Abstract
Obesity is a chronic disease associated with serious complications and increased mortality. Weight loss (WL) through lifestyle changes results in modest WL long-term possibly due to compensatory biological adaptations (increased appetite and reduced energy expenditure) promoting weight gain. Bariatric surgery was until recently the only intervention that consistently resulted in ≥ 15% WL and maintenance. Our better understanding of the endocrine regulation of appetite has led to the development of new medications over the last decade for the treatment of obesity with main target the reduction of appetite. The efficacy of semaglutide 2.4 mg/week—the latest glucagon-like peptide-1 (GLP-1) receptor analogue—on WL for people with obesity suggests that we are entering a new era in obesity pharmacotherapy where ≥15% WL is feasible. Moreover, the WL achieved with the dual agonist tirzepatide (GLP-1/glucose-dependent insulinotropic polypeptide) for people with type 2 diabetes and most recently also obesity, indicate that combining the GLP-1 with other gut hormones may lead to additional WL compared with GLP-1 receptor analogues alone and in the future, multi-agonist molecules may offer the potential to bridge further the efficacy gap between bariatric surgery and the currently available pharmacotherapies.
Keywords: Obesity, Pharmacotherapy, Bariatric surgery, Semaglutide, Tirzepatide, Liraglutide
This article is part of the Spotlight Issue on Obesity, Metabolism, and Diabetes.
1. Introduction
Obesity is a complex, chronic, progressive, and relapsing disease characterized by abnormal or excessive body fat that impairs health.1 It is one of the greatest global public health challenges, considering that 13% of the global population lives with obesity and that its prevalence has been tripled since 1975.2 Obesity drives the pathogenesis of multiple metabolic and mechanical complications including type 2 diabetes (T2D), hypertension, dyslipidaemia, sleep apnoea, cardiovascular disease, non-alcoholic fatty liver disease, infertility, and osteoarthritis3 which result in a decreased life expectancy of 5–20 years and increased healthcare costs.4–7
Lifestyle interventions are the cornerstones for the management of obesity, but even the most intensive programmes still commonly only achieve 5–10% weight loss (WL) and long-term weight maintenance remains a challenge.8 Although 5–10% WL reduces cardiometabolic risk factors it may not be enough to make a difference to the lives of people with BMI ≥35 kg/m2 (Class II obesity and above) and/or to reverse some obesity-related complications such as sleep apnoea and T2D.9,10 Until recently, pharmacotherapy to achieve and maintain ≥10% WL along with suitable tolerability and safety remained an unmet challenge. Bariatric surgery was the only intervention that consistently resulted in ≥15% WL and weight maintenance long-term.11 This amount of WL led to improved quality of life (QoL), significant health benefits and reduced mortality.11–13 Despite the considerable benefits of bariatric surgery, it is not feasible or scalable as a population-wide intervention. Recent clinical trials with advanced therapeutic candidates including new glucagon-like peptide-1 receptor agonists (GLP-1 RA) and dual agonists demonstrate that the gap of sustained WL between bariatric surgery and pharmacotherapy is gradually closing.14,15
Here, we review the available interventions for WL and weight maintenance with a focus on pharmacological therapies for obesity approved over the last decade as well as the emerging development of new obesity pharmacotherapies. We also discuss the future directions of obesity pharmacotherapy and the research priorities to support implementation of these treatments in clinical practice.
2. Obesity treatments
2.1. Lifestyle interventions
Lifestyle interventions usually include support for health-improving behavioural changes including diet, increasing physical activity, and reducing sedentary time. Moderate intensity lifestyle interventions such as 500–600 kcal deficit diet together with advice to increase physical activity to 150 min/week usually lead to a WL of 2–5% at 12 months and weight maintenance remain a challenge.
Intensive lifestyle interventions, which often include partial or total meal replacements, intensive behavioural therapy (IBT), and/or structured exercise, can lead up to ≈10% WL at the end of the first year.16,17
In the DROPLET study, a community delivered, low energy (810 kcal/day) total diet replacement programme with formula products as the sole food during the first 8 weeks followed by food reintroduction resulted in mean WL of 10.7 kg at 12 months compared with 3.1 kg at the usual care group in people with obesity.18 However, at the 3-year follow-up, mean WL was 6.2 kg at the intervention arm compared with 2.7 kg at the usual care19 with 24% of participants achieving ≥10% WL in the total diet replacement programme compared with 13% in usual care, suggesting that for this smaller number of patients this treatment was effective.19
Structured exercise programmes can usually add 1.5–3.5 kg WL to a dietary intervention, and they have multiple other health benefits such as improvement in body composition, physical function, and cardiorespiratory fitness.20 There is limited evidence on the effect of structured exercise on weight maintenance after weight-loss through diet,20 but a recent clinical trial showed that the addition of a 52-week structured but flexible aerobic exercise programme after a low-calorie diet programme (mean WL 12%) can lead to 4.1 kg less weight gain compared with people who continue with their usual physical activity.21 However, long-term maintenance of the recommended amount of moderate to vigorous physical activity can be challenging.22
The largest trial assessing the effectiveness of intensive lifestyle interventions was the Look AHEAD study, where 5145 people with obesity and T2D were randomized to receive intensive lifestyle support (intervention group) vs. a structured education programme (usual care group).23 The intensive lifestyle group lost 8.6% of their initial body weight at 1 year and 4.7% at 8 years while the usual care arm lost 0.7% at 1 year and 2.1% at 8 years.8 In the intensive lifestyle group, 37.7% of study participants achieved ≥10% WL at 1-year post-operatively, and 39.3% of them (324/2144, 15.1% of the total population at the intensive lifestyle group) were able to maintain ≥10% WL for 8 years.8
Overall, the Look AHEAD intervention did not demonstrate a reduction in cardiovascular events or a reduction at the risk for new onset heart failure or atrial fibrillation.24–26 However, in a post-hoc analysis, those participants in the intervention arm who lost ≥10% of their baseline body weight during the first year (early good responders) had 20% lower risk for cardiovascular events over a 10 years follow-up period.27 Similarly, a 10% decrease in BMI over the first year in participants at the Look AHEAD study was associated with a 31% lower risk of incident heart failure, when a 10% decrease in BMI over a 4-year follow-up period was associated with a 20% lower risk of incident heart failure.25 These results suggest that ≥10% WL is associated with cardiovascular benefits for people with obesity and T2D. Moreover, the REVERSE-AF study also showed that ≥10% weight reduction results in 88% reversal from persistent to paroxysmal or no atrial fibrillation.28
In general, weight regain is common after lifestyle interventions and approximately 80% of weight lost is expected to be regained over the next 5 years.29 Only 10–25% of individuals who will undergo different intensity lifestyle interventions will be able to lose and maintain ≥10% WL long-term. The rest of individuals could be considered non-responders to the lifestyle intervention and will need further interventions to achieve and maintain significant WL.
2.1.1. Why is it so challenging to maintain weight loss with lifestyle changes?
The weight regain after significant WL with lifestyle changes is not simply attributable to the loss of motivation or compliance from the patients. Instead, it is driven by potent biological mechanisms that stimulate food intake and reduce energy expenditure on the background of an ‘obesogenic’ environment, where ultra-processed, high-calorie foods are easily accessible, physical activity is reduced and sedentary time increased.30,31 The major drivers of weight regain after treatments that caused significant WL, include the persistence of a lower resting metabolic rate (RMR) (metabolic adaptation), the lower energy consumption during weight-bearing activities, and the persistence of increased appetite, probably mediated through long-lasting increased orexigenic and decreased anorexic signals.30,31 Overall, the increased appetite and the reduced energy expenditure during the weight-reduced state results in a feeling of constant and exhausting effort to maintain the achieved WL and to prevent the seemingly unavoidable weight regain over time.30,32
RMR is mainly determined by body composition and accounts for 60–70% of 24 h total energy expenditure in humans.31,33 However, RMR in response to WL is often reduced to a greater extent than would be expected based on the measured changes in body composition. This physiological mechanism is called ‘metabolic adaptation’ and is one of the reasons why the body resists further WL and individuals regain weight so easily.33 For example, the participants of the ‘Biggest Loser’ television program lost, on average, 40% of their body weight, and their mean RMR decreased by 610 kcal/day—this was on average 275 kcal/day lower compared with what was expected based on their body composition.34 This metabolic adaptation persisted 6 years later despite regaining two-thirds of the lost weight.33,34
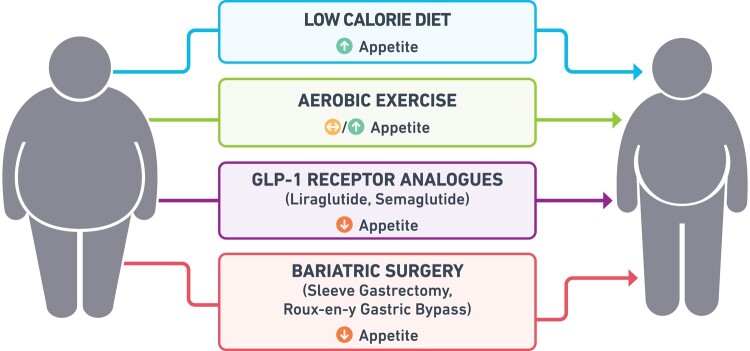
In addition, most people who manage to lose ≥10% of their body weight through low-calorie diet experience an increase in their appetite compared with baseline (Figure 1).35 Potential mediators of the increased appetite are the elevated levels of the hunger hormone ghrelin as well as the reduction in leptin and perhaps also satiety gut hormones such as peptide YY (PYY), amylin, and cholecystokinin.35 These changes remain even after 52 weeks from the completion of the low-calorie diet and despite that participants experience weight regain, for instance, 5.5 kg at the end of the cited study.35 Studies assessing the appetite-related responses during and after single bouts of continuous aerobic exercise indicate that subjective feelings of appetite are transiently suppressed during exercise in people with obesity; but energy intake is minimally affected.36,37 Regarding the chronic effects of aerobic exercise on appetite parameters, the results are inconsistent—however, a small increase in hunger at fasting state with a subsequent increase in satiety post-meal and without significant increase on energy intake has been found in a recent systematic review and meta-analysis.36,38
Figure 1.
The effect of different weight loss interventions [low-calorie diet, exercise, pharmacotherapy (GLP-1 receptor analogues), and bariatric surgery] on appetite.
Increased appetite likely plays a quantitatively greater role on weight regain than the decreased energy expenditure because the feedback circuits controlling long-term energy intake have greater overall strength compared with the feedback circuits controlling energy expenditure.30,32 So, if we consider obesity as a disease of dysregulated appetite where the increased hunger and/or the reduced satiety are the main symptoms,39 then lifestyle interventions which usually result in increase of appetite may not effectively treat the symptoms of the underlying disease and this will lead in the majority of the cases to weight regain long-term despite the successful initial WL. However, WL maintenance can occur in a smaller number of people who despite WL with lifestyle interventions do not experience increased appetite, suggesting that lifestyle interventions can also be considered as effective obesity treatments in a small number of patients and that there is individual variability in response to different lifestyle interventions regarding appetite signals and weight regain.31,40
Anti-obesity medications can also effectively treat obesity by counteracting the increased drive to eat and the impaired satiation associated with WL by lifestyle changes and could help with further WL and weight maintenance.41–43 However, as with most management techniques for chronic diseases, obesity relapses if the treatment is stopped.44
2.2. The example of bariatric surgery
Bariatric surgery is a collective term for surgical treatment of obesity. It is so far the most successful existing approach for safe and effective obesity treatment and results in sustained WL while at the same time reduces appetite.11,45 In the Swedish obese subjects (SOS) study, bariatric surgery was able to induce and maintain ≥15% mean WL over 20 years follow-up (compared with 1% WL at the usual care group).11 This amount of sustained WL was associated with improvement in QoL and obesity-related complications, reduction by 53% in fatal and 33% in total cardiovascular events, and reduction by 29% in mortality compared with the usual care group,11,12,46,47 providing a WL threshold associated with multiple clinically important benefits. Moreover, bariatric surgery in SOS study reduced the risk for new onset heart failure by 35% and for new diagnosis of atrial fibrillation by 29% compared with the usual care group.48,49 The benefits of bariatric surgery on reducing cardiovascular events, cardiovascular death, all-cause mortality and new onset heart failure compared with the non-surgical management are consistent across multiple observational, matched-cohort studies, especially in people with obesity and T2D.50,51
The most commonly performed bariatric procedures in the SOS study were gastric band and vertical banded gastroplasty with fewer patients undergoing Roux-en-Y gastric bypass (RYGB).11 Today, the two most commonly performed bariatric procedures (≈85% of bariatric procedures worldwide) are sleeve gastrectomy (SG) and RYGB.52 RYGB results in 30% WL over the first post-operative year and a sustained 25–27% WL long-term.11,53 SG leads to 25% WL at the first post-operative years and around 20% WL long-term.53 WL and WL maintenance after bariatric surgery are achieved as a consequence of voluntary reduced food intake due to reduced appetite (Figure 1) rather than through restriction, malabsoprtion or increased energy expenditure.54 One of the potential mediators for the reduced appetite and food intake after bariatric surgery is the changes in the peripheral signals of body weight regulation (how the gut communicates with the brain) through alteration of gut anatomy. More specifically, both RYGB and SG substantially increase the secretion of multiple satiety gut hormones after food intake, including GLP-1 and PYY and when the action of these hormones was blocked after RYGB, the food intake increased by 20%,55 supporting a role of these gut hormones in early post-prandial satiety.
