/or mylohyoid to enhance the advancement of the UA. Studies in which individual analysis for GA with or without hyoid surgery or tongue base reduction were selected.
Eight papers suggest a positive impact of GA on OSA outcomes; however, evidence for genioplasty and genioglossus advancement is limited by heterogeneity in patient selection and surgical technique.1793 A case series by Song et al. evaluated the individual role of GA and identified a mean AHI reduction from 37.6 (24.2) to 20.4 (15.1) (41.7% reduction).1794 After GA with HS, mean AHI reduced from 34.5 (22.1) to 15.3 (17.6) (55.7% reduction).1794
One of the inherent limitations of studying GA outcomes is variation in patient selection, and available data on clinical characteristics and candidacy for surgery is limited. For example, Chen et al. selected subjects who declined CPAP1795 as compared to Wootten et al. who selected pediatric subjects who failed adenotonsillectomy (T&A).1796 Other authors had stricter criteria and indicated GA only after cephalometry,1792,1797,1798 or DISE.1799
Despite the absence of clear practice guidelines, several predictive factors have been described. Riley et al. suggested that patients with normal skeletal development are the best candidates for GA; however, patients with mandibular deficiency can be candidates.1800 Troell et al. suggested a positive correlation between the length of advancement and AHI improvement.1801 Kezirian et al. found BMI and AHI to be predictors of outcome.1802 Consequently, Foltán et al. excluded patients with an BMI over 30.1803 Vilaseca et al. observed that AHI severity predicted outcomes, and best results were seen in patients with mild OSA.1798
Finally, another important factor is the difference in the follow-up periods, for surgical benefit may wane with time. There is significant heterogeneity among selected papers, varying from 31792 to 12 months.1795 There is no long-term follow-up data for GA.
While limited, the available data suggests that GA surgery can have a positive impact on AHI; however, there is significant heterogeneity in patient selection criteria, use of simultaneous procedures, operative techniques, and length of follow-up. (Table IX.D.5.b)
TABLE IX.D.5.b.
Evidence on genioglossus advancement for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Song et al.1804 | 2016 | 2a | Systematic review and meta-analysis | 1. GA (n = 24) 2. GA-HS (n = 50) |
1. AHI 2. LSAT |
1. GA: 45.7% AHI reduction, (p < 0.005). No significant LSAT improvement. 2. GA-HS: 55.7% AHI reduction (p < 0.001). LSAT improved from 80% to 88% (p < 0.002) |
| Kuscu et al.1805 | 2015 | 4 | Case series | 1. GA (n = 17) | AHI, ESS, minimum, and mean O2 saturation variation | 53% success rate. Statistically significant differences in AHI, mean SpO2 and ESS score |
| Chen et al.1795 | 2014 | 4 | Nonrandomized prospectivecohort study | 1. UPPP (n = 27) 2. UPPP + GA (n = 24) 3. UPPP +TBA (n = 26) |
Sleep stage, AHI, LSAT, CT90 | All significantly improve mean AHI, LSAT, and CT90 scores. No statistical comparison made |
| Foltan et al.1803 | 2007 | 4 | Case series | 1. GA (n = 31) | ESS, RDI, ODI, duration of the desaturation, basal O2 saturation, and average desaturation variation | 74% success rate. Improved ESS, RDI, ODI (p < 0.01) |
| Richard et al.1799 | 2006 | 4 | Case–control | 1. Multilevel surgery with GA (n = 14) 2. Multilevel surgery without GA (n = 8) |
1. AHI 2. ODI 3. ESS |
No significant difference outcomes between groups |
| Riley et al.1797 | 1993 | 4 | Case series | 1. GA-HS (n = 6) 2. GA-HS + UPPP (n = 233) 3. UPPP (n = 10) |
Success rate | 1. 66% success 2. 60% success 3. 80% success No statistical comparison performed |
| Riley et al.1792 | 1986 | 4 | Case series | 1. GA-HS (n = 5) | 1. AHI, RDI, success | 1. 60% success 2. AHI decreased for all patients 3. RDI decreased for all patients by 46%–85% |
Abbreviations: AHI, apnea hypopnea index; CT90, percentage of time with saturation below 90%; ESS, Epworth sleepiness scale; GA, genioglossus advancement; HS, hyoid surgery; LSAT, lowest oxygen saturation; ODI, oxygen desaturation index; RDI, respiratory disturbance index; UPPP, uvulopalatopharyngoplasty.
IX.D.5.c |. Tongue suture suspension
Surgical intervention for OSA must target the area of collapse to improve outcomes.1806,1807 Evidence found that those with hypopharyngeal or tongue base collapse did not respond to palatal surgery alone and had poorer outcomes with success rates as low as 5%.1634,1808 In 2006, approximately 19% of sleep surgery was performed to target the hypopharynx including tongue base.1809
Tongue suture suspension has been developed to increase the anterior to posterior space of the hypopharynx with minimal morbidity and recovery time. The surgery involves placing a bone anchored screw into the lingual cortex of the mandibular symphysis, placing an anchoring nonabsorbable suture through the base of the tongue and securing it to the screw. Initially, a technique was utilized which placed a screw directly through the oral mucosa along the lingual surface of the mandible. This technique allowed for a minimally invasive, reversible intervention. Modifications have since been made involving a submental cutaneous approach, positioning a suspension suture in the mandibular symphysis without the use of a screw with successful outcomes.1810 For the purposes of this review, surgical success, unless otherwise specified, is defined by Sher’s criteria as (1) a 50% decrease in Respiratory Disturbances Index (RDI) or AHI and (2) RDI or AHI < 20.1634
The largest SR to date1811 estimated surgical success of isolated tongue suspension (TS) to be 36.6%, ranging from 0% to 87%.1812–1814 However, it is important to note that TS is uncommonly performed as an isolated surgery and is more often performed in combination with or following palate surgery, and therefore, success rate estimations quoted in the literature more often reflect MLS. When combined with UPPP, TS success rates increased to 62.3%, ranging from 40% to 81%.1815,1816
The main challenge with TS surgery is titrating the tension on the tongue to optimize the anterior to posterior diameter of the hypopharynx without causing discomfort or tissue strangulation. Overall complications from this procedure are low at about 8%,1811 including sialadenitis, pain, and infection. There were reports of mandibular fractures with early use of bone anchored screws; however, modifications using adapted screws1812 or a no-screw technique1810 have not reported fracture complications. There has been concern regarding longevity of surgical outcomes due to risk of suture migration given that most of the studies published have limited follow-up between 2 months and 2 years.
Success rates vary based on the patient selection criteria. Across studies, surgical success for isolated tongue suture suspension ranged from 21% to 75% when average BMI of the selected sample was less than 30, but trended toward 30%–32% when average BMI exceeded 30.1811 When looking at the subjective QOL outcomes after TS, one small study of 23 patients found no significant change in subjective sleepiness or QOL 2 months after surgery.1814 One review found significantly higher success rates in symptom improvement; however, the study included shorter postoperative follow-up periods from 3 to 12 months.1817
Overall, previous SRs1811,1817 have supported a grade C recommendation for TS. The wide variation in success rates across studies illustrates the importance of careful patient selection. (Table IX.D.5.c)
TABLE IX.D.5.c.
Evidence for tongue suture suspension for OSA
| Study | Year | LOE 1–5 | Study design | Study groups | Outcome | Conclusion |
|---|---|---|---|---|---|---|
| Handler et al.1811 | 2014 | 2a | Systematic review and meta-analysis | TS (6 studies, n = 82) TS + UPPP (8 studies, n = 167) |
AHI | TS: Mean preop AHI: 32, postop: 19, 41% AHI reduction, 36.6% successa TS + UPPP: mean preop AHI: 44, postop: 17, 62% AHI reduction, 62.3% success |
| Kezirian and Goldberg1802 | 2006 | 2a | Systematic review | TS (6 studies, n = 77) |
AHI ESS |
1. Significant change in AHI. Mean preop AHI: 33–45, postop: 17–24. 20%–57%successa 2. Three to six studies reported sig. improved ESS |
| Bostanci and Turhan1817 | 2016 | 2a | Systematic review | 1. TS (7 studies, n = 113) 2. TS + UPPP (10 studies, n = 300) |
AHI ESS |
TS: significant change in mean AHI preop: 18–45, postop: 5–28. 48% successb (11%–77%). Significant decrease in ESS TS + UPPP: sig change in mean AHI preop: 29–53, postop: 9–24. 70% successb (20%–89%). Significant decrease in ESS |
| Woodson et al.1818 | 2010 | 2b | Prospective case series | TS for moderate to severe OSA (n = 42) | AHI, ESS, FOSQ, | Significant change in mean AHI preop: 35, postop: 27 Sig. change in ESS preop 12, postop: 8 Sig change in FOSQ preop: 16, postop:18 |
| Tsou et al.1819 | 2018 | 4 | Prospective case series | Patients with large tongues, small tonsils who underwent UPPP and TS (n = 36) | AHI REM sleep Sleep efficiency |
Significant change in mean AHI preop: 25, postop: 18. 66% successa No change in ESS Increased REM and sleep efficiency in success group |
| Thomas et al.1816 | 2003 | 1b | Randomized trial | UPPP with 1. TA (n = 9) or 2. GA (n = 8) |
AHI ESS |
Group 1. Significant change in mean ESS preop: 12, postop: 4. 57% (4/7) successa Group 2. Significant improvement in ESS preop: 13, postop: 5. 50% (2/4) successa |
Abbreviations: AHI, apnea hypopnea index; ESS, Epworth sleepiness scale; FOSQ, Functional Outcomes of Sleep Questionnaire; GA, genioglossus advancement, hyoid myotomy; OSA, obstructive sleep apnea; TS, tongue suspension; UPPP, uvulopalatopharyngoplasty.
Success based on Sher’s criteria = reduction in AHI ≥ 50% with final AHI < 20/h.
Success not defined.
IX.D.5.d |. Hyoid suspension
In 1996, Sher et al. revealed that overall surgical success of UPPP decreased from 52% of those with retropalatal collapse to 5% for those with mainly hypopharyngeal collapse.1634 Consequently, it became clear that the field of sleep surgery required additional surgical interventions to try and address hypopharyngeal obstruction. One technique included anterior repositioning of the hyoid to increase the anteroposterior dimension of the hypopharynx and subsequently reduce tongue base obstruction.1820 Hyoid suspension or hyoidthyroidpexy can be performed alone or in combination with other surgeries including genioglossus advancement or maxillomandibular ostomy to target hypopharyngeal obstruction.
Hyoid suspension was first described in 1984, at which time, the hyoid bone was isolated from the strap musculature attachments and suspended to the inferior border of the mandible using fascia lata.1821 This technique was then modified to isolate the hyoid from the suprahyoid musculature and suspend the center of hyoid arch anteroinferiorly to the thyroid lamina, which was found to have less morbidity.1822
In the largest review of isolated hyoid myotomy and suspension to date, AHI improved by 38% (range of 7%–83%) for a mean AHI reduction from 37 to 23.1804 In this study, three of the four studies evaluating subjective QOL found modest improvement in sleepiness, as measured by the ESS. Another study found that surgical success, as defined by Sher’s criteria,1634 ranged from 17% to 87% across studies evaluating isolated hyoid suspension.1551,1798,1802,1820,1823 For the studies in which the sample population’s average BMI fell below 30, success rates ranged from 52% to 78%1551,1798,1820; however, success rates decreased to 17% when the population’s BMI was above 30,1823 illustrating the impact of careful patient selection on surgical success rates.
Given concern for immediate obstruction upon induction and extubation, hyoid myotomy, and suspension under local anesthesia was developed. One study of 32 patients undergoing hyoid suspension and uvulopalatal flap under local anesthesia found a 78% success rate by decreasing RDI by 50% with postoperative RDI<20.1551 Symptomatically, ESS scores decreased from an average of 14 to 8. Complications included mild intraoperative pain, transient dysphagia with aspiration, seroma, and infection. However, the higher success rate may have been due to careful patient selection for surgery under local anesthesia given the patient sample had lower average BMI as compared to other studies.
In practice, it is common to perform MLS as isolated hypopharyngeal obstruction is rare in OSA. Several studies have shown significantly higher success rates when combined with palatal surgery.1670,1802,1820 One study found that surgical success of hyoid suspension increased from 22% to 78% with concomitant tonsillectomy.1670 Similarly, ESS decreased by 45% with concomitant tonsillectomy as compared to 27% without.
There has yet to be an RCT evaluating efficacy of hyoid suspension as an isolated procedure or as part of an MLS. There is significant heterogeneity across studies due to large variation in patient inclusion criteria, surgical interventions, duration of follow-up, and outcome measures. Consequently, there are no published meta-analyses evaluating hyoid suspension outcomes. (Table IX.D.5.d)
TABLE IX.D.5.d.
Evidence on hyoid suspension for OSA
| Study | Year | LOE (1A-5) | Study design | Study groups | Clinical endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Kezirian1802 | 2006 | 2a | Systematic review of retrospective studies | 1. HS (4 studies, n = 101) 2. HS + GA and palate surgery (7 studies, n = 328) |
AHI EDS |
1. Preop AHI: 32–48, Postop: 15–37. Sig decrease in 2/4 studies. 17%–78% success. EDS with sig decrease in ¾ studies. 2. Preop AHI: 27–71, Postop: 10–57. Sig decrease in 2/7 studies. 18%–77% success. EDS with sig decrease in ¾ studies. |
| Verse1670 | 2015 | 2a | Prospective controlled clinical trial | UPPP with 1. Lingual tonsillectomy (n = 58) 2. HS+RFA (n = 50) |
AHI | 1. Mean AHI preop: 35, AHI postop: 17, 59% success. ESS decreased from 10 to 6, (p < 0.001). 2. Mean AHI preop: 50, AHI postop: 29, 54% success. ESS decreased from 12 to 8, (p < 0.001) No significant difference between groups. |
| Verse1824 | 2006 | 2b | Prospective cohort study |
Multilevel surgery 1. With HS (n = 23) 2. Without HS (n = 12) |
AHI ESS |
1. Mean preop AHI: 39, postop: 21, (p < 0.001). ESS decreased from 9 to 7, (p < 0.05). 2. No significant decrease in AHI. ESS showed decrease from 9 to 4, (p < 0.05). |
| Song1804 | 2016 | 2a | Systematic review and metaanalysis | Isolated HS (9 studies, n = 101) | AHI ESS |
Mean AHI reduced 38% from 37 to 23 (p < 0.001). 7%–83% reduction in AHI Mean ESS decrease from 10 to 3 (p < 0.003). |
| Askar1825 | 2019 | 4 | Case series | DISE confirmed hypopharyngeal collapse (n = 21) | AHI | Mean AHI decreased from 48 to 12, (p < 0.001). |
Success based on Sher’s criteria = reduction in AHI ≥ 50% with final AHI < 20/h.
Abbreviations: AHI: apnea hypopneaiIndex; DISE = drug-induced sleep endoscopy; EDS = Excessive daytime sleepiness; ESS = Epworth sleepiness scale; HS = hyoid suspension; RFA = radiofrequency ablation; Sig = signficant
IX.D.5.e |. Lingual tonsillectomy
It is important for the practitioner to perform an assessment of the potential for lingual tonsil hypertrophy in retroglossal airway obstruction. This may be accomplished by indirect examination with a flexible laryngoscope, cross-sectional imaging with either CT or MRI, or DISE. The practitioner may be guided by a grading system for lingual tonsillar hypertrophy such as suggested by Friedman et al.556 Patients with Friedman grade 3 or 4 would be more likely to obstruct. A limitation to the Friedman grading system is that one cannot assess depth of tonsillar tissue, or, alternatively, the component of tongue base enlargement and retroglossal airway compromise that is rather due to macroglossia. Often lingual tonsillectomy is performed in conjunction with palate surgery as a form of multilevel airway surgery.
Many tools for performing lingual tonsillectomy have been reported, including: CO2 laser, electrocautery, harmonic scalpel, coblation, radiofrequency ablation (RFA), and a microdebrider. There are several methods of visualizing the base of tongue and lingual tonsils including direct visualization with a laryngoscope or indirect approach with an angled endoscope, operating microscope, or more recently, TORS with a DaVinci robot (Intuitive Surgical; Sunnyvale, CA). The robotic approach offers superior visualization, instrument access, and more precise tissue resection which can result in larger volumes of tissue removal.1826 Friedman et al. found TORS to be superior to RFA of the tongue base and submucosal minimally invasive lingual incision in terms of AHI reduction and surgical success rates.1827 Coblation and TORS appear to have similar success rates, though no prospective, randomized trial has been performed to date. There is a suggestion in the literature that coblation is less painful than electrocautery, but no prospective, randomized trial has been performed to assess this.1828
When base of tongue obstruction from lingual tonsils is seen, evidence suggests that there is an additional benefit to lingual tonsillectomy over palate-level surgery alone.1829,1830 Success rates (traditionally defined as a reduction of AHI > 50% and a postoperative AHI < 20) across multiple studies are in the range of 66.4%–68.4% versus 40.7% for UPPP alone. This is also true for other measures of success, such as a lower ESS (reduction of 5.4–7.1 points) and improvement in SaO2 nadir (increase of 5.1%–5.4%). Complications of lingual tonsillectomy are rare, but include bleeding, dysphagia, globus sensation, dysgeusia, tongue numbness and soreness, and scarring.1831 There is a body of evidence that suggests that outcomes after sleep surgery, including lingual tonsillectomy, are better with BMI < 30, preoperative AHI < 60, and absence of lateral velopharyngeal wall collapse on DISE.1832
It should be emphasized that there is no clear phenoptype for successful base of tongue surgery, including patients with lingual tonsil hypertrophy. The studies examined in these SRs have much heterogeneity, both in patient evaluation, and in surgical technique. As there are no RCTs for this surgery, a strong recommendation cannot be given. However, lingual tonsillectomy can be recommended with a preponderance of benefit over harm for adult OSA patients with lingual tonsil hypertrophy (Friedman grade 3 or 4). The individual practitioner must weigh the considerations of anatomy, severity of OSA, BMI, surgical training, and familiarity with available surgical equipment when deciding whether to proceed with lingual tonsillectomy. (Table IX.D.5.e)
TABLE IX.D.5.e.
Evidence on lingual tonsillectomy for OSA
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Miller et al.1829 | 2017 | 2a | SR and meta-analysis |
Adult patients with OSA (n = 353) | 1. AHI 2. Epworth Sleepiness Scale 3.O2 nadir 4. Snoring visual analogue scale 5. Surgical success rate (AHI reduction >50% and to <20 events/h) 6. Cure rate (AHI < 5) |
TORS BOT reduction decreases AHI (44.3 ± 22.4 to 17.8 ± 16.5, p < 0.01) and symptoms of sleepiness (ESS: 12.9 ± 5.4 to 5.8 ± 37, p < 0.01) and is considered successful in a majority (68.4%) of cases. |
| Samutsakorn et al.1830 | 2017 | 2a | SR and meta-analysis | Adult patients with OSA with multilevel obstruction (n = 107) |
1. AHI 2. O2 nadir 3. Epworth Sleepiness Scale |
Lingual tonsillectomy with palatal surgery reduces AHI by 18.5 events/h (p = 0.006), increases O2 nadir (mean increase: 5.3, p = 0.05), and reduces daytime sleepiness (mean reduction: 5.4, p = 0.001) in select patients with OSA. |
IX.D.5.f |. Epiglottis surgery
The role of the epiglottis in adult OSA has been studied comparatively little in the adult arena. With the increasing prevalence of DISE, sleep surgeons have come to recognize that epiglottic collapse can play a role in airway obstruction. However, data that is available is conflicting. One early study by Catalfumo et al. showed a 12% incidence of epiglottic collapse during awake endoscopy.1833 A systemic review by Torre et al. found a much broader potential range of epiglottic collapse in OSA, ranging from 15% to 73.5%.1834 A more recent study by Vonk et al. showed that epiglottic collapse noted on DISE may be subject to body position. In 324 patients, a floppy epiglottis was noted in 18.5%, but when rotated to the lateral head or trunk position, only one patient had evidence of obstruction.1835
Whether or not identification of epiglottic collapse on DISE is relevant is also of debate. Kwon et al. found epiglottic obstruction during DISE in 43.7% of patients undergoing MLS. No epiglottic intervention was performed. There was no difference in success rates in those that had epiglottic obstruction (44.4%) and those that did not (40.7%).1836 Primary epiglottic obstruction may be a reason for CPAP intolerance. Dedhia et al. found that 15% of adult patients who were CPAP failures had DISE findings of primary epiglottic obstruction.1837 It is prudent for the DISE surgeon to determine whether epiglottic collapse is from a posterior displacement of the epiglottis due to concurrent collapse of the tongue base or whether epiglottic collapse occurs in isolation. This may help guide surgical decision-making.
A variety of epiglottic procedures have been described including epiglottoplasty,1838 partial epiglottectomy,1839 and epiglottopexy.1840 Golz et al.1839 used laser partial epiglottidectomy alone as a treatment for OSA in 27 adult patients with epiglottic-malacia, and reported a significant reduction of RDI from 45 ± 14.6 to 14 ± 5.1 events/h, and an improvement in SaO2 nadir from 66% to 95%. These procedures are typically done in conjunction with base of tongue surgery and/or palatal surgery, making it difficult to assess the contribution from the epiglottis to a patient’s outcome.1841,1842 While there is no clear guidance from the adult literature as to when to include the epiglottis in surgical planning, there is clear recognition amongst sleep surgeons that the epiglottis has a role to play in OSA. Surgical procedures to ameliorate that component are well described in the literature and should be considered in surgical planning.
IX.D.6 |. Maxillary and mandibular surgery
IX.D.6.a |. Maxillary expansion
Maxillary expansion as a treatment for OSA has mostly been studied in the pediatric population with only a few studies in adults. It is an orthopedic modality used by orthodontists to expand the palate by laterally forcing apart the two halves of the maxillary bone. During this process, the intermaxillary suture is stretched and an osteogenic process ensues. By activating an expansion screw, Rapid Maxillary Expansion (RME) has the effect of both widening the bony alveolar housing for teeth and increasing the maxillary dental arch length. It can be used to correct dental malocclusions, including posterior crossbites, dental crowding, and transversely deficient maxillary arches. Etiology of transversely deficient maxillae includes genetic predisposition, parafunctional oral habits, and inadequate labial/buccal force applied by the tongue during craniofacial development. This last etiology can result from increased nasal resistance, leading to mouth breathing, due to an inferior position of the tongue.1843 Craniofacial anomalies that are common in patients with OSA include mandibular deficiency, narrowed posterior airway space, steep mandibular plane angle, and long anterior facial height.234,1844 Benefits of maxillary expansion include increased nasal cavity volume, improved ability to breathe through the nose,1845–1847 and increased width of the palate resulting in enhanced oral cavity volume.1848 This allows the tongue to posture appropriately (antero-superiorly) at rest and while swallowing. An additional benefit of maxillary expansion is the increased tension of the palatal tissues, reducing laxity and tissue collapse in the OP.1849,1850 RME is effective in the treatment of OSA, with improvements/reductions in the apnea/hypopnea index, or AHI.1851–1855
Technique
Micro-Implant Rapid Palatal Expander (MARPE) describes an appliance which employs dental microimplants to engage the maxillary bone for more skeletal expansion than dental tipping. An MARPE allows an orthodontist to apply transverse forces directly to the skeleton through the use of micro-implants which engage both halves of the maxilla. While MARPE design, case selection, and protocol is essential to success, it allows the clinician to expand the maxillae in much older patients. Skeletally borne expanders allow for a more translational expansion while reducing bone bending and buccal tooth tipping.1856 When buttressing forces of the maxillary sutures are too strong to be overcome by RME alone, surgical interventions can guarantee successful expansion. These adjunctive procedures are known as Surgically Assisted Rapid Palatal Expansion (SARPE) and Distraction Osteogenesis Maxillary Expansion (DOME). They involve surgical corticotomies and with initial activation of the expander intraoperatively to confirm intermaxillary separation.1857 Further activation of the expander is delayed for a short latency period (approximately 1 week), which allows a healing callous to form in the suture. A callous has been shown to readily ossify, so expansion (0.25–1 mm a day) is then resumed after the latency period is complete.1858
Patients with OSA and maxillary constriction are the best candidates for RME.1859,1860 RME was shown up to result in a 70% reduction in AHI in children, and in 95% reduction in AHI when performed in addition to T&A or when tonsils are not hypertrophied. Children with small or surgically removed tonsils benefit more from expansion than do those with large tonsils that undergo RME without T&A.1851 (Table IX.D.6.a)
TABLE IX.D.6.a.
Summary table for maxillary expansion for OSA
| Study | Year | LOE | Study design | Study group(s) | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|
| Adults with OSA | ||||||
| Vinha et al.1855 | 2016 | 3a | Case series | 16 adults with maxillary transverse deficiency and OSA | AHI reduction of 56.2%; RDI reduction of 55%; ESS improvement; reduced desaturation and microarousals | SARME promotes an improvement in OSA symptoms, decreases the rates of respiratory disturbances, microarousal, and desaturation, and reduces daytime sleepiness. |
| Yoon et al.1857 | 2019 | 3a | Retrospective cohort study | 75 adult patients with OSA and narrow maxilla/nasal floor | Reduction of AHI from 17 to8 (53% change) | DOME treatment reduced the severity of OSA, refractory nasal obstruction, daytime somnolence, and increased the percentage of REM sleep. |
| Pediatric patients with OSA | ||||||
| Camacho et al.1851 | 2017 | 2a | Systematic review and meta-analysis | 314 children with transverse maxillary deficiency and OSA | AHI decrease of 70%; cure rate (AHI <1/h) for 90 patients was 26%; improved LSAT; more improvement in children after T&A or small tonsils | Improvement in AHI and lowest oxygen saturation has consistently been seen in children undergoing RME. |
| Machado et al.1853 | 2016 | 2a | Systematic review and meta-analysis | 215 children 0–12 year-old with OSA | Mean change in AHI after follow up was −6.9 | RME is an effective treatment for OSA. |
| Vale et al.1854 | 2017 | 2a | Systematic review and meta-analysis | Children younger than 18 years old with OSA that underwent RME | Statistically significant reduction in AHI after RME | RME reduced AHI in children with OSA, making RME therapy an appropriate alternative treatment option for these patients. |
| Cistulli et al.1852 | 1998 | 3a | Case study | 10 young adults with mild to moderate OSA and maxillary constriction | 90% of participants had improved snoring and hypersomnolence. Reduction in AHI from 19 to 7 | RME may be a useful treatment alternative for selected adults with OSA. |
| Quo et al.1860 | 2017 | 3a | Retrospective study | 45 children age 3–14 years with sleep disordered breathing | The majority of children showed improvement in sleep scores and symptoms after bimaxillary expansion | Bimaxillary expansion is a treatment option for improving respiratory parameters in children with sleep disordered breathing |
IX.D.6.b |. Maxillomandibular advancement
MMA is one of the most effective anatomic surgical interventions for OSA.1800 The earlier indications for MMA included moderate to severe OSA, morbid obesity, severe mandibular deficiency, and failure of other forms of therapy.1861 Current workup of OSA prior to surgery includes the use of DISE. Concentric collapse of the velum is a contraindication for hypoglossal nerve stimulator implantation and lateral pharyngeal wall collapse is difficult to manage with soft tissue surgery alone.1800 MMA is effective in addressing circumferential velum and lateral oropharyngeal wall collapse patterns.1800,1861
Previous algorithms considered MMA if patients had inadequate response to phase I MLS that involved soft tissue surgical approaches such as the uvulopalatal flap and genioglossus advancement.1862 The phased approach remains a reasonable option, with expansion of phase 1 procedures to include various modifications of UPPP, lingual tonsillectomy, maxillary expansion, and UA stimulation. In the contemporary approach for surgical decision making, MMA can be recommended prior to other procedures in: (1) patients with any degree of OSA, with dentofacial deformity, or (2) patients presenting with CCC of the velum and/or lateral oropharyngeal wall collapse as seen on DISE, or (3) after failure of one or more of these surgeries.567,1566,1862
MMA results in a high surgical success rate and modest cure rate. Surgical success for sleep procedures is defined as a reduction in the AHI by more than 50%, or with a less than 20 events/h. Cure is defined as AHI < 5.1863 Holty et al. performed the largest MA by examining 22 studies with 627 patients who underwent MMA for OSA and reported a mean decrease in AHI from 63.9 to 9.5 events/h. The surgical success and cure rates were 86.0% and 43.2%, respectively.1867 Another large MA performed by Zaghi et al. which included 45 studies with 528 patients and showed that mean postoperative changes in AHI and RDI after MMA were 47.8 and 44.4 events/h, respectively. They reported success and cure rates of 85.5% and 38%, respectively.1566
MMA for OSA is typically accomplished with maxilla and mandible advancements of 8–14 mm.1862 Such numbers need to be interpreted with caution as the landmarks can be inconsistent, and rotations of the maxillomandibular complex to achieve facial balance may further skew the interpretation. Barrera et al. examined differences in anatomic dimensions between patients with and without OSA.567 Skeletal and soft tissue dimensions were measured with cephalometry and MRI. Compared to controls, patients with OSA demonstrated: (1) increased mandibular plane-hyoid (MP-H) distance, (2) increased tongue volume, and (3) smaller posterior airway space. GottsaunderWolf et al. performed an SR of posterior airway changes following MMA using lateral cephalometry and CBCT.1863 In five studies, where a third reported ranges for maxillary and mandibular advancements, there was an increase of 8.1°–9.8° for SNA and 9.5°–11.0° for SNB.
Caples et al.1686 reviewed surgical modifications of the UA for treatment of OSA in 2010 to update the AASM on UA surgery. Of 1383 studies reviewed, nine studies on MMA were included. MMA resulted in the greatest reduction in AHI of 87% (95% CI 80%–92%), with a mean postoperative AHI of 7.7.
Secondary measures, such as sleepiness or QOL, were reported in one study as a reduction in the Epworth score from 17.8 to 4.71864 and another reported significant decline in mean BP at 6 months after surgery.1865
There is a need to better characterize surgical morbidity and adverse events after MMA. As MMA is generally performed in patients significantly older than corrective jaw surgery (orthognathic) patients, rates of malocclusion, malunion, and prolonged paresthesia are likely to be higher than seen in orthognathic surgery. SRs report the rates of major complications such as airway compromise at 1%, and other complications such as malunion at 5%. A less described but frequently encountered side effect from MMA surgery is nasal obstruction requiring corrective surgery. With classic MMA techniques, persistent nasal obstruction is reported to be as high as 18.7% from 379 patients over 15 years.1686 Nasal obstruction rates can be reduced to 6.5% with modifications including pre-MMA maxillary expansion, concurrent septoplasty, piriformplasty, and emphasis on postoperative nasal and sinus rinsing.1866 There is a need to update evidence on adverse events in contemporary MMA. (Table IX.D.6.b)
TABLE IX.D.6.b.
Summary table for maxillomandibular advancement
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusions |
|---|---|---|---|---|---|---|
| Caples et al.1686 | 2010 | 2a | Systematic review and meta-analysis | Review of 1383 studies with nine MMA studies | MMA has low surgical morbidity and adverse events Improvement in AHI is greatest with MMA | Overall reduction in AHI of 87% (95% CI 80%–92%) with a mean postoperative AHI of 7.7. |
| Liu et al.1800 | 2019 | 2b | Retrospective study | Patient selection criteria reviewed based on retrospective experience | Historical review of MMA surgery at Stanford | Outlines the contemporary approach for MMA in the treatment of OSA. It details a stepwise approach for the procedure. |
| Riley et al.1861 | 1990 | 2b | Retrospective study | 40 adults with OSA evaluated based on AHI | MMA has a higher success rate than genioglossal advancement and hyoid suspension | MMA is effective in treating moderate to severe OSA. |
| Barrera1862 | 2018 | 2b | Systematic review | Clinical technique and systemic review based on AHI outcomes | MMA success increases with advancements over 10 mm Success rate over 86% | This review highlights the variety of skeletal surgery offered for the treatment of OSA, including MMA as the gold-standard. |
| Zaghi et al.1566 | 2016 | 2a | Systematic review and meta-analysis | Success and cure rate | Forty-five studies and 518 patients with surgical success and cure rates of 85.5% and 38%, respectively | Meta-analysis of MMA showing surgical success with surgery. |
| Gottsauner-Wolf et al.1863 | 2018 | 2a | Systematic review and meta-analysis | Review of 15 publications with average advancements compared | Average advancement of the mandible were 8.1–9.8 mm and 9.5–11.0 mm | Systematic review analyzed posterior airway changes after MMA for OSA. They showed significant advancements for both the maxilla and mandible. |
| Holty and Guilleminault1867 | 2010 | 2a | SR and MA | MMA (n = 627 adults) | AHI, ESS, QOL | MMA sig improved AHI (63.9–9.5), reduced daytime symptoms and improved QOL. Success and cure rates were 86.0% and 43.2%, respectively. Younger, lower weight, and AHI and greater maxillary advancement were predictive of increased surgical success. Major complication rate: 1.0%, Minor complication rate: 3.1%, |
| Camacho et al.1868 | 2019 | 2a | SR and MA | MMA (n = 120) | AHI, ESS, LSAT | Improvements in AHI, sleepiness, and lowest oxygen saturation were maintained in the long term (4 to <8 years). Mean AHI increased to moderate OSA in the very long term (≥8 years). |
| Awad et al.1793 | 2019 | 2a | Systematic review | Review of 414 studies (125 studies on MMA) | MMA has highest success and cure rates | This systematic review analyzed current trends in skeletal surgery for treatment of OSA. They report that MMA is the most widely studied and efficacious multilevel surgery for OSA. |
IX.D.7 |. Hypoglossal nerve stimulation (HNS)
HNS is an emerging surgical treatment for OSA. A few companies have produced HNS systems, but only the Inspire II (Inspire Medical Systems, MN, USA) has obtained FDA approval. The Apnex device (Apnex Medical, MN USA) produced promising Phase II data, but failed to complete a Phase III trial.1869 The ImThera device (LivaNova, London UK) has shown promising results in a Phase II trial and is currently under investigation in an ongoing phase III trial.1870 The Nyxoah Genio device is a bilateral HNS system with external battery with preliminary data showing improvement in AHI post-implant.1871 Therefore, the bulk of literature regarding candidacy, efficacy, and usage of HNS comes from cohorts implanted with the Inspire II device.
IX.D.7.a |. Candidacy for HNS
Current clinical candidacy criteria for HNS or UA stimulation are based on early feasibility studies and Stimulation Therapy for Apnea Reduction (STAR) trial which formed the basis for Federal Drug Administration (FDA) candidacy recommendations for Inspire II (Inspire Medical Systems, MN, USA).
Criteria for patient enrollment in the pivotal STAR trial were based on feasibility trials that suggested improved outcomes with BMI ≤ 32 kg/m2 and AHI ≤ 50 events/h1872 along with two small studies (N of 7 and 21 patients) that suggested HNS was not effective in patients with palate level CCC on DISE prior to implantation. A statistically significant difference in AHI reduction was seen in the group without palate CCC leading the authors to conclude the absence of CCC can predict HNS success and DISE was recommended as a tool for patient selection.1872,1873 The STAR trial involved a multi-institutional single group trial with 126 patients who were not adherent to CPAP with enrollment criteria: BMI < 32 kg/m2, with AHI greater than 20 and less than 50, central/mixed apnea events less than 25% of all apneic events, AHI in non-supine position > 10 events/h. Patients with tonsil size 3 or 41645 or palate CCC on DISE were excluded from the trial. The trial demonstrated therapy success based on the Sher criteria (≥50% reduction in AHI from baseline and post-treatment AHI <20) in 66% of the patients.382 Effectiveness of HNS therapy in patients with a larger AHI range (>15 and <65 events/h), and BMI <35 kg/m2 along with absence of palate CCC on DISE was further demonstrated by several post-approval outcomes studies, meta-analyses, and ADHERE registry.1488,1489,1548,1874–1876
Current candidacy criteria for HNS require further evaluation. Mwenge et al. evaluated effectiveness of HNS in 13 patients with wider inclusion criteria for AHI and BMI 25–40 kg/m2 and did not use DISE as a screening tool. Successful AHI reduction was demonstrated in 69% (nine out of 13) of patients at 12 months after implantation. Kezirian et al. looked at HNS in patients with selection criteria AHI 20–100 events/h, BMI < 40 kg/m2 and reported that 55% (17 out of 31) patients achieved Sher treatment success criteria.1869
FDA indications for HNS do not consider BMI as a definitive candidacy criterion. The STAR trial only included patients with BMI <32 kg/m2, thus this criterion has persisted for post-approval HNS patient selection by some insurance coverage policies. Data from most recent ADHERE post-implant follow-up studies have suggested an inverse association of BMI and HNS therapy effectiveness.1488,1876 However, the appropriate cutoff for BMI level is not clear. Kezirian et al. demonstrated that patients with BMI <35 had greater AHI reduction with HNS. Huntley et al. found no difference in HNS therapy success rates between BMI >32 and less than equal to sign 32 kg/m2 groups.1489
Current evidence demonstrates effectiveness of the HNS therapy in patients with absence of CCC. However, evidence level for CCC as a predictor of HNS outcomes is low quality and based on two very small case series.1872,1873 There are no multi-institutional studies demonstrating HNS therapy success rate in larger population of patients with CCC.
HNS therapy is indicated for treatment of a subset of adult patients 18 years of age and older with moderate-to-severe OSA (15 ≤ AHI ≤ 65 events/h) of which <25% of events are central/mixed apneas, who have failed or cannot tolerate PAP treatment and do not have a CCC at the soft palate level. Current evidence supports HNS therapy success in patients with BMI ≤ 35 kg/m2. As of 2021, the current HNS implant is not FDA approved for entry into many types of MRI, including no body MRI and only 1.5T scanners for head and neck MRI. This was expanded in 2022 to allow for full body MRI in 1.5T scanners.
When considering HNS therapy, clinical judgment should be used to assess patients’ candidacy based on a comprehensive sleep medicine history, polysomnographic findings, UA anatomy and pathophysiology, body mass and fat distribution, medical and psychiatric comorbidities, physical and cognitive limitations, comorbid sleep disorders, other available treatment alternatives, presence of other implantable devices, occupation, and anticipated needs for MRI imaging.
IX.D.7.b |. Efficacy of HNS
The phase III trial STAR cohort demonstrated median AHI reduction by 68% from 29.3 to 9.0.382 The randomized controlled therapy withdrawal portion of the STAR trial demonstrated that responders to HNS reverted to baseline OSA levels when the therapy was removed, and improved again when it was restarted.1877 Over a period of 5 years, the STAR trial cohort demonstrated significant improvements in objective measures of OSA such as AHI, ODI, and percentage of sleep spent under 90% saturation383,815,1878 as well as significant improvements in subjective daytime sleepiness (ESS), snoring level (bed partner visual analog score), and sleep-related quality of life (FOSQ). ESS decreased by 4.4 units on average with 78% of subjects below 10, indicating normal daytime sleepiness.383,815,1547,1878,1879 At the 5-year mark, 75% of the remaining cohort met Sher’s criteria. When including the last known values for those lost to follow up, the surgical success rate was approximately 63%.383
Post-approval single-center and multi-institutional cohort studies further confirm significant improvement in both objective and subjective measures with HNS. In the phase IV German Post-Market Study (GPMS), median AHI decreased from 28.6 to 10 at 3 years. In addition, 67% of the original cohort demonstrated an AHI < 10.1548,1880,1881 An ongoing prospective observational study (ADHERE Registry), which serves as a registry of Inspire patients around the world, demonstrates significant improvements in AHI and ESS, as well as better treatment adherence than PAP. Mean AHI decreased from 35.6 to 10.2 and ESS from 11.9 to 7.5.1882 Overall, 69% met Sher’s criteria at 12 months.1876
Several retrospective case–control studies observed that HNS outperformed UPPP and transoral robotic tonguebase surgery, with regard to significant improvement in both AHI and Sher’s criteria.1883,1884 Prior sleep surgery does not influence outcomes of HNS, and HNS non-responders can likewise be considered for further therapy, including surgery, if needed to optimize care.1885–1887 Smaller retrospective cohorts show that HNS improves sleep architecture with lower arousal index, reduced light (N1) sleep, and increased deeper (N2 and N3) sleep.1888,1889
Several SRs have demonstrated that HNS is effective in improving moderate-to-severe OSA.1874,1875,1890,1891 Constantino et al., when analyzing the major cohorts of Inspire, Apnex, and ImThera, found that a majority of patients met Sher’s criteria at rates of 72%, 55%, and 77%, respectively, for each system at 12 months.1890 In a review of 600 Inspire patients, Kent et al. observed that 77% met Sher’s criteria and 42% obtained OSA cure with an AHI < 5, while the ESS decreased to less than 10 in 75% of included patients.1891
Despite the potential for HNS to improve OSA, there remain subsets of nonresponders who fail to meet predetermined AHI improvement. Evidence demonstrates nonresponders may still benefit from HNS. In the ADHERE registry, even though 31% did not meet Sher’s criteria at 12 months, over 90% of patients were satisfied with their HNS, and would choose it again.1876 The German Post-Market Study cohort demonstrated that among AHI nonresponders, the ESS and FOSQ significantly improved and over 90% continued to utilize their device.1548,1881,1892
Investigation continues into eligibility criteria for HNS since the literature postulates that a wider variety of patients may benefit from HNS than were included in the STAR trial. In a retrospective case series of 31 Veterans Affairs patients, of whom 61% did not meet the FDA’s proposed criteria, Sher’s criteria was met in 90%, with 72% achieving an AHI less than 5.1893 In addition, patients with isolated retropalatal collapse achieve similar results from HNS when compared to patients with other airway collapse patterns.1894 An analysis of the ADHERE registry found that patients 65 years and older are more likely to have greater AHI reductions and higher device usage.1895 Yet, previous ADHERE studies call into question whether age is an independent factor in outcomes.1876 A German cohort found no differences in outcomes between patients younger and older than 65 years.1896 Female sex and lower BMI have been suggested to increase odds of reaching surgical success.1876 In contrast, a retrospective case–control study found equivalent results in patients with BMI above and below 32.1489 A smaller retrospective series demonstrated that higher baseline AHI is associated with greater overall AHI reductions.1897
The impact of HNS on common complications of OSA is currently under investigation. Within the STAR cohort, Inspire responders had reduced HRV suggesting lower sympathetic tone and improved CV health.1898 In non-diabetic patients, HNS reduced insulin resistance and improved oral glucose tolerance.1899 A retrospective study comparing ADHERE and a PAP cohort found HNS improved systolic and mean arterial pressures (MAPs) of baseline hypertensive patients, whereas PAP improved pressure measures in all groups, especially diastolic and MAP.1900 (Table IX.D.7.b)
TABLE IX.D.7.b.
Evidence on HNS therapy for OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoint | Conclusions |
|---|---|---|---|---|---|---|
| Kent et al.1891 | 2019 | 2a | SR and meta-analysis of prospective and retrospective self-controlled cohort studies | Inspire implantation (n = 584) | 1. AHI 2. ESS 3. FOSQ 4. O2 nadir |
HNS leads to significant improvements in objective and subjective measures of OSA and QOL. AHI decreased by mean 25.2 (6 months) and 16.5 (12 months). ESS decreased to <10 in 75% patients. 77% met Sher’s criteria and 42% obtained OSA cure. Greater improvement seen in patients with greater pre-operative OSA. |
| Costantino et al.1890 | 2020 | 4 | SR and meta-analysis of prospective self-controlled cohort studies | HNS patients: Inspire: 239 ImThera: 59 Apnex: 52 | 1. AHI 2. ESS 3. ODI |
HNS is safe and effective for moderate-to-severe OSA. Inspire and ImThera had surgical success rate ≥ 75%. AHI reduction of 56% (Inspire), 54% (ImThera), and 44% (Apnex) at 12 months and 59% (Inspire) at 60 months. ESS and ODI significantly improved. |
| Kompelli et al.1875 | 2018 | 4 | SR and meta-analysis of self-controlled cohort studies | HNS patients (381) | 1. AHI 2. ESS 3. ODI 4. FOSQ 5. SAQLI |
HNS is safe and effective treatment for CPAP refractory OSA with significant improvements in objective and subjective measures. Mean AHI reduction of 23.5 and 21.2 at 6 and 12 months, respectively. |
| Certal et al.1874 | 2015 | 4 | SR and meta-analysis of self-controlled cohort studies | HNS patients (200) | 1. AHI 2. ODI 3. ESS |
HNS is safe and effective treatment of moderate-to-severe OSA in patients who failed CPAP. Clinically significant decreases in mean AHI, ODI, and ESS as well as QOL through 12 months. |
| Strollo et al.382 | 2014 | 2b | STAR trial Prospective, self-controlled cohort study; randomized controlled therapy-withdrawal trial |
Inspire implantation (n = 126) STAR cohort | 1. AHI 2. ODI 3. ESS 4. FOSQ 5. % sleep under 90% SaO2 |
HNS results in clinically significant reductions in OSA severity and subjective OSA measures at 1 year, confirmed by withdrawal trial. More favorable effect with BMI 32 or lower and AHI 50 or less. |
| Woodson et al.1877 | 2014 | 1b | STAR trial Prospective, randomized controlled therapy withdrawal study |
Inspire responders therapy withdrawn therapy maintained STAR cohort | 1. AHI 2. ODI 3. ESS 4. FOSQ 5. Snoring |
Sleep parameters revert to baseline when therapy is withdrawn and improve when restarted. HNS benefit continued through 18 months. |
| Strollo et al.815 | 2015 | 2b | STAR trial cont. Prospective cohort study |
Inspire implantation at 12 and 18 months (n = 124) STAR cohort | 1. AHI 2. ODI 3. ESS 4. FOSQ 5. % sleep under 90% SaO2 |
HNS produces consistent and sustained therapy response in OSA severity, self-reported sleepiness, and QOL over 18 months. |
| Soose et al.1547 | 2016 | 2b | STAR trial cont. Prospective, self-controlled cohort study |
Inspire implantation at 24 months (n = 111) STAR cohort |
1. ESS 2. FOSQ 3. Snoring |
HNS produces clinically meaningful, significant improvements in subjective OSA measurements that are consistent through 2 years. |
| Woodson et al.1878 | 2016 | 2b | STAR trial cont. Prospective, self-controlled cohort study |
Inspire implantation at 36 months (n = 116) STAR cohort |
1. AHI 2. ODI 3. ESS 4. FOSQ 5. % sleep under 90% SaO2 |
HNS has lasting significant improvements in objective and subjective sleep measures at 36 months for CPAP intolerant, moderate-to-severe OSA patients |
| Gillespie et al.1879 | 2017 | 2b | STAR trial cont. Prospective, self-controlled cohort study |
Inspire implantation at 48 months (n = 91) STAR cohort |
1. ESS 2. FOSQ 3. Snoring |
HNS maintains sustained benefit on ESS, FOSQ, and snoring through 48 months. |
| Woodson et al.383 | 2018 | 2b | STAR trial cont. Prospective, self-controlled cohort study |
Inspire implantation at 5 years (n = 97) STAR cohort |
1. ESS 2. FOSQ 3. Snoring 4. AHI 5. ODI |
At 5 years, HNS produces clinically meaningful and significant improvements in objective and subjective OSA measures. Adverse outcomes are rare and benign. Non-responders had higher AHI and ODI, and lower FOSQ scores at baseline. |
| Dedhia et al.1898 | 2019 | 4 | Prospective, self-controlled cohort study | Inspire responders (n = 32) STAR cohort |
1. SDNN 2. LF 3. HF |
HNS significantly improves heart rate variability during sleep similar to PAP usage suggesting lower sympathetic tone and improved cardiovascular health. |
| Thaler et al.1876 | 2019 | 2b | Prospective, self-controlled cohort study | Inspire implantation (n = 640) ADHERE registry |
1. AHI 2. Treatment success 3. ESS 4. Objective therapy use |
HNS meets surgical success per Sher criteria in 83% and 69% of patients at 6 and 12 months, respectively. HNS significantly reduces AHI and ESS. Female sex and lower BMI increase odds of surgical success. |
| Steffen et al.1881 | 2020 | 2b | Prospective, multi-institutional self-controlled cohort study | Inspire implantation (n = 41) German Post-Market Study cohort (G-PMS) |
1. AHI 2. ODI 3. ESS |
HNS produces long-term control of objective and subjective OSA measures in CPAP-intolerant patients that met FDA inclusion criteria. 67% patients demonstrated AHI < 10/h at 3 years. Median AHI dropped from 28.6 to 10 at 3 years. |
| Withrow et al.1895 | 2019 | 2b | Prospective cohort study | Inspire implantation 1. <65 years (n = 365) 2. ≥65 years (n = 235) ADHERE |
1. AHI 2. ESS 3. Therapy usage |
Age ≥ 65 is independent factor for HNS success, with higher AHI reduction and therapy usage in older adults. It is safe and well tolerated among older adults. |
| Boon et al.1882 | 2018 | 2b | Prospective, self-controlled cohort study | Inspire implantation (n = 301) ADHERE |
1. Adverse events 2. AHI 3. ESS 4. Adherence |
HNS provides objective success and satisfaction from surgeons and patients. Mean AHI decreased from 35.6 to 10.2, and mean ESS decreased from 11.9 to 7.5. Adverse events were rare and benign. |
| Steffen et al.1548 | 2018 | 2b | Prospective, multiinstitutional self-controlled cohort study | Inspire implantation (n = 60) German Post-Market Study cohort (G-PMS) |
1. AHI 2. ODI 3. ESS 4. FOSQ |
HNS is effective therapy with durable results through 12 months. Median AHI decreased from 28.6 to 9.5 at 12 months. 73% patients met Sher criteria. Usage approached 40 h/week. Nonresponders’ ESS and FOSQ significantly improved, and all continued to utilize device. |
| Hasselbacher et al.1892 | 2018 | 2b | Prospective, multiinstitutional self-controlled cohort study | Inspire implantation (n = 60) G-PMS |
1. ESS 2. FOSQ 3. Snoring 4. User experience |
HNS leads to significantly improved daytime sleepiness and functioning, 73% and 59% reaching normal responses on ESS and FOSQ, respectively. Subjective improvement correlates with higher usage. 96% patient satisfaction. |
| Heiser, Maurer et al.1880 | 2017 | 2b | Self-controlled cohort study | Inspire implantation (n = 60) G-PMS |
1. AHI 2. ODI 3. ESS 4. FOSQ 5. Therapy usage |
HNS achieved significant improvement in objective and subjective OSA measures at 6 months. 68% met Sher criteria, with average AHI reduction of 61%. Adherence was significantly higher than CPAP. |
| Shah et al.1884 | 2018 | 4 | Retrospective case–control study | Sleep surgery patients 1. Inspire (n = 20) 2. UPPP (n = 20) |
1. AHI 2. ESS |
HNS produced greater decrease in post-op AHI versus UPPP. 100% of HNS patients met Sher criteria versus 40% of UPPP patients. Previous sleep surgery did not have effect on AHI in either group. |
| Huntley et al.1883 | 2019 | 4 | Retrospective case–control study | Sleep surgery patients with BOT collapse 1. Inspire (n = 76) 2. TORS BOT resection (n = 24) |
1. PSG outcomes 2. Complications 3. LOS and readmission |
HNS significantly outperformed TORS for surgical success, AHI, O2 nadir, hospital LOS, and no readmissions. 87% HNS patients reached Sher’s criteria and 59% reached cure. HNS has largely replaced TORS for BOT obstruction at their institution. |
| Sarber et al.1893 | 2020 | 4 | Retrospective case series | Veterans undergoing HNS (n = 31) | 1. PSG outcomes 2. ESS 3. Perioperative outcomes |
In veterans with several medical and psychiatric comorbidities (61% did not meet FDA criteria), HNS significantly improved objective and subjective sleep measures, albeit higher complication risk. Sher criteria met in 90% of patients. 72% patients reached cure. No statistical difference in those with and without psychiatric disorders. |
| Walia et al.1900 | 2020 | 3b | Retrospective cohort study | OSA patients 1. HNS (n = 278) 2. PAP (n = 517) |
1. Blood pressure 2. ESS |
In matched patients, PAP had greater improvements in diastolic and mean arterial pressure. Only baseline hypertensive patients had BP improvement with HNS. HNS improved ESS to a greater degree. |
| Kezirian et al.1886 | 2019 | 2b | Self-controlled cohort study ADHERE registry | Inspire implantation 1. No prior sleep surgery (n = 217) 2. Prior sleep surgery (n = 82) |
1. AHI | Previous upper airway sleep surgery is not associated with HNSefficacy HNS. These patients should be considered for HNS. |
| Bohorquez et al.1888 | 2020 | 4 | Retrospective case series | Inspire responders (n = 35) | 1. Sleep architecture from PSG parameters 2. BMI |
HNS responders had significant improvement in sleep architecture: reductions in N1 and arousal index, increases in N2 and N3. No changes in REM. |
| Evans et al.1897 | 2020 | 4 | Retrospective case series | Inspire implantation (n = 25) | Pre-operative clinical predictors of HNS success | Higher starting AHI was positively associated greater AHI reduction. |
| Mahmoud et al.1894 | 2019 | 4 | Retrospective case series | Inspire implantation 1. Retropalatal collapse (n = 25) 2. Any other collapse (n = 57) |
1. AHI 2. Nadir O2 |
Isolated retropalatal collapse performed similarly to other collapse types. HNS produced significant decreases in mean AHI and oxygen nadir. |
| Steffen et al.1899 | 2019 | 4 | Prospective case series | Non-diabetic Inspire implantation (n = 25) | 1. AHI 2. ODI 3. ESS/FOSQ 4. Glucose metabolism 5. Appetite markers |
HNS significantly improves AHI, ODI, and subjective measures of OSA. At 12 months, HgbA1C and BMI were similar, but insulin resistance and oral glucose tolerance improved. Hedonic food drive improved. |
| Huntley et al.1885 | 2018 | 3b | Retrospective case–control study | Inspire implantation 1. No prior sleep surgery (n = 141) 2. Prior “palate” surgery (n = 23) |
1. AHI 2. O2 nadir 3. ESS |
Patients with persistent moderate-to-severe OSA after prior palate surgery should be considered for HNS. No statistical differences in outcomes in patients with and without prior palate surgery. |
| Huntley et al.1489 | 2018 | 3b | Retrospective case–control study | Inspire implantation 1. BMI > 32 (n = 40) 2. BMI < 32 (n = 113) |
1. AHI 2. O2 nadir 3. ESS |
Patients with BMI >32 had statistically similar outcomes with HNS compared to those with BMI <32. Over 92% patients met Sher criteria in both cohorts, and over 55% obtained cure. |
| Zhu et al.1896 | 2018 | 4 | Prospective cohort study | Inspire implantation 1. Age >64 years (n = 31) 2. Age <65 years (n = 31) |
1. AHI 2. ODI 3. ESS |
HNS leads to significant reductions in AHI, ODI, and ESS in older patients, statistically similar to younger patients. |
| Hofauer et al.1889 | 2017 | 4 | Case series | Inspire implantation (n = 26) | 1. Sleep architecture from PSG parameters | HNS significantly decreased N1 sleep time and arousals. |
| Steffen et al.1887 | 2019 | 4 | Prospective case series | Inspire nonre-sponders 1. UAS followed by UPPP (n = 7) 2. UAS preceded by UPPP (n = 10) 3. UAS alone (n = 8) |
1. AHI 2. ODI 3. ESS |
UPPP should be considered in HNS nonresponders if DISE demonstrates palatal collapse, as significant improvement in AHI, ODI, and ESS can be achieved in these patients. |
| Friedman et al.1870 | 2016 | 4 | Self-controlled cohort study | ImThera implantation (n = 46) Phase II study | 1. AHI 2. ODI 3. Arousal index 4. ESS 5. Sleep apnea quality of life index |
ImThera produced significant decreases in objective and subjective measures. Mean AHI decreased from 34.9 to 25.4 in all patients. In responders, mean AHI decreased from 35.7 to 0.5. Only 35% met Sher’s criteria. 67 adverse events occurred. |
| Kezirian et al.1869 | 2014 | 4 | Self-controlled cohort study | Apnex implantation (n = 31) | 1. PSG parameters 2. Compliance 3. Subjective improvement 4. Safety |
Apnex produced significant improvements in objective and subjective measures of OSA. Mean AHI improved from 45.4 to 25.3. 55% met Sher’s criteria. |
IX.D.7.c |. Titration of HNS
For hypoglossal nerves stimulation (HNS) therapy, proper titration of the electrical parameters is critical to comfort, use, and successful long-term outcomes. HNS therapy is an adjustable medical device that can be titrated in the clinic or sleep laboratory setting to optimize effectiveness and comfort longitudinally. HNS settings can be adjusted to maintain adequate disease control, as body weight or other pathophysiologic factors change over time. Prior studies have shown a graded increase in airflow and multilevel airway measurements with increasing stimulation amplitudes until a plateau is reached, balancing patient discomfort with therapeutic efficacy.1901–1905 Although increasing amplitude may be associated with improved UA opening, a threshold is reached where further increases in amplitude are associated with therapy discomfort and subsequently detrimental to sleep-onset and sleep-maintenance.
Most research on HNS titration refers to the Inspire implantable HNS system (Inspire Medical Systems, MN, USA). The STAR trial established the foundation for the current titration protocol which consists of: (1) device activation four weeks post-implant, (2) initial patient self-titration and accommodation period, followed by (3) laboratory-based overnight titration PSG 2–6 months postoperatively.1904 Device activation is recommended with standard default stimulation settings (bipolar electrode configuration [+–+], pulse width = 90 μs, rate=33 Hz), confirmation of proper system function, and determination of the functional threshold – defined as the lowest amplitude at which tongue protrusion passes the mandibular incisors.382,1906 Initial amplitude is set at or just below the functional threshold and includes a patient-control range of ten 0.1 V increments (e.g., functional threshold = 1.0 V with a range of 0.8–1.7 V with 0.1 V increments).1907 Patients are asked to progressively increase the amplitude on their remote every few nights at home. This allows self-titration by patients to optimize their symptoms while simultaneously balancing their comfort with the device.
When assessing treatment response, two types of PSG have been reported: (1) titration PSG which consists of an in-lab study with real-time therapy adjustments made by a sleep technician according to established laboratory protocols akin to CPAP titration studies, and (2) full-night efficacy PSG, either in-lab or via home portable monitoring, which measures the sleep outcomes at one setting all night. Although the STAR trial and German post-market study included titration studies between 2 and 6 months, the manuscripts reported outcomes from the subsequent full-night efficacy studies.1880 Reports from the multicenter ADHERE global clinical registry, however, represent a combination of titration study data and full-night efficacy studies depending on the center and individual insurance coverage.1488,1876 Three studies have reported HNS results from postoperative titration studies.1546,1894,1908 As expected, the results from the titration studies showed more improvement in the final AHI when compared to full-night outcomes studies. In summary, variability exists in outcomes reporting based on study type. This variability underscores the importance of distinguishing between titration and full-night outcomes and the need for more fullnight efficacy data in understanding long-term therapy outcomes.1909
For HNS patients with suboptimal OSA response or problems with device adherence, examination and implementation of best-practice approaches for device data download, patient education, targeted advanced titration and programming adjustments, and close clinical follow-up, may optimize long-term outcomes. For refractory cases with persistent OSA, there may be a role for awake endoscopy or DISE to further titrate therapy.1910–1912 Industry-sponsored recommendations including sleep laboratory titration protocols, as well as office-based therapy troubleshooting and advanced titration protocols are available; however, peer-reviewed practice guidelines are still in development. Additional research is needed to determine which titration protocols improve outcomes and which approaches have the most meaningful effects. (Table IX.D.7.c)
TABLE IX.D.7.c.
Clinical studies with titration parameters of hypoglossal nerve stimulation (HNS)
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Strollo et al.382 | 2014 | 2b | Multicenter prospective series of OSA patients CPAP intolerant and oral appliance therapy who underwent HNS implantation. | 126 moderate to severe OSA patients who underwent HNS implantation | Device use, sleep study parameters: AHI, ODI, ESS, FOSQ. Objective outcomes measured by in lab PSG (non-titration) at 12 months postoperatively | HNS provides significant improvement in OSA severity. AHI decreased from 32.0 ± 11.8 to 15.3 ± 16.1 |
| Kent et al.1546 | 2016 | 4 | Single center retrospective case series of OSA patients CPAP intolerant and oral appliance therapy who underwent HNS. | 20 patients with moderate to severe OSA who underwent HNS implantation | Objectively measured device use, adverse events, ESS, sleep study parameters: AHI, ODI. Objective outcomes were taken from the 2-month postoperative in-lab titration PSG | HNS can successfully be implemented with significant improvement in sleep outcomes. AHI decreased from 33.3 ± 13.0 to 5.1±4.3 |
| Huntley et al.1908 | 2017 | 4 | Comparison of two centers retrospective review of their OSA patients treated with HNS. | 1. 48 Moderate to severe OSA patients who underwent HNS implantation 2. 49 Moderate to severe OSA patients who underwent HNS implantation at a different medical center |
Comparison between two different implant centers HNS results: Objectively measured device use, adverse events, ESS, sleep study parameters: AHI, ODI. Objective outcomes were taken from the 2-month postoperative in-lab titration PSG | Study demonstrates reproducible improvement multiple sleep characteristics. Notably, AHI decreased from 35 ± 17.3 to 6 ± 8.3 |
| Mahmoud et al.1894 | 2018 | 4 | Single center retrospective series of OSA patients CPAP intolerant and oral appliance therapy who underwent HNS. Compared patients who had undergone prior surgery for OSA and those who had not. | 1. 30 Moderate to severe OSA patients with persistent OSA despite prior airway surgery who then underwent HNS implantation 2. 17 Patients with moderate to severe OSA who had not undergone prior airway surgery for OSA | Objectively measured device use, adverse events, ESS, sleep study parameters: AHI, ODI. Objective outcomes were taken from the 2-month postoperative in-lab titration PSG | Prior airway surgery had no effect on ultimate outcomes. Overall, AHI decreased from 39.3±2.8 to 3.9±1.2 |
Abbreviations: AHI, apnea-hypopnea index; ESS, Epworth Sleep Study; FOSQ, Functional Outcomes of Sleep Questionnaire; HNS, hypoglossal nerve stimulation; ODI, oxygen desaturation index; OSA, obstructive sleep apnea.
IX.D.7.d |. Adherence in HNS therapy
HNS outcomes are directly tied to therapy use – also referred to as therapy compliance or adherence. Furthermore, therapy adherence is influenced by therapy side effects, which include various device-related, stimulationrelated, and/or psychosocial-related factors. Long-term adherence to OSA medical device therapies remains one of the biggest challenges in the treatment of OSA. The STAR trial reported 86% of participants using the therapy nightly at the 12-month mark; however, this adherence data represented self-report rather than objective analysis.382
Technology advances allowed for objective therapy use data to be downloaded from the pulse generator using the telemetry unit and clinician programmer. Early published objective adherence data was limited to total hours of therapy usage since the last device interrogation (reported as mean hours per night or week of use). At a mean 7.8 months follow-up, Kent et al. reported mean objective adherence of 7.0 ± 2.2 h/night.1546 Heiser et al. reported similar high adherence rates with a mean of 6.6 ± 2.7 h/night at 12 months.1880 Comparing HNS outcomes of 97 consecutive patients at two centers, Huntley et al. also reported favorable adherence in the first year with 63.4% and 78.8% of the participants at the respective centers using the therapy over 40 h/week.1908
Therapy side effects with negative potential impact on adherence have been reported in multiple studies. In the STAR trial, discomfort due to electrical stimulation was the most common non-serious adverse event reported and improved across the 5-year trial – 40% of patients noted an episode of discomfort during the first year but only 3.5% of patients reported discomfort during the fifth year.382,383 Tongue abrasion from tongue movement against an adjacent tooth was reported in 21% of patients in the first year and was reduced to 4.1% of patients in the fifth year. The reduction in adverse stimulation-related side effects over time suggests a potential role for long-term therapy accommodation that includes patient education, therapy troubleshooting, targeted reprogramming, and close clinical follow-up.
More recently, Heiser et al. reported on 508 HNS patients from the multicenter international ADHERE registry with a mean objective nightly use of 6.4 ± 2.0 h/night at the first post-titration time point (n = 344 patients; average = 137±77 days postoperatively) and 5.7 ± 2.2 h/night at the final visit (n = 229 patients; average = 386 ± 136 days postoperatively).1488 At both the post-titration and final visits, 23% of patients had a treatment-related adverse event, which most commonly consisted of stimulation-related discomfort, tongue abrasion, or insomnia/arousal. Furthermore, using a logistic regression model they showed increasing therapy adherence was associated with increasing age, lower BMI, and increasing AHI.1488 Thaler et al. published the results of 1017 patients implanted with the HNS as part of the ADHERE registry.1876 After 12-months, mean objective therapy use was 5.6 ± 2.1 h/night. Stimulation-related discomfort remained the most commonly reported adverse event and while adverse events typically decreased from their 6to 12-month time points, insomnia/arousals was the only event that increased (3% vs. 5% of patients). These patients would likely benefit from advanced titration programs to increase the comfort of the device but there is a scarcity of evidence to guide practicioners.
More studies are needed to examine which specific treatment factors and patient factors most strongly impact therapy adherence and outcomes. For example, akin to other medical devices, comorbid insomnia may be a negative predictor of HNS therapy adherence and may benefit from concomitant insomnia and OSA management. Future investigations of HNS therapy should focus on patient education, patient selection, and post-implant troubleshooting algorithms to further optimize therapy comfort and adherence. Additionally, technology advances could provide clinicians with more detailed and granular, and even remotely accessed, nightly adherence data for better longitudinal care. (Table IX.D.7.d)
TABLE IX.D.7.d.
Clinical studies looking at adherence and related adverse events of hypoglossal nerve stimulation (HNS)
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Strollo et al.382 | 2014 | 2b | Multicenter prospective series of OSA patients CPAP intolerant and oral appliance therapy who underwent HNS implantation | 126 moderate to severe OSA patients who underwent HNS implantation. STAR trial | Subjective adherence use. Adverse events at 12 months postoperatively | 86% of patients used device daily, 93% reported device usage at least 5 days/week. Long-term adverse events: 40% with stimulation discomfort and 21% with tongue soreness |
| Kent et al.1546 | 2016 | 4 | Single center retrospective case series of OSA patients CPAP intolerant and oral appliance therapy who underwent HNS | 20 patients with moderate to severe OSA who underwent HNS implantation | Objectively measured device use and adverse events at average follow-up of 7.8 months | Mean time from surgery to last follow up = 7.6 ± 3.3 months; Mean adherence = 7.0 ±2.2 h/night Long-term adverse events: 15% dry mouth; 5% with tongue abrasions |
| Heiser et al.1880 | 2017 | 4 | Single center prospective series of OSA patients CPAP intolerant and oral appliance therapy who underwent HNS | 31 patients with moderate to severe OSA who underwent HNS implantation | Objectively measured device use and adverse events 12 months after surgery | Mean adherence = 6.6 ± 2.7 h/night. No long-term adverse events reported |
| Huntley et al.1908 | 2017 | 4 | Two center retrospective review of OSA patients treated with HNS | 1. 48 Patients with moderate to severe OSA who underwent HNS implantation as a single center 2. 49 Patients with moderate to severe OSA who underwent HNS implantation at a different medical center |
Comparison between two different implant centers HNS results: objectively measured device use and adverse events | Mean weekly adherence between centers: 63.4% and 78.8% used device >40 h/week (43.75 ± 11.60 and 48.00 ± 10.24 h/weekly) at 8.6 and 11.4 months postimplantation. Long-term adverse events: 4% patients had dry mouth, 3% had headaches, 2% with incisional discomfort, and 1% with tongue abrasion |
| Hasselbacher et al.1892 | 2018 | 4 | Multicenter, prospective case series of HNS patients | 60 patients with moderate to severe OSA who underwent HNS implantation | Objectively measured device use and long-term adverse events at 12 months postoperatively | Mean adherence = 5.6 ± 2.1 h/night. No long-term adverse events reported |
| Woodson et al.383 | 2018 | 4 | Multicenter, prospective case series of HNS patients | 97 OSA patients treated with HNS, all of which were enrolled in the STAR trial | Objective device use and adverse events at 60 months after implantation | Median adherence = 6.6 ± 2.7 h/night. Compared to 12 months, stimulation discomfort and tongue abrasions decreased significantly (81–5 and 28–2 patients) |
| Heiser et al.1488 | 2019 | 4 | Multicenter, postmarketing observational, propsective case series of HNS patients | 508 patients with OSA treated with HNS | Objectively measured device use and adverse events taken 12 months after implantation | 32% Complication rate with tongue discomfort being most common at 8%–12%. Average adherence = 5.7±2.2 h/night |
| Thaler et al.1876 | 2020 | 4 | Multicenter, postmarketing observational, prospective case series of HNS patients | 1017 patients with OSA treated with HNS | Objectively measured device use and adverse events 12 months after implantation | Average adherence = 5.6 ± 2.1 h/night. Most common adverse event being stimulation discomfort (12% at posttitration visit and then 8% at 12 month follow-up) |
Abbreviations: AHI, apnea-hypopnea index; ESS, Epworth Sleepiness Scale; HNS, hypoglossal nerve stimulation; ODI, oxygen desaturation index; OSA, obstructive sleep apnea.
IX.D.8 |. Multilevel surgery
MLS for adults with OSA was popularized in the late 1990s following the recognition that single level surgery (with UPPP) was successful in a minority of unselected cases.1634,1913 This was found to be due to persistent retropalatal collapse but also untreated additional sites of obstruction.1643,1914 As acceptance of the concept of multilevel UA collapse grew,1584,1915 many centers around the world accordingly developed protocols to surgically treat multiple levels of the UA simultaneously. These protocols are collectively referred to as MLS and generically consist of the simultaneous surgical treatment of more than one site of UA obstruction in patients with OSA.
Initially MLS included nasal, retropalatal, and hypopharyngeal sites. Given the widespread adoption of DISE and the VOTE classification, an updated definition might include the nose, palate, oropharyngeal lateral walls, tongue base, and epiglottis as potential subsites. For instance, genioglossus advancement (GGA) with hyoid suspension (HS) was previously categorized as hypopharyngeal surgery only but this combination of surgeries has effects on the tongue base and epiglottis and therefore can be considered MLS. MMA also meets the definition of MLS. Similarly, HNS has the unique capability of treating palatal, tongue base, and epiglottis levels of collapse through palatoglossal and glossopharyngeal coupling.
Surgery for OSA is not considered first line therapy of OSA in adults. Patients pursue MLS for salvage of untreated or incompletely treated OSA due to PAP therapy intolerance. Therefore, comparing response rates to PAP effectiveness rather than efficacy is more appropriate.1916 Defining surgical success is most typically by PSG parameters but recognizing improvement in QOL, bedpartner reports of snoring and ideally reduced long-term health risk are of importance as well.
When interpreting the results of various MLS protocols over the past 20 years, it is important to recognize that not only have the thresholds for defining surgical success evolved over time, the core definition of a hypopnea has changed and the AHI has replaced the RDI as the outcome measure of choice.1917,1918 Currently, the most accepted definition of surgical success are the Sher criteria: achieving a 50% decrease in AHI and to less than 20 events/h. However, there is a movement toward using more objective outcomes (e.g., ODI) and/or a 50% AHI decrease to under 15 events/h into the mild OSA range, which correlates with long-term health risk and mortality.114,118,433,434,638,642,660,683 The selection of patients has largely shifted from awake staging systems assigned by physical exam and awake flexible laryngoscopy (Friedman stage, Fujita classification, Muller maneuver) to dynamic assessment under DISE.
Overall the literature on MLS is predominantly comprised of case series and lower quality prospective and retrospective cohort studies. Note that many smaller and lower quality studies are not included in the references due to citation limits. The exceptions are a randomized, sham-placebo controlled trial examining the effects of RFA and an RCT examining medical management versus MLS.1497,1919 The studies are heterogeneous in terms of patient selection, procedures, and outcome reporting. Even in papers using the same general procedure, there remains significant variability between studies such as the palatopharyngoplasty technique used or number of RFA sessions.
Overall, surgical success rates based on AHI/RDI improvements are in the 60%–70% range.1811,1817,1830,1913,1920,1921 Table IX.D.8 describes specific and heterogeneous success rates with a given MLS protocol. Most typically, postoperative AHI/RDI is determined within the first 12 months following surgery; however, a small number of studies have demonstrated longer-term efficacy.1922–1927 Most studies additionally compare pre- and postoperative nocturnal oximetry data including average SpO2, O2 nadir, and time spent below 88% which similarly show significant improvements overall.
TABLE IX.D.8.
Evidence on the efficacy of multilevel surgery
| Study | Year | LOE | Study design | Study group | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Riley et al.1797 | 1993 | 4 | Retrospective case series | UPPP, GGA and HS (Phase I) (n = 306) | RDI (<20/h), normalized oxygenation | Phase 1 MLS is effective (76.5% success rate) |
| Elasfour A et al.1947 | 1998 | 2b | Cohort study | 1. UPPP (n = 11) 2. UPPP with MLG (n = 18) Groups assigned based levels of negative pressure (mesopharynx vs. mesopharynx and esophagus) |
AHI (50% reduction) | Group 1 success (72.7%) higher than group 2 (61.1%) but group assignment based on presence of multiple levels of negative pressure |
| Friedman M et al1786 | 2003 | 2b | Retrospective cohort study | 1. UPPP with tongue RFA (n = 143) 2. UPPP alone (n = 134) |
AHI, AI, O2 nadir, ESS, snoring | The addition of tongue RFA seems to benefit Friedman stage II and III patients |
| Woodson BT et al1497 | 2003 | 1b | RCT | 1. Tongue and palate RFA (n = 30) 2. CPAP (n = 30) 3. Sham-placebo RFA (n = 30) |
ESS, FOSQ, SNORE25 median reaction time, AHI | RFA improved reaction time, QOL, ESS vs. baseline. RFA improved QOL, airway volume, AI, ArI. No difference in QOL and ESS improvement in CPAP vs. RFA. Mild side effects in RFA vs. sham |
| Baisch et al.1928 | 2006 | 2b | Retrospective cohort study | Tongue RFA and/or modified UPF or palate RFA and/or tonsillectomy and/or septoplasty or septorhinoplasty and/or inderior turbinate RFA 1. with HS (n = 67) 2. without HS (n = 16) |
AHI, ArI, SpO2 | AHI, ArI, SpO2, and ESS all significantly improved overall. Inclusion of hyoid suspension improved AHI success rate |
| Verse et al.1824 | 2006 | 2b | Prospective cohort study | UPF, tonsillectomy, tongue RFA and nasal surgery if needed 1. with HS (n = 45) 2. without HS (n = 15) |
ESS and AHI; O2 nadir, mean SpO2, ArI | Group 1 showed improvements in all outcomes, group 2 showed improvements in arousal index and ESS only |
| Friedman et al.1948 | 2007 | 4 | Case series | Nasal surgery, Pillar implant, tongue RFA (n = 145) | AHI, ESS, snoring VAS, pain levels, narcotic use, complications | All outcome measures significantly improved with 47.5% cure rate |
| Lin et al.1920 | 2008 | 4 | SR and MA (predominantly level four studies) | Surgery for OSA at least two sites: nose, oropharynx, hypopharynx (n = 1978) | AHI decrease of 50% and less than 20/h, complication rate, ESS, snoring VAS, AI, sleep architecture, O2 nadir | Surgical success 66.4%, complication rate 14.6%. All outcome measures significantly improved |
| Friedman et al.1788 | 2008 | 2b | Retrospective cohort study | UPPP or Pillar implants and/or nasal surgery with 1. Tongue RFA (n = 48) 2. Submucosal minimally invasive lingual excision (n = 48) |
AHI, O2 nadir, AI, snoring, ESS, complications | Group 2 had higher success rate (64.6% vs. 41.7%) but had higher complication rate and morbidity |
| van den Broek et al.1787 | 2008 | 2b | Retrospective cohort study | 1. UPPP (n = 37) 2. UPPP with tongue RFA (n = 38) |
AHI (Sher criteria), subjective improvement, AI, ODI, mean SpO2), adverse events | Mild improvement in success rate in group 2 (49% vs. 42%) without significant added morbidity |
| Eun et al.1949 | 2009 | 2b | Cohort study | UPPP and tongue RFA in 1. REM related OSA (n = 28) 2. Non-REM related OSA (n = 62) |
AHI (Sher criteria) | Group1 had higher success rate (50% vs. 35.5%), however there were significant baseline differences between groups |
| Fernandez et al.1791 | 2009 | 2b | Prospective cohort study, randomized | 1. UPPP with TBS (n = 29) 2. UPPP with tongue RFA (n = 28) |
AHI (>15 and 50% drop), ESS | Success rates comparable between groups (57.1% and 51.7%) but significantly worse in obese patients |
| Ceylan et al.1496 | 2009 | 2b | Prospective cohort study | 1. Tongue and palate RFA (n = 26) 2. Nasal CPAP (n = 21) |
ESS, AHI, T90, O2 nadir | No difference in treatment success between groups |
| Kezirian et al.1932 | 2010 | 2b | Prospective cohort study | UPPP, tonsillectomy, GGA ± HS (n = 30) | AHI (50% decrease and to <15/h), CRP, IL6, homocysteine, insulin resistance, leptin, FOSQ | 43% surgical response rate. Significantly improved mean AHI, FOSQ. Significant reduction in CRP in responders, otherwise no differences |
| Friedman et al.1827 | 2012 | 2b | Cohort study | 1. TORS with ZPP (n = 27) 2. Submucosal coblation of tongue base with ZPP (n = 22) 3. Tongue RFA with ZPP (n = 24) |
Complications, pain, ESS, snoring, AHI, O2 nadir | Complications, pain and hospital LOS no different. ESS and snoring improved in all groups. Return to diet and activity longest in group 1. Success rate highest in group 1 (66.7% vs. 45.5% vs. 20.8%) |
| van Maanen et al.1950 | 2012 | 2b | Retrospective cohort series | Influence of positional OSA following 1. UPPP or Z-PP withBOT surgery (n = 94) 2. BOT surgery alone (n = 36) |
AHI, AI | Sleep position not correlated with outcomes |
| Li et al.1951 | 2013 | 2b | Cohort study | 1. UPPP with TBS (n = 45) 2. UPPP (n = 33) |
AHI, ESS, O2 nadir | Success rates significantly greater in group 1 |
| Plzak et al1952 | 2013 | 2b | Cohort study | 1. UPPP (n = 35) 2. UPPP with tongue RFA (n = 79) |
AHI, ESS, ODI, mean SpO2, snoring, adverse events | All measures significantly improved except mean SpO2. Success rate better in group 2 (51.7% vs. 41.9%) |
| van Maanen et al.1953 | 2014 | 3b | Case–control study | UPPP or ZPP ± tongue RFA in patients with 1. Non-positional OSA (n = 71) 2. Positional OSA (n = 68) |
AHI | Significant improvement is AHI though greater in non-positional OSA |
| Handler et al.1811 | 2014 | 4 | SR of case series | 1. TBS 2. TBS and UPPP 3. GGA and UPPP 4. GGA, HS, and UPPP |
Surgical success rate | Group 1 success 36.6%, group 2 62.3%, groups 3 and 4 61.1%. No significant difference between groups 2 and ¾ |
| Verse et al.1670 | 2015 | 2b | Prospective cohort study | 1. UPPP and lingual tonsillectomy (n = 58) 2. UPPP, tongue RFA and HS (n = 50) |
AHI | Greater reductions in AHI in group 1 |
| Bostanci et al.1817 | 2016 | 4 | SR of case series and cohort studies | 1. TBS (Repose) (n = 62) 2. Modified TBS (n = 51) 3. UPPP with TBS (Repose) (n = 176) 4. UPPP with modified TBS (n = 124) |
AHI | Success rates higher in group 2 compared to group 1. Success higher and not different in groups 3 and 4 |
| Salapatas et al.1954 | 2016 | 4 | Retrospective case series | Various nasal procedures, palatal stiffening, and tongue RFA (n = 601) | AHI, ESS, snoring intensity | AHI: 19.8 ± 5.9/h to12.7 ± 7.6/h (p < 0.0001), 45.9% surgical success; ESS: 12.1 ± 4.8 to 6.8 ± 2.9 (p < 0.001); mean snoring intensity 8.8 ± 0.8 to 4.0 ± 2.1 (p < 0.001). |
| Li et al.1955 | 2016 | 2b | Retrospective cohort study | 1. Relocation pharyngoplasty ± coblation endoscopic lingual lightening (n = 30) 2. Relocation pharyngoplasty (n = 60) |
AHI, complications | Surgical response rate greater in MLS group, no increase in complications |
| Brietzke et al.1939 | 2017 | 2b | Retrospective cohort study, national database | 1. UPPP alone (n = 7559) 2. UPPP with nasal surgery (n = 5219) 3. UPPP with hypopharyngeal surgery (n = 1164) 4. UPPP with nasal surgery and hypopharyngeal surgery (n = 691) |
Complications, postoperative CPAP orders (implying surgical failure) | MLS (groups 2–4) had higher rates of postoperative bleeding and postoperative CPAP orders |
| Song et al.1794 | 2017 | 4 | SR and MA of predominantly case series | 1. Standard genioplasty (n = 27) 2. Modified genioplasty (n = 10) 3. GTA (n = 24) 4. GTA + HS (n = 50) |
AHI, O2 nadir | Group 1 and 4 improved AHI and O2 nadir significantly, groups 3 improved AHI significantly |
| Samutsakorn et al.1830 | 2018 | 4 | SR and MA of case series | Lingual tonsillectomy with palate surgery (n = 107) | AHI, ESS, O2 nadir | All outcome measures improved significantly |
| Mulholland et al.1921 | 2019 | 4 | SR and MA | Multilevel palate and tongue base surgery (n = 1806) | AHI | MLS results in significant improvements in OSA severity |
| Missale et al.1956 | 2019 | 2b | Cohort study | ESP or barbed sus- pension/relocation pharyngoplasty ± nasal surgery (n = 70) | AHI, ESS, intraoperative anterio-posterior pharyngeal width | All measures significantly improved. Pharyngeal measurements correlated with success |
| MacKay et al.1919 | 2020 | 1b | RCT | mUPPP and tongue RFA (n = 51) vs. medical management (weight loss, positional) (n = 51) | AHI, ESS | Surgery more effective than medical management in PAP failure |
Abbreviations: AHI, apnea hypopnea index; AI, apnea index; ArI, arousal index; BITR, bilateral inferior turbinate reduction; ESS, Epworth Sleepiness Scale; GGA, genioglossal advancement; GTA, genial tubercle advancement; HS, hyoid suspension; LT, lingual tonsillectomy; MLG, midline glossectomy; RDI, respiratory disturbance index; RFA, radiofrequency ablation; T88, time spent with SpO2 <88%; T90, time spent with SpO2 <90%; TBS, tongue base suspension; TORS, transoral robotic surgery; UPF, uvulopalatal flap; UPPP, uvulopalatalpharyngoplasty.
Snoring and QOL measures, most commonly the ESS, demonstrate significant improvements after MLS. Most studies describe raw ESS score changes rather than improvement below established thresholds for defining pathologic sleepiness. Finally, a smaller number of studies report significant improvements in other metrics such as arousal index,1497,1824,1928,1929 sleep architecture,1798,1920,1929–1931 CRP,1932 airway cross-sectional area,1933–1936 and volume changes,1497,1937 reflux symptoms,928 swallowing function,1938 and reaction time.1497,1926,1936 Notably absent are CV outcomes measures.
A few studies attempted to compare the efficacy of single level and MLS surgery. None of these are RCTs and therefore are fundamentally flawed to answer this question. Most of these studies assigned patients to single or MLS based on classification systems such as Fujita or Friedman, where inherently easier to treat patients may be more likely to receive single level surgery. Some studies showed greater efficacy with MLS, while others showed superiority of single level surgery. This question is not as relevant at the present time given the trend towards individualization of treatment selection using strategies such as DISE and endotyping.
MLS for OSA is effective at reducing polysomnographic measures of OSA severity, bedpartner reports of snoring, and QOL measures. Patients should be adequately counseled regarding success rates of specific MLS protocols. Procedure selection should be tailored to levels of UA collapse, patient goals, and surgeon comfort/experience.
Safety of MLS
All patients with OSA are at an increased risk of perioperative complications, and this is especially true for UA surgery such as MLS for OSA. Contributors to this increased risk include muscle relaxation from general anesthesia, respiratory suppression, and heightened arousal threshold from narcotics, UA edema, and difficulty tolerating PAP postoperatively. It therefore stands to reason that MLS may increase risk by extending general anesthesia time, increasing narcotic requirements and worsening UA edema. Patients requiring MLS likely also have more severe OSA.
Multiple studies have shown significantly increased complication rates and pain scores in MLS when compared to single level surgery, represented by UPPP alone.1939–1941 However, in one large national database analysis, Baker et al. did not find increased complications between UPPP alone and UPPP combined with tongue base or nasal surgery but did identify differences in length of hospital stay.1942 Another consideration is the risk-benefit of staged versus simultaneous UA surgery. One study found no difference in complications between staged and simultaneous MLS, but did find higher healthcare expenses in the staged group.1943
Concurrent nasal surgery may theoretically increase complication rates by temporarily promoting open-mouth breathing and abolishing the nasal-ventilatory reflex. Busaba compared single stage UPPP with nasal surgery to staged UPPP and nasal surgery and found no difference in complication rates.1944 Similarly Pang reviewed MLS cases with and without nasal surgery and found no difference in complication rates.1945
Postoperative disposition is another peri-operative safety concern. Although the biggest risk following MLS may occur around postoperative day 3 coinciding with REM rebound, peaking edema, and pain (and therefore narcotic use), most postoperative patients are admitted for overnight observation for one night only.1521 Same-day discharge versus overnight admission for nasal surgery with UPPP showed no difference in complication rates.1946 There are no studies reporting on the safety of same-day discharge following combined palate and hypopharyngeal surgery.
As mentioned, MLS is a heterogeneous collection of protocols, some of which are minimally invasive. For instance, RFA treatments of the palate and tongue base are routinely performed on an outpatient basis due to the limited risk profile. One study even reported performing GGA with hyoid myotomy and uvulopalatal flap under local anesthesia with no difference in complication rates compared to when performed in the OR.1550
Overall, complications – and especially major complications – are relatively uncommon as demonstrated in a large single series of MLS patients.1559 Due to wide-ranging morbidity of various procedures, reporting a single, generalized complication rate is misleading. The reader is therefore encouraged to review specific protocols to evaluate common adverse events.
Guidelines for the perioperative care of OSA surgery patients are well summarized by Ravesloot et al. although these are largely based on low quality studies and expert opinion and specifically does not address MLS.1521 Therefore, developing guidelines for perioperative care of patients undergoing MLS represents an opportunity for further work. (Table IX.D.8)
IX.D.9 |. Durability of surgery: long-term results
Evidence regarding the durability of response to surgical intervention for OSA is limited. While short-term effects of sleep surgery have been well-studied, there tends to be a lack of long-term follow-up data in studies of this population. There are no SRs that examine long-term efficacy of surgical interventions.
