Abstract
An epidemic of obesity has affected large portions of the world, increasing the risk of developing many different age-associated diseases, including cancer, cardiovascular disease, and diabetes. In contrast with the prevailing notion that “a calorie is just a calorie,” there are clear differences, within and between individuals, in the metabolic response to different macronutrient sources. Recent findings challenge this oversimplification; calories from different macronutrient sources or consumed at different times of day have metabolic effects beyond their value as fuel. Here, we summarize discussions conducted at a recent NIH workshop which brought together experts in calorie restriction, macronutrient composition, and time-restricted feeding to discuss how dietary composition and feeding schedule impact whole-body metabolism, longevity and healthspan. These discussions may provide insights into the long-sought molecular mechanisms engaged by calorie restriction to extend lifespan, lead to novel therapies, and potentially inform the development of a personalized food-as-medicine approach to healthy aging.
Keywords: calorie restriction, protein restriction, methionine, isoleucine, ketogenesis, time-restricted feeding, fasting
eTOC blurb
Mihaylova et al. summarize discussions at a recent NIH workshop focused on the regulation of healthy aging by dietary composition and feeding schedule, and defined questions and challenges that must be addressed to translate these research findings to the clinic.
Introduction
For the last century, calorie restriction (CR) has remained the gold-standard for interventions that can extend the lifespan and healthspan of model organisms including flies, rodents, and even non-human primates1. While some metabolic benefits of CR in healthy non-obese humans have been identified2–4, historically, a CR diet has been viewed too abstemious and difficult to be broadly adopted by humans. As a result, one of the “holy grails” of aging research is finding a way to harness the benefits of CR without the need to restrict calories5,6.
Despite several successes in identifying molecules that extend fly and mouse lifespan, including rapamycin, an inhibitor of the mTOR (mechanistic Target of Rapamycin) protein kinase7–12, we do not yet have CR-in-a-pill per se, and there remain deep mysteries surrounding the physiological and molecular mechanisms by which CR functions. For example, while CR and similar diets may converge on reduced activity of mTOR13, there are substantial differences between CR and rapamycin – in flies, rapamycin extends lifespan across a broad range of nutrient intakes12, while in mice the molecular impact of CR and rapamycin treatment are extremely different14–16. In addition, putative geroprotectors may have side effects in humans that limit their potential benefits – for example, while rapamycin robustly extends mouse lifespan, it is FDA-approved as an immunosuppressant, and most laboratory studies are performed in barrier facilities with minimal or no pathogen burden. Rapamycin also causes metabolic disruptions that may be deleterious in humans – and thus the safety of putative geroprotectors in humans must be assessed, as well as their potential efficacy and utility across biological aging17.
In contrast to this limited track record, CR and dietary interventions that seek to mimic some of the benefits of CR – including restriction of protein or specific amino acids, ketogenic diets, and time-restricted feeding – have a strong pre-clinical track record in model organisms, strong associations with decreased rates of age-related disease and mortality in human longitudinal studies, and emerging data from observational human studies and randomized clinical trials1. As such, while some have criticized so-called “anti-aging” diets, many researchers, including those represented at a recent NIH workshop (Table 1), believe that there is much to learn from the study of dietary interventions that improve healthspan in both model organisms and in humans.
Table 1: Workshop Agenda.
Workshop schedule, with talk titles, speakers and key references and background reading.
| NIH Workshop on “Dietary Composition, Time-Restricted Feeding and Associated Metabolic Reprogramming in Healthspan and Longevity Regulation” August 22–23, 2022 | ||
|---|---|---|
| Session 1: Nutrient sensing, macronutrient restriction and dietary composition manipulation in healthspan and longevity regulation | ||
| Talk Title | Speaker | Key References |
| When a calorie is not just a calorie: The regulation of healthy aging by dietary protein and branched-chain amino acids | Dudley Lamming, Ph.D. | 44,69–71,79 |
| Effects of methionine restriction on metabolic and cognitive health | Mirela Delibegovic, Ph.D. | 65 |
| The influence of ketogenic diets on lifespan and healthspan | Jon Ramsey, Ph.D. | 87,89–91 |
| The impact of ketosis on gene expression in multiple tissues of mice | Gino Cortopassi, Ph.D., | 94,95 |
| Session Ila: Interactions between circadian rhythms and dietary interventions in healthspan promotion | ||
| Talk Title | Speaker | Key References |
| Bioenergetics and the Healthful Response to Time Restricted Feeding | Joseph Bass, M.D., Ph.D. | 108 |
| Time-Restricted Feeding and Circadian Mediated Regulation of Age-linked Cardiometabolic Disorders | Girish Melkani, Ph.D. | 110,111,113 |
| Mechanisms of metabolic remodeling during intermittent fasting | Rajat Singh, Ph.D. | 119,120 |
| A dietary molecule as TRF mimetic to promote healthy aging | Zheng Chen, Ph.D. | 101–105 |
| Session Ilb: Interactions between circadian rhythms and dietary interventions in healthspan promotion | ||
| Talk Title | Speaker | Key References |
| Time to live healthier and longer? The tale of mice on Time-Restricted Feeding | Amandine Chaix, Ph.D. | 96–98 |
| Circadian regulation of Drosophila feeding behavior | William Ja, Ph.D. | 116,117 |
| You are when you eat: mechanisms underlying lifespan extension due to intermittent time-restricted feeding in Drosophila | Michele Shirasu-Hiza, Ph.D. | 116 |
| Session III: Macronutrient manipulation in tissue homeostasis and organ function | ||
| Talk Title | Speaker | Key References |
| Influence of diet and nutrient abundance on mammalian intestinal homeostasis | Maria Mihaylova, Ph. D. | 122–124 |
| Branched chain amino acids selectively promote cardiac hypertrophy | Mary Latimer, Ph. D. | 75 |
| Immunometabolic Checkpoints of Inflammation: Lessons from CALERIE | Vishwa Deep Dixit, DVM, Ph. D. | 3,38 |
| Session IV: Translational promises and challenges of dietary interventions to enhance human healthspan | ||
| Talk Title | Speaker | Key References |
| Nutrient and metabolic determinants of human health span—from bench to bedside and back | Anna Thalacker-Mercer, Ph. D. | 82,83 |
| Throwing a Monkey Wrench into Translational Diet Studies | Julie Mattison, Ph. D. | 19,24–27 |
| Opportunities and challenges in incorporating concepts from circadian rhythms and Time-Restricted Eating into lifestyle to increase human healthspan | Satchidananda Panda, Ph.D. | 126–132,134 |
Here, we summarize the presentations and discussions that took place at a NIH Workshop on “Dietary Composition, Time-Restricted Feeding and Associated Metabolic Reprogramming in Healthspan and Longevity Regulation” on August 22–23, 2022. Insights into the dietary regulation of aging and health discussed at this workshop across a range of organisms are summarized in part in Figure 1. We provide a list of provocative questions that will need to be considered by future research (Table 2) to address challenges in the field (Figure 2). Finally, we discuss how the studies discussed here and future work will provide new insights into the molecular regulation of aging and age-related diseases (Figure 3), and may also help optimize dietary interventions that benefit health and longevity for all humans.
Figure 1. Key findings from discussed studies on caloric and nutrient restriction paradigms in fly and mammalian studies.
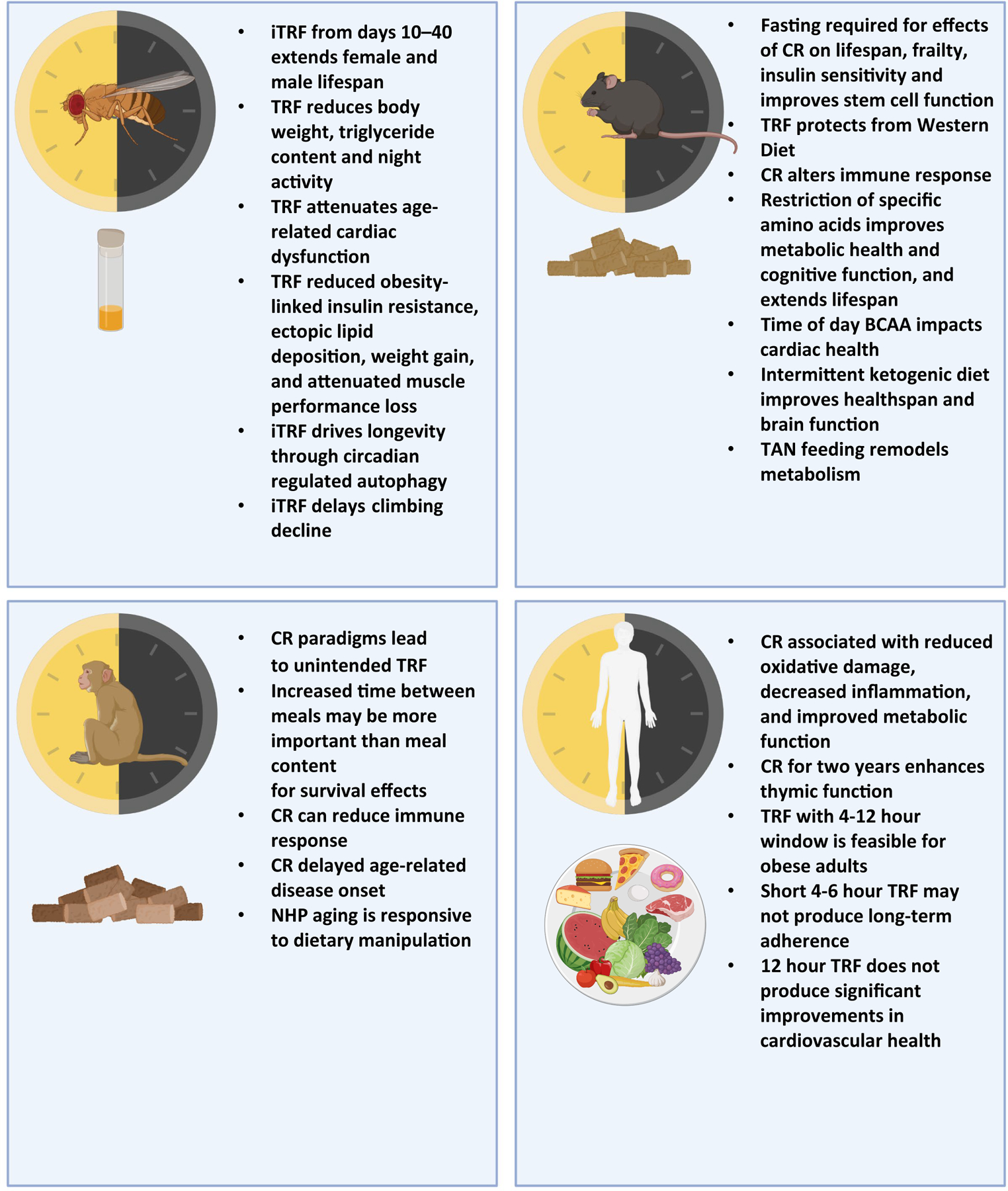
This schematic summarizes some of the key takeaways from presenters of the NIH workshop, summarizing major findings of studies involving time-restricted feeding, fasting and other feeding paradigms across files, mice, nonhuman primates (NHPs) and humans. In flies, several feeding paradigms were described. Time-Restricted Feeding (TRF) in flies can reduce triglyceride content and improve cardiac and skeletal muscle health under aging and obesity. Intermittent fasting regiment (iTRF) from 10–40 days of age extends lifespan of flies 10–15%, however applied too early or late in life can lead to negative consequences for fly health. Additionally, iTRF can delay age-related climbing inability and intestinal dysfunction. In mice, many different time-restricted and nutrient type-restricted studies were described. In particular, the fasting component of Caloric Restriction (CR) is necessary for many of the improvements observed with CR like insulin sensitivity, lifespan extension and reduced frailty. Fasting can improve intestinal stem cell function and serine and glycine deprivation affects muscle stem cells. TRF can protect from obesogenic, western diets and intermittent ketogenic diets improve healthspan measurements. Twice a day feeding in the dark cycle (TAN) remodels metabolic tissues and increases energy expenditure. Multiple amino acid-restricted diets were discussed; restriction of either methionine or branched-chain amino acids (BCAAs) improves metabolic health and cognition, and extends lifespan. In NHPs, meal spacing matters for healthspan measurements and fasting in between meals can improve survival. In humans, adherence to various forms of caloric restriction and time-restricted feeding can be challenging. While some healthy people with obesity can tolerate 4–12 hour feeding windows, shorter windows do not produce long-term adherence. Restricting eating to 12 hours also did not produce significant improvements in cardiovascular health. Caloric restriction sustained over two years can improve thymic function and reduce oxidative damage, however long-term effects of extended caloric restriction on immune cell function remains to be further elucidated. (Parts of this figure were created with BioRender.com)
Meeting report
CR studies in non-human primates
Calorie restriction (CR) strongly preserves healthspan and extends the lifespan of diverse model organisms18–22. To assess the applicability of these studies to humans, CR has been studied in rhesus macaques, a non-human primate that shares 93% genetic homology with humans23, for over 30 years. Two separate studies, based at the NIA and at the University of Wisconsin-Madison (UW), reported different CR effects on overall lifespan. Specifically, survival time was improved in UW CR monkeys compared to their controls, while NIA CR monkeys experienced no survival advantage compared to their controls24,25. Careful consideration of the similar but distinct study designs pointed to features that may account for this effect, including the age of onset, diet composition, and feeding regimen, including the time of day of feeding and the length of time between meals19,26.
