Abstract
Successful development of sequence-specific siRNA (small interfering RNA)-based drugs requires an siRNA design that functions consistently in different organisms. Utilizing the CAPSID program previously developed by our group, we here designed siRNAs against mammalian target of rapamycin (mTOR) that are entirely complementary among various species and investigated their multispecies-compatible gene-silencing properties. The mTOR siRNAs markedly reduced mTOR expression at both the mRNA and protein levels in human, mouse, and monkey cell lines. The reduction in mTOR expression resulted in inactivation of both mTOR complex I and II signaling pathways, as confirmed by reduced phosphorylation of p70S6K (70-kDa ribosomal protein S6 kinase), 4EBP1 (eIF4E-binding protein 1), and AKT, and nuclear accumulation of FOXO1 (forkhead box O1), with consequent cell-cycle arrest, proliferation inhibition, and autophagy activation. Moreover, interfering with mTOR activity in vivo using mTOR small-hairpin RNA-expressing recombinant adeno-associated virus led to significant antitumor effects in xenograft and allograft models. Thus, the present study demonstrates that cross-species siRNA successfully silences its target and readily produces multispecies-compatible phenotypic alterations—antitumor effects in the case of mTOR siRNA. Application of cross-species siRNA should greatly facilitate the development of siRNA-based therapeutic agents.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-0998-1) contains supplementary material, which is available to authorized users.
Keywords: Small-interfering RNA, Cross-species activity, Mammalian target of rapamycin, CAPSID, Tumor therapeutics
Introduction
RNA interference (RNAi) is a post-transcriptional, sequence-specific mechanism by which small-interfering RNAs (siRNAs) comprising 19- to 26-nucleotide RNA duplexes knock down the expression of target genes [1, 2]. The potential of siRNA-based agents as a next-generation therapeutic modality has been extensively investigated in a wide variety of human diseases, including cancer, ocular degeneration, and virus-associated diseases [3–5]. In theory, any target gene can be modulated to achieve therapeutic efficacy by applying a properly designed siRNA.
Validating the therapeutic potential of siRNA in various cell-culture systems and animal models remains a prerequisite for clinical application. In this context, the strict sequence-specific property of siRNA would limit the clinical applicability of siRNAs designed and tested to work in non-human models; thus, designing single siRNA molecules with cross-species activity is crucial for efficient preclinical and clinical testing. Recently, we developed the novel program, CAPSID (Convenient Application Program for siRNA Design), to design siRNAs by primarily identifying highly conserved sequences across multiple targets [6]. Using the CAPSID program simultaneously running multiple viral genome sequences, we successfully designed siRNAs that were universally effective against different serotypes of enteroviruses with sequence variations [6, 7]. The nature of CAPSID—an siRNA design based on conserved patterns-optimization from multiple sequences—thus allows extraction of siRNAs with multispecies-compatible gene-silencing activity.
Mammalian target of rapamycin (mTOR) has been identified as an attractive target for several human diseases, including cancer, diabetes, aging, and pain [8–11]. mTOR function is mediated by two distinct multimeric complexes in which mTOR is the common major player: mTOR complex 1 (mTORC1) and mTORC2; the available evidence indicates that mTORCs are differentially regulated [12, 13]. Activation of mTORC1 following mTOR phosphorylation at Ser2448 leads to subsequent phosphorylation of 4EBP1 (eIF4E-binding protein 1) and p70S6K (70-kDa ribosomal protein S6 kinase), resulting in cap-dependent translation. Additionally, mTORC1 interrupts autophagy induction by inactivating Ulk1 (unc-51 [C. elegans]-like kinase 1) through phosphorylation [14]. In contrast, mTORC2 is activated through phosphorylation of mTOR at Ser2481, leading to subsequent phosphorylation of AKT at Ser473. Phosphorylation of FOXO1 (forkhead box O1), also a downstream event, leads to the translocation of FOXO1 from the nucleus to the cytosol. Thus, activation of the mTOR-signaling pathway is closely associated with multiple cellular responses, including cell growth, cell survival, and cell motility [12, 13]. Indeed, the mTOR-mediated signaling pathway was found to be constitutively upregulated in a broad spectrum of tumors [9, 10, 13]. Rapamycin and its analogues, temsirolimus and everolimus, which inhibit mTORC1 via FK507-binding protein 12 (FKBP12), have recently been developed as anticancer drugs for clinical use. However, these drugs block only mTORC1 and actually promote mTORC2 activity because of negative feedback loop inactivation, thus limiting their antitumor potency. Overcoming this limitation requires simultaneous inactivation of both mTORC activities, making mTOR itself an excellent target.
Recombinant adeno-associated virus (rAAV) has been identified as one of the most promising tools for in vivo delivery of gene-based drugs. The advantages of rAAV are well characterized, and include a lack of pathogenicity and toxicity, the ability to infect both dividing and non-dividing cells, low host immune response, and long-lasting transgene expression [15–17]. rAAV vectors have been approved by the Food and Drug administration for Phase I/II clinical trials in various types of human diseases [18]. By incorporating siRNA sequence information as short-hairpin RNA (shRNA) into the rAAV genome, the corresponding siRNAs can be readily expressed by rAAV vectors.
In this study, we sought to improve the efficacy of siRNA-based drugs and aid the development process by designing siRNA with cross-species activity. Utilizing CAPSID, which extracts siRNAs from conserved sequence patterns in a multi-sequence information context, we designed siRNAs targeting mTOR and determined their multispecies-compatible RNAi potency and underlying mechanisms. The cross-species antitumor effects of mTOR siRNA were further investigated in vivo in xenograft and allograft models using rAAV-mediated expression of mTOR shRNA.
