Abstract
Phosphorus (P), an essential macronutrient required for plant growth and development, is often limiting in natural and agro-climatic environments. To cope with heterogeneous or low phosphate (Pi) availability, plants have evolved an array of adaptive responses facilitating optimal acquisition and distribution of Pi. The root system plays a pivotal role in Pi-deficiency-mediated adaptive responses that are regulated by a complex interplay of systemic and local Pi sensing. Cross-talk with sugar, phytohormones, and other nutrient signaling pathways further highlight the intricacies involved in maintaining Pi homeostasis. Transcriptional regulation of Pi-starvation responses is particularly intriguing and involves a host of transcription factors (TFs). Although PHR1 of Arabidopsis is an extensively studied MYB TF regulating subset of Pi-starvation responses, it is not induced during Pi deprivation. Genome-wide analyses of Arabidopsis have shown that low Pi stress triggers spatiotemporal expression of several genes encoding different TFs. Functional characterization of some of these TFs reveals their diverse roles in regulating root system architecture, and acquisition and utilization of Pi. Some of the TFs are also involved in phytohormone-mediated root responses to Pi starvation. The biological roles of these TFs in transcriptional regulation of Pi homeostasis in model plants Arabidopsis thaliana and Oryza sativa are presented in this review.
Keywords: Pi deficiency, Model plants, Pi-deficiency responses, Pi acquisition, Mobilization, Transcriptional regulation
Introduction
Phosphorus (P), a key element for plant growth, is absorbed by root system in the form of inorganic phosphate (Pi) from soil matrices [1]. Due to slow diffusion rates in rhizospheres and/or fixation as immobile organic Pi or inorganic complexes, it is often a limiting nutrient leading to loss of crop productivity. Deciphering intricate adaptive mechanisms that plants have evolved for coping with heterogeneous or low Pi availability would be pivotal for developing crop plants amenable for enhanced Pi acquisition and use under low Pi conditions [2–4]. Model plants (Arabidopsis thaliana, Medicago truncatula, Oryza sativa, Phaseolus vulgaris, Solanum lycopersicum, Zea mays) are often used for elucidation of Pi-deficiency-mediated responses and several reviews have provided valuable insights [3–14]. Pi deficiency triggers an array of adaptive responses (altered root system, accumulation of anthocyanins, attenuated plant growth, secretion of organic acids, phosphatases and nucleases) facilitating enhanced Pi acquisition and utilization by plants [10, 15]. Genome-wide analyses using Affymetrix microarrays, suppression subtractive hybridization, and deep-sequencing techniques have led to the identification of several Pi-starvation-responsive (PSR) genes involved in acquisition, mobilization, and substitution of Pi, metabolic pathways, signal transduction, transcriptional regulation, and many processes related to growth and development [16–23]. In this review, we evaluate the regulatory influence of functionally characterized PSR genes encoding TFs from A. thaliana and O. sativa on various developmental, metabolic, and/or molecular responses that affect Pi homeostasis.
TFs regulating Pi-starvation responses
The identification and characterization of PHO regulon in unicellular prokaryotic and eukaryotic organisms and PSR1 in Chlamydomonas [24–28] provided the impetus to look for similar regulatory and signaling mechanisms in plant species. Increased Pi transport upon Pi starvation in roots and cultured cells of plants suggested the presence of a system analogous to the PHO regulon of yeast [29, 30]. Promoter analysis of a tomato phosphate starvation-induced (TPSI1) gene further revealed the presence of a cis-regulatory element (CACGTG/T) that was similar to the one found in the promoters of PSR genes of yeast [31–33]. Therefore, efforts were focused on the identification of TFs that could have positive or negative regulatory control on the hosts of PSR genes in plants. Transcriptome analyses revealed several PSR genes encoding TFs that were responsive to Pi deprivation and exhibited differential spatiotemporal expression profiles [16–18, 23, 34, 35]. However, transcriptome analysis alone is not sufficient for defining the potential roles of TFs in the maintenance of Pi homeostasis. It is essential to functionally characterize them by either suppressing (T-DNA knock-down or RNAi gene silencing) or overexpressing their genes for elucidation of their biological roles in acquisition and mobilization of Pi [36–38]. TFs non-responsive to different Pi regimes could also exert a regulatory control on the subset of molecular events that govern Pi homeostasis [22, 39, 40]. Here, accounts of several genes of Arabidopsis and rice encoding different TFs that have been characterized and shown to play a role in the maintenance of Pi homeostasis are presented
TFs constitutively induced irrespective of Pi status
Arabidopsis
PHR1 (At4g28610): This is one of the 15 members of the MYB-CC gene family in Arabidopsis and is localized in the nucleus irrespective of Pi status [39]. Gel mobility shift assay revealed the binding of PHR1 as a dimer to an imperfect palindromic 8-bp sequence (GNATATNC) called P1BS (PHR1-binding sequence) found in the promoters of many PSR genes [16, 18, 39, 41]. The P1BS motif has also been identified near MYCS, an arbuscular mycorrhizas (AM)-specific motif, and both motifs are pivotal for activation of AM-induced Pi transporters [42]. Promoter deletion analyses of Pi transporters from Arabidopsis (Pht1;4) and barley (HvPht1;1) have provided further evidence towards the role of this cis-regulatory motif in transcriptional regulation of PSR genes [43, 44]. SIZ1, PHO2, and microRNAs are important components of PHR1-mediated regulation of PSR genes [45, 46]. Interestingly, though, Pi-starvation-suppressed genes do not show enrichment with the P1BS motif [22].
PHR1-LIKE1 (PHL1): This is phylogenetically a close relative of PHR1 and together these two functionally redundant TFs act as key integrators of both specific and generic Pi-starvation responses [22]. The expression of PHL1 (At5g29000) overlapped with that of PHR1 in both shoots and roots under varying Pi regime [22].
Rice
OsPHR1 and OsPHR2: These are two homologs of AtPHR1 in rice that were isolated based on amino acid sequence identity, and their role as transcriptional activators was demonstrated by yeast two-hybrid assay [47]. Similar to AtPHR1, neither OsPHR1 (AK063486) nor OsPHR2 (AK100065) are responsive to Pi deprivation [47].
TFs induced by Pi deprivation
Arabidopsis
MYB62: This is a member of R2R3 type MYB TF family in Arabidopsis normally comprising three repeats of 52 amino acids, i.e., R1, R2, and R3 domains that bind DNA in a sequence-specific manner [48]. MYB62 (At1g68320) is induced specifically in the shoots during long-term Pi deprivation [16].
ZAT6: In Arabidopsis, Cys-2/His-2 (C2H2)-type zinc finger proteins (ZFPs) represent one of the largest families of putative transcriptional regulators [49]. ZAT6 along with most of the other C2H2-type zinc finger proteins contain a DLN-box/EAR motif in their C-terminus which functions as a transcription repression domain [37, 50]. Transcriptome analysis revealed the expression of ZAT6 (At5g04340) in shoots of long-term Pi-deprived seedlings [16], and a subsequent study showed a noticeable increase in its expression in both roots and shoots of Pi-deprived young seedlings [37].
WRKY6 and WRKY75: The Arabidopsis WRKY family comprises 74 members and some of them have been implicated in plant responses to biotic and abiotic stresses [36, 51, 52]. The C2H2 zinc finger domain of WRKY proteins regulates the spatiotemporal expression of their target genes by binding to TTGAC/T (W box) elements [51, 53]. One of the characteristic features of WRKY proteins is the ability of auto-and cross-regulating their own promoters and other WRKYs, respectively [54]. Promoters of many of the PSR genes have one or more W boxes [36]. For instance, two WRKY box (W-box) cis-elements (TTGACC/T) are found in Pht1;5 promoter [55]. Pi deficiency affects induction of both WRKY6 (At1g62300) and WRKY75 (At5g13080) [36, 52].
bHLH32: The basic helix-loop-helix (bHLH) proteins are a superfamily of TFs that bind as dimers to specific DNA target sites and are critical regulatory components in transcriptional networks. The bHLH motif consists of about 60 amino acids with two functionally distinct regions; the basic region at the N-terminal end of the domain facilitates DNA binding and the HLH region acts as a dimerization domain at the C-terminal end. BHLH32 (At3g25710) encodes bHLH TF [56], and microarray analysis showed its induction in leaves and roots of seedlings deprived of Pi for 48 h [34].
PRD (At1g79700): Transcriptomic and quantitative RT-PCR analyses showed Pi-deficiency-mediated regulation of genes encoding AP2/ERF TF PRD (phosphate root development) belonging to the AINTEGUMENTA-like (AIL) gene family [57, 58].
HRS1 (At1g13300): Bioinformatic analysis revealed that HRS1 and its six homologs (HHO1 to HHO6) encode G2-like TFs in Arabidopsis. G2-like TFs have a DNA binding domain originally identified in maize Golden 2 protein [59]. Transcriptome analysis showed the induction of HRS1 during long-term Pi deprivation [16]. HRS1 promoter-driven GUS activity was detected in root hairs under Pi-replete conditions, and Pi deficiency further augmented the reporter activity [60].
Rice
OsPTF1: This TF from rice is characterized by a basic helix-loop-helix (bHLH) domain. The expression of OsPTF1 (AY238991) was induced in Pi-deprived roots, while it remained constitutive in shoots [61].
Effects of mutation in TFs
The effects of mutation in TF on various morphophysiological and molecular responses are often used as yardsticks for evaluating its role in regulating the adaptive responses of the plant to different Pi regime [4, 62–65]. Here, we present an overview on the effects of a mutation in different TFs on Pi content and root traits (Table 1), developmental responses and accumulation of anthocyanin (Table 2), and expression of PSR genes (Table 3).
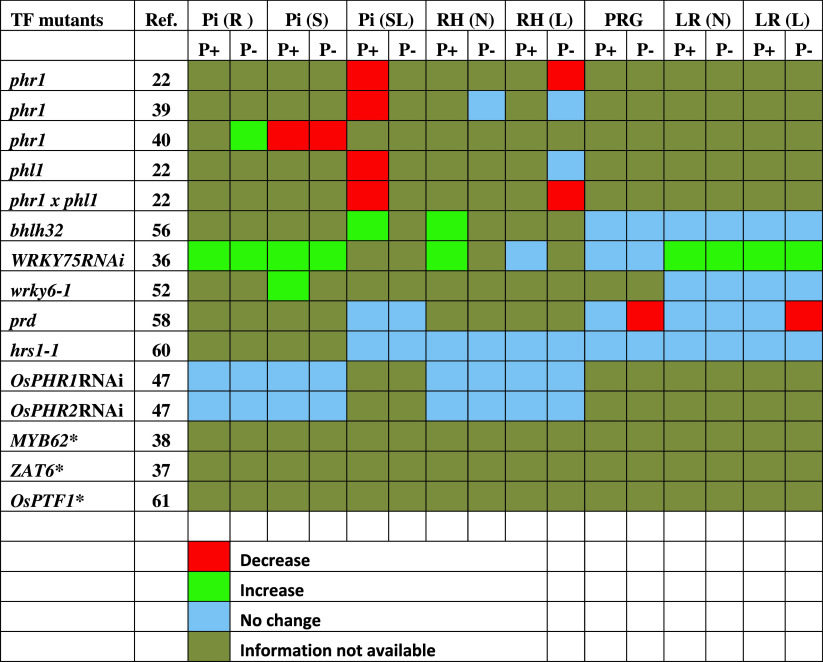
Table 1.