At the workshop, Dr. Julie Mattison discussed follow-up rodent studies at the NIA, which imposed the monkey diets and feeding strategies on mice and discovered that an increased fasting duration between meals improved health and survival independent of diet composition26. Increased fasting time was also associated with the upregulation of genomic pathways associated with a pro-longevity response27. The importance of considering time of feeding and fasting in the CR setting was highlighted in a recent study using automated feeders, which found that CR mice consumed their entire day’s meal in about two hours; a typical CR regimen thus unintentionally imposes a long fast between meals28. Feeding mice a low energy diet that eliminated this fasting period blocked the positive effects of CR on insulin sensitivity, frailty, cognitive performance, and longevity29, demonstrating that the prolonged time between meals is required for many of the benefits of a CR diet. Moreover, daily fasting alone – imposed upon mice by making them eat an ad libitum quantity of food each day in only 3 hours – recapitulated the metabolic effects and many of the transcriptional effects of a CR diet in liver and inguinal white adipose tissue29.
Dr. Mattison concluded by noting that, while rhesus monkeys are a valuable translational model for human aging and share many of the characteristic phenotypes, there are a number of “monkey wrenches.” These include many of the same caveats found in rodent studies, such as the need to account for sex differences, age of onset, site differences, diet composition and variability between food batches, treatment of the control group, and the feeding regimen. Additionally, the relatively long lifespan of rhesus macaques poses a challenge for longitudinal studies and highlights the need for validating biomarkers. Yet, despite the complexities, Dr. Mattison concluded that non-human primates offer our greatest opportunity to better understand aging and age-related disease processes.
CR studies in humans/CALERIE
Despite the beneficial effects of 40% CR in many strains of mice, it has been observed that this degree of restriction can lead to reduced lifespan in some strains of mice30. The negative effects of CR include increased mortality from influenza virus31, death from polymicrobial sepsis32, impaired immunity to parasitic infections33, and the death of thymocytes34 in mice. Four years of CR in primates has been shown to reduce immune response35. Thus, a great unknown in the CR field is whether CR can delay aging without causing immunological tradeoffs, prevent age-related diseases, and extend lifespan in humans. This is particularly relevant now, as the popular press surrounding the results of CR in model organisms has led an increasing number of people to adopt CR or CR-mimetic strategies for themselves.
Almost two decades ago, a diverse set of NIH-funded researchers undertook the ambitious CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) studies (CALERIE-I and CALERIE-II) to determine if two years of a CR diet would slow aging and protect against age-related diseases in humans36,37. Dr. Vishwa Deep Dixit gave an update at the workshop on his laboratory’s recent work from CALERIE-II participants. Dixit and colleagues hypothesized that negative energy balance in humans, without systems tradeoffs, will reveal new immunometabolic checkpoints to lower inflammation and enhance healthspan. Analyses of healthy humans aged 25–45 years of age, revealed that sustained CR for two years enhanced thymic function, as measured by reduction of ectopic lipid, increased functional thymic volume, and increase in recent thymic emigrants in CD4 and CD8 T cells in blood3. Analyses of abdominal subcutaneous adipose tissue revealed that CR in humans activates transcriptional programs implicated in mitochondrial metabolism, anti-inflammatory responses, and longevity (Figure 1). His laboratory identified the gene encoding macrophage derived protein PLA2G7 (group VII A platelet activating factor acetylhydrolase) as significantly inhibited by CR in humans3. Using mouse models, Dr. Dixit’s lab showed that reducing PLA2G7 had beneficial effects, decreasing inflammaging via inhibition of NLRP3 inflammasome and protecting mice from thymic involution and metabolic dysfunction3.
Dr. Dixit also reported on the identification of a CR-inhibited adipokine, SPARC (matricellular protein, secreted protein acidic and rich in cysteine). SPARC is a critical regulator of inflammation, converting anti-inflammatory macrophages into a pro-inflammatory phenotype. In mice, depletion of adipocyte SPARC decreased inflammaging and extended healthspan38. Dr. Dixit summarized by noting that his lab has demonstrated that – while it remains unknown if CR extends human lifespan, CR is clearly relevant to human physiology and immunobiology, and may be an important way to harness immunometabolic checkpoints of inflammation and longevity. These data also demonstrated that the simple reduction of calories by 15%, without alteration in meal timings or frequency, was sufficient to reduce oxidative stress, inflammation and improve metabolic function in humans.
Protein and amino acid restriction
CR proportionally decreases the consumption of dietary macronutrients, including protein, and it has long been hypothesized that reduced intake of specific macronutrients might account for the benefits of a CR diet. Recently there has been great interest in the role of dietary protein. Most dietary advice for humans recommends increased protein consumption, especially for active individuals and those who are middle aged or elderly39,40. However, long-term large prospective and retrospective clinical trials have shown that dietary protein intake is correlated with rates of multiple age-related diseases, including cancer, cardiovascular disease, and diabetes in humans, as well as increased mortality in those under 65 years of age41–43. Two short-term randomized clinical trials of protein restriction (PR) similarly found that PR reduces weight and adipose mass, and improve blood glucose regulation, in overweight individuals and people with type 2 diabetes44,45.
Restriction of protein has been shown to extend the lifespan of both flies46–49 and rodents20,50. Restriction of dietary protein also improves metabolic health in mice and rats50–52. One potential contributor to the variability in human studies has been variation in protein source, which has been shown to affect longevity in rats53. A possible explanation for the effect of different protein sources on health and longevity is that different protein sources have distinct amino acid profiles. This naturally begs the question of which, if any, amino acids reduced in a PR diet are responsible for the benefits of a PR diet.
Methionine Restriction
Many researchers have attributed the benefits of a vegan diet to the consumption of plant proteins that are naturally lower in the essential amino acid methionine than red meat54,55, although this has been disputed56. As discussed by Dr. Mirela Delibegovic, methionine restriction (MR) in rodents – achieved by reducing methionine levels from 0.86% of the diet to 0.172% – dramatically decreases body weight and adiposity, improves insulin sensitivity relative to animals on a control diet, and extends lifespan57–59. MR and methionine depleted diets have also shown efficacy in restoring metabolic health to genetic and diet-induced models of obesity, promoting reduced weight and adiposity, decreasing or reversing hepatic steatosis, and normalizing glucose homeostasis60–63. Beneficial metabolic effects, notably increased fat oxidation, have also been seen in short-term clinical trials of MR in humans64.
However, while in this respect MR mimics the effects of a CR diet, MR-fed mice have ad libitum access to food. The Delibegovic lab has shown that MR in aged 12-month-old mice significantly increases food intake, yet completely reverses age-induced alterations in body weight, adiposity, physical activity, and glucose tolerance to the levels observed in healthy 2-month-old control-fed mice65. Similarly, a methionine depleted diet leads to dramatic reductions in body weight and adiposity, and improved glucose tolerance, in both 6-month-old and 22-month-old mice66.
In unpublished work discussed by Dr. Delibegovic, her laboratory assessed the ability of MR to improve cognitive and motor function in aged mice using rotarod and Y-maze tests. As expected, aging led to decreased locomotor function and cognitive performance in control-diet fed mice; relative to these controls, aged MR-fed mice had improved locomotor and cognitive performance. They next asked if MR could improve cognitive function and performance in a mouse model of Alzheimer’s disease. Using Tg4510 mice, a model of frontotemporal dementia with overexpression of mutant human Tau protein, they found that MR significantly improved performance in behavioural tests, without alterations in metabolic outcomes, suggesting the cognitive benefits of MR are directly regulated by the diet (Figure 1).
Branched-chain amino acid restriction
The branched-chain amino acids (BCAAs) leucine, isoleucine, and valine are elevated in the blood of both humans and rodent models of obesity and diabetes and can be predictive of the future development of type 2 diabetes67,68. At the workshop, Dr. Dudley Lamming discussed recent work from his laboratory examining the effects of BCAA restriction on the healthspan and longevity of mice. The Lamming lab has found that restricting dietary BCAAs by 67% promoted metabolic health in wild-type C57BL/6J mice when started in midlife and led to a 30% increase in lifespan and a reduction in frailty in male, but not female, wild-type mice when they underwent lifelong feeding69. BCAAs are strong agonists of mTORC1, and BCAA-restricted male, but not female mice had reduced mTORC1 activity in skeletal muscle and liver.
While the BCAAs have typically been considered as a group, emerging evidence suggests that each of the three BCAAs has distinct metabolic roles. Dr. Lamming’s group recently showed that dietary isoleucine is a key regulator of metabolic health, and that reduction of dietary isoleucine is both necessary and sufficient for the metabolic benefits of a PR diet70. At the workshop, Dr. Lamming highlighted unpublished work showing that specifically restricting isoleucine by 67% reduces frailty and extends the lifespan of both male and female mice71. Further, Dr. Lamming’s recent work and work from other groups has found that specifically restricting valine also benefits the metabolic health of mice72 (Figure 1).
While the benefits of BCAA restriction for the healthy aging of humans remains unknown, recent studies suggest that short-term restriction of BCAAs promotes insulin sensitivity73. Dietary levels of isoleucine are associated with body mass index in humans70, while blood levels of isoleucine are predictive of increased mortality risk74. Dr. Lamming suggested that reducing dietary BCAAs or isoleucine, or developing pharmaceuticals that mimic these effects, may hold potential as a translatable intervention to promote healthy aging.
Interestingly, the time-of-day when BCAAs are consumed may impact their effect on health. Dr. Mary Latimer discussed her research on how the time of day of BCAA consumption influences cardiometabolic and cardiovascular outcomes. She found that feeding mice a BCAA-enriched meal at the end of the active period (i.e., the last 4hrs of the dark phase) rapidly increased cardiac protein synthesis and mass, as well as cardiomyocyte size75. In contrast, consuming an identical BCAA-enriched meal at the beginning of the active period (i.e., first 4hrs of the dark phase) had no effect. Dr. Latimer also observed greater activation of mTOR signaling in the heart by BCAAs fed at the end of the active period. Pharmacological inhibition of mTOR signaling with rapamycin blocked BCAA-induced augmentation of cardiac mass and cardiomyocyte size, while repetitive consumption of BCAA-enriched meals at the end of the active period accelerated adverse cardiac remodeling and contractile dysfunction in mice subjected to transverse aortic constriction. Thus, in addition to the quantity of BCAAs consumed, the timing of BCAA consumption has implications for cardiac health and disease.
