Abstract
Gut microbiota is essential for maintaining local and systemic immune homeostasis in the presence of bacterial challenges. It has been demonstrated that microbiota play contrasting roles in cancer development as well as anticancer immunity. Cancer immunotherapy, a novel anticancer therapy that relies on the stimulation of host immunity, has suffered from a low responding rate and incidence of severe immune‐related adverse events (irAEs). Previous studies have demonstrated that the diversity and composition of gut microbiota were associated with the heterogeneity of therapeutic effects. Therefore, alteration in microbiota taxa can lead to improved clinical outcomes in immunotherapy. In this review, we determine whether microbiota composition or microbiota‐derived metabolites are linked to responses to immunotherapy and irAEs. Moreover, we discuss various approaches to improve immunotherapy efficacy or reduce toxicities by modulating microbiota composition.
Keywords: cancer, gut microbiota, immunotherapy, immune‐related adverse events, predominant bacteria, precision medicine
The role of gut microbiota in cancer immunotherapy. Through manipulation of commensals in cancer patients by diet interventions, fecal microbial transplant, prebiotics, probiotics and bacteria consortia, host antitumor immunity can be enhanced by dominance of “beneficial” bacteria in gut lumen and their metabolites. Increased effector T cells and induction of regulatory T cells (Tregs) can be seen in gut‐associated lymphoid tissue, which leads to improved clinical outcomes of cancer immunotherapy with lower incidence of immune‐related adverse events (fecal microbiota transplant; perforin; granzyme B; Treg).
Highlights
Gut microbiota has been acknowledged as key regulators in host‐mediated anticancer immune response especially during immunotherapy, and antibiotics‐induced dysbiosis often leads to resistance to immunotherapy and poor clinical outcomes.
Although the mechanisms underlying gut microbiota‐mediated potentiating efficacy while ameliorating side effects of immunotherapy differs across bacteria genus and immunotherapy types, it's generally via enhancing anticancer immunity and remodulating the tumor microenvironment.
We reviewed the commensal bacteria profiles associated with responders to immunotherapy in various cancer types, characterized by a high diversity with abundance of specific species, which may help predict patients; sensitivity to immunotherapy before treatment.
Since the causal relationship between individual gut microbiota and response to immunotherapy, we propose gut microbiota act as the future therapeutic target and adjuvant in personalized anticancer regimen, and reviewed the current dilemma and potential strategies to manipulate gut microbiota towards “beneficial bacteria.”
INTRODUCTION
Globally, multifactorial cancer is the second leading cause of mortality [1], and immune systems are intricately linked to tumor growth [2]. In healthy individuals, abnormal cells can be recognized and removed by immune cells rapidly through immune surveillance. However, cancer cells can escape from host immunity, proliferate and form an intra‐tumoral immunosuppressive environment [3]. Immunotherapy, including adoptive cell therapy (ACT), cancer vaccines, cytokines, oncolytic virus therapies and immune checkpoint inhibitor (ICI) therapy, has dramatically changed the treatment landscape of cancers [4]. Different from conventional anticancer therapies like chemotherapy and radiotherapy, it retards tumor growth indirectly by unleashing and enhancing host Antitumor immune response. Clinical breakthroughs have been achieved through immunotherapy in various tumor types, including hematologic malignancies, melanoma, nonsmall‐cell lung cancer (NSCLC), renal cell cancer (RCC), hepatocellular carcinoma (HCC), and gastrointestinal (GI) cancer [5].
However, immunotherapy is only effective for a small share of patients, and most patients fail immunotherapy due to primary refractoriness and acquired resistance due to the undesired immune infiltrates in tumor microenvironment (TME) [6]. Besides, treatment paradigms are complicated by immune‐related adverse effects, inhibiting the rapid development and clinical application of immunotherapy. Therefore, recent advances in cancer immunotherapy have focused on normalizing the immune defects in TME as well as amplifying antitumor immunity via metabolic reprogramming and combination with other antitumor approaches.
The commensal microbiota has an intricate relationship between cancer and anticancer therapies in a balance between proinflammation and anti‐inflammation function. Emerging evidence has suggested that gut microbiota and its metabolites can significantly contribute to the efficacy and/or toxicity of immune‐related interventions. Specific microbiota profiling is associated with either improved or defective antitumor immunity between responders and nonresponders. Thus, manipulating gut microbiota towards the dominance of “beneficial” bacteria might be a new therapeutic strategy and a novel biomarker to improve and predict the clinical outcomes of cancer patients receiving immunotherapy (Figure 1).
Figure 1.
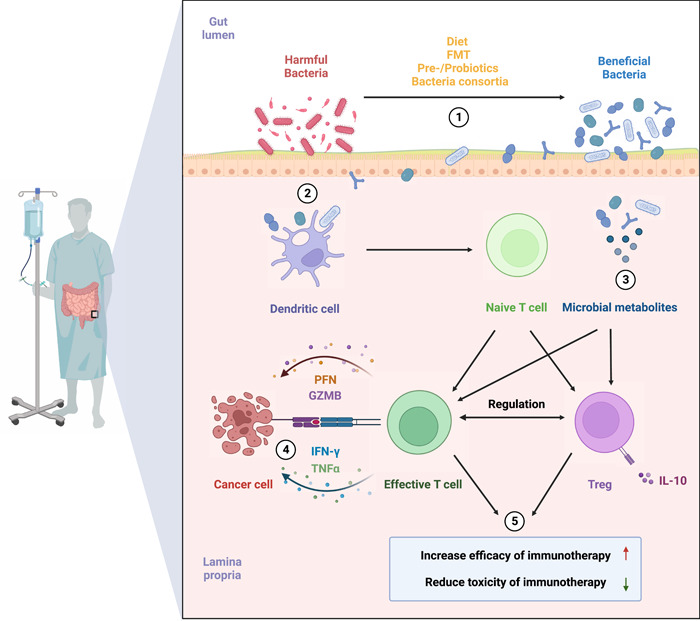
The role of gut microbiota in cancer immunotherapy. Through manipulation of commensals in cancer patients by diet interventions, fecal microbial transplant, prebiotics, probiotics and bacteria consortia, host antitumor immunity can be enhanced by dominance of “beneficial” bacteria in gut lumen and their metabolites. Increased effector T cells and induction of Tregs can be seen in GALT, which leads to improved clinical outcomes of cancer immunotherapy with lower incidence of immune‐related adverse events. FMT, fecal microbiota transplant; GALT, gut‐associated lymphoid tissue; GZMB, granzyme B; IFN‐γ, interferon‐γ; IL‐10, interleukin‐10; PFN, perforin; TNF‐α, tumor necrosis factor‐α; Treg, regulatory T cell.
THE CANCER‐MICROBIOTA‐IMMUNE AXIS
The dual role of gut microbiota in cancer
Gut microbiota differs widely even among healthy individuals [7]. They reside on intestinal epithelial barriers and are symbiotic with the host. The complex microbial ecosystem is crucial in human health, and disruption of which is associated with chronic inflammation, auto‐immune diseases, heart failure, and even cancer [8, 9] (Figure 2). Previous studies have indicated commensal microbiota plays contrasting roles in cancer initiation, progression, and metastasis.
Figure 2.
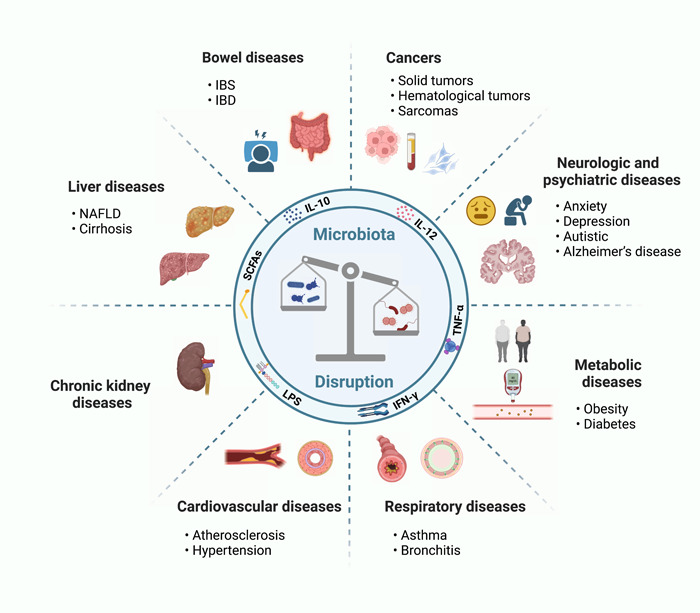
Human microbiota disruption contributes to various diseases including cancers, bowel diseases, liver diseases, chronic kidney diseases, cardiovascular diseases, respiratory diseases, metabolic diseases, and neurologic and psychiatric diseases. IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IFN‐γ, interferon‐γ; IL‐10, interleukin‐10; IL‐12, interleukin‐12; LPS, lipopolysaccharides; NAFLD, nonalcoholic fatty liver disease; SCFAs, short‐chain fatty acids; TNF‐α, tumor necrosis factor‐α.
In 1987, a study observed that patients with chronic infection of Salmonella typhi were related to an enhanced risk of death induced by hepatobiliary cancer [10]. In 1994, the World Health Organization designated Helicobacter pylori as a class I biological carcinogen and acknowledged it as a crucial pathological factor in gastric adenocarcinoma [11]. Other commensals found in the colon and pancreatic cancers also had the capacity to protumorigenic effects [12]. Researchers have found that the reduction of bacterial load could prominently reduce colorectal tumor growth [13]. Besides these well‐elucidated oncogenic species, disruption of gut microbiota, namely dysbiosis, can also act as a key driver to cancer initiation [14]. The mechanism underlying carcinogenesis caused by gut microbiota includes its producing toxic metabolites, inducing inflammation milieu, and suppressing antitumor immunity, which leads to genomic instability, DNA damage, and immune escape in tumor tissue [15, 16]. Recent studies have envisioned other potential mechanisms, including regulating hormones in circulation or through a reciprocal route called the gut‐brain axis, which further explains how gut microbiota induces dysplasia in distal organs and systems outside the gastrointestinal tract [17].
Conversely, other studies have supported that intact intestinal microbiota served as a protective role against cancer. A causal relationship was observed between antibiotic use and the increased incidence of breast cancer in mice and humans [18, 19]. Iida et al. showed commensal bacteria exert anticancer properties partially through priming tumor‐associated innate myeloid cells for pro‐inflammatory cytokine production, which could alter inflammation at the gut mucous site and in the TME [20]. Through human‐into‐mice fecal microbiota transfer (FMT), Erick et al. found gut microbiota could significantly alleviate pancreatic adenoma (PDAC) tumor growth in mice [21]. These studies suggested that gut microbiota might retard tumor growth partially through altering intratumoral microbial composition and shaping the TME. Interestingly, a recent study showed that gut microbiota and its postbiotics make a contribution to intestinal and immune homeostasis in healthy individuals by modulating function and self‐renewal of intestinal stem cells (ISCs) [22]. Additionally, it can improve immune surveillance through tumor adjuvanticity and antigenicity [23].
Induction of acute inflammatory reactions often stimulates the antitumor immune responses by promoting the maturation of dendritic cells (DC) and effector T cells, and chronic inflammation facilitates tumor progression and treatment resistance [24]. While some bacteria may activate the immune system and thus generate inflammation and oxidative stress, they can also promote tumor development, especially under the context of persistent inflammation, potentially leading to a complex interplay in cancer progression. Infection with H. pylori chronically activates disrupted gastric mucosal immune response which leads to accumulation of immunosuppressive cells and finally stomach cancer. Intestinal microbiota or microbial products are essential for inflammatory bowel disease to induce colorectal cancer (CRC), and the use of a common antimicrobial additive may exacerbate colonic inflammation and increase the risk of colitis‐associated colon tumorigenesis in a microbiota‐dependent manner [25, 26]. Further research is needed to fully understand these interactions and elucidate how to adjust the composition of the commensal species to achieve a balance between immune activation and immunosuppression.