Effects of mutation in TFs on Pi content and different root traits
The effects of mutation in TFs on Pi content in roots (R), shoot (S), seedlings (SL), number [RH (N)] and length [RH (L)] of root hairs, primary root growth (PRG), and number [LR (N)] and length [LR (L)] of lateral roots in the mutants were compared with their corresponding wild-types grown under identical conditions
* The effects of overexpression have been reported but no data available for loss-of-function mutant
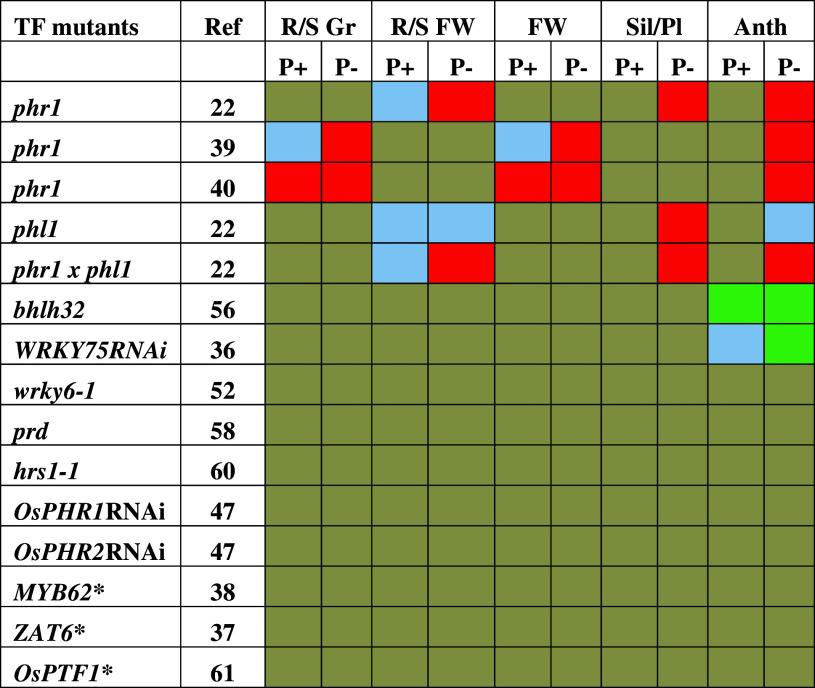
Table 2.
Effects of mutation in TF on developmental and metabolic traits
The effects of mutation in TFs on root/shoot growth ratio (R/S Gr), root/shoot fresh weight ratio (R/S FW), fresh weight (FW), number of siliques/plant (Sil/Pl), and anthocyanin accumulation (Anth) in the mutants were compared with their corresponding wild-types grown under identical conditions. The color code is the same as shown for Table 1
* The effects of overexpression have been reported but no data available for loss-of-function mutant
Table 3.
Effects of mutation in Arabidopsis TFs on molecular responses
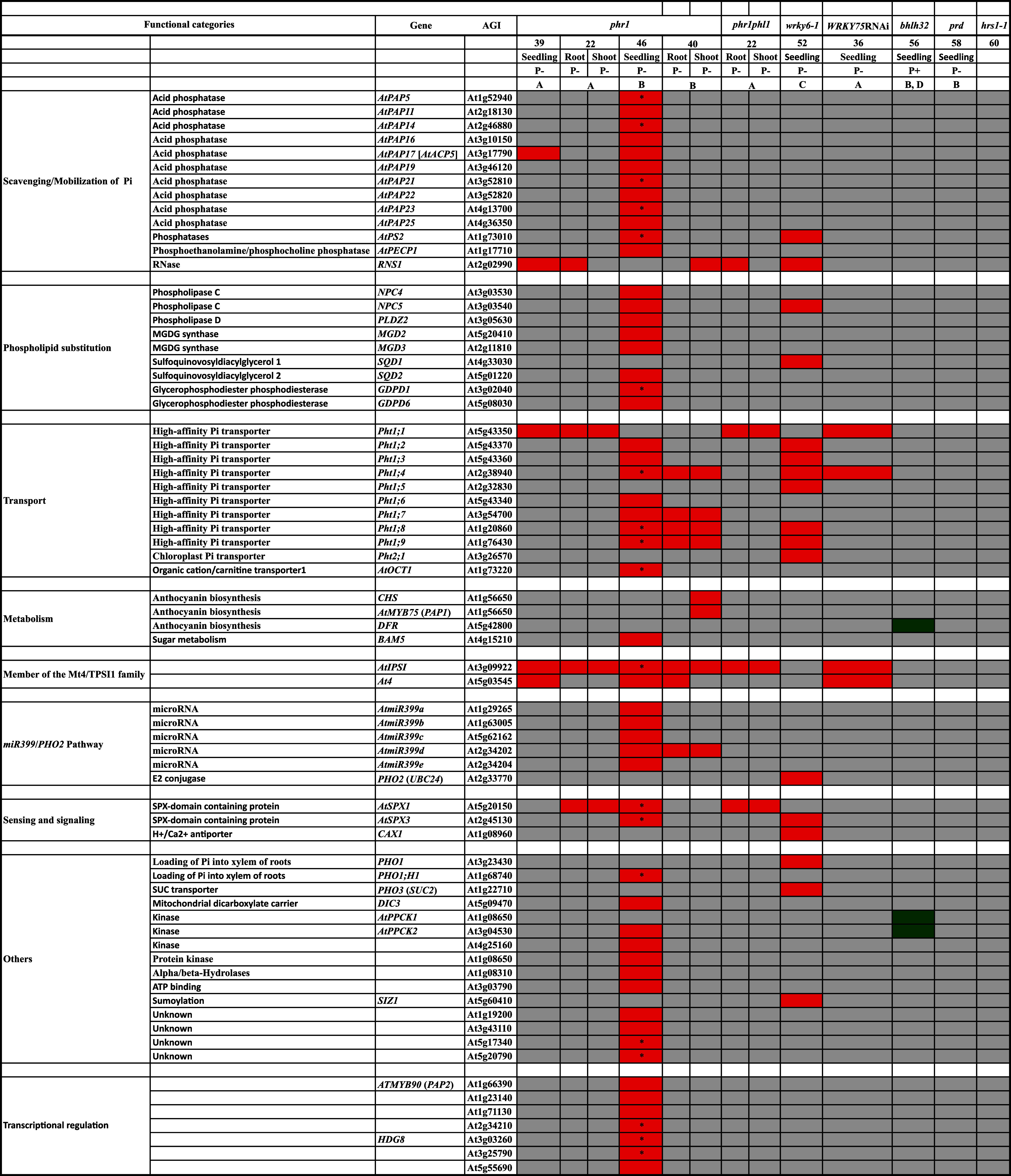
The effects of mutation in Arabidopsis TFs on molecular responses were compared with their corresponding wild-types grown under identical conditions. The molecular responses were detected by one or more molecular techniques involving Northern analysis (A), qRT-PCR (B), microarray (C), and/or RT-PCR (D). The color code is the same as used for Table 1
* Overlap in the suppressive effects of P−phr1 and P+pho2 on the expression of PSR genes [46]
Effects of mutation in TF on Pi content
The phr1 mutant isolated from an EMS mutagenized population showed significant reduction in Pi content of P+ seedlings compared to wild-type [22, 39]. Relatively, P+ phl1 mutant showed rather moderate reduction in Pi levels, while a more accentuated drop in Pi content was observed in P+ double mutant phr1 phl1. This suggested a prevalence of partial functional redundancy between these two MYB-CC family genes [22]. Interestingly, a phr1 mutant isolated from T-DNA insertion gene knock-out pools contained almost twice the amount of Pi in roots and 30 % less in shoots of Pi-deficient seedlings compared to the wild-type [40]. Since the phenotype of phr1 resembles the pho1 mutant defective in Pi loading, the likely involvement of PHR1 in both acquisition and mobilization of Pi from root to shoot can be assumed. Earlier study had shown a pivotal role of PHO1 in Pi loading to the xylem during Pi deprivation and a modest involvement of its homolog PHO1;H1 [66]. This suggested a likely regulatory effect of PHR1 on PHO1. However, contrary to this assumption, Pi-starvation-mediated induction of PHO1;H1, but not that of PHO1, was impaired in phr1 [66]. PHO1 appears thus to be regulated by a mechanism yet to be identified [40]. Irrespective of the Pi regime, significant increases in Pi content were noticed in roots and shoots of WRKY75RNAi plants [36]. The bhlh32 mutant seedlings revealed higher Pi content than the wild-type only under Pi replete condition [56]. A similar trend of higher Pi content in P+ shoots was observed for wrky6-1 [52], whereas, Pi content was comparable to the wild-type in prd and hrs1-1 mutants, and RNAi lines of OsPHR1 and OsPHR2 grown under different Pi regime [47, 58, 60].
The regulatory role of MYB62, if any, in Pi acquisition and accumulation could not be elucidated due to difficulties in identifying homozygous T-DNA insertion mutants or developing RNAi-lines [38]. Similarly, RNAi suppression of ZAT6 yielded lethal phenotype and therefore the effect of loss-of-function of this gene could not be functionally characterized [37]. Information on analysis of loss-of-function mutant of OsPTF1 is not yet available. Overall, the TFs characterized so far show variable regulatory influence on Pi contents of the mutant seedlings. Table 1 summarizes the effect of a mutation in TF on Pi content of roots, shoots, and/or seedlings grown under P+ and P− conditions. These functionally characterized TFs represent only a handful of those that have been identified from different transcriptome analyses of spatiotemporal responses to Pi deficiency in Arabidopsis and rice [16–18, 23, 34, 35, 67, 68]. Characterization of other TFs identified from these microarray databases could further add to our present understanding of their influence on Pi contents in roots and shoots of the plants grown under different Pi regime.
Effects of mutation in TF on local Pi-responsive root traits
Since mutation in different TFs led to variable effects on the spatial distribution of Pi, it is reasonable to assume that one or more of the root traits that play a pivotal role in Pi acquisition and mobilization is under their regulation. Here, we present a comparative analysis of the effects of mutation in different TFs on root traits that are responsive to local Pi availability.
Root hairs
Root hairs mediate the uptake of Pi by expanding the available absorptive surface area of root system thereby facilitating exploration of a larger soil area [69]. In Arabidopsis, there is only sparse development of root hairs under P+ condition whereas Pi deficiency triggers significant increases in both their number and length [69–74]. The significance of root hairs in Pi acquisition has been demonstrated by the conspicuous presence of depletion zones of 32P around root hairs [75], and by comparing Pi uptake efficacies of wild-type and a hairless Arabidopsis mutant [76]. Rapid elongation of Arabidopsis root hairs in areas of agar Petri plates containing a low concentration of Pi demonstrated the cell autonomous perception of local Pi availability independent of plant Pi status [5, 7]. Even though exogenous application of cytokinin resulted in the suppression of some of the PSR genes in the roots, it did not repress the Pi-starvation-mediated stimulation of root hair length [77]. This does suggest the existence of independent regulatory mechanisms governing systemic and local Pi sensing responses. Table 1 gives a comparative account of the effect of mutation in TFs on root hair development under different Pi regime. Although increases in root hair length and number of phr1 were comparable to the wild-type when starved of Pi for 7 days [39], significant reduction in root hair length was detected in both phr1 and phr1phl1 during prolonged Pi deprivation for 12 days [22]. This suggested that PHR1 acts as a positive regulator of root hair elongation at least during long-term Pi deprivation and thus exerts some control on local Pi-dependent responses. Root hair number was significantly higher in WRKY75RNAi and bhlh32 when grown under P+ condition [36, 56]. These studies highlighted the negative regulatory effect of some of the TFs (WRKY75 and BHLH32) on root hair development generating increased total available root surface area for Pi acquisition, resulting in elevated Pi content in the seedlings. Although expression of HRS1 was induced in root hair zone and in root hair cells during Pi deprivation, root hair development in hrs1-1 did not differ from wild-type irrespective of Pi status [60]. The lack of obvious phenotypic variations between the wild-type and RNAi lines of OsPHR1 and OsPHR2 [47] also suggested that there was no effect on root hair development in these transgenics. At present, the regulatory roles of ZAT6, MYB62, WRKY6, PRD, and OsPTF1 on root hair development under different Pi regime are not known and merit further studies [37, 38, 52, 58, 61].
