Abstract
Taurine, a naturally occurring sulfur-containing amino acid, has attracted significant attention in recent years due to its potential health benefits. Found in various foods and often used in energy drinks and supplements, taurine has been studied extensively to understand its impact on human physiology. Determining its exact functional roles represents a complex and multifaceted topic. We provide an overview of the scientific literature and present an analysis of the effects of taurine on various aspects of human health, focusing on aging and cardiovascular pathophysiology, but also including athletic performance, metabolic regulation, and neurological function. Additionally, our report summarizes the current recommendations for taurine intake and addresses potential safety concerns. Evidence from both human and animal studies indicates that taurine may have beneficial cardiovascular effects, including blood pressure regulation, improved cardiac fitness, and enhanced vascular health. Its mechanisms of action and antioxidant properties make it also an intriguing candidate for potential anti-aging strategies.
Keywords: aging, 2-aminoethanesulfonic acid, cardiovascular risk, energy drinks, inflammation, metabolism, oxidative stress, supplements, tauric acid, taurine
1. Introduction
Taurine (2-aminoethanesulfonic acid, also known as tauric acid) is a non-protein amino acid found in various animal tissues, especially in the brain, heart, and skeletal muscles. It is also present in several foods, such as meat, fish, dairy products, and energy drinks.
The main aim of this review is to summarize the key functional roles played by taurine in aging and in cardiovascular pathophysiology, especially based on the most recent findings in these fields. Specifically, taurine has been linked to, antioxidant activity, anti-inflammatory effects, and blood pressure regulation, with major implications for human health.
2. Nomenclature, Chemistry, and Biochemistry
The name taurine derives from the Latin taurus (cognate to Ancient Greek ταῦρος, “taûros”) meaning bull or ox: indeed, taurine was first isolated from the bile of the ox, Bos taurus, in 1827 by the German scientists Leopold Gmelin and Friedrich Tiedemann [1]. Early studies focused on its presence in animal tissues, where it was found in high concentrations in the brain, heart, and skeletal muscles. Later on, in 1846, the English chemist Edmund Ronalds confirmed the presence of taurine in human bile [2]. Taurine is detected in high concentrations in oxidative tissues, characterized by a high number of mitochondria, and in lower concentrations in glycolytic tissues [3,4,5,6]. The taurine content in various human tissues is reported in Table 1; over the years, researchers have explored its role in various physiological processes, leading to an increased understanding of its significance in human health.
Table 1.
| Tissue | Content in μmol/L (Liquid) or μmol/g (Solid) |
|---|---|
| Bile | ~200 |
| Plasma | 50–100 |
| Leukocytes and platelets | 10–50 |
| Retina | 30–40 |
| Heart | 6–25 |
| Brain | 0.8–20 |
| Skeletal muscle | 2.2–5.4 |
| Kidney | 1.4–1.8 |
| Liver | 0.3–2 |
| Erythrocytes | 0.05–0.08 |
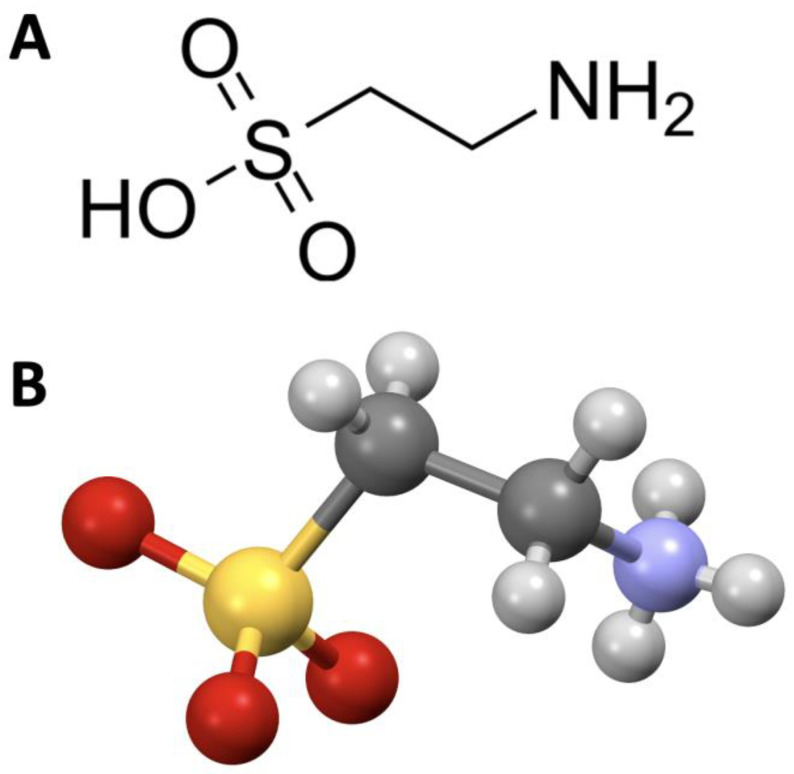
Chemically, taurine is classified as a beta-amino acid, and its molecular formula is C2H7NO3S (Molecular Weight, MW: 125.15). Structurally, it is characterized by an amino group (NH2) and a sulfonic acid group (SO3H) attached to the beta carbon (Figure 1); unlike other amino acids, taurine lacks a chiral center, meaning it is optically inactive; its relatively simple structure allows it to perform diverse functions within the body.
Figure 1.
Chemical structure (A) and call-and-stick model (B) of taurine.
While the human body can synthesize taurine to some extent, dietary intake is essential to maintain optimal levels. Foods rich in taurine include meat, fish, poultry, and dairy products. Vegetarians and vegans may have a lower taurine intake due to their dietary restrictions [12], but the significance of this in terms of deficiency remains unclear.
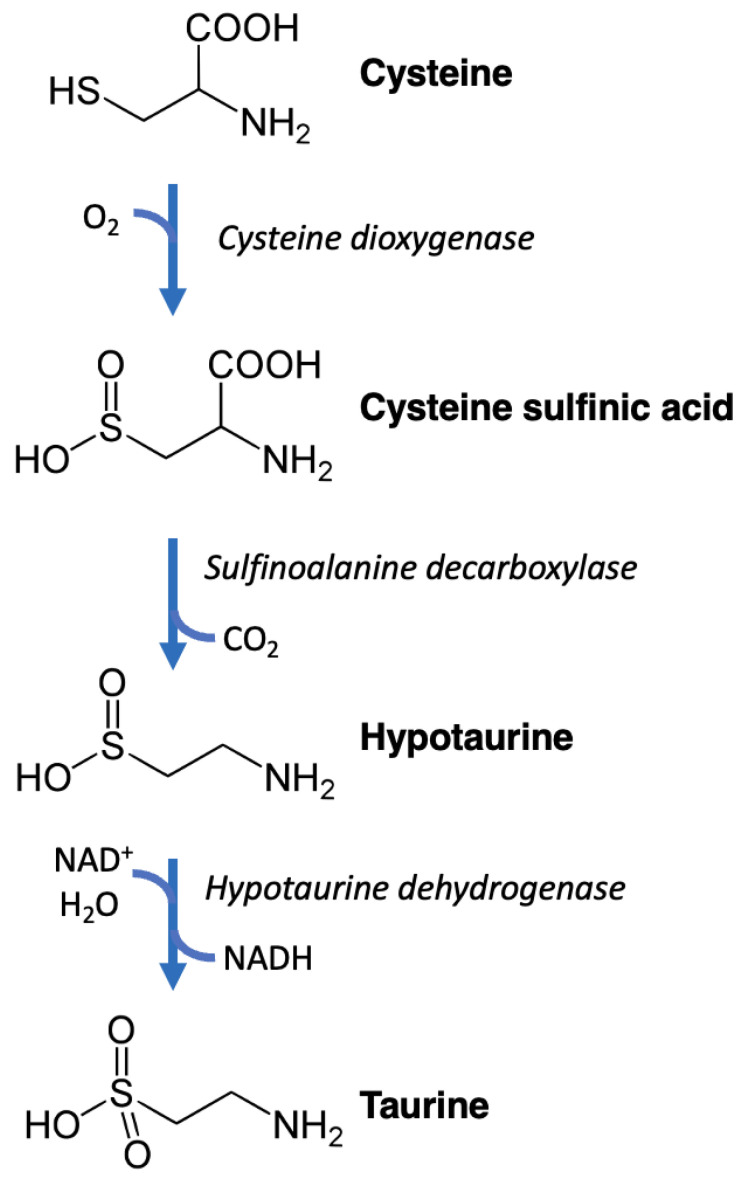
Taurine is synthesized in humans in the liver mainly via the “cysteine sulfinic pathway” (Figure 2). Cysteine dioxygenase oxidizes cysteine to form cysteine sulfinic acid, which is then decarboxylated by cysteine sulfinic acid decarboxylase to obtain hypotaurine, which is then oxidized by hypotaurine dioxygenase to form taurine [13,14,15,16,17,18]. An alternative pathway is trans-sulfuration, in which homocysteine is converted into cystathionine, which is then transformed into hypotaurine by cystathionine gamma-lyase, cysteine dioxygenase, and cysteine sulfinic acid decarboxylase, and finally oxidized to form taurine [19,20,21].
Figure 2.
Representation of the chemical reactions of the cysteine sulfinic pathway leading to taurine synthesis.
Taurine has been extensively studied to determine its effects on human health. In terms of cellular function, taurine is primarily found in the intracellular fluid of many tissues, where it plays a vital role in a number of physiological processes [22,23,24,25,26,27,28]. It acts as an osmolyte, regulating cell volume and maintaining cell integrity [29,30]. In the liver, taurine is conjugated with bile acids, forming bile salts that aid in fat digestion and absorption in the intestines [31,32,33,34]. These processes are crucial for lipid metabolism and absorption of fat-soluble vitamins [35].
Taurine has also been shown to be involved in calcium (Ca2+) signaling, modulation of ion channels, and neurotransmission, affecting neural excitability and synaptic transmission. Intriguingly, this amino acid exhibits important antioxidant properties, protecting cells from oxidative and nitrosative stress by scavenging free radicals and reactive oxygen species (ROS) [36,37,38,39,40,41,42,43,44]. These antioxidant actions certainly contribute to its potential benefits in terms of neuroprotection and cardiovascular health [45]. In fact, taurine is highly concentrated in the brain and several studies indicate that taurine might act as a neurotransmitter or neuromodulator, influencing neurotransmitter release and receptor function, affecting cognitive processes, mood, behavior, memory, learning, and anxiety regulation [46,47,48,49,50,51].
Taurine has been thought to be essential for the development and survival of neural cells and to protect them under cell-damaging conditions, indeed in the brain stem taurine regulates vital functions, including cardiovascular control and arterial blood pressure. Its neuroprotective effects involve also reducing neuronal apoptosis and inflammation [46], making it a subject of interest in research on neurodegenerative diseases and brain injuries and offering benefits during stroke recovery [52,53,54,55,56]. Premature infants are vulnerable to taurine deficiency because they lack some of the enzymes needed to synthesize cysteine and taurine. However, human breast milk contains high levels of taurine which is sufficient for newborns; formula milk is often supplemented with taurine, although evidence is mixed as to whether this strategy is actually beneficial or not [57,58,59,60,61,62]. Nevertheless, further studies are needed to fully understand taurine’s neurological effects.