However, not every person with severe and complex obesity wants or is fit enough to undergo surgery, and there is no surgical capacity to operate on every person that qualifies for bariatric surgery. So, pharmacotherapies mimicking some of the post-operative physiological changes after bariatric surgery would be a logical approach to try to achieve similar WL.
2.3. Approved pharmacotherapies for obesity over the last decade
Numerous obesity medications targeting appetite and reward centres have been tried in the past to support WL but the majority has been withdrawn due to safety concerns.56–58 For example, sibutramine was withdrawn due to increased risk of cardiovascular events (myocardial infarction and non-fatal stroke) in people with obesity and pre-existing cardiovascular conditions when rimonabant was withdrawn due to increased risk of psychiatric adverse events including depressed mood disorders, anxiety, and suicide.57,58 More recently, lorcaserin was withdrawn from the market because of a signal of increased cancer risk.59 Nevertheless, the better understanding over the last years of the peripheral and central signals and mechanisms involved in WL and WL maintenance have contributed to the development of more effective and safe weight-loss medications. Orlistat, which was approved in 1999 for obesity treatment have demonstrated its safety and efficacy in multiple trials, but it has, at best, modest effect (WL 3–5%) as result of reduced absorption of ingested fat and behavioural changes (to avoid steatorrhea).60
Over the past decade, several agents that act by reducing hunger or promote satiation have been approved by regulatory authorities worldwide for chronic weight management, including phentermine plus topiramate (approved only in the USA), bupropion plus naltrexone, and the GLP-1 RAs liraglutide 3 mg and semaglutide 2.4 mg.
2.3.1. Phentermine-topiramate
Phentermine-topiramate (PHEN-TPM) is an oral medication approved for obesity treatment in the USA, but not in Europe due to concerns about the medication’s long-term cardiovascular safety. The fixed-dose combination approved in the USA contains PHEN doses from 3.75 to 15 mg and TPM doses from 23 to 92 mg for daily administration.
PHEN is a sympathomimetic amine which acts as an appetite suppressant via the central nervous system.61 It is indicated for short-term use in weight management in USA, however, long-term data are not available. TPM is an anticonvulsant indicated for use in the treatment of migraine and epilepsy.61 One of the known effects of TPM is also a decrease in appetite.
In people without diabetes, 56 weeks of PHEN-TPM 15/92 extended release (ER) in combination with 500 kcal/day deficit diet resulted in 10.9% WL compared with 1.6% with placebo and 32.3% of participants achieved more than ≥15% WL.62 Similar results were reported at the 2-year follow-up of PHEN-TPM 15/92 ER63 (Table 1).
Table 1.
Large multi-centre clinical trials for approved obesity pharmacotherapies over last decade and tirzepatide in populations without diabetes (or with majority of participants without diabetes)
| Lifestyle intervention | Comparator | Duration (week) | BMI baseline (drug/comparator) | WL (drug/comparator) ETD vs. comparator |
≥5% WL (%) (drug/comparator) |
≥ 10% WL (%) (drug/comparator) |
≥ 15% WL (%) (drug/comparator) |
Comment | |
|---|---|---|---|---|---|---|---|---|---|
|
Phentermine/Topiramate ER
3.75/23, 7.5/46, or 15/92 |
|||||||||
| Gadde, 201164 CONQUER (7.5/46 and 15/92) |
500 kcal/day deficit diet + advise for PA | vs. placebo | 56 | 36.6/36.7 | −7.8% to −9.8%/−1.2% ETD: −6.6% to – 8.6% |
62–70/21 | 37–48/7 | NR | 15.6% of participants had T2D |
| Allison, 201262 EQUIP (3.75/23 and 15/92) |
500 kcal/d deficit diet + advise for PA | vs. placebo | 56 | 41.9–42.6/42.0 | −5.1% to −10.9%/−1.56% ETD: −3.54% to −9.34% |
44.9–66.7/17.3 | 18.8–47.2/7.4 | 7.3–32.3/3.4 | |
| Garvey 201263 SEQUEL (7.5/46 and 15/92) |
500 kcal/d deficit diet + advise for PA | vs. placebo | 108 | 36.1–36.2/36 | −9.3% to −10.5%/−1.8% ETD: −7.5% to –9.7% |
75.2–79.3/30 | 50.3–53.9/11.5 | 24.2–31.9/6.6 | |
|
Naltrexone/Buproprion SR
16/360 or 32/360 |
|||||||||
| Greenway 201065 COR-I (16/360 and 32/360) |
500 kcal/d deficit diet + advise for PA | vs. placebo | 56 | 36.1–36.2/36.2 | −5.0% to −6.1%/−1.3% ETD: - 3.7% to −4.8% |
39–48/16 | 20–25/7 | 9–12/2 | |
| Apovian 201366 COR-II (32/360) |
500 kcal/d deficit diet + advise for PA | vs. placebo | 56 | 36.2/36.1 | −6.4%/−1.2% ETD: −5.2% |
50.5/17.1 | 28.3/5.7 | 13.5/2.4 | Primary outcome was at 28 weeks |
| Wadden 201167 COR-BMOD (32/360) |
IBT-28 group sessions plus calorie deficit diet and advise for PA | vs. placebo | 56 | 36.3/37 | −9.3%/−5.1% ETD: −4.2% |
66.4/42.5 | 41.5/20.2 | 29.1/10.9 | |
| Liraglutide 3mg | |||||||||
| Pi-Sunyer 201568 SCALE-Obesity |
500 kcal deficit diet + advise for PA | vs. placebo | 56 | 38.3/38.3 | −8.0%/−2.6% ETD: −5.4% |
63.2/27.1 | 33.1/10.6 | 14.4/3.5 | |
| Le Roux 201769 SCALE-pre-diabetes |
500 kcal deficit diet + advise for PA | vs. placebo | 160 | 38.8/39.0 | −6.1%/−1.9% ETD: −4.3% |
49.6/23.7 | 24.8/9.9 | 11/3.1 | 79% reduction at the risk of developing diabetes in people with pre-diabetes |
| Wadden 202070 SCALE-IBT |
IBT-23 brief sessions plus diet 1200–1800 kcal/d + advise for PA | vs. placebo | 56 | 39.3/38.7 | −7.5%/−4.0% ETD: −3.4% |
61.5/38.8 | 30.5/19.8 | 18.1/8.9 | |
| Wadden 2013 41 SCALE −Maintenance |
500kcal deficit diet after achieving ≥5% WL with 1200–1400kcal diet | vs. placebo | 56 | 36/35.2 | −6.2%/−0.2% ETD: −6.1% |
50.5/21.8 | 26.1/6.3 | NR | Weight maintenance study—participants randomized after they have achieved ≥5% weight loss with diet |
| Blackman 201671 SCALE sleep apnea |
500 kcal deficit diet + advise for PA | vs. placebo | 32 | 38.9/39.4 | −5.7%/−1.6% ETD: −4.2% |
46.3/18.5 | 23.4/1.7 | NR | Apnoea hypopnea Index (events/hour) reduced by 6.1 events/hour |
| Kelly 202072 Liraglutide for Adolescents with Obesity |
Individualized counselling on healthy nutrition + advise for PA for 60 min/d | vs. placebo | 56 | 35.3/35.8 | −2.65%/ +2.37% ETD: −5.01% |
43.3/18.7 | 26.1/8.1 | NR | Participants were adolescents 12 to <18 years old |
| Semaglutide 2.4mg a | |||||||||
| Wilding, 202115 STEP-1 |
500 kcal/day deficit diet + advise for PA | vs. placebo | 68 | 37.8/38.0 | −14.9%/−2.4% ETD: −12.4% |
86.4/31.5 | 69.1/12 | 50.5/4.9 | |
| Wadden 201373 STEP-3 |
Low-calorie diet (1000–1200 kcal) for 8 weeks and IBT (30 counselling sessions) | vs. placebo | 68 | 38.1/37.8 | −16%/−5.7% ETD: −10.3% |
86.6/47.6 | 75.3/27 | 55.8/13.2 | |
| Rubino, 202174 STEP-4 |
500 kcal/day deficit diet + advise for PA | vs. placebo | 0 →68 20→68 |
38.4 34.5/34.1 |
−17.4%/−5.0% −7.9%/ +6.9% ETD: −14.8% | 88.7/47.6 | 79/20.4 | 63.7/9.2 | Medication withdrawal study—all participants received Semaglutide 2.4 mg for 20 weeks and then randomized to placebo vs. Semaglutide 2.4 mg for next 48 weeks |
| Kadowaki 202275 STEP-6 | 500 kcal/day deficit diet + advise for PA | vs. placebo | 68 | 86.9/90.2 | −13.2%/−2.1% ETD: −11.06% |
83/21 | 61/5 | 41/3 | Study in East Asian population (Japan, South Korea) − 25% of participants had T2D |
| Rubino, 202276 STEP-8 |
500 kcal/day deficit diet + advise for PA | vs. liraglutide 3mg | 68 | 37/37.2 | −15.8%/−6.4% ETD: −9.4% |
87.2/58.1 | 70.9/25.6 | 55.6/12.0 | |
| Tirzepatide 5–15mg a | |||||||||
| Jastreboff A, 2022 SURMOUNT-177 |
500 kcal/day deficit diet and advise for PA | vs. placebo | 72 | 37.4–38.2/38.2 | −15.0% to −20.9%/−3.1% ETD: −11.9% to −17.8% |
85.1–90.9/34.5 | 68.5–83.5/18.8 | 48.0–70.6/8.8 | |
WL, weight loss; ER, extended release; SR, slow release; ETD, estimated treatment difference; PA, physical activity; IBT, intensive behavioural therapy; T2D, type 2 diabetes.
aData presented as treatment-policy estimand.
In people with T2D, 56 weeks of PHEN-TPM ER 15/92 reduces body weight by 9.6% compared with 2.6% with placebo and improved HbA1c by 1.6% (17.5 mmol/mol) compared with 1.2% (13.1 mmol/mol) with placebo78 (Table 2). The most commonly reported adverse events are upper respiratory tract infection, constipation, insomnia, paraesthesia, sinusitis, taste change and dry mouth.62–64 PHEN-TPM has also a warning of birth defects (cleft lip and palate) in the offspring of pregnant women taking the medication, due to the known teratogenic effect of TPM.
Table 2.
