Abstract
Populations with common physical diseases – such as cardiovascular diseases, cancer and neurodegenerative disorders – experience substantially higher rates of major depressive disorder (MDD) than the general population. On the other hand, people living with MDD have a greater risk for many physical diseases. This high level of comorbidity is associated with worse outcomes, reduced adherence to treatment, increased mortality, and greater health care utilization and costs. Comorbidity can also result in a range of clinical challenges, such as a more complicated therapeutic alliance, issues pertaining to adaptive health behaviors, drug‐drug interactions and adverse events induced by medications used for physical and mental disorders. Potential explanations for the high prevalence of the above comorbidity involve shared genetic and biological pathways. These latter include inflammation, the gut microbiome, mitochondrial function and energy metabolism, hypothalamic‐pituitary‐adrenal axis dysregulation, and brain structure and function. Furthermore, MDD and physical diseases have in common several antecedents related to social factors (e.g., socioeconomic status), lifestyle variables (e.g., physical activity, diet, sleep), and stressful live events (e.g., childhood trauma). Pharmacotherapies and psychotherapies are effective treatments for comorbid MDD, and the introduction of lifestyle interventions as well as collaborative care models and digital technologies provide promising strategies for improving management. This paper aims to provide a detailed overview of the epidemiology of the comorbidity of MDD and specific physical diseases, including prevalence and bidirectional risk; of shared biological pathways potentially implicated in the pathogenesis of MDD and common physical diseases; of socio‐environmental factors that serve as both shared risk and protective factors; and of management of MDD and physical diseases, including prevention and treatment. We conclude with future directions and emerging research related to optimal care of people with comorbid MDD and physical diseases.
Keywords: Depression, physical diseases, comorbidity, cardiovascular diseases, cancer, inflammation, lifestyle factors, childhood trauma, collaborative care, digital technologies
Major depressive disorder (MDD) is prevalent within the general population, with an approximate global point prevalence of 4.7% 1 . In populations with common physical diseases – such as cardiovascular diseases 2 , 3 , cancer 4 and neurodegenerative disorders 5 , 6 , 7 , 8 – this prevalence is much higher, with several meta‐analyses reporting MDD rates of up to 41% in selected physical diseases 2 , 3 , 4 , 5 , 6 , 7 , 8 . This relationship is often bidirectional, with both observational and some Mendelian randomization studies demonstrating that MDD and physical diseases can be predictors and outcomes of each other 9 , 10 , 11 , 12 , 13 , 14 .
There are a range of potential explanations for the high level of comorbidity between MDD and physical diseases 15 , 16 , 17 , 18 . Shared genetic and biological pathways suggest that there are numerous pathological mechanisms implicated in both MDD and physical diseases that may increase risk or exacerbate comorbidity 15 , 16 . Furthermore, there are several shared antecedent social, lifestyle and life event risk factors for MDD and physical diseases 17 , 18 . In addition, factors precipitated by one disease can increase the risk of another. For example, motivational impairments present in MDD may affect the ability to exercise and maintain a healthy diet, resulting in an increased risk of physical diseases.
The consequences of this high level of comorbidity are far reaching, with evidence supporting worse outcomes 19 , reduced adherence to treatment 20 , increased mortality 21 , and increased health care utilization and costs 22 , 23 , 24 , 25 , 26 . MDD poses a substantial disease burden, ranking second among leading causes of years lived with disability according to the Global Burden of Disease Study 27 . Using data from the Danish registry and previously published methods 28 , more than one third of the total nonfatal burden (34.4%) in people with MDD was due to comorbid physical diseases, such as respiratory diseases (e.g., asthma and chronic obstructive pulmonary disorder), pain‐related conditions, cardiovascular diseases, and gastrointestinal disorders.
Comorbidity of MDD and physical diseases also introduces several clinical challenges that are often not apparent within the published literature, in which clinical populations can be highly selected. These include a higher prevalence of other mediating or moderating disorders such as substance abuse and personality disorders, a more complicated therapeutic alliance, issues pertaining to adaptive health behaviors 29 , drug‐drug interactions and adverse events induced by medications used for physical and mental diseases.
This paper draws on meta‐analyses and Mendelian randomization studies, as well as on randomized controlled trials (RCTs) where appropriate, to provide a detailed, up‐to‐date overview of: a) the epidemiology of the comorbidity of MDD and physical diseases, including prevalence and bidirectional risk; b) shared biological pathways implicated in the pathogenesis of MDD and physical diseases, c) socio‐environmental factors that serve as shared risk and protective factors; d) clinical management of MDD and physical diseases, including considerations regarding prevention and treatment; and e) future directions and emerging research related to optimal care of people with comorbid MDD and physical diseases.
While this review focuses on, and primarily refers to, MDD and its relation to physical diseases, it is also informed by evidence concerning closely related constructs, such as elevated depressive symptoms, as well as by studies that investigate depression but have not used formalized DSM‐5/ICD‐11 diagnoses of MDD. Furthermore, we use the term “physical diseases” throughout to refer to non‐psychiatric and non‐communicable diseases discussed in the review. We do, however, acknowledge that this is an imperfect definition, as MDD itself can also be considered a physical disease with well‐observed physical mechanisms (as discussed in the paper) and clinical manifestations.
EPIDEMIOLOGY OF THE COMORBIDITY OF MAJOR DEPRESSIVE DISORDER AND SPECIFIC PHYSICAL DISEASES
In this section, we provide an overview of the association between MDD and specific physical diseases as emerging from meta‐analytic data.
MDD has been identified as a risk factor for several physical diseases (see Figure 1), with much evidence suggesting a bidirectional relationship. We explore this further using results from Mendelian randomization studies, which use genetic variation as a natural experiment to investigate the causal relations between potentially modifiable risk factors and health outcomes 30 . This method is arguably less susceptible to known limitations of observational studies such as confounding or reverse causation 30 , thus complementing the extensive observational literature in this area.
Figure 1.
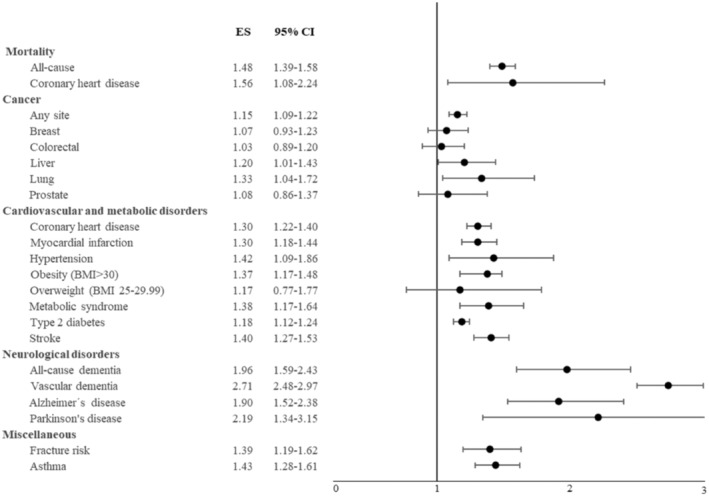
Meta‐analytic data on the risk for mortality and physical diseases among individuals with major depressive disorder compared to people without this condition. ES – effect size (risk ratio or odds ratio), BMI – body mass index (see also supplementary information).
MDD is also highly prevalent in a range of physical diseases (see Figure 2), with an approximate mean aggregate point prevalence of 25%. While this is higher than the general population 1 , 31 , meta‐analyses that have synthesized prevalence estimates often report high heterogeneity (with I2 typically higher than 90%) 32 , 33 , 34 , suggesting that prevalence is highly variable. The influence of factors such as disease stage, severity, setting (e.g., hospital vs. community), timing (e.g., immediate vs. years after disease onset), measurement methods (e.g., self‐report, clinical diagnosis, clinician rating), and definition of MDD used (e.g., clinical cut‐offs vs. elevated symptoms) in determining these estimates should be considered. Such factors are explored in the following disease‐specific sections.
Figure 2.
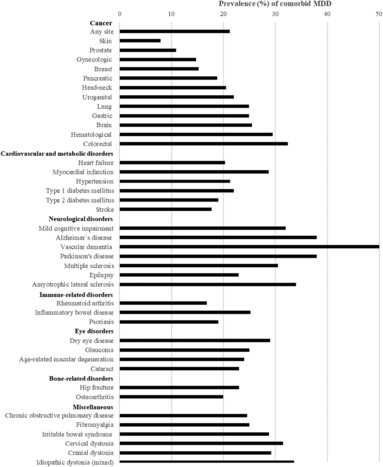
Point prevalence of comorbid major depressive disorder (MDD) in physical diseases, using estimates from published meta‐analyses (see also supplementary information).
Cardiovascular diseases
The point prevalence of MDD after myocardial infarction is reported to be 28.7%, while it is 17.7% after stroke 33 , 35 . Prevalence rates of MDD are influenced by the severity of the comorbid disease 36 . For example, in people with heart failure, MDD rates range from 11% in people with less functional impairment (class 1 according to the New York Heart Association) to 42% in those with severe impairment (class 4) 36 .
Many guidelines and position statements, such as those of the American Heart Association and the European Society of Cardiology 2 , 37 , consider MDD a potentially modifiable risk factor for cardiovascular diseases. Indeed, several meta‐analyses of prospective cohort studies have reported that baseline MDD increases the risk of future cardiovascular events 38 , 39 , 40 , 41 . While previous meta‐analyses have raised concerns regarding a variety of potential confounders 39 , a recent meta‐analysis of Danish registry cohorts that accounted for these confounders reported that MDD diagnosis was associated with higher risk of subsequent ischemic heart disease (hazard ratio, HR: 1.63, 95% CI: 1.36‐1.95) and stroke (HR: 1.94, 95% CI: 1.63‐2.30) 11 . On the other hand, baseline ischemic heart disease (HR: 1.79, 95% CI: 1.43‐2.23) and stroke (HR: 2.62, 95% CI: 2.09‐3.29) were associated with subsequent MDD, demonstrating a bidirectional relationship 11 .
Recent Mendelian randomization studies have indicated that the genetic liability for MDD is associated with an increased risk for coronary artery disease (odds ratio, OR: 1.26, 95% CI: 1.10‐1.43) 42 , small vessel stroke (OR: 1.33, 95% CI: 1.08‐1.65) 43 , and myocardial infarction (OR: 1.15, 95% CI: 1.07‐1.23) 44 , while there is a null association between genetic liability for cardiovascular diseases and subsequent increased MDD risk (see Figure 3) 42 , 43 , 44 .
Figure 3.
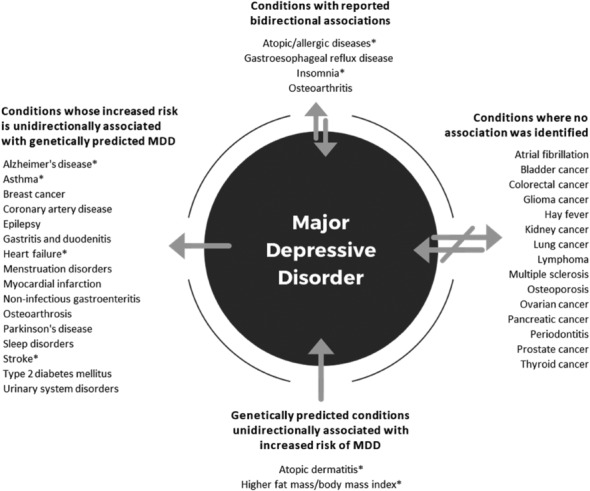
Association between major depressive disorder (MDD) and physical diseases according to Mendelian randomization studies. Asterisks indicate conditions where the evidence is mixed (see also supplementary information).
In people with cardiovascular diseases and stroke survivors, MDD is associated with increased health care costs and unplanned rehospitalizations 23 , 25 , an increased risk of atrial fibrillation and chest pain 2 , and a significant decrease in quality of life 45 , 46 . Furthermore, MDD occurring after a cardiovascular event is associated with poorer adherence to treatments and adaptive lifestyle changes 20 , including attendance and completion of rehabilitation 47 , which improves after resolution of depressive symptoms 48 .
Diabetes mellitus
The point prevalence of MDD is high in both type 1 (22%) and type 2 (19%) diabetes mellitus 32 . People with MDD have a higher risk of type 2 diabetes (risk ratio, RR: 1.18, 95% CI: 1.12‐1.24) 49 and people with type 2 diabetes have a higher risk of MDD (RR: 1.15, 95% CI: 1.02‐1.30) 50 . Previous meta‐analyses of prospective cohort studies suggest a bidirectional association between MDD and type 2 diabetes. However, recent Mendelian randomization studies suggest a unidirectional relationship, with genetic liability for MDD associated with increased risk of type 2 diabetes 42 , 51 .
Comorbid MDD in people with type 2 diabetes is associated with poorer adherence to diabetes treatment 52 and self‐care activities (e.g., exercise, healthy eating) 53 , 54 , increased health care costs 22 , 54 , reduced glycemic control 55 , 56 , and increased hospital admissions and complications 57 , 58 , 59 , 60 . A recent meta‐analysis reported that baseline MDD is associated with an increased risk of incident diabetes‐related complications (HR: 1.14, 95% CI: 1.07‐1.21) 57 . The risk of functional disability is also substantially increased in people with comorbid MDD and diabetes compared to individuals with one disease 58 .
Furthermore, comorbid MDD and diabetes may increase the risk of other physical diseases 59 . For example, a prospective study reported that individuals with diabetes and comorbid MDD had an increased risk of dementia (HR: 2.69, 95% CI: 1.77‐4.07) compared to individuals with diabetes only 41 , 59 .
Metabolic syndrome
The metabolic syndrome includes insulin resistance, central obesity, impaired glucose tolerance, raised triglycerides, reduced high density lipoprotein (HDL) cholesterol, non‐alcoholic fatty liver disease, and hypertension 61 . It is a major risk factor for developing both type 2 diabetes and cardiovascular diseases, as well as for premature mortality 62 .
There is a bidirectional association between MDD and the metabolic syndrome. People with MDD are 1.38 (95% CI: 1.17‐1.64) times more likely than the general population to develop the metabolic syndrome 63 , while people with the metabolic syndrome are 1.49 (95% CI: 1.20‐1.87) times more likely to develop MDD 10 . This association exists in both adults and older people 64 . However, a Mendelian randomization study suggests that genetically predicted MDD is positively associated with the risk of the metabolic syndrome, but that genetically predicted metabolic syndrome is not associated with the risk of MDD 65 .
Individual components of the metabolic syndrome, such as obesity, may also have a bidirectional association with MDD. Meta‐analyses of prospective observational studies report that baseline MDD increases the risk of developing obesity (RR: 1.37, 95% CI: 1.17‐1.48), and baseline obesity increases the risk of onset of future MDD (RR: 1.18, 95% CI: 1.04‐1.35) 66 . However, several recent Mendelian randomization studies have shown that genetically predicted increased body mass index and fat mass are associated with an increased risk of MDD, while the reverse is not true 67 , 68 , 69 , 70 .
Emerging studies also suggest that the metabolic profile can influence the association between obesity and MDD, with a recent meta‐analysis of cross‐sectional studies reporting that metabolically unhealthy obesity was associated with a 30% to 83% increased risk of MDD, whereas obesity with a favorable metabolic profile was not associated with an increase of that risk 71 . Furthermore, one cohort study found that, while the metabolic syndrome overall was not associated with the resolution of MDD symptoms, abnormal circulating triglycerides and cholesterol were associated with a lower likelihood of symptom resolution 72 . This is in keeping with another small case‐control study which found an association between low HDL cholesterol and poorer MDD prognosis 73 .
Cancer
Large meta‐analyses have estimated the point prevalence of MDD in people with cancer to be around 21% 4 , 74 , 75 . However, this estimate is highly variable depending on a range of factors related to disease course (e.g., early vs. advanced stages), treatment time point (acute treatment vs. survivorship), and assessment method (self‐reported or clinical diagnosis) 4 , 74 , 75 .
A previous meta‐analysis demonstrated that prevalence rates of MDD are generally highest during the acute phases of the disease and during treatment (estimates between 14% and 27%) 4 . Prevalence rates at 2‐ and 5‐year post‐treatment generally return to similar estimates as the general population or healthy controls 76 , 77 .
Previous meta‐analyses and large cohort studies have also identified that the prevalence of MDD can substantially vary based on cancer type 78 , 79 , 80 . While there is some inconsistency between studies, hematological, gastrointestinal, lung and gynecological cancers are often identified as having a higher MDD prevalence compared to other types of cancer 78 , 79 , 80 .