As we will discuss below in a dedicated paragraph, taurine has been associated with several benefits especially on the cardiovascular system, including blood pressure regulation, anti-inflammatory effects, and improvements in endothelial function; overall, these properties contribute to its potential in reducing the risk of cardiovascular diseases [63,64,65].
3. Taurine and Cardiovascular Health
Taurine plays a crucial role in cardiovascular physiology. Numerous studies have investigated the potential cardioprotective effects of taurine, focusing on its impact on blood pressure, cardiac contractility, and vascular function. It may help reduce blood pressure in individuals with hypertension and improve endothelial function, leading to enhanced vascular health. Its antioxidant properties may also reduce the risk of cardiovascular diseases such as atherosclerosis and heart failure [66,67].
As we will see in detail in the paragraphs below, the main cardiovascular effects of taurine are attributed to a number of underlying mechanisms. For instance, its modulation of ion channels, including Ca2+ and potassium (K+) channels, influences cardiac electrical activity and vascular tone. Its role in Ca2+ homeostasis also impacts myocardial contractility and relaxation. Additionally, the antioxidant properties of taurine, for which the exact underlying mechanisms remain unclear, might help protect against oxidative stress, a factor involved in the pathophysiology of cardiovascular disease. Interestingly, two taurine-containing modified uridines, 5-taurinomethyluridine (τm5u) and 5-taurinomethyl-2-thiouridine (τm5s2u) have been identified in mitochondrial tRNA: these conjugates could be associated with the actions of taurine as an antioxidant [68,69,70,71]. Another proposed mechanism is the stabilization of intracellular levels of antioxidant enzymes like superoxide dismutase (SOD) and glutathione [72,73].
Taurine has been also implicated in metabolic regulation, particularly in relation to glucose and lipid metabolism [74,75]. Various studies indicate that taurine might help improve insulin sensitivity, making it beneficial for individuals with type 2 diabetes (T2D) or those at risk of developing the condition [76,77,78,79]. A recent preclinical study has shown that taurine can rescue pancreatic β-cell stress by stimulating α-cell trans-differentiation [80]. Additionally, taurine may aid in reducing triglyceride levels and improving lipid profiles [81,82,83,84,85], potentially lowering the risk of cardiovascular diseases and metabolic syndrome.
Preclinical investigations have provided valuable insights into the cardiovascular effects of taurine. In models of hypertension, heart failure, and atherosclerosis, taurine supplementation has consistently been shown to improve cardiac function, reduce blood pressure, and enhance vascular health. At the same time, human studies investigating taurine’s cardiovascular effects have also yielded promising results. Clinical trials have demonstrated its potential to reduce blood pressure, improve left ventricular function, and enhance exercise capacity in individuals with heart failure.
3.1. Taurine and Cardiac Function
Taurine accounts for ~50% of the total free amino acids in the heart; it has been shown to enhance cardiac contractility and improve heart function in both human and animal models. Animal studies have revealed that taurine deficiency induces atrophic cardiac remodeling [86], whilst taurine supplementation can increase myocardial contractility, stroke volume, and cardiac output [87,88,89,90,91,92,93]. In humans, taurine has been associated with improvements in the left ventricular function and exercise tolerance [94,95,96,97,98]. Notably, in 1985 taurine was approved as treatment for patients with heart failure in Japan [96].
The beneficial effects of taurine on Ca2+ and sodium (Na+) handling [89,90,99,100,101,102,103], myocardial energetics [104,105], and cellular signaling pathways (including glucose transport, 3-phosphoinositide-dependent protein kinase-1, AKT, sirtuin 1 (SIRT1), FOXO3, p38, NFkappaB, and others) [106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] are thought to underlie its major cardioprotective effects. Other mechanisms include the promotion of natriuresis and diuresis, most likely via an osmoregulatory activity in the kidney, a regulation of vasopressin release, and a modulation of the atrial natriuretic factor secretion [122,123,124,125]. In addition, taurine has been shown to attenuate the actions of angiotensin II on its downstream signaling pathways, on Ca2+ transport, and on protein synthesis [113].
3.2. Taurine and Vascular Function
The endothelium, a single layer of cells lining the blood vessels, plays a crucial role in vascular health. Taurine has been shown to improve the endothelial function by promoting nitric oxide (NO) production and reducing endothelial dysfunction [126]. Enhanced endothelial function contributes to better vascular relaxation, reduced inflammation, and improved blood flow, which may benefit cardiovascular health and reduce the risk of atherosclerosis and cardiovascular events [127,128,129,130].
The ability of taurine to regulate ion channels [131,132], modulate Ca2+ homeostasis [133,134,135], and enhance endothelial function [136,137,138,139,140] may contribute to its antihypertensive properties. Additionally, its antioxidant activity [54,126,141,142,143] may help protect blood vessels from oxidative stress, further contributing to its beneficial effects on blood pressure regulation.
Both human and animal studies have demonstrated that taurine supplementation can lead to a modest reduction in blood pressure [144,145,146,147]. Despite the fact that the effects of taurine on a healthy endothelium remain controversial, with some investigators showing an enhancement of the endothelium-dependent relaxation in response to acetylcholine [148] and other reports not confirming these findings [145,149], its beneficial action on a dysfunctional endothelium is more consistent [130,140,144]. A synergistic action in terms of cell survival has been experimentally shown [150] when combining taurine with another well-established enhancer of vascular function, i.e., L-arginine [129,151,152,153].
Strikingly, in a recent clinical trial, 120 patients with T2D were randomly allocated to take either 1 g of taurine or placebo three times per day for an 8-week period; taurine-supplemented patients displayed a significant decrease in serum insulin and HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) compared to the placebo group accompanied by a significant decline in several markers of inflammation, oxidative stress, and endothelial dysfunction [154]. A meta-analysis published in 2018 concluded that the ingestion of taurine can reduce blood pressure to a clinically relevant magnitude, without any major adverse side effects [155]. However, future studies are warranted to establish the exact effects of oral taurine supplementation on targeted pathologies and the optimal supplementation doses and periods.
3.3. Taurine and Athletic Performance
The presence of taurine in many energy drinks and sports supplements (~750–1000 mg in a can of 240 mL) is most likely due to its purported role in enhancing athletic performance. However, these energy drinks also contain caffeine, which has been previously linked to perceived energy boosts [156,157].
Some studies suggest that taurine may improve exercise capacity, reduce muscle damage, and alleviate exercise-induced oxidative stress. Its potential to increase muscle contractility and decrease fatigue has garnered interest among athletes. Nevertheless, conflicting findings warrant caution in interpreting these claims and several concerns on the use and abuse of energy drinks have been raised [158,159,160,161,162,163].
4. Taurine and Aging
4.1. Taurine and Longevity
Levels of taurine have been shown to decline as we age, and offsetting this loss with a taurine supplement might delay the development of age-related health problems [164,165,166,167]. Indeed, as shown in a Science paper recently published, when mice received taurine supplements, their lifespans increased by approximately 10% compared to the control group [168]. Mice in the taurine group also seemed healthier, with improvements in muscle endurance and strength. Researchers fed mice between 15 and 30 mg of taurine per day depending on their age. These doses would be equivalent to 3 to 6 g of taurine for an 80-kg body weight, which is within the safe limits according to European Food Safety Authority recommendations [169,170].
Taurine was also shown to shape the gut microbiota of mice and positively affect the restoration of intestinal homeostasis [171], suggesting that it could be harnessed to re-establish a normal microenvironment and to treat or prevent gut dysbiosis.
Beneficial effects on some hallmarks of aging were observed in Caenorhabditis elegans worms and middle-aged rhesus monkeys (Macaca mulatta) [172]. The taurine-fed worms lived longer and were healthier than the controls. The monkeys had lower body weights, reduced signs of liver damage, and denser bones [168].
Consistent with these data, a previous study conducted using data from the Korea National Health and Nutrition Examination Survey (KNHANES) had shown that taurine supplementation can decrease the cardiometabolic risk in male elderly subjects aged 75 and older [173]. Similarly, a double-blind study conducted in 24 women randomly assigned to receive taurine (1.5 g) or placebo (1.5 g of starch) for 16 weeks revealed that taurine supplementation prevented the decrease in SOD plasma levels [141], suggesting taurine as a potential strategy to control oxidative stress during the aging process.
4.2. Taurine and Cell Senescence
Cell senescence represents one of the fundamental mechanisms of aging [174,175]. Senescent cells are characterized by the cell cycle arrest, decreased susceptibility to apoptosis, and release of a particular set of cytokines, known as senescence-associated secretory phenotype (SASP) [176,177,178]. Despite preventing malignant transformation, accumulation of senescent cells negatively affects tissue functionality [179,180].
Multiple evidence demonstrates that the age-dependent decrease in the taurine content is associated with cell senescence. For instance, metabolomic analyses of human umbilical vein endothelial cells (HUVECs) at different passages have revealed a correlation between lower levels of taurine and HUVECs senescence [181].
In vitro, taurine mitigated replicative aging of bone marrow-derived multipotent stromal cells and restored their osteogenic differentiation potential at late passages [182]. Deletion of Slc6a6 (sodium- and chloride-dependent taurine transporter) resulted in a drastic shortening of the lifespan of mice [168,183]; specifically, Slc6a6 knockout mice exhibited a high expression of senescence markers p16 and p21, mirrored by a high expression of senescence-associated beta-galactosidase (SA-β-Gal) activity in the bones and liver. Treatment of Slc6a6 knockout mice with senolytics increased their lifespan, suggesting a causative link between cell senescence and taurine deficiency [168]. In line with these results, taurine supplementation for 10 months in aged wild type mice led to a reduction of senescent cells by a factor of two in the brain, gut and muscle, and almost by a factor of three in the liver and fat [168]. Some investigators indicate that taurine deficiency may induce cell senescence via activation of SMAD3 and β-catenin [184].
4.3. Taurine and Unfolded Protein Response
Loss of proteostasis is one of the hallmarks of aging. The burden of misfolded proteins increases with age due to the accumulation of somatic mutations, dysregulation of splicing, loss of chaperone activity, and malfunctioning autophagy [174,185]. Accumulation of misfolded proteins in the endoplasmic reticulum (ER) triggers an unfolded protein response (UPR) and ER stress, eventually resulting in cell death [186].
Knockout of Slc6a6 triggers UPR in the murine skeletal muscle, as demonstrated by unbiased RNA sequencing and by the direct measurement of ER stress-associated proteins content [183]. In drosophila, taurine’s beneficial effects on lifespan were totally abrogated by the silencing of Erol1 or Xbp1 genes; the products of these genes play crucial role in resolving ER stress [187]. Taurine cotreatment also prevented detrimental consequences of UPR during glucose deprivation or cisplatin toxicity [188,189].
4.4. Taurine and Telomere Attrition
Telomere attrition limits cell ability to proliferate endlessly [190,191,192]. The enzyme telomerase reverse transcriptase (TERT) prevents critical shortening of telomere length [174]. In vitro studies have shown that taurine can increase the TERT expression in dental-pulp-derived stem cells, thus maintaining their chondrogenic differentiation potential [193]. In line with this observation, a correlation was reported between the liver telomere length and the plasma levels of taurine in mice [194]. Taurine was also shown to mitigate detrimental consequences of telomere attrition; for instance, taurine supplementation prevented premature death of D. rerio with Tert deficiency [168].
