Abstract
Objective
To investigate potential interactive effects of fine particulate matter (PM2.5) and ozone (O3) on daily mortality at global level.
Design
Two stage time series analysis.
Setting
372 cities across 19 countries and regions.
Population
Daily counts of deaths from all causes, cardiovascular disease, and respiratory disease.
Main outcome measure
Daily mortality data during 1994-2020. Stratified analyses by co-pollutant exposures and synergy index (>1 denotes the combined effect of pollutants is greater than individual effects) were applied to explore the interaction between PM2.5 and O3 in association with mortality.
Results
During the study period across the 372 cities, 19.3 million deaths were attributable to all causes, 5.3 million to cardiovascular disease, and 1.9 million to respiratory disease. The risk of total mortality for a 10 μg/m3 increment in PM2.5 (lag 0-1 days) ranged from 0.47% (95% confidence interval 0.26% to 0.67%) to 1.25% (1.02% to 1.48%) from the lowest to highest fourths of O3 concentration; and for a 10 μg/m3 increase in O3 ranged from 0.04% (−0.09% to 0.16%) to 0.29% (0.18% to 0.39%) from the lowest to highest fourths of PM2.5 concentration, with significant differences between strata (P for interaction <0.001). A significant synergistic interaction was also identified between PM2.5 and O3 for total mortality, with a synergy index of 1.93 (95% confidence interval 1.47 to 3.34). Subgroup analyses showed that interactions between PM2.5 and O3 on all three mortality endpoints were more prominent in high latitude regions and during cold seasons.
Conclusion
The findings of this study suggest a synergistic effect of PM2.5 and O3 on total, cardiovascular, and respiratory mortality, indicating the benefit of coordinated control strategies for both pollutants.
Introduction
Numerous epidemiological studies have reported the adverse effects of exposure to ambient air pollution on human health, such as premature mortality and morbidity from major cardiopulmonary diseases.1 2 3 4 Among all air pollutants, fine particulate matter (PM2.5, particulate matter with aerodynamic diameter ≤2.5 μm) and ozone (O3) are among the most studied, given their harmful impacts on human health and high levels in many parts of the world. Additionally, these are the only two air pollutants included in the Global Burden of Disease study as independent risk factors for mortality in humans.5 As reported by the Global Burden of Disease 2019 study, exposure to ambient PM2.5 and O3 accounted for 4.1 million and 0.36 million attributable deaths globally in 2019.6 Although abundant evidence links PM2.5 and O3 with human health outcomes,7 8 studies have mostly treated the health impacts of PM2.5 or O3 separately, and sometimes they consider the other pollutant as a potential confounder while neglecting potential interactive effects. Experimental studies have proposed the biological plausibility of the combined effects of PM2.5 and O3 through pathways involving lung inflammation, oxidative stress, macrophage activity, and endothelial function.9 Considering the substantial disease burden attributable to these two air pollutants, it is of great scientific and public health importance to determine whether they have an interactive relationship. Understanding these interactive effects should provide a scientific knowledge base to incentivise governments to develop strategies on coordinated control of these two important air pollutants.10
In recent decades, although an increasing number of epidemiological studies have discovered an interaction between particulate matter and O3, no consensus has been reached.11 12 13 14 For example, a time series study in Shanghai reported that high exposure to inhalable particles (PM10, particulate matter with aerodynamic diameter ≤10 μm) enhanced the associations of O3 with mortality.12 A study in Moscow also found increased mortality risks from all causes, ischaemic heart disease, and cerebrovascular disease associated with PM10 at concurrent high exposures to O3.14 A similar study in Hong Kong, however, observed that exposure to O3 could mitigate the adverse effects of particulate air pollution on cardiovascular and respiratory morbidity13; another study in Seoul also found decreased associations of O3 on stroke mortality with higher exposure to PM10.11 Several factors could explain the discrepancies, including differences in methodologies used to assess interactive effects, heterogeneity in study regions and health outcomes, and insufficient study samples to achieve enough statistical power. For example, previous studies have relied on the significance of the interaction term of PM2.5 and O3 to evaluate the presence of interactive effects.15 The estimates from regression models with interaction terms, however, would be incomparable for most other studies that only reported estimates for a single pollutant. Also, the characteristics of populations and pollution types (eg, chemical compositions of particles) can differ. Moreover, previous studies have primarily examined the interaction between PM10 and O3 because of the difficulties in measuring PM2.5. With the availability of an expanded monitoring network of PM2.5 and its growing importance in the Global Burden of Disease study, additional investigations are required to understand better the association between PM2.5, O3, and mortality.
In this study, we used multiple statistical approaches to systematically examine whether PM2.5 and O3 have an interactive effect on total, cardiovascular, and respiratory mortality, and we investigated potential factors that might influence this association at a global level, such as region and season. We used data from the Multi-Country Multi-City (MCC) Collaborative Research Network (https://mccstudy.lshtm.ac.uk/), which collects vast time series data in the specialty of environmental health. Using this dataset, the research group has previously estimated significant mortality hazards of short term exposure to PM2.5 and O3 across multiple countries and regions.16 17
Methods
Data collection
We collected daily air pollution data and mortality records from the MCC database, as described previously.18 19 Data on daily PM2.5 and O3 concentrations were available for 372 cities across 19 countries and regions. Supplementary eFigure 1 and eFigure 2 show the geographical locations of the cities, along with the corresponding average annual concentrations of PM2.5 and O3. Mortality data were obtained from the national or regional death registries of each country and region. Causes of death were classified using ICD-10 (international classification of diseases, 10th revision) codes. In each location, mortality is represented by daily counts due to either non-external causes (A00-R99) or, when not available, all causes. We also collected mortality data on two major causes from 14 countries: cardiovascular disease (I00-I99) and respiratory disease (J00-J99).20 Mortality data on cardiovascular and respiratory diseases were not available in Australia, Chile, Germany, Israel, and Romania.
