Abstract
In the last three decades, ablation of atrial fibrillation (AF) has become an evidence-based safe and efficacious treatment for managing the most common cardiac arrhythmia. In 2007, the first joint expert consensus document was issued, guiding healthcare professionals involved in catheter or surgical AF ablation. Mounting research evidence and technological advances have resulted in a rapidly changing landscape in the field of catheter and surgical AF ablation, thus stressing the need for regularly updated versions of this partnership which were issued in 2012 and 2017. Seven years after the last consensus, an updated document was considered necessary to define a contemporary framework for selection and management of patients considered for or undergoing catheter or surgical AF ablation. This consensus is a joint effort from collaborating cardiac electrophysiology societies, namely the European Heart Rhythm Association, the Heart Rhythm Society, the Asia Pacific Heart Rhythm Society, and the Latin American Heart Rhythm Society .
Keywords: Atrial fibrillation, Catheter ablation, Surgical ablation
Table of contents
1. Introduction
1.1. Preamble
1.2. Organization of the writing committee
1.3. Methods
1.4. Document review and approval
1.5. Scope of the document
2. Classification—atrial fibrillation pathophysiology
2.1. Definitions
2.2. Classifications
2.3. Natural history of atrial fibrillation and atrial fibrillation progression
2.4. Pathophysiology of atrial fibrillation
2.4.1. Genetics of atrial fibrillation
2.4.2. Molecular basis of atrial fibrillation
2.4.3. Mechanisms of atrial fibrillation initiation and maintenance
2.4.3.1. Role of triggers and automaticity
2.4.3.2. Role of focal and rotational activity and spiral waves
2.4.3.3. Role of multi-wavelet reentry
2.4.3.4. Role of endocardial–epicardial asynchrony
2.4.4. Structural and electrical remodelling in atrial fibrillation
2.4.4.1. Structural remodelling
2.4.4.2. Electrical remodelling
2.4.5. Autonomic nervous system and its role in atrial fibrillation pathophysiology
3. Anatomical considerations—implications for catheter ablation
3.1. The pulmonary veins—typical anatomy and variants
3.2. Pulmonary vein epicardial connections
3.3. Fossa ovalis—interatrial septum (implications for transseptal puncture)
3.4. Architecture of left atrial musculature
3.5. Coronary sinus—vein of Marshall
3.6. Superior vena cava
3.7. Autonomic ganglionated plexi
3.8. Pericardial reflections
3.9. Phrenic nerves
3.10. Esophagus
4. Indications for catheter ablation of atrial fibrillation
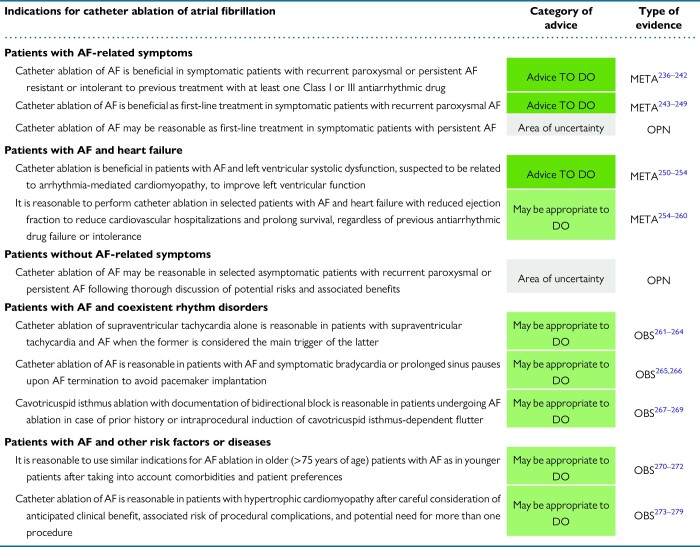
4.1. Catheter ablation in patients with atrial fibrillation–related symptoms
4.2. Catheter ablation in patients with atrial fibrillation and heart failure
4.3. Catheter ablation in patients without atrial fibrillation–related symptoms
4.4. Patients with atrial fibrillation and coexistent rhythm disorders
4.4.1. Atrial fibrillation and supraventricular tachycardia
4.4.2. Atrial fibrillation and sinus node dysfunction
4.4.3. Atrial fibrillation and atrial flutter
4.5. Atrial fibrillation with other risk factors or diseases
4.5.1. Older patients with atrial fibrillation
4.5.2. Atrial fibrillation and hypertrophic cardiomyopathy
4.5.3. Patients with atrial fibrillation and obesity—physical inactivity—obstructive sleep apnoea
5. Atrial fibrillation risk factors and preprocedural management
5.1. Atrial fibrillation risk factors
5.1.1. Hypertension
5.1.2. Obesity
5.1.3. Obstructive sleep apnoea
5.1.4. Alcohol consumption
5.1.5. Physical inactivity
5.1.6. Diabetes mellitus
5.1.7. Smoking
5.1.8. Cardiovascular comorbidities
5.2. Preprocedural management
5.2.1. Preprocedural predictors of atrial fibrillation recurrences
5.2.1.1. Atrial fibrillation type and duration
5.2.1.2. Left atrial size
5.2.1.3. Electrocardiographic predictors
5.2.1.4. Preprocedural imaging of atrial structure
5.2.2. Preprocedural pharmacological treatment
5.2.2.1. Preprocedural anticoagulation
5.2.2.2. Preprocedural antiarrhythmic drug treatment
5.2.3. Imaging for exclusion of thrombus
5.2.3.1. Candidates for thrombus screening prior to ablation
5.2.3.2 Imaging modalities for thrombus exclusion
6. Mapping and ablation tools for atrial fibrillation catheter ablation
6.1. Mapping tools
6.1.1. Invasive mapping tools
6.1.1.1. Electroanatomical contact mapping
6.1.1.2. Non-contact mapping
6.1.1.3. Spatiotemporal dispersion mapping
6.1.2. Non-invasive mapping tools
6.1.2.1. Electrocardiographic imaging
6.1.2.2. Magnetic resonance imaging fibrosis guidance
6.2. Ablation tools
6.2.1. Radiofrequency ablation
6.2.2. Cryoablation and ultra-low temperature cryoablation
6.2.2.1. Conventional cryoballoon technologies
6.2.2.2. Ultra-low temperature cryoablation
6.2.3. Pulsed field ablation
6.2.3.1. Biophysics and mechanisms
6.2.3.2. Efficiency and safety—key advantages
6.2.3.3. Efficacy of pulsed field ablation
6.2.4. Laser ablation
6.3. Robotic and magnetic catheter navigation
6.4. Future developments
6.4.1. Mapping tools
6.4.2. Ablation tools
7. Procedural management and techniques
7.1. Anesthesia and ventilation during atrial fibrillation ablation
7.1.1. General anesthesia vs. sedation
7.1.2. Ventilation
7.2. Vascular access
7.3. Continuous arterial blood pressure monitoring
7.4. Anticoagulation during atrial fibrillation ablation
7.5. Transseptal puncture
7.6. The use of intracardiac echocardiography
7.7. Fluoroless ablation
7.8. Esophageal temperature management
8. Ablation strategies
8.1. Pulmonary vein isolation
8.1.1. Endpoint of pulmonary vein isolation
8.1.2. Pulmonary vein isolation using radiofrequency energy
8.1.2.1. Electrogram parameters and impedance change
8.1.2.2. Lesion quality indicators
8.1.2.3. Waiting phase
8.1.2.4. Adenosine testing
8.1.2.5. First-pass isolation
8.1.2.6. Loss of pace capture along pulmonary vein isolation line
8.1.2.7. Inducibility of atrial fibrillation after pulmonary vein isolation
8.1.3. Pulmonary vein isolation with cryoballoon ablation
8.2. Adjunctive ablation targets beyond pulmonary vein isolation
8.2.1. Cavotricuspid isthmus
8.2.2. Linear lesions
8.2.3. Complex fractionated atrial electrogram ablation
8.2.4. Stepwise approach to atrial fibrillation ablation
8.2.5. Left atrial posterior wall isolation
8.2.6. Substrate ablation
8.2.7. Vein of Marshall ablation
8.2.8. Ablation of non-pulmonary vein triggers
8.2.9. Ganglionated plexi ablation
9. Postprocedural management
9.1. Sheath removal—hemostasis achievement
9.2. Duration of hospitalization—same-day discharge
9.3. Postprocedural pharmacological management
9.3.1. Anticoagulants
9.3.1.1. Early postprocedural (the first 2 months)
9.3.1.2. Late postprocedural (more than 2 months)
9.3.1.3. Candidates to discontinue anticoagulation
9.3.1.4. Targeted anticoagulation (on demand) postablation
9.3.2. Antiarrhythmic drug treatment
9.3.3. Proton pump inhibitors
9.3.4. Anti-inflammatory agents
9.4. Rhythm monitoring following catheter ablation
9.4.1. Continuous postablation rhythm monitoring
9.4.2. Intermittent postablation rhythm monitoring
9.4.3. Practical considerations on postablation rhythm monitoring
9.5. Early recurrences after ablation—postablation blanking period
9.5.1. Incidence and pathophysiology of early recurrence after atrial fibrillation ablation
9.5.2. Duration of blanking period
9.5.3. Management of early recurrences after catheter ablation
9.5.3.1. Electrical cardioversion
9.5.3.2. Early reablation
9.6. Patient follow-up following catheter ablation
9.7. Atrial tachycardia following atrial fibrillation ablation
9.7.1. Incidence—underlying mechanisms
9.7.2. Management
10. Ablation outcome and efficacy
10.1. Acute procedural success
10.2. Atrial fibrillation recurrence endpoints
10.3. Atrial fibrillation burden endpoints
10.4. Atrial fibrillation progression endpoints
10.5. Atrial fibrillation–related symptoms
10.6. Quality of life assessment
11. Complications
11.1. General considerations
11.2. Factors associated with procedural complication rate
11.2.1. Procedural volume
11.2.2. Type of energy source
11.2.3. Role of ablation protocols
11.2.4. Time course of complications and implications for discharge practice
11.3. Presentation, treatment, and prevention of specific complications
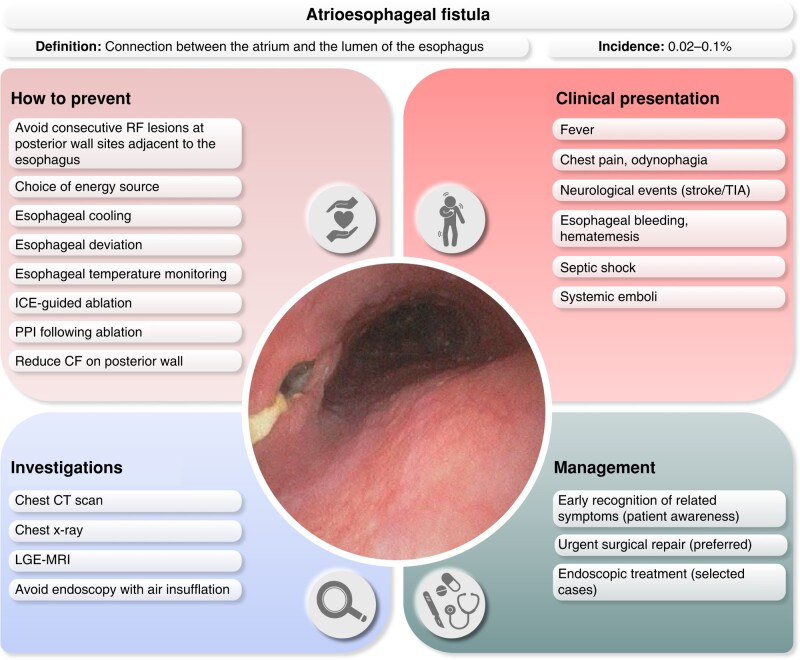
11.3.1. Esophageal perforation
11.3.2. Periprocedural thromboembolic events
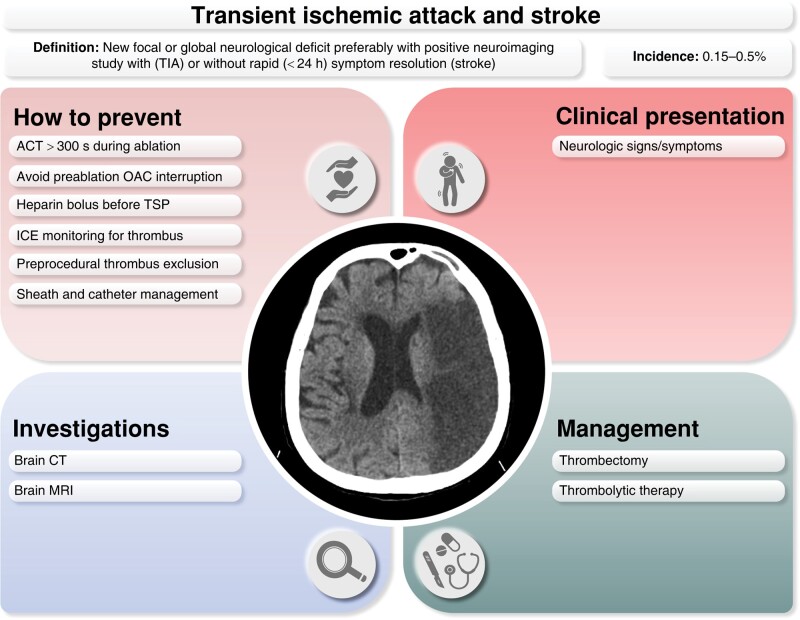
11.3.3. Asymptomatic cerebral lesions
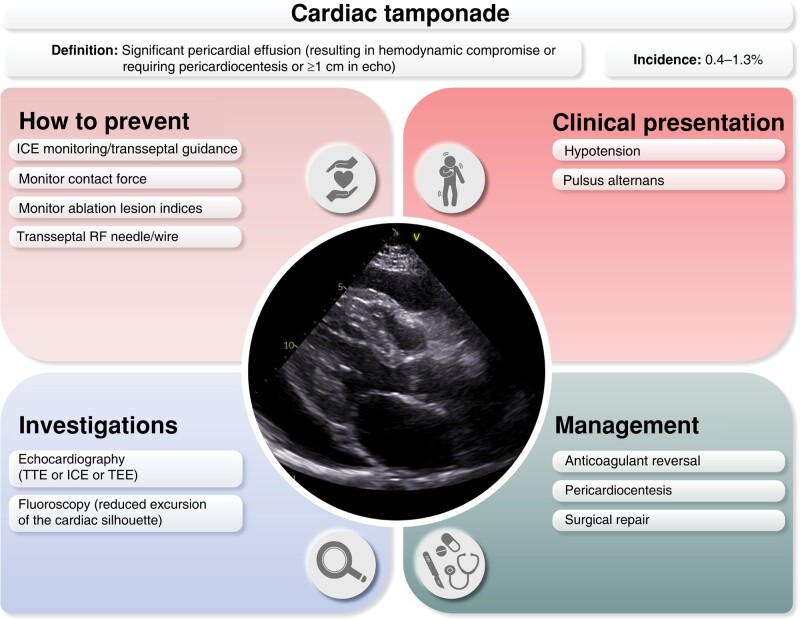
11.3.4. Cardiac tamponade
11.3.5. Pulmonary vein stenosis
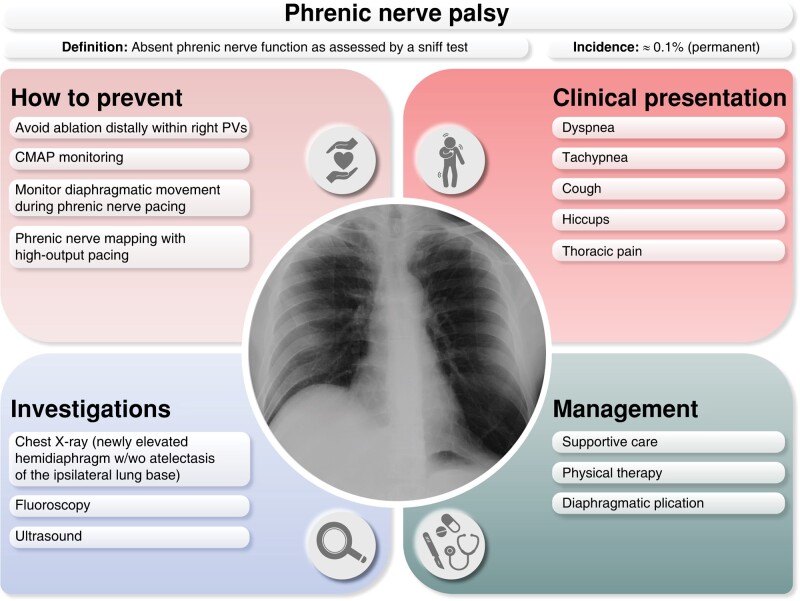
11.3.6. Phrenic nerve palsy
11.3.7. Vascular complications
11.3.8. Other complications of AF ablation
11.3.8.1. Air embolism
11.3.8.2. Acute coronary artery stenosis and occlusion
11.3.8.3. Mitral valve trauma and curvilinear catheter entrapment
11.3.8.4. Stiff left atrial syndrome
11.3.8.5. Gastric hypomotility
12. Surgical and hybrid atrial fibrillation ablation
12.1. Technology and techniques
12.1.1. Energy sources
12.1.1.1. Radiofrequency energy
12.1.1.2. Cryoenergy
12.1.2. Specific ablation tools
12.1.2.1. Radiofrequency ablation tools
12.1.2.2. Cryoablation tools
12.1.3. Procedural targets and lesion sets
12.1.3.1. Pulmonary vein isolation
12.1.3.2. Isolation of the left atrial posterior wall
12.1.3.3. Right and left atrial linear lesions
12.1.3.4. Ganglionated plexi ablation
12.1.3.5. Ligament of Marshall
12.1.3.6. Left atrial appendage exclusion
12.2. Concomitant surgical ablation of atrial fibrillation
12.2.1. Efficacy of concomitant atrial fibrillation surgery
12.2.2. Safety of concomitant atrial fibrillation surgery
12.2.3. Optimal lesion set in patients undergoing left atrial open procedures
12.2.4. Optimal lesion set in patients undergoing non-left atrial open procedures
12.3. Stand-alone surgical ablation of atrial fibrillation
12.3.1. Stand-alone Cox maze procedure
12.3.2. Minimally invasive surgical—hybrid atrial fibrillation ablation
12.3.2.1. Thoracoscopic surgical approach
12.3.2.2. Hybrid convergent procedure
12.3.3. Clinical evidence—comparison of catheter and surgical ablation
12.3.3.1. Paroxysmal atrial fibrillation
12.3.3.2. Persistent and long-standing persistent atrial fibrillation
12.4. Left atrial appendage exclusion
13. Training and institutional requirements for atrial fibrillation ablation
13.1. Training requirements
13.1.1. Appropriate selection of patients
13.1.2. Technical knowledge required
13.1.3. Training of non-medical team members
13.1.4. Completion of training
13.1.5. Maintaining competence
13.2. Institutional requirements
13.2.1. Staff
13.2.2. Equipments and facilities
13.2.3. Follow-up and other requirements
14. Areas for future research
14.1. Basic translational science
14.2. Risk factor modification
14.3. Patient selection—personalized management
14.4. Energy sources—ablation tools
14.5. Ablation strategies
14.6. Endpoints and outcomes after ablation
Supplementary material
Data availability
References
1. Introduction
1.1. Preamble
In the last three decades, ablation of atrial fibrillation (AF) has become an evidence-based safe and efficacious treatment for managing the most common cardiac arrhythmia. In 2007, the first joint expert consensus document was issued, guiding healthcare professionals involved in catheter or surgical AF ablation.1 Mounting research evidence and technological advances have resulted in a rapidly changing landscape in the field of catheter and surgical AF ablation, thus stressing the need for regularly updated versions of this partnership, which was issued in 2012 and 2017.2,3 Seven years after the last consensus, an updated document was considered necessary to define a contemporary framework for selection and management of patients considered for or undergoing catheter or surgical AF ablation. This consensus is a joint effort from collaborating cardiac electrophysiology societies, namely the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS).
1.2. Organization of the writing committee
The EHRA, as the leading society, nominated the chair of the document, and each of the partner societies nominated a co-chair. The writing group was defined based on a list of representatives put forward by each organization. The members were qualified in order of preference provided that they did not meet any of the following: part-time employment or salary from a related company, significant stock ownership, holding of a patent which generates significant revenues, and receipt of significant royalties for intellectual property related to the topic of the scientific paper. The entire group comprised 44 members and was appointed to section writing teams based on preference and expertise, aiming to cover specific content. All members provided disclosure statements to assess potential conflicts of interest. Details are available in the Supplementary material.
1.3. Methods
A detailed survey including 140 questions was sent to all members, aiming to capture common practice and preferences in the care of patients undergoing AF ablation. After a comprehensive literature search, evaluation of existing evidence, and consideration of the survey results, practical advice was proposed by the writing group in five sections (indications, preprocedural management, ablation strategies, procedural, and postprocedural management). The writing group had face-to-face meetings and web-based conference calls discussing proposed guidance and pertinent supporting evidence, while consensus modifications were made based on raised comments, thus compiling a final list of clinical advice for the voting process. During voting, each member had the option to agree, disagree, or abstain. Every proposed advice was included only if the voting results (excluding abstention) were at least 80% in support. In total, the suggested clinical advice has been approved by an average of 94% of the writing committee members.
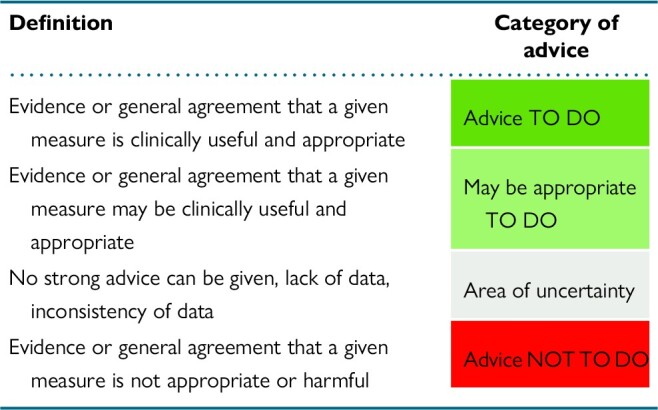
It should be emphasized that the current document is not intended as a guideline and aims to document the current expert consensus in the dedicated narrow field of catheter and surgical AF ablation. Healthcare professionals should refer to the latest guidelines for overall structured management of AF patients.4,5 In this consensus document, a colour-coded classification of proposed clinical advice was used. Classification of different categories of advice and the respective definitions are presented in Table 1. Furthermore, the evidence supporting each advice has been classified in different categories based on the type, quality, and quantity of respective sources (Table 2).
Table 1.
Colour-coded classification of different categories of advice and respective definition
|
Table 2.
Classification of different types of evidence and respective criteria
| Type of evidence—abbreviation | Criteria |
|---|---|
| META |
|
| RAND |
|
| OBS |
|
| OPN |
|
RCT, randomized clinical trial.
1.4. Document review and approval
The draft document was subjected to a peer review process by a review committee whose members were assigned by each of the partner societies. All peer reviewers were requested to complete a declaration of interest and were not allowed to own stocks or stock options or any type of financial interest in a company marketing electrophysiologic products. Each partnering organization has officially reviewed and endorsed the final document.
1.5. Scope of the document
The objective of this consensus document is to provide practical guidance and set standards in the selection and management (preprocedural, procedural, and postprocedural) of patients considered for or undergoing AF ablation. Specific sections are devoted to AF pathophysiology, anatomical considerations, evaluation and management of complications, training, and institutional requirements for AF ablation. The terms and abbreviations used in the consensus statement are summarized in Table 3.
Table 3.
Abbreviations
| Term (abbreviation) | Definition |
|---|---|
| AAD | Antiarrhythmic drug |
| ACC | American College of Cardiology |
| AEF | Atrioesophageal fistula |
| AF | Atrial fibrillation |
| AFl | Atrial flutter |
| AHA | American Heart Association |
| AI | Ablation index |
| ANS | Autonomic nervous system |
| APD | Action potential duration |
| APHRS | Asia Pacific Heart Rhythm Society |
| ASD | Atrial septum defect |
| AT | Atrial tachycardia |
| ATP | Adenosine triphosphate |
| AVNRT | Atrioventricular nodal reentry tachycardia |
| AVRT | Atrioventricular reentry tachycardia |
| BMI | Body mass index |
| BP | Blood pressure |
| CABG | Coronary artery bypass graft |
| CCS | Canadian Cardiovascular Society |
| CCT | Cardiac computed tomography |
| CF | Contact force |
| CFAE | Complex fractionated atrial electrogram |
| CMR | Cardiac magnetic resonance |
| CNS | Cardiac nervous system |
| CPAP | Continuous positive airway pressure |
| CRT-D | Cardiac resynchronization therapy defibrillator |
| CS | Coronary sinus |
| CSANZ | Cardiac Society of Australia and New Zealand |
| CTI | Cavotricuspid isthmus |
| DAT | Diagnosis to ablation time |
| DOAC | Direct oral anticoagulant |
| EAM | Electroanatomical mapping |
| ECG | Electrocardiogram |
| ECGI | Electrocardiographic imaging |
| EHRA | European Heart Rhythm Association |
| ERP | Effective refractory period |
| ESC | European Society of Cardiology |
| FTI | Force time integral |
| GCV | Great cardiac vein |
| GP | Ganglionated plexi |
| HCM | Hypertrophic cardiomyopathy |
| HF | Heart failure |
| HFJV | High-frequency jet ventilation |
| HFLTV | High-frequency low tidal volume |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HRS | Heart Rhythm Society |
| ICD | Implantable cardiac defibrillator |
| ICE | Intracardiac echocardiography |
| ICM | Implantable cardiac monitor |
| INR | International normalized ratio |
| LA | Left atrium |
| LAA | Left atrial appendage |
| LAHRS | Latin American Heart Rhythm Society |
| LAPW | Left atrial posterior wall |
| LGE | Late gadolinium enhancement |
| LoE | Level of evidence |
| LIPV | Left inferior pulmonary vein |
| LMWH | Low molecular weight heparin |
| LSI | Lesion size index |
| LSPV | Left superior pulmonary vein |
| LVEF | Left ventricular ejection fraction |
| MRI | Magnetic resonance imaging |
| OSA | Obstructive sleep apnoea |
| PFA | Pulsed field ablation |
| PFO | Patent foramen ovale |
| PN | Phrenic nerve |
| PPI | Proton pump inhibitor |
| PV | Pulmonary vein |
| PVI | Pulmonary vein isolation |
| PWI | Posterior wall isolation |
| QoL | Quality of life |
| RA | Right atrium |
| RCT | Randomized clinical trial |
| RF | Radiofrequency |
| RSPV | Right superior pulmonary vein |
| SVC | Superior vena cava |
| SVT | Supraventricular tachycardia |
| TEE | Transesophageal echocardiography |
| TIA | Transient ischemic attack |
| TTI | Time to isolation |
| UFH | Unfractionated heparin |
| VKA | Vitamin K antagonist |
| VoM | Vein of Marshall |
2. Classification—atrial fibrillation pathophysiology
2.1. Definitions
Atrial fibrillation is the most common supraventricular arrhythmia characterized by rapid, disorganized atrial electrical activation leading to ineffective atrial contraction. The diagnosis of clinical AF requires rhythm documentation with an electrocardiogram (ECG) tracing. Electrocardiographic characteristics of AF include:
absence of distinct P waves on the surface ECG;
irregular atrial activations with an atrial cycle length that is usually <200 ms; and
‘absolutely’ irregular R–R intervals [when atrioventricular (AV) conduction is not impaired].
By convention, an AF episode is defined as an arrhythmia that has the ECG characteristics of AF and persists for at least 30 s in an ECG recording (or the duration of a 12-lead ECG).5 While the 30 s duration has been employed in previous published consensus statements, it is important to recognize that this duration of AF has not been associated with clinically meaningful outcomes or pathophysiological processes. While it has been proposed that 30 s of atrial tachyarrhythmia may be a harbinger of more advanced or clinically relevant disease, recent evidence suggests that may not be the case.6 Moreover, the 30 s sustained AF episode duration was defined in the era of non-invasive intermittent rhythm monitoring, and its relevance is unknown when applied to continuous rhythm monitoring [cardiac implantable electronic devices, implantable cardiac monitors (ICMs), or wearable devices (e.g. ECG-tracking smartwatches)].7
2.2. Classifications
Although there are several classification systems for AF, for this consensus document, we have continued to endorse the duration-based AF classification system employed by the American College of Cardiology/American Heart Association (AHA)/HRS, the Canadian Cardiovascular Society, the Cardiac Society of Australia and New Zealand, and ESC, with slight modifications (Table 4).5,8–11 This classification system broadly categorized AF into four clinical patterns, based on the clinical assessment of AF episode duration and persistence: (i) paroxysmal AF, defined as a continuous AF episode lasting longer than 30 s but terminating spontaneously or with intervention within 7 days of onset; (ii) persistent AF, defined as a continuous AF episode lasting longer than 7 days but <1 year; (iii) long-standing persistent AF, defined as continuous AF ≥1 year in duration, in patients where rhythm control management is being pursued; and (iv) permanent AF, defined as AF for which a therapeutic decision has been made not to pursue sinus rhythm (SR) restoration.
Table 4.
Proposed classification of atrial fibrillation
| Duration-based classification |
|---|
Paroxysmal—continuous AF episode lasting longer than 30 s but terminating spontaneously or with intervention within 7 days of onset
|
Persistent—continuous AF episode lasting longer than 7 days but <1 year
|
| Long-standing persistent—continuous AF episode lasting longer than 1 year, in whom rhythm control management is being pursued |
| Permanent—AF for which a therapeutic decision has been made not to pursue sinus rhythm restoration |
AF, atrial fibrillation.
It is important to recognize that permanent AF represents a therapeutic attitude on the part of a patient and the treating physician rather than on any inherent pathophysiological attribute of the AF. If a rhythm control strategy is recommended after re-evaluation, the AF should be redesignated as paroxysmal, persistent, or long-standing persistent AF. Early paroxysmal AF is defined as a continuous AF episode lasting longer than 30 s but terminating within 24 h of onset either spontaneously or with intervention. The 24 h duration was chosen based on the knowledge that important changes in AF-related electrical and structural remodelling occur over time frames as short as 24 h,12,13 leading to reductions in cardioversion14,15 and catheter ablation efficacy.16 Similarly, AF episodes >24 h have been associated with increased risk of ischemic stroke or systemic embolism, as well as increased cardiovascular hospitalization, all-cause hospitalization, and all-cause mortality.17–19 Early persistent AF is defined as continuous AF of more than 7 days of duration but <3 months of duration. Within the context of AF ablation and clinical trials of AF ablation, early persistent AF defines a population of patients in whom better outcomes of AF ablation are anticipated when compared with persistent AF of more than 3 months of duration.
A duration-based AF classification is a relatively straightforward schema that can be employed to standardize reporting, characterize the severity of disease, define patient populations in clinical trials of catheter and surgical ablation of AF, and form the basis of therapeutic recommendations regarding invasive arrhythmia management. However, it is important to recognize that clinical assessment of AF episode duration often underestimates the temporal persistence of AF when compared with long-term ECG monitoring, often leading to misclassification between paroxysmal and persistent AF.20,21 In addition, AF is a chronic progressive disease, evolving often from short paroxysms of AF to more frequent exacerbations of longer-lasting persistent AF. If both paroxysmal and persistent episodes are present, the classification should be defined based on the predominant AF pattern during the preceding 6 months.
2.3. Natural history of atrial fibrillation and atrial fibrillation progression
Atrial fibrillation is a chronic progressive disease characterized by exacerbations and remissions. Early in its course, AF is predominantly an isolated electrical disorder, triggered by rapid discharges originating mainly from the pulmonary veins (PVs), either secondary to enhanced automaticity or triggered activity from afterdepolarizations. These triggered impulses initiate and maintain AF through sustained rapid firing with secondary disorganization into fibrillatory waves. Although reentry is not usually sustained in a normal atrium, the presence of a vulnerable substrate can perpetuate AF through electrical heterogeneity [e.g. regional differences in conduction velocity, action potential duration (APD), and refractory period], with functional conduction abnormalities promoting reentrant activity and stabilizing reentrant circuits. Moreover, the cumulative effect of these intermittent AF episodes is electrical, contractile, and structural remodelling, with fibrosis promoting reentry through structural conduction abnormalities, and chamber dilatation promoting reentry. This atrial structural remodelling and worsening of atrial cardiomyopathy promote sustained arrhythmia and underpin the progression from paroxysmal to persistent forms of AF.22
While a wealth of experimental data exists regarding structural and functional atrial changes that contribute to the development, maintenance, and progression of AF, considerably less data exist regarding the natural history of AF. The reported rate of AF progression to non-paroxysmal AF types varies substantially due to differences in patient characteristics and comorbidities, study design (retrospective vs. prospective), follow-up duration (progression appears to be non-linear), and arrhythmia monitoring technology (e.g. most used intermittent rhythm assessments, which underestimate progression).7,22,23 Within these limitations, a proportion of patients presenting with their first AF episode will remain free of further recurrence, particularly if they are young and free of comorbidities at the time of index presentation.22,24–26 A metaanalysis of 47 studies reported that the incidence of progression from paroxysmal to non-paroxysmal AF was 7.1 per 100 patient-years of follow-up, with higher incidence in studies with shorter follow-up duration.23 In a relatively young and healthy population at low risk of AF progression, 7.4% of patients with symptomatic paroxysmal AF receiving first-line antiarrhythmic drug (AAD) therapy experienced an episode of persistent AF over a 3 year follow-up as documented by continuous rhythm monitoring with implantable cardiac device.27 A recent loop recorder study of 417 paroxysmal AF patients with 2.2 years of follow-up demonstrated progression to persistent or permanent AF in 8.4% (∼3.8% annually).28 For longer duration studies, the rate of progression has been reported to be 22–36% at 10 years.24,29,30 Importantly, while AF progression has been associated with worse outcomes, it is unclear whether progression is responsible for or merely a marker of a worse underlying substrate.31,32
Predictors associated with progression from paroxysmal to persistent AF include increasing age, the presence of structural cardiac pathology [left atrial (LA) dilatation], and an increasing burden of modifiable risk factors and concomitant risk conditions such as hypertension, diabetes mellitus, obesity, heart failure (HF), coronary artery disease, chronic kidney disease, chronic obstructive pulmonary disease, prior transient ischemic attack (TIA) or stroke, and obstructive sleep apnoea (OSA).25,30,33–36 Several biomarkers have also been associated with AF progression.28,37
2.4. Pathophysiology of atrial fibrillation
2.4.1. Genetics of atrial fibrillation
Atrial fibrillation is a complex disease where both environmental and genetic factors contribute to disease pathogenesis. Studies have shown familial aggregation and heritability of AF.38,39 After accounting for established clinical risk factors, individuals with a first-degree relative with AF have a 40% increased risk for AF development.40
The first rare pathogenic variant linked to familial AF was found in the Kv1.7 voltage-gated potassium channel.41 Since then, further variants have been identified in genes encoding potassium channels,42–48 sodium channel,49–51 and other non-channel proteins52,53 in patients and families with AF. In addition, genome-wide association studies comparing AF patients with the general population have associated a common variant at the 4q25 locus, a non-coding region of the genome near the gene PITX2, with a 60% increased risk of developing AF.54 Further genome-wide association studies have associated single nucleotide polymorphisms at more than 140 loci with AF.55–58 Single nucleotide polymorphisms identified by genome-wide association studies account for ∼22% of the risk of developing AF.59
Polygenic risk scores derived from these single nucleotide polymorphisms have been associated with stroke, outcomes after AF ablation or cardioversion, and response to certain rate and rhythm control medications.60 Larger, prospective, multi-ethnic studies will be necessary before clinical application of these scores can be considered.
It may be reasonable to refer patients with onset of AF earlier than 45 years old without any identifiable risk factors to an inherited arrhythmia clinic for consideration of genetic testing and family screening.60 The 2022 EHRA/HRS/APHRS/LAHRS expert consensus statement on the state of genetic testing for cardiac diseases supports analysis of specific genetic variants (SCN5A, KCNQ1, MYL4, and truncating TTN) in index patients in whom the diagnosis of familial AF is established, based on examination of the patient's clinical history, family history, and ECG characteristics.61 Currently, there is no role for routine clinical genetic testing in older patients presenting with AF in the absence of familial disease.61
2.4.2. Molecular basis of atrial fibrillation
Atrial fibrillation triggers resulting from ectopic activity within the atria are linked to spontaneous diastolic Ca2+-release from the sarcoplasmic reticulum via leaky ryanodine receptor channels. Early afterdepolarizations due to loss-of-function mutations in outward potassium channels, or gain-of-function mutations in inward calcium channels leading to a reduced repolarization reserve, have also been linked to spontaneous ectopic activity.62,63 The canine PVs have been shown to have smaller inward rectifier K+ current (IK1) and L-type Ca2+ current (ICa,L), as well as larger delayed rectifier K+ currents, compared with the LA cells.63
Conduction abnormalities have a role in AF pathophysiology, presumably by increasing susceptibility to reentry and maintenance of AF. The most important determinants of conduction are as follows: (i) structural integrity of atrial tissue, often disrupted by fibrosis; (ii) effective cell-to-cell coupling, principally determined by connexin hemichannels in intercalated disks; and (iii) integrity of the rapid phase-0 Na+ current (INa), which provides the electrical energy for conduction.63,64
2.4.3. Mechanisms of atrial fibrillation initiation and maintenance
2.4.3.1. Role of triggers and automaticity
Atrial fibrillation is initiated by triggers and then sustained by distinct mechanisms for longer durations. Ectopic activity, particularly occurring in the PVs, has been shown to have a central role in initiation of AF.65 Variances in the ion channels and the structure of PV tissue predispose to ectopic activity by (i) reducing APD leading to reentry and (ii) increasing delayed afterdepolarizations (DADs) due to aberrant Ca2+-release leading to spontaneous ectopy.63,66 Clinically, PVs are noted to have smaller electrogram voltages, slower conduction, shorter effective refractory period (ERP), and a greater vulnerability to AF induction during programmed electrical stimulation.67 Embryologically, the posterior wall of the LA has the same origin as the PVs and therefore is considered to have a similar arrhythmogenic role.68 Other sites of triggered activity include the superior vena cava (SVC), the ligament of Marshall, and the LA appendage (LAA), although atrial sites beyond PVs are less clearly linked to AF initiation.69
2.4.3.2. Role of focal and rotational activity and spiral waves
The concept of small rapidly rotating circuits postulates that fibrillatory conduction is maintained by AF-perpetuating drivers or localized regions that activate faster compared with the surrounding atrial tissue.70,71 Rotational and focal drivers of AF have been identified near regions of fibrosis by optical mapping of ex-vivo animal hearts, ex-vivo human atria, and in-vivo human atria.72–74
Unfortunately, the tools required to demonstrate rotational and focal drivers of AF are limited by the complexity of assessing intracardiac electrograms during fibrillatory conduction, particularly in reference to the accurate identification of local activation timings.75–77
2.4.3.3. Role of multi-wavelet reentry
The multiple wavelet concept was initially proposed by Garrey,78 refined by Moe et al.79 with computer modelling studies, and later supported by Allessie and colleagues80 with mapping of AF in canine atria and human atria.81 The multiple wavelet theory proposes that multiple AF-perpetuating wavelets self-replenish by collision, facilitated by structural obstacles and conduction dissociation between the endocardial and epicardial surfaces of the atrial wall. This theory implies that extensive ablation is required to limit the surface area of conduction and resolve constant replenishment of fibrillatory wavelets. Recent mechanistic evidence from computational models also suggests that smaller areas for fibrillatory waves to propagate are associated with improved long-term postablation outcomes in persistent AF.82
2.4.3.4. Role of endocardial–epicardial asynchrony
Recent data have found that despite the relatively thin-walled atria, the complex LA anatomy has a structure that, combined with the progression of intramural fibrosis, can contribute to AF maintenance by providing a larger three-dimensional (3D) substrate that increases the probability of intramural reentry and AF maintenance. Preclinical and clinical surgical high-density mapping studies have found that activation is often asynchronous and dissociated during AF, likely exacerbated by slow conduction and intramural conduction delay and block.74,83,84 These findings have been confirmed in right atrium (RA) recordings in humans with AF undergoing cardiac surgical procedures85–88 and in LA simultaneous endo-epicardial recordings of patients undergoing catheter ablation of AF.89,90 Such findings further increase the complex nature of AF and may explain why mapping from the endocardium or epicardium alone has failed to identify the true underlying mechanism of AF.
In summary, the presently available data suggest that both ectopic activity and reentry play important roles in AF initiation and maintenance of fibrillatory conduction. Moreover, localized driver sites may have a role in AF maintenance independent of the initiating mechanism. The specific mechanisms and determinants remain to be elucidated, along with their implications for therapy.
2.4.4. Structural and electrical remodelling in atrial fibrillation
2.4.4.1. Structural remodelling
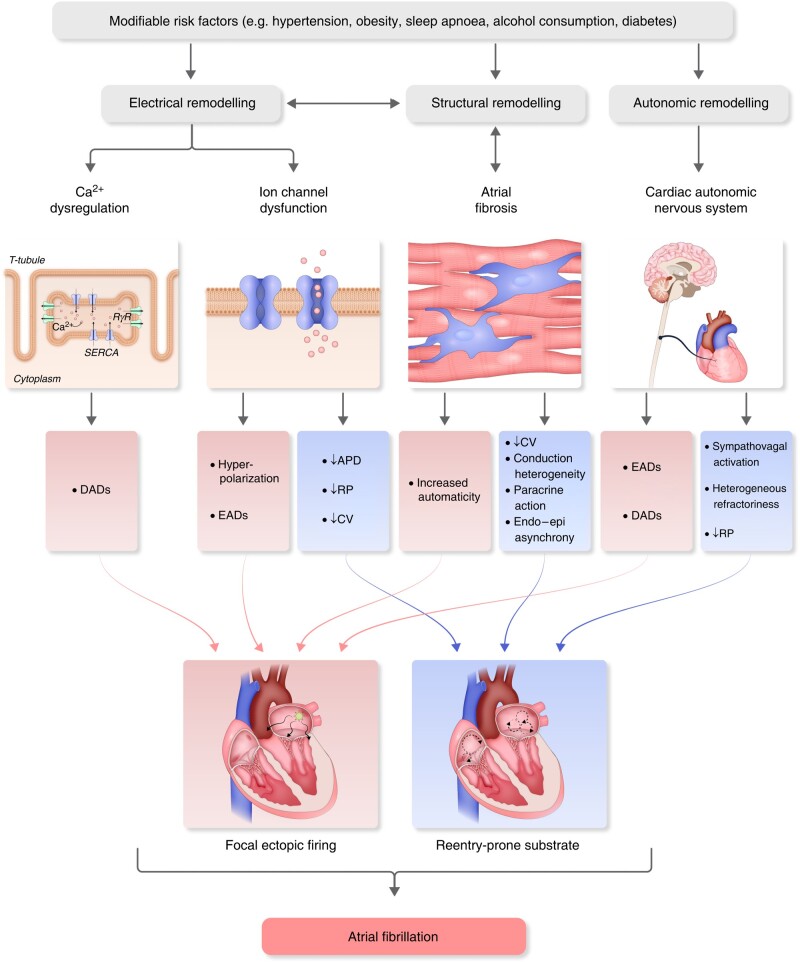
The atria of patients with AF often show evidence of structural remodelling. The easiest type of structural change to recognize is LA enlargement, which is seen in many AF patients and correlates with disease progression and outcomes.91,92 Atrial enlargement provides more atrial tissue to harbour disordered wavelets or drivers and also correlates with the presence of fibrosis.93 Atrial fibrosis can be a result of the electrical remodelling of AF, AF-related risk factors, or a fibrotic atrial cardiomyopathy.64,93–96 The mechanisms of fibrosis and its consequences comprise many phenomena at molecular, cellular, organelle, and tissue levels.97 At the molecular level, dynamic changes occur in the genome, the transcriptome, and the signalling pathways underlying the generation of profibrotic molecules.98 Cellular changes involve interactions among the various cardiac cells, including myocytes, fibroblasts or myofibroblasts, and inflammatory cells such as macrophages and neutrophils.98,99 Tissue changes relate to the dynamics of scar, angiogenesis, electrical conduction, and contractility.100 Fibrosis may also increase the number of fibroblasts, promoting AF by altering the electrophysiological behaviour of cardiomyocytes coupled to fibroblasts through cardiomyocyte–fibroblast interactions99,101 (Figure 1).
Figure 1.
Pathophysiological mechanisms of atrial fibrillation. APD, action potential duration; CV, conduction velocity; DADs, delayed afterdepolarizations; EADs, early afterdepolarizations; RP, refractory period; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase.
Atrial fibrosis results in heterogeneous electrical conduction and repolarization and may facilitate multiple wavelet reentry or anchor driver regions.102 Clinically, identification of atrial fibrosis has been challenging, with promising techniques including detection of increased signal intensity on gadolinium-enhanced magnetic resonance imaging (MRI)103 or identification of low amplitude electrical signals at invasive electrophysiology study,104,105 although a mismatch between these techniques has been suggested106 (Section 5.2.1.4.).
Another potentially important factor in AF-related atrial remodelling is fatty infiltration, which is known to increase in several pathophysiological conditions and is regarded as arrhythmogenic.107,108 Epicardial fatty infiltration occurs with obesity and has been associated with AF via structural and electrical remodelling of the atria, via direct infiltration of adipose tissue into the atrial tissue, and via indirect mechanisms through paracrine modulators resulting in inflammation and oxidative stress109,110 (Section 5.2.1.4.).
Myocardial infiltration by amyloid deposits may also disturb atrial conduction in cardiac amyloidosis.111 Patients with long-standing AF and rheumatic heart disease have a very high prevalence of atrial amyloidosis.112 Isolated atrial amyloidosis is more prevalent than amyloid light chain (AL) amyloidosis or wild-type (senile) transthyretin cardiac amyloidosis, with a prevalence of >90% in the ninth decade.113 Pathophysiologic association between amyloidosis and AF is still under investigation but is considered to relate to structural abnormalities similar to atrial fibrosis.
2.4.4.2. Electrical remodelling
Electrical remodelling in AF patients involves shortened atrial refractory periods from down-regulation of Ca2+ currents, shortened repolarization and hyperpolarization of atrial cells from increased outward K+ currents, and conduction slowing from altered expression and localization of connexins between myocytes114 (Figure 1). Oxidative stress, atrial dilatation, microRNAs, inflammation, and myofibroblast activation also have a role in electrical remodelling.64
Electrical remodelling, manifested as shortening of atrial refractoriness, develops within the first few days of AF.100,115 Several ion channel modifications underlying such electrical changes have been described in animal models and humans.114,116–118 Dominant frequency of AF is shown to increase gradually after AF onset, stabilizing within 2 weeks. These dominant frequency changes are associated with down-regulation of ICaL and INa and up-regulation of IK1, along with corresponding mRNA or protein changes. Interstitial fibrosis develops at 6–12 months, highlighting increasing tendency of AF to persist over time.119,120 Sustained AF shortens APD and ERP, decreasing the wavelength and facilitating the acceleration and stabilization of sustained reentry. The primary determinants of APD shortening are the decrease in ICaL and increase in IK1.119 Rapid atrial rates can activate fibroblasts and increase collagen gene activity, promoting fibrosis and structural remodelling.121
2.4.5. Autonomic nervous system and its role in atrial fibrillation pathophysiology
The electrophysiology of the heart is highly influenced by the autonomic nervous system (ANS; Section 3.7.). Initiation and termination of AF episodes have been linked to changes and abnormalities in cardiac autonomic tone.122–124 At the whole heart and cellular levels, both extrinsic and intrinsic autonomic modulations have been shown to produce early or DADs that trigger ectopic firing and contribute to AF maintenance.125–130
Autonomic interventions have been shown to modulate AF occurrence. A small randomized trial of vagal stimulation via the tragus reduced AF burden over 6 months.131 This effect may be mediated by up-regulation of small conductance calcium-activated potassium channels in the stellate ganglion.132 Spinal cord stimulation has also demonstrated a protective effect on AF inducibility in a tachypacing model.133
Due to the inter-relationship between the sympathetic and parasympathetic ANS components, it is not possible to perform selective modulation of the parasympathetic or the sympathetic nervous system alone with direct ablation at ganglionated plexi (GP) sites. However, ablation targeting GP sites has been shown to modulate cardiac autonomic tone and AF inducibility.134–137 Due to their anatomic location in proximity to the PVs, these GP sites may actually be ablated during a standard PV isolation (PVI) procedure.
3. Anatomical considerations—implications for catheter ablation
3.1. The pulmonary veins—typical anatomy and variants
Atrial fibrillation is regarded as a primarily LA arrhythmia, mainly because AF episodes are initiated most commonly by atrial extrasystoles emanating from the PVs. Since the ground-breaking publication of Haïssaguerre et al.,65 multiple studies have shown that unique anatomic features of the PV myocardial sleeves or extensions enable focal automaticity.66,138 In addition to the enhanced focal activity of the PV themselves, anisotropic, heterogeneous conduction in the PV antra creates an environment prone to microreentrant activity, acting like a ‘repeater’ augmenting single ectopics into a burst of fibrillatory activity or PV tachycardia139,140 (Section 2.4.3.).
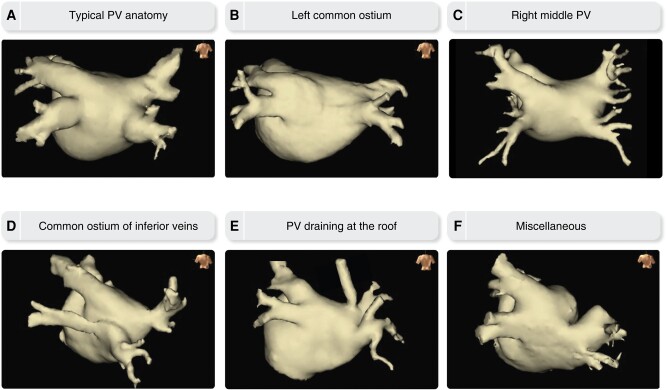
The entrance of the PVs to the LA is located on the superior–posterior part, with the inferior PVs entering the LA inferiorly but also posteriorly to the superior PVs. The typical PV branching pattern comprises four separate PV ostia, with a pair of superior and inferior PVs on the left and right posterior aspect. Most common PV variants include a common trunk (either short or long) of the left-sided PVs and an additional (middle) PV on the right side.141,142 Rarely, other atypical variations in PV anatomy may be encountered including an accessory PV draining at the LA roof, a common superior or inferior conjoined vein, and three or even all four PVs entering LA together with a common trunk141–143 (Figure 2).
Figure 2.
Typical PV anatomy and common variants. PV, pulmonary vein.
Myocardial sleeves extend into the PVs ∼2–3 cm from the PV–LA junction, often taking a spiralling course.144 Additionally, the thickness of the LA wall in the region of the PV antra varies from 2 mm (posterior wall) up to 8 mm at the ridge separating the left superior PV (LSPV) from the LAA.68,141,145 This variance in target lesion depth is one of the challenges in safely achieving transmural and durable PVI.146
3.2. Pulmonary vein epicardial connections
Besides the knowledge of typical PV anatomy and related variants, it is also critical to understand the concept of epicardial connections between PVs and other adjacent atrial structures as it can strongly influence short-term and long-term achievement of PVI. Although difficult to evaluate, their overall prevalence appears to be as high as 13.5%.147 The presence of underlying structural heart disease or a patent foramen ovale (PFO) is associated with a higher prevalence of epicardial connections, whereas a left common trunk is associated with absence of epicardial connections.147,148 Several studies have reported the anatomical distribution and functional impact of these epicardial connections.147–151
More than half of epicardial connections are located in the left PVs and are mediated by the ligament of Marshall.147 As described hereafter, the ligament of Marshall is an epicardial structure containing the vein of Marshall (VoM), the Marshall myocardial bundle, and autonomic nerves. Post-mortem studies have revealed that, unlike other atrial tracts, the ligament of Marshall is distinctly segregated and insulated from the underlying LA myocardium and connects directly to the coronary sinus (CS) musculature and the LA free wall at the level of the left inferior PV (LIPV).152,153
Epicardial connections are also located in the right PVs connecting them with the RA or less frequently with distinct areas of the LA. In the former, epicardial connections are supported by muscular strands that connect the muscular sleeves of the right PVs to the RA.153–155 Epicardial connections between the right PV and the posterior wall of the LA have also been described suggesting variants of the septopulmonary bundle that link the right carina with the posterior wall.147,148,150,156
3.3. Fossa ovalis—interatrial septum (implications for transseptal puncture)
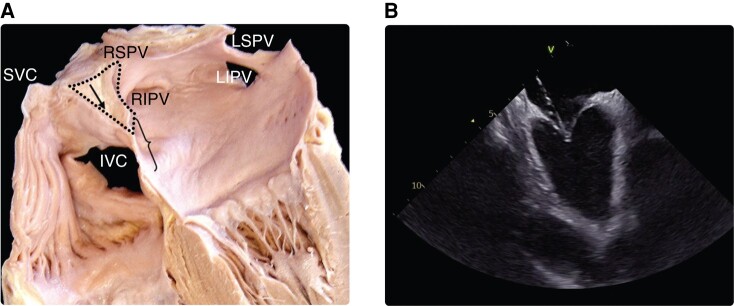
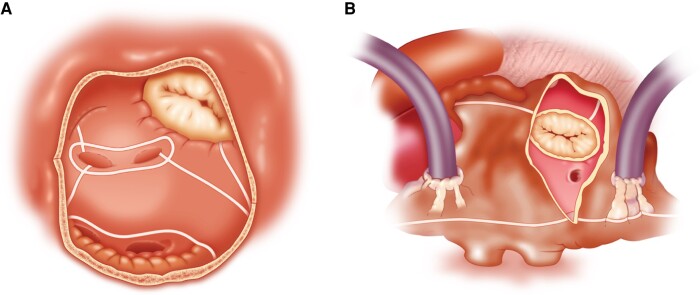
During cardiac development, a complex advancement, growth, and migration of atrial tissue forming the septum primum and then the septum secundum allow the formation of the interatrial septum, which eventually separates left from RA.145,157 During this process, the fossa ovalis is formed, which is where the septum primum overlies the septum secundum. The fossa ovalis represents the thinnest part of the septum and thus is the ideal location for transseptal puncture.145,157,158 It has an average vertical diameter of 18.5 ± 6.9 mm and an average horizontal diameter of 10.0 ± 2.4 mm.159 The septal area located superiorly (cranially) to the fossa ovalis is formed by an infolded groove of the atrial wall between the SVC and the right PVs and contains extracardiac adipose tissue.159 Inadvertent puncture of this area must be avoided since it may result in interatrial septum dissection, atrial wall hematoma, or tamponade160 (Figure 3).
Figure 3.
(A) Anatomy of interatrial septum and optimal site of transseptal puncture (demarcated with a brace). Black arrow in the dotted area shows the infolded groove of the atrial wall between the SVC and the right PVs filled with extracardiac fat tissue. (B) Intracardiac echo view of typical tenting before transseptal crossing. Modified from Tzeis et al.159 IVC, inferior vena cava; LIVP, left inferior pulmonary vein; LSVP, left superior pulmonary vein; PV, pulmonary vein; RIVP, right inferior pulmonary vein; RSVP, right superior pulmonary vein; SVC, superior vena cava
In ∼25–28% of patients, the two membranes that comprise the fossa ovalis do not fuse, so that a PFO is present. This defect varies considerably in size, from a more slit like formation to defects of 19 mm size, with a mean reported PFO diameter of 5 mm.161–163 Although the fossa ovalis is considered to be the optimal site for transseptal puncture, crossing the septum via a PFO during AF catheter ablation has several limitations, since the PFO is located very cranially and anteriorly at the septum, thus impeding access to the caudal parts of the LA (including the inferior PVs) and the right superior PV (RSPV), where a steep turn is needed to enter. Hence, some operators prefer to perform transseptal puncture inferior and posterior to a present PFO. Several observational studies have shown that use of a PFO to gain access in the LA during AF catheter ablation does not adversely affect ablation efficacy when compared with needle-assisted LA access.164,165 However, the presence of a large and/or compliant PFO has been reported as independent predictor of PVI failure and increased arrhythmia recurrence rate following AF catheter ablation.166
In contrast, ‘true’ atrial septum defects (ASDs) are usually located at the site of a transseptal puncture and offer a very convenient access to the LA and the PV regions. However, an ASD with a relevant left-to-right shunt results in RA volume load with subsequent increased arrhythmogenic remodelling. The latter should be taken into account when individualizing AF ablation approach, since in the presence of an ASD, the RA is likely implicated in AF initiation and maintenance and thus should be evaluated as potential ablation target.158,167
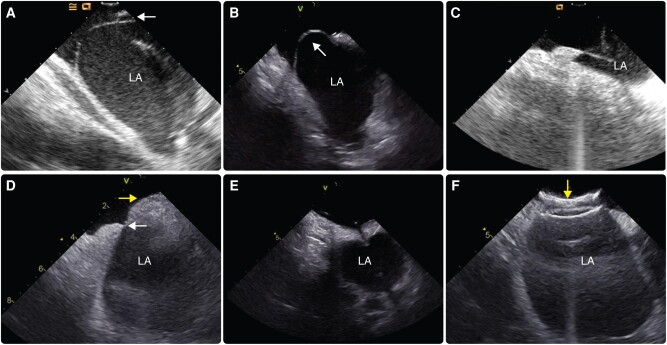
The rare variant of an atrial septum aneurysm (∼1–2% of patients) can complicate transseptal puncture. Most commonly, the aneurysm comprises a ‘floppy septum’, which means that true crossing of the septum requires pushing the transseptal needle almost to or even beyond the most left lateral boundaries of the LA, risking a perforation of the LA. Available technologies that facilitate crossing of the septum in challenging anatomies are presented in Section 7.5.157,158,161,167,168 Anatomic variations of interatrial septum and clinical settings that may be encountered during transseptal puncture are presented in Figure 4.
Figure 4.
Anatomic variations of the interatrial septum that may be encountered during transseptal puncture. (A) Patent foramen ovale (white arrow); (B) septal aneurysm with large excursion towards the right atrium (white arrow); (C) tenting of floppy septum from transseptal needle close to the left atrial wall; (D) very small fossa ovalis (white arrow) in a patient with lipomatous septal hypertrophy (yellow arrow); (E) standard transseptal needle crossing a pericardial patch; (F) atrial septal closure device (yellow arrow) covering almost all of the interatrial septum. LA, left atrium.
Some patients with AF may have had prior surgical or percutaneous ASD closure. Surgical closure of an ASD with a stitch typically does not impede subsequent transseptal puncture. Use of a pericardial patch to close the ASD may impede crossing of the septum, but there is often room to cross above or below the patch. Direct puncture through the patch with a radiofrequency (RF) needle is also feasible. Percutaneous closure devices can pose more of a challenge. Typically, there is a room inferior–posterior to most ASD closure devices for transseptal access through the native septum using the usual transseptal tools.169 Occasionally (Figure 4F) an ASD device may cover the entire septum. Crossing through an ASD closure device has been described but should be reserved for highly experienced centers.170
3.4. Architecture of left atrial musculature
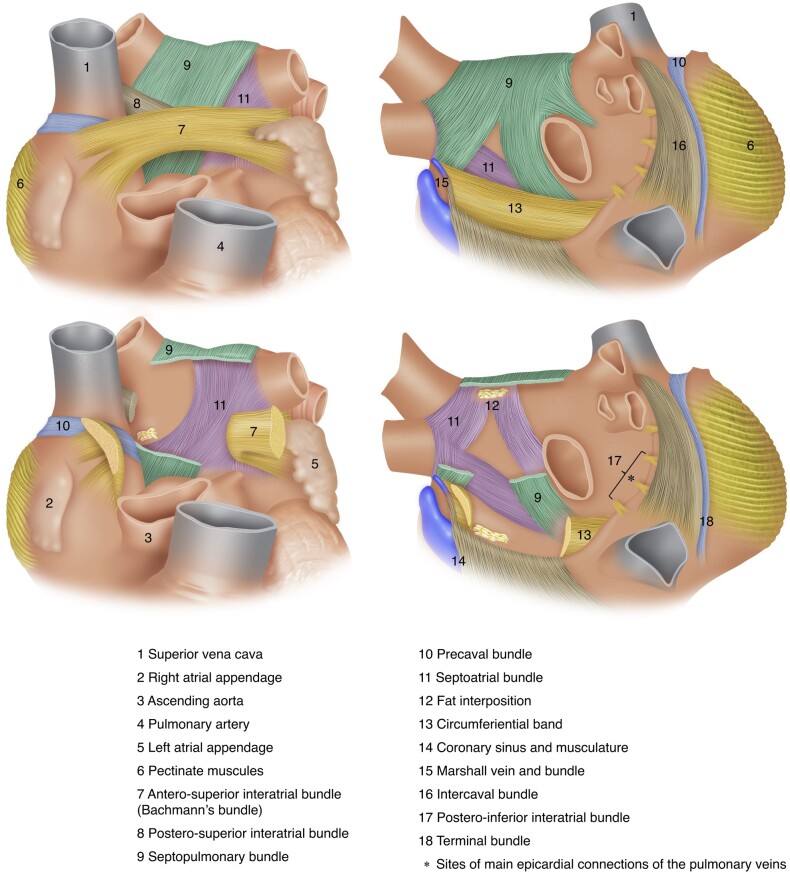
The orientation of the major atrial muscular bundles has been recognized from anatomical dissections, with mostly circular bundles around the ostia of the PVs, AV valves, and LAA.171 The body of the LA is comprised of the venous component located posteriorly, the septum, and the vestibular portion, which forms the ‘LA outlet’.155 The vestibule partly forms the mitral isthmus located between the orifice of the LIPV and the annular attachment of the mitral valve.155,172 Several anatomical isthmuses can be identified between these native obstacles, which have the potential for supporting reentry.173–175 The body of the LA has relatively smooth wall with a complex architecture of overlapping myofibres of different orientation. The most prominent interatrial muscular connection is the Bachmann's bundle comprised of atrial myocardial strands aligned in a parallel fashion. It extends from the right of the SVC orifice, crosses the interatrial groove, and courses along the anterior wall of the LA until the LAA where it divides into two branches that encircle it.171 The superior part continues along the left lateral ridge and the inferior part towards the atrial vestibule and then merge into the musculature of the lateral and inferoposterior atrial wall176 (Figure 5).
Figure 5.
Architecture of atrial musculature. Upper left: main atrial muscular bundles from anterior view. Lower left: transection of the Bachmann's bundle, postero-superior interatrial bundle, and the septopulmonary bundle enables visualization of the septoatrial bundle. Upper right: main atrial muscular bundles from posterior view with slight rightward tilting—the stars denote epicardial connections of the right PVs with the right atrium and left atrium posterior wall. Lower right: transection of the septopulmonary bundle coursing epicardially enables visualization of the septoatrial bundle and neighbouring fat inter-position. PV, pulmonary vein.
In 1920, Papez177 first described the septopulmonary bundle and the septoatrial bundle. This terminology directly reflects their different course through the LA components previously described. The two bundles arise from the septum, but the septoatrial bundle preferentially covers the LA body (as well as the LAA and the vestibule), while the septopulmonary bundle mainly encircles the PVs. Both bundles course along the dome and the posterior wall, where the septopulmonary bundle epicardially overlaps the septoatrial bundle to form a bilayer architecture. Until recently, these bundles were not considered to be separated by a layer of insulating tissue.154,171,177,178 Recently, the septopulmonary bundle has been described to be separated from the LA body by fat inter-position. This intervening fat layer may act as an insulation preventing transmission of ablation energy to the epicardially situated septopulmonary bundle and thus impairing the achievement of durable PVI, complete roof line, or posterior wall isolation (PWI).179
3.5. Coronary sinus—vein of Marshall
The coronary venous system, with the CS located at its most proximal part, drains ∼85% of the venous flow into the RA. The great cardiac vein (GCV) ascends into the left AV groove, where it passes close to the circumflex artery and under the cover of the LAA. The CS has an individualized musculature separated from the LA myocardium by fat, with sparse connections to the posterior wall via discrete muscular tracts.180 The juncture between the GCV and the CS is marked by the entrance of the VoM.181
The CS-VoM musculature has an arbourized layout. A primary bundle running epicardially along the vein displays secondary bundles insulated into fibro-fatty tissue. Following an epi-endocardial course, these secondary bundles join at the bottom with the LA free wall myocardium.180,182–184 A muscular continuum is observed from the CS to the left PVs, using the VoM as a hub: its primary bundle is connected to the CS musculature near the Vieussens valve, while its secondary bundles are connected to the left PV sleeves at the ridge.
The VoM is an embryological remnant of the left upper caval system resulting from the involution of the left anterior cardinal vein.185 This vestigial structure is separated into two portions: (i) the extracardiac portion, named the ligament of Marshall, is contained in a fold of pericardium, occluded in almost all cases and associated with branches of extrinsic cardiac nerves and (ii) the intracardiac part that extends from the left lateral ridge (between LAA and left PVs) to the CS, maintaining patency at different distance from its connection with the CS, forming the VoM (also known as oblique vein of the LA). The VoM has an epicardial myocardial sleeve (the Marshall bundle) and neighbours with closely associated autonomic nerve fibres and fat.182,183,186 The Marshall bundle is an insulated muscular structure that connects to the LA myocardium at the level of the left PVs with limited connections to the underlying myocardium along its epicardial course. Several studies have demonstrated that the muscular fibres of the VoM and adjacent structures have a multi-faceted proarrhythmic potential, since they may be the source of focal activities, part of reentry circuits and autonomic modulators.187–191 Being co-localized with arrhythmogenic structures, the VoM may represent an ablation target beyond PVI during AF catheter ablation (Section 8.2.7.).
The VoM has close anatomical relationship with the mitral isthmus, located between the mitral annulus and the LIPV ostium155,192 with practical implications during ablation attempts at the mitral isthmus either for LA substrate modification or for the treatment of perimitral flutter.172,193 Achievement of mitral isthmus block may prove challenging not only due to mitral isthmus wall thickness but also due to its complex anatomy including: (i) the thick left lateral atrial wall, rarely exceeding 4 mm,152,194 (ii) the VoM,195,196 and (iii) the GCV with its musculature extending over 2–40 mm, either at the anchored or free wall of the vessel.197
3.6. Superior vena cava
Apart from the PVs, the SVC also exhibits myocardial sleeves that extend as much as 4–5 cm cranially into the vein.145,198 Increased length of SVC myocardial sleeves and increased SVC diameter are reported as independent predictors of SVC firing in AF patients undergoing catheter ablation.199 However, the SVC myocardium has different origin than the myocardial sleeves of the PVs, and hence, the arrhythmogenic potential of the SVC is not prominent. This seems to be especially true for the influent or antral region of the SVC, which is not known to have such anisotropic or heterogenous conduction properties as the PV antral region.200 Several studies have reported that the SVC acts as an extra-PV trigger in 2–6% of patients.198,201 In such settings, SVC isolation is usually attempted. Superior vena cava isolation can be complicated by sinus node dysfunction due to close vicinity of the sinus node to the lateral influx of the SVC into the RA. Delivery of RF energy should be avoided in the sinus node region at the base of the right atrial appendage joining the SVC, and ablation should be interrupted if sinus acceleration or deceleration is observed. Furthermore, collateral damage may occur to the neighbouring right phrenic nerve (PN), which should be clearly delineated with high-output pacing prior to ablation202 (Figure 6; Section 3.9.).
Figure 6.
Course of the right phrenic nerve in relation to neighbouring structures in different projections (A: right anterior oblique; B: right lateral; C: right posterior oblique)—reconstruction from computed tomography scan. IVC, inferior vena cava; LA, left atrium; RA, right atrium; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava.
3.7. Autonomic ganglionated plexi
The cardiac nervous system (CNS) plays a crucial role in arrhythmogenesis and more specifically in the initiation and maintenance of AF. The CNS is divided into the extrinsic and the intrinsic CNSs.145,202–204 The extrinsic CNS consists of sympathetic and parasympathetic components and includes neurons in the brain and spinal cord and nerves directed to the heart.205 The extrinsic parasympathetic fibres are carried almost entirely within the vagus nerve.206 The extrinsic sympathetic fibres are largely derived from the autonomic ganglia along the cervical and thoracic spinal cord.204,206 The intrinsic ANS includes autonomic nerve fibres once they enter the pericardial sac, forming a complex network composed of GPs, concentrated within epicardial fat pads.207,208 These GPs function as integration centers between extrinsic and intrinsic cardiac ANSs and contain predominantly parasympathetic, as well as sympathetic neurons.203,204,209
Ganglionated plexi are most commonly located at the anterior–superior LA (close to the SVC–RA junction and the anterior aspect of the RSPV), at the inferior–posterior RA/LA junction (adjacent to the interatrial groove), at the lateral-posterior (close to LIPV) and lateral-superior LA (between LAA and LSPV), and in proximity to the VoM.202–204,209
Localization of GPs is feasible with nuclear imaging studies and intraprocedurally with high-frequency stimulation to elicit a vagal response.208,210–213 However, due to their common localization close to the PVs, it is estimated that the GPs are ‘collaterally ablated’ in 20–50% of AF patients undergoing wide antral circumferential PVI. In line with this, a substantial proportion of patients display signs of autonomic modulation, e.g. changes in mean heart rate or heart rate variability, following PVI, a finding which has not been observed in pulsed field ablation (PFA)-treated patients.214,215 Some studies have shown that such an increase in resting heart rate after PVI is associated with a more favourable prognosis.216–218
3.8. Pericardial reflections
Although less frequent than in ventricular arrhythmia management, pericardial access is sometimes required for the treatment of atrial arrhythmias. Alternative to the conventional endocardial ablation, hybrid strategies have been proposed to improve the transmurality of lesions created during AF ablation with favourable impact on arrhythmia outcome219–221 (Section 12). In selected patients, epicardial approach might be an option for second or third ablation strategy to achieve transmural block in areas with protected epicardial connections.222–224 Therefore, it is important to familiarize with the anatomy of the pericardium and its anatomic characteristics that impair accessibility in specific areas of the LA during epicardial mapping and ablation. The normal pericardium is a double-layered sac consisting of an outer fibrous envelope and an inner serous sac (divided into a visceral layer and a parietal layer) that is invaginated by the heart. The visceral layer is reflected from the heart back onto the parietal layer along the great vessels including the aorta, pulmonary artery, proximal PVs, and vena cavae. These reflections define recesses and sinuses that constrain catheter manipulation. Therefore, epicardial mapping of the anterior wall or the mitral annulus is unimpeded, whereas the network of pericardial sinuses at the posterior wall limits the catheter from crossing the dome, roof, and carina on both sides.224,225
There are three sinuses in the pericardial space. The superior sinus is situated along the right side of the ascending aorta. The transverse sinus is located behind the great vessels and has the LA dome as an anterior boundary. Its exploration allows access to the antero-superior aspect of the LA. The oblique sinus extends behind the LA between the four PVs. Its exploration allows access to most of the inferior part of the dome and the posterior wall. However, superiorly, the oblique sinus is separated from the transverse sinus by the pericardial reflection connecting left and right PVs. Thus, the middle part of the dome remains inaccessible for epicardial mapping.
3.9. Phrenic nerves
Ablation-induced damage of the PNs (mainly the right one) is a possible complication of AF catheter ablation (Section 11). The anatomical relationship of the right PN to the right PVs is complex, due to the course of the PN in between the RA and LA: cranio-caudally, coming from the lateral aspect of the SVC, it runs between both atria along the antero-septal portion of the RSPV and turns then via the posterior RA to the lateral RA, where it crosses very often the crista terminalis145,202 (Figure 6). Thus, damage occurs most frequently while isolating the RSPV, especially while using balloon devices.226,227 There are several potential reasons why a (transient or permanent) palsy of the right PN may occur significantly more often with balloon-shaped than point-by-point RF ablation. First, balloon devices are—by their shape and technical design—placed inside the PVs, and ablation energy is also delivered (in part) inside the PVs.226 Thus, the PN, which runs along the PV, is more often comprised within the most distal extensions of the ablation lesion. Furthermore, the balloon is inflated in the PV with the purpose to obtain maximum contact and occlusion of the PV by the balloon. Therefore, the PV tissue is circumferentially stretched and the PV diameter is enlarged, placing the PN closer to the ablation lesion. Proposed measures to prevent the occurrence of PN palsy/paralysis are reported in Section 11.226,228 Larger diameter of the right PVs and a flat angle between the right PV and the LA body are reported to predict PN damage during PVI, whereas an enlarged LA is potentially protective.228
There is also an anatomic relationship between the left PN and the LAA, but damage to the nerve is rare when using endocardial ablation techniques. This is because the PN remains along the whole course on the pericardial surface and does not enter the pericardial space or the epicardium, so that the distance between the endocardial surface close to the PVs and the left PN is usually more than 7–10 mm. Localization and mapping of the left PN with high-output pacing is feasible and avoids its inadvertent injury during LAA isolation using RF or cryoballoon ablation.229 During surgical/epicardial ablation, protective measures similar to those taken during endocardial PVI for the right PN are recommended.
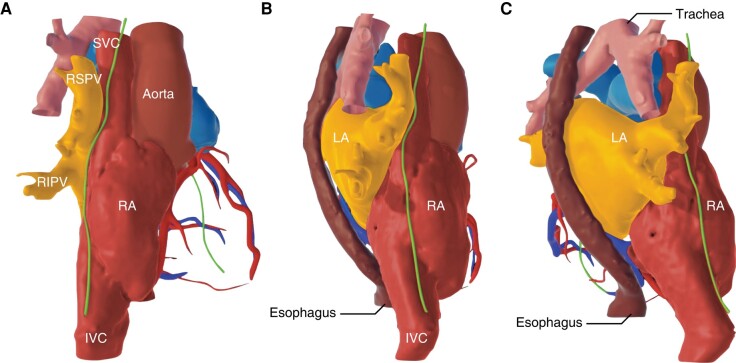
3.10. Esophagus
Thermal injury to the esophagus by ablation energy is one of the most dangerous and frequently fatal complications of AF ablation230 (Section 11). The anatomic course of the esophagus is variable but is more commonly closer to the left PVs145,202,230–233 (Figure 6). However, it should be kept in mind that the esophagus is a mobile structure, and its relative position may change intraprocedurally especially when the patient remains under conscious sedation, allowing esophageal peristalsis to occur.234 In 67% of patients undergoing AF catheter ablation, the esophagus shifts sideways by ≥2 cm, while in 4%, there is a lateral movement exceeding 4 cm.235 Furthermore, the location, size, and shape of the esophagus may be affected by the presence of common esophageal abnormalities such as hiatal hernia.
Apart from the distance between the esophagus and the LA posterior wall (LAPW), another anatomical factor that influences the probability of thermal esophageal injury is the presence of pericardial fat pads around each PV that are located between the LA and the esophagus and may protect against esophageal lesions during ablation.230,232,233 Most of the inferior PVs are not covered by fat pads.232
Furthermore, the movement of the esophagus may be restricted by surrounding mediastinal structures, like the descending aorta or the spine. In these cases, if the LA wall is ‘tented’ by the ablation catheter posteriorly towards the esophagus, the latter remains entrapped, so that the full impact of the applied energy is absorbed by the esophageal wall. If the ablation also damages the arterioles supplying the esophagus, impairing blood flow to the affected esophageal tissue, the resultant ulcerations may not heal and may progress to perforation and fistulaization to the pericardium and/or to the LA232,233 (Section 11).
4. Indications for catheter ablation of atrial fibrillation
|
This section presents the consensus of the writing group on the indications for catheter ablation of AF. Suggested advice has been formulated based on the presence of AF-related symptoms and the duration-dependent type of AF (Section 2) but also in specific patient groups. Advice pertaining to the management of patients with persistent AF is also applicable to those with long-standing persistent AF. The writing group decided not to issue a separate set of advice for long-standing persistent AF due to lack of specific evidence and a high degree of similarity with the management of persistent AF patients.
The final decision regarding patient eligibility for catheter ablation should be refined on an individualized basis, considering factors that influence rhythm outcome including among others age, duration of AF episodes, comorbidities, atrial dilatation, and presence of fibrosis. Furthermore, the selection of catheter ablation vs. AADs for rhythm control may also depend on the underlying clinical setting, which may limit the use of several AADs and/or may reinforce the need for SR maintenance due to associated prognostic benefit. Therefore, the selection of optimal management strategy should be guided by a balanced analysis of the potential clinical benefits of reducing AF burden, the likelihood of achieving it, and the associated risk of complications. Finally, patient preferences should be taken into consideration in a shared decision-making process.
4.1. Catheter ablation in patients with atrial fibrillation–related symptoms
Patients with AF may experience different types of symptoms including palpitations, dyspnea, dizziness, fatigue, pre-syncope, and syncope. The presence and intensity of AF symptoms may vary significantly even in the same patient. Several symptom scales [EHRA score, Canadian Cardiovascular Society Severity of Atrial Fibrillation (CCS-SAF) scale] have been developed to assess AF-related symptoms in a more standardized approach.280,281 The documentation of correlation between symptoms and underlying rhythm in patients with intermittent AF is challenging, since patient symptomatology is not specific and may be attributed to coexistent cardiovascular conditions or AF risk factors.282 Symptom rhythm correlation is low in patients with persistent AF especially in the presence of comorbidities such as HF and diabetes.283,284 These considerations need to be taken into account when assessing patients’ symptomatic status before tailoring management approach.
Several multicenter randomized clinical trials (RCTs) have demonstrated the superiority of catheter ablation over AADs in patients with paroxysmal or persistent AF resistant or intolerant to AADs, in reducing AF recurrences and improving symptoms and quality of life (QoL).236–242
Implementation of an early rhythm control strategy in patients with AF and concomitant cardiovascular conditions is associated with improved cardiovascular outcomes.285 Antiarrhythmic agents have a modest efficacy in preventing AF recurrences with significant adverse event rates.286,287 Observational data have shown that invasive intervention early in the natural course of AF results in favourable outcome, with shorter ‘diagnosis-to-ablation’ time related to lower likelihood of arrhythmia recurrence, repeat ablation, and cardiovascular hospitalization.288–290 However, a recent RCT enrolling 100 symptomatic paroxysmal or persistent AF patients demonstrated that a strategy of AAD therapy with 12 month delay in catheter ablation had no impact on arrhythmia-free survival or AF burden over 12 month postablation follow-up when compared with an early ablation strategy (within 1 month).291 This study provides reassurance that an initial approach of medical therapy and risk factor management may be reasonable without compromising ablation outcomes. This approach takes into consideration the highly variable natural history of paroxysmal AF (Section 2.3.).
Several prospective multicenter RCTs have evaluated cryoballoon ablation as first-line treatment in symptomatic paroxysmal AF and demonstrated that it significantly reduces atrial tachyarrhythmia recurrences and improves patients’ QoL with similar risk of adverse events when compared with AAD treatment243–245,292 (Table 5).
Table 5.
Randomized controlled clinical trials comparing catheter ablation vs. antiarrhythmic drugs as first-line treatment in patients with symptomatic AF
| RAAFT-1248 | MANTRA-PAF246 | RAAFT-2247 | STOP-AF243 | EARLY-AF244 | CRYO-FIRST245 | |
|---|---|---|---|---|---|---|
| Year of publication | 2005 | 2012 | 2014 | 2021 | 2021 | 2021 |
| Sample size (ablation vs. AADs) | 32 vs. 35 | 146 vs. 148 | 66 vs. 61 | 104 vs. 99 | 154 vs. 149 | 107 vs. 111 |
| Mean age (SD), years (ablation vs. AADs) | 53 (8) vs. 54 (8) | 56 (9) vs. 54 (10) | 56 (9) vs. 54 (12) | 60 (11) vs. 62 (11) | 58 (12) vs. 60 (11) | 51 (13) vs. 54 (13) |
| Mean LA diameter (SD), mm (ablation vs. AAD) | 41 (8) vs. 42 (7) | 40 (6) vs. 40 (5) | 40 (5) vs. 43 (5) | 39 (6) vs. 38 (5) | 40 (5) vs. 38 (7) | 37 (6) vs. 38 (5) |
| Mean LVEF (SD), % (ablation vs. AAD) | 53 (5) vs. 54 (6) | LVEF >60% in 80 vs. 82% | 61 (5) vs. 61 (7) | 61 (6) vs. 61 (6) | 60 (7) vs. 60 (8) | 63 (5.4) vs. 64 (5.4) |
| Paroxysmal AF (%) (ablation vs. AADs) | 97 vs. 95 | 100 vs. 100 | 99 vs. 97 | 100 vs. 100 | 96 vs. 94 | 100 vs. 100 |
| Ablation type | Radiofrequency | Radiofrequency | Radiofrequency | Cryoballoon | Cryoballoon | Cryoballoon |
| Ablation strategy | PVI | PV encirclement plus roof line, additional ablation lesions allowed | PVI, additional ablation lesions allowed | PVI | PVI | PVI |
| Acute PVI rate (%) | 100 | 87 | 98 | 100 | 100 | |
| Rhythm monitoring protocol |
|
|
|
|
|
|
| Primary endpoint—definition | First recurrence of AF >15 s | AF burden and cumulative burden | First recurrence of AF/AFL/AT >30 s | Initial failure of the procedure; subsequent AF surgery or LA ablation; AF/AFL/AT ≥30 s during ambulatory monitoring or ≥10 s on a 12-lead ECG; cardioversion or Class I or III AAD outside the 90 day blanking period (ablation group only) | First recurrence of AF/AFL/AT ≥30 s or AAD initiation | Free from any AF/AFL/AT >30 s |
| Follow-up (years) | 1 | 2 | 2 | 1 | 1 | 1 |
| Recurrence of any atrial tachyarrhythmia (%) (ablation vs. AADs) | 13 vs. 63a | 15 vs. 29b | 54.5 vs. 72.1 | 20.2 vs. 35.4 | 42.9 vs. 67.8 | 17.8 vs. 32.4 |
| Treatment effect | 0.56 (0.35–0.90) | 0.57 (0.36–0.91) | 0.48 (0.35–0.66) | 0.48 (0.26–0.86) | ||
| Serious AEs—no. of patients (%) (ablation vs. AADs) | 13.7 vs. 10.8 (NS) | 9.1 vs.4.9 | 14 vs. 14 (NS) | 3.2 vs. 4.0 (N.S) | 24.3 vs. 33.3 (NS) | |
| Findings/comments | RF ablation superior to AAD | No significant difference between RF ablation and AADs in the cumulative AF burden over a period of 2 yearsc | RF ablation superior to AAD | Cryoballoon ablation superior to AADs | Cryoballoon ablation superior to AADsd | Cryoballoon ablation superior to AADs |
AAD, antiarrhythmic drug; AE, adverse event; AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; LA, left atrium; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NS, non-significant; PVI, pulmonary vein isolation; RF, radiofrequency; SD, standard deviation; TTM, transtelephonic monitoring.
aSymptomatic AF recurrence, P < 0.001.
bRecurrence of any AF, P = 0.004.
cNo PVI documentation.
dContinuous cardiac rhythm monitoring.
The superiority of cryoballoon ablation over antiarrhythmic therapy in reducing arrhythmia burden was also verified in the 3 year follow-up of patients enrolled in the EARLY-AF trial with a strict monitoring protocol with implantable loop recorder and scheduled follow-up visits.27 A crucial question is whether the favourable impact of catheter ablation as first-line treatment in paroxysmal AF patients is specific for cryoenergy ablation or represents a ‘class effect’ irrespective of the employed ablation technology. Prior trials of first-line RF catheter ablation demonstrated modest efficacy in arrhythmia outcome but were limited by high cross-over rates, inconsistent procedural endpoints, and lack of procedural standardization246–248 (Table 5). A pooled analysis concluded that RF catheter ablation resulted in significantly higher freedom from AF recurrence compared with AAD therapy in AAD-naïve paroxysmal AF patients.249 Furthermore, randomized comparison of cryoballoon ablation with RF ablation has demonstrated similar safety and efficacy in arrhythmia outcome in drug-refractory paroxysmal AF patients.293,294
Recent data have indicated that in addition to traditional physical symptoms, AF may be associated with significant adverse impact on mental health. An observational study found that over one-third of patients referred for AF management demonstrated severe psychological distress.295 A recent randomized trial indicated significant improvements in psychological distress maintained at 12 months associated with catheter ablation but not with active medical therapy.296
In the real world, RF ablation has greater heterogeneity in procedural results and is less reproducible than cryoablation in paroxysmal AF patients.297 Furthermore, the center's annual AF ablation caseload is an independent predictor of procedural success only in RF-treated paroxysmal AF patients.297 Despite variant needs in gaining experience and maintaining skills, an annual operator volume of at least 25 AF ablation procedures and an annual hospital volume of 50 AF ablation cases have been associated with improved procedural outcome.298,299 Therefore, procedural volumes should be taken into account when selecting the type of ablation technology to perform first-line catheter ablation.
The value of catheter ablation as first-line rhythm control therapy in persistent AF patients has not been specifically evaluated. Although the relative efficacy of catheter ablation in reducing AF burden and first AF recurrence is similar in paroxysmal and persistent AF types, extrapolation of the beneficial impact of first-line catheter ablation from the paroxysmal to the persistent patient group needs further verification.240,300 Nevertheless, the discrimination between paroxysmal and persistent AF may be challenging, and some patients may present with both paroxysmal and persistent AF episodes. In addition, some patients may present with an early stage of persistent AF, which is associated with fewer arrhythmia relapses following ablation compared with longer-lasting persistent AF. Suggested advice for catheter ablation in patients with paroxysmal or persistent AF in relation to the presence of AF-related symptoms is presented in Figure 7.
Figure 7.
Suggested advice for catheter ablation in patients with paroxysmal or persistent AF in relation to the presence of AF-related symptoms. AAD, antiarrhythmic drug; AF, atrial fibrillation.
4.2. Catheter ablation in patients with atrial fibrillation and heart failure
Atrial fibrillation and HF frequently coexist and potentiate each other in a vicious circle (AF begets HF and HF begets AF). Several studies have evaluated potential benefits of AF catheter ablation in patients with HF (Table 6). The favourable impact of catheter ablation in patients with AF and impaired left ventricular systolic function extends beyond rhythm outcome and may frequently result in left ventricular ejection fraction (LVEF) improvement. In the CAMERA-MRI trial, 68 patients with persistent AF and non-ischemic cardiomyopathy were randomized to catheter ablation or medical rate control.250 All patients had cardiac magnetic resonance (CMR) before enrolment to assess LVEF and late gadolinium enhancement (LGE), indicative of underlying ventricular fibrosis. Patients randomized to catheter ablation had significantly greater LVEF improvement compared with the rate control group, while LVEF normalization was achieved in 58% of patients postablation. These results were maintained during long-term follow-up.251 The study findings suggest that left ventricular dysfunction was at least partly attributed to arrhythmia-mediated cardiomyopathy and could be reverted with SR maintenance achieved by catheter ablation. On the other hand, patients with more advanced HF are more likely to have established myocardial dysfunction due to structural alterations, pathophysiologically unrelated to AF, which is thus not reversible by catheter ablation (Table 6).
Table 6.
Randomized controlled clinical trials comparing catheter ablation vs. medical therapy in patients with atrial fibrillation and heart failure with reduced ejection fraction
| PABA-CHF301 | MacDonald et al.302 | ARC-HF303 | CAMTAF252 | AATAC255 | CAMERA-MRI250 | AMICA304 | CASTLE-AF256 | CABANA subanalysis257 | RAFT-AF253 | CASTLE HTx260 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of publication | 2008 | 2011 | 2013 | 2014 | 2016 | 2017 | 2019 | 2018 | 2021 | 2022 | 2023 |
| Sample size | 81 | 41 | 52 | 50 | 203 | 66 | 140 | 363 | 778 | 411 | 194 |
| Mean age (years) | 60.5 ± 8 | 63 ± 7 | 63 ± 9 | 57 ± 11 | 61 ± 11 | 61 ± 10 | 65 ± 8 | 64 ± 5 | 68 ± 8 | 67 ± 8 | 64 ± 11 |
| AF type | Parox: 52% Pers or LS-pers: 48% |
Pers: 100% | Pers: 100% | Pers: 100% | Pers: 100% | Pers: 100% | Pers: 76.4% LS-pers: 23.6% |
Parox: 32.5% Pers: 38.3% LS-pers: 29.2% |
Parox: 31.6% Pers: 55.3% LS-pers: 13.1% |
Parox: 7.3% Pers: 69.3% LS-pers: 23.4% |
Parox: 30% Pers: 56%, LS-pers: 14% |
| NYHA | NYHA II and III: 100% | NYHA II: 10% NYHA III: 90% |
NYHA II: 52% NYHA III: 48% |
NYHA II: 46% NYHA III: 54% |
NYHA II and III: 100% | Average NYHA: 2.5±0.6 | NYHA II: 39% NYHA III: 61% |
NYHA I: 11% NYHA II: 60% NYHA III: 28% NYHA IV: 1% |
NYHA II: 76.1% NYHA III: 23.7% NYHA IV: 0.3% |
NYHA II: 67% NYHA III: 33% |
NYHA II: 31% NYHA III: 55%, NYHA IV: 14% |
| Baseline LVEF | 28 ± 8% | 39 ± 11% | 24 ± 8% | 33 ± 10% | 29 ± 7% | 33 ± 9% | 26 ± 9% | 32 ± 9% | 55 ± 8% LVEF ≤35%: 8% |
41 ± 15% LVEF ≤45%: 58% |
27 ± 6% |
| Ischemic etiology | 71% | 49% | 33% | 26% | 64% | 0% | 50% | 46% | 21.9% | 31.4% | 39% |
| LA diameter (mm) | 48 ± 6 | 48 ± 7 | 51 ± 10 | 47 ± 5 | 48 ± 7 | 51 ± 6 | 49 ± 7 | – | 46 ± 6 | 49 ± 7 | |
| Follow-up (years) | 0.5 | 0.5 | 1 | 0.5 | 2 | 0.5 | 1 | 3.1 ± 1.6 | 5 | 3.2 ± 1.8 | 1.5 ± 0.5 |
| Control arm—therapy | AV node ablation plus BiV pacing | Rate control | Rate control | Rate control | Rate control Amiodarone |
Rate control | Best medical therapy (rate or rhythm control) | Medical therapy (rate or rhythm control) | Medical therapy (rate or rhythm control) | Rate control | Guideline-directed medical therapy |
| Primary outcome | Composite of LVEF, 6 min walk test distance, and MLWHF score | LVEF | Change in peak O2 consumption | LVEF | Freedom from AF/AFL/AT >30 s off AADs | Change in LVEF at 6 months | Absolute increase in LVEF | Composite of all-cause mortality and HF hospitalization | Composite of all-cause mortality, disabling stroke, serious bleeding, and cardiac arrest | Composite of all-cause mortality, and HF events | Composite of death from any cause, LVAD implantation, or urgent heart transplantation |
| Mean change in LVEF (ablation vs. control) | 8 ± 8 vs. 1 ± 4%, P < 0.001 |
4.5 ± 11.1 vs. 2.8 ± 6.7%, P = 0.6 | 10.9 ± 11.5 vs. 5.4 ± 8.5% P = 0.055 |
8.1 vs. −3.6%, P < 0.001 |
8.1 ± 4 vs. 6.2 ± 5, P = 0.02 | 18.3 vs. 4.4%, P < 0.0001 | 8.8 vs. 7.3% P = 0.36 |
8.0 vs. 0.2% P = 0.005 |
10.1 ± 1.2 vs. 3.8 ± 1.2% P = 0.017 (24 months) |
7.8 ± 7.6 vs. 1.4 ± 7.2 (12 months) |
|
| Rhythm outcome (ablation vs. control) | 12 vs. 100% in AF | 50 vs. 100% in AF | 88 vs. 8% in SR | 19 vs. 100% in AF (at 6 months) | 70 vs. 34% free from AF | 25 vs. 100% in AF (at 6 months) | 73.5 vs. 50% in SR (at 1 year) | 63.1 vs. 21.7% in SR (at 5 years) | 44 vs. 28% w/o AF recurrence (at 5 years) | 85.6 vs. 12.9% in SR (at 2 years) | 31.4 ± 33.3% vs 8.6 ± 26.3% AF burden reduction at 12 months |
| Main findings | Improved composite endpoint | No LVEF improvement | Significant increase in peak O2 consumption | LVEF improvement | Reduction in AF recurrence, unplanned hospitalizations, and mortality | LVEF improvement | No LVEF improvement | Reduction in all-cause death or HF hospitalization | Reduction in the primary composite, reduction in all-cause mortality, and improvement in QoL | Similar primary outcome (P = 0.066) Increase in LVEF |
Reduction in the primary composite endpoint |
AAD, antiarrhythmic drug; AF, atrial fibrillation; AFl, atrial flutter; AT, atrial tachycardia; AV, atrioventricular; BiV, biventricular; HF, heart failure; LA, left atrium; LS-pers, long-standing persistent; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MLWHF, Minnesota Living with Heart Failure; NYHA, New York Heart Association; Parox, paroxysmal; Pers, persistent; QoL, quality of life; SR, sinus rhythm.