4.5. Taurine and Sirtuins
Sirtuins are a family of proteins that possess either mono-ADP-ribosyltransferase or deacetylase activity [195,196]. Sirtuins regulate many signaling pathways, mostly connecting them with a metabolic state of the organism [197,198]. Their expression is decreased with age and their activation or overexpression is associated with increased longevity [199,200].
Taurine was shown to activate cytoplasmic SIRT1 in the liver, heart, and brain [121,201,202,203,204]. In these tissues, taurine-mediated upregulation of SIRT1 activity was associated with the prevention of organ dysfunction. For instance, in the heart, taurine promoted p53 inhibition via its deacetylation by SIRT1, resulting in a diminished apoptosis rate; of note, the protective effects of taurine were lost after cotreatment with a specific SIRT1 inhibitor [202].
Molecular docking modeling suggests that taurine activates SIRT1 via direct interaction with the protein; interestingly, taurine was predicted to bind another region of SIRT1 compared to the SIRT1 potent agonist resveratrol. Although the latter binds to the 289–304 amino acid sequence, taurine requires a pocket formed by amino acid 441–445 [121].
4.6. Taurine and Stem Cells
Depletion of stem cell pools is notably associated with aging and age-related disorders, leading to a gradual decline in organ functions and their healing capacities after damage [174,205,206,207]. Mounting data show that taurine increases the survival of stem cells, increases their regenerative capacity, and maintains stemness [208]. Notably, knocking out Slc6a6 abrogates the development of embryonic stem cells, again pointing to the crucial role of taurine [209]. Several studies demonstrate the beneficial effects of taurine treatment on neural stem cells and stem cells involved in bone and cartilage development [193,210,211,212,213,214]; moreover, it has also been suggested that taurine may promote development of skeletal muscles [215].
5. Recommended Intake and Safety Concerns
Currently, there are no established dietary reference intakes (DRIs) for taurine [216]. However, it is generally believed that the typical Western diet provides sufficient taurine for most people [217,218]. Specific populations, such as vegetarians or vegans, may have a lower taurine intake, but evidence of deficiency remains limited [219,220].
The normal dietary levels of taurine can vary depending on an individual’s diet and specific food choices. Taurine is a naturally occurring amino acid found in various foods [219,221,222,223,224], including seaweed, fish, meat, and some dairy products (Table 2); the average daily intake of taurine from the typical diet is estimated to be around 40 to 400 milligrams (mg) per day in adults.
Table 2.
Taurine content in foods.
| Food | Average Content in mg/100 g |
|---|---|
| Pyropia tenera (red algae, seaweed, dried) | 979 |
| Scallops (raw) | 827 |
| Mussels (raw) | 655 |
| Porphyra haitanensis (seaweed, dried) | 646 |
| Clams (raw) | 520 |
| Oysters (raw) | 507 |
| Octopus (raw) | 388 |
| Squid (raw) | 356 |
| Turkey (raw), dark meat | 306 |
| Chicken (raw), dark meat | 169 |
| White fish (raw) | 151 |
| Pork (raw) | 61 |
| Salami (cured) | 59 |
| Ham (baked) | 50 |
| Lamb (raw), dark meat | 47 |
| Beef (raw) | 43 |
| Tuna (canned) | 42 |
| Shrimps (raw) | 39 |
| Goat’s milk (pasteurized) | 6.8 |
| Egg yolk | 3.7 |
| Yogurt | 3.3 |
| Cow’s milk (pasteurized) | 2.4 |
| Ice cream | 1.9 |
Foods that contain the highest levels of taurine come from the sea and include seaweed and shellfish; for instance, taurine represents ~80% of the total amino acid content of pacific oyster (Crassostrea gigas) [225].
Regarding standard supplemental doses, taurine supplements are available in various forms, including capsules, tablets, and energy drinks. The recommended dosage of taurine as a dietary supplement might vary based on the specific product and its intended use. In general, most taurine supplements are available in doses ranging from 500 mg to 2000 mg per serving. It is important to note that individual responses to dietary supplements can differ, and the appropriate dose for a person may depend on various factors, including age, weight, overall health status, and underlying medical conditions. For this reason, it is advisable to follow the recommended dosage provided on the supplement’s packaging or as advised by a healthcare professional.
Overall, taurine is considered generally safe for most individuals when consumed in moderate amounts, as found in the average diet. However, as with any dietary supplement, moderation is key, and excessive consumption of taurine supplements beyond recommended doses may lead to potential side effects, including gastrointestinal disturbances (such as nausea, vomiting, and diarrhea) and neurological symptoms (dizziness, tremors, and headache) [226,227,228]. Moreover, caution should be used because of the potential interactions between taurine supplements and certain medications, particularly those having analogous effects (e.g., lowering blood pressure), targeting similar signaling pathways (e.g., Ca2+, angiotensin), and used to modulate heart or central nervous system functions. medications or [49,50,229]. Pregnant and lactating women, as well as individuals with specific health conditions, such as bipolar disorder, epilepsy, or kidney problems, should exercise caution and consult healthcare professionals before taking taurine supplements.
A risk assessment study conducted by Shao and colleagues, based on toxicological evidence from several clinical trials testing taurine supplementation, established the upper level of taurine supplementation at 3 g per day [230]. The only adverse effects noted in this study after consuming a 3 g dose of taurine were gastrointestinal disorders. Notably, the minimum dose used in these trials was 3 g/day, much greater than the usual intake of taurine from a normal diet (<0.4 g/day).
6. Conclusions
Taurine has a diverse array of functions in human health. From its origins in animal tissues to its roles in aging, cardiovascular health, neuroprotection, and cellular function, taurine continues to capture the attention of researchers and health professionals alike. Recent findings specifically suggest that taurine is a promising cardioprotective agent, offering potential benefits for cardiovascular health in both human and animal studies. However, its role in reducing cardiovascular risk warrants further investigation, including large-scale clinical trials, making it an intriguing subject for ongoing research and potential therapeutic applications. Further research is also needed to fully elucidate its mechanisms of action and confirm its efficacy in different settings including longevity. An adequate dietary intake of taurine through a balanced diet is recommended, and caution should be exercised when considering taurine supplementation, especially at high doses.
Acknowledgments
We thank Xujun Wang, for his helpful discussion.
Author Contributions
Conceptualization, G.S.; methodology, U.K. and P.M.; writing—original draft preparation, U.K., F.V., P.M. and S.S.J.; writing—review and editing, A.L. and G.S.; supervision, G.S.; funding acquisition, U.K., F.V., S.S.J. and G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
The Santulli’s Lab is currently supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1-TR002556-06, UM1-TR004400) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). U.K. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190). F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST915561). S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tiedemann F., Gmalin L. Einige neue Bestandtheile der Galle des Ochsen. Ann. Phys. 1827;85:326–337. doi: 10.1002/andp.18270850214. [DOI] [Google Scholar]
- 2.Garrod A. Lectures on the Chemistry of Pathology and Therapeutics: Showing the Application of the Science of Chemistry to the Discovery, Treatment, and Cure of Disease. Lancet. 1848;52:333–336. [Google Scholar]
- 3.Baliou S., Adamaki M., Ioannou P., Pappa A., Panayiotidis M.I., Spandidos D.A., Christodoulou I., Kyriakopoulos A.M., Zoumpourlis V. Protective role of taurine against oxidative stress (Review) Mol. Med. Rep. 2021;24:605. doi: 10.3892/mmr.2021.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jong C.J., Sandal P., Schaffer S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules. 2021;26:4913. doi: 10.3390/molecules26164913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen S.H., Andersen M.L., Cornett C., Gradinaru R., Grunnet N. A role for taurine in mitochondrial function. J. Biomed. Sci. 2010;17((Suppl. S1)):S23. doi: 10.1186/1423-0127-17-S1-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Luca A., Pierno S., Camerino D.C. Taurine: The appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen J.G., Smith L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968;48:424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- 8.Hayes K.C., Sturman J.A. Taurine in metabolism. Annu. Rev. Nutr. 1981;1:401–425. doi: 10.1146/annurev.nu.01.070181.002153. [DOI] [PubMed] [Google Scholar]
- 9.Sole M.J., Jeejeebhoy K.N. Conditioned nutritional requirements and the pathogenesis and treatment of myocardial failure. Curr. Opin. Clin. Nutr. Metab. Care. 2000;3:417–424. doi: 10.1097/00075197-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hansen S.H. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab. Res. Rev. 2001;17:330–346. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 11.Wojcik O.P., Koenig K.L., Zeleniuch-Jacquotte A., Costa M., Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208:19–25. doi: 10.1016/j.atherosclerosis.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laidlaw S.A., Shultz T.D., Cecchino J.T., Kopple J.D. Plasma and urine taurine levels in vegans. Am. J. Clin. Nutr. 1988;47:660–663. doi: 10.1093/ajcn/47.4.660. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein C.L., Haschemeyer R.H., Griffith O.W. In vivo studies of cysteine metabolism. Use of D-cysteinesulfinate, a novel cysteinesulfinate decarboxylase inhibitor, to probe taurine and pyruvate synthesis. J. Biol. Chem. 1988;263:16568–16579. doi: 10.1016/S0021-9258(18)37428-3. [DOI] [PubMed] [Google Scholar]