Primary root length
Several studies demonstrated the influence of Pi deprivation on remodeling of RSA [72, 73, 78–80]. RSA is a pivotal part of plant adaptation to heterogeneous distribution of Pi in soils, and is an overall representation of the spatial growth responses of ontogenetically distinct embryonic and post-embryonically developed primary and lateral roots, respectively. RSA exhibits extensive developmental plasticity that augments its exploratory capacity for foraging top soils rich in Pi [81–83]. To determine specifically the influence of local Pi availability on primary root growth, Arabidopsis seedlings were grown on vertically orientated agar Petri plates containing patches of different Pi supply. The growth of primary root was inhibited upon encountering a Pi-deficient patch suggesting an influence of local Pi availability on this trait [84]. Low Pi-mediated inhibition of primary root growth is caused by reduction of cell elongation followed by progressive exhaustion of meristematic cells, resulting in a shift from an indeterminate to a determinate developmental program [85–88]. In fact, the magnitude of gene expression triggered by low Pi has been shown to be specified by root meristem activity [89]. Transgenic Arabidopsis expressing quiescent center (QC) identity marker QC46::uidA was used for determining whether low Pi condition has any effect on QC activity [88]. The study revealed that alteration in QC preceded loss of meristematic activity in the primary root, which led to the assumption that QC could act as a sensor of Pi deficiency that eventually affects meristem maintenance. Table 1 presents a comparative account of the effects of mutation in TFs on different root traits under P+ and P− conditions. An inhibitory effect on primary root growth was pronounced in P− prd seedlings suggesting a positive regulatory effect of PRD on Pi-deficiency-mediated growth response of primary root, which was consistent with its expression specifically in the apex of the primary root [58]. An impairment of local Pi sensing ability of Arabidopsis primary root grown under P–Fe-condition is well documented [90, 91]. Fe is a strong Pi chelator and therefore there is a rationale for an antagonistic interaction between them [1, 92–95]. Elevated concentration of Fe in Pi-deficient plants resulting in coordinated suppression of iron transporter IRT1 in roots and induction of FER1 (encoding a protein involved in iron storage in the chloroplast) in leaves point to a link between Pi-deficiency-mediated molecular responses and Fe transport, accumulation, and homeostasis [16, 73, 96]. Therefore, it was hypothesized that primary root growth inhibition during Pi deficiency may be a consequence of elevated Fe availability and its presumed toxicity [91, 96]. In contrast, the root growth response of pdr2 (conditional short root mutant) under conditions where Fe2+ was made bioavailable at each Pi concentration (0–2.5 mM Pi) revealed that the sensitization of the primary root growth of the mutant to an inhibitory effect of Pi deprivation is largely independent of external Fe availability [97]. These studies thus provide differing views about the role of Fe in mediating local Pi-sensing responses of primary root. It would therefore be interesting to compare the growth of the primary root of pdr2 seedlings under both P− and P–Fe- conditions that may provide useful information towards the role of this TF, if any, in regulating Fe-mediated local Pi-sensing ability of this root trait. The majority of the TFs studied so far either did not show any influence (WRKY75RNAi, bhlh32, hrs1-1) or their effects are not known/reported (phr1, phl1, phr1 phl1, wrky6-1) on Pi-deficiency-mediated inhibition of primary root growth of Arabidopsis seedlings [22, 36, 39, 52, 56, 60]. Also, there was no noticeable phenotypic variation between OsPHR2RNAi and wild-type [47].
Lateral root development
Ontogenetically primary and lateral roots represent distinct entities, and, unlike primary roots, Pi-deficiency-mediated developmental responses of lateral roots are dependent on both sucrose and auxin [72]. Despite these differences between primary and lateral roots, the developmental response of the former has a substantial influence on the latter. For instance, lateral root formation could be stimulated by removal of primary root tip or ablation of root cap cells by the expression of diphtheria toxin [80, 98, 99]. Pi-deficiency-induced exhaustion of the meristematic activity in primary root could have a likely influence on auxin movement in root tip and consequently on branching of lateral root [7, 100]. Therefore, interaction between primary root growth and lateral root development is crucial for establishing RSA, which enhances its exploratory capacity particularly within the upper layers of soils. Significant reduction in lateral root length of Pi-deprived prd supported this assumption and highlighted its role in mediating root architectural responses to Pi deprivation [58]. In contrast, there were marked increases in number and length of lateral roots in WRKY75RNAi under both P+ and P− conditions despite having primary root growth comparable to the wild-type [36]. Broadly, the mutation in majority of the characterized TFs [phr1, phl1, phr1 phl1, bhlh32, hrs1-1, wrky6-1] either did not elicit any significant effect on Pi-deficiency-mediated developmental responses of lateral roots or it has not been reported [22, 39, 40, 52, 56, 60].
Reverse genetics approaches (T-DNA knock-down and RNAi gene silencing) led to the characterization of a few TFs (PHR1, WRKY75, BHLH32, PRD) that affected one or more of the root traits in Arabidopsis either during Pi deprivation or independent of Pi status [22, 36, 56, 58]. Among these TFs, elevated Pi content in bhlh32 and WRKY75RNAi seedlings could possibly be correlated with significant increases in their root hair numbers and/or accentuated development of lateral roots [36, 56]. A more direct role of other TFs in regulating Pi uptake and mobilization by regulating developmental responses of different root traits needs to be established. It would be interesting to investigate the functional interactions across these TFs, and whether their double or triple mutants could have any additive regulatory influence on the root traits and thereby on Pi content of the seedlings. This strategy may be helpful in developing transgenics that could have higher Pi-use efficiency under Pi-limiting condition.
Although genome-wide analyses of Arabidopsis led to the identification of an array of PSR genes [16, 17], none of these studies highlighted the role of local and systemic Pi sensing. To address this issue, a split-root experiment was designed facilitating exposure of two parts of the same root system to separate media differing in their Pi content [23]. This approach led to the identification of several PSR genes including TFs that were specific to either local or systemic Pi sensing. It is therefore anticipated that functional characterization of some of these TFs that are specifically responsive to local Pi availability could shed more light on local Pi sensing. A similar approach of using a split-root system would be an attractive option for dissecting TFs specific for local and systemic sensing in rice, which, unlike Arabidopsis, stimulates the growth of primary and adventitious roots during Pi deficiency [47, 61, 101]. On the whole, our present understanding of the TFs regulating local Pi-sensing responses has only begun to be unraveled in plants.
Potential cross-talk of TFs with phytohormone and sugar signaling pathways in mediating root responses to Pi deficiency
Modifications in RSA are characteristic responses of plants to Pi deficiency [83]. Among the phytohormones, ethylene has been implicated in regulating Pi-deficiency-mediated root hair development [55, 69, 102–104]. Ethylene also integrates with auxin signaling by modulating tissue-specific responses, biosynthesis, and transport of auxin [105]. Interactions between these two phytohormones have a synergistic effect on root hair development and root growth [105, 106]. Auxin plays a pivotal role in development of root system [107–109] and several studies have demonstrated a cross-talk between Pi sensing and auxin signaling [72, 78–80, 83, 86, 110–113]. Further, auxin has been implicated in promoting root growth by modulating the gibberellic acid (GA) response [114]. More conclusive evidence toward the cross-talk between GA and Pi starvation responses is now beginning to emerge [38, 115]. Active photosynthesis or supplementation of the nutrient medium with sucrose (Suc) or other metabolizable sugars is pivotal for Pi-deficiency-mediated developmental responses of root hairs and lateral roots [72]. Since mutations in BHLH32 and WRKY75 significantly affected root traits [36, 56], examining their cross-talk, if any, with one or more of the phytohormones and/or sugar signaling pathways would have broader implications in developing strategies for generating transgenics with a better adaptability towards Pi deficiency.
Effects of mutation in TF on systemic Pi sensing
Many Pi-deficiency-mediated adaptive responses are regulated by internal availability of Pi involving long-distance signaling as deduced from split-root experiments [116–118]. The roles of microRNAs and cation/H+ exchangers (CAX1 and CAX3) have been postulated in mediating shoot-to-root Pi signaling, thereby establishing a paradigm for systemic regulation of Pi homeostasis [46, 119–122]. The likelihood of Suc loading in phloem resulting in a shoot-derived systemic signal for regulating Pi-deficiency-mediated responses of root system has also been suggested [123]. Sucrose is also vital for activation of several PSR genes including Pht1;1, Pht1;4 [124–126], and therefore the effects of TFs on systemic responses could possibly be mediated through one or more of these signaling pathways. Here, we give a brief account of various systemically regulated developmental, metabolic, and molecular traits that are affected by mutation in different TFs.
Developmental
Retarded growth of roots and shoots resulting in attenuated fresh weight of the seedling is a typical developmental response to Pi deficiency [15]. Table 2 summarizes the effects of mutation in TFs on developmental responses of the mutants. Compared to the wild-type, there were marginal but significant reductions in both root/shoot growth ratios and total fresh weights of phr1 mutants, specifically under Pi-deprived condition [39]. There were no effects on these developmental traits either under P+ condition or when subjected to other nutrient deficiencies [39]. The study clearly suggested a likely role of PHR1 in regulating Pi-deficiency-mediated developmental responses. Similarly, a T-DNA tagged knock-out phr1 mutant also showed reductions in the fresh weights of shoot and roots and root/shoot ratios under both P+ and P− conditions compared to the wild-type [40]. Although reduced fresh weights and root/shoot ratios were also observed for both phr1 and phr1phl1 mutants during Pi deficiency, there was no significant effect on these development traits in phl1 irrespective of Pi regime [22]. When grown to maturity under Pi-deprivation condition, both single (phr1 and phl1) and double (phr1phl1) mutants senesced before flowering [22]. The study suggested a degree of functional redundancy between PHR1 and PHL1 and, importantly, the roles of these TFs as positive regulators in providing some protection against the stress imposed by Pi deficiency [22]. In contrast, under P− condition, wrky6-1 mutant showed better growth than the wild-type indicating WRKY6 to be a negative regulator of Pi-deficiency-mediated responses. At present, a majority of the TF mutants (WRKY75RNAi, bhlh32, prd, hrs1-1, OsPHR1RNAi, OsPHR2RNAi) have not been evaluated for their developmental responses under different Pi regime. For a holistic evaluation of the role of a particular TF in the maintenance of Pi homeostasis, it would be useful to compare developmental responses of the mutant with the wild-type from seedling to maturity stage under both Pi-deprived and Pi-replete conditions. In this context, it would be worthwhile to follow a quantitative framework that could be used routinely for inferring developmental responses of the wild-type and different TF mutants, which could be incorporated into a mechanistic model. Various growth parameters could be collected from dissected and living plants using nondestructive methods for precisely capturing the developmental responses of Arabidopsis [127]. Morphometric comparisons of vegetative and reproductive traits of wild-type and TF mutants could be achieved using software such as ImageJ (a Java image-processing program; http://rsb.info.nih.gov/ij), WINRHIZO [128], or EZ-RHIZO [129]. This would give a better perspective of the effects of mutations in different TFs on the growth and development of the mutants grown under different Pi conditions.
Metabolic adaptations
Plants have evolved an intricate mechanism of ameliorating adverse effects of Pi deprivation by modifying an array of metabolic responses [13]. Among metabolic adaptations, anthocyanin accumulation in shoots is a typical response to Pi deficiency [15] and it is likely to provide protection to nucleic acids and chloroplasts from UV and photo-inhibitory damage [130]. Microarray analysis of global gene expression during Pi deficiency revealed differential regulation of several genes involved in various steps of anthocyanin biosynthetic pathway leading to the synthesis of cyanidin, flavonoids, and pelargonidin [16]. The comparative effects of mutation in TFs on anthocyanin accumulation are presented in Table 2. Lack of anthocyanin accumulation during Pi deprivation was used as an effective strategy for screening phr1 and phr1phl1 mutants [22, 39, 40]. The effect on accumulation of anthocyanin was rather marginal in the phl1 mutant [22], whereas significant increases in accumulation were observed both under P+ and P− conditions in bhlh32 [56] and in Pi-deprived WRKY75RNAi seedlings [36]. These studies suggest the utility of this trait not only for screening the mutants but also for evaluating a likely positive [PHR1, PHL1] or negative [BHLH32, WRKY75] regulatory influence of TFs on Pi-deficiency-mediated responses. The effect on this trait has not been reported for other characterized mutants (wrky6-1, prd, hrs1-1, OsPHR1RNAi, OsPHR2RNAi) [47, 52, 58, 60].
