Abstract
Biological effects of high fluence low-power (HFLP) lasers have been reported for some time, yet the molecular mechanisms procuring cellular responses remain obscure. A better understanding of the effects of HFLP lasers on living cells will be instrumental for the development of new experimental and therapeutic strategies. Therefore, we investigated sub-cellular mechanisms involved in the laser interaction with human hepatic cell lines. We show that mitochondria serve as sub-cellular “sensor” and “effector” of laser light non-specific interactions with cells. We demonstrated that despite blue and red laser irradiation results in similar apoptotic death, cellular signaling and kinetic of biochemical responses are distinct. Based on our data, we concluded that blue laser irradiation inhibited cytochrome c oxidase activity in electron transport chain of mitochondria. Contrary, red laser triggered cytochrome c oxidase excessive activation. Moreover, we showed that Bcl-2 protein inhibited laser-induced toxicity by stabilizing mitochondria membrane potential. Thus, cells that either overexpress or have elevated levels of Bcl-2 are protected from laser-induced cytotoxicity. Our findings reveal the mechanism how HFLP laser irradiation interfere with cell homeostasis and underscore that such laser irradiation permits remote control of mitochondrial function in the absence of chemical or biological agents.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03321-z) contains supplementary material, which is available to authorized users.
Keywords: Phototoxicity, Apoptosis, Reactive oxygen species (ROS), High fluence low-power laser irradiation, Photobiomodulation, Mitochondrial membrane potential (ΔmΦ)
Introduction
Application of low-power lasers is gaining increasing attention in various fields of biomedical research and even clinically used technologies [1, 2]. Particularly, low-power (1–500 mW) visible or near-infrared spectrum (400–1000 nm) laser irradiation is emerging as a promising therapeutic approach, named low‐level light therapy (LLLT) or photobiomodulation (PBM) [1, 2]. Burst of research in this direction showed some benefits of PBM in wound healing, hair growth, Parkinson’s disease treatment, tissue regeneration, or reduce pain and inflammation [3–8]. However, reported therapeutic outcomes of clinical studies are varied and conflicting [4, 8–10]. Thus, PBM remains controversial as a therapeutic modality [11–13]. Indeed, underlying cellular mechanisms of PBM are not well understood [1, 2]. In addition, each PBM treatment varies in a large number of parameters, such as the wavelength, fluence, power density, pulse structure, and timing of the applied light [1, 2]. This in turn precludes efficient clinical transition of PBM.
Although low-power lasers are used in PBM, optical power density of used systems may reach significant values of kW/cm2 [1, 2, 14]. Using such high fluence low-power (HFLP) lasers in studies of molecular mechanisms of laser-cell interactions gained attention of researchers [1, 14]. It is worth noting that power densities above 50 kW/cm2 may initiate thermal interaction with the laser radiation for most of soft tissues [15–17]. Therefore, HFLP lasers used in PBM studies do not overcome such power densities to eliminate non-specific thermal interactions [1, 14]. In fact, numerous studies on laser photobiomodulation ascribed bewildering biological effects ranging from apoptosis to cell proliferation; for review, see [14]. However, there is a certain consensus on phototoxic effects of HFLP laser treatment revealing that depending on the dose low-power laser irradiation induces either apoptotic or necrotic cell death [2, 18–23]. In addition, similar HFLP laser irradiation is used in super-resolution localization microscopy [24, 25]. Thus, photodamage effects in live-cell super-resolution fluorescence microscopy represent a major drawback [19, 26]. Unfortunately, so far, live-cell super-resolution localization microscopy largely ignored possible phototoxic effects [26].
Recent studies proposed mitochondria as major sub-cellular “effector” of HFLP laser irradiation [2, 18–23]. Furthermore, it was postulated that cytochrome c oxidase (COX) is the crucial chromophore in the cellular response to PBM [1, 2, 27]. In fact, COX involvement in laser-mediated responses was done by matching the absorption spectra of COX to laser light range utilized in PBM [28]. Studies, using thorough functional approach to elucidate role of mitochondria and COX in laser treatment, are rather limited in numbers [18, 20, 23]. The vast majority of such reports are rather descriptive in nature [1, 2]. Thus, it is not surprising that the molecular or biophysical foundations for laser-induced cellular effects remain poorly understood.
Moreover, most studies focus their attention on the effects of red or near-infrared (NIR) lasers [2, 18–23], while comparison with other laser wavelengths is often omitted. HFLP laser studies also utilize broad spectrum of cell models of distinct lineages, which makes it difficult to compare obtained results [2, 18–23]. Surprisingly, very little is known about responses of hepatic cells to laser irradiation. We recently reported different doses of red laser irradiation led to activation of distinct biochemical signaling that triggers cell death pathways in hepatocyte-derived carcinoma Huh7 cells [21]. Here, we present a comparative study on deciphering molecular mechanisms of laser–cell interactions. We investigate photodamage effects of different laser wavelengths (398, 505, and 650 nm) on closely related cell lines (HepG2, Huh7, and Alexander cells) and focus on the kinetics of mitochondrial alterations and COX activity upon laser treatment and functional consequences of such alterations.
Materials and methods
Chemicals and antibodies
The following fluorescent probes were used: Cellular ROS/Superoxide Detection Assay Kit (Abcam, Cambridge, United Kingdom) to detect the generation of ROS and superoxide; and acridine orange (5 µg/ml) to monitor lysosomal integrity (Thermo Fisher Scientific, Waltham, MA, US); JC-1 (1 µM) to monitor mitochondrial membrane potential (Thermo Fisher Scientific, Waltham, MA, US); and VAD-FMK conjugated to FITC (FITC-VAD-FMK) to detect caspase-3 activation (Abcam, Cambridge, United Kingdom). To investigate mitochondrial ROS, cells were loaded with MitoTracker® red CM-H2XRos (reduced form of MitoTracker® red; 0.5 μM; Thermo Fisher Scientific, Waltham, MA, US) by incubating them for 15 min. Acetoxymethylester of calcein (calcein-AM, 1 μM) and ethidium homodimer (EthD-1, 4 μM) or propidium iodide (PI, 500 nM) was used to monitor cell viability (Thermo Fisher Scientific, Waltham, MA, US). Acridine orange (5 µg/ml) and LysoTracker® Red (200 nM) were used to monitor lysosomal integrity (Thermo Fisher Scientific, Waltham, MA, US); thioflavin T (5 µM) to monitor endoplasmic reticulum (ER) stress (Abcam, Cambridge, United Kingdom). To monitor mitochondria, dynamics cells were stained with MitoTracker Red (200 nM, Thermo Fisher Scientific, Waltham, MA, US). The cell-permeant NucRed Live 647 Ready Probes Reagent fluorescent nucleic acid stain or hoechst 33342 (Thermo Fisher Scientific, Waltham, MA, US) were used to label nucleus. The optimal incubation time for each probe was determined experimentally.
The following reagents were used: thapsigargin (1 µM, Sigma, St. Louis, MO, US) as the inducer of ER stress; Mito-TEMPO (20 μM, Sigma, St. Louis, MO, US) to scavenge mitochondrial ROS; staurosporine (2 μM, Abcam, Cambridge, United Kingdom) as a known inducer of apoptosis; H2O2 (500 μM) as a known ROS inducer; ionomycin (1 µM, LKT Labs, St Paul, MN, US) as a known inducer of mitochondrial permeability transition pore; ADDA-5 (15 µM, Sigma, St. Louis, MO, US) and KCN (5 mM, Sigma, St. Louis, MO, US) as inhibitors of cytochrome c oxidase; bezafibrate (1 mM, Sigma, St. Louis, MO, US) as activator of cytochrome c oxidase; ABT-737 (10 µM, Abcam, Cambridge, United Kingdom) as inhibitor of Bcl-2; WST-1 (Roche, Mannheim, Germany) and alamarBlue (Thermo Fisher Scientific, Waltham, MA, US) to assess cell viability.
The following antibodies were used: anti-Bcl-2, dilution 1:1000 (#15071, Cell Signaling Technology, Danvers, MA, US); anti-p53, dilution 1:1000 (#2527, Cell Signaling Technology, Danvers, MA, US); anti-β-actin, dilution 1:2000 (#10D10, Thermo Fisher Scientific, Waltham, MA, US); annexin V Alexa Fluor™ 488, dilution 1:100 (#V13245, Thermo Fisher Scientific, Waltham, MA, US); anti-mouse-HRP, dilution 1:10,000 (#G21040, Thermo Fisher Scientific, Waltham, MA, US); and anti-rabbit-HRP, dilution 1:10,000 (#G21234, Thermo Fisher Scientific, Waltham, MA, US).
Characterization of the laser system and high fluence low-power laser treatment
For irradiation of cells, we used previously published system [21] that was modified to have three wavelengths (Fig. S1A, B). Briefly, the optical circuit includes laser diode modules LDI-650-FP-20-E-18-SM04-FA-CW, LDI-520-FP-20-E-3-SM03-FA-CW, and LDI-405-FP-20-E-8-SM03-FA-CW (LasersCom, LLC, Belarus) with a fiber output of light at the wavelength of 650 (red), 505 (green), and 398 (blue) nm, respectively (Fig. S1C). As a delivery system for laser radiation, a single-mode optical fiber of the SMF-28 type (Corning, New York, US) with a reduced diameter at the output end (biconical optical taper) was utilized. Using for irradiation, such type of a fiber taper with a waist diameter of 15 ± 5 μm allows to influence not only individual cells, but also certain regions of cells, whose size exceeds the diameter of the waist [21]. Biconical optical tapers were produced by an apparatus for splicing optical fibers Fujikura FSM-100 M/P utilizing the previously published methodology [21, 29]. Furthermore, to eliminate the undesirable effects of optical radiation propagation, a protective coating of titanium dioxide (TiO2) was formed by the ion-sputtering method at the waist of the biconical taper. After the process of spraying the protective coating, the fiber optic taper was scraped a few millimeters from the narrowest point in the waist, and the fiber end was polished on a Bare Fiber Polisher (Krell Technologies, Neptune City, NJ, US). The output laser power for all wavelengths was kept of ~ 50 µW, which was measured by an optical power meter PM100D (Thorlabs, Newton, NJ, US). The divergence of laser radiation at wavelengths of 650 (red), 505 (green), and 398 (blue) at the taper end for the coordinates X and Y was determined by taking into account the waist radius of the Gaussian beam, where the intensity values fall to 1/e2 level (Fig. S1D). Assessment of the laser radiation divergence at the taper end was performed using a beam-profiling camera Spiricon SP620U (Ophir-Spiricon, LLC, North Logan, US). Based on the obtained data of the radiation divergence, the dependence of the power density on the distance for 50 µW was determined (Fig. S1E). The cells in selected area were irradiated with the irradiance of < 1 kW/cm2.
Cells were seeded in 35 mm tissue culture eppendorf cell imaging dishes (Eppendorf, Hamburg, Germany) 24 h before laser irradiation. Depending on the experiment, cells were labeled either prior laser irradiation or immediately after. Positioning of optical tapers in the closest proximity to the cells was performed using an Eppendorf micromanipulator (5171, Eppendorf, Wesseling-Berzdorf, Germany) that was connected to either a Nikon Diaphot 200 microscope (Nikon, Tokyo, Japan) or Olympus IX83 microscope (Olympus, Tokyo, Japan). Optical tapers were sterilized with 75% ethanol prior positioning to the cells.
Cell culture
Human hepatocellular carcinoma cell lines Huh7 obtained from the Japanese Collection of Research Bioresources (JCRB), HepG2 (American-Type Culture Collection, ATCC, Manassas, VA, US), and Alexander (PLC/PRF/5, ATCC) were cultured in EMEM medium (ATCC) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, US) as recommended by the supplier. Cultures were kept in a humidified 5% CO2 atmosphere at 37 °C.
Measurement of cellular viability
To analyze cytotoxic effects triggered by high fluence low-power laser treatment, we used fluorescent LIVE/DEAD® Viability/Cytotoxicity Kit (Thermo Fisher Scientific, Waltham, MA, US). This two-color fluorescence cell viability assay is based on the ability of calcein-AM to be retained within live cells, inducing an intense uniform green fluorescence and EthD-1 to bind the nuclei of damaged cells, thus producing a bright red fluorescence in dead cells [21, 30]. Cells were seeded in 35 mm tissue culture eppendorf cell imaging dishes (Eppendorf, Hamburg, Germany) 24 h before laser irradiation, then stained with calcein-AM (1 μM) and EthD-1 (4 μM) for 30 min. After labelling cells were exposed to laser light. Subsequently images were captured using Bio-Rad MRC-1024 laser scanning confocal microscope (Bio-Rad, Cambridge, MA, US). ImageJ software (NIH) was used for image processing. Fluorescence intensity of both dyes was measured at the respective timepoints and was normalized to total fluorescence 30 min after dye loading. Confirming the validity of the live/dead staining was also treated with 20% ethanol for 30 min and subsequent imaging.