Restriction of other amino acids
Of the other common amino acids, dietary threonine and tryptophan have recently been shown to regulate expression of the energy balance hormone FGF21 and restriction of threonine has been shown to retard the development of obesity-associated metabolic dysfunction76. Tryptophan restriction has also been reported to extend the lifespan of mice and rats77,78. Dr. Lamming reported that his lab has found that histidine restriction by 67% promotes leanness and metabolic health in young and old aged male mice, but did not extend lifespan; moreover, histidine-restricted mice have increased energy expenditure relative to control-fed animals, but this effect was not dependent on FGF2179.
Dr. Anna Thalacker-Mercer discussed the reliance of skeletal muscle stem/progenitor cells on the non-essential amino acids serine and glycine. Supplementation with glycine extends the lifespan of both male and female UM-HET3 mice, and perhaps of rats80,81, and Dr. Thalacker-Mercer previously demonstrated that glycine has a positive relationship with glucose disposal rate, a marker of insulin action, in humans82. She discussed recent work from her laboratory showing that with advancing age, these amino acids are reduced in both human and rodent models83. Furthermore, Dr. Thalacker-Mercer’s group has found that reduced serine or glycine availability impairs skeletal muscle stem/progenitor cell proliferation and leads to pronounced adipocyte accumulation in the skeletal muscle of aged mice following injury, hallmarks of aging skeletal muscle83. These results suggest that decreased serine and glycine availability in advanced age may underlie age-related muscle deterioration.
Ketogenic Diets
CR, intermittent fasting, and time-restricted feeding are all dietary interventions that include periods of fasting84. In mice, postprandial blood ketone levels can be significantly increased by eight hours of fasting85, and many scientists are interested in understanding how ketones may influence aging. Dr. Jon Ramsey discussed multiple mechanisms by which the ketone β-hydroxybutyrate may influence aging: by acting as an energy substrate and alternative to glucose, through receptor mediated effects on cells, through protein acetylation or β-hydroxybutyrylation, or via inhibition of histone deacetylases86. In their studies, Dr. Ramsey’s group has observed a 13.6% increase in median lifespan and a significant decrease in histiocytic sarcomas in mice maintained on a ketogenic diet87. In addition, motor function and memory were improved in aged mice consuming a ketogenic versus control diet87. Studies of mice on an intermittent ketogenic diet fed every other week also showed improvement in healthspan measurements in aged animals88.
These initial ketogenic diet and aging studies were completed in male mice, and a recent study showed that a ketogenic diet started in middle-aged female mice may also produce benefits, as demonstrated by increased spatial memory and exploratory behavior and increased mitochondrial mass in skeletal muscle89. These results are consistent with studies of male mice reporting increased mitochondrial number in gastrocnemius muscle90 and an increase in muscle mitochondrial biogenesis and preservation of skeletal muscle mass with advanced age in animals consuming a ketogenic diet91. This work supports the idea that maintenance of skeletal muscle mass and mitochondria with aging may contribute to improvements in motor function with a ketogenic diet.
β-hydroxybutyrate inhibits the NLRP3 inflammasome92 and a ketogenic diet mitigates age-related increases in pro-inflammatory cytokines87; this inhibition of inflammation may play a role in the extension of healthspan and longevity with a ketogenic diet. Additional discussion at the meeting highlighted the importance of isocaloric dietary approaches to determine the impact of sustained ketosis independent from diet-induced obesity or weight loss. In contrast to humans, where there is interest in ketogenic diets inducing satiety and weight loss, level of intake may predict outcomes in mice as these animals may become obese when provided ad libitum access to ketogenic diets, negating the beneficial effects by depleting adipose-resident γδ T cells and impairing metabolic health and glucose homeostasis93.
Dr. Gino Cortopassi discussed the impact of ketosis on brain function, including the results of four studies with carbohydrate versus ketogenic diet interventions from 13–26 months of age. Mouse brains were analyzed by RNAseq and these data supported a rise in synaptic pruning with age, while the ketogenic diet mitigated the microglial component of this pruning. Other ketogenic diet studies of 7-month duration supported the concept that this diet has a pro-synaptic and long-term potentiation effect, while a short 6-week ketogenic diet produced profound changes in hepatic gene expression and anti-inflammatory effects in brain. Dr. Cortopassi’s group recently identified potential “ketodrugs” that appear to mimic consumption of a ketogenic diet94, including analogs more potent than the parent ketodrug compound95. Such ketodrugs have an anti-inflammatory impact in the brain of similar potency to a ketogenic diet. Dr. Cortopassi concluded that these data support the idea that the anti-inflammatory actions of the ketogenic diet, β-hydroxybutyrate, and ketodrugs may underpin the increased effects of these interventions on longevity and functional preservation in mice.
Time-Restricted Feeding in rodents
Time-restricted feeding (TRF) refers to dietary interventions that limit consumption of food to a short daily window of time. In landmark studies, workshop speaker Dr. Satchidananda Panda previously found that mice were protected from isocaloric Western diet-induced metabolic disease if they were only permitted ad libitum food access for 8 hours per day, during their active phase96,97. In mice fed a standard diet, short-term TRF studies did not show a significant change in body weight, while an increase in muscle mass was often observed. Muscle mass preservation was sometimes (not always) observed in high-fat-fed mice on TRF98. Long-term TRF studies in mice fed a “standard diet” (or equivalent) showed an increase in both lifespan and healthspan in C57BL/6J mice. But CR, relative to TRF, showed better healthspan and lifespan outcomes26 and CR + TRF have an additive contribution to mouse lifespan99. Altogether, these rodent studies have highlighted the importance of quality, quantity, and timing of nutrition in healthspan.
At the workshop, Dr. Amandine Chaix highlighted that the majority of previously published TRF studies were conducted in young, C57BL/6J male mice, with the effects of TRF assessed after relatively short, 8–12-week interventions. Dr. Chaix then discussed recently published work investigating the effects of TRF in young versus middle-aged mice of both sexes100. Overall, 3-month-old and 12-month-old male C57BL/6J mice showed similar metabolic benefits from TRF. In contrast, female mice, independently of their age, did not show a difference in body weight changes between ad libitum fed and TRF-fed groups. Yet, females on TRF had better glucose tolerance and reduced hepatic steatosis suggesting that some benefits of TRF were independent from body weight changes. This study also highlights the critical importance of further understanding sexual dimorphism in diet and corresponding metabolic response in rodents and humans. In ongoing research, Dr. Chaix is investigating the lifelong health benefits of TRF initiated in middle-aged male mice upon pre-established obesity and metabolic imbalance. This study is also examining whether TRF can increase lifespan and sustain clock function through old age.
Dr. Zheng Chen discussed a pharmacological approach to realizing the benefits of TRF. The Chen laboratory identified Nobiletin, a natural polymethoxylated flavone, as a clock-enhancing small molecule that directly activates retinoid acid receptor-related orphan receptor (ROR) in the core oscillator101. Similar to TRF, Nobiletin confers broad beneficial effects in various disease and aging mouse models. The benefits of Nobiletin include fortifying mitochondrial respiration and cardiolipin synthesis in aging skeletal muscle102,103, and reducing Alzheimer’s disease pathology in mouse models104,105. During the workshop, Dr. Chen presented data suggesting that Nobiletin and TRF have functional synergism to improve certain key metabolites and hormones in naturally aged mice, acting together to strengthen circadian resilience and physiological homeostasis during healthy aging.
Dr. Joseph Bass discussed recent work from his laboratory on how circadian disruption alters energy balance and showed that the response to time-restricted feeding involves adipose thermogenesis. He discussed foundational work from his laboratory demonstrating that a high-fat diet feeding causes period lengthening in mice, demonstrating the ability of energy dense diets to disrupt timekeeping mechanisms in the body106. Restricting high-fat diet feeding to the dark period reduced weight gain compared to restricting high-fat diet to the light period, even if animals consumed the same amount of energy in calories, highlighting that the importance of time of day of food consumption to maintain energy balance107. In part, this is due to decreased energy expenditure in the light (inactive) cycle, leading to higher weight gain in mice fed during the day. Recent investigation of the molecular mechanisms underlying these effects by the Bass laboratory showed that ablation of the zinc finger protein 423 (ZFP423), specifically in adipose tissue, blunted the negative effects of daytime high-fat diet feeding in mice by increasing futile creatine cycling108. Conversely, adipocyte-specific overexpression of ZFP423 suppressed adipocyte thermogenesis and exaggerated weight gain even when eating occurred during the active phase (the dark period for mice). Thus, circadian control of adipocyte creatine metabolism drove the timing of diet-induced thermogenesis, and enhancement of adipocyte circadian rhythm through overexpression of the clock activator BMAL1 (brain and muscle Arnt-like protein-1) ameliorated metabolic complications during diet-induced obesity, reducing diet-induced increases in weight and adiposity, and improving glucose tolerance108.
Time-Restricted Feeding in flies
Dr. Girish Melkani discussed the implications of studies on the time of eating in humans, which have shown that late-night eaters and skipping breakfast have higher risk of heart disease after controlling for diet and lifestyle factors109. Aging is one of the highest risk factors for the development of several human diseases which are exacerbated by metabolic challenges. The mechanistic basis of metabolic and circadian dysregulation leading to aging and cardiometabolic disorders is largely unexplored. Using Drosophila as a model organism, his group has examined the effects of TRF on heart health (Figure 1). TRF-fed flies had reduced body weight, triglyceride content and significantly reduced night activity, while increasing daily sleep (Figure 1). TRF also attenuated age related cardiac dysfunction and RNA sequencing of hearts showed upregulated TCP chaperones and downregulated ETC components that may contribute to delays of age-dependent cardiac defects110. In addition, Dr. Melkani reported that TRF counteracted obesity-linked dysmetabolism and improved muscle performance by suppressing intramuscular fat deposits, mitochondrial defects, and markers of insulin resistance in Drosophila111 (Figure 1).
Dr. Melkani’s group has demonstrated a mechanistic basis for the TRF-mediated benefits to muscle in diet- and genetic-induced obesity by utilizing temporal transcriptomic data of muscle followed by genetic validations. His group has uncovered the involvement of distinct pathways in different obesity models for TRF-mediated muscle improvement112 (Figure 1). For example, TRF resulted in significant upregulation of Gnmt, Sardh and CG5955; genetic inhibition of these genes leads to skeletal muscle dysfunction, aberrant lipid accumulation and loss of TRF-mediated benefits. In contrast, flies with skeletal muscle-specific inhibition of Dgat2 retained muscle function during aging, a result that mimicked the benefits of TRF. Furthermore, de novo purine biosynthesis appeared to be upregulated as a consequence of diet-induced obesity, while AMPK signaling, glycogen metabolism, glycolysis, TCA cycle and ETC signaling were specifically upregulated in a genetic obesity model of TRF. TRF-mediated benefits in genetic-induced obesity were mediated via activation of AMPK, which led to increased ATP levels. Altogether, they identified/validated shared and distinct pathways in the regulation of aging under TRF113. The findings may pave the way for future TRF studies in cardiac and skeletal muscle, providing a natural and affordable form of alternative intervention for managing pathophysiological effects related to aging, metabolism and obesity.
Benefits from intermittent feeding are not always associated with longevity111, and Dr. William Ja discussed historical findings to extend lifespan in Drosophila via TRF114. In the past few years, new intermittent fasting regimens have been reported to extend Drosophila lifespan, such as the 2:5 diet where flies are fed for two consecutive days per week followed by 5 days of fasting115, as well as an intermittent fasting regimen (iTRF) that drives longevity through circadian autophagy116. At least with iTRF, longevity required matching of food access cycles to natural feeding rhythms, highlighting the importance of precise measurements of feeding behavior and its regulation. Diverse methods to quantify food intake in flies were discussed, including the use of an automated liquid feeding assay (Capillary Feeder, CAFE), which can provide more precise metrics, including meal frequency, timing, and satiety ratio to quantify how satisfying each type of diet is to flies117. Using these methods, Dr. Ja showed that flies ate more frequently during the light period, consistent with previous studies118, and that there was strong rhythmicity in food intake. They also observed fly strains with alternative, “two-peak” feeding rhythms. Using genetic approaches to knock out clock function in the gut specifically, they observed disruption of feeding behaviors but intact locomotor activity. His laboratory is now examining whether different feeding rhythms might alter the response to interventions.