Carbohydrate partitioning and accumulation of starch in leaf are typical responses associated with Pi deficiency [73, 74, 131]. Starch biosynthesis is triggered by accumulation of 3-PGA [17] which allosterically activates AGPase during Pi deficiency [132]. The enzyme AGPase catalyzes the first committed step in starch biosynthesis and its activity is subject to allosteric inhibition by Pi [132]. Sugar also plays a pivotal role in Pi-deficiency-mediated modulation of auxin-dependent lateral root development [72, 113]. The expression of several genes involved in starch biosynthesis is differentially regulated by Pi deprivation in Arabidopsis [17, 18]. However, the carbohydrate accumulation pattern in TF mutants has not been thoroughly examined, with the exception of a study [40] evaluating its distribution in shoot and root of a phr1 mutant grown under P+ and P− conditions. The study revealed significant decrease in accumulation of sugars (Fru and Glc) and starch with a concurrent attenuation in AGPase activity in Pi-deprived phr1 plants compared to the wild-type. The study highlighted the role of PHR1 in regulating carbon metabolism during Pi deficiency.
Molecular responses
TFs play a pivotal role in mediating adaptive responses to Pi deprivation by altering the expression of genes [4, 9, 10, 12]. A comparative account of the effects of mutation in different TFs on the expression of PSR genes belonging to various functional categories is provided in Table 3. Among the TF mutants characterized, phr1 has been the most extensively studied for its effect on the regulation of PSR genes in Arabidopsis [22, 39, 40, 46]. Unlike other characterized TFs in Arabidopsis and rice (WRKY75, ZAT6, MYB62, OsPTF1, OsMYB2P-1) that show significant induction in response to Pi deprivation [36–38, 61, 133], PHR1 is expressed irrespective of Pi regime [22, 39, 40, 46]. However, the expression of several PSR genes, belonging to different functional categories, is attenuated in phr1 mutants [22, 39, 40, 46].
One of the crucial adaptive responses to Pi deficiency is the ability of plants to trigger molecular machinery for scavenging/remobilization of available Pi. Purple acid phosphatases (PAP), members of the metallo-phosphoesterase family, comprise the largest class of plant APases that are involved in intra- and/or extra-cellular Pi scavenging and recycling during Pi deficiency and are considered to be attractive candidates for engineering Pi-efficient crops [13]. A family of 29 genes (AtPAP1–AtPAP29), sharing conserved domains of PAPs, has been identified in Arabidopsis genome [134] whose transcriptional expression is influenced by a wide spectrum of environmental and developmental cues [135]. Pi deficiency triggers the expression of AtPAP17 [16, 136], AtPAP11 [134], AtPAP5, AtPAP14, AtPAP22, AtPAP23, and AtPAP25 [16]. Northern blot analysis showed a reduction in the transcript levels of AtPAP17 [39]. In a subsequent study, a qRT-PCR analysis of Pi-deprived phr1 seedlings revealed suppression in the expression of several members (AtPAP5, AtPAP11, AtPAP14, AtPAP16, AtPAP17, AtPAP19, AtPAP21, AtPAP22, AtPAP23, AtPAP25) of this family [46]. RNAi-mediated suppression of OsPHR1 and OsPHR2 also resulted in the suppression of the Pi-starvation-inducible expression of OsPAP10 in rice [47]. Further, members of phosphatase family, i.e., AtPS2 in phr1 and wrky6-1 [46, 52] and AtPS2-1, and AtPS2-2 in WRKY75RNAi [36], showed down-regulation under Pi-deprived condition. Pi deficiency also induced ribonuclease gene AtRNS1, highlighting its role in Pi mobilization from RNA [16, 137]. Reduction in the expression of AtRNS1 in phr1 [22, 39, 40], phr1phl1 [22], and wrky6-1 [52] provided some evidence towards the role of some of the TFs as positive regulators of Pi scavenging and/or recycling during Pi deprivation.
Phospholipids in cell membranes comprise a large proportion of the P reserve. During Pi deficiency, phospholipids are hydrolyzed, releasing diacylglycerol (DAG) and Pi, and the former is converted into sulfolipids or galactolipids [138, 139]. Microarray analysis identified an array of Pi-deficiency-induced genes that are involved in this substitution process for maintaining Pi homeostasis [16]. Many of them (NPC4, NPC5, PLDZ2, MGD2, MGD3, SQD2) showed reduced expression in Pi-deprived phr1 compared to the wild-type [46]. Relatively, the effect on this substitution process was rather moderate in wrky6-1 as indicated by reduced expression of only NPC5 and SQD1 [52]. These studies further highlighted the positive regulatory effect of PHR1 on phospholipid substitution. The wider influence of PHR1 on Pi homeostasis by exerting a positive regulatory influence on the acquisition and mobilization of Pi was also substantiated by qRT-PCR analysis revealing reduction in the expression of seven out of nine members (Pht1;2, Pht1;3, Pht1;4, Pht1;6, Pht1;7, Pht1;8, Pht1;9) of the Pht1 family in Pi-deprived phr1 seedlings [46]. Another independent study corroborated the attenuated expression of some of the members (Pht1;4, Pht1;7, Pht1;8, Pht1;9) of the Pht1 family in roots and shoots of a P− phr1 mutant [40]. Contrary to these studies [40, 46], northern analysis of both Pi-deprived phr1 and phr1phl1 mutants showed lower transcript levels of Pht1;1 [22, 39]. The incongruity between these reports could possibly be due to a combination of factors including different analytical techniques (northern analysis and microarray/qRT-PCR) used for evaluating gene expression, variations in the experimental design [111], and/or the likelihood of certain elemental impurities in the agar [73] that would have been used for preparing the growth medium. A revisit to this problem could therefore provide a more pragmatic insight into the role of PHR1 in regulating Pi acquisition and mobilization mediated by one or more members of the Pht1 family. A repressing influence was also explicit in the majority of the members (Pht1;2, Pht1;3, Pht1;4, Pht1;5, Pht1;8, Pht1;9) of the Pht1 family in wrky6-1 grown under P− condition. Pi-deficiency-induced expression in majority of the Pht1 family members were affected in both phr1 and wrky6-1 except that of Pht1;5 in the latter mutant. Attenuated expression of Pht1;5 was also evident in WRKY75 RNAi under both P+ and P− conditions [55]. The presence of two WRKY box (W-box) cis-elements (TTGACC/T) in Pht1;5 promoter suggests a potential interaction with WRKY TFs [36, 55]. Unlike Pht1;1 and Pht1;4 that have been shown to play a role in Pi acquisition [140], Pht1;5 has been implicated in mobilizing Pi between sink and source organs and mediating a cross-talk between Pi homeostasis and ethylene signaling [55]. These studies thus point to the differential regulatory influence of both PHR1 and WRKY TFs on several members of the Pht1 family [22, 36, 39, 40, 46, 52]. A reduction in the expression of CHS and PAP1 in phr1 mutant [40] was consistent with impaired accumulation of anthocyanin during Pi deficiency [22, 39]. In contrast, an increase in the expression of DFR (dihydroflavonol reductase) in both P+ and P− bhlh32 mutant seedlings suggested a negative regulatory effect of BHLH32 on the metabolic responses of Arabidopsis grown under Pi-deprived condition [56]. Since there was an elevated Pi content in the bhlh32 mutant under Pi-replete condition, the negative regulatory effect of BHLH32 on anthocyanin accumulation could presumably be independent of Pi content [56]. In addition, P− phr1 mutant showed a reduced expression of BAM5 [46]; a gene involved in starch metabolism in Arabidopsis. Since during Pi deficiency plants accumulate sugars and starch in their leaves to provide a carbon pool for reallocation to the roots [123], the regulatory effect of PHR1 on BAM5 may have some indirect implications on the root responses to Pi deficiency. AtIPS1 and At4, members of the Pi-starvation-induced TPSI1/MT4 family, exhibited reductions in their expression in phr1 [22, 39, 40, 46], and WRKY75RNAi [36]. The transcript levels of AtIPS1 were also attenuated in both roots and shoots of phr1phl1 [22]. Since AtIPS1 and At4 are induced rapidly and specifically during Pi deficiency, they are useful candidates for deciphering Pi sensing mechanisms and the components of signal transduction pathways that integrate plant responses to Pi deficiency. In fact, this strategy was used successfully by generating Arabidopsis transgenic harboring GUS reporter gene under the control of IPS1 promoter that showed strong GUS activity under P− condition, and screening of the mutants with abnormal GUS activity led to the isolation and characterization of phr1 [39]. A loss-of-function mutant of At4 showed an aberration in the distribution of Pi from shoot to root during Pi deficiency resulting in greater accumulation of Pi in its shoots relative to the wild-type [141]. Since the conserved sequence in IPS1/At4 non-coding RNA has partial complementarity to miRNAs [121, 141, 142], thereby regulating the gene expression at the post-transcriptional level [143]. Notably, the sequence complementarity between a motif of IPS1 and miRNA is interrupted by a mismatched loop at the miRNA cleavage site resulting in ‘target mimicry’; a term coined for defining this mechanism of sequestering miRNAs [142]. Arabidopsis genome encodes six miR399 genes (a–f) that show induction during Pi deficiency [46, 119, 121, 144]. By employing deep sequencing, several other miRNAs have been identified that were either induced (miR156, miR399*, miR399, miR778, miR827, and miR2111) or suppressed (miR169, miR395, and miR398) by Pi deprivation [21]. There was an accentuated accumulation of Pi in P+ shoots of transgenic Arabidopsis overexpressing miR399, implicating its positive regulatory effect on Pi homeostasis [46, 119, 144, 145]. The mobility of miR399* and miR399 from scions to rootstocks across the graft junction suggested their possible role during Pi signaling [21].
Arabidopsis mutant pho2 accumulates up to threefold more Pi in leaves, often resulting in Pi toxicity as indicated by necrosis or chlorosis at the tip of older leaves [146–148], and several PSR genes (AtIPS1, At4, Pht1;8, and Pht1;9) remain induced in the mutant even under P+ condition [46]. Subsequently, map-based cloning identified PHO2 as E2 conjugase gene (At2g33770), and its potential orthologs were identified in rice, M. truncatula, and Populus trichocarpa, suggesting it to be conserved across taxonomically diverse species [46]. Pi-sufficient transgenic Arabidopsis overexpressing miR399 suppresses the expression of PHO2 thereby phenocopying pho2 [46]. The systemic regulation of PHO2 by long-distance movement of miR399s from shoot to roots was demonstrated by reciprocal grafting between transgenic Arabidopsis overexpressing miR399 and the wild-type [149, 150]. Together, these studies highlight the potential roles of Pi-responsive small RNAs in influencing the expression of the target genes involved in the adaptive responses to Pi deficiency. In P− phr1 mutant, the expressions of five miR399 genes (a–e) were reduced [46]. Another study confirmed the suppression of miR399d in roots and shoots of a Pi-deprived phr1 mutant along with an induction of PHO2 [40]. These studies showed that miR399 and PHO2 act downstream of PHR1 in the Pi-signaling network [40, 46].
A reduction in the expression of AtSPX1 in both phr1 and phr1phl1 [22, 46] and of AtSPX3 in phr1 and wrky6-1 [46] suggested the involvement of genes encoding SPX domain (SYG1, Pho81 and XPR1) that is similar to Pho81, which is a positive regulator of Pi sensing mechanism in yeast [151]. AtSPX1 positively regulates genes involved in Pi mobilization (phosphatases and ribonucleases), and AtSPX3 negatively regulates several PSR genes including AtSPX1 [152]. At present, the role of SPX family proteins in Pi signaling cascade is yet to be determined. Suppression of CAX1 in wrky6-1 [52] suggested a likely involvement of Ca2+ in the maintenance of Pi homeostasis [9]. In addition, phr1 showed attenuation in the expression of several other genes belonging to different functional categories, i.e., loading of Pi into xylem of roots (PHO;H1), mitochondrial dicarboxylate carrier (DIC3), kinases (AtPPCK1, and AtPPCK2), transcriptional regulation (AtMYB60, HDG8), and genes with unknown functions [46]. Both PHO1 and PHO3 were down-regulated in wrky6-1 but not in phr1 [52]. It is evident from the literature that phr1 has been extensively characterized [22, 39, 40, 46] compared to other TF mutants (wrky6-1, WRKY75RNAi, bhlh32, prd, hrs1-1) [36, 52, 56, 58, 60]. A lack of suppression of these TFs in phr1 suggests that they may be functioning independently of PHR1. Whether PHR1 interacts and/or is influenced by other Pi-responsive TF(s) in regulating Pi homeostasis are yet to be explored. Functional redundancy across 14 other PHR1-like genes in the regulation of Pi homeostasis has been proposed, and it merits further investigations (39). At present, the precise positions of other TFs in Pi sensing and signaling pathway and epistatic interactions across them, if any, have not yet been determined. A comprehensive molecular analysis of other Pi-responsive TFs is needed to build an integrated signaling model defining adaptive responses of plants to different Pi regimes.
