Abstract
Schizophrenia (SZ) places a tremendous burden on public health as one of the leading causes of disability and death. SZ patients are more prone to developing obesity than the general population from the clinical practice. The development of obesity frequently causes poor psychiatric outcomes in SZ patients. In turn, maternal obesity during pregnancy has been associated with an increased risk of SZ in offspring, suggesting that these two disorders may have shared neuropathological mechanisms. The gut microbiota is well known to serve as a major regulator of bidirectional interactions between the central nervous system and the gastrointestinal tract. It also plays a critical role in maintaining physical and mental health in humans. Recent studies have shown that the dysbiosis of gut microbiota is intimately associated with the onset of SZ and obesity through shared pathophysiological mechanisms, particularly the stimulation of immune inflammation. Therefore, gut microbiota may serve as a common biological basis for the etiology in both SZ and obesity, and the perturbed gut–brain axis may therefore account for the high prevalence of obesity in patients with SZ. On the basis of these findings, this review provides updated perspectives and intervention approaches on the etiology, prevention, and management of obesity in SZ patients by summarizing the recent findings on the role of gut microbiota in the pathogenesis of SZ and obesity, highlighting the role of gut‐derived inflammation.
Keywords: gut microbiota, obesity, prebiotics, probiotics, schizophrenia
This review provides updated perspectives on the etiology, prevention, and management of obesity in SZ patients by summarizing the recent findings on the role of gut microbiota in the pathogenesis of SZ and obesity, highlighting the role of gut‐derived inflammation.
Highlights
Obesity represents a major challenge to the clinical management of schizophrenia (SZ) and contributes to worse psychiatric outcomes in SZ.
Perturbations in gut–brain axis, particularly gut‐derived inflammation, are closely related to the pathogenesis of both SZ and obesity, suggesting that gut microbiota may be a potential hub linking these two disorders.
Gut microbiota is a modifiable component that may be used as a novel therapeutic option for treating obesity or improving psychiatric outcomes in SZ patients.
Future research on obesity in SZ includes understanding the therapeutic potential of the gut microbiota, its temporospatial dynamics, its mechanistic interaction with the host, and the crosstalk between the gut and the brain.
INTRODUCTION
Schizophrenia (SZ) is a chronic and severe psychiatric disorder with a lifetime prevalence of approximately 1% worldwide [1] and 0.6% in China [2]. SZ is defined as abnormalities in cognition, thinking, emotion, and behavior as well as incompatibility between mental activities and the external environment. It often appears at the age of adolescence and early adulthood. SZ patients usually face challenging circumstances as a result of a high recurrence rate, recurrent disabilities, extreme poverty, and a low treatment effectiveness, placing a significant burden on individuals, families, and societies [3].
Notably, a significant proportion of SZ patients experienced early onset of metabolic syndrome (MS), which worsened as the disease progresses [4, 5]. Among them, obesity is the most common characteristics of the MS in SZ. On the basis of a 6‐month follow‐up assessment, the prevalence of obesity in SZ was reported to be approximately 20% and with progression of the conditions, this prevalence could rise to 60% or even higher, which is significantly higher than the general population [6]. Clinically, obesity is associated with adverse psychiatric outcomes in SZ, in addition to poor physical health and a lower life expectancy [7, 8]. Moreover, the development of obesity has been shown to be linked to brain anatomical abnormalities in both healthy individuals [9] and patients with SZ [10, 11]. Importantly, all these obesity‐related brain regions are frequently disrupted in SZ [12], suggesting that two disorders may have shared neuropathological and pathophysiological mechanisms.
The “gut–brain axis” research and findings have laid the foundation for the interaction between gut microbiota and host health in recent years. Emerging evidence indicates that the dysbiosis of gut microbiota may play a significant role in the emergence of SZ and obesity. First, gut microbiome is essential for normal metabolic and immunological function of the host. Furthermore, perturbations in the gut microbiota have been reported to be linked to the pathogenesis of obesity by influencing glucose/lipid tolerance, insulin resistance, and low‐grade inflammation [13]. The pathophysiology of SZ has also been linked to similar processes [14]. Second, the dysbiosis of gut microbiota may promote the release of circulating proinflammatory cytokines, and some of which may pass through the blood–brain barrier (BBB) to cause neuroinflammation, such as microglia proliferation, which is associated with altered brain neurological substrates in both SZ and obesity [15]. In addition, gut microbial dysbiosis could also lead to the activation of the hypothalamic‐pituitary‐adrenal (HPA) axis, resulting in elevated circulating cortisol levels and decreased levels of brain‐derived neurotrophic factor [16], both of which are also associated with lower brain volume and poor cognitive performance in both patients with SZ and obesity. On the basis of the aforementioned theoretical framework, we propose that the high prevalence of obesity in SZ may be related to shared pathophysiological pathways, and that gut microbial dysbiosis may provide a common biological basis for the etiology of both SZ and obesity (Figure 1).
Figure 1.
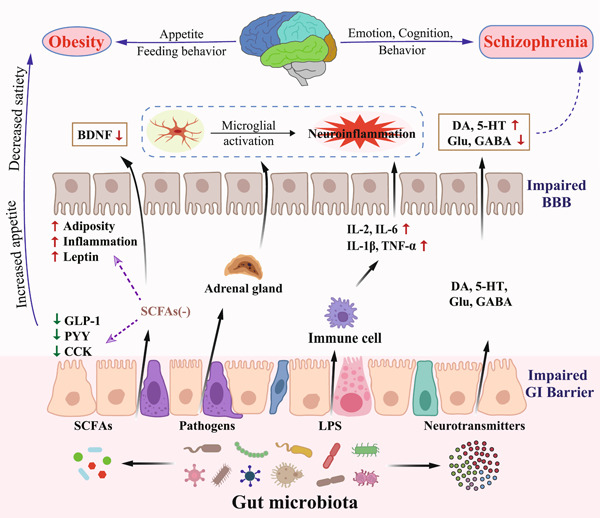
Role of gut microbiota in the pathophysiology of schizophrenia and obesity. The gut microbiota can convert dietary nutrients into metabolites, such as SCFAs, and neurotransmitters, such as DA, 5‐HT, Glu, and GABA. These metabolites have different peripheral and central effects that can alter host cognition, mood and behavior on the one hand, and modify host metabolism and central regulation of appetite on the other hand. The realization of these processes includes the direct crossing of the BBB, the stimulation of the afferent vagus nerve and the triggering of the intestinal endocrine/immune system and the HPA axis, ultimately leading to microglial activation and thus stimulation of upstream neural circuits. 5‐HT, serotonin; BBB, blood–brain barrier; BDNF, brain‐derived neurotrophic factor; CCK, cholecystokinin; DA, dopamine; GABA, γ‐aminobutyric acid; GI, gastrointestinal; GLP‐1, glucagon‐like peptide‐1; Glu, glutamate; IL‐1β, interleukin 1β; IL‐2, interleukin 2; IL‐6, interleukin 6; LPS, lipopolysaccharide; PYY, peptide YY; SCFAs, short‐chain fatty acids; TNF‐α, tumor necrosis factor α.
GUT–BRAIN AXIS AND GUT MICROBIOTA
The gut–brain axis is a bidirectional information exchange system that links the gastrointestinal (GI) track and brain. The gut microbiota is considered to be a key regulator of this axis, forming the Microbiota–Gut–Brain (MGB) axis [17]. On the one hand, gut microbiota can affect brain development through neural, immune, and endocrine pathways, regulating mood, cognition, and behavioral phenotypes of the host; on the other hand, the brain can also regulate GI function and homeostasis through neural and endocrine pathways, influencing the composition, structure, and function of gut microbiota.
Nearly 100 trillion microorganisms exist in human GI track. These microorganisms are significant to the development of the immune system, and are required for the host to maintain metabolic and physiological homeostasis [18, 19]. The intestinal microorganism is mainly composed of bacteria, followed by a smaller proportion of fungi, viruses, and archaea. The gut microbiota may be divided into six major phyla based on the metagenomic sequencing, including Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteria [20]. Among them, the two most prominent phyla are Firmicutes and Bacteroidetes, which account for about 70%–75% of the total gut microbiota. In addition, researchers have proposed three different enterotypes to classify the gut microbial community into different enterotypes, that is, enterotype of Bacteroides, Prevotella, and Ruminococcus [21]. Each enterotype is distinguished by relatively high levels of particular microbial genera that are functional relevance. For example, the enterotype‐Bacteroides is associated with a chronic high‐fat or high‐protein diet, while the enterotype‐Prevotella is associated with a high‐carbohydrate diet [22]. The proposal of these enterotypes may help us gain a better knowledge of how the distribution, composition, and structure of the gut microbiota impact human health and illness, notwithstanding the criticism surrounding this categorization system [23].
The gut microbiota is also dynamically influenced by a variety of factors, such as genetics, diet, metabolism, age, geography, antibiotic therapy, and stress [24, 25, 26]. Therefore, the structure of gut microbiome is also a good characterization of individual environmental history, leading to individual differences in disease risk, disease progression, and response to treatment. As an important internal environmental factor, gut microbiota is directly implicated in various pathophysiological processes of the host, including cytokine release, neurotransmitter production, tryptophan metabolism, and oxidative stress. This is accomplished by stimulating the upstream neural route via the vagus nerve, triggering the enteroendocrine/immune system and the HPA axis [27]. Additionally, the gut microbiota may also be involved in various pathophysiological processes associated with the disease onset and progression by affecting host gene expression or epigenetic modifications [28].
SCHIZOPHRENIA
Although the pathogenesis of SZ remains unclear, the interaction between genetic and environmental risk factors has been proposed as the potential cause leading to the development of SZ. Among them, the genetic basis determines the susceptibility of an individual, while environmental factors could be the initiating factors that determine whether an individual develops the disease or not. In the development and progression of SZ, the gut microbiota is also considered to be one of the key internal environmental factors.
Gut microbiota in SZ
There are numerous hypotheses for the pathogenesis of SZ, that is, the dopamine (DA) hypothesis, the glutamate (Glu) hypothesis, the 5‐hydroxytryptamine (5‐HT) hypothesis, the γ‐aminobutyric acid (GABA) hypothesis, and the dysconnection hypothesis [29, 30]. The DA hypothesis is one of those that is commonly considered to contribute to the etiology of SZ. This is demonstrated by the observation of the overactive dopaminergic neurons in patients with SZ and the evidence that most antipsychotic drugs reduce the psychiatric symptoms by blocking dopaminergic receptors. However, no single hypothesis can explain the complexity of SZ. In recent years, the association of gut microbiota with many psychiatric disorders has been recognized. Thus, the complexity of SZ can be comprehensively explained by the MGB axis, which links different hypotheses together (Figure 2).
Figure 2.
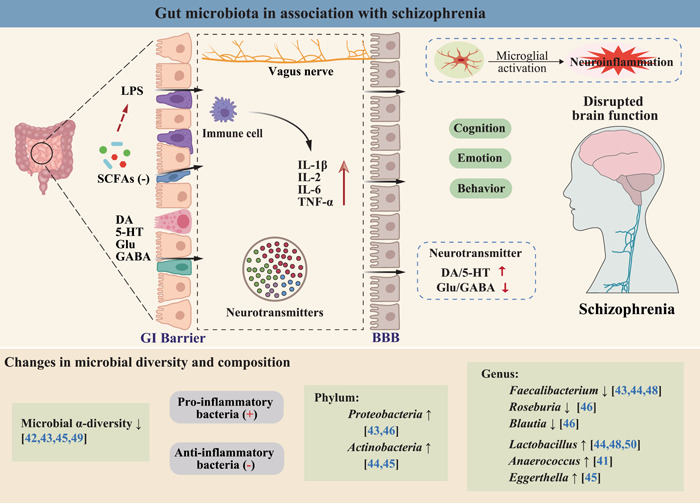
Gut microbiota in association with schizophrenia. 5‐HT, serotonin; BBB, blood–brain barrier; DA, dopamine; GABA, γ‐aminobutyric acid; GI, gastrointestinal; Glu, glutamate; IL‐1β, interleukin 1β; IL‐2, interleukin 2; IL‐6, interleukin 6; LPS, lipopolysaccharide; SCFAs, short‐chain fatty acids; TNF‐α, tumor necrosis factor α.
The dysregulation of several neurotransmitters, for example, the hyperactivation of DA and 5‐HT, an insufficient level of Glu and GABA in the brain, may contribute to the onset of SZ [31]. According to recent findings, the BBB and intestinal barrier are structurally and functionally comparable, and bioactive compounds originating from the gut microbiota can cross both barrier systems similarly, impacting brain function [32]. In fact, more than 90% of 5‐HT in the human body is synthesized in the intestine, and the gut microbiota contributes to the biosynthesis of 95% of 5‐HT in the colonic enterochromaffin cell. Additionally, the gut microbiota can release functional neurotransmitters (e.g., the DA, Glu, 5‐HT, and GABA), via digestion and breakdown of compounds from food. Some of these neurotransmitters can cross the BBB directly to influence the function of the central nervous system [33]. Furthermore, metabolites derived from the gut microbiota, such as indoles and short‐chain fatty acids (SCFAs), can influence host cognition, mood, and behavior directly or indirectly through their neuroactive properties and effects on other gut–brain signaling pathways, such as the immune and endocrine systems [34, 35, 36].
