Abstract
Introduction
Glucagon-like peptide 1 receptor agonists (GLP-1) elicit substantial reductions in glycemia and body weight in people with type 2 diabetes (T2D) and obesity, but existing data suggest the therapy must be continued indefinitely to maintain clinical improvements. Given the high cost and poor real-world persistence of GLP-1, an effective therapy that enables deprescription with sustained clinical improvements would be beneficial. Thus, the purpose of this real-world study was to assess the effect of GLP-1 deprescription on glycemia and body weight following co-therapy with carbohydrate restricted nutrition therapy (CRNT) supported via telemedicine in a continuous remote care model.
Methods
A retrospective, propensity score matched cohort study among patients with T2D at a telemedicine clinic was conducted. Patients in whom GLP-1 were deprescribed (DeRx; n = 154) were matched 1:1 with patients in whom GLP-1 were continued (Rx). HbA1c and body weight at enrollment in clinic (pre-CRNT), at date of deprescription or index date (derx/ID), and at 6 and 12 months (m) post-derx/ID were utilized in this study.
Results
No regression in weight was observed following deprescription with > 70% maintaining ≥ 5% weight loss 12 m post-derx/ID. HbA1c rose 6 m and 12 m post-derx/ID in both DeRx and Rx cohorts, but most patients maintained HbA1c < 6.5%. HbA1c and body weight measured 6 m and 12 m following derx/ID did not significantly differ between cohorts and were improved at derx/ID and at follow-up intervals compared to pre-CRNT.
Conclusion
These results demonstrate the potential for an alternate therapy, such as CRNT supported via telemedicine, to enable maintenance of weight loss and glycemia below therapeutic targets following discontinuation of GLP-1 therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-024-01547-0.
Keywords: Type 2 diabetes, Ketogenic diet, GLP-1 receptor agonists, Weight, Hemoglobin A1c, Deprescription, Real-world
Key Summary Points
| Why carry out this study? |
| In clinical trials, glucagon-like peptide 1 receptor agonists (GLP-1) have demonstrated significant reductions in glycemia and body weight among patients with type 2 diabetes and obesity with rapid regression of clinical improvements upon discontinuation of the medication even with persistent caloric restriction and exercise counseling, suggesting the drug must be continued indefinitely. |
| Cost and poor persistence of the GLP-1 therapy pose real-world challenges to maintaining improved health outcomes long-term, so therapies that enable deprescription with maintenance of clinical improvements are needed. |
| What was learned from the study? |
| Body weight did not rise in the 12 months following deprescription of GLP-1 therapy when patients continued carbohydrate restricted nutrition therapy supported via telemedicine in a continuous remote care model. |
| Hemoglobin A1c rose but on average remained below the diagnostic threshold for type 2 diabetes. |
| There was no difference between discontinued and continued GLP-1 therapy cohorts in body weight or HbA1c over 12 months following GLP-1 deprescription or matched index date. |
| This study informs clinical practice, showing that improved glycemia and weight loss can be maintained following GLP-1 deprescription among patients undergoing CRNT supported by continuous remote care, potentially mitigating the need for lifetime, continuous use of the pharmaceutical. |
Introduction
About one in seven adults in the USA lives with type 2 diabetes (T2D) [1], and 78% also live with excess weight or obesity [2]. Prevalence of T2D, excess weight, and obesity continues to grow [3, 4] alongside the cost of healthcare for these conditions [5, 6], particularly through introduction of high cost medications associated with significant weight loss, such as glucagon-like peptide 1 receptor agonists (GLP-1) [7, 8].
Recent pharmaceutical advancements among incretin mimetics like GLP-1 show great potential, having elicited substantial glucose-lowering effects in T2D [9–16] and weight loss nearing that which is achieved through surgical intervention among people with excess weight or obesity without T2D [17, 18]. However, clinical trial evidence to date demonstrates the high efficacy and high cost drugs must be continued indefinitely to sustain improved clinical outcomes [19, 20].