A large number of factors have been associated with a greater risk of MDD in people with cancer 81 . A recent systematic review identified a range of somatic (e.g., advanced cancer stage, comorbidities, pain), sociodemographic (e.g., female gender), social (e.g., low socioeconomic status, impaired social support), and psychiatric (e.g., previous history of MDD) factors that were commonly associated with increased MDD risk. Pre‐existing MDD and personality factors such as neuroticism were the most consistently associated 81 .
MDD may modestly increase the risk of cancer onset and mortality. A recent meta‐analysis reported that MDD and anxiety were associated with a significantly increased risk of cancer incidence (RR: 1.13, 95% CI: 1.06‐1.19) and cancer‐specific mortality (RR: 1.21, 95% CI: 1.16‐1.26) 82 . These estimates are similar to a previous meta‐analysis that examined MDD separately from anxiety 83 , 84 .
Mendelian randomization studies suggest that genetically predicted MDD is associated with a slightly increased risk of breast cancer (OR: 1.09, 95% CI: 1.02‐1.17), but not of a range of other cancer types 85 , 86 . Some studies have also reported that MDD may predict lower T‐cell cytokine expression and reduce treatment adherence or initiation 87 , 88 , while improvement in depressive symptoms has been associated with increased survival in people with cancer 89 .
Neurological diseases
MDD is associated with multiple neurological diseases. Meta‐analytic evidence from longitudinal studies indicates that MDD is a meaningful risk factor for future Alzheimer’s disease (RR: 1.90, 95% CI: 1.52‐2.38) 90 , all‐cause dementia (RR: 1.96, 95% CI: 1.59‐2.43) 90 , vascular dementia (RR: 2.71, 95% CI: 2.48‐2.97) 90 , and Parkinson’s disease (RR: 2.20, 95% CI: 1.87‐2.58) 91 . Some authors suggest that MDD may be considered a prodrome of these neurological diseases 92 .
Mendelian randomization studies provide further support to a unidirectional association for some neurological diseases, but not all. Genetically predicted MDD is a risk factor for Parkinson's disease and epilepsy, while there is no evidence for genetically predicted neurological diseases being a risk factor for MDD 93 , 94 . Two Mendelian randomization studies provided contrasting results for MDD and Alzheimer's disease 95 , 96 , and two studies found no association between genetically predicted MDD and multiple sclerosis 97 , 98 .
Meta‐analyses and reviews indicate an overall high point prevalence of MDD in Parkinson’s disease (38%) 34 , epilepsy (22.9%) 5 , migraine (up to 47.9%) 99 , multiple sclerosis (30.5%) 6 , mild cognitive impairment (32%) 7 , and Alzheimer's disease (41%) 8 . MDD is consistently associated with reduced quality of life across several neurological diseases 100 , as well as with increased disability and poorer functioning. For example, MDD is associated with increased seizure frequency in people with epilepsy and excessive daytime sleepiness in Parkinson's disease 101 , 102 .
Furthermore, MDD increases the risk for progression and chronicity 103 , 104 , 105 . For example, the presence of depressive symptoms is associated with faster progression from mild cognitive impairment to Alzheimer's disease 104 . A separate study reported similar results for migraine, where depressive symptoms dose‐dependently increased the risk of progression from episodic to chronic disease 105 .
Osteoporosis
A growing body of evidence shows that MDD is associated with poor bone health 106 , 107 , 108 , 109 . A meta‐analysis pooling the results of 21 cross‐sectional studies involving 1,842 participants with MDD and 17,401 controls found that MDD was associated with lower bone mineral density at the lumbar spine, femur and total hip, with small to medium effect sizes 110 .
A separate meta‐analysis also reported that MDD was prospectively associated with an increased annual bone loss rate of 0.35% (95% CI: 0.18‐0.53), and a 39% increased risk of fracture (RR: 1.39, 95% CI: 1.19‐1.62) 106 . Complicating this, the use of selective serotonin reuptake inhibitors (SSRIs) is independently associated with osteoporosis 107 .
A recent Mendelian randomization analysis failed to substantiate these findings, reporting that a genetic predisposition towards MDD showed no effect on bone mineral density or fracture risk, concluding that reverse causality or residual confounding may be at play 108 . In support to these latter data, there is some evidence that the prevalence of MDD is increased in those with osteoporosis, with a recent meta‐analysis reporting that 23% of older adults with osteoporosis also reported MDD 109 . MDD is also common following fractures, likely due to associated pain and reduced functional status 111 .
Mortality
While both MDD and several physical diseases are associated with independent increases in mortality, their coexistence compounds this risk. For example, a prospective analysis using the UK Biobank (N=499,830) reported that both MDD (HR: 1.26, 95% CI: 1.19‐1.33) and diabetes mellitus (HR: 1.62, 95% CI: 1.52‐1.72) independently increased the risk of mortality; however, the presence of both conditions amplified that risk (HR: 2.16, 95% CI: 1.94‐2.42) 112 . Furthermore, a recent umbrella review found that MDD increased all‐cause or cardiovascular‐related mortality in patients with several physical diseases (i.e., heart failure, coronary heart disease, stroke, cancer, chronic kidney disease, diabetes mellitus) 113 . The associations between MDD and all‐cause mortality among populations with cancer, post‐acute myocardial infarction, and heart failure showed the strongest level of evidence 113 . There is also evidence that increasing levels of psychological distress can confer greater risk of premature death owing to cardiovascular diseases 114 .
Research using Danish registers and the recently introduced life‐years lost metric 115 examined the overall reduction in life expectancy associated with MDD, and explored how different types of physical diseases contribute to this premature mortality 21 . Overall, men and women with MDD lost 8.27 (95% CI: 8.10‐8.47) and 6.40 (95% CI: 6.25‐6.55) years of life respectively, compared to age‐ and sex‐matched controls from the general population. The co‐occurrence of a mood disorder such as MDD and substance use disorders (e.g., alcohol use disorder) had a substantial further impact on premature mortality, with an additional ~6 years lost 116 . The contribution of comorbid cardiovascular disease to premature mortality in those with MDD was comparable in men and women (~1 year), while respiratory diseases accounted for further 0.71 and 0.99 years lost in men and in women respectively.
COVID‐19 and neuropsychiatric sequalae
A global 27.6% (95% CI: 25.1‐30.3) increase in MDD prevalence due to the COVID‐19 pandemic has been estimated 117 , although this finding remains controversial 118 . The long‐term psychiatric and physical disease consequences of the infection or “Long COVID” are currently unclear and an area of emerging research 119 , 120 .
Long COVID has been associated with new onset of a range of physical diseases (e.g., cardiovascular disease, type 2 diabetes) 120 . There also appears to be an increased risk of MDD as well as other mental disorders 121 . However, this risk may be transient and similar to non‐COVID severe respiratory infections 122 .
Furthermore, COVID‐19 infection has also been implicated in several biological processes relevant to MDD and associated physical diseases, such as immune activation, particularly in those with severe acute infection 120 , 123 . Neuroimaging studies in people who have recovered from the infection have also identified numerous small brain changes, including structural and functional alterations within the hippocampus 124 . Continued research is required to elucidate the potential neuropsychiatric sequelae of COVID‐19 infection.
SHARED RISK FACTORS
Lifestyle and behavioral risk factors
To fully understand the comorbidity between MDD and physical diseases, it is crucial to consider the role of health behaviors. In the general population, there is broad acceptance that adverse health behaviors, such as alcohol consumption, tobacco smoking, or illicit drug use can increase the risk of physical diseases and associated mortality 125 , 126 . Additionally, there is strong evidence that low physical activity, poor diet, and poor sleeping patterns are key drivers of subsequent physical diseases.
For instance, the World Health Organization's 2020 Physical Activity Guidelines presented moderate‐certainty evidence of a curvilinear dose‐response relationship between physical activity and risk of all‐cause mortality and multiple life‐threatening physical diseases, including cardiovascular diseases, diabetes mellitus and even cancers 127 . Similarly, striking data on the impact of eating patterns was provided by the 2016 Global Burden of Disease Study 128 , which identified “poor dietary habits” as one of the leading risk factors for mortality worldwide, with almost one fifth of all deaths attributable to it.
While the relationship between sleep and disease is non‐linear, there is a strong indication from large‐scale studies that sleeping problems are a risk factor for common physical diseases 129 , with either too short or too long sleep durations associated with increased mortality risk 130 .
These lifestyle factors are also likely to be a central driver of the heightened rates of physical diseases (and associated mortality) observed in MDD, especially when considering the extensive evidence that people with MDD are affected by the same lifestyle and behavioral health risks 131 , 132 . For instance, systematic reviews have found that people with MDD are significantly more likely to engage in excessive alcohol and tobacco use 131 , 132 , and have a higher total food intake and reduced diet quality 133 , higher levels of sedentary behavior 134 , and poorer sleep continuity and quality 135 , compared to non‐depressed people.
Despite the observed trends, the causality of the relationships between health behaviors and MDD is unclear and likely bidirectional. On the one hand, multiple independent meta‐analyses of prospective data have shown that physical inactivity, tobacco smoking, excessive alcohol consumption, impaired sleep, and poor diet at baseline are all associated with a subsequently increased risk of developing MDD 136 , 137 . On the other hand, developing MDD can have a pronounced detrimental impact on an individual's health behaviors, including sleep impairment, low motivation for physical activity, over/under‐eating, and a propensity to self‐medicate with tobacco, alcohol or substance use 138 , 139 .
MDD is also associated with reduced adherence to treatment for chronic diseases, which may further exacerbate disease outcomes 140 . Furthermore, certain medications used to treat MDD may induce behavioral risks. For instance, the appetite‐increasing effects of medications such as mirtazapine and quetiapine may partially account for the increased risk of obesity and cardiometabolic diseases among people treated with these medications 141 , 142 , while the sedative effects of agents such as mirtazapine and tricyclic antidepressants (e.g., amitriptyline, clomipramine) 138 could inhibit individuals from engaging in regular physical activity.
Stressful life events
Life stressors can have negative consequences on both mental and physical health across the lifespan. Research on early life stress – often referred to as childhood adversity or adverse childhood experiences – primarily focuses on experiences of maltreatment (e.g., abuse or neglect) and household dysfunction (e.g., domestic violence or parental mental illness) 143 , 144 . For instance, accumulating evidence from several meta‐analyses of both retrospective and prospective studies suggests that adverse childhood experiences are related to a more than two‐fold increase in the risk of developing MDD in adulthood 143 , 145 .
In parallel, a recent meta‐review of 16 meta‐analyses indicated moderate associations between adverse childhood experiences and respiratory diseases (d=0.44), gastrointestinal diseases (d=0.38), neurological diseases and pain (d=0.34), and cardiovascular diseases (d=0.32), as well as weak associations with cancer (d=0.24), diseases of the musculoskeletal system (d=0.21), and endocrine and metabolic diseases (d=0.17) in adulthood 144 .
Adverse childhood experiences are additionally associated with a higher likelihood of experiencing further severe stressful life events later in life (e.g., losing one's job or divorce) 146 , 147 , 148 , 149 . Notably, severe stressful life events frequently precede the onset of a first episode of MDD 150 . Furthermore, a meta‐analysis of six RCTs 151 suggests that, although severe stressful life events affect the prognosis of individ‐uals seeking treatment for MDD, these effects are largely shared with environmental factors (e.g., social support or employment status) that may be a consequence of the experience of trauma.
Severe stressful life events are also associated with an increased risk of physical diseases, particularly cardiovascular diseases 152 . Adults from the general population who experienced a stressful life event had a 1.1 to 1.6‐fold elevated risk of incident coronary heart disease and stroke 152 . Stressful life events can also act as a disease trigger among individuals at risk for cardiovascular diseases, and as a factor aggravating the prognosis of these diseases 152 . A further consideration is that physical diseases and their related symptoms (e.g., pain, fatigue), as well as treatment‐related factors (e.g., surgery, medication side effects), can be a stressful life event accompanied by feelings of grief, stress, shame, and other negative psychological states that can exacerbate or increase the risk of MDD.
It is important to note that not all individuals who experience life stress develop MDD and/or a physical disease 153 , 154 . Indeed, a meta‐analysis of cross‐sectional studies showed that resilience (i.e., the ability to successfully adapt to difficult, challenging or disruptive life events) significantly mediated the association between adverse childhood experiences and symptoms of MDD 155 . Likewise, social connection and belongingness, adaptive lifestyle behaviors, positive parenting, and supportive relationships from carers, friends and within the community are all resilience‐promoting factors that may have a protective effect on an individual's risk for MDD following adverse childhood experiences 156 , 157 .
Social risk factors
Reducing the burden of disease related to MDD and poor physical health requires the focus to move beyond individual risk and protective factors to consider the social determinants of health, i.e., “the conditions in which people are born, grow, live, work and age” 158 . In this context, risk and protective factors cluster and are interwoven at multiple levels. Some occur at different times, while others persist across the life course 159 .
There is clear evidence that both MDD and physical diseases are more common in people from disadvantaged backgrounds 160 , 161 . Both absolute poverty (i.e., level of income necessary to maintain basic living standards) and relative poverty or deprivation (i.e., level of income necessary to maintain minimum living standards relative to those of a society or country) have independent, adverse impacts on mental and physical health. Indigenous people, those from cultural or linguistic minorities, migrants or refugees, and people with a disability are more likely to experience socioeconomic disadvantage than other individuals in the community 162 . Intergenerational poverty and trauma are also common and confer an additional risk to family members of parents who live in poverty.
Other common social determinants that intersect with the aforementioned variables include gender inequality and restrictive gender norms, which, in many settings, privilege the male or masculine over the female or feminine 163 . Discrimination, marginalization and victimization linked to gender are associated with a greater risk of experiencing poor mental and physical health. This appears to be mediated through exposure to stress‐related experiences, but may be also driven by gender‐specific disparities in access to education, home ownership, and safety in the home and employment (women and girls), or an over‐representation in the criminal justice system and reduced access to health care (men and boys) 164 .
Racial, ethnic or sexual minority status is associated with higher rates of health problems, through experiences of discrimination and systemic biases 165 . Structural racism, cultural racism 166 and inter‐generational trauma can also impact on mental and physical health. As with gender norms, norms related to race become embedded in later childhood and adolescence, and the effects persist across the life course 167 .
The above social determinants exert their impacts on mental and physical health through multiple inter‐related mechanisms. Effects on health may be direct (e.g., through restricted access to quality nutrition) or mediated through individual (e.g., security provided by safe housing and/or neighborhoods), relational (e.g., exposure to parental stress in childhood; presence of positive peer relationships in adolescence), psychological (e.g., effects on self‐efficacy), or institutional (e.g., neighborhood disadvantage, access to health care) factors 168 . These factors interact in complex ways with other social determinants (e.g., gender inequality, exposure to hazardous work, child labor). For example, low social status because of poverty may be associated with discrimination and other disadvantages (e.g., exposure to violence, social isolation or loneliness), all of which are associated with poor mental and physical health 169 , 170 , 171 , 172 .
Such processes also have a developmental and transgenerational aspect 161 . The impacts of exposure to adversity may differ according to developmental periods, and health impacts may also vary by type of adversity. Children and adolescents raised in poverty may be less likely to accumulate the “health capital” that contributes to educational attainment, health literacy, a healthy parent‐child attachment style, positive peer relationships, the development of social and emotional skills, and the ability to parent later in their own life 173 . As a consequence, early life poverty contributes to intergenerational cycles of poverty and transmission of mental and physical health risks 173 . In contrast, protective factors such as access to resources (e.g., education), consistent relationships (i.e., supportive and stable families), and social and policy factors (e.g., access to affordable health care, social welfare) may assist individuals to overcome the impacts of adversity 174 .
SHARED BIOLOGICAL MECHANISMS
Several biological pathways are implicated in the pathogenesis of both MDD and physical diseases (see Figure 4). Here, we first provide a conceptual overview of how these shared pathways contribute to disease outcomes, and then discuss several prominently investigated biological mechanisms. Pathogenesis is unlikely to be driven by any singular pathway alone, but rather by the interaction of multiple pathways affecting both mental and physical health.
Figure 4.
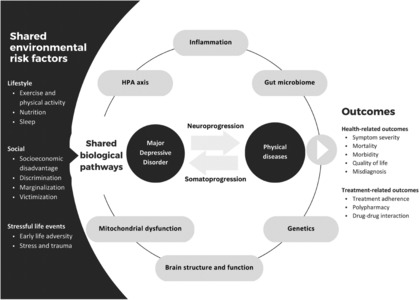
Environmental and biological factors influencing the comorbidity between major depressive disorder and physical diseases. HPA – hypothalamic‐pituitary‐adrenal.