We obtained daily concentrations of PM2.5 (24 hour average) and O3 (eight hours maximum) from fixed site monitoring stations and averaged these at city level. Daily mean temperature and relative humidity were obtained from the local meteorological bureau or other government sectors.18 Overall, the percentages of missing daily observations for all cause mortality, PM2.5, O3, and temperature were 0.20%, 10.02%, 8.04%, and 2.10%, which were omitted from subsequent analyses.
Statistical analysis
This study adopted a time series design, with random effects multilevel meta-analysis. We utilised the two stage analytical framework to assess the associations of PM2.5 and O3 with daily mortality, which is described elsewhere.16 21 In the first stage, the city specific model was built using quasi-Poisson generalised linear regressions, adjusting for long term time trends using a natural cubic spline function of time with seven degrees of freedom per year; an indicator variable for the day of the week; temperature using a natural spline function with six degrees of freedom; and relative humidity using the same spline function but with three degrees of freedom. We a priori selected the lags of air pollutants as the moving average of the present and previous day (lag 0-1), and the lag structure of temperature as the moving average of the present and previous three days (lag 0-3), in accordance with previous studies.7 16 21 In the second stage, we combined city specific estimates and their statistical uncertainty; a random effects multilevel meta-analytical model was used to fit the best linear unbiased predictions for exposure-health associations at both global and regional levels.22 The best linear unbiased predictions borrow information across units within the same hierarchical level and can provide more accurate estimates, especially in locations with small sample sizes. The weight assigned to each city is determined by an inverse function of within study standard errors (a function of both number of cases and variations in exposure) and between study heterogeneity. Potential heterogeneity across cities was assessed using Cochran Q tests and I2 statistics.
We adopted two established methods to test the potential interactive effects of PM2.5 and O3 on mortality. Firstly, we conducted stratified analyses based on fourths of PM2.5 and O3 concentrations to detect the interaction.23 24 To ensure comparability, the thresholds to differentiate strata of air pollutants (ie, 25th and 75th centiles) were based on the distribution of exposures in each city. Specifically, we categorised each day of PM2.5 and O3 concentrations based on cut-points for each pollutant at the lowest (≤25%) and highest (>75%) quartile. The first stage models were run for a given pollutant (eg, PM2.5) on each stratum of the other pollutant (eg, O3) at each location. Then, the second stage models were run separately for each stratum to pool the coefficients at regional and global levels. Lastly, we reported the pooled effect estimates of a given pollutant in subgroups with 25% lowest, 25-75%, and 75% highest concentrations of the other pollutant. P values for differences between strata were calculated based on likelihood ratio tests. Secondly, we examined the possible synergistic interactions between PM2.5 and O3 in their associations with mortality using the synergy index.25 26 To comply with the synergy index method, we classified concentrations of PM2.5 and O3 into low and high levels, with cut-points at the median values across all cities, and dummy variables were created to represent the combination of the 2×2 categories—namely, reference, low exposure to PM2.5 and low exposure to O3; relative risk01, low PM2.5 and high O3 exposures; relative risk10, high PM2.5 and low O3 exposures; and relative risk11, high PM2.5 and high O3 exposures. The synergy index is calculated as: (RR11−1)/(RR01−1+RR10−1), where RR is the relative risk of mortality associated with the air pollutants. A synergy index >1 denotes a synergistic interaction, whereas a synergy index <1 indicates an antagonistic interaction. Confidence intervals of synergy indices were estimated using a bootstrap simulation method.27 To illustrate the health impact of air pollutants, we calculated the annual number of deaths attributable to PM2.5 or O3, or both, at country level, based on the annual death numbers summed from city level time series data and the country specific population attributable risk percentage ([RR−1)/RR].
To further explore potential influencing factors in the interaction between PM2.5 and O3, we examined the interaction between these pollutants in their association with mortality in different seasons and regions. We defined the warm season in the northern hemisphere as April to September and the cold season as October to March—and the opposite for the southern hemisphere. A high latitude region was classified as cities with a latitude above the median (40.3° for the northern hemisphere) across all cities, and a low latitude region as cities with a latitude below the median. We further conducted stratified analyses by regions classified by the World Health Organization (see supplementary eTable 1), including the Western-Pacific Regional Office, the Regional Office for Europe, and the Regional Office for the Americas. P values for interactions were calculated based on likelihood ratio tests.
Several sensitivity analyses were used to test the robustness of our estimates. Firstly, we explored additional lags of air pollutants that were frequently used by previous short term studies,16 28 including the present day (lag 0) and moving average of the present day and previous two days (lag 0-2) or three days (lag 0-3) days. Secondly, we tested the influence of longer lags of temperature modelling (using a natural spline function with six degrees of freedom), including the present day and the moving average of the present and the previous 7, 14, and 21 days.
We conducted all statistical analyses in R software (version 3.3.1), using the mgcv and dlnm packages for fitting first stage models, and the mixmeta package for performing multilevel meta-analyses. Results are presented as percentage change in (central estimates) or relative risk of mortality, with respective 95% confidence intervals, per 10 μg/m3 increase in PM2.5 and O3 concentrations. P values <0.05 were considered significant in all statistical analyses.
Patient and public involvement
This was a multilocation collaboration using aggregated city level mortality and environmental data. No patients or members of the public were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. The primary obstacles to patient and public involvement were that the team was not trained to work with the public, we lacked permission to engage them, and the data and place where the research took place were restricted.
