Abstract
Arabidopsis thaliana plants with null mutations in the genes encoding the α and β subunits of the single heterotrimeric G protein are less and more sensitive, respectively, to O3 damage than wild-type Columbia-0 plants. The first peak of the bimodal oxidative burst elicited by O3 in wild-type plants is almost entirely missing in both mutants. The late peak is normal in plants lacking the Gβ protein but missing in plants lacking the Gα protein. Endogenous reactive oxygen species (ROS) are first detectable in chloroplasts of leaf epidermal guard cells. ROS production in adjacent cells is triggered by extracellular ROS signals produced by guard cell membrane-associated NADPH oxidases encoded by the AtrbohD and AtrbohF genes. The late, tissue damage–associated component of the oxidative burst requires only the Gα protein and arises from multiple cellular sources. The early component of the oxidative burst, arising primarily from chloroplasts, requires signaling through the heterotrimer (or the Gβγ complex) and is separable from Gα-mediated activation of membrane-bound NADPH oxidases necessary for both intercellular signaling and cell death.
INTRODUCTION
The oxidative burst, a transient increase in reactive oxygen species (ROS), predominantly superoxide (˙O2−) and hydrogen peroxide (H2O2), is among the first biochemical responses of plants to pathogen attack and abiotic stress (Mehdy, 1994; Lamb and Dixon, 1997; Alvarez et al., 1998; Baker and Orlandi, 1999). Although the deleterious effects of ROS have long been known, recognition of their role in cell signaling and regulation of gene expression is relatively recent and still poorly understood (Sauer et al., 2001; Droge, 2002; Ermak and Davies, 2002). The phytohormones auxin and abscisic acid (ABA) elicit the production of ROS (Pei et al., 2000; Joo et al., 2001; Zhang et al., 2001; Schopfer et al., 2002), as does a large variety of abiotic stresses, including extremes of temperature, high light levels, water deficit, herbicides, cycloheximide, amines, and air pollutants, such as ozone (O3), SO2, and NO2 (Allan and Fluhr, 1997; Tenhaken and Rubel, 1998; Scheel, 2002). ROS are essential for auxin and ABA signaling as well as for activating stress and defense responses (Levine et al., 1994; Alvarez et al., 1998; Pei et al., 2000; Joo et al., 2001; Zhang et al., 2001; Scheel, 2002). O3 represents an oxidative stress to living organisms and is a major atmospheric pollutant that damages crops, forests, and urban vegetation (Runeckles and Chevonne, 1992). The plant's response to O3 resembles the biotic defense response and includes a biphasic oxidative burst and induction of the hypersensitive response and systemic acquired resistance (Conklin and Last, 1995; Sharma et al., 1996; Sharma and Davis, 1997; Sandermann et al., 1998; Rao et al., 2000; Sandermann, 2000).
The emerging picture of the plant oxidative burst is complex (Allan and Fluhr, 1997; Overmyer et al., 2000; Scheel, 2002). There are multiple enzymatic sources of ROS in plants, both extracellular and intracellular, including cell wall peroxidases and amine oxidases, plasma membrane–bound NADPH oxidases, and intracellular oxidases and peroxidases in mitochondria, chloroplasts, peroxisomes, and nuclei (Allan and Fluhr, 1997; Bolwell and Wojtaszek, 1997; Bowler and Fluhr, 2000; Corpas et al., 2001; Laurenzi et al., 2001; Bolwell et al., 2002; del Rio et al., 2002; Scheel, 2002). Processes believed to be activated by the oxidative burst include homeostatic antioxidant defenses, physiological adaptations to stress, resistance to pathogens, and cell death (Levine et al., 1994; Jabs et al., 1996; Romeis et al., 1999; Grant and Loake, 2000; Mullineaux et al., 2000; Noctor et al., 2000; Overmyer et al., 2003). How various cellular ROS sources are activated and whether they play different roles in the stress response is not well understood (Mahalingam and Fedoroff, 2003).
Evidence is accumulating that heterotrimeric G protein signaling is involved in stress-associated physiological processes. Unlike animals, plants have a small number of heterotrimeric G proteins. The Arabidopsis thaliana genome encodes a single canonical α and β subunit and just two γ subunits (Jones and Assmann, 2004; Offermanns, 2003). Recent studies in rice (Oryza sativa) provide evidence that the heterotrimer is present in the plasma membrane, that the β and γ subunits form a tight complex, and that a significant fraction of the constituent proteins is present as free Gα monomers and as Gβγ complexes (Kato et al., 2004). The results of phenotypic analyses of Arabidopsis plants with T-DNA knockout insertion mutations in the GPA1 and AGB1 genes encoding the Gα and the Gβ subunits, respectively, show that the G protein affects, but is not essential for, auxin regulation of cell division (Ullah et al., 2001, 2003). Heterotrimeric G protein signaling to membrane-bound NADPH oxidase has been implicated in the development of disease resistance and the apoptotic hypersensitive response in rice (Suharsono et al., 2002). The α subunit of the single Arabidopsis heterotrimeric G protein is also involved in regulating stomatal closure in response to ABA (Wang et al., 2001).
We show here that mutations in the genes encoding the α and β subunits of the heterotrimeric G protein have markedly different effects on the O3 tolerance of Arabidopsis plants and that the genes are differentially expressed in plants during and after exposure to O3. We present evidence that the proteins serve different signaling functions in the course of the oxidative stress response to O3. We show that there is both a temporal and spatial progression of endogenous ROS signaling in leaves responding to O3 exposure, commencing with elevation of ROS levels in guard cell chloroplasts and membranes and later spreading to neighboring cells by means of extracellular ROS signals generated by membrane-bound AtrbohD and AtrbohF NADPH oxidases. Both the Gα and the Gβ proteins are necessary for the rapid initial component of the biphasic oxidative burst, but only the Gα protein is required for the late, cell death–associated component.
RESULTS
G Protein Mutations Affect the Sensitivity of Arabidopsis to O3 Damage
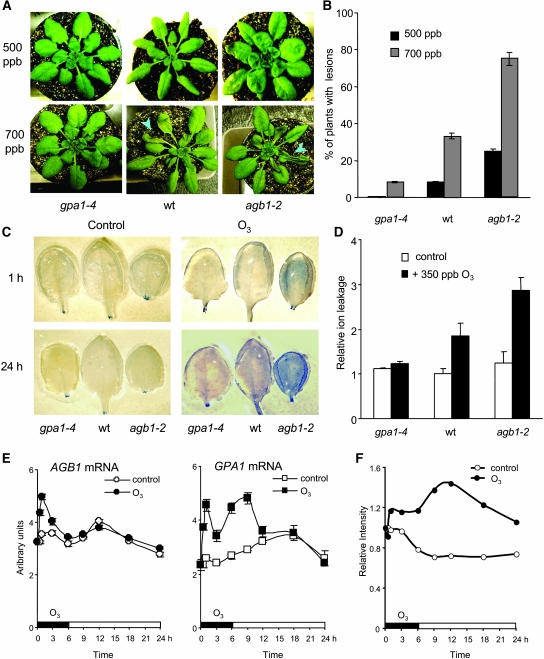
Arabidopsis plants with null mutations in the genes encoding the α (gpa1-4) and β (agb1-2) subunits of the G protein (Lease et al., 2001; Jones et al., 2003) exhibit different sensitivities to tissue damage from O3. We exposed wild-type and mutant plants to either 500 or 700 ppb O3 for 3 h, then examined the plants for visible lesions after 24 h. Wild-type plants showed few visible lesions after exposure to 500 ppb O3 but showed extensive tissue damage after exposure to 700 ppb (Figures 1A and 1B). Homozygous gpa1-4 plants showed less damage than wild-type plants, whereas homozygous agb1-2 plants showed more damage at both ozone levels (Jones et al., 2003; Booker et al., 2004). We also tested the O3 sensitivity of the gpa1-1 and gpa1-2 null alleles of the Gα gene, both in the Wassilewskija (Ws) ecotype (Jones et al., 2003), and the gpa1-1 allele of the Gβ gene in the Columbia-0 (Col-0) ecotype (Lease et al., 2001; Jones et al., 2003). Plants homozygous for either Gα allele were less sensitive to O3 damage than wild-type Ws plants, whereas agb1-1 homozygotes, like agb1-2 homozygotes, were more sensitive than Col-0 wild-type plants (see Supplemental Figure 1 online). Similar results were also obtained when tissue damage was monitored in leaves by vital dye staining with Trypan Blue or by ion leakage, an indicator of plasma membrane damage, during and after exposure to 350 ppb O3 for 6 h (Figures 1C and 1D), a dose which causes no visible damage to the relatively O3-resistant Col-0 ecotype. Thus, mutant plants lacking the Gα protein are more resistant, whereas mutant plants lacking the Gβ protein are less resistant to O3 damage than wild-type plants. Because the O3 sensitivities of Ws and Col-0 plants are markedly different (Tamaoki et al., 2003), subsequent experiments were performed using the gpa1-4 and agb1-2 alleles, both of which are in the Col-0 background.