Large multi-centre clinical trials for approved obesity pharmacotherapies over last decade and tirzepatide in people with type 2 diabetes
| Lifestyle change | Comparator | Background therapy | Duration (week) | BMI baseline (drug/comparator) | Weight loss (drug/comparator) ETD vs. comparator |
HbA1c (%) baseline (drug/comparator) |
HbA1c (%) change (drug/comparator) |
≥ 10% WL (%) (drug/comparator) |
≥ 15% WL (%) (drug/comparator) |
HbA1c ≤ 7% (drug/comparator) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phentermine/Topiramate ER 15/92 | |||||||||||
| Garvey 201478 OB-202/DM-230 |
500 kcal/day deficit diet and advise for PA | vs. placebo | Diet ± oral glucose lowering meds | 56 | 35.5/35.2 | −9.6%/−2.6% ETD: −7% | 8.8/8.5 | −1.6%/−1.2% ETD: −0.4% | 37/9 | NR | 53/40 |
| Naltrexone/Buproprion SR 32/360 | |||||||||||
| Hollander 201379 COR-Diabetes |
500 kcal/d deficit diet + advise for PA | vs. placebo | Not on or stable dose glucose-lowering meds | 56 | 36.7/36.3 | −5.0%/−1.8% ETD: −3.2% |
8.0/8.0 | −0.6%/−0.1% ETD: −0.5% |
26.3/8.0 | 44.1/26.3 | |
| Liraglutide 3mg | |||||||||||
| Davies 201580 SCALE Diabetes |
500 kcal/d deficit diet + advise for PA | vs. placebo vs. liraglutide 1.8 mg |
diet + exercise or ≤ 3 oral glucose-lowering meds | 56 | 37.1/37.4 37.1/37.4 |
−6.0%/−2.0% ETD: −4% −6.0%/−4.7% ETD: −1.3% |
7.9/7.9 7.9/8.0 |
−1.3/−0.3 ETD: −0.93 −1.3/−1.1 ETD: −0.2 |
25.2/6.7 25.2/15.9 |
NR | 69.2/27.2 69.2/66.7 |
| Garvey 202081 SCALE-Insulin |
IBT-23 sessions | vs. placebo | Basal insulin + ≤ 2 oral glucose-lowering meds |
56 | 35.9/35.3 | −5.8%/−1.5% ETD: −4.3% |
7.9/8.0 | −1.1/−0.6 ETD: −0.5 |
22.8/6.6 | NR | NR |
| Semaglutide 2.4 mg a | |||||||||||
| Davies 202182 STEP-2 |
500 kcal/d deficit diet + advise for PA | vs. placebo vs. semaglutide 1 mg |
diet + exercise or ≤ 3 oral glucose-lowering meds | 68 | 35.9/35.9 35.9/35.3 |
−9.64%/−3.42% ETD: −6.21% − 9.64%/−6.99% ETD: −2.65% |
8.1/8.1 8.1/8.1 |
−1.6/−0.4 ETD: −1.2 −1.6/−1.5 ETD: −0.2 |
45.6/8.2 45.6/28.7 |
25.8/3.2 25.8/13.7 |
78.5/26.5 78.5/72.3 |
| Tirzepatide 5–15 mg | |||||||||||
| Rosenstock 202114 SURPASS-1a |
No new diet or exercise programme | vs. placebo | Diet and exercise | 40 | 31.5– 32.2/31.7 | −6.3 to −7.8 kg/−1.0 kg ETD: −5.3 kg to −6.8 kg |
7.80–7.97/8.05 | −1.69 to −1.75/−0.09 ETD: −1.6 to −1.66 |
27 to 38/0 | 12 to 23/0 | 78–85/23 |
| Frias 202183 SURPASS-2a |
Not described | vs. semaglutide 1mg | Metformin ≥ 1500 mg/d |
40 | 33.8– 34.5/34.2 | −7.6 to −11.2 kg/−5.7 kg ETD: −1.9 to −5.5 kg |
8.26–8.32/8.25 | −2.01 to −2.3/−1.86 ETD: −0.15 to −0.45 |
34–57/24 | 15 to 36/8 | 82 to 86/79 |
| Ludvik 202184 SURPASS-3a |
Not described | vs. insulin degludec | Metfromin ± SGLT-2i | 52 | 33.4–33.7/33.4 | −7 to −11.3 kg/ +1.9 kg ETD: −8.9 to −13.2 kg |
8.17–8.21/8.12 | −1.85 to −2.14/−1.25 ETD: −0.60 to −0.89 |
35–58/3 | 12 to 35/0 | 79–83/58 |
| Del Prato 2021 85 SURPASS-4a |
Not described | vs. insulin glargine | ≤ 3 oral glucose –lowering meds | 52 | 32.5–32.8/32.5 | −6.4 to −10.6 kg/ +1.7 kg ETD: −8.1 to −12.3kg |
8.52–8.60/8.51 | −2.11 to −2.41/−1.39 ETD: −0.72 to −1.02 |
32–59/2 | 13–33/ < 1 | 75–85/49 |
| Dahl 202186 SURPASS-5a |
Not described | vs. placebo | Insulin glargine ± metformin | 40 | 33.4–33.6/33.2 | −5.4 to −8.8 kg/ +1.6 kg ETD: −7.1 to −10.5 kg |
8.23–8.36/8.37 | −2.11 to −2.40/−0.86 ETD: −1.24 to −1.53 |
21–42/1 | 7–24/0 | 85–90/35 |
| Inagaki N87 SURPASS J-mono (Japanese population) |
Not described | vs. dulaglutide 0.75mg | Diet and exercise | 52 | 28–28.6/27.8 | −5.4 to −9.4 kg/−0.4kga ETD: −4.9 to −8.9 kg |
8.2/8.2 | −2.24 to −2.57/−1.27a ETD: −1.1 to −1.5 |
34–67/3b | 16–45/ < 1b | 94–99/67b |
| Kadowaki T88 SURPASS J-combob (Japanese population) |
Not described | No comparator | 1 oral glucose-lowering med | 52 | 27.6–28.4 | −3.8 to −10.3 kgb | 8.5–8.6 | −2.5 to −3b | 20–64b | 7–41b | 93–98b |
WL, weight loss; ER, extended release; SR: slow release; ETD, estimated treatment difference; PA, physical activity; IBT, intensive behavioural therapy; T2D, type 2 diabetes; SGLT-2i: sodium-glucose co-transporters inhibitor.
aData presented as treatment-policy estimand.
bData presented as efficacy estimand, as there is no available data for treatment-policy estimand.
The improvement in weight with PHEN/TPM was associated with improvements in QoL89 and cardiometabolic risk factors.62–64 A retrospective analysis of PHEN used concurrently with TPM, either separately or in fixed-dose combination showed a trend for a lower rate of major adverse cardiovascular events (MACE) and other cardiovascular outcomes among those receiving PHEN/TPM (including the fixed-dose combination) than among the unexposed cohort (HR: 0.24; 95% CI, 0.03 to 1.70 for fixed-dose PHEN-TPM).90 However, the cardiovascular safety of this treatment requires evaluation in an adequately powered outcome trial.
2.3.2. Naltrexone-buproprion
Naltrexone is an opioid receptor antagonist that is approved for the treatment of alcohol and opioid dependence.91 Bupropion is a dopamine and norepinephrine reuptake inhibitor that was first approved for the treatment of depression and later for smoking cessation.91 The exact mechanism whereby the naltrexone/bupropion (NB) combination leads to WL is not fully understood, but it is likely that it promotes satiety, reduces food intake and may enhance energy expenditure through actions at the hypothalamus and mesolimbic dopamine circuit. Participants in clinical trials who responded to NB as an obesity treatment reported feeling less hungry and more full compared with those receiving placebo and they found it easier to control their food cravings.65
In people without diabetes, NB 32/360 together with a 500 kcal deficit diet resulted in 6.3% WL at 52 weeks compared with 0.9% in the placebo group.65 In COR-I and COR-II studies, 25–28.3% of those on NB 32/360 achieved ≥10% WL.65,66 Additionally, NB 32/360 in combination with an IBT program and diet resulted in 9.3% WL vs. 5.1% with placebo.67 In people with obesity and T2D, NB 32/360 resulted in 5% mean WL at 56 weeks compared with 1.8% with placebo.79 26.3% of participants receiving NB 32/360 achieved ≥10% WL and HbA1c was reduced by 0.6% compared with 0.1% in those receiving placebo.79
The most common adverse events with NB 32/360 are nausea, headache, constipation, dry mouth, anxiety, dizziness, hypertension, and vomiting.65,79 The NB is contraindicated in people with epilepsy as buproprion is associated with dose-related risk of seizures.
Physical function and self-esteem improved with NB 32/360 compared with placebo.92,93 The WL achieved with NB 32/360 was also associated with improvements in some cardiometabolic risk factors such as HDL and hepatic insulin resistance markers, however the systolic blood pressure (SBP) and diastolic blood pressure improved more in the placebo group compared with the NB 32/360.66
A clinical trial on cardiovascular outcomes for NB was terminated early due to early release of the interim analysis performed after 50% of planned events (HR 0.88; adjusted 99.7% CI 0.57–1.34 compared with placebo).94 So, the cardiovascular safety of NB remains uncertain and will require further revaluation in adequately powered outcome trials.