Lifestyle intervention, as the cornerstone of T2D and obesity care, may serve as an effective combination and sequential therapy to pharmaceuticals to enable eventual deprescription—particularly among interventions demonstrated to elicit significant weight loss, regression of prediabetes to normoglycemia, and remission of T2D, such as carbohydrate restricted nutrition therapy (CRNT) [21, 22]. To date, no studies have assessed the use of CRNT as an adjunct lifestyle intervention with GLP-1 and its effect on maintaining outcomes after discontinuation of GLP-1.
Virta Health, a nationwide telemedicine clinic in the USA, specializes in treating adults with T2D, prediabetes, and obesity through a medically supervised intervention focused on delivering CRNT. Patients engage with this system through a mobile health application (app) which offers educational resources, tracking of biomarkers, direct communication with a healthcare team that includes health coaches and licensed medical professionals, and an optional social community for peer interaction. Using real-world data from the clinic, we assessed the impact of GLP-1 deprescription (DeRx) on glycemia and body weight among people with T2D and excess weight or obesity compared to a matched cohort of patients who continued (Rx) GLP-1 therapy.
Methods
Study Population and Design
This retrospective, real-world analysis utilized de-identified data obtained from medical records among patients of Virta Health. The use of de-identified data, in compliance with the Health Insurance Portability and Accountability Act (HIPAA) standards, exempts this study from the need for ethics committee approval, as it does not involve identifiable human subjects. Patients in the clinic are initially counseled to achieve and sustain nutritional ketosis (blood beta-hydroxybutyrate (BHB) 0.5–3.0 mmol/L). The initial guidance is to restrict carbohydrate less than 30 g per day (or less than 50 g if consuming a vegan eating pattern), protein intake around 1.5 g/kg of reference body weight, and fat intake is titrated to achieve satiety while enabling weight loss if that is a goal of the patient. Level of carbohydrate restriction and ketones are later individualized on the basis of the patient’s personal carbohydrate tolerance and health goals. Patients were encouraged to continue CRNT for the entire period while they are under care in the telemedicine clinic. Frequency of follow-up with patients regarding biomarkers and the nutrition therapy is individualized on the basis of patient outreach and health need and can be as often as daily. Weight is tracked regularly using a cellular-connected scale (Body Trace BT003, New York, USA) which automatically uploads data to the app. Additionally, patients are advised to consistently upload their fingerstick blood glucose and BHB measurements to track their treatment progression. As a component of the clinic’s care protocol, patients who are enrolled in the clinic are encouraged to complete regular laboratory assessments, including hemoglobin A1c (HbA1c), in line with the recommended frequency by care standards.
In this study, we identified patients with a diagnosis of T2D who were established on GLP-1 therapy prior to enrollment in the clinic where they then initiated CRNT as a co-therapy and whose GLP-1 was subsequently deprescribed following improved glycemia to HbA1c < 6.5% within 3–9 months of beginning CRNT (GLP-1 DeRx cohort) to assess change in glycemia and weight following deprescription. To assess HbA1c and weight changes after deprescription compared to continued GLP-1 therapy, a matched cohort of patients who were established on GLP-1 prior to enrollment and improved glycemia to HbA1c < 6.5 but remained on GLP-1 therapy concurrent with CRNT (GLP-1 Rx cohort) was identified.
Outcomes and Study Measures
The retrospective analysis primarily aimed to assess change in HbA1c and body weight 6 and 12 months following GLP-1 deprescription. This study also aimed to determine if HbA1c or body weight differed in the year following deprescription or index date (derx/ID) between the GLP-1 DeRx and GLP-1 Rx cohorts. In addition to HbA1c and body weight, diabetes medication data, demographics and app data, including gender, age, race and ethnicity, and app-uploaded fingerstick blood BHB (a biomarker of adherence to the CRNT) were obtained from medical records for this analysis.