Outcomes that determine durability of treatment include symptoms, somnographic variables, and comorbidities. These parameters are not uniformly examined across the literature when assessing for long-term response.1686 In addition, surgery can have a variable effects on the durability of different subjective outcomes within the same patient compared to its effect on objective polysomnographic criteria.1665 Surgical success is traditionally defined as >50% reduction in AHI to a level less than 20,1501,1507 although this is not synonymous with complete resolution of disease.1957 This definition of “success” has been met with criticism,1957 citing possible sub-therapeutic responses.
One Cochrane review examined the efficacy of any surgical intervention for OSA compared to non-surgical or no intervention.1483 Of the studies included in this review, duration of follow-up for surgical patients was heterogeneous, with a mean duration of 11 months (range: 3 months −4 years). Few studies of surgical intervention for OSA include long-term data beyond 4 years, with longest follow-up at 8 years.1958
Durability of response to pharyngeal and palatal surgeries has been shown to be variable. About half of patients who undergo such procedures are reported to have persistent improvement in clinical and polysomnographic OSA parameters at time of long-term follow-up, which ranges from 3 to 8 years.812,1507,1958 MMA, whether performed as an isolated procedure or as a secondary phased intervention in a multiphase approach, has been associated with more consistent longstanding improvement of OSA parameters for 2–6 years after surgery in upwards of 90% of patients.1501,1959 Data for HNS has demonstrated up to a 75% surgical success rate at 5-year follow-up.1890
Sleep surgeries targeting structures of the UA have been reported to show better long-term outcomes for patients with mild-to-moderate OSA,1958,1960 especially in those with clearly identifiable obstructive anatomic variants.1961 A >50% improvement of AHI in mild to moderate OSA is more likely to result in AHI reduction to levels closer to the normal range. The greater propensity for relapse in patients with severe OSA may be due to the higher preexisting burden of disease in this population, as well as presence of comorbid conditions, such as obesity.1962,1963 Although a greater clinical effect of surgery on reduction of AHI has been noted for patients with more severe OSA,1501,1959 somnographic parameters can still remain above the normal range, thus necessitating continued treatment. Surgeries targeting skeletal structures and HNS have demonstrated longer-lasting success rates in patients with moderate-to-severe OSA at 2–6 years.1890,1959
Response to surgery tends to degrade over time, and up to half of patients treated with surgery for OSA can eventually relapse.1507,1665,1958 Relapse has been suggested to be related, in part, to weight gain, interval development of illness, or medication changes, although it can still occur without clear cause.1482,1962 The majority of failures tend to be apparent between 6 and 12 months after surgery. Improved outcomes and longer persistence of response have been shown with multiphase and multilevel surgeries that address multiple anatomic sites rather than single-site surgery.1665,1686,1921,1959
There is a paucity of data examining the long-term effects of sleep surgery on morbidity and mortality.1686 Short-term improvements in symptoms, somnographic variables, and comorbid parameters1964,1965 have been wellreported in these patients; however, the best way of measuring longstanding impact has yet to be determined. Surgical treatment of OSA has been suggested to have positive effects on CV outcomes and on mortality,1498,1964 although the evidence is limited and should be interpreted cautiously.
The variety of surgical interventions available, along with the clinical and anatomic variability of patients undergoing these procedures, makes it difficult to generalize durability of response. Newer surgical techniques, such as hypoglossal nerve stimulator implantation, are also just beginning to amass longitudinal evidence based on therapeutic AHI. Improvements in surgical techniques, such as expansion palatopharyngoplasty, have led to increased success of short-term surgical response rates,1966 and long-term data will be needed for these updated techniques.
Surgery has the potential to provide long-lasting benefit, but long-term follow-up is required as OSA is a chronic disease. The variability of response and the risk of relapse noted in the current literature reinforce the necessity of evidence-driven patient selection and directed counseling about the expected goals and range of outcomes. (Table IX.D.9)
TABLE IX.D.9.
Summary of evidence for durability of response to surgery
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion | ||
|---|---|---|---|---|---|---|---|---|
| He et al.812 | 2019 | 2a | SR and meta-analysis (two RCTs and nine cohort studies) | Adults who underwent UPPP or its modification for OSA and had short-term (n = 285) and/or long-term (n = 368) follow-up (at least 34 months) with objective sleep study results | AHI | 1) Despite surgical efficacy decreasing over time (67.3% short-term and 44.35% long-term), UPPP surgeries are effective. 2) BMI, lowest O2 sat, and proportion of sleep time with O2 sat <90% were potentially predictive of long-term response. |
||
| Costantino et al.1890 | 2019 | 2a | SR and meta-analysis of cohort studies | Adult patients (n = 350) who underwent hypoglossal nerve stimulation (HNS) for treatment of moderate-to-severe OSA | AHI, ESS, adverse events | 1) At 5-year follow up: surgical success rate was 75%, mean AHI reduction was 18.00, and ESS mean reduction was 5.27 (for Inspire HNS). 2) 6% of patients with serious device-related adverse events. |
||
| Janson et al.1958 | 1997 | 2b | Cohort study | Patients (n = 34) with OSA who underwent UPPP with (n = 25) or without (n = 9) long-term follow-up | Symptoms, AHI (responders with >50% AHI reduction and AHI ≤10) | 1) 48% were responders at long-term (4–8 year) follow up. 2) Responders had lower preoperative AHI. 3) Some initially successfully treated patients (n = 4, 16%) relapsed in the long term. |
||
| Riley et al.1959 | 2000 | 2b | Cohort study | Patients (n = 40) with severe OSA who underwent phase II (skeletal and soft tissue) surgical reconstruction for sleep apnea | PSG variables (RDI, LSAT), QOL, complications | 90% of patients showed persistent clinical success (improved quality of life assessments and polysomnography equivalent to CPAP patients; or postoperative RDI < 20 with at least 50% reduction and LSAT levels equivalent to nasal CPAP patients) at time of long-term follow-up (mean follow-up 50.7 ± 31.9 months). | ||
| Boot et al.1665 | 2000 | 2b | Cohort study | Patients (n = 58) with OSA who underwent UPPP with long-term follow up data (11–74 months, median 34 months) | Snoring, excessive daytime sleepiness, ODI | 1) Response to UPPP for OSA decreases progressively over years after surgery. 2) UPPP in combination with tonsillectomy more effective than UPPP alone. |
||
| Lee et al.1964 | 2018 | 3b | Retrospective case–control study | 1) Adults with OSA who underwent UPPP (n = 22,213) 2) Adults with OSA who did not undergo surgical intervention (n = 170,103) 3) Matched control group of adults without OSA (n = 961,590) |
Newly diagnosed MI, CHF, and AF | UPPP, regardless of its effects on AHI, can significantly reduce risk of CHF (hazard ratio [HR]: 1.17 [1.10–1.24] without surgery to 0.76 [0.60–0.96]) and AF (HR: 1.39 [1.32–1.46] without surgery to 1.12 [0.94–1.32]) in patients with OSA for up to 8 years after surgery. | ||
| Walker-Engström et al.1507 | 2002 | 1a | Randomized controlled trial | Patients with mild-to-moderate OSA treated with oral appliance (OA, n = 45) vs. UPPP (n = 43) | AI, AHI (success defined as >50% reduction of AI/AHI), symptoms, compliance, need for complementary treatment, adverse effects | 1) Success rate at 4-year follow-up was higher in OA group vs. UPPP (81% vs. 53%), but effectiveness of OA partly invalidated by its long-term compliance of 62%. 2) Success rate of UPPP decreased from 1 to 4 year follow-up: 70% to 53% (AI), and from 60% to 35% (AHI). |
||
Abbreviations: ESS, Epworth Sleepiness Scale; lateral PP, lateral pharyngoplasty; LAUP, laser-assisted uvulopalatoplasty; LSAT, low oxyhemoglobin desaturation; OA, oral appliance; ODI, nocturnal oxygen desaturation index; TA, tongue advancement (mandibular osteotomy); TCRTA, temperature-controlled radiofrequency tissue ablation; TS, tongue suspension; RDI, respiratory disturbance index; UPPP, uvulopalatopharyngoplasty.
IX.D.10 |. Tracheotomy
Tracheostomy, a surgical procedure in which an opening is created in the trachea to bypass an UA obstruction, was described in 1969 for the treatment of OSA. Until that time, there were no other treatments for OSA or its medical sequelae of pulmonary and systemic hypertension, disabling daytime sleepiness, and decreased mental function.1967
During the 1970s, the medical management of OSA with continuous PAP was not widely used or available and many articles evaluated the efficacy of tracheostomy for OSA. Guilleminault et al.1968 reported on 268 patients seen between 1972 and 1978 who were classified as “predominantly OSA” based on clinical symptoms and PSG. Of the 72 patients that underwent tracheostomies, there were 50 patients with follow-up; these patients had a mean preoperative AI >60 events/h and had postoperative PSGs carried out 6 weeks to 3 months after surgery. For all patients, the AI decreased to below 5 events/h and elimination of daytime sleepiness and fatigue. Patients, when asked, responded that they would opt for surgery again, based on their improvement in symptoms.
Simmons et al.1969 reported on 14 patients (12 adults and 2 children) with OSA where no specific anatomical cause was found. Indications for surgery were hypertension, loss of livelihood and social contacts, and inability to drive due to disabling hypersomnolence for adults, and for children were emotional and learning problems and enuresis. Tracheostomy was successful in all 14 patients after medical management had failed. Several additional articles describe resolution of hypersomnolence, hemodynamic abnormalities, cardiac arrhythmias, sleep cycle disruptions, and arterial blood gas abnormalities associated with OSA, as well as resolution of obstructive events with tracheostomy.1970–1977
In 1980, CPAP was developed by Dr. Colin Sullivan who applied pressure through the nose to keep the UA open. As CPAP use has become more prevalent, it has evolved into the initial treatment for many patients with OSA. However, there are many patients who do not tolerate CPAP, while others who continue to have hypersomnolence, morning headaches, and CV disease secondary to OSA despite CPAP use.
To determine if tracheostomy results increased survival for patients with OSA, Partinen et al.1978 evaluated the long-term outcome of 198 OSA patients undergoing tracheostomy compared to individuals treated with weight loss and better sleep hygiene. The patients were seen between 1972 and 1980 and received tracheostomy (n = 71) or recommendations for weight loss (n = 127). At 5-year follow-up, all deaths (14) had occurred in the non-surgical group, and this group also had a higher 5-year crude vascular mortality rate. He et al.1979 studied 385 male OSA patients for over a decade and found that those with an AI > 20 events/h had a greater mortality rate than those with an AI < 20/h. For patients with an AI > 20/h, none of the patients treated with tracheostomy (N = 19) or nasal CPAP (N = 4) died.
An SR of the literature by Camacho et al.1980 identified 10 relevant studies that had reported outcomes for AI, AHI, ODI, effect on daytime sleepiness, and mortality. Tracheostomy was found to significantly decrease AI, ODI, sleepiness, and CV mortality in both obese and non-obese patients with OSA.
Mild complications from tracheostomy include wound infections, necrosis of skin flaps after permanent tracheostomy, and an increase in pulmonary secretions resulting in pneumonia or bronchitis. Respiratory distress secondary to mucous plugging and presence of granulation tissue requiring operative or procedural intervention have been reported. There was one report of the severe complication of tracheal innominate fistula, which resulted in death.1968,1971,1974
Tracheostomy is difficult for some patients to adjust to and can result in depression and psychosocial problems.1968,1981 To counter this, some patients may wear a scarf or high collared shirt to cover the tracheostomy tube during the day while it is plugged. The tracheostomy can also restrict activities of daily living and social activities.
Tracheostomy has been shown to be an effective surgical procedure for patients with OSA and OHS. It can result in resolution of OSA-associated conditions such as sleep stage abnormalities, pulmonary and systemic hypertension, hypersomnolence, and poor QOL. Mortality has also been shown to decrease in OSA patients who have had tracheostomy versus those treated with medical therapies. Currently, tracheostomy is reserved for those patients who are unable to use CPAP, or have not benefited from CPAP and do not have addressable identifiable UA abnormalities but are amenable to surgery.
IX.D.11 |. Bariatric surgery
Obesity, as defined by a BMI ≥ 30 kg/m2, continues to be a worldwide epidemic. According to the most recent WHO data, the prevalence of worldwide obesity has tripled since 1975. In 2016, more than 1.9 billion adults worldwide were overweight with 650 million categorized as obese.1982 The prevalence of OSA amongst obese adults is high (55%–90%), with a positive correlation between OSA and increasing BMI.1983–1985 Of the various treatment options available for OSA in obese patients, bariatric surgery has been shown to improve various co-morbidities including OSA.194,195,1986
Several RCTs have been completed evaluating the impacts of bariatric surgery on OSA.194,1987,1988 Two of these primarily compare the results of diet, exercise, and intense nutritional care versus the results of bariatric surgery.194,1988 These two studies revealed that the surgical group achieved significantly higher weight loss than the nutrition group, but there was no significant difference in their AHI values. Furthermore, there was found to be no significant difference in their ability to be weaned from a PAP system.1988 The RCTs listed may have been limited by their method of bariatric surgical intervention as both utilized gastric banding. However, SRs show that procedures that are both restrictive as well as malabsorptive (such as roux-en-y surgery), where the stomach anatomy and transit time are altered, are more efficacious in reducing OSA when compared to purely restrictive procedures like gastric banding.1989,1990
More recent SRs suggest that there is significant reduction in AHI levels following bariatric surgery. One SR compared intensive lifestyle interventions to bariatric surgery with regards to their effect on OSA. Those undergoing bariatric surgery had a two times greater reduction in AHI that those in the intensive lifestyle intervention group.1963 Several of the studies included other OSA endpoints, specifically the ESS scores and nocturnal SaO2 nadir.1963,1983,1987,1991,1992 In a study comparing bariatric surgery with CPAP treatment, both groups had similar decreases in ESS.1987 Two other studies, and one SR, noted a reduction in the ESS after bariatric surgery,1963,1983,1991 while one study noted a decrease in the percent time with nocturnal SaO2 below 90%.1983 Additionally, an SR noted an increase in the nocturnal mean and nadir SaO2 following bariatric surgery.1992
There are general trends noted amongst all these studies. The most important is that bariatric surgery often results in a significant reduction in AHI levels. However, OSA often persists following bariatric surgery. There is little data about the ability to discontinue PAP use once significant weight reduction has occurred. (Table IX.D.11)
TABLE IX.D.11.
Evidence for bariatric surgery and OSA
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Ashrafian et al.195 | 2015 | 1c | Systematic review | 1. Bariatric surgery 2. Non-surgical weight loss |
AHI before and after intervention | 1. Surgical intervention associated with significant reduction in post op AHI (but significant heterogeneity). 2. Non-surgical intervention also associated with significant post op reduction in AHI (but significant heterogeneity). |
| Bakker et al.1987 | 2018 | 2b | 1 RCT, low quality, did not meet power | 1. Laparascopic gastric banding 2. CPAP |
AHI at 9 and 18 months after intervention | No difference in AHI off CPAP between groups at 9 or 18 months. |
| Buchwald et al.1993 | 2004 | 1c | Systematic review | Meta-analysis of improvement/resolution of OSA in patients undergoing bariatric surgery | Improvement/resolution of OSA | 83.6%–85.7% resolution/improvement in OSA among total patient population, but significant heterogeneity of studies, with inclusion of RCTs, non-randomized CTs, and uncontrolled case series. Also included all types of bariatric procedures. |
| Dixon et al.194 | 2012 | 1b | 1 RCT | 1. Diet, exercise, behavioral program 2. Laprascopic adjustable gastric banding | Reduction in AHI at 2 years | Surgical group achieved significantly greater weight loss but no difference in AHI reduction. Patterns suggest that much of benefit to AHI is associated with mild to moderate weight loss with diminishing returns with further weight loss. |
| Feigel-Guiller et al.1988 | 2015 | 1b | 1 RCT | 1. Intensive nutritional care 2. Laprascopic adjustable gastric banding |
Rate of PAP weaning (AHI < 20) at years 1 and 3 | Surgical group achieved significantly greater weight loss at years 1 and 3, but no difference in ability to wean from PAP at years 1 and 3. |
| Greenburg et al.1994 | 2009 | 1c | Systematic review | Meta-analysis of improvement/resolution of OSA in patients undergoing bariatric surgery | 1. BMI reduction 2. AHI reduction |
Significant heterogeneity between studies. Significant reduction in BMI and AHI seen, but AHI was still moderate. Bariatric surgery improves, but does not resolve OSA. |
| Haines et al.1991 | 2007 | 2c | Prospective uncontrolled trial | Patients with OSA undergoing bariatric surgery | 1. BMI reduction 2. RDI reduction at 6–42 months after surgery |
Significant reduction in BMI and RDI, but RDI remained moderate. Bariatric surgery improves, but does not resolve OSA. |
| Hariri et al.1995 | 2018 | 2c | Retrospective review | 1. Obesity classes (I, II, III) 2. Sleeve gastrectomy versus Roux-en-Y Bypass 3. Pre-op AHI severity (mild, med, severe); All 3 evaluated at 6 months and 1 year |
Subjective postop OSA remission (STOP-BANG <2 and discontinuation of CPAP) | No difference in subjective OSA remission between obesity class groups at 6 or 12 months. No difference in subjective OSA remission by type of surgery at 6 or 12 months. No difference in subjective OSA remission by pre-op AHI severity. |
| Lettieri et al.1983 | 2008 | 2c | Prospective uncontrolled trial | Patients with OSA undergoing bariatric surgery | AHI severity (mild, mod, severe) at 1 year post bariatric surgery | Significant change in BMI, ESS score, and AHI postop at 1 year, but AHI remained moderate. Severity improved in 50%. |
| Miras et al.1996 | 2018 | 2c | Retrospective review | All patients in the National Bariatric Surgery Registry (NBRS) from 2000 to 2015 totaling 50,782 patients | Prevalence of OSA before and after bariatric surgery recorded years 1–5 postoperatively | 19.7% of patients had diagnosed sleep apnea prior to bariatric surgery (79.8% with no diagnosis or indication of OSA). Postoperatively, the prevalence dropped to 10%. However, there are no specific numbers listed for sleep apnea results post-procedure. In general, a decrease in the prevalence of OSA reported after bariatric surgery. |
| Quintas-Neves et al.1989 | 2016 | 2a | Systematic review | Review of 22 cohort studies involving pre- and postoperative OSA in the setting of bariatric surgery | Improvement/resolution of OSA. Type of bariatric surgery and impacts on OSA | Significant reduction in BMI and AHI values following bariatric surgery with short-term follow-up (1–2 years). Greater reduction in AHI values in those undergoing restrictive and malabsorptive procedures (roux-en-y) vs. restrictive alone (gastric banding) |
| Sarkhosh et al.1990 | 2013 | 2a | Systematic review | Sixty-nine studies reviewed: three RCTs, 11 controlled trials, 55 case series | Type of bariatric surgery and impacts on OSA | Significant improvement or resolution in OSA following bariatric surgery. Interventions with a malabsoprtive mechanism, which alter the gut anatomy and transit time, are more efficacious in impacting in obstructive sleep apnea. |
| Wong et al.1963 | 2018 | 2a | Systematic review | Meta-analysis of improvement/resolution of OSA in patients undergoing bariatric surgery. 15 studies included in analysis | Improvement and resolution of OSA following bariatric surgery. Secondary measures such as BMI, ESS, and comparisons to intensive lifestyle interventions were performed | Significant reduction in AHI following bariatric surgery with greater reduction noted in those with higher presurgical AHI. Two times greater reduction in AHI in bariatric surgery group compared to intensive lifestyle interventions. 97.5% of bariatric patients still had residual OSA (AHI > 5). Also noted no correlation between amount of weight lost and change in AHI. |
| Zhang et al.1992 | 2019 | 2a | Systematic review | Systematic review of improvement/resolution of OSA in patients undergoing bariatric surgery. 15 studies included: 13 prospective, one RCT, and one retrospective | Improvement and resolution of OSA following bariatric surgery. Primary endpoints included AHI, mean SaO2, and nadir SaO2 | Improvement of mean and nadir SaO2 following bariatric surgery. Significant reduction in AHI between pre- and post-surgical period. Significant heterogeneity noted. |
IX.E |. Surgical Efficacy for OSA Symptoms and Comorbidities
IX.E.1 |. Surgery and daytime sleepiness and quality of life
Sleepiness is arguably the most common daytime complaint of OSA sufferers.1997,1998 It is attributed to the recurrent arousals and desaturations that lead to sleep fragmentation and decreased slow wave sleep. Assessing sleepiness with patient-reported scales such as the ESS, Functional Outcomes of Sleep Questionnaire (FOSQ), and Calgary SAQLI are critical. Scores are often not associated with the AHI.1999,2000
Evidence supports that UA surgery reduces sleepiness. Palatal surgery outcomes are the most widely published. The traditional UPPP with and without tonsillectomy was found to be significantly more effective at reducing ESS compared to no treatment.1688 Other palatal techniques and hypopharyngeal surgeries have also been effective at alleviating sleepiness.1666,1802,1804,1817,1920,1966,2002–2004 Studies on HNS and MMA report significant improvements lasting beyond 5 and 10 years, respectively, with normalization of ESS and sleep-related QOL scores (FOSQ and SAQLI).1868,1874,1890,2005–2007 Nasal surgery significantly improved ESS despite not having a large effect on AHI.545,906,1660,1961,2008 Less invasive techniques such as RF and soft palate implants may have benefit on sleepiness.1737,1746,1782,2009–2012 Tracheostomy resolves sleepiness in most, but not all, OSA patients.1980 The only surgical technique that has been labelled ineffective in this regard is LAUP.1483
Certain factors appear to further improve the effect of surgery on sleepiness. Repeated treatments in cases of minimally invasive procedures can achieve longerterm results.2013 Targeting multiple levels is more effective than single-site procedures, in the short-term.2012,2014 Steward et al. showed that ESS and FOSQ significantly improved with five sessions of multilevel (tongue and soft palate) TCRFTA compared to three single-level (tongue) sessions.2015
Surgery has been compared against non-surgical interventions in a few randomized controlled studies. Mackay et al. recently reported that surgery (combined modified UPPP and minimally invasive tongue volume reduction) was significantly more effective in lowering ESS (12.4–5.3) compared to ongoing medical management (11.1–10.5) over 6 months.1919 The aim of this trial was to assess whether modified UPPP and minimally invasive tongue reduction was beneficial for those with moderate to severe OSA.1919 This was a multicenter, open-label RCT to assess the benefits of surgical intervention versus medical management (instruction on mitigating reversible causes of inspiratory flow resistance – weight loss, bed positioning, etc.). Symptomatic adults (age 18–70 years) with an AHI > 15 were enrolled with 51 patients in each group (n = 102). Primary outcomes were reduction in AHI and Epworth Sleepiness Score (ESS).
The mean AHI was reduced from 47.9 to 20.8/h at 6 months in the surgery cohort and decreased from 45.3 to 34.5/h for the medical management group. The mean ESS was 12.4–5.3 at 6 months in the surgery group and marginally decreased from 11.1 to 10.5 in the medical management group (p < 0.001). Four percent in the surgery group had serious adverse events. Of note, only seven of 51 medical management patients attempted CPAP during the trial, indicating that 44 patients in the control arm were not receiving active treatment, perhaps contributing to differences between groups.
Collectively, the observed benefits from surgery are seen in both objective and subjective findings. However, it is unclear what truly is a significant reduction in AHI and thus such changes should be interpreted in the context of patient reports and/or hard outcomes.
Walker-Engstrom et al. found similar score improvements for sleep and vitality in both UPPP and OA therapy groups with mild-moderate OSA after a year.2016 Woodson et al. conducted a trial of mild–moderate OSA patients who underwent multilevel TCRFTA (palate and tongue base) versus CPAP versus sham.1497 ESS and FOSQ scores of both CPAP and TCRFTA treatment groups improved significantly compared to sham, although the CI included zero.1497,2017 Vicini et al. demonstrated that both MMA and APAP reduced ESS scores equally 1 year after commencing treatment, with no statistical difference between the groups.1503
Vigilance
Vigilance, a surrogate marker for sleepiness, has been reported as an outcome measure in a few studies. Multilevel TCRFTA (palate and tongue base) significantly improved reaction time testing compared with baseline, while CPAP did not.1497 Faster reaction time correlated with a greater number of levels and sessions of TCRFTA.2015 Modified UPPP was shown to significantly improve alertness through better Oxford sleep resistance (OSLER) test scores compared to the untreated group.1666 The proportion of patients who passed the 40-min test after surgery rose from 41% to 91%.1666 Boyd et al. found that patients performed better in a psychomotor vigilance task that measures sustained attention over 10 min after MMA.2005 MMA and CPAP improved vigilance to the same extent from baseline in 24 patients who had been treated with CPAP initially, and then underwent MMA.2018
Sexual function
Two small prospective studies on UPPP have shown postoperative increase in testosterone levels and normalization of decreased libido, as well as significant improvement in the International Index of Erectile Function.2019,2020
Psychological symptoms
Prospective cohort studies and case series have shown improvement or resolution in anxiety, depression, hostility, and daytime dysfunction after OSA surgery.2014,2021–2024 Ishman et al. found that the reduction in sleepiness scores, and not the OSA severity, is predictive of improvement in depressive scores using the Beck Depression Inventory-II.2014 Li et al., however, did not find any correlation between mood improvement and changes in polysomnographic parameters or ESS scores.2023 There is a possibility that psychological symptoms improve at least in part due to the patient’s perception of his/her disease being treated, and not purely due to change in AHI.2022
General quality of life
Holty and Guilleminault reported that MMA reduced symptoms such as irritability, morning headaches, memory loss, and impaired concentration in most patients.1867 Skeletal advancement surgery can improve SF-36 Health Survey scores, with 66.7%–100% of patients returning to normal levels postoperatively.2005,2006 SF-36 scores similarly improved in patients who underwent modified UPPP, and not in the untreated group.1666
The Cochrane review by Sundaram et al. in 2005 concluded that symptoms and QOL were not significantly improved with surgery.1483 The review however was not comprehensive and did not include all types of sleep surgery such as MMA and many newer surgical techniques. (Table IX.E.1)
TABLE IX.E.1.
Evidence for surgery and improved daytime sleepiness and quality of life
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Farrar et al.2009 | 2008 | 1a | SR and MA | Isolated radiofrequency ablation of the palate, tongue base, or both | ESS, RDI, LSAT | Radiofrequency ablation reduces ESS and RDI levels. ESS decreased by 31%. RDI reduced by 31% in short-term and 45% in long-term. |
| Franklin et al.2017 | 2009 | 1a | SR | 1. Surgery 2. Sham or no treatment |
ESS, AHI, QOL | Laser-assisted uvulopalatoplasty or radiofrequency ablation has no effect on daytime sleepiness, apnea reduction, and QOL. |
| Gao et al.2010 | 2019 | 1a | SR and network MA | Minimally invasive treatments including surgery | AHI, ESS | Simple surgical procedures improve AHI and ESS insignificantly. |
| Ishii et al.545 | 2015 | 1a | SR and MA | Nasal surgery including endoscopic sinus surgery | AHI, RDI, ESS | Significant improvements in ESS and RDI after nasal surgery, but no significant improvements in AHI. |
| Kezirian and Goldberg1802 | 2006 | 1a | SR | Hypopharyngeal surgeries | PSG, ESS, QOL | Hypopharyngeal surgery has success rates of 35%–62%. Improved ESS and FOSQ were seen. |
| Stuck et al.1688 | 2018 | 1a | SR | UPPP ± tonsillectomy | PSG, ESS, sexual function, cardiac parameters morbidity and mortality | UPPP ± tonsillectomy reduced AHI and ESS, and improved sexual function. AHI change was 18.59. ESS change was 5.37. |
| Sundaram et al.1483 | 2005 | 1a | SR | 1. Surgical intervention for OSA 2. Other surgical or nonsurgical interventions, or no intervention |
PSG, sleepiness, QOL | Surgery has inconsistent effects. There is no convincing evidence to support surgical treatment of OSA. |
| Zaghi et al.2007 | 2016 | 1a | SR and MA | MMA | AHI, RDI, LSAT, ESS | MMA is an effective treatment for OSA. Success rate of 85.5%; cure rate of 38.5%. ESS decreased from 13.5 to 3.2. |
| Atef et al.2013 | 2005 | 1b | RCT | 1. Bipolar radiofrequency volumetric tissue reduction of the palate 2. LAUP |
AHI | Bipolar radiofrequency volumetric tissue reduction of the palate required more sessions to achieve a favorable outcome, but benefit was also maintained for longer. |
| Baba et al.1782 | 2015 | 1b | SR and MA | Isolated TCRFTA of the soft palate, base of tongue or both | ESS, RDI, LSAT, SF-36 | TCRFTA can reduce RDI and sleepiness when directed at the base of tongue or as a multilevel procedure. |
| Browaldh et al.1666 | 2016 | 1b | RCT | 1. Modified UPPP including tonsillectomy 2. No treatment |
ESS, SF-36, vigilance | ESS, SF-36, vigilance improved after surgery. |
| Holmlund et al.1737 | 2014 | 1b | RCT | 1. Radiofrequency of the soft palate 2. Sham surgery |
AHI, ESS, snoring | Radiofrequency surgery of the soft palate has no effect on daytime sleepiness or apnea frequency. |
| Li et al.906 | 2011 | 1b | SR and MA | Nasal surgery including endoscopic sinus surgery | AHI, ESS | Nasal surgery can effectively reduce daytime sleepiness, but is limited in reducing OSA severity. |
| Mackay et al.1919 | 2020 | 1b | RCT | 1. Combined modified UPPP and radiofrequency of the tongue 2. Ongoing medical management |
ESS, PSG | Combined palatal and tongue surgery, compared with medical management, reduced AHI. and ESS at 6 months. Mean AHI and ESS baseline-adjusted between-group difference was −17.6 and −6.7, respectively. |
| Sharma et al.1961 | 2019 | 1b | SR and descriptive MA | 1. Rhinological surgical intervention 2. Non-surgical and/or non-rhinological surgical intervention |
AHI, RDI, ESS | Nasal surgery may have limited benefit on OSA, although improvements in sleep parameters and ESS are seen. ESS changed by 3.9. |
| Vicini et al.1503 | 2010 | 1b | RCT | 1. MMA 2. Auto-titrating positive airway pressure |
AHI, ESS | Both groups had similar improvement of mean AHI and ESS. |
| Walker-Engstrom et al.2016 | 2000 | 1b | RCT | 1. UPPP 2. MAD |
PSG, Minor Symptoms Evaluation-Profile (MSE-P) | QOL improved in both groups 1 year after intervention. The dental group had superior PSG values, but lower contentment. |
| Woodson et al.1497 | 2003 | 1b | RCT | 1. TCRFTA 2. CPAP 3. Sham-placebo |
QOL, ESS, PSG, reaction time | TCRFTA improved reaction time, OSA-specific QOL and ESS compared to baseline. TCRFTA improved reaction time, while CPAP did not. |
| Wu et al.1660 | 2017 | 1b | MA | Isolated nasal surgery | AHI, ESS | AHI and ESS improve significantly after isolated nasal surgery, but AHI demonstrated only slight improvement. |
| Bostanci and Turhan1817 | 2016 | 2a | SR | Tongue base suspension (TBS) techniques ± UPPP | AHI, LSAT, ESS | Tongue base suspension has some efficacy, with or without UPPP. ESS improved with tongue base suspension. |
| Choi et al.1746 | 2013 | 2a | MA | Pillar implant for mild to moderate OSA | ESS, AHI, snoring | The Pillar implant reduced ESS and AHI significantly for mild to moderate OSA. |
| Holty and Guilleminault1867 | 2010 | 2a | SR and MA | MMA | PSG, ESS, QOL | MMA improved AHI, reduced daytime symptoms and improved QOL. Success and cure rates were 86.0% and 43.2%, respectively. |
| Wang et al.2008 | 2019 | 2a | SR and MA | Nasal surgery ± endoscopic sinus surgery | AHI, ODI, LSAT, ESS | Isolated nasal and sinus surgery can reduce daytime sleepiness in OSA. ESS change was 3.79. There was no significant improvement on objective parameters. |
| Conradt et al.2018 | 1998 | 2b | Cohort | Patients were treated with CPAP before undergoing MMA | PSG, vigilance | MMA has positive effects on sleep, respiration and vigilance which are comparable to CPAP. |
| Klonoff et al.2022 | 1987 | 2b | Cohort | 1. UPPP 2. Coronary bypass surgery |
Psychological assessment battery, PSG | Anxiety and depression are lower after both surgeries, possibly by virtue of the surgery resolving the disease. |
| Xiao et al.2024 | 2016 | 2b | Cohort | 1. Nasal surgery for patients with nasal obstruction 2. No surgery for patients without nasal obstruction |
PSG, PSQI, Symptom Check List-90 | Nasal surgery reduced daytime dysfunction scores on PSQI, anxiety, and hostility scores. |
| Boyd et al.2005 | 2019 | 4 | Case series | MMA | ESS, QOL, AHI, psychomotor vigilance testing | MMA improved all measures. AHI decreased by 81.3%. ESS change was 8.5. 66.7% had normal FOSQ after surgery compared to 10% before surgery. |
| Camacho et al.1980 | 2014 | 4 | SR and MA | Tracheostomy | AI, AHI, ODI, sleepiness | Tracheostomy significantly decreases AI, AHI, and sleepiness. AI change was 83.47. Mean AHI and ODI was in the moderate range post tracheostomy. |
| Camacho et al.1868 | 2019 | 4 | SR and MA | MMA | AHI, ESS, LSAT | Improvements in AHI, sleepiness, and lowest oxygen saturation were maintained in the long-term (4 to <8 years). Mean AHI increased to moderate OSA in the very long term (≥8 years). |
| Certal et al.1874 | 2015 | 4 | SR and MA | Hypoglossal nerve stimulation therapy | AHI, ODI, ESS, QOL | Hypoglossal nerve stimulation improves AHI, ODI, and ESS fairly consistently over 12 months. There was improvement in QOL. |
| Costantino et al.1890 | 2020 | 4 | SR and MA | Hypoglossal nerve stimulation therapy | AHI, ESS, ODI | Hypoglossal nerve stimulation achieves high surgical success rates of up to 76.9% (ImThera) at 12 months, and 75% (Inspire) at 60 months. ESS improved with all devices. |
| Dahlof et al.2021 | 2000 | 4 | Case series | UPPP | Psychiatric symptoms, dexamethasone suppression test, PSG | Sleep disturbance and reduced daytime alertness may be at least one of the factors behind depressive symptoms in OSA. Relapse occurred at 6 months, but symptoms were still less than baseline. |
| Ishman et al.2014 | 2014 | 4 | Case series | Any OSA surgery | ESS, Beck Depression Index, RDI | Surgery, especially multilevel surgery, significantly reduces depression, and sleepiness scores. 77.3% and 75.0% had resolution of sleepiness and depression, respectively. |
| Justin et al.2002 | 2016 | 4 | SR and MA | TORS | AHI, LSAT, ESS | TORS appears to be a promising procedure as part of multilevel surgery. AHI decreased by 24.0. Success achieved in 48.2%. ESS decreased by 7.2. |
| Li et al.2023 | 2004 | 4 | Case series | Extended uvulopalatal flap | RDI, LSAT, 5-Item Mental Health scale (MH-5), ESS | Extended uvulopalatal flap can mildly improve, but not normalize, mood. Success rate was 79.8%. The improvement is not purely due to changes in PSG or ESS. |
| Lin et al.1920 | 2008 | 4 | SR and MA | Multilevel surgery involving at least two levels (nose, oropharynx, hypopharynx) | PSG, ESS, QOL | Multilevel surgery can improve outcomes. Success rate was 66.4%. ESS improved in 23/26 groups and changed by 43.0%. |
| Llewellyn et al.2011 | 2019 | 4 | SR and MA | Cautery-assisted palatal stiffening operation | AHI, ODI, LSAT, ESS | Cautery-assisted palatal stiffening operation improved respiratory parameters. ESS improved from 11.8 to 5.1. |
| Meccariello et al.2003 | 2017 | 4 | SR and MA | TORS | AHI, LSAT, ESS | TORS appears to be a promising procedure as part of multilevel surgery. Failure rate is 36.1%. ESS decreased significantly. |
| Murphey et al.2004 | 2015 | 4 | SR and MA | Glossectomy | AHI, LSAT, ESS | Glossectomy significantly improves sleep outcomes and ESS as part of multilevel surgery. AHI decreased by 27.81. Success achieved in 59.56%. ESS decreased by 5.49. |
| Pang et al.1966 | 2018 | 4 | MA | Palate surgeries | AHI, ESS, QOL | Palate surgery improved AHI and ESS. AHI change was 19.9. Success rate was 67.5%. ESS change was 5.8. |
| Santamaria et al.2019 | 1988 | 4 | Case series cross-sectional study, then prospective cohort study | UPPP | Serum testosterone, interview on sexual function | Increased testosterone, and return of libido and sexual functioning to normal range was seen after UPPP. |
| Shin et al.2020 | 2013 | 4 | Cohort | 1. UPPP 2. CPAP 3. MAD |
International Index of Erectile Function (KIIEF5), SAQLI, ESS, AHI, LSAT | Erectile dysfunction may improve after UPPP. ESS, AHI, LSAT improved significantly in the UPPP group. |
| Song et al.1804 | 2016 | 4 | SR and MA | Hyoid surgery | AHI, ESS | Isolated hyoid surgery reduced OSA severity and improved sleepiness. Hyothyroidopexy achieved the largest AHI reduction. |
| Steward et al.2015 | 2004 | 4 | Case series | TCRFTA of tongue and palate | ESS, FOSQ, reaction time | The addition of more sessions and levels of TCRFTA result in further improvement of ESS, QOL and reaction time. |
| Tsui et al.2006 | 2020 | 4 | Cohort | 1. Surgery (sagittal split ramus osteotomy/mandibular distraction osteogenesis) 2. Matched controls without OSA |
ESS, QOL | ESS improved in the surgical arm. QOL scores in the surgical groups improved to the level of the control group. QOL was similar between the two surgical arms. |
| Veer et al.2012 | 2014 | 4 | SR | Radiofrequency ablation | AHI, ESS | Radiofrequency ablation can improve AHI and ESS. Absolute reduction could not be calculated due to paucity of available data. |
Abbreviations: AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale; FOSQ, Functional Outcomes of Sleep Questionnaire; LAUP, laser-assisted uvulopalatoplasty; LSAT, lowest oxygen saturation; MA, meta-analysis; MAD, mandibular advancement device; MMA, maxillomandibular advancement; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; QOL, quality of life; RCT, randomized controlled trial; RDI, respiratory disturbance index; SAQLI, Calgary Sleep Apnea Quality of Life Index; SF-36, 36-Item Short Form; SR, systematic review; TCRFTA, temperature-controlled radiofrequency tissue ablation; UPPP, uvulopalatopharyngoplasty.
IX.E.2 |. Surgery to improve OSA severity
The AHI is the most popular parameter for measuring OSA severity and surgical outcomes. The definition of surgical success based on AHI change varies across studies, with the most common being Sher’s criteria (a reduction in AHI of ≥50% and to a value of <20) which was used in most of the studies reviewed.1634 However, newer metrics such as the sleep apnea-specific hypoxia and composite scores may better capture sleep apnea disease burden.2025,2026
Nasal surgery has a limited ability to improve respiratory parameters,545,906,2008 although Ishii et al. and Wu et al. found small improvements in RDI and AHI, respectively.545,1660 Palatal surgeries, one of the most commonly performed OSA surgeries, have a reputation for not consistently lowering AHI even in mild to moderate OSA.1686 UPPP was cited as having a success rate of 40.7% in an unselected patient population; success rates reached 52.3% in cases with solely retropalatal obstruction.1634 A recent MA in 2018 by Pang et al. reported an overall pooled success rate of 67.5% for palate surgeries based on patients without hypopharyngeal collapse.1966 New palatal techniques may further improve the success of procedures targeting retropalatal obstruction.1690,1722,2027 However, even the simple tonsillectomy can achieve 100% success in a targeted population, that is, mild-moderate OSA patients with enlarged tonsils.335
Hypopharyngeal techniques were developed to deal with retroglossal obstruction, but they have unpredictable success rates of between 35% and 62%, even when combined with UPPP.1802,1811,1817 TORS for tongue base resection and lingual tonsillectomy demonstrate improved LSATs and success rates between 48.2% and 68.4%.1829,2002,2003 In general, tongue base resection and advancement techniques have better results than minimally invasive tongue base reduction with RF.1790,1811,2004
There is inconsistent evidence on minimally invasive procedures.1736,1737,2010 Some studies report significant improvement in RDI and AI after both single and multilevel RF procedures.1497,1782,2009 Multiple sessions may be required and data reports on short follow-up periods of 6 months.1736,2013 Techniques such as cautery-assisted palatal stiffening operation (CAPSO) and the Pillar procedure have also been reported to reduce AHI and ODI.1746,2011
HNS has shown statistically significant reductions of approximately 50% in both AHI and ODI at 12 months, success rates 70–75%, with similar efficacy persisting to 60 months.1874,1890 Its adherence data may be better than that of CPAP (median nightly usage of 5.8 h, with self-reported nightly device use in 80% at 5 years).1890 A withdrawal study that showed return of OSA severity to baseline after the device was turned off for at least a week confirmed its efficacy.1877
MMA and tracheostomy have consistently high success rates.1501,1686,2028 The cornerstone of skeletal surgery that expands pharyngeal dimensions at all levels, Holty and Guilleminault first reported a pooled success rate of 86.0% (p < 0.001) after MMA, with mean AHI dropping from 63.9 to 9.5.1867,2029 Cure (AHI < 5) was achieved in 43.2%.1867 MMA has classically been a second-line surgery in the older Stanford protocol. Elshaug et al. reported that the pooled success rate of patients rose from 55% after phase I surgeries to 86% after phase II surgery.1957 However, MMA can be highly effective as either primary or second-line surgery.1686,2007 Limited data on other types of skeletal surgery suggests efficacy in selected patients too.1794,1804,2030,2031 Bypassing the UA with a tracheostomy as a last resort can dramatically reduce the AI (73.0 ± 27.1 to 0.2 ± 1.2/h).1980
Multilevel surgeries can achieve decent outcomes in OSA which is frequently a multilevel disease.1830,1920,1921 Using Sher’s criteria, the success rate of MLS was 66.4% (59.2% if MMA was excluded).1920 Certain techniques such as tongue base procedures may have better results as part of MLS.1811 Mackay et al. showed MLS reduced AHI (47.9–20.8) significantly more than ongoing medical management (45.3–34.5), with more patients (26% vs. 8%) in the surgical arm achieving an AHI <10.1919 That said, additional levels of surgery do not always yield further AHI reductions.2032
It is prudent to be mindful that initial efficacy may decrease with time due to tissue laxity and non-anatomical factors. The surgical response of UPPP decreased from 67.3% in the short-term to 44.35% after at least 34 months.812 The improvement in AHI post-MMA persists with 89% of patients maintaining surgical successes at a mean of 44 months, but mean AHI increased to the moderate range after 8 years.1867,1868
The MA by Sundaram et al. concluded that surgery was less successful than non-surgical interventions, stating that TCRFTA and UPPP had poorer AHI outcomes compared to CPAP and OA therapy.1483,1497,1507 However, Vicini et al. compared the most consistently successful OSA surgery to date, MMA, against APAP and showed both groups had marked AHI reduction after a year, without a statistically significant difference between the groups.1503
It can be argued that CPAP adherence is highly variable, so surgery may have an overall better outcome in some patients.1495 Surgery is an option to reduce OSA severity in CPAP-intolerant patients. When appropriately selected, some surgeries can have high success rates. Overall the literature is limited by lack of comparative analyses and long-term outcomes. (Table IX.E.2)
TABLE IX.E.2.
Evidence for surgery to improve OSA severity
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Back et al.1736 | 2009 | 1a | SR | Radiofrequency ablation of the soft palate | PSG, snoring | Effectiveness of radiofrequency ablation of the soft palate is uncertain. |
| Camacho et al.2033 | 2017 | 1a | SR and MA | LAUP | AHI, LSAT | AHI worsened in 44%. LAUP should be performed with caution or not performed at all given its unfavorable results. |
| Caples et al.1686 | 2010 | 1a | SR and MA | Various surgeries (MMA, soft palate procedures, LAUP, radiofrequency ablation, soft palate implants, multilevel simultaneous, and phased surgeries) | AHI | MMA results in substantial and consistent reductions in AHI. Pharyngeal surgeries are less consistent. Newer pharyngeal techniques and multilevel procedures appear promising. |
| Gao et al.2010 | 2019 | 1a | SR and network MA | Minimally invasive treatments including surgery | AHI, ESS | Simple surgical procedures improve AHI and ESS insignificantly. |
| Farrar et al.2009 | 2008 | 1a | SR and MA | Isolated radiofrequency ablation of the palate, tongue base, or both | ESS, RDI, LSAT | Radiofrequency ablation reduces ESS and RDI levels. ESS decreased by 31%. RDI reduced by 31% in short-term and 45% in long-term. |
| He et al.812 | 2019 | 1a | SR and MA | UPPP and its modifications | AHI, ODI, LSAT, and CT90 | Surgical response decreased from 67.3% to 44.35% from short to long-term (≥34 months). |
| Ishii et al.545 | 2015 | 1a | SR and MA | Nasal surgery including endoscopic sinus surgery | AHI, RDI, ESS | Significant improvements in ESS and RDI after nasal surgery, but no significant improvements in AHI. |
| Kezirian and Goldberg1802 | 2006 | 1a | SR | Hypopharyngeal surgeries | PSG, ESS, QOL | Hypopharyngeal surgery has success rates of 35%–62%. Improved ESS and FOSQ were seen. |
| Rotenberg et al.1495 | 2016 | 1a | SR | 1. CPAP 2. Surgery |
Adherence rates of CPAP | The majority of patients are not adherent to CPAP. Surgery may provide a better overall outcome. |
| Sundaram et al.1483 | 2005 | 1a | SR | 1. Surgical intervention for OSA 2. Other surgical or nonsurgical interventions, or no intervention |
PSG, sleepiness, QOL | Surgery has inconsistent effects. There is no convincing evidence to support surgical treatment of OSA. |
| Zaghi et al.2007 | 2016 | 1a | SR and MA | MMA | AHI, RDI, LSAT, ESS | MMA is an effective treatment for OSA. Success rate of 85.5%; cure rate of 38.5%. ESS decreased from 13.5 to 3.2. |
| Atef et al.2013 | 2005 | 1b | RCT | 1. Bipolar radiofrequency volumetric tissue reduction of the palate 2. LAUP |
AHI | Bipolar radiofrequency volumetric tissue reduction of the palate required more sessions to achieve a favorable outcome, but benefit was also maintained for longer. |
| Baba et al.1782 | 2015 | 1b | SR and MA | Isolated TCRFTA of the soft palate, base of tongue, or both | ESS, RDI, LSAT, SF-36 | TCRFTA can reduce RDI and sleepiness when directed at the base of tongue or as a multilevel procedure. |
| Elshaug et al.1957 | 2007 | 1b | MA | Phase I and II upper airway surgeries | AHI | Phase II surgeries have a higher success rate than Phase I (86% vs. 55%). |
| Holmlund et al.1737 | 2014 | 1b | RCT | 1. Radiofrequency of the soft palate 2. Sham surgery |
AHI, ESS, snoring | Radiofrequency surgery of the soft palate has no effect on daytime sleepiness or apnea frequency. |
| Li et al.906 | 2011 | 1b | SR and MA | Nasal surgery including endoscopic sinus surgery | AHI, ESS | Nasal surgery can effectively reduce daytime sleepiness, but is limited in reducing OSA severity. |
| Mackay et al.1919 | 2020 | 1b | RCT | 1. Combined modified UPPP and radiofrequency of the tongue 2. Ongoing medical management |
ESS, PSG | Combined palatal and tongue surgery, compared with medical management, reduced AHI. and ESS at 6 months. Mean AHI and ESS baseline-adjusted between-group difference was −17.6 and −6.7, respectively. |
| Pirklbauer et al.2028 | 2011 | 1b | SR | MMA | AHI, QOL | MMA can be a successful primary intervention for OSA. |
| Vicini et al.1503 | 2010 | 1b | RCT | 1. MMA 2. Auto-titrating positive airway pressure |
AHI, ESS | Both groups had similar improvement of mean AHI and ESS. |
| Walker-Engstrom et al.1507 | 2002 | 1b | RCT | 1. UPPP 2. MAD |
AHI, AI, ODI | Success rate with MAD (81%) was significantly higher than with UPPP (53%). |
| Woodson et al.1497 | 2003 | 1b | RCT | 1. TCRFTA 2. CPAP 3. Sham-placebo |
QOL, ESS, PSG, reaction time | TCRFTA improved reaction time, OSA-specific QOL and ESS compared to baseline. TCRFTA improved reaction time, while CPAP did not. |
| Woodson et al.1877 | 2014 | 1b | RCT | 1. Hypoglossal nerve stimulation turned off 2. Hypoglossal nerve stimulation turned on |
AHI, ODI, arousal index, ESS, FOSQ | OSA returned to baseline severity in the withdrawal arm. Improvement in OSA severity and QOL are due to electrical stimulation of the hypoglossal nerve. |
| Wu et al.1660 | 2017 | 1b | MA | Isolated nasal surgery | AHI, ESS | AHI and ESS improve significantly after isolated nasal surgery, but AHI demonstrated only slight improvement. |
| Bostanci and Turhan1817 | 2016 | 2a | SR | Tongue base suspension (TBS) techniques ± UPPP | AHI, LSAT, ESS | Tongue base suspension has some efficacy, with or without UPPP. ESS improved with tongue base suspension. |
| Choi et al.1746 | 2013 | 2a | MA | Pillar implant for mild to moderate OSA | ESS, AHI, snoring | The Pillar implant reduced ESS and AHI significantly for mild to moderate OSA. |
| John et al.1501 | 2018 | 2a | SR and MA | MMA | AHI, RDI, ESS, LSAT | MMA achieved 100% surgical success. |
| Holty and Guilleminault1867 | 2010 | 2a | SR and MA | MMA | PSG, ESS, QOL | MMA improved AHI, reduced daytime symptoms and improved QOL. Success and cure rates were 86.0% and 43.2%, respectively. |
| Mulholland et al.1921 | 2019 | 2a | SR and MA | Multilevel palate and tongue base surgery | AHI, LSAT | Multilevel (non-MMA) surgeries can significantly reduce AHI. Mean AHI change of 23.67. |
| Pang et al.1690 | 2016 | 2a | SR and MA | 1. Expansion sphincter pharyngoplasty 2. Other palate surgeries |
AHI | The surgical success rate of expansion sphincter pharyngoplasty was 86.3%. |
| Sher et al.1634 | 1996 | 2a | SR and MA | Various upper airway surgeries (nasal surgeries, UPPP, retrolingual operations, tracheostomy) | PSG | UPPP has at best <50% chance of success. |
| Wang et al.2008 | 2019 | 2a | SR and MA | Nasal surgery ± endoscopic sinus surgery | AHI, ODI, LSAT, ESS | Isolated nasal and sinus surgery can reduce daytime sleepiness in OSA. ESS change was 3.79. There was no significant improvement on objective parameters. |
| Camacho et al.335 | 2016 | 3a | SR and MA | Tonsillectomy | PSG, ESS | Tonsillectomy can have high success (85.2%) and cure (57.4%) rates in patients with large tonsils. |
| Noller et al.2030 | 2017 | 3a | SR and MA | Mandibular advancement surgeries | AHI, LSAT | Isolated mandibular advancement is efficacious for adult patients with mandibular insufficiency. |
| Volner et al.1722 | 2017 | 3b | SR and MA | Transpalatal advancement pharyngoplasty | AHI, LSAT | Transpalatal advancement pharyngoplasty reduced AHI and LSAT significantly. AHI reduced by 64.8%. LSAT improved by 3.55 points. |
| Binar and Karakoc2027 | 2018 | 4 | SR and MA | Anterior palatoplasty | AHI | The surgical success rate of anterior palatoplasty was 60.6%. |
| Camacho et al.1980 | 2014 | 4 | SR and MA | Tracheostomy | AI, AHI, ODI, sleepiness | Tracheostomy significantly decreases AI, AHI, and sleepiness. AI change was 83.47. Mean AHI and ODI was in the moderate range post tracheostomy. Sleepiness decreased significantly. |
| Camacho et al.1868 | 2019 | 4 | SR and MA | MMA | AHI, ESS, LSAT | Improvements in AHI, sleepiness, and lowest oxygen saturation were maintained in the long-term (4 to <8 years). Mean AHI increased to moderate OSA in the very long term (≥8 years). |
| Certal et al.1874 | 2015 | 4 | SR and MA | Hypoglossal nerve stimulation therapy | AHI, ODI, ESS, QOL | Hypoglossal nerve stimulation improves AHI, ODI, and ESS fairly consistently over 12 months. There was improvement in QOL. |
| Costantino et al.1890 | 2020 | 4 | SR and MA | Hypoglossal nerve stimulation therapy | AHI, ESS, ODI | Hypoglossal nerve stimulation achieves high surgical success rates of up to 76.9% (ImThera) at 12 months, and 75% (Inspire) at 60 months. ESS improved with all devices. |
| Handler et al.1811 | 2014 | 4 | SR | Tongue advancement surgeries ± UPPP | AHI | Tongue suspension is effective and safe as part of multilevel surgery. Success rate is 36.6% as a standalone procedure. |
| Justin et al.2002 | 2016 | 4 | SR and MA | TORS | AHI, LSAT, ESS | TORS appears to be a promising procedure as part of multilevel surgery. AHI decreased by 24.0. Success achieved in 48.2%. ESS decreased by 7.2. |
| Lin et al.1920 | 2008 | 4 | SR and MA | Multilevel surgery involving at least two levels (nose, oropharynx, hypopharynx) | PSG, ESS, QOL | Multilevel surgery can improve outcomes. Success rate was 66.4%. ESS improved in 23/26 groups and changed by 43.0%. |
| Llewellyn et al.2011 | 2019 | 4 | SR and MA | Cautery-assisted palatal stiffening operation | AHI, ODI, LSAT, ESS | Cautery-assisted palatal stiffening operation improved respiratory parameters. ESS improved from 11.8 to 5.1. |
| Meccariello et al.2003 | 2017 | 4 | SR and MA | TORS | AHI, LSAT, ESS | TORS appears to be a promising procedure as part of multilevel surgery. Failure rate is 36.1%. ESS decreased significantly. |
| Miller et al.1829 | 2017 | 4 | SR and MA | TORS tongue base reduction | AHI, LSAT, ESS, snoring | The surgical success rate of TORS for tongue base reduction was 68.4%. Cure rate was 23.8%. |
| Minni et al.2032 | 2020 | 4 | Case series | UPPP and barbed reposition pharyngoplasty ± hyoid suspension | AHI, ODI, ESS | Barbed reposition pharyngoplasty was more effective than UPPP. Hyoid suspension added benefit when performed with UPPP, and not with barbed reposition pharyngoplasty. |
| Murphey et al.2004 | 2015 | 4 | SR and MA | Glossectomy | AHI, LSAT, ESS | Glossectomy significantly improves sleep outcomes and ESS as part of multilevel surgery. AHI decreased by 27.81. Success achieved in 59.56%. ESS decreased by 5.49. |
| Pang et al.1966 | 2018 | 4 | MA | Palate surgeries | AHI, ESS, QOL | Palate surgery improved AHI and ESS. AHI change was 19.9. Success rate was 67.5%. ESS change was 5.8. |
| Samutsakorn et al1830 | 2018 | 4 | SR and MA | Lingual tonsillectomy with palatal surgery | AHI, LSAT, ESS | Lingual tonsillectomy and palatal surgery may benefit selected patients. |
| Song et al.1804 | 2016 | 4 | SR and MA | Hyoid surgery | AHI, ESS | Isolated hyoid surgery reduced OSA severity and improved sleepiness. Hyothyroidopexy achieved the largest AHI reduction. |
| Song et al.1794 | 2017 | 4 | SR and MA | Genial tubercle advancement and genioplasty | AHI, LSAT, ESS | Standard genioplasty, and genial tubercle advancement with and without hyoid suspension can improve AHI and LSAT. |
| Stuck et al.1790 | 2002 | 4 | Case series | Radiofrequency volumetric tissue reduction of the tongue base | RDI, ESS | 33% were cured. 55.6% showed a reduction in RDI of >20%. ESS decreased from 7.9 to 4.9. |
| Tsui et al.2031 | 2016 | 4 | SR | Mandibular distraction osteogenesis | AHI, RDI | Mandibular distraction osteogenesis can achieve success in adults with retrognathic mandibles. |
Abbreviations: AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; CT90, percentage of sleep time with oxyhemoglobin saturation <90%; ESS, Epworth Sleepiness Scale; FOSQ, Functional Outcomes of Sleep Questionnaire; LAUP, laser-assisted uvulopalatoplasty; LSAT, lowest oxygen saturation; MA, meta-analysis; MAD, mandibular advancement device; MMA, maxillomandibular advancement; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PSG, polysomnography; QOL, quality of life; RCT, randomized controlled trial; RDI, respiratory disturbance index; SR, systematic review; TCRFTA, temperature-controlled radiofrequency tissue ablation; TORS, transoral robotic surgery; UPPP, uvulopalatopharyngoplasty.
IX.E.3 |. Surgery and mortality risk
Few studies to date have focused on the impact of surgical procedures for OSA on all-cause mortality and survival. A retrospective cohort study of OSAS patients including those treated with UPPP, CPAP, or weight loss demonstrated a lower likelihood of all-cause death in UPPP patients when compared to untreated patients.2034 After adjusting for age, sex, BMI, AHI, and select medical comorbidities, the adjusted HR for all-cause mortality in the UPPP group was 0.14 (95% CI, 0.04–0.50).2034 The likelihood of OSA-related mortality was also lower in UPPP patients than in untreated patients (adjusted HR of 0).
A retrospective case–control study investigated mortality in heavy snorers including 256 with OSA who underwent UPPP compared to that of a control group of 744 patients without snoring or SDB who underwent nasal surgery.2035 During a follow-up period of 5–9 years, the long-term all-cause mortality rate was not different between the OSA patients treated with UPPP (3.9%) and the nasal surgery control group (3.9%, p = 0.99). Given the increased mortality risk of OSA, the comparable mortality between the UPPP and the control groups may indicate that UPPP treatment of OSA conferred a survival benefit in OSA patients.
Few studies have compared OSA patient survival or mortality between surgically and medically treated patients. The largest retrospective cohort study on this topic studied survival in veterans with OSA treated with UPPP (n = 2072) or provision of CPAP (n = 18,754).1498 When comparing all-cause mortality rates between the two groups, the CPAP group had a statistically significant higher mortality rate (7.1%) than did the UPPP group (3.4%). On average, the UPPP group had an overall survival of 22 days longer than did the CPAP group. After adjusting for known confounders of mortality, patients in the CPAP group had a 31% greater probability of mortality at any time during the study period than did patients in the UPPP group.
In another retrospective cohort study of OSA patients, 5-year survival and mortality were compared amongst 149 UPPP and 126 CPAP (confirmed active use) patients.2036 The 5-year survival probability did not differ between the UPPP (0.94 ± 0.24, mean ± SD) and CPAP (0.95 ± 0.34) groups. On subset analysis of patients with an AI > 20, there were similarly no difference in 5-year survival probability between the UPPP (0.92 ± 0.17) and CPAP groups (0.90 ± 0.14). There was also no statistically significant difference in all-cause mortality rates between the two groups at 4.0% for the UPPP patients and 2.4% for the CPAP patients.
Taken together, retrospective cohort studies indicate that sleep apnea surgery may confer a mortality and survival benefit. (Table IX.E.3)
TABLE IX.E.3.
Evidence for surgery and mortality risk
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Marti et al.2034 | 2002 | 2c | Retrospective cohort (outcomes) study | OSA patients (N = 475) who underwent UPPP, weight loss, CPAP, or no treatment | Mortality (all cause and OSA-related) | Surgery reduces all-cause and OSA-related mortality compared to no treatment. |
| Lysdahl and Haraldsson2035 | 2000 | 2b | Retrospective matched cohort | Heavy snorers including OSA (n = 256) patients non-heavy snorer nasal surgery patients (n = 744) | Mortality (all cause) | Mortality was no different between OSA patients treated with UPPP and non-OSA nasal surgery. |
| Weaver et al.1498 | 2004 | 2b | Retrospective cohort database study | Veterans with OSA managed with UPPP (n = 2072) or provision of CPAP (n = 18,754) | Mortality (all cause), survival | Survival and mortality were better for UPPP patients than for patients prescribed CPAP. |
| Keenan et al.2036 | 2002 | 2c | Retrospective cohort (outcomes) study | OSA patients (N = 362), including those treated with UPPP (n = 149) and nasal CPAP with active use (n = 126) | All-cause mortality, survival | All-cause mortality and 5-year survival were no different between UPPP and CPAP patients. |
IX.E.4 |. Surgery and hypertension improvement
An MA of 30 different randomized controlled studies demonstrated clinically relevant reductions in BP for those patients treated with CPAP.1209 Whether similar clinically relevant reductions in BP can be achieved in patients who have undergone sleep apnea surgery will be addressed in this section.
There have been no Cochrane reviews of the effect of sleep apnea surgery on BP. The data outlined include mostly case series with risks for bias and limitations from lack of control groups.2037
The most recent study by Chin et al. examined 89 patients who underwent multilevel soft tissue surgery of which 40 were identified as responders, as defined by a post-surgical AHI of less than 20 events/h.2038 In these responders, a statistically significant reduction in morning and nocturnal SBP was noted. DBP reduction was not statistically significant. In 2017, Fehrm et al. designed an RCT to assess BP changes in patients undergoing modified UPPP in comparison to a control group.2039 The study included 65 patients of which 32 initially underwent the surgery and 33 remained in the control arm with the use of CPAP therapy. BP measurements were taken after surgery and 6 months postoperatively. After 6 months, the control arm was also offered the modified UPPP, and both groups were followed for a total of 24 months. SBP and DBP measurements were significantly reduced in the surgical treatment arm at 6 months, with a −9.4 mmHg difference in SBP and −6.4 mmHg in DBP. This change in BP was greater than in the initial CPAP control group, which demonstrated only a −2.6 and −2.0 mmHg drop in both SBP and DBP, respectively. After the CPAP control arm was offered surgery at 6 months, at the time of the 24-month follow-up, there was persistent improvement in BP in both groups that underwent surgery. In 2014, Rotenberg et al. published his experience with soft tissue palatal surgery ± nasal surgery in a cohort of 126 patients. In regards to BP, a statistically significant reduction in SBP was seen (p < 0.001) in the surgical group with a reduction noted from 143.2 to 134.5 mmHg.1499 Finally, one study evaluated a cohort of 18 patients who underwent soft tissue surgery with the use of 24-h ambulatory BP monitoring – considered superior to in-office spot BP monitoring.1714 This study demonstrated statistically significant improvements in nocturnal SBP, DBP, and MAP measurements after surgery at 6 months.
A multicenter prospective cohort study on 30 patients undergoing MMA surgery demonstrated notable findings regarding QOL measures and AHI reductions.2005 In regards to BP, a non-significant reduction in mean diastolic BP and in systolic BP were noted. Another study on patients undergoing MMA was completed in 2015 by Islam et al.2040 This study looked at 45 patients that underwent the surgery, and in the 85% who were determined to have surgical success (AHI < 15% or 50% reduction from the index AHI), significant reductions in both SBP and DBP were seen, particularly in patients with previously diagnosed hypertension treated with medications. In the 10 patients with a history of hypertension treated with medications, the reductions in mean SBP, mean DBP, and MAP, were −6, −10, and −9 mmHg, respectively. Those patients who did not meet the definition of surgical success had significant reductions in DBP alone. Regarding the long-term effect of BP reduction in surgically managed patients, Boyd et al. studied 30 patients that underwent MMA surgery and determined that over a mean of 6.6 years of follow-up (range, 2.1–11.2 years), statistically significant reductions were seen in DBP with non-statistically significant reductions in SBP.2041 Notably the extent of BP improvements seen in these small surgical studies are substantial, along the lines seen with effective BP medications and larger than reported with CPAP.1209,2039 More research is needed to understand the long-term pathophysiologic effects of sleep surgery on BP control. (Table IX.E.4)
TABLE IX.E.4.
Evidence for blood pressure change after surgery for OSA
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Fehrm et al.2039 | 2017 | 1b | Randomized controlled trial | 65 patients 1. UPPP (n = 32) 2. CPAP (n = 33) Crossover of CPAP group at 6 months |
SBP and DBP at 6–24 months | Larger SBP and DBP reductions seen in surgical group compared to CPAP group. (SBP difference −9.4 mmHg and DBP difference −6.4 mmHg postsurgery vs. −2.6 mmHg SBP and −2.0 mmHg DBP with CPAP) |
| Chin et al.2038 | 2019 | 4 | Case series | 89 patients that underwent multilevel ST surgery | Serum leptin, cholesterol, SBP, DBP, and triglycerides | Morning SBP improvements in surgical responders (mean difference −7.2 mmHg after surgery). Nocturnal BP change was not significant. |
| De Paula Soares et al.1714 | 2014 | 4 | Case series | 18 patients underwent ST surgery | ABPM before and 6 months after surgery | SBP, DBP, and MAP during sleep were significantly decreased after surgery. (SBP: −7.4 mmHg, DBP: −4.2 mmHg, MAP: −5.3 mmHg). |
| Rotenberg et al.1499 | 2014 | 4 | Case series | A total of patients underwent ST surgery ± nasal surgery | AHI, ESS, SAQLI, SBP | SBP significantly reduced after surgery. (SBP difference −8.7 mmHg after surgery vs. no difference with CPAP). |
| Boyd et al.2005 | 2019 | 4 | Case series | 30 patients that underwent MMA | ESS, FOSQ, AHI, PVT, SBP, DBP, and CRP | Significant DBP reduction seen (mean difference −3.3 mmHg). |
| Islam et al.2040 | 2015 | 4 | Case series | 45 patients that underwent MMA | Pre and PO SBP and DBP measurements | SBP significantly reduced after surgery (mean difference −3.8 mmHg). DBP change was not significant. |
| Boyd et al.2041 | 2015 | 4 | Case series | 30 patients that underwent MMA followed for >2 years (average 6 years) | AHI, ESS, FOSQ, SBP, and DBP | Reductions in DBP were seen in patients followed for at least >2 years (mean difference −4.7 mmHg). |
Abbreviations: ABPM, 24 h ambulatory blood pressure monitoring; AHI, apnea/hypopnea index; CRP, C reactive protein; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; FOSQ, functional outcomes of sleep questionnaire; LOE, level of evidence; MAP, mean arterial pressure; MMA, maxillomandibular advancement; PO, postoperative; PVT, psychomotor vigilance testing; SAQLI, sleep apnea quality of life index; SBP, systolic blood pressure; ST, soft tissue.
IX.E.5 |. Surgery and cardiovascular outcomes
Patients with OSA have increased inflammatory, metabolic, hemodynamic, and autonomic dysfunction, which contribute to increased risk of CV disease.110 The obstruction of the airway with resultant hypoxemia cause increased sympathetic activity, which leads to downstream effects to the CV system through heart rate and BP elevations. These deleterious effects have been linked to increases in HF, CAD, and all-cause mortality.641,642
Treatment with CPAP has been evaluated in an attempt to discern its effect on the incidence of CV events such as MI, acute coronary syndrome, and stroke. The results of these studies have been mixed, but overall, they do suggest CV benefits with CPAP treatment for OSA.38,118,1249
One SR analyzed a total of 33 articles on the effects of sleep surgery on CV outcomes.1687 This study determined that the overall evidence regarding CV outcomes imply a benefit; however, the studies were mostly case series, and larger randomized prospective trials were recommended to more definitively understand the role of sleep surgery in reducing long-term CV consequences associated with OSA.
A recent study in 2018 by Lee et al. examined the entire population of South Korea over a 7-year time frame via their national healthcare database and found that 22,231 UPPP patients had a statistically significant reduction in rates of CHF and AF in comparison to untreated patients with OSA (n = 170,085).1964 Regarding MI, the incidence in the untreated OSA group was higher than in the UPPP group, but lacked statistical significance.
A variety of secondary markers of CV function and/or stress are associated with CV dysfunction. These markers include CRP, serum leptin, BP, cholesterol, left and right ventricular function, nitric oxide (NO) levels, and HRV. These surrogate markers of CV outcomes are used in lieu of long-term follow-up, given the challenges with identifying differences in incidence of HF, MI, or stroke, which can take large cohorts and several years of follow-up not yet done in sleep surgery research.
Studies have examined the role of pharyngeal soft tissue surgery and the reduction of CV marker levels such as CRP and highly sensitive C-reactive protein (hs-CRP), serum leptin, trigylicerides, cholesterol, NO, and carotid artery intima-media thickness (CIMT). A case–control study done by Binar et al. demonstrated a significant difference in hs-CRP in patients undergoing pharyngeal surgery.1693 A total of 23 patients were followed after surgery, and the reduction in hs-CRP, which was significant, was only seen in the post-surgical patients that were able to obtain an AHI < 5/h, which occurred in only 26%. Lee et al., in a study of 30 patients undergoing lateral expansion pharyngoplasty, found reductions in hs-CRP in patients irrespective of post-surgical AHI; however, this only reached significance in the group of surgical patients with severe OSA prior to intervention.2042
Nocturnal HRV is a marker of autonomic dysfunction brought on by nightly hypoxemia from OSA and is considered a significant risk factor for CV dysfunction. Dehdia et al. evaluated UA stimulation therapy and its effect on nocturnal HRV in a cohort of 32 patients that underwent a repeat sleep study 12 months after their stimulation device was implanted, in addition to a withdrawal period.1898 This study demonstrated that HRV was improved between the patient’s baseline sleep study to the 12-month treatment study, and these effects did not change during the 1-week therapy withdrawal period. Studies that examined HRV in patient’s undergoing pharyngeal surgery showed that in patients with post-surgical AHI of less than 20/h, significant reductions in HRV were seen.1705,2043
CIMT, assessed via ultrasound or MRI, has been used as a screening tool for arteriosclerosis and risk stratification for future cardiac events.2044 CIMT, which is often used to monitor the effects of cardiac drugs such as lipid lowering agents and anti-hypertensive medications, has also been used as a surrogate cardiac marker. Peng et al. demonstrated in 52 patients that underwent UPPP and nasal surgery, that CIMT thickness, cholesterol, triglycerides, and BP were all significantly reduced.2045 This effect was mirrored in a group of patients that elected to start CPAP, and furthermore, changes in CIMT were not seen in the group that did not pursue surgery or CPAP therapy. A similar study, published in 2019 by Zhan et al., demonstrated that in 53 patients undergoing UPPP surgery, a variety of cardiac markers, such as CIMT, arterial stiffness parameters, and echocardiography, improved after UPPP surgery.1965 The surgical success rate in the group was 60.4%, and in this group, the reduction in the abovementioned parameters was demonstrated, including flow velocity reductions across the aortic and pulmonary valves on echocardiography.
In summary, CV events are a known risk factor of OSA, particularly for patients with moderate or severe disease. For OSA patients unable to tolerate CPAP therapy, one potential goal of surgical management is the reduction of cardiac risk factors in an effort to improve cardiac outcomes. Caution must be taken in reviewing the published literature regarding the reduction of CV risk for this group given the lack of rigorous long-term trials; however, the current research has demonstrated improvements in cardiac outcomes, and multiple surrogate markers of cardiac outcomes have been positively affected by adequate sleep surgery. (Table IX.E.5)
TABLE IX.E.5.
Evidence for surgery and cardiovascular markers and outcomes
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Halle et al.1687 | 2017 | 2a | Systematic review of cohort studies and case series | Multiple studies on different surgical interventions | Nineteen different cardiovascular endpoints assessed | Data is limited secondary to weak study design (level 4 evidence) but surgery showed improvements in CV outcomes. |
| Lee et al.1964 | 2018 | 2b | Cohort study | 170,103 patients with OSA (no surgery), 22, 213 with OSA and had UPPP; propensity score matched controls: 961,590 no OSA | Newly diagnosed MI, CHF, and AF | UPPP was associated with lower incidences of CHF and AF. MI incidence rate was not significant. |
| Zhan et al.1965 | 2019 | 4 | Case series | 53 patients that underwent UPPP surgery | CIMT, arterial stiffness, echocardiography, and PSG | ST surgery improves parameters associated with carotid arteriosclerosis. |
| Chin et al.2038 | 2019 | 4 | Case series | 89 patients that underwent multilevel ST surgery | Serum leptin, cholesterol, SBP, DBP, and triglycerides | Cardiovascular markers such as total cholesterol, triglycerides, and leptin improved with successful surgery. |
| Dedhia et al.1898 | 2019 | 4 | Case series | 33 patients with UAS device | Nocturnal heart rate variability | Pts with UAS had reduced nocturnal heart rate variability. |
| Suslu et al.1705 | 2017 | 4 | Case series | 28 patients that underwent lateral expansion pharyngoplasty | Nocturnal heart rate variability | Autonomic variables included HRV were decreased in the patients who achieved surgical success. |
| Binar et al.1693 | 2017 | 3b | Case–control study | 23 patients that underwent lateral expansion pharyngoplasty vs. 28 patients on CPAP | hs-CRP, AHI, ESS | Only surgical patients that had AHI <5 after surgery had significant hs-CRP reductions. |
| Peng et al.2045 | 2016 | 3b | Case–control study | 52 patients underwent nasal surgery and UPPP, 12 had CPAP only | AHI, ESS, CIMT cholesterol | Six months after surgery significant changes in CIMT and with successful surgery. |
| Lin et al.2046 | 2014 | 3b | Case–control study | 15 healthy patients compared to 35 OSA pts undergoing UPPP | AHI, serum leptin, NOx | Patients with surgical success had normalization in leptin and NOx. |
| Choi et al.2043 | 2012 | 3b | Case–control study | 14 patients with unsuccessful ST surgery and 22 pts with successful ST surgery | BMI, PSG data, HRV | HRV significantly decreased in the successful surgical group. |
| Lee et al.2042 | 2011 | 4 | Case series | 30 patients underwent lateral expansion pharyngoplasty | BMI, ESS, AHI, and hs-CRP before and 6 months after surgery | Significant reductions in hs-CRP was noted regardless of AHI reduction. |
| Kinoshita et al.2047 | 2006 | 4 | Case series | 15 patients that underwent UPPP surgery | BMI, AHI, cholesterol, BP, and CRP | Significant reduction in CRP seen. |
| Zoha et al.2048 | 1992 | 4 | Case series | 19 patients undergoing UPPP surgery | AHI, left and right ventricular EF | 91% of patient had global improvements in EF. |
| Partinen et al.2049 | 1990 | 3b | Case series–control study | 71 patients that underwent tracheostomy vs. 127 recommended weight loss | Stroke, MI, CAD, Mortality, BP | Significant differences in mortality and cardiovascular events at 7 years of follow-up. |
Abbreviations: AF, atrial fibrillation; AHI, apnea/hypopnea index; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CHF, congestive heart failure; CIMT, carotid intima-media thickness; CRP, C reactive protein; EF, ejection fraction; ESS, Epworth Sleepiness Scale; HRV, heart rate variability; hs-CRP, high sensitive c-reactive protein; MI, myocardial infarction; NOx, nitric oxide derivative; PSG, polysomnography; ST, soft tissue; UAS, upper airway stimulator; UPPP, uvulopalatopharyngoplasty.
IX.E.6 |. Surgery and cognitive measures
It is well recognized that OSA is associated with neurocognitive impairment. Among the various cognitive functions, attention, memory, and executive function are most commonly affected by this chronic disorder.829,846,849,2050,2051 There is compelling evidence that surgical treatment reduces OSA severity and daytime sleepiness.1686,1921,1966,2052 Furthermore, in children, multiple studies have evaluated the efficacy of T&A for OSA on cognitive function.2053,2054 This section will focus on the neurocognitive effects of sleep surgery in adults suffering from OSA (Table IX.E.6).
TABLE IX.E.6.
Evidence for surgery and cognitive measures
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Lojander et al.2058 | 1999 | 2b | RCT | OSA patients (n = 49) 1. UPPP ± MO with HS 2. CPAP 3. Conservative measures |
Comprehensive NP battery (3 and 12 months FS) | No difference in cognitive function between UPPP and control group. Patients with CPAP showed a better visual memory than controls. |
| Klonoff et al.2022 | 1987 | 3b | Cohort | 1. UPPP (n = 11) 2. Controls without OSA scheduled for cardiac surgery (n = 11) |
Comprehensive NP battery (3 months FS) | UPPP improved memory, executive, and language functions, as well as IQ. Both groups experienced similar improvements. |
| Boyd et al.2005 | 2019 | 4 | Cohort | MMA (n = 30) | Attention (6 months FS) | MMA improved response time and reduced errors. |
| Dahlof et al.2055 | 2002 | 4 | Cohort | UPPP (n = 51) | 1. General intelligence 2. Learning and memory 3. Executive function (6 months FS) |
UPPP improved learning, visuospatial, and executive functions. No correlation between NP findings and surgical outcome. |
| Lin et al.2056 | 2016 | 4 | Cohort | 1. Multilevel surgery (n = 21) 2. Controls without OSA (n = 15) |
1. Comprehensive NP battery (3 months FS) 2. Gray matter volume measured by volumetric MRI |
Surgery improved attention, visuospatial, and executive domains. No correlation between NP findings and gray matter volume. |
Abbreviations: CPAP, continuous positive airway pressure; FS, following surgery; HS, hyoid suspension; IQ, intelligence quotient; LOE, level of evidence; MMA, maxillomandibular advancement; MRI, magnetic resonance imaging; MO, mandibular osteotomy; NP, neuropsychological; RCT, randomized controlled trial; UPPP, uvulopalatopharyngoplasty.
Klonoff et al. were the first to assess neurocognitive function in adult patients undergoing surgery for OSA.2022 Although notable improvements in cognitive function were found after UPPP, similar results were observed in a control group of patients scheduled for cardiac surgery, suggesting that these changes were not disease-specific. Since then, two other studies have demonstrated improvements in cognitive function, albeit in different domains, after UPPP with or without tongue base surgery.2055,2056 Additionally, a multicenter study on MMA has shown positive effects on neurocognitive performance by means of an attention test.2005 Other cognitive domains were not examined. In general, the findings of these cohort studies are quite heterogeneous, showing improvements in different domains. Based on this limited evidence, surgical treatment might be effective to counteract some of the neurocognitive deficits associated with OSA. This might be consistent with previous research on CPAP therapy.1281,2057
There is one RCT available on the neurocognitive effect of OSA surgery.2058 In this study, 49 patients were offered CPAP or MLS based on clinical findings. Each group was subsequently randomized to active treatment or conservative measures consisting of weight reduction and alcohol avoidance. All patients were administered a neuropsychological test battery at baseline, and 3 and 12 months after treatment. The results revealed a significant improvement in visual memory among CPAP users; however, there were no differences in cognitive function between surgical patients and controls. Importantly, surgical patients did not achieve a satisfactory reduction in OSA severity and the study was limited by its small sample size and lack of executive function testing.
Lin et al. investigated morphologic brain changes in OSA patients following multilevel surgical treatment using volumetric MRI.2056 According to their results, surgery decreased aberrant gray matter volumes of the precuneus, insula, and cerebellum, but did not change the anterior cingulate gyrus, which is mainly involved in CV control. Notably, these changes were not associated with neurocognitive outcome after surgery.
Overall, the evidence supporting the efficacy of OSA surgery in terms of neurocognitive outcome is sparse and inconclusive with heterogeneous findings due to the use of diverse neuropsychological test batteries. Therefore, Décary et al. proposed a standardized neurocognitive assessment for patients with OSA, covering executive function, attention, memory, and general intelligence.2059 Confounding factors, such as age, cognitive reserve, and surgical response, can complicate comparison between studies. Future research addressing these caveats is warranted to evaluate the efficacy of surgical treatment on neurocognitive function in OSA patients. (Table IX.E.6)
IX.E.7 |. Surgery and cerebrovascular disease
If untreated, OSA can have grave medical consequences. These adverse effects are often more profound in the cerebrovascular than in the CV system.647 Hence, ischemic stroke represents an important clinical endpoint for evaluating efficacy of OSA treatment.26,38
Several studies have demonstrated that surgical treatment for OSA can improve surrogate endpoints of stroke, including BP,1714,1865,1877,2039,2041,2060 lipid profile,2061,2062 platelet volume,2063,2064 CRP,2042,2065 inflammatory cytokines,2066–2068 matrix metalloproteinases,2069 CIMT,2070 HRV,1898,2043 and endothelial function.2071,2072 However, few studies have examined the effect of OSA surgery on incident stroke (Table IX.E.7).
TABLE IX.E.7.
Evidence for surgery and cerebrovascular disease
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Partinen and Guilleminault2073 | 1990 | 4 | Cohort (retrospective) |
OSA patients 1. Tracheostomy (n = 71) 2. Weight loss recommendation (n = 127) |
1. Stroke/TIA 2. Myocardial infarction 3. Mortality (7 years follow-up) |
Lower mortality and CV morbidity in tracheostomy group. |
| Marti et al.2034 | 2002 | 2b | Cohort (retrospective) | OSA patients 1. UPPP (n = 88) 2. CPAP (n = 124) 3. Successful weight loss (n = 134) 4. No intervention (n = 98) |
Mortality (4–9 years follow-up) | Higher CV mortality in group without treatment. No significant differences between UPPP, CPAP, and weight loss groups. No CV events in UPPP group. |
| Chen et al.2074 | 2015 | 2b | Cohort (retrospective) | OSA patients 1. UPPP (n = 5635) 2. No intervention (n = 4704) |
Stroke/TIA (1 year follow-up) | Significantly lower incidence of stroke in UPPP group. |
Abbreviations: CPAP, continuous positive airway pressure; CV, cardiovascular; LOE, level of evidence; OSA, obstructive sleep apnea; TIA, transient ischemic attack; UPPP, uvulopalatopharyngoplasty.
Partinen and Guilleminault evaluated CV events in patients with OSA treated by tracheostomy or weight loss counseling.2073 Despite an increased prevalence of CV disease in the tracheostomy group, these patients had significantly lower vascular mortality than the conservatively treated patients did after accounting for age, BMI, and OSA severity. Furthermore, during the 7-year follow-up, the incidence of stroke was significantly lower in the tracheostomy than in the weight loss group. Using the Taiwan National Health Insurance database, Chen et al. explored the 1-year incidence of stroke among patients with OSA who underwent UPPP versus those who did not receive any treatment.2074 As a result, the authors found an adjusted RR of 0.45 for stroke in the UPPP group compared with the noninterventional group. Because some members of the latter group engaged in undocumented OSA treatment, this beneficial effect of surgery may have been underestimated. In another retrospective study comparing UPPP with CPAP, weight reduction, and no treatment, patients receiving treatment had significantly lower CV mortality than patients without treatment.2034 Statistical differences between treatment groups were not noted.
Besides this empirical research, cost-effectiveness models have indicated protective effects of HNS on CV outcomes. Based on data of the STAR trial, Pietzsch et al. estimated a substantial risk reduction of stroke with HNS (10-year RR 0.75).2075 Another model based on realworld data revealed a similar benefit of HNS over a 10-year period (RR 0.76; number needed to treat 55.6 for stroke).2076
In summary, there is limited evidence that surgical treatment for OSA reduces the risk of CVAs. Most research on this topic has been conducted with surrogate endpoints for stroke. Only one retrospective study was identified that evaluated the direct effect of conventional OSA surgery on incident stroke.2074 Analogous to CPAP-therapy,26,38 large randomized trials with rigorous study design are needed to delineate the effects of surgery on cerebrovascular morbidity and mortality in patients with OSA. (Table IX.E.7)
IX.E.8 |. Surgery and work productivity
Untreated OSA patients often have daytime symptoms that can affect work productivity. Ulfberg et al. compared OSA patients with non-snorers and found that OSA patients had more difficulty doing their job because of tiredness/sleepiness (OR = 37), and had large or very large difficulties with concentrating on (OR = 7.5) and learning (OR = 9.1) new tasks.2077 Mulgrew et al. examined patients with suspected OSA and reported that excessively sleepy patients with an ESS ≥18 had significantly worse time management, mental–interpersonal relationships, and work output than those whose ESS was ≤5.1322 Given that many OSA patients complain of excessive daytime sleepiness, poor work productivity is a highly probable consequence.
A negative impact on the economy may manifest in the form of the cost of days absent from work because of OSA and the cost of disability or work duty modifications.1024,2078 In a large study of 19,438 OSA patients from the Danish National Patient Registry, Jennum et al. showed that OSA patients have higher hospitalization rates than non-OSA controls.133 OSA patients also have greater total direct (expenses for medical visits, hospitalizations, and medication) and indirect (loss of income due to disease-related work disability) costs up to 8 years prior to and after diagnosis.133 The study reported that CPAP and surgery could reduce workplace costs, but not to the level 2 years prior to treatment initiation, and CPAP was comparatively more effective at doing so than surgery.133 T&A and UPPP were listed as examples of surgeries undergone by the population; no sub-analysis of specific surgeries was performed.
The handful of studies analyzing the impact of OSA treatment on work productivity are mostly focused on CPAP.1024 Zhang et al. is perhaps the only study that examined the impact of surgery on work ability using a Work Ability Index (WAI) questionnaire.2079 Minimally invasive MLS was performed on the inferior nasal conchae, soft palate, tonsils, and tongue base of 51 patients. Statistically significant improvements in polysomnogram parameters (e.g., AHI, lowest saturations, microarousal index), ESS, as well as better WAI scores were noted 1 year after the surgery.2079 WAI scores improved from 37.76 ± 4.46 to 40.00 ± 4.53 (maximum score being 49 points; a higher score indicating better work ability).2079 The study observed that the change in the WAI score was influenced by the change in ESS, and patients whose occupation was classified as “mental work” improved more than those who did “manual work.”2079
Extrapolation from improvements seen in sleepiness, vigilance, reaction time, perceived general well-being, and reduced errors or lapses2005 after the majority of OSA surgeries suggests that surgery could lead to improved work productivity. However, in the presence of scant hard evidence demonstrating this link, the efficacy of surgery on work productivity is not certain. (Table IX.E.8)
TABLE IX.E.8.
Evidence for surgery to improve work productivity
| Study | Year | LOE | Study design | Study groups | Clinical end-point | Conclusion |
|---|---|---|---|---|---|---|
| Jennum and Kjellberg133 | 2011 | 2b | Cross-sectional controlled study | 1. Snoring 2. Sleep apnea 3. Obesity hypoventilation syndrome 4. Matched controls without sleep-disordered breathing |
Direct and indirect economic costs, mortality | Sleep-disordered breathing has major socioeconomic consequences for the individual patient and society. Surgery can reduce workplace costs. |
| Zhang et al.2079 | 2020 | 4 | Case series | Minimally invasive surgery | PSG, ESS, Work Ability Index | PSG parameters and patients’ perception of their ability to work improved significantly. |
Abbreviations: ESS, Epworth Sleepiness Scale; PSG, polysomnography.
