Abstract
Separation of cells and organelles by bilayer membranes is a fundamental principle of life. Cellular membranes contain a baffling variety of proteins, which fulfil vital functions as receptors and signal transducers, channels and transporters, motors and anchors. The vast majority of membrane-bound proteins contain bundles of α-helical transmembrane domains. Understanding how these proteins adopt their native, biologically active structures in the complex milieu of a membrane is therefore a major challenge in today’s life sciences. Here, we review recent progress in the folding, unfolding and refolding of α-helical membrane proteins and compare the molecular interactions that stabilise proteins in lipid bilayers. We also provide a critical discussion of a detergent denaturation assay that is increasingly used to determine membrane-protein stability but is not devoid of conceptual difficulties.
Keywords: Hydrophobic effect, Hydrogen bonds, van der Waals forces, Aromatic interactions, Ion pairs, Salt bridges, Two-stage model, Lateral pressure profile, Hydrophobic mismatch, Lipid bilayers, Detergent micelles
Introduction
Almost every biological process depends on proteins. As versatile as the biological functions of proteins are, so are their physicochemical and structural features. Over the past decades, substantial progress has been made in our understanding of the thermodynamics and kinetics of the folding of water-soluble proteins [1]. Concomitantly, tremendous efforts in structural biology have yielded tens of thousands of different three-dimensional protein structures (http://www.rcsb.org/pdb/statistics/contentGrowthChart.do?content=total&seqid=100). By contrast, we are only beginning to understand the forces that govern the folding of membrane-embedded proteins, which are notoriously underrepresented in protein structure databases, with a total of only 207 unique high-resolution structures available as of 3 November 2009 (http://blanco.biomol.uci.edu/Membrane_Proteins_xtal.html#Latest). This is in sharp contrast to the biological and pharmacological significance of membrane proteins, as they account for roughly 30% of open reading frames in the human genome [2] and represent more than 50% of drug targets in the human body [3]. Elucidating how integral membrane proteins adopt, maintain and modulate their native fold in the complex environment of a lipid bilayer (Fig. 1) is one of the most challenging endeavours in the life sciences and, at the same time, a prerequisite for innovative approaches in pharmacology.
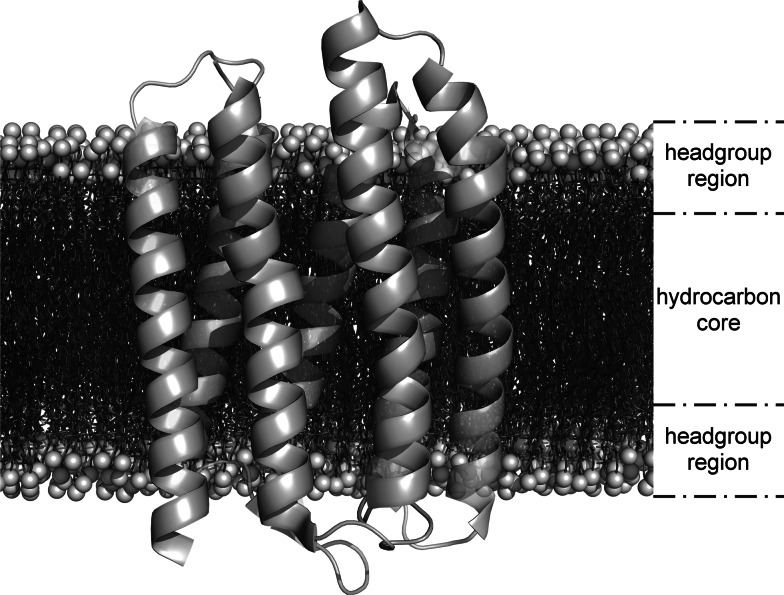
Fig. 1.
Seven-helix phototaxis receptor sensory rhodopsin II in a phospholipid bilayer. A high-resolution protein structure (PDB 1jgj) obtained by X-ray crystallography [182] was superimposed onto a bilayer manually assembled from 3,648 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (POPC, Molfile from Avanti Polar Lipids, Alabaster, AL) monomers. Each of the two headgroup regions is about 15 Å thick, whereas the hydrocarbon core is about 30 Å in thickness
This paper reviews recent advances in research efforts aimed at shedding light on the molecular forces that control the folding of α-helical membrane proteins. We first provide a short survey of soluble-protein folding (“Brief survey of soluble-protein folding”) for comparison and an introduction to membrane-protein folding (“Introduction to membrane-protein folding: the two-stage model”), which then is discussed in more detail with respect to membrane insertion (“First stage: membrane insertion and secondary structure formation”) and interhelical interactions (“Second stage: helix–helix interactions”). Some peculiar features of lipid membranes that affect protein folding are considered as well (“Lipid bilayers as hosts of integral membrane proteins”). We close with a synopsis of our current understanding of membrane-protein folding (“The forces governing membrane-protein folding”) and a critical discussion of experimental approaches to studying protein stability in lipid membranes (“Determining the stability of α-helical membrane proteins”).
Brief survey of soluble-protein folding
When a completely unfolded water-soluble protein folds into a rather compact, biologically active conformation (Fig. 2), a dramatic loss in polypeptide chain entropy needs to be overcome by favourable interactions within the protein and between the protein and its solvent [4, 5]. Covalent contributions to protein stability may come from peptidyl–prolyl isomerisation, disulphide bridges as well as a range of co- and posttranslational protein modifications such as methylation, phosphorylation or glycosylation. However, the major players in stabilising the native fold of a protein are of non-covalent character. These are the hydrophobic effect, hydrogen bonds, van der Waals forces, aromatic interactions and ion pairs/salt bridges.
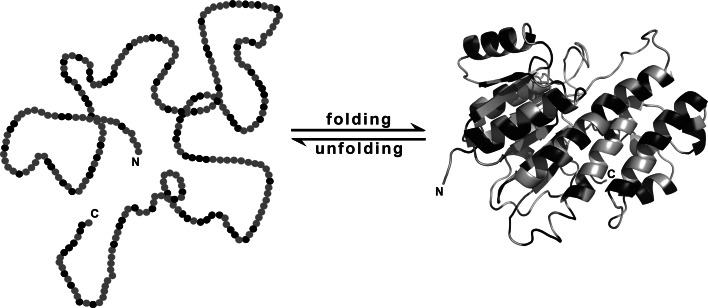
Fig. 2.
Schematic representation of water-soluble protein folding, exemplified using human carbonyl reductase 1 (276 residues). In an idealised scenario, a completely unfolded state devoid of specific intramolecular interactions and stable structure (left) is in equilibrium with a folded, biologically active state (right; PDB 1wma). Note that the unfolded states of several proteins have been shown to retain considerable amounts of secondary structure and long-range contacts [181] and that folding and unfolding reactions may involve kinetic and equilibrium intermediates [183]. In both panels, hydrophilic amino acid residues (Arg, Asn, Asp, Gln, Glu, His, Lys) are shown in black, whereas others are coloured grey
The hydrophobic effect is a major driving force in the folding of water-soluble proteins [1, 6]. Accordingly, establishing intramolecular contacts between hydrophobic (apolar) protein residues leads to a release of water and a concomitant large gain in entropy. Thus, burying hydrophobic polypeptide segments in a protein’s interior promotes the formation of a hydrophobic protein core [7], whose compactness can be increased by partial secondary structure formation through backbone hydrogen bonding [8]. Under certain conditions, some proteins indeed populate a denatured (i.e., biologically inactive) hydrophobically collapsed state displaying a lose arrangement of secondary structure elements often referred to as molten globule [9].
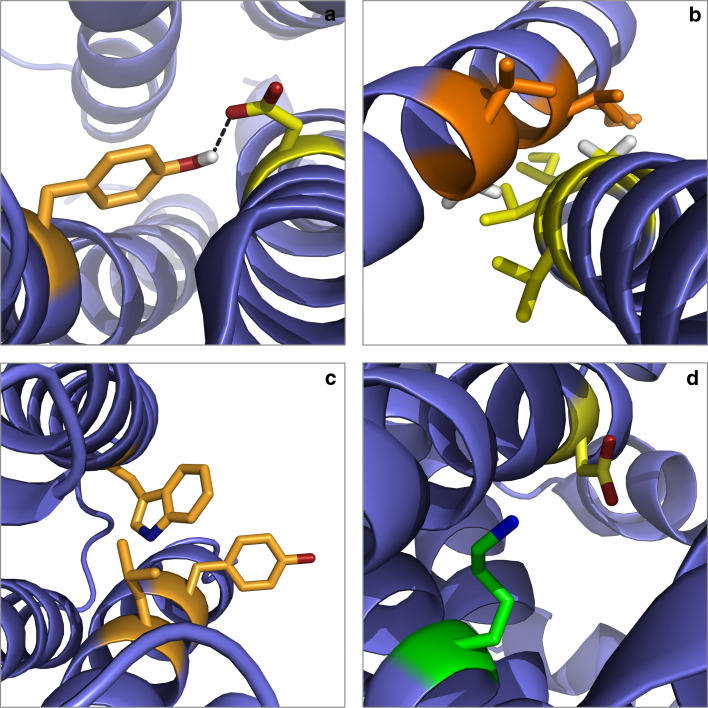
On top of the rather unspecific hydrophobic collapse, adoption of the native (i.e., biologically active) conformation necessitates a number of specific interactions, which are exemplified in Fig. 3: (a) In many cases, hydrogen bonds between amino acid side chains are essential for protein stability [10]. In the coiled-coil homodimer of the GCN4-leucine zipper (GCN4-LZ) from yeast (Fig. 3a), two Asn residues create an interhelical hydrogen bond that is involved in dimer formation [11]. (b) Short atom–atom distances in a compactly folded protein enable van der Waals forces to contribute substantially to protein stability [12]. A typical example is provided by helices 7 and 8 of the E. coli Hsc20 J-type co-chaperone (Fig. 3b), where bulky, branched amino acid side chains interdigitate to create a large interhelical contact area in an antiparallel coiled coil [13]. (c) Aromatic amino acid residues contribute in several ways to protein folding and protein–protein contacts [14]. Interactions are possible between two aromatic systems (π–π), as is the case with helices 1 and 2 in the de novo-designed protein α2D [15] (Fig. 3c), or between basic and aromatic amino acids (cation–π) [16, 17]. (d) Salt bridges between oppositely charged residues may offer a large gain in stability, as is demonstrated by His31 and Asp70 of T4 lysozyme [18] (Fig. 3d). In brief, nature employs a broad repertoire of covalent and non-covalent interactions to create an enormous number of different proteins.
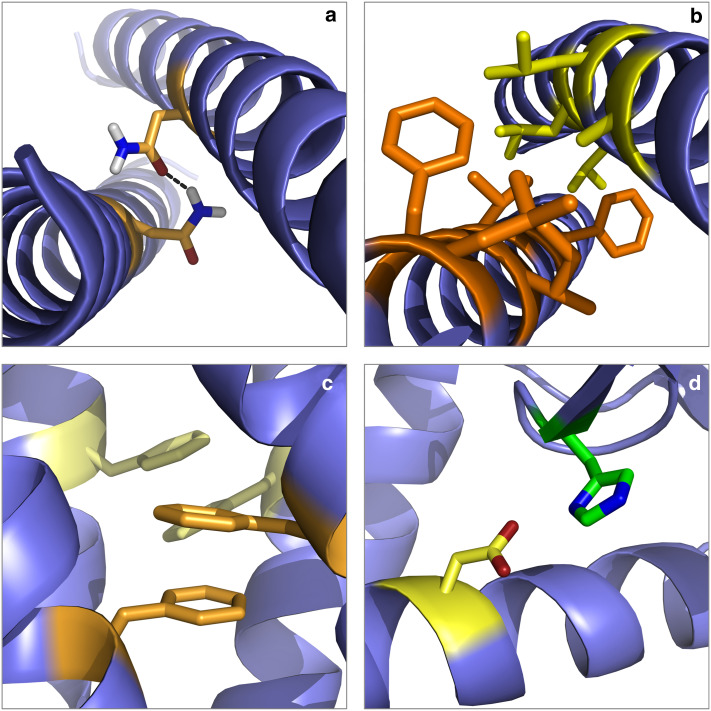
Fig. 3.
Specific interactions in water-soluble protein folding. Colour code for residues shown in atomic detail is red for oxygen, blue for nitrogen and white for hydrogen. a Interhelical hydrogen bonds. The homodimer of the GCN4-leucine zipper from Saccharomyces cerevisiae (PDB 2zta) contains an interhelical Asn16–Asn16 (orange) hydrogen bond (dashed line). b van der Waals contacts. Large side chains of helices 7 (orange) and 8 (yellow) interdigitate to form extensive van der Waals contacts in E. coli J-type co-chaperone HSC20 (PDB 1fpo). This example illustrates also the burial of hydrophobic side chains from water, which is a manifestation of the hydrophobic effect. c Aromatic-aromatic interactions. Two Phe10–Phe29 pairs are involved in π–π interactions in the homodimeric de novo-designed protein α2D (PDB 1qp6). d Salt bridges. A salt bridge encompassing His31 (green) and Asp70 (yellow) stabilises T4 lysozyme (PDB 2lzm)
Introduction to membrane-protein folding: the two-stage model
The environment in which integral membrane proteins reside and function differs fundamentally from that encountered by water-soluble proteins. A frequently heard view is that the hydrophobic effect is absent in the hydrocarbon core of a lipid bilayer. Conversely, a “lipophobic effect” is not at play either since the early suggestion that membrane proteins might be somewhat like “inside-out” water-soluble proteins with hydrophilic cores and hydrophobic surfaces [19] was refuted when the first high-resolution crystal structures [20, 21] revealed that the proteinaceous interiors of compactly folded membrane proteins are as hydrophobic as those of water-soluble proteins. The residues exposed on lipid-facing surfaces of membrane proteins, however, are even more hydrophobic on average than those buried in the protein core [22, 23], which clearly sets them apart from water-soluble proteins (Fig. 4).