To analyze toxicity of cytochrome c oxidase inhibitors, we utilized either WST-1 assay (Roche, Mannheim, Germany) or alamarBlue (Thermo Fisher Scientific, Waltham, MA, US). Cells were seeded onto 96-well plates at a density of 10,000 cells per well and treated with different cytochrome c oxidase inhibitors of indicated concentrations. 24 h after the treatment, WST-1 reagent was added to each well and incubated for 2 h at 37 °C to form formazan. The absorbance was measured using a TECAN microplate reader SpectraFluor Plus (TECAN, Mannedorf, Switzerland) at 450 nm. Readings were done in quadruplicates; three independent experiments were performed for each measurement. Alternatively, 24 h after the treatment, alamarBluer reagent was added to each well and incubated for 2 h at 37 °C. Fluorescence (using an excitation between 530 and 560 and an emission at 590 nm) was measured using a TECAN microplate reader SpectraFluor Plus (TECAN, Mannedorf, Switzerland). Readings were done in quadruplicates; three independent experiments were performed for each measurement.
Detection of mitochondrial reactive oxygen species (ROS)
Mitochondria-specific ROS were assessed as described previously [21, 31]. Briefly, cells were seeded in 35 mm tissue culture eppendorf cell imaging dishes (Eppendorf, Hamburg, Germany) 24 h before laser irradiation then were loaded with 0.5 µM MitoTracker® red CM-H2XRos for 15 min at 37 °C in the dark, irradiated with different laser wavelengths and imaged using Bio-Rad MRC-1024 laser scanning confocal microscope. Quantitative analysis of MitoTracker® red CM-H2XRos fluorescence imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (Bio-Rad, Cambridge, MA, US).
Quantification of mitochondrial membrane potential
Mitochondrial membrane potential (ΔmΦ) was assessed utilizing previously described methodology [21, 31, 32]. Cells were stained with 1 µM JC-1 probe, irradiated with different laser wavelengths, and imaged using Bio-Rad MRC-1024 laser scanning confocal microscope. JC-1 has advantages over other cationic dyes in that it can selectively enter into mitochondria and reversibly change color from red to green, as the membrane potential decreases. In healthy cells with high mitochondrial ΔmΦ, JC-1 spontaneously forms complexes known as J-aggregates with intense red fluorescence. On the other hand, in apoptotic or unhealthy cells with low ΔmΦ, JC-1 remains in the monomeric form, which shows only green fluorescence. The ratio of green-to-red fluorescence is dependent only on the membrane potential, but not on other factors such as mitochondrial size, shape, and density, which may influence single-component fluorescence signals [33, 34]. Quantitative analysis of ΔmΦ imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (Bio-Rad, Cambridge, MA, US).
Apoptosis assay
The Dead Cell Apoptosis Kit (Thermo Fisher Scientific, Waltham, MA, US) was used to measure early apoptosis by detecting phosphatidylserine expression and membrane permeability. Following laser irradiation, cells were stained with Dead Cell Apoptosis Kit according to the manufacturer’s instructions. Phosphatidylserine expression as an early sign of apoptosis was determined by the binding of Alexa Fluor 488 Annexin V, propidium iodide was used to differentiate necrotic cells. Hoechst 33342 was used as nucleus staining. After staining, cells were fixed in 4% paraformaldehyde for 10 min at room temperature. As positive control 2 µM, staurosporine for 3 h was used. Labeled cells were then imaged using high-resolution spinning disk confocal microscopy IXplore SpinSR (Olympus, Tokyo, Japan). Fluorescence images were taken with the acquisition software cellSens (Olympus, Tokyo, Japan). ImageJ software (NIH) was used for image processing.
Caspase-3 activity assay
As an apoptosis parameter, caspase-3 activation was detected using the caspase-3 inhibitor VAD-FMK conjugated to FITC (FITC-VAD-FMK) as a marker. FITC-VAD-FMK is cell permeable, nontoxic, and irreversibly binds to activated caspases in apoptotic cells. After 40 min post laser irradiation, cells were loaded with FITC-VAD-FMK (Abcam, Cambridge, United Kingdom) according to the manufacturer’s instructions. Following the staining, cells were then imaged using high-resolution spinning disk confocal microscopy IXplore SpinSR (Olympus, Tokyo, Japan). Fluorescence intensity was measured using ImageJ software (NIH). As a positive control, cells were treated with 2 μM staurosporine for 3 h.
Lysosomal stability assessment
After laser treatment, cells were stained with 5 µg/ml acridine orange (AO, Thermo Fisher Scientific, Waltham, MA, US). Once inside the lysosomes, the metachromatic AO sensitizes the lysosomal membrane to photo-oxidation by blue light. Loss of lysosomal integrity can be measured as a ‘loss of red fluorescence’. The accompanying decrease in fluorescence intensity was analyzed by Bio-Rad MRC-1024 laser scanning confocal microscope. In addition, to evaluate the integrity of the lysosomal compartment, we used LysoTracker Red. LysoTracker Red is highly selective for acidic organelles (e.g., lysosomes), and its fluorescent intensity reflects accumulation in such structures [35]. If there is ongoing lysosomal membrane permeabilization, it leads to loss of accumulated LysoTracker Red fluorescence signal. The decrease in fluorescence intensity was analyzed by Bio-Rad MRC-1024 laser scanning confocal microscope.
Thioflavin T assay
Thioflavin T assay was used to assess misfolded or unfolded proteins in ER stress [36]. Thioflavin T is a small molecule exhibiting enhanced fluorescence when it binds to protein aggregates. Thus, thioflavin T-fluorescence correlates directly with established indicators of unfolded protein response activation which allows the detection and quantification of endoplasmic reticulum stress in live cells [36]. After experimental laser treatment, cellular media were replaced by media containing 5 µM thioflavin T for 30 min. Thioflavin T fluorescence intensities were assessed using Bio-Rad MRC-1024 laser scanning confocal microscope with 450/485-nm excitation/emission filters set as previously described [36].
Evaluation of mitochondrial permeability transition pore (mPTP)
mPTP opening was assessed using established technique by loading cells with 1 μM calcein AM (green) in the presence of 1 mM cobalt chloride for 30 min before laser treatment [37]. Quenching of free calcein by cobalt chloride allows observing mitochondrial integrity as an mPTP indicator evaluation of mitochondrial permeability transition pore [37, 38]. mPTP is a cyclophilin D-dependent pore complex which forms in the inner mitochondrial membrane (IMM) and results in mitochondrial permeability [39]. The calcein/cobalt assay utilizes calcein AM to label the entire cell, followed by a treatment with cobalt chloride to quench the calcein fluorescence outside of the mitochondrial matrix [37, 38]. Co2+ cannot penetrate IMM and thus mitochondrial matrix exhibits green fluorescence. When IMM is damaged, the calcein fluorescence is quenched [37, 38]. Loss of mitochondrial integrity or mPTP was evaluated from calcein fluorescence intensity levels obtained from spinning disk confocal microscopy IXplore SpinSR (Olympus, Tokyo, Japan). Mitochondria were labeled with MitoTracker Red. Hoechst 33342 was used as nucleus staining. ImageJ software (NIH) was used for image processing.
Cell extracts and western immunoblot analysis
Aliquots of whole cell lysates [40, 41] containing equal amounts of protein were obtained using lysis buffer RIPA. Protein samples were subjected to SDS-PAGE electrophoresis, transferred to PVDF membranes. The membranes were blocked with 5% (w/v) nonfat dried milk for 1 h, then incubated with various specific primary antibodies listed in Supplemental Table 2 at 4 °C overnight and detected as described [40, 41]. All antibodies used in the study are summarized in section Chemicals and antibodies.
Transient transfection
Huh7 and Alexander cells were transfected with plasmid GFP-Bcl2 (Plasmid 17999, provided by Clark Distelhorst [42]) purchased from Addgene Watertown, MA, US. Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, US) was used as a transfection reagent. Transfection was performed according manufacturer recommendation. Transfection was conducted in the presence of OptiMEM (Thermo Fisher Scientific, Waltham, MA, US) supplemented with 10% FBS, 100 U of penicillin G and 100 μg of streptomycin per ml. Transfections were done in duplicate wells and at least in three independent experiments. Transfection efficiency was monitored by immunoblot analysis and spinning disk confocal microscopy IXplore SpinSR (Olympus, Tokyo, Japan).
Confocal microscopy
For standard analysis, Nikon Diaphot 200 microscope (Nikon, Tokyo, Japan) in combination with Bio Rad MRC-1024 confocal laser scanning imaging system (Bio-Rad, Cambridge, MA, US) has been used in this work for the visualization of cells. Eppendorf micromanipulator 5171 (Eppendorf, Wesseling-Berzdorf, Germany), connected to Nikon Diaphot 200 microscope (Nikon, Tokyo, Japan), was used for precise positioning of taper. Fluorescence images were taken with the acquisition software Lasersharp 2000 v5.2 (BioRad, Hercules, CA). ImageJ software (NIH) was used for image processing and quantification. Quantitative analysis of ΔmΦ and MitoTracker® red CM-H2XRos fluorescence was performed utilizing LaserSharp 2000 software (Bio-Rad, Cambridge, MA, US). Analysis of mitochondria circularity, semi-quantitative analysis of calcein, ethidium homodimer, caspase-3 inhibitor, and LysoTracker Red fluorescence was done with ImageJ software (NIH).
To visualize in great detail, the morphological changes of cells upon laser treatment, we utilized brand new high-resolution spinning disk confocal microscopy IXplore SpinSR (Olympus, Tokyo, Japan). Labeled cells were then imaged using high-resolution spinning disk confocal microscope. Fluorescence images were taken with the acquisition software cellSens (Olympus, Tokyo, Japan). ImageJ software (NIH) was used for image processing.
Spinning disk super-resolution microscopy
IXplore SpinSR Olympus super-resolution imaging system (Olympus, Tokyo, Japan) has been used in this work for the mitochondria dynamics upon laser treatment. Mitochondria were labeled with MitoTracker Red. To obtain maximal possible resolution, we utilized silicone immersion objective UPLSAPO100XS (Olympus, Tokyo, Japan). Fluorescence images were taken with the acquisition software cellSens (Olympus, Tokyo, Japan). ImageJ software (NIH) was used for image processing and quantification.
Statistical analysis
Quantitative results are present as mean ± SEM. The statistical significance of differences between the groups was determined using ANOVA Fisher’s LSD and Newman–Keuls tests. Differences were considered statistically significant at *p < 0.05.
For quantitative fluorescence microscopy analysis (analysis of mitochondria dynamics, mitochondrial ROS detection, ΔmΦ), we used rigorously defined guidelines for accuracy and precision quantification [43]. The sample size determination was based on a statistical method described in [44], which determines sample size for 95% confidence interval and 0.9 statistical power equal to 30. Therefore, n = 30 cells were used in quantification.
Results
Acute laser damage of hepatocyte-derived cell lines
It becomes evident that some doses of HFLP laser treatment exert phototoxic effects [1, 14, 22]. We recently reported different doses of red laser irradiation led to the activation of distinct biochemical signaling that triggers cell death pathways in Huh7 [21]. Indeed, we developed a laser system allowing direct irradiation of the cell layer in aqueous solutions [21]. A scheme of the experimental setup is shown in Fig. S1A. Our previous research showed that laser power of ~ 50 µW induces apoptosis, whereas 1 mW irradiation results in necrosis [21]. We proposed a tentative link between mitochondria depolarization and laser-induced cellular responses (Fig. S1B) [21]. However, we did not reveal precise cellular ‘effectors’ of laser irradiation. In addition, there was no comparison with other laser wavelengths. Moreover, a majority of HFLP laser studies utilize broad spectrum of cell models originating from different lineages [2, 18–23]. Therefore, we expanded our study to three commonly used in photobiomodulation laser wavelength ranges (398, 505, and 650 nm) and utilized comparative analysis of cellular responses on closely related cell lines (HepG2, Huh7, and Alexander cells). Here, we utilized only one laser power of ~ 50 µW with corresponding irradiance of < 1 kW/cm2. We selected laser power that is one of the most frequently used in biomedical applications [1, 2, 4, 5]. In addition, selected irradiance corresponds to super-resolution localization microscopy conditions [24, 25].