Materials and methods
Cell culture
HeLa, SK-Hep1, A549, NCI-H292, MDA-MB231, B16-F10, SCC7, Cos7, and OMK cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) with 10 % fetal bovine serum (Gibco, Middleton, WI, USA), GlutaMAX-1 (2 mM), and penicillin (100 IU/ml)/streptomycin (50 μg/ml), and maintained at 37 °C under a humidified 5 % carbon dioxide atmosphere.
siRNA/shRNA preparation and treatment
siRNAs targeting mTOR were designed from a set of human (NM_004958), monkey (XR_014791), mouse (NM_020009), and rat (NM_019906) mTOR sequences using the CAPSID program (http://cms.ulsan.ac.kr/capsid). The siRNA score for each siRNA was calculated based on the number of siRNA design criteria described in the previous study [6]. siRNAs were synthesized by Dharmacon Inc. (Lafayette, CO, USA). As a negative control, control siRNA (5′-AUUCUAUCACUAGCGUGAC-3′) provided from Dharmacon Inc. was hired. Either control or mTOR siRNAs seemed unlikely to induce off-target effects, which were confirmed by limited NCBI BLAST search, no cytotoxicity in all the cell lines examined in the present study, and no induction of immunostimulatory gene (interferon-β; oligoadenylate synthetase) expressions in HeLa cells (data not shown). Recombinant adeno-associated virus 2 (rAAV2) vectors expressing mTOR no. 4 or control mall-hairpin RNA (shRNA) driven by an H1 promoter was constructed as described previously [19]. The shRNA sequences against mTOR (derived from mTOR siRNA no. 4) were 5′-GAT CCG AAT GTT GAC CAA TGC TAT TTC AAG AGA ATA GCA TTG GTC AAC ATT CTT TTT TGG AAA AGC T-3′ (sense) with a BamHI linker, and 5′-AGC TTT TCC AAA AAA GAA TGT TGA CCA ATG CTA TTC TCT TGA AAT AGC ATT GGT CAA CAT TCG-3′ (antisense) with a HindIII linker. Nucleotides specific for mTOR are underlined. The shRNA sequences against control were identical with the sense/antisense shRNAs for mTOR except the shRNA portions corresponding to control siRNA sequences. rAAV production and titration were carried out as described in a previous report [19].
Real-time reverse transcription-polymerase chain reaction
Total RNA was extracted using the TRIzol reagent (Invitrogen) and reverse-transcribed with Superscript III (Invitrogen) using oligo-dT primers (Invitrogen) at 55 °C. A real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed using SyBr Green I and the following primers: mouse-mTOR, 5′-CCA CTG TGC CAG AAT CCA TC-3′ (sense) and 5′-GAG AAA TCC CGA CCA GTG AG-3′ (antisense); human/monkey-mTOR, 5′-CCA CAG TGC CAG AAT CTA TT-3′ (sense) and 5′-GAG AAG TCC CGA CCA GTG AG-3′ (antisense); and β-actin (universal), 5′-TGA AGA TCA AGA TCA TTG CTC-3′ (sense) and 5′-TGA AGA TCA AGA TCA TTG CTC-3′ (antisense). Cycling conditions consisted of 3 min at 95 °C (initial denaturation/polymerase activation), followed by 39 cycles of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C.
5′ Rapid amplification of cDNA ends and DNA sequencing
To detect specific target mTOR mRNA cleavage, 5′ rapid amplification of cDNA ends (RACE) was performed according to the GeneRacer manual (Invitrogen) with some modifications. When sequence-specific mRNA cleavage by mTOR siRNA occurs, a PCR product of 210 bp in size would be obtained. Briefly, 5 μg of total RNA was ligated to 250 ng of GeneRACER RNA adaptor using T4 ligase (NEB, Ipswich, MA, USA). RT was carried out using mTOR siRNA no. 4 specific antisense primers (5′-GAT GAG CAG CTC AAC TTG CGT TGG-3′). The RT product was amplified using a GeneRACER-specific sense primer (5′-CGA CTG GAG CAC GAG GAC ACT G-3′) and an mTOR-specific antisense primer (5′-TCC TCG TTC GGG ATC GCT TGT TGC TGC C-3′). A second round of nested PCR employed a GeneRACER-specific nested sense primer (5′-GGA CAC TGA CAT GGA CTG AAG G-3′) and an mTOR-specific nested antisense primer (5′-GTG TCC ATC AGC CTC CAG TTC AGC-3′). PCR was performed with HighFidelity Taq polymerase using the following cycling conditions: 95 °C for 3 min, followed by 20 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, with a final extension step of 72 °C for 10 min. PCR products were cloned and sequenced.
Immunoblot analyses
Proteins were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with Tris-buffered saline (TBS) containing 0.1 % Tween-20 and 5 % (w/v) bovine serum albumin, membranes were incubated with various primary antibodies and appropriate secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Bands were detected using an enhanced chemiluminescence (ECL) system. mTOR, phospho-mTOR(S2448), p70S6K, phospho-S6K(T389), 4EBP1, phospho-4EBP1 (T37/46), AKT, and phospho-AKT(S473) antibodies were obtained from Cell Signaling (Boston, MA, USA). The β-actin antibody was from Sigma (St. Louis, MO, USA).
Translocation of GFP-FOXO1
Subcellular localization of FOXO1 was determined by monitoring the green fluorescent protein (GFP) signal of GFP-FOXO1 as described previously [20]. HeLa cells were cotransfected using Lipofectamine plus (Invitrogen) with a GFP-FOXO1 expression plasmid (1 μg; Addgene plasmid 17551) and 100 nM of either control siRNA or mTOR siRNA. Seventeen hours after transfection, cells were stained with Hoechest 33342 (1 μg/ml) for 10 min and observed under the fluorescence microscope. The degree of the relocalization of GFP-FOXO1 from cytosols to nuclei was quantified by determining the GFP signals in each compartment.
Evaluation of cap-dependent translation
Cap-dependent translation was determined using a bicistronic reporter system, in which the activity of the firefly luciferase gene (under the control of cap-dependent translation) relative to that of the Renilla luciferase gene (under the control of an internal ribosome entry site) was calculated and expressed as a ratio.
Transmission electron microscopy
HeLa cells transfected with siRNAs were harvested and embedded in 2 % agar containing 10 mM MgCl2. The solidified cell pellet was fixed in 4 % glutaraldehyde at 4 °C, sectioned, and stained with 1 % uranyl acetate and/or lead citrate. Stained sections were imaged and photographed with a JEOL 100CX transmission electron microscope.
Cell cycle and cell index analysis
HeLa cells were transfected with either control siRNA or mTOR siRNA. For cell cycle analysis, the cells were trypsinized at 48 h and fixed with ice-cold ethanol. The cells were then incubated with 0.05 % propidium iodide and analyzed using a Becton-Dickinson FACSCalibur flow cytometer with CellQuest software.
For cell index analysis, the cells transfected with siRNAs were plated on an E-Plate96 after 48 h and incubated for 30 min at room temperature. The cells were then placed in a Real-Time Cell Analyzer Station. The dynamic cell index was monitored using the xCELLigence System (Roche Applied Science, Mannheim, Germany), according to the supplier's instructions. Dynamic cell index values were calculated and plotted graphically.
