Abstract
With the increasing prevalence of energy metabolism disorders such as diabetes, cardiovascular disease, obesity, and anorexia, the regulation of feeding has become the focus of global attention. The gastrointestinal tract is not only the site of food digestion and absorption but also contains a variety of appetite-regulating signals such as gut-brain peptides, short-chain fatty acids (SCFAs), bile acids (BAs), bacterial proteins, and cellular components produced by gut microbes. While the central nervous system (CNS), as the core of appetite regulation, can receive and integrate these appetite signals and send instructions to downstream effector organs to promote or inhibit the body’s feeding behaviour. This review will focus on the gut-brain axis mechanism of feeding behaviour, discussing how the peripheral appetite signal is sensed by the CNS via the gut-brain axis and the role of the central “first order neural nuclei” in the process of appetite regulation. Here, elucidation of the gut-brain axis mechanism of feeding regulation may provide new strategies for future production practises and the treatment of diseases such as anorexia and obesity.
Keywords: gut-brain axis, food intake regulation, gut-brain peptides, gut microbes, bacterial metabolites, appetite
1. Introduction
As one of the basic physiological activities of humans, feeding is essential for maintaining the body’s vital activities and energy homeostasis. If the organism’s long-term energy intake is less than its energy expenditure, a number of dysfunctions can be triggered, which in severe cases can be life-threatening. The regulation of body feeding is influenced by a variety of factors, including (i) Food quality, freshness, and composition, which affect intake; even the simplest salt requirement can affect feeding by influencing food salinity. (ii) External environmental pressures: approximately 35 to 60 percent of people reported that stress increased their food intake, while 25 to 40 percent reported that stress decreased their food intake, depending on the type of stress they were under [1]. (iii) The physiological state of the organism, such as the late gestation period of the mother, where the uterine contents compress the gastrointestinal tract, causing contraction of the gastrointestinal tract, resulting in a decrease in feeding and a significant increase in food intake after delivery. Previous studies may have focused more on the effects of external factors on foraging and lacked an in-depth investigation of the mechanisms.
For the past few years, the gut-brain axis involved in food intake regulation has increasingly become a leading research issue, and great progress has been made at the genetic, metabolic, and neural pathway levels involved in the regulation of food intake. The intestinal tract is able to fully perceive the nutritional status of the organism after ingestion and transmit it via the gut-brain axis to the brain, which in turn regulates food intake and maintains the body’s energy metabolism [2]. As key appetite signalling molecules, gut-brain peptides, gut microbes, and their metabolites play an important role in this process [3]. This review explains the role of “first-order neural nuclei” in the brain’s feeding regulatory circuit and details how gut-brain peptides, gut microbes, and their metabolites control the organism’s feeding behaviour.
Compared to previous articles that focused solely on the effects of gut-brain peptides on food intake [4], this article provides a more comprehensive review of the pathways involved in the regulation of feeding behaviour through microbe-gut-brain interactions. We believe that this analysis will lay the foundation not only for future research aimed at exploring new mechanisms of appetite regulation by the gut-brain axis but also for future livestock production practices.
2. The Role of “First-Order Neural Nuclei” in Food Intake Regulation
The gut-brain axis is a bidirectional communication system formed by the CNS and the gastrointestinal tract, involving humoral pathways such as gut-brain peptides, gut microbial metabolites, and cytokines, and neural pathways such as the vagus nerve, spinal nerve, and autonomic nervous system, which can condition many physiological functions such as behavioural regulation and immune response [5]. As the starting point of the brain’s feeding regulation circuit, the “first-order neural nuclei“ play an indispensable role in energy homeostasis mediated by the gut-brain axis.
2.1. Arcuate Nucleus (ARC)
The ARC of the hypothalamus, as one of the most important nuclei for sensing the energy levels of the organism, contains two peptide-producing neurons responsible for appetite regulation: The neuropeptide Y/agouti-related peptide (NPY/AgRP) neurons and the pro-opiomelanocortin (POMC) neurons [6]. Because of the abundant blood supply and the weak blood-brain barrier in the third ventricle, NPY/AgRP neurons and POMC neurons can directly sense a variety of signalling molecules in body fluids involved in appetite regulation; hence, the ARC is often considered the ‘first-order neural nuclei’ in humoral regulatory pathways [7].The ARC integrates appetite-regulating signalling molecules from the circulation and then sends hunger or satiety signals via neural projections to other nuclei within the CNS, which together form a complex feeding regulatory circuit that in turn stimulates or inhibits feeding behaviour in organisms [8].
In addition to producing AgRP, 90% of AgRP neurons co-express the neuropeptide NPY, which is why they are also called NPY/AgRP neurons. Activation of AgRP neurons promotes feeding behaviour even under conditions of appetite suppression and decreases neural activity in the anorexigenic Parabrachial nucleus (PBN) [9]. Dopamine receptor D1 (Drd1) is also expressed on AgRP neurons, and upregulation of Drd1 activity induces the ingestion of high-fat and high-sugar foods [10]. Dynamin-related protein1 (Drpl), a key protein, mediates mitochondrial fission and fatty acid oxidation in AgRP neurons as an important mechanism for AgRP neurons to promote food intake [11]. Killing AgRP neurons in adult mice using diphtheria toxin by Luquet et al. resulted in decreased appetite, whereas knocking out these neurons in young mice did not affect feeding [12], elaborating that a compensatory mechanism is formed in young mice to maintain food intake.
POMC proteins act as precursors for a wide range of functional peptides [13], which are converted to α-Melanocyte-stimulating hormone (α-MSH), β-MSH, γ-MSH, β-endorphin and Adrenocorticotropic hormone (ACTH) by the cleavage of Prohormone convertase 2 (PC2) and 1/3 (PC1/3) [14]. Among these, MSH acts as an important activating ligand for melanocortin receptor-4 (MC4R) and MC3R, and activated MC3/4R can reduce appetite and increase energy expenditure [15]. In contrast, during energy deprivation, AgRP peptides secreted by AgRP neurons act as inverse agonists of MC4R, reducing MC4R activity and promoting feeding behaviour [16]. It is widely accepted in the field that POMC neurons reduce appetite and induce satiety; however, it is noteworthy that cannabinoid-activated POMC neurons promote ingestion by inducing the release of appetite-stimulating β-endorphin rather than α-MSH [17].
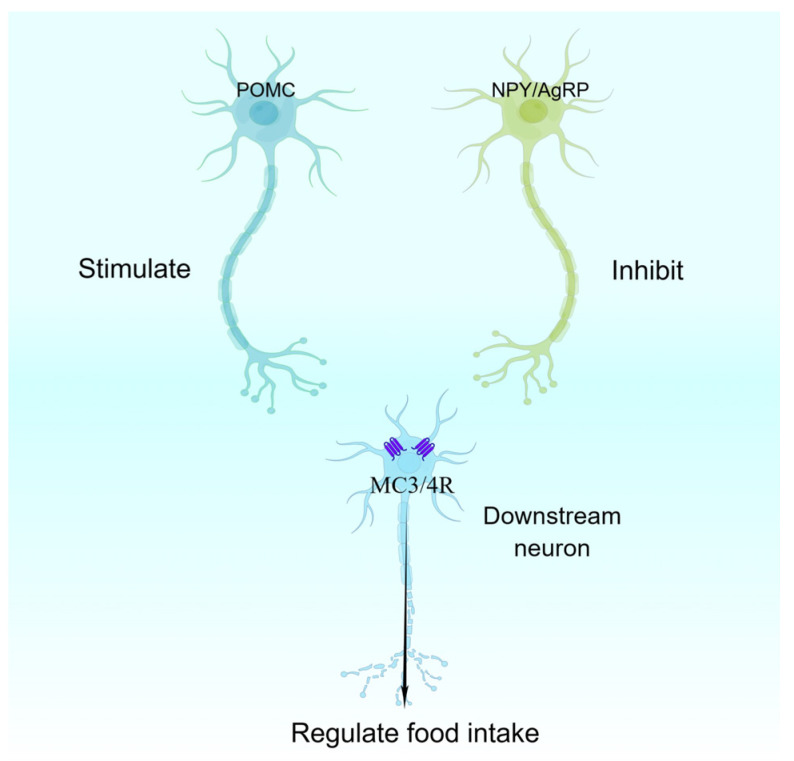
POMC neurons, NPY/AgRP neurons, and downstream neurons expressing MC3/4R together form the well-known central melanocortin system, which plays an important role in the maintenance of organismal energy homeostasis [16] (Figure 1).
Figure 1.
Central melanocortin system. AgRP, agouti-related peptide; NPY, neuropeptide Y; MC3/4R, melanocortin receptor-3/4; POMC, pro-opiomelanocortin.
2.2. Nucleus Tractus Solitarii (NTS)
The vagus nerve is an important link between the peripheral organs and the brainstem NTS, connecting the gut and other organs at one end, sensing the energy state of the organism, and the NTS at the other end, integrating nutritional signals from the gastrointestinal tract and thus regulating feeding behaviour and energy metabolism [18]. The gastrointestinal system releases nutritional signals such as glucagon-like peptide-1 (GLP-1), Ghrelin, and cholecystokinin (CCK), depending on the nutritional status of the body, while the vagal afferent nerve terminals express GLP-1 receptors, Ghrelin receptors, and CCK receptors, and the appropriate nutrient signals bind to these receptors, which are then transmitted to the brain via the vagus nerve to control appetite [19], so the NTS is often referred to as the “first-order nuclei” of the vagal afferent nerve to the CNS. Due to research innovations, researchers discovered neuropod cells in the intestinal wall, which form synaptic connections with the vagus nerve and transfer with the help of glutamate as a neurotransmitter, allowing the brain to respond more quickly and accurately to intestinal signals [20,21]. In addition to vagal connections, the NTS also directly senses nutrient molecules in the humoral circulation as well as neural signals projected from the forebrain, which integrate multiple energy state signals and transfer them to other nuclei to jointly control energy metabolism [22]. Upregulation of calcitonin receptor neuron activity in the NTS was found to induce non-aversive feeding inhibition [23]; stimulation of NTS POMC neurons also rapidly enhanced organ satiety [24]; chemical activation of NTS A2 neurons via projections to the paraventricular nucleus of the hypothalamus (PVN); however, not the bed nucleus of the stria terminalis (BNST), reduced food intake [25]. Overall, it is generally accepted that the vagal-brain neural circuit mediates postprandial satiety signals, which cause feeding behaviour to cease [26]. However, Chen et al. recently found that upregulation of the activity of neurons co-expressing NPY and catecholamines in the NTS occurs in response to hunger signals that elicit food intake [27].