- 14.Drake M.R., De La Rosa J., Stipanuk M.H. Metabolism of cysteine in rat hepatocytes. Evidence for cysteinesulphinate-independent pathways. Biochem. J. 1987;244:279–286. doi: 10.1042/bj2440279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Y.C., Ding S.T., Lee Y.H., Wang Y.C., Huang M.F., Liu I.H. Taurine homeostasis requires de novo synthesis via cysteine sulfinic acid decarboxylase during zebrafish early embryogenesis. Amino Acids. 2013;44:615–629. doi: 10.1007/s00726-012-1386-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D., Fan J., Liu H., Qiu G., Cui S. Testosterone enhances taurine synthesis by upregulating androgen receptor and cysteine sulfinic acid decarboxylase expressions in male mouse liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2023;324:G295–G304. doi: 10.1152/ajpgi.00076.2022. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson K.R., Madl J.E., Clements J.R., Wu J.Y., Larson A.A., Beitz A.J. Colocalization of taurine- and cysteine sulfinic acid decarboxylase-like immunoreactivity in the cerebellum of the rat with monoclonal antibodies against taurine. J. Neurosci. 1988;8:4551–4564. doi: 10.1523/JNEUROSCI.08-12-04551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S., Sahoo B.M., Banik B.K. Biological Effects and Mechanisms of Taurine in Various Therapeutics. Curr. Drug Discov. Technol. 2023;online ahead of print doi: 10.2174/1570163820666230525101353. [DOI] [PubMed] [Google Scholar]
- 19.Sbodio J.I., Snyder S.H., Paul B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019;176:583–593. doi: 10.1111/bph.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons C.R., Liu Q., Huang Q., Hao Q., Begley T.P., Karplus P.A., Stipanuk M.H. Crystal structure of mammalian cysteine dioxygenase. A novel mononuclear iron center for cysteine thiol oxidation. J. Biol. Chem. 2006;281:18723–18733. doi: 10.1074/jbc.M601555200. [DOI] [PubMed] [Google Scholar]
- 21.Park E., Park S.Y., Cho I.S., Kim B.S., Schuller-Levis G. A Novel Cysteine Sulfinic Acid Decarboxylase Knock-Out Mouse: Taurine Distribution in Various Tissues with and without Taurine Supplementation. Pt 1Adv. Exp. Med. Biol. 2017;975:461–474. doi: 10.1007/978-94-024-1079-2_37. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Peng Q., Shang J., Dong W., Wu S., Guo X., Xie Z., Chen C. The role of taurine in male reproduction: Physiology, pathology and toxicology. Front. Endocrinol. 2023;14:1017886. doi: 10.3389/fendo.2023.1017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen C., Li F., Zhang L., Duan Y., Guo Q., Wang W., He S., Li J., Yin Y. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol. Nutr. Food Res. 2019;63:e1800536. doi: 10.1002/mnfr.201800536. [DOI] [PubMed] [Google Scholar]
- 24.Spriet L.L., Whitfield J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:96–101. doi: 10.1097/MCO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 25.Wu G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids. 2020;52:329–360. doi: 10.1007/s00726-020-02823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oja S.S., Saransaari P. Taurine and epilepsy. Epilepsy Res. 2013;104:187–194. doi: 10.1016/j.eplepsyres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Rosca A.E., Vladareanu A.M., Mirica R., Anghel-Timaru C.M., Mititelu A., Popescu B.O., Caruntu C., Voiculescu S.E., Gologan S., Onisai M., et al. Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential. J. Clin. Med. 2022;11:666. doi: 10.3390/jcm11030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Y., Li X., Liu Y., Gao J., Tao J. The molecular targets of taurine confer anti-hyperlipidemic effects. Life Sci. 2021;278:119579. doi: 10.1016/j.lfs.2021.119579. [DOI] [PubMed] [Google Scholar]
- 29.Schousboe A., Pasantes-Morales H. Role of taurine in neural cell volume regulation. Can. J. Physiol. Pharmacol. 1992;70:S356–S361. doi: 10.1139/y92-283. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J., Du X., Li J., Yamagata N., Xu B. Taurine Boosts Cellular Uptake of Small D-Peptides for Enzyme-Instructed Intracellular Molecular Self-Assembly. J. Am. Chem. Soc. 2015;137:10040–10043. doi: 10.1021/jacs.5b06181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falany C.N., Johnson M.R., Barnes S., Diasio R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 1994;269:19375–19379. doi: 10.1016/S0021-9258(17)32178-6. [DOI] [PubMed] [Google Scholar]
- 32.Murakami S., Fujita M., Nakamura M., Sakono M., Nishizono S., Sato M., Imaizumi K., Mori M., Fukuda N. Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin. Exp. Pharmacol. Physiol. 2016;43:372–378. doi: 10.1111/1440-1681.12534. [DOI] [PubMed] [Google Scholar]
- 33.Bellentani S., Pecorari M., Cordoma P., Marchegiano P., Manenti F., Bosisio E., De Fabiani E., Galli G. Taurine increases bile acid pool size and reduces bile saturation index in the hamster. J. Lipid Res. 1987;28:1021–1027. doi: 10.1016/S0022-2275(20)38617-X. [DOI] [PubMed] [Google Scholar]
- 34.Batta A.K., Salen G., Shefer S., Tint G.S., Dayal B. The effect of tauroursodeoxycholic acid and taurine supplementation on biliary bile acid composition. Hepatology. 1982;2:811–816. doi: 10.1002/hep.1840020612. [DOI] [PubMed] [Google Scholar]
- 35.de Aguiar Vallim T.Q., Tarling E.J., Edwards P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim M.A., Eraqi M.M., Alfaiz F.A. Therapeutic role of taurine as antioxidant in reducing hypertension risks in rats. Heliyon. 2020;6:e03209. doi: 10.1016/j.heliyon.2020.e03209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degim Z., Celebi N., Sayan H., Babul A., Erdogan D., Take G. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids. 2002;22:187–198. doi: 10.1007/s007260200007. [DOI] [PubMed] [Google Scholar]
- 38.Chang C.Y., Shen C.Y., Kang C.K., Sher Y.P., Sheu W.H., Chang C.C., Lee T.H. Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol. Appl. Pharmacol. 2014;279:351–363. doi: 10.1016/j.taap.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Wen C., Li F., Guo Q., Zhang L., Duan Y., Wang W., Li J., He S., Chen W., Yin Y. Protective effects of taurine against muscle damage induced by diquat in 35 days weaned piglets. J. Anim. Sci. Biotechnol. 2020;11:56. doi: 10.1186/s40104-020-00463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S.H., Seo H., Kwon D., Yuk D.Y., Jung Y.S. Taurine Ameliorates Tunicamycin-Induced Liver Injury by Disrupting the Vicious Cycle between Oxidative Stress and Endoplasmic Reticulum Stress. Life. 2022;12:354. doi: 10.3390/life12030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niknahad H., Mehrabani P.S., Arjmand A., Alidaee S., Mazloomi S., Ahmadi P., Abdoli N., Saeed M., Rezaei M., Ommati M.M., et al. Cirrhosis-induced oxidative stress in erythrocytes: The therapeutic potential of taurine. Clin. Exp. Hepatol. 2023;9:79–93. doi: 10.5114/ceh.2023.126028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Q., Zhang L., Yin Y., Gong S., Yang Y., Chen S., Han M., Duan Y. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants. 2022;11:1391. doi: 10.3390/antiox11071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali S.N., Arif A., Ansari F.A., Mahmood R. Cytoprotective effect of taurine against sodium chlorate-induced oxidative damage in human red blood cells: An ex vivo study. Amino Acids. 2022;54:33–46. doi: 10.1007/s00726-021-03121-5. [DOI] [PubMed] [Google Scholar]
- 44.Askwith T., Zeng W., Eggo M.C., Stevens M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp. Neurol. 2012;233:154–162. doi: 10.1016/j.expneurol.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faghfouri A.H., Seyyed Shoura S.M., Fathollahi P., Shadbad M.A., Papi S., Ostadrahimi A., Faghfuri E. Profiling inflammatory and oxidative stress biomarkers following taurine supplementation: A systematic review and dose-response meta-analysis of controlled trials. Eur. J. Clin. Nutr. 2022;76:647–658. doi: 10.1038/s41430-021-01010-4. [DOI] [PubMed] [Google Scholar]
- 46.Rafiee Z., Garcia-Serrano A.M., Duarte J.M.N. Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes. Nutrients. 2022;14:1292. doi: 10.3390/nu14061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochoa-de la Paz L.D., Martinez-Davila I.A., Miledi R., Martinez-Torres A. Modulation of human GABArho1 receptors by taurine. Neurosci. Res. 2008;61:302–308. doi: 10.1016/j.neures.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Hilgier W., Oja S.S., Saransaari P., Albrecht J. Taurine prevents ammonia-induced accumulation of cyclic GMP in rat striatum by interaction with GABAA and glycine receptors. Brain Res. 2005;1043:242–246. doi: 10.1016/j.brainres.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 49.Frosini M., Sesti C., Dragoni S., Valoti M., Palmi M., Dixon H.B., Machetti F., Sgaragli G. Interactions of taurine and structurally related analogues with the GABAergic system and taurine binding sites of rabbit brain. Br. J. Pharmacol. 2003;138:1163–1171. doi: 10.1038/sj.bjp.0705134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto-Kitsukawa S., Okuyama S., Aihara H. Enhancing effect of taurine on the rat caudate spindle. I: Interaction of taurine with the nigro-striatal dopamine system. Pharmacol. Biochem. Behav. 1988;31:411–416. doi: 10.1016/0091-3057(88)90367-X. [DOI] [PubMed] [Google Scholar]
- 51.Kontro P., Oja S.S. Release of taurine, GABA and dopamine from rat striatal slices: Mutual interactions and developmental aspects. Neuroscience. 1988;24:49–58. doi: 10.1016/0306-4522(88)90310-7. [DOI] [PubMed] [Google Scholar]
- 52.Jakaria M., Azam S., Haque M.E., Jo S.H., Uddin M.S., Kim I.S., Choi D.K. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223. doi: 10.1016/j.redox.2019.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramirez-Guerrero S., Guardo-Maya S., Medina-Rincon G.J., Orrego-Gonzalez E.E., Cabezas-Perez R., Gonzalez-Reyes R.E. Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship. Front. Mol. Neurosci. 2022;15:937789. doi: 10.3389/fnmol.2022.937789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seol S.I., Kim H.J., Choi E.B., Kang I.S., Lee H.K., Lee J.K., Kim C. Taurine Protects against Postischemic Brain Injury via the Antioxidant Activity of Taurine Chloramine. Antioxidants. 2021;10:372. doi: 10.3390/antiox10030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh S.J., Lee H.J., Jeong Y.J., Nam K.R., Kang K.J., Han S.J., Lee K.C., Lee Y.J., Choi J.Y. Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci. Rep. 2020;10:15551. doi: 10.1038/s41598-020-72755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu K., Zhu R., Jiang H., Li B., Geng Q., Li Y., Qi J. Taurine inhibits KDM3a production and microglia activation in lipopolysaccharide-treated mice and BV-2 cells. Mol. Cell Neurosci. 2022;122:103759. doi: 10.1016/j.mcn.2022.103759. [DOI] [PubMed] [Google Scholar]