Figure 1.
Plant Responses to O3 and G Protein Gene Expression Levels.
(A) to (D) Ozone sensitivity of wild-type and G protein mutant plants.
(A) and (B) Photographs of plants with and without leaf damage ([A], arrowheads) and the percentage of 4-week-old plants with visible lesions 24 h after a 3-h exposure to O3 at the indicated concentrations (B).
(C) Cell death 1 and 24 h after the onset of a 6-h exposure to 350 ppb ozone. Leaves were stained with Trypan Blue D (see Methods).
(D) Ion leakage (see Methods) was assayed 24 h after a 6-h exposure of 4-week-old plants to 350 ppb O3. Ion leakage is reported as the ratio of the value measured in O3-exposed plants to the value obtained in control plants maintained in ambient air (n = 4); raw values are given in Supplemental Table 1 online.
(E) and (F) Four-week-old wild-type plants were exposed to 350 ppb ozone for 6 h.
(E) Gα and Gβ transcript levels were measured by RT-PCR.
(F) Gα protein was detected by protein gel blotting with Gα antibodies; the blots were scanned and the values normalized by the amount present at the initial time point.
The GPA1 and AGB1 genes show different expression profiles after O3 exposure. The abundance of both transcripts increased rapidly, peaking at ∼1 h after the onset of O3 exposure (Figure 1E). The GPA1 gene showed a second later peak in its transcript profile not observed for the AGB1 gene. GPA1 protein levels, detected with anti-GPA1 antibodies, increased both early in the treatment and again between 9 and 12 h after the onset of O3 exposure (Figure 1F). The late rise in GPA1 transcript and protein abundance, together with the marked resistance of gpa1-4 mutant plants to O3-induced tissue damage and cell death, suggest that Gα's role in cell death response is separable from its role in the heterotrimeric G protein.
G Protein Mutations Affect the O3-Induced Oxidative Burst
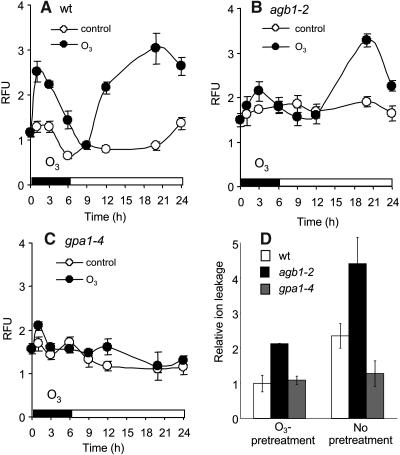
The oxidative burst has been implicated both in cell death and in the enhancement of plant resistance to pathogen attack and abiotic stresses (Levine et al., 1994; Jabs et al., 1997; Alvarez et al., 1998; Orozco-Cardenas et al., 2001; Suharsono et al., 2002). We found that a 6-h exposure of wild-type plants to 350 ppb O3, a level that caused tissue damage as judged by ion leakage, but produced few visible lesions, evoked a marked biphasic oxidative burst (Figure 2A). We measured H2O2 as catalase-inhibitable fluorescence generated upon incubation of cell extracts with the fluorogenic substrate 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCFDA) as described in Methods. Leaf H2O2 production increased rapidly after the onset of O3 exposure, reaching a first peak after 1 to 1.5 h. It then declined to a minimum at 6 to 9 h and rose to a second peak between 12 and 24 h. Neither peak was detectable in gpa1-4 plants (Figure 2C). The first peak was minimal in agb1-2 plants, but the second peak was of the same or greater magnitude than in wild-type plants (Figure 2B). Hence, heterotrimeric G protein signaling is necessary to elicit the early peak, and either both subunits are necessary or the action of one is contingent on the presence of the other. However, unlike the early component of the oxidative burst, the late component is of comparable magnitude in agb1-2 and wild-type plants, indicating that it requires only the Gα protein.
Figure 2.
O3-Induced Oxidative Burst in Wild-Type and Mutant Plants.
(A) to (C) Wild-type (A), agb1-2 (B), and gpa1-4 (C) plants were exposed to 350 ppb O3 for 6 h (closed circles) or air (open circles), then maintained in O3-free air; leaves were collected and frozen at the indicated times. ROS were measured by H2DCFDA fluorescence; values were normalized by the total protein concentration in leaf extracts, and relative fluorescence units (RFU) were calculated as a ratio of the value obtained at the indicated time to the control value at time 0 (n = 5).
(D) Mutant and wild-type plants (4 weeks old) were exposed to O3 for 2 h, transferred to O3-free air for 2 h, and then exposed to 700 ppb ozone for 3 h, following which leaves were collected and assayed for ion leakage as described in Methods, reported as the ratio of O3-exposed/control value (raw values are given in Supplemental Table 1 online).
Biochemical changes that occur within the first few hours after O3 exposure increase the ability of plants to withstand subsequent oxidative stress. When exposed briefly to 350 ppb O3 (2 h), then tested for their ability to withstand a damaging dose of 700 ppb O3 2 h later, wild-type plants showed virtually no tissue damage, in contrast with what was observed without such a pretreatment (Figure 2D). Plants lacking the Gα protein exhibited little tissue damage with or without pretreatment. Plants lacking the Gβ protein were more sensitive to damage from O3 than either wild-type or gpa1-4 plants even with pretreatment but still responded to pretreatment. Thus, G protein signaling contributes to activating the protective response, but it is not solely responsible for it.
ROS Arise from Multiple Cellular Sources
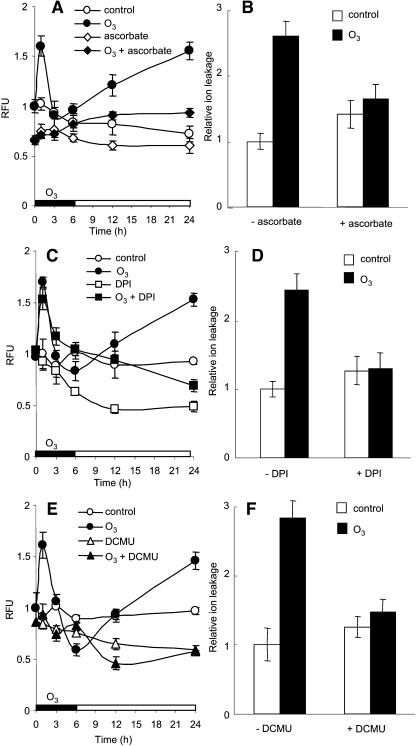
We used ROS scavengers and inhibitors to investigate the sources of the O3-induced oxidative burst. Ascorbate, a primary antioxidant in plants, suppressed both the early and late ROS peaks (Figure 3A) and prevented O3-induced cell death (Figure 3B). This has been attributed to ascorbate's ability to scavenge ROS produced by the dissolution of ozone in the apoplastic fluid (Luwe et al., 1993). Pretreatment with the flavin oxidase inhibitor diphenylene iodonium (DPI) before O3 exposure completely eliminated the second ROS peak but depressed the first peak only slightly (Figure 3C). DPI also suppressed O3-induced cell death, as judged by ion leakage (Figure 3D). Hence, flavin oxidases are largely or entirely responsible for the second cell death–associated ROS peak. DPI effects only a slight reduction in the initial peak (Figure 3C), suggesting that a different, DPI-insensitive ROS-generating system is responsible for most of the first component of the oxidative burst.
Figure 3.
The Effect of Ascorbate, DPI, and DCMU on ROS Production and Cell Death after O3 Exposure.
Four-week-old wild-type Col-0 plants were pretreated with 50 mM ascorbate in MES buffer (pH 6.8) ([A] and [B]), 20 μM DPI in 0.2% DMSO ([C] and [D]), or 10 μM DCMU in 0.05% EtOH for 4 h ([E] and [F]), then exposed to 350 ppb ozone for 6 h. RFU, relative fluorescence units.
(A), (C), and (E) Plants were harvested and frozen at the indicated times, and ROS were quantified by DCF fluorescence.
(B), (D), and (F) Plants were pretreated and exposed to O3 as in (A), (C), and (E), respectively; ion leakage was measured after 24 h as described in Methods, reported as the ratio of O3-exposed/control value (error bars represent ±se, n = 3; raw values are given in Supplemental Table 1 online).