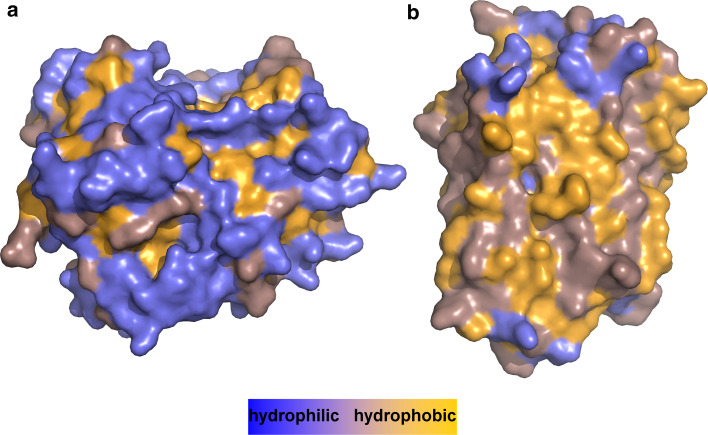
Fig. 4.
Differences in the hydrophobicity of surface areas between globular water-soluble and α-helical membrane proteins of similar size. Colour code is blue for hydrophilic surfaces, orange for hydrophobic ones and dark salmon for residues that are in between. a Human carbonyl reductase 1 (PDB 1wma, 276 residues) presents mainly hydrophilic residues to its aqueous environment and shields hydrophobic ones in its core. b Bacteriorhodopsin from Halobacterium salinarum (PDB 1c3w, 231 residues) exposes mostly hydrophobic residues to its lipid bilayer environment, whereas hydrophilic residues are found in the rather small regions that are in contact with lipid headgroups and water. Note that the interiors of membrane proteins are as hydrophobic as those of water-soluble proteins (not shown, see [20, 22])
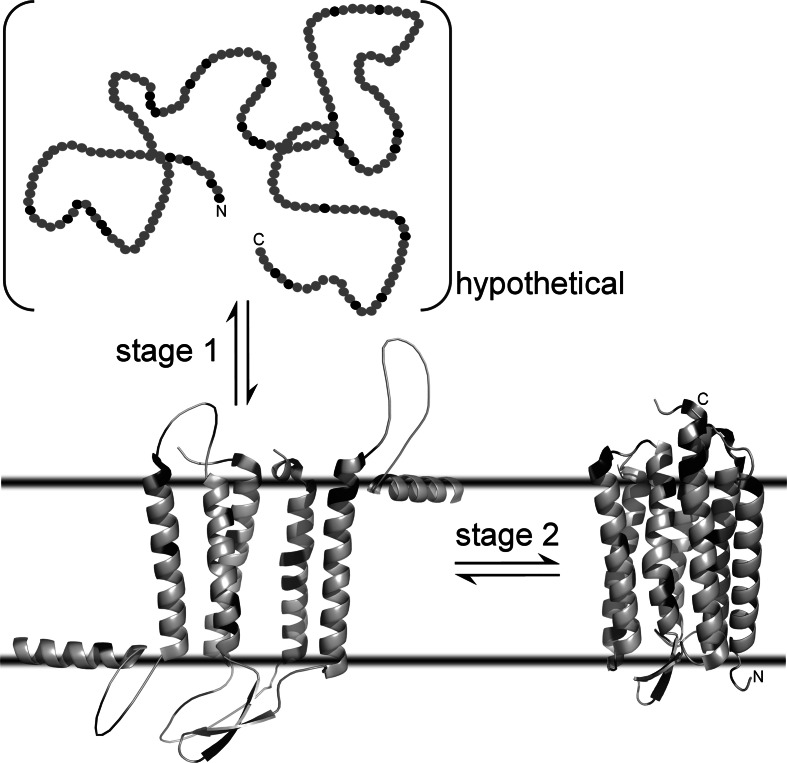
Depending on their predominant secondary structure composition, integral membrane proteins are divided into two classes. Proteins composed of β-sheets form membrane-spanning barrels called porins that are found only in the outer membranes of gram-negative bacteria, mitochondria and chloroplasts [24, 25]. By contrast, the vast majority of integral membrane proteins consist of bundles of transmembrane α-helices, and these proteins are the subject of the present review. The folding of α-helical membrane proteins has been conceptualised by a two-stage model [26] as illustrated in Fig. 5. The first stage (discussed in “First stage: membrane insertion and secondary structure formation”) comprises insertion of a polypeptide chain into a lipid bilayer and simultaneous secondary structure formation. During the second stage (treated in “Second stage: helix–helix interactions”), prearranged secondary structure elements associate with each other within the membrane and give rise to the final protein fold including tertiary and quaternary contacts.
Fig. 5.
Schematic representation of the two-stage model of membrane-protein folding, exemplified using bacteriorhodopsin from Halobacterium salinarum (231 residues). In stage 1, an unfolded polypeptide chain (top) is inserted into a lipid bilayer to form a loose, biologically inactive bundle rich in α-helical secondary structure (bottom left). In stage 2, interhelical interactions give rise to a compactly folded, biologically active state (bottom right, PDB 1c3w). Note that a water-soluble unfolded state (top) does not exist for most α-helical membrane proteins (see “Determining the stability of α-helical membrane proteins”). In these cases, stage 1 corresponds to the translocon-mediated membrane insertion of nascent polypeptide chains. Also, the depiction of the bilayer-bound denatured state (bottom left) is schematic, as no high-resolution structure is available. In all panels, hydrophilic amino acid residues (Arg, Asn, Asp, Gln, Glu, His, Lys) are shown in black, whereas others are coloured grey
First stage: membrane insertion and secondary structure formation
Membrane insertion: the hydrophobic effect
What destines a polypeptide chain to insert into a lipid bilayer as an integral membrane protein? It is the hydrophobic effect: the percentage of apolar residues is much higher in integral membrane proteins than in their water-soluble counterparts [22, 23]. Therefore, membrane proteins cannot bury most of their hydrophobic surfaces by folding around a hydrophobic core (Fig. 4b). Hence, membrane proteins avidly associate with lipid bilayers both in vivo [27, 28] and in vitro [29] and tend to aggregate in aqueous solutions.
The dominant role of hydrophobicity in determining whether a polypeptide chain inserts into an artificial or biological membrane is underscored by the reliability with which transmembrane sequences can be predicted on the basis of their hydrophobicity alone. A number of different hydrophobicity scales have been developed to this end [30–34]. These scales have in common that they are based on free-energy increments for partitioning of each of the 20 proteinogenic amino acids between an aqueous phase and a lipid bilayer or model system.
Bulk hydrocarbon phases
Unsurprisingly, hydrophobicity scales derived from bulk-phase partitioning experiments confirmed that apolar side chains are more lipophilic than polar or even charged ones [30–32]. More importantly, it turned out that partitioning of the polar peptide bond from water into octanol is as unfavourable as that of a charged side chain [32].
However, bulk hydrocarbon phases represent poor mimics of the highly anisotropic and heterogeneous environment of a lipid bilayer. Pure lipid bilayers, and even more biological membranes with a high fraction of lipid-embedded proteins, are not entirely hydrophobic [35]. Along the bilayer normal, hydrophobicity increases gradually from the aqueous phase through the headgroup region and into the hydrocarbon core. Moreover, membranes underlie high thermal disorder and are chemically heterogeneous, especially in the region around the lipid headgroup and the interface separating it from the acyl chain core (Fig. 1) [35]. Tremendous, depth-dependent pressures are encountered in lipid bilayers, which are not present in bulk phases (see “The forces governing membrane-protein folding” and “Determining the stability of α-helical membrane proteins”).
Lipid bilayer interfaces
To overcome the shortcomings of bulk-phase partitioning scales, Wimley and White [33] developed a scale for the partitioning of short peptides into the interface of simple model membranes composed of the zwitterionic phospholipid 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (POPC). Qualitatively, they observed the same trend obtained from bulk-phase scales, but the partitioning free energies were only half the magnitude. This is due to the higher relative static permittivity (relative dielectric constant) of the interfacial region and the ensuing incomplete desolvation of the peptides as compared with bulk hydrocarbon phases [33]. In order to account for both transmembrane insertion and interfacial partitioning, the octanol and the interfacial partitioning scales were combined into a downloadable tool for the prediction of transmembrane segments from sequence information (Membrane Protein Explorer, MPEx, http://blanco.biomol.uci.edu/mpex/).
Biological membranes
Although hydrophobicity is the major determinant of membrane partitioning, only a few α-helical membrane proteins can insert autonomously into artificial lipid bilayers or biological membranes [36]. In vivo, the vast majority of membrane proteins are inserted cotranslationally by a transmembrane protein complex called translocon [28]. Von Heijne and co-workers [34] established a biological hydrophobicity scale for protein insertion into the endoplasmic reticulum (ER) membrane. To this end, they supplemented E. coli leader peptidase, a protein normally containing two transmembrane helices, with an additional transmembrane domain (termed H1) that consisted of a combination of Ala and Leu residues and additionally 1 of the 20 proteinogenic amino acids as target residue. H1 was positioned between two N-linked glycosylation sites that served as probes for ER membrane insertion. Then, in vitro protein translation was performed in ER microsomes derived from dog pancreas. When H1 became inserted as a transmembrane helix into the ER membrane, only one of its flanking glycosylation sites was accessible from the lumen and could be glycosylated. When, by contrast, H1 was not inserted but instead translocated across the ER membrane, both glycosylation sites were accessible to luminal glycosyl transferase. After translation, the fractions of membrane-inserted H1 and membrane-translocated H1 were quantified by sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis (PAGE), and free energies of partitioning into the ER membrane were calculated.
This so-called biological partitioning scale matched the octanol scale strikingly well [32]. The good agreement between a crude, seemingly isotropic octanol scale and a complex, anisotropic biological scale might appear surprising at first glance. However, it should be kept in mind that water-saturated octanol does not present a pure hydrocarbon phase but contains considerable amounts of water. For instance, the free energy of Arg partitioning from water into pure cyclohexane is six times higher (more unfavourable) than that for partitioning into water-saturated octanol [31, 32]. In a similar way, recent molecular dynamics (MD) simulations imply that the free-energy cost of membrane penetration of Arg is substantially reduced by rather large amounts of water that is dragged into the membrane interior at membrane-protein contents typical of biological membranes [37].
The above results imply that the translocon-mediated insertion of transmembrane domains obeys the physicochemical principles established with the aid of simple model systems [38, 39]. In other words, the translocon as a water-filled pore enables nascent polypeptide chains to directly interact with membrane lipids so they can either remain in the aqueous environment inside the pore for translocation or laterally slip out of the translocon for insertion into the ER membrane [34]. In a subsequent paper, von Heijne and co-workers refined the biological hydrophobicity scale by considering position dependencies of side chains in a transmembrane helix along the bilayer normal [40]. Taken together, these experiments not only constitute a major breakthrough in our understanding of in vivo membrane-protein insertion and the inner workings of the translocon complex, but have also furnished an improved tool for the prediction of transmembrane domains from polypeptide sequences named ΔG Prediction Server (http://syrah.cbr.su.se/DGpred/index.php?p=home). This biological partitioning scale was later also incorporated into the MPEx tool mentioned in the preceding section.
Secondary structure formation: intrahelical hydrogen bonds
Even the most hydrophobic polypeptides could not insert into lipid bilayers without concomitant secondary structure formation. As mentioned in the previous section (see also “Interhelical hydrogen bonds”), desolvation of the polar peptide bond upon partitioning from water into octanol is energetically unfavourable, bringing about a change in Gibbs free energy of +8.4 kJ/mol per residue [32]. However, the loss of hydrogen bonds between the polypeptide backbone and water molecules can largely be offset by the formation of intramolecular hydrogen bonds, i.e., adoption of secondary structure. This is known as partitioning–folding coupling of transmembrane domains and has been reviewed elsewhere [39, 41, 42]. An important corollary of this coupling phenomenon is that it is virtually impossible to completely unfold a transmembrane helix. On the one hand, the apolar side chains of a typical transmembrane domain render it too hydrophobic to be extracted from its lipidic surrounding. On the other hand, the energetic penalty incurred on desolvation of the polypeptide backbone forces it to assume regular secondary structure as long as it remains embedded in a hydrophobic, water-depleted environment. The free-energy increase associated with unravelling an α-helix in an alkane phase has been estimated to be +17 kJ/mol per amino acid residue. Thus, unfolding a 20-residue transmembrane helix within a lipid bilayer would be accompanied by a prohibitively high free-energy penalty of +340 kJ/mol [39, 43, 44].
Second stage: helix–helix interactions
After and probably already during membrane insertion and secondary structure formation, transmembrane helices interact with one another to give rise to tertiary and quaternary contacts (Fig. 5, right). This chapter discusses the molecular forces underlying the second stage of membrane-protein folding [26], whereas the following chapter deals with its fine-tuning by the lipid bilayer in which it takes place.
