Abstract
Pulmonary disease can refer to the disease of the lung itself or the pulmonary manifestations of systemic diseases, which are often connected to the malfunction of the immune system. Regulatory T (Treg) cells have been shown to be important in maintaining immune homeostasis and preventing inflammatory damage, including lung diseases. Given the increasing amount of evidence linking Treg cells to various pulmonary conditions, Treg cells might serve as a therapeutic strategy for the treatment of lung diseases and potentially promote lung transplant tolerance. The most potent and well-defined Treg cells are Foxp3-expressing CD4+ Treg cells, which contribute to the prevention of autoimmune lung diseases and the promotion of lung transplant rejection. The protective mechanisms of Treg cells in lung disease and transplantation involve multiple immune suppression mechanisms. This review summarizes the development, phenotype and function of CD4+Foxp3+ Treg cells. Then, we focus on the therapeutic potential of Treg cells in preventing lung disease and limiting lung transplant rejection. Furthermore, we discussed the possibility of Treg cell utilization in clinical applications. This will provide an overview of current research advances in Treg cells and their relevant application in clinics.
Keywords: Foxp3, Immunosuppression, Lung disease, Regulatory T cells, TGF-β
Introduction
Pulmonary disease can refer to either the lung disease or the respiratory symptoms of systemic diseases. There are different kinds of lung diseases in human beings, the most common type of pulmonary disease is bronchitis, which is an inflammation of the air passages. Pulmonary disease can be caused by a variety of factors, including infection, exposure to toxins or irritants and smoking. According to the aetiology, pulmonary diseases can be categorized into immune-related lung diseases (i.e. bronchial asthma and allergic rhinitis), lung diseases caused by air pollution and smoking (i.e. chronic obstructive pulmonary disease, which encompasses obstructive emphysema and chronic bronchitis), lung tumors, infectious lung diseases (such as pneumonia, tuberculosis, etc.), lung manifestations of systemic diseases, etc [1].
The lung contains the highest density of blood vessels in the human body, which link the respiratory system to the outside world [2]. It would cause serious damage and even be lethal if a human lung disease manifests. Treatment for pulmonary disease depends on the specific type and severity of the condition. In some cases, medication including oral, intravenous drip, and inhalation therapy may be necessary to manage symptoms [3]. In other cases, surgery may be necessary to remove damaged tissue or correct a problem with lung transplantation when appropriate.
It has become increasingly apparent that lung disorders are often connected to the malfunction of the immune system [4,5]. Regulatory T (Treg) cells have an important role in maintaining immune balance in the body through immune regulation and immunosuppression. Treg cells are a subpopulation of CD4+ T cells that are critical for maintaining self-tolerance and preventing autoimmune disease [6,7]. Treg cells were initially characterized as CD4+CD25+ T cells, which constitute 5–10% of peripheral CD4+ T cells in humans [8]. Furthermore, Foxp3 has been identified as the main transcription factor that distinguishes this lineage of thymic Treg cells [8,9].
There is growing evidence that Treg cells are involved in the development and progression of a number of different lung disorders [10,11]. For example, it has been shown that Treg cells are inappropriately activated in the lungs of patients with asthma and chronic obstructive pulmonary disease (COPD) [11–13]. In addition, Treg cells are deficient in patients with idiopathic pulmonary fibrosis (IPF) [14,15]. However, Treg cells have been shown a controversial role in cancer progression. Treg cell infiltration into tumors is a contributor to poor prognosis via hindering effective immune response against tumor cells in non-small cell lung cancer (NSCLC) [16,17].
Given the growing body of evidence linking Treg cells to various lung disorders, Treg cell function manipulation might be a potential therapeutic strategy for treating lung disorders. Moreover, the expanded role of Treg cells in inflammation regulation serves as a rationale for methods to increase Treg cell populations [18]. Therefore, it is hoped that a better understanding of the role of Treg cells in lung disease will lead to more effective treatments for these conditions. In this review, we summarize the characteristics of Treg cells and discuss the regulatory role of Treg cells against lung diseases and transplant rejection. We have summarized the mechanisms of action and therapeutic studies of Treg cells in lung diseases to provide readers with a clearer and more structured overview of the content (Table 1). This will provide an overview of current research advances in Treg cells and their relevant application in clinics.
Table 1. The mechanisms of action and therapeutic studies of Treg cells in lung diseases.
| Disease | Type of Treg cells | Main way | Main profile | Outcome | Reference |
|---|---|---|---|---|---|
| Asthma | CD4+ Treg cells, Foxp3+ Treg cells | TGF-β, IL-10, DLL4-Notch signalling pathway | ↓ ILC2, Th1 cells, Th2 cells, Th17 cells, Granulocytes | Inhibition | [153,158–162] |
| ARDS | Foxp3+ Treg cells | TGF-β, IL-10 | ↓ Lung pyroptosis, Th17 cells | Inhibition | [168,170,171,175] |
| IPF | CD4+Foxp3+ Treg cells | TGF-β | ↑ TGF-β1 | Early: Promotion | [174,181] |
| ↓ Th17 cells, CXCL10, Fibrosis | Late stage: Inhibition | ||||
| COPD | Foxp3+ Treg cells | TGF-β, IL-10 | ↓ HIF-1α, TNF-α, IL-1β, IL-17A, RORγt | Inhibition | [13,34,169,184] |
| CF | Foxp3+ Treg cells | TGF-β, IL-10 | ↓ IL-6, IL-8, IL-17A | Inhibition | [186,193] |
| Lung cancer | CD4+Foxp3+ Treg cells, CD25+ Treg cells | TGF-β, IL-10 | ↑ HIF-1α, CTLA4, CD39, GITR, B7H1, IFN-γ, CXCL13 | Promotion | [17,199,202–204,208–210,212–215] |
| ↓ CD8+ T cells, NK cells, IL-2 | |||||
| Bronchitis | Nrp-1+ Treg cells | TGF-β, IL-10 | ↑ IL-35, Glentiy-1 | Inhibition | [161,235,236,322–325] |
| ↓ IL-17, TIM-3, Macrophages, MHC class II molecules | |||||
| Pneumonia | Foxp3+CD39+ Treg cells | TGF-β, IL-10, IL-35 | ↑ EGF, BLIMP1 | Inhibition | [240,243,245,247,252,258] |
| ↓ CD4+ and CD8+ T cells, B cells, DCs, NK cells, Neutrophils, Eosinophils | |||||
| Pulmonary embolus | CD4+Foxp3+ Treg cells | TGF-β | ↑ SPARC, Monocytes | Inhibition | [264] |
Abbreviations: ARDS, acute respiratory distress syndrome; BCL6, B-cell lymphoma 6; BLIMP1, B-lymphocyte-induced maturation protein 1; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; CTLA4, cytotoxic T-lymphocyte-associated protein 4; CXCL10, C-X-C motif chemokine ligand 10; CXCL13, C-X-C motif chemokine ligand 13; DC, dendritic cells; DLL4, delta-like 4; EGF, epidermal growth factor; GITR, glucocorticoid-induced tumour necrosis factor receptor; HIF-1α, hypoxia-inducible factor 1α; IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-10, interleukin-10; IL-17A, interleukin 17A; IL-35, interleukin-35; ILC2, Type 2 innate lymphocytes; IPF, idiopathic pulmonary fibrosis; MHC, major histocompatibility complex; NK, natural killer; SPARC, scalable processor architecture; TGF-β, transforming growth factor β; Th1, Type 1 T helper; Th2, Type 2 T helper; Th17, Type 17 T helper; TIM-3, T-cell immunoglobulin and mucin domain-containing protein 3; TNF-α, tumor necrosis factor alpha.
Biology of Treg cells
Origin and development of Treg cells
Treg cells are a specialized subset of T cells that play an indispensable role in maintaining the body’s immune homeostasis and preventing autoimmunity. These cells are characterized as CD4+Foxp3+ Treg cells [19]. Foxp3, discovered as a marker molecule of Treg cells, is a member of the forkhead transcription factor family, which plays a crucial role in the immune regulation of the human body [20,21]. Foxp3 mutation can hinder the development and normal function of Treg cells, resulting in the development of immune dysregulation and autoimmune diseases [22–26]. Apart from its role as a marker molecule of Treg cells, Foxp3 also serves as a regulatory factor that can modulate the activity of Treg cells by regulating other genes including Il2, Ifng and Ctla4 [23,26].
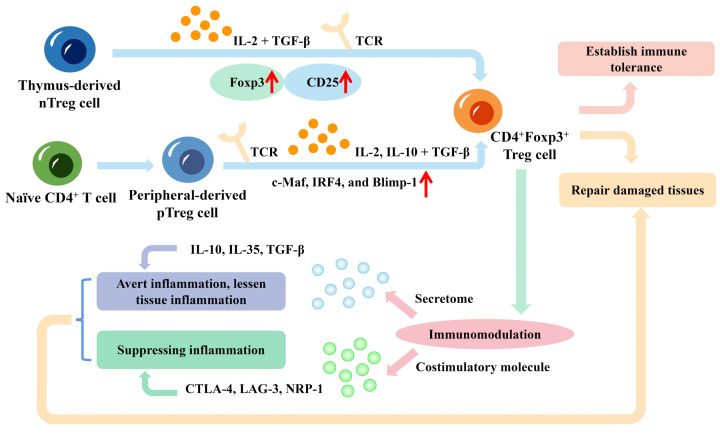
CD4+Foxp3+ Treg cells can be classified into two major types based on their developmental origins: thymus-derived Treg cells (tTreg cells) and peripherally-derived Treg cells (pTreg cells) [24,27]. As shown in Figure 1, the majority of CD4+Foxp3+ Treg cells develop in the thymus from precursors that were born in the bone marrow. These cells migrate to the thymus, where they recognize self-antigens presented by thymic epithelial cells, ultimately differentiating into CD4+Foxp3+ Treg cells [28,29]. The thymus-generated Treg cells are also referred to as naturally developed Treg cells (nTreg cells), which express high levels of CD25 and Foxp3 [28–30]. The expression of Foxp3 is initiated during development in the thymus but is not yet stable. The long-term persistence and differentiation of this type of tTreg cell in peripheral tissues are largely dependent on the assistance of TGF-β [28,31–34]. The differentiation of tTreg cells is driven by signals mediated by the T-cell receptor (TCR) and by cytokines such as interleukin (IL)-2 and transforming growth factor-β (TGF-β) [35]. Once they mature, tTreg cells migrate to the periphery where they can suppress the activation of autoreactive T cells.
Figure 1. The origin and function of CD4+Foxp3+ regulatory T (Treg) cells.
Thymus-derived regulatory cells (tTreg cells), which express high levels of CD25 and forkhead box protein P3 (Foxp3), develop in the thymus from precursors that were born in the bone marrow. These cells migrate to the thymus and differentiate into CD4+Foxp3+ Treg cells. The differentiation of tTreg cells is driven by signals mediated by the T-cell receptor (TCR) and by cytokines such as interleukin (IL)-2 and transforming growth factor-beta (TGF-β). The peripheral original peripherally-derived Treg cells (pTreg cells) are induced or generated from naïve CD4+ T cells in response to the combination of TCR signalling and exposure to TGF-β as well as IL-2 and IL-10. Transcription factors such as c-Maf, interferon regulatory factor 4 (IRF4), and B-lymphocyte-induced maturation protein 1 (Blimp-1) also play important roles in pTreg cell differentiation. Effector Treg cells have been implicated in immunomodulation, tissue repair, and immune tolerance. Treg cells can act as an immunomodulator via cytokines and cell-cell contact. These cells can generate anti-inflammatory factors IL-10, IL-35 and TGF-β to avert inflammation and lessen tissue inflammation. Treg cells have inhibitory molecules, for instance, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3) and neuropilin-1 (NRP-1), which aid in suppressing inflammation.
The peripheral original pTreg cells are induced or generated from naïve CD4+ T cells in response to antigenic stimulation. Their differentiation is induced by the combination of TCR signalling and exposure to TGF-β and other cytokines, such as IL-2 and IL-10 [26,27,36]. These cytokines activate downstream signalling pathways that lead to the up-regulation of Foxp3. Foxp3-transformed T cells require T-cell receptor (TCR) stimulation to exert inhibitory effects. In addition, other transcription factors such as c-Maf, IRF4, and Blimp-1 also play important roles in pTreg cell differentiation [37]. When expressed, Foxp3 will also promote the development and function of Treg cells in a CD28-dependent manner, which can be further stimulated by TGF-β for increased Foxp3 expression in Treg cells [36,38–40]. It can be concluded that TGF-β has a crucial role in inducing the development of Foxp3+ Treg cells (Figure 1). pTreg cells can arise from multiple subsets of peripheral CD4+ T cells, including naïve T cells, memory T cells, and even type 1 T helper (Th1) and type 17 T helper (Th17) cells, etc [41].
Both tTreg and pTreg cells are important for maintaining immune tolerance and preventing autoimmunity [42]. These CD4+Foxp3+ Treg cells can modulate the balance and stability of the immune system within the body by regulating the timing and magnitude of immune responses, not just to autoimmunity but also to other antigens [42–45]. The CD4+Foxp3+ Treg cells not only have a vital role in immune regulation but also serve as a benchmark for disease diagnosis and treatment. When a disease manifests, the proportion of CD4+Foxp3+ Treg cells decreases, and the expression of Foxp3 is reduced [46]. Furthermore, augmenting the expression of Foxp3 or other pathways in vivo may increase the number of CD4+Foxp3+ Treg cells, resulting in better therapeutic results [47–49].
Effector CD4+Foxp3+ Treg cells
The effector CD4+Foxp3+ Treg cells not only possess a regulatory function but also exhibit certain effector functions, such as the ability to secrete cytokines or migrate to inflamed tissues. Effector CD4+Foxp3+ Treg cells have been implicated in a variety of immune processes, including immunomodulation, repairing damaged tissues, establishing immune tolerance, etc (Figure 1). They can suppress the immune response of innate and adaptive immune cells, including conventional T cells, B cells and natural killer (NK) cells. In general, Treg cells exert their immunomodulatory effects on various types of immune cells through three mechanisms: soluble factor mediation (such as anti-inflammatory cytokines, FLG-2, adenosine, granzyme and perforin), inhibitory receptor engagement and growth factor competition [50,51]. These three approaches often work in conjunction, thereby strengthening the immune regulation of Treg cells.
Specifically, the Foxp3+ Treg cell can secrete different types of cytokines (IL-10, IL-35 and TGF-β) to suppress immune activation, particularly in instances where inflammation takes place in an organ or tissue. IL-10 can suppress the activation of antigen-presenting cells (APCs) by inhibiting CD28 tyrosine phosphorylation, leading to a decrease in the immune response of CD4+ T cells [52]. Furthermore, IL-10 can inhibit effector T cells and B cells, as well as down-regulate major histocompatibility complex class I (MHC-I) expression, resulting in reduced inflammation [53]. Similarly, TGF-β is a multi-functional cytokine with potent anti-inflammatory properties that inhibits the differentiation of effector T cells, thereby exerting immunosuppressive effects [54–56]. IL-35, on the other hand, induces the proliferation of CD4+CD25+ regulatory T cells and inhibits Th17 differentiation [57]. Although it can be produced by Treg cells through strong stimulation, IL-35 efficacy in humans is still limited [57]. Treg cells can also secrete FLG-2, adenosine, granzyme A/B and perforin to exert immunosuppressive effects. FLG-2 can hinder T-cell polarization toward Th2 and simultaneously down-regulate Th1 and Th17 immune responses [58]. It can also inhibit dendritic cell (DC) maturation by reducing the expression of CD80 and MHC II molecules [59], and binding to the FcγRIIB receptor expressed by APCs to exert an inhibitory effect [60]. Cells surrounded by adenosine can facilitate the transformation of pro-inflammatory immune cells that are activated by ATP into anti-inflammatory cells through CD39 and CD73, thereby reversing the pro-inflammatory environment [61]. Granzyme A/B and perforin interaction rely on contact with effector cells to function. Perforin can form transportive perforin channels after contacting effector cell membranes, while granzyme A/B can enter effector cells either through perforin channels or via mannose-6-phosphate receptor-dependent mechanisms [53,62]. This leads to the cleavage of caspases and activation of intracellular enzymes, which results in apoptosis of effector cells and immune response inhibition [63,64]. Granzyme B is highly expressed by Treg cells in tumor diseases, and it can induce apoptosis of NK cells and CD8+ T cells [53,65]. Moreover, studies have shown that granzyme B can help build tolerance in transplants [66].
Treg cells employ inhibitory receptors including cytotoxic T lymphocyte-associated protein 4 (CTLA-4), neuropilin-1 (NRP-1), Galectin-1, LAG-3 and TIM-3 to exert immunosuppressive effects, which aid in suppressing inflammation [67]. CTLA-4 is constitutively expressed on Treg cells and can effectively suppress autoreactive T cells [68]. CTLA-4 and CD28 are cognate receptors that bind to the B7 molecule, but CTLA-4 has a higher affinity for B7 than CD28 [69,70], leading to the inhibition of T-cell activation via arresting the cell cycle, transmitting inhibitory signals to slow down the secretion of IL-2 and decreasing the expression of B7 molecules [71,72]. NRP-1 mediates the formation of an immune synapse between T cells and DCs, limiting the access of effector T cells to APCs and promoting the interaction between Treg cells and naïve DCs [73,74]. Galectin-1 is up-regulated upon Treg activation and, when combined with specific cell surface receptors (such as CD2, CD3, CD4, CD7 and CD43), can block the secretion of pro-inflammatory cytokines (IL-2 and IFN-γ) and enhance the secretion of anti-inflammatory cytokine IL-10, which will induce cycle arrest and enhance apoptosis of activated T cells [75–77]. LAG-3, a homologue of CD4, has a stronger affinity for MHC class II molecules, which can inhibit DC maturation and immune stimulation [78,79]. Such inhibition will further inhibit the activation of T cells. At present, little is known about TIM-3, but its existence has been detected in Treg cells. TIM-3+ Treg cells can suppress effector Th17 cells and show high expression of IL-10, CTLA-4, LAG-3 and other factors [80]. Targeted therapy using TIM-3+ Treg cells has potential in cancer treatment [81].
Treg cells can exert immunosuppressive effects by engaging in competition for growth factors, with IL-2 being the primary growth factor involved. IL-2 plays a crucial role in the development, survival, and function of Treg cells, as well as in the activation of T cells [82–87]. Thus, competition arises between Treg cells and effector T cells for IL-2. Treg cells express CD25 and form a high-affinity IL-2 trimeric receptor, enabling them to compete with effector T cells for IL-2 in the local environment [88]. If effector T cells lack access to IL-2, they will be unable to proliferate and undergo apoptosis [89].
The immunomodulatory effect of Foxp3+ Treg cells can be utilized in treating human autoimmune diseases, for some autoimmune disorders can lead to allergic reactions caused by hypersensitivity to antigens [90]. Apart from their crucial role in regulating the immune system, Foxp3+ Treg cells have been found to help repair and heal various tissues such as muscle, bone, lung tissue, heart, kidney tissue, and more [91–107]. When tissue damage occurs, Treg cells can produce anti-inflammatory factors like IL-10 to combat inflammation, as well as secrete growth factors such as amphiregulin and keratinocyte growth factor (KGF) to promote tissue repair and regeneration. Treg cells can also inhibit the function of INF-γ and use IL-33 to regenerate Treg cells and increase their numbers, speeding up the repair process. They can induce macrophage differentiation and suppress adverse cell factors through cell contact and other methods. Furthermore, de Candia et al. found that Treg cells can act as critical metabolic sensors controlling the immune system, by which to restore organismal homeostasis in metabolic disorders [108]. However, the immunosuppressive nature of these cells can create conditions that lead to immune escape, as seen in cancer where they aid cancer cells in evading the immune system. Consequently, cancer cells can proliferate rapidly leading to uncontrolled growth and progression of the disease [109–111].