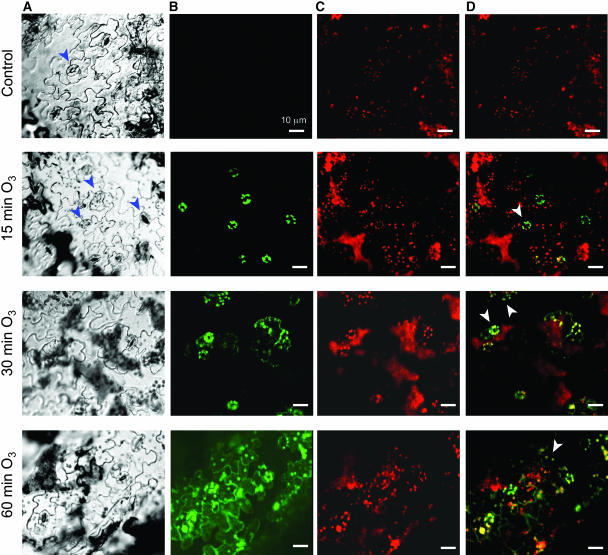
Because ABA and light stress are known to elicit chloroplast ROS production (Karpinski et al., 1999; Zhang et al., 2001), we tested the effect of DCMU, an electron transport inhibitor, on the O3-induced oxidative burst. DCMU pretreatment inhibited both the early and the late O3-induced ROS peaks and cell death, implicating chloroplastic ROS sources (Figures 3E and 3F). To further investigate the cellular sources of the early ROS peak, we exposed wild-type plants to 350 ppb O3 for various times, prepared and loaded epidermal peels with the H2DCFDA, and examined them by confocal microscopy. ROS fluorescence was first observed in the chloroplasts of stomatal guard cells and was detectable within 5 min (Figure 4; see Supplemental Figure 2 online). By 30 min, ROS-induced fluorescence was evident at the periphery of the epidermal pavement cells in immediate contact with guard cells that had fluorescing chloroplasts, suggesting that the guard cells were signaling to neighboring cells. By 60 min, there were large patches of fluorescing epidermal cells around the stomata, and these showed peripheral and chloroplast fluorescence as well as a more diffuse, cytoplasmic fluorescence.
Figure 4.
Fluorescence Microscopic Imaging of ROS in Epidermal Cells.
Four-week-old wild-type plants were exposed to 350 ppb O3 for the indicated times. Epidermal peels were loaded with H2DCFDA for 5 min, washed, and examined by confocal microscopy. For each time point, the panels show the cell structure of the tissue (A), H2DCFDA fluorescence (B), fluorescence (C), and H2DCFDA and chloroplast fluorescence images superimposed (D). Blue arrowheads indicate guard cells; white arrowheads indicate ROS-producing chloroplasts in guard cells (15 and 30 min) and epidermal cells (30 and 60 min). Experiments were repeated at least five times with similar results.
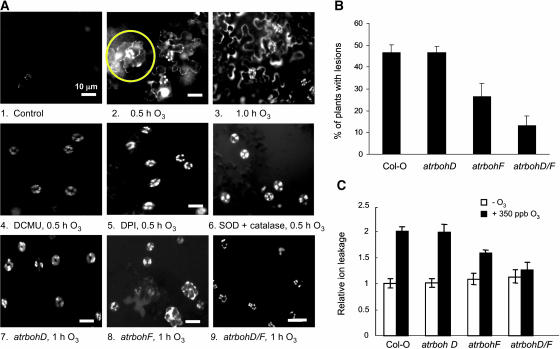
Disrupting electron transport with DCMU inhibited guard cell chloroplast fluorescence (Figure 5A, 4); inhibiting flavin oxidases with DPI did not (Figure 5A, 5). Both DPI and DCMU treatment completely suppressed ROS-induced fluorescence in epidermal cells adjacent to guard cells, consistent with the postulated role of ROS in intercellular signaling (Levine et al., 1994; Allan and Fluhr, 1997). Further evidence that intercellular signaling requires ROS is provided by the ability of extracellular catalase and superoxide dismutase (SOD) to suppress the development of ROS fluorescence in pavement cells adjacent to stomatal guard cells (Figure 5A, 6). Insertion mutations in the AtrbohD and AtrbohF genes, which have been shown to be responsible for ROS production in response to pathogens (Torres et al., 2002), decreased the appearance of ROS fluorescence in cells adjacent to guard cells (Figure 5A, 7 to 9). The occasional small patches of ROS-fluorescent cells observed in the atrbohF single mutant are completely absent in the atrbohD atrbohF double mutant (Figure 5A, 9). We conclude that activation of both chloroplastic enzymes and membrane-bound NADPH oxidases in guard cells initiates the early component of the O3-induced oxidative burst and that both signals are required to elicit ROS production in adjacent cells.
Figure 5.
Fluorescence Microscopy Imaging of ROS in Epidermal Tissue.
(A) Four-week-old wild-type plants were exposed to O3-free air (1) or 350 ppb O3 for 0.5 h (2) or 1 h (3) or pretreated with 10 μM DCMU in 0.05% EtOH for 2 h (4) or 20 μM DPI in 0.2% DMSO (5) then exposed to 350 ppb O3 for 0.5 h. Epidermal peels were loaded with H2DCFDA for 5 min, washed, and examined by fluorescence microscopy. The yellow circle in (2) highlights a patch of fluorescing cells adjacent to a guard cell with highly fluorescent chloroplasts. (6) Epidermal peels were pretreated with SOD (400 units/mL) and catalase (300 units/mL), exposed to ozone for 0.5 h, then loaded with H2DCFDA for 5 min, washed, and examined. (7) to (9) Plants homozygous for the indicated mutations were exposed to O3 for 1 h, and then epidermal peels were prepared as for wild-type plants.
(B) The percentage of 4-week-old wild-type and atrboh mutant plants with visible lesions 24 h after a 3-h exposure to 700 ppb O3.
(C) Ion leakage (see Methods) was assayed 24 h after a 6-h exposure of 4-week-old plants to 350 ppb O3 (raw values are given in Supplemental Table 1 online). Experiments were repeated at least five times with similar results.
Both inhibitor studies and genetic evidence from mutants show that extracellular ROS produced by guard cell NADPH oxidases encoded by the AtrbohD and AtrbohF genes propagate the ROS signal to neighboring cells to elicit the complete early component of the oxidative burst. Figure 5B further shows that the atrbohF single mutant and the atrbohD atrbohF double mutant are less susceptible to O3-induced tissue damage, as measured by ion leakage, than are wild-type plants or plants of the same ecotype that are homozygous for the atrbohD mutation. The observation that the double mutant consistently shows less damage by either criterion and that both single mutants show reduced ROS signaling between epidermal cells indicates that both proteins are involved. This is in agreement with the observation that DPI decreases O3-induced tissue damage and indicates that the AtrbohD- and AtrbohF-generated ROS contribute to cell death (Figure 3D). However, it is also possible that atrbohD and atrbohF mutations interfere with cell death by interfering with intercellular propagation of the ROS signal.
The Early Component of the Oxidative Burst Requires Both Gα and Gβ, but the Late Component Requires Only the Gα Subunit
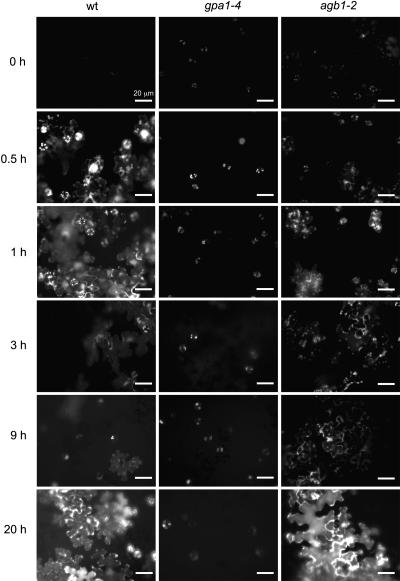
We used fluorescence microscopy of H2DCFDA-loaded epidermal peels to compare the evolution of the oxidative burst in wild-type, gpa1-4, and agb1-2 plants during and after exposure to 350 ppb O3 for 6 h. In wild-type plants, the early component of the oxidative burst peaked at 1 h, then subsided by 3 h (Figure 6, wt). The late ROS burst began to develop at ∼9 h and arose from the epidermal cells but did not emanate from stomatal guard cells. Large patches of intensely fluorescent epidermal cells were observed at 20 h after the onset of O3 exposure, coincident with cell death and the development of large lesions in plants exposed to high O3 levels (Figure 1). O3 exposure of gpa1-4 plants caused some intensification of guard cell chloroplast fluorescence; ROS fluorescence was not observed in guard cell membranes or in neighboring epidermal cells. Consistent with results obtained in whole leaf extracts (Figure 2), ROS fluorescence was also not detected in gpa1-4 plants at later times. Thus, the initial chloroplastic component of the oxidative burst was markedly attenuated in plants lacking the Gα protein, and the late component was completely absent. Moreover, gpa1-4 plants show no membrane-associated fluorescence either early or late in the oxidative stress response.