Immunomodulation function of gut microbiota in adaptive and innate cancer immune response
The intricate crosstalk between the commensal microbiota and the host immune system begins at the gut epithelial. Through ligation of pathogen‐associated molecular patterns (PAMPs) derived from gut microbes and pattern recognition receptors (PRRs) presented on a variety of intestinal epithelial cells and innate immune cells, antigen‐presenting cells like DCs are activated, and pro‐inflammatory cytokines are widely produced. Activation and translocation of DCs from gut‐associated lymphoid tissue (GALT) to mesenteric lymph nodes boost the stimulation of naïve CD4+ and CD8+ T cells. Studies have shed light on how gut microbiota affect the generation, differentiation, and infiltration of adaptive and innate immune cells in tumor tissues [27]. In murine models of CRC and melanoma, oral gavage with commensal Clostridiales strains potently induced antitumor immunity via potentiating infiltration and activation of intratumoral CD8+ T cells, with therapeutic efficacy superior to anti‐PD‐1 therapy alone [28]. Commensal microbial community could also positively influence patients' outcomes after tumor resection through activating CD8+ T cells‐dependent antitumor response [29].
Previous studies have stated that microbiota‐derived modulators via PRRs have been linked to cancer initiation and development through the activation of innate immune pathways [30, 31]. However, other studies reported that gut microbiota could also affect innate immunity in a beneficial way against cancer. Gut commensals were observed to enhance antitumor T cell immunity by activating DCs via toll‐like receptor 4 (TLR4) signaling in melanoma mice receiving radiation [32]. Besides, the prevalence of specific gut bacteria induced by vancomycin treatment was also associated with potentiation of cytotoxic T cell response via stimulating interleukin 12 (IL‐12) secretion by CD8α+ DCs in mice models of lung and cervical cancer [33]. Lam et al. proposed that gut microbiota could reprogram innate the immune landscape in TME by skewing intratumoral mononuclear phagocytes (MPs) toward immunostimulatory monocytes and DCs through regulating the natural killer (NK) cell‐DC axis and type I interferon (IFN‐I) signaling. These MPs tended to skew toward protumorigenic macrophages when gut microbiota was depleted [34]. Intact commensal bacteria were also found to support immune surveillance in mice with lung carcinoma partially through enhancing γδT17 cell response [35].
IMPACT OF THE GUT MICROBIOTA ON CANCER IMMUNOTHERAPY
Involvement of gut microbiota to cancer immunotherapy
In addition to influencing tumor development, the commensal bacteria can also influence treatment outcomes. Preliminary studies have explained the engagement and contribution of gut microbiota to reprogramming anticancer immune responses, presenting a rationale for a novel field, “oncomicrobiotics,” which is focused on how commensal microbiota influences the host‐cancer equilibrium [36].
Through the gut wall where immune cells are located, the gut microbiota interacts with the immune system, which allows it to affect gut immunity as well as immune responses in distal mucosal sites via circulation and systemic metabolism [37]. Antitumor therapies like chemotherapy, radiotherapy, and immunotherapy can damage the integrity of the physical gut epithelial barrier, causing translocation or accumulation of specific microbiota, which leads to alteration in the constitution of the commensal microbiota. Upon Rag2–/–γc–/– mice receiving total body irradiation (TBI) before ACT, an augmented antitumor immunity was seen due to systemic liberation of Lipopolysaccharides (LPS), a common metabolite produced by Gram‐ bacteria) and microbial translocation permitted by TBI‐induced mucosal barrier injury [32]. In mice treated with antibodies against cytotoxic T lymphocyte‐associated antigen 4 (CTLA‐4), the equilibrium between intraepithelial lymphocytes (IELs) and ileal epithelial cells (IECs) was compromised, resulting in a damaged intestinal tract and accumulation of Bacteroides fragilis and Bacteroides cepacian (which later found to be associated with preferred clinical outcomes) [38].
Dysbiosis of intestinal contents could also affect the clinical outcome of cancer immunotherapy since gut microbiota and host immunity are mutualistic [39]. It is common practice to prescribe antibiotics to patients undergoing cancer treatments to prevent or alleviate opportunistic infections. Beyond its antibacterial effects, evidence have shown that antibiotics are the most common cause of dysbiosis, leading to a detrimental impact on T cell‐based immunotherapies by changing the composition or decreasing the diversity of the gut microbiota [40]. Perturbations of gut microbiota in mice receiving an antibiotic cocktail (vancomycin, imipenem, and neomycin) lead to impaired function of tumor‐infiltrating myeloid‐derived cells and poorer response to CpG‐oligonucleotide immunotherapy [20]. Administration of broad‐spectrum antibiotics, respectively, in murine models of MCA205 sarcomas, Ret melanoma, and MC38 colon cancer all resulted in the compromised antitumor effect of CTLA‐4‐specific antibodies [38]. Retrospective analyses have been conducted in cancer patients based on observations in these preclinical models to determine whether antibiotic premedication could impact the outcomes of ICI treatment. A recent study showed that malignancy patients receiving ICI had adverse outcomes (objective remission rate [ORR], progression‐free survival [PFS], and overall survival [OS]) related to antibiotics administration [41].
Relationship between gut microbiota composition and efficacy of immunotherapy
ICI therapy
Anti‐programmed cell death protein 1 (PD1)/programmed cell death ligand 1 (PD‐L1) and anti‐CTLA‐4 monoclonal antibodies (mAbs) are currently the most widely used ICI therapies. Despite bringing hope to cancer patients who were defined as uncurable before, current ICI therapies are limited by low initial response rate and a long‐term loss of antitumor efficacy. Many preclinical and clinical studies have suggested that interindividual differences in the composition of the commensal microbiota might account for the significant heterogeneity in the success of ICI treatments. The baseline stool samples collected from patients being treated with mAbs were analyzed using shotgun DNA sequencing, 16S rRNA sequencing, and metabolomics, and studies found that decreased diversity or richness of fecal bacterial composition was correlated with lower response rate and worse patient survival [42]. In addition to the diversity of the gut microbiota, further studies have identified that the accumulation of specific bacteria species or strains in the gut microbiota ecosystem was also associated with higher therapeutic efficacy of immunotherapies and enhanced antitumor T cell immunity.
C57BL/6 mice harboring different commensal microbes from Taconic Farms (TAC) and Jackson Laboratory (JAX) were treated with anti‐PD1 mAb, respectively, and JAX‐fed mice (relatively abundant in Bifidobacterium) showed far higher response rate to the treatment. This phenotype could be transferred to TAC‐fed mice by cohousing or fecal transplant [43]. Matson et al. identified that Bifidobacterium longum, Enterococcus faecium, and Collinsella aerofaciens were more prevalent in responding versus nonresponding metastatic melanoma patients receiving anti‐PD‐1 blockade, and FMT from responders to germ‐free (GF) mice bearing melanoma could potently facilitate antitumor immune responses and restore therapeutic efficacy of anti‐PD‐L1 blockade in mice [44], indicating that gut microbiota composition was at least partially involved in the efficacy of ICI therapies. Metastatic melanoma patients with high Faecalibacterium abundance were prone to benefit from anti‐PD‐1 and anti‐CTLA‐4 therapies with a significantly prolonged PFS compared to those with the dominance of Bacteroidales in the gut microbiota [45, 46]. In patients with NSCLC and RCC, sequencing analysis of feces showed that the prevalence of Akkermansia muciniphila was associated with favored clinical responses to ICI therapies [47]. Baseline stool samples of patients with advanced‐stage GI cancer collected before and during anti‐PD‐1/PD‐L1 treatment presented an elevation of the Prevotella/Bacteroides ratio in patients with preferred clinical outcomes. Besides, it was shown that a subset of responders was significantly enriched in Prevotella, Ruminococcaceae, and Lachnospiraceae genus [48]. In addition, Combination of anti‐PD‐1‐based immunotherapy with traditional Chinese medicine Gegen Qinlian decoction (GQD) eradicated colorectal cancer in mice by remodeling the gut microbiota portraited by a high abundance of Bacteroides acidifaciens and increasing the level of CD8 T cells and IFN‐γ [49].
Chimeric antigen receptor T cells (CARTs) and allogeneic hematopoietic cell transplantation (allo‐HCT) therapy
ICI treatment has achieved great success in numerous solid tumor types. However, for hematological malignancies, CARTs targeting CD19 and allo‐HCT have been demonstrated as prototype and innovation of T cell‐based anticancer therapies, respectively [50]. A growing understanding of how gut microbiota composition affects allo‐HCT and CART immunotherapy is emerging. A great diversity of commensal bacteria taxa with the dominance of Eubacterium limosum was related to a lower risk of progression and relapse after stem cell transplantation [51], while abundance in Enterococcus induced by administration of broad‐spectrum antibiotic was linked with exacerbated graft‐versus‐host disease (GVHD) and unfavorable OS [52]. Smith et al. observed that distinct dominance of gut microbiota before CD19‐CART treatment led to different outcomes in patients with B cell malignancies. Oscillospiraceae, Lachnospiraceae, and Ruminococcaceae were enriched in patients achieving a complete response (CR), while the increased presentation of Peptostreptococcaceae was associated with resistance to anti‐CD19 CAR‐T cells [53]. To corroborate the result, a two‐center study was conducted by the same team later, and researchers found that a higher abundance of Ruminococcus, along with Bacteroides and Faecalibacterium, were also correlated with higher efficacy and no toxicity development in CART therapy [54]. Notably, “favorable” gut microbiota identified in CAR‐T therapy such as Ruminococcaceae, Faecalibacterium, and Lachnospiraceae, were consistent with the “beneficial” bacteria capable of promoting the efficacy of ICI treatment. However, specific bacterial taxa of the Bacteroides genus showed increased efficacy of CAR‐T immunotherapy in contrast to its role of reducing anticancer immunity in ICI immunotherapy (Table 1).
Table 1.
Modulation of specific microbiota taxa in cancer immunotherapy.
| Gut microbiota taxa | Model | Immunotherapy | Cancer | Major findings | Author/Year | Ref |
|---|---|---|---|---|---|---|
| Akkermansia muciniphila | Human and mice | Anti‐PD‐1/PD‐L1 | NSCLC, RCC |
|
Routy et al., 2018 | [47] |
| Ruminococcaceae | Human | Anti‐PD‐1/PD‐L1 | GI cancer | Associated with distinct bacterial pathways including fermentation to SCFAs, unsaturated fatty acid biosynthesis, vitamin and starch biosynthesis | Peng et al., 2020 | [48] |
| Human | Anti‐CD19 CAR‐T | B cell lymphoma and leukemia | Improve antitumor effects of CAR‐T cell infusion | Smith et al., 2018 | [53] | |
| Ruminococcus | Human | Anti‐CD19 CAR‐T | B cell lymphoma and leukemia |
|
Smith et al., 2022 | [54] |
| Faecalibacterium | human | Anti‐PD‐1 and Anti‐CTLA‐4 | Melanoma |
|
Gopalakrishnan et al., 2018 | [46] |
| Human | Anti‐CD19 CAR‐T | B cell lymphoma and leukemia |
|
Smith et al., 2022 | [54] | |
| Bifidobacterium | Mice | Anti‐PD‐1 | Melanoma |
|
Sivan et al., 2015 | [43] |
| Bifidobacterium longum | Human | Anti‐PD‐1 | Melanoma |
|
Matson et al., 2018 | [44] |
| Prevotella | Human | Anti‐PD‐1/PD‐L1 | GI cancer |
|
Peng et al., 2020 | [48] |
| Lachnospiraceae | Human | Anti‐PD‐1/PD‐L1 | GI cancer | Associated with distinct bacterial pathways including fermentation to SCFAs, unsaturated fatty acid biosynthesis, vitamin and starch biosynthesis | Peng et al., 2020 | [48] |
|
Lachnospiraceae Collinsella aerofaciens |
Human | Anti‐CD19 CAR‐T | B cell malignancies | Improve antitumor effects of CAR‐T cell infusion | Smith et al., 2018 | [53] |
| Human | Anti‐PD‐1 | Melanoma |
|
Matson et al., 2018 | [44] | |
| Enterococcus faecium | Human | Anti‐PD‐1 | Melanoma |
|
Matson et al., 2018 | [44] |
| Oscillospiraceae | Human | Anti‐CD19 CAR‐T | B cell malignancies | Improve antitumor effects of CAR T cell infusion | Smith et al., 2018 | [53] |
Abbreviations: CAR‐T, chimeric antigen receptor T‐cell; CTLA‐4, cytotoxic T lymphocyte‐associated antigen 4; DC, dendritic cell; GI cancer, gastrointestinal cancer; ICI, immune checkpoint inhibitor; NSCLC, nonsmall cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death ligand 1; RCC, renal cell cancer; SCFAs, short‐chain fatty acids; TME, tumor microenvironment.