- 57.Verner A., Craig S., McGuire W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007;2007:CD006072. doi: 10.1002/14651858.CD006072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wharton B.A., Morley R., Isaacs E.B., Cole T.J., Lucas A. Low plasma taurine and later neurodevelopment. Arch. Dis. Child. Fetal. Neonatal Ed. 2004;89:F497–E498. doi: 10.1136/adc.2003.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao S.L., Jiang H., Niu S.P., Wang X.H., Du S. Effects of Taurine Supplementation on Growth in Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Indian J. Pediatr. 2018;85:855–860. doi: 10.1007/s12098-018-2609-0. [DOI] [PubMed] [Google Scholar]
- 60.Dhillon S.K., Davies W.E., Hopkins P.C., Rose S.J. Effects of dietary taurine on auditory function in full-term infants. Adv. Exp. Med. Biol. 1998;442:507–514. doi: 10.1007/978-1-4899-0117-0_61. [DOI] [PubMed] [Google Scholar]
- 61.Gaull G.E. Taurine in human milk: Growth modulator or conditionally essential amino acid? J. Pediatr. Gastroenterol. Nutr. 1983;2((Suppl. S1)):S266–S271. doi: 10.1097/00005176-198300201-00040. [DOI] [PubMed] [Google Scholar]
- 62.Furukawa T., Fukuda A. Maternal taurine as a modulator of Cl− homeostasis as well as of glycine/GABA(A) receptors for neocortical development. Front. Cell Neurosci. 2023;17:1221441. doi: 10.3389/fncel.2023.1221441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamori Y., Sagara M., Arai Y., Kobayashi H., Kishimoto K., Matsuno I., Mori H., Mori M. Taurine Intake with Magnesium Reduces Cardiometabolic Risks. Pt 2Adv. Exp. Med. Biol. 2017;975:1011–1020. doi: 10.1007/978-94-024-1079-2_80. [DOI] [PubMed] [Google Scholar]
- 64.Sagara M., Murakami S., Mizushima S., Liu L., Mori M., Ikeda K., Nara Y., Yamori Y. Taurine in 24-h Urine Samples Is Inversely Related to Cardiovascular Risks of Middle Aged Subjects in 50 Populations of the World. Adv. Exp. Med. Biol. 2015;803:623–636. doi: 10.1007/978-3-319-15126-7_50. [DOI] [PubMed] [Google Scholar]
- 65.Zulli A., Lau E., Wijaya B.P., Jin X., Sutarga K., Schwartz G.D., Learmont J., Wookey P.J., Zinellu A., Carru C., et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: Association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. 2009;53:1017–1022. doi: 10.1161/HYPERTENSIONAHA.109.129924. [DOI] [PubMed] [Google Scholar]
- 66.Oudit G.Y., Trivieri M.G., Khaper N., Husain T., Wilson G.J., Liu P., Sole M.J., Backx P.H. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation. 2004;109:1877–1885. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 67.Swiderski J., Sakkal S., Apostolopoulos V., Zulli A., Gadanec L.K. Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients. 2023;15:2562. doi: 10.3390/nu15112562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wada T., Shimazaki T., Nakagawa S., Otuki T., Kurata S., Suzuki T., Watanabe K., Saigo K. Chemical synthesis of novel taurine-containing uridine derivatives. Nucleic Acids Res. Suppl. 2002;2:11–12. doi: 10.1093/nass/2.1.11. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki T., Suzuki T., Wada T., Saigo K., Watanabe K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002;21:6581–6589. doi: 10.1093/emboj/cdf656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fakruddin M., Wei F.Y., Suzuki T., Asano K., Kaieda T., Omori A., Izumi R., Fujimura A., Kaitsuka T., Miyata K., et al. Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease. Cell Rep. 2018;22:482–496. doi: 10.1016/j.celrep.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 71.Kirino Y., Goto Y., Campos Y., Arenas J., Suzuki T. Specific correlation between the wobble modification deficiency in mutant tRNAs and the clinical features of a human mitochondrial disease. Proc. Natl. Acad. Sci. USA. 2005;102:7127–7132. doi: 10.1073/pnas.0500563102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higuchi M., Celino F.T., Shimizu-Yamaguchi S., Miura C., Miura T. Taurine plays an important role in the protection of spermatogonia from oxidative stress. Amino Acids. 2012;43:2359–2369. doi: 10.1007/s00726-012-1316-9. [DOI] [PubMed] [Google Scholar]
- 73.Tabassum H., Rehman H., Banerjee B.D., Raisuddin S., Parvez S. Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin. Chim. Acta. 2006;370:129–136. doi: 10.1016/j.cca.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Miyazaki T., Ito T., Baseggio Conrado A., Murakami S. Editorial for Special Issue on “Regulation and Effect of Taurine on Metabolism”. Metabolites. 2022;12:795. doi: 10.3390/metabo12090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Carvalho F.G., Batitucci G., Abud G.F., de Freitas E.C. Taurine and Exercise: Synergistic Effects on Adipose Tissue Metabolism and Inflammatory Process in Obesity. Adv. Exp. Med. Biol. 2022;1370:279–289. doi: 10.1007/978-3-030-93337-1_27. [DOI] [PubMed] [Google Scholar]
- 76.De Carvalho F.G., Munoz V.R., Brandao C.F.C., Simabuco F.M., Pavan I.C.B., Nakandakari S., Pauli J.R., De Moura L.P., Ropelle E.R., Marchini J.S., et al. Taurine upregulates insulin signaling and mitochondrial metabolism in vitro but not in adipocytes of obese women. Nutrition. 2022;93:111430. doi: 10.1016/j.nut.2021.111430. [DOI] [PubMed] [Google Scholar]
- 77.Brons C., Spohr C., Storgaard H., Dyerberg J., Vaag A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur. J. Clin. Nutr. 2004;58:1239–1247. doi: 10.1038/sj.ejcn.1601955. [DOI] [PubMed] [Google Scholar]
- 78.Nakaya Y., Minami A., Harada N., Sakamoto S., Niwa Y., Ohnaka M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am. J. Clin. Nutr. 2000;71:54–58. doi: 10.1093/ajcn/71.1.54. [DOI] [PubMed] [Google Scholar]
- 79.Anuradha C.V., Balakrishnan S.D. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can. J. Physiol. Pharmacol. 1999;77:749–754. doi: 10.1139/y99-060. [DOI] [PubMed] [Google Scholar]
- 80.Sarnobat D., Moffett R.C., Ma J., Flatt P.R., McClenaghan N.H., Tarasov A.I. Taurine rescues pancreatic beta-cell stress by stimulating alpha-cell transdifferentiation. Biofactors. 2023;49:646–662. doi: 10.1002/biof.1938. [DOI] [PubMed] [Google Scholar]
- 81.Tagawa R., Kobayashi M., Sakurai M., Yoshida M., Kaneko H., Mizunoe Y., Nozaki Y., Okita N., Sudo Y., Higami Y. Long-Term Dietary Taurine Lowers Plasma Levels of Cholesterol and Bile Acids. Int. J. Mol. Sci. 2022;23:1793. doi: 10.3390/ijms23031793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo J., -Gao Y., Cao X., Zhang J., Chen W. Cholesterollowing effect of taurine in HepG2 cell. Lipids Health Dis. 2017;16:56. doi: 10.1186/s12944-017-0444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yokogoshi H., Mochizuki H., Nanami K., Hida Y., Miyachi F., Oda H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. J. Nutr. 1999;129:1705–1712. doi: 10.1093/jn/129.9.1705. [DOI] [PubMed] [Google Scholar]
- 84.Balkan J., Kanbagli O., Hatipoglu A., Kucuk M., Cevikbas U., Aykac-Toker G., Uysal M. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci. Biotechnol. Biochem. 2002;66:1755–1758. doi: 10.1271/bbb.66.1755. [DOI] [PubMed] [Google Scholar]
- 85.Zhang M., Bi L.F., Fang J.H., Su X.L., Da G.L., Kuwamori T., Kagamimori S. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–271. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 86.Pansani M.C., Azevedo P.S., Rafacho B.P., Minicucci M.F., Chiuso-Minicucci F., Zorzella-Pezavento S.G., Marchini J.S., Padovan G.J., Fernandes A.A., Matsubara B.B., et al. Atrophic cardiac remodeling induced by taurine deficiency in Wistar rats. PLoS ONE. 2012;7:e41439. doi: 10.1371/journal.pone.0041439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mozaffari M.S., Tan B.H., Lucia M.A., Schaffer S.W. Effect of drug-induced taurine depletion on cardiac contractility and metabolism. Biochem. Pharmacol. 1986;35:985–989. doi: 10.1016/0006-2952(86)90087-0. [DOI] [PubMed] [Google Scholar]
- 88.Lake N. Loss of cardiac myofibrils: Mechanism of contractile deficits induced by taurine deficiency. Am. J. Physiol. 1993;264:H1323–H1326. doi: 10.1152/ajpheart.1993.264.4.H1323. [DOI] [PubMed] [Google Scholar]
- 89.Satoh H., Nakatani T., Tanaka T., Haga S. Cardiac functions and taurine’s actions at different extracellular calcium concentrations in forced swimming stress-loaded rats. Biol. Trace Elem. Res. 2002;87:171–182. doi: 10.1385/BTER:87:1-3:171. [DOI] [PubMed] [Google Scholar]
- 90.Franconi F., Martini F., Stendardi I., Matucci R., Zilletti L., Giotti A. Effect of taurine on calcium levels and contractility in guinea-pig ventricular strips. Biochem. Pharmacol. 1982;31:3181–3185. doi: 10.1016/0006-2952(82)90547-0. [DOI] [PubMed] [Google Scholar]
- 91.Schaffer S.W., Seyed-Mozaffari M., Kramer J., Tan B.H. Effect of taurine depletion and treatment on cardiac contractility and metabolism. Prog. Clin. Biol. Res. 1985;179:167–175. [PubMed] [Google Scholar]
- 92.Kaplan J.L., Stern J.A., Fascetti A.J., Larsen J.A., Skolnik H., Peddle G.D., Kienle R.D., Waxman A., Cocchiaro M., Gunther-Harrington C.T., et al. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS ONE. 2018;13:e0209112. doi: 10.1371/journal.pone.0209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samadi M., Haghi-Aminjan H., Sattari M., Hooshangi Shayesteh M.R., Bameri B., Armandeh M., Naddafi M., Eghbal M.A., Abdollahi M. The role of taurine on chemotherapy-induced cardiotoxicity: A systematic review of non-clinical study. Life Sci. 2021;265:118813. doi: 10.1016/j.lfs.2020.118813. [DOI] [PubMed] [Google Scholar]
- 94.Ahmadian M., Dabidi Roshan V., Ashourpore E. Taurine Supplementation Improves Functional Capacity, Myocardial Oxygen Consumption, and Electrical Activity in Heart Failure. J. Diet. Suppl. 2017;14:422–432. doi: 10.1080/19390211.2016.1267059. [DOI] [PubMed] [Google Scholar]
- 95.Ahmadian M., Roshan V.D., Aslani E., Stannard S.R. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther. Adv. Cardiovasc. Dis. 2017;11:185–194. doi: 10.1177/1753944717711138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azuma J., Sawamura A., Awata N., Ohta H., Hamaguchi T., Harada H., Takihara K., Hasegawa H., Yamagami T., Ishiyama T., et al. Therapeutic effect of taurine in congestive heart failure: A double-blind crossover trial. Clin. Cardiol. 1985;8:276–282. doi: 10.1002/clc.4960080507. [DOI] [PubMed] [Google Scholar]
- 97.Beyranvand M.R., Khalafi M.K., Roshan V.D., Choobineh S., Parsa S.A., Piranfar M.A. Effect of taurine supplementation on exercise capacity of patients with heart failure. J. Cardiol. 2011;57:333–337. doi: 10.1016/j.jjcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 98.Azuma J., Sawamura A., Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn. Circ. J. 1992;56:95–99. doi: 10.1253/jcj.56.95. [DOI] [PubMed] [Google Scholar]