Dr. Mimi Shirasu-Hiza discussed night feeding, which in humans is associated with multitude of metabolic diseases. Her laboratory tested many different dietary regimens before identifying a dietary intervention, iTRF, that promoted longevity and healthspan in flies116. iTRF consists of a 6-hour morning feeding window, followed by fasting for 20 hours, and then refeeding for 28 hours before fasting for an additional 20 hours. Applying iTRF from 10 to 40 days of age extended lifespan by 15–20%. Consistent with previous work115, iTRF applied too early or extended past day 45 was found to be deleterious for fly health. While the iTRF experiments were primarily conducted in female flies, Dr. Shirasu-Hiza also observed similar, slightly more modest benefits for males (e.g., 10–15% lifespan extension).
iTRF delayed several age-associated phenotypes, including climbing activity decline and intestinal dysfunction. iTRF was distinct from CR, as the average food intake during iTRF is higher than that in CR flies; importantly, the effects of iTRF are additive with CR for life extension. iTRF also enhanced circadian gene expression compared to ad libitum fed controls, and proper clock function was necessary for the effects of iTRF-mediated life extension. Highlighting the importance of circadian timing in the health benefits of TRF, only night-centered TRF extended lifespan while day-centered TRF did not. Moreover, night-specific expression of atg1 on an ad libitum diet was sufficient for an extension of lifespan similar to iTRF-mediated lifespan extension and the lifespan extension caused by night-specific atg1 expression was not further extended by iTRF. Importantly, day-specific atg1 expression was not sufficient to extend lifespan. These results suggest that the health benefits of circadian-aligned (night-centered) TRF are mediated by enhancement of both circadian clock function and autophagy.
Intermittent fasting
Dr. Rajat Singh discussed mechanisms of metabolic remodeling during intermittent fasting, focusing on interactions between food, timing, and autophagy in mice. He described an isocaloric intervention, in which animals were fed two times a day in the light cycle (TAD); his lab found that autophagy activation and flux increased in a biphasic manner following fasting periods between the two meals compared to regular ad libitum mice, where autophagy increased only at the end of the light cycle and stayed low during the dark cycle119. Increased levels of FGF21 correlated with the first autophagy phase, during the light cycle in TAD animals, but interestingly did not with the second phase in the dark cycle. Conversely, insulin levels increased following the second meal administered right before the dark cycle in the TAD animals but not following the first light cycle meal. Coordinated activation of autophagy in the hypothalamus-liver and hypothalamus-fat axes appeared to be required for remodeling in these peripheral tissues as demonstrated by loss of these benefits in tissue-specific autophagy deficient mice. Collectively, these findings suggest that the two meals a day regiment can segregate anabolic and catabolic processes and modulate autophagy in multiple tissues to drive systemic metabolic benefits120.
Dr. Singh then discussed new work from his lab comparing TAD feeding during daytime versus twice a day feeding in the dark cycle (TAN). His laboratory found that nocturnal meal spacing resulted in greater weight loss and lower adiposity compared to the daytime twice a day group. Interestingly, TAN feeding remodeled subcutaneous fat and in particular vascularity and nerve density. Post-prandially, TAN-fed mice had consistently increased energy expenditure; while brown fat and eWAT activation did not drive this weight loss, it may be driven by beiging of iWAT. Collectively, subcutaneous tissue remodeling and increased energy expenditure regulated body composition changes in TAN-fed mice. There may also be a potential neuronal humoral axis driven by TAN feeding that induces browning and possibly enervation in the subcutaneous fat depot. It remains to be seen how humans may respond to the once versus twice a day feeding as opposed to more frequent but lower calorie meals.
Dr. Maria Mihaylova discussed key early findings of dietary effects on aging and in particular, epithelial biology. She next talked about the importance of adult stem cells in maintaining tissue homeostasis and function and how they are impacted by aging121,122. One of the key efforts of her research is understanding nutrient sensing and utilization across different tissues and cell types. The remainder of her talk focused on mammalian intestinal stem and progenitor cells and their response to nutrient perturbations. Intestinal stem cells can directly sense nutrient deprivation in the form of fasting123 and adapt to CR over time124. Her work stemmed from biochemical analysis of nutrient responses in crypts enriched in stem and progenitor cells, which responded robustly to nutrient deprivation when mice were subjected to 16 to 24 hours of fasting. She also stated the necessity of understanding how fasting affects adult stem cells, given that many of the life-extending caloric restriction regimens entrain mice to consume food in short periods of time, while the reminder of time mice are subjected to extended periods of fasting.
Dr. Mihaylova discussed how mammalian intestinal stem cells induced a fatty acid oxidation program in response to fasting, which allowed them to switch their metabolism to better utilize exogenous lipids. Interestingly, fasting primed stem and progenitor cells to proliferate better in nutrient-rich culture conditions, which mimics a refeeding state following the fast. This may also be the case in vivo, where stem and progenitor cells may be primed to proliferate faster upon refeeding, perhaps leading to extension of villi for more effective nutrient absorption under nutrient rich states. Along with collaborators, Dr. Mihaylova showed that with age stem cell function declines and stem and progenitor cell proliferation is also reduced, leading to decreased epithelial turnover and worsened responses to tissue injury. She hypothesized that some of the age-dependent effects may result in part due to metabolic changes and inflexibility in aged stem and progenitor cells compared to young stem cells. The Mihaylova laboratory, with collaborators, is now developing several approaches to measure cellular metabolic signatures and heterogeneity at or near the single cell level. She hopes that in the future, these approaches will help capture the diverse cellular responses to dietary interventions across young and aged tissues.
Time-restricted feeding studies in humans.
Dr. Satchidananda Panda closed out the workshop by giving an overview of opportunities and challenges in leveraging the concept of time-restricted feeding to increase human healthspan. Many (not all) studies, carried out in sensitized conditions of feeding energy-dense diet (high fat, high sucrose, high fructose etc. diets), found that TRF prevented or reduced the risks for many age-related chronic diseases that affect diverse organ systems and brain regions98. In addition, therapeutic or regression studies in middle-aged mice or genetic models of metabolic or age-related diseases have yielded many benefits of TRF. TRF also increased survival from LPS challenge100.
TRF may potentially benefit humans who spread their calorie intake over a long period of the 24-hour day. As TRF is based on the concept of circadian rhythm, which is affected by changes in eating time125, specifically breakfast or dinner time, it is necessary to assess eating time over a few days. However, the current gold standard methods for collecting human nutrition data – 24 hour dietary recall and food frequency questionnaires – were not designed to capture daily eating window and day-to-day variations reliably. So, new methods or survey instruments are needed to capture human eating time behavior.
The Panda laboratory developed a simple app – myCircadianClock – to determine the eating window from several days of self-recorded meal timing logs. They used mid 95%ile eating window calculated from at least one week (preferably 2–3 weeks) of food records, which can account for habitual and day-to-day mealtime variations. They found that adults working regular hours had a 95% eating window of ~14 hours and 45min, and only <10% of adults had an eating window of less than 12 hours126,127. By combining app data with actigraphy-based sleep measurement, they found that 80% of adults ate or drank energy-containing food/beverages within 1 hour of waking up, and 50% of adults ate or drank within 2h before bedtime. Therefore, app-based multiple day food records integrating sleep time are needed for collecting circadian-relevant meal timing in humans.
Several pilot or feasibility studies have shown that short-term (<12 weeks) TRF with a target eating window ranging from 4 to 12 hours was feasible among healthy overweight, obese, or those with metabolic diseases128. Most of these studies reported some health benefits relating to improvements in metabolic health; modest weight loss, improved glucose tolerance, blood pressure, plasma lipids, and markers of chronic inflammation. However, TRF with 4 – 6 hour target windows produced mild to moderate adverse events and may not have long-term adherence. Although the 8 hour target window appeared doable in 3–6 months studies, objective data on eating time was not available for most studies. In one study129, where objective data was available, participants started with 8 hours, but more than 50% drifted towards 10 hours by the end of 3 months of intervention. In 10 hour TRF, participants adhered for 5–6 days a week, and long-term adherence (at 1y; 9 months after study completion) was ~70%130. 12 hour TRF did not produce significant improvement in cardiometabolic disease risk factors131. Altogether, 8–10 hour TRF may offer a pragmatic eating window for long-term adherence.
Although the goal of TRF in humans is not explicitly to reduce energy intake, TRF of 10 hours or less also resulted in inadvertent calorie reduction between 5–20%. In addition, some TRF studies also resulted in an improvement in diet quality and a reduction in snacking131,132. However, health improvements in TRF studies appeared to be disproportionately larger than those expected from the observed weight loss130 or no change in weight133.
As TRF inadvertently causes CR in humans and rodent CR studies inadvertently include TRF, an obvious question is whether humans in CR studies also inadvertently adopt TRF? A systematic analysis of multiple days of meal records from 2-year long CR study, CALERIE II, found no significant change in the eating window134; their baseline eating window was >12 hours and was not significantly reduced by CR. These health benefits found in TRF studies resulted when the participants’ baseline eating window was 12 hours or longer and they reduced their eating window by >3 hours. The findings raise the question of whether adherence to CR and the benefits of CR can be improved among humans who eat >12 hours by incorporating 8–10 hour TRF as a tool to follow CR.
Solutions and Challenges
There is a growing awareness that the long-held paradigm that “a calorie is a calorie is a calorie” is misleading, and that calories from different macronutrient sources impact metabolism and health beyond their simple caloric value. While this has previously been shown to be true for carbohydrates and fats135, recent work has shown that protein has a critical impact, with low-protein, high-carbohydrate diets promoting metabolic health and longevity in rodents and low-protein diets promoting leanness, glycemic control and insulin sensitivity in humans44,45. Presentations at the workshop further highlighted this concept through discussions on how energy intake, the precise macronutrient composition of the diet, and daily eating time regulates healthy aging in model organisms ranging from the humble fruit fly up to mice, non-human primates, and even humans. Following the workshop, participants listed many important open questions relating to dietary interventions in aging and age-related diseases that are summarized in part in Table 2, and also further discussed below. Many of these open questions relate to challenges that will need to be overcome in order to translate the research discussed at the workshop to the clinic (Figure 2).
Table 2:
Provocative questions in dietary interventions to promote healthspan and longevity.
| Nutrient sensing, macronutrient restriction and dietary composition manipulation |
|
| Circadian rhythms and dietary interventions in healthspan promotion |
|
| Macronutrient manipulation in tissue homeostasis and organ function |
|
| Translational promises and challenges of dietary interventions to enhance human healthspan |
|
Figure 2. Challenges for translating dietary and pharmaceutical interventions into the clinic.