Relationship between gut microbiota composition and immune‐related toxicities
As a novel anticancer agent, immunotherapy can activate the host immunity against cancer and shift the inherent immunosuppression tone in TME. However, the enhanced systemic immune response not only exerts effects on tumor tissues but also on normal tissues, causing colitis, hepatitis, pneumonitis, and GVHD, collectively known as immune‐related adverse events (irAEs) [55]. Searching for an intervention that concurrently improves clinical effects and prevents adverse events of immunotherapy has long been a concerning question. Commensal bacteria are pivotal in alleviating overstimulation of the immune system, which induces immune tolerance by potentiating induction of regulatory T cells (Tregs) at mucosal barrier sites and producing immunomodulatory metabolites into circulation [56]. Gut microbiota can maintain host homeostasis in the gut lumen and the rest. Manipulation of commensals might be the key to the question. The intestinal Blautia genus was associated with improved overall patient survival while preventing the incidence of lethal GVHD in patients undergoing allo‐HCT [57]. In a preclinical murine model, changes in gut microbiota composition due to anti‐CTLA‐4 treatment were linked to the severity of intestinal lesions, and recolonization of antibiotics‐treated mice with Bacteroides cepacia and Bacteroides fragilis was observed to be capable of uncoupling efficacy and toxicity of CTLA‐4 blockade [38]. Two prospective studies of patients with metastatic melanoma reported that the overrepresentation of Bacteroidetes phylum in baseline stool samples of patients was related to the resistance to the onset of ipilimumab‐induced colitis [45, 58]. A previous study focused on patients with advanced NSCLC showed that the suppressive role of Lactobacillaceae, Raoultella, and Akkermensia species in the development of irAEs was observed during anti‐PD‐1/PD‐L1 treatment [59]. Interestingly, although capable of attenuating immune‐related toxicities, clinical outcomes of immunotherapies were not improved by the Bacteroidetes phylum and Akkermensia species.
The beneficial mechanism mediated by gut microbiota in cancer immunotherapy
The human microbiota produces a wide range of genes, and the microbiota encodes peptides that mimic tumor neoantigens [60]. In preclinical murine models, upon stimulation of antimicrobial antigen immunity, bacterial epitope‐specific T cells expand, enter circulation, and transport into the TME, facilitating immune response at distant sites by producing chemokines, expressing CD40L or cross‐reactivity of tumor antigen‐specific T cells [61]. A number of studies have demonstrated the ability of gut microbes to stimulate systemic innate immune responses via interacting with PRRs that mediate anti‐inflammatory or pro‐immune effects [62]. Upon exposure to PAMP which present on microbial surfaces, Antigen‐presenting cells (APCs) located at the gut mucosa or elsewhere upregulate pro‐inflammatory genes such as tumor necrosis factor‐α (TNF‐α), IL‐12, and IFN in a PRR‐dependent manner, thereby promoting Th1/Tc1 immune responses against cancer. Notably, a recent study has proposed that specific gut bacterial species may also promote antitumor immunity by suppressing the expression of PD‐L2 and its binding partner in T cells [63].
Inosine produced by a subset of intestinal bacteria increases antitumor immunity through enhancing Th1 differentiation and effector function of naïve T cells expressing A2AR [64]. It can also feed effector T cells that infiltrate into TME depletion of glucose by cancer cells [65]. A positive correlation was found between anti‐PD‐1/PD‐L1 responses and bacteria that produce short‐chain fatty acids (SCFAs) like Eubacterium, Lactobacillus, and Streptococcus [48]. Possible mechanisms of immunostimulatory action of gut microbiota in CAR‐T infusion also relied on the microbial metabolites (including bile acid metabolites [66], tryptophan metabolites [67], and SCFAs [68]) and bacterial‐derived membrane fractions (e.g., lipoteichoic acid and LPS) which exert strong influences on T cells via host receptors and other targeted molecules, so hypothetically can modulate anticancer immunity in T‐cell based CAR‐T therapy as well (Figure 3) [50].
Figure 3.
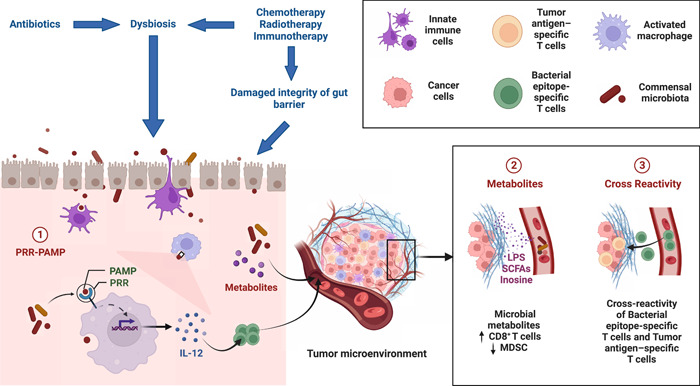
The mechanisms underlying the immunostimulatory involvement of gut microbiota in cancer immunotherapy. Anticancer therapies such as chemotherapy, radiotherapy and immunotherapy can increase permeability of gut epithelial, translocation of bacteria and dysbiosis. During cancer therapy, antibiotics are sometimes used to treat opportunistic infection, which can also lead to disruption of gut microbiota. The mechanism of immunostimulation by gut microbiota includes ligation of PRR and PAMP, release of microbial metabolites such as SCFAs, LPS and inosine, and cross‐reactivity of bacteria epitope‐specific T cells and tumor antigen‐specific T cells. LPS, Lipopolysaccharides; MDSC, myeloid‐derived suppressor cells; PAMP, pathogen‐associated molecular patterns; PRR, pattern recognition receptor; SCFAs, short‐chain fatty acids.
APPLICATIONS OF GUT MICROBIOTA: SERVING AS NEW THERAPEUTIC TOOLS AND BIOMARKERS FOR IMMUNOTHERAPY
New therapeutic strategies to improve cancer immunotherapy
Cancer patients commonly receive conventional therapies like chemotherapies and radiotherapies before being treated with immunotherapies, which could disrupt gut commensals unfavorably. Thus, manipulating gut microbiota composition to a status of optimal biodiversity and signature before immune‐related interventions might be a new and effective therapeutic tool. Recent studies have raised awareness of the importance of a population‐specific approach to microbiota‐based combination treatments due to the co‐diversification of the gut microbiota with humans [69]. Therefore, interventions will have to be adapted according to the age, lifestyle, comorbidities, comedications, and genetic inheritance of patients for an optimized personal therapy (Table 2).
Table 2.
Strategies to modulate gut microbiota in cancer immunotherapy and the corresponding clinical trials.
|
ClinicalTrials.gov Identifier |
Study title | Cancer types | Intervention | Objective | Status |
|---|---|---|---|---|---|
| NCT04645680 |
Diet and Immune Effects Trial: DIET—A Randomized Double Blinded Dietary Intervention Study in Patients With Metastatic Melanoma Receiving Immunotherapy |
Melanoma | Dietary Intervention: Two different diets vary in fiber content | To assess effect of high‐in‐fiber diet on efficacy and toxicity of immunotherapy drugs pembrolizumab or nivolumab | Recruiting |
| NCT04866810 | The Effect of Diet and Exercise on Immunotherapy and the Microbiome (EDEN) | Melanoma | Dietary Intervention: A plant‐based, high‐fiber diet and a change for high intensity exercise | To see if nutritional intake and physical activity change the gut microbiota in people with melanoma receiving relatlimab and nivolumab immunotherapy | Not Applicable |
| NCT05251389 | FMT to Convert Response to Immunotherapy | Melanoma | Procedure; Fecal Microbial Transplantation | To investigate the influence of FMT on efficacy and toxicity of immunotherapy | Recruiting |
| NCT04163289 |
Preventing Toxicity in Renal Cancer Patients Treated With Immunotherapy Using Fecal Microbiota Transplantation (PERFORM) |
Renal Cell Carcinoma | Drug: Fecal Microbiota Transplant | To study the safety of FMT combination treatment with ipilimumab and nivolumab, and whether reduce incidence of immune‐related toxicities in patients | Recruiting |
| NCT04056026 | A Single Dose FMT Infusion as an Adjunct to Keytruda for Metastatic Mesothelioma | Mesothelio‐ma | Biological: Fecal Microbiota Transplant | To see if FMT can increase efficacy of an immunotherapy drug Keytruda | Completed |
| NCT04116775 | Fecal Microbiota Transplant and Pembrolizumab for Men With Metastatic Castration Resistant Prostate Cancer | Prostate Cancer | Biological: Fecal Microbiota Transplant | To evaluate anticancer effect of FMT in pembrolizumab treatment | Recruiting |
| NCT05303493 | Camu‐Camu Prebiotic and Immune Checkpoint Inhibition in Patients With Nonsmall Cell Lung Cancer and Melanoma | Advanced NSCLC Stage IV and Melanoma Stage IV |
Probiotics drug: Camu Camu Capsules |
To evaluate the safety and tolerability of Camu Camu prebiotic in addition to ICI in patients with NSCLC and melanoma | Recruiting |
| NCT05220124 | A Study of Probiotics Administration in the Immunotherapy of Urothelial Bladder Carcinoma | Bladder Urothelial Carcinoma | Probiotics drug: Live Combined (Bifidobacterium, Lac‐tobacillus, and Enterococcus Capsules) | To evaluate clinical benefits of probiotics in immunotherapy | Recruiting |
| NCT04699721 | Clinical Study of Neoadjuvant Chemotherapy and Immunotherapy Combined With Probiotics in Patients With Potential/Resectable NSCLC | Nonsmall Cell Lung Cancer Stage III |
Probiotics drug: Bifidobacterium trifidum live powder |
To evaluate safety and effect of neoadjuvant chemotherapy and immunotherapy combined with probiotics | Recruiting |
Abbreviations: CAR‐T, chimeric antigen receptor T‐cell; CTLA‐4, cytotoxic T lymphocyte‐associated antigen 4; DC, dendritic cell; GI cancer, gastrointestinal cancer; ICI, immune checkpoint inhibitor; NSCLC, nonsmall cell lung cancer; PD‐1, programmed cell death protein 1; PD‐L1, programmed cell death ligand 1; RCC, renal cell cancer; SCFAs, short‐chain fatty acids; TME, tumor microenvironment.
Dietary intervention
As is known to all, “you are what you eat,” which means long‐term dietary habits affect the composition and activity of microbes residing in the human gut from the scientific aspect. Notably, studies have also indicated that the gut microbiota rapidly respond to dietary changes, even when these alterations occur over a brief period [70]. This underscores the potential of intervening cancer patients' diet as a plausible strategy to suppress tumor growth. Accumulating evidence have shown that patients with a diet high in sugar and fat were correlated with poor response to immunotherapy, while consumption of sufficient dietary fibers and salt led to improved clinical outcomes [71]. Microbial fermentation of dietary fibers produces SCFAs like butyrate and propionate, which can interact with the gut wall and assist in maintaining intestinal immune homeostasis as the main metabolites produced by the gut microbiota of long‐term responders to ICI therapy [72]. High salt condition was associated with significant tumor regression. It enhanced anticancer immunity in tumor‐bearing mice, indicating that salt intake might be a diet‐related factor to affect the efficacy of immunotherapy [73]. Therefore, a diet high in fibers and salt as well as low in fat and sugar can be recommended for cancer patients receiving immunotherapy. A few studies have claimed that distinct diets could result in predictable shifts in existing host bacterial taxa [74]. Higher fiber intake was associated with increased Provotella, and higher salt intake led to a lower abundance of Bacteroides and Proteobacteria, while upregulated Firmicutes in a mouse model [75]. Ketogenic diet could upregulate the abundance of commensal Eisenbergiella massiliensis which are strongly correlated with the serum concentration of principle ketone body, 3‐hydroxybutyrate (3‐HB) and induce T‐cell based antineoplastic effect in a 3‐HB dependent manner, thereby promoting ICI efficacy and increase overall survival rate in mice with colorectal cancer [76]. For further development of dietary interventions, more studies are needed to determine how dietary interventions can modulate gut microbiota composition and enhance anticancer immune response, as well as the underlying mechanism.