- 99.Yamauchi-Takihara K., Azuma J., Kishimoto S., Onishi S., Sperelakis N. Taurine prevention of calcium paradox-related damage in cardiac muscle. Its regulatory action on intracellular cation contents. Biochem. Pharmacol. 1988;37:2651–2658. doi: 10.1016/0006-2952(88)90259-6. [DOI] [PubMed] [Google Scholar]
- 100.Henry E.F., MacCormack T.J. Taurine protects cardiac contractility in killifish, Fundulus heteroclitus, by enhancing sarcoplasmic reticular Ca2+ cycling. J. Comp. Physiol. B. 2018;188:89–99. doi: 10.1007/s00360-017-1107-4. [DOI] [PubMed] [Google Scholar]
- 101.Gates M.A., Morash A.J., Lamarre S.G., MacCormack T.J. Intracellular taurine deficiency impairs cardiac contractility in rainbow trout (Oncorhynchus mykiss) without affecting aerobic performance. J. Comp. Physiol. B. 2022;192:49–60. doi: 10.1007/s00360-021-01407-4. [DOI] [PubMed] [Google Scholar]
- 102.Satoh H., Sperelakis N. Taurine inhibition of fast Na+ current in embryonic chick ventricular myocytes. Eur. J. Pharmacol. 1992;218:83–89. doi: 10.1016/0014-2999(92)90150-3. [DOI] [PubMed] [Google Scholar]
- 103.Oz E., Erbas D., Gelir E., Aricioglu A. Taurine and calcium interaction in protection of myocardium exposed to ischemic reperfusion injury. Gen. Pharmacol. 1999;33:137–141. doi: 10.1016/S0306-3623(98)00284-5. [DOI] [PubMed] [Google Scholar]
- 104.Wong A.P., Niedzwiecki A., Rath M. Myocardial energetics and the role of micronutrients in heart failure: A critical review. Am. J. Cardiovasc. Dis. 2016;6:81–92. [PMC free article] [PubMed] [Google Scholar]
- 105.Dragan S., Buleu F., Christodorescu R., Cobzariu F., Iurciuc S., Velimirovici D., Xiao J., Luca C.T. Benefits of multiple micronutrient supplementation in heart failure: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019;59:965–981. doi: 10.1080/10408398.2018.1540398. [DOI] [PubMed] [Google Scholar]
- 106.Razzaghi A., Choobineh S., Gaeini A., Soori R. Interaction of exercise training with taurine attenuates infarct size and cardiac dysfunction via Akt-Foxo3a-Caspase-8 signaling pathway. Amino Acids. 2023;55:869–880. doi: 10.1007/s00726-023-03275-4. [DOI] [PubMed] [Google Scholar]
- 107.Li C., Zhou Y., Niu Y., He W., Wang X., Zhang X., Wu Y., Zhang W., Zhao L., Zheng H., et al. Deficiency of Pdk1 drives heart failure by impairing taurine homeostasis through Slc6a6. FASEB J. 2023;37:e23134. doi: 10.1096/fj.202300272R. [DOI] [PubMed] [Google Scholar]
- 108.Li S., Wang D., Zhang M., Zhang C., Piao F. Taurine Ameliorates Apoptosis via AKT Pathway in the Kidney of Diabetic Rats. Adv. Exp. Med. Biol. 2022;1370:227–233. doi: 10.1007/978-3-030-93337-1_22. [DOI] [PubMed] [Google Scholar]
- 109.Li M., Gao Y., Wang Z., Wu B., Zhang J., Xu Y., Han X., Phouthapane V., Miao J. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front. Immunol. 2022;13:927215. doi: 10.3389/fimmu.2022.927215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu C., He P., Guo Y., Tian Q., Wang J., Wang G., Zhang Z., Li M. Taurine attenuates neuronal ferroptosis by regulating GABA(B)/AKT/GSK3beta/beta-catenin pathway after subarachnoid hemorrhage. Free Radic. Biol. Med. 2022;193:795–807. doi: 10.1016/j.freeradbiomed.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Das J., Vasan V., Sil P.C. Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2012;258:296–308. doi: 10.1016/j.taap.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 112.Wei C., Ding X., Liu C., Pei Y., Zhong Y., Sun W. Mechanism of taurine in alleviating myocardial oxidative stress in rats after burn through p38 MAPK signaling pathway. Minerva Med. 2019;110:472–475. doi: 10.23736/S0026-4806.19.05982-2. [DOI] [PubMed] [Google Scholar]
- 113.Azuma M., Takahashi K., Fukuda T., Ohyabu Y., Yamamoto I., Kim S., Iwao H., Schaffer S.W., Azuma J. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur. J. Pharmacol. 2000;403:181–188. doi: 10.1016/S0014-2999(00)00483-0. [DOI] [PubMed] [Google Scholar]
- 114.Takatani T., Takahashi K., Uozumi Y., Matsuda T., Ito T., Schaffer S.W., Fujio Y., Azuma J. Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem. Biophys. Res. Commun. 2004;316:484–489. doi: 10.1016/j.bbrc.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 115.Sedaghat M., Choobineh S., Ravasi A.A. Taurine with combined aerobic and resistance exercise training alleviates myocardium apoptosis in STZ-induced diabetes rats via Akt signaling pathway. Life Sci. 2020;258:118225. doi: 10.1016/j.lfs.2020.118225. [DOI] [PubMed] [Google Scholar]
- 116.Ghosh J., Das J., Manna P., Sil P.C. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: Role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol. Appl. Pharmacol. 2009;240:73–87. doi: 10.1016/j.taap.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 117.Yousuf M., Shamsi A., Mohammad T., Azum N., Alfaifi S.Y.M., Asiri A.M., Mohamed Elasbali A., Islam A., Hassan M.I., Haque Q.M.R. Inhibiting Cyclin-Dependent Kinase 6 by Taurine: Implications in Anticancer Therapeutics. ACS Omega. 2022;7:25844–25852. doi: 10.1021/acsomega.2c03479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feng X., Hu W., Hong Y., Ruan L., Hu Y., Liu D. Taurine Ameliorates Iron Overload-Induced Hepatocyte Injury via the Bcl-2/VDAC1-Mediated Mitochondrial Apoptosis Pathway. Oxid. Med. Cell. Longev. 2022;2022:4135752. doi: 10.1155/2022/4135752. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Zhao D., Zhang X., Feng Y., Bian Y., Fu Z., Wu Y., Ma Y., Li C., Wang J., Dai J., et al. Taurine Alleviates LPS-Induced Acute Lung Injury by Suppressing TLR-4/NF-kappaB Pathway. Adv. Exp. Med. Biol. 2022;1370:63–72. doi: 10.1007/978-3-030-93337-1_6. [DOI] [PubMed] [Google Scholar]
- 120.Wu G., San J., Pang H., Du Y., Li W., Zhou X., Yang X., Hu J., Yang J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon. 2022;215:17–27. doi: 10.1016/j.toxicon.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Kp A.D., Shimoga Janakirama A.R., Martin A. SIRT1 activation by Taurine: In vitro evaluation, molecular docking and molecular dynamics simulation studies. J. Nutr. Biochem. 2022;102:108948. doi: 10.1016/j.jnutbio.2022.108948. [DOI] [PubMed] [Google Scholar]
- 122.Mozaffari M.S., Patel C., Abdelsayed R., Schaffer S.W. Accelerated NaCl-induced hypertension in taurine-deficient rat: Role of renal function. Kidney Int. 2006;70:329–337. doi: 10.1038/sj.ki.5001503. [DOI] [PubMed] [Google Scholar]
- 123.Li W., Yang J., Lyu Q., Wu G., Lin S., Yang Q., Hu J. Taurine attenuates isoproterenol-induced H9c2 cardiomyocytes hypertrophy by improving antioxidative ability and inhibiting calpain-1-mediated apoptosis. Mol. Cell. Biochem. 2020;469:119–132. doi: 10.1007/s11010-020-03733-7. [DOI] [PubMed] [Google Scholar]
- 124.Gentile S., Bologna E., Terracina D., Angelico M. Taurine-induced diuresis and natriuresis in cirrhotic patients with ascites. Life Sci. 1994;54:1585–1593. doi: 10.1016/0024-3205(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 125.Dlouha H., McBroom M.J. Atrial natriuretic factor in taurine-treated normal and cardiomyopathic hamsters. Proc. Soc. Exp. Biol. Med. 1986;181:411–415. doi: 10.3181/00379727-181-42273. [DOI] [PubMed] [Google Scholar]
- 126.Guizoni D.M., Vettorazzi J.F., Carneiro E.M., Davel A.P. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide. 2020;94:48–53. doi: 10.1016/j.niox.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 127.Dharmashankar K., Widlansky M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015;7:719–741. doi: 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gambardella J., Khondkar W., Morelli M.B., Wang X., Santulli G., Trimarco V. Arginine and Endothelial Function. Biomedicines. 2020;8:277. doi: 10.3390/biomedicines8080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fennessy F.M., Moneley D.S., Wang J.H., Kelly C.J., Bouchier-Hayes D.J. Taurine and vitamin C modify monocyte and endothelial dysfunction in young smokers. Circulation. 2003;107:410–415. doi: 10.1161/01.CIR.0000046447.72402.47. [DOI] [PubMed] [Google Scholar]
- 131.El Idrissi A., Okeke E., Yan X., Sidime F., Neuwirth L.S. Taurine regulation of blood pressure and vasoactivity. Adv. Exp. Med. Biol. 2013;775:407–425. doi: 10.1007/978-1-4614-6130-2_31. [DOI] [PubMed] [Google Scholar]
- 132.Yildiz O., Ulusoy K.G. Effects of taurine on vascular tone. Amino Acids. 2022;54:1527–1540. doi: 10.1007/s00726-022-03198-6. [DOI] [PubMed] [Google Scholar]
- 133.Hagiwara K., Kuroki G., Yuan P.X., Suzuki T., Murakami M., Hano T., Sasano H., Yanagisawa T. The effect of taurine on the salt-dependent blood pressure increase in the voltage-dependent calcium channel beta 3-subunit-deficient mouse. J. Cardiovasc. Pharmacol. 2003;41((Suppl. S1)):S127–S131. [PubMed] [Google Scholar]
- 134.Meldrum M.J., Tu R., Patterson T., Dawson R., Jr., Petty T. The effect of taurine on blood pressure, and urinary sodium, potassium and calcium excretion. Adv. Exp. Med. Biol. 1994;359:207–215. doi: 10.1007/978-1-4899-1471-2_21. [DOI] [PubMed] [Google Scholar]
- 135.Sun B., Maruta H., Ma Y., Yamashita H. Taurine Stimulates AMP-Activated Protein Kinase and Modulates the Skeletal Muscle Functions in Rats via the Induction of Intracellular Calcium Influx. Int. J. Mol. Sci. 2023;24:4125. doi: 10.3390/ijms24044125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ra S.G., Choi Y., Akazawa N., Kawanaka K., Ohmori H., Maeda S. Effects of Taurine Supplementation on Vascular Endothelial Function at Rest and After Resistance Exercise. Adv. Exp. Med. Biol. 2019;1155:407–414. doi: 10.1007/978-981-13-8023-5_38. [DOI] [PubMed] [Google Scholar]
- 137.Katakawa M., Fukuda N., Tsunemi A., Mori M., Maruyama T., Matsumoto T., Abe M., Yamori Y. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens. Res. 2016;39:848–856. doi: 10.1038/hr.2016.86. [DOI] [PubMed] [Google Scholar]
- 138.Guizoni D.M., Freitas I.N., Victorio J.A., Possebom I.R., Araujo T.R., Carneiro E.M., Davel A.P. Taurine treatment reverses protein malnutrition-induced endothelial dysfunction of the pancreatic vasculature: The role of hydrogen sulfide. Metabolism. 2021;116:154701. doi: 10.1016/j.metabol.2021.154701. [DOI] [PubMed] [Google Scholar]
- 139.Casey R.G., Gang C., Joyce M., Bouchier-Hayes D.J. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J. Vasc. Res. 2007;44:31–39. doi: 10.1159/000097893. [DOI] [PubMed] [Google Scholar]