Lung Treg cells
Lung-specific Treg cells are specialized to function within the lungs. Different subtypes of regulatory T cells including CD4+CD25+ Treg cells, Foxp3+ Treg cells and type 1 regulatory T cells (Tr1 cells, which secret high levels of IL-10 but lack the expression of Foxp3), pTreg cells play an important role in maintaining immune tolerance in the respiratory system by suppressing inflammation and preventing tissue damage [112,113]. They achieve this by releasing anti-inflammatory cytokines and by directly inhibiting the activity of other immune cells. IL-10 secreted by lung Treg cells and Tr1 cells can inhibit the production of pro-inflammatory cytokines IL-5 and IL-13, which play important roles in respiratory diseases such as asthma. Lung Treg cells can inhibit the activation of type 2 innate lymphocytes (ILC2s) via direct inducible costimulator (ICOS) induction, by which to suppress the production of IL-5 and IL-13 [114,115]. Moreover, lung Treg cells can secrete amphiregulin, a growth factor that stimulates the proliferation and differentiation of epithelial cells and helps to repair damaged lung tissue [99,116,117]. Faustino et al. found that ST2+ Treg cells in the lung can act as a negative regulator of early innate γδT cell response by elevating Ebi3, by which to reduce allergen-inducing inflammation in the lung [118]. During acute immune rejection after lung transplantation, there is an increase in the number of Treg cells, which function as immune suppressors. Their role is to decrease immune rejection, thereby contributing to the successful integration of the transplanted lung tissue and enabling transplant recipients to live in harmony with their new lungs [119,120].
Conversion between Treg cells and other CD4+ T-cell subsets
Treg cell is one of the subsets of CD4+ T cells, which can convert to each other under specific conditions. The conversion between Treg cells and other CD4+ T cell subsets is a complex and dynamic process that can occur in response to various environmental signals. Under certain conditions, Treg cells can convert into other CD4+ T cell subsets such as Th1, type 2 T helper (Th2), Th17 or T follicular helper (Tfh) cells. Conversely, other CD4+ T cell subsets can convert into Treg cells (Figure 2). This conversion is driven by changes in the expression of transcription factors such as Foxp3, T-bet, GATA3, RORγt and BCL6, which are critical regulators of T-cell differentiation [121]. The conversion between Treg cells and other CD4 T-cell subsets plays an important role in maintaining immune homeostasis and can have significant implications for the development of homeostasis in the immune system. However, the mechanisms underlying Treg cell conversion and the factors that regulate this process are still not fully understood and require further investigation.
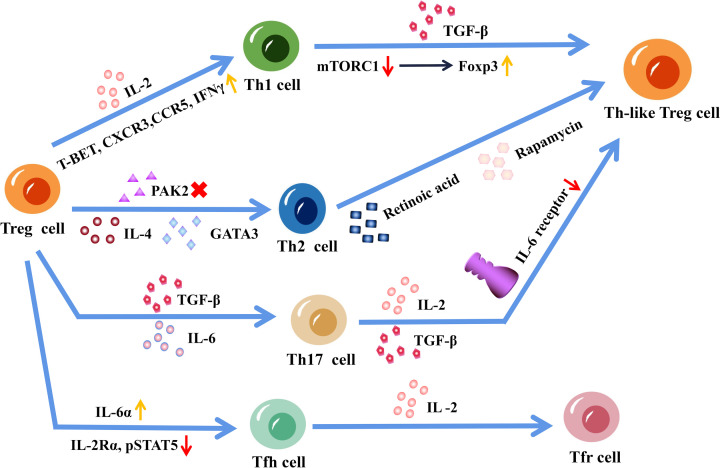
Figure 2. Conversion between regulatory T (Treg) cells and other CD4+ T-cell subsets.
Under interleukin (IL)-2 stimulation, the expression level of T-BET, C-X-C Motif Chemokine Receptor 3 (CXCR3), C-C Motif Chemokine Receptor 5 (CCR5) and interferon-γ (IFN-γ) was up-regulated, and Treg cells were transformed into type 1 T helper (Th1) cells. In the presence of transforming growth factor-beta (TGF-β), the reduction of mammalian target of rapamycin complex 1 (mTORC1) promoted forkhead box protein P3 (Foxp3) expression and the transformation of Th1 cells into Th1-like Treg cells. IL-4 promotes the conversion of Treg cells to type 2 T helper (Th2) cells, and when p21-activated kinase 2 (Pak2) cannot be expressed, Treg cells express GATA binding protein 3 (GATA3) and Th2-related factors, thereby converting Treg cells into Th2 cells. Under all-trans-retinoic acid and rapamycin stimulation, memory Th2 cells can be transferred to Foxp3+ T cells via TGF-β. IL-6 and TGF-β can induce the transformation of naturally developed treg (nTreg) cells to type 17 T helper (Th17) cells, and IL-2 and TGF-β down-regulate IL-6 receptor expression in Treg cells, which are resistant to the transformation of Treg cells into Th17 cells. Under the influence of IL-6α, IL-12, and IL-21, Treg cells can be transferred into T follicular helper (Tfh) cells. This transformation not only induces the expression of IL-6α but also leads to a reduction in the expression of IL-2Rα and phosphorylated STAT-5. IL-2 can promote the transformation of Tfh cells into follicular Treg (Tfr) cells.
In humans, stimulating Treg cells with IL-12 will result in the up-regulation of T-BET, C-X-C Motif Chemokine Receptor (CXCR)3, C-C Motif Chemokine Receptor (CCR)5 and interferon-γ (IFN-γ), which induces a Th1-like Treg phenotype via up-regulating the expression [122,123]. Koch et al. found that activation of signal transducer and activator of transcription (STAT)-1 by effector T cell-derived IFN-γ induced T-bet and CXCR3 expression in Treg cells. However, this conversion was abortive due to delayed induction of IL-12 receptor component IL-12 Rβ, which prevented Treg cells from completing STAT-4-dependent Th1 cell differentiation in acute type 1 inflammatory response [124]. There has been little research on the direct conversion of Th1 cells to Treg cells. Kanamori et al. established a method for Treg inducing by resting Th1 cells without T-cell receptor ligation before stimulation in the presence of TGF-β. They found that Foxp3 expression was promoted with reduced mammalian target of rapamycin complex 1 (mTORC1) activity in Th1 cells [125]. The conversion between Th2 and Treg cells is a complex process that is influenced by various factors including genetic and epigenetic programming. Memory Th2 cells were able to shift to Foxp3+ T cells by TGF-β when stimulated in the presence of all-trans retinoic acid and rapamycin [126]. In contrast, IL-4 was found able to promote the development of Th2 cells from Treg cells [127]. In the absence of signal transduction activator (Pak2) expression in Treg cells, these cells can express the transcription factor GATA3 along with Th2-related cytokines, which shifts Treg cells into Th2 cells [128]. It was found that IL-6 can prompt the conversion of nTreg cells into Th17 cells in a TGF-β-dependent manner. IL-6-induced IL-17-producing cells can self-induce even without exogenous TGF-β [129]. However, Zheng et al. found that Treg cells induced by IL-2 and TGF-β are resistant to Th17 transformation. For IL-2 and TGF-β down-regulated IL-6 receptor expression in the induced Treg cells [130]. Treg cells were found to convert into functional Tfh cells in Peyer’s patches and in atherosclerosis, which correlates with increased IL-6α expression as well as decreased IL-2Rα and phosphorylated STAT-5 expression [131–133]. IL-2 can convert Tfh cells to follicular Treg cells in patients with systemic lupus erythematosus [134]. Indicating that Treg cells and other CD4+ T cell subsets can convert to each other, however, it is a complex process that is influenced by various factors, including the cytokine environment and the expression of specific transcription factors. Further research is needed to fully understand the mechanisms that regulate this conversion and its implications for immune function and disease.
Costimulatory molecules on Foxp3+ Treg cells
Costimulatory molecules play an active role in co-signaling with the initial activating signals, contributing to T-cell differentiation and fate. They are critical for the complex control of Treg cell development and function [135,136]. There are several costimulatory molecules including CD28, CTLA-4, ICOS, programmed cell death 1 (PD-1), T-cell immunoglobulin and mucin domain (TIM)-1, TIM-3, glucocorticoid-induced tumor necrosis factor receptor (GITR) and OX40 expressing on Treg cells (Figure 3).
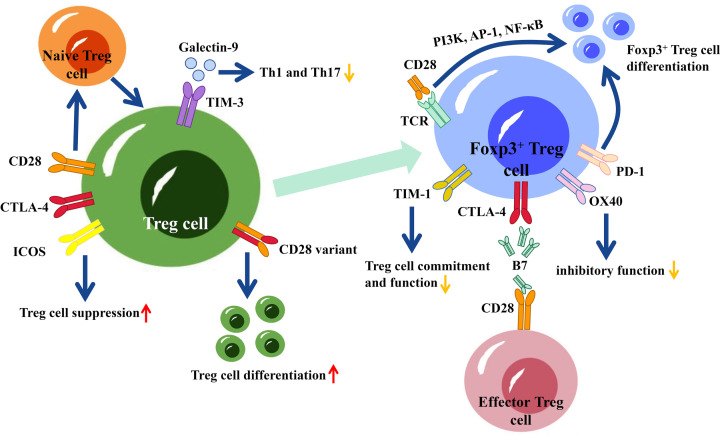
Figure 3. Co-stimulatory molecules and their effects on forkhead box P3 (Foxp3)+ regulatory T (Treg) cells.
CD28, Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and inducible costimulator (ICOS) are involved in Treg cell activation. ICOS has the potential to enhance the suppressive capabilities of Treg cells, while CD28 plays a role in facilitating the conversion of naïve Treg cells into mature Treg cells. Additionally, a variant of CD28 can trigger the differentiation of Treg cells. T-cell immunoglobulin and mucin domain (TIM)-3 is consistently present on natural Treg cells, and it engages with Galectin-9 to diminish the secretion of type 1 T helper (Th1) and Th17 cytokines while increasing the proportion of Treg cells. CD28 and T-cell receptor (TCR) co-stimulate the differentiation of Foxp3+ Treg cells by activating phosphoinositide 3-kinases (PI3K), activator protein 1 (AP-1) and nuclear factor kappa-B (NF-κB) signaling pathways. CTLA-4 is more predominantly present in Foxp3+ Treg cells, and the affinity of the CTLA-4 receptor on Foxp3+ Treg cells is higher than that of CD28, enabling Treg cells to bind more easily to cytokines such as B7 in the physiological environment. Programmed cell death 1 (PD-1) is able to assist in the differentiation and maintenance of Foxp3+ Treg cells. OX40 signaling inhibits the TGF-β-mediated induction of Treg cells. T-cell immunoglobulin and mucin domain (TIM)-1 is involved in the differentiation of Th1, Th2, and Th17 cells while inhibiting both the commitment to and suppressive function of the Treg cell phenotype.
The immunoglobulin-related receptor family, which includes CD28, CTLA-4, ICOS and other proteins, can promote Treg cell activation [69,137]. CD28 is critical to the development of naïve Treg cells in the thymus [136]. CD28 and TCR costimulate the differentiation of Foxp3+ Treg cells by activating PI3K, AP-1, NF-κB and other related signaling pathways [137,138]. Foxp3+ Treg cells express CTLA-4, which is homologous to the CD28 on effector T cells, resulting in a competitive relationship between the two [69]. Compared with CD28, CTLA-4 is more predominantly present in Foxp3+Treg cells, and the affinity of the CTLA-4 receptor on Foxp3+ Treg cells is higher than that of CD28, enabling Treg cells to bind more susceptible to cytokines such as B7 in the physiological environment [69,70,137]. However, CTLA-4 can dimerize with CD28, resulting in the formation of a high-affinity CD28 variant that can participate in positive co-stimulation, leading to the activation of Treg cell differentiation [69,139]. The costimulatory molecule ICOS plays a role in promoting IL-2-induced cell survival and proliferation, enhancing the inhibitory activity of Treg cells [140].
In addition to CTLA-4, PD-1 also plays a central role in both the differentiation and maintenance of Foxp3+ Treg cells. This results in an increased generation of Treg cells and enhances their suppressor function with sustained Foxp3 expression [141]. However, PD-1-mediated inhibitory effects were demonstrated independently of CTLA-4 [142]. Additionally, specific blockade of the PDL-1-B7-1 pathway leads to a reduction in the Treg population, indicating its involvement in Treg homeostasis. TIM-3 is constitutively expressed on natural Treg cells and can interact with Galectin-9 for Treg function [143]. Blocking TIM-3 reduces the suppressive ability of natural Treg cells and stops the formation of allospecific adaptive Treg cells, preventing or cancelling induced peripheral tolerance [144,145]. Therefore, administering soluble Galectin-9 will decrease the production of Th1 and Th17 cytokines and increase the proportion of Treg cells, which will further promote allograft survival [136,146]. GITR is considered a promising therapeutic target in cancer and autoimmune disease, for it can activate signaling pathways. GITR is also thought to be involved in the regulation of Foxp3+ Treg cells due to its constitutive expression on Treg cells. However, the precise role of GITR is still unknown, especially in transplantation [136].
OX40 and TIM-1 signaling negatively affect Treg cells. OX40 signaling can inhibit the TGF-β-mediated induction of Foxp3+ Treg cells from both naïve CD4+ T cells and CD4+ T effector cells [147,148]. OX40 ligation will result in reduced Foxp3 expression, while OX40 stimulation causes the abrogation of natural Foxp3+ Treg cell suppressive function [149]. Nevertheless, OX40 is not implicated in the homeostatic regulation of Treg cells [148]. TIM-1 is involved in the differentiation of Th1, Th2 and Th17 cells while inhibiting both the commitment to and suppressive function of the Treg cell phenotype [150,151].
The role of Treg cells in human lung diseases
Asthma
Asthma is a chronic heterogeneous disease and is characterized by heightened inflammation and mediator release caused by overactive Th2 cells [152]. The pathogenesis of asthma is marked by an abnormal Th cell immune response, where Treg cells have impaired immunosuppressive function, while effector Th cells are overreactive [153]. It was found that Treg/Th17 cell imbalance is a promoter of asthma development [154]. In human allergic asthma, there is an inflammatory environment characterized by high levels of the chemokine C-C motif chemokine ligand (CCL)20, which can hinder the TGF-β-induced differentiation of Treg cells while facilitating the migration of CCR6+ Treg cells. Treg cells expressing CCR6 have the potential to promote inflammation and exacerbate allergic asthma. Therefore, inhibiting the CCR6-CCL20 pathway may be advantageous in preventing the transformation of Treg cells specific to house dust mites into Th17-like cells. [155,156].
In contrast with Th cells, Treg cells are major contributors to the regulation of asthma by negatively modulating Th1, Th2 and Th17 cells, which helps to prevent the overactivity of these cells [157]. Treg cells prevent airway inflammation by producing the anti-inflammatory cytokines IL-10 and TGF-β, which can inhibit the proliferation of ILC2s and granulocytes, thereby regulating autoimmune effects [158,159]. The suppression of Th2 inflammation by Treg cells is achieved through the action of the transcription factor BCL6, which hinders the expression of GATA3, a transcription factor necessary for the production of IL-4 and IL-13. Under inflammatory conditions, Treg cells promote their stability by expressing BCL6 [153,159]. Ding et al. found that down-regulation of the expression of glucocorticoid-induced tumour necrosis factor receptor ligand (GITRL) on the surface of DCs will induce tolerogenic Treg cell skewing in asthma, which further facilitated the apoptosis of pathogenic Th2 and Th17 cells [157]. Yao et al. found that the suppressive function and stability of Treg cells are enhanced by androgen receptor signaling, which down-regulates ST2 expression on Treg cells and reduces allergen-induced IL-33 expression in airway epithelial cells. In addition, Treg cells were demonstrated to improve airway remodeling and restore Th1/Th2 balance through the DLL4-Notch signaling pathway [160,161].
In the context of catabolism, there exists an enzyme known as heme oxygenase 1 (HO-1) with anti-inflammatory properties. Increasing HO-1 transcription has the capacity to enhance the expression of Foxp3 and IL-10 within Treg cells, along with an increase in the transcription of TGF-β1. Additionally, HO-1 can induce the up-regulation of Foxp3 and anti-inflammatory cytokines in cells that did not previously express them. This mechanism serves as a protective response against airway inflammation in asthma and is mediated by Treg cells, IL-10, and membrane-bound TGF-β1 [162]. Furthermore, there is the discovery of a novel interleukin cytokine, IL-38, which shares functional similarities with IL-36Ra [163]. While IL-38 can be secreted by various cell types, no existing literature confirms its production by Treg cells. It appears to be a widespread anti-inflammatory factor. Notably, in the presence of elevated periostin concentrations, IL-38 is inversely correlated with the percentage of CD4+CD25highFoxp3+ Treg and CD4+CD25+ T cells. This suggests a potential connection between IL-38 and suppressive Treg cells in the context of asthma development. However, further research is needed to elucidate the mechanism through which these two factors collaborate to inhibit bronchial inflammation in asthma [164,165].
Acute respiratory distress syndrome (ARDS)
ARDS is a pulmonary inflammatory disorder characterized by cytokine-induced inflammation [166]. ARDS development is linked to an imbalance between pro- and anti-inflammatory factors in the lungs, and an elevated Th17/Treg ratio is a risk factor for early ARDS onset [167,168]. It was found that secretory phosphoprotein 1 (SPP1) suppresses VHL expression, thereby decreasing the ubiquitination and degradation of hypoxia-inducible factor 1α (HIF-1α), resulting in an increase in the Th17/Treg ratio and exacerbation of inflammation in ARDS [167,169].
Luteolin, which is derived from certain fruits and vegetables such as apples and broccoli, has a preventive effect by reducing lung inflammation and injury. It was found that luteolin can activate Treg cells to reduce the symptoms of ARDS [170]. When induced by luteolin, there is accelerated differentiation of CD4+CD25+ T cells into CD4+CD25+Foxp3+ Treg cells in BALF and serum, which is accompanied by an increase in IL-10 production [171]. Treg cells have been found to alleviate caspase-11-mediated inflammatory cell death in lung tissue [170]. Curcumin is the active component found in the herb turmeric, which has been found to have anti-inflammatory properties that enhance the ability of Treg cells to combat ARDS [172]. Similar to luteolin, curcumin increases the rate of differentiation of initial CD4+ T cells into CD4+CD25+Foxp3+ Treg cells.