In vivo tumor models
Male, 5- to 7-week-old BLAB/c nude mice from Orient Bio Inc. (Seongnam, Korea) were utilized. All animal procedures were approved by the Institutional Animal Care and Use Committee of this Institution. HeLa or B16-F10 cells were infected with rAAV-mTOR shRNA or rAAV-control shRNA at a multiplicity of infection (MOI) or 300 (HeLa) or 1000 (B16-F10). After incubating for 1 day, 200 μl of transduced HeLa (5 × 106) or B16-F10 (1 × 106) cells was subcutaneously injected into nude mice. For systemic administration of rAAV2-shRNAs, NCI-H292 cells (1 × 107) were implanted into the shoulders. After 11 days, mice were intravenously administered viruses [1.5 × 1011 vector genomes (v.g.)/mouse]. Tumor diameter and body weight were measured every 1–3 days; tumor volume was calculated by the formula (length × width × height) × (π/6). After killing animals at designated times, tumors were collected and subjected to further analysis.
Statistical analysis
Statistics were performed using GraphPad Instat (GraphPad Software, La Jolla, CA, USA). “n” indicated how many independent assays were carried out for each study. Standard deviation (SD) was calculated from the values of these independent assays. The data were reported as the mean ± SD. Statistical analyses between the experimental groups were performed using a Student’s t test and two-way analysis of variance. Values of p < 0.05 were considered to indicate statistical significance. *p < 0.05, **p < 0.01.
Results
Efficient multispecies-compatible gene-silencing by mTOR siRNAs designed for cross-species activity
To design single siRNA molecules exhibiting cross-species activity, we used the CAPSID program to screen siRNA candidates from completely conserved sequence (CCS) patterns in multiple species scores [6]. The CAPSID employed hierarchical filters based on sequence specificities and thermodynamic properties of the queries, and yielded possible siRNA candidates. A total of seven CCS patterns were extracted in a set of mTOR genes from human, monkey, mouse, and rat. On the basis of siRNA scores and RNA secondary structure (data not shown) in the target regions, we initially selected four candidates (mTOR siRNA no. 1–4) and ultimately employed siRNA no. 4 of the highest siRNA score for further studies (Table 1). All mTOR siRNAs (nos. 1–4) sharply downregulated both mRNA and protein levels of mTOR in HeLa cells compared to control siRNA (Fig. 1a). The mTOR siRNA-mediated decrease in mTOR protein was clearly noticed at both 24 and 48 h following siRNA treatment, and there was no non-specific response over time in the presence of control siRNA (Fig. 1b). Utilizing modified 5′ RACE and sequencing techniques, we further verified that target mRNA was specifically cleaved through RNAi between positions 10 and 11 of the siRNA guide strand to generate a 210-bp PCR product (Fig. 1c) [21]. The cross-species activity of mTOR siRNA was examined in several cell lines of human, primate, and murine origin. mTOR siRNA treatment markedly reduced target gene expression at both mRNA and protein levels in all cell lines originated from various species (Fig. 2). Collectively, the data provide clear evidence that mTOR siRNAs designed to be cross-species reactive indeed induced multispecies-compatible target gene-silencing through RNAi.
Table 1.
Sequences of cross-species siRNA candidates
| Name | Target sequence (5′ → 3′) | Position (nucleotides) | Target | siRNA-score |
|---|---|---|---|---|
| mTOR siRNA no. 1 | GGAGUCUACUCGCUUCUAU | 253 | HEAT | 9.5 |
| mTOR siRNA no. 2 | GAAGAAGGUCACUGAGGAU | 5677 | FAT | 9 |
| mTOR siRNA no. 3 | ACAACCUCCAGGAUACACU | 5772 | FAT | 8.5 |
| mTOR siRNA no. 4 | GAAUGUUGACCAAUGCUAU | 7221 | Kinase | 13 |
Fig. 1.
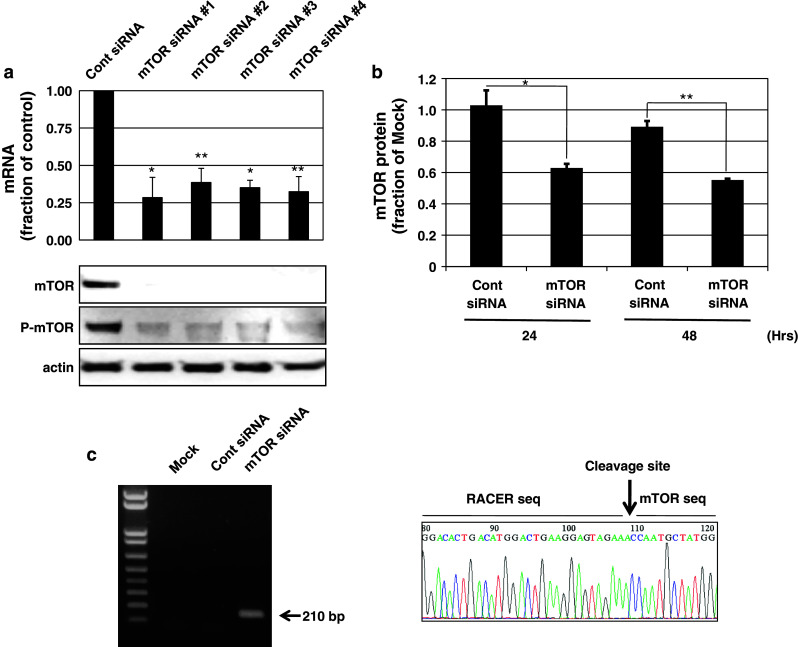
Gene-silencing effects of siRNA candidates targeting mTOR in HeLa cells. a mTOR mRNA and protein levels were measured by real-time RT-PCR (upper panel) and immunoblot analysis (lower panel). HeLa cells were transfected with mTOR-targeting siRNA or control siRNA at 100 nM using Oligofectamine. After 24 h, total RNA was isolated for RT, or whole-cell lysates were prepared for immunoblot analyses (n = 3). Real-time PCR was carried out using iQ SYBR Green Supermix as described in “Materials and methods.” b mTOR protein levels were monitored at 24 and 48 h. Band densities were analyzed with a densitometer (LAS-4000, n = 3). c To detect specific target mTOR mRNA cleavage, modified 5′RACE designed to amplify cleaved mTOR mRNA as final PCR product was performed (n = 2). Total RNA from cells transfected with the corresponding siRNAs was ligated to the GeneRACER RNA adaptor. Sequence-specific mRNA cleavage by mTOR siRNA was confirmed by both the presence of 210-bp PCR products as indicated by arrow and sequencing analysis for this PCR product. Each value represents the mean ± SD. *p < 0.05, **p < 0.01
Fig. 2.