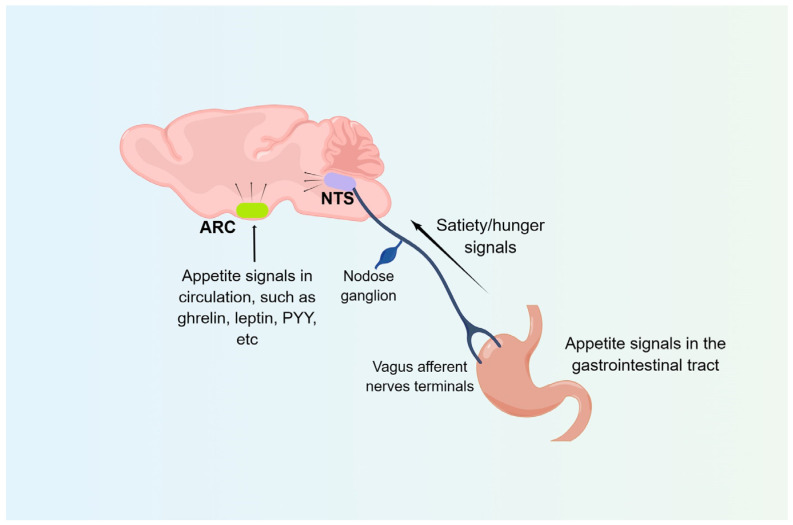
In summary, the ARC and NTS, the two “first-order neural nuclei” that are the starting point of the appetite control loop in the brain, can respond to satiety/hunger signals from the gastrointestinal tract to regulate the body’s food intake and energy balance (Figure 2).
Figure 2.
Food intake regulatory pathways. The ARC receives humoral signals, while the NTS mainly receives signals from the gastrointestinal tract via the vagus afferent nerves. ARC, arcuate nucleus; NTS, nucleus tractus solitarii; PYY, peptide YY.
3. The Effect of Typical Gut-Brain Peptides on Food Intake Regulation
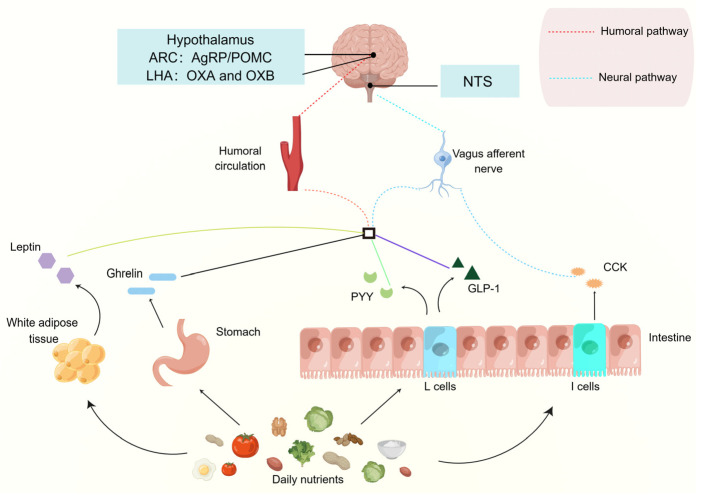
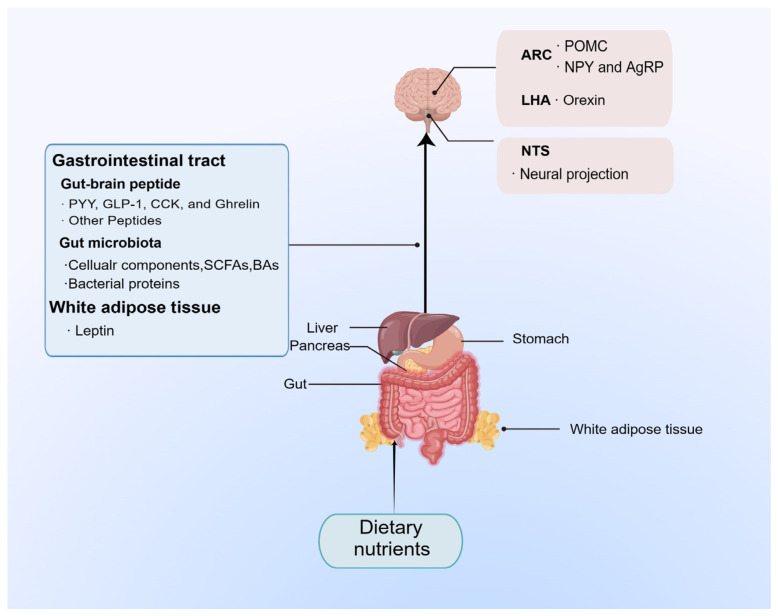
In the gut-brain axis, the gastrointestinal tract plays an essential role, directly contacting and digesting food, sensing changes in the body’s nutritional needs, and releasing gut-brain peptides, a signalling molecule that is transferred into the circulation to manipulate the activity of the associated appetite neurons, thereby maintaining the energy homeostasis of the organism (Figure 3).
Figure 3.
Diagram of Gut-brain peptides regulate appetite. The nutrients in the diet can stimulate the corresponding tissues and organs to secrete gut-brain peptides into the circulation and then transmit appetite signals to the brain through neural and humoral pathways, mediating the feeding behaviour of the organism. AgRP, agouti-related peptide; ARC, arcuate nucleus; CCK, cholecystokinin; GLP-1, glucagon-like peptide-1; LHA, lateral hypothalamic area; NPY, neuropeptide Y; NTS, nucleus tractus solitarii; OXA, orexin A; OXB, orexin B; PYY, peptide YY; POMC, pro-opiomelanocortin.
3.1. Orexin
Orexin A (OXA) and OXB, a pro-feeding gut-brain peptide, are formed by enzymatic cleavage of the precursor molecule prepro-orexin (PPO) and secreted mainly from the lateral hypothalamic area (LHA) [28]. Molecular and immunohistochemical techniques have demonstrated that the orexin receptor (OXR) is widely distributed throughout the brain, with the most abundant expression in the hypothalamus [29]. OXR is a member of the G protein-coupled receptor (GPR) family and has two receptor subtypes, OX1R and OX2R. OX1R has a higher affinity for OXA, while OX2R has a similar affinity for both [28]. OXR binds to its corresponding ligand and initiates intracellular signalling pathways that regulate feeding, neuroendocrine, and sleep/wake behaviour in mammals [30]. Experiments have shown that ventricular injections of both OXA and OXB stimulate food intake; however, OXA has a significantly greater feeding effect than OXB [31], a process that is blocked by OXR antagonists, inducing satiety and reducing body weight [32]. Microinjection of exogenous OXA into the Central amygdala (Cea) increases the intake of high-fat foods without affecting the normal food intake; however, this effect is partially blocked by Drd1 antagonists [33], suggesting that OXA (Cea) is also involved in the composition of the hedonic feeding loop. Endogenous androgens, possibly testosterone, reduce glucose-deprivation-induced feeding behaviour in animals by down-regulating the activity of OXA neurons [34]. The researchers found that orexin-induced feeding behaviour is mainly mediated by the NPY system in the ARC and that the use of NPY receptor antagonists to some degree reversed the feeding-promoting effects of Orexin [29]. Furthermore, a study by Morello et al. showed that there is a significant negative correlation between OXA and α-MSH in obese mice due to OXA binding to the OX1R on POMC neurons and inhibiting POMC gene transcription through a series of signalling events, which in turn reduces α-MSH production [35]. The central function of Orexin has been extensively studied; however, its role in peripheral tissues such as the gut, fat, and liver are becoming a source of discoveries and new research. In the future, a deeper understanding of the mechanism of action of orexin and its downstream effects will bring different insights into improving the health and nutrition of the organism.
3.2. Ghrelin
Ghrelin, also known as growth hormone secretagogue peptide, is produced by X/A-type (rodents) and P/D1-type (humans) digestive tract mucosal cells [36] and plays a wide range of roles in food intake control, gastrointestinal inflammation, and cardiovascular regulation [37]. The Ghrelin gene encodes for the production of Ghrelin precursor proteinogen (Preproghrelin), consisting of 117 amino acids, and this peptide produces different subproducts in response to a series of enzymes, of which acylated Ghrelin (Acyl-ghrelin, AG) and deacylated Ghrelin (Des- acyl-ghrelin, DAG) are the most abundant, both consisting of 28 amino acids [38]. Ghrelin’s N-terminal serine-3 (Ser-3) is acylated with n-octanoic acid under the mediation of Ghrelin O-acyltransferase (GOAT), a modification that is essential for Ghrelin to recognise and activate relevant receptors [39]. The Ghrelin receptor (Growth hormone secretagogue receptor, GHSR), a member of the GPR family, can heterodimerize with other weight-regulating GPRs such as MC3R, Drd1, and OX1R, which interact to jointly regulate the energy homeostasis of the organism [40].
Under normal physiological conditions, ghrelin levels in the body increase gradually during fasting, peaking before feeding and decreasing rapidly after feeding, and there is considerable evidence that this change is closely related to circulating nutrients [41], such as glucose, amino acids, fatty acids, and trace elements. Studies have shown that Ghrelin has the ability to promote food intake, increase body weight, and accelerate energy expenditure in animals. Mice injected subcutaneously with the GHSR agonist JMV 1843 for 10 days showed a significant increase in food intake and a significant increase in body weight compared to mice injected with saline [42]. Feeding tryptophan to weanling pigs resulted in increased plasma Ghrelin levels as well as significantly enhanced feeding activity at 2 h, 8 h, and 24 h [43]. It was found that Ghrelin’s appetite-stimulating mechanism is achieved mainly through two feeding regulatory pathways: humoral and neural [7]. Secreted ghrelin reaches the ARC via the humoral circulation and activates the GHSR on NPY/AgRP neurons, significantly increasing the activity of these neurons and inhibiting the activity of anorexigenic neurons, thereby increasing food intake and body weight [44]. Notably, however, GHSR was not expressed on POMC neurons, suggesting that inhibition of POMC neurons is achieved indirectly via the inhibitory neurotransmitter γ-aminobutyric acid (GABA) released by NPY/AgRP neurons [45]. Ghrelin also binds to the GHSR on vagal afferent nerves, transmitting hunger signals to brain regions involved in energy regulation and stimulating feeding behaviour in animals [7]. In addition, Bruschetta et al. reported that Ghrelin reduces hypothalamic α-MSH levels by stimulating AgRP neurons to secrete prolyl carboxypeptidase (PCRP), which in turn produces a stronger appetitive drive [46].
3.3. CCK
CCK is mainly secreted by type I cells of the small intestinal mucosa in amounts proportional to dietary protein and lipid levels and is widely distributed in the gastrointestinal tract and the central and peripheral nervous systems. Researches has shown that CCK acts as a satiety signal and can perform a variety of biological functions, including stimulating pancreatic secretion, contracting the gallbladder, and delaying gastric emptying [47]. CCK exists in a variety of active molecules due to differences in translation and processing, with CCK-58, CCK-8, and CCK-33 being the major molecular forms [48]. The presence of two CCK receptor subtypes, the CCK-1 receptor (CCK-1R) and the CCK-2R, can be observed in the organism. In rats, CCK-1R is distributed in the pancreas, gallbladder, vagal afferent nerve, and certain areas of the brain and is mainly involved in the regulation of feeding; CCK-2R is expressed in the gastric mucosa, CNS, and vagus nerve [49]. In a study by L. Wang et al., dietary soy protein may trigger the secretion of CCK through activation of the calcium-sensing receptor (CaSR) and intracellular Ca2+/TRPM5 pathway, resulting in a decrease in appetite [50]. A more rapid release of CCK was observed when spinach extract was given orally to rats, which in turn induced an earlier sensation of satiety, an effect that may arise from the higher content of flavonoids in spinach [51]. As well, intraperitoneal injection of CCK in blind cavefish, Astyanax fasciatus mexicanus, significantly reduced feed intake compared to the saline-injected group [52].