ARDS is a severe form of acute lung injury (ALI) [173]. As evidenced by their accumulation in the BALF of ALI patients, Treg cells promote inflammation regression in ALI patients by inducing cytokine TGF-β1 and neutrophil apoptosis. Furthermore, Treg cells can modulate fibrocyte recruitment to the lung in LPS-induced injury, which reduces fibroplasia in ALI patients [168]. In a mouse model of ALI, Treg cells suppressed fibroblast recruitment along the CXCL12/CXCR4 axis, thereby reducing fibrotic proliferation [174]. Ge Gen Scutellaria Tang (GQD), an herbal remedy for inflammatory lung diseases, has been shown to improve the degree of lung damage in ALI. GQD stimulates Treg cell activity and promotes the expression of CYP1A1 and cytokine TGF-β while reducing the level of Th17 cells. Such Treg cell stimulation further restores the Treg/Th17 balance, thus reducing the degree of pulmonary edema [175].
Idiopathic pulmonary fibrosis (IPF)
IPF is a chronic and progressive pulmonary disease characterized by the gradual scarring of lung tissue, leading to a decline in lung function [174,176,177]. In IPF patients, Treg cells fail to suppress the secretion of Th1 and Th2 cytokines, resulting in a deficiency of Treg cells [178]. The presence of PD-1 on the surface of CD4+ T cells, along with the production of TGF-β1 and IL-17A, can inhibit Treg cell transformation and promote IPF development [179]. The development of IPF is exacerbated by a significant increase in the number of activated Treg cells in the peripheral blood of patients. Likewise, quiescent Treg cells generated in the thymus can undergo proliferation and differentiate into activated Treg cells, which also exhibit immunosuppressive properties and enhance the population of cytokine-secreting CD45RA−CD25+ cells [180].
Treg cells have a dual role in pulmonary fibrosis depending on the disease stage. In the early stage, they contribute to the development of fibrosis by promoting TGF-β1 release, reducing the Th17 response, stimulating the aggregation of fibroblasts, increasing the deposition of collagen and participating in the progression of pulmonary fibrosis [181]. Conversely, in the late stage, Treg cells have a protective function by suppressing the production of CXCL10 and fibroblasts, thereby decreasing fibroblast recruitment and mitigating the extent of pulmonary fibrosis [174,181]. Treg cells also mediate the transfer of proteins FGF9 and CCL2 to ameliorate bleomycin-induced pulmonary fibrosis [177].
Chronic obstructive pulmonary disease (COPD)
COPD is a severe inflammatory disease characterized by heightened pro-inflammatory cytokine levels and increased reactive oxygen species in the peripheral blood [182]. An imbalance in the ratio of Th17 to Treg cells plays a significant role in the initiation and development of the inflammatory response in COPD [156]. This imbalance results in a decrease in the expression of intracellular Foxp3 mRNA, which in turn affects the secretion of anti-inflammatory factors like IL-10 and TGF-β by Treg cells. In the absence of adequate IL-10 levels, IL-23 levels increase, thereby accelerating the differentiation of Th17 cells. Th17 cells, which express RORγt, release the pro-inflammatory cytokines IL-17A, IL-17F and IL-22 [183]. These cytokines induce the production of neutrophil growth factor GM-CSF, chemokines CXCL1 and CXCL8, and antimicrobial peptides by epithelial cells to promote neutrophil accumulation in the airway [184].
In clinical practice, the administration of oral N-acetylcysteine (NAC) can reduce cytokine activity and prevent neutrophil aggregation, which reduces inflammation, in the lungs. These effects are evident through increased levels of IL-10 in COPD patients, modulation of Th1/Th2 cell balance, and increased differentiation of CD4+ T cells into Treg cells [182]. In COPD patients, the inflammatory cytokine IL-17 is responsible for directing neutrophil aggregation to the airways [183]. This effect is synergized by HIF-1α and RORγt protein expression. While NAC treatment can help alleviate the inflammatory environment of COPD by reducing HIF-1α expression [13,34]. Xuanbai Chengqi decoction (XBCQ) is an herbal medicine used to treat COPD patients in the exacerbation stage. Moderate doses of XBCQ can restore Treg/Th17 balance, suppress Treg cell deficiency in the lung, reduce the expression of pro-inflammatory factors such as IL-1β and TNF-α, inhibit inflammatory infiltration, and significantly improve lung injury caused by COPD [184]. Furthermore, administration of the anticholinergic drug tiotropium bromide and the long-acting β2 agonist olodaterol to PBMCs from COPD patients has been shown to elevate the proportion of Foxp3+ Treg cells and reduce the proportion of T cells that express both acetylcholine IL-17A and acetylcholine RORγt in PBMCs. This effectively controls the development of inflammation in COPD [169].
Cystic fibrosis (CF)
CF is an autosomal recessive genetic disorder resulting from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [185]. The hallmark of CF is chronic unremitting lung disease, a major cause of morbidity and mortality in CF patients. This condition is characterized by chronic infections, immune dysfunction, and dysregulated airway inflammatory responses, evidenced by decreased Treg cell activity and excessive inflammatory reactions [186,187]. Treg cells, especially CD4+CD25+ Treg cells, play a crucial role in maintaining the balance between anti-inflammatory and pro-inflammatory mediators, thereby stabilizing the immune system [187,188]. These cells help prevent excessive inflammation by inhibiting the hyperactivity of certain T cell subsets, such as Th17 or Th2 cells [189]. However, in CF lung disease, there is an elevation in pro-inflammatory cytokines such as IL-6, IL-8, tumor necrosis factor-α and IL-1β, along with a decrease in anti-inflammatory cytokine IL-10 and anti-inflammatory mediator lipoxin levels [190]. This dysregulation of Th17/Th2 cells contributes to the development of chronic lung disease in CF [189]. Moreover, the deficiency in the number or function of Treg cells may also contribute to CF lung disease. Studies have shown that compared with healthy controls, patients with CF lung disease have significantly lower Treg cell counts in their peripheral blood, with a reduced proportion of Treg cells in CD4+ cells [190]. In the context of Pseudomonas aeruginosa infection, CFTR and Pseudomonas aeruginosa act as major mediators of Treg cell functional impairment in patients with CF lung disease [188]. Interestingly, Treg cells in patients with Type 1 diabetes are dysfunctional, leading to the secretion of the pro-inflammatory factor IL-17 [191].
The positive correlation between the number of Treg cells and lung function in CF patients suggests that restoring T-cell responses can be achieved by increasing Treg cell numbers and/or enhancing their function. One approach is by providing chemically modified Foxp3 mRNA or enhancing indoleamine 2,3-dioxygenase (IDO) activity, as demonstrated in a recent study showing the association between IDO dysfunction and Treg cell dysregulation in Cftr−/− mice [188]. This presents a promising treatment strategy for CF lung disease. Another potential therapeutic approach involves direct modulation of T-cell responses to counteract the excessive inflammatory response observed in CF [192]. As CF is a systemic disease that affects the absorption of fat-soluble vitamins A and D, treating CF patients with 1,25(OH)2-vitamin D3 has shown positive effects. This treatment leads to increased IL-10 levels in Treg cells, resulting in elevated expression of TGF-β1 transcripts and proportion of TGF-β on CD4+CD25+ cells, and decreased Th2 responses in CD4+ T cells [193]. Furthermore, highly effective CFTR modulators have been developed to improve the quality of life for patients with multiple CFTR mutations. Treatment with Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) has been shown to down-regulate blood levels of pro-inflammatory factors IL-6, IL-8 and IL-17A and up-regulate the percentages of Treg cells in CF patients [186]. ELX/TEZ/IVA, the first approved triple CFTR modulator therapy, effectively addresses inflammation and immune dysregulation caused by CFTR deficiency [194]. These findings provide hope for more effective and targeted therapies in managing CF lung disease.
Lung cancer
Based on their histological features, lung cancer can be categorized into two histological types: NSCLC and small cell lung cancer (SCLC). NSCLC is the most prevalent form, constituting approximately 80% of lung cancer cases. NSCLC further subdivides into adenocarcinoma, large cell carcinoma and squamous cell carcinoma [195,196]. While Treg cells play a role in suppressing inflammation in immune responses, they also possess dual functions of aiding cancer cells in evading immune surveillance in cancer immunity and promoting both tissue repair and immune suppression [197]. Treg cells were found to hinder effective anti-tumor immunity in patients with tumors, and contribute to the development of diverse malignancies, including NSCLC [198].
In typical physiological conditions, effector T cells engage with activated APCs, presenting processed antigenic peptides through MHC molecules. Subsequently, neighboring T cells interact with the antigen/MHC complex via the TCR, leading to additional stimulatory signals, including the involvement of CD28 proteins on T cells in conjunction with B7 proteins found on the surface of APCs. This heightened interaction enhances T-cell activation and promotes an effective immune response [199]. However, as the immune response wanes, the expression of molecules such as CTLA-4 and PD-1 on the surface of CD8+ T cells increases. These cooperative inhibitory molecules interact with ligands in the tumor microenvironment (TME), ultimately inducing T-cell exhaustion [196]. Treg cells that express TIM-3 exhibit heightened activation and greater immunosuppressive activity compared with TIM-3-negative Treg cells. Consequently, the co-expression of TIM-3 and PD-1 on Treg cells serves as a marker for the depletion of CD8+ T cells in human tumors [200].
Treg cells employ various mechanisms to exert their role of maintaining immune self-tolerance and suppressing the activation of effector T cells. One such mechanism is Treg cells homing into tumour tissues, guided by the gradient of CCR4 ligands such as CCL17 and CCL22. Once there, they release immunosuppressive cytokines such as TGF-β and IL-10, which serve to facilitate immune evasion by tumors through the inhibition of effector T-cell activation and function [201,202]. Another important mechanism involves the interaction of CTLA-4 produced by Treg cells with the B7 protein on the surface of APCs. This interaction can impair the immune system’s ability to mount an effective anti-tumor response. CTLA-4 expressed by effector Treg cells binds to CD80 or CD86 molecules on APCs with higher affinity than CD28 molecules, resulting in a more potent inhibitory signal to APCs and rendering effector T cells unresponsive. This interaction can be blocked by anti-CTLA-4 antibodies, thereby restoring the anti-tumor immune function of immune cells [17,199,203]. Third, Treg cells can directly inhibit the anti-tumor activity of immune cells by interacting with NK cells and DCs. The high-affinity receptor CD25 on the surface of effector Treg cells enables them to deprive IL-2, inhibiting the activation and tumor-clearing function of NK cells and CD8+ T cells [204]. In the context of lymph nodes draining from lung tumours, Treg cells prevent the activation of T cells and their recognition of tumor antigen proteins presented by DCs. Treg cells achieve this by hindering the expression of stimulatory proteins on the surface of DCs [205,206]. Within mediastinal lymph nodes, this inhibition occurs in a spatially coordinated manner and relies on MHC II interactions between Treg cells and type 1 DCs [207]. Furthermore, Treg cells within tumor-associated tertiary lymphoid structures (TA-TLS), acting as potent T-cell suppressors, often express high levels of the enzyme CD39. Together with CD73, this enzyme generates adenosine, which further promotes tumour growth and immune evasion [208,209].
The TME plays a pivotal role in the initiation and progression of tumors, and the development of tumors relies on an appropriate TME. Interactions between tumor cells and the surrounding stroma within the TME are a significant source of intratumoural heterogeneity [210,211]. In NSCLC, the TME of patients with lymph node metastasis or advanced disease stages exhibits elevated production of TGF-β. This promotes the infiltration of effector Treg cells into the TME and increases the expression of inhibitory molecules such as GITR and PD-L1 on the surface of APCs, thereby enhancing the immunosuppressive function of Treg cells. Moreover, within the TME, Treg cells are recruited by CCL20 produced by tumor cells, which bind to the receptor CCR6. Additionally, heightened glycolytic activity in cancer cells provides ample fatty acids and lactic acid, fueling the proliferation of Treg cells [17,212,213]. In the mediastinal lymph nodes of cancer patients, increased levels of IFN-γ promote the transformation of Treg cells into Th1-like effector Treg cells. This transformation increases their interaction with type 1DCs, ultimately inhibiting the activity of CD8+ T cells. The chemokine CXCL13 is predominantly expressed in advanced CD8+ T cells and Treg cells. When co-expressed with its receptor CXCR5, it can enhance tumor evasion and invasion [210,214]. It was found that vascular endothelial growth factor (VEGF), a growth factor involved in angiogenesis, contributes to immunosuppression within the TME. VEGF-A exerts its immunosuppressive effect by increasing the number of immature dendritic cells, promoting Treg cell recruitment through VEGFR-2 binding, and enhancing Treg cell infiltration in the TME by binding to NRP-1 [198]. Additionally, FOXC1, a transcription factor, promotes the expression of LINC00301 in NSCLC. LINC00301 promotes TGF-β1 secretion in lung tumors to attract Treg cells, which will further result in immunosuppression via facilitating the inhibition of CD8+ T cell differentiation and HIF-1α up-regulation [215].
Lung cancer immunotherapy presents significant challenges, with tumor cells having the ability to evade immune surveillance and dampen overall immune responses through the secretion of immunosuppressive cytokines by Treg cells and reduced expression of MHC molecules [216]. However, there are potential avenues for intervention in lung cancer treatment. Anti-CCR4 antibodies can be employed to hinder the recruitment of Treg cells, anti-CD25 antibodies like docetaxel and cyclophosphamide can be used to reduce the number of Treg cells, and anti-TGF-β drugs can be employed to counteract the immunosuppressive effects of cytokines secreted by Treg cells within the TME [199,217,218]. Dasatinib, an antitumor medication, has been shown in clinical settings to inhibit the differentiation and function of CD4+CD25+ Treg cells in peripheral blood, leading to a reduction in the inhibition of CD8+ T cells [219]. Epacadostat, an inhibitor of the immunomodulatory molecule indoleamine 2,3-dioxygenase-1, when combined with dasatinib in the treatment of NSCLC, reduces Treg cells in the lung TME and reactivates immunomodulatory mechanisms while suppressing tumor immunosuppression caused by Treg cells [220]. The administration of anti-PD-1 antibodies boosts effector T-cell activation within tumors and prevents Treg cell differentiation. In NSCLC, the combination of anti-PD-1 and dasatinib has been discovered to completely inhibit the proliferation and differentiation of tumor-infiltrating Treg cells, reduce Treg cell levels, induce tumor regression, and elicit immune memory [221].
Swelling and inflammation in the main passages (bronchial tubes) that carry air to the lungs (bronchitis)
It has been discovered that acute bronchitis, an inflammation of the bronchi in the lungs, is associated with an imbalance in the Th17/Treg ratio in children. This imbalance is indicated by an increase in serum levels of IL-17 and IL-22 cytokines, as well as a decrease in serum levels of IL-10 and TGF-β. This suggests that the Th17/Treg ratio plays a role in the development of acute bronchitis [222,223]. Dysfunctional Treg cells are a significant factor in severe viral bronchiolitis in infants, as defective Treg cells produce lower levels of CD39 compared with healthy Treg cells, and healthy Treg cells are capable of blocking allergen-induced HMGB1 translocation [224]. Respiratory syncytial virus (RSV), a common respiratory virus, is a leading cause of bronchitis and pneumonia in infants and the elderly [225,226]. Infants infected with RSV show decreased levels of activated Treg cells, as well as significantly reduced levels of alarmin IL-33 and increased levels of Th1, Th2, and pro-inflammatory cytokines [227]. Studies have indicated that following RSV infection, there is an excessive secretion of IL-8, resulting in increased levels of Th17-related factors, including IL-17, IL-23 and RORγt. However, there is minimal alteration in the Treg subpopulation, and the levels of IL-10, TGF-β, and FOXP3 decrease. This shift in the Th17/Treg ratio contributes to symptoms like bronchial inflammation, collapse and deformation, underscoring the impact of Treg cell numbers on the development of bronchial inflammation [228–231]. These findings highlight the significance of Treg cell quantity in the context of bronchial swelling and inflammation.
Furthermore, the research findings suggest that bronchiolitis obliterans syndrome (BOS) is linked to a reduction in Treg cell levels. These Treg cells employ a non-contact immunosuppressive mechanism by secreting immunosuppressive cytokines like TGF-β and IL-10, thereby promoting tolerance and resolving inflammation [232,233]. Additionally, BOS patients exhibit increased senescent CD28null T cells and natural killer T-like cells in their peripheral blood [234]. TGF-β exerts its inhibitory effects by suppressing the cytotoxic activity of NK cells and the expression of IFN-γ and T-bet. It also hinders macrophage phagocytic function by down-regulating receptors responsible for the phagocytosis of bacteria, senescent cells, and apoptotic cells. Furthermore, TGF-β dampens antigen presentation by macrophages, reducing the expression of MHC class II molecules and inflammatory cytokines, thereby achieving immunosuppression. Treg cells contribute to immune suppression through both direct cell-to-cell contact and the production of cytokines like galectin-1 and IL-35 [235,236]. They also inhibit the activation and functional roles of T cells generated in antigen-specific immune responses, thereby fostering immune tolerance to self-antigens and suppressing autoimmune reactions.
Lung infection (pneumonia)
Pneumonia stands as a significant global health concern, being one of the leading causes of morbidity and mortality worldwide and representing the top infectious cause of death [237]. It is an inflammatory lung disease primarily caused by pathogens, leading to the release of excessive inflammatory cytokines and rendering individuals susceptible to secondary infections for an extended period [238]. The development of pneumonia can disrupt the balance between Th17 cells and Treg cells, leading to acute inflammatory damage in the lungs, potentially progressing into severe conditions like ARDS or sepsis [239].
Treg cells can critically function as immunosuppressors in infectious diseases by producing abundant anti-inflammatory cytokines like TGF-β, IL-10 and IL-35 [240]. Additionally, Foxp3+CD39+ Treg cells can help eliminate inflammasome-mediated pro-inflammatory responses by hydrolyzing ATP [241]. Treg cells also contribute to the reduction of lung inflammation and promote lung repair by interacting with macrophages and alveolar epithelial cells [242]. Moreover, in healthy tissues, Treg cells are essential for maintaining immune homeostasis and are capable of assembling rapidly in response to viral attacks in the lungs to aid in tissue repair. They actively promote tissue regeneration following injury by producing repair mediators like EGF receptor ligand-deregulated proteins [243]. Overall, the multifaceted functions of Treg cells serve to regulate an overactive immune system and support the maintenance and repair of healthy tissues in various contexts.
Mycoplasma pneumoniae (MP) is a common extracellular pathogen responsible for up to 40% of community-acquired pneumonia (CAP) cases in children. MP attaches to ciliated epithelial cells of the respiratory mucosa, activating various signaling pathways and causing lung damage [244,245]. The severity of lung injury in patients with MP pneumonitis is associated with an imbalance between Treg and Th17 cells [246]. In MP pneumonia patients, Treg cells exert anti-inflammatory effects by inhibiting immune cells, such as CD4+ and CD8+ T cells, B cells, dendritic cells, and NK cells, through contact-dependent inhibition and the release of anti-inflammatory cytokines IL-10 and TGF-β1 [245,247].
Pseudomonas aeruginosa (PA) is known to cause chronic and acute lung infections in immunodeficient or immunosuppressed patients, leading to inflammation, elevated levels of pro-inflammatory cytokines and chemokines, and substantial neutrophil recruitment [240,248]. In mouse lung tissue, PA infection can result in increased inflammation, up-regulation of pro-inflammatory cytokines such as TNF-α, IL-17 and IL-6, and an amplified Th17 cell response, despite CAP being an infrequent outcome of PA infection [237,249]. However, the involvement of Treg cells in the host response against CAP has not been directly proven [250].