Fecal microbiota transplantation (FMT)
By transplanting fecal material from healthy donors to recipients, FMT directly modulates the gut microbiota profiling, which can promote the recolonization of bacteria that exhibit health‐enhancing properties, restore microbial diversity in the gastrointestinal tract, and improve clinical outcomes of immunotherapy [77]. In preclinical models, tumor regression has been demonstrated in mice receiving FMT from patients responding to ICI treatment [78]. In a first‐in‐human clinical trial, the feasibility and safety of FMT treatment in immunotherapy were confirmed with 3 out of 10 patients with anti‐PD‐1‐refractory metastatic melanoma responding to anti‐PD‐1 blockade after FMT from previous responding patients to nonresponding patients, and none of the 10 patients developed severe irAEs during FMT treatment [79]. Another phase I clinical trial enlightened by the first one also focused on the clinical benefit of FMT together with anti–PD‐1 in metastatic melanoma patients who failed immunotherapy, reporting that 2 out of 15 patients achieved partial response while one achieved CR. Despite the fact that all patients in this clinical trial experienced at least one irAE, the degree is minimal [80]. A recent multicenter phase I trial combining healthy donor FMT with the PD‐1 inhibitors showed a more promising result that 13 out of 20 patients with advanced melanoma (previously untreated) responded to the combined treatment including four CR, although 5 out of 20 patients reported grade 3 irAEs during the combination therapy. Safety of FMT as first‐line setting was also ensured for no severe irAEs occurred when FMT was conducted alone. However, it's noteworthy that the similarity between the gut microbiome of donor and recipient only gradually increased over time in responders [81].
Defined commensal strains
Besides immune modulation, convenience, and low price, there might be safety risks of transferring chronic diseases like obesity, pathogens, and carcinogenesis during FMT treatment, which makes administering defined commensal strains as exogenous probiotics a preferred alternative for FMT. Developing specific microbiota‐based combinatory treatment has been shown to improve the overall response rate of immunotherapy. Integration of inosine supplementation with checkpoint‐blockade therapy and adoptive T‐cell therapy has led to delayed tumor growth and survival in mice [65], and inosine‐producing microbes like Bifidobacterium pseudolongum might represent a novel and efficacious way to deliver the molecule and enhance its accumulation in the TME, which could be added to the diets of cancer patients to improve the efficacy of ICI treatment [82]. Tanoue et al., have identified a rare commensal consortium isolated from healthy human feces, consisting of 11 bacterial strains with 7 Bacteroidales and 4 nonbacteroidales species, and supplementation with theses 11 strains could enhance both spontaneous and ICI‐mediated antitumour immunity via induction of IFN‐γ‐producing CD8+ T cells in mice intestine without causing colitis [83]. In addition to the colonization of gut with defined commensal species of remarkable clinical benefits in enhancing cancer treatment outcomes, modulation of intestinal bacteria gene circuits yields engineered bacteria, such as Escherichia coli (E. coli), have recently been presented as innovative anticancer agents by stimulating both innate and adaptive immunity, either alone or as adjuvants when combined with other modalities [84]. Programming immunotherapeutic Escherichia coli loaded with anti‐CD47 blocking nanobody could augment activation of intratumoral T cells and effectively shrink tumor size in multiple syngeneic murine tumor models, meanwhile another preclinical study showed engineered E. coli Nissle 1917 strain potentiated efficacy of PD‐L1 blockade mediated by increasing L‐ arginine mediated T cells production and activation [85, 86].
Prebiotics and probiotics
Prebiotics are nondigestible food ingredients that can serve as nutrients for gut microbes. Studies have investigated that selective enhancement of gut bacteria can improve anticancer therapy outcomes. For example, inulin can stimulate the growth of Faecalibacterium and Bifidobacterium species which were associated with improved efficacy of immunotherapy before [78]. Novel classes of prebiotics have been discovered to potentiate the antitumor effect of the anti‐PD‐1/anti‐PD‐L1 blockade in mouse models. Ginseng polysaccharides, a main component of Panax ginseng, sensitized the tumor to ICI therapy through increasing the microbial metabolites valeric acid and reshaping gut microbial composition from nonresponders towards that of responders in combination with ICI treatment [87]. Diosgenin, a natural steroidal saponin with similar activities to prebiotics, promoted antitumor effects of PD‐1 mAb by modulating intestinal microbiota with upregulation of Clostridiales, Lactobacillus, and Sutterella, and downregulation of Bacteroides [88].
Probiotics, including the commonly found Lactobacillus and Bifidobacterium species, are live microorganisms that are generally acknowledged as a promoter of the host's health in a positive manner [89]. It is reported that cancer patients are prone to self‐administered probiotics as adjuvant to the cancer immunotherapy. Preclinical and clinical studies have reported that probiotic intervention, including Lactobacillus casei BL23, Lactobacillus plantarum A, or combination of Bifidobacterium lactis Bl‐04 and Lactobacillus acidophilus NCFM, demonstrated remarkable antitumor immune effect and was capable of restoring the imbalance gut microbial profile [90, 91, 92], which confirmed its potential therapeutic benefits to fight cancer. Furthermore, Sivan et al. found that treating preclinical melanoma with PD‐L1‐specific antibodies while oral gavage with Bifidobacterium could nearly abolish tumor growth [43]. Another preclinical research revealed that probiotic Enterococcus facilitated the efficacy of checkpoint inhibitor immunotherapy by secreting orthologs of the NlpC/p60 peptidoglycan hydrolase, indicating that screening microbiota species genetically coded with peptidoglycan remodeling activity may obtain potential probiotic candidates capable of enhancing cancer immunotherapy [93]. A recent study reported that oral gavage of a frequently used probiotic Lactobacillus reuteri in specific‐pathogen‐free mice challenged with melanoma bolstered the ICI treatment efficacy via promoting the production of probiotic‐released indole‐3‐aldehyde, and such beneficial effect was further recapitulated in melanoma patients [94]. However, there are still safety concerns about over‐the‐counter probiotics use in patients receiving ICI therapy for cancer. On the one hand, probiotic supplements might not be effective if exogenous bacteria colonize poorly in the host intestines and last only a short period. On the other hand, an assessment of commercially available probiotics in murine models of melanoma and CRC showed defective antitumor response to treatment with anti‐PD‐L1 and increased tumor outgrowth [95, 96].
Novel biomarkers to predict host response to immunotherapy
Previous studies have shown that different fingerprints were detected between responders and nonresponders to ICI therapies based on gut microbiota profiling [14], indicating the potential of utilizing gut microbiota composition and its metabolites to predict the clinical outcomes of immunotherapy. Research have been conducted on gut microbiota as a biomarker for immunotherapy response in recent years, stratifying responders versus nonresponders, as well as predicting the incidence and severity of immune‐related toxicities in a minimal invasive and easy way.
Relative enrichment of certain bacteria species (e.g., Akkermansia muciniphila [47], Bifidobacterium longum [43], Bacteroides fragilis [38], and Ruminococcaceae family [45]) was associated with favorable prognosis of PD‐1/PDL‐1/CTLA‐4 inhibitors. Another relative abundance of bacteria (e.g., Roseburia intestinalis and B. thetaiotaomicron [97]) was reported negatively related to immunotherapy responses. The genetic diversity of bacteria in the gut might serve as an early predictor of the effectiveness of anti‐CD19 CAR T‐cell therapy in patients with large B cell lymphoma, and a high diversity might favor better outcomes [98].
Further study has observed enhanced peptidoglycan biosynthesis in patients who demonstrated a long‐term response to CAR‐T, while upregulated nonoxidative branch of the pentose phosphate pathway was associated with increased incidence of toxicities, indicating metabolites from bacterial taxa might also serve as biomarkers [50]. Therefore, establishing a multi‐parameter model which not only relies on commensal microbial composition and its metabolites level but also takes variables like tumor genomics, comorbidities, age, and germline genetics into consideration might be the future tendency to comprehensively predict whether patients are sensitive to immunotherapies.
DISCUSSION
Previous studies have shed light on the relationship between the gut, the immune system, the hypothalamic‐pituitary‐adrenal (HPA) axis, and the autonomic nervous system [99]. Consumption of certain food such as dietary fibers will increase the production of short chain fatty acids such as butyrate, which are generated by anaerobic bacteria during fermentation. This will in turn influence the production of neuropeptide such as neuropeptide Y (NPY), and then significantly affect the suppression or activation of certain immune cells [100]. The spleen, a pivotal organ in the immune system, serves as a crucial interface facilitating communication between the brain and the immune system [101]. Use of antibiotic cocktail (ABX) in cancer patients leads to a poor response to T‐cell based immunotherapies via affecting the diversity as well as composition of gut microbiota, but the underlying mechanism remains unclear. Strong correlations were recently observed after a 14‐day ABX treatment between gut microbiota components, spleen weight, splenic cell components and yield of certain compounds in the spleen, brain and plasma, which infers that depletion of microbiota following antibiotics application may affect host immunity through altering the spleen and brain function [102]. More investigations need to be conducted on how gut microbiota influence the immune system through the gut‐brain axis or gut‐microbiota‐spleen‐brain axis.
The vagus nerve, as a major component of the parasympathetic nervous system with dense innervation of the gut, is capable of regulating microbiota‐gut‐brain axis in a bidirectional way [103]. After sensing and transferring the microbiota metabolites information through its afferents to the brain, the vagus nerve fibers subsequently initiate the activation of anti‐inflammatory responses, reduce gut permeability, and thus regulate microbiota composition [104]. Previous studies have also reported that activation of the vagus nerve, namely vagus nerve stimulation (VNS), has potent anti‐inflammatory properties and antitumor effect via triggering antitumor immune cells or upregulating the cholinergic anti‐inflammatory pathway, inferring its potential to establish a favorable intestinal microenvironment for cancer immunotherapy while reducing the occurrence and severity of colitogenic side effects [105, 106, 107]. These findings highlight the importance of VNS in cancer pathology and immunotherapy, suggesting that more investigation should be conducted in the future to unravel the clinical efficacy of a combinatory therapy of VNS and immunotherapy, which may serve as a promising anticancer treatment regimen with the advantages of prescribing less dose of immunotherapeutic drugs to patients, reducing their pain, and suppressing tumor growth effectively in a synergistic way.
While there have been significant research on how the gut dysbiosis can influence the initiation and development of tumors, it is equally important to understand whether intestinal or extraintestinal malignancies promote compositional shifts of the commensal microbiota to their own benefit. During the onset of tumorigenesis, a sustained gut dysbiosis with the hallmark of the Clostridium species were recently observed in both human and mice harbored with various cancer types, accompanied with stress ileopathy due to decrease in parasympathetic signaling in the ileal mucosa. Besides, the inhibition of Clostridium species by antibiotics vancomycin could overtly prevent cancer‐induced ileopathy, restore intestinal homeostasis and control tumor progression [108]. In addition, whatever anticancer regimens patients received during treatment, such as chemotherapy, radiation therapy, surgery and even immunotherapy itself, could drive a convert on the diversity and profile of microbiota [109], which may in turn influence the efficacy of anticancer therapies. This bidirectional relationship between tumors and the gut microbiota is an intriguing and complex research field, and a broader comprehension on this filed will contribute valuable insights into cancer biology, anticancer treatment strategies and side effect management.