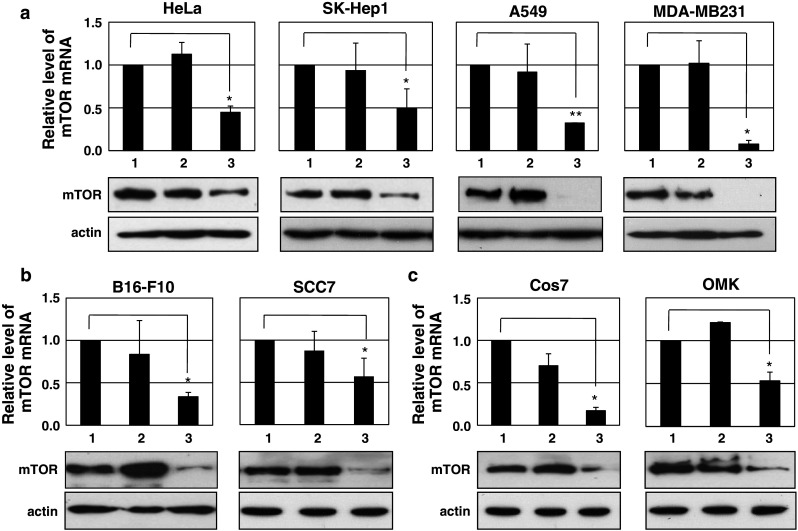
Cross-species activity of mTOR siRNA in cell lines. Cross-species effects of mTOR siRNA on mTOR gene expression were determined by real-time RT-PCR (upper panel graph; n = 3) and immunoblot analysis (lower panel; n ≥ 2) in cell lines of human (a), murine (b), and monkey (c) origin. Experimental details are provided in Fig. 1a. Lane 1 mock-treated, lane 2 control siRNA-treated, lane 3 mTOR siRNA no. 4-treated. Each value represents the mean ± SD. *p < 0.05, **p < 0.01
Significant interference of the mTOR signaling pathway by mTOR siRNA
To investigate the effects of mTOR siRNA on the mTORC1 signaling pathway, we examined the expression of mTOR protein and the activities of downstream substrates by immunoblot analysis. mTOR siRNA treatment decreased the levels of both Ser2448-phosphorylated mTOR and total mTOR (Fig. 3a). mTOR siRNA dramatically reduced the levels of phospho-p70S6K(Thr389) and phospho-4EBP1(Thr37/46)—the active forms of mTORC1 downstream signaling substrates—without affecting total levels of the corresponding proteins (Fig. 3a). The activation of the mTORC1 pathway has also been identified to promote the cap-dependent translation through 4EBP1 regulation [8]. Cap-dependent translation by mTORC1 was decreased by 84 ± 8 % compared to the control following mTOR siRNA treatment (Fig. 3b). The decrease in the rate of cap-dependent translation induced by mTOR siRNA was similar to that caused by rapamycin (89 ± 4 %), a well-known mTORC1 inhibitor.
Fig. 3.
Inhibition of the mTORC1 signaling pathway in HeLa cells transfected with mTOR siRNA. a HeLa cells were transfected with mTOR siRNA no. 4, and experiments were performed as detailed in Fig. 1a. Immunoblot analyses were carried out to determine the amount of phospho-mTOR(S2448), phospho-S6K(T389), and phospho-4EBP1(T37/46), as well as the total amount of the corresponding proteins (n = 3). b Cap-dependent translation was determined in HeLa cells transfected with both siRNA and a bicistronic reporter plasmid expressing firefly luciferase (cap-dependent translation) and Renilla luciferase (under the control of an internal ribosome entry site), as described in “Materials and methods” (n = 3). Each value represents the mean ± SD. *p < 0.05
The interfering effect of mTOR siRNA on mTORC2 signaling was investigated by determining the degree of phospho-mTOR(S2481) and phospho-AKT(S473) by immunoblot analysis. As shown in Fig. 4a, mTOR siRNA treatment decreased both phospho-mTOR and the resultant levels of phospho-AKT. The total amount of AKT protein remained unchanged, as was the case for mTORC1 downstream substrates. Another typical mTOR2 downstream phenotype, accumulation of phospho-FOXO1 in the cytosol, was determined by monitoring GFP signals after transfecting an expression vector for GFP-FOXO1 fusion protein. As expected, most GFP signal was observed in the cytosol in the presence of control siRNA (Fig. 4b). By contrast, GFP-FOXO1 was mostly located in nuclei after mTOR siRNA treatment, indicating inhibition of FOXO1 phosphorylation because of inactivation of mTORC2 signaling. The ratio of nuclear to cytosolic FOXO1 was 16 ± 7 % in control siRNA-transfected cells and 72 ± 14 % in mTOR siRNA transfected cells. Taken together with the results shown in Fig. 3, the data presented in Fig. 4 indicate that the knockdown of mTOR expression by mTOR siRNA profoundly interferes with the entire mTOR signaling pathway, composed of mTORC1 and mTORC2.
Fig. 4.
Inhibition of the mTORC2 signaling pathway in HeLa cells transfected with mTOR siRNA. a The expression levels of phosphorylated mTOR(S2481) and AKT(S473) as well as the total amount of the corresponding proteins were examined in cells treated as described in Fig. 3a (n = 3). b FOXO1 distribution was evaluated in HeLa cells by monitoring the GFP signal 17 h after transfecting with a GFP-FOXO1 fusion protein expression vector and corresponding siRNA (n = 2). Nuclei were stained with Hoechst 33258, and images were observed by fluorescence microscopy using excitation wavelengths of 490 nm (for GFP) and 360 nm (for Hoechst). Each value represents the mean ± SD. **p < 0.01
Altered mTOR-associated cellular responses following mTOR siRNA treatment
The effect of mTOR siRNA-mediated inhibition of mTOR signaling on cellular properties was initially determined by examining cell-cycle distribution by flow cytometry. mTOR siRNA treatment markedly increased the proportion of cells in G1 phase and decreased those in S/M phase; the proportion of G1-phase cells was increased by 20 ± 3 % and that of S/M-phase cells was decreased by 19 ± 9 % in mTOR siRNA-transfected cells compared to the corresponding proportions in the control group (Fig. 5a, Supplemental Fig. 1). The extent of the G1 arrest induced by mTOR siRNA seemed to be similar or somewhat higher than that induced by rapamycin [22, 23]. Cell growth and cytotoxicity were also monitored by real-time using the xCELLigence System and expressed as cell indexes. Following mTOR siRNA treatment, cell indexes were gradually reduced and only reached 49 ± 6 % of those in control siRNA-treated cells 140 h post-transfection (Fig. 5b). The effect of mTOR siRNA treatment on autophagy was examined by observing autophagic vesicle formation by transmission electron microscopy. Typical autophagic vesicles of distinct size with double-layered membranes were readily detectable in cells treated with mTOR siRNA, but not in those treated with control siRNA (Fig. 5c). Thus, these data strongly suggest that mTOR siRNA treatment significantly interferes with mTOR-associated cellular responses, namely cell growth and autophagy, because of inactivation of the mTOR signaling pathway.