X |. PEDIATRIC OSA: DIFFERENCES IN EVALUATION AND MANAGEMENT
X.A |. Evaluation for Pediatric OSA
Pediatric is distinct from adult OSA in epidemiology, pathophysiology, diagnosis, and treatment. Less common than in adults, OSA has an estimated prevalence of 1%–3% in children.2080 Untreated pediatric OSA is associated with hypertension,2081 autonomic dysfunction,2082 attention-deficit/hyperactivity disorder,2083 neurobehavioral impairment,2084,2085 and poor QOL.2086 Comorbidities in children that are associated with an increased risk of OSA include Down syndrome, craniofacial anomalies, neuromuscular disorders, mucopolysaccharidoses, and obesity.2080
X.A.1 |. Diagnosis by history
Snoring, apneic pauses, and gasping or snorting during sleep are the cardinal features of OSA in children and routine screening for snoring is recommended by the AAP.2087,2088 Though daytime sleepiness is also associated with childhood OSA, it is less common than in adults. Other symptoms common in children with OSA include nocturnal enuresis, daytime attention deficit, or hyperactivity,2083,2089–2092 learning difficulties and poor school performance.2080 However, while these symptoms in combination with habitual snoring are suggestive of childhood OSA, clinical evaluation alone is inadequate in establishing a diagnosis of OSA. For this reason, attended overnight PSG remains the gold standard and primary recommended method for diagnosis and severity grading of OSA in children.2088 However, due to the limitations of attended PSG in children including lack of availability, high cost, and poor tolerance, a clinical diagnosis of obstructive SDB can be made by clinical history and physical exam.
X.A.2 |. Sleep disordered breathing and validated subjective measures
When a diagnosis of OSA based on PSG is not possible, a diagnosis of SDB is often made based on clinical evaluation alone. Symptom screening may be further supplemented with validated questionnaires for symptoms of SDB or to assess sleep-related QOL.2093–2095 Among these, the Sleep-Related Breathing Disorder scale of the Pediatric Sleep Questionnaire (SRBD-PSQ) and the OSA-18 are two of the most commonly used and cited questionnaires (Table X.A). A recent MA of the accuracy of the SRBD-PSQ in diagnosing OSA in children showed a sensitivity of 0.72 [95%CI 0.68, 0.77], specificity of 0.59 [95%CI 0.56, 0.63], and an overall fair diagnostic accuracy (AUC) of 0.73 [95%CI 0.63, 0.82].2096 In general, questionnaires that have been validated against PSG have demonstrated variable sensitivities and specificities depending on the diagnostic cut-offs used, with frequently poor diagnostic accuracy.2094,2097 However, these questionnaires do have clinical utility in measuring subjective disease burden and sleep-related QOL. For example, the SRBD-PSQ score has been shown to correlate better than PSG with other measures of sleep-related morbidity (e.g., executive dysfunction, behavior, QOL, sleepiness) as well as response to treatment.2098
TABLE X.A.
Commonly cited surveys validated in children for assessment of obstructive sleep apnea or sleep-disordered breathing
| Name | Respondent | Age (years) | Items | Response type | Scoring | Purpose | Sensitivity/specificity against PSG (%) |
|---|---|---|---|---|---|---|---|
| SRBD-PSQ2112 | Caregiver | 2–18 | 22 | Yes/no/don’t know | >7 Considered positive screen | Screening for symptoms of SDB | 50–85/13–72 |
| OSA-182113 | Caregiver | 0.5–12 | 18 | 7-pt Likert | Range 7–126 0–60 mild 60–80 moderate >80 severe |
Sleep-related quality of life impact | 55–93/25–84 |
Abbreviation: SRBD-PSQ, Sleep-Related Breathing Disorder Scale of the Pediatric Sleep Questionnaire.
X.A.3 |. Physical examination
As a standard part of the head and neck exam, tonsillar hypertrophy is described according to the Brodsky scale,1645 though awake tonsil size assessment has not generally correlated well with either baseline OSA severity2099–2101 or response to AT.2102 Macroglossia should be noted and may be more significant in children with craniofacial syndromes. Other physical exam findings associated with OSA in children include adenoidal facies, nasal obstruction due to turbinate hypertrophy or septal deviation, mouth breathing, micrognathia, midface hypoplasia, and a narrow high-arched palate. General muscle tone and neurologic status should also be noted. Symptoms of stertor or stridor while awake may prompt flexible awake endoscopy during the clinic visit.
X.A.4 |. Objective testing
Unlike sleep testing in adults where home sleep testing is an accepted method of OSA diagnosis, no home sleep tests (HSTs) have been shown to be reliable or valid in children for routine clinical use. Therefore, attended overnight PSG remains the recommended method for diagnosis of OSA in children.2103 The AASM recommends the use of pediatric respiratory scoring rules for any children less than 18 years of age, but there is an option to use adult scoring rules for teenagers at least 13 years of age.12 OSA is diagnosed when SDB is accompanied by an abnormal PSG with an obstructive AHI > 1. Although there is no universal consensus on cutoffs for OSA severity in children, several studies use an AHI of 1 to <5 for mild,5 to <10 for moderate, and ≥10 for severe OSA.
According to the American Academy of OtolaryngologyHead and Neck Surgery Clinical Practice Guidelines for Tonsillectomy in Children, T&A may be recommended as treatment for SDB in the absence of PSG if clinical history and physical exam are congruent with the diagnosis.2104 Further recommendations include obtaining PSG in children with SDB if they exhibit obesity, Down syndrome, craniofacial abnormalities, neuromuscular disorders, sickle cell disease, or mucopolysaccharidoses.
X.A.5 |. Drug-induced sleep endoscopy in children
First described and widely used in the evaluation of adults with OSA, DISE is increasingly used in children with OSA. While the indications for DISE in adults are well established, the indications in children remain controversial.2105 DISE has been described in the evaluation of surgically naïve children2106,2107 as well as in those who have failed T&A2108 to help guide surgical decision-making. Patterns of obstruction observed in children are not as well established as in adults and vary by age and comorbidities.2109 While most adult rating scales like the VOTE scale focus primarily on dynamic obstruction at the soft palate, lateral OP, base of tongue, and epiglottis, it is widely acknowledged that the nasal airway, adenoid hypertrophy, and arytenoid cartilages can also be important sources of obstruction in children.2110,2111 (Table X.A)
X.B |. Adenotonsillectomy as First-Line Treatment for Pediatric OSA
The management of pediatric OSA depends on several factors including the severity of the sleep disorder, associated daytime and nighttime symptoms, and comorbidities. Adenotonsillar hypertrophy is the main contributor to OSA in the majority of healthy children.2104 As such, T&A is the first-line surgical treatment for OSA in children. This is the recommendation of guidelines published by the American Academy of Otolaryngology-Head and Neck Surgery (AAOHNS),2104 the AAP,2088 and the AASM.2114 Evidence in RCTs and SRs2053,2054,2115 has shown that T&A results in improvements in PSG parameters as well as daytime and nighttime symptoms, QOL, and behavior. While T&A should be recommended in children diagnosed with OSA by PSG, T&A is frequently recommended for SDB based on clinical history and examination findings alone, and has been shown to result in significant improvements in neurocognitive and QOL outcomes. However, there is increasing awareness that some children may see improvement and resolution of OSA without surgical intervention and may benefit from medical treatment or observation alone.
The most significant and widely referenced study on the management of OSA in children is the Childhood Adenotonsillectomy Trial (CHAT).2054 The CHAT study was a well-designed and rigorously conducted RCT with wide geographic and racial representation for the US, as well as high follow-up rates. It included 464 children (ages 5–9 years) with OSA randomized to T&A or watchful waiting with supportive care (observation). The study reported significantly greater improvements in the T&A versus observation groups in symptoms, behavior, QOL, and PSG outcomes. However, there was no difference in objective testing for attention and executive function between the groups. Normalization of PSG was much higher in the T&A 153/194 (79%) versus the observation group 93/203 (46%).2054 At baseline, mean AHI scores were 6.9 (SD 5.7) in the T&A group and 6.6 (SD 5.6) in the observation group. At 7 months, the mean AHI score in the T&A compared with the observation group was 1.6 (SD 3.0) versus 5.9 (SD 10.1), respectively. Children with more severe baseline OSA showed greater improvement after T&A compared to watchful waiting, but they were also less likely to show PSG normalization after T&A. Similarly, fewer African American children and those with obesity had normalization of AHI, irrespective of the assigned treatment.
Several observational cohort studies reported significant improvements after T&A for OSA in children.2116–2118 A multicenter retrospective study of 578 children in six pediatric sleep centers in the US and Europe was reported by Bhattacharjee et al.2116 All children had a T&A for OSA with pre- and postoperative PSG and 50% were obese. T&A resulted in a significant reduction in AHI from a mean 18.2 to 6.4, but only 27% had complete resolution of OSA (AHI < 1). Older age (>7 years), and those with chronic asthma or obesity were more likely to have persistent OSA. Most children had an improvement but not resolution in AHI after T&A. This study2116 and several others have reported persistent OSA after T&A in children with severe OSA, obesity, Down syndrome, craniofacial, genetic, and neuromuscular disorders.2117,2119
Two meta-analyses of outcomes of T&A for OSA were published in the last 5 years.2053,2115 Both included data from the CHAT study and reported significant improvements in PSG parameters, symptoms, QOL, and behavior in children with OSA undergoing T&A compared to observation. Chinnadurai et al.,2053 in an MA of three studies on outcomes of T&A for OSA, reported a significant 5-point difference in improvement in AHI after T&A versus observation. However, two studies included in this MA were small cohort trials.2053 Venekamp et al.2115 reported on three studies with 562 children but were unable to combine the results because of substantial differences in the groups of children studied.2115 They reported significant improvements following T&A versus observation but mostly in healthy, normal-weight children. They noted that these benefits were modified by comorbid conditions, such as craniofacial, neuromuscular, genetic, and metabolic disorders and that demographic characteristics, particularly obesity, can lead to more severe baseline OSA and a less significant response to T&A.2115
There is an ongoing debate about the efficacy and timing of T&A for mild OSA (AHI <5). Trosman et al. studied children with mild OSA, including a variety of comorbidities such as obesity, craniofacial disorders, and hypotonia. Of 62 children, 19 had a T&A, and the rest were observed.2120 T&A resulted in a significant improvement in AHI over observation in the non-syndromic, nonobese children that was not seen in the observation group. Indeed, several children in the observation group experienced worsening AHI. Volsky et al.2121 studied 64 children with mild OSA and compared 30 who underwent T&A with 34 in the observation group. Outcomes were measured subjectively with QOL instruments. Baseline QOL measures improved significantly 4 and 8 months after T&A. There was no change in QOL measures for the observation group at 4 months but some improvement at 8 months. There is a paucity of outcomes data in children with significant comorbidities and mild OSA and it remains unknown if T&A should be a first-line treatment in these children. There is additional concern that T&A for mild OSA can lead to weight gain especially in obese children that can worsen the severity of the sleep disorder.2122 Furthermore, the role of CPAP therapy as an alternative to T&A, especially in obese children, has not been studied extensively and better patient-focused outcome measures are needed.
As an alternative to T&A for treatment of pediatric OSA, medical treatment has been proposed including nasal steroids and oral montelukast.2123–2125 An MA including five studies that evaluated montelukast as stand-alone treatment for pediatric OSA found a 55% improvement in AHI. A separate analysis of two studies found a 70% improvement in AHI when treatment with montelukast was combined with nasal steroid therapy.2126 However, these studies did not compare medical treatment to standard surgical treatment and measured only short-term outcomes (typically 3–6 months). The long-term impact of these medical treatments and their potential side effects is unknown. In addition, montelukast now has an FDA black box warning due to risk of serious adverse mental health side effects.2127
In children with persistent OSA symptoms after T&A, PSG is indicated. Options for treatment include noninvasive positive pressure therapy, medical therapy as described above, or further surgical treatment. DISE is being increasingly explored in the treatment of persistent OSA in children after T&A.2109 Surgical therapy in these T&A failures can be dictated by findings on DISE and may commonly include revision T&A, lingual tonsillectomy, supraglottoplasty, or nasal surgery. 2128 Future directions include the increasing application of HNS in children who fail T&A. Initial studies in adolescents with Down syndrome with persistent severe OSA after T&A showed median reduction of AHI by 85%.2129
In summary, there is strong evidence to support T&A as the first line treatment of OSA in children. However, in children with mild to moderate OSA and mild symptoms, a period of observation or medical therapy may be appropriate. There is a paucity of research on the best management of children with mild OSA or in children with significant comorbidities and OSA. Persistent OSA in children may be evaluated with DISE which can further dictate medical or surgical therapy. (Table X.B)
TABLE X.B.
Evidence on adenotonsillectomy for pediatric OSA
| Study | Year | LOE | Study design | Study groups | Clinical endpoints | Conclusion |
|---|---|---|---|---|---|---|
| Marcus et al.2054 | 2013 | 1b | RCT | Children with OSA followed up for 7-months: 1. T&A 2. Observation |
PSG, behavior, quality of life, symptoms, neurobehavioral outcomes | Improvement in both groups. Significantly greater improvement in the surgical group. No improvement in neurobehavioral outcomes in either group |
| Bhattacharjee et al.2116 | 2010 | 2b | Observational cohort | Children with OSA with before and after T&A PSG data | Resolution of OSA | Significant improvement in AHI but resolution in only 27% |
| Chinnadurai et al.2053 | 2017 | 1a | SR of RCTs | Children with OSA with T&A versus observation | Improvement/resolution of OSA | T&A compared to observation leads to short-term improvement in sleep outcomes in children with OSA |
| Venekamp et al.2115 | 2015 | 1a | SR of RCTs | Children with OSA with T&A vs. observation or CPAP | Improvement/resolution of OSA | T&A compared to observation or CPAP leads to benefits in PSG parameters quality of life, symptoms and behavior |
| Trosman et al.2120 | 2016 | 3a | Observational cohort | Children with mild OSA | PSG | T&A compared to observation leads to a significantly greater improvement in AHI especially in non-obese, non-syndromic children |
| Volsky et al.2121 | 2014 | 3b | Observational cohort | Children with mild OSA | OSA-18 quality of life scores | Quality of life significantly improves in children with mild OSA after T&A with less improvement with observation |
| Kheirandish-Gozal et al.2123 | 2014 | 3b | Observational cohort | Children with mild OSA treated with intranasal corticosteroid and oral montelukast | PSG in subset; symptoms | Beneficial effects in >80% of children |
| Liming et al.2125 | 2019 | 2b | SR of observational cohorts | Children with mild OSA treated with intranasal corticosteroid and oral montelukast | PSG | Treatment with montelukast and intranasal steroids or montelukast alone is beneficial for short-term management of mild pediatric OSA |
Abbreviations: T&A, tonsillectomy with or without adenoidectomy; SR, systematic review.
XI |. KNOWLEDGE GAPS AND RESEARCH OPPORTUNITIES
There is continued rapid growth in knowledge and advancements within the field of SDB. Growing evidence demonstrates that OSA results from individualized features of physiology and symptomatology. The far-reaching independent effects of untreated OSA on cardiometabolic comorbidities, health outcomes, and mortality are unresolved. The next phase of investigation requires determining optimal paradigms for diagnosis and management of OSA by translating large-scale analyses into personalized care.
The ICS:OSA outlines areas where consistent evidence supports screening, diagnosis, and treatment for OSA while at the same time identifies important knowledge gaps. Specially, there is a need to demonstrate that the treatments for OSA have meaningful impact on outcomes other than sleep health and patient satisfaction.
XI.A |. Phenotypes and Metrics of Disease
The contributors to adult OSA are multiple and complex. Integrated knowledge of sleep architecture, ventilatory control, UA anatomy, insomnia, and neuromuscular control is necessary to appreciate the complexities of the disease and determine the best steps for management.
XI.A.1 |. AHI and the need for alternative metrics of OSA
The ICS:OSA demonstrates a variability in OSA phenotypes and disease sequelae. Yet, the historical paradigm for stratification of OSA severity has centered primarily on the AHI as the outcome metric. Utility of a single outcome metric, whereas simple, does not adequately predict the multitude of clinical endpoints attributed to OSA. A recent publication from the Sleep Research Society summarizes the strengths and weaknesses of AHI as the main metric of OSA.2025
Traditionally, AHI is the summary metric that defines OSA presence and severity. It represents the focal measure of UA instability in a sleep study2130 and of treatment efficacy. A 1999 consensus statement suggested that categories of mild (<5 events/h), moderate (5–15 events/h), and severe (>30 events/h) for AHI were convenient and appropriate markers for clinical severity for the use of CPAP therapy.2131 At the time, these experts-derived cutoffs were not determined based on strong evidence. While definitions and scoring of hypopneas have changed, these cutoff AHI values are still used for categorical severity and treatment goals.
Problems with AHI as the primary metric of OSA disease include its lack of correlation with symptoms and sleepiness severity and issues with consistency of reporting. AHI has been demonstrated to have poor correlation with sleepiness, neurocognitive function, and QOL.421,954,2132–2135 There are issues with consistency and reproducibility of the AHI metric. A night-to-night variability of AHI in the short term is related to variation in sleep stages, sleeping position, environment, and arousals.2136–2138 Interscorer reliability of AHI when the scoring is tightly controlled within a sleep center is high.2139 However, when applied to clinical practice, sleep laboratories may use a variety of AHI metrics depending on insurance payor edicts or updates in clinical scoring rules (i.e., hypopnea definitions). This leads to variability in interpretation of AHI.
While AHI was the starting point and is the best studied metric of OSA, it falls short as a unifying metric that captures both OSA severity and outcome prognostication. Alternative determinants of disease severity may involve combinations of other metrics. The ODI is defined by desaturations on pulse oximetry and is associated with the potential for more objective scoring.732 Arousal index, area under the SaO2 curve to account for hypoxic exposure, apnea–hypopnea length, and inter-apneic interval are currently experimental but suggest associations with clinically meaningful outcomes. Disease burden has been associated with metrics such as: hypoxic burden, which may contribute to CV mortality2140; arousal intensity, which has implications for memory disturbance2141,2142; and specific blood biomarkers, which may demonstrate inflammatory responses to chronic intermittent hypoxia.2143–2145 Thus, the optimal metric to identify OSA severity may differ between individuals depending on time of onset and burden of disease, and may be outcome-specific. Further research is required to identify measures that capture potential heterogeneity in mechanisms underlying the origins of OSA and individual responses to hypoxia.2146–2148
Future definitions of OSA severity will likely merge the AHI with other quantitative and semiquantitative dimensions linking OSA pathophysiology and symptomatology.2149,2150 An integrative approach may better determine and stratify patients who will benefit from OSA treatment.
XI.A.2 |. Phenotypes of disease
Initial clustering and multi-dimensional analyses of OSA demonstrate it is a heterogeneous disorder with three clinical “phenotypes” proposed: (1) Disturbed sleep, (2) Minimally symptomatic, and (3) Excessively sleepy.1998,2150–2152 Other studies have identified additional phenotypic groups, but these three have been identified across multiple populations. Pien et al. studied changes in symptoms related to sleepiness and fatigue, insomnia, and apneic events in these groups and found that the effect of PAP treatment differed by phenotypic group.
OSA phenotypes may also be associated with different levels of CV risk. Analyses of OSA symptom subtypes and CV disease showed a higher risk of incident CV disease and CV mortality in the “excessively sleepy” when compared with other clusters.640,1205
Another proposed clinical phenotype is the distinction between positional patients who primarily manifest OSA when in the supine position compared to nonpositional patients who demonstrate significant SDB in all positions.41 Understanding the differences in OSA disease and treatment outcomes between positional and non-positional patients has important implications for multi-modal treatment discussions.
Physiologic phenotypes of disease describe the heterogeneity of anatomic and non-anatomic contributors to disease such as UA collapsibility (measured with Pcrit), airway dilator muscle tone changes in sleep (measured with EMG), respiratory triggers for awakening (arousal thresholds), and sensitivity of the ventilatory control system (loop gain). The PALM scale (Pcrit, arousal threshold, loop gain, and muscle responsiveness) has been proposed to categorize OSA patients and target therapies to pathologic mechanism(s) of disease.36
Understanding the implications of these clustered traits and OSA phenotypes on treatment outcomes and other health risks will be an important area for individualized therapy.
Research opportunities include:
Determining which combination of PSG and symptom-based metrics capture OSA severity, inform prognosis and determine CV and mortality risks.
Determine accessible biomarkers for OSA that can be used to determine treatment responses.
Understand the differences between OSA patients who have significant daytime sleepiness compared to those without sleepiness.
Predict risks and symptoms associated with REM-related OSA, especially in those with mild OSA (AHI 5–15 events/h).
Delineate the mechanisms behind how obesity relates to OSA and individual OSA severity prognosis with weight management.
Determine the genetic associations of SDB.
Examine utility of interventions to alter loop-gain and arousal thresholds as adjunctive treatments for OSA.
Understand the role of sleep position and clinical outcomes.
XI.B |. OSA Screening and Diagnosis
The impact of OSA screening in primary care settings is not settled in terms of cost, effectiveness, and outcomes at the population level. Support exists for the utility and performance of the STOP-BANG as a screening tool, but evidence on the long-term health impacts of screening is lacking. Understanding health risks and behaviors that can be modified for benefit will define the optimal screening and testing strategies.
Different testing modalities now exist and given the prevalence and concern for OSA diagnosis, improving testing access and convenience is an emerging market. Home sleep studies improve convenience. Understanding the limitations of home sleep testing interpretations compared to in-lab sleep studies or oximetry testing will allow practitioners to choose the optimal testing and management strategies. Additionally, there are various consumer sleep technologies including nearables, wearables used for sleep tracking with variable capabilities. Devices assessments for potential benefits, disadvantages, and needs for validation are currently ongoing.2153
Research opportunities include:
Assess the cost-utility tradeoff for routine screening in primary care settings.
Create programs for inclusive and equitable access to OSA testing and decision making.
Understand the predictors for false-negative home sleep studies that warrant follow-up and repeat testing. Determine who requires in-lab testing for diagnosis.
Analyze the financial tradeoffs of using home sleep study testing versus in-lab sleep studies in specific high-risk populations.
Optimize screening paradigms to determine those at highest risk for medical and behavioral consequences of untreated OSA.
Systems to improve access to OSA testing.
Understand the role, utility, and value of consumer sleep technologies for sleep tracking.
XI.C |. OSA Therapy
XI.C.1 |. Benefits of PAP
In the face of conflicting data between cohort and RCT studies on the effect of PAP therapy on cardiometabolic health outcomes and mortality risk, it is difficult to interpret the overall benefits of PAP therapy. Pack et al. summarized the biases and limitations associated with the negative RCT studies on the effects of PAP therapy and CV events. Specifically, RCT studies were secondary prevention studies and all trials excluded OSA subjects with excessive sleepiness who are increased risk for CV events and most likely to benefit from treatment.1205 RCTs also had inadequate adherence to PAP therapy and on secondary analysis of data in adherent patients, CPAP benefit was found to be consistent with prior epidemiological studies.1206
Research opportunities include:
Incorporate real-world PAP usage patterns to better understand modifications to health risks and mortality risks.
Identify the patient phenotypes that benefit most from PAP therapy.
Understand the efficacy of MADs, weight reduction, and positional sleep on altering health risks.
XI.C.2 |. PAP adherence
PAP remains the first-line therapy for OSA treatment, but efficacy is limited by adherence. “Big data” analyses of CPAP telemonitoring data provide new opportunities to understand adherence and real-world use patterns. Yet, current dichotomization of PAP use into adherence versus non-adherence based on pre-set thresholds (i.e., >4 h per night on >70% of nights over a rolling 30-day period) ignores the potential for linear dose-dependent relationships between CPAP use and improved clinical outcomes and creates barriers to reimbursement in patients who may be receiving meaningful clinical benefit despite suboptimal use.1098 The use of the 4-h, 70% threshold may be contributing to reduced access to treatment by rendering users below this threshold ineligible for coverage. Thus, further work is required to identify clinically meaningful adherence thresholds for PAP use, which may be outcome-dependent and vary in the context of patient treatment goals.
Furthermore, identifying factors that influence PAP adherence can inform innovations for optimization of PAP use and motivate behavior change programs. Data suggests disparities exist with PAP adherence across socioeconomic and racial groups.1095–1097 Predictors of adherence include sociodemographics, comorbid conditions, OSA severity and perceived benefit of treatment, and behavioral factors, which may explain up to 50% of variance in PAP adherence.1106,1117 Interventions targeting modifiable predictors have informed protocols such as patient education and engagement programs, and optimization of device design. However, there remains an opportunity to identify predictors for non-adherence to improve patient expectation management and promote early consideration of alternative treatment options.
Research opportunities include:
Reexamine current PAP use threshold definitions and identify clinically meaningful adherence thresholds.
Determine predictors of PAP adherence and design protocols to automate and inform providers about patients at-risk for low adherence levels.
Quality initiatives to utilize telehealth or EMRintegrated systems to provide feedback to patients about PAP use and importance of PAP adherence.
Understand the relationship between rhinitis, and sinusitis with PAP adherence.
Identify and disseminate the most common modifiable changes to influence PAP adherence such as nasal obstruction, humidification, insomnia, PAP desensitization.
XI.C.3 |. Surgery and definitions of success
Based on the AASM clinical practice guideline by Kent et al., referral for sleep surgery evaluation should be strongly considered for adults with OSA and BMI < 40 who are intolerant or unaccepting of PAP, and referral for bariatric surgery evaluation for adults with obesity (BMI > 35) who are intolerant or unaccepting of PAP.1506
Classic definitions of surgical success are based on specific levels of AHI reduction (i.e., Sher’s criteria) are limiting and do not fully capture the benefits of surgery in patients who cannot use PAP. A refocus on measures of QOL, symptoms, snoring sound reduction, and long-term health risk reduction that are important to patients2154 will be vital to understanding the full effects of surgery. Standardized approaches for surgical outcome reporting will be important and should quantify AHI reduction with standard analyses2026 along with considerations for T90, ODI, AI, and sleep related symptoms. Specifically, much of the surgical literature is based on small case series with shortterm outcomes instead of prospectively designed trials. Standardized methodology for outcomes reporting is critical to allow for meta-analyses and evaluation of combined data.
Further work is also required to better understand which PAP alternatives are best for individual patients. Traditionally, surgery is performed after PAP has been trialed; however, in candidates with amenable anatomy, primary surgery may have a high probability of success. Further work is needed to understand the best candidates for primary surgery and each surgery type.
XI.C.4 |. Surgical outcome
Comparisons between surgical outcomes and PAP outcomes are lacking. Specifically, postsurgical AHI improvement has rarely been compared to real-world PAP outcomes. Results on a titration sleep study do not adequately reflect PAP adherence and effectiveness. Larger prospective studies are needed to compare long-term outcomes in patients who receive surgical therapy versus PAP therapy.
Limitations of literature on surgery includes variability in procedural choice and techniques, non-standardized reporting of outcomes, small and heterogeneous populations, and many uncontrolled cohort studies. Recent advances include the addition of surgical techniques that reduce long-term risks for dysphagia and efforts to understand which anatomic and clinical features respond best to surgical interventions.
Anatomic surgery may not lead to OSA resolution in all patients in terms of achieving AHI < 5 events/h. However, the reduction in obstructive events, improvement in hypoxic burden, improvements in QOL, and potential for health risk reduction are factors essential to surgical counseling.
Research opportunities include:
Examine the thresholds of BMI for surgical candidacy and understanding the relationship between BMI and surgical success.
Determine predictors for surgical success for each surgical approach.
Determine candidates for each surgery type and candidates for surgery as first-line treatment for OSA.
Develop protocols to determine how to use DISE and other diagnostic tools to improve surgical outcomes.
Determine the best practices approach for surgical selection based on clinical exam, treatment goals, PSG results, anatomy, DISE, and other factors related to OSA.
Understand surgical outcomes in terms of OSA reduction, daytime sleepiness, snoring, and sleep quality.
Understand the long-term outcomes of surgery on hypertension, CV disease, neurocognitive function, and mortality.
Determine the role of surgery as adjunctive therapy to medical treatments for OSA.
Evaluate management of HNS and determine how to resolve those with suboptimal response.
Determine best-practice approaches for HNS advanced titration, programming adjustments, and clinical follow-up. Determine which titration protocols improve outcomes and which approaches have the most meaningful effects.
XI.D |. Longitudinal OSA Care
OSA is a chronic disease that requires different modalities of therapy that may evolve over time. Long-term follow-up is necessary to assess for continued therapeutic response. As new medical and surgical treatment options for OSA are introduced, there is a need for large-scale studies to identify long-term efficacy and determine who is at risk for disease recurrence. The optimal care models for long-term management of OSA are unknown. Frequency of sleep studies has not yet been well established. Furthermore, while changes in OSA severity have been demonstrated in different age, weight, and comorbidity groups, the longitudinal course of OSA in a single patient with variations in these factors over time is not fully known. Therefore, further collaboration is required to identify follow-up protocols that are sensitive to changes in OSA over the disease course.
Research opportunities include:
Optimize paradigms for longitudinal testing that include risk factors for disease progression or recurrence.
Validate and incorporate consumer sleep technologies for tracking OSA as a chronic disease in a clinically meaningful manner.
Understand the impact and roles of OSA treatment on CV and neurocognitive comorbidities. Specifically, define OSA as a modifiable risk factor for each disease such as: diabetes, hypertension, CAD, AF, HF, stroke, memory loss, dementia, and cancer.
Examine the mechanisms that underly the association between OSA severity and cancer risk.
Efficacy and candidacy for alternative therapies including myofunctional therapy, PT, and oxygen therapy.
XI.E |. Patient-Centered Models of Care
It is evident from the data presented in the ICS:OSA that medical and surgical therapy provide variable benefits. Identification of an optimal OSA treatment may prove challenging for patients given the multitude of therapeutic options and lack of clarity regarding optimal treatment paradigms. Improved shared decision-making processes and decision tools will be important to help patients identify goals in OSA treatment and allow providers to recommend specific treatments that meet individual goals and values.2154
It is essential for providers to comprehensively counsel patients about options for OSA treatment. Shared-decision making is especially important with patients who have failed first-line PAP therapy, given the variety of alternative treatments and high levels of decisional conflict in considering alternatives.2155 Prior studies have demonstrated that shared decision-making reduced decision delay, improved time to OSA therapy initiation, improved treatment compliance, and reduced decisional regret for parents of children with OSA.2156–2158
Multiple components of optimal OSA treatment require adherence to therapies such as PAP, HNS, MAD, weight management, and sleep hygiene. There is a need to understand patient-specific behaviors and influencers of behavioral change and motivation. Collaboration is key to furthering understanding, providing individualized care.
Research opportunities include:
Translating information from large datasets into personalized medicine.
Improve the equity and access to knowledge about OSA and treatment options.
Developing patient-facing decision support tools to navigate the options for OSA therapy and the risk and benefit tradeoffs.
Align individual clinical factors and patient values with treatment options.
Supplementary Material
Sex as a Contributing Factor for OSA.
Aggregate grade of evidence:
C (Level 2b: 3 studies; Level 2c: 17 studies; Level 3b: 16 studies; Level 4: 19 studies).
Obesity as a Contributing Factor for OSA.
Aggregate grade of evidence:
C (Level 1: 5 studies; Level 2: 11 studies; Level 3: 13 studies; Level 5: 2 studies).
Craniofacial Anatomy as a Contributor for OSA.
Aggregate grade of evidence:
C (Level 3a: 1 study; Level 3b: 21 studies; Level 4: 3 studies).
Questionnaires for OSA Screening and Quality of Life Evaluation.
Aggregate quality of evidence:
C (Level 1a: 5 studies; Level 1b: 4 studies; Level 2a: 3 studies; Level 2b: 7 studies.
Benefit:
Validated questionnaires offer simple, point-of-care options for screening in suspected OSA to prioritize patients needing confirmatory sleep study testing and to evaluate functional status and health-related QOL in patients with OSA.
Harm:
Minimal to none.
Cost:
No financial burden to patients. Clinic and patient time required to administer and collect responses are required.
Benefits-harm assessment:
Preponderance of benefit over harm. Low risk of harm with additional sleep study testing in patients with false positive responses. Low risk that a false negative response may lead to delay in testing and further management.
Value judgments:
Validated screening questionnaires can enhance diagnosis and treatment rates of OSA in a cost-effective manner. Validated functional outcomes and health-status QOL questionnaires serve as important tools in evaluating the behavioral and functional consequences of OSA and in measuring response to treatment.
Recommendation level:
Recommendation.
Intervention:
Validated clinical questionnaires can be used to identify patients at high risk for OSA, measure responses to treatment, and evaluate the health-related QOL. The STOP-BANG using a cutoff score of 5 may be used to screen patients for OSA and identify those needing sleep study testing. The FOSQ may be used to assess both functional outcomes in patients with OSA and to follow change in status over time and after treatment.
Widespread Screening for OSA in the Primary Care Setting.
Aggregate quality of evidence:
C: (Level 2a: 6 studies; Level 2b: 6 studies; Level 2c: 1 study).
Benefit:
Screening for OSA has the potential for earlier diagnosis and treatment which may be associated with reduction of symptoms.
Harm:
Potential for unnecessary diagnostic testing. There is very little evidence on the benefits of widespread screening for OSA.
Cost:
Low cost of screening and time required for screening.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Primary care screening may enhance diagnosis and treatment rates at the population level, although no evidence has shown the long-term health benefits of screening.
Recommendation level:
Option to screen for OSA in asymptomatic patients.
Intervention:
Screening for OSA in the primary care setting is optional. Further studies are required to determine the long-term health impact of widespread screening and health risk prevention, which is not proven. STOP-BANG questionnaire has consistently higher sensitivity compared with other screening questionnaires.
Screening for OSA in Perioperative Patients.
Aggregate quality of evidence:
C (Level 2a: 1 study; Level 2b: 11 studies; Level 3b: 1 study; Level 4: 1 study).
Benefit:
Preoperative screening for OSA can identify high-risk patients in order to guide perioperative management and prioritize patients needing sleep study testing.
Harm:
Time required to implement screening system and the potential for over testing patients for sleep apnea (false positives on screening).
Cost:
Low costs associated with screening patients for OSA using questionnaires but an increase in indirect costs for additional testing and monitoring may occur.
Benefits-harm assessment:
Strongly favors screening for the diagnosis of OSA in the preoperative setting due to effects on perioperative management.
Value judgments:
The evidence favoring preoperative screening of OSA for perioperative planning.
Recommendation level:
Recommendation to screen for OSA in the preoperative setting.
Intervention:
Patients who are undergoing surgery benefit from preoperative screening for OSA. Based on the evidence reviewed, the STOP-BANG questionnaire is recommended as a screening tool due to its high sensitivity.
Patient Reported Symptoms Related to OSA: Snoring, Gasping, and Excessive Daytime Sleepiness.
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 7 studies; Level 2b: 4 studies; Level 5: 1 study).
Benefit:
Symptoms of snoring, gasping, and excessive daytime sleepiness are linked to OSA.
Harm:
Potential for over-testing for OSA.
Cost:
Minimal to none.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
Patients can benefit from identification and treatment of OSA. Reporting of OSA symptoms may identify patients requiring further testing. Symptoms are not sufficient for diagnosis.
Recommendation level:
Recommendation.
Intervention:
Patients reporting symptoms of snoring, gasping, and daytime sleepiness should be further evaluated for OSA.
Use of Imaging for OSA Diagnosis.
Aggregate quality of evidence:
C (Level 2a: 5 studies; Level 2b: 3 studies).
Benefit:
Imaging allows for visualization of the anatomical sites of obstruction. Imaging may be a potential diagnostic tool for OSA as PSGs are expensive and time consuming.
Harm:
Unnecessary radiation exposure and poor diagnostic accuracy.
Cost:
Cost and time to obtain imaging.
Benefits-harm assessment:
Harms outweigh the benefits for OSA diagnosis.
Value judgments:
Lateral cephalometry is helpful for treatment planning in orthognathic surgery for OSA but not as a diagnostic tool for OSA.
Recommendation level:
Recommendation against use of imaging for OSA diagnosis.
Intervention:
Routine imaging is not useful as a diagnostic tool for OSA. Imaging may aid in specific treatment planning for the OSA patient.
Home Sleep Apnea Testing for Diagnosis of OSA.
Aggregate quality of evidence:
A (11 Level 1a studies; 13 Level 1b; 3 Level 2a; 14 Level 2b studies; 1 Level 2c; and 3 Level 3b studies).
Benefit:
HSAT with type III devices have good diagnostic accuracy in selected patients. HSATs are useful in populations with a high prevalence of OSA or when combined with validated sleep questionnaire(s) that enhance the pre-test probability of moderate to severe OSA.
Harm:
Specificity of HSAT is lower in mild OSA patients. AHI, recording time, and sleep position affect the accuracy of HSAT. The moderate false negative rate seen with HSAT can potentially delay diagnosis and treatment.
Cost:
A home-based diagnostic pathway for OSA is associated with lower costs than a laboratorybased pathway from insurance payer’s perspective. There is substantial gap in the literature to guide home-based testing decision making and cost-benefit tradeoffs.
Benefits-harm assessment:
In patients with high pretest probability of OSA, HSAT can diagnose OSA. The benefits outweigh the harms. HSAT is valued for testing convenience, potential cost savings and allows for increased access to testing. Certain populations may benefit from PSG over HSAT testing.
Value judgments:
Sleep study data represent only one component of the OSA diagnosis. Clinical history and examination are as important and are complimentary to the sleep study.
Recommendation level:
Recommendation.
Intervention:
Home sleep apnea testing (HSAT) may be used for the evaluation of patients with a high pre-test probability for obstructive sleep apnea. Inadequate or inconclusive results on HSAT may require PSG testing.
Indications for in-Lab Polysomnogram Versus Hsat for OSA Diagnosis.
Aggregate quality of evidence:
For patients with hFrEF:
Grade C (Level 1b: 3 studies; Level 2b: 1 study; Level 3b: 1 study)
For patients with COPD:
Grade C (Level 1b: 1 study, Level 3b: 1 study)
For patients following stroke:
Grade D (Level 2b: 1 study)
For patients with technically inadequate or normal initial HSAT:
Grade C (Level 2b: 1 study)
Benefit:
Attended PSG can differentiate obstructive versus central events, which is important for hFrEF and post-stroke patients. PSG can also detect hypoventilation, essential for patients with COPD to provide accurate diagnosis of OSA that may be missed on HSAT.
Harm:
PSG may limit accessibility for testing when compared to HSAT which may lead to delays in diagnosis.
Cost:
PSG is more expensive than HSAT.
Benefits-harm assessment:
Benefits outweigh the harms as PSG more accurately diagnoses OSA in patients with significant co-morbidities including hFrEF, COPD, or stroke. PSG may be useful in patients with technically inadequate HSATs and in a minority of those with normal HSAT testing.
Value judgments:
Missed or delayed diagnoses of OSA will lead to adverse consequences particularly in patients with significant co-morbidities associated with OSA.
Recommendation level:
Recommend (in hFrEF, COPD, inadequate, or normal HSAT). Option (stroke).
Intervention:
PSG is indicated for the diagnosis of OSA in patients with hFrEF, COPD, or those with inadequate HSAT results. Patients with a high pretest probability and negative HSAT results should be offered repeat testing with in-lab PSG.
Oximetry for OSA Diagnosis.
Aggregate quality of evidence:
For the general population:
Grade C (Level 1b: 2 studies; Level 2b: 3 studies; Level 3b: 1 study)
For patients with hFrEF:
Grade C (Level 1b: 2 studies; Level 3b: 1 study)
For patients with COPD:
Grade C (Level 1b: 1 study; Level 2b: 1 study, Level 3b: 1 study)
For patients with atrial fibrillation:
Grade D (Level 2b; 1 study)
For patients following stroke:
Grade D (Level 2b: 2 studies; Level 3b: 1 study)
Benefit:
Overnight oximetry is less costly intervention than PSG for the diagnosis of SDB and is readily available. The use of oximetry may allow early diagnosis of SDB and in turn may lead to more timely treatment of OSA with PAP.
Harm:
Include risks of missed diagnoses and inability of overnight oximetry to differentiate obstructive vs. central events. Overnight oximetry is also unable to detect hypoventilation. Variability in interpretation of overnight oximetry between providers is recognized and may lead to inaccurate treatments. Small patient samples in some of the studies reduced generalizability of the results.
Cost:
There was no formal cost assessment in the studies reviewed.
Benefits-harm assessment:
Missed diagnoses with oximetry may lead to harm from undiagnosed OSA in patients with heart failure, atrial fibrillation, COPD, or stroke. The inability to distinguish between obstructive and central events, coupled with variability in interpretation of studies, can also lead to incorrect treatments. Combined, these harms outweigh the benefits of overnight oximetry to diagnose OSA. For the general population, balance of benefit and harm.
Value judgments:
Missed diagnoses of OSA will lead to adverse consequences of OSA as it would lead to incorrect or delayed treatment.
Recommendation level:
Recommend against (heart failure, atrial fibrillation, COPD, and stroke); Option (general population).
Intervention:
Oximetry is not recommended for the diagnosis of OSA in patients with heart failure, atrial fibrillation, COPD, or stroke. Oximetry may be an option for the general population without comorbidities, but more evidence is required.
Screening for OSA in Patients with Cardiovascular Disease.
Aggregate quality of evidence:
C (Level 1b: 1 study; Level 2a: 3 studies; Level 2b: 16 studies; Level 2c: 1 study; Level 3b: 1 study).
Benefit:
Earlier diagnosis and treatment of OSA may improve overall health and reduce the risk of all-cause mortality and cardiovascular morbidity and mortality.
Harm:
Inconvenience of screening and testing.
Cost:
Costs associated with screening and diagnosis for OSA.
Benefits-harm assessment:
Moderately favors screening for OSA in patients with a history of cardiovascular disease.
Value judgments:
There is moderate quality evidence that OSA leads to cardiovascular disease, but low quality evidence that treatment with CPAP reduces this risk. The harm/costs of diagnosis and treatment are low and the benefits outweigh the harms.
Recommendation level:
Recommendation.
Intervention:
Screen for OSA in patients with a history of cardiovascular disease.
Evaluation of OSA in Patients with Coronary Artery Disease.
Aggregate quality of evidence:
B (Level 1a: 3 studies; Level 1b: 4 studies; Level 2b: 10 studies; Level 3a: 3 studies; Level 3b: 3 studies).
Benefit:
OSA is very likely associated with coronary artery disease and myocardial ischemia and outcomes in these patients.
Harm:
Inconvenience associated with evaluation and testing for OSA.
Cost:
Low cost of evaluating and testing patients for OSA.
Benefits-harm assessment:
Benefit of identifying OSA in patients with CAD outweigh the low harms and cost of testing.
Value judgments:
Patients of CAD are at high risk for OSA.
Recommendation level:
Recommendation.
Intervention:
Patients with CAD and/or a history of MI should be evaluated for OSA.
Evaluation of OSA in Patients with Hypertension.
Aggregate quality of evidence:
C (Level 3b: 6 studies; see Table VII.B).
Benefit:
OSA is a treatable secondary cause of refractory hypertension.
Harm:
There is minimal harm associated with evaluation and testing for OSA, other than inconvenience to the patient.
Cost:
Low to moderate cost of evaluation and testing.
Benefits-harm assessment:
The benefits of identifying OSA in patients with refractory hypertension may outweigh the inconvenience and cost of testing.
Value judgments:
In patients with resistant or refractory hypertension, identification of comorbid OSA is indicated because OSA treatment may aid in blood pressure management.
Recommendation level:
Recommendation
Intervention:
Patients should be screened for OSA if they have persistent resistant or refractory hypertension after antihypertensive medication treatment has been optimized.
Evaluation of OSA in Patients with Atrial Fibrillation.
Aggregate quality of evidence:
C (Level 1b: 5 studies; Level 2b: 5 studies; Level 3b: 6 studies; Level 4: 5 studies).
Benefit:
Evaluation for OSA prior to treatment of AF may permit timely OSA treatment and may reduce the risk of AF recurrence.
Harm:
Inconvenience of OSA evaluation and testing.
Cost:
Low cost associated with screening and testing AF patients for OSA.
Benefits-harm assessment:
Weakly favors screening for OSA in patients undergoing treatment of paroxysmal or persistent AF with cardioversion or catheter ablation procedures.
Value judgments:
The overall quality of evidence favoring diagnosis of OSA solely for the prevention of recurrent atrial fibrillation is generally weak, with no adequately powered randomized clinical trials.
Recommendation level:
Recommendation.
Intervention:
Patients who are undergoing cardioversion or catheter ablation for atrial fibrillation may benefit from screening for OSA.
Evaluation of OSA in Patients with Heart Failure.
Aggregate quality of evidence:
C (Level 1b: 1 study; Level 2b: 4 studies.)
Benefit:
Earlier diagnosis and treatment of OSA in patients with heart failure may improve cardiac function and potential impact outcomes.
Harm:
The potential inconvenience of screening and diagnosing OSA with sleep studies. Inconvenience and noncompliance of potential therapy.
Cost:
Low to moderate cost associated with screening and testing for OSA in patients with HF.
Benefits-harm assessment:
Benefits of earlier diagnosis and management of OSA in those with HF outweighs the harms of screening and testing for OSA.
Value judgments:
Diagnosis and subsequent treatment of OSA in patients with HF improve OSA symptoms and may improve cardiac function. The potential benefits of diagnosis outweigh the low risks of harm.
Recommendation level:
Recommendation.
Intervention:
Patients with heart failure should be screened and evaluated for OSA.
Evaluation of OSA in Patients with Cerebrovascular Disease.
Aggregate quality of evidence:
C (Level 2a: 10 studies; Level 2b: 1 study; Level 3b: 1 study).
Benefit:
Earlier diagnosis of OSA may improve overall health and cognitive status and reduce the risk of incident and recurrent stroke and stroke mortality. Patients with stroke have a high pre-test probability of OSA.
Harm:
Inconvenience of evaluation and sleep testing for OSA diagnosis.
Cost:
Low cost associated with evaluation and testing patients for OSA.
Benefits-harm assessment:
Moderately favors screening for OSA to reduce incidence of stroke and recurrence of stroke in patients presenting with stroke.
Value judgments:
The overall quality of evidence favoring diagnosis of OSA to prevent incident and recurrent stroke and stroke mortality is modest.
Recommendation level:
Recommendation.
Intervention:
Recommend patients presenting with TIA, ischemic, or hemorrhagic stroke undergo clinical evaluation for OSA.
Association between OSA and Pulmonary Hypertension.
Aggregate grade of evidence:
C (Level 2a: 2 studies; Level 2b: 4 studies).
Evaluation of OSA in Patients with COPD.
Aggregate quality of evidence:
B (Level 2A: 1 study; Level 2b: 7 studies; Level 4: 2 studies).
Benefit:
Patients with comorbid OSA and COPD are at risk for increased morbidity and mortality compared with either disease alone.
Harm:
There is minimal harm to screening for OSA other than the inconvenience of testing.
Cost:
There are low to moderate costs to testing for OSA.
Benefits-harm assessment:
The benefits of OSA evaluation of patients with comorbid COPD outweigh the harm.
Value judgments:
There is overwhelming evidence about the worsened clinical outcomes in patients with both COPD and OSA compared to either COPD or OSA alone.
Recommendation level:
Recommendation
Intervention:
Recommend evaluation for OSA in patients with COPD.
Evaluation of OSA in Patients with Cognitive Impairment.
Aggregate quality of evidence:
C (Level 2a: 9 studies; Level 3a: 7 studies).
Benefit:
Evaluation and treatment of OSA has the potential to improve select domains of cognitive function and may slow the incidence of cognitive decline.
Harm:
The potential harms of OSA screening and testing are low.
Cost:
There are low to moderate costs associated with sleep study testing.
Benefits-harm assessment:
The benefits of diagnosis and treatment for OSA outweigh the potential harm.
Value judgments:
Evidence suggests that OSA is associated with neurocognitive impairment and decline. Most evidence suggests that OSA may represent a modifiable risk factor for dementia, but conflicting and inconsistent data exist.
Recommendation level:
Recommendation.
Intervention:
Recommend evaluation and testing for OSA in those at high risk for cognitive decline or impairment.
Association between Allergic Rhinitis and OSA.
Aggregate grade of evidence:
B (Level 1b: 3 studies; Level 2b: 1 study).
Association between Nasal Obstruction and OSA.
Aggregate grade of evidence:
B (Level 1b: 3 studies; Level 2b: 1 study).
Association between Chronic Rhinosinusitis and OSA.
Aggregate grade of evidence:
C (Level 2a: 8 studies; Level 2b: 5 studies; Level 2c: 1 study).
Association between Gerd and OSA.
Aggregate grade of evidence:
B (Level 1b: 2 studies; Level 2b: 7 studies; Level 2c: 1 study).
Gerd Treatment with PPI Improves Snoring and Sleep Symptoms, not OSA Severity.
Aggregate grade of evidence:
C (Level 1b: 1 study; Level 2a: 1 study).
OSA Treatment Improves Gerd Symptoms.
Aggregate grade of evidence:
C (Level 1b: 1 study; Level 2b: 1 study).
Association between OSA and Insomnia.
Aggregate grade of evidence:
C (Level 2: 3 studies; Level 4: 12 studies).
Association between Rem Sleep Behavior Disorder and OSA.
Aggregate grade of evidence:
C (Level 3b: 4 studies; Level 4: 2 studies).
Association between RLS and OSA.
Aggregate grade of evidence:
C (Level 3b: 1 study; Level 4: 2 studies).
Association between PLMS/PLMD and OSA.
Aggregate grade of evidence:
C (Level 2b: 1 study; Level 3b: 8 studies; Level 4: 2 studies).
Association between OSA and Narcolepsy.
Aggregate grade of evidence:
B (Level 2b: 1 study; Level 2c: 3 studies; Level 3b: 4 studies).
CPAP for OSA.
Aggregate quality of evidence:
A (Level 1a: 7 studies; Level 1b: 5 studies; Level 2a: 3 studies).
Benefit:
CPAP reduces disease severity and sleepiness and likely has beneficial effects on blood pressure, quality of life, and cognitive impairment.
Harm:
Inconvenience and minor discomforts associated with PAP therapy. Variable adherence levels.
Cost:
Low cost associated with CPAP device, replacement supplies, and follow-up visits. Direct cost to patients depends on insurance coverage.
Benefits-harm assessment:
CPAP therapy for the treatment of OSA is associated with greater benefit than harm.
Value judgments:
CPAP therapy is effective for the treatment for obstructive sleep apnea and improves multiple factors related to sleep and daytime functioning.
Recommendation level:
Strong recommendation.
Intervention:
CPAP therapy should be employed for treatment of OSA.
APAP for OSA.
Aggregate quality of evidence:
B (Level 1a: 2 studies; Level 1b: 4 studies).
Benefit:
Reduction of sleep apnea severity, sleepiness, and improvement in measures of quality of life.
Harm:
Inconvenience and minor discomforts associated with PAP therapy. Similar to CPAP.
Cost:
Low cost associated with APAP device, replacement supplies, and follow-up visits. Direct cost to patients depends on insurance coverage.
Benefits-harm assessment:
APAP therapy for the treatment of OSA is associated with greater benefit than harm.
Value judgments:
APAP therapy is effective for the treatment for obstructive sleep apnea and improves multiple factors related to sleep and daytime functioning. For appropriately selected patients newly diagnosed with OSA, APAP can be initiated in lieu of in-lab titration of PAP therapy with consideration for patient value preferences and resource utilization.
Recommendation level:
Recommendation
Intervention:
APAP may be employed for treatment of OSA.
Use of Bilevel PAP for OSA.
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 2 studies).
Benefit:
BPAP may be beneficial for patients who require high treatment pressure and those who are unable to tolerate CPAP at high pressure settings.
Harm:
Studies show no difference in PAP adherence with BPAP compared with CPAP. Side effects are similar to CPAP.
Cost:
BPAP devices are associated with higher costs compared with CPAP devices.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Studies show no significant difference between BPAP, CPAP, and APAP for the improvement in OSA severity, sleepiness, and quality of life. BPAP also confers no significant advantage over CPAP or APAP in improved adherence, except as a potential therapy option for patients nonadherent to CPAP.
Recommendation level:
Option
Intervention:
BPAP should not be used over CPAP for initial treatment of routine OSA. BPAP is an option when the treatment pressure requirements are greater than can be delivered via CPAP or when a patient is unable to tolerate CPAP at high pressure settings.
Nasal Mask Interfaces for PAP Therapy.
Aggregate quality of evidence:
B (Level 1a: 2 studies; Level 1b: 1 study; Level 2b: 12 studies; Level 4: 3 studies).
Benefit:
Better control of OSA, better adherence to CPAP therapy, less sleepiness associated with use of nasal mask and nasal pillow interfaces compared to oronasal masks.
Harm:
Lower rates of CPAP adherence among oronasal mask users may be associated with greater likelihood of OSA treatment abandonment. Not all users can tolerate nasal masks.
Cost:
Low1076 cost differences. Cost of different types of masks are not substantively different.
Benefits-harm assessment:
Preponderance of benefit of nasal interfaces over harm, given consistent evidence from small RCTs and observational studies.
Value judgments:
The preponderance of evidence favors the initial use of nasal interfaces (nasal masks or nasal pillows) over oronasal masks. Oronasal and oral masks may be appropriate for a select group of patients who are intolerant to nasal masks, or who have large air leaks during sleep due to mouth opening, but these studies have not been performed.
Recommendation level:
Recommendation.
Intervention:
In general, nasal interfaces should be utilized initially in patients naïve to CPAP therapy.
Use of Objective Measures of PAP Adherence.
Aggregate quality of evidence:
B (Level 2b: 7 studies).
Benefit:
Objective PAP adherence provides a direct measure of PAP usage times in order to understand and improve PAP adherence with interventions.
Harm:
Harm associated with PAP use and monitoring is low. In some, adherence data has implications for PAP payment and coverage.
Cost:
Minimal additional financial cost – objective measures are incorporated into most PAP machines.
Benefits-harm assessment:
Benefit outweighs harm.
Value judgments:
PAP adherence levels are important to measure and follow. The current definitions of adherence may not be optimally matched to outcomes.
Recommendation level:
Recommendation.
Intervention:
Recommend objective measurement of PAP use over subjective queries.
Predictors of PAP Adherence.
Aggregate grade of evidence:
C (Level 2b: 12 studies; Level 4: 10 studies).
Educational Interventions to Improve PAP Adherence.
Aggregate quality of evidence:
A (Level 1a: 2; Level 1b: 7).
Benefit:
Increased CPAP adherence in analysis of systematic reviews.
Harm:
Minimal, time required for education.
Cost:
Low to moderate; variable costs of different educational interventions.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
Educational therapies are overall low-cost, time-limited interventions that can be used to potentially increase adherence in certain CPAP users; however, variation in educational therapies across trials limits specific recommendations for optimal type of education.
Recommendation level:
Recommendation.
Intervention:
Recommend patient education to increase PAP adherence.
Supportive Interventions to Improve PAP Adherence.
Aggregate quality of evidence:
A (Level 1a: 1; Level 1b: 13).
Benefit:
Increased CPAP adherence.
Harm:
Minimal.
Cost:
Moderate to potentially high depending on type of supportive intervention and resources required.
Benefits-harm assessment:
Benefit outweighs the harm.
Value judgments:
Supportive interventions provide additional encouragement and reinforcement through various strategies that may increase CPAP usage by a modest amount. Supportive mechanisms are variable between studies and consideration of costs and resources required for support platforms should be assessed.
Recommendation level:
Strong Recommendation
Intervention:
Supportive interventions may be used to increase CPAP adherence if resources are available.
Behavioral Interventions to Improve PAP Adherence.
Aggregate quality of evidence:
A (Level 1a: 2; Level 1b: 6).
Benefit:
Behavioral interventions lead to increased CPAP adherence.
Harm:
Minimal, time required for intervention.
Cost:
Moderate to high; variable costs of different behavioral interventions including time, resources, and healthcare personnel. Interventions may require development of infrastructure.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Behavioral interventions utilize different psychotherapeutic techniques derived from various models of health behavior change which have a positive impact on CPAP adherence. Populations that benefit most from behavioral interventions are not well understood and interventions may be burdensome for patients and resource intensive.
Recommendation level:
Option.
Intervention:
Consideration should be given to using behavioral therapies to increase adherence in CPAP users; however, it is unclear which group of OSA patients will most benefit from behavioral therapies.
Management of Aerophagia for PAP Adherence.
Aggregate grade of evidence:
B (Level 1b: 1).
ClaustrophobiaManagement to Improve PAP Adherence.
Aggregate quality of evidence:
D (Level 4: 3 studies).
Benefit:
Unclear. Reducing claustrophobic tendencies may lead to a potential increase in CPAP use.
Harm:
Time and resources needed to attend therapy sessions.
Cost:
Cost of therapy sessions may have out of pocket fees.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Exposure or desensitization therapy could be used to reduce claustrophobic symptoms associated with PAP, but further studies are needed.
Recommendation level:
No recommendation.
Intervention:
No specific recommendation can be made about the use of exposure or desensitization therapy to treat claustrophobic tendencies to optimize PAP use.
Humidification to Improve PAP Adherence.
Aggregate quality of evidence:
B (Level 1a: 3; Level 2b: 2; Level 3b: 1).
Benefit:
Potential benefit in reduction of PAPrelated side effects. Three systematic reviews showed no clinical improvement in adherence or symptom scores, while one cohort study showed greater adherence with humidification.
Harm:
Possibility for excess condensation of water into the PAP circuit or into the user’s face, nose, or mouth. Inconvenience of purchasing additional supplies and cleaning the humidification system.
Cost:
Low cost of humidification system and supplies.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Although the evidence was inconclusive, humidification could be appropriate for some patients to consider based on their preference and symptoms.
Recommendation level:
Option.
Intervention:
Heated humidification may be considered in select OSA patients that report nasal congestion, dry nose or mouth, and sore throat.
Nasal Obstruction Limits PAP Adherence.
Aggregate grade of evidence:
C (Level 2b: 4 studies).
Treatment of Nasal Obstruction to Improve PAP Adherence.
Aggregate quality of evidence:
C (Level 1a: 1 study; Level 1b: 2 studies; Level 2b: 4 studies; Level 2c: 1 study; Level 3a: 1 study).
Benefit:
Objective and subjective nasal obstruction is associated with lower PAP adherence. Treating nasal obstruction either medically or surgically may improve PAP adherence.
Harm:
Complications of individual management options (i.e., oronasal mask discomfort, nasal sprays side effects/inconvenience of use, turbinoplasty or septoplasty surgery risks, and complications).
Cost:
Ranges from low cost (humidification, nasal spray) to high cost (surgery).
Benefits-harm assessment:
In patients with symptomatic nasal obstruction, there is a preponderance of benefit over harm for medical nasal therapy; balance of benefit and harm for surgical nasal therapy for improving PAP adherence.
Value judgments:
Treating nasal obstruction can promote PAP adherence and optimize OSA therapy outcomes.
Recommendation level:
Nasal obstruction treatment with topical medical therapies to improve PAP adherence: Recommendation.
Surgery for nasal obstruction to improve PAP adherence: Option.
Intervention:
Nasal obstruction treatment with topical medical therapies can improve PAP adherence. Surgery for nasal obstruction is an option to improve PAP adherence.
Treatment of TECSA.
Aggregate quality of evidence:
B (Level 1b: 1 study; Level 1c: 2 studies; Level 3b: 5 studies; Level 4: 2 studies).
Benefit:
ASV improves AHI more than CPAP in patients with TECSA.
Harm:
ASV is currently contraindicated for those with central sleep apnea related to HFrEF (LVEF < 45%).
Cost:
Higher costs associated with ASV compared to CPAP.
Benefits-harm assessment:
Benefits exceed harm in patients without HFrEF.
Value judgments:
In the majority of cases, TECSA resolves spontaneously with CPAP use. The evidence compares ASV to CPAP at time of diagnosis in most studies, and includes both patients with persistent-TECSA and those likely to improve on CPAP alone.
Recommendation level:
Recommendation
Intervention:
ASV can be used if TECSA is severe or if TECSA persists with CPAP use.
Insomnia Treatment to Improve PAP Adherence.
Aggregate quality of evidence:
B (Level 1b: 5 studies).
Benefit:
Treatment of adult patients with insomnia/insomnia symptoms and OSA via CBTi delivered by trained therapists may improve insomnia symptoms and increase use of PAP therapy.
Harm:
Low harms associated with CBTi including time required for therapy and risk for persistent symptoms.
Cost:
There are no published data on costs of CBTi in patients with OSA. May depend on insurance policy coverage.
Benefits-harm assessment:
Benefit outweighs harm.
Value judgments:
Small number of studies; no studies of pharmacotherapy for insomnia in patients with OSA.
Recommendation level:
Recommendation
Intervention:
Patients with insomnia and OSA should be offered CBTi for improvement in insomnia symptoms and PAP adherence.
PAP to Improve Daytime Sleepiness.
Aggregate quality of evidence:
A (Level 1: 3 studies).
Benefit:
Treatment with CPAP leads to modest reductions in the ESS and improved performance on MWT or OSLER.
Harm:
The potential harms of CPAP are inconvenience and therapy-related side effects (including, but not limited to, sleep disruption, airway dryness, nasal congestion, and aerophagia).
Cost:
CPAP treatment for OSA is associated with a low-to-moderate cost for CPAP equipment and visits for maintenance.
Benefits-harm assessment:
The benefits of PAP therapy for reducing daytime sleepiness outweigh the harms associated with PAP.
Value judgments:
Successful treatment of OSA with CPAP leads to improved daytime alertness, with improvements in subjective sleepiness and objective wakefulness.
Recommendation level:
Strong recommendation.
Intervention:
Patients with OSA and daytime sleepiness should be offered PAP therapy as it can effectively reduce daytime sleepiness related to OSA.
PAP for OSA Severity.
Aggregate quality of evidence:
A (Level 1a: 3 studies).
Benefit:
Treatment with CPAP improves AHI compared to placebo tablet, no intervention, conservative management, positional therapy, sham CPAP, sham surgery, or nasal dilator strips.
Harm:
The potential harms of CPAP are inconvenience and therapy-related side effects (including, but not limited to, sleep disruption, airway dryness, nasal congestion, and aerophagia).
Cost:
CPAP treatment for OSA is associated with a low-to-moderate costs for CPAP equipment and visits for maintenance.
Benefits-harm assessment:
The benefits of PAP therapy on reducing the AHI outweigh the harms associated with CPAP.
Value judgments:
There is strong evidence that treatment of OSA with CPAP leads to reductions in AHI.
Recommendation level:
Strong recommendation.
Intervention:
Patients should be offered PAP therapy to reduce OSA severity. There are greater reductions in AHI in patients with severe OSA.
PAP Therapy and Mortality Risk.
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 2 studies; Level 2a: 1 study).
Benefit:
Treatment with CPAP is not consistently associated with reduction in cardiovascular and all-cause mortality in available studies, although CPAP adherence was variable and severe OSA and excessively sleepy patients were excluded in the RCT studies. Modest reductions in mortality have been demonstrated in non-randomized studies.
Harm:
The potential harms of CPAP are inconvenience and therapy-related side effects (including, but not limited to, sleep disruption, airway dryness, nasal congestion, and aerophagia).
Cost:
Low costs for PAP equipment and visits for maintenance.
Benefits-harm assessment:
Based on the best available evidence, benefits are unclear and harms are minimal for PAP therapy in reducing cardiovascular and all-cause mortality.
Value judgments:
Successful treatment of OSA with CPAP may lead to reduced cardiovascular and all-cause mortality. Observational cohort studies support the use of PAP to reduce mortality, although the RCT data has not shown this benefit.
Recommendation level:
Option.
Intervention:
PAP therapy may be considered for OSA patients as an option to reduce mortality. There is potential for the reduction in cardiovascular and all-cause mortality with PAP therapy in patients with severe OSA, excessive daytime sleepiness, and adherent PAP use.
PAP Therapy and Mortality Risk.
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 2 studies; Level 2a: 1 study).
Benefit:
Treatment with CPAP is not consistently associated with reduction in cardiovascular and all-cause mortality in available studies, although CPAP adherence was variable and severe OSA and excessively sleepy patients were excluded in the RCT studies. Modest reductions in mortality have been demonstrated in non-randomized studies.
Harm:
The potential harms of CPAP are inconvenience and therapy-related side effects (including, but not limited to, sleep disruption, airway dryness, nasal congestion, and aerophagia).
Cost:
Low costs for PAP equipment and visits for maintenance.
Benefits-harm assessment:
Based on the best available evidence, benefits are unclear and harms are minimal for PAP therapy in reducing cardiovascular and all-cause mortality.
Value judgments:
Successful treatment of OSA with CPAP may lead to reduced cardiovascular and all-cause mortality. Observational cohort studies support the use of PAP to reduce mortality, although the RCT data has not shown this benefit.
Recommendation level:
Option.
Intervention:
PAP therapy may be considered for OSA patients as an option to reduce mortality. There is potential for the reduction in cardiovascular and all-cause mortality with PAP therapy in patients with severe OSA, excessive daytime sleepiness, and adherent PAP use.
PAP for Hypertension Control.
Aggregate quality of evidence:
A (Level 1a: metaanalyses including 54 RCTs).
Benefit:
Successful treatment of OSA with CPAP leads to a modest reduction BP in subjects with HTN, especially in those with severe OSA. Antihypertensive medications will likely still be required. The reduction in BP may be greater in subjects with resistant HTN and those who report daytime sleepiness.
Harm:
The harms associated with treatment of OSA with CPAP therapy are minimal.
Cost:
Low cost involved with CPAP equipment and visits for maintenance.
Benefits-harm assessment:
The benefits of treating patients with moderate to severe OSA with PAP to reduce BP outweigh risks of treatment.
Value judgments:
Successful treatment of OSA with CPAP leads to reduction in BP. Antihypertensive medications will likely still be required.
Recommendation level:
Strong recommendation.
Intervention:
Patients with OSA, particularly those with moderate to severe OSA and/or resistant hypertension, should be offered CPAP to help decrease BP.
PAP for Control of Atrial Fibrillation.
Aggregate quality of evidence:
C (Level 1b: 1 study; Level 2b: 6 studies; Level 4: 4 studies).
Benefit:
Treatment of OSA with PAP therapy may reduce the recurrence of atrial fibrillation following restoration of sinus rhythm with electrical cardioversion or catheter ablation procedures. Based on a single RCT, there is no evidence that CPAP reduces AF burden in patients with paroxysmal AF who have not undergone cardioversion or ablation procedures. The impact of OSA treatments other than PAP on risk of recurrent AF has not been reported.
Harm:
Inconvenience and side effects of CPAP therapy.
Cost:
Cost of treating patients for OSA.
Benefits-harm assessment:
The harm of OSA diagnosis and treatment in patients with AF are low; however, the evidence for treatment to reduce AF recurrence is mixed. Balance of benefit and harm.
Value judgments:
The overall quality of evidence favoring diagnosis and treatment of OSA solely for the prevention of recurrent atrial fibrillation without cardioversion or ablation is generally weak, with no adequately powered randomized clinical trials. Patients with paroxysmal AF did not demonstrate AF burden reduction with CPAP therapy.
Recommendation level:
Option.
Intervention:
Patients who are undergoing cardioversion or catheter ablation for atrial fibrillation may benefit from adjunctive treatment of OSA with CPAP to prevent AF recurrence. Shared decision-making regarding CPAP treatment is suggested to weigh individual patient symptoms, preferences, and comorbidities.
PAP for OSA after CVA.
Aggregate quality of evidence:
B (Level 1b: 9 studies; Level 2a: 2 studies; Level 2b: 2 studies).
Benefit:
Treatment of OSA with CPAP may improve overall health and cognitive status and reduce the risk of recurrent stroke and stroke mortality.
Harm:
Side effects and inconvenience of CPAP therapy.
Cost:
Low cost associated with PAP therapy and maintenance.
Benefits-harm assessment:
Preponderance of benefit over harm for the use of CPAP in stroke patients.
Value judgments:
The overall evidence favors diagnosis and treatment of OSA to improve neurologic recovery and functional status after stroke. Treatment may also prevent recurrent stroke and reduce risk of stroke mortality, although the evidence is mixed.
Recommendation level:
Recommendation
Intervention:
Patients may benefit from OSA treatment with CPAP within the acute stroke phase after a TIA, ischemic, or hemorrhagic stroke.
PAP for Improving Clinical Outcomes in Heart Failure with Reduced Ejection Fraction (HFrEF).
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 7 studies; Level 2b: 5 studies; Level 3b: 2 studies).
Benefit:
Treatment of OSA with CPAP in HFrEF results in direct amelioration of systolic function, stroke volume, and daytime sleepiness, as well as systolic blood pressure, heart rate, daytime sympathetic nerve activity, and improved heart rate variability.
Harm:
Inconvenience of use. Evidence for CPAP and reduction in mortality and event free survival is limited.
Cost:
Low to moderate costs are associated with PAP machine and supplies. There may be additional costs related to provider visits to monitor for treatment efficacy and adherence.
Benefits-harm assessment:
There may be a benefit conferred by the use of CPAP to treat patients with moderate-to-severe OSA and HFrEF.
Value judgments:
Treatment of OSA with CPAP in HFrEF is successful in improving LVEF, daytime sleepiness, and other factors that influence heart failure management. There is little high-quality evidence to support or dispute the treatment of moderate-to-severe OSA in patients with HFrEF to reduce mortality. Balanced with low risk of harm, it may be appropriate to offer patients with moderate-to-severe OSA and concomitant HFrEF treatment with CPAP.
Recommendation level:
Option.
Intervention:
Patients with HFrEF and symptomatic OSA may benefit from CPAP therapy.
PAP for Improving Clinical Outcomes in Heart Failure with Preserved Ejection Fraction (HFpEF).
Aggregate quality of evidence:
C (Level 1b: 3 studies; Level 2b: 5 studies).
Benefit:
Treatment of OSA with CPAP in HFpEF resulted in improvement in LV diastolic function and reduced LV and intraventricular septal thickness.
Harm:
Inconvenience of use associated with CPAP.
Cost:
Low costs are associated with PAP machine and supplies. There may be additional costs related to provider visits to monitor for treatment efficacy and adherence.
Benefits-harm assessment:
Benefits of PAP therapy outweighs the harms in this population.
Value judgments:
Treatment of OSA in HFpEF results in improved diastolic dysfunction and its surrogates such as IV septum and LV thickness. Evidence on impact of CPAP on event-free survival is limited.
Recommendation level:
Option.
Intervention:
Patients with HFpEF and OSA may benefit from CPAP therapy.
PAP for Cardiovascular Event Risk Reduction in Coronary Artery Disease.
Aggregate quality of evidence:
B (Level 1a: 2 studies; Level 1b: 4 studies; Level 2a: 2 studies; Level 2b: 5 studies) mix of positive and negative findings.
Benefit:
PAP therapy in patients with OSA reduced fatal and non-fatal cardiac events in cohort studies. However, RCT studies did not uniformly show this benefit. CV risk reduction may be related to PAP adherence levels on secondary analyses.
Harm:
Minor effects associated with discomfort and inconvenience of PAP. Variable adherence rates on PAP.
Cost:
Low costs are associated with PAP machine and supplies and provider visits to monitor for treatment efficacy and adherence.
Benefits-harm assessment:
Low harm associated with OSA treatment with CPAP. The evidence for benefit is mixed. Balance of benefit and harm.
Value judgments:
There is moderate quality evidence that OSA leads to cardiovascular disease, but low-quality evidence that treatment with CPAP reduces this risk. RCT trial design may underestimate the effects of CPAP.
Recommendation level:
Option.
Intervention:
CPAP use in patients with CAD does not clearly reduce CV risk in all patients. Further studies are required to identify the best candidates for improved outcomes. Shared decision-making regarding CPAP treatment is suggested to weigh individual patient symptoms, preferences, and comorbidities.
PAP to Improve Cognitive Measures.
Aggregate quality of evidence:
Cognitively intact patients:
B (Level 1a: 2 studies; Level 1b: 2 studies; Level 2a: 1 study; Level 2b: 2 studies).
Cognitively impaired patients:
C (Level 1b: 1 study; Level 2a: 1 study; Level 2b: 1 study).
Benefit:
In cognitively intact patients PAP treatment offers a small degree of benefit in a limited number of domains. In cognitively impaired patients PAP treatment may offer benefit based on a few short-term and observational studies. Effects of PAP therapy on longitudinal progression of cognitive impairment is unknown.
Harm:
Harms associated with PAP use are minimal.
Cost:
Low to moderate cost associated with CPAP treatment for OSA.
Benefits-harm assessment:
Balance of potential for benefits and the low risk of harm.
Value judgments:
Most studies are limited by short treatment periods. Research in cognitively intact patients may have ceiling effects limiting the measurable effect size. While the current evidence is limited for cognitively-impaired patients, there are few other options available that may delay cognitive decline. High-quality longitudinal studies are needed to better understand which patient populations may obtain cognitive benefits from CPAP therapy.
Recommendation level:
Option.
Intervention:
CPAP therapy may be considered in OSA patients (including those with dementia/MCI) to potentially improve select domains of cognition and reduce cognitive decline.
PAP to Improve Glycemic Control.
Aggregate quality of evidence:
B (Level 1a: 2 studies; Level 1b: 3 studies; Level 2a: 1 study; Level 2b: 3).
Benefit:
Treatment of OSA with PAP therapy may improve insulin resistance; however, systematic reviews have failed to show consistent improvements in glycemic control with PAP therapy.
Harm:
Inconvenience and side effects of CPAP therapy.
Cost:
Low cost of PAP therapy for treatment of OSA.
Benefits-harm assessment:
Balance of benefit to harm for PAP treatment in insulin resistant patients to improve glycemic control.
Value judgments:
The overall quality of evidence for the treatment of OSA with PAP therapy to improve insulin resistance is generally weak, with no adequately powered randomized clinical trials. Only one study showed significant reduction in HgA1c. It is unknown whether greater PAP adherence could lead to better glycemic control.
Recommendation level:
Option.
Intervention:
Patients with OSA and insulin resistance may benefit from adjunctive treatment of OSA with PAP to improve insulin resistance. Further studies are needed to identify the relationship between PAP therapy and glycemic control.
PAP to Reduce Motor Vehicle Accidents.
Aggregate quality of evidence:
B (Level 2a: 3 studies; Level 2b: 1 study).
Benefit:
High potential benefit exists for patients with OSA who adhere to PAP therapy and also for public health and safety, as driving accidents pose a risk to others on the road.
Harm:
Patients may have difficulty tolerating PAP therapy or experience side effects.
Cost:
Lack of insurance coverage may result in moderate financial burden for the patient. The average cost of diagnosis and treatment with CPAP was estimated at $1190 per patient per year.132
Benefits-harm assessment:
Benefits to the individual and public health clearly outweigh potential harms, which include minor adverse effects of PAP therapy and potential costs.
Value judgments:
The choice of whether or not to use PAP therapy to reduce driving related accidents must be weighed against harms to public health and safety. Those in high-risk workplace settings may be mandated by the employer to adhere to PAP therapy. The value of adherence has been shown in clinical trials to benefit both the individual, the employer and to payers who may reap reductions in overall healthcare costs and in costs related to accidents.
Recommendation level:
Recommendation.
Intervention:
PAP therapy in OSA patients is recommended to reduce motor vehicle accidents.
PAP to Improve Work Productivity.
Aggregate quality of evidence:
C (Level 2c: 5 studies).
Benefit:
Improvements in OSA-related symptoms may be related to improvements in work productivity, work-related absenteeism, mental demands, and inter-personal relationships.
Harm:
Minor risks associated with PAP therapy and inconvenience or intolerance.
Cost:
Low to moderate costs associated with PAP equipment and visits.
Benefits-harm assessment:
Balance of benefit over harm. Low risk of harm, but the evidence to show clear benefit for CPAP impact on work productivity is limited.
Value judgments:
Although the aggregate grade of evidence is low, the potential benefits of CPAP in improving work productivity through reductions in OSA-related symptoms should be greatly valued by the patient, employers, and other stakeholders that support healthcare or benefit related costs.
Recommendation level:
Option.
Intervention:
In patients with OSA and sleepiness who experience work-related absenteeism, reduced productivity, or work-related accidents, treatment of OSA with CPAP should be considered.
PAP to Improve COPD Outcomes and OSA in Overlap Syndrome.
Aggregate quality of evidence:
B (Level 2A: 1 study; Level 2b: 7 studies; Level 4: 2 studies).
Benefit:
Patients with comorbid OSA and COPD are at risk for increased morbidity and mortality. CPAP therapy seems to mitigate these risks.
Harm:
The potential harms of CPAP are inconvenience (use nightly, maintenance/cleaning) and therapy-related side effects (including but not limited to discomfort of equipment, sleep disruption, airway dryness, nasal congestion, skin abrasion, and aerophagia).
Cost:
There are low to moderate for treatment with PAP therapy, depending on the type of testing and PAP device used.
Benefits-harm assessment:
The benefits of OSA treatment of patients with comorbid COPD outweigh the harm.
Value judgments:
There is overwhelming evidence about the worsened clinical outcomes in patients with both COPD and OSA compared to either COPD or OSA alone. CPAP therapy seems to mitigate these risks, but randomized clinical trials are needed, including those that assess for benefits in the subsets of patients with OSA and COPD (e.g., those with severe daytime gas exchange abnormalities, specifically chronic hypercapnia). Studies evaluating the role of bilevel PAP in these patients are much needed as well.
Recommendation level:
Recommendation
Intervention:
Treatment with PAP therapy is recommended in patients with concomitant COPD and OSA.
Tongue Retention Devices for OSA.
Aggregate quality of evidence:
C (Level 1a: 1 study; Level 1b: 3 studies; Level 2b: 1 study; Level 3b: 6 studies; Level 4: 5 studies).
Benefit:
Improved AHI, ODI, and ESS.
Harm:
Side effects including tongue numbness, tongue pain, tooth or gum pain, and dry mouth.
Cost:
Low to moderate cost associated with device. Variable cost which is dependent on device type.
Benefits-harm assessment:
Balance of benefit over harm.
Value judgments:
TRD can be used for OSA but has suboptimal effectiveness and tolerance, and objective verification of response is recommended.
Recommendation level:
Option.
Intervention:
Tongue retention devices can be used to treat OSA, though other treatment options, including other oral appliances, demonstrate better effectiveness and tolerability.
Mandibular Repositioning Devices for OSA.
Aggregate quality of evidence:
A (Level 1a: 8 studies).
Benefit:
Improved AHI, ODI, arousal index, ESS, and blood pressure.
Harm:
Side-effects include risk for tooth movement and pain associated with use.
Cost:
Device cost is moderate and coverage varies by practice location.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
MRD is considered an effective treatment option for OSA for reducing OSA severity and improving sleep symptoms. Effectiveness of therapy is variable.
Recommendation level:
Strong recommendation.
Intervention:
MRD is an effective treatment option in selected patients with OSA.
MRD Compared to CPAP for OSA.
Aggregate quality of evidence:
A (Level 1a: 5 studies).
Benefit:
MRD and CPAP are associated with similar outcomes for quality of life improvement, improved blood pressure, and cognitive function. MRD has potential for improved compliance rates compared to CPAP.
Harm:
Inconsistent efficacy in AHI reduction with MRD relative to CPAP.
Cost:
Device cost is moderate and coverage varies by practice location.
Benefits-harm assessment:
Balance of benefit over harm.
Value judgments:
CPAP more effectively reduces OSA severity, but MRD is associated with improved compliance. Patient preference and likelihood of device use should be considered in choosing MRD versus CPAP for OSA therapy.
Recommendation level:
Option.
Intervention:
MRD is an effective alternative to CPAP in OSA.
Positional Therapy with Sleep Position Trainers for Positional OSA.
Aggregate quality of evidence:
B (Level 1: 6 studies; Level 2: 6 studies).
Benefit:
New generation sleep position trainers are effective in reducing OSA severity and symptoms in those with positional OSA.
Harm:
Serious side effects of newer generation positional devices have not been reported. Older TBT techniques are associated with back discomfort. Risk for variable efficacy in treating OSA and variable compliance. Studies are limited to date (small, short-term and examine a limited range of outcomes).
Cost:
Cost and coverage vary based on insurance.
Benefits-harm assessment:
Benefits outweigh the harms.
Value judgments:
New generation positional therapy device use for positional OSA can be effective without serious side effects. It can be used as primary treatment or in combination with other treatments and may have better adherence than classic positional therapy with TBT.
Recommendation level:
Recommendation.
Intervention:
For positional OSA, positional therapy is an effective intervention. New generation sleep position therapy may have better adherence rates than classic positional therapy (TBT).
Weight Loss for Improvement in OSA Severity.
Aggregate quality of evidence:
B (Level 2a: 2 studies; Level 2b: 9 studies).
Benefit:
Medical weight loss is effective in reducing OSA severity.
Harm:
No deaths were reported in any of the trials. There was no difference in reported adverse events.
Cost:
Cost analysis not performed. Low to moderate dependent on recommended restricted calorie plan and exercise regimen.
Benefits-harm assessment:
Preponderance of benefit over harm. Studies demonstrate that weight loss is effective at reducing the AHI.
Value judgments:
Small number of RCTs with small numbers of subjects demonstrated effectiveness of weight loss.
Recommendation level:
Recommendation.
Intervention:
Weight loss programs should be suggested in overweight or obese patients with OSA.
Medical Treatment of Nasal Obstruction and Sinusitis for OSA Severity.
Aggregate quality of evidence:
B: Level 1A (2 studies); Level 1B (13 studies); Level 3B (3 studies); Level 4 (11 studies).
Benefit:
Improvements in sleepiness and small reductions in AHI and O2 saturations.
Harm:
Risks associated with topical nasal corticosteroid use include: epistaxis, nasal discomfort/burning, dryness, crusting, foul taste, headache, and sore throat.
Cost:
Low monthly cost associated with topical nasal corticosteroid use.
Benefits-harm assessment:
Balance of benefit and harm due to mixed evidence on changes in OSA severity.
Value judgments:
Topical nasal corticosteroids are associated with improvement in nasal obstruction and sleep symptoms in allergic rhinitis patients with OSA and may produce a small change in AHI.
Recommendation level:
Topical corticosteroids:
Option.
Topical decongestants:
Recommend against.
Oral antihistamines and leukotriene inhibitors:
No recommendation due to insufficient evidence.
Intervention:
Topical nasal corticosteroids are an option for treatment of allergic rhinitis and as adjunct therapy for OSA. While adverse events and side effect profiles of topical nasal corticosteroids are minimal, the small reductions seen in objective sleep parameters suggests that they should not be used as definitive therapy for OSA.
Nasal Dilators for Treatment of OSA.
Aggregate grade of evidence:
B (Level 1a: 1 study; Level 2b: 5 studies; Level 3b: 1 study; Level 4: 7 studies).
Use of Surfactants for Treatment of OSA.
Aggregate grade of evidence:
C (Level 2b: 1 study; Level 3b: 2 studies).
Supplemental Oxygen for Treatment of OSA.
Aggregate quality of evidence:
B (Lavel 1b: 8 studies; Lavel 1a: 1 study).
Benefit:
Oxygen improves oxygen saturation in patients with OSA. May reduce blood pressure in certain patient populations receiving higher levels of oxygen supplementation.
Harm:
Risk of carbon dioxide retention and respiratory acidosis.
Cost:
Moderate to high cost of treatment with supplemental oxygen and related to increased health care utilization.
Benefits-harm assessment:
Harms exceed benefit.
Value judgments:
Oxygen therapy improves oxygen saturation in patients with OSA but does not benefit sleep quality, daytime functioning, duration, or frequency of apnea and hypopnea events. Inconsistent effects of treatment with supplemental oxygen on blood pressure are reported.
Recommendation level:
Recommend against.
Intervention:
Oxygen therapy should not be used as a treatment for OSA.
Myofunctional Therapy for OSA Treatment.
Aggregate grade of evidence:
B (Level 1: 5 studies).
Evaluation of PAP Non-Adherence for Surgical Candidacy.
Aggregate grade of evidence:
B (Level 1b: 4 studies; Level 3b: 5 studies; Level 5: 3 studies).
BMI and Candidacy for Sleep Surgery.
Aggregate quality of evidence:
C (Level 2a: 3 studies; Level 2b: 1 study; Level 3b: 3 studies; Level 4: 4 studies).
Benefit:
Improvement in sleep apnea severity and symptoms, as well as CPAP tolerance after sleep surgery.
Harm:
Higher rates of surgical failure and likelihood for persistent sleep apnea in patients who are morbidly obese. Risks specific to surgery type, increased perioperative risks related to obesity and respiratory complications, and long-term surgical complications.
Cost:
Moderate cost associated with surgery, recovery, and time off work.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Properly selected patients with appropriate anatomy can have substantial improvement in OSA severity regardless of their BMI; insufficient data exists to determine surgical success in patients with BMI > 40 kg/m2.
Recommendation level:
Option.
Intervention:
BMI level should be considered in determining surgical candidacy.
Surgery as Primary Treatment for OSA.
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 2 studies; Level 2a: 2 studies; Level 2b: 2 studies; Level 3b: 4 studies).
Benefit:
Proceeding to surgery first in appropriate patients may result in resolution of OSA and related health issues and avoids the cost and inconvenience of using CPAP long-term.
Harm:
Postoperative pain, possible surgical failure, and delay of appropriate treatment for OSA; perioperative risks and complications.
Cost:
Moderate cost of surgery and recovery compared to CPAP device costs.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Certain patients with appropriate anatomic parameters may benefit from proceeding directly to surgery rather than attempting CPAP first. However, conservative medical treatment should still be the default given the lack of strong evidence favoring surgery over CPAP and low risk related to CPAP use.
Recommendation level:
Option.
Intervention:
Primary surgery for OSA may be considered in specific patients with appropriate anatomic features such as tonsil hypertrophy or craniofacial abnormalities. In this group, shared decision-making regarding the risks, benefits, and tradeoffs of primary surgery versus CPAP treatment is suggested to weigh individual patient symptoms, preferences, and comorbidities.
OSA and Difficult Airway Management.
Aggregate quality of evidence:
B (Level 2a: 1 study; Level 2b: 3 studies; Level 3a: 1 study; Level 3b: 3 studies).
Benefit:
Reduce rates of respiratory compromise and intraoperative loss of airway.
Harm:
Increased time and potential increased cost of additional equipment necessary for intubation.
Cost:
Low, dependent on necessary time and equipment for securing the airway.
Benefits-harm assessment:
Benefits outweigh harm for the consideration of difficult airway management plans in patients undergoing surgery for OSA.
Value judgments:
Overwhelming consistent evidence from observational studies show an association between OSA and difficult perioperative airway management.
Recommendation level:
Recommendation.
Intervention:
Contingency plans for difficult airway management, such as those provided by the ASA,1520 should be implemented in OSA patients undergoing surgery.
Perioperative Sedatives and Opiates and Acute Upper Airway Obstruction in OSA.
Aggregate quality of evidence:
C (Level 2b: 1 study, 1 case report).
Benefit:
Reduced risk of oversedation and respiratory depression.
Harm:
Inadequate anesthesia or patient discomfort/anxiety in the perioperative period.
Cost:
Decreased cost associated with judicious use of pharmacologic agents.
Benefits-harm assessment:
Benefits outweigh harm as judicious use of sedating medications preserves airway patency in OSA patients.
