Abstract
The intricacy of diseases, shaped by intrinsic processes like immune system exhaustion and hyperactivation, highlights the potential of immune renormalization as a promising strategy in disease treatment. In recent years, our primary focus has centered on γδ T cell-based immunotherapy, particularly pioneering the use of allogeneic Vδ2+ γδ T cells for treating late-stage solid tumors and tuberculosis patients. However, we recognize untapped potential and optimization opportunities to fully harness γδ T cell effector functions in immunotherapy. This review aims to thoroughly examine γδ T cell immunology and its role in diseases. Initially, we elucidate functional differences between γδ T cells and their αβ T cell counterparts. We also provide an overview of major milestones in γδ T cell research since their discovery in 1984. Furthermore, we delve into the intricate biological processes governing their origin, development, fate decisions, and T cell receptor (TCR) rearrangement within the thymus. By examining the mechanisms underlying the anti-tumor functions of distinct γδ T cell subtypes based on γδTCR structure or cytokine release, we emphasize the importance of accurate subtyping in understanding γδ T cell function. We also explore the microenvironment-dependent functions of γδ T cell subsets, particularly in infectious diseases, autoimmune conditions, hematological malignancies, and solid tumors. Finally, we propose future strategies for utilizing allogeneic γδ T cells in tumor immunotherapy. Through this comprehensive review, we aim to provide readers with a holistic understanding of the molecular fundamentals and translational research frontiers of γδ T cells, ultimately contributing to further advancements in harnessing the therapeutic potential of γδ T cells.
Subject terms: Lymphocytes, Innate immunity, Adaptive immunity, Tumour immunology, Immunological disorders
Introduction
Until now, cancer remains one of the biggest challenges for human health.1 The underlying cause is that cancer cells originate from healthy cells, which results in highly similar molecular fingerprints. This similarity makes it difficult for the immune system to recognize and efficiently kill transformed cells in a timely manner. Simultaneously, the unique microenvironment created by the transformed cells progressively attenuates immune functions, leading ultimately to immune escape.2–4 The imbalanced or exhausted immune system is widely acknowledged as one of the key physiological hallmarks of tumor patients, including reduced numbers of total leukocytes, dysfunctional γδ T cell subsets, and increased proportions of exhausted CD8+ T cells and Tregs, among others.5–10 Since 2016, we have been at the forefront of translating the application of allogeneic γδ T cells, specifically the Vγ9Vδ2+ γδ T subset, from the laboratory to clinical practice, with the aim of renormalizing the dysfunctional immune system in patients with advanced solid tumor11,12 or multidrug-resistant tuberculosis (MDR-TB).13 In a comprehensive study involving 132 patients diagnosed with various types of cancer (including liver, lung, pancreatic, breast, and others), we administered a total of 414 cell infusions. Through this investigation, we not only established the safety of transferring allogeneic Vγ9Vδ2+ γδ T cells (abbreviated as Vδ2 T cells below) generated from healthy donors’ PBMCs after in vitro expansion but also demonstrated their clinical efficacy in extending patient survival and improving quality of life.11 Nevertheless, while conducting this investigator-initiated clinical trial, it became apparent that patients’ responses to allogeneic Vδ2 T cell therapy varied, with some demonstrating favorable outcomes, while others experienced only modest improvement. This highlights the need to uncover the underlying factors that contribute to the failure of infused cells in inducing an immune response against cancer cells, particularly the adverse effects of the tumor microenvironment. In this comprehensive review, we explore the origin and fate of γδ T cells, their subsets, their relevance to various diseases including infections, autoimmune diseases, and cancer, as well as their functional differences, vulnerability, and transition within these contexts. Additionally, based on our insights and updated knowledge, we discuss and propose viable strategies for the application of allogeneic γδ T cells as promising immunotherapy for various diseases.
γδ T cells vs αβ T cells
In the realm of cellular immunity, γδ T cells stand apart from their αβ T cell counterparts due to their distinct attributes, which intricately shape their roles in both pathogenesis of diseases and the field of immunotherapy. αβ T cells, forming the predominant subset of CD3+ T cells within the immune repertoire, predominantly recognize peptide antigens presented by major histocompatibility complex (MHC) molecules. In contrast, γδ T cells adopt an alternative T cell receptor (TCR) architecture, consisting of γ and δ chains, granting them the ability to perceive a wider array of antigens in MHC-independent manner. This remarkable property encompasses recognition of both exogenous and endogenous antigens, spanning foreign as well as self-antigens.8,9,14
This dichotomy between γδ and αβ T cells extends across numerous dimensions, encompassing TCR structural variances, thymic developmental trajectories, mechanisms of antigen identification and presentation, activation cues, their roles in pathological conditions, and their applications in immunotherapy. The structural divergence of the γδ TCR and αβ TCR serves as the bedrock for distinguishing the functional roles these two T cell subsets assume within immune responses. This distinction becomes particularly evident when delving into their respective thymic development, as elaborated upon in forthcoming sections. Specifically, while the developmental journey of αβ T cells entails stages of double-negative (DN), double-positive (DP), and single-positive (SP) prior to dissemination into the circulation, γδ T cells follow a distinct course. The latter either exit the thymus during the DN (DN2-DN3) phase or progress through DN and DP or DN, DP, and SP stages before embarking into circulation. This unique developmental pattern equips γδ T cells with a greater spectrum of immune functions, spanning both innate and adaptive roles, along with diversified capacities in antigen recognition and presentation.
Distinctly stratified in their antigen recognition, αβ T cells operate within the confines of MHC-dependent recognition, whereas γδ T cells extend their sensing capabilities to include stress-induced antigens, phosphoantigens, and other non-peptidic molecules, all while circumventing the need for MHC mediation. Yet, the most pivotal divergence lies in their activation mechanisms and antigen presentation capabilities. While activation of αβ T cells necessitates a dual input of signals—antigen recognition and co-stimulation—to orchestrate immune responses, γδ T cells can be activated by a singular signal,15 such as a phosphoantigen. Notably, within the realm of antigen presentation, a specific subset of human γδ T cells, the Vδ2 T cells, takes on the role of professional antigen-presenting cells (APCs), a function beyond the purview of αβ T cells. This unique attribute situates γδ T cells as key regulators of immune functions within the broader immune cell landscape.
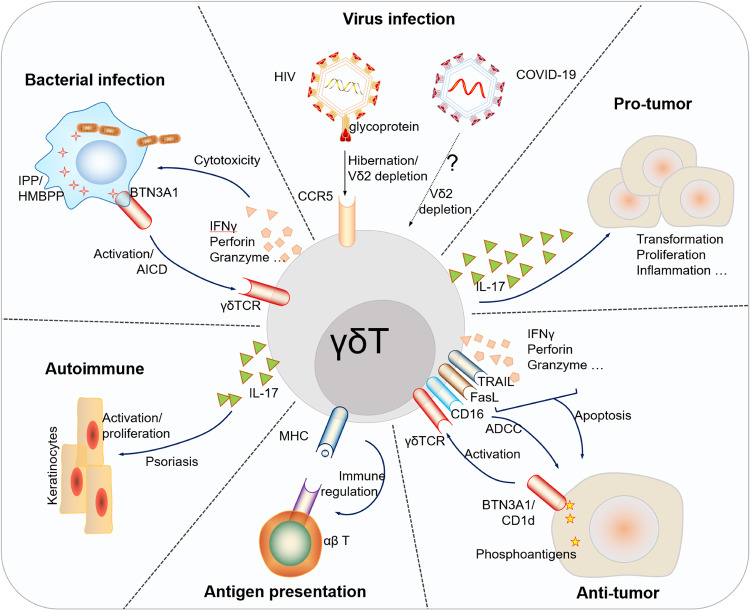
In terms of disease, αβ T cells are well-known for their adaptive immune responses and are critical in combating infections and mounting antigen-specific immune responses.9,16–18 They are highly specialized and undergo clonal expansion upon encountering specific antigens, however, the frequency of cells among αβ T cells which can recognize a given peptide antigen is extremely low. In contrast, γδ T cells exhibit characteristics of both innate and adaptive immunity. They can rapidly respond to various pathogens through their innate-like receptors, allowing for early immune defense even in the absence of prior antigen exposure.9,14 Moreover, γδ T cells are actively involved in tissue surveillance at barrier sites and contribute to the maintenance of tissue homeostasis.10,19 In disease settings, γδ T cells have been implicated in both protective and pathogenic roles. Their ability to respond rapidly to infections and produce cytokines enables them to contribute to pathogen clearance. However, dysregulation of γδ T cell activation and function has been associated with the development of autoimmune diseases, where these cells can recognize self-antigens and contribute to tissue damage.7,20
In the field of immunotherapy, αβ T cells have been extensively studied fundamentally and clinically, and the paradigm is chimeric antigen receptor (CAR)-T cell therapy, which targets specific antigens on cancerous or autoreactive immune cells.21–24 In comparison, γδ T cells are relatively less explored but show great potential. Due to their innate-like features, γδ T cells have the capacity to recognize and eliminate tumor cells without prior sensitization. This makes them attractive candidates for immunotherapeutic strategies, including administration of freshly expanded11,12 and genetically modified γδ T cells (e.g. CAR-γδ T).25–27
The multifaceted functions and extensive antigen recognition abilities of γδ T cells, coupled with their unique properties such as innate and adaptive-like traits and the capacity to identify stress-induced molecules, render them invaluable in the context of diseases and as prime candidates for immunotherapeutic strategies. To benefit further research on the mechanisms underlying γδ T cell roles in disease and on the optimization of its therapeutic potential, we comprehensively reviewed the origin and fate, γδ T cell subsets, and their roles in diseases and immunotherapy.
Chronological milestones of γδ T cell research
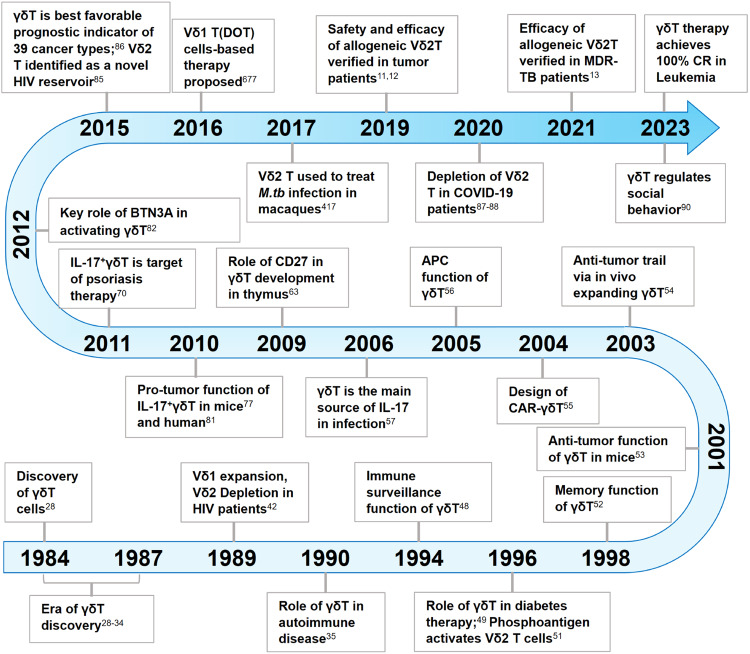
To help readers better establish a whole picture about the discovery and the roles of γδ T cells in diseases, we summarize the milestones about γδ T cells since their discovery from the beginning of 1980s (Fig. 1). In fact, it came as a big surprise when the existence of a second set of rearranging TCR genes was discovered. In 1984, γδ T cells were first reported by Tonegawa et al.,28 with significant contributions from Adrian C. Hayday.28,29 Until 1987, important work on identifying γ and δ chains and their rearrangements marked a key era in γδ T cell discovery.28–34 These foundational discoveries have laid the groundwork for comprehending their distinctive attributes and have opened doors for more extensive investigations into their functional roles within the immune system and across diverse disease contexts. Starting in 1989, studies began to unravel the involvement of γδ T cells in autoimmune diseases and anti-infection immunity.35–44 Notably, in human immunodeficiency virus (HIV) infected patients, a shift in the γδ T cell subtypes was observed, with an expansion of Vδ1+ and depletion of Vδ2+ subtype, leading to an inversion of Vδ2/Vδ1 ratios in circulating γδ T cells.42,45–47 These findings provided important insights into the dysregulation of γδ T cell populations in specific diseases.
Fig. 1.
Chronological Milestones of γδ T cell research from its discovery in 1984 till 2023. HIV human immunodeficiency virus, CAR chimeric antigen receptor, APC antigen-presenting cell, IL-17 interleukin 17, BTN3A butyrophilin 3A, M.tb mycobacterium tuberculosis, MDR-TB multidrug-resistant tuberculosis, CR complete remission, COVID-19 coronavirus disease of 2019
In the following years, the multifaceted functions of γδ T cells were further elucidated. In 1994, the role of intraepithelial γδ T cells in immune surveillance and tissue repair was reported,48 highlighting their significance in monitoring and maintaining the integrity of damaged epithelial tissues. Additionally, the therapeutic potential of γδ T cells was demonstrated in mouse models of diabetes and allergic airway inflammation.49,50 Furthermore, the discovery of the activation of Vδ2 T cells by so-called ‘phosphoantigens’ opened avenues for exploring the unique activation mechanisms of γδ T cells,51 and the memory function52 demonstrated the adaptive immunity of γδ T cells.
From 2001 to 2010, several milestones were achieved in the field of γδ T cell research. The anti-tumor function of γδ T cells was discovered in murine models, leading to their use as immunotherapy for lymphoid malignancies.53,54 Genetic modification techniques were applied to enhance the cytotoxicity of γδ T cells, pioneering the design of CAR-γδ T cells.55 Furthermore, γδ T cells were found to serve as professional APCs,56 expanding our understanding of their immune regulatory functions. During this period, IL-17-producing γδ T cells gained great attention,57–67 particularly in the context of infectious and inflammatory diseases. Their crucial roles in immune responses to pathogens, such as Mycobacterium tuberculosis (M.tb) and Escherichia coli, were elucidated.57–60 Moreover, the association between γδ T cells and autoimmune inflammation was established,62,68 further underscoring their diverse functions in immune homeostasis.
In the subsequent years, research efforts focused on IL-17-producing γδ T cells and their implications in human diseases. Their involvement in psoriasis, as both necessary and sufficient for plaque formation, identified IL-17-producing γδ T cells as promising therapeutic target.69–75 Notably, the pro-tumor role played by IL-17+γδ T cells in both mice76–79 and humans80,81 has significantly enhanced our understanding of the involvement of γδ T cells in tumorigenesis. A significant breakthrough was the discovery of BTN3A182 as a sensing molecule for phosphoantigens which added substantially to our understanding of the mechanisms of activation of human γδ T cells. Furthermore, the identification of memory γδ T cells revealed their adaptive functions and immune memory capabilities.83,84 Additionally, γδ T cells’ adverse role as a host for HIV latent infection85 and their prognostic value in various types of cancer86 highlighted their broader clinical significance.
In recent years, groundbreaking advancements have been made in the clinical application of γδ T cells. Studies from our group demonstrated the safety and efficacy of allogeneic Vδ2 T cells derived from healthy donors in the treatment of late-stage lung and liver cancer patients,11,12 pointing towards the potential of off-the-shelf Vδ2 T cell products in cancer immunotherapy. Furthermore, the successful application of γδ T cells in treating MDR-TB13 and their functional attenuation in COVID-19 patients87–89 expanded our understanding of their therapeutic potential in combating challenging infectious diseases. Strikingly, the regulatory role of γδ T cells in the social behavior of mice90 and the achievement of 100% complete remission in leukemia patients (NCT03533816) who received γδ T cell therapy have heightened our anticipation regarding the physiological functions of γδ T cells.
In summary, the chronological progression of γδ T cell research has revealed their diverse functions and therapeutic implications in autoimmune diseases, infections, and cancers. From understanding their roles in immune surveillance and tissue repair to their applications in immunotherapy and disease management, γδ T cells have emerged as important players in the field of immunology. Continued research in this area holds great promise for the development of novel therapeutic strategies and improved patient outcomes.
γδ T cell origin and development
γδ T cell origin
Like αβ T cells, γδ T cells develop in the thymus from progenitor T cells originating from bone marrow hematopoietic stem cells. They are considered the earliest T cell subset in vertebrates. In murine models, γδ T cell development in the thymus has been extensively studied,91–93 revealing that DN (CD4−CD8−) cells expressing TCR-γδ commit to the γδ lineage without undergoing DP (CD4+CD8+) selection. Conversely, for αβ T cells, DN cells expressing the pre-TCR (TCR‐β paired with the invariant pre‐TCR‐α chain) develop through DP and then differentiate into SP (CD4+ or CD8+) cells.16,92,94–98 Evidence suggests that γδ T cell development in the human thymus follows a similar pattern,93 although the regulatory mechanisms, including signaling, factors, and molecular processes controlling V(D)J rearrangement, require further confirmation. Often, these mechanisms are investigated based on findings from murine studies.95 Nonetheless, more and more insights into the development of human γδ T cells in the thymus are gradually accumulating.
During thymic development, γδ T cells precede αβ T cells in ontogeny, and γδ TCR rearrangements occur early in embryonic stages in mice and humans.99–101 γδ versus αβ T cell commitment depends on TCR signal strength and Notch signaling.102–104 In mice, strong TCR signaling without Notch signal induces γδ lineage commitment, while low TCR signal strength with strong Notch signaling promotes αβ lineage.105–107 Notch signaling alone is insufficient to determine γδ/αβ commitment. Intrinsic signals from the TCR complex, along with trans-conditioning by different thymocyte subsets, also contribute to this process.108
In humans, sustained Notch signaling is required for γδ T cell development, mediated by specific Notch receptor–ligand interactions, particularly Jagged2/Notch3 signaling.109,110 Human γδ T cell differentiation involves a Notch-independent DN pathway generating mature DN and SP (CD8+) γδ T cells, and a Notch-dependent DP pathway producing immature CD4+ SP cells followed by DP γδ T cells. The postnatal human thymus exhibits DN, DP, and SP TCRγδ+ populations, highlighting heterogeneity.97,103,111 Although only a small fraction of γδ T cells co-express either CD8 or CD4 (SP) on their surface, with CD8+γδ T cell population being the most abundant, this implies a fraction of γδ T cells undergo the similar DP to SP development route as αβ T cell since they share the same co-receptor CD8 and CD4. This observation is puzzling since unlike αβ T cells, γδ T cell mediated recognition is MHC non-restricted, therefore, the exact role of CD8 or CD4 expression on γδ T cells and precise ontogenesis of thymic γδ T cells awaits further elucidation. Notably, growing evidence has revealed that circulating γδ T cells also express high level of CD56, endorsing γδ T cells phenotypically similar to natural killer T-cells (NKT), which mature in thymus at the DP stage. Whether or not and how CD56+γδ T (γδNKT) cells112,113 mature at the DP stage remain mysterious and to be fundamentally resolved. Collectively, the above discussions are briefly sketched in Fig. 2. Additionally, activated extrathymic γδ T cells express Notch receptors, regulating effector functions. Inhibiting Notch signaling has been shown to impair the anti-tumor cytotoxicity of γδ T cells, providing further evidence of its significance in both thymic development and overall function.114 The human γδ T cell repertoire undergoes diversification at birth, with the Vδ1+ subset dominating in cord blood. However, as individuals mature into adulthood, this repertoire becomes more constrained, and the Vγ9Vδ2 subset takes precedence in peripheral blood, constituting 75% or more of the γδ T cell population.99 Additionally, the Vδ1+ subset was also found to be enriched in the post-natal thymus, demonstrating thymic rearrangement and expression of TRG and TRD genes.115 This finding supports previous conclusions regarding the TCR repertoire of γδ T cells that develop in the human thymus. Altogether, understanding γδ T cell development illuminates their roles in immune surveillance and responses, providing insights into regulatory mechanisms and heterogeneity within this T cell subset.
Fig. 2.
The possible mechanisms of human γδ T development and fate decision in thymus. In circulation, the phenotypes of γδ T cells at least include naïve γδ T cells, IFNγ-producing γδ T cells (γδT1), IL-17-producing γδ T cells (γδT17), IFNγ/IL4-producing γδ T cells (γδNKT or CD56+γδT), very rare CD4+γδ T cells, and CD8+γδ T cells
γδ-TCR V(D)J recombination
Overall, γδTCR expression was detected by 14 days of gestation in murine100 and by eight weeks of fetal development in human.116 They constitute the initial T cell lineage to undergo development within the thymus and then migrate to various tissues, where they serve as swift producers of effector cytokines like IFNγ and IL-17, crucial for barrier defense. The divergence between γδ and αβ T cells takes place during their development in the thymus at the DN stage. At this stage, thymocytes evolve into two distinct T cell lineages based on the expression of either γδ or αβ TCRs.117–120 Most of the γδ T cells remain DN and develop into mature γδ T cells before they egress from the thymus.
The generation of a diverse TCR repertoire involves the V(D)J recombination of the four TCR loci. This recombination occurs at different stages of thymocyte development, with TRB, TRG, and TRD loci rearranging in the CD34+ stages, and TRA rearranging in the DP stage.121,122 Rearrangement of the TRG locus happens earlier and is potentially completed earlier than the TRB locus, indicating sequential and overlapping rearrangement windows. The human TRG locus consists of 14 TRGV genes (of which only six are functionally expressed; Vγ2-5, Vγ8, Vγ9) and 5 TRGJ genes (JP1, JP, J1, JP2, J2), which can associate with one of two TRGC elements.9 During fetal development, central TRGV elements are predominantly rearranged, while postnatal thymocytes mainly use distal TRGV and TRGJ segments with TRGC2.123,124 The TRD locus contains eight TRDV segments, of which TRDV4-8 also have TRA designation due to their location within the TRA locus. The usage of V segments in V(D)J recombination changes during development, with fetal thymocytes favoring downstream TRDV and TRDJ segments and a shift towards more upstream elements occurring later in life.95,124,125 It should be marked here that one major distinction of γδ T cells from conventional αβ T cells, is the diversity of TCR sequences endowed by the recombination activating gene (RAG)-mediated V(D)J recombination of TCRδ (TRD) locus (TRDV, TRDD, TRDJ) and TCRγ (TRG) locus (TRGV, TRGJ), similar to the TCRβ locus (TRB) and TCRα (TRA) of αβ T cells.9 Despite the low number of functionally expressed Vγ and Vδ genes (see above), theoretically, γδ T cells can generate up to 10^17-10^18 γδTCRs due to non-germline encoded variability occurring during recombination,14,126 compared with αβ TCRs, which can generate 10^15-10^18 αβTCRs. However, in reality, most of the peripheral Vδ2 T cells display semi-invariant TCR repertoires, using the same Vγ9 gene segments in both cord and adult blood.127,128 This may be due to continuous microbial exposures after birth, leading to the focusing of Vγ9Vδ2 T cell repertoire among individuals.14,128,129 Moreover, the reduction of γδTCR diversity in cancer patients130 suggests that tumor antigen recognition can also result in clonal focusing of the γδ TCR repertoire.
In human, the incorporation of nucleotides during V(D)J recombination varies between embryonic, fetal, and postnatal γδ thymocytes. Fetal thymocytes, characterized by delayed induction of terminal deoxynucleotidyl transferase (TdT), exhibit highly invariant germline-encoded complementarity-determining region-3 (CDR3) sequences in γδ T cells generated during early development. The expression of TdT is regulated by the RNA-binding protein LIN28B, which is abundantly expressed in fetal γδ T cells and acts as an inhibitor of TdT. In the absence of TdT, short homology repeats present in certain V/D/J segments can facilitate recombination, resulting in the formation of specific germline-encoded sequences in fetal γδ thymocytes. This differential regulation of TdT and the utilization of short homology repeats are responsible for the generation of invariant/public cytomegalovirus (CMV)-reactive CDR3 sequences and the acquisition of effector functions in the fetal γδ T cell repertoire. These distinct characteristics are attributed to the intrinsic properties of fetal hematopoietic stem and precursor cells, characterized by high expression of LIN28B, and are dependent on the HSPC/LIN28B axis within the human fetal thymus.115,124,131,132 Notably, γδ-TCR recombination involves strict regulation, the allelic exclusion, which refers to the process of achieving monoallelic expression of a gene. While biallelic rearrangements have been observed at the TRD locus, they are less frequent and mostly represent incomplete or out-of-frame rearrangements. In contrast, functional rearrangements at both TRG alleles suggest allelic inclusion for this locus, allowing the expression of two different γ-chains on the same cell.133–135
γδ-TCR V(D)J recombination signaling
The factors and molecular processes governing V(D)J recombination at the TRD and TRG loci in humans are not fully understood, but studies in mice suggest IL7R signaling, E proteins (HEB and E2A), Notch signaling, and transcription factors MYB and RUNX1 play crucial or important roles in regulation of TRD/TRG rearrangement.91,102–104,109,136,137 For IL7R signaling, its role in regulating Trg rearrangement has been mainly documented in murine. In human, however, even though it has been implicated in the regulation of TRG rearrangement as well, further evidence is required. IL7R signaling induces histone acetylation, chromatin accessibility, transcription, and rearrangement at the Trg locus through IL7-induced recruitment of STAT5 to the Trg enhancer Eγ.138–140 E proteins (HEB and E2A) play a crucial role in regulating V(D)J recombination at the TRG and TRD loci. They can induce recombination at the human TRG and TRD loci in non-lymphoid cells, likely by controlling accessibility at recombination signal sequence (RSS) sites.141–143 Notch signaling, in addition to its positive effects on TCR rearrangement, can negatively control the process by inhibiting E protein function and promoting degradation of E2A, and can upregulate MYB and RUNX1, which are involved in promoting chromatin accessibility and germline transcription at the TRG and TRD loci.144,145 These pathways and transcription factors are interconnected, as shown by Notch-mediated induction of MYB and RUNX1, which in turn regulate the accessibility and transcriptional activity of the TRG and TRD loci. MYB and RUNX1 can promote chromatin accessibility by recruiting histone-modifying enzymes and chromatin remodeling complexes. Additionally, epigenetic modifications and lineage-specific factors may also play roles in regulating V(D)J recombination.91,95,141 Overall, the regulation of V(D)J recombination at the TRG and TRD loci involves a complex interplay of various signaling pathways, transcription factors, epigenetic modifications, and lineage-specific factors. Further research is still needed to fully understand the precise mechanisms underlying the regulation of TCR gene rearrangement in humans.
γδ selection and fate decision
As one subset of T lymphocytes, γδ T cells also develop from hematopoietic stem and progenitor cells (HSPCs) found in the bone marrow or fetal liver. These HSPCs migrate to the thymus as multipotent thymus seeding progenitors (TSPs) and undergo a complex differentiation process under the influence of the thymic microenvironment. TSPs can also develop into other cell types such as natural killer (NK) cells and dendritic cells (DCs) under specific culture conditions.95,98,110,121,146 Notch signaling, triggered by interaction with Notch ligands on thymic epithelial cells (TECs), leads to the progression of TSPs to the early T cell precursors (ETPs) stage,102,109–111,147 accompanied by the upregulation of genes like GATA3146 and Interleukin 7 receptor (IL7R)104,111,136 crucial for T cell development. ETPs exhibit limited potential to develop into other cell lineages,104,109,147,148 and the transcription factors BCL11B and GATA3 further promote the T cell lineage while suppressing alternative cell fates.146,149 The upregulation of CD1 and recent identification of CD44 loss150 serve as markers of irreversible commitment to the T cell lineage. It is noteworthy to mention that CD44dim expression is observed in normal uncommitted ETPs. The loss of CD44, manifested in terms of gene and protein levels, takes place during the double-negative (DN) stage prior to CD1a surface expression.150 Consequently, the downregulation of CD44 has been recognized as a pivotal and accurate indicator of T-cell commitment (Fig. 2).95 IL7 signaling, induced by TEC-derived IL7, supports the proliferation and survival of T lineage cells,104,137 as evident from IL7R-deficient patients151 lacking T cells. Once committed, T lineage cells can differentiate into either αβ or γδ lineage T cells but lose their potential to develop into non-T lineage cells. Determining the exact stage at which bi-potent progenitors commit to either αβ or γδ lineage has been challenging in human, and the precise definition of these lineages has been ambiguous as well. Thus the γδ T cell receptor has been used as a reliable marker for γδ fate, since no unique surface marker other than TCR has been identified for γδ T cells, and the limited enriched cell surface markers in particular developmental stages are different between murine and human.93,95 Additional complexity arises from the observation that human γδ lineage cells can differentiate through a transient DP stage.103 Lastly, human fetal γδ T cells exhibit a phosphoantigen-reactive TCR repertoire, but the role of endogenous phosphoantigens is uncertain. Ligand-independent TCR signaling, analogous to pre-TCR signaling, potentially influences γδ lineage commitment in humans. Even more complexity is added by the fact that trans-rearrangements between TCR loci have been identified, giving rise to rare αβ T cells which express a Vγ instead of a Vβ gene.152,153, Furthermore, allelic exclusion between TCR αβ vs. γδ genes is not complete, since small number of T cells simultaneously expressing functional αβ and γδ TCRs are present in healthy donors and patients with autoimmune diseases.154 Excitingly, application of advanced technology such as single-cell transcriptome and proteome will significantly benefit the establishment of clear lineage-specific gene expression signatures and the identification of unique surface markers, which will promisingly promote our understanding about γδ T cell fate determination in thymus.93,155,156 For instance, a recent research using single-cell RNA sequencing (scRNA-seq) and high-dimensional flow cytometry has provided an updated insight into the developmental trajectory of Vγ9Vδ2 T cells within the postnatal thymus.157 This trajectory has been delineated into three discrete stages, characterized by the acquisition of functionality and substantial alterations in the expression patterns of transcription factors, chemokines, and surface markers. Specifically, these stages are demarcated as follows: stage 1 cells, identifiable by CD4+CD161−/low markers; stage 2 cells, characterized as CD4−CD161−; and stage 3 cells, distinguished by CD4−CD161+ markers. This work offered a foundational understanding for future investigations into influential factors shaping the development of human γδ T cells in thymus, and particularly enhanced our comprehension of the molecular mechanisms steering human Vγ9Vδ2 T cell development, which would potentially facilitate Vγ9Vδ2 T cell-based immunotherapy in the context of diseases like cancer and infections.
γδ cell fate decision signaling
About the regulation signaling of γδ cell fate decision, serval molecular mechanisms are involved, mainly including TCR signaling, Notch signaling, IL7R signaling, and the transcriptional regulation network (Fig. 2). For the role of TCR signaling158 in deciding αβ fate, two models were proposed: instructive (strength of TCR signaling determines fate) and stochastic (random occurrence from DP to SP).159 For γδ fate decision, it appears to be predetermined rather than randomly occurred in mice based on DN thymocytes expressing high levels of SOX13 or IL7R.160,161 Studies indicate that lineage choice is also determined by the TCR signal strength rather than TCR type. γδ cells exhibit stronger TCR signaling compared to αβ cells, which influences gene expression and cell fate.105,107,119,162 This has been confirmed through manipulations of signal strength, where the γδ TCR activates stronger MAPK signaling, resulting in prolonged ERK activation and stabilization of EGR1.105,163–165 Differences in downstream components and the abundance of γδ TCR contribute to signal intensity. Similar mechanisms are suggested to operate in human thymocytes, where chromatin changes and AP-1 motifs are associated with γδ commitment.166,167 TCR signaling prevents the transition to the αβ lineage and instead induces γδ-like cells in thymus. Upregulation of EGR transcription factors and ID3 further support the role of signal strength as an instructive factor.105,167 While the instructive model likely applies to human γδ T cell development, further research is needed to confirm its validity.
Notch signaling and IL7R signaling play distinct roles in γδ T cell development, with species-specific requirements.102–104,109–111,114,136,138,145,147,148 In mice, Notch signaling promotes αβ lineage development, while in humans, evidence suggests its involvement in favoring the γδ lineage. Notch ligands, particularly JAG2, support γδ T cell development, while DLL1 and DLL4 contribute to αβ lineage development. The molecular mechanisms underlying the preference for γδ fate remain unclear, but Notch signaling counteracts the αβ lineage transcription factor BCL11B. On the other hand, IL7 signaling exhibits species-specific effects. In mice, deficiencies in the IL7 pathway significantly impair γδ lineage development, while the impact on αβ lineage is moderate. In humans, even though several studies indicated that inhibiting IL7R disrupts αβ lineage development but allows reduced γδ differentiation,95,104,137 the in-depth role of IL7R signaling in human γδ lineage commitment requires further investigation.
Identifying the transcription factors involved in establishing γδ fate has been a challenging task as well. Although a transcriptional signature of mouse γδ thymocytes has been described, many factors were also found in other T cell types.95 EGR1-3 and ID3 are potential regulators induced by TCR signaling, with ID3 inhibiting T lineage commitment and TRD rearrangements.105,107,163,168 SOX13 is involved in γδT17 differentiation, while RUNX3’s specific functions in γδ lineage commitment remain unclear. Other factors, such as NR4A1-3, ETV5, KLF2, RELB, HES1, and ZBTB16, are selectively upregulated in human γδ lineage thymocytes.167,169,170 Epigenetic regulation varies between αβ and γδ committed cells, with γδ T cells exhibiting extensive chromatin remodeling.
In conclusion, γδ cell fate regulation involves intricate interplay among TCR, Notch, and IL7R signaling pathways, along with a complex transcriptional network. While TCR signaling’s instructive role is evident, species-specific differences in Notch and IL7R signaling add complexity. Crucial transcription factors like EGR1-3, ID3, and SOX13 contribute to γδ lineage determination, accompanied by significant epigenetic modulation.171 However, challenges and species-specific variations highlight the ongoing need for deeper research into human γδ T cell development.