Moreover, the association between SZ and MS has become an emerging issue to be addressed in the management of SZ. According to the clinical data from our group, patients with first‐episode SZ exhibited glucolipid metabolic abnormalities before receiving antipsychotic medication [37]. The use of antipsychotics further worsened the insulin resistance and related metabolic disorders in SZ patients [38]. Moreover, the discontinuation of antipsychotic medication was not associated with improvement of glucolipid metabolic disturbance in these patients [39]. These findings suggest that SZ itself may involve pathophysiological processes that affect the body's glucolipid metabolism. Of note, existing studies suggest that gut microbiota and its metabolites can affect the body's glucolipid metabolism by regulating host gut hormone secretion, immunological inflammation, and insulin sensitivity [13]. Thus, dysbiosis of the gut microbiota is also thought to be associated with the high prevalence of MS in SZ.
Evidence of disturbed gut microbiota from clinical studies
The dysbiosis of gut microbiota in patients with SZ has been documented in numerous clinical studies. Initially, a metagenomic sequencing analysis showed that the oropharyngeal microbiota of schizophrenic and healthy populations differed dramatically at the phylum and genus levels [40]. Subsequently, several studies compared the differences in gut microbial composition between schizophrenic and healthy populations. These results indicated that the diversity and composition of the gut microbiota were significantly altered in patients with SZ [41, 42, 43, 44, 45] and that specific bacteria may serve as biomarkers to distinguish SZ patients from healthy populations [46, 47]. Additionally, the severity of psychiatric symptoms and responsiveness to subsequent treatment in first‐episode SZ patients were strongly correlated with the relative abundance of Lactobacillus [48]. Moreover, the gut microbiota of SZ patients differed significantly between the acute and remission phases [47, 49]. Patients with acute exacerbation exhibited higher abundance of Bacteroides and lower abundance of Prevotella than those in remission, which was strongly correlated with the severity of their psychiatric symptoms [49]. Likewise, the gut microbiota differed significantly between patients with first‐episode drug‐naïve and those with chronic medication [50], suggesting that antipsychotics may also impact and reshape the gut microbial profiles. Furthermore, Zheng et al. [42] found that transplantation of fecal microbiota from SZ patients into germ‐free mice reduced Glu levels in the hippocampus of the mice, suggesting that the gut microbiota itself can influence the brain neurochemistry associated with the onset of SZ. Similarly, transplantation of fecal microbiota from drug‐free patients with SZ into specific pathogen‐free mice has been reported to induce SZ‐like behaviors [51]. Of note, these mice also showed an elevation in the kynurenine‐kynurenic acid pathway of tryptophan degradation in both periphery and brain, suggesting that changes in the gut microbial composition may contribute to the onset of SZ by manipulating tryptophan‐kynurenine metabolism [51]. Together, these results suggest that alterations in the gut microbiota may be an important driver in the development of SZ (Table 1).
Table 1.
Summary of schizophrenia‐related gut microbial alterations reported in clinical studies.
| Author (Year) | Study design | Population characteristics | Intervention | Clinical findings (vs. HCs) |
|---|---|---|---|---|
| Evidence for: Low‐grade inflammation mediated by gut microbial dysbiosis | ||||
| Zheng et al. (2019) [42] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Chinese population: 63 SZ, 69 HCs Female: 33%, 48% Age (years): 43.49 ± 1.68, 39.99 ± 1.62 BMI (kg/m2): 22.90 ± 0.32, 23.16 ± 0.33 Illness duration (N/A) Antipsychotics (58 SZ) |
N/A |
Microbial α‐diversity ↓ Veillonellaceae and PANSS scores (−) Lachnospiraceae and PANSS scores (+) Microbial panel (Aerococcaceae, Bifidobacteriaceae, Brucellaceae, Pasteurellaceae, and Rikenellaceae) for discriminating SZ from HCs (ROC = 0.769) |
| Zhang et al. (2020) [43] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Chinese population: 10 FSZ, 16 HCs Female: 40%, 44% Age (years): 37.6 ± 7.2, 35.8 ± 6.8 BMI (kg/m2): 23.3 ± 6.8, 22.3 ± 6.5 |
N/A |
Microbial α‐diversity ↓ Phylum: Proteobacteria ↑ Genus: Faecalibacterium and Lachnospiraceae ↓ |
| Li et al. (2020) [44] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Chinese population: 82 SZ, 80 HCs Female: 44%, 51% Age (years): 42.15 ± 13.13, 41.03 ± 14.34 BMI (kg/m2): 24.48 ± 4.33, 23.03 ± 3.05 Illness duration (N/A) Antipsychotics (75 SZ) |
N/A |
Microbial α‐diversity (ns) Phylum: Actinobacteria ↑; Firmicutes ↓ Genus: Collinsella, Lactobacillus, Succinivibrio, Mogibacterium, Corynebacterium ↑; Adlercreutzia, Anaerostipes, Ruminococcus and Faecalibacterium ↓. Succinivibrio and PANSS scores (+) Corynebacterium and PANSS scores (−) |
| Xu et al. (2020) [45] |
Cross‐sectional study Fecal samples Shotgun sequencing 16S rRNA sequencing |
Chinese population: Discovery: 40 SZ, 40 HCs Female: 50%, 50%. BMI: N/A Age (years): 35 ± 11, 34 ± 9 Validation: 44 SZ, 44 HCs Female: 36%, 36% Age (years): 35 ± 11, 35 ± 11 BMI (kg/m2): 22.0 ± 3.2, 23.1 ± 3.7 Illness duration (N/A) Antipsychotics (N/A) |
N/A |
Microbial α‐diversity ↓ Phylum: Actinobacteria ↑ Genus: Eggerthella and Megasphaera ↑; Enterococcus ↓ Species: Akkermansia muciniphila, Bifidobacterium adolescentis, Clostridium perfringens, Lactobacillus gasseri and Megasphaera elsdeniis↑ |
| Shen et al. (2018) [46] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Chinese population: 64 SZ, 53 HCs Female: 44%, 34% Age (years): 42 ± 11, 39 ± 14 BMI (kg/m2): 23.49 ± 3.8, 23.14 ± 2.8 Illness duration ≤ 10 years Antipsychotics >6 months |
N/A |
Microbial α‐diversity (ns) Phylum: Proteobacteria ↑ Genus: Succinivibrio, Megasphaera, Collinsella, Clostridium, Klebsiella, Methanobrevibacter ↑; Blautia, Coprococcus, Roseburia ↓. Microbial panel discriminating SZ from HCs (ROC = 0.837) |
| Schwarz et al. (2018) [48] |
Cross‐sectional and 12 month follow‐up Fecal samples Shotgun sequencing |
Finnish population: 28 FEP, 16 HCs Female: 43%, 50% Age (years): 25.9 ± 5.5, 27.8 ± 6.0 BMI (kg/m2): 23.8 ± 4.3, 23.9 ± 3.1 |
N/A |
Genus: Lactobacillus, Tropheryma, Halothiobacillus, Saccharophagus, Ochrobactrum, Deferribacter and Halorubrum ↑; Anabaena, Nitrosospira, Gallionella, Bacteroides, Ruminococcus, and Faecalibacterium ↓ Lactobacillus and psychotic symptoms (+) Lactobacillus and treatment response (−) (up to 12 months) |
| Zhang et al. (2018) [49] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Chinese population: 12 aSZ, 13 rSZ Female: 50%, 46% Age (years): 36.5 ± 8.9, 36.2 ± 8.2 BMI (kg/m2): 23.7 ± 3.2, 24.2 ± 2.7 Duration (years): 8.0 ± 6.9, 9.3 ± 6.4 Antipsychotics >6 months |
N/A |
aSZ (vs. rSZ): Microbial α‐diversity ↓ Genus: Bacteroides ↑, Prevotella ↓ Genus Prevotella and PANSS scores (−) |
| Evidence against: Low‐grade inflammation mediated by gut microbial dysbiosis | ||||
| Nguyen et al. (2018) [41] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
American population: 25 SZ, 25 HCs Female: 40%, 44% Age (years): 52.9 ± 11.2, 54.7 ± 10.7 BMI (kg/m2): 31.8 ± 5.4, 28.9 ± 4.0 Illness duration >10 years Antipsychotics (21 SZ) |
N/A |
Microbial α‐diversity (ns) Phylum: Proteobacteria ↓ Genus: Anaerococcus ↑; Haemophilus, Sutterella, Clostridium ↓. Family Ruminococcaceae and Negative symptoms (−) |
| Ma et al. (2020) [50] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Chinese population: (40 FSZ, 85 TSZ), 69 HCs Female: 46%, 46% Age (years): 24.19 ± 6.18, 23.14 ± 3.20 BMI: 18–25 kg/m2 Antipsychotics (TSZ) > 3 months Illness duration (TSZ) > 1 year |
N/A |
FSZ (vs. HCs): Microbial α‐diversity (ns) Genus: Actinobacillus, Fusobacterium, Megasphaera, SMB53 ↓; Escherichia ↑ TSZ (vs. FSZ and HCs): Microbial α‐diversity ↓ Genus: Escherichia, Enterococcus, Lactobacillus, Shigella, Streptococcus ↑ |
Abbreviations: aSZ, acute schizophrenia; BMI, body mass index; FEP, first‐episode psychosis; FSZ, first‐episode drug‐naïve schizophrenia; HCs, healthy controls; PANSS, positive and negative syndrome scale; ROC, receiver operating characteristic; rRNA, ribosomal RNA; rSZ, remission schizophrenia; SZ, schizophrenia; TSZ, chronically antipsychotic‐treated schizophrenia.
Treatment resistance in SZ may also be related to the MGB axis. In clinical practice, although most patients with SZ respond well to available treatment regimens, approximately 30% of patients still respond poorly to both typical and atypical antipsychotics and exhibit persistent psychotic symptoms, a condition known as treatment‐resistant schizophrenia (TRS) [52]. The mechanisms underlying treatment resistance are still unknown, but one study found that the addition of minocycline treatment improved working memory, lack of volition, and anxiety‐depression symptoms in patients with TRS [53]. Therefore, it is speculated that treatment resistance may also be related to the MGB axis. However, it is important to note that while the effects on other symptoms (e.g., depression and anxiety) in TRS patients through modulation of the gut microenvironment are promising, the available evidence for the effects of probiotics on their psychiatric symptoms is scarce and unsatisfactory [54], making it difficult to draw conclusions. In a double‐blind randomized controlled trial on individuals with SZ, treatment with Lactobacillus and Bifidobacterium did not result in improved psychiatric symptoms than the placebo group [55]. The reasons for this negative result may come from two sources: on the one hand, the role of gut microbiota in the pathogenesis of SZ has not been revealed at the strain level, that is, whether the pathogenesis of SZ is associated with specific strains of bacteria or the pooled effect of multiple microbiota; on the other hand, the physical structure and neural substrate of the brain have been irreversibly altered in schizophrenic patients, and the alterations cannot be reversed simply by modulating the gut microbiota.
OBESITY
Gut microbiota in obesity
The etiology of obesity involves a complex interplay of host genetics, social environment, dietary choices, and psychosomatic variables. Despite the established link between the development of obesity and an imbalance of energy intake and expenditure, the maintenance of the body's energy homeostasis is highly regulated by complex and coordinated hormones in the gut (Figure 3). Multiple hormones have contributed to the regulation of energy balance, including leptin receiving feedback from the adipocyte itself, ghrelin secreted by the gastric mucosa, and several brain–gut peptides that regulate appetite, such as glucagon‐like peptide‐1 (GLP‐1), peptide YY, and cholecystokinin [56]. The gut microbiota is an important internal environmental factor that regulates host metabolism, intestinal hormone secretion, immune inflammatory status, insulin sensitivity, and food intake, all processes that are closely associated with the development of obesity [56]. On the one hand, the gut microbiota is involved in food digestion and nutrient absorption, and is highly influenced by dietary patterns and food preferences [57]. On the other hand, the gut microbiota can convert dietary nutrients into various metabolites, such as SCFAs and indoles, and these microbiota‐derived metabolites could further regulate appetite and feeding behavior of the host after being absorbed into the blood [58, 59]. In addition, the gut microbiota can affect the energy balance by altering the capacity for energy acquisition and storage [60, 61]. Therefore, the gut microbiota is considered to be a key regulator during the development of obesity.
Figure 3.
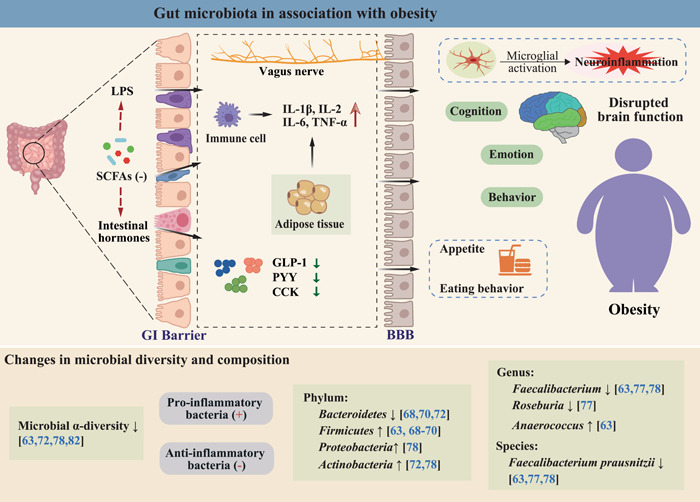
Gut microbiota in association with obesity. BBB, blood–brain barrier; CCK, cholecystokinin; GI, gastrointestinal; GLP‐1, glucagon‐like peptide‐1; IL‐1β, interleukin 1β; IL‐2, interleukin 2; IL‐6, interleukin 6; LPS, lipopolysaccharide; PYY, peptide YY; SCFAs, short‐chain fatty acids; TNF‐α, tumor necrosis factor α.