Statistical Methods
To adjust for confounders and minimize bias, propensity score matching was used to match each patient in the GLP-1 DeRx cohort (reference cohort) 1:1 with a patient in the GLP-1 Rx cohort. The reference cohort was matched using propensity scores estimated from a multivariate regression model and the nearest neighbor method without any replacement. For matching, enrollment and index date covariates included age, gender, race and ethnicity, HbA1c, body mass index (BMI), number of diabetes medications, and distribution of follow-up HbA1c and weight data availability and the GLP-1 drug prescribed prior to enrollment in the clinic. To assess balance between the cohorts after matching, baseline covariates were assessed using analysis of variance (ANOVA) or chi-squared test and standardized differences between cohorts.
Longitudinal and between matched cohort differences in HbA1c and weight were assessed at enrollment in the clinic (pre-CRNT), derx/ID, and 6 and 12 months post-derx/ID using linear mixed effects models. Additionally, we repeated the analyses in two medication subgroups with sufficient sample size (semaglutide and dulaglutide). Recognizing the effect of diabetes medications on HbA1c and body weight, we included the number of diabetes medications for each patient at enrollment and derx/ID in the propensity score matching to adjust for confounding factors. Further, two sensitivity analyses were performed: (1) after removing patients on sodium/glucose cotransporter 2 (SGLT2) inhibitors, and (2) after removing patients on any diabetes medication other than metformin.
We assessed longitudinal changes in BHB in the matched cohorts using two different methods. First, the daily BHB measurements were compiled as count data where percentage days of logging BHB ≥ 0.3 mM (indicative of carbohydrate restriction and low levels of nutritional ketosis) were calculated for the four main time intervals: enrollment to derx/ID, derx/ID ± 3 months, and 6 ± 3 and 12 ± 3 months post-derx/ID. We then used generalized estimating equations (GEE) with an unstructured correlation matrix, logarithmic link, and Poisson distribution to assess longitudinal changes and rate of change in frequency of BHB ≥ 0.3 mM between the two cohorts. Second, mean BHB was calculated for the four main time intervals and a linear mixed effect model was used to assess longitudinal changes in mean BHB and the rate of mean BHB changes between the two cohorts.
All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria) version 4.2.2 (2022-10-31) and IBM SPSS statistics (version 29.0.1.0). Two-sided p values less than 0.05 were considered statistically significant.
Results
Following GLP-1 deprescription in 154 individuals meeting inclusion criteria for the primary cohort, HbA1c increased at 6 months (0.4% [95% CI 0.2, 0.6], p < 0.001) and 12 months (0.6% [95% CI 0.3, 0.8]) compared to time of deprescription, though the mean remained within the non-diabetic range (6.0% at 6 months; 6.2% at 12 months). Weight did not significantly increase at 6 or 12 months following deprescription (p > 0.05). Compared to pre-CRN therapy, HbA1c and weight remained significantly lower up to 12 months following deprescription.
Patient characteristics of the propensity score matched cohorts are described in Table 1. No significant differences were observed between matched cohorts, and cohorts were balanced according to absolute standardized differences. Within the GLP-1 DeRx cohort, the medication most frequently utilized by patients prior to enrollment in the clinic was dulaglutide (43.5%), followed by semaglutide (29.2%), liraglutide (17.5%), and exenatide (7.1%); following deprescription, one patient was prescribed no diabetes medication, 132 patients continued on only metformin, and 21 patients continued on a diabetes medication other than metformin. The mean duration of care in both cohorts was at least 18 months.
Table 1.