Respiratory syncytial virus (RSV) is a significant cause of viral pneumonia and bronchiolitis in children worldwide. RSV’s ability to evade normal immune memory underscores the critical role of effective T-cell responses in clearing RSV infections [251]. During RSV infection of the lungs, Foxp3+ Treg cells exert a critical role in maintaining immune homeostasis and can suppress inflammation in healthy individuals. In response to viral infection of the respiratory tract, Treg cells in the lung produce the anti-inflammatory cytokine IL-10 through the transcriptional regulator B lymphocyte-inducible maturation protein 1 (BLIMP1), thus regulating excessive immune responses [252]. Studies investigating respiratory syncytial virus models have shown that the depletion of Treg cells can lead to delayed migration of CD8+ T cell subpopulations [253]. Similarly, in studies using Influenza A virus models in mice, infected individuals exhibit a marked induction of CD4+Foxp3+ T cells, but no significant effects on mortality, viral clearance, or lung tissue cellularity have been demonstrated [253,254].
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus Severe Acute Respiratory Syndrome Coronavirus 2, is characterized by a crucial role of cytokine storms in its pathogenesis. An excessive immune response fails to clear the virus but instead exacerbates respiratory distress and damages the heart and other organs [255–257]. Normally, Treg cells act as the first line of defense against viral infections and uncontrolled inflammation, inhibiting CD4+ and CD8+ T cell responses and reducing infiltration of neutrophils, NK cells and eosinophils [258]. However, severe COVID-19 patients exhibit increased levels of cytokines like IL-1β, IL-6, IL-7, IL-10 and IL-17, as well as the chemokine IL-8, accompanied by deficiencies in the regulation of pro-inflammatory responses by Treg cells due to reduced Treg cell numbers and decreased Treg/Th17 cell ratios [259,260]. It was demonstrated that the inhibition of Treg cell differentiation by STAT-3-mediated signaling further exacerbates cytokine storms and promotes immunopathological responses [261]. Therefore, Treg cells act as crucial modulators of immune responses during pneumonia, ensuring an appropriate balance between inflammation and resolution, ultimately supporting effective defense and recovery.
Blocked lung artery (pulmonary embolus)
Deep vein thrombosis refers to the condition in which blood clots develop in the deep veins of the body [262]. This condition may lead to serious complications such as pulmonary embolism, post-thrombotic syndrome, and chronic thromboembolic pulmonary hypertension [263]. Treg cells have been found to accumulate in thrombosed veins, where they form the matricellular acid- and cysteine-rich protein SPARC (secreted protein acidic and rich in cysteine) within the clot, regulate matrix metalloproteinase activity, recruit monocytes and respond to TGF-β cytokine to facilitate clot regression [264]. The study has delved into the phenotype of Treg cells within thrombi and the walls of thrombosed veins, revealing that Treg cells in the vicinity became activated upon recruitment. This activation was evident through the significant up-regulation of CD69 expression in Treg cells, with levels steadily increasing as the thrombus aged. The specific mechanisms of action of Treg cells in pulmonary embolism have not yet been thoroughly studied, and research in this area is ongoing. However, Treg cells are generally known for their immunosuppressive and anti-inflammatory functions, which might potentially help prevent excessive inflammation and immune-mediated damage to lung tissue through immune modulation. Treg cells are potentially active in preventing excessive inflammation and immune-related damage to lung tissue. They achieve this regulation of immunity by producing cytokines like IL-10 and TGF-β to dampen the inflammatory response, thereby potentially reducing inflammation linked with pulmonary embolism [265,266]. Furthermore, Treg cells may contribute to tissue repair and regeneration [267,268] and could play a role in repairing damaged lung tissue in cases of pulmonary embolism. Additionally, Treg cells have the capacity to curtail excessive immune activation [19,269]. In the pulmonary vascular system, pulmonary embolism might trigger an immune response due to the presence of a thrombus or embolus. Treg cells can help counteract an overload of the immune response brought about by pulmonary embolism. It’s crucial to emphasize that the precise mechanisms through which Treg cells function in pulmonary embolism may differ among individuals and according to the unique circumstances of the disease. Additional research is required to gain a comprehensive understanding of Treg cell involvement in pulmonary embolism and to explore their potential application in therapeutic strategies.
The role of Treg cells following lung transplantation
Although lung transplantation is currently the only treatment for end-stage lung disease [270], the survival rate after lung transplantation is significantly lower compared with that of other organs. This is due to the high immunogenicity of the lung and continuous exposure to the external environment [271]. In the early 1980s, the technical obstacles of lung transplantation were overcome. However, the main challenge in achieving survival in lung transplant patients is chronic rejection known as bronchiolitis obliterans (BO) [272]. Approximately 50% of the patients who survive more than three months post-transplantation are expected to develop this complication. BO is a chronic rejection response that can occur one to several years after lung transplantation, leading to progressive fibrosis of the small airways and a gradual reduction in lung ventilation [273]. Following lung transplantation, chronic rejection occurs at the location where type V collagen (COLV) is exposed [274]. COLV is a small fibrous collagen that is expressed by small airway epithelial cells and is located in the peribronchial and perivascular tissues of the lung [275]. After lung transplantation, matrix metalloproteinases can cleave collagen, which exposes COLV and releases its antigen fragment. This results in increased levels of COLV in patients with BO after transplantation. This antigen fragment plays a role in the development of BO. In addition to COLV, K-α1-tubulin also contributes to autoimmune-mediated BO development and chronic rejection [276]. IL-17 is associated with the development of post-transplant BO, and the autoantibodies to COLV and K-α1 tubulin in lung transplant recipients are IL-17 dependent. In mouse studies, the inhibition of IL-17 resulted in decreased levels of autoantibodies to COLV and K-α1 tubulin [276,277]. In addition, there was a significant increase in the level of IL-17 in the bronchial lavage fluid of lung transplant patients, suggesting that IL-17 may have a key role in the development of BO following lung transplantation [278].
The majority of evidence suggests that the adaptive immune response in transplanted lung tissue can result in the primary risk factor for BO after lung transplantation, which is the damage caused by autoimmune mediators [276]. One of the key features of inflammatory diseases is an imbalance in the Th1/Th17 response, often accompanied by a reduction and/or alteration in regulatory Treg cells [169,279]. Recent research has demonstrated that maintaining a balance between Treg cells and Th17 cells is a potential strategy for preventing autoimmune diseases and inhibiting the development of BO after transplantation (Figure 4).
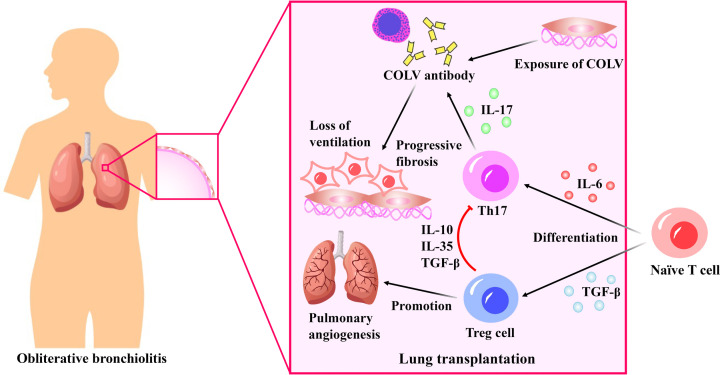
Figure 4. The role of regulatory T (Treg) cells following lung transplantation.
After lung transplantation, type V collagen (COLV) exposure leads to the production of antibodies that lead to pulmonary fibrosis and breathing difficulties. COLV antibodies are dependent on interleukin (IL)-17 produced by Th17 cells. naïve T cells will differentiate into Th17 cells in the presence of IL-6, which will further promote COLV antibody production. While TGF-β can promote the transformation of naïve T cells into Treg cells, which can produce cytokines such as IL-10, IL-35, and transforming growth factor-beta (TGF-β) to inhibit Th17 activity. In addition, Treg cells can promote pulmonary angiogenesis.
The differentiation of Treg cells and Th17 cells is dependent on the presence of TGF-β. When present in low concentrations along with IL-6, TGF-β and IL-6 induce the production of transcription factors RORγt and RORα in T cells, which activate the initial T cells to differentiate into pro-inflammatory Th17 cells. At the same time, they inhibit the expression of Foxp3, the transcription factor of Treg cells. However, in high concentrations, TGF-β can up-regulate the expression of Foxp3, resulting in the differentiation of anti-inflammatory Treg cells [280]. Moreover, TGF-β expression in Treg cells helps maintain their anti-inflammatory function following migration from the thymus [281]. Disrupting the balance of Treg/Th17 cells can cause a range of inflammatory reactions that harm the body [169]. Romagnani et al. have shown that Th17 and Treg cells come from the same precursor, and inhibiting the production of Th17 cells can promote the development of Treg cells, thus effectively preventing the occurrence of autoimmune diseases by the secretion of IL-10, IL-35 and TGF-β [282].
It was shown that lung transplant patients with BO display a systemic activation of T cells, including Th1, Th2, and Treg cells, in comparison with healthy individuals. However, in comparison to those developing BO, patients with stable BO exhibit a higher presence of Treg and Th2 cells in their blood, bronchi and alveolar lavage fluids [232]. Moreover, Treg cells have an important role in regulating pulmonary angiogenesis. D’Alessio et al. showed that pulmonary angiogenesis was significantly reduced in mice with depleted Treg cells, but the amount of angiogenesis was almost fully restored after the adoptive transfer of homologous Treg cells [283]. This provides further evidence of the involvement of Treg cells in preventing the onset of BO after lung transplantation. Once autoimmunity and alloimmunity start, they become difficult to suppress, and the absence or damage of Treg cells after transplantation leads to insufficient numbers of Treg cells in the body, which cannot exert immunosuppressive effects [284]. Meloni et al. have revealed that patients with BO have lower levels of Treg cells in their blood compared with healthy lung transplant patients, indicating defects in the Treg cell population [285]. Moreover, infection, acute rejection, or other damage can impair the function of Treg cells or convert them into other effector cells. Some studies have shown that in vitro-induced Treg cells rapidly lose Foxp3 expression activity after transfer to living organisms[286]. Currently, there is only one clinical trial utilizing Treg cells in the treatment of post-lung transplant rejection registered in ClinicalTrials.gov (NCT00340951), and the outcomes of this research have not yet been disclosed. Hence, utilizing Treg cells for treating BO after lung transplantation is still a major focus of research.
Conclusion and further directions
Lung diseases can have multiple causes and result in high morbidity and mortality rates. Lung transplantation is currently the only viable treatment option for end-stage lung disease. However, the development of BO caused by chronic rejection after transplantation is the primary limitation of this procedure. Recent research on Treg cells has highlighted their potential significance in treating lung diseases.
Currently, numerous studies have indicated the involvement of Treg cells and Th17 cells in various lung diseases, including asthma, ARDS, COPD, lung cancer and pulmonary infectious diseases, etc. Treg cells and Th17 cells represent two opposing T cell subsets, with Th17 cells promoting inflammation and Treg cells playing a crucial role in maintaining self-tolerance. When the balance between Treg and Th17 cells is disrupted, inflammation may occur. Therefore, the balance of Treg and Th17 cells can be regulated by adjusting the expression of cytokines such as IL-17A, IL-6, IL-23, IL-33 and Foxp3 to achieve the goal of treating lung diseases. In the case of BO, the disease is caused by autoimmune-mediated injury caused by the adaptive immune response of the transplanted lung tissue. The balance between Treg and Th17 cells is critical in preventing autoimmune diseases and inhibiting the onset of BO after transplantation. As a result, the combined action of TGF-β and related cytokines, such as IL-2, IL-6 and IL-21, can help regulate and maintain the balance of Treg and Th17 cells, thereby suppressing the occurrence of BO.
For example, studies have shown that asthma has Th2 (Th2 cells play a central role) and non-Th2 (Th17 and Th1 cells play an important role) phenotypes [287], Treg cells exhibit a significant inhibitory effect on Th cell responses, demonstrated by Treg cells with the TIGIT phenotype selectively inhibiting Th1 and Th17 cell responses [288]. As a result, Treg cells play a crucial role in regulating conditions like asthma. Moreover, the balance between Th17 and Treg cells, represented by the Th17/Treg ratio, has a significant impact on the early onset of ARDS, making its regulation crucial for ARDS treatment [289]. Studies have indicated that transplanting Treg cells into ARDS model mice can effectively reduce pro-inflammatory cytokine levels in the alveoli [290]. Treg cells also play a vital role in ALI regression, as they can induce apoptosis of neutrophils, which helps reduce fibrocyte recruitment and ALI fibrosis [291,292]. In the context of COPD, Th17 cells produce pro-inflammatory factors like IL-17, while Treg cells release anti-inflammatory cytokines such as IL-10 and TGF-β, effectively controlling lung inflammation [293]. In NSCLC, plasma levels of IL-35 are higher in NSCLC patients compared with healthy individuals [294]. IL-35, secreted by Treg cells, exerts an immunosuppressive effect by inhibiting T-cell proliferation and function [295,296]. However, Treg cells also contribute to immune escape and tumour growth by inhibiting the host immune response through TGF-β and IL-35 [297,298]. Understanding the role of IL-35 and Treg cells in NSCLC is essential for gaining valuable insights into potential treatment approaches [299].
In terms of treatment, it was shown that Treg cells can be used in the clinical treatment of GVHD [300]. There are two primary sources of Treg cells: endogenous Treg cells in the patient’s body, which can be increased in quantity through induction, and donor-derived Treg cells, which are primarily suitable for preventing and inhibiting rejection after transplantation. Donor Treg cells can be extracted and amplified in vitro through selective procedures for use in patients (i.e., allogeneic use) [301]. However, this method does not apply to the treatment of autoimmune diseases.
Due to their immunomodulatory and anti-inflammatory characteristics, Treg cells play a pivotal role in managing lung diseases. Augmenting Treg cell numbers or enhancing their function can effectively inhibit inflammation and slow down the progression of conditions such as autoimmune lung diseases, asthma, ARDS, chronic obstructive pulmonary disease, cystic fibrosis, pneumonia, etc [170,182,186,237,302–304]. Conversely, for certain lung diseases like lung cancer, restraining the proliferation and differentiation of Treg cells is essential to reduce tumour burden and increase anti-tumor immune response, thus achieving therapeutic outcomes [220,305,306]. Additionally, by understanding the extent of fibrosis progression, managing the balance of Treg cells within the body, and strategically timing their deployment, it becomes possible to exert finer control over Treg cells, offering potential benefits in the management of IPF [174]. Studies have suggested that harnessing Treg cells for the treatment of lung diseases and lung transplantation represents a breakthrough in enhancing therapy and carries significant research implications. However, substantial challenges remain to be addressed, such as the need for large-scale in vitro cultivation, the acquisition of antigen-specific Treg cells, balancing quantity and function, and producing substantial numbers of functional Treg cells. Currently, there are three main methods. The first is in vivo expansion, achieved by promoting the growth of Treg cells. For instance, the use of IL-2 and TNF superfamily member 15-immunoglobulin combination or the drug RGI-2001 can increase the number of Treg cells for in vivo expansion [307–309]. The second method involves in vitro expansion. Various techniques are currently used for in vitro Treg cell amplification, such as cord blood-based amplification and rapamycin-based amplification [310–313]. The third method to increase the number of Treg cells is genetic engineering. The expanded polyclonal Treg cells can be genetically modified to synthesize chimeric antigen receptors or artificial cell receptors, resulting in a large number of antigen-specific Treg cells [314–316].
It was shown that the delicate balance in the human body enables minor environmental changes to trigger Treg cell responses, and modifications in the cytokine environment can impede Treg cell reconstruction, altering their efficacy [317]. Thus, in the large-scale expansion of Treg cells, the culture environment necessitates pre-treatment. The common approach involves incorporating relevant cytokines, such as IL-2 and IL-15, into the growth medium, which can sustain Treg cell stability and boost their expansion. Treatments involving rapamycin, TGF-β, and trans-retinoic acid can curb the growth of contaminated cells, resulting in higher Treg cell purity [318–320]. Additionally, donor-derived Treg cells are susceptible to interference from various endogenous factors in the treatment of rejection after transplantation, which can affect the reconstruction of these cells. This effect can be regulated through a low dose of IL-2, thereby sustaining Treg cell homeostasis [321]. In summary, pre-treatment of the culture environment is essential before in vitro induction to ensure smooth induction of cells without damaging Treg cells.
In conclusion, research on the Treg cells and their clinical application is rapidly developing. Numerous studies suggest that the utilization of Treg cells in the treatment of lung diseases and lung transplantation is a breakthrough in improving treatment and has great research significance. However, there are still major challenges to overcome. How to conduct large-scale in vitro culture, obtain antigen-specific Treg cells, reconcile quantity and function, and produce a large number of functional Treg cells? These issues require further investigation and improvement. As a result, utilizing Treg cells for the clinical treatment of lung diseases and lung transplantation is a hot research area with vast prospects and far-reaching significance.
Abbreviations
- ALI
acute lung injury
- APC
antigen-presenting cell
- ARDS
acute respiratory distress syndrome
- BLIMP1
B lymphocyte-inducible maturation protein 1
- BO
bronchiolitis obliterans
- BOS
bronchiolitis obliterans syndrome
- CAP
community-acquired pneumonia
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CNS
central nervous system
- COLV
Type V collagen
- COPD
chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CXCR
C-X-C motif chemokine receptor
- DC
dendritic cell
- ELX/TEZ/IVA
elexacaftor/tezacaftor/ivacaftor
- GITR
glucocorticoid-induced tumor necrosis factor receptor
- GITRL
glucocorticoid-induced tumour necrosis factor receptor ligand
- GQD
Ge Gen Scutellaria Tang
- HIF-1α
hypoxia-inducible factor 1α
- HO-1
heme oxygenase 1
- ICOS
inducible costimulator
- IDO
indoleamine 2,3-dioxygenase
- IFN-γ
interferon-γ
- IL
interleukin
- ILC2s
Type 2 innate lymphocytes
- IPF
idiopathic pulmonary fibrosis
- KGF
keratinocyte growth factor
- LAG-3
lymphocyte activation gene 3
- MHC-I
major histocompatibility complex class I
- MP
mycoplasma pneumoniae
- mTORC1
mammalian target of rapamycin complex 1
- NAC
N-acetylcysteine
- NK
natural killer
- NRP-1
Neuropilin-1
- NSCLC
non-small cell lung cancer
- nTreg cell
naturally developed Treg cell
- PA
Pseudomonas aeruginosa
- PBMC
peripheral blood mononuclear cell
- PD-1
programmed cell death 1
- pTreg cell
peripheral original peripherally-derived Treg cell
- RSV
respiratory syncytial virus
- SCLC
small cell lung cancer
- SPP1
secretory phosphoprotein 1
- STAT
signal transducer and activator of transcription
- TCR
T-cell receptor
- Tfh
T follicular helper
- Tfr
follicular Treg
- TGF-β
transforming growth factor-beta
- Th1
Type 1 T helper
- Th17
Type 17 T helper
- Th2
Type 2 T helper
- TIM
T-cell immunoglobulin and mucin domain
- TME
tumor microenvironment
- Tr1 cell
Type 1 regulatory T cell
- Treg
Regulatory T
- tTreg cell
thymus-derived regulatory cell
- VEGF
vascular endothelial growth factor
- XBCQ
Xuanbai Chengqi decoction
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by grants from NSFC [grant number 81900919]; the Natural Science Foundation of Guangdong Province [grant numbers 2022A1515011817 and 2017A030313105]; the Characteristic Innovation Project of Ordinary Colleges and Universities in Guangdong Province [grant numbers 2023KTSCX097]; the Special Fund for Science and Technology Innovation Strategy of Guangdong Province [grant number pdjh2023b0388]; and the National College Student Innovation and Entrepreneurship Training Program [grant number 202314278012].