As a modern form of immunotherapy, the combination of cancer vaccines and standard therapies can also enhance antitumor immune responses. However, the unsatisfactory therapeutic efficacy of cancer vaccines in phase III clinical trials has greatly inhibited its development and clinical application. One possible reason is the phenomenon of “immunosenescence” happening in the elderly, who were the majority of cancer patients, suggesting that finding a potent adjuvant in the formulation of cancer vaccines to help restore and stimulate the host immune system is an important tool to improve clinical efficacy. Despite the lack of precise data on cancer vaccines, there has been an apparent correlation between microbiota and vaccination effectiveness against several pathogens, indicating that microbiota might serve as a natural adjuvant to enhance the action of cancer vaccines by providing inflammatory cytokines (IL‐12, IL‐1β, and IFN‐γ) and PAMPs to activate the immune system during vaccination [110, 111]. A study in the murine model has taken one step forward by using microbiota as a real cancer vaccine adjuvant and reported enhanced immune response against tumors [112]. Oncolytic virus, as a new class of immunotherapy agents, has played a pivotal role in cancer treatment by directly killing tumor cells and inducing immunity when used as gene vectors carrying specific checkpoint antibodies. Preclinical studies have shown a plausible relationship between intestinal flora and efficacy of oncolytic virus‐mediated immunotherapy. Yi et al., suggested that gut microbiota may synergistically increase the antitumoral activity of oncolytic virus via the common IFN‐mediated pathway in colorectal cancer [113]. What's more, the antitumor effect of oncolytic adenovirus Ad5D24‐CpG (Ad‐CpG) was recently found to at least partially attribute to gut microbiota, and Bifidobacterium supplementation could hamper tumor progression and intratumoral Treg infiltration in melanoma mice receiving Ad‐CpG treatment [114]. Sarcomas are rare and heterogeneous mesenchymal neoplasms derived from the bone or soft tissues associated with several challenges due to their less immunogenic portrait than other tumor types. Although Immunotherapy has revolutionized cancer treatment, immuno‐oncology agents have not yet been approved for patients with sarcomas attributed to the limitations of low tumor mutation burden and the immunosuppressive TME [115]. However, several research have indicated that immunotherapy may represent an efficient therapeutic strategy for this group of diseases, including cancer vaccines, immune checkpoint blockade and adoptive cell transfer [116]. Future studies investigating novel immunotherapy strategies in rare cancer types like sarcomas should incorporate the analysis and intervention of commensal bacteria, which will allow for a better understanding of the gut microbiota involved in sensitivity and treatment resistance to immune‐oncology agents.
Regarding the preliminary research and clinical intervention of gut microbiota in immunotherapy, there are still some issues to be resolved. For example, current tools to explore the effect of gut microbiota mainly include the antibiotics treatment models or germ‐free (GF) mouse models, among which the GF mice were widely acknowledged as the gold standard due to their complete microbial depletion and ability to be exclusively colonized with defined microbes [117]. However, concerns have been raised regarding the impaired development of the immune system in GF mice due to a lack of early immune education, which potentially impacts their applicability in microbiota studies in terms of immune‐related conditions, including cancer immunotherapy [118]. In addition, GF mouse models are expensive to acquire and maintain, thus antibiotics treatment may present as a cost‐effective and easily accessible alternative to germ‐free models with less limitation. In addition, as for microbiota modulation approaches, the conventional screening measure is unable to detect side effects of FMT [119], necessitating meticulous collection of stools from healthy donors to prevent potential infection caused by transfer of pathogenic bacteria during FMT. Using defined bacterium as daily medication might face technological obstacles like finding optimal culture conditions and encapsulation which allows large‐scale manufacture in vivo and preserved biological activities before it takes effect in our gut.
Microbiota effects are not likely dependent on one species and might be caused by diverse microbiota compositions, and future research should focus on identifying a group of microbe consortium with great benefit to optimizing cancer immunotherapy. Comprehensive mapping of the biological effects and modes of action of prebiotics and probiotics for each cancer type still has not been well‐elucidated, which inhibits their application in individual precision medicine (Figure 4). Therefore, the development of cheap, rapid and accurate testing techniques on patient serum and stool samples, which combines meta‐transcriptomic sequencing, metagenomic or metabolomic analysis, will help elucidate the underlying molecular mechanism involved in different therapeutic responses and propose novel therapeutic targets for cancer patients with microbiota‐related immunotherapy resistance.
Figure 4.
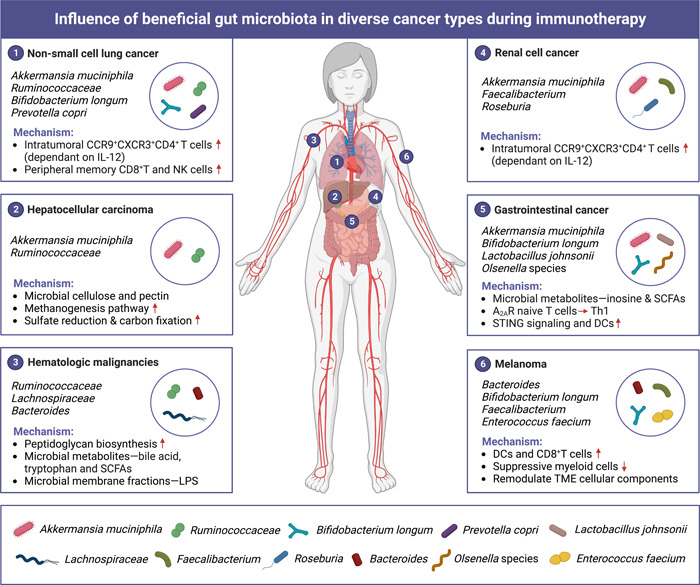
Influence of beneficial gut microbiota in diverse cancer types during immunotherapy. Gut microbiota has contrast role in cancer initiation and development, which not only affects gastrointestinal (GI) cancer locally, but also impacts cancer developed in distal organs including nonsmall‐cell lung cancer, hepatocellular carcinoma, hematologic malignancies, renal cell cancer, and melanoma.
CONCLUSION
Gut microbiota serves as pivotal intermediate between the gut, brain, spleen and immune system, maintaining homeostasis of the organic whole. Unfavored intestinal flora leads to intestinal or extraintestinal malignancies, hampers anticancer immunity and participates in shaping the immunosuppressive TME. Accumulating studies have observed that the diversity and composition of host gut microbiota were associated with the efficacy of immunotherapy as well as the incidence of irAEs, which shed lights on utilizing microbiota as novel biomarkers to predict patients' response to immunotherapy and targeting microbiota as potential anticancer agents alone or as adjuvant. This highlights the importance of investigating precise and safe approaches which could alter microbiota profile in cancer patients towards a more diverse composition in favor of “beneficial bacteria.” Meanwhile, more studies are needed to comprehensively mapping the blueprints of beneficial commensal strains across various cancer types and tracking the dynamic change of gut microbiota throughout the treatment course, and focus on the complex role of gut microbiota in new form of immunotherapy in the future.
AUTHOR CONTRIBUTIONS
Yutian Zou, Minqing Wu, and Xiaoming Xie conceived the idea and edited the manuscript. Jindong Xie, Manqing Liu, and Xinpei Deng collected the data and wrote the manuscript. Jindong Xie and Manqing Liu drew the figures. Yuhui Tang, Shaoquan Zheng, Xueqi Ou, and Hailin Tang validated and supervised the manuscript. All authors have read the final manuscript and approved it for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We thank BioRender for giving high‐quality clipart icons (https://biorender.com). This work was supported by grants from the National Natural Science Foundation of China (82173366).
Xie, Jindong , Liu Manqing, Deng Xinpei, Tang Yuhui, Zheng Shaoquan, Ou Xueqi, Tang Hailin, Xie Xiaoming, Wu Minqing, and Zou Yutian. 2024. “Gut Microbiota Reshapes Cancer Immunotherapy Efficacy: Mechanisms and Therapeutic Strategies.” iMeta 3, e156. 10.1002/imt2.156
Jindong Xie, Manqing Liu, and Xinpei Deng contributed equally.
Contributor Information
Xiaoming Xie, Email: xiexm@sysucc.org.cn.
Minqing Wu, Email: wumq@sysucc.org.cn.
Yutian Zou, Email: zouyt@sysucc.org.cn.
DATA AVAILABILITY STATEMENT
Supplementary information (graphical abstract, slides, videos, Chinese translated version, and update materials) is available online DOI or http://www.imeta.science/.
REFERENCES
- 1. Bray, Freddie , Ferlay Jacques, Soerjomataram Isabelle, Siegel Rebecca L., Torre Lindsey A., and Jemal Ahmedin. 2018. “Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.” CA: A Cancer Journal for Clinicians 68: 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Hanahan, Douglas , and Weinberg Robert A.. 2011. “Hallmarks of Cancer: The Next Generation.” Cell 144: 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3. Singh, Raghwendra Pratap , Bashir Hilal, and Kumar Rashmi. 2021. “Emerging Role of Microbiota in Immunomodulation and Cancer Immunotherapy.” Seminars in Cancer Biology 70: 37–52. 10.1016/j.semcancer.2020.06.008 [DOI] [PubMed] [Google Scholar]
- 4. Zhang, Yuan‐Yuan , and Zhang Zemin. 2020. “The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor‐Infiltrating Immune Cells and Their Therapeutic Implications.” Cellular & Molecular Immunology 17: 807–821. 10.1038/s41423-020-0488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma, Padmanee , Hu‐Lieskovan Siwen, Wargo Jennifer A., and Ribas Antoni. 2017. “Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy.” Cell 168: 707–723. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan, Chong‐Xian , Liu Hongtao, Robins Elizabeth, Song Wenru, Liu Delong, Li Zihai, and Zheng Lei. 2020. “Next‐Generation Immuno‐Oncology Agents: Current Momentum Shifts in Cancer Immunotherapy.” Journal of Hematology & Oncology 13: 29. 10.1186/s13045-020-00862-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Human Microbiome Project Consortium . 2012. “Structure, Function and Diversity of the Healthy Human Microbiome.” Nature 486: 207–214. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gomaa, Eman Zakaria . 2020. “Human Gut Microbiota/Microbiome in Health and Diseases: A Review.” Antonie Van Leeuwenhoek 113: 2019–2040. 10.1007/s10482-020-01474-7 [DOI] [PubMed] [Google Scholar]
- 9. Chen, An‐Tian , Zhang Jian, and Zhang Yuhui. 2023. “Gut Microbiota in Heart Failure and Related Interventions.” iMeta 2: e125. 10.1002/imt2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Welton, John C. , Marr John S., and Friedman Stephen M.. 1979. “Association Between Hepatobiliary Cancer and Typhoid Carrier State.” The Lancet 313: 791–794. 10.1016/s0140-6736(79)91315-1 [DOI] [PubMed] [Google Scholar]
- 11. Roviello, Giandomenico , Iannone Luigi Francesco, Bersanelli Melissa, Mini Enrico, and Catalano Martina. 2022. “The Gut Microbiome and Efficacy of Cancer Immunotherapy.” Pharmacology & Therapeutics 231: 107973. 10.1016/j.pharmthera.2021.107973 [DOI] [PubMed] [Google Scholar]