Figure 6.
Fluorescence Imaging of ROS in Col-0, gpa1-4, and agb1-2 Epidermal Tissue.
Four-week-old plants were exposed to 350 ppb O3, then transferred to O3-free air. Epidermal peels were prepared at the indicated times as described in the legend to Figure 5 and examined by fluorescence microscopy. Experiments were repeated at least five times with similar results.
Guard cell chloroplasts of the agb1-2 mutant showed no increase in ROS production after 30 min of O3 exposure. After 1 h, there were small patches of fluorescent epidermal cells, and chloroplast ROS fluorescence was detected in both guard cells and adjacent epidermal cells. At 3 and 9 h, agb1-2 plants showed large patches of weakly fluorescing epidermal cells. Chloroplast fluorescence in epidermal cells, which have fewer but larger chloroplasts than guard cells in Arabidopsis (Pyke and Leech, 1994), was more intense in the agb1-2 mutant than in wild-type plants. At 20 h after the onset of O3 exposure, the cellular distribution of ROS fluorescence in agb1-2 plants resembled that in wild-type plants and often was more intense. We conclude that both the Gα and Gβ subunits of the heterotrimeric G protein are required to trigger fully the initial component of the oxidative burst but that only the Gα protein is required for the late oxidative burst associated with cell death. We further conclude that the resistance of the gpa1-4 mutant to O3-induced cell damage is attributable to the defect in Gα-mediated activation of cell death–associated late component of the oxidative burst.
Leaves of gpa1-4 plants exhibit a slight increase in the chloroplastic ROS signal 0.5 h after the onset of O3 exposure. This suggests that the Gβ protein (or the Gβγ complex) can itself activate chloroplastic ROS production. The weakness of the ROS signal may be due to the low activity (or stability) of the Gβ protein (or the Gβγ complex) in the absence of the Gα protein or it may have other causes. Because the late component of the oxidative burst develops normally in agb1-2 plants, we conclude that the Gα/NADPH-oxidase signaling pathway is intact in agb1-2 mutant plants and that its activation does not depend on either the presence of the Gβ protein or the formation of the heterotrimeric G protein.
DISCUSSION
Our results show that G protein signaling is required to activate the intracellular sources of ROS that contribute to the first component of the biphasic, stress-elicited oxidative burst in Arabidopsis. We make this inference from the observation that the first component of the biphasic oxidative burst is highly attenuated or absent in leaves of mutant plants lacking either the Gα protein or the Gβ protein. Hence, maximal rapid activation of intracellular ROS production requires signaling through the heterotrimeric G protein. This, in turn, shows that the ROS produced by dissolution of O3 in the apoplastic fluid do not themselves penetrate cells to activate ROS-generating systems. Rather, the extracellular ROS activate the heterotrimeric G protein either directly or indirectly. The actual oxidation target may be the G protein itself, a receptor, or either a protein or a small molecule that generates a further signal.
Our results further show that the α and β subunits of the Arabidopsis heterotrimeric G protein act both synergistically and separately in activating different intracellular ROS-generating systems. Guard cell chloroplasts are the first detectable ROS source in wild-type plants. A modest early O3 increase in ROS production by guard cell chloroplasts is observed in gpa1-4 mutant plants but not in agb1-2 mutant plants. This observation suggests that the Gβ protein, presumably as part of the Gβγ complex (Kato et al., 2004), activates the chloroplastic source of ROS, albeit weakly. However, full activity of Gβ protein (or possibly its stability or that of the Gβγ complex) depends on the presence of the Gα protein.
By contrast, ROS production is not observed in chloroplasts early in the O3 response of agb1-2 mutant plants. Instead, there is a modest increase in peripheral, presumably membrane-associated ROS production early in the response. However, the agb1-2 mutant develops the late, DPI-inhibitable ROS peak. Thus, Gα signals to targets in membranes, but not in chloroplasts, and Gα signaling is independent of the ability to form the heterotrimer. Taken together, these observations imply that the Gα and Gβγ signals act both separately and synergistically.
The initial production of endogenous ROS is rapid: guard cell chloroplast fluorescence, as well as some membrane fluorescence, is already quite marked by 15 min after the onset of O3 exposure (Figure 4). We have observed H2DCFDA fluorescence within 5 min after the onset of O3 exposure and within a minute after the addition of H2O2 to epidermal peels preloaded with H2DCFDA (see Supplemental Figure 2 online), strongly suggesting that the initial phase of the oxidative burst is triggered directly by G protein–mediated signaling. It has been reported that guard cell ROS production can be detected within seconds after the application of H2O2 to tobacco (Nicotiana tabacum) epidermal peels (Allan and Fluhr, 1997).
Late Cell Death Component of the Oxidative Burst
The late component of the oxidative burst does not occur in the gpa1-4 mutant, but the timing and magnitude of the late component of the oxidative burst are unaffected by the absence of the Gβ protein. Thus, of the three G protein subunits, the Gα subunit is both necessary and sufficient for the late oxidative burst. The late component of the oxidative burst clearly differs from the early component in its cellular origins. The Gα-stimulated membrane-associated ROS production makes only a small contribution to the early, largely chloroplast-generated component of the oxidative burst in whole leaf tissue but is responsible for most or all of the late burst. Hence, the cell death signaling system is intact in plants that express the Gα, but not the Gβ, protein.
However, because many hours elapse between the initial rapid component of the oxidative burst and the late component, the G protein may act both directly, as discussed below, and indirectly through its effect on gene expression. The differences in the expression profiles of the Gα and Gβ genes also suggest that the genes themselves respond to different regulatory signals: the Gα gene is expressed both early and later in the stress response, whereas the Gβ gene is expressed only during the early part of the response. The O3 resistance of the gpa1-4 mutant is consistent with the postulated role of the Gα protein in activating membrane-bound NADPH oxidases to produce damaging levels of ROS (Suharsono et al., 2002). The association of cell death with ROS production by membrane-bound NADPH oxidases is further supported by the observation that mutants lacking either the AtrbohD, the AtrbohF, or both NADPH oxidase proteins lack the late oxidative burst (J.H. Joo and N.V. Fedoroff, unpublished data) and that atrbohF mutants and atrbohD atrbohF double mutants are more resistant to O3 than wild-type plants of the same ecotype (Figure 5B). These results extend previous observations, made at lower concentrations of O3, that the gpa1-4 mutant does not exhibit the epinastic response of wild-type leaves (Booker et al., 2004).
Molecular connections between the Gα protein and the NADPH oxidase are not yet completely defined, but it has been reported that Gα acts through the small GTPase Rac and Rop, a small RHO-like protein (Baxter-Burrell et al., 2002; Suharsono et al., 2002). There is evidence that Rac is activated by phosphatidic acid (PA) (Suharsono et al., 2002; Park et al., 2004), and it has also been reported that Gα interacts with and inhibits phospholipase Dα (Zhao and Wang, 2004). The PA generated by phospholipase Dα upon activation and dissociation of Gα inhibits the activity of the ABI1- and ABI2-encoded phosphatases, which negatively regulate ABA signaling through the stress-activated MAPK cascade (Zhang et al., 2004). The observations that PA both increases and decreases ROS-associated cell death may find its explanation in the balance between the signaling and cell death roles of ROS (Zhang et al., 2003; Park et al., 2004).
Intercellular Signaling in the Early Component of the Oxidative Burst
ROS produced by membrane-associated NADPH oxidases are necessary for the propagation of the early component of the oxidative burst from its source in guard cells to neighboring cells. Elevated ROS levels are detectable in the membranes and chloroplasts of epidermal pavement cells in immediate contact with each guard cell within 30 min. By 1 h after the onset of O3 exposure, there are large patches of pavement cells, all showing ROS production from chloroplasts, membranes, and other cytoplasmic sources.