CRediT Author Contribution
Peizhen Lao: Writing—original draft. Jingyi Chen: Writing—original draft. Longqian Tang: Writing—original draft. Jiwen Zhang: Writing—original draft. Yuxi Chen: Writing—original draft. Yuyin Fang: Writing—original draft. Xingliang Fan: Conceptualization, Supervision, Funding acquisition, Writing—review & editing.
References
- 1.Liao S.X., Sun P.P., Gu Y.H., Rao X.M., Zhang L.Y. and Ou-Yang Y. (2019) Autophagy and pulmonary disease. Ther. Adv. Respir. Dis. 13, 1753466619890538 10.1177/1753466619890538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan A., Agarwal S., Clauss M., Britt N.S. and Dhillon N.K. (2020) Extracellular vesicles: novel communicators in lung diseases. Respir. Res. 21, 175 10.1186/s12931-020-01423-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pramanik S., Mohanto S., Manne R., Rajendran R.R., Deepak A., Edapully S.J.et al. (2021) Nanoparticle-based drug delivery system: the magic bullet for the treatment of chronic pulmonary diseases. Mol. Pharm. 18, 3671–3718 10.1021/acs.molpharmaceut.1c00491 [DOI] [PubMed] [Google Scholar]
- 4.Boe D.M., Boule L.A. and Kovacs E.J. (2017) Innate immune responses in the ageing lung. Clin. Exp. Immunol. 187, 16–25 10.1111/cei.12881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer K.C. (2004) Lung infections and aging. Ageing Res. Rev. 3, 55–67 10.1016/j.arr.2003.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondelkova K., Vokurkova D., Krejsek J., Borska L., Fiala Z. and Ctirad A. (2010) Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica. (Hradec. Kralove) 53, 73–77 10.14712/18059694.2016.63 [DOI] [PubMed] [Google Scholar]
- 7.Corthay A. (2009) How do regulatory T cells work? Scand. J. Immunol. 70, 326–336 10.1111/j.1365-3083.2009.02308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu M., Wang Y.M., Wang Y., Zhang G.Y., Zheng G., Yi S.et al. (2016) Regulatory T cells in kidney disease and transplantation. Kidney Int. 90, 502–514 10.1016/j.kint.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z.et al. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 10.1111/j.0105-2896.2006.00427.x [DOI] [PubMed] [Google Scholar]
- 10.Zhao H., Wu L., Yan G., Chen Y., Zhou M., Wu Y.et al. (2021) Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct. Target Ther. 6, 263 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldman A.D., Fritz J.M. and Lenardo M.J. (2020) A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh R., Alape D., de Lima A., Ascanio J., Majid A. and Gangadharan S.P. (2019) Regulatory T cells in respiratory health and diseases. Pulm Med. 2019, 1907807 10.1155/2019/1907807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J. and Sun Y. (2020) Role of regulatory T cells in disturbed immune homeostasis in patients with chronic obstructive pulmonary disease. Front. Immunol. 11, 723 10.3389/fimmu.2020.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racanelli A.C., Kikkers S.A., Choi A.M.K. and Cloonan S.M. (2018) Autophagy and inflammation in chronic respiratory disease. Autophagy 14, 221–232 10.1080/15548627.2017.1389823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Geffen C., Deissler A., Quante M., Renz H., Hartl D. and Kolahian S. (2021) Regulatory immune cells in idiopathic pulmonary fibrosis: friends or foes? Front Immunol. 12, 663203 10.3389/fimmu.2021.663203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteside T.L. (2015) The role of regulatory T cells in cancer immunology. Immunotargets Ther. 4, 159–171 10.2147/ITT.S55415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J., Bi G., Shan G., Jin X., Bian Y. and Wang Q. (2022) Tumor-associated regulatory T cells in non-small-cell lung cancer: current advances and future perspectives. J. Immunol. Res. 2022, 4355386 10.1155/2022/4355386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proto J.D., Doran A.C., Gusarova G., Yurdagul A. Jr, Sozen E., Subramanian M.et al. (2018) Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity 49, 666e6–677e6 10.1016/j.immuni.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S., Yamaguchi T., Nomura T. and Ono M. (2008) Regulatory T cells and immune tolerance. Cell 133, 775–787 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 20.Hill J.A., Feuerer M., Tash K., Haxhinasto S., Perez J., Melamed R.et al. (2007) Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 27, 786–800 10.1016/j.immuni.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S., Wing K. and Miyara M. (2007) Regulatory T cells - a brief history and perspective. Eur. J. Immunol. 37, S116–S123 10.1002/eji.200737593 [DOI] [PubMed] [Google Scholar]
- 22.Bhartiya D., Singh P., Sharma D. and Kaushik A. (2022) Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues. Stem Cell Rev. Rep. 18, 1718–1727 10.1007/s12015-021-10243-6 [DOI] [PubMed] [Google Scholar]
- 23.Morikawa H. and Sakaguchi S. (2014) Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 259, 192–205 10.1111/imr.12174 [DOI] [PubMed] [Google Scholar]
- 24.Thornton A.M. and Shevach E.M. (2019) Helios: still behind the clouds. Immunology 158, 161–170 10.1111/imm.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudensky A.Y. (2011) Regulatory T cells and Foxp3. Immunol. Rev. 241, 260–268 10.1111/j.1600-065X.2011.01018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori S., Nomura T. and Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 27.Shevach E.M. and Thornton A.M. (2014) tTregs, pTregs, and iTregs: similarities and differences. Immunol. Rev. 259, 88–102 10.1111/imr.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wing J.B., Tanaka A. and Sakaguchi S. (2019) Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50, 302–316 10.1016/j.immuni.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 29.Nishizuka Y. and Sakakura T. (1969) Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 166, 753–755 10.1126/science.166.3906.753 [DOI] [PubMed] [Google Scholar]
- 30.Piccirillo C.A., d'Hennezel E., Sgouroudis E. and Yurchenko E. (2008) CD4+Foxp3+ regulatory T cells in the control of autoimmunity: in vivo veritas. Curr. Opin. Immunol. 20, 655–662 10.1016/j.coi.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N.et al. (2003) Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M.O., Sanjabi S. and Flavell R.A. (2006) Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 10.1016/j.immuni.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 33.Laurence A., Tato C.M., Davidson T.S., Kanno Y., Chen Z., Yao Z.et al. (2007) Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K. and Ohkura N. (2020) Regulatory T cells and human disease. Annu. Rev. Immunol. 38, 541–566 10.1146/annurev-immunol-042718-041717 [DOI] [PubMed] [Google Scholar]
- 35.Lee W. and Lee G.R. (2018) Transcriptional regulation and development of regulatory T cells. Exp. Mol. Med. 50, e456 10.1038/emm.2017.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvaraj R.K. and Geiger T.L. (2007) A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J. Immunol. 178, 7667–7677 10.4049/jimmunol.178.12.7667 [DOI] [PubMed] [Google Scholar]
- 37.Cosovanu C. and Neumann C. (2020) The many functions of Foxp3+ regulatory T cells in the intestine. Front. Immunol. 11, 600973 10.3389/fimmu.2020.600973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider-Schaulies J. and Beyersdorf N. (2018) CD4+ Foxp3+ regulatory T cell-mediated immunomodulation by anti-depressants inhibiting acid sphingomyelinase. Biol. Chem. 399, 1175–1182 10.1515/hsz-2018-0159 [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M.et al. (1998) Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969–1980 10.1093/intimm/10.12.1969 [DOI] [PubMed] [Google Scholar]
- 40.Floess S., Freyer J., Siewert C., Baron U., Olek S., Polansky J.et al. (2007) Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 5, e38 10.1371/journal.pbio.0050038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X. and Zhu J. (2020) CD4 T helper cell subsets and related human immunological disorders. Int. J. Mol. Sci. 21, 8011 10.3390/ijms21218011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi S. (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6, 345–352 10.1038/ni1178 [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S., Powrie F. and Ransohoff R.M. (2012) Re-establishing immunological self-tolerance in autoimmune disease. Nat. Med. 18, 54–58 10.1038/nm.2622 [DOI] [PubMed] [Google Scholar]
- 44.Szurek E., Cebula A., Wojciech L., Pietrzak M., Rempala G., Kisielow P.et al. (2015) Differences in Expression Level of Helios and Neuropilin-1 Do Not Distinguish Thymus-Derived from Extrathymically-Induced CD4+Foxp3+ Regulatory T Cells. PloS ONE 10, e0141161 10.1371/journal.pone.0141161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiPaolo R.J. and Shevach E.M. (2009) CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur. J. Immunol. 39, 234–240 10.1002/eji.200838772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yongjun C., Nan Q., Yumeng S., Xiaowen J. and Weibo W. (2021) Dioscin alleviates hashimoto's thyroiditis by regulating the SUMOylation of IRF4 to promote CD4(+)CD25(+)Foxp3(+) treg cell differentiation. Autoimmunity 54, 51–59 10.1080/08916934.2020.1855428 [DOI] [PubMed] [Google Scholar]
- 47.Liang C.L., Lu W., Qiu F., Li D., Liu H., Zheng F.et al. (2021) Paeoniflorin ameliorates murine lupus nephritis by increasing CD4(+)Foxp3(+) Treg cells via enhancing mTNFalpha-TNFR2 pathway. Biochem. Pharmacol. 185, 114434 10.1016/j.bcp.2021.114434 [DOI] [PubMed] [Google Scholar]
- 48.Wang R. and Huang K. (2020) CCL11 increases the proportion of CD4+CD25+Foxp3+ Treg cells and the production of IL-2 and TGF-beta by CD4+ T cells via the STAT5 signaling pathway. Mol. Med. Rep. 21, 2522–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J., Lu Y., Wu W. and Feng Y. (2021) Taurine promotes the production of CD4(+)CD25(+)FOXP3(+) Treg cells through regulating IL-35/STAT1 pathway in a mouse allergic rhinitis model. Allergy Asthma Clin. Immunol. 17, 59 10.1186/s13223-021-00562-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bayati F., Mohammadi M., Valadi M., Jamshidi S., Foma A.M. and Sharif-Paghaleh E. (2021) The therapeutic potential of regulatory T cells: challenges and opportunities. Front. Immunol. 11, 10.3389/fimmu.2020.585819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.d'Hennezel E. and Piccirillo C.A. (2011) Analysis of human FOXP3+ Treg cells phenotype and function. Methods Mol. Biol. 707, 199–218 10.1007/978-1-61737-979-6_13 [DOI] [PubMed] [Google Scholar]
- 52.Palomares O., Martín-Fontecha M., Lauener R., Traidl-Hoffmann C., Cavkaytar O., Akdis M.et al. (2014) Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun. 15, 511–520 10.1038/gene.2014.45 [DOI] [PubMed] [Google Scholar]
- 53.Arce-Sillas A., Álvarez-Luquín D.D., Tamaya-Domínguez B., Gomez-Fuentes S., Trejo-García A., Melo-Salas M.et al. (2016) Regulatory T cells: molecular actions on effector cells in immune regulation. J. Immunol. Res. 2016, 1720827 10.1155/2016/1720827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batlle E. and Massagué J. (2019) Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 10.1016/j.immuni.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorelik L., Constant S. and Flavell R.A. (2002) Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195, 1499–1505 10.1084/jem.20012076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas D.A. and Massagué J. (2005) TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 8, 369–380 10.1016/j.ccr.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 57.Huang A., Cheng L., He M., Nie J., Wang J. and Jiang K. (2017) Interleukin-35 on B cell and T cell induction and regulation. J. Inflamm. 14, 16 10.1186/s12950-017-0164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J., Vuitton D.A., Muller N., Hemphill A., Spiliotis M., Blagosklonov O.et al. (2015) Deletion of Fibrinogen-like Protein 2 (FGL-2), a Novel CD4+ CD25+ Treg Effector Molecule, Leads to Improved Control of Echinococcus multilocularis Infection in Mice. PLoS Negl. Trop Dis. 9, e0003755 10.1371/journal.pntd.0003755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu J., Yan J., Rao G., Latha K., Overwijk W.W., Heimberger A.B.et al. (2016) The Duality of Fgl2 - secreted immune checkpoint regulator versus membrane-associated procoagulant: therapeutic potential and implications. Int. Rev. Immunol. 35, 325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H., Shalev I., Manuel J., He W., Leung E., Crookshank J.et al. (2008) The FGL2-FcgammaRIIB pathway: a novel mechanism leading to immunosuppression. Eur. J. Immunol. 38, 3114–3126 10.1002/eji.200838338 [DOI] [PubMed] [Google Scholar]
- 61.Beavis P.A., Stagg J., Darcy P.K. and Smyth M.J. (2012) CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 33, 231–237 10.1016/j.it.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 62.Osinska I., Popko K. and Demkow U. (2014) Perforin: an important player in immune response. Cent Eur. J. Immunol. 39, 109–115 10.5114/ceji.2014.42135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trapani J.A. (2001) Granzymes: a family of lymphocyte granule serine proteases. Genome Biol. 2, REVIEWS3014 10.1186/gb-2001-2-12-reviews3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metkar S.S., Wang B., Ebbs M.L., Kim J.H., Lee Y.J., Raja S.M.et al. (2003) Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J. Cell Biol. 160, 875–885 10.1083/jcb.200210158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao X., Cai S.F., Fehniger T.A., Song J., Collins L.I., Piwnica-Worms D.R.et al. (2007) Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27, 635–646 10.1016/j.immuni.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 66.Gondek D.C., Devries V., Nowak E.C., Lu L.F., Bennett K.A., Scott Z.A.et al. (2008) Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J. Immunol. 181, 4752–4760 10.4049/jimmunol.181.7.4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vignali D.A., Collison L.W. and Workman C.J. (2008) How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou T.Z., Qureshi O.S. and Sansom D.M. (2019) Measuring CTLA-4-dependent suppressive function in regulatory T cells. Methods Mol. Biol. 1899, 87–101 10.1007/978-1-4939-8938-6_7 [DOI] [PubMed] [Google Scholar]
- 69.Rowshanravan B., Halliday N. and Sansom D.M. (2018) CTLA-4: a moving target in immunotherapy. Blood 131, 58–67 10.1182/blood-2017-06-741033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goronzy J.J. and Weyand C.M. (2008) T-cell co-stimulatory pathways in autoimmunity. Arthritis Res. Ther. 10, S3 10.1186/ar2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedline R.H., Brown D.S., Nguyen H., Kornfeld H., Lee J., Zhang Y.et al. (2009) CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 206, 421–434 10.1084/jem.20081811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker L.S. (2013) Treg and CTLA-4: two intertwining pathways to immune tolerance. J. Autoimmun. 45, 49–57 10.1016/j.jaut.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy S., Bag A.K., Singh R.K., Talmadge J.E., Batra S.K. and Datta K. (2017) Multifaceted role of neuropilins in the immune system: potential targets for immunotherapy. Front Immunol. 8, 1228 10.3389/fimmu.2017.01228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarris M., Andersen K.G., Randow F., Mayr L. and Betz A.G. (2008) Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413 10.1016/j.immuni.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blaser C., Kaufmann M., Muller C., Zimmermann C., Wells V., Mallucci L.et al. (1998) Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur. J. Immunol. 28, 2311–2319 [DOI] [PubMed] [Google Scholar]
- 76.Perillo N.L., Pace K.E., Seilhamer J.J. and Baum L.G. (1995) Apoptosis of T cells mediated by galectin-1. Nature 378, 736–739 10.1038/378736a0 [DOI] [PubMed] [Google Scholar]
- 77.Camby I., Le Mercier M., Lefranc F. and Kiss R. (2006) Galectin-1: a small protein with major functions. Glycobiology 16, 137R–157R 10.1093/glycob/cwl025 [DOI] [PubMed] [Google Scholar]
- 78.Andrews L.P., Marciscano A.E., Drake C.G. and Vignali D.A. (2017) LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 276, 80–96 10.1111/imr.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang B., Workman C., Lee J., Chew C., Dale B.M., Colonna L.et al. (2008) Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 180, 5916–5926 10.4049/jimmunol.180.9.5916 [DOI] [PubMed] [Google Scholar]
- 80.Gautron A.S., Dominguez-Villar M., de Marcken M. and Hafler D.A. (2014) Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur. J. Immunol. 44, 2703–2711 10.1002/eji.201344392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu J.F., Wu L., Yang L.L., Deng W.W., Mao L., Wu H.et al. (2018) Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 37, 44 10.1186/s13046-018-0713-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu A., Zhu L., Altman N.H. and Malek T.R. (2009) A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 30, 204–217 10.1016/j.immuni.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ai T., Zhang J., Wang X., Zheng X., Qin X., Zhang Q.et al. (2018) DNA methylation profile is associated with the osteogenic potential of three distinct human odontogenic stem cells. Signal Transduct. Target Ther. 3, 1 10.1038/s41392-017-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thornton A.M., Donovan E.E., Piccirillo C.A. and Shevach E.M. (2004) Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J. Immunol. 172, 6519–6523 10.4049/jimmunol.172.11.6519 [DOI] [PubMed] [Google Scholar]
- 85.Fan M.Y., Low J.S., Tanimine N., Finn K.K., Priyadharshini B., Germana S.K.et al. (2018) Differential roles of IL-2 signaling in developing versus mature Tregs. Cell Rep. 25, 1204e4–1213e4 10.1016/j.celrep.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng G., Yu A., Dee M.J. and Malek T.R. (2013) IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J. Immunol. 190, 1567–1575 10.4049/jimmunol.1201218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gasteiger G. and Kastenmuller W. (2012) Foxp3+ Regulatory T-cells and IL-2: The Moirai of T-cell Fates? Front Immunol. 3, 179 10.3389/fimmu.2012.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson B.H. (2004) IL-2, regulatory T cells, and tolerance. J. Immunol. 172, 3983–3988 10.4049/jimmunol.172.7.3983 [DOI] [PubMed] [Google Scholar]
- 89.Pandiyan P., Zheng L., Ishihara S., Reed J. and Lenardo M.J. (2007) CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8, 1353–1362 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- 90.Dominguez-Villar M. and Hafler D.A. (2018) Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673 10.1038/s41590-018-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Munoz-Rojas A.R. and Mathis D. (2021) Tissue regulatory T cells: regulatory chameleons. Nat. Rev. Immunol. 21, 597–611 10.1038/s41577-021-00519-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panduro M., Benoist C. and Mathis D. (2016) Tissue Tregs. Annu. Rev. Immunol. 34, 609–633 10.1146/annurev-immunol-032712-095948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang T.T., Yuan J., Zhu Z.F., Zhang W.C., Xiao H., Xia N.et al. (2012) Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 107, 232 10.1007/s00395-011-0232-6 [DOI] [PubMed] [Google Scholar]