- 12. Kroemer, Guido , and Zitvogel Laurence. 2018. “Cancer Immunotherapy in 2017: The Breakthrough of the Microbiota.” Nature Reviews Immunology 18: 87–88. 10.1038/nri.2018.4 [DOI] [PubMed] [Google Scholar]
- 13. Bullman, Susan , Pedamallu Chandra S., Sicinska Ewa, Clancy Thomas E., Zhang Xiaoyang, Cai Diana, Neuberg Donna, et al. 2017. “Analysis of Fusobacterium Persistence and Antibiotic Response in Colorectal Cancer.” Science 358: 1443–1448. 10.1126/science.aal5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinato, David J. 2020. “Antibiotic‐Induced Dysbiosis as a Putative Actionable Driver of Cancer Immunity in Renal Cell Carcinoma.” European Urology 78: 207–208. 10.1016/j.eururo.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 15. Gur, Chamutal , Ibrahim Yara, Isaacson Batya, Yamin Rachel, Abed Jawad, Gamliel Moriya, Enk Jonatan, et al. 2015. “Binding of the Fap2 Protein of Fusobacterium Nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors From Immune Cell Attack.” Immunity 42: 344–355. 10.1016/j.immuni.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis, Petra , Hold Georgina L., and Flint Harry J.. 2014. “The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer.” Nature Reviews Microbiology 12: 661–672. 10.1038/nrmicro3344 [DOI] [PubMed] [Google Scholar]
- 17. Matson, Vyara , Chervin Carolina Soto, and Gajewski Thomas F.. 2021. “Cancer and the Microbiome‐Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy.” Gastroenterology 160: 600–613. 10.1053/j.gastro.2020.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blaser, Martin . 2011. “Antibiotic Overuse: Stop the Killing of Beneficial Bacteria.” Nature 476: 393–394. 10.1038/476393a [DOI] [PubMed] [Google Scholar]
- 19. Rossini, Anna , Rumio Cristiano, Sfondrini Lucia, Tagliabue Elda, Morelli Daniele, Miceli Rosalba, Mariani Luigi, Palazzo Marco, Ménard Sylvie, and Balsari Andrea. 2006. “Influence of Antibiotic Treatment on Breast Carcinoma Development in Proto‐Neu Transgenic Mice.” Cancer Research 66: 6219–6224. 10.1158/0008-5472.Can-05-4592 [DOI] [PubMed] [Google Scholar]
- 20. Iida, Noriho , Dzutsev Amiran, Stewart C. Andrew, Smith Loretta, Bouladoux Nicolas, Weingarten Rebecca A., Molina Daniel A., et al. 2013. “Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment.” Science 342: 967–970. 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riquelme, Erick , Zhang Yu, Zhang Liangliang, Montiel Maria, Zoltan Michelle, Dong Wenli, Quesada Pompeyo, et al. 2019. “Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes.” Cell 178: 795–806.e12. 10.1016/j.cell.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma, Ning , Chen Xiyue, Johnston Lee J., and Ma Xi. 2022. “Gut Microbiota‐Stem Cell Niche Crosstalk: A New Territory for Maintaining Intestinal Homeostasis.” iMeta 1: e54. 10.1002/imt2.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zitvogel, Laurence , Ayyoub Maha, Routy Bertrand, and Kroemer Guido. 2016. “Microbiome and Anticancer Immunosurveillance.” Cell 165: 276–287. 10.1016/j.cell.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 24. Zhao, Hua‐Kan , Wu Lei, Yan Guifang, Chen Yu, Zhou Mingyue, Wu Yongzhong, and Li Yongsheng. 2021. “Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention.” Signal Transduction and Targeted Therapy 6: 263. 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bain, Calum C. , and Mowat Allan McI. 2014. “Macrophages in Intestinal Homeostasis and Inflammation.” Immunological Reviews 260: 102–117. 10.1111/imr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang, Hai‐Xia , Wang Weicang, Romano Kymberleigh A., Gu Min, Sanidad Katherine Z., Kim Daeyoung, Yang Jun, et al. 2018. “A Common Antimicrobial Additive Increases Colonic Inflammation and Colitis‐Associated Colon Tumorigenesis in Mice.” Science Translational Medicine 10(443): eaan4116. 10.1126/scitranslmed.aan4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sepich‐Poore, Gregory D. , Zitvogel Laurence, Straussman Ravid, Hasty Jeff, Wargo Jennifer A., and Knight Rob. 2021. “The Microbiome and Human Cancer.” Science 371(6536): eabc4552. 10.1126/science.abc4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montalban‐Arques, Ana , Katkeviciute Egle, Busenhart Philipp, Bircher Anna, Wirbel Jakob, Zeller Georg, Morsy Yasser, et al. 2021. “Commensal Clostridiales Strains Mediate Effective Anti‐Cancer Immune Response Against Solid Tumors.” Cell Host & Microbe 29: 1573–1588.e7. 10.1016/j.chom.2021.08.001 [DOI] [PubMed] [Google Scholar]
- 29. Zhou, Cheng‐Bei , Zhou Yilu, and Fang Jingyuan. 2021. “Gut Microbiota in Cancer Immune Response and Immunotherapy.” Trends in Cancer 7: 647–660. 10.1016/j.trecan.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 30. Coleman, Olivia I. , Lobner Elena M., Bierwirth Sandra, Sorbie Adam, Waldschmitt Nadine, Rath Eva, Berger Emanuel, et al. 2018. “Activated ATF6 Induces Intestinal Dysbiosis and Innate Immune Response to Promote Colorectal Tumorigenesis.” Gastroenterology 155: 1539–1552.e12. 10.1053/j.gastro.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 31. Pushalkar, Smruti , Hundeyin Mautin, Daley Donnele, Zambirinis Constantinos P., Kurz Emma, Mishra Ankita, Mohan Navyatha, et al. 2018. “The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression.” Cancer Discovery 8: 403–416. 10.1158/2159-8290.Cd-17-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paulos, Chrystal M. , Wrzesinski Claudia, Kaiser Andrew, Hinrichs Christian S., Chieppa Marcello, Cassard Lydie, Palmer Douglas C., et al. 2007. “Microbial Translocation Augments the Function of Adoptively Transferred Self/Tumor‐Specific CD8+ T Cells Via TLR4 Signaling.” Journal of Clinical Investigation 117: 2197–2204. 10.1172/jci32205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uribe‐Herranz, Mireia , Bittinger Kyle, Rafail Stavros, Guedan Sonia, Pierini Stefano, Tanes Ceylan, Ganetsky Alex, et al. 2018. “Gut Microbiota Modulates Adoptive Cell Therapy Via CD8α Dendritic Cells and IL‐12.” JCI Insight 3(4): e94952. 10.1172/jci.insight.94952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lam, Khiem C. , Araya Romina E., Huang April, Chen Quanyi, Di Modica Martina, Rodrigues Richard R., Lopès Amélie, et al. 2021. “Microbiota Triggers STING‐type I IFN‐Dependent Monocyte Reprogramming of the Tumor Microenvironment.” Cell 184: 5338–5356.e21. 10.1016/j.cell.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng, Min , Qian Liting, Shen Guodong, Bian Geng, Xu Tingjuan, Xu Weiping, Shen Gan, and Hu Shilian. 2014. “Microbiota Modulate Tumoral Immune Surveillance in Lung Through a γδT17 Immune Cell‐Dependent Mechanism.” Cancer Research 74: 4030–4041. 10.1158/0008-5472.Can-13-2462 [DOI] [PubMed] [Google Scholar]
- 36. Daillère, Romain , Vétizou Marie, Waldschmitt Nadine, Yamazaki Takahiro, Isnard Christophe, Poirier‐Colame Vichnou, Duong Connie P. M., et al. 2016. “Enterococcus Hirae and Barnesiella Intestinihominis Facilitate Cyclophosphamide‐Induced Therapeutic Immunomodulatory Effects.” Immunity 45: 931–943. 10.1016/j.immuni.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 37. Hone Lopez, Sara , Jalving Mathilde, Fehrmann Rudolf S. N., Nagengast Wouter B., de Vries Elisabeth G. E., and de Haan Jacco J.. 2022. “The Gut Wall's Potential as a Partner for Precision Oncology in Immune Checkpoint Treatment.” Cancer Treatment Reviews 107: 102406. 10.1016/j.ctrv.2022.102406 [DOI] [PubMed] [Google Scholar]
- 38. Vétizou, Marie , Pitt Jonathan M., Daillère Romain, Lepage Patricia, Waldschmitt Nadine, Flament Caroline, Rusakiewicz Sylvie, et al. 2015. “Anticancer Immunotherapy by CTLA‐4 Blockade Relies on the Gut Microbiota.” Science 350: 1079–1084. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pitt, Jonathan M. , Vétizou Marie, Waldschmitt Nadine, Kroemer Guido, Chamaillard Mathias, Boneca Ivo Gomperts, and Zitvogel Laurence. 2016. “Fine‐Tuning Cancer Immunotherapy: Optimizing the Gut Microbiome.” Cancer Research 76: 4602–4607. 10.1158/0008-5472.Can-16-0448 [DOI] [PubMed] [Google Scholar]
- 40. Ghanem, Sassine , Kim Charles, Dutta Dibyendu, Salifu Moro, and Lim Seah H.. 2021. “Antimicrobial Therapy During Cancer Treatment: Beyond Antibacterial Effects.” Journal of Internal Medicine 290: 40–56. 10.1111/joim.13238 [DOI] [PubMed] [Google Scholar]
- 41. Wu, Qing , Liu Junjin, Wu Sumei, and Xie Xianhe. 2021. “The Impact of Antibiotics on Efficacy of Immune Checkpoint Inhibitors in Malignancies: A Study Based on 44 Cohorts.” International Immunopharmacology 92: 107303. 10.1016/j.intimp.2020.107303 [DOI] [PubMed] [Google Scholar]
- 42. Zitvogel, Laurence , Ma Yuting, Raoult Didier, Kroemer Guido, and Gajewski Thomas F.. 2018. “The Microbiome in Cancer Immunotherapy: Diagnostic Tools and Therapeutic Strategies.” Science 359: 1366–1370. 10.1126/science.aar6918 [DOI] [PubMed] [Google Scholar]
- 43. Sivan, Ayelet , Corrales Leticia, Hubert Nathaniel, Williams Jason B., Aquino‐Michaels Keston, Earley Zachary M., Benyamin Franco W., et al. 2015. “Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti‐PD‐L1 Efficacy.” Science 350: 1084–1089. 10.1126/science.aac4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matson, Vyara , Fessler Jessica, Bao Riyue, Chongsuwat Tara, Zha Yuanyuan, Alegre Maria‐Luisa, Luke Jason J., and Gajewski Thomas F.. 2018. “The Commensal Microbiome is Associated With anti‐PD‐1 Efficacy in Metastatic Melanoma Patients.” Science 359: 104–108. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaput, Nathalie , Lepage Patrica, Coutzac Clélia, Soularue Émilie, Le Roux Karine, Monot Céline, Boselli Lisa, et al. 2017. “Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated With Ipilimumab.” Annals of Oncology 28: 1368–1379. 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- 46. Gopalakrishnan, Vancheswaran , Spencer Christine N., Nezi Luigi, Reuben Alexandre, Andrews Miles C., Karpinets Tatiana V., Prieto P. A., et al. 2018. “Gut Microbiome Modulates Response to Anti‐PD‐1 Immunotherapy in Melanoma Patients.” Science 359: 97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Routy, Bertrand , Le Chatelier Emmanuelle, Derosa Lisa, Duong Connie P. M., Alou Maryam Tidjani, Daillère Romain, Fluckiger Aurélie, et al. 2018. “Gut Microbiome Influences Efficacy of PD‐1‐Based Immunotherapy Against Epithelial Tumors.” Science 359: 91–97. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 48. Peng, Zhi , Cheng Siyuan, Kou Yan, Wang Ziqi, Jin Rong, Hu Han, Zhang Xiaotian, et al. 2020. “The Gut Microbiome is Associated With Clinical Response to Anti‐PD‐1/PD‐L1 Immunotherapy in Gastrointestinal Cancer.” Cancer Immunology Research 8: 1251–1261. 10.1158/2326-6066.Cir-19-1014 [DOI] [PubMed] [Google Scholar]