2.3.3. Currently approved pharmacotherapies for obesity based on gut hormones
2.3.3.1. GLP-1 receptor agonists
GLP-1 is an incretin hormone secreted predominantly from L cells located in the small intestine in response to food intake.95 In addition to the glucose-lowering actions such as stimulation of glucose-induced insulin secretion, delay in gastric emptying and inhibition of glucagon secretion, exogenous GLP-1 infusion in humans resulted recurrently in reduced calorie intake, reduced appetite and effects on the reward system without direct changes in energy expenditure.96–98
GLP-1 RAs have been developed initially for the treatment of T2D, however due to their efficacy in inducing WL and reducing appetite, they have been repurposed in higher doses as treatments for obesity. Exogenous GLP-1 and GLP-1 RAs may predominantly access the brain via the leaks in the blood–brain barrier, where the underlying neuronal tissue shows a dense expression of GLP-1 receptors. In animal experiments, the effect of GLP-1 RAs on food intake is entirely dependent on central nervous system mechanisms,99 and in humans this is supported by imaging experiments.100,101
2.3.3.2. Liraglutide 3 mg
In 2014, liraglutide 3 mg once daily became the first GLP-1 RA to be approved for the treatment of adults with obesity and in 2021 it was also approved for adolescents ≥12 years old.72 Liraglutide 3 mg is a long-acting GLP-1 RA and mechanistic studies have demonstrated that it increases post-prandial satiety and fullness, reduces hunger and prospective food consumption and decreases ad libitum food intake at lunch by ≈16% (136 kcal).43 Moreover, the mean 24 h energy expenditure was reduced by ≈5% (139 kcal), which was mainly explained by the decrease in food intake and body weight (Figure 1).43
Liraglutide 3 mg in combination with a 500 kcal/day deficit diet resulted in 6.1–8% WL in adults without diabetes (Table 1).68,70 For people with T2D, liraglutide 3 mg resulted in 5.8%—6% WL compared with 1.5–2% WL with placebo (Table 2).80,81 HbA1c reduced by 1.6% with liraglutide 3 mg and 0.4% with placebo.80,81
Liraglutide 3 mg is also effective for weight maintenance after initial WL through diet intervention. In SCALE-Maintenance study, after a 5% WL obtained via a diet over 4–12 weeks, liraglutide 3 mg resulted in further 6.1% WL at 56 weeks compared with placebo (6.2 vs. 0.2%).41
The most common side effects include mild to moderate gastrointestinal problems such as nausea, diarrhoea, constipation and (more rarely) vomiting which occurred primarily within the first 4–8 weeks after initiation of liraglutide treatment.68,80 Pancreatitis, a rare side effect of GLP-1 RA was reported in 0.7% of participants at liraglutide 3 mg arm [0.3 events per 100 person-years of observation (PYO)] at the 3-year follow-up of SCALE-Prediabetes trial compared with 0.3% of participants at placebo arm (0.1 events per 100 PYO) and the vast majority of the cases were graded as mild.69
The QoL and especially the physical function improved more with use of liraglutide 3 mg compared with placebo, with people achieving ≥15% WL having the most benefit.102 In addition, WL with liraglutide 3 mg improves also multiple other obesity-related complications, including reduction in T2D incidence by 79% in people with pre-diabetes after 3 years of treatment compared with placebo69 and reduction in apnoea-hypopnea index in people with sleep apnoea (however, without change in requirement of continuous positive airway pressure support).71
Liraglutide 1.8 mg (dose approved for T2D treatment) was shown to reduce cardiovascular events in patients with T2D and established cardiovascular disease (LEADER trial) followed for up to 5 years.103 On the other hand, in a 24-week clinical trial in people with chronic heart failure (left ventricular ejection fraction ≤45%, n = 241) with and without diabetes, liraglutide 1.8 mg once daily did not improve the left ventricular systolic function compared with placebo and raised some concerns regarding cardiac safety of the medication at this population.104 However, a subanalysis of LEADER trial [including 1667 people with T2D and heart failure at baseline, New York Heart Association (NYHA) functional Class I–III] found that the beneficial effects of liraglutide 1.8 mg vs. placebo on major cardiovascular events and mortality were consistent in people with and without heart failure and there was no difference between the two groups in hospitalization for heart failure, suggesting that liraglutide 1.8mg use is safe for people with T2D and heart failure (NYHA Class I–III).105
Liraglutide 3 mg (dose approved for obesity treatment) has been shown to improve multiple cardiometabolic risk factors, but studies to evaluate cardiovascular outcomes in people with obesity have not been done. An analysis of data from SCALE programme demonstrated that liraglutide 3 mg use was not associated with excess cardiovascular risk compared with comparators (HR 0.42, 95% CI 0.17–1.08), however the confidence interval was wide.106
2.3.3.3. Semaglutide 2.4 mg
Semaglutide 2.4 mg once weekly is a new long-acting GLP-1 RA which was approved in 2021 for treatment of obesity in the USA and Europe. Semaglutide 2.4 mg reduces energy intake in people with obesity during an ad libitum lunch by 35% (−224 kcal vs. placebo) through appetite reduction.42 People received semaglutide reported reduced hunger, increase in fullness and satiety, better control of eating and fewer and weakened food cravings.42 Moreover, the rate of gastric emptying was reduced as well as the prospective food consumption for participants receiving semaglutide 2.4 mg.42
In clinical trials, 56 weeks of semaglutide 2.4 mg in combination with a 500 kcal/day deficit diet resulted in 14.9% WL vs. 2.4% with placebo in people without diabetes.15 50.5% of those receiving semaglutide 2.4 mg achieved ≥15% WL and 32% achieved ≥20% WL compared with 4.9 and 1.7%, respectively at the placebo group (Table 1).15 In direct comparison with liraglutide 3 mg, semaglutide 2.4 mg results in more than double WL (Table 1, STEP-8).76 Additionally, 68 weeks of semaglutide 2.4 mg in combination with IBT and a low-calorie diet during the first 8 weeks resulted in 16% WL vs. 5.7% in the placebo group.73
In people with T2D, WL with semaglutide 2.4 mg once weekly was 9.6 vs. 7% with semaglutide 1 mg once weekly (dose approved for T2D treatment) and 3.4% with placebo (Table 2).82 HbA1c was reduced by 1.6% at 68 weeks with semaglutide 2.4 mg compared with 1.5% with semaglutide 1 mg and 0.4% with placebo (Table 2).82
Why semaglutide and liraglutide are less effective in people with T2D compared with populations without diabetes is not fully understood, but the different population demographics in the T2D clinical trials (higher percentage of men), the use of background glucose-lowering medications that can contribute to weight gain (such as sulphonylureas) and the reduction of glycosuria due to improvement of glycaemic control with GLP-1 RAs may contribute to these results.107
WL with semaglutide was associated with improvements in QoL and participants in the semaglutide group were more likely to have clinically meaningful within-person improvements in physical function than with placebo.15 Mild to moderate gastrointestinal problems such as nausea, vomiting and diarrhoea were the most common side effects of semaglutide.15,108 Adverse events leading to discontinuation of semaglutide 2.4 mg ranged between 3 and 7% in STEP programme.15,73–76,82 Acute pancreatitis was reported in 0.2% of participants at the STEP-1 trial (0.2 events per 100 PYO).15
Despite the improvement in multiple cardiovascular risk factors with semaglutide 2.4 mg, the cardiovascular safety profile has not yet been confirmed in people with obesity. A large clinical trial (SELECT, NCT03574597) is currently taking place to assess the effect of semaglutide on major cardiovascular outcomes in people with obesity and established cardiovascular disease. However, in SUSTAIN 6 study, semaglutide 1 mg in people with T2D and established cardiovascular disease resulted in reduction of MACE by 26% compared with placebo (mainly due to reduction in stroke incidence), providing some reassurance.109 In SUSTAIN-6, there was no effect of semaglutide 1 mg compared with placebo on hospitalization for heart failure,109 but the impact of semaglutide 2.4 mg in people with heart failure with preserved ejection fraction and obesity will be assessed at the ongoing STEP-HFpEF (NCT04788511) and STEP-HFpEF DM (NCT04916470) trials.
Finally, oral semaglutide—an approved treatment for T2D at the doses of 7 and 14 mg once daily—is currently undergoing phase 3 trials as treatment for obesity at the dose of 50 mg once daily. In a phase 2, dose-finding trial, 40 mg of oral semaglutide in people with T2D resulted in 6.9% WL compared with 6.4% with injectable semaglutide 1 mg and 1.2% with placebo.110
2.3.4. Summary of approved pharmacotherapies for obesity management
Until 2021, the approved pharmacotherapies for chronic weight management (PHEN/TPM, buproprion/naltrexone, liraglutide 3 mg, orlistat) could result in 5–10% mean WL and WL maintenance when combined with moderate intensity lifestyle interventions. In 2021, Semaglutide 2.4 mg once weekly became the first approved obesity pharmacotherapy which leads to ≈15% WL in people without diabetes when combined with moderate intensity lifestyle interventions, which almost doubles the effectiveness on WL over previous obesity pharmacotherapies with a good tolerability and safety profile in clinical trials.
3. Emerging treatments for obesity
3.1. Gut hormones-dual agonists and triple agonists
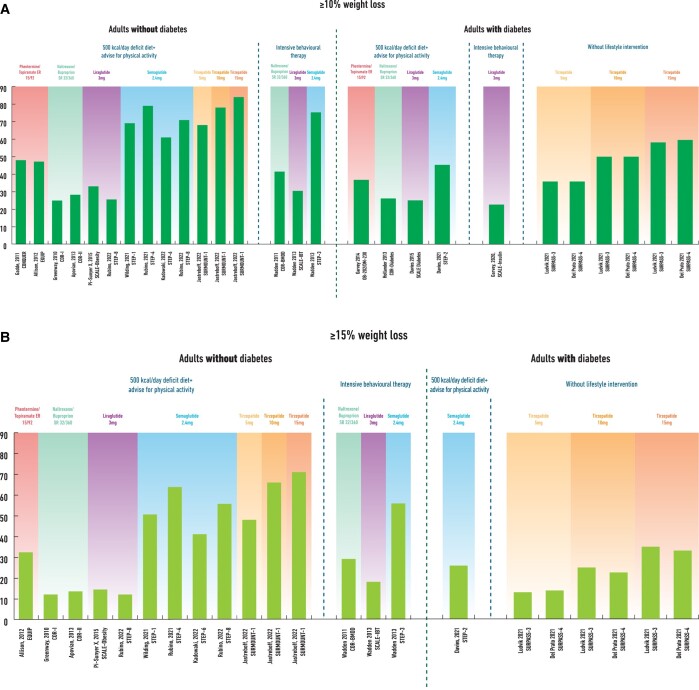
Although GLP-1 RAs and especially semaglutide are effective treatments for obesity and T2D, there is still significant efficacy gap regarding WL between the currently available pharmacotherapies and bariatric surgery (Figures 2A and B). Based on the example of bariatric surgery where multiple gut hormones are increased post-operatively, the combination of GLP-1 with other gut hormones such as glucose-dependent insulinotropic peptide (GIP), glucagon, amylin, and PYY may complement and enhance further the GLP-1 effect leading to additional WL, increased energy expenditure and improved metabolic outcomes.111
Figure 2.
The efficacy gap in weight loss between lifestyle interventions, approved pharmacotherapies for obesity over last decade (plus tirzepatide) and bariatric surgery in adults without type 2 diabetes (Figure 2A) and in adults with type 2 diabetes (Figure 2B). Data in Figure 2A are based on published clinical trials used (A) lifestyle interventions (500 kcal deficit diet plus advise for physical activity or intensive behavioural therapy) plus placebo (green background), 15,62,65–68,70 (B) liraglutide 3 mg,68,70 naltrexone/buproprion 32/360,65–67 phentermine/topiramate 15/92,62 semaglutide 2.4 mg,15,74–76 and tirzepatide 5 and 15 mg77 plus lifestyle interventions (approved obesity pharmacotherapies plus tirzepatide, orange background) or c) sleeve gastrectomy112 and Roux-en-Y gastric bypass112 (bariatric surgery, blue background) in adults without type 2 diabetes. Data in Figure 2B are based on published clinical trials used a) lifestyle interventions (500 kcal deficit diet plus advise for physical activity or intensive behavioural therapy) plus placebo (green background),78–80,82 (B) liraglutide 3 mg,80,81 naltrexone/buproprion 32/360,79 phentermine/topiramate 15/92,78 semaglutide 2.4 mg82 plus lifestyle interventions, and tirzepatide 5 mg and 15mg84,85,87 (approved obesity pharmacotherapies plus tirzepatide, orange background) or c) sleeve gastrectomy113 and Roux-en-Y gastric bypass113 (bariatric surgery, blue background) in adults with type 2 diabetes.
Tirzepatide is the first dual co-agonist (acting on GLP-1/GIP receptors) which has been approved for treatment of T2D based on the findings of the clinical trials from the SURPASS programme. Moreover, tirzepatide is currently undergoing an extensive programme of phase 3 clinical trials as treatment for obesity (SURMOUNT). The recently published SURMOUNT-1 trial randomized 2539 participants with obesity (without diabetes) to three different doses of tirzepatide (5, 10, and 15 mg, n = 1896) or placebo (n = 643) with follow-up 72 weeks and showed that tirzepatide 5–15 mg results in 15–20.9% WL when combined with moderate intensity lifestyle interventions compared with 3.1% WL with placebo.77
Other dual co-agonists acting on GLP-1/amylin receptors and GLP-1/glucagon receptors are also being assessed in early phase clinical trials as potential treatments for obesity and metabolic complications.114,115 Moreover, triple agonists, for example, GLP-1/GIP/glucagon agonists are being explored as potential treatments, although data from clinical trials are limited and in early stage.