Evidence of disturbed gut microbiota from clinical studies
High‐fat diets have long been recognized to be associated with an increased prevalence of obesity. Notably, high‐fat diets induce weight gain along with negative impact on the gut microbiota via alteration on its composition, diversity and structure [62, 63, 64]. Generally, a high α‐diversity is regarded as a sign of “good” health, while loss of diversity occurs in most pathogenic conditions, such as obesity and type 2 diabetes [65, 66]. In addition, obese individuals have shown significant alterations in the composition of the gut microbiota. Early in 2005, a pioneering study revealed decreased phylum Bacteroidetes and increased phylum Firmicutes in genetically obese mice, thus positing a role of microbial dysbiosis in obesity [67]. Subsequently, this finding was also confirmed in humans, that is, a more widespread Bacteroides was found in individuals with normal weight, while an increased abundance of the Firmicutes was found in obese individuals [68, 69, 70, 71, 72]. However, other studies have reported conflicting results, suggesting bacterial composition at phylum level cannot be recapitulated in different studies and various conditions. These reports included an increase of Bacteroidetes in overweight individuals [73], an increase of Bacteroidetes and a decrease of Firmicutes in obese individuals [74], and no difference in the two major phyla (Bacteroidetes and Firmicutes) between obese and lean individuals [75]. Importantly, the obesity‐associated microbiota has been shown to be more capable of acquiring and storing energy than that of their lean counterparts [60, 61], and this phenotype can be transferred through fecal microbiota transplants (FMTs) [60], resulting in the rapid weight gain in recipient mice even under diet with low fat. This indicates that changes in the gut microbiota may not simply be a consequence of obesity but may have an active role in its pathogenesis.
Moreover, the enrichment of proinflammatory bacteria (e.g., Enterobacter cloacae, Prevotella genus) and the depletion of anti‐inflammatory bacteria (e.g., Clostridium species) have been constantly reported in obese individuals [76, 77, 78]. An imbalance of typical proinflammatory Gram‐negative bacteria and anti‐inflammatory SCFA‐producing bacteria might be a potential driver for the chronic low‐grade inflammation seen in obesity [79, 80, 81]. Nevertheless, this concept has only been confirmed in animal studies and in associative nature in humans, and strong evidence is still lacking. Additionally, patients with severe obesity even showed dysbiosis in gut microbial function, and the success of bariatric surgery in these patients has increasingly been shown to be associated with improved function of the major gut microbiota [82, 83]. Of note, changes in microbial function are also closely related to altered microbial composition, as Turnbaugh et al. [72] found that the microbiota enriched with Bacteroidetes had higher levels of functional diversity, while those enriched in Firmicutes had lower levels of functional diversity. Taken together, these findings suggest that dysbiosis of the gut microbiota may be an important driver in the development of obesity (Table 2).
Table 2.
Summary of obesity‐related gut microbial alterations reported in clinical studies.
| Author (year) | Study design | Population characteristics | Intervention | Clinical findings (vs. lean/nonobese or baseline) |
|---|---|---|---|---|
| Evidence for: Low‐grade inflammation mediated by gut microbial dysbiosis | ||||
| Andoh et al. (2016) [63] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Japanese population: 10 obese, 10 lean Female: 50%, 50% Mean age (years): 41, 45 BMI (kg/m2): 38.1 ± 3.5, 16.6 ± 1.0 |
N/A |
Microbial α‐diversity ↓ Phylum: Firmicutes ↑; Fusobacteria ↑ Genus: Alistipes, Anaerococcus, Coprococcus, Fusobacterium, and Parvimonas ↑; Bacteroides, Faecalibacterium, Desulfovibrio, Lachnoanaerobaculum, Olsenella ↓ Anti‐inflammatory bacteria species: Faecalibacterium prausnitzii ↓ Proinflammatory bacteria species: Bacteroides vulgatus ↑ |
| Turnbaugh et al. (2009) [72] |
Comparative study Fecal samples 16S rRNA sequencing Shotgun sequencing |
European or African Female: 31 monozygotic twin pairs, 23 dizygotic twin pairs. Age range: 21–32 years Concordant for leanness (BMI: 18.5–24.9 kg/m2) or obesity (BMI ≥ 30 kg/m2) |
N/A |
Microbial α‐diversity ↓ Phylum Firmicutes (ns) Phylum Bacteroidetes ↓ Phylum Actinobacteria ↑ F/B ratio ↑ |
| Haro et al. (2015) [77] |
Clincal trial Fecal samples 16S rRNA sequencing |
20 obese men: Age: 63.3 ± 2.0 years BMI: 32.2 ± 0.5 kg/m2 |
Two dietary interventions: ① Med (n = 10) ② LFHCC (n = 10) duration: 1 year |
Med diet: Genus: Prevotella ↓; Roseburia, Oscillospira ↑ Species: Parabacteroides distasonis ↑ LFHCC diet: Genus: Prevotella ↑; Roseburia ↓ Species: Faecalibacterium prausnitzii ↑ |
| Lee et al. (2019) [78] |
Clincal trial Fecal samples 16S rRNA sequencing |
12 obese women: Age: 52.5 (32–62) years BMI: 37.0 (31.0–40.5) kg/m2 |
BS: ① MWL (n = 4) ② AGB (n = 4) ③ RYGB (n = 4) |
RYGB (at 10% weight‐loss): Microbial α‐diversity ↑ Phylum: Proteobacteria ↑; Actinobacteria ↑ Species: Faecalibacterium prausnitzii ↑ |
| Aron‐Wisnewsky et al. (2019) [82] |
Clinical trial Fecal samples Shotgun sequencing |
61 severe obese women: Age: 36.9 ± 9.86 years BMI: 45.6 ± 5.23 kg/m2 |
BS: ① AGB (n = 20) ② RYGB (n = 41) |
Baseline: Microbial gene richness ↓ 1‐year postsurgery: Microbial gene richness ↑ |
| Evidence for: Increased phylum Firmicutes and/or decreased phylum Bacteroidetes (increased F/B ratio) | ||||
| Koliada et al. (2017) [68] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
Ukrainian population: 11 obese, 16 overweight 27 normal, 7 underweight Female: 75% Mean age: 44.2 years |
N/A |
Phylum Firmicutes ↑ Phylum Bacteroidetes ↓ F/B ratio ↑ |
|
Rahat‐Rozenbloom et al. (2014) [69] |
Observational study Faecal samples Rectal dialysis bag 16S rRNA sequencing |
University of Toronto: 11 overweight, 11 lean Female: 45%, 45% Age (years): 42.5 ± 3.9, 35.8 ± 4.2 BMI (kg/m2): 30.1 ± 0.8, 22.6 ± 0.6 |
N/A |
Phylum Firmicutes ↑ Phylum Bacteroidetes (ns) F/B ratio ↑ |
| Ley et al. (2006) [70] |
Clinical trial Fecal samples 16S rRNA sequencing |
12 obese Age range: 21–65 years BMI range: 30–43 kg/m2 2 lean (as blank control) Mean BMI: 23 kg/m2 Mean age: 34 years |
Weight‐loss diets: ① FAT‐R (n = 6) ② CARB‐D (n = 6) duration: 1 year |
Baseline (obese vs. lean): Phylum Firmicutes ↑ Phylum Bacteroidetes ↓ F/B ratio ↑ 1‐year diet‐treatment: Phylum Firmicutes ↓ Phylum Bacteroidetes ↑ F/B ratio ↓ |
| Evidence against: Increased phylum Firmicutes and/or decreased phylum Bacteroidetes (increased F/B ratio) | ||||
| Schwiertz et al. (2010) [73] |
Cross‐sectional study Fecal samples 16S rRNA sequencing |
University of Giessen and Marburg: 30 obese, 35 overweight, 33 lean Female: 65%. Age: 47 ± 13 years |
N/A |
Proportion of Firmicutes ↓ Proportion of Bacteroidetes ↑ F/B ratio ↓ |
| Kellerer et al. (2019) [74] |
Clincal trial Fecal samples 16S rRNA sequencing |
German population: 17 morbidly obese, 17 nonobese Female: 82%, 82% Age (years): 41.8 ± 9.1, 41.7 ± 9.7 BMI (kg/m2): 52.5 (47.0–56.8), 21.5 (19.6–23.3). BMI‐LSG: 39.1 (32.6–44.0) kg/m2 Nonobese as blank control |
BS: LSG Follow‐up: 6‐month |
Baseline (obese vs. nonobese): Phylum Firmicutes ↓ Phylum Bacteroidetes ↑ F/B ratio ↓ LSG (6‐month follow‐up): Phylum Firmicutes ↑ Phylum Bacteroidetes ↓ F/B ratio ↑ |
| Duncan et al. (2008) [75] |
Clincal trial Fecal samples 16S rRNA sequencing |
North of Scotland: 29 obese men (BMI > 30 kg/m2) 14 nonobese (BMI < 30 kg/m2) Balanced cross‐over design: (on 23 obese individuals) |
Weight‐loss diets: ① LC for 4 weeks ② MC for 4 weeks duration: 8 weeks |
Cross‐sectional and clincal trial: Phylum Firmicutes (ns) Phylum Bacteroidetes (ns) |
Abbreviations: AGB, adjustable gastric banding; BMI, body mass index; BS, Bariatric surgery; CARB‐R, carbohydrate‐restricted; FAT‐R, fat‐restricted; LC, high‐protein low carbohydrate, ketogenic; LFHCC, a low‐fat, high‐complex carbohydrates diet; LSG, laparoscopic sleeve gastrectomy; MC, high‐protein moderate‐carbohydrate, non‐ketogenic; Med, Mediterranean diet; MWL, medical weight loss; rRNA, ribosomal RNA; RYGB, Roux‐en‐Y‐gastric bypass.
OBESITY IN SCHIZOPHRENIA
Obesity is particularly prevalent in SZ, with nearly 40%–60% of patients being obese, a rate significantly higher than that in the general population [6, 84, 85]. SZ patients with obesity tend to have poorer cognitive performance and physical condition, as well as shorter life expectancy than their lean counterparts [7]. Thus, obesity is recognized as one of the strongest contributors to adverse psychiatric outcomes in SZ [12]. Previously, our group assessed the cognitive function of patients with SZ using the MATRICS Consensus Cognitive Battery and found that combined metabolic disorders, such as obesity, primarily affect patients' working memory and information processing speed [86], and that these cognitive deficits further have an impact on their social functioning and quality of life [87].
Factors involved in the pathophysiology of obesity in SZ
Until now, the exact mechanisms underlying the high prevalence of obesity in SZ patients are not fully understood. Among the factors contributing to this high prevalence, the complex interplay between unhealthy lifestyle, adverse effects of antipsychotics, and the inherent pathophysiological mechanisms of SZ are the most cited explanations [84, 88]. SZ patients tend to have unhealthy lifestyles, including alcohol consumption, smoking, poor diet, lack of exercise, and have more difficult social circumstances. In particular, dietary patterns are the key determinant shaping the composition of the gut microbiota. For example, a high‐calorie diet for just 3 days has been reported to result in an increased abundance of Firmicutes and decreased abundance of Bacteriodetes in human [89]. Likewise, there is additional evidence that the composition of the gut microbiota changes within 24 h of a high‐fat diet [22]. Interestingly, recent studies indicate that the antipsychotic‐induced weight gain may also be attributable to alterations in the gut microbiome [90, 91]. In line with this evidence, preliminary findings in germ‐free mice suggested that olanzapine could induce weight gain and promote a shift towards “obesogenic” microbial profile (i.e., increased Firmicutes while decreased Bacteroides) only after the colonization of gut microbiota [92]. Similar findings were also observed in antipsychotic‐treated human studies for both children and adults [93, 94]. As risperidone treatment progressed, the gut microbial composition changed dynamically, with a significant positive correlation between the Firmicutes/Bacteroides ratio and the increase in BMI [94]. Taken together, these data suggest that both dietary patterns and antipsychotic medication may interact with and reshape the microbial profiles, leading to rapid weight gain in patients with SZ. Thus, future research should distinguish those from the innate microbiota of SZ patient, which is crucial for a more thorough understanding of the relationship between gut microbiota and SZ.
Notably, studies also showed that medication‐free SZ patients have impaired fasting glucose, insulin resistance and elevated cortisol levels compared to the healthy population [95, 96]. Moreover, siblings of these patients are at a high risk for MS, which is not dependent on the action of any antipsychotic medication [97]. Similarly, epidemiological research has reported that up to 44.8% of medication‐free SZ patients are obese or overweight, significantly higher than that of healthy controls (36.6%) [98]. This evidence indicates that SZ itself is an independent risk factor for obesity and that it may entail certain pathophysiological processes that have an impact on host metabolism. Conversely, maternal obesity during pregnancy has also been associated with an increased risk of SZ in the offspring [99], further suggesting that the two disorders may share certain pathophysiological mechanisms.