Interhelical hydrogen bonds
Hydrogen bonds are donated by hydrogen atoms covalently bound to a strongly electronegative atom (e.g., N–H or O–H) and accepted by another electronegative atom with at least one non-bonded pair of electrons (e.g., C=O). Thus, hydrogen bonds basically represent electrostatic interactions between dipoles, but they additionally bear some hallmarks of covalent bonds. Most notably, hydrogen bonds are directional, meaning that their strength strongly depends on the angle and distance between donor and acceptor.
It has been estimated that a hydrogen bond within a lipid bilayer could offer a free-energy contribution to protein stability of −20 kJ/mol [41]. This is because of two factors, both of which are due to the low static permittivity (dielectricity) of the membrane’s hydrocarbon core [45]. First, polar interactions experience low shielding in an apolar environment and thus are stronger than in a polar surrounding. Second, intra- and interhelical hydrogen bonds are promoted because of the low abundance of competing water molecules in the hydrocarbon core of a lipid bilayer [46]. This has drawn considerable attention to the role of interhelical hydrogen bonds in stabilising membrane proteins. Strikingly, some mutations in membrane proteins are pathogenic because they result in misfolding caused by inappropriate hydrogen bonding [47, 48].
Designed peptide dimer
Interhelical hydrogen bonds can have decisive influence on the stability of integral membrane proteins. GCN4-LZ is a water-soluble coiled coil that dimerises by virtue of a heptad repeat motif (see also “Brief survey of soluble-protein folding” and Fig. 3a) [11]. Hydrophobic interactions in the dimer interface are supported by an interhelical hydrogen bond between Asn16 of one helix and Asn16 of the other helix. By replacing polar residues outside the dimer interface by apolar ones, the groups of Engelman [49] and DeGrado [50] created two membrane-embedded variants of GCN4-LZ as a model system to analyse helix–helix interactions in hydrophobic environments. Both GCN4-LZ variants still self-associated, albeit with impaired oligomerisation specificity, as both dimers and trimers were found to co-exist in equilibrium. Upon substitution of the hydrogen-bonding Asn by a Val residue, which cannot form hydrogen bonds, GCN4-LZ was found incapable of self-association within the membrane [50].
Transmembrane helix dimer
A solution NMR structure of the ζζ homodimer (ζζ), a component of the T-cell receptor (TCR) complex, exemplifies how interhelical hydrogen bonds shape a naturally occurring transmembrane dimer [51]. The ζζ helix interface is dominated by the polar residues Asp6, Tyr12 and Thr17. Each Tyr12–Thr17 pair forms an interhelical side-chain hydrogen bond that brackets the helix bundle. Deletion of a single hydroxyl group in one ζ strand by a Tyr17-to-Phe17 mutation caused a drop in dimerisation efficiency. Previous mutation experiments had suggested that both dimerisation of ζ and association of ζζ with TCRα strongly depend on Asp6 [52]. A high-resolution structure unveiled that Asp6 initiates a complex hydrogen bond network [51] that involves at least one water molecule, the carbonyl of Cys2, which is located directly above Asp6, and the backbone amide of Asp6 in the opposite strand. The hydrogen bond network around Asp6 might create a polar binding site for an Arg residue of TCRα, which could be responsible for the highly specific association behaviour of the TCR complex [51, 52].
Polytopic membrane proteins
Interhelical hydrogen bonds have been identified in a number of polytopic membrane proteins, but their impact on overall stability remains controversial. Figure 6a depicts the hydrogen bond between Tyr185 of helix 6 and Asp212 of helix 7 in bacteriorhodopsin (bR). On average, helix–helix interfaces in membrane proteins contain only one interhelical hydrogen bond [53]. Lau and Bowie [54] established an approach to quantify the stability of diacylglycerol kinase (DAGK) by reversibly denaturing it through titration with SDS. When the Bowie group [55] applied this approach to assess the effect of Ala substitutions in 24 positions of the second transmembrane helix of bacteriorhodopsin, they found that polar and apolar residues make similar contributions to protein stability, indicating that hydrogen bonds do not play a dominant role. Furthermore, double-mutant cycle experiments [56] revealed that the average contribution to protein stability of each out of eight interhelical hydrogen bonds in bacteriorhodopsin was only −2.5 kJ/mol.
Fig. 6.
Specific interactions in membrane-protein folding. Colour code for residues shown in atomic detail is red for oxygen, blue for nitrogen and white for hydrogen. a Interhelical hydrogen bonds. In bacteriorhodopsin from Halobacterium salinarum (PDB 1c3w), Tyr185 (orange) of helix 6 and Asp212 (yellow) of helix 7 form an interhelical hydrogen bond (dashed line). b van der Waals contacts. Small residues (orange and yellow) increase the homodimer interface and allow for extensive van der Waals contacts in human glycophorin A (PDB 1afo). Hydrogens are shown for Gly residues only. c Aromatic–aromatic interactions. In subunit I of aberrant ba 3-cytochrome c oxidase from Thermus thermophilus (PDB 1ehk), Trp110 of helix 4 interacts with Tyr23 and Leu27 of helix 1, although it is partly exposed to the lipid bilayer. d Salt bridges. Lys358 and Asp237 are crucial for membrane insertion of lac permease from E. coli (PDB 2v8n) and have been suggested to form a salt bridge within the protein’s transmembrane region [95, 96]
The discrepancy between this value and the estimated maximum of −20 kJ/mol [41] might originate from the limited degrees of freedom in orienting two interacting transmembrane helices with respect to one another, as hydrogen bond strength sharply decreases when the distance or angle between donor and acceptor deviates from its optimal value [57]. Furthermore, favourable enthalpic interactions on hydrogen bonding may be partly offset by an unfavourable entropy loss resulting from tight interhelical interactions. From an evolutionary viewpoint, rigid transmembrane helix bundles clamped by hydrogen bonds also appear undesirable, because selection is on function rather than stability [56]. However, we also want to point out potential caveats in the use of SDS to denature integral membrane proteins [58, 59], which will be discussed in detail in “Determining the stability of α-helical membrane proteins”.
Interhelical backbone hydrogen bonds
An interesting but controversial idea is that interhelical hydrogen bonds in membrane proteins can be established not exclusively between amino acid side chains but also between polypeptide backbones. Some authors [60, 61] propose that, under conditions of close helix–helix approach (see “van der Waals interactions and tight packing”), hydrogen bonds between backbone Cα–H and O=C might add to protein stability, whereas others contest this view [62].
Overall, interhelical hydrogen bonds in integral membrane proteins undoubtedly bear structural and functional relevance [49–51, 53, 63]. However, a picture emerges in which their free-energy contributions to protein stability are rather modest [55, 56]. The question then remains as to which other forces may drive helix–helix interactions in lipid bilayers.
van der Waals interactions and tight packing
van der Waals forces (also referred to as London dispersion forces) are a generic property of matter resulting from mutually induced, temporary dipolar moments. Thus, van der Waals forces are always attractive, but their strength decreases sharply with distance, so that two or more moieties have to get in close contact in order to experience significant van der Waals attraction.
The GXXXG motif
The discovery by the Engelman group [64, 65] of a transmembrane helix dimerisation motif composed of exclusively apolar and small amino acids has steered attention to the importance of van der Waals forces in membrane-protein folding. Homodimerisation of the single-pass membrane protein glycophorin A (GpA) suspended in detergent micelles responded sensitively to mutations affecting the sequence Leu75-Ile76-X-X-Gly79-Val80-X-X-Gly83-Val84-X-X-Thr87 [64]. The same motif was also found to facilitate dimerisation of poly-Leu transmembrane segments [65]. The results of both studies could later be explained by the three-dimensional structure of GpA [66], part of which is reproduced in Fig. 6b. Gly79 and Gly83 from one helix form a cavity that is filled by Val80 and Val84 from the other helix, thus enabling short helix–helix distances around the dimerisation motif. This enlarges the direct contact interface of the two helices, which is further extended by formation of a right-handed coil with a crossing angle of −40° [66]. In vivo data revealed Val80 and Val84 to be dispensable for stability [67] and suggested the GXXXG sequence to be the crucial dimerisation motif in GpA. However, as was pointed out already in the original report [64], the GXXXG motif per se is usually not sufficient for high-affinity interactions, which further depends on the sequence context [68]. Nevertheless, the GXXXG motif has been found in a large set of single- and multispanning membrane proteins [69], appearing in as many as 12.5% of all transmembrane helices listed in non-redundant databases [70]. Thus, the motif seems to promote interhelical van der Waals contacts that are fine-tuned by neighbouring residues to avoid non-specific helix–helix interactions.
Small residues and tight helix packing
Small residues in helix–helix interfaces can enlarge interaction surfaces by a “knobs-into-holes” packing and thus provide an opportunity for more extensive van der Waals contacts [71, 72]. Burial of small residues has two additional entropic advantages. First, the loss in conformational entropy is much smaller when fixing small, unbranched rather than large, branched side chains in a helix–helix interface [73]. Second, the close helix approach reduces the lipid-exposed protein surface and releases bound or orientated lipid molecules to diffuse more freely in the membrane [74]. In a computational study on seven α-helical transmembrane proteins, Eilers et al. [75] found that interacting surfaces are indeed enlarged by tight packing, but the authors also caution that tight packing is a general feature of helix bundles in both membrane and soluble proteins [75]. The difference lies in the way tight packing is accomplished. The helix–helix interfaces of membrane proteins display a high percentage of small residues, whereas those of water-soluble proteins are rich in long hydrophobic side chains [75, 76]. Recent experimental results [77] on MS1, a membrane-soluble derivative of GCN4-LZ (see “Interhelical hydrogen bonds”), have shed more light on the involvement of small residues in transmembrane helix association. All four wild-type residues at heptad position a (Val/Asn/Val/Val) were simultaneously substituted by either Gly, Ala, Val or Ile residues. Analytical ultracentrifugation showed that MS1-Gly remained dimeric and that MS1-Ala revealed a monomer–dimer equilibrium, whereas the larger-substitution mutants MS1-Val and MS1-Ile could not dimerise anymore. Thiol/disulphide exchange experiments confirmed that association strength decreased in the order Gly > Ala > Val > Ile. In addition, molecular mechanical force field simulations indicated the presence of Cα–H···O hydrogen bonds enabled by the close approach of the MS1 helices (see “Interhelical hydrogen bonds”).
However, others have argued that tight helix–helix packing in membrane proteins is in conflict with structural flexibility and that, in the absence of the hydrophobic effect, a driving force for tight packing might be missing [78]. Using a Voronoi computational procedure, Frömmel and co-workers [78] found average packing to be similar in 20 transmembrane helix bundles and 25 water-soluble proteins. The discrepancy between this observation and the results cited in the preceding paragraph might, among other reasons, be due to the limited structural information available for integral membrane proteins. It is to be hoped that a rapidly growing number of α-helical membrane-protein structures will offer a more detailed view and make statistical analysis more meaningful in the near future.
Quantitative contributions of van der Waals forces
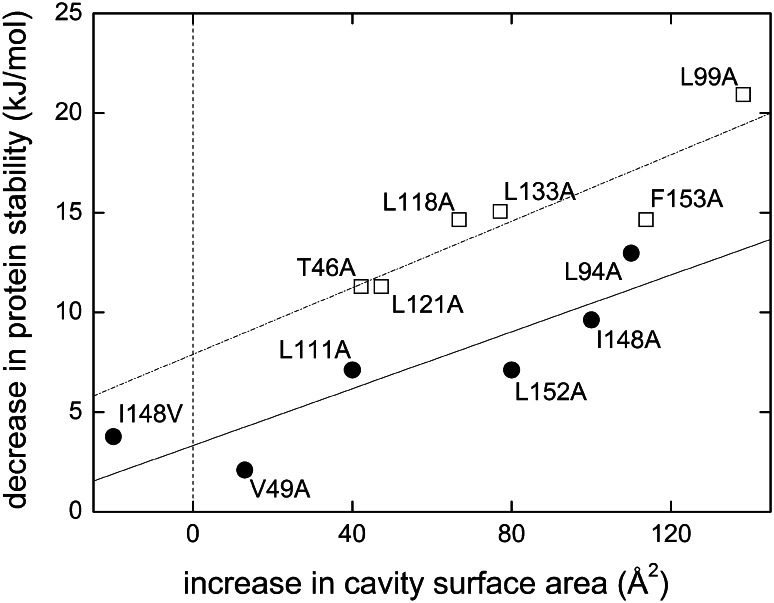
The Bowie laboratory [79] has recently used the above-mentioned SDS denaturation assay to quantify the contribution of van der Waals forces to membrane-protein stability. Figure 7 illustrates how protein stability decreases in a roughly linear fashion as cavity surface area increases upon large-to-small side-chain substitutions in the cores of bacteriorhodopsin and T4 lysozyme [80], which served as a representative water-soluble protein. In this plot, the y-axis intercept can be taken as a measure of the hydrophobic contribution to protein stability, which is expectedly smaller in bacteriorhodopsin than in T4 lysozyme. By contrast, the slope, which correlates with the strength of van der Waals forces, is virtually identical, indicating that van der Waals forces per unit cavity surface area contribute equally to stability in membrane and soluble proteins. Thus, the authors suggest [79] that dispersion forces are more prominent in membrane proteins not because van der Waals interactions are stronger when referred to a given area of buried surface, but simply because membrane proteins bury larger surface areas when compared with water-soluble proteins [81].