Catheter ablation in HF with reduced ejection fraction (HFrEF) patients may also have a beneficial impact on patient prognosis. A pooled analysis of randomized data concluded that rhythm control strategy reduces hospitalizations and confers a survival benefit in HFrEF patients when implemented with catheter ablation but not with antiarrhythmic medications.305 The CASTLE-AF study enrolled patients with paroxysmal or persistent AF and HF [New York Heart Association (NYHA) Class II or above, and LVEF < 35%], and implantable cardiac defibrillator (ICD), who were unresponsive, intolerant, or unwilling to take AAD.306 Patients were randomized to catheter ablation or medical treatment with rate or rhythm control. Fewer patients in the catheter ablation group had primary endpoint events (death from any cause or hospitalization for worsening HF) at a follow-up of 3.2 years [28.5 vs 44.6%; hazard ratio (HR), 0.62; P = 0.007]. Mortality was also significantly reduced in the catheter ablation group (13.4 vs. 25.0%; HR, 0.53; P = 0.01). Therefore, these results were strongly supportive that catheter ablation may favourably affect prognosis in this population, despite study limitations related to sample size, strict selection criteria, generalizability of findings, lack of blinded randomization, and treatment allocation. In the AATAC prospective RCT, catheter ablation was also shown to significantly reduce all-cause mortality in ICD/CRT-D recipients with persistent AF and HFrEF (LVEF < 40%) when compared with amiodarone treatment.255 In the recent CASTLE HTx trial, catheter ablation plus optimal medical therapy in patients with symptomatic AF and end-stage HFrEF referred for heart transplantation evaluation significantly reduced the composite of death from any cause, implantation of a left ventricular assist device, or urgent heart transplantation than medical therapy alone after a median follow-up of 18 months (8 vs. 30%; HR, 0.24; P < 0.001).260
Proper patient selection is crucial for maximizing benefit from AF catheter ablation in HFrEF patients. Several indicators may help guide this decision (Table 7).
Table 7.
Characteristics associated with LVEF recovery in response to AF catheter ablation in patients with impaired left ventricular systolic function
| Characteristics | Evidence |
|---|---|
| Lower NYHA class | Lower NYHA Class (I and II) at presentation is a predictor of significant LVEF recovery following AF ablation when compared with higher NYHA Class (III and IV) in patients with HFrEF256 |
| Non-ischemic etiology | Non-ischemic HF etiology is a significant predictor of LVEF improvement after AF ablation in patients with HFrEF256 |
| Persistent AF | Persistent AF is an independent predictor of LVEF improvement and left ventricular reverse remodelling after AF ablation in patients with impaired LVEF307–310 |
| Narrow QRS | Narrow QRS (≤120 ms) is an independent predictor of LVEF recovery after AF ablation in patients with impaired LVEF307,308 |
| Absence of CMR-detected atrial fibrosis | Extent of atrial fibrosis is inversely correlated to LVEF response following AF catheter ablation in patients with HFrEF311 |
| Absence of CMR-detected ventricular fibrosis | Absence of ventricular fibrosis is an independent predictor of LVEF normalization after AF catheter ablation in patients with non-ischemic cardiomyopathy and persistent AF250 |
| Post-cardioversion EF and NYHA improvement | Improvement in functional status and/or LVEF after cardioversion is indicative of underlying tachyarrhythmia-mediated cardiomyopathy and a favourable response to catheter ablation in HFrEF patients |
| Absence of severe atrial dilatation | Absence of severe atrial dilatation (LAVI ≤ 50 mL/m2) is an independent predictor of LVEF recovery after AF ablation in patients with impaired LVEF307,308 |
| AF preceding HF or simultaneous AF and HF diagnosis | Patients with simultaneous AF and HF diagnosis or AF history preceding HF diagnosis are more likely to present normalization of LVEF and resolution of HF symptoms following catheter ablation252,312 |
AF, atrial fibrillation; CMR, cardiovascular magnetic resonance; HF, heart failure; HFrEF, HF with reduced ejection fraction; LA, left atrial; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Higher NYHA class (III/IV), ischemic HF etiology, paroxysmal AF type, prolonged QRS duration (>120 ms), severe LA dilatation [left atrial volume index (LAVI) >50 mL/m2], and atrial and ventricular fibrosis are predictors of lack of LVEF recovery following catheter ablation in patients with impaired left ventricular systolic function.253,256,307,308,311,313
It is also important to determine the relative chronologic sequence of AF and HF presentation, since patients who develop HF first have a worse prognosis, are less likely to present AF-mediated tachymyopathy and have a poorer outcome after AF ablation.252,312,314 The Antwerp score based on four simple parameters (wide QRS, known HF etiology, severe atrial dilatation, and paroxysmal AF) has been shown to predict left ventricular systolic function recovery after AF ablation in HF patients.307 Recently, this score was externally validated in a large multicenter study yielding good discrimination and calibration.308 Nevertheless, even in the presence of less favourable characteristics, some patients with AF and HF may experience improvement in LV systolic function and clinical outcome following SR restoration.
Registry and observational data suggest that catheter ablation significantly reduces arrhythmia recurrences and the risk of cardiovascular events compared with drug therapy in HF patients across all LVEF subgroups, even in HF patients with preserved ejection fraction (HFpEF).313,315–321 In a prespecified subanalysis of the CABANA trial in patients with baseline HF symptoms (NYHA Class ≥II, 79% with LVEF ≥50%), catheter ablation conferred significant improvement in arrhythmia recurrence, QoL, and survival when compared with pharmacological therapy.257 In the subgroup of HFpEF patients (LVEF ≥ 50%), ablation reduced mortality by 60% compared with drug therapy.257 A small, randomized trial demonstrated that AF catheter ablation significantly improved invasive hemodynamic parameters, exercise capacity, and QoL outcomes when compared with medical therapy in patients with HFpEF and concomitant AF.322 Adequately powered prospective RCTs are needed to provide more robust clinical data.
4.3. Catheter ablation in patients without atrial fibrillation–related symptoms
The main objective of AF catheter ablation is symptom amelioration and reduction in arrhythmia recurrences. Pertinent benefit, beyond symptomatic control, might justify eligibility of truly asymptomatic AF patients for catheter ablation.
A key issue in the management of patients without symptoms while remaining in AF is the exclusion of a pseudo-asymptomatic status. Up to 77% of these patients may experience subjective symptomatic amelioration,323 improvement in functional class, and decrease in brain natriuretic peptide levels with SR restoration following electrical cardioversion.324 Therefore, in asymptomatic patients, especially at younger age, a cardioversion is worth attempting to assess potential symptomatic improvement that would enhance patient eligibility for catheter ablation due to reclassification in the symptomatic category.
Atrial fibrillation has significant hemodynamic consequences that may lead to HF and worsen patient prognosis, such as loss of atrial contribution to cardiac output, rapid and irregular heart rate, and loss of heart rate adaptation to metabolic demands. Heart rate irregularity results in inefficient cardiac cycles due to inadequate ventricular filling, contributes to hemodynamic impairment, and worsens left ventricular systolic and diastolic functions.325 An irregularly paced ventricular rhythm following AV node ablation decreases cardiac output and increases pulmonary wedge pressure when compared with a regular rhythm at the same average cycle length.326 Therefore, SR maintenance might confer benefit due to prevention of abovementioned AF-mediated hemodynamic sequelae even in the absence of coexisting symptoms.
Apart from symptomatic improvement, catheter ablation is also effective in delaying AF progression from paroxysmal to persistent type (Section 2). Atrial fibrillation progression to longer-lasting types has an impact on patient outcome since non-paroxysmal AF is associated with significantly increased risk of thromboembolism, HF, hospital admissions, and mortality compared with paroxysmal AF.32,327,328 In the ATTEST trial, catheter ablation significantly delayed AF progression compared with AADs in patients with drug-refractory paroxysmal AF.329 Furthermore, in the 3 year follow-up of the EARLY-AF trial, first-line treatment of paroxysmal AF patients with cryoballoon ablation was associated with significantly lower incidence of persistent AF [HR, 0.25; 95% confidence inetrval (CI), 0.09–0.70] when compared with AAD therapy using continuous cardiac monitoring.27 Therefore, beyond symptom control, catheter ablation may have a favourable impact by limiting disease progression especially when implemented in early stages of AF natural course.
The largest trial assessing potential prognostic benefits of catheter ablation is the CABANA trial that enrolled 2204 symptomatic patients with AF aged 65 years and older or younger than 65 years with 1 or more risk factors for stroke.330 Patients were randomized to catheter ablation or AAD/rate control therapy. In the intention-to-treat analysis, catheter ablation did not significantly reduce the primary composite endpoint of death, disabling stroke, serious bleeding, or cardiac arrest compared with medical therapy over a median follow-up of 48.5 months. However, the study was limited by high crossover rates, while the per-protocol analysis demonstrated significant differences in favour of catheter ablation (P = 0.046). In addition, the composite secondary endpoint of death from any cause or cardiovascular hospitalization occurred significantly less frequently in the catheter ablation group than in the medical therapy group. Despite these considerations and caveats, the study findings do not support the use of catheter ablation to improve prognosis in the general population of asymptomatic patients with AF. However, in the CABANA trial, the clinical outcome of ablation vs. AAD therapy demonstrated an age-based variation with the largest relative and absolute prognostic benefit seen in patients younger than 65 years, suggesting that selected patient subgroups may have clinical outcome benefit from catheter ablation.331 Furthermore, a prespecified analysis of the EAST-AFNET 4 trial showed that an early systematic rhythm control strategy (mainly with AADs) confers a similar degree of outcome benefit in symptomatic and asymptomatic AF patients.332
4.4. Patients with atrial fibrillation and coexistent rhythm disorders
4.4.1. Atrial fibrillation and supraventricular tachycardia
Paroxysms of AF are commonly triggered by ectopic beats from the PVs.65 However, other types of supraventricular tachycardia (SVT), such as AV nodal reentry tachycardia (AVNRT), focal atrial tachycardia (AT), or AV reentry tachycardia (AVRT) may trigger AF, especially in younger patients.333 The incidence of AF in patients with paroxysmal SVT is higher than in age-matched normal populations, while 12% of patients with known SVT also experience AF episode within 12 months of follow-up.334 Furthermore, a small subgroup of patients who are referred for AF catheter ablation, ranging from 7.6 to 10.1%, also have inducible SVT.261,262 Sciarra et al.261 reported that the role of SVT as AF trigger could be verified in 42.3% of patients with AF and inducible SVT, as evidenced by spontaneous conversion of SVT to AF. In these patients, elimination of the SVT only, without AF catheter ablation, may be sufficient for a favourable rhythm outcome with freedom from AF recurrences ranging from 70 to 92.3% during follow-up.261,263,264 Observational trials have demonstrated an age-related increase in the risk of AF recurrence in patients with coexistent SVT and AF following ablation of the SVT only, with age over 50 years indicative of high AF recurrence rate.264,335 Therefore, in patients with coexistent SVT and AF, preferably in the younger age group, and only when the former is considered the main trigger of the latter, it is reasonable to simplify the ablation strategy to elimination of the SVT only. Atrial fibrillation ablation would then be deferred depending upon AF recurrence following SVT ablation.
4.4.2. Atrial fibrillation and sinus node dysfunction
Symptomatic prolonged sinus pauses are common upon AF termination and may be aggravated by AV node–blocking agents and AADs. This often leads to the indication for pacemaker implantation. Atrial fibrillation catheter ablation is effective in preventing both AF recurrences and sinus pauses upon AF termination, likely due to autonomic modulation.265 Chen et al.266 reported that 95.3% of patients with paroxysmal, AF-related, symptomatic prolonged sinus pauses who underwent AF catheter ablation no longer needed a pacemaker and had significantly higher freedom from AF recurrences and tachycardia-related hospitalizations compared with those treated with permanent pacemaker implantation and AADs. Although sinus node dysfunction in the presence of paroxysmal AF is mainly attributed to electrical remodelling,336 underlying sinus node structural remodelling may also be present in a few cases.337,338 A minority of patients with coexistent AF and sinus node dysfunction will still require permanent pacemaker implantation following catheter ablation due to underlying structural alteration of the sinus node.339 However, the vagal denervation that occurs with catheter ablation results in a higher resting heart rate, which may also help a patient compensate for coexistent sinus node dysfunction, even if AF recurs. Atrial fibrillation recurrence rate was significantly higher in patients requiring pacemaker implantations after AF ablation than those who did not.339,340
4.4.3. Atrial fibrillation and atrial flutter
Cavotricuspid isthmus (CTI)-dependent atrial flutter (AFl) is frequent in patients with AF, either spontaneously or during type Ic AAD or amiodarone therapy.267 The two arrhythmias have mechanistic and pathophysiological linkage with short bursts of AF frequently preceding and triggering AFl development.341 Scharf et al.267 reported that spontaneous or pacing induced AFl occurrence during AF ablation procedure is predictive of symptomatic AFl during post-PVI follow-up, with 24% of patients who did not undergo CTI ablation during the PVI procedure experiencing symptomatic AFl recurrence during a mean follow-up of 609 ± 252 days. These findings are supportive of CTI ablation in case of AFl occurrence during AF ablation procedure. Contradictory findings have also been reported. Wazni et al.268 in a trial conducted at the beginning of the AF ablation era advocated that PVI only, without CTI ablation, suppressed both AF and typical AFl recurrences. However, in this patient series, CTI block reduced early AFl recurrences, since 55% of patients not receiving CTI ablation experienced episodes of typical AFl within the first 8 weeks following catheter ablation and 20% needed electrical cardioversion.268 Based on the concept that PV ectopics are main triggers of typical AFl, the CRAFT trial tested the hypothesis whether cryoballoon PVI was superior to CTI ablation as first-line therapy in patients with typical AFl without prior AF documentation. The primary efficacy outcome measure (time to first recurrence of sustained symptomatic atrial arrhythmia) was similar between the compared groups, although patients subjected to PVI had a five-fold higher likelihood of flutter recurrence within 1 year (10 vs. 2%, P = 0.07).342
In recent catheter ablation trials, recurrence rates are not negligible, and therefore, patients with both AF and typical AFl may still be prone to AFl recurrence following PVI since even short bursts of AF may trigger AFl. In addition, CTI ablation reduces the likelihood of AFl recurrence if AAD is administered following AF catheter ablation.
Non–CTI-dependent AFl is also encountered following AF ablation especially after extensive ablation lesion sets in the context of persistent AF ablation.339,343 However, these types of macroreentrant ATs may resolve spontaneously in some patients, and therefore, catheter ablation should be deferred for several months and beyond the blanking period unless non–CTI-dependent AFl episodes are recurrent, highly symptomatic and resistant to AADs and cardioversion (Section 9).
4.5. Atrial fibrillation with other risk factors or diseases
4.5.1. Older patients with atrial fibrillation
Some centers may withhold ablation therapy in older patients.344 This reluctance stems from a perceived less favourable risk to benefit ratio of catheter ablation in elderly patients. Two recent metaanalyses of observational studies demonstrated similar AF ablation success rates with a significantly higher risk of complications in patients >75 years when compared with younger ones.270,271 However, contradictory results have also been reported. Data from a Danish nationwide cohort study reported a similar incidence of periprocedural complications and AF relapse in patients ≥75 years subjected to catheter ablation when compared with patients aged 65–74 years.272
At present, it is unclear whether a specific technology of AF catheter ablation should be preferred in elderly patients due to associated enhanced safety profile. In a propensity-matched comparison of older patients ≥75 years, cryoballoon ablation was associated with similar efficacy and safety, but with shorter procedural time when compared with RF ablation.345 Furthermore, a subanalysis of the CABANA trial found no prognostic benefit of catheter ablation (CA) in patients ≥75 years of age, with similar rates of complications and AF recurrences postablation.331
4.5.2. Atrial fibrillation and hypertrophic cardiomyopathy
Atrial fibrillation is highly prevalent in patients with hypertrophic cardiomyopathy (HCM).346,347 These patients often have limited options for antiarrhythmic therapy, due to hypertrophy and underlying structural heart disease. However, AF is often poorly tolerated and impairs clinical outcome in HCM patients, thus stressing the need to pursue SR maintenance in many patients.348
Several studies have evaluated the efficacy of catheter ablation in HCM patients with AF. Three metaanalyses have reported significantly lower freedom from AF/AT recurrences in patients with as compared to those without HCM after single and multiple catheter ablations.273,274,279 Recent studies have shown that catheter ablation has comparable efficacy in HCM patients as compared to the general patient population when treating paroxysmal AF.275,276 However, results are poorer in patients with persistent AF.273,275,276 Therefore, early invasive intervention, before progression of AF and/or underlying atrial substrate, is of primary importance in HCM patients to increase success rates.
Non-PV triggers are commonly involved in AF pathophysiology in HCM patients and are documented in many patients with arrhythmia recurrence following catheter ablation, thus supporting the concept of extensive ablation lesion sets to increase success rate.277 However, adjunctive ablation beyond PVI was not associated with additional benefit in a large multicenter cohort of HCM patients undergoing AF catheter ablation.276 The use of RF vs. cryoballoon ablation has no impact on procedural outcome among HCM patients.276 Furthermore, the risk of major procedural complications appears to be increased in HCM patients when compared with the general AF population.276 Despite a temporal decline in the incidence of procedural complications in HCM patients, real-world data verify the still high periprocedural morbidity and mortality.278
4.5.3. Patients with atrial fibrillation and obesity—physical inactivity—obstructive sleep apnoea
Obesity and physical inactivity are associated with increased risk of AF349 and reduced efficacy of AF ablation350,351 (Section 5.1.2.). Obesity also increases the risk of complications of catheter ablation and increases radiation to both the patient and personnel352 (Section 11). Comprehensive management of these modifiable risk factors improves the outcome of catheter ablation353–356 (Section 5.1.). However, weight reduction and improvement in cardiorespiratory fitness requisites lengthy efforts with slow yielding results that may be difficult to sustain in long term. Furthermore, prolonged delays from AF diagnosis to catheter ablation adversely affect success rates.288–290 Therefore, catheter ablation of AF should not be deferred in obese or physical inactive patients who have initiated lifestyle interventions and are showing progress towards their pertinent lifestyle goals. Individualized risk–benefit assessment is needed in patients with morbid obesity [body mass index (BMI) >40 kg/m2] due to a higher complication rate and lower long-term freedom from AF.350–352 Evaluation at a comprehensive weight loss clinic may be useful to determine eligibility for medications or surgical approaches to facilitate weight loss (Section 5.1.2.).
Obstructive sleep apnoea is associated with AF,355 and up to 45% of patients referred for AF ablation have OSA.356 Patients with OSA have a significantly increased risk of AF recurrence following catheter ablation compared with those without OSA.357–360 Treatment with continuous positive airway pressure (CPAP) appears to significantly reduce the risk of AF recurrence or progression in patients with AF and OSA.361,362 Continuous positive airway pressure therapy also results in reversal of atrial remodelling in AF patients.362 For these reasons, some centers are reluctant to perform AF ablation before OSA evaluation and potential initiation of CPAP treatment. The rate of AF recurrence following PVI is similar between CPAP-treated OSA patients and non-OSA patients.357 In addition, PVI considerably reduces the burden of paroxysmal AF in OSA patients, but the use of CPAP following ablation has not been shown to further reduce the risk of AF recurrence in a recent randomized study, which was though lacking sufficient statistical power (Section 5.1.3.).363 Finally, there are no controlled studies comparing AF ablation followed by OSA treatment vs. OSA treatment followed by AF ablation if needed. At present time, there is no evidence supporting the concept that CPAP may completely prevent AF recurrences and the need for catheter ablation at follow-up.
5. Atrial fibrillation risk factors and preprocedural management
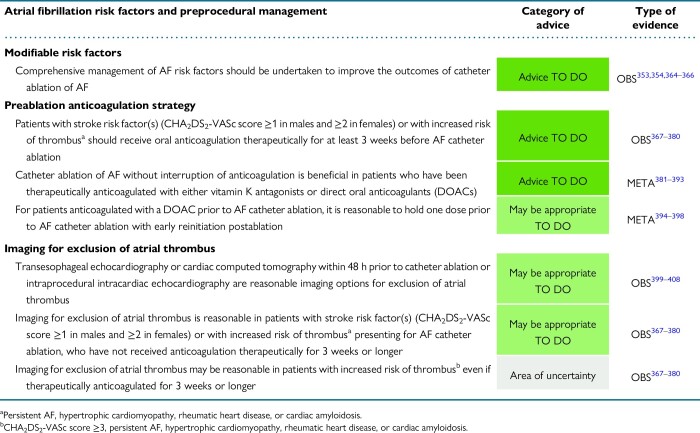
|
5.1. Atrial fibrillation risk factors
Several risk factors for AF development and recurrence following catheter ablation have been identified, many of which are modifiable. These include traditional modifiable risk factors such as hypertension409–412 and diabetes,364,413–417 but also emerging factors such as obesity,109,351,418,419 metabolic syndrome,420–422 physical inactivity,423–427 OSA,358,359,428,429 alcohol consumption,365,430,431 and smoking.366,432 There is compelling evidence to suggest that management of these risk factors has the potential to reduce AF burden and improve the outcomes of ablation strategies to maintain SR. In practice, although there have been variable results when targeting risk factors in isolation, comprehensive management in specific risk factor management clinics has been shown to be effective in conferring tangible clinical improvements.353,354 In addition, there are cardiovascular comorbidities that warrant specific treatments and may have a role in improving the outcomes of catheter ablation. The recently described HEAD2TOES schema with targets for secondary prevention of AF is presented in Figure 8.433 Below we discuss the evidence pertaining to each of these modifiable factors (see Supplementary material online, Table S1).
Figure 8.
Risk factors and respective targets for AF prevention in patients considered for or undergoing AF ablation—the HEAD2TOES schema (green light: established evidence; orange light: evolving evidence). AF, atrial fibrillation; AHI, apnoea–hypopnoea index; BMI, body mass index; CPAP, continuous positive airway pressure; HbA1c, glycated haemoglobin; HTN, hypertension.
5.1.1. Hypertension
Hypertension is one of the most significant risk factors for AF development.434–436 It increases left ventricular wall thickness and diastolic dysfunction, which mediate adverse atrial remodelling associated with increased LA pressure, wall thickness, fibrotic changes, and dilatation. In addition, the increased activation of the renin-angiotensin-aldosterone system in hypertensive patients mediates atrial fibrosis and electrophysiological remodelling thus promoting AF.437
Discrepant results have been reported regarding the impact of uncontrolled hypertension on AF ablation outcome. In an observational study including 531 patients who underwent AF catheter ablation, uncontrolled hypertension was significantly associated with postablation arrhythmia recurrence after confounder adjustment.412 In contrast, a registry analysis showed that patients with a diagnosis of hypertension, without information regarding the efficiency of antihypertensive management, had similar rhythm outcome after catheter ablation to those without hypertension.438 In the SMAC-AF randomized trial, short-term aggressive blood pressure (BP) treatment (target systolic BP ≤120 mmHg) for a median duration of 3.5 months before scheduled AF catheter ablation in patients with hypertension did not reduce arrhythmia recurrence following ablation when compared with standard BP treatment (target systolic BP <140 mmHg).439 Although treating modest hypertension in isolation has not proven to be of benefit, when undertaken in the setting of a comprehensive risk factor management programme in overweight and obese individuals, it has been associated with higher rate of SR maintenance after catheter ablation.353
Renal artery denervation, a procedure developed for the treatment of resistant hypertension, has also a potential antiarrhythmic role. In a small, randomized study of 27 patients with drug-resistant hypertension scheduled for AF catheter ablation, combined renal artery denervation and PVI resulted in significant BP reduction and a higher freedom from AF recurrence at 12 months compared with PVI only.440 In the larger, multicenter ERADICATE-AF RCT, 302 patients with hypertension resistant to at least one antihypertensive medication undergoing paroxysmal AF ablation were randomized to catheter ablation alone or ablation plus renal denervation.441 Addition of renal denervation to catheter ablation significantly increased freedom from AF recurrence at 12 months when compared with ablation alone. The underlying mechanisms explaining the favourable impact of renal artery denervation on AF burden have not been clarified. It has been postulated that this effect may be due to BP control itself or to direct antiarrhythmic actions of renal artery denervation including reduction in central sympathetic output and attenuation of atrial structural and electrophysiological remodelling.442
5.1.2. Obesity
Obesity is a pandemic and contributes significantly to the increasing prevalence of AF worldwide. The correlation between obesity and AF is well-recognized.443 A metaanalysis of 51 studies reported that for every 5-unit increase in BMI, there was a 29% greater excess risk of incident AF and a 13% increased likelihood of AF recurrence following ablation.444 Related mechanisms include structural and electrical atrial remodelling.109,445,446 In addition, cardiac imaging studies have shown that obesity is associated with increased epicardial and pericardial fat depositions adjacent to the LA.447 Increased pericardial and epicardial fat is associated with AF, likely through direct fatty infiltration of the LA and/or paracrine effects attributable to released cytokines and chemokines.448
Based on the findings of the ESC-EHRA AF Ablation Long-Term Registry, patients with BMI over 30 kg/m2 had 1.2-fold increased likelihood of AF recurrence following catheter ablation when compared with overweight patients.351 A single-center retrospective study enrolling 2715 consecutive patients undergoing AF catheter ablation concluded that BMI over 35 kg/m2 was an independent predictor of worse postablation rhythm outcome.419 In an observational study that categorized patients who underwent ablation by BMI, AF recurrence was higher in all high-BMI groups when compared with normal-weight controls.350
The role of weight loss in patients undergoing AF ablation has also been examined in several studies. The ARREST-AF study evaluated the value of a comprehensive risk factor management approach in patients undergoing AF ablation and showed that aggressive risk factor management achieved significantly greater weight loss and increased freedom from AF.353 In contrast, a small non-randomized study in patients with morbid obesity and long-standing persistent AF undergoing AF ablation reported that weight loss did not improve either AF symptom severity or freedom from AF recurrence at 1-year follow-up.449 The prospective SORT-AF trial randomized patients with symptomatic AF and BMI 30–40 kg/m2 undergoing AF ablation to a supervised structured weight management programme or to usual care on the day of the procedure and evaluated potential impact on rhythm outcome assessed by invasive monitoring.450 The primary endpoint of AF burden did not differ between compared groups probably due to a high rate of non-compliance in the intervention group, a modest weight loss achieved, and a rather short postablation follow-up.
Bariatric surgery may also have a positive impact on postablation AF recurrence rate in patients with morbid obesity. In a retrospective cohort study of 239 patients with morbid obesity, bariatric surgery prior to AF ablation was associated with reduced risk of AF recurrence and reduced rate of repeat AF ablation. Prospective RCTs are needed to confirm the positive impact of surgical weight loss procedures.451
5.1.3. Obstructive sleep apnoea
Obstructive sleep apnoea is a chronic condition characterized by recurrent pharyngeal collapse leading to repetitive interruption of ventilation during sleep. It is increasingly recognized as a critical risk factor in a variety of cardiovascular conditions and has recently been shown to double the risk of incident AF.452 Both the acute effects of apnoeic episodes and the chronic effects of long-term OSA contribute to the increased risk of AF. The transient hypoxaemia associated with pharyngeal collapse is postulated to mediate changes in atrial ERP acutely and subsequently to enhance susceptibility to AF induction and maintenance.453 In the long-term, OSA mediates significant hemodynamic changes resulting in increased LA pressures and LA enlargement.454 Obstructive sleep apnoea is also known to induce a systemic inflammatory and pro-thrombotic state, which increase the likelihood of fibrotic changes and electrophysiological remodelling within the atria.455
Mounting evidence suggests that OSA is also associated with worse outcomes following catheter ablation.358,359,428,429 Metaanalyses report that patients with OSA have a significantly increased risk (ranging from 25 to 70%) for AF recurrence following AF catheter ablation.359,428 Observational studies and metaanalyses concluded that the use of CPAP as a treatment strategy for OSA is associated with improved patient outcome following ablation.456,457 A recent study in patients with OSA and AF demonstrated that CPAP therapy reverses electrical remodelling as documented by high-density RA mapping.362 Randomized evidence on the impact of CPAP treatment on arrhythmia outcome after ablation is sparse. In a recent study, 83 patients with paroxysmal AF and OSA undergoing PVI were randomized to either CPAP or standard of care without any difference in postablation AF recurrence as documented by implantable loop recorders. Of note, this was a small study and probably without adequate statistical power to detect subtle treatment differences.363
Importantly, treatment of OSA in the context of a comprehensive risk factor management strategy has been associated with improved ablation outcomes.353,354 In the ARREST-AF study, patients were offered therapy if the apnoea–hypopnoea index (AHI) was ≥30/h or if it was >20/h with resistant hypertension or problematic daytime sleepiness.353 However, proponents of treating sleep apnoea are increasingly utilizing therapy with an AHI ≥15/h in patients with AF.362 This latter trigger for treatment is being prospectively evaluated in the SNORE-AF study (ACTRN12621001213831).
5.1.4. Alcohol consumption
Long-term alcohol intake is associated with incident AF in a dose-dependent manner.458 High quantities of alcohol consumption (more than three standard drinks per day) increase the risk of AF development by almost 35%, with a more significant effect in males. However, there is limited association with incident AF in those who limit alcohol consumption to less than one standard drink per day. Proposed mechanisms underlying this association include pleiotropic effects of alcohol on atrial electrical properties: conduction slowing and shortening of the atrial ERP,459 alterations in autonomic nervous control of the heart,460 and an increase in circulating plasma free fatty acids, which have been shown to be arrhythmogenic.461 Structural LA remodelling may also be implicated in the association of alcohol consumption with AF development, since chronic alcohol consumption has been identified as a predictor of LA enlargement.462 Furthermore, chronic alcohol consumption is closely associated with other independent AF risk factors including hypertension, obesity, and OSA.
Several studies support a relationship between alcohol consumption and AF recurrence following ablation. In a study of patients with paroxysmal AF undergoing ablation, those consuming alcohol had higher AF recurrence rates after first catheter ablation compared with those who did not, though this difference was attenuated after repeat ablations.430 In an observational study of symptomatic patients with paroxysmal AF, alcohol consumption was an independent predictor of low-voltage zones assessed by LA voltage mapping and AF recurrence following catheter ablation.431 Contradictory results regarding potential association of alcohol consumption with substrate remodelling (atrial low voltage and conduction slowing) have been reported.459,463 The association of alcohol consumption with adverse postablation rhythm outcome has also been validated in studies using objective markers of alcohol use, such as ethyl glucuronide levels in hair, thus overcoming potential bias in patient self-reporting.464
Several studies have evaluated the impact of alcohol abstinence on clinical outcome after AF ablation either alone or in the context of a comprehensive risk factor management. Alcohol reduction of ≥1% from baseline to 1 year follow-up is independently associated with a lower risk of postablation arrhythmia recurrence.365 Therefore, it is reasonable to reduce alcohol consumption to fewer than 30 g/week (three standard drinks) in individuals undergoing catheter ablation of AF as part of a comprehensive management of risk factors.353,354
5.1.5. Physical inactivity
Physical activity and exercise are linked with cardiovascular health. Increasing evidence supports that sedentary lifestyle increases the risk of incident AF.465 Regular light-to-moderate exercise has been shown to reduce the risk of AF development.466 However, the relationship between physical exercise and incident AF does not appear to be linear. Several cohort studies have shown that exercise intensity has a U-shaped relationship with AF, with highly active subjects exhibiting increased risk of incident AF compared with moderately active individuals.466–468 Regular moderate-intensity exercise would, therefore, appear to be the key in reducing the risk of AF.
Physical activity is also important in secondary AF prevention. In the CARDIO-FIT study, cardiorespiratory fitness (a surrogate for physical activity) measured by metabolic equivalents was associated with reduced AF burden and symptom severity in obese individuals with symptomatic AF. Each unit increase in metabolic equivalent was associated with 13% decline in the risk of AF recurrence.469 In a recent prospective RCT (ACTIVE-AF), implementation of a supervised exercise-based intervention with progressively increased aerobic exercise up to 210 min per week significantly reduced arrhythmia recurrence by 50% and improved symptom severity when compared with usual care.470
Several studies have evaluated the impact of physical activity and cardiorespiratory fitness on clinical outcome following catheter ablation (see Supplementary material online, Table S1). Higher cardiorespiratory fitness measured with the use of exercise stress test is associated with reduced arrhythmia recurrence and mortality following ablation.423 In the subgroup of highly trained athletes, several rather small observational studies have shown that catheter ablation is similarly effective as in the general population.424,426,471,472 The largest observational study in 144 athletes undergoing ablation found a similar arrhythmia recurrence rate following PVI when compared with a matched cohort of non-athletes.424 In a randomized study including persistent AF patients treated with AF ablation, participation of patients in an exercise-based cardiac rehabilitation programme improved exercise capacity after a 6-month follow-up without associated reduction in AF recurrence when compared with usual care.427 The latter finding may be due to insufficient sample size and limited patient follow-up. Based on the existing evidence, individuals undergoing catheter ablation of AF should follow a training programme of at least moderate aerobic exercise for a minimum of 210 min per week to improve rhythm outcome.469
5.1.6. Diabetes mellitus
A number of studies have confirmed diabetes mellitus as an independent risk factor for AF.473 Through increased production of reactive oxygen species and advanced glycation end-products, diabetes has been shown to result in fibrotic changes as well as ion channel and gap junction remodelling within the atria.474 These changes increase conduction heterogeneity, reduce conduction velocity, and prolong APD, promoting an electrophysiological milieu that favours AF development.
The association between diabetes mellitus and AF recurrence following ablation has been demonstrated in several studies. Metabolic syndrome, which encompasses disorders of BP, fasting sugar state, body weight, and lipids, has been associated with poor AF catheter ablation outcomes.420–422 In a prospective study including 1496 patients with non-paroxysmal AF undergoing catheter ablation, metabolic syndrome was associated with higher AF recurrence rates.422 In the German Ablation Registry, diabetic patients experienced a similar rate of AF recurrence when compared with those without diabetes at 12 months of follow-up.414 In contrast, another observational study of 2504 patients undergoing AF ablation concluded, after propensity-matched analysis, that diabetes was an independent predictor of postablation AF recurrence.415 Smaller observational studies have also demonstrated the detrimental impact of diabetes mellitus on catheter ablation outcome.416
In addition, preablation glycaemic control has been shown to affect arrhythmia recurrences after ablation.364 Patients with glycated haemoglobin (HbA1c) >9% were more than twice as likely to experience AF recurrence following AF ablation compared with those with an HbA1c < 7. In multivariate analysis, improved glycaemic control prior to ablation, defined as >10% reduction in HbA1c during the last 12 months prior to ablation, was an independent predictor of arrhythmia-free survival after ablation.364 Therefore, in the context of a multidisciplinary risk factor management approach, optimized glycaemic control should be set as a treatment objective in diabetic patients undergoing AF ablation to improve rhythm outcome.
5.1.7. Smoking
Several long-term prospective observational cohort studies have identified smoking as an independent predictor of incident AF.475–477 Most of these studies showed that the risk was higher in those who continued smoking compared with those who were able to quit. Proposed implicated mechanisms include increased sympathetic tone, oxidative stress, inflammation, and atrial fibrosis. In the presence of AF, smoking increases the risk of thromboembolism and mortality, even after adjusting for well-recognized risk factors used in stroke risk stratification schemes.478 In a matched case–control study including AF patients with a low stroke risk (CHA2DS2-VASc score 0 in men or 1 in women), smoking was the only independent predictor associated with ischemic stroke. These findings provide strong evidence that smoking cessation should be an important part of AF risk factor management.479
There are limited data exploring the relationship between smoking and AF ablation. In a small study of 59 patients who underwent PVI, smoking was associated with a three-fold relative risk of AF recurrence.432 In another retrospective study of persistent AF patients undergoing ablation, smokers had a significantly higher incidence of non-PV triggers compared with non-smokers without associated difference in long-term ablation outcomes.366 Implementation of smoking cessation in the context of a structured risk factor management programme significantly improves long-term outcome in symptomatic AF patients undergoing AF ablation.480
5.1.8. Cardiovascular comorbidities
Cardiovascular disease contributes to the development of AF. There is considerable evidence supporting the association of AF with HF and valvular heart disease. In patients with severe rheumatic mitral stenosis, reduction of chronic stretch after mitral commissurotomy results in reversal of atrial structural remodelling and associated conduction abnormalities.481 In HFrEF patients, HF-directed therapy reduces AF recurrence, cardiovascular hospitalization, and mortality.482–484 Although not specifically studied in the context of AF catheter ablation, optimizing therapy directed at these underlying conditions, when indicated, may improve AF ablation outcomes. Therefore, guideline-recommended HF treatment should be undertaken in patients undergoing catheter ablation of AF.
5.2. Preprocedural management
5.2.1. Preprocedural predictors of atrial fibrillation recurrences
Atrial fibrillation recurrence following catheter ablation is not uncommon and remains a notable problem.485 Several preprocedural factors are associated with increased risk of AF recurrences, including modifiable comorbidities (Section 5.1.), AF type and duration, LA size, and abnormal atrial substrate as detected by ECG and cardiac imaging.5 Consideration of these predictors of postablation rhythm outcome is important to drive patient selection for AF ablation.
5.2.1.1. Atrial fibrillation type and duration
The association of AF type with postablation recurrence rates has been widely investigated. Despite variation associated with the type and intensity of postablation rhythm monitoring, postablation arrhythmia recurrence rate is lower in patients with paroxysmal when compared with those with persistent AF.243–245,366,486–490 Apart from AF type, the time interval from AF diagnosis to ablation (DAT) is a predictor of postablation AF recurrence.288,290,491–494 Each year increase in DAT increases the risk of AF recurrence by 20% after adjustment for baseline comorbidities and medications.491 A metaanalysis of 6 observational studies with a total of 4950 patients demonstrated that DAT <1 year was associated with a lower AF recurrence rate (relative risk: 0.73) compared with DAT >1 year.288
5.2.1.2. Left atrial size
Several studies showed that LA size is an independent preprocedural predictor of AF recurrence following AF ablation.409,495–498 A linear relationship has been reported between the increase in LA anteroposterior diameter and the mean predicted proportion of patients with AF recurrence after AF ablation.409 Although linear LA measurements are widely used in everyday practice and clinical trials, they may underestimate LA dilatation, since LA enlargement is asymmetric, mainly occurring in the medial-lateral and superior-inferior axes and to a lesser extent in the anteroposterior axis, due to constrainment of the LA within the thoracic cavity. Left atrial volume is a more accurate indicator of LA size, and it has been shown to independently predict AF recurrence following catheter ablation in a metaanalysis of 13 studies.496 Left atrial dilatation is suggestive of underlying atrial remodelling and correlates with AF progression and presence of fibrosis (Section 2).91–93
5.2.1.3. Electrocardiographic predictors
Electrocardiography is a widely available and inexpensive tool for evaluation of atrial substrate.499,500 Several P-wave indices have been associated with AF recurrences following catheter ablation.501–505 In a recent metaanalysis of 14 studies with 1674 patients, maximal P-wave duration and P-wave dispersion were shown to predict postablation AF recurrences.506 Prolonged P-wave duration on amplified 12-lead surface ECG also correlates with the extent of LA low-voltage substrate, and a cutoff value ≥150 ms was shown to identify persistent AF patients at increased risk for arrhythmia recurrence following PVI.507,508 In addition, several signal-averaged P-wave parameters, including total filtered P-wave duration, have been proposed to reflect the extent of underlying atrial remodelling and predict postablation AF recurrences.499,500,509 However, signal-averaged ECG measurements are rarely used in everyday practice.
5.2.1.4. Preprocedural imaging of atrial structure
Preprocedural documentation of atrial structural changes is useful to identify patients with advanced atrial remodelling and AF progression, who are less likely to have a favourable response to catheter ablation. Preprocedural imaging may provide relevant prognostic information with implications for guiding selection of patients considered more suitable candidates for ablation. Atrial fibrosis is the primary structural change associated with atrial cardiomyopathy, AF progression, and persistence and can be detected and quantified by LGE-MRI.99,103,510–513 In the DECAAF study, the extent of preablation fibrosis as assessed by LGE-MRI was an independent predictor of arrhythmia recurrence following AF catheter ablation.103 Implementation of this imaging modality requires experience in atrial LGE imaging and specific image sequences. Lack of reproducibility in atrial fibrosis assessment based on LGE measurement has limited its widespread adoption. Furthermore, discrepancies have been reported in the extent and distribution of fibrotic areas documented by LGE-MRI when compared with low-voltage areas identified during LA catheter-based mapping.106,514
Cardiac computed tomography (CCT) may be used to quantify LA epicardial adipose tissue, which is related to AF recurrence after catheter ablation. Several observational studies and a metaanalysis have shown that epicardial adipose tissue volume or thickness have a negative impact on AF ablation outcomes.515–519 Discrepant results have also been reported.520,521 In a retrospective observational study, enhanced attenuation of posterior LA adipose tissue, as an imaging marker of local inflammation, was associated with increased risk of AF recurrence in patients undergoing catheter ablation.521
Preprocedural imaging may also be useful for anatomic modelling to guide the ablation procedure. Though most patients have typical PV anatomy (Section 3.1.), unusual PV variants (PV draining at the roof, common trunk) occur and may influence choice of ablation approach and modality (single shot vs. point-by-point ablation). While postablation imaging is no longer routinely performed to check for PV stenosis in the absence of symptoms, preprocedural imaging in patients who have undergone prior RF ablation may be reasonable to identify unrecognized significant stenosis and/or avoid ablation in areas of mild-moderate PV narrowing.
5.2.2. Preprocedural pharmacological treatment
5.2.2.1. Preprocedural anticoagulation
Based on currently used stroke risk assessment that guides decision-making on eligibility for antithrombotic treatment, patients with AF and stroke risk factor(s) (CHA2DS2-VASc score ≥1 in males and ≥2 in females) who are scheduled for AF catheter ablation should receive oral anticoagulation therapeutically for at least 3 weeks prior to ablation.9,522 This panel of experts shares the opinion that a minimum of 3-week therapeutic anticoagulation before AF catheter ablation is also beneficial in patients with the lowest CHA2DS2-VASc score (0 in males and 1 in females) if they are considered to have increased risk of thrombus due to persistent AF type or specific underlying heart disease (HCM, rheumatic heart disease, and cardiac amyloidosis; Section 5.2.3.1.).
In the pre-DOAC era, several trials validated the superiority of performing catheter ablation without warfarin interruption.381–383 COMPARE was a large, randomized trial that demonstrated that undergoing AF ablation on uninterrupted vitamin K antagonist (VKA) compared with VKA interruption and bridging with low molecular weight heparin (LMWH) resulted in a significantly lower incidence of thromboembolic events and was associated with a lower minor bleeding risk.384 Importantly, there was no increased incidence of major bleeding in the uninterrupted group. In the VENTURE-AF, RE-CIRCUIT, AXAFA-AFNET 5, and ELIMINATE-AF trials, patients undergoing AF ablation on uninterrupted rivaroxaban, dabigatran, apixaban, and edoxaban, respectively, were compared with patients undergoing AF ablation on uninterrupted warfarin.385–388 The primary endpoint used in these studies varied slightly, as did the outcomes. In the VENTURE-AF, the primary endpoint of major bleeding did not differ between the rivaroxaban and warfarin groups385; in the RE-CIRCUIT, the primary endpoint of major bleeding occurred significantly less frequently with dabigatran compared with warfarin386; in the AXAFA-AFNET 5 and the ELIMINATE-AF, the primary composite outcome of all-cause death, stroke, or major bleeding did not differ between the DOAC (apixaban or edoxaban, respectively) and warfarin groups.387,388 Metaanalyses have documented a significant relative risk reduction in major bleeding (50–55%) with uninterrupted DOAC when compared with uninterrupted warfarin strategy at the time of AF ablation.389,390 Taken together, these studies provide strong evidence in favour of the use of uninterrupted anticoagulation with either DOACs or VKA during AF ablation procedures.
Several randomized trials have shown comparable efficacy and safety of a minimally interrupted DOAC anticoagulation strategy, skipping a single dose at the day of the procedure, when compared with uninterrupted strategy.394–398 A recent metaanalysis including 2168 patients reported similar rate of adverse clinical events (major bleeding, thromboembolic events) with minimally interrupted (holding morning DOAC dose on the day of the procedure without any LMWH bridging) when compared with an uninterrupted DOAC strategy.523 However, there was no sign of lower bleeding rates with preprocedural DOAC interruption.523 The randomized trials supporting the minimally (single dose) interrupted DOAC strategy had several limitations: most were single-center, mainly performed in Asian populations, and the sample size was insufficient to document non-inferiority. It is though acknowledged that conduction of an adequately powered randomized trial comparing the two preprocedural anticoagulation strategies based on standard sample size calculations is rather unrealistic due to a prohibitive sample size related to the very low event rate.
A survey of the writing group showed that 47% of the members routinely implement an uninterrupted anticoagulation strategy when performing AF catheter ablation, while 53% use a minimally interrupted anticoagulation approach (single skipped DOAC dose).
5.2.2.2. Preprocedural antiarrhythmic drug treatment
Many patients undergoing AF ablation are already on prior AAD treatment when scheduled for AF ablation. Optimal handling of AAD before ablation has not been clarified. Observational data support that failure of amiodarone to restore and maintain SR prior to AF ablation is not associated with poor procedural outcome.524 A retrospective observational study in 180 consecutive patients undergoing their first ablation procedure demonstrated a similar rate of symptomatic AF recurrences in patients undergoing ablation while taking AAD when compared with those who were not on an AAD at the time of ablation, at 6 months and at the end of follow-up (mean 24 months).525
Prior trials of persistent AF ablation employing extensive substrate ablation evaluated the impact of AAD continuation on intraprocedural SR restoration and procedural outcome. In a retrospective study of persistent AF patients undergoing a stepwise AF ablation, preprocedural amiodarone prolonged AF cycle length during catheter ablation and reduced substrate ablation needed to achieve SR without favourable impact on long-term outcome.526 A multicenter prospective randomized study of long-standing persistent AF patients on amiodarone therapy undergoing PVI plus substrate ablation also demonstrated that amiodarone continuation during ablation significantly reduced the procedure, RF, and fluoroscopy times.527 However, amiodarone continuation was associated with significantly increased late recurrence rates, which was attributed to AAD-mediated masking of non-PV triggers.527 In the absence of definitive evidence suggesting an impact of AAD continuation at the time of AF ablation on procedural outcome, pertinent recommendations cannot be issued.
5.2.3. Imaging for exclusion of thrombus
5.2.3.1. Candidates for thrombus screening prior to ablation
The presence of atrial thrombus is a contraindication for catheter ablation due to associated risk of procedural thromboembolic complications. In this context, patients undergoing catheter ablation should be screened to rule out the presence of thrombus. A survey of the writing group showed that 59% of the members routinely employ imaging for thrombus exclusion in all patients undergoing AF ablation irrespective of presenting rhythm, AF type, and prior anticoagulation. However, the adoption of uninterrupted periprocedural anticoagulation strategy has reduced substantially the rate of periprocedural stroke thus calling into question the need for routine screening of all patients undergoing AF ablation.399,528,529 Furthermore, several baseline factors increase the risk of thrombus detection and/or procedural thromboembolic event, supporting the adoption of a selective, individualized strategy of thrombus surveillance in patients undergoing AF ablation.
The writing group suggests that imaging for thrombus exclusion is reasonable in patients who are considered eligible for anticoagulation before AF catheter ablation (CHA2DS2-VASc score ≥1 in males and ≥2 in females, persistent AF, HCM, cardiac amyloidosis, or rheumatic heart disease—see below) but have not received anticoagulation therapeutically for 3 weeks or longer.
In the context of maximizing procedural safety, screening for thrombus may be reasonable even if patients have received therapeutic anticoagulation for 3 weeks or longer prior to catheter ablation in the presence of any of the following factors.
High CHA2DS2-VASc score. In an early trial assessing the value of systematic screening with transesophageal echocardiography (TEE) before AF catheter ablation, the prevalence of thrombus or sludge was shown to increase with increasing CHADS2 score.530 In a recent trial of consecutive DOAC-treated AF patients, higher CHA2DS2-VASc score significantly predicted the presence of TEE-detected LA thrombus before catheter ablation or scheduled electrical cardioversion.367 A recent metaanalysis of 35 studies assessing the prevalence of LA thrombus in adequately anticoagulated patients with AF/AFl undergoing TEE before cardioversion or AF catheter ablation demonstrated a significantly higher prevalence of LA thrombus in patients with CHA2DS2-VASc scores ≥3 when compared with those with scores ≤2 (6.3 vs. 1.1%, P < 0.001).368 Therefore, preprocedural imaging for thrombus exclusion may be a reasonable approach in patients with CHA2DS2-VASc score ≥3 scheduled for AF catheter ablation even if adequately treated with therapeutic oral anticoagulation for at least 3 weeks.
Persistent atrial fibrillation. Multiple anticoagulation trials have validated that persistent AF patients, even if adequately anticoagulated, are more likely to experience thromboembolic events when compared with those with paroxysmal AF after adjustment for baseline variables.328,369–371 In an older retrospective study of 1058 AF patients undergoing systematic screening with TEE to rule out atrial thrombus before AF ablation, patients with persistent AF had a 3% incidence of LAA thrombus when compared with 0.5% in patients with paroxysmal AF presenting in normal SR.530 In a multicenter retrospective study of 414 consecutive AF patients undergoing TEE before scheduled electrical cardioversion or ablation, LAA thrombus was documented in 15 patients and 93.3% of those had persistent AF.367 In a recent prospective registry, 900 patients with at least 3 weeks of prior uninterrupted therapeutic anticoagulation underwent AF ablation without any type of imaging screening. In total, four (0.32%) thromboembolic complications were documented, and all occurred in patients with persistent AF.529 In a recent metaanalysis of 14 653 adequately anticoagulated patients with AF/AFl undergoing TEE before cardioversion or AF catheter ablation, non-paroxysmal AF was associated with a four-fold higher LA thrombus prevalence when compared with paroxysmal AF.368 Based on the abovementioned evidence, persistent AF patients have an increased risk of LA thrombus irrespective of their anticoagulation status.
Hypertrophic cardiomyopathy—rheumatic heart disease—cardiac amyloidosis. Atrial fibrillation patients with HCM have significantly higher incidence of ischemic stroke compared with those without HCM.372 Hypertrophic cardiomyopathy patients with AF categorized as low risk based on their CHA2DS2-VASc score (0 in men or 1 in women) have a significantly greater stroke risk than AF patients without HCM and a CHA2DS2-VASc score of 2.372 In a large cohort of patients with AF undergoing TEE, HCM patients had a significantly higher risk of LA thrombus than matched control subjects (8.8 vs. 4.1%; P < 0.001) despite high rates of anticoagulation at the time of TEE and continuously for 1 month prior.380
Several small observational studies have shown that patients with rheumatic mitral stenosis have a substantially increased incidence of LA thrombus as documented by TEE even when in SR, varying from 2.4 to 25%.373–375
Patients with cardiac amyloidosis have an increased incidence of intracardiac thrombus, even in the absence of AF/AFl, due to the amyloid infiltration, which results in atrial enlargement, mechanical dysfunction, and blood stasis.376,377 In a series of 116 autopsies, intracardiac thrombus was identified in 33% of amyloidosis cases, with AL amyloidosis and AF being independently associated with thromboembolism.376 In a single-center, retrospective analysis of patients referred for elective direct cardioversion for atrial arrhythmias, patients with cardiac amyloidosis had 10 times higher rate of TEE-documented LA/LAA thrombus when compared with a matched cohort (28.5 vs. 2.5%) even if anticoagulated for ≥3 weeks before TEE.378 In a more recent observational study, LA thrombus was present in 14% of patients with cardiac amyloidosis referred for electrical cardioversion, despite prior anticoagulation, mainly with DOACs.379
Based on the abovementioned evidence, patients with HCM, rheumatic heart disease, or cardiac amyloidosis are considered as high risk for stroke, and therefore, routine preprocedural screening for thrombus exclusion may be reasonable irrespective of their CHA2DS2-VASc score or previous anticoagulation.
5.2.3.2. Imaging modalities for thrombus exclusion
Several imaging modalities may be used for exclusion of atrial thrombus in patients undergoing AF ablation. The selection of a particular imaging tool is based on patient's characteristics, physician preference and expertise, institutional availabilities, and cost. Available options are discussed below.
Transesophageal echocardiography. Transesophageal echocardiography has long been used in the preablation setting since it enables reliable exclusion of atrial thrombus and assessment of LA size, functional parameters, and valvular disorders. In an early large prospective intraoperative study, TEE had a sensitivity and specificity of 100 and 99%, respectively, for identifying atrial thrombi when compared with direct visual inspection of LA content.531 However, TEE is a semi-invasive procedure requiring sedation and esophageal intubation, occasionally limited by subjective estimates, and is not devoid of complications (0.18–2.8%), which may be associated with major morbidity (0.2%) and rarely mortality (<0.01–0.02%).532,533 Transesophageal echocardiography prior to the ablation procedure may lengthen the procedure and general anesthesia time, while it can prove helpful in guiding transseptal puncture.
Cardiac computed tomography. Delayed phase CCT is a useful and reliable imaging modality for exclusion of atrial thrombus. Incorporation of late acquisition protocols reduces false-positive rates by providing a time delay to allow enhanced LAA contrast opacification and differentiate whether a low attenuation region is due to thrombus or circulatory stasis and low blood flow. A metaanalysis of 22 studies demonstrated that CCT had a sensitivity and specificity of 0.99 and 0.94, respectively, vs. TEE.407 Delayed imaging CCT protocols significantly improved specificity when compared with early imaging protocols.406,407 A recent prospective cohort analysis evaluating optimal time delay for late phase CCT protocols demonstrated that even a 3-min delay may be associated with false-positive results, while a 6-min delayed acquisition protocol is optimal due to associated 100% specificity.408 Related disadvantages include (i) the risk of contrast-induced nephropathy, which is low in patients with normal renal function and reversible in most cases, and (ii) the related radiation exposure, which is though relatively low (<3 mSv) with contemporary technology computed tomography (CT) scans.406,534,535
Another prerequisite for recommending CCT for exclusion of atrial thrombus prior to AF ablation is its performance within 48 h prior to ablation to prevent the likelihood of de novo thrombus formation in the waiting period between screening and ablation.
Cardiac magnetic resonance imaging. Cardiac MRI enables imaging of atrial anatomical features and structural changes that are important for procedural planning (Section 5.2.1.4.). It also has a favourable diagnostic performance for assessment of atrial thrombus.536–538 A recent metaanalysis of four CMR studies reported a sensitivity and specificity of 0.80 and 0.98, respectively, when compared with TEE.407 However, the existing trials supporting the role of CMR for thrombus exclusion are limited, single-center, rather small in number, heterogeneous in type of MRI sequence used, and with uncertain reproducibility. Large-scale studies with standardized and consistent CMR protocols are needed to support the value of MRI as reasonable imaging option for exclusion of atrial thrombus in the preablation setting.
Intracardiac echocardiography. Intracardiac echocardiography (ICE) is increasingly used as an alternative to TEE for exclusion of LAA thrombus at the time of AF ablation.400 In an early study comparing ICE with TEE in patients undergoing AFl ablation, ICE showed very high correlation with TEE for detection of LA stunning.401 Since then, several prospective studies comparing ICE with TEE for detection of LAA and/or LA thrombus have indicated that ICE is as effective as TEE.402,403 The ICE-CHIP study compared ICE with TEE and observed a non-significant trend to increased thrombus detection with TEE.404 However, ICE imaging was performed from the RA, and it is now well-established that optimal LAA views are obtained when the ICE catheter is positioned in the right ventricle or the pulmonary artery.405 Intracardiac echocardiography may also have a role for LA thrombus screening after a recent equivocal or even negative TEE in patients undergoing AF ablation.405 In a prospective multicenter registry of 6186 patients undergoing AF ablation on uninterrupted DOAC anticoagulation, ICE was used to screen for LA/LAA thrombi. In this population with mean CHAD2S2-VASc score of 2.9, no thrombi were observed and only one TIA occurred.399 In practices familiar with ICE, it is reasonable to use ICE, with imaging from the RV inflow tract or pulmonary artery, instead of TEE to screen for LA/LAA thrombi prior to ablation. Since ICE may be used at some centers to guide transseptal puncture and monitor for complications, use of ICE to screen for thrombi may save procedural time and cost (compared with additional TEE or CCT performance).
A survey of the writing group showed that 59% of the writing group members mainly use TEE for exclusion of LA thrombus, 18% use ICE, and 23% use cardiac CT.
6. Mapping and ablation tools for atrial fibrillation catheter ablation
6.1. Mapping tools
6.1.1. Invasive mapping tools
6.1.1.1. Electroanatomical contact mapping
Mapping and ablation require precise navigation within the chamber of interest. Electroanatomical mapping (EAM) systems allow for 3D visualization of the anatomy of any heart chamber, delineation of AT/AFL circuits, catheter positioning, and catheter manipulation without use of fluoroscopy. Accurate anatomical reconstruction of the 3D shell of the chamber of interest is of the utmost importance. However, inaccuracies are caused by continuous motion of the heart, patient breathing, and body movement. All available EAM systems have a set of algorithms to account for movement (including respiratory gating). Mapping can be performed either with point-by-point acquisition using the ablation catheter or more frequently using dedicated multielectrode catheters.
In general, there are two types of EAM systems. Impedance-based systems utilize a transthoracic electrical field for catheter localization, which is created by surface patch electrodes that emit high-frequency electrical signal in three orthogonal axes. Drawbacks include that the field is non-linear, impedance is affected by changes in tissue properties, and the co-ordinate system (patches) can move. Magnetic field–based systems, on the other hand, are not affected by tissue differences and are inherently linear and stable over time. Magnetic sensors are needed to visualize catheters, so map creation is only possible with sensor-enabled catheters. The co-ordinate system is often linked to the fluoroscopy unit under the patient and can be distorted by metal (bed, flat detector, ICDs, etc.).
Several EAM systems are currently on the market. The CARTO 3 (Biosense Webster, Irvine, CA, USA) is a hybrid system, which uses magnetic technology for localization of dedicated mapping and ablation catheters and current-based technology for visualization of electrodes and shaft of other electrophysiology catheters. Integration of ICE into the CARTO mapping system (CARTOSOUND, Biosense Webster) enables the construction of the 3D shell of the chamber of interest from a series of ICE-acquired chamber contours with associated reduction in fluoroscopy time even to zero.539,540
The Ensite NavX (Precision) EAM system (Abbott, Chicago, IL, USA) uses both voltage and impedance data for localization of proprietary and non-proprietary mapping and ablation catheters. In addition, the system integrates magnetic information for dynamic optimization of 3D models (field scaling) when sensor-enabled catheters are used. The latest generation of the Ensite NavX (Ensite X) EAM system uses magnetic-based data from sensor-enabled catheters to create 3D maps, also maintaining the option to rely on impedance data for map creation and catheter visualization.
The Rhythmia system (Boston Scientific, Marlborough, MA, USA) uses a combination of magnetic and impedance-based location technology and allows for automated high-density mapping using a dedicated steerable 64-electrode mini basket catheter (Figure 9).
Figure 9.
Multielectrode mapping catheters.
Multimodality approaches allow for image integration of pre-acquired CCTs and MRIs with real-time 3D EAM maps or ICE imaging. Accuracy is critically dependent on fusion quality (including the use of fiducial points in both images). In addition, 3D rotational angiography images can be merged with live two-dimensional fluoroscopy and thus obviate the need for pre-interventional imaging. The quality of EAM acquired with multielectrode catheters has generally reduced the need for fusion with pre-acquired images due to the higher resolution of the former and errors associated with the latter.
The quality of electrogram acquisition is crucial for accurate annotation of signals, particularly in low-voltage areas and diseased atrial tissue. Furthermore, mapping catheters now have multiple bipoles with smaller electrodes for signal recording, allowing faster acquisition of high-quality signals (Figure 9). Synthesizing all this information from multiple electrograms requires mathematical algorithms, which automate electrogram annotation and timing.
Newer modules are helping operators to delineate complex tachycardias. A new module in the latest CARTO 3 platform provides an algorithm, which computes conduction velocity based on collected electrograms and applies a global best-fit solution when displaying wave propagation (Coherent Mapping).541 Activation direction mapping in the latest generation EnSite X system uses ‘omnipolar’ EGMs correcting for voltage differences caused by directionality of the electrical wavefront in relation to the mapping electrodes. Using these corrected electrograms, the system can compute a beat-by-beat vector direction of the wavefront and present the information in a propagation map.542 The incremental value of both coherent and omnipolar mapping in the management of complex atrial tachyarrhythmias needs to be proven in prospective studies.
Electroanatomical mapping systems have attempted to incorporate algorithms for mapping persistent AF. CARTOFINDER is an algorithm used to map focal and rotational sources during AF, with clinical trials pending.543–545 Both CARTO and Ensite include optional algorithms for automated detection of complex fractionated atrial electrograms (CFAE) during AF and tagging of respective areas on the 3D anatomical map. Other algorithms are in development (Section 6.4.).
The evolution of ablation indicators (Section 8.1.2.2.) incorporating power, ablation duration, and contact force (CF) in one formula to assess lesion quality has further leveraged EAM systems. Radiofrequency-based ablation incorporating CF-sensing ablation catheters combined with quality lesion indicators allows for automated tagging of ablation points to interactively review the position and quality of RF lesions.
Using EAM systems has proven to reduce fluoroscopic duration and dose.546–548 However, studies assessing the clinical benefit of safety and efficacy in various arrhythmias have initially shown mixed results.549–553 The implementation of the CLOSE protocol in RF-based AF catheter ablation has proven both effective and safe554–559 and consequently standardized the PVI procedure using the CARTO mapping system (Section 8.1.2.2.).
The cost of the ablation procedure is inevitably increased when using EAM systems with high complexity (multipolar catheters, mapping system upgrades). This cost needs to be weighed against the benefits described above. However, the future role of 3D EAM systems in PVI procedures using single-shot or large footprint ablation catheters is at present unclear.
6.1.1.2. Non-contact mapping
Most EAM systems require contact between the mapping catheter and cardiac tissue to accurately record cardiac potentials. It is difficult, however, to achieve stable, complete contact of multipolar or even single point catheters on the cardiac surface, and therefore, interpolation algorithms are required to smooth out the electrical data collected. This limits both the spatial and temporal accuracy of such recordings and can obscure finer, detailed patterns of activation. It is also difficult to obtain a global, instantaneous, panoramic view of a chamber's activation sequence when recordings of the various chamber segments are collected sequentially in a stepwise fashion.
Non-contact mapping utilizes a large, multipolar catheter positioned within the chamber of interest to record global far-field and near-field unipolar electrograms. These electrograms are then typically fed into a mathematical algorithm, which can interpolate and extrapolate global activation based on the ‘ground-truth’ of the recorded unipoles.560 The resulting activation patterns can then be projected onto a map surface.
One system (AQMap, Acutus Medical) utilizes a multi-splined, 48 electrode catheter, equipped with ultrasound transducers for reconstruction of the anatomic surface, which records unipolar electrograms and then uses an inverse algorithm to calculate the ‘charge density’ on each point of the map surface.560 Charge density is calculated from the local unipolar voltage but filters out putative far-field effects to provide a higher resolution local electrical activation. Early results have suggested that complex atrial activations during AF can be identified, but the additive effect of targeting these activations in the persistent AF ablation strategy is not well-established.561 Furthermore, the system loses accuracy when the cardiac surface of interest is 40 mm or more from the catheter.562
Electrographic flow mapping (Ablamap, Ablacon) utilizes a large basket catheter to record unipolar electrograms, which are then interpolated and processed into a beat-by-beat electrical intensity map.563 As the electrical intensity changes beat-by-beat in AF, the Horn–Schunk iterative algorithm calculates the flow pattern seen during AF over time. Regions with divergent flow patterns can be labelled as ‘sources’, which can then be targeted for ablation. The recently announced FLOW-AF trial randomized persistent or long-standing persistent AF patients with recurrent, symptomatic AF despite at least one prior AF ablation procedure to PVI plus electrographic flow-guided source ablation or to PVI only. Adjunctive ablation of electrographic flow sources resulted in significant improvement in 1 year freedom from AF.564
6.1.1.3. Spatiotemporal dispersion mapping
Mapping and ablation of regions exhibiting CFAEs was pioneered many years ago by the work of Nademanee et al.565 However, the STAR-AF II trial did not show an incremental benefit in rhythm outcome when CFAE ablation was performed in addition to PVI during AF ablation.566 Several studies have shown promising results regarding the efficacy and safety of AF ablation guided by spatiotemporal electrogram dispersion.567–569 Seitz et al. described an ablation approach where regions displaying spatiotemporal ‘dispersion’ were targeted.567 Dispersion areas were visually identified and defined as clusters of electrograms, either fractionated or non-fractionated, that displayed interelectrode time and space dispersion at a minimum of three adjacent bipoles such that activation spans the AF cycle length. These electrograms could be continuously fractionated, burst fractionated, or of very rapid cycle length. Areas displaying spatiotemporal electrogram dispersion can be identified either manually or automatically with the use of machine and deep learning algorithms (VOLTA VX1) that perform real-time analysis of electrograms recorded by multipolar catheters and then annotate areas of interest on the 3D anatomical shell, which represent potential ablation targets.570 The TAILORED AF trial (NCT04702451) is comparing an ablation strategy targeting areas of spatiotemporal dispersion identified with the use of this artificial intelligence software algorithm in combination with PVI to PVI alone in patients with persistent AF and will provide further data on the efficacy of this approach. Stability and reproducibility of identified target sites displaying spatiotemporal dispersion needs to be documented.
6.1.2. Non-invasive mapping tools
6.1.2.1. Electrocardiographic imaging
In recent years, a drive towards better understanding of AF mechanisms has resulted in the emergence of new forms of AF mapping technology, which employ the principle of phase mapping, a mathematical approach for the assessment of spatial and temporal periodicity in tissue electrical activity and identification of periodic rotations or ‘rotors’.571 Optical mapping work in animal models provides compelling evidence for the existence of rotors and their role in AF perpetuation, and these insights served as the basis for clinical translation to AF mapping.572
Electrocardiographic imaging or ECGI mapping is a non-invasive phase-mapping approach, which utilizes a 252-body surface electrode array and patient-specific heart-torso geometries to display virtual cardiac potentials on the epicardial surface. Activation mapping in AF allows identification of reentrant and focal activities generated from unipolar electrograms combined with phase-mapping analysis. Potential advantages over conventional invasive mapping systems include non-invasive, simultaneous, global characterization of biatrial electrical activity, albeit at a lower mapping resolution.573 Three-dimensional imaging acquisition is central to the technique for generation of individualized anatomical models of the atria and torso volume conductor. Computed tomography imaging is most commonly utilized due to its speed, widespread availability, and high-resolution imaging with the obvious disadvantage of exposure to ionizing radiation.574 Magnetic resonance imaging provides better soft tissue delineation albeit at a lower resolution, while eliminating radiation risk.512 However, higher costs and longer scan times limit its applicability. The recently developed imageless ECGI overcomes the need for CT or MRI imaging by estimating the cardiac geometry and location inside the patient's thorax based on electrical, statistical, and thoracic geometrical information.575
The ECGI technique was first applied in a study of continuous biatrial activation mapping validated against invasively generated electroanatomical maps.576 In this study, multiple concurrent wavelets were identified as the most common pattern of activation and ablation near ECGI-identified critical sites resulted in restoration of SR. Using commercially available mapping systems, unstable reentry circuits with varying spatiotemporal activity were described as the predominant sustaining mechanism in persistent AF patients.577,578 In patients with ablation-induced AF termination, arrhythmia-free survival was 85% at 1 year.577 In the AFACART study, an ablation strategy consisting of targeted ablation of AF drivers and PVI, followed by LA linear ablation if AF persisted, resulted in 77% freedom from AF at 1 year in a cohort of persistent AF patients with continuous AF duration less than 1 year.579 Driver-only ablation resulted in AF termination in 64% of patients.579 The TARGET-AF1 trial reported a 65% freedom from recurrent AF/AT at 1 year in persistent AF patients undergoing PVI plus ECGI-guided ablation with a high rate of AF termination during driver ablation.580
More recently, ECGI findings indicative of AF complexity, including number, distribution, and density pattern of AF reentrant sites, have been associated with AF termination during ablation.581,582 The ongoing STRATIFY trial will further evaluate the role of ECGI-based assessment of AF complexity for prediction of ablation efficacy (NCT04578275).
Inherent limitations of ECGI relate to electrode density and mapping resolution. Transformation of electrograms using phase-mapping is a complex process, and an obvious disadvantage is the limited ability for raw signal analysis by the operator prior to transformation. In addition, ECGI mapping is costly and time consuming. Finally, the lack of a gold standard for validating the existence of rotors/drivers and conflicting results using ECGI vs. other phase-mapping approaches have fuelled scepticism about validity and reproducibility.571,577,578
Nevertheless, ECGI remains the only modality capable of simultaneous biatrial electrical characterization in AF. Further assessment in randomized trials as well as technological improvements to streamline workflows is needed before it can be incorporated as a valid tool in the routine invasive management of AF patients.
6.1.2.2. Magnetic resonance imaging fibrosis guidance
Magnetic resonance imaging has been used to identify areas of atrial fibrosis in patients scheduled for AF catheter ablation.105,583,584 However, adequate spatial resolution remains problematic in the thin-walled LA, and reproducibility of the different imaging techniques across centers remains low (Section 5.2.1.4.). Several RCTs have failed to document that ablation of MRI-detected fibrotic areas provides incremental benefit in postablation rhythm outcome (Section 8.2.6.).
6.2. Ablation tools
6.2.1. Radiofrequency ablation
Radiofrequency catheter ablation is a widely employed thermal-based technique with documented beneficial effect on rhythm outcome when compared with medical therapy in paroxysmal and persistent AF patients.236–238 Initial studies reported on PVI using non-irrigated catheters with RF delivery in temperature-controlled mode. Since the introduction of irrigated catheters, RF is most often delivered in power-controlled mode with conventional power settings between 20 and 40 W. The positive impact of CF measurement on procedural and RF time and recurrence rates585–588 has resulted in the adoption and widespread use of irrigated CF-sensing catheters and additionally facilitated the development of algorithms aimed at real-time assessment of lesion quality including the force time integral (FTI),589 lesion size index (LSI),590 and ablation index (AI)558,591 (Section 8.1.2.2.). Within the last years, the employment of point-by-point workflows using CF-sensing catheters and focusing on optimized and contiguous lesions has resulted in improved outcomes for paroxysmal AF, with high first-pass isolation rates and 1 year success rates.554,555,558,559
More recently, focus has centered on enhanced lesion formation for durable PVI, with increased power delivery proposed to improve lesion quality and reduce procedure times. Experimental studies have demonstrated shallower and wider lesions with higher power and shorter duration lesions,592,593 and several clinical studies have underscored the enhanced procedural efficiency and preserved safety profile associated with 40–50 W ablation in power-controlled mode.594–599 In a recent small RCT comparing high-power (50 W) vs. standard power (30 W anterior/25 W posterior wall) RF ablation in patients undergoing PVI, the former resulted in significantly shorter time to achieve PVI, higher freedom from arrhythmias at 12 months, and a trend towards increased asymptomatic cerebral emboli.600 Furthermore, power-controlled ablation at 70 W over 5–7 s is associated with significantly greater procedural efficiency, fewer AF recurrences, and a similar safety profile to conventional power protocol (30–40 W for 20–40 s).601,602 The absence of use of AI or LSI to standardize the lesion set in these latter studies may limit the reproducibility of the results. Care should be exercised using higher power at the posterior wall due to potential inadvertent overshoot and ‘heat stacking’ when applying consecutive lesions in close proximity, although it is also possible that a high-power short-duration (HPSD) protocol may be safer over the esophagus due to less depth of penetration (Section 11.3.1.).599,603 Based on recent RCT findings, HPSD ablation may be associated with increased risk of asymptomatic cerebral emboli (Section 11.2.3.).
The reduced accuracy of tissue temperature feedback during high-power irrigated ablation has led to the development of novel catheters equipped with multiple thermocouples capable of accurate, real-time tissue temperature monitoring, allowing RF delivery in temperature-controlled mode using low irrigation flow rates (QDOT Micro, Biosense Webster; DiamondTemp, Medtronic). The randomized DIAMOND-AF study demonstrated similar safety and efficacy of the DiamondTemp ablation system compared with standard CF-guided ablation with higher overall power delivery and reduced procedure times using temperature-controlled ablation.604 The CF-sensing QDOT catheter is capable of energy delivery of up to 50 W in ‘QMODE’ with a recent study supporting the safety and efficacy of this modality with a first-pass isolation rate of 92% and no esophageal injury on postprocedural endoscopy.605 Furthermore, this catheter is capable of very high power delivery at 90 W over 4 s (QMODE+). Several pre-clinical studies report a predominant resistive form of tissue heating with 90 W/4 s ablation with a high rate of contiguity and transmurality.592,606–608 In contrast, in a recent canine study, lesion size was smallest with RF applications at 90 W/4 s, followed by 50 W/10 s and greatest with 30 W/30 s.609 The QDOT-FAST study demonstrated the feasibility and safety of 90 W/4 s ablation in paroxysmal AF patients undergoing PVI.610 The safety profile of this ablation workflow was further supported in a study showing lack of esophageal injury.611 More recently, a small non-randomized study reported significantly reduced procedural times with 90 W/4 s vs conventional 25–40 W ablation with a similar safety profile.612 In contrast, a further comparative study suggested an overall similar procedure time (with time being lost due to a lower rate of first-pass isolation with 90 W/4 s ablation protocol).606 In the multicenter randomized POWER PLUS trial, first-time PVI with very HPSD (90 W/4 s) RF ablation resulted in a significant but modest reduction in procedure time with similar safety and 6-month arrhythmia recurrence rate when compared with 35/50 W AI-guided conventional ablation.613 Furthermore, despite the potential of HPSD ablation to alleviate the issue of catheter instability, when it does occur it may have a greater impact on lesion formation particularly in areas of increased tissue thickness such as the carina.
In summary, contiguous, point-by-point RF ablation for PVI has evolved into a clinically safe and efficacious procedure. With high-power or very high-power ablation (in temperature-controlled or power-controlled mode), procedural times of close to or less than one hour are achievable with similar safety and efficacy profiles.596,606 In the absence of definite randomized data on long-term outcomes, the decision to opt for novel higher power strategies may come down to operator preference or patient profile (shorter procedure times are preferable in awake patients, whereas low fluid delivery may be advantageous in patients with HF). Large-scale randomized trials are needed to determine the long-term efficacy of such novel strategies.
A multielectrode RF balloon catheter (HELIOSTAR, Biosense Webster) has also been used for PVI.614 This single-shot ablation device is compatible with a 3D EAM system (CARTO) and is equipped with 10 irrigated, flexible electrodes that independently deliver RF energy, thus allowing customization of energy delivery in a focal, segmental, or circumferential approach. Furthermore, the use of an integrated, intraluminal, circular diagnostic catheter enables real-time recording of PV electrograms. Observational studies have demonstrated that PVI with this multielectrode RF balloon catheter may have favourable safety and effectiveness in paroxysmal AF patients.615–618
Another recent development in the field of RF ablation is a larger footprint, single-tip ablation device. Instead of a 3–4 mm, irrigated terminal tip, this catheter employs a lattice spherical structure, which is 9 mm in diameter, and the surface of the sphere contains nine mini-electrodes (0.7 mm), which also contain thermocouples.619 The system delivers temperature-controlled, contact sensing–facilitated RF with saline irrigation sprayed from the center of the catheter, which does not impact temperature sensing at the surface of the catheter in contact with the tissue. The system delivers high-current RF to achieve a uniform current cloud on the lattice spherical surface.619 In an initial pilot study, the system demonstrated a very high incidence of durable PVI and linear lesion block.619 The system has also incorporated PFA offering versatility in type of delivered energy.620
6.2.2. Cryoablation and ultra-low temperature cryoablation
6.2.2.1. Conventional cryoballoon technologies
Cryotherapy, or the use of freezing temperatures to elicit a specific tissue response, has a long history of safe and effective use in medicine. After open cardiac surgery applications had developed in the 1960s and 70s, the first clinical experience using a focal tip cryoablation catheter—targeting the AV node—was described in 2001.621 Finally, the development of a balloon-based cryothermy in the beginning of the 21st century has led to a striking uptake in cryoablation for AF ablation. A single-shot balloon design delivers significant benefits over a focal design. Firstly, it allows procedural simplification (no need for mapping systems, potential time gains), and secondly, it blocks antegrade flow from the targeted PV, thereby eliminating balloon heating by the blood pool and greatly enhancing cryoablation efficacy. However, a caveat related to the absence of mapping system in the cryoballoon procedural workflow is the increased fluoroscopy exposure.622
All currently available cryoballoon catheters have an open inner lumen to allow insertion of a guidewire or a diagnostic catheter and use N2O as a coolant, exploiting the Joule–Thomson gas expansion effect to achieve temperatures down to a theoretical minimum of −89°C. The first-generation cryoballoon (Artic Front, Medtronic, Inc) showed superiority over AADs in a randomized setting.239 However, over 80% of patients with AF recurrence after first-generation cryoballoon ablation showed PV reconnection at the time of repeat ablation and over 50% had reconnection of more than one PV.623 The second-generation cryoballoon (Arctic Front Advance, Medtronic, Inc.) was introduced in 2012 and incorporates a modified refrigerant injection system characterized by eight injection jets in a more distal balloon position. Thus, a more homogeneous cooling of the complete distal balloon hemisphere, including the distal tip, is achieved.
More recently, the POLARx (Boston Scientific) cryoballoon catheter received approval, and clinical experience has accumulated. A recent multicenter registry has validated the procedural safety and efficacy of this cryoballoon for the treatment of patients with paroxysmal AF.624 Despite similarities in ablation technique and catheter design, important differences exist when compared with other cryoballoons.625,626 In the COMPARE-CRYO trial, 201 symptomatic paroxysmal AF patients undergoing their first PVI procedure were randomized to cryoballoon ablation using either the POLARx or the Arctic Front catheter and were monitored with an ICM. The freedom from arrhythmia recurrence at 12 months was similar in both groups, but the use of the POLARx balloon resulted in significantly higher rate of PN palsies that did not recover within 24 h.627
The FIRE AND ICE trial compared energy modalities for AF ablation and randomized 762 patients with drug-refractory paroxysmal AF to treatment with either the second-generation cryoballoon or conventional RF using a prospective multicenter design. The study showed cryoballoon ablation to be non-inferior to RF ablation with respect to its primary endpoints of efficacy and safety and reported a possible reduction in re-hospitalization or reablation in secondary analyses.294,628 Phrenic nerve injury was the most commonly reported complication at discharge in the cryoballoon ablation group (2.7%). Permanent PN palsy was reported in 0.3% of patients. More recently, the CIRCA-DOSE trial evaluated contemporary approaches to PVI using latest generation technology in both the cryoballoon ablation as well as the RF ablation arms using an ICM for postablation rhythm monitoring.622 In this trial, no difference in 1 year efficacy (freedom from atrial tachyarrhythmia) was confirmed between the compared groups, whereas continuous monitoring showed median AF burden reduction of >99% with both ablation technologies.
6.2.2.2. Ultra-low temperature cryoablation
An innovative approach using highly compressed liquid nitrogen allows a change from the liquid to the gaseous phase without the associated volume expansion and thus without the associated problems of vapour lock when using liquid nitrogen in closed circuit catheters.629 The implications are that an ablation energy source with a far wider therapeutic margin can be used—liquid nitrogen boils at −189 °C—and that cryocatheter design is no longer constrained by the need for occlusion.
Currently, a single platform using this technology is commercially available in the EU (iCLAS, Adagio Inc) and is undergoing clinical evaluation in the USA (IDE # G180263). The iCLAS system allows the use of variable shape catheters that enable rapid reconfiguration of the catheter to the desired target tissue. The recently published first in man CRYOCURE-2 observational study reported promising acute procedural results and 12-month freedom from atrial arrhythmias in a mixed paroxysmal and persistent AF population.630 Potential synergies between ultra-low temperature cryoballoon and PFA may exist, such as guaranteed tissue contact and elimination of microbubbles. Evidence for this strategy is currently limited, and a trial assessing its usefulness is underway (NCT05408754).
6.2.3. Pulsed field ablation
6.2.3.1. Biophysics and mechanisms
In contrast to RF or cryothermy, irreversible cardiac electroporation is considered a non-thermal energy source, meaning the cell death induction is not dependent on thermal processes. Instead of exposing cells to a thermal insult, electrical fields are applied to the cells leading to a disruption in cell membrane integrity and function.631 This short-term disruption then leads eventually to cellular death and replacement fibrosis. Ablation using irreversible electroporation is more commonly referred to as PFA.
The exact mechanism of PFA-induced cell death is not known.631 Application of electrical fields of sufficient strength will lead to accumulating charge on cell membranes, which can result in development of nanopores in the membrane surface and increased membrane permeability. This permeability disrupts the intracellular and extracellular concentration gradients required for cellular homeostasis. If the electrical field application is sufficiently long, alterations in cellular pH, generation of reactive oxygen species, release of mitochondrial cytosome c, and other processes all result in a progression to cellular apoptosis combined with some immediate cellular necrosis.632–634 These processes occur over days to weeks and lead eventually to replacement fibrosis over 4–8 weeks. Unlike thermal ablation, PFA does not permanently disrupt the local tissue extracellular matrix structure or vascular supply,635 which is a critical element in why PFA may not result in as much collateral damage to non-cardiac tissues.631
Electrical field ablation was pioneered in the 1980s when Scheinman et al.636 delivered a full defibrillator shock through a catheter in the heart. The investigators achieved heart block, but the accompanying heat and barotrauma sidelined direct current ablation and paved the way for RF ablation. The key technological advance for today's PFA is that a ‘large charge’ can be broken up into a series of multiple applications of very brief duration. Since the cardiac cell is like a capacitor that can store charge, the intensity of the electrical field will depend on the total duration of the exposure (number of repeated applications) in addition to the actual voltage delivered.631
Key parameters for PFA include voltage, pulse width, waveform (biphasic vs. monophasic), and polarity (bipolar vs. monopolar). Increasing voltage will not only increase treatment effect but can also generate unwanted heating, gaseous microbubbles, and barotrauma.633,637 Most systems approved or in development today are utilizing voltages of 500–2000 V peak-to-peak for each application. Monopolar PFA delivers energy from a single catheter to a return ground patch. Bipolar PFA delivers energy between adjacent electrodes and is more suited to larger, efficient, multipolar ablation catheters. Pulse width is also critical for treatment effect and minimization of gaseous emboli, muscle contraction, and unwanted heat generation. Most systems are utilizing pulse widths in the microsecond range. Delivery of pulses (typically 10–20) usually occurs in a series called a ‘packet’ or ‘train’ and then multiple trains (1–7) may be delivered over several seconds.
Although PFA is supposed to be non-thermal, the recipes used for ablation today encroach on the thermal threshold. For biphasic, bipolar pulses, there can be 5–40°C rises in tissue surface temperature.631,638,639 However, these rises are for such brief duration (a few milliseconds) that significant thermal damage does not occur. Contact between the tissue and catheter is still required for optimal PFA delivery.640 Whether CF is required for optimal PFA delivery is still an open question.
6.2.3.2. Efficiency and safety—key advantages
Since PFA can be delivered over several milliseconds and several packets can be delivered within seconds, procedural efficiency is one of the key advantages of this energy source. Furthermore, PFA can be easily delivered in large footprint (so-called ‘single shot’) devices, which can achieve large lesions around the PVs and on the posterior wall with ease. Even in the early evaluation studies, where operators were very early in their learning curve, LA dwell times were only 60–90 min or less.641,642 Now that some systems are available commercially, early registries report average procedure times close to or even less than one hour for AF ablation.643,644
The safety profile of PFA also makes it very promising for cardiac ablation. With thermal ablation, there has always been a low, but detectable, risk of collateral damage to the lung, esophagus, and PN. Esophageal damage can lead to fatal complications such as an atrial-oesophageal fistula (AEF; Section 11.3.1.). Early preclinical data have suggested that the field threshold for damaging cardiac cells is much lower than that required for smooth muscle (esophagus),645 vascular (veins and arteries),635 and nerve cells.646 Adipose tissue is an excellent insulator for electricity, and even thin fat layers separating esophageal tissue from the LA may have a significant protective effect.631 Preclinical data have confirmed that clinical PFA systems approved or under development do not cause long-term esophageal damage.638,647,648 Esophageal temperature rises are not seen during ablation over the esophagus in humans.642 While acute PN capture can occur during PFA applications, PN palsy is very rare.643 Even when PN palsy occurs, it typically recovers within a few hours.649 PFA also does not appear to cause PV stenosis.643,650 Skeletal muscle contraction was a problem with early versions of PFA, but with the implementation of more optimized waveforms (biphasic especially), this risk is reduced, and most studies have shown that PFA can be safely performed without the use of paralytics.641,642,651
Microbubble formation has been observed with most PFA systems. It is unclear whether this is due to unwanted heat generation, an electrolytic effect on water, or displacement of nitrogen gas.633 The size of the bubbles appears to be small (<40 μm),652 and if gaseous, they should spontaneously resorb prior to causing significant cerebral ischemia. Early studies have suggested a low rate of silent cerebral emboli on cerebral MRI post-PFA ablation (3%), but further studies are required to confirm the potential risk of these bubbles.641,653 High Joule monophasic pulse deliveries, e.g., can cause very large volumes of these bubbles and have been associated with ST segment elevation and MRI lesions indicating embolic ischemia.654
Coronary arterial spasm has been reported with PFA deliveries in proximity to coronary vessels655,656 (Section 11.2.2.). The spasm persisted even after delivery was terminated and required injection of intracoronary nitroglycerin to terminate the process. Cough has also been frequently reported even in anesthetized patients, which may be due to field stimulation of the J receptors within the PVs or due to bronchial stimulation.631 In large cohorts of unselected patients, the safety profile of PFA was consistent with preferential tissue ablation.643,644
6.2.3.3. Efficacy of pulsed field ablation
Pulsed field ablation can be delivered from a variety of different catheter shapes and styles. It can be delivered from larger, multipolar catheters creating a ‘large footprint’ ablation. It can also be delivered from balloon-style devices. Finally, it can also be delivered from standard point-by-point RF-style catheters (3.5 mm tip) and even larger tip catheters (like the 9 mm lattice sphere).657,658 Pulsed field ablation deliveries can cause myocardial cell stunning and disappearance of electrical signals. Therefore, acute disappearance of electrograms cannot necessarily predict long-term success. Repetitive applications of PFA around the veins may push the field penetration (and therefore lesion depth) to achieve better results.642
Early studies using a pentaspline multielectrode PFA catheter have shown that optimized biphasic, bipolar PFA deliveries can achieve very high rates of durable PVI at a 3-month remapping procedure.641 Few 1-year follow-up studies have been published to date. A pooled analysis of three non-randomized prospective studies reported a 78.5% freedom from any atrial arrhythmia at 1 year in paroxysmal AF patients.651 Multicenter registries (MANIFEST-PV and EU-PORIA) using this pentaspline multielectrode PFA catheter reported 1-year arrhythmia-free survival of 78.1 and 74%, respectively, in real-world mixed paroxysmal and persistent AF populations undergoing PVI, without a standardized rhythm monitoring protocol.644,659
Recent evidence supports the efficacy and safety of other PFA systems. In the large-scale, prospective, multicenter PULSED AF trial, PFA resulted in 100% acute PVI rate with a low rate of primary safety adverse events (0.7% without PV, esophageal, or PN complications) and 12-month clinical success rates consistent with those reported in thermal catheter ablation studies with similarly rigorous rhythm monitoring (66.2% in paroxysmal AF and 55.1% in persistent AF).660 A biphasic PFA system with a variable-loop circular catheter integrated with a 3D mapping system showed a 71% 1-year atrial arrhythmia freedom without device-related serious adverse events in a paroxysmal AF patient population.661 A focal 9 mm lattice-tip catheter able to deliver both RF and PFA recently demonstrated 78% freedom from atrial arrhythmias at 12 months with primary safety endpoint rate of 0.6% in a mixed paroxysmal and persistent AF patient population. Invasive remapping demonstrated PVI durability in 97% of PVs and 91% of all deployed linear lesions using the optimized waveform.620
In the recent ADVENT trial, 607 patients with drug-refractory paroxysmal AF were randomized either to PFA with the pentaspline catheter or to thermal ablation (either CF-sensing RF or cryoballoon ablation). After a 12-month follow-up, PFA was shown to be non-inferior to thermal ablation in respect to efficacy (composite of acute procedural and chronic success) and safety (device-related and procedure-related serious adverse events).662 Results of the SINGLE SHOT CHAMPION (NCT05534581) and BEAT AF (NCT05159492) RCTs will shed further light on the long-term efficacy and safety of PFA for AF ablation when compared with RF and cryoballoon ablation.
6.2.4. Laser ablation
A laser balloon ablation system transmits light energy through a balloon filled with deuterium oxide (‘heavy water’) to perform PVI. The lumen of the 16 Fr catheter contains a fiber optic endoscope that allows PVI under direct visualization. The balloon is quite compliant, allowing a variable inflation diameter (25–32 mm), depending on PV size. Once inflated, the operator can visualize the edge of the balloon and the PV antra. The laser can then be delivered in a 30° arc around the antrum of the vein. Energy can be titrated from 5.5 to 12 W for 20–30 s depending on the thickness of the tissue and the proximity to the esophagus.663 A newer development is the ability for the catheter to rotate the laser arc 360° around the PV in a continuous sweep to avoid gaps and reduce procedure times compared with the segmental lesions delivered with the old system.663
Several studies sought to compare the safety and efficacy of laser balloon ablation with RF or cryoballoon ablation. In an early multicenter, prospective RCT, laser ablation resulted in similar 1-year freedom from AF when compared with wide-area circumferential RF ablation in persistent AF patients.664 Evidence from both comparative and randomized trials and from a metaanalysis demonstrated similar efficacy and safety of laser balloon compared with cryoballoon ablation.665–667 How laser will fit in a post-PFA world remains to be seen.
6.3. Robotic and magnetic catheter navigation
The concept of remote catheter navigation was developed many years ago and was quite promising for some time. The benefit was that operators could reduce radiation exposure for themselves (and possibly the patient) and reduce the risk of occupational injury associated with wearing medical protective gear. The systems fell into two main categories: (i) magnetically assisted catheter control, such as the Niobe (Stereotaxis Inc., USA) and the Magnetecs system and (ii) robotic assisted catheter control, such as the Sensei robotic catheter system (Hansen Medical, USA) and the Amigo remote catheter system (Catheter Precision, USA). As AF ablation procedures have become shorter (single-shot technologies) and radiation exposure has become very low (electroanatomic mapping, ‘zero’ fluoroscopy techniques), the use of these remote navigation systems has become more niche and is not being widely adopted. Evidence from non-randomized trials and metaanalyses demonstrates that AF ablation guided by remote magnetic navigation is associated with similar efficacy as manual navigation but showed reduced periprocedural complications, reduced fluoroscopy time, and prolonged procedure time.668–670 The high cost of installation and disposables is a key barrier to wider adoption. In a post-PFA world when procedural times will be further reduced, the advantage of such systems for AF ablation will be further limited.
6.4. Future developments
6.4.1. Mapping tools
Future mapping catheters are being developed, which will allow for accommodation of larger numbers and smaller electrodes to increase the resolution of maps. Three-dimensional printing of electrodes is also allowing large numbers of electrodes to be placed on flexible surfaces. Already the Orion basket mapping catheter (Boston Scientific) exemplifies this technology. Future grid and basket designs will be developed.
The development of the ‘near’ unipole reference is a new advance, which will be expanded in multiple catheters. This was first seen on the Sphere 9 catheter where the indifferent electrode is placed on the shaft of the catheter, close to the mapping elements, rather than at Wilson's Central Terminal. This produces a unipolar signal with less far-field artefact. Future basket designs will also feature algorithms, which can measure far-field signals and subtract them from unipolar recordings. This will allow for cleaner, localized unipolar recordings, which may enhance accuracy in defining propagation of wavefronts and identifying local arrhythmia sources.
Artificial intelligence will be further incorporated into mapping system algorithms to help identify critical zones for arrhythmia initiation or perpetuation, particularly for complex arrhythmias like AF (Section 6.1.1.3.). The main limitation to this approach is the ‘black box’ nature of artificial intelligence algorithms, which may limit operator acceptance.
6.4.2. Ablation tools
Combined thermal/pulsed field modalities may overcome several limitations of the currently available PFA systems. Combined pulsed field cryoablation using ultra-low cryothermy can create deeper lesions by delivering subtherapeutic cryoablation to create ice, which acts as an electrical insulator. Large voltages of PFA can then be delivered without causing heating, bubbles, or muscle contraction because of the ice on the catheter.671 Combined RF-PFA may allow for preconditioning of the tissue with low-dose RF, dropping local impedance, and increasing intracellular fluid, which could allow for increased PFA efficacy.
Even subtherapeutic doses of PFA can cause electrical stunning of cardiomyocytes such that signals disappear very quickly. Disappearance of signals, however, does not guarantee a fully developed lesion. Repeated deliveries may be used to achieve durability, but this is still empiric. Other electrogram characteristics or new lesion assessment technologies (such as optical assessment of tissue birefringence) may be required to acutely assess whether a fully transmural lesion has been developed with PFA.