3.2. GLP-1/GIP combinations for people with obesity and T2D
GIP is a 42-amino acid peptide secreted by endocrine K cells in the duodenum and jejunum in response to nutrient ingestion and is an incretin hormone. In people without diabetes, GIP stimulates insulin secretion but does not change glucagon release during hyperglycaemia, whereas it increases glucagon release without affecting insulin secretion during hypoglycaemia.116,117 In the context of T2D, the ability of GIP to stimulate insulin secretion and to ameliorate glycaemia is impaired.118
Regarding the effect of GIP on appetite, it has only been assessed in a few acute studies in humans.101,119,120 Unlike GLP-1, exogenous GIP infusion does not seem to reduce appetite and the combination of GLP-1 with GIP in people with and without T2D does not lead to any more significant appetite reduction compared with GLP-1 alone.101
On this background, it came as a surprise that the GLP-1/GIP co-agonist, tirzepatide, had glucose-lowering and weight-loss activities that exceeded those of GLP-1 RA comparators in clinical trials.83,121 Tirzepatide is a once weekly unimolecular agonist of both GLP-1 and GIP receptors which has a deliberate bias towards GIP over GLP-1 activity (it possesses five-fold increased relative potency at human GIP receptor when compared with GLP-1 receptor).122 This has been provided as a potential explanation for the more prominent effects on weight and glucose compared with the more balanced dual agonists targeting GLP-1 and GIP receptors equally (although this is difficult to understand given that GIP alone has no effect on food intake in man). Further research is required to understand the mechanisms mediating the effects of tirzepatide on weight and glycaemia. Compared with GLP-1, however, tirzepatide acts as a biased agonist with little beta-arrestin recruitment and receptor internalization, which may explain the superior activity on target cells.123
The phase 3 trials with tirzepatide doses 5–15 mg in people with T2D who are overweight or have obesity (SURPASS programme) have shown marked improvements both on WL and glycaemia, despite that tirzepatide in SURPASS programme was not combined with lifestyle changes (as primary outcome was improvement in glycaemia rather than WL), such as in the SCALE and STEP programmes. In SURPASS-3 study, 52 weeks of tirzepatide 15 mg led to mean WL of 11.3 kg (treatment-policy estimand) compared with 1.9 kg weight gain with insulin degludec and 35% of those on tirzepatide 15 mg were able to achieve ≥15% WL compared with 0% at the insulin degludec group (Table 2).84 Moreover, HbA1c reduced with tirzepatide 15 mg by 2.14% (treatment-policy estimand) in SURPASS-3.84 Similar findings regarding WL and glycaemic improvement after 52 weeks of tirzepatide use were also reported at the SURPASS-4 trial (Table 2). 85
In direct comparison with semaglutide 1 mg (dose for treatment of T2D), tirzepatide 15 mg led to 5.5 kg more WL at 40 weeks and 36% of people achieved ≥15% WL compared with 8% with semaglutide 1 mg (SURPASS-2).83 Tirzepatide 15 mg resulted also in an HbA1c reduction of 0.45% more compared with semaglutide 1 mg (Table 2).83
The safety and efficacy of tirzepatide as treatment for obesity in people without diabetes has been assessed at the recently published SURMOUNT-1 clinical trial. The study found that tirzepatide 5–15 mg together with a moderate intensity lifestyle intervention for 72 weeks resulted in 30–57% of participants achieving ≥20% WL and 15–36% ≥ 25% WL compared with 3 and 1.5% with placebo, respectively.77 The physical function was improved more with tirzepatide compared with placebo at the SURMOUNT-1 study and the mean reduction in total body fat mass was 33.9% with tirzepatide compared with 8.2% with placebo.77 Between participants with pre-diabetes, 95.3% had reverted to normoglycaemia in the tirzepatide group at 72 weeks, when compared with 61.9% of participants in the placebo group.77
Nausea, diarrhea, vomiting, and constipation were the most commonly reported side effects both in SURPASS programme and in SURMOUNT-1 study. Most of them were minor to moderate in severity and temporary. Tirzepatide did not increase the risk of hypoglycaemia in SURPASS programme unless it was combined with insulin or sulfonylureas.14,85,86 Decreased appetite was also a commonly reported side effect. Adverse events leading to discontinuation of medication ranged from 3–11% with tirzepatide 5 mg to 7–11% with tirzepatide 15 mg at SURPASS programme and between 4.3 and 7.1% at SURMOUNT-1.14,77,83–86 In SURMOUNT-1, the incidence of pancreatitis was low and similar between tirzepatide and placebo group (0.2% in each group), when cholecystitis was reported more frequently with tirzepatide compared with placebo (the overall incidence was still low, < 0.6%), possibly due to the considerable weight reduction with the medication.77
Cardiometabolic risk factors (SBP, total cholesterol, HDL, and LDL) are all improved with tirzepatide in people with and without T2D.14,77,85 In SURPASS-4 study, people with T2D and increased cardiovascular risk were randomized to tirzepatide 5–15 mg (997 participants) or insulin glargine (1005 participants) for at least 52 weeks, with treatment continued for a maximum of 104 weeks aiming to provide some initial evidence on cardiovascular safety of tirzepatide.85 An adjudicated 4-point MACE (cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina) occurred in 109 participants and was not increased on tirzepatide compared with glargine (HR 0.74, 95% CI 0.51–1.08).85 Additionally, a pre-specified cardiovascular meta-analysis including all seven randomized controlled trials from the SURPASS programme compared the time to first occurrence of a 4-point MACE (cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina) between pooled tirzepatide groups and control groups.124 Overall, 142 participants (109 from the SURPASS-4 study) had at least one MACE-4 event. The hazard ratios comparing tirzepatide vs. controls were 0.80 (95% CI, 0.57–1.11) for MACE-4; 0.90 (95% CI, 0.50–1.61) for cardiovascular death; and 0.80 (95% CI, 0.51–1.25) for all-cause death.124 These results suggest that that there is no excess cardiovascular risk with tirzepatide, however the definite impact of tirzepatide on cardiovascular disease in people with T2D will be addressed in the ongoing SURPASS-CVOT study (NCT04255433). The impact of tirzepatide in people with heart failure with preserved ejection fraction and obesity will also be assessed at the SUMMIT trial (NCT04847557).
Overall, the GLP-1/GIP co-agonist tirzepatide, is the first approved treatment for T2D which at the higher dose (15 mg) consistently leads to >10% mean WL in studies with at least 52 weeks follow-up (Table 2), despite that at the SURPASS programme there was no additional lifestyle intervention. Moreover, in people with obesity (without diabetes) tirzepatide 10 and 15 mg when combined with moderate intensity lifestyle interventions can lead to ≈20% mean WL with good tolerability and similar side effect profile to that of GLP-1 RAs.
4. A new era in obesity pharmacotherapy
The WL achieved with semaglutide 2.4 mg in people with obesity and the dual agonist tirzepatide in people with T2D and/or obesity suggests that we are entering a new era in obesity pharmacotherapy where ≥15% WL and maintenance is feasible (Figure 3A and B).
Figure 3.
Categorical weight loss (≥10% in Figure 3A, ≥ 15% in Figure 3B) in large clinical trials with the approved obesity pharmacotherapies over the last decade (plus tirzepatide) in people with and without type 2 diabetes (studies with follow-up 52–72 weeks).
These obesity treatments will be presented to patients only if the healthcare providers understand that obesity is a chronic disease which is difficult to treat with lifestyle changes alone and the management usually requires a multimodal treatment strategy including pharmacotherapy.107 Both clinicians and public or private payers need to understand that sustained WL requires lifelong treatment of obesity as otherwise weight regain will occur when treatment is discontinued.44 There are however important research priorities over the next years to allow the new obesity pharmacotherapies to become more widely acceptable and available.
4.1. The cardiovascular safety of new pharmacotherapies for obesity
In the past, multiple WL medications have been withdrawn due to safety concerns.56–58 Today, good quality evidence is lacking regarding the cardiovascular benefits of the approved pharmacotherapies for obesity as discussed before, although the results of the cardiovascular outcome trials conducted in people with T2D are likely to also apply to people with obesity. Currently, the SELECT study is taking place and will assess the effect of semaglutide 2.4 mg on cardiovascular outcomes in people with obesity and established cardiovascular disease but without diabetes. Moreover, SURPASS-CVOT study will compare dulaglutide vs. tirzepatide on cardiovascular outcomes in people with T2D and established cardiovascular disease and will provide evidence on whether the dual GLP-1/GIP co-agonist is as safe as GLP-1 RAs. Future studies will be needed to establish the safety and benefits of the new medications, including the new combinations of gut hormones.
4.2. How we can best combine the new pharmacotherapies with lifestyle interventions to achieve weight loss targets and healthy weight maintenance?
The majority of the clinical trials combine pharmacotherapies for obesity with moderate intensity lifestyle changes. However, intensive lifestyle interventions including low-calorie diets and structured exercise may be used to support WL in clinical practice. How to best combine an intensive lifestyle intervention with pharmacotherapies for obesity treatment requires further investigation.
A single-centre clinical trial assessed the efficacy in weight maintenance of liraglutide 3 mg and/or a 52-week structured exercise programme vs. placebo and continuing with usual physical activity, in people who achieved at least 5% WL through an 8-week low-calorie diet (‘responders’ to low-calorie diet).21 The combination of liraglutide 3 mg with a structured exercise programme (n = 49) resulted in 15.7% mean total WL at 60 weeks after initiation of the low-calorie diet with 49% of participants achieving ≥15% WL and 32% achieving ≥20% WL.21 In participants receiving placebo and continuing with usual activity (n = 49) after at least 5% WL with the 8-week low-calorie diet, mean WL at 60 weeks was 6.2% and only 10% of participants were able to achieve ≥15% WL.21 Importantly, the addition of the exercise programme also caused a greater loss of fat mass, preservation of lean mass, improved cardiorespiratory fitness and overall improvement in cardiometabolic risk factors compared with the WL obtained with liraglutide 3 mg alone.21 The WL achieved in this trial (at 60 weeks) with the combination of liraglutide 3 mg plus structured exercise for weight maintenance in people who were ‘responders’ to the initial 8-week low-calorie diet is similar to the WL reported with semaglutide 2.4 mg once weekly at 68 weeks when combined with a 500 kcal deficit diet15 (STEP-1 study, Table 1) and significantly more than the 8% WL in SCALE-Obesity where liraglutide 3 mg was combined with 500 kcal deficit diet and advise for physical activity (Table 1).68
On the other hand, in the STEP-3 study where semaglutide 2.4 mg was combined with IBT and an 8-week low-calorie diet,15,73 the WL achieved at 68 weeks was similar to the WL reported when Semaglutide 2.4 mg was combined with a 500 kcal/day deficit diet15 (16 vs. 14.9%) and less when compared with patients who could tolerate Semaglutide 2.4 mg in the STEP 4 study (16 vs. 17.4%).74 These results suggest the possibility that intensive lifestyle treatments may not be able to provide additional WL to effective obesity medications such as semaglutide and tirzepatide. However, lean muscle mass loss remains a challenge, because with effective obesity treatments patients consume small amount of calories and may not be enough space in their diet to ensure adequate protein intake, thus rendering them catabolic and burning muscle mass. The addition of exercise to these effective pharmacological interventions may further improve body composition, physical function, and cardiorespiratory fitness.21 Further studies are necessary to help us understand how to best combine the new pharmacotherapies with different lifestyle interventions not only to maximize WL, but also to optimize body composition and health benefits.
Moreover, the sustainability of WL achieved through different combinations of intensive lifestyle and pharmacotherapy needs to be assessed with long-term studies, together with the changes in body composition, appetite, and energy expenditure over time.