Gut‐derived inflammation as a potential hub linking SZ and obesity
In recent years, the “gut–brain axis” research has drawn increasing attention, in particular the interaction between gut microbiota and host health. Gut microbial dysbiosis has been observed in both patients with SZ and obesity (Figures 2 and 3), and a perturbed gut–brain axis is closely related to the pathogenesis of the two disorders. As noted in Tables 1 and 2, both obesity and SZ were associated with a decrease in microbial diversity, and both were characterized by a decrease in anti‐inflammatory bacteria with butyrate‐producing capacity (e.g., genera Faecalibacterium and Roseburia) and an increase in many proinflammatory Gram‐negative bacteria as well as other pathogenic bacteria (e.g., phylum Proteobacteria and genus Anaerococcus). Since inflammatory processes are involved in the pathophysiology of both obesity and SZ, we hypothesize that gut microbiota‐derived inflammatory signaling may serve as a potential hub linking SZ and obesity, which may be associated with the high prevalence of obesity in SZ.
Inflammation in obesity
Obesity is a state of low‐grade inflammation characterized by macrophage infiltration in adipose tissue and release of cytokines, such as tumor necrosis factor α (TNF‐α) and interleukin 6 (IL‐6) that are produced by macrophages to activate inflammatory signaling and thereby enhance the systemic low‐grade inflammation in obesity [100]. Importantly, this low‐grade inflammation can impair insulin sensitivity causing insulin resistance and hyperinsulinemia, which further exacerbates obesity [101, 102]. In turn, obesity further exacerbates low‐grade inflammation through the secretion of proinflammatory cytokines by adipocytes, thus creating a vicious cycle (Figure 4). Thus, although the causal pathways underpinning the relationship between obesity and inflammation have not been fully identified, obesity and inflammation are certainly causally related to each other.
Figure 4.
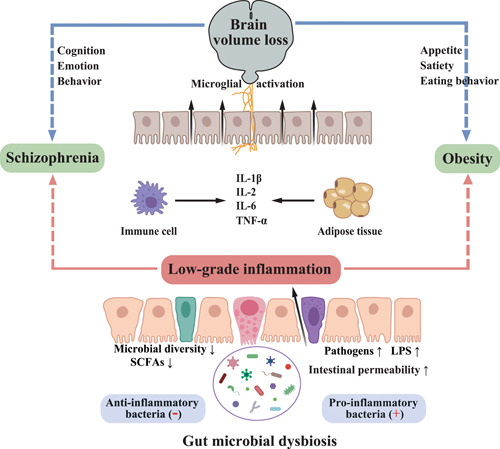
Gut‐derived inflammation as a potential hub linking schizophrenia and obesity. Gut microbial dysbiosis has been observed in both SZ and obesity. It leads to impaired intestinal barrier and thereby increased transport of pathogens and LPS to the circulatory system, resulting in the production of proinflammatory cytokines and elevated inflammation in the circulation. Peripheral cytokine signaling can induce neuroinflammation, leading to structural remodeling of the brain (manifested as brain volume loss), which can lead to changes in cognition, emotion, and behavior, as well as changes in appetite, satiety and eating behavior. IL‐1β, interleukin 1β; IL‐2, interleukin 2; IL‐6, interleukin 6; LPS, lipopolysaccharide; SCFAs, short‐chain fatty acids; SZ, schizophrenia; TNF‐α, tumor necrosis factor α.
In clinical study, increased proinflammatory molecules have been consistently observed in obesity. This includes positive correlations between body measurements such as BMI, waist circumference and waist‐to‐hip ratio and C‐reactive protein (CRP) levels [103], and between adipocyte size and levels of TNF‐α, IL‐6, and high‐sensitivity CRP [104]. Although most of the studies were based on correlations and did not establish a causal relationship between inflammation and obesity, they did support the likely interplay between obesity and inflammation. In turn, a 10% weight loss over 1 year has also been shown to reduce plasma concentrations of several proinflammatory cytokines in obese women [105]. Moreover, the interaction between inflammation and obesity is also shown by the ability of anti‐inflammatory treatments to improve obesity‐associated insulin resistance. These treatment strategies include IL‐1‐receptor antagonist [106], IL‐1β‐neutralizing antibody [107], TNF‐α blockade [108], and anti‐inflammatory drug [109]. Altogether, these findings highlight pivotal roles of inflammation in the development of obesity and the opportunities for interventions.
Inflammation in schizophrenia
Inflammation has long been suggested to play a critical role in the pathogenesis of SZ (Figure 4). Although SZ typically appears in early adulthood, its neuronal disruption is proposed to begin as early as in utero [110]. Indeed, many of the currently proposed early risk factors for SZ are associated with in utero adversities, such as maternal infections and malnutrition during pregnancy, and obstetric complications leading to fetal hypoxia [111, 112, 113]. These hypoxic and infectious factors could stimulate maternal and thereby fetal inflammatory responses, leading to the early “hits” for the pathogenesis of SZ, as the developing brain is highly sensitive to the effects of inflammation. After a series of early hits in utero, SZ may potentially be characterized by a chronic inflammatory state. This evidence includes peripheral and neuroinflammation being increasingly reported in patients with SZ, often already at illness onset. Such studies in SZ have found higher levels of proinflammatory molecules in the cerebrospinal fluid of SZ patients than in controls [114]. Studies of cytokines in the peripheral blood also showed similar results, that is, higher levels of proinflammatory cytokines in both first‐episode SZ and relapsed patients than in healthy controls [115]. In addition, changes in some inflammatory markers may correlate with disease progression. For instance, increased baseline proinflammatory markers, such as IL‐6 and CRP, have been identified as risk factors for poor treatment response at 3‐month follow‐up and worse clinical outcome at 1‐year follow‐up in patients with first‐episode psychosis (FEP) [116, 117].
Notably, inflammation also appears to affect the neurotransmitter system in SZ, which has long been a major focus of research into the neurobiology of SZ. For example, administration of IL‐1β at birth affects dopaminergic neurons in adult mice [118], short exposure to IL‐6 reduces the survival of serotonergic neurons in the fetal brain [119], and chronic exposure to interferon‐α reduces the release of striatal DA in association with anhedonia‐like behavior [120], a typically negative symptom in chronic SZ. Nevertheless, a critical discussion of these findings should mention that increases in proinflammatory cytokines are not specific to SZ and have been observed in other psychiatric disorders; and that the effects of confounding factors, such as body mass index and medication, should also be considered.
Gut microbiota and inflammation
The gut microbiota encompasses a diverse community of bacteria that plays an essential role in maintaining host metabolic and immune homeostasis, in part by regulating the permeability of the gut (Figure 4). Certain beneficial bacteria, such as Faecalibacterium species, could produce specific enzymes that enable the fermentation of indigestible carbohydrates in dietary fiber into SCFAs, and these SCFAs play a role in maintaining the integrity of the gut wall and inhibiting inflammation [80, 121, 122]. On the other hand, some harmful bacteria, particularly Gram‐negative members, including lipopolysaccharide producers and pathogens, can increase intestinal permeability and facilitate LPS transport to the circulatory system [123]. LPS is an endotoxin in the cell wall of Gram‐negative bacteria, and its binding to Toll‐like receptor‐4 could induce the body's immune inflammatory reaction [124]. Under normal conditions, the gut barrier could minimize the movement of LPS from the bowels into the circulation. Factors such as diet or pathogenic bacteria, may promote a “leaky gut,” where LPS leaves the gut and enters the bloodstream [125, 126]. In response, the body produces proinflammatory cytokines and other mediators that effectively initiate an inflammatory response [127].
This gut microbiota‐derived inflammation has been observed in both obesity and SZ. Recently, a review in JAMA pointed out that the depletion of certain anti‐inflammatory butyrate‐producing bacteria and the enrichment of proinflammatory bacteria are shared characteristics of the gut microbiota in patients with psychiatric disorders, including SZ [128]. Similarly, increased level of bacterial LPS has been observed in obesity, and perturbation in the gut microbiota and changes in intestinal permeability have been identified as potential triggers of obesity‐related low‐grade inflammation [102]. Moreover, high levels of peripheral proinflammatory cytokines could further induce neuroinflammation that leads to the neurodegeneration and structural remodeling of the brain, which is consistently observed in both obesity and SZ [129]. Considering the causal effect of inflammation on the development of obesity and SZ, we propose that gut microbiota‐derived inflammation may be a trigger for obesity in SZ, which may also explain the significant risk of SZ among adult offspring born to obese mothers.
Gut microbiota: A promising therapeutic target for obesity in SZ
Several interventions are potentially able to restore the gut microbial balance to treat obesity in SZ patients (Figure 5). Among them, dietary modification remains the primary intervention. Changes in diet have been reported to explain 57% of the total structural variation in gut microbiota [130], and different dietary components directly shape different gut microbial compositions [131, 132]. For example, fiber is one of the main dietary components that consists of nondigestible carbohydrates. Fermentation of nondigestible carbohydrates by the gut microbiota has been shown to increase the production of SCFAs, which have multiple beneficial effects on host metabolic homeostasis, such as increasing gut microbial diversity and reducing obesity‐associated inflammation [59]. In addition to diet, the use of pre/probiotics is also one of the most widely used strategies to modulate the gut microbiota. In the past 10 years, the beneficial effects of probiotics on weight loss have been extensively demonstrated in individuals with diabetes and obesity [133, 134, 135]. Recently, two randomized clinical trials have also demonstrated for the first time that probiotics plus dietary fiber can effectively reduce the antipsychotic‐induced weight gain and metabolic disturbance in SZ patients [136, 137]. And such favorable effects were associated with an increased abundance of gut microbiota [137]. Similarly, FMT from lean donors has been shown to have beneficial effects on insulin sensitivity in obese individuals [138, 139], accompanied by increased levels of butyrate‐producing bacteria [138]. In addition, Liang et al. also demonstrated that SZ patients with enterotype‐Prevotella (characterized by high abundance of Proteobacteria and Firmicutes) have a higher risk of obesity [140], suggesting that dysbiosis of the gut microbiota may be an essential component in the development of obesity in SZ. Altogether, these findings suggest that the gut microbiota would be a promising therapeutic target for obesity in SZ.
Figure 5.
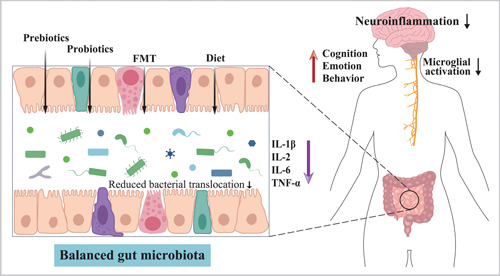
Potential therapeutic options for restoring gut homeostasis and treating obesity in schizophrenia. Administration of probiotics, prebiotics and fecal microbiota transplants leads to a beneficial gut microbiota composition, reduced bacterial translocation and reduced systemic inflammation. The ensuing reduction in proinflammatory cytokines may lead to reduced microglial activation, improved cognition, and reduced appetite and eating behavior. FMT, fecal microbiota transplants; IL‐1β, interleukin 1β; IL‐2, interleukin 2; IL‐6, interleukin 6; TNF‐α, tumor necrosis factor α.
On the other hand, these interventions may also potentially improve the brain volume loss associated with both obesity and SZ (Figure 5). In the general population, weight gain‐related brain volume loss is a major cause of poor cognitive performance in obesity. Similarly, SZ patients with obesity typically experience a worse psychiatric outcome than those with normal weight [12]. On the basis of this evidence, it is tempting to infer that development of obesity may increase the severity of brain volume loss associated with the core symptoms of SZ, leading to a worse disease process. In line with this hypothesis, several studies on SZ patients have shown that obese individuals have more severe brain volume loss in both white matter and gray matter compared with their lean counterparts [10, 11]. More recently, McWhinney et al. [141] replicated the associations between obesity and brain structure in a large multicenter sample from the ENIGMA‐SZ working group and further demonstrated that almost all obesity‐associated brain regions were also associated with SZ. Fortunately, longitudinal studies have confirmed that obesity‐related brain volume loss are reversible through early intervention for weight loss [142, 143]. Thus, these therapeutic approaches targeting the gut microbiota may not only improve obesity and obesity‐associated low‐grade inflammation, but may also ameliorate the brain volume loss associated with clinical, cognitive, and functional outcomes in SZ.
SUMMARY AND FUTURE DIRECTIONS
In summary, gut microbial dysbiosis has been detected in both SZ and obesity, and perturbations in gut–brain axis, particularly gut‐derived inflammation, are closely related to the pathogenesis of both disorders. This suggests that gut microbiota may be a potential hub linking SZ and obesity, and the microbiota‐derived inflammatory signaling may explain the high prevalence of obesity in SZ. Given that obesity presents a significant challenge in the clinical management of SZ and that gut microbiota is a modifiable component, gut microbiota may represent a novel therapeutic option for treating obesity or improving psychiatric outcomes in SZ patients. Nevertheless, the mechanisms underlying the critical link between obesity and SZ remain to be fully elucidated, and with various psychosocial and other biological factors involved, the role of the gut microbiota requires further exploration and consideration before being adopted as a therapeutic target. Moreover, most current research has concentrated on the role of gut microbiota in maintaining mental health or influencing disease severity, and few studies have been conducted directly on schizophrenic patients with obesity. In addition, there are limitations to the existing studies that require special attention.