Real-time guidance of ablation procedures with magnetic resonance systems has been proposed for some time, and early feasibility studies have been performed.672 However, the approach has been limited by the size of the MRI system, the current inability of an operator to function comfortably in the environment, and the limited spatial resolution of the various catheters within real-time, non-processed MRI imaging. As the resolution of systems improve and the size of MRI machines decreases, this may eventually become a possible way to perform ablation without any risk of fluoroscopic exposure. Current mapping systems, however, are already enabling near-zero fluoro procedures and could slow down development of MRI-guided ablation systems.
Carbon beam or other high-energy, heavy ion beams may be used to non-invasively beam radiation into specific cardiac structures to achieve ablation. Preliminary preclinical data show that beams can be targeted to the AV node, the PV–atrial junction, and the left ventricle.673 While the non-invasive nature of the ablation is enticing, the complexity and cost of installing such systems (such as MRI guidance) is sure to be a limiting factor.
7. Procedural management and techniques
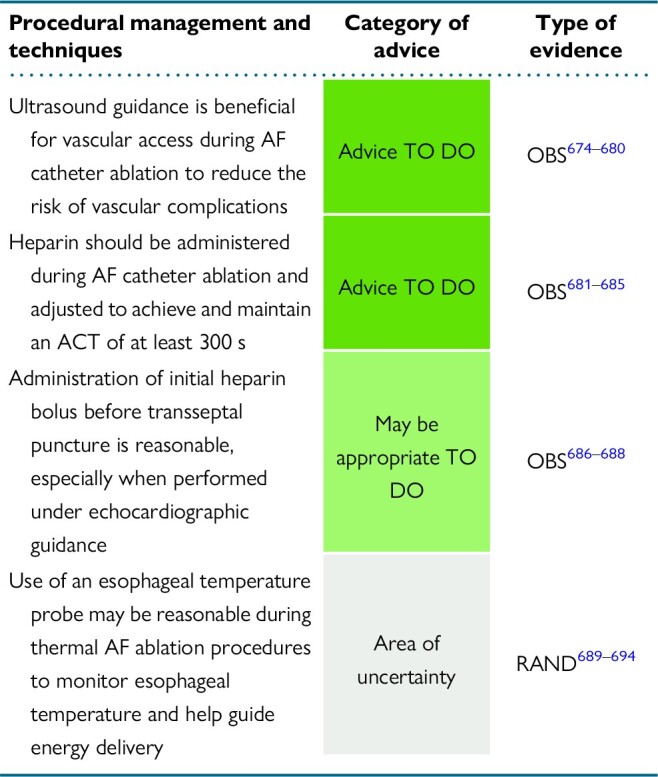
|
7.1. Anesthesia and ventilation during atrial fibrillation ablation
An AF ablation procedure can be performed under general anesthesia, deep sedation, or conscious sedation based on patient and procedural characteristics, physician experience, anesthesia availability, and institutional protocols. A multidisciplinary approach, involving electrophysiologists and anesthesiologists, is necessary to develop a safe and effectively structured anesthesia protocol.
7.1.1. General anesthesia vs. sedation
General anesthesia is the most commonly used anesthetic method in patients undergoing AF ablation. Under deep sedation, the anesthesia depth approaches that of general anesthesia, and in most centers an anaesthesiologist, a second physician, or a specially trained nurse is required to be present.695 For conscious sedation, patients are able to respond purposefully to verbal commands.
An analysis of the National Anesthesia Clinical Outcomes Registry that included 51 070 cases of AF ablations from 2013 to 2018 showed that 94% of cases were performed under general anesthesia in the USA.696 In addition, the worldwide EHRA survey in 2021 showed that the most commonly used anesthetic technique was general anesthesia (40.5%), followed by conscious sedation (32.0%) and deep sedation (27.5%). However, this varied by continent, and in Europe, conscious sedation was still the most commonly used technique (38%). Between 2010 and 2019, the proportion of procedures performed under general anesthesia and deep sedation increased by 4.4 and 4.8%, respectively, whereas the use of conscious sedation decreased by 9.2%.695
In addition to alleviating pain and anxiety, an important goal for anesthesia during AF ablation is to minimize patient movement as this improves catheter stability. Therefore, general anesthesia and deep sedation have frequently been preferred. A prior prospective study randomized 257 patients undergoing ablation for paroxysmal AF to either conscious sedation or general anesthesia and demonstrated significantly improved 17-month ablation efficacy with general anesthesia. General anesthesia was also associated with shorter fluoroscopy and procedure times.697 Other retrospective studies and a metaanalysis have also observed better outcomes when general anesthesia is used compared with conscious sedation, and this finding was associated with improved CF and greater first-pass isolation.697–700 General anesthesia has also been found to be as safe as conscious sedation in terms of total complications and serious adverse events.700 With the increasing use of cryoablation for PVI, a number of studies have demonstrated the feasibility of conscious sedation for this technique with similar efficacy and complication rates to general anesthesia, but with significantly reduced total procedure duration due to reduced anesthetic time.701–703 With the emergence of PFA, there may be a swing back towards the use of general anesthesia to reduce PFA-related pain and prevent discomfort due to contraction of the diaphragm.641–643,704–706 However, recent studies have documented the safety and efficacy of deep sedation protocols during AF ablation with PFA.705,706 A survey of the writing group showed that 52.8% of the writing group members use general anesthesia during AF ablation procedures, 27.8% use deep sedation, and 19.4% use conscious sedation.
7.1.2. Ventilation
Catheter–tissue CF and catheter stability are critically influenced by respiration. An early study demonstrated greater CF when ablation was performed during periods of apnoea with implications for ablation time to achieve PVI and acute reconnection rates.707 Ventilation modulation has been employed in several studies to improve catheter stability and contact. Beyond using periods of apnoea, techniques have included high-frequency jet ventilation (HFJV) and high-frequency low tidal volume (HFLTV) ventilation. High-frequency jet ventilation has been shown to improve catheter stability.708,709 A recent prospective registry indicated that use of HFJV in patients undergoing PVI for paroxysmal AF using CF catheters was associated with decreased arrhythmia recurrence without appreciable increase in adverse procedural events.708 High-frequency jet ventilation is most suitable for patients with normal pulmonary physiology and chest wall compliance. Hypotension requiring administration of vasopressors is significantly more frequent during HFJV cases when compared with those using standard ventilation.708 Complications that have been described with the use of HFJV have included airway dehydration, inadequate oxygenation and ventilation, respiratory acidosis, barotrauma, gastric distension, and aspiration.710,711 Both due to these potential complications and lack of widespread availability of dedicated ventilators, adoption of HFJV during PVI has been relatively limited.
A simpler alternative strategy that has recently been described is the use of conventional ventilators to deliver HFLTV ventilation. Several studies demonstrated that HFLTV ventilation was associated with improved catheter CF and stability, higher first-pass PVI rate, and shorter total procedural and RF times without an increase in complications.712–714 This technique has been more widely adopted due to its ease of use. A recent large prospective multicenter registry enrolling paroxysmal AF patients undergoing catheter ablation demonstrated that HFLTV ventilation improved freedom from atrial arrhythmia recurrence, AF-related symptoms, and AF-related hospitalizations in comparison with standard ventilation.715
A survey of the writing group showed that 5.6% of the writing group members routinely use HFJV and 29% routinely use HFLTV ventilation during RF ablation procedures.
7.2. Vascular access
Femoral venous access for AF ablation may be obtained using anatomical markers or under ultrasound guidance. Significant vascular complications that may occur include inadvertent arterial puncture, arteriovenous fistula, pseudoaneurysm formation, access site hematoma, and retroperitoneal bleed716,717 (Section 11.3.6.). When traditional anatomical marking is used for vascular access, an inferior approach is associated with increased risk of femoral pseudoaneurysm and arteriovenous fistula, while a superior approach may be associated with an increased risk of retroperitoneal bleeding. Evidence from observational studies and metaanalyses has indicated that use of ultrasound-guided vascular access significantly reduced the risks of vascular complications, postprocedural pain, and prolonged bruising.675,678–680 A multicenter RCT comparing ultrasound-guided venipuncture vs. an anatomically guided approach was terminated early due to substantially lower than expected complication rates.676 Nevertheless, analysis of data collected demonstrated that first-pass success in gaining femoral vein access was higher in the ultrasound-guided group, while puncture time, extra puncture attempts, inadvertent arterial puncture, and unsuccessful cannulation were all significantly lower in the ultrasound-guided group.676 In an era where AF ablations are increasingly performed on uninterrupted anticoagulation, the risks associated with vascular complications need to be minimized. Therefore, preventive measures including ultrasound-guided venipuncture should be implemented routinely.
A survey of the writing group showed that 75.7% of the writing group members routinely use ultrasound guidance for vascular access during AF catheter ablation.
7.3. Continuous arterial blood pressure monitoring
Continuous arterial BP monitoring via an intraarterial line is utilized in many laboratories to monitor patients undergoing AF ablation (39.5% of the writing group members routinely use invasive arterial BP monitoring during AF catheter ablation). Limited data comparing outcomes with invasive vs. non-invasive BP monitoring exist. A retrospective multicentre study of 362 patients having AF ablation under general anesthesia found no difference in complication rates between the invasive and the non-invasive BP monitoring groups.718 In theory, an arterial line may provide critical early indication to the presence of a major complication such as pericardial tamponade. Whether this justifies the routine use of invasive hemodynamic monitoring or would indeed improve outcomes is not established. In patients with impaired ventricular function, hemodynamic instability, and significant comorbidities, the use of invasive BP monitoring may be reasonable on an individualized basis.
7.4. Anticoagulation during atrial fibrillation ablation
Meticulous sheath handling and optimal intraprocedural anticoagulation with unfractionated heparin (UFH) is critical to prevent thromboembolic complications and the development of silent cerebral infarction.681,682,684,685,719 A single non-randomized study evaluated the impact of flushing the transseptal sheath prior to vascular entry using 2 U/cc heparin concentration when compared with a flush containing 1000 U/cc heparin on the incidence of thrombus formation on the transseptal sheath.720 Intracardiac echocardiography was used to screen for thrombus. Patients having received a low-dose heparin flush prior to intravenous access had a significantly higher incidence of thrombus formation compared with the high-dose heparin group (9 vs. 1%) within 5–15 min of entering the LA. Notably however, the procedures were not performed on uninterrupted oral anticoagulant (OAC), heparin was not administered until after the second transseptal crossing, the initial UFH bolus was at least 5000 units, and the target activated clotting time (ACT) was only 250–300. It remains unclear whether a strategy of heparinized saline infusion of sheaths is important in the context of a contemporary anticoagulation strategy. Nevertheless, 84% of the writing group members reported using heparinized sheath irrigation.
Most of the evidence regarding UFH administration during AF ablation was derived from patients taking VKA. Studies that investigated the use of UFH in patients on uninterrupted DOAC have shown that higher amounts of intraprocedural UFH were needed to achieve target ACT and not all DOACs interact with UFH in the same way.721–726 Post hoc analysis of RE-CIRCUIT showed that patients on dabigatran required similar amounts of UFH to achieve therapeutic ACTs compared with VKA, while other studies indicated that more UFH was needed in patients taking factor Xa antagonists.723–726 From literature review, a great amount of variability exists across different practices on intraprocedural UFH dosing protocols. Specific dosing regimens should be tailored to the patient population, medication use, and the last dose of OAC as these factors impact the amount of UFH needed to achieve therapeutic anticoagulation.726–728
A metaanalysis of 19 studies involving 7150 patients concluded that patients with ACT > 300 s during AF catheter ablation had significantly reduced risk of thromboembolic complications without increased risk of bleeding when compared with those with ACT < 300 s, irrespective of the type of oral anticoagulation used periprocedurally.683 A survey of the writing group showed that 61% of the members employ a target ACT > 300 s during AF ablation, while 34% a value >350 s.
Evidence supports initial heparin bolus administration before transseptal puncture. Observational studies in patients undergoing AF ablation have demonstrated that UFH administration before transseptal puncture is associated with a reduced incidence of ICE-detected thrombus when compared with those receiving UFH after transseptal puncture.686,687 A prospective observational study in 280 patients undergoing AF ablation under VKA treatment reported that compliance to a periprocedural anticoagulation protocol including UFH administration before transseptal puncture, maintenance of therapeutic preprocedural INR, and consistent procedural ACT levels >300 s resulted in significantly reduced incidence of silent cerebral ischemia after ablation.688 In addition, the increasing use of ICE significantly decreases the risk of transseptal puncture associated bleeding. A survey of the writing group showed that 74% of the members administer initial UFH bolus before transseptal puncture.
In the event when anticoagulation needs to be reversed due to intraprocedural complications such as cardiac perforation and cardiac tamponade, UFH can be reversed with protamine administration. This was validated by an RCT showing that protamine expedites vascular hemostasis after AF ablation.729 If bleeding stops, reversal of OAC is not suggested to protect against periprocedural thromboembolic risk. If bleeding persists despite protamine administration, fresh frozen plasma can be administered in warfarin-treated patients, idarucizumab to reverse dabigatran, and andexanet for reversal of Factor Xa inhibitors.730,731 If specific reversal agents are not available, prothrombin complex concentrates (Factors II, VII, IX, and X) can be administered to achieve immediate hemostasis and should be preferred over recombinant activated Factor VIIa due to the latter's prominent procoagulant effect.732
7.5. Transseptal puncture
Transseptal puncture can now be performed with several different technologies. In addition to the conventional needle, transseptal access can also be gained using an RF needle or a needle-free technique. Several RCTs have compared the RF needle with a standard approach. These studies found that transseptal puncture with an RF needle was associated with significantly shorter time required for transseptal LA access, shorter fluoroscopy requirement, lower rate of transseptal failure, and fewer visible plastic shavings after needle advancement. Complication rates did not differ.733,734 However, one of these studies had a transseptal failure rate of 28% and an incidence of visible plastic shavings of 33%, which are not consistent with the actual very low incidence of these two events in clinical practice.733 Furthermore, the time savings of 20 s to several minutes seem insignificant in a clinical or lab usage context. Neither study addressed the additional cost associated with use of the RF needle. Indeed, uptake of the RF needle has varied widely and in many countries is not in routine use. An observational study also found that use of the RF transseptal needle was associated with a lower incidence of MRI-confirmed silent cerebral lesions.735
The needle-free transseptal approach can be achieved with the use of specific wires. A transseptal wire (Safesept, Pressure Products, San Pedro, CA, USA) is safe and effective in gaining LA access without a need for transseptal needle or exchange for a standard guidewire.736 In a large, retrospective single-center analysis, it was shown to significantly reduce the risk of transseptal puncture-related cardiac tamponade.737 A newer technology is an RF wire that can be used to cross the septum and provide support for the transseptal sheath (Versacross, Boston Scientific). The RF wire forms a pigtail end that can be advanced into the SVC to guide initial sheath placement. When pulled into the sheath, the wire straightens out, and upon ‘tenting’ of the septum, RF is applied to the tip of the wire and the wire is advanced into the LA, reforming the pigtail end that can be advanced into a PV and allow the sheath to be atraumatically advanced. This system can be useful in cases of redo ablation or prior ASD closures to prevent the ‘jumping’ across the septum that may occur with standard needles.
With circumferential RF ablation, both the single (two sheaths via one transseptal puncture site) and double transseptal (each sheath via a separate transseptal puncture site) approaches have been used (50% of the writing group members use single and 50% double transseptal access). A prospective study comparing single vs. double transseptal in patients undergoing AF ablation revealed no difference in procedure time, fluoroscopy time, complication rates, or AF recurrence between the two approaches.738
The use of steerable sheaths during AF ablation facilitates catheter navigation and manipulation and is associated with increased catheter stability.739 In an earlier prospective RCT, the use of steerable sheath significantly reduced arrhythmia recurrences 6 months after AF ablation and was the only independent predictor of rhythm outcome.740 The introduction of steerable sheaths that can be visualized on 3D electroanatomical maps facilitates fluoroless understanding of their positioning. Integration of visualizable steerable sheaths in AF ablation workflows has been shown to reduce fluoroscopy exposure when compared with the use of conventional steerable sheaths.741–744
7.6. The use of intracardiac echocardiography
The use of ICE during AF ablation offers multiple benefits in different stages of the procedure. As already presented in detail (Section 5.3.2.2.), ICE is useful to screen for LA/LAA thrombus at the time of catheter ablation. Intracardiac echocardiography use has also a favourable impact on procedural duration and safety. Observational studies and two metaanalyses indicated that ICE use in AF ablation was associated with significant reductions in fluoroscopy time, procedure time, and complication rates compared with AF ablation without ICE.745–748 In a propensity score–matched analysis, ICE was associated with a significantly lower incidence of complications and repeat ablation.747 A retrospective analysis of a national representative database including 299 152 patients undergoing AF ablation over a 14-year period reported that the use of ICE was significantly increased over the years and led to significant reduction in complication rate, in-hospital mortality, and length of hospital stay.749 A more recent propensity score–matched analysis from a nationwide database validated the favourable impact of ICE use on in-hospital mortality, readmission rate, and length of stay without increase in healthcare-associated cost.750
Intracardiac echocardiography may also be used as an adjunct to AF ablation tools to guide safe and efficient energy delivery. Direct visualization of the LAPW and the adjacent esophagus may guide titration of power and duration at these high-risk areas to reduce the risk of collateral damage during energy delivery.751 Intracardiac echocardiography use allows real-time visualization of PV anatomy preventing inadvertent intra-PV RF energy delivery that increases the risk of PV stenosis. Intracardiac echocardiography is also useful in validating proper PV occlusion during cryoballoon ablation either with colour-flow Doppler assessment of PV leakage or with evaluation of microbubble backflow to the LA after saline injection in the internal lumen of the cryoballoon.752–754 The latter approach is feasible, safe, and useful in patients with contraindication to iodinated contrast medium.755 Intracardiac echocardiography has been used to measure LA wall thickness in different segments of the PV periphery and accordingly adjust target AI during RF energy delivery.756 Employment of a tailored AI protocol based on ICE-measured LA wall thickness significantly increased acute procedural success and freedom from AF recurrence following PVI in paroxysmal AF patients compared with an FTI protocol.756
Factors limiting the adoption of ICE use in routine AF ablation workflow include associated increase in procedural cost and the need for a second operator or multitasking by a single operator. A survey of the writing group showed that 47.4% of the members routinely use ICE during AF ablation. Therefore, in practices familiar with ICE, it is reasonable to use ICE to exclude thrombi and enhance procedural safety and efficiency during AF ablation.
7.7. Fluoroless ablation
Radiation exposure during catheter ablation of AF can cause potential delayed complications both in patients and operators that include acute and subacute skin injury, cataract, and malignancy.757 In addition, wearing of lead over time can lead to orthopaedic injuries (back pain, disc herniations) in operators and laboratory staff. Traditionally, many steps during an AF catheter ablation require fluoroscopy, including catheter positioning, transseptal puncture, PV angiography, and ablation. Studies have shown that the lifetime risk of excess fatal malignancies normalized to 60 min of fluoroscopy was 0.07% for female and 0.1% for male patients and that obese patients receive more than twice the effective radiation dose of normal-weight ones during AF ablation procedures.758,759
Fluoroscopy times were frequently in excess of 60 min in the initial years of AF ablation. However, a single-center analysis of over 2300 AF ablations indicated that fluoroscopy times and doses have dramatically decreased over a 12 year period.760 Indeed, today, fluoroscopy times and doses generally average fewer than 10 min and 1000 mGycm2 for RF AF ablation procedures predominantly associated with positioning of diagnostic catheters and transseptal puncture.761 The use of advanced 3D mapping systems has largely obviated the need for fluoroscopy after LA access has been achieved. When considering low-dose pulsed fluoroscopy of 2–5 min duration with collimation,762,763 the effective radiation dose is as low as 1 mSv, equivalent to ∼4 months background radiation. Whether zero fluoroscopy meaningfully reduces the risk associated with 3–5 min of fluoroscopy remains unproven. Electrophysiologists should be familiar with measures that reduce radiation exposure of patients and cath lab personnel, which include, but are not limited to, fluoroscopy system customization, workflow adaptations (frame rate, collimation, cine, projection angle, sensitive areas), and shielding measures.764,765
Nevertheless, in recent years, there has been an initiative to perform zero-fluoroscopy AF ablation. A number of studies have demonstrated that fluoroless transseptal puncture and AF ablation can be performed with TEE and/or ICE guidance with similar procedural duration, acute success rate, procedural complication rate, and 1-year AF recurrence rate to a minimal fluoroscopy approach.766–774 In up to 37% of patients in some series, complete fluoroless ablation could not be achieved, and minimal rescue fluoroscopy was needed to confirm catheter location and to assess for potential complications.767,769,770
The increasing use of cryoablation has again resulted in significantly longer fluoroscopy times, when compared with RF ablation, with reported times of ∼20 min.294 Fluoroless cryoballoon ablation has not been widely adopted both because of the need to identify balloon positioning at the PV ostium and to prove occlusion of the vein with contrast injection. A single observational study of 50 patients found that fluoroless cryoablation is achievable with similar outcomes to a fluoro-guided procedure, but this approach is not in wide clinical usage.775 A survey of the writing group showed that 18.4% of the members routinely perform fluoroless RF ablation.
7.8. Esophageal temperature management
Animal models and clinical series have documented that esophageal perforation develops in the presence of underlying esophageal tissue injury.776,777 Therefore, because of the rarity of AEF occurrence, endoscopically detected esophageal lesions are considered as a surrogate indicator for potential development of AEF.
Studies evaluating the relationship between measured esophageal heating during RF AF ablation and detection of esophageal ulceration on postprocedural endoscopy have yielded divergent results. In a cohort of patients undergoing their first RF ablation under continuous esophageal temperature monitoring using an infrared thermography system, peak esophageal temperature was predictive of thermal esophageal lesions detected by postablation endoscopy.691 A retrospective study of 43 patients who underwent high-power (50 W) short-duration (6–7 s) ablation found no difference in peak esophageal temperatures measured on the multielectrode S-Cath probe (Circa Scientific, LLC, Englewood, CO, USA), between those patients who developed compared with those who did not develop esophageal abnormalities (including small ulcers, non-bleeding erosions, erythema, and esophagitis). However, it was not determined whether the peak temperatures occurred in anatomic relationship to the esophagus.692 A metaanalysis of studies reporting prevalence and prevention of endoscopically detected esophageal lesions following AF ablation found a lesion prevalence of 11% and no difference with or without the use of esophageal temperature monitoring.693
Since then, a prospective randomized study of 86 patients has found no difference in new endoscopically detected esophageal lesions when comparing ablation with vs. without luminal esophageal temperature monitoring (S-Cath, Circa Scientific, LLC, Englewood, CO, USA), with an overall prevalence of 9%.689 However, ablation was not terminated until the esophageal temperature reached 42°C. Achievement of an esophageal temperature of 42°C was predictive of esophageal lesions raising the possibility that an approach limiting temperature rise to more conservative levels may potentially be effective in preventing esophageal lesion formation. In another prospective RCT, esophageal temperature monitoring using an intraluminal probe (SensiTherm™, FIAB, Firenze, Italy) had no significant impact on the incidence of endoscopically diagnosed esophageal lesions. The total prevalence of esophageal lesions was 10%, and peak temperature measured by the thermoprobe did not correlate with the incidence of esophageal lesions.690 In a consecutive series of 120 patients undergoing high-power (50 W), short-duration RF ablation, the endoscopic detection of ulceration was compared between an initial group with use of a Circa esophageal temperature probe (maximum allowable temperature of 39°C) and a second group without esophageal temperature monitoring.694 The overall incidence of new endoscopically detected lesions was only 2.5% with no difference between the groups. The authors used a series of measures to avoid overheating the esophagus such as not performing contiguous lesions over the esophagus and allowing time between lesions for cooling, suggesting that this approach may be most important. Based on the existing evidence, the use of esophageal temperature monitoring during AF ablation has not resulted in reduced risk of endoscopically detected esophageal lesions. Esophageal temperature probes with varied numbers of temperature sensors and varied temporal responsiveness are available for clinical use,778,779 but the esophagus is broad relative to the spatial resolution of even multisensor temperature probes, and severe esophageal temperature rise may remain undetected when the sensor is >2 cm away from the ablation catheter.780
Mechanical esophageal deviation has been reported, but its use has been limited to a small number of patients at a limited number of centers.781 Significant esophageal deviation related trauma has been reported when trying to achieve the extent of mechanical esophageal deviation required to avoid esophageal heating, and it remains unclear whether the benefits of esophageal deviation exceed the risks.781
Esophageal cooling has also been evaluated for reducing the severity of esophageal heating. A systematic review of four RCTs found that esophageal cooling reduced the risk of severe esophageal injury during AF catheter ablation.782 A single-center study randomized 188 patients undergoing RF ablation to either active esophageal cooling at 4 °C using the ensoETM device (Attune Medical, USA) or standard practice with a single-sensor temperature probe. Esophageal endoscopy was performed in 120 patients 1 week following ablation and demonstrated significantly higher occurrence of thermal injury in the control group when compared with those receiving esophageal protection.783 The use of esophageal cooling has also been shown to improve postprocedural freedom from AF recurrences.784 The challenge with these studies is that the AEF is such a rare complication that it is unlikely any RCT will show a true difference in that endpoint. In a retrospective analysis of RF ablation cases from 30 US hospitals, the rate of AEF was significantly lower in the group of 14 224 patients who received active esophageal cooling when compared with the control cohort of 10 962 patients who underwent RF ablation without esophageal cooling but under esophageal temperature monitoring in >90% of cases (0 vs. 0.146%).785
Additional strategies that may be considered for limiting severe esophageal heating include altering ablation lesion set to avoid ablation of atrial tissue directly overlying the esophagus,786 avoiding higher CF during LAPW ablation,787 and avoiding consecutive ablation lesions at sites with risk of esophageal injury.780 Use of PFA may also mitigate this risk.
A survey of the writing group showed that 50% of the members routinely use an esophageal temperature probe during catheter ablation procedures to monitor esophageal temperature during energy delivery. Furthermore, as a strategy to avoid severe esophageal heating during RF catheter ablation, 84.2% of the members avoid high CF during energy delivery at the posterior wall, 76.3% reduce ablation power and/or duration at the posterior wall, 63.2% avoid consecutive lesions at sites with risk of esophageal injury, 10.5% use mechanical esophageal deviation, and 2.6% use esophageal cooling.
8. Ablation strategies
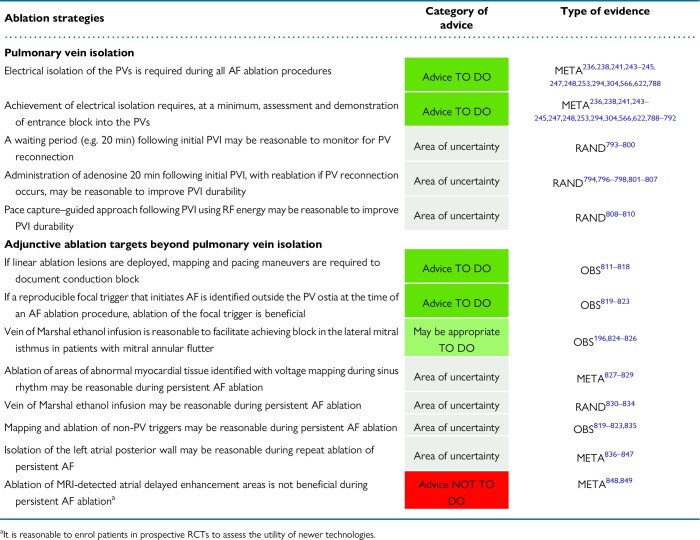
|
8.1. Pulmonary vein isolation
8.1.1. Endpoint of pulmonary vein isolation
Pulmonary vein isolation is the cornerstone of AF ablation and is required during all AF ablation procedures. The endpoint of PVI is achievement of electrical disconnection between the PVs and the LA. This disconnection can be verified by documenting the absence of wavefront propagation from the LA to the PV (entrance block) and/or from the PV to the LA (exit block). Pulmonary vein entrance block is confirmed with disappearance or dissociation of PV potentials recorded usually with a multipolar catheter. Pulmonary vein exit block is verified in the presence of non-conducted spontaneous PV activity (isolated PV ectopics, PV tachycardia, or PV AF) or during non-conducted PV pacing. During pacing from the vein to assess PV to LA conduction, it is important to verify local PV capture (usually recorded on a multipolar catheter) and to avoid inadvertent far-field capture of the LAA (when pacing anteriorly in the left PVs) or SVC (when pacing anteriorly in the RSPV) that could erroneously suggest the presence of persistent electrical connection.850,851 Pacing the posterior and proximal aspect of the PVs is a simple method to avoid far-field capture of these structures. Differential pacing maneuvers, catheter placement in adjacent structures, and gradual decrease of pacing output to demonstrate loss of far-field capture have also been proposed to differentiate far-field from near-field capture.851 Following PVI, it may not be possible to demonstrate PV sleeve capture in up to 20% of patients. This finding correlated with PV entrance block and with adenosine-proof isolation.852
Initial studies of segmental PVI using non-irrigated catheters reported persistent PV to LA conduction in almost 40% of cases in the presence of entrance block, stressing the need to include exit block documentation in the PVI procedural endpoint.853 However, recent studies of circumferential PVI using contemporary RF ablation technology have indicated that unidirectional exit conduction in the presence of documented entrance block is extremely infrequent.790 Duytschaever et al.791 reported a 0.6% prevalence of residual PV–LA conduction after proven entrance block.
Few studies have also assessed the impact of bidirectional vs. unidirectional (entrance only) block on acute PV reconnection rate and long-term arrhythmia outcome after PVI. Chen et al.792 showed that bidirectional block of the PV–LA junction is associated with reduced intraprocedural reconnection incidence compared with unidirectional block. However, in a retrospective cohort analysis, inability to demonstrate exit block was not associated with increased risk of PV reconnection in redo procedures.790
The reported very low rates of persistent PV–LA conduction in the presence of entrance block using contemporary ablation technology indicate that this finding alone is an adequate procedural endpoint during PVI. However, exit block documentation may prove useful when verification of entrance block is ambiguous.
8.1.2. Pulmonary vein isolation using radiofrequency energy
8.1.2.1. Electrogram parameters and impedance change
Changes in electrogram morphology have been proposed as indicators of lesion transmurality during RF energy delivery to achieve PVI.589,854–857 Elimination of the negative component of the atrial unipolar electrogram during PVI procedure following the contiguous ‘point-by-point’ approach was demonstrated to be a marker of transmural lesion creation in both animal and human studies.858,859 However, electrogram morphology-guided ablation has yielded variable long-term outcomes when compared with ablation guided by contemporary lesion quality indicators (Section 8.1.2.2.).860,861 In a recent study using AI and CLOSE protocol-guided PVI, change in the unipolar electrogram was not found to correlate with RF markers of an adequate lesion. Changes in unipolar electrogram morphology indicative of transmurality are completed within 5–7 s of energy application, well before completion of AI-guided delivery of high-quality lesion.862,863
Electrode impedance has long been proposed as an indicator of electrode–tissue contact and lesion size.864 Insufficient impedance fall (<10 Ω) has been associated with LA to PV conduction recovery.865 However, the quantitative relationship between real-time contact and impedance drop is complex and varies according to parameters including absolute force and catheter orientation.866–870 Local impedance monitoring using an ablation catheter with microelectrodes incorporated into the catheter tip (IntellaNav Mifi OI™, Boston Scientific) may improve the utility of impedance monitoring for lesion prediction.871–873 Further work is needed to refine the precise roles of catheter and generator-derived biophysical parameters to reliably predict lesion formation and the impact on clinical outcome.
8.1.2.2. Lesion quality indicators
The advent of irrigated RF catheters with incorporated CF-sensing mechanisms has seen the parallel development of real-time lesion prediction algorithms integrating biophysical data (power, temperature, duration of RF delivery, and CF) to provide an estimate of critical lesion characteristics including area, depth, and continuity.874 Early experience of CF and its impact on electrical reconnection after PVI was reported in multiple prospective studies including TOCCATA, EFFICAS I, and EFFICAS II, revealing a higher likelihood for reconnection with lower CF and FTI values achieved.875–878 In the EFFICAS II study, lesion contiguity was associated with more durable PVI.878 Although important first steps, these FTI-based studies did not incorporate RF power or regional variation in LA wall thickness. In addition to these parameters, catheter stability, contact angle, and respiration are important determinants of RF lesion formation.875,879–882
The AI is a marker of lesion quality that incorporates CF, time, and power in a weighted formula. It has provided accurate estimation of lesion depth in animal studies883 and a strong correlation with impedance drop during LA ablation.884 Although attractive to standardize workflow, none of the available lesion prediction tools has yet incorporated real-time measurement of atrial wall thickness to guide RF delivery and provide relatively crude estimates of transmurality.756,885
The CLOSE protocol refers to PV encirclement using CF-sensing catheter targeting an interlesion distance ≤6 mm and AI ≥400 at posterior/inferior walls and ≥550 at roof/anterior wall.558 The proof-of-concept AI targets have been associated with high first-pass isolation rates and both low rates of acute PV reconnection and atrial tachyarrhthmia recurrence in prospective studies. In a pilot study, the incidences of first-pass and adenosine-proof isolation were both 98%, and single-procedure success was 91.3% at 1 year.558 Strict application of criteria for contiguity and AI in CLOSE-guided PVI was shown to improve procedural and 1-year outcome over conventional CF-guided PVI.559,886,887 In the CLOSE to CURE study, PVI using the CLOSE protocol resulted in significant reduction in the atrial tachyarrhthmia burden (documented by implanted cardiac monitor), which was maintained during longer follow-up.555
A recent randomized study indicated that the optimal interlesion distance in AI-guided ablation may be less than the 5–6 mm incorporated in the CLOSE protocol with an interlesion distance of 3–4 mm providing higher first-pass isolation with lower AI targets and shorter procedure duration.888 Optimal interlesion distance may also vary according to the anatomic region being ablated.889 High-power short-duration circumferential PVI (50 W at all sites) using a standard CLOSE protocol approach has been shown to reduce both total procedural duration and RF time, without increasing the complication rate compared with lower power settings.595,596,694,890–892
The LSI is another proprietary multi-parametric index incorporating time, power, CF, and impedance during ablation, which also predicts the extent of myocardial tissue lesions. Further studies on the value of quality lesion indications for PVI and ablation beyond the PVs are warranted.
A survey of the writing group showed that 82% of the members routinely use lesion quality indicators to guide energy delivery during PVI with RF ablation.
8.1.2.3. Waiting phase
In the non–CF-monitoring ablation era, early detection (within 30–60 min with or without adenosine challenge) of PV reconnection and adjuvant ablation of PV reconnection sites was reported to reduce AF recurrence rate after PVI.793,801,893–896 Others demonstrated that immediate ablation of early detected reconnection may not improve the long-term outcomes despite the association of acute PV reconnection with late AF recurrence.797 More recently, the use of the aforementioned lesion quality prediction tools has called into question the necessity of a waiting phase after initial PVI. It is now known that suboptimal tissue–catheter CF during RF delivery can be associated with spontaneous early reconnection or dormant conduction after PVI.897 In the CIRCA-DOSE study, using contemporary AF ablation technologies, spontaneous reconnection was elicited in 5.4% of PVs in 16.0% of patients and was significantly more prevalent among patients treated with CF-RF ablation when compared with cryoballoon ablation (22.3 vs. 12.8%, P = 0.03).798 While CF catheters were used in this study, AI, interlesion distance and other key features of the CLOSE protocol were not. Interestingly, acute intraprocedural PV reconnection, even when eliminated by adjuvant ablation, was associated with significantly higher arrhythmia recurrence rate only in the cryoablation group and not in the RF group. The implications of these differences remain uncertain, and the overall recurrence rates between the two approaches did not differ.798
Several studies have specifically evaluated whether the incorporation of a waiting period in the procedural workflow improves arrhythmia outcome among patients undergoing RF PVI using contemporary technology. A multicenter randomized study assessing potential impact of a 30 min waiting period and/or adenosine triphosphate (ATP) testing after PVI on long-term 3 year outcome demonstrated no improvement in freedom from AF recurrence when using any of these strategies.799 Another prospective multicenter study randomized consecutive paroxysmal AF patients to AI-guided PVI with vs. without a 20-min waiting period and also found similar rates of arrhythmia recurrence at 1-year follow-up.800
In the context of these data and taking into consideration that a waiting period considerably prolongs procedure duration without documented improvement in arrhythmia-free outcomes, its incorporation in contemporary procedural workflows is no longer considered necessary. However, the value of a waiting phase after PVI with newly introduced ablation protocols or energy sources, including PFA, merits further assessment.
A survey of the writing group showed that 57% of the members employ a waiting period of at least 20 min following initial PVI when performing RF ablation with CF-sensing catheters.
8.1.2.4. Adenosine testing
Intravenous adenosine (or ATP) can be used to unmask dormant conduction across circumferential PV ablation lines.798,802,898,899 Adenosine dose and the time elapsed since initial PVI are determinants of adenosine-induced PV reconnection.896,900 Adenosine is given as a rapid bolus followed by saline bolus at a dose required to achieve at least one blocked P-wave or a sinus pause >3 s798,803,901 with 12–18 mg of adenosine being sufficient to achieve AV block in most patients.803
Although some data suggest that use of adenosine to identify dormant conduction and guide further ablation may improve outcomes,802 contradictory results have also been reported.805,806 The routine use of adenosine has not been consistently associated with improved outcomes when compared with a no-adenosine strategy.805 A recent study indicated that patients without spontaneous or adenosine-provoked PV reconnection had better outcomes than those with acute reconnection despite undergoing further ablation. Although the authors suggested that efforts should be directed towards ensuring an ideal ablation lesion at the first attempt to achieve durable PVI, this finding may also point to anatomic variations that render durable isolation more difficult to achieve.798
In the CIRCA-DOSE study using CF catheters and FTI but not AI or the CLOSE protocol, adenosine-mediated reconnection was observed in 5.7% of PVs in 17.2% of patients and was significantly more common after CF-RF ablation when compared with cryoballoon ablation (31.3 vs. 10.2%, P < 0.001).798 Adenosine-mediated reconnection was associated with higher AF recurrence rates in the cryoballoon-treated patients but not with use of RF when additional ablation was performed to achieve PVI. Studies using the CLOSE protocol have indicated significantly higher rates of adenosine-proof isolation compared with a standard approach to PVI (97 vs. 82%), and this translated into improved outcomes.559 Furthermore, a multicenter randomized study evaluating potential benefit derived from employment of ATP testing and/or prolonged waiting periods after PVI reported no significant differences in freedom from AF recurrence over standard care.799
Taking into consideration (i) the high rate of adenosine-proof PVI with contemporary RF ablation technology including the CLOSE protocol, (ii) the lack of documented benefit on long-term outcomes derived from adenosine testing post-PVI when contemporary RF technology is used, (iii) the questionable value of adjunctive ablation at adenosine-unmasked reconnection sites for long-term outcomes, and (iv) the increment in procedural time and cost when employing adenosine testing, the routine use of adenosine testing post-PVI is not a requirement.
A survey of the writing group showed that 21.6% of the members routinely employ adenosine testing after initial PVI when performing RF ablation with CF-sensing catheters.
8.1.2.5. First-pass isolation
First-pass isolation is defined as achievement of PVI upon completion of the encirclement of ipsilateral PVs. First-pass isolation is an indicator of high-quality lesion set with favourable impact on procedural outcome. In a real-world setting, first-pass isolation is highly predictive of 12-month clinical success after CF-guided ablation in paroxysmal AF patients,902 while the absence of first-pass isolation is associated with inferior PVI durability and AF ablation outcomes.903 CLOSE protocol-guided PVI is associated with higher incidence of first-pass isolation of the PVs and higher single-procedure arrhythmia-free survival at 1 year when compared with conventional CF-guided PVI.558,559,887 First-pass isolation is associated with reduced likelihood of acute PV reconnection, and therefore whenever achieved, the waiting phase post-PVI may be obviated.
8.1.2.6. Loss of pace capture along pulmonary vein isolation line
The pace capture approach is an adjunctive technique to evaluate the integrity of a circumferential ablation lesion set.807–809,904–906 In this method, bipolar pacing at a high output (10 mA, 2 ms pulse width) is attempted along the ablation line.808,809,904 The sites of local LA capture during SR are identified and ablated further until local capture is lost. The pertinent procedural endpoint is PVI with absence of pace capture along the entire circumferential PVI line.808,906
An RCT including paroxysmal AF patients revealed that the rate of freedom from AF was higher with a pace-guided approach than the conventional method at 12 months as well as after a 5-year follow-up.808 However, other studies have not reproduced these findings.809
A survey of the writing group showed that 31.6% of the members routinely perform pace capture testing along the ablation line after initial PVI when performing RF ablation.
8.1.2.7. Inducibility of atrial fibrillation after pulmonary vein isolation
Electrophysiological and pharmacological stimulation approaches are sometimes performed to test for inducibility of AF following PVI. Generally, stimulation protocols consist of rapid atrial pacing and/or high-dose isoproterenol infusion, which can vary widely between centers. Inducible AF has been defined as anything from 30 s to 10 min of AF with no clear consensus on this. In the event of inducible AF, several studies have tested the value of additional ablation targeting atrial tissue displaying CFAE and low-voltage areas.223 However, AF meeting the above definitions can be induced in up to 49.5% of patients with no history of clinical AF.907 Inducibility is dependent on the induction protocol, the number of induction attempts, and the definition of inducibility. Contradictory results have been reported regarding the prognostic value of non-inducibility or change in inducibility status after AF catheter ablation on long-term freedom from recurrent arrhythmias.908–911
A survey of the writing group showed that 15.8% of the members routinely employ AF inducibility after initial PVI when performing RF ablation.
8.1.3. Pulmonary vein isolation with cryoballoon ablation
The cryoballoon is a double layer balloon that is introduced into the LA via a steerable sheath (Section 6.2.2.1.). Navigation to the individual PV is achieved by a circular mapping catheter advanced through the central catheter lumen and can be used to map PV potentials and thereby document PVI. Exceptionally, a stiff guidewire may be used if balloon positioning is difficult. To completely occlude the PV, the balloon is positioned in alignment with the PV axis, and specific maneuvers with the steerable sheath are performed (hockey stick, pull down). The degree of occlusion may be verified by injection of contrast agent through the central lumen. Commonly, a four-step grading score is used to describe the degree of occlusion912 although other imaging modalities such as TEE or ICE may be used to reduce fluoroscopy exposure to near zero (Section 7.6.).913 Invasive pressure monitoring through the central lumen has been described as a reliable tool to assess PV occlusion.914 Very recently, wide band dielectric imaging, a non-fluoroscopic imaging modality, was reported to accurately assess PV occlusion and guide cryoballoon-based PVI.915
Various dosing strategies have been proposed. In animal experiments, single applications with 120, 180, and 240 s freezing times led to transmural lesions and a high rate of durable PVI.916,917 In the randomized FIRE and ICE trials, a bonus application was added to the 240 s index application.294 In a more recent randomized study, no differences in PVI durability nor in clinical outcome were observed after 2 × 120 vs. 2 × 240 s cryolesions per PV.293,918 Other studies showed that an empiric bonus application does not improve outcome, if PVI occurs within 75 s after starting the cryoapplication (time to isolation—TTI).919,920 Findings from a metaanalysis endorse the use of a single freeze application approach, the latter resulting in shorter procedure times and a lower adverse event rate without compromising efficacy.921
The optimal freeze duration is subject to controversy. Since side effects at adjacent structures, such as the PN and the esophagus, usually occur beyond 180 s, shorter application times may be desirable to maximize safety.922 However, data from remapping studies indicate a higher rate of durable PVI after single 240 s freeze applications compared with 180 s without associated increase in complication rates.923 Alternatively, individualized dosing strategies are used, where the cryoapplication duration consists of the TTI plus a fixed time interval. In two randomized comparisons of a single 180 s fixed cryoapplication protocol compared with a TTI plus 60–90 s-guided approach, no differences in freedom from atrial tachyarrhythmias were seen.924,925
Most commonly, the TTI is used as a marker of adequate lesion formation. In addition, the slope of the temperature curve, the minimal temperature, and the thaw time have been reported to be associated with durable PVI.926–928 On the other hand, achievement of balloon temperatures <−60°C (using the Artic Front device) may prompt termination of energy delivery to avoid collateral damage. In clinical practice, the procedure is usually concluded after the last energy application and documentation of PVI. However, a waiting time of 20 min or provocation maneuvers such as adenosine testing to assess LA to PV reconduction has been evaluated (Sections 8.1.2.3. and 8.1.2.4.).
In patients with variant PV anatomy, e.g. common trunks, PVI using the 28 mm cryoballoon may be more challenging. In patients with short common trunks, sequential treatment of the individual branches is usually performed. In patients with long common trunks, a segmental approach with different balloon orientations (superior, inferior) may be applied. In various studies, a similar clinical outcome was reported following cryoballoon ablation in patients with standard PV anatomy when compared with those with common PV ostium.929–931 However, contradictory results have also been reported.932
A survey of the writing group showed that 55% of the writing group members employ cryoablation dosing algorithms to modify cryolesion duration based on real-time monitoring of elimination of PV potentials and 55% stop prematurely the deployment of cryolesion after the first 60 s if elimination of PV potentials has not been achieved. In the absence of real-time recording of PV potentials during cryoballoon ablation, 43.8% of the writing group members deliver a cryolesion of 180 s, 9.4% of 210 s, and 47% of 240 s duration.
8.2. Adjunctive ablation targets beyond pulmonary vein isolation
8.2.1. Cavotricuspid isthmus
Catheter ablation is the recommended treatment for the management of patients with CTI-dependent AFl due to the high success rates associated with low risk of procedural complications.269 In the CF catheter ablation era, lesion quality indices can be employed to standardize lesion deployment and procedural workflow during CTI ablation.933–935
A survey of the writing group shows that 92.1% of the writing group members perform CTI ablation in patients with prior history or intraprocedural induction of CTI-dependent AFl during AF catheter ablation. A suggested approach regarding CTI ablation in patients undergoing AF ablation and pertinent supporting evidence are presented in Section 4.4.3.
8.2.2. Linear lesions
The origins of linear ablation for AF lie in the Cox maze procedure and its subsequent iterations (Section 12). The most common sites for linear ablation are the LA roof joining the superior aspects of the PV encircling lesion sets, the region of tissue between the anteroinferior aspect of the left PV encirclement and the lateral mitral annulus (the ‘mitral isthmus’), and an anterior line between the anterior mitral valve annulus and either the right PV encirclement (most common) or to the roof line or to the left PV encirclement.
The incremental benefit of linear ablation beyond PVI to prevent AF recurrence has not been demonstrated in prospective RCTs, although it is indicated for the interventional management of macroreentrant AT, which may be encountered either during an AF catheter ablation procedure or during follow-up.811,812 Incomplete linear ablation, i.e. delivering lesions in a linear pattern without achieving block, has the potential to be proarrhythmic and create the substrate for left ATs and therefore should be avoided.815,818
The STAR-AF II study reported no improvement in ablation efficacy with linear ablation (lateral mitral line and roof line) in addition to PVI over PVI alone in patients with persistent AF.566 It should be noted that in the subgroup of patients allocated to the PVI plus lines group, bidirectional block across both roof and mitral lines was achieved in 74% of patients. In a STAR-AF II subanalysis, freedom from arrhythmia recurrence was similar among patients with as compared to those without complete linear block.814 These data indicate that empirical linear ablation does not confer incremental benefit over PVI alone among persistent AF patients, irrespective of the quality of the deployed linear lesion and the achievement of bidirectional block.814 Evidence from metaanalyses also support this conclusion.936,937 Further prospective, multicenter studies of linear ablation with durable bidirectional conduction block may be warranted to establish its role in selected patients with AF.938
A survey of the writing group showed that 0% of the members routinely performs empiric linear ablation (other than to isolate the posterior wall) during ablation of paroxysmal AF and 13.2% of the members when performing ablation of persistent AF.
8.2.3. Complex fractionated atrial electrogram ablation
Complex fractionated atrial electrogram represent low-voltage (0.06–0.25 mV peak-to-peak bipolar amplitude), fractionated, high-frequency electrograms recorded during AF and were proposed to represent sites of potential drivers for AF thus serving as a potential target for catheter ablation. This approach was widely adopted for both paroxysmal and persistent AF.939 However, the pathophysiologic mechanisms underpinning the creation and stability of CFAE and their contribution to AF maintenance were never clarified.940 Furthermore, there was no universally accepted definition allowing for standardization.
The multicenter prospective STAR-AF II study randomized 589 patients with persistent AF to PVI plus linear ablation (259 patients), PVI plus ablation of CFAE (identified by automated software in the mapping system, 263 patients), or PVI alone (67 patients) and demonstrated no benefit of either of these approaches over PVI alone.566 The CHASE AF study randomized 205 patients to PVI alone or PVI plus CFAE ablation. The latter group also underwent linear ablation if atrial macroreentry occurred. There was no significant improvement in arrhythmia-free survival with addition of CFAE ablation.941 A meta-analysis comprising 1415 patients from 13 studies concluded that despite acceptable procedural safety, CFAE ablation did not improve arrhythmia-free survival in paroxysmal, persistent, or long-lasting persistent AF.942 In a recent, large meta-regression and trial sequential analysis, CFAE ablation was shown to be ineffective as an adjunctive strategy in persistent AF ablation, and further study of this ablation approach was considered futile.936 The enthusiasm for CFAE ablation to treat AF has therefore waned and should be avoided in most cases to avoid proarrhythmic lesions.
8.2.4. Stepwise approach to atrial fibrillation ablation
The stepwise approach, which incorporated both linear ablation and defragmentation to target termination of persistent AF either directly or via intermediate AT, has gradually fallen out of favour. The procedure demonstrated early promise in patients with persistent and long-standing persistent AF particularly when achievement of SR was used as an endpoint.242,943 However, subsequent studies have not reproduced these initial results reporting poor 5-year clinical outcomes (20.1% single procedure and 55.9% multiple procedure arrhythmia-free survival at 5 years).944 Recurrence rates of atrial tachyarrhythmias are high, reflecting the proarrhythmic effect of either incomplete linear ablation and/or iatrogenic islands of atrial scar caused by CFAE ablation, which may serve as anchors or isthmus borders for macroreentry and localized reentry.944 In a recent metaanalysis, a stepwise strategy for persistent AF ablation had no significant impact on freedom from atrial arrhythmia recurrences.936
8.2.5. Left atrial posterior wall isolation
The LAPW shares a common embryological origin with the PVs and shares some of the PV arrhythmogenic properties.945,946 Extensive parasympathetic neural plexi located at the LAPW and extending to the PV antrum may also contribute to the initiation and maintenance of AF.207,208,947 Therefore, electrical isolation of the LAPW as an adjunct to PVI seems to be a reasonable approach to increase the success rate of catheter ablation in AF patients.
Several catheter ablation techniques have been proposed for achievement of PWI including: (i) posterior wall box isolation (circumferential PVI with deployment of roof and inferior lines connecting the superior and inferior margins of PV rings, respectively), (ii) single ring isolation (en-bloc encirclement of PVs and posterior wall),948,949 and (iii) posterior wall debulking (extensive focal ablation of the posterior wall without linear lesion deployment).836 Cryoballoon ablation has also been used for PWI, although adjuvant RF ablation may be needed in up to 45.5% of patients.950–953 Posterior wall ablation is also feasible with a pentaspline PFA catheter.657,954,955 In the PersAFOne study, PFA ablation under ICE guidance resulted in low-voltage posterior wall homogeneity with first pass in all 24 patients without primary safety events. Interestingly, invasive remapping 2–3 months postablation demonstrated no evidence of conduction through the posterior wall in 100% of patients and partial voltage recovery of the PW-ablated area in 3 of 21 patients.657
Prior trials investigating PWI plus PVI in comparison with PVI alone in patients with AF have yielded conflicting results (Table 8). Although smaller non-randomized, retrospective trials showed promising results, more recent large prospective RCTs have demonstrated negative results. Yu et al.956 randomized 113 patients to PVI alone or PVI plus posterior LA isolation and an anterior line and demonstrated no improvement in outcome. The POBI-AF trial randomized 217 patients with persistent AF to PVI alone or PVI plus posterior wall box isolation, the latter defined as voltage abatement <0.1 mV, bidirectional block of the roof line, and documentation of both entrance and exit block. Sixty-nine percent of the posterior LA isolation group also underwent an anterior line. Using intermittent Holter monitoring, the reported freedom from any documented AF without AADs was similar in the PVI alone and the PVI plus PWI groups.837 In the recent CAPLA study, 338 patients with symptomatic persistent AF undergoing first-time RF ablation were randomized to either PVI (wide antral circumferential) plus PWI (roof and floor line deployment plus ablation of earliest electrograms within the box if needed) or PVI alone. Contact force–sensing catheters were used with specific lesion quality targets, and the follow-up monitoring was intense (twice daily ECG transmissions). There was no difference in the primary study endpoint with 53.3% freedom from AF at 12 months in the PVI group as compared to 54.1% in the PVI + PWI group.838
Table 8.
Clinical trials comparing left atrial posterior wall isolation plus PVI vs. PVI alone in AF patients undergoing catheter ablation
| Study | Study design | Number of patients | Ablation strategy | Outcome |
|---|---|---|---|---|
| Wong et al.957 | RCT | 67 persistent AF patients (PVI + PWI: 39, PVI: 28) | PVI vs. PVI + PWI PWI: box with additional ablation lesions within the box as needed |
No difference in atrial arrhythmia recurrence rate between the PVI + PWI and PVI only groups at a median follow-up of 12.4 ± 3.0 months (25.6 vs. 28.6%; P = 0.79) |
| Kistler et al.838 | RCT | 338 symptomatic persistent AF patients (first ablation) (PVI + PWI: 170, PVI: 168) | PVI (wide antral circumferential) plus PWI (roof and floor lines deployment plus ablation of earliest electrograms within the box if needed) or PVI alone | No difference in the primary study endpoint at 12 months, with 52.4% freedom from recurrent atrial arrhythmia after a single ablation procedure without AADs in the PVI + PWI group as compared to 53.6% in the PVI group (P = 0.98) |
| Jiang et al.839 | Pooled analysis of 26 studies (9 RCTs) | 3287 paroxysmal and persistent AF patients receiving PVI + PWI | PWI: both box and non-box ablation lesions | In persistent AF, adjunctive PWI was associated with substantially lower recurrence of all atrial arrhythmias (risk ratio: 0.74; 95% CI: 0.62–0.90, P < 0.001) and AF (risk ratio: 0.67; 95% CI: 0.50–0.91, P = 0.01), particularly when only randomized data were examined PVI + PWI using a non-box lesion was associated with significantly less recurrence of AF (OR: 0.30; 95% CI: 0.22–0.41). |
| Jankelson et al.958 | Consecutive series | 321 paroxysmal AF patients (PVI: 214; PVI + PWI: 107) | PVI vs. PVI + PWI PWI consisted of a roof line connecting the LSPV and RSPV along with a low posterior line connecting the inferior PVs |
Recurrence at 1 year: PVI group: 14% vs. PVI + PWI group: 15% (P = 0.96) |
| Ahn et al.953 | RCT | 100 persistent AF patients undergoing first ablation (PVI only: 50 vs. PVI + PWI: 50) with cryoballoon | PWI: additional cryoballoon ablation lesions at 9–13 different locations on the LAPW. | Atrial tachyarrhythmia recurrence during a mean follow-up of 457.9 ± 61.8 days: PVI only: 46% PVI + PWI: 24%, P = 0.035 |
| Sirico et al.840 | Consecutive series | 73 persistent and long-standing persistent AF patients receiving PWI + PVI | PWI: roof line joining the 2 superior PVs and inferior line linking the 2 inferior PVs | PWI + PVI was able to reduce the mean atrial arrhythmic burden by more than 50% compared with preablation, reporting very low levels (≤5%) over 2 years |
| Tokioka et al.841 | Consecutive series | 181 persistent AF patients (PVI only: 91 vs. PVI + PWI: 90) | PWI: Pentaray was placed at the posterior wall to record electrical potentials Endpoint was defined as the absence of electrical activity and inability to capture outside the posterior wall during pacing with the Pentaray catheter with 5 mA output from the posterior LA |
At a median follow-up of 19 months: AF recurrence: PVI only: 47.3% PVI + PWI: 31.1% (P = 0.35) Persistent AF recurrence: PVI only: 20.9% PVI + PWI: 5.6% (P = 0.002) |
| Pothineni et al.842 | Consecutive series | 196 paroxysmal (61%) and persistent (39%) AF patients undergoing repeat ablation (PVRI: 93; PWI ± PVRI: 103) | PVRI vs. PWI ± PVRI PWI consisted of linear lesions across the LA roof and floor connecting the previous circumferential lesion sets that were used for left and right PVI, with additional lesions at sites of earliest activation within the ‘box’ if needed |
Freedom from atrial arrhythmias off AADs at 1 year: PVRI: 69.9% vs. PWI ± PVRI: 43.7% (P = 0.5) |
| Salih et al.843 | Metaanalysis of 6 studies | 1334 persistent AF patients (PVI: 663; PVI + PWI: 671) | PVI vs. PVI + PWI | At 21.6 ± 13 months: AF recurrence rate: PVI only: 29.1% PVI + PWI: 19.8%, risk ratio: 0.64; 95% CI: 0.42–0.97, P < 0.04 Atrial arrhythmia recurrence rate: PVI only: 41.1% PVI + PWI: 30.8%, risk ratio: 0.75; 95% CI: 0.60–0.94, P < 0.01 |
| Sutter et al.844 | Retrospective study | 558 persistent AF patients undergoing initial and repeat ablation (PVI: 255, PVI + PWI: 78, PVI + lines: 225) | PVI vs. PVI + PWI vs. PVI + lines PWI: linear ablation along the LA roof to connect LSPV and RSPV and linear ablation along the LA floor to connect inferior PVs Lines: one or more of the following: mitral isthmus, LA roof, or cavotricuspid isthmus line |
Sinus rhythm at 6 months: PVI: 73.9% vs. PVI + lines: 72.2% vs. PVI + PWI: 57.7% |
| Yamaji et al.845 | RCT | Persistent AF patients without LA low-voltage area Electrophysiological test subgroup: 57 (+PWI: 24; −PWI: 33) |
+PWI: PVI + PWI + SVCI + CTIA −PWI: PVI + SVCI + CTIA PWI: roof line joining the two superior PVs and inferior line connecting the two inferior PVs |
AF/AT recurrence at median 62.7 weeks: +PWI: 25% vs. −PWI: 15% (P = 0.311) |
| Lee et al.837 | RCT | 207 persistent AF patients (PVI: 105; PVI + PWI: 102) | PVI vs. PVI + PWI PWI: roof line joining the two superior PVs and inferior line connecting the two inferior PVs with touch-up ablation at the PW if needed to achieve exit block (additional anterior line at the physician's discretion) |
Freedom from atrial arrhythmia without AAD at 1 year: PVI: 50.5% vs. PVI + PWI: 55.9% (P = 0.522) |
| McLellan et al.846 | Consecutive series | 161 persistent AF patients undergoing circumferential PVI followed by PWI (no-adenosine challenge: 107, adenosine challenge: 54)a | PWI: roof and inferior wall lines with the endpoint of bidirectional block | Adenosine-induced reconnection of the PW was demonstrated in 17% Freedom from recurrent atrial arrhythmia at 19 ± 8 months: adenosine challenge: 65% vs. no-adenosine challenge: 40% (P < 0.01) |
| Bai et al.836 | Prospective non-randomized trial | 52 persistent AF patients (PVI only: 20; PVI + PWI: 32) All patients underwent a second procedure 3 months after the first procedure |
PWI: PVI was extended to the CS and to the left side of the interatrial septum, along with extensive ablations on the LAPW At 3 months, electrophysiology study was performed in all patients to confirm durability of the PWI and PVI |
Freedom from atrial arrhythmia without AADs at 1, 2, and 3 year follow-ups: PVI: 20, 15, and 10%, respectively PVI + PWI: 65, 50, and 40%, respectively, P < 0.001 |
| Kim et al.847 | RCT | 120 persistent AF patients (PVI + lines: 60 vs. PVI + lines + PWI: 60) | Roof, anterior perimitral and CTI lines with conduction block were performed in all patients PWI: additional posterior inferior line connecting inferior PVs |
Recurrence at 1 year: PVI + lines: 36.7% vs. PVI + lines + PWI: 16.7%, P = 0.02 |
AAD, antiarrhythmic drug; AF, atrial fibrillation; CI, confidence interval; CS, coronary sinus; CTIA, cavotricuspid isthmus ablation; LA, left atrium; LAPW, left atrial posterior wall; LSPV, left superior pulmonary vein; OR, odds ratio; PV, pulmonary vein; PVI, pulmonary vein isolation; PVRI, pulmonary vein reisolation; PWI, posterior wall isolation; RCTs, randomized controlled trials; RSPV, right superior pulmonary vein; SVCI, superior vena cava isolation.
aAdenosine challenge to assess dormant conduction in the PVs and PW.
One explanation for the lack of incremental benefit from catheter ablation of the LAPW could be the inability to achieve durable electrical isolation. Pertinent challenges stem from (i) the significant variation in thickness of the septopulmonary bundle that is the dominant structure in the LAPW, (ii) difficulties in achieving transmural lesion at the LAPW roof due to insulation of the epicardial muscular bundle by fat inter-position,179 and (iii) a tendency to lower the power and duration of energy delivery during LAPW ablation to prevent thermal injury to the neighbouring esophagus.959,960 In fact, LAPW reconnection rates have been reported to be as high as 40–100%, with predominantly posterior location, while an association has been shown between LAPW reconnection and elevated esophageal temperature during the index procedure.960–962 Alternate explanations for lack of demonstrated benefit from PWI include the following: (i) PVI alone using a wide antral isolation strategy already encompasses much of the LAPW, potentially leaving little additional benefit from roof and inferior lines; (ii) the contribution of the posterior LA to persistent AF mechanism is not universal, and a ‘one size fits all’ approach may be ineffective; or (iii) survival of epicardial LAPW tissue. This allows the possibility that posterior LA isolation may have a role in a specific group of persistent AF patients. In a recent subgroup analysis of the CAPLA study, it was found that patients with short cycle length posterior LA activity did derive a benefit from PWI, indicating that this may be a determinant of which patients will have improved outcomes with this additional step.963
Hybrid ablation has also been used to target LAPW as part of an ablation strategy in patients with persistent and long-standing persistent AF (Section 12.3.3.2.).
A survey of the writing group showed that 15.8% of the members perform PWI during first-time and 26.3% when performing redo ablation of paroxysmal AF. Furthermore, 31.6% of the writing group members perform PWI during first-time and 65.8% when performing redo ablation of persistent AF.
8.2.6. Substrate ablation
A range of conditions has been demonstrated to promote the development of abnormal atrial substrate.964 These include classically recognized factors associated with AF such as hypertension, HF,965 diabetes, and advanced age.966 Recently, other conditions have been shown to drive atrial substrate development such as obesity,109,967 sleep disordered breathing,362 excess alcohol intake,459,968 and prolonged high-intensity training in certain athletic sports.969 The pathophysiologic mechanisms underlying areas of abnormal electrical substrate include regional fibrosis, loss of cellular coupling due to loss of connexin, inflammation, and adipocyte infiltration into tissues.108,518 These changes promote AF initiation and maintenance (Section 2.4.—Figure 1).
Electroanatomical mapping and cardiac MRI have both been utilized to define the atrial substrate, albeit using quite different surrogates of atrial fibrosis—bipolar voltage and late gadolinium signal intensity, respectively. Ablation guided by electroanatomical voltage mapping is a patient-tailored approach targeting low-voltage areas, either by encirclement leading to isolation or direct ablation of the entire low-voltage area.829,970 Endpoints of this approach include local voltage reduction, elimination of fractionated electrograms, and regional isolation. Preliminary observational studies suggested the potential for favourable outcome.836,971–973 Several randomized trials have evaluated whether adjuvant ablation of low-voltage areas may provide incremental benefit on rhythm outcome among paroxysmal or persistent AF patients. In the VOLCANO trial including 398 patients with paroxysmal AF and the STABLER-SR II trial including 300 patients with persistent AF, low-voltage area ablation in addition to PVI did not improve arrhythmia-free survival.827,974 However, the recent STABLE-SR-III trial randomized 438 older patients with paroxysmal AF to PVI plus low-voltage area ablation or PVI alone and showed a significant incremental benefit derived by low-voltage area ablation in this patient population.975 In all trials, LA voltage mapping was performed during SR with a low-voltage cutoff <0.5 mV.
A recent prospective RCT presented evidence supporting the concept of substrate ablation in persistent AF patients.828 The ERASE AF study enrolled 324 patients who were randomized to PVI only (163 patients) or PVI plus substrate modification (161 patients). Substrate modification was only performed in the subset of patients (34%) found to have low-voltage regions (voltage threshold 0.5 mV) during SR mapping. The primary study endpoint (first recurrence of an atrial arrhythmia >30 s after a single procedure) was reached in 50% of PVI only patients and 35% of PVI plus substrate modification group at 12 months (HR = 0.62, 95% CI = 0.43–0.88, P = 0.006).828
Despite recent encouraging results, methodologic challenges inherent to the strategy of low voltage–guided substrate modification remain. Voltage measurements are not only dependent on rhythm status (AF vs. SR), size, and configuration of the recording electrodes and catheter–tissue contact but can also vary up to three-fold according to atrial rate and wavefront directionality.976 Furthermore, voltage parameters indicative of abnormal substrate lack objective definition and low-voltage cutoffs vary considerably among different investigators. A one size fits all voltage cutoff does not consider regional variations in atrial wall thickness, nor again the nature of the recording electrodes. Ultimately, identification of substrate may require a more sophisticated analysis incorporating not only voltage but also electrogram morphology and possibly measures of regional atrial conduction.
Cardiac MRI-LGE has been used to identify and localize cardiac fibrotic areas in a variety of cardiac diseases, including AF.92,105,512 Attempts to ‘calibrate’ electroanatomic voltage mapping using MRI-LGE have reported LA voltages between 0.2 and 0.45 mV as demarcating LA scar.977 Correlations of variable strength between LA voltage mapping and atrial histology have been reported.105,512,978 The DECAAF study reported that the severity of MRI-defined atrial fibrosis was a predictor of AF recurrence following AF catheter ablation, thereby supporting a role for MRI-LGE in the preprocedural evaluation of atrial substrate (Section 5.2.1.4.). This requires considerable experience of atrial LGE imaging, specific imaging sequences, and access to a reproducible image processing workflow, which to date has limited the widespread uptake of this technique.
In the ALICIA trial, 155 symptomatic, drug-refractory AF patients (54% paroxysmal AF) undergoing first or repeat ablation were randomized to either PVI or PVI plus MRI-guided ablation of fibrotic areas by either homogenization or isolation.848 Fibrotic areas outside the PV antra were identified in only half of the patients, and their ablation did not reduce arrhythmia recurrence rate at 1 year of follow-up.848 In the recent much larger DECAAF-II trial, 843 persistent AF patients were randomized to either MRI-guided fibrosis ablation plus PVI or PVI alone. The primary composite of atrial arrhythmia recurrence or repeat ablation did not differ between the two groups after a median follow-up period of 273 days (43.0 vs. 46.1%; P = 0.63). Furthermore, there was a significantly higher occurrence of the primary safety composite outcome in the fibrosis-guided ablation plus PVI group (2.2 vs. 0%, P = 0.001), largely driven by higher ischemic stroke events.849 Therefore, one should avoid additional ablation based on MRI-detected fibrosis pending development of better MRI resolution and future studies.
A survey of the writing group showed that 0% of the members perform ablation of MRI-detected or voltage mapping-detected abnormal atrial myocardial areas during first-time and 18.4% during redo ablation of paroxysmal AF. Furthermore, 13.2% of the writing group members perform ablation of MRI- or voltage mapping-detected abnormal atrial myocardial areas during first-time and 31.6% during redo ablation of persistent AF.
8.2.7. Vein of Marshall ablation
The VoM is an embryological remnant of the left upper caval system that possesses arrhythmogenic potential and has been proposed as a target during AF catheter ablation979 (Section 3.5.). Ethanol infusion into the VoM has been proposed as an adjunctive ablation strategy in persistent AF, acting not only by eliminating this arrhythmogenic structure but also providing collateral benefits including autonomic modulation and partial ablation of LA areas that are routinely targeted during circumferential isolation of left PVs and lateral mitral isthmus line deployment.825 In a large cohort of consecutive patients treated with ethanol infusion in the VoM, the reported feasibility was almost 90% during the first attempt, with previous CS ablation reported as the only predictor of failure, while the reported complication rate was 2.0%.980
The VENUS trial was a prospective RCT that evaluated potential incremental benefit derived by VoM ethanol infusion in addition to an extensive ablation procedure containing many components of the stepwise approach. In this study, the majority of patients received mitral isthmus ablation, LAPW isolation, and CFAE ablation. The study demonstrated that adjunctive VoM ethanol infusion significantly improved the off-AAD arrhythmia-free survival (49.2 vs. 38%, P = 0.04).830 Although these data are encouraging, the standard ablation procedure in this study was extensive, non-standardized, with significant differences between the compared groups and included, at the operator's discretion, empiric linear ablation, CFAE ablation, and LAPW isolation most frequently in combination. In a VENUS substudy, the favourable impact of VoM ethanol infusion was potentiated when performed in high-volume centers and when perimitral block was achieved.833 The benefit of VoM ethanol infusion when added to PVI has yet to be shown to improve outcomes over PVI alone.
In a recently published randomized trial, VoM ethanol infusion as the first step in mitral isthmus linear ablation was shown to significantly reduce the number of RF applications needed to achieve mitral isthmus block.826 Pambrun et al.193 recently reported results of the ‘Marshall-PLAN’ procedure for persistent AF. This procedure adds VoM ethanol infusion to a lesion set including PVI, linear lesions (posterior mitral line, roof line, and CTI line), LA ridge, ‘saddle’ (between the LSPV and the LAA), and extensive CS ablation. Implementation of this ablation strategy in an observational cohort of 75 consecutive patients with persistent AF (duration 9 ± 11 months) resulted in a 72% freedom from arrhythmia recurrence at 12 months off-AAD after a single procedure.834 A randomized trial comparing the Marshall-PLAN ablation approach with PVI only in persistent AF patients is ongoing (NCT 04681872).
A survey of the writing group shows that 5.3% of the members employ VoM ethanol infusion when performing first-time persistent AF ablation and 26.3% during redo ablation of persistent AF.
8.2.8. Ablation of non-pulmonary vein triggers
Pulmonary vein isolation is a highly effective procedure in patients with paroxysmal AF, in whom spontaneous PV firing is frequently the only trigger for AF paroxysms.981 However, recurrence of arrhythmia has been reported in up to 20% of paroxysmal AF patients in the presence of isolated PVs.982,983 These observations have driven approaches towards identification and targeting of non-PV triggers. Non-PV triggers have been described originating from specific anatomic regions including the LAPW, SVC, CS, VoM, crista terminalis, interatrial septum, and LAA.984 In addition, persistent left SVC has also been reported as a site of AF triggers.985
Electrical LAA isolation has been proposed as a strategy to eliminate potential LAA triggers with varying reported success. In the prospective, randomized BELIEF trial, 173 patients with long-standing persistent AF were randomized to either empirical endocardial LAA electrical isolation plus extensive ablation (PVI plus ablation of PW, part of the LA septum, non-PV triggers, and SVC) vs. extensive ablation alone at the index procedure. At 12-month follow-up, patients with LAA isolation had a higher freedom from atrial arrhythmias.986 A large propensity score–matched study and a metaanalysis of nine studies in non-paroxysmal AF patients undergoing catheter ablation concluded that LAA isolation significantly increased freedom from all atrial arrhythmia recurrence without increased risk of acute procedural complications.987,988 In a metaanalysis of seven studies assessing impact of LAA isolation on AF recurrence utilizing various approaches, including ablation, surgery, and ligation by Lariat, LAA isolation was shown to be associated with a significantly lower rate of AF/AT recurrence.989 The authors concluded that further randomized studies were nevertheless required to confirm safety and efficacy of this approach. However, the multicenter prospective randomized aMAZE trial in 610 patients with symptomatic persistent and long-standing persistent AF did not show a benefit in arrhythmia-free survival with addition of LAA ligation with the Lariat epicardial suture device on top of PVI (404 patients) vs. PVI alone (206 patients).990
Several studies have reported a high incidence of LAA thrombus formation and increased risk of thromboembolism after endocardial LAA isolation using RF energy despite adequate OAC therapy.991–993 Intracardiac thrombus formation is identified in one-fifth of patients undergoing wide area LAA isolation and the respective rate of stroke/TIA is 6–9.8%.991,992 Interventional LAA occlusion may be protective against thromboembolism in this clinical setting.991 In a non-randomized study of 166 patients with durable LAA isolation, interventional LAA occlusion was associated with significant reduction in thromboembolic complications when compared with OAC therapy.994 Randomized trials are needed to document the efficacy of this preventive approach. Considering the modest evidence supporting the value of LAA isolation as a stand-alone adjunct to PVI in persistent AF patients and the associated increased risk of thrombus formation and thromboembolism, this ablation strategy may be only justified during redo ablation procedures in persistent AF patients and after informing the patient for the need of permanent thromboprophylaxis or mechanical closure of the LAA.
Routine identification and ablation of non-PV triggers is limited by the absence of standardized induction protocols, differences in trigger definition, and paucity of prospective randomized studies indicating a benefit of this approach either in denovo or repeat procedures. Induction protocols have used varying amounts of isoprenaline up to 20 μg/min or higher and burst pacing to induce AF followed by cardioversion to initiate trigger activity. Variable trigger definitions have been proposed, including triggers resulting in AF paroxysms or repetitive focal activity even isolated atrial ectopics. In a randomized study of persistent AF patients, empiric ablation of common non-PV trigger sites in addition to PVI did not improve outcome compared with PVI combined with ablation of only documented non-PV triggers.835 Prevalence of triggered ectopics has varied widely according to population and technique used.995–997 More data are needed to establish a consensus on the characterization and ablation of non-PV triggers.
A survey of the writing group showed that 31.6% of the writing group members employ mapping and ablation of non-PV triggers during first-time and 68.4% during redo ablation of paroxysmal AF, while 34.2% during initial ablation of persistent AF and 73% during redo ablation of persistent AF. Furthermore, 83.8% of writing group members employ mapping and ablation of non-PV triggers in redo AF ablation procedures when all PVs remain isolated. In addition, 0% of the writing group members perform LAA isolation during first-time persistent AF ablation and 5.3% when performing redo persistent AF ablation.
8.2.9. Ganglionated plexi ablation
The cardiac ANS plays an important role in the initiation and maintenance of AF.123,126,208,210,211,998–1005 The GP, containing the cardiac parasympathetic and sympathetic ganglia, are located on the epicardial aspect of the PV antra and are frequently ablated during PVI (Section 3.7.). Their functional localization is possible with high-frequency stimulation (cycle length 50 ms, 12–15 V, 10 ms pulse width), manifesting as sinus bradycardia or AV nodal conduction delay or block.999,1005 However, the sensitivity of endocardial high-frequency stimulation to identify GP sites is not optimal.210
Ganglionated plexi ablation plus PVI has been variably reported to improve the outcome following AF ablation in some RCTs.1006,1007 However, in the prospective randomized AFACT study, adjunctive epicardial GP ablation during thoracoscopic AF surgery did not improve freedom from AF recurrence but was associated with an increased risk of major complications.1008 Due to the inconsistent RCT outcomes and the technical challenges associated with high-frequency stimulation, the evidence in support of this approach is modest.
A survey of the writing group showed that 2.7% of the members perform GP ablation during first-time ablation of paroxysmal or persistent AF ablation and 0% during redo ablation of paroxysmal or persistent AF.
9. Postprocedural management
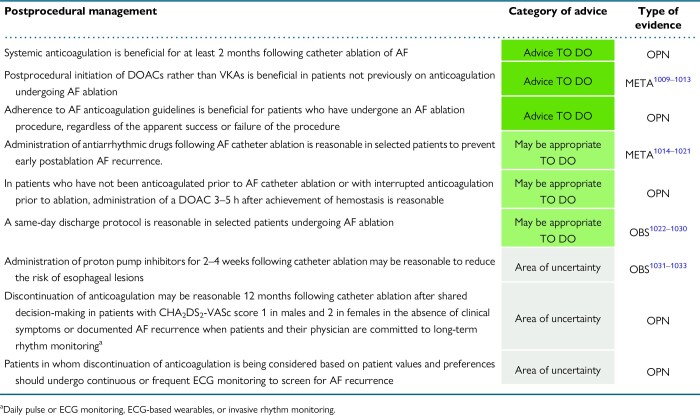
|
9.1. Sheath removal—hemostasis achievement
After completion of the ablation procedure, ensuring adequate hemostasis is of primary importance to reduce the risk of vascular complications. Sheaths can be removed after waning of heparin's anticoagulant effect or while the patient is on full anticoagulation. In the former case, sheaths should be removed when the ACT is <200–250 s or after reversal of heparin effect with protamine infusion. Two RCTs and a recent metaanalysis of five studies have consistently shown that the use of protamine after catheter ablation significantly expedites vascular hemostasis and patient ambulation by about 3 h without associated increase in vascular or thromboembolic complications.729,1034,1035 This favourable effect should be weighed against a 1.2% risk of adverse reaction to protamine often presented with profound hypotension.1036 A survey of the writing group showed that 57.9% of the members routinely use protamine to reverse heparin anticoagulation effect after completion of AF ablation.
A figure-of-eight suture technique (with the use of either a knot or a three-way stopcock to secure suture in place) has been proposed for achieving hemostasis after catheter ablation obviating the need for manual compression of the puncture site.1037–1039 This technique significantly reduces the time required for hemostasis and patient's post-procedure time in the electrophysiological lab, without associated increase in bleeding complications when compared with manual compression.1038,1039 Closure of venous access sites with specialized devices also shortens time to postablation hemostasis and patient ambulation and reduces the need for pain medications, without significant difference in the incidence of minor or major access site complications.1040
9.2. Duration of hospitalization—same-day discharge
Catheter ablation for AF has typically been performed as an in-patient procedure with at least one overnight stay. Given the increasing demand for AF ablation, same-day discharge protocols have increasingly been adopted to minimize health care resource utilization.1022–1024 Avoiding overnight hospital stay increases patient satisfaction and may also have benefits for the patients such as reduced risk of infection. Metaanalyses of observational studies have shown that same-day discharge was successful in >80% of the planned cases, and the reported safety outcomes were favourable. No differences in 30-day complications or 30-day readmissions were identified between the patients with same-day discharge compared with those with overnight hospital stay.1025,1026,1029 Moderate quality evidence from a recent randomized trial supports the safety of same-day discharge after cryoballoon AF ablation.1027 Feasibility and safety of same-day discharge has been reported even when implemented as default management strategy in consecutive patient cohorts.1028 Overall, same-day discharge after AF ablation appears to be a safe strategy in selected patients provided that appropriate institutional protocols and patient pathways are established to identify suitable patients and ensure adequate follow-up.1030 Eligibility criteria for same-day discharge include, but are not limited to, uncomplicated catheter ablation, at least 3–6 h of postprocedural monitoring, achievement of complete hemostasis, well-tolerated ambulation, normal vitals signs at discharge, and absence of symptoms or concerning comorbidities.1022 A standardized same-day discharge protocol based on specific eligibility criteria has been described, and its safety has been validated in a large multicenter prospective registry.1029
A survey of the writing group showed that 23.7% of the members implement a default strategy of same-day discharge, while 57.9% employ a same-day discharge management protocol in selected patients following AF catheter ablation.
9.3. Postprocedural pharmacological management
9.3.1. Anticoagulants
9.3.1.1. Early postprocedural (the first 2 months)
Anticoagulation is recommended for at least 2 months following catheter ablation for all patients regardless of CHA2DS2-VASc score in prior guidelines and consensus documents.5,1041 This is due to endothelial damage, an inflammatory state, and potential stunning of atrial myocardium following ablation and/or cardioversion. In patients not previously on anticoagulation, initiation of DOACs rather than VKA is preferred postablation because of the immediate effect that does not require bridging with UFH or LMWH.1009–1013 In patients who had not been anticoagulated or who did not take their last DOAC dose prior to the procedure, administration of the DOAC 3–5 h after sheath removal is advisable, provided there is no evidence of mechanical complications. In patients who require lifelong anticoagulation with VKA (e.g. mechanical heart valve or rheumatic heart disease), it is recommended that ablation be performed on uninterrupted VKA.299
9.3.1.2. Late postprocedural (more than 2 months)
The management of anticoagulation beyond the early postprocedural period after AF ablation remains controversial. Prior guidelines have recommended continuing anticoagulation based on the patient's stroke risk profile rather than the presumed success or failure of the ablation.5,1041
In the absence of high-quality evidence, long-term anticoagulation after AF ablation in patients with CHA2DS2-VASc score ≥2 for men or ≥3 for women is considered beneficial for a number of reasons: (i) recurrences of AF are common both early and late following AF ablation; (ii) asymptomatic AF is common and is even more common following than prior to AF ablation1042; (iii) there have been no large, randomized prospective trials that have assessed the safety of discontinuing anticoagulation in this patient population; (iv) while registry data suggested a lower risk for stroke in patients undergoing AF ablation compared with matched AF controls,1043,1044 the largest prospective randomized trial on AF ablation, the landmark CABANA trial, failed to show a reduction in the risk of subsequent stroke in patients undergoing ablation.1045 This is in line with a metaanalysis of randomized controlled trials of AF ablation vs. AAD treatment, which also did not find a significant benefit of ablation over AAD treatment with regard to the subsequent risk of stroke258; (v) several studies have shown a temporal dissociation between ischemic stroke and episodes of AF,1046–1048 which suggests that AF might be a marker of increased thromboembolic risk rather than a causal factor; and (vi) stroke risk is a lifelong consideration and increases with age such that patients many years from an apparently successful ablation will be at higher risk than when the decision to stop anticoagulation was made.
Arguments against the long-term management of postablation anticoagulation based solely on stroke risk score include the following: (i) patients in SR without evidence of AF have generally no indication for anticoagulation and (ii) long-term and possibly lifelong continuation of anticoagulation has a small, yet significantly increased risk of severe bleeding complications, which in some patients may outweigh the potential benefits on stroke prevention.
In the absence of RCTs comparing cessation vs. continuation of anticoagulation after AF ablation, several metaanalysis have summarized available data from non-randomized studies.1049–1051 In summary, a decreased thromboembolic risk and a favourable net clinical benefit from continued anticoagulation were generally seen in patients with CHA2DS2-VASc ≥2, while no significant benefit was found from continued anticoagulation in patients with a CHA2DS2-VASc ≤1. This is important when interpreting individual studies that did not show an increased stroke risk with discontinuation of anticoagulation after AF ablation. Many AF ablation cohorts are skewed towards enrolment of low-risk patients with CHA2DS2-VASc ≤1. Accordingly, the overall background stroke risk in those cohorts is low, and the number of potential high-risk patients with CHA2DS2-VASc ≥2 is often too small to show a disadvantage of discontinuation of anticoagulation.
The ongoing OCEAN trial (Optimal Anti-Coagulation for Enhanced-Risk Patients Post–Catheter Ablation for Atrial Fibrillation, NCT02168829) is enrolling subjects at risk for stroke as indicated by a CHA2DS2-VASc ≥1 and who have not had clinically apparent atrial arrhythmias for at least 12 months after their most recent AF ablation.1052 Eligible patients are randomized to anticoagulation with rivaroxaban 15 mg daily vs. aspirin 75–160 mg daily and followed for the primary composite endpoint of clinically overt stroke, systemic embolism, and covert stroke based on brain MRI during 3 years of follow-up. The results of OCEAN will provide important data to inform future management of anticoagulation after successful AF ablation. Additional clinical trials however will be needed to define the long-term stroke risk and the need for continued anticoagulation after AF ablation overall as well as for selected patient subgroups, especially in those with presumptive successful AF elimination after ablation.
9.3.1.3. Candidates to discontinue anticoagulation
Discontinuation of anticoagulation may be considered in several patient categories following AF catheter ablation.
Low-risk patients (CHA2DS2-VASc 0 in men and 1 in females). In low-risk patients, anticoagulation should be discontinued 2 months after ablation regardless of the ablation outcome. Based on current guidelines, risk-benefit assessment does not justify antithrombotic protection in these patients, irrespective of their rhythm status.522
Intermediate-risk patients (CHA2DS2-VASc 1 in men and 2 in females). In this patient category, discontinuation of anticoagulation may be considered 12 months following catheter ablation in the absence of clinical symptoms or electrocardiographically documented AF recurrence. The writing group suggests deferral of OAC discontinuation until the completion of 12 months following catheter ablation in this patient category, to increase the likelihood of selecting patients with truly successful AF elimination. A proposed prerequisite to maximize safety after discontinuation of anticoagulation is that both patients and their physicians are committed to long-term rhythm monitoring (daily pulse or ECG monitoring, digital heart rhythm devices, or invasive monitoring) to screen for AF recurrence and guide accordingly the reinitiation of anticoagulant treatment.
Higher risk patients (CHA2DS2-VASc ≥2 in men and ≥3 in women). In higher risk patients, anticoagulation should not be discontinued for several reasons mentioned above. However, if discontinuation of anticoagulation is being considered based on strong patient values and preferences and despite prior clarification of pertinent exposure to increased thromboembolic risk, patients should be placed under regular rhythm monitoring to screen for AF recurrence. As stated above, this may include daily pulse or ECG monitoring, digital wearable heart rhythm devices, or invasive monitoring, and selection of monitoring option should be individualized after detailed discussion with the patient. In case of documented AF recurrence, therapeutic anticoagulation should be reinitiated. In addition, LAA occlusion may be discussed as an alternative approach. The ongoing OPTION trial (NCT03795298) is a prospective, randomized study to determine if LAA closure is a reasonable alternative to OAC in patients after AF ablation.
9.3.1.4. Targeted anticoagulation (on demand) postablation
When assessing the need for continued anticoagulation after AF ablation beyond the blanking period, the key question is whether the ablation was successful in eliminating AF. Unfortunately, the answer to this question is difficult to ascertain in most patients. The exception is patients with implantable cardiac devices including pacemakers, ICDs, and ICM. In addition to the binary detection of episodes of AF recurrence, implantable devices can also quantify the burden and duration of AF episodes, both of which correlate with stroke risk.1053,1054 Most of the implantable cardiac devices have remote monitoring capabilities and could potentially be used to guide intermittent ‘on demand’ anticoagulation during periods of AF. This strategy could be attractive in patients with paroxysmal AF, especially in younger, active ones who may have a risk for bleeding complications related to everyday activities.
The first large, randomized trial, IMPACT, showed no benefit of such an intermittent on demand anticoagulation strategy over standard continued anticoagulation in 2718 ICD-patients with regard to thromboembolism and bleeding.1055 Among other factors, the use of VKA in the majority of patients, rather than DOACs, might have negatively affected the results of this study given the delay in achieving therapeutic anticoagulation. More recently, two small pilot studies have tested the strategy of an intermittent on demand anticoagulation with DOACs in device recipients. Using single-arm designs in 48 patients and 59 patients, such an approach was feasible and decreased anticoagulation utilization by 75 and 94%, respectively.1056,1057 The studies were not designed to assess the clinical outcomes of stroke or bleeding.
The concept of using continuous ECG monitoring by means of ICM or intensified non-invasive ECG monitoring using wearable devices is of potential interest as an adjunctive tool to guide anticoagulation after AF ablation. This strategy needs to be tested in prospective studies using appropriate cutoffs for AF burden and AF duration before it could be recommended for routine clinical practice. Furthermore, the strategy of ‘on demand’ anticoagulation is limited by the reported temporal dissociation between AF and stroke, which casts doubt on the value of guiding initiation and discontinuation of anticoagulation based on rhythm criteria.1046,1047 A prospective randomized study testing the strategy of intermittent vs. continuous DOAC administration based on symptoms and smartwatch-detected AF is currently underway (REACT-AF).
9.3.2. Antiarrhythmic drug treatment
Several prospective RCTs assessed the value of routine AAD administration in the immediate postablation period.1014–1019 EAST-AF was the largest study and randomized 2038 AF patients (68% paroxysmal) to 3 months of AAD treatment postablation or standard medical therapy without AAD. While more patients remained free from atrial arrhythmias during the 3-month blanking period in the AAD group (59.0 vs. 52.1%, P = 0.01), no difference was observed 1 year after ablation (69.5 vs. 67.8%, P = 0.38).1014 Aggregation of all studies in metaanalyses confirmed the effectiveness of short-term AAD therapy in preventing early but not late relapses after discontinuation.1020,1021 Given the psychological and financial burden of arrhythmia-related hospitalizations and cardioversions during the blanking period, short-term continuation of AAD for several months after the ablation should be considered in selected patients to prevent early AF recurrence, particularly those with persistent AF prior to ablation or who have tolerated antiarrhythmic medications prior to ablation. In others who experience AAD-related side effects, discontinuation after ablation is reasonable.
A survey of the writing group showed that 57.8% of the writing group members administer AADs during the blanking period as a strategy to prevent early AF recurrences after paroxysmal AF ablation and 86.8% after persistent AF ablation.
9.3.3. Proton pump inhibitors
Damage of the esophagus is one of the most feared complications of AF ablation. Esophageal lesions on routine endoscopy were found in 10–15% and ulcerations in about 5% of patients after AF ablation.693 Atrioesophageal fistulae occur in 0.016–0.1% of AF ablation procedures.1058–1067 It is hypothesized that AEF results from a double hit injury starting with a transmural ablation lesion extending through the atrial wall to the esophagus followed by subsequent ulcer erosion from gastroesophageal reflux (Section 11.3.1.).1068 Based on this presumed mechanism, administration of proton pump inhibitors (PPIs) to prevent ulceration has been widely adopted after LA ablation procedures.1031 Evidence to support or disprove this practice is limited. A preclinical study found that progression of esophageal ulcer and development of AEF after ablation were associated with reflux esophagitis.1032 A substudy of the MADE-PVI trial suggested a reduction in esophageal lesions as assessed by endoscopy in patients with preprocedural use of PPI.1033 However, in a large-scale, retrospective, propensity score-matched analysis, the use of PPI before or on the day of ablation was not associated with reduced mortality or severe esophageal injury within 30 days postablation.1069 Adequately powered clinical trials to establish the efficacy of pharmacological prophylaxis to reduce AEF are lacking and unlikely to be feasible given the low incidence of AEF.
Preclinical experience suggests a very low risk (if any) for esophageal injury647 with PFA, and no AEF has been reported with early clinical use so far.643,644 Accordingly, the value of PPI postablation treatment in PFA cases is less compelling.
A survey of the writing group showed that 79% of the members employ short-term administration of PPI as a strategy to prevent esophageal lesions following catheter ablation when using non-PFA energy sources, while 54% when using PFA.
9.3.4. Anti-inflammatory agents
Early AF recurrence in the first few weeks following AF ablation has been linked to inflammation induced by the ablation procedure. Routine anti-inflammatory treatment may reduce the incidence of early relapses of AF after ablation and potentially also long-term recurrence.
Colchicine has been studied for this purpose in two prospective randomized trials. A 3-month course of treatment with colchicine 0.5 mg twice daily compared with placebo resulted in a reduction of AF recurrences up to 90 days (16 vs. 34%, P = 0.01).1070 Interestingly, the 3-month treatment with colchicine also improved long-term outcomes with reduced recurrence rates at 1 year (31 vs. 50%, P = 0.01).1071 The benefit in terms of AF recurrence also translated into benefits in QoL and psychological score. However, these data have not been reproduced and are limited by small sample size.
Corticosteroids have also been used as a short-term treatment after AF ablation to reduce recurrences.1072–1076 Study designs were inconsistent with regard to the duration of steroid treatments (single dose vs. several days). Most of the studies showed a decrease in early recurrence rates until 3 months, but no difference with regard to late recurrence. It is also possible that steroids may limit ablative lesion healing. In view of the potential side effects of steroids, when applied over weeks or even months, as well as the limited and inconclusive data available, steroids after ablation should only be used cautiously and for short durations.
9.4. Rhythm monitoring following catheter ablation
Arrhythmia monitoring postablation is useful to detect asymptomatic postprocedural arrhythmia recurrences and determine the etiology of symptomatic palpitations. Palpitations may result from recurrent AF or other atrial tachyarrhythmia but may also result from atrial or ventricular premature beats and therefore are not an accurate predictor of AF recurrence.1077 In the CIRCA-DOSE trial, only 45% of the symptom-triggered activations were adjudicated as AFl or AF during the postablation continuous monitoring of paroxysmal AF patients using an ICM.7
Multiple studies have demonstrated that asymptomatic AF commonly occurs in patients following catheter ablation. Two studies reported that the proportion of asymptomatic AF events was 11–35% prior to and 53–65% after ablation.1078,1079 In another study assessing the correlation between symptoms and underlying rhythm following AF catheter ablation, 53.8% of recorded AF episodes were asymptomatic, with an increase in asymptomatic episodes from the acute to the chronic period after ablation.1080 In the DISCERN-AF study, continuous monitoring of symptomatic AF patients before and after catheter ablation with an ICM demonstrated that the ratio of asymptomatic to symptomatic AF episodes significantly increased from 1.1 before to 3.7 after ablation with 12% of patients having asymptomatic recurrences only.1042 In the CIRCA-DOSE study, the 1-year arrhythmia-free survival based on the presence of documented recurrence of either symptomatic or asymptomatic atrial tachyarrhythmia lasting >30 s on continuous cardiac monitoring was 52.6%, while the respective survival free from symptomatic only arrhythmia recurrences was 85.3%.7 Consequently, symptoms are not well correlated with postablation AF burden, stressing the need for postprocedural follow-up strategies consisting of continuous or intermittent ambulatory rhythm monitoring in addition to symptom-driven rhythm assessments.