Neuroprogression and somatoprogression
The term “neuroprogression” refers to the process of psychiatric disease acceleration and its underlying operative factors, including reduced neurogenesis and increased apoptosis as well as hypothalamic‐pituitary‐adrenal (HPA) axis dysfunction, immune and oxidative stress, and mitochondrial dysfunction. Its manifestations, such as impaired cognitive function and structural neuroimaging changes, and consequent deteriorating function and declining treatment response, tend to increase with stage 175 , 176 .
The same pathways (e.g., inflammation, oxidative stress, mitochondrial dysfunction) that are involved in neuroprogression of MDD have a parallel role in the genesis and progression of many physical comorbidities, including cardiovascular diseases and the metabolic syndrome. The term “somatoprogression” refers to these pathways and the accumulation of a physical comorbidity that often occurs in parallel to neuroprogression. This construct overlaps with that of allostatic load, which encompasses biological effects secondary to the aggregate burden of stress and wear and tear on the body 177 .
The above two parallel processes provide a theoretical foundation for the comorbidity across MDD and physical diseases. Understanding these processes also provides a mechanistic foundation for the construct of clinical staging 178 . Many of the individual elements of progression – such as inflammation 179 , oxidative stress 180 , and neurogenesis 181 – are also individually targetable and potentially plastic.
Genetics
Both MDD and several physical diseases have a substantial genetic component. For example, family and twin studies suggest that the genetic contribution to MDD accounts for approximately 37% of the variation in susceptibility 182 . Similar rates are estimated for physical diseases such as coronary artery disease (~43%) 183 and stroke (~38%) 184 . Furthermore, several large meta‐analyses of genome‐wide association studies (GWAS) have identified genetic loci associated with MDD 185 as well as with many physical diseases, such as obesity 186 , type 2 diabetes mellitus 187 , and heart disease 188 .
There are several shared genetic factors between MDD and physical diseases. For example, in a large UK study, significant genetic correlations were identified between MDD and body mass index, coronary artery disease, and type 2 diabetes mellitus 189 . The significant genetic overlap between MDD and cardiometabolic conditions, in particular coronary artery disease and obesity, has been confirmed in other studies 190 . In contrast, a large study by the Brainstorm Consortium reported little genetic overlap between common neurological diseases (such as Alzheimer's disease, epilepsy, multiple sclerosis, and Parkinson's disease) and psychiatric diseases including MDD 191 .
A recent systematic review identified 24 pleiotropic genes that are shared between mood disorders and cardiometabolic conditions 192 . Shared genetic pathways were detected between type 2 diabetes mellitus, cardiovascular disease, obesity and MDD, relating to axonal guidance (e.g., glycogen synthase kinase‐3 beta, insulin‐like growth factor‐1), corticotropin releasing hormone, and 5ʹ adenosine monophosphate‐activated protein kinase signaling 192 .
Hypothalamic‐pituitary‐adrenal axis
Stress is a major precipitating factor for the onset and progression of psychiatric disorders, including MDD. HPA axis dysregulation has been implicated in the onset, symptom profile, severity, chronicity, treatment response, and treatment resistance in MDD 193 , 194 , 195 , 196 . A large meta‐analysis reported that individuals with MDD tend to display elevated cortisol (d=0.33, 95% CI: 0.21‐0.45) and adrenocorticotropic hormone (ACTH) (d=0.27, 95% CI: 0.00‐0.54) levels 195 .
HPA axis dysregulation in MDD becomes more pervasive with age. For example, basal cortisol is elevated during all phases of the diurnal cycle in older adults with MDD (g=0.88, 95% CI: 0.60‐1.15) 197 . This is noteworthy, as late‐life MDD is associated with immune dysregulation and high rates of comorbid physical diseases 197 and consequent polypharmacy.
Mechanistically, the signal transduction of glucocorticoids is involved in an array of behavioral, cardiovascular, cognitive, immunological, metabolic and reproductive processes 198 , 199 . According to longitudinal data from a large cohort study 200 , increased levels of hair cortisol were predictive of MDD somatic symptoms. Furthermore, the results of a meta‐analysis 195 support the notion that HPA axis hyperactivity is a link between MDD and comorbid physical diseases, such as diabetes mellitus, dementia, coronary heart disease, and osteoporosis. This link seems to be particularly pronounced in people who present with melancholic or psychotic features 195 . It is, however, worth noting that there are several other pathways involved in the stress response that may be relevant to the comorbidity between MDD and some physical diseases, including the renin‐angiotensin system 201 .
Unfortunately, despite the apparently common co‐occurrence of HPA axis dysregulation, MDD and comorbid physical diseases, few clinical studies have specifically investigated their interplay. Exclusion criteria have been often applied to people with both MDD and a comorbid physical disease in clinical trials.
There is some indication that sex‐specific differences in HPA axis dysregulation exist in humans. However, the relevant evidence is somewhat contradictory (possibly due to variability in menstrual cycle stage, health, age, or stress modality) 202 . This area is still largely under‐researched.
Inflammation
It is generally appreciated that MDD is associated with inflammation 203 , at least in a proportion of individuals (~30‐50%) 204 . In large meta‐analyses, MDD has been related to the up‐ or down‐regulation of acute‐phase reactants 205 , cytokines 206 and chemokines 207 . Low‐grade inflammation – as indexed by a concentration of C‐reactive protein (CRP) higher than 3 mg/L – is more likely in individuals with depression than in matched controls, occurring in around a quarter of the former according to a large meta‐analysis (OR: 1.46, 95% CI: 1.22‐1.75) 205 .
Chronic low‐grade inflammation is also a feature of a variety of physical diseases (e.g., cardiovascular, metabolic and respiratory diseases; cancer, osteoporosis, arthritis) as well as of other serious mental disorders 208 , 209 , 210 , 211 . In both atherosclerotic conditions and depressive episodes, a pro‐inflammatory state can be induced by hypercortisolemia, reduced paraoxonase‐1 levels, as well as reduced HDL and elevated low‐density lipoprotein (LDL) cholesterol, leading to endothelial injury and the downstream release of interleukin‐6 (IL‐6), CRP, tumor necrosis factor‐alpha (TNFα), and soluble endothelial adhesion molecules 211 . Activated immune cells release IL‐1β, stimulating the production of interferon gamma and TNFα, which are commonly elevated in MDD, cardiovascular diseases, metabolic diseases such as diabetes mellitus, and autoimmune conditions such as rheumatoid arthritis 212 .
Data‐driven GWAS analysis supports the association between MDD and immune disorder liability. A recent study (N=500,199) found that MDD was positively correlated with Crohn's disease, ulcerative colitis, hyperthyroidism and asthma (Z‐scores: 0.09‐0.19, q<0.05) 213 . The most robust association was observed for asthma (OR: 1.25, 95% CI: 1.13‐1.37) 213 . IL‐4 is a major cytokine involved in asthma, and is associated with a T helper (Th)‐2 cell response 212 . In MDD, the induction of M1 macrophage cells may lead to IL‐4 production via the compensatory immune‐regulatory system (CIRS) Th‐2 response 212 . Another point of possible overlap is in elevation of highly pro‐inflammatory Th‐17 cells, which are implicated in autoimmune disorders 212 . Emerging evidence supports a role for Th‐17 cells in the genesis and progression of MDD 214 , 215 . This suggests that there may be a subgroup of MDD people with a “lymphoid immunophenotype” (adaptive immune response), contrasting with the innate‐immune response myeloid immunophenotype 204 .
Mitochondrial function and energy metabolism
Mitochondrial function is widely recognized as a factor in the pathophysiology of several psychiatric disorders, including MDD 216 , and a variety of physical conditions, such as metabolic diseases 217 , cardiovascular diseases 218 , and neurodegenerative disorders 219 .
Mitochondria are dynamic organelles that generate adenosine triphosphate (ATP) and are involved in calcium homeostasis, as well as playing key roles in the redox state of the cell and apoptosis. For example, mitochondrial dynamics substantially affect cardiomyocyte health, with multiple rodent studies showing that alterations to processes such as fusion and fission can lead to cardiomyopathy, hypertension, atherosclerosis and heart failure 220 . ATP production is also impaired in people with MDD compared with healthy controls 221 , 222 . Preclinical models of MDD suppress mitochondrial function 223 . In humans, there is evidence of reduced mitochondrial respiration 221 and neuroimaging evidence of decreased energy generation 224 in MDD.
Oxidative stress occurs when there is an excess of reactive oxygen species, which are predominantly produced by mitochondria during the process of respiration, and especially when respiration is inefficient. While reactive oxygen species are required by cells and play a role in processes such as cell signaling, a sustained excess of these species can cause damage to DNA and various cellular structures 225 . There is a wealth of evidence that oxidative stress is associated with both MDD 227 and several physical conditions, such as insulin resistance 217 and cardiovascular diseases 218 , 227 .
Mitophagy is the selective degradation of dysfunctional/damaged mitochondria, and is a crucial process for optimal cellular function and in the adaptation to cellular stress. Adequate mitophagy is not only required for optimal ATP production, but also to reduce oxidative stress, and impairments to mitophagy have been associated with both MDD and physical diseases such as cardiovascular diseases 228 , 229 and neurodegenerative disorders 230 . For example, insufficient mitophagy has been shown to have a role in the development of atherosclerosis, which is partly mitigated by inflammatory processes, and could contribute to cardiomyopathy, heart failure, and myocardial infarction 231 .
Gut microbiome
The gut microbiome, increasingly implicated in MDD and other psychiatric disorders 232 , as well as in several physical diseases, may potentially underpin their interactions. The microbiome affects the gut‐brain axis through several of the aforementioned shared mechanisms, i.e. regulating physiological homeostasis via the autonomic nervous system and the HPA axis, and signaling within and between the enteric and central nervous systems via neuromodulatory metabolites and immunomodulatory responses 233 .
There is overlap in the relevant mechanistic pathways across MDD and physical diseases. Prime amongst these is the physical maintenance of the tight‐junction integrity of the intestinal epithelium, which contains immune signaling pathways and is mediated by the microbiome and its metabolites 234 . Disruptions to the gut epithelial cell wall and transfer of microorganism‐associated molecular patterns, such as lipopolysaccharides (LPS), cause an immune cascade through the activation of toll‐like receptors (TLRs) and inflammatory responses, with flow‐on effects to blood‐brain barrier function and neuroinflammation 235 , 236 . In addition to a compelling body of pre‐clinical evidence 237 , plasma biomarkers of increased gut permeability, including LPS and zonulin, have been found in greater abundance in people with depressive disorders compared to healthy controls 238 .
Evidence of bacterial translocation from the gastrointestinal tract to systemic circulation has been observed within several organs and tissues and is considered contributory to a range of physical diseases. For example, atherosclerotic plaques have microbial communities resembling the gut and oral microbiomes. The resulting immune activation may contribute to the pathophysiology of plaques in the context of cardiovascular disease 239 . In the metabolic syndrome, systemic LPS activates a TLR4‐mediated inflammatory response and alters insulin signaling within white adipose tissue 240 . Increased osteoclastic activity and reduced bone mineral density have been observed following increased intestinal permeability in the context of osteoporosis 241 . Evidence of serum and plasma IgG against periodontal bacteria in human and animal studies of Alzheimer's disease has also supported the systemic and neurological relevance of the oral microbiome 242 .
Microbial metabolites – most notably, short‐chain fatty acids, trimethylamine N‐oxide and bile acids – have cell‐specific effects on the central nervous system as well as on peripheral organs involved in MDD comorbidities 233 . The strength of evidence for microbial causation varies across conditions, being relatively stronger in the metabolic syndrome. For example, germ‐free mice are resistant to the obesogenic effects of high‐fat diets 243 , whilst wild type and germ‐free mice experience metabolic alterations from microbiota‐modulating antibiotic and fecal microbiota transplant interventions 244 , 245 , 246 , 247 . However, this link is less established in osteoporosis and cancers outside of colorectal cancer 241 , 248 . Larger longitudinal cohort and intervention studies are required to translate pre‐clinical observations across all diseases.
Brain structure and function
Severe emotional distress can directly or indirectly (e.g., through functional reorganization of associated neural networks) affect neural substrates that are key in modulating depressive symptoms 249 , including hippocampus, amygdala, hypothalamus, insula, striatum, and medial and orbitofrontal as well as anterior cingulate cortices 250 , 251 , 252 . Physical diseases (e.g., stroke, brain tumors, multiple sclerosis, Alzheimer's disease and Parkinson's disease), as well as lesions or neurodegeneration induced by such diseases, can similarly affect these neural substrates via disease‐specific pathology or indirectly via elevated emotional distress (e.g., at time of diagnosis and adjustment).
Common neural circuitries can also emerge from shared underlying biological mechanisms. These constitute either common underlying mechanisms influencing the liability to both MDD and physical diseases, or mediating mechanisms in causal relationships between MDD and physical diseases. Autonomic, immunoinflammatory and neuroendocrine dysregulations influence the brain's homeostatic, cognitive, reward and emotional circuitries 253 . The insula, the hypothalamus (particularly the paraventricular nucleus) and the anterior cingulate cortex play a critical role in monitoring the body's homeostatic state. Deficiencies in immunological, glucocorticoid and metabolic (e.g., leptin, insulin) signaling affect the activity of these interoceptive regions and their connectivity with core emotional, cognitive and motivational brain regions 254 .
Alterations in interoceptive regions are associated with “sickness behavior”, characterized by lack of energy, weakness, hyperalgesia, loss of appetite and insomnia, commonly associated with both MDD and physical diseases such as cancer 255 , 256 , as well as symptoms of increased appetite, energy balance disturbances and hypersomnia, which are shared between atypical MDD and metabolic diseases including obesity, the metabolic syndrome and diabetes mellitus 15 , 257 . Deficiencies in endocrine and immunological signaling via interoceptive pathways can also lead to interruptions in dopamine signaling in the brain's reward and motivation circuitries 258 , most notably in the orbitofrontal and ventromedial prefrontal cortex, ventral tegmental area and ventral striatum 259 , 260 . An extensive literature implicates shared alterations in the reward circuitry in MDD, neurodegenerative disorders, and obesity 261 , 262 , 263 .
The interoceptive network receives afferent projections from the vagus nerve via the nucleus tractus solitarius and the thalamus 264 , thereby receiving information from respiratory, cardiac and gastric sources. A frontal‐vagal brain network – including the medulla of brainstem, hypothalamus, amygdala, insula, as well as dorsolateral prefrontal, anterior cingulate and orbitofrontal cortex – has been proposed to link cardiovascular diseases, metabolic diseases and MDD, because of its influence on the cardiovascular system, mood, appetite and sleep 265 .
Finally, hippocampal atrophy is shared across MDD and many physical diseases. Impairment of hippocampal neurogenesis, neuroplasticity and dendritic remodeling is critically linked to several physical conditions 266 , 267 , 268 . On the other hand, lower hippocampal volume is one of the most consistently reported structural brain abnormalities in MDD 250 , 269 . The hippocampus is part of the brain's default mode network. Grey matter and functional connectivity of this network are commonly affected in MDD and neurological diseases 270 , 271 .
CLINICAL MANAGEMENT
Diagnosis of comorbid MDD and physical diseases
Diagnosing comorbid MDD in people with physical diseases can be challenging, as several depressive symptoms overlap with symptoms of these diseases (e.g., fatigue, aching, sleep disturbances, appetite and weight changes), thus showing poorer sensitivity and specificity in this context. Furthermore, grief and distress due to physical diseases are frequent, particularly in severe disease states (e.g., terminal cancer), and can result in clinical difficulties to distinguish between adjustment reactions or “appropriate sadness” and MDD 272 . For example, a study reported that only half of individuals with MDD and diabetes mellitus were recognized as having depression during standard care and, out of those correctly identified, few received adequate treatment 273 .
Similar complexities are present for the appropriate diagnosis of physical diseases in people with MDD. This has been termed “diagnostic overshadowing”, describing the tendency for clinicians to misattribute physical symptoms (e.g., pain) to a person's mental disorder rather to a potential comorbid physical disease 274 .
Prevention of comorbid MDD
Interventions aimed to prevent MDD have been explored in people with at‐risk physical diseases. A Cochrane review 275 found very low‐certainty evidence from ten RCTs supporting the use of antidepressant medications in the prevention of MDD. Similar results have been reported by systematic reviews of trials exploring antidepressant medications as a means for preventing MDD related to administration of interferon alpha. However, due to the limited evidence base, tolerability and acceptability of preventive antidepressant use has not been rigorously assessed. Further research is required to ensure that the benefits of prophylactic interventions outweigh potential medical (e.g., side effects) and financial considerations.