In previous reports, peripheral CCK-mediated decreases in feeding activity were mainly mediated via the vagal pathway [53]. Decreasing vagal sensitivity to CCK in rats can cause dysregulation of food intake [54]. One hour after peripheral injection of CCK-8 in goldfish, the researchers found significantly higher levels of POMC mRNA in brain tissue, indicating that peripheral CCK signalling mediates appetite loss via vagal afferent neurotransmission to the hindbrain and subsequently through the POMC signalling pathway [55]. Fan et al. also demonstrated that POMC neurons are also expressed in the caudal part of the NTS and can be activated by electrical or CCK-induced stimulation of the vagal afferent nerve [56]. In addition, optogenetic activation of CCK-expressing neurons in the NTS, which send axonal projections to the PVN, induces satiety and reduces body weight in mice [57]. In short, CCK can be used as a general gastrointestinal hormone to regulate gastrointestinal motility, and it can also act as a neurotransmitter in the CNS. At present, there are many studies on the mechanism of CCK in regulating animal feeding but fewer reports on the application of CCK in production practice, so it is undoubtedly of great practical importance to intensify research in this area.
3.4. GLP-1
GLP-1, a product encoded by the proglucagon gene, is mainly synthesised and secreted by distal L-cells of the small intestine. Research has reported that GLP-1 is also produced in the brain by preproglucagon (PPG) neurons in the NTS and that this region serves as the major source of central endogenous GLP-1 [58]. In mouse models, GLP-1 receptor (GLP-1R) expression is observed in both the peripheral (pancreas, gastrointestinal tract, kidney, etc.) and CNS (hypothalamus, hippocampus, brainstem, etc.) [59]. It has been reported that active GLP-1 secreted from the small intestine crosses the blood-brain barrier but is rapidly degraded to inactive fragments in the circulation [60], demonstrating that only a minority of peripherally active GLP-1 reaches the CNS. The researchers then measured GLP-1 levels in brain tissue and found that GLP-1 levels in the brain were many times higher than in the circulation [58]. These examples all support the view that centrally active GLP-1 is predominantly derived from NTS PPG neurons and not from the peripheral circulation.
Intracerebroventricular injection of Exendin-4 (Ex-4), a GLP-1R-specific agonist, significantly reduced food intake and decreased body weight as well as hypothalamic and gastric ghrelin levels in rats [61], consistent with Hong et al., finding that Ex-4 inhibits Ghrelin secretion via the Mammalian target of Rapamycin (mTOR) signaling pathway [62]. GLP-1 analogues were found to increase POMC neurons’ activity in a time-dependent manner after intraperitoneal and subcutaneous injections in mice [63]. Moreover, NTS PPG neurons can send dense projections to multiple regions of the brain, e.g., dense mapping to the ARC region increases the frequency of action potential discharges of POMC neurons [64]; reverse tracing and immunohistochemical techniques have shown that inputs from NTS PPG neurons to the PVN mediate the generation of satiety in the organism [65]; and that central GLP-1 reduces the excitability of dopamine neurons in the ventral tegmental area (VTA) of the midbrain and decreases the intake of high-fat foods by inhibiting the hedonic pathway [66]. In conclusion, GLP-1R-expressing neuronal nuclei in the brain respond to both NTS PPG neurons and GLP-1 signals from the gastrointestinal tract to induce a feeling of satiety in the body.
3.5. Peptide YY (PYY)
PYY is an anorexigenic brain-gut peptide that is released in response to food intake, primarily by L-cells in the small intestine, and is distributed in sequentially increasing concentrations from the foregut to the hindgut [67]. Keire et al. found that PYY1–36, as the major form secreted from the distal small intestine of rats, is enzymatically converted in the circulation by dipeptidyl peptidase-4 (DPP4) to the more active molecular form PYY3–36 [68], which induces an enhanced feeling of satiety after a meal. However, when the C-terminus of PYY3–36 is removed, PYY-induced anorexia also disappears [69], demonstrating that the integrity of the C-terminus may be essential for PYY to maintain its biological activity. Five GPR isoforms of PPY have been identified, of which the Y2 receptor (Y2-R) has a higher affinity for PYY3–36 [70]. Adrian et al. observed low basal levels of PYY in the circulation; however, with the onset of feeding, its secretion is consistent with the nutrients in the diet and remains high for several hours after meal times [71].
Trials have revealed that oral administration of L-arginine upregulates the concentration of PYY in the postprandial circulation and reduces appetite as well as lipid intake [72]. Administration of PYY3–36 to mice significantly diminished food consumption, and the effect of injection at night is more obvious than during the day [73]. Co-administration of PYY3–36 with Ex-4 subcutaneously resulted in a significant increase in the number of c-fos-positive neurons in the brain compared to when the drugs were administered separately or alone [74], implicating that PYY and GLP-1 may modulate food intake in some synergistic manner [75]. Immunohistochemistry and in situ hybridization have revealed that hypothalamic Y2-R mRNA is abundant in NPY-expressing neurons [76], which can be activated by NNC0165-1273 (an analogue of PYY3–36), inducing a decrease in NPY/AgRP neurons activity and causing organismic satiety [77], an effect that is resisted when Y2-R is knocked out [78]. PYY3–36 also upregulates POMC neuronal activity by decreasing the activity level of NPY nerve terminals [79]. In addition, the vagus nerve partially mediates the satiety signalling of PYY, and PYY3–36 induces reduced appetite in rats by stimulating Y2-R on the vagal afferent nerve [80], and the anorexigenic effect of PYY is subsequently attenuated when the bilateral subphrenic vagus nerve is ablated [81].
3.6. Leptin
Leptin is an anorexigenic gut-brain peptide encoded by the ob gene and secreted mainly in white adipose tissue. The diverse distribution of the leptin receptor (LepR) allows leptin to exert pleiotropic effects, including regulation of food intake, pro-inflammatory immune response, cognition, reproduction, and many other functions [82]. LepRb, a subtype of the receptor, is abundantly expressed in brain regions associated with energy regulation [83] and causes a down-regulation of appetite in the organism. Evidence shows that POMC neurons and NPY/AgRP neurons in the ARC are one of the main targets of Leptin in the brain and that Leptin reduces food intake and increases the body’s energy expenditure by depolarising POMC neurons and hyperpolarising NPY/AgRP neurons [84]. Researchers have further investigated the signalling mechanism of Leptin and found that when Leptin binds to its corresponding receptor, it sequentially phosphorylates and activates Janus-activated kinase 2 (JAK2) and Signal transducer and activator of transcription 3 (STAT3), then pSTAT3 dissociates from LepR and enters the nucleus to bind to POMC and AgRP genes, promoting transcription of POMC mRNA and reducing levels of AgRP mRNA [85]. In addition, the LepRs are also expressed on the vagus nerve, and when these receptors are knocked out, increased food intake and weight are observed in animals [86].
Weight gain due to Leptin deficiency can be treated by exogenous injections of Leptin. However, in many people with obesity, Leptin levels are abnormally high compared to normal levels, and this has been attributed to the development of “leptin resistance” [87], i.e., impairment of the Leptin signalling pathway [88]. Moreover, Leptin is potentially associated with gastrointestinal hormones such as Grehlin, CCK, and GLP-1, which collectively exhibit synergistic or opposing appetite-regulating effects [89]; however, the complex feeding mechanisms involved need to be further investigated.
4. The Influence of Gut Microbes and Their Metabolites in Food Intake Regulation
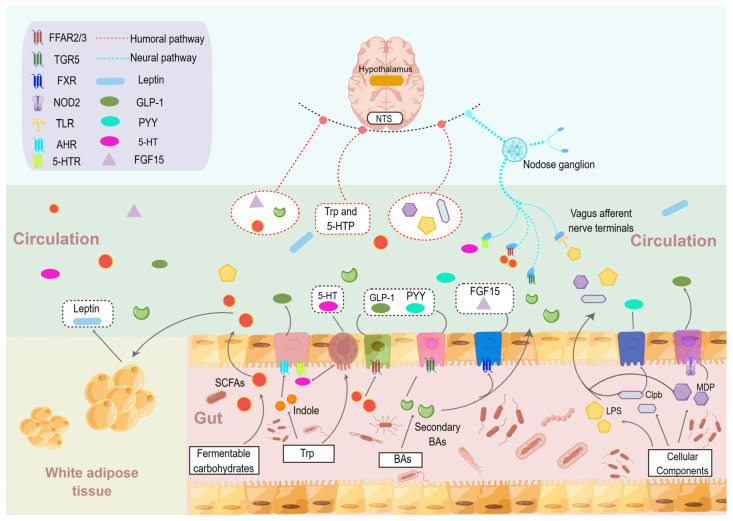
As the largest and most complex micro-ecosystem in the human body, gut microbes play a variety of important roles in the host. There is increasing evidence that gut microbes can communicate with the brain and play a key function in regulating the host’s feeding behaviour and energy homeostasis [90], with functional metabolites such as Indole, SCFAs, and BAs, as well as cellular components and bacterial proteins, serving as intermediate messengers to mediate the communication between the two (Figure 4).
Figure 4.
Gut microbe-brain communication in the regulation of food intake. Gut microbes rely mainly on their functional metabolites and cellular components to mediate host food intake through humoral, enterodocrine, and neural signalling pathways. AHR, aromatic hydrocarbon receptor; Clpb, caseinolytic peptidase B; FGF15, fibroblast growth factors 15; FXR, farnesoid X receptor; FFAR2/3, free fatty acid receptor 2/3; GLP-1, glucagon-like peptide-1; LPS, lipopolysaccharide; MDP, muramyl dipeptide; NOD2, nucleotide-binding oligomerization domain containing 2; NTS, nucleus tractus solitarii; PYY, peptide YY; SCFAs, short-chain fattyacids; TGR5, takeda G-protein-coupled receptors 5; TLR, Toll-like receptor; Trp, tryptophan; 5-HT, 5-hydroxy tryptamine; 5-HPT, 5-hydroxytryptophan; 5-HTR, 5-HT receptor.
4.1. SCFAs
Researchers have found that adding dietary fibre to meals could suppress appetite and improve body weight [91,92], and these changes were subsequently confirmed to be associated with SCFAs, a fermentation product of the colon [93]. In the study by Brown et al., although all three major SCFAs (acetic, propionic, and butyric) activated the free fatty acid receptor 2 (FFAR2)/GPR43 and FFAR3/GPR41, FFAR2 had a higher affinity for acetic acid, whereas propionic and butyric acids were potent FFAR3 agonists [94]. Both receptors are expressed in enteroendocrine L cells, while FFAR3 is also observed in the peripheral nervous system [95].