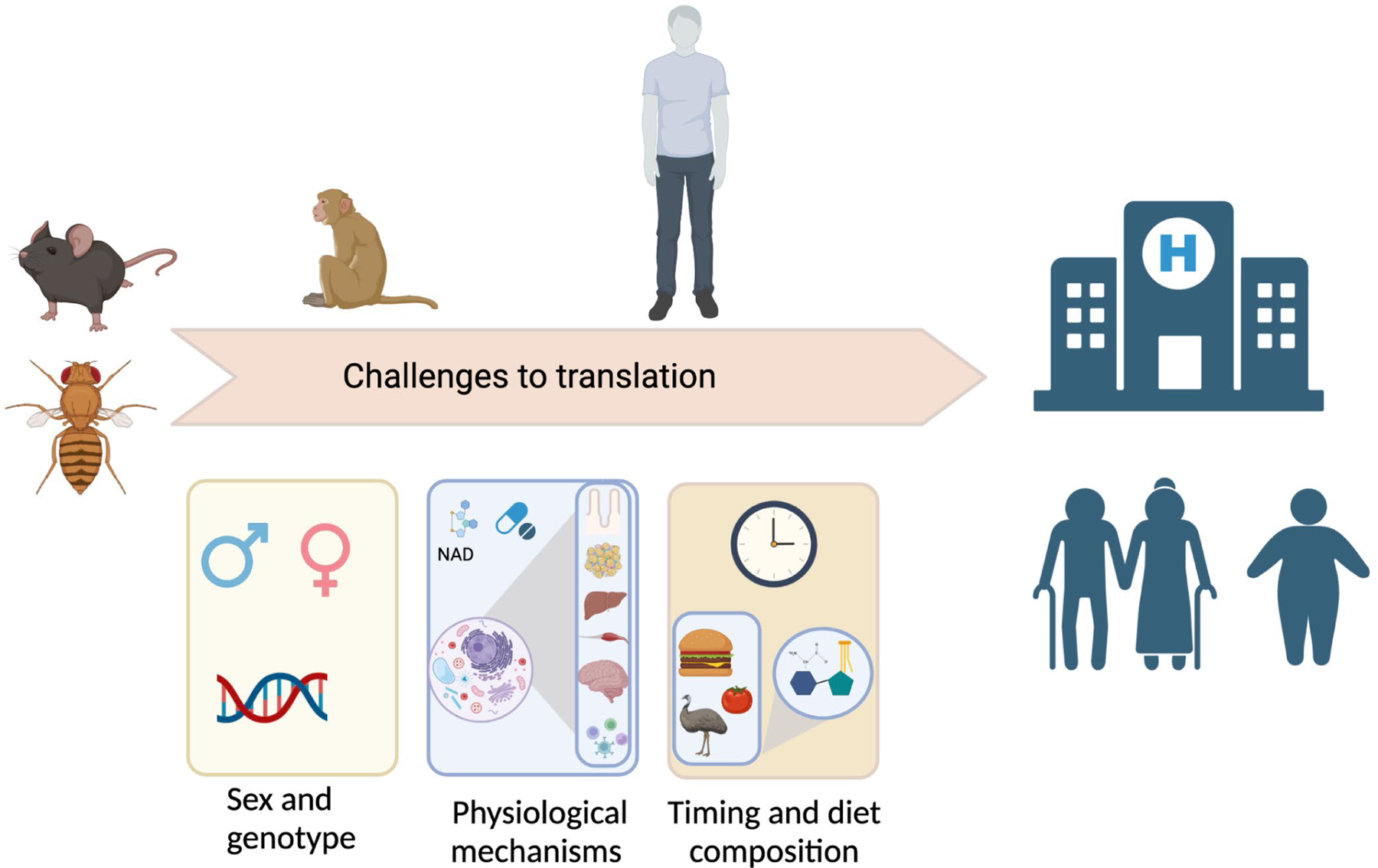
1) Genetic background and sex play a major role in the response to dietary and pharmaceutical interventions in the pre-clinical studies discussed here; 2) we are only beginning to understand the physiological and molecular mechanisms engaged by these interventions, and many questions remain; and 3) while it is clear that timing and diet composition are critically important regulators of metabolic health, the intersection of timing and diet composition has not been deeply explored. Finally, age, obesity and daily energy expenditure may be important factors in the response to different dietary interventions, and some interventions may be less beneficial or even deleterious in older individuals. This figure was created with BioRender.com.
The workshop participants all acknowledged the absolute need for more systematic assessment of the effect of sex and genetic background as key biological variables that could influence the responses to health promoting diet interventions. As the field accumulates a multitude of diet intervention studies and accompanying data sets, it will be necessary to create effective ways for big data and inter-study comparisons to account for a multitude of variables. Indeed, it was previously shown that the sex of the animal influenced the response to both CR and PR in mice51,136. In the work presented at this workshop, restriction of BCAAs and isoleucine was shown to have stronger effects on the frailty and longevity of male than female mice, highlighting the importance of studying interventions in both sexes. Genetic background has also recently been shown to be critical in the response to a panel of different healthy diets as well as CR, PR, and to high fat diet feeding30,51,136–138. Harnessing the power of natural genetic variation creates a unique opportunity to gain new insight into the physiological and molecular mechanisms that control the response to dietary components. It remains to be seen how the response to dietary interventions will differ in the heterogenous human population, as well as across the age spectrum.
There are significant unanswered questions regarding how dietary needs and the efficacy of interventions may change with age, and this is a challenge that will need to be met in order to successfully translate the interventions explored here to the clinic. Interventions that are only effective early in life are much less likely to be of broad clinical use as interventions that are effective starting later in mid-life or in old age. Calorie restriction, for example, is noticeably less effective in aged mice than in young animals139. A particular issue for PR is that aging in humans is associated with sarcopenia and frailty, and increasing – not decreasing – dietary protein in the elderly is widely recommended to preserve muscle mass140–142. It will therefore be important to study the long-term effect of these novel dietary interventions on muscle mass, quality, and frailty, as initiating PR or restriction of specific amino acids later in life could potentially be deleterious. While initiating PR or restriction of specific amino acids starting in old age has not been widely studied, BCAA restriction in mice starting at 16 months of age has not been observed to increase frailty69, while conversely PR starting around 22 months of age in mice resulted in a loss of lean mass in males51. Future studies in mice and in humans should thoroughly assess both the positive and negative benefits of dietary interventions starting at a wide array of ages.
Another important future question is understanding how interactions between macronutrients may contribute to the ultimate effects of these macronutrients on healthy aging. For example, it was recently shown that the type of carbohydrate is critical to the metabolic benefits of low protein, high carbohydrate diets143, and it is logical to assume that both carbohydrate and fat quality, as well as energy density, will have effects on healthy aging even if the impact of protein quality and quantity dominates. Micronutrients may also have an important role; in Drosophila it was recently shown that supplementation with dietary cholesterol – an essential micronutrient in this species – rescues the negative effects of a high protein, low carbohydrate diet on longevity144.
Speakers also discussed technical issues that may impact both the outcome and interpretation of studies. As discussed above, many of the effects of CR on healthspan and longevity appear to require the imposed prolonged fasting period between meals that CR-fed mice are incidentally subject to due to once-per-day feeding paradigms. The implementation of automated food dispensers in animal studies has already led to important new findings28,29,99, and may enable some of these effects to be deconvoluted in the future. Studies of CR in humans may similarly need to control for the period of time in which calories are consumed, as spreading the calories out over three meals a day may prove to be much less effective than one potentially larger meal once per day. A recent analysis of data from the CALERIE study found that while the reduction in calorie intake only explained 41% of the variance in weight loss between participants, eating interval length and timing of the first and last meals also significantly contributed to the variance in weight loss between study participants134.
Another very important potential confounder of diet studies is housing temperature; for instance, Dr. Bass described in this workshop how feeding during the light phase at thermoneutrality further increased weight gain compared to dark phase feeding. Most studies of mice are conducted at room temperature (22°C), which is cool for small rodents with little subcutaneous fat and thus leads to thermal stress and activation of the sympathetic nervous system. Conducting studies at thermoneutrality can reduce heightened cold-induced sympathetic activity and thereby unmask crucial, yet missing, regulators of dietary adaptation. Since humans are homeothermic and may not be typically subjected to thermal stress, insight gained from thermoneutrality experiments may provide a more direct bridge towards understanding the interplay between diet and physiology across species. More research is needed to understand exactly how much thermal stress rodents are subjected to at room temperature. It is also unknown how the thermal stresses humans are subject to vary with seasons, local climate, and socioeconomic status. Recent work has shown that other factors, such as relative humidity, can also alter metabolic stress and impact the response to diet145.
TRF studies have also highlighted the importance of metabolic regulation, nutrition timing, and health in the context of circadian alignment. Indeed, TRF studies suggest that even under isocaloric and identical macronutrient composition feeding, the timing of food intake can affect many parameters of metabolic measurements and health. A better understanding of the relationship between clock and metabolism will be crucial to optimize therapeutic approaches in free-living conditions, as well as care in hospitals where constant lighting or nutrition, such as parenteral nutrition, can result in circadian disruption. This dissociation between the light cycle and nutrition provided in the opposite phase violates alignment between clocks and feeding, which may lead to inflammatory conditions and insulin resistance. As we go forward, considering how and when both geroprotective diets and pharmaceuticals are best delivered relative to the circadian cycle should be tested in animal models, as this may be key to identifying the most efficacious way to promote human health.
Although a number of different nutrient sensitive pathways have been studied (Figure 3) in the context of CR, more molecular changes and potential mechanisms underlying TRF need to be explored, with an emphasis not just on the effects on the whole organism, but with an emphasis on tissue- and cell-specific effects and mechanisms. As but one potential mechanism not discussed here, recent studies have suggested that NAD+ metabolism may be a key regulator of the response to TRF and intermittent fasting, acting via the regulation of the sirtuin family of NAD+-dependent deacetylases146,147. The effect of these types of dietary intervention on hunger, and thus stress, must also be considered, although a recent analysis of data from the CALERIE study suggests perceived hunger is not significantly increased over the long-term in non-obese humans on a CR regimen148. TRF and other interventions might also potentially interact with geroprotective compounds, and little is known about the potential additive, synergistic, or even deleterious effects of combining such interventions. Initial animal studies have implicated several physiological mechanisms, including but not limited to autophagy, adipose tissue thermogenesis, integrative stress response, proteostasis and mitochondria function97,100,149. However, a comprehensive assessment of the effects of TRF across many tissues in mice – let alone humans – remains to be determined. The pleiotropic effects of TRF on multiple organ systems open new research avenues to identify tissue-specific and inter-organ communication mechanisms.
Figure 3. Molecular mechanisms of dietary restriction.