Preventive psychotherapy interventions have been similarly understudied. The previously cited Cochrane review 275 identified only one trial (N=193), which examined problem‐solving therapy in age‐related macular degeneration, and found lower odds for developing MDD compared with treatment‐as‐usual (OR: 0.43, 95% CI: 0.20‐0.95). A recent meta‐analysis of RCTs of psychotherapy – mostly cognitive‐behavioral therapy (CBT) – as a preventive intervention for MDD found positive results, including for a sub‐sample of people with physical diseases (n=11; RR: 0.71) 276 . A systematic review of five RCTs evaluating the effectiveness of psychotherapy in preventing MDD in adults with cancer found that it was superior to usual care (standardized mean difference, SMD: –0.23). In a cohort of people with breast cancer, findings were similarly favorable (SMD: –0.32) 277 .
However, a large RCT in people with cardiovascular disease and/or diabetes showed that there was no significant effect of a CBT‐based preventive program. Four risk factors predicted MDD at follow‐up: baseline anxiety and MDD symptoms, stressful life events, and the presence of three or more chronic diseases 278 . It may be that preventive programs will be more effective if targeted at high‐risk cohorts such as those with high subclinical depressive symptoms (indicated prevention) or other MDD risk factors (selective prevention).
In summary, proactive treatment to prevent MDD in at‐risk individuals with physical diseases may be a viable approach, but large high‐quality RCTs are needed.
Treatment of comorbid MDD
Among individuals with MDD and a physical disease, systematic reviews of RCTs have shown that antidepressants, compared to placebo, show effect sizes similar to or even larger (i.e., SMDs higher than 0.50) 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 than those for MDD without physical comorbidity, where SMDs range between 0.17 and 0.49 287 . Such effect sizes have been reported for MDD comorbid with cardiovascular diseases (e.g., coronary artery disease 281 , ischemic heart disease 282 , myocardial infaction 288 ), neurological diseases (e.g., multiple sclerosis 279 , Parkinson's disease 289 , stroke 290 , 291 ), diabetes mellitus 292 , cancer 286 , 293 , rheumatoid arthritis 280 and human immunodeficiency virus (HIV) infection 294 . Whether these larger effect sizes are due to differing biological processes, smaller placebo effects, or other reasons such as small‐study inflation, needs further study. Indeed, most meta‐analyses were based on a few small RCTs.
In other diseases – such as epilepsy, inflammatory bowel disease, traumatic brain injury, asthma and chronic obstructive pulmonary disease – few or no RCTs of antidepressant treatment for comorbid MDD have been conducted 295 , 296 , 297 , 298 , 299 , 300 , 301 , resulting in a sparse evidence base for treatment recommendations.
Many studies have demonstrated that psychotherapies 302 – including CBT 303 , 304 , 305 , mindfulness‐based interventions 306 , 307 , 308 , compassion‐focused therapies 309 , 310 and problem‐solving therapy 311 – effectively treat MDD in people with diseases such as cancer 307 , 308 , diabetes mellitus 312 , 313 , cardiovascular diseases 314 , 315 , 316 , 317 , 318 , HIV infection 319 , psoriasis 320 , multiple sclerosis 279 , 321 , 322 , inflammatory bowel disease 305 , chronic obstructive pulmonary disease 323 , 324 , 325 , and kidney failure 326 , 327 , 328 .
Regardless of intervention type, effect sizes are generally low to moderate 309 , and many individual studies are at risk of bias 309 , have low sample sizes, and use heterogenous research designs 310 . Findings concerning cardiovascular diseases are more robust, particularly in people with heart failure. An umbrella review concluded that there is sound evidence that psychotherapy can treat MDD in people with ischemic heart disease, based on the findings of four systematic reviews 318 . Similarly, in a scoping review of nine psychotherapy trials, seven showed significant reductions in MDD symptoms, although two did not maintain benefit at longer‐term follow‐up314.
Psychotherapy can also be delivered online or via telephone to people with physical diseases, with comparable outcomes to face‐to‐face delivery 303 , 304 , 322 , 329 , particularly if clinician‐guided 303 . These modalities have also been shown to be acceptable to individuals 330 , 331 , which is particularly important for those who may have mobility or accessibility difficulties 322 .
Effect of MDD treatments on physical disease outcomes
In addition to improving depressive symptoms, antidepressant medication may have positive effects on physical disease outcomes. For example, a recent umbrella review found that SSRIs may improve fasting glucose/HbA1c and pain 332 , and may reduce hospitalization rates in coronary artery disease 281 . Among individuals with diabetes mellitus, antidepressant treatment is reported by RCTs to improve glycemic control 292 , and is associated with lower mortality 333 and a lower risk for myocardial infarction 334 . Furthermore, antidepressants improve motor function and disability after stroke 290 , and motor symptoms in Parkinson's disease 289 .
There is also tentative evidence that psychotherapies may improve physical health‐related quality of life and fasting glucose/HbA1C 332 , and have a positive impact on physical outcomes in people with ischemic heart disease 318 . However, results are limited by the low quality of trials, and recent advances in medical care may have outweighed previously demonstrated benefits of psychotherapy 318 . A systematic review found that the effect of psychotherapy on disease activity in people with inflammatory bowel disease was not clear 305 .
A systematic review focusing on people with rheumatic conditions reported that CBT led to reduction of pain severity in four of seven studies, and to significant reduction of fatigue in one of four studies 329 . Psychotherapy may also lead to increased engagement in lifestyle behaviors that positively influence physical health 327 , 335 . For example, CBT has been found to improve medication adherence in people undergoing dialysis 327 . However, it is not yet known whether these changes translate into improved physical outcomes 327 .
Effect of physical disease treatments on MDD outcomes
Medications such as non‐steroidal anti‐inflammatory drugs (NSAIDs), statins, angiotensin‐converting enzyme (ACE) inhibitors, drugs acting on the renin‐angiotensin system, and cytokine inhibitors may yield additional positive effects when added to an antidepressant 179 , 336 , 337 , 338 , 339 , reducing depressive symptoms among individuals with a physical disease 338 , 339 . As a prominent example, a recent meta‐analysis found that anti‐inflammatory drugs improved depressive symptoms with a SMD of 0.64 (95% CI: 0.40‐0.88) when used as add‐on to antidepressants in MDD, and of 0.41 (95% CI: 0.22‐0.60) when used as monotherapy among people with a physical disease 338 . Furthermore, anti‐inflammatory add‐on to antidepressants in MDD improved response and remission rates 338 .
The most frequently studied anti‐inflammatory drugs are NSAIDs, cytokine inhibitors and statins. Several of these drugs (e.g., statins) target physical diseases that are disproportionally common in people with MDD (e.g., cardiovascular diseases and diabetes mellitus) 340 . The antidepressant effects of these drugs provide further support to the previously discussed shared biological mechanisms of MDD and physical diseases (e.g., inflammation, HPA axis activation, and mitochondrial dysfunction) 341 .
On the other hand, many commonly used treatments for physical diseases can induce depressive symptoms as a side effect 342 . A well‐known example is interferon or IL‐2 treatment, in which up to 80% of individuals develop depressive symptoms, often dominated by somatic/neurovegetative manifestations within the first weeks, and 25% develop a major depressive episode within 48 weeks 343 . The proposed mechanism is pro‐inflammatory and immune‐activating 344 , with administration of pro‐inflammatory cytokines representing one of the most robust human models of MDD 345 .
Adverse events and clinical considerations of management
Among individuals with physical diseases, it is important to balance the potential antidepressant effects of pharmacotherapy with possible side effects. The adverse event profile of any antidepressant must be tailored to the symptomatic and risk profile of the comorbid physical disease and the specific individual. Potential adverse events include weight gain and the related risk of developing or exacerbating diabetes mellitus (particularly relevant to tricyclic antidepressants and mirtazapine) 346 ; cardiac toxicity and QTc prolongation (highest risk with tricyclic antidepressants and lowest with sertraline) 347 , 348 ; impact on bone metabolism, increasing the risk for osteoporosis and fractures (especially with SSRIs) 349 ; and bleeding, which is further increased when combining multiple classes of medications (e.g., SSRIs and NSAIDs 350 ). Furthermore, clinicians need to consider potential drug‐drug interactions, which are divided into pharmacodynamic (more frequent with older antidepressants) and pharmacokinetic (e.g., affecting hepatic metabolism, with antidepressants often being dependent on cytochrome P450 metabolism) 351 .
Overall, the antidepressant treatment of MDD that is comorbid with a physical disease will benefit from interdisciplinary care (e.g., frequent discussions with the clinician responsible for the treatment of the physical disease), consideration of patient‐related factors (e.g., age, pain, polypharmacy, and previous antidepressant trials all affect choice of antidepressant drug), and ongoing management. Finally, psychotherapy trials have not systematically assessed adverse events or contraindications 352 , 353 , 354 . Therefore, psychotherapy intervention trials in individuals with physical diseases have thus far reported very few adverse events, but clinical monitoring is indicated 310 , 324 .
FUTURE DIRECTIONS AND CONCLUSIONS
This paper reviews the substantial evidence base documenting that MDD is highly prevalent in populations with a range of common physical diseases, and vice versa. This high level of comorbidity translates into poorer economic and treatment outcomes.
A range of mechanisms have been implicated in both MDD and comorbid physical diseases, suggesting shared pathophysiology. We have discussed prominent pathways, such as inflammation, the gut microbiome, mitochondrial function, brain structure and function, and the HPA axis. Additional pathways requiring further investigation are endothelial and autonomic dysfunction, leptin and insulin signaling, and biological aging 2 , 15 .
Shared mechanisms provide opportunities for treatment that may benefit both MDD and comorbid physical diseases, but may also inform the investigation of potential off‐label interventions and drug‐repurposing strategies. For example, statin therapy, commonly prescribed for cardiovascular diseases, is being trialed for MDD 355 , 356 . Metformin (a medication typically prescribed for type 2 diabetes mellitus) and candesartan (an angiotensin II receptor blocker) are also being trialed for depression 357 .
Similarly, there are a range of lifestyle, physiological, social and genetic risk factors that are shared by MDD and physical diseases 358 . Interventions that address these factors may improve both psychiatric and physical outcomes. An example is the developing evidence base to support the use of lifestyle approaches to mental health care. Clinical guidelines 359 increasingly suggest that lifestyle interventions should be a major component of MDD management. Of the lifestyle domains reviewed in one of these guidelines 360 , the strongest recommendations when treating MDD were for exercise, relaxation, and work‐directed, sleep and mindfulness‐based interventions. There was further evidence to support dietary and green space interventions, but fewer data from RCTs to support interventions targeting smoking, loneliness or social support.
Further to the need for additional intervention and prevention strategies is the need for new models addressing challenges to accessible care and integrating psychiatric and physical considerations 361 . Having MDD, as well as subthreshold depressive symptoms, that are comorbid with a physical health condition amplifies barriers to accessing and engaging in potentially helpful treatments and self‐management strategies 16 . Treatment needs are often multiplied, diverse and chronic, placing strain on health services and families, especially in low‐ and middle‐income countries, cultural and linguistic minorities, and First Nations people, and those in rural areas with scarce resources 362 . Innovative strategies to overcome these barriers, incorporating integrated care for physical health conditions (particularly cardiometabolic diseases) in MDD, are required 363 .
One example is the collaborative care model, usually involving a physician and at least one other health professional (and sometimes peer or carer supports) who communicate with each other and the individual with MDD in a structured and planned way, to optimize treatment and care 16 , 61 . Contact and follow‐up appointments are organized by a central coordinator (e.g., a case manager) who promotes self‐management strategies (e.g., symptom and treatment monitoring and management, goal setting, problem solving, healthy lifestyle habits, and stress management) 364 . There can also be emphasis on enhancing patient‐centered decision making, and consideration of patients’ broader recovery goals 16 , 365 . Including lived experience input may also strengthen and support the broader aims of person‐centered management.
Collaborative care interventions have shown positive effects for people with depressive symptoms and coronary heart disease 366 , breast cancer 367 , and diabetes mellitus 292 , 364 . Such interventions appear to be equally effective in delivering MDD care for people with and without physical diseases 368 . Their effect on physical health, however, varies depending on the specific condition 292 , 369 . Implementing collaborative care interventions also requires careful consideration of leadership and delivery resources, costs for ongoing care, and cultural context 370 .
A further potential way to extend the reach and scope of effective treatment and care is provided by digital technologies. eHealth and mHealth interventions range from multicomponent intensive psychosocial programs to briefer specific self‐management interventions (e.g., targeting exercise) 16 , 371 , 372 , 373 . They can be adapted to suit context and resource capabilities, although the majority of RCTs are being conducted in high‐income countries 372 .
A meta‐analysis of RCTs of digital interventions reported a significant moderate effect on depressive outcomes (g=–0.37, 95% CI: –0.60 to –0.14) 371 . Key predictors of significant effects were a two‐way “clinician‐patient communication loop”, coupled with progress monitoring and adjustment of treatment as well as self‐management strategies over time 362 , 371 . Successful interventions ranged from those delivered via phone to more complex ones delivered via web platforms, highlighting the adaptability of digital approaches 371 . At this stage, however, the number of trials on each comorbid physical disease varies, and results are inconsistent 371 , 372 . More attention also needs to be paid to the scalability and validation of digital interventions and how they can be better integrated into health services 362 , 372 .
In summary, there is now a substantial body of evidence documenting a shared biological and environmental pathogenesis between MDD and several physical diseases. Further efforts are required to develop prevention and intervention strategies that target these shared pathways. These include investigation of therapeutics that target overlapping biological mechanisms (e.g., statins, metformin, interventions on gut microbiome) and the integration of strategies that address risk factors such as lifestyle behavior (e.g., exercise, diet). Furthermore, research and implementation efforts are now required to accelerate the development and translation of transdiagnostic, interdisciplinary models of care that consider both psychiatric and somatic presentations.
ACKNOWLEDGEMENTS
M. Berk is supported by National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship and Leadership 3 Investigator grants (nos. 1156072 and 2017131). A.J. Walker is supported by a Trisno Family Fellowship, funded in part by an NHMRC Centres of Research Excellence grant (no. 1153607); L. Schmaal by a NHMRC Leadership 1 Investigator Grant (no. 2017962); L.J. Williams by a NHMRC Emerging Leadership Fellowship (no. 1174060); W. Marx by an NHMRC Investigator Grant (no. 2008971) and a Multiple Sclerosis Research Australia early‐career fellowship; J.J. McGrath by the Danish National Research Foundation; O. Plana‐Ripoll by a Lundbeck Foundation Fellowship (no. R345‐2020‐1588) and grants from Independent Research Fund Denmark (nos. 2066‐00009B and 1030‐00085B); A. O'Neil by an NHMRC Emerging Leader 2 Fellowship (no. 2009295), and J. Firth by a UK Research and Innovation Future Leaders Fellowship (no. MR/T021780/1). Supplementary information on this study is available at https://osf.io/j53aq/?view_only=d0d1ff8d25c94ff086cc95536ff68aa6.