Fig. 7.
Dependence of protein stability on cavity surface area in water-soluble and membrane proteins. Experimental data for T4 lysozyme (open squares, taken from [80]) and bacteriorhodopsin (filled circles, [79]) as well as linear regressions (dash-dotted and solid lines, respectively). The stabilities of both proteins decreased in a roughly linear fashion with an increase in cavity surface area caused by the large-to-small amino acid substitutions indicated in the figure. Similar slopes observed for the two proteins suggest that van der Waals forces contribute equally to the stabilities of water-soluble and membrane proteins. The y-axis intercepts are different because of the greater contribution of the hydrophobic effect in thermal unfolding of T4 lysozyme as compared with SDS denaturation of bacteriorhodopsin. Figure adapted with permission from [79]. Copyright 2009 American Chemical Society
Aromatic interactions
Aromatic side chains are peculiar in that they can participate in hydrophobic, van der Waals and weak polar interactions [16, 17]. Thus, they represent a versatile instrument for interactions between transmembrane helices. Several studies suggest that Phe, Trp and Tyr facilitate helix–helix association in transmembrane domains [82–84]. When placed in position i–3 of a GXXXG motif, Phe can enhance transmembrane domain interactions in model peptides derived from library screening [82]. In addition, the same motif is involved in the assembly of transmembrane helices from vesicular stomatitis virus G-protein [82]. Other library screens have shown that Trp deletion diminishes helix–helix interactions [83] and that Lys makes contacts with Phe, Trp and Tyr through cation–π interactions [84]. Despite having their share in coiled-coil interactions, aromatic residues are also supposed to stabilise parallel transmembrane helix bundles by aromatic clustering [85]. Owing to their large hydrophobic surface, they have been suggested to interact with neighbouring helices even when they are partly exposed to lipids [85], as illustrated in Fig. 6c. Finally, Trp and Tyr have been found at the termini of many transmembrane helices close to the hydrophobic–hydrophilic interface, where they are thought to vertically anchor the protein in the membrane through interactions with lipid headgroups [86–89]. Tamm and co-workers [90] examined both aromatic clustering and membrane anchoring in the β-barrel membrane protein OmpA. They observed that aromatic residues can interact with one another even when they are 7 Å apart. Among the aromatic amino acids, Tyr was found to exert the strongest contribution to OmpA stability [90]. Unfortunately, such a quantitative account is currently not available for other integral membrane proteins. Nevertheless, aromatic residues seem to fulfil diverse functions in stabilising α-helical membrane proteins, too.
Ion pairs/salt bridges
Electrostatic interactions between ion pairs are described by Coulomb’s law. Accordingly, charge–charge interactions are not directional, and their strength is inversely proportional to the square of the distance between the charged moieties as well as to the relative static permittivity (relative dielectric constant) of the medium. Salt bridges are oppositely charged ion pairs that are close enough in space to form hydrogen bonds on top of their purely electrostatic attraction.
The low-dielectric environment offered by the hydrocarbon core of a lipid membrane is expected to result in strong electrostatic interactions. However, charged residues such as Asp, Glu, Lys and Arg occur scarcely in integral membrane proteins [91, 92] because of their large unfavourable free energy of membrane insertion [32, 34]. Charge removal by protonation or deprotonation may alleviate this free-energy penalty [93]. This is also reflected by the fact that pK a values in the hydrocarbon core of a lipid bilayer or the microenvironment of a helix bundle differ dramatically, and in favour of the neutral species, from the corresponding values in aqueous solution [93]. Another way of reducing the free-energy cost is charge compensation by formation of intra- or interhelical salt bridges. Indeed, membrane insertion of poly-Leu transmembrane peptides containing one Lys and one Asp residue depends strongly on the position of the charged residues along the helical axis [94]. Complete insertion is possible only when the two oppositely charged residues are located at the same side of the helix and are separated by one helical turn, which allows intrahelical salt-bridge formation [94]. An example of an interhelical salt bridge is the Asp237–Lys358 ion pair of lac permease (lacY) from E. coli [95, 96], which is shown in Fig. 6d. However, it has to be emphasised that such interhelical salt bridges are very rare. More often, unpaired charged residues appear in extramembraneous regions or water-filled pores of ion channels and transporters, where they are of functional importance in controlling single-channel conductance, ion selectivity or voltage sensing [93]. For example, the voltage-sensing S4 helix of the voltage-dependent potassium channel Kv1.2 contains four Arg residues [97]. In many integral membrane proteins, charged residues are located in the intra- or extracellular headgroup region of the membrane and help in determining membrane-protein topology [98]. According to the “positive-inside rule”, the positively charged residues Arg and Lys are more abundant on the cytoplasmic side [99].
Lipid bilayers as hosts of integral membrane proteins
Integral membrane proteins and lipid bilayers in which they reside are subject to the influence of numerous parameters, including temperature, pressure, pH, ionic strength and co-solutes [100, 101]. For example, counterions and pH can affect membrane electrostatics [102–104], bilayer material properties [105, 106] and membrane hydration [107–109]. Here, we restrict ourselves to three topics that distinguish membrane from soluble proteins: the lateral pressure profile, hydrophobic match and mismatch and specific lipid effects.
The lateral pressure profile
Physical origin
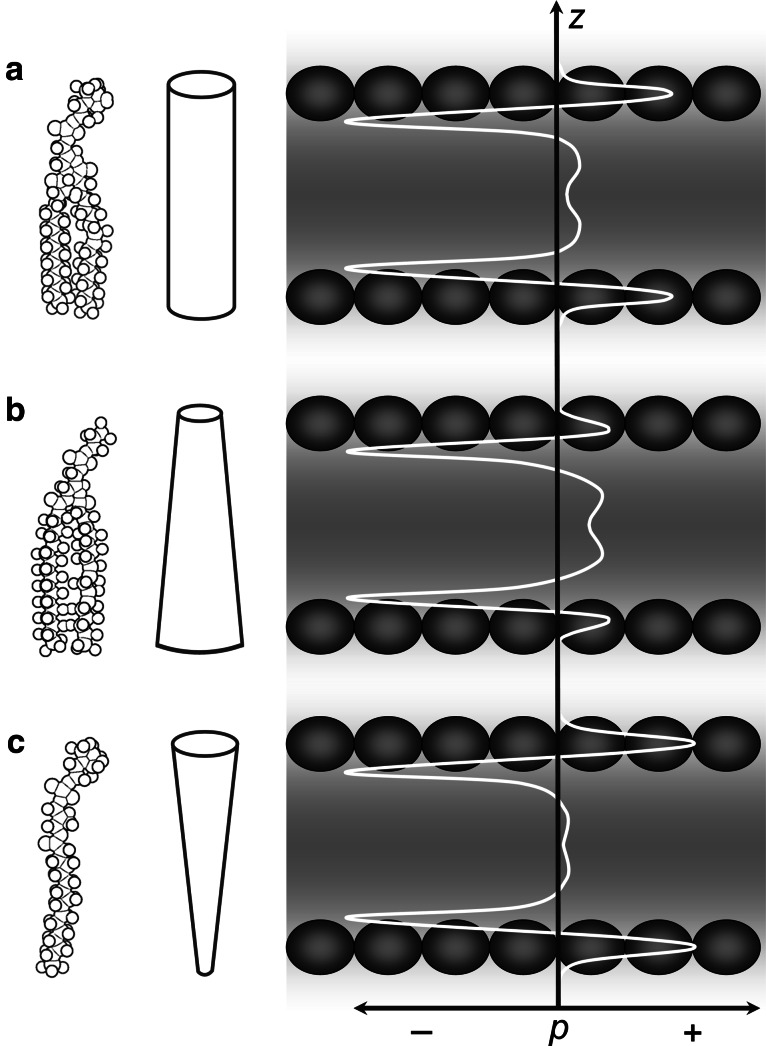
One of the most striking differences between membrane and soluble proteins is the pressure to which they are exposed. A protein that is suspended free in aqueous solution experiences isotropic pressure, whereas the pressure acting on a membrane protein varies dramatically along the bilayer normal. Owing to surface tension, the hydrophilic headgroup regions and the hydrophobic hydrocarbon core strive to minimise their contact area, thus generating strong negative pressure (pulling forces) acting laterally along the interfacial regions on both sides of a lipid bilayer [110, 111]. This interfacial tension is balanced by positive lateral pressure (pushing forces) arising in the headgroup regions and the hydrocarbon core, part of which stems from steric clash. In addition, the headgroup region experiences electrostatic repulsion between charged groups as well as loss of hydration water on compression. Within the hydrocarbon core, a further contribution to positive lateral pressure comes from stiffening of the lipid acyl chains, which incurs an entropic penalty. The dependence of lateral pressure, p, on membrane depth, z, is known as the lateral pressure profile, p(z) [112, 113]. A schematic profile typical of a POPC bilayer is shown in Fig. 8a. At mechanical equilibrium, the membrane neither expands nor contracts, and thus the pressure integral along the bilayer normal must be zero [112, 114, 115]. This means that the sum of the areas on the positive side of the pressure profile (in the two headgroup regions and the hydrocarbon core) must equal the sum of the areas on the negative side (in the two interfacial regions). However, local pressures can amount to several hundred atmospheres [116].
Fig. 8.
Schematic phospholipid shapes (left) and lateral pressure profiles (right) in bilayers composed of those lipids. Lateral pressure, p, is plotted versus bilayer depth, z. a In “cylindrical” lipids, like POPC, the headgroup and the acyl chains have similar area requirements. b Addition of lipids with small headgroups, such as POPE, redistributes positive lateral pressure from the headgroup regions to the hydrocarbon core. c Vice versa, addition of lipids with only one acyl chain (lysolipids), such as lysoPC, lowers the positive lateral pressure in the hydrocarbon core but augments it in the headgroup regions. The negative lateral pressure component is due to interfacial tension between the lipids’ acyl chains and their hydrated headgroups and thus remains virtually constant. At equilibrium, the pressure integral across the bilayer is always zero, i.e., the cumulative areas under the p(z) curves are equal for positive and negative pressure components
Unfortunately, a direct measurement of the local lateral pressure along the bilayer normal is not possible. Lateral pressure profiles such as those depicted in Fig. 8 may be obtained from MD simulations [117–119], though a recent approach relying on pressure-susceptible fluorescent probes that preferentially localise to different parts of a lipid bilayer holds promise for experimental confirmation [120–123].
Changes in lateral pressure profile
The exact shape of the lateral pressure profile depends on numerous parameters, including size, charge and hydration of the lipid headgroups, length and degree of saturation or branching of the acyl chains and presence of additives that decrease the interfacial tension [118, 119, 124–130]. For instance, addition of 1-palmitoyl-2-oleoyl-sn-glycero-phosphoethanolamine (POPE), whose headgroup is smaller than that of POPC, diminishes the lateral pressure in the headgroup regions. However, as the interfacial tension remains unaffected and must be matched by the sum of positive pressure contributions, the lateral pressure in the acyl chain core increases accordingly (Fig. 8b) [131, 132]. By contrast, incorporation of single-chain phospholipids (lysolipids) relieves the pressure in the hydrocarbon core and raises the pressure in the headgroup regions (Fig. 8c).
Implications for membrane-protein folding
The lateral pressure profile is anticipated to affect both membrane insertion (i.e., stage 1 in Fig. 5) and protein conformational equilibria (i.e., stage 2), which are associated with shape changes [112, 133]. For example, the presence of POPE or 1,2-dioleoyl-sn-glycero-phosphoethanolamine (DOPE) has been demonstrated to oppose transmembrane insertion of the self-inserting 20-residue peptide alamethicin [134, 135], which can be explained by decreased pressure in the headgroup regions and increased pressure in the hydrocarbon core. DOPE also shifts the equilibrium of membrane-spanning alamethicin towards the functionally active dimer [136], whose hour-glass shape is better compatible with high pressure in the acyl chain region. Likewise, DOPE and 1,2-dielaidoyl-sn-glycero-phosphoethanolamine (DEPE) promote association of the bacterial potassium channel KcsA to form the active homotetrameric structure [137, 138].
Hydrophobic match and mismatch
Bilayer thickness is another modulator of membrane-protein structure, stability and function. Thickness is determined by several factors, most notably, length and degree of saturation of lipid acyl chains [130], cholesterol content [139] and membrane proteins themselves [140]. Several membrane proteins have been shown to be most stable or active at an optimal bilayer thickness [141–144]. For instance, the activity of bacteriorhodopsin requires a minimal acyl chain length of 16–18 carbon atoms [145].
Hydrophobic match is achieved when the hydrophobic stretch of a transmembrane protein and the hydrophobic thickness of its host membrane are of equal length [146, 147]. Under conditions of hydrophobic mismatch, membrane proteins, the membrane or both may respond in a variety of ways to minimise the energetic penalty associated with exposure of hydrophobic moieties (both apolar amino acid residues and lipid acyl chains) [148]. Many α-helical membrane proteins have rather well-defined hydrophobic stretches because they possess aromatic residues (mainly Trp) that preferably localise to the bilayer interfaces between lipid headgroups and acyl chains (see “Aromatic interactions”) [86–88]. Fluorescence-spectroscopic experiments have shown that the microenvironment of Trp residues in KcsA is the same in PC bilayers ranging in thickness from 10 to 24 carbon atoms, implying that the drive to relieve hydrophobic mismatch is very strong [149].