Results
Descriptive statistics
This analysis included 19.3 million deaths for total or non-external causes of death from 372 cities across 19 countries and regions during the study period 1994 to 2020. Overall, 5.3 million and 1.9 million deaths were attributed to cardiovascular and respiratory diseases, respectively (table 1). The median annual mean concentrations of PM2.5 and O3 across the 372 cities were 11.4 μg/m3 (interquartile range 7.7-16.8 μg/m3) and 54.3 (39.9-68.9) μg/m3, respectively. Supplementary eFigure 3 presents boxplots for PM2.5 and O3 exposures at city level. The mean temperature and relative humidity levels were 13.7°C and 68.4%, respectively. On average, PM2.5 was weakly correlated with O3 (Spearman correlation coefficient rs=0.09), mean temperature (rs=0.20), and relative humidity (rs=0.16) and O3 was moderately correlated with mean temperature (rs=0.42) and negatively correlated with relative humidity (rs=−0.39). Supplementary eFigure 4 shows city specific correlations among air pollutants and temperature. The Spearman correlations between PM2.5 and O3 varied across cities, with a median rs of 0.14 (interquartile range −0.17-0.34).
Table 1.
Summary statistics on mortality and air pollution data in 372 cities across 19 countries and regions
| Country/region | No of cities | Period | No of deaths (000s) | Median (IQR) | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Cardiovascular | Respiratory | PM2.5 (μg/m3) | O3 (μg/m3) | ||||
| Australia | 3 | 2001-07 | 388.1 | NA | NA | 6.0 (4.3-8.4) | 28.8 (21.9-36.7) | |
| Canada | 25 | 1999-15 | 2207.3 | 678.1 | 188.7 | 6.7 (4.3-10.3) | 44.9 (32.7-57.8) | |
| Chile | 3 | 2008-14 | 282.7 | NA | NA | 20.7 (15.0-29.7) | 23.8 (12.0-32.2) | |
| China | 2 | 2013-15 | 248.7 | 103.4 | 31.1 | 49.7 (30.1-80.0) | 82.3 (49.9-121.5) | |
| Cyprus | 2 | 2010-19 | 21.0 | 7.2 | 1.9 | 17.3 (13.2-22.9) | 63.1 (46.8-76.3) | |
| Ecuador | 1 | 2014-18 | 44.5 | 10.7 | 5.6 | 16.5 (12.5-20.4) | 19.5 (15.1-26.1) | |
| Finland | 1 | 1994-14 | 153.3 | 57.4 | 9.7 | 12.6 (7.4-21.7) | 51.3 (40.0-63.0) | |
| France | 18 | 2008-15 | 884.8 | NA | 55.7 | 13.1 (9.0-20.0) | 69.6 (50.6-87.1) | |
| Germany | 11 | 2008-15 | 1051.8 | NA | NA | 12.0 (8.0-18.8) | 41.3 (24.0-57.0) | |
| Iran | 1 | 2013-15 | 146.0 | 63.0 | 13.0 | 36.3 (28.3-48.1) | 30.4 (19.7-49.2) | |
| Israel | 4 | 2003-20 | 374.3 | NA | NA | 14.1 (10.5-18.9) | 68.1 (54.5-80.6) | |
| Japan | 46 | 2012-15 | 1674.5 | 437.4 | 261.7 | 12.6 (8.3-18.2) | 56.8 (41.8-72.7) | |
| Norway | 1 | 2000-18 | 84.5 | 23.5 | 7.2 | 9.1 (6.6-12.6) | 43.1 (30.6-55.5) | |
| Portugal | 4 | 2004-18 | 457.7 | 140.3 | 51.3 | 8.4 (5.6-13.0) | 61.0 (48.4-73.1) | |
| Romania | 5 | 2009-16 | 112.0 | NA | NA | 12.7 (8.6-18.2) | 34.2 (21.9-47.7) | |
| Spain | 15 | 2009-13 | 405.8 | 119.9 | 50.3 | 10.6 (7.5-14.9) | 51.2 (38.4-62.6) | |
| Taiwan | 3 | 2007-14 | 506.1 | 117.4 | 58.9 | 32.3 (20.6-44.6) | 55.0 (40.5-72.4) | |
| United Kingdom | 28 | 2008-16 | 1946.8 | 597.6 | 287.7 | 9.3 (6.4-14.5) | 43.3 (31.2-55.2) | |
| United States | 199 | 1999-06 | 9313.7 | 2915.8 | 919.1 | 10.8 (7.3-15.8) | 56.9 (42.4-71.6) | |
| Pooled | 372 | 1994-20 | 19 261.1 | 5271.7 | 1942.0 | 11.4 (7.7-16.8) | 54.3 (39.9-68.9) | |
IQR=interquartile range; NA=not available; O3=ozone; PM2.5=fine particulate matter with aerodynamic diameter ≤2.5 μm.
Regression results
Among the 372 cities, we observed significant associations of exposure to PM2.5 and O3 with total mortality (see supplementary eFigure 5); a 10 μg/m3 increase in PM2.5 and O3 was associated with increments of 0.79% (95% confidence interval 0.66% to 0.91%) and 0.31% (95% confidence interval 0.25% to 0.37%) in total mortality. Heterogeneity among the city specific estimates was moderate for total mortality associated with PM2.5 and O3, with I2 statistics of 29.6% (Cochran Q=396.7, P<0.001) and 30.2% (Q=398.7, P<0.001), respectively. The corresponding heterogeneity results for PM2.5 and O3 were I2=28.3% (Cochran Q=340.3, P<0.001) and I2=18.3% (Cochran Q=335.1, P=0.004) for cardiovascular mortality and I2=23.8% (Cochran Q=342.8, P<0.001) and I2=18.1% (Cochran Q=333.4, P=0.007) for respiratory mortality.