γδ T cell migrate from thymus to periphery or tissue
After undergoing fate determination in the thymus, γδ T cells embark on a remarkable journey to the peripheral tissues, where they establish colonization, particularly in sites such as the skin, mucosa, and intestine.19 This intricate process involves a series of tightly regulated mechanisms governed by a multitude of regulatory molecules, signaling pathways, and cellular interactions.113,172,173 It is important to note here that the current understanding of γδ T cells from thymus to peripheral organs or circulation primarily relies on research conducted in mice, and there is a lack of extensive evidences in human. Once γδ T cells complete their maturation journey in the thymus, they exit the organ and enter the bloodstream, ready to embark on their migratory adventure. The migration of γδ T cells to specific tissues is orchestrated by a combination of chemotactic signals and adhesion molecules that guide them to their intended destinations.
In the context of skin colonization, the attraction of γδ T cells is mediated by chemokines produced by resident cells in the skin, most notably keratinocytes. These chemokines, including CCL20 (MIP-3α) and CCL27 (CTACK), act as potent chemoattractants for γδ T cells expressing specific chemokine receptors such as CCR6 and CCR10.174–178 The interaction between these chemokines and their corresponding receptors prompts the migration of γδ T cells towards the epidermal layer of the skin where they self-renew, allowing them to establish a resident population within the tissue.113,179,180 Similarly, the colonization of mucosal tissues, such as the respiratory and gastrointestinal tracts, involves a similar set of chemotactic cues. Epithelial cells lining the mucosal surfaces play a crucial role by producing specific chemokines, such as CCL20 and CXCL16, which serve as attractants for γδ T cells expressing the corresponding chemokine receptors.181 For instance, CCR6 and CXCR6 are expressed on γδ T cells and facilitate their migration towards mucosal tissues. These precise chemokine-receptor interactions are pivotal for the directed migration and successful colonization of γδ T cells in these particular tissue microenvironments.173,182 As for intestinal colonization, additional factors come into play. The gut-associated lymphoid tissue (GALT), present in the intestinal mucosa, creates a supportive environment for γδ T cell colonization. Within the GALT, specialized cells such as DCs and macrophages present antigens to γδ T cells, influencing their localization and activation within the intestinal tissue. Moreover, the intestinal epithelial cells produce various regulatory molecules, including cytokines and chemokines, which shape the migration patterns of γδ T cells in the gut. These signals, such as TGF-β, IL-15, and IL-7, contribute to the positioning and retention of γδ T cells within the intestinal tissue.113,173,181,183,184
During the process of positioning, migration, and colonization in specific tissues, certain signaling pathways play a critical role in guiding γδ T cells to navigate towards their desired tissue compartments. Adhesion molecules may participate in the adhesion and transmigration of γδ T cells across endothelial barriers during tissue homing. Selectins, integrins, and their corresponding ligands on γδ T cells and endothelial cells facilitate the rolling, firm adhesion, and subsequent diapedesis of γδ T cells into the peripheral tissues.173,185 These adhesion molecules provide the necessary interactions for the precise localization of γδ T cells within specific tissue microenvironments. Therefore, the migration and colonization of γδ T cells in peripheral tissues are complex processes regulated by a variety of chemotactic signals, adhesion molecules, and signaling pathways. The precise interplay between these factors guides γδ T cells towards their intended tissue destinations, such as the skin, mucosa, and intestine.10 Further investigation of these mechanisms in human will advance our understanding of γδ T cells in tissue-specific immune surveillance and responses, further enhancing the potential applications of γδ T cells in disease immunotherapy.
Collectively, during the process of migration and homing to various locations, diverse chemokine receptors on γδ T cells play a critical role in determining whether these cells circulate or become tissue-resident. Although existing insights into the function of chemokine receptors in γδ T cell migration are largely derived from gene-targeted knockout mouse models, such as the CCR9/CCL25 pathway guiding murine γδ T cells to the small intestine,186 it is reasonable to hypothesize a similar molecular mechanism in humans. Excitingly, recent research has turned its attention to the homing properties of human γδ T cells, with a specific focus on examining the functional significance of chemokine receptor expression in both healthy individuals and patients.187 In the peripheral blood, the predominant Vδ2 subset expresses CCR5, which serves as a receptor for CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES). Additionally, Vδ2 T cells express CXCR3, the receptor for CXCL10/CXCL11.188 CCR5 and CXCR3 are linked to Th1 cells, renowned for their cytokine production, including IFN-γ and TNF-α, upon activation.189 In contrast, the Vδ1 subset of peripheral blood γδ T cells demonstrates a distinct preference for CXCR1, the receptor for CXCL5/CXCL6/CXCL8.188,190 Notably, Vδ1 T cells, unlike their Vδ2 counterparts, express CCR2 and exhibit migratory responses to CCL2. Significantly altered expression of this chemokine is observed in various human tumors like lung, prostate, liver, or breast cancer.191 This divergence in chemokine receptor expression between Vδ1 and Vδ2 T cells underscores distinct homing mechanisms within tumors, suggesting chemotactic properties of γδ T cells are crucial for determining their effectiveness in immunotherapy.
In summary, after thymic fate determination, γδ T cells navigate from thymus to peripheral tissues, including skin, mucosa, and intestine. Chemotactic signals and adhesion molecules orchestrate this journey. In humans, chemokine receptors (CCR5, CXCR3, CXCR1) on γδ T cells demonstrate tissue-specific homing. By probing chemokine receptor profiles, we will be able to unlock insights into cancer immunotherapy with γδ T cell subsets (Vδ1, Vδ2) and their potential for selective targeting. Advances in understanding tissue-specific immune response help refine γδ T cell-based therapies clinically.
γδ T cell fate from embryo to adulthood and old age
The comprehensive developmental pathway of γδ T cells, spanning from early embryonic phases to adulthood in humans, remains incompletely elucidated. However, a wealth of available data is progressively unraveling the intricacies of this trajectory. Meanwhile, insights gleaned from murine studies also offer invaluable knowledge to infer and construct a plausible framework for the actual progression in humans. During murine embryonic development, γδTCR expression was detected by 14 days of gestation in murine100. In human, the Vγ9 and Vδ2 variable (V) gene segments are the first to undergo rearrangement in γ/δ T cell development, and detectable at 5 to 6 weeks of gestation in the fetal liver192 and after 8 weeks in thymus116. At the mid-gestation (20-30 weeks), Vδ2 T cells become the predominant in the γδ T cell repertoire and is capable of producing IFN-γ193–195. However, as gestation progresses, there is an increase in the generation of Vδ1+ T cells, which ultimately make up the majority of the γδ repertoire in cord blood and the pediatric thymus.115,193,196,197 Therefore, Vδ2 T cells constitutes smaller proportion comparing with Vδ1+ T cells at birth.95,194,195 Nevertheless, there is a consensus that Vδ2 T cells undergo phenotypic maturation soon after birth194,198. Overall, γδ T cells are known to play vital protective roles throughout the lifespan, particularly in defense against infections and transformations. Their early maturation and functional development contribute to their ability to mount effective immune responses and provide immune surveillance against various pathogens and pathological processes.
Once γδ T cells have completed their maturation in the thymus and migrated into peripheral tissues, they embark on the process of aging. Although research on γδ T cells is limited, similar mechanisms observed in αβT cells may also apply to γδ T cells. We thus proposed that epigenetic regulation (e.g. DNA methylation, histone modifications)199,200 may play a pivotal role in the aging or exhaustion process of γδ T cells, contributing to their functional decline and altered immune responses. In aged T cells, global DNA hypomethylation and regional hypermethylation have been observed, affecting gene expression and cellular function.201–203 Alterations in the balance of histone acetylation and deacetylation, mediated by histone acetyltransferases and histone deacetylases, respectively, can impact T cell function, immune responses, and gene expression patterns. Additionally, specific microRNAs and long non-coding RNAs exhibit altered expression in aged T cells,204–207 influencing T cell differentiation, proliferation, and immune signaling pathways by targeting key genes involved in T cell function.200
The fate of γδ T cells during aging is also influenced by thymic involution, a gradual reduction in thymus size and output, leading to decreased production of new γδ T cells. This causes gradual reduction in the proportion of peripheral γδ T cells, particularly Vδ2 T cells, from childhood to adulthood and into old age.194,208,209 Consequently, the aging microenvironment, characterized by twelve hallmarks of aging,210 including changes in cytokine profiles and tissue-specific alterations, affects the localization and function of γδ T cells within tissues. The functional properties of γδ T cells also undergo changes with advancing age, including a decline in proliferative capacity, impaired cytokine production (e.g., IFN-γ and TNF-α), and alterations in receptor expression and signaling molecules.195,200
Moreover, following a thorough review of relevant literature models, we have succinctly outlined the developmental trends of circulating γδ T cells across the lifespan, ranging from embryonic stages to advanced age, as visually depicted in Fig. 3. Notably, the identification of γ/δTCR expression occurring around 5 to 8 weeks of gestation in the fetal liver and thymus has been reported,116,192 and subsequently, there is a noticeable shift in the predominant population, with Vδ2 T cells assuming dominance during mid-gestation (20-30 weeks) followed by a transition to Vδ1 T cell predominance at birth.95,193–195 Significantly, our most recent investigation209 unveils that the proportion of γδ T cells within the CD3+ T cell population reaches its zenith at 35 years of age. In this context, the proportion of Vδ2 T cells in the overall γδ T cell reaches a plateau within the age range of 20 to 35 years. Yet, the precise age at which the proportion of Vδ2 T cells surpasses that of Vδ1 T cells remains an enigma. Equally noteworthy, our research identifies the pivotal year of 45 as a checkpoint for γδ T cell aging. It is at this juncture that the Vδ2/Vδ1 ratio descends below 1, marking an association with immune aging and characterized by the hallmark of a reversed Vδ2/Vδ1 ratio. This discovery holds profound implications for our understanding of the aging immune system. Altogether, these age-related changes collectively affect the ability of γδ T cells to mount effective immune responses, immune surveillance, tissue homeostasis, and overall immune function.
Fig. 3.

Development waves of two major human γδ T cell subsets in circulation, inspired by models of literatures.95,242,746,747 This sketch depicts the variations in total γδT, Vδ2T, and Vδ1T cell populations from embryonic stages through adulthood and into old age. This representation is informed by the integration of our recently published data on γδ T cells, encompassing a cohort of 43,096 healthy individuals spanning an age range of 20 to 88 years209
γδ T cell subsets
Before delving into the discussion of γδ T cell subsets, it is essential to provide a brief overview of the fundamental knowledge regarding γδ T cells and the diversity of the γδTCR repertoire. The γδ T cells account for approximately 1–5% of total T cells in peripheral blood but much higher proportions are present in various human tissues such as the intestine (nearly 40%211) and skin (10–30%173,183,212,213). γδ T cells represent a unique subset of lymphoid cells that exhibit characteristics of both innate and adaptive immunity.83,183,214,215 Additionally, they are regarded as professional APCs capable of regulating their αβ counterparts.17,56,216,217 Furthermore, they can exhibit the function as a “signal processing hub,” receiving signals from and transmitting signals to other immune cells,16,218 such as B cells,219–222 dendritic cells,223–226 macrophages,227–230 NK cells,231–233 and αβ T cells56,234,235 making them an integral part of both innate and adaptive immunity.
Unlike T cells with αβ TCR, the antigen recognition of γδ T cells does not depend on the processing by APCs and subsequent presentation by MHC molecules; thus, they are considered non-MHC restricted.8,236,237 This feature of γδ T cells allows them to carry out unique functions compared to their αβ counterparts, resulting in a broader range of immune responses and broader protection. Although MHC-restricted γδ T cells have been discovered, they only constitute a small fraction of the γδ T cell population.238,239
Additionally, prior to focusing on the discourse concerning human γδ T cell subsets, we have provided a brief overview of the disparities in γδ T cell profiles between humans and mice. This includes distinctions in the γ/δ chains and their combinations, as well as disparities in distribution, thymic development and antigen recognition. This overview aims to provide readers with a straightforward understanding of the unique attributes of species-specific γδ T cells and subsets (Table 1).
Table 1.
An overview of the differences in γδ T cell profiles between humans and mice (based on the Tonegawa nomenclature715)
| Mouse (chains) | Distribution | Development in thymus | Recognition/antigen | Notes | Human (chains) | Distribution | Development in thymus | Recognition/antigen | Notes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vγ | Vδ | Vδ | Vγ | ||||||||
| Vγ1 |
Vδ5; Vδ6.3 |
Thymus, spleen, liver716 | Emerging in neonatal thymus, prevailing postnatally717,718 | Mycobacterial Hsp6036,719,720 | / | Vδ1 |
Vγ2;Vγ3 Vγ4;Vγ5 Vγ8;Vγ9 |
Epithelia, dermis, spleen, liver, rare in blood721 |
Mid-gestation onwards198 | MICA/B267,268, CD1c/d722,723,[254 Lipohexapeptides724,725 | Paired with diverse Vγ chains721 |
| Vγ2 | / | Rare | Present in a minority of postnatal thymic γδ T cells726 | MHC class II gene molecule I-Ek727 | / | Vδ2 | Vγ9 | Peripheral blood728 | Detectable 5 to 6 weeks in fetal liver192 | Phosphoantigens729, staphylococcal enterotoxin A730,731 | Vδ2/Vγ9 exclusively pairs728 |
| Vγ4 |
Vδ1 Vδ4; Vδ5; Vδ6; Vδ7 |
Blood, spleen, lung, lymph nodes726 | Emerging postnatally and then dominate thymic γδ T cells717,718 |
Diverse gut pathogens732, bacterial pathogens66, imiquimod436 |
Major γδT cell population in adult thymus, lymph nodes and spleen733 | Vδ3 |
Vγ2; Vγ3 |
Liver, gut epithelium, rare in blood734,735 | Predominant in late-fetal and neonatal blood736 | CD1d737 | Account for ∼0.2% of circulating T cells and respond to CD1d737 |
| Vγ5 | Vδ1 | Epidermis733,738 | Earliest T cells in murine fetal thymus at day 14100 | Stressed epithelial cells739 | Mainly γ chain in intestinal intraepithelial lymphocytes740,741 | Vδ5 | Vγ4 | / | Endothelial protein C receptor (EPCR)656 | Recognizing transformed cells via binding to endothelial protein C receptor.656 | |
| Vγ6 | Vδ1 | Uterus,vagina, tongue,placenta, testes, lung, kidney19 | Present in late fetal and newborn thymus742 | Commensal microbiota343 | A major proportion of γδ T cells in uterine tissue19 |
Vδ4; Vδ6; Vδ7; Vδ7 |
/ | Peripheral blood of lymphoma patients255 | / | / | |
| Vγ7 |
Vδ4; Vδ5; Vδ6 |
Intestinal mucosa717 | Thymic independent741,743,744 | Stressed intestinal epithelial cells48 | Paired with multiple Vδ chains745 | ||||||
δTCR chain-based taxonomy of human γδ T cells
In humans, there are three major γδ T cell subsets classified based on their TRDV genes, which are referred to as Vδ1+, Vδ2+, and Vδ3+. However, the Vδ2+ subset primarily pairs with Vγ9 TCR, making it the predominant γδ T cell population in circulating blood. In comparison with Vδ1+ T cells, which are generated in the human thymus a few months after birth, Vγ9Vδ2 cells develop at early stages of fetal development.123,194 Therefore, it is fair to speculate that Vγ9Vδ2 cells serve as the first line of defense and form an integral part of innate immunity9. On the other hand, the Vγ9-negative Vδ2+ subset has been reported to demonstrate properties of adaptive immunity.128
Vδ1+ cells are mainly located in the gut epithelium, skin, spleen, and liver, and only a small proportion is detectable in circulating blood. Pairings between Vδ1+ and Vγ chains are more flexible than the highly conserved Vγ9Vδ2 TCRs. Sequencing evidence strongly indicates that the TCR diversity of Vδ1+ cells mainly originates from TRD rather than TRG repertoires.128,214 Furthermore, strong clonotypic focusing of Vδ1+ cells is observed in most adults, and it comprises the private Vδ1+ T cell population exclusive to each adult.115,240 The above clonotyping and viral infection response studies on Vδ1+ and Vδ2+ cells all indicate that clonally selected Vδ1+ T cells exhibit adaptive immune cell characteristics such as “memory-like” features and rapid clonal expansion capacity, whereas semi-invariant Vγ9Vδ2 T cells align more with innate immunity.214,240,241
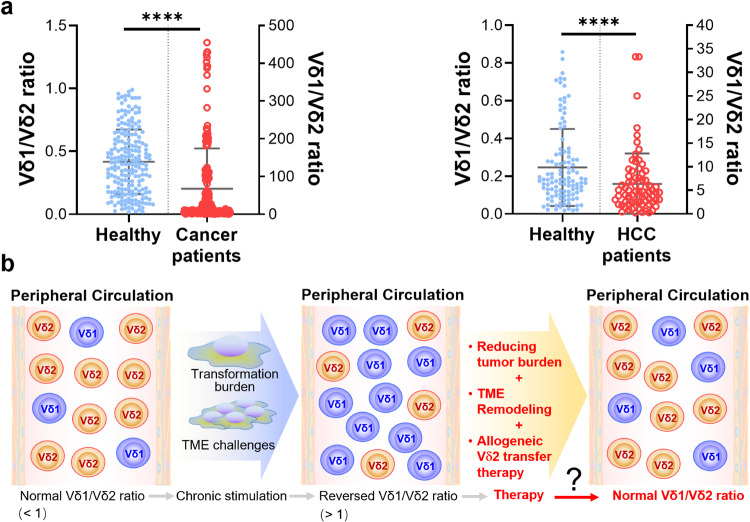
Since clonotypic expansions of non-Vγ9Vδ2 T cells (Vδ1+, Vγ9−Vδ2+, Vδ3+, etc.) take place in both diseased and healthy individuals, it is presumed that the non-Vγ9Vδ2 TCR repertoires “record” the immunological history (previous antigen challenges) of each individual.242 Interestingly, we243 and other groups244–246 observed an inverted Vδ1/Vδ2 ratio in the peripheral blood and/or tissues in cancers or infectious diseases187 (as shown in Fig. 4), this could be explained by the rapid clonal expansion of the “adaptive” Vδ1+ subset upon antigen challenges during tumor progression or infections, whereas the “innate” Vγ9Vδ2 population does not thrive under chronic antigen stimulation247 and undergoes activation-induced cell death (AICD).248 Therefore, a holistic treatment approach encompassing tumor burden reduction, TME remodeling, and the adoptive transfer of allogeneic Vδ2+ cells holds promise for re-establishing host immunity and preserving the normal Vδ1/Vδ2 ratio. Nevertheless, further scientific evidence is required to certify this hypothesis.
Fig. 4.
An inverted Vδ1/Vδ2 ratio in the peripheral blood of various solid tumor patients, including those with liver, lung, breast, pancreatic, kidney, and other types of cancer. a In healthy populations, the Vδ1/Vδ2 ratio is usually less than 1. However, in cancer patients, including those with hepatocellular carcinoma (HCC), this ratio is reversed, and it becomes far greater than 1 according to our previous work.243,748 b A hypothetic sketch suggests that the normal Vδ1/Vδ2 ratio is skewed by the burden of transformation and the challenges posed by the tumor microenvironment (TME), resulting in a disordered ratio. Available therapy approaches provide alternatives for re-modulating the TME to achieve the normalization of the Vδ1/Vδ2 ratio and subsequently immune function
In contrast to the Vδ1+ and Vδ2+ subsets, the Vδ3+ subset is rarely detected in the peripheral blood of healthy individuals but is enriched in the liver and gut epithelium.17,193,249–251 Interestingly, Vδ3+ cells recognize similar ligands as the Vδ1+ subset.251 Moreover, like the Vδ1+ subset, they showed an increased expression of CD16 molecules, which are low-affinity IgG Fc region receptors (FcγRIII), and were capable of orchestrating antibody-dependent cellular cytotoxicity (ADCC) in the PBMCs of individuals infected with Plasmodium falciparum.252 Similar cytotoxic phenotypes or clonal expansion have also been observed in CMV253 and hepatitis C Virus infections,254 suggesting its role in combating infections. Furthermore, recent studies have demonstrated the infiltration or expansion of this subset in tumors, suggesting its potential role in mediating anti-tumor immune responses.255–258 Additionally, in vitro expanded Vδ3+ T cells have shown the ability to induce maturation and IgM secretion by B cells.259 However, because of its rarity, there is limited evidence available to clearly delineate the functional role of the Vδ3+ subset. Therefore, further research is needed to fully unravel the functional role that the Vδ3+ subset plays under physiological and pathological conditions.
In this article, we primarily limit our discussion to Vδ1+ and Vδ2+ (Vγ9Vδ2 subtype unless specified otherwise) T cells, due to their abundance in the literature and experimental/clinical applicability.
Vδ1+ T cells
Vδ1 T cells are players of adaptive immunity. Vδ1+ T cell relies on γδTCR- and natural killer receptors (NKR)-mediated recognition of tumor antigens or stress signals, similar to Vδ2 T cell. However, there are significant differences between the two subtypes. Specifically, Vδ1 TCR recognizes MHC-like proteins of the CD1 family, such as CD1c and CD1d,260–265 Annexin A2,266 and MHC class I chain-related protein A and B (MICA/B),267,268 which are mostly upregulated in transformed cells and virus infected cells. Evidence indicates δ1TCR has a much higher affinity toward CD1d than MICA/B.262,269 The drastic difference in their TCR ligand recognition patterns further implies non-redundant roles of Vδ1+ and Vδ2+ in establishing immune surveillance.270 Based on published studies, we can conclude that Vδ1+ T cells play a significant role in adaptive immunity among γδ T cell subsets.
Like Vδ2 T cells, Vδ1 T cells also highly express natural killer group 2 member D (NKG2D), which is a stress-sensing molecule that recognizes its cognate ligand MICA/B on the surface of the cancer cells. However, Vδ1+ TCR and NKG2D do not share binding sites on MICA/B, and the strength of NKG2D-MICA/B binding is 1000-fold stronger than that of Vδ1+TCR-MICA/B.269 Despite discrepancies in their antigen recognition, both Vδ2 and Vδ1 T cells rely on secretion of the perforin/granzyme-B mediated secretory and death receptor (TRAIL/TRAIL-R, Fas/FasL) pathways to execute their anti-tumor cytotoxic activity.
Vδ2+ T cells
Overall, activation and recognition of Vδ2 T cells are dependent on phosphoantigen presence. The ligand recognition by Vγ9Vδ2 T cells mainly falls into two groups, namely γδ TCR-mediated and NKR-mediated ones.8 Although γδ T cells were discovered almost four decades ago, knowledge of the exact molecular mechanism of antigen recognition by γδTCR is still rather limited, partly due to the low binding affinity to its ligands, which makes ligand identification difficult.271 Different from other γδ T subsets, Vδ2+ TCRs recognize phosphoantigens that accumulated in tumor cells due to their dysregulated mevalonate pathway.272–275 Notably, phosphoantigens do not directly bind to γδTCR, instead, they bind to the intracellular B30.2 domain of the butyrophilin family protein, BTN3A1.82,276 This binding then triggers a conformational change of BTN3A1, allowing its collaborator BTN2A1 to hinge onto the Vγ9 chain of the γδTCR, which then activates Vδ2 T cells.277–280 However, whether the Vδ2 chain of the Vγ9Vδ2 TCR is involved in the antigen recognition process is still elusive. In addition to the BTN3A1/BTN2A1-mediated phosphoantigen recognition, Vγ9Vδ2 TCR could also interact with the F1-ATPase, apolipoprotein A-1, or hMSH2, which are often abnormally upregulated in cancerous cells.281–283 Interestingly, rodents do not have a homologous γδTCR which can be activated by phosphoantigens. As a consequence, conventional mouse models are not suited to study the significance of phosphantigen-reactive γδ T cells in the context of cancer and infection. The recent discovery of a phosphoantigen-reactive Vγ9Vδ2 TCR in alpacas (Vicugna pacos) has established them as the first non-primate species with this feature.284 This introduces a novel model for Vγ9Vδ2 T-related research, complementing the existing nonhuman primate models.
Apart from TCR-mediated antigen recognition, NKR plays crucial roles in activating Vδ2 T cells and initiating tumor lysis. Specifically, NKG2D on Vδ2 T cells binds to MICA/B285–287 and UL16 Binding Proteins (ULBPs) of cancer cells,288,289 and the DNAX Accessory Molecule 1(DNAM1) on Vδ2+ binds to Nectin-like 5 of cancer cells, leading to perforin-granzyme axis mediated cancer cytotoxicity.290 Like NK cells, Vδ2 T cells also express CD16 and are capable of orchestrating ADCC upon binding to tumor-specific antibodies.291–293 Interestingly, this type of killing appears to be restricted to the Vδ2+ subtype but not Vδ1+ in an in vitro study.294 Conversely, it has been demonstrated that in patients with viral infections, in vivo expression of CD16 on Vδ1 T cells occurs.252,253 Therefore, understanding the differences in phenotypic characteristics and the underlying molecular mechanisms between the two subtypes helps in extrapolating their respective clinical advantages.
Effector subsets defined by cytokine release
The anti-tumor role of γδ T cells was first established by the seminal work of Hayday and his colleagues using TCRδ-deficient mice.53 Early studies suggested that γδ T cells serve as an early source of IFNγ and contribute to anti-tumor responses in various cancer types.295–299 However, recent advancements have unveiled that γδ T cells can also play pro-tumor roles in cancer. For instance, the pro-tumorigenic role of IL17-producing γδ T cells was validated in IL17 knockout mice which showed slower tumor progression in different models of cancers.76,79,300–303
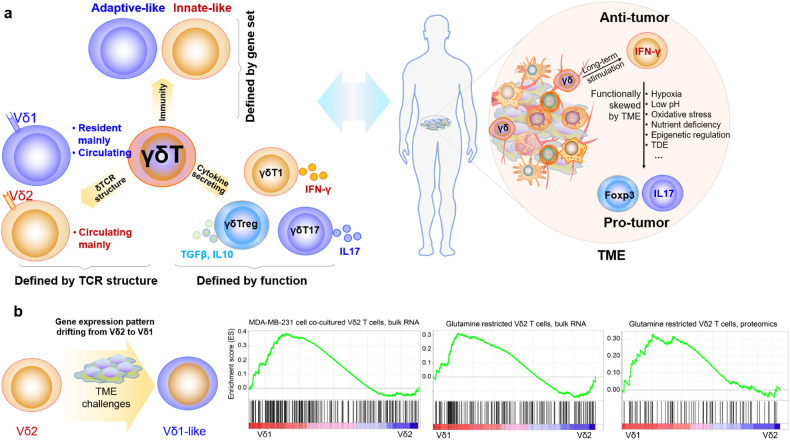
Given that the γδTCR chains do not exhibit a distinct functional bias within the tissue microenvironment, they are insufficient for classifying the immune function of γδ T cells. Therefore, alternative approaches have been employed to functionally define subsets of γδ T cells based on their immune response functions, particularly their ability to release cytokines. Two major effector subsets of γδ T cells can be categorized based on their ability to produce specific cytokines. γδT1 cells, which produce IFN-γ (IFN-γ+γδ T cells), mainly playing anti-tumor function. γδT17 cells, which produce IL-17 (IL-17+γδ T cells), leading to pro-tumor and autoimmune diseases.11,16,304 Notably, γδNKT cells, which produce both IL-4 and IFN-γ, have also received increasing attention.98,112 About their development in thymus, both IFN-γ-producing subsets (γδT1 and γδNKT) has been shown to rely on strong signals from the TCRs, whereas γδT17 cells have been reported to develop even in the absence of TCR ligand selection.63,112,162,305
Actually, the functional propensities of each subset of γδ T cells are highly context-dependent, as they could be modulated by their immediate environment (as shown in Fig. 5a). Specifically, cytokines produced by γδ T cells under distinct circumstances help to define their functions more precisely. γδT1 mediate intracellular pathogen clearance and elicit anti-tumor immunity, whereas γδT17 provide protection against extracellular bacteria and fungi infections. Another less well-characterized functional subset of γδ T cells that carries out regulatory functions in cancer or inflammatory diseases has been identified as γδTreg.306–311 This population is induced upon receiving Inflammatory signals in the TME and could potentially sabotage the anti-tumor phenotype of γδ T cells while reprogramming them into γδTreg.306,307,312 This subset has been identified as CD73+Foxp3+Vδ1+ T cell in the PBMC or tumor specimen of breast cancer patients313 and tumor-infiltrated CD39+Foxp3+γδT in colon cancer. Both CD39+ and CD73+ γδTreg possess immune-regulatory functions.314,315 Lastly, a minor subtype of γδ T cells that could initiate a Th2-like response (IL-4 production) under pathological conditions has been identified.43,316 The above evidence further supports the functional plasticity of γδ T cells is context-dependent.8,17,317,318
Fig. 5.
The γδTCR chains are insufficient to classify γδ T immune function. a Three ways to subclassify γδ T cells are δTCR structure-based, cytokine secretion-based, or gene expression pattern (or immunity)-based. To our knowledge, in the context of the TME, a function-based γδ T taxonomy would be more objective than the TCR-based approach to describe their pro- or anti-tumor functions, since the immune function tends to be switchable (the right sketch). b Interestingly, we observed that, in the context of TME challenges, the gene expression pattern of Vδ2 T cell can be skewed toward that of Vδ1+ T cell (the left sketch graph). For example, our group recently discovered that cancer cell coincubation or amino acid (glutamine) stress, which are the common features of the TME, can skew Vδ2 T cells towards a Vδ1-like T cells (at the gene expression level) (the right graph)
Furthermore, accumulating evidence reveals that the immune function of both Vδ1+ (generally pro-tumor) and Vδ2+ (generally anti-tumor) subsets is plastic and depends on the specific cytokine milieu. Vδ2 T cells could be skewed toward IL17-producing γδT17 when stimulated with a cytokine cocktail of IL-1-β, TGF-β, IL-6, and IL-23 in vitro,81 and they can also be induced into FOXP3-expressing Treg in the presence of TGFβ1, IL-15, and antigen stimulation.319,320 In the additional presence of the epigenetic modifier Vitamin C, the FOXP3 locus is specifically demethylated, in line with regulatory function.320 An early study showed that IL4 could negatively impact γδ T cell-mediated tumor immunity, skewing γδ T cell population toward the IL-10-secreting Vδ1+ instead of the IFNγ-secreting Vδ2+ subset.312 Clinically, both IL17-producing Vδ2+ and the IFNγ-producing Vδ1 T cells have been found in cancers,321–323 and distinctive cytotoxic hallmark patterns were found on Vδ1+ and Vδ2 T cell subsets, respectively.324 Moreover, intrahepatic γδ T cells are mainly comprised of polyclonal Vδ1+ subsets that are phenotypically distinct from those in the matching blood, implying functional plasticity of the Vδ1+ T cells.241 Importantly, Hayday’s group correlated Vδ1+ but not Vδ2 T cells with better outcomes in the patient with triple-negative breast cancer (TNBC), suggesting a protective role of a subset of Vδ1+ T cells.325 By analyzing RNA sequencing data, we observed a shift from Vδ2+ to Vδ1+ subset gene expression profiles in in vitro expanded Vδ2+ cells after co-culturing with MDA-MB-231 TNBC cell line. A similar shift was also observed when Vδ2+ cells were cultured under the glutamine (one of the main nutrients deprived in TME) deficient condition (Fig. 5b). These phenotypes indicate the plasticity of Vδ2 T cells, once again demonstrating that a TCR-based classification is insufficient to describe the functional signatures of γδ T cells in the TME. Therefore, one cannot simply classify Vδ1+ and Vδ2+ subsets’ functions based on their respective TCR signatures, since the properties of γδ T cells in tumorigenesis may be pleiotropic depending on the tumor type and stages.9,321
Additionally, beyond tumors, distinct functional heterogeneity and plasticity have been observed among γδ T cell subsets, which can play either protective60,84,326,327 or detrimental328–330 roles in the context of infections and autoimmune diseases. Hence, a thorough understanding of the intricate functional behaviors and phenotypic variations of γδ T cell subsets is crucial to elucidate their roles in diverse disease contexts. Therefore, in the subsequent subsections, we proceeded to provide a comprehensive discussion on IFNγ-producing γδ T (γδT1), IL-17-producing γδ T (γδT17), regulatory γδ T (γδ Treg), and antigen presenting γδ T cells (γδ TAPC).