Hydrophobic mismatch can affect conformational equilibria and conformation-dependent activities provided that the conformations involved differ in their hydrophobic stretch. At positive hydrophobic mismatch (i.e., when the protein hydrophobic stretch exceeds the thickness of the unperturbed lipid bilayer), small transmembrane peptides or single helices of multispanning α-helical membrane proteins can tilt [150], as has been suggested for KcsA in thin bilayers [149]. Similarly, the mechanosensitive channel of large conductance, MscL, opens in thin membranes by helix tilting [151, 152]. In contrast to peptides and single helices, tilting of multispanning membrane proteins as a whole has not been observed. In such cases, as well as under conditions of negative hydrophobic mismatch, the bilayer may deform or undergo local changes in lipid composition to match the protein’s hydrophobic stretch [148, 153]. Furthermore, both positive and negative hydrophobic mismatch can be relieved by protein oligomerisation, which minimises the apolar surface of protein or lipid exposed to water, as has been shown for bacteriorhodopsin in both thick and thin membranes [154]. Association sets in not before the bilayer’s hydrophobic thickness is 4 Å longer or 10 Å shorter than the hydrophobic stretch of bacteriorhodopsin [155].
Specific lipid effects
Besides determining generic bilayer properties such as charge density, lateral pressure profile and hydrophobic thickness, lipids may also influence membrane proteins through specific protein–lipid interactions. Lipids can act as co-factor that facilitate the folding or stabilise the structure of membrane proteins [129, 156]. An example is provided by the homotrimeric α-helical membrane protein DAGK from E. coli, which requires 1,2-dioleoyl-sn-glycero-phosphoglycerol (DOPG) for proper folding. The finding [157] that DOPG, but not equally negatively charged 1,2-dioleoyl-sn-glycero-phosphoserine (DOPS), augments the rate and yield of DAGK folding speaks in favour of a specific lipid rather than a generic charge effect. Cardiolipin, a four-chain lipid primarily found in the inner mitochondrial membrane, avidly binds to the large mitochondrial membrane protein bovine cytochrome c oxidase [158] and is essential for its function [159–161]. It has been suggested [160] that cardiolipin is explicitly required for association of cytochrome c oxidase subunits IVa and IVb. Several membrane-protein crystal structures show tightly bound lipid molecules and provide valuable insights into how these specifically interact with membrane proteins [156, 162].
Likewise, the function of KvAP channel, a voltage-dependent K+-channel from the archaebacterium Aeropyrum pernix [163], depends on certain lipid species. KvAP senses voltage with the aid of Arg-containing structures located at the membrane interface and pointing into the membrane interior [164, 165]. MD simulations of KvAP predicted that these positively charged “voltage-sensor paddles” electrostatically interact with negatively charged lipid phosphate groups [166]. Later on it was confirmed experimentally that the functional state of KvAP requires POPE or 1-palmitoyl-2-oleoyl-sn-glycero-phosphoglycerol (POPG) [167] and that phosphate groups play a crucial role, as their enzymatic removal disrupts function [168].
The forces governing membrane-protein folding
One of the central recurrent questions in the field of membrane-protein folding is how a protein embedded in the apolar environment of a lipid bilayer can compensate for the absence of the hydrophobic effect in order to assume a stable native fold. From “Second stage: helix–helix interactions”, it is clear that interhelical hydrogen and van der Waals contacts have their share in determining membrane-protein stability. However, hydrogen bonds are less strong than might have been expected, and therefore the focus has recently been shifting to van der Waals forces. When normalised with respect to buried surface area, van der Waals forces are not stronger than in water-soluble proteins, but they play a more dominant role because membrane proteins bury a greater amount of surface area. By contrast, salt bridges are of minor importance for the stability of most integral membrane proteins, whereas aromatic residues are involved in diverse interactions whose relative contributions remain to be established. In summary, it seems that extensive van der Waals packing, a moderate number of rather weak hydrogen bonds and possibly aromatic interactions contribute to the stability of integral membrane proteins.
How can this combination of modestly strong interactions compensate for the absence of the hydrophobic effect, which makes such a large favourable contribution to the stability of water-soluble proteins? The answer might be that this is the wrong question to ask. Whereas it is true that the hydrophobic effect could not contribute as much to intramembrane helix–helix interactions (stage 2 in Fig. 5) as to soluble-protein folding, its role in forcing a predominantly apolar polypeptide chain into a lipid bilayer (stage 1) and the ensuing consequences cannot be overrated. On top of the hydrophobic effect, soluble proteins need to establish favourable specific interactions in order to overcome the huge entropic cost of drastically reducing the conformational flexibility of their polypeptide chains on folding. By contrast, the transmembrane domains of integral membrane proteins are tightly bound to a lipid bilayer and are forced to assume regular secondary structures by the partitioning–folding coupling (see “Secondary structure formation: intrahelical hydrogen bonds”). Consequently, membrane proteins have much less chain entropy to gain from abandoning their native folds, since large parts of their polypeptide chains will experience motional and conformational confinement also in membrane-embedded denatured states. Moreover, differences in the lateral pressure (see “The lateral pressure profile”) exerted on different parts of membrane-bound proteins add further restrictions to vertical movements and conformational changes. Finally, even in the apolar milieu of a lipid bilayer, the hydrophobic effect might still make a significant contribution to helix–helix interactions in situations of hydrophobic mismatch (see “Hydrophobic match and mismatch”). In conclusion, integral membrane proteins might get along with fewer specific protein–protein interactions than water-soluble proteins simply because of the numerous restrictions imposed upon them by their lipid bilayer environment, into which they partition as dictated by the hydrophobic effect. This view is also supported by the difficulties encountered in unfolding integral membrane proteins out of lipid bilayers or detergent micelles, which we discuss in the last chapter.
Determining the stability of α-helical membrane proteins
In light of the enormous importance of membrane-bound receptors, channels, transporters and enzymes involved in a plethora of physiological and pathological processes, it appears unfortunate that our current understanding of the folding of α-helical membrane proteins is so limited, especially when compared with the rapid progress made during the past decades on soluble-protein folding. Two principal obstacles are to be blamed for this discrepancy. First, on a rather general note, most integral membrane proteins are difficult to produce by heterologous expression [169] in quantities and purities sufficient for biophysical and structural studies. Second, their pronounced hydrophobicity poses problems not only during routine laboratory work, but specifically in efforts aimed at determining the stability of integral membrane proteins. This, in turn, is primarily due to the partitioning–folding coupling discussed in “Secondary structure formation: intrahelical hydrogen bonds”. Accordingly, the transmembrane domains of α-helical membrane proteins are too hydrophobic to leave the lipid bilayer or other membrane-mimetic system used. At the same time, however, they cannot unravel within the bilayer because of the prohibitive energetic cost of exposing unsaturated backbone hydrogen bond donors and acceptors to a low-dielectric medium. Consequently, elevated temperature or high concentrations of chemical denaturants (such as urea or guanidine hydrochloride, GdnHCl) may unfold water-exposed, extramembraneous loops and possibly abrogate helix–helix interactions (stage 2 in Fig. 5), but they are, in general, incapable of completely solubilising and unfolding entire α-helical membrane proteins (stage 1) [58, 59].
Unfolding and refolding by soluble chemical denaturants
Several attempts at reversibly unfolding α-helical membrane proteins into high concentrations of urea or GdnHCl have been made. A promising candidate was DAGK from E. coli, which displayed a loss of both secondary and tertiary structure in acidic GdnHCl [170]. However, quantitative refolding into lipid bilayers could not be achieved, which impaired determination of thermodynamic protein-stability data. Ironically, the opposite was observed for two other membrane proteins from E. coli. Fractions of monomeric lac permease could be solubilised in 5 M urea after overexpression [171]. Intriguingly, the solubilised protein was mainly α-helical and remained soluble in phosphate buffer for a week. However, once inserted into E. coli membranes, lac permease could not be converted back into its soluble form. Similar results were described for melibiose permease [172].
This is in contrast with a number of seminal papers reporting the successful, fully reversible unfolding of β-barrel membrane proteins (porins or outer membrane proteins, OMPs), which form water-filled pores and possess primary sequences composed of alternating hydrophobic–hydrophilic patterns. This peculiarity renders them hydrophilic enough to come off from the membrane and become solubilised as unfolded monomers in aqueous solution in the presence of high concentrations of denaturants commonly used for soluble proteins [24]. Therefore, virtually complete and reversible unfolding was possible from various detergent micelles or lipid bilayers into a urea-solubilised state that is independent of the initial environment of the folded protein [90, 173–175], thus offering unique insights into the molecular interactions that stabilise β-barrel membrane proteins.
Denaturation and renaturation by SDS
In order to render α-helical membrane proteins amenable to protein-stability assays, Lau and Bowie [54] resorted to the use of the strongly denaturing, anionic detergent SDS rather than more traditional chemical denaturants like urea or GdnHCl. This approach has since been employed successfully by several laboratories [55, 56, 79, 176–178] and has furnished some of the most spectacular findings covered in this review (see “First stage: membrane insertion and secondary structure formation”, “Second stage: helix–helix interactions” and Fig. 7) [55, 176, 177]. Besides enabling the reversible denaturation and renaturation of α-helical membrane proteins, a particular advantage of this approach over unfolding induced by water-soluble denaturants like urea or GdnHCl is that the SDS-denatured state is much closer to the membrane-bound denatured state present (though to a very small percentage) under physiological conditions.
Notwithstanding these assets, the SDS denaturation assay suffers from a decisive drawback as compared with unfolding experiments on soluble proteins. Whereas SDS potently denatures integral membrane proteins, it does not necessarily unfold them. The terms denaturation and unfolding are sometimes used interchangeably, although they may refer to quite dissimilar processes (see [179] for a recent review). In its most general meaning, denaturation denotes any process by which a protein loses its native biological activity. This comprises, but is in no way limited to, complete unravelling of the polypeptide chain. Minor conformational changes, oligomer dissociation, aggregation, covalent modifications or clogging (of membrane channels or transporters) can entail denaturation, as well. Unfolding, by contrast, refers to a complete loss of regular secondary, tertiary and quaternary structure and should, in an ideal scenario, give rise to a polypeptide chain that behaves like a random coil (Fig. 2, left). Although this is not strictly the case for some (if not most) water-soluble proteins [180, 181], ensembles of urea- or GdnHCl-induced unfolded conformations serve as common reference states for unfolding and refolding studies exploring the influence on protein stability of amino acid composition (sequence) or environmental conditions, including pH, ionic strength, temperature, ligand concentration and so on.
Importantly, such a common reference state does not exist for SDS-induced denaturation of integral membrane proteins. A denatured, only partly unfolded membrane protein remains tightly associated with mixed micelles composed of SDS and lipid or detergent from its host bilayer or micelle. Thus, changes in the protein sequence (mutagenesis) or membrane-mimetic system affect not only the native state but also the denatured state, so that free-energy differences derived from such experiments cannot be assigned unambiguously to stabilisation or destabilisation of the native state. Interactions of the denatured state with detergent and lipid in mixed micelles are almost impossible to account for quantitatively, because the exact composition of these micelles is difficult to determine [55, 177] and is further affected by the presence of protein. As an example, consider the use of different phospholipids to modulate the lateral pressure profile (see “The lateral pressure profile” and Fig. 8) and asses its influence on membrane-protein stability. Then, the outcome of an SDS denaturation experiment will depend not only on changes in stability of the native state in SDS-free bilayers or micelles, but also on interactions of the denatured state with lipids and detergents in mixed micelles as well as on the susceptibility of different lipids and detergents to solubilisation or micellisation. The latter complication is of particular relevance to the present example, since lipids with small headgroups like POPE, which are used to increase lateral chain pressure, are anticipated to be more reluctant to partition into highly curved micellar structures than are single-chain lipids like lysoPC, which decrease lateral chain pressure.
In summary, this is not to say that the SDS denaturation assay is without merits. In fact, it is probably the best the protein-folding field currently has to offer for determining the stability of integral α-helical membrane proteins. There is no doubt that SDS denaturation has provided and will continue to provide unique insights into the forces that govern the folding of this important class of proteins, but one should keep in mind the caveats inherent in this technique.