Table 2 displays the stratified associations of PM2.5 and O3 (lag 0-1) with total, cardiovascular, and respiratory mortality. The associations of both PM2.5 and O3 generally increased in higher centiles of co-pollutant strata. Interactions between PM2.5 and O3 on total mortality were significant (P<0.001). Specifically, the effect estimates for a 10 μg/m3 increase in PM2.5 was 0.47% (95% confidence interval 0.26% to 0.67%), 0.70% (0.53% to 0.87%), and 1.25% (1.02% to 1.48%) in strata of O3 in the lowest fourth (≤25%), 25-75% centile, and highest fourth (>75%), respectively. Meanwhile, the associations of O3, for a 10 μg/m3 increment, increased from 0.04% (−0.09% to 0.16%) to 0.19% (0.10% to 0.28%) and 0.29% (0.18% to 0.39%) in strata of PM2.5 in the lowest fourth, 25-75% centile, and highest fourth, respectively. Although the interactions between PM2.5 and O3 were not statistically significant for cardiovascular and respiratory mortality (P>0.05), the trend of larger estimates in strata of higher concentrations of co-pollutant remained consistent.
Table 2.
Percentage changes (central estimates) in total, cardiovascular, and respiratory mortality associated with a 10 μg/m3 increase in PM2.5 and O3 in analyses stratified by level of co-pollutant
| Endpoints by pollutant and co-pollutant strata | Estimates (95% CI) | P value* |
|---|---|---|
| Total mortality | ||
| PM2.5: | ||
| ≤25% O3 | 0.47 (0.26 to 0.67) | <0.001 |
| 25-75% O3 | 0.70 (0.53 to 0.87) | |
| >75% O3 | 1.25 (1.02 to 1.48) | |
| O3: | ||
| ≤25% PM2.5 | 0.04 (−0.09 to 0.16) | <0.001 |
| 25-75% PM2.5 | 0.19 (0.10 to 0.28) | |
| >75% PM2.5 | 0.29 (0.18 to 0.39) | |
| Cardiovascular mortality | ||
| PM2.5: | ||
| ≤25% O3 | 0.52 (0.23 to 0.81) | 0.01 |
| 25-75% O3 | 0.56 (0.25 to 0.87) | |
| >75% O3 | 1.08 (0.63 to 1.53) | |
| O3: | ||
| ≤25% PM2.5 | 0.21 (−0.02 to 0.44) | 0.69 |
| 25-75% PM2.5 | 0.24 (0.11 to 0.38) | |
| >75% PM2.5 | 0.30 (0.10 to 0.50) | |
| Respiratory mortality | ||
| PM2.5: | ||
| ≤25% O3 | 0.98 (0.49 to 1.48) | 0.53 |
| 25-75% O3 | 0.96 (0.57 to 1.36) | |
| >75% O3 | 1.27 (0.61 to 1.93) | |
| O3: | ||
| ≤25% PM2.5 | −0.11 (−0.48 to 0.26) | 0.30 |
| 25-75% PM2.5 | 0.09 (−0.15 to 0.34) | |
| >75% PM2.5 | 0.15 (−0.15 to 0.46) | |
CI=confidence interval; O3=ozone; PM2.5=fine particulate matter with aerodynamic diameter ≤2.5 μm.
P values for interactions were obtained from likelihood ratio tests. P<0.05 indicates significant between group differences.
Table 3 depicts the interactions between PM2.5 and O3 on total, cardiovascular, and respiratory mortality using the synergy index method. When compared with the low PM2.5 and low O3 exposure group, the relative risk in the other three groups (low-high exposures, high-low, and high-high) were all >1 for all three endpoints, indicating stronger associations between a certain pollutant and mortality at higher levels of co-pollutant. In the additive interaction model, the joint estimate for PM2.5 and O3 was larger than the sum of the individual estimates, indicating a synergistic interaction. For example, the individual relative risk for PM2.5 and O3 on total mortality was 1.007 (95% confidence interval 1.005 to 1.009) and 1.001 (0.998 to 1.003), respectively, whereas the joint estimate was 1.015 (1.013 to 1.018), with a synergy index of 1.92 (95% confidence interval 1.46 to 3.33). The synergy indices for PM2.5 and O3 on cardiovascular and respiratory mortality were 1.37 (0.92 to 5.56) and 1.35 (0.90 to 9.21), indicating additive interactions. Across all countries, the attributable number of deaths for the interaction between exposure to PM2.5 and O3 was 37 651 across all countries (see supplementary eFigure 6), which is larger than the sum of the individual estimates (18 113 for PM2.5 and 3649 for O3).
Table 3.
Synergy index and relative risk for exposures to PM2.5 and O3 on total, cardiovascular, and respiratory mortality
| Endpoint by category | Relative risk* (95% CI) |
|---|---|
| Total mortality | |
| Low-low | Reference |
| Low-high | 1.001 (0.998 to 1.003) |
| High-low | 1.007 (1.005 to 1.009) |
| High-high | 1.015 (1.013 to 1.018) |
| Synergy index | 1.92 (1.46 to 3.33) |
| Cardiovascular mortality | |
| Low-low | Reference |
| Low-high | 1.002 (0.998 to 1.006) |
| High-low | 1.006 (1.002 to 1.010) |
| High-high | 1.011 (1.006 to 1.016) |
| Synergy index | 1.37 (0.92 to 5.56) |
| Respiratory mortality | |
| Low-low | Reference |
| Low-high | 1.001 (0.994 to 1.008) |
| High-low | 1.012 (1.005 to 1.019) |
| High-high | 1.018 (1.012 to 1.025) |
| Synergy index† | 1.35 (0.90 to 9.21) |
CI=confidence interval; O3=ozone; PM2.5=fine particulate matter with aerodynamic diameter ≤2.5 μm.
Risk for certain strata of low/high levels of air pollutants to days with low levels for both pollutants. Low and high designations were based on whether levels were above or below the median values of air pollutants across cities. First exposure relates to PM2.5 and second to O3; low-high=relative risk (RR)01; high-low=RR10; high-high=RR11.