IFNγ-producing γδ T (γδT1): anti-tumor role and plasticity
An infiltrated or circulatory IFNγ-producing γδ1+ T cell population has been considered a positive prognostic marker in cancers.8,297,307 For instance, Dieli’s group observed a positive correlation between the frequency of γδ TILs in the tumor specimen and the 5-year patient prognosis in 557 colorectal cancer (CRC) patients.331 However, this conclusion was challenged by evidence indicating that proinflammatory γδ17 may contribute to cancer development in various tumor models.80 Similarly, immunosuppressive γδTreg has been found to positively correlate with the progression of CRC314 and breast cancer.332 A recent discovery has also shown that the conversion of IFNγ-producing γδ T to IL17-producing ones occurs as CRC progresses,333 underscoring their functional plasticity shaped by the TME.334 Furthermore, it is still unclear whether tumor-infiltrated γδ T cells come from the original tissue-resident ones (characterized with surface markers CD69 and CD103335–337) or peripheral blood, or both.14 Hence, elucidating the functional diversity and plasticity of γδ T cells across various cancer types is necessary. Using the ‘deep deconvolution’ CIBERSORT algorithm,338 Gentles et al. conducted extensive transcriptomic analyses on tumor biopsy samples across 39 cancer types with over 18,000 samples and concluded that infiltrated-γδ T cell is the best prognostic immune cell subset (out of 22) to predict favorable patient outcomes.86 However, a follow-up study with an optimized deconvolution strategy separating γδ T cells from NK and αβ T cells contested this conclusion, suggesting a much looser correlation between γδ TIL and cancer prognoses in 50 hematological and solid malignancies.339 Therefore, the application of spatiotemporal scRNA-seq or single-cell proteomics can enable the in-situ clarification of the functional contributions of individual γδ T subsets (γδ1, γδ17, and γδTreg, etc.) and their functional evolution in the TME. Recently, we carried out functional phenotyping of γδTILs of HCC patients by scRNA-seq and found low IL17A but high IFNG expression in γδTILs (mostly Vδ1+), implying cytotoxic effector function of γδTILs in HCC.243 Since γδ T cells display heterogeneity across cancer types or even among individuals, more sophisticated and thorough studies are needed to truly shed light on the functional discrepancies and plasticity of γδ T cells and facilitate their clinical applications. Moreover, deciphering the molecular mechanisms underlying the spatial and temporal functional pleiotropy of γδ T cells, specifically the signature effector functionalities of individual subsets, can help develop intervention strategies to skew the function of γδ T cells in cancer patients towards an anti-tumorigenic effect.
IL-17-producing γδ T (γδT17): pro-tumor and pro-inflammatory role
Different from mice, the IL17-producing γδT17 population is scarcely found in healthy individuals but undergoes rapid expansion in proinflammatory milieu such as acute infections81 and cancers.80,321,340–342 The evidence indicates that circulating and/or tissue-resident γδT17 cells promote the metastasis of breast tumors,302 the progression of liver cancer79 and lung cancer,343 and are associated with poorer prognoses in patients with colon80 and gall-bladder cancers.340 IL-1β, an inflammatory cytokine secreted by myeloid lineage cells in the TME, has been found to skew γδ T functional polarization toward γδT17 subtype in various cancer models.78,301,302 Importantly, a randomized, double-blinded trial on 10,061 patients, dubbed as “CANTOS” study, demonstrated IL-1β antibody inhibition could greatly decrease both the incidence and mortality rate of lung cancer.344 This evidence further supports the pro-tumorigenic functions of γδT17. Moreover, IL17-mediated interactions between γδ T and myeloid lineage cells facilitate cancer progression. For instance, γδT17 recruits immunosuppressive myeloid-derived suppressor cells (MDSCs) into the TME.76,79,80 A recent study even demonstrated that commensal microbiota could promote IL17 secretion in lung-resident γδ T cells, which then promote tumor progression.343 Interestingly, evidence indicates that the presence of γδT17 is essential for the efficacy of chemotherapy by facilitating the recruitment of IFNγ-producing cytotoxic CD8+ TILs.345 Therefore, further evidence is required to elaborate the role(s) of γδT17 in cancers.
γδT17 cells are involved in both proinflammatory diseases and infections. They contribute to tissue inflammation and immune dysregulation in conditions like autoimmune disorders.7,20 In infections, they actively participate in pathogen clearance by producing IL-17, IFN-γ, and other proinflammatory cytokines, while activating immune cells such as macrophages and neutrophils.57,59,346 However, dysregulated activation of γδT17 cells can lead to tissue damage71,347 and chronic inflammation,309 even autoimmune diseases like psoriasis.69,313,348 Understanding their intricate regulation network is important for developing effective treatment regiments.
In conclusion, gaining further insights into the thymic development process and the diverse array of factors within the immediate microenvironment surrounding γδ T cells is essential for a comprehensive understanding of the functional evolution and plasticity exhibited by distinct subsets of γδ T cells, whether characterized by their TCR chains or the cytokines they release, as discussed earlier. This enhanced understanding has the potential to significantly improve our interpretation of the roles γδ T cells play in both normal physiological processes and pathological conditions. Consequently, it can aid in the development of more effective immunotherapies based on harnessing the potential of γδ T cells.
Unveiling novel effector functions: regulatory (γδTreg) and antigen-presenting (γδTAPC) roles
Accumulating evidence has unveiled the multifaceted roles of γδ T cells in humans, extending beyond their roles in anti-/pro-tumor or anti-/pro-inflammation responses. They also exhibit crucial functions as regulatory immune cells known as γδTreg and as γδTAPC involved in the process of antigen recognition. Notably, emerging research suggests that effector γδ T cells can transition into γδTreg under specific microenvironmental conditions.306–311 Previously, we had thoroughly reviewed the regulatory functions of γδ T cells,349,350 particularly Vδ1 and Vδ2 subsets, it was demonstrated that these subsets can be induced to express FoxP3 and execute regulatory functions in the presence of TGF-β, IL-2, and IL-15.351 Similar to conventional Tregs, human γδ T cells employ various molecules such as GM-CSF, IL-10, TGF-β, IL-17, CD39, CD73, and checkpoint receptors as part of their immunosuppressive mechanisms.350 Notably, the Vδ1 subset, majorly tissue-resident, displays a propensity to convert into γδTregs, as indicated by the expression of CD73+ and CD39+ phenotypes in cancer patients, although consistent Foxp3 expression has not been universally observed.313–315 Our research (Fig. 5b), alongside reported literatures,319,352 supports the notion that Vδ2 T cells can also be skewed towards γδTreg under specific microenvironmental cues, such as the presence of TGFβ1, IL-15, and antigen stimulation. Remarkably, Vitamin C has been identified as a catalyst for the conversion of Vδ2 T cells into Foxp3+γδTreg.320 Taken together, above work underlines the substantial functional plasticity of γδ T cell subsets, with their effector functions subject to modulation by microenvironmental factors.
On a separate note, a distinctive feature of human γδ T cells, notably the Vδ2 subset, is their capability to serve as professional APC to transmit antigen signals to αβT cells, including CD4+ and CD8+ T cells. The antigen-presenting function of Vδ2 T cells was initially reported by Brandes in 2005,56 emphasizing the immunological importance of Vδ2 T cells in adaptive immunity regulation. Subsequent studies proposed that the APC function of human blood-derived γδ T cells is precisely regulated spatially and temporally, requiring pre-sensitization with specific antibody-coated target cells for full APC functionality.353 Furthermore, it was demonstrated that the APC function of γδ T cells can be compromised in conditions such as sepsis, resulting in impaired activation of CD4+ T cells. Conversely, γδ T cells from healthy individuals retain normal APC function.354 This observation aligns with our findings indicating that allogeneic Vδ2 T cells from healthy donors demonstrate promising clinical effectiveness in solid tumor patients.11,12 Our research also indicated that the infusion of allogeneic Vδ2 T cells can increase the proportions of CD4+ and CD8+ T cells in the blood of most patients (refer to Fig. 7d), consistent with the APC function of Vδ2 T cells, which can promote αβT cell proliferation.354 It is this APC function that positions the adoptive transfer of Vδ2 T cells as a promising strategy for tumor immunotherapy. Therefore, the exploration of how to effectively exploit the potential of Vδ2 T cells for the utmost benefit of patients requires further investigation. Specifically, a deeper understanding of the underlying molecular regulatory mechanisms of γδTAPC is imperative.
Fig. 7.
The correlation between tumor-infiltrating γδ T cells and cancer pathogenesis, along with the clinical potential of allogeneic γδ T cells in tumor immunotherapy. According to our recent TCGA-based bioinformatics analysis (a–c) and revisiting our previous clinical trial data records (d),11,12 allogeneic γδ T cell indicates the future of tumor immunotherapy. a The abundance of γδ T cells in normal and tumor tissue depends on the type of organ (‘n’ represents normal). b Correlation analysis between TRDC and programmed cell death (PCD) gene sets show variability across cancers. Nevertheless, pyroptosis and PANoptosis of γδ T cells appear to be more correlated with cancers than other PCD pathways. PCD gene set lists are provided in the supplemental file. The correlation coefficient is represented by “r”. c The infiltrated γδ T cells (TRDC) can serve as an indicator for the overall survival (OS) in a very small fraction of cancer types (11/33), and only correlate with better OS in 9/33 cancers. d Our clinical trials have shown that allogeneic Vδ2 T therapy significantly increases the percentages of CD4+ and CD8+ T cells in certain patients with solid tumors (represented by red lines in the graph, each line representing data from an individual patient). e A sketch graph of allogeneic γδ T cell-based cancer immunotherapy is presented, along with potential challenges and γδ Tplus strategy (γδ T + mAb, bispecific Ab, chemo, radio, intervention, etc.) in clinical application. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. ACC Adrenocortical carcinoma, BLCA Bladder Urothelial Carcinoma, BRCA Breast invasive carcinoma, CESC Cervical squamous cell carcinoma, CHOL Cholangio carcinoma, COAD Colon adenocarcinoma, DLBC Lymphoid Neoplasm Diffuse Large B-cell Lymphoma, ESCA Esophageal squamous cell carcinoma, GBM Glioblastoma multiforme, HNSC Head and neck squamous cell carcinoma, KICH Kidney chromophobe, KIRC Kidney renal clear cell carcinoma, KIRP Kidney renal papillary cell carcinoma, LAML Acute myeloid leukemia, LGG Brain lower grade glioma, LIHC Liver hepatocellular carcinoma, LUAD Lung adenocarcinoma, LUSC Lung squamous cell carcinoma, MESO Mesothelioma, OV Ovarian serous cystadenocarcinoma, PAAD Pancreatic adenocarcinoma, PCPG Pheochromocytoma and paraganglioma, PRAD Prostate adenocarcinoma, READ Rectum adenocarcinoma, SARC Sarcoma, SKCM Skin cutaneous melanoma, STAD Stomach adenocarcinoma, TGCT Testicular germ cell tumors, THCA Thyroid carcinoma, THYM Thymoma, UCEC Uterine corpus endometrial carcinoma, UCS Uterine carcinosarcoma, UVM Uveal melanoma
γδ T cell and diseases
Accumulating evidence now strongly affirms the multifaceted role of γδ T cells in the pathogenesis and progression of a multitude of diseases. This encompasses infections initiated by pathogens such as viruses and bacteria, autoimmune disorders, tumor, and more. To begin, we provide a brief overview of the contributions of γδ T cells to these diseases, including their function as APCs, as depicted in Fig. 6.
Fig. 6.
Brief sketch depicts the major roles of human γδ T cells in the immune regulation, pathogenesis and progression of diverse diseases (representative mechanisms shown). AICD activation induced cell death, ADCC antibody-dependent cellular cytotoxicity, HMBPP (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate, IPP Isopentenyl pyrophosphate; The ‘?’ means the molecular mechanism is not clear yet
γδ T cell in infectious diseases
γδ T cells play protective roles in infectious diseases. Unlike conventional αβ T cells, which recognize peptide antigens presented by MHC molecules on APCs, γδ T cells have unique TCRs that allows them to recognize a wide variety of peptide or non-peptide antigens, including microbial products, stress-induced molecules, and self-antigens. Once activated, γδ T cells initiate a rapid immune response against pathogens by directly recognizing conserved molecular patterns expressed by various microbes, such as lipopolysaccharides (LPS), lipoteichoic acid (LTA), via pattern recognition receptors, and phosphoantigens via the TCR. Afterward, activated γδ T cells exhibit cytotoxic capabilities and directly eliminate infected cells by releasing cytotoxic molecules, such as perforin and granzymes, which induce apoptosis in target cells. This cytotoxicity is particularly important for controlling intracellular pathogens, including viruses and certain intracellular bacteria. Furthermore, γδ T cells are potent producers of various anti-infection cytokines, including IFN-γ, IL-17, and IL-22. These cytokines play key roles in recruiting and activating other immune cells, such as neutrophils, dendritic cells, macrophages, and NK cells, to eliminate pathogens and promote tissue repair. Additionally, γδ T cells interact with other immune cells, including αβ T cells, B cells, and NK cells, through the secretion of modulatory cytokines or direct cell-to-cell contact. These interactions help shape the intricate immune network and optimize the innate and adaptive immune responses against pathogens, facilitating their rapid clearance.
γδ T cells in M.tb infection
TB is a highly contagious airborne disease caused by the M.tb infection. According to the “Global Tuberculosis Report 2022” by the World Health Organization (WHO), TB is the leading cause of death globally attributed to a single infectious bacterium, second only to COVID-19.355 The progression of TB heavily depends on the ability of M.tb to evade and manipulate the host immune responses.356–359 TB could evade host immune surveillance and exploit host macrophages and other immune cells, aiding its evolution within the human host.360–363 Early studies have shown peripheral expansion of γδ T cells following TB infection364 and demonstrated that resident pulmonary lymphocytes express high levels of γδTCR, suggesting their crucial role in fighting against TB infection at the frontline.40,246,365,366 Additionally, high-throughput immune repertoire sequencing has the potential to provide fresh insights into the roles of γδ T cells in TB,246 including the identification of new M.tb proteins as potential ligands that bind to γδTCR, thereby activating γδ T cell-mediated immunity.367 Moreover, γδ T cell could recognize a wide range of non-peptidic antigen such as phospho- and lipid-antigens, maximizing its protective role against M.tb infection.368–370 It has been shown that both IFN-γ and IL-17A/IL-17F-mediated immunity are crucial for γδ T cells to fulfill their roles in curbing Mycobacterium pathogenesis.371–375
Interestingly, the Vγ9Vδ2 T cell subset but not others expands shortly after birth and exhibits potent cytotoxic functions, serving as a protective mechanism against sudden microbial exposure such as M.tb in newborns.376 Early studies have indicated the presence of memory-like responses in Vδ2 T cells following Bacille Calmette-Guérin (BCG) vaccination. Given that BCG is a mycobacterial strain like M.tb, it is speculated that TB infection could elicit similar immune responses in Vδ2 T cells.377 Therefore, Chen and colleagues further investigated the adaptive immune response of γδ T cells in TB-infected primates, suggesting that immunizing Vδ2 T cells could be a promising strategy for TB vaccine development.378–383 Based on these findings, we conducted a groundbreaking clinical trial utilizing allogeneic Vδ2 T cell therapy in the treatment of MDR-TB. The results showed a reduction in M.tb load and the healing of pulmonary lesions, indicating an enhancement of the host’s immune defenses.13 Furthermore, studies have demonstrated that co-administration of phosphoantigens with IL-2, resulting in the expansion of the Vδ2+ subset, improves the treatment outcome of TB in macaques.384,385
Recently, a study showcased the expansion of a distinctive subset of NK-like CD8+ γδ T cells (predominantly Vδ1+) during chronic M.tb infection. This subset was found to be functionally and clonally distinct from the well-studied pAg-reactive Vδ2 T cells that expand during acute Mtb infection.386 Moreover, it has been observed that lung tissue-resident γδ T cells in TB patients primarily consist of the Vδ1+ subset, rather than the Vδ2+ subset,246 which raises the question of whether circulating Vδ2 T cells could infiltrate lung tissue and eliminate M.tb-infected cells. Therefore, conducting further research to unravel the mechanisms underlying γδ T cell-mediated immune responses in TB, particularly the functional diversity of each subset in peripheral and local inflammatory sites, could make a significant contribution to the advancement of γδ T cell immunotherapy for TB.
γδ T cells in human immunodeficiency virus (HIV) infection
HIV is a retrovirus characterized by its composition of two copies of positive-sense single-stranded RNA. Its primary targets are CD4+ T cells, namely helper T cells. HIV attaches to CD4 receptors on the surface of these cells, along with co-receptors such as CCR5 or CXCR4, facilitating its entry into the CD4+ T cells. Once inside the CD4+ T cell, the viral RNA will be reverse transcribed into DNA and thus integrated into the host cell’s DNA, permanently becoming part of the cell’s genetic material. Following integration, the virus exploits the host cell’s machinery to produce viral proteins and replicate the viral RNA, resulting in the formation of new viral particles. Subsequently, these newly formed viral particles are released from the infected CD4+ T cell, capable of infecting other CD4+ T cells and various immune cell subsets like DCs and macrophages. This widespread infection and subsequent destruction of immune cells contribute to the progressive deterioration of the patient’s immune system.387,388 The cumulative impact of HIV weakens the immune system’s ability to mount effective immune responses, rendering individuals more susceptible to opportunistic infections, such as TB and other complications.389,390 Regarding the impact of HIV on the γδ T cell subset, early studies have shown a depletion in the Vδ2 subset, along with an increased level of the Vδ1+ subset. As a result, an inverted Vδ1/Vδ2 ratio was detected in HIV infected primates.46,187,391–395 Previous study indicated HIV envelope protein gp120 could bind to integrin α4β7 and CCR5 on Vδ2 T cells and activate caspases-dependent apoptosis, ultimately inducing the AICD of Vδ2+ subset.244 Interestingly, the Vδ1+ subset is spared from HIV virus-mediated killing in patients due to its lack of the CCR5 receptor, which is involved in this mechanism.396 Furthermore, functional profiling has revealed that γδ T cells in HIV patients with rapid disease progression produce higher levels of IL-17 but not IFNγ. This observation is also positively correlated with γδ T cell activation, indicating the crucial role that γδ17 plays in HIV pathogenesis.393 Additionally, the role of γδ1 T cells in controlling HIV virus was demonstrated through the production of IFNγ in HIV-exposed seronegative individuals, highlighting the specific immune response against the HIV Gag peptide. This finding strongly suggests that γδ1 T cells play a crucial role in mediating the immune defense against HIV.397
Furthermore, NKp30+Vδ1+ T produced high levels of CCL3, CCL4, and CCL5 to suppress the replication of HIV-1 within CD4+/CCR5+ human lymphoid cells.398 Vδ1+ subset, isolated from the PBMCs of both HIV-1 infected patients and healthy donors, secreted both IFNγ and IL-17 upon stimulation with Candida albicans. On the other hand, Vδ2+ subset secreted IFNγ and IL-17 in response to mycobacterial or phosphoantigens. These findings suggest a nonredundant role for these two γδ T subsets in HIV patients, as they play vital roles in fighting against opportunistic infections and partially compensating for the loss of CD4+ T cells in HIV-infected patients.61 Additionally, there is an increased production of IFN-γ and TNFα in Vδ1+, while the reverse is true for Vδ2+ in HIV-infected patients. This further suggests the compensating role that Vδ1+ might play in rescuing the loss of Vδ2+ in these patients.399 Given the significant correlation between the loss of Vδ2+ cells and HIV progression, there has been considerable interest in exploring methods to restore and enhance the antiviral effector functions of γδ T cells.400 Interestingly, the loss of the circulating Vδ2+ population and its ability to secrete IFNγ could be restored and closely correlated with the increase in CD4+ T cell count in chronic HIV-infected patients who received highly active antiretroviral therapy (HAART). This finding further supports the possibility of utilizing the quantity and quality of Vδ2 T cells as a convenient biomarker to assess the effectiveness of HAART treatment in patients.401
Hence, there is a natural inclination to consider the clinical restoration and reconstitution of γδ T cell population in HIV patients as a potential beneficial approach for disease control. Successful in vitro expansion of γδ T cells from HIV+ donors was accomplished using zoledronate/IL-2, demonstrating cytotoxic effects towards malignant cells.402 Furthermore, a prior study explored the clinical application potential of ex vivo expanded Vδ2 T cells derived from HIV patients, revealing their effectiveness in suppressing virus replication in autologous infected CD4+ T cells.403 Encouragingly, recent advancements in single-cell transcriptomics on the PBMCs of HIV patients have provided an opportunity to gain a deeper understanding of the functional roles and evolutionary dynamics of γδ T cells in the context of HIV infection.404,405 While ongoing research and further clinical trials are necessary, γδ T cell immunotherapy holds great promise as a distinct and innovative approach in the treatment of HIV.406
γδ T cells in COVID-19 infection
COVID-19 is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has rapidly evolved into a global pandemic.407 SARS-CoV-2 belongs to the coronavirus genus and is an enveloped, single-stranded ribonucleic acid (RNA) virus.408,409 The virus gains entry into the host cell by binding its spike protein (S protein) to the angiotensin-converting enzyme 2 (ACE2) receptor on the surface of host cells. This attachment is followed by membrane fusion, allowing the injection of viral RNA into the host cell. Once inside, the viral RNA takes control of the host cell’s machinery, leading to the production of new viral particles. Subsequently, the virus can infect other cells, contributing to the further spread of the infection. Moreover, SARS-CoV-2 exhibits higher mutation rates in comparison to DNA viruses. Mutations in the viral genome arise during the replication process of the virus and facilitate immune (both innate and adaptive) evasion of this virus.410–413 Since SARS-CoV-2 infection can lead to dysregulated immune responses, including an excessive release of pro-inflammatory cytokines, such as IL-6, IL-17, IFNγ, and IL1β, often referred to as a “cytokine storm”.414–416 This cytokine dysregulation can impact both innate and adaptive immunity and lead to the dysfunction and exhaustion of immune cells and tissue damage. Several studies demonstrated the impact of COVID 19 on the number and function of γδ T cells in the peripheral blood.87,89,417–419 For instance, compared to healthy donors, patients with COVID-19 exhibit a significant decrease in the Vδ2+ subset of γδ T cells and an inverted Vδ1/Vδ2 ratio. A comprehensive immune profiling on moderate to severe COVID-19 patients suggested an overall increase in innate immune cells (monocytes, neutrophils, and eosinophils) while a reduction in T cell population.88,420 Subsequent functional analysis demonstrated decreased secretion of IFN-γ and elevated secretion of IL-17A, along with increased expression of PD-1, in peripheral γδ T cells of patients. Considering that the Vδ2+ subset is the main source of IFN-γ, these findings imply that excessive inflammation in COVID-19 patients could potentially lead to reduced responsiveness or AICD of peripheral Vδ2 T cells and/or their migration towards inflammatory lungs. This notion is further substantiated by the considerably higher levels of IFN-γ observed in tissues compared to blood samples.89 The role of Vδ1+ T cells in COVID-19 has received limited attention due to its low presence in the peripheral blood. However, Vδ1+ is the predominant subset of tissue-associated γδ T cells, known for their swift responses against pathogens. A recent study highlighted the rapid activation and expansion of peripheral Vδ1+ T cells in nonhuman primates during SARS-CoV-2 infection. Notably, Vδ1+ T cells from both peripheral and Bronchoscopy and Bronchoalveolar Lavage (BAL) fluid were skewed toward IL-17-producing functionality, suggesting viral suppressing and proinflammatory role it plays. This observation is further supported by a positive correlation between the frequency of circulating Vδ1+ T cells and the viral load in BAL fluid during the early phase of infection.421 Given the known presence of γδ T cells, particularly the Vδ1+ subset, in lung tissue and their ability to exhibit distinct physiological or pathological functions based on the local microenvironment,246,323,422 their involvement in mediating the clearance or disease progression of COVID-19 is not surprising. These findings collectively indicate the active participation of both Vδ1+ and Vδ2+ subsets in the control of SARS-CoV-2. Recent advancements in single-cell multi-omics techniques applied to samples from COVID-19 patients have provided valuable data for the further evaluation of the functional and developmental characteristics of both peripheral and tissue-resident γδ T cell populations.423–425 These studies have the potential to enhance our understanding of the precise functional evolution, developmental trajectory, and γδTCR clonotypic variations of each γδ T cell subset, both in circulating and local inflammation sites, throughout the course of SARS-CoV-2 infection. In summary, further research is needed to delve deeper into these aspects and gain comprehensive insights into the role of γδ T cells in the context of SARS-CoV-2 infection.
γδ T cell in autoimmune disease
Unlike conventional αβ T cells, which recognize peptides presented by MHC molecules on APCs, γδ T cells do not rely on MHC presentation for antigen recognition. As a result, γδ T cells have a much wider capacity for antigen recognition and can respond to non-peptide antigens, including stress-induced molecules and microbial elements. Given their broad recognition capabilities, it is reasonable to speculate on the role that γδ T cells may play in the development of autoimmunity. In instances where the immune system becomes dysregulated and mistakenly launches attacks on the body’s own healthy cells and tissues as if they were foreign invaders, the involvement of γδ T cells could be significant.
γδ T cells in psoriasis
Psoriasis is a chronic autoimmune skin disorder that affects as much as 2–3% of the world’s population and characterized by the formation of red, inflamed skin patches covered with silvery scales, known as psoriasis plaques. The overproduction of IL-17 in psoriatic lesions is one of the primary factors contributing to the dysregulated immune system that leads to the development of psoriasis.7,20 IL-17 functions as a pro-inflammatory cytokine, inducing inflammation and recruiting immune cells in the skin. Additionally, it stimulates the proliferation and activation of keratinocytes, the predominant cell type in the epidermis, leading to the characteristic inflamed, thickened, and scaly appearance of the skin. In addition to CD4+ T cells that produce IL-17, γδT17 cells have emerged as another significant source of IL-17, playing a pivotal role in driving the progression of psoriasis.69,71,73,74 Mice deficient of Sox13, a key transcription factor regulating γδT17 differentiation, led to selective deficiency of γδT17 cells during thymic development and are protected from psoriasis-like dermatitis.75 Furthermore, γδT17 cells have been found to exhibit a higher abundance in psoriatic skin lesions compared to healthy skin.426,427 These cells possess the ability to release cytokines, such as IL-17 and IFN-γ, which contribute to inflammation and the proliferation of keratinocytes. As a result, this process leads to the formation of the characteristic plaques seen in psoriasis.179,428 Multiple immune receptors signaling pathways348,429–433 and metabolic enzymes or mediators313,330,434,435 have been found to promote the differentiation of γδT17 cells in psoriasis. For instance, our research group discovered that GLS1-mediated glutaminolysis is essential for γδT17 cell differentiation and keratinocyte proliferation, thereby contributing to the pathogenesis of psoriasis.313 Additionally, we discovered that mTOR1 and mTOR2 signaling pathways regulate the differentiation of γδT17 and are dysregulated in psoriasis-like mouse model.434 Recently, it has been shown that γδT17 cells exhibit dynamic trafficking patterns, moving to and from lymph nodes and sites of skin inflammation.436,437
Additionally, a study demonstrated that peripheral but not tissue-resident γδ T cells could regulate neutrophil expansion and recruitment in the pathogenesis of psoriatic arthritis, suggesting the complementary role γδ T cells plays in exacerbating the disease progression.438 Conversely, other specialized immune cells also participate in facilitating the differentiation of γδT17 in psoriasis. For instance, certain microbial components, such as mannan, could activate macrophages, leading to the secretion of TNF-α. This, in turn, stimulates local γδ T cells to produce IL-17A.439 Moreover, nociceptive sensory neurons establish close contact with dermal DCs and regulate their production of IL-23, which plays a crucial role in the differentiation of dermal γδT17 cells.440
Considering the crucial role played by IL-17, produced by both Th17 and γδT17 cells in the pathogenesis of psoriasis, recent therapeutic strategies have primarily focused on reducing IL-17 production, counteracting its effects by corresponding inhibitors or antibodies, or limit the chemotaxis of Th17 and γδT17 cells.429,441–448 However, adverse side effects such as neurological diseases, infections, and liver dysfunction have been reported.449–451 This may be partly attributed to the significant role IL-17 plays in combating certain pathogens, particularly fungal infections. Blocking IL-17 partially compromises the immune system, increasing the likelihood of opportunistic infections.451
Notably, it has been demonstrated that excessive dietary cholesterol exacerbates γδT17-cell-mediated psoriasis.176 Furthermore, evidence indicated that feeding mice with a western diet, characterized by high fat and simple sugar content or high fat diet alone can induce psoriasiform dermatitis by promoting the accumulation of dermal γδT17 cells.452,453 This suggests that dietary interventions could serve as an alternative approach for controlling psoriasis. Furthermore, the application of scRNA-seq on the skin samples from patients holds the potential to shed light on the role that γδ T cells might play in the etiology and progression of psoriasis, providing valuable insights into their intricate involvement in the disease.454–457 Altogether, psoriasis is an autoimmune skin disorder driven by IL-17 overproduction, involving both Th17 and γδT17 cells. These cells induce inflammation and keratinocyte proliferation, contributing to plaques. Therapies targeting IL-17 face challenges due to its dual role in immunity. As our understanding of γδ T cells’ involvement grows, new treatment approaches are emerging for improved psoriasis management.
γδ T cells in inflammatory bowel diseases (IBDs)
γδ T cells have been implicated in the pathogenesis of inflammatory bowel disease (IBD) characterized by chronic inflammation of the gastrointestinal (GI) tract, including Crohn’s disease and ulcerative colitis.10,458 In the gut mucosa, γδ T cells belong to a group of non-classical intraepithelial lymphocytes (IELs) residing within the intestinal epithelium. γδ IELs are present in higher numbers compared to other tissues and are considered essential regulators of intestinal homeostasis and immune responses.459,460 Moreover, their unique anatomical position enables them to act as the first-line defenders against intestinal pathogen invasion.10 An increase γδ T cell frequency in the diseased intestinal mucosa has been reported in the IBD patients.460,461 γδ T cells actively contribute to a multifaceted immunoregulatory role in coordinating both innate and acquired immune responses, thereby preserving the integrity of epithelial tissues. Early study indicated a protective role γδ IEL plays in a chemical-induced acute colitis model.462–465 Moreover, recent study has indicated that γδ IELs could promote the viability of Paneth cells, which locate in the small intestine and are responsible for executing antimicrobial functions in Crohn’s disease.466 Notably, an early study demonstrated a decreased frequency of the intestinal CD8+ γδ T cell subset (mainly Vδ1+) in both the peripheral blood and the gut of patients with IBD. This functionally distinct subset exhibits cytotoxicity and produces IFN-γ and TNF-α instead of IL-17.467 On the other hand, γδ T cells have been shown to exacerbate chronic ulcerative colitis.468 Moreover, γδ IELs have been found to contribute to the excessive shedding of apoptotic enterocytes into the intestinal lumen, which is characterized in IBDs and is linked with disease reoccurrence.469 These findings underscore the intricate interplay between γδ T cells and the immune response in IBDs and highlights the need for further research to uncover their precise mechanisms.
Emerging evidence also indicates that the gut microbiota closely regulates intestinal immune homeostasis. Intestinal γδ T cells actively interact with and respond to the gut microbiota, adjusting their functions accordingly.184,343,470–472 For instance, the gut microbiota produces short-chain fatty acids (SCFAs), which can suppress IL-17 production by intestinal γδ T cells in patients with IBDs.473 Conversely, genetic mutations can increase susceptibility to IBDs by disrupting the regulation of immune responses to pathogenic stimuli.474–476 For instance, mutations in genes such as NOD2, a cytosolic bacterial sensor, have been identified as high-risk factors for Crohn’s disease.477–480 When these genes are mutated, the recognition of gut microbiota by intestinal intraepithelial lymphocytes (IELs), including γδ T cells, becomes dysregulated, leading to the inflammatory pathologies observed in IBDs.481–483
In conclusion, intestinal γδ T cells synergistically collaborate with the local immune microenvironment and epithelial cells to uphold symbiosis with the gut microbiota and mount immune responses against invading pathogens. Disruption of this intricate collaboration can lead to IBDs, other intestinal disorders, and even cancers. The recent application of scRNA-seq technology on clinical samples of IBDs has shed light on the complex interaction network among various immune cell subsets at the site of inflammation.484–488 This advancement aids in further comprehending the interplay between γδ T cells, the microbiota, and the pathogenesis of IBDs. Additionally, identifying the elusive antigens recognized by γδTCRs in the gut also holds promise for discovering novel therapeutic targets.184
γδ T cells in multiple sclerosis (MS)
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system (CNS), characterized by inflammation, demyelination, and damage to nerve fibers. Its cause involves a combination of genetic and environmental factors.489–492 Experimental autoimmune encephalomyelitis (EAE) is an animal model used to study MS, where immunization with myelin antigens induces an autoimmune response against the CNS.493 In both MS and EAE, the immune system mistakenly attacks the protective myelin sheath, leading to inflammation and disruption of nerve signals. This results in diverse neurological symptoms, including muscle weakness, sensory disturbances, coordination problems, and cognitive impairments. Early Studies have demonstrated the enrichment and functional characteristics of γδ T cells in the MS and EAE lesions, as well as in the cerebrospinal fluid and peripheral blood of both patients and animal models.494–499 Notably, the role of γδ T cells in MS and EAE is controversial, as there is evidence supporting both their protective326,500 and pathogenic functions.501–503 For instance, γδ T cells regulate the production of IFNγ by T cells infiltrating the CNS and the absence of γδ T cells in TCRδ−/− mice resulted in a more severe course of EAE, like what is observed in mice deficient in IFNγ. This suggests that γδ T cells are important regulators of CNS inflammation and necessary for adequate production of IFNγ in the CNS, which is crucial for the recovery from EAE.326 Conversely, conflicting evidence indicated a pathogenic role of γδ T cells instead in CNS inflammation and autoimmunity.68,504–507 For instance, IL-1 and IL-23 promote the differentiation of γδT17 cells, which in turn amplifies Th17 responses and contributes to the development of EAE autoimmunity.62 Furthermore, it has been shown that IL-23-activated γδ T cells can suppress Foxp3+Treg cells, thereby inhibiting the Treg cell-mediated suppression of effector T cell Th17 responses. This disruption in Treg cell function leads to enhanced pathology in EAE.68
Recent research advancements have unveiled the regulatory role of γδ T cells in the meninges, the protective membranes surrounding the brain, in modulating brain functions. Under normal conditions, meningeal T cells that produce IFN-γ are involved in the regulation of social behavior,508 while meningeal-resident γδT17 cells play a role in modulating anxiety-like behavior,509 synaptic plasticity, and short-term memory510. Under pathological conditions, γδT17 plays pivotal role in ischemic brain injury65 and EAE model,511 migrating from the intestine to the meninges after injury and participating in the regulation of aberrant brain functions.471 Emerging evidence suggests that changes in the composition and structure of the gut microbiota can have a significant impact on the development and functioning of the host immune system, potentially leading to inflammation in the CNS. One compelling example comes from a mouse model where it was demonstrated that Lactobacillus acidipiscis-induced γδ Treg cells can mitigate experimental EAE by suppressing the development of encephalomyelitic Th1 and Th17 cells.512 Furthermore, recent research has shown that psychosocial stress can lead to a reduction in Lactobacillus johnsonii within the gut microbiota. This reduction, in turn, promotes the differentiation of intestinal γδ T cells into γδT17 cells and their accumulation in the colon. Subsequently, these γδT17 cells migrate to the meninges, establishing a gut-brain axis that mediates the observed depressive behavior.90,513 Therefore, a deeper understanding of the regulatory roles played by the gut microbiota could potentially facilitate the development of precise intervention strategies aimed at reconstituting or modifying the microbiota in the treatment of MS.