In most investigations, the participants' demographic parameters were not sufficiently characterized. Apart from race, age, and gender, additional factors need to be included, such as body mass index, smoking, alcohol consumption, exercise, diet, usage of antibiotics, or other drugs having endocrine or immunomodulatory effects. For instance, eating disorders (e.g., anorexia, bulimia, and binge eating disorder) are particularly prevalent in both SZ and obesity. The eating patterns and food preferences are important determinants of gut microbiota. However, it is unclear how eating disorders affect the specific gut microbiota in these patients. Additionally, existing evidence have shown that antipsychotic medications have a major impact on the gut microbiota and thereby dramatically raise the risk of obesity [144]. Thus, future studies would benefit from this consideration. Similarly, the clinical features of the disease are not well evaluated, including the age of onset, duration of illness, classification and severity of symptoms, risk of suicide, and the presence of other chronic conditions. For example, most previous studies have not further explored potential differences in gut microbial diversity and composition between individuals with predominantly positive symptoms in SZ and those with predominantly negative symptoms. Given that those patients with the two symptom clusters present with distinctly diverse clinical presentations, it is necessary to clarify the inherent connection between these clinical features and specific microbial taxa. Moreover, most previous studies had a cross‐sectional design with small sample sizes, and there was considerable heterogeneity in fecal sample collection, storage, and analysis methods across studies, which may affect the outcomes as well [145]. To assure sample and data quality, a systematic protocol for handling stool samples should be developed.
The interaction between gut microbiota and host health is dynamic and variable. To enhance comparison between studies and facilitate meta‐analyses, it is crucial to accurately identify and profile personal and disease‐related characteristics linked with gut microbiota. More large‐scale and multiomics studies combining host genomics, microbiomics, metabolomics, and brain connectomics are also required to fully understand the complex operating mechanisms of the MGB axis. Although studies on the gut microbiota in SZ have recently accumulated, research on gut microbiota in SZ patients with obesity and its relationship with brain imaging features is still in its infancy. Future research should therefore focus more on changes in the gut microbiota before and after the development of obesity in SZ patients and assess how the dynamics of these microbiota–host interactions and the resulting alterations in gut–brain communication, such as gut‐derived metabolites and inflammatory markers, further come to influence brain structure and function. In addition, clinical translation of these findings is needed to consider interventions targeting gut microbiota as potential therapies to improve the clinical outcomes of SZ patients. This will lay a solid theoretical platform for revealing the pathophysiology of obesity in SZ patients and pave the way for the creation of fresh methods for the prevention and treatment of obesity in SZ patients.
AUTHOR CONTRIBUTIONS
Xiaoli Wu and Liwei Xie designed this frame of the manuscript. Hui Wu drafted the manuscript. Hui Wu, Yaxi Liu, Jie Wang, Shengyun Chen, and Liwei Xie edited the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Guangdong Province (Grant No. 2020A1515011288) to Xiaoli Wu, National Natural Science Foundation of China (Grant Nos. 81900797 and 82072436), and Guangdong Basic and Applied Basic Research Foundation (Grant No. 2020B1515020046) to Liwei Xie.
Wu, Hui , Liu Yaxi, Wang Jie, Chen Shengyun, Xie Liwei, and Wu Xiaoli. 2023. “Schizophrenia and obesity: May the gut microbiota serve as a link for the pathogenesis?” iMeta 2, e99. 10.1002/imt2.99
Contributor Information
Liwei Xie, Email: xielw@gdim.cn.
Xiaoli Wu, Email: wuxiaoli@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
Supplementary materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.
REFERENCES
- 1. McCutcheon, Robert A. , Reis Marques Tiago, and Howes Oliver D.. 2020. “Schizophrenia—An Overview.” JAMA Psychiatry 77: 201–10. 10.1001/jamapsychiatry.2019.3360 [DOI] [PubMed] [Google Scholar]
- 2. Huang, Yueqin , Wang Yu, Wang Hong, Liu Zhaorui, Yu Xin, Yan Jie, Yu Yaqin, et al. 2019. “Prevalence of Mental Disorders in China: A Cross‐sectional Epidemiological Study.” The Lancet Psychiatry 6: 211–24. 10.1016/s2215-0366(18)30511-x [DOI] [PubMed] [Google Scholar]
- 3. Cloutier, Martin , Sanon Aigbogun Myrlene, Guerin Annie, Nitulescu Roy, Ramanakumar Agnihotram V., Kamat Siddhesh A., DeLucia Michael, et al. 2016. “The Economic Burden of Schizophrenia in the United States in 2013.” The Journal of Clinical Psychiatry 77: 764–71. 10.4088/JCP.15m10278 [DOI] [PubMed] [Google Scholar]
- 4. Nettis, Maria Antonietta , Pergola Giulio, Kolliakou Anna, O'Connor Jennifer, Bonaccorso Stefania, David Anthony, Gaughran Fiona, et al. 2019. “Metabolic‐Inflammatory Status as Predictor of Clinical Outcome at 1‐year Follow‐up in Patients with First Episode Psychosis.” Psychoneuroendocrinology 99: 145–53. 10.1016/j.psyneuen.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 5. Penninx, Brenda W. J. H. , and Lange Sjors M. M.. 2018. “Metabolic Syndrome in Psychiatric Patients: Overview, Mechanisms, and Implications.” Dialogues in Clinical Neuroscience 20: 63–73. 10.31887/DCNS.2018.20.1/bpenninx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strassnig, Martin , Kotov Roman, Cornaccio Danielle, Fochtmann Laura, Harvey Philip D., and Bromet Evelyn J.. 2017. “Twenty‐Year Progression of Body Mass Index in a County‐Wide Cohort of People with Schizophrenia and Bipolar Disorder Identified at Their First Episode of Psychosis.” Bipolar Disorders 19: 336–43. 10.1111/bdi.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bora, E. , Akdede B. B., and Alptekin K.. 2017. “The Relationship Between Cognitive Impairment in Schizophrenia and Metabolic Syndrome: A Systematic Review and Meta‐Analysis.” Psychological Medicine 47: 1030–40. 10.1017/s0033291716003366 [DOI] [PubMed] [Google Scholar]
- 8. Bocarsly, Miriam E. , Fasolino Maria, Kane Gary A., LaMarca Elizabeth A., Kirschen Gregory W., Karatsoreos Ilia N., McEwen Bruce S., and Gould Elizabeth. 2015. “Obesity Diminishes Synaptic Markers, Alters Microglial Morphology, and Impairs Cognitive Function.” Proceedings of the National Academy of Sciences 112: 15731–6. 10.1073/pnas.1511593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willette, Auriel A. , and Kapogiannis Dimitrios. 2015. “Does the Brain Shrink as the Waist Expands? Ageing Research Reviews 20: 86–97. 10.1016/j.arr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolenič, Marián , Španiel Filip, Hlinka Jaroslav, Matějka Martin, Knytl Pavel, Šebela Antonín, Renka Jiří, and Hajek Tomas. 2020. “Higher Body‐Mass Index and Lower Gray Matter Volumes in First Episode of Psychosis.” Frontiers in Psychiatry 11: 556759. 10.3389/fpsyt.2020.556759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hidese, Shinsuke , Ota Miho, Matsuo Junko, Ishida Ikki, Yokota Yuuki, Hattori Kotaro, Yomogida Yukihito, and Kunugi Hiroshi. 2021. “Association Between Obesity and White Matter Microstructure Impairments in Patients with Schizophrenia: A Whole‐Brain Magnetic Resonance Imaging Study.” Schizophrenia Research 230: 108–10. 10.1016/j.schres.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 12. Minichino, Amedeo , Ando' Agata, Francesconi Marta, Salatino Adriana, Delle Chiaie Roberto, and Cadenhead Kristin. 2017. “Investigating the Link Between Drug‐Naive First Episode Psychoses (FEPs), Weight Gain Abnormalities and Brain Structural Damages: Relevance and Implications for Therapy.” Progress in Neuro‐Psychopharmacology and Biological Psychiatry 77: 9–22. 10.1016/j.pnpbp.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 13. Baothman, Othman A. , Zamzami Mazin A., Taher Ibrahim, Abubaker Jehad, and Abu‐Farha Mohamed. 2016. “The Role of Gut Microbiota in the Development of Obesity and Diabetes.” Lipids in Health and Disease 15: 108. 10.1186/s12944-016-0278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müller, Norbert . 2018. “Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations.” Schizophrenia Bulletin 44: 973–82. 10.1093/schbul/sby024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He, Ying , Kosciolek Tomasz, Tang Jinsong, Zhou Yao, Li Zongchang, Ma Xiaoqian, Zhu Qiyun, et al. 2018. “Gut Microbiome and Magnetic Resonance Spectroscopy Study of Subjects at Ultra‐High Risk for Psychosis May Support the Membrane Hypothesis.” European Psychiatry 53: 37–45. 10.1016/j.eurpsy.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 16. Misiak, Błażej , Łoniewski Igor, Marlicz Wojciech, Frydecka Dorota, Szulc Agata, Rudzki Leszek, and Samochowiec Jerzy. 2020. “The HPA Axis Dysregulation in Severe Mental Illness: Can We Shift the Blame to Gut Microbiota? Progress in Neuro‐Psychopharmacology and Biological Psychiatry 102: 109951. 10.1016/j.pnpbp.2020.109951 [DOI] [PubMed] [Google Scholar]
- 17. Cryan, John F. , O'Riordan Kenneth J., Cowan Caitlin S.M., Sandhu Kiran V., Bastiaanssen Thomaz F. S., Boehme Marcus, Codagnone Martin G., et al. 2019. “The Microbiota–Gut–Brain Axis.” Physiological Reviews 99: 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 18. Thaiss, Christoph A. , Zmora Niv, Levy Maayan, and Elinav Eran. 2016. “The Microbiome and Innate Immunity.” Nature 535: 65–74. 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- 19. Dabke, Kruttika , Hendrick Gustaf, and Devkota Suzanne. 2019. “The Gut Microbiome and Metabolic Syndrome.” Journal of Clinical Investigation 129: 4050–7. 10.1172/JCI129194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckburg, Paul B. , Bik Elisabeth M., Bernstein Charles N., Purdom Elizabeth, Dethlefsen Les, Sargent Michael, Gill Steven R., Nelson Karen E., and Relman David A.. 2005. “Diversity of the Human Intestinal Microbial Flora.” Science 308: 1635–8. 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arumugam, Manimozhiyan , Raes Jeroen, Pelletier Eric, Le Paslier Denis, Yamada Takuji, Mende Daniel R., Fernandes Gabriel R., et al. 2011. “Enterotypes of the Human Gut Microbiome.” Nature 473: 174–80. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu, Gary D. , Chen Jun, Hoffmann Christian, Bittinger Kyle, Chen Ying‐Yu, Keilbaugh Sue A., Bewtra Meenakshi, et al. 2011. “Linking Long‐Term Dietary Patterns with Gut Microbial Enterotypes.” Science 334: 105–8. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costea, Paul I. , Hildebrand Falk, Arumugam Manimozhiyan, Bäckhed Fredrik, Blaser Martin J., Bushman Frederic D., de Vos Willem M., et al. 2018. “Enterotypes in the Landscape of Gut Microbial Community Composition.” Nature Microbiology 3: 8–16. 10.1038/s41564-017-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothschild, Daphna , Weissbrod Omer, Barkan Elad, Kurilshikov Alexander, Korem Tal, Zeevi David, Costea Paul I., et al. 2018. “Environment Dominates Over Host Genetics in Shaping Human Gut Microbiota.” Nature 555: 210–5. 10.1038/nature25973 [DOI] [PubMed] [Google Scholar]
- 25. Rinninella, Emanuele , Raoul Pauline, Cintoni Marco, Franceschi Francesco, Miggiano Giacinto, Gasbarrini Antonio, and Mele Maria. 2019. “What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem Across Age, Environment, Diet, and Diseases.” Microorganisms 7: 14. 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin, Deng , Wang Ran, Luo Junjie, Ren Fazheng, Gu Zhenglong, Zhao Yiqiang, and Zhao Liang. 2020. “The Core and Distinction of the Gut Microbiota in Chinese Populations Across Geography and Ethnicity.” Microorganisms 8: 1579. 10.3390/microorganisms8101579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao, Binbin , Fan Yajuan, Ma Qingyan, He Xiaoyan, Zhou Lina, Zhu Feng, Wang Wei et al. 2019. “Advances in Research Related to Gut Microbiota and the Pathogenesis of Schizophrenia.” China Journal of Psychiatry 52: 357–60. 10.3760/cma.j.issn.1006-7884.2019.05.011 [DOI] [Google Scholar]