9.4.1. Continuous postablation rhythm monitoring
Continuous rhythm monitoring includes ICM, pacemakers, or ICDs and allows for continuous, remote, long-term monitoring in asymptomatic and symptomatic individuals. Pacemakers and ICDs with an atrial lead may record intracardiac atrial electrograms and detect atrial high-rate episodes as an indicator of AF occurrence. The positive predictive value of recorded atrial high-rate episodes varies upon the programmed rate and duration thresholds, with false-positive rates of 17.3% for episodes lasting >6 min and 3.3% with threshold duration >6 h.1081 Long-term subcutaneous ICM can facilitate continuous AF monitoring based on R–R interval analysis over a time period of up to 4.5 years.1082,1083 These continuous ECG monitoring devices have been used in several studies to evaluate the results of catheter or surgical AF ablation.7,244,1084,1085 Although ICM hold promise for the determination of AF burden in the long term, AF detection algorithms are primarily based on R–R interval regularity, and pertinent limitations include reduced specificity due to undersensing of beats, oversensing of myopotentials, and irregular atrial and ventricular premature beats, as well as limited memory resulting in electrograms not being retrievable to verify the correct rhythm diagnosis.1086,1087 Continuous rhythm monitoring devices are more expensive, require implantation, and may not be available in all healthcare settings. A continuous rhythm monitoring strategy, although invasive, overcomes many of the limitations of intermittent monitoring in assessing arrhythmia recurrence and offers the opportunity to determine the most accurate estimate of AF ablation outcomes.1086–1088 In those patients in whom the decision is made to continue long-term anticoagulation regardless of ablation outcome, the cost and effort of continuous rhythm monitoring is likely not warranted.
9.4.2. Intermittent postablation rhythm monitoring
Intermittent rhythm monitoring includes standard 12-lead ECGs, ambulatory patch or electrode ECG monitors, transtelephonic monitoring systems, and patient and automatically activated external recorders.1089,1090 The wide availability of direct-to-consumer mobile health devices for heart rate and rhythm assessment equipped with either ECG-based or photoplythesmography-based technology has increased the availability of rhythm monitoring options in the postablation setting.1091,1092 In a study conducted after AF ablation, a smartphone-based single-lead system was compared with transtelephonic monitor ECGs with 100% sensitivity and 97% specificity in detecting AF or AFl.1090 A pilot randomized study demonstrated that the use of a self-monitoring strategy with an ECG-based hand-held device for rhythm assessment in patients after AF ablation resulted in a similar rate of AF detection and less requirement for additional ECG monitoring when compared with the standard-of-care follow-up practice.1093 Furthermore, long-term intermittent monitoring with an ECG-based hand-held device was shown to be significantly more effective in detecting AF recurrences after AF ablation when compared with short, continuous (Holter) heart rhythm monitoring.1094 The potential of integrating similar monitoring paradigms in the postablation care of AF patients is being evaluated in a multicenter international project.1095
Intermittent monitoring is limited by reduced sensitivity in detecting sporadic arrhythmias, resulting in underdetection of recurrences, which inflates estimates of arrhythmia-free survival. Such misclassification errors likely affect the accuracy and precision of comparative risk estimates. In a secondary analysis of the CIRCA-DOSE trial enrolling paroxysmal AF patients undergoing catheter ablation, the sensitivity for detecting postablation arrhythmia recurrences was shown to increase with the intensity of intermittent rhythm monitoring.7 Commonly employed intermittent monitoring protocols (three short-duration 24 and 48 h ambulatory Holter ECG monitors) failed to detect a considerable proportion of recurrences (sensitivity 15.8 and 24.5%, respectively) and demonstrated poor agreement with the true AF burden.7 Based on computational simulation, an intermittent postablation monitoring with a minimum cumulative duration of 28 days on an annual basis, using serial longer term (7-day and 14-day) ambulatory ECG devices, provides a reasonable arrhythmia detection (sensitivity nearly 60%) and quantification of AF burden (nearly 80% agreement) when compared with the gold standard of continuous monitoring with ICM.7 In a recent systematic review, intermittent monitoring was associated with detection of significantly less atrial arrhythmia recurrences than continuous monitoring in paroxysmal AF, but not in persistent AF or paroxysmal-persistent combined arms.1096
9.4.3. Practical considerations on postablation rhythm monitoring
The suggested pattern and intensity of postablation rhythm monitoring should be tailored based on whether patient management is part of routine clinical care or part of a clinical research trial. Monitoring strategies implemented during routine clinical care may be less strict and standardized than in clinical trials, since documentation of asymptomatic arrhythmia recurrences in everyday practice does not affect decision-making in postablation management except in patients where discontinuation of anticoagulation is considered or in the presence of impaired ventricular function (Section 9.3.1.3.). In this context, as part of routine clinical care, rhythm status should be assessed during regular follow-up within 2–3 months after ablation with a minimum standard of a 12-lead ECG. In the absence of symptoms, all patients should be evaluated on an annual basis thereafter with a 12-lead ECG in every follow-up visit (Section 9.6.). In case of arrhythmia symptoms, some type of intermittent rhythm monitoring is suggested. Intensity and type of monitoring should be individualized based on symptom severity, frequency, availability of monitoring tools, associated cost, and patient preferences.
In the clinical trial setting, it is evident that continuous invasive monitoring represents the gold standard of postablation monitoring and intermittent monitoring of prolonged duration with longer term ambulatory ECG devices stands as best alternative.7 However, their standardized employment in clinical trials would increase substantially the cost of trial conduction and would prevent consistency in trial reporting and comparisons with historical controls. In addition, the availability of longer term ambulatory ECG devices is limited in several practices thus impairing widespread implementation of prolonged duration monitoring regimens. Furthermore, prolonged duration intermittent rhythm monitoring can be burdensome for patients and may result in reduced compliance.
Based on the above considerations, the writing group suggests that, in the clinical trial setting and in the absence of invasive monitoring, a minimum of 24-hour continuous Holter type monitor should be considered every 3 months for the first year following catheter ablation, preferably in combination with symptom-based monitoring. Where available, longer duration recordings with 7-day or 14-day continuous monitoring are preferable.
9.5. Early recurrences after ablation—postablation blanking period
9.5.1. Incidence and pathophysiology of early recurrence after atrial fibrillation ablation
Recurrences of atrial tachyarrhythmia (AF or AFl or AT) may occur in the initial weeks to months after catheter ablation, leading to unplanned hospitalizations or emergency department visits.1097 Some of these early recurrences may resolve with time. Therefore, employment of an initial blanking or blinding period is recommended when reporting efficacy outcomes.1098 Recurrence of any type of atrial tachyarrhythmia during that period is not counted as treatment failure, and invasive treatment like repeat ablation is usually not considered. However, the underlying pathophysiological mechanisms responsible for early re-currence of AF, without late AF occurrence, are not well understood.1099
Short-term processes lasting hours to days and long-term processes lasting weeks to months may be operative during the initial period after AF ablation. These processes may be proarrhythmic or antiarrhythmic. Short-term processes include ischemia, myocardial necrosis, oxidative stress, and myocardial edema.1100–1102 Long-term processes include local and systemic inflammation,1101–1105 nerve sprouting after neural damage,1106 proliferative tissue repair, and scar maturation.1102,1107–1109 Better understanding of the underlying mechanisms for early recurrence and delayed response to ablation will potentially lead to identification of therapeutic targets for AF ablation. Furthermore, the duration of blanking period can also be better defined.
The incidence of early recurrences after AF ablation is highly dependent on the type and intensity of implemented monitoring protocol (Section 9.4.). As a result, there is remarkable variability in the reported incidence of early recurrences after AF ablation, which ranges from 16 to 67%.1110–1112 In a prespecified analysis of the CIRCA-DOSE study, the rate of early postablation recurrences documented by continuous rhythm monitoring was 61%, with a median interval of 12 days between the index ablation procedure and the first early recurrence.1110 Several studies have shown similar incidence of early recurrences between RF and cryoballoon ablation.1110,1113
Multiple predictors for early recurrences after AF ablation have been identified, and many are also predictive of late recurrences and long-term treatment failure.1114–1123 Baseline characteristics predictive of early recurrences after AF ablation include older age, female gender, presence of structural heart disease, longer AF duration prior to ablation, non-paroxysmal AF, higher CHA2DS2-VASc scores, larger LA size, impaired renal function, HF, and presence of LA epicardial adipose tissue.518,1118–1124 Acute procedural predictors for early recurrences after AF ablation include incomplete PVI and multiple AF foci.1125–1127 Other predictors include markers of inflammation and increased levels of C-reactive protein and homocystein.1128,1129
9.5.2. Duration of blanking period
The blanking period following catheter ablation has been introduced to blind monitoring and efficacy assessment during the initial postablation phase during which detected recurrences do not necessarily indicate treatment failure. It should be noted that the absence of early recurrences during the blanking period is strongly predictive of freedom from late recurrence. Calkins et al.1117 reported that patients free from AF recurrence during the 3-month blanking period have 90% likelihood of remaining free from AF recurrence at a 12-month follow-up or longer (89% negative predictive value for paroxysmal and 91% for persistent AF). On the other hand, the predictive value of an early recurrence for a late recurrence is highly variable. Special characteristics of early recurrences after AF ablation have been identified to be more predictive of later recurrence and bear important implications for the optimal blanking period. Increasing number of early recurrences within the blanking period is predictive of late recurrence. In a study involving 300 patients undergoing AF ablation with PVI and elimination of non-PV triggers, patients experiencing multiple early recurrences spanning the initial 6 week postablation period had lower long-term ablation success compared with those with isolated or no early recurrences.1130 A single-center study of 196 consecutive patients undergoing AF ablation using continuous monitoring during follow-up demonstrated that the higher the burden of AF recurrences during the blanking period, the higher the likelihood of long-term AF recurrence.1131
Multiple studies have shown that the timing of early recurrences within the blanking period is crucial in the prediction of long-term ablation failure. In a study involving 331 patients undergoing cryoballoon ablation for AF, all patients who experienced early recurrence in the second half of the 3-month blinding period developed late recurrence of AF afterwards.1132 In a retrospective analysis of 3681 AF patients treated with cryoballoon ablation, early recurrence within 1 month after ablation was shown to significantly predict the occurrence of long-term arrhythmia recurrences.1133 In the ADVICE trial, 401 patients with paroxysmal AF undergoing PVI were followed for 12 months with transtelephonic monitoring.1134 Early recurrence of atrial tachyarrhythmia occurred in 44.6% of patients, and the risk of late recurrence varied significantly according to the timing of the early recurrence. One year freedom from AF recurrence was 77.2% in patients without early recurrence compared with 62.6, 36.4 and 7.8% in patients with early recurrence in the first, second, and third month after ablation, respectively.1134 In a prespecified substudy of the CIRCA-DOSE trial, occurrences of early recurrence in the first, second, and third month of blanking period were associated with 4.9, 26.8, and 63.4 times higher likelihood of late recurrence of atrial tachyarrhythmia.1110 Early recurrences occurring later than 52 days following catheter ablation had a 95% specificity for predicting late recurrence.1110 Several studies have also shown that the risk of late recurrence is inversely related to the timing of early recurrence within the blanking period.1135–1137
With the current evidence, a consensus among the members of this writing group has been reached to recommend an 8-week blanking period after AF ablation. This cutoff was agreed while placing emphasis to minimize misclassification of patients with early recurrences that are not indicative of treatment failure and their pertinent exposure to an unnecessary need for redo ablation procedure. The writing group supports the use of the revised 8-week blanking period in future clinical trial design.
9.5.3. Management of early recurrences after catheter ablation
Pharmacological management has been shown to prevent early arrhythmia recurrences following catheter ablation (Section 9.3.2.). Early postablation recurrences may be a transient finding in some patients. Therefore, aggressive management may be unnecessary due to increased likelihood of spontaneous remission. However, a watchful waiting approach with rate control medication allows persistence of AF facilitating the atrial remodelling process. Delayed implementation of rhythm control interventions contributes to long-term failure of SR maintenance.1138 Based on this concept, prompt management of early recurrences is favoured to pursue SR maintenance.
9.5.3.1. Electrical cardioversion
Several studies have evaluated the impact of electrical cardioversion of early recurrences after AF ablation on long-term arrhythmia-free survival, providing conflicting results. Reported inconsistency may be due to variance in AF type, timing of cardioversion in relation to recurrence onset, intensity of rhythm monitoring during follow-up, and definition of AF recurrence.
In a study of 55 patients who underwent AF catheter ablation and required electrical cardioversion for persistent AF or AFl, 84% of patients experienced recurrence during a mean follow-up of 15 months.1139 No difference in outcome was observed for early (within 90 days of ablation procedure) or late (90–180 days following ablation procedure) cardioversion. In a retrospective study of 180 patients (60% persistent AF) who underwent electrical cardioversion due to early AF recurrence (within 7 days) following RF AF ablation, successful electrical cardioversion occurred in two-thirds of patients but had no impact on long-term rhythm outcome compared with unsuccessful cardioversion.1140
In contrast, other studies reported beneficial effect of timely electrical cardioversion of early recurrence after AF ablation. In a large propensity score–matched cohort of patients with early recurrence following catheter ablation, successful electrical cardioversion was associated with significant reduction in the 1-year AF recurrence rate.1141 In a prospective cohort, early cardioversion of postablation recurrences was associated with a favourable long-term rhythm outcome.1142 In a study of patients undergoing surgical AF ablation, postoperative implementation of an intensive rhythm control strategy, including systematic use of cardioversion, led to a significantly higher proportion of patients maintained in SR during follow-up.1143 Timely cardioversion of early recurrences after catheter ablation also impacts long-term rhythm outcome. In a retrospective analysis of 384 consecutive patients with persistent arrhythmia following catheter ablation, early cardioversion within 30 days of arrhythmia recurrence was an independent predictor of SR maintenance.1144
With the current evidence, it is reasonable to consider cardioversion in patients with early recurrence after catheter ablation, especially within 30 days of arrhythmia onset. If early AF recurs after cardioversion, pharmacological pretreatment and waiting several weeks for inflammation to subside before repeat cardioversion are reasonable.
9.5.3.2. Early reablation
Early reablation is another possible treatment option for early recurrences after catheter ablation. Few studies have evaluated the impact of early reablation on long-term rhythm outcome. In a retrospective study of 302 consecutive AF patients, early reablation within the first month after the index procedure was shown to significantly reduce the incidence of further recurrences with an associated increase in the total number of procedures over the entire follow-up.1145 In the STOP-AF trial, 245 patients with paroxysmal AF were randomized to either medical therapy or cryoballoon ablation. Early AF recurrence within the first 3 months after ablation occurred in 51.5% of patients and was significantly associated with late recurrence. Early reablation was independently associated with lower risk of late recurrence. However, patient allocation to reablation was non-randomized, and nearly half of patients with early recurrence not receiving early reablation did not develop late recurrence.1146
Despite the efficacy of early reablation in reducing the incidence of late recurrences, the rationale of implementing an invasive procedure with inherent risks and associated costs to treat a potentially transient arrhythmia is debated. Therefore, the writing group suggests that reablation of atrial tachyarrhythmia recurrences within the blanking period is not recommended unless they are recurrent, highly symptomatic, and resistant to AADs and cardioversion.
9.6. Patient follow-up following catheter ablation
After undergoing AF ablation, all patients should be seen in follow-up within 2–3 months. Thereafter, all patients should be assessed on an annual basis by physicians (family physicians, internists, cardiologists, or cardiac electrophysiologists) with a minimum standard of a 12-lead ECG in the absence of symptoms. Patients experiencing arrhythmia-related symptoms should undergo additional intermittent rhythm monitoring (Section 9.4.3.). Comprehensive management of AF patients based on the ‘Atrial Fibrillation Better Care’ (ABC) pathway is recommended.5 ‘A’ stands for Anticoagulation/Avoid stroke, ‘B’ stands for Better symptom management, and ‘C’ stands for Cardiovascular and Comorbidity optimization. The implementation of ABC pathway has been shown to reduce health-related costs, improve cardiovascular outcome, and reduce cardiovascular and all-cause mortality when compared with usual care.1147–1152 A similar integrated management of patients following catheter ablation is suggested.
9.7. Atrial tachycardia following atrial fibrillation ablation
9.7.1. Incidence—underlying mechanisms
The incidence of AT following AF ablation varies from less than 5 to 40% and is associated with the strategy and extent of prior ablation.343,811,815,1153–1161 Atrial tachycardias after AF ablation can be due to a focal (automatic or triggered activity) or reentrant mechanism (macroreentrant or microreentrant). They are frequently associated with reconnection of previously isolated PVs1153,1162 and may be focal, from the PV itself, or due to reentry between multiple sites of reconnection. In a recent multicenter study using high-resolution mapping, 7% of AT after AF ablation were PV-gap reentrant ATs with distinct circuits and two critical isthmuses at the entrance and exit gaps of previous PVI lines.1163
Macroreentrant AT is the most common form and is seen with higher incidence after extensive LA ablation.811,1164,1165 Linear ablation combined with PVI may result in reentrant AT because of conduction gaps and non-transmural ablation lesions.172,174 Complex fractionated atrial electrogram–based ablation is also associated with high AT incidence.939,1166
The incidence of AT after cryoballoon ablation is 3–11%, and more than half of these ATs are macroreentrant.1167–1172 Cryoballoon ablation may result in more antral and generous posterior LA debulking during PVI compared with RF,1173,1174 narrowing the posterior wall isthmus regions and potentially increasing the likelihood of macroreentrant tachycardias.
Many patients present with recurrent AT after prior surgical ablation, with macroreentry responsible for the majority of AT mechanisms; CTI flutter represents 24–32%, mitral flutter 18–32%, and roof-dependent flutter 12–16% of AT during follow-up catheter ablation procedures.1175,1176
9.7.2. Management
Management of AT post-AF ablation depends on the pattern and timing of occurrence, type of prior ablation, and intensity of symptoms. Atrial tachycardias often occur during the blanking period after ablation without necessarily predicting procedural failure.343 Atrioventricular nodal agents should be maximized to achieve ventricular rate control. In the case of severe symptoms, earlier intervention may be required. Class III AADs may be preferred if pharmacologic treatment of postablation AT is needed. Electrical cardioversion is generally the first step for symptomatic persistent AT occurring early after AF ablation. If AT recurs soon after an early cardioversion, it may be worth waiting at least 2 weeks for ablation-related inflammation to subside before performing a repeat cardioversion. Up to a third of ATs have been reported to resolve in the first 3 months after AF ablation.343,1164 However, after this time frame, it is reasonable to pursue an ablation strategy if pharmacological control is ineffective or not desired.
It is beyond the scope of this document to provide insights into invasive management of AT following AF catheter ablation. In general, a multi-level strategy with assessment of tachycardia ECG characteristics, CS, and biatrial activation pattern using multipolar diagnostic catheters and ultra-high-density atrial mapping complemented by entrainment maneuvers is suggested to unravel underlying AT mechanism, which is the key to ablation success.1177–1180 Despite pertinent challenges in ablation of AT after AF ablation (difficulty in achieving transmurality, epicardial-dependent tachycardias, and safety concerns in specific areas), recent studies have shown very promising results in acute AT termination and long-term SR maintenance.1181,1182
10. Ablation outcome and efficacy
10.1. Acute procedural success
Pulmonary vein isolation is the cornerstone of AF ablation. Electrical isolation of the PVs is recommended during all AF ablation procedures, and isolation should be minimally confirmed by assessment of entry block within the PVs (Section 8.1.1.).
Due to the high recurrence rate observed in patients with persistent and long-standing persistent AF with PVI alone, efforts were made to identify additive strategies to improve the outcomes of AF ablation. These strategies have included linear RF lesions in the LA and RA, CFAE ablation, GP ablation, ablation of non-PV triggers, isolation of the LAA, ablation of fibrotic areas identified by voltage mapping or MRI, PWI, ablation of rotational activity, and VoM alcohol ablation.566,830,838,843,848,849,986,988,1183–1185 Up to now, none of these strategies have been broadly adopted. Therefore, in persistent AF, ablation beyond PVI is of unclear benefit. However, if linear ablation lesions are deployed during AF ablation procedures, then confirmation of bidirectional block with mapping and pacing maneuvers is a required procedural endpoint (Section 8).
10.2. Atrial fibrillation recurrence endpoints
Since the first AF ablation consensus statement published in 2007, AF ablation success has been defined in a dichotomous manner by the absence of any atrial arrhythmia lasting >30 s off AADs. Overwhelming evidence indicates that this 30 s cutoff does not correlate with symptom severity, is not associated with cardiovascular outcomes, and results in marked underestimation of treatment efficacy.19 There is still uncertainty around the duration of AF leading to an increased risk of stroke.19,1053,1186 A recent secondary analysis of the CIRCA-DOSE trial reported that a 1 h duration threshold of postablation AF recurrence is associated with subsequent patient clinical outcome, since longer AF recurrences resulted in significantly increased healthcare utilization and impaired disease-specific QoL.1187
Until we have more data on duration thresholds of AF recurrence associated with patient clinical outcome, we continue to recommend reporting the 30 s threshold data to allow comparison with earlier literature. Furthermore, it seems rational to move towards reporting AF burden to define ablation outcomes in a more granular fashion rather than necessarily considering a procedure as successful or unsuccessful based on any single cutoff value (Section 10.3.). It also remains important to report all categories of recurrence transparently, such as freedom from symptomatic atrial arrhythmias, AF recurrence separately from other atrial arrhythmias, single and multiple procedure success rates, and success on and off antiarrhythmic therapy. Success rate should be reported at 1 year and after single and multiple procedures.
10.3. Atrial fibrillation burden endpoints
Given the challenges of achieving 100% AF freedom, AF burden has emerged as an important endpoint of AF ablation. Although it is best measured with continuous monitoring (via ICM, pacemakers, or ICDs), it can also be assessed with intermittent external monitoring. However, commonly employed short-duration (24 and 48 h) ambulatory monitors may overestimate the true AF burden. Computational simulation of different monitoring strategies demonstrated that intermittent monitoring duration is inversely related to observed AF burden. A reasonable assessment of true AF burden is achieved by at least 28 days of annual cumulative intermittent non-invasive monitoring using serial longer term (7-day and 14-day) ambulatory ECG devices.7
Recent data have indicated the clinical relevance of reporting AF burden as a procedural endpoint of catheter ablation. By studying AF burden, a striking AF reduction is often observed following ablation despite AF recurrences being recorded.622 Reduced AF burden following AF ablation is also associated with improvement in QoL.1188 In CASTLE-AF, a trial of patients with AF and HFrEF randomized to catheter ablation or drug therapy, a 50% lower AF burden at 6 months was associated with a decrease in the primary endpoint of all-cause mortality and HF hospitalization and a reduction in all-cause mortality. However, AF recurrence as a dichotomous variable (defined as a 30 s or more AF recording) was not predictive of the primary composite outcome or mortality.1189 In a recent subanalysis of the CIRCA-DOSE study, postablation burden >0.1% was associated with significantly increased risk of healthcare utilization (emergency room visit, all-cause hospitalization, cardioversion, and repeat ablation).1187 It seems unlikely however that a single AF burden cutoff point accurately reflects each of the endpoints of symptom severity, health care utilization, and cardiovascular outcomes. It is probable that the relationship between AF burden and these endpoints will vary between patients dependent on other factors (e.g. CHAD2S2-VASc score for thromboembolic risk).
Based on the above considerations, reporting AF burden as the outcome of AF ablation trials is strongly advised especially in trials with prolonged cumulative intermittent or continuous postablation rhythm monitoring.
10.4. Atrial fibrillation progression endpoints
Progression from paroxysmal to persistent and permanent AF occurs in some patients, and achievement of rhythm control gets more difficult as AF progresses to the persistent stage and beyond (Section 2.3.). In the ATTEST study, AF ablation was superior to AADs in delaying progression from paroxysmal to persistent AF.1190 In the CABANA trial, catheter ablation was shown to have a significant impact on the natural history of AF and protect against AF progression to persistent and long-standing persistent types.1191 More recently, the EARLY-AF trial provided longer term follow-up in 303 patients with paroxysmal AF randomized to first-line rhythm control therapy with either cryoballoon ablation or antiarrhythmic medications.27 After 3 years, patients in the cryoablation group were less likely to progress to persistent AF compared with patients treated with AADs (1.9 vs. 7.4%; HR 0.25, 95% CI, 0.09–0.70)27 (Section 4.1.). Although not widely reported in clinical trials, the reduction in AF progression with ablation is an important metric.
10.5. Atrial fibrillation–related symptoms
Although reported in trials, the endpoint of AF-related symptoms is difficult to clearly assess. Even in patients with highly symptomatic AF, as many as half of all episodes may occur without associated symptoms. The ratio of asymptomatic to symptomatic episodes increases up to four-fold postablation, perhaps due to shorter AF durations, slower ventricular rate, or autonomic modulation after the procedure1042 (Section 9.4.). Double-blind treatment allocation is not easily feasible in trials evaluating the effect of AF ablation, and therefore, improvement in symptoms can also partially be related to a placebo effect. Moreover, symptomatic and asymptomatic episodes often coexist in the same patient. Nevertheless, since AF ablation partly serves as a treatment primarily for symptom amelioration, it is relevant to report AF-related symptoms, keeping in perspective that symptoms should not serve as a surrogate to assess AF burden nor other clinical endpoints such as stroke, hospitalization, and mortality.
10.6. Quality of life assessment
Quality of life should remain an important endpoint for AF ablation studies, but not necessarily the primary endpoint. Quality of life is limited by treatment expectancy bias. Quality of life can be measured both using well-established scales like the SF-36 and EQ5D, but also using more specific scales such as the AFEQT, MAFSI, AFSS, or Symptom Severity Score.1041 Atrial fibrillation–specific scales are associated with increased sensitivity and are more effective in discriminating between patients with successful and failed ablation.1041 Studies using both general and specific scales showed improvement in QoL with catheter ablation over AAD.1192–1195 The CAPTAF trial, using QoL as primary endpoint, concluded that QoL improvement was greater with ablation compared with AAD, despite the fact that freedom from AF and number of cardioversions were similar in both groups; however, AF burden was reduced to a greater extent in the ablation group compared with the AAD group.1188 In a CIRCA-DOSE subanalysis, significant impairment in AF-specific QoL following catheter ablation was demonstrated only in patients with postablation AF episode durations >24 h or AF burdens >0.1% when compared with patients without AF recurrence.1187
11. Complications
11.1. General considerations
Catheter ablation for AF is a complex electrophysiology procedure. Due to its invasive nature requiring vascular access, catheter manipulation, and energy delivery in the LA, which is thin-walled and neighbours organs potentially susceptible to thermal damage, AF ablation has a relevant complication rate. This is particularly important because in most cases, the aim of the procedure is mainly symptomatic improvement.
Major complications are usually defined as complications that result in permanent injury or death, require intervention for treatment, and prolong or require hospitalization. The rate of complications after AF ablation lies, as consistently reported by administrative databases, large registries and randomized trials, in the range of 2.5–8%.298,1045,1065,1066,1196–1199 In-hospital deaths are very rare. Contemporary in-hospital death rates (in experienced units) are usually in the range of 0.05–0.1%.1066,1196–1200 Although cumulative experience and technical advances would be expected to lead to a significant decrease in the procedural complication rate, reports of time trends of the complication rates provide conflicting results.1045,1066,1196,1197,1199 A recent pooled analysis of adjudicated safety outcomes exclusively from RCTs demonstrated a significant decrease in the overall rate of complications related to AF catheter ablation in the more recent period (2018–2022) when compared with the preceding 5-year period (3.8 vs. 5.3%, respectively).1198 Importantly, some complications such as pericardial tamponade, stroke, or esophageal perforation may be severe or immediately life-threatening and require urgent or emergent management. For this reason, awareness of the different complications and knowledge of their presentation pattern and management are mandatory.
Several studies have assessed sex-based differences in AF ablation adverse events. In an observational cohort study of 58 960 patients undergoing AF ablation from 2016 to 2020, female gender was independently associated with a higher risk of hospitalization >1 day and major and any adverse events.1201 This gender disparity in AF complication rates has been shown to persist over time and may be attributed to higher burden of associated comorbidities, delayed referral for catheter ablation, higher rate of non-paroxysmal AF type among females as well as anatomical differences between genders.1202,1203
The main complications of catheter ablation for AF are listed in Table 9. In this section, the presentation, investigation, treatment as well as methods to prevent these complications will be discussed.
Table 9.
Main complications of catheter ablation of atrial fibrillation
| Complication type | Complication rate, % |
|---|---|
| Periprocedural death | 0.05–0.1 |
| Atrioesophageal fistula | 0.02–0.1 |
| Periprocedural thromboembolic event | 0.15–0.5 |
| Cardiac tamponade | 0.4–1.3 |
| Severe pulmonary vein stenosis | 0–0.5 |
| Permanent phrenic nerve palsy | 0.08–0.1 |
| Vascular complications | 1–4a |
| Asymptomatic acute cerebral lesions | 5–30 |
aWithout ultrasound-guided vascular puncture.
11.2. Factors associated with procedural complication rate
11.2.1. Procedural volume
Reports consistently demonstrate a correlation between procedural volume and safety outcomes in catheter ablation of AF. Overall complication rates1196,1204,1205 and early mortality1200,1205 after catheter ablation of AF are higher in low-volume than in high-volume centers. Although the annual center caseload cutoff for the definition of low-volume and high-volume centers may vary between studies, the effect remains consistent. The magnitude of this effect is substantial. Particularly for early mortality, high-volume centers are reported to have rates as low as one-third of those reported by low-volume centers.1196,1200,1204 Thus, operator experience appears to be the most critical factor to decrease complications. Indeed, no other technological or procedural aspect has been reported to be associated with such a decisive reduction of complications. These data strongly emphasize the need for structured education and training in the field of AF ablation (see Section 13).
11.2.2. Type of energy source
Radiofrequency and cryoenergy have been used in the last two decades in the majority of AF ablations. Apart from these, PFA is a novel emerging and promising energy source that is expected to gain a significant role in the coming years. Other sources have been applied during the last two decades for AF ablation but have not found a way into broad clinical application.
Despite obvious differences between the main energy sources, the complication rates between RF and cryoablation do not seem to differ significantly, although the type of complications differ.293,294,1206,1207 Persistent PN palsy following PVI is observed almost exclusively after cryoablation, whereas esophageal perforation is, in the vast majority, a consequence of RF ablation.1060 The respective data for PFA are still limited, but the existing evidence indicates an overall complication rate that is similar to the other two energy sources.643,644,651,662 Due to the specific effect of electroporation on cardiomyocytes, adverse extracardiac effects such as esophageal damage are expected to be significantly limited, if not absent, after PFA (Section 11.3.1.).
11.2.3. Role of ablation protocols
Radiofrequency ablation had been initially performed with power settings of 30–35 W at the anterior LA wall and reduced power of 20–25 W at the posterior wall to reduce complications such as cardiac perforation and damage to the esophagus. In recent years, ablation protocols with increased power have been introduced. These are based on power settings of 50 W up to 90 W with respective limitation of the maximal duration of energy application at each ablation site. Initial concerns of a potentially increased complication rate due to the higher energy power were not confirmed. Indeed, existing data confirm the safety of this approach, albeit without indication of any considerable reduction in complication rates.601,1208–1210 In particular, there is no indication for an increased rate of esophageal damage, although related impact, either positive or negative, would be difficult to detect given the rarity of this complication.597 A recent RCT comparing higher power (40 W) short-duration vs. lower power (25 W) longer duration ablation on posterior wall with specific AI targets demonstrated an equivalent risk of esophageal thermal injury (4.5%) as documented by postablation endoscopy.1211
Recent trials suggest that the type of implemented RF ablation protocol may have an impact on the rate of postablation asymptomatic cerebral emboli. In a prospective randomized trial, HPSD (70 W for 9–10 s) RF ablation for PVI was associated with significantly higher rate of MRI-detected subclinical strokes when compared with conventional AI-guided (25–40 W) ablation.602 Another smaller RCT comparing high vs. standard power RF ablation for PVI also demonstrated a trend towards more asymptomatic cerebral emboli with HPSD ablation.1212
11.2.4. Time course of complications and implications for discharge practice
With increasing experience and optimization of workflows, same-day discharge of patients has been implemented for many different interventional cardiac procedures. Although traditionally patients stayed in the hospital for at least one night after AF catheter ablation, several centers moved to same-day discharge, since the majority of relevant complications occurs in the first few hours after the procedure.1213 Indeed, several reports from different hospital settings demonstrate the safety of same-day discharge.1024,1026,1214 Interestingly, these reports pertain to both cryoablation and RF ablation.1026 Thus, with respect to complications, same-day discharge after an uneventful AF ablation appears safe provided that specific criteria are met (Section 9.2.).
11.3. Presentation, treatment, and prevention of specific complications
11.3.1. Esophageal perforation
Esophageal injury is a rare but lethal complication of AF catheter ablation.1063 It occurs with a time delay after the procedure with a reported incidence, which varies from 0.016 to 0.1%.1060–1067 The respective incidence in large surveys enrolling more than 100 000 AF ablations ranges from 0.016 to 0.026%.1060–1062 In the largest, multinational POTTER-AF registry enrolling a total of 553 729 catheter ablation procedures in 214 centers, the incidence of AEF was 0.025%. Also noteworthy is that the incidence of AEF varied markedly between centers (maximum of 0.4%, minimum 0.0066%; P < 0.01), implicating some aspect of modifiable ablation technique in the occurrence. The median time from catheter ablation to symptom onset and to AEF diagnosis was 18 (range: 0–60) and 21 (range: 2–63) days, respectively.1060
Three main types of esophageal injury are observed: AEF, atrial-pericardial fistula, and esophageal hematoma. These types of complications are caused by thermal damage to the esophagus that is in close vicinity to the posterior LA wall.1215 It is observed almost exclusively after RF ablation, but rare cases of esophageal perforation after cryoablation have been described.1059 In the POTTER-AF registry, the incidence of AEF was significantly higher in RF as compared to cryoballoon ablation (0.038 vs. 0.0015%, P < 0.0001). Pulsed field ablation is described to have a specific effect on cardiac myocytes and is expected to be associated with a substantially lower risk of esophageal injury. Initial clinical data with MRI imaging seem to corroborate this assumption,1216 but definite conclusions cannot be drawn yet due to the very low incidence of this complication and the limited number of procedures performed so far with PFA.
Notably, esophageal lesions detected during routine endoscopy as potential precursors of perforation are common after ablation and lie in the 10% range,690 but only a small minority will advance to esophageal perforation. The most common symptoms of esophageal perforation are fever, chest pain or odynophagia, and neurological events (septic emboli), but patients can present with esophageal bleeding, hematemesis, systemic emboli, septic shock, or death1060 (Figure 10).
Figure 10.
Prevention, clinical presentation investigation, and management of atrio-oesophageal fistula. CF, contact force; CT, computed tomography; ICE, intracardiac echocardiography; LGE, late gadoliniun enhancement; MRI, magnetic resonance imaging; PPI, proton pump inhibitor; RF, radiofrequency; TIA, transient ischemic attack.
Chest CT with intravenous contrast is the preferred modality to document the diagnosis of AEF.1060 Typical findings include air in the mediastinum or contrast extravasation to the pericardium, mediastinum, or esophagus. However, a normal chest CT scan does not rule out the presence of an AEF, and therefore in case of high clinical suspicion, ongoing vigilance and repeat imaging are recommended to ensure prompt diagnosis and timely intervention. An LGE-MRI is also useful for documentation of AEF diagnosis.1217 If AEF is suspected, a barium swallow is contraindicated as entry of barium into the circulation could be fatal. Furthermore, endoscopy with air insufflation should be avoided in patients with symptoms suggestive of AEF, due to the risk of massive, life-threatening air embolism. This is particularly important in a patient with acute gastrointestinal bleeding during the postablation period, when endoscopy is often the first diagnostic test performed on an emergency basis. However, esophageal endoscopy with CO2 insufflation may be performed with relative safety, usually in patients with high-risk features but negative initial chest CT scan, since CO2 is rapidly absorbed into the blood with minimal risk of gaseous embolism. Early recognition of an AEF is critical, and thus, it is important to inform patients of warning related symptoms and to advise them to contact their AF ablation center directly in case of occurrence (Figure 10).
Several approaches have been proposed for reducing the risk of this complication, including visualization of the course of the esophagus by integration of the CCT or CMR images in the 3D mapping systems or by ICE, avoiding ablation or reducing CF and ablation power in the vicinity of the esophagus or at the LAPW or by employing esophageal temperature monitoring, esophageal cooling, or deviation.1215,1218,1219 However, the impact of these preventive measures has not been clearly documented (Section 7.8.).690,782–785 A widely used strategy is the routine use of PPIs for a limited period following the procedure. Nevertheless, there is no substantial evidence for the benefit of this practice (Section 9.3.3.). Given the rarity of the complication, conclusive evidence will be difficult to obtain.
Treatment of an AEF is a medical emergency that requires urgent surgical repair.1060,1220,1221 Case series have reported an 83–100% mortality without surgical repair compared with a 34% mortality with surgical repair.1220,1221 Several case reports have been published describing favourable outcomes with esophageal stent placement for treatment of an esophageal perforation or an esophageal pericardial fistula.1221–1224 In the POTTER-AF registry, overall mortality in patients with AEF was 65.8% and was significantly lower following surgical (51.9%) or endoscopic treatment (56.5%) compared with conservative management (89.5%).1060
In summary, esophageal perforation is a rare but unpredictable and immediately life-threatening complication. Prompt diagnosis and surgical treatment are typically needed. Awareness of patients and physicians is of paramount importance.
11.3.2. Periprocedural thromboembolic events
Thromboembolic events are one of the most significant complications of AF ablation (Figure 11). These manifest in almost all cases as strokes or TIA. In contemporary large series, the incidence of stroke or TIA after catheter ablation lies in the range of 0.15–0.5%.1066,1196,1198,1199 Thromboembolic events typically occur within 24 h of the ablation procedure, with the high-risk period extending for the first 2 weeks following ablation.1225,1226 Potential reasons of thromboembolic complications include the development of thrombi on or within sheaths and ablation catheters introduced into the LA, char formation at the tip of the ablation catheter, mobilization of a preexisting LA thrombus, and electrical cardioversion during the procedure. Therefore, a strict anticoagulation protocol during the procedure with heparin administration (even before transseptal puncture), regular ACT measurements, and maintenance of an ACT of at least 300 s, as well as meticulous attention to sheath management are recommended (Section 7.4.). Routine imaging screening for the presence of atrial thrombus reduces the rate of thromboembolic complications (Section 5.2.3.).
Figure 11.
Prevention, clinical presentation, investigation, and management of transient ischemic attack/stroke in the postablation setting. ACT, activated clotting time; CT, computed tomography; ICE, intracardiac echocardiography; MRI, magnetic resonance imaging; OAC, oral anticoagulant; TIA, transient ischemic attack; TSP, transseptal puncture.
Diagnosis of thromboembolic events is usually straightforward. The manifestations depend on the location of the occlusion within the arterial tree. Treatment also varies according to the location of the embolus and, importantly for cerebral embolic events, the time interval between symptom onset and diagnosis. Peripheral arterial embolization might be amenable to surgical thrombectomy, whereas cerebral embolization has traditionally been managed conservatively. There is however growing interest in aggressive early management of such events, using either thrombolytic drugs or percutaneous interventional techniques. The involvement of neurologists and interventional radiologists with experience in the interventional treatment of the cerebral arterial tree is of major importance.
11.3.3. Asymptomatic cerebral lesions
As recognized in recent years, catheter ablation for AF results in asymptomatic acute cerebral lesions that can be detected by high-resolution diffusion-weighted brain MRI. Hyperintensity in T2-weighted fluid attenuated inverse recovery sequence (FLAIR positivity) is useful in differentiating acute from chronic cerebral ischemic lesions.1227 The prevalence can be as high as 30% without difference between patients on VKA and patients on DOACs.387,388,1228 These lesions are considered silent ischemic cerebral lesions since no grossly detectable symptoms are present. Recent data support that HPSD ablation protocols may increase the risk of asymptomatic cerebral emboli (Section 11.2.3.).1212
Subtle cognitive dysfunction has been reported early (3 months) after AF ablation when compared with patients undergoing SVT ablation or patients being treated medically.1229 In another study with longer follow-up, early postablation cognitive dysfunction was transient with complete recovery at 12 months of follow-up. Indeed, a higher percentage of ablation treated patients demonstrated cognitive improvement at 12 months compared with medically treated patients.1230 There are multiple potential mechanisms by which early post AF ablation cognitive dysfunction may occur, but several studies have found no relationship between asymptomatic cerebral lesions on MRI and cognitive decline.1230–1232
11.3.4. Cardiac tamponade
Cardiac tamponade remains the most frequent, potentially life-threatening complication of AF catheter ablation. In recent large surveys, the reported incidence varies from 0.4 to 1.3%.1066,1196–1199 Women seem to have a higher risk for tamponade than men.1203,1233 The substantially higher incidence of cardiac tamponade during AF ablation compared with other cardiac electrophysiology procedures can be attributed to a number of procedural differences, including the need for transseptal puncture, extensive intracardiac catheter manipulation and ablation, and the need for systemic anticoagulation during the procedure. The most common causes of cardiac perforation leading to cardiac tamponade during AF ablation are (i) misdirected transseptal puncture with the puncture performed too posteriorly exiting the RA into the pericardium before entering the LA or with the puncture advanced too much and exiting the LA via the roof, LAA, or the lateral LA wall (Section 3.3.); (ii) direct LA mechanical trauma during catheter manipulation and ablation; and (iii) overheating during RF energy delivery, with or without the development of a steam pop. Excessive power, temperature, and force applied at the tip of the catheter might also contribute.
The need for periprocedural and intraprocedural anticoagulation with heparin infusion to achieve an ACT >300 s may increase the volume of bleeding if perforation occurs. Concerns of increased bleeding risk related to uninterrupted anticoagulation have not been confirmed. Previous studies showed that uninterrupted VKA anticoagulation did not result in higher incidence of tamponade compared with interrupted VKA anticoagulation therapy with bridging heparin.381,382,819,997,1234,1235 Several RCTs comparing uninterrupted DOAC therapy with uninterrupted VKA anticoagulation demonstrated the safety of a periprocedural regimen with uninterrupted DOACs386,388,1236; this anticoagulation regimen has become current standard in most high-volume centers (Section 5.2.).
The impact of technical aspects of the ablation procedure to the risk of tamponade is not clear. A randomized study reported substantially lower tamponade rates in procedures performed with cryoballoon ablation compared with RF energy,294 but observational data do not confirm this finding.1206 Although it was anticipated that the introduction of CF-sensing catheters would reduce the rate of tamponade, this was not confirmed in clinical trials.1237 The use of ICE is important for early diagnosis but also prevention of cardiac tamponade. In a large nationwide cohort study including more than 100 000 patients who underwent AF ablation, the absence of intraprocedural ICE use was associated with 4.85-fold increased risk for cardiac perforation1238 (Section 7.6.).
Cardiac tamponade presents either as an abrupt or as a gradual BP decrease (Figure 12). In the latter case, administration of fluid might return BP to normal before further subsequent decline. It is vital that operators and staff be vigilant to the development of cardiac tamponade, as a delay in diagnosis can be fatal. Due to the immediately life-threatening character of this complication, if not managed appropriately, the development of hypotension during an AF ablation procedure should be assumed to indicate tamponade until proven otherwise. An early sign of cardiac tamponade is a reduced or absent excursion of the cardiac silhouette on fluoroscopy with a simultaneous BP fall. The diagnosis is confirmed by immediate echocardiography. Importantly, the presentation of cardiac tamponade might be delayed and can occur any time from an hour after the procedure to weeks later.1239 The incidence of delayed tamponade was 0.2% in a worldwide survey report.1239 Most, but not all, patients presented with warning symptoms, and some presented with hypotension and shock.
Figure 12.
Prevention, clinical presentation, investigation, and management of periprocedural cardiac tamponade. ICE, intracardiac echocardiography; RF, radiofrequency; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Early recognition and rapid appropriate treatment of cardiac tamponade is mandatory to prevent irreversible deterioration in perfusion of the brain and other organs. In a dedicated worldwide survey, cardiac tamponade was reported to be the most frequent cause of periprocedural death, with 25% of all fatalities occurring in association with this complication.1240 Most cardiac tamponades can be managed successfully by immediate percutaneous drainage. Percutaneous drainage is best achieved by subxiphoid Seldinger puncture of the pericardial sac and placement of an intra-pericardial catheter, such as a pigtail catheter. The puncture can be performed either with fluoroscopic guidance based on anatomic landmarks or with echocardiographic guidance.1241 Usually, BP promptly increases after initial aspiration. Once the pericardial space has been drained, the patient needs to be monitored for ongoing bleeding with the drainage catheter left in place. Continuation of bleeding after aspiration of a substantial amount of blood indicates an extensive perforation that may need surgical repair. Although these are the minority of cases,1242,1243 it is for this reason that AF ablation procedures should only be performed in hospitals equipped or prepared to manage these types of emergencies with access to emergency surgical support. Several case series have reported the feasibility and safety of immediate direct autotransfusion of the blood aspirated from the pericardial space through a femoral vein, without the use of a cell saver system, to reduce the need for allotransfusion following emergency pericardiocentesis in patients undergoing cardiac electrophysiology procedures.1244,1245 Reversal of anticoagulation with protamine may be helpful to stop bleeding, but it may also lead to thrombus formation in the pigtail catheter if bleeding has not stopped. Therefore, protamine should be administered once the rate of aspiration decreases significantly. The drainage catheter is usually left in place for at least 12 h following placement. However, observational studies have shown that early removal of the pericardial drain within the electrophysiology laboratory, after exclusion of blood reaccumulation, is safe and effective in reducing in-hospital stay and the need for analgesia when compared with delayed drain removal.1244,1246 In patients anticoagulated with warfarin, fresh frozen plasma may be administered. Specific reversal agents for DOACs are available and provide the opportunity to immediately reverse the anticoagulant effect but do not seem to play any substantial role in clinical practice (Section 7.4.).
11.3.5. Pulmonary vein stenosis
Pulmonary vein stenosis is a well-recognized complication of AF ablation that results from thermal injury to the PVs. With the transition from ostial to antral ablation and the increased awareness that energy delivery within the PVs should be avoided, the rate of this complication has reduced significantly so that it is currently exceedingly rare. In large contemporary series of AF ablations, the reported incidence of severe PV stenosis is 0–0.5%.1066,1198,1247 Nevertheless, cases of asymptomatic PV stenosis or moderate PV narrowing may not be taken into account. Pulmonary vein stenosis has been described for both point-by-point RF ablation as well as cryoballoon ablation.789,1248–1251 There are limited data regarding the impact of RF power on the rate of PV stenosis.597,610,1252 The highest risk for PV stenosis is associated with RF ablation close to the PV orifices and/or within the PVs, with significantly higher incidence compared with antral ablation.789 Ablation within the PVs should be avoided but can occur due to shifts in the 3D electroanatomic map, respiratory motion, poor catheter stability, and/or operator inexperience.
Symptoms usually occur weeks to months after the ablation procedure and include dyspnea, hemoptysis, cough, (recurrent) pulmonary infections or pneumonia, and chest pain.1250,1253–1255 These may lead to misdiagnoses such as pneumonia, pulmonary embolism, or even lung cancer; therefore, patients should be informed about the importance of returning to the ablation center if such signs or symptoms develop. According to the percentage reduction of the luminal diameter, the severity of PV stenosis is generally defined as mild (<50%), moderate (50–70%), or severe (>70%). Notably, patients with severe stenosis of a single PV may remain asymptomatic.1255
Diagnosis is made by CT angiographic imaging, MRI, perfusion scans, TEE, or invasive PV angiography. The preferred imaging modality is MRI or CT angiography because they allow precise visualization of the location and severity of PV narrowing. Additional advantage of MRI is the option of simultaneous assessment of pulmonary perfusion data.
Treatment of PV stenosis is difficult. Interventional treatment is indicated in the presence of symptoms. Asymptomatic or mildly symptomatic PV stenoses should be managed conservatively with watchful waiting, given that symptomatic amelioration has been observed after PV stenosis or occlusion without treatment due to the formation of collateral vessels.1256 For symptomatic patients, PV angioplasty should be considered. The dilation procedure is often complex, especially if the target PV is completely occluded as evidenced by lack of visualization using either direct angiography via the LA or anterogradely via pulmonary artery angiography. Electroanatomic 3D mapping with registration of the anatomy of the LA and the PVs, as well as fusion with the reconstructed LA from the imaging scan before the index procedure, enables a precise localization of the occluded PV.1257 Baseline CT angiography or MRI is more helpful in defining the PV anatomy.
Many PV stenoses are rigid and difficult to dilate. Even after acutely successful angioplasty, PV restenosis occurs in up to 50% of cases.1250,1254,1255,1258 Percutaneous treatment of PV stenosis with stenting is associated with reduced risk of restenosis when compared with balloon angioplasty, particularly with the use of larger diameter and drug-eluting stents.1250,1254,1255,1258 Nevertheless, even after stenting, restenosis rates are high.1254,1255,1259 There are only limited data on the role of surgical treatment of PV stenosis. Connecting the patch to the proximal end of the stenosis is challenging because this end is buried in the lung parenchyma. Given this difficulty and the excessive risk, there is no evidence for recommending surgical treatment in patients with recurrent PV stenosis after AF ablation. Even for patients with recurrent severe and persistent problems due to restenosis despite interventional treatment, recurrent infection and hemoptysis are uncommon and manageable, and the need for lobectomy or pneumonectomy is very rare.1254 Therefore, repeat percutaneous intervention is the treatment of choice for cases of PV restenosis after angioplasty.
11.3.6. Phrenic nerve palsy
Phrenic nerve palsy is a significant complication of AF ablation and results from direct PN injury. The right PN is most commonly affected because it descends in close proximity to sites of ablation in the SVC and both right-sided PVs. It courses slightly further from the right inferior PV, so that injury during treatment of this vein is less common than that occurring with RSPV ablation. Injury of the left PN may also occur during ablation of the LAA due to its course anterior to the base of the LAA (Section 3.9.).
Phrenic nerve palsy is observed with all technologies of thermal AF ablation, but the vast majority of cases occurs after cryoablation.244,294,1206,1260 With cryoballoon ablation, most PN injuries are transient and resolve before the end of the procedure.1261 Based on recent PFA registries, the occurrence of PN palsy following ablation with the pentaspline, multielectrode PFA catheter is exceedingly rare.643,644
In patients with persistent PN palsy, recovery of nerve function may occur within weeks and in the vast majority by 12 months, although 18–24 months might be required in some patients.1248,1262 In a large multinational registry enrolling 17 356 patients undergoing cryoballoon-based PVI, PN injury recovered in 97.0% of patients at 12 months, with only 0.1% of the overall population showing permanent PNI.1261 In recent large surveys, the reported incidence of permanent PN palsy ranges from 0.08 to 0.1%.1066,1198,1261
Several mechanisms have been proposed to explain the increased incidence of PN injury after balloon-based AF ablation. First, wedging or exerting force to direct the balloon into the RSPV for complete PV occlusion can distort the anatomy and decrease the distance between the RSPV endocardium and the right PN.227 Second, a small balloon size relative to PV diameter can increase the likelihood of distal ablation in the vein. Studies have shown a higher risk of PN injury associated with the smaller 23 mm balloon compared with the larger 28 mm balloon, the latter resulting in more proximal energy application.239,1248 The smaller balloon is potentially advanced further within the PV, causing distortion of the anatomy, creating a higher susceptibility to PN thermal injury. Third, the use of additional freeze cycles can increase the risk of dose-dependent nerve palsy.1169 Phrenic nerve palsy can also occur during antral ablation using RF energy. This likely results from thermal injury to the PN as it courses anterior to the right PVs. Another common scenario of PN palsy is during electrical isolation of the SVC using point-by-point RF ablation (Section 3.9.).
Phrenic nerve palsy can be asymptomatic but typically causes dyspnea, tachypnea, cough, hiccups, and thoracic pain (Figure 13). The diagnosis is suggested when newly elevated hemidiaphragm with or without atelectasis of the ipsilateral lung base is observed on postprocedural chest x-ray. When suspected, diaphragm excursion should be evaluated using fluoroscopy (sniff test) or ultrasound to confirm the diagnosis.
Figure 13.
Prevention, clinical presentation, investigation, and management of phrenic nerve palsy. CMAP, compound motor action potential; PV, pulmonary vein; w/wo, with or without.
A number of strategies have been employed to prevent PN palsy. These include limiting ablation to antral regions with various balloon maneuvers; preablation high-output pacing to establish whether the PN can be captured from the proposed ablation site before energy delivery; PN mapping with anatomic tagging of its course using an EAM system to guide safe deployment of ablation lesions; and monitoring of diaphragmatic excursion with abdominal palpation, fluoroscopy, or intracardiac ultrasound while pacing the PN from the SVC or subclavian vein during ablation.1263 Monitoring the effects of right PN pacing is now considered a standard part of cryoballoon ablation and should also be considered during RF energy delivery at the anterior part of the rights PVs and during SVC isolation (Figure 6). Finally, diaphragmatic electromyography for direct monitoring of diaphragmatic compound motor action potentials during ablation is a technique for early detection of PN palsy that has been reported to reduce incidence of palsy.1264,1265 Compound motor action potentials are recorded using body surface electrodes, esophageal electrodes, or a diagnostic catheter positioned in the hepatic vein. A decrease in the amplitude of the myopotential by 30% is more sensitive than abdominal palpation for predicting the subsequent reduction in diaphragmatic excursion and PN palsy.1266 Energy delivery should be interrupted immediately at the first sign of PN injury.
There is no active treatment known to facilitate PN healing. In highly symptomatic patients, physical therapy of intercostal muscles and scalenes can improve breathing. In patients with permanent nerve palsy, surgical treatment with diaphragmatic plication can improve dyspnea and functional status.
11.3.7. Vascular complications
Vascular complications are the most common major complications of catheter ablation for AF and include groin hematoma, pseudoaneurysm of the femoral artery, arteriovenous fistula, and retroperitoneal bleeding. Current estimates of incidence range from 1 to 4%.1066,1196–1198 The incidence of vascular complications that result from AF ablation is lower than those reported for ventricular tachycardia (VT) ablation, in which femoral arterial access is used in many cases.1267,1268
Most groin hematomas can be managed conservatively or with ultrasound-guided compression. However, complications such as femoral pseudoaneurysm, arteriovenous fistula, and retroperitoneal bleeding might require blood transfusion and/or surgical or percutaneous repair, which leads to increased morbidity and prolonged hospital stay.1269 Rarely, a large dense hematoma can lead to neurological sequelae.
The incidence of these complications may be related to the number and size of the venous sheaths used, insertion of an arterial pressure line, and perhaps to the intensity of anticoagulation management before, during, and after the procedure. Recent randomized studies did not provide any indication for increased risk of vascular complications under uninterrupted DOAC compared with uninterrupted VKA anticoagulation.387
The approach used for femoral venous access may affect the risk of vascular complications. When an inferior approach to femoral vein access is used, small medial branches of the femoral artery, which can run across and superficial to the femoral vein, might be penetrated before entry to the femoral vein, possibly leading to a femoral pseudoaneurysm and arteriovenous fistula. When a superior approach is used, there is an increased risk of retroperitoneal bleeding.
Several studies have consistently demonstrated the safety and the beneficial effect of ultrasound-guided puncture for vascular access. This is an easy-to-learn technique that requires standard equipment and significantly reduces vascular complications in electrophysiology procedures.674–677 For this reason, ultrasound guidance is recommended for vascular access during AF catheter ablation to reduce the risk of vascular complications (Section 7.2.).
11.3.8. Other complications of AF ablation
Apart from the aforementioned serious complications, catheter ablation for AF may lead to several other complications, some of which may be significant.
11.3.8.1. Air embolism
Air embolism may occur acutely during an AF ablation procedure. The most common cause is introduction of air via the transseptal sheath, either through the infusion line or due to suction when catheters are removed. Immediate diagnosis and treatment are based on clinical suspicion and depend on the site of embolization within the vascular tree. A common presentation of air embolism during AF ablation is acute inferior ischemia and/or complete AV block as a result of the preferential downstream migration of air emboli into the right coronary artery. Supportive care usually results in complete resolution of symptoms and signs within minutes. However, pacing and cardiopulmonary resuscitation might be needed if the hypotension and AV block persist, but almost always patients recover completely.1270 Air embolism to the cerebral vasculature can be associated with altered mental status, seizure, and focal neurological signs. Treatment should be initiated immediately if cerebral air embolism is suspected. The most important initial step is to maximize cerebral perfusion by the administration of fluids and supplemental oxygen, which increases the rate of nitrogen absorption from air bubbles. For large air emboli, it might be beneficial to briefly suspend the patient in a head-down position.1271
To prevent air embolism, it is imperative that all infusion lines are monitored closely for bubbles. When catheters are removed, they should be withdrawn slowly to minimize suction effects, and the fluid column within the sheath should be aspirated simultaneously. Particular care is advised when inserting and removing balloon catheters through large sheaths.1272
11.3.8.2. Acute coronary artery stenosis and occlusion
Injury to the coronary arteries during AF ablation is rare. The circumflex artery is in close proximity to the lateral LA and can potentially be injured during ablation at sites adjacent to its course within the CS, the lateral mitral isthmus, or the base of the LAA. Coronary artery injury can manifest as ventricular fibrillation or with features of acute myocardial infarction with ST segment changes occurring during ablation.1273,1274 Immediate coronary angiography reveals the occlusion site and facilitates revascularization (Section 11.2.2.).
The sinus node artery originates from the proximal circumflex artery in one-third of cases and then courses along the anterior LA and then the septal SVC and could therefore be susceptible to injury during ablation. Ablation at the anterior LA and septal RA has been reported to result in injury of the sinus node artery presenting with sinus arrest during or within 1 h of ablation without evidence of other ECG changes associated with coronary occlusion.1273,1275 Permanent pacemaker insertion may be required to treat this complication.
Emphasis should be placed on recent reports of severe coronary spasm during catheter ablation with the pentaspline PFA catheter.644,656 This adverse event mostly occurs during PFA application adjacent to a coronary artery (proximity-related). More rarely, a generalized coronary spasm has been described even when ablating remotely to a coronary artery. This adverse event can be mitigated by nitroglycerin administration before PFA applications at high-risk areas. However, it remains unclear whether nitroglycerine pretreatment will eliminate any direct coronary artery injury from PFA.1276 In general, these findings raise caution on the use of the pentaspline catheter for PFA delivery in proximity to a coronary artery, as during CTI or mitral isthmus ablation.655,656
11.3.8.3. Mitral valve trauma and curvilinear catheter entrapment
Entrapment of a circular multielectrode mapping catheter by the mitral valve apparatus is an uncommon but established complication of AF ablation.1277–1281 It results from inadvertent positioning of a multielectrode catheter close to the mitral valve or into the left ventricle, often during attempts to position the catheter into the LIPV or when using such catheters to create electroanatomic maps of the LA. This complication should be suspected when attempts to reposition the catheter into another PV are met with resistance. When suspected, it is important to confirm the diagnosis with echocardiography. One option is to administer high-dose adenosine to cause AV block, thereby relieving tension in the mitral apparatus and freeing up the catheter tip.1282 Although successful freeing of the catheter has also been reported with gentle clockwise catheter manipulation and advancing the sheath into the ventricle, there have also been a number of cases reported in which the mitral valve apparatus and/or papillary muscles are torn during attempts to free the catheter.1278,1281,1283,1284 There have also been several cases reported in which the distal tip of the circular catheter broke off during attempts at catheter removal and had to be subsequently removed either with a snare or with an open surgical procedure.1279,1280,1283 In these cases, if gentle attempts to free the catheter fail, elective surgical removal of the catheter should be performed. To prevent this complication, circular and multispline catheters should be manipulated with extreme caution near the mitral valve. Furthermore, extreme vigilance is warranted during catheter manipulation at the vicinity of mechanical mitral valves due to increased risk of entrapment. In case of entrapment of a multispline catheter in a mechanical mitral valve, extensive traction increases the risk of mechanical valve damage or shearing of catheter splines. Different techniques to release entrapped multipolar catheters using the ablation catheter have been proposed.1285–1287
11.3.8.4. Stiff left atrial syndrome
First described after mitral valve surgery, stiff LA syndrome was later recognized as a rare complication of LA catheter ablation, typically after multiple ablations.1288–1291 Extensive LA ablation has been associated with worsening of echocardiographicaly measured LA stiffness.1292 Symptoms include unexplained dyspnea and signs of right HF. Diagnostic findings include new or worsening pulmonary hypertension, LA diastolic abnormalities, LA hypertension, and large V waves on LA pressure or pulmonary capillary wedge pressure tracings.1291 The complication appears to be associated with extensive LA ablation particularly in patients with small LA size, high LA pressures, preexisting severe LA scarring, and comorbidities such as diabetes and OSA.1289
Most patients show symptomatic improvement after diuretic therapy, which appears to be more effective for this syndrome than for other forms of pulmonary hypertension.1293 In contrast, another study reported a case of stiff LA syndrome after AF catheter ablation that failed with furosemide and spironolactone, but which responded to sildenafil.1294
11.3.8.5. Gastric hypomotility
Gastric hypomotility may occur in the setting of AF ablation due to inadvertent injury of the anterior vagal esophageal plexus usually when RF energy is applied to the LAPW.1295–1297 Endoscopically detected gastric hypomotility has also been reported in 10–18% of patients undergoing cryoballoon AF ablation.1298–1300 Common symptoms include nausea, vomiting, bloating, and abdominal pain developing within a few hours to a few weeks after the ablation procedure.1301–1303 Symptomatic but also asymptomatic gastric problems may be frequent after ablation.1302,1303 The time to recovery is variable, with some patients recovering within 2 weeks, but others requiring a much more protracted time to recovery, occasionally >3 months.1295
Diagnostic evaluation can include endoscopy or a barium swallow to look for residual food after an overnight fast, abdominal CT scan that shows marked gastric dilation, or real-time MRI to assess gastric motility and pyloric spasm.1304
Management of this complication depends on the severity of symptoms and whether gastric hypomotility or pylorospasm predominates. Dietary modification with small, low-fat, and low-fiber meals may be adequate to alleviate symptoms. Pharmacological treatment can be used to relieve symptoms (antiemetics) and to promote gastric contractility. In the latter category, several agents have been proposed including erythromycin, domperidone, and metoclopramide.1305,1306 Metoclopramide treatment should not extend beyond 12 weeks due to associated risk of movement disorders. Domperidone has a substantially lower risk of central nervous system side effects, but it has been associated with QT prolongation.1306 In patients with predominant pylorospasm, intrapyloric injection of botulinum toxin or different types of surgical pyloric interventions have been proposed as treatment options.1307
12. Surgical and hybrid atrial fibrillation ablation
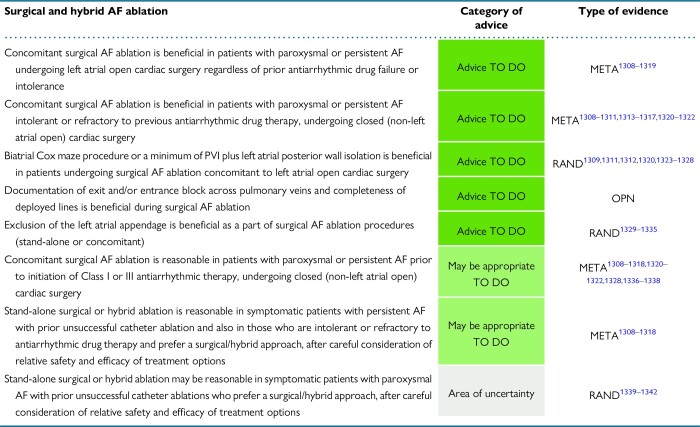
|
12.1. Technology and techniques
Radiofrequency ablation and cryoablation are the two dominant technologies used today due to their safety and efficacy profiles and will be the only ones discussed in this section. While there have not been any RCTs to compare the efficacy of one ablation technology with another, these technologies have had proven clinical efficiency over the last two decades.
To date, a prospective, multicenter, non-RCT, AtriCure Bipolar Radiofrequency Ablation of Permanent Atrial Fibrillation, has resulted in specific FDA approval for surgical treatment of AF.1343 This device was used on patients with non-paroxysmal AF undergoing concomitant coronary artery bypass graft (CABG) and/or valve procedures and Cox maze IV ablation and resulted in a 76% freedom from AF recurrence off AADs at 6 months with a major peri-operative adverse event rate of 9%.
Surgical ablation procedures for AF can be grouped into three different groups: (i) a full, biatrial Cox maze procedure; (ii) PVI or posterior LA isolation alone, or (iii) PVI combined with an extended LA lesion set. All surgical AF ablation approaches are combined with LAA exclusion. At present, it is recommended that the term ‘Cox maze procedure’ is appropriately used only to refer to a biatrial lesion set including specific transmural lesions that extend between non-conductive tissues (valve annulus or vena cava or another lesion; Figure 14).1344 The best late rhythm outcomes have been shown with the full biatrial Cox maze procedure, while a certain subgroup of patients, such as those with paroxysmal AF, have reasonable results with more limited lesion sets.1345
Figure 14.
Lesion sets of the Cox maze IV procedure. (A) Left atrial lesion set including: (i) left atriotomy, (ii) ablation around the left-sided PVs, (iii) ablation around the right-sided PVs, (iv) posterior wall box lesion, (v) line connecting left PV lesion to excluded LAA, (vi) line connecting box lesion to mitral annulus, (vii) cryoablation to the epicardial ostial region of the coronary sinus (not shown). (B) Right atrial lesion set including: (i) right atriotomy extending over crista terminalis, (ii) line from the atriotomy to the superior and inferior vena cava posterior to the crista terminalis (to avoid injury to the sinoatrial node), (iii) line connecting the atriotomy to the tricuspid annulus (2 o’ clock relative to the valve), and (iv) line connecting the right atriotomy to the right atrial appendage. LAA, left atrial appendage; PV, pulmonary vein.
12.1.1. Energy sources
12.1.1.1. Radiofrequency energy
Radiofrequency energy can be delivered by either unipolar or bipolar electrodes, which can be either dry or irrigated. Irrigation helps to deliver RF energy uniformly and to prevent char formation by keeping temperatures cooler at the tissue interface.1346 Unipolar RF ablation works by delivering RF energy from the probe directly to the tissue. The unipolar devices do not provide surgeons with transmurality indicators. In contrast, bipolar RF can be either directional or constrained, and transmurality can be implied by the manufacturer's dose–response algorithms. The directional bipolar devices have two side-by-side electrodes that are applied to the tissue surface, with the energy passing through the tissue between them. The constrained bipolar devices consist of a clamp with two jaws, which are applied on opposite sides of atrial tissue. The energy passes through the tissue between the two jaws. When the conductance falls to a stable minimum, transmurality is inferred.
Unlike bipolar RF devices, unipolar ones have failed to consistently create transmural lesions and have a risk of forming endocardial char or thrombus.1347–1350 Both unipolar and directional bipolar RF energy sources have had difficulty creating transmural lesions when used from the epicardial surface on the beating heart.1351 This difficulty is due to the circulating intracavitary blood flow, which produces convective cooling. To overcome this problem, devices have employed suction to pull the atrial tissue into apposition, thus partially ameliorating the circulating heat sink. Radiofrequency ablation with constrained bipolar devices has allowed for faster and more efficient ablation due to direct contact with the tissue. Since the tissue is ablated between the jaws of a clamp, the energy delivery is focused and isolated from the surrounding circulating intracavitary blood reservoir, allowing for more effective creation of lesions on both the beating and arrested heart.
Factors that affect lesion size and depth are power, impedance, ablation duration, temperature, and CF.1352–1355 The generators of the irrigated and non-irrigated bipolar RF clamps produce power transmitted to the electrodes, and these data are used to predict the transmurality of the lesion. The generators of irrigated clamps do this by measuring the impedance between electrodes, varying the power according to the impedance, and terminating power delivery once the feedback programme detects a steady state plateau.1346 On the other hand, the generators of non-irrigated clamps measure conductance and continue ablation until a stable low conductance is reached. Voltage is varied according to the conductance, resulting in a safe delivery of energy to the tissue.1352 Care should be taken to clean the electrodes after every two to three ablations with the non-irrigated clamps because char decreases conductance, which can result in non-transmural lesions. Importantly, in a human heart ex-vivo model, a double ablation without unclamping improved lesion transmurality. Epicardial fat and muscle thickness can also decrease conductance and limit ablation depth.1352 The ablation duration affects the tissue temperature profile. Cardiac muscle exposed to temperature of 55°C or higher for more than a few seconds will show irreversible coagulation necrosis.1356 Lastly, adequate but not excessive CF is needed to achieve a reliable transmural lesion.788,1357
12.1.1.2. Cryoenergy
Cryoablation has been used since the 1960s to ablate cardiac tissue. It is the second most common ablation technology used for surgical ablation. In contrast to RF energy, cryothermy creates homogenous scars in a non-directional pattern. Cryoablation is safe because cold temperatures do not denature proteins and thus preserves fibrous tissue and the extracellular matrix, which makes it an ideal technology for ablation around valvular tissue.1358,1359 Temperature, probe size, frequency, duration of ablation, and the cryogen cooling agent are all factors that determine the lesion's volume and depth.1360 The cryoablation probes deliver very low temperatures to cause irreversible cell death and actively measure the probe–tissue interface temperature through a thermocouple. The potential disadvantages are the relatively longer time to create a lesion (2–3 min) and the difficulty creating a lesion on the beating heart due to the heat sink effect created from circulating intracavitary blood.1361,1362 Due to this, cryoablation should not be used to create epicardial lesions off cardiopulmonary bypass. To create a reliable uniform and continuous cryolesion, a critical lethal temperature of <−30°C must be reached during ablation.1360
12.1.2. Specific ablation tools
12.1.2.1. Radiofrequency ablation tools
Unipolar devices. Unipolar RF devices come in varying lengths and can measure the electrode interface temperature with or without a suction stabilization device to enhance tissue contact. They can either be irrigated or non-irrigated. Despite the variety of unipolar devices, as mentioned above, they have had limited success in creating transmural lesions consistently.1348,1349,1363 None have been FDA-approved for surgical treatment of AF. The only FDA-approved unipolar device is for hybrid therapy of persistent and long-standing persistent AF (EPi-Sense Guided Coagulation System with Visitrax, AtriCure, Inc.) and is described below (Section 12.3.2.2.).
Bipolar clamp devices. The only ablation device with FDA approval for the treatment of AF during concomitant cardiac procedures is the bipolar, non-irrigated RF clamp (Isolator Synergy clamp, AtriCure Inc., Mason, OH, USA). In a chronic animal study using this device with a single application, all lesions produced were transmural.1364 However, in clinical experience, multiple applications are needed to achieve exit block. In a recent human ex-vivo heart explant model, a single application resulted in only 65% of lesions being transmural throughout their entire length. Inability to achieve transmurality was related to the increased thickness of atrial tissue and the presence of epicardial fat. Application of two successive ablations without unclamping resulted in 100% lesion transmurality.1352 In comparison, irrigated bipolar RF clamps, Cardioblate BP2 and LP (Medtronic Inc., Minneapolis, MN, USA), use a similar algorithm to provide real-time measurements of lesion transmurality based on impedance. This device has also been studied in porcine models and showed a high rate of lesion transmurality. Using the same ex-vivo human heart model, it has also been shown that a double application without unclamping results in significantly increased rate of lesion transmurality compared with single application (92 vs. 74%).1365 Most recently, another bipolar RF ablation device (Isolator Synergy EnCompass Clamp, AtriCure Inc., Mason, OH, USA) has been tested experimentally and has been shown to produce reliable transmurality and isolation of the entire posterior LA wall and all four PVs with a single application in an in-vivo beating heart model.1366 Further clinical trials will be needed to test its clinical performance.
Directional (non-clamp) devices. There are several directional unipolar and bipolar RF devices, with or without suction assistance, that can be applied either on the epicardium or endocardium. The ablation times range from 10 to 40 s per the manufacturer's instructions with the highest risk of ablation gaps at the end of the devices; thus, continuous lesions should be overlapped to increase transmurality. The two non–suction-assisted bipolar RF devices are the Isolator pen (AtriCure, Inc.) and Coolrail device (AtriCure, Inc.). In both acute and chronic animal models, the creation of transmural lesions has been inferior to bipolar RF clamps.1363,1367,1368 Furthermore, the Coolrail device should be used with caution as it has been associated with a few case reports of AEFs following AF ablation.1369 Rinsing the pericardium with saline may be used to prevent AEF during ablation with the Coolrail device.
The two suction-assisted RF devices on the market are the Cobra Fusion 150 (AtriCure, Inc.) and EPi-Sense Coagulation System with VisiTrax (AtriCure, Inc.). The Cobra Fusion device has both unipolar and bipolar RF energy delivery capabilities. During ablation delivery, suction should be maintained at −500 mmHg for 1–2 min depending on the tissue thickness and desired temperature setting per the manufacturer's instructions. In an acute porcine beating heart model, delivery of two separate applications (initial bipolar followed by unipolar energy without disrupting the suction) from an epicardial approach resulted in 68% rate of lesion transmurality.1348 The EPi-Sense device is a 3 cm long, suction-assisted, irrigated unipolar RF device. The lesion transmurality of this device has been variable in multiple animal studies from 15 to 100%.1363,1370 However, its clinical performance has been validated in the treatment of patients with persistent and long-standing persistent AF in the setting of a minimally invasive hybrid approach (Section 12.3.3.2.).219
12.1.2.2. Cryoablation tools
There are two available cryogen sources on the market, nitrous oxide and argon, which have been tested and shown to be efficacious in animal and donor human transplant heart models.1358,1371,1372 Nitrous oxide has a higher heat absorption than argon. The argon device (Cardioblate CryoFlex, Medtronic Inc.) reaches a minimum temperature of −160°C. The two nitrous oxide devices (cryoFORM and cryoICE, AtriCure, Inc.) reach minimal probe temperatures of −50 to −70°C. Both companies have designed long malleable disposable probes to adjust to the complex atrial anatomy. In a chronic ovine model using the cryoICE (AtriCure, Inc.) device, transmurality was achieved in almost all atrial lesions (98%) performed endocardially.1373 Similarly, in a chronic canine model, use of the CryoFlex clamp and probe (Medtronic Inc.) resulted in 93% tissue section transmurality of all LAA and PV lesions and 84% tissue section transmurality in all Cox maze linear lesions.1374 There have been no surgical cryoablation devices that have yet received an FDA indication for the treatment of AF, but there are ongoing clinical trials with both the nitrous oxide and argon devices.
12.1.3. Procedural targets and lesion sets
There are insufficient high-quality data on what should be the most important targets during surgical ablation. This section will review what is known from retrospective case series and the few randomized trials that have been performed.
12.1.3.1. Pulmonary vein isolation
As with catheter ablation, PVI is a foundational part of all surgical AF ablation procedures. Although documentation of exit and/or entrance block across PVs is preferred, it is infrequently performed. Intraoperative mapping has documented complex activation patterns both in the LA and RA in patients with long-standing persistent AF undergoing surgery for AF and mitral valve disease, indicating that a simplified approach with PVI alone may not be adequate during concomitant surgical ablation.1375 Similar to catheter ablation of persistent AF, surgical PVI alone has had disappointing late results. In a single-center cohort of consecutive patients with medically refractory symptomatic AF, a minimally invasive surgical approach employing PVI combined only with ablation of autonomic ganglionated plexi and ligament of Marshall resulted in a single-procedural success rate of 37.8% after a 5-year follow-up using ECG and transtelephonic monitoring.1376 Retrospective observational data suggest that surgical ablation with PVI alone is inferior to the biatrial maze procedure in patients with persistent or long-standing persistent AF.1377 One randomized trial on non-paroxysmal AF patients undergoing mitral valve surgery reported similar rate of freedom from AF with PVI as compared to the biatrial maze procedure.1345 However, the study was underpowered to adequately answer the question of which lesion set was more efficacious.
12.1.3.2. Isolation of the left atrial posterior wall
Isolation of the entire LAPW and all four PVs is the most important part of surgical ablation procedures. In a large retrospective study of patients undergoing the Cox maze IV procedure, failure to isolate the entire posterior LA resulted in only 33% freedom from AF off AADs at 5 years compared with a 66% freedom in patients who underwent posterior LA isolation (P = 0.017).1326 An incomplete lesion set is even more impactful in patients undergoing mitral valve surgery. In such patients, the failure to isolate the entire posterior LA during a Cox maze procedure was the only independent predictor of procedural failure and resulted in 6.7-fold increased risk of AF recurrence.1378 Due to anticipated improvement in rhythm outcome with LA PWI, a minimum of PVI plus LA PWI should be performed in patients undergoing surgical AF ablation.
12.1.3.3. Right and left atrial linear lesions
Linear lesions interrupting the CTI in the RA and the mitral isthmus in the LA aim to prevent macroreentrant tachycardias. Catheter ablation data suggest that macroreentry is the predominant mechanism of ATs in patients with prior history of mitral valve surgery. In many cases macroreentrant circuits are located in the RA, but left-sided circuits may also occur particularly if a concomitant Maze procedure was performed.1379 In a single-center analysis of consecutive persistent AF patients undergoing thoracoscopic ablation, adjunctive CTI ablation significantly increased freedom from atrial tachyarrhythmia recurrence.1380 The superior-inferior vena cava ablation line anchors the RA isthmus line and thus serves an important role in preventing late RA flutter. Documentation of completeness is beneficial in all deployed linear lesions during surgical AF ablation.
12.1.3.4. Ganglionated plexi ablation
There has been interest in GP ablation in stand-alone surgical AF ablation procedures. An epicardial antral PVI and posterior box lesion including the ligament of Marshall results in collateral ablation of most atrial GP. Therefore, it is difficult to evaluate the additional role of GP ablation on top of epicardial PVI plus PWI using bipolar clamps. The only randomized study examining the efficacy and safety of additional GP ablation in patients undergoing thoracoscopic surgery showed no incremental benefit in AF recurrence rate and a significantly higher rate of major procedural complications in patients randomized to GP ablation.1008 Therefore, with the exception of a clinical trial setting, GP ablation should not be performed during surgical AF ablation.
12.1.3.5. Ligament of Marshall
There are no data from the surgical literature to support the ligament of Marshall as a target for ablation. However, this structure is usually divided while isolating the left PVs during surgical ablation procedures.
12.1.3.6. Left atrial appendage exclusion
Exclusion of the LAA is a standard part of all surgical AF ablation procedures and is discussed in detail in Section 12.4.
12.2. Concomitant surgical ablation of atrial fibrillation
Patients undergoing cardiac surgery frequently have concomitant AF which if untreated has been shown to increase the risk of postoperative ischemic stroke and to negatively impact long-term survival.1327,1336,1381 Surgical ablation of AF combined with LAA exclusion or excision restores SR and atrial contraction and reduces the risk of thromboembolism. In this section, the efficacy, safety, and optimal lesion set of concomitant AF ablation during cardiac surgical procedures are discussed. It is noteworthy that in patients eligible for cardiac surgery and concomitant AF ablation, it is often challenging to differentiate whether patient reported symptoms are related to underlying cardiac disease or coexistent AF.
12.2.1. Efficacy of concomitant atrial fibrillation surgery
Concomitant AF surgery has been shown to increase SR maintenance rate in multiple randomized and non-randomized trials.1308–1310,1328,1336 A metaanalysis of 23 RCTs demonstrated that AF ablation concomitant to cardiac surgery results in increased freedom from AF at 12 months.1382 Several trials have demonstrated a reduced incidence of stroke at 5 years postoperatively.1311,1312,1320,1337,1338 Improvement of long-term survival after concomitant surgical AF ablation has not been proved by an RCT. However, an analysis of the US Society of Thoracic Surgeons AF database with propensity matching showed that concomitant surgical AF ablation was associated with a reduction in 30-day mortality.1308 In addition, several retrospective and propensity-matched studies as well as large national registries have demonstrated that the performance of surgical AF ablation concomitant with other cardiac procedures (particularly mitral valve surgery and CABG) was associated with improved long-term survival.1313–1318,1321,1322 In a retrospective propensity score–matched analysis of a nationwide registry, concomitant surgical ablation for AF in patients undergoing isolated CABG was shown to significantly improve long-term survival rates.1322 Improved QoL at a long-term postoperative follow-up period has also been demonstrated in patients who underwent AF surgery with SR restoration.1383,1384
12.2.2. Safety of concomitant atrial fibrillation surgery
Several studies, including RCTs, have demonstrated that concomitant AF surgery is safe and does not increase operative mortality.1313,1314,1327,1337,1385 Although a propensity score–matching study showed an increased incidence of acute kidney injury after AF surgery, the associated long-term risks were offset by the significant survival benefit derived from the concomitant Cox maze procedure.1386
Postoperative atrial tachyarrhythmias and new permanent pacemaker implantation are the typical complications potentially related to AF surgery. Incomplete linear lesions with residual conduction and inappropriate surgical techniques are mainly responsible for postoperative occurrence of predominantly macroreentrant ATs.1387 Intraoperative verification of conduction block, particularly to ensure PVI, may reduce the incidence of AT due to incomplete ablation.1388
Increased incidence of new permanent pacemaker implantation after the Cox maze procedure has been demonstrated in many studies.1308,1382,1389,1390 However, in a recent large European registry of patients undergoing valve surgery, surgical AF ablation was not associated with increased need for permanent pacemaker implantation.1391 Sinus node dysfunction requiring permanent pacemaker implantation can occur in up to 10% of patients after the Cox maze procedure for non-paroxysmal AF and may be a result of unmasking preexisting sick sinus syndrome.1392 In addition, mechanical or thermal injury to the sinus or AV node and interruption of the conduction system arterial supply are the main intraoperative reasons for postoperative bradycardia and in-hospital permanent pacemaker implantation.1392 Multidisciplinary collaboration between cardiothoracic surgeons and electrophysiologists, proper training on ablation techniques, and deployment of complete linear lesions may reduce the incidence of postoperative atrial tachyarrhythmias and permanent pacemaker implantation and enhance patient outcomes.1337,1393
12.2.3. Optimal lesion set in patients undergoing left atrial open procedures
The biatrial Cox maze procedure is the preferred procedure for surgical AF ablation during open LA procedures and achieves high rates of AF conversion to SR and freedom from AF recurrence.1327,1385,1394 However, recognizing that surgical training and experience may vary across centers, the writing group suggests that biatrial Cox maze procedure or a minimum of PVI plus LA PWI is required in patients undergoing surgical ablation concomitant to LA open cardiac surgery. The lesion set of the Cox maze IV procedure is shown in Figure 14.
12.2.4. Optimal lesion set in patients undergoing non-left atrial open procedures
Fewer patients undergoing non-LA open procedures, such as aortic valve replacement or CABG, have undergone concomitant AF ablation compared with those with LA open procedures, because of the necessity of adding an LA incision to perform ablation in the LA. Epicardial PVI alone has been performed more often than a biatrial maze procedure in AF patients undergoing non-LA open procedures, and this might have led to biased analyses of the data.1395 A dilated LA has been shown to be associated with worse AF-free and event-free survivals after PVI for patients with paroxysmal AF undergoing non-LA open cardiac surgery.1396 Several studies have shown that biatrial maze procedure is associated with superior rhythm outcome and a lower risk of adverse events and long-term overall mortality compared with LA lesion sets following surgical AF ablation concomitant to non-mitral valve surgery.1390,1397–1399
However, many surgeons are reluctant to increase procedural complexity and risks by performing AF ablation through an atriotomy.1400 Therefore, selection of PVI combined with PWI or other modified procedure should be individualized in patients undergoing surgical ablation concomitant to closed (non-LA open) cardiac surgery. Further clinical studies are needed to clarify the optimal lesion set in these patients and to further elucidate associated risks and benefits.
12.3. Stand-alone surgical ablation of atrial fibrillation
12.3.1. Stand-alone Cox maze procedure
The largest series of stand-alone Cox maze IV procedures (236 consecutive patients, 59% prior failed catheter ablation, median duration of preoperative AF 6.2 years) demonstrated very high late efficacy in SR maintenance (89 and 77% freedom from recurrent atrial tachyarrhythmias at 5 and 10 years, respectively), irrespective of AF type or surgical approach (median sternotomy vs. minimally invasive approach), without 30-day mortality.1323 Lapenna et al.1324 reported on 59 patients undergoing stand-alone Cox maze procedure with similar excellent early and late outcomes with 84% of patients remaining in SR at 7 years, without 30-day mortality or late strokes. In 133 patients undergoing minimally invasive, stand-alone Cox maze procedure (78% long-standing persistent AF), Ad et al.1325 reported a 73% freedom from atrial tachyarrhythmias off AADs at 5 years with only one late stroke and no associated mortality. These case series demonstrate the low mortality and excellent late outcomes achieved by Cox maze procedure as stand-alone treatment in a challenging group of patients with the majority having long-standing persistent AF of long duration. It is important to point out that these procedures were done with cardiopulmonary bypass, which may explain the safety of this procedure. Unfortunately, there have been no prospective multicenter trials of the stand-alone treatment of AF with the Cox maze procedure.
12.3.2. Minimally invasive surgical—hybrid atrial fibrillation ablation
Minimally invasive techniques have been introduced in AF surgery aiming to reduce surgical invasiveness while maintaining efficacy in rhythm outcome. These approaches combine sternotomy-sparing minimal surgical incisions with different access sites, endoscopic visualization, with or without catheter ablation at the same or at a different stage (hybrid ablation). The reduced invasiveness of these techniques compared with the surgical Cox maze procedure has rendered these approaches more attractive to patients and surgeons. Evidence in different AF patient categories is accumulating to establish efficacy and safety. However, comparison of different study results is limited by non-uniformity in patient populations and lack of standardized surgical technique, ablation technology and deployed lesion sets.
During the last decade, the ‘hybrid’ approach, consisting of a combined surgical-percutaneous catheter ablation strategy, has garnered increasing acceptance in clinical practice, with promising rhythm outcomes.221,1401–1408 A key aspect of this treatment strategy is that it harmonizes epicardial and endocardial ablation components to effectively target key drivers of AF, including the PVs and the LAPW. There are different surgical modalities to achieve the target of PVI and LA PWI. In this section, we discuss the two main techniques currently employed in clinical practice.
Hybrid ablation combines expertise from the surgical and electrophysiology teams to achieve an optimal result. Coordination and collaboration among the multidisciplinary team members are paramount to a successful programme. The timing of the epicardial and endocardial stage of the hybrid ablation procedure varies based on institutional practice. In the single-stage model, epicardial and endocardial procedures can occur back-to-back in the same suite or separate suites or over sequential days. Completion of both phases in the same session prolongs procedural time, which may add additional risk in patients with comorbidities. For dual-staged programs, the epicardial component typically occurs in the cardiac operating room, and the endocardial component is scheduled ∼2–4 months later in the electrophysiology laboratory, aiming to complement the epicardial component with touch-up lesions, ensuring isolation of PVs, LAPW, and completeness of epicardially deployed linear lesions. The impact of different procedural timing on patient outcomes is unknown. The minimally invasive surgical part of the hybrid procedure is most frequently performed using a video-assisted thoracoscopic surgical approach or with the ‘convergent’ approach.
12.3.2.1. Thoracoscopic surgical approach
The thoracoscopic approach is performed under general anesthesia with double-lumen endotracheal tube placement for selective lung ventilation. On the right side, a camera port is placed in the fifth intercostal space midaxillary line, a 5 or 10 mm working port in the sixth or seventh intercostal space anterior axillary line, and a 5 mm working port in the third intercostal space anterior axillary line. The pericardium is opened anterior to the PN. Blunt dissection is used to open the transverse and oblique sinuses. Antral isolation of the right PVs as a pair is performed with repetitive applications using a bipolar RF clamp (Section 12.1.2.). The same port incisions are made on the left side but placed more posteriorly. The pericardium is opened posterior to the PN. In patients with severe chronic obstructive pulmonary disease, thoracoscopic epicardial isolation of the PVs can be performed only on the left, and the right PVs subsequently isolated from the endocardium to avoid bilateral sequential lung deflation. An alternative would also be a convergent procedure using subxiphoid access along with Lariat closure of the LAA. After PVI documentation, a roof line (connecting both superior PVs) and an inferior line (connecting both inferior PVs) are made epicardially using directional ablation devices (Section 12.1.2.) to create box isolation of the LAPW (Figure 15). As an alternative, one epicardial box lesion including the posterior LA wall and the PVs can be created using only the irrigated bipolar biparietal Cardioblate Gemini-S (Medtronic Inc.) RF ablation system by performing two clamp lesions from the right and left sides that overlap in the middle of the posterior wall (Figure 15).
Figure 15.
Posterior view of the left atrium showing epicardial lesion sets during thoracoscopic surgical AF ablation: pulmonary vein isolation with connecting roof and inferior lines (A), en-bloc pulmonary vein and posterior wall isolation using the Cardioblate Gemini-S (Medtronic Inc.) RF ablation system (B), and posterior wall ablation using the convergent approach (C). AF, atrial fibrillation; RF, radiofrequency.
Additional ablation lesions may be deployed, such as circular lesion of the SVC, linear lesion connecting both caval veins and mitral isthmus line. Endocardial touch-up ablation to achieve bidirectional block can be delivered in the same or subsequent stage with catheter ablation. Furthermore, in patients with prior history or intraprocedural induction of CTI-dependent flutter, the CTI is ablated endocardially.
Left atrial appendage exclusion should also be performed in all patients during minimally invasive surgical AF ablation to reduce thromboembolic risk (Section 12.4.). Through an incision at the third to fourth intercostal space or via a completely thoracoscopic approach, the surgeon can easily reach the LAA and exclude it. Left atrial appendage exclusion must be performed at the very base of the structure, as to avoid leaving behind residual stump. Incomplete removal of the LAA is associated with increased risk of local thrombus formation and embolization.
Since the thoracoscopic surgical AF ablation lesions are exclusively epicardial, one can perform the procedure without the need for anticoagulation during and after the procedure. Therefore, stand-alone thoracoscopic surgical AF ablation is the best option for patients who have bleeding diathesis (particularly central nervous system bleeds) precluding the anticoagulation needed during and after endocardial catheter ablation. Additional suitable patient subsets include those without the ability to achieve LA access endocardially (large atrial septal occlusion device, interrupted inferior vena cava) or those with LAA thrombus.
12.3.2.2. Hybrid convergent procedure
The hybrid convergent procedure was first described in 2009, and clinical outcomes were published in 2010 by Kiser et al.1409 Since then, the employment of this technique has been reported in numerous studies, and subsequent modifications have been developed to maximize safety and improve clinical outcomes.221,1401–1408,1410–1414 This is a minimally invasive, closed-chest procedure performed on the beating heart that combines epicardial RF ablation focused on the LAPW followed by complementary endocardial catheter ablation. Epicardial ablation is performed under endoscopic visualization using a closed-irrigation, unipolar RF catheter device (EPi-Sense Guided Coagulation System with Visitrax, AtriCure, Inc.). The device is inserted via a small subxiphoid incision using a pericardioscopic cannula (Subtle, AtriCure, Inc.) to reach the LAPW and is manoeuvred in the pericardial space using the cannula and endoscope. During ablation, epicardial tissue is suctioned by vacuum onto the RF coil on one side of the device such that RF energy is only applied towards the heart and thus stabilizes the device on the atrium and optimizes energy delivery. Each lesion is created by a 90 s application of alternating current via an impedance-based power control algorithm with a maximum output of 30 W. Lesions are overlapped across the entire LAPW to promote contiguity and thus minimize gaps. The epicardial stage aims to debulk as much of the LAPW as can be accessed and is limited at the superior margin of the lesion set by the oblique sinus (Section 3.8.). Posterior segments of the PV ostia/antra may also be reached and ablated in most cases (Figure 15). The endocardial component supplements the epicardial lesions around the pericardial reflections and any incompletely ablated posterior wall areas and addresses any remaining gaps between the PV and the posterior wall lesion set ensuring electrical isolation of the PVs. The endocardial component can also include a CTI line and additional substrate modification. A key difference between thoracoscopic ablation and the hybrid convergent procedure is that PVI is performed epicardially using bipolar RF clamps in the former, while in the latter, it is achieved endocardially with catheter ablation.