The clinical implications of γδ T cells in MS are currently being investigated, with their presence and activation at the lesion sites and in peripheral blood suggesting their potential as biomarkers for monitoring disease progression. Furthermore, γδ T cells have been associated with specific clinical features of MS, such as cognitive impairment and disability progression. Advancing our understanding of the role of γδ T cells in MS may facilitate the development of targeted therapeutic strategies.
γδ T cells in diabetes
Diabetes is intricately associated with autoimmune diseases, particularly within the realm of type 1 diabetes (T1D). In this specific subtype, the immune system mounts an attack on and ultimately annihilates the insulin-producing pancreatic β cells.514 Because insulin is an essential hormone responsible for regulating blood glucose, T1D leads to a shortage of insulin production, which in turn leads to elevated blood glucose levels. The immune response seen in T1D predominantly involves conventional T cells, namely the CD4+ helper and CD8+ cytotoxic T cells.515
Conversely, γδ T cells bridge innate and adaptive immunity by secreting cytokines or acting as antigen-presenting cells. They are thought to regulate T and B cell responses in T1D. Notably, γδ TCRs possess a broader antigen recognition repertoire than αβ TCRs and are MHC-unrestricted, enabling them to directly recognize T1D-associated antigens.
Early studies indicated deficient αβ but not γδ TCR thymocyte development in the non-obese diabetic (NOD) mouse model, suggesting distinct regulation of these T cell populations in diabetic milieu.516,517 Moreover, thymic αβ/γδ-lineage decision skews towards αβ in diabetes-prone NOD mice, revealing thymic selection anomalies.518
Notably, γδ T cells in NOD mice recognize processed insulin like αβ counterparts.519 Aerosolized insulin induces regulatory CD8+γδ T cells in NOD mice, preventing diabetes onset.49 Furthermore, reduced CD8+ and CD8−γδ T cells were observed in prediabetic individuals.520 A longitudinal study established the temporal association between γδ T cell percentage and the onset of T1D. Cumulatively, these studies provide further endorsement for the regulatory and hence protective role played by γδ T cells in T1D.521–523
However, the introduction of TCRδ-deficiency onto the NOD mouse background shields them from T1D, thereby hinting at the pathogenic role of γδ T cells.524 Moreover, a recent study illuminated the dualistic, both protective and pathogenic, role that γδ T cells enact in T1D contingent upon their functional subsets.525 As such, the precise role of γδ T cells in diabetes remains to be clarified and may pivot on specific contextual factors. Additionally, the involvement of γδ T cells in type 2 diabetes (T2D)526,527 remains relatively unexplored, with a scarcity of available literature to facilitate in-depth discussions. This situation underscores the need for additional investigations to shed light on this aspect.
γδ T cells in cancers
Elements of tumor microenvironment (TME) attenuate γδ T cell functions
It has been well acknowledged that the TME is detrimental to the T cell-mediated tumor immunosurveillance.2,3,528 The functional polarization of γδ T cells by various TME elements results in their pleiotropic effector functions in cancers (Fig. 3a).529 Here, we briefly list some well-known TME features contributing to the modulation of T cell immunity.
Epigenetic regulation
Recently, it has been discovered that epigenetic and transcriptional regulations have an impact on the functional differentiation of γδ T cells.171,317,530 In our recent review, we elaborated on epigenetic modulators in the TME that can initiate a functional shift in infiltrated T cells.531 For example, lactate, alpha KG, and acetyl-coa can regulate various histone modifications, thus affecting transcription factor(s) binding. This can result in either the “silencing” or “activation” of gene expression in γδ T cells, similar but through different regulatory transcription factors or cytokines when compared with αβ T cells.317,532 However, most of the previous studies were conducted using mice models, and there are significant differences in the functional regulation and differentiation of γδ T cells in mice and humans. Therefore, more studies using human samples are needed.
Hypoxia
Similar to αβ T cells, γδ T cells primarily rely on glycolysis rather than mitochondrial respiration to carry out their effector responses. This metabolic shift occurs as naïve cells differentiate into effector cells. However, TME poses challenges for both glycolysis and oxygen availability, severely impairing the anti-tumor effector function, survival capacity, and proliferation/differentiation potential of naïve γδ T cells. In a brain tumor model, a hypoxic TME was found to impair the effector function of γδ T cells, while αβ T cells were unaffected.533 Conversely, Siegers et al. reported enhanced cytotoxicity but reduced proliferation of γδ T cells under hypoxic conditions in vitro.534 These seemingly contradictory observations can be attributed to the heterogeneity of the TME across various cancer types. Therefore, further research is crucial to unravel the complex interactions and design therapeutic regimens that are tailored to the specific TME characteristics of individual cancer patients.
Oxidative stress
Dysregulated Reactive oxygen species (ROS) in TME have long been considered to negatively impact T cell-mediated anti-tumor immunity.535 Nonetheless, tumor-associated neutrophils-derived ROS could restrain the pro-tumoral effect of γδT17 cells.536 Further understanding of the roles of ROS in tuning γδ T cell functions might benefit their clinical application.537
Exosomes
Tumor-derived exosomes (TDE) play an important role in the development of tumor immune escape.538 The TDE has been shown to regulate the pro- or anti-tumor responses of γδ T cells.539 Ni et al. showed that cancer cell-secreted exosomes upregulated the immunosuppressive CD73+Vδ1+ TILs (Treg) population via exosome-embedded lncRNA SNHG16 in breast tumors.313 Moreover, a study showed that tumor-derived exosomes could induce MDSC-directed γδ T exhaustion.539 Interestingly, a study published by Tu’s group showed that, exosomes derived from Vδ2 T cells exhibit strong anti-tumor potentiality as well.540 However, how to utilize exosomes (tumor- or immune cell derived) to further potentiate clinical efficacy of adoptive transferred allogeneic Vδ2 T cells remains to be fully addressed.
Treg
Tumor-infiltrated CD4+Treg has been shown to inhibit the anti-tumor immunity of γδ T cells in HCC through the secretion of TGFβ and IL-10.541 Additionally, tumor-derived TGFβ can induce the differentiation of immunosuppressive CD39+ γδTreg cells in colorectal cancer (CRC).314 Circulating neutrophils542–544 and MDSCs545 in the TME can also restrain the anti-tumor response of γδ T cells.546 Therefore, fully deciphering the immune landscape of TME and elaborating the interactions between immunosuppressive cell populations and γδ T cells ensure further understanding of γδ T cell functions in TME.
Checkpoint molecules
In the context of TME, another important feature is the elevated expression of checkpoint molecules, which are involved in dampening the effector capabilities of tumor-infiltrating γδ T cells. The principal co-inhibitory molecules expressed in T cells predominantly encompass PD1 (Programmed Cell Death Protein 1), LAG3 (Lymphocyte-Activation Gene 3), CTLA4 (Cytotoxic T-Lymphocyte Associated Protein 4), TIM3 (or HAVCR2; T cell immunoglobulin and mucin-domain containing-3), TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domains), BTLA (B and T lymphocyte attenuator), B7-H3 (CD276), and others. These pivotal checkpoint molecules have been documented to assume crucial roles in curtailing T cell cytotoxic functions.547–549 In the case of γδ T cells, these checkpoint molecules also govern cellular effector function. For instance, PD1 and TIM3 can differentially modulate the anti-tumor activity of specific subsets of murine γδ T cells, namely Vγ6+ and Vγ4+ cells, which produce IL-17A.550 In human, the exhaustion of intratumoral γδ T cells correlates with the expression of various immune checkpoints such as PD1, TIGIT, TIM3, CTLA4, and CD39.551,552 As for BTLA, it negatively regulates human Vδ2 T cell proliferation553 and curbs γδ T cell numbers and sustains normal frequencies of γδ T cell subsets. As a results, it maintains the equilibrium of γδ T cell populations and controls inflammatory responses in mice.554
In the case of B7-H3, an immunoregulatory protein belonging to the B7 family, it is expressed on T cells. B7-H3 can suppress the cytotoxicity of human Vδ2 T cells by downregulating the expressions of IFN-γ, perforin, and granzyme B.555 Intriguingly, TIM3 not only fulfills roles in modulating the function of γδ T cells in tumors but also reduces inflammatory reactions of γδ T cells. Consequently, this leads to a reduced susceptibility to malaria infection and minimized malaria symptoms in children.556
Furthermore, according to our work, we propose that LAG3 holds promise as a target checkpoint in solid tumor, particularly in HCC. This assumption is grounded in our published data, which indicate that LAG3, rather than other molecules such as TIM3 and PD1, is notably upregulated in HCC-infiltrating γδ T cells. Additionally, a similar phenotype can be induced through glutamine restriction.243 Nonetheless, given the intricate nature of the TME, multiple checkpoint molecules, as opposed to a single entity, contribute to impairing the effector function of γδ T cells. Thus, we propose that a prospective strategy for tumor immunotherapy shall involve the simultaneous blockade of multiple checkpoint targets and the adoptive transfer of γδ T cells derived from healthy donors.
γδ T cells in hematological cancers
Hematologic cancer, also known as hematological malignancy or blood cancer, encompasses a diverse group of neoplastic disorders affecting the blood, bone marrow, and lymphatic system. This category includes leukemia, lymphoma, and multiple myeloma. These malignancies originate from aberrant growth and differentiation of blood cells, leading to perturbations in normal hematopoiesis and hematologic function.557–559 Hematologic cancer has multifaceted causes, encompassing genetic560–564, environmental,565,566 and lifestyle factors,567 etc. Viral infections, for example, have been associated with a higher risk of specific hematologic cancers.243,568,569 Additionally, autoimmune diseases, immunodeficiency disorders, and chronic inflammatory conditions can heighten the susceptibility to hematologic cancer.570–574 Together, these factors contribute to the development of hematologic malignancies. Although progress has been made in the long-term survival of the patients,575 the inherent complexity and heterogeneity of hematologic cancer makes it difficult to develop universal treatment strategies. In the context of hematological malignancies, observations have been made regarding functional deficiencies of γδ T cells.576 Studies have demonstrated the dual roles of γδ T cells, exhibiting both anti-tumor577–581 and pro-tumor582 functions. However, it is important to note that these functional outcomes are highly dependent on the context and functional characteristics of the γδ T cell subsets involved.
Early studies focused on stimulating in vivo or ex vivo expansion of γδ T cells of patients.583 Nevertheless, one of the main drawbacks of using autologous γδ T cells in cancer treatment is the compromised function of γδ T cells in cancer patients, not to mention the systemic side effects of the drugs used to stimulate γδ T cell proliferation, such as zoledronate,584,585 which ultimately leads to limited clinical benefits.54,586–589 Furthermore, γδ T cell recognition of malignant cells does not depend on MHC presentation, meaning they would theoretically not recognize the recipient as “non-self” and mount immune attacks.9,14 This unique property provides an advantage in utilizing allogeneic γδ T cells for cancer treatment and bypassing the graft-versus-host effects associated with MHC-mismatched αβ T cells.590,591 Additionally, early clinical observations indicated that increased γδ T cell levels (particularly the Vδ1+ subset) predicted long-term disease-free survival in acute leukemia patients following Allogeneic stem cell transplantation (ASCT).592–595 These findings prompted successful attempts to utilize haploidentical stem cell transplantation (HSCT) in treating pediatric patients with acute leukemia (NCT01810120). The approach involved depleting αβ T and B cells using antibodies while preserving only the mature immune-competent γδ T cell and NK cell populations. In this study, during the early post-transplantation period, the Vδ1+ and Vδ2+ subsets were predominantly composed of central-memory cells. Interestingly, the differentiation status persisted in the Vδ2+ subtype even six months after transplantation, while the Vδ1+ subtype exhibited a drastic decrease in central-memory cells but an increase in terminally differentiated cells by the sixth month. Furthermore, a significant increase in the percentage of the Vδ1+ subset, accompanied by a decrease in the Vδ2+ subset, was demonstrated, suggesting diverse functional roles between these two subsets.596 A follow-up study further demonstrated a 5-year probability of chronic graft-versus-host disease (GVHD)-free, relapse-free survival (GRFS) at 71%, comparable to that of HLA-matched donor HSCT recipients, indicating long-term benefits of allogeneic γδ T cells graft.597 A similar clinical trial was conducted in adults with hematological malignancies.598 In all cases, early reconstitution of γδ T cells was observed after HSCT, along with prognostic benefits such as reduced risk of infections and improved event-free survival, emphasizing their functional roles following allogeneic HSCT for leukemia.595,599–601 Currently, multiple clinical trials are underway to directly transfer allogeneic γδ T cells to exert a graft-versus-leukemia (GVL) effect, either Vδ1+ or Vδ2+ subset (phase I clinical trial NCT03790072, NCT03533816), to patients with hematologic cancer.602,603 Additionally, an intriguing and promising prospect of applying allogeneic γδ T cell immunotherapy is the treatment of patients with malignant γδ T cell transformation, such as hepatosplenic γδ T cell lymphoma (HSGDTL),604–607 primary cutaneous γδ T cell lymphoma (PCGDTL),608,609 and acute lymphoblastic leukemia (γδ T-ALL).8,610–612
Other than HSCT, another promising immune cell-based immunotherapy to treat hematological cancers is CAR-T cell Therapy.23,613 CAR T therapy is a groundbreaking evolution in cell-based immunotherapy pioneered by Dr. Carl June in treating hematological malignancies, such as chronic and acute leukemia like acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) etc. CD19-directed CAR-T cell therapy has demonstrated remarkable efficacy, with an overall remission rate (OR) as high as 81% within 3 months in the treatment of B-Cell Lymphoblastic Leukemia.614,615 This efficacy is attributed to the efficient recognition and binding of CD19 CAR-T cells to CD19-expressing malignant B cells, leading to their targeted destruction.21,22,616–619 However, the application of CAR-T therapy is hindered by systemic side effects, such as cytokine storm syndrome, a systemic immune dysregulation that can result in multiorgan failure if left untreated.415,620 Additionally, neurotoxicity has been reported in over 60% of patients receiving CAR-T therapy.621–623 This neurotoxicity is mainly caused by the ability of CAR-T cells to trigger cytokine release syndrome (CRS) and migrate to the central nervous system (CNS), targeting and compromising the integrity of the blood brain barrier (BBB),624 which enable them to further interact with neuronal cells and activate brain-resident immune cells, leading to an inflammatory response and subsequent neurotoxicity.
Recently, CAR-transduced γδ T cell-based immunotherapy has garnered attention due to several unique advantages. Firstly, γδ T cells exhibit a wide recognition spectrum for tumor-associated antigens, encompassing stress-induced ligands, phosphoantigens, and non-peptide antigens. This expanded recognition capacity positions γδ T cells favorably compared to αβ T cells in the realm of cancer immunotherapy. Additionally, γδ T cells can exert tumor-toxicity through direct engagement with cancer cells or by activating other immune cells. Furthermore, their MHC-unrestricted recognition of malignant cells reduces the likelihood of graft-versus-host disease (GVHD)599 and enables them to overcome immune evasion strategies employed by cancers, such as downregulated MHC molecule expression. Although CAR γδ T cell immunotherapy is still in its early stages of development, several promising studies have emerged. For instance, studies demonstrated reduced tumor burden in mice using CD19-specific CAR-T cells in leukemia model.625,626 Furthermore, recent research by Pablo et al. showcased the efficacy of allogeneic CD123 CAR-Delta One T (DOT, Vδ1+) cells, which target the interleukin-3α chain receptor (CD123) expressed on acute myeloid leukemia (AML) blasts, in the treatment of AML in mice.627 Additionally, allogeneic CD20-targeted CAR Vδ1+ γδ T cells, specifically designed to target the B-cell-restricted CD20 antigen, exhibited anti-tumor activity in a B-cell lymphoma mouse model. A phase I trial is currently underway to evaluate the efficacy of these CAR T cells in patients with relapsed/refractory B-cell malignancies (NCT04735471).628
To conclude, hematologic cancers encompass a wide array of neoplastic disorders affecting the blood, bone marrow, and lymphatic system. Their complex etiology involves genetic, environmental, and lifestyle factors, with viral infections and immune-related conditions contributing to susceptibility. Advances have improved patient survival, yet the intricate nature of these malignancies hinders universal treatment strategies. Observations on γδ T cells reveal their dual roles in cancer, but context-specific functions underscore the need for deeper understanding. Allogeneic γδ T cells show promise, as seen in HSCT trials, offering advantages over conventional approaches. Furthermore, CAR-γδ T cell therapy emerges with expanded recognition and potential benefits. Despite progress, in-depth investigations, including single-cell transcriptome analysis,629–631 remain crucial to fully exploit γδ T cells’ potential and advance targeted therapies for hematologic malignancies.
γδ T cells in solid tumors
γδ T cells hold great potential as a novel immunotherapeutic approach for not only hematological cancers but also solid tumors. While they increasingly exhibit remarkable potential in the immunotherapy of hematological malignancies as discussed above, γδ T cells also represent the future in solid tumor immunotherapy. It has been recognized that γδ T cells possess a remarkable ability to identify stress-induced antigens on tumor cells, even in scenarios involving low mutational burdens or MHC defects,256 rendering them a valuable approach for solid tumor therapy. Tumor cells frequently downregulate HLA class I (MHC-I) to evade the immune response, which impedes the conventional activation of CD8+ T cells. This distinctive trait of γδ T cells becomes particularly advantageous in augmenting the scope of existing T cell-based immunotherapies. For instance, T cell receptor-engineered T cell (TCR-T) therapy primarily focuses on antigens presented through HLA class I molecules. However, in cancers where HLA class I is deficient, TCR-T cells might encounter challenges in recognizing antigens. Integrating the autonomous antigen recognition capability of γδ T cells, independent of HLA class I, into the development of the γδ TCR-T will enable evasion of immune evasion mechanisms caused by diminished HLA expression in cancer cells. Additionally, the identification of individuals with tumors lacking HLA class I would enable the personalized utilization of CAR- or TCR-γδ T cells, ensuring treatment alignment with the tumor’s immune characteristics.
The unique MHC-independent recognition also offers the advantage of reduced immune rejection, rendering allogeneic adoptive γδ T cell transfer a safer therapeutic approach.11,12,587 Additionally, γδ T cells exhibit efficient APC capabilities, effectively activating other immune cells to mediate tumor clearance.56,217,632 By functioning both as tumor-specific effectors and potent APCs, γδ T cells hold significant promise in the field of immunotherapy for both hematological cancers and solid tumors.
Currently, there are two main categories of γδ T cell-based therapies which include γδ T cell engagers and adoptive γδ T cell transfer9. The cell engagers strategy primarily involves the use of mono- or bispecific antibodies to connect γδ T cells with their targets, leading to highly specific tumor lysis. For example, the use of an agonistic BTN3A1 antibody, which binds to BTN3A1+ cancer cells, triggers phosphoantigen-like Vγ9Vδ2 T activation and tumor recognition82,633 A phase I/IIA clinical trial of this strategy is currently underway (NCT04243499). Additionally, bispecific antibodies designed to bind both the γδ TCR (mainly Vγ9) and cancer-specific target molecules, such as HER2,634 CD123,635 EGFR,636 CD40,637 and CD1d,638 are also being developed. Interestingly, bispecific antibodies targeting both cancer phosphoantigen-sensing Vγ9 TCR and CD3 binding domains have demonstrated enhanced effectiveness in αβ T cell-mediated cancer-killing, indicating that Vγ9 TCRs act as a “cancer detector” and recruit αβ T cells to the tumor microenvironment.639 Furthermore, in addition to γδ T cells, novel bispecific engager strategies640 might also simultaneously recruit other innate-like effector cells.
On the other hand, adoptive cell therapy is further divided into two types, naturally expanded or genetically modified γδ T cell strategy.641 Early studies showed limited success in using in vivo synthetic phosphoantigen-stimulated or cancer patient-derived ex vivo expanded autologous γδ T cell transfer,14,583,587 mainly due to the impaired immunity of patients. Therefore, the focus has shifted to allogeneic γδ T cell transfer. Early attempts were made in hematological malignancies using allogeneic stem cell transplantation depleted for αβT cells627,628or haploidentical γδ T cells,603 which showed reasonably high objective response (OR) rate with limited side effects. Therefore, our research team pioneered the first clinical allogeneic Vδ2 T cells transfer on 132 patients with various terminal solid tumors. The observed clinical benefits through a total of 414 cell infusions established a proof-of-concept for the application of allogeneic Vδ2 T cells in solid tumor treatment.11,12 Additionally, clinical applications of allogeneic Vδ1+ T (DOT) cells showed promising results in hematological malignancies.642,643 Unlike the Vδ2+ subtype, Vδ1+ T cells seem to resist AICD,248,279 which might provide persistent protection. A previous study also showed the superior tumor cytotoxicity of Vδ1+ over Vδ2+ in a mouse xenograft tumor model.644 Therefore, further functional comparisons between these two subtypes could help gain insights into their respective clinical benefits.
Along with the natural expansion strategy, CARs-transduced γδ T cells have been developed with well-known targets on solid cancers, such as GPC327 and NKG2D ligand.645 However, it has been found that the cancer cell cytotoxicity of CAR- Vγ9Vδ2 T gradually diminishes, raising concerns about Vγ9Vδ2 clinical persistence.646 Another type of CAR modification is to fuse γδ TCR with αβ T cells, namely γδ TCR-engineered T cells.271,647,648 This strategy was deployed in various cancer models,649 and phase I clinical trials are ongoing. Table 1 lists ongoing or completed clinical trials on allogeneic γδ T cell-based cancer therapy. Although promising preclinical results were demonstrated, further evidence is needed to establish both the safety and efficacy profile of these genetically modified γδ T cell strategies. Finally, adoptive γδ T cell transfer could be applied in combination with immune checkpoint inhibitors (ICIs: PD-1, CTLA4, LAG3, etc.) to maximize cytotoxic potency and avoid exhaustion.247,650 Nonetheless, both γδ T cell engager and adoptive γδ T cell transfer strategies require further clinical validations.
Allogeneic γδ T cells: off-the-shelf medicine for tumor immunotherapy
For cancer patients, the impaired function of γδ T cells and the difficulty in expanding circulating Vδ2 T cells for autologous immune cell therapy have been observed in our preclinical studies. Additionally, the tumor microenvironment not only suppresses the function of γδ T cells but also reduces their cell number with programmed cell death, including AICD, playing a crucial role in the reduction of infiltrated γδ T cells, according to our published work.243 However, it is difficult to conclude the abundance of TME-infiltrated γδ T cells between normal and cancer tissue using TCGA-based data analysis (Fig. 7a). Notably, TCGA analysis suggested a significant correlation between TRDC and the gene sets of pyroptosis and PANoptosis in most carcinomas (Fig. 7b), indicating programmed cell death of γδ T cells in the TME. Nevertheless, this analysis cannot determine which subset of γδ T cells is more tolerant in the TME.
Additionally, although infiltrated-γδ T cells have been identified as the most favorable indicator for good prognosis in 25 types of cancers,86 over-mining of TCGA data may lead to biased and controversial conclusions.339 Therefore, we conducted a straightforward assay to investigate the relationship between TRDC and the overall survival (OS) of pan-cancer patients based on the TCGA database. We found that, among 33 types of cancers, TRDC is correlated with good prognosis (better OS) of only 9 types of cancers, and two types has worse OS (Fig. 7c). However, TCGA database-based bioinformatics can still provide some valuable directional cues for understanding γδ T cell immunity in the context of the TME. We anticipate that the latest scRNA-seq technology will help uncover the comprehensive signatures of γδ T cells in both healthy individuals and tumor patients.
Even though γδ T cells are numerically reduced and functionally impaired in the TME, intra-tumoral infiltration is positively correlated with good prognosis among most cancers. γδ T cells, particularly allogeneic γδ T cells-based immunotherapy, represent a new alternative treatment for cancer patients. For example, adoptive transfer of in vitro-expanded allogeneic Vδ2 T cells not only controls tumor progression and achieves remission in some patients with solid tumors,11,12 but may also serve as APCs to elevate the percentages of CD4+ and CD8+ T cells (red dots/lines in Fig. 7d) according to our study. Meanwhile, one of the major functions of Vδ2 T cells is to secrete IFNγ, which plays a crucial role in regulating αβT cells as well. Preliminary evaluation based on our clinical data showed that most of those patients who had elevated CD4+ and/or CD8+ T cells had an extended overall survival, suggesting that the regulation of αβT cell percentage might be a response indicator for allogeneic Vδ2 T cell therapy. Notably, this endorses that although γδ T cells recognize and kill target cells in a MHC-independent manner, MHCs of γδ T cells functionally play crucial roles in regulating other types of immune cells, such as αβT cells. Remarkably, that MHC-independent recognition pattern highlighted the unique advantage of no acute graft-versus-host disease (GVHD) of γδ T cells. Together, we believe that the unique advantages of γδ T cell-based cancer immunotherapy cannot be replaced by other types of immunotherapies, and represents a key future for tumor immunotherapy.
Additionally, it is interesting to highlight the similarity between γδ T cells and NK cells in their recognition and elimination of stressed or transformed cells, encompassing cancer cells and pathogen-infected cells, through an array of activating and inhibitory receptors. Notably, NK cells have been comprehensively reviewed recently.651,652 Overall, both γδ T and NK cells exhibit a broader spectrum of tumor cell recognition compared to conventional αβ T cells. The main difference between the two cell types refers to the expression of the γδ TCR which is missing on the CD3-negative NK cells. As a consequence, both cell types identify stress-induced ligands via activating and inhibitory NK receptors, but only γδ T cells recognize tumor cells on the basis of enhanced phosphoantigen production. While γδ T cells act independently of MHC restriction, the activation of NK cells is intimately regulated by receptors which sense dysregulated HLA class I expression and/or stress-induced ligands on cancer cells. The use of either cell type mitigates risks of alloreactivity and graft-versus-host disease (GvHD).601,651,653 Diverse innate cytotoxicity receptors on their cell surfaces equip them to detect a wide range of cancer antigens. These qualities support the development of allogeneic cell therapies involving γδ T cells or NK cells, with applicability to diverse malignancies.652 At present, both γδ T cells and NK cells are extensively explored in CAR-based therapies.26,279,651,654 However, variations may exist in their antigen recognition. Particularly noteworthy is that γδ T cells, but not NK cells, serve as professional antigen-presenting cells,56,354 exerting pivotal roles in regulating immune responses in cancers. Despite shared features between these two immune cell types, distinctions in antigen recognition mechanisms and immune attributes might influence the precision of CAR-NK and CAR-γδT therapies when targeting malignancies. The choice of CAR antigens and the characteristics of the tumor microenvironment can impact treatment efficacy. Nonetheless, in-depth research is indispensable to fully comprehend the potential of γδ T and NK cells in the context of targeting malignancies.
Finally, in order to provide a comprehensive overview of the current advancements in allogeneic γδ T cell-based immunotherapy for cancer, we have compiled and presented a summary of registered clinical trials available on the clinicaltrials.gov website, as shown in Table 2.
Table 2.
Summary of allogeneic γδ T cell-based cancer therapy in clinical trials
| Tumor type | NCT | Status | Therapy | Recruit number | Start date | Location | Supplementary |
|---|---|---|---|---|---|---|---|
| Breast |
NCT 03183206 |
Completed | Procedure: Cryosurgery or IRE surgery. Biological: Allogeneic γδ T cell. Other: Allogeneic γδ T cells/A Cryosurgery or IRE. | 100 | June, 2017 | Guangdong, China |
~1.5 × 108 γδ T cells every 2 wk. Cell culture: RPMI-1640, IL-2, IL15, zoledronate, vitamin C |
| Liver |
NCT 03183219 |
30 | June, 2017 | Guangdong, China | |||
| Lung |
NCT 03183232 |
30 | June, 2017 | Guangdong, China | |||
| Pancreatic |
NCT 03180437 |
62 | June, 2017 | Guangdong, China | |||
| Malignant Solid Tumor |
NCT 04765462 |
Recruiting | Allogeneic γδ T cells | 60 | March, 2021 | Beijing, China | In the process of a dose escalation trial |
|
AML; ALL; Myelodysplastic Syndromes; |
NCT 04764513 |
Recruiting | Ex-vivo expanded γδ T cell infusion | 20 | Sept, 2021 | Beijing, China | |
| AML |
NCT 04008381 |
Unknown | Ex-vivo Expanded γδ T Lymphocytes | 38 | Sept, 2019 | Wuhan, China | |
| AML |
NCT 05358808 |
Recruiting | Ex-Vivo Expanded Allogeneic γδ T-lymphocytes (TCB-008) | 148 | Aug, 2022 | UK | 7 × 10^7 or 7 × 10^8 cells |
| AML |
NCT 03790072 |
Completed | Ex-vivo Expanded γδ T-lymphocytes (OmnImmune®) | 10 | Nov, 2018 | Czechia | Dose escalation |
| Glioblastoma |
NCT 05664243 |
Not yet recruiting | Autologous/ Allogeneic genetically modified γδ T cells | 120 | Jan 2023 | Alabama, US | / |
| AML |
NCT 05015426 |
Recruiting | γδ T-Cell Infusion | 32 | Aug, 2021 | Florida, US | Dose escalation |
|
Neuroblastoma; Refractory/Relapsed Neuroblastoma |
NCT 05400603 |
Not yet recruiting | Ex Vivo Expanded Allogeneic γδ T Cells in Combination with chemotherapy | 24 | Nov, 2022 | Georgia, US | Dose escalation |
|
Non-Hodgkin’s Lymphoma (NHL); Peripheral T Cell Lymphoma (PTCL) |
NCT 04696705 |
Recruiting | Ex-vivo expanded allogeneic γδT cells | 10 | Dec, 2020 | Tianjin, China | Dose escalation |
| HCC |
NCT 04518774 |
Unknown | Ex-vivo expanded allogeneic γδT cells | 8 | Aug, 2020 | Beijing, China | |
|
AML, CML, ALL Myelodysplastic Syndromes |
NCT 03533816 |
100%CR in AML declaimed | Expanded/Activated γδ T-cell Infusion | 38 | Jan, 2020 | Kansas, US |
1, 3, or 10 × 10^6 cells/kg. Cell expansion: CliniMACS-Prodigy |
|
Cancer;Malignancy; Refractory/Relapsed Cancer |
NCT 05302037 |
Not yet recruiting | Allogeneic NKG2DL-targeting CAR-grafted γδ T Cells (CTM-N2D) | 9 | April 2022 | Singapore | Four infusions: 1 × 10^7, 1 × 10^8, 3 × 10^8 or 1 × 10^9 per infusion every 7 days |
| Colorectal; TNBC; Sarcoma; Prostate; Nasopharyngeal Carcinoma; Gastric |
NCT 04107142 |
Unknown | Biological: Adoptive Cell Transfer of NKG2DL-targetting CAR-grafted γδ T cell | 10 | Dec, 2019 | Malaysia | “3 + 3” dose escalation: 3 × 10^8 - 3 × 10^9 |
| Lymphoma |
NCT 04735471 |
Recruiting | Anti-CD20 Allogeneic CAR- γδ T with chemotherapy | 78 | March, 2021 | US | “3 + 3” Dose Escalation |
| B-cell Leukemia |
NCT 04439721 |
Unknown | Allogenic γδT Cell infusion agent | 5 | May, 2020 | Jiangsu, China | 0.5 × 10^6-8 × 10^7γδT /kg, once |
| Non-Hodgkin’s Lymphoma |
NCT 05554939 |
Recruiting | Allogenic CD19-CAR-γδT cell with chemotherapy | 30 | Dec, 2022 | Beijing, China | “3 + 3” Dose Escalation |
|
Leukemia Lymphoma |
NCT 02656147 |
Unknown | Allogeneic Anti-CD19-CAR γδT | 48 | Oct, 2017 | Beijing, China | Dose escalation |
Note: Conclusion of these study is not available yet except NCT03533816
Future prospects
Based on our previous clinical observations,11,12 it has been found that allogeneic Vδ2 T cell transfer, derived from healthy donors and administered to cancer patients, is safe. However, it has also been observed that only a fraction of patients responded well to the treatment. Therefore, we have summarized a few key challenges that need to be addressed in order to ensure successful allogeneic γδ T cell clinical applications (as depicted in Fig. 7e).