- 28. Alam, Reza , Abdolmaleky Hamid M., and Zhou Jin‐Rong. 2017. “Microbiome, Inflammation, Epigenetic Alterations, and Mental Diseases.” American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 174: 651–60. 10.1002/ajmg.b.32567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valton, Vincent , Romaniuk Liana, Douglas Steele J., Lawrie Stephen, and Seriès Peggy. 2017. “Comprehensive Review: Computational Modelling of Schizophrenia.” Neuroscience & Biobehavioral Reviews 83: 631–46. 10.1016/j.neubiorev.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 30. Stahl, Stephen M . 2018. “Beyond the Dopamine Hypothesis of Schizophrenia to Three Neural Networks of Psychosis: Dopamine, Serotonin, and Glutamate.” CNS Spectrums 23: 187–91. 10.1017/s1092852918001013 [DOI] [PubMed] [Google Scholar]
- 31. Yang, Albert , and Tsai Shih‐Jen. 2017. “New Targets for Schizophrenia Treatment Beyond the Dopamine Hypothesis.” International Journal of Molecular Sciences 18: 1689. 10.3390/ijms18081689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daneman, Richard , and Rescigno Maria. 2009. “The Gut Immune Barrier and the Blood–Brain Barrier: Are They so Different? Immunity 31: 722–35. 10.1016/j.immuni.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 33. Sherwin, Eoin , Sandhu Kiran V., Dinan Timothy G., and Cryan John F.. 2016. “May the Force Be With You: The Light and Dark Sides of the Microbiota–Gut–Brain Axis in Neuropsychiatry.” CNS Drugs 30: 1019–41. 10.1007/s40263-016-0370-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaglin, Mathilde , Rhimi Moez, Philippe Catherine, Pons Nicolas, Bruneau Aurélia, Goustard Bénédicte, Daugé Valérie, et al. 2018. “Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats.” Frontiers in Neuroscience 12: 216. 10.3389/fnins.2018.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nøhr, M. K. , Egerod K. L., Christiansen S. H., Gille A., Offermanns S., Schwartz T. W., and Møller M.. 2015. “Expression of the Short Chain Fatty Acid Receptor GPR41/FFAR3 in Autonomic and Somatic Sensory Ganglia.” Neuroscience 290: 126–37. 10.1016/j.neuroscience.2015.01.040 [DOI] [PubMed] [Google Scholar]
- 36. Dalile, Boushra , Van Oudenhove Lukas, Vervliet Bram, and Verbeke Kristin. 2019. “The Role of Short‐Chain Fatty Acids in Microbiota–Gut–Brain Communication.” Nature Reviews Gastroenterology & Hepatology 16: 461–78. 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
- 37. Wu, Xiaoli , Huang Zeping, Wu Renrong, Zhong Zhiyong, Wei Qinling, Wang Houliang, Diao Feici, et al. 2013. “The Comparison of Glycometabolism Parameters and Lipid Profiles Between Drug‐Naïve, First‐Episode Schizophrenia Patients and Healthy Controls.” Schizophrenia Research 150: 157–62. 10.1016/j.schres.2013.07.051 [DOI] [PubMed] [Google Scholar]
- 38. Wu, Xiaoli , Huang Zeping, Han Hongying, Zhong Zhiyong, Gan Zhaoyu, Guo Xiaofeng, Diao Feici, et al. 2014. “The Comparison of Glucose and Lipid Metabolism Parameters in Drug‐Naïve, Antipsychotic‐treated, and Antipsychotic Discontinuation Patients with Schizophrenia.” Neuropsychiatric Disease and Treatment 10: 1361–8. 10.2147/ndt.S63140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu, Xiaoli , Qin Feng, Wang Jihui, and Zhao Jingping. 2015. “Lipid Metabolism Abnormality Among Patients with Schizophrenia and Efect of Antipsychotics Withdrawal on It.” Chinese Journal of Neuromedicine 14: 156–61. 10.3760/cma.j.issn.1671-8925.2015.02.011 [DOI] [Google Scholar]
- 40. Castro‐Nallar, Eduardo , Bendall Matthew L., Pérez‐Losada Marcos, Sabuncyan Sarven, Severance Emily G., Dickerson Faith B., Schroeder Jennifer R., Yolken Robert H., and Crandall Keith A.. 2015. “Composition, Taxonomy and Functional Diversity of the Oropharynx Microbiome in Individuals with Schizophrenia and Controls.” PeerJ 3: e1140. 10.7717/peerj.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen, Tanya T. , Kosciolek Tomasz, Maldonado Yadira, Daly Rebecca E., Martin Averria Sirkin, McDonald Daniel, Knight Rob, and Jeste Dilip V.. 2019. “Differences in Gut Microbiome Composition Between Persons with Chronic Schizophrenia and Healthy Comparison Subjects.” Schizophrenia Research 204: 23–9. 10.1016/j.schres.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng, Peng , Zeng Benhua, Liu Meiling, Chen Jianjun, Pan Junxi, Han Yu, Liu Yiyun, et al. 2019. “The Gut Microbiome from Patients with Schizophrenia Modulates the Glutamate‐glutamine‐GABA Cycle and Schizophrenia‐relevant Behaviors in Mice.” Science Advances 5: eaau8317. 10.1126/sciadv.aau8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang, Xue , Pan Li‐Ya, Zhang Zhe, Zhou Yuan‐Yue, Jiang Hai‐Yin, and Ruan Bing. 2020. “Analysis of Gut Mycobiota in First‐Episode, Drug‐Naïve Chinese Patients with Schizophrenia: A Pilot Study.” Behavioural Brain Research 379: 112374. 10.1016/j.bbr.2019.112374 [DOI] [PubMed] [Google Scholar]
- 44. Li, Shijia , Zhuo Min, Huang Xia, Huang Yuanyuan, Zhou Jing, Xiong Dongsheng, Li Jiahui, et al. 2020. “Altered Gut Microbiota Associated with Symptom Severity in Schizophrenia.” PeerJ 8: e9574. 10.7717/peerj.9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu, Ruihuan , Wu Bingbing, Liang Jingwen, He Fusheng, Gu Wen, Li Kang, Luo Yi, et al. 2020. “Altered Gut Microbiota and Mucosal Immunity in Patients with Schizophrenia.” Brain, Behavior, and Immunity 85: 120–7. 10.1016/j.bbi.2019.06.039 [DOI] [PubMed] [Google Scholar]
- 46. Shen, Yang , Xu Jintian, Li Zhiyong, Huang Yichen, Yuan Ye, Wang Jixiang, Zhang Meng, Hu Songnian, and Liang Ying. 2018. “Analysis of Gut Microbiota Diversity and Auxiliary Diagnosis as a Biomarker in Patients with Schizophrenia: A Cross‐sectional Study.” Schizophrenia Research 197: 470–7. 10.1016/j.schres.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 47. Pan, Rubing , Zhang Xulai, Gao Jiaqi, Yi Weizhuo, Wei Qiannan, and Su Hong. 2020. “Analysis of the Diversity of Intestinal Microbiome and its Potential Value as a Biomarker in Patients with Schizophrenia: A Cohort Study.” Psychiatry Research 291: 113260. 10.1016/j.psychres.2020.113260 [DOI] [PubMed] [Google Scholar]
- 48. Schwarz, Emanuel , Maukonen Johanna, Hyytiäinen Tiina, Kieseppä Tuula, Orešič Matej, Sabunciyan Sarven, Mantere Outi, et al. 2018. “Analysis of Microbiota in First Episode Psychosis Identifies Preliminary Associations with Symptom Severity and Treatment Response.” Schizophrenia Research 192: 398–403. 10.1016/j.schres.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 49. Zhang, Yanwu , Lijun Bai, Qiang Cheng, Xulai Zhang, Jiaojiao Gao, Jun Duan, Zhihan Xu, et al. 2018. “High‐throughput Sequencing Analysis of Gut Microbiota in Patients with Schizophrenia in the Stage of Onset and Remission.” Chinese Journal of Nervous and Mental Disorders 44: 705–9. 10.3969/j.issn.1002-0152.2018.12.001 [DOI] [Google Scholar]
- 50. Ma, Xiaoqian , Asif Huma, Dai Lulin, He Ying, Zheng Wenxiao, Wang Dong, Ren Honghong, et al. 2020. “Alteration of the Gut Microbiome in First‐Episode Drug‐Naïve and Chronic Medicated Schizophrenia Correlate with Regional Brain Volumes.” Journal of Psychiatric Research 123: 136–44. 10.1016/j.jpsychires.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 51. Zhu, Feng , Guo Ruijin, Wang Wei, Ju Yanmei, Wang Qi, Ma Qingyan, Sun Qiang, et al. 2020. “Transplantation of Microbiota from Drug‐free Patients with Schizophrenia Causes Schizophrenia‐like Abnormal Behaviors and Dysregulated Kynurenine Metabolism in Mice.” Molecular Psychiatry 25: 2905–18. 10.1038/s41380-019-0475-4 [DOI] [PubMed] [Google Scholar]
- 52. Harvey, Philip D. , and Rosenthal Jennifer B.. 2016. “Treatment Resistant Schizophrenia: Course of Brain Structure and Function.” Progress in Neuro‐Psychopharmacology and Biological Psychiatry 70: 111–6. 10.1016/j.pnpbp.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 53. Kelly, Deanna L. , Sullivan Kelli M., McEvoy Joseph P., McMahon Robert P., Wehring Heidi J., Gold James M., Liu Fang, et al. 2015. “Adjunctive Minocycline in Clozapine‐treated Schizophrenia Patients with Persistent Symptoms.” Journal of Clinical Psychopharmacology 35: 374–81. 10.1097/jcp.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minichino, Amedeo , Brondino Natascia, Solmi Marco, Del Giovane Cinzia, Fusar‐Poli Paolo, Burnet Philip, Cipriani Andrea, and Lennox Belinda R.. 2021. “The Gut‐microbiome as a Target for the Treatment of Schizophrenia: A Systematic Review and Meta‐analysis of Randomised Controlled Trials of Add‐on Strategies.” Schizophrenia Research 234: 58–70. 10.1016/j.schres.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 55. Dickerson, Faith B. , Stallings Cassie, Origoni Andrea, Katsafanas Emily, Savage Christina L. G., Schweinfurth Lucy A. B., Goga Joshana, et al. 2014. “Effect of Probiotic Supplementation on Schizophrenia Symptoms and Association with Gastrointestinal Functioning: A Randomized, Placebo‐Controlled Trial.” The Primary Care Companion for CNS Disorders 16: 278–85. 10.4088/PCC.13m01579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Torres‐Fuentes, Cristina , Schellekens Harriët, Dinan Timothy G., and Cryan John F.. 2017. “The Microbiota–Gut–Brain Axis in Obesity.” The Lancet Gastroenterology & Hepatology 2: 747–56. 10.1016/s2468-1253(17)30147-4 [DOI] [PubMed] [Google Scholar]
- 57. Gentile, Christopher L. , and Weir Tiffany L.. 2018. “The Gut Microbiota at the Intersection of Diet and Human Health.” Science 362: 776–80. 10.1126/science.aau5812 [DOI] [PubMed] [Google Scholar]
- 58. Fetissov, Sergueï O . 2017. “Role of the Gut Microbiota in Host Appetite Control: Bacterial Growth to Animal Feeding Behaviour.” Nature Reviews Endocrinology 13: 11–25. 10.1038/nrendo.2016.150 [DOI] [PubMed] [Google Scholar]
- 59. Sandhu, Kiran V. , Sherwin Eoin, Schellekens Harriët, Stanton Catherine, Dinan Timothy G., and Cryan John F.. 2017. “Feeding the Microbiota–Gut–Brain Axis: Diet, Microbiome, and Neuropsychiatry.” Translational Research 179: 223–44. 10.1016/j.trsl.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 60. Turnbaugh, Peter J. , Ley Ruth E., Mahowald Michael A., Magrini Vincent, Mardis Elaine R., and Gordon Jeffrey I.. 2006. “An Obesity‐associated Gut Microbiome with Increased Capacity for Energy Harvest.” Nature 444: 1027–31. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 61. Krajmalnik‐Brown, Rosa , Ilhan Zehra‐Esra, Kang Dae‐Wook, and DiBaise John K.. 2012. “Effects of Gut Microbes on Nutrient Absorption and Energy Regulation.” Nutrition in Clinical Practice 27: 201–14. 10.1177/0884533611436116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang, Peng , Yu Yinghua, Qin Yanfang, Zhou Yuan, Tang Renxian, Wang Qingling, Li Xiangyang, et al. 2019. “Alterations to the Microbiota–Colon–Brain Axis in High‐fat‐diet‐induced Obese Mice Compared to Diet‐resistant Mice.” The Journal of Nutritional Biochemistry 65: 54–65. 10.1016/j.jnutbio.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 63. Andoh, Akira , Nishida Atsushi, Takahashi Kenichiro, Inatomi Osamu, Imaeda Hirotsugu, Bamba Shigeki, Kito Katsuyuki, Sugimoto Mitsushige, and Kobayashi Toshio. 2016. “Comparison of the Gut Microbial Community Between Obese and Lean Peoples Using 16S Gene Sequencing in a Japanese Population.” Journal of Clinical Biochemistry and Nutrition 59: 65–70. 10.3164/jcbn.15-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kong, Ling Chun , Holmes Bridget A., Cotillard Aurelie, Habi‐Rachedi Fatiha, Brazeilles Rémi, Gougis Sophie, Gausserès Nicolas, et al. 2014. “Dietary Patterns Differently Associate with Inflammation and Gut Microbiota in Overweight and Obese Subjects.” PLoS ONE 9: e109434. 10.1371/journal.pone.0109434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Human Microbiome Project Consortium . 2012. “Structure, Function and Diversity of the Healthy Human Microbiome.” Nature 486: 207–14. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Le Chatelier, Emmanuelle , Nielsen Trine, Qin Junjie, Prifti Edi, Hildebrand Falk, Falony Gwen, Almeida Mathieu, et al. 2013. “Richness of Human Gut Microbiome Correlates with Metabolic Markers.” Nature 500: 541–6. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 67. Ley, Ruth E. , Bäckhed Fredrik, Turnbaugh Peter, Lozupone Catherine A., Knight Robin D., and Gordon Jeffrey I.. 2005. “Obesity Alters Gut Microbial Ecology.” Proceedings of the National Academy of Sciences 102: 11070–5. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koliada, Alexander , Syzenko Ganna, Moseiko Vladislav, Budovska Liudmyla, Puchkov Kostiantyn, Perederiy Vyacheslav, Gavalko Yuriy, et al. 2017. “Association Between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population.” BMC Microbiology 17: 120. 10.1186/s12866-017-1027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rahat‐Rozenbloom, S. , Fernandes J., Gloor G. B., and Wolever T. M. S.. 2014. “Evidence for Greater Production of Colonic Short‐chain Fatty Acids in Overweight Than Lean Humans.” International Journal of Obesity 38: 1525–31. 10.1038/ijo.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ley, Ruth E. , Turnbaugh Peter J., Klein Samuel, and Gordon Jeffrey I.. 2006. “Human Gut Microbes Associated with Obesity.” Nature 444: 1022–3. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 71. Crovesy, Louise , Masterson Daniele, and Rosado Eliane Lopes. 2020. “Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review.” European Journal of Clinical Nutrition 74: 1251–62. 10.1038/s41430-020-0607-6 [DOI] [PubMed] [Google Scholar]