- 94.Weirather J., Hofmann U.D., Beyersdorf N., Ramos G.C., Vogel B., Frey A.et al. (2014) Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 115, 55–67 10.1161/CIRCRESAHA.115.303895 [DOI] [PubMed] [Google Scholar]
- 95.Gandolfo M.T., Jang H.R., Bagnasco S.M., Ko G.J., Agreda P., Satpute S.R.et al. (2009) Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 76, 717–729 10.1038/ki.2009.259 [DOI] [PubMed] [Google Scholar]
- 96.do Valle Duraes F., Lafont A., Beibel M., Martin K., Darribat K., Cuttat R.et al. (2020) Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight 5, e130651 10.1172/jci.insight.130651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bansal S.S., Ismahil M.A., Goel M., Zhou G., Rokosh G., Hamid T.et al. (2019) Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139, 206–221 10.1161/CIRCULATIONAHA.118.036065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ikeno Y., Ohara D., Takeuchi Y., Watanabe H., Kondoh G., Taura K.et al. (2020) Foxp3+ regulatory T cells inhibit CCl(4)-induced liver inflammation and fibrosis by regulating tissue cellular immunity. Front Immunol. 11, 584048 10.3389/fimmu.2020.584048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J., Tan J., Martino M.M. and Lui K.O. (2018) Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol. 9, 585 10.3389/fimmu.2018.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhuang R., Meng Q., Ma X., Shi S., Gong S., Liu J.et al. (2022) CD4(+)FoxP3(+)CD73(+) regulatory T cell promotes cardiac healing post-myocardial infarction. Theranostics 12, 2707–2721 10.7150/thno.68437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kinsey G.R., Sharma R., Huang L., Li L., Vergis A.L., Ye H.et al. (2009) Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 20, 1744–1753 10.1681/ASN.2008111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J., Li X., Yuan Q., Wang C., Xu L., Wei X.et al. (2021) The semaphorin 4A-neuropilin 1 axis alleviates kidney ischemia reperfusion injury by promoting the stability and function of regulatory T cells. Kidney Int. 100, 1268–1281 10.1016/j.kint.2021.08.023 [DOI] [PubMed] [Google Scholar]
- 103.Burzyn D., Kuswanto W., Kolodin D., Shadrach J.L., Cerletti M., Jang Y.et al. (2013) A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 10.1016/j.cell.2013.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lam A.J., MacDonald K.N., Pesenacker A.M., Juvet S.C., Morishita K.A., Bressler B.et al. (2019) Innate control of tissue-reparative human regulatory T cells. J. Immunol. 202, 2195–2209 10.4049/jimmunol.1801330 [DOI] [PubMed] [Google Scholar]
- 105.Zhang C., Li L., Feng K., Fan D., Xue W. and Lu J. (2017) ‘Repair’ Treg cells in tissue injury. Cell. Physiol. Biochem. 43, 2155–2169 10.1159/000484295 [DOI] [PubMed] [Google Scholar]
- 106.Mock J.R., Dial C.F., Tune M.K., Gilmore R.C., O'Neal W.K., Dang H.et al. (2020) Impact of regulatory T cells on Type 2 alveolar epithelial cell transcriptomes during resolution of acute lung injury and contributions of IFN-gamma. Am. J. Respir. Cell Mol. Biol. 63, 464–477 10.1165/rcmb.2019-0399OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dial C.F., Tune M.K., Doerschuk C.M. and Mock J.R. (2017) Foxp3(+) regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am. J. Respir. Cell Mol. Biol. 57, 162–173 10.1165/rcmb.2017-0019OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Candia P., Procaccini C., Russo C., Lepore M.T. and Matarese G. (2022) Regulatory T cells as metabolic sensors. Immunity 55, 1981–1992 10.1016/j.immuni.2022.10.006 [DOI] [PubMed] [Google Scholar]
- 109.Qiu Y., Ke S., Chen J., Qin Z., Zhang W., Yuan Y.et al. (2022) FOXP3+ regulatory T cells and the immune escape in solid tumours. Front Immunol. 13, 982986 10.3389/fimmu.2022.982986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishikawa H. and Sakaguchi S. (2010) Regulatory T cells in tumor immunity. Int. J. Cancer 127, 759–767 10.1002/ijc.25429 [DOI] [PubMed] [Google Scholar]
- 111.Wang J., Gong R., Zhao C., Lei K., Sun X. and Ren H. (2023) Human FOXP3 and tumour microenvironment. Immunology 168, 248–255 10.1111/imm.13520 [DOI] [PubMed] [Google Scholar]
- 112.Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D.et al. (2010) The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 10.1038/ni.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu J., Ji X., Wang L., Sun F., Huang C., Peng H.et al. (2022) Interleukin-27 ameliorates allergic asthma by alleviating the lung Th2 inflammatory environment. Int. J. Mol. Med. 49, 86 10.3892/ijmm.2022.5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aron J.L. and Akbari O. (2017) Regulatory T cells and type 2 innate lymphoid cell-dependent asthma. Allergy 72, 1148–1155 10.1111/all.13139 [DOI] [PubMed] [Google Scholar]
- 115.Fan X., Xu Z.B., Li C.L., Zhang H.Y., Peng Y.Q., He B.X.et al. (2021) Mesenchymal stem cells regulate type 2 innate lymphoid cells via regulatory T cells through ICOS-ICOSL interaction. Stem Cells 39, 975–987 10.1002/stem.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poole J.A., Nordgren T.M., Heires A.J., Nelson A.J., Katafiasz D., Bailey K.L.et al. (2020) Amphiregulin modulates murine lung recovery and fibroblast function following exposure to agriculture organic dust. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L180–L191 10.1152/ajplung.00039.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halvorsen E.C., Franks S.E., Wadsworth B.J., Harbourne B.T., Cederberg R.A., Steer C.A.et al. (2019) IL-33 increases ST2(+) Tregs and promotes metastatic tumour growth in the lungs in an amphiregulin-dependent manner. Oncoimmunology 8, e1527497 10.1080/2162402X.2018.1527497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Faustino L.D., Griffith J.W., Rahimi R.A., Nepal K., Hamilos D.L., Cho J.L.et al. (2020) Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat. Immunol. 21, 1371–1383 10.1038/s41590-020-0785-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neujahr D.C., Cardona A.C., Ulukpo O., Rigby M., Pelaez A., Ramirez A.et al. (2009) Dynamics of human regulatory T cells in lung lavages of lung transplant recipients. Transplantation 88, 521–527 10.1097/TP.0b013e3181b0e719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madsen C.B., Norgaard A., Iversen M. and Ryder L.P. (2010) Elevated mRNA levels of CTLA-4, FoxP3, and granzyme B in BAL, but not in blood, during acute rejection of lung allografts. Transpl. Immunol. 24, 26–32 10.1016/j.trim.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 121.Koizumi S.I. and Ishikawa H. (2019) Transcriptional regulation of differentiation and functions of effector T regulatory cells. Cells-Basel 8, 939 10.3390/cells8080939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dominguez-Villar M., Baecher-Allan C.M. and Hafler D.A. (2011) Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17, 673–675 10.1038/nm.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kitz A. and Dominguez-Villar M. (2017) Molecular mechanisms underlying Th1-like Treg generation and function. Cell. Mol. Life Sci. 74, 4059–4075 10.1007/s00018-017-2569-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koch M.A., Thomas K.R., Perdue N.R., Smigiel K.S., Srivastava S. and Campbell D.J. (2012) T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity 37, 501–510 10.1016/j.immuni.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kanamori M., Nakatsukasa H., Ito M., Chikuma S. and Yoshimura A. (2018) Reprogramming of Th1 cells into regulatory T cells through rewiring of the metabolic status. Int. Immunol. 30, 357–373 10.1093/intimm/dxy043 [DOI] [PubMed] [Google Scholar]
- 126.Kim B.-S., Kim I.-K., Park Y.-J., Kim Y.-S., Kim Y.-J., Chang W.-S.et al. (2010) Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc. Natl. Acad. Sci. 107, 8742–8747 10.1073/pnas.0911756107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pelly V.S., Coomes S.M., Kannan Y., Gialitakis M., Entwistle L.J., Perez-Lloret J.et al. (2017) Interleukin 4 promotes the development of ex-Foxp3 Th2 cells during immunity to intestinal helminths. J. Exp. Med. 214, 1809–1826 10.1084/jem.20161104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Hagan K.L., Miller S.D. and Phee H. (2017) Pak2 is essential for the function of Foxp3+ regulatory T cells through maintaining a suppressive Treg phenotype. Sci. Rep. 7, 17097 10.1038/s41598-017-17078-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu L., Kitani A., Fuss I. and Strober W. (2007) Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 178, 6725–6729 10.4049/jimmunol.178.11.6725 [DOI] [PubMed] [Google Scholar]
- 130.Zheng S.G., Wang J. and Horwitz D.A. (2008) Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 180, 7112–7116 10.4049/jimmunol.180.11.7112 [DOI] [PubMed] [Google Scholar]
- 131.Tsuji M., Komatsu N., Kawamoto S., Suzuki K., Kanagawa O., Honjo T.et al. (2009) Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 10.1126/science.1169152 [DOI] [PubMed] [Google Scholar]
- 132.Gaddis D.E., Padgett L.E., Wu R., McSkimming C., Romines V., Taylor A.M.et al. (2018) Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun. 9, 1095 10.1038/s41467-018-03493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xie M.M. and Dent A.L. (2018) Unexpected help: follicular regulatory T cells in the germinal center. Front Immunol. 9, 1536 10.3389/fimmu.2018.01536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hao H., Nakayamada S., Yamagata K., Ohkubo N., Iwata S., Inoue Y.et al. (2021) Conversion of T follicular helper cells to T follicular regulatory cells by interleukin-2 through transcriptional regulation in systemic lupus erythematosus. Arthritis Rheumatol. 73, 132–142 10.1002/art.41457 [DOI] [PubMed] [Google Scholar]
- 135.Bour-Jordan H. and Bluestone J.A. (2009) Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol. Rev. 229, 41–66 10.1111/j.1600-065X.2009.00775.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magee C.N., Boenisch O. and Najafian N. (2012) The role of costimulatory molecules in directing the functional differentiation of alloreactive T helper cells. Am. J. Transplant. 12, 2588–2600 10.1111/j.1600-6143.2012.04180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Esensten J.H., Helou Y.A., Chopra G., Weiss A. and Bluestone J.A. (2016) CD28 costimulation: from mechanism to therapy. Immunity 44, 973–988 10.1016/j.immuni.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boomer J.S. and Green J.M. (2010) An enigmatic tail of CD28 signaling. Cold Spring Harb. Perspect. Biol. 2, a002436 10.1101/cshperspect.a002436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rohr J., Guo S., Huo J., Bouska A., Lachel C., Li Y.et al. (2016) Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia 30, 1062–1070 10.1038/leu.2015.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kornete M., Mason E.S., Girouard J., Lafferty E.I., Qureshi S. and Piccirillo C.A. (2015) Th1-Like ICOS+ Foxp3+ Treg Cells Preferentially Express CXCR3 and Home to beta-Islets during Pre-Diabetes in BDC2.5 NOD Mice. PloS ONE 10, e0126311 10.1371/journal.pone.0126311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K.et al. (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blazar B.R., Carreno B.M., Panoskaltsis-Mortari A., Carter L., Iwai Y., Yagita H.et al. (2003) Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 171, 1272–1277 10.4049/jimmunol.171.3.1272 [DOI] [PubMed] [Google Scholar]
- 143.Boenisch O., D'Addio F., Watanabe T., Elyaman W., Magee C.N., Yeung M.Y.et al. (2010) TIM-3: a novel regulatory molecule of alloimmune activation. J. Immunol. 185, 5806–5819 10.4049/jimmunol.0903435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sabatos C.A., Chakravarti S., Cha E., Schubart A., Sánchez-Fueyo A., Zheng X.X.et al. (2003) Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4, 1102–1110 10.1038/ni988 [DOI] [PubMed] [Google Scholar]
- 145.Sanchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X.X., Sabatos C.A.et al. (2003) Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4, 1093–1101 10.1038/ni987 [DOI] [PubMed] [Google Scholar]
- 146.He W., Fang Z., Wang F., Wu K., Xu Y., Zhou H.et al. (2009) Galectin-9 significantly prolongs the survival of fully mismatched cardiac allografts in mice. Transplantation 88, 782–790 10.1097/TP.0b013e3181b47f25 [DOI] [PubMed] [Google Scholar]
- 147.So T. and Croft M. (2007) Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J. Immunol. 179, 1427–1430 10.4049/jimmunol.179.3.1427 [DOI] [PubMed] [Google Scholar]
- 148.Vu M.D., Xiao X., Gao W., Degauque N., Chen M., Kroemer A.et al. (2007) OX40 costimulation turns off Foxp3+ Tregs. Blood 110, 2501–2510 10.1182/blood-2007-01-070748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kroemer A., Xiao X., Vu M.D., Gao W., Minamimura K., Chen M.et al. (2007) OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J. Immunol. 179, 5584–5591 10.4049/jimmunol.179.8.5584 [DOI] [PubMed] [Google Scholar]
- 150.Degauque N., Mariat C., Kenny J., Zhang D., Gao W., Vu M.D.et al. (2008) Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J. Clin. Invest. 118, 735–741 10.1172/JCI32562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xiao S., Najafian N., Reddy J., Albin M., Zhu C., Jensen E.et al. (2007) Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J. Exp. Med. 204, 1691–1702 10.1084/jem.20062498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holgate S.T., Holloway J., Wilson S., Howarth P.H., Haitchi H.M., Babu S.et al. (2006) Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J. Allergy Clin. Immunol. 117, 496–506, quiz 7 10.1016/j.jaci.2006.01.039 [DOI] [PubMed] [Google Scholar]
- 153.Chen W., Cao Y., Zhong Y., Sun J. and Dong J. (2022) The mechanisms of effector Th cell responses contribute to Treg cell function: new insights into pathogenesis and therapy of asthma. Front. Immunol. 13, 862866 10.3389/fimmu.2022.862866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jing W., Wang W. and Liu Q. (2019) Passive smoking induces pediatric asthma by affecting the balance of Treg/Th17 cells. Pediatr. Res. 85, 469–476 10.1038/s41390-019-0276-0 [DOI] [PubMed] [Google Scholar]
- 155.Shen X., Zhang H., Xie H., Chen L., Li S., Zheng J.et al. (2021) Reduced CCR6(+)IL-17A(+)Treg Cells in Blood and CCR6-Dependent Accumulation of IL-17A(+)Treg Cells in Lungs of Patients With Allergic Asthma. Front. Immunol. 12, 710750 10.3389/fimmu.2021.710750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lopes F., Tibério I., Leme A. and Fairclough L. (2022) Editorial: The importance of Th17/Treg imbalance in asthma and COPD development and progression. Front. Immunol. 13, 1025215 10.3389/fimmu.2022.1025215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding F., Liu B., Niu C., Wang T., Wang Y., Geng G.et al. (2020) Low-dose LPS induces tolerogenic treg skewing in asthma. Front. Immunol. 11, 2150 10.3389/fimmu.2020.02150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liang P., Peng S., Zhang M., Ma Y., Zhen X. and Li H. (2017) Huai Qi Huang corrects the balance of Th1/Th2 and Treg/Th17 in an ovalbumin-induced asthma mouse model. Biosci. Rep. 37, BSR20171071 10.1042/BSR20171071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gandhi V.D., Cephus J.Y., Norlander A.E., Chowdhury N.U., Zhang J., Ceneviva Z.J.et al. (2022) Androgen receptor signaling promotes Treg suppressive function during allergic airway inflammation. J. Clin. Invest. 132, e153397 10.1172/JCI153397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao Y.E., Qin C.C., Yang C.M. and Huang T.X. (2022) γδT17/γδTreg cell subsets: a new paradigm for asthma treatment. J. Asthma:Off. J. Assoc. Care Asthma 59, 2028–2038 10.1080/02770903.2021.1980585 [DOI] [PubMed] [Google Scholar]
- 161.Huang M.T., Dai Y.S., Chou Y.B., Juan Y.H., Wang C.C. and Chiang B.L. (2009) Regulatory T cells negatively regulate neovasculature of airway remodeling via DLL4-Notch signaling. J. Immunol. 183, 4745–4754 10.4049/jimmunol.0804371 [DOI] [PubMed] [Google Scholar]
- 162.Xia Z.W., Xu L.Q., Zhong W.W., Wei J.J., Li N.L., Shao J.et al. (2007) Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor- 1. Am. J. Pathol. 171, 1904–1914 10.2353/ajpath.2007.070096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zou Y., Xu S., Xiao Y., Qiu Q., Shi M., Wang J.et al. (2018) Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J. Clin. Invest. 128, 4510–4524 10.1172/JCI97965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chu M., Chu I.M., Yung E.C., Lam C.W., Leung T.F., Wong G.W.et al. (2016) Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma. Molecules 21, 10.3390/molecules21070933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xie L., Huang Z., Li H., Liu X., Zheng S. and Su W. (2019) IL-38: a new player in inflammatory autoimmune disorders. Biomolecules 9, 345 10.3390/biom9080345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xie K., Chen Y.Q., Chai Y.S., Lin S.H., Wang C.J. and Xu F. (2021) HMGB1 suppress the expression of IL-35 by regulating Naïve CD4+ T cell differentiation and aggravating Caspase-11-dependent pyroptosis in acute lung injury. Int. Immunopharmacol. 91, 107295 10.1016/j.intimp.2020.107295 [DOI] [PubMed] [Google Scholar]
- 167.Chen L., Yang J., Zhang M., Fu D., Luo H. and Yang X. (2023) SPP1 exacerbates ARDS via elevating Th17/Treg and M1/M2 ratios through suppression of ubiquitination-dependent HIF-1α degradation. Cytokine 164, 156107 10.1016/j.cyto.2022.156107 [DOI] [PubMed] [Google Scholar]
- 168.Yu Z.X., Ji M.S., Yan J., Cai Y., Liu J., Yang H.F.et al. (2015) The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care 19, 82 10.1186/s13054-015-0811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thomas R., Qiao S. and Yang X. (2023) Th17/Treg imbalance: implications in lung inflammatory diseases. Int. J. Mol. Sci. 24, 4865 10.3390/ijms24054865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Z.T., Zhang D.Y., Xie K., Wang C.J. and Xu F. (2021) Luteolin activates Tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury. Int. Immunopharmacol. 99, 107914 10.1016/j.intimp.2021.107914 [DOI] [PubMed] [Google Scholar]