Qualified donor selection
One challenge is if and how to match donors with recipients to guarantee therapeutic benefits. Due to their MHC-independence, HLA-matching may not be required, but there are as yet scarce data available to judge whether full allogeneic mismatch or haplo-identical transfers are preferable. For clinical application of allogeneic Vδ1+ T cells, the matching strategy might be solved by sequencing the γδTCR on donor γδ T cells to selectively expand subclones with strong functional activities,271,655 which can recognize and attack the tumor-associated antigens (TAAs) and/or neoantigens of the patients. Interestingly, evidence has shown that human γδTCR displayed cross-reactivity with CMV-infected cells and tumor cells,656–658 implying that previous infection history of the donors might partially affect the effectiveness of donor γδ T cells towards cancer patients. For Vδ2 T cells, however, the strategy may focus on examination of the expression level of tumor-derived phosphoantigens, which helps the physician decide what type of cancers or which individual patient is more suitable for this therapy. Notably, during the past few years, our group developed a strategy for examining the immune phenotypes of circulating immune cells based on flow cytometry assay, which can help analyze the function of each cell population. This approach enables us to perform donor-recipient matching. Altogether, further exploration of functional “biomarkers” can help develop personalized and precision treatment regimens to maximize the efficacy of γδ T-based cell therapy.
Vδ1+ vs Vδ2+: two branches of tumor immunotherapy
Another challenge is which γδ T subtype is more effective for tumor therapy. Careful evaluations of chemotaxis ability, durability, and tumor-cytotoxicity need to be established for both hematological and solid cancers to compare the clinical benefit and safety of Vδ1 and Vδ2 T cell subsets. For instance, although both subtypes share a suite of chemokine receptors on their surface, CCR5 is restricted to Vδ2 T cells, while CXCR1 is mainly expressed on Vδ1+ cells of circulating blood.188,190 In addition, tumor-infiltrating Vδ1+ cells highly express CXCR3.308 These findings suggest different tissue migratory patterns of Vδ1+ and Vδ2+ subsets when they receive inflammatory signals. Furthermore, dysregulated profiles of chemokine and chemokine receptor expression in γδ T cells can contribute to disease progression.659 Notably, adverse factors in the TME discussed above might “manipulate” γδ T cell migration patterns toward a pro-tumorigenic one. Since the chemokine landscape helps determine immune cell chemotactic migration and retention within the TME, which further shapes the pro- or anti-tumor responses in a spatiotemporal manner,660,661 a thorough understanding of γδ T cell chemokine receptor profiles and factors orchestrating γδ T cell chemotaxis, especially the tumor trafficking properties of both Vδ1+ and Vδ2+ subsets, might benefit the advancement of allogeneic γδ T cell-based cancer immunotherapy.
Clinical efficacy evaluation
Clinical efficacy evaluation in tumor cell therapy mainly involves the applications of common criteria, Response Evaluation Criteria in Solid Tumors (RECIST), including assessments of objective tumor response (tumor size, volume, or radiographic imaging) that is applied to classify responses as complete response, partial response, stable disease, or progressive disease, overall survival (OS), progression-free survival (PFS), quality of life (QoL), adverse events (AEs), and biomarker analysis. These parameters provide insights into treatment response, patient outcomes, safety, and the therapy’s impact on the patient’s well-being. By employing rigorous scientific methodologies, researchers and clinicians can make evidence-based decisions regarding the efficacy of tumor cell therapy in immunotherapy. However, comprehensive approaches are needed to assess the long-term persistence and functionality of γδ T cells in vivo, including their ability to establish durable memory responses and exhibit APC-like properties, which is crucial to understand their roles in shaping overall patient immunity in addition to tumor cell killing. Previously, we used immunophenotypes to assess the immune status of patients before and after allogeneic γδ T cell transfer, which can reveal significant perturbation in their immune profile (as shown in Fig. 7d). Utilizing advanced immunological techniques such as single-cell multi-omics spatiotemporal analyses and robust experimental models, researchers can gain deeper insights into the immunological mechanisms and therapeutic potential of γδ T cells, further paving the way for enhanced patient care and tailored immunotherapeutic strategies.
Cross-talk between γδ T cell and microbiota
The microbiota plays a crucial role in regulating T cell immunity.662 The dynamic interaction between commensal microbiota and T cells influences the maturation, differentiation, and effector function of T cells in various lymphoid tissues and organs.663–665 The microbiota provides essential signals and antigens for T cell activation and differentiation.666–669 Notably, specific bacterial species can induce the production of Tregs, contributing to immune tolerance and counteracting excessive inflammation.670 Microbiota-derived metabolites, such as SCFAs, play a role in promoting immune cell differentiation and function.667,671–675 Particularly during early life, the microbiota aids in the maturation of T cells and shapes their functional repertoire. Imbalances in gut microbiota composition, known as dysbiosis, are associated with alterations in T cell populations and functions, leading to immune dysregulation and increased susceptibility to diseases, including cancer, infections, and autoimmune disorders.676,677 Importantly, the interaction between the microbiota and T cells is reciprocal, with both components collaborating to establish a delicate balance critical for maintaining immune homeostasis. For instance, antibiotic (ABX) treatment or a low dietary fiber intake can induce alterations in the gut microbiota, which can contribute to cancer resistance to ICIs.678–686 The effectiveness of ICI immunotherapies is closely linked to the gut microbiome.686–689 Additionally, fecal microbiota transplantation (FMT) from responders has been shown to enhance the efficacy of anti-PD-1 therapy in cancer patients.690,691 Moreover, FMT from healthy donors could also be beneficial for patients with refractory ICI-induced colitis.692 Therefore, targeting the microbiota has emerged as a new and complementary treatment approach for cancer and autoimmune diseases.693–695 Recent studies have also indicated that the response and toxicity of CD19-CAR-T cell cancer immunotherapy are associated with the gut microbiome.696 Maintaining a non-antibiotic-disrupted gut microbiome is essential for the clinical efficacy of CD19-CAR-T cell cancer immunotherapy.697
Given the abundance of γδ T cells in peripheral tissues such as the skin, intestines, and lungs, which are also rich in commensal microbiota known to closely regulate γδ T cell functions,470,473,698 it is crucial to assess the impact of the commensal microbiota on the differentiation and effector functions of γδ T cells. This evaluation is essential for formulating effective therapeutic strategies.
Paths for further improving γδ T cell therapy efficacy
Long-term transfer
A more important concern is how to increase the clinical efficacy of allogeneic Vδ1 or Vδ2 T cell-based cancer immunotherapy, as well as how to re-energize γδ T cells or maintain their long-term persistence. In our study, we discovered a drastic loss of Vδ2 T donor population 2 weeks after cell transfer, implying apoptotic cell death and exhaustion of donor cells. Since only those cancer patients who received multiple infusions had a higher probability to have better life quality and to survive longer, we thus propose that applying adoptive transfer regularly over extended time periods, at least until the time point of complete tumor remission or normalization of serum tumor makers, might be required. Moreover, we anticipate that allogeneic Vδ2 T cells will be an optimal clinical medicine for postoperative immune reconstitution of cancer patients, because of their dual properties of combining potent cytotoxicity with the ability to present antigens.
Engineering modifications of γδ T cells
Although our published research indicates the promising clinical efficacy of allogeneic γδ T cells derived from healthy donors,11,12 it is important to acknowledge the challenges posed by the exhaustion of tumor-infiltrating γδ T cells.243 This phenomenon serves as a reminder that even infused allogeneic γδ T cells could experience functional depletion upon infiltrating the complex tumor microenvironment. In light of this, engineering modifications of γδ T cells present a compelling avenue to surmount this hurdle. These modifications offer an innovative strategy to create off-the-shelf products endowed with enhanced anti-tumor activity and prolonged survival within the tumor microenvironment.
In the current landscape, multiple approaches to engineering γδ T cells have emerged, each holding considerable potential. For instance, CAR-γδ T cells,25–27,699,700 leveraging chimeric antigen receptors to confer γδ T cells with the ability to be more specifically target tumor-associated antigens, are one of frontiers of engineering γδ T cells. The representative applications of CAR-γδ T cells in clinical are briefly summarized in Table 2. On a different front, the creation of Gene-Modified Chemotherapy-Resistant γδ T cells701–703 equips these cells with the resilience to withstand the cytotoxic effects of chemotherapy agents, rendering them more effective agents for combination therapies, and the related clinical trial is posted either (NCT05664243). Another noteworthy advancement is the development of γδ T Cell Bispecific Antibody Adapters.634,635,637,640,704,705 These adapters bridge TCRs of γδ T cells and surface antigens tumor cells,279 facilitating direct and potent interactions between the two cell types in patients. Alternative paths to engineering γδ T cells like transferring γδ TCRs to αβ T cells,706 γδ TCR-T Cells (genetic modifications of the TCR),707 are also proposed and under investigation either. As for Antibody-Coupled γδ T Cells, which is based on the newly emerged Antibody-Coupled T Cell Receptor technique by utilizing the power of antibody-antigen interactions to enhance the targeting precision of γδ T cells toward tumor cells, are currently not documented yet.
The clinical significance of these engineered modifications is substantial. They offer the potential to overcome the limitations posed by exhaustion within the tumor microenvironment, amplifying the therapeutic impact of γδ T cells in cancer treatment. Looking ahead, the future applications of engineered γδ T cells extend beyond cancer. The lessons learned from these strategies could pave the way for novel therapies in infectious diseases, autoimmune disorders, and more. As research in this field progresses, engineered γδ T cells hold the promise of revolutionizing the landscape of immunotherapy, ushering in a new era of targeted and potent treatments.
Allogeneic γδ T plus existing therapeutic regimens
An additional strategy to further elevate the clinical efficacy of allogeneic γδ T cells is to combine them with other cancer treatment strategies such as chemotherapies and metformin527,533,708,709 which may help to relieve the TME pressures on donor γδ T cells and enhance their efficacy and persistence in the long-term. According to our previous work,243 TME-challenged γδ T cells express higher levels of lymphocyte activation gene 3 (LAG3) rather than other types of immune checkpoint molecules, and we thus propose that combination of allogeneic γδ T cell plus anti-LAG3 mAb will further greatly enhance the efficacy. Given the fact that PDL1 is routinely upregulated in tumor cells, the triple combo medicine γδ T cell, anti-LAG3 mAb, and anti-PD1 or anti-PDL1 mAb should be a better choice. Furthermore, in the context of TME stress, infused γδ T cells would gradually lose their chemotactic capability and thus could not migrate toward the tumor site. In this respect, various formats of bispecific antibodies are in development which will initiate a new direction for γδ T cell application.704 Additionally, combinations with other treatments including radiotherapy, interventional therapy, agonistic anti-BTN3A mAb, bispecific antibodies, or intratumoral application of zoledronate also greatly expand horizons of clinical applications of allogeneic γδ T cells.335,633,636,710
Allogeneic γδ T plus FMT
The gut microbiota, which plays a critical role in shaping the immune system and influencing diverse physiological processes such as tumorigenesis,693,711–714 has been demonstrated to orchestrate with immune responses of γδ T cells.470 The understanding of immune remodulation of gut microbiota emphasizes the potential of FMT as a complementary treatment alongside γδ T cells in tumor therapy. By transferring gut microbiota from a healthy donor to the recipient’s gastrointestinal tract, FMT takes advantage of the microbiota’s ability to impact tumor development and response to therapy.679,693,712–714 Currently, the exact mechanisms underlying FMT’s effects in tumor therapy are not fully understood but likely involve the interplay between the gut microbiota, immune cells, and the tumor microenvironment. The gut microbiota has been implicated in regulating immune cell activation, differentiation, and function, including γδ T cells.470 Therefore, the incorporation of FMT as an adjunctive treatment strategy could provide a promising avenue for improving the outcomes of allogeneic γδ T cell-based tumor immunotherapy.
Detour the remaining technical roadblocks for γδ T cells
In the realm of functional research, the progress of functional research on γδ T cells has considerably lagged behind that of αβ T cells, primarily due to the absence of a specific gene conditional knockout mouse model for γδ T cells. This absence can be attributed to multiple factors, including the intricate and less understood nature of γδ T cell development within the thymus. Unlike αβ T cells, which have a well-defined developmental pathway and specific markers, γδ T cell development is characterized by its complexity and limited understandings. The process involves multiple subsets and distinct genetic programs influenced by TCR gene rearrangement, signaling networks, and interactions with thymic stromal cells, as discussed above.
The lack of a definitive marker or transcription factor91,95,141,145 exclusive to γδ T cells poses challenges in designing gene conditional knockout models specific to this subset. Furthermore, γδ T cells represent a smaller population within the thymus compared to αβ T cells, complicating the generation of targeted knockout models. Their lower abundance and absence of unique markers or genes pose difficulties in selectively targeting and manipulating their development using current conditional knockout strategies. Furthermore, the intricate interaction between γδ T cells and the thymic microenvironment adds another layer of complexity to the situation. Thymic stromal cells play a crucial role in supporting γδ T cell development and maturation through various signaling pathways and interactions. Disrupting a specific gene in thymic stromal cells may have unintended consequences on multiple T cell subsets, including αβ T cells, making it challenging to achieve selective knockout of γδ T cells.
Despite the challenges involved, ongoing efforts are being made to overcome the existing technical obstacles and establish targeted gene knockout mouse models for γδ T cells. The Kamiya group recently reported a novel approach for generating specific gene knockout mice in γδ T cells, introducing a detour paradigm.90 Their strategy involves the creation of mice with targeted gene deficiencies, followed by the isolation of γδ T cells from these mice and subsequent adoptive transfer into TCRγ-KO mice. This approach enables the development of specific gene knockout mouse models to study the function of a particular gene in γδ T cells. While this method has its limitations, it provides researchers with a valuable tool to explore the role of specific genes in the context of γδ T cell biology. Meanwhile, the ongoing progress in the development of humanized mouse models offers possibilities for in-depth investigating γδ T cells as well. This entails the utilization of appropriate murine models to investigate human γδ T cells, for instance the human TCR transgenic mice. Complementing this approach, it may be advantageous to incorporate transgenic expression of human BTN molecules. These combined efforts are poised to provide valuable insights into the complex biology of γδ T cells in a context closely mirroring human immunology.
Closing remarks
The field of immunotherapy is continuously advancing, and among the emerging therapeutic strategies, allogeneic γδ T cells transfer have gained significant attention as a promising avenue for future immunotherapies. The unique properties of γδ T cells, such as their potent cytotoxicity, ability to recognize a broad range of antigens in MHC-independent manner, and potential for immunomodulation, make them attractive candidates for combating various diseases. To fully harness the therapeutic potential of γδ T cells, a deeper understanding of their underlying molecular mechanisms is essential. One area of research that warrants further exploration is thymus development, which plays a crucial role in shaping the repertoire and functional diversity of γδ T cells. Investigating the intricate processes involved in γδ T cell maturation and selection within the thymus will shed light on their ontogeny and help unravel the complex interplay between different subsets of γδ T cells.
Another aspect that requires closer examination is the plasticity of effector functions in γδ T cells, particularly in the context of disease microenvironment. γδ T cells possess the ability to exhibit diverse effector phenotypes, including cytotoxicity, cytokine production, and immunoregulatory functions. Understanding the factors that govern the plasticity of γδ T cell effector functions and the molecular cues that drive their differentiation into specific functional subsets will be crucial for optimizing their therapeutic applications.
In the realm of clinical trials, the integration of γδ T cell-based immunotherapy as adjuvant applications holds great promise. Combining γδ T cell therapy with existing treatment regimens, such as chemotherapy or checkpoint blockade, has the potential to synergistically enhance anti-tumor responses and improve patient outcomes. Additionally, exploring innovative strategies like FMT, which can modulate the gut microbiome and influence γδ T cell functionality, may further enhance the therapeutic efficacy of γδ T cell-based immunotherapies.
In conclusion, the evolving landscape of immunotherapy highlights allogeneic γδ T cell transfer as a promising avenue for future treatments. Leveraging γδ T cells’ unique attributes, such as their versatile antigen recognition and immunomodulatory potential, presents exciting therapeutic possibilities. To unlock their full potential, a deeper comprehension of γδ T cell development and plasticity is imperative. Investigating thymus-driven maturation and understanding effector function plasticity within disease contexts will guide their optimal use. Integrating γδ T cell therapy into clinical approaches, including synergistic combinations with existing treatments and innovative strategies like microbiome modulation, holds great potential. This ongoing scientific exploration promises personalized and effective immunotherapies. By unraveling the intricacies of γδ T cell biology, interactions with microenvironments, and their therapeutic applications, we are poised to revolutionize precision medicine and fully harness γδ T cells’ therapeutic prowess.
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (82002787, Y.H.; 32270950, Y.Z.W.), the Key Program of the National Natural Science Foundation of China (32030036, Z.N.Y.), and the Startup Foundation of the Zhuhai People’s Hospital (YNXM20210305, Y.Z.W.). Y.S.L. is supported by the Major International (Regional) Joint Research Program of the National Natural Science Foundation of China (81920108027). D.K. is supported by Deutsche Forschungsgemeinschaft (Ka 502/19-3).
Author contributions
Y.H. and Y.Z.W. concepted, drafted and revised the review, and prepared figures. D.K. critically review and revised the article. Y.S.L., X.Z., Z.N.Y. and L.G.L. provided suggestions or resources. Q.L.H. compiled literatures and prepared tables. All authors have read and approved the article.
Competing interests
D.K. is a member of the Scientific Advisory Boards of ImCheck Therapeutics, Lava Therapeutics, In8Bio, PhosphoGam. The other authors declare no conflict of interest on this work.
Footnotes
These authors contributed equally: Yi Hu, Qinglin Hu
Contributor Information
Zhinan Yin, Email: tzhinan@jnu.edu.cn.
Dieter Kabelitz, Email: dietrich.kabelitz@uksh.de.
Yangzhe Wu, Email: tyzwu@jnu.edu.cn.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403. doi: 10.1016/j.ccell.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 3.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021;21:785–797. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almagro J, et al. Tissue architecture in tumor initiation and progression. Trends Cancer. 2022;8:494–505. doi: 10.1016/j.trecan.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Papotto PH, Ribot JC, Silva-Santos B. IL-17(+) gammadelta T cells as kick-starters of inflammation. Nat. Immunol. 2017;18:604–611. doi: 10.1038/ni.3726. [DOI] [PubMed] [Google Scholar]
- 8.Silva-Santos B, Mensurado S, Coffelt S. B. gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer. 2019;19:392–404. doi: 10.1038/s41568-019-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mensurado S, Blanco-Dominguez R, Silva-Santos B. The emerging roles of gammadelta T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 2023;20:178–191. doi: 10.1038/s41571-022-00722-1. [DOI] [PubMed] [Google Scholar]
- 10.Ribot JC, Lopes N, Silva-Santos B. gammadelta T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2021;21:221–232. doi: 10.1038/s41577-020-00452-4. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol. Immunol. 2021;18:427–439. doi: 10.1038/s41423-020-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alnaggar M, et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J. Immunother. Cancer. 2019;7:36. doi: 10.1186/s40425-019-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang J, et al. Allogeneic Vgamma9Vdelta2 T-cell therapy promotes pulmonary lesion repair: an open-label, single-arm pilot study in patients with multidrug-resistant tuberculosis. Front. Immunol. 2021;12:756495. doi: 10.3389/fimmu.2021.756495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebestyen Z, et al. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 2020;19:169–184. doi: 10.1038/s41573-019-0038-z. [DOI] [PubMed] [Google Scholar]
- 15.Deseke M, Prinz I. Ligand recognition by the γδ TCR and discrimination between homeostasis and stress conditions. Cell Mol. Immunol. 2020;17:914–924. doi: 10.1038/s41423-020-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat. Rev. Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, et al. T cells in health and disease. Sig. Transduct. Target Ther. 2023;8:235. doi: 10.1038/s41392-023-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu G, et al. Comparing mouse and human tissue-resident gammadelta T cells. Front. Immunol. 2022;13:891687. doi: 10.3389/fimmu.2022.891687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papotto PH, Reinhardt A, Prinz I, Silva-Santos B. Innately versatile: gammadelta17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 2018;87:26–37. doi: 10.1016/j.jaut.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter DL, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.June CH, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 24.Ellebrecht CT, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirzaei HR, et al. Prospects for chimeric antigen receptor (CAR) gammadelta T cells: a potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 2016;380:413–423. doi: 10.1016/j.canlet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wawrzyniecka PA, et al. Chimeric antigen receptor T cells for gamma-delta T cell malignancies. Leukemia. 2022;36:577–579. doi: 10.1038/s41375-021-01385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makkouk A, et al. Allogeneic Vδ1 gamma delta T cells engineered with glypican-3 (GPC3)-specific CAR expressing soluble IL-15 have enhanced antitumor efficacy against hepatocellular carcinoma in preclinical models. J. Clin. Oncol. 2021;39:e14511–e14511. doi: 10.1200/JCO.2021.39.15_suppl.e14511. [DOI] [Google Scholar]
- 28.Saito H, et al. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature. 1984;309:757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- 29.Hayday AC, et al. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985;40:259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- 30.Lefranc M-P, Rabbitts T. Two tandemly organized human genes encoding the T-cell γ constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985;316:464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- 31.Murre C, et al. Human γ-chain genes are rearranged in leukaemic T cells and map to the short arm of chromosome 7. Nature. 1985;316:549–552. doi: 10.1038/316549a0. [DOI] [PubMed] [Google Scholar]
- 32.Bank I, et al. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986;322:179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- 33.Brenner MB, et al. Identification of a putative second T-cell receptor. Nature. 1986;322:145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- 34.Borst J, et al. A T-cell receptor γ/CD3 complex found on cloned functional lymphocytes. Nature. 1987;325:683–688. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- 35.Born W, et al. Recognition of a peptide antigen by heat shock-reactive γδ T lymphocytes. Science. 1990;249:67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien RL, et al. Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 37.Holoshttz J, et al. Isolation of CD4-CD8-mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989;339:226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- 38.Janis EM, Kaufmann SH, Schwartz RH, Pardoll DM. Activation of γδ T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989;244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 39.Modlin RL, et al. Lymphocytes bearing antigen-specific γδ T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 40.Augustin A, Kubo RT, Sim G-K. Resident pulmonary lymphocytes expressing the γ/δ T-cell receptor. Nature. 1989;340:239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 41.Haregewoin A, Soman G, Horn RC, Finberg RW. Human γδ + T cells respond to mycobacterial heat-shock protein. Nature. 1989;340:309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- 42.Autran B, et al. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clin. Exp. Immunol. 1989;75:206. [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrick DA, et al. Differential production of interferon-γ and interleukin-4 in response to Th1-and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 44.Morita CT, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 45.De Paoli P, et al. A subset of γδ lymphocytes is increased during HIV‐1 infection. Clin. Exp. Immunol. 1991;83:187–191. doi: 10.1111/j.1365-2249.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Maria A, et al. Selective increase of a subset of T cell receptor γδ T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J. Infect. Dis. 1992;165:917–919. doi: 10.1093/infdis/165.5.917. [DOI] [PubMed] [Google Scholar]
- 47.Boullier S, Cochet M, Poccia F, Gougeon M-L. CDR3-independent gamma delta V delta 1 + T cell expansion in the peripheral blood of HIV-infected persons. J. Immunol. 1995;154:1418–1431. doi: 10.4049/jimmunol.154.3.1418. [DOI] [PubMed] [Google Scholar]
- 48.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 49.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 γδ T cells that prevent murine insulin-dependent diabetes. J. Exp. Med. 1996;184:2167–2174. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuany-Amorim C, et al. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 51.Fournié JJ, Bonneville M. Stimulation of γδ T cells by phosphoantigens. Res. Immunol. 1996;147:338–347. doi: 10.1016/0923-2494(96)89648-9. [DOI] [PubMed] [Google Scholar]
- 52.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. 1998;161:1045–1054. doi: 10.4049/jimmunol.161.2.1045. [DOI] [PubMed] [Google Scholar]
- 53.Girardi M, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 54.Wilhelm M, et al. T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 55.Rischer M, et al. Human γδ T cells as mediators of chimaeric‐receptor redirected anti‐tumour immunity. Br. J. Haematol. 2004;126:583–592. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 56.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 57.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 58.Umemura M, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 59.Shibata K, et al. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 60.Peng M, et al. Interleukin 17-producing γδ T cells increased in patients with active pulmonary tuberculosis. Cell Mol. Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenoglio D, et al. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113:6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- 62.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-γ-and interleukin 17–producing γδ T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas JD, et al. CCR6 and NK1. 1 distinguish between IL‐17 A and IFN‐γ‐producing γδ effector T cells. Eur. J. Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 65.Shichita T, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 66.Martin B, et al. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 67.Cho JS, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petermann F, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pantelyushin S, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J. Immunol. 2011;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai Y, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J. Immunol. 2011;187:5026–5031. doi: 10.4049/jimmunol.1101817. [DOI] [PubMed] [Google Scholar]
- 73.Becher B, Pantelyushin S. Hiding under the skin: Interleukin-17–producing γδ T cells go under the skin? Nat. Med. 2012;18:1748–1750. doi: 10.1038/nm.3016. [DOI] [PubMed] [Google Scholar]
- 74.Krueger JG. Hiding under the skin: a welcome surprise in psoriasis. Nat. Med. 2012;18:1750–1751. doi: 10.1038/nm.3025. [DOI] [PubMed] [Google Scholar]
- 75.Gray EE, et al. Deficiency in IL-17-committed Vγ4 + γδ T cells in a spontaneous Sox13-mutant CD45. 1+ congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 2013;14:584–592. doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rei M, et al. Murine CD27(-) Vgamma6(+) gammadelta T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl Acad. Sci. USA. 2014;111:E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wakita D, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010;40:1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 78.Carmi Y, et al. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J. Immunol. 2011;186:3462–3471. doi: 10.4049/jimmunol.1002901. [DOI] [PubMed] [Google Scholar]
- 79.Ma S, et al. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- 80.Wu P, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caccamo N, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 82.Harly C, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheridan BS, et al. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy AG, et al. Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection. J. Immunol. 2014;192:3697–3708. doi: 10.4049/jimmunol.1303420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soriano-Sarabia N, et al. Peripheral Vgamma9Vdelta2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015;11:e1005201. doi: 10.1371/journal.ppat.1005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gentles AJ, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rijkers G, Vervenne T, van der Pol P. More bricks in the wall against SARS-CoV-2 infection: involvement of gamma9delta2 T cells. Cell Mol. Immunol. 2020;17:771–772. doi: 10.1038/s41423-020-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carissimo G, et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat. Commun. 2020;11:5243. doi: 10.1038/s41467-020-19080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jouan Y, et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J. Exp. Med. 2020;217:1–9. doi: 10.1084/jem.20200872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu X, et al. Dectin-1 signaling on colonic gammadelta T cells promotes psychosocial stress responses. Nat. Immunol. 2023;24:625–636. doi: 10.1038/s41590-023-01447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hosokawa H, Rothenberg EV. How transcription factors drive choice of the T cell fate. Nat. Rev. Immunol. 2021;21:162–176. doi: 10.1038/s41577-020-00426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumaria N, Martin S, Pennington DJ. Developmental origins of murine gammadelta T-cell subsets. Immunology. 2019;156:299–304. doi: 10.1111/imm.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rothenberg EV. Single-cell insights into the hematopoietic generation of T-lymphocyte precursors in mouse and human. Exp. Hematol. 2021;95:1–12. doi: 10.1016/j.exphem.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol. Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 95.Boehme L, Roels J, Taghon T. Development of gammadelta T cells in the thymus - a human perspective. Semin Immunol. 2022;61:101662. doi: 10.1016/j.smim.2022.101662. [DOI] [PubMed] [Google Scholar]
- 96.Munoz-Ruiz M, Sumaria N, Pennington DJ, Silva-Santos B. Thymic determinants of gammadelta T cell differentiation. Trends Immunol. 2017;38:336–344. doi: 10.1016/j.it.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Shah DK, Zuniga-Pflucker JC. An overview of the intrathymic intricacies of T cell development. J. Immunol. 2014;192:4017–4023. doi: 10.4049/jimmunol.1302259. [DOI] [PubMed] [Google Scholar]
- 98.Pellicci DG, Koay HF, Berzins SP. Thymic development of unconventional T cells: how NKT cells, MAIT cells and gammadelta T cells emerge. Nat. Rev. Immunol. 2020;20:756–770. doi: 10.1038/s41577-020-0345-y. [DOI] [PubMed] [Google Scholar]
- 99.McVay LD, Carding SR. Generation of human gammadelta T-cell repertoires. Crit. Rev. Immunol. 1999;19:431–460. [PubMed] [Google Scholar]
- 100.Carding SR, et al. Developmentally regulated fetal thymic and extrathymic T-cell receptor gamma delta gene expression. Genes Dev. 1990;4:1304–1315. doi: 10.1101/gad.4.8.1304. [DOI] [PubMed] [Google Scholar]
- 101.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin. Immunol. 2006;121:251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Washburn T, et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/S0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 103.Van Coppernolle S, et al. Notch induces human T-cell receptor gammadelta+ thymocytes to differentiate along a parallel, highly proliferative and bipotent CD4 CD8 double-positive pathway. Leukemia. 2012;26:127–138. doi: 10.1038/leu.2011.324. [DOI] [PubMed] [Google Scholar]
- 104.Garcia-Peydro M, de Yebenes VG, Toribio ML. Notch1 and IL-7 receptor interplay maintains proliferation of human thymic progenitors while suppressing non-T cell fates. J. Immunol. 2006;177:3711–3720. doi: 10.4049/jimmunol.177.6.3711. [DOI] [PubMed] [Google Scholar]
- 105.Haks MC, et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Zarin P, et al. Enforcement of gammadelta-lineage commitment by the pre-T-cell receptor in precursors with weak gammadelta-TCR signals. Proc. Natl Acad. Sci. USA. 2014;111:5658–5663. doi: 10.1073/pnas.1312872111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayes SM, Li L, Love PE. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 108.Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nat. Immunol. 2007;8:137–144. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- 109.Garcia-Peydro M, de Yebenes VG, Toribio ML. Sustained Notch1 signaling instructs the earliest human intrathymic precursors to adopt a gammadelta T-cell fate in fetal thymus organ culture. Blood. 2003;102:2444–2451. doi: 10.1182/blood-2002-10-3261. [DOI] [PubMed] [Google Scholar]
- 110.Van de Walle I, et al. Specific Notch receptor-ligand interactions control human TCR-alphabeta/gammadelta development by inducing differential Notch signal strength. J. Exp. Med. 2013;210:683–697. doi: 10.1084/jem.20121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Van de Walle I, et al. An early decrease in Notch activation is required for human TCR-alphabeta lineage differentiation at the expense of TCR-gammadelta T cells. Blood. 2009;113:2988–2998. doi: 10.1182/blood-2008-06-164871. [DOI] [PubMed] [Google Scholar]
- 112.Buus TB, Odum N, Geisler C, Lauritsen JPH. Three distinct developmental pathways for adaptive and two IFN-gamma-producing gammadelta T subsets in adult thymus. Nat. Commun. 2017;8:1911. doi: 10.1038/s41467-017-01963-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fiala GJ, Gomes AQ, Silva-Santos B. From thymus to periphery: molecular basis of effector gammadelta-T cell differentiation. Immunol. Rev. 2020;298:47–60. doi: 10.1111/imr.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gogoi D, Dar AA, Chiplunkar SV. Involvement of Notch in activation and effector functions of gammadelta T cells. J. Immunol. 2014;192:2054–2062. doi: 10.4049/jimmunol.1300369. [DOI] [PubMed] [Google Scholar]
- 115.Di Lorenzo B, Ravens S, Silva-Santos B. High-throughput analysis of the human thymic Vdelta1(+) T cell receptor repertoire. Sci. Data. 2019;6:115. doi: 10.1038/s41597-019-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McVay LD, et al. The generation of human gammadelta T cell repertoires during fetal development. J. Immunol. 1998;160:5851–5860. doi: 10.4049/jimmunol.160.12.5851. [DOI] [PubMed] [Google Scholar]