Value judgments:
Observational studies show an association between increased opioid use and airway instability in the perioperative period.
Recommendation level:
Recommendation.
Intervention:
Sedating and opiate medications should be used cautiously in the preoperative and intraoperative settings for patients with OSA undergoing upper airway surgery.
Steroids for Airway Edema Management after Upper Airway Surgery.
Aggregate quality of evidence:
D (Expert opinion).
Benefit:
Reduced upper airway edema in the postoperative period with systemic steroid use.
Harm:
Adverse effects of acute systemic steroid administration including hyperglycemia, hypertension, acid reflux, immune suppression, and restlessness.
Cost:
Increased cost of medication.
Benefits-harm assessment:
Theoretical benefit and possible harm associated with systemic steroid use.
Value judgments:
Little evidence and reasoning from first principles suggest that upper airway edema occurs after soft tissue and MMA surgeries in the immediate postoperative period, which could potentiate increased airway obstruction and respiratory failure.
Recommendation level:
Option
Intervention:
Systemic steroid administration in the perioperative period can be considered to reduce upper airway edema unless contraindicated. Optimal dosage based on expert opinion includes once preoperatively and several doses postoperatively.
Perioperative CPAP use and Sleep Surgery.
Aggregate quality of evidence:
B (Level 1b: 2 studies).
Benefit:
Reduced postoperative cardiopulmonary complications with decreased likelihood of need for intensive monitored care with the use of perioperative CPAP.
Harm:
Rare procedure risk of subcutaneous, orbital, and/or intracranial emphysema in postoperative patients related to sinus and skull base surgery.
Cost:
Low, dependent on length and type of PAP therapy prescribed.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
A limited number of studies show benefit of perioperative CPAP in patients with OSA undergoing surgery. Limited evidence for PAP management after upper airway surgery. Limited evidence for PAP management after upper airway surgery is predominantly based on expert opinion.
Recommendation level:
Option.
Intervention:
Patients with OSA already receiving PAP should continue therapy preoperatively unless otherwise contraindicated or not tolerated. PAP therapy can be considered on a case-by-case basis postoperatively depending on the severity of OSA, nature of the surgery, and tolerance.
Postoperative Disposition after Sleep Surgery.
Aggregate grade of evidence:
C (1 Level 1b; 12 Level 2b studies).
Opioids for Pain Control after Sleep Surgery.
Aggregate quality of evidence:
B (Level 1a: 4 studies; Level 1b: 5 studies).
Benefit:
The use of non-opioid analgesic interventions reduces opioid usage and postoperative respiratory complications after sleep surgery.
Harm:
Risk of inadequate analgesia and consequent effect on quality of life and hemodynamic instability.
Cost:
Variable, dependent on medication selection, and dosage.
Benefits-harm assessment:
Slight benefit of opioid avoidance as oversedation can lead to dangerous postoperative complications however this is not well-studied.
Value judgments:
OSA patients are susceptible to respiratory obstruction in the postoperative period. Therefore, opioid-sparing pain management techniques are desirable but not wellestablished in existing literature.
Recommendation level:
Option.
Intervention:
Opioids should be used judiciously in the postoperative pain regimen of OSA patients. When intravenous and high dose opioids are used, consider pulse-oximetry monitoring in the immediate postoperative period. Multimodal analgesic regimens, including local anesthetic infiltration, non-steroid anti-inflammatory drugs, acetaminophen, and dexamethasone, should be considered to achieve adequate analgesia and reduce opioid requirements.
Physical Examination for Level of Obstruction Assessment and Surgical Planning.
Aggregate grade of evidence:
C (Level 2b: 5 studies; Level 2c: 3 studies; Level 3a: 4 studies; Level 4: 4 studies; Level 5: 5 studies).
DISE Findings Associated with Surgical Outcomes.
Aggregate quality of evidence:
C (Level 2b: 2 studies; Level 4: 6 studies).
Benefit:
Selected DISE findings are associated with outcomes of upper airway surgery and hypoglossal nerve stimulation. Other DISE findings are not clearly associated with outcomes.
Harm:
Additional risk of anesthesia if done as a stand-alone procedure; potential but small increase in anesthetic risk if performed at the same time as a planned surgery. The use of DISE does not always lead to improved surgical outcomes.
Cost:
Increased time and effort to perform DISE before surgery.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Recent multicenter studies have identified specific DISE findings associated with outcomes of upper airway surgery and hypoglossal nerve stimulation. Additional research can evaluate whether newer palate surgery techniques can address potential limitations of upper airway surgery, such as oropharyngeal lateral wall-related obstruction.
Recommendation level:
Option.
Intervention:
DISE can be used to assess the upper airway for areas of collapse and can guide surgical intervention in selected cases.
DISE to Improve Treatment Outcomes.
Aggregate grade of evidence:
C (Level 2b: 1 study; Level 4: 4 studies).
Sinus Surgery for OSA Treatment.
Aggregate quality of evidence:
C (Level 3a: 1 study; Level 4: 6 studies).
Benefit:
Possible small decrease in AHI and improvement in sleep symptoms with sinus surgery.
Harm:
Risks of endoscopic sinus surgery including pain, bleeding, infection, orbital injury, cerebrospinal fluid leak, etc. Continued need for adjunctive OSA therapies.
Cost:
Moderate to high costs associated with sinus surgery and indirect costs of time off work and recovery period.
Benefits-harm assessment:
Preponderance of harm over benefit.
Value judgments:
While sinus surgery has been shown to improve sleep symptoms in chronic rhinosinusitis patients, sinus surgery alone should not be performed to significantly improve or cure sleep apnea.
Recommendation level:
Recommendation against.
Intervention:
While endoscopic sinus surgery may improve subjective sleep quality in patients with chronic rhinosinusitis and concurrent SDB, SDB alone is not an indication to perform sinus surgery.
Nasal Surgery for OSA.
Aggregate quality of evidence:
Grade B (Level 1b: 1 study; Level 2a: 3 studies; Level 2b: 3 studies; Level 4: 3 studies).
Benefit:
Nasal surgery is associated with improved subjective nasal breathing, improved ESS, but only small improvements in AHI and RDI.
Harm:
Risks and complications related to nasal surgery; persistent sleep apnea is likely for patients with moderate to severe OSA.
Cost:
High costs for surgery and postoperative care.
Benefits-harm assessment:
Balance of benefit and harm due to the evidence showing no significant changes in AHI and improvements in sleepiness symptoms. Benefits may outweigh harm for patients with symptomatic nasal obstruction.
Value judgments:
Nasal surgery to treat nasal obstruction that is refractory to medical management can improve ESS and RDI. Nasal surgery alone should not be used to cure moderate to severe OSA.
Recommendation level:
Option.
Intervention:
Consider nasal surgery for patients with concurrent nasal obstruction and OSA for symptom improvement and PAP tolerance. Decision to treat should be based on clinical exam and goals of therapy.
Tonsillectomy for Treatment of OSA.
Aggregate quality of evidence:
C (Level 3a: 1 study; Level 3b: 6 studies; Level 4: 10 studies).
Benefit:
Palatine tonsillectomy improves the severity of OSA in patients with tonsil hypertrophy.
Harm:
Consequences of postoperative healing include pain and dysphagia and required time off work for recovery. Low risk for postoperative bleeding requiring treatment.
Cost:
Moderate cost of surgical treatment and postoperative care including time off work.
Benefits-harm assessment:
Benefits outweigh low risk of harm. Tonsillectomy for OSA patients with tonsil hypertrophy can improve OSA severity.
Value judgments:
Adults with tonsil hypertrophy can benefit from tonsillectomy to improve OSA severity and daytime sleepiness. Upon anatomic assessment, tonsillectomy can be combined with palatoplasty techniques, however the additional benefit of palatoplasty in those with tonsil hypertrophy requires further study.
Recommendation level:
Recommendation.
Intervention:
Assess for tonsil hypertrophy in patients with OSA and consider tonsillectomy as a treatment option for OSA especially in those with grade 3 or 4 tonsils and in those who cannot tolerate CPAP.
Uvulopalatopharyngoplasty for OSA.
Aggregate quality of evidence:
B (Level 1: 1 study; Level 2: 2 studies; Level 3: 3 studies).
Benefit:
Significant reductions in AHI, sleepiness and OSA severity are seen after UPPP. Candidates for surgery have typically demonstrated CPAP intolerance.
Harm:
Overall success rates and amount of AHI reduction after traditional UPPP are variable and based on short-term studies. Side effects of UPPP include acute postoperative pain and temporary dysphagia. Complications include postoperative bleeding, acute airway edema, nasopharyngeal stenosis, globus, and velopharyngeal insufficiency.
Cost:
Moderate cost for surgery. Indirect costs associated with time-off work after surgery.
Benefits-harm assessment:
Benefits outweigh the risks of harm in patients who cannot use CPAP.
Value judgments:
UPPP has the ability to improve OSA severity and daytime sleepiness. Surgery outcomes depend on patient selection. Modifications in UPPP surgical techniques may have lower morbidity compared to traditional UPPP.
Recommendation level:
Recommendation.
Intervention:
UPPP can be effective as a treatment for OSA in a properly selected patients who cannot tolerate CPAP therapy. Decision for surgery requires shared decision making to assess patient symptoms, clinical features of disease, and individual goals and values for therapy.
Expansion Sphincter Pharyngoplasty for OSA.
Aggregate quality of evidence:
C (Level 2: 2 studies; Level 3: 1 study; Level 4: 13 studies).
Benefit:
Reduction in AHI and OSA severity after surgery. Rate of complications may be less than traditional UPPP surgery. Small studies suggest improvements in CRP levels with effective palatoplasty surgery.
Harm:
Moderate side effects and risks include acute postoperative pain, temporary dysphagia, risk for postoperative bleeding, velopharyngeal insufficiency, globus sensation, and persistent OSA. Minimal long-term complications.
Cost:
Moderate cost for surgery. Indirect cost includes time-off of work after surgery.
Benefits-harm assessment:
Balance of benefit and harm. Potential for benefits outweigh risks for harm in patients with OSA who cannot use CPAP.
Value judgments:
ESP has the potential to significantly decrease OSA severity and improve daytime sleepiness. ESP has been shown to improve circumferential velopharyngeal collapse seen on DISE and may have lower complication rates compared to traditional UPPP. Surgery outcomes will depend on patient selection. Low quality evidence suggests outcomes may be better than traditional UPPP. Surgical approach and technique may vary by surgeon and depend on anatomic features.
Recommendation level:
Option.
Intervention:
Expansion sphincter pharyngoplasty can be offered as a surgical approach to treat velopharyngeal and oropharyngeal collapse in OSA patients who cannot use CPAP.
Lateral Pharyngoplasty for OSA.
Aggregate grade of evidence:
B (Level 1b: 2 studies; Level 2b: 5 studies; Level 3b: 1 study; Level 4: 1 study).
Transpalatal Advancement for Treatment of OSA.
Aggregate grade of evidence:
D (Level 3: 2 studies; Level 4: 2 studies).
Radiofrequency Palatoplasty for OSA Treatment.
Aggregate quality of evidence:
B (Level 1b: 4 studies; Level 2a: 1 study; Level 2b: 5 studies; Level 3a: 7 studies; Level 3b: 4 studies; Level 4: 2 studies).
Benefit:
Minimally invasive surgery that can be performed in the clinic. Reduction in snoring in the short term.
Harm:
Short-term postoperative pain with relatively low complication rates. Risks for superficial mucosal erosion, edema, oronasal fistula, and sloughing.
Cost:
Moderate cost of equipment and surgical fees which may not be covered by insurance; minimal time off of work.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
RF palatoplasty may provide short-term benefits in selected OSA patients who prefer minimally invasive treatment. Patients should be aware that improvement in sleep apnea and snoring may be limited and repeated treatments may be needed.
Recommendation level:
Option.
Intervention:
RF palatoplasty is an option with short-term efficacy for patients with primary snoring or mild OSA.
Pillar Implants for OSA Treatment.
Aggregate quality of evidence:
B (Level 1a: 1 study; Level 1b: 4 studies; Level 4: 12 studies).
Benefit:
Easy to perform in the office under local anesthesia with excellent patient tolerance and limited morbidity. Small changes in AHI, improved ESS and snoring in certain patients with snoring or mild to moderate OSA.
Harm:
Self-limited mild pain, foreign body sensation, and swallowing difficulties for 72 h, risk for ulceration, and implant extrusion.
Cost:
Moderate to high, often not covered by insurance. Minimal time off work.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Short-term improvements in symptoms and AHI in patients with BMI <32 with mild to moderate OSA. There is minimal morbidity and efficacy is variable.
Recommendation level:
Option.
Intervention:
Pillar implants can be considered in patients with primary snoring or mild to moderate OSA and suspected soft palate collapse for improvements in snoring, sleepiness, and small changes in AHI.
Palate Suture Suspension for Treatment of OSA.
Aggregate grade of evidence:
C (Level 1: 1 study; Level 3: 1 study; Level 4: 9 studies).
Tongue Base Radiofrequency for OSA Treatment.
Aggregate grade of evidence:
C (Level 3: 1 study; Level 4: 11 studies).
Genioglossus Advancement for OSA Treatment.
Aggregate grade of evidence:
C (Level 2a: 1 study; Level 4: 6 studies).
Tongue Suture Suspension for OSA Treatment.
Aggregate grade of evidence:
C (Level 1b: 1 study; Level 2a: 3 studies; Level 2b: 1 study; Level 4: 1 study).
Hyoid Suspension for Treatment of OSA.
Aggregate grade of evidence:
C (Level 2a: 3 studies; Level 2b: 1 study; Level 4: 1 study).
Lingual Tonsillectomy for Treatment of OSA.
Aggregate quality of evidence:
B (Level 2a: 2 studies).
Benefit:
Lingual tonsillectomy results in reduction of AHI, an increase in O2 saturation nadir and improved sleepiness.
Harm:
Potential complications of surgery include: bleeding, dysphagia and globus sensation, dysgeusia, tongue numbness, pain, and pharyngeal scarring.
Cost:
Moderate to high costs associated with surgery and postoperative healing time (typically 2–3 weeks of time off from work).
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
The addition of lingual tonsillectomy can provide additional benefit to palate-level surgery for select OSA patients with multilevel obstruction involving the tongue base. Patients must have clear evidence of lingual tonsil hypertrophy and associated obstruction at the tongue base. This may be assessed by indirect exam, cross-sectional imaging, or drug-induced sleep endoscopy.
Recommendation level:
Option.
Intervention:
Lingual tonsillectomy is an option for properly selected OSA patients. Specifically lingual tonsillectomy can be considered when lingual tonsillar hypertrophy causes a component of upper airway obstruction in OSA patients who cannot use CPAP.
Maxillary Expansion for OSA Treatment.
Aggregate grade of evidence:
Adult OSA:
D (Level 3: 2 studies).
Pediatric OSA:
C (Level 2: 3 studies; Level 3: 2 studies).
Maxillomandibular Advancement for OSA Treatment.
Aggregate quality of evidence:
B (Level 2a: 6 studies; Level 2b: 3 studies).
Benefit:
MMA offers significant AHI reduction and improvement in ESS.
Harm:
Serious adverse events are rare. MMA is a lengthy and technically challenging procedure with surgical risks for dental malocclusion, facial neurosensory deficits, and cosmetic changes.
Cost:
High costs related to surgery, and visits. Indirect costs: time off work, recovery time varies from 4 to 6 weeks, may need orthodontic management before and after surgery often with out-of-pocket costs.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
MMA surgery is effective for patients with moderate to severe OSA in lowering AHI. It may improve the oxyhemoglobin saturation as well as systemic blood pressure.
Recommendation level:
Recommendation.
Intervention:
MMA is an effective treatment for patients with moderate to severe OSA who have failed CPAP. It is indicated in patients with skeletal-dental abnormalities and craniofacial anomaly.
Hypoglossal Nerve Stimulation for Treatment of OSA.
Aggregate quality of evidence:
B (Level 1b: 1 study; Level 2a: 4 studies; Level 2b: 22 studies; Level 3a: 1 study; Level 3b: 7 studies; Level 4: 16 studies).
Benefit:
Improvement in objective and subjective measures of OSA.
Harm:
Surgical risks, device malfunction, potential for nonresponse requiring additional therapy, MRI limitations.
Cost:
Moderate to high. HNS may be cost effective through mitigation of OSA complications when considering patient population non-adherent to PAP, but no studies of HNS impact on long-term major adverse health consequences of OSA have been published that would permit cost-utility analysis.
Benefits-harm assessment:
Preponderance of benefit over harm
Value judgments:
HNS can significantly improve moderate to severe OSA in patients that are nonadherent to PAP. Approximately 70%–75% of patients will respond as defined by AHI (>50% AHI reduction and overall AHI < 20) and up to half may have a complete response (AHI < 5). Follow-up with full night studies or HST for long-term therapeutic AHI measures is recommended.
Recommendation level:
Recommendation.
Intervention:
HNS is an effective therapy for select patients with moderate-to-severe OSA that are intolerant of PAP and meet clinical criteria for therapy.
HNS Titration for OSA.
Aggregate grade of evidence:
D (Level 2b: 1 study; Level 4: 3 studies).
HNS Adherence.
Aggregate grade of evidence:
C (Level 2b: 1 study; Level 4: 7 studies).
Multilevel Airway Surgery for Treatment of OSA.
Aggregate quality of evidence:
B (Level 1b: 1 study; Level 2b: 30 studies; Level 3b: 2 studies; Level 4: 81 studies).
Benefit:
Effective at reducing OSA severity and improving quality of life in PAP-intolerant patients.
Harm:
Increased pain and possibly increased complications compared to single level surgery and medical treatments for OSA.
Cost:
Moderate to high costs associated with surgery and time off work for recovery.
Benefits-harm assessment:
Slight preponderance of benefit over harm.
Value judgments:
Properly selected patients and procedure combinations can result in improved OSA severity and symptoms. Data on efficacy of MLS over single-level surgery is mixed.
Recommendation level:
Option.
Intervention:
MLS may be considered in patients intolerant of PAP therapy. Patients should receive adequate counseling expectations regarding the published success rates and complications and shared-decision making to create a mutual treatment plan.
Long-Term Response to Surgery for OSA.
Aggregate grade of evidence:
C (Level 1a: 1 study; Level 2a: 2 studies; Level 2b: 3 studies; Level 3b: 2 studies). Evidence grade is based on studies with mean follow-up duration greater than 2 years.
Bariatric Surgery to Improve OSA.
Aggregate quality of evidence:
B (Level 1: 5 studies; Level 2: 9 studies).
Benefit:
OSA severity improves after bariatric surgery when there is significant weight reduction.
Harm:
Bariatric surgery is an invasive procedure with potential risks. Residual sleep disordered breathing may persist following bariatric surgery.
Cost:
High costs associated with surgery, hospitalization, and time off work for follow-up visits and recovery.
Benefits-harm assessment:
Benefits outweigh the harms.
Value judgments:
Bariatric surgery in obese patients with OSA can lead to a significant reduction in the OSA severity and other obesityrelated comorbidities. Surgery is associated with improved OSA-related symptoms and may improve candidacy for other sleep apnea surgeries if PAP cannot be tolerated. Durability of OSA outcomes requires further investigation.
Recommendation level:
Recommendation
Intervention:
Bariatric surgery is effective in reducing OSA severity in obese patients.
Surgery to Improve Sleep Related Quality of Life.
Aggregate quality of evidence:
B (Level 1a: 8 studies; Level 1b: 11 studies; Level 2a: 4 studies; Level 2b: 3 studies; Level 4: 20 studies).
Benefit:
Improved sleepiness, reduced daytime symptoms, and improved quality of life.
Harm:
Risks specific to surgery type and potential for complications.
Cost:
Moderate to high costs related to surgery and hospital care. Indirect costs: Time off work after surgery.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
Properly selected surgical interventions can lead to improvement in symptoms of SDB with low risk for harm.
Recommendation level:
Recommendation.
Intervention:
OSA surgery may be offered to patients who cannot use CPAP. Surgery has the ability to improve sleep-related and general quality of life measures in properly selected patients.
Surgery to Improve OSA Severity.
Aggregate quality of evidence:
B (Level 1a: 11 studies; Level 1b: 12 studies; Level 2a: 8 studies; Level 3a: 2 studies; Level 3b: 1 study; Level 4: 19 studies).
Benefit:
Improved sleep parameters such as the apnea-hypopnea index, oxygen desaturation index, lowest oxygen saturation has been reported after surgery. Reduced OSA severity may improve risk for OSA-associated comorbidities.
Harm:
Side effects depend on the site of surgery. Some of the more common risks include: bleeding, aspiration, airway edema, stenosis, velopharyngeal insufficiency, dysphagia, globus sensation, numbness, change in taste, numbness, damaged teeth, respiratory compromise, worsened OSA, death.
Cost:
Moderate to high costs for surgery and postoperative care. Indirect cost of time off for recovery.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
Properly selected surgical interventions for patients can lead to significant reductions in OSA severity with low risks for harm.
Recommendation level:
Recommendation.
Intervention:
Assessment and discussion of surgical candidacy should be offered to patients who cannot use CPAP. Surgery has the ability to reduce OSA severity.
Surgery for Mortality Risk Reduction.
Aggregate quality of evidence:
Grade C (Level 2b: 2 studies; Level 2c: 2 studies).
Benefit:
Potential for reduced mortality when compared to no treatment. Mortality and survival may be equivalent to provision of CPAP.
Harm:
Risks related to specific surgery types. Rare perioperative mortality risk (see Table X).
Cost:
Moderate costs related to surgery and recovery.
Benefits-Harm assessment:
Balance of benefit and harm.
Value judgments:
OSA surgery may improve mortality outcomes compared to no treatment based on retrospective cohort data.
Recommendation level:
Option.
Intervention:
Consider surgical treatment in patients with moderate to severe OSA who cannot use CPAP to improve mortality risk.
Sleep Surgery for Hypertension Management.
Aggregate quality of evidence:
C (Level 1b: 1 study; Level 4: 6 studies).
Benefit:
Reductions are seen in systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure in patients who have undergone successful sleep apnea surgery (soft tissue and MMA surgery).
Harm:
Risks specific to surgery type, potential for surgical complications, and ineffective postsurgical response.
Cost:
Moderate costs associated with surgery and time for recovery.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
OSA patients with treatment resistant hypertension and CPAP intolerance may benefit from sleep apnea surgery to lower blood pressure. The durability and long-term effects are unknown.
Recommendation level:
Option.
Intervention:
Current data suggests that when surgery is successful in treating OSA, then meaningful reductions in blood pressure may be seen. Surgery can be considered in CPAP-intolerant patients.
Surgery for Improved Cardiovascular Outcomes and Markers of Disease.
Aggregate quality of evidence:
C (Level 2a: 1 study; Level 2b: 1 study; Level 3b: 5 studies; Level 4: 7 studies).
Benefit:
Reductions in the incidence of heart failure and atrial fibrillation are seen after soft tissue surgery. Improvements in surrogate markers of cardiovascular dysfunction such as CRP, HRV, serum leptin, NOx, cholesterol, and arterial stiffness are seen after successful sleep surgery.
Harm:
Potential for surgical complications and ineffective post-surgical response.
Cost:
Moderate costs related to surgery and time for recovery.
Benefits-harm assessment:
Balanced benefit and harm.
Value judgments:
Evidence suggests successful surgery is associated with protective effects to the cardiovascular system. One large retrospective study demonstrated improvements in some cardiovascular outcomes. The durability and long-term effects are unknown.
Recommendation level:
Option.
Intervention:
Sleep apnea surgery is an option to improve markers of cardiovascular disease in those who cannot use CPAP.
Surgery to Improve Cognitive Measures.
Aggregate grade of evidence:
C (Level 2b: 1 study; Level 3b: 1 study; Level 4: 3 studies; conflicting evidence).
Surgery to Reduce Cerebrovascular Disease Risk.
Aggregate grade of evidence:
C (Level 2b: 2 studies; Level 4: 1 study).
Surgery to Improve Work Productivity.
Aggregate quality of evidence:
D (Level 2b: 1 study; Level 4: 1 study).
Benefit:
Reduced direct and indirect economic costs related to absenteeism, medical expenses, and work duty modifications. Reduced sleepiness and improved alertness may translate to better work productivity.
Harm:
Risks related to surgery and complications.
Cost:
Direct costs: Moderate costs specific to surgery and hospitalization; indirect costs: time-off work after surgery.
Benefits-harm assessment:
Balance of benefit and harm.
Value judgments:
Properly selected surgical interventions can be successful with low risk of harm. Evidence on direct relationship to workplace productivity is limited.
Recommendation level:
No recommendation.
Intervention:
Surgery may improve daytime sleepiness but the evidence demonstrating significant improvements in work productivity is limited.
Adenotonsillectomy as Treatment for Pediatric OSA.
Aggregate quality of evidence:
B (Level 1a: 2 studies; Level 1b: 1 study; Level 2b: 2 studies; Level 3a: 1 study; Level 3b: 2 studies).
Benefit:
Improved postoperative sleep parameters, symptoms, quality-of-life, and behavior.
Harm:
Risk of bleeding, possible dehydration, parental days off work for postoperative care.
Cost:
Moderate costs related to surgery and postoperative care.
Benefits-harm assessment:
Preponderance of benefit over harm.
Value judgments:
Adenotonsillectomy is associated with improved OSA severity, sleep symptoms, quality of life and behavior measures.
Recommendation level:
Recommendation.
Intervention:
Tonsillectomy with or without adenoidectomy should be offered in children with OSA.
ACKNOWLEDGMENTS
We sincerely thank Ms. Julia Martinez and Ms. Ofeibia Laud-Darku for their efforts in the preparation of this manuscript. Mr. Christopher Stave for literature search assistance. We also thank Richard Orlandi, MD and Timothy Smith, MD for their encouragement, support, and advice during development of this work.
List of abbreviations
- AAOHNS
American Academy of Otolaryngology-Head and Neck Surgery
- AAP
American Academy of Pediatrics
- AASM
American Academy of Sleep Medicine
- AD
Alzheimer’s disease
- AF
atrial fibrillation
- AHI
apnea hypopnea index
- AI
apnea index
- APAP
auto-titrating positive airway pressure
- AR
allergic rhinitis
- ARI
arousal index
- ASA
American Society of Anesthesiologists
- ASV
adaptive servo-ventilation
- ATS
American Thoracic Society
- AUC
area under the curve
- BMI
body mass index
- BP
blood pressure
- BPAP
bilevel positive airway pressure
- BUR
auto-backup rate
- BVS
bilevel ventilatory support
- BVS-S
spontaneous bilevel ventilatory support
- BVS-ST
spontaneous-timed bilevel ventilatory support
- BVS-VTPS
volume-targeted bilevel ventilatory support
- CAD
coronary artery disease
- CAHI
residual central AHI
- CBCT
cone beam computed tomography
- CBT
cognitive behavioral therapy
- CBTi
cognitive behavioral therapy for insomnia
- CCC
complete concentric collapse
- CEA
cost effectiveness analysis
- CHAT
Childhood Adenotonsillectomy Trial
- CHF
congestive heart failure
- CI
confidence interval
- CIH
cyclical intermittent hypoxia
- CIMT
carotid artery intima-media thickness
- CMS
Centers for Medicaid and Medicare Services
- CO2
carbon dioxide
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- CRP
C-reactive protein
- CRS
chronic rhinosinusitis
- CSA
central sleep apnea
- CT
computerized tomography
- CV
cardiovascular
- CVA
cerebrovascular accident
- DBP
diastolic blood pressure
- DISE
drug-induced sleep endoscopy
- DM
diabetes mellitus
- DOME
Distraction Osteogenesis Maxillary Expansion
- ECG
electrocardiogram
- EEG
electroencephalogram
- EF
ejection fraction
- EMG
electromyography
- EOG
electro-oculography
- EPAP
expiratory positive airway pressure
- ESP
expansion sphincter pharyngoplasty
- ESS
Epworth sleepiness scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- FSS
Friedman scoring system
- FTP
Friedman tongue position
- GDM
gestational diabetes mellitus
- GERD
gastroesophageal reflux disease
- GLP-1 RA
glucagon-like peptide-1 receptor agonists
- HF
heart failure
- HfpEF
heart failure with preserved ejection fraction
- HfrEF
heart failure with reduced ejection fraction
- HNS
hypoglossal nerve stimulation
- HR
hazards ratio
- HRQOL
health-related quality of life
- HRV
heart rate variability
- hs-CRP
highly sensitive C-reactive protein
- HSAT
home sleep apnea test
- HST
home sleep test
- ICU
intensive care unit
- IFL
inspiratory flow limitation
- IPS
inspiratory pressure support
- LAUP
laser-assisted uvulopalatoplasty
- LES
lower esophageal sphincter
- LOE
level of evidence
- LP
lateral pharyngoplasty
- LSAT
lowest oxygen saturation
- LVEF
left ventricular ejection fraction
- MA
meta-analysis
- MAD
mandibular advancement device
- MAP
mean arterial pressure
- MARPE
Micro-Implant Rapid Palatal Expander
- MC
Mallampati classification
- MCI
mild cognitive impairment
- MCII
multichannel intraluminal impedance
- MI
myocardial infarction
- MLS
multilevel surgery
- MMA
maxillomandibular advancement
- MP-H
mandibular plane-hyoid
- mPAP
mean pulmonary arterial pressure
- MRD
mandibular repositioning device
- MRI
magnetic resonance imaging
- MSLT
multiple sleep latency test
- MT
myofunctional therapy
- MWT
Maintenance of Wakefulness Test
- NHANES
National Health and Nutrition Examination Survey
- NO
nitric oxide
- NPV
negative predictive value
- NREM
non-rapid eye movement
- NT
narcolepsy type
- OA
oral appliance
- ODI
oxygen desaturation index
- OHS
obesity hypoventilation syndrome
- ONF
oronasal fistula
- OP
oropharynx
- OR
odds ratio
- OS
overlap syndrome
- OSA
obstructive sleep apnea
- OSAS
obstructive sleep apnea syndrome
- OSLER
Oxford Sleep Resistance Test
- P-SAP
Perioperative Sleep Apnea Prediction Score
- PACU
post-anesthesia care unit
- PAH
pulmonary arterial hypertension
- PAP
positive airway pressure
- PAT
peripheral arterial tone
- PCI
percutaneous coronary intervention
- Pcrit
upper airway critical closing pressure
- PH
pulmonary hypertension
- PLM
periodic limb movement
- POSA
positional obstructive sleep apnea
- PPV
positive predictive value
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROM
patient reported outcome measure
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- PT
positional therapy
- QALY
quality-adjusted life year
- QOL
quality of life
- QSQ
Quebec Sleep Questionnaire
- RBD
REM sleep behavior disorder
- RCT
randomized controlled trial
- RDI
respiratory disturbance index
- REI
respiratory event index
- RERA
respiratory-event related arousal
- RF
radiofrequency
- RFA
radiofrequency ablation
- RFS
reflux finding score
- RLS
restless leg syndrome
- RME
Rapid Maxillary Expansion
- RR
relative risk
- RSI
Reflex Symptom Index
- SaO2
oxygen saturation
- SAQLI
Sleep Apnea Quality of Life Index
- SARPE
Surgically Assisted Rapid Palatal Expansion
- SBP
systolic blood pressure
- SD
standard deviation
- SDB
sleep-disordered breathing
- SF-36
36-item Short Form Health Survey
- SGA
small for gestational age
- SHHS
Sleep Heart Health Study
- SMD
standardized mean difference
- SNA
sella, nasion, A point angle
- SNB
sella, nasion, B point angle
- SR
systematic review
- SRBD-PSQ
Sleep-Related Breathing Disorder scale of the Pediatric Sleep Questionnaire
- STAR
Stimulation Therapy for Apnea Reduction
- STEMI
ST-segment elevation myocardial infarction
- T&A
adenotonsillectomy T2DM type 2 diabetes mellitus
- T88
time spent with oxygen saturation less than 88%
- T90
time spent with oxygen saturation less than 90%
- TBRF
tongue base radiofrequency
- TBT
tennis-ball technique
- TCRFTA
temperature-controlled radiofrequency tissue ablation
- TECSA
treatment-emergent central sleep apnea
- TIA
transient ischemic attack
- TMD
temporomandibular disorder
- TMJ
temporomandibular joint
- TORS
transoral robotic surgery
- TPA
transpalatal advancement pharyngoplasty
- TRD
tongue retention device
- TS
tongue suspension
- TST
total sleep time
- UA
upper airway
- UARS
upper airway resistance syndrome
- UAS
upper airway stimulator
- UES
upper esophageal sphincter
- UPPP
uvulopalatopharyngoplasty
- VAS
visual analog scale
- VP
velopharynx
- VPI
velopharyngeal Insufficiency
- WAI
Work Ability Index
- WHO
World Health Organization
Footnotes
Author disclosures are provided as a supplemental file.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Gastaut H, Tassinari CA, Duron B. Polygraphic study of diurnal and nocturnal (hypnic and respiratory) episodal manifestations of Pickwick syndrome. Rev Neurol (Paris) 1965;112(6):568–579. [PubMed] [Google Scholar]
- 2.Jung R, Kuhlo W. Neurophysiological studies of abnormal night sleep and the Pickwickian syndrome. Prog Brain Res 1965;18:140–159. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1(8225):862–865. [DOI] [PubMed] [Google Scholar]
- 4.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol 2016;6(1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 5.Rudmik L, Smith TL. Development of an evidence-based review with recommendations using an online iterative process. Int Forum Allergy Rhinol 2011;1(6):431–437. [DOI] [PubMed] [Google Scholar]
- 6.Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol 2018;8(2):108–352. [DOI] [PubMed] [Google Scholar]
- 7.Wang EW, Zanation AM, Gardner PA, et al. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol 2019;9(S3):S145–S365. [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62(10):e1–e34. [DOI] [PubMed] [Google Scholar]
- 9.Oxford Centre for Evidence-Based Medicine (CEBM) – levels of evidence (March 2009). Accessed. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009
- 10.American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics 2004;114:874–877. [DOI] [PubMed] [Google Scholar]
- 11.Berry RBea. The AASM Manual for the Scoring of Sleep and Associated Events Version 2.6. Vol Version 2.62020
- 12.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redline S, Kapur VK, Sanders MH, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Resp Crit Care 2000;161(2):369–374. [DOI] [PubMed] [Google Scholar]
- 14.Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep 2015;38(12):1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansukhani MP, Kolla BP, Wang Z, Morgenthaler TI. Effect of varying definitions of hypopnea on the diagnosis and clinical outcomes of sleep-disordered breathing: a systematic review and meta-analysis. J Clin Sleep Med 2019;15(5):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirotsu C, Haba-Rubio J, Andries D, et al. Effect of three hypopnea scoring criteria on OSA prevalence and associated comorbidities in the general population. J Clin Sleep Med 2019;15(2):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budhiraja R, Javaheri S, Parthasarathy S, Berry RB, Quan SF. The association between obstructive sleep apnea characterized by a minimum 3 percent oxygen desaturation or arousal hypopnea definition and hypertension. J Clin Sleep Med 2019;15(9):1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Won CHJ, Reid M, Sofer T, et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep 2020;43(5):zsz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort the atherosclerosis risk in communities-sleep heart health study. Circulation 2015;132(14):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med 2016;27–28:54–58. [DOI] [PubMed] [Google Scholar]
- 21.Brown LK. Use it or lose it: medicare’s new paradigm for durable medical equipment coverage? Chest 2010;138(4):785–789. [DOI] [PubMed] [Google Scholar]
- 22.Bliwise D, Bliwise NG, Kraemer HC, Dement W. Measurement error in visually scored electrophysiological data - respiration during sleep. J Neurosci Methods 1984;12(1):49–56. [DOI] [PubMed] [Google Scholar]
- 23.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep 1998;21(7):749–757. [DOI] [PubMed] [Google Scholar]
- 24.Rashid NHA, Zaghi S, Scapuccin M, Camacho M, Certal V, Capasso R. The value of oxygen desaturation index for diagnosing obstructive sleep apnea: a systematic review. Laryngoscope 2021;131(2):440–447. [DOI] [PubMed] [Google Scholar]
- 25.Linz D, Kadhim K, Brooks AG, et al. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int J Cardiol 2018;272:155–161. [DOI] [PubMed] [Google Scholar]
- 26.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. New Engl J Med 2016;375(10):919–931. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labarca G, Campos J, Thibaut K, Dreyse J, Jorquera J. Do T90 and SaO(2) nadir identify a different phenotype in obstructive sleep apnea? Sleep Breath 2019;23(3):1007–1010. [DOI] [PubMed] [Google Scholar]
- 29.Muraki I, Tanigawa T, Yamagishi K, et al. Nocturnal intermittent hypoxia and C reactive protein among middle-aged community residents: a cross-sectional survey. Thorax 2010;65(6):523–527. [DOI] [PubMed] [Google Scholar]
- 30.Yilmaz Avci A, Avci S, Lakadamyali H, Can U. Hypoxia and inflammation indicate significant differences in the severity of obstructive sleep apnea within similar apnea-hypopnea index groups. Sleep Breath 2017;21(3):703–711. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra RK, Kirsch DB, Kristo DA, et al. Polysomnography for obstructive sleep apnea should include arousal-based scoring: an American academy of sleep medicine position statement. J Clin Sleep Med 2018;14(7):1245–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev 2017;35:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182(2):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Coverage Determination (NCD) for Continuous Positive Airway Pressure (CPAP) Therapy For Obstructive Sleep Apnea (OSA) (240.4) Centers for Medicare & Medicaid Services. Published 2008. 2020. Accessed May 7, 2020. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=226&ncdver=3&SearchType=Advanced&CoverageSelection=Both&NCSelection=NCA%257CCAL%257CNCD%257CMEDCAC%257CTA%257CMCD&ArticleType=SAD%257CEd&PolicyType=Both&s=-&AdvSearchName=3%257C [Google Scholar]
- 35.Ye LC, Plan GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014;44(6):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vavougios GD, Natsios G, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res 2016;25(1):31–38. [DOI] [PubMed] [Google Scholar]
- 38.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea a randomized controlled trial. JAMA 2012;307(20):2161–2168. [DOI] [PubMed] [Google Scholar]
- 39.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med 2019;15(2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep 1984;7(2):110–114. [DOI] [PubMed] [Google Scholar]
- 41.Ravesloot MJL, Vonk PE, Maurer JT, Oksenberg A, de Vries N. Standardized framework to report on the role of sleeping position in sleep apnea patients. Sleep Breath 2021;25(4):1717–1728. [DOI] [PubMed] [Google Scholar]
- 42.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients – anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest 1997;112(3):629–639. [DOI] [PubMed] [Google Scholar]
- 43.Sunnergren O, Brostrom A, Svanborg E. Positional sensitivity as a confounder in diagnosis of severity of obstructive sleep apnea. Sleep Breath 2013;17(1):173–179. [DOI] [PubMed] [Google Scholar]
- 44.Kim KT, Cho YW, Kim DE, Hwang SH, Song ML, Motamedi GK. Two subtypes of positional obstructive sleep apnea: supine-predominant and supine-isolated. Clin Neurophysiol 2016;127(1):565–570. [DOI] [PubMed] [Google Scholar]
- 45.Heinzer R, Petitpierre NJ, Marti-Soler H, Haba-Rubio J. Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort. Sleep Med 2018;48:157–162. [DOI] [PubMed] [Google Scholar]
- 46.Yingjuan M, Siang WH, Alvin TKL, Poh HP. Positional therapy for positional obstructive sleep apnea. Sleep Med Clin 2019;14(1):119. [DOI] [PubMed] [Google Scholar]
- 47.McSharry DG, Saboisky JP, DeYoung P, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep 2014;37(3):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis 1982;126(5):758–762. [DOI] [PubMed] [Google Scholar]
- 49.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res 2001;10(4):253–264. [DOI] [PubMed] [Google Scholar]
- 50.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath 2012;16(2):519–526. [DOI] [PubMed] [Google Scholar]
- 51.Haba-Rubio J, Janssens JP, Rochat T, Sforza E. Rapid eye movement-related disordered breathing - clinical and polysomnographic features. Chest 2005;128(5):3350–3357. [DOI] [PubMed] [Google Scholar]
- 52.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movementrelated sleep-disordered breathing influence of age and gender. Chest 2008;134(6):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath 2008;12(3):259–264. [DOI] [PubMed] [Google Scholar]
- 54.Goh DYT, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Resp Crit Care 2000;162(2):682–686. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Resp Crit Care 2000;161(5):1465–1472. [DOI] [PubMed] [Google Scholar]
- 56.Chervin RD, Aldrich MS. The relation between multiple sleep latency test findings and the frequency of apneic events in REM and Non-REM sleep. Chest 1998;113(4):980–984. [DOI] [PubMed] [Google Scholar]
- 57.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep 2002;25(3):307–314. [PubMed] [Google Scholar]
- 58.Lee SA, Paek JH, Han SH. REM-related sleep-disordered breathing is associated with depressive symptoms in men but not in women. Sleep Breath 2016;20(3):995–1002. [DOI] [PubMed] [Google Scholar]
- 59.Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep Med 2011;12(9):827–831. [DOI] [PubMed] [Google Scholar]
- 60.Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med 2010;181(9):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan A, Harrison SL, Kezirian EJ, et al. Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in Osteoporotic Fractures in Men (MrOS) sleep study. J Clin Sleep Med 2013;9(3):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su CS, Liu KT, Panjapornpon K, Andrews N, Foldvary-Schaefer N. Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med 2012;8(3):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 1991;143(6):1300–1303. [DOI] [PubMed] [Google Scholar]
- 64.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009;108(5):246–249. [PMC free article] [PubMed] [Google Scholar]
- 65.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004;291(16):2013–2016. [DOI] [PubMed] [Google Scholar]
- 66.Cho N, Joo S, Kim J, et al. Relation of habitual snoring with components of metabolic syndrome in Korean adults. Diabetes Res Clin Pract 2006;71(3):256–263. [DOI] [PubMed] [Google Scholar]
- 67.Sogebi OA, Oyewole EA, Olusoga-Peters OO. Sleep disordered breathing (SDB) experiences associated with snoring in adult Nigerians. Afr Health Sci 2011;11(3):309–314. [PMC free article] [PubMed] [Google Scholar]
- 68.Wali SO, Abaalkhail BA. Prevalence and predictors of habitual snoring in a sample of Saudi middle-aged adults. Saudi Med J 2015;36(8):920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med 2001;161(12):1514–1519. [DOI] [PubMed] [Google Scholar]
- 70.Medicine AAoS. AASM Manual for the Scoring of Sleep and Associated Events: Summary of Updates in Version 2.6 Published 2020. Accessed June 2021. https://j2vjt3dnbra3ps7ll1clb4q2-wpengine.netdna-ssl.com/wp-content/uploads/2020/01/Summary-of-Updates-in-v2.6-1.pdf
- 71.Guilleminault C, Stoohs R, Shiomi T, Kushida C, Schnittger I.Upper airway resistance syndrome, nocturnal blood pressure monitoring, and borderline hypertension. Chest 1996;109(4):901–908. [DOI] [PubMed] [Google Scholar]
- 72.Gold AR, Gold MS, Harris KW, Espeleta VJ, Amin MM, Broderick JE. Hypersomnolence, insomnia and the pathophysiology of upper airway resistance syndrome. Sleep Med 2008;9(6):675–683. [DOI] [PubMed] [Google Scholar]
- 73.Guilleminault C, Palombini L, Poyares D, Chowdhuri S. Chronic insomnia, postmenopausal women, and sleep disordered breathing: part 1. Frequency of sleep disordered breathing in a cohort. J Psychosom Res 2002;53(1):611–615. [DOI] [PubMed] [Google Scholar]
- 74.Stoohs RA, Knaack L, Blum HC, Janicki J, Hohenhorst W. Differences in clinical features of upper airway resistance syndrome, primary snoring, and obstructive sleep apnea/hypopnea syndrome. Sleep Med 2008;9(2):121–128. [DOI] [PubMed] [Google Scholar]
- 75.Kristo DA, Lettieri CJ, Andrada T, Taylor Y, Eliasson AH. Silent upper airway resistance syndrome: prevalence in a mixed military population. Chest 2005;127(5):1654–1657. [DOI] [PubMed] [Google Scholar]
- 76.de Godoy LB, Luz GP, Palombini LO, et al. Upper airway resistance syndrome patients have worse sleep quality compared to mild obstructive sleep apnea. PLoS One 2016;11(5):e0156244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Douglas NJ. Upper airway resistance syndrome is not a distinct syndrome. Am J Resp Crit Care 2000;161(5):1413–1415. [DOI] [PubMed] [Google Scholar]
- 78.Gold AR, Dipalo F, Gold MS, O’Hearn D. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest 2003;123(1):87–95. [DOI] [PubMed] [Google Scholar]
- 79.So SJ, Lee HJ, Kang SG, Cho CH, Yoon HK, Kim L. A comparison of personality characteristics and psychiatric symptomatology between upper airway resistance syndrome and obstructive sleep apnea syndrome. Psychiatry Investig 2015;12(2):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guilleminault C, Kirisoglu C, Poyares D, et al. Upper airway resistance syndrome: a long-term outcome study. J Psychiatr Res 2006;40(3):273–279. [DOI] [PubMed] [Google Scholar]
- 81.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014;146(5):1387–1394. [DOI] [PubMed] [Google Scholar]
- 82.Littleton SW, Mokhlesi B. The pickwickian syndrome-obesity hypoventilation syndrome. Clin Chest Med 2009;30(3):467–478. vii-viii. [DOI] [PubMed] [Google Scholar]
- 83.Masa JF, Pepin JL, Borel JC, Mokhlesi B, Murphy PB, Sanchez-Quiroga MA. Obesity hypoventilation syndrome. Eur Respir Rev 2019;28(151):180097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.BaHammam AS. Prevalence, clinical characteristics, and predictors of obesity hypoventilation syndrome in a large sample of Saudi patients with obstructive sleep apnea. Saudi Med J 2015;36(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balachandran JS, Masa JF, Mokhlesi B. Obesity hypoventilation syndrome epidemiology and diagnosis. Sleep Med Clin 2014;9(3):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masa JF, Corral J, Alonso ML, et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick study. Am J Respir Crit Care Med 2015;192(1):86–95. [DOI] [PubMed] [Google Scholar]
- 87.Piper A Obesity hypoventilation syndrome: weighing in on therapy options. Chest 2016;149(3):856–868. [DOI] [PubMed] [Google Scholar]
- 88.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165(9):1217–1239. [DOI] [PubMed] [Google Scholar]
- 89.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- 90.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 91.Quan SF, Howard BV, Iber C, et al. The sleep heart health study: design, rationale, and methods. Sleep 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 92.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep 2000;23(4):S122–S126. [PubMed] [Google Scholar]
- 93.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 2004;170(10):1108–1113. [DOI] [PubMed] [Google Scholar]
- 94.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med 2004;169(2):168–173. [DOI] [PubMed] [Google Scholar]
- 95.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177(9):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010;11(5):441–446. [DOI] [PubMed] [Google Scholar]
- 97.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3(4):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7(8):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003;167(9):1181–1185. [DOI] [PubMed] [Google Scholar]
- 100.Basner M, Muller U, Elmenhorst EM. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep 2011;34(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Redline S, Tishler PV. The genetics of sleep apnea. Sleep Med Rev 2000;4(6):583–602. [DOI] [PubMed] [Google Scholar]
- 102.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE Jr. Craniofacial structure and obstructive sleep apnea syndrome – a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop 1996;109(2):163–172. [DOI] [PubMed] [Google Scholar]
- 103.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014;189(3):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson DA, Guo N, Rueschman M, Wang R, Wilson JG, Redline S. Prevalence and correlates of obstructive sleep apnea among African Americans: the Jackson Heart Sleep Study. Sleep 2018;41(10):zsy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the multi-ethnic study of atherosclerosis (MESA). Sleep 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee RWW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep 2010;33(8):1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwab RJ, Pasirstein M, Kaplan L, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 2006;173(4):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suen C, Wong J, Ryan CM, et al. Prevalence of undiagnosed obstructive sleep apnea among patients hospitalized for cardiovascular disease and associated in-hospital outcomes: a scoping review. J Clin Med 2020;9(4):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Budhiraja R, Budhiraja P, Quan SF. Sleep-disordered breathing and cardiovascular disorders. Respir Care 2010;55(10):1322–1332, Discussion 1330–1322. [PMC free article] [PubMed] [Google Scholar]
- 110.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009;373(9657):82–93. [DOI] [PubMed] [Google Scholar]
- 111.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S. Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 2017;136(19):1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 2001;154(1):50–59. [DOI] [PubMed] [Google Scholar]
- 113.Khan A, Patel NK, O’Hearn DJ, Khan S. Resistant hypertension and obstructive sleep apnea. Int J Hypertens 2013;2013:193010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med 2009;179(12):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000;283(14):1829–1836. [DOI] [PubMed] [Google Scholar]
- 116.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353(19):2034–2041. [DOI] [PubMed] [Google Scholar]
- 117.Rice TB, Foster GD, Sanders MH, et al. The relationship between obstructive sleep apnea and self-reported stroke or coronary heart disease in overweight and obese adults with type 2 diabetes mellitus. Sleep 2012;35(9):1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 119.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 2011;140(2):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur Respir J 2020;55(4):1901104. [DOI] [PubMed] [Google Scholar]
- 121.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 2008;133(2):496–506. [DOI] [PubMed] [Google Scholar]
- 122.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009;169(17):1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kline CE, Reboussin DM, Foster GD, et al. The effect of changes in cardiorespiratory fitness and weight on obstructive sleep apnea severity in overweight adults with type 2 diabetes. Sleep 2016;39(2):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Billings ME, Johnson DA, Simonelli G, et al. Neighborhood walking environment and activity level are associated with OSA: the multi-ethnic study of atherosclerosis. Chest 2016;150(5):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr 2011;158(5):789–795.e781. [DOI] [PubMed] [Google Scholar]
- 126.Wang R, Dong Y, Weng J, et al. Associations among neighborhood, race, and sleep apnea severity in children. A six-city analysis. Ann Am Thorac Soc 2017;14(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guglielmi O, Lanteri P, Garbarino S. Association between socioeconomic status, belonging to an ethnic minority and obstructive sleep apnea: a systematic review of the literature. Sleep Med 2019;57:100–106. [DOI] [PubMed] [Google Scholar]
- 128.Sharifi M, Sequist TD, Rifas-Shiman SL, et al. The role of neighborhood characteristics and the built environment in understanding racial/ethnic disparities in childhood obesity. Prev Med 2016;91:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hajat A, Hsia C, O’Neill MS. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep 2015;2(4):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Billings ME, Gold D, Szpiro A, et al. The association of ambient air pollution with sleep apnea: the multi-ethnic study of atherosclerosis. Ann Am Thorac Soc 2019;16(3):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen YL, Liu WT, Lee KY, Chuang HC, Chen HW, Chuang KJ. Association of PM2.5 with sleep-disordered breathing from a population-based study in Northern Taiwan urban areas. Environ Pollut 2017;233:109–113. [DOI] [PubMed] [Google Scholar]
- 132.Sullivan F Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. American Academy of Sleep Medicine Published 2016. Accessed June 2021. https://aasm.org/resources/pdf/sleep-apnea-economic-crisis.pdf
- 133.Jennum P, Kjellberg J. Health, social and economical consequences of sleep-disordered breathing: a controlled national study. Thorax 2011;66(7):560–566. [DOI] [PubMed] [Google Scholar]
- 134.Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999;22(6):749–755. [DOI] [PubMed] [Google Scholar]
- 135.Kapur VK, Redline S, Nieto J, Young TB, Newman AB, Henderson JA. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002;25(3):289–296. [PubMed] [Google Scholar]
- 136.Vo TN, Kats AM, Langsetmo L, et al. Association of sleep-disordered breathing with total healthcare costs and utilization in older men: the outcomes of sleep disorders in older men (MrOS Sleep) study. Sleep 2020;43(1):zsz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004;27(3):453–458. [DOI] [PubMed] [Google Scholar]
- 138.Kim RD, Kapur VK, Redline-Bruch J, et al. An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea. Sleep 2015;38(7):1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Streatfeild J, Hillman D, Adams R, Mitchell S, Pezzullo L. Cost-effectiveness of continuous positive airway pressure therapy for obstructive sleep apnea: health care system and societal perspectives. Sleep 2019;42(12). [DOI] [PubMed] [Google Scholar]
- 140.Billings ME, Kapur VK. Medicare long-term CPAP coverage policy: a cost-utility analysis. J Clin Sleep Med 2013; Oct 15;9(10):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guest JF, Helter MT, Morga A, Stradling JR. Cost-effectiveness of using continuous positive airway pressure in the treatment of severe obstructive sleep apnoea/hypopnoea syndrome in the UK. Thorax 2008;63(10):860–865. [DOI] [PubMed] [Google Scholar]
- 142.Donovan LM, Kapur VK. Screening commercial drivers for sleep apnea: are profits and public safety aligned? Sleep 2020;43(4):zsaa043. [DOI] [PubMed] [Google Scholar]
- 143.Chhatre S, Chang YHA, Gooneratne NS, Kuna S, Strollo P, Jayadevappa R. Association between adherence to continuous positive airway pressure treatment and cost among medicare enrollees. Sleep 2020;43(1):zsz188. [DOI] [PubMed] [Google Scholar]
- 144.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163(3 pt 1):608–613. [DOI] [PubMed] [Google Scholar]
- 145.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001;163(3 pt 1):685–689. [DOI] [PubMed] [Google Scholar]
- 146.Fietze I, Laharnar N, Obst A, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - results of SHIP-trend. J Sleep Res 2019;28(5):e12770. [DOI] [PubMed] [Google Scholar]
- 147.Huang TY, Lin BM, Redline S, Curhan GC, Hu FB, Tworoger SS. Type of menopause, age at menopause, and risk of developing obstructive sleep apnea in postmenopausal women. Am J Epidemiol 2018;187(7):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest 2001;119(1):62–69. [DOI] [PubMed] [Google Scholar]
- 149.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest 2004;125(1):127–134. [DOI] [PubMed] [Google Scholar]
- 150.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep 1997;20(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Neruntarat C, Chantapant S. Prevalence of sleep apnea in HRH Princess Maha Chakri Srinthorn Medical Center, Thailand. Sleep Breath 2011;15(4):641–648. [DOI] [PubMed] [Google Scholar]
- 152.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994;149(3 pt 1):722–726. [DOI] [PubMed] [Google Scholar]
- 153.Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J 2011;37(5):1137–1143. [DOI] [PubMed] [Google Scholar]
- 154.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003;167(9):1186–1192. [DOI] [PubMed] [Google Scholar]
- 155.Subramanian S, Jayaraman G, Majid H, Aguilar R, Surani S. Influence of gender and anthropometric measures on severity of obstructive sleep apnea. Sleep Breath 2012;16(4):1091–1095. [DOI] [PubMed] [Google Scholar]
- 156.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA 2003;289(17):2230–2237. [DOI] [PubMed] [Google Scholar]
- 157.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med 1995;151(5):1459–1465. [DOI] [PubMed] [Google Scholar]
- 158.Alotair H, Bahammam A. Gender differences in Saudi patients with obstructive sleep apnea. Sleep Breath 2008;12(4):323–329. [DOI] [PubMed] [Google Scholar]
- 159.Vagiakis E, Kapsimalis F, Lagogianni I, et al. Gender differences on polysomnographic findings in Greek subjects with obstructive sleep apnea syndrome. Sleep Med 2006;7(5):424–430. [DOI] [PubMed] [Google Scholar]
- 160.Yukawa K, Inoue Y, Yagyu H, et al. Gender differences in the clinical characteristics among Japanese patients with obstructive sleep apnea syndrome. Chest 2009;135(2):337–343. [DOI] [PubMed] [Google Scholar]
- 161.Gabbay IE, Lavie P. Age- and gender-related characteristics of obstructive sleep apnea. Sleep Breath 2012;16(2):453–460. [DOI] [PubMed] [Google Scholar]
- 162.Leppanen T, Kulkas A, Duce B, Mervaala E, Toyras J. Severity of individual obstruction events is gender dependent in sleep apnea. Sleep Breath 2017;21(2):397–404. [DOI] [PubMed] [Google Scholar]
- 163.Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath 2018;22(1):241–249. [DOI] [PubMed] [Google Scholar]
- 164.Mano M, Hoshino T, Sasanabe R, et al. Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health 2019;16(6):1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Z, Cheng J, Yang W, Zou H, Su C, Miao J. Gender differences in clinical manifestations and polysomnographic findings in Chinese patients with obstructive sleep apnea. Sleep Breath 2020; Sep 24(3):1019–1026. [DOI] [PubMed] [Google Scholar]
- 166.Resta O, Carpagnano GE, Lacedonia D, et al. Gender difference in sleep profile of severely obese patients with obstructive sleep apnea (OSA). Resp Med 2005;99(1):91–96. [DOI] [PubMed] [Google Scholar]
- 167.Subramanian S, Hesselbacher S, Mattewal A, Surani S. Gender and age influence the effects of slow-wave sleep on respiration in patients with obstructive sleep apnea. Sleep Breath 2013;17(1):51–56. [DOI] [PubMed] [Google Scholar]
- 168.Cho SH, Jeon JY, Jang KS, et al. Gender-specific cephalometric features related to obesity in sleep apnea patients: trilogy of soft palate-mandible-hyoid bone. Maxillofac Plast Reconstr Surg 2019;41(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sutherland K, Lee RWW, Chan TO, Ng S, Hui DS, Cistulli PA. Craniofacial phenotyping in Chinese and Caucasian patients with sleep apnea: influence of ethnicity and sex. J Clin Sleep Med 2018;14(7):1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med 2002;166(10):1388–1395. [DOI] [PubMed] [Google Scholar]
- 171.Mohsenin V Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep Med 2003;4(6):523–529. [DOI] [PubMed] [Google Scholar]
- 172.Segal Y, Malhotra A, Pillar G. Upper airway length may be associated with the severity of obstructive sleep apnea syndrome. Sleep Breath 2008;12(4):311–316. [DOI] [PubMed] [Google Scholar]
- 173.Brooks LJ, Strohl KP. Size and mechanical properties of the pharynx in healthy men and women. Am Rev Respir Dis 1992;146(6):1394–1397. [DOI] [PubMed] [Google Scholar]
- 174.Huang KT, Chin CH, Tseng CC, et al. The influence of obesity on different genders in patients with obstructive sleep apnea. Scientific World Journal 2014;2014:487215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Harada Y, Oga T, Chihara Y, et al. Differences in associations between visceral fat accumulation and obstructive sleep apnea by sex. Ann Am Thorac Soc 2014;11(3):383–391. [DOI] [PubMed] [Google Scholar]
- 176.Lim YH, Choi J, Kim KR, et al. Sex-specific characteristics of anthropometry in patients with obstructive sleep apnea: neck circumference and waist-hip ratio. Ann Otol Rhinol Laryngol 2014;123(7):517–523. [DOI] [PubMed] [Google Scholar]
- 177.Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO(2) matter? Chest 2000;117(2):454–459. [DOI] [PubMed] [Google Scholar]
- 178.Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J Appl Physiol (1985) 2013;114(1):52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jordan AS, Catcheside PG, Orr RS, O’Donoghue FJ, Saunders NA, McEvoy RD. Ventilatory decline after hypoxia and hypercapnia is not different between healthy young men and women. J Appl Physiol (1985) 2000;88(1):3–9. [DOI] [PubMed] [Google Scholar]
- 180.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol (1985) 2005;99(5):2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med 2000;162(5):1627–1632. [DOI] [PubMed] [Google Scholar]
- 182.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol (1985) 2000;89(1):192–199. [DOI] [PubMed] [Google Scholar]
- 183.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med 2004;98(10):984–989. [DOI] [PubMed] [Google Scholar]
- 184.Polesel DN, Nozoe KT, Tufik SB, et al. Gender differences in the application of anthropometric measures for evaluation of obstructive sleep apnea. Sleep Sci 2019;12(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Perri RA, Kairaitis K, Wheatley JR, Amis TC. Anthropometric and craniofacial sexual dimorphism in obstructive sleep apnea patients: is there male-female phenotypical convergence? J Sleep Res 2015;24(1):82–91. [DOI] [PubMed] [Google Scholar]
- 186.Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest 2003;123(5):1544–1550. [DOI] [PubMed] [Google Scholar]
- 187.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 2009;135(4):957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep 2010;33(4):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284(23):3015–3021. [DOI] [PubMed] [Google Scholar]
- 190.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the sleep heart health study. Arch Intern Med 2002;162(8):893–900. [DOI] [PubMed] [Google Scholar]
- 191.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985) 2005;99(4):1592–1599. [DOI] [PubMed] [Google Scholar]
- 192.Ashrafian H, le Roux CW, Rowland SP, et al. Metabolic surgery and obstructive sleep apnoea: the protective effects of bariatric procedures. Thorax 2012;67(5):442–449. [DOI] [PubMed] [Google Scholar]
- 193.Peromaa-Haavisto P, Tuomilehto H, Kössi J, et al. Obstructive sleep apnea: the effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med 2017;35:85–90. [DOI] [PubMed] [Google Scholar]
- 194.Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012;308(11):1142–1149. [DOI] [PubMed] [Google Scholar]
- 195.Ashrafian H, Toma T, Rowland SP, et al. Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses. Obes Surg 2015;25(7):1239–1250. [DOI] [PubMed] [Google Scholar]
- 196.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017– 2018. NCHS Data Brief 2020(360):1–8. [PubMed] [Google Scholar]
- 197.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303(3):235–241. [DOI] [PubMed] [Google Scholar]
- 198.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis 1990;141(5 Pt 1):1228–1231. [DOI] [PubMed] [Google Scholar]
- 199.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea. Proc Am Thorac Soc 2008;5(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995;152(5 Pt 1):1673–1689. [DOI] [PubMed] [Google Scholar]
- 201.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 1992;89(5):1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li Y, Lin N, Ye J, Chang Q, Han D, Sperry A. Upper airway fat tissue distribution in subjects with obstructive sleep apnea and its effect on retropalatal mechanical loads. Resp Care 2012;57(7):1098–1105. [DOI] [PubMed] [Google Scholar]
- 203.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non–rapid eye movement sleep. Am J Resp Crit Care 2002;165(7):945–949. [DOI] [PubMed] [Google Scholar]
- 204.Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Resp Crit Care 2014;190(8):930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 1993;148(2):462–466. [DOI] [PubMed] [Google Scholar]
- 206.Pahkala R, Seppä J, Ikonen A, Smirnov G, Tuomilehto H. The impact of pharyngeal fat tissue on the pathogenesis of obstructive sleep apnea. Sleep Breath 2014;18(2):275–282. [DOI] [PubMed] [Google Scholar]
- 207.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003;168(5):522–530. [DOI] [PubMed] [Google Scholar]
- 208.Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014;37(10):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jang M-S, Kim HY, Dhong H-J, et al. Effect of parapharyngeal fat on dynamic obstruction of the upper airway in patients with obstructive sleep apnea. Am J Resp Crit Care 2014;190(11):1318–1321. [DOI] [PubMed] [Google Scholar]
- 210.Chen H-C, Wang C-J, Lo Y-L, et al. Parapharyngeal fat pad area at the subglosso-supraglottic level is associated with corresponding lateral wall collapse and apnea-hypopnea index in patients with obstructive sleep apnea: a pilot study. Sci Rep 2019;9(1):17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep 2007;30(2):179–186. [DOI] [PubMed] [Google Scholar]
- 212.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 1984;130(2):175–178. [DOI] [PubMed] [Google Scholar]
- 213.Shimura R, Tatsumi K, Nakamura A, et al. Fat accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest 2005;127(2):543–549. [DOI] [PubMed] [Google Scholar]
- 214.Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest 2015;148(5):1193–1203. [DOI] [PubMed] [Google Scholar]
- 215.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. New Engl J Med 2014;370(24):2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jamieson A, Guilleminault C, Partinen M, Quera-Salva MA. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep 1986;9(4):469–477. [DOI] [PubMed] [Google Scholar]
- 217.Hochban W, Brandenburg U. Morphology of the viscerocranium in obstructive sleep apnoea syndrome–cephalometric evaluation of 400 patients. J Craniomaxillofac Surg 1994;22(4):205–213. [DOI] [PubMed] [Google Scholar]
- 218.Frohberg U, Naples RJ, Jones DL. Cephalometric comparison of characteristics in chronically snoring patients with and without sleep apnea syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80(1):28–33. [DOI] [PubMed] [Google Scholar]
- 219.Lowe AA, Fleetham JA, Adachi S, Ryan CF. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop 1995;107(6):589–595. [DOI] [PubMed] [Google Scholar]
- 220.Battagel JM, L’Estrange PR. The cephalometric morphology of patients with obstructive sleep apnoea (OSA). Eur J Orthod 1996;18(6):557–569. [DOI] [PubMed] [Google Scholar]
- 221.Tsai HH, Ho CY, Lee PL, Tan CT. Cephalometric analysis of nonobese snorers either with or without obstructive sleep apnea syndrome. Angle Orthod 2007;77(6):1054–1061. [DOI] [PubMed] [Google Scholar]
- 222.Riha RL, Brander P, Vennelle M, Douglas NJ. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep 2005;28(3):315–320. [PubMed] [Google Scholar]
- 223.Johal A, Patel SI, Battagel JM. The relationship between craniofacial anatomy and obstructive sleep apnoea: a casecontrolled study. J Sleep Res 2007;16(3):319–326. [DOI] [PubMed] [Google Scholar]
- 224.Chi L, Comyn FL, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J 2011;38(2):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gungor AY, Turkkahraman H, Yilmaz HH, Yariktas M. Cephalometric comparison of obstructive sleep apnea patients and healthy controls. Eur J Dent 2013;7(1):48–54. [PMC free article] [PubMed] [Google Scholar]
- 226.Banhiran W, Wanichakorntrakul P, Metheetrairut C, Chiewvit P, Planuphap W. Lateral cephalometric analysis and the risks of moderate to severe obstructive sleep-disordered breathing in Thai patients. Sleep Breath 2013;17(4):1249–1255. [DOI] [PubMed] [Google Scholar]
- 227.Costa ESRA, dos Santos Gil NA. Craniofacial skeletal architecture and obstructive sleep apnoea syndrome severity. J Craniomaxillofac Surg 2013;41(8):740–746. [DOI] [PubMed] [Google Scholar]
- 228.Sakat MS, Sütbeyaz Y, Yüceler Z, Kantarci M, Kilic K, Kurt S. Cephalometric measurements with multislice computed tomography in patients with obstructive sleep apnea syndrome. J Craniofac Surg 2016;27(1):82–86. [DOI] [PubMed] [Google Scholar]
- 229.Neelapu BC, Kharbanda OP, Sardana HK, et al. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med Rev 2017;31:79–90. [DOI] [PubMed] [Google Scholar]
- 230.Seto BH, Gotsopoulos H, Sims MR, Cistulli PA. Maxillary morphology in obstructive sleep apnoea syndrome. Eur J Orthod 2001;23(6):703–714. [DOI] [PubMed] [Google Scholar]
- 231.Johal A, Conaghan C. Maxillary morphology in obstructive sleep apnea: a cephalometric and model study. Angle Orthod 2004;74(5):648–656. [DOI] [PubMed] [Google Scholar]
- 232.Kuzucu I, Parlak IS, Baklaci D, Guler I, Kum RO, Ozcan M. Morphometric evaluation of the pterygoid hamulus and upper airway in patients with obstructive sleep apnea syndrome. Surg Radiol Anat 2020;42(5):489–496. [DOI] [PubMed] [Google Scholar]
- 233.Conway WA, Bower GC, Barnes ME. Hypersomnolence and intermittent upper airway obstruction. Occurrence caused by micrognathia. JAMA 1977;237(25):2740–2742. [PubMed] [Google Scholar]
- 234.Cistulli PA. Craniofacial abnormalities in obstructive sleep apnoea: implications for treatment. Respirology 1996;1(3):167–174. [DOI] [PubMed] [Google Scholar]
- 235.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med 2003;167(10):1427–1432. [DOI] [PubMed] [Google Scholar]
- 236.Lam B, Ip MS, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax 2005;60(6):504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miyao E, Noda A, Miyao M, Yasuma F, Inafuku S. The role of malocclusion in non-obese patients with obstructive sleep apnea syndrome. Intern Med 2008;47(18):1573–1578. [DOI] [PubMed] [Google Scholar]
- 238.Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest 1995;108(2):375–381. [DOI] [PubMed] [Google Scholar]
- 239.Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnoea: multiple comparisons of cephalometric variables of obese and non-obese patients. J Craniomaxillofac Surg 2000;28(4):204–212. [DOI] [PubMed] [Google Scholar]
- 240.Paoli JR, Lauwers F, Lacassagne L, Tiberge M, Dodart L, Boutault F. Craniofacial differences according to the body mass index of patients with obstructive sleep apnoea syndrome: cephalometric study in 85 patients. Br J Oral Maxillofac Surg 2001;39(1):40–45. [DOI] [PubMed] [Google Scholar]
- 241.Yu X, Fujimoto K, Urushibata K, Matsuzawa Y, Kubo K. Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest 2003;124(1):212–218. [DOI] [PubMed] [Google Scholar]
- 242.Xu L, Keenan BT, Wiemken AS, et al. Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea. Sleep 2020;43(5):zsz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997;155(1):186–192. [DOI] [PubMed] [Google Scholar]
- 244.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med 2001;163(4):947–950. [DOI] [PubMed] [Google Scholar]
- 245.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med 1995;151(3 pt 1):682–687. [DOI] [PubMed] [Google Scholar]
- 246.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med 1995;122(3):174–178. [DOI] [PubMed] [Google Scholar]
- 247.Douglas NJ, Luke M, Mathur R. Is the sleep apnoea/hypopnoea syndrome inherited? Thorax 1993;48(7):719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gislason T, Johannsson JH, Haraldsson A, et al. Familial predisposition and cosegregation analysis of adult obstructive sleep apnea and the sudden infant death syndrome. Am J Respir Crit Care Med 2002;166(6):833–838. [DOI] [PubMed] [Google Scholar]
- 249.Pillar G, Lavie P. Assessment of the role of inheritance in sleep apnea syndrome. Am J Respir Crit Care Med 1995;151(3 pt 1):688–691. [DOI] [PubMed] [Google Scholar]
- 250.Chi L, Comyn FL, Keenan BT, et al. Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep 2014;37(10):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Johannsdottir B, Thorarinsson F, Thordarson A, Magnusson TE. Heritability of craniofacial characteristics between parents and offspring estimated from lateral cephalograms. Am J Orthod Dentofacial Orthop 2005;127(2):200–207; quiz 260–201. [DOI] [PubMed] [Google Scholar]
- 252.Liang J, Cade BE, Wang H, et al. Comparison of heritability estimation and linkage analysis for multiple traits using principal component analyses. Genet Epidemiol 2016;40(3):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gao X, Azarbarzin A, Keenan BT, et al. Heritability of heart rate response to arousals in twins. Sleep 2017;40(6): zsx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ibrahim LH, Jacono FJ, Patel SR, et al. Heritability of abnormalities in cardiopulmonary coupling in sleep apnea: use of an electrocardiogram-based technique. Sleep 2010;33(5):643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Thomas RJ, Mietus JE, Peng CK, et al. Relationship between delta power and the electrocardiogram-derived cardiopulmonary spectrogram: possible implications for assessing the effectiveness of sleep. Sleep Med 2014;15(1):125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rizzatti FG, Mazzotti DR, Mindel J, et al. Defining extreme phenotypes of obstructive sleep apnea across international sleep centers. Chest 2020;158(3):1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114(7):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110(6):1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet 2003;72(2):340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Palmer LJ, Buxbaum SG, Larkin EK, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med 2004;169(12):1314–1321. [DOI] [PubMed] [Google Scholar]
- 261.Liang J, Cade BE, He KY, et al. Sequencing analysis at 8p23 identifies multiple rare variants in DLC1 associated with sleep-related oxyhemoglobin saturation level. Am J Hum Genet 2019;105(5):1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Varvarigou V, Dahabreh IJ, Malhotra A, Kales SN. A review of genetic association studies of obstructive sleep apnea: field synopsis and meta-analysis. Sleep 2011;34(11):1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cade BE, Chen H, Stilp AM, et al. Genetic associations with obstructive sleep apnea traits in Hispanic/Latino Americans. Am J Respir Crit Care Med 2016;194(7):886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cade BE, Lee J, Sofer T, et al. Whole-genome association analyses of sleep-disordered breathing phenotypes in the NHLBI TOPMed program. Genome Med 2021; Aug 26 13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chen H, Cade BE, Gleason KJ, et al. Multiethnic meta-analysis identifies RAI1 as a possible obstructive sleep apnea-related quantitative trait locus in men. Am J Respir Cell Mol Biol 2018;58(3):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Patel SR, Goodloe R, De G, et al. Association of genetic loci with sleep apnea in European Americans and African-Americans: the candidate gene association resource (CARe). PLoS One 2012;7(11):e48836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Elsea SH, Girirajan S. Smith-Magennis syndrome. Eur J Hum Genet 2008;16(4):412–421. [DOI] [PubMed] [Google Scholar]
- 268.Smith AC, Dykens E, Greenberg F. Sleep disturbance in Smith-Magenis syndrome (del 17 p11.2). Am J Med Genet 1998;81(2):186–191. [PubMed] [Google Scholar]
- 269.Cade BE, Chen H, Stilp AM, et al. Associations of variants In the hexokinase 1 and interleukin 18 receptor regions with oxyhemoglobin saturation during sleep. PLoS Genet 2019;15(4):e1007739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang H, Cade BE, Sofer T, et al. Admixture mapping identifies novel loci for obstructive sleep apnea in Hispanic/Latino Americans. Hum Mol Genet 2019;28(4):675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhu X, Tang H, Risch N. Admixture mapping and the role of population structure for localizing disease genes. Adv Genet 2008;60:547–569. [DOI] [PubMed] [Google Scholar]
- 272.Veatch OJ, Bauer CR, Keenan BT, et al. Characterization of genetic and phenotypic heterogeneity of obstructive sleep apnea using electronic health records. BMC Med Genomics 2020;13(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pendergrass SA, Brown-Gentry K, Dudek SM, et al. The use of phenome-wide association studies (PheWAS) for exploration of novel genotype-phenotype relationships and pleiotropy discovery. Genet Epidemiol 2011;35(5):410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Strausz S, Ruotsalainen S, Ollila HM, et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur Respir J 2021;57(5):2003091. [DOI] [PubMed] [Google Scholar]
- 275.Ferris BG Jr, Mead J, Opie LH. Partitioning of respiratory flow resistance in man. J Appl Physiol 1964;19:653–658. [DOI] [PubMed] [Google Scholar]
- 276.Magliulo G, Iannella G, Ciofalo A, et al. Nasal pathologies in patients with obstructive sleep apnoea. Acta Otorhinolaryngol Ital 2019;39(4):250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Georgalas C The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol 2011;268(9):1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Awad MI, Kacker A. Nasal obstruction considerations in sleep apnea. Otolaryngol Clin North Am 2018;51(5):1003–1009. [DOI] [PubMed] [Google Scholar]
- 279.Desfonds P, Planes C, Fuhrman C, Foucher A, Raffestin B. Nasal resistance in snorers with or without sleep apnea: effect of posture and nasal ventilation with continuous positive airway pressure. Sleep 1998;21(6):625–632. [DOI] [PubMed] [Google Scholar]
- 280.Craig TJ, Ferguson BJ, Krouse JH. Sleep impairment in allergic rhinitis, rhinosinusitis, and nasal polyposis. Am J Otolaryngol 2008;29(3):209–217. [DOI] [PubMed] [Google Scholar]
- 281.Reinberg A, Gervais P, Levi F, Smolensky M, Del Cerro L, Ugolini C. Circadian and circannual rhythms of allergic rhinitis: an epidemiologic study involving chronobiologic methods. J Allergy Clin Immunol 1988;81(1):51–62. [DOI] [PubMed] [Google Scholar]
- 282.Rundcrantz H Postural variations of nasal patency. Acta Otolaryngol 1969;68(5):435–443. [DOI] [PubMed] [Google Scholar]
- 283.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985) 1988;64(2):789–795. [DOI] [PubMed] [Google Scholar]
- 284.Susarla SM, Thomas RJ, Abramson ZR, Kaban LB. Biomechanics of the upper airway: changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg 2010;39(12):1149–1159. [DOI] [PubMed] [Google Scholar]
- 285.Mickelson SA. Nasal surgery for obstructive sleep apnea syndrome. Otolaryngol Clin North Am 2016;49(6):1373–1381. [DOI] [PubMed] [Google Scholar]
- 286.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med 1996;153(1):255–259. [DOI] [PubMed] [Google Scholar]
- 287.Fitzpatrick MF, McLean H, Urton AM, Tan A, O’Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J 2003;22(5):827–832. [DOI] [PubMed] [Google Scholar]
- 288.Hollowell DE, Suratt PM. Mandible position and activation of submental and masseter muscles during sleep. J Appl Physiol (1985) 1991;71(6):2267–2273. [DOI] [PubMed] [Google Scholar]
- 289.McNicholas WT, Coffey M, Boyle T. Effects of nasal airflow on breathing during sleep in normal humans. Am Rev Respir Dis 1993;147(3):620–623. [DOI] [PubMed] [Google Scholar]
- 290.Berry RB, Kouchi KG, Bower JL, Light RW. Effect of upper airway anesthesia on obstructive sleep-apnea. Am J Resp Crit Care 1995;151(6):1857–1861. [DOI] [PubMed] [Google Scholar]
- 291.Douglas NJ, White DP, Weil JV, Zwillich CW. Effect of breathing route on ventilation and ventilatory drive. Respir Physiol 1983;51(2):209–218. [DOI] [PubMed] [Google Scholar]
- 292.White DP, Cadieux RJ, Lombard RM, Bixler EO, Kales A, Zwillich CW. The effects of nasal anesthesia on breathing during sleep. Am Rev Respir Dis 1985;132(5):972–975. [DOI] [PubMed] [Google Scholar]
- 293.Lundberg J Airborne nitric oxide: inflammatory marker and aerocrine messenger in man. Acta Physiol Scand 1996;157(S633):4–27. [DOI] [PubMed] [Google Scholar]
- 294.Djupesland PG, Chatkin JM, Qian W, et al. Aerodynamic influences on nasal nitric oxide output measurements. Acta Otolaryngol 1999;119(4):479–485. [DOI] [PubMed] [Google Scholar]
- 295.Haight JS, Djupesland PG. Nitric oxide (NO) and obstructive sleep apnea (OSA). Sleep Breath 2003;7(2):53–62. [DOI] [PubMed] [Google Scholar]
- 296.Brander PE, Soirinsuo M, Lohela P. Nasopharyngeal symptoms in patients with obstructive sleep apnea syndrome. Effect of nasal CPAP treatment. Respiration 1999;66(2):128–135. [DOI] [PubMed] [Google Scholar]
- 297.Lofaso F, Coste A, d’Ortho MP, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J 2000;16(4):639–643. [DOI] [PubMed] [Google Scholar]
- 298.Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope 1992;102(10):1150–1152. [DOI] [PubMed] [Google Scholar]
- 299.Lavie P, Fischel N, Zomer J, Eliaschar I. The effects of partial and complete mechanical occlusion of the nasal passages on sleep structure and breathing in sleep. Acta Otolaryngol 1983;95(1–2):161–166. [DOI] [PubMed] [Google Scholar]
- 300.Wilhoit SC, Suratt PM. Effect of nasal obstruction on upper airway muscle activation in normal subjects. Chest 1987;92(6):1053–1055. [DOI] [PubMed] [Google Scholar]
- 301.Carskadon MA, Bearpark HM, Sharkey KM, et al. Effects of menopause and nasal occlusion on breathing during sleep. Am J Respir Crit Care Med 1997;155(1):205–210. [DOI] [PubMed] [Google Scholar]
- 302.Suratt PM, Turner BL, Wilhoit SC. Effect of intranasal obstruction on breathing during sleep. Chest 1986;90(3):324–329. [DOI] [PubMed] [Google Scholar]
- 303.Zwillich CW, Pickett C, Hanson FN, Weil JV. Disturbed sleep and prolonged apnea during nasal obstruction in normal men. Am Rev Respir Dis 1981;124(2):158–160. [DOI] [PubMed] [Google Scholar]
- 304.Olsen KD, Kern EB, Westbrook PR. Sleep and breathing disturbance secondary to nasal obstruction. Otolaryngol Head Neck Surg 1981;89(5):804–810. [DOI] [PubMed] [Google Scholar]
- 305.Cassisi NJ, Biller HF, Ogura JH. Changes in arterial oxygen tension and pulmonary mechanics with the use of posterior packing in epistaxis: a preliminary report. Laryngoscope 1971;81(8):1261–1266. [DOI] [PubMed] [Google Scholar]
- 306.Cole P, Haight JS. Mechanisms of nasal obstruction in sleep. Laryngoscope 1984;94(12 Pt 1):1557–1559. [PubMed] [Google Scholar]
- 307.Slocum CW, Maisel RH, Cantrell RW. Arterial blood gas determination in patients with anterior packing. Laryngoscope 1976;86(6):869–873. [DOI] [PubMed] [Google Scholar]
- 308.Friedman M, Maley A, Kelley K, et al. Impact of nasal obstruction on obstructive sleep apnea. Otolaryngol Head Neck Surg 2011;144(6):1000–1004. [DOI] [PubMed] [Google Scholar]
- 309.Armengot M, Hernandez R, Miguel P, Navarro R, Basterra J. Effect of total nasal obstruction on nocturnal oxygen saturation. Am J Rhinol 2008;22(3):325–328. [DOI] [PubMed] [Google Scholar]
- 310.Turhan M, Bostanci A, Akdag M, Dinc O. A comparison of the effects of packing or transseptal suture on polysomnographic parameters in septoplasty. Eur Arch Otorhinolaryngol 2013;270(4):1339–1344. [DOI] [PubMed] [Google Scholar]
- 311.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol (1985) 2005;99(6):2440–2450. [DOI] [PubMed] [Google Scholar]
- 312.Katsantonis GP, Moss K, Miyazaki S, Walsh J. Determining the site of airway collapse in obstructive sleep apnea with airway pressure monitoring. Laryngoscope 1993;103(10):1126–1131. [DOI] [PubMed] [Google Scholar]
- 313.Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med 1986;315(21):1327–1331. [DOI] [PubMed] [Google Scholar]
- 314.Rodenstein DO, Dooms G, Thomas Y, et al. Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax 1990;45(10):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Suratt PM, Dee P, Atkinson RL, Armstrong P, Wilhoit SC. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis 1983;127(4):487–492. [DOI] [PubMed] [Google Scholar]
- 316.Pae EK, Lowe AA, Fleetham JA. A role of pharyngeal length in obstructive sleep apnea patients. Am J Orthod Dentofacial Orthop 1997;111(1):12–17. [DOI] [PubMed] [Google Scholar]
- 317.Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 2002;165(1):71–77. [DOI] [PubMed] [Google Scholar]
- 318.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med 2000;162(2 pt 1):740–748. [DOI] [PubMed] [Google Scholar]
- 319.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis 1993;148(5):1385–1400. [DOI] [PubMed] [Google Scholar]
- 320.Anastassov GE, Trieger N. Edema in the upper airway in patients with obstructive sleep apnea syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86(6):644–647. [DOI] [PubMed] [Google Scholar]
- 321.Schwab RJ. Properties of tissues surrounding the upper airway. Sleep 1996;19(10):S170–S174. [DOI] [PubMed] [Google Scholar]
- 322.Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J 1989;2(7):613–622. [PubMed] [Google Scholar]
- 323.Bradley TD, Rutherford R, Grossman RF, et al. Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis 1985;131(6):835–839. [DOI] [PubMed] [Google Scholar]
- 324.Ingman T, Nieminen T, Hurmerinta K. Cephalometric comparison of pharyngeal changes in subjects with upper airway resistance syndrome or obstructive sleep apnoea in upright and supine positions. Eur J Orthod 2004;26(3):321–326. [DOI] [PubMed] [Google Scholar]
- 325.Stauffer JL, Buick MK, Bixler EO, et al. Morphology of the uvula in obstructive sleep apnea. Am Rev Respir Dis 1989;140(3):724–728. [DOI] [PubMed] [Google Scholar]