Characteristics of the matched cohorts
| GLP-1 deprescription cohort (n = 154) | Continued GLP-1 therapy cohort (n = 154) | |
|---|---|---|
| Age, mean (SD), years | 55.9 (8.7) | 55.3 (8.4) |
| Gender, n (%) | ||
| Female | 77 (50.0) | 90 (58.4) |
| Male | 77 (50.0) | 64 (41.6) |
| Race and ethnicity (n,%) | ||
| Non-Hispanic White | 100 (64.9) | 108 (70.1) |
| Non-Hispanic Black or African American | 9 (5.8) | 19 (12.3) |
| Hispanic | 29 (18.8) | 19 (12.3) |
| Non-Hispanic American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or Multiple Races | 7 (4.5) | 8 (5.2) |
| Enrollment BMI mean (SD), kg/m2 | 35.7 (6.9) | 36.5 (7.2) |
| Enrollment HbA1c mean (SD), % | 7.3 (1.2) | 7.4 (1.4) |
| Distribution of diabetes medication classes at deprescription or index date (n, %) | ||
| GLP-1 | 0 (0) | 154 (100) |
| SGLT2i | 10 (6.5) | 0 (0) |
| Sulfonylureas | 1 (0.6) | 0 (0) |
| DPP4 | 4 (2.5) | 0 (0) |
| Insulin | 4 (2.6) | 0 (0) |
| Thiazolidinediones | 2 (1.3) | 3 (1.9) |
| Metformin | 149 (96.8) | 49 (31.8) |
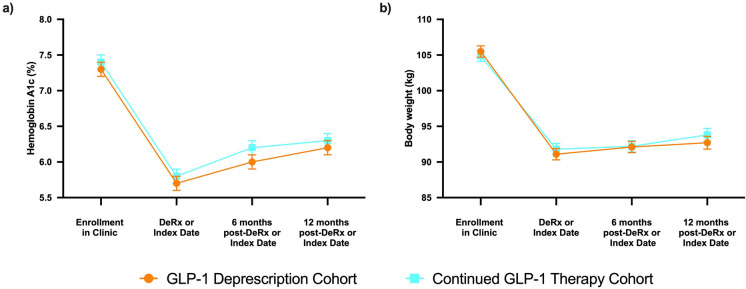
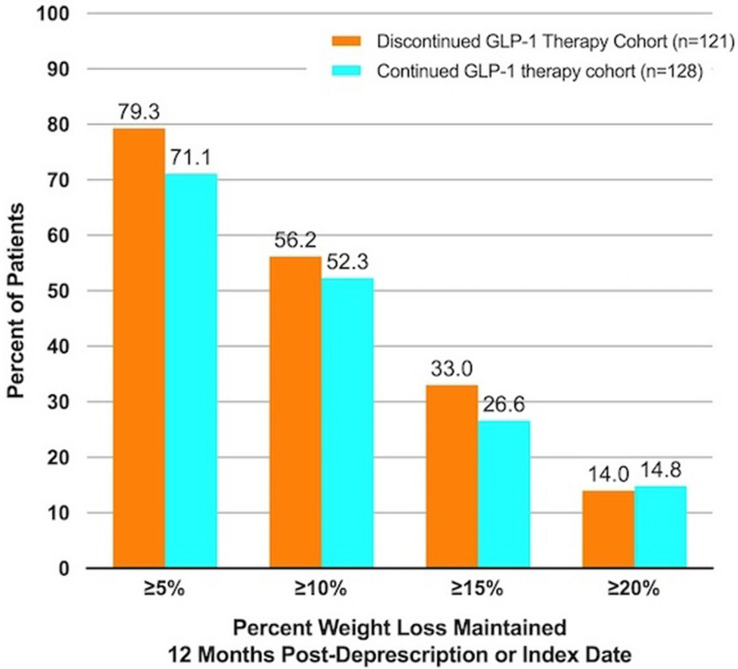
HbA1c and body weight measured 6 and 12 months following derx/ID did not significantly differ between cohorts (Fig. 1). In all cohorts, HbA1c and body weight improved significantly at time of derx/ID and at follow-up intervals compared to levels at enrollment in the clinic, prior to adding CRNT as co-therapy. In both the DeRx and Rx cohorts, HbA1c at 6 and 12 months follow-up rose relative to derx/ID (p < 0.001). HbA1c for most individuals in both cohorts remained below 6.5% up to 12 months following derx/ID (DeRx, 64.8%; Rx, 64.1%), including 20.4%, and 20.3% of the GLP-1 DeRx, and Rx cohorts who maintained normoglycemia (HbA1c < 5.7%) 12 months following derx/ID. No significant change in body weight following derx/ID was observed in any cohort (p values > 0.05). More than 70% of patients in each of the matched cohorts maintained at least 5% body weight loss 12 months following derx/ID (Fig. 2). Subgroup analyses of semaglutide and dulaglutide were consistent with the full cohort and HbA1c and body weight changes by medication are described in Supplementary Fig. 1. Sensitivity analyses removing patients who were prescribed (1) SGLT2 inhibitors and (2) any diabetes medication other than metformin, from the analysis, were consistent with the overall findings.