Regulatory mechanisms governing Pi signaling in rice are also beginning to emerge through functional characterization of OsPHR1 and OsPHR2 [47], OsPTF1 [61], OsMYB2P-1 [133], and OsSPX1 [153, 154]. Although there were no significant differences in Pi content and the phenotypic traits (primary root length and root hairs) between RNAi-silenced lines of OsPHR1 and OsPHR2 and the wild-type, there was distinct Pi-deficiency-mediated suppression in the expression of OsIPS1, OsIPS2, OsPAP10, and OsSQD2 [47]. The study thus assigned a role for OsPHR1 and OsPHR2 in regulating a subset of PSR genes. The RNAi lines of OsSPX1 (OsSPX1-Ri) exhibited severe signs of toxicity due to over-accumulation of Pi probably as a consequence of elevated expression of Pi transporters OsPT2 and OsPT8 [153]. In another study, it was demonstrated that OsSPX1 acts as a negative regulator of OsPHR2 which suggested a likely involvement of the SPX domain on the maintenance of Pi homeostasis in rice [154]. The role of OsSPX1 in optimizing plant growth under Pi deficiency condition by participating in a negative feedback loop could thus be hypothesized [153, 154]. The suppression of OsMYB2P-1 by RNAi also accentuated sensitivity towards Pi deficiency in Pi-deprived transgenic rice as indicated by their growth inhibition, lower shoot biomass, reduced Pi contents in roots and shoots, and reduced expression of OsPT6, OsPT8, and OsPT10 [133]. Irrespective of the Pi regime, the expression of some of the PSR genes was either suppressed (OsmiR399a, OsmiR399j, OsIPS1, OsSQD, OsPAP10) or enhanced (OsPHO2) in RNAi OsMYB2P-1 transgenic plants compared to the wild-type [133]. Together, these studies highlight the significant regulatory influence of some of the characterized rice TFs (OsPHR1, OsPHR2, OsSPX1, OsPTF1, OsMYB2P-1) on various morphophysiological and molecular adaptive responses to Pi deficiency.
Overexpression of TFs for enhanced Pi-use efficiency
Diminishing Pi rock reserves and low Pi-use efficiency have been major concerns around the world and have led to several research studies for developing sustainable strategies for Pi-use efficiency. Several Pi-use-efficient genotypes of agronomic crop species have been identified among landraces and wild ancestors that can sustain optimal growth in Pi-deficient soils [155–161]. Often, this process of selecting Pi-use-efficient genotypes is influenced by various environmental factors and has met with a rather modest success [162, 163]. Therefore, efforts are directed towards dissecting the genetic and molecular basis of Pi-use efficiency in plants [158, 164]. Diverse responses exhibited by plants to Pi deficiency suggest an occurrence of multiple control points that are likely involved in defining Pi-use efficiency. Traditional plant breeding approaches along with genomic studies have revealed that Pi-use efficiency is governed by an array of genes in quantitative trait loci (QTLs), whose cloning could be expedited by developing near-isogenic lines (NILs) and recombinant inbred lines (RILs) in conjunction with next generation sequencing [11, 165–168]. An alternative approach employed has been to manipulate functionally characterized PSR genes that have been implicated in Pi acquisition. Among the PSR genes, the pivotal role of high-affinity Pi transporters Pht1;1 and Pht1;4 in Pi uptake in Arabidopsis was demonstrated by analyzing single [169] and double mutants [140]. Therefore, overexpression of these genes encoding Pi transporters provided an attractive paradigm to make the plant more Pi-use efficient. Consistent with this assumption, overexpression of Pht1;1 in Pi-deprived tobacco-cultured cells elevated the Pi uptake rate resulting in higher biomass [170]. Likewise, transgenic rice plants overexpressing high-affinity Pi transporters from rice (OsPT1) and tobacco (NtPT1) accumulated significantly higher amounts of Pi compared to non-transformed controls [171, 172]. However, overexpression of HvPht1;1 did not result in increased Pi uptake or higher Pi accumulation in transgenic barley under any of the conditions tested [173]. This suggested that overexpression of individual PSR genes may not always be ideal for enhanced Pi acquisition and accumulation. Several TFs have been functionally characterized in Arabidopsis and rice that have been shown to regulate a subset of PSR genes including members of the Pht1 family [22, 39, 40, 46, 47, 52, 61, 133]. Therefore, their overexpression could be relatively more promising in achieving higher Pi-use efficiency. In fact, overexpression of Dof1 TF from maize in Arabidopsis resulted not only in increased nitrogen content in the transgenic plants but also conferred tolerance towards nitrogen deficiency, emphasizing the benefits of TFs in improving nutrient acquisition and use [174]. The feasibility of increasing Pi content under both P+ and P− conditions in the shoots of transgenic Arabidopsis was aptly demonstrated by overexpressing PHR1 under a strong constitutive 35S promoter [40]. A significant increases in the expression of miR399d, a decline in the levels of the target PHO2, and an elevated expression of Pht1;7 in P+ and P− shoots of PHR1 overexpressing lines resulted in an enhanced accumulation of Pi in the transgenic shoot [40]. Significant accumulation of Pi in shoots of P+ rice transgenic plants could also be achieved by overexpressing OsPHR2 which was attributed to an elevated expression of OsPT9 in both roots and shoots [47]. Further, accentuated growth of primary and adventitious roots and root hairs of P+ OsPHR2 overexpressing plants revealed the potential role of OsPHR2 in mediating developmental responses of the root system during Pi deprivation [47]. In addition, significant induction in the expression of OsIPS1, OsIPS2, OsPAP10, and OsSQD2 in both roots and shoots of P+ OsPHR2 overexpressing plants suggested the onset of molecular responses that are normally triggered during Pi deficiency. However, transcript abundance of OsPHO2 did not change despite a significant increase in the transcripts of OsmiR399 (OsmiR399a, OsmiR399d, OsmiR399f, and OsmiR399j) in P+ OsPHR2 overexpressing line [47]. This pointed to different mechanisms that could be operating downstream of miR399 in regulating Pi sensing and signaling pathways in Arabidopsis and rice.
RSA plays a pivotal role in soil exploration and mobilization of Pi from Pi-deficient zones in rhizospheres [11], and even modest changes in root growth-related parameters could have a significant influence on the Pi uptake ability of plants [101]. Therefore, it would be worthwhile to identify TFs whose overexpression could trigger appreciable changes in different root traits particularly under Pi-deficient condition. In this regard, overexpression of OsPTF1 showed promising results as evidenced by significantly higher total root length and root surface area, Pi content, root and shoot biomass, tillering ability, and panicle weight during growth in hydroponics, soil pot, and field experiments under Pi-deprived conditions [61]. Likewise, overexpression of Pi-deficiency-inducible OsMYB2P-1 in rice and Arabidopsis made them more tolerant to Pi deficiency [133]. In contrast, overexpression of OsSPX1 caused a retarded growth phenotype irrespective of Pi regime [153] and is thus an unlikely candidate for developing Pi-use-efficient plants. Overexpression of AtSPX1 induced increased transcript levels of acid phosphatases (PAP2, PAP17) and RNS1 under both P+ and P− conditions [152], and therefore transgenic Arabidopsis could possibly be more efficient in mobilizing Pi from soil to the root system. These studies clearly reflect that overexpression of a particular TF in Arabidopsis generating desirable/undesirable traits for higher Pi-use efficiency does not always guarantee a similar result for its orthologs in rice, or vice versa.
Some of the overexpressors were also developed as an alternative technique for the functional characterization of TFs whose RNAi-mediated suppression was either lethal [37] or failed to suppress the expression of the target gene [38]. Under both P+ and P− conditions, there were significant increases in lateral root length and root:shoot ratio, and elevated uptake of Pi and total Pi concentration in roots and shoots of ZAT6 overexpressing (ZOe) plants [37]. The expression of several PSR genes (At4, AtIPS1, AtPS2-1, AtPS2-2, AtACP5, Pht1;1 and Pht1;4), involved in mobilization, acquisition, and translocation of Pi, were attenuated to varying levels during Pi deficiency in ZOe plants compared to the wild-type plants [37]. Among the PSR genes, AtIPSI contains a motif with sequence complementarity to miR399 and overexpression of AtIPSI triggers accumulation of PHO2 mRNA resulting in lower shoot Pi content [142]. Pi-deprived ZOe plants not only showed reduced expression of AtIPSI but also an increased Pi content in shoots and roots, suggesting a likely cross-talk of ZAT6 with one or more components of the PHR1-miR399-PHO2 pathway. Unlike ZOe, the overexpression of MYB62 resulted in lower shoot Pi content, reductions in the lengths of primary and lateral roots, and systemic suppression of several PSR genes [38]. MYB62 overexpressing plants also revealed a GA-deficient phenotype that could be partially restored upon exogenous application of GA [38]. This study provided some insight into a potential role of Pi-responsive TF in regulating Pi-deficiency-mediated responses through a phytohormone signaling pathway. It is anticipated that more Pi-responsive TFs will be identified in the near future whose manipulations would exert a significant impact on Pi-use efficiency of plants.
Other regulatory mechanisms governing Pi-starvation responses
Apart from transcriptional regulation by various TFs, other mechanisms also exert significant influences in regulating Pi-starvation responses. For instance, it was demonstrated that PHR1 could be sumoylated by SUMO E3 ligase SIZ1 [45]. Unlike the phr1 mutant, the loss-of-function mutant of SIZ1 exhibited hypersensitive responses during Pi starvation on different traits of the root system [45]. This suggests that some of the regulatory effects of SIZ1 on Pi-starvation-mediated responses are independent of PHR1. Endoplasmic reticulum (ER)-localized phosphate transporter traffic facilitator (PHF1) has also been shown to play a role in mediating the trafficking of PHT1 family proteins [175]. PHF1 is homologous to the yeast SEC12 protein which is involved in mobilization of cargo proteins in the secretory pathway [175]. PHF1 is induced during Pi deficiency and the phf1 mutant showed impaired uptake, transport, and accumulation of Pi, and increased resistance towards arsenate due to the defect in targeting the high-affinity Pi transporter PHT1;1 to the plasma membrane [175].
Epigenetic regulation mediated by histones equally plays a crucial role in remodeling the chromatin, thereby facilitating the association of TF with DNA resulting in transcription [176]. ARP6 (actin-related protein 6) is a component of the SWR1 (switch/sucrose non-fermentable-related, SWI/SNF) chromatin remodeling complex that regulates transcription via deposition of the H2A.Z histone variant into the chromatin [177, 178]. Loss-of-function mutants of ARP6 in Arabidopsis revealed a pleiotropic phenotype with several developmental defects, including altered leaf development, reduced fertility, and early flowering [179]. The arp6-1 and arp6-2 mutants grown under P+ condition showed severely impaired Pi uptake rate, lower Pi accumulation, increased acid phosphatase activity, and induction of several PSR genes [74]. ChIP analysis revealed higher enrichment of H2A.Z for a subset of PSR genes under P+ condition, suggesting that ARP6-mediated H2A.Z deposition might maintain a subset of PSR genes in a repressed transcriptional state. The study thus provided evidence towards the role of ARP6 in mediating H2A.Z deposition facilitating a chromatin-level control of PSR genes, thereby affecting Pi-starvation responses [74]. Overall, these studies highlight a complex network of regulatory mechanisms that operate in concert to maintain Pi homeostasis under different Pi regimes.