- 72. Turnbaugh, Peter J. , Hamady Micah, Yatsunenko Tanya, Cantarel Brandi L., Duncan Alexis, Ley Ruth E., Sogin Mitchell L., et al. 2009. “A Core Gut Microbiome in Obese and Lean Twins.” Nature 457: 480–4. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schwiertz, Andreas , Taras David, Schäfer Klaus, Beijer Silvia, Bos Nicolaas A., Donus Christiane, and Hardt Philip D.. 2010. “Microbiota and SCFA in Lean and Overweight Healthy Subjects.” Obesity 18: 190–5. 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 74. Kellerer, Teresa , Brandl Beate, Büttner Janine, Lagkouvardos Ilias, Hauner Hans, and Skurk Thomas. 2019. “Impact of Laparoscopic Sleeve Gastrectomy on Gut Permeability in Morbidly Obese Subjects.” Obesity Surgery 29: 2132–43. 10.1007/s11695-019-03815-6 [DOI] [PubMed] [Google Scholar]
- 75. Duncan, S. H. , Lobley G. E., Holtrop G., Ince J., Johnstone A. M., Louis P., and Flint H. J.. 2008. “Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss.” International Journal of Obesity 32: 1720–4. 10.1038/ijo.2008.155 [DOI] [PubMed] [Google Scholar]
- 76. Fei, Na , and Zhao Liping. 2013. “An Opportunistic Pathogen Isolated from the Gut of an Obese Human Causes Obesity in Germfree Mice.” The ISME Journal 7(4): 880–4. 10.1038/ismej.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haro, Carmen , Montes‐Borrego Miguel, Rangel‐Zúñiga Oriol A., Alcalá‐Díaz Juan F., Gómez‐Delgado Francisco, Pérez‐Martínez Pablo, Delgado‐Lista Javier, et al. 2016. “Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population.” The Journal of Clinical Endocrinology & Metabolism 101: 233–42. 10.1210/jc.2015-3351 [DOI] [PubMed] [Google Scholar]
- 78. Lee, Clare J. , Florea Liliana, Sears Cynthia L., Maruthur Nisa, Potter James J., Schweitzer Michael, Magnuson Thomas, Clark Jeanne M., et al. 2019. “Changes in Gut Microbiome After Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial.” Obesity Surgery 29: 3239–45. 10.1007/s11695-019-03976-4 [DOI] [PubMed] [Google Scholar]
- 79. Chambers, Edward S. , Preston Tom, Frost Gary, and Morrison Douglas J.. 2018. “Role of Gut Microbiota‐Generated Short‐chain Fatty Acids in Metabolic and Cardiovascular Health.” Current Nutrition Reports 7: 198–206. 10.1007/s13668-018-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guo, Pingting , Zhang Ke, Ma Xi, and He Pingli. 2020. “Clostridium Species as Probiotics: Potentials and Challenges.” Journal of Animal Science and Biotechnology 11: 24. 10.1186/s40104-019-0402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cani, Patrice D. , Bibiloni Rodrigo, Knauf Claude, Waget Aurélie, Neyrinck Audrey M., Delzenne Nathalie M., and Burcelin Rémy. 2008. “Changes in Gut Microbiota Control Metabolic Endotoxemia‐induced Inflammation in High‐Fat Diet‐Induced Obesity and Diabetes in Mice.” Diabetes 57: 1470–81. 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 82. Aron‐Wisnewsky, Judith , Prifti Edi, Belda Eugeni, Ichou Farid, Kayser Brandon D., Dao Maria Carlota, Verger Eric O., et al. 2019. “Major Microbiota Dysbiosis in Severe Obesity: Fate After Bariatric Surgery.” Gut 68: 70–82. 10.1136/gutjnl-2018-316103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Debédat, Jean , Clément Karine, and Aron‐Wisnewsky Judith. 2019. “Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery.” Current Obesity Reports 8: 229–42. 10.1007/s13679-019-00351-3 [DOI] [PubMed] [Google Scholar]
- 84. Manu, P. , Dima L., Shulman M., Vancampfort D., De Hert M., and Correll C. U.. 2015. “Weight Gain and Obesity in Schizophrenia: Epidemiology, Pathobiology, and Management.” Acta Psychiatrica Scandinavica 132: 97–108. 10.1111/acps.12445 [DOI] [PubMed] [Google Scholar]
- 85. Annamalai, Aniyizhai , Kosir Urska, and Tek Cenk. 2017. “Prevalence of Obesity and Diabetes in Patients with Schizophrenia.” World Journal of Diabetes 8: 390–6. 10.4239/wjd.v8.i8.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen, Shengyun , Xia Xiaowei, Deng Chao, Wu Xiuhua, Han Zili, Tao Jiong, and Wu Xiaoli. 2020. “The Correlation Between Metabolic Syndrome and Neurocognitive and Social Cognitive Performance of Patients with Schizophrenia.” Psychiatry Research 288: 112941. 10.1016/j.psychres.2020.112941 [DOI] [PubMed] [Google Scholar]
- 87. Chen, Shengyun , Wen Fei, Zhao Chongbang, Zhang Dongmei, and Wu Xiaoli. 2020. “Effect of Cognitive Impairment on Social Function and Quality of Life in Chronic Schizophrenia.” National Medical Journal of China 100: 351–6. 10.3760/cma.j.issn.0376-2491.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 88. Schizophrenia Collaborative Group of the Psychiatric Branch of the Chinese Medical Association . 2020. “Chinese Expert Consensus on the Management of Metabolic Syndrome in Patients with Schizophrenia.” Chinese Journal of Psychiatry 53: 3–10. 10.3760/cma.j.issn.1006-7884.2020.01.002. [DOI] [Google Scholar]
- 89. Jumpertz, Reiner , Le Duc Son, Turnbaugh Peter J., Trinidad Cathy, Bogardus Clifton, Gordon Jeffrey I., and Krakoff Jonathan. 2011. “Energy‐balance Studies Reveal Associations Between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans.” The American Journal of Clinical Nutrition 94: 58–65. 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen, Anderson , Park Tae Yang, Li Kevin J., and DeLisi Lynn E.. 2020. “Antipsychotics and the Microbiota.” Current Opinion in Psychiatry 33: 225–30. 10.1097/yco.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 91. Singh, Raghunath , Stogios Nicolette, Smith Emily, Lee Jiwon, Maksyutynsk Kateryna, Au Emily, Wright David C., et al. 2022. “Gut Microbiome in Schizophrenia and Antipsychotic‐induced Metabolic Alterations: A Scoping Review.” Therapeutic Advances in Psychopharmacology 12: 204512532210965. 10.1177/20451253221096525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Morgan, Andrew P. , Crowley James J., Nonneman Randal J., Quackenbush Corey R., Miller Cheryl N., Ryan Allison K., Bogue Molly A., et al. 2014. “The Antipsychotic Olanzapine Interacts with the Gut Microbiome to Cause Weight Gain in Mouse.” PLoS ONE 9: e115225. 10.1371/journal.pone.0115225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yuan, Xiuxia , Zhang Peifen, Wang Yaping, Liu Yafei, Li Xue, Kumar Bachoo Upshant, Hei Gangrui, et al. 2018. “Changes in Metabolism and Microbiota After 24‐week Risperidone Treatment in Drug Naïve, Normal Weight Patients with First Episode Schizophrenia.” Schizophrenia Research 201: 299–306. 10.1016/j.schres.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 94. Bahr, S. M. , Tyler B. C., Wooldridge N., Butcher B. D., Burns T. L., Teesch L. M., Oltman C. L., et al. 2015. “Use of the Second‐Generation Antipsychotic, Risperidone, and Secondary Weight Gain Are Associated with an Altered Gut Microbiota in Children.” Translational Psychiatry 5: e652. 10.1038/tp.2015.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen, D. C. , Du X. D., Yin G. Z., Yang K. B., Nie Y., Wang N., Li Y. L., et al. 2016. “Impaired Glucose Tolerance in First‐Episode Drug‐Naïve Patients with Schizophrenia: Relationships with Clinical Phenotypes and Cognitive Deficits.” Psychological Medicine 46: 3219–30. 10.1017/s0033291716001902 [DOI] [PubMed] [Google Scholar]
- 96. Verma, Swapna K. , Subramaniam Mythily, Liew Alvin, and Poon Lye Yin. 2009. “Metabolic Risk Factors in Drug‐Naive Patients with First‐episode Psychosis.” The Journal of Clinical Psychiatry 70: 997–1000. 10.4088/JCP.08m04508 [DOI] [PubMed] [Google Scholar]
- 97. Enez Darcin, Aslı , Yalcin Cavus Sercin, Dilbaz Nesrin, Kaya Hasan, and Dogan Eylem. 2015. “Metabolic Syndrome in Drug‐Naïve and Drug‐free Patients with Schizophrenia and in Their Siblings.” Schizophrenia Research 166: 201–6. 10.1016/j.schres.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 98. Tian, Yang , Wang Dongmei, Wei Gaoxia, Wang Jiesi, Zhou Huixia, Xu Hang, Dai Qilong, et al. 2021. “Prevalence of Obesity and Clinical and Metabolic Correlates in First‐Episode Schizophrenia Relative to Healthy Controls.” Psychopharmacology 238: 745–53. 10.1007/s00213-020-05727-1 [DOI] [PubMed] [Google Scholar]
- 99. Khandaker, G. M. , Dibben C. R. M., and Jones P. B.. 2012. “Does Maternal Body Mass Index During Pregnancy Influence Risk of Schizophrenia in the Adult Offspring? Obesity Reviews 13: 518–27. 10.1111/j.1467-789X.2011.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lazar, Mitchell A . 2005. “How Obesity Causes Diabetes: Not a Tall Tale.” Science 307: 373–5. 10.1126/science.1104342 [DOI] [PubMed] [Google Scholar]
- 101. Cox, Amanda J. , West Nicholas P., and Cripps Allan W.. 2015. “Obesity, Inflammation, and the Gut Microbiota.” The Lancet Diabetes & Endocrinology 3: 207–15. 10.1016/s2213-8587(14)70134-2 [DOI] [PubMed] [Google Scholar]
- 102. Cani, Patrice D. , Amar Jacques, Iglesias Miguel Angel, Poggi Marjorie, Knauf Claude, Bastelica Delphine, Neyrinck Audrey M., et al. 2007. “Metabolic Endotoxemia Initiates Obesity and Insulin Resistance.” Diabetes 56: 1761–72. 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 103. Choi, J. , Joseph L., and Pilote L.. 2013. “Obesity and C‐Reactive Protein in Various Populations: A Systematic Review and Meta‐analysis.” Obesity Reviews 14: 232–44. 10.1111/obr.12003 [DOI] [PubMed] [Google Scholar]
- 104. Bahceci, M. , Gokalp D., Bahceci S., Tuzcu A., Atmaca S., and Arikan S.. 2007. “The Correlation Between Adiposity and Adiponectin, Tumor Necrosis Factor α, Interleukin‐6 and High Sensitivity C‐Reactive Protein Levels. Is Adipocyte Size Associated with Inflammation in Adults? Journal of Endocrinological Investigation 30: 210–4. 10.1007/bf03347427 [DOI] [PubMed] [Google Scholar]
- 105. Marfella, Raffaele , Esposito Katherine, Siniscalchi Mario, Cacciapuoti Federico, Giugliano Francesco, Labriola Domenico, Ciotola Myriam, et al. 2004. “Effect of Weight Loss on Cardiac Synchronization and Proinflammatory Cytokines in Premenopausal Obese Women.” Diabetes Care 27: 47–52. 10.2337/diacare.27.1.47 [DOI] [PubMed] [Google Scholar]
- 106. Malozowski, Saul , and Sahlroot Jon Todd. 2007. “Interleukin‐1‐receptor Antagonist in Type 2 Diabetes Mellitus.” New England Journal of Medicine 357: 302–3. Author reply 303. 10.1056/NEJMc071324 [DOI] [PubMed] [Google Scholar]