- 117.Ciofani M, et al. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 118.Ciofani M, Zuniga-Pflucker JC. Determining gammadelta versus alphass T cell development. Nat. Rev. Immunol. 2010;10:657–663. doi: 10.1038/nri2820. [DOI] [PubMed] [Google Scholar]
- 119.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J. Exp. Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prinz I, et al. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat. Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 121.Weerkamp F, et al. Human thymus contains multipotent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood. 2006;107:3131–3137. doi: 10.1182/blood-2005-08-3412. [DOI] [PubMed] [Google Scholar]
- 122.Ktorza S, et al. CD34-positive early human thymocytes: T cell receptor and cytokine receptor gene expression. Eur. J. Immunol. 1995;25:2471–2478. doi: 10.1002/eji.1830250910. [DOI] [PubMed] [Google Scholar]
- 123.Krangel MS, Yssel H, Brocklehurst C, Spits H. A distinct wave of human T cell receptor gamma/delta lymphocytes in the early fetal thymus: evidence for controlled gene rearrangement and cytokine production. J. Exp. Med. 1990;172:847–859. doi: 10.1084/jem.172.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tieppo P, et al. The human fetal thymus generates invariant effector gammadelta T cells. J. Exp. Med. 2020;217:e20190834. doi: 10.1084/jem.20190580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papadopoulou M, et al. TCR sequencing reveals the distinct development of fetal and adult human Vgamma9Vdelta2 T cells. J. Immunol. 2019;203:1468–1479. doi: 10.4049/jimmunol.1900592. [DOI] [PubMed] [Google Scholar]
- 126.Elliott JF, et al. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988;331:627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- 127.Casorati G, De Libero G, Lanzavecchia A, Migone N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J. Exp. Med. 1989;170:1521–1535. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davey MS, et al. The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(−) subsets. Nat. Commun. 2018;9:1760. doi: 10.1038/s41467-018-04076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fisch P, et al. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 130.Chen H, et al. Profiling the pattern of the human T-cell receptor gammadelta complementary determinant region 3 repertoire in patients with lung carcinoma via high-throughput sequencing analysis. Cell Mol. Immunol. 2019;16:250–259. doi: 10.1038/cmi.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y, et al. The role of short homology repeats and TdT in generation of the invariant gamma delta antigen receptor repertoire in the fetal thymus. Immunity. 1995;3:439–447. doi: 10.1016/1074-7613(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 132.Kallemeijn MJ, et al. Next-generation sequencing analysis of the human TCRgammadelta+ T-cell repertoire reveals shifts in Vgamma- and Vdelta-usage in memory populations upon aging. Front. Immunol. 2018;9:448. doi: 10.3389/fimmu.2018.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Couedel C, et al. Allelic exclusion at the TCR delta locus and commitment to gamma delta lineage: different modalities apply to distinct human gamma delta subsets. J. Immunol. 2004;172:5544–5552. doi: 10.4049/jimmunol.172.9.5544. [DOI] [PubMed] [Google Scholar]
- 134.Asnafi V, et al. Analysis of TCR, pT alpha, and RAG-1 in T-acute lymphoblastic leukemias improves understanding of early human T-lymphoid lineage commitment. Blood. 2003;101:2693–2703. doi: 10.1182/blood-2002-08-2438. [DOI] [PubMed] [Google Scholar]
- 135.Davodeau F, et al. Surface expression of two distinct functional antigen receptors on human gamma delta T cells. Science. 1993;260:1800–1802. doi: 10.1126/science.8390096. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Garcia S, et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7Ralpha gene expression in early human thymopoiesis and leukemia. J. Exp. Med. 2009;206:779–791. doi: 10.1084/jem.20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pallard C, et al. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–535. doi: 10.1016/S1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 138.Ye SK, et al. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 2001;15:813–823. doi: 10.1016/S1074-7613(01)00230-8. [DOI] [PubMed] [Google Scholar]
- 139.Ye SK, et al. Induction of germline transcription in the TCRgamma locus by Stat5: implications for accessibility control by the IL-7 receptor. Immunity. 1999;11:213–223. doi: 10.1016/S1074-7613(00)80096-5. [DOI] [PubMed] [Google Scholar]
- 140.Wagatsuma K, et al. STAT5 orchestrates local epigenetic changes for chromatin accessibility and rearrangements by direct binding to the TCRgamma locus. J. Immunol. 2015;195:1804–1814. doi: 10.4049/jimmunol.1302456. [DOI] [PubMed] [Google Scholar]
- 141.Roels J, et al. Transcriptional dynamics and epigenetic regulation of E and ID protein encoding genes during human T cell development. Front. Immunol. 2022;13:960918. doi: 10.3389/fimmu.2022.960918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghosh JK, Romanow WJ, Murre C. Induction of a diverse T cell receptor gamma/delta repertoire by the helix-loop-helix proteins E2A and HEB in nonlymphoid cells. J. Exp. Med. 2001;193:769–776. doi: 10.1084/jem.193.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Langerak AW, et al. Basic helix-loop-helix proteins E2A and HEB induce immature T-cell receptor rearrangements in nonlymphoid cells. Blood. 2001;98:2456–2465. doi: 10.1182/blood.V98.8.2456. [DOI] [PubMed] [Google Scholar]
- 144.Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez-Caparros A, et al. Notch signaling controls transcription via the recruitment of RUNX1 and MYB to enhancers during T cell development. J. Immunol. 2019;202:2460–2472. doi: 10.4049/jimmunol.1801650. [DOI] [PubMed] [Google Scholar]
- 146.Van de Walle I, et al. GATA3 induces human T-cell commitment by restraining Notch activity and repressing NK-cell fate. Nat. Commun. 2016;7:11171. doi: 10.1038/ncomms11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Garbe AI, et al. Differential synergy of Notch and T cell receptor signaling determines alphabeta versus gammadelta lineage fate. J. Exp. Med. 2006;203:1579–1590. doi: 10.1084/jem.20060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Smedt M, et al. Different thresholds of Notch signaling bias human precursor cells toward B-, NK-, monocytic/dendritic-, or T-cell lineage in thymus microenvironment. Blood. 2005;106:3498–3506. doi: 10.1182/blood-2005-02-0496. [DOI] [PubMed] [Google Scholar]
- 149.Ha VL, et al. The T-ALL related gene BCL11B regulates the initial stages of human T-cell differentiation. Leukemia. 2017;31:2503–2514. doi: 10.1038/leu.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cante-Barrett K, et al. Loss of CD44(dim) expression from early progenitor cells marks T-cell lineage commitment in the human thymus. Front. Immunol. 2017;8:32. doi: 10.3389/fimmu.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B( + )NK(+) severe combined immunodeficiency. Nat. Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 152.Hinz T, et al. Cell-surface expression of transrearranged Vgamma-cbeta T-cell receptor chains in healthy donors and in ataxia telangiectasia patients. Br. J. Haematol. 2000;109:201–210. doi: 10.1046/j.1365-2141.2000.01962.x. [DOI] [PubMed] [Google Scholar]
- 153.Davodeau F, et al. Surface expression of functional T cell receptor chains formed by interlocus recombination on human T lymphocytes. J. Exp. Med. 1994;180:1685–1691. doi: 10.1084/jem.180.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Edwards SC, et al. A population of proinflammatory T cells coexpresses alphabeta and gammadelta T cell receptors in mice and humans. J. Exp. Med. 2020;217:e20190834. doi: 10.1084/jem.20190834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zeng Y, et al. Single-cell RNA sequencing resolves spatiotemporal development of pre-thymic lymphoid progenitors and thymus organogenesis in human embryos. Immunity. 2019;51:930–948.e936. doi: 10.1016/j.immuni.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 156.Sanchez Sanchez G, et al. Identification of distinct functional thymic programming of fetal and pediatric human gammadelta thymocytes via single-cell analysis. Nat. Commun. 2022;13:5842. doi: 10.1038/s41467-022-33488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perriman L, et al. A three-stage developmental pathway for human Vγ9Vδ2 T cells within the postnatal thymus. Sci. Immunol. 2023;8:eabo4365. doi: 10.1126/sciimmunol.abo4365. [DOI] [PubMed] [Google Scholar]
- 158.Shah K, Al-Haidari A, Sun J, Kazi JU. T cell receptor (TCR) signaling in health and disease. Sig. Transduct. Target Ther. 2021;6:412. doi: 10.1038/s41392-021-00823-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Spidale NA, et al. Interleukin-17-producing gammadelta T cells originate from SOX13(+) progenitors that are independent of gammadeltaTCR signaling. Immunity. 2018;49:857–872.e5. doi: 10.1016/j.immuni.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Melichar HJ, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 162.Munoz-Ruiz M, et al. TCR signal strength controls thymic differentiation of discrete proinflammatory gammadelta T cell subsets. Nat. Immunol. 2016;17:721–727. doi: 10.1038/ni.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bain G, et al. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat. Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 164.Lee SY, et al. Noncanonical mode of ERK action controls alternative alphabeta and gammadelta T cell lineage fates. Immunity. 2014;41:934–946. doi: 10.1016/j.immuni.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Murphy LO, et al. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 166.Yukawa M, et al. AP-1 activity induced by co-stimulation is required for chromatin opening during T cell activation. J. Exp. Med. 2020;217:e20182009. doi: 10.1084/jem.20182009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roels J, et al. Distinct and temporary-restricted epigenetic mechanisms regulate human alphabeta and gammadelta T cell development. Nat. Immunol. 2020;21:1280–1292. doi: 10.1038/s41590-020-0747-9. [DOI] [PubMed] [Google Scholar]
- 168.Rivera RR, et al. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/S1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 169.Sagar, et al. Deciphering the regulatory landscape of fetal and adult gammadelta T-cell development at single-cell resolution. EMBO J. 2020;39:e104159. doi: 10.15252/embj.2019104159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pennington DJ, et al. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat. Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 171.Schmolka N, Wencker M, Hayday AC, Silva-Santos B. Epigenetic and transcriptional regulation of gammadelta T cell differentiation: programming cells for responses in time and space. Semin Immunol. 2015;27:19–25. doi: 10.1016/j.smim.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 172.Kagnoff MF. Current concepts in mucosal immunity. III. Ontogeny and function of gamma delta T cells in the intestine. Am. J. Physiol. 1998;274:G455–G458. doi: 10.1152/ajpgi.1998.274.3.G455. [DOI] [PubMed] [Google Scholar]
- 173.Nielsen MM, Witherden DA, Havran W. L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jin Y, et al. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J. Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu W, et al. Skin gammadelta T cells and their function in wound healing. Front. Immunol. 2022;13:875076. doi: 10.3389/fimmu.2022.875076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Frascoli M, et al. Skin gammadelta T cell inflammatory responses are hardwired in the thymus by oxysterol sensing via GPR183 and calibrated by dietary cholesterol. Immunity. 2023;56:562–575.e566. doi: 10.1016/j.immuni.2023.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marshall AS, et al. Skin-Resident gammadelta T cells exhibit site-specific morphology and activation states. J. Immunol. Res. 2019;2019:9020234. doi: 10.1155/2019/9020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu Y, et al. Epidermal resident gammadelta T cell development and function in skin. Cell. Mol. Life Sci. CMLS. 2021;78:573–580. doi: 10.1007/s00018-020-03613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Castillo-Gonzalez R, Cibrian D, Sanchez-Madrid F. Dissecting the complexity of gammadelta T-cell subsets in skin homeostasis, inflammation, and malignancy. J. Allergy Clin. Immunol. 2021;147:2030–2042. doi: 10.1016/j.jaci.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 180.Cruz MS, Diamond A, Russell A, Jameson JM. Human alphabeta and gammadelta T cells in skin immunity and disease. Front. Immunol. 2018;9:1304. doi: 10.3389/fimmu.2018.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fischer MA, Golovchenko NB, Edelblum K. L. gammadelta T cell migration: separating trafficking from surveillance behaviors at barrier surfaces. Immunol. Rev. 2020;298:165–180. doi: 10.1111/imr.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McCarthy NE, Eberl M. Human gammadelta T-cell control of mucosal immunity and inflammation. Front. Immunol. 2018;9:985. doi: 10.3389/fimmu.2018.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu. Rev. Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 184.Rampoldi F, Prinz I. Three layers of intestinal gammadelta T cells talk different languages with the microbiota. Front. Immunol. 2022;13:849954. doi: 10.3389/fimmu.2022.849954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Johnson MD, Witherden DA, Havran WL. The role of tissue-resident T cells in stress surveillance and tissue maintenance. Cells. 2020;9:686. doi: 10.3390/cells9030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Uehara S, Song K, Farber JM, Love PE. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3(high)CD69+ thymocytes and gammadeltaTCR+ thymocytes preferentially respond to CCL25. J. Immunol. 2002;168:134–142. doi: 10.4049/jimmunol.168.1.134. [DOI] [PubMed] [Google Scholar]
- 187.Poggi A, et al. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood. 2004;103:2205–2213. doi: 10.1182/blood-2003-08-2928. [DOI] [PubMed] [Google Scholar]
- 188.Glatzel A, et al. Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J. Immunol. 2002;168:4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- 189.Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kabelitz D, Wesch D. Features and functions of gamma delta T lymphocytes: focus on chemokines and their receptors. Crit. Rev. Immunol. 2003;23:339–370. doi: 10.1615/CritRevImmunol.v23.i56.10. [DOI] [PubMed] [Google Scholar]
- 191.Lança T, et al. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J. Immunol. 2013;190:6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 192.McVay LD, Carding SR. Extrathymic origin of human gamma delta T cells during fetal development. J. Immunol. 1996;157:2873–2882. doi: 10.4049/jimmunol.157.7.2873. [DOI] [PubMed] [Google Scholar]
- 193.Dimova T, et al. Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc. Natl Acad. Sci. USA. 2015;112:E556–E565. doi: 10.1073/pnas.1412058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Willcox CR, Davey MS, Willcox BE. Development and selection of the human Vgamma9Vdelta2(+) T-cell repertoire. Front. Immunol. 2018;9:1501. doi: 10.3389/fimmu.2018.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu W, Lau ZWX, Fulop T, Larbi A. The aging of gammadelta T cells. Cells. 2020;9:1181. doi: 10.3390/cells9051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J. Immunol. 1994;153:3979–3988. doi: 10.4049/jimmunol.153.9.3979. [DOI] [PubMed] [Google Scholar]
- 197.Ribot JC, et al. Human gammadelta thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 2014;192:2237–2243. doi: 10.4049/jimmunol.1303119. [DOI] [PubMed] [Google Scholar]
- 198.Parker CM, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J. Exp. Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019;19:573–583. doi: 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat. Immunol. 2021;22:687–698. doi: 10.1038/s41590-021-00927-z. [DOI] [PubMed] [Google Scholar]
- 201.Tserel L, et al. Age-related profiling of DNA methylation in CD8 + T cells reveals changes in immune response and transcriptional regulator genes. Sci. Rep. 2015;5:13107. doi: 10.1038/srep13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Urban LA, et al. The impact of age-related hypomethylated DNA on immune signaling upon cellular demise. Trends Immunol. 2021;42:464–468. doi: 10.1016/j.it.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao J, et al. DNA methylation of T lymphocytes as a therapeutic target: implications for rheumatoid arthritis etiology. Front. Immunol. 2022;13:863703. doi: 10.3389/fimmu.2022.863703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang S, et al. MicroRNA profile of circulating CD4 + T cells in aged patients with atherosclerosis obliterans. BMC Cardiovasc. Disord. 2022;22:172. doi: 10.1186/s12872-022-02616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim C, Ye Z, Weyand CM, Goronzy J. J. miR-181a-regulated pathways in T-cell differentiation and aging. Immun. Ageing. 2021;18:28. doi: 10.1186/s12979-021-00240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Taheri M, et al. Emerging role of non-coding RNAs in regulation of T-lymphocyte function. Front. Immunol. 2021;12:756042. doi: 10.3389/fimmu.2021.756042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wells AC, Pobezinskaya EL, Pobezinsky LA. Non-coding RNAs in CD8 T cell biology. Mol. Immunol. 2020;120:67–73. doi: 10.1016/j.molimm.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Argentati K, et al. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J. Leukoc. Biol. 2002;72:65–71. doi: 10.1189/jlb.72.1.65. [DOI] [PubMed] [Google Scholar]
- 209.Jia Z, et al. Immune-ageing evaluation of peripheral T and NK lymphocyte subsets in Chinese healthy adults. Phenomics. 2023;3:360–374. doi: 10.1007/s43657-023-00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lopez-Otin C, et al. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 211.Ullrich R, et al. gamma delta T cells in the human intestine express surface markers of activation and are preferentially located in the epithelium. Cell Immunol. 1990;128:619–627. doi: 10.1016/0008-8749(90)90053-T. [DOI] [PubMed] [Google Scholar]
- 212.Toulon A, et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bos JD, et al. T-cell receptor gamma delta bearing cells in normal human skin. J. Invest. Dermatol. 1990;94:37–42. doi: 10.1111/1523-1747.ep12873333. [DOI] [PubMed] [Google Scholar]
- 214.Ravens S, et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2017;18:393–401. doi: 10.1038/ni.3686. [DOI] [PubMed] [Google Scholar]
- 215.Silva-Santos B, Strid J. gammadelta T cells get adaptive. Nat. Immunol. 2017;18:370–372. doi: 10.1038/ni.3705. [DOI] [PubMed] [Google Scholar]
- 216.Melandri D, et al. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 2018;19:1352–1365. doi: 10.1038/s41590-018-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Brandes M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc. Natl Acad. Sci. USA. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chan KF, Duarte JDG, Ostrouska S, Behren A. gammadelta T cells in the tumor microenvironment-interactions with other immune cells. Front. Immunol. 2022;13:894315. doi: 10.3389/fimmu.2022.894315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vermijlen D, et al. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J. Immunol. 2007;178:4304–4314. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 221.Wen L, et al. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non alpha/beta” T cells. J. Exp. Med. 1996;183:2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caccamo N, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J. Immunol. 2006;177:5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 223.Conti L, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J. Immunol. 2005;174:252–260. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 224.Devilder MC, et al. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J. Immunol. 2006;176:1386–1393. doi: 10.4049/jimmunol.176.3.1386. [DOI] [PubMed] [Google Scholar]
- 225.Caccamo N, et al. gammadelta T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis. Eur. J. Immunol. 2006;36:2681–2690. doi: 10.1002/eji.200636220. [DOI] [PubMed] [Google Scholar]
- 226.Martino A, Poccia F. Gamma delta T cells and dendritic cells: close partners and biological adjuvants for new therapies. Curr. Mol. Med. 2007;7:658–673. doi: 10.2174/156652407782564345. [DOI] [PubMed] [Google Scholar]
- 227.Dalton JE, Pearson J, Scott P, Carding SR. The interaction of gamma delta T cells with activated macrophages is a property of the V gamma 1 subset. J. Immunol. 2003;171:6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- 228.Ferrero E, et al. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leucocyte subpopulations: focus on gammadelta cells. Immunology. 2003;108:365–374. doi: 10.1046/j.1365-2567.2003.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Spencer CT, et al. Granzyme A produced by gamma(9)delta(2) T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS Pathog. 2013;9:e1003119. doi: 10.1371/journal.ppat.1003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dieli F, et al. Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 2000;30:1512–1519. doi: 10.1002/(SICI)1521-4141(200005)30:5<1512::AID-IMMU1512>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 231.Maniar A, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nussbaumer O, Gruenbacher G, Gander H, Thurnher M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by gammadelta T lymphocytes. Blood. 2011;118:2743–2751. doi: 10.1182/blood-2011-01-328526. [DOI] [PubMed] [Google Scholar]
- 233.Liu M, et al. gammadeltaT cells suppress liver fibrosis via strong cytolysis and enhanced NK cell-mediated cytotoxicity against hepatic stellate cells. Front. Immunol. 2019;10:477. doi: 10.3389/fimmu.2019.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Roberts NA, et al. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36:427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat. Rev. Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 236.Willcox BE, Willcox C. R. gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat. Immunol. 2019;20:121–128. doi: 10.1038/s41590-018-0304-y. [DOI] [PubMed] [Google Scholar]
- 237.Vermijlen D, et al. gammadelta T cell responses: how many ligands will it take till we know? Semin Cell Dev. Biol. 2018;84:75–86. doi: 10.1016/j.semcdb.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 238.Benveniste PM, et al. Generation and molecular recognition of melanoma-associated antigen-specific human gammadelta T cells. Sci. Immunol. 2018;3:eaav4036. doi: 10.1126/sciimmunol.aav4036. [DOI] [PubMed] [Google Scholar]
- 239.Kierkels GJJ, et al. Identification of a tumor-specific allo-HLA-restricted gammadeltaTCR. Blood Adv. 2019;3:2870–2882. doi: 10.1182/bloodadvances.2019032409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Davey MS, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat. Commun. 2017;8:14760. doi: 10.1038/ncomms14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hunter S, et al. Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol. 2018;69:654–665. doi: 10.1016/j.jhep.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fichtner AS, Ravens S, Prinz I. Human gammadelta TCR Repertoires in Health and Disease. Cells. 2020;9:800. doi: 10.3390/cells9040800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Carbone A, Vaccher E, Gloghini A. Hematologic cancers in individuals infected by HIV. Blood. 2022;139:995–1012. doi: 10.1182/blood.2020005469. [DOI] [PubMed] [Google Scholar]
- 244.Li H, Pauza CD. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood. 2011;118:5824–5831. doi: 10.1182/blood-2011-05-356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gioia C, et al. Lack of CD27-CD45RA-V gamma 9 V delta 2 + T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J. Immunol. 2002;168:1484–1489. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- 246.Ogongo P, et al. Differential skewing of donor-unrestricted and gammadelta T cell repertoires in tuberculosis-infected human lungs. J. Clin. Invest. 2020;130:214–230. doi: 10.1172/JCI130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen D, et al. gammadelta T cell exhaustion: opportunities for intervention. J. Leukoc. Biol. 2022;112:1669–1676. doi: 10.1002/JLB.5MR0722-777R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Correia DV, et al. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 249.Dunne MR, et al. Persistent changes in circulating and intestinal gammadelta T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS One. 2013;8:e76008. doi: 10.1371/journal.pone.0076008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kenna T, et al. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 2004;113:56–63. doi: 10.1016/j.clim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 251.Rice MT, et al. Recognition of the antigen-presenting molecule MR1 by a Vdelta3(+) gammadelta T cell receptor. Proc. Natl Acad. Sci. USA. 2021;118:e2110288118. doi: 10.1073/pnas.2110288118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Leon-Lara X, et al. Evidence for an adult-like type 1-immunity phenotype of Vdelta1, Vdelta2 and Vdelta3 T cells in ghanaian children with repeated exposure to Malaria. Front. Immunol. 2022;13:807765. doi: 10.3389/fimmu.2022.807765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tuengel J, et al. Characterization of adaptive-like gammadelta T cells in ugandan infants during primary cytomegalovirus infection. Viruses. 2021;13:1987. doi: 10.3390/v13101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ravens S, et al. Human gammadelta T cell receptor repertoires in peripheral blood remain stable despite clearance of persistent Hepatitis C virus infection by direct-acting antiviral drug therapy. Front. Immunol. 2018;9:510. doi: 10.3389/fimmu.2018.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wang L, et al. The feature of distribution and clonality of TCR gamma/delta subfamilies T cells in patients with B-cell non-Hodgkin lymphoma. J. Immunol. Res. 2014;2014:241246. doi: 10.1155/2014/241246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.de Vries NL, et al. gammadelta T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature. 2023;613:743–750. doi: 10.1038/s41586-022-05593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Melo AM, et al. Tissue distribution of gammadelta T cell subsets in oesophageal adenocarcinoma. Clin. Immunol. 2021;229:108797. doi: 10.1016/j.clim.2021.108797. [DOI] [PubMed] [Google Scholar]
- 258.Gherardin NA, et al. gammadelta T cells in merkel cell carcinomas have a proinflammatory profile prognostic of patient survival. Cancer Immunol. Res. 2021;9:612–623. doi: 10.1158/2326-6066.CIR-20-0817. [DOI] [PubMed] [Google Scholar]
- 259.Petrasca A, Melo AM, Breen EP, Doherty DG. Human Vdelta3(+) gammadelta T cells induce maturation and IgM secretion by B cells. Immunol. Lett. 2018;196:126–134. doi: 10.1016/j.imlet.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 260.Spada FM, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J. Exp. Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Uldrich AP, et al. CD1d-lipid antigen recognition by the gammadelta TCR. Nat. Immunol. 2013;14:1137–1145. doi: 10.1038/ni.2713. [DOI] [PubMed] [Google Scholar]
- 262.Luoma AM, et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity. 2013;39:1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Luoma AM, Castro CD, Adams EJ. gammadelta T cell surveillance via CD1 molecules. Trends Immunol. 2014;35:613–621. doi: 10.1016/j.it.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Roy S, et al. Molecular analysis of lipid-reactive Vdelta1 gammadelta T cells identified by CD1c tetramers. J. Immunol. 2016;196:1933–1942. doi: 10.4049/jimmunol.1502202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hayday A, Vantourout P. A long-playing CD about the gammadelta TCR repertoire. Immunity. 2013;39:994–996. doi: 10.1016/j.immuni.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 266.Marlin R, et al. Sensing of cell stress by human gammadelta TCR-dependent recognition of annexin A2. Proc. Natl Acad. Sci. USA. 2017;114:3163–3168. doi: 10.1073/pnas.1621052114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 268.Groh V, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl Acad. Sci. USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xu B, et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc. Natl Acad. Sci. USA. 2011;108:2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Adams EJ, Gu S, Luoma AM. Human gamma delta T cells: evolution and ligand recognition. Cell Immunol. 2015;296:31–40. doi: 10.1016/j.cellimm.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Grunder C, et al. gamma9 and delta2CDR3 domains regulate functional avidity of T cells harboring gamma9delta2TCRs. Blood. 2012;120:5153–5162. doi: 10.1182/blood-2012-05-432427. [DOI] [PubMed] [Google Scholar]
- 272.Gober HJ, et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Benzaid I, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011;71:4562–4572. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- 274.Ashihara E, et al. Isopentenyl pyrophosphate secreted from Zoledronate-stimulated myeloma cells, activates the chemotaxis of gammadeltaT cells. Biochem. Biophys. Res. Commun. 2015;463:650–655. doi: 10.1016/j.bbrc.2015.05.118. [DOI] [PubMed] [Google Scholar]
- 275.Tanaka Y, et al. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 276.Sandstrom A, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rigau M, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gammadelta T cells. Science. 2020;367:eaay5516. doi: 10.1126/science.aay5516. [DOI] [PubMed] [Google Scholar]
- 278.Karunakaran MM, et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vgamma9Vdelta2 TCR and is essential for phosphoantigen sensing. Immunity. 2020;52:487–498.e486. doi: 10.1016/j.immuni.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kabelitz D, et al. Cancer immunotherapy with gammadelta T cells: many paths ahead of us. Cell Mol. Immunol. 2020;17:925–939. doi: 10.1038/s41423-020-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yang Y, et al. A structural change in butyrophilin upon phosphoantigen binding underlies phosphoantigen-mediated Vgamma9Vdelta2 T cell activation. Immunity. 2019;50:1043–1053.e1045. doi: 10.1016/j.immuni.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 281.Scotet E, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 282.Chen H, et al. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J. Biol. Chem. 2008;283:12528–12537. doi: 10.1074/jbc.M708067200. [DOI] [PubMed] [Google Scholar]
- 283.Dai Y, et al. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J. Biol. Chem. 2012;287:16812–16819. doi: 10.1074/jbc.M111.327650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fichtner AS, et al. Alpaca (Vicugna pacos), the first nonprimate species with a phosphoantigen-reactive Vgamma9Vdelta2 T cell subset. Proc. Natl Acad. Sci. USA. 2020;117:6697–6707. doi: 10.1073/pnas.1909474117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 286.Rincon-Orozco B, et al. Activation of V gamma 9V delta 2 T cells by NKG2D. J. Immunol. 2005;175:2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 287.Wrobel P, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand. J. Immunol. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 288.Lanca T, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115:2407–2411. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 289.Simoes AE, Di Lorenzo B, Silva-Santos B. Molecular determinants of target cell recognition by human gammadelta T cells. Front. Immunol. 2018;9:929. doi: 10.3389/fimmu.2018.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Toutirais O, et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur. J. Immunol. 2009;39:1361–1368. doi: 10.1002/eji.200838409. [DOI] [PubMed] [Google Scholar]
- 291.Tokuyama H, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs-rituximab and trastuzumab. Int. J. Cancer. 2008;122:2526–2534. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 292.Capietto AH, Martinet L, Fournie JJ. Stimulated gammadelta T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J. Immunol. 2011;187:1031–1038. doi: 10.4049/jimmunol.1100681. [DOI] [PubMed] [Google Scholar]
- 293.Gertner-Dardenne J, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–4884. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 294.Fisher JP, et al. Neuroblastoma killing properties of Vdelta2 and Vdelta2-negative gammadeltaT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 2014;20:5720–5732. doi: 10.1158/1078-0432.CCR-13-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Street SE, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Liu Z, et al. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J. Immunol. 2008;180:6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 297.Gao Y, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jarry U, et al. Stereotaxic administrations of allogeneic human Vgamma9Vdelta2 T cells efficiently control the development of human glioblastoma brain tumors. Oncoimmunology. 2016;5:e1168554. doi: 10.1080/2162402X.2016.1168554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pereboeva L, Harkins L, Wong S, Lamb LS. The safety of allogeneic innate lymphocyte therapy for glioma patients with prior cranial irradiation. Cancer Immunol. Immunother. 2015;64:551–562. doi: 10.1007/s00262-015-1662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Patin EC, et al. Type I IFN receptor signaling controls IL7-dependent accumulation and activity of protumoral IL17A-producing gammadelta T cells in breast cancer. Cancer Res. 2018;78:195–204. doi: 10.1158/0008-5472.CAN-17-1416. [DOI] [PubMed] [Google Scholar]
- 301.Kimura Y, et al. IL-17A-producing CD30(+) Vdelta1 T cells drive inflammation-induced cancer progression. Cancer Sci. 2016;107:1206–1214. doi: 10.1111/cas.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Coffelt SB, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kulig P, et al. IL17A-Mediated Endothelial Breach Promotes Metastasis Formation. Cancer Immunol Res. 2016;4:26–32. doi: 10.1158/2326-6066.CIR-15-0154. [DOI] [PubMed] [Google Scholar]
- 304.Parker ME, Ciofani M. Regulation of gammadelta T cell effector diversification in the thymus. Front. Immunol. 2020;11:42. doi: 10.3389/fimmu.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jensen KD, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Fleming C, Morrissey S, Cai Y, Yan J. gammadelta T cells: unexpected regulators of cancer development and progression. Trends Cancer. 2017;3:561–570. doi: 10.1016/j.trecan.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lo Presti E, Dieli F, Meraviglia S. Tumor-infiltrating gammadelta T lymphocytes: pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front. Immunol. 2014;5:607. doi: 10.3389/fimmu.2014.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ye J, et al. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer Res. 2013;73:6137–6148. doi: 10.1158/0008-5472.CAN-13-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yao YE, Qin CC, Yang CM, Huang TX. gammadeltaT17/gammadeltaTreg cell subsets: a new paradigm for asthma treatment. J. Asthma. 2022;59:2028–2038. doi: 10.1080/02770903.2021.1980585. [DOI] [PubMed] [Google Scholar]
- 310.Yang X, et al. Tofacitinib restores the balance of gammadeltaTreg/gammadeltaT17 cells in rheumatoid arthritis by inhibiting the NLRP3 inflammasome. Theranostics. 2021;11:1446–1457. doi: 10.7150/thno.47860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Xuan L, et al. Regulatory gammadelta T cells induced by G-CSF participate in acute graft-versus-host disease regulation in G-CSF-mobilized allogeneic peripheral blood stem cell transplantation. J. Transl. Med. 2018;16:144. doi: 10.1186/s12967-018-1519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mao Y, et al. A new effect of IL-4 on human gammadelta T cells: promoting regulatory Vdelta1 T cells via IL-10 production and inhibiting function of Vdelta2 T cells. Cell Mol. Immunol. 2016;13:217–228. doi: 10.1038/cmi.2015.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ni C, et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+gammadelta1 Treg cells. Sig. Transduct. Target Ther. 2020;5:41. doi: 10.1038/s41392-020-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hu G, et al. Tumor-infiltrating CD39(+)gammadeltaTregs are novel immunosuppressive T cells in human colorectal cancer. Oncoimmunology. 2017;6:e1277305. doi: 10.1080/2162402X.2016.1277305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chabab G, et al. Identification of a regulatory Vdelta1 gamma delta T cell subpopulation expressing CD73 in human breast cancer. J. Leukoc. Biol. 2020;107:1057–1067. doi: 10.1002/JLB.3MA0420-278RR. [DOI] [PubMed] [Google Scholar]
- 316.Seo N, Tokura Y, Furukawa F, Takigawa M. Down-regulation of tumoricidal NK and NK T cell activities by MHC Kb molecules expressed on Th2-type gammadelta T and alphabeta T cells coinfiltrating in early B16 melanoma lesions. J. Immunol. 1998;161:4138–4145. doi: 10.4049/jimmunol.161.8.4138. [DOI] [PubMed] [Google Scholar]
- 317.Schmolka N, et al. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory gammadelta T cell subsets. Nat. Immunol. 2013;14:1093–1100. doi: 10.1038/ni.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chitadze G, Oberg HH, Wesch D, Kabelitz D. The ambiguous role of gammadelta T lymphocytes in antitumor immunity. Trends Immunol. 2017;38:668–678. doi: 10.1016/j.it.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 319.Casetti R, et al. Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J. Immunol. 2009;183:3574–3577. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- 320.Kouakanou L, et al. Vitamin C supports conversion of human gammadelta T cells into FOXP3-expressing regulatory cells by epigenetic regulation. Sci. Rep. 2020;10:6550. doi: 10.1038/s41598-020-63572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lo Presti E, et al. Squamous cell tumors recruit gammadelta T cells producing either IL17 or IFNgamma depending on the tumor stage. Cancer Immunol. Res. 2017;5:397–407. doi: 10.1158/2326-6066.CIR-16-0348. [DOI] [PubMed] [Google Scholar]