Fig. 5.
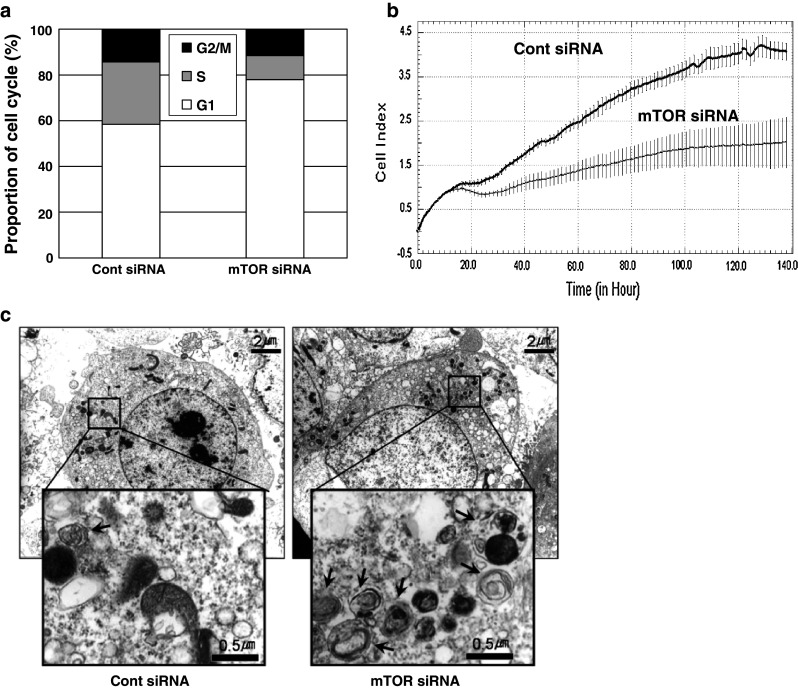
Changes in cell growth and autophagy induced by mTOR siRNA treatment. HeLa cells were transfected with siRNAs, as described in Fig. 1a, and analyzed after 48 h. a Cells were fixed in cold ethanol, stained with 0.05 % propidium iodide, and analyzed by flow cytometry with the aid of the CellQuest Program (n = 3). b Cells in 96-well plates were continuously monitored with an xCELLigence real-time cell analyzer system for 140 h (n = 2). Y axis means cell index that represents the properties of cell proliferation and viability. c Cells were prepared for transmission electron microscopy analysis and examined for the presence of autophagic vesicles. Black arrows indicate autophagic vesicles
Multispecies-compatible antitumor effects of mTOR shRNA-expressing rAAV in vivo
To verify the antitumor effects of mTOR siRNA in vivo, we first generated rAAVs expressing mTOR shRNA along with GFP as a reporter gene (rAAV-mTOR shRNA) (Supplemental Fig. 2a, b). rAAV-mTOR shRNA decreased mTOR mRNA levels by 33 % at 2 days and 72 % at 4 days post-infection compared to rAAV-Cont shRNA (Supplemental Fig. 2c). Similarly, mTOR protein expression was substantially decreased, and inactivation for mTORC1 and mTORC2 was also confirmed by a reduction in both phospho-mTORs and phospho-p70S6K levels (Supplemental Fig. 2d).
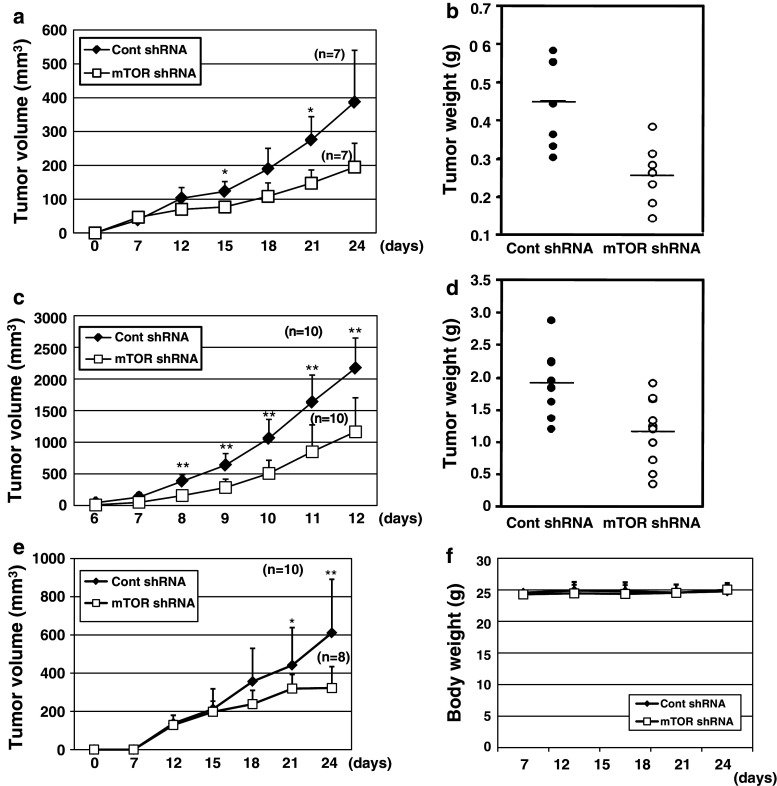
The cross-species antitumor activity of mTOR siRNA was determined in the human-origin HeLa xenograft model and the mouse-origin B16-F10 allograft model. Tumor growth rates were greatly retarded in tumor cells transduced with rAAV-mTOR shRNA regardless of cell origin (Fig. 6a, c). Final tumor volumes in HeLa and B16-F10 models with rAAV-mTOR shRNA were reduced by 50 ± 18 % and 51 ± 24 %, respectively, compared to shRNA control group. Actual tumor weights with rAAV-mTOR shRNA treatment were decreased by approximately 36 ± 9 % in HeLa-xenografted animals and by 60 ± 27 % in B16-F10-xenografted animals (Fig. 6b, d). Moreover, mTOR protein levels were reduced in cells of both rAAV-mTOR shRNA-transduced cell lines (data not shown), as further confirmed by analysis of tumors at the end of the experimental period (Supplemental Fig. 3a, b). Target-specific mTOR mRNA cleavage was also confirmed using xenografted HeLa tumors by modified 5′ RACE (Supplemental Fig. 3c) and sequencing analysis for this PCR product (data not shown).