Single-center and multicenter studies have reported 66–95% freedom from atrial tachyarrhythmias at 1 year following the hybrid convergent procedure,221,1401–1404,1406,1408,1415 with 52–81% arrhythmia-free survival without the use of AADs.1401,1403–1405,1415 Randomized controlled trial data are reported in detail in Section 12.3.3. Gained experience with hybrid convergent ablation has identified potential adverse events that can be mitigated. Thermal injury to the esophagus can be avoided by careful device orientation, esophageal temperature monitoring, and prophylactic irrigation of the pericardial space. Late pericardial effusion due to Dressler's syndrome and cardiac tamponade can be prevented by pericardial drains1403,1412 and prophylactic medications (colchicine, steroids, and/or nonsteroidal anti-inflammatory drugs).1416,1417 Timely diagnosis of pericardial effusion is facilitated by patient education on symptoms and transthoracic echo surveillance at ∼2–4 weeks.1416 Complications can arise from both epicardial and endocardial procedures, but greater experience has been associated with reduced procedural complications.1414
Several energy sources and variant lesion sets (apart from PVI and LAPW ablation) have been incorporated in convergent endocardial ablation workflows. One large study reported the use of endocardial cryoballoon in hybrid convergent procedures with favourable safety and efficacy.1418 The availability of PFA can improve safety and durability of endocardial lesions delivered at the posterior wall and thus potentially increase success rates. Given the likely role of LAA in persistent AF pathophysiology,819 limited studies have also combined the hybrid convergent approach with epicardial placement of a clip or stapled excision of the LAA reporting favourable initial results.1406,1419–1421
12.3.3. Clinical evidence—comparison of catheter and surgical ablation
Several RCTs have compared efficacy and safety of surgical ablation (minimally invasive or hybrid) with catheter ablation in mostly persistent and long-standing persistent AF patients. Existing data and pertinent advice are reported below.
12.3.3.1. Paroxysmal atrial fibrillation
Randomized controlled clinical trial data evaluating the efficacy of any type of stand-alone surgical AF ablation in paroxysmal AF patients are limited. The FAST trial was a head-to-head randomized comparison of catheter ablation vs. epicardial thoracoscopic surgery (bipolar RF ablation without standardized procedural workflow) in a total of 124 patients who had drug-refractory, symptomatic AF (66% paroxysmal AF) with prior failed catheter ablation or at high risk for failure (dilated LA).1339 The primary efficacy study endpoint (freedom from atrial tachyarrhythmias >30 s off AADs at 12 month follow-up) was significantly higher in the surgical as compared to the catheter ablation group (66 vs. 37%, P = 0.002). In the subgroup analysis, surgical ablation showed superior efficacy in the paroxysmal but not in the persistent AF patient subgroup. The adverse event rate at 12 months was significantly higher in the surgical ablation group mainly due to increased procedural complications. After a mean follow-up period of 7.0 years from randomization, atrial tachyarrhythmia recurrence was still significantly lower with surgical ablation (56%) compared with catheter ablation (87%), without any difference in the primary clinical composite endpoint (death, myocardial infarction, or cerebrovascular event).1340 In a smaller RCT of 64 patients with previous failed catheter ablation (59% paroxysmal AF), minimally invasive surgical ablation (thoracoscopic approach using bipolar RF clamp and targeting PVI, posterior box isolation, and GP ablation) resulted in a significantly lower atrial tachyarrhythmia recurrence rate at 12-months of follow-up as documented by ICM, with an associated significant increase in serious adverse events when compared with catheter ablation.1341
Only one RCT has compared minimally invasive surgical ablation (thoracoscopic approach using irrigated bipolar RF clamp and targeting PVI only with adjunctive LAA ligation) with catheter ablation as primary invasive AF treatment. The study included 52 patients with drug-refractory paroxysmal or early persistent (<3 months duration) AF with ICM for rhythm assessment during follow-up. Single-procedure arrhythmia-free survival off AADs after 2 years was similar in the catheter ablation when compared with the surgical ablation group (56 vs. 29%, respectively, P = 0.059), while a greater proportion of patients in the catheter ablation group had a low AF burden (<0.5%) at 2 years. Procedure-related major complications occurred more often with the surgical than with the catheter ablation approach (20.8 vs. 0%, P = 0.029).1342
In paroxysmal AF patients, the primary therapeutic target for any type of ablation strategy remains PVI. The following factors have been taken into account when determining the role of stand-alone surgical or hybrid ablation in symptomatic paroxysmal AF patients: (i) paucity of RCT data in paroxysmal AF patients, (ii) discrepancy in reported rhythm outcome benefit when compared with catheter ablation, (iii) consistent reporting of higher complication rate with surgical ablation compared with catheter ablation, (iv) lack of pathophysiological evidence to support ablation targets beyond PVI in paroxysmal AF patients that may be achieved more efficiently with surgical approaches, and (v) efficiency of catheter ablation in achieving durable PVI while ensuring reduced hospital stay and more rapid patient recovery.
12.3.3.2. Persistent and long-standing persistent atrial fibrillation
Several RCTs have evaluated the role of minimally invasive surgical or hybrid ablation in comparison with catheter ablation in symptomatic patients with persistent or long-standing persistent AF as primary ablative therapy.
In the CASA-AF trial, 120 patients with symptomatic long-standing persistent AF without prior ablation were randomized to surgical ablation (thoracoscopic approach using bipolar RF clamp and targeting PVI, posterior box isolation, and GP ablation) or catheter ablation (PVI, posterior box isolation, CTI, and mitral isthmus line).1422 At 12 months, 26% of patients in the surgical ablation arm and 28% of patients in the catheter ablation arm were free from atrial tachyarrhythmias, off AADs, after a single procedure as documented by invasive cardiac rhythm monitoring (P = 0.84). A similar percentage of patients experienced an arrhythmia burden reduction of ≥75% as well as procedure-related serious complications within 30 days of the intervention in both compared arms. Surgical ablation was associated with significantly higher costs and fewer quality-adjusted life-years than catheter ablation. Based on the study findings, the authors concluded that they found no evidence to suggest stand-alone thoracoscopic surgical ablation as first-line invasive therapy in patients with symptomatic long-standing persistent AF refractory to AADs.
In the CONVERGE trial, 153 patients with symptomatic persistent or long-lasting persistent AF (mean duration 4.4 ± 4.7 years) were randomized 2:1 to undergo PVI plus PWI with a hybrid thoracoscopic/endocardial approach (99 patients) or PVI plus roof line (no PWI) plus CTI line using a percutaneous, fully endocardial approach (50 patients). There was a significantly higher 12-month freedom from AF in the absence of new or increased dosage of previously failed or intolerant AADs (primary endpoint) in the hybrid arm compared with the endocardial arm (68 vs. 50%; P = 0.036).219 However, there was a higher major adverse event rate of 7.8% in the hybrid group compared with a 0% incidence in the endocardial group. The reported efficacy superiority of the hybrid as compared to the catheter ablation approach should be acknowledged in the context of relevant limitations such as the non-uniform ablation targets in compared groups (no empirical PWI in the catheter ablation group) and comparison of a hybrid (epicardial/endocardial) double approach vs. a single catheter ablation.
Recently, the HARTCAP-AF trial randomized 41 symptomatic, ablation-naive patients with persistent or long-standing persistent AF to an epicardial surgical ablation performed thoracoscopically (bipolar RF ablation) with occlusion/removal of the LAA combined with percutaneous endocardial ablation (one stage) vs. percutaneous endocardial catheter ablation, with optional repeated catheter ablation(s).1423 Hybrid ablation resulted in significantly more patients in SR off AADs at 12 months of follow-up compared with catheter ablation (89 vs. 41%, P = 0.002), without increasing the number of serious adverse events (21 vs. 14%, P = 0.685).
The recent, multicenter CEASE-AF trial randomized a total of 154 patients with drug-refractory, symptomatic persistent, or long-standing persistent AF in a 2:1 ratio to either a staged hybrid ablation or catheter ablation with potential repeat ablation, which was not considered a primary effectiveness failure. The hybrid ablation procedure included thoracoscopic RF ablation (PVI, PWI) and LAA exclusion with second-stage endocardial catheter ablation performed 3–6 months later. In the catheter ablation arm, PVI was mandatory while additional ablation was left to physician's discretion (only 40.2% received posterior wall ablation). Only 11.5% of patients in the endocardial ablation arm underwent a second catheter ablation procedure. The primary efficacy endpoint (freedom from AF/AFL/AT >30 s off all Class I/III AADs except those at doses previously failed) was significantly higher in the hybrid group as compared to the catheter ablation group (71.6 vs. 39.2%, P < 0.001) with similar major complication rates.1424
There is no RCT directly comparing minimally invasive epicardial surgical ablation alone vs. hybrid ablation. A systematic metaanalysis of 41 studies (published until November 2016) reporting outcomes of these two types of ablation strategies in a total of 2737 patients concluded that single-procedure survival free from atrial tachyarrhythmias without AADs was similar between epicardial-alone and hybrid approaches both at 12 months (epicardial alone 72 vs. hybrid 63%) and at 24 months (69 and 57%, respectively). Interestingly, hybrid ablation was associated with higher rate of major complications, while transdiaphragmatic access and use of unipolar RF were associated with lower success rates when compared with thoracoscopic access and bipolar RF, respectively.1425
A limitation of hybrid AF ablation that restrains its wider applicability is the higher rate of complications compared with percutaneous catheter ablation. This is not surprising given the added complexity and duration of combining surgical and catheter-based procedures, particularly when done at the same session. The reported rate of procedural-related serious adverse events in the abovementioned RCTs as well as in observational studies is usually in the range of 8–20%.1422,1423,1426,1427 These findings emphasize the importance of assembling an experienced multidisciplinary hybrid team consisting of a cardiologist, electrophysiologist, and surgeon as discussed above. Patients should be informed of the risks and benefits of a hybrid vs. a percutaneous ablation approach prior to undergoing an AF ablation procedure. Continued advances in ablation technologies and surgical and catheter-based approaches are anticipated to further improve patient outcome and reduce complications from hybrid ablation procedures.
12.4. Left atrial appendage exclusion
The LAA is the site of thrombus location in 90% of non-rheumatic AF patients with stroke and is a well-documented target for stroke reduction in patients with AF.1428 Multiple percutaneous and surgical techniques have been proposed for LAA elimination. Recent evidence supports the value of percutaneous LAA occlusion devices as an alternative to anticoagulants in AF patients.1429,1430 Surgical management of the LAA has an established role for stroke risk reduction in AF patients as a part of surgical/hybrid AF ablation, as an adjunct to concomitant cardiac surgery and, more rarely, as a stand-alone treatment. Early evaluation of the Cox maze III procedure suggested a reduction in late stroke after surgery.1431,1432 Other retrospective series subsequently suggested a lower-than-expected incidence of late neurologic events after a Cox maze procedure, independent of the preoperative CHA2DS2-VASc score or long-term warfarin use.1433,1434 The reduction in stroke has been attributed to both SR restoration and LAA elimination.
Historically, the most common techniques for exclusion of the LAA have been internal ligation, excision, or stapling at the base.1435–1438 Unfortunately, the efficacy of internal ligation and stapled excision or exclusion have been poor in late follow-up.1439–1441 While surgical excision has been shown to be effective, there has been concern for bleeding complications, especially in elderly patients with friable tissue. A more recent technique has been the use of external clips placed either under direct visualization or thoracoscopically at the base of the appendage (AtriClip, AtriCure, Inc.).1442–1446 The first AtriClip exclusion device was FDA approved in 2009 for the occlusion of the LAA in patients undergoing other open cardiac surgical procedures. In a large prospective non-randomized trial, the EXCLUDE trial, 60 of 61 patients had a successful LAA exclusion at the 3-month follow-up with a first-generation AtriClip device.1447 Subsequently, the long-term results from a prospective device trial reported that all 36 patients were without stroke, and there was 100% LAA occlusion confirmed by imaging at 3 years without thrombi, reperfusion, or residual neck stump of >1 cm.1448 In a recent larger series of 291 patients undergoing epicardial deployment of AtriClip device during open-heart surgery, the LAA was successfully excluded at 3 years in all patients.1449 Furthermore, the subgroup of patients with LAA occlusion who discontinued OAC during follow-up had a 87.5% relative risk reduction in ischemic stroke when compared with the expected rate in patients with similar CHA2DS2-VASc score.1449
Since then, several iterations have been made with the most recent devices, Pro-V and FLEX-V AtriClip (AtriCure, Inc.), receiving FDA approval in 2016 and 2018, respectively. Several studies have established the safety and long-term efficacy of stand-alone minimally invasive or thoracoscopic LAA occlusion with the AtriClip (AtriCure, Inc.) device in patients who either cannot be anticoagulated or who are not candidates for a transcatheter approach.1329–1334,1450,1451 The role of concomitant surgical LAA occlusion, in addition to OAC use, is best supported by a large RCT on LAA occlusion (LAAOS III).1335 Over 4800 patients with AF undergoing cardiac surgery were randomized to LAA occlusion (amputation, stapling, or suturing) or no treatment and were followed for a mean period of 3.8 years, with 76.8% of the participants continuing their OAC treatment. At 3 years, there were significantly fewer strokes and systemic emboli in the occluded as compared to the non-occluded cohort (4.8 vs. 7%, P = 0.001).
13. Training and institutional requirements for atrial fibrillation ablation
For patients to have safe and effective AF ablation treatment, clinicians need to be adequately trained and work in an institution with appropriate facilities and support. Before performing AF ablation, clinicians and institutions need to have formally assessed and recorded that they have the training, standard operating procedures, and facilities to:
select appropriate patients for treatment,
deliver the treatment in a safe and cost-effective way,
manage common complications (e.g. postprocedural pain, hematoma),
manage or have arrangements to manage rare complications (e.g. cardiac perforation and tamponade, AEF, stroke),
ensure adequate patient follow-up,
record and audit their results and outcomes, and
respond to incidents, errors, and complications and modify their practice to reduce the probability of recurrence.
The cost, efficiency, and access to catheter ablation are important considerations. It is therefore unrealistic to expect every clinician and institution to provide the same level of facility and care. More complex cases with higher risk should be treated by clinicians with a greater level of experience and training, in institutions with greater support. Conversely, many lower risk patients undergoing simple PVI will not require the same level of support. As long as centers and clinicians can demonstrate that they are able to deliver all of these fundamental quality metrics outlined above, then it is reasonable for them to perform catheter ablation of AF.
13.1. Training requirements
Atrial fibrillation ablation is not performed by a doctor or surgeon alone; it is a procedure involving a multidisciplinary team. Patients may interact with and depend on many of the clinicians in this team, and therefore, training, competence, and access to facilities for all of these team members need to be considered.
13.1.1. Appropriate selection of patients
Patients who are suitable for AF ablation are likely to be identified by clinicians who do not perform AF ablation, including specialist nurses. However, before being scheduled for a procedure, patients should have had the opportunity to meet a doctor who is competent to perform the procedure, with knowledge of the outcomes and other treatment options in order to allow the patient to make an informed decision. Patients should also understand any limitations of the facilities available to them, and physicians should be able to advise patients when a more complex level of care is needed. The treatment options for AF, AF ablation, and factors that influence outcomes are discussed in detail in previous sections. Trainees should have demonstrated similar knowledge and quality of consent as their supervisors before selecting and consenting patients independently. Clinical staff involved in preparing patients for their procedure should also be aware of clinical features that may give them an increased risk or poorer outcome from an ablation, and members of the team like specialist nurses performing pre-admission assessment should be competent to identify such risk factors and alert the rest of the team.
13.1.2. Technical knowledge required
The doctor performing the procedure should have a thorough understanding and appropriate training (with formal documentation of this if appropriate) of:
current indications for AF ablation (Section 4),
relevant anatomy (Section 3),
advantages and disadvantages of different technologies and techniques for AF ablation (Sections 6 and 8),
success rates for ablation in different patient groups (Sections 8 and 10),
appropriate postoperative management and follow-up of patients (Section 9), and
prevention, clinical presentation, and management of procedural complications (Section 11).
Physicians should also have been through a formal and documented training programme with their progress logged and signed off by an appropriate supervisor. They should have demonstrated a knowledge of the areas required and competence to perform independently:
achieve venous access (including use of vascular ultrasound)
perform transseptal puncture
identify and isolate the PVs (including validating PVI on electrophysiological tracings and performing differential pacing maneuvers)
be competent in using and interpreting 3D mapping systems
understand biophysics of RF, cryothermy and other energy sources, energy selection, and application
achieve hemostasis post-procedure; this may include use of figure-of-eight sutures and/or vascular closure devices
identify and drain a pericardial effusion
Atrial fibrillation ablation now comes in several forms, which range from PVI to complex AT ablation. It is recognized that some clinicians may work in a service that just performs PVI with single-shot technologies and refers patients with rhythms other than AF to other electrophysiologists. The writing group suggests that all physicians involved in AF ablation, even when focusing on single-shot PVI, should also have attained basic competence in mapping and ablation procedures that are required for treatment of coexistent arrhythmias, e.g. typical AFl, atypical AFl, or SVT. In case of non-availability of 3D mapping systems, patients with AT post-AF ablation should be referred to appropriately equipped institutions.
13.1.3. Training of non-medical team members
The other members of the team performing ablation should have training appropriate to their roles. These roles may be varied but whatever their role, their training and competence should be recorded and assessed. Important roles fulfilled by non-medical members of the team may include the following:
selecting patients—understanding indications and characteristics adversely affecting outcomes of AF ablation
managing electrophysiology equipment
managing analgesia/sedation—appropriate and safe sedation training
assisting in management of life-threatening complications (e.g. tamponade)—training and rehearsal of procedures and use of equipment
performing patient follow-up
identification of complications or postablation arrhythmia recurrence
13.1.4. Completion of training
Numbers of procedures required to achieve competence are very difficult to define because different clinicians will progress at different rates. It is recommended that trainees should have completed a training programme, and their supervisor/trainer should be able to take responsibility for a trainee and confirm that the trainee is competent to perform the procedures they intend to undertake as an independent practitioner.
The writing group suggests that the minimum required practical experience with active participation includes:
50 AF ablation procedures,
20 CTI flutters, and
10 non–CTI-dependent focal or reentrant tachycardias.
These numbers are consistent with the 2015 ACC/AHA/HRS Advanced Training Statement on Clinical Cardiac Electrophysiology and the Level 2 EHRA Certified Electrophysiology Specialist requirements.1452 Further skills and knowledge may be required depending on the practice of the trainee/physician.
13.1.5. Maintaining competence
It is well recognized that both physician and institutional procedure volumes are associated with improved patient outcomes. Even if physicians have received training for catheter ablation, it is important that they are performing these procedures regularly and continue a programme of self-education to ensure that they are aware of the most current evidence and thinking on AF and its management.
Actual procedure numbers continue to be difficult to define because some clinicians will require longer training and more procedures to maintain their performance than others. Analysis of early practice suggested that individual and institutional volumes of <25 and <50 AF ablations per year, respectively, were associated with worse outcomes.298 More recent evidence suggests that procedure numbers are less important for institutions using cryoballoon ablation, with studies failing to show a significant difference between high-volume and low-volume centers.297,1453 The reality is probably more nuanced than simply a distinction between high-volume and low-volume centers because, although outcomes may not be statistically different, high-volume centers will manage more complex, high-risk cases.1454 Therefore, we would recommend that rather than using procedure numbers as a crude assessment of competence, all centers performing AF ablation should be able to demonstrate their procedure outcomes and compliance with the recommendations in this consensus document.
There is evidence of improved performance with team-based simulations and loss of performance when this is discontinued.1455,1456 It is therefore strongly suggested that all members of the ablation team take part in regular rehearsals or simulations to practice management of emergencies and rarely seen complications like pericardial effusion. This ensures that not only all team members are aware of the plan and their role in it, but also that the necessary equipment is available.
13.2. Institutional requirements
13.2.1. Staff
Institutions should have sufficient trained staff to provide pre-admission counselling, AF ablation, and postoperative support and follow-up. These roles should ideally not all be carried out by the physician performing the procedure, to ensure that other members of the team are appropriately trained to support patients in the absence of that physician. If an institution is not able to offer 24/7 care to patients, patients should be able to access care in the event of an emergency (even if it involves attending an emergency room) and know what those arrangements are. Staff should be aware of common complications after AF ablation and to triage them appropriately.
13.2.2. Equipments and facilities
Atrial fibrillation ablation in selected patients can be performed safely in institutions without cardiothoracic surgical services.1214,1453 In a retrospective, non-randomized, propensity-matched analysis of Medicare beneficiaries aged 65 years and older, the presence or absence of on-site cardiothoracic surgery was not associated with 30-day rate of cardiac perforation, cardiothoracic surgery, rehospitalization, and death after AF ablation.1457 However, in this study, hospitals without cardiothoracic surgery accounted for just 2% of total ablations indicating that in the USA, this remains uncommon. When AF ablation is performed in centers without cardiothoracic surgery services, it is recommended that transfer arrangements and checklists should be in place, and patients should be aware of the potential need to be transferred to a cardiothoracic center in case of emergency.
All institutions should have the following minimum equipment list to perform AF ablation:
ultrasound for vascular access,
echocardiography, including TEE,
fluoroscopic X-ray imaging,
3D mapping or a single-shot PV ablation technology, and
pericardial drainage equipment and anticoagulant reversal.
Institutions performing AT ablation should have access to a 3D mapping technology.
13.2.3. Follow-up and other requirements
Institutions should have arrangements for patient follow-up. Follow-up intervals and duration are discussed in Section 9. Follow-up does not always need to take place face-to-face. Digital ECG recording systems can facilitate remote phone or video consultation follow-up when the patient and the physician both feel this is appropriate. It is important that this follow-up system should be used to record AF ablation outcomes. These results should be audited, and the institution has formal arrangements for identifying and responding to serious complications. The outcomes of physicians and their teams should be regularly reviewed and arrangements in place to identify and manage poor performance. Institutions should have a culture and system in place that encourages reporting of poor outcomes and responding to this by avoiding individual blame, rather aiming to understand and correct the system failures that have led to poor performance and confirm that appropriate changes have resulted in improved outcomes.
If there is a regional or national audit database, centers should submit their data to those, including their complication rates.
14. Areas for future research
There has been significant progress in the safety and efficacy of AF ablation as well as significant advances in the technologies used to perform ablation. However, many critical questions remain unanswered, especially as we enter a new era in energy delivery with the advent of PFA (Table 10).
Table 10.
Unanswered questions in AF ablation
| Topic | Questions |
|---|---|
| Basic/translational science |
|
| Risk factor modification |
|
| Patient selection—personalized management |
|
| Energy sources—ablation tools |
|
| Ablation strategies |
|
| Endpoints and outcomes after ablation |
|
AF, atrial fibrillation; LA, left atrial; OSA, obstructive sleep apnoea; PFA, pulsed field ablation; PV, pulmonary vein; PVI, PV isolation.
14.1. Basic translational science
The importance of basic and translational research to better understand the mechanisms of AF should not be underestimated. It should be recognized that even after a century of research, the mechanisms of AF have not been fully elucidated, hampering our ability to develop better clinical tools for treating AF. The debate continues over the primacy of the multiple wavelet hypothesis vs. focal sources of AF.1458 While prior attempts to map with phase-mapping and other technologies have not resulted in meaningful improvements in AF ablation, emerging technologies continue to offer promise. It is also possible that persistent AF has multiple mechanisms, which may vary in different patients and substrates. Investigation into more personalized AF treatment strategies, based on clinical and electrophysiologic measurements, that minimize tissue destruction should be encouraged. Whether we can map AF in a way that leads to changes in ablation strategies with an impact on short-term and long-term outcomes perhaps remains one of the largest unanswered questions in AF ablation.
14.2. Risk factor modification
Recent evidence has highlighted the importance of risk factor modification for improving the outcome after AF ablation and preventing long-term AF recurrences (Section 5.1.). Optimal strategies for maintaining weight loss and risk factor modification long term and its effect on late AF recurrence should be investigated. Longer term (>10 year) outcome after ablation of AF should also be investigated to determine which patients benefit most from early intervention. It has also become apparent that an underlying fibrotic atrial myopathy underlies AF progression in many patients. Pharmacologic approaches to minimize the progression of atrial remodelling and fibrosis may be important for improving long-term freedom from AF after ablation.
14.3. Patient selection—personalized management
A key step in AF ablation is optimization of patient selection. Several variables are predictive of ablation outcome (Section 5.2.1.). There have been significant advances in our understanding of LA substrate and its relation to ablation outcomes. The DECAAF study highlighted the value of MRI-detected fibrosis for predicting outcomes after ablation.103 However, these findings have not been widely reproduced or employed. More recent studies have highlighted the promise of machine learning to predict outcomes following ablation.1459,1460 Development of a personalized approach to identify optimal AF ablation candidates and predict procedural outcome is necessary to advance precision medicine approach in the care of AF patients.
To date, patient selection and indications for ablation of AF have focused on those with symptoms and left ventricular systolic dysfunction. However, recent data supporting improved outcomes with early rhythm control in asymptomatic persons raise the question as to whether ablation may improve long-term outcomes in those without symptoms.332 Determining whether ablation can improve outcomes in persons with asymptomatic AF will require relatively large RCTs.
14.4. Energy sources—ablation tools
In clinical practice across the world, cryothermy and RF remain the predominant modes of energy delivery for AF ablation. As both of these technologies develop, the best approach to lesion delivery still remains unclear. Pulsed field ablation has the potential to change that by providing safer and more efficient lesion delivery. Utilization of PFA is rapidly growing, and larger multicenter experiences are reassuring.643,644 Additional investigation will be required to determine whether PFA results in similar or better long-term outcomes compared with cryoballoon and RF ablation.662 While PFA may reduce the risk of significant PN palsy, esophageal injury, and PV stenosis, does it permanently impair GP? If not, what are the implications for longer term efficacy?1461 Does PFA perform as well on non-PV targets with similar safety or are there additional safety concerns as has been recently highlighted with coronary vasospasm?656 Finally, if PFA does provide more reliable and facile ablation, will easier ablation result in more atrial myocardium being ablated and thus increased risk for low-compliance complications of AF ablation such as stiff LA syndrome? Early data suggest that PFA does not engender changes that favour restrictive physiology,1180 but more data are needed, particularly in patients undergoing extensive substrate modification.
14.5. Ablation strategies
While PVI remains the gold standard for the treatment of paroxysmal AF, PV reconnection after ablation remains a common problem and the major reason for recurrence after ablation. Novel energy sources and approaches to minimize PV reconnection after ablation will be essential for determining the true effectiveness for durable PVI on freedom from AF. This is critical before other adjunctive strategies can be investigated.
Outcomes following ablation of persistent AF are suboptimal. Despite many clinical trials, no adjunctive ablation strategy has been shown to be consistently superior to PVI alone. Delineation of optimal method(s) for ablation of persistent AF beyond PVI remains a priority in future research in AF ablation. Advances in computational power and machine learning may also allow better characterization of the AF substrate and appropriate targets beyond PVI. Personalized computational modelling has been evaluated to help ‘personalize’ ablation and pre-determine ablation targets.1462 Future studies will need to prospectively evaluate both clinical and machine learning risk stratification schemes, and randomized studies will be needed to test personalized approaches to AF ablation. As with any new technology, reproducibility across centers will be essential.
The combined approach of hybrid ablation has shown some value for improved outcomes in patients with persistent AF and more advanced atrial substrates (Section 12). However, the morbidity of such procedures is generally higher than catheter ablation, and outcomes depend on surgical tools and experience. Studies to identify the best candidates, tools, and approach to hybrid ablation are needed. Future studies should also compare catheter ablation with hybrid approaches, ideally involving similar lesion sets and follow-up. Hybrid approaches that involve two procedures (surgical ablation followed by catheter ablation) should ideally be compared with two catheter ablations. Furthermore, the optimal timing between different stages of the hybrid procedure should be investigated.
While interventional catheter-based approaches to treat AF dominate our current approach, non-invasive methods for treating AF will undoubtedly be developed in the future. Stereotactic body radiotherapy has been demonstrated to be effective for refractory VT and is growing in use.1463 Stereotactic body radiotherapy has also been used in a pilot study to treat AF in humans,1464 and such techniques will only improve with safer targeting and radiation technology. Carbon and proton beam ablation may allow more accurate targeting and lower radiation dose to surrounding tissues.1465 Further research into non-invasive ablation of AF should be encouraged.
14.6. Endpoints and outcomes after ablation
Testing different ablation strategies, evaluating the impact of new technologies, and accurately understanding the impact of ablation require reporting and evaluation of standard, pragmatic, and meaningful measures of arrhythmia suppression. While it is generally agreed that 30 s of sustained atrial arrhythmia has limited value from a disease burden and patient perspective, there still is no consensus on what the optimal efficacy endpoint should be for AF ablation (Section 10.2.). While AF burden may be an ideal measure,7 at present, it requires either extended monitoring to provide periodic samples of AF burden or an implanted device to measure truly continuous AF burden (Section 10.3.). However, this status quo may change as wearable technologies evolve (Section 9.4.).1466,1467 A key goal for the field should be the identification of a universal, pragmatic, and meaningful efficacy endpoint for AF ablation that impacts outcome.
Measuring outcomes is also challenging in asymptomatic patients; while hard outcomes such as mortality and stroke would provide the strongest support for ablation in asymptomatic patients, other outcomes such as exercise tolerance and QoL improvements would be important to ascertain.
Supplementary Material
Contributor Information
Stylianos Tzeis, Department of Cardiology, Mitera Hospital, 6, Erythrou Stavrou Str., Marousi, Athens, PC 151 23, Greece.
Edward P Gerstenfeld, Section of Cardiac Electrophysiology, University of California, San Francisco, CA, USA.
Jonathan Kalman, Department of Cardiology, Royal Melbourne Hospital, Melbourne, Australia; Department of Medicine, University of Melbourne and Baker Research Institute, Melbourne, Australia.
Eduardo B Saad, Electrophysiology and Pacing, Hospital Samaritano Botafogo, Rio de Janeiro, Brazil; Cardiac Arrhythmia Service, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Alireza Sepehri Shamloo, Department of Electrophysiology, Heart Center at University of Leipzig, Leipzig, Germany.
Jason G Andrade, Department of Medicine, Vancouver General Hospital, Vancouver, British Columbia, Canada.
Chirag R Barbhaiya, NYU Grossman School of Medicine, New York, NY, USA.
Tina Baykaner, Division of Cardiology and Cardiovascular Institute, Stanford University, Stanford, CA, USA.
Serge Boveda, Heart Rhythm Management Department, Clinique Pasteur, Toulouse, France; Universiteit Brussel (VUB), Brussels, Belgium.
Hugh Calkins, Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Ngai-Yin Chan, Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong Special Administrative Region, China.
Minglong Chen, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Shih-Ann Chen, Heart Rhythm Center, Taipei Veterans General Hospital, Taipei, and Cardiovascular Center, Taichung Veterans General Hospital, Taichung, Taiwan.
Nikolaos Dagres, Deutsches Herzzentrum der Charité, Berlin, Germany.
Ralph J Damiano, Division of Cardiothoracic Surgery, Department of Surgery, Washington University School of Medicine, Barnes-Jewish Hospital, St. Louis, MO, USA.
Tom De Potter, Cardiovascular Center, OLV Hospital Aalst, Belgium.
Isabel Deisenhofer, Department of Electrophysiology, German Heart Center Munich, Technical University of Munich (TUM) School of Medicine and Health, Munich, Germany.
Nicolas Derval, IHU LIRYC, Electrophysiology and Heart Modeling Institute, Cardiac Electrophysiology and Stimulation Department, Fondation Bordeaux Université and Bordeaux University Hospital (CHU), Pessac-Bordeaux, France.
Luigi Di Biase, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA.
Mattias Duytschaever, Department of Cardiology, AZ Sint-Jan Hospital, Bruges, Belgium.
Katia Dyrda, Department of Medicine, Montreal Heart Institute, Université de Montréal, Montreal, Canada.
Gerhard Hindricks, Deutsches Herzzentrum der Charité, Berlin, Germany.
Meleze Hocini, IHU LIRYC, Electrophysiology and Heart Modeling Institute, Cardiac Electrophysiology and Stimulation Department, Fondation Bordeaux Université and Bordeaux University Hospital (CHU), Pessac-Bordeaux, France.
Young-Hoon Kim, Division of Cardiology, Korea University College of Medicine and Korea University Medical Center, Seoul, Republic of Korea.
Mark la Meir, Cardiac Surgery Department, Vrije Universiteit Brussel, Universitair Ziekenhuis Brussel, Brussels, Belgium.
Jose Luis Merino, La Paz University Hospital, Idipaz, Universidad Autonoma, Madrid, Spain; Hospital Viamed Santa Elena, Madrid, Spain.
Gregory F Michaud, Massachusetts General Hospital and Harvard Medical School, MA, USA.
Andrea Natale, Texas Cardiac Arrhythmia Institute, St. David’s Medical Center, Austin, TX, USA; Case Western Reserve University, Cleveland, OH, USA; Interventional Electrophysiology, Scripps Clinic, San Diego, CA, USA; Department of Biomedicine and Prevention, Division of Cardiology, University of Tor Vergata, Rome, Italy.
Isabelle Nault, Institut Universitaire de Cardiologie et de Pneumologie de Quebec (IUCPQ), Quebec, Canada.
Santiago Nava, Departamento de Electrocardiología, Instituto Nacional de Cardiología ‘Ignacio Chávez’, Ciudad de México, México.
Takashi Nitta, Department of Cardiovascular Surgery, Nippon Medical School, Tokyo, Japan.
Mark O’Neill, Cardiovascular Directorate, St. Thomas’ Hospital and King’s College, London, UK.
Hui-Nam Pak, Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea.
Jonathan P Piccini, Duke University Medical Center, Durham, NC, USA.
Helmut Pürerfellner, Ordensklinikum Linz Elisabethinen, Linz, Austria.
Tobias Reichlin, Department of Cardiology, Inselspital Bern, Bern University Hospital, University of Bern, Bern, Switzerland.
Luis Carlos Saenz, International Arrhythmia Center, Cardioinfantil Foundation, Bogota, Colombia.
Prashanthan Sanders, Centre for Heart Rhythm Disorders, University of Adelaide and Royal Adelaide Hospital, Adelaide, Australia.
Richard Schilling, Barts Heart Centre and Welbeck Heart Health, London, UK.
Boris Schmidt, Cardioangiologisches Centrum Bethanien, Medizinische Klinik III, Agaplesion Markuskrankenhaus, Frankfurt, Germany.
Gregory E Supple, Cardiac Electrophysiology Section, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA.
Kevin L Thomas, Duke University Medical Center, Durham, NC, USA.
Claudio Tondo, Department of Clinical Electrophysiology and Cardiac Pacing, Centro Cardiologico Monzino, IRCCS, Milan, Italy; Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
Atul Verma, McGill University Health Centre, McGill University, Montreal, Canada.
Elaine Y Wan, Department of Medicine, Division of Cardiology, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, USA.
Supplementary material
Supplementary material is available at Europace online.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007;9:335–79. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632–96.e21. [DOI] [PubMed] [Google Scholar]
- 3. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017;33:369–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6. Diederichsen SZ, Haugan KJ, Brandes A, Lanng MB, Graff C, Krieger D, et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol 2019;74:2771–81. [DOI] [PubMed] [Google Scholar]
- 7. Aguilar M, Macle L, Deyell MW, Yao R, Hawkins NM, Khairy P, et al. Influence of monitoring strategy on assessment of ablation success and postablation atrial fibrillation burden assessment: implications for practice and clinical trial design. Circulation 2022;145:21–30. [DOI] [PubMed] [Google Scholar]
- 8. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 9. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol 2020;36:1847–948. [DOI] [PubMed] [Google Scholar]
- 10. NHFA CSANZ Atrial Fibrillation Guideline Working Group; Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ 2018;27:1209–66. [DOI] [PubMed] [Google Scholar]
- 11. Cheung CC, Nattel S, Macle L, Andrade JG. Management of atrial fibrillation in 2021: an updated comparison of the current CCS/CHRS, ESC, and AHA/ACC/HRS guidelines. Can J Cardiol 2021;37:1607–18. [DOI] [PubMed] [Google Scholar]
- 12. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 13. Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation 1998;98:2202–9. [DOI] [PubMed] [Google Scholar]
- 14. Crijns HJ, van Wijk LM, van Gilst WH, Kingma JH, van Gelder IC, Lie KI. Acute conversion of atrial fibrillation to sinus rhythm: clinical efficacy of flecainide acetate. Comparison of two regimens. Eur Heart J 1988;9:634–8. [DOI] [PubMed] [Google Scholar]
- 15. Suttorp MJ, Kingma JH, Jessurun ER, Lie AHL, van Hemel NM, Lie KI. The value of class IC antiarrhythmic drugs for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. J Am Coll Cardiol 1990;16:1722–7. [DOI] [PubMed] [Google Scholar]
- 16. Andrade JG, Deyell MW, Verma A, Macle L, Champagne J, Leong-Sit P, et al. Association of atrial fibrillation episode duration with arrhythmia recurrence following ablation: a secondary analysis of a randomized clinical trial. JAMA Netw Open 2020;3:e208748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boriani G, Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, et al. Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke 2011;42:1768–70. [DOI] [PubMed] [Google Scholar]
- 18. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 19. Chew DS, Li Z, Steinberg BA, O’Brien EC, Pritchard J, Bunch TJ, et al. Arrhythmic burden and the risk of cardiovascular outcomes in patients with paroxysmal atrial fibrillation and cardiac implanted electronic devices. Circ Arrhythm Electrophysiol 2022;15:e010304. [DOI] [PubMed] [Google Scholar]
- 20. Charitos EI, Purerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63:2840–8. [DOI] [PubMed] [Google Scholar]
- 21. Andrade JG, Yao RRJ, Deyell MW, Hawkins NM, Rizkallah J, Jolly U, et al. Clinical assessment of AF pattern is poorly correlated with AF burden and post ablation outcomes: a CIRCA-DOSE sub-study. J Electrocardiol 2020;60:159–64. [DOI] [PubMed] [Google Scholar]
- 22. De With RR, Erkuner O, Rienstra M, Nguyen BO, Korver FWJ, Linz D, et al. Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE V. Europace 2020;22:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blum S, Meyre P, Aeschbacher S, Berger S, Auberson C, Briel M, et al. Incidence and predictors of atrial fibrillation progression: a systematic review and metaanalysis. Heart Rhythm 2019;16:502–10. [DOI] [PubMed] [Google Scholar]
- 24. Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Ilstrup DM, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med 1987;317:669–74. [DOI] [PubMed] [Google Scholar]
- 25. Pappone C, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Sacchi S, et al. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm 2008;5:1501–7. [DOI] [PubMed] [Google Scholar]
- 26. Simantirakis EN, Papakonstantinou PE, Kanoupakis E, Chlouverakis GI, Tzeis S, Vardas PE. Recurrence rate of atrial fibrillation after the first clinical episode: a prospective evaluation using continuous cardiac rhythm monitoring. Clin Cardiol 2018;41:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2022;388:105–16. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen BO, Weberndorfer V, Crijns HJ, Geelhoed B, Ten Cate H, Spronk H, et al. Prevalence and determinants of atrial fibrillation progression in paroxysmal atrial fibrillation. Heart 2022;109:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Potpara TS, Stankovic GR, Beleslin BD, Polovina MM, Marinkovic JM, Ostojic MC, et al. A 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation: implications of arrhythmia progression on prognosis: the Belgrade atrial fibrillation study. Chest 2012;141:339–47. [DOI] [PubMed] [Google Scholar]
- 30. Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P, et al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm 2017;14:801–7. [DOI] [PubMed] [Google Scholar]
- 31. Piccini JP, Passman R, Turakhia M, Connolly AT, Nabutovsky Y, Varma N. Atrial fibrillation burden, progression, and the risk of death: a case-crossover analysis in patients with cardiac implantable electronic devices. Europace 2019;21:404–13. [DOI] [PubMed] [Google Scholar]
- 32. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 33. Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149:489–96. [DOI] [PubMed] [Google Scholar]
- 34. Parkash R, Green MS, Kerr CR, Connolly SJ, Klein GJ, Sheldon R, et al. The association of left atrial size and occurrence of atrial fibrillation: a prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am Heart J 2004;148:649–54. [DOI] [PubMed] [Google Scholar]
- 35. Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J 2008;29:2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vitolo M, Proietti M, Imberti JF, Bonini N, Romiti GF, Mei DA, et al. Factors associated with progression of atrial fibrillation and impact on all-cause mortality in a cohort of European patients. J Clin Med 2023;12:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De With RR, Marcos EG, Dudink E, Spronk HM, Crijns H, Rienstra M, et al. Atrial fibrillation progression risk factors and associated cardiovascular outcome in well-phenotyped patients: data from the AF-RISK study. Europace 2020;22:352–60. [DOI] [PubMed] [Google Scholar]
- 38. Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet 2005;118:179–84. [DOI] [PubMed] [Google Scholar]
- 39. Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, et al. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J 2006;27:708–12. [DOI] [PubMed] [Google Scholar]
- 40. Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA 2010;304:2263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 2003;299:251–4. [DOI] [PubMed] [Google Scholar]
- 42. Olesen MS, Bentzen BH, Nielsen JB, Steffensen AB, David JP, Jabbari J, et al. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC Med Genet 2012;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet 2004;75:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mann SA, Otway R, Guo G, Soka M, Karlsdotter L, Trivedi G, et al. Epistatic effects of potassium channel variation on cardiac repolarization and atrial fibrillation risk. J Am Coll Cardiol 2012;59:1017–25. [DOI] [PubMed] [Google Scholar]
- 45. Sinner MF, Pfeufer A, Akyol M, Beckmann BM, Hinterseer M, Wacker A, et al. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG). Eur. Heart J 2008;29:907–14. [DOI] [PubMed] [Google Scholar]
- 46. Olesen MS, Refsgaard L, Holst AG, Larsen AP, Grubb S, Haunso S, et al. A novel KCND3 gain-of-function mutation associated with early-onset of persistent lone atrial fibrillation. Cardiovasc Res 2013;98:488–95. [DOI] [PubMed] [Google Scholar]
- 47. Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB, et al. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J 2013;34:1517–25. [DOI] [PubMed] [Google Scholar]
- 48. Tsai CT, Hsieh CS, Chang SN, Chuang EY, Juang JM, Lin LY, et al. Next-generation sequencing of nine atrial fibrillation candidate genes identified novel de novo mutations in patients with extreme trait of atrial fibrillation. J Med Genet 2015;52:28–36. [DOI] [PubMed] [Google Scholar]
- 49. Olesen MS, Yuan L, Liang B, Holst AG, Nielsen N, Nielsen JB, et al. High prevalence of long QT syndrome-associated SCN5A variants in patients with early-onset lone atrial fibrillation. Circ Cardiovasc Genet 2012;5:450–9. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, et al. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li RG, Wang Q, Xu YJ, Zhang M, Qu XK, Liu X, et al. Mutations of the SCN4B-encoded sodium channel beta4 subunit in familial atrial fibrillation. Int J Mol Med 2013;32:144–50. [DOI] [PubMed] [Google Scholar]
- 52. Feghaly J, Zakka P, London B, MacRae CA, Refaat MM. Genetics of atrial fibrillation. J Am Heart Assoc 2018;7:e009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pessente GD, Sacilotto L, Calil ZO, Olivetti NQS, Wulkan F, de Oliveira TGM, et al. Effect of occurrence of Lamin A/C (LMNA) genetic variants in a cohort of 101 consecutive apparent “Lone AF” patients: results and insights. Front Cardiovasc Med 2022;9:823717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448:353–7. [DOI] [PubMed] [Google Scholar]
- 55. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 2018;50:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roselli C, Rienstra M, Ellinor PT. Genetics of atrial fibrillation in 2020: GWAS, genome sequencing, polygenic risk, and beyond. Circ Res 2020;127:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee JY, Kim TH, Yang PS, Lim HE, Choi EK, Shim J, et al. Korean atrial fibrillation network genome-wide association study for early-onset atrial fibrillation identifies novel susceptibility loci. Eur Heart J 2017;38:2586–94. [DOI] [PubMed] [Google Scholar]
- 58. Low SK, Takahashi A, Ebana Y, Ozaki K, Christophersen IE, Ellinor PT, et al. Identification of six new genetic loci associated with atrial fibrillation in the Japanese population. Nat Genet 2017;49:953–8. [DOI] [PubMed] [Google Scholar]
- 59. Weng LC, Choi SH, Klarin D, Smith JG, Loh PR, Chaffin M, et al. Heritability of atrial fibrillation. Circ Cardiovasc Genet 2017;10:e001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shoemaker MB, Shah RL, Roden DM, Perez MV. How will genetics inform the clinical care of atrial fibrillation? Circ Res 2020;127:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilde AAM, Semsarian C, Márquez MF, Shamloo AS, Ackerman MJ, Ashley EA, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Europace 2022;24:1307–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 2010;7:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res 2020;127:51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–68. [DOI] [PubMed] [Google Scholar]
- 65. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 66. Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol 2003;551:801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence S, et al. Electroanatomic properties of the pulmonary veins: slowed conduction, low voltage and altered refractoriness in AF patients. J Cardiovasc Electrophysiol 2011;22:1083–91. [DOI] [PubMed] [Google Scholar]
- 68. Elbatran AI, Anderson RH, Mori S, Saba MM. The rationale for isolation of the left atrial pulmonary venous component to control atrial fibrillation: a review article. Heart Rhythm 2019;16:1392–8. [DOI] [PubMed] [Google Scholar]
- 69. Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017;14:1087–96. [DOI] [PubMed] [Google Scholar]
- 70. Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation 2001;103:2631–6. [DOI] [PubMed] [Google Scholar]
- 71. Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation 2004;110:3181–6. [DOI] [PubMed] [Google Scholar]
- 72. Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000;101:194–9. [DOI] [PubMed] [Google Scholar]
- 73. Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 1992;355:349–51. [DOI] [PubMed] [Google Scholar]
- 74. Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, et al. Atrial fibrillation driven by micro-anatomic intramural reentry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm 2011;8:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zaman JAB, Sauer WH, Alhusseini MI, Baykaner T, Borne RT, Kowalewski CAB, et al. Identification and characterization of sites where persistent atrial fibrillation is terminated by localized ablation. Circ Arrhythm Electrophysiol 2018;11:e005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baykaner T, Rogers AJ, Meckler GL, Zaman J, Navara R, Rodrigo M, et al. Clinical implications of ablation of drivers for atrial fibrillation: a systematic review and metaanalysis. Circ Arrhythm Electrophysiol 2018;11:e006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Garrey WE. Auricular fibrillation. Physiol Rev 1924;4:215–50. [Google Scholar]
- 79. Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200–20. [DOI] [PubMed] [Google Scholar]
- 80. Kirchhof C, Chorro F, Scheffer GJ, Brugada J, Konings K, Zetelaki Z, et al. Regional entrainment of atrial fibrillation studied by high-resolution mapping in open-chest dogs. Circulation 1993;88:736–49. [DOI] [PubMed] [Google Scholar]
- 81. Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous biatrial high-density (510-512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation 2015;132:2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kamali R, Kump J, Ghafoori E, Lange M, Hu N, Bunch TJ, et al. Area available for atrial fibrillation to propagate is an important determinant of recurrence after ablation. JACC Clin Electrophysiol 2021;7:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eckstein J, Maesen B, Linz D, Zeemering S, van Hunnik A, Verheule S, et al. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc Res 2011;89:816–24. [DOI] [PubMed] [Google Scholar]
- 84. Zhang L, van Schie MS, Knops P, Taverne Y, de Groot NMS. A novel diagnostic tool to identify atrial endo-epicardial asynchrony using signal fingerprinting. Hellenic J Cardiol 2023:S1109-9666(23)00123-9. [DOI] [PubMed] [Google Scholar]
- 85. Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, et al. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J 2014;35:86–97. [DOI] [PubMed] [Google Scholar]
- 86. Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol 2010;3:606–15. [DOI] [PubMed] [Google Scholar]
- 87. Parameswaran R, Teuwen CP, Watts T, Nalliah CJ, Royse A, Goldblatt J, et al. Functional atrial endocardial-epicardial dissociation in patients with structural heart disease undergoing cardiac surgery. JACC Clin Electrophysiol 2020;6:34–44. [DOI] [PubMed] [Google Scholar]
- 88. Parameswaran R, Kalman JM, Royse A, Goldblatt J, Larobina M, Watts T, et al. Endocardial-epicardial phase mapping of prolonged persistent atrial fibrillation recordings: high prevalence of dissociated activation patterns. Circ Arrhythm Electrophysiol 2020;13:e008512. [DOI] [PubMed] [Google Scholar]
- 89. Hong KL, Baley J, Baranchuk A, Bisleri G, Glover BM. Epicardial electrical activation during atrial fibrillation: looking at the other side of the coin. JACC Case Rep 2019;1:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jiang R, Buch E, Gima J, Upadhyay GA, Nayak HM, Beaser AD, et al. Feasibility of percutaneous epicardial mapping and ablation for refractory atrial fibrillation: insights into substrate and lesion transmurality. Heart Rhythm 2019;16:1151–9. [DOI] [PubMed] [Google Scholar]
- 91. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 92. Mahnkopf C, Badger TJ, Burgon NS, Daccarett M, Haslam TS, Badger CT, et al. Evaluation of the left atrial substrate in patients with lone atrial fibrillation using delayed-enhanced MRI: implications for disease progression and response to catheter ablation. Heart Rhythm 2010;7:1475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58:2225–32. [DOI] [PubMed] [Google Scholar]
- 94. Kottkamp H. Fibrotic atrial cardiomyopathy: a specific disease/syndrome supplying substrates for atrial fibrillation, atrial tachycardia, sinus node disease, AV node disease, and thromboembolic complications. J Cardiovasc Electrophysiol 2012;23:797–9. [DOI] [PubMed] [Google Scholar]
- 95. Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis A, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol 2015;26:484–92. [DOI] [PubMed] [Google Scholar]
- 96. Chrispin J, Ipek EG, Habibi M, Yang E, Spragg D, Marine JE, et al. Clinical predictors of cardiac magnetic resonance late gadolinium enhancement in patients with atrial fibrillation. Europace 2017;19:371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ben Amar B, Bianca C. Towards a unified approach in the modeling of fibrosis: a review with research perspectives. Phys Life Rev 2016;17:61–85. [DOI] [PubMed] [Google Scholar]
- 98. Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol 2014;29:20–7. [DOI] [PubMed] [Google Scholar]
- 99. Xintarakou A, Tzeis S, Psarras S, Asvestas D, Vardas P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. Europace 2020;22:342–51. [DOI] [PubMed] [Google Scholar]
- 100. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 101. Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 2011;89:744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fedorov VV, Hansen BJ. A secret marriage between fibrosis and atrial fibrillation drivers. JACC Clin Electrophysiol 2018;4:30–2. [DOI] [PubMed] [Google Scholar]
- 103. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 104. Spragg DD, Khurram I, Zimmerman SL, Yarmohammadi H, Barcelon B, Needleman M, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm 2012;9:2003–9. [DOI] [PubMed] [Google Scholar]
- 105. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen J, Arentz T, Cochet H, Müller-Edenborn B, Kim S, Moreno-Weidmann Z, et al. Extent and spatial distribution of left atrial arrhythmogenic sites, late gadolinium enhancement at magnetic resonance imaging, and low-voltage areas in patients with persistent atrial fibrillation: comparison of imaging vs. electrical parameters of fibrosis and arrhythmogenesis. Europace 2019;21:1484–93. [DOI] [PubMed] [Google Scholar]
- 107. Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J 2017;38:53–61. [DOI] [PubMed] [Google Scholar]
- 108. Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol 2020;76:1197–211. [DOI] [PubMed] [Google Scholar]
- 109. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 110. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J 2017;38:1294–302. [DOI] [PubMed] [Google Scholar]
- 111. Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002;106:2091–7. [DOI] [PubMed] [Google Scholar]
- 112. Leone O, Boriani G, Chiappini B, Pacini D, Cenacchi G, Martin Suarez S, et al. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J 2004;25:1237–41. [DOI] [PubMed] [Google Scholar]
- 113. Steiner I, Hajkova P. Patterns of isolated atrial amyloid: a study of 100 hearts on autopsy. Cardiovasc Pathol 2006;15:287–90. [DOI] [PubMed] [Google Scholar]
- 114. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–99. [DOI] [PubMed] [Google Scholar]
- 115. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 116. Caballero R, de la Fuente MG, Gomez R, Barana A, Amoros I, Dolz-Gaiton P, et al. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol 2010;55:2346–54. [DOI] [PubMed] [Google Scholar]
- 117. Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, et al. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 1999;85:428–36. [DOI] [PubMed] [Google Scholar]
- 119. Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, et al. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Deshmukh A, Barnard J, Sun H, Newton D, Castel L, Pettersson G, et al. Left atrial transcriptional changes associated with atrial fibrillation susceptibility and persistence. Circ Arrhythm Electrophysiol 2015;8:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Burstein B, Qi XY, Yeh YH, Calderone A, Nattel S. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovasc Res 2007;76:442–52. [DOI] [PubMed] [Google Scholar]
- 122. Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precedethe onset of postoperative atrial fibrillation. J Am Coll Cardiol 2003;42:1262–8. [DOI] [PubMed] [Google Scholar]
- 123. Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–9. [DOI] [PubMed] [Google Scholar]
- 124. Tomita T, Takei M, Saikawa Y, Hanaoka T, Uchikawa S, Tsutsui H, et al. Role of autonomic tone in the initiation and termination of paroxysmal atrial fibrillation in patients without structural heart disease. J Cardiovasc Electrophysiol 2003;14:559–64. [DOI] [PubMed] [Google Scholar]
- 125. Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol 2005;45:1878–86. [DOI] [PubMed] [Google Scholar]
- 126. Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2:624–31. [DOI] [PubMed] [Google Scholar]
- 127. Zhou J, Scherlag BJ, Edwards J, Jackman WM, Lazzara R, Po SS. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol 2007;18:83–90. [DOI] [PubMed] [Google Scholar]
- 128. Patterson E, Jackman WM, Beckman KJ, Lazzara R, Lockwood D, Scherlag BJ, et al. Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol 2007;18:1067–75. [DOI] [PubMed] [Google Scholar]
- 129. Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation 2000;101:1185–91. [DOI] [PubMed] [Google Scholar]
- 130. Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 2008;1:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Stavrakis S, Stoner JA, Humphrey MB, Morris L, Filiberti A, Reynolds JC, et al. TREAT AF (transcutaneous electrical vagus nerve stimulation to suppress atrial fibrillation): a randomized clinical trial. JACC Clin Electrophysiol 2020;6:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shen MJ, Hao-Che C, Park HW, George Akingba A, Chang PC, Zheng Z, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm 2013;10:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bernstein SA, Wong B, Vasquez C, Rosenberg SP, Rooke R, Kuznekoff LM, et al. Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm 2012;9:1426–33.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, et al. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm 2009;6:1257–64. [DOI] [PubMed] [Google Scholar]
- 135. Katritsis D, Giazitzoglou E, Sougiannis D, Goumas N, Paxinos G, Camm AJ. Anatomic approach for ganglionic plexi ablation in patients with paroxysmal atrial fibrillation. Am J Cardiol 2008;102:330–4. [DOI] [PubMed] [Google Scholar]
- 136. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm 2011;8:672–8. [DOI] [PubMed] [Google Scholar]
- 137. Nakagawa H, Scherlag BJ, Wu R, Po S, Lockwood D, Yokoyama K, et al. Addition of selective ablation of autonomic ganglia to pulmonary vein antrum isolation for treatment of paroxysmal and persistent atrial fibrillation. Circulation 2004;110:543. [Google Scholar]
- 138. Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879–86. [DOI] [PubMed] [Google Scholar]
- 139. Jaïs P, Hocini M, Macle L, Choi KJ, Deisenhofer I, Weerasooriya R, et al. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 2002;106:2479–85. [DOI] [PubMed] [Google Scholar]
- 140. Cherry EM, Ehrlich JR, Nattel S, Fenton FH. Pulmonary vein reentry--properties and size matter: insights from a computational analysis. Heart Rhythm 2007;4:1553–62. [DOI] [PubMed] [Google Scholar]
- 141. Bonczar M, Piątek-Koziej K, Wolska J, Tomala O, Stitou EA, Pękala J, et al. Variations in human pulmonary vein ostia morphology: a systematic review with metaanalysis. Clin Anat 2022;35:906–26. [DOI] [PubMed] [Google Scholar]
- 142. Anselmino M, Blandino A, Beninati S, Rovera C, Boffano C, Belletti M, et al. Morphologic analysis of left atrial anatomy by magnetic resonance angiography in patients with atrial fibrillation: a large single center experience. J Cardiovasc Electrophysiol 2011;22:1–7. [DOI] [PubMed] [Google Scholar]
- 143. Cheruiyot I, Munguti J, Olabu B, Gichangi P. A metaanalysis of the relationship between anatomical variations of pulmonary veins and atrial fibrillation. Acta Cardiol 2020;75:1–9. [DOI] [PubMed] [Google Scholar]
- 144. Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation 1966;34:412–22. [DOI] [PubMed] [Google Scholar]
- 145. Rivaud MR, Blok M, Jongbloed MRM, Boukens BJ. How Cardiac Embryology Translates into Clinical Arrhythmias. J Cardiovasc Dev Dis 2021;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Teres C, Soto-Iglesias D, Penela D, Jáuregui B, Ordoñez A, Chauca A, et al. Left atrial wall thickness of the pulmonary vein reconnection sites during atrial fibrillation redo procedures. Pacing Clin Electrophysiol 2021;44:824–34. [DOI] [PubMed] [Google Scholar]
- 147. Barrio-Lopez MT, Sanchez-Quintana D, Garcia-Martinez J, Betancur A, Castellanos E, Arceluz M, et al. Epicardial connections involving pulmonary veins: the prevalence, predictors, and implications for ablation outcome. Circ Arrhythm Electrophysiol 2020;13:e007544. [DOI] [PubMed] [Google Scholar]
- 148. Perez-Castellano N, Villacastin J, Salinas J, Vega M, Moreno J, Doblado M, et al. Epicardial connections between the pulmonary veins and left atrium: relevance for atrial fibrillation ablation. J Cardiovasc Electrophysiol 2011;22:149–59. [DOI] [PubMed] [Google Scholar]
- 149. Barrio-Lopez MT, Castellanos E, Ortiz M, Arceluz M, Lazaro C, Salas J, et al. Atrial mapping during pulmonary vein pacing to detect conduction gaps in a second pulmonary vein isolation procedure. J Interv Card Electrophysiol 2018;53:195–205. [DOI] [PubMed] [Google Scholar]
- 150. Yoshida K, Baba M, Shinoda Y, Harunari T, Tsumagari Y, Koda N, et al. Epicardial connection between the right-sided pulmonary venous carina and the right atrium in patients with atrial fibrillation: a possible mechanism for preclusion of pulmonary vein isolation without carina ablation. Heart Rhythm 2019;16:671–8. [DOI] [PubMed] [Google Scholar]
- 151. Cabrera JA, Ho SY, Climent V, Fuertes B, Murillo M, Sanchez-Quintana D. Morphological evidence of muscular connections between contiguous pulmonary venous orifices: relevance of the interpulmonary isthmus for catheter ablation in atrial fibrillation. Heart Rhythm 2009;6:1192–8. [DOI] [PubMed] [Google Scholar]
- 152. Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J 2008;29:356–62. [DOI] [PubMed] [Google Scholar]
- 153. Ho SY, Cabrera JA, Sanchez-Quintana D. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol 2012;5:220–8. [DOI] [PubMed] [Google Scholar]
- 154. Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc Res 2002;54:325–36. [DOI] [PubMed] [Google Scholar]
- 155. Ho SY, Sanchez-Quintana D. The importance of atrial structure and fibers. Clin Anat 2009;22:52–63. [DOI] [PubMed] [Google Scholar]
- 156. Patel PJ, D’Souza B, Saha P, Chik WW, Riley MP, Garcia FC. Electroanatomic mapping of the intercaval bundle in atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:1262–7. [DOI] [PubMed] [Google Scholar]
- 157. Anderson RH, Spicer DE, Brown NA, Mohun TJ. The development of septation in the four-chambered heart. Anat Rec 2014;297:1414–29. [DOI] [PubMed] [Google Scholar]
- 158. Klimek-Piotrowska W, Hołda MK, Koziej M, Piątek K, Hołda J. Anatomy of the true interatrial septum for transseptal access to the left atrium. Ann Anat 2016;205:60–4. [DOI] [PubMed] [Google Scholar]
- 159. Tzeis S, Andrikopoulos G, Deisenhofer I, Ho SY, Theodorakis G. Transseptal catheterization: considerations and caveats. Pacing Clin Electrophysiol 2010;33:231–42. [DOI] [PubMed] [Google Scholar]
- 160. Meier D, Antiochos P, Herrera-Siklody C, Eeckhout E, Delabays A, Tzimas G, et al. Interatrial septum dissection and atrial wall hematoma following transseptal puncture: a systematic review of the literature. Catheter Cardiovasc Interv 2020;96:424–31. [DOI] [PubMed] [Google Scholar]
- 161. Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001;38:613–23. [DOI] [PubMed] [Google Scholar]
- 162. Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20. [DOI] [PubMed] [Google Scholar]
- 163. Benvenuti F, Meucci F, Vuolo L, Nistri R, Pracucci G, Picchioni A, et al. Relation between the size of patent foramen ovale and the volume of acute cerebral ischemic lesion in young patients with cryptogenic ischemic stroke. Neurol Sci 2022;43:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Knecht S, Wright M, Lellouche N, Nault I, Matsuo S, O’Neill MD, et al. Impact of a patent foramen ovale on paroxysmal atrial fibrillation ablation. J Cardiovasc Electrophysiol 2008;19:1236–41. [DOI] [PubMed] [Google Scholar]
- 165. Miyazaki S, Shah AJ, Nault I, Wright M, Jadidi AS, Forclaz A, et al. Impact of patent foramen ovale on left atrial linear lesions in the context of atrial fibrillation ablation. J Cardiovasc Electrophysiol 2011;22:846–50. [DOI] [PubMed] [Google Scholar]
- 166. Barrio-Lopez MT, Castellanos E, Betancur A, Zorita B, Medina J, Losada N, et al. The presence of a large patent foramen ovale reduces acute and chronic success in atrial fibrillation ablation. J Interv Card Electrophysiol 2022;64:705–13. [DOI] [PubMed] [Google Scholar]
- 167. Almendarez M, Alvarez-Velasco R, Pascual I, Alperi A, Moris C, Avanzas P. Transseptal puncture: review of anatomy, techniques, complications and challenges, a critical view. Int J Cardiol 2022;351:32–38. [DOI] [PubMed] [Google Scholar]
- 168. De Ponti R, Cappato R, Curnis A, Della Bella P, Padeletti L, Raviele A, et al. Trans-septal catheterization in the electrophysiology laboratory: data from a multicenter survey spanning 12 years. J Am Coll Cardiol 2006;47:1037–42. [DOI] [PubMed] [Google Scholar]
- 169. Li X, Wissner E, Kamioka M, Makimoto H, Rausch P, Metzner A, et al. Safety and feasibility of transseptal puncture for atrial fibrillation ablation in patients with atrial septal defect closure devices. Heart Rhythm 2014;11:330–5. [DOI] [PubMed] [Google Scholar]
- 170. Santangeli P, Di Biase L, Burkhardt JD, Horton R, Sanchez J, Bailey S, et al. Transseptal access and atrial fibrillation ablation guided by intracardiac echocardiography in patients with atrial septal closure devices. Heart Rhythm 2011;8:1669–75. [DOI] [PubMed] [Google Scholar]
- 171. Wang K, Ho SY, Gibson DG, Anderson RH. Architecture of atrial musculature in humans. Br Heart J 1995;73:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Jais P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004;110:2996–3002. [DOI] [PubMed] [Google Scholar]
- 173. Derval N, Takigawa M, Frontera A, Mahida S, Konstantinos V, Denis A, et al. Characterization of complex atrial tachycardia in patients with previous atrial interventions using high-resolution mapping. JACC Clin Electrophysiol 2020;6:815–26. [DOI] [PubMed] [Google Scholar]
- 174. Hocini M, Jais P, Sanders P, Takahashi Y, Rotter M, Rostock T, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation 2005;112:3688–96. [DOI] [PubMed] [Google Scholar]
- 175. Ho SY. Normal and abnormal atrial anatomy relevant to atrial flutters: areas of physiological and acquired conduction blocks and delays predisposing to reentry. Card Electrophysiol Clin 2022;14:375–84. [DOI] [PubMed] [Google Scholar]
- 176. van Campenhout MJ, Yaksh A, Kik C, de Jaegere PP, Ho SY, Allessie MA, et al. Bachmann’s bundle: a key player in the development of atrial fibrillation? Circ Arrhythm Electrophysiol 2013;6:1041–6. [DOI] [PubMed] [Google Scholar]
- 177. Papez J. Heart musculature of the atria. Am J Anat 1920;27:255–85. [Google Scholar]
- 178. Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:1525–33. [DOI] [PubMed] [Google Scholar]
- 179. Pambrun T, Duchateau J, Delgove A, Denis A, Constantin M, Ramirez FD, et al. Epicardial course of the septopulmonary bundle: anatomical considerations and clinical implications for roof line completion. Heart Rhythm 2021;18:349–57. [DOI] [PubMed] [Google Scholar]
- 180. Chauvin M, Shah DC, Haissaguerre M, Marcellin L, Brechenmacher C. The anatomic basis of connections between the coronary sinus musculature and the left atrium in humans. Circulation 2000;101:647–52. [DOI] [PubMed] [Google Scholar]
- 181. von Ludinghausen M, Ohmachi N, Besch S, Mettenleiter A. Atrial veins of the human heart. Clin Anat 1995;8:169–89. [DOI] [PubMed] [Google Scholar]
- 182. Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen PS, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol 2000;36:1324–7. [DOI] [PubMed] [Google Scholar]
- 183. Makino M, Inoue S, Matsuyama TA, Ogawa G, Sakai T, Kobayashi Y, et al. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J Cardiovasc Electrophysiol 2006;17:594–9. [DOI] [PubMed] [Google Scholar]
- 184. Von Lüdinghausen M, Ohmachi N, Boot C. Myocardial coverage of the coronary sinus and related veins. Clin Anat 1992;5:1–15. [Google Scholar]
- 185. xMarshall J. On the development of the great anterior veins in man and mammalia; including an account of certain remnants of foetal structure found in the adult, a comparative view of these great veins in the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philos Trans Roy Soc Lond 1850;140:133–70. [Google Scholar]
- 186. DeSimone CV, Noheria A, Lachman N, Edwards WD, Gami AS, Maleszewski JJ, et al. Myocardium of the superior vena cava, coronary sinus, vein of Marshall, and the pulmonary vein ostia: gross anatomic studies in 620 hearts. J Cardiovasc Electrophysiol 2012;23:1304–9. [DOI] [PubMed] [Google Scholar]
- 187. Baez-Escudero JL, Keida T, Dave AS, Okishige K, Valderrabano M. Ethanol infusion in the vein of Marshall leads to parasympathetic denervation of the human left atrium: implications for atrial fibrillation. J Am Coll Cardiol 2014;63:1892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hwang C, Chen PS. Ligament of Marshall: why it is important for atrial fibrillation ablation. Heart Rhythm 2009;6:S35–40. [DOI] [PubMed] [Google Scholar]
- 189. Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation 2000;101:1503–5. [DOI] [PubMed] [Google Scholar]
- 190. Ulphani JS, Arora R, Cain JH, Villuendas R, Shen S, Gordon D, et al. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol 2007;293:H1629–35. [DOI] [PubMed] [Google Scholar]
- 191. Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol 1999;10:636–48. [DOI] [PubMed] [Google Scholar]
- 192. Wittkampf FH, van Oosterhout MF, Loh P, Derksen R, Vonken EJ, Slootweg PJ, et al. Where to draw the mitral isthmus line in catheter ablation of atrial fibrillation: histological analysis. Eur Heart J 2005;26:689–95. [DOI] [PubMed] [Google Scholar]
- 193. Pambrun T, Denis A, Duchateau J, Sacher F, Hocini M, Jaïs P, et al. Marshall bundles elimination, pulmonary veins isolation and Lines completion for anatomical ablation of persistent atrial fibrillation: Marshall-PLAN case series. J Cardiovasc Electrophysiol 2019;30:7–15. [DOI] [PubMed] [Google Scholar]
- 194. Becker AE. Left atrial isthmus: anatomic aspects relevant for linear catheter ablation procedures in humans. J Cardiovasc Electrophysiol 2004;15:809–12. [DOI] [PubMed] [Google Scholar]
- 195. Baez-Escudero JL, Morales PF, Dave AS, Sasaridis CM, Kim YH, Okishige K, et al. Ethanol infusion in the vein of Marshall facilitates mitral isthmus ablation. Heart Rhythm 2012;9:1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Takigawa M, Vlachos K, Martin CA, Bourier F, Denis A, Kitamura T, et al. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace 2020;22:1252–60. [DOI] [PubMed] [Google Scholar]
- 197. Pambrun T, Derval N, Duchateau J, Denis A, Chauvel R, Tixier R, et al. Epicardial course of the musculature related to the great cardiac vein: anatomical considerations and clinical implications for mitral isthmus block after vein of Marshall ethanol infusion. Heart Rhythm 2021;18:1951–8. [DOI] [PubMed] [Google Scholar]
- 198. Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation 2000;102:67–74. [DOI] [PubMed] [Google Scholar]
- 199. Nyuta E, Takemoto M, Sakai T, Mito T, Masumoto A, Todoroki W, et al. Importance of the length of the myocardial sleeve in the superior vena cava in patients with atrial fibrillation. J Arrhythm 2021;37:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Depes D, Mennander A, Paavonen T, Kholová I. Autonomic nerves in myocardial sleeves around caval veins: potential role in cardiovascular mortality? Cardiovasc Pathol 2022;59:107426. [DOI] [PubMed] [Google Scholar]
- 201. Miyazaki S, Taniguchi H, Kusa S, Ichihara N, Nakamura H, Hachiya H, et al. Factors predicting an arrhythmogenic superior vena cava in atrial fibrillation ablation: insight into the mechanism. Heart Rhythm 2014;11:1560–6. [DOI] [PubMed] [Google Scholar]
- 202. Corradi D, Callegari S, Gelsomino S, Lorusso R, Macchi E. Morphology and pathophysiology of target anatomical sites for ablation procedures in patients with atrial fibrillation: part II: pulmonary veins, caval veins, ganglionated plexi, and ligament of Marshall. Int J Cardiol 2013;168:1769–78. [DOI] [PubMed] [Google Scholar]
- 203. Wickramasinghe SR, Patel VV. Local innervation and atrial fibrillation. Circulation 2013;128:1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kusayama T, Wan J, Yuan Y, Chen PS. Neural mechanisms and therapeutic opportunities for atrial fibrillation. Methodist Debakey Cardiovasc J 2021;17:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol 2005;209:425–38. [DOI] [PubMed] [Google Scholar]
- 206. Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 2014;114:1004–21. [DOI] [PubMed] [Google Scholar]
- 207. Hou Y, Zhou Q, Po SS. Neuromodulation for cardiac arrhythmia. Heart Rhythm 2016;13:584–92. [DOI] [PubMed] [Google Scholar]
- 208. Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247:289–98. [DOI] [PubMed] [Google Scholar]
- 209. Kim MY, Sandler BC, Sikkel MB, Cantwell CD, Leong KM, Luther V, et al. Anatomical distribution of ectopy-triggering plexuses in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008715. [DOI] [PubMed] [Google Scholar]
- 210. Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2009;20:1186–9. [DOI] [PubMed] [Google Scholar]
- 211. Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol 2005;13:37–42. [DOI] [PubMed] [Google Scholar]
- 212. Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 2006;3:387–96. [DOI] [PubMed] [Google Scholar]
- 213. Stirrup J, Gregg S, Baavour R, Roth N, Breault C, Agostini D, et al. Hybrid solid-state SPECT/CT left atrial innervation imaging for identification of left atrial ganglionated plexi: technique and validation in patients with atrial fibrillation. J Nucl Cardiol 2020;27:1939–50. [DOI] [PubMed] [Google Scholar]
- 214. Lemoine MD, Mencke C, Nies M, Obergassel J, Scherschel K, Wieboldt H, et al. Pulmonary vein isolation by pulsed-field ablation induces less neurocardiac damage than cryoballoon ablation. Circ Arrhythm Electrophysiol 2023;16:e011598. [DOI] [PubMed] [Google Scholar]
- 215. Musikantow DR, Neuzil P, Petru J, Koruth JS, Kralovec S, Miller MA, et al. Pulsed field ablation to treat atrial fibrillation: autonomic nervous system effects. JACC Clin Electrophysiol 2023;9:481–93. [DOI] [PubMed] [Google Scholar]
- 216. von Olshausen G, Saluveer O, Schwieler J, Drca N, Bastani H, Tapanainen J, et al. Sinus heart rate post pulmonary vein ablation and long-term risk of recurrences. Clin Res Cardiol 2021;110:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yu HT, Kim TH, Uhm JS, Kim JY, Joung B, Lee MH, et al. Prognosis of high sinus heart rate after catheter ablation for atrial fibrillation. Europace 2017;19:1132–9. [DOI] [PubMed] [Google Scholar]
- 218. Goff ZD, Laczay B, Yenokyan G, Sivasambu B, Sinha SK, Marine JE, et al. Heart rate increase after pulmonary vein isolation predicts freedom from atrial fibrillation at 1 year. J Cardiovasc Electrophysiol 2019;30:2818–22. [DOI] [PubMed] [Google Scholar]
- 219. DeLurgio DB, Crossen KJ, Gill J, Blauth C, Oza SR, Magnano AR, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [DOI] [PubMed] [Google Scholar]
- 220. Makati KJ, Sood N, Lee LS, Yang F, Shults CC, DeLurgio DB, et al. Combined epicardial and endocardial ablation for atrial fibrillation: best practices and guide to hybrid convergent procedures. Heart Rhythm 2021;18:303–12. [DOI] [PubMed] [Google Scholar]
- 221. Kress DC, Erickson L, Choudhuri I, Zilinski J, Mengesha T, Krum D, et al. Comparative effectiveness of hybrid ablation versus endocardial catheter ablation alone in patients with persistent atrial fibrillation. JACC Clin Electrophysiol 2017;3:341–9. [DOI] [PubMed] [Google Scholar]
- 222. Reddy VY, Neuzil P, D’Avila A, Ruskin JN. Isolating the posterior left atrium and pulmonary veins with a “box” lesion set: use of epicardial ablation to complete electrical isolation. J Cardiovasc Electrophysiol 2008;19:326–9. [DOI] [PubMed] [Google Scholar]
- 223. Piorkowski C, Kronborg M, Hourdain J, Piorkowski J, Kirstein B, Neudeck S, et al. Endo-/epicardial catheter ablation of atrial fibrillation: feasibility, outcome, and insights into arrhythmia mechanisms. Circ Arrhythm Electrophysiol 2018;11:e005748. [DOI] [PubMed] [Google Scholar]
- 224. Tung R. Percutaneous epicardial ablation of atrial fibrillation. Card Electrophysiol Clin 2020;12:371–81. [DOI] [PubMed] [Google Scholar]
- 225. D’Avila A, Scanavacca M, Sosa E, Ruskin JN, Reddy VY. Pericardial anatomy for the interventional electrophysiologist. J Cardiovasc Electrophysiol 2003;14:422–30. [DOI] [PubMed] [Google Scholar]
- 226. Smith NM, Segars L, Kauffman T, Olinger AB. Using anatomical landmark to avoid phrenic nerve injury during balloon-based procedures in atrial fibrillation patients. Surg Radiol Anat 2017;39:1369–75. [DOI] [PubMed] [Google Scholar]
- 227. Okumura Y, Henz BD, Bunch TJ, Dalegrave C, Johnson SB, Packer DL. Distortion of right superior pulmonary vein anatomy by balloon catheters as a contributor to phrenic nerve injury. J Cardiovasc Electrophysiol 2009;20:1151–7. [DOI] [PubMed] [Google Scholar]
- 228. Ströker E, de Asmundis C, Saitoh Y, Velagić V, Mugnai G, Irfan G, et al. Anatomic predictors of phrenic nerve injury in the setting of pulmonary vein isolation using the 28-mm second-generation cryoballoon. Heart Rhythm 2016;13:342–51. [DOI] [PubMed] [Google Scholar]
- 229. Romero J, Natale A, Lakkireddy D, Cerna L, Diaz JC, Alviz I, et al. Mapping and localization of the left phrenic nerve during left atrial appendage electrical isolation to avoid inadvertent injury in patients undergoing catheter ablation of atrial fibrillation. Heart Rhythm 2020;17:527–34. [DOI] [PubMed] [Google Scholar]
- 230. Gupta T, Cheema N, Randhawa A, Sahni D. Translational anatomy of the left atrium and esophagus as relevant to the pulmonary vein antral isolation for atrial fibrillation. Surg Radiol Anat 2020;42:367–76. [DOI] [PubMed] [Google Scholar]
- 231. Bunch TJ, May HT, Crandall BG, Weiss JP, Bair TL, Osborn JS, et al. Intracardiac ultrasound for esophageal anatomic assessment and localization during left atrial ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2013;24:33–9. [DOI] [PubMed] [Google Scholar]
- 232. Jang SW, Kwon BJ, Choi MS, Kim DB, Shin WS, Cho EJ, et al. Computed tomographic analysis of the esophagus, left atrium, and pulmonary veins: implications for catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2011;32:1–6. [DOI] [PubMed] [Google Scholar]
- 233. Sarairah SY, Woodbury B, Methachittiphan N, Tregoning DM, Sridhar AR, Akoum N. Esophageal thermal injury following cryoballoon ablation for atrial fibrillation. JACC Clin Electrophysiol 2020;6:262–8. [DOI] [PubMed] [Google Scholar]
- 234. Lemola K, Sneider M, Desjardins B, Case I, Han J, Good E, et al. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation 2004;110:3655–60. [DOI] [PubMed] [Google Scholar]
- 235. Good E, Oral H, Lemola K, Han J, Tamirisa K, Igic P, et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol 2005;46:2107–10. [DOI] [PubMed] [Google Scholar]
- 236. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 237. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349–61. [DOI] [PubMed] [Google Scholar]
- 238. Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 239. Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013;61:1713–23. [DOI] [PubMed] [Google Scholar]
- 240. Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol 2020;75:3105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Mont L, Bisbal F, Hernández-Madrid A, Pérez-Castellano N, Viñolas X, Arenal A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicenter, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 243. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 244. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 245. Kuniss M, Pavlovic N, Velagic V, Hermida JS, Healey S, Arena G, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021;23:1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Cosedis Nielsen C, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–95. [DOI] [PubMed] [Google Scholar]
- 247. Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 248. Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634–40. [DOI] [PubMed] [Google Scholar]