- 322.Sureshbabu SK, Chaukar D, Chiplunkar SV. Hypoxia regulates the differentiation and anti-tumor effector functions of gammadeltaT cells in oral cancer. Clin. Exp. Immunol. 2020;201:40–57. doi: 10.1111/cei.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wu Y, et al. A local human Vdelta1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat. Cancer. 2022;3:696–709. doi: 10.1038/s43018-022-00376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Pizzolato G, et al. Single-cell RNA sequencing unveils the shared and the distinct cytotoxic hallmarks of human TCRVdelta1 and TCRVdelta2 gammadelta T lymphocytes. Proc. Natl Acad. Sci. USA. 2019;116:11906–11915. doi: 10.1073/pnas.1818488116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wu Y, et al. An innate-like Vdelta1(+) gammadelta T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med. 2019;11:eaax9364. doi: 10.1126/scitranslmed.aax9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ponomarev ED, et al. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J. Immunol. 2004;173:1587–1595. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- 327.Conti HR, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J. Exp. Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Park SG, et al. T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity. 2010;33:791–803. doi: 10.1016/j.immuni.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cui Y, et al. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J. Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shibata S, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from gammadelta-T cells. Nat. Commun. 2015;6:7687. doi: 10.1038/ncomms8687. [DOI] [PubMed] [Google Scholar]
- 331.Meraviglia S, et al. Distinctive features of tumor-infiltrating gammadelta T lymphocytes in human colorectal cancer. Oncoimmunology. 2017;6:e1347742. doi: 10.1080/2162402X.2017.1347742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ma C, et al. Tumor-infiltrating gammadelta T lymphocytes predict clinical outcome in human breast cancer. J. Immunol. 2012;189:5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Reis BS, et al. TCR-Vgammadelta usage distinguishes protumor from antitumor intestinal gammadelta T cell subsets. Science. 2022;377:276–284. doi: 10.1126/science.abj8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mensurado S, Silva-Santos B. Battle of the gammadelta T cell subsets in the gut. Trends Cancer. 2022;8:881–883. doi: 10.1016/j.trecan.2022.08.006. [DOI] [PubMed] [Google Scholar]
- 335.Zakeri N, et al. Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma. Nat. Commun. 2022;13:1372. doi: 10.1038/s41467-022-29012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Khairallah C, Chu TH, Sheridan BS. Tissue adaptations of memory and tissue-resident gamma delta T cells. Front. Immunol. 2018;9:2636. doi: 10.3389/fimmu.2018.02636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Steinbach K, Vincenti I, Merkler D. Resident-memory T cells in tissue-restricted immune responses: for better or worse? Front. Immunol. 2018;9:2827. doi: 10.3389/fimmu.2018.02827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Newman AM, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Tosolini M, et al. Assessment of tumor-infiltrating TCRVgamma9Vdelta2 gammadelta lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology. 2017;6:e1284723. doi: 10.1080/2162402X.2017.1284723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Patil RS, et al. IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer. 2016;139:869–881. doi: 10.1002/ijc.30134. [DOI] [PubMed] [Google Scholar]
- 341.Rutkowski MR, et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Van Hede D, et al. Human papillomavirus oncoproteins induce a reorganization of epithelial-associated gammadelta T cells promoting tumor formation. Proc. Natl Acad. Sci. USA. 2017;114:E9056–E9065. doi: 10.1073/pnas.1712883114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Jin C, et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell. 2019;176:998–1013.e1016. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ridker PM, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 345.Ma Y, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hamada S, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Xu R, et al. TNFR2(+) regulatory T cells protect against bacteremic pneumococcal pneumonia by suppressing IL-17A-producing gammadelta T cells in the lung. Cell Rep. 2023;42:112054. doi: 10.1016/j.celrep.2023.112054. [DOI] [PubMed] [Google Scholar]
- 348.Cai Y, et al. A critical role of the IL-1beta-IL-1R signaling pathway in skin inflammation and psoriasis pathogenesis. J. Invest. Dermatol. 2019;139:146–156. doi: 10.1016/j.jid.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kabelitz D, Peters C, Wesch D, Oberg HH. Regulatory functions of γδ T cells. Int. Immunopharmacol. 2013;16:382–387. doi: 10.1016/j.intimp.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 350.Peters C, Kabelitz D, Wesch D. Regulatory functions of γδ T cells. Cell Mol. Life Sci. 2018;75:2125–2135. doi: 10.1007/s00018-018-2788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Peters C, Häsler R, Wesch D, Kabelitz D. Human Vδ2 T cells are a major source of interleukin-9. Proc. Natl Acad. Sci. USA. 2016;113:12520–12525. doi: 10.1073/pnas.1607136113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Peters C, Oberg HH, Kabelitz D, Wesch D. Phenotype and regulation of immunosuppressive Vδ2-expressing γδ T cells. Cell Mol. Life Sci. 2014;71:1943–1960. doi: 10.1007/s00018-013-1467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Himoudi N, et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J. Immunol. 2012;188:1708–1716. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 354.Yang XW, et al. Impairment of antigen-presenting function of peripheral γδ T cells in patients with sepsis. Clin. Exp. Immunol. 2022;207:104–112. doi: 10.1093/cei/uxab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Bagcchi S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe. 2023;4:e20. doi: 10.1016/S2666-5247(22)00359-7. [DOI] [PubMed] [Google Scholar]
- 356.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Chandra P, Grigsby SJ, Philips JA. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022;20:750–766. doi: 10.1038/s41579-022-00763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Behr M, Schurr E, Gros P. TB: screening for responses to a vile visitor. Cell. 2010;140:615–618. doi: 10.1016/j.cell.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 359.Lienhardt C, et al. Global tuberculosis control: lessons learnt and future prospects. Nat. Rev. Microbiol. 2012;10:407–416. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 360.Cambier CJ, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annu. Rev. Pathol. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 362.Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159:1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 363.Kumar D, et al. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell. 2010;140:731–743. doi: 10.1016/j.cell.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 364.Vorkas CK, et al. Mucosal-associated invariant and gammadelta T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight. 2018;3:e121899. doi: 10.1172/jci.insight.121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kulicke CA, Lewinsohn DA, Lewinsohn DM. Clonal enrichments of Vdelta2- gammadelta T cells in Mycobacterium tuberculosis-infected human lungs. J. Clin. Invest. 2020;130:68–70. doi: 10.1172/JCI133119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Maertzdorf J, et al. Mycobacterium tuberculosis invasion of the human lung: first contact. Front. Immunol. 2018;9:1346. doi: 10.3389/fimmu.2018.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Li Y, et al. Identification of the ligands of TCRγδ by screening the immune repertoire of γδT cells from patients with tuberculosis. Front. Immunol. 2019;10:2282. doi: 10.3389/fimmu.2019.02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.D’Souza MP, et al. Casting a wider net: immunosurveillance by nonclassical MHC molecules. PLoS Pathog. 2019;15:e1007567. doi: 10.1371/journal.ppat.1007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Van Rhijn I, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat. Immunol. 2013;14:706–713. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.De Libero G, Singhal A, Lepore M, Mori L. Nonclassical T cells and their antigens in tuberculosis. Cold Spring Harb. Perspect. Med. 2014;4:a018473. doi: 10.1101/cshperspect.a018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Martinez-Barricarte R, et al. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018;3:eaau6759. doi: 10.1126/sciimmunol.aau6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Philippot Q, et al. Human IL-23 is essential for IFN-gamma-dependent immunity to mycobacteria. Sci. Immunol. 2023;8:eabq5204. doi: 10.1126/sciimmunol.abq5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ogishi M, et al. Inherited human ITK deficiency impairs IFN-gamma immunity and underlies tuberculosis. J. Exp. Med. 2023;220:e20220484. doi: 10.1084/jem.20220484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ogishi M, et al. Inherited PD-1 deficiency underlies tuberculosis and autoimmunity in a child. Nat. Med. 2021;27:1646–1654. doi: 10.1038/s41591-021-01388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Okada S, et al. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349:606–613. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Papadopoulou M, et al. Fetal public Vgamma9Vdelta2 T cells expand and gain potent cytotoxic functions early after birth. Proc. Natl Acad. Sci. USA. 2020;117:18638–18648. doi: 10.1073/pnas.1922595117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. 1998;161:1045–1054. doi: 10.4049/jimmunol.161.2.1045. [DOI] [PubMed] [Google Scholar]
- 378.Chen ZW. Protective immune responses of major Vgamma2Vdelta2 T-cell subset in M. tuberculosis infection. Curr. Opin. Immunol. 2016;42:105–112. doi: 10.1016/j.coi.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Shen L, et al. Immunization of Vgamma2Vdelta2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl Acad. Sci. USA. 2019;116:6371–6378. doi: 10.1073/pnas.1811380116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Shen Y, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Shen L, et al. Fast-acting gammadelta T-cell subpopulation and protective immunity against infections. Immunol. Rev. 2020;298:254–263. doi: 10.1111/imr.12927. [DOI] [PubMed] [Google Scholar]
- 382.Qaqish A, et al. Adoptive transfer of phosphoantigen-specific gammadelta T cell subset attenuates mycobacterium tuberculosis infection in nonhuman primates. J. Immunol. 2017;198:4753–4763. doi: 10.4049/jimmunol.1602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Chen ZW. Multifunctional immune responses of HMBPP-specific Vgamma2Vdelta2 T cells in M. tuberculosis and other infections. Cell Mol. Immunol. 2013;10:58–64. doi: 10.1038/cmi.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Shen H, et al. Adjunctive Zoledronate + IL-2 administrations enhance anti-tuberculosis Vgamma2Vdelta2 T-effector populations, and improve treatment outcome of multidrug-resistant tuberculosis(1) Emerg. Microbes Infect. 2022;11:1790–1805. doi: 10.1080/22221751.2022.2095930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Chen CY, et al. Phosphoantigen/IL2 expansion and differentiation of Vgamma2Vdelta2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog. 2013;9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Roy Chowdhury R, et al. NK-like CD8(+) gammadelta T cells are expanded in persistent Mycobacterium tuberculosis infection. Sci. Immunol. 2023;8:eade3525. doi: 10.1126/sciimmunol.ade3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat. Rev. Dis. Primers. 2015;1:15035. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 388.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 389.Bruchfeld J, Correia-Neves M, Kallenius G. Tuberculosis and HIV coinfection. Cold Spring Harb. Perspect. Med. 2015;5:a017871. doi: 10.1101/cshperspect.a017871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018;16:80–90. doi: 10.1038/nrmicro.2017.128. [DOI] [PubMed] [Google Scholar]
- 391.Li H, et al. Association between Vgamma2Vdelta2 T cells and disease progression after infection with closely related strains of HIV in China. Clin. Infect. Dis. 2008;46:1466–1472. doi: 10.1086/587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Hinz T, et al. T cell receptor gamma delta repertoire in HIV-1-infected individuals. Eur. J. Immunol. 1994;24:3044–3049. doi: 10.1002/eji.1830241219. [DOI] [PubMed] [Google Scholar]
- 393.Li Z, et al. Distortion of memory Vdelta2 gammadelta T cells contributes to immune dysfunction in chronic HIV infection. Cell Mol. Immunol. 2015;12:604–614. doi: 10.1038/cmi.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Rossol R, et al. Increase in Vdelta1+ gammadelta T cells in the peripheral blood and bone marrow as a selective feature of HIV-1 but not other virus infections. Br. J. Haematol. 1998;100:728–734. doi: 10.1046/j.1365-2141.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 395.Harris LD, et al. Mechanisms underlying gammadelta T-cell subset perturbations in SIV-infected Asian rhesus macaques. Blood. 2010;116:4148–4157. doi: 10.1182/blood-2010-05-283549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Li H, Pauza CD. The alpha4beta7 integrin binds HIV envelope but does not mediate bystander killing of gammadelta T cells. Blood. 2012;120:698–699. doi: 10.1182/blood-2012-03-420117. [DOI] [PubMed] [Google Scholar]
- 397.Restrepo C, et al. HIV Gag-specific immune response mediated by double negative (CD3(+)CD4(-)CD8(-)) T cells in HIV-exposed seronegative individuals. J. Med. Virol. 2013;85:200–209. doi: 10.1002/jmv.23447. [DOI] [PubMed] [Google Scholar]
- 398.Hudspeth K, et al. Engagement of NKp30 on Vdelta1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood. 2012;119:4013–4016. doi: 10.1182/blood-2011-11-390153. [DOI] [PubMed] [Google Scholar]
- 399.Dobmeyer TS, et al. Reciprocal alterations of Th1/Th2 function in gammadelta T-cell subsets of human immunodeficiency virus-1-infected patients. Br. J. Haematol. 2002;118:282–288. doi: 10.1046/j.1365-2141.2002.03555.x. [DOI] [PubMed] [Google Scholar]
- 400.Li H, et al. Depletion and dysfunction of Vgamma2Vdelta2 T cells in HIV disease: mechanisms, impacts and therapeutic implications. Cell Mol. Immunol. 2013;10:42–49. doi: 10.1038/cmi.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Martini F, et al. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vgamma9Vdelta2 T cells in chronically infected patients undergoing structured treatment interruption. J. Infect. Dis. 2002;186:847–850. doi: 10.1086/342410. [DOI] [PubMed] [Google Scholar]
- 402.Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy. 2012;14:173–181. doi: 10.3109/14653249.2011.623693. [DOI] [PubMed] [Google Scholar]
- 403.Garrido C, et al. Gammadelta T cells: an immunotherapeutic approach for HIV cure strategies. JCI Insight. 2018;3:e120121. doi: 10.1172/jci.insight.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kazer SW, et al. Integrated single-cell analysis of multicellular immune dynamics during hyperacute HIV-1 infection. Nat. Med. 2020;26:511–518. doi: 10.1038/s41591-020-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wang S, et al. An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerg. Microbes Infect. 2020;9:2333–2347. doi: 10.1080/22221751.2020.1826361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Pauza CD, et al. gammadelta T cells in HIV disease: past, present, and future. Front. Immunol. 2014;5:687. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.El-Sadr WM, Vasan A, El-Mohandes A. Facing the new Covid-19 reality. N. Engl. J. Med. 2023;388:385–387. doi: 10.1056/NEJMp2213920. [DOI] [PubMed] [Google Scholar]
- 408.V’Kovski P, et al. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Zhu N, et al. A novel Coronavirus from patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Cai Y, et al. Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants. Science. 2021;373:642–648. doi: 10.1126/science.abi9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Vogel G. New subvariants are masters of immune evasion. Science. 2022;376:679–680. doi: 10.1126/science.adc9448. [DOI] [PubMed] [Google Scholar]
- 412.Thorne LG, et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature. 2022;602:487–495. doi: 10.1038/s41586-021-04352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Wang Q, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286.e278. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yang L, et al. The signal pathways and treatment of cytokine storm in COVID-19. Sig. Transduct. Target Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Fajgenbaum DC, June CH. Cytokine storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Cron RQ, Caricchio R, Chatham WW. Calming the cytokine storm in COVID-19. Nat. Med. 2021;27:1674–1675. doi: 10.1038/s41591-021-01500-9. [DOI] [PubMed] [Google Scholar]
- 417.Carter MJ, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 418.Odak I, et al. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine. 2020;57:102885. doi: 10.1016/j.ebiom.2020.102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Wilk AJ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Lucas C, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Fears AC, et al. The dynamics of gammadelta T cell responses in nonhuman primates during SARS-CoV-2 infection. Commun. Biol. 2022;5:1380. doi: 10.1038/s42003-022-04310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Wang X, et al. Host-derived lipids orchestrate pulmonary gammadelta T cell response to provide early protection against influenza virus infection. Nat. Commun. 2021;12:1914. doi: 10.1038/s41467-021-22242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Su Y, et al. Multi-Omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183:1479–1495.e1420. doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Huang L, et al. Dynamic blood single-cell immune responses in patients with COVID-19. Sig. Transduct. Target Ther. 2021;6:110. doi: 10.1038/s41392-021-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Ren X, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:1895–1913.e1819. doi: 10.1016/j.cell.2021.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Jameson JM. gammadelta T cells: a disappearing act with a big reveal. J. Exp. Med. 2018;215:2962–2963. doi: 10.1084/jem.20181960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Sandrock I, et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing gammadelta T cells. J. Exp. Med. 2018;215:3006–3018. doi: 10.1084/jem.20181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Jee MH, Mraz V, Geisler C, Bonefeld C. M. gammadelta T cells and inflammatory skin diseases. Immunol. Rev. 2020;298:61–73. doi: 10.1111/imr.12913. [DOI] [PubMed] [Google Scholar]
- 429.Bugaut H, Aractingi S. Major role of the IL17/23 axis in psoriasis supports the development of new targeted therapies. Front. Immunol. 2021;12:621956. doi: 10.3389/fimmu.2021.621956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kim SH, et al. Pellino-1 promotes intrinsic activation of skin-resident IL-17A-producing T cells in psoriasis. J. Allergy Clin. Immunol. 2023;151:1317–1328. doi: 10.1016/j.jaci.2022.12.823. [DOI] [PubMed] [Google Scholar]
- 431.Ueharaguchi Y, et al. Thromboxane A(2) facilitates IL-17A production from Vgamma4(+) gammadelta T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018;142:680–683.e682. doi: 10.1016/j.jaci.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 432.Zheng T, et al. p38alpha signaling in Langerhans cells promotes the development of IL-17-producing T cells and psoriasiform skin inflammation. Sci. Signal. 2018;11:eaao1685. doi: 10.1126/scisignal.aao1685. [DOI] [PubMed] [Google Scholar]
- 433.Cibrian D, et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 2016;17:985–996. doi: 10.1038/ni.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yang Q, et al. Roles of mTORC1 and mTORC2 in controlling gammadelta T1 and gammadelta T17 differentiation and function. Cell Death Differ. 2020;27:2248–2262. doi: 10.1038/s41418-020-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cai Y, et al. Differential roles of the mTOR-STAT3 signaling in dermal gammadelta T cell effector function in skin inflammation. Cell Rep. 2019;27:3034–3048.e3035. doi: 10.1016/j.celrep.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Ramirez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc. Natl Acad. Sci. USA. 2015;112:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Liu N, et al. Dynamic trafficking patterns of IL-17-producing gammadelta T cells are linked to the recurrence of skin inflammation in psoriasis-like dermatitis. EBioMedicine. 2022;82:104136. doi: 10.1016/j.ebiom.2022.104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Nguyen CT, et al. Peripheral gammadelta T cells regulate neutrophil expansion and recruitment in experimental psoriatic arthritis. Arthritis Rheumatol. 2022;74:1524–1534. doi: 10.1002/art.42124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Khmaladze I, et al. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc. Natl Acad. Sci. USA. 2014;111:E3669–E3678. doi: 10.1073/pnas.1405798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Riol-Blanco L, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:157–161. doi: 10.1038/nature13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Chen S, et al. Selective targeting of PI3Kdelta suppresses human IL-17-producing T cells and innate-like lymphocytes and may be therapeutic for IL-17-mediated diseases. J. Autoimmun. 2020;111:102435. doi: 10.1016/j.jaut.2020.102435. [DOI] [PubMed] [Google Scholar]
- 442.Pinget GV, et al. Immune modulation of monocytes dampens the IL-17(+) gammadelta T cell response and associated psoriasis pathology in mice. J. Invest. Dermatol. 2020;140:2398–2407.e2391. doi: 10.1016/j.jid.2020.03.973. [DOI] [PubMed] [Google Scholar]
- 443.Soley BDS, et al. B(1) and B(2) kinin receptor blockade improves psoriasis-like disease. Br. J. Pharmacol. 2020;177:3535–3551. doi: 10.1111/bph.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Zhang S, et al. Hyperforin ameliorates imiquimod-induced psoriasis-like murine skin inflammation by modulating IL-17A-producing gammadelta T cells. Front. Immunol. 2021;12:635076. doi: 10.3389/fimmu.2021.635076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Mortier C, et al. RORgammat inhibition ameliorates IL-23 driven experimental psoriatic arthritis by predominantly modulating gammadelta-T cells. Rheumatology (Oxford) 2023;62:3169–3178. doi: 10.1093/rheumatology/kead022. [DOI] [PubMed] [Google Scholar]
- 446.Cibrian D, et al. Targeting L-type amino acid transporter 1 in innate and adaptive T cells efficiently controls skin inflammation. J. Allergy Clin. Immunol. 2020;145:199–214.e111. doi: 10.1016/j.jaci.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 447.Han Y, et al. IL-38 ameliorates skin inflammation and limits IL-17 production from gammadelta T cells. Cell Rep. 2019;27:835–846.e835. doi: 10.1016/j.celrep.2019.03.082. [DOI] [PubMed] [Google Scholar]
- 448.Getschman AE, et al. Protein engineering of the chemokine CCL20 prevents psoriasiform dermatitis in an IL-23-dependent murine model. Proc. Natl Acad. Sci. USA. 2017;114:12460–12465. doi: 10.1073/pnas.1704958114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wang J, et al. Adverse events associated with anti-IL-17 agents for psoriasis and psoriatic arthritis: a systematic scoping review. Front. Immunol. 2023;14:993057. doi: 10.3389/fimmu.2023.993057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Lonnberg AS, Zachariae C, Skov L. Targeting of interleukin-17 in the treatment of psoriasis. Clin. Cosmet Investig. Dermatol. 2014;7:251–259. doi: 10.2147/CCID.S67534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br. J. Dermatol. 2017;177:47–62. doi: 10.1111/bjd.15015. [DOI] [PubMed] [Google Scholar]
- 452.Shi Z, et al. Short-term exposure to a western diet induces psoriasiform dermatitis by promoting accumulation of IL-17A-producing gammadelta T cells. J. Invest. Dermatol. 2020;140:1815–1823. doi: 10.1016/j.jid.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Nakamizo S, et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing gammadelta T cells. Sci. Rep. 2017;7:14076. doi: 10.1038/s41598-017-14292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Bielecki P, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature. 2021;592:128–132. doi: 10.1038/s41586-021-03188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Kim J, et al. Single-cell transcriptomics applied to emigrating cells from psoriasis elucidate pathogenic versus regulatory immune cell subsets. J. Allergy Clin. Immunol. 2021;148:1281–1292. doi: 10.1016/j.jaci.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Reynolds G, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science. 2021;371:eaba6500. doi: 10.1126/science.aba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Liu Y, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling. Sci. Immunol. 2022;7:eabl9165. doi: 10.1126/sciimmunol.abl9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Fukushima K, et al. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor gamma/delta in inflammatory bowel disease. Gastroenterology. 1991;101:670–678. doi: 10.1016/0016-5085(91)90524-O. [DOI] [PubMed] [Google Scholar]
- 459.Catalan-Serra I, Sandvik AK, Bruland T, Andreu-Ballester JC. Gammadelta T cells in Crohn’s disease: a new player in the disease pathogenesis? J. Crohns Colitis. 2017;11:1135–1145. doi: 10.1093/ecco-jcc/jjx039. [DOI] [PubMed] [Google Scholar]
- 460.Yeung MM, et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.McVay LD, et al. Changes in human mucosal gamma delta T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol. Med. 1997;3:183–203. doi: 10.1007/BF03401672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Hoffmann JC, et al. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut. 2001;48:489–495. doi: 10.1136/gut.48.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Inagaki-Ohara K, et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J. Immunol. 2004;173:1390–1398. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 464.Chen Y, et al. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl Acad. Sci. USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Tsuchiya T, et al. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J. Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 466.Matsuzawa-Ishimoto Y, et al. The gammadelta IEL effector API5 masks genetic susceptibility to Paneth cell death. Nature. 2022;610:547–554. doi: 10.1038/s41586-022-05259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Kadivar M, Petersson J, Svensson L, Marsal J. CD8alphabeta+ gammadelta T cells: a novel T cell subset with a potential role in inflammatory bowel disease. J. Immunol. 2016;197:4584–4592. doi: 10.4049/jimmunol.1601146. [DOI] [PubMed] [Google Scholar]
- 468.Nanno M, et al. Exacerbating role of gammadelta T cells in chronic colitis of T-cell receptor alpha mutant mice. Gastroenterology. 2008;134:481–490. doi: 10.1053/j.gastro.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 469.Hu MD, et al. gammadelta intraepithelial lymphocytes facilitate pathological epithelial cell shedding via CD103-mediated granzyme release. Gastroenterology. 2022;162:877–889.e877. doi: 10.1053/j.gastro.2021.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Papotto PH, Yilmaz B, Silva-Santos B. Crosstalk between gammadelta T cells and the microbiota. Nat. Microbiol. 2021;6:1110–1117. doi: 10.1038/s41564-021-00948-2. [DOI] [PubMed] [Google Scholar]
- 471.Benakis C, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Tedesco D, et al. Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic gammadelta T-cell receptor-positive cells and pathogenesis of cholestatic liver disease. Gastroenterology. 2018;154:2178–2193. doi: 10.1053/j.gastro.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Dupraz L, et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal gammadelta T cells. Cell Rep. 2021;36:109332. doi: 10.1016/j.celrep.2021.109332. [DOI] [PubMed] [Google Scholar]
- 474.Mirkov MU, Verstockt B, Cleynen I. Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol. Hepatol. 2017;2:224–234. doi: 10.1016/S2468-1253(16)30111-X. [DOI] [PubMed] [Google Scholar]
- 475.Cleynen I, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Coulombe F, Behr MA. Crohn’s disease as an immune deficiency? Lancet. 2009;374:769–770. doi: 10.1016/S0140-6736(09)61576-2. [DOI] [PubMed] [Google Scholar]
- 477.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 478.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 479.Hampe J, et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn’s disease: a cohort study. Lancet. 2002;359:1661–1665. doi: 10.1016/S0140-6736(02)08590-2. [DOI] [PubMed] [Google Scholar]
- 480.van Heel DA, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet. 2005;365:1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 481.Jiang W, et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J. Exp. Med. 2013;210:2465–2476. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Ramanan D, et al. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Petnicki-Ocwieja T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl Acad. Sci. USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Smillie CS, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730.e722. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Martin JC, et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508.e1420. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Boland BS, et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci. Immunol. 2020;5:eabb4432. doi: 10.1126/sciimmunol.abb4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Parikh K, et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49–55. doi: 10.1038/s41586-019-0992-y. [DOI] [PubMed] [Google Scholar]
- 488.Mitsialis V, et al. Single-cell analyses of colon and blood reveal distinct immune cell signatures of ulcerative colitis and Crohn’s disease. Gastroenterology. 2020;159:591–608.e510. doi: 10.1053/j.gastro.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Attfield KE, et al. The immunology of multiple sclerosis. Nat. Rev. Immunol. 2022;22:734–750. doi: 10.1038/s41577-022-00718-z. [DOI] [PubMed] [Google Scholar]
- 490.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 491.Filippi M, et al. Multiple sclerosis. Nat. Rev. Dis. Primers. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 492.Charabati M, Wheeler MA, Weiner HL, Quintana FJ. Multiple sclerosis: neuroimmune crosstalk and therapeutic targeting. Cell. 2023;186:1309–1327. doi: 10.1016/j.cell.2023.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br. J. Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Sobel RA, Kuchroo VK. The immunopathology of acute experimental allergic encephalomyelitis induced with myelin proteolipid protein. T cell receptors in inflammatory lesions. J. Immunol. 1992;149:1444–1451. doi: 10.4049/jimmunol.149.4.1444. [DOI] [PubMed] [Google Scholar]
- 495.Wucherpfennig KW, et al. T cell receptor V alpha-V beta repertoire and cytokine gene expression in active multiple sclerosis lesions. J. Exp. Med. 1992;175:993–1002. doi: 10.1084/jem.175.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Hvas J, et al. Gamma delta T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J. Neuroimmunol. 1993;46:225–234. doi: 10.1016/0165-5728(93)90253-U. [DOI] [PubMed] [Google Scholar]
- 497.Stinissen P, et al. Increased frequency of gamma delta T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J. Immunol. 1995;154:4883–4894. doi: 10.4049/jimmunol.154.9.4883. [DOI] [PubMed] [Google Scholar]
- 498.Gao YL, Rajan AJ, Raine CS, Brosnan C. F. gammadelta T cells express activation markers in the central nervous system of mice with chronic-relapsing experimental autoimmune encephalomyelitis. J. Autoimmun. 2001;17:261–271. doi: 10.1006/jaut.2001.0547. [DOI] [PubMed] [Google Scholar]
- 499.Jensen MA, Dayal A, Arnason BG. Cytokine secretion by deltagamma and alphabeta T cells in monophasic experimental autoimmune encephalomyelitis. J. Autoimmun. 1999;12:73–80. doi: 10.1006/jaut.1998.0263. [DOI] [PubMed] [Google Scholar]
- 500.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J. Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 501.Blink SE, Miller SD. The contribution of gammadelta T cells to the pathogenesis of EAE and MS. Curr. Mol. Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Blink SE, et al. gammadelta T cell subsets play opposing roles in regulating experimental autoimmune encephalomyelitis. Cell Immunol. 2014;290:39–51. doi: 10.1016/j.cellimm.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Van Kaer L, et al. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol. Immunol. 2019;16:531–539. doi: 10.1038/s41423-019-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Rajan AJ, Klein JD, Brosnan CF. The effect of gammadelta T cell depletion on cytokine gene expression in experimental allergic encephalomyelitis. J. Immunol. 1998;160:5955–5962. doi: 10.4049/jimmunol.160.12.5955. [DOI] [PubMed] [Google Scholar]
- 505.Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J. Immunol. 1996;157:941–949. doi: 10.4049/jimmunol.157.2.941. [DOI] [PubMed] [Google Scholar]
- 506.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33 - 35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR delta chain gene. Eur. J. Immunol. 1999;29:4060–4071. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 507.Odyniec A, et al. Gammadelta T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J. Immunol. 2004;173:682–694. doi: 10.4049/jimmunol.173.1.682. [DOI] [PubMed] [Google Scholar]
- 508.Filiano AJ, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Alves de Lima K, et al. Meningeal gammadelta T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 2020;21:1421–1429. doi: 10.1038/s41590-020-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Ribeiro M, et al. Meningeal gammadelta T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. 2019;4:eaay5199. doi: 10.1126/sciimmunol.aay5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.McGinley AM, et al. Interleukin-17A serves a priming role in autoimmunity by recruiting IL-1beta-producing myeloid cells that promote pathogenic T cells. Immunity. 2020;52:342–356.e346. doi: 10.1016/j.immuni.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 512.Ren S, et al. Lactobacillus acidipiscis induced regulatory gamma delta T cells and attenuated experimental autoimmune encephalomyelitis. Front. Immunol. 2021;12:623451. doi: 10.3389/fimmu.2021.623451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Flemming A. Dectin-1 on colonic gammadelta T cells mediates vulnerability to psychosocial stress. Nat. Rev. Immunol. 2023;23:271. doi: 10.1038/s41577-023-00876-8. [DOI] [PubMed] [Google Scholar]
- 514.Popoviciu MS, et al. Type 1 diabetes mellitus and autoimmune diseases: a critical review of the association and the application of personalized medicine. J. Pers. Med. 2023;13:422. doi: 10.3390/jpm13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Pugliese A. Autoreactive T cells in type 1 diabetes. J. Clin. Invest. 2017;127:2881–2891. doi: 10.1172/JCI94549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Feng N, Vegh P, Rothenberg EV, Yui MA. Lineage divergence at the first TCR-dependent checkpoint: preferential γδ and impaired αβ T cell development in nonobese diabetic mice. J. Immunol. 2011;186:826–837. doi: 10.4049/jimmunol.1002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Godfrey DI, Kinder SJ, Silvera P, Baxter AG. Flow cytometric study of T cell development in NOD mice reveals a deficiency in alphabetaTCR+CDR-CD8- thymocytes. J. Autoimmun. 1997;10:279–285. doi: 10.1006/jaut.1997.0129. [DOI] [PubMed] [Google Scholar]
- 518.Mingueneau M, et al. Thymic negative selection is functional in NOD mice. J. Exp. Med. 2012;209:623–637. doi: 10.1084/jem.20112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Zhang L, et al. Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9-23. J. Autoimmun. 2010;34:478–484. doi: 10.1016/j.jaut.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Kretowski A, et al. Gammadelta T-cells alterations in the peripheral blood of high risk diabetes type 1 subjects with subclinical pancreatic B-cells impairment. Immunol. Lett. 1999;68:289–293. doi: 10.1016/S0165-2478(99)00066-8. [DOI] [PubMed] [Google Scholar]
- 521.Lang FP, et al. The temporal association between gamma delta T cells and the natural history of insulin-dependent diabetes. J. Autoimmun. 1993;6:107–119. doi: 10.1006/jaut.1993.1009. [DOI] [PubMed] [Google Scholar]
- 522.Zubkiewicz-Kucharska A, Noczyńska A. Abnormal distribution of gamma-delta T lymphocytes and their subsets in type 1 diabetes. Adv. Clin. Exp. Med. 2016;25:665–671. doi: 10.17219/acem/60714. [DOI] [PubMed] [Google Scholar]
- 523.Han G, et al. Interleukin-17-producing gammadelta+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-beta. Immunology. 2010;129:197–206. doi: 10.1111/j.1365-2567.2009.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Markle JG, et al. γδ T cells are essential effectors of type 1 diabetes in the nonobese diabetic mouse model. J. Immunol. 2013;190:5392–5401. doi: 10.4049/jimmunol.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.O’Brien RL, et al. A distinctive γδ T cell repertoire in NOD mice weakens immune regulation and favors diabetic disease. Biomolecules. 2022;12:1406. doi: 10.3390/biom12101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Buysschaert M, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal γδ T-cell number: a prospective case-series study. Br. J. Dermatol. 2014;171:155–161. doi: 10.1111/bjd.12886. [DOI] [PubMed] [Google Scholar]
- 527.Mu X, et al. Glucose metabolism controls human gammadelta T-cell-mediated tumor immunosurveillance in diabetes. Cell Mol. Immunol. 2022;19:944–956. doi: 10.1038/s41423-022-00894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Zheng L, et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. 2021;374:abe6474. doi: 10.1126/science.abe6474. [DOI] [PubMed] [Google Scholar]
- 529.Lopes N, et al. Distinct metabolic programs established in the thymus control effector functions of gammadelta T cell subsets in tumor microenvironments. Nat. Immunol. 2021;22:179–192. doi: 10.1038/s41590-020-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Jojic V, et al. Identification of transcriptional regulators in the mouse immune system. Nat. Immunol. 2013;14:633–643. doi: 10.1038/ni.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Li Y, Wu Y, Hu Y. Metabolites in the tumor microenvironment reprogram functions of immune effector cells through epigenetic modifications. Front. Immunol. 2021;12:641883. doi: 10.3389/fimmu.2021.641883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Mukasa R, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Park JH, et al. Tumor hypoxia represses gammadelta T cell-mediated antitumor immunity against brain tumors. Nat. Immunol. 2021;22:336–346. doi: 10.1038/s41590-020-00860-7. [DOI] [PubMed] [Google Scholar]