Expanding the experimental repertoire for α-helical membrane proteins
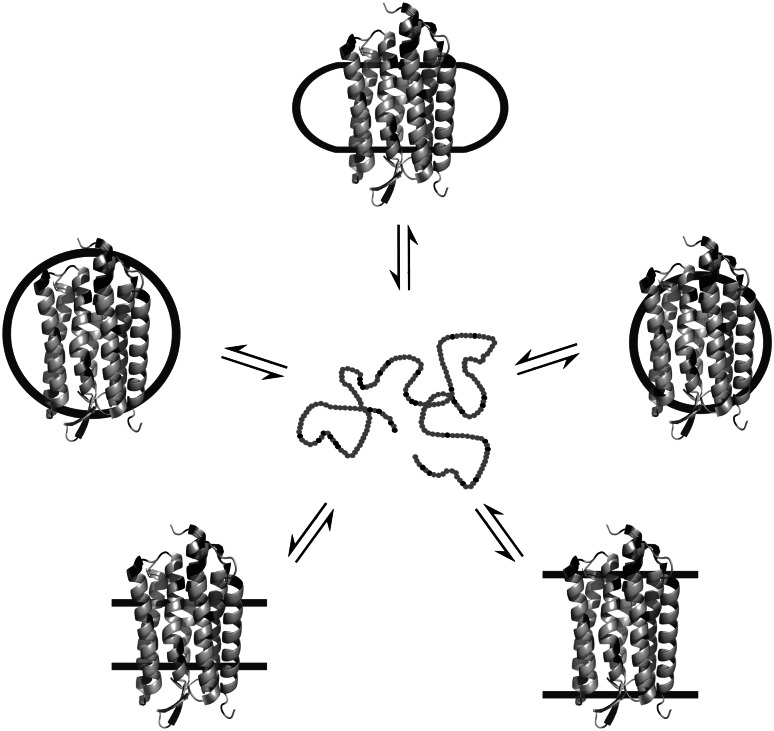
An alternative, idealised experimental setup for scrutinising the forces that contribute to the stability of membrane proteins might look like the scheme in Fig. 9, which is inspired by Tamm’s work on β-barrel membrane proteins (see “Unfolding and refolding by soluble chemical denaturants”) [90, 173–175]. The water-soluble, completely unfolded state depicted in the centre of this scheme is certainly a poor approximation of the membrane-bound denatured state found under physiological conditions. However, physiological relevance is less of an issue here than is the availability of a common reference state. The latter would allow for a direct comparison of protein stabilities in different environments by subtracting the unfolding Gibbs free energy determined in one membrane-mimetic system from the corresponding value measured in another system. This cancels the contribution of the common reference state and thus provides the difference in native-state Gibbs free energy between the two environments. In order for this paradigm to become applicable to α-helical membrane proteins, conditions must be found under which a hydrophobic polypeptide chain can be reversibly extracted from a membrane-mimetic system and unfolded by a chemical denaturant.
Fig. 9.
Idealised scenario of an α-helical membrane protein that assumes a monomeric, largely unstructured state in aqueous solution upon reversible unfolding out of different membrane-mimetic systems by a chemical denaturant. Membrane-mimetic systems shown are bicelles (top), micelles of different hydrophobic thicknesses (centre) and lipid bilayers of different hydrophobic thicknesses or lateral pressure profiles (bottom). The protein structure shown is from Halobacterium salinarum bacteriorhodopsin (PDB 1c3w), which was chosen purely for illustrative purposes
Acknowledgments
We thank Dr. Carolyn Vargas (Molecular Biophysics, University of Kaiserslautern, Germany) for helpful comments on the manuscript. All figures except Fig. 7 and Fig. 8 were created using the molecular visualisation system PyMOL. We are grateful to the team of DeLano Scientific (Palo Alto, CA) for providing this invaluable software package on an open-source basis and to Dr. Tobias Werther [Leibniz Institute of Molecular Pharmacology (FMP), Berlin, Germany] for introducing us to PyMOL. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to SK (grant no. KE 1478/1-1).
References
- 1.Fersht AR. From the first protein structures to our current knowledge of protein folding: delights and scepticisms. Nat Rev Mol Cell Biol. 2008;9:650–654. doi: 10.1038/nrm2446. [DOI] [PubMed] [Google Scholar]
- 2.Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overington JP, Al Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 4.Haber E, Anfinsen CB. Side-chain interactions governing the pairing of half-cystine residues in ribonuclease. J Biol Chem. 1962;237:1839–1844. [PubMed] [Google Scholar]
- 5.Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 6.Dill KA, Ozkan SB, Shell MS, Weikl TR. The protein folding problem. Annu Rev Biophys. 2008;37:289–316. doi: 10.1146/annurev.biophys.37.092707.153558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pace CN, Shirley BA, McNutt M, Gajiwala K. Forces contributing to the conformational stability of proteins. FASEB J. 1996;10:75–83. doi: 10.1096/fasebj.10.1.8566551. [DOI] [PubMed] [Google Scholar]
- 8.Ptitsyn OB. Stages in the mechanism of self-organization of protein molecules. Dokl Akad Nauk SSSR. 1973;210:1213–1215. [PubMed] [Google Scholar]
- 9.Dolgikh DA, Gilmanshin RI, Brazhnikov EV, Bychkova VE, Semisotnov GV, Venyaminov SY, Ptitsyn OB. α-Lactalbumin: compact state with fluctuating tertiary structure? FEBS Lett. 1981;136:311–315. doi: 10.1016/0014-5793(81)80642-4. [DOI] [PubMed] [Google Scholar]
- 10.Myers JK, Pace CN. Hydrogen bonding stabilizes globular proteins. Biophys J. 1996;71:2033–2039. doi: 10.1016/S0006-3495(96)79401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Stites WE. Energetics of side chain packing in staphylococcal nuclease assessed by systematic double mutant cycles. Biochemistry. 2001;40:14004–14011. doi: 10.1021/bi011268l. [DOI] [PubMed] [Google Scholar]
- 13.Cupp-Vickery JR, Vickery LE. Crystal structure of Hsc20, a J-type co-chaperone from Escherichia coli . J Mol Biol. 2000;304:835–845. doi: 10.1006/jmbi.2000.4252. [DOI] [PubMed] [Google Scholar]
- 14.Burley SK, Petsko GA. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science. 1985;229:23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- 15.Hill RB, DeGrado WF. Solution structure of α2D, a nativelike de novo designed protein. J Am Chem Soc. 1998;120:1138–1145. doi: 10.1021/ja9733649. [DOI] [Google Scholar]
- 16.Waters ML. Aromatic interactions in model systems. Curr Opin Chem Biol. 2002;6:736–741. doi: 10.1016/S1367-5931(02)00359-9. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty DA. Cation-π interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science. 1996;271:163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- 18.Weaver LH, Matthews BW. Structure of bacteriophage T4 lysozyme refined at 1.7 Å resolution. J Mol Biol. 1987;193:189–199. doi: 10.1016/0022-2836(87)90636-X. [DOI] [PubMed] [Google Scholar]
- 19.Engelman DM, Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci USA. 1980;77:5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deisenhofer J, Epp O, Miki K, Huber R, Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 Å resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis . J Mol Biol. 1984;180:385–398. doi: 10.1016/S0022-2836(84)80011-X. [DOI] [PubMed] [Google Scholar]
- 21.Rees DC, Komiya H, Yeates TO, Allen JP, Feher G. The bacterial photosynthetic reaction center as a model for membrane proteins. Annu Rev Biochem. 1989;58:607–633. doi: 10.1146/annurev.bi.58.070189.003135. [DOI] [PubMed] [Google Scholar]
- 22.Rees DC, DeAntonio L, Eisenberg D. Hydrophobic organization of membrane proteins. Science. 1989;245:510–513. doi: 10.1126/science.2667138. [DOI] [PubMed] [Google Scholar]
- 23.Samatey FA, Xu C, Popot J-L. On the distribution of amino acid residues in transmembrane α-helix bundles. Proc Natl Acad Sci USA. 1995;92:4577–4581. doi: 10.1073/pnas.92.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamm LK, Hong H, Liang B. Folding and assembly of β-barrel membrane proteins. Biochim Biophys Acta. 2004;1666:250–263. doi: 10.1016/j.bbamem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Hong H, Joh NH, Bowie JU, Tamm LK. Methods for measuring the thermodynamic stability of membrane proteins. Methods Enzymol. 2009;455:213–236. doi: 10.1016/S0076-6879(08)04208-0. [DOI] [PubMed] [Google Scholar]
- 26.Popot J-L, Engelman DM. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990;29:4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 28.Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- 29.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Fauchère J-L, Pliška V. Hydrophobic parameters π of amino acid side chains from the partitioning of N-acetyl-amino acid amides. Eur J Med Chem Chim Ther. 1983;18:369–375. [Google Scholar]
- 31.Radzicka A, Pedersen L, Wolfenden R. Influences of solvent water on protein folding: free energies of solvation of cis and trans peptides are nearly identical. Biochemistry. 1988;27:4538–4541. doi: 10.1021/bi00412a047. [DOI] [PubMed] [Google Scholar]
- 32.Wimley WC, Creamer TP, White SH. Solvation energies of amino acid side chains and backbone in a family of host–guest pentapeptides. Biochemistry. 1996;35:5109–5124. doi: 10.1021/bi9600153. [DOI] [PubMed] [Google Scholar]
- 33.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 34.Hessa T, Kim H, Bihlmaier K, Lundin C, Boekel J, Andersson H, Nilsson I, White SH, von Heijne G. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–381. doi: 10.1038/nature03216. [DOI] [PubMed] [Google Scholar]
- 35.Wiener MC, White SH. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure. Biophys J. 1992;61:434–447. doi: 10.1016/S0006-3495(92)81849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roosild TP, Greenwald J, Vega M, Castronovo S, Riek R, Choe S. NMR structure of Mistic, a membrane-integrating protein for membrane protein expression. Science. 2005;307:1317–1321. doi: 10.1126/science.1106392. [DOI] [PubMed] [Google Scholar]
- 37.Johansson AC, Lindahl E. Protein contents in biological membranes can explain abnormal solvation of charged and polar residues. Proc Natl Acad Sci USA. 2009;106:15684–15689. doi: 10.1073/pnas.0905394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Heijne G. Formation of transmembrane helices in vivo—is hydrophobicity all that matters? J Gen Physiol. 2007;129:353–356. doi: 10.1085/jgp.200709740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White SH, von Heijne G. How translocons select transmembrane helices. Annu Rev Biophys. 2008;37:23–42. doi: 10.1146/annurev.biophys.37.032807.125904. [DOI] [PubMed] [Google Scholar]
- 40.Hessa T, Meindl-Beinker NM, Bernsel A, Kim H, Sato Y, Lerch-Bader M, Nilsson I, White SH, von Heijne G. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 2007;450:1026–1030. doi: 10.1038/nature06387. [DOI] [PubMed] [Google Scholar]
- 41.White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 42.White SH, von Heijne G. Transmembrane helices before, during, and after insertion. Curr Opin Struct Biol. 2005;15:378–386. doi: 10.1016/j.sbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Tal N, Ben-Shaul A, Nicholls A, Honig B. Free-energy determinants of α-helix insertion into lipid bilayers. Biophys J. 1996;70:1803–1812. doi: 10.1016/S0006-3495(96)79744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Tal N, Honig B, Miller C, McLaughlin S. Electrostatic binding of proteins to membranes. Theoretical predictions and experimental results with charybdotoxin and phospholipid vesicles. Biophys J. 1997;73:1717–1727. doi: 10.1016/S0006-3495(97)78203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popot J-L, Engelman DM. Helical membrane protein folding, stability, and evolution. Annu Rev Biochem. 2000;69:881–922. doi: 10.1146/annurev.biochem.69.1.881. [DOI] [PubMed] [Google Scholar]
- 46.Bowie JU. Solving the membrane protein folding problem. Nature. 2005;438:581–589. doi: 10.1038/nature04395. [DOI] [PubMed] [Google Scholar]
- 47.Smith SO, Smith CS, Bormann BJ. Strong hydrogen bonding interactions involving a buried glutamic acid in the transmembrane sequence of the neu/erbB-2 receptor. Nat Struct Biol. 1996;3:252–258. doi: 10.1038/nsb0396-252. [DOI] [PubMed] [Google Scholar]
- 48.Therien AG, Grant FE, Deber CM. Interhelical hydrogen bonds in the CFTR membrane domain. Nat Struct Biol. 2001;8:597–601. doi: 10.1038/89631. [DOI] [PubMed] [Google Scholar]
- 49.Zhou FX, Cocco MJ, Russ WP, Brunger AT, Engelman DM. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat Struct Biol. 2000;7:154–160. doi: 10.1038/81919. [DOI] [PubMed] [Google Scholar]
- 50.Choma C, Gratkowski H, Lear JD, DeGrado WF. Asparagine-mediated self-association of a model transmembrane helix. Nat Struct Biol. 2000;7:161–166. doi: 10.1038/72440. [DOI] [PubMed] [Google Scholar]
- 51.Call ME, Schnell JR, Xu C, Lutz RA, Chou JJ, Wucherpfennig KW. The structure of the ζζ transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell. 2006;127:355–368. doi: 10.1016/j.cell.2006.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell. 2002;111:967–979. doi: 10.1016/S0092-8674(02)01194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adamian L, Liang J. Interhelical hydrogen bonds and spatial motifs in membrane proteins: polar clamps and serine zippers. Proteins. 2002;47:209–218. doi: 10.1002/prot.10071. [DOI] [PubMed] [Google Scholar]
- 54.Lau FW, Bowie JU. A method for assessing the stability of a membrane protein. Biochemistry. 1997;36:5884–5892. doi: 10.1021/bi963095j. [DOI] [PubMed] [Google Scholar]
- 55.Faham S, Yang D, Bare E, Yohannan S, Whitelegge JP, Bowie JU. Side-chain contributions to membrane protein structure and stability. J Mol Biol. 2004;335:297–305. doi: 10.1016/j.jmb.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 56.Joh NH, Min A, Faham S, Whitelegge JP, Yang D, Woods VL, Bowie JU. Modest stabilization by most hydrogen-bonded side-chain interactions in membrane proteins. Nature. 2008;453:1266–1270. doi: 10.1038/nature06977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker EN, Hubbard RE. Hydrogen bonding in globular proteins. Prog Biophys Mol Biol. 1984;44:97–179. doi: 10.1016/0079-6107(84)90007-5. [DOI] [PubMed] [Google Scholar]