4.3. The long-term clinical effectiveness and cost-effectiveness of new pharmacotherapies
Similar to treatments for other chronic diseases such as hypertension or diabetes, at the moment that the treatment for obesity stops the disease will relapse and body weight will increase again, as was seen in the STEP 1 extension study.44 In this exploratory analysis, a representative subset of participants (n = 327) who completed 68 weeks of treatment with semaglutide 2.4 mg in STEP-1 trial and achieved mean WL of 17.3% of their baseline weight, underwent an off-treatment extension (including lifestyle intervention) for an additional year.44 One year after withdrawal of semaglutide 2.4 mg and lifestyle intervention, participants regained two-thirds of their prior WL resulting in final total WL of 5.6% from baseline weight.44 Cardiometabolic improvements seen with semaglutide treatment were also reverted towards baseline at the end of the off-treatment extension period for most parameters.44
Additionally, in the STEP-4 trial, adults who were overweight or had obesity completed a 20-week run-in of weekly treatment with subcutaneous semaglutide, 2.4 mg, with a mean WL of 10.6%, and then were randomized to continued treatment with subcutaneous semaglutide vs. placebo for an additional 48 weeks.74 At the end of the trial, people receiving semaglutide 2.4 mg lost a further 7.9% of their body weight whereas those on placebo regained 6.9% of their body weight (almost 70% of the weight lost).74
These results suggest that ongoing treatment is required to maintain improvements in weight, although this topic so far has been poorly explored. Perhaps, an initial WL could be maintained long-term with lower doses of anti-obesity medications. For example, 1.2 mg liraglutide was sufficient to maintain WL for a year after an initial 12% WL obtained with a low-calorie diet.125
Currently, there is a lack of long-term data on the effectiveness and safety of obesity pharmacotherapies and on the improvement in obesity-related comorbidities (so far the longest follow-up study is for liraglutide 3 mg up to 3 years after initiation of treatment).69 The release of the STEP-5 study results (presented but not published yet) demonstrate that 104 weeks of semaglutide 2.4 mg once weekly results in 15.2% WL compared with 2.6% with placebo, with 36.1% of participants in semaglutide group achieving ≥20% WL at the end of the study.126
Studies in real-world settings with long-term follow-up on the new obesity pharmacotherapies may also provide detailed information about the cost-effectiveness of these interventions and may facilitate their approval by public and private payers for long-term use. Long-term data will provide additional information on the potential long-term side effects of obesity pharmacotherapy including adverse events due to significant long-term WL. WL through bariatric surgery has been associated with increased risk for fractures127 and increased risk of self-harm behaviors128—whether these complications will be observed with the new pharmacotherapies need further investigation.
In summary, further evidence on the long-term safety (especially cardiovascular safety), clinical effectiveness and cost-effectiveness of the new pharmacotherapies for obesity in real-world settings will support their wider use and acceptability by healthcare professionals, individuals living with obesity and healthcare systems.
5. Other gut hormones in the pipeline as potential future therapeutic candidates
5.1. Amylin analogues
Amylin is a pancreatic β-cell hormone that is co-secreted with insulin in response to food intake.129 It functions as a satiety signal, acting upon brain regions involved in both homeostatic and hedonic appetite regulation; it also slows the gastric emptying, and thereby suppresses post-prandial glucagon responses to meals.129
Cagrilintide is a weekly subcutaneous amylin analogue that is under development as treatment for obesity. In a phase 2 study, people with obesity were randomly assigned to cagrilintide 0.3–4.5 mg, liraglutide 3.0 mg, or placebo for 26 weeks.114 Cagrilintide led to dose-dependent weight reductions and greater WL at all doses compared with placebo at 26 weeks.114 WL with cagrilintide 4.5 mg (10.8%) was greater than with liraglutide 3.0 mg (9.0%) or placebo (3.0%).114 Cagrilintide 4.5 mg resulted in ≥10% WL in 53.5% of participants and ≥15% WL in 18.7% of participants.114 Gastrointestinal disorders were the most common adverse events, primarily nausea.114
Different doses of cagrilintide were also evaluated in a phase 1b study in combination with semaglutide 2.4 mg. At 20 weeks, cagrilintide 2.4 mg and semaglutide 2.4 mg led to 17.1% WL compared with 9.8% loss with semaglutide 2.4 mg plus placebo.130 This increased WL was not accompanied by worsening tolerability, suggesting that the two apparently complementary mechanisms of action may be combined for potential additive WL. Further clinical trials assessing the safety and efficacy of the combination of semaglutide plus cagrilintide as treatment of obesity are expected to take place over next years.
5.2. Glucagon agonists
Glucagon is a 29-amino acid peptide that is secreted from pancreatic α-cells in response to low levels of blood glucose or increasing levels of amino acids. It increases blood glucose through stimulation of glycogenolysis in the liver, but it also reduces food intake, increases satiety and possibly energy expenditure.131
The concept of GLP-1/glucagon co-agonists includes the concurrent activation of the GLP-1 receptors leading to decreased energy intake and the glucagon receptors causing increased energy expenditure and reduced energy intake. Animal studies with potent GLP-1/glucagon co-agonists were promising, however the results of the clinical studies in humans for the dual GLP-1/glucagon receptor agonist cotadutide, currently under development, were less impressive.115,132 In a phase 2b, randomized, double-blind study, adults who were overweight or had obesity with T2D were randomized to receive cotadutide 100 , 200 or 300 μg; placebo; or open-label liraglutide 1.8 mg for 54 weeks.115 The body weight reduction with cotatutide 300 μg was 5.02% at week 54 (compared with −0.68% in placebo group and −3.33% with liraglutide 1.8 mg) and 15.5% of people on cotadutide 300 μg achieved ≥10% WL. Cotadutide also significantly lowered HbA1c by 1.03–1.19% at week 54 while reduction with placebo was 0.45 and 1.17% with liraglutide 1.8 mg.115 Gastrointestinal disorders, including diarrhoea, nausea, and vomiting, were the most commonly reported adverse events with cotadutide at any tested dose and more patients stopped cotatutide due to side effects compared with placebo or liraglutide 1.8 mg.115 However, glucagon is also thought to increase hepatic lipid oxidation and inhibit lipogenesis and ad hoc analysis of this trial with cotatutide demonstrated improvements in hepatic parameters and supports further evaluation of cotadutide in non-alcoholic steatohepatitis.115
Another GLP-1/glucagon receptor dual agonist (SAR425899) was recently evaluated in single-ascending dose and multiple-ascending dose phase 1 trials where it was given once a day over 28 days.133 At the highest maintenance doses tested, there was a reduction of HbA1c by 0.54–0.59% when given to patients who were overweight or had obesity with T2D, and mean WL of 2.4–5.5 kg over the 28 days.133 SAR425899 was generally well tolerated, with treatment-emergent adverse effects of reduced appetite and nausea.133
Regarding triple agonists, evidence from experimental studies in animals suggests that the addition of GIP activity into dual GLP-1 and glucagon receptor agonism provides improved WL and glycaemic control while protecting against the diabetogenic risk of chronic glucagon agonism.133,134 Importantly, the addition of the GIP component may allow an increased potency of the agonist at the glucagon receptor. However, very limited data is available from clinical trials—a phase 1 clinical study in healthy individuals (lean to overweight) with a new unimolecular GLP-1, GIP, and glucagon receptor triagonist (SAR441255) showed that the triagonist improved glycaemic control during a mixed-meal tolerance test and was well tolerated.134 Additionally, a recent abstract presented the results of a 12-week, phase 1 study, where the safety and tolerability of multiple doses of another GLP-1/GIP/glucagon co-agonist (LY3437943) compared with placebo were assessed in 72 people with T2D. The triple co-agonist showed similar safety and tolerability profile to other incretins and led to placebo-adjusted reduction in HbA1C of up to 1.56% and to placebo-adjusted weight reduction of up to 8.96 kg.135 Further trials in larger populations of people with T2D and obesity are required to confirm the therapeutic potential of GLP-1/GIP/glucagon receptor triagonists.
5.3. PYY analogues
PYY is a peptide hormone that is co-secreted with GLP-1, particularly from distal epithelial L cells in the gut in response to food intake. PYY3–36 is the active form of the peptide and acts as a satiety hormone, suppressing food intake via activation of Y2 receptors in the hypothalamus. Infusion of PYY3–36 in people with obesity causes a 30% reduction in food intake.136 A recent phase 1 study investigating a long-acting PYY3–36 analogue demonstrates a reduction of 38–55% in food intake vs. placebo at 30 days after initiation of treatment and WL of 2.87–3.58 kg compared with placebo.137
The co-administration of GLP-1 and PYY3-36 in humans also reduced energy intake compared with placebo and more than the sum of the individual infusions, demonstrating a synergistic effect.138,139 A long-acting PYY analogue in combination with semaglutide is now being assessed in a phase 2 study as treatment for obesity (NCT04969939).
5.4. Summary of potential future obesity treatments based on gut hormones
Except of the approved GLP-1 RAs for weight management and the GLP-1/GIP co-agonist tirzeparide which has completed phase 3 trials as treatment for T2D and/or obesity, multiple other gut hormones such as amylin, glucagon and PYY are being tested in early phase clinical trials as potential treatments for obesity and obesity-related complications, either as monotherapies (amylin, PYY) or in combination with GLP-1 as dual (GLP-1/amylin, GLP-1/PYY, and GLP-1/glucagon) and triple co-agonists (GLP-1/GIP/glucagon). A phase 3 trial assessing the efficacy and safety of the combination of semaglutide 2.4 mg with cagrilintide 2.4 mg once weekly for people with obesity and T2D is expected to start at the last trimester of 2022 (REDEFINE 2, NCT05394519). In the near future, there is a real prospect of the above described gut hormone combinations to deliver improved weight-related outcomes over the currently available treatments for obesity and T2D.
6. Obesity treatments not based on gut hormones
It is common practice in chronic diseases such as hypertension or diabetes to target multiple mechanisms to achieve optimal disease management. Similarly, in obesity, despite that research has mainly focused in combinations of gut hormones, new treatments targeting different pathways such as Setmelanotide and Bimagrumab have also been developed.
Setmelanotide is an melanocortin-4 receptor agonist that reduces bodyweight and hunger in individuals with ultra-rare obesity genetic disorders caused by leptin receptor (LEPR) or pro-opiomelanoctin (POMC) deficiency (80% of participants in POMC trial and 45% of participants in LEPR trial achieved at least 10% WL after 1 year of medication use).140 Individuals with these genetic variants have severe hunger (hyperphagia) and early-onset severe obesity resulting from disruption at the melanocortin pathway, which plays pivotal part in body weight regulation.140 Setmelanotide has now been approved in the USA and Europe for chronic weight management in patients 6 years and older with obesity resulting from POMC, PCSK1, or LEPR deficiency confirmed by genetic testing.
Bimagrumab is a fully human monoclonal antibody that binds the activin type 2 receptors and prevents the actions of natural ligands, including myostatin and activin A, that otherwise negatively regulate skeletal muscle growth.141,142 In phase 2 clinical trials, 48 weeks of treatment with Bimagrumab in individuals living with obesity and T2D resulted in WL of 6.5% compared with 0.5% (−0.18 kg) in the placebo group, with a reduction in total body fat mass by 20.5% (−7.49 kg) and an increase in the lean mass by 3.6%, suggesting that Bimagrumab could be a potential treatment for sarcopenic obesity. HbA1c fell by 0.76% compared with a rise by 0.04% with placebo.141 Dietary intake based on 24 h recall did not differ from baseline at the end of the study, suggesting that increase in energy expenditure could be a mechanism of action for the WL with this medication.141
Overall, Setmelanotide is considered the first approved personalized treatment for obesity (for individuals with confirmed ultra-rare obesity genetic disorders) when the early phase trials with Bimagrumab provide evidence that we may succeed in improving the quality of WL and preserve lean mass in the treatment of obesity.
7. Conclusion
WL ≥10% and maintenance is challenging with lifestyle changes alone due to compensatory increases in appetite and reduction in energy expenditure. Bariatric surgery is currently the most effective intervention for sustained WL ≥20% and leads to multiple health benefits, but surgical procedures are difficult to scale to treat the entire population. Over the last decade, a number of medications have been approved for chronic weight management in people with obesity but Semaglutide 2.4 mg once weekly (a new GLP-1 RA) is the first one which leads to ≈15% WL (in people without diabetes). Moreover, the WL achieved in people with T2D and/or obesity at phase 3 clinical trials with the recently approved for T2D management dual agonist tirzepatide suggests that combination of gut hormones may lead to additional WL compared with GLP-1 RAs alone. Judging from the results of clinical trials in individuals with T2D, these treatments may also have beneficial cardiovascular effects. Additional research assessing long-term safety, effectiveness, and cost-effectiveness of these new pharmacotherapies (semaglutide 2.4 mg and tirzepatide) in trials and real-world settings will help us to understand better their position in the weight management algorithms for people with obesity and/or T2D. Furthermore, novel pharmacological interventions combining GLP-1 with other gut hormones are currently under development and may offer in the future the potential to bridge further the efficacy gap between bariatric surgery and the currently available pharmacotherapies.