Calculated as [RR11−1]/[(RR10-1)+(RR01−1)].
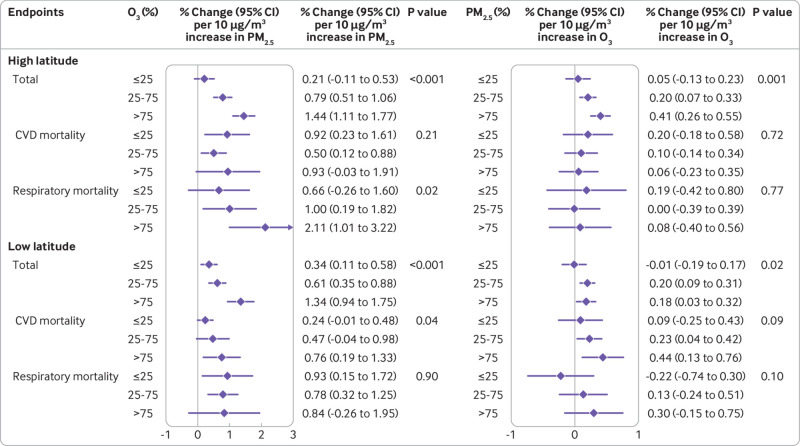
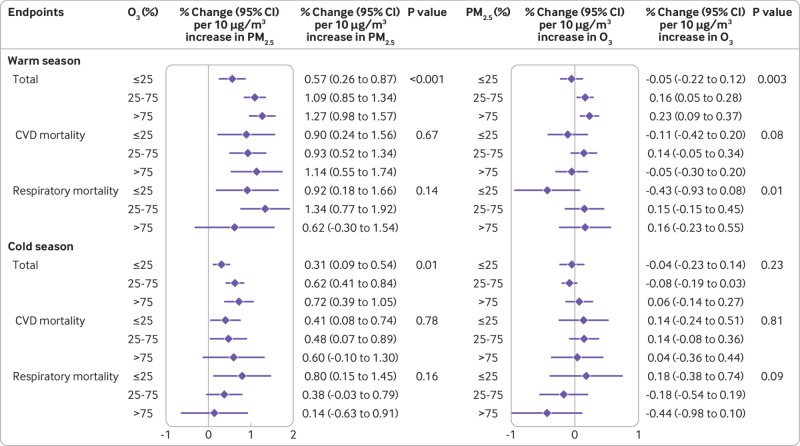
In the stratified analysis by high or low latitude region (fig 1), we observed larger associations of PM2.5 with total mortality with higher exposure to O3 in both regions (P<0.001). In the high latitude region, PM2.5 had a larger estimated impact on respiratory mortality with higher exposure to O3 (P=0.015); in the low latitude region, this interaction was only observed for cardiovascular mortality (P=0.041). The estimated associations of O3 with cardiovascular mortality were larger with higher exposure to PM2.5 in the low latitude region, although the between group difference was not significant (P=0.092). In contrast, in the high latitude region we only observed larger associations of O3 on total mortality with higher exposure to PM2.5 (P=0.001). Figure 2 shows the stratified results by season. Associations of PM2.5 were larger for total mortality with higher exposure to O3 in both the warm season (P<0.001) and the cold season (P=0.008). For O3, the association with total mortality (P=0.002) was larger only with higher exposure to PM2.5 in the warm season; no evidence of interaction was observed for any mortality endpoints in the cold season. Stratified analyses by WHO regions revealed comparable effect estimates and similar interaction patterns for the associations between PM2.5 or O3 and mortality (see supplementary eTable 2), with larger associations between PM2.5 and mortality with higher exposure to O3 across the Western-Pacific Regional Office, the Regional Office for Europe, and the Regional Office for the Americas.
Fig 1.
Percentage changes (central estimates and 95% confidence interval) in total, cardiovascular, and respiratory mortality associated with a 10 μg/m3 increase in PM2.5 and O3 in stratified analyses and in high and low latitude regions. High latitude (n=186), latitude above median (absolute value of 40.3°) across all cities; low latitude (n=186), latitude below median across all cities. P values for interactions were obtained from likelihood ratio tests. CI=confidence interval; CVD=cardiovascular mortality; O3=ozone; PM2.5=fine particular matter with an aerodynamic diameter ≤2.5 μm
Fig 2.
Percentage changes (central estimates and 95% confidence interval) in total, cardiovascular, and respiratory mortality associated with a 10 μg/m3 increase in PM2.5 and O3 in stratified analyses and in warm and cold seasons. Warm season is March to August for the northern hemisphere and September to February for the southern hemisphere; Cold season is September to February for the northern hemisphere and March to August for the southern hemisphere. P values for interactions were obtained from likelihood ratio tests. CI=confidence interval; CVD=cardiovascular mortality; O3=ozone; PM2.5=fine particular matter with an aerodynamic diameter ≤2.5 μm
Supplementary eTable 3 summarises the interaction between PM2.5 and O3 in the associations with total mortality stratified by region and season. The association of PM2.5 with total mortality was positive and statistically significant for both regions and seasons, but the association of O3 was generally not statistically significant. Nevertheless, we consistently found larger joint effects of PM2.5 and O3 than the sum of their individual effects in the additive interaction models for both regions and seasons. The synergy index of PM2.5 and O3 was 2.59 (95% confidence interval 1.59 to 6.87) in the high latitude region and 1.49 (1.01 to 5.46) in the low latitude region. For stratification by season, a larger synergy index was found in the cold season (1.86, 1.09 to 4.22) than in the warm season (1.68, 1.28 to 3.01).