- 49. Lv, Ji , Jia Yitao, Li Jing, Kuai Wentao, Li Yang, Guo Fang, Xu Xinjian, et al. 2019. “Gegen Qinlian Decoction Enhances the Effect of PD‐1 Blockade in Colorectal Cancer With Microsatellite Stability by Remodelling the Gut Microbiota and the Tumour Microenvironment.” Cell Death & Disease 10: 415. 10.1038/s41419-019-1638-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schubert, Maria‐Luisa , Rohrbach Roman, Schmitt Michael, and Stein‐Thoeringer Christoph K.. 2021. “The Potential Role of the Intestinal Micromilieu and Individual Microbes in the Immunobiology of Chimeric Antigen Receptor T‐Cell Therapy.” Frontiers in Immunology 12: 670286. 10.3389/fimmu.2021.670286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peled, Jonathan U. , Devlin Sean M., Staffas Anna, Lumish Melissa, Khanin Raya, Littmann Eric R., Ling Lilan, et al. 2017. “Intestinal Microbiota and Relapse After Hematopoietic‐Cell Transplantation.” Journal of Clinical Oncology 35: 1650–1659. 10.1200/jco.2016.70.3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stein‐Thoeringer, Christoph K. , Nichols Katherine B., Lazrak A., Docampo Melissa D., Slingerland Ann E., Slingerland J. B., Clurman A. G., et al. 2019. “Lactose Drives Enterococcus Expansion to Promote Graft‐Versus‐Host Disease.” Science 366: 1143–1149. 10.1126/science.aax3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith, Melody , Littmann Eric R., Slingerland John B., Clurman Annelie, Slingerland Ann E., Taur Ying, Schluter Jonas, et al. 2018. “Intestinal Microbiota Composition Prior to CAR T Cell Infusion Correlates With Efficacy and Toxicity.” Blood 132: 3492. 10.1182/blood-2018-99-118628 [DOI] [Google Scholar]
- 54. Smith, Melody , Dai Anqi, Ghilardi Guido, Amelsberg Kimberly V., Devlin Sean M., Pajarillo Raymone, Slingerland John B., et al. 2022. “Gut Microbiome Correlates of Response and Toxicity Following Anti‐CD19 CAR T Cell Therapy.” Nature Medicine 28: 713–723. 10.1038/s41591-022-01702-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baxi, Shrujal , Yang Annie, Gennarelli Renee L., Khan Niloufer, Wang Ziwei, Boyce Lindsay, and Korenstein Deborah. 2018. “Immune‐Related Adverse Events for Anti‐PD‐1 and Anti‐PD‐L1 Drugs: Systematic Review and Meta‐Analysis.” Bmj 360: k793. 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Belkaid, Yasmine , and Hand Timothy W.. 2014. “Role of the Microbiota in Immunity and Inflammation.” Cell 157: 121–141. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jenq, Robert R. , Taur Ying, Devlin Sean M., Ponce Doris M., Goldberg Jenna D., Ahr Katya F., Littmann Eric R., et al. 2015. “Intestinal Blautia is Associated with Reduced Death from Graft‐versus‐Host Disease.” Biology of Blood and Marrow Transplantation 21: 1373–1383. 10.1016/j.bbmt.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dubin, Krista , Callahan Margaret K., Ren Boyu, Khanin Raya, Viale Agnes, Ling Lilan, No Daniel, et al. 2016. “Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint‐Blockade‐Induced Colitis.” Nature Communications 7: 10391. 10.1038/ncomms10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hakozaki, Taiki , Richard Corentin, Elkrief Arielle, Hosomi Yukio, Benlaïfaoui Myriam, Mimpen Iris, Terrisse Safae, et al. 2020. “The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non‐Small Cell Lung Cancer.” Cancer Immunology Research 8: 1243–1250. 10.1158/2326-6066.Cir-20-0196 [DOI] [PubMed] [Google Scholar]
- 60. Zhou, Pei , Hu Yawen, Wang Xiaoyan, Shen Luxuan, Liao Xinghao, Zhu Yajuan, Yu Jiadong, et al. 2022. “Microbiome in Cancer: An Exploration of Carcinogenesis, Immune Responses and Immunotherapy.” Frontiers in Immunology 13: 877939. 10.3389/fimmu.2022.877939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fluckiger, Aurélie , Daillère Romain, Sassi Mohamed, Sixt Barbara Susanne, Liu Peng, Loos Friedemann, Richard Corentin, et al. 2020. “Cross‐Reactivity Between Tumor MHC Class I‐Restricted Antigens and an Enterococcal Bacteriophage.” Science 369: 936–942. 10.1126/science.aax0701 [DOI] [PubMed] [Google Scholar]
- 62. Thaiss, Christoph A. , Zmora Niv, Levy Maayan, and Elinav Eran. 2016. “The Microbiome and Innate Immunity.” Nature 535: 65–74. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- 63. Park, Joon Seok , Gazzaniga Francesca S., Wu Meng, Luthens Amalia K., Gillis Jacob, Zheng Wen, LaFleur Martin W., et al. 2023. “Targeting PD‐L2‐RGMb Overcomes Microbiome‐Related Immunotherapy Resistance.” Nature 617: 377–385. 10.1038/s41586-023-06026-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mager, Lukas F. , Burkhard Regula, Pett Nicola, Cooke Noah C. A., Brown Kirsty, Ramay Hena, Paik Seungil, et al. 2020. “Microbiome‐Derived Inosine Modulates Response to Checkpoint Inhibitor Immunotherapy.” Science 369: 1481–1489. 10.1126/science.abc3421 [DOI] [PubMed] [Google Scholar]
- 65. Wang, Ting‐Ting , Gnanaprakasam J. N. Rashida, Chen Xuyong, Kang Siwen, Xu Xuequn, Sun Hua, Liu Lingling, et al. 2020. “Inosine is an Alternative Carbon Source for CD8+‐T‐cell Function Under Glucose Restriction.” Nature Metabolism 2: 635–647. 10.1038/s42255-020-0219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hang, Saiyu , Paik Donggi, Yao Lina, Kim Eunha, Trinath Jamma, Lu Jingping, Ha Soyoung, et al. 2019. “Bile Acid Metabolites Control TH17 and Treg Cell Differentiation.” Nature 576: 143–148. 10.1038/s41586-019-1785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rothhammer, Veit , and Quintana Francisco J.. 2019. “The Aryl Hydrocarbon Receptor: An Environmental Sensor Integrating Immune Responses in Health and Disease.” Nature Reviews Immunology 19: 184–197. 10.1038/s41577-019-0125-8 [DOI] [PubMed] [Google Scholar]
- 68. Docampo, Melissa D. , Stein‐Thoeringer Christoph K., Lazrak Amina, Burgos da Silva Marina D., Cross Justin, and van den Brink Marcel R. M.. 2018. “Expression of the Butyrate/Niacin Receptor, GPR109a on T Cells Plays an Important Role in a Mouse Model of Graft Versus Host Disease.” Blood 132: 61. 10.1182/blood-2018-99-118783 [DOI] [Google Scholar]
- 69. Suzuki, Taichi A. , Fitzstevens J. Liam, Schmidt Victor T., Enav Hagay, Huus Kelsey E., Mbong Ngwese Mirabeau, Grießhammer Anne, et al. 2022. “Codiversification of Gut Microbiota With Humans.” Science 377: 1328–1332. 10.1126/science.abm7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. David, Lawrence A. , Maurice Corinne F., Carmody Rachel N., Gootenberg David B., Button Julie E., Wolfe Benjamin E., Ling Alisha V., et al. 2014. “Diet Rapidly and Reproducibly Alters the Human Gut Microbiome.” Nature 505: 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Szczyrek, Michał , Bitkowska Paulina, Chunowski Patryk, Czuchryta Paulina, Krawczyk Paweł, and Milanowski Janusz. 2021. “Diet, Microbiome, and Cancer Immunotherapy—A Comprehensive Review.” Nutrients 13: 2217. 10.3390/nu13072217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Botticelli, Andrea , Vernocchi Pamela, Marini Federico, Quagliariello Andrea, Cerbelli Bruna, Reddel Sofia, Del Chierico Federica, et al. 2020. “Gut Metabolomics Profiling of Non‐Small Cell Lung Cancer (NSCLC) Patients Under Immunotherapy Treatment.” Journal of Translational Medicine 18: 49. 10.1186/s12967-020-02231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Willebrand, Ralf , Hamad Ibrahim, Van Zeebroeck Lauren, Kiss Máté, Bruderek Kirsten, Geuzens Anneleen, Swinnen Dries, et al. 2019. “High Salt Inhibits Tumor Growth by Enhancing Anti‐Tumor Immunity.” Frontiers in Immunology 10: 1141. 10.3389/fimmu.2019.01141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh, Rasnik K. , Chang Hsin‐Wen, Yan Di, Lee Kristina M., Ucmak Derya, Wong Kirsten, Abrouk Michael, et al. 2017. “Influence of Diet on the Gut Microbiome and Implications for Human Health.” Journal of Translational Medicine 15: 73. 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Naqvi, Salma , Asar Turky Omar, Kumar Vikas, Al‐Abbasi Fahad A., Alhayyani Sultan, Kamal Mohammad Amjad, and Anwar Firoz. 2021. “A Cross‐Talk Between Gut Microbiome, Salt and Hypertension.” Biomedicine & Pharmacotherapy 134: 111156. 10.1016/j.biopha.2020.111156 [DOI] [PubMed] [Google Scholar]
- 76. Ferrere, Gladys , Tidjani Alou Maryam, Liu Peng, Goubet Anne‐Gaëlle, Fidelle Marine, Kepp Oliver, Durand Sylvère, et al. 2021. “Ketogenic Diet and Ketone Bodies Enhance the Anticancer Effects of PD‐1 Blockade.” JCI Insight 6(2): e145207. 10.1172/jci.insight.145207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pham, Fiona , Moinard‐Butot Fabien, Coutzac Clélia, and Chaput Nathalie. 2021. “Cancer and Immunotherapy: A Role for Microbiota Composition.” European Journal of Cancer 155: 145–154. 10.1016/j.ejca.2021.06.051 [DOI] [PubMed] [Google Scholar]
- 78. Araujo, Daniel V. , Watson Geoffrey A., Oliva Marc, Heirali Alya, Coburn Bryan, Spreafico Anna, and Siu Lillian L.. 2021. “Bugs as Drugs: The Role of Microbiome in Cancer Focusing on Immunotherapeutics.” Cancer Treatment Reviews 92: 102125. 10.1016/j.ctrv.2020.102125 [DOI] [PubMed] [Google Scholar]
- 79. Baruch, Erez N. , Youngster Ilan, Ben‐Betzalel Guy, Ortenberg Rona, Lahat Adi, Katz Lior, Adler Katerina, et al. 2021. “Fecal Microbiota Transplant Promotes Response in Immunotherapy‐Refractory Melanoma Patients.” Science 371: 602–609. 10.1126/science.abb5920 [DOI] [PubMed] [Google Scholar]
- 80. Davar, Diwakar , Dzutsev Amiran K., McCulloch John A., Rodrigues Richard R., Chauvin Joe‐Marc, Morrison Robert M., Deblasio Richelle N., et al. 2021. “Fecal Microbiota Transplant Overcomes Resistance to Anti‐PD‐1 Therapy in Melanoma Patients.” Science 371: 595–602. 10.1126/science.abf3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Routy, Bertrand , Lenehan John G., Miller Wilson H., Jamal Rahima, Messaoudene Meriem, Daisley Brendan A., Hes Cecilia, et al. 2023. “Fecal Microbiota Transplantation Plus Anti‐PD‐1 Immunotherapy in Advanced Melanoma: A Phase I Trial.” Nature Medicine 29: 2121–2132. 10.1038/s41591-023-02453-x [DOI] [PubMed] [Google Scholar]
- 82. Allen‐Vercoe, Emma , and Coburn Bryan. 2020. “A Microbiota‐Derived Metabolite Augments Cancer Immunotherapy Responses in Mice.” Cancer Cell 38: 452–453. 10.1016/j.ccell.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 83. Tanoue, Takeshi , Morita Satoru, Plichta Damian R., Skelly Ashwin N., Suda Wataru, Sugiura Yuki, Narushima Seiko, et al. 2019. “A Defined Commensal Consortium Elicits CD8 T Cells and Anti‐Cancer Immunity.” Nature 565: 600–605. 10.1038/s41586-019-0878-z [DOI] [PubMed] [Google Scholar]
- 84. Gurbatri, Candice R. , Arpaia Nicholas, and Danino Tal. 2022. “Engineering Bacteria as Interactive Cancer Therapies.” Science 378: 858–864. 10.1126/science.add9667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Canale, Fernando P. , Basso Camilla, Antonini Gaia, Perotti Michela, Li Ning, Sokolovska Anna, Neumann Julia, et al. 2021. “Metabolic Modulation of Tumours with Engineered Bacteria for Immunotherapy.” Nature 598: 662–666. 10.1038/s41586-021-04003-2 [DOI] [PubMed] [Google Scholar]