REFERENCES
- 1. Ferrari A, Somerville A, Baxter A et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med 2013;43:471‐81. [DOI] [PubMed] [Google Scholar]
- 2. Vaccarino V, Badimon L, Bremner JD et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J 2020;41:1687‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Q‐E, Zhou A‐M, Han YP et al. Poststroke depression and risk of recurrent stroke: a meta‐analysis of prospective studies. Medicine 2019;98:e17235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krebber A, Buffart L, Kleijn G et al. Prevalence of depression in cancer patients: a meta‐analysis of diagnostic interviews and self‐report instruments. Psychooncology 2014;23:121‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott AJ, Sharpe L, Hunt C et al. Anxiety and depressive disorders in people with epilepsy: a meta‐analysis. Epilepsia 2017;58:973‐82. [DOI] [PubMed] [Google Scholar]
- 6. Boeschoten RE, Braamse AM, Beekman AT et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta‐analysis. J Neurol Sci 2017;372:331‐41. [DOI] [PubMed] [Google Scholar]
- 7. Ismail Z, Elbayoumi H, Fischer CE et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta‐analysis. JAMA Psychiatry 2017;74:58‐67. [DOI] [PubMed] [Google Scholar]
- 8. Leung DK, Chan WC, Spector A et al. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta‐analysis. Int J Geriatr Psychiatry 2021;36:1330‐44. [DOI] [PubMed] [Google Scholar]
- 9. Luppino FS, de Wit LM, Bouvy PF et al. Overweight, obesity, and depression: a systematic review and meta‐analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220‐9. [DOI] [PubMed] [Google Scholar]
- 10. Pan A, Keum N, Okereke OI et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta‐analysis of epidemiological studies. Diabetes Care 2012;35:1171‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wium‐Andersen MK, Wium‐Andersen IK, Prescott EIB et al. An attempt to explain the bidirectional association between ischaemic heart disease, stroke and depression: a cohort and meta‐analytic approach. Br J Psychiatry 2020;217:434‐41. [DOI] [PubMed] [Google Scholar]
- 12. Zhang F, Rao S, Baranova A. Shared genetic liability between major depressive disorder and osteoarthritis. Bone Joint Res 2022;11:12‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai L, Bao Y, Fu X et al. Causal links between major depressive disorder and insomnia: a Mendelian randomisation study. Gene 2021;768:145271. [DOI] [PubMed] [Google Scholar]
- 14. Cao H, Li S, Baranova A et al. Shared genetic liability between major depressive disorder and atopic diseases. Front Immunol 2021;12:665160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milaneschi Y, Simmons WK, van Rossum EF et al. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry 2019;24:18‐33. [DOI] [PubMed] [Google Scholar]
- 16. Gold SM, Köhler‐Forsberg O, Moss‐Morris R et al. Comorbid depression in medical diseases. Nat Rev Dis Primers 2020;6:69. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . World mental health report: transforming mental health for all. Geneva: World Health Organization, 2022. [Google Scholar]
- 18. Arango C, Dragioti E, Solmi M et al. Risk and protective factors for mental disorders beyond genetics: an evidence‐based atlas. World Psychiatry 2021;20:417‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho CS, Feng L, Fam J et al. Coexisting medical comorbidity and depression: multiplicative effects on health outcomes in older adults. Int Psychogeriatr 2014;26:1221‐9. [DOI] [PubMed] [Google Scholar]
- 20. Goldstein CM, Gathright EC, Garcia S. Relationship between depression and medication adherence in cardiovascular disease: the perfect challenge for the integrated care team. Patient Prefer Adherence 2017;11:547‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weye N, Momen NC, Christensen MK et al. Association of specific mental disorders with premature mortality in the Danish population using alternative measurement methods. JAMA Netw Open 2020;3:e206646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Egede LE, Walker RJ, Bishu K et al. Trends in costs of depression in adults with diabetes in the United States: Medical Expenditure Panel Survey, 2004‐2011. J Gen Intern Med 2016;31:615‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baumeister H, Haschke A, Munzinger M et al. Inpatient and outpatient costs in patients with coronary artery disease and mental disorders: a systematic review. Biopsychosoc Med 2015;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welch CA, Czerwinski D, Ghimire B et al. Depression and costs of health care. Psychosomatics 2009;50:392‐401. [DOI] [PubMed] [Google Scholar]
- 25. Jiang W, Alexander J, Christopher E et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001;161:1849‐56. [DOI] [PubMed] [Google Scholar]
- 26. Christensen MK, McGrath JJ, Momen N et al. The health care cost of comorbidity in individuals with mental disorders: a Danish register‐based study. Aust N Z J Psychiatry 2023;57:914‐22. [DOI] [PubMed] [Google Scholar]
- 27. GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022;9:137‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weye N, Santomauro DF, Agerbo E et al. Register‐based metrics of years lived with disability associated with mental and substance use disorders: a register‐based cohort study in Denmark. Lancet Psychiatry 2021;8:310‐9. [DOI] [PubMed] [Google Scholar]
- 29. Berk M, Berk L, Dodd S et al. The sick role, illness cognitions and outcomes in bipolar disorder. J Affect Disord 2013;146:146‐9. [DOI] [PubMed] [Google Scholar]
- 30. Davies NM, Holmes MV, Smith GD. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim GY, Tam WW, Lu Y et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018;8:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farooqi A, Gillies C, Sathanapally H et al. A systematic review and meta‐analysis to compare the prevalence of depression between people with and without type 1 and type 2 diabetes. Prim Care Diabetes 2022;16:1‐10. [DOI] [PubMed] [Google Scholar]
- 33. Feng L, Li L, Liu W et al. Prevalence of depression in myocardial infarction: a PRISMA‐compliant meta‐analysis. Medicine 2019;98:e14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cong S, Xiang C, Zhang S et al. Prevalence and clinical aspects of depression in Parkinson's disease: a systematic review and meta‐analysis of 129 studies. Neurosci Biobehav Rev 2022;141:104749. [DOI] [PubMed] [Google Scholar]
- 35. Mitchell AJ, Sheth B, Gill J et al. Prevalence and predictors of post‐stroke mood disorders: a meta‐analysis and meta‐regression of depression, anxiety and adjustment disorder. Gen Hosp Psychiatry 2017;47:48‐60. [DOI] [PubMed] [Google Scholar]
- 36. Rutledge T, Reis VA, Linke SE et al. Depression in heart failure: a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006;48:1527‐37. [DOI] [PubMed] [Google Scholar]
- 37. Lichtman JH, Froelicher ES, Blumenthal JA et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350‐69. [DOI] [PubMed] [Google Scholar]
- 38. Gan Y, Gong Y, Tong X et al. Depression and the risk of coronary heart disease: a meta‐analysis of prospective cohort studies. BMC Psychiatry 2014;14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763‐74. [DOI] [PubMed] [Google Scholar]
- 40. Van der Kooy K, Van Hout H, Marwijk H et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007;22:613‐26. [DOI] [PubMed] [Google Scholar]
- 41. Dragioti E, Radua J, Solmi M et al. Impact of mental disorders on clinical outcomes of physical diseases: an umbrella review assessing population attributable fraction and generalized impact fraction. World Psychiatry 2023;22:86‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang B, Yuan S, Xiong Y et al. Major depressive disorder and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Diabetologia 2020;63:1305‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cai H, Cai B, Zhang H et al. Major depression and small vessel stroke: a Mendelian randomization analysis. J Neurol 2019;266:2859‐66. [DOI] [PubMed] [Google Scholar]
- 44. Li GH‐Y, Cheung C‐L, Chung AK‐K et al. Evaluation of bi‐directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol Med 2022;52:1765‐76. [DOI] [PubMed] [Google Scholar]
- 45. Cai W, Mueller C, Li YJ et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta‐analysis. Ageing Res Rev 2019;50:102‐9. [DOI] [PubMed] [Google Scholar]
- 46. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002;52:253‐64. [DOI] [PubMed] [Google Scholar]
- 47. Rao A, Zecchin R, Newton PJ et al. The prevalence and impact of depression and anxiety in cardiac rehabilitation: a longitudinal cohort study. Eur J Prevent Cardiol 2020;27:478‐89. [DOI] [PubMed] [Google Scholar]
- 48. Bauer LK, Caro MA, Beach SR et al. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. Am J Cardiol 2012;109:1266‐71. [DOI] [PubMed] [Google Scholar]
- 49. Graham EA, Deschenes SS, Khalil MN et al. Measures of depression and risk of type 2 diabetes: a systematic review and meta‐analysis. J Affect Disord 2020;265:224‐32. [DOI] [PubMed] [Google Scholar]
- 50. Mezuk B, Eaton WW, Albrecht S et al. Depression and type 2 diabetes over the lifespan: a meta‐analysis. Diabetes Care 2008;31:2383‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tao H, Fan S, Zhu T et al. Psychiatric disorders and type 2 diabetes mellitus: a bidirectional Mendelian randomization. Eur J Clin Invest 2023;53:e13893. [DOI] [PubMed] [Google Scholar]
- 52. Gonzalez JS, Peyrot M, McCarl LA et al. Depression and diabetes treatment nonadherence: a meta‐analysis. Diabetes Care 2008;31:2398‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin EH, Katon W, Von Korff M et al. Relationship of depression and diabetes self‐care, medication adherence, and preventive care. Diabetes Care 2004;27:2154‐60. [DOI] [PubMed] [Google Scholar]
- 54. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000;160:3278‐85. [DOI] [PubMed] [Google Scholar]
- 55. Richardson LK, Egede LE, Mueller M et al. Longitudinal effects of depression on glycemic control in veterans with type 2 diabetes. Gen Hosp Psychiatry 2008;30:509‐14. [DOI] [PubMed] [Google Scholar]
- 56. Lustman PJ, Anderson RJ, Freedland KE et al. Depression and poor glycemic control: a meta‐analytic review of the literature. Diabetes Care 2000;23:934‐42. [DOI] [PubMed] [Google Scholar]
- 57. Nouwen A, Adriaanse M, van Dam K et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta‐analysis. Diabet Med 2019;36:1562‐72. [DOI] [PubMed] [Google Scholar]
- 58. Egede LE. Diabetes, major depression, and functional disability among US adults. Diabetes Care 2004;27:421‐8. [DOI] [PubMed] [Google Scholar]
- 59. Katon WJ, Lin EH, Williams LH et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med 2010;25:423‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cohen A. Addressing comorbidity between mental disorders and major noncommunicable diseases: background technical report to support implementation of the WHO European Mental Health Action Plan 2013‐2020 and the WHO European Action Plan for the Prevention and Control of Noncommunicable Diseases 2016‐2025. Copenhagen: World Health Organization Regional Office for Europe, 2017. [Google Scholar]
- 61. Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet 2005;366:1059‐62. [DOI] [PubMed] [Google Scholar]
- 62. Mottillo S, Filion KB, Genest J et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta‐analysis. J Am Coll Cardiol 2010;56:1113‐32. [DOI] [PubMed] [Google Scholar]
- 63. Moradi Y, Albatineh AN, Mahmoodi H et al. The relationship between depression and risk of metabolic syndrome: a meta‐analysis of observational studies. Clin Diabetes Endocrinol 2021;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Repousi N, Masana MF, Sanchez‐Niubo A et al. Depression and metabolic syndrome in the older population: a review of evidence. J Affect Disord 2018;237:56‐64. [DOI] [PubMed] [Google Scholar]
- 65. Zhang M, Chen J, Yin Z et al. The association between depression and metabolic syndrome and its components: a bidirectional two‐sample Mendelian randomization study. Transl Psychiatry 2021;11:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mannan M, Mamun A, Doi S et al. Is there a bi‐directional relationship between depression and obesity among adult men and women? Systematic review and bias‐adjusted meta analysis. Asian J Psychiatry 2016;21:51‐66. [DOI] [PubMed] [Google Scholar]
- 67. van den Broek N, Treur JL, Larsen JK et al. Causal associations between body mass index and mental health: a Mendelian randomisation study. J Epidemiol Community Health 2018;72:708‐10. [DOI] [PubMed] [Google Scholar]
- 68. Casanova F, O'Loughlin J, Martin S et al. Higher adiposity and mental health: causal inference using Mendelian randomization. Hum Mol Genet 2021;30:2371‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tyrrell J, Mulugeta A, Wood AR et al. Using genetics to understand the causal influence of higher BMI on depression. Int J Epidemiol 2019;48:834‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Speed MS, Jefsen OH, Børglum AD et al. Investigating the association between body fat and depression via Mendelian randomization. Transl Psychiatry 2019;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Malmir H, Mirzababaei A, Moradi S et al. Metabolically healthy status and BMI in relation to depression: a systematic review of observational studies. Diabetes Metab Syndr 2019;13:1099‐103. [DOI] [PubMed] [Google Scholar]
- 72. Virtanen M, Ferrie JE, Akbaraly T et al. Metabolic syndrome and symptom resolution in depression: a 5‐year follow‐up of older adults. J Clin Psychiatry 2017;78:11776. [DOI] [PubMed] [Google Scholar]
- 73. Lehto SM, Niskanen L, Tolmunen T et al. Low serum HDL‐cholesterol levels are associated with long symptom duration in patients with major depressive disorder. Psychiatry Clin Neurosci 2010;64:279‐83. [DOI] [PubMed] [Google Scholar]
- 74. Zhu J, Fang F, Sjölander A et al. First‐onset mental disorders after cancer diagnosis and cancer‐specific mortality: a nationwide cohort study. Ann Oncol 2017;28:1964‐9. [DOI] [PubMed] [Google Scholar]
- 75. Mitchell AJ, Chan M, Bhatti H et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncol 2011;12:160‐74. [DOI] [PubMed] [Google Scholar]
- 76. Mitchell AJ, Ferguson DW, Gill J et al. Depression and anxiety in long‐term cancer survivors compared with spouses and healthy controls: a systematic review and meta‐analysis. Lancet Oncol 2013;14:721‐32. [DOI] [PubMed] [Google Scholar]
- 77. Brandenbarg D, Maass SW, Geerse OP et al. A systematic review on the prevalence of symptoms of depression, anxiety and distress in long‐term cancer survivors: implications for primary care. Eur J Cancer Care 2019;28:e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Linden W, Vodermaier A, MacKenzie R et al. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord 2012;141:343‐51. [DOI] [PubMed] [Google Scholar]
- 79. Riedl D, Schuessler G. Prevalence of depression and cancer – a systematic review. Z Psychosom Med Psychother 2022;68:74‐86. [DOI] [PubMed] [Google Scholar]
- 80. Caruso R, Nanni M, Riba M et al. Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol 2017;56:146‐55. [DOI] [PubMed] [Google Scholar]
- 81. Riedl D, Schüßler G. Factors associated with and risk factors for depression in cancer patients – A systematic literature review. Transl Oncol 2022;16:101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang Y‐H, Li J‐Q, Shi J‐F et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta‐analysis of cohort studies. Mol Psychiatry 2020;25:1487‐99. [DOI] [PubMed] [Google Scholar]
- 83. Pinquart M, Duberstein P. Depression and cancer mortality: a meta‐analysis. Psychol Med 2010;40:1797‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jia Y, Li F, Liu Y et al. Depression and cancer risk: a systematic review and meta‐analysis. Public Health 2017;149:138‐48. [DOI] [PubMed] [Google Scholar]
- 85. Zhu G‐L, Xu C, Yang K‐B et al. Causal relationship between genetically predicted depression and cancer risk: a two‐sample bi‐directional Mendelian randomization. BMC Cancer 2022;22:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen X, Kong J, Diao X et al. Depression and prostate cancer risk: a Mendelian randomization study. Cancer Med 2020;9:9160‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lutgendorf SK, Lamkin DM, DeGeest K et al. Depressed and anxious mood and T‐cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun 2008;22:890‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Colleoni M, Mandala M, Peruzzotti G et al. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet 2000;356:1326‐7. [DOI] [PubMed] [Google Scholar]
- 89. Giese‐Davis J, Collie K, Rancourt KM et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol 2011;29:413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stafford J, Chung WT, Sommerlad A et al. Psychiatric disorders and risk of subsequent dementia: systematic review and meta‐analysis of longitudinal studies. Int J Geriatr Psychiatry 2022;37:10.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang S, Mao S, Xiang D et al. Association between depression and the subsequent risk of Parkinson's disease: a meta‐analysis. Prog Neuropsychopharmacol Biol Psychiatry 2018;86:186‐92. [DOI] [PubMed] [Google Scholar]
- 92. Invernizzi S, Simoes Loureiro I, Kandana Arachchige KG et al. Late‐life depression, cognitive impairment, and relationship with Alzheimer's disease. Dement Geriatr Cogn Disord 2021;50:414‐24. [DOI] [PubMed] [Google Scholar]
- 93. Mulugeta A, Zhou A, King C et al. Association between major depressive disorder and multiple disease outcomes: a phenome‐wide Mendelian randomisation study in the UK Biobank. Mol Psychiatry 2020;25:1469‐76. [DOI] [PubMed] [Google Scholar]