In sensitivity analysis (see supplementary eTable 4), compared with the main models (lag 0-1), the alternative lags of air pollutants generally yielded slightly smaller but comparable estimates for both PM2.5 and O3, although some marginally statistically significant estimates were observed for O3 in the 25% lowest fourth of PM2.5. Supplementary eTable 5 summarises the sensitivity analysis using different temperature modelling. With longer temperature lags, the associations of both PM2.5 and O3 on total mortality generally increased but were mostly consistent, and the trend of larger estimates with higher exposure to co-pollutant remained.
Discussion
This study found positive interactive associations between PM2.5 and O3, with larger mortality estimates linked to higher exposure of co-pollutants in stratified analyses and larger synergistic interactions based on the synergy index. We also observed varied interactive associations on different mortality endpoints in different regions and seasons. Our findings suggest the need for coordinated control strategies for these two air pollutants.
Two established methods were used to examine the existence of an interaction between PM2.5 and O3 across 372 cities worldwide. Similar and consistent results were found for positive interactions between the two pollutants. We examined the interactions by stratified analyses, which have the advantage of simplicity and fewer variables that enable quantitative comparison of associations in exposure-specific strata. As an example, the percentage change in total mortality associated with a 10 μg/m3 increment in PM2.5 was 0.47%, 0.70%, and 1.25% with low, middle, and high levels of exposure to O3, showing a clear pattern on the positive and significant interaction between PM2.5 and O3. Previous studies have found similar results. One study, for example, observed larger associations of PM10 (for a 10 μg/m3 increase) from 0.21% to 0.37% in the strata with higher O3 concentrations, for strata classified according to 5% and 95% percentiles.12 Also, we used the synergy index to quantify the interactive effects. The synergy indices were 1.93, 1.37, and 1.36 for total, cardiovascular, and respiratory mortality, indicating synergistic interaction between PM2.5 and O3 compared with the sum of the individual associations. Earlier studies have used such methods. For example, one study reported a higher joint effect of PM2.5 and O3 on risk of preterm birth, with an adjusted relative risk of 3.63 that was larger than the independent risks (0.99 for PM2.5 and 1.34 for O3).29
Using stratified analyses, we identified regional and seasonal factors that may modify the interactions between PM2.5 and O3 with mortality. Interactive associations were larger in cities located at higher latitudes. The current analysis adjusted for temperature at short term scales, and our previous investigations have indicated larger individual associations of PM2.5 on mortality in cities with lower long term (ie, annual mean) temperature levels.16 As high latitude areas usually have lower ambient temperatures, there may be potential synergistic interactions between low temperatures and air pollutants, findings that are supported by animal studies on low temperature or cold stress with PM2.5.30 31 Furthermore, previous environmental studies have reported varied compositions of PM2.5 in regions at different latitudes, influenced by factors such as geographical and climate conditions, socioeconomic status, and emissions from local industries.32 33 These differences in PM2.5 composition have the potential to modify the associations between PM2.5 and mortality and its interaction with O3.34 In addition, the photochemical reactions that produce O3 also produce other reactive compounds, such as peroxyacetyl nitrate, the concentrations of which are correlated with O3, and hence O3 may capture some of the effects.35 Differences in the amount of these correlated toxic compounds may result in variations in the O3 effect size. However, PM2.5 and O3 levels exhibit distinct seasonal patterns.36 Typically, in winter, stagnant atmospheric conditions and meteorological inversions (where the normal decrease in temperature with increasing altitude is inverted, causing warmer air to be trapped near the ground by a layer of cooler air above it) contribute to severe haze, resulting in increased PM2.5 related pollution; conversely, in summer, intense solar radiation promotes the photochemical generation of O3.37 38 Despite the expected negative PM2.5-O3 correlations in winter and summer, our study identified noteworthy interactions between the two air pollutants in both warm and cold seasons. Moreover, while the effect estimates of PM2.5 and O3 with mortality were generally larger in the warm season, the results from the synergy index revealed larger PM2.5-O3 interactions during the cold season. This observation could be attributed to the seasonal differences in PM2.5 composition and different dispersion conditions of air pollutants.39 Additionally, O3 may act as a surrogate or have synergistic effects with other air pollutants (eg, nitrogen oxides) during the cold season.40 Furthermore, the cold season observation aligns with the finding of larger associations in regions characterised by higher latitudes and lower temperatures. Nevertheless, additional studies are warranted to replicate these findings, encompassing larger populations, diverse geographical regions, and additional complementary methodologies.
Policy implications
The findings of this study have important policy implications. Firstly, our study provides compelling evidence for the synergistic interaction between PM2.5 and O3 in their associations with mortality across hundreds of cities worldwide. These findings suggest that previous risk assessments, which solely accounted for individual associations of air pollutants, may have underestimated the true impact on disease burden. Therefore, our study emphasises the necessity for a more comprehensive analytical framework to consider the interactive effects of air pollutants in future scientific research and policy making scenarios. In terms of air pollution regulation, strategies to achieve synergistic control of PM2.5 and O3 are urgently needed, such as targeted end-of-pipe control measures or energy structure transformation. The resulting health benefits from these efforts would serve as a strong motivator to drive further action. Finally, from an individual’s perspective, our study highlights the importance of personal protection against synergistic effects of air pollution. The implications of this research extend to public health and global policies for air pollution control, with the potential to deliver health benefits not only to patients but also to vulnerable populations, such as those residing in high latitude or colder areas. Ultimately, these findings have far reaching implications for general populations worldwide, and emphasise the need for concerted efforts.
Limitations of this study
This study has some limitations. Most of the cities studied are located in the northern hemisphere, and data from South Asia, Africa, and Latin America were sparse. Thus caution is needed when generalising our results to other areas, and the findings may not be fully applicable to rural settings. Also, the sample size for total mortality was larger than that for cardiovascular or respiratory mortality, and we lacked information on other causes of death, which introduced some uncertainty to our estimates for cause specific mortality. Given the utilisation of diverse data sources in this multi-country study, the potential for misclassification of causes of death cannot be entirely ruled out.