- 326.Campana L, Eckert DJ, Patel SR, Malhotra A. Pathophysiology & genetics of obstructive sleep apnoea. Indian J Med Res 2010;131:176–187. [PMC free article] [PubMed] [Google Scholar]
- 327.Jara SM, Weaver EM. Association of palatine tonsil size and obstructive sleep apnea in adults. Laryngoscope 2018;128(4):1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zonato AI, Bittencourt LR, Martinho FL, Junior JF, Gregorio LC, Tufik S. Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope 2003;113(6):973–980. [DOI] [PubMed] [Google Scholar]
- 329.Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope 1999;109(12):1901–1907. [DOI] [PubMed] [Google Scholar]
- 330.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64(21):2222–2231. [DOI] [PubMed] [Google Scholar]
- 331.Cahali MB, Soares CF, Dantas DA, Formigoni GG. Tonsil volume, tonsil grade and obstructive sleep apnea: is there any meaningful correlation? Clinics (Sao Paulo) 2011;66(8):1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yagi H, Nakata S, Tsuge H, et al. Morphological examination of upper airway in obstructive sleep apnea. Auris Nasus Larynx 2009;36(4):444–449. [DOI] [PubMed] [Google Scholar]
- 333.Dahlqvist J, Dahlqvist A, Marklund M, Berggren D, Stenlund H, Franklin KA. Physical findings in the upper airways related to obstructive sleep apnea in men and women. Acta Otolaryngol 2007;127(6):623–630. [DOI] [PubMed] [Google Scholar]
- 334.Thulesius HL, Thulesius HO, Jessen M. Pharyngometric correlations with obstructive sleep apnea syndrome. Acta Otolaryngol 2004;124(10):1182–1186. [DOI] [PubMed] [Google Scholar]
- 335.Camacho M, Li D, Kawai M, et al. Tonsillectomy for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2016;126(9):2176–2186. [DOI] [PubMed] [Google Scholar]
- 336.Woodson BT, Naganuma H. Comparison of methods of airway evaluation in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 1999;120(4):460–463. [PubMed] [Google Scholar]
- 337.Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Resp Crit Care 2000;161(2):347–352. [DOI] [PubMed] [Google Scholar]
- 338.Genta PR, Schorr F, Eckert DJ, et al. Upper airway collapsibility is associated with obesity and hyoid position. Sleep 2014;37(10):1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Verin E, Tardif C, Buffet X, et al. Comparison between anatomy and resistance of upper airway in normal subjects, snorers and OSAS patients. Resp Physiol 2002;129(3):335–343. [DOI] [PubMed] [Google Scholar]
- 340.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Resp Crit Care 2002;165(2):260–265. [DOI] [PubMed] [Google Scholar]
- 341.Schorr F, Kayamori F, Hirata RP, et al. Different craniofacial characteristics predict upper airway collapsibility in Japanese-Brazilian and white men. Chest 2016;149(3):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Brennick MJ, Delikatny J, Pack AI, et al. Tongue fat infiltration in obese versus lean zucker rats. Sleep 2014;37(6):1095–U1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wang SH, Keenan BT, Wiemken A, et al. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index the importance of tongue fat. Am J Resp Crit Care 2020;201(6):718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Khoo M Determinants of ventilatory instability and variability. Respir Phsiol 2000;122:167–182. [DOI] [PubMed] [Google Scholar]
- 345.Hlastala MP, Berger AJ. Chemical control of breathing. In: Physiology of Respiration 2nd ed. Oxford University Press; 2001:151, 155. [Google Scholar]
- 346.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001;163(5):1181–1190. [DOI] [PubMed] [Google Scholar]
- 347.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol 2007;581(pt 1):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Malhotra A, Jordan AS. Did fat boy Joe need hormone replacement? Sleep 2006;29(1):16–18. [PubMed] [Google Scholar]
- 349.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 2010;181(2):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90(1):47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Javaheri S A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 1999;23:985–987. [DOI] [PubMed] [Google Scholar]
- 352.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med 2009;5(3):205–211. [PMC free article] [PubMed] [Google Scholar]
- 353.Badr MS, Skatrud JB, Dempsey JA. Effect of chemoreceptor stimulation and inhibition on total pulmonary resistance in humans during NREM sleep. J Appl Physiol 1994;76(4):1682–1692. [DOI] [PubMed] [Google Scholar]
- 354.Badr MS. Pathophysiology of upper airway obstruction during sleep. Clin Chest Med 1998;19(1):21–32. [DOI] [PubMed] [Google Scholar]
- 355.Joosten SA, Landry SA, Sands SA, et al. Dynamic loop gain increases upon adopting the supine body position during sleep in patients with obstructive sleep apnoea. Respirology 2017;22(8):1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Younes M Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 2003;168(6):645–658. [DOI] [PubMed] [Google Scholar]
- 357.Ainslie PN, Lucas SJ, Burgess KR. Breathing and sleep at high altitude. Respir Physiol Neurobiol 2013;188(3):233–256. [DOI] [PubMed] [Google Scholar]
- 358.Bradley TD, Floras JS. Pathophysiologic and therapeutic implications of sleep apnea in congestive heart failure. J Card Fail 1996;2(3):223–240. [DOI] [PubMed] [Google Scholar]
- 359.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. New Engl J Med 2005;353:2025–2033. [DOI] [PubMed] [Google Scholar]
- 360.Hudgel DW, Devadatta P, Quadri M, Sioson ER, Hamilton H. Mechanism of sleep-induced periodic breathing in convalescing stroke patients and healthy elderly subjects. Chest 1993;104(5):1503–1510. [DOI] [PubMed] [Google Scholar]
- 361.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med 1998;158(4):1142–1149. [DOI] [PubMed] [Google Scholar]
- 362.Deacon-Diaz N, Malhotra A. Inherent vs. induced loop gain abnormalities in obstructive sleep apnea. Front Neurol 2018;9:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Li Y, Ye J, Han D, et al. Physiology-based modeling may predict surgical treatment outcome for obstructive sleep apnea. J Clin Sleep Med 2017;13(9):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Li Y, Ye J, Han D, et al. The effect of upper airway surgery on loop gain in obstructive sleep apnea. J Clin Sleep Med 2019;15(6):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Joosten SA, Leong P, Landry SA, et al. Loop gain predicts the response to upper airway surgery in patients with obstructive sleep apnea. Sleep 2017;40(7). [DOI] [PubMed] [Google Scholar]
- 366.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 2008;162(2):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol 2012;590(5):1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Inoue Y, Takata K, Sakamoto I, Hazama H, Kawahara R. Clinical efficacy and indication of acetazolamide treatment on sleep apnea syndrome. Psychiatry Clin Neurosci 1999;53(2):321–322. [DOI] [PubMed] [Google Scholar]
- 369.Orr JE, Sands SA, Edwards BA, et al. Measuring loop gain via home sleep testing in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2018;197(10):1353–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sands SA, Edwards BA, Terrill PI, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2018;197(9):1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J 2015;45(2):408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Haponik E, Smith P, Bohlman M, Allan R, Goldman S, Bleecker E. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis 1983;127:221–226. [DOI] [PubMed] [Google Scholar]
- 373.Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol 1992;73(3):1058–1066. [DOI] [PubMed] [Google Scholar]
- 374.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 1996;19(10):827–853. [DOI] [PubMed] [Google Scholar]
- 375.Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol (1985) 2014;116(3):325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Badr MS. Effect of ventilatory drive on upper airway patency in humans during NREM sleep. Respir Physiol 1996;103:1–10. [DOI] [PubMed] [Google Scholar]
- 377.Saboisky JP, Jordan AS, Eckert DJ, et al. Recruitment and ratecoding strategies of the human genioglossus muscle. J Appl Physiol 2010;109(6):1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Saboisky JP, Stashuk DW, Hamilton-Wright A, et al. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med 2012;185(3):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Powell GL, Rice A, Bennett-Cross SJ, Fregosi RF. Respiration-related discharge of hyoglossus muscle motor units in the rat. J Neurophysiol 2014;111(2):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. J Appl Physiol 1992;73(6):2462–2469. [DOI] [PubMed] [Google Scholar]
- 381.Tangel D, Mezzanotte WS, White DP. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol 1991;70(6):2574–2581. [DOI] [PubMed] [Google Scholar]
- 382.Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370(2):139–149. [DOI] [PubMed] [Google Scholar]
- 383.Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018;159(1):194–202. [DOI] [PubMed] [Google Scholar]
- 384.Malhotra A Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med 2014;370(2):170–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Mathew OP. Upper airway negative-pressure effects on respiratory activity of upper airway muscles. J Appl Physiol 1984;56(2):500–505. [DOI] [PubMed] [Google Scholar]
- 386.Mathew OP. Maintenance of upper airway patency. J Pediatr 1985;106(6):863–869. [DOI] [PubMed] [Google Scholar]
- 387.Mathew OP, Abuosba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol 1982;52(2):438–444. [DOI] [PubMed] [Google Scholar]
- 388.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol (Lond) 1991;436:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis 1993;148(3):597–605. [DOI] [PubMed] [Google Scholar]
- 390.Berry RB, White DP, Roper J, et al. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol 2003;94(5):1875–1882. [DOI] [PubMed] [Google Scholar]
- 391.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 2007;579(Pt 2):515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Li Y, Owens RL, Sands S, et al. The effect of Donepezil on arousal threshold and apnea-hypopnea index. A randomized, double-blind, cross-over study. Ann Am Thorac Soc 2016;13(11):2012–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Taranto-Montemurro L, Messineo L, Sands SA, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med 2019;199(10):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Pillar G, Malhotra A, Fogel R, et al. Upper airway muscle responsiveness to rising PCO2 during NREM sleep. J Appl Physiol 2000;89:1275–1282. [DOI] [PubMed] [Google Scholar]
- 395.Malhotra A, Pillar G, Fogel R, Beauregard J, Edwards J, White DP. Upper-airway collapsibility: measurements and sleep effects. Chest 2001;120(1):156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Malhotra A, Fogel R, Edwards J, Shea S, White D. Local mechanisms drive genioglossus muscle activation in obstructive sleep apnea. Am J Respir Crit Care Med 2000;161:1746–1749. [DOI] [PubMed] [Google Scholar]
- 397.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 1997;20(8):654–675. [DOI] [PubMed] [Google Scholar]
- 398.Younes M Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169(5):623–633. [DOI] [PubMed] [Google Scholar]
- 399.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J 2008;31(6):1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120(12):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc 2015;12(5):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ratnavadivel R, Stadler D, Windler S, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnea. Thorax 2010;65:107–112. [DOI] [PubMed] [Google Scholar]
- 403.Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: implications for obstructive sleep apnoea. Respir Res 2001;2(5):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Horner RL. Pathophysiology of obstructive sleep apnea. J Cardiopulm Rehabil Prev 2008;28(5):289–298. [DOI] [PubMed] [Google Scholar]
- 405.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383(9918):736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Malhotra A, Pillar G, Fogel R, Beauregard J, White D. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med 2000;162(3):1058–1062. [DOI] [PubMed] [Google Scholar]
- 407.Schwarz PB, Peever JH. Noradrenergic control of trigeminal motoneurons in sleep: relevance to sleep apnea. Adv Exp Med Biol 2010;669:281–284. [DOI] [PubMed] [Google Scholar]
- 408.Schwarz PB, Peever JH. Dopamine triggers skeletal muscle tone by activating D1-like receptors on somatic motoneurons. J Neurophysiol 2011;106(3):1299–1309. [DOI] [PubMed] [Google Scholar]
- 409.Schwarz PB, Yee N, Mir S, Peever JH. Noradrenaline triggers muscle tone by amplifying glutamate-driven excitation of somatic motoneurones in anaesthetized rats. J Physiol 2008;586(23):5787–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Gleeson K, Zwillich CW, WHite DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 1990;142:295–300. [DOI] [PubMed] [Google Scholar]
- 411.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2014;190(11):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Younes M Pharyngeal anatomy and severity of obstructive apnea. Am J Respir Crit Care Med 2004;170(6):716. [DOI] [PubMed] [Google Scholar]
- 413.Kobayashi I, Perry A, Rhymer J, et al. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol 1996;80(5):1595–1604. [DOI] [PubMed] [Google Scholar]
- 414.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49(5):565–571. [DOI] [PubMed] [Google Scholar]
- 415.Edwards BA, Sands SA, Owens RL, et al. The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep 2016;39(11):1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Li Y, Orr J, Jen R, et al. Is there a threshold that triggers cortical arousals in obstructive sleep apnea. Sleep 2019;42(6):zsz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Loewen A, Ostrowski M, Laprairie J, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep 2009;32(10):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Saboisky J, Eckert D, Malhotra A. Stable breathing through deeper sleeping. Thorax 2010;65(2):95–96. [DOI] [PubMed] [Google Scholar]
- 419.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med 2009;151(10):696–702. [DOI] [PubMed] [Google Scholar]
- 420.Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest 2020;157(2):403–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Weaver EM, Woodson BT, Steward DL. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg 2005;132(2):255–262. [DOI] [PubMed] [Google Scholar]
- 422.Senaratna CV, Perret JL, Matheson MC, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2017;36:116–124. [DOI] [PubMed] [Google Scholar]
- 423.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One 2015;10(12):e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Coutinho Costa J, Rebelo-Marques A, Machado JN, et al. Validation of NoSAS (neck, obesity, snoring, age, sex) score as a screening tool for obstructive sleep apnea: analysis in a sleep clinic. Pulmonology 2019;25(5):263–270. [DOI] [PubMed] [Google Scholar]
- 425.Hong C, Chen R, Qing S, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing: a hospital-based retrospective study in China. J Clin Sleep Med 2018;14(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med 2016;4(9):742–748. [DOI] [PubMed] [Google Scholar]
- 427.Peng M, Chen R, Cheng J, Li J, Liu W, Hong C. Application value of the NoSAS score for screening sleep-disordered breathing. J Thorac Dis 2018;10(8):4774–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Rong Y, Wang S, Wang H, et al. Validation of the NoSAS score for the screening of sleep-disordered breathing in a sleep clinic. Can Respir J 2020;2020:4936423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth 2010;57(5):423–438. [DOI] [PubMed] [Google Scholar]
- 430.Amra B, Rahmati B, Soltaninejad F, Feizi A. Screening questionnaires for obstructive sleep apnea: an updated systematic review. Oman Med J 2018;33(3):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev 2017;36:57–70. [DOI] [PubMed] [Google Scholar]
- 432.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 2013;62(7):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 434.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 435.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med 2001;2(6):477–491. [DOI] [PubMed] [Google Scholar]
- 436.Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, Tomlinson GA. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev 2014;18(4):321–331. [DOI] [PubMed] [Google Scholar]
- 437.Billings ME, Rosen CL, Auckley D, et al. Psychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: the HomePAP study. Sleep 2014;37(12):2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med 1998;158(2):494–503. [DOI] [PubMed] [Google Scholar]
- 439.Flemons WW, Reimer MA. Measurement properties of the Calgary sleep apnea quality of life index. Am J Respir Crit Care Med 2002;165(2):159–164. [DOI] [PubMed] [Google Scholar]
- 440.Lacasse Y, Godbout C, Sériès F. Independent validation of the sleep apnoea quality of life index. Thorax 2002;57(6):483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Silva GE, Goodwin JL, Vana KD, Quan SF. Obstructive sleep apnea and quality of life: comparison of the SAQLI, FOSQ, and SF-36 questionnaires. Southwest J Pulm Crit Care 2016;13(3):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20(10):835–843. [PubMed] [Google Scholar]
- 443.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 444.Abma IL, van der Wees PJ, Veer V, Westert GP, Rovers M. Measurement properties of patient-reported outcome measures (PROMs) in adults with obstructive sleep apnea (OSA): a systematic review. Sleep Med Rev 2016;28:18–31. [DOI] [PubMed] [Google Scholar]
- 445.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 446.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire - A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108(5):812–821. [DOI] [PubMed] [Google Scholar]
- 447.Chung F, Abdullah HR, Liao P. STOP-bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest 2016;149(3):631–638. [DOI] [PubMed] [Google Scholar]
- 448.Rosenthal LD, Dolan DC. The Epworth sleepiness scale in the identification of obstructive sleep apnea. J Nerv Ment Dis 2008;196(5):429–431. [DOI] [PubMed] [Google Scholar]
- 449.Bohning N, Schultheiss B, Eilers S, Penzel T, Bohning W, Schmittendorf E. Comparability of pulse oximeters used in sleep medicine for the screening of OSA. Physiol Meas 2010;31(7):875–888. [DOI] [PubMed] [Google Scholar]
- 450.de Vries CEE, de Raaff CAL, Ruys AT, de Vries N, Hilgevoord AAJ, van Wagensveld BA. Validity of a simple sleep monitor for diagnosing OSA in bariatric surgery patients. Surg Obes Relat Dis 2018;14(7):1020–1025. [DOI] [PubMed] [Google Scholar]
- 451.Dimitrov L, Macavei V. Can screening tools for obstructive sleep apnea predict postoperative complications? A systematic review of the literature. J Clin Sleep Med 2016;12(9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yaddanapudi SS, Pineda MC, Boorman DW, Bryne RE, Hing KL, Sharma S. High-resolution pulse oximetry (HRPO): a costeffective tool in screening for obstructive sleep apnea (OSA) in acute stroke and predicting outcome. J Stroke Cerebrovasc 2018;27(11):2986–2992. [DOI] [PubMed] [Google Scholar]
- 453.Tan A, Yin JDC, Tan LWL, van Dam RM, Cheung YY, Lee CH. Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med 2016;27–28:66–71. [DOI] [PubMed] [Google Scholar]
- 454.Tan A, Yin JSDC, Tan LDWL, van Dam RM, Cheung YY, Lee CH. Using the Berlin Questionnaire to predict obstructive sleep apnea in the general population. J Clin Sleep Med 2017;13(3):427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Kee K, Dixon J, Shaw J, et al. Comparison of commonly used questionnaires to identify obstructive sleep apnea in a high-risk population. J Clin Sleep Med 2018;14(12):2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth sleepiness scales. J Clin Sleep Med 2011;7(5):467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Luo JM, Huang R, Zhong X, Xiao Y, Zhou J. STOP-Bang questionnaire is superior to Epworth sleepiness scales, Berlin questionnaire, and STOP questionnaire in screening obstructive sleep apnea hypopnea syndrome patients. Chin Med J (Peking) 2014;127(17):3065–3070. [PubMed] [Google Scholar]
- 458.Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults evidence report and systematic review for the US preventive services task force. JAMA 2017;317(4):415–433. [DOI] [PubMed] [Google Scholar]
- 459.Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol 2012;30(11):1160–1177. [DOI] [PubMed] [Google Scholar]
- 460.Hobbs FDR, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9(40):1. [DOI] [PubMed] [Google Scholar]
- 461.Valenstein M, Vijan S, Zeber JE, Boehm K, Buttar A. The costutility of screening for depression in primary care. Ann Intern Med 2001;134(5):345–360. [DOI] [PubMed] [Google Scholar]
- 462.Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg 2008;74(9):834–838. [PubMed] [Google Scholar]
- 463.O’Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg 2004;14(1):23–26. [DOI] [PubMed] [Google Scholar]
- 464.Ramachandran SK, Kheterpal S, Consens F, et al. Derivation and validation of a simple perioperative sleep apnea prediction score. Anesth Analg 2010;110(4):1007–1015. [DOI] [PubMed] [Google Scholar]
- 465.Chan MTV, Chew YW, Seet E, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. JAMA 2019;321(18):1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Hai F, Porhomayon J, Vermont L, Frydrych L, Jaoude P, El-Solh AA. Postoperative complications in patients with obstructive sleep apnea: a meta-analysis. J Clin Anesth 2014;26(8):591–600. [DOI] [PubMed] [Google Scholar]
- 467.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth 2012;109(6):897–906. [DOI] [PubMed] [Google Scholar]
- 468.Opperer M, Cozowicz C, Bugada D, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the society of anesthesia and sleep medicine task force on preoperative preparation of patients with sleep-disordered breathing. Anesth Analg 2016;122(5):1321–1334. [DOI] [PubMed] [Google Scholar]
- 469.Chudeau N, Raveau T, Carlier L, et al. The STOP-BANG questionnaire and the risk of perioperative respiratory complications in urgent surgery patients: a prospective, observational study. Anaesth Crit Care Pain Med 2016;35(5):347–353. [DOI] [PubMed] [Google Scholar]
- 470.Chung F, Liao P, Farney R. Correlation between the STOP-bang score and the severity of obstructive sleep apnea. Anesthesiology 2015;122(6):1436–1437. [DOI] [PubMed] [Google Scholar]
- 471.Cote GA, Hovis CE, Hovis RM, et al. A screening instrument for sleep apnea predicts airway maneuvers in patients undergoing advanced endoscopic procedures. Clin Gastroenterol Hepatol 2010;8(8):660–665.e661. [DOI] [PubMed] [Google Scholar]
- 472.Fernandez-Bustamante A, Bartels K, Clavijo C, et al. Preoperatively screened obstructive sleep apnea is associated with worse postoperative outcomes than previously diagnosed obstructive sleep apnea. Anesth Analg 2017;125(2):593–602. [DOI] [PubMed] [Google Scholar]
- 473.Gokay P, Tastan S, Orhan ME. Is there a difference between the STOP-BANG and the Berlin obstructive sleep apnoea syndrome questionnaires for determining respiratory complications during the perioperative period? J Clin Nurs 2016;25(9–10):1238–1252. [DOI] [PubMed] [Google Scholar]
- 474.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg 2011;112(1):113–121. [DOI] [PubMed] [Google Scholar]
- 475.Pereira H, Xara D, Mendonca J, Santos A, Abelha FJ. Patients with a high risk for obstructive sleep apnea syndrome: postoperative respiratory complications. Rev Port Pneumol 2013;19(4):144–151. [DOI] [PubMed] [Google Scholar]
- 476.Seet E, Chua M, Liaw CM. High STOP-BANG questionnaire scores predict intraoperative and early postoperative adverse events. Singapore Med J 2015;56(4):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Vasu TS, Doghramji K, Cavallazzi R, et al. Obstructive sleep apnea syndrome and postoperative complications: clinical use of the STOP-BANG questionnaire. Arch Otolaryngol Head Neck Surg 2010;136(10):1020–1024. [DOI] [PubMed] [Google Scholar]
- 478.Fouladpour N, Jesudoss R, Bolden N, Shaman Z, Auckley D. Perioperative complications in obstructive sleep apnea patients undergoing surgery: a review of the legal literature. Anesth Analg 2016;122(1):145–151. [DOI] [PubMed] [Google Scholar]
- 479.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015;122(3):659–665. [DOI] [PubMed] [Google Scholar]
- 480.D’Apuzzo MR, Browne JA. Obstructive sleep apnea as a risk factor for postoperative complications after revision joint arthroplasty. J Arthroplasty 2012;27(8):95–98. [DOI] [PubMed] [Google Scholar]
- 481.Lockhart EM, Willingham MD, Abdallah AB, et al. Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med 2013;14(5):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Lyons PG, Zadravecz FJ, Edelson DP, Mokhlesi B, Churpek MM. Obstructive sleep apnea and adverse outcomes in surgical and nonsurgical patients on the wards. J Hosp Med 2015;10(9):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest 2013;144(3):903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg 2013;23(11):1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Proczko MA, Stepaniak PS, de Quelerij M, et al. STOP-Bang and the effect on patient outcome and length of hospital stay when patients are not using continuous positive airway pressure. J Anesth 2014;28(6):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of anesthesia and sleep medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016;123(2):452–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Abdelsattar ZM, Hendren S, Wong SL, Campbell DA Jr, Ramachandran SK. The impact of untreated obstructive sleep apnea on cardiopulmonary complications in general and vascular surgery: a cohort study. Sleep 2015;38(8):1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Sankar A, Dixon PR, Sivanathan L, Memtsoudis SG, de Almeida JR, Singh M. Cost-effectiveness analysis of preoperative screening strategies for obstructive sleep apnea among patients undergoing elective inpatient surgery. Anesthesiology 2020;133(4):787–800. [DOI] [PubMed] [Google Scholar]
- 489.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology 2008;108(5):822–830. [DOI] [PubMed] [Google Scholar]
- 490.Verbraecken J, Hedner J, Penzel T. Pre-operative screening for obstructive sleep apnoea. Eur Respir Rev 2017;26(143):160012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Subramani Y, Wong J, Nagappa M, Chung F. The benefits of perioperative screening for sleep apnea in surgical patients. Sleep Med Clin 2017;12(1):123–135. [DOI] [PubMed] [Google Scholar]
- 492.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth 2012;108(5):768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Farney RJ, Walker BS, Farney RM, Snow GL, Walker JM. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med 2011;7(5):459–465B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Acar HV, Yarkan Uysal H, Kaya A, Ceyhan A, Dikmen B. Does the STOP-Bang, an obstructive sleep apnea screening tool, predict difficult intubation? Eur Rev Med Pharmacol Sci 2014;18(13):1869–1874. [PubMed] [Google Scholar]
- 495.Chia P, Seet E, Macachor JD, Iyer US, Wu D. The association of pre-operative STOP-BANG scores with postoperative critical care admission. Anaesthesia 2013;68(9):950–952. [DOI] [PubMed] [Google Scholar]
- 496.Corso RM, Petrini F, Buccioli M, et al. Clinical utility of preoperative screening with STOP-Bang questionnaire in elective surgery. Minerva Anestesiol 2014;80(8):877–884. [PubMed] [Google Scholar]
- 497.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2006;104(5):1081–1093; quiz 1117–1088. [DOI] [PubMed] [Google Scholar]
- 498.Deflandre E, Degey S, Brichant JF, Poirrier R, Bonhomme V. Development and validation of a morphologic obstructive sleep apnea prediction score: the DES-OSA score. Anesth Analg 2016;122(2):363–372. [DOI] [PubMed] [Google Scholar]
- 499.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun YM. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 2012;114(5):993–1000. [DOI] [PubMed] [Google Scholar]
- 500.Khiani VS, Salah W, Maimone S, Cummings L, Chak A. Sedation during endoscopy for patients at risk of obstructive sleep apnea. Gastrointest Endosc 2009;70(6):1116–1120. [DOI] [PubMed] [Google Scholar]
- 501.Mehta PP, Kochhar G, Kalra S, et al. Can a validated sleep apnea scoring system predict cardiopulmonary events using propofol sedation for routine EGD or colonoscopy? A prospective cohort study. Gastrointest Endosc 2014;79(3):436–444. [DOI] [PubMed] [Google Scholar]
- 502.Setaro J, Reinsel R, Brun D. Preoperative screening for obstructive sleep apnea and outcomes in PACU. J Perianesth Nurs 2019;34(1):66–73. [DOI] [PubMed] [Google Scholar]
- 503.Lyons PG, Zadravecz FJ, Edelson DP, Mokhlesi B, Churpek MM. In response to “obstructive sleep apnea and adverse outcomes in surgical and nonsurgical patients on the wards”. J Hosp Med 2016;11(2):157. [DOI] [PubMed] [Google Scholar]
- 504.Lechner M, Breeze CE, Ohayon MM, Kotecha B. Snoring and breathing pauses during sleep: interview survey of a United Kingdom population sample reveals a significant increase in the rates of sleep apnoea and obesity over the last 20 years – data from the UK sleep survey. Sleep Med 2019;54:250–256. [DOI] [PubMed] [Google Scholar]
- 505.Ustun B, Westover MB, Rudin C, Bianchi MT. Clinical prediction models for sleep apnea: the importance of medical history over symptoms. J Clin Sleep Med 2016;12(2):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Johnson DA, Sofer T, Guo N, Wilson J, Redline S. A sleep apnea prediction model developed for African Americans, the Jackson heart sleep study. J Clin Sleep Med 2020;16(7):1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: the rational clinical examination systematic review. JAMA 2013;310(7):731–741. [DOI] [PubMed] [Google Scholar]
- 508.van der Spuy I, Zhao G, Karunanayake C, Pahwa P. Predictors of sleep apnea in the Canadian population. Can Respir J 2018;2018:6349790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Senthilvel E, Auckley D, Dasarathy J. Evaluation of sleep disorders in the primary care setting: history taking compared to questionnaires. J Clin Sleep Med 2011;7(1):41–48. [PMC free article] [PubMed] [Google Scholar]
- 510.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993;103(1):30–36. [DOI] [PubMed] [Google Scholar]
- 511.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 2005;28(3):309–314. [PubMed] [Google Scholar]
- 512.Li N, Wang J, Wang D, et al. Correlation of sleep microstructure with daytime sleepiness and cognitive function in young and middle-aged adults with obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2019;276(12):3525–3532. [DOI] [PubMed] [Google Scholar]
- 513.Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 514.Van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int 2002;90(3):11–15. [DOI] [PubMed] [Google Scholar]
- 515.Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci 1996;51(3):M108–115. [DOI] [PubMed] [Google Scholar]
- 516.Chung JH, Moon HS, Park SY, Kim KR, Cho SH, Kim YT. Effect of nocturnal hypoxia on nocturia in patients with obstructive sleep apnea. Int Neurourol J 2019;23(2):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Schatzl G, Temml C, Schmidbauer J, Dolezal B, Haidinger G, Madersbacher S. Cross-sectional study of nocturia in both sexes: analysis of a voluntary health screening project. Urology 2000;56(1):71–75. [DOI] [PubMed] [Google Scholar]
- 518.Zhou J, Xia S, Li T, Liu R. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath 2020;24(4):1293–1298. [DOI] [PubMed] [Google Scholar]
- 519.Finamore P, Scarlata S, Laudisio A, et al. Occurrence of nocturia is not mediated by nocturnal hypoxia length and severity in patients with sleep-disordered breathing. Sleep Med 2018;45:69–73. [DOI] [PubMed] [Google Scholar]
- 520.Umlauf MG, Chasens ER. Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev 2003;7(5):403–411. [DOI] [PubMed] [Google Scholar]
- 521.Umlauf MG, Chasens ER, Greevy RA, Arnold J, Burgio KL, Pillion DJ. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep 2004;27(1):139–144. [DOI] [PubMed] [Google Scholar]
- 522.Wang T, Huang W, Zong H, Zhang Y. The efficacy of continuous positive airway pressure therapy on nocturia in patients with obstructive sleep apnea: a systematic review and meta-analysis. Int Neurourol J 2015;19(3):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Norman D, Bardwell WA, Loredo JS, Ancoli-Israel S, Heaton RK, Dimsdale JE. Caffeine intake is independently associated with neuropsychological performance in patients with obstructive sleep apnea. Sleep Breath 2008;12(3):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Aurora RN, Crainiceanu C, Caffo B, Punjabi NM. Sleep-disordered breathing and caffeine consumption: results of a community-based study. Chest 2012;142(3):631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Bardwell WA, Ziegler MG, Ancoli-Israel S, et al. Does caffeine confound relationships among adrenergic tone, blood pressure and sleep apnoea? J Sleep Res 2000;9(3):269–272. [DOI] [PubMed] [Google Scholar]
- 526.Robinson GV, Pepperell JC, Davies RJ, Stradling JR. Caffeine levels following treatment of obstructive sleep apnoea. Thorax 2003;58(9):801–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Shie DY, Tsou YA, Tai CJ, Tsai MH. Impact of obesity on uvulopalatopharyngoplasty success in patients with severe obstructive sleep apnea: a retrospective single-center study in Taiwan. Acta Otolaryngol 2013;133(3):261–269. [DOI] [PubMed] [Google Scholar]
- 528.Ahbab S, Ataoglu HE, Tuna M, et al. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; evaluation of possible linkage. Med Sci Monit 2013;19:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Chang ET, Yang MC, Wang HM, Lai HL. Snoring in a sitting position and neck circumference are predictors of sleep apnea in Chinese patients. Sleep Breath 2014;18(1):133–136. [DOI] [PubMed] [Google Scholar]
- 530.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J 1990;3(5):509–514. [PubMed] [Google Scholar]
- 531.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Park KM, Shin KJ, Ha SY, et al. Risk factors for obstructive sleep apnea in non-obese Korean patients: significance of body weight. Sleep Biol Rhythms 2014;12(3):162–168. [Google Scholar]
- 533.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep-apnea and snoring in 1001 middle-aged men. Thorax 1991;46(2):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Yildirim Y, Yilmaz S, Guven M, et al. Evaluation of anthropometric and metabolic parameters in obstructive sleep apnea. Pulm Med 2015;2015:189761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Agha B, Johal A. Facial phenotype in obstructive sleep apnea-hypopnea syndrome: a systematic review and meta-analysis. J Sleep Res 2017;26(2):122–131. [DOI] [PubMed] [Google Scholar]
- 536.Khoo SM, Tan WC, Ng TP, Ho CH. Risk factors associated with habitual snoring and sleep-disordered breathing in a multiethnic Asian population: a population-based study. Respir Med 2004;98(6):557–566. [DOI] [PubMed] [Google Scholar]
- 537.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med 1997;127(8 pt 1):581–587. [DOI] [PubMed] [Google Scholar]
- 538.Yusoff MF, Baki MM, Mohamed N, et al. Obstructive sleep apnea among express bus drivers in Malaysia: important indicators for screening. Traffic Inj Prev 2010;11(6):594–599. [DOI] [PubMed] [Google Scholar]
- 539.Skomro RP, Kryger MH. Clinical presentations of obstructive sleep apnea syndrome. Prog Cardiovasc Dis 1999;41(5):331–340. [DOI] [PubMed] [Google Scholar]
- 540.Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep 1993;16(2):118–122. [PubMed] [Google Scholar]
- 541.Mihaicuta S, Topirceanu A, Udrescu L. SAS score: an innovative prediction model for sleep apnea syndrome. Am J Resp Crit Care 2015;191:A5617. [Google Scholar]
- 542.Santos RB, Silva WA, Parise BK, et al. Accuracy of global and/or regional anthropometric measurements of adiposity in screening sleep apnea: the ELSA-Brasil cohort. Sleep Med 2019;63:115–121. [DOI] [PubMed] [Google Scholar]
- 543.Fung E, Hong P, Moore C, Taylor SM. The effectiveness of modified cottle maneuver in predicting outcomes in functional rhinoplasty. Plast Surg Int 2014;2014:618313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Sugiura T, Noda A, Nakata S, Yasuda Y. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration 2007;74(1):56–60. [DOI] [PubMed] [Google Scholar]
- 545.Ishii L, Roxbury C, Godoy A, Ishman S, Ishii M. Does nasal surgery improve OSA in patients with nasal obstruction and OSA? A meta-analysis. Otolaryngol Head Neck Surg 2015;153(3):478–478. [DOI] [PubMed] [Google Scholar]
- 546.Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol 1987;13(2):149–156. [DOI] [PubMed] [Google Scholar]
- 547.Friedman M, Hamilton C, Samuelson CG, Lundgren ME, Pott T. Diagnostic value of the Friedman tongue position and Mallampati classification for obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg 2013;148(4):540–547. [DOI] [PubMed] [Google Scholar]
- 548.Yu JL, Rosen I. Utility of the modified Mallampati grade and Friedman tongue position in the assessment of obstructive sleep apnea. J Clin Sleep Med 2020;16(2):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Bins S, Koster TD, de Heij AH, et al. No evidence for diagnostic value of Mallampati score in patients suspected of having obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2011;145(2):199–203. [DOI] [PubMed] [Google Scholar]
- 550.Amra B, Pirpiran M, Soltaninejad F, Penzel T, Fietze I, Schoebel C. The prediction of obstructive sleep apnea severity based on anthropometric and Mallampati indices. J Res Med Sci 2019;24:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Ruangsri S, Jorns TP, Puasiri S, Luecha T, Chaithap C, Sawanyawisuth K. Which oropharyngeal factors are significant risk factors for obstructive sleep apnea? An age-matched study and dentist perspectives. Nat Sci Sleep 2016;8:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Lin HC, Lai CC, Lin PW, et al. Clinical prediction model for obstructive sleep apnea among adult patients with habitual snoring. Otolaryngol Head Neck Surg 2019;161(1):178–185. [DOI] [PubMed] [Google Scholar]
- 553.Subramanian S, Hesselbacher SE, Aguilar R, Surani SR. The NAMES assessment: a novel combined-modality screening tool for obstructive sleep apnea. Sleep Breath 2011;15(4):819–826. [DOI] [PubMed] [Google Scholar]
- 554.Sundman J, Fehrm J, Friberg D. Low inter-examiner agreement of the Friedman staging system indicating limited value in patient selection. Eur Arch Otorhinolaryngol 2018;275(6):1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Banhiran W, Junlapan A, Assanasen P, Chongkolwatana C. Physical predictors for moderate to severe obstructive sleep apnea in snoring patients. Sleep Breath 2014;18(1):151–158. [DOI] [PubMed] [Google Scholar]
- 556.Friedman M, Yalamanchali S, Gorelick G, Joseph NJ, Hwang MS. A standardized lingual tonsil grading system: interexaminer agreement. Otolaryngol Head Neck Surg 2015;152(4):667–672. [DOI] [PubMed] [Google Scholar]
- 557.Tang JA, Friedman M. Incidence of lingual tonsil hypertrophy in adults with and without obstructive sleep apnea. Otolaryngol Head Neck Surg 2018;158(2):391–394. [DOI] [PubMed] [Google Scholar]
- 558.Torre C, Zaghi S, Camacho M, Capasso R, Liu SY. Hypopharyngeal evaluation in obstructive sleep apnea with awake flexible laryngoscopy: validation and updates to Cormack-Lehane and Modified Cormack-Lehane scoring systems. Clin Otolaryngol 2018;43(3):823–827. [DOI] [PubMed] [Google Scholar]
- 559.Riley R, Guilleminault C, Herran J, Powell N. Cephalometric analyses and flow-volume loops in obstructive sleep apnea patients. Sleep 1983;6(4):303–311. [DOI] [PubMed] [Google Scholar]
- 560.Armalaite J, Lopatiene K. Lateral teleradiography of the head as a diagnostic tool used to predict obstructive sleep apnea. Dentomaxillofac Radiol 2016;45(1):20150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology 2009;110(4):928–939. [DOI] [PubMed] [Google Scholar]
- 562.Guijarro-Martinez R, Swennen GR. Cone-beam computerized tomography imaging and analysis of the upper airway: a systematic review of the literature. Int J Oral Maxillofac Surg 2011;40(11):1227–1237. [DOI] [PubMed] [Google Scholar]
- 563.Hsu WE, Wu TY. Comparison of upper airway measurement by lateral cephalogram in upright position and CBCT in supine position. J Dent Sci 2019;14(2):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Chen H, Aarab G, de Ruiter MH, de Lange J, Lobbezoo F, van der Stelt PF. Three-dimensional imaging of the upper airway anatomy in obstructive sleep apnea: a systematic review. Sleep Med 2016;21:19–27. [DOI] [PubMed] [Google Scholar]
- 565.Liu SY, Huon LK, Lo MT, et al. Static craniofacial measurements and dynamic airway collapse patterns associated with severe obstructive sleep apnoea: a sleep MRI study. Clin Otolaryngol 2016;41(6):700–706. [DOI] [PubMed] [Google Scholar]
- 566.Singh M, Tuteja A, Wong DT, et al. Point-of-care ultrasound for obstructive sleep apnea screening: are we there yet? A systematic review and meta-analysis. Anesth Analg 2019;129(6):1673–1691. [DOI] [PubMed] [Google Scholar]
- 567.Barrera JE, Pau CY, Forest VI, Holbrook AB, Popelka GR. Anatomic measures of upper airway structures in obstructive sleep apnea. World J Otorhinolaryngol Head Neck Surg 2017;3(2):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Jafari B, Mohsenin V. Polysomnography. Clin Chest Med 2010;31(2):287. [DOI] [PubMed] [Google Scholar]
- 569.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The new sleep scoring manual-the evidence behind the rules. J Clin Sleep Med 2007;3(2):107–107. [Google Scholar]
- 570.Corral-Penafiel J, Pepin JL, Barbe F. Ambulatory monitoring in the diagnosis and management of obstructive sleep apnoea syndrome. Eur Respir Rev 2013;22(129):312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Claman D, Sunwoo B. Improving accuracy of home sleep apnea testing. J Clin Sleep Med 2017;13(1):9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13(3):479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med 2011;7(5):531–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Pang KP, Gourin CG, Terris DJ. A comparison of polysomnography and the WatchPAT in the diagnosis of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;137(4):665–668. [DOI] [PubMed] [Google Scholar]
- 575.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med 2003;4(5):435–442. [DOI] [PubMed] [Google Scholar]
- 576.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest 2003;123(3):695–703. [DOI] [PubMed] [Google Scholar]
- 577.O’Donnell CP, Allan L, Atkinson P, Schwartz AR. The effect of upper airway obstruction and arousal on peripheral arterial tonometry, in obstructive sleep apnea. Am J Resp Crit Care 2002;166(7):965–971. [DOI] [PubMed] [Google Scholar]
- 578.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep 2005;28(4):499–521. [DOI] [PubMed] [Google Scholar]
- 579.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007;3(7):737. [PMC free article] [PubMed] [Google Scholar]
- 580.Masa JF, Corral J, Pereira R, et al. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax 2011;66(7):567–573. [DOI] [PubMed] [Google Scholar]
- 581.Garg N, Rolle AJ, Lee TA, Prasad B. Home-based diagnosis of obstructive sleep apnea in an Urban population. J Clin Sleep Med 2014;10(8):879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Setty AR. Underestimation of sleep apnea with home sleep apnea testing compared to in-laboratory sleep testing. J Clin Sleep Med 2017;13(4):531–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.El Shayeb M, Topfer LA, Stafinski T, Pawluk L, Menon D. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. Can Med Assoc J 2014;186(1):E25–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Abraham WT, Trupp RJ, Phillilps B, et al. Validation and clinical utility of a simple in-home testing tool for sleep-disordered breathing and arrhythmias in heart failure: results of the sleep events, arrhythmias, and respiratory analysis in congestive heart failure (SEARCH) study. Congest Heart Fail 2006;12(5):241–247; quiz 248–249. [DOI] [PubMed] [Google Scholar]
- 585.Abrahamyan L, Sahakyan Y, Chung S, et al. Diagnostic accuracy of level IV portable sleep monitors versus polysomnography for obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 2018;22(3):593–611. [DOI] [PubMed] [Google Scholar]
- 586.Kotzian ST, Schwarzinger A, Haider S, Saletu B, Spatt J, Saletu MT. Home polygraphic recording with telemedicine monitoring for diagnosis and treatment of sleep apnoea in stroke (HOPES Study): study protocol for a single-blind, randomised controlled trial. BMJ Open 2018;8(1):e018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Saletu MT, Kotzian ST, Schwarzinger A, Haider S, Spatt J, Saletu B. Home sleep apnea testing is a feasible and accurate method to diagnose obstructive sleep apnea in stroke patients during in-hospital rehabilitation. J Clin Sleep Med 2018;14(9):1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med 2010;6(2):196–204. [PMC free article] [PubMed] [Google Scholar]
- 589.Masa JF, Corral J, Pereira R, et al. Effectiveness of sequential automatic-manual home respiratory polygraphy scoring. Eur Respir J 2013;41(4):879–887. [DOI] [PubMed] [Google Scholar]
- 590.Corral J, Sanchez-Quiroga MA, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med 2017;196(9):1181–1190. [DOI] [PubMed] [Google Scholar]
- 591.Douglas JA, Chai-Coetzer CL, McEvoy D, et al. Guidelines for sleep studies in adults - a position statement of the Australasian Sleep Association. Sleep Med 2017;36:S2–S22. [DOI] [PubMed] [Google Scholar]
- 592.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep 2012;35(6):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Almeida G, Duran-Cantolla J, Guevara JC, et al. Automated analysis for the diagnosis of sleep apnoeas in patientes with suspected disease. Validity of a new respiratory polygraphy system. Eur Respir J 2019;54:PA821. [Google Scholar]
- 594.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature - an evidence review cosponsored by the American academy of sleep medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest 2003;124(4):1543–1579. [DOI] [PubMed] [Google Scholar]
- 595.Chesson AL, Ferber RA, Fry JM, et al. The indications for polysomnography and related procedures. Sleep 1997;20(6):423–487. [DOI] [PubMed] [Google Scholar]
- 596.Garcia-Diaz E, Quintana-Gallego E, Ruiz A, et al. Respiratory polygraphy with actigraphy in the diagnosis of sleep apnea-hypopnea syndrome. Chest 2007;131(3):725–732. [DOI] [PubMed] [Google Scholar]
- 597.Ayappa I, Norman RG, Seelall V, Rapoport DM. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med 2008;4(1):26–37. [PMC free article] [PubMed] [Google Scholar]
- 598.Skomro RP, Cjevre J, Reid J, et al. Outcomes of home-based diagnosis and treatment of obstructive sleep apnea. Chest 2010;138(2):257–263. [DOI] [PubMed] [Google Scholar]
- 599.Bravata DM, McClain V, Austin C, et al. Diagnosing and managing sleep apnea in patients with chronic cerebrovascular disease: a randomized trial of a home-based strategy. Sleep Breath 2017;21(3):713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Fitzpatrick M, Rac VE, Mitsakakis N, et al. SIESTA - Home sleep study with BresoDx for obstructive sleep apnea: a randomized controlled trial. Sleep Med 2020;65:45–53. [DOI] [PubMed] [Google Scholar]
- 601.Hui DS, Ng SS, To KW, et al. A randomized controlled trial of an ambulatory approach versus the hospital-based approach in managing suspected obstructive sleep apnea syndrome. Sci Rep 2017;7:45901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography - a randomized validation study. Ann Intern Med 2007;146(3):157–166. [DOI] [PubMed] [Google Scholar]
- 603.Guerrero A, Embid C, Isetta V, et al. Management of sleep apnea without high pretest probability or with comorbidities by three nights of portable sleep monitoring. Sleep 2014;37(8):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Ferber R, Millman R, Coppola M, et al. Portable recording in the assessment of obstructive sleep-apnea. Sleep 1994;17(4):378–392. [DOI] [PubMed] [Google Scholar]
- 605.Morales CR, Hurley S, Wick LC, et al. In-home, self-assembled sleep studies are useful in diagnosing sleep apnea in the elderly. Sleep 2012;35(11):1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011;34(6):695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Araujo I, Marques F, Andre S, et al. Diagnosis of sleep apnea in patients with stable chronic heart failure using a portable sleep test diagnostic device. Sleep Breath 2018;22(3):749–755. [DOI] [PubMed] [Google Scholar]
- 608.Aurora RN, Patil SP, Punjabi NM. Portable sleep monitoring for diagnosing sleep apnea in hospitalized patients with heart failure. Chest 2018;154(1):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.de Vries GE, van der Wal HH, Kerstjens HAM, et al. Validity and predictive value of a portable two-channel sleep-screening tool in the identification of sleep apnea in patients with heart failure. J Card Fail 2015;21(10):848–855. [DOI] [PubMed] [Google Scholar]
- 610.Savage HO, Khushaba RN, Zaffaroni A, et al. Development and validation of a novel non-contact monitor of nocturnal respiration for identifying sleep-disordered breathing in patients with heart failure. ESC Heart Fail 2016;3(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Chang Y, Xu LY, Han F, et al. Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. J Clin Sleep Med 2019;15(4):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Jen R, Orr JE, Li Y, et al. Accuracy of WatchPAT for the diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. COPD 2020;17(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 2010;6(2):131–137. [PMC free article] [PubMed] [Google Scholar]
- 614.Chernyshev OY, McCarty DE, Moul DE, et al. A pilot study: portable out-of-center sleep testing as an early sleep apnea screening tool in acute ischemic stroke. Nat Sci Sleep 2015;7:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Zeidler MR, Santiago V, Dzierzewski JM, Mitchell MN, Santiago S, Martin JL. Predictors of obstructive sleep apnea on polysomnography after a technically inadequate or normal home sleep test. J Clin Sleep Med 2015;11(11):1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Pataka A, Kalamaras G, Vlachogianni E, Argyropoulou P. Combination of oximetry and sleep questionnaires as screening tools for CPAP initiation in patients with obstructive sleep apnea. Pulmonology 2019;25(3):137–142. [DOI] [PubMed] [Google Scholar]
- 617.Christensson E, Franklin KA, Sahlin C, et al. Can STOP-Bang and pulse oximetry detect and exclude obstructive sleep apnea? Anesth Analg 2018;127(3):736–743. [DOI] [PubMed] [Google Scholar]
- 618.Hang LW, Wang HL, Chen JH, et al. Validation of overnight oximetry to diagnose patients with moderate to severe obstructive sleep apnea. BMC Pulm Med 2015;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Sharma S, Mather PJ, Chowdhury A, et al. Sleep overnight monitoring for apnea in patients hospitalized with heart failure (SOMA-HF Study). J Clin Sleep Med 2017;13(10):1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.del Campo F, Hornero R, Zamarron C, Abasolo DE, Alvarez D. Oxygen saturation regularity analysis in the diagnosis of obstructive sleep apnea. Artif Intell Med 2006;37(2):111–118. [DOI] [PubMed] [Google Scholar]
- 621.Erdenebayar U, Park JU, Jeong P, Lee KJ. Obstructive sleep apnea screening using a Piezo-electric sensor. J Korean Med Sci 2017;32(6):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Alakuijala A, Salmi T. Predicting obstructive sleep apnea with periodic snoring sound recorded at home. J Clin Sleep Med 2016;12(7):953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Ward NR, Cowie MR, Rosen SD, et al. Utility of overnight pulse oximetry and heart rate variability analysis to screen for sleep-disordered breathing in chronic heart failure. Thorax 2012;67(11):1000–1005. [DOI] [PubMed] [Google Scholar]
- 624.Sharma S, Mather P, Efird JT, et al. Photoplethysmographic signal to screen sleep-disordered breathing in hospitalized heart failure patients feasibility of a prospective clinical pathway. JACC Heart Fail 2015;3(9):725–731. [DOI] [PubMed] [Google Scholar]
- 625.Andrés-Blanco AM, Alvarez D, Crespo A, et al. Assessment of automated analysis of portable oximetry as a screening test for moderate-to-severe sleep apnea in patients with chronic obstructive pulmonary disease. PLoS One 2017;12(11):e0188094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Lajoie AC, Series F, Bernard S, et al. Reliability of home nocturnal oximetry in the diagnosis of overlap syndrome in COPD. Respiration 2020;99(2):132–139. [DOI] [PubMed] [Google Scholar]
- 627.Scott AS, Baltzan MA, Wolkove N. Examination of pulse oximetry tracings to detect obstructive sleep apnea in patients with advanced chronic obstructive pulmonary disease. Can Respir J 2014;21(3):171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Aaronson JA, van Bezeij T, van den Aardweg JG, van Bennekom CA, Hofman WF. Diagnostic accuracy of nocturnal oximetry for detection of sleep apnea syndrome in stroke rehabilitation. Stroke 2012;43(9):2491–2493. [DOI] [PubMed] [Google Scholar]
- 629.Lin SH, Branson C, Park L, Leung J, Doshi N, Auerbach SH. Oximetry as an accurate tool for identifying moderate to severe sleep apnea in patients with acute stroke. J Clin Sleep Med 2018;14(12):2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Ryan CM, Wilton K, Bradley TD, Alshaer H. In-hospital diagnosis of sleep apnea in stroke patients using a portable acoustic device. Sleep Breath 2017;21(2):453–460. [DOI] [PubMed] [Google Scholar]
- 631.Aarrestad S, Qvarfort M, Kleiven AL, Tollefsen E, Skjonsberg OH, Janssens JP. Diagnostic accuracy of simple tools in monitoring patients with chronic hypoventilation treated with non-invasive ventilation; a prospective cross-sectional study. Resp Med 2018;144:30–35. [DOI] [PubMed] [Google Scholar]
- 632.Bauters F, Rietzschel ER, Hertegonne KBC, Chirinos JA. The link between obstructive sleep apnea and cardiovascular disease. Curr Atheroscler Rep 2016;18(1):1. [DOI] [PubMed] [Google Scholar]
- 633.Ge X, Han F, Huang Y, Zhang Y, Yang T, Bai C, Guo X. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PLoS One 2013;8(7):e69432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Javaheri S, Blackwell T, Ancoli-Israel S, et al. Sleep-disordered breathing and incident heart failure in older men. Am J Resp Crit Care 2016;193(5):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Wang X, Zhang Y, Dong Z, Fan J, Nie S, Wei Y. Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: a systematic review and meta-analysis. Respir Res 2018;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol 2015;309(7):H1101–H1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Rosen D, Roux FJ, Shah N. Sleep and breathing in congestive heart failure. Clin Chest Med 2014;35(3):521–534. [DOI] [PubMed] [Google Scholar]
- 638.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med 2001;163(1):19–25. [DOI] [PubMed] [Google Scholar]
- 639.Fu YQ, Xia YY, Yi HL, Xu HJ, Guan J, Yin SK. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017;21(1):181–189. [DOI] [PubMed] [Google Scholar]
- 640.Labarca G, Dreyse J, Salas C, Letelier F, Jorquera J. A validation study of four different cluster analyses of OSA and the incidence of cardiovascular mortality in a Hispanic population. Chest 2021;160(6):2266–2274. [DOI] [PubMed] [Google Scholar]
- 641.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure the sleep heart health study. Circulation 2010;122(4):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Bouzerda A Cardiovascular risk and obstructive sleep apnea. Pan Afr Med J 2018;29:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Nakashima H, Kurobe M, Minami K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2015;4(1):75–84. [DOI] [PubMed] [Google Scholar]
- 645.Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep 2015;38(5):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment a cohort study. Ann Intern Med 2012;156(2):115–122. [DOI] [PubMed] [Google Scholar]
- 647.Catalan-Serra P, Campos-Rodriguez F, Reyes-Nunez N, et al. Increased incidence of stroke, but not coronary heart disease, in elderly patients with sleep apnea. Stroke 2019;50(2):491–494. [DOI] [PubMed] [Google Scholar]
- 648.Gonzaga C, Bertolami A, Bertolami M, Amodeo C, Calhoun D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens 2015;29(12):705–712. [DOI] [PubMed] [Google Scholar]
- 649.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011;57(2):119–127. [DOI] [PubMed] [Google Scholar]
- 650.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012;5(5):720–728. [DOI] [PubMed] [Google Scholar]
- 651.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment a prospective observational study. Am J Resp Crit Care 2012;186(9):909–916. [DOI] [PubMed] [Google Scholar]
- 652.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 2010;14(2):131–136. [DOI] [PubMed] [Google Scholar]
- 653.Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and allcause mortality in an adult population: MESA. Atherosclerosis 2011;219(2):963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA 2017;318(2):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med 2009;122(12):1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015;11(4):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Ip MSM, Lam B, Ng MMT, Lam WK, Tsang KWT, Lam KSL. Obstructive sleep apnea is independently associated with insulin resistance. Am J Resp Crit Care 2002;165(5):670–676. [DOI] [PubMed] [Google Scholar]
- 658.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J 2004;25(9):728–734. [DOI] [PubMed] [Google Scholar]
- 659.Patt BT, Jarjoura D, Haddad DN, et al. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am J Resp Crit Care 2010;182(12):1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342(19):1378–1384. [DOI] [PubMed] [Google Scholar]
- 661.Phillips CL, McEwen BJ, Morel-Kopp MC, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea: a randomised, placebo-controlled crossover study. Thorax 2012;67(7):639–644. [DOI] [PubMed] [Google Scholar]
- 662.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 2004;286(1):H388–H393. [DOI] [PubMed] [Google Scholar]
- 663.Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: a systematic review and meta-analysis. Respirology 2015;20(6):889–895. [DOI] [PubMed] [Google Scholar]
- 664.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest 2008;133(4):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Konishi T, Kashiwagi Y, Funayama N, et al. Obstructive sleep apnea is associated with increased coronary plaque instability: an optical frequency domain imaging study. Heart Vessels 2019;34(8):1266–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Kuniyoshi FHS, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 2008;52(5):343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep-apnea. J Clin Invest 1995;96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Franklin KA, Nilsson JB, Sahlin C, Naslund U. Sleep-apnea and nocturnal angina. Lancet 1995;345(8957):1085–1087. [DOI] [PubMed] [Google Scholar]
- 669.Mooe T, Franklin KA, Wiklund U, Rabben T, Holmstrom K. Sleep-disordered breathing and myocardial ischemia in patients with coronary artery disease. Chest 2000;117(6):1597–1602. [DOI] [PubMed] [Google Scholar]
- 670.Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med 2011;7(6):616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Lee CH, Sethi R, Li RG, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation 2016;133(21):2008–2017. [DOI] [PubMed] [Google Scholar]
- 672.Qu H, Guo M, Zhang Y, Shi DZ. Obstructive sleep apnea increases the risk of cardiac events after percutaneous coronary intervention: a meta-analysis of prospective cohort studies. Sleep Breath 2018;22(1):33–40. [DOI] [PubMed] [Google Scholar]
- 673.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med 2016;194(5):613–620. [DOI] [PubMed] [Google Scholar]
- 674.Wu X, Lv S, Yu X, Yao L, Mokhlesi B, Wei Y. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest 2015;147(3):708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Xie J, Kuniyoshi FHS, Covassin N, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc 2016;5(8):e003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Xie J, Kuniyoshi FHS, Covassin N, et al. Excessive daytime sleepiness independently predicts increased cardiovascular risk after myocardial infarction. J Am Heart Assoc 2018;7(2):e007221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief 2015(220):1–8. [PubMed] [Google Scholar]
- 678.Drager LF, Genta PR, Pedrosa RP, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol 2010;105(8):1135–1139. [DOI] [PubMed] [Google Scholar]
- 679.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011;58(5):811–817. [DOI] [PubMed] [Google Scholar]
- 680.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017;69(7):841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Mansukhani MP, Covassin N, Somers VK. Apneic sleep, insufficient sleep, and hypertension. Hypertension 2019;73(4):744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Min HJ, Cho YJ, Kim CH, et al. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J 2015;56(5):1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012;307(20):2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Xia W, Huang Y, Peng B, et al. Relationship between obstructive sleep apnoea syndrome and essential hypertension: a dose-response meta-analysis. Sleep Med 2018;47:11–18. [DOI] [PubMed] [Google Scholar]
- 685.Acosta-Castro P, Hirotsu C, Marti-Soler H, et al. REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J 2018;52(2): 1702484. [DOI] [PubMed] [Google Scholar]
- 686.Kapur VK, Resnick HE, Gottlieb DJ. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep 2008;31(8):1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 687.Ren R, Li Y, Zhang J, et al. Obstructive sleep apnea with objective daytime sleepiness is associated with hypertension. Hypertension 2016;68(5):1264–1270. [DOI] [PubMed] [Google Scholar]
- 688.Wang Q, Zhang C, Jia P, et al. The association between the phenotype of excessive daytime sleepiness and blood pressure in patients with obstructive sleep apnea-hypopnea syndrome. Int J Med Sci 2014;11(7):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Nabe B, Lies A, Pankow W, Kohl FV, Lohmann FW. Determinants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoea. J Sleep Res 1995;4(S1):97–101. [DOI] [PubMed] [Google Scholar]
- 690.Wolf J, Hering D, Narkiewicz K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res 2010;33(9):867–871. [DOI] [PubMed] [Google Scholar]
- 691.Sasaki N, Ozono R, Yamauchi R, et al. Age-related differences in the mechanism of nondipping among patients with obstructive sleep apnea syndrome. Clin Exp Hypertens 2012;34(4):270–277. [DOI] [PubMed] [Google Scholar]
- 692.Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S, Calhoun DA. Refractory hypertension: evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension 2015;66(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Dudenbostel T, Siddiqui M, Oparil S, Calhoun DA. Refractory hypertension: a novel phenotype of antihypertensive treatment failure. Hypertension 2016;67(6):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 695.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 2004;125(1):112–117. [DOI] [PubMed] [Google Scholar]
- 696.Martínez-García MA, Navarro-Soriano C, Torres G, et al. Beyond resistant hypertension. Hypertension 2018;72(3):618–624. [DOI] [PubMed] [Google Scholar]
- 697.Vongpatanasin W Resistant hypertension: a review of diagnosis and management. JAMA 2014;311(21):2216–2224. [DOI] [PubMed] [Google Scholar]
- 698.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001;19(12):2271–2277. [DOI] [PubMed] [Google Scholar]
- 699.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007;131(2):453–459. [DOI] [PubMed] [Google Scholar]
- 700.Sapiña-Beltrán E, Torres G, Benitez I, et al. Prevalence, characteristics, and association of obstructive sleep apnea with blood pressure control in patients with resistant hypertension. Ann Am Thorac Soc 2019;16(11):1414–1421. [DOI] [PubMed] [Google Scholar]
- 701.Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014;10(8):835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Johnson DA, Thomas SJ, Abdalla M, et al. Association between sleep apnea and blood pressure control among blacks: Jackson heart sleep study. Circulation 2019;139:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016;149(3):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Abumuamar AM, Dorian P, Newman D, Shapiro CM. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol 2018;41(5):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Bazan V, Grau N, Valles E, et al. Obstructive sleep apnea in patients with typical atrial flutter prevalence and impact on arrhythmia control outcome. Chest 2013;143(5):1277–1283. [DOI] [PubMed] [Google Scholar]
- 706.Bitter T, Langer C, Vogt J, Lange M, Horstkotte D, Oldenburg O. Sleep-disordered breathing in patients with atrial fibrillation and normal systolic left ventricular function. Dtsch Arztebl Int 2009;106(10):164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 2009;10(2):212–216. [DOI] [PubMed] [Google Scholar]
- 708.Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10(3):331–337. [DOI] [PubMed] [Google Scholar]
- 709.Szymanski FM, Filipiak KJ, Platek AE, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations - a long-term prospective, cross-sectional cohort study. Sleep Breath 2015;19(3):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Traaen GM, Overland B, Aakeroy L, et al. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int J Cardiol Heart Vasc 2020;26:100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Bitter T, Nolker G, Vogt J, Prinz C, Horstkotte D, Oldenburg O. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: the independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol 2012;23(1):18–25. [DOI] [PubMed] [Google Scholar]
- 712.Hojo R, Fukamizu S, Miyazawa S, Kawamura I, Sakurada H, Hiraoka M. The relationship between obstructive sleep apnea and recurrence of atrial fibrillation after pulmonary vein isolation using a contact force-sensing catheter. J Interv Card Electrophysiol 2019;54(3):209–215. [DOI] [PubMed] [Google Scholar]
- 713.Kawakami H, Nagai T, Fujii A, et al. Apnea-hypopnea index as a predictor of atrial fibrillation recurrence following initial pulmonary vein isolation: usefulness of type-3 portable monitor for sleep-disordered breathing. J Interv Card Electrophysiol 2016;47(2):237–244. [DOI] [PubMed] [Google Scholar]
- 714.Mazza A, Bendini MG, Cristofori M, et al. Baseline apnoea/hypopnoea index and high-sensitivity C-reactive protein for the risk of recurrence of atrial fibrillation after successful electrical cardioversion: a predictive model based upon the multiple effects of significant variables. Europace 2009;11(7):902–909. [DOI] [PubMed] [Google Scholar]
- 715.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J 2008;29(13):1662–1669. [DOI] [PubMed] [Google Scholar]
- 716.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110(4):364–367. [DOI] [PubMed] [Google Scholar]
- 717.Porthan KM, Melin JH, Kupila JT, Venho KKK, Partinen MM. Prevalence of sleep apnea syndrome in lone atrial fibrillation - A case-control study. Chest 2004;125(3):879–885. [DOI] [PubMed] [Google Scholar]
- 718.Kwon Y, Gharib SA, Biggs ML, et al. Association of sleep characteristics with atrial fibrillation: the multi-ethnic study of atherosclerosis. Thorax 2015;70(9):873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing – the sleep heart health study. Am J Resp Crit Care 2006;173(8):910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men outcomes of sleep disorders in older men (MrOS Sleep) study. Arch Intern Med 2009;169(12):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Chao TF, Liu CJ, Chen SJ, et al. Incidence and risk of atrial fibrillation in sleep-disordered breathing without coexistent systemic disease - nationwide longitudinal cohort study. Circ J 2014;78(9):U2182–U2280. [DOI] [PubMed] [Google Scholar]
- 722.Lin GM, Colangelo LA, Lloyd-Jones DM, et al. Association of sleep apnea and snoring with incident atrial fibrillation in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2015;182(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Kendzerska T, Gershon AS, Atzema C, et al. Sleep apnea increases the risk of new hospitalized atrial fibrillation a historical cohort study. Chest 2018;154(6):1330–1339. [DOI] [PubMed] [Google Scholar]
- 724.Cadby G, McArdle N, Briffa T, et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest 2015;148(4):945–952. [DOI] [PubMed] [Google Scholar]
- 725.May AM, Blackwell T, Stone PH, et al. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Resp Crit Care 2016;193(7):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Tung P, Levitzky YS, Wang R, et al. Obstructive and central sleep apnea and the risk of incident atrial fibrillation in a community cohort of men and women. J Am Heart Assoc 2017;6(7):e004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Matiello M, Nadal M, Tamborero D, et al. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnoea patients. Europace 2010;12(8):1084–1089. [DOI] [PubMed] [Google Scholar]
- 728.Jongnarangsin K, Chugh A, Good E, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2008;19(7):668–672. [DOI] [PubMed] [Google Scholar]
- 729.Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2(6):e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010;3(5):445–451. [DOI] [PubMed] [Google Scholar]
- 731.Monahan K, Brewster J, Wang L, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol 2012;110(3):369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment. JAMA Cardiol 2018;3(6):532–540. [DOI] [PubMed] [Google Scholar]
- 733.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009;54(19):1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Linz D, Brooks AG, Elliott AD, et al. Variability of sleep apnea severity and risk of atrial fibrillation the VARIOSA-AF study. JACC Clin Electrophysiol 2019;5(6):692–701. [DOI] [PubMed] [Google Scholar]
- 735.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 736.Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Mortality 1999–2017 on CDC WONDER Online Database. Data are from the Multiple Cause of Death Files, 1999–2017, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program Accessed January 7, 2019. http://wonder.cdc.gov/ucd-icd10.html
- 737.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34(19):1424–1431. [DOI] [PubMed] [Google Scholar]
- 738.Khattak HK, Hayat F, Pamboukian SV, Hahn HS, Schwartz BP, Stein PK. Obstructive sleep apnea in heart failure: review of prevalence, treatment with continuous positive airway pressure, and prognosis. Tex Heart Inst J 2018;45(3):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007;9(3):251–257. [DOI] [PubMed] [Google Scholar]
- 740.Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010;121(14):1598–1605. [DOI] [PubMed] [Google Scholar]
- 741.Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015;36(23):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007;49(15):1625–1631. [DOI] [PubMed] [Google Scholar]
- 743.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003;348(13):1233–1241. [DOI] [PubMed] [Google Scholar]
- 744.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005;60(9):781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Egea CJ, Aizpuru F, Pinto JA, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med 2008;9(6):660–666. [DOI] [PubMed] [Google Scholar]
- 746.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 2004;169(3):361–366. [DOI] [PubMed] [Google Scholar]
- 747.Sharma S, Fox H, Aguilar F, et al. Auto positive airway pressure therapy reduces pulmonary pressures in adults admitted for acute heart failure with pulmonary hypertension and obstructive sleep apnea. The ASAP-HF Pilot Trial. Sleep 2019;42(7):zsz100. [DOI] [PubMed] [Google Scholar]
- 748.Arikawa T, Toyoda S, Haruyama A, et al. Impact of obstructive sleep apnoea on heart failure with preserved ejection fraction. Heart Lung Circ 2016;25(5):435–441. [DOI] [PubMed] [Google Scholar]
- 749.Wachter R, Luthje L, Klemmstein D, et al. Impact of obstructive sleep apnoea on diastolic function. Eur Respir J 2013;41(2):376–383. [DOI] [PubMed] [Google Scholar]
- 750.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation 2005;112(3):375–383. [DOI] [PubMed] [Google Scholar]
- 751.Servantes DM, Javaheri S, Kravchychyn ACP, et al. Effects of exercise training and CPAP in patients with heart failure and OSA: a preliminary study. Chest 2018;154(4):808–817. [DOI] [PubMed] [Google Scholar]
- 752.Labarca G, Dreyse J, Drake L, Jorquera J, Barbe F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: systematic review and meta-analysis. Sleep Med Rev 2020;52:101312. [DOI] [PubMed] [Google Scholar]
- 753.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70(6):776–803. [DOI] [PubMed] [Google Scholar]
- 754.Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008;133(3):690–696. [DOI] [PubMed] [Google Scholar]
- 755.Lyons OD, Ryan CM. Sleep apnea and stroke. Can J Cardiol 2015;31(7):918–927. [DOI] [PubMed] [Google Scholar]
- 756.Mohammad Y, Almutlaq A, Al-Ruwaita A, Aldrees A, Alsubaie A, Al-Hussain F. Stroke during sleep and obstructive sleep apnea: there is a link. Neurol Sci 2019;40(5):1001–1005. [DOI] [PubMed] [Google Scholar]
- 757.Wang X, Ouyang YY, Wang Z, Zhao G, Liu LG, Bi YP. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013;169(3):207–214. [DOI] [PubMed] [Google Scholar]
- 758.Wu ZS, Chen FH, Yu F, Wang Y, Guo ZD. A meta-analysis of obstructive sleep apnea in patients with cerebrovascular disease. Sleep Breath 2018;22(3):729–742. [DOI] [PubMed] [Google Scholar]
- 759.Mansukhani MP, Bellolio MF, Kolla BP, Enduri S, Somers VK, Stead LG. Worse outcome after stroke in patients with obstructive sleep apnea: an observational cohort study. J Stroke Cerebrovasc 2011;20(5):401–405. [DOI] [PubMed] [Google Scholar]
- 760.Davis AP, Billings ME, Longstreth WT Jr, Khot SP. Early diagnosis and treatment of obstructive sleep apnea after stroke: are we neglecting a modifiable stroke risk factor? Neurol Clin Pract 2013;3(3):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Khot SP, Davis AP, Crane DA, et al. Effect of continuous positive airway pressure on stroke rehabilitation: a pilot randomized sham-controlled trial. J Clin Sleep Med 2016;12(7):1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Birkbak J, Clark AJ, Hulvej N. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review. J Clin Sleep Med 2014;10(1):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Culebras A, Anwar S. Sleep apnea is a risk factor for stroke and vascular dementia. Curr Neurol Neurosci Rep 2018;18(8):53. [DOI] [PubMed] [Google Scholar]
- 764.Dong RF, Dong ZL, Liu HM, Shi FK, Du JF. Prevalence, risk factors, outcomes, and treatment of obstructive sleep apnea in patients with cerebrovascular disease: a systematic review. J Stroke Cerebrovasc 2018;27(6):1471–1480. [DOI] [PubMed] [Google Scholar]
- 765.Li M, Hou WS, Zhang XW, Tang ZY. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol 2014;172(2):466–469. [DOI] [PubMed] [Google Scholar]
- 766.McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother 2018;18(7):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology 2019;92(7):e648–e654. [DOI] [PubMed] [Google Scholar]
- 768.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46(4):903–975. [DOI] [PubMed] [Google Scholar]
- 769.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med 1976;85(6):714–719. [DOI] [PubMed] [Google Scholar]
- 771.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Martinez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 2006;27(9):1106–1113. [DOI] [PubMed] [Google Scholar]
- 772.Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev 2016;21(5):591–598. [DOI] [PubMed] [Google Scholar]
- 773.Javaheri S, Javaheri S, Javaheri A. Sleep apnea, heart failure, and pulmonary hypertension. Curr Heart Fail Rep 2013;10(4):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009;104(9):1300–1306. [DOI] [PubMed] [Google Scholar]
- 775.Minic M, Granton JT, Ryan CM. Sleep disordered breathing in group 1 pulmonary arterial hypertension. J Clin Sleep Med 2014;10(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Nagaoka M, Goda A, Takeuchi K, et al. Nocturnal hypoxemia, but not sleep apnea, is associated with a poor prognosis in patients with pulmonary arterial hypertension. Circ J 2018;82(12):3076–3081. [DOI] [PubMed] [Google Scholar]
- 777.Sun X, Luo J, Xiao Y. Continuous positive airway pressure is associated with a decrease in pulmonary artery pressure in patients with obstructive sleep apnoea: a meta-analysis. Respirology 2014;19(5):670–674. [DOI] [PubMed] [Google Scholar]
- 778.Flenley DC. Sleep in chronic obstructive lung-disease. Clin Chest Med 1985;6(4):651–661. [PubMed] [Google Scholar]
- 779.Donovan LM, Feemster LC, Udris EM, et al. Poor outcomes among patients with chronic obstructive pulmonary disease with higher risk for undiagnosed obstructive sleep apnea in the LOTT cohort. J Clin Sleep Med 2019;15(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Du W, Liu J, Zhou J, Ye D, OuYang Y, Deng Q. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005–2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis 2018;13:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Resp Crit Care 2003;167(1):7–14. [DOI] [PubMed] [Google Scholar]
- 782.Starr P, Agarwal A, Singh G, et al. Obstructive sleep apnea with chronic obstructive pulmonary disease among medicare beneficiaries. Ann Am Thorac Soc 2019;16(1):153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Shawon MSR, Perret JL, Senaratna CV, Lodge C, Hamilton GS, Dharmage SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev 2017;32:58–68. [DOI] [PubMed] [Google Scholar]
- 784.Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea–clinical significance of weight loss. Sleep Med Rev 2013;17(5):321–329. [DOI] [PubMed] [Google Scholar]
- 785.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165(20):2408–2413. [DOI] [PubMed] [Google Scholar]
- 786.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141(11):846–850. [DOI] [PubMed] [Google Scholar]
- 787.Phillips BG, Hisel TM, Kato M, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens 1999;17(9):1297–1300. [DOI] [PubMed] [Google Scholar]
- 788.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol 2000;279(1):H234–H237. [DOI] [PubMed] [Google Scholar]
- 789.Chin K, Shimizu K, Nakamura T, et al. Changes in intraabdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 1999;100(7):706–712. [DOI] [PubMed] [Google Scholar]
- 790.Collen J, Lettieri CJ, Eliasson A. Postoperative CPAP use impacts long-term weight loss following bariatric surgery. J Clin Sleep Med 2015;11(3):213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Basoglu OK, Zou D, Tasbakan MS, et al. Change in weight and central obesity by positive airway pressure treatment in obstructive sleep apnea patients: longitudinal data from the ESADA cohort. J Sleep Res 2018;27(6): e12705. [DOI] [PubMed] [Google Scholar]
- 792.Garcia JM, Sharafkhaneh H, Hirshkowitz M, Elkhatib R, Sharafkhaneh A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res 2011;12(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Myllyla M, Kurki S, Anttalainen U, Saaresranta T, Laitinen T. High adherence to CPAP treatment does not prevent the continuation of weight gain among severely obese OSAS patients. J Clin Sleep Med 2016;12(4):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Redenius R, Murphy C, O’Neill E, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med 2008;4(3):205–209. [PMC free article] [PubMed] [Google Scholar]
- 795.Tachikawa R, Ikeda K, Minami T, et al. Changes in energy metabolism after continuous positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med 2016;194(6):729–738. [DOI] [PubMed] [Google Scholar]
- 796.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol 1996;271(6 pt 1):E1036–E1043. [DOI] [PubMed] [Google Scholar]
- 797.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax 2015;70(3):258–264. [DOI] [PubMed] [Google Scholar]
- 798.West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: a randomised controlled trial. Sleep Med 2009;10(9):1056–1058. [DOI] [PubMed] [Google Scholar]
- 799.Kanbay A, Demir NC, Tutar N, et al. The effect of CPAP therapy on insulin-like growth factor and cognitive functions in obstructive sleep apnea patients. Clin Respir J 2017;11(4):506–513. [DOI] [PubMed] [Google Scholar]
- 800.Palm A, Berne C, Igelstrom H, Asenlof P, Janson C, Lindberg E. The impact of continuous positive airway pressure on circulating IGF-1 in patients with obstructive sleep apnea. J Clin Sleep Med 2018;14(3):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep-apnea. Am Rev Respir Dis 1993;148(2):462–466. [DOI] [PubMed] [Google Scholar]
- 802.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med 2009;180(8):788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 2008;5(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172(1):114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Lankford DA, Proctor CD, Richard R. Continuous positive airway pressure (CPAP) changes in bariatric surgery patients undergoing rapid weight loss. Obes Surg 2005;15(3):336–341. [DOI] [PubMed] [Google Scholar]
- 806.Kim H, Kim MS, Lee JE, et al. Treatment outcomes and compliance according to obesity in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol 2013;270(11):2885–2890. [DOI] [PubMed] [Google Scholar]
- 807.Chan AS, Cistulli PA. Oral appliance treatment of obstructive sleep apnea: an update. Curr Opin Pulm Med 2009;15(6):591–596. [DOI] [PubMed] [Google Scholar]
- 808.Holley AB, Lettieri CJ, Shah AA. Efficacy of an adjustable oral appliance and comparison with continuous positive airway pressure for the treatment of obstructive sleep apnea syndrome. Chest 2011;140(6):1511–1516. [DOI] [PubMed] [Google Scholar]
- 809.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 2015;11(7):773–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014;10(2):215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Mayer G, Meier-Ewert K. Cephalometric predictors for orthopaedic mandibular advancement in obstructive sleep apnoea. Eur J Orthod 1995;17(1):35–43. [DOI] [PubMed] [Google Scholar]
- 812.He M, Yin GP, Zhan SY, et al. Long-term efficacy of uvulopalatopharyngoplasty among adult patients with obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2019;161(3):401–411. [DOI] [PubMed] [Google Scholar]
- 813.Kandasamy T, Wright ED, Fuller J, Rotenberg BW. The incidence of early post-operative complications following uvulopalatopharyngoplasty: identification of predictive risk factors. J Otolaryngol Head Neck Surg 2013;42(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope 2004;114(3):454–459. [DOI] [PubMed] [Google Scholar]
- 815.Strollo PJ Jr, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep 2015;38(10):1593–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Almendros I, Garcia-Rio F. Sleep apnoea, insulin resistance and diabetes: the first step is in the fat. Eur Respir J 2017;49(4):1700179. [DOI] [PubMed] [Google Scholar]
- 817.Trinh MD, Plihalova A, Gojda J, et al. Obstructive sleep apnoea increases lipolysis and deteriorates glucose homeostasis in patients with type 2 diabetes mellitus. Sci Rep 2021;11(1):3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Mesarwi O, Polak J, Jun J, Polotsky VY. Sleep disorders and the development of insulin resistance and obesity. Endocrinol Metab Clin North Am 2013;42(3):617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160(6):521–530. [DOI] [PubMed] [Google Scholar]
- 820.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol 2012;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Azman M, Sani A, Kamaruddin NA. Insulin resistance using HOMA model in obstructive sleep apnea: a cross sectional study. Ann Saudi Med 2014;34(6):476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med 2014;190(2):218–225. [DOI] [PubMed] [Google Scholar]
- 823.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 2007;175(8):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Lindberg E, Theorell-Haglow J, Svensson M, Gislason T, Berne C, Janson C. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest 2012;142(4):935–942. [DOI] [PubMed] [Google Scholar]
- 825.Michalek-Zrabkowska M, Macek P, Martynowicz H, et al. Obstructive sleep apnea as a risk factor of insulin resistance in nondiabetic adults. Life (Basel) 2021;11(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Murphy AM, Thomas A, Crinion SJ, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J 2017;49(4):1601731. [DOI] [PubMed] [Google Scholar]
- 827.Liu Y, Zou J, Li X, et al. Effect of the interaction between obstructive sleep apnea and lipoprotein (a) on insulin resistance: a large-scale cross-sectional study. J Diabetes Res 2019;2019:9583286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Aurora RN, Punjabi NM. Obstructive sleep apnea, sleepiness, and glycemic control in type 2 diabetes. J Clin Sleep Med 2019;15(5):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology 2013;18(1):61–70. [DOI] [PubMed] [Google Scholar]
- 830.Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: insights from a metareview and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev 2018;38:39–49. [DOI] [PubMed] [Google Scholar]
- 831.Zhu X, Zhao Y. Sleep-disordered breathing and the risk of cognitive decline: a meta-analysis of 19,940 participants. Sleep Breath 2018;22(1):165–173. [DOI] [PubMed] [Google Scholar]
- 832.Bubu OM, Andrade AG, Umasabor-Bubu OQ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev 2020;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Emamian F, Khazaie H, Tahmasian M, et al. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci 2016;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Mubashir T, Abrahamyan L, Niazi A, et al. The prevalence of obstructive sleep apnea in mild cognitive impairment: a systematic review. BMC Neurol 2019;19(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Gaeta AM, Benítez ID, Jorge C, et al. Prevalence of obstructive sleep apnea in Alzheimer’s disease patients. J Neurol 2020;267(4):1012–1022. [DOI] [PubMed] [Google Scholar]
- 836.Gronewold J, Haensel R, Kleinschnitz C, Frohnhofen H, Hermann DM. Sleep-disordered breathing in hospitalized geriatric patients with mild dementia and its association with cognition, emotion and mobility. Int J Environ Res Public Health 2019;16(5):863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Lee JE, Yang SW, Ju YJ, Ki SK, Chun KH. Sleep-disordered breathing and Alzheimer’s disease: a nationwide cohort study. Psychiatry Res 2019;273:624–630. [DOI] [PubMed] [Google Scholar]