Contributor Information
Dimitris Papamargaritis, Diabetes Research Centre, Leicester General Hospital, University of Leicester College of Medicine Biological Sciences and Psychology, Leicester LE5 4PW, UK.
Carel W le Roux, Diabetes Complications Research Centre, Conway Institute, University College Dublin, Dublin 4, Ireland; Diabetes Research Centre, Ulster University, Coleraine BT52 1SA, UK.
Jens J Holst, Department of Biomedical Sciences and the NNF Center for Basic Metabolic Research, University of Copenhagen Panum Institute, Copenhagen 2200, Denmark.
Melanie J Davies, Diabetes Research Centre, Leicester General Hospital, University of Leicester College of Medicine Biological Sciences and Psychology, Leicester LE5 4PW, UK.
Funding
This work was supported by the NIHR Leicester Biomedical Research Centre. D.P. is funded through an National Institute for Health and Care Research (NIHR) Clinical Lectureship.
Data availability
Data derived from sources in the public domain. Reference details are provided in full.
References
- 1. Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017;18:715–723. [DOI] [PubMed] [Google Scholar]
- 2. WHO . Obesity and Overweight.
- 3. Haslam DW, James WP. Obesity. Lancet 2005;366:1197–1209. [DOI] [PubMed] [Google Scholar]
- 4. Biener A, Cawley J, Meyerhoefer C. The high and rising costs of obesity to the US health care system. J Gen Intern Med 2017;32:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. Jama 2003;289:187–193. [DOI] [PubMed] [Google Scholar]
- 6. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cawley J, Biener A, Meyerhoefer C, Ding Y, Zvenyach T, Smolarz BG, Ramasamy A. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm 2021;27:354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wadden TA, Bantle JP, Blackburn GL, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Dutton GR, Egan C, Evans M, Foreyt JP, Sengardi SG, Gregg EW, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Montgomery B, Nathan DM, Nelson J, Patricio J, Peters A, Pi-Sunyer FX, Pownall H, Rickman AD, Vitolins M, Walkup MP, West DS, Williamson D, Wing RR, Wyatt H, Yanovski SZ. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tahrani AA, Morton J. Benefits of weight loss of 10% or more in patients with overweight or obesity: a review. Obesity (Silver Spring ) 2022;30:802–840. [DOI] [PubMed] [Google Scholar]
- 10. Cefalu WT, Bray GA, Home PD, Garvey WT, Klein S, Pi-Sunyer FX, Hu FB, Raz I, Van Gaal L, Wolfe BM, Ryan DH. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors’ expert forum. Diabetes Care 2015;38:1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sjöström L. Review of the key results from the Swedish obese subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219–234. [DOI] [PubMed] [Google Scholar]
- 12. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 13. Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 14. Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021;398:143–155. [DOI] [PubMed] [Google Scholar]
- 15. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989. [DOI] [PubMed] [Google Scholar]
- 16. Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Welsh P, Kean S, Ford I, McConnachie A, Messow CM, Sattar N, Taylor R. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–355. [DOI] [PubMed] [Google Scholar]
- 17. Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring ) 2006; 14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Astbury NM, Aveyard P, Nickless A, Hood K, Corfield K, Lowe R, Jebb SA. Doctor referral of overweight people to low energy total diet replacement treatment (DROPLET): pragmatic randomised controlled trial. BMJ 2018;362:k3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Astbury NM, Edwards RM, Ghebretinsea F, Shanyinde M, Mollison J, Aveyard P, Jebb SA. Extended follow-up of a short total diet replacement programme: results of the doctor referral of overweight people to low energy total diet replacement treatment (DROPLET) randomised controlled trial at 3 years. Int J Obes (Lond ) 2021;45:2432–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bellicha A, van Baak MA, Battista F, Beaulieu K, Blundell JE, Busetto L, Carraça EV, Dicker D, Encantado J, Ermolao A, Farpour-Lambert N, Pramono A, Woodward E, Oppert JM. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: an overview of 12 systematic reviews and 149 studies. Obes Rev 2021;22(Suppl 4):e13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, Svane MS, Bandholm T, Bojsen-Møller KN, Blond MB, Jensen JB, Stallknecht BM, Holst JJ, Madsbad S, Torekov SS. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med 2021;384:1719–1730. [DOI] [PubMed] [Google Scholar]
- 22. Unick JL, Gaussoin SA, Hill JO, Jakicic JM, Bond DS, Hellgren M, Johnson KC, Peters AL, Coday M, Kitzman DW, Bossart S, Wing RR. Four-year physical activity levels among intervention participants with type 2 diabetes. Med Sci Sports Exerc 2016;48:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (action for health in diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628. [DOI] [PubMed] [Google Scholar]
- 24. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pandey A, Patel KV, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, Johnson KC, McGuire DK, Bertoni AG, Kitzman D, Berry JD. Association of intensive lifestyle intervention. Fitness, and body mass Index with risk of heart failure in overweight or obese adults with type 2, diabetes Mellitus: an analysis from the Look AHEAD trial. Circulation 2020;141:1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CE, Vetter M, Mantzoros CS, Jeffery RW, Soliman EZ. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J 2015;170:770–777.e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, Evans M, Foreyt J, Foster G, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jeffery RW, Johnson KC, Kitabchi AE, Knowler WC, Kriska A, Lang W, Lewis CE, Montez MG, Nathan DM, Neiberg RH, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl PM, Safford M, Stewart K, Trence D, Wadden TA, Wing RR, Yanovski SZ. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, McEvoy RD, Abhayaratna WP, Kalman JM, Lau DH, Sanders P. PREVEntion and regReSsive effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace 2018;20:1929–1935. [DOI] [PubMed] [Google Scholar]
- 29. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am 2018;102:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aronne LJ, Hall KD, J MJ, Leibel RL, Lowe MR, Rosenbaum M, Klein S. Describing the weight-reduced state: physiology, behavior, and interventions. Obesity (Silver Spring ) 2021;29(Suppl 1):S9–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Busetto L, Bettini S, Makaronidis J, Roberts CA, Halford JCG, Batterham RL. Mechanisms of weight regain. Eur J Intern Med 2021;93:3–7. [DOI] [PubMed] [Google Scholar]
- 32. Polidori D, Sanghvi A, Seeley RJ, Hall KD. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity (Silver Spring ) 2016; 24:2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ravussin E, Smith SR, Ferrante AW Jr. Physiology of energy expenditure in the weight-reduced state. Obesity (Silver Spring ) 2021; 29(Suppl 1):S31–s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after “the biggest loser” competition. Obesity (Silver Spring ) 2016; 24:1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–1604. [DOI] [PubMed] [Google Scholar]
- 36. Dorling J, Broom DR, Burns SF, Clayton DJ, Deighton K, James LJ, King JA, Miyashita M, Thackray AE, Batterham RL, Stensel DJ. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: the modulating effect of adiposity, sex, and habitual physical activity. Nutrients 2018;10:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Douglas JA, King JA, Clayton DJ, Jackson AP, Sargeant JA, Thackray AE, Davies MJ, Stensel DJ. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int J Obes (Lond ) 2017;41:1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beaulieu K, Blundell JE, van Baak MA, Battista F, Busetto L, Carraça EV, Dicker D, Encantado J, Ermolao A, Farpour-Lambert N, Pramono A, Woodward E, Bellicha A, Oppert JM. Effect of exercise training interventions on energy intake and appetite control in adults with overweight or obesity: a systematic review and meta-analysis. Obes Rev 2021;22(Suppl 4):e13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grannell A, Fallon F, Al-Najim W, le Roux C. . Obesity and responsibility: is it time to rethink agency? Obes Rev 2021;22:e13270. [DOI] [PubMed] [Google Scholar]
- 40. Iepsen EW, Lundgren J, Holst JJ, Madsbad S, Torekov SS. Successful weight loss maintenance includes long-term increased meal responses of GLP-1 and PYY3-36. Eur J Endocrinol 2016;174:775–784. [DOI] [PubMed] [Google Scholar]
- 41. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond ) 2013; 37:1443–1451. [DOI] [PubMed] [Google Scholar]
- 42. Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab 2021;23:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond ) 2014; 38:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilding JP, Batterham RL, Davies M, Gaal LFV, Kandler K, Konakli K, Lingvay I, McGowan BM, Kalayci Oral T, Rosenstock J, Wadden TA, Wharton S, Yokote K, Kushner RF. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab 2022;24:1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007;246:780–785. [DOI] [PubMed] [Google Scholar]
- 46. Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- 47. Carlsson LMS, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Svensson PA, Taube M, Carlsson B, Peltonen M. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med 2020;383:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjöström L, Karason K. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol 2016;68:2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jamaly S, Carlsson L, Peltonen M, Jacobson P, Karason K. Surgical obesity treatment and the risk of heart failure. Eur Heart J 2019;40:2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, Nissen SE. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. Jama 2019;322:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eliasson B, Liakopoulos V, Franzén S, Näslund I, Svensson AM, Ottosson J, Gudbjörnsdottir S. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol 2015;3:847–854. [DOI] [PubMed] [Google Scholar]
- 52. Angrisani L, Santonicola A, Iovino P, Ramos A, Shikora S, Kow L. Bariatric Surgery Survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg 2021;31:1937–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arterburn D, Wellman R, Emiliano A, Smith SR, Odegaard AO, Murali S, Williams N, Coleman KJ, Courcoulas A, Coley RY, Anau J, Pardee R, Toh S, Janning C, Cook A, Sturtevant J, Horgan C, McTigue KM. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med 2018;169:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 2013;10:575–584. [DOI] [PubMed] [Google Scholar]
- 55. Svane MS, Jørgensen NB, Bojsen-Møller KN, Dirksen C, Nielsen S, Kristiansen VB, Toräng S, Wewer Albrechtsen NJ, Rehfeld JF, Hartmann B, Madsbad S, Holst JJ. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond ) 2016;40:1699–1706. [DOI] [PubMed] [Google Scholar]
- 56. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov 2022;21:201–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010;363:905–917. [DOI] [PubMed] [Google Scholar]
- 58. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007;370:1706–1713. [DOI] [PubMed] [Google Scholar]
- 59. Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer risk associated with Lorcaserin: the FDA's review of the CAMELLIA-TIMI 61 trial. N Engl J Med 2020;383:1000–1002. [DOI] [PubMed] [Google Scholar]
- 60. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 2016;315:2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith SM, Meyer M, Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacother 2013;47:340–349. [DOI] [PubMed] [Google Scholar]
- 62. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring ) 2012; 20:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–1352. [DOI] [PubMed] [Google Scholar]
- 65. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376:595–605. [DOI] [PubMed] [Google Scholar]
- 66. Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Kim D, Dunayevich E. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring ) 2013;21:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring ) 2011;19:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 69. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, Ortiz RV, Wilding JPH, Skjøth TV, Manning LS, Pi-Sunyer X. 3 Years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–1409. [DOI] [PubMed] [Google Scholar]
- 70. Wadden TA, Tronieri JS, Sugimoto D, Lund MT, Auerbach P, Jensen C, Rubino D. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring) 2020; 28:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond) 2016;40:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, Mastrandrea LD, Prabhu N, Randomized ASA. Controlled trial of liraglutide for adolescents with obesity. N Engl J Med 2020;382:2117–2128. [DOI] [PubMed] [Google Scholar]