The available evidence suggests that SCFAs maintain energy homeostasis in the body either by entering the brain via the somatic circulation, stimulating the secretion of gut-brain peptides, or mediating the afferent transmission of appetite signals via the vagus nerve [96]. PET-CT scans of mice injected intraperitoneally with 11C-acetate solution show that acetate crossed the blood-brain barrier to reach the ARC region and induced elevated neuronal activity in POMC as well as decreased AgRP neuronal activity [97]. At the same time, acetate also stimulates Leptin secretion from the white adipose tissue through a FFAR2-dependent mechanism [98], causing changes in the activity of appetite-related neurons in the brain. Psichas et al., injected propionate into the colon, which caused activation of FFAR2 on intestinal L cells, and observed elevated levels of PYY and GLP-1 in plasma from the rat jugular vein and mouse portal vein [99]. In research by Larraufie et al., butyric acid, the most potent agonist of anorexigenic peptides in SCFAs, increased the expression of the PYY gene 120-fold, whereas propionic acid and acetic acid increased it by 40-fold and 2-fold, respectively [100]. Oral (not intravenous) butyric acid significantly reduced the excitability of hypothalamic NPY-expressing neurons and the number of c-fos-positive neurons in the NTS and dorsal vagal complex (DVC); however, had no effect on the activity of POMC neurons [101]. In addition, the vagal afferent nerves are an important aspect of appetite regulation, and the researchers found that knocking out FFAR3 on the terminals of the vagal afferent nerve led to an increase in food intake in mice [102]. Failure of butyric acid-treated groups to reduce cumulative food intake in comparison with controls after subdiaphragmatic vagus nerve transection in mice [101], implying that the vagus nerve is essential for butyrate-induced satiety in the organism. At present, there is no doubt that SCFAs act as a satiety signal in vivo; however, the signal transduction mechanisms mediated by them are still at the primary research stage, and exploring the detailed mechanisms of SCFAs will help us better understand their effects on appetite regulation.
4.2. BAs
Elementary BAs are converted from cholesterol in the liver, released into the intestinal lumen during the digestion of chyme by the organism, and converted to secondary BAs such as deoxycholic acid (DCA) and lithocholic acid (LCA) by uncoupling and dehydroxylation in the presence of bile salt hydrolase-expressing intestinal microorganisms such as Clostridium spp., Lactobacillus spp., Enterococcus spp., and so on [103]. Recent research has revealed that in addition to affecting lipid metabolism and inflammatory response, BAs also play a role in regulating appetite and energy homeostasis [104], where farnesoid X receptor (FXR) and takeda G-protein-coupled receptor 5 (TGR5) act as crucial receptors to mediate BAs’ function.
The research demonstrated that BAs are potent agonists that promote the release of GLP-1 and PYY, which upregulate the activity of TGR5 in intestinal L-cells, significantly increasing colonic levels of GLP-1 (a 3.5-fold increase compared to control) and PYY (a 2.9-fold increase compared to control) [105]. While knocking out TGR5, small intestinal perfusion with BAs did not induce secretion of the anorexigenic peptides GLP-1 and PPY [106]. Meanwhile, BAs also triggers FXR in the distal small intestine to secrete fibroblast growth factor (FGF) 15 into the circulation and cross the blood-brain barrier to interact with AgRP/NPY neuronal FGF receptors, subsequently leading to a series of signal transduction events to diminish AgRP/NPY gene expression [107], which indicates that the BAs-FXR-FGF15 pathway is an instrumental part of maintaining energy homeostasis in the organisation. Additionally, the presence of BAs in the brain was also observed by Higashi et al. and was positively correlated with the level of BAs in the plasma [108], leading to the hypothesis that BAs in the peripheral circulation could cross the blood-brain barrier into the brain. Using RNA Scope brain expression profiling, TGR5 mRNA was found to be highly expressed in the ARC area of the hypothalamus, and oral or intravenous administration of the TGR5 agonist INT-777 to mice abolished feeding behaviour by inhibiting AgRP/NPY mRNA expression and neuropeptide release [109]. Furthermore, the vagus nerve is also involved in BAs mediating satiety, and TGR5 on it transduces afferent DCA anorexic signals that specifically trigger the activation of POMC anorexic neurons in the hypothalamus, causing a reduction in food intake [110].
4.3. Tryptophan-Derived Metabolites
Tryptophan (Trp) is an essential amino acid and the only one with an indole structure, mainly provided by dietary protein. Agus et al. reported that Trp in the gut, under the direct or indirect regulation of the flora, can generate various active molecules mainly through three metabolic pathways (the serotonin pathway, the indole pathway, and the kynurenine pathway), which in turn mediate the body’s metabolism, immunity, and gastrointestinal function [111].
Tryptophan hydroxylase (TPH) serves as the key rate-limiting enzyme in the serotonin pathway, with two isoforms, TPH1 (mainly found in enterochromaffin cells, ECs) and TPH2 (mainly found in the brain), in which the enzyme hydroxylates Trp to produce 5-hydroxytryptophan (5-HTP), which is then converted through a series of metabolic pathways to serotonin (5-hydroxy tryptamine, 5-HT) [112]. Meanwhile, gut microbes are also major participants in regulating 5-HT production [113], e.g., SCFAs produced by the flora increase the expression level of ECs TPH1 mRNA, which in turn accelerates the biosynthesis of 5-HT [114], and other studies have demonstrated that spore-forming microbes from mice and humans can also promote 5-HT production [115]. The fact that 5-HT, whether of gut or brain origin, induces satiety in the organism has long been undisputed [116]. In vitro assays showed that 5-HT (30 μM or 100 μM) significantly upregulated the ability of the mouse small intestinal endocrine cell line STC-1 to release GPL-1 compared to control, whereas it did not induce the above changes when a non-specific 5-HT receptor antagonist was used [117], suggesting that there may be a reciprocal mechanism by which 5-HT may be involved in the secretion of GLP-1. Although gut-derived 5-HT does not cross the blood-brain barrier, its precursors, Try and 5-HTP, do, indirectly influencing central 5-HT function and production [118]. Existing studies have shown that the ARC, PVN, and neuronal nuclei expressing 5-HT1A receptor(5-HT1AR), 5-HT1BR, 5-HT2AR, and 5-HT2CR are the main targets of 5-HT in the brain, mediating the feeding behaviour of the organism. Activation of 5-HT1BR significantly inhibits the ingestive effects of NPY/AgRP neurons and increases the feeling of satiety in the body [119]. In total, 5-HT also binds to 5-HT2CR on anorexigenic neurons, POMC, upregulating the level of central α-MSH biosynthesis [120]. In addition, the vagus nerve acts as a sensory transducer that transmits 5-HT signals from the gut to the brain [121]. The diversity of 5-HT receptors and their widespread distribution mean that 5-HT has a wide range of biological functions. Current research points to a number of 5-HT receptors being involved in the regulation of feeding, but which receptors play a major role and how they work together need to be further confirmed.
Previous research has shown that up to 85 strains can encode tryptophanase genes, and tryptophan can be fermentatively produced in these colonies to form indole, a ligand for aromatic hydrocarbon receptors (AHR) [122]. Enhanced GLP-1 secretion was observed after stimulation of the mouse GLUTag cell line using Ficz, an AHR agonist [123]. This is consistent with the results of Chimerel et al., that indole induces a rapid release of GLP-1 from intestinal L-cells in the short term but inhibits its secretion in the long term, a phenomenon that appears to be related to the production and use of adenosine triphosphate in the cells [124]. The above results indicate that indole may mediate host appetite by affecting the secretion of gut-brain peptides. In addition, indole and its derivatives may affect the balance of human gut microbes by inhibiting fungal growth and modulating flora motility, colonisation and biofilm formation [122]; however, it is not clear whether this flora balance is relevant to host appetite regulation.
4.4. Bacterial Proteins and Cellular Components of Gut Microbe
Further research has shown that gut microbes produce bacterial proteins that affect brain areas involved in energy regulation, as caseinolytic peptidase B (Clpb) protein homologs act as conformational antigen-mimetic of α-MSH [125], i.e., Clpb has homology with the amino acid sequence of α-MSH. Clpb protein homologs can be produced by flora, which includes E. coli [126,127]. Measurement of Clpb levels in healthy and anorexic populations using immunoassays showed that Clpb levels were higher in anorexic patients and did not differ significantly between patient subgroups [128], and reduced levels of anti-Clpb immunoglobulin M (IgM) and IgG were observed in patient plasma, so it was hypothesised that the enhanced satiety sensation mediated by Clpb may result from decreased levels of humoral immunity [129]. Macrogene sequencing has shown that the relative abundance of the Clpb-producing phylum and family is decreased in obesity patients [130]. Treatment of ob/ob mice with Hafnia alvei, a potential probiotic that secretes Clpb protein, significantly decreased food intake as well as weight and fat gain [131,132]; however, did not produce the above changes when given to a Clpb-deficient strain [133]. Manon Dominique et al. added equal calories of bovine serum proteins, D-fructose, and oleic acid to E. coli cultures and found that the supplementary protein group upregulated bacterial Clpb mRNA expression [134], suggesting that protein-mediated satiety may be related to bacterial secretion of Clpb. The researchers then explored the mechanism of Clpb-induced anorexia and found that Clpb increased PYY secretion in primary cultured cells of rat intestinal mucosa in a dose-dependent manner [134,135], demonstrating that Clpb activates the appetite signalling pathway mediated by PYY to suppress food intake. Moreover, the presence of Clpb in the hypothalamus of rodents and humans can be observed using protein immunoblotting [136]. Clpb injected intraperitoneally reaches the hypothalamus via the systemic circulation and increases the number of c-fos-positive neurons in anorexic neurons, with repeated injections causing a reduction in food intake in mice [137].
In parallel, other trials have demonstrated that Lipopolysaccharide (LPS) and Muramyl dipeptide (MDP) serve as modulators of food intake. As a major component of the outer membrane of Gram-negative bacteria, LPS binds to Toll-like receptors (TLRs) and then undergoes a series of signalling pathways that ultimately trigger metabolic dysfunction and disease development in the body [138]. It was found by Breen et al. that LPS reduced appetite and body weight and increased the incidence of inflammation and mortality in mice [139], whereas injection of agmatine into the brain ventricles normalised LPS-mediated disorders, including anorexia nervosa [140]. In broiler chickens injected intraperitoneally with LPS, a decrease in the levels of AgRP mRNA and NPY mRNA was clearly observed, while the expression of anorexigenic neuropeptides was unchanged [141], implying that the decrease in the expression levels of AgRP and NPY genes might be one of the main reasons for the anorexia induced by LPS. Further studies uncovered that blockade of the mTOR signalling pathway with rapamycin attenuated LPS-induced appetite reduction and suppression of AgRP gene expression [142], demonstrating that LPS can exert its anorectic effects through the mTOR-AgRP pathway. LPS can also increase the excitability of vagal afferent nerves through a TLR4-dependent mechanism [143], yet cutting subphrenic vagal afferent nerves did not increase food intake [144]. These findings provide support for the idea that LPS can co-regulate the energy metabolism of the organisation through both neural and humoral pathways. As an agonist of the nucleotide-binding oligomerization domain-containing 2 (NOD2) pattern recognition receptor, MDP is considered a marker of bacterial proliferation in the host organism. In previous reports, MDP was shown to significantly increase satiety [145], which was attributed to the presence of NOD2 receptor expression on mouse intestinal L-cells, leading to a significant increase in GPL-1 secretion when treated with MDP derivatives under normoglycaemic conditions [146]. Additionally, Gabanyi et al. observed that certain regions of the brain can be targeted by radioisotope-labelled MDP and that specific knockout of the hypothalamic NOD2 receptor upregulates appetite and body weight in mice [147], confirming that hypothalamic neurons can directly sense changes in gut microbes and adjust the organism’s appetite accordingly.