- 838.Ju Y-e, Lopez O, Redline S, Stein P. Obstructive sleep apnea increases risk of incident dementia in community-dwelling older adults. Neurology 2013;80(7):P03.098–P003.098. [Google Scholar]
- 839.Lutsey PL, Bengtson LG, Punjabi NM, et al. Obstructive sleep apnea and 15-year cognitive decline: the atherosclerosis risk in communities (ARIC) study. Sleep 2016;39(2):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Lutsey PL, Misialek JR, Mosley TH, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the atherosclerosis risk in communities study. Alzheimers Dement 2018;14(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Wang G, Goebel JR, Li C, Hallman HG, Gilford TM, Li W. Therapeutic effects of CPAP on cognitive impairments associated with OSA. J Neurol 2020;267(10):2823–2828. [DOI] [PubMed] [Google Scholar]
- 842.Cross N, Lampit A, Pye J, Grunstein RR, Marshall N, Naismith SL. Is obstructive sleep apnoea related to neuropsychological function in healthy older adults? A systematic review and meta-analysis. Neuropsychol Rev 2017;27(4):389–402. [DOI] [PubMed] [Google Scholar]
- 843.Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch Clin Neuropsychol 2016;31(2):186–193. [DOI] [PubMed] [Google Scholar]
- 844.Vaessen TJ, Overeem S, Sitskoorn MM. Cognitive complaints in obstructive sleep apnea. Sleep Med Rev 2015;19:51–58. [DOI] [PubMed] [Google Scholar]
- 845.Kilpinen R, Saunamäki T, Jehkonen M. Information processing speed in obstructive sleep apnea syndrome: a review. Acta Neurol Scand 2014;129(4):209–218. [DOI] [PubMed] [Google Scholar]
- 846.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep 2013;36(2):203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Saunamäki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand 2007;115(1):1–11. [DOI] [PubMed] [Google Scholar]
- 848.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc 2004;10(5):772–785. [DOI] [PubMed] [Google Scholar]
- 849.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep 2003;26(3):298–307. [DOI] [PubMed] [Google Scholar]
- 850.Fulda S, Schulz H. Cognitive dysfunction in sleep-related breathing disorders: a meta-analysis. Sleep Res Online 2003;5(1):13–43. [Google Scholar]
- 851.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med 2014;10(4):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2012;186(2):190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013;187(1):99–105. [DOI] [PubMed] [Google Scholar]
- 854.Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med 2014;15(7):742–748. [DOI] [PubMed] [Google Scholar]
- 855.Brenner R, Kivity S, Peker M, et al. Increased risk for cancer in young patients with severe obstructive sleep apnea. Respiration 2019;97(1):15–23. [DOI] [PubMed] [Google Scholar]
- 856.Pataka A, Bonsignore MR, Ryan S, et al. Cancer prevalence is increased in females with sleep apnoea: data from the ESADA study. Eur Respir J 2019;53(6):1900091. [DOI] [PubMed] [Google Scholar]
- 857.Jara SM, Phipps AI, Maynard C, Weaver EM. The association of sleep apnea and cancer in veterans. Otolaryngol Head Neck Surg 2020;162(4):581–588. [DOI] [PubMed] [Google Scholar]
- 858.Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med 2014;15(9):1016–1020. [DOI] [PubMed] [Google Scholar]
- 859.Kendzerska T, Leung RS, Hawker G, Tomlinson G, Gershon AS. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014;186(13):985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Chen JC, Hwang JH. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med 2014;15(7):749–754. [DOI] [PubMed] [Google Scholar]
- 861.Fang HF, Miao NF, Chen CD, Sithole T, Chung MH. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep Apnea: a nationwide nested case-control study. J Cancer 2015;6(11):1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Chen CY, Hu JM, Shen CJ, et al. Increased incidence of colorectal cancer with obstructive sleep apnea: a nationwide population-based cohort study. Sleep Med 2020;66:15–20. [DOI] [PubMed] [Google Scholar]
- 863.Sillah A, Watson NF, Schwartz SM, Gozal D, Phipps AI. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018;29(10):987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Faiz SA, Balachandran D, Hessel AC, et al. Sleep-related breathing disorders in patients with tumors in the head and neck region. Oncologist 2014;19(11):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Saesen K, van der Veen J, Buyse B, Nuyts S. Obstructive sleep apnea in head and neck cancer survivors. Support Care Cancer 2020;29(1):279–287. [DOI] [PubMed] [Google Scholar]
- 866.Martinez-Garcia MA, Martorell-Calatayud A, Nagore E, et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur Respir J 2014;43(6):1661–1668. [DOI] [PubMed] [Google Scholar]
- 867.Dal Molin M, Brant A, Blackford AL, et al. Obstructive sleep apnea and pathological characteristics of resected pancreatic ductal adenocarcinoma. PLoS One 2016;11(10):e0164195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Huang HY, Lin SW, Chuang LP, et al. Severe obstructive sleep apnea associated with higher risk of mortality in stage III and IV lung cancer. J Clin Sleep Med 2020. Online ahead of print. [PubMed] [Google Scholar]
- 869.Labarca G, Jorquera J, Dreyse J, Salas C, Letelier F. Hypoxemic features of obstructive sleep apnea and the risk of mortality: a cluster analysis. Sleep Breath 2021;25(1):95–103. [DOI] [PubMed] [Google Scholar]
- 870.Gaoatswe G, Kent BD, Corrigan MA, et al. Invariant natural killer T cell deficiency and functional impairment in sleep apnea: links to cancer comorbidity. Sleep 2015;38(10):1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Freitas LS, Silveira AC, Martins FC, et al. Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. Sleep Breath 2020;24(4):1463–1472. [DOI] [PubMed] [Google Scholar]
- 872.Liu Y, Lao M, Chen J, et al. Short-term prognostic effects of circulating regulatory T-Cell suppressive function and vascular endothelial growth factor level in patients with non-small cell lung cancer and obstructive sleep apnea. Sleep Med 2020;70:88–96. [DOI] [PubMed] [Google Scholar]
- 873.Jarrar Y, Zihlif M, Al Bawab AQ, Sharab A. Effects of intermittent hypoxia on expression of glucose metabolism genes in MCF7 breast cancer cell line. Curr Cancer Drug Targets 2020;20(3):216–222. [DOI] [PubMed] [Google Scholar]
- 874.Zhu H, Wang D, Zhang L, et al. Upregulation of autophagy by hypoxia-inducible factor-1alpha promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep 2014;32(3):935–942. [DOI] [PubMed] [Google Scholar]
- 875.Malec V, Gottschald OR, Li S, Rose F, Seeger W, Hanze J. HIF-1 alpha signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radic Biol Med 2010;48(12):1626–1635. [DOI] [PubMed] [Google Scholar]
- 876.Cubillos-Zapata C, Hernandez-Jimenez E, Avendano-Ortiz J, et al. Obstructive sleep apnea monocytes exhibit high levels of vascular endothelial growth factor secretion, augmenting tumor progression. Mediators Inflamm 2018;2018:7373921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Martinez CA, Kerr B, Jin C, Cistulli PA, Cook KM. Obstructive sleep apnea activates HIF-1 in a hypoxia dose-dependent manner in HCT116 colorectal carcinoma cells. Int J Mol Sci 2019;20(2):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878.Kang Y, Greaves B, Perry RR. Effect of acute and chronic intermittent hypoxia on DNA topoisomerase II alpha expression and mitomycin C-induced DNA damage and cytotoxicity in human colon cancer cells. Biochem Pharmacol 1996;52(4):669–676. [DOI] [PubMed] [Google Scholar]
- 879.Cooper C, Liu GY, Niu YL, Santos S, Murphy LC, Watson PH. Intermittent hypoxia induces proteasome-dependent downregulation of estrogen receptor alpha in human breast carcinoma. Clin Cancer Res 2004;10(24):8720–8727. [DOI] [PubMed] [Google Scholar]
- 880.Martinive P, Defresne F, Bouzin C, et al. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: implications for anticancer therapies. Cancer Res 2006;66(24):11736–11744. [DOI] [PubMed] [Google Scholar]
- 881.Chen A, Sceneay J, Godde N, et al. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene 2018;37(31):4214–4225. [DOI] [PubMed] [Google Scholar]
- 882.Liu L, Liu W, Wang L, Zhu T, Zhong J, Xie N. Hypoxiainducible factor 1 mediates intermittent hypoxia-induced migration of human breast cancer MDA-MB-231 cells. Oncol Lett 2017;14(6):7715–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J 2012;39(1):215–217. [DOI] [PubMed] [Google Scholar]
- 884.Graves EE, Vilalta M, Cecic IK, et al. Hypoxia in models of lung cancer: implications for targeted therapeutics. Clin Cancer Res 2010;16(19):4843–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Guo X, Liu Y, Kim JL, et al. Effect of cyclical intermittent hypoxia on Ad5CMVCre induced solitary lung cancer progression and spontaneous metastases in the KrasG12D+; p53fl/fl; myristolated p110fl/fl ROSA-gfp mouse. PLoS One 2019;14(2):e0212930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Gaustad JV, Simonsen TG, Roa AMA, Rofstad EK. Tumors exposed to acute cyclic hypoxia show increased vessel density and delayed blood supply. Microvasc Res 2013;85:10–15. [DOI] [PubMed] [Google Scholar]
- 887.Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol 2013;186(3):303–307. [DOI] [PubMed] [Google Scholar]
- 888.Cubillos-Zapata C, Avendano-Ortiz J, Hernandez-Jimenez E, et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur Respir J 2017;50(4): 1700833. [DOI] [PubMed] [Google Scholar]
- 889.Gallego-Martin T, Farre R, Almendros I, Gonzalez-Obeso E, Obeso A. Chronic intermittent hypoxia mimicking sleep apnoea increases spontaneous tumorigenesis in mice. Eur Respir J 2017;49(2):1602111. [DOI] [PubMed] [Google Scholar]
- 890.Huang MH, Zhang XB, Wang HL, et al. Intermittent hypoxia enhances the tumor programmed death ligand 1 expression in a mouse model of sleep apnea. Ann Transl Med 2019;7(5):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.Craig TJ, Hanks CD, Fisher LH. How do topical nasal corticosteroids improve sleep and daytime somnolence in allergic rhinitis? J Allergy Clin Immun 2005;116(6):1264–1266. [DOI] [PubMed] [Google Scholar]
- 892.Stuck BA, Czajkowski J, Hagner AE, et al. Changes in daytime sleepiness, quality of life, and objective sleep patterns in seasonal allergic rhinitis: a controlled clinical trial. J Allergy Clin Immunol 2004;113(4):663–668. [DOI] [PubMed] [Google Scholar]
- 893.Young T, Finn L, Kim H, et al. Nasal obstruction as a risk factor for sleep-disordered breathing. J Allergy Clin Immun 1997;99(2):S757–S762. [DOI] [PubMed] [Google Scholar]
- 894.Lavigne F, Petrof BJ, Johnson JR, et al. Effect of topical corticosteroids on allergic airway inflammation and disease severity in obstructive sleep apnoea. Clin Exp Allergy 2013;43(10):1124–1133. [DOI] [PubMed] [Google Scholar]
- 895.Kiely JL, Nolan P, McNicolas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with coexisting rhinitis. Thorax 2004;59(1):50–55. [PMC free article] [PubMed] [Google Scholar]
- 896.Santos CB, Hanks C, McCann J, Lehman EB, Pratt E, Craig TJ. The role of montelukast on perennial allergic rhinitis and associated sleep disturbances and daytime somnolence. Allergy Asthma Proc 2008;29(2):140–145. [DOI] [PubMed] [Google Scholar]
- 897.Golden S, Teets SJ, Lehman EB, et al. Effect of topical nasal azelastine on the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Ann Allergy Asthma Immunol 2000;85(1):53–57. [DOI] [PubMed] [Google Scholar]
- 898.Silvoniemi P, Suonpaa J, Sipila J, Grenman R, Erkinjuntti M. Sleep disorders in patients with severe nasal obstruction due to septal deviation. Acta Otolaryngol 1997;117:199–201. [DOI] [PubMed] [Google Scholar]
- 899.An YS, Li YR, Kang D, et al. The effects of nasal decongestion on obstructive sleep apnoea. Am J Otolaryngol 2019;40(1):52–56. [DOI] [PubMed] [Google Scholar]
- 900.Clarenbach CF, Kohler M, Senn O, Thurnheer R, Bloch KE. Does nasal decongestion improve obstructive sleep apnea? J Sleep Res 2008;17(4):444–449. [DOI] [PubMed] [Google Scholar]
- 901.Djupesland PG, Skatvedt O, Borgersen AK. Dichotomous physiological effects of nocturnal external nasal dilation in heavy snorers: the answer to a rhinologic controversy? Am J Rhinol 2001;15(2):95–103. [DOI] [PubMed] [Google Scholar]
- 902.Kerr P, Millar T, Buckle P, Kryger M. The importance of nasal resistance in obstructive sleep-apnea syndrome. J Otolaryngol 1992;21(3):189–195. [PubMed] [Google Scholar]
- 903.McLean HA, Urton AM, Driver HS, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J 2005;25(3):521–527. [DOI] [PubMed] [Google Scholar]
- 904.Pevernagie D, Hamans E, Van Cauwenberge P, Pauwels R. External nasal dilation reduces snoring in chronic rhinitis patients: a randomized controlled trial. Eur Respir J 2000;15(6):996–1000. [DOI] [PubMed] [Google Scholar]
- 905.de Aguiar Vidigal T, Martinho Haddad FL, Gregorio LC, Poyares D, Tufik S, Azeredo Bittencourt LR. Subjective, anatomical, and functional nasal evaluation of patients with obstructive sleep apnea syndrome. Sleep Breath 2013;17(1):427–433. [DOI] [PubMed] [Google Scholar]
- 906.Li HY, Wang PC, Chen YP, Lee LA, Fang TJ, Lin HC. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy 2011;25(1):45–49. [DOI] [PubMed] [Google Scholar]
- 907.Yamasaki A, Levesque PA, Lindsay RW. Improvement in snoring-related quality-of-life outcomes after functional nasal surgery. Facial Plast Surg Aesthet Med 2020;22(1):25–35. [DOI] [PubMed] [Google Scholar]
- 908.Bosco G, Perez-Martin N, Morato M, Racionero MA, Plaza G. Nasal surgery may improve upper airway collapse in patients with obstructive sleep apnea: a drug-induced sleep endoscopy study. J Craniofac Surg 2020;31(1):68–71. [DOI] [PubMed] [Google Scholar]
- 909.Li HY, Lee LA, Wang PC, Fang TJ, Chen NH. Can nasal surgery improve obstructive sleep apnea: subjective or objective? Am J Rhinol Allergy 2009;23(6):E51–E55. [DOI] [PubMed] [Google Scholar]
- 910.Nakata S, Noda A, Yasuma F, et al. Effects of nasal surgery on sleep quality in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol 2008;22(1):59–63. [DOI] [PubMed] [Google Scholar]
- 911.Shuaib SW, Undavia S, Lin J, Johnson CM, Stupak HD. Can functional septorhinoplasty independently treat obstructive sleep apnea? Plast Reconstr Surg 2015;135(6):1554–1565. [DOI] [PubMed] [Google Scholar]
- 912.Hisamatsu K, Kudo I, Makiyama K. The effect of compound nasal surgery on obstructive sleep apnea syndrome. Am J Rhinol Allergy 2015;29(6):e192–e196. [DOI] [PubMed] [Google Scholar]
- 913.Lenders H, Schaefer J, Pirsig W. Turbinate hypertrophy in habitual snorers and patients with obstructive sleep-apnea - findings of acoustic rhinometry. Laryngoscope 1991;101(6):614–618. [DOI] [PubMed] [Google Scholar]
- 914.Alt JA, DeConde AS, Mace JC, Steele TO, Orlandi RR, Smith TL. Quality of life in patients with chronic rhinosinusitis and sleep dysfunction undergoing endoscopic sinus surgery a pilot investigation of comorbid obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg 2015;141(10):873–881. [DOI] [PubMed] [Google Scholar]
- 915.Alt JA, Ramakrishnan VR, Platt MP, Schlosser RJ, Storck T, Soler ZM. Impact of chronic rhinosinusitis on sleep: a controlled clinical study. Int Forum Allergy Rhinol 2019;9(1):16–22. [DOI] [PubMed] [Google Scholar]
- 916.Orb Q, Orlandi RR, Alt JA. Sleep dysfunction and its association to chronic rhinosinusitis: updated review. Laryngoscope Invest 2017;2(2):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Sunderram J, Weintraub M, Black K, et al. Chronic rhinosinusitis is an independent risk factor for OSA in world trade center responders. Chest 2019;155(2):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Jiang RS, Liang KL, Hsin CH, Su MC. The impact of chronic rhinosinusitis on sleep-disordered breathing. Rhinology 2016;54(1):75–79. [DOI] [PubMed] [Google Scholar]
- 919.Rotenberg BW, Pang KP. The impact of sinus surgery on sleep outcomes. Int Forum Allergy Rhinol 2015;5(4):329–332. [DOI] [PubMed] [Google Scholar]
- 920.Tosun F, Kemikli K, Yetkin S, Ozgen F, Durmaz A, Gerek M. Impact of endoscopic sinus surgery on sleep quality in patients with chronic nasal obstruction due to nasal polyposis. J Craniofac Surg 2009;20(2):446–449. [DOI] [PubMed] [Google Scholar]
- 921.Yalamanchali S, Cipta S, Waxman J, Pott T, Joseph N, Friedman M. Effects of endoscopic sinus surgery and nasal surgery in patients with obstructive sleep apnea. Otolaryngol Head Neck Surg 2014;151(1):171–175. [DOI] [PubMed] [Google Scholar]
- 922.Alt JA, Sautter NB, Mace JC, Detwiller KY, Smith TL. Antisomnogenic cytokines, quality of life, and chronic rhinosinusitis: a pilot study. Laryngoscope 2014;124(4):E107–E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.Altintas A, Soylu A, Yegin Y, Celik M, Kaya KH. Impact of laryngopharyngeal reflux on the levels of depression and anxiety in patients with obstructive sleep apnea syndrome. J Craniofac Surg 2017;28(2):E121–E124. [DOI] [PubMed] [Google Scholar]
- 924.Caparroz F, Campanholo M, Stefanini R, et al. Laryngopharyngeal reflux and dysphagia in patients with obstructive sleep apnea: is there an association? Sleep Breath 2019;23(2):619–626. [DOI] [PubMed] [Google Scholar]
- 925.Chan KK, Ing AJ, Laks L, Cossa G, Rogers P, Birring SS. Chronic cough in patients with sleep-disordered breathing. Eur Respir J 2010;35(2):368–372. [DOI] [PubMed] [Google Scholar]
- 926.Elhennawi DM, Ahmed MR, Abou-Halawa AS. Correlation of obstructive sleep apnoea and laryngopharyngeal reflux: phmetry study. Clin Otolaryngol 2016;41(6):758–761. [DOI] [PubMed] [Google Scholar]
- 927.Eryilmaz A, Erisen L, Demir UL, et al. Management of patients with coexisting obstructive sleep apnea and laryngopharyngeal reflux disease. Eur Arch Otorhinolaryngol 2012;269(12):2575–2580. [DOI] [PubMed] [Google Scholar]
- 928.Kim SJ, Kim H, Jeong JI, Hong SD, Chung SK, Dhong HJ. Changes in the reflux symptom index after multilevel surgery for obstructive sleep apnea. Clin Exp Otorhinolaryngol 2017;10(3):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Lee JS, Heo SJ, Kim JS, Ahn D, Sohn JH, Kim H. Relationship between the severity of laryngopharyngeal reflux and sleep apnea: using drug-induced sleep endoscopy (DISE). Eur Arch Otorhinolaryngol 2018;275(1):219–224. [DOI] [PubMed] [Google Scholar]
- 930.Magliulo G, Iannella G, Polimeni A, et al. Laryngopharyngeal reflux in obstructive sleep apnoea patients: literature review and meta-analysis. Am J Otolaryngol 2018;39(6):776–780. [DOI] [PubMed] [Google Scholar]
- 931.Morse CA, Quan SF, Mays MZ, Green C, Stephen G, Fass R. Is there a relationship between obstructive sleep apnea and gastroesophageal reflux disease? Clin Gastroenterol Hepatol 2004;2(9):761–768. [DOI] [PubMed] [Google Scholar]
- 932.Qu Y, Ye JY, Han DM, et al. Esophageal functional changes in obstructive sleep apnea/hypopnea syndrome and their impact on laryngopharyngeal reflux disease. Chin Med J (Engl) 2015;128(16):2162–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933.Rodrigues MM, Dibbern RS, Santos VJB, Passeri LA. Influence of obesity on the correlation between laryngopharyngeal reflux and obstructive sleep apnea. Braz J Otorhinolaryngol 2014;80(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934.Sundar KM, Daly SE, Pearce MJ, Alward WT. Chronic cough and obstructive sleep apnea in a community-based pulmonary practice. Cough 2010;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Teklu M, Gouveia CJ, Yalamanchili A, et al. Predicting obstructive sleep apnea status with the reflux symptom index in a sleep study population. Laryngoscope 2020;130(12):E952–E957. [DOI] [PubMed] [Google Scholar]
- 936.Xavier SD, Eckley CA, Duprat AC, et al. Temporal association between respiratory events and reflux in patients with obstructive sleep apnea and laryngopharyngeal reflux. J Clin Sleep Med 2019;15(10):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Caparroz FA, Campanholo MAT, Regina CG, et al. Clinical and polysomnographic predictors of laryngopharyngeal reflux in obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2019;85(4):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Gilani S, Quan SF, Pynnonen MA, Shin JJ. Obstructive sleep apnea and gastroesophageal reflux: a multivariate populationlevel analysis. Otolaryngol Head Neck Surg 2016;154(2):390–395. [DOI] [PubMed] [Google Scholar]
- 939.Kim Y, Lee YJ, Park JS, et al. Associations between obstructive sleep apnea severity and endoscopically proven gastroesophageal reflux disease. Sleep Breath 2018;22(1):85–90. [DOI] [PubMed] [Google Scholar]
- 940.Rassameehiran S, Klomjit S, Hosiriluck N, Nugent K. Meta-analysis of the effect of proton pump inhibitors on obstructive sleep apnea symptoms and indices in patients with gastroesophageal reflux disease. Proc (Bayl Univ Med Cent) 2016;29(1):3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Shepherd K, Orr W. Mechanism of gastroesophageal reflux in obstructive sleep apnea: airway obstruction or obesity? J Clin Sleep Med 2016;12(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942.Xavier SD, Moraes JP, Eckley CA. Prevalence of signs and symptoms of laryngopharyngeal reflux in snorers with suspected obstructive sleep apnea. Braz J Otorhinolaryngol 2013;79(5):589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Bjornsdottir E, Janson C, Sigurdsson JF, et al. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep 2013;36(12):1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Bjorvatn B, Pallesen S, Gronli J, Sivertsen B, Lehmann S. Prevalence and correlates of insomnia and excessive sleepiness in adults with obstructive sleep apnea symptoms. Percept Mot Skills 2014;118(2):571–586. [DOI] [PubMed] [Google Scholar]
- 945.Cho YW, Kim KT, Moon HJ, Korostyshevskiy VR, Motamedi GK, Yang KI. Comorbid insomnia with obstructive sleep apnea: clinical characteristics and risk factors. J Clin Sleep Med 2018;14(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Gupta MA, Knapp K. Cardiovascular and psychiatric morbidity in obstructive sleep apnea (OSA) with insomnia (sleep apnea plus) versus obstructive sleep apnea without insomnia: a case-control study from a nationally representative US sample. PLoS One 2014;9(3):e90021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Krakow B, Melendrez D, Ferreira E, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 2001;120(6):1923–1929. [DOI] [PubMed] [Google Scholar]
- 948.Lang CJ, Appleton SL, Vakulin A, et al. Co-morbid OSA and insomnia increases depression prevalence and severity in men. Respirology 2017;22(7):1407–1415. [DOI] [PubMed] [Google Scholar]
- 949.Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel a high rate of comorbid insomnia and obstructive sleep apnea. Chest 2013;144(2):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 950.Nguyen XL, Chaskalovic J, Rakotonanahary D, Fleury B. Insomnia symptoms and CPAP compliance in OSAS patients: a descriptive study using data mining methods. Sleep Med 2010;11(8):777–784. [DOI] [PubMed] [Google Scholar]
- 951.Sivertsen B, Bjornsdottir E, Overland S, Bjorvatn B, Salo P. The joint contribution of insomnia and obstructive sleep apnoea on sickness absence. J Sleep Res 2013;22(2):223–230. [DOI] [PubMed] [Google Scholar]
- 952.Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med 2004;5(5):449–456. [DOI] [PubMed] [Google Scholar]
- 953.Stelzer FG, Garcia E, Schorr F, Barea LM, Barros HT. Prevalence of chronic insomnia in patients with obstructive sleep apnea. Braz J Psychiatry 2021;43(4):370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Tasbakan MS, Gunduz C, Pirildar S, Basoglu OK. Quality of life in obstructive sleep apnea is related to female gender and comorbid insomnia. Sleep Breath 2018;22(4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 955.Wallace DM, Wohlgemuth WK. Predictors of insomnia severity index profiles in United States veterans with obstructive sleep apnea. J Clin Sleep Med 2019;15(12):1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Zhang Y, Ren R, Lei F, et al. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2019;45:1–17. [DOI] [PubMed] [Google Scholar]
- 957.Sweetman A, McEvoy RD, Smith S, et al. The effect of cognitive and behavioral therapy for insomnia on week-to-week changes in sleepiness and sleep parameters in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. Sleep 2020;43(7):zsaa002. [DOI] [PubMed] [Google Scholar]
- 958.Sweetman A, Lack L, McEvoy RD, et al. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med Rev 2021;60:101519. [DOI] [PubMed] [Google Scholar]
- 959.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019;142:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960.Boeve BF, Dickson DW, Olson EJ, et al. Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med 2007;8(1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep 2005;28(2):203–206. [DOI] [PubMed] [Google Scholar]
- 962.Li SX, Lam SP, Zhang JH, et al. A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med 2016;21:114–120. [DOI] [PubMed] [Google Scholar]
- 963.Gabryelska A, Roguski A, Simpson G, Maschauer EL, Morrison I, Riha RL. Prevalence of obstructive sleep apnoea in REM behaviour disorder: response to continuous positive airway pressure therapy. Sleep Breath 2018;22(3):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964.Koo DL, Lee JY, Nam H. Difference in severity of sleep apnea in patients with rapid eye movement sleep behavior disorder with or without parkinsonism. Sleep Med 2018;49:99–104. [DOI] [PubMed] [Google Scholar]
- 965.Rodrigues RND, Rodrigues AAADES, Pratesi R, Krieger J. Outcome of restless legs severity after continuous positive air pressure (CPAP) treatment in patients affected by the association of RLS and obstructive sleep apneas. Sleep Med 2006;7(3):235–239. [DOI] [PubMed] [Google Scholar]
- 966.Silva C, Peralta AR, Bentes C. The urge to move and breathe - the impact of obstructive sleep apnea syndrome treatment in patients with previously diagnosed, clinically significant restless legs syndrome. Sleep Med 2017;38:17–20. [DOI] [PubMed] [Google Scholar]
- 967.Aritake-Okada S, Namba K, Hidano N, et al. Change in frequency of periodic limb movements during sleep with usage of continuous positive airway pressure in obstructive sleep apnea syndrome. J Neurol Sci 2012;317(1–2):13–16. [DOI] [PubMed] [Google Scholar]
- 968.Ren R, Huang GP, Zhang JH, et al. Age and severity matched comparison of gender differences in the prevalence of periodic limb movements during sleep in patients with obstructive sleep apnea. Sleep Breath 2016;20(2):821–827. [DOI] [PubMed] [Google Scholar]
- 969.Iriarte J, Murie-Fernandez M, Toledo E, et al. Sleep structure in patients with periodic limb movements and obstructive sleep apnea syndrome. J Clin Neurophysiol 2009;26(4):267–271. [DOI] [PubMed] [Google Scholar]
- 970.Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med 2006;2(3):281–287. [PubMed] [Google Scholar]
- 971.Haba-Rubio J, Staner L, Krieger J, Macher JP. Periodic limb movements and sleepiness in obstructive sleep apnea patients. Sleep Med 2005;6(3):225–229. [DOI] [PubMed] [Google Scholar]
- 972.Drakatos P, Higgins S, Pengo MF, et al. Derived arterial stiffness is increased in patients with obstructive sleep apnea and periodic limb movements during sleep. J Clin Sleep Med 2016;12(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Murase K, Hitomi T, Hamada S, et al. The additive impact of periodic limb movements during sleep on inflammation in patients with obstructive sleep apnea. Ann Am Thorac Soc 2014;11(3):375–382. [DOI] [PubMed] [Google Scholar]
- 974.Wu MN, Lai CL, Liu CK, et al. Basal sympathetic predominance in periodic limb movements in sleep after continuous positive airway pressure. Sleep Breath 2018;22(4):1005–1012. [DOI] [PubMed] [Google Scholar]
- 975.Xie J, Chahal CAA, Covassin N, et al. Periodic limb movements of sleep are associated with an increased prevalence of atrial fibrillation in patients with mild sleep-disordered breathing. Int J Cardiol 2017;241:200–204. [DOI] [PubMed] [Google Scholar]
- 976.Jokubauskas L, Baltrusaityte A. Relationship between obstructive sleep apnoea syndrome and sleep bruxism: a systematic review. J Oral Rehabil 2017;44(2):144–153. [DOI] [PubMed] [Google Scholar]
- 977.Lopes AJD, Cunha TCA, Monteiro MCM, Serra-Negra JM, Cabral LC, Simamoto PC. Is there an association between sleep bruxism and obstructive sleep apnea syndrome? A systematic review. Sleep Breath 2020;24(3):913–921. [DOI] [PubMed] [Google Scholar]
- 978.Chiaro G, Maestri M, Riccardi S, et al. Sleep-related rhythmic movement disorder and obstructive sleep apnea in five adult patients. J Clin Sleep Med 2017;13(10):1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Bugalho P, Mendonca M, Barbosa R, Salavisa M. The influence of sleep disordered breathing in REM sleep behavior disorder. Sleep Med 2017;37:210–215. [DOI] [PubMed] [Google Scholar]
- 980.Lakshmanan S, Thompson NR, Pascoe M, et al. Impact of positive airway pressure on international restless legs syndrome score in sleep disordered breathing. J Clin Med 2019;8(12):2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Loewen A, Siemens A, Hanly P. Sleep disruption in patients with sleep apnea and end-stage renal disease. J Clin Sleep Med 2009;5(4):324–329. [PMC free article] [PubMed] [Google Scholar]
- 982.Xie J, Covassin N, Chahal AA, et al. Effect of adaptive servoventilation on periodic limb movements in sleep in patients with heart failure. Am J Cardiol 2019;123(4):632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Longstreth WT, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep 2007;30(1):13–26. [DOI] [PubMed] [Google Scholar]
- 984.Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet 2001;68(3):686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med 2018;43:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the innsbruck narcolepsy cohort. J Clin Sleep Med 2013;9(8):805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Sansa G, Iranzo A, Santamaria J. Obstructive sleep apnea in narcolepsy. Sleep Med 2010;11(1):93–95. [DOI] [PubMed] [Google Scholar]
- 988.Black J, Reaven NL, Funk SE, et al. Medical comorbidity in narcolepsy: findings from the burden of narcolepsy disease (BOND) study. Sleep Med 2017;33:13–18. [DOI] [PubMed] [Google Scholar]
- 989.Jennum P, Thorstensen EW, Pickering L, Ibsen R, Kjellberg J. Morbidity and mortality of middle-aged and elderly narcoleptics. Sleep Med 2017;36:23–28. [DOI] [PubMed] [Google Scholar]
- 990.Sullivan SS. Narcolepsy in adolescents. Adolesc Med State Art Rev 2010;21(3):542–555, x-xi. [PubMed] [Google Scholar]
- 991.Chervin RD, Aldrich MS. Sleep onset REM periods during multiple sleep latency tests in patients evaluated for sleep apnea. Am J Respir Crit Care Med 2000;161(2 Pt 1):426–431. [DOI] [PubMed] [Google Scholar]
- 992.George CF, Feldman N, Zheng Y, et al. A 2-week, polysomnographic, safety study of sodium oxybate in obstructive sleep apnea syndrome. Sleep Breath 2011;15(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993.Poli F, Ricotta L, Vandi S, et al. Catathrenia under sodium oxybate in narcolepsy with cataplexy. Sleep Breath 2012;16(2):427–434. [DOI] [PubMed] [Google Scholar]
- 994.Seeck-Hirschner M, Baier PC, von Freier A, Aldenhoff J, Goder R. Increase in sleep-related breathing disturbances after treatment with sodium oxybate in patients with narcolepsy and mild obstructive sleep apnea syndrome: two case reports. Sleep Med 2009;10(1):154–155. [DOI] [PubMed] [Google Scholar]
- 995.Series F, Series I, Cormier Y. Effects of enhancing slow-wave sleep by gamma-hydroxybutyrate on obstructive sleep apnea. Am Rev Respir Dis 1992;145(6):1378–1383. [DOI] [PubMed] [Google Scholar]
- 996.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep 2013;36(6):835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013;70(1–2):22–26. [DOI] [PubMed] [Google Scholar]
- 998.Filardi M, Demir N, Pizza F, et al. Prevalence and neurophysiological correlates of sleep disordered breathing in pediatric type 1 narcolepsy. Sleep Med 2020;65:8–12. [DOI] [PubMed] [Google Scholar]
- 999.Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol 2001;86(5):494–507; quiz 507–498. [DOI] [PubMed] [Google Scholar]
- 1000.Izci B, Vennelle M, Liston WA, Dundas KC, Calder AA, Douglas NJ. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J 2006;27(2):321–327. [DOI] [PubMed] [Google Scholar]
- 1001.Pamidi S, Kimoff RJ. Maternal sleep-disordered breathing. Chest 2018;153(4):1052–1066. [DOI] [PubMed] [Google Scholar]
- 1002.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol 2010;115(1):77–83. [DOI] [PubMed] [Google Scholar]
- 1003.Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol 2017;129(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep 2005;28(10):1299–1305. [DOI] [PubMed] [Google Scholar]
- 1005.Louis JM, Koch MA, Reddy UM, et al. Predictors of sleepdisordered breathing in pregnancy. Am J Obstet Gynecol 2018;218(5):521.e521–521.e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax 2014;69(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1007.Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev 2021;58:101436. [DOI] [PubMed] [Google Scholar]
- 1008.Bourjeily G, Danilack VA, Bublitz MH, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med 2017;38:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep 2014;37(5):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010.Liu L, Su G, Wang S, Zhu B. The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: a systematic review and meta-analysis. Sleep Breath 2019;23(2):399–412. [DOI] [PubMed] [Google Scholar]
- 1011.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep 2013;36(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Stajic D, Ilic D, Vukovic J, Baturan B, Ilic A, Milovancev A. The effect of continuous positive airway pressure treatment on hypertensive disorder in pregnant women with obstructive sleep apnea. Sleep Breath 2021;26(1):297–305. [DOI] [PubMed] [Google Scholar]
- 1013.Facco FM. Sleep Disordered Breathing, Obesity and Pregnancy Study (SOAP) ClinicalTrials.gov. Published 2022. Updated March 8, 2022. Accessed March 8, 2022. https://clinicaltrials.gov/ct2/show/NCT02086448
- 1014.Humphries A, Mirjalili SA, Tarr GP, Thompson JMD, Stone P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med 2019;32(23):3923–3930. [DOI] [PubMed] [Google Scholar]
- 1015.Dominguez JE, Street L, Louis J. Management of obstructive sleep apnea in pregnancy. Obstet Gynecol Clin North Am 2018;45(2):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.Tamisier R, Fabre F, O’Donoghue F, Levy P, Payen JF, Pepin JL. Anesthesia and sleep apnea. Sleep Med Rev 2018;40:79–92. [DOI] [PubMed] [Google Scholar]
- 1017.Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gynecol 2017;60(2):405–417. [DOI] [PubMed] [Google Scholar]
- 1018.Zaremba S, Mueller N, Heisig AM, et al. Elevated upper body position improves pregnancy-related OSA without impairing sleep quality or sleep architecture early after delivery. Chest 2015;148(4):936–944. [DOI] [PubMed] [Google Scholar]
- 1019.Amnakkittikul S, Chirakalwasan N, Wanitcharoenkul E, et al. Postpartum resolution of obstructive sleep apnea in women with gestational diabetes and the relationship with glucose metabolism. Acta Diabetol 2018;55(7):751–754. [DOI] [PubMed] [Google Scholar]
- 1020.Edwards N, Blyton DM, Hennessy A, Sullivan CE. Severity of sleep-disordered breathing improves following parturition. Sleep 2005;28(6):737–741. [DOI] [PubMed] [Google Scholar]
- 1021.Reid J, Glew RA, Skomro R, et al. Sleep disordered breathing and gestational hypertension: postpartum follow-up study. Sleep 2013;36(5):717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.Ponce S, Pastor E, Orosa B, et al. The role of CPAP treatment in elderly patients with moderate obstructive sleep apnoea: a multicentre randomised controlled trial. Eur Respir J 2019;54(2):1900518. [DOI] [PubMed] [Google Scholar]
- 1023.Zhao YY, Wang R, Gleason KJ, et al. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: best apnea interventions for research (BestAIR) trial. Sleep 2017;40(4):zsx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024.Wickwire EM, Albrecht JS, Towe MM, et al. The impact of treatments for OSA on monetized health economic outcomes: a systematic review. Chest 2019;155(5):947–961. [DOI] [PubMed] [Google Scholar]
- 1025.Zheng DN, Xu Y, You SJ, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular endpoint randomised trial and meta-analysis. EClinicalMedicine 2019;11:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.Ning Y, Zhang TS, Wen WW, et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath 2019;23(1):77–86. [DOI] [PubMed] [Google Scholar]
- 1027.Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J 2018;39(24): 2291. [DOI] [PubMed] [Google Scholar]
- 1028.Labarca G, Reyes T, Jorquera J, Dreyse J, Drake L. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: systematic review and meta-analysis. Clin Respir J 2018;12(8):2361–2368. [DOI] [PubMed] [Google Scholar]
- 1029.Gupta A, Shukla G, Afsar M, et al. Role of positive airway pressure therapy for obstructive sleep apnea in patients with stroke: a randomized controlled trial. J Clin Sleep Med 2018;14(4):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Congrete S, Bintvihok M, Thongprayoon C, et al. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: A meta-analysis. J Evid Based Med 2018;11(3):145–151. [DOI] [PubMed] [Google Scholar]
- 1031.Joyeux-Faure M, Baguet JP, Barone-Rochette G, et al. Continuous positive airway pressure reduces night-time blood pressure and heart rate in patients with obstructive sleep apnea and resistant hypertension: the RHOOSAS randomized controlled trial. Front Neurol 2018;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Abuzaid AS, Al Ashry HS, Elbadawi A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol 2017;120(4):693–699. [DOI] [PubMed] [Google Scholar]
- 1033.Campos-Rodriguez F, Gonzalez-Martinez M, Sanchez-Armengol A, et al. Effect of continuous positive airway pressure on blood pressure and metabolic profile in women with sleep apnoea. Eur Respir J 2017;50(2):1700257. [DOI] [PubMed] [Google Scholar]
- 1034.Hoyos CM, Yee BJ, Wong KK, Grunstein RR, Phillips CL. Treatment of sleep apnea with CPAP lowers central and peripheral blood pressure independent of the timeof-day: a randomized controlled study. Am J Hypertens 2015;28(10):1222–1228. [DOI] [PubMed] [Google Scholar]
- 1035.Ip S, D’Ambrosio C, Patel K, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev 2012;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036.Bloch KE, Huber F, Furian M, et al. Autoadjusted versus fixed CPAP for obstructive sleep apnoea: a multicentre, randomised equivalence trial. Thorax 2018;73(2):174–184. [DOI] [PubMed] [Google Scholar]
- 1037.Lebret M, Rotty MC, Argento C, et al. Comparison of auto- and fixed-continuous positive airway pressure on air leak in patients with obstructive sleep apnea: data from a randomized controlled trial. Can Respir J 2019;2019:6310956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038.Wimms AJ, Kelly JL, Turnbull CD, et al. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Resp Med 2020;8(4):349–358. [DOI] [PubMed] [Google Scholar]
- 1039.Pepin JL, Tamisier R, Baguet JP, et al. Fixed-pressure CPAP versus auto-adjusting CPAP: comparison of efficacy on blood pressure in obstructive sleep apnoea, a randomised clinical trial. Thorax 2016;71(8):726–733. [DOI] [PubMed] [Google Scholar]
- 1040.Sarac S, Afsar GC, Oruc O, Topcuoglu OB, Salturk C, Peker Y. Impact of patient education on compliance with positive airway pressure treatment in obstructive sleep apnea. Med Sci Monit 2017;23:1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med 2007;3(7):706–712. [PMC free article] [PubMed] [Google Scholar]
- 1042.Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment-emergent central sleep apnea. Ann Thorac Med 2016;11(3):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Selim B, Ramar K. Advanced positive airway pressure modes: adaptive servo ventilation and volume assured pressure support (vol 13, pg 839, 2016). Expert Rev Med Devices 2016;13(9):878–878. [DOI] [PubMed] [Google Scholar]
- 1044.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servoventilation for central sleep apnea in systolic heart failure. New Engl J Med 2015;373(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Knitter J, Bailey OF, Poongkunran C, et al. Comparison of physiological performance of four adaptive servo ventilation devices in patients with complex sleep apnea. Am J Resp Crit Care 2019;199(7):925–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Kuzniar TJ, Patel S, Nierodzik CL, Smith LC. Comparison of two servo ventilator devices in the treatment of complex sleep apnea. Sleep Med 2011;12(6):538–541. [DOI] [PubMed] [Google Scholar]
- 1047.Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest 2007;132(6):1839–1846. [DOI] [PubMed] [Google Scholar]
- 1048.Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med 2011;7(2):187–195. [PMC free article] [PubMed] [Google Scholar]
- 1049.Dellweg D, Kerl J, Hoehn E, Wenzel M, Koehler D. Randomized controlled trial of noninvasive positive pressure ventilation (NPPV) versus servoventilation in patients with CPAP-induced central sleep apnea (complex sleep apnea). Sleep 2013;36(8):1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Huseini T, Mcardle N, Jasper E, et al. The use and efficacy of adaptive servo-ventilation. J Sleep Res 2017;26:69–69. [DOI] [PubMed] [Google Scholar]
- 1051.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep 2007;30(4):468–475. [DOI] [PubMed] [Google Scholar]
- 1052.Morgenthaler TI, Kuzniar TJ, Wolfe LF, Willes L, McLain WC, Goldberg R. The complex sleep apnea resolution study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep 2014;37(5):U927–U297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1053.Pepin JLD, Woehrle H, Liu DQ, et al. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med 2018;14(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1054.Su M, Zhang X, Huang M, Ding N. Adaptive pressure support servoventilation: a novel treatment for residual sleepiness associated with central sleep apnea events. Sleep Breath 2011;15(4):695–699. [DOI] [PubMed] [Google Scholar]
- 1055.Yoshimura C, Toyoshima H, Matsumoto T, et al. Adaptive servo-ventilation (Asv) therapy improves long-term prognosis in patients with complex sleep apnea syndrome (Comp Sas) better than cheyne-stokes respiration (Csr)-central sleep apnea (Csa). J Sleep Res 2017;26:71–71. [Google Scholar]
- 1056.Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med 2014;10(6):637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.Aurora RN, Bista SR, Casey KR, et al. Updated adaptive servoventilation recommendations for the 2012 AASM guideline: “the treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses”. J Clin Sleep Med 2016;12(5):757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1058.Bachour A, Vitikainen P, Maasilta P. Rates of initial acceptance of PAP masks and outcomes of mask switching. Sleep Breath 2016;20(2):733–738. [DOI] [PubMed] [Google Scholar]
- 1059.Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst Rev 2006;2006(4):CD005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060.Andrade RGS, Viana FM, Nascimento JA, et al. Nasal vs oronasal CPAP for OSA treatment: a meta-analysis. Chest 2018;153(3):665–674. [DOI] [PubMed] [Google Scholar]
- 1061.Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One 2013;8(5):e64382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1062.Anderson FE, Kingshott RN, Taylor DR, Jones DR, Kline LR, Whyte KF. A randomized crossover efficacy trial of oral CPAP (Oracle) compared with nasal CPAP in the management of obstructive sleep apnea. Sleep 2003;26(6):721–726. [DOI] [PubMed] [Google Scholar]
- 1063.Ebben MR, Narizhnaya M, Segal AZ, Barone D, Krieger AC. A randomised controlled trial on the effect of mask choice on residual respiratory events with continuous positive airway pressure treatment. Sleep Med 2014;15(6):619–624. [DOI] [PubMed] [Google Scholar]
- 1064.Ebben MR, Oyegbile T, Pollak CP. The efficacy of three different mask styles on a PAP titration night. Sleep Med 2012;13(6):645–649. [DOI] [PubMed] [Google Scholar]
- 1065.Goh KJ, Soh RY, Leow LC, et al. Choosing the right mask for your Asian patient with sleep apnoea: a randomized, crossover trial of CPAP interfaces. Respirology 2019;24(3):278–285. [DOI] [PubMed] [Google Scholar]
- 1066.Khanna R, Kline LR. A prospective 8 week trial of nasal interfaces vs. a novel oral interface (Oracle) for treatment of obstructive sleep apnea hypopnea syndrome. Sleep Med 2003;4(4):333–338. [DOI] [PubMed] [Google Scholar]
- 1067.Massie CA, Hart RW. Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest 2003;123(4):1112–1118. [DOI] [PubMed] [Google Scholar]
- 1068.Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax 1998;53(4):290–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069.Rowland S, Aiyappan V, Hennessy C, et al. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med 2018;14(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1070.Ryan S, Garvey JF, Swan V, Behan R, McNicholas WT. Nasal pillows as an alternative interface in patients with obstructive sleep apnoea syndrome initiating continuous positive airway pressure therapy. J Sleep Res 2011;20(2):367–373. [DOI] [PubMed] [Google Scholar]
- 1071.Teo M, Amis T, Lee S, Falland K, Lambert S, Wheatley J. Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep 2011;34(7):951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.Zhu X, Wimms AJ, Benjafield AV. Assessment of the performance of nasal pillows at high CPAP pressures. J Clin Sleep Med 2013;9(9):873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073.Beecroft J, Zanon S, Lukic D, Hanly P. Oral continuous positive airway pressure for sleep apnea: effectiveness, patient preference, and adherence. Chest 2003;124(6):2200–2208. [DOI] [PubMed] [Google Scholar]
- 1074.Bettinzoli M, Taranto-Montemurro L, Messineo L, et al. Oronasal masks require higher levels of positive airway pressure than nasal masks to treat obstructive sleep apnea. Sleep Breath 2014;18(4):845–849. [DOI] [PubMed] [Google Scholar]
- 1075.Deshpande S, Joosten S, Turton A, et al. Oronasal masks require a higher pressure than nasal and nasal pillow masks for the treatment of obstructive sleep apnea. J Clin Sleep Med 2016;12(9):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076.Laaka A, Hollmen M, Bachour A. Evaluation of CPAP mask performance during 3 years of mask usage: time for reconsideration of renewal policies? BMJ Open Respir Res 2021;8(1):e001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1077.Calero G, Farre R, Ballester E, Hernandez L, Daniel N, Canal JMM. Physiological consequences of prolonged periods of flow limitation in patients with sleep apnea hypopnea syndrome. Resp Med 2006;100(5):813–817. [DOI] [PubMed] [Google Scholar]
- 1078.Meurice J, Paquereau J, Denjean A, Patte F, Series F. Influence of correction of flow limitation on continuous positive airway pressure efficiency in sleep apnoea/hypopnoea syndrome. Eur Respir J 1998;11(5):1121–1127. [DOI] [PubMed] [Google Scholar]
- 1079.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 2008;4(2): 157. [PMC free article] [PubMed] [Google Scholar]
- 1080.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep 2008;31(1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1081.Bureau M, Series F. Comparison of two in-laboratory titration methods to determine effective pressure levels in patients with obstructive sleep apnoea. Thorax 2000;55(9):741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082.McArdle N, Grove A, Devereux G, Mackay-Brown L, Mackay T, Douglas N. Split-night versus full-night studies for sleep apnoea/hypopnoea syndrome. Eur Respir J 2000;15(4):670–675. [DOI] [PubMed] [Google Scholar]
- 1083.Sanders MH, Montserrat JM, Farre R, Givelber RJ. Positive pressure therapy: a perspective on evidence-based outcomes and methods of application. Proc Am Thorac Soc 2008;5(2):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084.Patel NP, Ahmed M, Rosen I. Split-night polysomnography. Chest 2007;132(5):1664–1671. [DOI] [PubMed] [Google Scholar]
- 1085.Hoffstein V, Mateika S. Predicting nasal continuous positive airway pressure. Am J Respir Crit Care Med 1994;150(2):486–488. [DOI] [PubMed] [Google Scholar]
- 1086.Ebben MR, Narizhnaya M, Krieger AC. A new predictive model for continuous positive airway pressure in the treatment of obstructive sleep apnea. Sleep Breath 2017;21(2):435–442. [DOI] [PubMed] [Google Scholar]
- 1087.Rowley JA, Tarbichi AG, Badr MS. The use of a predicted CPAP equation improves CPAP titration success. Sleep Breath 2005;9(1):26–32. [DOI] [PubMed] [Google Scholar]
- 1088.Fitzpatrick MF, Alloway CE, Wakeford TM, MacLean AW, Munt PW, Day AG. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med 2003;167(5):716–722. [DOI] [PubMed] [Google Scholar]
- 1089.Lugo VM, Garmendia O, Suarez-Giron M, et al. Comprehensive management of obstructive sleep apnea by telemedicine: clinical improvement and cost-effectiveness of a Virtual Sleep Unit. A randomized controlled trial. PLoS One 2019;14(10):e0224069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090.Masa JF, Jimenez A, Duran J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med 2004;170(11):1218–1224. [DOI] [PubMed] [Google Scholar]
- 1091.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147(4):887–895. [DOI] [PubMed] [Google Scholar]
- 1092.Centers for Medicare & Medicaid Services. National Coverage Determination (NCD) for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (240.4) 2008. Published March 13, 2008. Accessed April 1, 2021. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?ncdid=226&ncdver=3&keyword=CPAP&keywordType=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&contractOption=all&sortBy=relevance&bc=AAAAAAQAAAAA&KeyWordLookUp=Doc&KeyWordSearchType=Exact
- 1093.Centers for Medicare & Medicaid Services. Local Coverage Determination (LCD): Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea (L33718) 2020. Published January 1, 2020. Accessed April 1, 2021. https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33718
- 1094.Centers for Medicare & Medicaid Services. Local Coverage Article: Positive Airway Pressure (PAP) Devices for the Treatment of Obstructive Sleep Apnea - Policy Article (A52467) 2020. Published April 3, 2020. Accessed April 1, 2021. https://www.cms.gov/medicare-coverage-database/details/article-details.aspx?articleid=52467&ver=35&keyword=CPAP&keywordType=starts&areaId=all&docType=NCA,CAL,NCD,MEDCAC,TA,MCD,6,3,5,1,F,P&contractOption=all&sortBy=relevance&bc=AAAAAAQAAAAA&KeyWordLookUp=Doc&KeyWordSearchType=Exact
- 1095.Borker PV, Carmona E, Essien UR, et al. Neighborhoods with greater prevalence of minority residents have lower CPAP adherence. Am J Respir Crit Care Med 2021;204(3):339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1096.Patel SR, Bakker JP, Stitt CJ, Aloia MS, Nouraie SM. Age and sex disparities in adherence to CPAP. Chest 2021;159(1):382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097.Platt AB, Field SH, Asch DA, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep 2009;32(6):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30(6):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1099.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest 1993;103(6):1675–1680. [DOI] [PubMed] [Google Scholar]
- 1100.Meurice JC, Dore P, Paquereau J, et al. Predictive factors of long-term compliance with nasal continuous positive airway pressure treatment in sleep apnea syndrome. Chest 1994;105(2):429–433. [DOI] [PubMed] [Google Scholar]
- 1101.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest 1995;107(2):375–381. [DOI] [PubMed] [Google Scholar]
- 1102.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest 1996;109(6):1470–1476. [DOI] [PubMed] [Google Scholar]
- 1103.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep 2003;26(3):308–311. [DOI] [PubMed] [Google Scholar]
- 1104.Means MK, Lichstein KL, Edinger JD, et al. Changes in depressive symptoms after continuous positive airway pressure treatment for obstructive sleep apnea. Sleep Breath 2003;7(1):31–42. [DOI] [PubMed] [Google Scholar]
- 1105.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS). Sleep Med Rev 2003;7(1):81–99. [DOI] [PubMed] [Google Scholar]
- 1106.Aloia MS, Arnedt JT, Stepnowsky C, Hecht J, Borrelli B. Predicting treatment adherence in obstructive sleep apnea using principles of behavior change. J Clin Sleep Med 2005;1(4):346–353. [PubMed] [Google Scholar]
- 1107.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 2011;15(6):343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1108.Bakker JP, O’Keeffe KM, Neill AM, Campbell AJ. Ethnic disparities in CPAP adherence in New Zealand: effects of socioeconomic status, health literacy and self-efficacy. Sleep 2011;34(11):1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109.Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep 2011;34(12):1653–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110.Billings ME, Rosen CL, Wang R, et al. Is the relationship between race and continuous positive airway pressure adherence mediated by sleep duration? Sleep 2013;36(2):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep 2007;30(3):320–324. [PubMed] [Google Scholar]
- 1112.Schwartz SW, Sebastiao Y, Rosas J, Iannacone MR, Foulis PR, Anderson WM. Racial disparity in adherence to positive airway pressure among US veterans. Sleep Breath 2016;20(3):947–955. [DOI] [PubMed] [Google Scholar]
- 1113.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep 2004;27(1):134–138. [DOI] [PubMed] [Google Scholar]
- 1114.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159(4 pt 1):1108–1114. [DOI] [PubMed] [Google Scholar]
- 1115.Pelletier-Fleury N, Rakotonanahary D, Fleury B. The age and other factors in the evaluation of compliance with nasal continuous positive airway pressure for obstructive sleep apnea syndrome. A Cox’s proportional hazard analysis. Sleep Med 2001;2(3):225–232. [DOI] [PubMed] [Google Scholar]
- 1116.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest 2002;121(2):430–435. [DOI] [PubMed] [Google Scholar]
- 1117.Stepnowsky CJ, Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep 2002;25(7):758–762. [DOI] [PubMed] [Google Scholar]
- 1118.Stepnowsky CJ, Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med 2002;3(3):239–247. [DOI] [PubMed] [Google Scholar]
- 1119.Lloberes P, Marti S, Sampol G, et al. Predictive factors of quality-of-life improvement and continuous positive airway pressure use in patients with sleep apnea-hypopnea syndrome: study at 1 year. Chest 2004;126(4):1241–1247. [DOI] [PubMed] [Google Scholar]
- 1120.Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J 2004;24(3):461–465. [DOI] [PubMed] [Google Scholar]
- 1121.Stepnowsky CJ, Marler MR, Palau J, Annette Brooks J. Socialcognitive correlates of CPAP adherence in experienced users. Sleep Med 2006;7(4):350–356. [DOI] [PubMed] [Google Scholar]
- 1122.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax 2010;65(9):829–832. [DOI] [PubMed] [Google Scholar]
- 1123.Wallace DM, Shafazand S, Aloia MS, Wohlgemuth WK. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med 2013;9(9):885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Wallace DM, Sawyer AM, Shafazand S. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath 2018;22(1):5–15. [DOI] [PubMed] [Google Scholar]
- 1125.Schoch OD, Baty F, Niedermann J, Rudiger JJ, Brutsche MH. Baseline predictors of adherence to positive airway pressure therapy for sleep apnea: a 10-year single-center observational cohort study. Respiration 2014;87(2):121–128. [DOI] [PubMed] [Google Scholar]
- 1126.Budhiraja R, Kushida CA, Nichols DA, et al. Impact of randomization, clinic visits, and medical and psychiatric cormorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J Clin Sleep Med 2016;12(3):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Dzierzewski JM, Wallace DM, Wohlgemuth WK. Adherence to continuous positive airway pressure in existing users: self-efficacy enhances the association between continuous positive airway pressure and adherence. J Clin Sleep Med 2016;12(2):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128.Jacobsen AR, Eriksen F, Hansen RW, et al. Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One 2017;12(12):e0189614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1129.Hoshino T, Sasanabe R, Tanigawa T, et al. Effect of rapid eye movement-related obstructive sleep apnea on adherence to continuous positive airway pressure. J Int Med Res 2018;46(6):2238–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Liou HYS, Kapur VK, Consens F, Billings ME. The effect of sleeping environment and sleeping location change on positive airway pressure adherence. J Clin Sleep Med 2018;14(10):1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Philip P, Bioulac S, Altena E, et al. Specific insomnia symptoms and self-efficacy explain CPAP compliance in a sample of OSAS patients. PLoS One 2018;13(4):e0195343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1132.Baron CE, Smith TW, Baucom BR, et al. Relationship partner social behavior and continuous positive airway pressure adherence: the role of autonomy support. Health Psychol 2020;39(4):325–334. [DOI] [PubMed] [Google Scholar]
- 1133.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep 2007;30(5):635–640. [DOI] [PubMed] [Google Scholar]
- 1134.Wozniak DR, Lasserson TJ, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2014(1):CD007736. [DOI] [PubMed] [Google Scholar]
- 1135.Chervin RD, Theut S, Bassetti C, Aldrich MS. Compliance with nasal CPAP can be improved by simple interventions. Sleep 1997;20(4):284–289. [DOI] [PubMed] [Google Scholar]
- 1136.Falcone VA, Damiani MF, Quaranta VN, Capozzolo A, Resta O. Polysomnograph chart view by patients: a new educational strategy to improve CPAP adherence in sleep apnea therapy. Resp Care 2014;59(2):193–198. [DOI] [PubMed] [Google Scholar]
- 1137.Hwang D, Chang JW, Benjafield AV, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The tele-OSA randomized trial. Am J Respir Crit Care Med 2018;197(1):117–126. [DOI] [PubMed] [Google Scholar]
- 1138.Pengo MF, Czaban M, Berry MP, et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. J Thorac Dis 2018;10:S160–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Roecklein KA, Schumacher JA, Gabriele JM, Fagan C, Baran AS, Richert AC. Personalized feedback to improve CPAP adherence in obstructive sleep apnea. Behav Sleep Med 2010;8(2):105–112. [DOI] [PubMed] [Google Scholar]
- 1140.Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2012;35(4):477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1141.Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Resp Crit Care 1999;159(4):1096–1100. [DOI] [PubMed] [Google Scholar]
- 1142.Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna S . A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med 2013;9(6):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143.DeMolles DA, Sparrow D, Gottlieb DJ, Friedman R. A pilot trial of a telecommunications system in sleep apnea management. Med Care 2004;42(8):764–769. [DOI] [PubMed] [Google Scholar]
- 1144.Hoet F, Libert W, Sanida C, Van den Broecke S, Bruyneel AV, Bruyneel M. Telemonitoring in continuous positive airway pressure-treated patients improves delay to first intervention and early compliance: a randomized trial. Sleep Med 2017;39:77–83. [DOI] [PubMed] [Google Scholar]
- 1145.Mendelson M, Vivodtzev I, Tamisier R, et al. CPAP treatment supported by telemedicine does not improve blood pressure in high cardiovascular risk OSA patients: a randomized, controlled trial. Sleep 2014;37(11):1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146.Munafo D, Hevener W, Crocker M, Willes L, Sridasome S, Muhsin M. A telehealth program for CPAP adherence reduces labor and yields similar adherence and efficacy when compared to standard of care. Sleep Breath 2016;20(2):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1147.Pepin JL, Jullian-Desayes I, Sapene M, et al. Multimodal remote monitoring of high cardiovascular risk patients with OSA initiating CPAP a randomized trial. Chest 2019;155(4):730–739. [DOI] [PubMed] [Google Scholar]
- 1148.Stepnowsky CJ, Palau JJ, Marler MR, Gifford AL. Pilot randomized trial of the effect of wireless telemonitoring on compliance and treatment. J Med Internet Res 2007;9(2):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149.Stepnowsky C, Edwards C, Zamora T, Barker R, Agha Z. Patient perspective on use of an interactive website for sleep apnea. Int J Telemed Appl 2013;2013:239382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150.Turino C, de Batlle J, Woehrle H, et al. Management of continuous positive airway pressure treatment compliance using telemonitoring in obstructive sleep apnoea. Eur Respir J 2017;49(2):1601128. [DOI] [PubMed] [Google Scholar]
- 1151.Aloia MS, Di Dio L, Ilniczky N, Perlis ML, Greenblatt DW, Giles DE. Improving compliance with nasal CPAP and vigilance in older adults with OAHS. Sleep Breath 2001;5(1):13–21. [DOI] [PubMed] [Google Scholar]
- 1152.Bakker JP, Wang R, Weng J, et al. Motivational enhancement for increasing adherence to CPAP a randomized controlled trial. Chest 2016;150(2):337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1153.Lai AYK, Fong DYT, Lam JCM, Weaver TE, Ip MSM. The efficacy of a brief motivational enhancement education program on CPAP adherence in OSA a randomized controlled trial. Chest 2014;146(3):600–610. [DOI] [PubMed] [Google Scholar]
- 1154.Dantas APT, Winck JC, Figueiredo-Braga M. Adherence to APAP in obstructive sleep apnea syndrome: effectiveness of a motivational intervention. Sleep Breath 2015;19(1):327–334. [DOI] [PubMed] [Google Scholar]
- 1155.Olsen S, Smith SS, Oei TPS, Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol 2012;80(1):151–163. [DOI] [PubMed] [Google Scholar]
- 1156.Wang WH, He GP, Wang MR, Liu LH, Tang HQ. Effects of patient education and progressive muscle relaxation alone or combined on adherence to continuous positive airway pressure treatment in obstructive sleep apnea patients. Sleep Breath 2012;16(4):1049–1057. [DOI] [PubMed] [Google Scholar]
- 1157.Watson NF, Mystkowski SK. Aerophagia and gastroesophageal reflux disease in patients using continuous positive airway pressure: a preliminary observation. J Clin Sleep Med 2008;4(5):434–438. [PMC free article] [PubMed] [Google Scholar]
- 1158.Shirlaw T, Hanssen K, Duce B, Hukins C. A randomized crossover trial comparing autotitrating and continuous positive airway pressure in subjects with symptoms of aerophagia: effects on compliance and subjective symptoms. J Clin Sleep Med 2017;13(7):881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med 2006;12(6):409–413. [DOI] [PubMed] [Google Scholar]
- 1160.Edmonds JC, Yang H, King TS, Sawyer DA, Rizzo A, Sawyer AM. Claustrophobic tendencies and continuous positive airway pressure therapy non-adherence in adults with obstructive sleep apnea. Heart Lung 2015;44(2):100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Edinger JD, Radtke RA. Use of in vivo desensitization to treat a patient’s claustrophobic response to nasal CPAP. Sleep 1993;16(7):678–680. [PubMed] [Google Scholar]
- 1162.McCrae CS, Rowe MA, Dautovich ND, et al. Sleep hygiene practices in two community dwelling samples of older adults. Sleep 2006;29(12):1551–1560. [DOI] [PubMed] [Google Scholar]
- 1163.Means MK, Edinger JD. Graded exposure therapy for addressing claustrophobic reactions to continuous positive airway pressure: a case series report. Behav Sleep Med 2007;5(2):105–116. [DOI] [PubMed] [Google Scholar]
- 1164.Koutsourelakis I, Vagiakis E, Perraki E, et al. Nasal inflammation in sleep apnoea patients using CPAP and effect of heated humidification. Eur Respir J 2011;37(3):587–594. [DOI] [PubMed] [Google Scholar]
- 1165.Zhu D, Wu M, Cao Y, et al. Heated humidification did not improve compliance of positive airway pressure and subjective daytime sleepiness in obstructive sleep apnea syndrome: a meta-analysis. PLoS One 2018;13(12):e0207994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Kennedy B, Lasserson TJ, Wozniak DR, Smith I. Pressure modification or humidification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2019;12(12):CD003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1167.Palm A, Midgren B, Theorell-Haglow J, et al. Factors influencing adherence to continuous positive airway pressure treatment in obstructive sleep apnea and mortality associated with treatment failure - a national registry-based cohort study. Sleep Med 2018;51:85–91. [DOI] [PubMed] [Google Scholar]
- 1168.Wiest GH, Harsch IA, Fuchs FS, et al. Initiation of CPAP therapy for OSA: does prophylactic humidification during CPAP pressure titration improve initial patient acceptance and comfort? Respiration 2002;69(5):406–412. [DOI] [PubMed] [Google Scholar]
- 1169.Varendh M, Andersson M, Bjornsdottir E, et al. Nocturnal nasal obstruction is frequent and reduces sleep quality in patients with obstructive sleep apnea. J Sleep Res 2018;27(4):e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1170.Li HY, Engleman H, Hsu CY, et al. Acoustic reflection for nasal airway measurement in patients with obstructive sleep apnea-hypopnea syndrome. Sleep 2005;28(12):1554–1559. [DOI] [PubMed] [Google Scholar]
- 1171.Värendh M, Andersson M, Bjornsdottir E, et al. PAP treatment in patients with OSA does not induce long-term nasal obstruction. J Sleep Res 2019;28(5):e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172.Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung 2019;197(2):115–121. [DOI] [PubMed] [Google Scholar]
- 1173.Van Ryswyk E, Anderson CS, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnea and cardiovascular disease. Sleep 2019;42(10):zsz152. [DOI] [PubMed] [Google Scholar]
- 1174.Skirko JR, James KT, Shusterman DJ, Weaver EM. Association of allergic rhinitis with change in nasal congestion in new continuous positive airway pressure users. JAMA Otolaryngol Head Neck Surg 2020;146(6):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1175.Cisternas A, Aguilar F, Montserrat JM, et al. Effects of CPAP in patients with obstructive apnoea: is the presence of allergic rhinitis relevant? Sleep Breath 2017;21(4):893–900. [DOI] [PubMed] [Google Scholar]
- 1176.Balsalobre L, Pezato R, Mangussi-Gomes J, et al. What is the impact of positive airway pressure in nasal polyposis? An experimental study. Int Arch Otorhinolaryngol 2019;23(2):147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177.La Mantia I, Andaloro C. Effectiveness of intranasal sodium hyaluronate in mitigating adverse effects of nasal continuous positive airway pressure therapy. Am J Rhinol Allergy 2017;31(6):364–369. [DOI] [PubMed] [Google Scholar]
- 1178.Ryan S, Doherty LS, Nolan GM, McNicholas WT. Effects of heated humidification and topical steroids on compliance, nasal symptoms, and quality of life in patients with obstructive sleep apnea syndrome using nasal continuous positive airway pressure. J Clin Sleep Med 2009;5(5):422–427. [PMC free article] [PubMed] [Google Scholar]
- 1179.Charakorn N, Hirunwiwatkul P, Chirakalwasan N, Chaitusaney B, Prakassajjatham M. The effects of topical nasal steroids on continuous positive airway pressure compliance in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 2017;21(1):3–8. [DOI] [PubMed] [Google Scholar]
- 1180.Camacho M, Riaz M, Capasso R, et al. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta-analysis. Sleep 2015;38(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181.Kempfle JS, BuSaba NY, Dobrowski JM, Westover MB, Bianchi MT. A cost-effectiveness analysis of nasal surgery to increase continuous positive airway pressure adherence in sleep apnea patients with nasal obstruction. Laryngoscope 2017;127(4):977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med 2018;13(2):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1183.Javaheri S, Goetting MG, Khayat R, Wylie PE, Goodwin JL, Parthasarathy S. The performance of two automatic servoventilation devices in the treatment of central sleep apnea. Sleep 2011;34(12):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1184.Heider K, Arzt M, Lerzer C, et al. Adaptive servo-ventilation and sleep quality in treatment emergent central sleep apnea and central sleep apnea in patients with heart disease and preserved ejection fraction. Clin Res Cardiol 2018;107(5):421–429. [DOI] [PubMed] [Google Scholar]
- 1185.Javaheri S, Winslow D, McCullough P, Wylie P, Kryger MH. The use of a fully automated automatic adaptive servoventilation algorithm in the acute and long-term treatment of central sleep apnea. Chest 2015;148(6):1454–1461. [DOI] [PubMed] [Google Scholar]
- 1186.Neu D, Balkissou AD, Mairesse O, Pefura-Yone EW, Noseda A. Complex sleep apnea at auto-titrating CPAP initiation: prevalence, significance and predictive factors. Clin Respir J 2017;11(2):200–209. [DOI] [PubMed] [Google Scholar]
- 1187.Alessi CA, Fung CH, Dzierzewski JM, et al. Randomized controlled trial of an integrated approach to treating insomnia and improving the use of positive airway pressure therapy in veterans with comorbid insomnia disorder and obstructive sleep apnea. Sleep 2021;44(4):zsaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1188.Ong JC, Crawford MR, Dawson SC, et al. A randomized controlled trial of CBT-I and PAP for obstructive sleep apnea and comorbid insomnia: main outcomes from the MATRICS study. Sleep 2020;43(9):zsaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1189.Sweetman A, Lack L, Bastien C. Co-morbid insomnia and sleep apnea (COMISA): prevalence, consequences, methodological considerations, and recent randomized controlled trials. Brain Sci 2019;9(12):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1190.Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep 2019;42(12):zsz178. [DOI] [PubMed] [Google Scholar]
- 1191.Sweetman A, Lack L, McEvoy RD, et al. Cognitive behavioural therapy for insomnia reduces sleep apnoea severity: a randomised controlled trial. ERJ Open Res 2020;6(2):00161–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1192.Bjorvatn B, Berge T, Lehmann S, Pallesen S, Saxvig IW. No effect of a self-help book for insomnia in patients with obstructive sleep apnea and comorbid chronic insomnia - a randomized controlled trial. Front Psychol 2018;9:2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193.Zhang XJ, Li QY, Wang Y, Xu HJ, Lin YN. The effect of non-benzodiazepine hypnotics on sleep quality and severity in patients with OSA: a meta-analysis. Sleep Breath 2014;18(4):781–789. [DOI] [PubMed] [Google Scholar]
- 1194.Luyster FS, Strollo PJ, Thunstrom E, Peker Y. Long-term use of continuous positive airway pressure therapy in coronary artery disease patients with nonsleepy obstructive sleep apnea. Clin Cardiol 2017;40(12):1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep 1997;20(4):278–283. [DOI] [PubMed] [Google Scholar]
- 1196.Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med 2013;188(5):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1197.Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy a retrospective analysis. Chest 2018;153(4):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1198.Kuna ST, Shuttleworth D, Chi L, et al. Web-based access to positive airway pressure usage with or without an initial financial incentive improves treatment use in patients with obstructive sleep apnea. Sleep 2015;38(8):1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1199.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 2006;61(5):430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev 2009;13(6):427–436. [DOI] [PubMed] [Google Scholar]
- 1201.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006;29(3):381–401. [DOI] [PubMed] [Google Scholar]
- 1202.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006(1):CD001106. [DOI] [PubMed] [Google Scholar]
- 1203.Sanchez-de-la-Torre M, Sanchez-de-la-Torre A, Bertran S, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med 2020;8(4):359–367. [DOI] [PubMed] [Google Scholar]
- 1204.Huang ZW, Liu ZH, Luo Q, et al. Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial. Am J Hypertens 2015;28(3):300–306. [DOI] [PubMed] [Google Scholar]
- 1205.Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med 2019;200(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1206.Pack AI, Magalang UJ, Singh B, Kuna ST, Keenan BT, Maislin G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias. Sleep 2021;44(2):zsaa229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1207.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007;50(2):417–423. [DOI] [PubMed] [Google Scholar]
- 1208.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea a systematic review and meta-analysis. JAMA 2015;314(21):2280–2293. [DOI] [PubMed] [Google Scholar]
- 1209.Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 2014;145(4):762–771. [DOI] [PubMed] [Google Scholar]
- 1210.Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014;32(12):2341–2350; discussion 2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1211.Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2016;18(2):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1212.Mansukhani MP, Somers VK, Shafazand S. PAP and cardiovascular events in adults with sleep apnea: is PAP useful? J Clin Sleep Med 2017;13(12):1487–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1213.Bakker JP, Edwards BA, Gautam SP, et al. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnea severity (vol 10, pg 365, 2014). J Clin Sleep Med 2014;10(6):711–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1214.Turnbull F, Blood Pressure Lowering Treatment Trialists C. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362(9395):1527–1535. [DOI] [PubMed] [Google Scholar]
- 1215.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62(4):300–305. [DOI] [PubMed] [Google Scholar]
- 1216.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107(20):2589–2594. [DOI] [PubMed] [Google Scholar]
- 1217.Srivali N, Chahal AC, Mansukhani MP, Mandrekar J, Somers VK, Caples SM. The effect of positive airway pressure treatment of obstructive and central sleep apnea on the recurrence of atrial fibrillation/flutter postintervention. J Clin Sleep Med 2019;15(10):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1218.Caples SM, Mansukhani MP, Friedman PA, Somers VK. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol 2019;278:133–136. [DOI] [PubMed] [Google Scholar]
- 1219.McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Resp Med 2014;2(10):804–812. [DOI] [PubMed] [Google Scholar]
- 1220.Aaronson JA, Hofman WF, van Bennekom CAM, et al. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med 2016;12(4):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1221.Bravata DM, Concato J, Fried T, et al. Auto-titrating continuous positive airway pressure for patients with acute transient ischemic attack a randomized feasibility trial. Stroke 2010;41(7):1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1222.Kim Y, Koo YS, Lee HY, Lee SY. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis. PLoS One 2016;11(1):e0146317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1223.King S, Cuellar N. Obstructive sleep apnea as an independent stroke risk factor: a review of the evidence, stroke prevention guidelines, and implications for neuroscience nursing practice. J Neurosci Nurs 2016;48(3):133–142. [DOI] [PubMed] [Google Scholar]
- 1224.McKee Z, Wilson RD, Auckley DH. Evaluation of an OSA risk stratifying and treatment protocol during inpatient rehabilitation of post-stroke patients. Sleep Breath 2020;24(2):513–521. [DOI] [PubMed] [Google Scholar]
- 1225.Parra O, Sanchez-Armengol A, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res 2015;24(1):47–53. [DOI] [PubMed] [Google Scholar]
- 1226.Parra O, Sanchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011;37(5):1128–1136. [DOI] [PubMed] [Google Scholar]
- 1227.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke 2011;42(4):1062–1067. [DOI] [PubMed] [Google Scholar]
- 1228.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 1998;97(21):2154–2159. [DOI] [PubMed] [Google Scholar]
- 1229.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999;160(4):1101–1106. [DOI] [PubMed] [Google Scholar]
- 1230.Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation 1998;98(21):2269–2275. [DOI] [PubMed] [Google Scholar]
- 1231.Aggarwal S, Nadeem R, Loomba RS, Nida M, Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol 2014;37(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232.Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol 2005;45(12):2008–2011. [DOI] [PubMed] [Google Scholar]
- 1233.Gilman MP, Floras JS, Usui K, Kaneko Y, Leung RS, Bradley TD. Continuous positive airway pressure increases heart rate variability in heart failure patients with obstructive sleep apnoea. Clin Sci (Lond) 2008;114(3):243–249. [DOI] [PubMed] [Google Scholar]
- 1234.Yoshinaga K, Burwash IG, Leech JA, et al. The effects of continuous positive airway pressure on myocardial energetics in patients with heart failure and obstructive sleep apnea. J Am Coll Cardiol 2007;49(4):450–458. [DOI] [PubMed] [Google Scholar]
- 1235.Hall AB, Ziadi MC, Leech JA, et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation 2014;130(11):892–901. [DOI] [PubMed] [Google Scholar]
- 1236.Ferrier KA, Neill AM, O’Meeghan T, Richards M, Campbell AJ. Continuous positive airway pressure in heart failure patients with obstructive sleep apnoea. Intern Med J 2008;38(11):829–836. [DOI] [PubMed] [Google Scholar]
- 1237.Johnson CB, Beanlands RS, Yoshinaga K, et al. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can J Cardiol 2008;24(9):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1238.Malone S, Liu PP, Holloway R, Rutherford R, Xie A, Bradley TD. Obstructive sleep apnoea in patients with dilated cardiomyopathy: effects of continuous positive airway pressure. Lancet 1991;338(8781):1480–1484. [DOI] [PubMed] [Google Scholar]
- 1239.Smith LA, Vennelle M, Gardner RS, et al. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J 2007;28(10):1221–1227. [DOI] [PubMed] [Google Scholar]
- 1240.Lisi E, Faini A, Bilo G, et al. Diastolic dysfunction in controlled hypertensive patients with mild-moderate obstructive sleep apnea. Int J Cardiol 2015;187:686–692. [DOI] [PubMed] [Google Scholar]
- 1241.Cloward TV, Walker JM, Farney RJ, Anderson JL. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with nasal continuous positive airway pressure. Chest 2003;124(2):594–601. [DOI] [PubMed] [Google Scholar]
- 1242.Akar Bayram N, Ciftci B, Durmaz T, et al. Effects of continuous positive airway pressure therapy on left ventricular function assessed by tissue Doppler imaging in patients with obstructive sleep apnoea syndrome. Eur J Echocardiogr 2009;10(3):376–382. [DOI] [PubMed] [Google Scholar]
- 1243.Alchanatis M, Paradellis G, Pini H, Tourkohoriti G, Jordanoglou J. Left ventricular function in patients with obstructive sleep apnoea syndrome before and after treatment with nasal continuous positive airway pressure. Respiration 2000;67(4):367–371. [DOI] [PubMed] [Google Scholar]
- 1244.Dursunoglu N, Dursunoglu D, Ozkurt S, et al. Effects of CPAP on left ventricular structure and myocardial performance index in male patients with obstructive sleep apnoea. Sleep Med 2007;8(1):51–59. [DOI] [PubMed] [Google Scholar]
- 1245.Glantz H, Johansson MC, Thunstrom E, et al. Effect of CPAP on diastolic function in coronary artery disease patients with nonsleepy obstructive sleep apnea: a randomized controlled trial. Int J Cardiol 2017;241:12–18. [DOI] [PubMed] [Google Scholar]
- 1246.Shivalkar B, Van de Heyning C, Kerremans M, et al. Obstructive sleep apnea syndrome: more insights on structural and functional cardiac alterations, and the effects of treatment with continuous positive airway pressure. J Am Coll Cardiol 2006;47(7):1433–1439. [DOI] [PubMed] [Google Scholar]
- 1247.Craig S, Kylintireas I, Kohler M, et al. Effect of CPAP on cardiac function in minimally symptomatic patients with OSA: results from a subset of the MOSAIC randomized trial. J Clin Sleep Med 2015;11(9):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1248.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J 2006;28(3):596–602. [DOI] [PubMed] [Google Scholar]
- 1249.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Resp Crit Care 2007;176(12):1274–1280. [DOI] [PubMed] [Google Scholar]
- 1250.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005;127(6):2076–2084. [DOI] [PubMed] [Google Scholar]
- 1251.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol 2007;50(14):1310–1314. [DOI] [PubMed] [Google Scholar]
- 1252.da Silva Paulitsch F, Zhang L. Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: a meta-analysis of randomized trials. Sleep Med 2019;54:28–34. [DOI] [PubMed] [Google Scholar]
- 1253.Andrade AG, Bubu OM, Varga AW, Osorio RS. The relationship between obstructive sleep apnea and Alzheimer’s disease. J Alzheimers Dis 2018;64:S255–S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1254.Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol 2019;18(3):296–306. [DOI] [PubMed] [Google Scholar]
- 1255.Perez-Cabezas V, Ruiz-Molinero C, Jimenez-Rejano JJ, Gonzalez-Medina G, Galan-Mercant A, Martin-Valero R. Continuous positive airway pressure treatment in patients with Alzheimer’s disease: a systematic review. J Clin Med 2020;9(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1256.Liguori C, Mercuri NB, Izzi F, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep 2017;40(5). 10.1093/sleep/zsx011 [DOI] [PubMed] [Google Scholar]
- 1257.Wang CN, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 2020;45(1):104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1258.Lipford MC, Flemming KD, Calvin AD, et al. Associations between cardioembolic stroke and obstructive sleep apnea. Sleep 2015;38(11):1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1259.Huang X, Tang S, Lyu XJ, Yang CQ, Chen XP. Structural and functional brain alterations in obstructive sleep apnea: a multimodal meta-analysis. Sleep Med 2019;54:195–204. [DOI] [PubMed] [Google Scholar]
- 1260.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci 2016;39(8):552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1261.Lajoie AC, Lafontaine AL, Kimoff RJ, Kaminska M. Obstructive sleep apnea in neurodegenerative disorders: current evidence in support of benefit from sleep apnea treatment. J Clin Med 2020;9(2):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1262.Xie LL, Kang HY, Xu QW, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1263.O’Hara R, Luzon A, Hubbard J, Zeitzer JM. Sleep apnea, apolipoprotein epsilon 4 allele, and TBI: mechanism for cognitive dysfunction and development of dementia. J Rehabil Res Dev 2009;46(6):837–850. [DOI] [PubMed] [Google Scholar]
- 1264.Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018;86:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265.Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 2002;131(1–2):135–144. [DOI] [PubMed] [Google Scholar]
- 1266.Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev 2013;17(5):341–347. [DOI] [PubMed] [Google Scholar]
- 1267.Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for improvements in sleepiness, cognition, mood, and quality of life in elderly patients with OSA: systematic review and meta-analysis of randomized controlled trials. Chest 2020;158(2):751–764. [DOI] [PubMed] [Google Scholar]
- 1268.Pan YY, Deng Y, Xu X, Liu YP, Liu HG. Effects of continuous positive airway pressure on cognitive deficits in middle-aged patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Chin Med J (Engl) 2015;128(17):2365–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1269.Yan B, Jin Y, Hu Y, Li S. Effects of continuous positive airway pressure on elderly patients with obstructive sleep apnea: a meta-analysis. Med Sci (Paris) 2018;34(Focus issue F1):66–73. [DOI] [PubMed] [Google Scholar]
- 1270.Pan WH, Kastin AJ. Can sleep apnea cause Alzheimer’s disease? Neurosci Biobehav Rev 2014;47:656–669. [DOI] [PubMed] [Google Scholar]
- 1271.Richards KC, Gooneratne N, Dicicco B, et al. CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc 2019;67(3):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1272.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the apnea positive pressure long-term efficacy study (APPLES). Sleep 2012;35(12):1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1273.Berlowitz DJ, Shafazand S. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). J Clin Sleep Med 2013;9(5):516–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1274.Wang ML, Wang C, Tuo M, et al. Cognitive effects of treating obstructive sleep apnea: a meta-analysis of randomized controlled trials. J Alzheimers Dis 2020;75(3):705–715. [DOI] [PubMed] [Google Scholar]
- 1275.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc 2008;56(11):2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1276.Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med 2009;5(4):305–309. [PMC free article] [PubMed] [Google Scholar]
- 1277.Wang Y, Cheng C, Moelter S, et al. One year of continuous positive airway pressure adherence improves cognition in older adults with mild apnea and mild cognitive impairment. Nurs Res 2020;69(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1278.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84(19):1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1279.Skiba V, Novikova M, Suneja A, McLellan B, Schultz L. Use of positive airway pressure in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med 2020;16(6):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1280.Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1281.Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest 2015;148(5):1214–1223. [DOI] [PubMed] [Google Scholar]
- 1282.McMillan A, Bratton DJ, Faria R, et al. A multicentre randomised controlled trial and economic evaluation of continuous positive airway pressure for the treatment of obstructive sleep apnoea syndrome in older people: PREDICT. Health Technol Assess 2015;19(40):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1283.Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J 2015;46(1):142–151. [DOI] [PubMed] [Google Scholar]
- 1284.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med 2005;165(4):447–452. [DOI] [PubMed] [Google Scholar]
- 1285.Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath 2005;9(4):176–180. [DOI] [PubMed] [Google Scholar]
- 1286.Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab 2012;97(11):4212–4218. [DOI] [PubMed] [Google Scholar]
- 1287.Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung 2009;187(1):17–22. [DOI] [PubMed] [Google Scholar]
- 1288.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007;62(11):969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1289.Martinez-Ceron E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med 2016;194(4):476–485. [DOI] [PubMed] [Google Scholar]