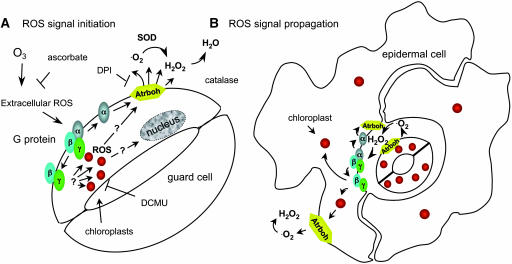
Several kinds of evidence support the interpretation that ROS are either the intercellular signals or part of the intercellular signaling system, as diagrammed in Figure 7. Addition of SOD and catalase to reduce extracellular superoxide and hydrogen peroxide suppresses ROS production in epidermal cells adjacent to guard cells. DPI, a flavin oxidase inhibitor, abrogates the increase in ROS in cells adjacent to guard cells but does not affect ROS production by guard cell chloroplasts. Finally, mutations in both the AtrbohD and AtrbohF genes, both of which have been implicated in the oxidative burst triggered by pathogens and ABA (Torres et al., 2002; Kwak et al., 2003), each markedly reduces fluorescence in cells adjacent to guard cells, whereas a double mutant plant exhibits only chloroplast fluorescence (Figure 5). Thus, ROS production by membrane-bound NADPH oxidases is required for activating cytoplasmic ROS production in neighboring cells but not in guard cells. The gpa1-4 mutant lacking the Gα protein exhibits no membrane fluorescence at any time during the oxidative stress response, implying that the Gα is absolutely required for activation of the DPI-inhibitable membrane-bound ROS-generating system and underscores its importance in communicating to adjacent epidermal cells. We conclude that membrane-bound NADPH oxidases participate in ROS signaling early in the oxidative stress response, in addition to their independent role in cell death–associated ROS production (Dat et al., 2003). The observation that the guard cell chloroplast oxidative burst is activated even when the membrane-bound enzymes are inhibited or absent implies that communication of the oxidative stress signal to chloroplasts does not depend on activation of the membrane-associated oxidases.
Figure 7.
Diagram of ROS Signaling in Leaf Epidermal Tissue Exposed to O3.
(A) Oxidative stress activates G protein signaling to guard cell chloroplasts and membranes. Ascorbate, a ROS scavenger, suppresses accumulation of the initial oxidative species from O3, whereas DCMU inhibits electron transport in the chloroplasts.
(B) The diagram shows signal propagation in the early oxidative burst. Activation of membrane-bound NADPH oxidases (AtrbohD and AtrbohF), inhibitable by DPI, produces extracellular superoxide (˙O2−), which rapidly dismutates to hydrogen peroxide (H2O2). Reduction of extracellular ROS by SOD and catalase prevents propagation of the ROS signal to epidermal cells adjacent to guard cells. ROS signaling to neighboring cells is represented as occurring by the same mechanisms.
Several observations suggest that the chloroplastic ROS signal contributes to activating the membrane-associated NADPH oxidases in intercellular signaling during the early component of the oxidative burst. DCMU suppresses chloroplastic ROS production but does not interfere with ROS production in the cytoplasm and membranes of guard cells. Nonetheless, DCMU largely suppresses the spread of the ROS signal between cells, even when cells are supplemented with NADPH, the electron donor for the membrane-bound oxidases (see Supplemental Figure 3 online). Thus, the early ROS burst from chloroplasts appears to be necessary for propagation of the ROS signal throughout the epidermal layer during the initial component of the oxidative burst.
These results establish that ROS signaling from the chloroplast is central to the O3-induced oxidative stress response, as it has been shown to be for light and wound stress signaling (Fryer et al., 2002, 2003; Chang et al., 2004). Analysis of gene expression profiles during the O3-induced oxidative stress response has revealed the coordinate upregulation and downregulation of genes coding for proteins that are targeted to and function in chloroplasts (Mahalingam et al., 2005). Strikingly, there is a set of rapidly upregulated nuclear genes whose promoters show a significant overrepresentation of the light-regulated T box motif (ACTTG) first identified through analysis of the nuclear gene encoding a subunit of the chloroplast glyceraldehyde-3-phosphate dehydrogenase gene (Chan et al., 2001; Mahalingam et al., 2005). Because light stress induces ROS production, redox signals are likely common intermediaries in chloroplast signaling to nuclei in both light and oxidative stress (Fryer et al., 2002). The sharp delimitation of the subcellular ROS-generating compartments suggests that ROS signals are rapidly quenched in the reducing environment of the cell. This, in turn, suggests that redox signals are either transmitted between cellular compartments by redox-sensitive proteins, such as protein disulfide isomerases, or translated into other types of signals by redox-sensitive proteins, such as redox-regulated kinases.
Biochemical changes that occur early in the oxidative stress response increase the ability of plants to withstand subsequent stress. Exposure of plants to a brief, nondamaging dose of O3 decreases subsequent cell damage from a higher O3 dose. The agb1-2 plants were more sensitive to tissue damage from O3 than wild-type plants; preliminary observations suggest that they exhibit a weaker transcriptional response for several genes that encode enzymes involved in redox homeostasis, such as ascorbate peroxidase and glutathione S-transferase (J.H. Joo and N.V. Fedoroff, unpublished data). It has been reported that mutations that decrease catalase activity increase the susceptibility of tobacco to oxidative burst–triggered cell death in response to high light stress (Dat et al., 2003). Thus, it is likely that early heterotrimeric G protein–mediated signaling contributes to activating the changes in gene expression that have been designated the defense response or cross-tolerance (Bowler and Fluhr, 2000).
However, there are also G protein–independent signals in the oxidative stress response, as evidenced by the fact that the agb1-2 mutant developed some cross tolerance in these experiments. Preliminary transcript analysis indicates that some O3-responsive genes are relatively unaffected by the G protein mutations, whereas others are markedly affected (J.J. Joo and N.V. Fedoroff, unpublished data) and stress MAPKs are activated after O3 exposure in both G protein mutants, albeit somewhat less markedly than in wild-type plants (S. Wang and N.V. Fedoroff, unpublished data). The hypersensitivity of the agb1-2 mutant to O3 damage may be explained, therefore, by a suboptimal activation of defense response genes, combined with an unaltered or somewhat intensified Gα-mediated cell death response. However, it is also possible that the Gβ protein (or the Gβγ complex) serves as a negative regulator of cell death, actively inhibiting expression of cell death genes or activation of cell death proteins.
Stress Signaling to Chloroplasts
Although there is ample evidence supporting the central importance of the photosynthetic apparatus in stress responses to excess light and biotic and abiotic stresses, how signals reach the chloroplasts is not understood (Mullineaux et al., 2000; Mullineaux and Karpinski, 2002). This observation of a markedly attenuated early oxidative burst in plants with G protein mutations implies that the ROS generated upon dissolution of O3 in the plant's apoplastic fluid do not enter the cell and act directly on chloroplasts, nor do they directly activate membrane-bound NADPH oxidases. One possibility is that the G protein itself is redox regulated. It has been reported, for example, that two mammalian Gα proteins, Gαi and Gαo, are directly regulated by ROS (Nishida et al., 2000). H2O2 treatment of the Gα subunit activates it, as judged by the H2O2 dose-dependent increase in its affinity for GTP, and activates Gβγ signaling through PI(3) kinase to downstream kinases (Nishida et al., 2000). Alternatively or in addition, ROS may initiate signaling by directly modifying other membrane-bound proteins that normally respond to hormone signals, such as ABA. It has been reported that H2O2, whose production is activated by ABA, activates plasma membrane–bound Ca2+ channels (Pei et al., 2000). Also, phosphatases generally contain active site sulfhydryl residues that are very sensitive to oxidation (Rhee et al., 2000), and both the ABI1 and ABI2 phosphatases have been reported to be inactivated by oxidation (Meinhard and Grill, 2001; Meinhard et al., 2002). The ABI1 and ABI2 phosphatases are also potential intermediaries in Gα protein signaling because Gα regulates phospholipase D activity, which in turn regulates the activity of the ABI-encoded phosphatases that negatively regulate signaling through the stress-activated MAPK kinase cascade (Zhang et al., 2004).
The role of the Gβ protein (or the Gβγ complex), which appears to be required for activating ROS production by chloroplasts, is a challenging area for further investigation. The Gβγ complex is known to have many targets in mammalian systems (Offermanns, 2003), among which are calcium channels (Herlitze et al., 1996; Hummer et al., 2003; Wolfe et al., 2003). Calcium signaling is central to plant stress responses (Knight et al., 1996, 1997; Klusener et al., 2002), and recent work on PPF1, a chloroplast-localized Ca2+ ion channel, suggests that it functions to inhibit programmed cell death in apical meristems (Li et al., 2004). However, there are many calcium channels in plants cells, and whether heterotrimeric G proteins participate in their regulation remains to be elucidated (White, 2000).