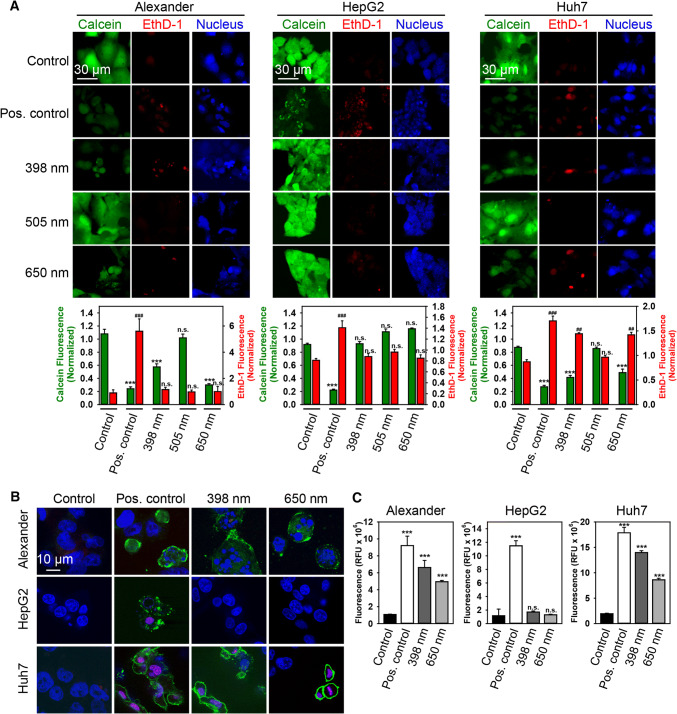
First of all, we investigated acute laser damage utilizing the LIVE/DEAD® Viability/Cytotoxicity Kit-based approach that we successfully used previously [21]. Consistent with the previous reports [2, 18–23], blue (398 nm) and red (650 nm) laser irradiation did significantly affect the viability of Huh7 and Alexander cells within the first 60 min of exposure (Figs. 1a and S2A). Interestingly, green (505 nm) laser irradiation did not induce cell death in both Huh7 and Alexander cells (Figs. 1a and S2A). Surprisingly, HepG2 cells were resistant to laser-induced cell death at any laser wavelength (Figs. 1a and S2A). It is worth noting here that necrosis evaluation analysis revealed significant increase in both propidium iodide and EthD-1 staining only in Huh7 cells (Fig. S2B, C).
Fig. 1.
HFLP laser irradiation induces acute cell death of Huh7 and Alexander cells but not HepG2. a Cell viability was detected by the fluorescent live/dead cell assay kit (Thermo Fisher Scientific). Alexander, HepG2 and Huh7 cells were treated with different wavelengths (398, 505 and 650 nm) of laser (50 µW) for 1 h. Cells were loaded with calcein-AM (green) and ethidium homodimer (red) images were acquired by laser scanning confocal microscopy. NucRed™ Live 647 ReadyProbes™ Reagent (blue) was used for nucleus labelling. Control cells were untreated. As a positive control, cells were treated with 20% ethanol for 30 min. ImageJ software (NIH) was used for image processing and quantification. Fluorescence intensity of both dyes was measured and was normalized to total fluorescence of non-irradiated spot. Data are expressed as mean ± SEM (n = 3), Newman–Keuls test for calcein–AM comparison **p < 0.01, ***p < 0.001; Newman–Keuls test for ethidium homodimer comparison ###p < 0.001. b Huh7, HepG2, and Alexander cells were treated with different wavelengths (398 and 650 nm) of (50 µW) laser irradiation for 40 min and then labeled with hoechst 33342 nuclear stain (blue), annexin V (green) and PI (red). Cells treated with 2 μM staurosporine for 3 h served as a positive control. Merge of blue and red gives magenta color. Labeled cells were then imaged using spinning disk confocal microscopy. c Huh7, HepG2 and Alexander cells were treated with different wavelengths (398 and 650 nm) of (50 µW) laser irradiation for 40 min and then incubated with caspase-3 inhibitor VAD-FMK conjugated to FITC (FITC-VAD-FMK). Following the staining, cells were analyzed using a spinning disk confocal microscopy. Fluorescence intensities were analyzed with ImageJ (NIH). Data are expressed as mean ± SEM (n = 3), ***p < 0.001. Cells treated with 2 μM staurosporine for 3 h served as a positive control
Exposure of Huh7 and Alexander cells to either blue or red-laser-induced early signs of apoptosis (Fig. 1b). Translocation of phosphatidylserine to the outer cell membrane leaflet was detected by binding of annexin V without any concomitant increase in membrane permeability as shown by propidium iodide exclusion (Fig. 1b). In agreement with cytotoxicity results, we found no signs of neither annexin V nor propidium iodide positive staining in HepG2 cells under laser treatment (Fig. 1b). Assessment of caspase-3 activity in cells treated with either blue or red laser showed that, indeed, both wavelengths induced apoptotic cell death in Huh7 and Alexander cells, which was evident already 40 min after exposure to 50 µW of laser irradiation (Fig. 1c). This constellation suggested apoptotic cell death triggered by blue and red laser irradiation. Again, HepG2 cells were insensitive to laser irradiation in terms of increased caspase-3 activity (Fig. 1c).
HFLP laser effect on dynamics and functioning of mitochondria
Emerging evidence suggest that mitochondria are the most probable subcellular acceptors of laser irradiation [2, 18–23]. Recent studies suggest that mitochondrial dysfunction induced by laser irradiation is the main cause of laser-induced cytotoxicity [2, 18–23]. Indeed, the very thorough and recent review on laser-induced cellular effects summarized that cells/tissues (namely muscle, brain, heart, and nerve) with high numbers of mitochondria tended to respond more efficiently to laser treatment [22]. Surprisingly, little is known about effects of laser irradiation on physiological functions of hepatic cells [2, 18–23]. Our previous study showed that red HFLP laser irradiation could induce cell apoptosis via the mitochondrial pathway in Huh7 cells [21]. Thus, to further validate the role of mitochondria in hepatic cell injury triggered by laser irradiation, we analyzed responses of three cell lines to three laser wavelengths.
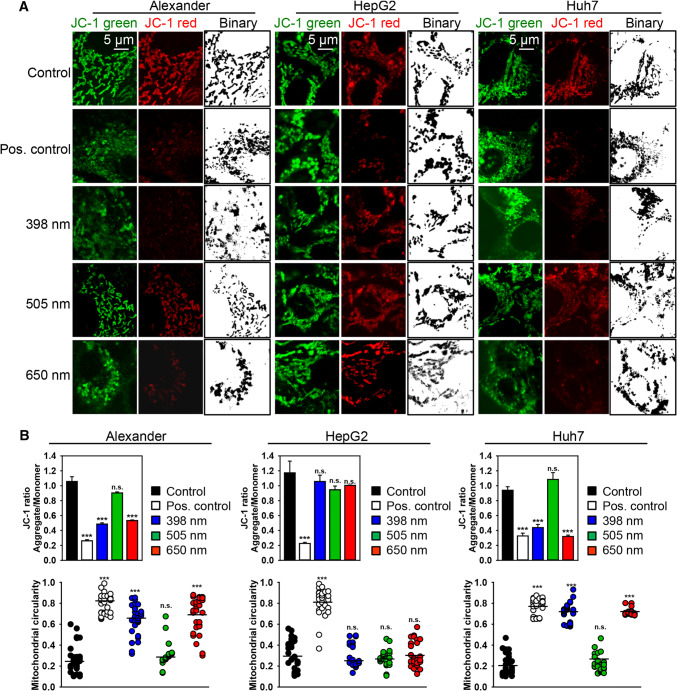
The representative images show that the decrease in JC-1 red fluorescence intensity (Figs. 2a and S3A) corresponds closely to the increase in mitochondrial circularity (Fig. 2b) after red and blue HFLP laser treatment, indicating that HFLP irradiation induces a decrease in ΔmΦ along with mitochondrial dysfunction in Alexander and Huh7 cells. Binarized confocal images and circularity assessment clearly show massive fission on mitochondria (Fig. 2). Green laser wavelength did not induce any noticeable changes in mitochondrial membrane potential and dynamics in Alexander and Huh7 cells (Figs. 2 and S3A). Similar to the cytotoxicity results, HepG2 showed no signs of mitochondria dysfunction upon either wavelengths exposure (Figs. 2 and S3A).
Fig. 2.
HFLP laser irradiation affects mitochondria dynamics. a HepG2, Huh7 and Alexander cells were irradiated with different wavelengths of laser (398, 505 and 650 nm; 50 µW) for 30 min, then stained with 1 μM JC-1 and analyzed by laser scanning confocal microscopy. Red fluorescent images of dye aggregates indicate high- ΔmΦ mitochondria. Confocal imaging with binarized pictures of the original images shows mitochondrial fragmentation in Huh7 and Alexander cells treated with laser (398 and 650 nm) in comparison with controls. As a positive control, cells were treated with 20% ethanol for 20 min. b Quantitative analysis of ΔmΦ and mitochondrial circularity imaged in (a). ImageJ software (NIH) was used for image processing and quantification. JC-1 data are expressed as mean ± SEM (n = 3), ***p < 0.001. Pre-processed images of mitochondria stained with JC-1 as described in (a) were subjected to morphometric analysis for mitochondrial circularity. Morphometric analysis was performed using ImageJ (NIH). The mitochondrial circularity data expressed as mean of n = 30–50 cells; ***p < 0.001
Summarizing this part, we can conclude that red and blue laser irradiation triggered ΔmΦ collapse and massive mitochondrial fission in Alexander and Huh7 cells (Fig. 2). These data support findings of apoptosis (Fig. 1b, c) triggered by red and blue laser in Alexander and Huh7 cells. Importantly, during apoptosis, mitochondria undergo permeabilization of the mitochondrial outer membrane (MOMP) and massive fission [45, 46]. This results in the formation of small, round organelles that tend to aggregate around the nucleus [45, 46]. Therefore, we further analyzed kinetics of mitochondria shape and mitochondrial membrane potential upon laser treatment. As far as HepG2 were not responsible to laser treatment, we further focused on responses of Huh7 and Alexander cells. Moreover, it is evident here that green wavelength of laser of such power and irradiance had not significant effects on functionality of Huh7 and Alexander cells. Thus, we studied effects of blue and red laser light.
To clearly see the kinetics of mitochondria shape, we utilized brand new super-resolution spinning disk microscopy. The principle of such technique is similar to structured illumination microscopy (SIM) and achieves a spatial resolution of 120 nm [47, 48]. The advantage of this system is that it is 10 × faster than conventional SIM, because signals are recovered by optical demodulation through the stripe pattern of the disk [47, 48].
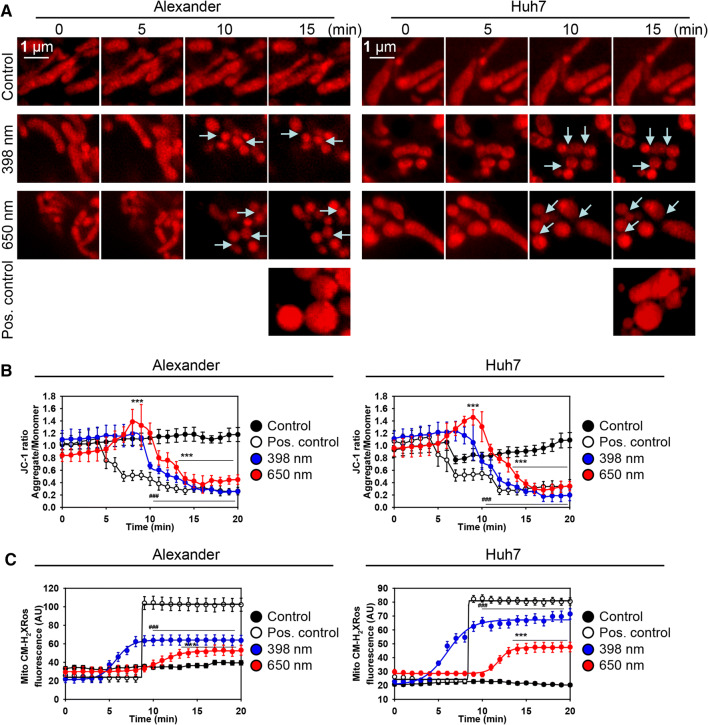
Indeed, time-laps super-resolution images showed clearly fission of mitochondria upon both red and blue laser wavelengths treatment in Huh7 and Alexander cells (Figs. 3a and S3B). Already, 10 min after laser irradiation (both blue and red), mitochondria exhibited small size and round shape in comparison to control (Figs. 3a and S3B). This is a pre-requisite of apoptotic events in cells [45, 46]. Importantly, treatment of Huh7 and Alexander cells with 20% ethanol for 15 min, as a positive control, resulted in formation of swollen round mitochondria (Figs. 3a and S3B). Indeed, relatively high doses of ethanol (more than 10%) are known to induce primary necrosis (defined as immediate cell death appearing after severe death stimuli) in hepatocytes [49, 50], that is accompanied by swelling of the mitochondrial matrix [51].