- 249. Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and metaanalysis. Europace 2015;17:370–8. [DOI] [PubMed] [Google Scholar]
- 250. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol 2017;70:1949–61. [DOI] [PubMed] [Google Scholar]
- 251. Sugumar H, Prabhu S, Costello B, Chieng D, Azzopardi S, Voskoboinik A, et al. Catheter ablation versus medication in atrial fibrillation and systolic dysfunction: late outcomes of CAMERA-MRI study. JACC Clin Electrophysiol 2020;6:1721–31. [DOI] [PubMed] [Google Scholar]
- 252. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–8. [DOI] [PubMed] [Google Scholar]
- 253. Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes A, et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation 2022;145:1693–704. [DOI] [PubMed] [Google Scholar]
- 254. Romero J, Gabr M, Alviz I, Briceno D, Diaz JC, Rodriguez D, et al. Improved survival in patients with atrial fibrillation and heart failure undergoing catheter ablation compared to medical treatment: a systematic review and metaanalysis of randomized controlled trials. J Cardiovasc Electrophysiol 2022;33:2356–66. [DOI] [PubMed] [Google Scholar]
- 255. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 256. Sohns C, Zintl K, Zhao Y, Dagher L, Andresen D, Siebels J, et al. Impact of left ventricular function and heart failure symptoms on outcomes post ablation of atrial fibrillation in heart failure: CASTLE-AF trial. Circ Arrhythm Electrophysiol 2020;13:e008461. [DOI] [PubMed] [Google Scholar]
- 257. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter ablation versus medical therapy for atrial fibrillation: a systematic review and metaanalysis of randomized controlled trials. Circ Arrhythm Electrophysiol 2019;12:e007414. [DOI] [PubMed] [Google Scholar]
- 259. Briceño DF, Markman TM, Lupercio F, Romero J, Liang JJ, Villablanca PA, et al. Catheter ablation versus conventional treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: a systematic review and metaanalysis of randomized controlled trials. J Interv Card Electrophysiol 2018;53:19–29. [DOI] [PubMed] [Google Scholar]
- 260. Sohns C, Fox H, Marrouche NF, Crijns H, Costard-Jaeckle A, Bergau L, et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380–9. [DOI] [PubMed] [Google Scholar]
- 261. Sciarra L, Rebecchi M, De Ruvo E, De Luca L, Zuccaro LM, Fagagnini A, et al. How many atrial fibrillation ablation candidates have an underlying supraventricular tachycardia previously unknown? Efficacy of isolated triggering arrhythmia ablation. Europace 2010;12:1707–12. [DOI] [PubMed] [Google Scholar]
- 262. Katritsis DG, Giazitzoglou E, Wood MA, Shepard RK, Parvez B, Ellenbogen KA. Inducible supraventricular tachycardias in patients referred for catheter ablation of atrial fibrillation. Europace 2007;9:785–9. [DOI] [PubMed] [Google Scholar]
- 263. Delise P, Gianfranchi L, Paparella N, Brignole M, Menozzi C, Themistoclakis S, et al. Clinical usefulness of slow pathway ablation in patients with both paroxysmal atrioventricular nodal reentrant tachycardia and atrial fibrillation. Am J Cardiol 1997;79:1421–3. [DOI] [PubMed] [Google Scholar]
- 264. Torbey E, Karam B, Sleiman E, Tabet R, Kirk M, Donaldson D, et al. Incidence and risk factors for atrial fibrillation recurrence after ablation of nodal and atrioventricular reentrant tachycardia: a metaanalysis. Cureus 2020;12:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Inada K, Yamane T, Tokutake K, Yokoyama K, Mishima T, Hioki M, et al. The role of successful catheter ablation in patients with paroxysmal atrial fibrillation and prolonged sinus pauses: outcome during a 5-year follow-up. Europace 2014;16:208–13. [DOI] [PubMed] [Google Scholar]
- 266. Chen YW, Bai R, Lin T, Salim M, Sang CH, Long DY, et al. Pacing or ablation: which is better for paroxysmal atrial fibrillation-related tachycardia-bradycardia syndrome? Pacing Clin Electrophysiol 2014;37:403–11. [DOI] [PubMed] [Google Scholar]
- 267. Scharf C, Veerareddy S, Ozaydin M, Chugh A, Hall B, Cheung P, et al. Clinical significance of inducible atrial flutter during pulmonary vein isolation in patients with atrial fibrillation. J Am Coll Cardiol 2004;43:2057–62. [DOI] [PubMed] [Google Scholar]
- 268. Wazni O, Marrouche NF, Martin DO, Gillinov AM, Saliba W, Saad E, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation 2003;108:2479–83. [DOI] [PubMed] [Google Scholar]
- 269. Pérez FJ, Schubert CM, Parvez B, Pathak V, Ellenbogen KA, Wood MA. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a metaanalysis. Circ Arrhythm Electrophysiol 2009;2:393–401. [DOI] [PubMed] [Google Scholar]
- 270. Prasitlumkum N, Tokavanich N, Trongtorsak A, Cheungpasitporn W, Kewcharoen J, Chokesuwattanaskul R, et al. Catheter ablation for atrial fibrillation in the elderly >75 years old: systematic review and metaanalysis. J Cardiovasc Electrophysiol 2022;33:1435–49. [DOI] [PubMed] [Google Scholar]
- 271. Kawamura I, Aikawa T, Yokoyama Y, Takagi H, Kuno T. Catheter ablation for atrial fibrillation in elderly patients: systematic review and a metaanalysis. Pacing Clin Electrophysiol 2022;45:59–71. [DOI] [PubMed] [Google Scholar]
- 272. Nielsen J, Kragholm KH, Christensen SB, Johannessen A, Torp-Pedersen C, Kristiansen SB, et al. Periprocedural complications and one-year outcomes after catheter ablation for treatment of atrial fibrillation in elderly patients: a nationwide Danish cohort study. J Geriatr Cardiol 2021;18:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Providencia R, Elliott P, Patel K, McCready J, Babu G, Srinivasan N, et al. Catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and metaanalysis. Heart 2016;102:1533–43. [DOI] [PubMed] [Google Scholar]
- 274. Zhao DS, Shen Y, Zhang Q, Lin G, Lu YH, Chen BT, et al. Outcomes of catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a systematic review and metaanalysis. Europace 2016;18:508–20. [DOI] [PubMed] [Google Scholar]
- 275. Dinshaw L, Münkler P, Schäffer B, Klatt N, Jungen C, Dickow J, et al. Ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: treatment strategy, characteristics of consecutive atrial tachycardia and long-term outcome. J Am Heart Assoc 2021;10:e017451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Creta A, Elliott P, Earley MJ, Dhinoja M, Finlay M, Sporton S, et al. Catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a European observational multicenter study. Europace 2021;23:1409–17. [DOI] [PubMed] [Google Scholar]
- 277. Santangeli P, Di Biase L, Themistoclakis S, Raviele A, Schweikert RA, Lakkireddy D, et al. Catheter ablation of atrial fibrillation in hypertrophic cardiomyopathy: long-term outcomes and mechanisms of arrhythmia recurrence. Circ Arrhythm Electrophysiol 2013;6:1089–94. [DOI] [PubMed] [Google Scholar]
- 278. Rozen G, Elbaz-Greener G, Marai I, Andria N, Hosseini SM, Biton Y, et al. Utilization and complications of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Heart Assoc 2020;9:e015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Ezzeddine FM, Agboola KM, Hassett LC, Killu AM, Del-Carpio Munoz F, DeSimone CV, et al. Catheter ablation of atrial fibrillation in patients with and without hypertrophic cardiomyopathy: systematic review and metaanalysis. Europace 2023;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Dorian P, Guerra PG, Kerr CR, O’Donnell SS, Crystal E, Gillis AM, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol 2009;2:218–24. [DOI] [PubMed] [Google Scholar]
- 281. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace 2007;9:1006–23. [DOI] [PubMed] [Google Scholar]
- 282. Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation 2012;125:2933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Sugishita K, Shiono E, Sugiyama T, Ashida T. Diabetes influences the cardiac symptoms related to atrial fibrillation. Circ J 2003;67:835–8. [DOI] [PubMed] [Google Scholar]
- 284. Hermans ANL, Gawalko M, Slegers DPJ, Andelfinger N, Pluymaekers N, Verhaert DVM, et al. Mobile app-based symptom-rhythm correlation assessment in patients with persistent atrial fibrillation. Int J Cardiol 2022;367:29–37. [DOI] [PubMed] [Google Scholar]
- 285. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 286. Freemantle N, Lafuente-Lafuente C, Mitchell S, Eckert L, Reynolds M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011;13:329–45. [DOI] [PubMed] [Google Scholar]
- 287. Valembois L, Audureau E, Takeda A, Jarzebowski W, Belmin J, Lafuente-Lafuente C. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2019;9:Cd005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Chew DS, Black-Maier E, Loring Z, Noseworthy PA, Packer DL, Exner DV, et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and metaanalysis of observational studies. Circ Arrhythm Electrophysiol 2020;13:e008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Kawaji T, Shizuta S, Yamagami S, Aizawa T, Komasa A, Yoshizawa T, et al. Early choice for catheter ablation reduced readmission in management of atrial fibrillation: impact of diagnosis-to-ablation time. Int J Cardiol 2019;291:69–76. [DOI] [PubMed] [Google Scholar]
- 290. Bisbal F, Alarcón F, Ferrero-De-Loma-Osorio A, González-Ferrer JJ, Alonso-Martín C, Pachón M, et al. Diagnosis-to-ablation time in atrial fibrillation: a modifiable factor relevant to clinical outcome. J Cardiovasc Electrophysiol 2019;30:1483–90. [DOI] [PubMed] [Google Scholar]
- 291. Kalman JM, Al-Kaisey AM, Parameswaran R, Hawson J, Anderson RD, Lim M, et al. Impact of early vs. delayed atrial fibrillation catheter ablation on atrial arrhythmia recurrences. Eur Heart J 2023;44:2447–54. [DOI] [PubMed] [Google Scholar]
- 292. Andrade JG, Wazni OM, Kuniss M, Hawkins NM, Deyell MW, Chierchia GB, et al. Cryoballoon ablation as initial treatment for atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:914–30. [DOI] [PubMed] [Google Scholar]
- 293. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 294. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 295. Walters TE, Wick K, Tan G, Mearns M, Joseph SA, Morton JB, et al. Psychological distress and suicidal ideation in patients with atrial fibrillation: prevalence and response to management strategy. J Am Heart Assoc 2018;7:e005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Al-Kaisey AM, Parameswaran R, Bryant C, Anderson RD, Hawson J, Chieng D, et al. Atrial fibrillation catheter ablation vs medical therapy and psychological distress: a randomized clinical trial. JAMA 2023;330:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi F, et al. Results from a multicenter comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace 2017;19:48–57. [DOI] [PubMed] [Google Scholar]
- 298. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128:2104–12. [DOI] [PubMed] [Google Scholar]
- 299. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Monahan KH, Bunch TJ, Mark DB, Poole JE, Bahnson TD, Al-Khalidi HR, et al. Influence of atrial fibrillation type on outcomes of ablation vs. drug therapy: results from CABANA. Europace 2022;24:1430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008;359:1778–85. [DOI] [PubMed] [Google Scholar]
- 302. MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 2011;97:740–7. [DOI] [PubMed] [Google Scholar]
- 303. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–903. [DOI] [PubMed] [Google Scholar]
- 304. Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol 2019;12:e007731. [DOI] [PubMed] [Google Scholar]
- 305. Chen S, Pürerfellner H, Meyer C, Acou WJ, Schratter A, Ling Z, et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2020;41:2863–73. [DOI] [PubMed] [Google Scholar]
- 306. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 307. Bergonti M, Spera F, Tijskens M, Bonomi A, Saenen J, Huybrechts W, et al. A new prediction model for left ventricular systolic function recovery after catheter ablation of atrial fibrillation in patients with heart failure: the ANTWOORD Study. Int J Cardiol 2022;358:45–50. [DOI] [PubMed] [Google Scholar]
- 308. Bergonti M, Ascione C, Marcon L, Pambrun T, Della Rocca DG, Ferrero TG, et al. Left ventricular functional recovery after atrial fibrillation catheter ablation in heart failure: a prediction model. Eur Heart J 2023;44:3327–5. [DOI] [PubMed] [Google Scholar]
- 309. Kawaji T, Shizuta S, Aizawa T, Yamagami S, Kato M, Yokomatsu T, et al. Impact of catheter ablation for atrial fibrillation on cardiac disorders in patients with coexisting heart failure. ESC Heart Fail 2021;8:670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Okada M, Tanaka N, Oka T, Tanaka K, Ninomiya Y, Hirao Y, et al. Clinical significance of left ventricular reverse remodeling after catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction. J Cardiol 2021;77:500–8. [DOI] [PubMed] [Google Scholar]
- 311. Kirstein B, Neudeck S, Gaspar T, Piorkowski J, Wechselberger S, Kronborg MB, et al. Left atrial fibrosis predicts left ventricular ejection fraction response after atrial fibrillation ablation in heart failure patients: the Fibrosis-HF study. Europace 2020;22:1812–21. [DOI] [PubMed] [Google Scholar]
- 312. Tsuji A, Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, et al. Impact of the temporal relationship between atrial fibrillation and heart failure on prognosis after ablation. Circ J 2020;84:1467–74. [DOI] [PubMed] [Google Scholar]
- 313. Ishiguchi H, Yoshiga Y, Shimizu A, Ueyama T, Fukuda M, Kato T, et al. Long-term events following catheter-ablation for atrial fibrillation in heart failure with preserved ejection fraction. ESC Heart Fail 2022;9:3505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Smit MD, Moes ML, Maass AH, Achekar ID, Van Geel PP, Hillege HL, et al. The importance of whether atrial fibrillation or heart failure develops first. Eur J Heart Fail 2012;14:1030–40. [DOI] [PubMed] [Google Scholar]
- 315. von Olshausen G, Benson L, Dahlström U, Lund LH, Savarese G, Braunschweig F. Catheter ablation for patients with atrial fibrillation and heart failure: insights from the Swedish Heart Failure Registry. Eur J Heart Fail 2022;24:1636–46. [DOI] [PubMed] [Google Scholar]
- 316. Shiraishi Y, Kohsaka S, Ikemura N, Kimura T, Katsumata Y, Tanimoto K, et al. Catheter ablation for patients with atrial fibrillation and heart failure with reduced and preserved ejection fraction: insights from the KiCS-AF multicenter cohort study. Europace 2023;25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Gu G, Wu J, Gao X, Liu M, Jin C, Xu Y. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction: a metaanalysis. Clin Cardiol 2022;45:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Yamauchi R, Morishima I, Okumura K, Kanzaki Y, Morita Y, Takagi K, et al. Catheter ablation for non-paroxysmal atrial fibrillation accompanied by heart failure with preserved ejection fraction: feasibility and benefits in functions and B-type natriuretic peptide. Europace 2021;23:1252–61. [DOI] [PubMed] [Google Scholar]
- 319. Rordorf R, Scazzuso F, Chun KRJ, Khelae SK, Kueffer FJ, Braegelmann KM, et al. Cryoballoon ablation for the treatment of atrial fibrillation in patients with concomitant heart failure and either reduced or preserved left ventricular ejection fraction: results from the Cryo AF Global Registry. J Am Heart Assoc 2021;10:e021323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Aldaas OM, Lupercio F, Darden D, Mylavarapu PS, Malladi CL, Han FT, et al. Meta-analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol 2021;142:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Black-Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018;15:651–7. [DOI] [PubMed] [Google Scholar]
- 322. Chieng D, Sugumar H, Segan L, Tan C, Vizi D, Nanayakkara S, et al. Atrial fibrillation ablation for heart failure with preserved ejection fraction. JACC Heart Fail 2023;11:646–58. [DOI] [PubMed] [Google Scholar]
- 323. Ganapathy AV, Monjazeb S, Ganapathy KS, Shanoon F, Razavi M. “Asymptomatic” persistent or permanent atrial fibrillation: a misnomer in selected patients. Int J Cardiol 2015;185:112–3. [DOI] [PubMed] [Google Scholar]
- 324. Shin DI, Jaekel K, Schley P, Sause A, Müller M, Fueth R, et al. Plasma levels of NT-pro-BNP in patients with atrial fibrillation before and after electrical cardioversion. Z Kardiol 2005;94:795–800. [DOI] [PubMed] [Google Scholar]
- 325. Stojadinović P, Deshraju A, Wichterle D, Fukunaga M, Peichl P, Kautzner J, et al. The hemodynamic effect of simulated atrial fibrillation on left ventricular function. J Cardiovasc Electrophysiol 2022;33:2569–77. [DOI] [PubMed] [Google Scholar]
- 326. Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol 1997;30:1039–45. [DOI] [PubMed] [Google Scholar]
- 327. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and metaanalysis. Eur Heart J 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 328. Link MS, Giugliano RP, Ruff CT, Scirica BM, Huikuri H, Oto A, et al. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the ENGAGE AF-TIMI 48 trial (effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48). Circ Arrhythm Electrophysiol 2017;10:e004267. [DOI] [PubMed] [Google Scholar]
- 329. Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Gellér L, Kalējs O, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Bahnson TD, Giczewska A, Mark DB, Russo AM, Monahan KH, Al-Khalidi HR, et al. Association between age and outcomes of catheter ablation versus medical therapy for atrial fibrillation: results from the CABANA trial. Circulation 2022;145:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns H, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2022;43:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Sauer WH, Alonso C, Zado E, Cooper JM, Lin D, Dixit S, et al. Atrioventricular nodal reentrant tachycardia in patients referred for atrial fibrillation ablation: response to ablation that incorporates slow-pathway modification. Circulation 2006;114:191–5. [DOI] [PubMed] [Google Scholar]
- 334. Hamer ME, Wilkinson WE, Clair WK, Page RL, McCarthy EA, Pritchett EL. Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. J Am Coll Cardiol 1995;25:984–8. [DOI] [PubMed] [Google Scholar]
- 335. Dagres N, Clague JR, Lottkamp H, Hindricks G, Breithardt G, Borggrefe M. Impact of radiofrequency catheter ablation of accessory pathways on the frequency of atrial fibrillation during long-term follow-up; high recurrence rate of atrial fibrillation in patients older than 50 years of age. Eur Heart J 2001;22:423–7. [DOI] [PubMed] [Google Scholar]
- 336. Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu LF, Scavée C, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 2003;108:1172–5. [DOI] [PubMed] [Google Scholar]
- 337. Akoum N, McGann C, Vergara G, Badger T, Ranjan R, Mahnkopf C, et al. Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant. J Cardiovasc Electrophysiol 2012;23:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Calkins H. Should catheter ablation be the preferred approach for treatment of atrial fibrillation related symptomatic sinus node dysfunction? Pacing Clin Electrophysiol 2014;37:401–2. [DOI] [PubMed] [Google Scholar]
- 339. Merino JL. Slow conduction and flutter following atrial fibrillation ablation: proarrhythmia or unmasking effect of radiofrequency application? J Cardiovasc Electrophysiol 2006;17:516–9. [DOI] [PubMed] [Google Scholar]
- 340. Hwang TH, Yu HT, Kim TH, Uhm JS, Kim JY, Joung B, et al. Permanent pacemaker implantations after catheter ablation in patients with atrial fibrillation associated with underlying sinus node dysfunction. Korean Circ J 2020;50:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol 2008;51:779–86. [DOI] [PubMed] [Google Scholar]
- 342. Gupta D, Ding WY, Calvert P, Williams E, Das M, Tovmassian L, et al. Cryoballoon pulmonary vein isolation as first-line treatment for typical atrial flutter. Heart 2023;109:364–71. [DOI] [PubMed] [Google Scholar]
- 343. Chugh A, Oral H, Lemola K, Hall B, Cheung P, Good E, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2005;2:464–71. [DOI] [PubMed] [Google Scholar]
- 344. Chen J, Hocini M, Larsen TB, Proclemer A, Sciaraffia E, Blomström-Lundqvist C. Clinical management of arrhythmias in elderly patients: results of the European Heart Rhythm Association survey. Europace 2015;17:314–7. [DOI] [PubMed] [Google Scholar]
- 345. Ikenouchi T, Nitta J, Nitta G, Kato S, Iwasaki T, Murata K, et al. Propensity-matched comparison of cryoballoon and radiofrequency ablation for atrial fibrillation in elderly patients. Heart Rhythm 2019;16:838–45. [DOI] [PubMed] [Google Scholar]
- 346. Guttmann OP, Rahman MS, O’Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart 2014;100:465–72. [DOI] [PubMed] [Google Scholar]
- 347. Rowin EJ, Orfanos A, Estes NAM, Wang W, Link MS, Maron MS, et al. Occurrence and natural history of clinically silent episodes of atrial fibrillation in hypertrophic cardiomyopathy. Am J Cardiol 2017;119:1862–5. [DOI] [PubMed] [Google Scholar]
- 348. Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, et al. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation 2017;136:2420–36. [DOI] [PubMed] [Google Scholar]
- 349. Asad Z, Abbas M, Javed I, Korantzopoulos P, Stavrakis S. Obesity is associated with incident atrial fibrillation independent of gender: a metaanalysis. J Cardiovasc Electrophysiol 2018;29:725–32. [DOI] [PubMed] [Google Scholar]
- 350. Sivasambu B, Balouch MA, Zghaib T, Bajwa RJ, Chrispin J, Berger RD, et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol 2018;29:239–45. [DOI] [PubMed] [Google Scholar]
- 351. Glover BM, Hong KL, Dagres N, Arbelo E, Laroche C, Riahi S, et al. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart 2019;105:244–50. [DOI] [PubMed] [Google Scholar]
- 352. Shoemaker MB, Muhammad R, Farrell M, Parvez B, White BW, Streur M, et al. Relation of morbid obesity and female gender to risk of procedural complications in patients undergoing atrial fibrillation ablation. Am J Cardiol 2013;111:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 354. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 355. Zhang D, Ma Y, Xu J, Yi F. Association between obstructive sleep apnea (OSA) and atrial fibrillation (AF): a dose-response metaanalysis. Medicine 2022;101:e29443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Szymanski FM, Filipiak KJ, Platek AE, Hrynkiewicz-Szymanska A, Kotkowski M, Kozluk E, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations—a long-term prospective, cross-sectional cohort study. Sleep Breath 2015;19:849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Li L, Wang ZW, Li J, Ge X, Guo LZ, Wang Y, et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a metaanalysis of observational studies. Europace 2014;16:1309–14. [DOI] [PubMed] [Google Scholar]
- 358. Kawakami H, Nagai T, Fujii A, Uetani T, Nishimura K, Inoue K, et al. Apnea-hypopnea index as a predictor of atrial fibrillation recurrence following initial pulmonary vein isolation: usefulness of type-3 portable monitor for sleep-disordered breathing. J Interv Card Electrophysiol 2016;47:237–244. [DOI] [PubMed] [Google Scholar]
- 359. Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011;108:47–51. [DOI] [PubMed] [Google Scholar]
- 360. Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331–7. [DOI] [PubMed] [Google Scholar]
- 361. Li X, Zhou X, Xu X, Dai J, Chen C, Ma L, et al. Effects of continuous positive airway pressure treatment in obstructive sleep apnea patients with atrial fibrillation: a metaanalysis. Medicine 2021;100:e25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Nalliah CJ, Wong GR, Lee G, Voskoboinik A, Kee K, Goldin J, et al. Impact of CPAP on the atrial fibrillation substrate in obstructive sleep apnea: the SLEEP-AF study. JACC Clin Electrophysiol 2022;8:869–77. [DOI] [PubMed] [Google Scholar]
- 363. Hunt TE, Traaen GM, Aakerøy L, Bendz C, Øverland B, Akre H, et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: a randomized controlled trial. Heart Rhythm 2022;19:1433–41. [DOI] [PubMed] [Google Scholar]
- 364. Donnellan E, Aagaard P, Kanj M, Jaber W, Elshazly M, Hoosien M, et al. Association between preablation glycemic control and outcomes among patients with diabetes undergoing atrial fibrillation ablation. JACC Clin Electrophysiol 2019;5:897–903. [DOI] [PubMed] [Google Scholar]
- 365. Takahashi Y, Nitta J, Kobori A, Sakamoto Y, Nagata Y, Tanimoto K, et al. Alcohol consumption reduction and clinical outcomes of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2021;14:e009770. [DOI] [PubMed] [Google Scholar]
- 366. Cheng WH, Lo LW, Lin YJ, Chang SL, Hu YF, Hung Y, et al. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J Cardiovasc Electrophysiol 2018;29:699–706. [DOI] [PubMed] [Google Scholar]
- 367. Bertaglia E, Anselmino M, Zorzi A, Russo V, Toso E, Peruzza F, et al. NOACs and atrial fibrillation: incidence and predictors of left atrial thrombus in the real world. Int J Cardiol 2017;249:179–83. [DOI] [PubMed] [Google Scholar]
- 368. Lurie A, Wang J, Hinnegan KJ, McIntyre WF, Belley-Côté EP, Amit G, et al. Prevalence of left atrial thrombus in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2021;77:2875–86. [DOI] [PubMed] [Google Scholar]
- 369. Lip GY, Frison L, Grind M. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med 2008;264:50–61. [DOI] [PubMed] [Google Scholar]
- 370. Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J 2013;34:2464–71. [DOI] [PubMed] [Google Scholar]
- 371. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015;36:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Jung H, Sung JH, Yang PS, Jang E, Yu HT, Kim TH, et al. Stroke risk stratification for atrial fibrillation patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2018;72:2409–11. [DOI] [PubMed] [Google Scholar]
- 373. Manjunath CN, Srinivasa KH, Panneerselvam A, Prabhavathi B, Ravindranath KS, Rangan K, et al. Incidence and predictors of left atrial thrombus in patients with rheumatic mitral stenosis and sinus rhythm: a transesophageal echocardiographic study. Echocardiography 2011;28:457–60. [DOI] [PubMed] [Google Scholar]
- 374. Saidi SJ, Motamedi MH. Incidence and factors influencing left atrial clot in patients with mitral stenosis and normal sinus rhythm. Heart 2004;90:1342–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Ahmed K, Rehman Memon A, Liaquat H Sr, Mujtaba M, Parkash C, Sultan FAT, et al. The frequency of left atrial thrombus on transthoracic echocardiogram in patients with mitral stenosis. Cureus 2020;12:e7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007;116:2420–6. [DOI] [PubMed] [Google Scholar]
- 377. Feng D, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009;119:2490–7. [DOI] [PubMed] [Google Scholar]
- 378. El-Am EA, Dispenzieri A, Melduni RM, Ammash NM, White RD, Hodge DO, et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol 2019;73:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Touboul O, Algalarrondo V, Oghina S, Elbaz N, Rouffiac S, Hamon D, et al. Electrical cardioversion of atrial arrhythmias with cardiac amyloidosis in the era of direct oral anticogulants. ESC Heart Fail 2022;9:3556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Burczak DR, Julakanti RR, Kara Balla A, Scott CG, Geske JB, Ommen SR, et al. Risk of left atrial thrombus in patients with hypertrophic cardiomyopathy and atrial fibrillation. J Am Coll Cardiol 2023;82:278–9. [DOI] [PubMed] [Google Scholar]
- 381. Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Horton R, Gallinghouse GJ, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation 2010;121:2550–6. [DOI] [PubMed] [Google Scholar]
- 382. Santangeli P, Di Biase L, Horton R, Burkhardt JD, Sanchez J, Al-Ahmad A, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: evidence from a metaanalysis. Circ Arrhythm Electrophysiol 2012;5:302–11. [DOI] [PubMed] [Google Scholar]
- 383. Wazni OM, Beheiry S, Fahmy T, Barrett C, Hao S, Patel D, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio: comparison of strategies of anticoagulation management in the periprocedural period. Circulation 2007;116:2531–4. [DOI] [PubMed] [Google Scholar]
- 384. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of coumadin in preventing thromboembolism in atrial fibrillation (AF) patients undergoing catheter ablation (COMPARE) randomized trial. Circulation 2014;129:2638–44. [DOI] [PubMed] [Google Scholar]
- 385. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–36. [DOI] [PubMed] [Google Scholar]
- 387. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbüchel H, Mont L, et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Cardoso R, Knijnik L, Bhonsale A, Miller J, Nasi G, Rivera M, et al. An updated metaanalysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Heart Rhythm 2018;15:107–15. [DOI] [PubMed] [Google Scholar]
- 390. Romero J, Cerrud-Rodriguez RC, Alviz I, Diaz JC, Rodriguez D, Arshad S, et al. Significant benefit of uninterrupted DOACs versus VKA during catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2019;5:1396–405. [DOI] [PubMed] [Google Scholar]
- 391. Di Biase L, Callans D, Haeusler KG, Hindricks G, Al-Khalidi H, Mont L, et al. Rationale and design of AXAFA-AFNET 5: an investigator-initiated, randomized, open, blinded outcome assessment, multicentre trial to comparing continuous apixaban to vitamin K antagonists in patients undergoing atrial fibrillation catheter ablation. Europace 2017;19:132–8. [DOI] [PubMed] [Google Scholar]
- 392. Romero J, Cerrud-Rodriguez RC, Diaz JC, Michaud GF, Taveras J, Alviz I, et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: a systematic review and metaanalysis of randomized controlled trials. Europace 2018;20:1612–20. [DOI] [PubMed] [Google Scholar]
- 393. Di Biase L, Lakkireddy D, Trivedi C, Deneke T, Martinek M, Mohanty S, et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm 2015;12:1162–8. [DOI] [PubMed] [Google Scholar]
- 394. Yu HT, Shim J, Park J, Kim TH, Uhm JS, Kim JY, et al. When is it appropriate to stop non-vitamin K antagonist oral anticoagulants before catheter ablation of atrial fibrillation? A multicenter prospective randomized study. Eur Heart J 2019;40:1531–7. [DOI] [PubMed] [Google Scholar]
- 395. Reynolds MR, Allison JS, Natale A, Weisberg IL, Ellenbogen KA, Richards M, et al. A Prospective randomized trial of apixaban dosing during atrial fibrillation ablation: the AEIOU trial. JACC Clin Electrophysiol 2018;4:580–8. [DOI] [PubMed] [Google Scholar]
- 396. Nakamura K, Naito S, Sasaki T, Take Y, Minami K, Kitagawa Y, et al. Uninterrupted vs. interrupted periprocedural direct oral anticoagulants for catheter ablation of atrial fibrillation: a prospective randomized single-center study on postablation thromboembolic and haemorrhagic events. Europace 2019;21:259–67. [DOI] [PubMed] [Google Scholar]
- 397. Ando M, Inden Y, Yoshida Y, Sairaku A, Yanagisawa S, Suzuki H, et al. Differences in prothrombotic response between the uninterrupted and interrupted apixaban therapies in patients undergoing cryoballoon ablation for paroxysmal atrial fibrillation: a randomized controlled study. Heart Vessels 2019;34:1533–41. [DOI] [PubMed] [Google Scholar]
- 398. Nagao T, Suzuki H, Matsunaga S, Nishikawa Y, Harada K, Mamiya K, et al. Impact of periprocedural anticoagulation therapy on the incidence of silent stroke after atrial fibrillation ablation in patients receiving direct oral anticoagulants: uninterrupted vs. interrupted by one dose strategy. Europace 2019;21:590–7. [DOI] [PubMed] [Google Scholar]
- 399. Patel K, Natale A, Yang R, Trivedi C, Romero J, Briceno D, et al. Is transesophageal echocardiography necessary in patients undergoing ablation of atrial fibrillation on an uninterrupted direct oral anticoagulant regimen? Results from a prospective multicenter registry. Heart Rhythm 2020;17:2093–9. [DOI] [PubMed] [Google Scholar]
- 400. Wang Y, Zhao Y, Zhou K, Zei PC, Wang Y, Cheng H, et al. Intracardiac echocardiography is a safe and effective alternative to transesophageal echocardiography for left atrial appendage thrombus evaluation at the time of atrial fibrillation ablation: the ICE-TEE study. Pacing Clin Electrophysiol 2023;46:3–10. [DOI] [PubMed] [Google Scholar]
- 401. Morton JB, Sanders P, Sparks PB, Morgan J, Kalman JM. Usefulness of phased-array intracardiac echocardiography for the assessment of left atrial mechanical “stunning” in atrial flutter and comparison with multiplane transesophageal echocardiography(*). Am J Cardiol 2002;90:741–6. [DOI] [PubMed] [Google Scholar]
- 402. Baran J, Stec S, Pilichowska-Paszkiet E, Zaborska B, Sikora-Frąc M, Kryński T, et al. Intracardiac echocardiography for detection of thrombus in the left atrial appendage: comparison with transesophageal echocardiography in patients undergoing ablation for atrial fibrillation: the Action-Ice I study. Circ Arrhythm Electrophysiol 2013;6:1074–81. [DOI] [PubMed] [Google Scholar]
- 403. Tsyganov A, Shapieva A, Sandrikov V, Fedulova S, Mironovich S, Dzeranova A, et al. Transesophageal vs. intracardiac echocardiographic screening in patients undergoing atrial fibrillation ablation with uninterrupted rivaroxaban. BMC Cardiovasc Disord 2017;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Saksena S, Sra J, Jordaens L, Kusumoto F, Knight B, Natale A, et al. A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: the intracardiac echocardiography guided cardioversion helps interventional procedures study. Circ Arrhythm Electrophysiol 2010;3:571–7. [DOI] [PubMed] [Google Scholar]
- 405. Sriram CS, Banchs JE, Moukabary T, Moradkhan R, Gonzalez MD. Detection of left atrial thrombus by intracardiac echocardiography in patients undergoing ablation of atrial fibrillation. J Interv Card Electrophysiol 2015;43:227–36. [DOI] [PubMed] [Google Scholar]
- 406. Yu S, Zhang H, Li H. Cardiac computed tomography versus transesophageal echocardiography for the detection of left atrial appendage thrombus: a systemic review and metaanalysis. J Am Heart Assoc 2021;10:e022505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Vira T, Pechlivanoglou P, Connelly K, Wijeysundera HC, Roifman I. Cardiac computed tomography and magnetic resonance imaging vs. transoesophageal echocardiography for diagnosing left atrial appendage thrombi. Europace 2019;21:e1–10. [DOI] [PubMed] [Google Scholar]
- 408. Spagnolo P, Giglio M, Di Marco D, Cannaò PM, Agricola E, Della Bella PE, et al. Diagnosis of left atrial appendage thrombus in patients with atrial fibrillation: delayed contrast-enhanced cardiac CT. Eur Radiol 2021;31:1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, et al. Preprocedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 2007;28:836–41. [DOI] [PubMed] [Google Scholar]
- 410. Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol 2010;21:1071–8. [DOI] [PubMed] [Google Scholar]
- 411. Arya A, Hindricks G, Sommer P, Huo Y, Bollmann A, Gaspar T, et al. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace 2010;12:173–80. [DOI] [PubMed] [Google Scholar]
- 412. Santoro F, Di Biase L, Trivedi C, Burkhardt JD, Paoletti Perini A, Sanchez J, et al. Impact of uncontrolled hypertension on atrial fibrillation ablation outcome. JACC Clin Electrophysiol 2015;1:164–73. [DOI] [PubMed] [Google Scholar]
- 413. Anselmino M, Matta M, D’Ascenzo F, Pappone C, Santinelli V, Bunch TJ, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and metaanalysis. Europace 2015;17:1518–25. [DOI] [PubMed] [Google Scholar]
- 414. Bogossian H, Frommeyer G, Brachmann J, Lewalter T, Hoffmann E, Kuck KH, et al. Catheter ablation of atrial fibrillation and atrial flutter in patients with diabetes mellitus: who benefits and who does not? Data from the German ablation registry. Int J Cardiol 2016;214:25–30. [DOI] [PubMed] [Google Scholar]
- 415. Creta A, Providencia R, Adragao P, de Asmundis C, Chun J, Chierchia G, et al. Impact of type-2 diabetes mellitus on the outcomes of catheter ablation of atrial fibrillation (European observational multicenter study). Am J Cardiol 2020;125:901–6. [DOI] [PubMed] [Google Scholar]
- 416. Wang A, Truong T, Black-Maier E, Green C, Campbell KB, Barnett AS, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus. Heart Rhythm O2 2020;1:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Deshmukh A, Ghannam M, Liang J, Saeed M, Cunnane R, Ghanbari H, et al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:1232–9. [DOI] [PubMed] [Google Scholar]
- 418. De Maat GE, Mulder BA, Berretty WL, Al-Jazairi MIH, Tan ES, Wiesfeld ACP, et al. Obesity is associated with impaired long-term success of pulmonary vein isolation: a plea for risk factor management before ablation. Open Heart 2018;5:e000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Winkle RA, Mead RH, Engel G, Kong MH, Fleming W, Salcedo J, et al. Impact of obesity on atrial fibrillation ablation: patient characteristics, long-term outcomes, and complications. Heart Rhythm 2017;14:819–27. [DOI] [PubMed] [Google Scholar]
- 420. Chang SL, Tuan TC, Tai CT, Lin YJ, Lo LW, Hu YF, et al. Comparison of outcome in catheter ablation of atrial fibrillation in patients with versus without the metabolic syndrome. Am J Cardiol 2009;103:67–72. [DOI] [PubMed] [Google Scholar]
- 421. Tang RB, Dong JZ, Liu XP, Long DY, Yu RH, Kalifa J, et al. Metabolic syndrome and risk of recurrence of atrial fibrillation after catheter ablation. Circ J 2009;73:438–43. [DOI] [PubMed] [Google Scholar]
- 422. Mohanty S, Mohanty P, Di Biase L, Bai R, Pump A, Santangeli P, et al. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J Am Coll Cardiol 2012;59:1295–301. [DOI] [PubMed] [Google Scholar]
- 423. Donnellan E, Wazni OM, Harb S, Kanj M, Saliba WI, Jaber WA. Higher baseline cardiorespiratory fitness is associated with lower arrhythmia recurrence and death after atrial fibrillation ablation. Heart Rhythm 2020;17:1687–93. [DOI] [PubMed] [Google Scholar]
- 424. Mandsager KT, Phelan DM, Diab M, Baranowski B, Saliba WI, Tarakji KG, et al. Outcomes of pulmonary vein isolation in athletes. JACC Clin Electrophysiol 2020;6:1265–74. [DOI] [PubMed] [Google Scholar]
- 425. Liu MB, Lee JZ, Klooster L, Petty SA, Scott LR. Influence of endurance sports on atrial fibrillation ablation outcomes. J Arrhythm 2022;38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Koopman P, Nuyens D, Garweg C, La Gerche A, De Buck S, Van Casteren L, et al. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace 2011;13:1386–93. [DOI] [PubMed] [Google Scholar]
- 427. Kato M, Ogano M, Mori Y, Kochi K, Morimoto D, Kito K, et al. Exercise-based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: a randomized controlled clinical trial. Eur J Prev Cardiol 2019;26:1931–40. [DOI] [PubMed] [Google Scholar]
- 428. Congrete S, Bintvihok M, Thongprayoon C, Bathini T, Boonpheng B, Sharma K, et al. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: a metaanalysis. J Evid Based Med 2018;11:145–51. [DOI] [PubMed] [Google Scholar]
- 429. Matiello M, Nadal M, Tamborero D, Berruezo A, Montserrat J, Embid C, et al. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnoea patients. Europace 2010;12:1084–9. [DOI] [PubMed] [Google Scholar]
- 430. Takigawa M, Takahashi A, Kuwahara T, Takahashi Y, Okubo K, Nakashima E, et al. Impact of alcohol consumption on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation. J Am Heart Assoc 2016;5:e004149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Qiao Y, Shi R, Hou B, Wu L, Zheng L, Ding L, et al. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J Am Heart Assoc 2015;4:e002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Fukamizu S, Sakurada H, Takano M, Hojo R, Nakai M, Yuba T, et al. Effect of cigarette smoking on the risk of atrial fibrillation recurrence after pulmonary vein isolation. J Arrhythm 2010;26:21–9. [Google Scholar]
- 433. Elliott AD, Middeldorp ME, Van Gelder IC, Albert CM, Sanders P. Author correction: epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol 2023;20:429. [DOI] [PubMed] [Google Scholar]
- 434. Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 435. Kim D, Yang PS, Kim TH, Jang E, Shin H, Kim HY, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol 2018;72:1233–45. [DOI] [PubMed] [Google Scholar]
- 436. Okin PM, Hille DA, Larstorp AC, Wachtell K, Kjeldsen SE, Dahlof B, et al. Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension 2015;66:368–73. [DOI] [PubMed] [Google Scholar]
- 437. Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res 2018;122:352–68. [DOI] [PubMed] [Google Scholar]
- 438. Zylla MM, Hochadel M, Andresen D, Brachmann J, Eckardt L, Hoffmann E, et al. Ablation of atrial fibrillation in patients with hypertension-an analysis from the German Ablation Registry. J Clin Med 2020;9:2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Parkash R, Wells GA, Sapp JL, Healey JS, Tardif JC, Greiss I, et al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation: a randomized, open-label clinical trial (SMAC-AF [substrate modification with aggressive blood pressure control]). Circulation 2017;135:1788–98. [DOI] [PubMed] [Google Scholar]
- 440. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60:1163–70. [DOI] [PubMed] [Google Scholar]
- 441. Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA 2020;323:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Hohl M, Selejan SR, Wintrich J, Lehnert U, Speer T, Schneider C, et al. Renal denervation prevents atrial arrhythmogenic substrate development in CKD. Circ Res 2022;130:814–28. [DOI] [PubMed] [Google Scholar]
- 443. Al-Kaisey AM, Kalman JM. Obesity and atrial fibrillation: epidemiology, pathogenesis and effect of weight loss. Arrhythm Electrophysiol Rev 2021;10:159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, et al. Obesity and the risk of incident, postoperative, and postablation atrial fibrillation: a metaanalysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol 2015;1:139–52. [DOI] [PubMed] [Google Scholar]
- 445. Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 446. Munger TM, Dong YX, Masaki M, Oh JK, Mankad SV, Borlaug BA, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol 2012;60:851–60. [DOI] [PubMed] [Google Scholar]
- 447. Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol 2011;57:1745–51. [DOI] [PubMed] [Google Scholar]
- 448. Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res 2014;102:205–13. [DOI] [PubMed] [Google Scholar]
- 449. Mohanty S, Mohanty P, Natale V, Trivedi C, Gianni C, Burkhardt JD, et al. Impact of weight loss on ablation outcome in obese patients with longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:246–53. [DOI] [PubMed] [Google Scholar]
- 450. Gessler N, Willems S, Steven D, Aberle J, Akbulak RO, Gosau N, et al. Supervised obesity reduction trial for AF ablation patients: results from the SORT-AF trial. Europace 2021;23:1548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Donnellan E, Wazni OM, Kanj M, Baranowski B, Cremer P, Harb S, et al. Association between preablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace 2019;21:1476–83. [DOI] [PubMed] [Google Scholar]
- 452. Youssef I, Kamran H, Yacoub M, Patel N, Goulbourne C, Kumar S, et al. Obstructive sleep apnea as a risk factor for atrial fibrillation: a metaanalysis. J Sleep Disord Ther 2018;7:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011;8:1436–43. [DOI] [PubMed] [Google Scholar]
- 454. Holtstrand Hjalm H, Fu M, Hansson PO, Zhong Y, Caidahl K, Mandalenakis Z, et al. Association between left atrial enlargement and obstructive sleep apnea in a general population of 71-year-old men. J Sleep Res 2018;27:252–8. [DOI] [PubMed] [Google Scholar]
- 455. Iwasaki YK, Kato T, Xiong F, Shi YF, Naud P, Maguy A, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol 2014;64:2013–23. [DOI] [PubMed] [Google Scholar]
- 456. Qureshi WT, Nasir UB, Alqalyoobi S, O’Neal WT, Mawri S, Sabbagh S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol 2015;116:1767–73. [DOI] [PubMed] [Google Scholar]
- 457. Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Lévy P, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol 2018;3:532–40. [DOI] [PubMed] [Google Scholar]
- 458. Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, et al. Alcohol and incident atrial fibrillation—a systematic review and metaanalysis. Int J Cardiol 2017;246:46–52. [DOI] [PubMed] [Google Scholar]
- 459. Voskoboinik A, Wong G, Lee G, Nalliah C, Hawson J, Prabhu S, et al. Moderate alcohol consumption is associated with atrial electrical and structural changes: insights from high-density left atrial electroanatomic mapping. Heart Rhythm 2019;16:251–9. [DOI] [PubMed] [Google Scholar]
- 460. Mandyam MC, Vedantham V, Scheinman MM, Tseng ZH, Badhwar N, Lee BK, et al. Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol 2012;110:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Khawaja O, Bartz TM, Ix JH, Heckbert SR, Kizer JR, Zieman SJ, et al. Plasma free fatty acids and risk of atrial fibrillation (from the cardiovascular health study). Am J Cardiol 2012;110:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, et al. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc 2016;5:e004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Sagawa Y, Nagata Y, Miwa N, Yamaguchi T, Watanabe K, Kaneko M, et al. Alcohol consumption is associated with postablation recurrence but not changes in atrial substrate in patients with atrial fibrillation: insight from a high-density mapping study. J Am Heart Assoc 2022;11:e025697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Barmano N, Charitakis E, Kronstrand R, Walfridsson U, Karlsson JE, Walfridsson H, et al. The association between alcohol consumption, cardiac biomarkers, left atrial size and reablation in patients with atrial fibrillation referred for catheter ablation. PLoS One 2019;14:e0215121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a metaanalysis. J Cardiovasc Electrophysiol 2016;27:1021–9. [DOI] [PubMed] [Google Scholar]
- 466. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 2008;118:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Jin MN, Yang PS, Song C, Yu HT, Kim TH, Uhm JS, et al. Physical activity and risk of atrial fibrillation: a nationwide cohort study in general population. Sci Rep 2019;9:13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Morseth B, Graff-Iversen S, Jacobsen BK, Jørgensen L, Nyrnes A, Thelle DS, et al. Physical activity, resting heart rate, and atrial fibrillation: the Tromsø study. Eur Heart J 2016;37:2307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 470. Elliott AD, Verdicchio CV, Mahajan R, Middeldorp ME, Gallagher C, Mishima RS, et al. An exercise and physical activity program in patients with atrial fibrillation: the ACTIVE-AF randomized controlled trial. JACC Clin Electrophysiol 2023;9:455–65. [DOI] [PubMed] [Google Scholar]
- 471. Calvo N, Mont L, Tamborero D, Berruezo A, Viola G, Guasch E, et al. Efficacy of circumferential pulmonary vein ablation of atrial fibrillation in endurance athletes. Europace 2010;12:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Decroocq M, Ninni S, Klein C, Machuron F, Verbrugge E, Klug D, et al. No impact of sports practice before or after atrial fibrillation ablation on procedure efficacy in athletes: a case-control study. Europace 2019;21:1833–42. [DOI] [PubMed] [Google Scholar]
- 473. Aune D, Feng T, Schlesinger S, Janszky I, Norat T, Riboli E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and metaanalysis of cohort studies. J Diabetes Complicat 2018;32:501–11. [DOI] [PubMed] [Google Scholar]
- 474. Liu C, Fu H, Li J, Yang W, Cheng L, Liu T, et al. Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu Kardiyol Derg 2012;12:543–50. [DOI] [PubMed] [Google Scholar]
- 475. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. Smoking and incidence of atrial fibrillation: results from the atherosclerosis risk in communities (ARIC) study. Heart Rhythm 2011;8:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo diet and cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol 2010;25:95–102. [DOI] [PubMed] [Google Scholar]
- 477. Zhu W, Yuan P, Shen Y, Wan R, Hong K. Association of smoking with the risk of incident atrial fibrillation: a metaanalysis of prospective studies. Int J Cardiol 2016;218:259–66. [DOI] [PubMed] [Google Scholar]
- 478. Albertsen IE, Rasmussen LH, Lane DA, Overvad TF, Skjoth F, Overvad K, et al. The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation: insights from the Danish diet, cancer, and health study. Chest 2014;145:559–66. [DOI] [PubMed] [Google Scholar]
- 479. Kwon S, Kim TJ, Choi EK, Ahn HJ, Lee E, Lee SR, et al. Predictors of ischemic stroke for low-risk patients with atrial fibrillation: a matched case-control study. Heart Rhythm 2021;18:702–8. [DOI] [PubMed] [Google Scholar]
- 480. Pathak RK, Middeldorp ME, Stolcman S, Willoughby S, Mahajan R, Lau D, et al. Aggressive risk factor REduction STudy: implications for the substrate for atrial fibrillation (ARREST-AF substrate study). Circ J 2015;132:S115–6. [Google Scholar]
- 481. John B, Stiles MK, Kuklik P, Brooks AG, Chandy ST, Kalman JM, et al. Reverse remodeling of the atria after treatment of chronic stretch in humans: implications for the atrial fibrillation substrate. J Am Coll Cardiol 2010;55:1217–26. [DOI] [PubMed] [Google Scholar]
- 482. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brügemann J, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 483. Pandey AK, Okaj I, Kaur H, Belley-Cote EP, Wang J, Oraii A, et al. Sodium-glucose co-transporter inhibitors and atrial fibrillation: a systematic review and metaanalysis of randomized controlled trials. J Am Heart Assoc 2021;10:e022222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in heart failure-assessment of reduction in mortality and morbidity (CHARM) program. J Am Coll Cardiol 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 485. Cheng WH, Lo LW, Lin YJ, Chang SL, Hu YF, Hung Y, et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm 2019;16:1327–33. [DOI] [PubMed] [Google Scholar]
- 486. Sawhney N, Anousheh R, Chen WC, Narayan S, Feld GK. Five-year outcomes after segmental pulmonary vein isolation for paroxysmal atrial fibrillation. Am J Cardiol 2009;104:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Uchiyama T, Miyazaki S, Taniguchi H, Komatsu Y, Kusa S, Nakamura H, et al. Six-year follow-up of catheter ablation in paroxysmal atrial fibrillation. Circ J 2013;77:2722–7. [DOI] [PubMed] [Google Scholar]
- 488. Gokoglan Y, Mohanty S, Gunes MF, Trivedi C, Santangeli P, Gianni C, et al. Pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: more than a decade of follow-up. Circ Arrhythm Electrophysiol 2016;9:e003660. [DOI] [PubMed] [Google Scholar]
- 489. Hung Y, Lo LW, Lin YJ, Chang SL, Hu YF, Chung FP, et al. Characteristics and long-term catheter ablation outcome in long-standing persistent atrial fibrillation patients with non-pulmonary vein triggers. Int J Cardiol 2017;241:205–11. [DOI] [PubMed] [Google Scholar]
- 490. Wynn GJ, El-Kadri M, Haq I, Das M, Modi S, Snowdon R, et al. Long-term outcomes after ablation of persistent atrial fibrillation: an observational study over 6 years. Open Heart 2016;3:e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Chew DS, Jones KA, Loring Z, Black-Maier E, Noseworthy PA, Exner DV, et al. Diagnosis-to-ablation time predicts recurrent atrial fibrillation and rehospitalization following catheter ablation. Heart Rhythm O2 2022;3:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. De Greef Y, Schwagten B, Chierchia GB, de Asmundis C, Stockman D, Buysschaert I. Diagnosis-to-ablation time as a predictor of success: early choice for pulmonary vein isolation and long-term outcome in atrial fibrillation: results from the Middelheim-PVI Registry. Europace 2018;20:589–95. [DOI] [PubMed] [Google Scholar]
- 493. Baysal E, Okşul M, Burak C, Yalin K, Soysal AU, Yalman H, et al. Decreasing time between first diagnosis of paroxysmal atrial fibrillation and cryoballoon ablation positively affects long-term consequences. J Interv Card Electrophysiol 2022;65:365–72. [DOI] [PubMed] [Google Scholar]
- 494. Takamiya T, Nitta J, Inaba O, Sato A, Inamura Y, Murata K, et al. Impact of diagnosis-to-ablation time on non-pulmonary vein triggers and ablation outcomes in persistent atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:1251–8. [DOI] [PubMed] [Google Scholar]
- 495. D’Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a metaanalysis. Int J Cardiol 2013;167:1984–9. [DOI] [PubMed] [Google Scholar]
- 496. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a metaanalysis. Europace 2018;20:33–42. [DOI] [PubMed] [Google Scholar]
- 497. Costa FM, Ferreira AM, Oliveira S, Santos PG, Durazzo A, Carmo P, et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int J Cardiol 2015;184:56–61. [DOI] [PubMed] [Google Scholar]
- 498. Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and metaanalysis of observational studies. Europace 2012;14:638–45. [DOI] [PubMed] [Google Scholar]
- 499. Blanche C, Tran N, Rigamonti F, Burri H, Zimmermann M. Value of P-wave signal averaging to predict atrial fibrillation recurrences after pulmonary vein isolation. Europace 2013;15:198–204. [DOI] [PubMed] [Google Scholar]
- 500. Yugo D, Kuo MJ, Hu YF, Liu CM, Lin YJ, Chang SL, et al. Dynamic changes in signal-averaged P wave after catheter ablation of atrial fibrillation. J Chin Med Assoc 2022;85:549–53. [DOI] [PubMed] [Google Scholar]
- 501. Salah A, Zhou S, Liu Q, Yan H. P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arq Bras Cardiol 2013;101:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Pranata R, Yonas E, Vania R. Prolonged P-wave duration in sinus rhythm preablation is associated with atrial fibrillation recurrence after pulmonary vein isolation-a systematic review and metaanalysis. Ann Noninvasive Electrocardiol 2019;24:e12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Nakatani Y, Sakamoto T, Yamaguchi Y, Tsujino Y, Kataoka N, Kinugawa K. P-wave vector magnitude predicts recurrence of atrial fibrillation after catheter ablation in patients with persistent atrial fibrillation. Ann Noninvasive Electrocardiol 2019;24:e12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Müller-Edenborn B, Chen J, Allgeier J, Didenko M, Moreno-Weidmann Z, Neumann FJ, et al. Amplified sinus-P-wave reveals localization and extent of left atrial low-voltage substrate: implications for arrhythmia freedom following pulmonary vein isolation. Europace 2020;22:240–9. [DOI] [PubMed] [Google Scholar]
- 505. Moreno-Weidmann Z, Müller-Edenborn B, Jadidi AS, Bazan-Gelizo V, Chen J, Park CI, et al. Easily available ECG and echocardiographic parameters for prediction of left atrial remodeling and atrial fibrillation recurrence after pulmonary vein isolation: a multicenter study. J Cardiovasc Electrophysiol 2021;32:1584–93. [DOI] [PubMed] [Google Scholar]
- 506. Liu P, Lv T, Yang Y, Gao Q, Zhang P. Use of P wave indices to evaluate efficacy of catheter ablation and atrial fibrillation recurrence: a systematic review and metaanalysis. J Interv Card Electrophysiol 2022;65:827–40. [DOI] [PubMed] [Google Scholar]
- 507. Jadidi A, Müller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier J, et al. The duration of the amplified sinus-P-wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol 2018;4:531–43. [DOI] [PubMed] [Google Scholar]
- 508. Koutalas E, Kallergis E, Nedios S, Kochiadakis G, Kanoupakis E. P-wave duration as a marker of atrial remodeling in patients referred to ablation for atrial fibrillation: a new stratification tool emerging? Hellenic J Cardiol 2023. [DOI] [PubMed] [Google Scholar]
- 509. Okumura Y, Watanabe I, Ohkubo K, Ashino S, Kofune M, Hashimoto K, et al. Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal-averaged P-wave duration. Pacing Clin Electrophysiol 2007;30:304–13. [DOI] [PubMed] [Google Scholar]
- 510. Kuppahally SS, Akoum N, Badger TJ, Burgon NS, Haslam T, Kholmovski E, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J 2010;160:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Akkaya M, Higuchi K, Koopmann M, Burgon N, Erdogan E, Damal K, et al. Relationship between left atrial tissue structural remodelling detected using late gadolinium enhancement MRI and left ventricular hypertrophy in patients with atrial fibrillation. Europace 2013;15:1725–32. [DOI] [PubMed] [Google Scholar]
- 512. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Ambale-Venkatesh B, Lima JA. Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol 2015;12:18–29. [DOI] [PubMed] [Google Scholar]
- 514. Nairn D, Eichenlaub M, Müller-Edenborn B, Huang T, Lehrmann H, Nagel C, et al. Differences in atrial substrate localization using late gadolinium enhancement-magnetic resonance imaging, electrogram voltage, and conduction velocity: a cohort study using a consistent anatomical reference frame in patients with persistent atrial fibrillation. Europace 2023;25:euad278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Nakahara S, Hori Y, Kobayashi S, Sakai Y, Taguchi I, Takayanagi K, et al. Epicardial adipose tissue-based defragmentation approach to persistent atrial fibrillation: its impact on complex fractionated electrograms and ablation outcome. Heart Rhythm 2014;11:1343–51. [DOI] [PubMed] [Google Scholar]
- 516. Chao TF, Hung CL, Tsao HM, Lin YJ, Yun CH, Lai YH, et al. Epicardial adipose tissue thickness and ablation outcome of atrial fibrillation. PLoS One 2013;8:e74926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Masuda M, Mizuno H, Enchi Y, Minamiguchi H, Konishi S, Ohtani T, et al. Abundant epicardial adipose tissue surrounding the left atrium predicts early rather than late recurrence of atrial fibrillation after catheter ablation. J Interv Card Electrophysiol 2015;44:31–7. [DOI] [PubMed] [Google Scholar]
- 518. Sepehri Shamloo A, Dagres N, Dinov B, Sommer P, Husser-Bollmann D, Bollmann A, et al. Is epicardial fat tissue associated with atrial fibrillation recurrence after ablation? A systematic review and metaanalysis. Int J Cardiol Heart Vasc 2019;22:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, et al. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J 2011;75:2559–65. [DOI] [PubMed] [Google Scholar]
- 520. Vroomen M, Olsthoorn JR, Maesen B, L’Espoir V, La Meir M, Das M, et al. Quantification of epicardial adipose tissue in patients undergoing hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg 2019;56:79–86. [DOI] [PubMed] [Google Scholar]
- 521. El Mahdiui M, Simon J, Smit JM, Kuneman JH, van Rosendael AR, Steyerberg EW, et al. Posterior left atrial adipose tissue attenuation assessed by computed tomography and recurrence of atrial fibrillation after catheter ablation. Circ Arrhythm Electrophysiol 2021;14:e009135. [DOI] [PubMed] [Google Scholar]
- 522. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 523. van Vugt SPG, Westra SW, Volleberg R, Hannink G, Nakamura R, de Asmundis C, et al. Meta-analysis of controlled studies on minimally interrupted vs. continuous use of non-vitamin K antagonist oral anticoagulants in catheter ablation for atrial fibrillation. Europace 2021;23:1961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Yadav R, Brilliant J, Akhtar T, Milstein J, Sampognaro JR, Marine J, et al. Relationship between amiodarone response prior to ablation and 1-year outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2023;34:860–8. [DOI] [PubMed] [Google Scholar]
- 525. Dukes JW, Chilukuri K, Scherr D, Marine JE, Berger RD, Nazarian S, et al. The effect of antiarrhythmic medication management on atrial fibrillation ablation outcomes. J Cardiovasc Electrophysiol 2013;24:882–7. [DOI] [PubMed] [Google Scholar]
- 526. Miwa Y, Minamiguchi H, Bhandari AK, Cannom DS, Ho IC. Amiodarone reduces the amount of ablation during catheter ablation for persistent atrial fibrillation. Europace 2014;16:1007–14. [DOI] [PubMed] [Google Scholar]
- 527. Mohanty S, Di Biase L, Mohanty P, Trivedi C, Santangeli P, Bai R, et al. Effect of periprocedural amiodarone on procedure outcome in patients with longstanding persistent atrial fibrillation undergoing extended pulmonary vein antrum isolation: results from a randomized study (SPECULATE). Heart Rhythm 2015;12:477–83. [DOI] [PubMed] [Google Scholar]
- 528. Efremidis M, Bazoukis G, Vlachos K, Prappa E, Megarisiotou A, Dragasis S, et al. Safety of catheter ablation of atrial fibrillation without pre- or periprocedural imaging for the detection of left atrial thrombus in the era of uninterrupted anticoagulation. J Arrhythm 2021;37:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Diab M, Wazni OM, Saliba WI, Tarakji KG, Ballout JA, Hutt E, et al. Ablation of atrial fibrillation without left atrial appendage imaging in patients treated with direct oral anticoagulants. Circ Arrhythm Electrophysiol 2020;13:e008301. [DOI] [PubMed] [Google Scholar]
- 530. Puwanant S, Varr BC, Shrestha K, Hussain SK, Tang WH, Gabriel RS, et al. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol 2009;54:2032–9. [DOI] [PubMed] [Google Scholar]
- 531. Manning WJ, Weintraub RM, Waksmonski CA, Haering JM, Rooney PS, Maslow AD, et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med 1995;123:817–22. [DOI] [PubMed] [Google Scholar]
- 532. Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921–64. [DOI] [PubMed] [Google Scholar]
- 533. Daniel WG, Erbel R, Kasper W, Visser CA, Engberding R, Sutherland GR, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991;83:817–21. [DOI] [PubMed] [Google Scholar]
- 534. Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, McDonald RJ, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med 2020;2:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Stocker TJ, Deseive S, Leipsic J, Hadamitzky M, Chen MY, Rubinshtein R, et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J 2018;39:3715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Kitkungvan D, Nabi F, Ghosn MG, Dave AS, Quinones M, Zoghbi WA, et al. Detection of LA and LAA thrombus by CMR in patients referred for pulmonary vein isolation. JACC Cardiovasc Imaging 2016;9:809–18. [DOI] [PubMed] [Google Scholar]
- 537. Rathi VK, Reddy ST, Anreddy S, Belden W, Yamrozik JA, Williams RB, et al. Contrast-enhanced CMR is equally effective as TEE in the evaluation of left atrial appendage thrombus in patients with atrial fibrillation undergoing pulmonary vein isolation procedure. Heart Rhythm 2013;10:1021–7. [DOI] [PubMed] [Google Scholar]
- 538. Ohyama H, Hosomi N, Takahashi T, Mizushige K, Osaka K, Kohno M, et al. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke 2003;34:2436–9. [DOI] [PubMed] [Google Scholar]
- 539. Brooks AG, Wilson L, Chia NH, Lau DH, Alasady M, Leong DP, et al. Accuracy and clinical outcomes of CT image integration with Carto-Sound compared to electro-anatomical mapping for atrial fibrillation ablation: a randomized controlled study. Int J Cardiol 2013;168:2774–82. [DOI] [PubMed] [Google Scholar]
- 540. Di Biase L, Zou F, Lin AN, Grupposo V, Marazzato J, Tarantino N, et al. Feasibility of three-dimensional artificial intelligence algorithm integration with intracardiac echocardiography for left atrial imaging during atrial fibrillation catheter ablation. Europace 2023;25:euad211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Vicera JJB, Lin YJ, Lee PT, Chang SL, Lo LW, Hu YF, et al. Identification of critical isthmus using coherent mapping in patients with scar-related atrial tachycardia. J Cardiovasc Electrophysiol 2020;31:1436–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Deno DC, Bhaskaran A, Morgan DJ, Goksu F, Batman K, Olson GK, et al. High-resolution, live, directional mapping. Heart Rhythm 2020;17:1621–8. [DOI] [PubMed] [Google Scholar]
- 543. Unland R, Bergau L, El Hamriti M, Guckel D, Piran M, Fink T, et al. Find me if you can: first clinical experience using the novel CARTOFINDER algorithm in a routine workflow for atrial fibrillation ablation. J Clin Med 2021;10:2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Honarbakhsh S, Schilling RJ, Dhillon G, Ullah W, Keating E, Providencia R, et al. A novel mapping system for panoramic mapping of the left atrium: application to detect and characterize localized sources maintaining atrial fibrillation. JACC Clin Electrophysiol 2018;4:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545. Chang TY, Lin CY, Lin YJ, Wu CI, Chang SL, Lo LW, et al. Long-term outcome of patients with long-standing persistent atrial fibrillation undergoing ablation guided by a novel high-density panoramic mapping system: a propensity score matching study. Heart Rhythm O2 2022;3:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546. Sporton SC, Earley MJ, Nathan AW, Schilling RJ. Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: a prospective randomized study. J Cardiovasc Electrophysiol 2004;15:310–5. [DOI] [PubMed] [Google Scholar]
- 547. Estner HL, Deisenhofer I, Luik A, Ndrepepa G, von Bary C, Zrenner B, et al. Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX). Europace 2006;8:583–7. [DOI] [PubMed] [Google Scholar]
- 548. Scaglione M, Biasco L, Caponi D, Anselmino M, Negro A, Di Donna P, et al. Visualization of multiple catheters with electroanatomical mapping reduces X-ray exposure during atrial fibrillation ablation. Europace 2011;13:955–62. [DOI] [PubMed] [Google Scholar]
- 549. Martinek M, Nesser HJ, Aichinger J, Boehm G, Purerfellner H. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol 2007;30:1215–23. [DOI] [PubMed] [Google Scholar]
- 550. Caponi D, Corleto A, Scaglione M, Blandino A, Biasco L, Cristoforetti Y, et al. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome?: a randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace 2010;12:1098–104. [DOI] [PubMed] [Google Scholar]
- 551. Bertaglia E, Bella PD, Tondo C, Proclemer A, Bottoni N, De Ponti R, et al. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace 2009;11:1004–10. [DOI] [PubMed] [Google Scholar]
- 552. Della Bella P, Fassini G, Cireddu M, Riva S, Carbucicchio C, Giraldi F, et al. Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. J Cardiovasc Electrophysiol 2009;20:258–65. [DOI] [PubMed] [Google Scholar]
- 553. Kistler PM, Rajappan K, Harris S, Earley MJ, Richmond L, Sporton SC, et al. The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: a prospective randomized study. Eur Heart J 2008;29:3029–36. [DOI] [PubMed] [Google Scholar]
- 554. Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht S, et al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicenter VISTAX trial. Europace 2020;22:1645–52. [DOI] [PubMed] [Google Scholar]
- 555. Duytschaever M, De Pooter J, Demolder A, El Haddad M, Phlips T, Strisciuglio T, et al. Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: the CLOSE to CURE study. Heart Rhythm 2020;17:535–43. [DOI] [PubMed] [Google Scholar]
- 556. De Pooter J, Strisciuglio T, El Haddad M, Wolf M, Phlips T, Vandekerckhove Y, et al. Pulmonary vein reconnection no longer occurs in the majority of patients after a single pulmonary vein isolation procedure. JACC Clin Electrophysiol 2019;5:295–305. [DOI] [PubMed] [Google Scholar]
- 557. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A, et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol 2018;11:e006576. [DOI] [PubMed] [Google Scholar]
- 558. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Y, et al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 559. Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace 2018;20:f419–27. [DOI] [PubMed] [Google Scholar]
- 560. Grace A, Willems S, Meyer C, Verma A, Heck P, Zhu M, et al. High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight 2019;4:e126422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Willems S, Verma A, Betts TR, Murray S, Neuzil P, Ince H, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF trial. Circ Arrhythm Electrophysiol 2019;12:e007233. [DOI] [PubMed] [Google Scholar]
- 562. Shi R, Parikh P, Chen Z, Angel N, Norman M, Hussain W, et al. Validation of dipole density mapping during atrial fibrillation and sinus rhythm in human left atrium. JACC Clin Electrophysiol 2020;6:171–81. [DOI] [PubMed] [Google Scholar]
- 563. Haines DE, Kong MH, Ruppersberg P, Haeusser P, Avitall B, Szili Torok T, et al. Electrographic flow mapping for atrial fibrillation: theoretical basis and preliminary observations. J Interv Card Electrophysiol 2022;66:1015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Reddy VY, Neuzil P, Langbein A, Petru J, Funasako M, Dinshaw L, et al. AB-453070-2 FLOW-AF: a randomized controlled trial of electrographic flow-guided ablation in redo patients with non-paroxysmal atrial fibrillation. Heart Rhythm 2023;20:S1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565. Nademanee K, Schwab MC, Kosar EM, Karwecki M, Moran MD, Visessook N, et al. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J Am Coll Cardiol 2008;51:843–9. [DOI] [PubMed] [Google Scholar]
- 566. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 567. Seitz J, Bars C, Theodore G, Beurtheret S, Lellouche N, Bremondy M, et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J Am Coll Cardiol 2017;69:303–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568. Lin R, Zeng C, Xu K, Wu S, Qin M, Liu X. Dispersion-guided ablation in conjunction with circumferential pulmonary vein isolation is superior to stepwise ablation approach for persistent atrial fibrillation. Int J Cardiol 2019;278:97–103. [DOI] [PubMed] [Google Scholar]
- 569. Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 2016;9:e002962. [DOI] [PubMed] [Google Scholar]
- 570. Seitz J, Durdez TM, Albenque JP, Pisapia A, Gitenay E, Durand C, et al. Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation. J Cardiovasc Electrophysiol 2022;33:2250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol 2012;60:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572. Gray RA, Jalife J, Panfilov AV, Baxter WT, Cabo C, Davidenko JM, et al. Mechanisms of cardiac fibrillation. Science 1995;270:1222–3; author reply 1224–5. [PubMed] [Google Scholar]
- 573. Salinet J, Molero R, Schlindwein FS, Karel J, Rodrigo M, Rojo-Alvarez JL, et al. Electrocardiographic imaging for atrial fibrillation: a perspective from computer models and animal experiments to clinical value. Front Physiol 2021;12:653013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med 2004;10:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Molero R, González-Ascaso A, Climent AM, Guillem MS. Robustness of imageless electrocardiographic imaging against uncertainty in atrial morphology and location. J Electrocardiol 2023;77:58–61. [DOI] [PubMed] [Google Scholar]
- 576. Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ Jr, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation 2010;122:1364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, et al. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–8. [DOI] [PubMed] [Google Scholar]
- 578. Lim HS, Hocini M, Dubois R, Denis A, Derval N, Zellerhoff S, et al. Complexity and distribution of drivers in relation to duration of persistent atrial fibrillation. J Am Coll Cardiol 2017;69:1257–69. [DOI] [PubMed] [Google Scholar]
- 579. Knecht S, Sohal M, Deisenhofer I, Albenque JP, Arentz T, Neumann T, et al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace 2017;19:1302–9. [DOI] [PubMed] [Google Scholar]
- 580. Honarbakhsh S, Dhillon G, Abbass H, Waddingham PH, Dennis A, Ahluwalia N, et al. Noninvasive electrocardiographic imaging-guided targeting of drivers of persistent atrial fibrillation: the TARGET-AF1 trial. Heart Rhythm 2022;19:875–84. [DOI] [PubMed] [Google Scholar]
- 581. Gao X, Lam AG, Bilchick KC, Darby A, Mehta N, Mason PK, et al. The use of non-invasive mapping in persistent AF to predict acute procedural outcome. J Electrocardiol 2019;57S:S21–6. [DOI] [PubMed] [Google Scholar]
- 582. Rodrigo M, Climent AM, Hernandez-Romero I, Liberos A, Baykaner T, Rogers AJ, et al. Noninvasive assessment of complexity of atrial fibrillation: correlation with contact mapping and impact of ablation. Circ Arrhythm Electrophysiol 2020;13:e007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583. Badger TJ, Adjei-Poku YA, Marrouche NF. MRI in cardiac electrophysiology: the emerging role of delayed-enhancement MRI in atrial fibrillation ablation. Future Cardiol 2009;5:63–70. [DOI] [PubMed] [Google Scholar]
- 584. Bisbal F, Calvo M, Trucco E, Arbelo E, Berruezo A, Mont L. Left atrial tachycardia after atrial fibrillation ablation: can magnetic resonance imaging assist the ablation? Can J Cardiol 2015;31:104.e1–3. [DOI] [PubMed] [Google Scholar]
- 585. Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 586. Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 587. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak R, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Andrade JG, Monir G, Pollak SJ, Khairy P, Dubuc M, Roy D, et al. Pulmonary vein isolation using “contact force” ablation: the effect on dormant conduction and long-term freedom from recurrent atrial fibrillation—a prospective study. Heart Rhythm 2014;11:1919–24. [DOI] [PubMed] [Google Scholar]
- 589. Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace 2014;16:660–7. [DOI] [PubMed] [Google Scholar]
- 590. Whitaker J, Fish J, Harrison J, Chubb H, Williams SE, Fastl T, et al. Lesion index-guided ablation facilitates continuous, transmural, and durable lesions in a porcine recovery model. Circ Arrhythm Electrophysiol 2018;11:e005892. [DOI] [PubMed] [Google Scholar]
- 591. Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJ, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace 2017;19:775–83. [DOI] [PubMed] [Google Scholar]
- 592. Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa M, et al. High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol 2018;29:1570–5. [DOI] [PubMed] [Google Scholar]
- 593. Borne RT, Sauer WH, Zipse MM, Zheng L, Tzou W, Nguyen DT. Longer duration versus increasing power during radiofrequency ablation yields different ablation lesion characteristics. JACC Clin Electrophysiol 2018;4:902–8. [DOI] [PubMed] [Google Scholar]
- 594. Kyriakopoulou M, Wielandts JY, Strisciuglio T, El Haddad M, Pooter J, Almorad A, et al. Evaluation of higher power delivery during RF pulmonary vein isolation using optimized and contiguous lesions. J Cardiovasc Electrophysiol 2020;31:1091–8. [DOI] [PubMed] [Google Scholar]
- 595. Berte B, Hilfiker G, Russi I, Moccetti F, Cuculi F, Toggweiler S, et al. Pulmonary vein isolation using a higher power shorter duration CLOSE protocol with a surround flow ablation catheter. J Cardiovasc Electrophysiol 2019;30:2199–204. [DOI] [PubMed] [Google Scholar]
- 596. Chen S, Schmidt B, Bordignon S, Urbanek L, Tohoku S, Bologna F, et al. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: procedural data, lesion analysis, and initial results from the FAFA AI high power study. J Cardiovasc Electrophysiol 2019;30:2724–31. [DOI] [PubMed] [Google Scholar]
- 597. Winkle RA, Mohanty S, Patrawala RA, Mead RH, Kong MH, Engel G, et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm 2019;16:165–9. [DOI] [PubMed] [Google Scholar]
- 598. Winkle RA, Moskovitz R, Hardwin Mead R, Engel G, Kong MH, Fleming W, et al. Atrial fibrillation ablation using very short duration 50 W ablations and contact force sensing catheters. J Interv Card Electrophysiol 2018;52:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599. Wielandts JY, Kyriakopoulou M, Almorad A, Hilfiker G, Strisciuglio T, Phlips T, et al. Prospective randomized evaluation of high power during CLOSE-guided pulmonary vein isolation: the POWER-AF study. Circ Arrhythm Electrophysiol 2021;14:e009112. [DOI] [PubMed] [Google Scholar]
- 600. Lee AC, Voskoboinik A, Cheung CC, Yogi S, Tseng ZH, Moss JD, et al. A randomized trial of high vs standard power radiofrequency ablation for pulmonary vein isolation: SHORT-AF. JACC Clin Electrophysiol 2023;9:1038–47. [DOI] [PubMed] [Google Scholar]
- 601. Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler V, et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace 2020;22:388–93. [DOI] [PubMed] [Google Scholar]
- 602. Castrejón-Castrejón S, Martínez Cossiani M, Jáuregui-Abularach M, Basterra Sola N, Ibáñez Criado JL, Osca Asensi J, et al. POWER FAST III trial investigators. Multicenter prospective comparison of conventional and high-power short duration radiofrequency application for pulmonary vein isolation: the high-power short-duration radiofrequency application for faster and safer pulmonary vein ablation (POWER FAST III) trial. J Interv Card Electrophysiol 2023;66:1889–99. [DOI] [PubMed] [Google Scholar]
- 603. Leung LWM, Akhtar Z, Sheppard MN, Louis-Auguste J, Hayat J, Gallagher MM. Preventing esophageal complications from atrial fibrillation ablation: a review. Heart Rhythm O2 2021;2:651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604. Kautzner J, Albenque JP, Natale A, Maddox W, Cuoco F, Neuzil P, et al. A novel temperature-controlled radiofrequency catheter ablation system used to treat patients with paroxysmal atrial fibrillation. JACC Clin Electrophysiol 2021;7:352–63. [DOI] [PubMed] [Google Scholar]
- 605. Almorad A, Wielandts JY, El Haddad M, Knecht S, Tavernier R, Kobza R, et al. Performance and safety of temperature- and flow-controlled radiofrequency ablation in ablation index-guided pulmonary vein isolation. JACC Clin Electrophysiol 2021;7:408–9. [DOI] [PubMed] [Google Scholar]
- 606. Bortone A, Albenque JP, Ramirez FD, Haïssaguerre M, Combes S, Constantin M, et al. 90 vs 50-watt radiofrequency applications for pulmonary vein isolation: experimental and clinical findings. Circ Arrhythm Electrophysiol 2022;15:e010663. [DOI] [PubMed] [Google Scholar]
- 607. Leshem E, Zilberman I, Tschabrunn CM, Barkagan M, Contreras-Valdes FM, Govari A, et al. High-power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol 2018;4:467–79. [DOI] [PubMed] [Google Scholar]
- 608. Takigawa M, Kitamura T, Martin CA, Fuimaono K, Datta K, Joshi H, et al. Temperature- and flow-controlled ablation/very-high-power short-duration ablation vs conventional power-controlled ablation: comparison of focal and linear lesion characteristics. Heart Rhythm 2021;18:553–61. [DOI] [PubMed] [Google Scholar]
- 609. Nakagawa H, Ikeda A, Sharma T, Govari A, Ashton J, Maffre J, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high power-short duration and moderate power-moderate duration: effects of thermal latency and contact force on lesion formation. Circ Arrhythm Electrophysiol 2021;14:e009899. [DOI] [PubMed] [Google Scholar]
- 610. Reddy VY, Grimaldi M, De Potter T, Vijgen JM, Bulava A, Duytschaever MF, et al. Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol 2019;5:778–86. [DOI] [PubMed] [Google Scholar]
- 611. Halbfass P, Wielandts JY, Knecht S, Le Polain de Waroux JB, Tavernier R, De Wilde V, et al. Safety of very high-power short-duration radiofrequency ablation for pulmonary vein isolation: a two-centre report with emphasis on silent esophageal injury. Europace 2022;24:400–5. [DOI] [PubMed] [Google Scholar]
- 612. Richard Tilz R, Sano M, Vogler J, Fink T, Saraei R, Sciacca V, et al. Very high-power short-duration temperature-controlled ablation versus conventional power-controlled ablation for pulmonary vein isolation: the fast and furious – AF study. Int J Cardiol Heart Vasc 2021;35:100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. O’Neill L, El Haddad M, Berte B, Kobza R, Hilfiker G, Scherr D, et al. Very high-power ablation for contiguous pulmonary vein isolation: results from the randomized POWER PLUS trial. JACC Clin Electrophysiol 2022;9:511–22. [DOI] [PubMed] [Google Scholar]
- 614. Reddy VY, Schilling R, Grimaldi M, Horton R, Natale A, Riva S, et al. Pulmonary vein isolation with a novel multielectrode radiofrequency balloon catheter that allows directionally tailored energy delivery: short-term outcomes from a multicenter first-in-human study (RADIANCE). Circ Arrhythm Electrophysiol 2019;12:e007541. [DOI] [PubMed] [Google Scholar]
- 615. Dhillon GS, Honarbakhsh S, Di Monaco A, Coling AE, Lenka K, Pizzamiglio F, et al. Use of a multielectrode radiofrequency balloon catheter to achieve pulmonary vein isolation in patients with paroxysmal atrial fibrillation: 12-month outcomes of the RADIANCE study. J Cardiovasc Electrophysiol 2020;31:1259–69. [DOI] [PubMed] [Google Scholar]
- 616. Almorad A, Del Monte A, Della Rocca DG, Pannone L, Ramak R, Overeinder I, et al. Outcomes of pulmonary vein isolation with radiofrequency balloon vs. cryoballoon ablation: a multi-centric study. Europace 2023;25:euad252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Schilling R, Dhillon GS, Tondo C, Riva S, Grimaldi M, Quadrini F, et al. Safety, effectiveness, and quality of life following pulmonary vein isolation with a multielectrode radiofrequency balloon catheter in paroxysmal atrial fibrillation: 1-year outcomes from SHINE. Europace 2021;23:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Del Monte A, Almorad A, Pannone L, Della Rocca DG, Bisignani A, Monaco C, et al. Pulmonary vein isolation with the radiofrequency balloon catheter: a single center prospective study. Europace 2023;25:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619. Reddy VY, Anter E, Rackauskas G, Peichl P, Koruth JS, Petru J, et al. Lattice-tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythm Electrophysiol 2020;13:e008718. [DOI] [PubMed] [Google Scholar]
- 620. Reddy VY, Peichl P, Anter E, Rackauskas G, Petru J, Funasako M, et al. A focal ablation catheter toggling between radiofrequency and pulsed field energy to treat atrial fibrillation. JACC Clin Electrophysiol 2023;9:1786–801. [DOI] [PubMed] [Google Scholar]
- 621. Dubuc M, Khairy P, Rodriguez-Santiago A, Talajic M, Tardif JC, Thibault B, et al. Catheter cryoablation of the atrioventricular node in patients with atrial fibrillation: a novel technology for ablation of cardiac arrhythmias. J Cardiovasc Electrophysiol 2001;12:439–44. [DOI] [PubMed] [Google Scholar]
- 622. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 623. Furnkranz A, Chun KR, Nuyens D, Metzner A, Koster I, Schmidt B, et al. Characterization of conduction recovery after pulmonary vein isolation using the “single big cryoballoon” technique. Heart Rhythm 2010;7:184–90. [DOI] [PubMed] [Google Scholar]
- 624. Martin CA, Tilz RRR, Anic A, Defaye P, Luik A, de Asmundis C, et al. Acute procedural efficacy and safety of a novel cryoballoon for the treatment of paroxysmal atrial fibrillation: results from the POLAR ICE study. J Cardiovasc Electrophysiol 2023;34:833–40. [DOI] [PubMed] [Google Scholar]
- 625. Tilz RR, Meyer-Saraei R, Eitel C, Fink T, Sciacca V, Lopez LD, et al. Novel cryoballoon ablation system for single shot pulmonary vein isolation- the prospective ICE-AGE-X study. Circ J 2021;85:1296–304. [DOI] [PubMed] [Google Scholar]
- 626. Assaf A, Bhagwandien R, Szili-Torok T, Yap SC. Comparison of procedural efficacy, balloon nadir temperature, and incidence of phrenic nerve palsy between two cryoballoon technologies for pulmonary vein isolation: a systematic review and metaanalysis. J Cardiovasc Electrophysiol 2021;32:2424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627. Reichlin T, Kaueffer T, Knecht S, Madaffari A, Badertscher P, Maurhofer J, et al. Comparison of the PolarX and the Arctic Front cryoballoon for pulmonary vein isolation in patients with paroxysmal atrial fibrillation (COMPARE CRYO). Presented at ESC Congress 2023, Late Breaking Sciences in Atrial Fibrillation Session 2023. [DOI] [PubMed]
- 628. Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629. Bredikis A, Wilber DJ. Cryoablation of Cardiac Arrhythmias. 1st ed. Philadelphia, PA: Elsevier/Saunders; 2011. [Google Scholar]
- 630. De Potter T, Klaver M, Babkin A, Iliodromitis K, Hocini M, Cox J, et al. Ultra-low temperature cryoablation for atrial fibrillation: primary outcomes for efficacy and safety: the cryocure-2 study. JACC Clin Electrophysiol 2022;8:1034–9. [DOI] [PubMed] [Google Scholar]
- 631. Verma A, Asivatham SJ, Deneke T, Castellvi Q, Neal RE 2nd. Primer on pulsed electrical field ablation: understanding the benefits and limitations. Circ Arrhythm Electrophysiol 2021;14:e010086. [DOI] [PubMed] [Google Scholar]
- 632. Ruzgys P, Novickij V, Novickij J, Satkauskas S. Influence of the electrode material on ROS generation and electroporation efficiency in low and high frequency nanosecond pulse range. Bioelectrochemistry 2019;127:87–93. [DOI] [PubMed] [Google Scholar]
- 633. Rubinsky L, Guenther E, Mikus P, Stehling M, Rubinsky B. Electrolytic effects during tissue ablation by electroporation. Technol Cancer Res Treat 2016;15:NP95–103. [DOI] [PubMed] [Google Scholar]
- 634. Xie F, Varghese F, Pakhomov AG, Semenov I, Xiao S, Philpott J, et al. Ablation of myocardial tissue with nanosecond pulsed electric fields. PLoS One 2015;10:e0144833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat 2007;6:307–12. [DOI] [PubMed] [Google Scholar]
- 636. Scheinman MM, Morady F, Hess DS, Gonzalez R. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA 1982;248:851–5. [PubMed] [Google Scholar]
- 637. van den Bos W, Scheffer HJ, Vogel JA, Wagstaff PG, de Bruin DM, de Jong MC, et al. Thermal energy during irreversible electroporation and the influence of different ablation parameters. J Vasc Interv Radiol 2016;27:433–43. [DOI] [PubMed] [Google Scholar]
- 638. Stewart MT, Haines DE, Miklavcic D, Kos B, Kirchhof N, Barka N, et al. Safety and chronic lesion characterization of pulsed field ablation in a porcine model. J Cardiovasc Electrophysiol 2021;32:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639. Meckes D, Emami M, Fong I, Lau DH, Sanders P. Pulsed-field ablation: computational modeling of electric fields for lesion depth analysis. Heart Rhythm O2 2022;3:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640. Verma A, Howard BT, Tzou WT, Mattison LM, Kos B, Miklavcic D, et al. Effects of tissue proximity on cardiac lesion formation using pulsed field ablation. Heart Rhythm 2022;19:S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. [DOI] [PubMed] [Google Scholar]
- 642. Verma A, Boersma L, Haines DE, Natale A, Marchlinski FE, Sanders P, et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrhythm Electrophysiol 2022;15:e010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A, et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644. Schmidt B, Bordignon S, Neven K, Reichlin T, Blaauw Y, Hansen J, et al. EUropean real-world outcomes with pulsed field ablatiOn in patients with symptomatic atRIAl fibrillation: lessons from the multicentre EU-PORIA registry. Europace 2023;25:euad185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645. Maor E, Ivorra A, Rubinsky B. Non thermal irreversible electroporation: novel technology for vascular smooth muscle cells ablation. PLoS One 2009;4:e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646. Li W, Fan Q, Ji Z, Qiu X, Li Z. The effects of irreversible electroporation (IRE) on nerves. PLoS One 2011;6:e18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647. Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck ED, et al. Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 2020;13:e008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648. Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, et al. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm 2019;16:754–64. [DOI] [PubMed] [Google Scholar]