5. Conclusions and Prospect
In general, the gut-brain axis serves as an inevitable chain in regulating the food intake of organisms, in which the gut-brain peptides, intestinal microorganisms, and their metabolites perform significant actions (Figure 5). Gut-brain peptides such as Ghrelin, CCK, and Leptin can enter the CNS directly through the humoral pathway to influence the function of appetitive neurons or indirectly via the vagus nerve to regulate the organism’s feeding behaviour. Gut microbes, however, rely mainly on their functional metabolites and cellular components, such as SCFAs, MDP, and BAs, to cross the blood-brain barrier to reach the brain, pass through vagal afferent neural pathways, or stimulate enteroendocrine cells to release the PPY and GPL-1 pathways, which work together to regulate food intake and energy homeostasis in the body.
Figure 5.
Diagram of the gut-brain axis that regulates food intake. Gut-brain peptides, gut microbes, and their metabolites transmit the nutritional state of the body to the brain through the gut-brain axis pathway and thus regulate the food intake of the body by affecting the activity of appetite related neurons. AgRP, agouti-related peptide; ARC, arcuate nucleus; BAs, bile acids; CCK, cholecystokinin; GLP-1, glucagon-like peptide-1; LHA, lateral hypothalamic area; NPY, neuropeptide Y; NTS, nucleus tractus solitarii; PYY, peptide YY; POMC, pro-opiomelanocortin; SCFAs, short-chain fatty acids.
As technology advances, research into the gut-brain axis involved in appetite regulation is becoming increasingly popular; however, there are three points that deserve particular attention in future research: (i) The majority of existing studies have focused on gut-brain peptides working separately, and there is a lack of exploration to unify them. Trials have indicated that greater anorexic impulses are produced when PYY and GLP-1 are co-administered [75]; in the meantime, Blanco et al., also noted that Ghrelin attenuates the satiety mediated by CCK, PYY, and GLP-1 [148]. Enhancing the study of gut-brain peptide interactions and uncovering the complex mechanisms involved may have unexpected effects on the regulation of appetite. (ii) Bacterial flora is one of the most abundant in the organism, with a mass of about 1–1.5 kg and a number of 1013–1014, which is about 10 times the number of cells in the human body. Many articles have reported that gut microbes affect the CNS; however, the mechanisms involved are lacking. Future research on flora and appetite will not only simply show whether gut microbes regulate ingestion but, more importantly, analyse the pathways through which they influence appetite regulation. (iii) Fecal microbiota transplantation (FMT) can be used to intervene and adjust the composition of the gut microbiome to regulate the host’s feeding behaviour; however, clinical trials of FMT are still in the exploratory stage and face significant challenges. To begin with, the use of FMT has been associated with toxic side effects and even fatalities [149]; furthermore, the structural composition of the feacal flora is complex, and studies have shown that only specific flora in the donor are functioning, so it is essential to tap into the key flora [150]; and lastly, from a moral, ethical, and psychological standpoint, the use of FMT is not acceptable to the general public. FMT research is an inevitable trend; finding the “potential probiotics” in FMT may be an effective strategy for microbial-based appetite treatment. In conclusion, there is no doubt that a significant role is played by the gut-brain axis in the organism, and further exploration of the gut-brain mechanism will be of great revelation and application value.
Author Contributions
S.L. wrote the manuscript with input and guidance from Y.S., M.L., S.C., B.L., D.L., Z.W., H.S. and Y.C. helped to collect and analyse the literature. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Financial support for this research was provided by the Modern Agro-industry Technology Research System of China (CARS-34) and the Science and Technology Innovation Team of Henan Province High Quality Forage and Animal Health (No.22IRTSTHN022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Packard A.E.B., Ghosal S., Herman J.P., Woods S.C., Ulrich-Lai Y.M. Chronic Variable Stress Improves Glucose Tolerance in Rats with Sucrose-Induced Prediabetes. Psychoneuroendocrinology. 2014;47:178–188. doi: 10.1016/j.psyneuen.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mithieux G. Crosstalk between Gastrointestinal Neurons and the Brain in the Control of Food Intake. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28:739–744. doi: 10.1016/j.beem.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Fetissov S.O., Averina O.V., Danilenko V.N. Neuropeptides in the Microbiota-Brain Axis and Feeding Behavior in Autism Spectrum Disorder. Nutrition. 2019;61:43–48. doi: 10.1016/j.nut.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Roh E., Choi K.M. Hormonal Gut–Brain Signaling for the Treatment of Obesity. Int. J. Mol. Sci. 2023;24:3384. doi: 10.3390/ijms24043384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 6.Vohra M.S., Benchoula K., Serpell C.J., Hwa W.E. AgRP/NPY and POMC Neurons in the Arcuate Nucleus and Their Potential Role in Treatment of Obesity. Eur. J. Pharmacol. 2022;915:174611. doi: 10.1016/j.ejphar.2021.174611. [DOI] [PubMed] [Google Scholar]
- 7.Wen S., Wang C., Gong M., Zhou L. An Overview of Energy and Metabolic Regulation. Sci. China Life Sci. 2019;62:771–790. doi: 10.1007/s11427-018-9371-4. [DOI] [PubMed] [Google Scholar]
- 8.Bouret S.G. Development of Hypothalamic Circuits That Control Food Intake and Energy Balance. In: Harris R.B.S., editor. Appetite and Food Intake: Central Control. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2017. [PubMed] [Google Scholar]
- 9.Essner R.A., Smith A.G., Jamnik A.A., Ryba A.R., Trutner Z.D., Carter M.E. AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J. Neurosci. 2017;37:8678–8687. doi: 10.1523/JNEUROSCI.0798-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q., Tang Q., Purohit N.M., Davenport J.B., Brennan C., Patel R.K., Godschall E., Zwiefel L.S., Spano A., Campbell J.N., et al. Food-Induced Dopamine Signaling in AgRP Neurons Promotes Feeding. Cell Rep. 2022;41:111718. doi: 10.1016/j.celrep.2022.111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin S., Yoon N.A., Liu Z.-W., Song J.E., Horvath T.L., Kim J.D., Diano S. Drp1 Is Required for AgRP Neuronal Activity and Feeding. eLife. 2021;10:e64351. doi: 10.7554/eLife.64351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 13.Clark A.J.L., Lowry P. 60 YEARS OF POMC: POMC: The Consummate Peptide Hormone Precursor. J. Mol. Endocrinol. 2016;56:E1–E2. doi: 10.1530/JME-16-0016. [DOI] [PubMed] [Google Scholar]
- 14.Dores R.M., Baron A.J. Evolution of POMC: Origin, Phylogeny, Posttranslational Processing, and the Melanocortins. Ann. N. Y. Acad. Sci. 2011;1220:34–48. doi: 10.1111/j.1749-6632.2010.05928.x. [DOI] [PubMed] [Google Scholar]
- 15.Tao Y.-X. Mutations in the Melanocortin-3 Receptor (MC3R) Gene: Impact on Human Obesity or Adiposity. Curr. Opin. Investig. Drugs. 2010;11:1092–1096. [PubMed] [Google Scholar]
- 16.Biebermann H., Kühnen P., Kleinau G., Krude H. The Neuroendocrine Circuitry Controlled by POMC, MSH, and AGRP. Handb. Exp. Pharmacol. 2012;209:47–75. doi: 10.1007/978-3-642-24716-3_3. [DOI] [PubMed] [Google Scholar]
- 17.Koch M., Varela L., Kim J.G., Kim J.D., Hernández-Nuño F., Simonds S.E., Castorena C.M., Vianna C.R., Elmquist J.K., Morozov Y.M., et al. Hypothalamic POMC Neurons Promote Cannabinoid-Induced Feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki Y., Yada T. Vagal Afferents Sense Meal-Associated Gastrointestinal and Pancreatic Hormones: Mechanism and Physiological Role. Neuropeptides. 2012;46:291–297. doi: 10.1016/j.npep.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Miller G.D. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. Am. J. Lifestyle Med. 2019;13:586–601. doi: 10.1177/1559827617716376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaelberer M.M., Rupprecht L.E., Liu W.W., Weng P., Bohórquez D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020;43:337–353. doi: 10.1146/annurev-neuro-091619-022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., Bohórquez D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science. 2018;361:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng W., Gordian D., Ludwig M.Q., Pers T.H., Seeley R.J., Myers M.G. Hindbrain Circuits in the Control of Eating Behaviour and Energy Balance. Nat. Metab. 2022;4:826–835. doi: 10.1038/s42255-022-00606-9. [DOI] [PubMed] [Google Scholar]
- 23.Cheng W., Gonzalez I., Pan W., Tsang A.H., Adams J., Ndoka E., Gordian D., Khoury B., Roelofs K., Evers S.S., et al. Calcitonin Receptor Neurons in the Mouse Nucleus Tractus Solitarius Control Energy Balance via the Non-Aversive Suppression of Feeding. Cell Metab. 2020;31:301–312.e5. doi: 10.1016/j.cmet.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan C., Zhou J., Feng Q., Zhang J.-E., Lin S., Bao J., Wu P., Luo M. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J. Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy S., Collis Glynn M., Dixon T.N., Grill H.J., McNally G.P., Ong Z.Y. Nucleus of the Solitary Tract A2 Neurons Control Feeding Behaviors via Projections to the Paraventricular Hypothalamus. Neuropsychopharmacology. 2023;48:351–361. doi: 10.1038/s41386-022-01448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lartigue G. Role of the Vagus Nerve in the Development and Treatment of Diet-Induced Obesity. J. Physiol. 2016;594:5791–5815. doi: 10.1113/JP271538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J., Cheng M., Wang L., Zhang L., Xu D., Cao P., Wang F., Herzog H., Song S., Zhan C. A Vagal-NTS Neural Pathway That Stimulates Feeding. Curr. Biol. 2020;30:3986–3998.e5. doi: 10.1016/j.cub.2020.07.084. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H., Williams S.C., Richardson J.A., Kozlowski G.P., Wilson S., et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell. 1998;92:573–585. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 29.Milbank E., López M. Orexins/Hypocretins: Key Regulators of Energy Homeostasis. Front. Endocrinol. 2019;10:830. doi: 10.3389/fendo.2019.