Fig. 1.
Comparison of HbA1c and body weight. Longitudinal and between-group change in estimated mean a hemoglobin A1c (HbA1c, %) and b body weight (kg) from enrollment to 12 months following deprescription or index date in GLP-1 deprescription and continued GLP-1 therapy cohorts
Fig. 2.
Proportion of patients maintaining weight loss targets at 12 months post-deprescription or index date by cohort
Frequency of achieving BHB ≥ 0.3 mM via CRNT declined more rapidly among the GLP-1 Rx cohort compared to the DeRx cohort (p = 0.037), and mean BHB of the GLP-1 Rx cohort was lower compared to the DeRx cohort at all time intervals (p < 0.05; Supplementary Fig. 2).
Discussion
Results of this real-world analysis demonstrate that GLP-1 can be discontinued without weight regain following initiation of successful co-therapy with carbohydrate restricted nutrition within this care model. While glycemia increased marginally, the mean remained below the diagnostic threshold for diabetes. No differences in glycemia or body weight were observed up to 12 months following deprescription of GLP-1 compared to a matched cohort in whom GLP-1 therapy was continued. Taken together, these results suggest that CRNT supported via telemedicine in a continuous remote care model may be used in combination with GLP-1 therapy to enable stepping off GLP-1 therapy in some individuals, and continuing CRNT (often with concurrent metformin therapy) may provide an effective maintenance therapy, particularly for weight loss. More frequent maintenance of nutritional ketosis achieved through CRNT (indicating more consistent carbohydrate restriction) was observed in the DeRx cohort during the pre-deprescription time interval compared to the GLP-1 Rx cohort, suggesting adherence to CRNT may assist clinical decision-making regarding the feasibility of deprescription for individual patients.
The STEP 1 trial extension showed rapid regression in glycemia and body weight following withdrawal of semaglutide administered in conjunction with a physical activity and caloric restriction lifestyle intervention [19]. One year after therapy withdrawal, participants regained 64% of weight lost and 80% of the decline in HbA1c that had been achieved. A similar regression in weight as well as fasting plasma glucose among individuals with T2D following the withdrawal of a GLP-1 drug was observed in the SCALE trial [23]. Results from the present real-world analysis contrast prior research, showing less regression of outcomes—only 15% of the body weight lost and 36% of the HbA1c decline achieved with combination GLP-1 and CRNT prior to GLP-1 deprescription was regained in the year following discontinuation of the medication, despite being in a group with more progressive insulin resistance. Specifically, among those deprescribed semaglutide, there was no regression in body weight 1 year following discontinuation and a 40% regression in the HbA1c decline. Although the effects of GLP-1 therapy as an adjunct to lifestyle intervention prior to enrollment in the clinic are unknown, it is reasonable to expect GLP-1 therapy resulted in HbA1c and body weight reductions prior to those achieved by adding CRNT and continuous remote care, suggesting the overall regression in HbA1c and body weight in the context of GLP-1 deprescription and continued CRNT may be less than what can be observed in these data.
The STEP 4 and SURMOUNT 4 trials assessed withdrawal of GLP-1 therapy, but not lifestyle intervention, and showed regain of about half of the weight lost during combination therapy over the next 11–12 months while lifestyle intervention was continued [20, 24]. The lifestyle intervention studied in these trials focused on caloric restriction and exercise with monthly in-person or telephone counseling, while the lifestyle intervention in the present real-world study focuses primarily on carbohydrate restriction and eating until satiety with continuous remote support, suggesting that the type of nutrition therapy and degree of support utilized as a combination and sequential therapy may play a role in the ability to maintain weight loss following discontinuation of the GLP-1.