Fig. 3.
IXplore SpinSR Olympus super-resolution microscopy reveals kinetics of mitochondria fragmentation upon HFLP laser irradiation. a Representative time-lapse super-resolution images of mitochondria in living Huh7 and Alexander cells stained with MitoTracker® red. Cells exposed to HFLP laser (398 and 650 nm; 50 µW) for indicated time periods. As a positive control, cells were treated with 20% ethanol for 15 min. Arrows show cites of mitochondria fission. b Kinetics of ΔmΦ upon HFLP laser irradiation. Huh7 and Alexander cells were labeled with 1 μM JC-1. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) for indicated time periods. Quantitative analysis of ΔmΦ imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), t = 0 timepoint served as control, ***p < 0.001. As a positive control, cells were treated with 20% ethanol. c Kinetics of mitochondrial ROS accumulation upon HFLP laser irradiation. Huh7 and Alexander cells were labeled with MitoTracker® red CM-H2XRos. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) for indicated time periods. Quantitative analysis of MitoTracker® red CM-H2XRos fluorescence imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), t = 0 timepoint served as control, ***p < 0.001. Non-irradiated cells treated with H2O2 (0.5 mM) were used as a ROS positive control
Furthermore, we analyzed kinetics of mitochondrial membrane potential by time-lapse ratiometric JC-1 confocal analysis (Figs. S4 and S5). Indeed, quantification of ratiometric JC-1 imaging showed that blue and red laser irradiation resulted in rapid dissipation of ΔmΦ in Huh7 and Alexander cells after 10 min of laser exposure (Fig. 3b). Interestingly, red laser irradiation induced a spike of hyperpolarization of mitochondria between 5th and 10th minute of laser treatment in both cell lines (Fig. 3b). In contrast to that blue laser had no effect on hyperpolarization and induced only rapid dissipation of ΔmΦ in both cell lines (Fig. 3b). To explore the state of mitochondrial ROS generation upon laser irradiation, we stained Huh7 and Alexander cells with mitochondrial ROS-specific derivative of MitoTracker® red CM-H2XRos and assessed quantitatively kinetics of ROS accumulation using confocal microscopy (Fig. 3c). Indeed, blue laser irradiation induced higher levels of mitochondrial ROS accumulation in both cell lines in comparison with red laser irradiation (Fig. 3c). In addition, blue laser resulted in faster accumulation kinetic of mitochondrial ROS in comparison with red laser irradiation (Fig. 3c).
Lysosomes and ER stress do not initiate HFLP laser-induced photodamage in Huh7 and Alexander cells
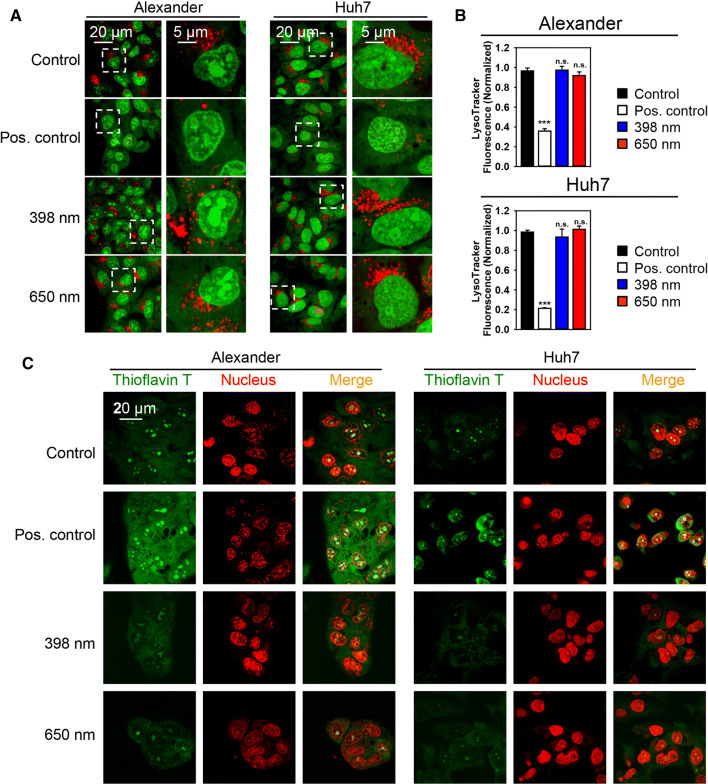
Summarizing aforementioned experimental data together with similar previously published studies [2, 18–23], we can point toward mitochondria as potential ‘effectors’ of laser irradiation. However, there are other possibilities that the laser irradiation may excite distinct molecular dynamics, for example, ER stress or lysosomal leakage. ER stress and lysosomal destabilization are known to induce cell death in a mitochondrion-dependent fashion [52–55]. Therefore, we analyzed whether blue and red laser irradiation results in either lysosomal destabilization or ER stress at the timepoint of treatment when we observed massive dissipation of ΔmΦ. Indeed, acridine orange staining (Fig. 4a) and LysoTracker Red fluorescent intensity assessment (Fig. 4b) showed no significant signs of lysosomal damage upon both red and blue laser irradiation.
Fig. 4.
HFLP laser irradiation does not affect lysosomal integrity and does not induce ER stress. a Huh7 and Alexander cells were stained with 5 µg/ml acridine orange (AO), then exposed to HFLP laser (398 and 650 nm; 50 µW) for 15 min and analyzed by laser scanning confocal microscopy. AO stains DNA in nucleus and RNA in nucleoli in green. Lysosomal acidic vesicles demonstrate exclusively red fluorescence. As a positive control, cells were treated with 20% ethanol for 20 min. b Huh7 and Alexander cells were stained with LysoTracker Red, then exposed to HFLP laser (398 and 650 nm; 50 µW) for 15 min and analyzed by laser scanning confocal microscopy. Fluorescence intensities were analyzed with ImageJ (NIH). Data are expressed as mean ± SEM (n = 3), ***p < 0.001. As a positive control, cells were treated with 20% ethanol for 20 min. c Huh7 and Alexander cells were stained with 5 µM Thioflavin T (green), then exposed to HFLP laser (398 and 650 nm; 50 µW) for 15 min and analyzed by laser scanning confocal microscopy. NucRed™ Live 647 ReadyProbes™ Reagent (red) was used for nucleus labelling. Corresponding overlay of Thioflavin T and nucleus staining is shown on merged images. Cells treated with 1 µM thapsigargin for 12 h served as a positive control
The accumulation of misfolded or unfolded proteins represents a reliable marker of ER stress [36, 56]. Cell may undergo apoptosis if the accumulation of misfolded or unfolded proteins is not resolved [56]. Thus, to qualify the effects of laser treatment on protein aggregation, we stained Huh7 and Alexander cells with specific thioflavin T dye. Thioflavin T is a fluorescence dye that reacts specifically with aggregated proteins and is used for the determination of their aggregations [36]. Indeed, thioflavin T analysis revealed no significant changes in accumulation of unfolded proteins after 15 min of either blue or red laser treatment (Fig. 4c). Importantly, 15 min laser irradiation by either blue or red wavelength resulted in dramatic dissipation of ΔmΦ in Huh7 and Alexander cells (Fig. 3b). Taking these data together, one can conclude that neither lysosomal dysfunction nor ER stress trigger initiation of cellular responses upon blue or red laser irradiation. Thus, it is evident that mitochondria play a pivotal role in the laser-induced cellular damage.
Laser irradiation modulates the activity of mitochondrial respiratory chain in Huh7 and Alexander cells
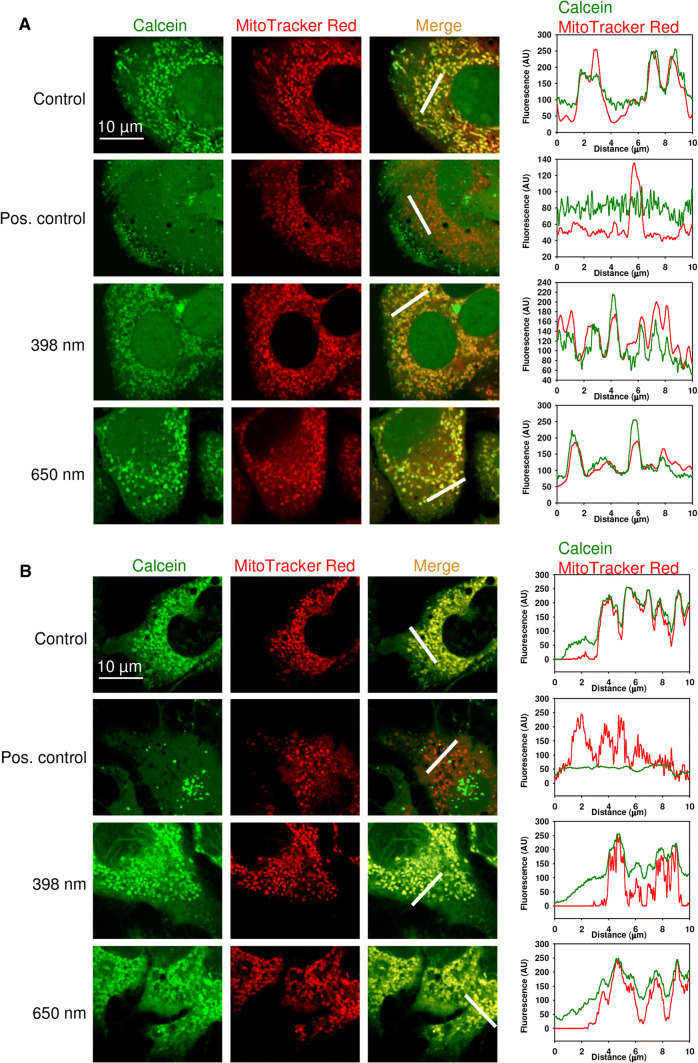
Furthermore, to verify the role of mitochondria dysfunction in laser-induced damage, we analyzed mPTP opening upon laser treatment in Huh7 and Alexander cells. To assess this question, we utilized technique based on calcein AM loading and Co2+ quenching [37, 38]. Under physiological conditions calcein freely diffuses to all subcellular compartments, contrary its quencher (Co2+) is excluded from the mitochondrial matrix. As a consequence, mitochondria appear as brightly fluorescent spots under confocal microscopy [37, 38]. When cells are treated with ionophores that promote either inner mitochondrial membrane (IMM) permeabilization or mPTP opening, Co2+ gain access to the mitochondrial matrix. The latter results in calcein signal quenching in mitochondria [37, 38].
As shown in Fig. 5 and Figure S6, in control cells, calcein fluorescence remained constant and co-localizes with mitochondria, while cells treated with ionomycin had a significant fluorescence reduction and dissipation of calcien fluorescence from mitochondrial compartments. Blue or red laser treatment were comparable to the control fluorescence of calcein, that co-localized with mitochondria in Huh7 and Alexander cells (Figs. 5 and S6). These data indicate that neither blue nor red laser treatment resulted in mPTP opening, which is dependent on IMM permeabilization. Importantly, blue and red laser irradiation resulted in rapid dissipation of ΔmΦ (Figs. 2b, 3b), excessive mitochondrial ROS production (Fig. 3c) and massive fission of mitochondria (Figs. 2b, 3a and S3B) in Huh7 and Alexander cells. Moreover, blue and red laser treatment triggered apoptosis in Huh7 and Alexander cells (Fig. 1b). All these data summarized together indicate that blue and red laser irradiation results in permeabilization of the mitochondrial outer membrane which leads to apoptosis.
Fig. 5.