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soya S., Sakurai T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020;14:691. doi: 10.3389/fnins.2020.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards C.M., Abusnana S., Sunter D., Murphy K.G., Ghatei M.A., Bloom S.R. The Effect of the Orexins on Food Intake: Comparison with Neuropeptide Y, Melanin-Concentrating Hormone and Galanin. J. Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 32.Gotter A.L., Webber A.L., Coleman P.J., Renger J.J., Winrow C.J. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin Receptor Function, Nomenclature and Pharmacology. Pharmacol. Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- 33.Jin T., Jiang Z., Luan X., Qu Z., Guo F., Gao S., Xu L., Sun X. Exogenous Orexin-A Microinjected Into Central Nucleus of the Amygdala Modulates Feeding and Gastric Motility in Rats. Front. Neurosci. 2020;14:274. doi: 10.3389/fnins.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takamata A., Nishimura Y., Oka A., Nagata M., Kosugi N., Eguchi S., Negishi H., Morimoto K. Endogenous Androgens Diminish Food Intake and Activation of Orexin A Neurons in Response to Reduced Glucose Availability in Male Rats. Nutrients. 2022;14:1235. doi: 10.3390/nu14061235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morello G., Imperatore R., Palomba L., Finelli C., Labruna G., Pasanisi F., Sacchetti L., Buono L., Piscitelli F., Orlando P., et al. Orexin-A Represses Satiety-Inducing POMC Neurons and Contributes to Obesity via Stimulation of Endocannabinoid Signaling. Proc. Natl. Acad. Sci. USA. 2016;113:4759–4764. doi: 10.1073/pnas.1521304113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schalla M.A., Stengel A. The Role of the Gastric Hormones Ghrelin and Nesfatin-1 in Reproduction. Int. J. Mol. Sci. 2021;22:11059. doi: 10.3390/ijms222011059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao Z.-T., Luo Q. Molecular Mechanisms and Health Benefits of Ghrelin: A Narrative Review. Nutrients. 2022;14:4191. doi: 10.3390/nu14194191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Massadi O., López M., Tschöp M., Diéguez C., Nogueiras R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017;40:167–180. doi: 10.1016/j.tins.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 40.Price M.L., Ley C.D., Gorvin C.M. The Emerging Role of Heterodimerisation and Interacting Proteins in Ghrelin Receptor Function. J. Endocrinol. 2021;252:R23–R39. doi: 10.1530/JOE-21-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunez-Salces M., Li H., Feinle-Bisset C., Young R.L., Page A.J. The Regulation of Gastric Ghrelin Secretion. Acta Physiol. 2021;231:e13588. doi: 10.1111/apha.13588. [DOI] [PubMed] [Google Scholar]
- 42.Holubová M., Spolcová A., Demianová Z., Sýkora D., Fehrentz J.A., Martinez J., Stofková A., Jurčovičová J., Drápalová J., Lacinová Z., et al. Ghrelin Agonist JMV 1843 Increases Food Intake, Body Weight and Expression of Orexigenic Neuropeptides in Mice. Physiol. Res. 2013;62:435–444. doi: 10.33549/physiolres.932488. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H., Yin J., Li D., Zhou X., Li X. Tryptophan Enhances Ghrelin Expression and Secretion Associated with Increased Food Intake and Weight Gain in Weanling Pigs. Domest. Anim. Endocrinol. 2007;33:47–61. doi: 10.1016/j.domaniend.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Chen S.-R., Chen H., Zhou J.-J., Pradhan G., Sun Y., Pan H.-L., Li D.-P. Ghrelin Receptors Mediate Ghrelin-Induced Excitation of Agouti-Related Protein/Neuropeptide Y but Not pro-Opiomelanocortin Neurons. J. Neurochem. 2017;142:512–520. doi: 10.1111/jnc.14080. [DOI] [PubMed] [Google Scholar]
- 45.Kim J.D., Leyva S., Diano S. Hormonal Regulation of the Hypothalamic Melanocortin System. Front. Physiol. 2014;5:480. doi: 10.3389/fphys.2014.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruschetta G., Jin S., Kim J.D., Diano S. Prolyl Carboxypeptidase in Agouti-Related Peptide Neurons Modulates Food Intake and Body Weight. Mol. Metab. 2018;10:28–38. doi: 10.1016/j.molmet.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caron J., Domenger D., Dhulster P., Ravallec R., Cudennec B. Protein Digestion-Derived Peptides and the Peripheral Regulation of Food Intake. Front. Endocrinol. 2017;8:85. doi: 10.3389/fendo.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moran T.H., Kinzig K.P. Gastrointestinal Satiety Signals II. Cholecystokinin. Am. J. Physiol. -Gastrointest. Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- 49.Wren A.M., Bloom S.R. Gut Hormones and Appetite Control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 50.Wang L., Ding L., Zhu W., Hang S. Soybean Protein Hydrolysate Stimulated Cholecystokinin Secretion and Inhibited Feed Intake through Calcium-Sensing Receptors and Intracellular Calcium Signalling in Pigs. Food Funct. 2021;12:9286–9299. doi: 10.1039/D1FO01596F. [DOI] [PubMed] [Google Scholar]
- 51.Panda V., Shinde P. Appetite Suppressing Effect of Spinacia Oleracea in Rats: Involvement of the Short Term Satiety Signal Cholecystokinin. Appetite. 2017;113:224–230. doi: 10.1016/j.appet.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 52.Penney C.C., Volkoff H. Peripheral Injections of Cholecystokinin, Apelin, Ghrelin and Orexin in Cavefish (Astyanax Fasciatus Mexicanus): Effects on Feeding and on the Brain Expression Levels of Tyrosine Hydroxylase, Mechanistic Target of Rapamycin and Appetite-Related Hormones. Gen. Comp. Endocrinol. 2014;196:34–40. doi: 10.1016/j.ygcen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Broberger C., Holmberg K., Shi T.J., Dockray G., Hökfelt T. Expression and Regulation of Cholecystokinin and Cholecystokinin Receptors in Rat Nodose and Dorsal Root Ganglia. Brain Res. 2001;903:128–140. doi: 10.1016/S0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- 54.Ndjim M., Poinsignon C., Parnet P., Le Dréan G. Loss of Vagal Sensitivity to Cholecystokinin in Rats Born with Intrauterine Growth Retardation and Consequence on Food Intake. Front. Endocrinol. 2017;8:65. doi: 10.3389/fendo.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang K.S., Yahashi S., Azuma M., Matsuda K. The Anorexigenic Effect of Cholecystokinin Octapeptide in a Goldfish Model Is Mediated by the Vagal Afferent and Subsequently through the Melanocortin- and Corticotropin-Releasing Hormone-Signaling Pathways. Peptides. 2010;31:2130–2134. doi: 10.1016/j.peptides.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Fan W., Ellacott K.L.J., Halatchev I.G., Takahashi K., Yu P., Cone R.D. Cholecystokinin-Mediated Suppression of Feeding Involves the Brainstem Melanocortin System. Nat. Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 57.D’Agostino G., Lyons D.J., Cristiano C., Burke L.K., Madara J.C., Campbell J.N., Garcia A.P., Land B.B., Lowell B.B., Dileone R.J., et al. Appetite Controlled by a Cholecystokinin Nucleus of the Solitary Tract to Hypothalamus Neurocircuit. eLife. 2016;5:e12225. doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt M.K., Richards J.E., Cook D.R., Brierley D.I., Williams D.L., Reimann F., Gribble F.M., Trapp S. Preproglucagon Neurons in the Nucleus of the Solitary Tract Are the Main Source of Brain GLP-1, Mediate Stress-Induced Hypophagia, and Limit Unusually Large Intakes of Food. Diabetes. 2019;68:21–33. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gribble F.M., Reimann F. Metabolic Messengers: Glucagon-like Peptide 1. Nat. Metab. 2021;3:142–148. doi: 10.1038/s42255-020-00327-x. [DOI] [PubMed] [Google Scholar]
- 60.Holst J.J., Deacon C.F. Glucagon-like Peptide-1 Mediates the Therapeutic Actions of DPP-IV Inhibitors. Diabetologia. 2005;48:612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- 61.Lu K., Chen X., Deng X., Long J., Yan J. Potential Role of Hypothalamic and Plasma Ghrelin in the Feeding Behavior of Obese Type 2 Diabetic Rats with Intraventricular Glucagon-Like Peptide-1 Receptor Agonist Intervention. Obes. Facts. 2021;14:10–20. doi: 10.1159/000509956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong X., Zhang H., Liang H., Li D., Huang J., Li Z., Jiang S., Zhang W., Xu G. Exendin-4 Decreases Ghrelin Levels through MTOR Signaling. Mol. Cell. Endocrinol. 2016;437:201–212. doi: 10.1016/j.mce.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 63.Dong Y., Carty J., Goldstein N., He Z., Hwang E., Chau D., Wallace B., Kabahizi A., Lieu L., Peng Y., et al. Time and Metabolic State-Dependent Effects of GLP-1R Agonists on NPY/AgRP and POMC Neuronal Activity in Vivo. Mol. Metab. 2021;54:101352. doi: 10.1016/j.molmet.2021.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh I., Wang L., Xia B., Liu J., Tahiri A., El Ouaamari A., Wheeler M.B., Pang Z.P. Activation of Arcuate Nucleus Glucagon-like Peptide-1 Receptor-Expressing Neurons Suppresses Food Intake. Cell Biosci. 2022;12:178. doi: 10.1186/s13578-022-00914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katsurada K., Maejima Y., Nakata M., Kodaira M., Suyama S., Iwasaki Y., Kario K., Yada T. Endogenous GLP-1 Acts on Paraventricular Nucleus to Suppress Feeding: Projection from Nucleus Tractus Solitarius and Activation of Corticotropin-Releasing Hormone, Nesfatin-1 and Oxytocin Neurons. Biochem. Biophys. Res. Commun. 2014;451:276–281. doi: 10.1016/j.bbrc.2014.07.116. [DOI] [PubMed] [Google Scholar]
- 66.Chen X.-Y., Chen L., Yang W., Xie A.-M. GLP-1 Suppresses Feeding Behaviors and Modulates Neuronal Electrophysiological Properties in Multiple Brain Regions. Front. Mol. Neurosci. 2021;14:793004. doi: 10.3389/fnmol.2021.793004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adrian T.E., Ferri G.L., Bacarese-Hamilton A.J., Fuessl H.S., Polak J.M., Bloom S.R. Human Distribution and Release of a Putative New Gut Hormone, Peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 68.Keire D.A., Whitelegge J.P., Souda P., Faull K.F., Bassilian S., Reidelberger R.D., Haver A.C., Reeve J.R. PYY(1-36) Is the Major Form of PYY in Rat Distal Small Intestine: Quantification Using High-Resolution Mass Spectrometry. Regul. Pept. 2010;165:151–157. doi: 10.1016/j.regpep.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lafferty R.A., Flatt P.R., Irwin N. C-Terminal Degradation of PYY Peptides in Plasma Abolishes Effects on Satiety and Beta-Cell Function. Biochem. Pharmacol. 2018;158:95–102. doi: 10.1016/j.bcp.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Østergaard S., Kofoed J., Paulsson J.F., Madsen K.G., Jorgensen R., Wulff B.S. Design of Y2 Receptor Selective and Proteolytically Stable PYY3-36 Analogues. J. Med. Chem. 2018;61:10519–10530. doi: 10.1021/acs.jmedchem.8b01046. [DOI] [PubMed] [Google Scholar]