Fig. 6.
Antitumor effects of rAAV expressing mTOR shRNA with cross-species activity. HeLa (a, b) and B16-F10 (c, d) cells were infected with rAAV expressing mTOR shRNA or control shRNA and implanted into nude mice, as described in “Materials and methods.” Tumor growth and body weight were monitored every 1–3 days. After killing the animals, tumors were removed, weighed, and prepared for further analysis. e, f Eleven days after implanting NCI-H292 cells into the shoulders of nude mice, 1.5 × 1011 v.g. of either virus was intravenously injected. There was no morbidity change or animal death during the animal studies. Each value represents the mean ± SD. n the number of animal. *p < 0.05, **p < 0.01
The therapeutic potential of mTOR siRNA was additionally investigated by intravenously administering rAAV-mTOR shRNA in the H292 xenograft model. With only a single administration of virus, tumor growth was significantly delayed, as reflected in the estimated 49 ± 11 % decrease in tumor volume (Fig. 6e). Inhibition of tumor growth by rAAV-mTOR shRNA was similar to that induced by administration of the anti-cancer drug paclitaxel (data not shown). Notably, there was no significant change in body weight after rAAV injection. Plus, H&E staining analysis indicated that the normal hepatic architecture was well preserved in virus-experienced animals (Supplemental Fig. 3d). Also, other tissues of these animals, such as heart, kidney, spleen, and lung, did not show any sign of histological alteration (data not shown), indicating that this treatment is minimally toxic. Collectively, the results suggest that administration of cross-species mTOR siRNA via rAAV can induce potent multispecies-compatible antitumor activities.
Discussion
This study provides clear evidence that (1) an mTOR siRNA sequence extracted by CAPSID efficiently silences gene expression through a RNAi mechanism in a multispecies-compatible manner; (2) mTOR siRNA treatment establishes a cytostatic status in cancer cells by interfering with both mTORC1 and mTORC2 activities; and (3) cross-species antitumor effects can be achieved in vivo by introducing mTOR siRNA via rAAV. Collectively, our data thus demonstrate that acquiring cross-species siRNA assures a universally effective RNAi response among species as the first step in the development of siRNA-based agents.
Unlike chemical drugs, siRNA therapeutics were limited by the sequence specificity of siRNA targeting mechanisms. One of the essential features of siRNA designed by CAPSID is that the siRNA sequence is entirely complementary to multiple target genes [6, 24]. In theory, siRNAs with cross-species activity would be extracted by simultaneously screening RNA sequence information from various species, including human, monkey, and mouse. However, prior to this report, no available software allowed for the simultaneous selection of siRNA candidates that targeted conserved regions from multiple sequences. Without this capability, there is a high likelihood that siRNA selected based on the sequence information of one species would have target sequence mismatches in other species. siRNA target sequence mismatches can demolish RNAi activity or induce off-target effects, both of which are major problems in siRNA applications [25, 26]. However, cross-species siRNA overcomes this limitation. Because it is based on a simultaneous sequence analysis of multiple species, including humans, a single siRNA designed by CAPSID can be readily utilized throughout all phases of drug development, from cell- and animal-based evaluation systems to clinical trials. Moreover, off-target effects by cross-species siRNA in one species would be more applicable to predicting off-target effects in another species. Thus, cross-species siRNA should facilitate the development of siRNA therapeutics.
Several previous reports have directly addressed the application of cross-species RNAi. To understand gene expression profiling associated with anhydrobiosis of the nematode Aphelenchus avenae, which is refractile to the RNAi technique, researchers treated another nematode, Panagrolaimus superbus, with siRNAs designed based on A. avenae [27]. In addition, a universal method for cross-species RNAi rescue in Drosophila was also developed as a feasible means for validating large-scale RNAi screening results [28]. Similar to our previous studies [7], Kumar et al. [29] also reported that a single siRNA induced antiviral activities against two different flaviviruses.
The cross-species activity of mTOR siRNA was clearly noticed at both the mRNA and protein levels across all cell lines originated from various species. Yet, the degree of gene knockdown varied among different cells. The degree of gene knockdown analyzed by real-time PCR varied among different cells, ranging from a high of 92 ± 4 % in human MDA-MB231 cells to a low of 47 ± 10 % in monkey OMK cells at the mRNA level. We believe there are several explanations for these differences in silencing efficacy. One of the major reasons for this discrepancy could be the accessibility of target RNA; this could be due to the difference in ordered structure of target RNA itself or in complexed architecture with accessory proteins. In addition, it would be possibly due to the difference in the endogenous expression level of mTOR mRNA among various cell lines. Plus, knockdown efficacy could be influenced by experimental factors, such as transfection efficiency or culture condition.
In the present study, we further expanded and confirmed the application of cross-species RNAi using mTOR as a target of therapeutic siRNA in cancer models. Achieving strong antitumor potency through modulation of the mTOR signaling pathway requires inactivation of both mTORC1 and mTORC2. Cross-species mTOR siRNAs markedly silenced mTOR, which is an essential positive regulator in both mTORC complexes. As a result, mTOR signaling in its entirety was interrupted, as evidenced by cell-cycle arrest, growth retardation, and induction of autophagy. Knocking down the entire mTOR signaling pathway further led to antitumor effects in vivo with minimal toxicity. In agreement with our results, several reports have consistently shown that inhibition of both mTORC1 and mTORC2 signaling by siRNA or inhibitors can induce potent antitumor activity [30, 31]. PP242, an inhibitor of the active site of mTOR in mTORC1/2, was shown to cause the death of human leukemia cells and delay the onset of leukemia [32]. AZD8055, a novel ATP-competitive inhibitor of mTOR, induced antitumor activity in vitro and in vivo [33]. Treatment with siRNA against mTOR resulted in downregulation of the mTOR signaling pathway and exerted antitumor activity in a human colorectal cancer model [34].
Given their distinct roles, other components of mTORC1 and mTORC2 complexes could be excellent targets of cross-species siRNAs, designed as part of efforts to develop siRNA-based therapeutic agents in various human diseases, such as diabetes and muscular disorders. For example, specific inhibition of mTORC1 using siRNA against Raptor, a component of mTORC1, has been shown to increase lipolysis and decrease fat accumulation in cells [35]. siRNA targeting Rictor, a component of mTORC2, was reported to regulate myoblast differentiation [36]. In this regard, cross-species siRNAs for other mTORC components would expand the potential range of the mTOR signaling pathway as a target in the development of therapeutic agents. Moreover, cross-species siRNAs offer potential therapeutic strategies for many other pathological conditions considered amenable to the application of siRNA therapeutics [5].