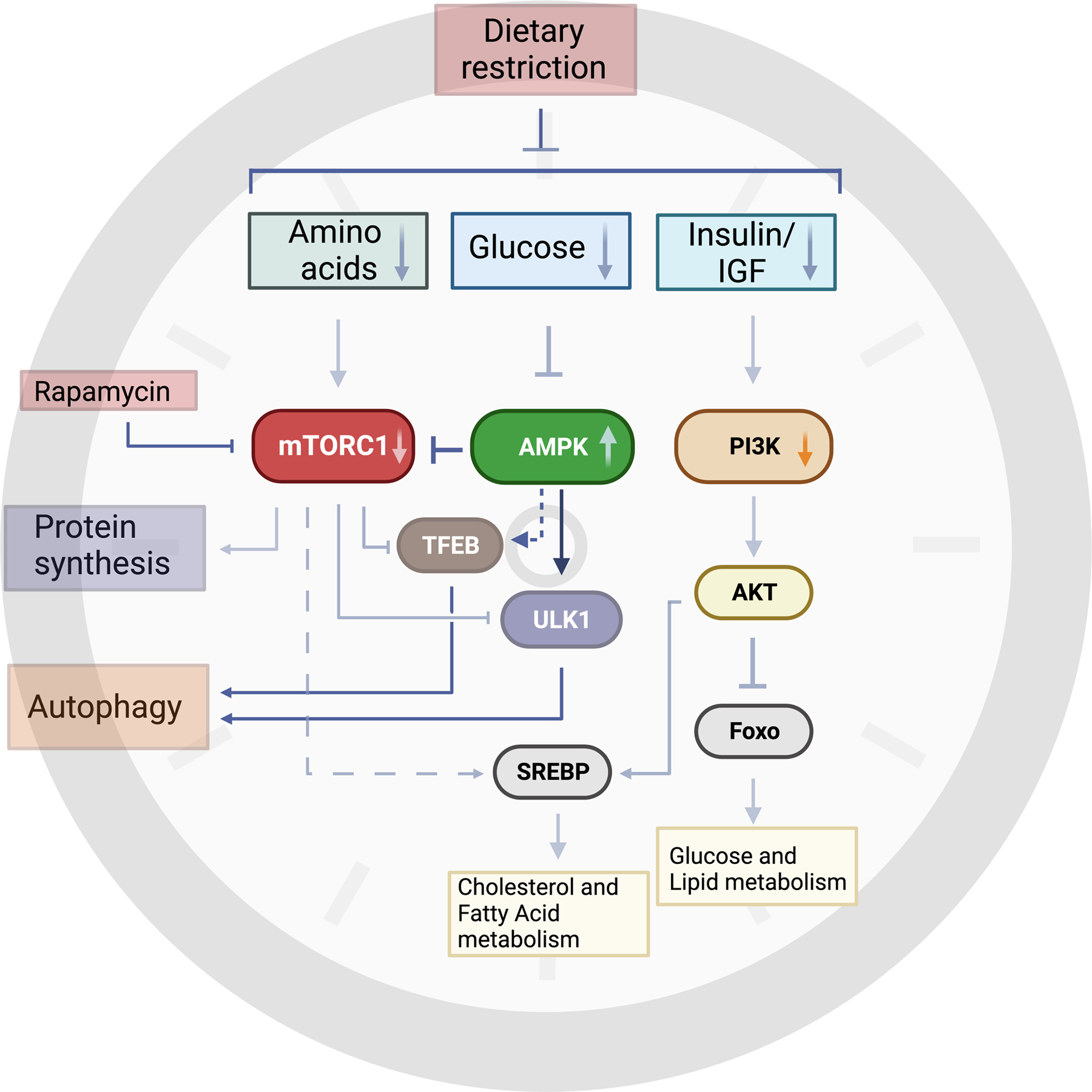
Dietary restriction encompasses reduced consumption of macronutrients such as carbohydrates and amino acids to alter their blood levels and, subsequently, insulin and IGF levels. These changes are sensed across different cell types and impinge on several conserved nutrient sensors, such as mTOR complex 1 (mTORC1) and AMP- activated protein kinase (AMPK). Reduced mTORC1 activity, due to lower levels of certain amino acids, leads to decreased protein synthesis and ribosomal biogenesis. AMPK acts as a sensor of cellular energy by sensing changes in intracellular AMP, ADP and ATP levels. Glucose deprivation activates AMPK which in turn can phosphorylate and regulate several downstream substrates. AMPK and mTORC1 both converge on regulating autophagy in opposing ways. AMPK-dependent phosphorylation of Unc-51-like kinase 1 (ULK1) is required for mitophagy, a specific type of autophagy that involves degrading damaged mitochondria that may be impaired in aged tissues. Dietary restriction of carbohydrates or overall calorie restriction can also reduce metabolic activity through PI3K and AKT pathways. Reduced AKT activity will increase forkhead box protein O (Foxo)-dependent transcriptional programs involved in glucose and lipid signaling. Additionally, mTORC1 and AKT regulate sterol regulatory element-binding protein (SREBP) 1 and 2, which regulate fatty acid and cholesterol metabolism, respectively. Inhibition of mTORC1 through Rapamycin can mimic some of the beneficial effects of dietary restriction, but it remains to be seen if Rapamycin affects different tissues and cell types to the same extent. This figure was created with BioRender.com.
The combination of TRF and CR in health indices is an active research area. In a weight loss treatment clinic in the US, when obese individuals were advised to follow CR, the addition of 8 hour TRF resulted in a further reduction in energy intake and improved cardiometabolic risks and brain health150. However, in another CR study in China, where the baseline eating window was 10 hours, reducing the eating window to 8 hours or following habitual eating window of 10 hours did not produce any significantly different health benefits between CR (10 hour eating window) and CR+ 8 hour eating window arms151. These results suggest TRF may benefit individuals who have a baseline eating window of >12 hours.
An important caveat when discussing all of these interventions is that the effectiveness of these dietary and timing regimens on healthy aging and longevity has not been tested in humans. Indeed, this is even true in the case of CR – while the CALERIE study was very ambitious, lifespan was not an outcome tracked in this two-year-long study. All studies of TRF, PR, and similar interventions thus far have been even shorter. Particularly in the absence of reliable biomarkers of aging, a major challenge will be showing that interventions that work well in flies and mice will also extend healthy aging in non-human primates and eventually, humans who may have complex regional or cultural dietary preferences, supplement intake and variable day to day energy balance.
Finally, there has been great interest for decades in designing small molecular mimetics of some of the effects of CR. While it remains to be seen if this is truly possible, small molecule mimetics of TRF, such as Nobiletin suggest that small molecules mimicking at least portions of CR – in this case, strengthening circadian rhythms – is indeed possible. It is exciting to start considering the possibility of future individualized precision nutrition in which optimized combination of dietary, drugs and timing considerations will be tailored to individual patients for successful improvement in health and longevity.
Conclusions
A recent NIH workshop on “Dietary Composition, Time-Restricted Feeding and Associated Metabolic Reprogramming in Healthspan and Longevity Regulation” brought together some of the leading experts who are investigating how dietary composition and restricting feeding times can promote healthy aging and even increase longevity in model organisms, and who discussed some of the initial results on translating findings from these studies from the bench to the dining table and the clinic. Recent results in this rapidly moving field have overthrown the century old paradigm that the effects of dietary components are solely mediated by their caloric value; instead, the new paradigm is that “a calorie is more than just a calorie,” with calories from different amino acids, dietary carbohydrates, or consumed at different times of day having different metabolic and molecular impacts. Further research in this area is critical to understanding not only the metabolic effects of distinct macronutrients, but to gain new insights into the long-standing problem of identifying the molecular mechanisms by which CR promotes longevity. Many of these interventions are already being translated into humans in the dining room or the metabolic kitchen but identifying molecular mechanisms that allow us to mimic the effects of restricted diets, and to understand why the effects of a given diet varies between people, will allow the development of new classes of geroprotective agents.
ACKNOWLEDGEMENTS
We thank Dr. Yih-Woei Fridell of the National Institute on Aging for organizing the meeting, as well as the NIA Division of Aging Biology for their support. We thank Dr. Gino Cortopassi for his edits and suggestions. The graphical abstract and figures were created with BioRender.com. The Mihaylova lab is supported in part by the NIA (R00AG054760), Office of the NIH Director (DP2CA271361), the American Federation of Aging Research, the V Foundation, Pew Biomedical Scholar award and startup funds from the Ohio State University. The Delibegovic lab is funded by British Heart Foundation, Diabetes UK, BBSRC, NHS Grampian, Tenovus Scotland and the Development Trust (University of Aberdeen). Jon Ramsey is supported by NIA PO1AG062817, R21AG064290, and R21AG071156. Research support for Joseph Bass was from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01DK127800, R01DK113011, R01DK090625, and R01DK050203 and the National Institute on Aging (NIA) grant R01AG065988 and P01AG011412, as well as the University of Chicago Diabetes Research and Training Center grant P30DK020595. This work was supported by the National Institutes of Health (NIH) grants AG065992 to GCM and AG068550 to GCM and SP, as well as UAB Startup funds 3123226 and 3123227 to GCM. Rajat Singh is supported by NIH grants RF1AG043517, R01AG065985, R01DK123327, R56AG074568, and P01AG031782. ZC is primarily funded by The Welch Foundation (AU-1731–20190330) and NIH/NIA (R01AG065984, R56AG063746, RF1AG061901, and R56AG076144). Amandine Chaix is supported by NIA grant R01AG065993. WWJ is supported by the NIH (R01DC020031). Mimi Shirasu-Hiza is supported by NIH R01 R35GM127049, NIH R01 AG045842, and NIH R21 NS122366. The research in Dixit Lab was supported in part by NIH grants AG031797, AG045712, P01AG051459, AR070811, AG076782, AG073969, AG068863 and Cure Alzheimer’s Fund (CAF). Anna Thalacker-Mercer is supported by the NIH/NIA (AG075059, AG058630), NIAMS (AR071133), NHLBI (HL153460), pilot and feasibility funds from the NIDDK funded UAB Nutrition Obesity Research Center (DK056336) and the NIA funded UAB Nathan Shock Center (AG050886), and startup funds from UAB. JAM is supported by the Intramural Research Program, NIA, NIH. The Panda laboratory is supported by the NIH (R01CA236352, R01CA258221, RF1AG068550, P30CA014195), the Wu Tsai Human Performance Alliance and the Joe and Clara Tsai Foundation. The Lamming lab is supported in part by the NIA (AG056771, AG062328, AG061635, and AG081482), the NIDDK (DK125859), startup funds from UW-Madison, the U.S. Department of Veterans Affairs (I01-BX004031), and this work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
DECLARATION OF INTERESTS
DWL has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. SP is the author of the books “The Circadian Code” and “The Circadian Diabetes Code.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green CL, Lamming DW, and Fontana L (2022). Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol 23, 56–73. 10.1038/s41580-021-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, et al. (2016). Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY: ) 8, 1416–1431. 10.18632/aging.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spadaro O, Youm Y, Shchukina I, Ryu S, Sidorov S, Ravussin A, Nguyen K, Aladyeva E, Predeus AN, Smith SR, et al. (2022). Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677. 10.1126/science.abg7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, and Ravussin E (2018). Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab 27, 805–815 e804. 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane MA, Mattison J, Ingram DK, and Roth GS (2002). Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics. Microsc Res Tech 59, 335–338. 10.1002/jemt.10214. [DOI] [PubMed] [Google Scholar]
- 6.Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, and Roth GS (2004). Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci 1019, 412–423. 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 7.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. (2011). Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66, 191–201. 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strong R, Miller RA, Bogue M, Fernandez E, Javors MA, Libert S, Marinez PA, Murphy MP, Musi N, Nelson JF, et al. (2020). Rapamycin-mediated mouse lifespan extension: Late-life dosage regimes with sex-specific effects. Aging Cell 19, e13269. 10.1111/acel.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, and Lamming DW (2016). Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice. J Gerontol A Biol Sci Med Sci 71, 876–881. 10.1093/gerona/glw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, Tung H, Vizzini N, Chen B, Smith K, et al. (2016). Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5. 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, and Partridge L (2010). Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11, 35–46. 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MB, Hill CM, Bitto A, and Kaeberlein M (2021). Antiaging diets: Separating fact from fiction. Science 374, eabe7365. 10.1126/science.abe7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fok WC, Bokov A, Gelfond J, Yu Z, Zhang Y, Doderer M, Chen Y, Javors M, Wood WH 3rd, Zhang Y, et al. (2014). Combined treatment of rapamycin and dietary restriction has a larger effect on the transcriptome and metabolome of liver. Aging Cell 13, 311–319. 10.1111/acel.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fok WC, Livi C, Bokov A, Yu Z, Chen Y, Richardson A, and Perez VI (2014). Short-term rapamycin treatment in mice has few effects on the transcriptome of white adipose tissue compared to dietary restriction. Mech Ageing Dev 140, 23–29. 10.1016/j.mad.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fok WC, Zhang Y, Salmon AB, Bhattacharya A, Gunda R, Jones D, Ward W, Fisher K, Richardson A, and Perez VI (2013). Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci 68, 108–116. 10.1093/gerona/gls127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannick JB, and Lamming DW (2023). Targeting the biology of aging with mTOR inhibitors. Nat Aging 3, 642–660. 10.1038/s43587-023-00416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCay CM, Crowell MF, and Maynard LA (1935). The Effect of Retarded Growth Upon the Length of Life Span and Upon the Ultimate Body Size. The Journal of nutrition 10, 63–79. 10.1093/jn/10.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, and Anderson RM (2014). Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications 5, 3557. 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weindruch R, Walford RL, Fligiel S, and Guthrie D (1986). The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition 116, 641–654. 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 21.Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, and Guarente L (2002). Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348. 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 22.Gribble KE, and Welch DB (2013). Life-span extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera). J Gerontol A Biol Sci Med Sci 68, 349–358. 10.1093/gerona/gls170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhesus Macaque GenomeS., Analysis C, Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, et al. (2007). Evolutionary and biomedical insights from the rhesus macaque genome. Science 316, 222–234. 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 24.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, and Weindruch R (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204. 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. (2012). Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK, and de Cabo R (2019). Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab 29, 221–228 e223. 10.1016/j.cmet.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aon MA, Bernier M, Mitchell SJ, Di Germanio C, Mattison JA, Ehrlich MR, Colman RJ, Anderson RM, and de Cabo R (2020). Untangling Determinants of Enhanced Health and Lifespan through a Multi-omics Approach in Mice. Cell Metab 32, 100–116 e104. 10.1016/j.cmet.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, and Takahashi JS (2017). Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab 26, 267–277 e262. 10.1016/j.cmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak HH, Haws SA, Green CL, Koller M, Lavarias MT, Richardson NE, Yang SE, Dumas SN, Sonsalla M, Bray L, et al. (2021). Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat Metab 3, 1327–1341. 10.1038/s42255-021-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao CY, Rikke BA, Johnson TE, Diaz V, and Nelson JF (2010). Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95. 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner EM, Beli E, Clinthorne JF, and Duriancik DM (2011). Energy intake and response to infection with influenza. Annu Rev Nutr 31, 353–367. 10.1146/annurev-nutr-081810-160812. [DOI] [PubMed] [Google Scholar]