- 58.Renthal R. An unfolding story of helical transmembrane proteins. Biochemistry. 2006;45:14559–14566. doi: 10.1021/bi0620454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanley AM, Fleming KG. The process of folding proteins into membranes: challenges and progress. Arch Biochem Biophys. 2008;469:46–66. doi: 10.1016/j.abb.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Senes A, Ubarretxena-Belandia I, Engelman DM. The Cα–H···O hydrogen bond: a determinant of stability and specificity in transmembrane helix interactions. Proc Natl Acad Sci USA. 2001;98:9056–9061. doi: 10.1073/pnas.161280798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arbely E, Arkin IT. Experimental measurement of the strength of a Cα–H···O bond in a lipid bilayer. J Am Chem Soc. 2004;126:5362–5363. doi: 10.1021/ja049826h. [DOI] [PubMed] [Google Scholar]
- 62.Yohannan S, Faham S, Yang D, Grosfeld D, Chamberlain AK, Bowie JU. A Cα–H···O hydrogen bond in a membrane protein is not stabilizing. J Am Chem Soc. 2004;126:2284–2285. doi: 10.1021/ja0317574. [DOI] [PubMed] [Google Scholar]
- 63.Partridge AW, Melnyk RA, Deber CM. Polar residues in membrane domains of proteins: molecular basis for helix–helix association in a mutant CFTR transmembrane segment. Biochemistry. 2002;41:3647–3653. doi: 10.1021/bi0120502. [DOI] [PubMed] [Google Scholar]
- 64.Lemmon MA, Flanagan JM, Hunt JF, Adair BD, Bormann B-J, Dempsey CE, Engelman DM. Glycophorin A dimerization is driven by specific interactions between transmembrane α-helices. J Biol Chem. 1992;267:7683–7689. [PubMed] [Google Scholar]
- 65.Lemmon MA, Treutlein HR, Adams PD, Brünger AT, Engelman DM. A dimerization motif for transmembrane α-helices. Nat Struct Biol. 1994;1:157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- 66.MacKenzie KR, Prestegard JH, Engelman DM. A transmembrane helix dimer: structure and implications. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- 67.Brosig B, Langosch D. The dimerization motif of the glycophorin A transmembrane segment in membranes: importance of glycine residues. Protein Sci. 1998;7:1052–1056. doi: 10.1002/pro.5560070423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doura AK, Kobus FJ, Dubrovsky L, Hibbard E, Fleming KG. Sequence context modulates the stability of a GxxxG-mediated transmembrane helix–helix dimer. J Mol Biol. 2004;341:991–998. doi: 10.1016/j.jmb.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 69.Kim S, Jeon TJ, Oberai A, Yang D, Schmidt JJ, Bowie JU. Transmembrane glycine zippers: physiological and pathological roles in membrane proteins. Proc Natl Acad Sci USA. 2005;102:14278–14283. doi: 10.1073/pnas.0501234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senes A, Gerstein M, Engelman DM. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with β-branched residues at neighboring positions. J Mol Biol. 2000;296:921–936. doi: 10.1006/jmbi.1999.3488. [DOI] [PubMed] [Google Scholar]
- 71.Crick F. The packing of α-helices: simple coiled-coils. Acta Crystallogr. 1953;6:689–697. doi: 10.1107/S0365110X53001964. [DOI] [Google Scholar]
- 72.Langosch D, Heringa J. Interaction of transmembrane helices by a knobs-into-holes packing characteristic of soluble coiled coils. Proteins. 1998;31:150–159. doi: 10.1002/(SICI)1097-0134(19980501)31:2<150::AID-PROT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 73.MacKenzie KR, Engelman DM. Structure-based prediction of the stability of transmembrane helix–helix interactions: the sequence dependence of glycophorin A dimerization. Proc Natl Acad Sci USA. 1998;95:3583–3590. doi: 10.1073/pnas.95.7.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Helms V. Attraction within the membrane. Forces behind transmembrane protein folding and supramolecular complex assembly. EMBO Rep. 2002;3:1133–1138. doi: 10.1093/embo-reports/kvf245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eilers M, Shekar SC, Shieh T, Smith SO, Fleming PJ. Internal packing of helical membrane proteins. Proc Natl Acad Sci USA. 2000;97:5796–5801. doi: 10.1073/pnas.97.11.5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adamian L, Liang J. Helix–helix packing and interfacial pairwise interactions of residues in membrane proteins. J Mol Biol. 2001;311:891–907. doi: 10.1006/jmbi.2001.4908. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Kulp DW, Lear JD, DeGrado WF. Experimental and computational evaluation of forces directing the association of transmembrane helices. J Am Chem Soc. 2009;131:11341–11343. doi: 10.1021/ja904625b. [DOI] [PubMed] [Google Scholar]
- 78.Hildebrand PW, Rother K, Goede A, Preissner R, Frömmel C. Molecular packing and packing defects in helical membrane proteins. Biophys J. 2005;88:1970–1977. doi: 10.1529/biophysj.104.049585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joh NH, Oberai A, Yang D, Whitelegge JP, Bowie JU. Similar energetic contributions of packing in the core of membrane and water-soluble proteins. J Am Chem Soc. 2009;131:10846–10847. doi: 10.1021/ja904711k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eriksson AE, Baase WA, Zhang X-J, Heinz DW, Blaber M, Baldwin EP, Matthews BW. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992;255:178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- 81.Oberai A, Joh NH, Pettit FK, Bowie JU. Structural imperatives impose diverse evolutionary constraints on helical membrane proteins. Proc Natl Acad Sci USA. 2009;106:17747–17750. doi: 10.1073/pnas.0906390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unterreitmeier S, Fuchs A, Schäffler T, Heym RG, Frishman D, Langosch D. Phenylalanine promotes interaction of transmembrane domains via GxxxG motifs. J Mol Biol. 2007;374:705–718. doi: 10.1016/j.jmb.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 83.Ridder A, Skupjen P, Unterreitmeier S, Langosch D. Tryptophan supports interaction of transmembrane helices. J Mol Biol. 2005;354:894–902. doi: 10.1016/j.jmb.2005.09.084. [DOI] [PubMed] [Google Scholar]
- 84.Johnson RM, Hecht K, Deber CM. Aromatic and cation–π interactions enhance helix–helix association in a membrane environment. Biochemistry. 2007;46:9208–9214. doi: 10.1021/bi7008773. [DOI] [PubMed] [Google Scholar]
- 85.Adamian L, Nanda V, DeGrado WF, Liang J. Empirical lipid propensities of amino acid residues in multispan alpha helical membrane proteins. Proteins. 2005;59:496–509. doi: 10.1002/prot.20456. [DOI] [PubMed] [Google Scholar]
- 86.Roth M, Arnoux B, Ducruix A, Reiss-Husson F. Structure of the detergent phase and protein–detergent interactions in crystals of the wild-type (strain Y) Rhodobacter sphaeroides photochemical reaction center. Biochemistry. 1991;30:9403–9413. doi: 10.1021/bi00103a003. [DOI] [PubMed] [Google Scholar]
- 87.Wallin E, Tsukihara T, Yoshikawa S, von Heijne G, Elofsson A. Architecture of helix bundle membrane proteins: an analysis of cytochrome c oxidase from bovine mitochondria. Protein Sci. 1997;6:808–815. doi: 10.1002/pro.5560060407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 89.Yau W-M, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 90.Hong H, Park S, Jimenez RH, Rinehart D, Tamm LK. Role of aromatic side chains in the folding and thermodynamic stability of integral membrane proteins. J Am Chem Soc. 2007;129:8320–8327. doi: 10.1021/ja068849o. [DOI] [PubMed] [Google Scholar]
- 91.Ulmschneider MB, Sansom MS, Di Nola A. Properties of integral membrane protein structures: derivation of an implicit membrane potential. Proteins. 2005;59:252–265. doi: 10.1002/prot.20334. [DOI] [PubMed] [Google Scholar]
- 92.Gratkowski H, Lear JD, DeGrado WF. Polar side chains drive the association of model transmembrane peptides. Proc Natl Acad Sci USA. 2001;98:880–885. doi: 10.1073/pnas.98.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cymes GD, Ni Y, Grosman C. Probing ion-channel pores one proton at a time. Nature. 2005;438:975–980. doi: 10.1038/nature04293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chin C-N, von Heijne G. Charge pair interactions in a model transmembrane helix in the ER membrane. J Mol Biol. 2000;303:1–5. doi: 10.1006/jmbi.2000.4122. [DOI] [PubMed] [Google Scholar]
- 95.King SC, Hansen CL, Wilson TH. The interaction between aspartic acid 237 and lysine 358 in the lactose carrier of Escherichia coli . Biochim Biophys Acta. 1991;1062:177–186. doi: 10.1016/0005-2736(91)90390-T. [DOI] [PubMed] [Google Scholar]
- 96.Dunten RL, Sahin-Tóth M, Kaback HR. Role of the charge pair aspartic acid-237–lysine-358 in the lactose permease of Escherichia coli . Biochemistry. 1993;32:3139–3145. doi: 10.1021/bi00063a028. [DOI] [PubMed] [Google Scholar]
- 97.Long SB, Campbell EB, MacKinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 98.von Heijne G. Membrane-protein topology. Nat Rev Mol Cell Biol. 2006;7:909–918. doi: 10.1038/nrm2063. [DOI] [PubMed] [Google Scholar]
- 99.von Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986;5:3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hope MJ, Cullis PR. Effects of divalent cations and pH on phosphatidylserine model membranes: a 31P NMR study. Biochem Biophys Res Commun. 1980;92:846–852. doi: 10.1016/0006-291X(80)90780-9. [DOI] [PubMed] [Google Scholar]
- 101.Cevc G, Seddon JM, Hartung R, Eggert W. Phosphatidylcholine–fatty acid membranes. I. Effects of protonation, salt concentration, temperature and chain-length on the colloidal and phase properties of mixed vesicles, bilayers and nonlamellar structures. Biochim Biophys Acta. 1988;940:219–240. doi: 10.1016/0005-2736(88)90197-6. [DOI] [PubMed] [Google Scholar]
- 102.Brockman H. Dipole potential of lipid membranes. Chem Phys Lipids. 1994;73:57–79. doi: 10.1016/0009-3084(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 103.Clarke RJ, Lüpfert C. Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: a basis for the Hofmeister effect. Biophys J. 1999;76:2614–2624. doi: 10.1016/S0006-3495(99)77414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou J, Thorpe IF, Izvekov S, Voth GA. Coarse-grained peptide modeling using a systematic multiscale approach. Biophys J. 2007;92:4289–4303. doi: 10.1529/biophysj.106.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rand RP, Sengupta S. Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta. 1972;255:484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- 106.Morein S, Andersson A-S, Rilfors L, Lindblom G. Wild-type Escherichia coli cells regulate the membrane lipid composition in a “window” between gel and non-lamellar structures. J Biol Chem. 1996;271:6801–6809. doi: 10.1074/jbc.271.12.6801. [DOI] [PubMed] [Google Scholar]
- 107.McIntosh TJ. Hydration properties of lamellar and non-lamellar phases of phosphatidylcholine and phosphatidylethanolamine. Chem Phys Lipids. 1996;81:117–131. doi: 10.1016/0009-3084(96)02577-7. [DOI] [PubMed] [Google Scholar]
- 108.Parsegian VA, Rand RP. Membrane interaction and deformation. Ann N Y Acad Sci. 1983;416:1–12. doi: 10.1111/j.1749-6632.1983.tb35175.x. [DOI] [PubMed] [Google Scholar]
- 109.Zhao W, Róg T, Gurtovenko AA, Vattulainen I, Karttunen M. Atomic-scale structure and electrostatics of anionic palmitoyloleoylphosphatidylglycerol lipid bilayers with Na+ counterions. Biophys J. 2007;92:1114–1124. doi: 10.1529/biophysj.106.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coster HG, Simons R. Energy of formation of bimolecular lipid membranes. Biochim Biophys Acta. 1968;163:234–239. doi: 10.1016/0005-2736(68)90102-8. [DOI] [PubMed] [Google Scholar]
- 111.Jähnig F. What is the surface tension of a lipid bilayer membrane? Biophys J. 1996;71:1348–1349. doi: 10.1016/S0006-3495(96)79336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cantor RS. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 113.Marsh D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys J. 2007;93:3884–3899. doi: 10.1529/biophysj.107.107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Evans EA, Waugh R. Mechano-chemistry of closed, vesicular membrane systems. J Colloid Interf Sci. 1977;60:286–298. doi: 10.1016/0021-9797(77)90288-0. [DOI] [Google Scholar]
- 115.Marsh D. Lateral pressure in membranes. Biochim Biophys Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 116.Chiu S-W, Clark M, Balaji V, Subramaniam S, Scott HL, Jakobsson E. Incorporation of surface tension into molecular dynamics simulation of an interface: a fluid phase lipid bilayer membrane. Biophys J. 1995;69:1230–1245. doi: 10.1016/S0006-3495(95)80005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cantor RS. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J Phys Chem B. 1997;101:1723–1725. doi: 10.1021/jp963911x. [DOI] [Google Scholar]
- 118.Frink LJ, Frischknecht AL. Density functional theory approach for coarse-grained lipid bilayers. Phys Rev E: Stat Nonlinear Soft Matter Phys. 2005;72:041923. doi: 10.1103/PhysRevE.72.041923. [DOI] [PubMed] [Google Scholar]
- 119.Terama E, Ollila OH, Salonen E, Rowat AC, Trandum C, Westh P, Patra M, Karttunen M, Vattulainen I. Influence of ethanol on lipid membranes: from lateral pressure profiles to dynamics and partitioning. J Phys Chem B. 2008;112:4131–4139. doi: 10.1021/jp0750811. [DOI] [PubMed] [Google Scholar]