We found that a single systemic administration of mTOR shRNA-expressing rAAV induced efficient antitumor effects, producing results comparable to those achieved by multiple administrations of paclitaxel (data not shown). This suggests that mTOR downregulation by mTOR shRNA-expressing rAAV effectively induces prolonged inhibition of mTORC1/2 function and further implies that rAAV efficiently delivers mTOR shRNA in vivo. We also recently reported persistent antitumor effects of rAAV-delivered siRNA, monitored using micro-PET imaging [37]. Kota et al. [38] demonstrated that systemic administration of micro RNA-26a using rAAV induced antitumor effects in liver cancer. Sun et al. [39] showed that rAAV encoding shRNA specific for the androgen receptor exerted strong gene-silencing activity in prostate cancer models. Furthermore, exciting progress has been reported for rAAV vectors used against other disease modalities, such as retinal disorders, muscular dystrophy, and neuronal disorders [18, 40–42]. We also demonstrated that decreased levels of GTP cyclohydrolase I (GCH1) induced by shGCH1-expressing rAAV relieved pain in an animal model [19]. Taken together, these observations suggest the promise of rAAV in therapeutic applications of RNAi technology.
In conclusion, we provide evidence that a single, multispecies-compatible mTOR siRNA can establish anti-mTOR activity by directly interfering with the mTOR signaling pathway and show that delivering mTOR siRNA via rAAV leads to potent cross-species antitumor effects in vivo. Because they can be universally utilized throughout the entire siRNA-based drug development process, from the first preliminary testing step to clinical trials, cross-species siRNAs could greatly facilitate the development of siRNA-based therapeutic agents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1 Cell cycle analysis of control siRNA or mTOR siRNA-treated cells. After subjecting HeLa cells with either control siRNA or mTOR siRNA for 48 h, the cells were trypsinized and fixed with ice-cold ethanol. The cells were then incubated with 0.05% propidium iodide and analyzed using flow cytometer. (PPTX 82 kb)
Fig. 2 Construction and expression of rAAV-mTOR shRNA. a Schematic diagram of cassette for rAAV-shRNAs. rAAVs harvor bi-directional promoters to express simultaneously shRNA and GFP, enabling the tracking of shRNA expression; H1 promoter for shRNA expression and CMV promoter for GFP. b HeLa cells were infected with rAAV-cont shRNA or rAAV-mTOR shRNA at MOI 300 for 2 days. GFP expression was observed in the infected cells under fluorescence microscope. c Two and 4 days post infection, mTOR mRNA level was examinedby real-time RT-PCR. Relative amount of mRNA level of mTOR was calculated by comparing those in rAAV-cont shRNA. d Expression levels of mTOR and phospho-p70S6K, phospho-mTOR (S2482) was examined by immuno-blotting analysis. GFP expression representing successful virus infection and actin representing equal loading of protein samples were similar to both virus infections. (PPTX 649 kb)
Fig. 3 In vivo efficacy of rAAV-mTORshRNA on xenograft and allograft models. Experimental procedures were described Fig. 6. At either 24 (a; HeLa) or 12 (b; B16-F10) days, tumors were removed, weighted, and subjected to immuno-blotting analysis for mTOR expression. c To detect mTOR mRNA cleavage, modified 5′RACE was performed as described in Fig. 1c. All RNAs from xenografted HeLa tumors were used as templates for RT. Sequence-specific mRNA cleavage was confirmed by the presence of 210 bp PCR product as indicated by the arrow. d Liver tissues recovered from H292 xenograft-bearing mice were stained with H & E (PPTX 1118 kb)
Acknowledgments
This work was supported by grants to H. Lee from the ministry of Health and Welfare (A101819), Republic of Korea.
Conflict of interest
None.
Footnotes
J. Ahn and H.N. Woo are co-first authors.
References
- 1.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29(1):1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK RNA interference in the clinic: challenges and future directions. Nat Rev Cancer 11 (1):59–67 [DOI] [PMC free article] [PubMed]
- 4.Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12(5):329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HS, Ahn J, Jun EJ, Yang S, Joo CH, Kim YK, Lee H. A novel program to design siRNAs simultaneously effective to highly variable virus genomes. Biochem Biophys Res Commun. 2009;384(4):431–435. doi: 10.1016/j.bbrc.2009.04.143. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Ahn J, Jee Y, Seo IS, Jeon EJ, Jeon ES, Joo CH, Kim YK, Lee H. Universal and mutation-resistant anti-enteroviral activity: potency of small interfering RNA complementary to the conserved cis-acting replication element within the enterovirus coding region. J Gen Virol. 2007;88(Pt 7):2003–2012. doi: 10.1099/vir.0.82633-0. [DOI] [PubMed] [Google Scholar]
- 8.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strat. 2009;6(2):47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, Lumb BM, Hunt SP. A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci. 2009;29(47):15017–15027. doi: 10.1523/JNEUROSCI.3451-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 13.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–14115. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K, Kim WJ, Cho YH, Lee YI, Lee H, Jeong S, Cho ES, Chang SI, Moon SK, Kang BS, Kim YJ, Cho SH. Cancer gene therapy using adeno-associated virus vectors. Front Biosci. 2008;13:2653–2659. doi: 10.2741/2872. [DOI] [PubMed] [Google Scholar]
- 16.Danos O. AAV vectors for RNA-based modulation of gene expression. Gene Ther. 2008;15(11):864–869. doi: 10.1038/gt.2008.69. [DOI] [PubMed] [Google Scholar]
- 17.Couto LB, High KA. Viral vector-mediated RNA interference. Curr Opin Pharmacol. 2010;10(5):534–542. doi: 10.1016/j.coph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Lee WI, Lee YS, Kim DH, Chang JW, Kim SW, Lee H. Effective relief of neuropathic pain by adeno-associated virus-mediated expression of a small hairpin RNA against GTP cyclohydrolase 1. Mol Pain. 2009;5:67. doi: 10.1186/1744-8069-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Hughes G, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R, Jacobsen M, Smith GCM, Guichard S, Pass M. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2009;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 21.Lasham A, Herbert M, Wallant NC, Patel R, Feng S, Eszes M, Cao H, Reid G. A rapid and sensitive method to detect siRNA-mediated mRNA cleavage in vivo using 5′ RACE and a molecular beacon probe. Nucleic Acids Res. 2010;38:e19. doi: 10.1093/nar/gkp1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421(1):29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Chapuis N, Bardet V, Tamburini J, Gallay N, Willems L, Knight ZA, Shokat KM, Azar N, Viguie F, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D. PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase and mTOR, has antileukemic activity in AML. Leukemia. 2008;22(9):1698–1706. doi: 10.1038/leu.2008.144. [DOI] [PubMed] [Google Scholar]