Function of the Arabidopsis Heterotrimeric G Protein
Previous studies on null mutations in the Arabidopsis genes coding for the Gα and Gβ proteins have provided evidence that the G protein is involved in a pathway that determines sensitivity to auxin (Ullah et al., 2001, 2003) and in ABA-mediated inhibition of stomatal opening (Wang et al., 2001). Additional evidence that the heterotrimeric G protein is involved in ABA signaling comes from reports that Gα interacts directly with AtPirin1, a cupin domain protein, to regulate seed germination (Lapik and Kaufman, 2003) and with phospholipase Dα1, as noted above (Zhao and Wang, 2004). ROS production is necessary for both ABA and auxin signaling (Pei et al., 2000; Joo et al., 2001). Stomatal closure and plasma membrane calcium channel activation are reduced in atrbohD atrbohF double mutants but can be restored with H2O2, suggesting that ROS serve as second messengers in ABA signaling (Kwak et al., 2003). However, it has also been reported that ABA activates ROS production by guard cell chloroplasts, in addition to the membrane-bound NADPH oxidases (Zhang et al., 2001; Kwak et al., 2003).
These experiments reveal that oxidizing conditions trigger a pattern of rapid intracellular ROS production in guard cells that is similar to that seen with ABA. They show that activation of the membrane-bound NADPH oxidase system requires the presence of the Gα subunit of the heterotrimeric G protein, an observation consistent with the results of studies on the effects of Gα mutations on disease resistance in rice (Suharsono et al., 2002). They reveal that activation of chloroplast ROS production in response to oxidizing conditions is mediated by the heterotrimeric G protein and is required for intercellular signaling from guard cells to adjacent cells. They further show that intercellular signaling is mediated by ROS produced by the AtrbohD- and AtrbohF-encoded NADPH oxidases. Finally, these studies show that the late cell death–associated component of the oxidative burst requires only the Gα subunit of the heterotrimeric G protein.
These results suggest that the role of both G protein and ROS signaling in the oxidative stress response and in ABA-mediated responses is significantly more complex than presently envisioned. Oxidation at the cell surface either directly or indirectly activates the heterotrimeric G protein, subcomponents of which differentially activate ROS production in different cellular compartments. ROS produced at the cell surface by membrane-bound NADPH oxidases act on guard cell plasma membrane calcium channels (Kwak et al., 2003) and stimulate ROS production in adjacent cells. Thus, cell surface ROS both feed back to influence the physiological state of the guard cell and serve as an intercellular signal. Our results suggest that chloroplast ROS production also influences ROS production at the cell surface and intercellular ROS signal production. Chloroplast signaling to the nucleus is well known but poorly understood (Pfannschmidt et al., 2001; Pfannschmidt, 2003). In view of the fact that cells are highly reducing environments, it appears likely that redox signals arising from chloroplasts are translated into other types of signals either through redox-sensitive kinases (Aro and Ohad, 2003) or by activation of other signaling mechanisms.
METHODS
Plant Materials, Growth Conditions, and O3 Treatment
Arabidopsis thaliana Col-0 plants were used in this study; the gpa1-4 and agb1-2 are previously characterized transcript-null mutants in the Col-0 background (Jones et al., 2003; Ullah et al., 2003). dSpm transposon insertions in AtrbohD and AtrbohF genes in Col-0 plants were identified by Torres et al. (2002), who also constructed the atrbohD atrbohF double mutants.
Plants were grown in MetroMix 200 (Scotts-Sierra Horticultural Products Company, Marysville, OH) in 5-cm pots (50 per flat) at 65% humidity under fluorescent light at 30 W/m2/s with a 14-h-light/10-h-dark photoperiod for 4 to 4.5 weeks. For O3 exposure, 4-week-old plants were transferred to an O3 fumigation chamber and exposed to 350 ± 50 ppb O3 for 6 h or to 500 ± 50 ppb or 700 ± 50 ppb ozone for 3 h. The O3 levels were selected based on their ability to either alter physiological functions, including transcript levels with minimal tissue damage, or to differentiate among the G protein mutant and wild-type plants based on tissue damage. Ozone was generated using an ozone generator (model 2000; Jelight Company, Irvine, CA) and monitored with an ozone monitor (model 450; Advanced Pollution Instrumentation, San Diego, CA). Control plants were transferred to an adjacent chamber under identical growth conditions except for the O3 treatment. Entire rosettes were harvested at different times after ozone treatment, frozen, and kept at −80°C for subsequent analysis.
Quantification of Tissue Damage
To assess the formation of visible lesions, wild-type, agb1-2, and gpa1-4 plants were exposed to 500 or 700 ppb O3 for 3 h, then transferred to O3-free air. At 24 h after the onset of O3 exposure, plants were examined for visible lesions; plants with lesions were photographed with a digital camera (Progres 3012; Kontron America, San Diego, CA), and the number of plants showing visible lesions was recorded. To measure tissue damage by ion leakage, 18 leaves from two plants were collected at the times indicated in each figure, rinsed with distilled water, then shaken in 25 mL of distilled water on a rotary shaker at 100 rpm for 4 h at room temperature. The conductivity of the wash solution (μS/cm) was determined using a Corning 316 conductivity meter (Corning, Big Flats, NY). The total ion content was obtained by determining the conductivity of the same leaf-containing solution after autoclaving (raw average values for three replicate measurements are given in Supplemental Table 1 online). Ion leakage per milligram of wet weight was calculated by dividing the conductivity of the solution before autoclaving by the conductivity of the solution after autoclaving and dividing the value by sample weight. Relative ion leakage is the ratio of the value obtained with leaves from treated plants to the value obtained with leaves from untreated control plants. Cell death was detected histochemically by Trypan Blue staining. Detached leaves were covered with an alcoholic lactophenol Trypan Blue mixture (30 mL ethanol, 10 g phenol, 10 mL H2O, 10 mL glycerol, 10 mL of 10.8 M lactic acid, and 10 mg of Trypan Blue), placed in a boiling water bath for 3 min, left at room temperature for 1 h, then transferred to a chloral hydrate solution (2.5 g/mL), and boiled for 20 min for destaining.
ROS Assays
Frozen plant tissue was hand ground in liquid nitrogen; the powder was weighed and immediately taken up in 10 mM Tris-HCl buffer, pH 7.3. The extract was centrifuged twice at 15,000 rpm for 5 min. ROS production was assayed by adding 100 mM H2DCFDA in DMSO to a final concentration of 10 μM and measuring fluorescence using a VersaFluor fluorometer (Bio-Rad, Hercules, CA). Because there are indications that the H2DCFDA is not completely specific for ROS (Myhre et al., 2003), we performed each measurement on equal aliquots, to one of which we added catalase (300 units/mL). We then subtracted the catalase-insensitive background from each experimental value. Preliminary experiments (see Supplemental Figure 4 online) established that almost all of the ROS in the plant extracts was catalase-sensitive, indicating that the predominant ROS is H2O2. Total protein was quantified using a Bio-Rad DC protein assay kit. The average fluorescence value obtained from three successive measurements was divided by the protein content and expressed as relative fluorescence units per milligram of protein. These values were then expressed as a ratio of relative fluorescence units obtained with O3-exposed and control plants.
Microscopy
Fluorescence microscopic observations were performed as described, with slight modifications (Zhang et al., 2001). Four-week-old wild-type plants were exposed to 350 ppb O3 for the indicated times. Epidermal peels were removed from the abaxial surface of each leaf and placed in a small Petri dish containing 10 mM MES-KCl, pH 7.2, for 5 min. The cells of epidermal peels are viable and the guard cells are physiologically responsive under these conditions, as shown by vital dye staining, and the responsiveness of guard cells to light and ABA (see Supplemental Figure 5 online). For chemical treatment, epidermal tissues stabilized with 10 mM MES-KCl for 2 h were transferred to fresh buffer with and without DCMU (10 μM in 0.05% EtOH; Sigma-Aldrich, St. Louis, MO), DPI (20 μM in 0.2% DMSO; Sigma-Aldrich), NADPH (200 μM in H2O), SOD (400 units/mL; Sigma-Aldrich), and catalase (300 units/mL; Sigma-Aldrich) for another 2 h, then exposed to O3 for the time indicated in each experiment. The epidermal strips were then transferred to 100 μM H2DCFDA in 10 mM Tris-HCl, pH 7.2, in the dark for 5 min. Excess H2DCFDA was removed by washing with the same buffer. Fluorescence was observed with an Olympus FV300 laser scanning confocal microscope (Olympus America, Melville, NY), with the following settings: excitation, 488 nm; emission, 530 nm. Chloroplasts were excited with a green helium-neon laser with emission collected after filtering through a 660 long-pass filter. Images were taken and Olympus FV300 software was used for analysis (version 4.0). H2DCFDA fluorescence was also observed using a Zeiss Axioskop 7082 fluorescence microscope (Carl Zeiss, Thornwood, NY) with the following settings: excitation, 500 nm; emission, 535 nm. Images were captured with a SenSys CCD camera system, and IPLab software was used for analysis (version 1.1.5; Scanalytics, Fairfax, VA).