One potential reason dietary carbohydrate restriction, and nutritional ketosis in particular, may provide an advantage for weight loss maintenance following GLP-1 deprescription is through reduced hunger and appetite—an effect shared by both the drug and the nutrition therapy. Participants in a clinical trial evaluating the effects of the CRNT utilized in the present study reported reduced perceptions of hunger after 10 weeks of therapy concurrent with mean blood BHB of 0.6 mM [25], and blood BHB concentrations are associated with lower concentrations of the hunger hormone ghrelin and higher concentrations of satiety hormones glucagon-like peptide 1 and cholecystokinin [26].
Longitudinal changes in HbA1c did not differ between DeRx and Rx cohorts, though the frequency of achieving BHB ≥ 0.3 mM with CRNT and mean BHB was higher within the DeRx cohort. Carbohydrate restriction results in less glycemic variability [27], particularly post-meal—an effect similar to that which is achieved with GLP-1 therapy through delayed gastric emptying [28, 29]. Further, reduced carbohydrate intake may to some degree replace the need for glucose-dependent insulin secretion with GLP-1 therapy. Past research has also shown that more frequent maintenance of nutritional ketosis is associated with improvements in atherogenic dyslipidemia, glycemia, body weight, and markers of renal function [30–32]. Given the differences in BHB concentrations and frequency with which nutritional ketosis was maintained between the cohorts, blood BHB concentrations appear to support clinical decision-making regarding GLP-1 deprescription in real-world clinical practice in addition to supporting patients in their daily nutrition choices and may be a useful indicator of likelihood of success in maintaining clinical improvements upon deprescription.
Another noteworthy observation from this study was that patients established on GLP-1 therapy prior to enrollment in the clinic achieved 13% weight loss and 1.6% reduction in HbA1c following initiation of CRNT in combination with GLP-1 therapy. Further improvement in glycemia and weight elicited with this combination therapy exceeds effects observed in other real-world studies among those who switched to injectable semaglutide from less potent GLP-1 [33, 34]. Further, the weight loss achieved with carbohydrate restriction and GLP-1 combination therapy was on par or greater than weight loss observed in STEP 2 among people with T2D treated with 2.4 mg and 1.0 mg semaglutide [10] and in the real world across 10 clinics [35], suggesting there may be benefit to pairing GLP-1 with CRNT therapy to achieve greater weight loss when clinically indicated or to enable greater weight loss when higher doses are poorly tolerated.
Additionally, cost and side effects are important considerations in GLP-1 therapy, and may contribute to the rates of uptake, adherence, and persistence observed throughout the USA today. For example, real-world persistence of GLP-1 therapy at 1 year is approximately 50% [36]. This suggests multiple therapeutic options must be accessible to enable the desired clinical outcomes for individual patients with unique preferences and circumstances.
Strengths of this analysis include its use of real-world data from a nationwide clinic, broadening the applicability of its findings, and that it is, to the best of the authors’ knowledge, the first study to assess glycemia and weight outcomes following withdrawal of GLP-1 in T2D under free-living, real-world conditions. Use of real-world data also has limitations given its retrospective and observational nature, even though differences between cohorts were reduced using matched cohort analysis. Duration of GLP-1 use prior to enrollment in the clinic, response to GLP-1 and prior lifestyle therapy prior to enrollment in the clinic, and adherence to GLP-1 therapy could not be accounted for. Application of these findings is limited to the medications utilized by the patient population included in this analysis. Future research should evaluate the effect of GLP-1 deprescription including medications which have recently come to market as indicated for T2D or for excess weight or obesity without T2D and include data from time of GLP-1 therapy initiation.