- 649. Howard B, Haines DE, Verma A, Kirchhof N, Barka N, Onal B, et al. Characterization of phrenic nerve response to pulsed field ablation. Circ Arrhythm Electrophysiol 2022;15:e010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650. Howard B, Haines DE, Verma A, Packer D, Kirchhof N, Barka N, et al. Reduction in pulmonary vein stenosis and collateral damage with pulsed field ablation compared with radiofrequency ablation in a canine model. Circ Arrhythm Electrophysiol 2020;13:e008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 652. Chung EM, Banahan C, Patel N, Janus J, Marshall D, Horsfield MA, et al. Size distribution of air bubbles entering the brain during cardiac surgery. PLoS One 2015;10:e0122166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653. Reinsch N, Füting A, Höwel D, Bell J, Lin Y, Neven K. Cerebral safety after pulsed field ablation for paroxysmal atrial fibrillation. Heart Rhythm 2022;19:1813–8. [DOI] [PubMed] [Google Scholar]
- 654. Loh P, van Es R, Groen MHA, Neven K, Kassenberg W, Wittkampf FHM, et al. Pulmonary vein isolation with single pulse irreversible electroporation: a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008192. [DOI] [PubMed] [Google Scholar]
- 655. Gunawardene Melanie A, Schaeffer Benjamin N, Jularic M, Eickholt C, Maurer T, Akbulak Ruken Ö, et al. Coronary spasm during pulsed field ablation of the mitral isthmus line. JACC Clin Electrophysiol 2021;7:1618–20. [DOI] [PubMed] [Google Scholar]
- 656. Reddy VY, Petru J, Funasako M, Kopriva K, Hala P, Chovanec M, et al. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circulation 2022;146:1808–19. [DOI] [PubMed] [Google Scholar]
- 657. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami K, et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. [DOI] [PubMed] [Google Scholar]
- 658. Anić A, Phlips T, Brešković T, Koopman P, Girouard S, Mediratta V, et al. Pulsed field ablation using focal contact force-sensing catheters for treatment of atrial fibrillation: acute and 90-day invasive remapping results. Europace 2023;25:euad147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659. Turagam MK, Neuzil P, Schmidt B, Reichlin T, Neven K, Metzner A, et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF registry. Circulation 2023;148:35–46. [DOI] [PubMed] [Google Scholar]
- 660. Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, et al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation 2023;147:1422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661. Duytschaever M, De Potter T, Grimaldi M, Anic A, Vijgen J, Neuzil P, et al. Paroxysmal atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circ Arrhythm Electrophysiol 2023;16:e011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662. Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–71. [DOI] [PubMed] [Google Scholar]
- 663. Rovaris G, Ciconte G, Schiavone M, Mitacchione G, Gasperetti A, Piazzi E, et al. Second-generation laser balloon ablation for the treatment of atrial fibrillation assessed by continuous rhythm monitoring: the LIGHT-AF study. Europace 2021;23:1380–90. [DOI] [PubMed] [Google Scholar]
- 664. Schmidt B, Neuzil P, Luik A, Osca Asensi J, Schrickel JW, Deneke T, et al. Laser balloon or wide-area circumferential irrigated radiofrequency ablation for persistent atrial fibrillation: a multicenter prospective randomized study. Circ Arrhythm Electrophysiol 2017;10:e005767. [DOI] [PubMed] [Google Scholar]
- 665. Chun JKR, Bordignon S, Last J, Mayer L, Tohoku S, Zanchi S, et al. Cryoballoon versus laserballoon: insights from the first prospective randomized balloon trial in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2021;14:e009294. [DOI] [PubMed] [Google Scholar]
- 666. Ye W, Chen Q, Fan G, Zhou X, Wang X, Mao W, et al. Efficacy and safety of visually guided laser balloon versus cryoballoon ablation for paroxysmal atrial fibrillation: a systematic review and metaanalysis. Front Cardiovasc Med 2023;10:1229223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Schiavone M, Gasperetti A, Montemerlo E, Pozzi M, Sabato F, Piazzi E, et al. Long-term comparisons of atrial fibrillation ablation outcomes with a cryoballoon or laser-balloon: a propensity-matched analysis based on continuous rhythm monitoring. Hellenic J Cardiol 2022;65:1–7. [DOI] [PubMed] [Google Scholar]
- 668. Proietti R, Pecoraro V, Di Biase L, Natale A, Santangeli P, Viecca M, et al. Remote magnetic with open-irrigated catheter vs. manual navigation for ablation of atrial fibrillation: a systematic review and metaanalysis. Europace 2013;15:1241–8. [DOI] [PubMed] [Google Scholar]
- 669. Jia K, Jin Q, Liu A, Wu L. Remote magnetic navigation versus manual control navigation for atrial fibrillation ablation: a systematic review and metaanalysis. J Electrocardiol 2019;55:78–86. [DOI] [PubMed] [Google Scholar]
- 670. Li X, Bao Y, Jia K, Zhang N, Lin C, Wei Y, et al. Comparison of the mid-term outcomes of robotic magnetic navigation-guided radiofrequency ablation versus cryoballoon ablation for persistent atrial fibrillation. J Cardiovasc Dev Dis 2022;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Verma A, Feld GK, Cox JL, Dewland TA, Babkin A, De Potter T, et al. Combined pulsed field ablation with ultra-low temperature cryoablation: a pre-clinical experience. J Cardiovasc Electrophysiol 2022;34:2124–33. [DOI] [PubMed] [Google Scholar]
- 672. Hilbert S, Sommer P, Gutberlet M, Gaspar T, Foldyna B, Piorkowski C, et al. Real-time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series. Europace 2016;18:572–7. [DOI] [PubMed] [Google Scholar]
- 673. Lehmann HI, Graeff C, Simoniello P, Constantinescu A, Takami M, Lugenbiel P, et al. Feasibility study on cardiac arrhythmia ablation using high-energy heavy ion beams. Sci Rep 2016;6:38895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674. Ströker E, de Asmundis C, Kupics K, Takarada K, Mugnai G, De Cocker J, et al. Value of ultrasound for access guidance and detection of subclinical vascular complications in the setting of atrial fibrillation cryoballoon ablation. Europace 2019;21:434–9. [DOI] [PubMed] [Google Scholar]
- 675. Wynn GJ, Haq I, Hung J, Bonnett LJ, Lewis G, Webber M, et al. Improving safety in catheter ablation for atrial fibrillation: a prospective study of the use of ultrasound to guide vascular access. J Cardiovasc Electrophysiol 2014;25:680–5. [DOI] [PubMed] [Google Scholar]
- 676. Yamagata K, Wichterle D, Roubicek T, Jarkovsky P, Sato Y, Kogure T, et al. Ultrasound-guided versus conventional femoral venipuncture for catheter ablation of atrial fibrillation: a multicenter randomized efficacy and safety trial (ULTRA-FAST trial). Europace 2018;20:1107–14. [DOI] [PubMed] [Google Scholar]
- 677. Sharma PS, Padala SK, Gunda S, Koneru JN, Ellenbogen KA. Vascular complications during catheter ablation of cardiac arrhythmias: a comparison between vascular ultrasound guided access and conventional vascular access. J Cardiovasc Electrophysiol 2016;27:1160–6. [DOI] [PubMed] [Google Scholar]
- 678. Pellegrino PL, Di Monaco A, Santoro F, Grimaldi M, D’Arienzo G, Casavecchia G, et al. Near zero vascular complications using echo-guided puncture during catheter ablation of arrhythmias: a retrospective study and literature review. J Arrhythm 2022;38:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679. Sobolev M, Shiloh AL, Di Biase L, Slovut DP. Ultrasound-guided cannulation of the femoral vein in electrophysiological procedures: a systematic review and metaanalysis. Europace 2017;19:850–5. [DOI] [PubMed] [Google Scholar]
- 680. Wang TKM, Wang MTM, Martin A. Meta-analysis of ultrasound-guided vs conventional vascular access for cardiac electrophysiology procedures. J Arrhythm 2019;35:858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. Verma A, Debruyne P, Nardi S, Deneke T, DeGreef Y, Spitzer S, et al. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol 2013;6:835–42. [DOI] [PubMed] [Google Scholar]
- 682. Yu Y, Wang X, Li X, Zhou X, Liao S, Yang W, et al. Higher incidence of asymptomatic cerebral emboli after atrial fibrillation ablation found with high-resolution diffusion-weighted magnetic resonance imaging. Circ Arrhythm Electrophysiol 2020;13:e007548. [DOI] [PubMed] [Google Scholar]
- 683. Briceno DF, Villablanca PA, Lupercio F, Kargoli F, Jagannath A, Londono A, et al. Clinical impact of heparin kinetics during catheter ablation of atrial fibrillation: metaanalysis and meta-regression. J Cardiovasc Electrophysiol 2016;27:683–93. [DOI] [PubMed] [Google Scholar]
- 684. Maleki K, Mohammadi R, Hart D, Cotiga D, Farhat N, Steinberg JS. Intracardiac ultrasound detection of thrombus on transseptal sheath: incidence, treatment, and prevention. J Cardiovasc Electrophysiol 2005;16:561–5. [DOI] [PubMed] [Google Scholar]
- 685. Ren JF, Marchlinski FE, Callans DJ, Gerstenfeld EP, Dixit S, Lin D, et al. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast. J Cardiovasc Electrophysiol 2005;16:474–7. [DOI] [PubMed] [Google Scholar]
- 686. Bruce CJ, Friedman PA, Narayan O, Munger TM, Hammill SC, Packer DL, et al. Early heparinization decreases the incidence of left atrial thrombi detected by intracardiac echocardiography during radiofrequency ablation for atrial fibrillation. J Interv Card Electrophysiol 2008;22:211–9. [DOI] [PubMed] [Google Scholar]
- 687. Asbach S, Biermann J, Bode C, Faber TS. Early heparin administration reduces risk for left atrial thrombus formation during atrial fibrillation ablation procedures. Cardiol Res Pract 2011;2011:615087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688. Di Biase L, Gaita F, Toso E, Santangeli P, Mohanty P, Rutledge N, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm 2014;11:791–8. [DOI] [PubMed] [Google Scholar]
- 689. Meininghaus DG, Blembel K, Waniek C, Kruells-Muench J, Ernst H, Kleemann T, et al. Temperature monitoring and temperature-driven irrigated radiofrequency energy titration do not prevent thermally induced esophageal lesions in pulmonary vein isolation: a randomized study controlled by esophagoscopy before and after catheter ablation. Heart Rhythm 2021;18:926–34. [DOI] [PubMed] [Google Scholar]
- 690. Schoene K, Arya A, Grashoff F, Knopp H, Weber A, Lerche M, et al. Oesophageal probe evaluation in radiofrequency ablation of atrial fibrillation (OPERA): results from a prospective randomized trial. Europace 2020;22:1487–94. [DOI] [PubMed] [Google Scholar]
- 691. Deneke T, Nentwich K, Berkovitz A, Sonne K, Ene E, Pavlov B, et al. High-resolution infrared thermal imaging of the esophagus during atrial fibrillation ablation as a predictor of endoscopically detected thermal lesions. Circ Arrhythm Electrophysiol 2018;11:e006681. [DOI] [PubMed] [Google Scholar]
- 692. Ayoub T, El Hajjar AH, Singh Sidhu GD, Bhatnagar A, Zhang Y, Mekhael M, et al. Esophageal temperature during atrial fibrillation ablation poorly predicts esophageal injury: an observational study. Heart Rhythm O2 2021;2:570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693. Ha FJ, Han HC, Sanders P, Teh AW, O’Donnell D, Farouque O, et al. Prevalence and prevention of esophageal injury during atrial fibrillation ablation: a systematic review and metaanalysis. Europace 2019;21:80–90. [DOI] [PubMed] [Google Scholar]
- 694. Chen S, Schmidt B, Seeger A, Bordignon S, Tohoku S, Willems F, et al. Catheter ablation of atrial fibrillation using ablation index-guided high power (50 W) for pulmonary vein isolation with or without esophageal temperature probe (the AI-HP ESO II). Heart Rhythm 2020;17:1833–40. [DOI] [PubMed] [Google Scholar]
- 695. Garcia R, Waldmann V, Vanduynhoven P, Nesti M, Jansen de Oliveira Figueiredo M, Narayanan K, et al. Worldwide sedation strategies for atrial fibrillation ablation: current status and evolution over the last decade. Europace 2021;23:2039–45. [DOI] [PubMed] [Google Scholar]
- 696. Dada RS, Hayanga JWA, Woods K, Schwartzman D, Thibault D, Ellison M, et al. Anesthetic choice for atrial fibrillation ablation: a national anesthesia clinical outcomes registry analysis. J Cardiothorac Vasc Anesth 2021;35:2600–6. [DOI] [PubMed] [Google Scholar]
- 697. Di Biase L, Conti S, Mohanty P, Bai R, Sanchez J, Walton D, et al. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm 2011;8:368–72. [DOI] [PubMed] [Google Scholar]
- 698. Chikata A, Kato T, Yaegashi T, Sakagami S, Kato C, Saeki T, et al. General anesthesia improves contact force and reduces gap formation in pulmonary vein isolation: a comparison with conscious sedation. Heart Vessels 2017;32:997–1005. [DOI] [PubMed] [Google Scholar]
- 699. Martin CA, Curtain JP, Gajendragadkar PR, Begley DA, Fynn SP, Grace AA, et al. Improved outcome and cost effectiveness in ablation of persistent atrial fibrillation under general anesthetic. Europace 2018;20:935–42. [DOI] [PubMed] [Google Scholar]
- 700. Pang N, Gao J, Zhang N, Zhang B, Wang R. Comparison of the different anesthesia strategies for atrial fibrillation catheter ablation: a systematic review and metaanalysis. Cardiol Res Pract 2022;2022;1124372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701. Weinmann K, Heudorfer R, Lenz A, Aktolga D, Rattka M, Bothner C, et al. Safety of conscious sedation in electroanatomical mapping procedures and cryoballoon pulmonary vein isolation. Heart Vessels 2021;36:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702. Wasserlauf J, Knight BP, Li Z, Andrei AC, Arora R, Chicos AB, et al. Moderate sedation reduces lab time compared to general anesthesia during cryoballoon ablation for AF without compromising safety or long-term efficacy. Pacing Clin Electrophysiol 2016;39:1359–65. [DOI] [PubMed] [Google Scholar]
- 703. Tohoku S, Schmidt B, Bordignon S, Chen S, Bologna F, Chun JK. Initial clinical experience of pulmonary vein isolation using the ultra-low temperature cryoablation catheter for patients with atrial fibrillation. J Cardiovasc Electrophysiol 2022;33:1371–9. [DOI] [PubMed] [Google Scholar]
- 704. Schmidt B, Bordignon S, Tohoku S, Chen S, Bologna F, Urbanek L, et al. 5S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol 2022;15:e010817. [DOI] [PubMed] [Google Scholar]
- 705. Iacopino S, Colella J, Dini D, Mantovani L, Sorrenti PF, Malacrida M, et al. Sedation strategies for pulsed-field ablation of atrial fibrillation: focus on deep sedation with intravenous ketamine in spontaneous respiration. Europace 2023;25:euad230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706. Grimaldi M, Quadrini F, Caporusso N, Troisi F, Vitulano N, Delmonte V, et al. Deep sedation protocol during atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter. Europace 2023;25:euad222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707. Kumar S, Morton JB, Halloran K, Spence SJ, Lee G, Wong MC, et al. Effect of respiration on catheter-tissue contact force during ablation of atrial arrhythmias. Heart Rhythm 2012;9:1041–7.e1. [DOI] [PubMed] [Google Scholar]
- 708. Sivasambu B, Hakim JB, Barodka V, Chrispin J, Berger RD, Ashikaga H, et al. Initiation of a high-frequency jet ventilation strategy for catheter ablation for atrial fibrillation: safety and outcomes data. JACC Clin Electrophysiol 2018;4:1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709. Aizer A, Qiu JK, Cheng AV, Wu PB, Barbhaiya CR, Jankelson L, et al. Rapid pacing and high-frequency jet ventilation additively improve catheter stability during atrial fibrillation ablation. J Cardiovasc Electrophysiol 2020;31:1678–86. [DOI] [PubMed] [Google Scholar]
- 710. Fernandez-Bustamante A, Ibañez V, Alfaro JJ, de Miguel E, Germán MJ, Mayo A, et al. High-frequency jet ventilation in interventional bronchoscopy: factors with predictive value on high-frequency jet ventilation complications. J Clin Anesth 2006;18:349–56. [DOI] [PubMed] [Google Scholar]
- 711. Babapoor-Farrokhran S, Alzubi J, Port Z, Khraisha O, Mainigi SK. Utility of high-frequency jet ventilation in atrial fibrillation ablation. J Innov Card Rhythm Manag 2021;12:4590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712. Osorio J, Varley A, Kreidieh O, Godfrey B, Schrappe G, Rajendra A, et al. High-frequency, low-tidal-volume mechanical ventilation safely improves catheter stability and procedural efficiency during radiofrequency ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2022;15:e010722. [DOI] [PubMed] [Google Scholar]
- 713. Gabriels J, Donnelly J, Khan M, Anca D, Beldner S, Willner J, et al. High-frequency, low tidal volume ventilation to improve catheter stability during atrial fibrillation ablation. JACC Clin Electrophysiol 2019;5:1224–6. [DOI] [PubMed] [Google Scholar]
- 714. Kadado AJ, Gobeil K, Fakhoury F, Pervaiz A, Chalhoub F. Very low tidal volume, high-frequency ventilation in atrial fibrillation ablation: a systematic review. J Interv Card Electrophysiol 2022;64:539–43. [DOI] [PubMed] [Google Scholar]
- 715. Osorio J, Zei PC, Díaz JC, Varley AL, Morales GX, Silverstein JR, et al. High-frequency low-tidal-volume ventilation improves long-term outcomes in atrial fibrillation ablation: a multicenter prospective study. JACC Clin Electrophysiol 2023;9:1543–54. [DOI] [PubMed] [Google Scholar]
- 716. Hoyt H, Bhonsale A, Chilukuri K, Alhumaid F, Needleman M, Edwards D, et al. Complications arising from catheter ablation of atrial fibrillation: temporal trends and predictors. Heart Rhythm 2011;8:1869–74. [DOI] [PubMed] [Google Scholar]
- 717. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128:2104–12. [DOI] [PubMed] [Google Scholar]
- 718. Iqbal AM, Li KY, Aznaurov SG, Lugo RM, Venkataraman R, Gautam S. Catheter ablation for atrial fibrillation can be safely performed without invasive hemodynamic monitoring: a multi-center study. J Interv Card Electrophysiol 2022;64:743–9. [DOI] [PubMed] [Google Scholar]
- 719. Deneke T, Shin DI, Balta O, Bünz K, Fassbender F, Mügge A, et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm 2011;8:1705–11. [DOI] [PubMed] [Google Scholar]
- 720. Maleki K, Mohammadi R, Hart D, Cotiga D, Farhat N, Steinberg JS. Intracardiac ultrasound detection of thrombus on transseptal sheath: incidence, treatment, and prevention. J Cardiovasc Electrophysiol 2005;16:561–5. [DOI] [PubMed] [Google Scholar]
- 721. Zhang RF, Ma CM, Wang N, Yang MH, Li WW, Yin XM, et al. Appropriate intraprocedural initial heparin dosing in patients undergoing catheter ablation for atrial fibrillation receiving uninterrupted non-vitamin-K antagonist oral anticoagulant treatment. BMC Cardiovasc Disord 2021;21:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722. Benali K, Verain J, Hammache N, Guenancia C, Hooks D, Magnin-Poull I, et al. Running after activated clotting time values in patients receiving direct oral anticoagulants: a potentially dangerous race. Results from a prospective study in atrial fibrillation catheter ablation procedures. J Clin Med 2021;10:4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723. Hohnloser SH, Camm AJ, Cappato R, Diener HC, Heidbuchel H, Mont L, et al. Periprocedural anticoagulation in the uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation (ELIMINATE-AF) trial. Europace 2021;23:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724. Calkins H, Willems S, Verma A, Schilling R, Hohnloser SH, Okumura K, et al. Heparin dosing in uninterrupted anticoagulation with dabigatran vs. warfarin in atrial fibrillation ablation: RE-CIRCUIT study. Europace 2019;21:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725. Payne JE, Koerber SM, Bickel T, Ghadban R, Flaker G, Gautam S. Higher initial weight-based heparin dosing is required with direct oral anticoagulants during catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2020;58:185–91. [DOI] [PubMed] [Google Scholar]
- 726. Martin AC, Kyheng M, Foissaud V, Duhamel A, Marijon E, Susen S, et al. Activated clotting time monitoring during atrial fibrillation catheter ablation: does the anticoagulant matter? J Clin Med 2020;9:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727. Songqun H, Chunling W, Zhifu G, Xinmiao H, Jiang C. Effects of rivaroxaban on activated clotting time in catheter ablation for atrial fibrillation in Chinese patients. J Interv Card Electrophysiol 2020;59:509–16. [DOI] [PubMed] [Google Scholar]
- 728. Safani M, Tobias S, Shandling AH, Redmond K, Lee MY. Comprehensive intraprocedural unfractionated heparin protocol during catheter ablation of atrial fibrillation in the presence of direct oral anticoagulants and wide spectrum of body mass index. J Cardiovasc Pharmacol Ther 2021;26:349–58. [DOI] [PubMed] [Google Scholar]
- 729. Ghannam M, Chugh A, Dillon P, Alyesh D, Kossidas K, Sharma S, et al. Protamine to expedite vascular hemostasis after catheter ablation of atrial fibrillation: a randomized controlled trial. Heart Rhythm 2018;15:1642–7. [DOI] [PubMed] [Google Scholar]
- 730. Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511–20. [DOI] [PubMed] [Google Scholar]
- 731. Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med 2015;373:2413–24. [DOI] [PubMed] [Google Scholar]
- 732. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. [DOI] [PubMed] [Google Scholar]
- 733. Hsu JC, Badhwar N, Gerstenfeld EP, Lee RJ, Mandyam MC, Dewland TA, et al. Randomized trial of conventional transseptal needle versus radiofrequency energy needle puncture for left atrial access (the TRAVERSE-LA study). J Am Heart Assoc 2013;2:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734. Andrade JG, Macle L, Bennett MT, Hawkins NM, Essebag V, Champagne J, et al. Randomized trial of conventional versus radiofrequency needle transseptal puncture for cryoballoon ablation: the CRYO-LATS trial. J Interv Card Electrophysiol 2022;65:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735. Tokuda M, Yamashita S, Matsuo S, Kato M, Sato H, Oseto H, et al. Radiofrequency needle for transseptal puncture is associated with lower incidence of thromboembolism during catheter ablation of atrial fibrillation: propensity score-matched analysis. Heart Vessels 2018;33:1238–44. [DOI] [PubMed] [Google Scholar]
- 736. Chow AWC, Cobb V, Sepahpour A, McCready JW. Transseptal puncture performed with the new needle-free ‘SafeSept’ guidewire: a multicenter experience. J Interv Card Electrophysiol 2020;59:29–34. [DOI] [PubMed] [Google Scholar]
- 737. Maclean E, Mahtani K, Roelas M, Vyas R, Butcher C, Ahluwalia N, et al. Transseptal puncture for left atrial ablation: risk factors for cardiac tamponade and a proposed causative classification system. J Cardiovasc Electrophysiol 2022;33:1747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738. Stauber A, Kornej J, Sepehri Shamloo A, Dinov B, Bacevicius J, Dagres N, et al. Impact of single versus double transseptal puncture on outcome and complications in pulmonary vein isolation procedures. Cardiol J 2021;28:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739. Deyell MW, Wen G, Laksman Z, Bennett MT, Chakrabarti S, Yeung-Lai-Wah JA, et al. The impact of steerable sheaths on unblinded contact force during catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2020;57:417–24. [DOI] [PubMed] [Google Scholar]
- 740. Piorkowski C, Eitel C, Rolf S, Bode K, Sommer P, Gaspar T, et al. Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: a prospective, randomized study. Circ Arrhythm Electrophysiol 2011;4:157–65. [DOI] [PubMed] [Google Scholar]
- 741. Janosi K, Debreceni D, Janosa B, Bocz B, Simor T, Kupo P. Visualizable vs. standard, non-visualizable steerable sheath for pulmonary vein isolation procedures: randomized, single-center trial. Front Cardiovasc Med 2022;9:1033755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742. Rajendra A, Hunter TD, Morales GX, Zei P, Boo LM, Varley A, et al. Steerable sheath visualizable under 3D electroanatomical mapping facilitates paroxysmal atrial fibrillation ablation with minimal fluoroscopy. J Interv Card Electrophysiol 2023;66:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743. Fitzpatrick N, Mittal A, Galvin J, Jauvert G, Keaney J, Keelan E, et al. The impact of steerable sheath visualization during catheter ablation for atrial fibrillation. Europace 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744. Khalaph M, Sommer P, Lucas P, Guckel D, Fink T, Sciacca V, et al. First clinical experience using a visualized sheath for atrial fibrillation ablation. Pacing Clin Electrophysiol 2022;45:922–9. [DOI] [PubMed] [Google Scholar]
- 745. Goya M, Frame D, Gache L, Ichishima Y, Tayar DO, Goldstein L, et al. The use of intracardiac echocardiography catheters in endocardial ablation of cardiac arrhythmia: metaanalysis of efficiency, effectiveness, and safety outcomes. J Cardiovasc Electrophysiol 2020;31:664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746. Xu J, Gao Y, Liu C, Wang Y. Radiofrequency ablation for treatment of atrial fibrillation with the use of intracardiac echocardiography versus without intracardiac echocardiography: a metaanalysis of observational and randomized studies. J Cardiovasc Electrophysiol 2022;33:897–907. [DOI] [PubMed] [Google Scholar]
- 747. Pimentel RC, Rahai N, Maccioni S, Khanna R. Differences in outcomes among patients with atrial fibrillation undergoing catheter ablation with versus without intracardiac echocardiography. J Cardiovasc Electrophysiol 2022;33:2015–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748. La Greca C, Cirasa A, Di Modica D, Sorgato A, Simoncelli U, Pecora D. Advantages of the integration of ICE and 3D electroanatomical mapping and ultrasound-guided femoral venipuncture in catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2021;61:559–66. [DOI] [PubMed] [Google Scholar]
- 749. Isath A, Padmanabhan D, Haider SW, Siroky G, Perimbeti S, Correa A, et al. Does the use of intracardiac echocardiography during atrial fibrillation catheter ablation improve outcomes and cost? A nationwide 14-year analysis from 2001 to 2014. J Interv Card Electrophysiol 2021;61:461–8. [DOI] [PubMed] [Google Scholar]
- 750. Deshpande S, Sawatari H, Ahmed R, Nair RG, Khan H, Khanji MY, et al. Impact of intracardiac echocardiography on readmission morbidity and mortality following atrial fibrillation ablation. J Cardiovasc Electrophysiol 2022;33:2496–503. [DOI] [PubMed] [Google Scholar]
- 751. Ren JF, Chen S, Callans DJ, Jiang C, Marchlinski FE. Role of intracardiac echocardiography for catheter ablation of atrial fibrillation: reduction of complications and mortality. J Am Coll Cardiol 2020;75:1244–5. [DOI] [PubMed] [Google Scholar]
- 752. Catanzariti D, Maines M, Angheben C, Centonze M, Cemin C, Vergara G. Usefulness of contrast intracardiac echocardiography in performing pulmonary vein balloon occlusion during cryo-ablation for atrial fibrillation. Indian Pacing Electrophysiol J 2012;12:237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753. Makino Y, Mizutani Y, Yamashita D, Yonekawa J, Satake A, Kurobe M, et al. Cryoballoon ablation for atrial fibrillation without the use of a contrast medium: a combination of the intracardiac echocardiography and pressure wave monitoring guided approach. Heart Vessels 2022;37:765–74. [DOI] [PubMed] [Google Scholar]
- 754. Suzuki A, Fujiwara R, Asada H, Iwasa K, Miyata T, Song WH, et al. Peri-balloon leak flow velocity assessed by intracardiac echography predicts pulmonary vein electrical gap-intracardiac echography-guided contrast-free cryoballoon ablation. Circ J 2022;86:256–65. [DOI] [PubMed] [Google Scholar]
- 755. Kanda T, Masuda M, Kurata N, Asai M, Iida O, Okamoto S, et al. A saline contrast-enhanced echocardiography-guided approach to cryoballoon ablation. Pacing Clin Electrophysiol 2020;43:664–70. [DOI] [PubMed] [Google Scholar]
- 756. Motoike Y, Harada M, Ito T, Nomura Y, Nishimura A, Koshikawa M, et al. Wall thickness-based adjustment of ablation index improves efficacy of pulmonary vein isolation in atrial fibrillation: real-time assessment by intracardiac echocardiography. J Cardiovasc Electrophysiol 2021;32:1620–30. [DOI] [PubMed] [Google Scholar]
- 757. Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. J Am Coll Cardiol 2010;56:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758. Lickfett L, Mahesh M, Vasamreddy C, Bradley D, Jayam V, Eldadah Z, et al. Radiation exposure during catheter ablation of atrial fibrillation. Circulation 2004;110:3003–10. [DOI] [PubMed] [Google Scholar]
- 759. Ector J, Dragusin O, Adriaenssens B, Huybrechts W, Willems R, Ector H, et al. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol 2007;50:234–42. [DOI] [PubMed] [Google Scholar]
- 760. Voskoboinik A, Kalman ES, Savicky Y, Sparks PB, Morton JB, Lee G, et al. Reduction in radiation dose for atrial fibrillation ablation over time: a 12-year single-center experience of 2344 patients. Heart Rhythm 2017;14:810–16. [DOI] [PubMed] [Google Scholar]
- 761. Lee G, Hunter RJ, Lovell MJ, Finlay M, Ullah W, Baker V, et al. Use of a contact force-sensing ablation catheter with advanced catheter location significantly reduces fluoroscopy time and radiation dose in catheter ablation of atrial fibrillation. Europace 2016;18:211–8. [DOI] [PubMed] [Google Scholar]
- 762. Walters TE, Kistler PM, Morton JB, Sparks PB, Halloran K, Kalman JM. Impact of collimation on radiation exposure during interventional electrophysiology. Europace 2012;14:1670–3. [DOI] [PubMed] [Google Scholar]
- 763. Schneider R, Lauschke J, Schneider C, Tischer T, Glass A, Bänsch D. Reduction of radiation exposure during ablation of atrial fibrillation. Herz 2015;40:883–91. [DOI] [PubMed] [Google Scholar]
- 764. Heidbuchel H, Wittkampf FH, Vano E, Ernst S, Schilling R, Picano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace 2014;16:946–64. [DOI] [PubMed] [Google Scholar]
- 765. Sommer P, Sciacca V, Anselmino M, Tilz R, Bourier F, Lehrmann H, et al. Practical guidance to reduce radiation exposure in electrophysiology applying ultra low-dose protocols: a European heart rhythm association review. Europace 2023;25:euad191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766. Zei PC, Quadros KK, Clopton P, Thosani A, Ferguson J, Brodt C, et al. Safety and efficacy of minimal- versus zero-fluoroscopy radiofrequency catheter ablation for atrial fibrillation: a multicenter, prospective study. J Innov Card Rhythm Manag 2020;11:4281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767. Tahin T, Riba A, Nemeth B, Arvai F, Lupkovics G, Szeplaki G, et al. Implementation of a zero fluoroscopic workflow using a simplified intracardiac echocardiography guided method for catheter ablation of atrial fibrillation, including repeat procedures. BMC Cardiovasc Disord 2021;21:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768. Lyan E, Tsyganov A, Abdrahmanov A, Morozov A, Bakytzhanuly A, Tursunbekov A, et al. Nonfluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2018;41:611–9. [DOI] [PubMed] [Google Scholar]
- 769. Falasconi G, Penela D, Soto-Iglesias D, Jauregui B, Chauca A, Antonio RS, et al. A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J Interv Card Electrophysiol 2022;64:629–39. [DOI] [PubMed] [Google Scholar]
- 770. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol 2015;38:797–806. [DOI] [PubMed] [Google Scholar]
- 771. Lurie A, Amit G, Divakaramenon S, Acosta JG, Healey JS, Wong JA. Outcomes and safety of fluoroless catheter ablation for atrial fibrillation. CJC Open 2021;3:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772. Sommer P, Bertagnolli L, Kircher S, Arya A, Bollmann A, Richter S, et al. Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: experience from 1000 consecutive procedures. Europace 2018;20:1952–8. [DOI] [PubMed] [Google Scholar]
- 773. Enriquez A, Velasco A, Diaz JC, Sadek M, Osorio J, Zei P, et al. Fluoroless catheter ablation of atrial fibrillation: a step-by-step workflow. J Interv Card Electrophysiol 2023;66:1291–301. [DOI] [PubMed] [Google Scholar]
- 774. Ahn J, Shin DG, Han S-J, Lim HE. Safety and efficacy of intracardiac echocardiography–guided zero-fluoroscopic cryoballoon ablation for atrial fibrillation: a prospective randomized controlled trial. Europace 2023;25:euad086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775. Alyesh D, Venkataraman G, Stucky A, Joyner J, Choe W, Sundaram S. Acute safety and efficacy of fluoroless cryoballoon ablation for atrial fibrillation. J Innov Card Rhythm Manag 2021;12:4413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776. Halbfass P, Pavlov B, Müller P, Nentwich K, Sonne K, Barth S, et al. Progression from esophageal thermal asymptomatic lesion to perforation complicating atrial fibrillation ablation: a single-center registry. Circ Arrhythm Electrophysiol 2017;10:e005233. [DOI] [PubMed] [Google Scholar]
- 777. Ripley KL, Gage AA, Olsen DB, Van Vleet JF, Lau CP, Tse HF. Time course of esophageal lesions after catheter ablation with cryothermal and radiofrequency ablation: implication for atrio-esophageal fistula formation after catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:642–6. [DOI] [PubMed] [Google Scholar]
- 778. Tschabrunn CM, Silverstein J, Berzin T, Ellis E, Buxton AE, Josephson ME, et al. Comparison between single- and multisensor esophageal temperature probes during atrial fibrillation ablation: thermodynamic characteristics. Europace 2015;17:891–7. [DOI] [PubMed] [Google Scholar]
- 779. Turagam MK, Miller S, Sharma SP, Prakash P, Gopinathannair R, Lakkireddy P, et al. Differences in transient thermal response of commercial esophageal temperature probes: insights from an experimental study. JACC Clin Electrophysiol 2019;5:1280–8. [DOI] [PubMed] [Google Scholar]
- 780. Barbhaiya CR, Kogan EV, Jankelson L, Knotts RJ, Spinelli M, Bernstein S, et al. Esophageal temperature dynamics during high-power short-duration posterior wall ablation. Heart Rhythm 2020;17:721–7. [DOI] [PubMed] [Google Scholar]
- 781. Bhardwaj R, Naniwadekar A, Whang W, Mittnacht AJ, Palaniswamy C, Koruth JS, et al. Esophageal deviation during atrial fibrillation ablation: clinical experience with a dedicated esophageal balloon retractor. JACC Clin Electrophysiol 2018;4:1020–30. [DOI] [PubMed] [Google Scholar]
- 782. Hamed M, Elseidy SA, Abdelazeem M, Morcos R, Abdallah A, Sammour Y, et al. Role of esophageal cooling in the prevention of esophageal injury in atrial fibrillation catheter ablation: a systematic review and metaanalysis of randomized controlled trials. Europace 2023;25:euad080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783. Leung LWM, Bajpai A, Zuberi Z, Li A, Norman M, Kaba RA, et al. Randomized comparison of esophageal protection with a temperature control device: results of the IMPACT study. Europace 2021;23:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784. Joseph C, Nazari J, Zagrodzky J, Brumback B, Sherman J, Zagrodzky W, et al. Improved 1-year outcomes after active cooling during left atrial radiofrequency ablation. J Interv Card Electrophysiol 2023;66:1621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785. Sanchez J, Woods C, Zagrodzky J, Nazari J, Singleton MJ, Schricker A, et al. Atrioesophageal fistula rates before and after adoption of active esophageal cooling during atrial fibrillation ablation. JACC Clin Electrophysiol 2023;9:2558–70. [DOI] [PubMed] [Google Scholar]
- 786. Teres C, Soto-Iglesias D, Penela D, Falasconi G, Viveros D, Meca-Santamaria J, et al. Relationship between the posterior atrial wall and the esophagus: esophageal position and temperature measurement during atrial fibrillation ablation (AWESOME-AF). A randomized controlled trial. J Interv Card Electrophysiol 2022;65:651–61. [DOI] [PubMed] [Google Scholar]
- 787. Zhang X, Kuang X, Gao X, Xiang H, Wei F, Liu T, et al. RESCUE-AF in patients undergoing atrial fibrillation ablation: the RESCUE-AF trial. Circ Arrhythm Electrophysiol 2019;12:e007044. [DOI] [PubMed] [Google Scholar]
- 788. Reddy VY, Dukkipati SR, Neuzil P, Natale A, Albenque J-P, Kautzner J, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath contact force ablation catheter study for atrial fibrillation (TOCCASTAR) study. Circulation 2015;132:907–15. [DOI] [PubMed] [Google Scholar]
- 789. Arentz T, Jander N, von Rosenthal J, Blum T, Fürmaier R, Görnandt L, et al. Incidence of pulmonary vein stenosis 2 years after radiofrequency catheter ablation of refractory atrial fibrillation. Eur Heart J 2003;24:963–9. [DOI] [PubMed] [Google Scholar]
- 790. Mochizuki A, Nagahara D, Kamiyama N, Fujito T, Miura T. Revaluation of the significance of demonstrable exit block after radiofrequency pulmonary vein isolation. Circ Rep 2020;2:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791. Duytschaever M, De Meyer G, Acena M, El-Haddad M, De Greef Y, Van Heuverswyn F, et al. Lessons from dissociated pulmonary vein potentials: entry block implies exit block. Europace 2013;15:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792. Chen S, Meng W, Sheng He D, Chen G, Zhang F, Yan Y, et al. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol 2012;35:524–31. [DOI] [PubMed] [Google Scholar]
- 793. Wang X-h, Liu X, Sun Y-m, Gu J-n, Shi H-f, Zhou L, et al. Early identification and treatment of PV re-connections: role of observation time and impact on clinical results of atrial fibrillation ablation. Europace 2007;9:481–6. [DOI] [PubMed] [Google Scholar]
- 794. Jiang CY, Jiang RH, Matsuo S, Liu Q, Fan YQ, Zhang ZW, et al. Early detection of pulmonary vein reconnection after isolation in patients with paroxysmal atrial fibrillation: a comparison of ATP-induction and reassessment at 30 minutes postisolation. J Cardiovasc Electrophysiol 2009;20:1382–7. [DOI] [PubMed] [Google Scholar]
- 795. Cheema A, Dong J, Dalal D, Marine JE, Henrikson CA, Spragg D, et al. Incidence and time course of early recovery of pulmonary vein conduction after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:387–91. [DOI] [PubMed] [Google Scholar]
- 796. Brunelli M, Raffa S, Große A, Hanazawa K, Sammut M, Roos M, et al. Residual conduction after pulmonary vein isolation with a circular multielectrode radiofrequency ablation catheter: the role of adenosine and orciprenalin during a prolonged observation time. Int J Cardiol 2013;168:4122–31. [DOI] [PubMed] [Google Scholar]
- 797. Efremidis M, Letsas K, Giannopoulos G, Lioni L, Vlachos K, Asvestas D, et al. Early pulmonary vein reconnection as a predictor of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace 2015;17:741–6. [DOI] [PubMed] [Google Scholar]
- 798. Andrade JG, Deyell MW, Nattel S, Khairy P, Dubuc M, Champagne J, et al. Prevalence and clinical impact of spontaneous and adenosine-induced pulmonary vein reconduction in the contact-force vs. cryoballoon atrial fibrillation ablation (CIRCA-DOSE) study. Heart Rhythm 2020;17:897–904. [DOI] [PubMed] [Google Scholar]
- 799. Jiang R, Chen M, Yang B, Liu Q, Zhang Z, Zhang F, et al. Intraprocedural endpoints to predict durable pulmonary vein isolation: a randomized trial of four postablation techniques. Europace 2020;22:567–75. [DOI] [PubMed] [Google Scholar]
- 800. Sousa PA, Barra S, Adão L, Primo J, Khoueiry Z, Puga L, et al. Assessment of the need of a waiting period after pulmonary vein isolation with the ablation index software. J Cardiovasc Electrophysiol 2022;33:1725–33. [DOI] [PubMed] [Google Scholar]
- 801. Miller MA, d’Avila A, Dukkipati SR, Koruth JS, Viles-Gonzalez J, Napolitano C, et al. Acute electrical isolation is a necessary but insufficient endpoint for achieving durable PV isolation: the importance of closing the visual gap. Europace 2012;14:653–60. [DOI] [PubMed] [Google Scholar]
- 802. Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, et al. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicenter, randomised superiority trial. Lancet 2015;386:672–9. [DOI] [PubMed] [Google Scholar]
- 803. Kapa S, Killu A, Deshmukh A, Mulpuru SK, Asirvatham SJ. Dose-dependent pulmonary vein reconnection in response to adenosine: relevance of atrioventricular block during infusion. J Interv Card Electrophysiol 2016;47:117–23. [DOI] [PubMed] [Google Scholar]
- 804. Teunissen C, Clappers N, Kassenberg W, Hassink RJ, van der Heijden JF, Loh P. Time matters: adenosine testing immediately after pulmonary vein isolation does not substitute a waiting period. Europace 2017;19:1140–5. [DOI] [PubMed] [Google Scholar]
- 805. McLellan AJA, Kumar S, Smith C, Ling LH, Prabhu S, Kalman JM, et al. The role of adenosine challenge in catheter ablation for atrial fibrillation: a systematic review and metaanalysis. Int J Cardiol 2017;236:253–61. [DOI] [PubMed] [Google Scholar]
- 806. Kobori A, Shizuta S, Inoue K, Kaitani K, Morimoto T, Nakazawa Y, et al. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: the unmasking dormant electrical reconduction by adenosine triphosphate (UNDER-ATP) trial. Eur Heart J 2015;36:3276–87. [DOI] [PubMed] [Google Scholar]
- 807. Zeng LJ, Shi L, Tian Y, Wang YJ, Yin XD, Liu XQ, et al. Pace capture and adenosine triphosphate provocation are complementary rather than mutually exclusive methods to ensure durable pulmonary vein isolation. J Cardiovasc Electrophysiol 2019;30:815–23. [DOI] [PubMed] [Google Scholar]
- 808. Moser J, Sultan A, Luker J, Servatius H, Salzbrunn T, Altenburg M, et al. 5-Year outcome of pulmonary vein isolation by loss of pace capture on the ablation line versus electrical circumferential pulmonary vein isolation. JACC Clin Electrophysiol 2017;3:1262–71. [DOI] [PubMed] [Google Scholar]
- 809. Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto K, et al. Pace-capture-guided ablation after contact-force-guided pulmonary vein isolation: results of the randomized controlled DRAGON trial. Europace 2018;20:1451–8. [DOI] [PubMed] [Google Scholar]
- 810. Steven D, Sultan A, Reddy V, Luker J, Altenburg M, Hoffmann B, et al. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J Am Coll Cardiol 2013;62:44–50. [DOI] [PubMed] [Google Scholar]
- 811. Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:243–8. [DOI] [PubMed] [Google Scholar]
- 812. Ouyang F, Ernst S, Vogtmann T, Goya M, Volkmer M, Schaumann A, et al. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: critical isthmus block can prevent atrial tachycardia recurrence. Circulation 2002;105:1934–42. [DOI] [PubMed] [Google Scholar]
- 813. Mujović N, Marinković M, Marković N, Stanković G, Lip GYH, Blomstrom-Lundqvist C, et al. Persistency of left atrial linear lesions after radiofrequency catheter ablation for atrial fibrillation: data from an invasive follow-up electrophysiology study. J Cardiovasc Electrophysiol 2017;28:1403–14. [DOI] [PubMed] [Google Scholar]
- 814. Sanchez-Somonte P, Jiang CY, Betts TR, Chen J, Mantovan R, Macle L, et al. Completeness of linear or fractionated electrogram ablation in addition to pulmonary vein isolation on ablation outcome: a substudy of the STAR AF II trial. Circ Arrhythm Electrophysiol 2021;14:e010146. [DOI] [PubMed] [Google Scholar]
- 815. Chae S, Oral H, Good E, Dey S, Wimmer A, Crawford T, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol 2007;50:1781–7. [DOI] [PubMed] [Google Scholar]
- 816. Tzeis S, Luik A, Jilek C, Schmitt C, Estner HL, Wu J, et al. The modified anterior line: an alternative linear lesion in perimitral flutter. J Cardiovasc Electrophysiol 2010;21:665–70. [DOI] [PubMed] [Google Scholar]
- 817. Pappone C, Manguso F, Vicedomini G, Gugliotta F, Santinelli O, Ferro A, et al. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 2004;110:3036–42. [DOI] [PubMed] [Google Scholar]
- 818. Takagi T, Derval N, Duchateau J, Chauvel R, Tixier R, Marchand H, et al. Gaps after linear ablation of persistent atrial fibrillation (Marshall-PLAN): clinical implication. Heart Rhythm 2023;20:14–21. [DOI] [PubMed] [Google Scholar]
- 819. Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109–18. [DOI] [PubMed] [Google Scholar]
- 820. Ikenouchi T, Nitta J, Inaba O, Kono T, Murata K, Takamiya T, et al. Effect of isolation feasibility of non-pulmonary vein foci on efficacy of ablation for atrial fibrillation: comparison of the isolation and focal ablation methods. J Interv Card Electrophysiol 2022;65:441–51. [DOI] [PubMed] [Google Scholar]
- 821. Della Rocca DG, Di Biase L, Mohanty S, Trivedi C, Gianni C, Romero J, et al. Targeting non-pulmonary vein triggers in persistent atrial fibrillation: results from a prospective, multicenter, observational registry. Europace 2021;23:1939–49. [DOI] [PubMed] [Google Scholar]
- 822. Takamiya T, Nitta J, Inaba O, Sato A, Ikenouchi T, Murata K, et al. One-year outcomes after pulmonary vein isolation plus posterior wall isolation and additional non-pulmonary vein trigger ablation for persistent atrial fibrillation with or without contact force sensing: a propensity score-matched comparison. J Interv Card Electrophysiol 2020;59:585–93. [DOI] [PubMed] [Google Scholar]
- 823. Zhao Y, Di Biase L, Trivedi C, Mohanty S, Bai R, Mohanty P, et al. Importance of non-pulmonary vein triggers ablation to achieve long-term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm 2016;13:141–9. [DOI] [PubMed] [Google Scholar]
- 824. Nakashima T, Pambrun T, Vlachos K, Goujeau C, André C, Krisai P, et al. Impact of vein of Marshall ethanol infusion on mitral isthmus block: efficacy and durability. Circ Arrhythm Electrophysiol 2020;13:e008884. [DOI] [PubMed] [Google Scholar]
- 825. Valderrábano M, Liu X, Sasaridis C, Sidhu J, Little S, Khoury DS. Ethanol infusion in the vein of Marshall: adjunctive effects during ablation of atrial fibrillation. Heart Rhythm 2009;6:1552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826. Gillis K, O’Neill L, Wielandts JY, Hilfiker G, Almorad A, Lycke M, et al. Vein of Marshall ethanol infusion as first step for mitral isthmus linear ablation. JACC Clin Electrophysiol 2022;8:367–76. [DOI] [PubMed] [Google Scholar]
- 827. Yang G, Zheng L, Jiang C, Fan J, Liu X, Zhan X, et al. Circumferential pulmonary vein isolation plus low-voltage area modification in persistent atrial fibrillation: the STABLE-SR-II trial. JACC Clin Electrophysiol 2022;8:882–91. [DOI] [PubMed] [Google Scholar]
- 828. Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger Franz X, et al. Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evidence 2022;1:EVIDoa2200141. [DOI] [PubMed] [Google Scholar]
- 829. Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:22–30. [DOI] [PubMed] [Google Scholar]
- 830. Valderrábano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN, et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA 2020;324:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831. Huang L, Gao M, Lai Y, Guo Q, Li S, Li C, et al. The adjunctive effect for left pulmonary vein isolation of vein of Marshall ethanol infusion in persistent atrial fibrillation. Europace 2023;25:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832. Nakashima T, Pambrun T, Vlachos K, Goujeau C, André C, Krisai P, et al. Strategy for repeat procedures in patients with persistent atrial fibrillation: systematic linear ablation with adjunctive ethanol infusion into the vein of Marshall versus electrophysiology-guided ablation. J Cardiovasc Electrophysiol 2022;33:1116–24. [DOI] [PubMed] [Google Scholar]
- 833. Lador A, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN, et al. Determinants of outcome impact of vein of Marshall ethanol infusion when added to catheter ablation of persistent atrial fibrillation: a secondary analysis of the VENUS randomized clinical trial. Heart Rhythm 2021;18:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834. Derval N, Duchateau J, Denis A, Ramirez FD, Mahida S, André C, et al. Marshall bundle elimination, pulmonary vein isolation, and line completion for ANATOmical ablation of persistent atrial fibrillation (Marshall-PLAN): prospective, single-center study. Heart Rhythm 2021;18:529–37. [DOI] [PubMed] [Google Scholar]
- 835. Dixit S, Marchlinski FE, Lin D, Callans DJ, Bala R, Riley MP, et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol 2012;5:287–94. [DOI] [PubMed] [Google Scholar]
- 836. Bai R, Di Biase L, Mohanty P, Trivedi C, Dello Russo A, Themistoclakis S, et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm 2016;13:132–40. [DOI] [PubMed] [Google Scholar]
- 837. Lee JM, Shim J, Park J, Yu HT, Kim TH, Park JK, et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol 2019;5:1253–61. [DOI] [PubMed] [Google Scholar]
- 838. Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi S, et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839. Jiang X, Liao J, Ling Z, Meyer C, Sommer P, Futyma P, et al. Adjunctive left atrial posterior wall isolation in treating atrial fibrillation: insight from a large secondary analysis. JACC Clin Electrophysiol 2022;8:605–18. [DOI] [PubMed] [Google Scholar]
- 840. Sirico G, Sirico D, Montisci A, Cerrato E, Morosato M, Panigada S, et al. Contact-Force guided posterior wall isolation as an adjunctive ablation strategy for persistent atrial fibrillation. J Atr Fibrillation 2021;14:20200475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841. Tokioka S, Fukamizu S, Kimura T, Takahashi M, Kitamura T, Hojo R. The effect of posterior wall isolation for persistent atrial fibrillation on recurrent arrhythmia. J Cardiovasc Electrophysiol 2021;32:597–604. [DOI] [PubMed] [Google Scholar]
- 842. Pothineni NVK, Lin A, Frankel DS, Supple GE, Garcia FC, Lin D, et al. Impact of left atrial posterior wall isolation on arrhythmia outcomes in patients with atrial fibrillation undergoing repeat ablation. Heart Rhythm O2 2021;2:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843. Salih M, Darrat Y, Ibrahim AM, Al-Akchar M, Bhattarai M, Koester C, et al. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: a metaanalysis. J Cardiovasc Electrophysiol 2020;31:1394–402. [DOI] [PubMed] [Google Scholar]
- 844. Sutter JS, Lokhnygina Y, Daubert JP, Bahnson T, Jackson K, Koontz JI, et al. Safety and efficacy outcomes of left atrial posterior wall isolation compared to pulmonary vein isolation and pulmonary vein isolation with linear ablation for the treatment of persistent atrial fibrillation. Am Heart J 2020;220:89–96. [DOI] [PubMed] [Google Scholar]
- 845. Yamaji H, Higashiya S, Murakami T, Hina K, Kawamura H, Murakami M, et al. Efficacy of an adjunctive electrophysiological test-guided left atrial posterior wall isolation in persistent atrial fibrillation without a left atrial low-voltage area. Circ Arrhythm Electrophysiol 2020;13:e008191. [DOI] [PubMed] [Google Scholar]
- 846. McLellan AJA, Prabhu S, Voskoboinik A, Wong MCG, Walters TE, Pathik B, et al. Isolation of the posterior left atrium for patients with persistent atrial fibrillation: routine adenosine challenge for dormant posterior left atrial conduction improves long-term outcome. Europace 2017;19:1958–66. [DOI] [PubMed] [Google Scholar]
- 847. Kim JS, Shin SY, Na JO, Choi CU, Kim SH, Kim JW, et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: a prospective randomized clinical trial. Int J Cardiol 2015;181:277–83. [DOI] [PubMed] [Google Scholar]
- 848. Bisbal F, Benito E, Teis A, Alarcón F, Sarrias A, Caixal G, et al. Magnetic resonance imaging-guided fibrosis ablation for the treatment of atrial fibrillation: the ALICIA trial. Circ Arrhythm Electrophysiol 2020;13:e008707. [DOI] [PubMed] [Google Scholar]
- 849. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher L, et al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850. Ip JE, Markowitz SM, Cheung JW, Liu CF, Thomas G, Lessner SJ, et al. Method for differentiating left superior pulmonary vein exit conduction from pseudo-exit conduction. Pacing Clin Electrophysiol 2013;36:299–308. [DOI] [PubMed] [Google Scholar]
- 851. Vijayaraman P, Dandamudi G, Naperkowski A, Oren J, Storm R, Ellenbogen KA. Assessment of exit block following pulmonary vein isolation: far-field capture masquerading as entrance without exit block. Heart Rhythm 2012;9:1653–9. [DOI] [PubMed] [Google Scholar]
- 852. Squara F, Liuba I, Chik W, Santangeli P, Zado ES, Callans DJ, et al. Loss of local capture of the pulmonary vein myocardium after antral isolation: prevalence and clinical significance. J Cardiovasc Electrophysiol 2015;26:242–50. [DOI] [PubMed] [Google Scholar]
- 853. Gerstenfeld EP, Dixit S, Callans D, Rho R, Rajawat Y, Zado E, et al. Utility of exit block for identifying electrical isolation of the pulmonary veins. J Cardiovasc Electrophysiol 2002;13:971–9. [DOI] [PubMed] [Google Scholar]
- 854. Tada H, Oral H, Wasmer K, Greenstein R, Pelosi F, Knight BP, et al. Pulmonary vein isolation: comparison of bipolar and unipolar electrograms at successful and unsuccessful ostial ablation sites. J Cardiovasc Electrophysiol 2002;13:13–19. [DOI] [PubMed] [Google Scholar]
- 855. Tada H, Oral H, Knight BP, Ozaydin M, Chugh A, Scharf C, et al. Randomized comparison of bipolar versus unipolar plus bipolar recordings during segmental ostial ablation of pulmonary veins. J Cardiovasc Electrophysiol 2002;13:851–6. [DOI] [PubMed] [Google Scholar]
- 856. Michowitz Y, Buch E, Bourke T, Tung R, Bradfield J, Mathuria N, et al. Unipolar and bipolar electrogram characteristics predict exit block during pulmonary vein antral isolation. Pacing Clin Electrophysiol 2012;35:1294–301. [DOI] [PubMed] [Google Scholar]
- 857. Bortone A, Appetiti A, Bouzeman A, Maupas E, Ciobotaru V, Boulenc JM, et al. Unipolar signal modification as a guide for lesion creation during radiofrequency application in the left atrium: prospective study in humans in the setting of paroxysmal atrial fibrillation catheter ablation. Circ Arrhythm Electrophysiol 2013;6:1095–102. [DOI] [PubMed] [Google Scholar]
- 858. Bortone A, Brault-Noble G, Appetiti A, Marijon E. Elimination of the negative component of the unipolar atrial electrogram as an in vivo marker of transmural lesion creation: acute study in canines. Circ Arrhythm Electrophysiol 2015;8:905–11. [DOI] [PubMed] [Google Scholar]
- 859. Bortone A, Lagrange P, Cauchemez B, Durand C, Dieuzaide P, Prévot S, et al. Elimination of the negative component of the unipolar electrogram as a local procedural endpoint during paroxysmal atrial fibrillation catheter ablation using contact-force sensing: the UNIFORCE study. J Interv Card Electrophysiol 2017;49:299–306. [DOI] [PubMed] [Google Scholar]
- 860. Ejima K, Kato K, Okada A, Wakisaka O, Kimura R, Ishizawa M, et al. Comparison between contact force monitoring and unipolar signal modification as a guide for catheter ablation of atrial fibrillation: prospective multi-center randomized study. Circ Arrhythm Electrophysiol 2019;12:e007311. [DOI] [PubMed] [Google Scholar]
- 861. Fu G, He B, Wang B, Feng M, Du X, Liu J, et al. Unipolar electrogram-guided versus lesion size index-guided catheter ablation in patients with paroxysmal atrial fibrillation. J Cardiovasc Dev Dis 2022;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862. Coeman M, Haddad ME, Wol M, Choudhury R, Vandekerckhove Y, Choudhury R, et al. ‘CLOSE’-guided pulmonary vein isolation and changes in local bipolar and unipolar atrial electrograms: observations from the EP lab. J Atr Fibrillation 2018;10:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863. Tomlinson DR, Myles M, Stevens KN, Streeter AJ. Transmural unipolar electrogram change occurs within 7 s at the left atrial posterior wall during pulmonary vein isolation. Pacing Clin Electrophysiol 2019;42:922–9. [DOI] [PubMed] [Google Scholar]
- 864. Zheng X, Walcott GP, Hall JA, Rollins DL, Smith WM, Kay GN, et al. Electrode impedance: an indicator of electrode-tissue contact and lesion dimensions during linear ablation. J Interv Card Electrophysiol 2000;4:645–54. [DOI] [PubMed] [Google Scholar]
- 865. Chinitz JS, Kapur S, Barbhaiya C, Kumar S, John R, Epstein LM, et al. Sites with small impedance decrease during catheter ablation for atrial fibrillation are associated with recovery of pulmonary vein conduction. J Cardiovasc Electrophysiol 2016;27:1390–8. [DOI] [PubMed] [Google Scholar]
- 866. Reichlin T, Knecht S, Lane C, Kuhne M, Nof E, Chopra N, et al. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm 2014;11:194–201. [DOI] [PubMed] [Google Scholar]
- 867. Wakili R, Clauss S, Schmidt V, Ulbrich M, Hahnefeld A, Schüssler F, et al. Impact of real-time contact force and impedance measurement in pulmonary vein isolation procedures for treatment of atrial fibrillation. Clin Res Cardiol 2014;103:97–106. [DOI] [PubMed] [Google Scholar]
- 868. Park HS, Kim IC, Cho YK, Yoon HJ, Kim H, Nam CW, et al. Comparison of the efficacy between impedance-guided and contact force-guided atrial fibrillation ablation using an automated annotation system. J Arrhythm 2018;34:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869. Knecht S, Reichlin T, Pavlovic N, Schaer B, Osswald S, Sticherling C, et al. Contact force and impedance decrease during ablation depends on catheter location and orientation: insights from pulmonary vein isolation using a contact force-sensing catheter. J Interv Card Electrophysiol 2015;43:297–306. [DOI] [PubMed] [Google Scholar]
- 870. Yasumoto K, Egami Y, Kawanami S, Sugae H, Ukita K, Kawamura A, et al. The correlation between local impedance drop and catheter contact in clinical pulmonary vein isolation use. Pacing Clin Electrophysiol 2022;45:984–92. [DOI] [PubMed] [Google Scholar]
- 871. Segreti L, De Simone A, Schillaci V, Bongiorni MG, Pelargonio G, Pandozi C, et al. A novel local impedance algorithm to guide effective pulmonary vein isolation in atrial fibrillation patients: preliminary experience across different ablation sites from the CHARISMA pilot study. J Cardiovasc Electrophysiol 2020;31:2319–27. [DOI] [PubMed] [Google Scholar]
- 872. Hashimoto K, Tsuzuki I, Seki Y, Ibe S, Yamashita T, Miyama H, et al. Change in the local impedance and electrograms recorded by a micro-electrode tip catheter during initial atrial fibrillation ablation. Journal of Arrhythmia 2021;37:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873. Solimene F, Giannotti Santoro M, De Simone A, Malacrida M, Stabile G, Pandozi C, et al. Pulmonary vein isolation in atrial fibrillation patients guided by a novel local impedance algorithm: 1-year outcome from the CHARISMA study. J Cardiovasc Electrophysiol 2021;32:1540–8. [DOI] [PubMed] [Google Scholar]
- 874. Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol 2008;1:354–62. [DOI] [PubMed] [Google Scholar]
- 875. Kumar S, Morton JB, Lee J, Halloran K, Spence SJ, Gorelik A, et al. Prospective characterization of catheter-tissue contact force at different anatomic sites during antral pulmonary vein isolation. Circ Arrhythm Electrophysiol 2012;5:1124–9. [DOI] [PubMed] [Google Scholar]
- 876. Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 877. Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 878. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak R, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879. Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real-time contact force curve (force-time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol 2010;21:1038–43. [DOI] [PubMed] [Google Scholar]
- 880. Kuck K-H, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, et al. A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm 2012;9:18–23. [DOI] [PubMed] [Google Scholar]
- 881. Kumar S, Morton JB, Halloran K, Spence SJ, Lee G, Wong MCG, et al. Effect of respiration on catheter-tissue contact force during ablation of atrial arrhythmias. Heart Rhythm 2012;9:1041–47.e1. [DOI] [PubMed] [Google Scholar]
- 882. El Haddad E, Taghji P, Phlips T, Wolf M, Demolder A, Choudhury R, et al. Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation: identifying the weakest link in the ablation chain. Circ Arrhythm Electrophysiol 2017;10:e004867. [DOI] [PubMed] [Google Scholar]
- 883. Nakagawa H, Ikeda A, Govari A, Papaioannou T, Constantine G, Bar-Tal M, et al. Abstract 12104: prospective study using a new formula incorporating contact force, radiofrequency power and application time (force-power-time index) for quantifying lesion formation to guide long continuous atrial lesions in the beating canine heart. Circulation 2013;128:A12104–A12104. [Google Scholar]
- 884. Ullah W, Hunter RJ, Finlay MC, McLean A, Dhinoja MB, Sporton S, et al. Ablation index and surround flow catheter irrigation: impedance-based appraisal in clinical ablation. JACC Clin Electrophysiol 2017;3:1080–8. [DOI] [PubMed] [Google Scholar]
- 885. Teres C, Soto-Iglesias D, Penela D, Jáuregui B, Ordoñez A, Chauca A, et al. Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the ‘ablate by-LAW’ single-center study-a pilot study. Europace 2022;24:390–9. [DOI] [PubMed] [Google Scholar]
- 886. Kyriakopoulou M, Strisciuglio T, El Haddad M, De Pooter J, Almorad A, Van Beeumen K, et al. Evaluation of a simple technique aiming at optimizing point-by-point isolation of the left pulmonary veins: a randomized study. Europace 2019;21:1185–92. [DOI] [PubMed] [Google Scholar]
- 887. Berte B, Hilfiker G, Moccetti F, Schefer T, Weberndörfer V, Cuculi F, et al. Pulmonary vein isolation using ablation index vs. CLOSE protocol with a surround flow ablation catheter. Europace 2020;22:84–9. [DOI] [PubMed] [Google Scholar]
- 888. Hoffmann P, Diaz Ramirez I, Baldenhofer G, Stangl K, Mont L, Althoff TF. Randomized study defining the optimum target interlesion distance in ablation index-guided atrial fibrillation ablation. Europace 2020;22:1480–6. [DOI] [PubMed] [Google Scholar]
- 889. Kobayashi S, Fukaya H, Oikawa J, Saito D, Sato T, Matsuura G, et al. Optimal interlesion distance in ablation index-guided pulmonary vein isolation for atrial fibrillation. J Interv Card Electrophysiol 2021;62:123–31. [DOI] [PubMed] [Google Scholar]
- 890. Francke A, Taha NS, Scharfe F, Schoen S, Wunderlich C, Christoph M. Procedural efficacy and safety of standardized, ablation index guided fixed 50 W high-power short-duration pulmonary vein isolation and substrate modification using the CLOSE protocol. J Cardiovasc Electrophysiol 2021;32:2408–17. [DOI] [PubMed] [Google Scholar]
- 891. Francke A, Scharfe F, Schoen S, Wunderlich C, Christoph M. Reconnection patterns after CLOSE-guided 50 W high-power-short-duration circumferential pulmonary vein isolation and substrate modification-PV reconnection might no longer be an issue. J Cardiovasc Electrophysiol 2022;33:1136–45. [DOI] [PubMed] [Google Scholar]
- 892. Chen S, Schmidt B, Bordignon S, Tohoku S, Urban VC, Schulte-Hahn B, et al. Catheter ablation of atrial fibrillation using ablation index-guided high-power technique: Frankfurt AI high-power 15-month follow-up. J Cardiovasc Electrophysiol 2021;32:616–24. [DOI] [PubMed] [Google Scholar]
- 893. Jiang C-Y, Jiang R-H, Matsuo S, Liu Q, Fan Y-Q, Zhang Z-W, et al. Early detection of pulmonary vein reconnection after isolation in patients with paroxysmal atrial fibrillation: a comparison of ATP-induction and reassessment at 30 minutes postisolation. J Cardiovasc Electrophysiol 2009;20:1382–7. [DOI] [PubMed] [Google Scholar]
- 894. Cheema A, Dong J, Dalal D, Marine JE, Henrikson CA, Spragg D, et al. Incidence and time course of early recovery of pulmonary vein conduction after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:387–91. [DOI] [PubMed] [Google Scholar]
- 895. Brunelli M, Raffa S, Große A, Hanazawa K, Sammut M, Roos M, et al. Residual conduction after pulmonary vein isolation with a circular multielectrode radiofrequency ablation catheter: the role of adenosine and orciprenalin during a prolonged observation time. Int J Cardiol 2013;168:4122–31. [DOI] [PubMed] [Google Scholar]
- 896. Dallaglio PD, Betts TR, Ginks M, Bashir Y, Anguera I, Rajappan K. The role of adenosine in pulmonary vein isolation: a critical review. Cardiol Res Pract 2016;2016:8632509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897. le Polain de Waroux J-B, Weerasooriya R, Anvardeen K, Barbraud C, Marchandise S, De Meester C, et al. Low contact force and force-time integral predict early recovery and dormant conduction revealed by adenosine after pulmonary vein isolation. Europace 2015;17:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898. Datino T, Macle L, Qi XY, Maguy A, Comtois P, Chartier D, et al. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation 2010;121:963–72. [DOI] [PubMed] [Google Scholar]
- 899. Tokuda M, Matsuo S, Isogai R, Uno G, Tokutake K, Yokoyama K, et al. Adenosine testing during cryoballoon ablation and radiofrequency ablation of atrial fibrillation: a propensity score-matched analysis. Heart Rhythm 2016;13:2128–34. [DOI] [PubMed] [Google Scholar]
- 900. Ghanbari H, Jani R, Hussain-Amin A, Al-Assad W, Huether E, Ansari S, et al. Role of adenosine after antral pulmonary vein isolation of paroxysmal atrial fibrillation: a randomized controlled trial. Heart Rhythm 2016;13:407–15. [DOI] [PubMed] [Google Scholar]
- 901. Prabhu S, Mackin V, McLellan AJ, Phan T, McGlade D, Ling LH, et al. Determining the optimal dose of adenosine for unmasking dormant pulmonary vein conduction following atrial fibrillation ablation: electrophysiological and hemodynamic assessment. DORMANT-AF study. J Cardiovasc Electrophysiol 2017;28:13–22. [DOI] [PubMed] [Google Scholar]
- 902. Osorio J, Hunter TD, Rajendra A, Zei P, Silverstein J, Morales G. Predictors of clinical success after paroxysmal atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol 2021;32:1814–21. [DOI] [PubMed] [Google Scholar]
- 903. Ninomiya Y, Inoue K, Tanaka N, Okada M, Tanaka K, Onishi T, et al. Absence of first-pass isolation is associated with poor pulmonary vein isolation durability and atrial fibrillation ablation outcomes. J Arrhythm 2021;37:1468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904. Barbhaiya CR, Aizer A, Knotts R, Bernstein S, Park D, Holmes D, et al. Simultaneous pace-ablate during CARTO-guided pulmonary vein isolation with a contact-force sensing radiofrequency ablation catheter. J Interv Card Electrophysiol 2019;54:119–24. [DOI] [PubMed] [Google Scholar]
- 905. Kogawa R, Okumura Y, Watanabe I, Sonoda K, Sasaki N, Takahashi K, et al. Difference between dormant conduction sites revealed by adenosine triphosphate provocation and unipolar pace-capture sites along the ablation line after pulmonary vein isolation. Int Heart J 2016;57:25–9. [DOI] [PubMed] [Google Scholar]
- 906. Okumura Y, Watanabe I, Nagashima K, Sonoda K, Mano H, Sasaki N, et al. The effects of standard electrical PV isolation vs. “pace and ablate” on ATP-provoked PV reconnections. J Interv Card Electrophysiol 2014;40:39–45. [DOI] [PubMed] [Google Scholar]
- 907. Kumar S, Kalman JM, Sutherland F, Spence SJ, Finch S, Sparks PB. Atrial fibrillation inducibility in the absence of structural heart disease or clinical atrial fibrillation: critical dependence on induction protocol, inducibility definition, and number of inductions. Circ Arrhythm Electrophysiol 2012;5:531–6. [DOI] [PubMed] [Google Scholar]
- 908. Darma A, Daneschnejad SS, Gaspar T, Huo Y, Wetzel U, Dagres N, et al. Role of inducibility and its dynamic change in the outcome of catheter ablation of atrial fibrillation: a single center prospective study. J Cardiovasc Electrophysiol 2020;31:705–11. [DOI] [PubMed] [Google Scholar]
- 909. Santangeli P, Zado ES, Garcia FC, Riley MP, Lin D, Frankel DS, et al. Lack of prognostic value of atrial arrhythmia inducibility and change in inducibility status after catheter ablation of atrial fibrillation. Heart Rhythm 2018;15:660–5. [DOI] [PubMed] [Google Scholar]
- 910. Leong-Sit P, Robinson M, Zado ES, Callans DJ, Garcia F, Lin D, et al. Inducibility of atrial fibrillation and flutter following pulmonary vein ablation. J Cardiovasc Electrophysiol 2013;24:617–23. [DOI] [PubMed] [Google Scholar]
- 911. Millenaar D, Becker N, Pavlicek V, Wintrich J, Böhm M, Mahfoud F, et al. Inducibility of atrial fibrillation after catheter ablation predicts recurrences of atrial fibrillation: a metaanalysis. Pacing Clin Electrophysiol 2021;44:667–76. [DOI] [PubMed] [Google Scholar]
- 912. Neumann T, Vogt J, Schumacher B, Dorszewski A, Kuniss M, Neuser H, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol 2008;52:273–8. [DOI] [PubMed] [Google Scholar]
- 913. Alyesh D, Frederick J, Choe W, Sundaram S. Step by step: how to perform a fluoroless cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2022;33:2351–5. [DOI] [PubMed] [Google Scholar]
- 914. Siklódy CH, Minners J, Allgeier M, Allgeier HJ, Jander N, Keyl C, et al. Pressure-guided cryoballoon isolation of the pulmonary veins for the treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2010;21:120–5. [DOI] [PubMed] [Google Scholar]
- 915. Rottner L, Sinning C, Reissmann B, Schleberger R, Dinshaw L, Münkler P, et al. Wide-band dielectric imaging and the novel cryoballoon-occlusion tool to guide cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol 2020;13:e008507. [DOI] [PubMed] [Google Scholar]
- 916. Takami M, Lehmann HI, Misiri J, Parker KD, Sarmiento RI, Johnson SB, et al. Impact of freezing time and balloon size on the thermodynamics and isolation efficacy during pulmonary vein isolation using the second generation cryoballoon. Circ Arrhythm Electrophysiol 2015;8:836–45. [DOI] [PubMed] [Google Scholar]
- 917. Andrade JG, Dubuc M, Guerra PG, Landry E, Coulombe N, Leduc H, et al. Pulmonary vein isolation using a second-generation cryoballoon catheter: a randomized comparison of ablation duration and method of deflation. J Cardiovasc Electrophysiol 2013;24:692–8. [DOI] [PubMed] [Google Scholar]
- 918. Cheung CC, Deyell MW, Macle L, Verma A, Champagne J, Leong-Sit P, et al. Repeat atrial fibrillation ablation procedures in the CIRCA-DOSE study. Circ Arrhythm Electrophysiol 2020;13:e008480. [DOI] [PubMed] [Google Scholar]
- 919. Chun KR, Stich M, Fürnkranz A, Bordignon S, Perrotta L, Dugo D, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm 2017;14:495–500. [DOI] [PubMed] [Google Scholar]
- 920. Wissner E, Heeger CH, Grahn H, Reissmann B, Wohlmuth P, Lemes C, et al. One-year clinical success of a ‘no-bonus’ freeze protocol using the second-generation 28 mm cryoballoon for pulmonary vein isolation. Europace 2015;17:1236–40. [DOI] [PubMed] [Google Scholar]
- 921. Farkowski MM, Karlinski M, Barra S, Providencia R, Golicki D, Pytkowski M, et al. Effectiveness and safety of a single freeze strategy of cryoballoon ablation of atrial fibrillation: an EHRA systematic review and metaanalysis. Europace 2022;24:58–69. [DOI] [PubMed] [Google Scholar]
- 922. Bordignon S, Chen S, Bologna F, Thohoku S, Urbanek L, Willems F, et al. Optimizing cryoballoon pulmonary vein isolation: lessons from >1000 procedures- the Frankfurt approach. Europace 2021;23:868–77. [DOI] [PubMed] [Google Scholar]
- 923. Chen S, Schmidt B, Bordignon S, Perrotta L, Bologna F, Chun KRJ. Impact of cryoballoon freeze duration on long-term durability of pulmonary vein isolation: ICE re-map study. JACC Clin Electrophysiol 2019;5:551–9. [DOI] [PubMed] [Google Scholar]
- 924. Heeger CH, Popescu SS, Saraei R, Kirstein B, Hatahet S, Samara O, et al. Individualized or fixed approach to pulmonary vein isolation utilizing the fourth-generation cryoballoon in patients with paroxysmal atrial fibrillation: the randomized INDI-FREEZE trial. Europace 2022;24:921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925. Ferrero-de-Loma-Osorio Á, García-Fernández A, Castillo-Castillo J, Izquierdo-de-Francisco M, Ibáñez-Críado A, Moreno-Arribas J, et al. Time-to-effect-based dosing strategy for cryoballoon ablation in patients with paroxysmal atrial fibrillation: results of the plusONE multicenter randomized controlled noninferiority trial. Circ Arrhythm Electrophysiol 2017;10:e005318. [DOI] [PubMed] [Google Scholar]
- 926. Ciconte G, Mugnai G, Sieira J, Velagić V, Saitoh Y, Irfan G, et al. On the quest for the best freeze: predictors of late pulmonary vein reconnections after second-generation cryoballoon ablation. Circ Arrhythm Electrophysiol 2015;8:1359–65. [DOI] [PubMed] [Google Scholar]
- 927. Aryana A, Mugnai G, Singh SM, Pujara DK, de Asmundis C, Singh SK, et al. Procedural and biophysical indicators of durable pulmonary vein isolation during cryoballoon ablation of atrial fibrillation. Heart Rhythm 2016;13:424–32. [DOI] [PubMed] [Google Scholar]
- 928. Ghosh J, Martin A, Keech AC, Chan KH, Gomes S, Singarayar S, et al. Balloon warming time is the strongest predictor of late pulmonary vein electrical reconnection following cryoballoon ablation for atrial fibrillation. Heart Rhythm 2013;10:1311–7. [DOI] [PubMed] [Google Scholar]
- 929. Bose A, Chevli PA, Berberian G, Januszkiewicz J, Ahmad G, Hashmath Z, et al. Presence of a left common pulmonary vein and pulmonary vein anatomical characteristics as predictors of outcome following cryoballoon ablation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2021;62:409–17. [DOI] [PubMed] [Google Scholar]
- 930. Coutiño HE, Ströker E, Takarada K, Mugnai G, Abugattas JP, Sieira J, et al. Radiofrequency versus cryoballoon ablation for atrial fibrillation in the setting of left common pulmonary veins. Pacing Clin Electrophysiol 2019;42:1456–62. [DOI] [PubMed] [Google Scholar]
- 931. Yamaguchi M, Miyazaki S, Kajiyama T, Hada M, Nakamura H, Hachiya H, et al. Pulmonary vein isolation in patients with a left common pulmonary vein: comparison between second-generation cryoballoon and radiofrequency ablation. J Cardiol 2019;73:292–8. [DOI] [PubMed] [Google Scholar]
- 932. Shigeta T, Okishige K, Yamauchi Y, Aoyagi H, Nakamura T, Yamashita M, et al. Clinical assessment of cryoballoon ablation in cases with atrial fibrillation and a left common pulmonary vein. J Cardiovasc Electrophysiol 2017;28:1021–7. [DOI] [PubMed] [Google Scholar]
- 933. Asvestas D, Sousonis V, Kotsovolis G, Karanikas S, Xintarakou A, Sakadakis E, et al. Cavotricuspid isthmus ablation guided by force-time integral – a randomized study. Clin Cardiol 2022;45:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934. Sakama S, Yagishita A, Sakai T, Morise M, Ayabe K, Amino M, et al. Ablation index-guided cavotricuspid isthmus ablation with contiguous lesions using fluoroscopy integrated 3D mapping in atrial flutter. J Interv Card Electrophysiol 2022;64:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935. Sasaki T, Nakamura K, Inoue M, Minami K, Miki Y, Goto K, et al. Optimal local impedance drops for an effective radiofrequency ablation during cavo-tricuspid isthmus ablation. J Arrhythm 2020;36:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936. Sau A, Kapadia S, Al-Aidarous S, Howard J, Sohaib A, Sikkel MB, et al. Temporal trends and lesion sets for persistent atrial fibrillation ablation: a metaanalysis with trial sequential analysis and meta-regression. Circ Arrhythm Electrophysiol 2023;16:e011861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937. Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK, et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and metaanalysis. Europace 2018;20:f366–76. [DOI] [PubMed] [Google Scholar]
- 938. Bergonti M, Spera FR, Ferrero TG, Nsahlai M, Bonomi A, Boris W, et al. Anterior mitral line in patients with persistent atrial fibrillation and anterior scar: a multicenter matched comparison-The MiLine study. Heart Rhythm 2023;20:658–65. [DOI] [PubMed] [Google Scholar]
- 939. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. [DOI] [PubMed] [Google Scholar]
- 940. Lau DH, Maesen B, Zeemering S, Verheule S, Crijns HJ, Schotten U. Stability of complex fractionated atrial electrograms: a systematic review. J Cardiovasc Electrophysiol 2012;23:980–7. [DOI] [PubMed] [Google Scholar]
- 941. Vogler J, Willems S, Sultan A, Schreiber D, Lüker J, Servatius H, et al. Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol 2015;66:2743–52. [DOI] [PubMed] [Google Scholar]
- 942. Providência R, Lambiase PD, Srinivasan N, Ganesh Babu G, Bronis K, Ahsan S, et al. Is there still a role for complex fractionated atrial electrogram ablation in addition to pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation? Meta-analysis of 1415 patients. Circ Arrhythm Electrophysiol 2015;8:1017–29. [DOI] [PubMed] [Google Scholar]
- 943. O’Neill MD, Wright M, Knecht S, Jaïs P, Hocini M, Takahashi Y, et al. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J 2009;30:1105–12. [DOI] [PubMed] [Google Scholar]
- 944. Schreiber D, Rostock T, Fröhlich M, Sultan A, Servatius H, Hoffmann BA, et al. Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol 2015;8:308–17. [DOI] [PubMed] [Google Scholar]
- 945. Suenari K, Chen YC, Kao YH, Cheng CC, Lin YK, Chen YJ, et al. Discrepant electrophysiological characteristics and calcium homeostasis of left atrial anterior and posterior myocytes. Basic Res Cardiol 2011;106:65–74. [DOI] [PubMed] [Google Scholar]
- 946. Markides V, Schilling RJ, Ho SY, Chow AW, Davies DW, Peters NS. Characterization of left atrial activation in the intact human heart. Circulation 2003;107:733–9. [DOI] [PubMed] [Google Scholar]
- 947. Pauza DH, Skripka V, Pauziene N. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs 2002;172:297–320. [DOI] [PubMed] [Google Scholar]
- 948. Thiyagarajah A, Mahajan R, Iwai S, Griffin A, Mishima RS, Linz D, et al. Single ring isolation with inferior line sparing for atrial fibrillation: a proof-of-concept study. Circ Arrhythm Electrophysiol 2021;14:e009552. [DOI] [PubMed] [Google Scholar]
- 949. Thomas SP, Lim TW, McCall R, Seow SC, Ross DL. Electrical isolation of the posterior left atrial wall and pulmonary veins for atrial fibrillation: feasibility of and rationale for a single-ring approach. Heart Rhythm 2007;4:722–30. [DOI] [PubMed] [Google Scholar]
- 950. Bisignani A, Pannone L, Miraglia V, Sieira J, Iacopino S, Bala G, et al. Feasibility and safety of left atrial posterior wall isolation with a new Cryoballoon technology in patients with persistent atrial fibrillation. Pacing Clin Electrophysiol 2022;45:605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 951. Aryana A, Baker JH, Espinosa Ginic MA, Pujara DK, Bowers MR, O’Neill PG, et al. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: a multicenter experience. Heart Rhythm 2018;15:1121–9. [DOI] [PubMed] [Google Scholar]
- 952. Aryana A, Allen SL, Pujara DK, Bowers MR, O’Neill PG, Yamauchi Y, et al. Concomitant pulmonary vein and posterior wall isolation using cryoballoon with adjunct radiofrequency in persistent atrial fibrillation. JACC Clin Electrophysiol 2021;7:187–96. [DOI] [PubMed] [Google Scholar]
- 953. Ahn J, Shin DG, Han SJ, Lim HE. Does isolation of the left atrial posterior wall using cryoballoon ablation improve clinical outcomes in patients with persistent atrial fibrillation? A prospective randomized controlled trial. Europace 2022;24:1093–101. [DOI] [PubMed] [Google Scholar]
- 954. Gunawardene MA, Frommeyer G, Ellermann C, Jularic M, Leitz P, Hartmann J, et al. Left atrial posterior wall isolation with pulsed field ablation in persistent atrial fibrillation. J Clin Med 2023;12:6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955. Sohns C, Fink T, Braun M, Sciacca V, Piran M, Khalaph M, et al. Lesion formation following pulsed field ablation for pulmonary vein and posterior wall isolation. Pacing Clin Electrophysiol 2023;46:714–6. [DOI] [PubMed] [Google Scholar]
- 956. Yu HT, Shim J, Park J, Kim IS, Kim TH, Uhm JS, et al. Pulmonary vein isolation alone versus additional linear ablation in patients with persistent atrial fibrillation converted to paroxysmal type with antiarrhythmic drug therapy: a multicenter, prospective, randomized study. Circ Arrhythm Electrophysiol 2017;10:e004915. [DOI] [PubMed] [Google Scholar]
- 957. Wong K, Schricker AA, Nerlekar R, Feng Z, Sudat S, Cook K, et al. The posterior wall isolation for persistent atrial fibrillation high-power short duration (PEF-HOT) trial. JACC Clin Electrophysiol 2023;9:2166–8. [DOI] [PubMed] [Google Scholar]
- 958. Jankelson L, Garber L, Shulman E, Cohen RB, Peterson C, Wadhwani L, et al. Outcomes of posterior wall isolation with pulmonary vein isolation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2022;33:209–17. [DOI] [PubMed] [Google Scholar]
- 959. Sugumar H, Thomas SP, Prabhu S, Voskoboinik A, Kistler PM. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:345–52. [DOI] [PubMed] [Google Scholar]
- 960. Tran VN, Kusa S, Smietana J, Tsai WC, Bhasin K, Teh A, et al. The relationship between esophageal heating during left atrial posterior wall ablation and the durability of pulmonary vein isolation. Europace 2017;19:1664–9. [DOI] [PubMed] [Google Scholar]
- 961. Markman TM, Hyman MC, Kumareswaran R, Arkles JS, Santangeli P, Schaller RD, et al. Durability of posterior wall isolation after catheter ablation among patients with recurrent atrial fibrillation. Heart Rhythm 2020;17:1740–4. [DOI] [PubMed] [Google Scholar]
- 962. Kumar P, Bamimore AM, Schwartz JD, Chung EH, Gehi AK, Kiser AC, et al. Challenges and outcomes of posterior wall isolation for ablation of atrial fibrillation. J Am Heart Assoc 2016;5:e003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963. Segan L, Chieng D, Prabhu S, Hunt A, Watts T, Klys B, et al. Posterior wall isolation improves outcomes for persistent AF with rapid posterior wall activity: a CAPLA substudy. Clin Electrophysiol 2023;9:2536–46. [DOI] [PubMed] [Google Scholar]
- 964. Al-Kaisey AM, Parameswaran R, Kalman JM. Atrial fibrillation structural substrates: etiology, identification and implications. Arrhythm Electrophysiol Rev 2020;9:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965. Prabhu S, Voskoboinik A, McLellan AJA, Peck KY, Pathik B, Nalliah CJ, et al. Biatrial electrical and structural atrial changes in heart failure: electroanatomic mapping in persistent atrial fibrillation in humans. JACC Clin Electrophysiol 2018;4:87–96. [DOI] [PubMed] [Google Scholar]
- 966. Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 2004;44:109–16. [DOI] [PubMed] [Google Scholar]
- 967. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Wood JPM, Manavis J, et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 2021;7:630–41. [DOI] [PubMed] [Google Scholar]
- 968. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020;382:20–8. [DOI] [PubMed] [Google Scholar]
- 969. Trivedi SJ, Claessen G, Stefani L, Flannery MD, Brown P, Janssens K, et al. Differing mechanisms of atrial fibrillation in athletes and non-athletes: alterations in atrial structure and function. Eur Heart J Cardiovasc Imaging 2020;21:1374–83. [DOI] [PubMed] [Google Scholar]
- 970. Kottkamp H, Bender R, Berg J. Catheter ablation of atrial fibrillation: how to modify the substrate? J Am Coll Cardiol 2015;65:196–206. [DOI] [PubMed] [Google Scholar]
- 971. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- 972. Cutler MJ, Johnson J, Abozguia K, Rowan S, Lewis W, Costantini O, et al. Impact of voltage mapping to guide whether to perform ablation of the posterior wall in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:13–21. [DOI] [PubMed] [Google Scholar]
- 973. Yang G, Yang B, Wei Y, Zhang F, Ju W, Chen H, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2016;9:e003382. [DOI] [PubMed] [Google Scholar]
- 974. Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, et al. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO trial. J Am Heart Assoc 2020;9:e015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975. Chen H, Li C, Han B, Xiao F, Yi F, Wei Y, et al. Circumferential pulmonary vein isolation with vs without additional low-voltage-area ablation in older patients with paroxysmal atrial fibrillation: a randomized clinical trial. JAMA Cardiol 2023;8:765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976. Wong GR, Nalliah CJ, Lee G, Voskoboinik A, Prabhu S, Parameswaran R, et al. Dynamic atrial substrate during high-density mapping of paroxysmal and persistent AF: implications for substrate ablation. JACC Clin Electrophysiol 2019;5:1265–77. [DOI] [PubMed] [Google Scholar]
- 977. Kapa S, Desjardins B, Callans DJ, Marchlinski FE, Dixit S. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:1044–52. [DOI] [PubMed] [Google Scholar]
- 978. Takahashi Y, Yamaguchi T, Otsubo T, Nakashima K, Shinzato K, Osako R, et al. Histological validation of atrial structural remodelling in patients with atrial fibrillation. Eur Heart J 2023;44:3339–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979. Vlachos K, Derval N, Pambrun T, Duchateau J, Martin CA, Bazoukis G, et al. Ligament of Marshall ablation for persistent atrial fibrillation. Pacing Clin Electrophysiol 2021;44:782–91. [DOI] [PubMed] [Google Scholar]
- 980. Kamakura T, Derval N, Duchateau J, Denis A, Nakashima T, Takagi T, et al. Vein of Marshall ethanol infusion: feasibility, pitfalls, and complications in over 700 patients. Circ Arrhythm Electrophysiol 2021;14:e010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981. Gianni C, Mohanty S, Trivedi C, Di Biase L, Natale A. Novel concepts and approaches in ablation of atrial fibrillation: the role of non-pulmonary vein triggers. Europace 2018;20:1566–76. [DOI] [PubMed] [Google Scholar]
- 982. Mohanty S, Trivedi C, Horton P, Della Rocca DG, Gianni C, MacDonald B, et al. Natural history of arrhythmia after successful isolation of pulmonary veins, left atrial posterior wall, and superior vena cava in patients with paroxysmal atrial fibrillation: a multi-center experience. J Am Heart Assoc 2021;10:e020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983. Sørensen SK, Johannessen A, Worck R, Hansen ML, Hansen J. Radiofrequency versus cryoballoon catheter ablation for paroxysmal atrial fibrillation: durability of pulmonary vein isolation and effect on atrial fibrillation burden: the RACE-AF randomized controlled trial. Circ Arrhythm Electrophysiol 2021;14:e009573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984. Della Rocca DG, Tarantino N, Trivedi C, Mohanty S, Anannab A, Salwan AS, et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: implications of pathophysiology for catheter ablation. J Cardiovasc Electrophysiol 2020;31:2154–67. [DOI] [PubMed] [Google Scholar]
- 985. Hsu LF, Jaïs P, Keane D, Wharton JM, Deisenhofer I, Hocini M, et al. Atrial fibrillation originating from persistent left superior vena cava. Circulation 2004;109:828–32. [DOI] [PubMed] [Google Scholar]
- 986. Di Biase L, Burkhardt JD, Mohanty P, Mohanty S, Sanchez JE, Trivedi C, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol 2016;68:1929–40. [DOI] [PubMed] [Google Scholar]
- 987. Romero J, Di Biase L, Mohanty S, Trivedi C, Patel K, Parides M, et al. Long-term outcomes of left atrial appendage electrical isolation in patients with nonparoxysmal atrial fibrillation: a propensity score-matched analysis. Circ Arrhythm Electrophysiol 2020;13:e008390. [DOI] [PubMed] [Google Scholar]
- 988. Romero J, Gabr M, Patel K, Briceno D, Diaz JC, Alviz I, et al. Efficacy and safety of left atrial appendage electrical isolation during catheter ablation of atrial fibrillation: an updated metaanalysis. Europace 2021;23:226–37. [DOI] [PubMed] [Google Scholar]
- 989. Friedman DJ, Black-Maier EW, Barnett AS, Pokorney SD, Al-Khatib SM, Jackson KP, et al. Left atrial appendage electrical isolation for treatment of recurrent atrial fibrillation: a metaanalysis. JACC Clin Electrophysiol 2018;4:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990. Bavry A. Outcomes of adjunctive left atrial appendage ligation utilizing the LARIAT compared to pulmonary vein antral isolation alone - aMAZE. Presented by Dr. David J. Wilber at the american heart association virtual annual scientific sessions (AHA 2021), 2021.