- 24.Jun EJ, Won MA, Ahn J, Ko A, Moon H, Tchah H, Kim YK, Lee H. An antiviral small-interfering RNA simultaneously effective against the most prevalent enteroviruses causing acute hemorrhagic conjunctivitis. Invest Ophthalmol Vis Sci. 2011;52(1):58–63. doi: 10.1167/iovs.09-5051. [DOI] [PubMed] [Google Scholar]
- 25.Echeverri CJ, Perrimon N. High-throughput RNAi screening in cultured cells: a user’s guide. Nat Rev Genet. 2006;7(5):373–384. doi: 10.1038/nrg1836. [DOI] [PubMed] [Google Scholar]
- 26.Ahn J, Jun ES, Lee HS, Yoon SY, Kim D, Joo CH, Kim YK, Lee H. A small interfering RNA targeting coxsackievirus B3 protects permissive HeLa cells from viral challenge. J Virol. 2005;79(13):8620–8624. doi: 10.1128/JVI.79.13.8620-8624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reardon W, Chakrabortee S, Pereira TC, Tyson T, Banton MC, Dolan KM, Culleton BA, Wise MJ, Burnell AM, Tunnacliffe A Expression profiling and cross-species RNA interference (RNAi) of desiccation-induced transcripts in the anhydrobiotic nematode Aphelenchus avenae. BMC Mol Biol 11:6 [DOI] [PMC free article] [PubMed]
- 28.Kondo S, Booker M, Perrimon N. Cross-species RNAi rescue platform in Drosophila melanogaster. Genetics. 2009;183(3):1165–1173. doi: 10.1534/genetics.109.106567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P, Lee SK, Shankar P, Manjunath N. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 2006;3(4):e96. doi: 10.1371/journal.pmed.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 31.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 32.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, Vu C, Lilly MB, Mallya S, Ong ST, Konopleva M, Martin MB, Ren P, Liu Y, Rommel C, Fruman DA Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med 16 (2):205–213 [DOI] [PMC free article] [PubMed]
- 33.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Hughes G, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R, Jacobsen M, Smith GC, Guichard S, Pass M AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 70 (1):288–298 [DOI] [PubMed]
- 34.Zhang YJ, Dai Q, Sun DF, Xiong H, Tian XQ, Gao FH, Xu MH, Chen GQ, Han ZG, Fang JY. mTOR signaling pathway is a target for the treatment of colorectal cancer. Ann Surg Oncol. 2009;16(9):2617–2628. doi: 10.1245/s10434-009-0555-9. [DOI] [PubMed] [Google Scholar]
- 35.Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59(4):775–781. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu L, Houghton PJ. The mTORC2 complex regulates terminal differentiation of C2C12 myoblasts. Mol Cell Biol. 2009;29(17):4691–4700. doi: 10.1128/MCB.00764-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JY, Kim JH, Khim M, Lee HS, Jung JH, Moon DH, Jeong S, Lee H. Persistent anti-tumor effects via recombinant adeno-associated virus encoding herpes thymidine kinase gene monitored by PET-imaging. Oncol Rep. 2011;25(5):1263–1269. doi: 10.3892/or.2011.1190. [DOI] [PubMed] [Google Scholar]
- 38.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun A, Tang J, Terranova PF, Zhang X, Thrasher JB, Li B. Adeno-associated virus-delivered short hairpin-structured RNA for androgen receptor gene silencing induces tumor eradication of prostate cancer xenografts in nude mice: a preclinical study. Int J Cancer. 2010;126(3):764–774. doi: 10.1002/ijc.24778. [DOI] [PubMed] [Google Scholar]
- 40.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 41.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Taylor LE, Flanigan KM, Gastier-Foster JM, Astbury C, Kota J, Sahenk Z, Walker CM, Clark KR. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68(5):629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabrera-Salazar MA, Roskelley EM, Bu J, Hodges BL, Yew N, Dodge JC, Shihabuddin LS, Sohar I, Sleat DE, Scheule RK, Davidson BL, Cheng SH, Lobel P, Passini MA. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease. Mol Ther. 2007;15(10):1782–1788. doi: 10.1038/sj.mt.6300249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1 Cell cycle analysis of control siRNA or mTOR siRNA-treated cells. After subjecting HeLa cells with either control siRNA or mTOR siRNA for 48 h, the cells were trypsinized and fixed with ice-cold ethanol. The cells were then incubated with 0.05% propidium iodide and analyzed using flow cytometer. (PPTX 82 kb)
Fig. 2 Construction and expression of rAAV-mTOR shRNA. a Schematic diagram of cassette for rAAV-shRNAs. rAAVs harvor bi-directional promoters to express simultaneously shRNA and GFP, enabling the tracking of shRNA expression; H1 promoter for shRNA expression and CMV promoter for GFP. b HeLa cells were infected with rAAV-cont shRNA or rAAV-mTOR shRNA at MOI 300 for 2 days. GFP expression was observed in the infected cells under fluorescence microscope. c Two and 4 days post infection, mTOR mRNA level was examinedby real-time RT-PCR. Relative amount of mRNA level of mTOR was calculated by comparing those in rAAV-cont shRNA. d Expression levels of mTOR and phospho-p70S6K, phospho-mTOR (S2482) was examined by immuno-blotting analysis. GFP expression representing successful virus infection and actin representing equal loading of protein samples were similar to both virus infections. (PPTX 649 kb)
Fig. 3 In vivo efficacy of rAAV-mTORshRNA on xenograft and allograft models. Experimental procedures were described Fig. 6. At either 24 (a; HeLa) or 12 (b; B16-F10) days, tumors were removed, weighted, and subjected to immuno-blotting analysis for mTOR expression. c To detect mTOR mRNA cleavage, modified 5′RACE was performed as described in Fig. 1c. All RNAs from xenografted HeLa tumors were used as templates for RT. Sequence-specific mRNA cleavage was confirmed by the presence of 210 bp PCR product as indicated by the arrow. d Liver tissues recovered from H292 xenograft-bearing mice were stained with H & E (PPTX 1118 kb)