Future perspectives
Although TFs investigated thus far appear to regulate overlapping subsets of PSR genes, epistatic interactions across them in coordinating Pi deficiency responses are far from being elucidated. In planta molecular interactions of TFs in the regulatory pathway could be studied using inducible RNAi/overexpressor and/or chimeric repressor gene-silencing technology (CRES-T) by expressing a fusion of TFs and the EAR motif plant-specific repression domain (SRDX) [180]. Moreover, in vitro yeast two-hybrid and/or in vivo bimolecular fluorescence complementation (BiFC) assays could provide a better insight into the mechanisms governing the level of interactions across TFs involved in the maintenance of Pi homeostasis. Microarray analysis based on ChIP (ChIP-on-Chip) is also a potent tool for the identification of co-regulators that can interact with TFs [181–183]. A more holistic understanding of the regulation of Pi homeostasis could be achieved by looking into taxonomically diverse species. With the rapid advancement of next generation DNA sequencing technology, such as high-throughput 454 (microbead-based pyrosequencing), the identification of newer gene-networks in organisms with large genomes is in the offing [184]. Studies using high-resolution deep sequencing has further facilitated the identification of numerous small RNAs and their TF targets, which have been linked to novel functions during nutrient starvation as well as plant growth and developmental [21, 185]. In the recently sequenced soybean genome, about 12 % of the genes encode TFs, and their overall distribution pattern among other protein coding loci was found to be largely similar to A. thaliana [186]. Thus, the identification of novel regulatory components in agronomically important crop species provides exciting avenues for engineering transgenic crops with higher Pi-use efficiency for meeting the growing demands of food production in a sustainable manner.
Acknowledgments
This work was supported by USDA grant (to K.G.R.) and by the Ministry of Science and Technology, Department of Biotechnology, Government of India (Ramalingaswamy Fellowship [2009] to A.J.).
References
- 1.Marschner H. Mineral nutrition of higher plants. 2. Boston: Academic; 1995. [Google Scholar]
- 2.Lynch JP. Roots of the second green revolution. Aust J Bot. 2007;55:493–512. doi: 10.1071/BT06118. [DOI] [Google Scholar]
- 3.Lynch JP. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 2011;156:1041–1049. doi: 10.1104/pp.111.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Z, Shao C, Meng YJ, Wu P, Chen M. Phosphate signaling in Arabidopsis and Oryza sativa . Plant Sci. 2009;176:170–180. doi: 10.1016/j.plantsci.2008.09.007. [DOI] [Google Scholar]
- 5.Ticconi CA, Abel S. Short on phosphate: plant surveillance and countermeasures. Trends Plant Sci. 2001;9:548–555. doi: 10.1016/j.tplants.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- 7.Desnos T. Root branching responses to phosphate and nitrate. Curr Opin Plant Biol. 2008;11:82–87. doi: 10.1016/j.pbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Doerner P. Phosphate starvation signaling: a threesome controls systemic Pi homeostasis. Curr Opin Plant Biol. 2008;11:536–540. doi: 10.1016/j.pbi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Lin W-Y, Lin S-I, Chiou T-J. Molecular regulators of phosphate homeostasis in plants. J Exp Bot. 2009;60:1427–1438. doi: 10.1093/jxb/ern303. [DOI] [PubMed] [Google Scholar]
- 10.Rouached H, Arpat AB, Poirier Y. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol Plant. 2010;3:288–299. doi: 10.1093/mp/ssp120. [DOI] [PubMed] [Google Scholar]
- 11.Vance CP. Quantitative trait loci, epigenetics, sugars, and microRNAs: quaternaries in phosphate acquisition and use. Plant Physiol. 2010;154:582–588. doi: 10.1104/pp.110.161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XJ, Finnegan PM. Regulation of phosphate starvation responses in higher plants. Ann Bot. 2010;105:513–526. doi: 10.1093/aob/mcq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaxton WC, Tran HT. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011;156:1006–1015. doi: 10.1104/pp.111.175281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SE, Jakobsen I, Grønlund M, Smith FA. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011;156:1050–1057. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghothama KG. Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- 16.Misson J, Raghothama KG, Jain A, et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA. 2005;102:11934–11939. doi: 10.1073/pnas.0505266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morcuende R, Bari R, Gibon Y, et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007;30:85–112. doi: 10.1111/j.1365-3040.2006.01608.x. [DOI] [PubMed] [Google Scholar]
- 18.Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol. 2007;143:156–171. doi: 10.1104/pp.106.090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderon-Vazquez C, Ibarra-Laclette E, Caballero-Perez J, Herrera-Estrella L. Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant- and species-specific levels. J Exp Bot. 2008;59:2479–2497. doi: 10.1093/jxb/ern115. [DOI] [PubMed] [Google Scholar]
- 20.Venkatachalam P, Jain A, Sahi SV, Raghothama KG. Molecular cloning and characterization of phosphate (Pi) responsive genes in Gulf ryegrass (Lolium multiflorum L.): a Pi hyperaccumulator. Plant Mol Biol. 2009;69:1–21. doi: 10.1007/s11103-008-9401-x. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh L-C, Lin S-I, Shih A-C, Chen J-W, Lin W-Y, Tseng C-Y, Li W-H, Chiou T-J. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009;151:2120–2132. doi: 10.1104/pp.109.147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, et al. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis . PLoS Genet. 2010;6(9):e1001102. doi: 10.1371/journal.pgen.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thibaud M-C, Arrighi J-F, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis . Plant J. 2010;64:775–789. doi: 10.1111/j.1365-313X.2010.04375.x. [DOI] [PubMed] [Google Scholar]
- 24.Oshima Y. Regulatory circuits for gene expression: the metabolism of galactose and of phosphate. In: Strathern J, Jones E, Broach J, editors. The molecular biology of the yeast Saccharomyces: metabolism and gene expression. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1982. pp. 159–180. [Google Scholar]
- 25.Wanner BL. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt R, Ingraham J, Lin E, Low K, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbrager H, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington D.C.: American Society for Microbiology; 1996. pp. 1357–1381. [Google Scholar]
- 26.Lenburg ME, O’Shea EK. Signaling phosphate starvation. Trends Biochem Sci. 1996;21:383–387. [PubMed] [Google Scholar]
- 27.Wykoff DD, Grossman AR, Weeks DP, Usuda H, Shimogawara K. Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas . Proc Natl Acad Sci USA. 1999;96:15336–15341. doi: 10.1073/pnas.96.26.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouillon J-M, Persson BL. New aspects on phosphate sensing and signaling in Saccharomyces cerevisiae . FEMS Yeast Res. 2006;6:171–176. doi: 10.1111/j.1567-1364.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein AH, Baretlein DA, Danon A. Phosphate starvation stress: an experimental system for molecular analysis. Plant Mol Biol Rep. 1989;7:7–16. doi: 10.1007/BF02669241. [DOI] [Google Scholar]
- 30.Mimura T, Dietz K-J, Kaiser W, Schramm MJ, Kaiser G, Heber U. Phosphate transport across biomembranes and cytosolic phosphate homeostasis in barley leaves. Planta. 1990;180:139–146. doi: 10.1007/BF00193988. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa N, Saito H, Miura K, Paolo J, Magbanua V, Bun-ya M, Harashima S, Oshima Y. Structure and distribution of specific cis-elements for transcriptional regulation of PHO84 in Saccharomyces cerevisiae . Mol Gen Genet. 1995;249:406–416. doi: 10.1007/BF00287102. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Muchhal US, Raghothama KG. Differential expression of TPSI1, a phosphate starvation-induced gene in tomato. Plant Mol Biol. 1997;33:867–874. doi: 10.1023/A:1005729309569. [DOI] [PubMed] [Google Scholar]
- 33.Mukatira UT, Chuming L, Varadarajan DK, Raghothama KG. Negative regulation of phosphate starvation-induced genes. Plant Physiol. 2001;127:1854–1862. doi: 10.1104/pp.010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu P, Ma L, Hou X, Wang M, Wu Y, Liu F, Deng XW. Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 2003;132:1260–1271. doi: 10.1104/pp.103.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ. Changes in genes expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 2003;132:578–596. doi: 10.1104/pp.103.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devaiah BN, Karthikeyan AS, Raghothama KG. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis . Plant Physiol. 2007;143:1789–1801. doi: 10.1104/pp.106.093971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devaiah BN, Nagarajan VK, Raghothama KG. Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol. 2007;145:147–159. doi: 10.1104/pp.107.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis . Mol Plant. 2009;2:43–58. doi: 10.1093/mp/ssn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Gene Dev. 2001;15:2122–2133. doi: 10.1101/gad.204401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson L, Müller R, Nielsen TH. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana . Plant Cell Environ. 2007;30:1499–1512. doi: 10.1111/j.1365-3040.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 41.Franco-Zorrilla JM, Gonzalez E, Bustos R, Linhares F, Leyva A, Paz-Ares J. The transcriptional control of plant responses to phosphate limitation. J Exp Bot. 2004;55:285–293. doi: 10.1093/jxb/erh009. [DOI] [PubMed] [Google Scholar]
- 42.Chen A, Gu M, Sun S, Zhu L, Hong S, Xu G. Identification of two conserved cis-acting elements, MYCS and P1BS, involved in the regulation of mycorrhiza-activated phosphate transporters in eudicot species. New Phytol. 2011;189:1157–1169. doi: 10.1111/j.1469-8137.2010.03556.x. [DOI] [PubMed] [Google Scholar]
- 43.Schunmann PH, Richardson AE, Smith FW, Delhaize E. Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.) J Exp Bot. 2004;55:855–865. doi: 10.1093/jxb/erh103. [DOI] [PubMed] [Google Scholar]
- 44.Karthikeyan A, Ballachanda D, Raghothama K. Promoter deletion analysis elucidates the role of cis elements and 5′UTR intron in spatiotemporal regulation of AtPht1;4 expression in Arabidopsis . Physiol Plant. 2009;136:10–18. doi: 10.1111/j.1399-3054.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 45.Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan AS, Raghothama KG, Baek D, Koo YD, Jin JB, Bressan RA, Yun D-J, Hasegawa PM. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA. 2005;102:7760–7765. doi: 10.1073/pnas.0500778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bari R, Datt Pant B, Stitt M, Scheible W-R. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Jiao F, Wu Z, Li Y, Wang X, He X, Zhong W, Wu P. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol. 2008;146:1673–1686. doi: 10.1104/pp.107.111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana . Curr Opin Plant Biol. 2001;4:447–456. doi: 10.1016/S1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- 49.Englbrecht CC, Schoof H, Böhm S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics. 2004;5:39. doi: 10.1186/1471-2164-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakamoto H, Araki T, Meshi T, Iwabuchi M. Expression of a subset of the Arabidopsis Cys(2)/His(2)-type zinc-finger protein gene family under water stress. Gene. 2000;248:23–32. doi: 10.1016/S0378-1119(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 51.Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol. 2007;10:366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y-F, Li L-Q, Xu Q, Kong Y-H, Wang H, Wu W-H. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis . Plant Cell. 2009;21:3554–3566. doi: 10.1105/tpc.108.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP. Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol. 2011;156:1149–1163. doi: 10.1104/pp.111.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z-H, Nimmo GA, Jenkins GI, Nimmo HG. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis . Biochem J. 2007;405:191–198. doi: 10.1042/BJ20070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nole-Wilson S, Tranby T, Krizek BA. AINTEGUMENTA-like (AIL) genes are expressed in young tissues and may specify meristematic or division-competent states. Plant Mol Biol. 2005;57:613–628. doi: 10.1007/s11103-005-0955-6. [DOI] [PubMed] [Google Scholar]