- 71.Adrian T.E., Bacarese-Hamilton A.J., Smith H.A., Chohan P., Manolas K.J., Bloom S.R. Distribution and Postprandial Release of Porcine Peptide YY. J. Endocrinol. 1987;113:11–14. doi: 10.1677/joe.0.1130011. [DOI] [PubMed] [Google Scholar]
- 72.Amin A., Neophytou C., Thein S., Martin N.M., Alamshah A., Spreckley E., Bloom S.R., Murphy K.G. L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans. Obesity. 2018;26:1721–1726. doi: 10.1002/oby.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maroni M.J., Capri K.M., Cushman A.V., Deane H.V., Concepcion H., DeCourcey H., Seggio J.A. The Timing of Fasting Leads to Different Levels of Food Consumption and PYY3-36 in Nocturnal Mice. Hormones. 2020;19:549–558. doi: 10.1007/s42000-020-00221-x. [DOI] [PubMed] [Google Scholar]
- 74.Kjaergaard M., Salinas C.B.G., Rehfeld J.F., Secher A., Raun K., Wulff B.S. PYY(3-36) and Exendin-4 Reduce Food Intake and Activate Neuronal Circuits in a Synergistic Manner in Mice. Neuropeptides. 2019;73:89–95. doi: 10.1016/j.npep.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Dischinger U., Hasinger J., Königsrainer M., Corteville C., Otto C., Fassnacht M., Hankir M., Seyfried F.J.D. Toward a Medical Gastric Bypass: Chronic Feeding Studies With Liraglutide + PYY3-36 Combination Therapy in Diet-Induced Obese Rats. Front. Endocrinol. 2020;11:598843. doi: 10.3389/fendo.2020.598843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broberger C., Landry M., Wong H., Walsh J.N., Hökfelt T. Subtypes Y1 and Y2 of the Neuropeptide Y Receptor Are Respectively Expressed in Pro-Opiomelanocortin- and Neuropeptide-Y-Containing Neurons of the Rat Hypothalamic Arcuate Nucleus. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 77.Jones E.S., Nunn N., Chambers A.P., Østergaard S., Wulff B.S., Luckman S.M. Modified Peptide YY Molecule Attenuates the Activity of NPY/AgRP Neurons and Reduces Food Intake in Male Mice. Endocrinology. 2019;160:2737–2747. doi: 10.1210/en.2019-00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Batterham R.L., Cowley M.A., Small C.J., Herzog H., Cohen M.A., Dakin C.L., Wren A.M., Brynes A.E., Low M.J., Ghatei M.A., et al. Gut Hormone PYY(3-36) Physiologically Inhibits Food Intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 79.Batterham R.L., Bloom S.R. The Gut Hormone Peptide YY Regulates Appetite. Ann. N. Y. Acad. Sci. 2003;994:162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 80.Koda S., Date Y., Murakami N., Shimbara T., Hanada T., Toshinai K., Niijima A., Furuya M., Inomata N., Osuye K., et al. The Role of the Vagal Nerve in Peripheral PYY3-36-Induced Feeding Reduction in Rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 81.Abbott C.R., Monteiro M., Small C.J., Sajedi A., Smith K.L., Parkinson J.R.C., Ghatei M.A., Bloom S.R. The Inhibitory Effects of Peripheral Administration of Peptide YY(3-36) and Glucagon-like Peptide-1 on Food Intake Are Attenuated by Ablation of the Vagal-Brainstem-Hypothalamic Pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Obradovic M., Sudar-Milovanovic E., Soskic S., Essack M., Arya S., Stewart A.J., Gojobori T., Isenovic E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021;12:585887. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tartaglia L.A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G.J., Campfield L.A., Clark F.T., Deeds J., et al. Identification and Expression Cloning of a Leptin Receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 84.Friedman J.M., Mantzoros C.S. 20 Years of Leptin: From the Discovery of the Leptin Gene to Leptin in Our Therapeutic Armamentarium. Metabolism. 2015;64:1–4. doi: 10.1016/j.metabol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 85.Friedman J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019;1:754–764. doi: 10.1038/s42255-019-0095-y. [DOI] [PubMed] [Google Scholar]
- 86.de Lartigue G., Ronveaux C.C., Raybould H.E. Deletion of Leptin Signaling in Vagal Afferent Neurons Results in Hyperphagia and Obesity. Mol. Metab. 2014;3:595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y., Rui L. Leptin Signaling and Leptin Resistance. Front. Med. 2013;7:207–222. doi: 10.1007/s11684-013-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morris D.L., Cho K.W., Rui L. Critical Role of the Src Homology 2 (SH2) Domain of Neuronal SH2B1 in the Regulation of Body Weight and Glucose Homeostasis in Mice. Endocrinology. 2010;151:3643–3651. doi: 10.1210/en.2010-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tucker J.A.L., Bornath D.P.D., McCarthy S.F., Hazell T.J. Leptin and Energy Balance: Exploring Leptin’s Role in the Regulation of Energy Intake and Energy Expenditure. Nutr. Neurosci. 2022:1–9. doi: 10.1080/1028415X.2022.2161135. [DOI] [PubMed] [Google Scholar]
- 90.Rautmann A.W., de La Serre C.B. Microbiota’s Role in Diet-Driven Alterations in Food Intake: Satiety, Energy Balance, and Reward. Nutrients. 2021;13:3067. doi: 10.3390/nu13093067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma J., Huangfu W., Yang X., Xu J., Zhang Y., Wang Z., Zhu X., Wang C., Shi Y., Cui Y. “King of the Forage”-Alfalfa Supplementation Improves Growth, Reproductive Performance, Health Condition and Meat Quality of Pigs. Front. Vet. Sci. 2022;9:1025942. doi: 10.3389/fvets.2022.1025942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Z., Du Y., Li N., Geng H., Ali Q., Li X., Gao Y., Wang Y., Xing R., Wu J., et al. Effects of Alfalfa Meal on Quality and Function of Pork Meatballs. Food Sci. Nutr. 2022;10:2601–2610. doi: 10.1002/fsn3.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ali Q., Ma S., La S., Guo Z., Liu B., Gao Z., Farooq U., Wang Z., Zhu X., Cui Y., et al. Microbial Short-Chain Fatty Acids: A Bridge between Dietary Fibers and Poultry Gut Health—A Review. Anim. Biosci. 2022;35:1461–1478. doi: 10.5713/ab.21.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 95.Bolognini D., Dedeo D., Milligan G. Metabolic and Inflammatory Functions of Short-Chain Fatty Acid Receptors. Curr. Opin. Endocr. Metab. Res. 2021;16:1–9. doi: 10.1016/j.coemr.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu J., Lin S., Zheng B., Cheung P.C.K. Short-Chain Fatty Acids in Control of Energy Metabolism. Crit. Rev. Food Sci. Nutr. 2018;58:1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 97.Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., Anastasovska J., Ghourab S., Hankir M., Zhang S., et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zaibi M.S., Stocker C.J., O’Dowd J., Davies A., Bellahcene M., Cawthorne M.A., Brown A.J.H., Smith D.M., Arch J.R.S. Roles of GPR41 and GPR43 in Leptin Secretory Responses of Murine Adipocytes to Short Chain Fatty Acids. FEBS Lett. 2010;584:2381–2386. doi: 10.1016/j.febslet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 99.Psichas A., Sleeth M.L., Murphy K.G., Brooks L., Bewick G.A., Hanyaloglu A.C., Ghatei M.A., Bloom S.R., Frost G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int. J. Obes. 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larraufie P., Martin-Gallausiaux C., Lapaque N., Dore J., Gribble F.M., Reimann F., Blottiere H.M. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018;8:74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z., Yi C.-X., Katiraei S., Kooijman S., Zhou E., Chung C.K., Gao Y., van den Heuvel J.K., Meijer O.C., Berbée J.F.P., et al. Butyrate Reduces Appetite and Activates Brown Adipose Tissue via the Gut-Brain Neural Circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 102.Cook T.M., Gavini C.K., Jesse J., Aubert G., Gornick E., Bonomo R., Gautron L., Layden B.T., Mansuy-Aubert V. Vagal Neuron Expression of the Microbiota-Derived Metabolite Receptor, Free Fatty Acid Receptor (FFAR3), Is Necessary for Normal Feeding Behavior. Mol. Metab. 2021;54:101350. doi: 10.1016/j.molmet.2021.101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ridlon J.M., Harris S.C., Bhowmik S., Kang D.-J., Hylemon P.B. Consequences of Bile Salt Biotransformations by Intestinal Bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monteiro-Cardoso V.F., Corlianò M., Singaraja R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromol. Med. 2021;23:99–117. doi: 10.1007/s12017-020-08625-z. [DOI] [PubMed] [Google Scholar]
- 105.Christiansen C.B., Trammell S.A.J., Wewer Albrechtsen N.J., Schoonjans K., Albrechtsen R., Gillum M.P., Kuhre R.E., Holst J.J. Bile Acids Drive Colonic Secretion of Glucagon-like-Peptide 1 and Peptide-YY in Rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G574–G584. doi: 10.1152/ajpgi.00010.2019. [DOI] [PubMed] [Google Scholar]