HFLP laser irradiation does not induce mitochondrial inner membrane permeabilization. a Alexander cells were irradiated with different wavelengths of laser (398, 650 nm; 50 µW) for 30 min. Representative confocal microscopic images and linescans after incubation with calcein-AM (1 µM, green) and MitoTracker® red (200 nM, red) in the presence of Co2+ (1 mM), which quenches calcein fluorescence (green) outside of mitochondria. MitoTracker® red confirms the localization of calcein fluorescence in mitochondria. Cells treated with 1 µM ionomycin solution for 20 min served as a positive control. b Huh7 cells were irradiated with different wavelengths of laser (398, 650 nm; 50 µW) for 30 min. Representative confocal microscopic images and linescans after incubation with calcein-AM (1 µM, green) and MitoTracker® red (200 nM, red) in the presence of Co2+ (1 mM), which quenches calcein fluorescence (green) outside of mitochondria. Cells treated with ionomycin as described in (a) were used as a positive control
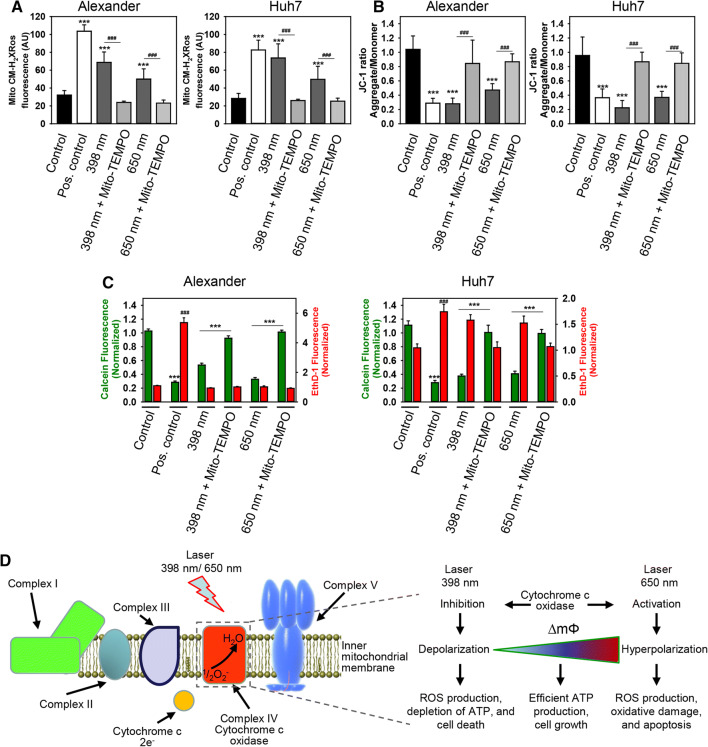
To further support role of mitochondria in laser-induced cell damage and subsequent death, we utilized mitochondria-targeted antioxidant mito-TEMPO to counteract phototoxicity. Mito-TEMPO is a compound, that is able to accumulate selectively in mitochondria [57]. This compound has been verified as potent mitochondria-targeted antioxidant with superoxide and alkyl radical scavenging properties [58–60]. First of all, mito-TEMPO inhibited blue and red laser-induced mitochondrial ROS accumulation in Huh7 and Alexander cells (Fig. 6a). Furthermore, supplementation of cell culture media with mito-TEMPO stabilized ΔmΦ and inhibited its dissipation induced by either blue or red laser irradiation (Fig. 6b). Finally, co-incubation with mito-TEMPO prevented laser-induced cell death in Huh7 and Alexander cells triggered by either blue or red laser irradiation (Fig. 6c). These results demonstrate a crucial role of mitochondria in laser-induced cell damage and suggest this organelle as a presumptive blue and red laser light ‘sensor’.
Fig. 6.
Mito-TEMPO prevented cell death induced under HFLP laser treatment. a Huh7 and Alexander cells were labeled with MitoTracker® red CM-H2XRos. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of Mito-TEMPO (20 µM) for 30 min. Quantitative analysis of MitoTracker® red CM-H2XRos fluorescence imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for control vs treatment comparison ***p < 0.001, Newman-Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. Non-irradiated cells treated with H2O2 (0.5 mM) were used as a ROS positive control. b Huh7 and Alexander cells were labeled with 1 μM JC-1. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of Mito-TEMPO (20 µM) for 30 min. Quantitative analysis of ΔmΦ imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for control vs treatment comparison ***p < 0.001, Newman-Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. As a positive control, cells were treated with 20% ethanol. c Huh7 and Alexander cells were labeled with calcein-AM (green) and ethidium homodimer (red). Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of Mito-TEMPO (20 µM) for 1 h. Control cells were untreated. As a positive control, cells were treated with 20% ethanol for 30 min. ImageJ software (NIH) was used for image processing and quantification. Fluorescence intensity of both dyes was measured and was normalized to total fluorescence of non-irradiated spot. Data are expressed as mean ± SEM (n = 3), Newman–Keuls test for control vs treatment comparison ***p < 0.001, Newman-Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. d Proposed model linking cytochrome c oxidase activity with the mitochondrial membrane potential and ROS production under healthy conditions and under HFLP laser treatment (398 and 650 nm)
Although end point effects (cytotoxicity, apoptosis execution) of blue and red laser irradiation of Huh7 and Alexander cells are similar, kinetics of mitochondrial ROS accumulation and dissipation of ΔmΦ showed significant differences upon blue and red laser treatment (Fig. 3b, c). Specifically, red laser treatment induced a spike of hyperpolarization of mitochondria, whereas blue laser—not (Fig. 3b). Blue laser resulted in higher levels and faster accumulation of mitochondrial ROS in comparison with red laser (Fig. 3c).
How may such substantially different kinetic events lead to similar phototoxic outcomes? Indeed, there is a model that links electron transport chain (ETC) activity, ΔmΦ, and the production of ROS [61]. According to this model, two distinct pathological conditions can lead to cell death regulated by mitochondrial dysfunction [61]. On one hand, strong cytochrome c oxidase (COX, the terminal oxidase of ETC [62]) inhibition leads to decreased ΔmΦ levels, energy depletion, ROS overproduction, and eventually cell death. On the other hand, hyperactivity of COX leads to a hyperpolarization of ΔmΦ, excessive ROS levels generation that results in extensive damage to the cell and triggers apoptosis [61]. Thus, we hypothesized that laser irradiation may change ETC activity via modulation of COX (Fig. 6d). We propose a tentative model of laser action on mitochondria, where blue laser inhibits COX resulting in cell death. Contrary red laser irradiation stimulates COX activity leading to hyperpolarization of ΔmΦ and subsequent apoptosis (Fig. 6d).
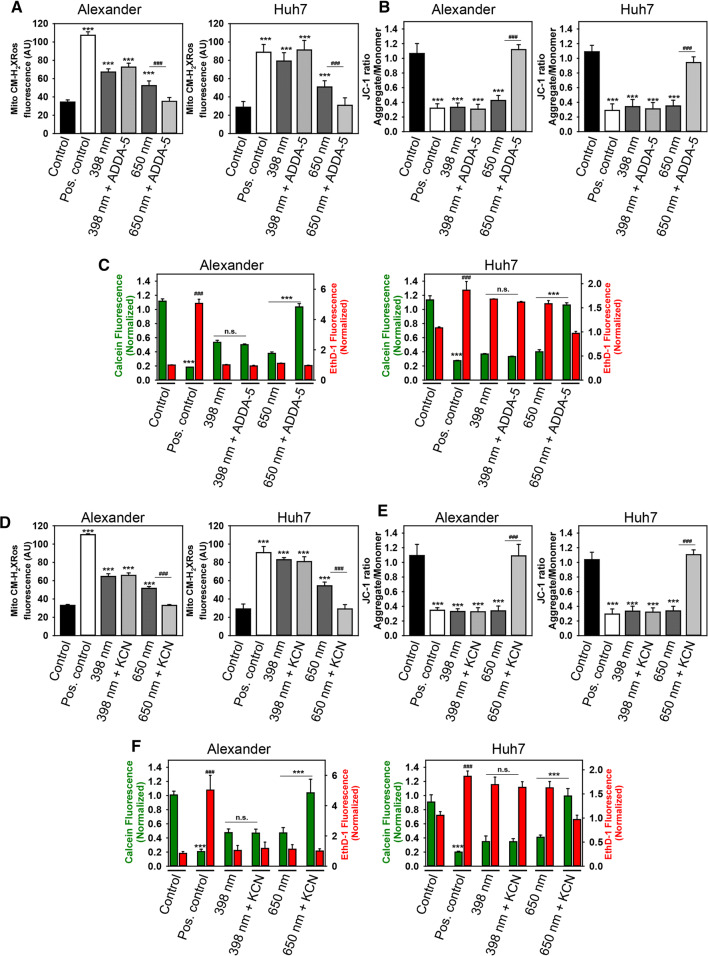
To verify our hypothesis, we employed non-selective (potassium cyanide, KCN [63]) and highly selective (ADDA-5 [64]) COX inhibitors. First, we experimentally selected appropriate concentrations of inhibitors by assessing their cytotoxicity (Fig. S7). Co-incubation of Huh7 and Alexander cells with either KCN or ADDA-5 inhibited red but not blue laser-induced mitochondrial ROS accumulation (Fig. 7a, d). Both inhibitors prevented ΔmΦ dissipation induced by red laser irradiation, whereas they did not stabilize ΔmΦ under blue laser treatment (Fig. 7b, e). Finally, the analysis of cell viability showed that when the activity of COX was inhibited by either KCN or ADDA-5, the cell death induced by red laser irradiation was completely prevented (Fig. 7c, f). However, although n.s. the trend is for cell survival for blue laser light in Alexander cells (Fig. 7c, f). These data are in line with the model proposed in [61] and our tentative scheme that links cytochrome c oxidase activity with the mitochondrial membrane potential and ROS production under healthy conditions and under HFLP laser treatment (Fig. 6d). To further validate our hypothesis, we utilized non-selective COX activator (bezafibrate, BZF). BZF has been shown to increase COX activity both in vitro and in vivo [65–67]. We assessed BZF cytotoxicity and selected treatment concentration of 1 mM that has minimal cytotoxic effects (Fig. S8A). Indeed, the treatment of Alexander and Huh7 cells with BZF resulted in mitochondrial hyperpolarization (Fig. S8B) and elevated levels of mitochondrial ROS (Fig. S8C). In fact, the irradiation of BZF-treated Alexander and Huh7 cells with blue laser prevented mitochondrial hyperpolarization (Fig. S8B) and normalized mitochondrial ROS accumulation (Fig. S8C). These date clearly confirm that blues laser irradiation results in COX inhibition.
Fig. 7.
Inhibition of cytochrome c oxidase protects from cell death induced by HFLP red laser. a Huh7 and Alexander cells were labeled with MitoTracker® red CM-H2XRos. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of ADDA-5 (15 µM) for 30 min. Quantitative analysis of MitoTracker® red CM-H2XRos fluorescence imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for control vs treatment comparison ***p < 0.001, Newman-Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. Non-irradiated cells treated with H2O2 (0.5 mM) were used as a ROS positive control. b Huh7 and Alexander cells were labeled with 1 μM JC-1. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of ADDA-5 (15 µM) for 30 min. Quantitative analysis of ΔmΦ imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for control vs treatment comparison ***p < 0.001, Newman–Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. As a positive control, cells were treated with 20% ethanol. c Huh7 and Alexander cells were labeled with calcein-AM (green) and ethidium homodimer (red). Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of ADDA-5 (15 µM) for 1 h. Control cells were untreated. As a positive control, cells were treated with 20% ethanol for 30 min. ImageJ software (NIH) was used for image processing and quantification. Fluorescence intensity of both dyes was measured and was normalized to total fluorescence of non-irradiated spot. Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for calcein-AM comparison ***p < 0.001, Newman-Keuls test for ethidium homodimer comparison ###p < 0.001. d Huh7 and Alexander cells were labeled with MitoTracker® red CM-H2XRos. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of KCN (5 mM) for 30 min. Cells analyzed as in (a). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for control vs treatment comparison ***p < 0.001, Newman-Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. e Huh7 and Alexander cells were labeled with 1 μM JC-1. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of KCN (5 mM) for 30 min. Cells analyzed as in (b). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for control vs treatment comparison ***p < 0.001, Newman-Keuls test for laser vs laser + Mito-TEMPO treatment comparison ###p < 0.001. f Huh7 and Alexander cells were labeled with calcein-AM (green) and ethidium homodimer (red). Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of KCN (5 mM) for 1 h. Cells analyzed as in (c). Data are expressed as mean ± SEM (n = 3), Newman-Keuls test for calcein-AM comparison ***p < 0.001, Newman-Keuls test for ethidium homodimer comparison ###p < 0.001
Summarizing the results, we can conclude that red laser induces activation of COX, on the contrary blue laser results in inhibition of COX. However, both excessive activation by red laser or inhibition by blue laser of COX lead to mitochondrial ROS overproduction, oxidative stress, and cell apoptosis.