- 58.Camacho-Cristóbal JJ, Rexach J, Conéjéro G, Al-Ghazi Y, Nacry P, Doumas P. PRD, an Arabidopsis AINTEGUMENTA-like gene, is involved in root architectural changes in response to phosphate starvation. Planta. 2008;228:511–522. doi: 10.1007/s00425-008-0754-9. [DOI] [PubMed] [Google Scholar]
- 59.Rossini L, Cribb L, Martin DJ, Langdale JA. The maize Golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell. 2001;13:1231–1244. doi: 10.1105/tpc.13.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H, Yang H, Wu C, Feng J, Liu X, Qin H, Wang D. Overexpressing HRS1 confers hypersensitivity to low phosphate-elicited inhibition of primary root growth in Arabidopsis thaliana . J Integr Plant Biol. 2009;51:382–392. doi: 10.1111/j.1744-7909.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 61.Yi K, Wu Z, Zhou J, Du L, Guo L, Wu Y, Wu P. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol. 2005;138:2087–2096. doi: 10.1104/pp.105.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valdés-López O, Hernández G. Transcriptional regulation and signaling in phosphorus starvation: what about legumes? J Integr Plant Biol. 2008;50:1213–1222. doi: 10.1111/j.1744-7909.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- 63.Yuan H, Liu D. Signaling components involved in plant responses to phosphate starvation. J Integr Plant Biol. 2008;50:849–859. doi: 10.1111/j.1744-7909.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 64.Nilsson L, Müller R, Nielsen TH. Dissecting the plant transcriptome and the regulatory responses to phosphate deprivation. Physiol Plant. 2010;139:129–143. doi: 10.1111/j.1399-3054.2010.01356.x. [DOI] [PubMed] [Google Scholar]
- 65.Péret B, Clément M, Nussaume L, Desnos T. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci. 2011;16:442–450. doi: 10.1016/j.tplants.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Stefanovic A, Ribot C, Rouached H, Wang Y, Chong J, Belbahri L, Delessert S, Poirier Y. Members of the PHO1 gene family show limited functional redundancy in phosphate transfer to the shoot, and are regulated by phosphate deficiency via distinct pathways. Plant J. 2007;50:982–994. doi: 10.1111/j.1365-313X.2007.03108.x. [DOI] [PubMed] [Google Scholar]
- 67.Wasaki J, Yonetani R, Kuroda S, et al. Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant Cell Environ. 2003;26:1515–1523. doi: 10.1046/j.1365-3040.2003.01074.x. [DOI] [Google Scholar]
- 68.Wasaki J, Shinano T, Onishi K, et al. Transcriptomic analysis indicates putative metabolic changes caused by manipulation of phosphorus availability in rice leaves. J Exp Bot. 2006;57:2049–2059. doi: 10.1093/jxb/erj158. [DOI] [PubMed] [Google Scholar]
- 69.Ma Z, Bielenberg DG, Brown KM, Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana . Plant Cell Environ. 2001;29:459–467. doi: 10.1046/j.1365-3040.2001.00695.x. [DOI] [Google Scholar]
- 70.Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538. doi: 10.1111/j.1365-3040.1996.tb00386.x. [DOI] [Google Scholar]
- 71.Lynch JP, Brown KM. Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil. 2001;237:225–237. doi: 10.1023/A:1013324727040. [DOI] [Google Scholar]
- 72.Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis . Plant Physiol. 2007;144:232–247. doi: 10.1104/pp.106.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain A, Poling MD, Smith AP, Nagarajan VK, Lahner B, Meagher RB, Raghothama KG. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol. 2009;150:1033–1049. doi: 10.1104/pp.109.136184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith AP, Jain A, Deal RB, Nagarajan VK, Poling MD, Raghothama KG, Meagher RB. Histone H2A.Z regulates the expression of several classes of phosphate starvation response genes but not as transcriptional activator. Plant Physiol. 2010;152:217–225. doi: 10.1104/pp.109.145532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhat KKS, Nye PH. Diffusion of phosphate to plant roots in soil: III. Depletion around onion roots without root hairs. Plant Soil. 1974;41:383–394. doi: 10.1007/BF00017265. [DOI] [Google Scholar]
- 76.Bates TR, Lynch JP. Plant growth and phosphorus accumulation of wild-type and two root hair mutants of Arabidopsis thaliana (Brassicaceae) Am J Bot. 2000;87:958–963. doi: 10.2307/2656994. [DOI] [PubMed] [Google Scholar]
- 77.Martín AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de la Peña A, Leyva A, Paz-Ares J. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis . Plant J. 2000;24:559–567. doi: 10.1046/j.1365-313x.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- 78.Williamson LC, Ribrioux S, Fitter AH, Leyser O. Phosphate availability regulates root system architecture in Arabidopsis . Plant Physiol. 2001;126:875–882. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Sompson J, Herrera-Estrella L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 2002;129:244–256. doi: 10.1104/pp.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis: identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol. 2005;137:681–691. doi: 10.1104/pp.104.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malamy JE, Ryan KS. Environmental regulation of lateral root initiation in Arabidopsis . Plant Physiol. 2001;127:899–909. doi: 10.1104/pp.010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neumann G, Massonneau A, Martinoia E, Römheld V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta. 1999;208:373–382. doi: 10.1007/s004250050572. [DOI] [Google Scholar]
- 83.López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–287. doi: 10.1016/S1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 84.Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis . Plant J. 2002;29:751–760. doi: 10.1046/j.1365-313X.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 85.Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- 86.Nacry P, Canivenc G, Muller B, Azmi A, Onckelen HV, Rossignol M, Doumas P. A role for auxin redistribution in the response of the root system architecture to phosphate starvation in Arabidopsis . Plant Physiol. 2005;138:2061–2074. doi: 10.1104/pp.105.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sánchez-Calderón L, López-Bucio J, Chacón-López A, Gutiérrez-Ortega A, Hernández-Abreu E, Herrera-Estrella L. Characterization of low phosphorus insensitive mutants reveals a cross talk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol. 2006;140:879–889. doi: 10.1104/pp.105.073825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana . Plant Cell Physiol. 2005;46:174–184. doi: 10.1093/pcp/pci011. [DOI] [PubMed] [Google Scholar]
- 89.Lai F, Thacker J, Li Y, Doerner P. Cell division activity determines the magnitude of phosphate starvation responses in Arabidopsis . Plant J. 2007;50:545–556. doi: 10.1111/j.1365-313X.2007.03070.x. [DOI] [PubMed] [Google Scholar]
- 90.Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T. Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet. 2007;39:792–796. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- 91.Ward JT, Lahner B, Yakubova E, David E, Salt DE, Raghothama KG. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008;147:1181–1191. doi: 10.1104/pp.108.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fox TC, Guerinot ML. Molecular biology of cation transport in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:669–696. doi: 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 1999;141:1–26. doi: 10.1046/j.1469-8137.1999.00331.x. [DOI] [Google Scholar]
- 95.Thimm O, Essigmann B, Kloska S, Altman T, Buckhout TJ. Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol. 2001;127:1030–1043. doi: 10.1104/pp.010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirsch J, Marin E, Floriani M, Chiarenza S, Richaud P, Nussaume L, Thibaud MC. Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie. 2006;88:1767–1771. doi: 10.1016/j.biochi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 97.Ticconi CA, Lucero RD, Sakhonwasee S, Adamson AW, Creff A, Nussaume L, Desnos T, Abel S. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc Natl Acad Sci USA. 2009;106:14174–14179. doi: 10.1073/pnas.0901778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torrey JG. Induction of lateral roots by indoleacetic acid and decapitation. Am J Bot. 1950;37:257–264. doi: 10.2307/2437843. [DOI] [Google Scholar]
- 99.Tsugeki R, Fedoroff N. Genetic ablation of root cap cells in Arabidopsis . Proc Natl Acad Sci USA. 1999;96:12941–12946. doi: 10.1073/pnas.96.22.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kramer EM, Bennett MJ. Auxin transport: a field in flux. Trends Plant Sci. 2006;11:382–386. doi: 10.1016/j.tplants.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 101.Wissuwa M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiol. 2003;133:1947–1958. doi: 10.1104/pp.103.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma Z, Baskin TI, Brown KM, Lynch JP. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol. 2003;131:1381–1390. doi: 10.1104/pp.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang YJ, Lynch JP, Brown KM. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J Exp Bot. 2003;54:2351–2361. doi: 10.1093/jxb/erg250. [DOI] [PubMed] [Google Scholar]
- 104.Nagarajan VK, Smith AA. Ethylene’s role in phosphate starvation signaling: more than just a root growth regulator. Plant Cell Physiol. 2012;53:277–286. doi: 10.1093/pcp/pcr186. [DOI] [PubMed] [Google Scholar]
- 105.Stepanova AN, Alonso JM. Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol. 2009;12:548–555. doi: 10.1016/j.pbi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 106.Osmont KS, Sibout R, Hardtke CS. Hidden branches: developments in root system architecture. Annu Rev Plant Biol. 2007;58:93–113. doi: 10.1146/annurev.arplant.58.032806.104006. [DOI] [PubMed] [Google Scholar]
- 107.Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis . Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inze D, Sandberg G, Casero PJ, et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- 110.Gilbert GA, Knight JD, Vance CP, Allan DL. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Ann Bot. 2000;85:921–928. doi: 10.1006/anbo.2000.1133. [DOI] [Google Scholar]
- 111.Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P. Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signalling. Plant Cell Environ. 2003;26:1053–1066. doi: 10.1046/j.1365-3040.2003.01030.x. [DOI] [Google Scholar]
- 112.Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J. 2004;37:801–814. doi: 10.1111/j.1365-313X.2004.02005.x. [DOI] [PubMed] [Google Scholar]
- 113.Pérez-Torres C-A, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell. 2008;20:3258–3272. doi: 10.1105/tpc.108.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellins response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- 115.Jiang C, Gao X, Liao L, Harberd NP, Fu X. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis . Plant Physiol. 2007;145:1460–1470. doi: 10.1104/pp.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu C, Muchhal US, Mukatira U, Kononowicz AK, Raghothama KG. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol. 1998;116:91–99. doi: 10.1104/pp.116.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burleigh SH, Harrison MJ. The down regulation of Mt4-like genes by phosphate fertilization occurs systemically and involves phosphate translocation to the shoots. Plant Physiol. 1999;119:241–248. doi: 10.1104/pp.119.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baldwin JC, Karthikeyan AS, Raghothama KG. LEPS2, a phosphorus starvation-induced novel acid phosphatase from tomato. Plant Physiol. 2001;125:728–737. doi: 10.1104/pp.125.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aung K, Lin S-I, Wu C-C, Huang Y-T, Su C-L, Chiou T-J. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 2006;141:1000–1011. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu JQ, Allan DL, Vance CP. Systemic signaling and local sensing of phosphate in common bean: cross-talk between photosynthate and microRNA399. Mol Plant. 2010;3:428–437. doi: 10.1093/mp/ssq008. [DOI] [PubMed] [Google Scholar]
- 121.Kuo H-F, Chiou T-J. The role of microRNAs in phosphorus deficiency signaling. Plant Physiol. 2011;156:1016–1024. doi: 10.1104/pp.111.175265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu T-Y, Aung K, Tseng C-Y, Chang T-Y, Chen Y-S, Chiou T-J. Vacuolar Ca2+/H+ transport activity is required for systemic phosphate homeostasis involving shoot-to-root signaling in Arabidopsis . Plant Physiol. 2011;156:1176–1189. doi: 10.1104/pp.111.175257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. J Exp Bot. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- 124.Franco-Zorrilla JM, Martín AC, Leyva A, Paz-Ares J. Interaction between phosphate starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol. 2005;138:847–857. doi: 10.1104/pp.105.060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu J, Samac DA, Bucciarelli B, Allan DL, Carroll P, Vance CP. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J. 2005;41:257–268. doi: 10.1111/j.1365-313X.2004.02289.x. [DOI] [PubMed] [Google Scholar]