- 86. Chowdhury, Sreyan , Castro Samuel, Coker Courtney, Hinchliffe Taylor E., Arpaia Nicholas, and Danino Tal. 2019. “Programmable Bacteria Induce Durable Tumor Regression and Systemic Antitumor Immunity.” Nature Medicine 25: 1057–1063. 10.1038/s41591-019-0498-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang, Ju‐Min , Liu Di, Wang Yuwei, Liu Liang, Li Jian, Yuan Jing, Jiang Zhihong, et al. 2022. “Ginseng Polysaccharides Alter the Gut Microbiota and Kynurenine/Tryptophan Ratio, Potentiating the Antitumour Effect of Antiprogrammed Cell Death 1/programmed Cell Death Ligand 1 (anti‐PD‐1/PD‐L1) Immunotherapy.” Gut 71: 734–745. 10.1136/gutjnl-2020-321031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dong, Meng‐Xue , Meng Zhefeng, Kuerban Kudelaidi, Qi Feilong, Liu Jiayang, Wei Yuxi, Wang Qian, et al. 2018. “Diosgenin Promotes Antitumor Immunity and PD‐1 Antibody Efficacy Against Melanoma by Regulating Intestinal Microbiota.” Cell Death & Disease 9: 1039. 10.1038/s41419-018-1099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Badgeley, Aja , Anwar Hina, Modi Karan, Murphy Paige, and Lakshmikuttyamma Ashakumary. 2021. “Effect of Probiotics and Gut Microbiota on Anti‐Cancer Drugs: Mechanistic Perspectives.” Biochimica et Biophysica Acta (BBA) ‐ Reviews on Cancer 1875: 188494. 10.1016/j.bbcan.2020.188494 [DOI] [PubMed] [Google Scholar]
- 90. Jacouton, Elsa , Chain Florian, Sokol Harry, Langella Philippe, and Bermúdez‐Humarán Luis G.. 2017. “Probiotic Strain Lactobacillus Casei BL23 Prevents Colitis‐Associated Colorectal Cancer.” Frontiers In Immunology 8: 1553. 10.3389/fimmu.2017.01553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hibberd, Ashley A. , Lyra Anna, Ouwehand Arthur C., Rolny Peter, Lindegren Helena, Cedgård Lennart, and Wettergren Yvonne. 2017. “Intestinal Microbiota is Altered in Patients With Colon Cancer and Modified by Probiotic Intervention.” BMJ Open Gastroenterology 4: e000145. 10.1136/bmjgast-2017-000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hu, Jing‐Tao , Wang Chunfeng, Ye Liping, Yang Wentao, Huang Haibin, Meng Fei, Shi Shaohua, and Ding Zhuang. 2015. “Anti‐Tumour Immune Effect of Oral Administration of Lactobacillus Plantarum to CT26 Tumour‐Bearing Mice.” Journal of Biosciences 40: 269–279. https://pubmed.ncbi.nlm.nih.gov/25963256 [DOI] [PubMed] [Google Scholar]
- 93. Griffin, Matthew E. , Espinosa Juliel, Becker Jessica L., Luo Ji‐Dung, Carroll Thomas S., Jha Jyoti K., Fanger Gary R., and Hang Howard C.. 2021. “Enterococcus Peptidoglycan Remodeling Promotes Checkpoint Inhibitor Cancer Immunotherapy.” Science 373: 1040–1046. 10.1126/science.abc9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bender, Mackenzie J. , McPherson Alex C., Phelps Catherine M., Pandey Surya P., Laughlin Colin R., Shapira Jake H., Medina Sanchez Luzmariel, et al. 2023. “Dietary Tryptophan Metabolite Released by Intratumoral Lactobacillus Reuteri Facilitates Immune Checkpoint Inhibitor Treatment.” Cell 186: 1846–1862.e26. 10.1016/j.cell.2023.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Spencer, Christine N. , McQuade Jennifer L., Gopalakrishnan Vancheswaran, McCulloch John A., Vetizou Marie, Cogdill Alexandria P., Khan Md A. Wadud, et al. 2021. “Dietary Fiber and Probiotics Influence the Gut Microbiome and Melanoma Immunotherapy Response.” Science 374: 1632–1640. 10.1126/science.aaz7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Arthur, Janelle C. , Gharaibeh Raad Z., Uronis Joshua M., Perez‐Chanona Ernesto, Sha Wei, Tomkovich Sarah, Mühlbauer Marcus, Fodor Anthony A., and Jobin Christian. 2013. “VSL#3 Probiotic Modifies Mucosal Microbial Composition but Does Not Reduce Colitis‐Associated Colorectal Cancer.” Scientific Reports 3: 2868. 10.1038/srep02868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gong, Jun , Chehrazi‐Raffle Alexander, Placencio‐Hickok Veronica, Guan Michelle, Hendifar Andrew, and Salgia Ravi. 2019. “The Gut Microbiome and Response to Immune Checkpoint Inhibitors: Preclinical and Clinical Strategies.” Clinical and Translational Medicine 8: 9. 10.1186/s40169-019-0225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saini, Neeraj , Chang Chia‐Chi, Strati Paolo, Nastoupil Loretta J., Westin Jason, Nair Ranjit, Fayad Luis, et al. 2020. “Gut Bacterial Diversity Associates with Efficacy of Anti‐CD19 CAR T‐Cell Therapy in Patients with Large B‐Cell Lymphoma.” Blood 136: 34–35. 10.1182/blood-2020-136756 [DOI] [Google Scholar]
- 99. Ben‐Shaanan, Tamar L. , Schiller Maya, Azulay‐Debby Hilla, Korin Ben, Boshnak Nadia, Koren Tamar, Krot Maria, et al. 2018. “Modulation of Anti‐Tumor Immunity by the Brain's Reward System.” Nature Communications 9: 2723. 10.1038/s41467-018-05283-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xavier, Joao B. , Young Vincent B., Skufca Joseph, Ginty Fiona, Testerman Traci, Pearson Alexander T., Macklin Paul, et al. 2020. “The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View.” Trends in Cancer 6: 192–204. 10.1016/j.trecan.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Buchmann Godinho, Douglas , da Silva Fiorin Fernando, Schneider Oliveira Mauro, Furian Ana Flavia, Rechia Fighera Michele, and Freire Royes Luiz Fernando. 2021. “The Immunological Influence of Physical Exercise on TBI‐Induced Pathophysiology: Crosstalk Between the Spleen, Gut, and Brain.” Neuroscience & Biobehavioral Reviews 130: 15–30. 10.1016/j.neubiorev.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 102. Wan, Xia‐Yun , Eguchi Akifumi, Sakamoto Akemi, Fujita Yuko, Yang Yong, Qu Youge, Hatano Masahiko, Mori Chisato, and Hashimoto Kenji. 2023. “Impact of Broad‐Spectrum Antibiotics on the Gut‐Microbiota‐Spleen‐Brain Axis.” Brain, Behavior, & Immunity ‐ Health 27: 100573. 10.1016/j.bbih.2022.100573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li, Yan‐Ting , Yuan Wenzhen, and Jin Weilin. 2023. “Vagus Innervation in the Gastrointestinal Tumor: Current Understanding and Challenges.” Biochimica et Biophysica Acta (BBA) ‐ Reviews on Cancer 1878: 188884. 10.1016/j.bbcan.2023.188884 [DOI] [PubMed] [Google Scholar]
- 104. Bonaz, Bruno , Bazin Thomas, and Pellissier Sonia. 2018. “The Vagus Nerve at the Interface of the Microbiota‐Gut‐Brain Axis.” Frontiers In Neuroscience 12: 49. 10.3389/fnins.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Das, Ritopa , Langou Sofia, Le Thinh T., Prasad Pooja, Lin Feng, and Nguyen Thanh D.. 2022. “Electrical Stimulation for Immune Modulation in Cancer Treatments.” Frontiers in Bioengineering and Biotechnology 9: 795300. 10.3389/fbioe.2021.795300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rawat, Jitendra K. , Roy Subhadeep, Singh Manjari, Guatam Swetlana, Yadav Rajnish K., Ansari Mohd Nazam, Aldossary Sara A., Saeedan Abdulaziz S., and Kaithwas Gaurav. 2019. “Transcutaneous Vagus Nerve Stimulation Regulates the Cholinergic Anti‐Inflammatory Pathway to Counteract 1, 2‐Dimethylhydrazine Induced Colon Carcinogenesis in Albino Wistar Rats.” Frontiers In Pharmacology 10: 353. 10.3389/fphar.2019.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Borovikova, Lyudmila V. , Ivanova Svetlana, Zhang Minghuang, Yang Huan, Botchkina Galina I., Watkins Linda R., Wang Haichao, Abumrad Naji, Eaton John W., and Tracey Kevin J.. 2000. “Vagus Nerve Stimulation Attenuates the Systemic Inflammatory Response to Endotoxin.” Nature 405: 458–462. https://pubmed.ncbi.nlm.nih.gov/10839541 [DOI] [PubMed] [Google Scholar]
- 108. Yonekura, Satoru , Terrisse Safae, Alves Costa Silva Carolina, Lafarge Antoine, Iebba Valerio, Ferrere Gladys, Goubet Anne‐Gaëlle, et al. 2022. “Cancer Induces a Stress Ileopathy Depending on β‐Adrenergic Receptors and Promoting Dysbiosis That Contributes to Carcinogenesis.” Cancer Discovery 12: 1128–1151. 10.1158/2159-8290.CD-21-0999 [DOI] [PubMed] [Google Scholar]
- 109. Helmink, Beth A. , Khan M. A. Wadud, Hermann Amanda, Gopalakrishnan Vancheswaran, and Wargo Jennifer A.. 2019. “The Microbiome, Cancer, and Cancer Therapy.” Nature Medicine 25: 377–388. 10.1038/s41591-019-0377-7 [DOI] [PubMed] [Google Scholar]
- 110. Cuzzubbo, Stefania , Mangsbo Sara, Nagarajan Divya, Habra Kinana, Pockley Alan Graham, and McArdle Stephanie E. B.. 2021. “Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments.” Frontiers in Immunology 11: 615240. 10.3389/fimmu.2020.615240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reens, Abigail L. , Cabral Damien J., Liang Xue, James E. Jr. Norton , Therien Alex G., Hazuda Daria J., and Swaminathan Gokul. 2021. “Immunomodulation by the Commensal Microbiome During Immune‐Targeted Interventions: Focus on Cancer Immune Checkpoint Inhibitor Therapy and Vaccination.” Frontiers in Immunology 12: 643255. 10.3389/fimmu.2021.643255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zebertavage, Lauren , Bambina Shelly, Shugart Jessica, Alice Alejandro, Zens Kyra D., Lauer Peter, Hanson Bill, Gough Michael J., Crittenden Marka R., and Bahjat Keith S. 2019. “A Microbial‐Based Cancer Vaccine for Induction Of EGFRvIII‐specific CD8+ T Cells and Anti‐Tumor Immunity.” PLoS One 14: e0209153. 10.1371/journal.pone.0209153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yi, Jia , Lin Peizhe, Li Qingbo, Zhang Ao, and Kong Xianbin. 2023. “A New Strategy for Treating Colorectal Cancer: Regulating the Influence of Intestinal Flora and Oncolytic Virus on Interferon.” Molecular Therapy ‐ Oncolytics 30: 254–274. 10.1016/j.omto.2023.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tripodi, Lorella , Feola Sara, Granata Ilaria, Whalley Thomas, Passariello Margherita, Capasso Cristian, Coluccino Ludovica, et al. 2023. “Bifidobacterium Affects Antitumor Efficacy of Oncolytic Adenovirus in a Mouse Model of Melanoma.” IScience 26: 107668. 10.1016/j.isci.2023.107668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Crombé, Amandine , Roulleau‐Dugage Mathtieu, and Italiano Antoine. 2022. “The Diagnosis, Classification, and Treatment of Sarcoma in This Era of Artificial Intelligence and Immunotherapy.” Cancer Communications 42: 1288–1313. 10.1002/cac2.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jeong, Sehan , Afroz Sharmin, Kang Donghyun, Noh Jeonghwan, Suh Jooyeon, Kim June Hyuk, You Hye Jin, Kang Hyun Guy, Kim Yi‐Jun, and Kim Jin‐Hong. 2023. “Sarcoma Immunotherapy: Confronting Present Hurdles and Unveiling Upcoming Opportunities.” Molecules and Cells 46: 579–588. 10.14348/molcells.2023.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kennedy, Elizabeth A. , King Katherine Y., and Baldridge Megan T.. 2018. “Mouse Microbiota Models: Comparing Germ‐Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria.” Frontiers In Physiology 9: 1534. 10.3389/fphys.2018.01534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Al‐Asmakh, Maha , and Zadjali Fahad. 2015. “Use of Germ‐Free Animal Models in Microbiota‐Related Research.” Journal of Microbiology and Biotechnology 25: 1583–1588. 10.4014/jmb.1501.01039 [DOI] [PubMed] [Google Scholar]
- 119. Gupta, Arjun , and Khanna Sahil. 2017. “Fecal Microbiota Transplantation.” Jama 318: 102. 10.1001/jama.2017.6466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary information (graphical abstract, slides, videos, Chinese translated version, and update materials) is available online DOI or http://www.imeta.science/.