Elevated expression of Bcl-2 protects from laser-induced cytotoxicity
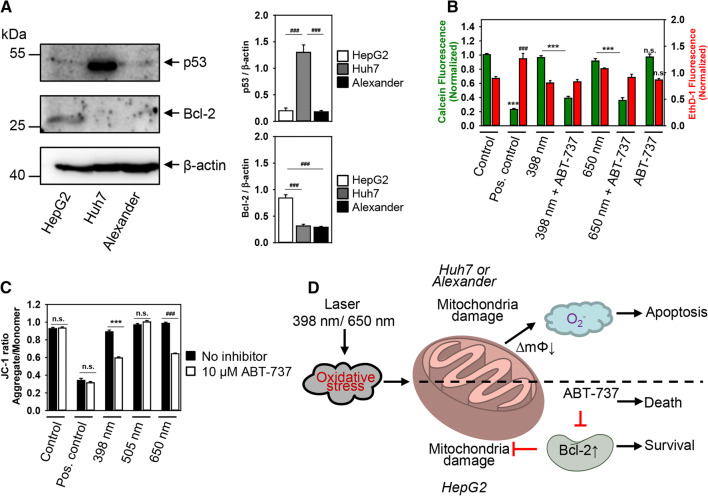
Our scheme nicely explained laser-induced cellular responses in Huh7 and Alexander cell, though it could not clarify why HepG2 were resistant to either laser wavelength. We hypothesized that such resistance of HepG2 could be explained by differences in expression of proteins that regulate mitochondria activity and apoptosis. Indeed, Huh7 cells showed the highest level of p53 protein expression, whereas HepG2 had elevated level of the anti-apoptotic Bcl-2 compared with Huh7 and Alexander cells as revealed by immunoblot analysis (Fig. 8a).
Fig. 8.
Inhibition of Bcl-2 sensitizes HepG2 to HFLP laser-induced death. a Bcl-2 and p53 were analyzed in whole cell lysates of HepG2, Huh7 and Alexander cells by immunoblotting; β-actin—control of equal protein loading. The graphs show densitometric quantification of p53 and Bcl-2 immunoblots. Data are expressed as mean ± SEM (n = 3), ###p < 0.001. b HepG2 cells were labeled with calcein AM (green) and ethidium homodimer (red). Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) in the presence or absence of ABT-737 (10 µM) for 1 h. Control cells were untreated. As a positive control, cells were treated with 20% ethanol for 30 min. ImageJ software (NIH) was used for image processing and quantification. Fluorescence intensity of both dyes was measured and was normalized to total fluorescence of non-irradiated spot. Data are expressed as mean ± SEM (n = 3), ***p < 0.001, ###p < 0.001. c HepG2 cells were labeled with 1 μM JC-1. Labeled cells were exposed to HFLP laser (398, 505 and 650 nm nm; 50 µW) in the presence or absence of ABT-737 (10 µM) for 30 min. Quantitative analysis of ΔmΦ imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), ***p < 0.001, ###p < 0.001. As a positive control, cells were treated with 20% ethanol. d Scheme of redox signaling activation in distinct cell lines after stimulation with different laser wavelengths. ∆mΦ—mitochondrial membrane potential
Bcl-2 and p53 are crucial proteins that control cell death programs through multiple pathways and participate in redox sensing via modulation of mitochondrial activity [68–71]. Huh7 cells show a large difference in total p53 expression in comparison with Alexander cell (Fig. 8a). It is worth noting here that p53 can translocate to the mitochondria and sensitize cells to oxidative stress [72]. However, Huh7 and Alexander cells show very similar cytotoxicity profile (Fig. 1) and mitochondrial dysfunction (Figs. 2, 3, 5) upon treatment to blue and red lasers. Moreover, although Huh7 express a mutated form of p53 that is abnormally stable and lacks transcriptional activity [73, 74], Huh7 cells show high sensitivity to laser-induced cytotoxicity (Fig. 1) and oxidative burst (Fig. 3). Therefore, we can postulate that p53 is very unlikely to mediate laser-induced cellular responses.
Contrary, Bcl-2 expression is similar in Huh7 and Alexander cells, but very distinct in HepG2 showing elevated levels (Fig. 8a). In general, it is well known that Bcl-2 family proteins act as global regulators of mitochondrial homeostasis [75]. Specifically, Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux [76]. As a result, cells with high levels of Bcl-2 are resistant to apoptosis-induced via dysregulation of mitochondrial function [77, 78]. Thus, we hypothesized that resistance of HepG2 to laser-induced cytotoxicity could be explained by elevated levels of Bcl-2 expression. To validate this, we used a potent Bcl-2 inhibitor (ABT-737 [79, 80]) to sensitize HepG2 cells to laser irradiation. Indeed, HepG2 cells co-incubated with ABT-737 showed significant cytotoxicity upon blue and red laser irradiation (Figs. 8b, S9). Importantly, only co-stimulation (blue or red laser in the presence of ABT-737) resulted in cell death of HepG2 (Figs. 8b). ABT-737 compound alone was not able to induce any significant toxicity (Figs. 8b, S8). Furthermore, either blue or red laser irradiation in the presence of ABT-737 resulted in dramatic dissipation of ΔmΦ (Fig. 8c).
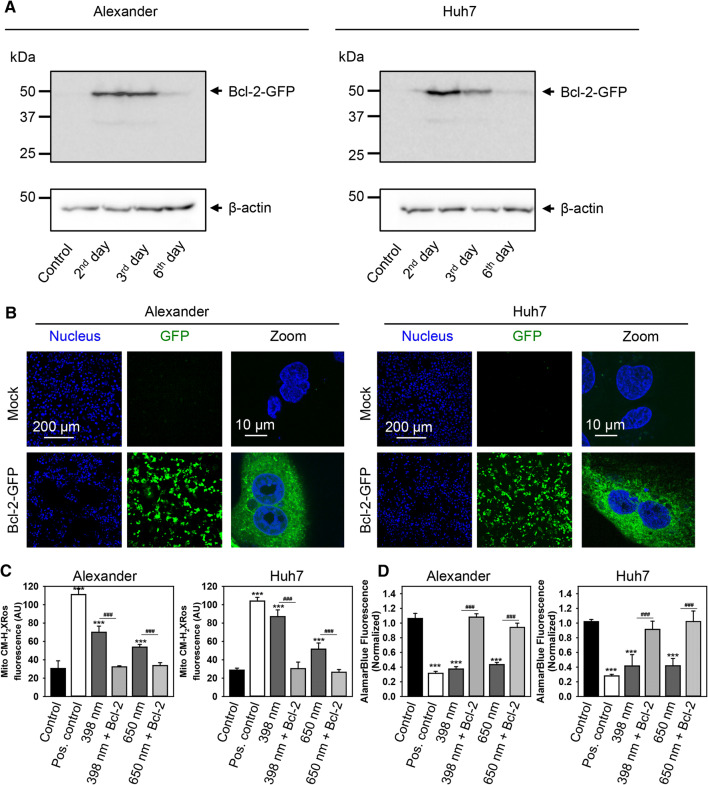
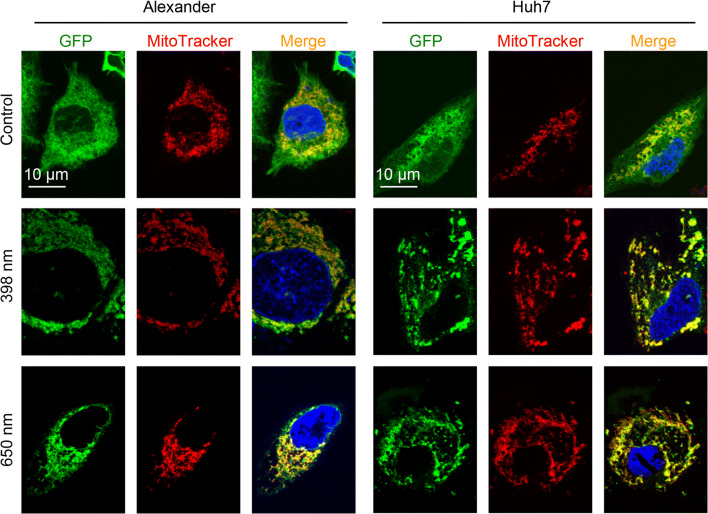
All these data together show that Bcl-2 contracts mitochondrial damage induced by blue or red laser irradiation and promotes cell survival (Fig. 8d). However, to further prove the role of Bcl-2 in cellular resistance to blue and red laser irradiation, we overexpressed Bcl-2 in Huh7 and Alexander cells. We utilized reliable GFP-Bcl-2 plasmid 17999, provided by Clark Distelhorst [42]. First of all, we checked transfection efficiency and stabilized the transfection protocol for Huh7 and Alexander cells (Fig. 9a, b). The stabile highest expression of Bcl-2 was detected after 2 days of transfection (Fig. 9a). Indeed, Huh7 and Alexander cells with overexpressed Bcl-2 protein showed no significant mitochondrial ROS accumulation upon blue or red laser treatment (Fig. 9c). In addition, cells with overexpressed Bcl-2 were resistant to cell death induced by either blue or red laser irradiation (Fig. 9d). It is worth noting here that upon no laser treatment overexpressed Bcl-2 resided in cytosolic and mitochondrial compartments in transfected Huh7 and Alexander cells (Fig. 10). Contrary upon blue and red laser treatment Bcl-2 translocated predominantly to mitochondria (Fig. 10). Indeed, it is well known that up-regulated Bcl-2 protects against apoptotic cell death by translocation to mitochondria [70, 81, 82]. In fact, Bcl-2 translocated to mitochondria antagonizes there pro-apoptotic Bax, Bak, and Bid proteins [81, 82]. Taken together, these data clearly confirmed a major role of Bcl-2 in regulation of mitochondrial stability and resistance to cytotoxicity upon blue and red laser irradiation.
Fig. 9.
Overexpression of Bcl-2 protects from cell death induced by HFLP laser. a Transient overexpression of Bcl-2 in transfected Alexander and Huh7 cells. Immunoblot detection of Bcl-2 in cells 2, 3, and 6 day post-transfection; β-actin—control of equal protein loading. b Overexpression of GFP-tagged Bcl-2 in transfected Alexander and Huh7 cells. Representative confocal images of GFP-tagged Bcl-2 in living Huh7 and Alexander cells 2 days post-transfection. Hoechst 33,342 stain (blue) was used for nucleus labelling. c Mock or GFP-tagged Bcl-2 transfected Huh7 and Alexander cells were labeled with MitoTracker® red CM-H2XRos. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) for 30 min. Quantitative analysis of MitoTracker® red CM-H2XRos fluorescence imaged by laser scanning confocal microscopy was performed utilizing LaserSharp 2000 software (BioRad). Data are expressed as mean ± SEM (n = 3), ***p < 0.001, ###p < 0.001. Non-irradiated cells treated with H2O2 (0.5 mM) were used as a ROS positive control. Mock or GFP-tagged Bcl-2 transfected Huh7 and Alexander cells were labeled with alamarBlue Cell Viability Reagent. Labeled cells were exposed to HFLP laser (398 and 650 nm; 50 µW) for 1 h. Control cells were untreated. As a positive control, cells were treated with 20% ethanol for 30 min. ImageJ software (NIH) was used for image processing and quantification. Fluorescence intensity of alamarBlue was measured and was normalized to total fluorescence of non-irradiated spot. Data are expressed as mean ± SEM (n = 3), ***p < 0.001, ###p < 0.001
Fig. 10.
Representative confocal images of GFP-tagged Bcl-2 translocation to mitochondria upon laser treatment in living Huh7 and Alexander cells GFP-tagged Bcl-2 transfected Huh7 and Alexander cells were exposed to HFLP laser (398 and 650 nm; 50 µW) for 20 min. Cells were labeled with MitoTracker® Red. Hoechst 33,342 stain (blue) was used for nucleus labelling
Discussion
Accumulative evidence suggests that even low power (~ 40–50 µW), but high fluence lasers may induce ROS production and phototoxicity in different cell types [18–20, 22, 23, 83]. Despite extensive research, precise biochemical mechanisms underlying cell-laser interaction are not well established [2, 14, 22]. Furthermore, the majority of studies focus on cellular responses to red and/or NIR light at 600–1000 nm wavelength [18–20, 22, 23, 83]. Only tiny fraction introduces comparative analysis of cellular effects in response to blue or green laser irradiation.