- 32.Sun D, Muthukumar AR, Lawrence RA, and Fernandes G (2001). Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol 8, 1003–1011. 10.1128/CDLI.8.5.1003-1011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristan DM (2007). Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 6, 817–825. 10.1111/j.1474-9726.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 34.Poetschke HL, Klug DB, Perkins SN, Wang TT, Richie ER, and Hursting SD (2000). Effects of calorie restriction on thymocyte growth, death and maturation. Carcinogenesis 21, 1959–1964. 10.1093/carcin/21.11.1959. [DOI] [PubMed] [Google Scholar]
- 35.Roecker EB, Kemnitz JW, Ershler WB, and Weindruch R (1996). Reduced immune responses in rhesus monkeys subjected to dietary restriction. J Gerontol A Biol Sci Med Sci 51, B276–279. 10.1093/gerona/51a.4.b276. [DOI] [PubMed] [Google Scholar]
- 36.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, et al. (2015). A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 70, 1097–1104. 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorling JL, van Vliet S, Huffman KM, Kraus WE, Bhapkar M, Pieper CF, Stewart T, Das SK, Racette SB, Roberts SB, et al. (2021). Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: highlights from CALERIE phase 2. Nutr Rev 79, 98–113. 10.1093/nutrit/nuaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu S, Sidorov S, Ravussin E, Artyomov M, Iwasaki A, Wang A, and Dixit VD (2022). The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity 55, 1609–1626 e1607. 10.1016/j.immuni.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuenca-Sanchez M, Navas-Carrillo D, and Orenes-Pinero E (2015). Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Advances in nutrition 6, 260–266. 10.3945/an.114.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Z, Nan F, Wang LY, Jiang H, Chen W, and Jiang Y (2020). Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr 39, 1724–1734. 10.1016/j.clnu.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, and van der Schouw YT (2010). Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33, 43–48. 10.2337/dc09-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. (2014). Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417. 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virtanen HEK, Voutilainen S, Koskinen TT, Mursu J, Kokko P, Ylilauri MPT, Tuomainen TP, Salonen JT, and Virtanen JK (2019). Dietary proteins and protein sources and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 109, 1462–1471. 10.1093/ajcn/nqz025. [DOI] [PubMed] [Google Scholar]
- 44.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, et al. (2016). Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell reports 16, 520–530. 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferraz-Bannitz R, Beraldo RA, Peluso AA, Dall M, Babaei P, Foglietti RC, Martins LM, Gomes PM, Marchini JS, Suen VMM, et al. (2022). Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial. Nutrients 14. 10.3390/nu14132670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruce KD, Hoxha S, Carvalho GB, Yamada R, Wang HD, Karayan P, He S, Brummel T, Kapahi P, and Ja WW (2013). High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp Gerontol 48, 1129–1135. 10.1016/j.exger.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mair W, Piper MD, and Partridge L (2005). Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 3, e223. 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, and Raubenheimer D (2008). Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A 105, 2498–2503. 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grandison RC, Piper MD, and Partridge L (2009). Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064. 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green CL, Pak HH, Richardson NE, Flores V, Yu D, Tomasiewicz JL, Dumas SN, Kredell K, Fan JW, Kirsh C, et al. (2022). Sex and genetic background define the metabolic, physiologic, and molecular response to protein restriction. Cell Metab 34, 209–226 e205. 10.1016/j.cmet.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ, and Le Couteur DG (2015). Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell reports 11, 1529–1534. 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, and Yu BP (1988). The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol 43, B5–12. 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- 54.McCarty MF, Barroso-Aranda J, and Contreras F (2009). The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Medical hypotheses 72, 125–128. 10.1016/j.mehy.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, Appleby PN, Key TJ, and Travis RC (2016). Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr 70, 306–312. 10.1038/ejcn.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacArthur MR, Mitchell SJ, Trevino-Villarreal JH, Grondin Y, Reynolds JS, Kip P, Jung J, Trocha KM, Ozaki CK, and Mitchell JR (2021). Total protein, not amino acid composition, differs in plant-based versus omnivorous dietary patterns and determines metabolic health effects in mice. Cell Metab 33, 1808–1819 e1802. 10.1016/j.cmet.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orentreich N, Matias JR, DeFelice A, and Zimmerman JA (1993). Low methionine ingestion by rats extends life span. The Journal of nutrition 123, 269–274. 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 58.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, and Smith-Wheelock M (2005). Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125. 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lees EK, Banks R, Cook C, Hill S, Morrice N, Grant L, Mody N, and Delibegovic M (2017). Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Scientific reports 7, 9977. 10.1038/s41598-017-10381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malloy VL, Perrone CE, Mattocks DA, Ables GP, Caliendo NS, Orentreich DS, and Orentreich N (2013). Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice. Metabolism 62, 1651–1661. 10.1016/j.metabol.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Ables GP, Perrone CE, Orentreich D, and Orentreich N (2012). Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One 7, e51357. 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu D, Yang SE, Miller BR, Wisinski JA, Sherman DS, Brinkman JA, Tomasiewicz JL, Cummings NE, Kimple ME, Cryns VL, and Lamming DW (2018). Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J 32, 3471–3482. 10.1096/fj.201701211R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haws SA, Yu D, Ye C, Wille CK, Nguyen LC, Krautkramer KA, Tomasiewicz JL, Yang SE, Miller BR, Liu WH, et al. (2020). Methyl-Metabolite Depletion Elicits Adaptive Responses to Support Heterochromatin Stability and Epigenetic Persistence. Mol Cell 78, 210–223 e218. 10.1016/j.molcel.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plaisance EP, Greenway FL, Boudreau A, Hill KL, Johnson WD, Krajcik RA, Perrone CE, Orentreich N, Cefalu WT, and Gettys TW (2011). Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab 96, E836–840. 10.1210/jc.2010-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, and Delibegovic M (2014). Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13, 817–827. 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haws SA, Leech CM, and Denu JM (2020). Metabolism and the Epigenome: A Dynamic Relationship. Trends Biochem Sci 45, 731–747. 10.1016/j.tibs.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Felig P, Marliss E, and Cahill GF Jr. (1969). Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 281, 811–816. 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 68.Trautman ME, Richardson NE, and Lamming DW (2022). Protein restriction and branched-chain amino acid restriction promote geroprotective shifts in metabolism. Aging Cell 21, e13626. 10.1111/acel.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, Finke M, Haider LR, Yu D, Flores V, et al. (2021). Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging 1, 73–86. 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu D, Richardson NE, Green CL, Spicer AB, Murphy ME, Flores V, Jang C, Kasza I, Nikodemova M, Wakai MH, et al. (2021). The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab 33, 905–922 e906. 10.1016/j.cmet.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green CL, Trautman ME, Babygirija R, Jain R, Chaiyakul K, Pak HH, Bleicher A, Novak G, Sonsalla MM, Yeh C-Y, et al. (2022). Dietary restriction of isoleucine increases healthspan and lifespan of genetically heterogeneous mice. bioRxiv, 2022.2010.2006.511051. 10.1101/2022.10.06.511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al. (2016). A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22, 421–426. 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karusheva Y, Koessler T, Strassburger K, Markgraf D, Mastrototaro L, Jelenik T, Simon MC, Pesta D, Zaharia OP, Bodis K, et al. (2019). Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr 110, 1098–1107. 10.1093/ajcn/nqz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deelen J, Kettunen J, Fischer K, van der Spek A, Trompet S, Kastenmuller G, Boyd A, Zierer J, van den Akker EB, Ala-Korpela M, et al. (2019). A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nature communications 10, 3346. 10.1038/s41467-019-11311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Latimer MN, Sonkar R, Mia S, Frayne IR, Carter KJ, Johnson CA, Rana S, Xie M, Rowe GC, Wende AR, et al. (2021). Branched chain amino acids selectively promote cardiac growth at the end of the awake period. J Mol Cell Cardiol 157, 31–44. 10.1016/j.yjmcc.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yap YW, Rusu PM, Chan AY, Fam BC, Jungmann A, Solon-Biet SM, Barlow CK, Creek DJ, Huang C, Schittenhelm RB, et al. (2020). Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nature communications 11, 2894. 10.1038/s41467-020-16568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Marte ML, and Enesco HE (1986). Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev 36, 161–171. 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 78.Segall PE, and Timiras PS (1976). Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Ageing Dev 5, 109–124. 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- 79.Flores V, Spicer AB, Sonsalla MM, Richardson NE, Yu D, Sheridan GE, Trautman ME, Babygirija R, Cheng EP, Rojas JM, et al. (2022). Regulation of metabolic health by dietary histidine in mice. The Journal of physiology. 10.1113/JP283261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller RA, Harrison DE, Astle CM, Bogue MA, Brind J, Fernandez E, Flurkey K, Javors M, Ladiges W, Leeuwenburgh C, et al. (2019). Glycine supplementation extends lifespan of male and female mice. Aging Cell 18, e12953. 10.1111/acel.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brind J, Malloy V, Augie I, Caliendo N, Vogelman JH, Zimmerman JA, and Orentreich N (2011). Dietary glycine supplementation mimics lifespan extension by dietary methionine restriction in Fisher 344 rats. The FASEB Journal 25, 528.522–528.522. 10.1096/fasebj.25.1_supplement.528.2. [DOI] [Google Scholar]
- 82.Thalacker-Mercer AE, Ingram KH, Guo F, Ilkayeva O, Newgard CB, and Garvey WT (2014). BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes 63, 791–800. 10.2337/db13-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gheller BJ, Blum JE, Lim EW, Handzlik MK, Hannah Fong EH, Ko AC, Khanna S, Gheller ME, Bender EL, Alexander MS, et al. (2021). Extracellular serine and glycine are required for mouse and human skeletal muscle stem and progenitor cell function. Molecular metabolism 43, 101106. 10.1016/j.molmet.2020.101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duregon E, Pomatto-Watson L, Bernier M, Price NL, and de Cabo R (2021). Intermittent fasting: from calories to time restriction. Geroscience 43, 1083–1092. 10.1007/s11357-021-00335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geisler CE, Hepler C, Higgins MR, and Renquist BJ (2016). Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr Metab (Lond) 13, 62. 10.1186/s12986-016-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newman JC, and Verdin E (2017). beta-Hydroxybutyrate: A Signaling Metabolite. Annu Rev Nutr 37, 51–76. 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, et al. (2017). A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab 26, 539–546 e535. 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, and Verdin E (2017). Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab 26, 547–557 e548. 10.1016/j.cmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pathak SJ, Zhou Z, Steffen D, Tran T, Ad Y, Ramsey JJ, Rutkowsky JM, and Baar K (2022). 2-month ketogenic diet preferentially alters skeletal muscle and augments cognitive function in middle aged female mice. Aging Cell 21, e13706. 10.1111/acel.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Z, Vidales J, Gonzalez-Reyes JA, Shibata B, Baar K, Rutkowsky JM, and Ramsey JJ (2021). A 1-Month Ketogenic Diet Increased Mitochondrial Mass in Red Gastrocnemius Muscle, but Not in the Brain or Liver of Middle-Aged Mice. Nutrients 13. 10.3390/nu13082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallace MA, Aguirre NW, Marcotte GR, Marshall AG, Baehr LM, Hughes DC, Hamilton KL, Roberts MN, Lopez-Dominguez JA, Miller BF, et al. (2021). The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell 20, e13322. 10.1111/acel.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. (2015). The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21, 263–269. 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldberg EL, Shchukina I, Asher JL, Sidorov S, Artyomov MN, and Dixit VD (2020). Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat Metab 2, 50–61. 10.1038/s42255-019-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomilov A, Allen S, Hui CK, Bettaieb A, and Cortopassi G (2018). Idebenone is a cytoprotective insulin sensitizer whose mechanism is Shc inhibition. Pharmacol Res 137, 89–103. 10.1016/j.phrs.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 95.Hui C, Tomilov A, Garcia C, Jiang X, Fash DM, Khdour OM, Rosso C, Filippini G, Prato M, Graham J, et al. (2020). Novel idebenone analogs block Shc’s access to insulin receptor to improve insulin sensitivity. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 132, 110823. 10.1016/j.biopha.2020.110823. [DOI] [PubMed] [Google Scholar]