RNA Preparation and Analysis
Leaf tissue for RNA isolation was harvested in liquid nitrogen and stored at −80°C. Total RNA was isolated with the Qiagen RNeasy plant mini kit (Valencia, CA), quantified spectrophotometrically at 260 nm. Reverse transcription and PCR were performed using 1 μg of total RNA and the Qiagen One-Step RT-PCR kit according to the manufacturer's instructions. PCR reactions were run for 25 or 28 cycles, which was determined in preliminary experiments to be in the linear range for these cDNA concentrations. Oligonucleotides for primers were purchased from Integrated DNA Technologies (Coralville, IA), using sequence data from the National Center for Biotechnology Information database. The following gene-specific primers were used for PCR amplification: GPA1, 5′-ATGGGCTTACTCTGCAGTA-3′ and 5′-CATAAAAGGCCAGCCTCCAGT-3′; AGB1, 5′-TCAAATCACTCTCCTGTGTCCTCC-3′ and 5′-TGTCTGTCTCCGAGCTCAAAGAACG-3′.
Protein Analysis
Membrane proteins were extracted using a buffer containing 50 mM Tris-HCl, pH 7.6, 1 mM EDTA, 1% Triton, 1% SDS, 5 mM DTT, and 1 mM PMSF and quantified using Bio-Rad's DC protein assay kit, which is compatible with Triton and SDS. Equal amounts of total membrane protein were loaded on a 12% polyacrylamide discontinuous gel (Bio-Rad mini electrophoresis system). After electrophoresis, proteins were transferred to Hybond-P PVDF membrane (Amersham, Piscataway, NJ) using a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). Immunoblotting was performed with rabbit polyclonal anti-Gα antibodies. After incubation with horseradish peroxidase–conjugated anti-rabbit IgG antibodies, proteins were detected using ECL Plus protein gel blotting detection reagents (Amersham) according to the manufacturer's instructions. The rabbit polyclonal antiserum was made against the peptide antigen NH2-CDETLRRRNLLEAGLL-CO2H linked to keyhole limpet hemocyanin via a thioether bond.
Supplementary Material
Acknowledgments
This work was supported by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service Grant 2002-35100-12139 to N.V.F. and by National Institute of General Medical Sciences (GM65989-01) and National Science Foundation (MCB-0209711) grants to A.M.J. We acknowledge Phillip Day for initial observations on G protein mutants, and we thank Robert Fluhr and Sally Assmann for critical comments. We thank Jonathan Jones for providing the seeds of atrbohD, atrbohF, and the double mutants.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Nina V. Fedoroff (nvf1@psu.edu).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.029603.
References
- Allan, A.C., and Fluhr, R. (1997). Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, M.E., Pennell, R.I., Meijer, P.J., Ishikawa, A., Dixon, R.A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. [DOI] [PubMed] [Google Scholar]
- Aro, E.M., and Ohad, I. (2003). Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 5, 55–67. [DOI] [PubMed] [Google Scholar]
- Baker, C.J., and Orlandi, E.W. (1999). Sources and effect of reactive oxygen species in plants. In Reactive Oxygen Species in Biological Systems: An Interdisciplinary Approach, D.L. Gilbert and C.A. Colton, eds (New York: Kluwer Academic Publishers), pp. 481–501.
- Baxter-Burrell, A., Yang, Z., Springer, P.S., and Bailey-Serres, J. (2002). RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296, 2026–2028. [DOI] [PubMed] [Google Scholar]
- Bolwell, G.P., Bindschedler, L.V., Blee, K.A., Butt, V.S., Davies, D.R., Gardner, S.L., Gerrish, C., and Minibayeva, F. (2002). The apoplastic oxidative burst in response to biotic stress in plants: A three-component system. J. Exp. Bot. 53, 1367–1376. [PubMed] [Google Scholar]
- Bolwell, G.P., and Wojtaszek, P. (1997). Mechanisms for the generation of reactive oxygen species in plant defence: A broad perspective. Physiol. Mol. Plant Pathol. 51, 347–366. [Google Scholar]
- Booker, F.L., Burkey, K.O., Overmyer, K., and Jones, A.M. (2004). Differential responses of G-protein Arabidopsis thaliana mutants to ozone. New Phytol. 162, 633–641. [DOI] [PubMed] [Google Scholar]
- Bowler, C., and Fluhr, R. (2000). The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 5, 241–246. [DOI] [PubMed] [Google Scholar]
- Chan, C.S., Guo, L., and Shih, M.C. (2001). Promoter analysis of the nuclear gene encoding the chloroplast glyceraldehyde-3-phosphate dehydrogenase B subunit of Arabidopsis thaliana. Plant Mol. Biol. 46, 131–141. [DOI] [PubMed] [Google Scholar]
- Chang, C.C., Ball, L., Fryer, M.J., Baker, N.R., Karpinski, S., and Mullineaux, P.M. (2004). Induction of ASCORBATE PEROXIDASE 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated with changes in photosynthesis. Plant J. 38, 499–511. [DOI] [PubMed] [Google Scholar]
- Conklin, P.L., and Last, R.L. (1995). Differential accumulation of antioxidant mRNAs in Arabidopsis thaliana exposed to ozone. Plant Physiol. 109, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas, F.J., Barroso, J.B., and del Rio, L.A. (2001). Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 6, 145–150. [DOI] [PubMed] [Google Scholar]
- Dat, J.F., Pellinen, R., Van De Cotte, B., Langebartels, C., Kangasjarvi, J., Inze, D., and Van Breusegem, F. (2003). Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 33, 621–632. [DOI] [PubMed] [Google Scholar]
- del Rio, L.A., Corpas, F.J., Sandalio, L.M., Palma, J.M., Gomez, M., and Barroso, J.B. (2002). Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J. Exp. Bot. 53, 1255–1272. [PubMed] [Google Scholar]
- Droge, W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. [DOI] [PubMed] [Google Scholar]
- Ermak, G., and Davies, K.J. (2002). Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 38, 713–721. [DOI] [PubMed] [Google Scholar]
- Fryer, M.J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P.M., and Baker, N.R. (2003). Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 33, 691–705. [DOI] [PubMed] [Google Scholar]
- Fryer, M.J., Oxborough, K., Mullineaux, P.M., and Baker, N.R. (2002). Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 53, 1249–1254. [PubMed] [Google Scholar]
- Grant, J.J., and Loake, G.J. (2000). Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 124, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze, S., Garcia, D.E., Mackie, K., Hille, B., Scheuer, T., and Catterall, W.A. (1996). Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380, 258–262. [DOI] [PubMed] [Google Scholar]
- Hummer, A., Delzeith, O., Gomez, S.R., Moreno, R.L., Mark, M.D., and Herlitze, S. (2003). Competitive and synergistic interactions of G protein beta(2) and Ca(2+) channel beta(1b) subunits with Ca(v)2.1 channels, revealed by mammalian two-hybrid and fluorescence resonance energy transfer measurements. J. Biol. Chem. 278, 49386–49400. [DOI] [PubMed] [Google Scholar]
- Jabs, T., Dietrich, R.A., and Dangl, J.L. (1996). Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273, 1853–1856. [DOI] [PubMed] [Google Scholar]
- Jabs, T., Tschope, M., Colling, C., Hahlbrock, K., and Scheel, D. (1997). Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. USA 94, 4800–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.M., and Assmann, S.M. (2004). Plants: The latest model system for G-protein research. EMBO Rep. 5, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.M., Ecker, J.R., and Chen, J.G. (2003). A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol. 131, 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, J.H., Bae, Y.S., and Lee, J.S. (2001). Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 126, 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski, S., Reynolds, H., Karpinska, B., Wingsle, G., Creissen, G., and Mullineaux, P. (1999). Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657. [DOI] [PubMed] [Google Scholar]
- Kato, C., Mizutani, T., Tamaki, H., Kumagai, H., Kamiya, T., Hirobe, A., Fujisawa, Y., Kato, H., and Iwasaki, Y. (2004). Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J. 38, 320–331. [DOI] [PubMed] [Google Scholar]
- Klusener, B., Young, J.J., Murata, Y., Allen, G.J., Mori, I.C., Hugouvieux, V., and Schroeder, J.I. (2002). Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 130, 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1997). Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M., Mori, I.C., Pei, Z.M., Leonhardt, N., Torres, M.A., Dangl, J.L., Bloom, R.E., Bodde, S., Jones, J.D., and Schroeder, J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, D., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Lapik, Y.R., and Kaufman, L.S. (2003). The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein alpha-subunit GPA1 and regulates seed germination and early seedling development. Plant Cell 15, 1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzi, M., Tipping, A.J., Marcus, S.E., Knox, J.P., Federico, R., Angelini, R., and McPherson, M.J. (2001). Analysis of the distribution of copper amine oxidase in cell walls of legume seedlings. Planta 214, 37–45. [DOI] [PubMed] [Google Scholar]