- 94. Yuan S, Tomson T, Larsson SC. Modifiable risk factors for epilepsy: a two‐sample Mendelian randomization study. Brain Behav 2021;11:e02098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Harerimana NV, Liu Y, Gerasimov ES et al. Genetic evidence supporting a causal role of depression in Alzheimer's disease. Biol Psychiatry 2022;92:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang J, Zuber V, Matthews PM et al. Sleep, major depressive disorder, and Alzheimer disease: a Mendelian randomization study. Neurology 2020;95:e1963‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Harroud A, Marrie RA, Fitzgerald KC et al. Mendelian randomization provides no evidence for a causal role in the bidirectional relationship between depression and multiple sclerosis. Mult Scler 2021;27:2077‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Binzer S, Jiang X, Hillert J et al. Depression and multiple sclerosis: a bidirectional Mendelian randomisation study. Mult Scler 2021;27:1799‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Antonaci F, Nappi G, Galli F et al. Migraine and psychiatric comorbidity: a review of clinical findings. J Headache Pain 2011;12:115‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Prisnie JC, Sajobi TT, Wang M et al. Effects of depression and anxiety on quality of life in five common neurological disorders. Gen Hosp Psychiatry 2018;52:58‐63. [DOI] [PubMed] [Google Scholar]
- 101. Feng F, Cai Y, Hou Y et al. Excessive daytime sleepiness in Parkinson's disease: a systematic review and meta‐analysis. Parkinsonism Relat Disord 2021;85:133‐40. [DOI] [PubMed] [Google Scholar]
- 102. Chen E, Sajatovic M, Liu H et al. Demographic and clinical correlates of seizure frequency: findings from the Managing Epilepsy Well Network database. J Clin Neurol 2018;14:206‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. McKay KA, Tremlett H, Fisk JD et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology 2018;90:e1316‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brendel M, Pogarell O, Xiong G et al. Depressive symptoms accelerate cognitive decline in amyloid‐positive MCI patients. Eur J Nucl Med Mol Imaging 2015;42:716‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ashina S, Serrano D, Lipton RB et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain 2012;13:615‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta‐analysis of prospective studies. Osteoporos Int 2018;29:1303‐12. [DOI] [PubMed] [Google Scholar]
- 107. Zhou C, Fang L, Chen Y et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta‐analysis. Osteoporos Int 2018;29:1243‐51. [DOI] [PubMed] [Google Scholar]
- 108. He B, Lyu Q, Yin L et al. Depression and osteoporosis: a Mendelian randomization study. Calcif Tissue Int 2021;109:675‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Heidari ME, Irvani SSN, Dalvand P et al. Prevalence of depression in older people with hip fracture: a systematic review and meta‐analysis. Int J Orthop Trauma Nurs 2021;40:100813. [DOI] [PubMed] [Google Scholar]
- 110. Schweiger JU, Schweiger U, Huppe M et al. Bone density and depressive disorder: a meta‐analysis. Brain Behav 2016;6:e00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Silverman SL, Shen W, Minshall ME et al. Prevalence of depressive symptoms in postmenopausal women with low bone mineral density and/or prevalent vertebral fracture: results from the Multiple Outcomes of Raloxifene Evaluation (MORE) study. J Rheumatol 2007;34:140‐4. [PubMed] [Google Scholar]
- 112. Prigge R, Wild SH, Jackson CA. Depression, diabetes, comorbid depression and diabetes and risk of all‐cause and cause‐specific mortality: a prospective cohort study. Diabetologia 2022;65:1450‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Machado MO, Veronese N, Sanches M et al. The association of depression and all‐cause and cause‐specific mortality: an umbrella review of systematic reviews and meta‐analyses. BMC Med 2018;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hockey M, Rocks T, Ruusunen A et al. Psychological distress as a risk factor for all‐cause, chronic disease‐ and suicide‐specific mortality: a prospective analysis using data from the National Health Interview Survey. Soc Psychiatry Psychiatr Epidemiol 2022;57:541‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Plana‐Ripoll O, Pedersen CB, Agerbo E et al. A comprehensive analysis of mortality‐related health metrics associated with mental disorders: a nationwide, register‐based cohort study. Lancet 2019;394:1827‐35. [DOI] [PubMed] [Google Scholar]
- 116. Plana‐Ripoll O, Musliner KL, Dalsgaard S et al. Nature and prevalence of combinations of mental disorders and their association with excess mortality in a population‐based cohort study. World Psychiatry 2020;19:339‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Santomauro DF, Herrera AMM, Shadid J et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID‐19 pandemic. Lancet 2021;398:1700‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. ten Have M, Tuithof M, van Dorsselaer S et al. Prevalence and trends of common mental disorders from 2007‐2009 to 2019‐2022: results from the Netherlands Mental Health Survey and Incidence Studies (NEMESIS), including comparison of prevalence rates before vs. during the COVID‐19 pandemic. World Psychiatry; 2023;22:275‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Penninx BW. Psychiatric symptoms and cognitive impairment in “Long COVID”: the relevance of immunopsychiatry. World Psychiatry 2021;20:357‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Davis HE, McCorkell L, Vogel JM et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023:21:133‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu E, Xie Y, Al‐Aly Z. Long‐term neurologic outcomes of COVID‐19. Nat Med 2022;28:2406‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Clift AK, Ranger TA, Patone M et al. Neuropsychiatric ramifications of severe COVID‐19 and other severe acute respiratory infections. JAMA Psychiatry 2022;79:690‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mazza MG, De Lorenzo R, Conte C et al. Anxiety and depression in COVID‐19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020;89:594‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Penninx BW, Benros ME, Klein RS et al. How COVID‐19 shaped mental health: from infection to pandemic effects. Nat Med 2022;28:2027‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Glantz S, Gonzalez M. Effective tobacco control is key to rapid progress in reduction of non‐communicable diseases. Lancet 2012;379:1269‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Parry CD, Patra J, Rehm J. Alcohol consumption and non‐communicable diseases: epidemiology and policy implications. Addiction 2011;106:1718‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bull FC, Al‐Ansari SS, Biddle S et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gakidou E, Afshin A, Abajobir AA et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Basnet S, Merikanto I, Lahti T et al. Associations of common chronic non‐communicable diseases and medical conditions with sleep‐related problems in a population‐based health examination study. Sleep Sci 2016;9:249‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta‐analysis. J Sleep Res 2009;18:148‐58. [DOI] [PubMed] [Google Scholar]
- 131. Fornaro M, Carvalho AF, De Prisco M et al. The prevalence, odds, predictors, and management of tobacco use disorder or nicotine dependence among people with severe mental illness: systematic review and meta‐analysis. Neurosci Biobehav Rev 2022;132:289‐303. [DOI] [PubMed] [Google Scholar]
- 132. Sullivan LE, Fiellin DA, O'Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med 2005;118:330‐41. [DOI] [PubMed] [Google Scholar]
- 133. Firth J, Stubbs B, Teasdale SB et al. Diet as a hot topic in psychiatry: a population‐scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry 2018;17:365‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schuch F, Vancampfort D, Firth J et al. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta‐analysis. J Affect Disord 2017;210:139‐50. [DOI] [PubMed] [Google Scholar]
- 135. Baglioni C, Nanovska S, Regen W et al. Sleep and mental disorders: a meta‐analysis of polysomnographic research. Psychol Bull 2016;142:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Firth J, Solmi M, Wootton RE et al. A meta‐review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020;19:360‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Li J, Wang H, Li M et al. Effect of alcohol use disorders and alcohol intake on the risk of subsequent depressive symptoms: a systematic review and meta‐analysis of cohort studies. Addiction 2020;115:1224‐43. [DOI] [PubMed] [Google Scholar]
- 138. Firth J, Siddiqi N, Koyanagi A et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6:675‐712. [DOI] [PubMed] [Google Scholar]
- 139. Brière FN, Rohde P, Seeley JR et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Compr Psychiatry 2014;55:526‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Grenard JL, Munjas BA, Adams JL et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta‐analysis. J Gen Intern Med 2011;26:1175‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Byrne P. Premature mortality of people with severe mental illness: a renewed focus for a new era. Ir J Psychol Med 2023;40:74‐83. [DOI] [PubMed] [Google Scholar]
- 142. Højlund M, Andersen K, Ernst MT et al. Use of low‐dose quetiapine increases the risk of major adverse cardiovascular events: results from a nationwide active comparator‐controlled cohort study. World Psychiatry 2022;21:444‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sahle BW, Reavley NJ, Li W et al. The association between adverse childhood experiences and common mental disorders and suicidality: an umbrella review of systematic reviews and meta‐analyses. Eur Child Adolesc Psychiatry 2022;31:1489‐99. [DOI] [PubMed] [Google Scholar]
- 144. Lovis‐Schmidt A, Schilling J, Pudschun C et al. Adverse childhood experiences and physical diseases in adulthood: a summary of meta‐analyses. Traumatology 2022; doi: 10.1037/trm0000412. [DOI] [Google Scholar]
- 145. Nelson J, Klumparendt A, Doebler P et al. Childhood maltreatment and characteristics of adult depression: meta‐analysis. Br J Psychiatry 2017;210:96‐104. [DOI] [PubMed] [Google Scholar]
- 146. Hammen C. Risk factors for depression: an autobiographical review. Annu Rev Clin Psychol 2018;14:1‐28. [DOI] [PubMed] [Google Scholar]
- 147. Cotter J, Drake RJ, Yung AR. Adulthood revictimization: looking beyond childhood trauma. Acta Psychiatr Scand 2016;134:368. [DOI] [PubMed] [Google Scholar]
- 148. Colman RA, Widom CS. Childhood abuse and neglect and adult intimate relationships: a prospective study. Child Abuse Negl 2004;28:1133‐51. [DOI] [PubMed] [Google Scholar]
- 149. Nelson EC, Heath AC, Madden PA et al. Association between self‐reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry 2002;59:139‐45. [DOI] [PubMed] [Google Scholar]
- 150. Monroe SM, Anderson SF, Harkness KL. Life stress and major depression: the mysteries of recurrences. Psychol Rev 2019;126:791‐816. [DOI] [PubMed] [Google Scholar]
- 151. Buckman JEJ, Saunders R, Arundell LL et al. Life events and treatment prognosis for depression: a systematic review and individual patient data meta‐analysis. J Affect Disord 2022;299:298‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 2018;15:215‐29. [DOI] [PubMed] [Google Scholar]
- 153. Cohen S, Murphy MLM, Prather AA. Ten surprising facts about stressful life events and disease risk. Annu Rev Psychol 2019;70:577‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry 2020;177:20‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Watters ER, Aloe AM, Wojciak AS. Examining the associations between childhood trauma, resilience, and depression: a multivariate meta‐analysis. Trauma Violence Abuse 2023;24:231‐44. [DOI] [PubMed] [Google Scholar]
- 156. Braithwaite EC, O'Connor RM, Degli‐Esposti M et al. Modifiable predictors of depression following childhood maltreatment: a systematic review and meta‐analysis. Transl Psychiatry 2017;7:e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Morris AS, Hays‐Grudo J. Protective and compensatory childhood experiences and their impact on adult mental health. World Psychiatry 2023;22:150‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. World Health Organization . A conceptual framework for action on the social determinants of health. Geneva: World Health Organization, 2010. [Google Scholar]
- 159. Marmot M. Social justice, epidemiology and health inequalities. Eur J Epidemiol 2017;32:537‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Patel V, Burns JK, Dhingra M et al. Income inequality and depression: a systematic review and meta‐analysis of the association and a scoping review of mechanisms. World Psychiatry 2018;17:76‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Suglia SF, Appleton AA, Bleil ME et al. Timing, duration, and differential susceptibility to early life adversities and cardiovascular disease risk across the lifespan: implications for future research. Prev Med 2021;153:106736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Aldridge RW, Story A, Hwang SW et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high‐income countries: a systematic review and meta‐analysis. Lancet 2018;391:241‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Heise L, Greene ME, Opper N et al. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet 2019;393:2440‐54. [DOI] [PubMed] [Google Scholar]
- 164. Williams DR, Lawrence JA, Davis BA et al. Understanding how discrimination can affect health. Health Serv Res 2019;54(Suppl. 2):1374‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Young C, Hanson C, Craig JC et al. Psychosocial factors associated with the mental health of indigenous children living in high income countries: a systematic review. Int J Equity Health 2017;16:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Williams DR, Etkins OS. Racism and mental health. World Psychiatry 2021;20:194‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Trent M, Dooley DG, Douge J et al. The impact of racism on child and adolescent health. Pediatrics 2019;144:e20191765. [DOI] [PubMed] [Google Scholar]
- 168. Yoshikawa H, Aber JL, Beardslee WR. The effects of poverty on the mental, emotional, and behavioral health of children and youth: implications for prevention. Am Psychol 2012;67:272‐84. [DOI] [PubMed] [Google Scholar]
- 169. Landstedt E, Almquist YB. Intergenerational patterns of mental health problems: the role of childhood peer status position. BMC Psychiatry 2019;19:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Schmitt MT, Branscombe NR, Postmes T et al. The consequences of perceived discrimination for psychological well‐being: a meta‐analytic review. Psychol Bull 2014;140:921‐48. [DOI] [PubMed] [Google Scholar]
- 171. Castellvi P, Miranda‐Mendizabal A, Pares‐Badell O et al. Exposure to violence, a risk for suicide in youths and young adults. A meta‐analysis of longitudinal studies. Acta Psychiatr Scand 2017;135:195‐211. [DOI] [PubMed] [Google Scholar]
- 172. Elovainio M, Hakulinen C, Pulkki‐Raback L et al. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health 2017;2:e260‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Cheng TL, Johnson SB, Goodman E. Breaking the intergenerational cycle of disadvantage: the three generation approach. Pediatrics 2016;137:e20152467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gartland D, Riggs E, Muyeen S et al. What factors are associated with resilient outcomes in children exposed to social adversity? A systematic review. BMJ Open 2019;9:e024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Moylan S, Maes M, Wray N et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 2013;18:595‐606. [DOI] [PubMed] [Google Scholar]
- 176. Ruiz NAL, Del Ángel DS, Olguín HJ et al. Neuroprogression: the hidden mechanism of depression. Neuropsychiatr Dis Treat 2018;14:2837‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Walker AJ, Kim Y, Price JB et al. Stress, inflammation, and cellular vulnerability during early stages of affective disorders: biomarker strategies and opportunities for prevention and intervention. Front Psychiatry 2014;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Scott J, Leboyer M, Hickie I et al. Clinical staging in psychiatry: a cross‐cutting model of diagnosis with heuristic and practical value. Br J Psychiatry 2013;202:243‐5. [DOI] [PubMed] [Google Scholar]
- 179. Salagre E, Fernandes BS, Dodd S et al. Statins for the treatment of depression: a meta‐analysis of randomized, double‐blind, placebo‐controlled trials. J Affect Disord 2016;200:235‐42. [DOI] [PubMed] [Google Scholar]
- 180. Berk M, Dean OM, Cotton SM et al. The efficacy of adjunctive N‐acetylcysteine in major depressive disorder: a double‐blind, randomized, placebo‐controlled trial. J Clin Psychiatry 2014;75:628‐36. [DOI] [PubMed] [Google Scholar]
- 181. Dean OM, Kanchanatawan B, Ashton M et al. Adjunctive minocycline treatment for major depressive disorder: a proof of concept trial. Aust N Z J Psychiatry 2017;51:829‐40. [DOI] [PubMed] [Google Scholar]
- 182. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta‐analysis. Am J Psychiatry 2000;157:1552‐62. [DOI] [PubMed] [Google Scholar]
- 183. Polderman TJ, Benyamin B, De Leeuw CA et al. Meta‐analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 2015;47:702‐9. [DOI] [PubMed] [Google Scholar]
- 184. Bevan S, Traylor M, Adib‐Samii P et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke 2012;43:3161‐7. [DOI] [PubMed] [Google Scholar]
- 185. Hyde CL, Nagle MW, Tian C et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet 2016;48:1031‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Locke AE, Kahali B, Berndt SI et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Dupuis J, Langenberg C, Prokopenko I et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nikpay M, Goel A, Won HH et al. A comprehensive 1000 Genomes‐based genome‐wide association meta‐analysis of coronary artery disease. Nat Genet 2015;47:1121‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hagenaars SP, Coleman JR, Choi SW et al. Genetic comorbidity between major depression and cardio‐metabolic traits, stratified by age at onset of major depression. Am J Med Genet B Neuropsychiatr Genet 2020;183:309‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wray NR, Ripke S, Mattheisen M et al. Genome‐wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018;50:668‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Brainstorm Consortium , Anttila V, Bulik‐Sullivan B et al. Analysis of shared heritability in common disorders of the brain. Science 2018;360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Amare AT, Schubert KO, Klingler‐Hoffmann M et al. The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies. Transl Psychiatry 2017;7:e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Juruena MF, Bocharova M, Agustini B et al. Atypical depression and non‐atypical depression: is HPA axis function a biomarker? A systematic review. J Affect Disord 2018;233:45‐67. [DOI] [PubMed] [Google Scholar]