- 171.Xie K., Chai Y.S., Lin S.H., Xu F. and Wang C.J. (2021) Luteolin regulates the differentiation of regulatory T cells and activates IL-10-dependent macrophage polarization against acute lung injury. J. Immunol. Res. 2021, 8883962 10.1155/2021/8883962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suresh M.V., Francis S., Aktay S., Kralovich G. and Raghavendran K. (2023) Therapeutic potential of curcumin in ARDS and COVID-19. Clin. Exp. Pharmacol. Physiol. 50, 267–276 10.1111/1440-1681.13744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.He Y.Q., Zhou C.C., Yu L.Y., Wang L., Deng J.L., Tao Y.L.et al. (2021) Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 163, 105224 10.1016/j.phrs.2020.105224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seyran M., Melanie S., Philip S., Amiq G. and Fabian B. (2022) Allies or enemies? The effect of regulatory T cells and related T lymphocytes on the profibrotic environment in bleomycin-injured lung mouse models Clin. Exp. Med. 231075–1088 10.1007/s10238-022-00945-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li W., Ding Z., Chen Y., Wang Y., Peng M., Li C.et al. (2022) Integrated pharmacology reveals the molecular mechanism of gegen qinlian decoction against lipopolysaccharide-induced acute lung injury. Front. Pharmacol. 13, 854544 10.3389/fphar.2022.854544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Spagnolo P., Kropski J.A., Jones M.G., Lee J.S., Rossi G., Karampitsakos T.et al. (2021) Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol. Therapeut. 222, 107798 10.1016/j.pharmthera.2020.107798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kamio K., Azuma A., Matsuda K., Usuki J., Inomata M., Morinaga A.et al. (2018) Resolution of bleomycin-induced murine pulmonary fibrosis via a splenic lymphocyte subpopulation. Respir. Res. 19, 71 10.1186/s12931-018-0783-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kotsianidis I., Nakou E., Bouchliou I., Tzouvelekis A., Spanoudakis E., Steiropoulos P.et al. (2009) Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 179, 1121–1130 10.1164/rccm.200812-1936OC [DOI] [PubMed] [Google Scholar]
- 179.Wang B., Bai W., Ma H. and Li F. (2021) Regulatory Effect of PD1/PD-Ligand 1 (PD-L1) on Treg cells in patients with idiopathic pulmonary fibrosis. Med. Sci. Monit. 27, e927577 10.12659/MSM.927577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hou Z., Ye Q., Qiu M., Hao Y., Han J. and Zeng H. (2017) Increased activated regulatory T cells proportion correlate with the severity of idiopathic pulmonary fibrosis. Respir. Res. 18, 170 10.1186/s12931-017-0653-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dai X., Yang Z., Zhang W., Liu S., Zhao Q., Liu T.et al. (2022) Identification of diagnostic gene biomarkers related to immune infiltration in patients with idiopathic pulmonary fibrosis based on bioinformatics strategies. Front. Med. 9, 959010 10.3389/fmed.2022.959010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu X., Hu Z. and Zhou H. (2021) N-Acetylcysteine Improves Inflammatory Response in COPD Patients by Regulating Th17/Treg Balance through Hypoxia Inducible Factor-1α Pathway. BioMed Res. Int. 2021, 6372128 10.1155/2021/6372128 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 183.Lourenço J.D., Ito J.T., Martins M.A., Tibério I. and Lopes F. (2021) Th17/Treg imbalance in chronic obstructive pulmonary disease: clinical and experimental evidence. Front. Immunol. 12, 804919 10.3389/fimmu.2021.804919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang Y., Li N., Li Q., Liu Z., Li Y., Kong J.et al. (2021) Xuanbai Chengqi Decoction Ameliorates Pulmonary Inflammation via Reshaping Gut Microbiota and Rectifying Th17/Treg Imbalance in a Murine Model of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstruct. Pulmonary Dis. 16, 3317–3335 10.2147/COPD.S337181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ingersoll S.A., Laval J., Forrest O.A., Preininger M., Brown M.R., Arafat D.et al. (2015) Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death-ligand 1. J. Immunol. (Baltimore, Md: 1950) 194, 5520–5528 10.4049/jimmunol.1500312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Westhölter D., Raspe J., Uebner H., Pipping J., Schmitz M., Straßburg S.et al. (2023) Regulatory T cell enhancement in adults with cystic fibrosis receiving Elexacaftor/Tezacaftor/Ivacaftor therapy. Front Immunol. 14, 1107437 10.3389/fimmu.2023.1107437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Anil N. and Singh M. (2014) CD4(+)CD25(high) FOXP3(+) regulatory T cells correlate with FEV1 in North Indian children with cystic fibrosis. Immunol. Invest. 43, 535–543 10.3109/08820139.2014.888447 [DOI] [PubMed] [Google Scholar]
- 188.Hector A., Schäfer H., Pöschel S., Fischer A., Fritzsching B., Ralhan A.et al. (2015) Regulatory T-cell impairment in cystic fibrosis patients with chronic pseudomonas infection. Am. J. Respir. Crit. Care Med. 191, 914–923 10.1164/rccm.201407-1381OC [DOI] [PubMed] [Google Scholar]
- 189.Westhölter D., Beckert H., Straßburg S., Welsner M., Sutharsan S., Taube C.et al. (2021) Pseudomonas aeruginosa infection, but not mono or dual-combination CFTR modulator therapy affects circulating regulatory T cells in an adult population with cystic fibrosis. J. Cystic Fibrosis: Off. J. Eur. Cystic Fibrosis Soc. 20, 1072–1079 10.1016/j.jcf.2021.05.001 [DOI] [PubMed] [Google Scholar]
- 190.McGuire J.K. (2015) Regulatory T cells in cystic fibrosis lung disease. More answers, more questions. Am. J. Respir. Crit. Care Med. 191, 866–868 10.1164/rccm.201502-0315ED [DOI] [PubMed] [Google Scholar]
- 191.Ziai S., Coriati A., Gauthier M.S., Rabasa-Lhoret R. and Richter M.V. (2014) Could T cells be involved in lung deterioration and hyperglycemia in cystic fibrosis? Diabetes Res. Clin. Pract. 105, 22–29 10.1016/j.diabres.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 192.Kushwah R., Gagnon S. and Sweezey N.B. (2013) Intrinsic predisposition of naïve cystic fibrosis T cells to differentiate towards a Th17 phenotype. Respir. Res. 14, 138 10.1186/1465-9921-14-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kreindler J.L., Steele C., Nguyen N., Chan Y.R., Pilewski J.M., Alcorn J.F.et al. (2010) Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J. Clin. Invest. 120, 3242–3254 10.1172/JCI42388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ridley K. and Condren M. (2020) Elexacaftor-tezacaftor-ivacaftor: the first triple-combination cystic fibrosis transmembrane conductance regulator modulating therapy. J. Pediatric Pharmacol. Therapeut. 25, 192–197 10.5863/1551-6776-25.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Duan M.C., Zhong X.N., Liu G.N. and Wei J.R. (2014) The Treg/Th17 paradigm in lung cancer. J. Immunol. Res. 2014, 730380 10.1155/2014/730380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang M., Ma J., Guo Q., Ding S., Wang Y. and Pu H. (2022) CD8(+) T cell-associated gene signature correlates with prognosis risk and immunotherapy response in patients with lung adenocarcinoma. Front. Immunol. 13, 806877 10.3389/fimmu.2022.806877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Glasner A., Rose S.A., Sharma R., Gudjonson H., Chu T., Green J.A.et al. (2023) Conserved transcriptional connectivity of regulatory T cells in the tumor microenvironment informs new combination cancer therapy strategies. Nat. Immunol. 24, 1020–1035 10.1038/s41590-023-01504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao Y., Guo S., Deng J., Shen J., Du F., Wu X.et al. (2022) VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: targeting the tumor microenvironment. Int. J. Biological Sci. 18, 3845–3858 10.7150/ijbs.70958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Principe D.R., Chiec L., Mohindra N.A. and Munshi H.G. (2021) Regulatory T-cells as an emerging barrier to immune checkpoint inhibition in lung cancer. Front. Oncol. 11, 684098 10.3389/fonc.2021.684098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang R., Sun L., Li C.F., Wang Y.H., Yao J., Li H.et al. (2021) Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 12, 832 10.1038/s41467-021-21099-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang P., Zhang X., Cui Y., Gong Z., Wang W. and Lin S. (2023) Revealing the role of regulatory T cells in the tumor microenvironment of lung adenocarcinoma: a novel prognostic and immunotherapeutic signature. Front. Immunol. 14, 1244144 10.3389/fimmu.2023.1244144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sarkar T., Dhar S. and Sa G. (2021) Tumor-infiltrating T-regulatory cells adapt to altered metabolism to promote tumor-immune escape. Curr. Res. Immunol. 2, 132–141 10.1016/j.crimmu.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tekguc M., Wing J.B., Osaki M., Long J. and Sakaguchi S. (2021) Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. PNAS 118, e2023739118 10.1073/pnas.2023739118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen Z., Tong L., Neo S.Y., Li S., Gao J., Schlisio S.et al. (2023) CD25(bright) NK cells display superior function and metabolic activity under regulatory T cell-mediated suppression. Oncoimmunology 12, 2175517 10.1080/2162402X.2023.2175517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ohue Y. and Nishikawa H. (2019) Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 110, 2080–2089 10.1111/cas.14069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Joshi N.S., Akama-Garren E.H., Lu Y., Lee D.Y., Chang G.P., Li A.et al. (2015) Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 10.1016/j.immuni.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zagorulya M., Yim L., Morgan D.M., Edwards A., Torres-Mejia E., Momin N.et al. (2023) Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity 56, 386.e10–405.e10 10.1016/j.immuni.2023.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sinjab A., Han G., Treekitkarnmongkol W., Hara K., Brennan P.M., Dang M.et al. (2021) Resolving the spatial and cellular architecture of lung adenocarcinoma by multiregion single-cell sequencing. Cancer Discov. 11, 2506–2523 10.1158/2159-8290.CD-20-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shevchenko I., Mathes A., Groth C., Karakhanova S., Müller V., Utikal J.et al. (2020) Enhanced expression of CD39 and CD73 on T cells in the regulation of anti-tumor immune responses. Oncoimmunology 9, 1744946 10.1080/2162402X.2020.1744946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen Z., Huang Y., Hu Z., Zhao M., Li M., Bi G.et al. (2021) Landscape and dynamics of single tumor and immune cells in early and advanced-stage lung adenocarcinoma. Clin. Transl. Med. 11, e350 10.1002/ctm2.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Quail D.F. and Joyce J.A. (2013) Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chelakkot C., Chelakkot V.S., Shin Y. and Song K. (2023) Modulating glycolysis to improve cancer therapy. Int. J. Mol. Sci. 24, 2606 10.3390/ijms24032606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dwivedi M., Tiwari S., Kemp E.H. and Begum R. (2022) Implications of regulatory T cells in anti-cancer immunity: from pathogenesis to therapeutics. Heliyon 8, e10450 10.1016/j.heliyon.2022.e10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jorgovanovic D., Song M., Wang L. and Zhang Y. (2020) Roles of IFN-γ in tumor progression and regression: a review. Biomarker Res. 8, 49 10.1186/s40364-020-00228-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sun C.C., Zhu W., Li S.J., Hu W., Zhang J., Zhuo Y.et al. (2020) FOXC1-mediated LINC00301 facilitates tumor progression and triggers an immune-suppressing microenvironment in non-small cell lung cancer by regulating the HIF1α pathway. Genome Medicine 12, 77 10.1186/s13073-020-00773-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lahiri A., Maji A., Potdar P.D., Singh N., Parikh P., Bisht B.et al. (2023) Lung cancer immunotherapy: progress, pitfalls, and promises. Mol. Cancer 22, 40 10.1186/s12943-023-01740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chang D.K., Peterson E., Sun J., Goudie C., Drapkin R.I., Liu J.F.et al. (2016) Anti-CCR4 monoclonal antibody enhances antitumor immunity by modulating tumor-infiltrating Tregs in an ovarian cancer xenograft humanized mouse model. Oncoimmunology 5, e1090075 10.1080/2162402X.2015.1090075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E.et al. (2015) Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, S185–S198 10.1016/j.semcancer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 219.Wei X., He L., Wang X., Lin M. and Dai J. (2020) Effects of dasatinib on CD8(+)T, Th1, and Treg cells in patients with chronic myeloid leukemia. J. Int. Med. Res. 48, 300060519877321 10.1177/0300060519877321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Domvri K., Petanidis S., Zarogoulidis P., Anestakis D., Charalampidis C., Tsavlis D.et al. (2022) Engineered hybrid Treg-targeted nanosomes restrain lung immunosuppression by inducing intratumoral CD8(+)T cell immunity. Int. J. Nanomed. 17, 4449–4468 10.2147/IJN.S346341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Redin E., Garmendia I., Lozano T., Serrano D., Senent Y., Redrado M.et al. (2021) SRC family kinase (SFK) inhibitor dasatinib improves the antitumor activity of anti-PD-1 in NSCLC models by inhibiting Treg cell conversion and proliferation. J. Immunotherapy Cancer 9, e001496 10.1136/jitc-2020-001496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kinkade S. and Long N.A. (2016) Acute bronchitis. Am. Fam. Physician 94, 560–565 [PubMed] [Google Scholar]
- 223.Tu Z., Xue H., Chen W., Cao L. and Zhang W. (2017) Changes of Treg and Th17 cells as well as cytokines in children with acute bronchitis. Exp. Therapeut. Med. 14, 3846–3850 10.3892/etm.2017.4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lynch J.P., Werder R.B., Curren B.F., Sikder M.A.A., Ullah A., Sebina I.et al. (2020) Long-lived regulatory T cells generated during severe bronchiolitis in infancy influence later progression to asthma. Mucosal Immunol. 13, 652–664 10.1038/s41385-020-0268-8 [DOI] [PubMed] [Google Scholar]
- 225.Qiu X., Xu S., Lu Y., Luo Z., Yan Y., Wang C.et al. (2022) Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev. 68, 37–53 10.1016/j.cytogfr.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 226.Shi T., Li N., He Y., Feng J., Mei Z., Du Y.et al. (2021) Th17/Treg cell imbalance plays an important role in respiratory syncytial virus infection compromising asthma tolerance in mice. Microb. Pathog. 156, 104867 10.1016/j.micpath.2021.104867 [DOI] [PubMed] [Google Scholar]
- 227.Christiaansen A.F., Syed M.A., Ten Eyck P.P., Hartwig S.M., Durairaj L., Kamath S.S.et al. (2016) Altered Treg and cytokine responses in RSV-infected infants. Pediatr. Res. 80, 702–709 10.1038/pr.2016.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Qin L., Feng J., Hu C., Li Y. and Niu R. (2016) Th17/Treg imbalance mediated by IL-8 in RSV-infected bronchial epithelial cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 41, 337–344 [DOI] [PubMed] [Google Scholar]
- 229.Gao M., Liu L.X., Wu F.L., Zhang X., Li Y.Y., Shi T.et al. (2017) The changes of Th17/Treg and related cytokines: IL-17, IL-23, IL-10, and TGF-beta in respiratory syncytial virus bronchiolitis rat model. Iran J. Allergy Asthma Immunol. 16, 386–395 [PubMed] [Google Scholar]
- 230.Zhu J., Liu X., Wang W., Ouyang X., Zheng W. and Wang Q. (2017) Altered expression of regulatory T and Th17 cells in murine bronchial asthma. Exp. Ther. Med. 14, 714–722 10.3892/etm.2017.4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang J.G., Chen X.J., Liu T. and Jiang S.J. (2016) FOXP3(+) associated with the pro-inflammatory regulatory T and T helper 17 effector cells in asthma patients. Exp. Ther. Med. 12, 2753–2758 10.3892/etm.2016.3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mamessier E., Lorec A.M., Thomas P., Badier M., Magnan A. and Reynaud-Gaubert M. (2007) T regulatory cells in stable posttransplant bronchiolitis obliterans syndrome. Transplantation 84, 908–916 10.1097/01.tp.0000281408.20686.cb [DOI] [PubMed] [Google Scholar]
- 233.Mora B.N., Boasquevisque C.H., Boglione M., Ritter J.M., Scheule R.K., Yew N.S.et al. (2000) Transforming growth factor-beta1 gene transfer ameliorates acute lung allograft rejection. J. Thorac. Cardiovasc. Surg. 119, 913–920 10.1016/S0022-5223(00)70086-9 [DOI] [PubMed] [Google Scholar]
- 234.Hodge G., Hodge S., Liu H., Nguyen P., Holmes-Liew C.L. and Holmes M. (2021) Bronchiolitis obliterans syndrome is associated with increased senescent lymphocytes in the small airways. J. Heart Lung Transplant. 40, 108–119 10.1016/j.healun.2019.12.005 [DOI] [PubMed] [Google Scholar]
- 235.Amin H.Z., Sasaki N. and Hirata K.I. (2017) Regulatory T cell immunity in atherosclerosis. Acta Med. Indones 49, 63–68 [PubMed] [Google Scholar]
- 236.Li X., Kang N., Zhang X., Dong X., Wei W., Cui L.et al. (2011) Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J. Immunol. 186, 6693–6700 10.4049/jimmunol.1002776 [DOI] [PubMed] [Google Scholar]
- 237.Wen L., Shi L., Kong X.L., Li K.Y., Li H., Jiang D.X.et al. (2022) Gut microbiota protected against pseudomonas aeruginosa pneumonia via restoring Treg/Th17 balance and metabolism. Front Cell Infect Microbiol. 12, 856633 10.3389/fcimb.2022.856633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bouras M., Asehnoune K. and Roquilly A. (2018) Contribution of dendritic cell responses to sepsis-induced immunosuppression and to susceptibility to secondary pneumonia. Front. Immunol. 9, 2590 10.3389/fimmu.2018.02590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee C.H., Choi Y., Seo S.Y., Kim S.H., Kim I.H., Kim S.W.et al. (2021) Addition of probiotics to antibiotics improves the clinical course of pneumonia in young people without comorbidities: a randomized controlled trial. Sci. Rep. 11, 926 10.1038/s41598-020-79630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li J., Chen T., Yuan C., Zhao G., Xu M., Li X.et al. (2017) Effect of intravenous immunoglobulin on the function of Treg cells derived from immunosuppressed mice with Pseudomonas aeruginosa pneumonia. PLoS ONE 12, e0176843 10.1371/journal.pone.0176843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhuang Q., Cai H., Yang M., Peng B., Luo Y., Zhang Y.et al. (2022) The association between regulatory T cell subpopulations and severe pneumonia post renal transplantation. J. Immunol. Res. 2022, 8720438 10.1155/2022/8720438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xiong Y., Zhong Q., Palmer T., Benner A., Wang L., Suresh K.et al. (2021) Estradiol resolves pneumonia via ERβ in regulatory T cells. JCI Insight 6, e133251 10.1172/jci.insight.133251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Morales-Nebreda L., Helmin K.A., Torres Acosta M.A., Markov N.S., Hu J.Y., Joudi A.M.et al. (2021) Aging imparts cell-autonomous dysfunction to regulatory T cells during recovery from influenza pneumonia. JCI Insight 6, e141690 10.1172/jci.insight.141690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wu H., Ding X., Zhao D., Liang Y. and Ji W. (2019) Effect of montelukast combined with methylprednisolone for the treatment of mycoplasma pneumonia. J. Int. Med. Res. 47, 2555–2561 10.1177/0300060518820412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Guo H., He Z., Li M., Wang T. and Zhang L. (2016) Imbalance of peripheral blood Th17 and Treg responses in children with refractory Mycoplasma pneumoniae pneumonia. J. Infect. Chemother. Off. J. Japan Soc. Chemother. 22, 162–166 10.1016/j.jiac.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 246.Kurata S., Osaki T., Yonezawa H., Arae K., Taguchi H. and Kamiya S. (2014) Role of IL-17A and IL-10 in the antigen induced inflammation model by Mycoplasma pneumoniae. BMC Microbiol. 14, 156 10.1186/1471-2180-14-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Odeh A.N. and Simecka J.W. (2016) Regulatory CD4+CD25+ T cells dampen inflammatory disease in murine mycoplasma pneumonia and promote IL-17 and IFN-γ responses. PLoS ONE 11, e0155648 10.1371/journal.pone.0155648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lou T.L., Ji T., Peng X., Ji W.W., Yuan L.X., Wang J.et al. (2021) Extract from tetrastigma hemsleyanum leaf alleviates Pseudomonas aeruginosa lung infection: network pharmacology analysis and experimental evidence. Front. Pharmacol. 12, 587850 10.3389/fphar.2021.587850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fujitani S., Sun H.Y., Yu V.L. and Weingarten J.A. (2011) Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest 139, 909–919 10.1378/chest.10-0166 [DOI] [PubMed] [Google Scholar]