- 1290.Abud R, Salgueiro M, Drake L, Reyes T, Jorquera J, Labarca G. Efficacy of continuous positive airway pressure (CPAP) preventing type 2 diabetes mellitus in patients with obstructive sleep apnea hypopnea syndrome (OSAHS) and insulin resistance: a systematic review and meta-analysis. Sleep Med 2019;62:14–21. [DOI] [PubMed] [Google Scholar]
- 1291.Martinez-Ceron E, Fernandez-Navarro I, Garcia-Rio F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev 2016;25:121–130. [DOI] [PubMed] [Google Scholar]
- 1292.Burks SV, Anderson JE, Bombyk M, et al. Nonadherence with employer-mandated sleep apnea treatment and increased risk of serious truck crashes. Sleep 2016;39(5):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1293.Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, Petridou ET. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev 2011;15(5):301–310. [DOI] [PubMed] [Google Scholar]
- 1294.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep 2010;33(10):1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1295.Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med 2001;134(11):1015–1023. [DOI] [PubMed] [Google Scholar]
- 1296.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med 1999;159(2):461–467. [DOI] [PubMed] [Google Scholar]
- 1297.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax 1998;53(5):341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1298.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001;164(6):939–943. [DOI] [PubMed] [Google Scholar]
- 1299.Barbe F, Sunyer J, de la Pena A, et al. Effect of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration 2007;74(1):44–49. [DOI] [PubMed] [Google Scholar]
- 1300.Cassel W, Ploch T, Becker C, Dugnus D, Peter JH, von Wichert P. Risk of traffic accidents in patients with sleep-disordered breathing: reduction with nasal CPAP. Eur Respir J 1996;9(12):2606–2611. [DOI] [PubMed] [Google Scholar]
- 1301.Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep 1996;19(5):378–381. [DOI] [PubMed] [Google Scholar]
- 1302.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Resp Crit Care 2000;161(3):857–859. [DOI] [PubMed] [Google Scholar]
- 1303.George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001;56(7):508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1304.Horstmann S, Hess CW, Bassetti C, Gugger M, Mathis J. Sleepiness-related accidents in sleep apnea patients. Sleep 2000;23(3):383–389. [PubMed] [Google Scholar]
- 1305.Karimi M, Hedner J, Habel H, Nerman O, Grote L. Sleep apnea related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish traffic accident registry data. Sleep 2015;38(3):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1306.Komada Y, Nishida Y, Namba K, Abe T, Tsuiki S, Inoue Y. Elevated risk of motor vehicle accident for male drivers with obstructive sleep apnea syndrome in the Tokyo metropolitan area. Tohoku J Exp Med 2009;219(1):11–16. [DOI] [PubMed] [Google Scholar]
- 1307.Krieger J, Meslier N, Lebrun T, et al. Accidents in obstructive sleep apnea patients treated with nasal continuous positive airway pressure - a prospective study. Chest 1997;112(6):1561–1566. [DOI] [PubMed] [Google Scholar]
- 1308.Yamamoto H, Akashiba T, Kosaka N, Ito D, Horie T. Long-term effects nasal continuous positive airway pressure on daytime sleepiness, mood and traffic accidents in patients with obstructive sleep apnoea. Resp Med 2000;94(1):87–90. [DOI] [PubMed] [Google Scholar]
- 1309.Barnes M, Houston D, Worsnop CJ, et al. A Randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Resp Crit Care 2002;165(6):773–780. [DOI] [PubMed] [Google Scholar]
- 1310.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Resp Crit Care 1998;157(3):858–865. [DOI] [PubMed] [Google Scholar]
- 1311.Scharf MB, Stover R, McDannold MD, Spinner O, Berkowitz DV, Conrad C. Outcome evaluation of long-term nasal continuous positive airway pressure therapy in obstructive sleep apnea. Am J Ther 1999;6(6):293–297. [DOI] [PubMed] [Google Scholar]
- 1312.Findley LJ, Fabrizio MJ, Knight H, Norcross BB, Laforte AJ, Suratt PM. Driving simulator performance in patients with sleep-apnea. Am Rev Respir Dis 1989;140(2):529–530. [DOI] [PubMed] [Google Scholar]
- 1313.George CFP, Boudreau AC, Smiley A. Effects of nasal CPAP on simulated driving performance in patients with obstructive sleep apnoea. Thorax 1997;52(7):648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1314.Hack M, Davies RJO, Mullins R, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax 2000;55(3):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1315.Orth M, Duchna HW, Leidag M, et al. Driving simulator and neuropsychological testing in OSAS before and under CPAP therapy (vol 26, pg 898, 2005). Eur Respir J 2006;27(1):242–242. [DOI] [PubMed] [Google Scholar]
- 1316.Suratt P, Findley, L. Effect of nasal CPAP treatment on automobile driving simulator performance and on self reported automobile accidents in subjects with sleep apnea (abstract). Am Rev Respir Dis 1992;145(A169):(Abstract). [Google Scholar]
- 1317.Turkington PM, Sircar M, Saralaya D, Elliott MW. Time course of changes in driving simulator performance with and without treatment in patients with sleep apnoea hypopnoea syndrome. Thorax 2004;59(1):56–59. [PMC free article] [PubMed] [Google Scholar]
- 1318.Minemura H, Akashiba T, Yamamoto H, et al. Traffic accidents in obstructive sleep apnea patients and effect of nasal CPAP treatment. Nihon Kyobu Shikkan Gakkai Zasshi 1993;31(9):1103–1108. [PubMed] [Google Scholar]
- 1319.Botokeky E, Freymond N, Gormand F, et al. Benefit of continuous positive airway pressure on work quality in patients with severe obstructive sleep apnea. Sleep Breath 2019;23(3):753–759. [DOI] [PubMed] [Google Scholar]
- 1320.Jurado-Gamez B, Guglielmi O, Gude-Sampedro F, Buela-Casal G. Effect of CPAP therapy on job productivity and psychosocial occupational health in patients with moderate to severe sleep apnea. Sleep Breath 2015;19(4):1293–1299. [DOI] [PubMed] [Google Scholar]
- 1321.Ulfberg J, Jonsson R, Edling C. Improvement of subjective work performance among obstructive sleep apnea patients after treatment with continuous positive airway pressure. Psychiatry Clin Neurosci 1999;53(6):677–679. [DOI] [PubMed] [Google Scholar]
- 1322.Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med 2007;9(1):42–53. [DOI] [PubMed] [Google Scholar]
- 1323.Glidewell RN, Renn BN, Roby E, Orr WC. Predictors and patterns of insomnia symptoms in OSA before and after PAP therapy. Sleep Med 2014;15(8):899–905. [DOI] [PubMed] [Google Scholar]
- 1324.Alawami M, Mustafa A, Whyte K, Alkhater M, Bhikoo Z, Pemberton J. Echocardiographic and electrocardiographic findings in patients with obesity hypoventilation syndrome. Intern Med J 2015;45(1):68–73. [DOI] [PubMed] [Google Scholar]
- 1325.Kessler R, Chaouat A, Schinkewitch P, et al. The obesityhypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 2001;120(2):369–376. [DOI] [PubMed] [Google Scholar]
- 1326.Kreivi HR, Italuoma T, Bachour A. Effect of ventilation therapy on mortality rate among obesity hypoventilation syndrome and obstructive sleep apnoea patients. ERJ Open Res 2020;6(2):00101–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1327.Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001;120(2):377–383. [DOI] [PubMed] [Google Scholar]
- 1328.Jennum P, Ibsen R, Kjellberg J. Social consequences of sleep disordered breathing on patients and their partners: a controlled national study. Eur Respir J 2014;43(1):134–144. [DOI] [PubMed] [Google Scholar]
- 1329.Castro-Anon O, de Llano LAP, Sanchez SD, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One 2015;10(2):e0117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1330.Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax 2012;67(8):727–734. [DOI] [PubMed] [Google Scholar]
- 1331.Patout M, Gagnadoux F, Rabec C, et al. AVAPS-AE versus ST mode: a randomized controlled trial in patients with obesity hypoventilation syndrome. Respirology 2020;25(10):1073–1081. [DOI] [PubMed] [Google Scholar]
- 1332.Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation - a randomized crossover trial. Chest 2006;130(3):815–821. [DOI] [PubMed] [Google Scholar]
- 1333.Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax 2009;64(8):719–725. [DOI] [PubMed] [Google Scholar]
- 1334.Howard ME, Piper AJ, Stevens B, et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax 2017;72(5):437–444. [DOI] [PubMed] [Google Scholar]
- 1335.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008;63(5):395–401. [DOI] [PubMed] [Google Scholar]
- 1336.Royer CP, Schweiger C, Manica D, Rabaioli L, Guerra V, Sbruzzi G. Efficacy of bilevel ventilatory support in the treatment of stable patients with obesity hypoventilation syndrome: systematic review and meta-analysis. Sleep Med 2019;53:153–164. [DOI] [PubMed] [Google Scholar]
- 1337.Soghier I, Brozek JL, Afshar M, et al. Noninvasive ventilation versus CPAP as initial treatment of obesity hypoventilation syndrome. Ann Am Thorac Soc 2019;16(10):1295–1303. [DOI] [PubMed] [Google Scholar]
- 1338.Bouloukaki I, Mermigkis C, Michelakis S, et al. The association between adherence to positive airway pressure therapy and long-term outcomes in patients with obesity hypoventilation syndrome: a prospective observational study. J Clin Sleep Med 2018;14(9):1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1339.Masa JF, Mokhlesi B, Benitez I, et al. Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet 2019;393(10182):1721–1732. [DOI] [PubMed] [Google Scholar]
- 1340.Corral J, Mogollon MV, Sanchez-Quiroga MA, et al. Echocardiographic changes with non-invasive ventilation and CPAP in obesity hypoventilation syndrome. Thorax 2018;73(4):361–368. [DOI] [PubMed] [Google Scholar]
- 1341.Masa JF, Mokhlesi B, Benitez I, et al. Cost-effectiveness of positive airway pressure modalities in obesity hypoventilation syndrome with severe obstructive sleep apnoea. Thorax 2020;75(6):459–467. [DOI] [PubMed] [Google Scholar]
- 1342.Masa JF, Benitez I, Sanchez-Quiroga MA, et al. Long-term noninvasive ventilation in obesity hypoventilation syndrome without severe OSA the pickwick randomized controlled trial. Chest 2020;158(3):1176–1186. [DOI] [PubMed] [Google Scholar]
- 1343.Mokhlesi B, Masa JF, Brozek JL, et al. Evaluation and management of obesity hypoventilation syndrome. An Official American Thoracic Society Clinical Practice Guideline (vol 200, pg e6, 2009). Am J Resp Crit Care 2019;200(10):1325–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1344.de Llano LAP, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration 2008;75(1):34–39. [DOI] [PubMed] [Google Scholar]
- 1345.Masa JF, Corral J, Caballero C, et al. Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax 2016;71(10):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1346.Carrillo A, Ferrer M, Gonzalez-Diaz G, et al. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Resp Crit Care 2012;186(12):1279–1285. [DOI] [PubMed] [Google Scholar]
- 1347.Chebib N, Nesme P, Freymond N, et al. Acute respiratory failure in obesity-hypoventilation syndrome managed in the ICU. Resp Care 2019;64(12):1545–1554. [DOI] [PubMed] [Google Scholar]
- 1348.Arellano-Maric MP, Hamm C, Duiverman ML, et al. Obesity hypoventilation syndrome treated with non-invasive ventilation: is a switch to CPAP therapy feasible? Respirology 2020;25(4):435–442. [DOI] [PubMed] [Google Scholar]
- 1349.Orfanos S, Jaffuel D, Perrin C, Molinari N, Chanez P, Palot A. Switch of noninvasive ventilation (NIV) to continuous positive airway pressure (CPAP) in patients with obesity hypoventilation syndrome: a pilot study. BMC Pulm Med 2017;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1350.Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest 2010;138(1):84–90. [DOI] [PubMed] [Google Scholar]
- 1351.Kakazu MT, Soghier I, Afshar M, et al. Weight loss interventions as treatment of obesity hypoventilation syndrome a systematic review. Ann Am Thorac Soc 2020;17(4):492–502. [DOI] [PubMed] [Google Scholar]
- 1352.Mandal S, Suh ES, Harding R, et al. Nutrition and exercise rehabilitation in obesity hypoventilation syndrome (NERO): a pilot randomised controlled trial. Thorax 2018;73(1):62–69. [DOI] [PubMed] [Google Scholar]
- 1353.Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology 1999;4(4):365–370. [DOI] [PubMed] [Google Scholar]
- 1354.Mezzanotte WS, Tangel DJ, Fox AM, Ballard RD, White DP. Nocturnal nasal continuous positive airway pressure in patients with chronic obstructive pulmonary-disease - influence on waking respiratory muscle function. Chest 1994;106(4):1100–1108. [DOI] [PubMed] [Google Scholar]
- 1355.Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J 2010;35(1):132–137. [DOI] [PubMed] [Google Scholar]
- 1356.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010;182(3):325–331. [DOI] [PubMed] [Google Scholar]
- 1357.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013;9(8):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1358.Singh G, Agarwal A, Zhang W, Kuo YF, Sultana R, Sharma G. Impact of PAP therapy on hospitalization rates in medicare beneficiaries with COPD and coexisting OSA. Sleep Breath 2019;23(1):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1359.Mapel DW, Dutro MP, Marton JP, Woodruff K, Make B. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res 2011;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1360.Duiverman ML. Noninvasive ventilation in stable hypercapnic COPD: what is the evidence? ERJ Open Res 2018;4(2):00012–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1361.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation a randomized clinical trial. JAMA 2017;317(21):2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1362.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;7(7):CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1363.Kuklisova Z, Tkacova R, Joppa P, Wouters E, Sastry M. Severity of nocturnal hypoxia and daytime hypercapnia predicts CPAP failure in patients with COPD and obstructive sleep apnea overlap syndrome. Sleep Med 2017;30:139–145. [DOI] [PubMed] [Google Scholar]
- 1364.Dort L, Remmers J. A combination appliance for obstructive sleep apnea: the effectiveness of mandibular advancement and tongue retention. J Clin Sleep Med 2012;8(3):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1365.Chang ET, Fernandez-Salvador C, Giambo J, et al. Tongue retaining devices for obstructive sleep apnea: a systematic review and meta-analysis. Am J Otolaryngol 2017;38(3):272–278. [DOI] [PubMed] [Google Scholar]
- 1366.Deane SA, Cistulli PA, Ng AT, Zeng B, Petocz P, Darendeliler MA. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial. Sleep 2009;32(5):648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1367.Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep 1991;14(6):546–552. [DOI] [PubMed] [Google Scholar]
- 1368.Cartwright RD. Predicting response to the tongue retaining device for sleep apnea syndrome. Arch Otolaryngol 1985;111(6):385–388. [DOI] [PubMed] [Google Scholar]
- 1369.Banhiran W, Durongphan A, Keskool P, Chongkolwatana C, Metheetrairut C. Randomized crossover study of tongueretaining device and positive airway pressure for obstructive sleep apnea. Sleep Breath 2019;24(3):1011–1018. [DOI] [PubMed] [Google Scholar]
- 1370.Lazard DS, Blumen M, Levy P, et al. The tongue-retaining device: efficacy and side effects in obstructive sleep apnea syndrome. J Clin Sleep Med 2009;5(5):431–438. [PMC free article] [PubMed] [Google Scholar]
- 1371.Johal A, Agha B. Ready-made versus custom-made mandibular advancement appliances in obstructive sleep apnea: a systematic review and meta-analysis. J Sleep Res 2018;27(6):e12660. [DOI] [PubMed] [Google Scholar]
- 1372.Petit FX, Pepin JL, Bettega G, Sadek H, Raphael B, Levy P. Mandibular advancement devices: rate of contraindications in 100 consecutive obstructive sleep apnea patients. Am J Respir Crit Care Med 2002;166(3):274–278. [DOI] [PubMed] [Google Scholar]
- 1373.Ngiam J, Balasubramaniam R, Darendeliler MA, Cheng AT, Waters K, Sullivan CE. Clinical guidelines for oral appliance therapy in the treatment of snoring and obstructive sleep apnoea. Aust Dent J 2013;58(4):408–419. [DOI] [PubMed] [Google Scholar]
- 1374.Heidsieck DS, de Ruiter MH, de Lange J. Management of obstructive sleep apnea in edentulous patients: an overview of the literature. Sleep Breath 2016;20(1):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1375.Cunali PA, Almeida FR, Santos CD, et al. Prevalence of temporomandibular disorders in obstructive sleep apnea patients referred for oral appliance therapy. J Orofac Pain 2009;23(4):339–344. [PubMed] [Google Scholar]
- 1376.Smith MT, Wickwire EM, Grace EG, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep 2009;32(6):779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1377.Gao X, Otsuka R, Ono T, Honda E, Sasaki T, Kuroda T. Effect of titrated mandibular advancement and jaw opening on the upper airway in nonapneic men: a magnetic resonance imaging and cephalometric study. Am J Orthod Dentofacial Orthop 2004;125(2):191–199. [DOI] [PubMed] [Google Scholar]
- 1378.Ishiyama H, Inukai S, Nishiyama A, et al. Effect of jawopening exercise on prevention of temporomandibular disorders pain associated with oral appliance therapy in obstructive sleep apnea patients: a randomized, double-blind, placebocontrolled trial. J Prosthodont Res 2017;61(3):259–267. [DOI] [PubMed] [Google Scholar]
- 1379.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev 2006(1):CD004435. [DOI] [PubMed] [Google Scholar]
- 1380.Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med 2015;3(11):869–878. [DOI] [PubMed] [Google Scholar]
- 1381.Kuhn E, Schwarz EI, Bratton DJ, Rossi VA, Kohler M. Effects of CPAP and mandibular advancement devices on health-related quality of life in OSA: a systematic review and meta-analysis. Chest 2017;151(4):786–794. [DOI] [PubMed] [Google Scholar]
- 1382.Araie T, Okuno K, Ono Minagi H, Sakai T. Dental and skeletal changes associated with long-term oral appliance use for obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2018;41:161–172. [DOI] [PubMed] [Google Scholar]
- 1383.Chen H, Eckert DJ, van der Stelt PF, et al. Phenotypes of responders to mandibular advancement device therapy in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Med Rev 2020;49:101229. [DOI] [PubMed] [Google Scholar]
- 1384.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax 2013;68(1):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1385.Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Breath 2018;22(3):555–568. [DOI] [PubMed] [Google Scholar]
- 1386.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJB. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest 2005;128(4):2130–2137. [DOI] [PubMed] [Google Scholar]
- 1387.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med 2009;5(5):428–430. [PMC free article] [PubMed] [Google Scholar]
- 1388.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope 2006;116(11):1995–2000. [DOI] [PubMed] [Google Scholar]
- 1389.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supinedependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med 2011;7(4):376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1390.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 1391.Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pepin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med 2017;13(6):813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1392.Benoist L, de Ruiter M, de Lange J, de Vries N. A randomized, controlled trial of positional therapy versus oral appliance therapy for position-dependent sleep apnea. Sleep Med 2017;34:109–117. [DOI] [PubMed] [Google Scholar]
- 1393.Berry RB, Uhles ML, Abaluck BK, et al. NightBalance sleep position treatment device versus auto-adjusting positive airway pressure for treatment of positional obstructive sleep apnea. J Clin Sleep Med 2019;15(7):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1394.Beyers J, Vanderveken OM, Kastoer C, et al. Treatment of sleep-disordered breathing with positional therapy: long-term results. Sleep Breath 2019;23(4):1141–1149. [DOI] [PubMed] [Google Scholar]
- 1395.de Ruiter MHT, Benoist LBL, de Vries N, de Lange J. Durability of treatment effects of the sleep position trainer versus oral appliance therapy in positional OSA: 12-month follow-up of a randomized controlled trial (vol 22, pg 441, 2017). Sleep Breath 2018;22(2):451–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1396.Eijsvogel MM, Ubbink R, Dekker J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med 2015;11(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397.Laub RR, Tonnesen P, Jennum PJ. A sleep position trainer for positional sleep apnea: a randomized, controlled trial. J Sleep Res 2017;26(5):641–650. [DOI] [PubMed] [Google Scholar]
- 1398.Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med 2014;10(8):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1399.Ravesloot MJL, Benoist L, van Maanen P, de Vries N. Novel positional devices for the treatment of positional obstructive sleep apnea, and how this relates to sleep surgery. Adv Otorhinolaryngol 2017;80:28–36. [DOI] [PubMed] [Google Scholar]
- 1400.van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep 2014;37(7):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1401.van Maanen JP, Meester KAW, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath 2013;17(2):771–779. [DOI] [PubMed] [Google Scholar]
- 1402.Van Maanen JP, Richard W, Van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res 2012;21(3):322–329. [DOI] [PubMed] [Google Scholar]
- 1403.Mok Y, Tan A, Hsu PP, et al. Comparing treatment effects of a convenient vibratory positional device to CPAP in positional OSA: a crossover randomised controlled trial. Thorax 2020;75(4):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1404.Dieltjens M, Vroegop AV, Verbruggen AE, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath 2015;19(2):637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1405.Srijithesh PR, Aghoram R, Goel A, Dhanya J. Positional therapy for obstructive sleep apnoea. Cochrane Database Syst Rev 2019;5(5):CD010990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1406.Desplan M, Mercier J, Sabate M, Ninot G, Prefaut C, Dauvilliers Y. A comprehensive rehabilitation program improves disease severity in patients with obstructive sleep apnea syndrome: a pilot randomized controlled study. Sleep Med 2014;15(8):906–912. [DOI] [PubMed] [Google Scholar]
- 1407.Fernandes JF, Araujo Lda S, Kaiser SE, Sanjuliani AF, Klein MR. The effects of moderate energy restriction on apnoea severity and CVD risk factors in obese patients with obstructive sleep apnoea. Br J Nutr 2015;114(12):2022–2031. [DOI] [PubMed] [Google Scholar]
- 1408.Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ 2009;339:b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1409.Maki-Nunes C, Toschi-Dias E, Cepeda FX, et al. Diet and exercise improve chemoreflex sensitivity in patients with metabolic syndrome and obstructive sleep apnea. Obesity (Silver Spring) 2015;23(8):1582–1590. [DOI] [PubMed] [Google Scholar]
- 1410.Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest 2015;148(5):1193–1203. [DOI] [PubMed] [Google Scholar]
- 1411.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009;179(4):320–327. [DOI] [PubMed] [Google Scholar]
- 1412.Lopez-Padros C, Salord N, Alves C, et al. Effectiveness of an intensive weight-loss program for severe OSA in patients undergoing CPAP treatment: a randomized controlled trial. J Clin Sleep Med 2020;16(4):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1413.Nerfeldt P, Nilsson BY, Udden J, Rossner S, Friberg D. Weight reduction improves nocturnal respiration in obese sleep apnoea patients-a randomized controlled pilot study. Obes Res Clin Pract 2008;2(2):71–142. [DOI] [PubMed] [Google Scholar]
- 1414.Papandreou C, Schiza SE, Bouloukaki I, et al. Effect of Mediterranean diet versus prudent diet combined with physical activity on OSAS: a randomised trial. Eur Respir J 2012;39(6):1398–1404. [DOI] [PubMed] [Google Scholar]
- 1415.Hudgel DW, Patel SR, Ahasic AM, et al. The role of weight management in the treatment of adult obstructive sleep apnea. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198(6):e70–e87. [DOI] [PubMed] [Google Scholar]
- 1416.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015;100(2):342–362. [DOI] [PubMed] [Google Scholar]
- 1417.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond) 2016;40(8):1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1418.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep 2012;35(11):1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1419.Christou GA, Katsiki N, Blundell J, Fruhbeck G, Kiortsis DN. Semaglutide as a promising antiobesity drug. Obes Rev 2019;20(6):805–815. [DOI] [PubMed] [Google Scholar]
- 1420.Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 2021;325(14):1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1421.SEDATIVE | Definition of SEDATIVE by Oxford Dictionary on Lexico.com also meaning of SEDATIVE. Published 2021. 2020. Accessed April 28, 2020. https://www.lexico.com/en/definition/sedative
- 1422.Cummiskey J, Guilleminault C, Delrio G, Silvestri R. The effects of flurazepam on sleep studies in patients with chronic obstructive pulmonary-disease. Chest 1983;84(2):143–147. [DOI] [PubMed] [Google Scholar]
- 1423.Mendelson WB, Garnett D, Gillin JC. Flurazepam-induced sleep-apnea syndrome in a patient with insomnia and mild sleep-related respiratory changes. J Nerv Ment Dis 1981;169(4):261–264. [DOI] [PubMed] [Google Scholar]
- 1424.Seda G, Tsai S, Lee-Chiong T. Medication effects on sleep and breathing. Clin Chest Med 2014;35(3):557. [DOI] [PubMed] [Google Scholar]
- 1425.Mason M, Cates CJ, Smith I. Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2015(7):CD011090. [DOI] [PubMed] [Google Scholar]
- 1426.Issa FG, Sullivan CE. Alcohol, snoring and sleep-apnea. J Neurol Neurosurg Psychiatry 1982;45(4):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1427.Kolla BP, Foroughi M, Saeidifard F, Chakravorty S, Wang Z, Mansukhani MP. The impact of alcohol on breathing parameters during sleep: a systematic review and meta-analysis. Sleep Med Rev 2018;42:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1428.Jordan AS, O’Donoghue FJ, Cori JM, Trinder J. Physiology of arousal in obstructive sleep apnea and potential impacts for sedative treatment. Am J Resp Crit Care 2017;196(7):814–821. [DOI] [PubMed] [Google Scholar]
- 1429.Sun H, Palcza J, Card D, et al. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med 2016;12(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1430.Wilhelm CP, de Shazo RD, Tamanna S, Ullah MI, Skipworth LB. The nose, upper airway, and obstructive sleep apnea. Ann Allergy Asthma Immunol 2015;115(2):96–102. [DOI] [PubMed] [Google Scholar]
- 1431.Schönhofer B, Franklin KA, Brünig H, Wehde H, Köhler D. Effect of nasal-valve dilation on obstructive sleep apnea. Chest 2000;118(3):587–590. [DOI] [PubMed] [Google Scholar]
- 1432.Liu HT, Lin YC, Kuan YC, et al. Intranasal corticosteroid therapy in the treatment of obstructive sleep apnea: a meta-analysis of randomized controlled trials. Am J Rhinol Allergy 2016;30(3):215–221. [DOI] [PubMed] [Google Scholar]
- 1433.Smith DF, Sarber KM, Spiceland CP, Ishman SL, Augelli DM, Romaker AM. Effects of medical therapy on mild obstructive sleep apnea in adult patients. J Clin Sleep Med 2019;15(7):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434.Alt JA, Ramakrishnan VR, Platt MP, et al. Sleep quality outcomes after medical and surgical management of chronic rhinosinusitis. Int Forum Allergy Rhinol 2017;7(2):113–118. [DOI] [PubMed] [Google Scholar]
- 1435.Acar M, Cingi C, Sakallioglu O, San T, Fatih Yimenicioglu M, Bal C. The effects of mometasone furoate and desloratadine in obstructive sleep apnea syndrome patients with allergic rhinitis. Am J Rhinol Allergy 2013;27(4):e113–116. [DOI] [PubMed] [Google Scholar]
- 1436.Tam YY, Shao IH, Wu CC, Hsieh ML. The impact of intranasal fluticasone on patients with obstructive sleep apnea: a prospective study. Braz J Otorhinolaryngol 2019;87(2):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1437.Meltzer EO, Munafo DA, Chung W, Gopalan G, Varghese ST. Intranasal mometasone furoate therapy for allergic rhinitis symptoms and rhinitis-disturbed sleep. Ann Allergy Asthma Immunol 2010;105(1):65–74. [DOI] [PubMed] [Google Scholar]
- 1438.Koutsourelakis I, Minaritzoglou A, Zakynthinos G, Vagiakis E, Zakynthinos S. The effect of nasal tramazoline with dexamethasone in obstructive sleep apnoea patients. Eur Respir J 2013;42(4):1055–1063. [DOI] [PubMed] [Google Scholar]
- 1439.Bahammam AS, Tate R, Manfreda J, Kryger MH. Upper airway resistance syndrome: effect of nasal dilation, sleep stage, and sleep position. Sleep 1999;22(5):592–598. [PubMed] [Google Scholar]
- 1440.Wenzel M, Schönhofer B, Siemon K, Köhler D. Nasal strips without effect on obstructive sleep apnea and snoring. Pneumologie 1997;51(12):1108–1110. [PubMed] [Google Scholar]
- 1441.Yagihara F, Lorenzi G, Santos-Silva R. Nasal dilator strip is an effective placebo intervention for severe obstructive sleep apnea. J Clin Sleep Med 2017;13(2):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1442.Camacho M, Malu OO, Kram YA, et al. Nasal dilators (Breathe Right Strips and NoZovent) for snoring and OSA: a systematic review and meta-analysis. Pulm Med 2016;2016:4841310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1443.Hoijer U, Ejnell H, Hedner J, Petruson B, Eng LB. The effects of nasal dilation on snoring and obstructive sleep-apnea. Arch Otolaryngol 1992;118(3):281–284. [DOI] [PubMed] [Google Scholar]
- 1444.Gosepath J, Amedee RG, Romantschuck S, Mann WJ. Breathe right nasal strips and the respiratory disturbance index in sleep related breathing disorders. Am J Rhinol 1999;13(5):385–389. [DOI] [PubMed] [Google Scholar]
- 1445.Metes A, Cole P, Hoffstein V, Miljeteig H. Nasal airway dilation and obstructed breathing in sleep. Laryngoscope 1992;102(9):1053–1055. [DOI] [PubMed] [Google Scholar]
- 1446.Schonhofer B, Kerl J, Suchi S, Kohler D, Franklin KA. Effect of nasal valve dilation on effective CPAP level in obstructive sleep apnea. Resp Med 2003;97(9):1001–1005. [DOI] [PubMed] [Google Scholar]
- 1447.Amaro ACS, Duarte FHG, Jallad RS, Bronstein MD, Redline S, Lorenzi G. The use of nasal dilator strips as a placebo for trials evaluating continuous positive airway pressure. Clinics 2012;67(5):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1448.Hoffstein V, Mateika S, Metes A. Effect of nasal dilation on snoring and apneas during different stages of sleep. Sleep 1993;16(4):360–365. [DOI] [PubMed] [Google Scholar]
- 1449.VanderTouw T, Crawford ABH, Wheatley JR. Effects of a synthetic lung surfactant on pharyngeal patency in awake human subjects. J Appl Physiol 1997;82(1):78–85. [DOI] [PubMed] [Google Scholar]
- 1450.Jokic R, Klimaszewski A, Mink J, Fitzpatrick MF. Surface tension forces in sleep apnea: the role of a soft tissue lubricant - a randomized double-blind, placebo-controlled trial. Am J Resp Crit Care 1998;157(5):1522–1525. [DOI] [PubMed] [Google Scholar]
- 1451.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol 2003;95(5):1761–1766. [DOI] [PubMed] [Google Scholar]
- 1452.Morrell MJ, Arabi Y, Zahn BR, Meyer KC, Skatrud JB, Badr MS. Effect of surfactant on pharyngeal mechanics in sleeping humans: implications for sleep apnoea. Eur Respir J 2002;20(2):451–457. [DOI] [PubMed] [Google Scholar]
- 1453.Zozula R, Rosen R. Compliance with continuous positive airway pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med 2001;7(6):391–398. [DOI] [PubMed] [Google Scholar]
- 1454.Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med 2013;9(3):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1455.Block AJ, Hellard DW, Cicale MJ. Snoring, nocturnal hypoxemia, and the effect of oxygen inhalation. Chest 1987;92(3):411–417. [DOI] [PubMed] [Google Scholar]
- 1456.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006;29(4):564–571. [DOI] [PubMed] [Google Scholar]
- 1457.Phillips BA, Schmitt FA, Berry DT, Lamb DG, Amin M, Cook YR. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest 1990;98(2):325–330. [DOI] [PubMed] [Google Scholar]
- 1458.Liao P, Wong J, Singh M, et al. Postoperative oxygen therapy in patients with OSA: a randomized controlled trial. Chest 2017;151(3):597–611. [DOI] [PubMed] [Google Scholar]
- 1459.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014;370(24):2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1460.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension 2006;47(5):840–845. [DOI] [PubMed] [Google Scholar]
- 1461.Turnbull CD, Sen D, Kohler M, Petousi N, Stradling JR. Effect of supplemental oxygen on blood pressure in obstructive sleep apnea (SOX). A randomized continuous positive airway pressure withdrawal trial. Am J Respir Crit Care Med 2019;199(2):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462.Sands SA, Edwards BA, Terrill PI, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J 2018;52(3):1800674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1463.Wang D, Wong KK, Rowsell L, Don GW, Yee BJ, Grunstein RR. Predicting response to oxygen therapy in obstructive sleep apnoea patients using a 10-minute daytime test. Eur Respir J 2018;51(1):1701587. [DOI] [PubMed] [Google Scholar]
- 1464.Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Resp Crit Care 2009;179(10):962–966. [DOI] [PubMed] [Google Scholar]
- 1465.Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep 2015;38(5):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1466.Verma RK, Johnson JJR, Goyal M, Banumathy N, Goswami U, Panda NK. Oropharyngeal exercises in the treatment of obstructive sleep apnoea: our experience. Sleep Breath 2016;20(4):1193–1201. [DOI] [PubMed] [Google Scholar]
- 1467.Ieto V, Kayamori F, Montes MI, et al. Effects of oropharyngeal exercises on snoring a randomized trial. Chest 2015;148(3):683–691. [DOI] [PubMed] [Google Scholar]
- 1468.Diaferia G, Santos-Silva R, Truksinas E, et al. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep Breath 2017;21(2):387–395. [DOI] [PubMed] [Google Scholar]
- 1469.Diaferia G, Badke L, Santos-Silva R, Bommarito S, Tufik S, Bittencourt L. Effect of speech therapy as adjunct treatment to continuous positive airway pressure on the quality of life of patients with obstructive sleep apnea. Sleep Med 2013;14(7):628–635. [DOI] [PubMed] [Google Scholar]
- 1470.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 2008;5(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1471.Phurrough S, Jacques L, Spencer F, Stiller J, Brechner R. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2) Centers for Medicare & Medicaid Services. March 13, 2008. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=204 [Google Scholar]
- 1472.Engleman HM, Martin SE, Douglas NJ. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax 1994;49(3):263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1473.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med 1994;149(1):149–154. [DOI] [PubMed] [Google Scholar]
- 1474.Stradling JR, Davies RJ. Is more NCPAP better? Sleep 2000;23(4):S150–S153. [PubMed] [Google Scholar]
- 1475.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005;128(2):624–633. [DOI] [PubMed] [Google Scholar]
- 1476.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 2011;34(1):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1477.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001;163(2):344–348. [DOI] [PubMed] [Google Scholar]
- 1478.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest 2006;130(6):1772–1778. [DOI] [PubMed] [Google Scholar]
- 1479.Aloia MS, Knoepke CE, Lee-Chiong T. The new local coverage determination criteria for adherence to positive airway pressure treatment: testing the limits? Chest 2010;138(4):875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1480.Krakow B, Ulibarri VA, Foley-Shea MR, Tidler A, McIver ND. Adherence and subthreshold adherence in sleep apnea subjects receiving positive airway pressure therapy: a retrospective study evaluating differences in adherence versus use. Respir Care 2016;61(8):1023–1032. [DOI] [PubMed] [Google Scholar]
- 1481.Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep 2011;34(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1482.Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010;33(10):1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1483.Sundaram S, Bridgman SA, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev 2005(4):CD001004. [DOI] [PubMed] [Google Scholar]
- 1484.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg 2016;45(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1485.Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002;127(1):13–21. [DOI] [PubMed] [Google Scholar]
- 1486.Choi JH, Cho SH, Kim SN, Suh JD, Cho JH. Predicting outcomes after uvulopalatopharyngoplasty for adult obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg 2016;155(6):904–913. [DOI] [PubMed] [Google Scholar]
- 1487.Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D. SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax 2013;68(9):846–853. [DOI] [PubMed] [Google Scholar]
- 1488.Heiser C, Steffen A, Boon M, et al. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur Respir J 2019;53(1):1801405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1489.Huntley C, Steffen A, Doghramji K, Hofauer B, Heiser C, Boon M. Upper airway stimulation in patients with obstructive sleep apnea and an elevated body mass index: a multi-institutional review. Laryngoscope 2018;128(10):2425–2428. [DOI] [PubMed] [Google Scholar]
- 1490.Camacho M, Teixeira J, Abdullatif J, et al. Maxillomandibular advancement and tracheostomy for morbidly obese obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2015;152(4):619–630. [DOI] [PubMed] [Google Scholar]
- 1491.Li HY, Wang PC, Lee LA, Chen NH, Fang TJ. Prediction of uvulopalatopharyngoplasty outcome: anatomy-based staging system versus severity-based staging system. Sleep 2006;29(12):1537–1541. [DOI] [PubMed] [Google Scholar]
- 1492.Martinho FL, Zonato AI, Bittencourt LR, et al. Obese obstructive sleep apnea patients with tonsil hypertrophy submitted to tonsillectomy. Braz J Med Biol Res 2006;39(8):1137–1142. [DOI] [PubMed] [Google Scholar]
- 1493.Vicente E, Marin JM, Carrizo S, Naya MJ. Tongue-base suspension in conjunction with uvulopalatopharyngoplasty for treatment of severe obstructive sleep apnea: long-term follow-up results. Laryngoscope 2006;116(7):1223–1227. [DOI] [PubMed] [Google Scholar]
- 1494.Chandrashekariah R, Shaman Z, Auckley D. Impact of upper airway surgery on CPAP compliance in difficult-to-manage obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2008;134(9):926–930. [DOI] [PubMed] [Google Scholar]
- 1495.Rotenberg BW, Vicini C, Pang EB, Pang KP. Reconsidering first-line treatment for obstructive sleep apnea: a systematic review of the literature. J Otolaryngol Head Neck Surg 2016;45:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1496.Ceylan K, Emir H, Kizilkaya Z, et al. First-choice treatment in mild to moderate obstructive sleep apnea: single-stage, multilevel, temperature-controlled radiofrequency tissue volume reduction or nasal continuous positive airway pressure. Arch Otolaryngol Head Neck Surg 2009;135(9):915–919. [DOI] [PubMed] [Google Scholar]
- 1497.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2003;128(6):848–861. [DOI] [PubMed] [Google Scholar]
- 1498.Weaver EM, Maynard C, Yueh B. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Head Neck Surg 2004;130(6):659–665. [DOI] [PubMed] [Google Scholar]
- 1499.Rotenberg BW, Theriault J, Gottesman S. Redefining the timing of surgery for obstructive sleep apnea in anatomically favorable patients. Laryngoscope 2014;124(4):S1–S9. [DOI] [PubMed] [Google Scholar]
- 1500.Senchak AJ, McKinlay AJ, Acevedo J, et al. The effect of tonsillectomy alone in adult obstructive sleep apnea. Otolaryngol Head Neck Surg 2015;152(5):969–973. [DOI] [PubMed] [Google Scholar]
- 1501.John CR, Gandhi S, Sakharia AR, James TT. Maxillomandibular advancement is a successful treatment for obstructive sleep apnoea: a systematic review and meta-analysis. Int J Oral Maxillofac Surg 2018;47(12):1561–1571. [DOI] [PubMed] [Google Scholar]
- 1502.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg 1993;51(7):742–747; discussion 748–749. [DOI] [PubMed] [Google Scholar]
- 1503.Vicini C, Dallan I, Campanini A, et al. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am J Otolaryngol 2010;31(1):14–20. [DOI] [PubMed] [Google Scholar]
- 1504.Liu SY, Huon LK, Powell NB, et al. Lateral pharyngeal wall tension after maxillomandibular advancement for obstructive sleep apnea is a marker for surgical success: observations from drug-induced sleep endoscopy. J Oral Maxillofac Surg 2015;73(8):1575–1582. [DOI] [PubMed] [Google Scholar]
- 1505.Yu MS, Ibrahim B, Riley RW, Liu SY. Maxillomandibular advancement and upper airway stimulation: extrapharyngeal surgery for obstructive sleep apnea. Clin Exp Otorhinolaryngol 2020;13(3):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1506.Kent D, Stanley J, Aurora RN, et al. Referral of adults with obstructive sleep apnea for surgical consultation: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2021;17(12):2507–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1507.Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, Ringqvist I. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest 2002;121(3):739–746. [DOI] [PubMed] [Google Scholar]
- 1508.Lojander J, Maasilta P, Partinen M, Brander PE, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest 1996;110(1):114–119. [DOI] [PubMed] [Google Scholar]
- 1509.Corso R, Russotto V, Gregoretti C, Cattano D. Perioperative management of obstructive sleep apnea: a systematic review. Minerva Anestesiol 2018;84(1):81–93. [DOI] [PubMed] [Google Scholar]
- 1510.Leong SM, Tiwari A, Chung F, Wong DT. Obstructive sleep apnea as a risk factor associated with difficult airway management - a narrative review. J Clin Anesth 2018;45:63–68. [DOI] [PubMed] [Google Scholar]
- 1511.Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med 2012;8(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1512.Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy a report from the multicenter perioperative outcomes group. Anesthesiology 2013;119(6):1360–1369. [DOI] [PubMed] [Google Scholar]
- 1513.Neligan PJ, Porter S, Max B, Malhotra G, Greenblatt EP, Ochroch EA. Obstructive sleep apnea is not a risk factor for difficult intubation in morbidly obese patients. Anesth Analg 2009;109(4):1182–1186. [DOI] [PubMed] [Google Scholar]
- 1514.Vest D, Lee D, Newcome K, Stamper H. A retrospective review of difficult intubations is obstructive sleep apnea a predictor? Clin Nurse Spec 2013;27(3):128–131. [DOI] [PubMed] [Google Scholar]
- 1515.Corso RM, Piraccini E, Calli M, et al. Obstructive sleep apnea is a risk factor for difficult endo tracheal intubation. Minerva Anestesiol 2011;77(1):99–100. [PubMed] [Google Scholar]
- 1516.Kim JA, Lee JJ. Preoperative predictors of difficult intubation in patients with obstructive sleep apnea syndrome. Can J Anaesth 2006;53(4):393–397. [DOI] [PubMed] [Google Scholar]
- 1517.Riley RW, Powell NB, Guilleminault C, Pelayo R, Troell RJ, Li KK. Obstructive sleep apnea surgery: risk management and complications. Otolaryngol Head Neck 1997;117(6):648–652. [DOI] [PubMed] [Google Scholar]
- 1518.Siyam MA, Benhamou D. Difficult endotracheal intubation in patients with sleep apnea syndrome. Anesth Analg 2002;95(4):1098–1102. [DOI] [PubMed] [Google Scholar]
- 1519.American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2014;120(2):268–286. [DOI] [PubMed] [Google Scholar]
- 1520.Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on management of the difficult airway. Anesthesiology 2013;118(2):251–270. [DOI] [PubMed] [Google Scholar]
- 1521.Ravesloot MJL, de Raaff CAL, van de Beek MJ, et al. Perioperative care of patients with obstructive sleep apnea undergoing upper airway surgery: a review and consensus recommendations. JAMA Otolaryngol Head Neck Surg 2019;145(8):751–760. [DOI] [PubMed] [Google Scholar]
- 1522.Connolly LA. Anesthetic management of obstructive sleep apnea patients. J Clin Anesth 1991;3(6):461–469. [DOI] [PubMed] [Google Scholar]
- 1523.Keamy MF, Cadieux RJ, Kofke WA, Kales A. The occurrence of obstructive sleep-apnea in a recovery room patient. Anesthesiology 1987;66(2):232–234. [DOI] [PubMed] [Google Scholar]
- 1524.Rafferty TD, Ruskis A, Sasaki C, Gee JB. Perioperative considerations in the management of tracheotomy for the obstructive sleep-apnea patient - 3 illustrative case-reports. Brit J Anaesth 1980;52(6):619–622. [DOI] [PubMed] [Google Scholar]
- 1525.Kim JA, Lee JJ, Jung HH. Predictive factors of immediate postoperative complications after uvulopalatopharyngoplasty. Laryngoscope 2005;115(10):1837–1840. [DOI] [PubMed] [Google Scholar]
- 1526.Esclamado RM, Glenn MG, Mcculloch TM, Cummings CW. Perioperative complications and risk-factors in the surgicaltreatment of obstructive sleep-apnea syndrome. Laryngoscope 1989;99(11):1125–1129. [DOI] [PubMed] [Google Scholar]
- 1527.Terris DJ, Clerk AA, Norbash AM, Troell RJ. Characterization of postoperative edema following laser-assisted uvulopalatoplasty using MRI and polysomnography: implications for the outpatient treatment of obstructive sleep apnea syndrome. Laryngoscope 1996;106(2):124–128. [DOI] [PubMed] [Google Scholar]
- 1528.Li KK, Riley RW, Powell NB, Zonato A, Troell R, Guilleminault C. Postoperative airway findings after maxillomandibular advancement for obstructive sleep apnea syndrome. Laryngoscope 2000;110(2):325–327. [DOI] [PubMed] [Google Scholar]
- 1529.Mickelson SA. Preoperative and postoperative management of obstructive sleep apnea patients. Otolaryngol Clin North Am 2007;40(4):877. [DOI] [PubMed] [Google Scholar]
- 1530.Iyer US, Koh KF, Chia NCH, Macachor J, Cheng A. Perioperative risk factors in obese patients for bariatric surgery: a Singapore experience. Singapore Med J 2011;52(2):94–99. [PubMed] [Google Scholar]
- 1531.Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation a review of 50,000 anesthetics. Anesthesiology 2009;110(4):891–897. [DOI] [PubMed] [Google Scholar]
- 1532.Ulnick KM, Debo RF. Postoperative treatment of the patient with obstructive sleep apnea. Otolaryngol Head Neck 2000;122(2):233–236. [DOI] [PubMed] [Google Scholar]
- 1533.Talei B, Cossu AL, Slepian R, Kacker A. Immediate complications related to anesthesia in patients undergoing uvulopalatopharyngoplasty for obstructive sleep apnea. Laryngoscope 2013;123(11):2892–2895. [DOI] [PubMed] [Google Scholar]
- 1534.Meoli AL, Rosen CL, Kristo D, et al. Upper airway management of the adult patient with obstructive sleep apnea in the perioperative period - avoiding complications. Sleep 2003;26(8):1060–1065. [DOI] [PubMed] [Google Scholar]
- 1535.Nagappa M, Mokhlesi B, Wong J, Wong DT, Kaw R, Chung F. The effects of continuous positive airway pressure on postoperative outcomes in obstructive sleep apnea patients undergoing surgery: a systematic review and meta-analysis. Anesth Analg 2015;120(5):1013–1023. [DOI] [PubMed] [Google Scholar]
- 1536.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc 2001;76(9):897–905. [DOI] [PubMed] [Google Scholar]
- 1537.Corso RM, Gregoretti C, Braghiroli A, Fanfulla F, Insalaco G. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: navigating through uncertainty. Anesthesiology 2014;121(3):664–665. [DOI] [PubMed] [Google Scholar]
- 1538.Liao P, Luo QW, Elsaid H, Kang WM, Shapiro CM, Chung F. Perioperative auto-titrated continuous positive airway pressure treatment in surgical patients with obstructive sleep apnea a randomized controlled trial. Anesthesiology 2013;119(4):837–847. [DOI] [PubMed] [Google Scholar]
- 1539.Mutter TC, Chateau D, Moffatt M, Ramsey C, Roos LL, Kryger M. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology 2014;121(4):707–718. [DOI] [PubMed] [Google Scholar]
- 1540.Rennotte MT, Baele P, Aubert G, Rodenstein DO. Nasal continuous positive airway pressure in the perioperative management of patients with obstructive sleep apnea submitted to surgery. Chest 1995;107(2):367–374. [DOI] [PubMed] [Google Scholar]
- 1541.de Araújo MTM, Bissoli NS, Gouvea SA, et al. CPAP therapy prevents increase in blood pressure after upper airway surgery for obstructive sleep apnoea. Sleep Breath 2013;17(4):1289–1299. [DOI] [PubMed] [Google Scholar]
- 1542.Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med 2013;6(1):50–54. [DOI] [PubMed] [Google Scholar]
- 1543.Choi DL, Reddy K, Weitzel EK, et al. Postoperative continuous positive airway pressure use and nasal saline rinses after endonasal endoscopic skull base surgery in patients with obstructive sleep apnea: a practice pattern survey. Am J Rhinol Allergy 2019;33(1):51–55. [DOI] [PubMed] [Google Scholar]
- 1544.Kezirian EJ, Weaver EM, Yueh B, Khuri SF, Daley J, Henderson WG. Risk factors for serious complication after uvulopalatopharyngoplasty. Arch Otolaryngol 2006;132(10):1091–1098. [DOI] [PubMed] [Google Scholar]
- 1545.Rocke D, Sharp S, Wiener D, Puscas L, Lee WT. Effectiveness of a postoperative disposition protocol for sleep apnea surgery. Am J Otolaryngol 2013;34(4):273–277. [DOI] [PubMed] [Google Scholar]
- 1546.Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016;155(1):188–193. [DOI] [PubMed] [Google Scholar]
- 1547.Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med 2016;12(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1548.Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter german post-market study. Laryngoscope 2018;128(2):509–515. [DOI] [PubMed] [Google Scholar]
- 1549.Huang HC, Lee LA, Fang TJ, Li HY, Lo CC, Wu JH. Transnasal butorphanol for pain relief after uvulopalatopharyngoplasty - a hospital-based, randomized study. Chang Gung Med J 2009;32(4):390–399. [PubMed] [Google Scholar]
- 1550.Neruntarat C Genioglossus advancement and hyoid myotomy under local anesthesia. Otolaryngol Head Neck Surg 2003;129(1):85–91. [DOI] [PubMed] [Google Scholar]
- 1551.Neruntarat C Hyoid myotomy with suspension under local anesthesia for obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2003;260(5):286–290. [DOI] [PubMed] [Google Scholar]
- 1552.Walker RP, Garrity T, Gopalsami C. Early polysomnographic findings and long-term subjective results in sleep apnea patients treated with laser-assisted uvulopalatoplasty. Laryngoscope 1999;109(9):1438–1441. [DOI] [PubMed] [Google Scholar]
- 1553.Walker RP, Griggdamberger MM, Gopalsami C, Totten MC. Laser-assisted uvulopalatoplasty for snoring and obstructive sleep-apnea - results in 170 patients. Laryngoscope 1995;105(9):938–943. [DOI] [PubMed] [Google Scholar]
- 1554.Wassmuth Z, Mair E, Loube D, Leonard D. Cautery-assisted palatal stiffening operation for the treatment of obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2000;123(1):55–60. [DOI] [PubMed] [Google Scholar]
- 1555.Cillo JE, Dattilo DJ. Early major medical complications after surgical management of obstructive sleep apnea: a retrospective cohort analysis and case series. J Oral Maxillofac Surg 2015;73(1):123–128. [DOI] [PubMed] [Google Scholar]
- 1556.Gessler EM, Bondy PC. Respiratory complications following tonsillectomy/UPPP: is step-down monitoring necessary? Ear Nose Throat J 2003;82(8):628–632. [PubMed] [Google Scholar]
- 1557.Hathaway B, Johnson JT. Safety of uvulopalatopharyngoplasty as outpatient surgery. Otolaryngol Head Neck 2006;134(4):542–544. [DOI] [PubMed] [Google Scholar]
- 1558.Mickelson SA, Hakim I. Is postoperative intensive care monitoring necessary after uvulopalatopharyngoplasty? Otolaryngol Head Neck 1998;119(4):352–356. [DOI] [PubMed] [Google Scholar]
- 1559.Pang KP, Siow JK, Tseng P. Safety of multilevel surgery in obstructive sleep apnea: a review of 487 cases. Arch Otolaryngol Head Neck Surg 2012;138(4):353–357. [DOI] [PubMed] [Google Scholar]
- 1560.Rotenberg B, Hu A, Fuller J, Bureau Y, Arra I, Sen M. The early postoperative course of surgical sleep apnea patients. Laryngoscope 2010;120(5):1063–1068. [DOI] [PubMed] [Google Scholar]
- 1561.Spiegel JH, Raval TH. Overnight hospital stay is not always necessary after uvulopalatopharyngoplasty. Laryngoscope 2005;115(1):167–171. [DOI] [PubMed] [Google Scholar]
- 1562.Strocker AM, Cohen AN, Wang MB. The safety of outpatient UPPP for obstructive sleep apnea: a retrospective review of 40 cases. Ear Nose Throat J 2008;87(8):466–468. [PubMed] [Google Scholar]
- 1563.Terris DJ, Fincher EF, Hanasono MM, Fee WE Jr, Adachi K. Conservation of resources: indications for intensive care monitoring after upper airway surgery on patients with obstructive sleep apnea. Laryngoscope 1998;108(6):784–788. [DOI] [PubMed] [Google Scholar]
- 1564.Vicini C, Dallan I, Canzi P, Frassineti S, La Pietra MG, Montevecchi F. Transoral robotic tongue base resection in obstructive sleep apnoea-hypopnoea syndrome: a preliminary report. ORL J Otorhinolaryngol Relat Spec 2010;72(1):22–27. [DOI] [PubMed] [Google Scholar]
- 1565.Nelson RE, Carter J, Anand AG. Hypopharyngeal airway surgery for obstructive sleep apnea: morbidity in the early postoperative period. J La State Med Soc 2015;167(1):11–16. [PubMed] [Google Scholar]
- 1566.Zaghi S, Holty JE, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016;142(1):58–66. [DOI] [PubMed] [Google Scholar]
- 1567.Glazer TA, Hoff PT, Spector ME. Transoral robotic surgery for obstructive sleep apnea perioperative management and postoperative complications. JAMA Otolaryngol Head Neck Surg 2014;140(12):1207–1212. [DOI] [PubMed] [Google Scholar]
- 1568.Macaluso RA, Reams C, Gibson WS, Vrabec DP, Matragrano A. Uvulopalatopharyngoplasty – postoperative management and evaluation of results. Ann Otol Rhinol Laryngol 1989;98(7):502–507. [DOI] [PubMed] [Google Scholar]
- 1569.Yang L, Sun DF, Wu Y, Han J, Liu RC, Wang LJ. Intranasal administration of butorphanol benefits old patients undergoing H-uvulopalatopharyngoplasty: a randomized trial. BMC Anesthesiol 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1570.Lee LA, Wang PC, Chen NH, et al. Alleviation of wound pain after surgeries for obstructive sleep apnea. Laryngoscope 2007;117(9):1689–1694. [DOI] [PubMed] [Google Scholar]
- 1571.Li L, Feng J, Xie SH, Geng LC. Preemptive submucosal infiltration with ropivacaine for uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 2014;151(5):874–879. [DOI] [PubMed] [Google Scholar]
- 1572.Xie GL, Chu QJ, Liu CL. Application of parecoxib in post-uvulopalatopharyngoplasty analgesia. J Int Med Res 2013;41(5):1699–1704. [DOI] [PubMed] [Google Scholar]
- 1573.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg 1992;75(6):940–946. [PubMed] [Google Scholar]
- 1574.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg 2002;95(2):461–466. [DOI] [PubMed] [Google Scholar]
- 1575.Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 1999;54(12):1136–1142. [DOI] [PubMed] [Google Scholar]
- 1576.Chawla S, Robinson S, Norton A, Esterman A, Taneerananon T. Peri-operative use of dexmedetomidine in airway reconstruction surgery for obstructive sleep apnoea. J Laryngol Otol 2010;124(1):67–72. [DOI] [PubMed] [Google Scholar]
- 1577.Diakos EA, Gallos ID, El-Shunnar S, Clarke M, Kazi R, Mehanna H. Dexamethasone reduces pain, vomiting and overall complications following tonsillectomy in adults: a systematic review and meta-analysis of randomised controlled trials. Clin Otolaryngol 2011;36(6):531–542. [DOI] [PubMed] [Google Scholar]
- 1578.Titirungruang C, Seresirikachorn K, Kasemsuwan P, Hirunwiwatkul P. The use of steroids to reduce complications after tonsillectomy: a systematic review and meta-analysis of randomized controlled studies. Eur Arch Otorhinolaryngol 2019;276(2):585–604. [DOI] [PubMed] [Google Scholar]
- 1579.Brinck ECV, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev 2018;12(12):CD012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1580.Chan AKM, Cheung CW, Chong YK. Alpha-2 agonists in acute pain management. Expert Opin Pharmacother 2010;11(17):2849–2868. [DOI] [PubMed] [Google Scholar]
- 1581.De Oliveira GS, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119(1):178–190. [DOI] [PubMed] [Google Scholar]
- 1582.Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev 2018;6(6):CD009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1583.Tolska H, Hamunen, K, Takala, A, Kontinen, VK. Systemic review of analgesics and dexamethasone for post-tonsillectomy pain in adults. Br J Anaesth 2019;123(2):e397–e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1584.Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Druginduced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014;124(3):797–802. [DOI] [PubMed] [Google Scholar]
- 1585.Denolf PL, Vanderveken OM, Marklund ME, Braem MJ. The status of cephalometry in the prediction of non-CPAP treatment outcome in obstructive sleep apnea patients. Sleep Med Rev 2016;27:56–73. [DOI] [PubMed] [Google Scholar]
- 1586.Hong S-N, Won T-B, Kim J-W, Lee CH, Rhee C-S. Upper airway evaluation in patients with obstructive sleep apnea. Sleep Med Res 2016;7(1):1–9. [Google Scholar]
- 1587.Kim ST, Choi JH, Jeon HG, Cha HE, Kim DY, Chung YS. Polysomnographic effects of nasal surgery for snoring and obstructive sleep apnea. Acta Otolaryngol 2004;124(3):297–300. [DOI] [PubMed] [Google Scholar]
- 1588.Osborn JL, Sacks R. Nasal obstruction. Am J Rhinol Allergy 2013;27:S7–S8. [DOI] [PubMed] [Google Scholar]
- 1589.Holmlund T, Franklin KA, Jaghagen EL, et al. Tonsillectomy in adults with obstructive sleep apnea. Laryngoscope 2016;126(12):2859–2862. [DOI] [PubMed] [Google Scholar]
- 1590.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: mallampati score as an independent predictor of obstructive sleep apnea. Sleep 2006;29(7):903–908. [DOI] [PubMed] [Google Scholar]
- 1591.Min YG, Jang YJ, Rhee CK, Kim CN, Hong SK. Correlation between anthropometric measurements of the oropharyngeal area and severity of apnea in patients with snoring and obstructive sleep apnea. Auris Nasus Larynx 1997;24(4):399–403. [DOI] [PubMed] [Google Scholar]
- 1592.Olszewska E, Woodson BT. Palatal anatomy for sleep apnea surgery. Laryngoscope Invest 2019;4(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1593.Dündar A, Ozunlu A, Sahan M, Ozgen F. Lingual tonsil hypertrophy producing obstructive sleep apnea. Laryngoscope 1996;106(9):1167–1169. [DOI] [PubMed] [Google Scholar]
- 1594.Friedman M, Salapatas AM, Bonzelaar LB. Updated Friedman staging system for obstructive sleep apnea. Adv Otorhinolaryngol 2017;80:41–48. [DOI] [PubMed] [Google Scholar]
- 1595.Delakorda M, Ovsenik N. Epiglottis shape as a predictor of obstruction level in patients with sleep apnea. Sleep Breath 2019;23(1):311–317. [DOI] [PubMed] [Google Scholar]
- 1596.Hormann K, Verse T. Laryngeal obstructive sleep apnea. In: Marion Philipp H, ed. Surgery for Sleep Disordered Breathing Springer, Berlin Heidelberg New York; 2005:107–114. [Google Scholar]
- 1597.Hsu PP, Tan BYB, Chan YH, Tay HN, Lu PKS, Blair RL. Clinical predictors in obstructive sleep apnea patients with computer-assisted quantitative videoendoscopic upper airway analysis. Laryngoscope 2004;114(5):791–799. [DOI] [PubMed] [Google Scholar]
- 1598.Fernández-Julián E, Garcia-Perez MA, Garcia-Callejo J, Ferrer F, Marti F, Marco J. Surgical planning after sleep versus awake techniques in patients with obstructive sleep apnea. Laryngoscope 2014;124(8):1970–1974. [DOI] [PubMed] [Google Scholar]
- 1599.Soares D, Folbe AJ, Yoo G, Badr MS, Rowley JA, Lin HS. Drug-induced sleep endoscopy vs awake Muller’s maneuver in the diagnosis of severe upper airway obstruction. Otolaryngol Head Neck Surg 2013;148(1):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1600.Yegïn Y, Celik M, Kaya KH, Koc AK, Kayhan FT. Comparison of drug-induced sleep endoscopy and Muller’s maneuver in diagnosing obstructive sleep apnea using the VOTE classification system. Braz J Otorhinolaryngol 2017;83(4):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1601.Borowiecki B, Pollak CP, Weitzman ED, Rakoff S, Imperato J. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope 1978;88(8 Pt 1):1310–1313. [DOI] [PubMed] [Google Scholar]
- 1602.Genta PR, Sands SA, Butler JP, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest 2017;152(3):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1603.Park D, Kim JS, Heo SJ. Obstruction patterns during druginduced sleep endoscopy vs natural sleep endoscopy in patients with obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg 2019;145(8):730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1604.Rojewski TE, Schuller DE, Clark RW, Schmidt HS, Potts RE. Synchronous video recording of the pharyngeal airway and polysomnograph in patients with obstructive sleep apnea. Laryngoscope 1982;92(3):246–250. [DOI] [PubMed] [Google Scholar]
- 1605.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci 1991;16(5):504–509. [DOI] [PubMed] [Google Scholar]
- 1606.Kezirian EJ. Drug-induced sleep endoscopy. Operative techniques in otolaryngology–head and neck surgery 2006;17:230–232. [Google Scholar]
- 1607.De Vito A, Carrasco Llatas M, Ravesloot MJ, et al. European position paper on drug-induced sleep endoscopy: 2017 update. Clin Otolaryngol 2018;43(6):1541–1552. [DOI] [PubMed] [Google Scholar]
- 1608.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172(11):1363–1370. [DOI] [PubMed] [Google Scholar]
- 1609.Fogel RB, Trinder J, Malhotra A, et al. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol 2003;550(Pt 3):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1610.Fogel RB, Trinder J, White DP, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol 2005;564(Pt 2):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1611.Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol 1994;476(1):141–151. [PMC free article] [PubMed] [Google Scholar]
- 1612.Shea SA, Edwards JK, White DP. Effects of sleep-wake transitions and REM sleep on genioglossal response to upper airway negative pressure. Am J Respir Crit Care Med 1998;157:A653. [DOI] [PubMed] [Google Scholar]
- 1613.Wheatley JR, Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. J Appl Physiol 1993;75(5):2117–2124. [DOI] [PubMed] [Google Scholar]
- 1614.Wheatley JR, White DP. The influence of sleep on pharyngeal reflexes. Sleep 1993;16(8):S87–S89. [DOI] [PubMed] [Google Scholar]
- 1615.Shteamer JW, Dedhia RC. Sedative choice in drug-induced sleep endoscopy: a neuropharmacology-based review. Laryngoscope 2017;127(1):273–279. [DOI] [PubMed] [Google Scholar]
- 1616.Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn 2000;9(3):370–386. [DOI] [PubMed] [Google Scholar]
- 1617.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001;24(12):726–731. [DOI] [PubMed] [Google Scholar]
- 1618.Hillman DR, Walsh JH, Maddison KJ, et al. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 2009;111(1):63–71. [DOI] [PubMed] [Google Scholar]
- 1619.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology 2005;103(3):470–477. [DOI] [PubMed] [Google Scholar]
- 1620.Rabelo FA, Braga A, Kupper DS, et al. Propofol-induced sleep: polysomnographic evaluation of patients with obstructive sleep apnea and controls. Otolaryngol Head Neck Surg 2010;142(2):218–224. [DOI] [PubMed] [Google Scholar]
- 1621.Berry S, Roblin G, Williams A, Watkins A, Whittet HB. Validity of sleep nasendoscopy in the investigation of sleep related breathing disorders. Laryngoscope 2005;115(3):538–540. [DOI] [PubMed] [Google Scholar]
- 1622.Murphy M, Bruno MA, Riedner BA, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep 2011;34(3):283–291a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1623.Abdullah VJ, Lee DL, Ha SC, van Hasselt CA. Sleep endoscopy with midazolam: sedation level evaluation with bispectral analysis. Otolaryngol Head Neck Surg 2013;148(2):331–337. [DOI] [PubMed] [Google Scholar]
- 1624.Genta PR, Eckert DJ, Gregorio MG, et al. Critical closing pressure during midazolam-induced sleep. J Appl Physiol (1985) 2011;111(5):1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1625.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand 2008;52(2):289–294. [DOI] [PubMed] [Google Scholar]
- 1626.Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 2004;101(5):1066–1076. [DOI] [PubMed] [Google Scholar]
- 1627.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol 2011;268:1233–1236. [DOI] [PubMed] [Google Scholar]
- 1628.Yoon BW, Hong JM, Hong SL, Koo SK, Roh HJ, Cho KS. A comparison of dexmedetomidine versus propofol during drug-induced sleep endoscopy in sleep apnea patients. Laryngoscope 2016;126(3):763–767. [DOI] [PubMed] [Google Scholar]
- 1629.Viana A, Zhao C, Rosa T, et al. The effect of sedating agents on drug-induced sleep endoscopy findings. Laryngoscope 2019;129(2):506–513. [DOI] [PubMed] [Google Scholar]
- 1630.Steinhart H, Kuhn-Lohmann J, Gewalt K, Constantinidis J, Mertzlufft F, Iro H. Upper airway collapsibility in habitual snorers and sleep apneics: evaluation with drug-induced sleep endoscopy. Acta Otolaryngol 2000;120(8):990–994. [DOI] [PubMed] [Google Scholar]
- 1631.Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 2010;136(4):393–397. [DOI] [PubMed] [Google Scholar]
- 1632.Rodriguez-Bruno K, Goldberg AN, McCulloch CE, Kezirian EJ. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg 2009;140(5):646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1633.Vroegop AV, Vanderveken OM, Wouters K, et al. Observer variation in drug-induced sleep endoscopy: experienced versus nonexperienced ear, nose, and throat surgeons. Sleep 2013;36(6):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1634.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19(2):156–177. [DOI] [PubMed] [Google Scholar]
- 1635.Abdullah VJ, Wing YK, van Hasselt CA. Video sleep nasendoscopy: the Hong Kong experience. Otolaryngol Clin North Am 2003;36(3):461–471, vi. [DOI] [PubMed] [Google Scholar]
- 1636.Iwanaga K, Hasegawa K, Shibata N, et al. Endoscopic examination of obstructive sleep apnea syndrome patients during drug-induced sleep. Acta Otolaryngol Suppl 2003(550):36–40. [DOI] [PubMed] [Google Scholar]
- 1637.Vicini C, De Vito A, Benazzo M, et al. The nose oropharynx hypopharynx and larynx (NOHL) classification: a new system of diagnostic standardized examination for OSAHS patients. Eur Arch Otorhinolaryngol 2012;269(4):1297–1300. [DOI] [PubMed] [Google Scholar]
- 1638.Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope 2007;117(8):1467–1473. [DOI] [PubMed] [Google Scholar]
- 1639.Blumen MB, Latournerie V, Bequignon E, Guillere L, Chabolle F. Are the obstruction sites visualized on drug-induced sleep endoscopy reliable? Sleep Breath 2015;19(3):1021–1026. [DOI] [PubMed] [Google Scholar]
- 1640.Hessel NS, Vries N. Increase of the apnoea-hypopnoea index after uvulopalatopharyngoplasty: analysis of failure. Clin Otolaryngol Allied Sci 2004;29(6):682–685. [DOI] [PubMed] [Google Scholar]
- 1641.Hsu YS, Jacobowitz O. Does sleep endoscopy staging pattern correlate with outcome of advanced palatopharyngoplasty for moderate to severe obstructive sleep apnea? J Clin Sleep Med 2017;13(10):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1642.Koutsourelakis I, Safiruddin F, Ravesloot M, Zakynthinos S, de Vries N. Surgery for obstructive sleep apnea: sleep endoscopy determinants of outcome. Laryngoscope 2012;122(11):2587–2591. [DOI] [PubMed] [Google Scholar]
- 1643.Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope 2012;122(2):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1644.Zhang P, Ye J, Pan C, Sun N, Kang D. The Role of obstruction length and height in predicting outcome of velopharyngeal surgery. Otolaryngol Head Neck Surg 2015;153(1):144–149. [DOI] [PubMed] [Google Scholar]
- 1645.Brodsky L Modern assessment of tonsils and adenoids. Pediatr Clin North Am 1989;36(6):1551–1569. [DOI] [PubMed] [Google Scholar]
- 1646.Green KK, Kent DT, D’Agostino MA, et al. Drug-induced sleep endoscopy and surgical outcomes: a multicenter cohort study. Laryngoscope 2019;129(3):761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1647.Huyett P, Kent DT, D’Agostino MA, et al. Drug-induced sleep endoscopy and hypoglossal nerve stimulation outcomes: a multicenter cohort study. Laryngoscope 2021;131(7):1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1648.Eichler C, Sommer JU, Stuck BA, Hormann K, Maurer JT. Does drug-induced sleep endoscopy change the treatment concept of patients with snoring and obstructive sleep apnea? Sleep Breath 2013;17(1):63–68. [DOI] [PubMed] [Google Scholar]
- 1649.Gillespie MB, Reddy RP, White DR, Discolo CM, Overdyk FJ, Nguyen SA. A trial of drug-induced sleep endoscopy in the surgical management of sleep-disordered breathing. Laryngoscope 2013;123(1):277–282. [DOI] [PubMed] [Google Scholar]
- 1650.Golbin D, Musgrave B, Succar E, Yaremchuk K. Clinical analysis of drug-induced sleep endoscopy for the OSA patient. Laryngoscope 2016;126(1):249–253. [DOI] [PubMed] [Google Scholar]
- 1651.Pang KP, Baptista PM, Olszewska E, et al. Does drug-induced sleep endoscopy affect surgical outcome? A multicenter study of 326 obstructive sleep apnea patients. Laryngoscope 2020;130(2):551–555. [DOI] [PubMed] [Google Scholar]
- 1652.Sukato DC, Abramowitz JM, Boruk M, Goldstein NA, Rosenfeld RM. Endoscopic sinus surgery improves sleep quality in chronic rhinosinusitis: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2018;158(2):249–256. [DOI] [PubMed] [Google Scholar]
- 1653.Gunhan K, Zeren F, Uz U, Gumus B, Unlu H. Impact of nasal polyposis on erectile dysfunction. Am J Rhinol Allergy 2011;25(2):112–115. [DOI] [PubMed] [Google Scholar]
- 1654.Jiang R-S, Liang K-L. The influence of functional endoscopic sinus surgery on sleep related outcomes in patients with chronic rhinosinusitis. Int J Otolaryngol 2019;2019:7951045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1655.Uz U, Günhan K, Yılmaz H, Ünlü H. The evaluation of pattern and quality of sleep in patients with chronic rhinosinusitis with nasal polyps. Auris Nasus Larynx 2017;44(6):708–712. [DOI] [PubMed] [Google Scholar]
- 1656.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2014;4(9):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1657.Little RE, Alt JA, Ramakrishnan VR, et al. Objective sleep measures after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2020;11(7):1056–1063. [DOI] [PubMed] [Google Scholar]
- 1658.Sériès F, St Pierre S, Carrier G. Surgical correction of nasal obstruction in the treatment of mild sleep apnoea: importance of cephalometry in predicting outcome. Thorax 1993;48(4):360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1659.Park CY, Hong JH, Lee JH, et al. Clinical effect of surgical correction for nasal pathology on the treatment of obstructive sleep apnea syndrome. PLoS One 2014;9(6):e98765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1660.Wu J, Zhao G, Li Y, et al. Apnea-hypopnea index decreased significantly after nasal surgery for obstructive sleep apnea: a meta-analysis. Medicine (Baltimore) 2017;96(5): e6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1661.Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J 2008;31(1):110–117. [DOI] [PubMed] [Google Scholar]
- 1662.Cai Y, Goldberg AN, Chang JL. The nose and nasal breathing in sleep apnea. Otolaryngol Clin North Am 2020;53(3):385–395. [DOI] [PubMed] [Google Scholar]
- 1663.Moxness MH, Nordgard S. An observational cohort study of the effects of septoplasty with or without inferior turbinate reduction in patients with obstructive sleep apnea. BMC Ear Nose Throat Disord 2014;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1664.Hu B, Han D, Li Y, Ye J, Zang H, Wang T. Polysomnographic effect of nasal surgery on positional and non-positional obstructive sleep apnea/hypopnea patients. Acta Otolaryngol 2013;133(8):858–865. [DOI] [PubMed] [Google Scholar]
- 1665.Boot H, van Wegen R, Poublon RML, Bogaard JM, Schmitz PIM, van der Meche FGA. Long-term results of uvulopalatopharyngoplasty for obstructive sleep apnea syndrome. Laryngoscope 2000;110(3):469–475. [DOI] [PubMed] [Google Scholar]
- 1666.Browaldh N, Bring J, Friberg D. SKUP(3) RCT; continuous study: changes in sleepiness and quality of life after modified UPPP. Laryngoscope 2016;126(6):1484–1491. [DOI] [PubMed] [Google Scholar]
- 1667.Stevenson EW, Turner GT, Sutton FD, Doekel RC, Pegram V, Hernandez J. Prognostic-significance of age and tonsillectomy in uvulopalatopharyngoplasty. Laryngoscope 1990;100(8):820–823. [DOI] [PubMed] [Google Scholar]
- 1668.Tetter N, Tschopp K. Contribution of hyoid and tonsillar procedures to outcome in multilevel surgery for obstructive sleep apnea syndrome. ORL J Otorhinolaryngol Relat Spec 2016;78(6):353–360. [DOI] [PubMed] [Google Scholar]
- 1669.Tschopp S, Tschopp K. Tonsil size and outcome of uvulopalatopharyngoplasty with tonsillectomy in obstructive sleep apnea. Laryngoscope 2019;129(12):E449–E454. [DOI] [PubMed] [Google Scholar]
- 1670.Verse T, Wenzel S, Brus J. Multi-level surgery for obstructive sleep apnea. Lingual tonsillectomy vs. hyoid suspension in combination with radiofrequency of the tongue base. Sleep Breath 2015;19(4):1361–1366. [DOI] [PubMed] [Google Scholar]
- 1671.Vidigal TA, Haddad FL, Cabral RF, et al. New clinical staging for pharyngeal surgery in obstructive sleep apnea patients. Braz J Otorhinolaryngol 2014;80(6):490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1672.Orr WC, Martin RJ. Obstructive sleep apnea associated with tonsillar hypertrophy in adults. Arch Intern Med 1981;141(8):990–992. [PubMed] [Google Scholar]
- 1673.Cahali MB, Soares CFP, Dantas ASD, Formigoni GGS. Objective and subjective tonsil volume and severity of obstrutive sleep apnea: is there a correlation in adults. Sleep Med 2009;10:S65–S66. [Google Scholar]
- 1674.Tan LTH, Tan AKL, Hsu PP, et al. Effects of tonsillectomy on sleep study parameters in adult patients with obstructive sleep apnea-a prospective study. Sleep Breath 2014;18(2):265–268. [DOI] [PubMed] [Google Scholar]
- 1675.Nakata S, Noda A, Yanagi E, Suzuki K, Yamamoto H, Nakashima T. Tonsil size and body mass index are important factors for efficacy of simple tonsillectomy in obstructive sleep apnoea syndrome. Clin Otolaryngol 2006;31(1):41–45. [DOI] [PubMed] [Google Scholar]
- 1676.Nakata S, Miyazaki S, Ohki M, et al. Reduced nasal resistance after simple tonsillectomy in patients with obstructive sleep apnea. Am J Rhinol 2007;21(2):192–195. [DOI] [PubMed] [Google Scholar]
- 1677.Verse T, Kroker BA, Pirsig W, Brosch S. Tonsillectomy as a treatment of obstructive sleep apnea in adults with tonsillar hypertrophy. Laryngoscope 2000;110(9):1556–1559. [DOI] [PubMed] [Google Scholar]
- 1678.Smith MM, Peterson E, Yaremchuk KL. The role of tonsillectomy in adults with tonsillar hypertrophy and obstructive sleep apnea. Otolaryngol Head Neck Surg 2017;157(2):331–335. [DOI] [PubMed] [Google Scholar]
- 1679.Stow NW, Sale PJP, Lee D, Joffe D, Gallagher RM. Simultaneous tonsillectomy and nasal surgery in adult obstructive sleep apnea: a pilot study. Otolaryngol Head Neck Surg 2012;147(2):387–391. [DOI] [PubMed] [Google Scholar]
- 1680.Miyazaki S, Itasaka Y, Tada H, Ishikawa K, Togawa K. Effectiveness of tonsillectomy in adult sleep apnea syndrome. Psychiatry Clin Neurosci 1998;52(2):222–223. [DOI] [PubMed] [Google Scholar]
- 1681.Houghton DJ, Camilleri AE, Stone P. Adult obstructive sleep apnoea syndrome and tonsillectomy. J Laryngol Otol 1997;111(9):829–832. [DOI] [PubMed] [Google Scholar]
- 1682.Cheong TH, Wang YT, Poh SC. Sleep apnoea syndrome – a report of 14 cases. Singapore Med J 1990;31(4):350–354. [PubMed] [Google Scholar]
- 1683.Aubert-Tulkens G, Hamoir M, Van den Eeckhaut J, Rodenstein DO. Failure of tonsil and nose surgery in adults with long-standing severe sleep apnea syndrome. Arch Intern Med 1989;149(9):2118–2121. [PubMed] [Google Scholar]
- 1684.Moser RJ, Rajagopal KR. Obstructive sleep-apnea in adults with tonsillar hypertrophy. Arch Intern Med 1987;147(7):1265–1267. [PubMed] [Google Scholar]
- 1685.Rubin AHE, Eliaschar I, Joachim Z, Alroy G, Lavie P. Effects of nasal surgery and tonsillectomy on sleep-apnea. Bull Eur Physiopathol Respir 1983;19(6):612–615. [PubMed] [Google Scholar]
- 1686.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010;33(10):1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1687.Halle TR, Oh MS, Collop NA, Quyyumi AA, Bliwise DL, Dedhia RC. Surgical treatment of OSA on cardiovascular outcomes: a systematic review. Chest 2017;152(6):1214–1229. [DOI] [PubMed] [Google Scholar]
- 1688.Stuck BA, Ravesloot MJL, Eschenhagen T, de Vet HCW, Sommer JU. Uvulopalatopharyngoplasty with or without tonsillectomy in the treatment of adult obstructive sleep apnea - a systematic review. Sleep Med 2018;50:152–165. [DOI] [PubMed] [Google Scholar]
- 1689.Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;137(1):110–114. [DOI] [PubMed] [Google Scholar]
- 1690.Pang KP, Pang EB, Win MT, Pang KA, Woodson BT. Expansion sphincter pharyngoplasty for the treatment of OSA: a systemic review and meta-analysis. Eur Arch Otorhinolaryngol 2016;273(9):2329–2333. [DOI] [PubMed] [Google Scholar]
- 1691.Rashwan MS, Montevecchi F, Cammaroto G, et al. Evolution of soft palate surgery techniques for obstructive sleep apnea patients: a comparative study for single-level palatal surgeries. Clin Otolaryngol 2018;43(2):584–590. [DOI] [PubMed] [Google Scholar]
- 1692.Babademez MA, Gul F, Teleke YC. Barbed palatoplasty vs. expansion sphincter pharyngoplasty with anterior palatoplasty. Laryngoscope 2020;130(4):E275–E279. [DOI] [PubMed] [Google Scholar]
- 1693.Binar M, Akcam T, Karakoc O, Sagkan RI, Musabak U, Gerek M. A new surgical technique versus an old marker: can expansion sphincter pharyngoplasty reduce C-reactive protein levels in patients with obstructive sleep apnea? Eur Arch Otorhinolaryngol 2017;274(2):829–836. [DOI] [PubMed] [Google Scholar]
- 1694.Guler I, Kuzucu I, Baklaci D, Kum RO, Kum NY, Ozcan M. Efficiency of expansion sphincter pharyngoplasty in the treatment of obstructive sleep apnea syndrome. Turk Arch Otorhinol 2018;56(4):206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1695.Hong SN, Kim HG, Han SY, et al. Indications for and outcomes of expansion sphincter pharyngoplasty to treat lateral pharyngeal collapse in patients with obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg 2019;145(5):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1696.Huntley C, Chou DW, Doghramji K, Boon M. Comparing upper airway stimulation to expansion sphincter pharyngoplasty: a single university experience. Ann Otol Rhinol Laryngol 2018;127(6):379–383. [DOI] [PubMed] [Google Scholar]
- 1697.Karakoc O, Binar M, Aydin U, Genc H, Akcam T, Gerek M. A tertiary center experience with velopharyngeal surgical techniques for treatment of snoring and obstructive sleep apnea. Auris Nasus Larynx 2018;45(3):492–498. [DOI] [PubMed] [Google Scholar]
- 1698.Lorusso F, Dispenza F, Modica DM, Gallina S. The role of modified expansion sphincter pharyngoplasty in multilevel obstructive sleep apnea syndrome surgery. Int Arch Otorhinolaryngol 2018;22(4):432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1699.Pang KP, Piccin O, Pang EB, Pang KA, Chan YH, Rotenberg BW. Combined expansion pharyngoplasty and anterior palatoplasty for the treatment of OSA. Indian J Otolaryngol 2016;68(4):528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1700.Despeghel AS, Mus L, Dick C, et al. Long-term results of a modified expansion sphincter pharyngoplasty for sleep-disordered breathing. Eur Arch Otorhinolaryngol 2017;274(3):1665–1670. [DOI] [PubMed] [Google Scholar]
- 1701.Plaza G, Baptista P, O’Connor-Reina C, Bosco G, Perez-Martin N, Pang KP. Prospective multi-center study on expansion sphincter pharyngoplasty. Acta Otolaryngol 2019;139(2):219–222. [DOI] [PubMed] [Google Scholar]
- 1702.Pang KP, Vicini C, Montevecchi F, et al. Long-term complications of palate surgery: a multicenter study of 217 patients. Laryngoscope 2020;130(9):2281–2284. [DOI] [PubMed] [Google Scholar]
- 1703.Liu SY, Hutz MJ, Poomkonsarn S, Chang CP, Awad M, Capasso R. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope 2020;130(12):E958–E962. [DOI] [PubMed] [Google Scholar]
- 1704.Bosco G, Perez-Martin N, Racionero MA, Plaza G. Expansion sphincter pharyngoplasty: usefulness of DISE. Acta Otorrinolaringol Esp (Engl Ed) 2019;70(4):215–221. [DOI] [PubMed] [Google Scholar]
- 1705.Suslu AE, Pamuk G, Pamuk AE, Ozer S, Jafarov S, Onerci TM. Effects of expansion sphincter pharyngoplasty on the apneahypopnea index and heart rate variability. J Oral Maxillofac Surg 2017;75(12):2650–2657. [DOI] [PubMed] [Google Scholar]
- 1706.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope 2003;113(11):1961–1968. [DOI] [PubMed] [Google Scholar]
- 1707.Cahali MB, Formigoni GGS, Gebrim EMMS, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep 2004;27(5):942–950. [DOI] [PubMed] [Google Scholar]
- 1708.Cahali MB. Revaluing the role of the tongue in obstructive sleep apnea. J Bras Pneumol 2019;45(4):e20190208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1709.Passos UL, Genta PR, Marcondes BF, Lorenzi G, Gebrim EMMS. State-dependent changes in the upper airway assessed by multidetector CT in healthy individuals and during obstructive events in patients with sleep apnea. J Bras Pneumol 2019;45(4):e20180264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1710.Huon LK, Liu SYC, Shih TTF, Chen YJ, Lo MT, Wang PC. Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol 2016;273(11):4021–4026. [DOI] [PubMed] [Google Scholar]
- 1711.Chi JC, Chiang RP, Chou TY, Shu CH, Shiao AS, Lin CM. The role of lateral pharyngoplasty in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2015;272(2):489–496. [DOI] [PubMed] [Google Scholar]
- 1712.Dizdar D, Civelek S, Calis ZA, Dizdar SK, Coskun BU, Vural A. Comparative analysis of lateral pharyngoplasty and uvulopalatopharyngoplasty techniques with polisomnography and epworth sleepiness scales. J Craniofac Surg 2015;26(7):e647–e651. [DOI] [PubMed] [Google Scholar]
- 1713.Carrasco-Llatas M, Marcano-Acuna M, Zerpa-Zerpa V, Dalmau-Galofre J. Surgical results of different palate techniques to treat oropharyngeal collapse. Eur Arch Otorhinolaryngol 2015;272(9):2535–2540. [DOI] [PubMed] [Google Scholar]
- 1714.de Paula Soares CF, Cavichio L, Cahali MB. Lateral pharyngoplasty reduces nocturnal blood pressure in patients with obstructive sleep apnea. Laryngoscope 2014;124(1):311–316. [DOI] [PubMed] [Google Scholar]
- 1715.Elzayat S, El-Sobki A, El-Deeb ME, Moussa HH. Managing obstructive sleep apnea patients with CPAP failure with a novel lateral pharyngoplasty as a stand-alone procedure. Am J Otolaryngol 2020;41(4):102500. [DOI] [PubMed] [Google Scholar]
- 1716.Tuncel U, Inancli HM, Kurkcuoglu SS, Enoz M. A comparison of unilevel and multilevel surgery in obstructive sleep apnea syndrome. Ear Nose Throat J 2012;91(8):E13–E18. [PubMed] [Google Scholar]
- 1717.Woodson BT, Toohill RJ. Transpalatal advancement pharyngoplasty for obstructive sleep-apnea. Laryngoscope 1993;103(3):269–276. [DOI] [PubMed] [Google Scholar]
- 1718.Woodson BT. Changes in airway characteristics after transpalatal advancement pharyngoplasty compared to uvulopalatopharyngoplasty (UPPP). Sleep 1996;19(10):S291–S293. [DOI] [PubMed] [Google Scholar]
- 1719.Woodson BT, Robinson S, Lim HJ. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharygoplasty. Otolaryngol Head Neck Surg 2005;133(2):211–217. [DOI] [PubMed] [Google Scholar]
- 1720.Shine NP, Lewis RH. The “Propeller” incision for transpalatal advancement pharyngoplasty: a new approach to reduce post-operative oronasal fistulae. Auris Nasus Larynx 2008;35(3):397–400. [DOI] [PubMed] [Google Scholar]
- 1721.Chan L, Kitpornchai L, Mackay S. Causative factors for complications in transpalatal advancement. Ann Otol Rhinol Laryngol 2020;129(1):18–22. [DOI] [PubMed] [Google Scholar]
- 1722.Volner K, Dunn B, Chang ET, et al. Transpalatal advancement pharyngoplasty for obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2017;274(3):1197–1203. [DOI] [PubMed] [Google Scholar]
- 1723.Powell NB, Riley RW, Troell RJ, Li K, Blumen MB, Guilleminault C. Radiofrequency volumetric tissue reduction of the palate in subjects with sleep-disordered breathing. Chest 1998;113(5):1163–1174. [DOI] [PubMed] [Google Scholar]
- 1724.Back L, Palomaki M, Piilonen A, Ylikoski J. Sleep-disordered breathing: radiofrequency thermal ablation is a promising new treatment possibility. Laryngoscope 2001;111(3):464–471. [DOI] [PubMed] [Google Scholar]
- 1725.Back LJ, Tervahartiala PO, Piilonen AK, Partinen MM, Ylikoski JS. Bipolar radiofrequency thermal ablation of the soft palate in habitual snorers without significant desaturations assessed by magnetic resonance imaging. Am J Respir Crit Care Med 2002;166(6):865–871. [DOI] [PubMed] [Google Scholar]
- 1726.Blumen MB, Dahan S, Wagner I, De Dieuleveult T, Chabolle F. Radiofrequency versus LAUP for the treatment of snoring. Otolaryngol Head Neck Surg 2002;126(1):67–73. [DOI] [PubMed] [Google Scholar]
- 1727.Emery BE, Flexon PB. Radiofrequency volumetric tissue reduction of the soft palate: a new treatment for snoring. Laryngoscope 2000;110(7):1092–1098. [DOI] [PubMed] [Google Scholar]
- 1728.Blumen MB, Vezina JP, Bequignon E, Chabolle F. Snoring intensity after a first session of soft palate radiofrequency: predictive value of the final result. Laryngoscope 2013;123(6):1556–1559. [DOI] [PubMed] [Google Scholar]
- 1729.Boudewyns A, Van De Heyning P. Temperature-controlled radiofrequency tissue volume reduction of the soft palate (somnoplasty) in the treatment of habitual snoring: results of a European multicenter trial. Acta Otolaryngol 2000;120(8):981–985. [DOI] [PubMed] [Google Scholar]
- 1730.Hukins CA, Mitchell IC, Hillman DR. Radiofrequency tissue volume reduction of the soft palate in simple snoring. Arch Otolaryngol Head Neck Surg 2000;126(5):602–606. [DOI] [PubMed] [Google Scholar]
- 1731.Hultcrantz E, Harder L, Loord H, et al. Long-term effects of radiofrequency ablation of the soft palate on snoring. Eur Arch Otorhinolaryngol 2010;267(1):137–142. [DOI] [PubMed] [Google Scholar]
- 1732.Sher AE, Flexon PB, Hillman D, et al. Temperature-controlled radiofrequency tissue volume reduction in the human soft palate. Otolaryngol Head Neck Surg 2001;125(4):312–318. [DOI] [PubMed] [Google Scholar]
- 1733.Stuck BA, Maurer JT, Hein G, Hormann K, Verse T. Radiofrequency surgery of the soft palate in the treatment of snoring: a review of the literature. Sleep 2004;27(3):551–555. [DOI] [PubMed] [Google Scholar]
- 1734.Stuck BA, Sauter A, Hormann K, Verse T, Maurer JT. Radiofrequency surgery of the soft palate in the treatment of snoring. A placebo-controlled trial. Sleep 2005;28(7):847–850. [DOI] [PubMed] [Google Scholar]
- 1735.Tatla T, Sandhu G, Croft CB, Kotecha B. Celon radiofrequency thermo-ablative palatoplasty for snoring – a pilot study. J Laryngol Otol 2003;117(10):801–806. [DOI] [PubMed] [Google Scholar]
- 1736.Back LJ, Hytonen ML, Roine RP, Malmivaara AO. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009;119(6):1241–1250. [DOI] [PubMed] [Google Scholar]
- 1737.Holmlund T, Levring-Jaghagen E, Franklin KA, Lindkvist M, Berggren D. Effects of Radiofrequency versus sham surgery of the soft palate on daytime sleepiness. Laryngoscope 2014;124(10):2422–2426. [DOI] [PubMed] [Google Scholar]