- 140.Moloney M.A., Casey R.G., O’Donnell D.H., Fitzgerald P., Thompson C., Bouchier-Hayes D.J. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diabetes Vasc. Dis. Res. 2010;7:300–310. doi: 10.1177/1479164110375971. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira Abud G., Giolo De Carvalho F., Batitucci G., Travieso S.G., Bueno Junior C.R., Barbosa Junior F., Marchini J.S., de Freitas E.C. Taurine as a possible antiaging therapy: A controlled clinical trial on taurine antioxidant activity in women ages 55 to 70. Nutrition. 2022;101:111706. doi: 10.1016/j.nut.2022.111706. [DOI] [PubMed] [Google Scholar]
- 142.Jong C.J., Azuma J., Schaffer S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids. 2012;42:2223–2232. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 143.Kang Y.J., Choi M.J. Liver Antioxidant Enzyme Activities Increase After Taurine in Ovariectomized Rats. Pt 2Adv. Exp. Med. Biol. 2017;975:1071–1080. doi: 10.1007/978-94-024-1079-2_85. [DOI] [PubMed] [Google Scholar]
- 144.Sun Q., Wang B., Li Y., Sun F., Li P., Xia W., Zhou X., Li Q., Wang X., Chen J., et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension. 2016;67:541–549. doi: 10.1161/HYPERTENSIONAHA.115.06624. [DOI] [PubMed] [Google Scholar]
- 145.Maia A.R., Batistas T.M., Victorio J.A., Clerici S.P., Delbin M.A., Carneiro E.M., Davel A.P. Taurine upplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS ONE. 2014;9:e105851. doi: 10.1371/journal.pone.0105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trachtman H., Del Pizzo R., Rao P., Rujikarn N., Sturman J.A. Taurine lowers blood pressure in the spontaneously hypertensive rat by a catecholamine independent mechanism. Am. J. Hypertens. 1989;2:909–912. doi: 10.1093/ajh/2.12.909. [DOI] [PubMed] [Google Scholar]
- 147.Scabora J.E., de Lima M.C., Lopes A., de Lima I.P., Mesquita F.F., Torres D.B., Boer P.A., Gontijo J.A. Impact of taurine supplementation on blood pressure in gestational protein-restricted offspring: Effect on the medial solitary tract nucleus cell numbers, angiotensin receptors, and renal sodium handling. J. Renin-Angiotensin-Aldosterone Syst. 2015;16:47–58. doi: 10.1177/1470320313481255. [DOI] [PubMed] [Google Scholar]
- 148.Abebe W., Mozaffari M.S. Effects of chronic taurine treatment on reactivity of the rat aorta. Amino Acids. 2000;19:615–623. doi: 10.1007/s007260070011. [DOI] [PubMed] [Google Scholar]
- 149.Sener G., Ozer Sehirli A., Ipci Y., Cetinel S., Cikler E., Gedik N., Alican I. Taurine treatment protects against chronic nicotine-induced oxidative changes. Fundam. Clin. Pharmacol. 2005;19:155–164. doi: 10.1111/j.1472-8206.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 150.Liang W., Yang Q., Wu G., Lin S., Yang J., Feng Y., Hu J. Effects of Taurine and L-Arginine on the Apoptosis of Vascular Smooth Muscle Cells in Insulin Resistance Hypertensive Rats. Pt 2Adv. Exp. Med. Biol. 2017;975:813–819. doi: 10.1007/978-94-024-1079-2_63. [DOI] [PubMed] [Google Scholar]
- 151.Forzano I., Avvisato R., Varzideh F., Jankauskas S.S., Cioppa A., Mone P., Salemme L., Kansakar U., Tesorio T., Trimarco V., et al. L-Arginine in diabetes: Clinical and preclinical evidence. Cardiovasc. Diabetol. 2023;22:89. doi: 10.1186/s12933-023-01827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Trimarco V., Izzo R., Lombardi A., Coppola A., Fiorentino G., Santulli G. Beneficial effects of L-Arginine in patients hospitalized for COVID-19: New insights from a randomized clinical trial. Pharmacol. Res. 2023;191:106702. doi: 10.1016/j.phrs.2023.106702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gambardella J., Fiordelisi A., Spigno L., Boldrini L., Lungonelli G., Di Vaia E., Santulli G., Sorriento D., Cerasuolo F.A., Trimarco V., et al. Effects of Chronic Supplementation of L-Arginine on Physical Fitness in Water Polo Players. Oxid. Med. Cell. Longev. 2021;2021:6684568. doi: 10.1155/2021/6684568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moludi J., Qaisar S.A., Kadhim M.M., Ahmadi Y., Davari M. Protective and therapeutic effectiveness of taurine supplementation plus low calorie diet on metabolic parameters and endothelial markers in patients with diabetes mellitus: A randomized, clinical trial. Nutr. Metab. 2022;19:49. doi: 10.1186/s12986-022-00684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Waldron M., Patterson S.D., Tallent J., Jeffries O. The Effects of Oral Taurine on Resting Blood Pressure in Humans: A Meta-Analysis. Curr. Hypertens. Rep. 2018;20:81. doi: 10.1007/s11906-018-0881-z. [DOI] [PubMed] [Google Scholar]
- 156.Gutierrez-Hellin J., Varillas-Delgado D. Energy Drinks and Sports Performance, Cardiovascular Risk, and Genetic Associations; Future Prospects. Nutrients. 2021;13:715. doi: 10.3390/nu13030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ozan M., Buzdagli Y., Eyipinar C.D., Baygutalp N.K., Yuce N., Oget F., Kan E., Baygutalp F. Does Single or Combined Caffeine and Taurine Supplementation Improve Athletic and Cognitive Performance without Affecting Fatigue Level in Elite Boxers? A Double-Blind, Placebo-Controlled Study. Nutrients. 2022;14:4399. doi: 10.3390/nu14204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kurtz J.A., VanDusseldorp T.A., Doyle J.A., Otis J.S. Taurine in sports and exercise. J. Int. Soc. Sports Nutr. 2021;18:39. doi: 10.1186/s12970-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pollard C.M., McStay C.L., Meng X. Public Concern about the Sale of High-Caffeine Drinks to Children 12 Years or Younger: An Australian Regulatory Perspective. Biomed Res. Int. 2015;2015:707149. doi: 10.1155/2015/707149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dawodu A., Cleaver K. Behavioural correlates of energy drink consumption among adolescents: A review of the literature. J. Child Health Care. 2017;21:446–462. doi: 10.1177/1367493517731948. [DOI] [PubMed] [Google Scholar]
- 161.Kaur A., Yousuf H., Ramgobin-Marshall D., Jain R., Jain R. Energy drink consumption: A rising public health issue. Rev. Cardiovasc. Med. 2022;23:83. doi: 10.31083/j.rcm2303083. [DOI] [PubMed] [Google Scholar]
- 162.Erdmann J., Wicinski M., Wodkiewicz E., Nowaczewska M., Slupski M., Otto S.W., Kubiak K., Huk-Wieliczuk E., Malinowski B. Effects of Energy Drink Consumption on Physical Performance and Potential Danger of Inordinate Usage. Nutrients. 2021;13:2506. doi: 10.3390/nu13082506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nuss T., Morley B., Scully M., Wakefield M. Energy drink consumption among Australian adolescents associated with a cluster of unhealthy dietary behaviours and short sleep duration. Nutr. J. 2021;20:64. doi: 10.1186/s12937-021-00719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kriebs A. Taurine levels modulate aging. Nat. Aging. 2023;3:758–759. doi: 10.1038/s43587-023-00465-3. [DOI] [PubMed] [Google Scholar]
- 165.Ferreira J. Systemic taurine decline drives aging. Lab Anim. 2023;52:175. doi: 10.1038/s41684-023-01226-w. [DOI] [Google Scholar]
- 166.Izquierdo J.M. Taurine as a possible therapy for immunosenescence and inflammaging. Cell. Mol. Immunol. 2023;online ahead of print doi: 10.1038/s41423-023-01062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barbiera A., Sorrentino S., Fard D., Lepore E., Sica G., Dobrowolny G., Tamagnone L., Scicchitano B.M. Taurine Administration Counteracts Aging-Associated Impingement of Skeletal Muscle Regeneration by Reducing Inflammation and Oxidative Stress. Antioxidants. 2022;11:1016. doi: 10.3390/antiox11051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh P., Gollapalli K., Mangiola S., Schranner D., Yusuf M.A., Chamoli M., Shi S.L., Lopes Bastos B., Nair T., Riermeier A., et al. Taurine deficiency as a driver of aging. Science. 2023;380:eabn9257. doi: 10.1126/science.abn9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vidal Valero M. Taurine supplement makes animals live longer—What it means for people is unclear. Nature. 2023;online ahead of print doi: 10.1038/d41586-023-01910-4. [DOI] [PubMed] [Google Scholar]
- 170.McGaunn J., Baur J.A. Taurine linked with healthy aging. Science. 2023;380:1010–1011. doi: 10.1126/science.adi3025. [DOI] [PubMed] [Google Scholar]
- 171.Qian W., Li M., Yu L., Tian F., Zhao J., Zhai Q. Effects of Taurine on Gut Microbiota Homeostasis: An Evaluation Based on Two Models of Gut Dysbiosis. Biomedicines. 2023;11:1048. doi: 10.3390/biomedicines11041048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Graham F. Daily briefing: Taurine makes animals live longer—But don’t binge on Red Bulls yet. Nature. 2023;online ahead of print doi: 10.1038/d41586-023-01948-4. [DOI] [PubMed] [Google Scholar]
- 173.Jun H., Choi M.J. Relationship Between Taurine Intake and Cardiometabolic Risk Markers in Korean Elderly. Adv. Exp. Med. Biol. 2019;1155:301–311. doi: 10.1007/978-981-13-8023-5_29. [DOI] [PubMed] [Google Scholar]
- 174.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 175.McHugh D., Gil J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Watanabe S., Kawamoto S., Ohtani N., Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108:563–569. doi: 10.1111/cas.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kumari R., Jat P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mohamad Kamal N.S., Safuan S., Shamsuddin S., Foroozandeh P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020;99:151108. doi: 10.1016/j.ejcb.2020.151108. [DOI] [PubMed] [Google Scholar]
- 180.Kowald A., Passos J.F., Kirkwood T.B.L. On the evolution of cellular senescence. Aging Cell. 2020;19:e13270. doi: 10.1111/acel.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yi S., Lin K., Jiang T., Shao W., Huang C., Jiang B., Li Q., Lin D. NMR-based metabonomic analysis of HUVEC cells during replicative senescence. Aging. 2020;12:3626–3646. doi: 10.18632/aging.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ji H., Zhao G., Luo J., Zhao X., Zhang M. Taurine postponed the replicative senescence of rat bone marrow-derived multipotent stromal cells in vitro. Mol. Cell. Biochem. 2012;366:259–267. doi: 10.1007/s11010-012-1304-0. [DOI] [PubMed] [Google Scholar]
- 183.Ito T., Yoshikawa N., Inui T., Miyazaki N., Schaffer S.W., Azuma J. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS ONE. 2014;9:e107409. doi: 10.1371/journal.pone.0107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ito T., Yamamoto N., Nakajima S., Schaffer S.W. Beta-Catenin and SMAD3 Are Associated with Skeletal Muscle Aging in the Taurine Transpoeter Knockout Mouse. Pt 1Adv. Exp. Med. Biol. 2017;975:497–502. doi: 10.1007/978-94-024-1079-2_39. [DOI] [PubMed] [Google Scholar]