Our study design was a time series analysis of short duration; thus we cannot provide information on the long term interactions between PM2.5 and O3. Potential measurement errors may be present, as exposure was based on city level averages and did not differentiate among heterogeneity in populations living in non-urban areas, air pollutant concentrations, or potential interactive effects, nor did the estimate deal with personal exposure. Previous studies have, however, found that assessing exposure at city level correlates strongly with temporal variation in personal exposure, thus utilising city level exposure assessment is a valid approach for time series studies focusing on short term exposure effects.41 42 Lastly, this study did not consider the impact of different PM2.5 compositions, as well as the interaction between temperature and other air pollutants (ie, nitrogen oxides and sulphur dioxide), which could influence the PM2.5-O3 interaction.
Conclusions
Regional and seasonal characteristics may modify the interaction between PM2.5 and O3 associated with different mortality outcomes, with more pronounced interactions in high latitude regions and during cold seasons. Our findings indicate a larger disease burden associated with exposure to PM2.5 and O3 than the sum of their individual contributions, which provide important evidence for future coordinated control of these air pollutants.
What is already known on this topic
Short term exposure to ambient fine particulate matter (PM2.5) and ozone (O3) has been linked to increased mortality, but studies evaluating interactive effects have been limited
Studies mostly have been conducted in a single city or country and adopted various designs and model variables, leading to heterogeneous results
What this study adds
This study found statistically significant synergistic associations between PM2.5 and O3 and total, cardiovascular, and respiratory mortality
The synergistic interactions between PM2.5 and O3 were more prominent in high latitude regions and during cold seasons
Web extra.
Extra material supplied by authors
Supplementary information: Additional eTables 1-5 and eFigures 1-6
Contributors: HK and AG are senior authors and contributed equally to this work. HK and AG designed the study. CL and RC are joint first authors with equal contribution. CL and RC coordinated the work, conducted the statistical analysis, and took the lead in drafting the manuscript and interpreting the results. FS, AMV-C, ST, EL, SA, DR, NR, MP, SB, JM, VG, MLB, and JS provided substantial scientific input in interpreting the results and drafting the manuscript. YG, PMC, NVO, JJKJ, AS, AE, FM, RR, YH, MH, CFSN, I-HH, AT, CI, YLG, S-CP, PM, and AZ provided the data and contributed to the revision of the submitted version of the manuscript. HK and AG are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: HK, RC, and CL were supported by the National Natural Science Foundation of China (92043301, 82030103, and 82103790). FS was supported by the Italian Ministry of University and Research, Department of Excellence project 2023-2027 ReDS “Rethinking Data Science” - Department of Statistics, Computer Science and Applications - University of Florence. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at URL www.icmje.org/disclosure-of-interest/ and declare: support from the National Natural Science Foundation of China and the Italian Ministry of University and Research; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (HK and AG) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We plan to engage the public in disseminating our research findings through various means, such as social media, newsletters, and conferences. The findings will also be disseminated through press releases by the research institutions of the contributing authors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
Data have been collected within the MCC (Multi-Country Multi-City) Collaborative Research Network (https://mccstudy.lshtm.ac.uk/) under a data sharing agreement and cannot be made publicly available. Researchers can refer to MCC participants listed as coauthors for information on accessing the data for each country.
References
- 1. Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med 2000;343:1742-9. 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 2. Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA 2004;292:2372-8. 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei Y, Wang Y, Di Q, et al. Short term exposure to fine particulate matter and hospital admission risks and costs in the Medicare population: time stratified, case crossover study. BMJ 2019;367:l6258. 10.1136/bmj.l6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian Y, Liu H, Wu Y, et al. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: time series study in 184 major Chinese cities. BMJ 2019;367:l6572. 10.1136/bmj.l6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223-49. 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen R, Yin P, Meng X, et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am J Respir Crit Care Med 2017;196:73-81. 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- 8. Yin P, Chen R, Wang L, et al. Ambient Ozone Pollution and Daily Mortality: A Nationwide Study in 272 Chinese Cities. Environ Health Perspect 2017;125:117006. 10.1289/EHP1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouthillier L, Vincent R, Goegan P, et al. Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1. Am J Pathol 1998;153:1873-84. 10.1016/S0002-9440(10)65701-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong C, Zhang Q, Zhang Y, et al. Impacts of climate change on future air quality and human health in China. Proc Natl Acad Sci U S A 2019;116:17193-200. 10.1073/pnas.1812881116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong YC, Lee JT, Kim H, Ha EH, Schwartz J, Christiani DC. Effects of air pollutants on acute stroke mortality. Environ Health Perspect 2002;110:187-91. 10.1289/ehp.02110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen GH, Song GX, Jiang LL, et al. Interaction between ambient particles and ozone and its effect on daily mortality. Biomed Environ Sci 2007;20:502-5. [PubMed] [Google Scholar]