- 194. Juruena MF, Gadelrab R, Cleare AJ et al. Epigenetics: a missing link between early life stress and depression. Prog Neuropsychopharmacol Biol Psychiatry 2021;109:110231. [DOI] [PubMed] [Google Scholar]
- 195. Stetler C, Miller GE. Depression and hypothalamic‐pituitary‐adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011;73:114‐26. [DOI] [PubMed] [Google Scholar]
- 196. Zajkowska Z, Gullett N, Walsh A et al. Cortisol and development of depression in adolescence and young adulthood – a systematic review and meta‐analysis. Psychoneuroendocrinology 2022;136:105625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Belvederi Murri M, Pariante C, Mondelli V et al. HPA axis and aging in depression: systematic review and meta‐analysis. Psychoneuroendocrinology 2014;41:46‐62. [DOI] [PubMed] [Google Scholar]
- 198. Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018;21:403‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Vreeburg SA, Hoogendijk WJ, van Pelt J et al. Major depressive disorder and hypothalamic‐pituitary‐adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 2009;66:617‐26. [DOI] [PubMed] [Google Scholar]
- 200. Iob E, Kirschbaum C, Steptoe A. Persistent depressive symptoms, HPA‐axis hyperactivity, and inflammation: the role of cognitive‐affective and somatic symptoms. Mol Psychiatry 2020;25:1130‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Vian J, Pereira C, Chavarria V et al. The renin‐angiotensin system: a possible new target for depression. BMC Med 2017;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Heck AL, Handa RJ. Sex differences in the hypothalamic‐pituitary‐adrenal axis' response to stress: an important role for gonadal hormones. Neuropsychopharmacology 2019;44:45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Berk M, Williams LJ, Jacka FN et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Felger JC, Miller AH. Identifying immunophenotypes of inflammation in depression: dismantling the monolith. Biol Psychiatry 2020;88:136‐8. [DOI] [PubMed] [Google Scholar]
- 205. Osimo EF, Baxter LJ, Lewis G et al. Prevalence of low‐grade inflammation in depression: a systematic review and meta‐analysis of CRP levels. Psychol Med 2019;49:1958‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kohler CA, Freitas TH, Maes M et al. Peripheral cytokine and chemokine alterations in depression: a meta‐analysis of 82 studies. Acta Psychiatr Scand 2017;135:373‐87. [DOI] [PubMed] [Google Scholar]
- 207. Leighton SP, Nerurkar L, Krishnadas R et al. Chemokines in depression in health and in inflammatory illness: a systematic review and meta‐analysis. Mol Psychiatry 2018;23:48‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Teixeira AL, Martins LB, Berk M et al. Severe psychiatric disorders and general medical comorbidities: inflammation‐related mechanisms and therapeutic opportunities. Clin Sci 2022;136:1257‐80. [DOI] [PubMed] [Google Scholar]
- 209. Miller AH. Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry 2020;19:108‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Halaris A. Inflammation‐associated co‐morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci 2017;31:45‐70. [DOI] [PubMed] [Google Scholar]
- 211. Walker AJ, Kim Y, Borissiouk I et al. Statins: neurobiological underpinnings and mechanisms in mood disorders. Neurosci Biobehav Rev 2021;128:693‐708. [DOI] [PubMed] [Google Scholar]
- 212. Maes M, Carvalho AF. The Compensatory Immune‐Regulatory Reflex System (CIRS) in depression and bipolar disorder. Mol Neurobiol 2018;55:8885‐903. [DOI] [PubMed] [Google Scholar]
- 213. Tylee DS, Lee YK, Wendt FR et al. An atlas of genetic correlations and genetically informed associations linking psychiatric and immune‐related phenotypes. JAMA Psychiatry 2022;79:667‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Beurel E, Lowell JA. Th17 cells in depression. Brain Behav Immun 2018;69:28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Slyepchenko A, Maes M, Köhler CA et al. T helper 17 cells may drive neuroprogression in major depressive disorder: proposal of an integrative model. Neurosci Biobehav Rev 2016;64:83‐100. [DOI] [PubMed] [Google Scholar]
- 216. Giménez‐Palomo A, Dodd S, Anmella G et al. The role of mitochondria in mood disorders: from physiology to pathophysiology and to treatment. Front Psychiatry 2021;12:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kim J‐A, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 2008;102:401‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Stamerra CA, Di Giosia P, Giorgini P et al. Mitochondrial dysfunction and cardiovascular disease: pathophysiology and emerging therapies. Oxid Med Cell Longev 2022;2022:9530007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 219. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443:787‐95. [DOI] [PubMed] [Google Scholar]
- 220. Uchikado Y, Ikeda Y, Ohishi M. Current understanding of the pivotal role of mitochondrial dynamics in cardiovascular diseases and senescence. Front Cardiovasc Med 2022;9:905072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Karabatsiakis A, Böck C, Salinas‐Manrique J et al. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl Psychiatry 2014;4:e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bansal Y, Kuhad A. Mitochondrial dysfunction in depression. Curr Neuropharmacol 2016;14:610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Rezin GT, Cardoso MR, Gonçalves CL et al. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem Int 2008;53:395‐400. [DOI] [PubMed] [Google Scholar]
- 224. Kennedy SH, Evans KR, Krüger S et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001;158:899‐905. [DOI] [PubMed] [Google Scholar]
- 225. Pizzino G, Irrera N, Cucinotta M et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017;2017:8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Mandal PK, Gaur S, Roy RG et al. Schizophrenia, bipolar and major depressive disorders: overview of clinical features, neurotransmitter alterations, pharmacological interventions, and impact of oxidative stress in the disease process. ACS Chem Neurosci 2022;13:2784‐802. [DOI] [PubMed] [Google Scholar]
- 227. Zullo A, Guida R, Sciarrillo R et al. Redox homeostasis in cardiovascular disease: the role of mitochondrial sirtuins. Front Endocrinol 2022;13:858330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Scaini G, Mason BL, Diaz AP et al. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: does inflammation play a role? Mol Psychiatry 2022;27:1095‐102. [DOI] [PubMed] [Google Scholar]
- 229. Diao RY, Gustafsson ÅB. Mitochondrial quality surveillance: mitophagy in cardiovascular health and disease. Am J Physiol Cell Physiol 2022;322:C218‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Fivenson EM, Lautrup S, Sun N et al. Mitophagy in neurodegeneration and aging. Neurochem Int 2017;109:202‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Li A, Gao M, Liu B et al. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis 2022;13:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. McGuinness AJ, Davis JA, Dawson SL et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry 2022;27:1920‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Cryan JF, O'Riordan KJ, Cowan CSM et al. The microbiota‐gut‐brain axis. Physiol Rev 2019;99:1877‐2013. [DOI] [PubMed] [Google Scholar]
- 234. Blachier F, Mariotti F, Huneau J‐F et al. Effects of amino acid‐derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007;33:547‐62. [DOI] [PubMed] [Google Scholar]
- 235. Sun L, Ma L, Ma Y et al. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 2018;9:397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Morris G, Fernandes BS, Puri BK et al. Leaky brain in neurological and psychiatric disorders: drivers and consequences. Aust N Z J Psychiatry 2018;52:924‐48. [DOI] [PubMed] [Google Scholar]
- 237. Kelly JR, Kennedy PJ, Cryan JF et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Stevens BR, Goel R, Seungbum K et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018;67:1555‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Ahmad AF, Dwivedi G, O'Gara F et al. The gut microbiome and cardiovascular disease: current knowledge and clinical potential. Am J Physiol Heart Circ Physiol 2019;317:H923‐38. [DOI] [PubMed] [Google Scholar]
- 240. Aron‐Wisnewsky J, Warmbrunn MV, Nieuwdorp M et al. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health – pathophysiology and therapeutic strategies. Gastroenterology 2021;160:573‐99. [DOI] [PubMed] [Google Scholar]
- 241. Contaldo M, Itro A, Lajolo C et al. Overview on osteoporosis, periodontitis and oral dysbiosis: the emerging role of oral microbiota. Appl Sci 2020;10:6000. [Google Scholar]
- 242. Jungbauer G, Stahli A, Zhu X et al. Periodontal microorganisms and Alzheimer disease – A causative relationship? Periodontol 2000. 2022;89:59‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rabot S, Membrez M, Bruneau A et al. Germ‐free C57BL/6J mice are resistant to high‐fat‐diet‐induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010;24:4948‐59. [DOI] [PubMed] [Google Scholar]
- 244. Cho I, Yamanishi S, Cox L et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Ridaura VK, Faith JJ, Rey FE et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Turnbaugh PJ, Ley RE, Mahowald MA et al. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027‐31. [DOI] [PubMed] [Google Scholar]
- 247. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019;129:4050‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Scott AJ, Alexander JL, Merrifield CA et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut 2019;68:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Padmanabhan JL, Cooke D, Joutsa J et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry 2019;86:749‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Schmaal L, Veltman DJ, van Erp TG et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016;21:806‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Schmaal L, Hibar D, Sämann PG et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017;22:900‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kaiser RH, Andrews‐Hanna JR, Wager TD et al. Large‐scale network dysfunction in major depressive disorder: a meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry 2015;72:603‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Duric V, Clayton S, Leong ML et al. Comorbidity factors and brain mechanisms linking chronic stress and systemic illness. Neural Plast 2016;2016:5460732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Berntson GG, Khalsa SS. Neural circuits of interoception. Trends Neurosci 2021;44:17‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Polityńska B, Pokorska O, Wojtukiewicz AM et al. Is depression the missing link between inflammatory mediators and cancer? Pharmacol Ther 2022;240:108293. [DOI] [PubMed] [Google Scholar]
- 256. Fraile‐Martinez O, Alvarez‐Mon MA, Garcia‐Montero C et al. Understanding the basis of major depressive disorder in oncological patients: biological links, clinical management, challenges, and lifestyle medicine. Front Oncol 2022;12:956923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Sen ZD, Danyeli LV, Woelfer M et al. Linking atypical depression and insulin resistance‐related disorders via low‐grade chronic inflammation: integrating the phenotypic, molecular and neuroanatomical dimensions. Brain Behav Immun 2021;93:335‐52. [DOI] [PubMed] [Google Scholar]
- 258. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013;14:609‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Felger JC. The role of dopamine in inflammation‐associated depression: mechanisms and therapeutic implications. Curr Top Behav Neurosci 2017;31:199‐219. [DOI] [PubMed] [Google Scholar]
- 260. Khanh DV, Choi Y‐H, Moh SH et al. Leptin and insulin signaling in dopaminergic neurons: relationship between energy balance and reward system. Front Psychol 2014;5:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Ng TH, Alloy LB, Smith DV. Meta‐analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry 2019;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Syan SK, McIntyre‐Wood C, Minuzzi L et al. Dysregulated resting state functional connectivity and obesity: a systematic review. Neurosci Biobehav Rev 2021;131:270‐92. [DOI] [PubMed] [Google Scholar]
- 263. Klein MO, Battagello DS, Cardoso AR et al. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol 2019;39:31‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655‐66. [DOI] [PubMed] [Google Scholar]
- 265. Iseger TA, van Bueren NE, Kenemans JL et al. A frontal‐vagal network theory for major depressive disorder: implications for optimizing neuromodulation techniques. Brain Stimul 2020;13:1‐9. [DOI] [PubMed] [Google Scholar]
- 266. Weerasinghe‐Mudiyanselage PD, Ang MJ, Kang S et al. Structural plasticity of the hippocampus in neurodegenerative diseases. Int J Mol Sci 2022;23:3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Morris G, Berk M, Puri BK. A comparison of neuroimaging abnormalities in multiple sclerosis, major depression and chronic fatigue syndrome (myalgic encephalomyelitis): is there a common cause? Mol Neurobiol 2018;55:3592‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Alosco ML, Hayes SM. Structural brain alterations in heart failure: a review of the literature and implications for risk of Alzheimer's disease. Heart Fail Rev 2015;20:561‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Gray JP, Müller VI, Eickhoff SB et al. Multimodal abnormalities of brain structure and function in major depressive disorder: a meta‐analysis of neuroimaging studies. Am J Psychiatry 2020;177:422‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Sha Z, Xia M, Lin Q et al. Meta‐connectomic analysis reveals commonly disrupted functional architectures in network modules and connectors across brain disorders. Cereb Cortex 2018;28:4179‐94. [DOI] [PubMed] [Google Scholar]
- 271. Crossley NA, Mechelli A, Scott J et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014;137:2382‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Lloyd‐Williams M. Difficulties in diagnosing and treating depression in the terminally ill cancer patient. Postgrad Med J 2000;76:555‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Katon WJ, Simon G, Russo J et al. Quality of depression care in a population‐based sample of patients with diabetes and major depression. Med Care 2004;42:1222‐9. [DOI] [PubMed] [Google Scholar]
- 274. Jones S, Howard L, Thornicroft G. ‘Diagnostic overshadowing’: worse physical health care for people with mental illness. Acta Psychiatr Scand 2008;118:169‐71. [DOI] [PubMed] [Google Scholar]
- 275. Kampling H, Baumeister H, Bengel J et al. Prevention of depression in adults with long‐term physical conditions. Cochrane Database Syst Rev 2021;3:CD011246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Cuijpers P, Pineda BS, Quero S et al. Psychological interventions to prevent the onset of depressive disorders: a meta‐analysis of randomized controlled trials. Clin Psychol Rev 2021;83:101955. [DOI] [PubMed] [Google Scholar]
- 277. Zahid J, Grummedal O, Madsen M et al. Prevention of depression in patients with cancer: a systematic review and meta‐analysis of randomized controlled trials. J Psychiatr Res 2020;120:113‐23. [DOI] [PubMed] [Google Scholar]
- 278. Pols A, Adriannse M, van Tulder M et al. Two‐year effectiveness of a stepped‐care depression prevention intervention and predictors of incident depression in primary care patients with diabetes type 2 and/or coronary heart disease and subthreshold depression: data from the Step‐Dep cluster randomised controlled trial. BMJ Open 2018;8:eo20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Fiest KM, Walker JR, Bernstein CN et al. Systematic review and meta‐analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult Scler Relat Disord 2016;5:12‐26. [DOI] [PubMed] [Google Scholar]
- 280. Fiest KM, Hitchon CA, Bernstein CN et al. Systematic review and meta‐analysis of interventions for depression and anxiety in persons with rheumatoid arthritis. J Clin Rheumatol 2017;23:425‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Tully PJ, Ang SY, Lee EJ et al. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev 2021;12:CD008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Ostuzzi G, Turrini G, Gastaldon C et al. Efficacy and acceptability of antidepressants in patients with ischemic heart disease: systematic review and meta‐analysis. Int Clin Psychopharmacol 2019;34:65‐75. [DOI] [PubMed] [Google Scholar]
- 283. Rayner L, Price A, Evans A et al. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev 2010;3:CD007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Price A, Rayner L, Okon‐Rocha E et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta‐analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2011;82:914‐23. [DOI] [PubMed] [Google Scholar]
- 285. Taylor D, Meader N, Bird V et al. Pharmacological interventions for people with depression and chronic physical health problems: systematic review and meta‐analyses of safety and efficacy. Br J Psychiatry 2011;198:179‐88. [DOI] [PubMed] [Google Scholar]
- 286. Ostuzzi G, Benda L, Costa E et al. Efficacy and acceptability of antidepressants on the continuum of depressive experiences in patients with cancer: systematic review and meta‐analysis. Cancer Treat Rev 2015;41:714‐24. [DOI] [PubMed] [Google Scholar]
- 287. Cipriani A, Furukawa TA, Salanti G et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet 2018;391:1357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Zhou W, Zhang Y, Meng H et al. Efficacy and safety of newer‐generation antidepressants for patients with myocardial infarction and depression: a meta‐analysis. Chin J Evid Based Med 2018;18:715‐20. [Google Scholar]