- 534.Siegers GM, Dutta I, Lai R, Postovit LM. Functional plasticity of gamma delta T cells and breast tumor targets in hypoxia. Front. Immunol. 2018;9:1367. doi: 10.3389/fimmu.2018.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Franchina DG, Dostert C, Brenner D. Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol. 2018;39:489–502. doi: 10.1016/j.it.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 536.Mensurado S, et al. Tumor-associated neutrophils suppress pro-tumoral IL-17+ gammadelta T cells through induction of oxidative stress. PLoS Biol. 2018;16:e2004990. doi: 10.1371/journal.pbio.2004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat. Rev. Cancer. 2022;22:280–297. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 538.Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Li L, et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral gammadelta T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38:2830–2843. doi: 10.1038/s41388-018-0627-z. [DOI] [PubMed] [Google Scholar]
- 540.Wang X, et al. Exosomes derived from Vdelta2-T cells control Epstein-Barr virus-associated tumors and induce T cell antitumor immunity. Sci. Transl. Med. 2020;12:eaaz3426. doi: 10.1126/scitranslmed.aaz3426. [DOI] [PubMed] [Google Scholar]
- 541.Yi Y, et al. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J. Hepatol. 2013;58:977–983. doi: 10.1016/j.jhep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 542.Sabbione F, et al. Neutrophils suppress gammadelta T-cell function. Eur. J. Immunol. 2014;44:819–830. doi: 10.1002/eji.201343664. [DOI] [PubMed] [Google Scholar]
- 543.Kalyan S, et al. Neutrophil uptake of nitrogen-bisphosphonates leads to the suppression of human peripheral blood gammadelta T cells. Cell Mol. Life Sci. 2014;71:2335–2346. doi: 10.1007/s00018-013-1495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Oberg HH, Wesch D, Kalyan S, Kabelitz D. Regulatory interactions between neutrophils, tumor cells and T cells. Front. Immunol. 2019;10:1690. doi: 10.3389/fimmu.2019.01690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Sacchi A, et al. Myeloid-derived suppressor cells specifically suppress IFN-gamma production and antitumor cytotoxic activity of Vdelta2 T cells. Front. Immunol. 2018;9:1271. doi: 10.3389/fimmu.2018.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Wesch D, Kabelitz D, Oberg HH. Tumor resistance mechanisms and their consequences on gammadelta T cell activation. Immunol. Rev. 2020;298:84–98. doi: 10.1111/imr.12925. [DOI] [PubMed] [Google Scholar]
- 547.Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002–1014. doi: 10.1016/S0140-6736(21)01206-X. [DOI] [PubMed] [Google Scholar]
- 548.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022;19:775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Demaria O, et al. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 550.Edwards SC, et al. PD-1 and TIM-3 differentially regulate subsets of mouse IL-17A-producing γδ T cells. J. Exp. Med. 2023;220:e20211431. doi: 10.1084/jem.20211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Rancan C, et al. Exhausted intratumoral Vδ2(-) γδ T cells in human kidney cancer retain effector function. Nat. Immunol. 2023;24:612–624. doi: 10.1038/s41590-023-01448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Brauneck F, et al. Bone marrow-resident Vδ1 T cells co-express TIGIT with PD-1, TIM-3 or CD39 in AML and Myeloma. Front. Med. 2021;8:763773. doi: 10.3389/fmed.2021.763773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Gertner-Dardenne J, et al. The co-receptor BTLA negatively regulates human Vγ9Vδ2 T-cell proliferation: a potential way of immune escape for lymphoma cells. Blood. 2013;122:922–931. doi: 10.1182/blood-2012-11-464685. [DOI] [PubMed] [Google Scholar]
- 554.Bekiaris V, et al. The inhibitory receptor BTLA controls γδ T cell homeostasis and inflammatory responses. Immunity. 2013;39:1082–1094. doi: 10.1016/j.immuni.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Lu H, et al. B7-H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology. 2020;9:1748991. doi: 10.1080/2162402X.2020.1748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Schofield L, et al. Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of γδ T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea. BMC Med. 2017;15:114. doi: 10.1186/s12916-017-0883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Zhang N, et al. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023;13:82. doi: 10.1038/s41408-023-00853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Brody H. Multiple myeloma. Nature. 2011;480:S33. doi: 10.1038/480S33a. [DOI] [PubMed] [Google Scholar]
- 559.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Papaemmanuil E, et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016;374:2209–2221. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Bejar R, et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Gaidzik VI, et al. RUNX1 mutations in acute myeloid leukemia are associated with distinct clinico-pathologic and genetic features. Leukemia. 2016;30:2160–2168. doi: 10.1038/leu.2016.126. [DOI] [PubMed] [Google Scholar]
- 563.Taylor J, Xiao W, Abdel-Wahab O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood. 2017;130:410–423. doi: 10.1182/blood-2017-02-734541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Metzeler KH, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN Favorable genetic category. Blood. 2011;118:6920–6929. doi: 10.1182/blood-2011-08-368225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Turner MC, et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020;70:460–479. doi: 10.3322/caac.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Pasqual E, et al. Association between radioactive iodine treatment for pediatric and young adulthood differentiated thyroid cancer and risk of second primary malignancies. J. Clin. Oncol. 2022;40:1439–1449. doi: 10.1200/JCO.21.01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Walter RB, Buckley SA, White E. Regular recreational physical activity and risk of hematologic malignancies: results from the prospective VITamins And lifestyle (VITAL) study. Ann. Oncol. 2013;24:1370–1377. doi: 10.1093/annonc/mds631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Kimani SM, et al. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV. 2020;7:e641–e651. doi: 10.1016/S2352-3018(20)30118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Noy A. HIV and lymphoma: from oncological futility to treatment. Lancet HIV. 2020;7:e598–e600. doi: 10.1016/S2352-3018(20)30227-7. [DOI] [PubMed] [Google Scholar]
- 570.Hemminki K, et al. Autoimmune diseases and hematological malignancies: exploring the underlying mechanisms from epidemiological evidence. Semin Cancer Biol. 2020;64:114–121. doi: 10.1016/j.semcancer.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 571.Ramadan SM, et al. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012;97:805–817. doi: 10.3324/haematol.2011.056283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Verhoeven D, Stoppelenburg AJ, Meyer-Wentrup F, Boes M. Increased risk of hematologic malignancies in primary immunodeficiency disorders: opportunities for immunotherapy. Clin. Immunol. 2018;190:22–31. doi: 10.1016/j.clim.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 573.Zhao H, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Sig. Transduct. Target Ther. 2021;6:263. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Craver BM, El Alaoui K, Scherber RM, Fleischman AG. The critical role of inflammation in the pathogenesis and progression of myeloid malignancies. Cancers (Basel). 2018;10:104. doi: 10.3390/cancers10040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Pulte D, Jansen L, Brenner H. Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century. Blood Cancer J. 2020;10:56. doi: 10.1038/s41408-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Rey J, et al. Natural killer and gammadelta T cells in haematological malignancies: enhancing the immune effectors. Trends Mol. Med. 2009;15:275–284. doi: 10.1016/j.molmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 577.Gertner-Dardenne J, et al. Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J. Immunol. 2012;188:4701–4708. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 578.Gomes AQ, et al. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood gammadelta T cells. Haematologica. 2010;95:1397–1404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Gundermann S, et al. A comprehensive analysis of primary acute myeloid leukemia identifies biomarkers predicting susceptibility to human allogeneic Vgamma9Vdelta2 T cells. J. Immunother. 2014;37:321–330. doi: 10.1097/CJI.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 580.Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy. 2012;14:1110–1118. doi: 10.3109/14653249.2012.700766. [DOI] [PubMed] [Google Scholar]
- 581.Bensussan A, Lagabrielle JF, Degos L. TCR gamma delta bearing lymphocyte clones with lymphokine-activated killer activity against autologous leukemic cells. Blood. 1989;73:2077–2080. doi: 10.1182/blood.V73.8.2077.2077. [DOI] [PubMed] [Google Scholar]
- 582.Schonefeldt S, et al. The diverse roles of gammadelta T cells in cancer: from rapid immunity to aggressive lymphoma. Cancers (Basel). 2021;13:6212. doi: 10.3390/cancers13246212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Fournie JJ, et al. What lessons can be learned from gammadelta T cell-based cancer immunotherapy trials? Cell Mol. Immunol. 2013;10:35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Kotian P, Boloor A, Sreenivasan S. Study of adverse effect profile of parenteral zoledronic acid in female patients with osteoporosis. J. Clin. Diagn. Res. 2016;10:OC04-06. doi: 10.7860/JCDR/2016/17061.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Jackson C, Freeman ALJ, Szlamka Z, Spiegelhalter DJ. The adverse effects of bisphosphonates in breast cancer: a systematic review and network meta-analysis. PLoS One. 2021;16:e0246441. doi: 10.1371/journal.pone.0246441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Dieli F, et al. Targeting human gammadelta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Nicol AJ, et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Kobayashi H, et al. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Merli P, et al. Immune modulation properties of zoledronic acid on TcRgammadelta T-lymphocytes after TcRalphabeta/CD19-depleted haploidentical stem cell transplantation: an analysis on 46 pediatric patients affected by acute leukemia. Front. Immunol. 2020;11:699. doi: 10.3389/fimmu.2020.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 591.Shlomchik WD. Graft-versus-host disease. Nat. Rev. Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 592.Godder KT, et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39:751–757. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]
- 593.Lamb LS, Jr., et al. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J. Hematother. 1996;5:503–509. doi: 10.1089/scd.1.1996.5.503. [DOI] [PubMed] [Google Scholar]
- 594.Lamb LS, Jr., et al. Influence of T cell depletion method on circulating gammadelta T cell reconstitution and potential role in the graft-versus-leukemia effect. Cytotherapy. 1999;1:7–19. doi: 10.1080/0032472031000141295. [DOI] [PubMed] [Google Scholar]
- 595.Perko R, et al. Gamma delta T cell reconstitution is associated with fewer infections and improved event-free survival after hematopoietic stem cell transplantation for pediatric leukemia. Biol. Blood Marrow Transplant. 2015;21:130–136. doi: 10.1016/j.bbmt.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Airoldi I, et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125:2349–2358. doi: 10.1182/blood-2014-09-599423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Locatelli F, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood. 2017;130:677–685. doi: 10.1182/blood-2017-04-779769. [DOI] [PubMed] [Google Scholar]
- 598.de Witte MA, et al. alphabeta T-cell graft depletion for allogeneic HSCT in adults with hematological malignancies. Blood Adv. 2021;5:240–249. doi: 10.1182/bloodadvances.2020002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Handgretinger R, Schilbach K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood. 2018;131:1063–1072. doi: 10.1182/blood-2017-08-752162. [DOI] [PubMed] [Google Scholar]
- 600.Minculescu L, et al. Granulocyte colony-stimulating factor effectively mobilizes TCR gammadelta and NK cells providing an allograft potentially enhanced for the graft-versus-leukemia effect for allogeneic stem cell transplantation. Front. Immunol. 2021;12:625165. doi: 10.3389/fimmu.2021.625165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Minculescu L, et al. Improved overall survival, relapse-free-survival, and less graft-vs.-host-disease in patients with high immune reconstitution of TCR gamma delta cells 2 months after allogeneic stem cell transplantation. Front. Immunol. 2019;10:1997. doi: 10.3389/fimmu.2019.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Vydra J, et al. A phase I trial of allogeneic gammadelta T lymphocytes from haploidentical donors in patients with refractory or relapsed acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2023;23:e232–e239. doi: 10.1016/j.clml.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Wilhelm M, et al. Successful adoptive transfer and in vivo expansion of haploidentical gammadelta T cells. J. Transl. Med. 2014;12:45. doi: 10.1186/1479-5876-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Hoard R, Shahin G, Andreca FD, Osswald M. Gamma-delta T-cell lymphoma following allogeneic stem cell transplant for primary myelofibrosis. Cureus. 2020;12:e10301. doi: 10.7759/cureus.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Pro B, Allen P, Behdad A. Hepatosplenic T-cell lymphoma: a rare but challenging entity. Blood. 2020;136:2018–2026. doi: 10.1182/blood.2019004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Song W, et al. Single cell profiling of gammadelta hepatosplenic T-cell lymphoma unravels tumor cell heterogeneity associated with disease progression. Cell Oncol. (Dordr) 2023;46:211–226. doi: 10.1007/s13402-022-00745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Makkouk A, et al. Off-the-shelf Vdelta1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer. 2021;9:e003441. doi: 10.1136/jitc-2021-003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Ramani NS, et al. Primary cutaneous gamma-delta (gamma/delta) T-cell lymphoma: an unusual case with very subtle histopathological findings. Am. J. Dermatopathol. 2016;38:e147–e149. doi: 10.1097/DAD.0000000000000608. [DOI] [PubMed] [Google Scholar]
- 609.Muhsen IN, et al. Clinical, diagnostic and prognostic characteristics of primary cutaneous gamma delta T-cell lymphomas. Clin. Hematol. Int. 2022;4:1–10. doi: 10.1007/s44228-022-00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Chen S, et al. The evolution of malignant and reactive gammadelta + T cell clones in a relapse T-ALL case after allogeneic stem cell transplantation. Mol. Cancer. 2013;12:73. doi: 10.1186/1476-4598-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Wang W, et al. Gamma-delta T-cell acute lymphoblastic leukemia/lymphoma: immunophenotype of three adult cases. J. Hematol. 2019;8:137–140. doi: 10.14740/jh535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Mirji G, Banavali S, Sengar M, Chiplunkar SV. Immunophenotype and function define TCRgammadelta + T-ALL as a distinct subgroup from TCRalphabeta + T-ALL patients. Leuk. Lymphoma. 2020;61:108–117. doi: 10.1080/10428194.2019.1650174. [DOI] [PubMed] [Google Scholar]
- 613.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Myers RM, et al. Humanized CD19-targeted chimeric antigen receptor (CAR) T cells in CAR-naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J. Clin. Oncol. 2021;39:3044–3055. doi: 10.1200/JCO.20.03458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front. Immunol. 2020;11:1973. doi: 10.3389/fimmu.2020.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Santomasso BD, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–971. doi: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Neelapu SS, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Parker KR, et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell. 2020;183:126–142 e117. doi: 10.1016/j.cell.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Deniger DC, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous gammadelta T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol. Ther. 2013;21:638–647. doi: 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Rozenbaum M, et al. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia. Front. Immunol. 2020;11:1347. doi: 10.3389/fimmu.2020.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Sanchez Martinez D, et al. Generation and proof-of-concept for allogeneic CD123 CAR-Delta One T (DOT) cells in acute myeloid leukemia. J. Immunother. Cancer. 2022;10:e005400. doi: 10.1136/jitc-2022-005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Nishimoto KP, et al. Allogeneic CD20-targeted gammadelta T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin Transl Immunology. 2022;11:e1373. doi: 10.1002/cti2.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Aoki T, et al. Single-cell transcriptome analysis reveals disease-defining T-cell subsets in the tumor microenvironment of classic Hodgkin lymphoma. Cancer Discov. 2020;10:406–421. doi: 10.1158/2159-8290.CD-19-0680. [DOI] [PubMed] [Google Scholar]
- 630.Zavidij O, et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer. 2020;1:493–506. doi: 10.1038/s43018-020-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Liu R, et al. Co-evolution of tumor and immune cells during progression of multiple myeloma. Nat. Commun. 2021;12:2559. doi: 10.1038/s41467-021-22804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Altvater B, et al. Activated human gammadelta T cells induce peptide-specific CD8 + T-cell responses to tumor-associated self-antigens. Cancer Immunol. Immunother. 2012;61:385–396. doi: 10.1007/s00262-011-1111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.De Gassart A, et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vgamma9Vdelta2 T cell-mediated antitumor immune response. Sci. Transl. Med. 2021;13:eabj0835. doi: 10.1126/scitranslmed.abj0835. [DOI] [PubMed] [Google Scholar]
- 634.Oberg HH, et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014;74:1349–1360. doi: 10.1158/0008-5472.CAN-13-0675. [DOI] [PubMed] [Google Scholar]
- 635.Ganesan R, et al. Selective recruitment of gammadelta T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia. 2021;35:2274–2284. doi: 10.1038/s41375-021-01122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.de Bruin RCG, et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vgamma9Vdelta2-T cells. Oncoimmunology. 2017;7:e1375641. doi: 10.1080/2162402X.2017.1375641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.de Weerdt I, et al. A bispecific antibody antagonizes prosurvival CD40 signaling and promotes Vgamma9Vdelta2 T cell-mediated antitumor responses in human B-cell malignancies. Cancer Immunol. Res. 2021;9:50–61. doi: 10.1158/2326-6066.CIR-20-0138. [DOI] [PubMed] [Google Scholar]
- 638.de Weerdt I, et al. A bispecific single-domain antibody boosts autologous Vgamma9Vdelta2-T cell responses toward CD1d in chronic lymphocytic leukemia. Clin. Cancer Res. 2021;27:1744–1755. doi: 10.1158/1078-0432.CCR-20-4576. [DOI] [PubMed] [Google Scholar]
- 639.van Diest E, et al. Gamma delta TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J. Immunother. Cancer. 2021;9:e003850. doi: 10.1136/jitc-2021-003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Lameris R, et al. A bispecific T cell engager recruits both type 1 NKT and Vgamma9Vdelta2-T cells for the treatment of CD1d-expressing hematological malignancies. Cell Rep. Med. 2023;4:100961. doi: 10.1016/j.xcrm.2023.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Garber K. gammadelta T cells bring unconventional cancer-targeting to the clinic - again. Nat. Biotechnol. 2020;38:389–391. doi: 10.1038/s41587-020-0487-2. [DOI] [PubMed] [Google Scholar]
- 642.Almeida AR, et al. Delta one T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/differentiation and preclinical proof of concept. Clin. Cancer Res. 2016;22:5795–5804. doi: 10.1158/1078-0432.CCR-16-0597. [DOI] [PubMed] [Google Scholar]
- 643.Di Lorenzo B, et al. Broad cytotoxic targeting of acute myeloid leukemia by polyclonal delta one T cells. Cancer Immunol. Res. 2019;7:552–558. doi: 10.1158/2326-6066.CIR-18-0647. [DOI] [PubMed] [Google Scholar]
- 644.Deniger DC, et al. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin. Cancer Res. 2014;20:5708–5719. doi: 10.1158/1078-0432.CCR-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Ang WX, et al. Electroporation of NKG2D RNA CAR improves Vgamma9Vdelta2 T cell responses against human solid tumor xenografts. Mol. Ther. Oncolytics. 2020;17:421–430. doi: 10.1016/j.omto.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Zhai X, et al. MUC1-Tn-targeting chimeric antigen receptor-modified Vgamma9Vdelta2 T cells with enhanced antigen-specific anti-tumor activity. Am. J. Cancer Res. 2021;11:79–91. [PMC free article] [PubMed] [Google Scholar]
- 647.Marcu-Malina V, et al. Redirecting alphabeta T cells against cancer cells by transfer of a broadly tumor-reactive gammadeltaT-cell receptor. Blood. 2011;118:50–59. doi: 10.1182/blood-2010-12-325993. [DOI] [PubMed] [Google Scholar]
- 648.Straetemans T, et al. GMP-grade manufacturing of T cells engineered to express a defined gammadeltaTCR. Front. Immunol. 2018;9:1062. doi: 10.3389/fimmu.2018.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Johanna I, et al. Evaluating in vivo efficacy - toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J. Immunother. Cancer. 2019;7:69. doi: 10.1186/s40425-019-0558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Gao Z, et al. Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment. Mol. Cancer. 2023;22:31. doi: 10.1186/s12943-023-01722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Ran GH, et al. Natural killer cell homing and trafficking in tissues and tumors: from biology to application. Sig. Transduct. Target Ther. 2022;7:205. doi: 10.1038/s41392-022-01058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Berrien-Elliott MM, Jacobs MT, Fehniger TA. Allogeneic natural killer cell therapy. Blood. 2023;141:856–868. doi: 10.1182/blood.2022016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Handgretinger R, Schilbach K. The potential role of γδ T cells after allogeneic HCT for leukemia. Blood. 2018;131:1063–1072. doi: 10.1182/blood-2017-08-752162. [DOI] [PubMed] [Google Scholar]
- 654.Gong Y, et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021;14:73. doi: 10.1186/s13045-021-01083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Ryan PL, et al. Heterogeneous yet stable Vdelta2(+) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc. Natl Acad. Sci. USA. 2016;113:14378–14383. doi: 10.1073/pnas.1611098113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Willcox CR, et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012;13:872–879. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 657.Halary F, et al. Shared reactivity of Vdelta2(neg) gammadelta T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Scheper W, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27:1328–1338. doi: 10.1038/leu.2012.374. [DOI] [PubMed] [Google Scholar]
- 659.Hammerich L, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. 2014;59:630–642. doi: 10.1002/hep.26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54:859–874. doi: 10.1016/j.immuni.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Mohseni AH, et al. Potential links between the microbiota and T cell immunity determine the tumor cell fate. Cell Death Dis. 2023;14:154. doi: 10.1038/s41419-023-05560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Legoux F, Salou M, Lantz O. MAIT cell development and functions: the microbial connection. Immunity. 2020;53:710–723. doi: 10.1016/j.immuni.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 664.Legoux F, et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366:494–499. doi: 10.1126/science.aaw2719. [DOI] [PubMed] [Google Scholar]
- 665.Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science380, eabo2296, (2023). [DOI] [PubMed]
- 666.Ji L, Hu X. Sweet memories of 8 empowered by butyrate. Immunity. 2019;51:201–203. doi: 10.1016/j.immuni.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 667.Bachem A, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8( + ) T cells. Immunity. 2019;51:285–297 e285. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 668.Schiering C, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Kim E, et al. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4(+) T cells. Immunity. 2022;55:145–158.e147. doi: 10.1016/j.immuni.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Ramanan D, et al. Regulatory T cells in the face of the intestinal microbiota. Nat. Rev. Immunol. 2023;23:1–14. doi: 10.1038/s41577-023-00890-w. [DOI] [PubMed] [Google Scholar]
- 671.Haghikia A, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022;43:518–533. doi: 10.1093/eurheartj/ehab644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Sun M, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Woo V, et al. Commensal segmented filamentous bacteria-derived retinoic acid primes host defense to intestinal infection. Cell Host Microbe. 2021;29:1744–1756 e1745. doi: 10.1016/j.chom.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Kim M, Kim CH. Regulation of humoral immunity by gut microbial products. Gut Microbes. 2017;8:392–399. doi: 10.1080/19490976.2017.1299311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Brown EM, Kenny DJ, Xavier RJ. Gut microbiota regulation of T cells during inflammation and autoimmunity. Annu. Rev. Immunol. 2019;37:599–624. doi: 10.1146/annurev-immunol-042718-041841. [DOI] [PubMed] [Google Scholar]
- 677.Miyauchi E, et al. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2023;23:9–23. doi: 10.1038/s41577-022-00727-y. [DOI] [PubMed] [Google Scholar]
- 678.Huang XZ, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8:e1665973. doi: 10.1080/2162402X.2019.1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Routy B, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 680.Pinato DJ, et al. Association between antibiotics and adverse oncological outcomes in patients receiving targeted or immune-based therapy for hepatocellular carcinoma. JHEP Rep. 2023;5:100747. doi: 10.1016/j.jhepr.2023.100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Hayase E, Jenq RR. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021;13:107. doi: 10.1186/s13073-021-00923-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Derosa L, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 2020;78:195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 683.Chalabi M, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 684.Pinato DJ, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Elkrief A, et al. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann. Oncol. 2019;30:1572–1579. doi: 10.1093/annonc/mdz206. [DOI] [PubMed] [Google Scholar]
- 686.Spencer CN, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Vetizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Sivan A, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Bender MJ, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862.e1826. doi: 10.1016/j.cell.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Baruch EN, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 691.Davar D, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Halsey TM, et al. Microbiome alteration via fecal microbiota transplantation is effective for refractory immune checkpoint inhibitor-induced colitis. Sci. Transl. Med. 2023;15:eabq4006. doi: 10.1126/scitranslmed.abq4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Fernandes MR, et al. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer. 2022;22:703–722. doi: 10.1038/s41568-022-00513-x. [DOI] [PubMed] [Google Scholar]
- 694.Derosa L, et al. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 2021;11:2396–2412. doi: 10.1158/2159-8290.CD-21-0236. [DOI] [PubMed] [Google Scholar]
- 695.Wang Y, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018;24:1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Smith M, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022;28:713–723. doi: 10.1038/s41591-022-01702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Stein-Thoeringer CK, et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 2023;29:906–916. doi: 10.1038/s41591-023-02234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Li Y, et al. Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent gammadelta T cells. Gut Microbes. 2022;14:2096994. doi: 10.1080/19490976.2022.2096994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Ganapathy T, Radhakrishnan R, Sakshi S, Martin S. CAR γδ T cells for cancer immunotherapy. Is the field more yellow than green? Cancer Immunol. Immunother. 2023;72:277–286. doi: 10.1007/s00262-022-03260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Wang Y, et al. CAR-modified Vγ9Vδ2 T cells propagated using a novel bisphosphonate prodrug for allogeneic adoptive immunotherapy. Int. J. Mol. Sci. 2023;24:10873. doi: 10.3390/ijms241310873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Lamb LS, Jr., et al. Engineered drug resistant γδ T cells kill glioblastoma cell lines during a chemotherapy challenge: a strategy for combining chemo- and immunotherapy. PloS One. 2013;8:e51805. doi: 10.1371/journal.pone.0051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Lamb LS, et al. A combined treatment regimen of MGMT-modified γδ T cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci. Rep. 2021;11:21133. doi: 10.1038/s41598-021-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Goswami T, et al. INB-200 phase I study of gene modified autologous gamma-delta (γδ) T cells in patients with newly diagnosed glioblastoma multiforme (GBM) receiving maintenance temozolomide (TMZ) J. Clin. Oncol. 2023;41:2007–2007. doi: 10.1200/JCO.2023.41.16_suppl.2007. [DOI] [Google Scholar]
- 704.Saura-Esteller J, et al. Gamma delta T-cell based cancer immunotherapy: past-present-future. Front. Immunol. 2022;13:915837. doi: 10.3389/fimmu.2022.915837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Guo Q, et al. TIM-3 blockade combined with bispecific antibody MT110 enhances the anti-tumor effect of γδ T cells. Cancer Immunol. Immunother. 2020;69:2571–2587. doi: 10.1007/s00262-020-02638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Dong R, Zhang Y, Xiao H, Zeng X. Engineering γδ T cells: recognizing and activating on their own way. Front. Immunol. 2022;13:889051. doi: 10.3389/fimmu.2022.889051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Li C, et al. Novel CD19-specific γ/δ TCR-T cells in relapsed or refractory diffuse large B-cell lymphoma. J. Hematol. Oncol. 2023;16:5. doi: 10.1186/s13045-023-01402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol. Immunother. 2007;56:1285–1297. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Todaro M, et al. Combining conventional chemotherapy and gammadelta T cell-based immunotherapy to target cancer-initiating cells. Oncoimmunology. 2013;2:e25821. doi: 10.4161/onci.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Lai AY, et al. Cutting Edge: bispecific gammadelta T cell engager containing heterodimeric BTN2A1 and BTN3A1 promotes targeted activation of Vgamma9Vdelta2(+) T cells in the presence of costimulation by CD28 or NKG2D. J. Immunol. 2022;209:1475–1480. doi: 10.4049/jimmunol.2200185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Antushevich H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta. 2020;503:90–98. doi: 10.1016/j.cca.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 712.Ting NL, Lau HC, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71:1412–1425. doi: 10.1136/gutjnl-2021-326264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Park EM, et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022;28:690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 714.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019;16:690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 715.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 716.Gerber DJ, et al. IL-4-producing γδ T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J. Immunol. 1999;163:3076–3082. doi: 10.4049/jimmunol.163.6.3076. [DOI] [PubMed] [Google Scholar]
- 717.Takagaki Y, et al. T cell receptor-gamma and-delta genes preferentially utilized by adult thymocytes for the surface expression. J. Immunol. 1989;142:2112–2121. doi: 10.4049/jimmunol.142.6.2112. [DOI] [PubMed] [Google Scholar]
- 718.Itohara S, et al. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc. Natl Acad. Sci. USA. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Fu Y-X, et al. In vivo response of murine gamma delta T cells to a heat shock protein-derived peptide. Proc. Natl Acad. Sci. USA. 1993;90:322–326. doi: 10.1073/pnas.90.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.O’brien RL, et al. Heat shock protein Hsp60-reactive gamma delta cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc. Natl Acad. Sci. USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Wesch D, Hinz T, Kabelitz D. Analysis of the TCR Vgamma repertoire in healthy donors and HIV-1-infected individuals. Int. Immunol. 1998;10:1067–1075. doi: 10.1093/intimm/10.8.1067. [DOI] [PubMed] [Google Scholar]
- 722.Porcelli S, et al. Recognition of cluster of differentiation 1 antigens by human CD4 − CD8 > − cytolytic T lymphocyte. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 723.Faure F, Jitsukawa S, Miossec C, Hercend T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood γ/δ cells. Eur. J. Immunol. 1990;20:703–706. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- 724.Vincent MS, et al. Apoptosis of Fashigh CD4+ synovial T cells by Borrelia-reactive Fas-ligandhigh γδ T cells in Lyme arthritis. J. Exp. Med. 1996;184:2109–2118. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Vincent MS, et al. Lyme arthritis synovial γδ T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J. Immunol. 1998;161:5762–5771. doi: 10.4049/jimmunol.161.10.5762. [DOI] [PubMed] [Google Scholar]
- 726.Bluestone J, et al. Repertoire development and ligand specificity of murine TCR gamma delta cells. Immunol. Rev. 1991;120:5–33. doi: 10.1111/j.1600-065X.1991.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 727.Schild H, et al. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 728.Bottino C, et al. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J. Exp. Med. 1988;168:491–505. doi: 10.1084/jem.168.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Constant P, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 730.Rust CJ, Verreck F, Vietor H, Koning F. Specific recognition of staphylococcal enterotoxin A by human T cells bearing receptors with the Vγ9 region. Nature. 1990;346:572–574. doi: 10.1038/346572a0. [DOI] [PubMed] [Google Scholar]
- 731.Loh EY, et al. Gene transfer studies of T cell receptor-gamma delta recognition. Specificity for staphylococcal enterotoxin A is conveyed by V gamma 9 alone. J. Immunol. 1994;152:3324–3332. doi: 10.4049/jimmunol.152.7.3324. [DOI] [PubMed] [Google Scholar]
- 732.Khairallah C, et al. A blend of broadly-reactive and pathogen-selected Vγ4 Vδ1 T cell receptors confer broad bacterial reactivity of resident memory γδ T cells. Mucosal. Immunol. 2022;15:176–187. doi: 10.1038/s41385-021-00447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu. Rev. Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 734.Kabelitz D, et al. Clonal expansion of Vγ3/Vδ3‐expressing γδ T cells in an HIV‐1/2‐negative patient with CD4 T‐cell deficiency. Br. J. Haematol. 1997;96:266–271. doi: 10.1046/j.1365-2141.1997.d01-2027.x. [DOI] [PubMed] [Google Scholar]
- 735.Kenna T, et al. Distinct subpopulations of γδ T cells are present in normal and tumor-bearing human liver. Clin Immunol. 2004;113:56–63. doi: 10.1016/j.clim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 736.van der Heiden M, et al. Characterization of the γδ T‐cell compartment during infancy reveals clear differences between the early neonatal period and 2 years of age. Immunol Cell Biol. 2020;98:79–87. doi: 10.1111/imcb.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Mangan BA, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J. Immunol. 2013;191:30–34. doi: 10.4049/jimmunol.1300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Asarnow DM, et al. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 739.Havran WL, Chien Y-H, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 740.Goodman T, Lefrançois L. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 741.Bonneville M, et al. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature. 1988;336:479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- 742.Lafaille JJ, et al. Junctional sequences of T cell receptor γδ genes: implications for γδ T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;59:859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- 743.Bandeira A, et al. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc. Natl Acad. Sci. USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Guy-Grand D, et al. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Kyes S, et al. Diversity in T-cell receptor gamma gene usage in intestinal epithelium. Proc. Natl Acad. Sci. USA. 1989;86:5527–5531. doi: 10.1073/pnas.86.14.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Prinz I, Silva-Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur. J. Immunol. 2013;43:1988–1994. doi: 10.1002/eji.201343759. [DOI] [PubMed] [Google Scholar]
- 747.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 748.Hu Y, et al. Apoptosis, pyroptosis, and ferroptosis conspiringly induce immunosuppressive hepatocellular carcinoma microenvironment and gammadelta T-cell imbalance. Front. Immunol. 2022;13:845974. doi: 10.3389/fimmu.2022.845974. [DOI] [PMC free article] [PubMed] [Google Scholar]