Conclusion
Results of this real-world analysis demonstrate that GLP-1 can be deprescribed without negative effects on glycemia and body weight following initiation of co-therapy with CRNT within this care model. These real-world data contrast clinical trial evidence in which rapid weight regain was observed following discontinuation of GLP-1 therapy even when traditional caloric restriction and physical activity counseling persisted and suggest that CRNT and continuous care may provide an appropriate glycemia and body weight maintenance therapy following deprescription, to mitigate the need for lifelong, continued GLP-1 therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Mitch Graves, Ed Jiang, and Pang Weirich for their assistance with data compilation and Caroline Roberts, Greeshma Shetty, and Jeff Stanley for sharing their clinical insight to inform this research.
Medical Writing and Editorial Assistance
The authors did not use any medical writing or editorial assistance for this article.
Author Contributions
Amy L McKenzie and Shaminie J Athinarayanan contributed to the concept, design, and planning of the study as well as interpretation of the study results. Shaminie J Athinarayanan analyzed the data. Amy L McKenzie drafted the manuscript and Shaminie J Athinarayanan reviewed.
Funding
This study and the Rapid Service Fee was funded by Virta Health.
Data Availability
Data may be obtained from a third party and are not publicly available.
Declarations
Conflict of Interest
Amy L McKenzie holds stock in Virta Health Corp. She was employed by Virta Health at initiation of the study; she is now affiliated with Abbott, Lingo Germany GmbH, Wiesbaden, Germany. Shaminie J Athinarayanan is employed and holds stock in Virta Health Corp.
Ethical Approval
This retrospective, real-world data exclusively utilized de-identified data and we determined that formal ethics approval is not required. All patient information used in this study was anonymized in accordance with the Health Insurance Portability and Accountability Act (HIPAA) regulations, ensuring that individual privacy rights and confidentiality are maintained. The study was compliant with the Helsinki Declaration of 1964, and its later amendments.
Footnotes
Prior Publication: A version of this manuscript was previously uploaded June 19, 2023, as a pre-print on medRxiv and is available at 10.1101/2023.06.18.23291518.
Amy L. McKenzie and Shaminie J. Athinarayanan contributed equally to this article.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 9 Mar 2022.
- 2.Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National and State Diabetes Trends. https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html. Accessed 17 May 2022.
- 4.National Center for Health Statistics. Table 26. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States, selected years 1988–1994 through 2015–2018. (Health, United States, 2019). https://www.cdc.gov/nchs/hus/data-finder.htm. Accessed 26 June 2023.
- 5.American Diabetes Association Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):dci180007. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawley J, Biener A, Meyerhoefer C, et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27(3):354–366. doi: 10.18553/jmcp.2021.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neilson LM, Munshi KD, Peasah SK, et al. Changes in type 2 diabetes medication utilization and costs in the United States, 2014–2019. Med Care. 2021;59:789–794. doi: 10.1097/MLR.0000000000001597. [DOI] [PubMed] [Google Scholar]
- 8.Heyward J, Christopher J, Sarkar S, Shin J, Kalyani RR, Alexander GC. Ambulatory noninsulin treatment of type 2 diabetes mellitus in the United States, 2015 to 2019. Diabetes Obes Metab. 2021;23(8):1843–1850. doi: 10.1111/dom.14408. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 10.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 11.Patti AM, Nikolic D, Magan-Fernandez A, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabetes Res Clin Pract. 2019;149:163–169. doi: 10.1016/j.diabres.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Patti AM, Rizvi AA, Giglio RV, Stoian AP, Ligi D, Mannello F. Impact of glucose-lowering medications on cardiovascular and metabolic risk in type 2 diabetes. J Clin Med. 2020;9(4):912. doi: 10.3390/jcm9040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giglio RV, Anca PS, Al-Rasadi K, et al. Novel therapeutical approaches to managing atherosclerotic risk. Int J Mol Sci. 2021;22(9):4633. doi: 10.3390/ijms22094633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzo M, Nikolic D, Banach M, et al. The effects of liraglutide on glucose, inflammatory markers, and lipoprotein metabolism: current knowledge and future perspective. Clin Lipidol. 2013;8(2):173–181. doi: 10.2217/clp.13.8. [DOI] [Google Scholar]