- 120.Langner M, Hui SW. Merocyanine 540 as a fluorescence indicator for molecular packing stress at the onset of lamellar-hexagonal transition of phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1999;1415:323–330. doi: 10.1016/S0005-2736(98)00185-0. [DOI] [PubMed] [Google Scholar]
- 121.Templer RH, Castle AJ, Curran AR, Rumbles G, Klug DR. Sensing isothermal changes in the lateral pressure in model membranes using di-pyrenyl phosphatidylcholine. Faraday Discuss. 1999;111:41–53. doi: 10.1039/a806472e. [DOI] [PubMed] [Google Scholar]
- 122.Kusube M, Tamai N, Matsuki H, Kaneshina S. Pressure-induced phase transitions of lipid bilayers observed by fluorescent probes Prodan and Laurdan. Biophys Chem. 2005;117:199–206. doi: 10.1016/j.bpc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 123.Boldyrev IA, Zhai X, Momsen MM, Brockman HL, Brown RE, Molotkovsky JG. New BODIPY lipid probes for fluorescence studies of membranes. J Lipid Res. 2007;48:1518–1532. doi: 10.1194/jlr.M600459-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cantor RS. Lipid composition and the lateral pressure profile in bilayers. Biophys J. 1999;76:2625–2639. doi: 10.1016/S0006-3495(99)77415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ly HV, Longo ML. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys J. 2004;87:1013–1033. doi: 10.1529/biophysj.103.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seddon JM, Cevc G, Kaye RD, Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984;23:2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- 127.McIntosh TJ, Simon SA. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 1986;25:4948–4952. doi: 10.1021/bi00365a034. [DOI] [PubMed] [Google Scholar]
- 128.Lewis RN, McElhaney RN. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylethanolamines. Biophys J. 1993;64:1081–1096. doi: 10.1016/S0006-3495(93)81474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 130.Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van den Brink-van der Laan E, Killian JA, de Kruijff B (2004) Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666:275–288 [DOI] [PubMed]
- 132.Bezrukov SM. Functional consequences of lipid packing stress. Curr Opin Colloid Interf Sci. 2000;5:237–243. doi: 10.1016/S1359-0294(00)00061-3. [DOI] [Google Scholar]
- 133.Cantor RS. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem Phys Lipids. 1999;101:45–56. doi: 10.1016/S0009-3084(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 134.Stankowski S, Schwarz G. Lipid dependence of peptide-membrane interactions. Bilayer affinity and aggregation of the peptide alamethicin. FEBS Lett. 1989;250:556–560. doi: 10.1016/0014-5793(89)80795-1. [DOI] [PubMed] [Google Scholar]
- 135.Lewis JR, Cafiso DS. Correlation between the free energy of a channel-forming voltage-gated peptide and the spontaneous curvature of bilayer lipids. Biochemistry. 1999;38:5932–5938. doi: 10.1021/bi9828167. [DOI] [PubMed] [Google Scholar]
- 136.Keller SL, Bezrukov SM, Gruner SM, Tate MW, Vodyanoy I, Parsegian VA. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993;65:23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Dalen A, Hegger S, Killian JA, de Kruijff B. Influence of lipids on membrane assembly and stability of the potassium channel KcsA. FEBS Lett. 2002;525:33–38. doi: 10.1016/S0014-5793(02)03061-2. [DOI] [PubMed] [Google Scholar]
- 138.van den Brink-van der Laan E, Chupin V, Killian JA, de Kruijff B (2004) Stability of KcsA tetramer depends on membrane lateral pressure. Biochemistry 43:4240–4250 [DOI] [PubMed]
- 139.Mouritsen OG, Zuckermann MJ. What’s so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- 140.Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G, Engelman DM. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci USA. 2004;101:4083–4088. doi: 10.1073/pnas.0307332101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johannsson A, Keightley CA, Smith GA, Richards CD, Hesketh TR, Metcalfe JC. The effect of bilayer thickness and n-alkanes on the activity of the (Ca2+ + Mg2+)-dependent ATPase of sarcoplasmic reticulum. J Biol Chem. 1981;256:1643–1650. [PubMed] [Google Scholar]
- 142.Caffrey M, Feigenson GW. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 1981;20:1949–1961. doi: 10.1021/bi00510a034. [DOI] [PubMed] [Google Scholar]
- 143.Montecucco C, Smith GA, Dabbeni-sala F, Johannsson A, Galante YM, Bisson R. Bilayer thickness and enzymatic activity in the mitochondrial cytochrome c oxidase and ATPase complex. FEBS Lett. 1982;144:145–148. doi: 10.1016/0014-5793(82)80588-7. [DOI] [PubMed] [Google Scholar]
- 144.Dumas F, Tocanne J-F, Leblanc G, Lebrun MC. Consequences of hydrophobic mismatch between lipids and melibiose permease on melibiose transport. Biochemistry. 2000;39:4846–4854. doi: 10.1021/bi992634s. [DOI] [PubMed] [Google Scholar]
- 145.Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids. 1994;73:159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 146.Jensen MØ, Mouritsen OG. Lipids do influence protein function—the hydrophobic matching hypothesis revisited. Biochim Biophys Acta. 2004;1666:205–226. doi: 10.1016/j.bbamem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 147.Mouritsen OG, Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984;46:141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Killian JA. Hydrophobic mismatch between proteins and lipids in membranes. Biochim Biophys Acta. 1998;1376:401–415. doi: 10.1016/s0304-4157(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 149.Williamson IM, Alvis SJ, East JM, Lee AG. Interactions of phospholipids with the potassium channel KcsA. Biophys J. 2002;83:2026–2038. doi: 10.1016/S0006-3495(02)73964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Planque MR, Goormaghtigh E, Greathouse DV, Koeppe II RE, Kruijtzer JA, Liskamp RM, de Kruijff B, Killian JA. Sensitivity of single membrane-spanning α-helical peptides to hydrophobic mismatch with a lipid bilayer: effects on backbone structure, orientation, and extent of membrane incorporation. Biochemistry. 2001;40:5000–5010. doi: 10.1021/bi000804r. [DOI] [PubMed] [Google Scholar]
- 151.Powl AM, East JM, Lee AG. Lipid–protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry. 2003;42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- 152.Sukharev S, Betanzos M, Chiang C-S, Guy HR. The gating mechanism of the large mechanosensitive channel MscL. Nature. 2001;409:720–724. doi: 10.1038/35055559. [DOI] [PubMed] [Google Scholar]
- 153.Dumas F, Lebrun MC, Tocanne J-F. Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett. 1999;458:271–277. doi: 10.1016/S0014-5793(99)01148-5. [DOI] [PubMed] [Google Scholar]
- 154.Botelho AV, Huber T, Sakmar TP, Brown MF. Curvature and hydrophobic forces drive oligomerization and modulate activity of rhodopsin in membranes. Biophys J. 2006;91:4464–4477. doi: 10.1529/biophysj.106.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lewis BA, Engelman DM. Bacteriorhodopsin remains dispersed in fluid phospholipid bilayers over a wide range of bilayer thicknesses. J Mol Biol. 1983;166:203–210. doi: 10.1016/S0022-2836(83)80006-0. [DOI] [PubMed] [Google Scholar]
- 156.Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim Biophys Acta. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 157.Seddon AM, Lorch M, Ces O, Templer RH, Macrae F, Booth PJ. Phosphatidylglycerol lipids enhance folding of an α helical membrane protein. J Mol Biol. 2008;380:548–556. doi: 10.1016/j.jmb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 158.Awasthi YC, Chuang TF, Keenan TW, Crane FL. Tightly bound cardiolipin in cytochrome oxidase. Biochim Biophys Acta. 1971;226:42–52. doi: 10.1016/0005-2728(71)90176-9. [DOI] [PubMed] [Google Scholar]
- 159.Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- 160.Sedlák E, Robinson NC. Phospholipase A2 digestion of cardiolipin bound to bovine cytochrome c oxidases alters both activity and quaternary structure. Biochemistry. 1999;38:14966–14972. doi: 10.1021/bi9914053. [DOI] [PubMed] [Google Scholar]
- 161.Abramovitch DA, Marsh D, Powell GL. Activation of beef-heart cytochrome c oxidase by cardiolipin and analogues of cardiolipin. Biochim Biophys Acta. 1990;1020:34–42. doi: 10.1016/0005-2728(90)90090-Q. [DOI] [PubMed] [Google Scholar]
- 162.Fyfe PK, McAuley KE, Roszak AW, Isaacs NW, Cogdell RJ, Jones MR. Probing the interface between membrane proteins and membrane lipids by X-ray crystallography. Trends Biochem Sci. 2001;26:106–112. doi: 10.1016/S0968-0004(00)01746-1. [DOI] [PubMed] [Google Scholar]
- 163.Hodgkin AL, Huxley AF. Propagation of electrical signals along giant nerve fibres. Proc R Soc London, Ser B. 1952;140:177–183. doi: 10.1098/rspb.1952.0054. [DOI] [PubMed] [Google Scholar]
- 164.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 165.Jiang Q-X, Wang D-N, MacKinnon R. Electron microscopic analysis of KvAP voltage-dependent K+ channels in an open conformation. Nature. 2004;430:806–810. doi: 10.1038/nature02735. [DOI] [PubMed] [Google Scholar]
- 166.Freites JA, Tobias DJ, von Heijne G, White SH. Interface connections of a transmembrane voltage sensor. Proc Natl Acad Sci USA. 2005;102:15059–15064. doi: 10.1073/pnas.0507618102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schmidt D, Jiang Q-X, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:775–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 168.Xu Y, Ramu Y, Lu Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature. 2008;451:826–829. doi: 10.1038/nature06618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Junge F, Schneider B, Reckel S, Schwarz D, Dötsch V, Bernhard F. Large-scale production of functional membrane proteins. Cell Mol Life Sci. 2008;65:1729–1755. doi: 10.1007/s00018-008-8067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nagy JK, Lonzer WL, Sanders CR. Kinetic study of folding and misfolding of diacylglycerol kinase in model membranes. Biochemistry. 2001;40:8971–8980. doi: 10.1021/bi010202n. [DOI] [PubMed] [Google Scholar]
- 171.Roepe PD, Kaback HR. Characterization and functional reconstitution of a soluble form of the hydrophobic membrane protein lac permease from Escherichia coli . Proc Natl Acad Sci USA. 1989;86:6087–6091. doi: 10.1073/pnas.86.16.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roepe PD, Kaback HR. Isolation and functional reconstitution of soluble melibiose permease from Escherichia coli . Biochemistry. 1990;29:2572–2577. doi: 10.1021/bi00462a020. [DOI] [PubMed] [Google Scholar]
- 173.Kleinschmidt JH, Wiener MC, Tamm LK. Outer membrane protein A of E. coli folds into detergent micelles, but not in the presence of monomeric detergent. Protein Sci. 1999;8:2065–2071. doi: 10.1110/ps.8.10.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hong H, Tamm LK. Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc Natl Acad Sci USA. 2004;101:4065–4070. doi: 10.1073/pnas.0400358101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hong H, Szabo G, Tamm LK. Electrostatic couplings in OmpA ion-channel gating suggest a mechanism for pore opening. Nat Chem Biol. 2006;2:627–635. doi: 10.1038/nchembio827. [DOI] [PubMed] [Google Scholar]
- 176.Otzen DE. Folding of DsbB in mixed micelles: a kinetic analysis of the stability of a bacterial membrane protein. J Mol Biol. 2003;330:641–649. doi: 10.1016/S0022-2836(03)00624-7. [DOI] [PubMed] [Google Scholar]
- 177.Curnow P, Booth PJ. Combined kinetic and thermodynamic analysis of α-helical membrane protein unfolding. Proc Natl Acad Sci USA. 2007;104:18970–18975. doi: 10.1073/pnas.0705067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Curnow P, Booth PJ. The transition state for integral membrane protein folding. Proc Natl Acad Sci USA. 2009;106:773–778. doi: 10.1073/pnas.0806953106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chang J-Y. Structural heterogeneity of 6 M GdmCl-denatured proteins: implications for the mechanism of protein folding. Biochemistry. 2009;48:9340–9346. doi: 10.1021/bi901417f. [DOI] [PubMed] [Google Scholar]
- 180.Dill KA, Shortle D. Denatured states of proteins. Annu Rev Biochem. 1991;60:795–825. doi: 10.1146/annurev.bi.60.070191.004051. [DOI] [PubMed] [Google Scholar]
- 181.Shortle D, Ackerman MS. Persistence of native-like topology in a denatured protein in 8 M urea. Science. 2001;293:487–489. doi: 10.1126/science.1060438. [DOI] [PubMed] [Google Scholar]
- 182.Luecke H, Schobert B, Lanyi JK, Spudich EN, Spudich JL. Crystal structure of sensory rhodopsin II at 2.4 angstroms: insights into color tuning and transducer interaction. Science. 2001;293:1499–1503. doi: 10.1126/science.1062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ptitsyn OB. Kinetic and equilibrium intermediates in protein folding. Protein Eng. 1994;7:593–596. doi: 10.1093/protein/7.5.593. [DOI] [PubMed] [Google Scholar]