- 106.Kuhre R.E., Wewer Albrechtsen N.J., Larsen O., Jepsen S.L., Balk-Møller E., Andersen D.B., Deacon C.F., Schoonjans K., Reimann F., Gribble F.M., et al. Bile Acids Are Important Direct and Indirect Regulators of the Secretion of Appetite- and Metabolism-Regulating Hormones from the Gut and Pancreas. Mol. Metab. 2018;11:84–95. doi: 10.1016/j.molmet.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu S., Marcelin G., Blouet C., Jeong J.H., Jo Y.-H., Schwartz G.J., Chua S. A Gut-Brain Axis Regulating Glucose Metabolism Mediated by Bile Acids and Competitive Fibroblast Growth Factor Actions at the Hypothalamus. Mol. Metab. 2018;8:37–50. doi: 10.1016/j.molmet.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Higashi T., Watanabe S., Tomaru K., Yamazaki W., Yoshizawa K., Ogawa S., Nagao H., Minato K., Maekawa M., Mano N. Unconjugated Bile Acids in Rat Brain: Analytical Method Based on LC/ESI-MS/MS with Chemical Derivatization and Estimation of Their Origin by Comparison to Serum Levels. Steroids. 2017;125:107–113. doi: 10.1016/j.steroids.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Perino A., Velázquez-Villegas L.A., Bresciani N., Sun Y., Huang Q., Fénelon V.S., Castellanos-Jankiewicz A., Zizzari P., Bruschetta G., Jin S., et al. Central Anorexigenic Actions of Bile Acids Are Mediated by TGR5. Nat. Metab. 2021;3:595–603. doi: 10.1038/s42255-021-00398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu X., Li J.-Y., Lee A., Lu Y.-X., Zhou S.-Y., Owyang C. Satiety Induced by Bile Acids Is Mediated via Vagal Afferent Pathways. JCI Insight. 2020;5:e132400. doi: 10.1172/jci.insight.132400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agus A., Planchais J., Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 112.Liu N., Sun S., Wang P., Sun Y., Hu Q., Wang X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021;22:7931. doi: 10.3390/ijms22157931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 114.Reigstad C.S., Salmonson C.E., Rainey J.F., Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Voigt J.-P., Fink H. Serotonin Controlling Feeding and Satiety. Behav. Brain Res. 2015;277:14–31. doi: 10.1016/j.bbr.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 117.Ripken D., van der Wielen N., Wortelboer H.M., Meijerink J., Witkamp R.F., Hendriks H.F.J. Nutrient-Induced Glucagon like Peptide-1 Release Is Modulated by Serotonin. J. Nutr. Biochem. 2016;32:142–150. doi: 10.1016/j.jnutbio.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 118.Tian P., O’Riordan K.J., Lee Y., Wang G., Zhao J., Zhang H., Cryan J.F., Chen W. Towards a Psychobiotic Therapy for Depression: Bifidobacterium Breve CCFM1025 Reverses Chronic Stress-Induced Depressive Symptoms and Gut Microbial Abnormalities in Mice. Neurobiol. Stress. 2020;12:100216. doi: 10.1016/j.ynstr.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heisler L.K., Jobst E.E., Sutton G.M., Zhou L., Borok E., Thornton-Jones Z., Liu H.Y., Zigman J.M., Balthasar N., Kishi T., et al. Serotonin Reciprocally Regulates Melanocortin Neurons to Modulate Food Intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 120.Tiligada E., Wilson J.F. Regulation of α-Melanocyte-Stimulating Hormone Release from Superfused Slices of Rat Hypothalamus by Serotonin and the Interaction of Serotonin with the Dopaminergic System Inhibiting Peptide Release. Brain Res. 1989;503:225–228. doi: 10.1016/0006-8993(89)91668-5. [DOI] [PubMed] [Google Scholar]
- 121.Jones L.A., Sun E.W., Martin A.M., Keating D.J. The Ever-Changing Roles of Serotonin. Int. J. Biochem. Cell Biol. 2020;125:105776. doi: 10.1016/j.biocel.2020.105776. [DOI] [PubMed] [Google Scholar]
- 122.Lee J.-H., Lee J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 123.Natividad J.M., Agus A., Planchais J., Lamas B., Jarry A.C., Martin R., Michel M.-L., Chong-Nguyen C., Roussel R., Straube M., et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018;28:737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 124.Chimerel C., Emery E., Summers D.K., Keyser U., Gribble F.M., Reimann F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014;9:1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fetissov S.O., Legrand R., Lucas N. Bacterial Protein Mimetic of Peptide Hormone as a New Class of Protein- Based Drugs. Curr. Med. Chem. 2019;26:546–553. doi: 10.2174/0929867324666171005110620. [DOI] [PubMed] [Google Scholar]
- 126.Ericson M.D., Schnell S.M., Freeman K.T., Haskell-Luevano C. A Fragment of the Escherichia Coli ClpB Heat-Shock Protein Is a Micromolar Melanocortin 1 Receptor Agonist. Bioorg. Med. Chem. Lett. 2015;25:5306–5308. doi: 10.1016/j.bmcl.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fetissov S.O., Hamze Sinno M., Coëffier M., Bole-Feysot C., Ducrotté P., Hökfelt T., Déchelotte P. Autoantibodies against Appetite-Regulating Peptide Hormones and Neuropeptides: Putative Modulation by Gut Microflora. Nutrition. 2008;24:348–359. doi: 10.1016/j.nut.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Breton J., Legrand R., Akkermann K., Järv A., Harro J., Déchelotte P., Fetissov S.O. Elevated Plasma Concentrations of Bacterial ClpB Protein in Patients with Eating Disorders. Int. J. Eat. Disord. 2016;49:805–808. doi: 10.1002/eat.22531. [DOI] [PubMed] [Google Scholar]
- 129.Molfino A., Amabile M.I., Imbimbo G., Emiliani A., Ramaccini C., Lahaye E., Takagi K., Fetissov S.O. Plasma Enterobacterial ClpB Levels and ClpB- and α-MSH-Reactive Immunoglobulins in Lung Cancer Patients with and without Anorexia. Nutrition. 2020;78:110952. doi: 10.1016/j.nut.2020.110952. [DOI] [PubMed] [Google Scholar]
- 130.Arnoriaga-Rodríguez M., Mayneris-Perxachs J., Burokas A., Pérez-Brocal V., Moya A., Portero-Otin M., Ricart W., Maldonado R., Fernández-Real J.-M. Gut Bacterial ClpB-like Gene Function Is Associated with Decreased Body Weight and a Characteristic Microbiota Profile. Microbiome. 2020;8:59. doi: 10.1186/s40168-020-00837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Legrand R., Lucas N., Dominique M., Azhar S., Deroissart C., Le Solliec M.-A., Rondeaux J., Nobis S., Guérin C., Léon F., et al. Commensal Hafnia Alvei Strain Reduces Food Intake and Fat Mass in Obese Mice-a New Potential Probiotic for Appetite and Body Weight Management. Int. J. Obes. 2020;44:1041–1051. doi: 10.1038/s41366-019-0515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucas N., Legrand R., Deroissart C., Dominique M., Azhar S., Le Solliec M.-A., Léon F., do Rego J.-C., Déchelotte P., Fetissov S.O., et al. Hafnia Alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity. Microorganisms. 2019;8:35. doi: 10.3390/microorganisms8010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tennoune N., Chan P., Breton J., Legrand R., Chabane Y.N., Akkermann K., Järv A., Ouelaa W., Takagi K., Ghouzali I., et al. Bacterial ClpB Heat-Shock Protein, an Antigen-Mimetic of the Anorexigenic Peptide α-MSH, at the Origin of Eating Disorders. Transl. Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dominique M., Breton J., Guérin C., Bole-Feysot C., Lambert G., Déchelotte P., Fetissov S. Effects of Macronutrients on the In Vitro Production of ClpB, a Bacterial Mimetic Protein of α-MSH and Its Possible Role in Satiety Signaling. Nutrients. 2019;11:2115. doi: 10.3390/nu11092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dominique M., Lucas N., Legrand R., Bouleté I.-M., Bôle-Feysot C., Deroissart C., Léon F., Nobis S., do Rego J.-C., Lambert G., et al. Effects of Bacterial CLPB Protein Fragments on Food Intake and PYY Secretion. Nutrients. 2021;13:2223. doi: 10.3390/nu13072223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dominique M., Boulete I., Bole-Feysot C., Leon F., Do Rego J.-C., Fetissov S.O., Déchelotte P., Lambert G., Legrand R., Lucas N. Rôle de la protéine bactérienne ClpB et d’un de ses fragments peptidiques dans la régulation de la prise alimentaire. Nutr. Clin. Métabolisme. 2019;33:23–24. doi: 10.1016/j.nupar.2019.01.258. [DOI] [Google Scholar]
- 137.Breton J., Tennoune N., Lucas N., Francois M., Legrand R., Jacquemot J., Goichon A., Guérin C., Peltier J., Pestel-Caron M., et al. Gut Commensal E. Coli Proteins Activate Host Satiety Pathways Following Nutrient-Induced Bacterial Growth. Cell Metab. 2016;23:324–334. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 138.Manco M., Putignani L., Bottazzo G.F. Gut Microbiota, Lipopolysaccharides, and Innate Immunity in the Pathogenesis of Obesity and Cardiovascular Risk. Endocr. Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 139.Breen D.M., Jagarlapudi S., Patel A., Zou C., Joaquim S., Li X., Kang L., Pang J., Hales K., Ziso-Qejvanaj E., et al. Growth Differentiation Factor 15 Neutralization Does Not Impact Anorexia or Survival in Lipopolysaccharide-Induced Inflammation. iScience. 2021;24:102554. doi: 10.1016/j.isci.2021.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taksande B.G., Chopde C.T., Umekar M.J., Kotagale N.R. Agmatine Attenuates Lipopolysaccharide Induced Anorexia and Sickness Behavior in Rats. Pharmacol. Biochem. Behav. 2015;132:108–114. doi: 10.1016/j.pbb.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 141.Yousefi M., Jonaidi H., Sadeghi B. Influence of Peripheral Lipopolysaccharide (LPS) on Feed Intake, Body Temperature and Hypothalamic Expression of Neuropeptides Involved in Appetite Regulation in Broilers and Layer Chicks. Br. Poult. Sci. 2021;62:110–117. doi: 10.1080/00071668.2020.1813254. [DOI] [PubMed] [Google Scholar]
- 142.Wang X.J., Li D., Jiao H.C., Zhao J.P., Lin H. Lipopolysaccharide Inhibits Hypothalamic Agouti-Related Protein Gene Expression via Activating Mechanistic Target of Rapamycin Signaling in Chicks. Gen. Comp. Endocrinol. 2021;313:113876. doi: 10.1016/j.ygcen.2021.113876. [DOI] [PubMed] [Google Scholar]
- 143.Pradhananga S., Tashtush A.A., Allen-Vercoe E., Petrof E.O., Lomax A.E. Protease-Dependent Excitation of Nodose Ganglion Neurons by Commensal Gut Bacteria. J. Physiol. 2020;598:2137–2151. doi: 10.1113/JP279075. [DOI] [PubMed] [Google Scholar]
- 144.Schwartz G.J., Plata-Salamán C.R., Langhans W. Subdiaphragmatic Vagal Deafferentation Fails to Block Feeding-Suppressive Effects of LPS and IL-1 Beta in Rats. Am. J. Physiol. 1997;273:R1193–R1198. doi: 10.1152/ajpregu.1997.273.3.R1193. [DOI] [PubMed] [Google Scholar]
- 145.Fosset S., Fromentin G., Rampin O., Lang V., Mathieu F., Tomé D. Pharmacokinetics and Feeding Responses to Muramyl Dipeptide in Rats. Physiol. Behav. 2003;79:173–182. doi: 10.1016/S0031-9384(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 146.Williams L., Alshehri A., Robichaud B., Cudmore A., Gagnon J. The Role of the Bacterial Muramyl Dipeptide in the Regulation of GLP-1 and Glycemia. Int. J. Mol. Sci. 2020;21:5252. doi: 10.3390/ijms21155252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gabanyi I., Lepousez G., Wheeler R., Vieites-Prado A., Nissant A., Wagner S., Moigneu C., Dulauroy S., Hicham S., Polomack B., et al. Bacterial Sensing via Neuronal Nod2 Regulates Appetite and Body Temperature. Science. 2022;376:eabj3986. doi: 10.1126/science.abj3986. [DOI] [PubMed] [Google Scholar]
- 148.Blanco A.M., Bertucci J.I., Valenciano A.I., Delgado M.J., Unniappan S. Ghrelin Suppresses Cholecystokinin (CCK), Peptide YY (PYY) and Glucagon-like Peptide-1 (GLP-1) in the Intestine, and Attenuates the Anorectic Effects of CCK, PYY and GLP-1 in Goldfish (Carassius Auratus) Horm. Behav. 2017;93:62–71. doi: 10.1016/j.yhbeh.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 149.Kawai K., Gebremeskel B.G., Acosta C.J. Systematic Review of Incidence and Complications of Herpes Zoster: Towards a Global Perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Merrick B., Allen L., Zain N.M.M., Forbes B., Shawcross D.L., Goldenberg S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020;2:100069. doi: 10.1016/j.infpip.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.