- 250.Neill D.R., Fernandes V.E., Wisby L., Haynes A.R., Ferreira D.M., Laher A.et al. (2012) T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice. PLoS Pathog. 8, e1002660 10.1371/journal.ppat.1002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mangodt T.C., Van Herck M.A., Nullens S., Ramet J., De Dooy J.J., Jorens P.G.et al. (2015) The role of Th17 and Treg responses in the pathogenesis of RSV infection. Pediatr. Res. 78, 483–491 10.1038/pr.2015.143 [DOI] [PubMed] [Google Scholar]
- 252.Yoo J.K., Kim T.S., Hufford M.M. and Braciale T.J. (2013) Viral infection of the lung: host response and sequelae. J. Allergy Clin. Immunol. 132, 1263–1276, quiz 77 10.1016/j.jaci.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Betts R.J., Prabhu N., Ho A.W., Lew F.C., Hutchinson P.E., Rotzschke O.et al. (2012) Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response. J. Virol. 86, 2817–2825 10.1128/JVI.05685-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chang Y.J., Kim H.Y., Albacker L.A., Baumgarth N., McKenzie A.N., Smith D.E.et al. (2011) Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 12, 631–638 10.1038/ni.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ochani R., Asad A., Yasmin F., Shaikh S., Khalid H., Batra S.et al. (2021) COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez. Med. 29, 20–36 [PubMed] [Google Scholar]
- 256.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S. and Mosayebi G. (2021) Cytokine profile and disease severity in patients with COVID-19. Cytokine 137, 155323 10.1016/j.cyto.2020.155323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.de Candia P., Prattichizzo F., Garavelli S. and Matarese G. (2021) T Cells: Warriors of SARS-CoV-2 Infection. Trends Immunol. 42, 18–30 10.1016/j.it.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Aygun H. (2022) Vitamin D can reduce severity in COVID-19 through regulation of PD-L1. Naunyn-Schmiedeberg's Arch. Pharmacol. 395, 487–494 10.1007/s00210-022-02210-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Martonik D., Parfieniuk-Kowerda A., Rogalska M. and Flisiak R. (2021) The role of Th17 response in COVID-19. Cells 10, 1550 10.3390/cells10061550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hasanvand A. (2022) COVID-19 and the role of cytokines in this disease. Inflammopharmacology 30, 789–798 10.1007/s10787-022-00992-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jafarzadeh A., Nemati M. and Jafarzadeh S. (2021) Contribution of STAT3 to the pathogenesis of COVID-19. Microb. Pathog. 154, 104836 10.1016/j.micpath.2021.104836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Paydar S., Sabetian G., Khalili H., Fallahi J., Tahami M., Ziaian B.et al. (2016) Management of deep vein thrombosis (DVT) prophylaxis in trauma patients. Bulletin Emergency Trauma 4, 1–7 [PMC free article] [PubMed] [Google Scholar]
- 263.Luther N., Shahneh F., Brähler M., Krebs F., Jäckel S., Subramaniam S.et al. (2016) Innate effector-memory T-cell activation regulates post-thrombotic vein wall inflammation and thrombus resolution. Circ. Res. 119, 1286–1295 10.1161/CIRCRESAHA.116.309301 [DOI] [PubMed] [Google Scholar]
- 264.Shahneh F., Grill A., Klein M., Frauhammer F., Bopp T., Schäfer K.et al. (2021) Specialized regulatory T cells control venous blood clot resolution through SPARC. Blood 137, 1517–1526 10.1182/blood.2020005407 [DOI] [PubMed] [Google Scholar]
- 265.Hoeppli R.E., Wu D., Cook L. and Levings M.K. (2015) The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front. Immunol. 6, 61 10.3389/fimmu.2015.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Van Linthout S., Miteva K. and Tschöpe C. (2014) Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 102, 258–269 10.1093/cvr/cvu062 [DOI] [PubMed] [Google Scholar]
- 267.Lei H., Schmidt-Bleek K., Dienelt A., Reinke P. and Volk H.D. (2015) Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front. Pharmacol. 6, 184 10.3389/fphar.2015.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wu J., Ren B., Wang D. and Lin H. (2022) Regulatory T cells in skeletal muscle repair and regeneration: recent insights. Cell Death Dis. 13, 680 10.1038/s41419-022-05142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Smigiel K.S., Srivastava S., Stolley J.M. and Campbell D.J. (2014) Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol. Rev. 259, 40–59 10.1111/imr.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bos S., Vos R., Van Raemdonck D.E. and Verleden G.M. (2020) Survival in adult lung transplantation: where are we in 2020? Curr. Opinion Organ Transplant. 25, 268–273 10.1097/MOT.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 271.Gauthier J.M., Harrison M.S., Krupnick A.S., Gelman A.E. and Kreisel D. (2019) The emerging role of regulatory T cells following lung transplantation. Immunol. Rev. 292, 194–208 10.1111/imr.12801 [DOI] [PubMed] [Google Scholar]
- 272.Moffatt S.D., Demers P., Robbins R.C., Doyle R., Wienacker A., Henig N.et al. (2005) Lung transplantation: a decade of experience. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 24, 145–151 10.1016/j.healun.2003.10.020 [DOI] [PubMed] [Google Scholar]
- 273.Weber D.J. and Wilkes D.S. (2013) The role of autoimmunity in obliterative bronchiolitis after lung transplantation. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L307–L311 10.1152/ajplung.00378.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yoshida S., Haque A., Mizobuchi T., Iwata T., Chiyo M., Webb T.J.et al. (2006) Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surgeons 6, 724–735 10.1111/j.1600-6143.2006.01236.x [DOI] [PubMed] [Google Scholar]
- 275.De Paepe A., Nuytinck L., Hausser I., Anton-Lamprecht I. and Naeyaert J.M. (1997) Mutations in the COL5A1 gene are causal in the Ehlers-Danlos syndromes I and II. Am. J. Hum. Genet. 60, 547–554 [PMC free article] [PubMed] [Google Scholar]
- 276.Burlingham W.J., Love R.B., Jankowska-Gan E., Haynes L.D., Xu Q., Bobadilla J.L.et al. (2007) IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J. Clin. Invest. 117, 3498–3506 10.1172/JCI28031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gelman A.E., Li W., Richardson S.B., Zinselmeyer B.H., Lai J., Okazaki M.et al. (2009) Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J. Immunol. (Baltimore, Md: 1950) 182, 3969–3973 10.4049/jimmunol.0803514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Cao H., Lan Q., Shi Q., Zhou X., Liu G., Liu J.et al. (2011) Anti-IL-23 antibody blockade of IL-23/IL-17 pathway attenuates airway obliteration in rat orthotopic tracheal transplantation. Int. Immunopharmacol. 11, 569–575 10.1016/j.intimp.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 279.Deng S., Xi Y., Wang H., Hao J., Niu X., Li W.et al. (2010) Regulatory effect of vasoactive intestinal peptide on the balance of Treg and Th17 in collagen-induced arthritis. Cell. Immunol. 265, 105–110 10.1016/j.cellimm.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 280.Benwell R.K. and Lee D.R. (2010) Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clin. Immunol. 134, 178–187 10.1016/j.clim.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 281.Noack M. and Miossec P. (2014) Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 13, 668–677 10.1016/j.autrev.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 282.Romagnani S., Maggi E., Liotta F., Cosmi L. and Annunziato F. (2009) Properties and origin of human Th17 cells. Mol. Immunol. 47, 3–7 10.1016/j.molimm.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 283.D'Alessio F.R., Zhong Q., Jenkins J., Moldobaeva A. and Wagner E.M. (2015) Lung angiogenesis requires CD4(+) forkhead homeobox protein-3(+) regulatory T cells. Am. J. Respir. Cell Mol. Biol. 52, 603–610 10.1165/rcmb.2014-0278OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Meloni F., Cascina A., Miserere S., Perotti C., Vitulo P. and Fietta A.M. (2007) Peripheral CD4(+)CD25(+) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplant. Proc. 39, 213–217 10.1016/j.transproceed.2006.10.227 [DOI] [PubMed] [Google Scholar]
- 285.Meloni F., Vitulo P., Bianco A.M., Paschetto E., Morosini M., Cascina A.et al. (2004) Regulatory CD4+CD25+ T cells in the peripheral blood of lung transplant recipients: correlation with transplant outcome. Transplantation 77, 762–766 10.1097/01.TP.0000116565.86752.6B [DOI] [PubMed] [Google Scholar]
- 286.Selvaraj R.K. and Geiger T.L. (2008) Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J. Immunol.(Baltimore, Md: 1950) 180, 2830–2838 10.4049/jimmunol.180.5.2830 [DOI] [PubMed] [Google Scholar]
- 287.Wechsler M.E., Rao V.V., Borelli A.N. and Anseth K.S. (2021) Engineering the MSC secretome: a hydrogel focused approach. Adv. Healthc. Mater. 10, e2001948 10.1002/adhm.202001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shevyrev D. and Tereshchenko V. (2019) Treg heterogeneity, function, and homeostasis. Front. Immunol. 10, 3100 10.3389/fimmu.2019.03100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hoshino M. and Dmochowski L. (1973) Electron microscope study of antigens in cells of mouse mammary tumor cell lines by peroxidase-labeled antibodies in sera of mammary tumor-bearing mice and of patients with breast cancer. Cancer Res. 33, 2551–2561 [PubMed] [Google Scholar]
- 290.Wils J.A. (1980) Moderately high-dosage methotrexate medication in metastatic breast cancer. Neth. J. Med. 23, 59–61 [PubMed] [Google Scholar]
- 291.Garibaldi B.T., D'Alessio F.R., Mock J.R., Files D.C., Chau E., Eto Y.et al. (2013) Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am. J. Respir. Cell Mol. Biol. 48, 35–43 10.1165/rcmb.2012-0198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Odintsova T.I. and Egorov Ts A. (1989) Isolation and characterization of gliadins from Aegilops squarrosa seeds. Biokhimiia 54, 396–408 [PubMed] [Google Scholar]
- 293.Ma R., Su H., Jiao K. and Liu J. (2023) Role of Th17 cells, Treg cells, and Th17/Treg imbalance in immune homeostasis disorders in patients with chronic obstructive pulmonary disease. Immun. Inflamm. Dis. 11, e784 10.1002/iid3.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kanazawa T., Asahi H., Hata H., Mochida K., Kagei N. and Stadecker M.J. (1993) Arginine-dependent generation of reactive nitrogen intermediates is instrumental in the in vitro killing of protoscoleces of Echinococcus multilocularis by activated macrophages. Parasite Immunol. 15, 619–623 10.1111/j.1365-3024.1993.tb00575.x [DOI] [PubMed] [Google Scholar]
- 295.Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M.et al. (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 10.1038/nature06306 [DOI] [PubMed] [Google Scholar]
- 296.Niedbala W., Wei X.Q., Cai B., Hueber A.J., Leung B.P., McInnes I.B.et al. (2007) IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 37, 3021–3029 10.1002/eji.200737810 [DOI] [PubMed] [Google Scholar]
- 297.Sasidharan Nair V. and Elkord E. (2018) Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol. Cell Biol. 96, 21–33 10.1111/imcb.1003 [DOI] [PubMed] [Google Scholar]
- 298.Masaki N., Ikeda H., Nishiyama K., Inoue T., Matayoshi Y. and Aozasa K. (1988) Results of radiation therapy of stage I-II non-Hodgkin’s lymphoma localized in the head and neck: Osaka University Hospital experience. Gan No Rinsho 34, 599–605 [PubMed] [Google Scholar]
- 299.Hao Y., Dong H., Li W., Lv X., Shi B. and Gao P. (2022) The molecular role of IL-35 in non-small cell lung cancer. Front. Oncol. 12, 874823 10.3389/fonc.2022.874823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhang L.F. and Xia C.Q. (2013) Ex vivo expansion of regulatory T cells for clinical applications against graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Chin. Med. J. 126, 4575–4582 [PubMed] [Google Scholar]
- 301.Riegel C., Boeld T.J., Doser K., Huber E., Hoffmann P. and Edinger M. (2020) Efficient treatment of murine acute GvHD by in vitro expanded donor regulatory T cells. Leukemia 34, 895–908 10.1038/s41375-019-0625-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Baeten P., Van Zeebroeck L., Kleinewietfeld M., Hellings N. and Broux B. (2022) Improving the efficacy of regulatory T cell therapy. Clin. Rev. Allergy Immunol. 62, 363–381 10.1007/s12016-021-08866-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pavón-Romero G.F., Parra-Vargas M.I., Ramírez-Jiménez F., Melgoza-Ruiz E., Serrano-Pérez N.H. and Teran L.M. (2022) Allergen immunotherapy: current and future trends. Cells 11, 212 10.3390/cells11020212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Xu Z., Jiang X., Dai X. and Li B. (2022) The dynamic role of FOXP3(+) Tregs and their potential therapeutic applications during SARS-CoV-2 infection. Front Immunol. 13, 916411 10.3389/fimmu.2022.916411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Madeddu C., Donisi C., Liscia N., Lai E., Scartozzi M. and Macciò A. (2022) EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: role of the tumor microenvironment. Int. J. Mol. Sci. 23, 6489 10.3390/ijms23126489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Van Damme H., Dombrecht B., Kiss M., Roose H., Allen E., Van Overmeire E.et al. (2021) Therapeutic depletion of CCR8(+) tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J. Immunother Cancer 9, e001749 10.1136/jitc-2020-001749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Guo W.W., Su X.H., Wang M.Y., Han M.Z., Feng X.M. and Jiang E.L. (2021) Regulatory T cells in GVHD therapy. Front. Immunol. 12, 697854 10.3389/fimmu.2021.697854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wolf D., Barreras H., Bader C.S., Copsel S., Lightbourn C.O., Pfeiffer B.J.et al. (2017) Marked in vivo donor regulatory T cell expansion via interleukin-2 and TL1A-Ig stimulation ameliorates graft-versus-host disease but preserves graft-versus-leukemia in recipients after hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. J. Am. Soc. Blood Marrow Transpl. 23, 757–766 10.1016/j.bbmt.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Duramad O., Laysang A., Li J., Ishii Y. and Namikawa R. (2011) Pharmacologic expansion of donor-derived, naturally occurring CD4(+)Foxp3(+) regulatory T cells reduces acute graft-versus-host disease lethality without abrogating the graft-versus-leukemia effect in murine models. Biol. Blood Marrow Transpl. J. Am. Soc. Blood Marrow Transpl. 17, 1154–1168 10.1016/j.bbmt.2010.11.022 [DOI] [PubMed] [Google Scholar]
- 310.Liu D.D., Hong W.C., Qiu K.Y., Li X.Y., Liu Y., Zhu L.W.et al. (2022) Umbilical cord blood: A promising source for allogeneic CAR-T cells. Front. Oncol. 12, 944248 10.3389/fonc.2022.944248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Eggenhuizen P.J., Ng B.H. and Ooi J.D. (2020) Treg enhancing therapies to treat autoimmune diseases. Int. J. Mol. Sci. 21, 7015 10.3390/ijms21197015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Parmar S., Liu X., Tung S.S., Robinson S.N., Rodriguez G., Cooper L.J.et al. (2014) Third-party umbilical cord blood-derived regulatory T cells prevent xenogenic graft-versus-host disease. Cytotherapy 16, 90–100 10.1016/j.jcyt.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hui Z., Zhang J., Zheng Y., Yang L., Yu W., An Y.et al. (2021) Single-cell sequencing reveals the transcriptome and TCR characteristics of pTregs and in vitro expanded iTregs. Front. Immunol. 12, 619932 10.3389/fimmu.2021.619932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Arjomandnejad M., Kopec A.L. and Keeler A.M. (2022) CAR-T regulatory (CAR-Treg) cells: engineering and applications. Biomedicines 10, 287 10.3390/biomedicines10020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Amini L., Greig J., Schmueck-Henneresse M., Volk H.D., Bézie S., Reinke P.et al. (2020) Super-Treg: toward a new era of adoptive Treg therapy enabled by genetic modifications. Front. Immunol. 11, 611638 10.3389/fimmu.2020.611638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Raffin C., Vo L.T. and Bluestone J.A. (2020) T(reg) cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 20, 158–172 10.1038/s41577-019-0232-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ikegawa S. and Matsuoka K.I. (2021) Harnessing Treg homeostasis to optimize posttransplant immunity: current concepts and future perspectives. Front. Immunol. 12, 713358 10.3389/fimmu.2021.713358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Fritsche E., Volk H.D., Reinke P. and Abou-El-Enein M. (2020) Toward an optimized process for clinical manufacturing of CAR-Treg cell therapy. Trends Biotechnol. 38, 1099–1112 10.1016/j.tibtech.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 319.Janssens I. and Cools N. (2020) Regulating the regulators: Is introduction of an antigen-specific approach in regulatory T cells the next step to treat autoimmunity? Cell. Immunol. 358, 104236 10.1016/j.cellimm.2020.104236 [DOI] [PubMed] [Google Scholar]
- 320.Romano M., Sen M., Scottà C., Alhabbab R.Y., Rico-Armada A., Lechler R.I.et al. (2021) Isolation and expansion of thymus-derived regulatory T cells for use in pediatric heart transplant patients. Eur. J. Immunol. 51, 2086–2092 10.1002/eji.202048949 [DOI] [PubMed] [Google Scholar]
- 321.Chawla A.S., Khalsa J.K., Dhar A., Gupta S., Umar D., Arimbasseri G.A.et al. (2020) A role for cell-autocrine interleukin-2 in regulatory T-cell homeostasis. Immunology 160, 295–309 10.1111/imm.13194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Murdoch J.R., Gregory L.G. and Lloyd C.M. (2014) gammadeltaT cells regulate chronic airway inflammation and development of airway remodelling. Clin. Exp. Allergy 44, 1386–1398 10.1111/cea.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rashedi I., Gomez-Aristizabal A., Wang X.H., Viswanathan S. and Keating A. (2017) TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated Treg induction via notch signaling. Stem Cells 35, 265–275 10.1002/stem.2485 [DOI] [PubMed] [Google Scholar]
- 324.Boonpiyathad T., Satitsuksanoa P., Akdis M. and Akdis C.A. (2019) Il-10 producing T and B cells in allergy. Semin. Immunol. 44, 101326 10.1016/j.smim.2019.101326 [DOI] [PubMed] [Google Scholar]
- 325.Lu X.X., McCoy K.S., Xu J.L., Hu W.K. and Chen H.B. (2013) Small interfering RNA targeting T-cell Ig mucin-3 decreases allergic airway inflammation and hyperresponsiveness. Inflammation 36, 582–591 10.1007/s10753-012-9580-0 [DOI] [PubMed] [Google Scholar]