- 126.Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis . Planta. 2007;225:907–918. doi: 10.1007/s00425-006-0408-8. [DOI] [PubMed] [Google Scholar]
- 127.Mündermann L, Erasmus Y, Lane B, Coen E, Prusinkiewicz P. Quantitative modeling of Arabidopsis development. Plant Physiol. 2005;139:960–968. doi: 10.1104/pp.105.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arsenault JL, Pouleur S, Messier C, Guay R. WinRHIZO, a root-measuring system with a unique overlap correction method. HortScience. 1995;30:906. [Google Scholar]
- 129.Armengaud P, Zambaux K, Hills A, Sulpice R, Pattison RJ, Blatt MR, Amtmann A. EZ-Rhizo: integrated software for the fast and accurate measurement of root system architecture. Plant J. 2009;57:945–956. doi: 10.1111/j.1365-313X.2008.03739.x. [DOI] [PubMed] [Google Scholar]
- 130.Zeng XQ, Chow WS, Su LJ, Peng XX, Peng CL. Protective effect of supplemental anthocyanins on Arabidopsis leaves under high light. Physiol Plant. 2010;138:215–225. doi: 10.1111/j.1399-3054.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- 131.Plaxton WC, Carswell MC. Metabolic aspects of the phosphorus starvation response in plants. In: Lerner HR, editor. Plants response to environmental stresses: from phytohormones to genome organization. New York: Marcel Dekker; 1999. pp. 349–372. [Google Scholar]
- 132.Ballicora MA, Iglesias AA, Preiss J. ADP–glucose pyrophosphorylase:a regulatory enzyme for plant starch synthesis. Photosynth Res. 2004;79:1–24. doi: 10.1023/B:PRES.0000011916.67519.58. [DOI] [PubMed] [Google Scholar]
- 133.Dai X, Wang Y, Yang A, Zhang W-H. OsMYB2P-1, a R2R3 MYB transcription factor, is involved in regulation of phosphate-starvation responses and root architecture in rice. Plant Physiol. 2012;159:169–183. doi: 10.1104/pp.112.194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li D, Zhu H, Liu K, Liu X, Leggewie G, Udvardi M. Purple acid phosphatases of Arabidopsis thaliana. Comparative analysis and differential regulation by phosphate deprivation. J Biol Chem. 2002;31:27772–27781. doi: 10.1074/jbc.M204183200. [DOI] [PubMed] [Google Scholar]
- 135.Tran HT, Hurley BA, Plaxton WC. Feeding hungry plants: the role of purple acid phosphatases in phosphate nutrition. Plant Sci. 2010;179:14–27. doi: 10.1016/j.plantsci.2010.04.005. [DOI] [Google Scholar]
- 136.del Pozo JC, Allona I, Rubio V, Leyva A, de la Pena A, Aragoncillo C, Paz-Ares J. A type 5 acid phosphates gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilizing/oxidative stress conditions. Plant J. 1999;19:579–589. doi: 10.1046/j.1365-313X.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 137.Bariola PA, MacIntosh GC, Green PJ. Regulation of S-like ribonuclease levels in Arabidopsis: antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol. 1999;119:331–342. doi: 10.1104/pp.119.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hartel H, Dormann P, Benning C. DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis . Proc Natl Acad Sci USA. 2000;97:10649–10654. doi: 10.1073/pnas.180320497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu B, Xu C, Benning C. Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth. Proc Natl Acad Sci USA. 2002;99:5732–5737. doi: 10.1073/pnas.082696499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shin H, Shin HS, Dewbre GR, Harrison MJ. Phosphate transporter in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low-and high-phosphate environments. Plant J. 2004;39:629–642. doi: 10.1111/j.1365-313X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- 141.Shin H, Shin H-S, Chen R, Harrison MJ. Loss of At4 function impacts phosphate distribution between the roots and the shoots during phosphate starvation. Plant J. 2006;45:712–726. doi: 10.1111/j.1365-313X.2005.02629.x. [DOI] [PubMed] [Google Scholar]
- 142.Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 143.Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes Dev. 2002;16:1616–1626. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis . Curr Biol. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 145.Chiou T-J, Aung K, Lin S, Wu C, Chiang S, Su C. Regulation of phosphate homeostasis by microRNA in Arabidopsis . Plant Cell. 2006;18:412–421. doi: 10.1105/tpc.105.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Allen E, Xie Z, Gustafson AM, Carrington JC. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 147.Delhaize E, Randall PJ. Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana . Plant Physiol. 1995;107:207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong B, Rengel Z, Delhaize E. Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana . Plant Physiol. 1998;205:251–256. doi: 10.1007/s004250050318. [DOI] [PubMed] [Google Scholar]
- 149.Lin S-I, Chiang S-F, Lin W-Y, Chen J-W, Tseng C-Y, Wu P-C, Chiou T-J. Regulatory network of microRNA399 and PHO2 by systemic signalling. Plant Physiol. 2008;147:732–746. doi: 10.1104/pp.108.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pant BD, Buhtz A, Kerr J, Schreiber WR. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wykoff DD, O’Shea EK. Phosphate transport and sensing in Saccharomyces cerevisiae . Genetics. 2001;159:1491–1499. doi: 10.1093/genetics/159.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Duan K, Yi K, Dang L, Huang H, Wu W, Wu P. Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J. 2008;54:965–975. doi: 10.1111/j.1365-313X.2008.03460.x. [DOI] [PubMed] [Google Scholar]
- 153.Wang C, Ying S, Huang H, Li K, Wu P, Shou H. Involvement of OsSPX1 in phosphate homeostasis in rice. Plant J. 2009;57:895–904. doi: 10.1111/j.1365-313X.2008.03734.x. [DOI] [PubMed] [Google Scholar]
- 154.Liu F, Wang Z, Ren H, Shen C, Li Y, Ling HQ, Wu C, Lian X, Wu P. OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J. 2010;62:508–517. doi: 10.1111/j.1365-313X.2010.04170.x. [DOI] [PubMed] [Google Scholar]
- 155.Whiteaker G, Gerloff GC, Gabelman WH, Lindgren D. Intraspecific differences in growth of beans at stress levels of phosphorus. J Am Soc Hort Sci. 1976;101:472–475. [Google Scholar]
- 156.Beebe S, Lynch J, Galwey N, Tohme J, Ochoa I. A geographical approach to identify phosphorus-efficient genotypes among landraces and wild ancestors of common bean. Euphytica. 1997;95:325–336. doi: 10.1023/A:1003008617829. [DOI] [Google Scholar]
- 157.Gahoonia TS, Nielsen NE. Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Euphytica. 1997;98:177–182. doi: 10.1023/A:1003113131989. [DOI] [Google Scholar]
- 158.Gahoonia TS, Nielsen NE. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil. 2004;262:55–62. doi: 10.1023/B:PLSO.0000037020.58002.ac. [DOI] [Google Scholar]
- 159.Wissuwa M, Ae N. Genotypic variation for phosphorus uptake from hardly soluble iron-phosphate in groundnut (Arachis hypogaea L.) Plant Soil. 1999;206:165–173. [Google Scholar]
- 160.Wissuwa M, Ae N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001;120:43–48. doi: 10.1046/j.1439-0523.2001.00561.x. [DOI] [Google Scholar]
- 161.Wissuwa M, Ae N. Genotypic differences in the presence of hairs on roots and gynophores of peanuts (Arachis hypogaea L.) and their significance for phosphorus uptake. J Expt Bot. 2001;52:1703–1710. doi: 10.1093/jexbot/52.361.1703. [DOI] [PubMed] [Google Scholar]
- 162.Singh SP, Urrea CA, Gutierrez JA, Garcia J. Selection for yield at two fertilizer levels in small seeded common bean. Can J Plant Sci. 1989;69:1011–1017. doi: 10.4141/cjps89-122. [DOI] [Google Scholar]
- 163.Lynch JP, Beebe SE. Adaptation of beans (Phaseolus vulgaris L.) to low phosphorus availability. HortScience. 1995;30:1165–1171. [Google Scholar]
- 164.Narang RA, Bruene A, Altman T. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 2000;124:1786–1799. doi: 10.1104/pp.124.4.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wissuwa M, Wegner J, Ae N, Yano M. Substitution mapping of Pup1:a major QTL increasing phosphorus uptake of rice. Theor Appl Genet. 2002;105:890–897. doi: 10.1007/s00122-002-1051-9. [DOI] [PubMed] [Google Scholar]
- 166.Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana . Plant Cell Environ. 2006;29:115–125. doi: 10.1111/j.1365-3040.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- 167.Heuer S, Lu X, Chin JH, Tanaka JP, Kanamori H, Matsumoto T, De Leon T, Ulat VJ, Ismail AM, Yano M, Wissuwa M. Comparative sequence analyses of the major quantitative trait locus Phosphorus uptake 1 (Pup1) reveal a complex genetic structure. Plant Biotechnol J. 2009;7:456–471. doi: 10.1111/j.1467-7652.2009.00415.x. [DOI] [PubMed] [Google Scholar]
- 168.Chin JH, Haefele SM, Gamuyao R, Ismail A, Wissuwa M, Heuer S. Development and application of gene based markers for the major rice QTL phosphorus uptake 1. Theor Appl Genet. 2010;120:1073–1086. doi: 10.1007/s00122-009-1235-7. [DOI] [PubMed] [Google Scholar]
- 169.Misson J, Thibaud MC, Bechtold N, Raghothama KG, Nussaume L. Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol Biol. 2004;55:727–741. doi: 10.1007/s11103-004-1965-5. [DOI] [PubMed] [Google Scholar]
- 170.Mitsukawa N, Okumura S, Shirano Y, Sato S, Kato T, Harashima S, Shibata D. Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc Natl Acad Sci USA. 1997;94:7098–7102. doi: 10.1073/pnas.94.13.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Seo HM, Jung Y, Song S, Kim Y, Kwon T, Kim DH, Jeung SJ, Yi YB, Yi G, Nam MH, et al. Increased expression of OsPT1, a high-affinity phosphate transporter, enhances phosphate acquisition in rice. Biotechnol Lett. 2008;30:1833–1838. doi: 10.1007/s10529-008-9757-7. [DOI] [PubMed] [Google Scholar]
- 172.Park MR, Baek SH, de los Reyes BG, Yun SJ. Overexpression of a high-affinity phosphate transporter gene from tobacco (NtPT1) enhances phosphate uptake and accumulation in transgenic rice plants. Plant Soil. 2007;292:259–269. doi: 10.1007/s11104-007-9222-8. [DOI] [Google Scholar]
- 173.Rae AL, Jarmey JM, Mudge SR, Smith FW. Over-expression of a high-affinity phosphate transporter in transgenic barley plants does not enhance phosphate uptake rates. Funct Plant Biol. 2004;31:141–148. doi: 10.1071/FP03159. [DOI] [PubMed] [Google Scholar]
- 174.Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T. Metabolic engineering with Dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. Proc Natl Acad Sci USA. 2004;101:7833–7838. doi: 10.1073/pnas.0402267101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.González E, Solano R, Rubio V, Leyva A, Paz-Ares J. PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis . Plant Cell. 2005;17:3500–3512. doi: 10.1105/tpc.105.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174. doi: 10.1074/jbc.272.45.28171. [DOI] [PubMed] [Google Scholar]
- 177.Krogan NJ, Keogh MC, Datta N, et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell. 2003;12:1565–1576. doi: 10.1016/S1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 178.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 179.Deal RB, Kandasamy MK, McKinney EC, Meagher RB. The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis . Plant Cell. 2005;17:2633–2646. doi: 10.1105/tpc.105.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mitsuda N, Ohme-Takagi M. Functional analysis of transcription factors in Arabidopsis . Plant Cell Physiol. 2009;50:1232–1248. doi: 10.1093/pcp/pcp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thibaud-Nissen F, Wu H, Richmond T, Redman JC, Johnson C, Green R, Arias J, Town CD. Development of Arabidopsis whole-genome microarrays and their application to the discovery of binding sites for the TGA2 transcription factor in salicylic acid-treated plants. Plant J. 2006;47:152–162. doi: 10.1111/j.1365-313X.2006.02770.x. [DOI] [PubMed] [Google Scholar]
- 182.Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell. 2007;19:731–749. doi: 10.1105/tpc.106.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zheng Y, Ren N, Wang H, Stromberg AJ, Perry SE. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-like15. Plant Cell. 2009;21:2563–2577. doi: 10.1105/tpc.109.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Margulies M, Egholm M, Altman WE, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR. Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol. 2009;150:1541–1555. doi: 10.1104/pp.109.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schmutz J, Cannon SB, Schlueter J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]