- 289. Mills KA, Greene MC, Dezube R et al. Efficacy and tolerability of antidepressants in Parkinson's disease: a systematic review and network meta‐analysis. Int J Geriatr Psychiatry 2018;33:642‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Su D, Zhang Y, Wang A et al. Efficacy and tolerability of selective serotonin reuptake inhibitors on promoting motor recovery after stroke: meta‐analysis of randomized controlled trials. Expert Rev Neurother 2021;21:1179‐89. [DOI] [PubMed] [Google Scholar]
- 291. Feng R, Wang P, Gao C et al. Effect of sertraline in the treatment and prevention of poststroke depression: a meta‐analysis. Medicine 2018;97:e13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. van der Feltz‐Cornelis C, Allen SF, Holt RIG et al. Treatment for comorbid depressive disorder or subthreshold depression in diabetes mellitus: systematic review and meta‐analysis. Brain Behav 2021;11:e01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Ostuzzi G, Matcham F, Dauchy S et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev 2018;4:CD011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Eshun‐Wilson I, Siegfried N, Akena DH et al. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev 2018;1:CD008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Maguire MJ, Marson AG, Nevitt SJ. Antidepressants for people with epilepsy and depression. Cochrane Database Syst Rev 2021;4:CD010682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Palmer SC, Natale P, Ruospo M et al. Antidepressants for treating depression in adults with end‐stage kidney disease treated with dialysis. Cochrane Database Syst Rev 2016;5:CD004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Mikocka‐Walus A, Prady SL, Pollok J et al. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev 2019;4:CD012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Pollok J, Van Agteren JEM, Carson‐Chahhoud KV. Pharmacological interventions for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018;12: CD012346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Zhuang J, Wang X, Xu L et al. Antidepressants for polycystic ovary syndrome. Cochrane Database Syst Rev 2013;5:CD008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Beedham W, Belli A, Ingaralingam S et al. The management of depression following traumatic brain injury: a systematic review with meta‐analysis. Brain Inj 2020;34:1287‐304. [DOI] [PubMed] [Google Scholar]
- 301. Tran L, Sharrad K, Kopsaftis Z et al. Pharmacological interventions for the treatment of psychological distress in patients with asthma: a systematic review and meta‐analysis. J Asthma 2021;58:759‐69. [DOI] [PubMed] [Google Scholar]
- 302. Miguel C, Karyotaki E, Ciharova M et al. Psychotherapy for comorbid depression and somatic disorders: a systematic review and meta‐analysis. Psychol Med 2023;53:2503‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Mehta S, Peynenburg VA, Hadjistavropoulos HD. Internet‐delivered cognitive behaviour therapy for chronic health conditions: a systematic review and meta‐analysis. J Behav Med 2019;42:169‐87. [DOI] [PubMed] [Google Scholar]
- 304. Cojocaru C, Popa C, Suciu N et al. The efficacy of cognitive‐behavioral therapy for treating major depressive disorder comorbid with chronic disease. Acta Mariseiensis Seria Medica 2021;67:12‐5. [Google Scholar]
- 305. Chen J, Chen X, Sun Y et al. The physiological and psychological effects of cognitive behavior therapy on patients with inflammatory bowel disease before COVID‐19: a systematic review. BMC Gastroenterol 2021;21:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Long J, Briggs M, Astin F. Overview of systematic reviews of mindfulness meditation‐based interventions for people with long‐term conditions. Adv Mind Body Med 2017;31:26‐36. [PubMed] [Google Scholar]
- 307. Piet J, Würtzen H, Zachariae R. The effect of mindfulness‐based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta‐analysis. J Consult Clin Psychol 2012;80:1007‐20. [DOI] [PubMed] [Google Scholar]
- 308. Cillessen L, Johannsen M, Speckens AEM et al. Mindfulness‐based interventions for psychological and physical health outcomes in cancer patients and survivors: a systematic review and meta‐analysis of randomized controlled trials. Psychooncology 2019;28:2257‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Kilic A, Hudson J, McCracken LM et al. A systematic review of the effectiveness of self‐compassion‐related interventions for individuals with chronic physical health conditions. Behav Ther 2021;52:607‐25. [DOI] [PubMed] [Google Scholar]
- 310. Austin J, Drossaert C, Schroevers M et al. Compassion‐based interventions for people with long‐term physical conditions: a mixed methods systematic review. Psychol Health 2020;36:16‐42. [DOI] [PubMed] [Google Scholar]
- 311. Frost R, Bauernfreund Y, Walters K. Non‐pharmacological interventions for depression/anxiety in older adults with physical comorbidities affecting functioning: systematic review and meta‐analysis. Int Psychogeriatr 2019;31:1121‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology 2011;36:1276‐86. [DOI] [PubMed] [Google Scholar]
- 313. Racaru S, Sturt J, Celik A. The effects of psychological interventions on diabetic peripheral neuropathy: a systematic review and meta‐analysis. Pain Manag Nurs 2021;22:302‐11. [DOI] [PubMed] [Google Scholar]
- 314. Zambrano J, Celano CM, Januzzi JL et al. Psychiatric and psychological interventions for depression in patients with heart disease: a scoping review. J Am Heart Assoc 2020;9:e018686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Thombs B, de Jonge P, Coyne J et al. Depression screening and patient outcomes in cardiovascular care. JAMA 2008;300:2161‐71. [DOI] [PubMed] [Google Scholar]
- 316. Berkman L, Blumenthal J, Burg M et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003;289:3106‐16. [DOI] [PubMed] [Google Scholar]
- 317. Freedland K, Carney R, Rich M et al. Cognitive behavior therapy for depression and self‐care in heart failure patients. JAMA Intern Med 2015;175:1773‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Biondi‐Zoccai G, Mazza M, Roever L et al. Evidence‐based psychotherapy in ischemic heart disease: umbrella review and updated meta‐analysis. In: Roncella A, Pristipino C (eds). Psychotherapy for ischemic heart disease. Cham: Springer, 2016:131‐58. [Google Scholar]
- 319. Van Luenen S, Garnefski N, Spinhoven P et al. The benefits of psychosocial interventions for mental health in people living with HIV: a systematic review and meta‐analysis. AIDS Behav 2018;22:9‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Sijercic I, Ennis N, Monson CM. A systematic review of cognitive and behavioral treatments for individuals with psoriasis. J Dermatolog Treat 2020;31:631‐8. [DOI] [PubMed] [Google Scholar]
- 321. Jones CD, Motl R, Sandroff BM. Depression in multiple sclerosis: is one approach for its management enough? Mult Scler Relat Disord 2021;51:102904. [DOI] [PubMed] [Google Scholar]
- 322. Ratajska A, Zurawski J, Healy B et al. Computerized cognitive behavioral therapy for treating depression in multiple sclerosis: a narrative review of current findings and future directions. Int J MS Care 2019;21:113‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Coventry PA, Gellatly JL. Improving outcomes for COPD patients with mild‐to‐moderate anxiety and depression: a systematic review of cognitive behavioural therapy. Br J Health Psychol 2008;13:381‐400. [DOI] [PubMed] [Google Scholar]
- 324. Fritzsche A, Clamor A, von Leupoldt A. Effects of medical and psychological treatment of depression in patients with COPD – a review. Respir Med 2011;105:1422‐33. [DOI] [PubMed] [Google Scholar]
- 325. Zhang X, Yin C, Tian W et al. Effects of cognitive behavioral therapy on anxiety and depression in patients with chronic obstructive pulmonary disease: a meta‐analysis and systematic review. Clin Respir J 2020;14:891‐900. [DOI] [PubMed] [Google Scholar]
- 326. Nadort E, Schouten RW, Witte SHS et al. Treatment of current depressive symptoms in dialysis patients: a systematic review and meta‐analysis. Gen Hosp Psychiatry 2020;67:26‐34. [DOI] [PubMed] [Google Scholar]
- 327. Cukor D, Ver Halen N, Asher D et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol 2014;25:196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Ahmad Othman A, Wan Jaafar WM, Zainuddin ZN et al. Effectiveness of cognitive behaviour therapy on depression among haemodialysis patients: a systematic review of literature. Cogent Psychol 2020;7:1. [Google Scholar]
- 329. Terpstra J, van der Vaart R, Ding J et al. Guided internet‐based cognitive behavioral therapy for patients with rheumatic conditions: a systematic review. Internet Interv 2021;26:100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Treanor CJ, Kouvonen A, Lallukka T et al. Acceptability of computerized cognitive behavioral therapy for adults: umbrella review. JMIR Ment Health 2021;8:e23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Alberts N, Hadjistavropoulos HD, Titov N et al. Patient and provider perceptions of internet‐delivered cognitive behavior therapy for recent cancer survivors. Support Care Cancer 2018;26:597‐603. [DOI] [PubMed] [Google Scholar]
- 332. Croatto G, Vancampfort D, Miola A et al. The impact of pharmacological and non‐pharmacological interventions on physical health outcomes in people with mood disorders across the lifespan: an umbrella review of the evidence from randomised controlled trials. Mol Psychiatry 2023;28:369‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Chen HM, Yang YH, Chen KJ et al. Antidepressants reduced risk of mortality in patients with diabetes mellitus: a population‐based cohort study in Taiwan. J Clin Endocrinol Metab 2019;104:4619‐25. [DOI] [PubMed] [Google Scholar]
- 334. Chen AC, Huang KL, Chen HM et al. Antidepressants and the risk of myocardial infarction among patients with diabetes: a population‐based cohort study. J Affect Disord 2021;294:109‐14. [DOI] [PubMed] [Google Scholar]
- 335. Glozier N, Christensen J, Naismith S et al. Internet‐delivered cognitive behavioural therapy for adults with mild to moderate depression and high cardiovascular disease risks: a randomised attention‐controlled trial. PLoS One 2013;8:e59139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Kessing LV, Rytgaard HC, Gerds TA et al. New drug candidates for depression – a nationwide population‐based study. Acta Psychiatr Scand 2019;139:68‐77. [DOI] [PubMed] [Google Scholar]
- 337. Kohler O, Gasse C, Petersen L et al. The effect of concomitant treatment with SSRIs and statins: a population‐based study. Am J Psychiatry 2016;173:807‐15. [DOI] [PubMed] [Google Scholar]
- 338. Kohler‐Forsberg O, Lydholm CN, Hjorthoj C et al. Efficacy of anti‐inflammatory treatment on major depressive disorder or depressive symptoms: meta‐analysis of clinical trials. Acta Psychiatr Scand 2019;139:404‐19. [DOI] [PubMed] [Google Scholar]
- 339. Kappelmann N, Lewis G, Dantzer R et al. Antidepressant activity of anti‐cytokine treatment: a systematic review and meta‐analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 2018;23:335‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Köhler‐Forsberg O, Otte C, Gold SM et al. Statins in the treatment of depression: hype or hope? Pharmacol Ther 2020;215:107625. [DOI] [PubMed] [Google Scholar]
- 341. Marrie RA, Bernstein CN. Psychiatric comorbidity in immune‐mediated inflammatory diseases. World Psychiatry 2021;20:298‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Qato DM, Ozenberger K, Olfson M. Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. JAMA 2018;319:2289‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Udina M, Castellvi P, Moreno‐Espana J et al. Interferon‐induced depression in chronic hepatitis C: a systematic review and meta‐analysis. J Clin Psychiatry 2012;73:1128‐38. [DOI] [PubMed] [Google Scholar]
- 344. Kovacs D, Kovacs P, Eszlari N et al. Psychological side effects of immune therapies: symptoms and pathomechanism. Curr Opin Pharmacol 2016;29:97‐103. [DOI] [PubMed] [Google Scholar]
- 345. DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev 2010;34:130‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Domecq JP, Prutsky G, Leppin A et al. Clinical review: Drugs commonly associated with weight change: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2015;100:363‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Taylor D. Antidepressant drugs and cardiovascular pathology: a clinical overview of effectiveness and safety. Acta Psychiatr Scand 2008;118:434‐42. [DOI] [PubMed] [Google Scholar]
- 348. Beach SR, Kostis WJ, Celano CM et al. Meta‐analysis of selective serotonin reuptake inhibitor‐associated QTc prolongation. J Clin Psychiatry 2014;75:e441‐9. [DOI] [PubMed] [Google Scholar]
- 349. Williams LJ, Berk M, Hodge JM et al. Selective serotonin reuptake inhibitors (SSRIs) and markers of bone turnover in men. Calcif Tissue Int 2018;103:125‐30. [DOI] [PubMed] [Google Scholar]
- 350. de Abajo FJ, Garcia‐Rodriguez LA. Risk of upper gastrointestinal tract bleed‐ing associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti‐inflammatory drugs and effect of acid‐suppressing agents. Arch Gen Psychiatry 2008;65:795‐803. [DOI] [PubMed] [Google Scholar]
- 351. Bahar MA, Kamp J, Borgsteede SD et al. The impact of CYP2D6 mediated drug‐drug interaction: a systematic review on a combination of metoprolol and paroxetine/fluoxetine. Br J Clin Pharmacol 2018;84:2704‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Klatte R, Strauss B, Fluckiger C et al. Defining and assessing adverse events and harmful effects in psychotherapy study protocols: a systematic review. Psychotherapy 2023;60:130‐48. [DOI] [PubMed] [Google Scholar]
- 353. Wong S, Chan J, Zhang D et al. The safety of mindfulness‐based interventions: a systematic review of randomized controlled trials. Mindfulness 2018;9:1344‐57. [Google Scholar]
- 354. Berk M, Parker G. The elephant on the couch: side‐effects of psychotherapy. Aust N Z J Psychiatry 2009;43:787‐94. [DOI] [PubMed] [Google Scholar]
- 355. De Giorgi R, Rizzo Pesci N, Quinton A et al. Statins in depression: an evidence‐based overview of mechanisms and clinical studies. Front Psychiatry 2021;12:702617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Otte C, Chae WR, Nowacki J et al. Simvastatin add‐on to escitalopram in patients with comorbid obesity and major depression (SIMCODE): study protocol of a multicentre, randomised, double‐blind, placebo‐controlled trial. BMJ Open 2020;10:e040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. El Massry M, Alaeddine LM, Ali L et al. Metformin: a growing journey from glycemic control to the treatment of Alzheimer's disease and depression. Curr Med Chem 2021;28:2328‐45. [DOI] [PubMed] [Google Scholar]
- 358. Domschke K. Prevention in psychiatry: a role for epigenetics? World Psychiatry 2021;20:227‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Malhi GS, Bell E, Bassett D et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 2021;55:7‐117. [DOI] [PubMed] [Google Scholar]
- 360. Marx W, Manger SH, Blencowe M et al. Clinical guidelines for the use of lifestyle‐based mental health care in major depressive disorder: World Federation of Societies for Biological Psychiatry (WFSBP) and Australasian Society of Lifestyle Medicine (ASLM) taskforce. World J Biol Psychiatry 2023;24:333‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Fusar‐Poli P, Correll CU, Arango C et al. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry 2021;20:200‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Shah A, Hussain‐Shamsy N, Strudwick G et al. Digital health interventions for depression and anxiety among people with chronic conditions: scoping review. J Med Internet Res 2022;24:e38030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Maj M, Stein DJ, Parker G et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020;19:269‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Wang Y, Hu M, Zhu D et al. Effectiveness of collaborative care for depression and HbA1c in patients with depression and diabetes: a systematic review and meta‐analysis. Int J Integr Care 2022;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Ali MK, Chwastiak L, Poongothai S et al. Effect of a collaborative care model on depressive symptoms and glycated hemoglobin, blood pressure, and serum cholesterol among patients with depression and diabetes in India: the INDEPENDENT randomized clinical trial. JAMA 2020;324:651‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open 2015;5:e009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Li M, Kennedy EB, Byrne N et al. Systematic review and meta‐analysis of collaborative care interventions for depression in patients with cancer. Psychooncology 2017;26:573‐87. [DOI] [PubMed] [Google Scholar]
- 368. Panagioti M, Bower P, Kontopantelis E et al. Association between chronic physical conditions and the effectiveness of collaborative care for depression: an individual participant data meta‐analysis. JAMA Psychiatry 2016;73:978‐89. [DOI] [PubMed] [Google Scholar]
- 369. Castelijns H, Eijsbroek V, Cees AT et al. Illness burden and physical outcomes associated with collaborative care in patients with comorbid depressive disorder in chronic medical conditions: a systematic review and meta‐analysis. Gen Hosp Psychiatry 2018;50:1‐14. [DOI] [PubMed] [Google Scholar]
- 370. Tuudah E, Foye U, Donetto S et al. Non‐pharmacological integrated interventions for adults targeting type 2 diabetes and mental health comorbidity: a mixed‐methods systematic review. Int J Integr Care 2022;22:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Maisto M, Diana B, Di Tella S et al. Digital interventions for psychological comorbidities in chronic diseases – a systematic review. J Pers Med 2021;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. White V, Linardon J, Stone J et al. Online psychological interventions to reduce symptoms of depression, anxiety, and general distress in those with chronic health conditions: a systematic review and meta‐analysis of randomized controlled trials. Psychol Med 2022;52:548‐73. [DOI] [PubMed] [Google Scholar]
- 373. Torous J, Bucci S, Bell IH et al. The growing field of digital psychiatry: current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry 2021;20:318‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]