In general, a number of previous studies demonstrated that HFLP laser irradiation-induced cell apoptosis via mitochondrial dysfunction [18–20, 22, 23, 83]. Mitochondria have been implicated to play a crucial role in both necrotic and apoptotic cell death [51, 84]. Indeed, we and others previously showed that it is possible to modulate mitochondria activity by laser triggering either apoptosis or necrosis depending on laser power used [20, 21]. In fact, in light of the previous studies, we and others identified mitochondria as one of cellular ‘effectors’ of laser irradiation [18–23, 83]. However, the question, whether laser-induced cellular responses are initiated at mitochondrial, remained unexplained.
First of all, here, we compared laser-induced responses in different hepatic cell lines. Moreover, we introduced analysis of cellular reactions to different wavelengths having the same power and irradiance. Not surprisingly, we found that different cell lines react differently to the same type of laser irradiation (Fig. 1a). HepG2 were resistant to laser-induced toxicity. Huh7 and Alexander cells showed similar cytotoxicity profile (Fig. 1). In fact, we found that only blue or red but not green laser irradiation resulted in apoptotic cell death of both Huh7 and Alexander cells (Fig. 1). Indeed, there are a limited number of studies analyzing green laser phototoxic effects [19, 22]. Most of published data in line with our findings emphasize non-harmful cellular responses (migration, differentiation) [22, 85, 86]. It is worth mentioning here that we do not exclude phototoxic effects of green laser with higher powers and/or fluencies. For instance, toxic effects of green laser have been observed in [19] with much higher irradiance, e.g., 3 kW/cm2 compared to ours < 1 kW/cm2.
Furthermore, we showed that blue and red laser irradiation result in dramatic depolarization of ΔmΦ and accompanied mitochondrial dysfunction in Huh7 and Alexander cells (Fig. 2). Mounting body of evidence from similar studies shows the same cellular responses to blue and red laser irradiation [18–20, 22, 23, 83]. However, red light has a photon energy which is smaller than blue light. Thus, there is an obvious question. How can photons with different energy result in similar cellular outcomes? Therefore, we assessed kinetics of mitochondrial potential changes upon laser irradiation. We found that red laser irradiation induced a spike of hyperpolarization of mitochondria, whereas blue laser resulted in rapid dissipation of ΔmΦ in Huh7 and Alexander cells (Fig. 3b). In addition, blue laser irradiation induced faster and higher levels of mitochondrial ROS accumulation in both cell lines in comparison with red laser irradiation (Fig. 3c).
In fact, enzymes of electron transport chain mediate mitochondrial membrane potentials [61, 62]. Hüttemann et al. proposed a model according to which both hypo- and hyperactive ETC complexes lead to excessive ROS production and cell death [61]. COX plays a central role in this model. When COX is inhibited, this results in the decrease of ΔmΦ levels, energy depletion, ROS overproduction, and eventually cell death. Hyperactivity of COX initially triggers a hyperpolarization of ΔmΦ, but then elevates ROS levels generation that results in extensive oxidative damage and cell death [61]. Importantly, a number of studies suggested that the mechanism of HFLP laser irradiation at the cellular level is based on the absorption of light by COX [22, 23, 28]. In fact, COX has two major absorption bands, one in the blue (~ 380–420 nm) and another red spectral region (∼ 600–660 nm), which consequently are the wavelengths most often used in PBM [1, 87]. In addition, there are other minor NIR (~ 800 nm) absorbance bands of COX [1, 87]. Thus, we hypothesized that blue laser may inhibit COX activity, whereas red laser results in hyperactivation of COX.
Currently, we found that co-incubation of Huh7 and Alexander cells with two structurally different inhibitors of COX inhibited red but not blue laser-induced mitochondrial ROS accumulation (Fig. 7a, d). In addition, specific COX inhibition by ADDA-5 prevented ΔmΦ dissipation induced by red laser irradiation, whereas it did not stabilize ΔmΦ under blue laser treatment (Fig. 7b, e). Furthermore, when the activity of COX was inhibited, cell death induced by red but not blue laser treatment was prevented in both Alexander and Huh7 cells (Fig. 7c, f). Our findings are strongly in line with those showing that blue spectral region (350–420 nm) laser irradiation leads to inhibition of COX [88–91]. Other studies revealed that red (600–700 nm) laser treatment resulted in a significant increase in COX activity [6, 92–94]. Furthermore, COX knockdown in human lung ASTC-a-1 cells completely inhibited red laser-induced apoptosis [23]. Taking together here presented data with aforementioned previously published studies, we can conclude, that COX plays a role of photoacceptor in cells with higher numbers of mitochondria. Blue laser irradiation leads to COX inhibition, whereas red laser treatment increases activity of COX (Fig. 6d). However, both excessive inhibition or activation of COX results in ETC dysregulation, elevated ROS production and execution of apoptosis (Fig. 6d).
In general, it has been postulated that different cell types exhibit a very distinct irradiation sensitivity [19, 22]. However, molecular mechanisms which drive sensitivity to laser irradiation were not fully elucidated. Here, we showed that elevated level of Bcl-2 expression mitigates laser-induced phototoxicity. HepG2 cells with high level of Bcl-2 protein were not sensitive to blue or red laser-induced cell death (Fig. 1a). In fact, pharmacological inhibition of Bcl-2 sensitized HepG2 to blue and red laser-induced mitochondrial depolarization (Fig. 8c) and subsequent death (Fig. 8b).
Mounting evidence suggests that elevated levels or overexpression of Bcl-2 protein prevents the decrease in mitochondrial membrane potential, subsequent ROS production, and oxidative stress-mediated cell death [70, 71, 77, 78, 95]. Mechanistically, Bcl-2 inhibits both the activator BH3-only proteins and the pore-forming proteins by mutual sequestration, in this way regulating proton flux and preventing apoptotic mitochondrial dysfunction [70, 76]. As a result, it was shown that overexpression of Bcl-2 in different cell lines was able to protect against oxidative stress induced by hydrogen peroxide, small ligands, and UV light [70, 71, 77, 96, 97]. In fact, numerous studies have pointed toward mitochondrial dysfunction as a reason for laser-induced cell death [18–20, 22, 23, 83]. However, only few evaluated mechanistic aspects of laser-induced cellular responses using genetic modifications [23]. Indeed, we confirmed protective role of Bcl-2 against laser-induced cell death by overexpression of Bcl-2 protein in Huh7 and Alexander cells (Fig. 9c, d).
Concluding remarks
In conclusions, our study unambiguously identifies mitochondria as a laser light “sensor” and “effector”. Furthermore, we show here that genetic background of cells, used as a models of laser irradiation studies, predisposes the outcomes. We demonstrate that despite blue and red laser irradiation resulting in similar apoptotic death—cellular signaling and kinetics of biochemical responses are distinct. Our data indicate that blue laser irradiation leads to inhibition of COX activity in ETC of mitochondria. Contrary, red laser triggers COX excessive activation. However, both processes lead to ROS overproduction, oxidative stress and apoptosis. Using pharmacological inhibition and genetic expression approaches, we revealed a crucial role of Bcl-2 protein on laser-induced cytotoxicity. Indeed, Bcl-2 protein inhibits laser-induced toxicity by stabilizing mitochondria membrane potential. In fact, our study not only shows different scenarios of laser-induced cell death, but also reveals an important interplay between mitochondrial dysfunction and apoptosis triggered by different lasers.
In summary, we have demonstrated that the knowledge of molecular mechanisms of laser-induced phototoxicity may provide a pharmacological basis to ameliorate them. Thus, our study may lead to the design of new laser-based approaches for remote control of mitochondrial ETC in the absence of chemical or biological agents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work is supported by Operational Programme Research, Development and Education financed by European Structural and Investment Funds and the Czech Ministry of Education, Youth and Sports (Project No. SOLID21—CZ.02.1.01/0.0/0.0/16_019/0000760), the Ministry of Science and Higher Education of the Russian Federation, goszadanie no 8.3134.2017/4.6 and MH CZ—DRO Institute for Clinical and Experimental Medicine—IKEM, IN 00023001.
Abbreviations
- HFLP
High fluence low-power
- ROS
Reactive oxygen species
- COX
Cytochrome c oxidase
- MOMP
Permeabilization of the mitochondrial outer membrane
- PMB
Photobiomodulation
- ETC
Electron transport chain
- IMM
Inner mitochondrial membrane
- SIM
Structured illumination microscopy
- ΔmΦ
Mitochondrial membrane potential
- ER
Endoplasmic reticulum
- mPTP
Mitochondrial permeability transition pore
- LLLT
Low‐level light therapy
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun SH, Kwok SJJ. Light in diagnosis, therapy and surgery. Nat Biomed Eng. 2017;1:0008. doi: 10.1038/s41551-016-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 5.Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC, Padwa BL, Hamblin MR, Barcellos-Hoff MH, Kulkarni AB, Mooney DJ. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci Transl Med. 2014;6(238):238ra269. doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci USA. 2003;100(6):3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Sousa MVP, Kawakubo M, Ferraresi C, Kaippert B, Yoshimura EM, Hamblin MR. Pain management using photobiomodulation: mechanisms, location, and repeatability quantified by pain threshold and neural biomarkers in mice. J Biophotonics. 2018;11(7):e201700370. doi: 10.1002/jbio.201700370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos L, Olmo-Aguado SD, Valenzuela PL, Winge K, Iglesias-Soler E, Arguelles-Luis J, Alvarez-Valle S, Parcero-Iglesias GJ, Fernandez-Martinez A, Lucia A. Photobiomodulation in Parkinson’s disease: a randomized controlled trial. Brain Stimul. 2019;12(3):810–812. doi: 10.1016/j.brs.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Lavery LA, Murdoch DP, Williams J, Lavery DC. Does anodyne light therapy improve peripheral neuropathy in diabetes? A double-blind, sham-controlled, randomized trial to evaluate monochromatic infrared photoenergy. Diabetes Care. 2008;31(2):316–321. doi: 10.2337/dc07-1794. [DOI] [PubMed] [Google Scholar]
- 10.Arnall DA, Nelson AG, Lopez L, Sanz N, Iversen L, Sanz I, Stambaugh L, Arnall SB. The restorative effects of pulsed infrared light therapy on significant loss of peripheral protective sensation in patients with long-term type 1 and type 2 diabetes mellitus. Acta Diabetol. 2006;43(1):26–33. doi: 10.1007/s00592-006-0207-5. [DOI] [PubMed] [Google Scholar]
- 11.Brosseau L, Robinson V, Wells G, Debie R, Gam A, Harman K, Morin M, Shea B, Tugwell P. Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.cd002049.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Ma J, Chen J, Shen B, Pei F, Kraus VB. The effectiveness of low-level laser therapy for nonspecific chronic low back pain: a systematic review and meta-analysis. Arthritis Res Ther. 2015;17:360. doi: 10.1186/s13075-015-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousefi-Nooraie R, Schonstein E, Heidari K, Rashidian A, Pennick V, Akbari-Kamrani M, Irani S, Shakiba B, Mortaz Hejri SA, Mortaz Hejri SO, Jonaidi A. Low level laser therapy for nonspecific low-back pain. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.cd005107.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolkova B, Uzhytchak M, Lynnyk A, Kubinova S, Dejneka A, Lunov O. A critical review on selected external physical cues and modulation of cell behavior: magnetic nanoparticles, non-thermal plasma and lasers. J Funct Biomater. 2019;10(1):2. doi: 10.3390/jfb10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsych Dis Treat. 2015;11:2191–2208. doi: 10.2147/Ndt.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denton ML, Foltz MS, Estlack LE, Stolarski DJ, Noojin GD, Thomas RJ, Eikum D, Rockwell BA. Damage thresholds for exposure to NIR and blue lasers in an in vitro RPE cell system. Invest Ophthalmol Vis Sci. 2006;47(7):3065–3073. doi: 10.1167/iovs.05-1066. [DOI] [PubMed] [Google Scholar]
- 17.Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, Dewhirst MW. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia. 2011;27(4):320–343. doi: 10.3109/02656736.2010.534527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, Xing D, Gao X, Chen WR. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol. 2009;218(3):603–611. doi: 10.1002/jcp.21636. [DOI] [PubMed] [Google Scholar]
- 19.Waldchen S, Lehmann J, Klein T, van de Linde S, Sauer M. Light-induced cell damage in live-cell super-resolution microscopy. Sci Rep. 2015;5:15348. doi: 10.1038/srep15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golovynska I, Golovynskyi S, Stepanov YV, Garmanchuk LV, Stepanova LI, Qu J, Ohulchanskyy TY. Red and near-infrared light induces intracellular Ca(2+) flux via the activation of glutamate N-methyl-d-aspartate receptors. J Cell Physiol. 2019;234:15989–16002. doi: 10.1002/jcp.28257. [DOI] [PubMed] [Google Scholar]