- 185.Kaushik S., Cuervo A.M. Proteostasis and aging. Nat. Med. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 186.Hetz C., Zhang K., Kaufman R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Du G., Liu Z., Yu Z., Zhuo Z., Zhu Y., Zhou J., Li Y., Chen H. Taurine represses age-associated gut hyperplasia in Drosophila via counteracting endoplasmic reticulum stress. Aging Cell. 2021;20:e13319. doi: 10.1111/acel.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang Y., Zhang Y., Liu X., Zuo J., Wang K., Liu W., Ge J. Exogenous taurine attenuates mitochondrial oxidative stress and endoplasmic reticulum stress in rat cardiomyocytes. Acta Biochim. Biophys. Sin. 2013;45:359–367. doi: 10.1093/abbs/gmt034. [DOI] [PubMed] [Google Scholar]
- 189.Chowdhury S., Sinha K., Banerjee S., Sil P.C. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors. 2016;42:647–664. doi: 10.1002/biof.1301. [DOI] [PubMed] [Google Scholar]
- 190.Ren Q., Zhang G., Dong C., Li Z., Zhou D., Huang L., Li W., Huang G., Yan J. Parental Folate Deficiency Inhibits Proliferation and Increases Apoptosis of Neural Stem Cells in Rat Offspring: Aggravating Telomere Attrition as a Potential Mechanism. Nutrients. 2023;15:2843. doi: 10.3390/nu15132843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gao Z., Daquinag A.C., Fussell C., Zhao Z., Dai Y., Rivera A., Snyder B.E., Eckel-Mahan K.L., Kolonin M.G. Age-associated telomere attrition in adipocyte progenitors predisposes to metabolic disease. Nat. Metab. 2020;2:1482–1497. doi: 10.1038/s42255-020-00320-4. [DOI] [PubMed] [Google Scholar]
- 192.Varzideh F., Gambardella J., Kansakar U., Jankauskas S.S., Santulli G. Molecular Mechanisms Underlying Pluripotency and Self-Renewal of Embryonic Stem Cells. Int. J. Mol. Sci. 2023;24:8386. doi: 10.3390/ijms24098386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mashyakhy M., Alkahtani A., Abumelha A.S., Sharroufna R.J., Alkahtany M.F., Jamal M., Robaian A., Binalrimal S., Chohan H., Patil V.R., et al. Taurine Augments Telomerase Activity and Promotes Chondrogenesis in Dental Pulp Stem Cells. J. Pers. Med. 2021;11:491. doi: 10.3390/jpm11060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gokarn R., Solon-Biet S., Youngson N.A., Wahl D., Cogger V.C., McMahon A.C., Cooney G.J., Ballard J.W.O., Raubenheimer D., Morris M.J., et al. The Relationship Between Dietary Macronutrients and Hepatic Telomere Length in Aging Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:446–449. doi: 10.1093/gerona/glx186. [DOI] [PubMed] [Google Scholar]
- 195.Xu H., Liu Y.Y., Li L.S., Liu Y.S. Sirtuins at the Crossroads between Mitochondrial Quality Control and Neurodegenerative Diseases: Structure, Regulation, Modifications, and Modulators. Aging Dis. 2023;14:794–824. doi: 10.14336/AD.2022.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grabowska W., Sikora E., Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18:447–476. doi: 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chang H.C., Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Watroba M., Dudek I., Skoda M., Stangret A., Rzodkiewicz P., Szukiewicz D. Sirtuins, epigenetics and longevity. Ageing Res. Rev. 2017;40:11–19. doi: 10.1016/j.arr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 200.Chen C., Zhou M., Ge Y., Wang X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020;187:111215. doi: 10.1016/j.mad.2020.111215. [DOI] [PubMed] [Google Scholar]
- 201.Abd Elwahab A.H., Ramadan B.K., Schaalan M.F., Tolba A.M. A Novel Role of SIRT1/ FGF-21 in Taurine Protection Against Cafeteria Diet-Induced Steatohepatitis in Rats. Cell. Physiol. Biochem. 2017;43:644–659. doi: 10.1159/000480649. [DOI] [PubMed] [Google Scholar]
- 202.Liu J., Ai Y., Niu X., Shang F., Li Z., Liu H., Li W., Ma W., Chen R., Wei T., et al. Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem. Biol. Interact. 2020;317:108972. doi: 10.1016/j.cbi.2020.108972. [DOI] [PubMed] [Google Scholar]
- 203.Sun Q., Hu H., Wang W., Jin H., Feng G., Jia N. Taurine attenuates amyloid beta 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochem. Biophys. Res. Commun. 2014;447:485–489. doi: 10.1016/j.bbrc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 204.Chou C.T., Lin W.F., Kong Z.L., Chen S.Y., Hwang D.F. Taurine prevented cell cycle arrest and restored neurotrophic gene expression in arsenite-treated SH-SY5Y cells. Amino Acids. 2013;45:811–819. doi: 10.1007/s00726-013-1524-y. [DOI] [PubMed] [Google Scholar]
- 205.Brunet A., Goodell M.A., Rando T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023;24:45–62. doi: 10.1038/s41580-022-00510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Oh J., Lee Y.D., Wagers A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guo J., Huang X., Dou L., Yan M., Shen T., Tang W., Li J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022;7:391. doi: 10.1038/s41392-022-01251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li X.W., Gao H.Y., Liu J. The role of taurine in improving neural stem cells proliferation and differentiation. Nutr. Neurosci. 2017;20:409–415. doi: 10.1080/1028415X.2016.1152004. [DOI] [PubMed] [Google Scholar]
- 209.Han X., Chesney R.W. Knockdown of TauT expression impairs human embryonic kidney 293 cell development. Adv. Exp. Med. Biol. 2013;776:307–320. doi: 10.1007/978-1-4614-6093-0_28. [DOI] [PubMed] [Google Scholar]
- 210.Huang X., Liu J., Wu W., Hu P., Wang Q. Taurine enhances mouse cochlear neural stem cell transplantation via the cochlear lateral wall for replacement of degenerated spiral ganglion neurons via sonic hedgehog signaling pathway. Cell Tissue Res. 2019;378:49–57. doi: 10.1007/s00441-019-03018-6. [DOI] [PubMed] [Google Scholar]
- 211.Zhou C., Zhang X., Xu L., Wu T., Cui L., Xu D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids. 2014;46:1673–1680. doi: 10.1007/s00726-014-1729-8. [DOI] [PubMed] [Google Scholar]
- 212.Gutierrez-Castaneda N.E., Gonzalez-Corona J., Griego E., Galvan E.J., Ochoa-de la Paz L.D. Taurine Promotes Differentiation and Maturation of Neural Stem/Progenitor Cells from the Subventricular Zone via Activation of GABA(A) Receptors. Neurochem. Res. 2023;48:2206–2219. doi: 10.1007/s11064-023-03883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hernandez-Benitez R., Ramos-Mandujano G., Pasantes-Morales H. Taurine stimulates proliferation and promotes neurogenesis of mouse adult cultured neural stem/progenitor cells. Stem Cell Res. 2012;9:24–34. doi: 10.1016/j.scr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 214.Yao X., Huang H., Li Z., Liu X., Fan W., Wang X., Sun X., Zhu J., Zhou H., Wei H. Taurine Promotes the Cartilaginous Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells In Vitro. Neurochem. Res. 2017;42:2344–2353. doi: 10.1007/s11064-017-2252-6. [DOI] [PubMed] [Google Scholar]
- 215.Miyazaki T., Honda A., Ikegami T., Matsuzaki Y. The role of taurine on skeletal muscle cell differentiation. Adv. Exp. Med. Biol. 2013;776:321–328. doi: 10.1007/978-1-4614-6093-0_29. [DOI] [PubMed] [Google Scholar]
- 216.Elango R. Tolerable Upper Intake Level for Individual Amino Acids in Humans: A Narrative Review of Recent Clinical Studies. Adv. Nutr. 2023;14:885–894. doi: 10.1016/j.advnut.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Garcia-Montero C., Fraile-Martinez O., Gomez-Lahoz A.M., Pekarek L., Castellanos A.J., Noguerales-Fraguas F., Coca S., Guijarro L.G., Garcia-Honduvilla N., Asunsolo A., et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients. 2021;13:699. doi: 10.3390/nu13020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Finicelli M., Di Salle A., Galderisi U., Peluso G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients. 2022;14:2956. doi: 10.3390/nu14142956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rana S.K., Sanders T.A. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986;56:17–27. doi: 10.1079/BJN19860082. [DOI] [PubMed] [Google Scholar]
- 220.Elshorbagy A., Jerneren F., Basta M., Basta C., Turner C., Khaled M., Refsum H. Amino acid changes during transition to a vegan diet supplemented with fish in healthy humans. Eur. J. Nutr. 2017;56:1953–1962. doi: 10.1007/s00394-016-1237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Caine J.J., Geracioti T.D. Taurine, energy drinks, and neuroendocrine effects. Clevel. Clin. J. Med. 2016;83:895–904. doi: 10.3949/ccjm.83a.15050. [DOI] [PubMed] [Google Scholar]
- 222.Stapleton P.P., Charles R.P., Redmond H.P., Bouchier-Hayes D.J. Taurine and human nutrition. Clin. Nutr. 1997;16:103–108. doi: 10.1016/S0261-5614(97)80234-8. [DOI] [PubMed] [Google Scholar]
- 223.Hwang E.S., Ki K.N., Chung H.Y. Proximate composition, amino Acid, mineral, and heavy metal content of dried laver. Prev Nutr. Food Sci. 2013;18:139–144. doi: 10.3746/pnf.2013.18.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Purchas R.W., Rutherfurd S.M., Pearce P.D., Vather R., Wilkinson B.H. Concentrations in beef and lamb of taurine, carnosine, coenzyme Q10, and creatine. Meat Sci. 2004;66:629–637. doi: 10.1016/S0309-1740(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 225.Zhao X., Li Q., Meng Q., Yue C., Xu C. Identification and expression of cysteine sulfinate decarboxylase, possible regulation of taurine biosynthesis in Crassostrea gigas in response to low salinity. Sci. Rep. 2017;7:5505. doi: 10.1038/s41598-017-05852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vidot H., Cvejic E., Carey S., Strasser S.I., McCaughan G.W., Allman-Farinelli M., Shackel N.A. Randomised clinical trial: Oral taurine supplementation versus placebo reduces muscle cramps in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2018;48:704–712. doi: 10.1111/apt.14950. [DOI] [PubMed] [Google Scholar]
- 227.Hladun O., Papaseit E., Martin S., Barriocanal A.M., Poyatos L., Farre M., Perez-Mana C. Interaction of Energy Drinks with Prescription Medication and Drugs of Abuse. Pharmaceutics. 2021;13:491. doi: 10.3390/pharmaceutics13101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rubio C., Camara M., Giner R.M., Gonzalez-Munoz M.J., Lopez-Garcia E., Morales F.J., Moreno-Arribas M.V., Portillo M.P., Bethencourt E. Caffeine, D-glucuronolactone and Taurine Content in Energy Drinks: Exposure and Risk Assessment. Nutrients. 2022;14:5103. doi: 10.3390/nu14235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McBroom M.J., Welty J.D. Comparison of taurine-verapamil interaction in hamsters and rats. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1985;80:217–219. doi: 10.1016/0742-8413(85)90045-3. [DOI] [PubMed] [Google Scholar]
- 230.Shao A., Hathcock J.N. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul. Toxicol. Pharmacol. 2008;50:376–399. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.