- 15.Nikolic D, Patti AM, Giglio RV, et al. Liraglutide improved cardiometabolic parameters more in obese than in non-obese patients with type 2 diabetes: a real-world 18-month prospective study. Diabetes Ther. 2022;13(3):453–464. doi: 10.1007/s13300-022-01217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoian AP, Sachinidis A, Stoica RA, Nikolic D, Patti AM, Rizvi AA. The efficacy and safety of dipeptidyl peptidase-4 inhibitors compared to other oral glucose-lowering medications in the treatment of type 2 diabetes. Metabolism. 2020;109:154295. doi: 10.1016/j.metabol.2020.154295. [DOI] [PubMed] [Google Scholar]
- 17.Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 18.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 19.Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553–1564. doi: 10.1111/dom.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie AL, Athinarayanan SJ, McCue JJ, et al. Type 2 diabetes prevention focused on normalization of glycemia: a two-year pilot study. Nutrients. 2021;13(3):749. doi: 10.3390/nu13030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. 2019;10:348. doi: 10.3389/fendo.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 24.Aronne LJ, Sattar N, Horn DB, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity. JAMA. 2024;331:e2324945. doi: 10.1001/jama.2023.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie AL, Hallberg SJ, Creighton BC, et al. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes. 2017;2(1):e5. doi: 10.2196/diabetes.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins C, Nymo S, Truby H, Rehfeld JF, Hunter GR, Gower BA. Association between ketosis and changes in appetite markers with weight loss following a very low-energy diet. Obesity. 2020;28(12):2331–2338. doi: 10.1002/oby.23011. [DOI] [PubMed] [Google Scholar]
- 27.Tay J, Thompson CH, Luscombe-Marsh ND, et al. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: a 2-year randomized clinical trial. Diabetes Obes Metab. 2018;20(4):858–871. doi: 10.1111/dom.13164. [DOI] [PubMed] [Google Scholar]
- 28.Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36(5):1396–1405. doi: 10.2337/dc12-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151:123–129. doi: 10.1016/j.regpep.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Athinarayanan SJ, Hallberg SJ, McKenzie AL, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):208. doi: 10.1186/s12933-020-01178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie AL, Athinarayanan SJ, Roberts CGP, et al. 312-OR: effect of nutritional ketosis trajectory on change in glycemia, weight, and atherogenic dyslipidemia over two years in people with type 2 diabetes. In: American Diabetes Association Scientific Sessions. 2023. p. 312-OR. (Diabetes; vol. 72 (Supplement 1)). 10.2337/db23-312-OR.
- 32.Athinarayanan SJ, Roberts CGP, Adams RN, et al. 410-P: Two-year (2y) eGFR slope in people with type 2 diabetes (T2D) receiving a very low carbohydrate diet (VLCD) intervention. American Diabetes Association Scientific Sessions. 2023. p. 410–P. (Diabetes; vol. 72 (Suppl_1)). 10.2337/db23-410-P.
- 33.Lingvay I, Kirk AR, Lophaven S, Wolden ML, Shubrook JH. Outcomes in GLP-1 RA-experienced patients switching to once-weekly semaglutide in a real-world setting: the retrospective observational EXPERT study. Diabetes Ther. 2021;12(3):879–896. doi: 10.1007/s13300-021-01010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crabtree TSJ, Adamson K, Reid H, et al. Injectable semaglutide and reductions in HbA1c and weight in the real world in people switched from alternative glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2022;24(7):1398–1401. doi: 10.1111/dom.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell W, Song X, Mohamed Y, et al. Medications and conditions associated with weight loss in patients prescribed semaglutide based on real-world data. Obesity. 2023;31(10):2482–2492. doi: 10.1002/oby.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss T, Yang L, Carr RD, et al. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Res Care. 2022;10(1):e002517. doi: 10.1136/bmjdrc-2021-002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available.