- 21.Lynnyk A, Lunova M, Jirsa M, Egorova D, Kulikov A, Kubinova S, Lunov O, Dejneka A. Manipulating the mitochondria activity in human hepatic cell line Huh7 by low-power laser irradiation. Biomed Opt Express. 2018;9(3):1283–1300. doi: 10.1364/BOE.9.001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zein R, Selting W, Hamblin MR. Review of light parameters and photobiomodulation efficacy: dive into complexity. J Biomed Opt. 2018;23(12):1–17. doi: 10.1117/1.JBO.23.12.120901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal. 2014;20(5):733–746. doi: 10.1089/ars.2013.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen GPC. Super-resolution microscopy demystified. Nat Cell Biol. 2019;21(1):72–84. doi: 10.1038/s41556-018-0251-8. [DOI] [PubMed] [Google Scholar]
- 25.Sahl SJ, Hell SW, Jakobs S. Fluorescence nanoscopy in cell biology. Nat Rev Mol Cell Biol. 2017;18(11):685–701. doi: 10.1038/nrm.2017.71. [DOI] [PubMed] [Google Scholar]
- 26.Artifacts of light Nat Methods. 2013;10(12):1135. doi: 10.1038/nmeth.2760. [DOI] [Google Scholar]
- 27.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 28.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol, B. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Lim KS, Harun SW, Arof H, Ahmad H. Fabrication and applications of microfiber. Sel Top Opt Fiber Technol. 2012 doi: 10.5772/2429. [DOI] [Google Scholar]
- 30.Papadopoulos NG, Dedoussis GV, Spanakos G, Gritzapis AD, Baxevanis CN, Papamichail M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177(1–2):101–111. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 31.Smolkova B, Lunova M, Lynnyk A, Uzhytchak M, Churpita O, Jirsa M, Kubinova S, Lunov O, Dejneka A. Non-thermal plasma, as a new physicochemical source, to induce redox imbalance and subsequent cell death in liver cancer cell lines. Cell Physiol Biochem. 2019;52(1):119–140. doi: 10.33594/000000009. [DOI] [PubMed] [Google Scholar]
- 32.Lunova M, Prokhorov A, Jirsa M, Hof M, Olzynska A, Jurkiewicz P, Kubinova S, Lunov O, Dejneka A. Nanoparticle core stability and surface functionalization drive the mTOR signaling pathway in hepatocellular cell lines. Sci Rep. 2017;7(1):16049. doi: 10.1038/s41598-017-16447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuliani T, Duval R, Jayat C, Schnebert S, Andre P, Dumas M, Ratinaud MH. Sensitive and reliable JC-1 and TOTO-3 double staining to assess mitochondrial transmembrane potential and plasma membrane integrity: interest for cell death investigations. Cytometry A. 2003;54(2):100–108. doi: 10.1002/cyto.a.10059. [DOI] [PubMed] [Google Scholar]
- 35.Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol. 1996;135(3):611–622. doi: 10.1083/jcb.135.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beriault DR. Werstuck GH (2013) Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound. Thioflavin T. Bba-Mol Cell Res. 1833;10:2293–2301. doi: 10.1016/j.bbamcr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, Porter GA., Jr The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell. 2011;21(3):469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76(2):725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. 2015;95(4):1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lunov O, Zablotskii V, Churpita O, Lunova M, Jirsa M, Dejneka A, Kubinova S. Chemically different non-thermal plasmas target distinct cell death pathways. Sci Rep. 2017;7(1):600. doi: 10.1038/s41598-017-00689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lunov O, Syrovets T, Rocker C, Tron K, Nienhaus GU, Rasche V, Mailander V, Landfester K, Simmet T. Lysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytes. Biomaterials. 2010;31(34):9015–9022. doi: 10.1016/j.biomaterials.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276(47):44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton N. Quantification and its applications in fluorescent microscopy imaging. Traffic. 2009;10(8):951–961. doi: 10.1111/j.1600-0854.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 44.Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR J. 2002;43(4):207–213. doi: 10.1093/ilar.43.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landes T, Martinou JC. Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochim Biophys Acta. 2011;1813(4):540–545. doi: 10.1016/j.bbamcr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi S. Resolution doubling using confocal microscopy via analogy with structured illumination microscopy. Jpn J Appl Phys. 2016;55(8):082501. doi: 10.7567/jjap.55.082501. [DOI] [Google Scholar]
- 48.Hayashi S, Okada Y. Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics. Mol Biol Cell. 2015;26(9):1743–1751. doi: 10.1091/mbc.E14-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin SJ, Reutelingsperger CPM, Mcgahon AJ, Rader JA, Vanschie RCAA, Laface DM, Green DR. Early redistribution of plasma-membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122(7):2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 2011;1813(4):616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3(9):a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boya P, Cohen I, Zamzami N, Vieira HL, Kroemer G. Endoplasmic reticulum stress-induced cell death requires mitochondrial membrane permeabilization. Cell Death Differ. 2002;9(4):465–467. doi: 10.1038/sj/cdd/4401006. [DOI] [PubMed] [Google Scholar]
- 54.Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jaattela M, Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197(10):1323–1334. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim CY, Zoncu R. The lysosome as a command-and-control center for cellular metabolism. J Cell Biol. 2016;214(6):653–664. doi: 10.1083/jcb.201607005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 57.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107(1):106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Le Tissier S, Peng T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang HL, Sedlic F, Bosnjak Z, Nilakantan V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radic Biol Med. 2010;49(10):1550–1560. doi: 10.1016/j.freeradbiomed.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu M, Liu H, Dudley SC., Jr Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107(8):967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu GR, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K. Lee I (2012) Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta-Bioenerg. 1817;4:598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sazanov LA. A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol. 2015;16(6):375–388. doi: 10.1038/nrm3997. [DOI] [PubMed] [Google Scholar]
- 63.Pearce LL, Bominaar EL, Hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J Biol Chem. 2003;278(52):52139–52145. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- 64.Oliva CR, Markert T, Ross LJ, White EL, Rasmussen L, Zhang W, Everts M, Moellering DR, Bailey SM, Suto MJ, Griguer CE. Identification of small molecule inhibitors of human cytochrome c oxidase that target chemoresistant glioma cells. J Biol Chem. 2016;291(46):24188–24199. doi: 10.1074/jbc.M116.749978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casarin A, Giorgi G, Pertegato V, Siviero R, Cerqua C, Doimo M, Basso G, Sacconi S, Cassina M, Rizzuto R, Brosel S, Dimauro S, Schon EA, Clementi M, Trevisson E, Salviati L. Copper and bezafibrate cooperate to rescue cytochrome c oxidase deficiency in cells of patients with SCO2 mutations. Orphanet J Rare Dis. 2012;7:21. doi: 10.1186/1750-1172-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yatsuga S, Suomalainen A. Effect of bezafibrate treatment on late-onset mitochondrial myopathy in mice. Hum Mol Genet. 2012;21(3):526–535. doi: 10.1093/hmg/ddr482. [DOI] [PubMed] [Google Scholar]
- 67.Bastin J, Aubey F, Rotig A, Munnich A, Djouadi F. Activation of peroxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients’ cells lacking its components. J Clin Endocrinol Metab. 2008;93(4):1433–1441. doi: 10.1210/jc.2007-1701. [DOI] [PubMed] [Google Scholar]
- 68.Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787(5):414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116(Pt 20):4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 70.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25(1):65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montero J, Letai A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018;25(1):56–64. doi: 10.1038/cdd.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koczor CA, Torres RA, Fields EJ, Boyd A, Lewis W. Mitochondrial matrix P53 sensitizes cells to oxidative stress. Mitochondrion. 2013;13(4):277–281. doi: 10.1016/j.mito.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR, Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990;87(5):1973–1977. doi: 10.1073/pnas.87.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell JK, Midkiff BR, Israelow B, Evans MJ, Lanford RE, Walker CM, Lemon SM, McGivern DR. Hepatitis C virus indirectly disrupts DNA damage-induced p53 responses by activating protein kinase R. MBio. 2017;8(2):e00121-00117. doi: 10.1128/mBio.00121-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hollville E, Carroll RG, Cullen SP, Martin SJ. Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol Cell. 2014;55(3):451–466. doi: 10.1016/j.molcel.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci USA. 1998;95(4):1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni Z, Wang B, Dai X, Ding W, Yang T, Li X, Lewin S, Xu L, Lian J, He F. HCC cells with high levels of Bcl-2 are resistant to ABT-737 via activation of the ROS-JNK-autophagy pathway. Free Radic Biol Med. 2014;70:194–203. doi: 10.1016/j.freeradbiomed.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 78.Mirkovic N, Voehringer DW, Story MD, McConkey DJ, McDonnell TJ, Meyn RE. Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene. 1997;15(12):1461–1470. doi: 10.1038/sj.onc.1201310. [DOI] [PubMed] [Google Scholar]
- 79.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15(4):1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hikita H, Takehara T, Shimizu S, Kodama T, Shigekawa M, Iwase K, Hosui A, Miyagi T, Tatsumi T, Ishida H, Li W, Kanto T, Hiramatsu N, Hayashi N. The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology. 2010;52(4):1310–1321. doi: 10.1002/hep.23836. [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- 82.Susnow N, Zeng LY, Margineantu D, Hockenbery DM. Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol. 2009;19(1):42–49. doi: 10.1016/j.semcancer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moon J, Yun J, Yoon YD, Park SI, Seo YJ, Park WS, Chu HY, Park KH, Lee MY, Lee CW, Oh SJ, Kwak YS, Jang YP, Kang JS. Blue light effect on retinal pigment epithelial cells by display devices. Integr Biol. 2017;9(5):436–443. doi: 10.1039/c7ib00032d. [DOI] [PubMed] [Google Scholar]
- 84.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ong WK, Chen HF, Tsai CT, Fu YJ, Wong YS, Yen DJ, Chang TH, Huang HD, Lee OK, Chien S, Ho JH. The activation of directional stem cell motility by green light-emitting diode irradiation. Biomaterials. 2013;34(8):1911–1920. doi: 10.1016/j.biomaterials.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mason MG, Nicholls P. Cooper CE (2014) Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: implications for non invasive in vivo monitoring of tissues. Biochim Biophys Acta. 1837;11:1882–1891. doi: 10.1016/j.bbabio.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen E. Inhibition of cytochrome oxidase and blue-light damage in rat retina. Graefes Arch Clin Exp Ophthalmol. 1993;231(7):416–423. doi: 10.1007/BF00919652. [DOI] [PubMed] [Google Scholar]
- 89.Chen E, Soderberg PG, Lindstrom B. Cytochrome oxidase activity in rat retina after exposure to 404 nm blue light. Curr Eye Res. 1992;11(9):825–831. doi: 10.1111/j.1755-3768.1993.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 90.Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005;280(22):21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- 91.King A, Gottlieb E, Brooks DG, Murphy MP, Dunaief JL. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem Photobiol. 2004;79(5):470–475. doi: 10.1111/j.1751-1097.2004.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 92.Hu WP, Wang JJ, Yu CL, Lan CC, Chen GS, Yu HS. Helium-neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J Invest Dermatol. 2007;127(8):2048–2057. doi: 10.1038/sj.jid.5700826. [DOI] [PubMed] [Google Scholar]
- 93.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62(8):607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 94.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 95.Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000;20(15):5680–5689. doi: 10.1128/MCB.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crawford MJ, Krishnamoorthy RR, Rudick VL, Collier RJ, Kapin M, Aggarwal BB, Al-Ubaidi MR, Agarwal N. Bcl-2 overexpression protects photooxidative stress-induced apoptosis of photoreceptor cells via NF-kappaB preservation. Biochem Biophys Res Commun. 2001;281(5):1304–1312. doi: 10.1006/bbrc.2001.4501. [DOI] [PubMed] [Google Scholar]
- 97.Lum MG, Nagley P. Two phases of signalling between mitochondria during apoptosis leading to early depolarisation and delayed cytochrome c release. J Cell Sci. 2003;116(8):1437–1447. doi: 10.1242/jcs.00320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.