- 73. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, Lingvay I, O'Neil PM, Rubino DM, Skovgaard D, Wallenstein SOR, Garvey WT. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 2021;325:1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, Rudofsky G, Tadayon S, Wadden TA, Dicker D. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 2021;325:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, Tobe K, Yamauchi T, Lim S. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol 2022;10:193–206. [DOI] [PubMed] [Google Scholar]
- 76. Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA 2022;327:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205–216. [DOI] [PubMed] [Google Scholar]
- 78. Garvey WT, Ryan DH, Bohannon NJ, Kushner RF, Rueger M, Dvorak RV, Troupin B. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care 2014;37:3309–3316. [DOI] [PubMed] [Google Scholar]
- 79. Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, Klassen P, Fujioka K. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013;36:4022–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB, DeFronzo RA. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687–699. [DOI] [PubMed] [Google Scholar]
- 81. Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, Skovgaard D, Sugimoto D, Jensen C, Mosenzon O. Efficacy and safety of liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: the SCALE insulin randomized controlled trial. Diabetes Care 2020;43:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, Rosenstock J, Shimomura I, Viljoen A, Wadden TA, Lingvay I. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021;397:971–984. [DOI] [PubMed] [Google Scholar]
- 83. Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–515. [DOI] [PubMed] [Google Scholar]
- 84. Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, Bray R, Rodríguez Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 2021;398:583–598. [DOI] [PubMed] [Google Scholar]
- 85. Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, Wiese RJ. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021;398:1811–1824. [DOI] [PubMed] [Google Scholar]
- 86. Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 2022;327:534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol 2022;10:623–633. [DOI] [PubMed] [Google Scholar]
- 88. Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol 2022;10:634–644. [DOI] [PubMed] [Google Scholar]
- 89. Kolotkin RL, Gadde KM, Peterson CA, Crosby RD. Health-related quality of life in two randomized controlled trials of phentermine/topiramate for obesity: what mediates improvement? Qual Life Res 2016;25:1237–1244. [DOI] [PubMed] [Google Scholar]
- 90. Ritchey ME, Harding A, Hunter S, Peterson C, Sager PT, Kowey PR, Nguyen L, Thomas S, Cainzos-Achirica M, Rothman KJ, Andrews EB, Anthony MS. Cardiovascular safety during and after use of phentermine and topiramate. J Clin Endocrinol Metab 2019;104:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Apovian CM. Naltrexone/bupropion for the treatment of obesity and obesity with type 2 diabetes. Future Cardiol 2016;12:129–138. [DOI] [PubMed] [Google Scholar]
- 92. Kolotkin RL, Chen S, Klassen P, Gilder K, Greenway FL. Patient-reported quality of life in a randomized placebo-controlled trial of naltrexone/bupropion for obesity. Clin Obes 2015;5:237–244. [DOI] [PubMed] [Google Scholar]
- 93. Halseth A, Shan K, Gilder K, Malone M, Acevedo L, Fujioka K. Quality of life, binge eating and sexual function in participants treated for obesity with sustained release naltrexone/bupropion. Obes Sci Pract 2018;4:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, Perez A, Smith SR. Effect of naltrexone-bupropion on Major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA 2016;315:990–1004. [DOI] [PubMed] [Google Scholar]
- 95. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439. [DOI] [PubMed] [Google Scholar]
- 96. Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rössner S, Hellström PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord 1999;23:304–311. [DOI] [PubMed] [Google Scholar]
- 97. Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Williams DL. The diverse effects of brain glucagon-like peptide 1 receptors on ingestive behaviour. Br J Pharmacol 2022;179:571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Farr OM, Upadhyay J, Rutagengwa C, DiPrisco B, Ranta Z, Adra A, Bapatla N, Douglas VP, Douglas KAA, Nolen-Doerr E, Mathew H, Mantzoros CS. Longer-term liraglutide administration at the highest dose approved for obesity increases reward-related orbitofrontal cortex activation in response to food cues: implications for plateauing weight loss in response to anti-obesity therapies. Diabetes Obes Metab 2019;21:2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Holst JJ. Treatment of type 2 diabetes and obesity on the basis of the incretin system: the 2021 banting medal for scientific achievement award lecture. Diabetes 2021;70:2468–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kolotkin RL, Fujioka K, Wolden ML, Brett JH, Bjorner JB. Improvements in health-related quality of life with liraglutide 3.0 mg compared with placebo in weight management. Clin Obes 2016;6:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, Nilsson B, Møller JE, Hjort J, Rasmussen J, Boesgaard TW, Schou M, Videbaek L, Gustafsson I, Flyvbjerg A, Wiggers H, Tarnow L. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail 2017;19:69–77. [DOI] [PubMed] [Google Scholar]
- 105. Marso SP, Baeres FMM, Bain SC, Goldman B, Husain M, Nauck MA, Poulter NR, Pratley RE, Thomsen AB, Buse JB. Effects of liraglutide on cardiovascular outcomes in patients with diabetes with or without heart failure. J Am Coll Cardiol 2020;75:1128–1141. [DOI] [PubMed] [Google Scholar]
- 106. Davies MJ, Aronne LJ, Caterson ID, Thomsen AB, Jacobsen PB, Marso SP. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab 2018;20:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2, diabetes: time to reframe the conversation. Lancet 2022;399:394–405. [DOI] [PubMed] [Google Scholar]
- 108. Wharton S, Calanna S, Davies M, Dicker D, Goldman B, Lingvay I, Mosenzon O, Rubino DM, Thomsen M, Wadden TA, Pedersen SD. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes Metab 2022;24:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 110. Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. Jama 2017;318:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Alexiadou K, Anyiam O, Tan T. Cracking the combination: gut hormones for the treatment of obesity and diabetes. J Neuroendocrinol 2019;31:e12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Capristo E, Panunzi S, De Gaetano A, Spuntarelli V, Bellantone R, Giustacchini P, Birkenfeld AL, Amiel S, Bornstein SR, Raffaelli M, Mingrone G. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. J Clin Endocrinol Metab 2018;103:2136–2146. [DOI] [PubMed] [Google Scholar]
- 113. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lau DCW, Erichsen L, Francisco AM, Satylganova A, le Roux CW, McGowan B, Pedersen SD, Pietiläinen KH, Rubino D, Batterham RL. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2, trial. Lancet 2021;398:2160–2172. [DOI] [PubMed] [Google Scholar]
- 115. Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, Hirshberg B, Ambery P. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care 2021;44:1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 2003;46:798–801. [DOI] [PubMed] [Google Scholar]
- 117. Khoo B, Tan TM. Combination gut hormones: prospects and questions for the future of obesity and diabetes therapy. J Endocrinol 2020;246:R65–r74. [DOI] [PubMed] [Google Scholar]
- 118. Christensen MB, Gasbjerg LS, Heimbürger SM, Stensen S, Vilsbøll T, Knop FK. GIP's involvement in the pathophysiology of type 2 diabetes. Peptides 2020;125:170178. [DOI] [PubMed] [Google Scholar]
- 119. Bergmann N, Lund A, Gasbjerg L, Meessen E, Andersen M, Bergmann S, Hartmann B, Holst J, Jessen L, Christensen M. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia 2019;62:665–675. [DOI] [PubMed] [Google Scholar]
- 120. Bergmann NC, Gasbjerg LS, Heimbürger SM, Krogh LSL, Dela F, Hartmann B, Holst JJ, Jessen L, Christensen MB, Vilsbøll T, Lund A, Knop FK. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake. Appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1, receptor agonist in men with type 2 diabetes. Diabetes Care 2020;43:588–596. [DOI] [PubMed] [Google Scholar]
- 121. Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, Gimeno RE, Milicevic Z, Robins D, Haupt A. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018;392:2180–2193. [DOI] [PubMed] [Google Scholar]
- 122. Coskun T, Sloop K, Loghin C, Alsina-Fernandez J, Urva S, Bokvist K, Cui X, Briere D, Cabrera O, Roell W. LY3298176, A novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 2018;18:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, Urva S, Emmerson PJ, Holst JJ, D'Alessio DA, Coghlan MP, Rosenkilde MM, Campbell JE, Sloop KW. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020;5:e140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 2022;28:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Iepsen EW, Lundgren J, Dirksen C, Jensen JE, Pedersen O, Hansen T, Madsbad S, Holst JJ, Torekov SS. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes (Lond ) 2015; 39:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. https://sciencehub.novonordisk.com/content/dam/hcpexperience/kol/en/congresses/ow/2021/ow21-step-5-primary-lb/pdfs/PPT_Garvey_Two_year_effect_semaglutide_2.4mg_STEP_5_no%20animations.pdf.
- 127. Ahlin S, Peltonen M, Sjöholm K, Anveden Å, Jacobson P, Andersson-Assarsson JC, Taube M, Larsson I, Lohmander LS, Näslund I, Svensson PA, Carlsson LMS. Fracture risk after three bariatric surgery procedures in Swedish obese subjects: up to 26 years follow-up of a controlled intervention study. J Intern Med 2020;287:546–557. [DOI] [PubMed] [Google Scholar]
- 128. Neovius M, Bruze G, Jacobson P, Sjöholm K, Johansson K, Granath F, Sundström J, Näslund I, Marcus C, Ottosson J, Peltonen M, Carlsson LMS. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol 2018;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dehestani B, Stratford NR, le Roux CW. Amylin as a future obesity treatment. J Obes Metab Syndr 2021;30:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Enebo LB, Berthelsen KK, Kankam M, Lund MT, Rubino DM, Satylganova A, Lau DCW. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet 2021;397:1736–1748. [DOI] [PubMed] [Google Scholar]
- 131. Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol 2010;6:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai LF, Robertson D, Jain M, Petrone M, Rondinone C, Hirshberg B, Jermutus LMEDI. , a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 2018;391:2607–2618. [DOI] [PubMed] [Google Scholar]
- 133. Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, Keil S, Haack T, Wagner M, Bossart M, Larsen PJ. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab 2019;21:120–128. [DOI] [PubMed] [Google Scholar]
- 134. Bossart M, Wagner M, Elvert R, Evers A, Hübschle T, Kloeckener T, Lorenz K, Moessinger C, Eriksson O, Velikyan I, Pierrou S, Johansson L, Dietert G, Dietz-Baum Y, Kissner T, Nowotny I, Einig C, Jan C, Rharbaoui F, Gassenhuber J, Prochnow HP, Agueusop I, Porksen N, Smith WB, Nitsche A, Konkar A. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab 2022;34:59–74.e10. [DOI] [PubMed] [Google Scholar]
- 135. https://diabetesjournals.org/diabetes/article/71/Supplement_1/340-OR/146500/340-OR-LY3437943-LY-a-Novel-Triple-GIP-GLP-1.
- 136. Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 2003;349:941–948. [DOI] [PubMed] [Google Scholar]
- 137. Tan TM, Minnion J, Khoo B, Ball LJ, Malviya R, Day E, Fiorentino F, Brindley C, Bush J, Bloom SR. Safety and efficacy of an extended-release peptide YY analogue for obesity: a randomized, placebo-controlled, phase 1 trial. Diabetes Obes Metab 2021;23:1471–1483. [DOI] [PubMed] [Google Scholar]
- 138. Neary N, Small C, Druce M, Park A, Ellis S, Semjonous N, Dakin C, Filipsson K, Wang F, Kent A. Peptide YY3-36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 2005;146:5120–5127. [DOI] [PubMed] [Google Scholar]
- 139. De Silva A, Salem V, Long C, Makwana A, Newbould R, Rabiner E, Ghatei M, Bloom S, Matthews P, Beaver J. The gut hormones PYY 3–36 And GLP-1 7,–36 Amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 2011; 14: 700–706. [Google Scholar]
- 140. Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, De Waele K, Farooqi IS, Gonneau-Lejeune J, Gordon G, Kohlsdorf K, Poitou C, Puder L, Swain J, Stewart M, Yuan G, Wabitsch M, Kühnen P. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol 2020;8:960–970. [DOI] [PubMed] [Google Scholar]
- 141. Heymsfield SB, Coleman LA, Miller R, Rooks DS, Laurent D, Petricoul O, Praestgaard J, Swan T, Wade T, Perry RG, Goodpaster BH, Roubenoff R. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw Open 2021;4:e2033457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F, Hatakeyama S, Trendelenburg AU, Morvan F, Richardson B, Glass DJ, Lach-Trifilieff E, Feige JN. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol 2012;32:2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data derived from sources in the public domain. Reference details are provided in full.