- 96.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. (2012). Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–860. 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chaix A, Zarrinpar A, Miu P, and Panda S (2014). Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005. 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chaix A, Manoogian ENC, Melkani GC, and Panda S (2019). Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu Rev Nutr 39, 291–315. 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acosta-Rodriguez V, Rijo-Ferreira F, Izumo M, Xu P, Wight-Carter M, Green CB, and Takahashi JS (2022). Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 376, 1192–1202. 10.1126/science.abk0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaix A, Deota S, Bhardwaj R, Lin T, and Panda S (2021). Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell reports 36, 109543. 10.1016/j.celrep.2021.109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, et al. (2016). The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab 23, 610–621. 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, Sun Y, Han L, Esser KA, Mileykovskaya E, et al. (2019). Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nature communications 10, 3923. 10.1038/s41467-019-11926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nohara K, Kim E, Wirianto M, Mileykovskaya E, Dowhan W, Chen Z, and Yoo SH (2020). Cardiolipin Synthesis in Skeletal Muscle Is Rhythmic and Modifiable by Age and Diet. Oxid Med Cell Longev 2020, 5304768. 10.1155/2020/5304768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim E, Nohara K, Wirianto M, Escobedo G Jr., Lim JY, Morales R, Yoo SH, and Chen Z (2021). Effects of the Clock Modulator Nobiletin on Circadian Rhythms and Pathophysiology in Female Mice of an Alzheimer’s Disease Model. Biomolecules 11. 10.3390/biom11071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wirianto M, Wang CY, Kim E, Koike N, Gomez-Gutierrez R, Nohara K, Escobedo G Jr., Choi JM, Han C, Yagita K, et al. (2022). The clock modulator Nobiletin mitigates astrogliosis-associated neuroinflammation and disease hallmarks in an Alzheimer’s disease model. FASEB J 36, e22186. 10.1096/fj.202101633R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, and Bass J (2007). High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–421. 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Arble DM, Bass J, Laposky AD, Vitaterna MH, and Turek FW (2009). Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17, 2100–2102. 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hepler C, Weidemann BJ, Waldeck NJ, Marcheva B, Cedernaes J, Thorne AK, Kobayashi Y, Nozawa R, Newman MV, Gao P, et al. (2022). Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 378, 276–284. 10.1126/science.abl8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, and Rimm EB (2013). Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 128, 337–343. 10.1161/CIRCULATIONAHA.113.001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gill S, Le HD, Melkani GC, and Panda S (2015). Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347, 1265–1269. 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, Le HD, Manor U, Panda S, and Melkani GC (2019). Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nature communications 10, 2700. 10.1038/s41467-019-10563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Livelo C, Guo Y, Abou Daya F, Rajasekaran V, Varshney S, Le HD, Barnes S, Panda S, and Melkani GC (2023). Time-restricted feeding promotes muscle function through purine cycle and AMPK signaling in Drosophila obesity models. Nature communications 14, 949. 10.1038/s41467-023-36474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Livelo C, Guo Y, and Melkani GC (2022). A Skeletal Muscle-Centric View on Time-Restricted Feeding and Obesity under Various Metabolic Challenges in Humans and Animals. Int J Mol Sci 24. 10.3390/ijms24010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kopeć S (1928). On the Influence of Intermittent Starvation on the Longevity of the Imaginal Stage of Drosophila Melanogaster. Journal of Experimental Biology 5, 204–211. 10.1242/jeb.5.3.204. [DOI] [Google Scholar]
- 115.Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, and Partridge L (2018). Short-Term, Intermittent Fasting Induces Long-Lasting Gut Health and TOR-Independent Lifespan Extension. Curr Biol 28, 1714–1724 e1714. 10.1016/j.cub.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ulgherait M, Midoun AM, Park SJ, Gatto JA, Tener SJ, Siewert J, Klickstein N, Canman JC, Ja WW, and Shirasu-Hiza M (2021). Circadian autophagy drives iTRF-mediated longevity. Nature 598, 353–358. 10.1038/s41586-021-03934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murphy KR, Park JH, Huber R, and Ja WW (2017). Simultaneous measurement of sleep and feeding in individual Drosophila. Nature protocols 12, 2355–2366. 10.1038/nprot.2017.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu K, Zheng X, and Sehgal A (2008). Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab 8, 289–300. 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martinez-Lopez N, Tarabra E, Toledo M, Garcia-Macia M, Sahu S, Coletto L, Batista-Gonzalez A, Barzilai N, Pessin JE, Schwartz GJ, et al. (2017). System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab 26, 856–871 e855. 10.1016/j.cmet.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martinez-Lopez N, Garcia-Macia M, Sahu S, Athonvarangkul D, Liebling E, Merlo P, Cecconi F, Schwartz GJ, and Singh R (2016). Autophagy in the CNS and Periphery Coordinate Lipophagy and Lipolysis in the Brown Adipose Tissue and Liver. Cell Metab 23, 113–127. 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brunet A, Goodell MA, and Rando TA (2023). Ageing and rejuvenation of tissue stem cells and their niches. Nat Rev Mol Cell Biol 24, 45–62. 10.1038/s41580-022-00510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keller A, Temple T, Sayanjali B, and Mihaylova MM (2021). Metabolic Regulation of Stem Cells in Aging. Curr Stem Cell Rep 7, 72–84. 10.1007/s40778-021-00186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mihaylova MM, Cheng CW, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, Abu-Remaileh M, Clavain L, Erdemir A, Lewis CA, et al. (2018). Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 22, 769–778 e764. 10.1016/j.stem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. (2012). mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495. 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Asher G, and Sassone-Corsi P (2015). Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92. 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 126.Gill S, and Panda S (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–798. 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta NJ, Kumar V, and Panda S (2017). A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One 12, e0172852. 10.1371/journal.pone.0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manoogian ENC, Chow LS, Taub PR, Laferrere B, and Panda S (2022). Time-restricted Eating for the Prevention and Management of Metabolic Diseases. Endocr Rev 43, 405–436. 10.1210/endrev/bnab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, Wang Q, Hodges JS, Esch N, Malaeb S, et al. (2020). Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans who are Overweight: A Feasibility Study. Obesity (Silver Spring) 28, 860–869. 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, and Taub PR (2020). Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab 31, 92–104 e105. 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phillips NE, Mareschal J, Schwab N, Manoogian ENC, Borloz S, Ostinelli G, Gauthier-Jaques A, Umwali S, Gonzalez Rodriguez E, Aeberli D, et al. (2021). The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 13. 10.3390/nu13031042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Malaeb S, Harindhanavudhi T, Dietsche K, Esch N, Manoogian ENC, Panda S, Mashek DG, Wang Q, and Chow LS (2020). Time-Restricted Eating Alters Food Intake Patterns, as Prospectively Documented by a Smartphone Application. Nutrients 12. 10.3390/nu12113396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, and Peterson CM (2018). Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27, 1212–1221 e1213. 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fleischer JG, Das SK, Bhapkar M, Manoogian ENC, and Panda S (2022). Associations between the timing of eating and weight-loss in calorically restricted healthy adults: Findings from the CALERIE study. Exp Gerontol 165, 111837. 10.1016/j.exger.2022.111837. [DOI] [PubMed] [Google Scholar]
- 135.Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, Goodwin S, Guo J, Howard L, Knuth ND, et al. (2015). Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab 22, 427–436. 10.1016/j.cmet.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, et al. (2016). Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab 23, 1093–1112. 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roy S, Sleiman MB, Jha P, Ingels JF, Chapman CJ, McCarty MS, Ziebarth JD, Hook M, Sun A, Zhao W, et al. (2021). Gene-by-environment modulation of lifespan and weight gain in the murine BXD family. Nat Metab 3, 1217–1227. 10.1038/s42255-021-00449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barrington WT, Wulfridge P, Wells AE, Rojas CM, Howe SYF, Perry A, Hua K, Pellizzon MA, Hansen KD, Voy BH, et al. (2018). Improving Metabolic Health Through Precision Dietetics in Mice. Genetics 208, 399–417. 10.1534/genetics.117.300536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hahn O, Drews LF, Nguyen A, Tatsuta T, Gkioni L, Hendrich O, Zhang Q, Langer T, Pletcher S, Wakelam MJO, et al. (2019). A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat Metab 1, 1059–1073. 10.1038/s42255-019-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beasley JM, LaCroix AZ, Neuhouser ML, Huang Y, Tinker L, Woods N, Michael Y, Curb JD, and Prentice RL (2010). Protein intake and incident frailty in the Women’s Health Initiative observational study. J Am Geriatr Soc 58, 1063–1071. 10.1111/j.1532-5415.2010.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paddon-Jones D, and Rasmussen BB (2009). Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 12, 86–90. 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paddon-Jones D, Short KR, Campbell WW, Volpi E, and Wolfe RR (2008). Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 87, 1562S–1566S. 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 143.Wali JA, Milner AJ, Luk AWS, Pulpitel TJ, Dodgson T, Facey HJW, Wahl D, Kebede MA, Senior AM, Sullivan MA, et al. (2021). Impact of dietary carbohydrate type and protein-carbohydrate interaction on metabolic health. Nat Metab 3, 810–828. 10.1038/s42255-021-00393-9. [DOI] [PubMed] [Google Scholar]
- 144.Zanco B, Mirth CK, Sgro CM, and Piper MD (2021). A dietary sterol trade-off determines lifespan responses to dietary restriction in Drosophila melanogaster females. eLife 10. 10.7554/eLife.62335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kasza I, Cuncannan C, Michaud J, Nelson D, Yen CE, Jain R, Simcox J, MacDougald OA, Parks BW, and Alexander CM (2022). “Humanizing” mouse environments: Humidity, diurnal cycles and thermoneutrality. Biochimie. 10.1016/j.biochi.2022.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Levine DC, Kuo HY, Hong HK, Cedernaes J, Hepler C, Wright AG, Sommars MA, Kobayashi Y, Marcheva B, Gao P, et al. (2021). NADH inhibition of SIRT1 links energy state to transcription during time-restricted feeding. Nat Metab 3, 1621–1632. 10.1038/s42255-021-00498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, et al. (2019). SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nature communications 10, 1886. 10.1038/s41467-019-09897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dorling JL, Bhapkar M, Das SK, Racette SB, Apolzan JW, Fearnbach SN, Redman LM, Myers CA, Stewart TM, Martin CK, and Group C.S. (2019). Change in self-efficacy, eating behaviors and food cravings during two years of calorie restriction in humans without obesity. Appetite 143, 104397. 10.1016/j.appet.2019.104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaix A, Lin T, Le HD, Chang MW, and Panda S (2019). Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab 29, 303–319 e304. 10.1016/j.cmet.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jamshed H, Steger FL, Bryan DR, Richman JS, Warriner AH, Hanick CJ, Martin CK, Salvy SJ, and Peterson CM (2022). Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern Med 182, 953–962. 10.1001/jamainternmed.2022.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, et al. (2022). Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N Engl J Med 386, 1495–1504. 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]