- 991. Fink T, Vogler J, Heeger CH, Sano M, Sciacca V, Reissmann B, et al. Impact of left atrial appendage closure on LAA thrombus formation and thromboembolism after LAA isolation. JACC Clin Electrophysiol 2020;6:1687–97. [DOI] [PubMed] [Google Scholar]
- 992. Rillig A, Tilz RR, Lin T, Fink T, Heeger CH, Arya A, et al. Unexpectedly high incidence of stroke and left atrial appendage thrombus formation after electrical isolation of the left atrial appendage for the treatment of atrial tachyarrhythmias. Circ Arrhythm Electrophysiol 2016;9:e003461. [DOI] [PubMed] [Google Scholar]
- 993. Kim YG, Shim J, Oh SK, Lee KN, Choi JI, Kim YH. Electrical isolation of the left atrial appendage increases the risk of ischemic stroke and transient ischemic attack regardless of postisolation flow velocity. Heart Rhythm 2018;15:1746–53. [DOI] [PubMed] [Google Scholar]
- 994. Zender N, Weise FK, Bordignon S, Herrmann E, Konstantinou A, Bologna F, et al. Thromboembolism after electrical isolation of the left atrial appendage: a new indication for interventional closure? Europace 2019;21:1502–8. [DOI] [PubMed] [Google Scholar]
- 995. Dixit S, Lin D, Frankel DS, Marchlinski FE. Catheter ablation for persistent atrial fibrillation: antral pulmonary vein isolation and elimination of nonpulmonary vein triggers are sufficient. Circ Arrhythm Electrophysiol 2012;5:1216–23; discussion 1223. [DOI] [PubMed] [Google Scholar]
- 996. Inoue K, Kurotobi T, Kimura R, Toyoshima Y, Itoh N, Masuda M, et al. Trigger-based mechanism of the persistence of atrial fibrillation and its impact on the efficacy of catheter ablation. Circ Arrhythm Electrophysiol 2012;5:295–301. [DOI] [PubMed] [Google Scholar]
- 997. Santangeli P, Di Biase L, Mohanty P, Burkhardt JD, Horton R, Bai R, et al. Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol 2012;23:687–93. [DOI] [PubMed] [Google Scholar]
- 998. Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 2010;121:2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 999. Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm 2009;6:S26–34. [DOI] [PubMed] [Google Scholar]
- 1000. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec 2000;259:353–82. [DOI] [PubMed] [Google Scholar]
- 1001. Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol 2006;47:1196–206. [DOI] [PubMed] [Google Scholar]
- 1002. Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation 2008;117:470–7. [DOI] [PubMed] [Google Scholar]
- 1003. Nishida K, Maguy A, Sakabe M, Comtois P, Inoue H, Nattel S. The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovasc Res 2011;89:825–33. [DOI] [PubMed] [Google Scholar]
- 1004. Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol 2014;64:823–31. [DOI] [PubMed] [Google Scholar]
- 1005. Stavrakis S, Nakagawa H, Po SS, Scherlag BJ, Lazzara R, Jackman WM. The role of the autonomic ganglia in atrial fibrillation. JACC Clin Electrophysiol 2015;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 2013;62:2318–25. [DOI] [PubMed] [Google Scholar]
- 1007. Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm 2013;10:1280–6. [DOI] [PubMed] [Google Scholar]
- 1008. Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, et al. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol 2016;68:1155–65. [DOI] [PubMed] [Google Scholar]
- 1009. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 1010. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 1011. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 1012. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 1013. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a metaanalysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 1014. Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi T, et al. Efficacy of antiarrhythmic drugs short-term use after catheter ablation for atrial fibrillation (EAST-AF) trial. Eur Heart J 2016;37:610–8. [DOI] [PubMed] [Google Scholar]
- 1015. Darkner S, Chen X, Hansen J, Pehrson S, Johannessen A, Nielsen JB, et al. Recurrence of arrhythmia following short-term oral amiodarone after catheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J 2014;35:3356–64. [DOI] [PubMed] [Google Scholar]
- 1016. Hayashi M, Miyauchi Y, Iwasaki YK, Yodogawa K, Tsuboi I, Uetake S, et al. Three-month lower-dose flecainide after catheter ablation of atrial fibrillation. Europace 2014;16:1160–7. [DOI] [PubMed] [Google Scholar]
- 1017. Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, et al. Antiarrhythmics after ablation of atrial fibrillation (5A study). Circulation 2009;120:1036–40. [DOI] [PubMed] [Google Scholar]
- 1018. Leong-Sit P, Roux JF, Zado E, Callans DJ, Garcia F, Lin D, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011;4:11–4. [DOI] [PubMed] [Google Scholar]
- 1019. Gu J, Liu X, Tan H, Zhou L, Gu J, Jiang W, et al. Extensive antiarrhythmic drugs after catheter ablation of persistent atrial fibrillation. Acta Cardiol 2012;67:407–14. [DOI] [PubMed] [Google Scholar]
- 1020. Chen W, Liu H, Ling Z, Xu Y, Fan J, Du H, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation-a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016;11:e0156121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021. Goldenberg GR, Burd D, Lodzinski P, Stabile G, Udell JA, Newman D, et al. Antiarrhythmic therapy as an adjuvant to promote post pulmonary vein isolation success—a metaanalysis. J Interv Card Electrophysiol 2016;47:171–6. [DOI] [PubMed] [Google Scholar]
- 1022. Deyell MW, Leather RA, Macle L, Forman J, Khairy P, Zhang R, et al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol 2020;6:609–19. [DOI] [PubMed] [Google Scholar]
- 1023. Creta A, Ventrella N, Providência R, Earley MJ, Sporton S, Dhillon G, et al. Same-day discharge following catheter ablation of atrial fibrillation: a safe and cost-effective approach. J Cardiovasc Electrophysiol 2020;31:3097–103. [DOI] [PubMed] [Google Scholar]
- 1024. Kowalski M, Parikh V, Salcido JR, Chalfoun N, Albano A, O’Neill PG, et al. Same-day discharge after cryoballoon ablation of atrial fibrillation: a multicenter experience. J Cardiovasc Electrophysiol 2021;32:183–90. [DOI] [PubMed] [Google Scholar]
- 1025. Tang PT, Davies M, Bashir Y, Betts TR, Pedersen M, Rajappan K, et al. Efficacy and safety of same-day discharge after atrial fibrillation ablation compared with postprocedural overnight stay: a systematic review and metaanalysis. Europace 2022;24:1569–84. [DOI] [PubMed] [Google Scholar]
- 1026. Jafry AH, Akhtar KH, Khan JA, Clifton S, Reese J, Sami KN, et al. Safety and feasibility of same-day discharge for catheter ablation of atrial fibrillation: a systematic review and metaanalysis. J Interv Card Electrophysiol 2022;65:803–11. [DOI] [PubMed] [Google Scholar]
- 1027. Sangrigoli R, Harding J, Venkataraman G, Tomaiko-Clark E, Bai R, Su W. Randomized prospective evaluation of same-day discharge after cryoballoon ablation of atrial fibrillation: results of the EASY PVI study. J Interv Card Electrophysiol 2023;66:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028. Deyell MW, Hoskin K, Forman J, Laksman ZW, Hawkins NM, Bennett MT, et al. Same-day discharge for atrial fibrillation ablation: outcomes and impact of ablation modality. Europace 2023;25:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029. Rajendra A, Osorio J, Diaz JC, Hoyos C, Rivera E, Matos CD, et al. Performance of the REAL-AF same-day discharge protocol in patients undergoing catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2023;9:1515–26. [DOI] [PubMed] [Google Scholar]
- 1030. Jimenez-Candil J, Hernandez Hernandez J, Cruz Galban A, Blanco F, Moriñigo JL, Sanchez García M, et al. Clinical and economic outcomes of a systematic same-day discharge programme after pulmonary vein isolation: comparison between cryoballoon vs. radiofrequency ablation. Europace 2023;25:euad265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031. Zellerhoff S, Lenze F, Eckardt L. Prophylactic proton pump inhibition after atrial fibrillation ablation: is there any evidence? Europace 2011;13:1219–21. [DOI] [PubMed] [Google Scholar]
- 1032. Yokoyama K, Nakagawa H, Seres KA, Jung E, Merino J, Zou Y, et al. Canine model of esophageal injury and atrial-esophageal fistula after applications of forward-firing high-intensity focused ultrasound and side-firing unfocused ultrasound in the left atrium and inside the pulmonary vein. Circ Arrhythm Electrophysiol 2009;2:41–9. [DOI] [PubMed] [Google Scholar]
- 1033. Cordes F, Ellermann C, Dechering DG, Frommeyer G, Kochhäuser S, Lange PS, et al. Preprocedural proton pump inhibition is associated with fewer peri-oesophageal lesions after cryoballoon pulmonary vein isolation. Sci Rep 2021;11:4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1034. Rolantova L, Bulava A, Eisenberger M, Chloubova I, Tothova V, Hanis J. Nurse-performed venous sheath removal in patients undergoing radiofrequency catheter ablation for atrial fibrillation: a randomised study. Eur J Cardiovasc Nurs 2019;18:332–9. [DOI] [PubMed] [Google Scholar]
- 1035. Kewcharoen J, Shah K, Bhardwaj R, Contractor T, Turagam MK, Mandapati R, et al. Periprocedural outcomes of protamine administration after catheter ablation of atrial fibrillation. Rev Cardiovasc Med 2022;23:34. [DOI] [PubMed] [Google Scholar]
- 1036. Chilukuri K, Henrikson CA, Dalal D, Scherr D, MacPherson EC, Cheng A, et al. Incidence and outcomes of protamine reactions in patients undergoing catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2009;25:175–81. [DOI] [PubMed] [Google Scholar]
- 1037. Traullé S, Kubala M, Doucy A, Quenum S, Hermida JS. Feasibility and safety of temporary subcutaneous venous figure-of-eight suture to achieve hemostasis after ablation of atrial fibrillation. Europace 2016;18:815–9. [DOI] [PubMed] [Google Scholar]
- 1038. Kumar V, Wish M, Venkataraman G, Bliden K, Jindal M, Strickberger A. A randomized comparison of manual pressure versus figure-of-eight suture for hemostasis after cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2019;30:2806–10. [DOI] [PubMed] [Google Scholar]
- 1039. Aytemir K, Canpolat U, Yorgun H, Evranos B, Kaya EB, Şahiner ML, et al. Usefulness of ‘figure-of-eight’ suture to achieve hemostasis after removal of 15-French calibre femoral venous sheath in patients undergoing cryoablation. Europace 2016;18:1545–50. [DOI] [PubMed] [Google Scholar]
- 1040. Natale A, Mohanty S, Liu PY, Mittal S, Al-Ahmad A, De Lurgio David B, et al. Venous vascular closure system versus manual compression following multiple access electrophysiology procedures. JACC Clin Electrophysiol 2020;6:111–24. [DOI] [PubMed] [Google Scholar]
- 1041. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042. Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173:149–56. [DOI] [PubMed] [Google Scholar]
- 1043. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JS, et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 2013;10:1272–7. [DOI] [PubMed] [Google Scholar]
- 1044. Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J 2016;37:2478–87. [DOI] [PubMed] [Google Scholar]
- 1045. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046. Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm 2011;8:1416–23. [DOI] [PubMed] [Google Scholar]
- 1047. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 1048. Camen S, Ojeda FM, Niiranen T, Gianfagna F, Vishram-Nielsen JK, Costanzo S, et al. Temporal relations between atrial fibrillation and ischemic stroke and their prognostic impact on mortality. Europace 2020;22:522–9. [DOI] [PubMed] [Google Scholar]
- 1049. Romero J, Cerrud-Rodriguez RC, Diaz JC, Rodriguez D, Arshad S, Alviz I, et al. Oral anticoagulation after catheter ablation of atrial fibrillation and the associated risk of thromboembolic events and intracranial hemorrhage: a systematic review and metaanalysis. J Cardiovasc Electrophysiol 2019;30:1250–7. [DOI] [PubMed] [Google Scholar]
- 1050. Proietti R, AlTurki A, Di Biase L, China P, Forleo G, Corrado A, et al. Anticoagulation after catheter ablation of atrial fibrillation: an unnecessary evil? A systematic review and metaanalysis. J Cardiovasc Electrophysiol 2019;30:468–78. [DOI] [PubMed] [Google Scholar]
- 1051. Liang JJ, Elafros MA, Mullen MT, Muser D, Hayashi T, Enriquez A, et al. Anticoagulation use and clinical outcomes after catheter ablation in patients with persistent and longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:823–32. [DOI] [PubMed] [Google Scholar]
- 1052. Verma A, Ha ACT, Kirchhof P, Hindricks G, Healey JS, Hill MD, et al. The optimal anti-coagulation for enhanced-risk patients post-catheter ablation for atrial fibrillation (OCEAN) trial. Am Heart J 2018;197:124–32. [DOI] [PubMed] [Google Scholar]
- 1053. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 1054. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–80. [DOI] [PubMed] [Google Scholar]
- 1055. Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J 2015;36:1660–8. [DOI] [PubMed] [Google Scholar]
- 1056. Waks JW, Passman RS, Matos J, Reynolds M, Thosani A, Mela T, et al. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dual-chamber pacemakers and implantable cardioverter-defibrillators: results from the tailored anticoagulation for non-continuous atrial fibrillation (TACTIC-AF) pilot study. Heart Rhythm 2018;15:1601–7. [DOI] [PubMed] [Google Scholar]
- 1057. Passman R, Leong-Sit P, Andrei AC, Huskin A, Tomson TT, Bernstein R, et al. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the rhythm evaluation for anticoagulation with continuous monitoring (REACT.COM) pilot study. J Cardiovasc Electrophysiol 2016;27:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1058. Barbhaiya CR, Kumar S, John RM, Tedrow UB, Koplan BA, Epstein LM, et al. Global survey of esophageal and gastric injury in atrial fibrillation ablation: incidence, time to presentation, and outcomes. J Am Coll Cardiol 2015;65:1377–8. [DOI] [PubMed] [Google Scholar]
- 1059. Piccini JP, Braegelmann KM, Simma S, Koneru JN, Ellenbogen KA. Risk of atrioesophageal fistula with cryoballoon ablation of atrial fibrillation. Heart Rhythm O2 2020;1:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060. Tilz RR, Schmidt V, Pürerfellner H, Maury P, Chun KJ, Martinek M, et al. A worldwide survey on incidence, management and prognosis of esophageal fistula formation following atrial fibrillation catheter ablation: the POTTER-AF study. Eur Heart J 2023;44:2458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1061. Barbhaiya Chirag R, Kumar S, Guo Y, Zhong J, John Roy M, Tedrow Usha B, et al. Global survey of esophageal injury in atrial fibrillation ablation. JACC Clin Electrophysiol 2016;2:143–50. [DOI] [PubMed] [Google Scholar]
- 1062. Gandjbakhch E, Mandel F, Dagher Y, Hidden-Lucet F, Rollin A, Maury P. Incidence, epidemiology, diagnosis and prognosis of atrio-oesophageal fistula following percutaneous catheter ablation: a French nationwide survey. Europace 2021;23:557–64. [DOI] [PubMed] [Google Scholar]
- 1063. Della Rocca DG, Magnocavallo M, Natale VN, Gianni C, Mohanty S, Trivedi C, et al. Clinical presentation, diagnosis, and treatment of atrioesophageal fistula resulting from atrial fibrillation ablation. J Cardiovasc Electrophysiol 2021;32:2441–50. [DOI] [PubMed] [Google Scholar]
- 1064. Dagres N, Kottkamp H, Piorkowski C, Doll N, Mohr F, Horlitz M, et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol 2006;17:1213–5. [DOI] [PubMed] [Google Scholar]
- 1065. Dagres N, Hindricks G, Kottkamp H, Sommer P, Gaspar T, Bode K, et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol 2009;20:1014–9. [DOI] [PubMed] [Google Scholar]
- 1066. Arbelo E, Brugada J, Blomström-Lundqvist C, Laroche C, Kautzner J, Pokushalov E, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J 2017;38:1303–16. [DOI] [PubMed] [Google Scholar]
- 1067. Markar SR, Koehler R, Low DE, Ross A. Novel multimodality endoscopic closure of postoperative esophageal fistula. Int J Surg Case Rep 2012;3:577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068. Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–55. [DOI] [PubMed] [Google Scholar]
- 1069. Ugata Y, Michihata N, Matsui H, Fushimi K, Yasunaga H. Impact of proton pump inhibitors on mortality and severe esophageal injury after catheter ablation for atrial fibrillation: a nationwide retrospective study using propensity score matching. Heart Vessels 2021;36:1730–8. [DOI] [PubMed] [Google Scholar]
- 1070. Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol 2012;60:1790–6. [DOI] [PubMed] [Google Scholar]
- 1071. Deftereos S, Giannopoulos G, Efremidis M, Kossyvakis C, Katsivas A, Panagopoulou V, et al. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm 2014;11:620–8. [DOI] [PubMed] [Google Scholar]
- 1072. Koyama T, Tada H, Sekiguchi Y, Arimoto T, Yamasaki H, Kuroki K, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010;56:1463–72. [DOI] [PubMed] [Google Scholar]
- 1073. Kim YR, Nam GB, Han S, Kim SH, Kim KH, Lee S, et al. Effect of short-term steroid therapy on early recurrence during the blanking period after catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2015;8:1366–72. [DOI] [PubMed] [Google Scholar]
- 1074. Iskandar S, Reddy M, Afzal MR, Rajasingh J, Atoui M, Lavu M, et al. Use of oral steroid and its effects on atrial fibrillation recurrence and inflammatory cytokines post ablation – the steroid AF study. J Atr Fibrillation 2017;9:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1075. Kim DR, Won H, Uhm JS, Kim JY, Sung JH, Pak HN, et al. Comparison of two different doses of single bolus steroid injection to prevent atrial fibrillation recurrence after radiofrequency catheter ablation. Yonsei Med J 2015;56:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1076. Won H, Kim JY, Shim J, Uhm JS, Pak HN, Lee MH, et al. Effect of a single bolus injection of low-dose hydrocortisone for prevention of atrial fibrillation recurrence after radiofrequency catheter ablation. Circ J 2013;77:53–9. [DOI] [PubMed] [Google Scholar]
- 1077. Vasamreddy CR, Dalal D, Dong J, Cheng A, Spragg D, Lamiy SZ, et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2006;17:134–9. [DOI] [PubMed] [Google Scholar]
- 1078. Janse PA, van Belle YL, Theuns DA, Rivero-Ayerza M, Scholten MF, Jordaens LJ. Symptoms versus objective rhythm monitoring in patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Eur J Cardiovasc Nurs 2008;7:147–51. [DOI] [PubMed] [Google Scholar]
- 1079. Piorkowski C, Kottkamp H, Tanner H, Kobza R, Nielsen JC, Arya A, et al. Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol 2005;16:1286–92. [DOI] [PubMed] [Google Scholar]
- 1080. Klemm HU, Ventura R, Rostock T, Brandstrup B, Risius T, Meinertz T, et al. Correlation of symptoms to ECG diagnosis following atrial fibrillation ablation. J Cardiovasc Electrophysiol 2006;17:146–50. [DOI] [PubMed] [Google Scholar]
- 1081. Kaufman ES, Israel CW, Nair GM, Armaganijan L, Divakaramenon S, Mairesse GH, et al. Positive predictive value of device-detected atrial high-rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm 2012;9:1241–6. [DOI] [PubMed] [Google Scholar]
- 1082. Hindricks G, Pokushalov E, Urban L, Taborsky M, Kuck KH, Lebedev D, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol 2010;3:141–7. [DOI] [PubMed] [Google Scholar]
- 1083. Xing LY, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Olesen MS, et al. Electrocardiographic markers of subclinical atrial fibrillation detected by implantable loop recorder: insights from the LOOP Study. Europace 2023;25:euad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084. Charitos EI, Ziegler PD, Stierle U, Graf B, Sievers HH, Hanke T. Long-term outcomes after surgical ablation for atrial fibrillation in patients with continuous heart rhythm monitoring devices. Interact Cardiovasc Thorac Surg 2015;21:712–21. [DOI] [PubMed] [Google Scholar]
- 1085. Perez-Castellano N, Fernandez-Cavazos R, Moreno J, Canadas V, Conde A, Gonzalez-Ferrer JJ, et al. The COR trial: a randomized study with continuous rhythm monitoring to compare the efficacy of cryoenergy and radiofrequency for pulmonary vein isolation. Heart Rhythm 2014;11:8–14. [DOI] [PubMed] [Google Scholar]
- 1086. Kapa S, Epstein AE, Callans DJ, Garcia FC, Lin D, Bala R, et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. J Cardiovasc Electrophysiol 2013;24:875–81. [DOI] [PubMed] [Google Scholar]
- 1087. Damiano RJ Jr, Lawrance CP, Saint LL, Henn MC, Sinn LA, Kruse J, et al. Detection of atrial fibrillation after surgical ablation: conventional versus continuous monitoring. Ann Thorac Surg 2016;101:42–7; discussion 47–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1088. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 1089. Goldenthal IL, Sciacca RR, Riga T, Bakken S, Baumeister M, Biviano AB, et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol 2019;30:2220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090. Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015;12:554–9. [DOI] [PubMed] [Google Scholar]
- 1091. Rizas KD, Freyer L, Sappler N, von Stülpnagel L, Spielbichler P, Krasniqi A, et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 2022;28:1823–30. [DOI] [PubMed] [Google Scholar]
- 1092. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar P, et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PubMed] [Google Scholar]
- 1093. Lambert CT, Patel D, Bumgarner JM, Kanj M, Cantillon D, Saliba W, et al. Atrial fibrillation future clinic. Novel platform to integrate smart device electrocardiogram into clinical practice. Cardiovasc Digit Health J 2021;2:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094. Hermans ANL, Gawalko M, Pluymaekers N, Dinh T, Weijs B, van Mourik MJW, et al. Long-term intermittent versus short continuous heart rhythm monitoring for the detection of atrial fibrillation recurrences after catheter ablation. Int J Cardiol 2021;329:105–12. [DOI] [PubMed] [Google Scholar]
- 1095. Gawałko M, Duncker D, Manninger M, van der Velden RMJ, Hermans ANL, Verhaert DVM, et al. The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: center and patient experiences. Europace 2021;23:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1096. Unni RR, Prager RT, Odabashian R, Zhang JJ, Fat Hing NN, Nery PB, et al. Rhythm-monitoring strategy and arrhythmia recurrence in atrial fibrillation ablation trials: a systematic review. CJC Open 2022;4:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097. Andrade JG, Khairy P, Verma A, Guerra PG, Dubuc M, Rivard L, et al. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2012;35:106–16. [DOI] [PubMed] [Google Scholar]
- 1098. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1099. Gottlieb LA, Dekker LRC, Coronel R. The blinding period following ablation therapy for atrial fibrillation: proarrhythmic and antiarrhythmic pathophysiological mechanisms. JACC Clin Electrophysiol 2021;7:416–30. [DOI] [PubMed] [Google Scholar]
- 1100. Opacic D, van Bragt KA, Nasrallah HM, Schotten U, Verheule S. Atrial metabolism and tissue perfusion as determinants of electrical and structural remodelling in atrial fibrillation. Cardiovasc Res 2016;109:527–41. [DOI] [PubMed] [Google Scholar]
- 1101. Lim HS, Schultz C, Dang J, Alasady M, Lau DH, Brooks AG, et al. Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:83–9. [DOI] [PubMed] [Google Scholar]
- 1102. Richter B, Gwechenberger M, Socas A, Zorn G, Albinni S, Marx M, et al. Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol 2012;101:217–25. [DOI] [PubMed] [Google Scholar]
- 1103. Sepehri Shamloo A, Bollmann A, Dagres N, Hindricks G, Arya A. Natriuretic peptides: biomarkers for atrial fibrillation management. Clin Res Cardiol 2020;109:957–66. [DOI] [PubMed] [Google Scholar]
- 1104. Liang JJ, Dixit S, Santangeli P. Mechanisms and clinical significance of early recurrences of atrial arrhythmias after catheter ablation for atrial fibrillation. World J Cardiol 2016;8:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105. Grégoire JM, Gilon C, Carlier S, Bersini H. Role of the autonomic nervous system and premature atrial contractions in short-term paroxysmal atrial fibrillation forecasting: insights from machine learning models. Arch Cardiovasc Dis 2022;115:377–87. [DOI] [PubMed] [Google Scholar]
- 1106. Scherschel K, Hedenus K, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW, et al. Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation. Sci Transl Med 2019;11:eaav7770. [DOI] [PubMed] [Google Scholar]
- 1107. Sasaki N, Okumura Y, Watanabe I, Nagashima K, Sonoda K, Kogawa R, et al. Transthoracic echocardiographic backscatter-based assessment of left atrial remodeling involving left atrial and ventricular fibrosis in patients with atrial fibrillation. Int J Cardiol 2014;176:1064–6. [DOI] [PubMed] [Google Scholar]
- 1108. McGann C, Kholmovski E, Blauer J, Vijayakumar S, Haslam T, Cates J, et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol 2011;58:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109. Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, et al. Recurrence of atrial fibrillation correlates with the extent of postprocedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging 2009;2:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110. Steinberg C, Champagne J, Deyell MW, Dubuc M, Leong-Sit P, Calkins H, et al. Prevalence and outcome of early recurrence of atrial tachyarrhythmias in the cryoballoon vs irrigated radiofrequency catheter ablation (CIRCA-DOSE) study. Heart Rhythm 2021;18:1463–70. [DOI] [PubMed] [Google Scholar]
- 1111. Charitakis E, Metelli S, Karlsson LO, Antoniadis AP, Rizas KD, Liuba I, et al. Comparing efficacy and safety in catheter ablation strategies for atrial fibrillation: a network metaanalysis. BMC Med 2022;20:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1112. Andrade JG, Khairy P, Verma A, Guerra PG, Dubuc M, Rivard L, et al. Early recurrence of atrial tachyarrhythmias following radiofrequency catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol 2012;35:106–16. [DOI] [PubMed] [Google Scholar]
- 1113. Baimbetov AK, Bizhanov KA, Jukenova AM, Aubakirova AT, Ualiyeva AY, Sagatov IY. Comparative effectiveness and safety of cryoablation versus radiofrequency ablation treatments for persistent atrial fibrillation. Am J Cardiol 2022;184:22–30. [DOI] [PubMed] [Google Scholar]
- 1114. Choi J-H, Kwon H-J, Kim HR, Park S-J, Kim JS, On YK, et al. Electrocardiographic predictors of early recurrence of atrial fibrillation. Ann Noninvasive Electrocardiol 2021;26:e12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1115. Ribeiro Da Silva M, Santos Silva G, Ribeiro Queiros P, Teixeira R, Almeida J, Fonseca P, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation: two sides of the same coin? Eur Heart J 2021;42:ehab724. [Google Scholar]
- 1116. Kim Yun G, Boo Ki Y, Choi J-I, Choi Yun Y, Choi Ha Y, Roh S-Y, et al. Early recurrence is reliable predictor of late recurrence after radiofrequency catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2021;7:343–51. [DOI] [PubMed] [Google Scholar]
- 1117. Calkins H, Gache L, Frame D, Boo LM, Ghaly N, Schilling R, et al. Predictive value of atrial fibrillation during the postradiofrequency ablation blanking period. Heart Rhythm 2021;18:366–73. [DOI] [PubMed] [Google Scholar]
- 1118. Filipovic K, Dittrich S, Scheurlen C, Arica Z, Erlhoefer S, Woermann J, et al. Validation of seven risk scores in a prospective and independent cohort: the challenge of predicting recurrence after atrial fibrillation ablation. Europace 2022;24:euac053.191. [Google Scholar]
- 1119. Dretzke J, Chuchu N, Agarwal R, Herd C, Chua W, Fabritz L, et al. Predicting recurrent atrial fibrillation after catheter ablation: a systematic review of prognostic models. Europace 2020;22:748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1120. Winkle RA, Jarman JW, Mead RH, Engel G, Kong MH, Fleming W, et al. Predicting atrial fibrillation ablation outcome: the CAAP-AF score. Heart Rhythm 2016;13:2119–25. [DOI] [PubMed] [Google Scholar]
- 1121. Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Bollmann A. The APPLE score – a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLOS ONE 2017;12:e0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1122. Jud FN, Obeid S, Duru F, Haegeli LM. A novel score in the prediction of rhythm outcome after ablation of atrial fibrillation: the SUCCESS score. Anatol J Cardiol 2019;21:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123. Mesquita J, Ferreira AM, Cavaco D, Moscoso Costa F, Carmo P, Marques H, et al. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure – ATLAS score. Europace 2018;20:f428–35. [DOI] [PubMed] [Google Scholar]
- 1124. Nielsen JC, Lin Y-J, de Oliveira Figueiredo MJ, Sepehri Shamloo A, Alfie A, Boveda S, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. Europace 2020;22:1147–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1125. Lee S-H, Tai C-T, Hsieh M-H, Tsai C-F, Lin Y-K, Tsao H-M, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2004;10:221–6. [DOI] [PubMed] [Google Scholar]
- 1126. Kuck K-H, Hoffmann BA, Ernst S, Wegscheider K, Treszl A, Metzner A, et al. Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: results from the gap-atrial fibrillation–German atrial fibrillation competence network 1 trial. Circ Arrhythm Electrophysiol 2016;9:e003337. [DOI] [PubMed] [Google Scholar]
- 1127. Liu H, Yuan P, Zhu X, Fu L, Hong K, Hu J. Is atrial fibrillation noninducibility by burst pacing after catheter ablation associated with reduced clinical recurrence? A systematic review and metaanalysis. J Am Heart Assoc 2020;9:e015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128. Okada M, Inoue K, Tanaka N, Masuda M, Furukawa Y, Hirata A, et al. Reappraising the role of baseline plasma C-reactive protein levels on recurrence after catheter ablation of persistent atrial fibrillation: insight from EARNEST-PVI trial. Eur Heart J 2021;42:ehab724. 0507. [Google Scholar]
- 1129. Charalampidis P, Teperikidis E, Boulmpou A, Papadopoulos CE, Potoupni V, Tsioni K, et al. Homocysteine as a predictor of paroxysmal atrial fibrillation-related events: a scoping review of the literature. Diagnostics 2022;12:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130. Liang JJ, Elafros MA, Chik WW, Santangeli P, Zado ES, Frankel DS, et al. Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: timing and frequency of early recurrences predicts long-term ablation success. Heart Rhythm 2015;12:2461–8. [DOI] [PubMed] [Google Scholar]
- 1131. Amankwah NA, Pothineni NVK, Guandalini G, Santangeli P, Schaller R, Supple GE, et al. Impact of atrial fibrillation recurrences during the blanking period following catheter ablation on long-term arrhythmia-free survival: a prospective study with continuous monitoring. J Interv Card Electrophysiol 2022;65:519–25. [DOI] [PubMed] [Google Scholar]
- 1132. Mugnai G, de Asmundis C, Hünük B, Ströker E, Velagic V, Moran D, et al. Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: predictive role of atrial arrhythmias occurring in the blanking period on the incidence of late recurrences. Heart Rhythm 2016;13:845–51. [DOI] [PubMed] [Google Scholar]
- 1133. Stabile G, Iacopino S, Verlato R, Arena G, Pieragnoli P, Molon G, et al. Predictive role of early recurrence of atrial fibrillation after cryoballoon ablation. Europace 2020;22:1798–804. [DOI] [PubMed] [Google Scholar]
- 1134. Willems S, Khairy P, Andrade JG, Hoffmann BA, Levesque S, Verma A, et al. Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation: insights from the ADVICE (adenosine following pulmonary vein isolation to target dormant conduction elimination) trial. Circ Arrhythm Electrophysiol 2016;9:e003909. [DOI] [PubMed] [Google Scholar]
- 1135. Alipour P, Azizi Z, Pirbaglou M, Ritvo P, Pantano A, Verma A, et al. Defining blanking period post-pulmonary vein antrum isolation. JACC Clin Electrophysiol 2017;3:568–76. [DOI] [PubMed] [Google Scholar]
- 1136. Themistoclakis S, Schweikert RA, Saliba WI, Bonso A, Rossillo A, Bader G, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008;5:679–85. [DOI] [PubMed] [Google Scholar]
- 1137. Noujaim C, Lim C, Mekhael M, Feng H, Chouman N, Younes H, et al. Identifying the prognostic significance of early arrhythmia recurrence during the blanking period and the optimal blanking period duration: insights from the DECAAF II study. Europace 2023;25:euad173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138. Cosio FG, Aliot E, Botto GL, Heidbüchel H, Geller CJ, Kirchhof P, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace 2007;10:21–7. [DOI] [PubMed] [Google Scholar]
- 1139. Chilukuri K, Dukes J, Dalal D, Marine JE, Henrikson CA, Scherr D, et al. Outcomes in patients requiring cardioversion following catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2010;21:27–32. [DOI] [PubMed] [Google Scholar]
- 1140. Ebert M, Stegmann C, Kosiuk J, Dinov B, Richter S, Arya A, et al. Predictors, management, and outcome of cardioversion failure early after atrial fibrillation ablation. Europace 2018;20:1428–34. [DOI] [PubMed] [Google Scholar]
- 1141. Dong Z, Du X, Hou XX, He L, Dong JZ, Ma CS. Effect of electrical cardioversion on 1-year outcomes in patients with early recurrence after catheter ablation for atrial fibrillation. Clin Cardiol 2021;44:1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142. Malasana G, Day JD, Weiss JP, Crandall BG, Bair TL, May HT, et al. A strategy of rapid cardioversion minimizes the significance of early recurrent atrial tachyarrhythmias after ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:761–6. [DOI] [PubMed] [Google Scholar]
- 1143. Sponga S, Leoni L, Buja G, Nalli C, Voisine P, Gerosa G. Role of an aggressive rhythm control strategy on sinus rhythm maintenance following intraoperative radiofrequency ablation of atrial fibrillation in patients undergoing surgical correction of valvular disease. J Cardiol 2012;60:316–20. [DOI] [PubMed] [Google Scholar]
- 1144. Baman TS, Gupta SK, Billakanty SR, Ilg KJ, Good E, Crawford T, et al. Time to cardioversion of recurrent atrial arrhythmias after catheter ablation of atrial fibrillation and long-term clinical outcome. J Cardiovasc Electrophysiol 2009;20:1321–5. [DOI] [PubMed] [Google Scholar]
- 1145. Lellouche N, Jaïs P, Nault I, Wright M, Bevilacqua M, Knecht S, et al. Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J Cardiovasc Electrophysiol 2008;19:599–605. [DOI] [PubMed] [Google Scholar]
- 1146. Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter sustained treatment of paroxysmal atrial fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol 2014;7:69–75. [DOI] [PubMed] [Google Scholar]
- 1147. Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (atrial fibrillation better care) pathway. Am J Med 2018;131:1359–66.e6. [DOI] [PubMed] [Google Scholar]
- 1148. Rivera-Caravaca JM, Roldán V, Martínez-Montesinos L, Vicente V, Lip GYH, Marín F. The atrial fibrillation better care (ABC) pathway and clinical outcomes in patients with atrial fibrillation: the prospective murcia AF project phase II cohort. J Gen Intern Med 2023;38:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149. Kotalczyk A, Guo Y, Stefil M, Wang Y, Lip GYH. Effects of the atrial fibrillation better care pathway on outcomes among clinically complex Chinese patients with atrial fibrillation with multimorbidity and polypharmacy: a report from the ChiOTEAF registry. J Am Heart Assoc 2022;11:e024319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150. Patel SM, Palazzolo MG, Murphy SA, Antman EM, Braunwald E, Lanz HJ, et al. Evaluation of the atrial fibrillation better care pathway in the ENGAGE AF-TIMI 48 trial. Europace 2022;24:1730–8. [DOI] [PubMed] [Google Scholar]
- 1151. Stevens D, Harrison SL, Kolamunnage-Dona R, Lip GYH, Lane DA. The atrial fibrillation better care pathway for managing atrial fibrillation: a review. Europace 2021;23:1511–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1152. Proietti M, Lip GYH, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Relation of outcomes to ABC (atrial fibrillation better care) pathway adherent care in European patients with atrial fibrillation: an analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) registry. Europace 2021;23:174–83. [DOI] [PubMed] [Google Scholar]
- 1153. Gerstenfeld EP, Callans DJ, Dixit S, Russo AM, Nayak H, Lin D, et al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 2004;110:1351–7. [DOI] [PubMed] [Google Scholar]
- 1154. Chugh A, Oral H, Good E, Han J, Tamirisa K, Lemola K, et al. Catheter ablation of atypical atrial flutter and atrial tachycardia within the coronary sinus after left atrial ablation for atrial fibrillation. J Am Coll Cardiol 2005;46:83–91. [DOI] [PubMed] [Google Scholar]
- 1155. Deisenhofer I, Estner H, Zrenner B, Schreieck J, Weyerbrock S, Hessling G, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace 2006;8:573–82. [DOI] [PubMed] [Google Scholar]
- 1156. Gerstenfeld EP, Callans DJ, Sauer W, Jacobson J, Marchlinski FE. Reentrant and nonreentrant focal left atrial tachycardias occur after pulmonary vein isolation. Heart Rhythm 2005;2:1195–202. [DOI] [PubMed] [Google Scholar]
- 1157. Gerstenfeld EP, Marchlinski FE. Mapping and ablation of left atrial tachycardias occurring after atrial fibrillation ablation. Heart Rhythm 2007;4:S65–72. [DOI] [PubMed] [Google Scholar]
- 1158. Lim TW, Koay CH, McCall R, See VA, Ross DL, Thomas SP. Atrial arrhythmias after single-ring isolation of the posterior left atrium and pulmonary veins for atrial fibrillation: mechanisms and management. Circ Arrhythm Electrophysiol 2008;1:120–6. [DOI] [PubMed] [Google Scholar]
- 1159. Anousheh R, Sawhney NS, Panutich M, Tate C, Chen WC, Feld GK. Effect of mitral isthmus block on development of atrial tachycardia following ablation for atrial fibrillation. Pacing Clin Electrophysiol 2010;33:460–8. [DOI] [PubMed] [Google Scholar]
- 1160. Wasmer K, Monnig G, Bittner A, Dechering D, Zellerhoff S, Milberg P, et al. Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm 2012;9:1660–6. [DOI] [PubMed] [Google Scholar]
- 1161. Karch MR, Zrenner B, Deisenhofer I, Schreieck J, Ndrepepa G, Dong J, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation 2005;111:2875–80. [DOI] [PubMed] [Google Scholar]
- 1162. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 2005;111:127–35. [DOI] [PubMed] [Google Scholar]
- 1163. Yamashita S, Takigawa M, Denis A, Derval N, Sakamoto Y, Masuda M, et al. Pulmonary vein-gap reentrant atrial tachycardia following atrial fibrillation ablation: an electrophysiological insight with high-resolution mapping. Europace 2019;21:1039–47. [DOI] [PubMed] [Google Scholar]
- 1164. Wojcik M, Berkowitsch A, Zaltsberg S, Hamm CW, Pitschner HF, Kuniss M, et al. Predictors of early and late left atrial tachycardia and left atrial flutter after catheter ablation of atrial fibrillation: long-term follow-up. Cardiol J 2015;22:557–66. [DOI] [PubMed] [Google Scholar]
- 1165. Mesas CE, Pappone C, Lang CC, Gugliotta F, Tomita T, Vicedomini G, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J Am Coll Cardiol 2004;44:1071–9. [DOI] [PubMed] [Google Scholar]
- 1166. Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606–12. [DOI] [PubMed] [Google Scholar]
- 1167. Mikhaylov EN, Bhagwandien R, Janse PA, Theuns DA, Szili-Torok T. Regular atrial tachycardias developing after cryoballoon pulmonary vein isolation: incidence, characteristics, and predictors. Europace 2013;15:1710–7. [DOI] [PubMed] [Google Scholar]
- 1168. Hermida A, Kubala M, Traulle S, Buiciuc O, Quenum S, Hermida JS. Prevalence and predictive factors of left atrial tachycardia occurring after second-generation cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:46–54. [DOI] [PubMed] [Google Scholar]
- 1169. Guhl EN, Siddoway D, Adelstein E, Voigt A, Saba S, Jain SK. Efficacy of cryoballoon pulmonary vein isolation in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:423–7. [DOI] [PubMed] [Google Scholar]
- 1170. Lyan E, Yalin K, Abdin A, Sawan N, Liosis S, Lange SA, et al. Mechanism, underlying substrate and predictors of atrial tachycardia following atrial fibrillation ablation using the second-generation cryoballoon. J Cardiol 2019;73:497–506. [DOI] [PubMed] [Google Scholar]
- 1171. Julia J, Chierchia GB, de Asmundis C, Mugnai G, Sieira J, Ciconte G, et al. Regular atrial tachycardias following pulmonary vein isolation for paroxysmal atrial fibrillation: a retrospective comparison between the cryoballoon and conventional focal tip radiofrequency techniques. J Interv Card Electrophysiol 2015;42:161–9. [DOI] [PubMed] [Google Scholar]
- 1172. Akerstrom F, Bastani H, Insulander P, Schwieler J, Arias MA, Jensen-Urstad M. Comparison of regular atrial tachycardia incidence after circumferential radiofrequency versus cryoballoon pulmonary vein isolation in real-life practice. J Cardiovasc Electrophysiol 2014;25:948–52. [DOI] [PubMed] [Google Scholar]
- 1173. Van Belle Y, Knops P, Janse P, Rivero-Ayerza M, Jessurun E, Szili-Torok T, et al. Electro-anatomical mapping of the left atrium before and after cryothermal balloon isolation of the pulmonary veins. J Interv Card Electrophysiol 2009;25:59–65. [DOI] [PubMed] [Google Scholar]
- 1174. Kenigsberg DN, Martin N, Lim HW, Kowalski M, Ellenbogen KA. Quantification of the cryoablation zone demarcated by pre- and postprocedural electroanatomic mapping in patients with atrial fibrillation using the 28-mm second-generation cryoballoon. Heart Rhythm 2015;12:283–90. [DOI] [PubMed] [Google Scholar]
- 1175. Gopinathannair R, Mar PL, Afzal MR, Di Biase L, Tu Y, Lakkireddy T, et al. Atrial tachycardias after surgical atrial fibrillation ablation: clinical characteristics, electrophysiological mechanisms, and ablation outcomes from a large, multicenter study. JACC Clin Electrophysiol 2017;3:865–74. [DOI] [PubMed] [Google Scholar]
- 1176. Huo Y, Schoenbauer R, Richter S, Rolf S, Sommer P, Arya A, et al. Atrial arrhythmias following surgical AF ablation: electrophysiological findings, ablation strategies, and clinical outcome. J Cardiovasc Electrophysiol 2014;25:725–38. [DOI] [PubMed] [Google Scholar]
- 1177. Takigawa M, Derval N, Frontera A, Martin R, Yamashita S, Cheniti G, et al. Revisiting anatomic macroreentrant tachycardia after atrial fibrillation ablation using ultrahigh-resolution mapping: implications for ablation. Heart Rhythm 2018;15:326–33. [DOI] [PubMed] [Google Scholar]
- 1178. Pascale P, Shah AJ, Roten L, Scherr D, Komatsu Y, Jadidi AS, et al. Pattern and timing of the coronary sinus activation to guide rapid diagnosis of atrial tachycardia after atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2013;6:481–90. [DOI] [PubMed] [Google Scholar]
- 1179. Arroyo R C, Latcu DG, Maeda S, Kubala M, Santangeli P, Garcia FC, et al. Coronary sinus activation and ECG characteristics of roof-dependent left atrial flutter after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2018;11:e005948. [DOI] [PubMed] [Google Scholar]
- 1180. Vlachos K, Efremidis M, Derval N, Martin CA, Takigawa M, Bazoukis G, et al. Use of high-density activation and voltage mapping in combination with entrainment to delineate gap-related atrial tachycardias post atrial fibrillation ablation. Europace 2021;23:1052–62. [DOI] [PubMed] [Google Scholar]
- 1181. Sundaram S, Choe W, Ryan Jordan J, Mullins N, Boorman C, Kessler EJ, et al. Catheter ablation of atypical atrial flutter: a novel 3D anatomic mapping approach to quickly localize and terminate atypical atrial flutter. J Interv Card Electrophysiol 2017;49:307–18. [DOI] [PubMed] [Google Scholar]
- 1182. Luik A, Schmidt K, Haas A, Unger L, Tzamalis P, Brüggenjürgen B. Ablation of left atrial tachycardia following catheter ablation of atrial fibrillation: 12-month success rates. J Clin Med 2022;11:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1183. Lupercio F, Lin AY, Aldaas OM, Romero J, Briceno D, Hoffmayer KS, et al. Role of adjunctive posterior wall isolation in patients undergoing atrial fibrillation ablation: a systematic review and metaanalysis. J Interv Card Electrophysiol 2020;58:77–86. [DOI] [PubMed] [Google Scholar]
- 1184. Kim MY, Coyle C, Tomlinson DR, Sikkel MB, Sohaib A, Luther V, et al. Ectopy-triggering ganglionated plexuses ablation to prevent atrial fibrillation: GANGLIA-AF study. Heart Rhythm 2022;19:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185. Gianni C, Mohanty S, Di Biase L, Metz T, Trivedi C, Gokoglan Y, et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm 2016;13:830–5. [DOI] [PubMed] [Google Scholar]
- 1186. Perera KS, Sharma M, Connolly SJ, Wang J, Gold MR, Hohnloser SH, et al. Stroke type and severity in patients with subclinical atrial fibrillation: an analysis from the asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and the atrial fibrillation reduction atrial pacing trial (ASSERT). Am Heart J 2018;201:160–3. [DOI] [PubMed] [Google Scholar]
- 1187. Andrade JG, Deyell MW, Macle L, Steinberg JS, Glotzer TV, Hawkins NM, et al. Healthcare utilization and quality of life for atrial fibrillation burden: the CIRCA-DOSE study. Eur Heart J 2023;44:765–76. [DOI] [PubMed] [Google Scholar]
- 1188. Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1189. Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L, et al. Atrial fibrillation burden and clinical outcomes in heart failure: the CASTLE-AF trial. JACC Clin Electrophysiol 2021;7:594–603. [DOI] [PubMed] [Google Scholar]
- 1190. Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Geller L, Kalejs O, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1191. Packer D, Monahan K, Piccini J, Al-Khalidi H, Silverstein A, Poole J, et al. Impact of treatment strategies for AF on the progression and regression of AF type in the CABANA trial. European Heart Journal 2020;41:ehaa946.0680. [Google Scholar]
- 1192. Gupta D, Vijgen J, Potter T, Scherr D, Van Herendael H, Knecht S, et al. Quality of life and healthcare utilisation improvements after atrial fibrillation ablation. Heart 2021;107:1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1194. Samuel M, Khairy P, Champagne J, Deyell MW, Macle L, Leong-Sit P, et al. Association of atrial fibrillation burden with health-related quality of life after atrial fibrillation ablation: substudy of the cryoballoon vs contact-force atrial fibrillation ablation (CIRCA-DOSE) randomized clinical trial. JAMA Cardiol 2021;6:1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195. Wazni O, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, et al. Quality of life after the initial treatment of atrial fibrillation with cryoablation versus drug therapy. Heart Rhythm 2022;19:197–205. [DOI] [PubMed] [Google Scholar]
- 1196. Wu L, Narasimhan B, Ho KS, Zheng Y, Shah AN, Kantharia BK. Safety and complications of catheter ablation for atrial fibrillation: predictors of complications from an updated analysis the national inpatient database. J Cardiovasc Electrophysiol 2021;32:1024–34. [DOI] [PubMed] [Google Scholar]
- 1197. Ngo L, Ali A, Ganesan A, Woodman R, Adams R, Ranasinghe I. Ten-year trends in mortality and complications following catheter ablation of atrial fibrillation. Eur Heart J Qual Care Clin Outcomes 2022;8:398–408. [DOI] [PubMed] [Google Scholar]
- 1198. Benali K, Khairy P, Hammache N, Petzl A, Da Costa A, Verma A, et al. Procedure-related complications of catheter ablation for atrial fibrillation. J Am Coll Cardiol 2023;81:2089–99. [DOI] [PubMed] [Google Scholar]
- 1199. Hsu JC, Darden D, Du C, Marine JE, Nichols S, Marcus GM, et al. Initial findings from the national cardiovascular data registry of atrial fibrillation ablation procedures. J Am Coll Cardiol 2023;81:867–78. [DOI] [PubMed] [Google Scholar]
- 1200. König S, Ueberham L, Schuler E, Wiedemann M, Reithmann C, Seyfarth M, et al. In-hospital mortality of patients with atrial arrhythmias: insights from the German-wide Helios hospital network of 161 502 patients and 34 025 arrhythmia-related procedures. Eur Heart J 2018;39:3947–57. [DOI] [PubMed] [Google Scholar]
- 1201. Mszar R, Friedman DJ, Ong E, Du C, Wang Y, Zeitler EP, et al. Sex-based differences in atrial fibrillation ablation adverse events. Heart 2023;109:595–605. [DOI] [PubMed] [Google Scholar]
- 1202. Forleo GB, Tondo C, De Luca L, Dello Russo A, Casella M, De Sanctis V, et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace 2007;9:613–20. [DOI] [PubMed] [Google Scholar]
- 1203. Ngo L, Ali A, Ganesan A, Woodman R, Adams R, Ranasinghe I. Gender differences in complications following catheter ablation of atrial fibrillation. Eur Heart J Qual Care Clin Outcomes 2021;7:458–67. [DOI] [PubMed] [Google Scholar]
- 1204. Tonchev IR, Nam MCY, Gorelik A, Kumar S, Haqqani H, Sanders P, et al. Relationship between procedural volume and complication rates for catheter ablation of atrial fibrillation: a systematic review and metaanalysis. Europace 2021;23:1024–32. [DOI] [PubMed] [Google Scholar]
- 1205. Cheung JW, Yeo I, Cheng EP, Ip JE, Thomas G, Liu CF, et al. Inpatient hospital procedural volume and outcomes following catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:1908–19. [DOI] [PubMed] [Google Scholar]
- 1206. Mörtsell D, Arbelo E, Dagres N, Brugada J, Laroche C, Trines SA, et al. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace 2019;21:581–9. [DOI] [PubMed] [Google Scholar]
- 1207. Buiatti A, von Olshausen G, Barthel P, Schneider S, Luik A, Kaess B, et al. Cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: an updated metaanalysis of randomized and observational studies. Europace 2017;19:378–84. [DOI] [PubMed] [Google Scholar]
- 1208. Ravi V, Poudyal A, Abid QU, Larsen T, Krishnan K, Sharma PS, et al. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: a systematic review and metaanalysis. Europace 2021;23:710–21. [DOI] [PubMed] [Google Scholar]
- 1209. Hansom SP, Alqarawi W, Birnie DH, Golian M, Nery PB, Redpath CJ, et al. High-power, short-duration atrial fibrillation ablation compared with a conventional approach: outcomes and reconnection patterns. J Cardiovasc Electrophysiol 2021;32:1219–28. [DOI] [PubMed] [Google Scholar]
- 1210. Mueller J, Halbfass P, Sonne K, Nentwich K, Ene E, Berkovitz A, et al. Safety aspects of very high power very short duration atrial fibrillation ablation using a modified radiofrequency RF-generator: single-center experience. J Cardiovasc Electrophysiol 2022;33:920–7. [DOI] [PubMed] [Google Scholar]
- 1211. Chieng D, Segan L, Sugumar H, Al-Kaisey A, Hawson J, Moore BM, et al. Higher power short duration vs. lower power longer duration posterior wall ablation for atrial fibrillation and esophageal injury outcomes: a prospective multicentre randomized controlled study (Hi-Lo HEAT trial). Europace 2023;25:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1212. Lee AC, Voskoboinik A, Cheung CC, Yogi S, Tseng ZH, Moss JD, et al. A randomized trial of high vs standard power radiofrequency ablation for pulmonary vein isolation. JACC Clin Electrophysiol 2023;9:1038–47. [DOI] [PubMed] [Google Scholar]
- 1213. Paul Nordin A, Drca N, Insulander P, Bastani H, Bourke T, Braunschweig F, et al. Low incidence of major complications after the first six hours post atrial fibrillation ablation: is same-day discharge safe and feasible in most patients? J Cardiovasc Electrophysiol 2021;32:2953–60. [DOI] [PubMed] [Google Scholar]
- 1214. Opel A, Mansell J, Butler A, Schwartz R, Fannon M, Finlay M, et al. Comparison of a high throughput day case atrial fibrillation ablation service in a local hospital with standard regional tertiary cardiac center care. Europace 2019;21:440–4. [DOI] [PubMed] [Google Scholar]
- 1215. Dagres N, Anastasiou-Nana M. Prevention of atrial-esophageal fistula after catheter ablation of atrial fibrillation. Curr Opin Cardiol 2011;26:1–5. [DOI] [PubMed] [Google Scholar]
- 1216. Cochet H, Nakatani Y, Sridi-Cheniti S, Cheniti G, Ramirez FD, Nakashima T, et al. Pulsed field ablation selectively spares the esophagus during pulmonary vein isolation for atrial fibrillation. Europace 2021;23:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1217. Marashly Q, Gopinath C, Baher A, Acharya M, Kheirkhahan M, Hardisty B, et al. Late gadolinium enhancement magnetic resonance imaging evaluation of post-atrial fibrillation ablation esophageal thermal injury across the spectrum of severity. J Am Heart Assoc 2021;10:e018924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1218. Di Biase L, Saenz LC, Burkhardt DJ, Vacca M, Elayi CS, Barrett CD, et al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol 2009;2:108–12. [DOI] [PubMed] [Google Scholar]
- 1219. Martinek M, Meyer C, Hassanein S, Aichinger J, Bencsik G, Schoefl R, et al. Identification of a high-risk population for esophageal injury during radiofrequency catheter ablation of atrial fibrillation: procedural and anatomical considerations. Heart Rhythm 2010;7:1224–30. [DOI] [PubMed] [Google Scholar]
- 1220. Singh SM, d’Avila A, Singh SK, Stelzer P, Saad EB, Skanes A, et al. Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm 2013;10:1591–7. [DOI] [PubMed] [Google Scholar]
- 1221. Mohanty S, Santangeli P, Mohanty P, Di Biase L, Trivedi C, Bai R, et al. Outcomes of atrioesophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol 2014;25:579–84. [DOI] [PubMed] [Google Scholar]
- 1222. Mohanty S, Santangeli P, Mohanty P, Di Biase L, Holcomb S, Trivedi C, et al. Catheter ablation of asymptomatic longstanding persistent atrial fibrillation: impact on quality of life, exercise performance, arrhythmia perception, and arrhythmia-free survival. J Cardiovasc Electrophysiol 2014;25:1057–64. [DOI] [PubMed] [Google Scholar]
- 1223. Eitel C, Rolf S, Zachäus M, John S, Sommer P, Bollmann A, et al. Successful nonsurgical treatment of esophagopericardial fistulas after atrial fibrillation catheter ablation: a case series. Circ Arrhythm Electrophysiol 2013;6:675–81. [DOI] [PubMed] [Google Scholar]
- 1224. Bunch TJ, Nelson J, Foley T, Allison S, Crandall BG, Osborn JS, et al. Temporary esophageal stenting allows healing of esophageal perforations following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol 2006;17:435–9. [DOI] [PubMed] [Google Scholar]
- 1225. Liu Y, Zhan X, Xue Y, Deng H, Fang X, Liao H, et al. Incidence and outcomes of cerebrovascular events complicating catheter ablation for atrial fibrillation. Europace 2016;18:1357–65. [DOI] [PubMed] [Google Scholar]
- 1226. Kosiuk J, Kornej J, Bollmann A, Piorkowski C, Myrda K, Arya A, et al. Early cerebral thromboembolic complications after radiofrequency catheter ablation of atrial fibrillation: incidence, characteristics, and risk factors. Heart Rhythm 2014;11:1934–40. [DOI] [PubMed] [Google Scholar]
- 1227. Deneke T, Jais P, Scaglione M, Schmitt R LDIB, Christopoulos G, Schade A, et al. Silent cerebral events/lesions related to atrial fibrillation ablation: a clinical review. J Cardiovasc Electrophysiol 2015;26:455–63. [DOI] [PubMed] [Google Scholar]
- 1228. Kimura T, Kashimura S, Nishiyama T, Katsumata Y, Inagawa K, Ikegami Y, et al. Asymptomatic cerebral infarction during catheter ablation for atrial fibrillation: comparing uninterrupted rivaroxaban and warfarin (ASCERTAIN). JACC Clin Electrophysiol 2018;4:1598–609. [DOI] [PubMed] [Google Scholar]
- 1229. Medi C, Evered L, Silbert B, Teh A, Halloran K, Morton J, et al. Subtle postprocedural cognitive dysfunction after atrial fibrillation ablation. J Am Coll Cardiol 2013;62:531–9. [DOI] [PubMed] [Google Scholar]
- 1230. Al-Kaisey AM, Parameswaran R, Bryant C, Anderson RD, Hawson J, Chieng D, et al. Impact of catheter ablation on cognitive function in atrial fibrillation: a randomized control trial. JACC Clin Electrophysiol 2023;9:1024–34. [DOI] [PubMed] [Google Scholar]
- 1231. Kato N, Muraga K, Hirata Y, Shindo A, Matsuura K, Ii Y, et al. Brain magnetic resonance imaging and cognitive alterations after ablation in patients with atrial fibrillation. Sci Rep 2021;11:18995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232. Haeusler KG, Eichner FA, Heuschmann PU, Fiebach JB, Engelhorn T, Blank B, et al. MRI-detected brain lesions and cognitive function in patients with atrial fibrillation undergoing left atrial catheter ablation in the randomized AXAFA-AFNET 5 trial. Circulation 2022;145:906–15. [DOI] [PubMed] [Google Scholar]
- 1233. Grecu M, Blomström-Lundqvist C, Kautzner J, Laroche C, Van Gelder IC, Jordaens L, et al. In-hospital and 12-month follow-up outcome from the ESC-EORP EHRA atrial fibrillation ablation long-term registry: sex differences. Europace 2020;22:66–73. [DOI] [PubMed] [Google Scholar]
- 1234. Santangeli P, Di Biase L, Al-Ahmad A, Horton R, Burkhardt JD, Sanchez JE, et al. Ablation for atrial fibrillation: termination of atrial fibrillation is not the end point. Card Electrophysiol Clin 2012;4:343–52. [DOI] [PubMed] [Google Scholar]
- 1235. Nairooz R, Sardar P, Payne J, Aronow WS, Paydak H. Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol 2015;187:426–9. [DOI] [PubMed] [Google Scholar]
- 1236. Ezekowitz MD, Pollack CV Jr, Halperin JL, England RD, VanPelt Nguyen S, Spahr J, et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J 2018;39:2959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1237. Lin H, Chen YH, Hou JW, Lu ZY, Xiang Y, Li YG. Role of contact force-guided radiofrequency catheter ablation for treatment of atrial fibrillation: a systematic review and metaanalysis. J Cardiovasc Electrophysiol 2017;28:994–1005. [DOI] [PubMed] [Google Scholar]
- 1238. Friedman DJ, Pokorney SD, Ghanem A, Marcello S, Kalsekar I, Yadalam S, et al. Predictors of cardiac perforation with catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2020;6:636–45. [DOI] [PubMed] [Google Scholar]
- 1239. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Delayed cardiac tamponade after radiofrequency catheter ablation of atrial fibrillation: a worldwide report. J Am Coll Cardiol 2011;58:2696–7. [DOI] [PubMed] [Google Scholar]
- 1240. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009;53:1798–803. [DOI] [PubMed] [Google Scholar]
- 1241. Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc 2002;77:429–36. [DOI] [PubMed] [Google Scholar]
- 1242. Bunch TJ, Asirvatham SJ, Friedman PA, Monahan KH, Munger TM, Rea RF, et al. Outcomes after cardiac perforation during radiofrequency ablation of the atrium. J Cardiovasc Electrophysiol 2005;16:1172–9. [DOI] [PubMed] [Google Scholar]
- 1243. Michowitz Y, Rahkovich M, Oral H, Zado ES, Tilz R, John S, et al. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circ Arrhythm Electrophysiol 2014;7:274–80. [DOI] [PubMed] [Google Scholar]
- 1244. Zhao Q, Li L, Liu N, Zhang M, Wu K, Ruan Y, et al. Early versus delayed removal of the pericardial drain in patients with cardiac tamponade complicating radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:597–603. [DOI] [PubMed] [Google Scholar]
- 1245. Zhao X, Liu JF, Su X, Long DY, Sang CH, Tang RB, et al. Direct autotransfusion in the management of acute pericardial tamponade during catheter ablation for atrial fibrillation: an imperfect but practical method. Front Cardiovasc Med 2022;9:984251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1246. Pedersen MEF, Leo M, Kalla M, Malhotra A, Stone M, Wong K, et al. Management of tamponade complicating catheter ablation for atrial fibrillation: early removal of pericardial drains is safe and effective and reduces analgesic requirements and hospital stay compared to conventional delayed removal. JACC Clin Electrophysiol 2017;3:367–73. [DOI] [PubMed] [Google Scholar]
- 1247. Raeisi-Giglou P, Wazni OM, Saliba WI, Barakat A, Tarakji KG, Rickard J, et al. Outcomes and management of patients with severe pulmonary vein stenosis from prior atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2018;11:e006001. [DOI] [PubMed] [Google Scholar]
- 1248. Andrade JG, Khairy P, Guerra PG, Deyell MW, Rivard L, Macle L, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm 2011;8:1444–51. [DOI] [PubMed] [Google Scholar]
- 1249. Fender EA, Packer DL, Holmes DR Jr. Pulmonary vein stenosis after atrial fibrillation ablation. EuroIntervention 2016;12:X31–4. [DOI] [PubMed] [Google Scholar]
- 1250. Saad EB, Marrouche NF, Saad CP, Ha E, Bash D, White RD, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med 2003;138:634–8. [DOI] [PubMed] [Google Scholar]
- 1251. Tokutake K, Tokuda M, Yamashita S, Sato H, Ikewaki H, Okajima E, et al. Anatomical and procedural factors of severe pulmonary vein stenosis after cryoballoon pulmonary vein ablation. JACC Clin Electrophysiol 2019;5:1303–15. [DOI] [PubMed] [Google Scholar]
- 1252. Kim J, Kim D, Yu HT, Kim TH, Joung B, Lee MH, et al. Revisiting symptomatic pulmonary vein stenosis after high-power short-duration radiofrequency ablation in patients with atrial fibrillation. Europace 2023;25:euad296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1253. Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000;36:468–71. [DOI] [PubMed] [Google Scholar]
- 1254. Fender EA, Widmer RJ, Hodge DO, Cooper GM, Monahan KH, Peterson LA, et al. Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation: presentation, management, and clinical outcomes. Circulation 2016;134:1812–21. [DOI] [PubMed] [Google Scholar]
- 1255. Fender EA, Widmer RJ, Hodge DO, Packer DL, Holmes DR Jr. Assessment and management of pulmonary vein occlusion after atrial fibrillation ablation. JACC Cardiovasc Interv 2018;11:1633–9. [DOI] [PubMed] [Google Scholar]
- 1256. Hilbert S, Paetsch I, Bollmann A, Jahnke C. Pulmonary vein collateral formation as a long-term result of post-interventional pulmonary vein stenosis. Eur Heart J 2016;37:2474. [DOI] [PubMed] [Google Scholar]
- 1257. Hilbert S, Sommer P, Bollmann A. Pulmonary vein dilatation in a case of total pulmonary vein occlusion: contemporary approach using a combination of 3D-mapping system and image integration. Catheter Cardiovasc Interv 2016;88:E227–32. [DOI] [PubMed] [Google Scholar]
- 1258. Holmes DR Jr, Monahan KH, Packer D. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv 2009;2:267–76. [DOI] [PubMed] [Google Scholar]
- 1259. Fender EA, Widmer RJ, Mahowald MK, Hodge DO, Packer DL, Holmes DR Jr. Recurrent pulmonary vein stenosis after successful intervention: prognosis and management of restenosis. Catheter Cardiovasc Interv 2020;95:954–8. [DOI] [PubMed] [Google Scholar]
- 1260. Mol D, Renskers L, Balt JC, Bhagwandien RE, Blaauw Y, van Driel V, et al. Persistent phrenic nerve palsy after atrial fibrillation ablation: follow-up data from The Netherlands Heart Registration. J Cardiovasc Electrophysiol 2022;33:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1261. Heeger CH, Sohns C, Pott A, Metzner A, Inaba O, Straube F, et al. Phrenic nerve injury during cryoballoon-based pulmonary vein isolation: results of the worldwide YETI registry. Circ Arrhythm Electrophysiol 2022;15:e010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1262. Sacher F, Monahan KH, Thomas SP, Davidson N, Adragao P, Sanders P, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol 2006;47:2498–503. [DOI] [PubMed] [Google Scholar]
- 1263. Yong Ji S, Dewire J, Barcelon B, Philips B, Catanzaro J, Nazarian S, et al. Phrenic nerve injury: an underrecognized and potentially preventable complication of pulmonary vein isolation using a wide-area circumferential ablation approach. J Cardiovasc Electrophysiol 2013;24:1086–91. [DOI] [PubMed] [Google Scholar]
- 1264. Franceschi F, Dubuc M, Guerra PG, Khairy P. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm 2011;8:1068–71. [DOI] [PubMed] [Google Scholar]
- 1265. Miyazaki S, Hachiya H, Taniguchi H, Nakamura H, Ichihara N, Usui E, et al. Prospective evaluation of bilateral diaphragmatic electromyograms during cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2015;26:622–8. [DOI] [PubMed] [Google Scholar]
- 1266. Mondésert B, Andrade JG, Khairy P, Guerra PG, Dyrda K, Macle L, et al. Clinical experience with a novel electromyographic approach to preventing phrenic nerve injury during cryoballoon ablation in atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:605–11. [DOI] [PubMed] [Google Scholar]
- 1267. Palaniswamy C, Kolte D, Harikrishnan P, Khera S, Aronow WS, Mujib M, et al. Catheter ablation of postinfarction ventricular tachycardia: ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014;11:2056–63. [DOI] [PubMed] [Google Scholar]
- 1268. Peichl P, Wichterle D, Pavlu L, Cihak R, Aldhoon B, Kautzner J. Complications of catheter ablation of ventricular tachycardia: a single-center experience. Circ Arrhythm Electrophysiol 2014;7:684–90. [DOI] [PubMed] [Google Scholar]
- 1269. Waigand J, Uhlich F, Gross CM, Thalhammer C, Dietz R. Percutaneous treatment of pseudoaneurysms and arteriovenous fistulas after invasive vascular procedures. Catheter Cardiovasc Interv 1999;47:157–64. [DOI] [PubMed] [Google Scholar]
- 1270. Kuwahara T, Takahashi A, Takahashi Y, Kobori A, Miyazaki S, Takei A, et al. Clinical characteristics of massive air embolism complicating left atrial ablation of atrial fibrillation: lessons from five cases. Europace 2012;14:204–8. [DOI] [PubMed] [Google Scholar]
- 1271. Krivonyak GS, Warren SG. Cerebral arterial air embolism treated by a vertical head-down maneuver. Catheter Cardiovasc Interv 2000;49:185–7. [DOI] [PubMed] [Google Scholar]
- 1272. Franzen OW, Klemm H, Hamann F, Koschyk D, von Kodolitsch Y, Weil J, et al. Mechanisms underlying air aspiration in patients undergoing left atrial catheterization. Catheter Cardiovasc Interv 2008;71:553–8. [DOI] [PubMed] [Google Scholar]
- 1273. Chugh A, Makkar A, Yen Ho S, Yokokawa M, Sundaram B, Pelosi F, et al. Manifestations of coronary arterial injury during catheter ablation of atrial fibrillation and related arrhythmias. Heart Rhythm 2013;10:1638–45. [DOI] [PubMed] [Google Scholar]
- 1274. Takahashi Y, Jaïs P, Hocini M, Sanders P, Rotter M, Rostock T, et al. Acute occlusion of the left circumflex coronary artery during mitral isthmus linear ablation. J Cardiovasc Electrophysiol 2005;16:1104–7. [DOI] [PubMed] [Google Scholar]
- 1275. Kitamura T, Fukamizu S, Arai K, Hojo R, Aoyama Y, Komiyama K, et al. Transient sinus node dysfunction following sinus node artery occlusion due to radiofrequency catheter ablation of the septal superior vena cava-right atrium junction. J Electrocardiol 2016;49:18–22. [DOI] [PubMed] [Google Scholar]
- 1276. Higuchi S, Im SI, Stillson C, Buck ED, Jerrell S, Schneider CW, et al. Effect of epicardial pulsed field ablation directly on coronary arteries. JACC Clin Electrophysiol 2022;8:1486–96. [DOI] [PubMed] [Google Scholar]
- 1277. Kesek M, Englund A, Jensen SM, Jensen-Urstad M. Entrapment of circular mapping catheter in the mitral valve. Heart Rhythm 2007;4:17–9. [DOI] [PubMed] [Google Scholar]
- 1278. Grove R, Kranig W, Coppoolse R, Lüdorff G, Wolff E, Warnecke H, et al. Demand for open heart surgery due to entrapment of a circular mapping catheter in the mitral valve in a patient undergoing atrial fibrillation ablation. Clin Res Cardiol 2008;97:628–9. [DOI] [PubMed] [Google Scholar]
- 1279. Lakkireddy D, Nagarajan D, Di Biase L, Vanga SR, Mahapatra S, Jared Bunch T, et al. Radiofrequency ablation of atrial fibrillation in patients with mitral or aortic mechanical prosthetic valves: a feasibility, safety, and efficacy study. Heart Rhythm 2011;8:975–80. [DOI] [PubMed] [Google Scholar]
- 1280. Zeljko HM, Mont L, Sitges M, Tolosana JM, Nadal M, Castella M, et al. Entrapment of the circular mapping catheter in the mitral valve in two patients undergoing atrial fibrillation ablation. Europace 2011;13:132–3. [DOI] [PubMed] [Google Scholar]
- 1281. Wu RC, Brinker JA, Yuh DD, Berger RD, Calkins HG. Circular mapping catheter entrapment in the mitral valve apparatus: a previously unrecognized complication of focal atrial fibrillation ablation. J Cardiovasc Electrophysiol 2002;13:819–21. [DOI] [PubMed] [Google Scholar]
- 1282. Kim EJ, Gerstenfeld EP, Pellegrini CN. Use of adenosine to release an entrapped catheter during ablation of premature ventricular complexes. JACC Case Rep 2021;3:610–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1283. Tavernier R, Duytschaever M, Taeymans Y. Fracture of a circular mapping catheter after entrapment in the mitral valve apparatus during segmental pulmonary vein isolation. Pacing Clin Electrophysiol 2003;26:1774–5. [DOI] [PubMed] [Google Scholar]
- 1284. Gurbuz O, Ercan A, Ozkan H, Kumtepe G, Karal IH, Ener S. Case report: paravalvular leak as a complication of percutaneous catheter ablation for atrial fibrillation. J Cardiothorac Surg 2014;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1285. Kawaji T, Kato M, Yokomatsu T. How to release PentaRay catheter entrapped in the hinge point of mechanical mitral valve? Europace 2020;22:204. [DOI] [PubMed] [Google Scholar]
- 1286. Yagishita A, Ayabe K, Sakama S, Morise M, Amino M, Ikari Y, et al. A novel technique to release a PentaRay entrapped in a mechanical mitral valve using an ablation catheter. JACC Clin Electrophysiol 2020;6:1597–8. [DOI] [PubMed] [Google Scholar]
- 1287. Lopes J, Sousa PA, Elvas L, Gonçalves L. Successful retrieval of a broken PentaRay catheter spine in a patient with mechanic mitral valve prosthesis. J Interv Card Electrophysiol 2021;61:625–6. [DOI] [PubMed] [Google Scholar]
- 1288. Cochet H, Scherr D, Zellerhoff S, Sacher F, Derval N, Denis A, et al. Atrial structure and function 5 years after successful ablation for persistent atrial fibrillation: an MRI study. J Cardiovasc Electrophysiol 2014;25:671–9. [DOI] [PubMed] [Google Scholar]
- 1289. Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8:1364–71. [DOI] [PubMed] [Google Scholar]
- 1290. Shoemaker MB, Hemnes AR, Robbins IM, Langberg JJ, Ellis CR, Aznaurov SG, et al. Left atrial hypertension after repeated catheter ablations for atrial fibrillation. J Am Coll Cardiol 2011;57:1918–9. [DOI] [PubMed] [Google Scholar]
- 1291. Welch TD, Coylewright M, Powell BD, Asirvatham SJ, Gersh BJ, Dearani JA, et al. Symptomatic pulmonary hypertension with giant left atrial v waves after surgical maze procedures: evaluation by comprehensive hemodynamic catheterization. Heart Rhythm 2013;10:1839–42. [DOI] [PubMed] [Google Scholar]
- 1292. Moon I, Lee SY, Lee E, Lee SR, Cha MJ, Choi EK, et al. Extensive left atrial ablation was associated with exacerbation of left atrial stiffness and dyspnea. J Cardiovasc Electrophysiol 2019;30:2782–9. [DOI] [PubMed] [Google Scholar]
- 1293. Witt C, Powell B, Holmes D, Alli O. Recurrent dyspnea following multiple ablations for atrial fibrillation explained by the “stiff left atrial syndrome”. Catheter Cardiovasc Interv 2013;82:E747–9. [DOI] [PubMed] [Google Scholar]
- 1294. Wong GR, Lau DH, Baillie TJ, Middeldorp ME, Steele PM, Sanders P. Novel use of sildenafil in the management of pulmonary hypertension due to post-catheter ablation ‘stiff left atrial syndrome’. Int J Cardiol 2015;181:55–6. [DOI] [PubMed] [Google Scholar]
- 1295. Lakkireddy D, Reddy YM, Atkins D, Rajasingh J, Kanmanthareddy A, Olyaee M, et al. Effect of atrial fibrillation ablation on gastric motility: the atrial fibrillation gut study. Circ Arrhythm Electrophysiol 2015;8:531–6. [DOI] [PubMed] [Google Scholar]
- 1296. Kuwahara T, Takahashi A, Takahashi Y, Kobori A, Miyazaki S, Takei A, et al. Clinical characteristics and management of periesophageal vagal nerve injury complicating left atrial ablation of atrial fibrillation: lessons from eleven cases. J Cardiovasc Electrophysiol 2013;24:847–51. [DOI] [PubMed] [Google Scholar]
- 1297. Knopp H, Halm U, Lamberts R, Knigge I, Zachäus M, Sommer P, et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm 2014;11:574–8. [DOI] [PubMed] [Google Scholar]
- 1298. Miyazaki S, Nakamura H, Taniguchi H, Hachiya H, Takagi T, Igarashi M, et al. Gastric hypomotility after second-generation cryoballoon ablation-unrecognized silent nerve injury after cryoballoon ablation. Heart Rhythm 2017;14:670–7. [DOI] [PubMed] [Google Scholar]
- 1299. Aksu T, Golcuk S, Guler TE, Yalin K, Erden I. Gastroparesis as a complication of atrial fibrillation ablation. Am J Cardiol 2015;116:92–7. [DOI] [PubMed] [Google Scholar]
- 1300. Shigeta T, Okishige K, Aoyagi H, Nishimura T, Nakamura RA, Ito N, et al. Clinical investigation of esophageal injury from cryoballoon ablation of persistent atrial fibrillation. Pacing Clin Electrophysiol 2019;42:230–7. [DOI] [PubMed] [Google Scholar]
- 1301. Bunch TJ, Ellenbogen KA, Packer DL, Asirvatham SJ. Vagus nerve injury after posterior atrial radiofrequency ablation. Heart Rhythm 2008;5:1327–30. [DOI] [PubMed] [Google Scholar]
- 1302. Miyazaki S, Taniguchi H, Kusa S, Komatsu Y, Ichihara N, Takagi T, et al. Factors associated with periesophageal vagal nerve injury after pulmonary vein antrum isolation. J Am Heart Assoc 2014;3:e001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1303. Pisani CF, Hachul D, Sosa E, Scanavacca M. Gastric hypomotility following epicardial vagal denervation ablation to treat atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:211–3. [DOI] [PubMed] [Google Scholar]
- 1304. Schwartz TW, Rehfeld JF, Stadil F, Larson LI, Chance RE, Moon N. Pancreatic-polypeptide response to food in duodenal-ulcer patients before and after vagotomy. Lancet 1976;307:1102–5. [DOI] [PubMed] [Google Scholar]
- 1305. Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 1990;322:1028–31. [DOI] [PubMed] [Google Scholar]
- 1306. Camilleri M, Atieh J. New developments in prokinetic therapy for gastric motility disorders. Front Pharmacol 2021;12:711500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1307. Zheng T, Camilleri M. Management of gastroparesis. Gastroenterol Hepatol (N Y) 2021;17:515–25. [PMC free article] [PubMed] [Google Scholar]
- 1308. Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, et al. Surgical ablation of atrial fibrillation in the United States: trends and propensity matched outcomes. Ann Thorac Surg 2017;104:493–500. [DOI] [PubMed] [Google Scholar]
- 1309. Budera P, Straka Z, Osmančík P, Vaněk T, Jelínek Š, Hlavička J, et al. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: final results of the PRAGUE-12 randomized multicenter study. Eur Heart J 2012;33:2644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1310. Nashef SAM, Fynn S, Abu-Omar Y, Spyt TJ, Mills C, Everett CC, et al. Amaze: a randomized controlled trial of adjunct surgery for atrial fibrillation. Eur J Cardiothorac Surg 2018;54:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1311. Osmancik P, Budera P, Talavera D, Hlavicka J, Herman D, Holy J, et al. Five-year outcomes in cardiac surgery patients with atrial fibrillation undergoing concomitant surgical ablation versus no ablation. The long-term follow-up of the PRAGUE-12 study. Heart Rhythm 2019;16:1334–40. [DOI] [PubMed] [Google Scholar]
- 1312. Wong JW, Mak KH. Impact of maze and concomitant mitral valve surgery on clinical outcomes. Ann Thorac Surg 2006;82:1938–47. [DOI] [PubMed] [Google Scholar]
- 1313. Lee R, McCarthy PM, Wang EC, Vaduganathan M, Kruse J, Malaisrie SC Jr, et al. Midterm survival in patients treated for atrial fibrillation: a propensity-matched comparison to patients without a history of atrial fibrillation. J Thorac Cardiovasc Surg 2012;143:1341–51; discussion 1350–1. [DOI] [PubMed] [Google Scholar]
- 1314. Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg 2018;155:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1315. Iribarne A, DiScipio AW, McCullough JN, Quinn R, Leavitt BJ, Westbrook BM, et al. Surgical atrial fibrillation ablation improves long-term survival: a multicenter analysis. Ann Thorac Surg 2019;107:135–42. [DOI] [PubMed] [Google Scholar]
- 1316. Kowalewski M, Pasierski M, Kołodziejczak M, Litwinowicz R, Kowalówka A, Wańha W, et al. Atrial fibrillation ablation improves late survival after concomitant cardiac surgery. J Thorac Cardiovasc Surg 2023;166:1656–68.e8. [DOI] [PubMed] [Google Scholar]
- 1317. Kim HJ, Kim YJ, Kim M, Yoo JS, Kim DH, Park DW, et al. Surgical ablation for atrial fibrillation during aortic and mitral valve surgery: a nationwide population-based cohort study. J Thorac Cardiovasc Surg 2022. [DOI] [PubMed] [Google Scholar]
- 1318. Suwalski P, Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: analysis from the polish national registry of cardiac surgery procedures (KROK). J Thorac Cardiovasc Surg 2019;157:1007–18.e4. [DOI] [PubMed] [Google Scholar]
- 1319. Gemelli M, Gallo M, Addonizio M, Van den Eynde J, Pradegan N, Danesi TH, et al. Surgical ablation for atrial fibrillation during mitral valve surgery: a systematic review and metaanalysis of randomized controlled trials. Am J Cardiol 2023;209:104–113. [DOI] [PubMed] [Google Scholar]
- 1320. Reston JT, Shuhaiber JH. Meta-analysis of clinical outcomes of maze-related surgical procedures for medically refractory atrial fibrillation. Eur J Cardiothorac Surg 2005;28:724–30. [DOI] [PubMed] [Google Scholar]
- 1321. Rankin JS, Lerner DJ, Braid-Forbes MJ, McCrea MM, Badhwar V. Surgical ablation of atrial fibrillation concomitant to coronary-artery bypass grafting provides cost-effective mortality reduction. J Thorac Cardiovasc Surg 2020;160:675–86.e13. [DOI] [PubMed] [Google Scholar]
- 1322. Suwalski P, Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur J Cardiothorac Surg 2020;57:691–700. [DOI] [PubMed] [Google Scholar]
- 1323. MacGregor RM, Bakir NH, Pedamallu H, Sinn LA, Maniar HS, Melby SJ, et al. Late results after stand-alone surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2022;164:1515–28.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1324. Lapenna E, De Bonis M, Giambuzzi I, Del Forno B, Ruggeri S, Cireddu M, et al. Long-term outcomes of stand-alone maze IV for persistent or long-standing persistent atrial fibrillation. Ann Thorac Surg 2020;109:124–31. [DOI] [PubMed] [Google Scholar]
- 1325. Ad N, Holmes SD, Friehling T. Minimally invasive stand-alone cox maze procedure for persistent and long-standing persistent atrial fibrillation: perioperative safety and 5-year outcomes. Circ Arrhythm Electrophysiol 2017;10:e005352. [DOI] [PubMed] [Google Scholar]
- 1326. Henn MC, Lancaster TS, Miller JR, Sinn LA, Schuessler RB, Moon MR, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2015;150:1168–76, 1178.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1327. Gammie JS, Haddad M, Milford-Beland S, Welke KF, Ferguson TB Jr, O’Brien SM, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 2008;85:909–14. [DOI] [PubMed] [Google Scholar]
- 1328. Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr, Moskowitz AJ, Voisine P, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1329. Mokracek A, Kurfirst V, Bulava A, Hanis J, Tesarik R, Pesl L. Thoracoscopic occlusion of the left atrial appendage. Innovations 2015;10:179–82. [DOI] [PubMed] [Google Scholar]
- 1330. Wang J-L, Zhou K, Qin Z, Cheng W-J, Zhang L-Z, Zhou Y-J. Minimally invasive thoracoscopic left atrial appendage occlusion compared with transcatheter left atrial appendage closure for stroke prevention in recurrent nonvalvular atrial fibrillation patients after radiofrequency ablation: a prospective cohort study. J Geriatr Cardiol 2021;18:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1331. Fu M, Qin Z, Zheng S, Li Y, Yang S, Zhao Y, et al. Thoracoscopic left atrial appendage occlusion for stroke prevention compared with long-term warfarin therapy in patients with nonvalvular atrial fibrillation. Am J Cardiol 2019;123:50–6. [DOI] [PubMed] [Google Scholar]
- 1332. Kim JY, Jeong DS, Park S-J, Park K-M, Kim JS, On YK. Long-term efficacy and anticoagulation strategy of left atrial appendage occlusion during total thoracoscopic ablation of atrial fibrillation to prevent ischemic stroke. Front Cardiovasc Med 2022;9:853299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1333. Branzoli S, Marini M, Guarracini F, Pederzolli C, Pomarolli C, D’Onghia G, et al. Epicardial standalone left atrial appendage clipping for prevention of ischemic stroke in patients with atrial fibrillation contraindicated for oral anticaogulation. J Cardiovasc Electrophysiol 2020;31:2187–91. [DOI] [PubMed] [Google Scholar]
- 1334. Cartledge R, Suwalski G, Witkowska A, Gottlieb G, Cioci A, Chidiac G, et al. Standalone epicardial left atrial appendage exclusion for thromboembolism prevention in atrial fibrillation. Interact Cardiovasc Thorac Surg 2022;34:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. [DOI] [PubMed] [Google Scholar]
- 1336. Quader MA, McCarthy PM, Gillinov AM, Alster JM, Cosgrove DM 3rd, Lytle BW, et al. Does preoperative atrial fibrillation reduce survival after coronary artery bypass grafting? Ann Thorac Surg 2004;77:1514–22; discussion 1522–4. [DOI] [PubMed] [Google Scholar]
- 1337. Ad N, Damiano RJ Jr, Badhwar V, Calkins H, La Meir M, Nitta T, et al. Expert consensus guidelines: examining surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2017;153:1330–54.e1. [DOI] [PubMed] [Google Scholar]
- 1338. Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg 1999;118:833–40. [DOI] [PubMed] [Google Scholar]
- 1339. Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23–30. [DOI] [PubMed] [Google Scholar]
- 1340. Castellá M, Kotecha D, van Laar C, Wintgens L, Castillo Y, Kelder J, et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. Europace 2019;21:746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1341. Pokushalov E, Romanov A, Elesin D, Bogachev-Prokophiev A, Losik D, Bairamova S, et al. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol 2013;24:1338–43. [DOI] [PubMed] [Google Scholar]
- 1342. Adiyaman A, Buist TJ, Beukema RJ, Smit JJJ, Delnoy P, Hemels MEW, et al. Randomized controlled trial of surgical versus catheter ablation for paroxysmal and early persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2018;11:e006182. [DOI] [PubMed] [Google Scholar]
- 1343. Philpott JM, Zemlin CW, Cox JL, Stirling M, Mack M, Hooker RL, et al. The ABLATE trial: safety and efficacy of Cox maze-IV using a bipolar radiofrequency ablation system. Ann Thorac Surg 2015;100:1541–6; discussion 1547–8. [DOI] [PubMed] [Google Scholar]
- 1344. McGilvray MMO, Barron L, Yates TE, Zemlin CW, Damiano RJ Jr. The Cox-maze procedure: what lesions and why. JTCVS Tech 2023;17:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1345. Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr, Moskowitz AJ, Voisine P, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1346. Hamner CE, Potter DD Jr, Cho KR, Lutterman A, Francischelli D, Sundt TM 3rd, et al. Irrigated radiofrequency ablation with transmurality feedback reliably produces Cox maze lesions in vivo. Ann Thorac Surg 2005;80:2263–70. [DOI] [PubMed] [Google Scholar]
- 1347. Schuessler RB, Lee AM, Melby SJ, Voeller RK, Gaynor SL, Sakamoto S-I, et al. Animal studies of epicardial atrial ablation. Heart Rhythm 2009;6:S41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1348. Saint LL, Lawrance CP, Okada S, Kazui T, Robertson JO, Schuessler RB, et al. Performance of a novel bipolar/monopolar radiofrequency ablation device on the beating heart in an acute porcine model. Innovations 2013;8:276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1349. Bugge E, Nicholson IA, Thomas SP. Comparison of bipolar and unipolar radiofrequency ablation in an in vivo experimental model. Eur J Cardiothorac Surg 2005;28:76–80; discussion 80–2. [DOI] [PubMed] [Google Scholar]
- 1350. Thomas SP, Guy DJR, Boyd AC, Eipper VE, Ross DL, Chard RB. Comparison of epicardial and endocardial linear ablation using handheld probes. Ann Thorac Surg 2003;75:543–8. [DOI] [PubMed] [Google Scholar]
- 1351. La Meir M. Surgical options for treatment of atrial fibrillation. Ann Cardiothorac Surg 2014;3:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1352. Khiabani AJ, MacGregor RM, Manghelli JL, Ruaengsri C, Carter DI, Melby SJ, et al. Bipolar radiofrequency ablation on explanted human hearts: how to ensure transmural lesions. Ann Thorac Surg 2020;110:1933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1353. Jaïs P, Haïssaguerre M, Shah DC, Takahashi A, Hocini M, Lavergne T, et al. Successful irrigated-tip catheter ablation of atrial flutter resistant to conventional radiofrequency ablation. Circulation 1998;98:835–8. [DOI] [PubMed] [Google Scholar]
- 1354. Haines DE. The biophysics and pathophysiology of lesion formation during radiofrequency catheter ablation. In Zipes DP, Jalife J, Stevenson WG (eds.), Cardiac Electrophysiology: From Cell to Bedside. 4th ed. Philadelphia: WB Saunders Co; 2005; 1018–27. [Google Scholar]
- 1355. Melby SJ, Zierer A, Voeller RK, Lall SC, Bailey MS, Moon MR, et al. Wide variations in energy delivery using an impedance-controlled algorithm in bipolar radiofrequency ablation: evidence against fixed time ablation. Innovations 2007;2:67–72. [DOI] [PubMed] [Google Scholar]
- 1356. Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94. [DOI] [PubMed] [Google Scholar]
- 1357. Varzaly JA, Chapman D, Lau DH, Edwards S, Louise J, Edwards J, et al. Contact force and ablation assessment of surgical bipolar radiofrequency clamps in the treatment of atrial fibrillation. Interact Cardiovasc Thorac Surg 2019;28:85–93. [DOI] [PubMed] [Google Scholar]
- 1358. Schill MR, Melby SJ, Speltz M, Breitbach M, Schuessler RB, Damiano RJ. Evaluation of a novel cryoprobe for atrial ablation in a chronic ovine model. Ann Thorac Surg 2017;104:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1359. Weimar T, Lee AM, Ray S, Schuessler RB, Damiano RJ. Evaluation of a novel cryoablation system: in vivo testing in a chronic porcine model. Innovations (Phila) 2012;7:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1360. Cox JL, Malaisrie SC, Churyla A, Mehta C, Kruse J, Kislitsina ON, et al. Cryosurgery for atrial fibrillation: physiologic basis for creating optimal cryolesions. Ann Thorac Surg 2021;112:354–62. [DOI] [PubMed] [Google Scholar]
- 1361. Masroor S, Jahnke M-E, Carlisle A, Cartier C, LaLonde J-P, MacNeil T, et al. Endocardial hypothermia and pulmonary vein isolation with epicardial cryoablation in a porcine beating-heart model. J Thorac Cardiovasc Surg 2008;135:1327–33.e5. [DOI] [PubMed] [Google Scholar]
- 1362. Aupperle H, Doll N, Walther T, Ullmann C, Schoon HA, Wilhelm Mohr F. Histological findings induced by different energy sources in experimental atrial ablation in sheep. Interact Cardiovasc Thorac Surg 2005;4:450–5. [DOI] [PubMed] [Google Scholar]
- 1363. Schuessler RB, Lee AM, Melby SJ, Voeller RK, Gaynor SL, Sakamoto S, et al. Animal studies of epicardial atrial ablation. Heart Rhythm 2009;6:S41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1364. MacGregor RM, Melby SJ, Schuessler RB, Damiano RJ. Energy sources for the surgical treatment of atrial fibrillation. Innovations (Phila) 2019;14:503–8. [DOI] [PubMed] [Google Scholar]
- 1365. Yates TA, McGilvray M, Schill MR, Barron L, Razo N, Roberts HG Jr, et al. Performance of an irrigated bipolar radiofrequency ablation clamp on explanted human hearts. Ann Thorac Surg 2023;116:307–13. [DOI] [PubMed] [Google Scholar]
- 1366. Yates T-A, McGilvray M, Razo N, McElligott S, Melby SJ, Zemlin C, et al. Efficacy of a novel bipolar radiofrequency clamp: an acute porcine model. Innovations (Phila) 2022;17:409–15. [DOI] [PubMed] [Google Scholar]
- 1367. Watanabe Y, Weimar T, Kazui T, Lee U, Schuessler RB, Damiano RJ Jr. Epicardial ablation performance of a novel radiofrequency device on the beating heart in pigs. Ann Thorac Surg 2014;97:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1368. Lee AM, Aziz A, Clark KL, Schuessler RB, Damiano RJ Jr. Chronic performance of a novel radiofrequency ablation device on the beating heart: limitations of conduction delay to assess transmurality. J Thorac Cardiovasc Surg 2012;144:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1369. Bringmans T, Verrijcken A, La Meir M, Rega F. Atrioesophageal fistula after epicardial ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2018;155:e19–21. [DOI] [PubMed] [Google Scholar]
- 1370. Kiser AC, Nifong LW, Raman J, Kasirajan V, Campbell N, Chitwood WR Jr. Evaluation of a novel epicardial atrial fibrillation treatment system. Ann Thorac Surg 2008;85:300–3. [DOI] [PubMed] [Google Scholar]
- 1371. Weimar T, Lee AM, Ray S, Schuessler RB, Damiano RJ Jr. Evaluation of a novel cryoablation system: in vivo testing in a chronic porcine model. Innovations (Phila) 2012;7:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1372. El Arid J-M, Sénage T, Toquet C, Al Habash O, Mugniot A, Baron O, et al. Human comparative experimental study of surgical treatment of atrial fibrillation by epicardial techniques. J Cardiothorac Surg 2013;8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1373. Schill MR, Melby SJ, Speltz M, Breitbach M, Schuessler RB, Damiano RJ Jr. Evaluation of a novel cryoprobe for atrial ablation in a chronic ovine model. Ann Thorac Surg 2017;104:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1374. Milla F, Skubas N, Briggs WM, Girardi LN, Lee LY, Ko W, et al. Epicardial beating heart cryoablation using a novel argon-based cryoclamp and linear probe. J Thorac Cardiovasc Surg 2006;131:403–11. [DOI] [PubMed] [Google Scholar]
- 1375. Nitta T, Ishii Y, Miyagi Y, Ohmori H, Sakamoto S-I, Tanaka S. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or reentrant activation as the mechanism in atrial fibrillation. J Thorac Cardiovasc Surg 2004;127:770–8. [DOI] [PubMed] [Google Scholar]
- 1376. Saini A, Hu YL, Kasirajan V, Han FT, Khan MZ, Wolfe L, et al. Long-term outcomes of minimally invasive surgical ablation for atrial fibrillation: a single-center experience. Heart Rhythm 2017;14:1281–8. [DOI] [PubMed] [Google Scholar]
- 1377. Gillinov AM, Bhavani S, Blackstone EH, Rajeswaran J, Svensson LG, Navia JL, et al. Surgery for permanent atrial fibrillation: impact of patient factors and lesion set. Ann Thorac Surg 2006;82:502–13; discussion 513–4. [DOI] [PubMed] [Google Scholar]
- 1378. Saint LL, Bailey MS, Prasad S, Guthrie TJ, Bell J, Moon MR, et al. Cox-maze IV results for patients with lone atrial fibrillation versus concomitant mitral disease. Ann Thorac Surg 2012;93:789–94; discussion 794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1379. Enriquez A, Santangeli P, Zado ES, Liang J, Castro S, Garcia FC, et al. Postoperative atrial tachycardias after mitral valve surgery: mechanisms and outcomes of catheter ablation. Heart Rhythm 2017;14:520–6. [DOI] [PubMed] [Google Scholar]
- 1380. Gwag HB, Jeong DS, Hwang JK, Park S-J, Park K-M, Kim JS, et al. Additional cavotricuspid isthmus ablation may reduce recurrent atrial tachyarrhythmia after total thoracoscopic ablation for persistent atrial fibrillation. Interact Cardiovasc Thorac Surg 2019;28:177–82. [DOI] [PubMed] [Google Scholar]
- 1381. Ngaage DL, Schaff HV, Mullany CJ, Sundt TM 3rd, Dearani JA, Barnes S, et al. Does preoperative atrial fibrillation influence early and late outcomes of coronary artery bypass grafting? J Thorac Cardiovasc Surg 2007;133:182–9. [DOI] [PubMed] [Google Scholar]
- 1382. McClure GR, Belley-Cote EP, Jaffer IH, Dvirnik N, An KR, Fortin G, et al. Surgical ablation of atrial fibrillation: a systematic review and metaanalysis of randomized controlled trials. Europace 2018;20:1442–50. [DOI] [PubMed] [Google Scholar]
- 1383. Johansson B, Houltz B, Berglin E, Brandrup-Wognsen G, Karlsson T, Edvardsson N. Short-term sinus rhythm predicts long-term sinus rhythm and clinical improvement after intraoperative ablation of atrial fibrillation. Europace 2008;10:610–7. [DOI] [PubMed] [Google Scholar]
- 1384. Grubitzsch H, Dushe S, Beholz S, Dohmen PM, Konertz W. Surgical ablation of atrial fibrillation in patients with congestive heart failure. J Card Fail 2007;13:509–16. [DOI] [PubMed] [Google Scholar]
- 1385. Saint LL, Damiano RJ Jr, Cuculich PS, Guthrie TJ, Moon MR, Munfakh NA, et al. Incremental risk of the Cox-maze IV procedure for patients with atrial fibrillation undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2013;146:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1386. Bakir NH, Khiabani AJ, MacGregor RM, Kelly MO, Sinn LA, Schuessler RB, et al. Concomitant surgical ablation for atrial fibrillation is associated with increased risk of acute kidney injury but improved late survival. J Thorac Cardiovasc Surg 2022;164:1847–57.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1387. Takahashi K, Miyauchi Y, Hayashi M, Iwasaki YK, Yodogawa K, Tsuboi I, et al. Mechanisms of postoperative atrial tachycardia following biatrial surgical ablation of atrial fibrillation in relation to the surgical lesion sets. Heart Rhythm 2016;13:1059–65. [DOI] [PubMed] [Google Scholar]
- 1388. Ishii Y, Nitta T, Kambe M, Kurita J, Ochi M, Miyauchi Y, et al. Intraoperative verification of conduction block in atrial fibrillation surgery. J Thorac Cardiovasc Surg 2008;136:998–1004. [DOI] [PubMed] [Google Scholar]
- 1389. Phan K, Xie A, Tsai YC, Kumar N, La Meir M, Yan TD. Biatrial ablation vs. left atrial concomitant surgical ablation for treatment of atrial fibrillation: a metaanalysis. Europace 2015;17:38–47. [DOI] [PubMed] [Google Scholar]
- 1390. Bogachev-Prokophiev AV, Afanasyev AV, Pivkin AN, Ovcharov MA, Zheleznev SI, Sharifulin RM, et al. A left atrial versus a biatrial lesion set for persistent atrial fibrillation ablation during open heart surgery. Eur J Cardiothorac Surg 2018;54:738–44. [DOI] [PubMed] [Google Scholar]
- 1391. Kowalewski M, Pasierski M, Finke J, Kołodziejczak M, Staromłyński J, Litwinowicz R, et al. Permanent pacemaker implantation after valve and arrhythmia surgery in patients with preoperative atrial fibrillation. Heart Rhythm 2022;19:1442–9. [DOI] [PubMed] [Google Scholar]
- 1392. Cox JL, Ad N, Churyla A, Malaisrie SC, Pham DT, Kruse J, et al. The maze procedure and postoperative pacemakers. Ann Thorac Surg 2018;106:1561–9. [DOI] [PubMed] [Google Scholar]
- 1393. Badhwar V, Rankin JS, Damiano RJ Jr, Gillinov AM, Bakaeen FG, Edgerton JR, et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2017;103:329–41. [DOI] [PubMed] [Google Scholar]
- 1394. Raanani E, Albage A, David TE, Yau TM, Armstrong S. The efficacy of the Cox/maze procedure combined with mitral valve surgery: a matched control study. Eur J Cardiothorac Surg 2001;19:438–42. [DOI] [PubMed] [Google Scholar]
- 1395. Li H, Lin X, Ma X, Tao J, Zou R, Yang S, Liu H, Hua P. Biatrial versus isolated left atrial ablation in atrial fibrillation: a systematic review and metaanalysis. Biomed Res Int 2018;2018:3651212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1396. Kainuma S, Mitsuno M, Toda K, Funatsu T, Nakamura T, Miyagawa S, et al. Dilated left atrium as a predictor of late outcome after pulmonary vein isolation concomitant with aortic valve replacement and/or coronary artery bypass grafting†. Eur J Cardiothorac Surg 2015;48:765–77; discussion 777. [DOI] [PubMed] [Google Scholar]
- 1397. Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a metaanalysis. J Thorac Cardiovasc Surg 2006;131:1029–35. [DOI] [PubMed] [Google Scholar]
- 1398. Kim HJ, Kim JB, Kim SO, Cho MS, Kim JK, Kim WK, et al. Long-term outcomes of surgical ablation for atrial fibrillation: impact of ablation lesion sets. JACC Asia 2021;1:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1399. Kainuma S, Mitsuno M, Toda K, Miyagawa S, Yoshikawa Y, Hata H, et al. Surgical ablation concomitant with nonmitral valve surgery for persistent atrial fibrillation. Ann Thorac Surg 2021;112:1909–20. [DOI] [PubMed] [Google Scholar]
- 1400. La Meir M, Gelsomino S, Nonneman B. The problem with concomitant atrial fibrillation in non-mitral valve surgery. Ann Cardiothorac Surg 2014;3:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1401. Civello K, Smith C, Boedefeld W. Combined endocardial and epicardial ablation for symptomatic atrial fibrillation: single center experience in 100+ consecutive patients. J Innov Card Rhythm Manag 2013;4:1–7. [Google Scholar]
- 1402. Gehi AK, Mounsey JP, Pursell I, Landers M, Boyce K, Chung EH, et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm 2013;10:22–8. [DOI] [PubMed] [Google Scholar]
- 1403. Gersak B, Jan M. Long-term success for the convergent atrial fibrillation procedure: 4-year outcomes. Ann Thorac Surg 2016;102:1550–7. [DOI] [PubMed] [Google Scholar]
- 1404. Gersak B, Zembala MO, Muller D, Folliguet T, Jan M, Kowalski O, et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg 2014;147:1411–6. [DOI] [PubMed] [Google Scholar]
- 1405. Gilligan D, Joyner C, Bundy G. Multidisciplinary collaboration for the treatment of atrial fibrillation: convergent procedure outcomes from a single center. J Innov Card Rhythm Manag 2013;4:1396–403. [Google Scholar]
- 1406. Tonks R, Lantz G, Mahlow J, Hirsh J, Lee LS. Short and intermediate term outcomes of the convergent procedure: initial experience in a tertiary referral center. Ann Thorac Cardiovasc Surg 2020;26:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1407. Toplisek J, Pernat A, Ruzic N, Robic B, Sinkovec M, Cvijic M, et al. Improvement of atrial and ventricular remodeling with low atrial fibrillation burden after hybrid ablation of persistent atrial fibrillation. Pacing Clin Electrophysiol 2016;39:216–24. [DOI] [PubMed] [Google Scholar]
- 1408. Zembala M, Filipiak K, Kowalski O, Buchta P, Niklewski T, Nadziakiewicz P, et al. Staged hybrid ablation for persistent and longstanding persistent atrial fibrillation effectively restores sinus rhythm in long-term observation. Arch Med Sci 2017;13:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1409. Kiser AC, Landers M, Horton R, Hume A, Natale A, Gersak B. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum 2010;13:E317–21. [DOI] [PubMed] [Google Scholar]
- 1410. Edgerton Z, Perini AP, Horton R, Trivedi C, Santangeli P, Bai R, et al. Hybrid procedure (endo/epicardial) versus standard manual ablation in patients undergoing ablation of longstanding persistent atrial fibrillation: results from a single center. J Cardiovasc Electrophysiol 2016;27:524–30. [DOI] [PubMed] [Google Scholar]
- 1411. Jan M, Zizek D, Gersak ZM, Gersak B. Comparison of treatment outcomes between convergent procedure and catheter ablation for paroxysmal atrial fibrillation evaluated with implantable loop recorder monitoring. J Cardiovasc Electrophysiol 2018;29:1073–80. [DOI] [PubMed] [Google Scholar]
- 1412. Gersak B, Pernat A, Robic B, Sinkovec M. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:1059–66. [DOI] [PubMed] [Google Scholar]
- 1413. Zannis K, Alam W, Sebag FA, Folliguet T, Bars C, Fahed M, et al. The convergent procedure: a hybrid approach for long lasting persistent atrial fibrillation ablation, the French experience. J Cardiovasc Surg (Torino) 2020;61:369–75. [DOI] [PubMed] [Google Scholar]
- 1414. Maclean E, Yap J, Saberwal B, Kolvekar S, Lim W, Wijesuriya N, et al. The convergent procedure versus catheter ablation alone in longstanding persistent atrial fibrillation: a single center, propensity-matched cohort study. Int J Cardiol 2020;303:49–53. [DOI] [PubMed] [Google Scholar]
- 1415. Kiser AC, Landers MD, Boyce K, Sinkovec M, Pernat A, Gersak B. Simultaneous catheter and epicardial ablations enable a comprehensive atrial fibrillation procedure. Innovations (Phila) 2011;6:243–7. [DOI] [PubMed] [Google Scholar]
- 1416. Lee LS. Subxiphoid minimally invasive epicardial ablation (convergent procedure) with left thoracoscopic closure of the left atrial appendage. Oper Tech Thorac Cardiovasc Surg 2019;23:152–65. [Google Scholar]
- 1417. Thosani AJ, Gerczuk P, Liu E, Belden W, Moraca R. Closed chest convergent epicardial-endocardial ablation of non-paroxysmal atrial fibrillation – a case series and literature review. Arrhythm Electrophysiol Rev 2013;2:65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1418. Makati K, Davoudi R, Giedrimas A, Irwin J, Sherman A, Gerogiannis I, et al. Safety and efficacy of a convergent hybrid procedure using cryo as endocardial energy source for the treatment of persistent and longstanding persistent atrial fibrillation. J Am Coll Cardiol 2020;75:424. [Google Scholar]
- 1419. Starck CT, Steffel J, Emmert MY, Plass A, Mahapatra S, Falk V, et al. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg 2012;15:416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1420. Lee LS. Subxiphoid minimally invasive epicardial ablation (convergent procedure) with left thoracoscopic closure of the left atrial appendage. Oper Tech Thorac Cardiovasc Surg 2018;23:152–65. [Google Scholar]
- 1421. Gegochkori N, Yang F, Miller A, Kulbak G, Jacobwitz I, Greenberg Y. Comparison of hybrid ablation for persistent atrial fibrillation with and without left atrial appendage closure: report of 1 year follow up. Presented at Venice Arrhythmias, Venice, Italy, 2019. J Interv Card Electrophysiol 2020;57:164. [Google Scholar]
- 1422. Haldar S, Khan HR, Boyalla V, Kralj-Hans I, Jones S, Lord J, et al. Catheter ablation vs. thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: CASA-AF randomized controlled trial. Eur Heart J 2020;41:4471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1423. van der Heijden CAJ, Weberndörfer V, Vroomen M, Luermans JG, Chaldoupi S-M, Bidar E, et al. Hybrid ablation versus repeated catheter ablation in persistent atrial fibrillation. JACC Clin Electrophysiol 2023;9:1013–23. [DOI] [PubMed] [Google Scholar]
- 1424. Doll N, Weimar T, Kosior DA, Bulava A, Mokracek A, Mönnig G, et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: a randomised, controlled trial. EClinicalMedicine 2023;61:102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1425. Pearman CM, Poon SS, Bonnett LJ, Haldar S, Wong T, Mediratta N, et al. Minimally invasive epicardial surgical ablation alone versus hybrid ablation for atrial fibrillation: a systematic review and metaanalysis. Arrhythm Electrophysiol Rev 2017;6:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1426. Bhatia NK, Shah RL, Deb B, Pong T, Kapoor R, Rogers AJ, et al. Mapping atrial fibrillation after surgical therapy to guide endocardial ablation. Circ Arrhythm Electrophysiol 2022;15:e010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1427. de Asmundis C, Chierchia GB, Mugnai G, Van Loo I, Nijs J, Czapla J, et al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: a single-center experience. Europace 2017;19:58–65. [DOI] [PubMed] [Google Scholar]
- 1428. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–9. [DOI] [PubMed] [Google Scholar]
- 1429. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. [DOI] [PubMed] [Google Scholar]
- 1430. Brouwer TF, Whang W, Kuroki K, Halperin JL, Reddy VY. Net clinical benefit of left atrial appendage closure versus warfarin in patients with atrial fibrillation: a pooled analysis of the randomized PROTECT-AF and PREVAIL studies. J Am Heart Assoc 2019;8:e013525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1431. Albåge A, Sartipy U, Kennebäck G, Johansson B, Scherstén H, Jidéus L; Swedish Arrhythmia Surgery Group . Long-term risk of ischemic stroke after the Cox-maze III procedure for atrial fibrillation. Ann Thorac Surg 2017;104:523–9. [DOI] [PubMed] [Google Scholar]
- 1432. Wang H, Han J, Wang Z, Yin Z, Liu Z, Jin Y, et al. A prospective randomized trial of the cut-and-sew maze procedure in patients undergoing surgery for rheumatic mitral valve disease. J Thorac Cardiovasc Surg 2018;155:608–17. [DOI] [PubMed] [Google Scholar]
- 1433. Ad N, Holmes SD, Massimiano PS, Rongione AJ, Fornaresio LM. Long-term outcome following concomitant mitral valve surgery and Cox maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2018;155:983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434. Pet M, Robertson JO, Bailey M, Guthrie TJ, Moon MR, Lawton JS, et al. The impact of CHADS2 score on late stroke after the Cox maze procedure. J Thorac Cardiovasc Surg 2013;146:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1435. Alqaqa A, Martin S, Hamdan A, Shamoon F, Asgarian KT. Concomitant left atrial appendage clipping during minimally invasive mitral valve surgery: technically feasible and safe. J Atr Fibrillation 2016;9:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1436. Suwalski P, Witkowska A, Drobiński D, Rozbicka J, Sypuła S, Liszka I, et al. Stand-alone totally thoracoscopic left atrial appendage exclusion using a novel clipping system in patients with high risk of stroke – initial experience and literature review. Kardiochir Torakochirurgia Pol 2015;12:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1437. Osmancik P, Budera P, Zdarska J, Herman D, Petr R, Fojt R, et al. Residual echocardiographic and computed tomography findings after thoracoscopic occlusion of the left atrial appendage using the AtriClip PRO device. Interact Cardiovasc Thorac Surg 2018;26:919–25. [DOI] [PubMed] [Google Scholar]
- 1438. Bedeir K, Warriner S, Kofsky E, Gullett C, Ramlawi B. Left atrial appendage epicardial clip (AtriClip): essentials and post-procedure management. J Atr Fibrillation 2019;11:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1439. Lee R, Vassallo P, Kruse J, Malaisrie SC, Rigolin V, Andrei A-C, et al. A randomized, prospective pilot comparison of 3 atrial appendage elimination techniques: internal ligation, stapled excision, and surgical excision. J Thorac Cardiovasc Surg 2016;152:1075–80. [DOI] [PubMed] [Google Scholar]
- 1440. Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924–9. [DOI] [PubMed] [Google Scholar]
- 1441. Vainrib AF, Harb SC, Jaber W, Benenstein RJ, Aizer A, Chinitz LA, et al. Left atrial appendage occlusion/exclusion: procedural image guidance with transesophageal echocardiography. J Am Soc Echocardiogr 2018;31:454–74. [DOI] [PubMed] [Google Scholar]
- 1442. van Laar C, Verberkmoes NJ, van Es HW, Lewalter T, Dunnington G, Stark S, et al. Thoracoscopic left atrial appendage clipping: a multicenter cohort analysis. JACC Clin Electrophysiol 2018;4:893–901. [DOI] [PubMed] [Google Scholar]
- 1443. Smith NE, Joseph J, Morgan J, Masroor S. Initial experience with minimally invasive surgical exclusion of the left atrial appendage with an epicardial clip. Innovations 2017;12:28–32. [DOI] [PubMed] [Google Scholar]
- 1444. Ad N, Massimiano PS, Shuman DJ, Pritchard G, Holmes SD. New approach to exclude the left atrial appendage during minimally invasive cryothermic surgical ablation. Innovations 2015;10:323–7. [DOI] [PubMed] [Google Scholar]
- 1445. Starck CT, Steffel J, Emmert MY, Plass A, Mahapatra S, Falk V, et al. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg 2012;15:416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1446. Toale C, Fitzmaurice GJ, Eaton D, Lyne J, Redmond KC. Outcomes of left atrial appendage occlusion using the AtriClip device: a systematic review. Interact Cardiovasc Thorac Surg 2019;29:655–62. [DOI] [PubMed] [Google Scholar]
- 1447. Ailawadi G, Gerdisch MW, Harvey RL, Hooker RL, Damiano RJ Jr, Salamon T, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011;142:1002–9, 1009.e1. [DOI] [PubMed] [Google Scholar]
- 1448. Emmert MY, Puippe G, Baumüller S, Alkadhi H, Landmesser U, Plass A, et al. Safe, effective and durable epicardial left atrial appendage clip occlusion in patients with atrial fibrillation undergoing cardiac surgery: first long-term results from a prospective device trial. Eur J Cardiothorac Surg 2014;45:126–31. [DOI] [PubMed] [Google Scholar]
- 1449. Caliskan E, Sahin A, Yilmaz M, Seifert B, Hinzpeter R, Alkadhi H, et al. Epicardial left atrial appendage AtriClip occlusion reduces the incidence of stroke in patients with atrial fibrillation undergoing cardiac surgery. Europace 2018;20:e105–14. [DOI] [PubMed] [Google Scholar]
- 1450. Zhang S, Cui Y, Li J, Tian H, Yun Y, Zhou X, et al. Concomitant transcatheter occlusion versus thoracoscopic surgical clipping for left atrial appendage in patients undergoing ablation for atrial fibrillation: a metaanalysis. Front Cardiovasc Med 2022;9:970847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1451. Branzoli S, Guarracini F, Marini M, D’Onghia G, Catanzariti D, Merola E, et al. Heart team for left appendage occlusion without the use of antithrombotic therapy: the epicardial perspective. J Clin Med 2022;11:6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1452. Zipes DP, Calkins H, Daubert JP, Ellenbogen KA, Field ME, Fisher JD, et al. 2015 ACC/AHA/HRS advanced training statement on clinical cardiac electrophysiology (a revision of the ACC/AHA 2006 update of the clinical competence statement on invasive electrophysiology studies, catheter ablation, and cardioversion). Circ Arrhythm Electrophysiol 2015;8:1522–51. [DOI] [PubMed] [Google Scholar]
- 1453. Hoffmann R, Parade U, Bauerle H, Winter KD, Rauschenbach U, Mischke K, et al. Safety and acute efficacy of cryoballoon ablation for atrial fibrillation at community hospitals. Europace 2021;23:1744–50. [DOI] [PubMed] [Google Scholar]
- 1454. Vassilikos VP, Pagourelias ED, Laroche C, Blomström-Lundqvist C, Kautzner J, Maggioni AP, et al. Impact of center volume on atrial fibrillation ablation outcomes in Europe: a report from the ESC EHRA EORP Atrial Fibrillation Ablation Long-Term (AFA LT) registry. Europace 2021;23:49–58. [DOI] [PubMed] [Google Scholar]
- 1455. Steinemann S, Berg B, Skinner A, DiTulio A, Anzelon K, Terada K, et al. In situ, multidisciplinary, simulation-based teamwork training improves early trauma care. J Surg Educ 2011;68:472–7. [DOI] [PubMed] [Google Scholar]
- 1456. Miller D, Crandall C, Washington C 3rd, McLaughlin S. Improving teamwork and communication in trauma care through in situ simulations. Acad Emerg Med 2012;19:608–12. [DOI] [PubMed] [Google Scholar]
- 1457. Friedman DJ, Pokorney SD, Khanna R, Goldstein L, Atwater BD, Bahnson TD, et al. Catheter ablation of atrial fibrillation with and without on-site cardiothoracic surgery. J Am Coll Cardiol 2019;73:2487–9. [DOI] [PubMed] [Google Scholar]
- 1458. Pope MTB, Kuklik P, Briosa EGA, Leo M, Mahmoudi M, Paisey J, et al. Impact of adenosine on wavefront propagation in persistent atrial fibrillation: insights from global noncontact charge density mapping of the left atrium. J Am Heart Assoc 2022;11:e021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1459. Tang S, Razeghi O, Kapoor R, Alhusseini MI, Fazal M, Rogers AJ, et al. Machine learning-enabled multimodal fusion of intra-atrial and body surface signals in prediction of atrial fibrillation ablation outcomes. Circ Arrhythm Electrophysiol 2022;15:e010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1460. Saglietto A, Gaita F, Blomstrom-Lundqvist C, Arbelo E, Dagres N, Brugada J, et al. AFA-Recur: an ESC EORP AFA-LT registry machine-learning web calculator predicting atrial fibrillation recurrence after ablation. Europace 2023;25:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1461. Stojadinovic P, Wichterle D, Peichl P, Nakagawa H, Cihak R, Haskova J, et al. Autonomic changes are more durable after radiofrequency than pulsed electric field pulmonary vein ablation. JACC Clin Electrophysiol 2022;8:895–904. [DOI] [PubMed] [Google Scholar]
- 1462. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WH, et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1463. Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017;377:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1464. Qian PC, Azpiri JR, Assad J, Gonzales Aceves EN, Cardona Ibarra CE, de la Pena C, et al. Noninvasive stereotactic radioablation for the treatment of atrial fibrillation: first-in-man experience. J Arrhythm 2020;36:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1465. Hohmann S, Deisher AJ, Konishi H, Rettmann ME, Suzuki A, Merrell KW, et al. Catheter-free ablation of infarct scar through proton beam therapy: tissue effects in a porcine model. Heart Rhythm 2020;17:2190–9. [DOI] [PubMed] [Google Scholar]
- 1466. Avram R, Ramsis M, Cristal AD, Nathan V, Zhu L, Kim J, et al. Validation of an algorithm for continuous monitoring of atrial fibrillation using a consumer smartwatch. Heart Rhythm 2021;18:1482–90. [DOI] [PubMed] [Google Scholar]
- 1467. Zhu L, Nathan V, Kuang J, Kim J, Avram R, Olgin J, et al. Atrial fibrillation detection and atrial fibrillation burden estimation via wearables. IEEE J Biomed Health Inform 2022;26:2063–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.