- 13. Qiu H, Yu IT, Tian L, et al. Effects of coarse particulate matter on emergency hospital admissions for respiratory diseases: a time-series analysis in Hong Kong. Environ Health Perspect 2012;120:572-6. 10.1289/ehp.1104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Revich B, Shaposhnikov D. The effects of particulate and ozone pollution on mortality in Moscow, Russia. Air Qual Atmos Health 2010;3:117-23. 10.1007/s11869-009-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo C, Yang HT, Chang LY, et al. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: a longitudinal cohort study. Diabetologia 2021;64:1298-308. 10.1007/s00125-021-05408-4. [DOI] [PubMed] [Google Scholar]
- 16. Liu C, Chen R, Sera F, et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med 2019;381:705-15. 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vicedo-Cabrera AM, Sera F, Liu C, et al. Short term association between ozone and mortality: global two stage time series study in 406 locations in 20 countries. BMJ 2020;368:m108. 10.1136/bmj.m108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 2015;386:369-75. 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo Y, Gasparrini A, Armstrong BG, et al. Temperature Variability and Mortality: A Multi-Country Study. Environ Health Perspect 2016;124:1554-9. 10.1289/EHP149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. WHO . International statistical classification of diseases and related health problems. World Health Organization, 2004. [Google Scholar]
- 21. Meng X, Liu C, Chen R, et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: multilocation analysis in 398 cities. BMJ 2021;372:n534. 10.1136/bmj.n534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sera F, Armstrong B, Blangiardo M, Gasparrini A. An extended mixed-effects framework for meta-analysis. Stat Med 2019;38:5429-44. 10.1002/sim.8362. [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Karvonen-Gutierrez CA, Gold EB, et al. Longitudinal Associations of Air Pollution With Body Size and Composition in Midlife Women: The Study of Women’s Health Across the Nation. Diabetes Care 2022;45:2577-84. 10.2337/dc22-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee W, Wu X, Heo S, et al. Associations between long term air pollution exposure and first hospital admission for kidney and total urinary system diseases in the US Medicare population: nationwide longitudinal cohort study. BMJ Med 2022;1:e000009. 10.1136/bmjmed-2021-000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575-9. 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 26. Rothman KJ. Modern Epidemiology. Little, Brown, 1986. [Google Scholar]
- 27. Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology 1996;7:286-90. 10.1097/00001648-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 28. Liu C, Cai J, Chen R, et al. Coarse Particulate Air Pollution and Daily Mortality: A Global Study in 205 Cities. Am J Respir Crit Care Med 2022;206:999-1007. 10.1164/rccm.202111-2657OC. [DOI] [PubMed] [Google Scholar]
- 29. Siddika N, Rantala AK, Antikainen H, et al. Synergistic effects of prenatal exposure to fine particulate matter (PM2.5) and ozone (O3) on the risk of preterm birth: A population-based cohort study. Environ Res 2019;176:108549. 10.1016/j.envres.2019.108549. [DOI] [PubMed] [Google Scholar]
- 30. Zhou J, Geng F, Xu J, et al. PM2.5 exposure and cold stress exacerbates asthma in mice by increasing histone acetylation in IL-4 gene promoter in CD4+ T cells. Toxicol Lett 2019;316:147-53. 10.1016/j.toxlet.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 31. Luo B, Shi H, Wang L, et al. Rat lung response to PM2.5 exposure under different cold stresses. Int J Environ Res Public Health 2014;11:12915-26. 10.3390/ijerph111212915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baxter LK, Duvall RM, Sacks J. Examining the effects of air pollution composition on within region differences in PM2.5 mortality risk estimates. J Expo Sci Environ Epidemiol 2013;23:457-65. 10.1038/jes.2012.114. [DOI] [PubMed] [Google Scholar]
- 33. Wang YS, Chang LC, Chang FJ. Explore Regional PM2.5 Features and Compositions Causing Health Effects in Taiwan. Environ Manage 2021;67:176-91. 10.1007/s00267-020-01391-5. [DOI] [PubMed] [Google Scholar]
- 34. Masselot P, Sera F, Schneider R, et al. Differential Mortality Risks Associated With PM2.5 Components: A Multi-Country, Multi-City Study. Epidemiology 2022;33:167-75. 10.1097/EDE.0000000000001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun M, Zhou Y, Wang Y, et al. Seasonal discrepancies in peroxyacetyl nitrate (PAN) and its correlation with ozone and PM2.5: Effects of regional transport from circumjacent industrial cities. Sci Total Environ 2021;785:147303. 10.1016/j.scitotenv.2021.147303. [DOI] [PubMed] [Google Scholar]
- 36. Fowler D, Brimblecombe P, Burrows J, et al. A chronology of global air quality. Philos Trans A Math Phys Eng Sci 2020;378:20190314. 10.1098/rsta.2019.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma Z, Hu X, Sayer AM, et al. Satellite-Based Spatiotemporal Trends in PM2.5 Concentrations: China, 2004-2013. Environ Health Perspect 2016;124:184-92. 10.1289/ehp.1409481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen L, Mickley LJ. Seasonal prediction of US summertime ozone using statistical analysis of large scale climate patterns. Proc Natl Acad Sci U S A 2017;114:2491-6. 10.1073/pnas.1610708114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cichowicz R, Wielgosiński G, Fetter W. Dispersion of atmospheric air pollution in summer and winter season. Environ Monit Assess 2017;189:605. 10.1007/s10661-017-6319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jhun I, Coull BA, Zanobetti A, Koutrakis P. The impact of nitrogen oxides concentration decreases on ozone trends in the USA. Air Qual Atmos Health 2015;8:283-92. 10.1007/s11869-014-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebelt ST, D’Souza RR, Yu H, Scovronick N, Moss S, Chang HH. Monitoring vs. modeled exposure data in time-series studies of ambient air pollution and acute health outcomes. J Expo Sci Environ Epidemiol 2023;33:377-85.. 10.1038/s41370-022-00446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strickland MJ, Darrow LA, Mulholland JA, et al. Implications of different approaches for characterizing ambient air pollutant concentrations within the urban airshed for time-series studies and health benefits analyses. Environ Health 2011;10:36. 10.1186/1476-069X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional eTables 1-5 and eFigures 1-6
Data Availability Statement
Data have been collected within the MCC (Multi-Country Multi-City) Collaborative Research Network (https://mccstudy.lshtm.ac.uk/) under a data sharing agreement and cannot be made publicly available. Researchers can refer to MCC participants listed as coauthors for information on accessing the data for each country.