- 107. Cavelti‐Weder, Claudia , Babians‐Brunner Andrea, Keller Cornelia, Stahel Marc A., Kurz‐Levin Malaika, Zayed Hany, Solinger Alan M., et al. 2012. “Effects of Gevokizumab on Glycemia and Inflammatory Markers in Type 2 Diabetes.” Diabetes Care 35: 1654–62. 10.2337/dc11-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dominguez, Helena , Storgaard Heidi, Rask‐Madsen Christian, Steffen Hermann Thomas, Ihlemann Nikolaj, Baunbjerg Nielsen Dorthe, Spohr Camilla, et al. 2005. “Metabolic and Vascular Effects of Tumor Necrosis Factor‐α Blockade with Etanercept in Obese Patients with Type 2 Diabetes.” Journal of Vascular Research 42: 517–25. 10.1159/000088261 [DOI] [PubMed] [Google Scholar]
- 109. Fleischman, Amy , Shoelson Steven E., Bernier Raquel, and Goldfine Allison B.. 2008. “Salsalate Improves Glycemia and Inflammatory Parameters In Obese Young Adults.” Diabetes Care 31: 289–94. 10.2337/dc07-1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stein, Kate , and Broome Matthew R.. 2015. “Neuroprogression in Schizophrenia: Pathways and Underpinning Clinical Staging and Therapeutic Corollaries.” Australian & New Zealand Journal of Psychiatry 49: 183–4. 10.1177/0004867414556321 [DOI] [PubMed] [Google Scholar]
- 111. Babulas, Vicki , Factor‐Litvak Pam, Goetz Raymond, Schaefer Catherine A., and Brown Alan S.. 2006. “Prenatal Exposure to Maternal Genital and Reproductive Infections and Adult Schizophrenia.” American Journal of Psychiatry 163: 927–9. 10.1176/ajp.2006.163.5.927 [DOI] [PubMed] [Google Scholar]
- 112. Eide, M. G. , Moster D., Irgens L. M., Reichborn‐Kjennerud T., Stoltenberg C., Skjærven R., Susser E., and Abel K.. 2013. “Degree of Fetal Growth Restriction Associated with Schizophrenia Risk in a National Cohort.” Psychological Medicine 43: 2057–66. 10.1017/s003329171200267x [DOI] [PubMed] [Google Scholar]
- 113. Xu, Ming‐Qing , Sun Wen‐Sheng, Liu Ben‐Xiu, Feng Guo‐Yin, Yu Lan, Yang Lawrence, He Guang, et al. 2009. “Prenatal Malnutrition and Adult Schizophrenia: Further Evidence from the 1959–1961 Chinese Famine.” Schizophrenia Bulletin 35: 568–76. 10.1093/schbul/sbn168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang, Alexandre K. , and Miller Brian J.. 2018. “Meta‐analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression.” Schizophrenia Bulletin 44: 75–83. 10.1093/schbul/sbx035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Goldsmith, D. R. , Rapaport M. H., and Miller B. J.. 2016. “A Meta‐analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder and Depression.” Molecular Psychiatry 21: 1696–709. 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fond, Guillaume , Lançon Christophe, Auquier Pascal, and Boyer Laurent. 2018. “C‐Reactive Protein as a Peripheral Biomarker in Schizophrenia. An Updated Systematic Review.” Frontiers in Psychiatry 9: 392. 10.3389/fpsyt.2018.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mondelli, Valeria , Ciufolini Simone, Belvederi Murri Martino, Bonaccorso Stefania, Di Forti Marta, Giordano Annalisa, Marques Tiago R., et al. 2015. “Cortisol and Inflammatory Biomarkers Predict Poor Treatment Response in First Episode Psychosis.” Schizophrenia Bulletin 41: 1162–70. 10.1093/schbul/sbv028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kabiersch, Alexa , Furukawa Hiromu, del Rey Adriana, and Besedovsky H. O.. 1998. “Administration of interleukin‐1 at Birth Affects Dopaminergic Neurons in Adult Mice.” Annals of the New York Academy of Sciences 840: 123–7. 10.1111/j.1749-6632.1998.tb09556.x [DOI] [PubMed] [Google Scholar]
- 119. Jarskog, L. Fredrik , Xiao Hong, Wilkie Mary Beth, Lauder Jean M., and Gilmore John H.. 1997. “Cytokine Regulation of Embryonic Rat Dopamine and Serotonin Neuronal Survival In Vitro.” International Journal of Developmental Neuroscience 15: 711–6. 10.1016/s0736-5748(97)00029-4 [DOI] [PubMed] [Google Scholar]
- 120. Felger, Jennifer C. , Mun Jiyoung, Kimmel Heather L., Nye Jonathon A., Drake Daniel F., Hernandez Carla R., Freeman Amanda A., et al. 2013. “Chronic Interferon‐α Decreases Dopamine 2 Receptor Binding and Striatal Dopamine Release in Association with Anhedonia‐like Behavior in Nonhuman Primates.” Neuropsychopharmacology 38: 2179–87. 10.1038/npp.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tan, Jian , McKenzie Craig, Potamitis Maria, Thorburn Alison N., Mackay Charles R., and Macia Laurence. 2014. “The Role of Short‐chain Fatty Acids in Health and Disease.” Advances in Immunology 121: 91–119. 10.1016/b978-0-12-800100-4.00003-9 [DOI] [PubMed] [Google Scholar]
- 122. Flint, Harry J. , Scott Karen P., Duncan Sylvia H., Louis Petra, and Forano Evelyne. 2012. “Microbial Degradation of Complex Carbohydrates in the Gut.” Gut Microbes 3: 289–306. 10.4161/gmic.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brandsma, Eelke , Kloosterhuis Niels J., Koster Mirjam, Dekker Daphne C., Gijbels Marion J. J., van der Velden Saskia, Ríos‐Morales Melany, et al. 2019. “A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis.” Circulation Research 124: 94–100. 10.1161/circresaha.118.313234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Qian, Cheng , and Cao Xuetao. 2013. “Regulation of Toll‐like Receptor Signaling Pathways in Innate Immune Responses.” Annals of the New York Academy of Sciences 1283: 67–74. 10.1111/j.1749-6632.2012.06786.x [DOI] [PubMed] [Google Scholar]
- 125. Graf, Daniela , Di Cagno Raffaella, Fåk Frida, Flint Harry J., Nyman Margareta, Saarela Maria, and Watzl Bernhard. 2015. “Contribution of Diet to the Composition of the Human Gut Microbiota.” Microbial Ecology in Health & Disease 26: 26164. 10.3402/mehd.v26.26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ghosh, Siddhartha S. , Wang Jing, Yannie Paul J., and Ghosh Shobha. 2020. “Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development.” Journal of the Endocrine Society 4: bvz039. 10.1210/jendso/bvz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fitzgerald, Katherine A. , and Kagan Jonathan C.. 2020. “Toll‐like Receptors and the Control of Immunity.” Cell 180: 1044–66. 10.1016/j.cell.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nikolova, Viktoriya L. , Smith Megan R. B., Hall Lindsay J., Cleare Anthony J., Stone James M., and Young Allan H.. 2021. “Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta‐analysis.” JAMA Psychiatry 78: 1343–54. 10.1001/jamapsychiatry.2021.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wu, Hui , Dai Guochao, Aizezi Muyeseer, Tang Juan, Zou Ke, Wu Yuhua, and Wu Xiaoli. 2022. “Gray Matter Reduction in Bilateral Insula Mediating Adverse Psychiatric Effects of Body Mass Index in Schizophrenia.” BMC Psychiatry 22: 639. 10.1186/s12888-022-04285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang, Chenhong , Zhang Menghui, Wang Shengyue, Han Ruijun, Cao Youfang, Hua Weiying, Mao Yuejian, et al. 2010. “Interactions Between Gut Microbiota, Host Genetics and Diet Relevant to Development of Metabolic Syndromes in Mice.” The ISME Journal 4: 232–41. 10.1038/ismej.2009.112 [DOI] [PubMed] [Google Scholar]
- 131. De Filippo, Carlotta , Cavalieri Duccio, Di Paola Monica, Ramazzotti Matteo, Poullet Jean Baptiste, Massart Sebastien, Collini Silvia, Pieraccini Giuseppe, and Lionetti Paolo. 2010. “Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa.” Proceedings of the National Academy of Sciences 107: 14691–6. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sonnenburg, Erica D. , Smits Samuel A., Tikhonov Mikhail, Higginbottom Steven K., Wingreen Ned S., and Sonnenburg Justin L.. 2016. “Diet‐induced Extinctions in the Gut Microbiota Compound Over Generations.” Nature 529: 212–5. 10.1038/nature16504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Marchesi, Julian R. , Adams David H., Fava Francesca, Hermes Gerben D. A., Hirschfield Gideon M., Hold Georgina, Quraishi Mohammed Nabil, et al. 2016. “The Gut Microbiota and Host Health: A New Clinical Frontier.” Gut 65: 330–9. 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhao, Liping , Zhang Feng, Ding Xiaoying, Wu Guojun, Lam Yan Y., Wang Xuejiao, Fu Huaqing, et al. 2018. “Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes.” Science 359: 1151–6. 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 135. Kassaian, Nazila , Feizi Awat, Aminorroaya Ashraf, Jafari Parvaneh, Ebrahimi Maryam Tajabadi, and Amini Masoud. 2018. “The Effects of Probiotics and Synbiotic Supplementation on Glucose and Insulin Metabolism in Adults with Prediabetes: A Double‐blind Randomized Clinical Trial.” Acta Diabetologica 55: 1019–28. 10.1007/s00592-018-1175-2 [DOI] [PubMed] [Google Scholar]
- 136. Huang, Jing , Kang Dongyu, Zhang Fengyu, Yang Ye, Liu Chenchen, Xiao Jingmei, Long Yujun, et al. 2022. “Probiotics Plus Dietary Fiber Supplements Attenuate Olanzapine‐induced Weight Gain in Drug‐Naïve First‐episode Schizophrenia Patients: Two Randomized Clinical Trials.” Schizophrenia Bulletin 48: 850–59. 10.1093/schbul/sbac044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Huang, Jing , Liu Chenchen, Yang Ye, Kang Dongyu, Xiao Jingmei, Long Yujun, Lang Bing, et al. 2022. “The Effects of Probiotics Plus Dietary Fiber on Antipsychotic‐induced Weight Gain: A Randomized Clinical Trial.” Translational Psychiatry 12: 185. 10.1038/s41398-022-01958-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vrieze, Anne , Van Nood Els, Holleman Frits, Salojärvi Jarkko, Kootte Ruud S., Bartelsman Joep F. W. M., Dallinga‐Thie Geesje M., et al. 2012. “Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome.” Gastroenterology 143: 913–916. 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 139. Kootte, Ruud S. , Levin Evgeni, Salojärvi Jarkko, Smits Loek P., Hartstra Annick V., Udayappan Shanti D., Hermes Gerben, et al. 2017. “Improvement of Insulin Sensitivity After Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition.” Cell Metabolism 26: 611–619.e6. 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 140. Liang, Ying , Shen Yang, Li Gaofei, Yuan Ye, Zhang Meng, and Gao Jiayu. 2022. “Schizophrenia Patients With Prevotella‐Enterotype Have a Higher Risk of Obesity.” Frontiers in Psychiatry 13: 864951. 10.3389/fpsyt.2022.864951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. McWhinney, Sean R. , Brosch Katharina, Calhoun Vince D., Crespo‐Facorro Benedicto, Crossley Nicolas A., Dannlowski Udo, Dickie Erin, et al. 2022. “Obesity and Brain Structure in Schizophrenia—ENIGMA Study in 3021 Individuals.” Molecular Psychiatry 27: 3731–37. 10.1038/s41380-022-01616-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mueller, Karsten , Möller Harald E., Horstmann Annette, Busse Franziska, Lepsien Jöran, Blüher Matthias, Stumvoll Michael, Villringer Arno, and Pleger Burkhard. 2015. “Physical Exercise in Overweight to Obese Individuals Induces Metabolic‐ and Neurotrophic‐related Structural Brain Plasticity.” Frontiers in Human Neuroscience 9: 372. 10.3389/fnhum.2015.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mansur, Rodrigo B. , Zugman Andre, Ahmed Juhie, Cha Danielle S., Subramaniapillai Mehala, Lee Yena, Lovshin Julie, et al. 2017. “Treatment with a GLP‐1R Agonist Over Four Weeks Promotes Weight Loss‐moderated Changes in Frontal–Striatal Brain Structures in Individuals with Mood Disorders.” European Neuropsychopharmacology 27: 1153–62. 10.1016/j.euroneuro.2017.08.433 [DOI] [PubMed] [Google Scholar]
- 144. Flowers, Stephanie A. , Baxter Nielson T., Ward Kristen M., Kraal A. Zarina, McInnis Melvin G., Schmidt Thomas M., and Ellingrod Vicki L.. 2019. “Effects of Atypical Antipsychotic Treatment and Resistant Starch Supplementation on Gut Microbiome Composition in a Cohort of Patients with Bipolar Disorder or Schizophrenia.” Pharmacotherapy 39: 161–70. 10.1002/phar.2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Costea, Paul I. , Zeller Georg, Sunagawa Shinichi, Pelletier Eric, Alberti Adriana, Levenez Florence, Tramontano Melanie, et al. 2017. “Towards Standards for Human Fecal Sample Processing in Metagenomic Studies.” Nature Biotechnology 35: 1069–76. 10.1038/nbt.3960 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.