- Lease, K.A., Wen, J., Li, J., Doke, J.T., Liscum, E., and Walker, J.C. (2001). A mutant Arabidopsis heterotrimeric G-protein beta subunit affects leaf, flower, and fruit development. Plant Cell 13, 2631–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Li, J., Wang, D.Y., Li, Q., Xu, Y.J., Cui, K.M., and Zhu, Y.X. (2004). PPF1 inhibits programmed cell death in apical meristems of both G2 pea and transgenic Arabidopsis plants possibly by delaying cytosolic Ca2+ elevation. Cell Calcium 35, 71–77. [DOI] [PubMed] [Google Scholar]
- Luwe, M., Takahama, U., and Heber, U. (1993). Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiol. 101, 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam, R., and Fedoroff, N. (2003). Stress response, cell death and signalling: The many faces of reactive oxygen species. Physiol. Plant. 119, 56–68. [Google Scholar]
- Mahalingam, R., Shah, N., Scrymgeour, A., and Fedoroff, N. (2005). Temporal evolution of the Arabidopsis oxidative stress response. Plant Mol. Biol., in press. [DOI] [PubMed]
- Mehdy, M.C. (1994). Active oxygen species in plant defense against pathogens. Plant Physiol. 105, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard, M., and Grill, E. (2001). Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 508, 443–446. [DOI] [PubMed] [Google Scholar]
- Meinhard, M., Rodriguez, P.L., and Grill, E. (2002). The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214, 775–782. [DOI] [PubMed] [Google Scholar]
- Mullineaux, P., Ball, L., Escobar, C., Karpinska, B., Creissen, G., and Karpinski, S. (2000). Are diverse signalling pathways integrated in the regulation of Arabidopsis antioxidant defence gene expression in response to excess excitation energy? Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux, P., and Karpinski, S. (2002). Signal transduction in response to excess light: Getting out of the chloroplast. Curr. Opin. Plant Biol. 5, 43–48. [DOI] [PubMed] [Google Scholar]
- Myhre, O., Andersen, J.M., Aarnes, H., and Fonnum, F. (2003). Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 65, 1575–1582. [DOI] [PubMed] [Google Scholar]
- Nishida, M., Maruyama, Y., Tanaka, R., Kontani, K., Nagao, T., and Kurose, H. (2000). G alpha(i) and G alpha(o) are target proteins of reactive oxygen species. Nature 408, 492–495. [DOI] [PubMed] [Google Scholar]
- Noctor, G., Veljovic-Jovanovic, S., and Foyer, C.H. (2000). Peroxide processing in photosynthesis: Antioxidant coupling and redox signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns, S. (2003). G-proteins as transducers in transmembrane signalling. Prog. Biophys. Mol. Biol. 83, 101–130. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas, M., Narvaez-Vasquez, J., and Ryan, C. (2001). Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13, 179–191. [PMC free article] [PubMed] [Google Scholar]
- Overmyer, K., Brosche, M., and Kangasjarvi, J. (2003). Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 8, 335–342. [DOI] [PubMed] [Google Scholar]
- Overmyer, K., Tuominen, H., Kettunen, R., Betz, C., Langebartels, C., Sandermann, H., Jr., and Kangasjarvi, J. (2000). Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12, 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J., Gu, Y., Lee, Y., and Yang, Z. (2004). Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol. 134, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt, T. (2003). Chloroplast redox signals: How photosynthesis controls its own genes. Trends Plant Sci. 8, 33–41. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt, T., Schutze, K., Brost, M., and Oelmuller, R. (2001). A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J. Biol. Chem. 276, 36125–36130. [DOI] [PubMed] [Google Scholar]
- Pyke, K.A., and Leech, R.M. (1994). A genetic analysis of chloroplast division and expansion in Arabidopsis thaliana. Plant Physiol. 104, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.V., Lee, H., Creelman, R.A., Mullet, J.E., and Davis, K.R. (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12, 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, S.G., Bae, Y.S., Lee, S.R., and Kwon, J. (2000). Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 2000, PE1. [DOI] [PubMed] [Google Scholar]
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J.D. (1999). Rapid Avr9- and Cf-9 -dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeckles, V.C., and Chevonne, B.I. (1992). Crop responses to ozone. In Surface Level Ozone Exposures and Their Effects on Vegetation, A.S. Lefohn, ed (Chelsea, MI: Lewis Publishers), pp. 189–270.
- Sandermann, H., Jr. (2000). Active oxygen species as mediators of plant immunity: Three case studies. Biol. Chem. 381, 649–653. [DOI] [PubMed] [Google Scholar]
- Sandermann, H.J., Ernst, E., Heller, W., and Langebartels, C. (1998). Ozone: An abiotic elicitor of plant defence reactions. Trends Plant Sci. 3, 47–49. [Google Scholar]
- Sauer, H., Wartenberg, M., and Hescheler, J. (2001). Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 11, 173–186. [DOI] [PubMed] [Google Scholar]
- Scheel, D. (2002). Oxidative burst and the role of reactive oxygen species in plant-pathogen interactions. In Oxidative Stress in Plants, D. Inze and M. Van Montagu, eds (New York: Taylor and Francis), pp. 137–153.
- Schopfer, P., Liszkay, A., Bechtold, M., Frahry, G., and Wagner, A. (2002). Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214, 821–828. [DOI] [PubMed] [Google Scholar]
- Sharma, Y.K., and Davis, K.R. (1997). The effects of ozone on antioxidant responses in plants. Free Radic. Biol. Med. 23, 480–488. [DOI] [PubMed] [Google Scholar]
- Sharma, Y.K., Leon, J., Raskin, I., and Davis, K.R. (1996). Ozone-induced responses in Arabidopsis thaliana: The role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc. Natl. Acad. Sci. USA 93, 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suharsono, U., Fujisawa, Y., Kawasaki, T., Iwasaki, Y., Satoh, H., and Shimamoto, K. (2002). The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 99, 13307–13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki, M., Matsuyama, T., Kanna, M., Nakajima, N., Kubo, A., Aono, M., and Saji, H. (2003). Differential ozone sensitivity among Arabidopsis accessions and its relevance to ethylene synthesis. Planta 216, 552–560. [DOI] [PubMed] [Google Scholar]
- Tenhaken, R., and Rubel, C. (1998). Induction of alkalinization and an oxidative burst by low doses of cycloheximide in soybean cells. Planta 206, 666–672. [Google Scholar]
- Torres, M.A., Dangl, J.L., and Jones, J.D. (2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J.G., Temple, B., Boyes, D.C., Alonso, J.M., Davis, K.R., Ecker, J.R., and Jones, A.M. (2003). The beta-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15, 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J.G., Young, J.C., Im, K.H., Sussman, M.R., and Jones, A.M. (2001). Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292, 2066–2069. [DOI] [PubMed] [Google Scholar]
- Wang, X.Q., Ullah, H., Jones, A.M., and Assmann, S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292, 2070–2072. [DOI] [PubMed] [Google Scholar]
- White, P.J. (2000). Calcium channels in higher plants. Biochim. Biophys. Acta 1465, 171–189. [DOI] [PubMed] [Google Scholar]
- Wolfe, J.T., Wang, H., Howard, J., Garrison, J.C., and Barrett, P.Q. (2003). T-type calcium channel regulation by specific G-protein betagamma subunits. Nature 424, 209–213. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Qin, C., Zhao, J., and Wang, X. (2004). Phospholipase D alpha1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA 101, 9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Wang, C., Qin, C., Wood, T., Olafsdottir, G., Welti, R., and Wang, X. (2003). The oleate-stimulated phospholipase D, PLDdelta, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 15, 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D.W., and Song, C.P. (2001). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J., and Wang, X. (2004). Arabidopsis phospholipase Dalpha1 interacts with the heterotrimeric G-protein alpha-subunit through a motif analogous to the DRY motif in G-protein-coupled receptors. J. Biol. Chem. 279, 1794–1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.