- 1738.Ferguson M, Smith TL, Zanation AM, Yarbrough WG. Radiofrequency tissue volume reduction: multilesion vs single-lesion treatments for snoring. Arch Otolaryngol Head Neck Surg 2001;127(9):1113–1118. [DOI] [PubMed] [Google Scholar]
- 1739.Back LJ, Liukko T, Rantanen I, et al. Radiofrequency surgery of the soft palate in the treatment of mild obstructive sleep apnea is not effective as a single-stage procedure: a randomized single-blinded placebo-controlled trial. Laryngoscope 2009;119(8):1621–1627. [DOI] [PubMed] [Google Scholar]
- 1740.De Kermadec H, Blumen MB, Engalenc D, Vezina JP, Chabolle F. Radiofrequency of the soft palate for sleep-disordered breathing: a 6-year follow-up study. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131(1):27–31. [DOI] [PubMed] [Google Scholar]
- 1741.Hofmann T, Schwantzer G, Reckenzaun E, Koch H, Wolf G. Radiofrequency tissue volume reduction of the soft palate and UPPP in the treatment of snoring. Eur Arch Otorhinolaryngol 2006;263(2):164–170. [DOI] [PubMed] [Google Scholar]
- 1742.Stuck BA. Radiofrequency-assisted uvulopalatoplasty for snoring: long-term follow-up. Laryngoscope 2009;119(8):1617–1620. [DOI] [PubMed] [Google Scholar]
- 1743.Balsevicius T, Uloza V, Sakalauskas R, Miliauskas S, Jarutiene I. Efficacy of radiofrequency treatment of the soft palate for patients with mild to moderate obstructive sleep apnea hypopnea syndrome: treatment protocol with nine lesions to the soft palate. Sleep Breath 2015;19(3):1003–1009. [DOI] [PubMed] [Google Scholar]
- 1744.Birkent H, Soken H, Akcam T, Karahatay S, Gerek M. The effect of radiofrequency volumetric tissue reduction of soft palate on voice. Eur Arch Otorhinolaryngol 2008;265(2):195–198. [DOI] [PubMed] [Google Scholar]
- 1745.Haraldsson PO, Karling J, Lysdahl M, Svanborg E. Voice quality after radiofrequency volumetric tissue reduction of the soft palate in habitual snorers. Laryngoscope 2002;112(7 Pt 1):1260–1263. [DOI] [PubMed] [Google Scholar]
- 1746.Choi JH, Kim SN, Cho JH. Efficacy of the Pillar implant in the treatment of snoring and mild-to-moderate obstructive sleep apnea: a meta-analysis. Laryngoscope 2013;123(1):269–276. [DOI] [PubMed] [Google Scholar]
- 1747.Friedman M, Schalch P, Joseph NJ. Palatal stiffening after failed uvulopalatopharyngoplasty with the pillar implant system. Laryngoscope 2006;116(11):1956–1961. [DOI] [PubMed] [Google Scholar]
- 1748.Friedman M, Schalch P, Lin HC, Kakodkar KA, Joseph NJ, Mazloom N. Palatal implants for the treatment of snoring and obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg 2008;138(2):209–216. [DOI] [PubMed] [Google Scholar]
- 1749.Goessler UR, Hein G, Verse T, Stuck BA, Hormann K, Maurer JT. Soft palate implants as a minimally invasive treatment for mild to moderate obstructive sleep apnea. Acta Otolaryngol 2007;127(5):527–531. [DOI] [PubMed] [Google Scholar]
- 1750.Maurer JT, Sommer JU, Hein G, Hormann K, Heiser C, Stuck BA. Palatal implants in the treatment of obstructive sleep apnea: a randomised, placebo-controlled single-centre trial. Eur Arch Otorhinolaryngol 2012;269(7):1851–1856. [DOI] [PubMed] [Google Scholar]
- 1751.Nordgard S, Stene BK, Skjostad KW. Soft palate implants for the treatment of mild to moderate obstructive sleep apnea. Otolaryngol Head Neck Surg 2006;134(4):565–570. [DOI] [PubMed] [Google Scholar]
- 1752.Steward DL, Huntley TC, Woodson BT, Surdulescu V. Palate implants for obstructive sleep apnea: multi-institution, randomized, placebo-controlled study. Otolaryngol Head Neck Surg 2008;139(4):506–510. [DOI] [PubMed] [Google Scholar]
- 1753.Walker RP, Levine HL, Hopp ML, Greene D, Pang K. Palatal implants: a new approach for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2006;135(4):549–554. [DOI] [PubMed] [Google Scholar]
- 1754.Nordgard S, Hein G, Stene BK, Skjostad KW, Maurer JT. Oneyear results: palatal implants for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;136(5):818–822. [DOI] [PubMed] [Google Scholar]
- 1755.Walker RP, Levine HL, Hopp ML, Greene D. Extended follow-up of palatal implants for OSA treatment. Otolaryngol Head Neck Surg 2007;137(5):822–827. [DOI] [PubMed] [Google Scholar]
- 1756.Neruntarat C Long-term results of palatal implants for obstructive sleep apnea. Eur Arch Otorhinolaryngol 2011;268(7):1077–1080. [DOI] [PubMed] [Google Scholar]
- 1757.Choi JH, Cho JH, Chung YS, Kim JW, Kim SW. Effect of the Pillar implant on snoring and mild obstructive sleep apnea: a multicenter study in Korea. Laryngoscope 2015;125(5):1239–1243. [DOI] [PubMed] [Google Scholar]
- 1758.Server EA, Alkan Z, Yigit O, Yasak AG. Long-term results of pillar implant procedure. Kulak Burun Bogaz Ihtis Derg 2016;26(5):258–264. [DOI] [PubMed] [Google Scholar]
- 1759.Bertoletti F, Indelicato A, Banfi P, Capolunghi B. Sleep apnoea/hypopnoea syndrome: combination therapy with the pillar palatal implant technique and continuous positive airway pressure (CPAP). A preliminary report. B-ENT 2009;5(4):251–257. [PubMed] [Google Scholar]
- 1760.Hu HC, Kuo CL, Tung TH, Chen SC, Li LP. Long-term results of palatal implantation for severe obstructive sleep apnea patients with prominent retropalatal collapse. J Chin Med Assoc 2018;81(9):837–841. [DOI] [PubMed] [Google Scholar]
- 1761.Gillespie MB, Wylie PE, Lee-Chiong T, Rapoport DM. Effect of palatal implants on continuous positive airway pressure and compliance. Otolaryngol Head Neck Surg 2011;144(2):230–236. [DOI] [PubMed] [Google Scholar]
- 1762.Friedman M, Vidyasagar R, Bliznikas D, Joseph NJ. Patient selection and efficacy of pillar implant technique for treatment of snoring and obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg 2006;134(2):187–196. [DOI] [PubMed] [Google Scholar]
- 1763.Cammaroto G, Montevecchi F, D’Agostino G, et al. Palatal surgery in a transoral robotic setting (TORS): preliminary results of a retrospective comparison between uvulopalatopharyngoplasty (UPPP), expansion sphincter pharyngoplasty (ESP) and barbed repositioning pharyngoplasty (BRP). Acta Otorhinolaryngol Ital 2017;37(5):406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1764.Vicini C, Meccariello G, Montevecchi F, et al. Effectiveness of barbed repositioning pharyngoplasty for the treatment of obstructive sleep apnea (OSA): a prospective randomized trial. Sleep Breath 2020;24(2):687–694. [DOI] [PubMed] [Google Scholar]
- 1765.Barbieri M, Missale F, Incandela F, et al. Barbed suspension pharyngoplasty for treatment of lateral pharyngeal wall and palatal collapse in patients affected by OSAHS. Eur Arch Otorhinolaryngol 2019;276(6):1829–1835. [DOI] [PubMed] [Google Scholar]
- 1766.Mantovani M, Rinaldi V, Torretta S, Carioli D, Salamanca F, Pignataro L. Barbed roman blinds technique for the treatment of obstructive sleep apnea: how we do it? Eur Arch Otorhinolaryngol 2016;273(2):517–523. [DOI] [PubMed] [Google Scholar]
- 1767.Mantovani M, Carioli D, Torretta S, Rinaldi V, Ibba T, Pignataro L. Barbed snore surgery for concentric collapse at the velum: the Alianza technique. J Craniomaxillofac Surg 2017;45(11):1794–1800. [DOI] [PubMed] [Google Scholar]
- 1768.Salamanca F, Costantini F, Mantovani M, et al. Barbed anterior pharyngoplasty: an evolution of anterior palatoplasty. Acta Otorhinolaryngol Ital 2014;34(6):434–438. [PMC free article] [PubMed] [Google Scholar]
- 1769.Pianta L, Bertazzoni G, Morello R, Perotti P, Nicolai P. Barbed expansion sphincter pharyngoplasty for the treatment of oropharyngeal collapse in obstructive sleep apnoea syndrome: a retrospective study on 17 patients. Clin Otolaryngol 2018;43(2):696–700. [DOI] [PubMed] [Google Scholar]
- 1770.El-Ahl MAS, El-Anwar MW. Expansion pharyngoplasty by new simple suspension sutures without tonsillectomy. Otolaryngol Head Neck Surg 2016;155(6):1065–1068. [DOI] [PubMed] [Google Scholar]
- 1771.Elbassiouny AMME. Modified barbed soft palatal posterior pillar webbing flap palatopharyngoplasty. Sleep Breath 2016;20(2):829–836. [DOI] [PubMed] [Google Scholar]
- 1772.Modica DM, Lorusso F, Presti G, Fasola S, Gallina S. Our assessment using palate postoperative problems score (PPOPS): tool for the evaluation of results in palatal surgery techniques. Indian J Otolaryngol 2019;71:766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1773.Rashwan MS, Montevecchi F, Firinua E, et al. Let’s know from our patients: PPOPS score for palate surgery evaluation/a pilot study. Eur Arch Otorhinolaryngol 2018;275(1):287–291. [DOI] [PubMed] [Google Scholar]
- 1774.Rinaldi V, Costantino A, Moffa A, et al. Postoperative pain and wound healing after coblation-assisted barbed anterior pharyngoplasty (CABAPh): an observational study. Indian J Otolaryngol Head Neck Surg 2019;71(2):1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1775.Askar SM, El-Anwar MW, Awad A. Modified anterior palatoplasty and double suspension sutures (with or without tonsillectomy) in selected patients with obstructive sleep apnea: a preliminary report. Sleep Breath 2018;22(3):789–795. [DOI] [PubMed] [Google Scholar]
- 1776.Askar SM, El-Anwar MW. Double suspension sutures: a simple surgical technique for selected cases of obstructive sleep apnoea: our experience with twenty-two patients. Clin Otolaryngol 2018;43(2):753–757. [DOI] [PubMed] [Google Scholar]
- 1777.Babademez MA, Gul F, Kale H, Sancak M. Technical update of barbed pharyngoplasty for retropalatal obstruction in obstructive sleep apnoea. J Laryngol Otol 2019;133(7):622–626. [DOI] [PubMed] [Google Scholar]
- 1778.Powell NB, Riley RW, Guilleminault C. Radiofrequency tongue base reduction in sleep-disordered breathing: a pilot study. Otolaryngol Head Neck Surg 1999;120(5):656–664. [DOI] [PubMed] [Google Scholar]
- 1779.Stuck BA, Kopke J, Hormann K, et al. Volumetric tissue reduction in radiofrequency surgery of the tongue base. Otolaryngol Head Neck Surg 2005;132(1):132–135. [DOI] [PubMed] [Google Scholar]
- 1780.Blumen MB, Coquille F, Rocchicioli C, Mellot F, Chabolle F. Radiofrequency tongue reduction through a cervical approach: a pilot study. Laryngoscope 2006;116(10):1887–1893. [DOI] [PubMed] [Google Scholar]
- 1781.den Herder C, Kox D, van Tinteren H, de Vries N. Bipolar radiofrequency induced thermotherapy of the tongue base: its complications, acceptance and effectiveness under local anesthesia. Eur Arch Otorhinolaryngol 2006;263(11):1031–1040. [DOI] [PubMed] [Google Scholar]
- 1782.Baba RY, Mohan A, Metta VV, Mador MJ. Temperature controlled radiofrequency ablation at different sites for treatment of obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Breath 2015;19(3):891–910. [DOI] [PubMed] [Google Scholar]
- 1783.Fibbi A, Ameli F, Brocchetti F, Mignosi S, Cabano ME, Semino L. Tongue base suspension and radiofrequency volume reduction: a comparison between 2 techniques for the treatment of sleep-disordered breathing. Am J Otolaryngol 2009;30(6):401–406. [DOI] [PubMed] [Google Scholar]
- 1784.Li KK, Powell NB, Riley RW, Guilleminault C. Temperaturecontrolled radiofrequency tongue base reduction for sleep-disordered breathing: long-term outcomes. Otolaryngol Head Neck Surg 2002;127(3):230–234. [DOI] [PubMed] [Google Scholar]
- 1785.Woodson BT, Nelson L, Mickelson S, Huntley T, Sher A. A multi-institutional study of radiofrequency volumetric tissue reduction for OSAS. Otolaryngol Head Neck Surg 2001;125(4):303–311. [DOI] [PubMed] [Google Scholar]
- 1786.Friedman M, Ibrahim H, Lee G, Joseph NJ. Combined uvulopalatopharyngoplasty and radiofrequency tongue base reduction for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg 2003;129(6):611–621. [DOI] [PubMed] [Google Scholar]
- 1787.van den Broek E, Richard W, van Tinteren H, de Vries N. UPPP combined with radiofrequency thermotherapy of the tongue base for the treatment of obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2008;265(11):1361–1365. [DOI] [PubMed] [Google Scholar]
- 1788.Friedman M, Soans R, Gurpinar B, Lin HC, Joseph N. Evaluation of submucosal minimally invasive lingual excision technique for treatment of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg 2008;139(3):378–384; discussion 385. [DOI] [PubMed] [Google Scholar]
- 1789.Riley RW, Powell NB, Li KK, Weaver EM, Guilleminault C. An adjunctive method of radiofrequency volumetric tissue reduction of the tongue for OSAS. Otolaryngol Head Neck Surg 2003;129(1):37–42. [DOI] [PubMed] [Google Scholar]
- 1790.Stuck BA, Maurer JT, Verse T, Hormann K. Tongue base reduction with temperature-controlled radiofrequency volumetric tissue reduction for treatment of obstructive sleep apnea syndrome. Acta Otolaryngol 2002;122(5):531–536. [DOI] [PubMed] [Google Scholar]
- 1791.Fernández-Julián E, Muñoz N, Achiques MT, García-Pérez MA, Orts M, Marco J. Randomized study comparing two tongue base surgeries for moderate to severe obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2009;140(6):917–923. [DOI] [PubMed] [Google Scholar]
- 1792.Riley RW, Powell NB, Guilleminault C. Inferior sagittal osteotomy of the mandible with hyoid myotomy-suspension - a new procedure for obstructive sleep-apnea. Otolaryngol Head Neck Surg 1986;94(5):589–593. [DOI] [PubMed] [Google Scholar]
- 1793.Awad M, Gouveia C, Zaghi S, Camacho M, Liu SYC. Changing practice: trends in skeletal surgery for obstructive sleep apnea. J Craniomaxillofac Surg 2019;47(8):1185–1189. [DOI] [PubMed] [Google Scholar]
- 1794.Song SA, Chang ET, Certal V, et al. Genial tubercle advancement and genioplasty for obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2017;127(4):984–992. [DOI] [PubMed] [Google Scholar]
- 1795.Chen SC, Shi S, Xia YH, et al. Changes in sleep characteristics and airway obstruction in OSAHS patients with multi-level obstruction following simple UPPP, UPPP-GA, or UPPP-TBA: a prospective, single-center, parallel group study. ORL J Otorhinolaryngol Relat Spec 2014;76(4):179–188. [DOI] [PubMed] [Google Scholar]
- 1796.Wootten CT, Shott SR. Evolving therapies to treat retroglossal and base-of-tongue obstruction in pediatric obstructive sleep apnea. Arch Otolaryngol 2010;136(10):983–987. [DOI] [PubMed] [Google Scholar]
- 1797.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg 1993;108(2):117–125. [DOI] [PubMed] [Google Scholar]
- 1798.Vilaseca I, Morello A, Montserrat JM, Santamaria J, Iranzo A. Usefulness of uvulopalatopharyngoplasty with genioglossus and hyoid advancement in the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2002;128(4):435–440. [DOI] [PubMed] [Google Scholar]
- 1799.Richard W, Kox D, den Herder C, van Tinteren H, de Vries N. One stage multilevel surgery (uvulopalatopharyngoplasty, hyoid suspension, radiofrequent ablation of the tongue base with/without genioglossus advancement), in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol 2007;264(4):439–444. [DOI] [PubMed] [Google Scholar]
- 1800.Liu SY, Awad M, Riley RW. Maxillomandibular advancement: contemporary approach at stanford. Atlas Oral Maxillofac Surg Clin North Am 2019;27(1):29–36. [DOI] [PubMed] [Google Scholar]
- 1801.Troell RJ, Riley RW, Powell NB, Li K. Surgical management of the hypopharyngeal airway in sleep disordered breathing. Otolaryngol Clin North Am 1998;31(6):979–1012. [DOI] [PubMed] [Google Scholar]
- 1802.Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg 2006;132(2):206–213. [DOI] [PubMed] [Google Scholar]
- 1803.Foltan R, Hoffmannova J, Pretl M, Donev F, Vlk M. Genioglossus advancement and hyoid myotomy in treating obstructive sleep apnoea syndrome - a follow-up study. J Craniomaxillofac Surg 2007;35(4–5):246–251. [DOI] [PubMed] [Google Scholar]
- 1804.Song SA, Wei JM, Buttram J, et al. Hyoid surgery alone for obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2016;126(7):1702–1708. [DOI] [PubMed] [Google Scholar]
- 1805.Kuscu O, Suslu AE, Ozer S, Gunaydin RO, Ogretmenoglu O, Onerci M. Sole effect of genioglossus advancement on apnea hypopnea index of patients with obstructive sleep apnea. Acta Otolaryngol 2015;135(8):835–839. [DOI] [PubMed] [Google Scholar]
- 1806.Kao YH, Shnayder Y, Lee KC. The efficacy of anatomically based multilevel surgery for obstructive sleep apnea. Otolaryngol Head Neck Surg 2003;129(4):327–335. [DOI] [PubMed] [Google Scholar]
- 1807.Kotecha BT, Hannan SA, Khalil HMB, Georgalas C, Bailey P. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol 2007;264(11):1361–1367. [DOI] [PubMed] [Google Scholar]
- 1808.Metes A, Hoffstein V, Mateika S, Cole P, Haight JSJ. Site of airway-obstruction in patients with obstructive sleep-apnea before and after uvulopalatopharyngoplasty. Laryngoscope 1991;101(10):1102–1108. [DOI] [PubMed] [Google Scholar]
- 1809.Kezirian EJ, Maselli J, Vittinghoff E, Goldberg AN, Auerbach AD. Obstructive sleep apnea surgery practice patterns in the United States: 2000 to 2006. Otolaryngol Head Neck Surg 2010;143(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1810.Omur M, Ozturan D, Elez F, Unver C, Derman S. Tongue base suspension combined with UPPP in severe OSA patients. Otolaryngol Head Neck Surg 2005;133(2):218–223. [DOI] [PubMed] [Google Scholar]
- 1811.Handler E, Hamans E, Goldberg AN, Mickelson S. Tongue suspension: an evidence-based review and comparison to hypopharyngeal surgery for OSA. Laryngoscope 2014;124(1):329–336. [DOI] [PubMed] [Google Scholar]
- 1812.DeRowe A, Gunther E, Fibbi A, et al. Tongue-base suspension with a soft tissue-to-bone anchor for obstructive sleep apnea: preliminary clinical results of a new minimally invasive technique. Otolaryngol Head Neck Surg 2000;122(1):100–103. [DOI] [PubMed] [Google Scholar]
- 1813.Woodson BT. A tongue suspension suture for obstructive sleep apnea and snorers. Otolaryngol Head Neck Surg 2001;124(3):297–303. [DOI] [PubMed] [Google Scholar]
- 1814.Woodson BT, DeRowe A, Hawke M, et al. Pharyngeal suspension suture with Repose bone screw for obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;122(3):395–401. [DOI] [PubMed] [Google Scholar]
- 1815.Sorrenti G, Piccin O, Latini G, Scaramuzzino G, Mondini S, Rinaldi Ceroni A. Tongue base suspension technique in obstructive sleep apnea: personal experience. Acta Otorhinolaryngol Ital 2003;23(4):274–280. [PubMed] [Google Scholar]
- 1816.Thomas AJ, Chavoya M, Terris DJ. Preliminary findings from a prospective, randomized trial of two tongue-base surgeries for sleep-disordered breathing. Otolaryngol Head Neck Surg 2003;129(5):539–546. [DOI] [PubMed] [Google Scholar]
- 1817.Bostanci A, Turhan M. A systematic review of tongue base suspension techniques as an isolated procedure or combined with uvulopalatopharyngoplasty in obstructive sleep apnea. Eur Arch Otorhinolaryngol 2016;273(10):2895–2901. [DOI] [PubMed] [Google Scholar]
- 1818.Woodson BT, Steward DL, Mickelson S, Huntley T, Goldberg A. Multicenter study of a novel adjustable tongueadvancement device for obstructive sleep apnea. Otolaryngol Head Neck Surg 2010;143(4):585–590. [DOI] [PubMed] [Google Scholar]
- 1819.Tsou YA, Huang CW, Wu TF, Hung LW, Chang WD. The effect of tongue base suspension with uvulopalatopharyngoplasty on sleep quality in obstructive sleep apnea. Sci Rep 2018;8(1):8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1820.den Herder C, van Tinteren H, de Vries N. Hyoidthyroidpexia: a surgical treatment for sleep apnea syndrome. Laryngoscope 2005;115(4):740–745. [DOI] [PubMed] [Google Scholar]
- 1821.Riley R, Guilleminault C, Powell N, Derman S. Mandibular osteotomy and hyoid bone advancement for obstructive sleepapnea – a case-report. Sleep 1984;7(1):79–82. [DOI] [PubMed] [Google Scholar]
- 1822.Riley RW, Powell NB, Guilleminault C. Obstructive sleep-apnea and the hyoid - a revised surgical-procedure. Otolaryngol Head Neck Surg 1994;111(6):717–721. [DOI] [PubMed] [Google Scholar]
- 1823.Bowden MT, Kezirian EJ, Utley D, Goode RL. Outcomes of hyoid suspension for the treatment of obstructive sleep apnea. Arch Otolaryngol 2005;131(5):440–445. [DOI] [PubMed] [Google Scholar]
- 1824.Verse T, Baisch A, Maurer JT, Stuck BA, Hörmann K. Multilevel surgery for obstructive sleep apnea: short-term results. Otolaryngol Head Neck Surg 2006;134(4):571–577. [DOI] [PubMed] [Google Scholar]
- 1825.Askar SM, El-Anwar MW, Awad A. Expansion hyoidthyroidpexy: combined hyoid surgery techniques for obstructive sleep apnea: all in one. Otolaryngol Head Neck Surg 2019;160(2):355–358. [DOI] [PubMed] [Google Scholar]
- 1826.O’Malley BW, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006;116(8):1465–1472. [DOI] [PubMed] [Google Scholar]
- 1827.Friedman M, Hamilton C, Samuelson CG, et al. Transoral robotic glossectomy for the treatment of obstructive sleep apnea-hypopnea syndrome. Otolaryngol Head Neck Surg 2012;146(5):854–862. [DOI] [PubMed] [Google Scholar]
- 1828.Cammaroto G, Montevecchi F, D’Agostino G, et al. Tongue reduction for OSAHS: TORSs vs coblations, technologies vs techniques, apples vs oranges. Eur Arch Otorhinolaryngol 2017;274(2):637–645. [DOI] [PubMed] [Google Scholar]
- 1829.Miller SC, Nguyen SA, Ong AA, Gillespie MB. Transoral robotic base of tongue reduction for obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2017;127(1):258–265. [DOI] [PubMed] [Google Scholar]
- 1830.Samutsakorn P, Hirunwiwatkul P, Chaitusaney B, Charakorn N. Lingual tonsillectomy with palatal surgery for the treatment of obstructive sleep apnea in adults: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2018;275(4):1005–1013. [DOI] [PubMed] [Google Scholar]
- 1831.Hoff PT, D’Agostino MA, Thaler ER. Transoral robotic surgery in benign diseases including obstructive sleep apnea: safety and feasibility. Laryngoscope 2015;125(5):1249–1253. [DOI] [PubMed] [Google Scholar]
- 1832.Lin HS, Rowley JA, Folbe AJ, Yoo GH, Badr MS, Chen W. Transoral robotic surgery for treatment of obstructive sleep apnea: factors predicting surgical response. Laryngoscope 2015;125(4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 1833.Catalfumo FJ, Golz A, Westerman ST, Gilbert LM, Joachims HZ, Goldenberg D. The epiglottis and obstructive sleep apnoea syndrome. J Laryngol Otol 1998;112(10):940–943. [DOI] [PubMed] [Google Scholar]
- 1834.Torre C, Camacho M, Liu SY, Huon LK, Capasso R. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope 2016;126(2):515–523. [DOI] [PubMed] [Google Scholar]
- 1835.Vonk PE, Ravesloot MJL, Kasius KM, van Maanen JP, de Vries N. Floppy epiglottis during drug-induced sleep endoscopy: an almost complete resolution by adopting the lateral posture. Sleep Breath 2020;24(1):103–109. [DOI] [PubMed] [Google Scholar]
- 1836.Kwon OE, Jung SY, Al-Dilaijan K, Min JY, Lee KH, Kim SW. Is epiglottis surgery necessary for obstructive sleep apnea patients with epiglottis obstruction? Laryngoscope 2019;129(11):2658–2662. [DOI] [PubMed] [Google Scholar]
- 1837.Dedhia RC, Rosen CA, Soose RJ. What is the role of the larynx in adult obstructive sleep apnea? Laryngoscope 2014;124(4):1029–1034. [DOI] [PubMed] [Google Scholar]
- 1838.Cassano M Endoscopic coblator-assisted epiglottoplasty in ‘obstructive sleep apnoea syndrome’ patients. Clin Otolaryngol 2017;42(5):1112–1114. [DOI] [PubMed] [Google Scholar]
- 1839.Golz A, Goldenberg D, Westerman ST, et al. Laser partial epiglottidectomy as a treatment for obstructive sleep apnea and laryngomalacia. Ann Otol Rhinol Laryngol 2000;109(12):1140–1145. [DOI] [PubMed] [Google Scholar]
- 1840.Oomen KPQ, Modi VK. Epiglottopexy with and without lingual tonsillectomy. Laryngoscope 2014;124(4):1019–1022. [DOI] [PubMed] [Google Scholar]
- 1841.Kayhan FT, Kaya KH, Koc AK, et al. Multilevel combined surgery with transoral robotic surgery for obstructive sleep apnea syndrome. J Craniofac Surg 2016;27(4):1044–1048. [DOI] [PubMed] [Google Scholar]
- 1842.Vicini C, Montevecchi F, Tenti G, Canzi P, Dallan I, Huntley TC. Transoral robotic surgery: tongue base reduction and supraglottoplasty for obstructive sleep apnea. Oper Tech Otolayngol Head Neck Surg 2012;23(1):45–47. [Google Scholar]
- 1843.Hershey HG, Stewart BL, Warren DW. Changes in nasal airway-resistance associated with rapid maxillary expansion. Am J Orthod Dentofac 1976;69(3):274–284. [DOI] [PubMed] [Google Scholar]
- 1844.Harvold EP, Tomer BS, Vargervik K, Chierici G. Primate experiments on oral respiration. Am J Orthod Dentofac 1981;79(4):359–372. [DOI] [PubMed] [Google Scholar]
- 1845.Baratieri C, Alves M, de Souza MMG, Araujo MTD, Maia LC. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am J Orthod Dentofac 2011;140(2):146–156. [DOI] [PubMed] [Google Scholar]
- 1846.Chang Y, Koenig LJ, Pruszynski JE, Bradley TG, Bosio JA, Liu DW. Dimensional changes of upper airway after rapid maxillary expansion: a prospective cone-beam computed tomography study. Am J Orthod Dentofac 2013;143(4):462–470. [DOI] [PubMed] [Google Scholar]
- 1847.Warren DW, Hershey HG, Turvey TA, Hinton VA, Hairfield WM. The nasal airway following maxillary expansion. Am J Orthod Dentofac 1987;91(2):111–116. [DOI] [PubMed] [Google Scholar]
- 1848.Iwasaki T, Saitoh I, Takemoto Y, et al. Improvement of nasal airway ventilation after rapid maxillary expansion evaluated with computational fluid dynamics. Am J Orthod Dentofac 2012;141(3):269–278. [DOI] [PubMed] [Google Scholar]
- 1849.Gorgulu S, Gokce SM, Olmez H, Sagdic D, Ors F. Nasal cavity volume changes after rapid maxillary expansion in adolescents evaluated with 3-dimensional simulation and modeling programs. Am J Orthod Dentofac 2011;140(5):633–640. [DOI] [PubMed] [Google Scholar]
- 1850.Vinha PP, Faria AC, Xavier SP, Christino M, de Mello FV. Enlargement of the pharynx resulting from surgically assisted rapid maxillary expansion. J Oral Maxillofac Surg 2016;74(2):369–379. [DOI] [PubMed] [Google Scholar]
- 1851.Camacho M, Chang ET, Song SJA, et al. Rapid maxillary expansion for pediatric obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2017;127(7):1712–1719. [DOI] [PubMed] [Google Scholar]
- 1852.Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep 1998;21(8):831–835. [DOI] [PubMed] [Google Scholar]
- 1853.Machado AJ, Zancanella E, Crespo AN. Rapid maxillary expansion and obstructive sleep apnea: a review and metaanalysis. Med Oral Patol Oral Cir Bucal 2016;21(4):E465–E469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1854.Vale F, Albergaria M, Carrilho E, et al. Efficacy of rapid maxillary expansion in the treatment of obstructive sleep apnea syndrome: a systematic review with meta-analysis. J Evid Based Dent Pract 2017;17(3):159–168. [DOI] [PubMed] [Google Scholar]
- 1855.Vinha PP, Eckeli AL, Faria AC, Xavier SP, de Mello FV. Effects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepiness. Sleep Breath 2016;20(2):501–508. [DOI] [PubMed] [Google Scholar]
- 1856.Carlson C, Sung J, McComb RW, Machado AW, Moon W. Microimplant-assisted rapid palatal expansion appliance to orthopedically correct transverse maxillary deficiency in an adult. Am J Orthod Dentofac 2016;149(5):716–728. [DOI] [PubMed] [Google Scholar]
- 1857.Yoon A, Guilleminault C, Zaghi S, Liu SYC. Distraction osteogenesis maxillary expansion (DOME) for adult obstructive sleep apnea patients with narrow maxilla and nasal floor. Sleep Med 2020;65:172–176. [DOI] [PubMed] [Google Scholar]
- 1858.Suri L, Taneja P. Surgically assisted rapid palatal expansion: a literature review. Am J Orthod Dentofac 2008;133(2):290–302. [DOI] [PubMed] [Google Scholar]
- 1859.Monini S, Malagola C, Villa MP, et al. Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch Otolaryngol 2009;135(1):22–27. [DOI] [PubMed] [Google Scholar]
- 1860.Quo SD, Hyunh N, Guilleminault C. Bimaxillary expansion therapy for pediatric sleep-disordered breathing. Sleep Med 2017;30:45–51. [DOI] [PubMed] [Google Scholar]
- 1861.Riley RW, Powell NB, Guilleminault C. Maxillary, mandibular, and hyoid advancement for treatment of obstructive sleep apnea: a review of 40 patients. J Oral Maxillofac Surg 1990;48(1):20–26. [DOI] [PubMed] [Google Scholar]
- 1862.Barrera JE. Skeletal surgery for obstructive sleep apnea. Sleep Med Clin 2018;13(4):549–558. [DOI] [PubMed] [Google Scholar]
- 1863.Gottsauner-Wolf S, Laimer J, Bruckmoser E. Posterior airway changes following orthognathic surgery in obstructive sleep apnea. J Oral Maxillofac Surg 2018;76(5):1093.e1–1093.e21. [DOI] [PubMed] [Google Scholar]
- 1864.Dattilo DJ, Drooger SA. Outcome assessment of patients undergoing maxillofacial procedures for the treatment of sleep apnea: comparison of subjective and objective results. J Oral Maxillofac Surg 2004;62(2):164–168. [DOI] [PubMed] [Google Scholar]
- 1865.Prinsell JR. Maxillomandibular advancement surgery in a sitespecific treatment approach for obstructive sleep apnea in 50 consecutive patients. Chest 1999;116(6):1519–1529. [DOI] [PubMed] [Google Scholar]
- 1866.Abdelwahab M, Poomkonsarn S, Ren X, et al. A comprehensive strategy for improving nasal outcomes after large maxillomandibular advancement for obstructive sleep apnea. Facial Plast Surg Aesthet Med 2021;23(6):437–442. [DOI] [PubMed] [Google Scholar]
- 1867.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2010;14(5):287–297. [DOI] [PubMed] [Google Scholar]
- 1868.Camacho M, Noller MW, Del Do M, et al. Long-term results for maxillomandibular advancement to treat obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg 2019;160(4):580–593. [DOI] [PubMed] [Google Scholar]
- 1869.Kezirian EJ, Goding GS, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res 2014;23(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1870.Friedman M, Jacobowitz O, Hwang MS, et al. Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: six-month results. Laryngoscope 2016;126(11):2618–2623. [DOI] [PubMed] [Google Scholar]
- 1871.Heiser C, Sommer JU, Hofauer B, et al. Bilateral vs unilateral hypoglossal nerve stimulation in patients with obstructive sleep apnea. OTO Open 2022;6(3):2473974x221109794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1872.Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 2012;122(7):1626–1633. [DOI] [PubMed] [Google Scholar]
- 1873.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013;9(5):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1874.Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2015;125(5):1254–1264. [DOI] [PubMed] [Google Scholar]
- 1875.Kompelli AR, Ni JS, Nguyen SA, Lentsch EJ, Neskey DM, Meyer TA. The outcomes of hypoglossal nerve stimulation in the management of OSA: a systematic review and meta-analysis. World J Otorhinolaryngol Head Neck Surg 2019;5(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1876.Thaler E, Schwab R, Maurer J, et al. Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy. Laryngoscope 2020;130(5):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1877.Woodson BT, Gillespie MB, Soose RJ, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg 2014;151(5):880–887. [DOI] [PubMed] [Google Scholar]
- 1878.Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg 2016;154(1):181–188. [DOI] [PubMed] [Google Scholar]
- 1879.Gillespie MB, Soose RJ, Woodson BT, et al. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg 2017;156(4):765–771. [DOI] [PubMed] [Google Scholar]
- 1880.Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngol Head Neck Surg 2017;156(2):378–384. [DOI] [PubMed] [Google Scholar]
- 1881.Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C. Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath 2020;24(3):979–984. [DOI] [PubMed] [Google Scholar]
- 1882.Boon M, Huntley C, Steffen A, et al. Upper airway stimulation for obstructive sleep apnea: results from the ADHERE registry. Otolaryngol Head Neck Surg 2018;159(2):379–385. [DOI] [PubMed] [Google Scholar]
- 1883.Huntley C, Topf MC, Christopher V, Doghramji K, Curry J, Boon M. Comparing upper airway stimulation to transoral robotic base of tongue resection for treatment of obstructive sleep apnea. Laryngoscope 2019;129(4):1010–1013. [DOI] [PubMed] [Google Scholar]
- 1884.Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018;39(3):266–270. [DOI] [PubMed] [Google Scholar]
- 1885.Huntley C, Vasconcellos A, Doghramji K, Hofauer B, Heiser C, Boon M. Upper airway stimulation in patients who have undergone unsuccessful prior palate surgery: an initial evaluation. Otolaryngol Head Neck Surg 2018;159(5):938–940. [DOI] [PubMed] [Google Scholar]
- 1886.Kezirian EJ, Heiser C, Steffen A, et al. Previous surgery and hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Head Neck Surg 2019;161(5):897–903. [DOI] [PubMed] [Google Scholar]
- 1887.Steffen A, Abrams N, Suurna MV, Wollenberg B, Hasselbacher K. Upper-airway stimulation before, after, or without uvulopalatopharyngoplasty: a two-year perspective. Laryngoscope 2019;129(2):514–518. [DOI] [PubMed] [Google Scholar]
- 1888.Bohorquez D, Mahmoud AF, Yu JL, Thaler ER. Upper airway stimulation therapy and sleep architecture in patients with obstructive sleep apnea. Laryngoscope 2020;130(4):1085–1089. [DOI] [PubMed] [Google Scholar]
- 1889.Hofauer B, Philip P, Wirth M, Knopf A, Heiser C. Effects of upper-airway stimulation on sleep architecture in patients with obstructive sleep apnea. Sleep Breath 2017;21(4):901–908. [DOI] [PubMed] [Google Scholar]
- 1890.Costantino A, Rinaldi V, Moffa A, et al. Hypoglossal nerve stimulation long-term clinical outcomes: a systematic review and meta-analysis. Sleep Breath 2020;24(2):399–411. [DOI] [PubMed] [Google Scholar]
- 1891.Kent DT, Carden KA, Wang L, Lindsell CJ, Ishman SL. Evaluation of hypoglossal nerve stimulation treatment in obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg 2019;145(11):1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1892.Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenter German post-market study. Eur Arch Otorhinolaryngol 2018;275(7):1913–1919. [DOI] [PubMed] [Google Scholar]
- 1893.Sarber KM, Chang KW, Epperson MV, et al. Hypoglossal nerve stimulation in veterans with obstructive sleep apnea. Laryngoscope 2020;130(9):2275–2280. [DOI] [PubMed] [Google Scholar]
- 1894.Mahmoud AF, Thaler ER. Outcomes of hypoglossal nerve upper airway stimulation among patients with isolated retropalatal collapse. Otolaryngol Head Neck Surg 2019;160(6):1124–1129. [DOI] [PubMed] [Google Scholar]
- 1895.Withrow K, Evans S, Harwick J, Kezirian E, Strollo P, Investigators AR. Upper airway stimulation response in older adults with moderate to severe obstructive sleep apnea. Otolaryngol Head Neck Surg 2019;161(4):714–719. [DOI] [PubMed] [Google Scholar]
- 1896.Zhu ZJ, Hofauer B, Wirth M, et al. Selective upper airway stimulation in older patients. Resp Med 2018;140:77–81. [DOI] [PubMed] [Google Scholar]
- 1897.Evans SS, Richman J, Cho DY, Withrow K. Increasing preoperative apnea severity improves upper airway stimulation response in OSA treatment. Laryngoscope 2020;130(2):556–560. [DOI] [PubMed] [Google Scholar]
- 1898.Dedhia RC, Shah AJ, Bliwise DL, et al. Hypoglossal nerve stimulation and heart rate variability: analysis of STAR trial responders. Otolaryngol Head Neck Surg 2019;160(1):165–171. [DOI] [PubMed] [Google Scholar]
- 1899.Steffen A, Chamorro R, Buyny L, et al. Upper airway stimulation in obstructive sleep apnea improves glucose metabolism and reduces hedonic drive for food. J Sleep Res 2019;28(5):e12794. [DOI] [PubMed] [Google Scholar]
- 1900.Walia HK, Thompson NR, Strohl KP, et al. Upper airway stimulation vs positive airway pressure impact on BP and sleepiness symptoms in OSA. Chest 2020;157(1):173–183. [DOI] [PubMed] [Google Scholar]
- 1901.ElShebiny T, Venkat D, Strohl K, Hans MG, Alonso A, Palomo JM. Hyoid arch displacement with hypoglossal nerve stimulation. Am J Resp Crit Care 2017;196(6):790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1902.Elshebiny T, Venkat D, Strohl M, Strohl K, Ponsky D, Palomo JM. Airway evaluation in response to hypoglossal nerve stimulation: a case report. J Dent Sleep Med 2017;4(1):15–17. [Google Scholar]
- 1903.Goding GS, Tesfayesus W, Kezirian EJ. Hypoglossal nerve stimulation and airway changes under fluoroscopy. Otolaryngol Head Neck Surg 2012;146(6):1017–1022. [DOI] [PubMed] [Google Scholar]
- 1904.Oliven A, Tov N, Geitini L, et al. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J 2007;30(4):748–758. [DOI] [PubMed] [Google Scholar]
- 1905.Steffen A, Kilic A, Konig IR, Suurna MV, Hofauer B, Heiser C. Tongue motion variability with changes of upper airway stimulation electrode configuration and effects on treatment outcomes. Laryngoscope 2018;128(8):1970–1976. [DOI] [PubMed] [Google Scholar]
- 1906.Vanderveken OM, Beyers J, Op de Beeck S, et al. Development of a clinical pathway and technical aspects of upper airway stimulation therapy for obstructive sleep apnea. Front Neurosci 2017;11;523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1907.Kezirian EJ. Acclimation settings with identical or similar sensation and functional thresholds. Laryngoscope 2016;126:S20–S21. [DOI] [PubMed] [Google Scholar]
- 1908.Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017;13(9):1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1909.Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol 2017;274(3):1727–1734. [DOI] [PubMed] [Google Scholar]
- 1910.Heiser C Advanced titration to treat a floppy epiglottis in selective upper airway stimulation. Laryngoscope 2016;126:S22–S24. [DOI] [PubMed] [Google Scholar]
- 1911.Meleca JB, Kominsky AH. Reconfiguration of upper airway stimulation devices utilizing awake endoscopy. Laryngoscope 2020;130(10):2494–2498. [DOI] [PubMed] [Google Scholar]
- 1912.Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015;45(1):129–138. [DOI] [PubMed] [Google Scholar]
- 1913.Lin HC, Weaver EM, Lin HS, Friedman M. Multilevel obstructive sleep apnea surgery. Adv Otorhinolaryngol 2017;80:109–115. [DOI] [PubMed] [Google Scholar]
- 1914.Kezirian EJ. Nonresponders to pharyngeal surgery for obstructive sleep apnea: insights from drug-induced sleep endoscopy. Laryngoscope 2011;121(6):1320–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1915.Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope 2011;121(12):2710–2716. [DOI] [PubMed] [Google Scholar]
- 1916.Weaver EM. Sleep apnea devices and sleep apnea surgery should be compared on effectiveness, not efficacy. Chest 2003;123(3):961–962; author reply 962. [DOI] [PubMed] [Google Scholar]
- 1917.Hobson JC, Robinson S, Antic NA, et al. What is “success” following surgery for obstructive sleep apnea? The effect of different polysomnographic scoring systems. Laryngoscope 2012;122(8):1878–1881. [DOI] [PubMed] [Google Scholar]
- 1918.Jung SY, Rhee EH, Al-Dilaijan KF, Kim SW, Min JY. Impact of AASM 2012 recommended hypopnea criteria on surgical outcomes for obstructive sleep apnea. Laryngoscope 2020;130(3):825–831. [DOI] [PubMed] [Google Scholar]
- 1919.MacKay S, Carney AS, Catcheside PG, et al. Effect of multilevel upper airway surgery vs medical management on the apnea-hypopnea index and patient-reported daytime sleepiness among patients with moderate or severe obstructive sleep apnea: the SAMS randomized clinical trial. JAMA 2020;324(12):1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1920.Lin HC, Friedman M, Chang HW, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2008;118(5):902–908. [DOI] [PubMed] [Google Scholar]
- 1921.Mulholland GB, Jeffery CC, Ziai H, et al. Multilevel palate and tongue base surgical treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2019;129(7):1712–1721. [DOI] [PubMed] [Google Scholar]
- 1922.Andsberg U, Jessen M. Eight years of follow-up–uvulopalatopharyngoplasty combined with midline glossectomy as a treatment for obstructive sleep apnoea syndrome. Acta Otolaryngol Suppl 2000;543:175–178. [DOI] [PubMed] [Google Scholar]
- 1923.Hou J, Yan J, Wang B, et al. Treatment of obstructive sleep apnea-hypopnea syndrome with combined uvulopalatopharyngoplasty and midline glossectomy: outcomes from a 5-year study. Respir Care 2012;57(12):2104–2110. [DOI] [PubMed] [Google Scholar]
- 1924.Neruntarat C Genioglossus advancement and hyoid myotomy: short-term and long-term results. J Laryngol Otol 2003;117(6):482–486. [DOI] [PubMed] [Google Scholar]
- 1925.Neruntarat C, Chantapant S. Radiofrequency surgery for the treatment of obstructive sleep apnea: short-term and long-term results. Otolaryngol Head Neck Surg 2009;141(6):722–726. [DOI] [PubMed] [Google Scholar]
- 1926.Steward DL, Weaver EM, Woodson BT. Multilevel temperature-controlled radiofrequency for obstructive sleep apnea: extended follow-up. Otolaryngol Head Neck Surg 2005;132(4):630–635. [DOI] [PubMed] [Google Scholar]
- 1927.Yüksel A, Ugur KS, Kizilbulut G, et al. Long-term results of one staged multilevel surgery with tongue suspension surgery or one level palatal surgery for treatment of moderate and severe obstructive sleep apnea. Eur Arch Otorhinolaryngol 2016;273(5):1227–1234. [DOI] [PubMed] [Google Scholar]
- 1928.Baisch A, Maurer JT, Hormann K. The effect of hyoid suspension in a multilevel surgery concept for obstructive sleep apnea. Otolaryngol Head Neck Surg 2006;134(5):856–861. [DOI] [PubMed] [Google Scholar]
- 1929.Sun X, Yi H, Cao Z, Yin S. Reorganization of sleep architecture after surgery for OSAHS. Acta Otolaryngol 2008;128(11):1242–1247. [DOI] [PubMed] [Google Scholar]
- 1930.Yang MC, Hsu YB, Lan MY, Lan MC. The comparison of multilevel surgery (hyoid myotomy and suspension with uvulopalatopharyngoplasty) with CPAP in moderate to severe OSAS patients. Eur Arch Otorhinolaryngol 2020;277(8):2349–2355. [DOI] [PubMed] [Google Scholar]
- 1931.Yi HL, Sun XQ, Chen B, et al. Z-palatopharyngoplasty plus genioglossus advancement and hyoid suspension for obstructive sleep apnea hypopnea syndrome. Otolaryngol Head Neck Surg 2011;144(3):469–473. [DOI] [PubMed] [Google Scholar]
- 1932.Kezirian EJ, Malhotra A, Goldberg AN, White DP. Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery. Laryngoscope 2010;120(7):1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1933.Emara TA, Omara TA, Shouman WM. Modified genioglossus advancement and uvulopalatopharyngoplasty in patients with obstructive sleep apnea. Otolaryngol Head Neck Surg 2011;145(5):865–871. [DOI] [PubMed] [Google Scholar]
- 1934.Hsu PP, Brett RH. Multiple level pharyngeal surgery for obstructive sleep apnoea. Singapore Med J 2001;42(4):160–164. [PubMed] [Google Scholar]
- 1935.Miller FR, Watson D, Boseley M. The role of the Genial Bone Advancement Trephine system in conjunction with uvulopalatopharyngoplasty in the multilevel management of obstructive sleep apnea. Otolaryngol Head Neck Surg 2004;130(1):73–79. [DOI] [PubMed] [Google Scholar]
- 1936.Steward DL. Effectiveness of multilevel (tongue and palate) radiofrequency tissue ablation for patients with obstructive sleep apnea syndrome. Laryngoscope 2004;114(12):2073–2084. [DOI] [PubMed] [Google Scholar]
- 1937.Chiffer RC, Schwab RJ, Keenan BT, Borek RC, Thaler ER. Volumetric MRI analysis pre- and post-transoral robotic surgery for obstructive sleep apnea. Laryngoscope 2015;125(8):1988–1995. [DOI] [PubMed] [Google Scholar]
- 1938.Rohrer JW, Eller R, Santillan PG, Barrera JE. Geniotubercle advancement with a uvulopalatal flap and its effect on swallow function in obstructive sleep apnea. Laryngoscope 2015;125(3):758–761. [DOI] [PubMed] [Google Scholar]
- 1939.Brietzke SE, Ishman SL, Cohen S, Cyr DD, Shin JJ, Kezirian EJ. National database analysis of single-level versus multilevel sleep surgery. Otolaryngol Head Neck Surg 2017;156(5):955–961. [DOI] [PubMed] [Google Scholar]
- 1940.Chan CY, Han HJ, Lye WK, Toh ST. Complications and pain in obstructive sleep apnoea - comparing single and multilevel surgery. Ann Acad Med Singapore 2018;47(3):101–107. [PubMed] [Google Scholar]
- 1941.Kezirian EJ, Weaver EM, Yueh B, et al. Incidence of serious complications after uvulopalatopharyngoplasty. Laryngoscope 2004;114(3):450–453. [DOI] [PubMed] [Google Scholar]
- 1942.Baker AB, Xiao CC, O’Connell BP, Cline JM, Gillespie MB. Uvulopalatopharyngoplasty: does multilevel surgery increase risk? Otolaryngol Head Neck Surg 2016;155(6):1053–1058. [DOI] [PubMed] [Google Scholar]
- 1943.Li HY, Wang PC, Hsu CY, Lee SW, Chen NH, Liu SA. Combined nasal-palatopharyngeal surgery for obstructive sleep apnea: simultaneous or staged? Acta Otolaryngol 2005;125(3):298–303. [DOI] [PubMed] [Google Scholar]
- 1944.Busaba NY. Same-stage nasal and palatopharyngeal surgery for obstructive sleep apnea: is it safe? Otolaryngol Head Neck Surg 2002;126(4):399–403. [DOI] [PubMed] [Google Scholar]
- 1945.Pang KP. One-stage nasal and multi-level pharyngeal surgery for obstructive sleep apnoea: safety and efficacy. J Laryngol Otol 2005;119(4):272–276. [DOI] [PubMed] [Google Scholar]
- 1946.Kieff DA, Busaba NY. Same-day discharge for selected patients undergoing combined nasal and palatal surgery for obstructive sleep apnea. Ann Otol Rhinol Laryngol 2004;113(2):128–131. [DOI] [PubMed] [Google Scholar]
- 1947.Elasfour A, Miyazaki S, Itasaka Y, Yamakawa K, Ishikawa K, Togawa K. Evaluation of uvulopalatopharyngoplasty in treatment of obstructive sleep apnea syndrome. Acta Otolaryngol Suppl 1998;537:52–56. [DOI] [PubMed] [Google Scholar]
- 1948.Friedman M, Lin HC, Gurpinar B, Joseph NJ. Minimally invasive single-stage multilevel treatment for obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2007;117(10):1859–1863. [DOI] [PubMed] [Google Scholar]
- 1949.Eun YG, Kwon KH, Shin SY, Lee KH, Byun JY, Kim SW. Multilevel surgery in patients with rapid eye movement-related obstructive sleep apnea. Otolaryngol Head Neck Surg 2009;140(4):536–541. [DOI] [PubMed] [Google Scholar]
- 1950.van Maanen JP, Ravesloot MJ, Witte BI, Grijseels M, de Vries N. Exploration of the relationship between sleep position and isolated tongue base or multilevel surgery in obstructive sleep apnea. Eur Arch Otorhinolaryngol 2012;269(9):2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1951.Li S, Wu D, Shi H. Treatment of obstructive sleep apnea hypopnea syndrome caused by glossoptosis with tongue-base suspension. Eur Arch Otorhinolaryngol 2013;270(11):2915–2920. [DOI] [PubMed] [Google Scholar]
- 1952.Plzak J, Zabrodsky M, Kastner J, Betka J, Klozar J. Combined bipolar radiofrequency surgery of the tongue base and uvulopalatopharyngoplasty for obstructive sleep apnea. Arch Med Sci 2013;9(6):1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1953.van Maanen JP, Witte BI, de Vries N. Theoretical approach towards increasing effectiveness of palatal surgery in obstructive sleep apnea: role for concomitant positional therapy? Sleep Breath 2014;18(2):341–349. [DOI] [PubMed] [Google Scholar]
- 1954.Salapatas AM, Bonzelaar LB, Hwang MS, et al. Impact of minimally invasive multilevel surgery on mild/moderate OSA. Otolaryngol Head Neck Surg 2016;155(4):695–701. [DOI] [PubMed] [Google Scholar]
- 1955.Li HY, Lee LA, Kezirian EJ. Efficacy of coblation endoscopic lingual lightening in multilevel surgery for obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg 2016;142(5):438–443. [DOI] [PubMed] [Google Scholar]
- 1956.Missale F, Fragale M, Incandela F, et al. Outcome predictors for non-resective pharyngoplasty alone or as a part of multilevel surgery, in obstructive sleep apnea-hypopnea syndrome. Sleep Breath 2019;24(4):1397–1406. [DOI] [PubMed] [Google Scholar]
- 1957.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep 2007;30(4):461–467. [DOI] [PubMed] [Google Scholar]
- 1958.Janson C, Gislason T, Bengtsson H, et al. Long-term follow-up of patients with obstructive sleep apnea treated with uvulopalatopharyngoplasty. Arch Otolaryngol 1997;123(3):257–262. [DOI] [PubMed] [Google Scholar]
- 1959.Riley RW, Powell NB, Li KK, Troell RJ, Guilleminault C. Surgery and obstructive sleep apnea: long-term clinical outcomes. Otolaryngol Head Neck Surg 2000;122(3):415–421. [DOI] [PubMed] [Google Scholar]
- 1960.Pang KP, Pang EB, Pang KA, Rotenberg B. Anterior palatoplasty in the treatment of obstructive sleep apnoea – a systemic review. Acta Otorhinolaryngol. Ital 2018;38(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1961.Sharma S, Wormald JCR, Fishman JM, Andrews P, Kotecha BT. Rhinological interventions for obstructive sleep apnoea – a systematic review and descriptive meta-analysis. J Laryngol Otol 2019;133(3):168–176. [DOI] [PubMed] [Google Scholar]
- 1962.Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical Guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis 2008;4(5): S109–S184. [DOI] [PubMed] [Google Scholar]
- 1963.Wong AM, Barnes HN, Joosten SA, et al. The effect of surgical weight loss on obstructive sleep apnoea: a systematic review and meta-analysis. Sleep Med Rev 2018;42:85–99. [DOI] [PubMed] [Google Scholar]
- 1964.Lee HM, Kim HY, Suh JD, et al. Uvulopalatopharyngoplasty reduces the incidence of cardiovascular complications caused by obstructive sleep apnea: results from the national insurance service survey 2007–2014. Sleep Med 2018;45:11–16. [DOI] [PubMed] [Google Scholar]
- 1965.Zhan X, Li L, Wu C, et al. Effect of uvulopalatopharyngoplasty (UPPP) on atherosclerosis and cardiac functioning in obstructive sleep apnea patients. Acta Otolaryngol 2019;139(9):793–797. [DOI] [PubMed] [Google Scholar]
- 1966.Pang KP, Plaza G, Baptista JP, et al. Palate surgery for obstructive sleep apnea: a 17-year meta-analysis. Eur Arch Otorhinolaryngol 2018;275(7):1697–1707. [DOI] [PubMed] [Google Scholar]
- 1967.Kuhlo W, Doll E, Franck MC. Successful management of Pickwickian syndrome using long-term tracheostomy. Dtsch Med Wochenschr 1969;94(24):1286–1290. [DOI] [PubMed] [Google Scholar]
- 1968.Guilleminault C, Simmons FB, Motta J, et al. Obstructive sleep-apnea syndrome and tracheostomy - long-term follow-up experience. Arch Intern Med 1981;141(8):985–989. [PubMed] [Google Scholar]
- 1969.Simmons FB, Guilleminault C, Dement WC, Tilkian AG, Hill M. Surgical management of airway obstructions during sleep. Laryngoscope 1977;87(3):326–338. [DOI] [PubMed] [Google Scholar]
- 1970.Aulakh PK, Westerman DE, Dedhia RC. The longest obstructive apnea you have ever seen: a patient with new-onset autonomic dysfunction. J Clin Sleep Med 2018;14(5):893–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1971.Haapaniemi JJ, Laurikainen EA, Halme P, Antila J. Long-term results of tracheostomy for severe obstructive sleep apnea syndrome. ORL J Otorhinolaryngol Relat Spec 2001;63(3):131–136. [DOI] [PubMed] [Google Scholar]
- 1972.Kim SH, Eisele DW, Smith PL, Schneider H, Schwartz AR. Evaluation of patients with sleep apnea after tracheotomy. Arch Otolaryngol 1998;124(9):996–1000. [DOI] [PubMed] [Google Scholar]
- 1973.Motta J, Guilleminault C, Schroeder JS, Dement WC. Tracheostomy and hemodynamic changes in sleep-induced apnea. Ann Intern Med 1978;89(4):454–458. [DOI] [PubMed] [Google Scholar]
- 1974.Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003;113(2):201–204. [DOI] [PubMed] [Google Scholar]
- 1975.Tilkian AG, Guilleminault C, Schroeder JS, Lehrman KL, Simmons FB, Dement WC. Sleep-induced apnea syndrome – prevalence of cardiac-arrhythmias and their reversal after tracheostomy. Am J Med 1977;63(3):348–358. [DOI] [PubMed] [Google Scholar]
- 1976.Weitzman ED, Kahn E, Pollak CP. Quantitative-analysis of sleep and sleep-apnea before and after tracheostomy in patients with the hypersomnia-sleep apnea syndrome. Sleep 1980;3(3–4):407–423. [DOI] [PubMed] [Google Scholar]
- 1977.Weitzman ED, Pollack CP, Borowiecki B. Hypersomnia-sleep apnea due to micrognathia – reversal by tracheoplasty. Arch Neurol 1978;35(6):392–395. [DOI] [PubMed] [Google Scholar]
- 1978.Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstructive sleep-apnea syndrome patients – mortality. Chest 1988;94(6):1200–1204. [DOI] [PubMed] [Google Scholar]
- 1979.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep-apnea – experience in 385 male-patients. Chest 1988;94(1):9–14. [PubMed] [Google Scholar]
- 1980.Camacho M, Certal V, Brietzke SE, Holty JE, Guilleminault C, Capasso R. Tracheostomy as treatment for adult obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope 2014;124(3):803–811. [DOI] [PubMed] [Google Scholar]
- 1981.Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse-effects of tracheostomy for sleep-apnea. JAMA 1981;246(4):347–350. [PubMed] [Google Scholar]
- 1982.Organization WH. Obesity and Overweight. Published 2020. Updated 03/03/2020 2020. Accessed March 3, 2020. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- 1983.Lettieri CJ, Eliasson AH, Andrada T, Khramtsov A, Raphaelson M, Kristo DA. Obstructive sleep apnea syndrome: are we missing an at-risk population? J Clin Sleep Med 2005;1(4):381–385. [PubMed] [Google Scholar]
- 1984.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care 2008;31(2):S303–S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1985.Rajala R, Partinen M, Sane T, Pelkonen R, Huikuri K, Seppalainen AM. Obstructive sleep apnoea syndrome in morbidly obese patients. J Intern Med 1991;230(2):125–129. [DOI] [PubMed] [Google Scholar]
- 1986.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13(1):58–61. [DOI] [PubMed] [Google Scholar]
- 1987.Bakker JP, Tavakkoli A, Rueschman M, et al. Gastric banding surgery versus continuous positive airway pressure for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2018;197(8):1080–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1988.Feigel-Guiller B, Drui D, Dimet J, et al. Laparoscopic gastric banding in obese patients with sleep apnea: a 3-year controlled study and follow-up after 10 years. Obes Surg 2015;25(10):1886–1892. [DOI] [PubMed] [Google Scholar]
- 1989.Quintas-Neves M, Preto J, Drummond M. Assessment of bariatric surgery efficacy on obstructive sleep apnea (OSA). Rev Port Pneumol (2006) 2016;22(6):331–336. [DOI] [PubMed] [Google Scholar]
- 1990.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013;23(3):414–423. [DOI] [PubMed] [Google Scholar]
- 1991.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery 2007;141(3):354–358. [DOI] [PubMed] [Google Scholar]
- 1992.Zhang YX, Wang WY, Yang CC, Shen JH, Shi ML, Wang B. Improvement in nocturnal hypoxemia in obese patients with obstructive sleep apnea after bariatric surgery: a meta-analysis. Obes Surg 2019;29(2):601–608. [DOI] [PubMed] [Google Scholar]
- 1993.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292(14):1724–1737. [DOI] [PubMed] [Google Scholar]
- 1994.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med 2009;122(6):535–542. [DOI] [PubMed] [Google Scholar]
- 1995.Hariri K, Kini SU, Herron DM, Fernandez-Ranvier G. Resolution of symptomatic obstructive sleep apnea not impacted by preoperative body mass index, choice of operation between sleeve gastrectomy and Roux-en-Y gastric bypass surgery, or severity. Obes Surg 2018;28(5):1402–1407. [DOI] [PubMed] [Google Scholar]
- 1996.Miras AD, Kamocka A, Patel D, et al. Obesity surgery makes patients healthier and more functional: real world results from the United Kingdom national bariatric surgery registry. Surg Obes Relat Dis 2018;14(7):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1997.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA 2020;323(14):1389–1400. [DOI] [PubMed] [Google Scholar]
- 1998.Keenan BT, Kim J, Singh B, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep 2018;41(3):zsx214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1999.Tam S, Woodson BT, Rotenberg B. Outcome measurements in obstructive sleep apnea: beyond the apnea-hypopnea index. Laryngoscope 2014;124(1):337–343. [DOI] [PubMed] [Google Scholar]
- 2000.Weaver EM, Kapur V, Yueh B. Polysomnography vs selfreported measures in patients with sleep apnea. Arch Otolaryngol Head Neck Surg 2004;130(4):453–458. [DOI] [PubMed] [Google Scholar]
- 2001.Sommer JU, Heiser C, Gahleitner C, et al. Tonsillectomy with uvulopalatopharyngoplasty in obstructive sleep apnea a two-center randomized controlled trial. Dtsch Arztebl Int 2016;113(1–2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2002.Justin GA, Chang ET, Camacho M, Brietzke SE. Transoral robotic surgery for obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2016;154(5):835–846. [DOI] [PubMed] [Google Scholar]
- 2003.Meccariello G, Cammaroto G, Montevecchi F, et al. Transoral robotic surgery for the management of obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2017;274(2):647–653. [DOI] [PubMed] [Google Scholar]
- 2004.Murphey AW, Kandl JA, Nguyen SA, Weber AC, Gillespie MB. The effect of glossectomy for obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2015;153(3):334–342. [DOI] [PubMed] [Google Scholar]
- 2005.Boyd SB, Chigurupati R, Cillo JE Jr, et al. Maxillomandibular advancement improves multiple health-related and functional outcomes in patients with obstructive sleep apnea: a multicenter study. J Oral Maxillofac Surg 2019;77(2):352–370. [DOI] [PubMed] [Google Scholar]
- 2006.Tsui WK, Yang Y, McGrath C, Leung YY. Improvement in quality of life after skeletal advancement surgery in patients with moderate-to-severe obstructive sleep apnoea: a longitudinal study. Int J Oral Maxillofac Surg 2020;49(3):333–341. [DOI] [PubMed] [Google Scholar]
- 2007.Zaghi S, Holty JEC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016;142(1):58–66. [DOI] [PubMed] [Google Scholar]
- 2008.Wang M, Liu SY, Zhou B, Li Y, Cui S, Huang Q. Effect of nasal and sinus surgery in patients with and without obstructive sleep apnea. Acta Otolaryngol 2019;139(5):467–472. [DOI] [PubMed] [Google Scholar]
- 2009.Farrar J, Ryan J, Oliver E, Gillespie MB. Radiofrequency ablation for the treatment of obstructive sleep apnea: a meta-analysis. Laryngoscope 2008;118(10):1878–1883. [DOI] [PubMed] [Google Scholar]
- 2010.Gao YN, Wu YC, Lin SY, Chang JZ, Tu YK. Short-term efficacy of minimally invasive treatments for adult obstructive sleep apnea: a systematic review and network meta-analysis of randomized controlled trials. J Formos Med Assoc 2019;118(4):750–765. [DOI] [PubMed] [Google Scholar]
- 2011.Llewellyn CM, Noller MW, Camacho M. Cautery-assisted palatal stiffening operation for obstructive sleep apnea: a systematic review and meta-analysis. World J Otorhinolaryngol Head Neck Surg 2019;5(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2012.Veer V, Yang WY, Green R, Kotecha B. Long-term safety and efficacy of radiofrequency ablation in the treatment of sleep disordered breathing: a meta-analysis. Eur Arch Otorhinolaryngol 2014;271(11):2863–2870. [DOI] [PubMed] [Google Scholar]
- 2013.Atef A, Mosleh M, Hesham M, Fathi A, Hassan M, Fawzy M. Radiofrequency vs laser in the management of mild to moderate obstructive sleep apnoea: does the number of treatment sessions matter? J Laryngol Otol 2005;119(11):888–893. [DOI] [PubMed] [Google Scholar]
- 2014.Ishman SL, Benke JR, Cohen AP, Stephen MJ, Ishii LE, Gourin CG. Does surgery for obstructive sleep apnea improve depression and sleepiness? Laryngoscope 2014;124(12):2829–2836. [DOI] [PubMed] [Google Scholar]
- 2015.Steward DL, Weaver EM, Woodson BT. A comparison of radiofrequency treatment schemes for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2004;130(5):579–585. [DOI] [PubMed] [Google Scholar]
- 2016.Walker-Engstrom ML, Wilhelmsson B, Tegelberg A, Dimenas E, Ringqvist I. Quality of life assessment of treatment with dental appliance or UPPP in patients with mild to moderate obstructive sleep apnoea. A prospective randomized 1-year follow-up study. J Sleep Res 2000;9(3):303–308. [DOI] [PubMed] [Google Scholar]
- 2017.Franklin KA, Anttila H, Axelsson S, et al. Effects and sideeffects of surgery for snoring and obstructive sleep apnea – a systematic review. Sleep 2009;32(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 2018.Conradt R, Hochban W, Heitmann J, et al. Sleep fragmentation and daytime vigilance in patients with OSA treated by surgical maxillomandibular advancement compared to CPAP therapy. J Sleep Res 1998;7(3):217–223. [DOI] [PubMed] [Google Scholar]
- 2019.Santamaria JD, Prior JC, Fleetham JA. Reversible reproductive dysfunction in men with obstructive sleep apnoea. Clin Endocrinol (Oxf) 1988;28(5):461–470. [DOI] [PubMed] [Google Scholar]
- 2020.Shin HW, Park JH, Park JW, et al. Effects of surgical vs. nonsurgical therapy on erectile dysfunction and quality of life in obstructive sleep apnea syndrome: a pilot study. J Sex Med 2013;10(8):2053–2059. [DOI] [PubMed] [Google Scholar]
- 2021.Dahlof P, Ejnell H, Hallstrom T, Hedner J. Surgical treatment of the sleep apnea syndrome reduces associated major depression. Int J Behav Med 2000;7(1):73–88. [Google Scholar]
- 2022.Klonoff H, Fleetham J, Taylor DR, Clark C. Treatment outcome of obstructive sleep apnea. Physiological and neuropsychological concomitants. J Nerv Ment Dis 1987;175(4):208–212. [DOI] [PubMed] [Google Scholar]
- 2023.Li HY, Huang YS, Chen NH, Fang TJ, Liu CY, Wang PC. Mood improvement after surgery for obstructive sleep apnea. Laryngoscope 2004;114(6):1098–1102. [DOI] [PubMed] [Google Scholar]
- 2024.Xiao Y, Han D, Zang H, Wang D. The effectiveness of nasal surgery on psychological symptoms in patients with obstructive sleep apnea and nasal obstruction. Acta Otolaryngol 2016;136(6):626–632. [DOI] [PubMed] [Google Scholar]
- 2025.Malhotra A, Ayappa I, Ayas N, et al. Metrics of sleep apnea severity: beyond the apnea-hypopnea index. Sleep 2021;44(7):zsab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2026.Kezirian EJ, Weaver EM, Criswell MA, de Vries N, Woodson BT, Piccirillo JF. Reporting results of obstructive sleep apnea syndrome surgery trials. Otolaryngol Head Neck Surg 2011;144(4):496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2027.Binar M, Karakoc O. Anterior palatoplasty for obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2018;158(3):443–449. [DOI] [PubMed] [Google Scholar]
- 2028.Pirklbauer K, Russmueller G, Stiebellehner L, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea syndrome: a systematic review. J Oral Maxillofac Surg 2011;69(6):e165–e176. [DOI] [PubMed] [Google Scholar]
- 2029.Fairburn SC, Waite PD, Vilos G, et al. Three-dimensional changes in upper airways of patients with obstructive sleep apnea following maxillomandibular advancement. J Oral Maxillofac Surg 2007;65(1):6–12. [DOI] [PubMed] [Google Scholar]
- 2030.Noller MW, Guilleminault C, Gouveia CJ, et al. Mandibular advancement for adult obstructive sleep apnea: a systematic review and meta-analysis. J Craniomaxillofac Surg 2017;45(12):2035–2040. [DOI] [PubMed] [Google Scholar]
- 2031.Tsui WK, Yang Y, Cheung LK, Leung YY. Distraction osteogenesis as a treatment of obstructive sleep apnea syndrome: a systematic review. Medicine (Baltimore) 2016;95(36):e4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2032.Minni A, Cialente F, Ralli M, et al. Uvulopalatopharyngoplasty and barbed reposition pharyngoplasty with and without hyoid suspension for obstructive sleep apnea hypopnea syndrome: a comparison of long-term functional results. Bosn J Basic Med Sci 2020;21(3):364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2033.Camacho M, Nesbitt NB, Lambert E, et al. Laser-assisted uvulopalatoplasty for obstructive sleep apnea: a systematic review and meta-analysis. Sleep 2017;40(3). [DOI] [PubMed] [Google Scholar]
- 2034.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J 2002;20(6):1511–1518. [DOI] [PubMed] [Google Scholar]
- 2035.Lysdahl M, Haraldsson PO. Long-term survival after uvulopalatopharyngoplasty in nonobese heavy snorers: a 5- to 9-year follow-up of 400 consecutive patients. Arch Otolaryngol Head Neck Surg 2000;126(9):1136–1140. [DOI] [PubMed] [Google Scholar]
- 2036.Keenan SP, Burt H, Ryan CF, Fleetham JA. Long-term survival of patients with obstructive sleep apnea treated by uvulopalatopharyngoplasty or nasal CPAP. Chest 1994;105(1):155–159. [DOI] [PubMed] [Google Scholar]
- 2037.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128(1):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2038.Chin CH, Lin PW, Lin HC, Friedman M, Lin MC. Effects of OSA surgery on leptin and metabolic profiles. Otolaryngol Head Neck Surg 2019;161(6):1048–1055. [DOI] [PubMed] [Google Scholar]
- 2039.Fehrm J, Friberg D, Bring J, Browaldh N. Blood pressure after modified uvulopalatopharyngoplasty: results from the SKUP(3) randomized controlled trial. Sleep Med 2017;34:156–161. [DOI] [PubMed] [Google Scholar]
- 2040.Islam S, Taylor CJ, Ormiston IW. Effects of maxillomandibular advancement on systemic blood pressure in patients with obstructive sleep apnoea. Br J Oral Maxillofac Surg 2015;53(1):34–38. [DOI] [PubMed] [Google Scholar]
- 2041.Boyd SB, Walters AS, Waite P, Harding SM, Song Y. Long-term effectiveness and safety of maxillomandibular advancement for treatment of obstructive sleep apnea. J Clin Sleep Med 2015;11(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2042.Lee LA, Huang CG, Chen NH, Wang CL, Fang TJ, Li HY. Severity of obstructive sleep apnea syndrome and highsensitivity C-reactive protein reduced after relocation pharyngoplasty. Otolaryngol Head Neck Surg 2011;144(4):632–638. [DOI] [PubMed] [Google Scholar]
- 2043.Choi JH, Yi JS, Lee SH, et al. Effect of upper airway surgery on heart rate variability in patients with obstructive sleep apnoea syndrome. J Sleep Res 2012;21(3):316–321. [DOI] [PubMed] [Google Scholar]
- 2044.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014;7(10):1025–1038. [DOI] [PubMed] [Google Scholar]
- 2045.Peng Y, Zhang L, Hu D, et al. Reduction of internal carotid artery intima-media thickness in patients with moderate- to-severe obstructive sleep apnea syndrome after nasal surgery and uvulopalatopharyngoplasty. Acta Otolaryngol 2016;136(5):514–521. [DOI] [PubMed] [Google Scholar]
- 2046.Lin CC, Wang YP, Lee KS, Liaw SF, Chiu CH. Effect of uvulopalatopharyngoplasty on leptin and endothelial function in sleep apnea. Ann Otol Rhinol Laryngol 2014;123(1):40–46. [DOI] [PubMed] [Google Scholar]
- 2047.Kinoshita H, Shibano A, Sakoda T, et al. Uvulopalatopharyngoplasty decreases levels of C-reactive protein in patients with obstructive sleep apnea syndrome. Am Heart J 2006;152(4):692.e691–695. [DOI] [PubMed] [Google Scholar]
- 2048.Zohar Y, Talmi YP, Frenkel H, et al. Cardiac function in obstructive sleep apnea patients following uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1992;107(3):390–394. [DOI] [PubMed] [Google Scholar]
- 2049.Partinen M, Guilleminault C. Daytime sleepiness and vascular morbidity at seven-year follow-up in obstructive sleep apnea patients. Chest 1990;97(1):27–32. [DOI] [PubMed] [Google Scholar]
- 2050.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleepdisordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol 2017;74(10):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2051.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep 2013;36(9):1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2052.Rojo-Sanchis C, Almerich-Silla JM, Paredes-Gallardo V, Montiel-Company JM, Bellot-Arcis C. Impact of bimaxillary advancement surgery on the upper airway and on obstructive sleep apnea syndrome: a meta-analysis. Sci Rep 2018;8(1):5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2053.Chinnadurai S, Jordan AK, Sathe NA, Fonnesbeck C, McPheeters ML, Francis DO. Tonsillectomy for obstructive sleep-disordered breathing: a meta-analysis. Pediatrics 2017;139(2):e20163491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2054.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2055.Dahlof P, Norlin-Bagge E, Hedner J, Ejnell H, Hetta J, Hallstrom T. Improvement in neuropsychological performance following surgical treatment for obstructive sleep apnea syndrome. Acta Otolaryngol 2002;122(1):86–91. [DOI] [PubMed] [Google Scholar]
- 2056.Lin WC, Huang CC, Chen HL, et al. Longitudinal brain structural alterations and systemic inflammation in obstructive sleep apnea before and after surgical treatment. J Transl Med 2016;14(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2057.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 2011;183(10):1419–1426. [DOI] [PubMed] [Google Scholar]
- 2058.Lojander J, Kajaste S, Maasilta P, Partinen M. Cognitive function and treatment of obstructive sleep apnea syndrome. J Sleep Res 1999;8(1):71–76. [DOI] [PubMed] [Google Scholar]
- 2059.Decary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep 2000;23(3):369–381. [PubMed] [Google Scholar]
- 2060.Pang KP, Pang EB, Pang KA, Vicini C, Chan YH, Rotenberg BW. Upper airway surgery for obstructive sleep apnea reduces blood pressure. Laryngoscope 2018;128(2):523–527. [DOI] [PubMed] [Google Scholar]
- 2061.Li L, Zhan X, Wang N, et al. Does airway surgery lower serum lipid levels in obstructive sleep apnea patients? A retrospective case review. Med Sci Monit 2014;20:2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2062.Qian Y, Zou J, Xu H, et al. Association of upper airway surgery and improved cardiovascular biomarkers and risk in OSA. Laryngoscope 2020;130(3):818–824. [DOI] [PubMed] [Google Scholar]
- 2063.Gunbey E, Karabulut I, Karabulut H, Zaim M. Impact of multilevel surgical treatment on mean platelet volume in patients with obstructive sleep apnea syndrome. J Craniofac Surg 2015;26(4):1287–1289. [DOI] [PubMed] [Google Scholar]
- 2064.Simsek G, Haytoglu S, Muluk NB, Arikan OK, Cortuk M, Kiraz K. Mean platelet volume decreases in adult patients with obstructive sleep apnea after uvulopalatal flap surgery. J Craniofac Surg 2015;26(7):2152–2154. [DOI] [PubMed] [Google Scholar]
- 2065.Friedman M, Bliznikas D, Vidyasagar R, Woodson BT, Joseph NJ. Reduction of C-reactive protein with surgical treatment of obstructive sleep apnea hypopnea syndrome. Otolaryngol Head Neck Surg 2006;135(6):900–905. [DOI] [PubMed] [Google Scholar]
- 2066.Constantinidis J, Ereliadis S, Angouridakis N, Konstantinidis I, Vital V, Angouridaki C. Cytokine changes after surgical treatment of obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol 2008;265(10):1275–1279. [DOI] [PubMed] [Google Scholar]
- 2067.Eun YG, Kim MG, Kwon KH, Shin SY, Cho JS, Kim SW. Short-term effect of multilevel surgery on adipokines and pro-inflammatory cytokines in patients with obstructive sleep apnea. Acta Otolaryngol 2010;130(12):1394–1398. [DOI] [PubMed] [Google Scholar]
- 2068.Mutlu M, Vuralkan E, Akin I, et al. Alteration of serum levels of inflammatory cytokines and polysomnographic indices after uvulopalatal flap surgery in obstructive sleep apnea. Ear Nose Throat J 2017;96(2):65–68. [DOI] [PubMed] [Google Scholar]
- 2069.Vuralkan E, Mutlu M, Firat IH, et al. Changes in serum levels of MDA and MMP-9 after UPF in patients with OSAS. Eur Arch Otorhinolaryngol 2014;271(5):1329–1334. [DOI] [PubMed] [Google Scholar]
- 2070.Peng YK, Zhang LC, Hu DF, et al. Reduction of internal carotid artery intima-media thickness in patients with moderate-to-severe obstructive sleep apnea syndrome after nasal surgery and uvulopalatopharyngoplasty. Acta Otolaryngol 2016;136(5):514–521. [DOI] [PubMed] [Google Scholar]
- 2071.Lee MY, Lin CC, Lee KS, et al. Effect of uvulopalatopharyngoplasty on endothelial function in obstructive sleep apnea. Otolaryngol Head Neck Surg 2009;140(3):369–374. [DOI] [PubMed] [Google Scholar]
- 2072.Yang HB, Wang Y, Dong MM. Effect of Hanuvulopalatopharyngoplasty on flow-mediated dilatation in patients with moderate or severe obstructive sleep apnea syndrome. Acta Otolaryngol 2012;132(7):769–772. [DOI] [PubMed] [Google Scholar]
- 2073.Partinen M, Guilleminault C. Daytime sleepiness and vascular morbidity at 7-year follow-up in obstructive sleep-apnea patients. Chest 1990;97(1):27–32. [DOI] [PubMed] [Google Scholar]
- 2074.Chen SY, Cherng YG, Lee FP, et al. Risk of cerebrovascular diseases after uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a nationwide cohort study. Medicine (Baltimore) 2015;94(41):e1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2075.Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR trial. Sleep 2015;38(5):735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2076.Pietzsch JB, Richter AK, Randerath W, et al. Clinical and economic benefits of upper airway stimulation for obstructive sleep apnea in a European setting. Respiration 2019;98(1):38–47. [DOI] [PubMed] [Google Scholar]
- 2077.Ulfberg J, Carter N, Talback M, Edling C. Excessive daytime sleepiness at work and subjective work performance in the general population and among heavy snorers and patients with obstructive sleep apnea. Chest 1996;110(3):659–663. [DOI] [PubMed] [Google Scholar]
- 2078.Leger D, Stepnowsky C. The economic and societal burden of excessive daytime sleepiness in patients with obstructive sleep apnea. Sleep Med Rev 2020;51:101275. [DOI] [PubMed] [Google Scholar]
- 2079.Zhang XQ, Liu LJ, Li XY, et al. Effect of minimally invasive surgery on the sleep quality and work ability of patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath 2020;25(2):829–834. [DOI] [PubMed] [Google Scholar]
- 2080.Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg 2011;145(1):S1–S15. [DOI] [PubMed] [Google Scholar]
- 2081.Teo DT, Mitchell RB. Systematic review of effects of adenotonsillectomy on cardiovascular parameters in children with obstructive sleep apnea. Otolaryngol Head Neck Surg 2013;148(1):21–28. [DOI] [PubMed] [Google Scholar]
- 2082.O’Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Sleep 2005;28(6):747–752. [DOI] [PubMed] [Google Scholar]
- 2083.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006;117(4):e769–e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2084.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics 2004;114(1):44–49. [DOI] [PubMed] [Google Scholar]
- 2085.Suratt PM, Barth JT, Diamond R, et al. Reduced time in bed and obstructive sleep-disordered breathing in children are associated with cognitive impairment. Pediatrics 2007;119(2):320–329. [DOI] [PubMed] [Google Scholar]
- 2086.Mitchell RB, Kelly J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol 2006;70(3):395–406. [DOI] [PubMed] [Google Scholar]
- 2087.Gozal D, Wang M, Pope DW Jr. Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics 2001;108(3):693–697. [DOI] [PubMed] [Google Scholar]
- 2088.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130(3):576–584. [DOI] [PubMed] [Google Scholar]
- 2089.Chervin RD, Archbold KH, Dillon JE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics 2002;109(3):449–456. [DOI] [PubMed] [Google Scholar]
- 2090.Galland BC, Dawes PJ, Tripp EG, Taylor BJ. Changes in behavior and attentional capacity after adenotonsillectomy. Pediatr Res 2006;59(5):711–716. [DOI] [PubMed] [Google Scholar]
- 2091.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep 2005;28(7):885–890. [DOI] [PubMed] [Google Scholar]
- 2092.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep 2001;24(3):313–320. [DOI] [PubMed] [Google Scholar]
- 2093.Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg 2004;131(6):827–832. [DOI] [PubMed] [Google Scholar]
- 2094.Burghard M, Brozek-Madry E, Krzeski A. Sleep disordered breathing in children - diagnostic questionnaires, comparative analysis. Int J Pediatr Otorhinolaryngol 2019;120:108–111. [DOI] [PubMed] [Google Scholar]
- 2095.Sen T, Spruyt K. Pediatric sleep tools: an updated literature review. Front Psychiatry 2020;11:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2096.Michelet D, Julien-Marsollier F, Vacher T, et al. Accuracy of the sleep-related breathing disorder scale to diagnose obstructive sleep apnea in children: a meta-analysis. Sleep Med 2019;54:78–85. [DOI] [PubMed] [Google Scholar]
- 2097.Patel AP, Meghji S, Phillips JS. Accuracy of clinical scoring tools for the diagnosis of pediatric obstructive sleep apnea. Laryngoscope 2020;130(4):1034–1043. [DOI] [PubMed] [Google Scholar]
- 2098.Rosen CL, Wang R, Taylor HG, et al. Utility of symptoms to predict treatment outcomes in obstructive sleep apnea syndrome. Pediatrics 2015;135(3):e662–e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2099.Howard NS, Brietzke SE. Pediatric tonsil size: objective vs subjective measurements correlated to overnight polysomnogram. Otolaryngol Head Neck Surg 2009;140(5):675–681. [DOI] [PubMed] [Google Scholar]
- 2100.Hwang SH, Guilleminault C, Park CS, Kim TW, Hong SC. Usefulness of adenotonsillar size for prediction of severity of obstructive sleep apnea and flow limitation. Otolaryngol Head Neck Surg 2013;149(2):326–334. [DOI] [PubMed] [Google Scholar]
- 2101.Nolan J, Brietzke SE. Systematic review of pediatric tonsil size and polysomnogram-measured obstructive sleep apnea severity. Otolaryngol Head Neck Surg 2011;144(6):844–850. [DOI] [PubMed] [Google Scholar]
- 2102.Tang A, Benke JR, Cohen AP, Ishman SL. Influence of tonsillar size on osa improvement in children undergoing adenotonsillectomy. Otolaryngol Head Neck Surg 2015;153(2):281–285. [DOI] [PubMed] [Google Scholar]
- 2103.Biederman J, Faraone SV, Monuteaux MC, Plunkett EA, Gifford J, Spencer T. Growth deficits and attentiondeficit/hyperactivity disorder revisited: impact of gender, development, and treatment. Pediatrics 2003;111(5 Pt 1):1010–1016. [DOI] [PubMed] [Google Scholar]
- 2104.Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update). Otolaryngol Head Neck Surg 2019;160:S1–S42. [DOI] [PubMed] [Google Scholar]
- 2105.Friedman NR, Parikh SR, Ishman SL, et al. The current state of pediatric drug-induced sleep endoscopy. Laryngoscope 2017;127(1):266–272. [DOI] [PubMed] [Google Scholar]
- 2106.Boudewyns A, Verhulst S, Maris M, Saldien V, Van de Heyning P. Drug-induced sedation endoscopy in pediatric obstructive sleep apnea syndrome. Sleep Med 2014;15(12):1526–1531. [DOI] [PubMed] [Google Scholar]
- 2107.Kirkham E, Ma CC, Filipek N, et al. Polysomnography outcomes of sleep endoscopy-directed intervention in surgically naive children at risk for persistent obstructive sleep apnea. Sleep Breath 2020;24(3):1143–1150. [DOI] [PubMed] [Google Scholar]
- 2108.Durr ML, Meyer AK, Kezirian EJ, Rosbe KW. Drug-induced sleep endoscopy in persistent pediatric sleep-disordered breathing after adenotonsillectomy. Arch Otolaryngol Head Neck Surg 2012;138(7):638–643. [DOI] [PubMed] [Google Scholar]
- 2109.Baldassari CM, Lam DJ, Ishman SL, et al. Expert consensus statement: pediatric drug-induced sleep endoscopy. Otolaryngol Head Neck Surg 2021;165(4):578–591. [DOI] [PubMed] [Google Scholar]
- 2110.Amos JM, Durr ML, Nardone HC, Baldassari CM, Duggins A, Ishman SL. Systematic review of drug-induced sleep endoscopy scoring systems. Otolaryngol Head Neck Surg 2018;158(2):240–248. [DOI] [PubMed] [Google Scholar]
- 2111.Wilcox LJ, Bergeron M, Reghunathan S, Ishman SL. An updated review of pediatric drug-induced sleep endoscopy. Laryngoscope Investig Otolaryngol 2017;2(6):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2112.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007;133(3):216–222. [DOI] [PubMed] [Google Scholar]
- 2113.Franco RA Jr, Rosenfeld RM, Rao M. First place–resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;123(1 pt 1):9–16. [DOI] [PubMed] [Google Scholar]
- 2114.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep 2011;34(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2115.Venekamp RP, Hearne BJ, Chandrasekharan D, Blackshaw H, Lim J, Schilder AGM. Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleepdisordered breathing in children. Cochrane Database Syst Rev 2015;2015(10):CD011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2116.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children a multicenter retrospective study. Am J Resp Crit Care 2010;182(5):676–683. [DOI] [PubMed] [Google Scholar]
- 2117.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg 2007;137(1):43–48. [DOI] [PubMed] [Google Scholar]
- 2118.Todd CA, Bareiss AK, McCoul ED, Rodriguez KH. Adenotonsillectomy for obstructive sleep apnea and quality of life: systematic review and meta-analysis. Otolaryngol Head Neck Surg 2017;157(5):767–773. [DOI] [PubMed] [Google Scholar]
- 2119.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Outcome of adenotonsillectomy in children with down syndrome and obstructive sleep apnoea. Arch Dis Child 2017;102(4):331. [DOI] [PubMed] [Google Scholar]
- 2120.Trosman SJ, Eleff DJ, Krishna J, Anne S. Polysomnography results in pediatric patients with mild obstructive sleep apnea: adenotonsillectomy vs. watchful waiting. Int J Pediatr Otorhinolaryngol 2016;83(4):25–30. [DOI] [PubMed] [Google Scholar]
- 2121.Volsky PG, Woughter MA, Beydoun HA, Derkay CS, Baldassari CM. Adenotonsillectomy vs observation for management of mild obstructive sleep apnea in children. Otolaryngol Head Neck Surg 2014;150(1):126–132. [DOI] [PubMed] [Google Scholar]
- 2122.Lewis TL, Johnson RF, Choi J, Mitchell RB. Weight gain after adenotonsillectomy: a case control study. Otolaryngol Head Neck Surg 2015;152(4):734–739. [DOI] [PubMed] [Google Scholar]
- 2123.Kheirandish-Gozal L, Bhattacharjee R, Bandla HPR, Gozal D. Antiinflammatory therapy outcomes for mild OSA in children. Chest 2014;146(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2124.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 2008;122(1):E149–E155. [DOI] [PubMed] [Google Scholar]
- 2125.Liming BJ, Ryan M, Mack D, Ahmad I, Camacho M. Montelukast and nasal corticosteroids to treat pediatric obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2019;160(4):594–602. [DOI] [PubMed] [Google Scholar]
- 2126.Liming BJ, Ryan M, Mack D, Ahmad I, Camacho M. Montelukast and nasal corticosteroids to treat pediatric obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2019;160(4):594–602. [DOI] [PubMed] [Google Scholar]
- 2127.FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair) Food & Drug Administration. Updated March 13, 2020. Accessed July 11, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxedwarning-about-serious-mental-health-side-effects-asthmaand-allergy-drug [Google Scholar]
- 2128.Arganbright JM, Lee JC, Weatherly RA. Pediatric druginduced sleep endoscopy: an updated review of the literature. World J Otorhinolaryngol Head Neck Surg 2021;7(3):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2129.Caloway CL, Diercks GR, Keamy D, et al. Update on hypoglossal nerve stimulation in children with down syndrome and obstructive sleep apnea. Laryngoscope 2020;130(4):E263–E267. [DOI] [PubMed] [Google Scholar]
- 2130.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22(5):667–689. [PubMed] [Google Scholar]
- 2131.Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest 1999;115(3):863–866. [DOI] [PubMed] [Google Scholar]
- 2132.Asghari A, Mohammadi F, Kamrava SK, Jalessi M, Farhadi M. Evaluation of quality of life in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol 2013;270(3):1131–1136. [DOI] [PubMed] [Google Scholar]
- 2133.Kang JM, Kang SG, Cho SJ, et al. The quality of life of suspected obstructive sleep apnea patients is related to their subjective sleep quality rather than the apnea-hypopnea index. Sleep Breath 2017;21(2):369–375. [DOI] [PubMed] [Google Scholar]
- 2134.Wu H, Yuan X, Wang L, Sun J, Liu J, Wei Y. The relationship between obstructive sleep apnea hypopnea syndrome and inflammatory markers and quality of life in subjects with acute coronary syndrome. Respir Care 2016;61(9):1207–1216. [DOI] [PubMed] [Google Scholar]
- 2135.Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance – the Apnea positive pressure long-term Efficacy Study (APPLES). Sleep 2011;34(3):303–314B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2136.Vazir A, Hastings PC, Papaioannou I, et al. Variation in severity and type of sleep-disordered breathing throughout 4 nights in patients with heart failure. Resp Med 2008;102(6):831–839. [DOI] [PubMed] [Google Scholar]
- 2137.Fietze I, Glos M, Zimmermann S, Penzel T. Long-term variability of the apnea-hypopnea index in a patient with mild to moderate obstructive sleep apnea. J Clin Sleep Med 2020;16(2):319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2138.Sforza E, Roche F, Chapelle C, Pichot V. Internight variability of apnea-hypopnea index in obstructive sleep apnea using ambulatory polysomnography. Front Physiol 2019;10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2139.Magalang UJ, Chen N-H, Cistulli PA, et al. Agreement in the scoring of respiratory events and sleep among international sleep centers. Sleep 2013;36(4):591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2140.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur Heart J 2019;40(14):1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2141.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010;137(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2142.Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One 2012;7(3):e34106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2143.Bhattacharjee R, Khalyfa A, Khalyfa AA, et al. Exosomal cargo properties, endothelial function and treatment of obesity hypoventilation syndrome: a proof of concept study. J Clin Sleep Med 2018;14(5):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2144.Gongol B, Shang F, He M, et al. Serum miR-92a is elevated in children and adults with obstructive sleep apnea. J Mol Biomark Diagn 2020;11(4). [PMC free article] [PubMed] [Google Scholar]
- 2145.Khalyfa A, Gozal D, Kheirandish-Gozal L. Plasma exosomes disrupt the blood-brain barrier in children with obstructive sleep apnea and neurocognitive deficits. Am J Respir Crit Care Med 2018;197(8):1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2146.Koch H, Schneider LD, Finn LA, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep 2017;40(11):zsx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2147.Smith JH, Baumert M, Nalivaiko E, McEvoy RD, Catcheside PG. Arousal in obstructive sleep apnoea patients is associated with ECG RR and QT interval shortening and PR interval lengthening. J Sleep Res 2009;18(2):188–195. [DOI] [PubMed] [Google Scholar]
- 2148.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep 2006;29(6):777–783. [DOI] [PubMed] [Google Scholar]
- 2149.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - new pathways for targeted therapy. Sleep Med Rev 2018;37:45–59. [DOI] [PubMed] [Google Scholar]
- 2150.Pien GW, Ye L, Keenan BT, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic sleep apnea cohort. Sleep 2018;41(3):zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2151.Kim J, Keenan BT, Lim DC, Lee SK, Pack AI, Shin C. Symptom-based subgroups of Koreans with obstructive sleep apnea. J Clin Sleep Med 2018;14(3):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2152.Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014;44(6):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2153.Khosla S, Deak MC, Gault D, et al. Consumer sleep technology: an American academy of sleep medicine position statement. J Clin Sleep Med 2018;14(5):877–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2154.Cai Y, Tripuraneni P, Gulati A, et al. Patient-defined goals for obstructive sleep apnea treatment. Otolaryngol Head Neck Surg 2022;167(4):791–798. 10.1177/01945998221075298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2155.Gulati A, Stephens EM, Cai Y, Chang JL. Characterizing decisional conflict in patients presenting to sleep surgery clinic and an exploration of resource limitations. Laryngoscope 2021;131(10):2384–2390. [DOI] [PubMed] [Google Scholar]
- 2156.Ishman SL, Tang A, Cohen AP, et al. Decision making for children with obstructive sleep apnea without tonsillar hypertrophy. Otolaryngol Head Neck Surg 2016;154(3):527–531. [DOI] [PubMed] [Google Scholar]
- 2157.Bergerons M, Duggins A, Chini B, Ishman SL. Clinical outcomes after shared decision-making tools with families of children with obstructive sleep apnea without tonsillar hypertrophy. Laryngoscope 2019;129(11):2646–2651. [DOI] [PubMed] [Google Scholar]
- 2158.Fung CH, Martin JL, Liang LJ, et al. Efficacy of a patient decision aid for improving person-centered decision-making by older adults with obstructive sleep apnea. J Clin Sleep Med 2021;17(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


