Collaborators
Kazuya Fujihara, Koji Hasegawa, Chika Hiraishi, Takanori Honda, Hisanori Horiuchi, Hisayuki Katsuyama, Akiko Kuwabara, Sadako Matsui, Kota Matsuki, Tetsuo Mianamino, Eriko Morishita, Yoko M. Nakao, Masahiro Natsuaki, Toshiyuki Nishikido, Akira Ohtake, Hiroaki Okazaki, Daisuke Sugiyama, Hayato Tada, Yutaka Taketani, Yoshikazu Tamori, Atsushi Tanaka, Yukiyo Yamamoto, Shizuya Yamashita, Hiroshi Yamato, Toru Yoshizumi
Systematic Review Members
Koichi Ando, Hiraku Chiba, Takahito Doi, Hiroki Emori, Kazuya Fujihara, Yoshifumi Fukushima, Aiko Hayashi, Aya Higashiyama, Aya Hirata, Satoshi Hirayama, Kyoko Inagaki, Takahiro Ishikawa, Naoyuki Iso-o, Kotaro Kanno, Yu Kataoka, Daisuke Kinoshita, Minako Kinuta, Kyoko Kohmo, Masaya Koshizaka, Yoshimi Kubota, Hisashi Makino, Koutatsu Maruyama, Masaki Matsubara, Wao Nakagawa-Tsutsui, Yasuo Nakai, Mariko Nakamoto, Aiko Narumi-Hyakutake, Tetsuo Nishikawa, Akihiro Nomura, Tomoyasu Oda, Masatsune Ogura, Hideo Oohira, Hirokazu Ohminami, Takeshi Okada, Hirofumi Okada, Fumitaka Okajima, Rie Okamoto, Hiroaki Okazaki, Sachiko Okazaki, Shizuko Omote, Atsuhito Saiki, Kaori Sakamoto, Mizuki Sata, Kayoko Sato, Yoshitaka Shiratori, Mayumi Shoji, Yuka Suganuma, Daisuke Suzuki, Harumitsu Suzuki, Nobuaki Suzuki, Hiroshi Takahashi, Yutaro Takahashi, Mikio Takanashi, Satoru Takase, Noriko Takebe, Yukinori Tamura, Kaki Tanaka, Katsunao Tanaka, Sarasa Tanaka, Yoshihiro Tanaka, Kousei Terada, Naoya Teramoto, Ayano Tsukagoshi-Yamaguchi, Hana Wakasa, Rie Yako, Eri Yamada, Masashi Yamamoto, Satoshi Yashiro, Yuya Yokota, Yukihiro Yoshimura, Zhang Yan
Peer Reviewers
Masahiro Akishita, Junya Ako, Hideaki Bujo, Masanori Emoto, Shinya Goto, Hiroyuki Hanada, Satoshi Hirayama, Naohisa Hosomi, Atsushi Hozawa, Katsunori Ikewaki, Tatsurou Ishida, Kouji Kajinami, Noriko Kameyama, Toru Kikuchi, Koichi Kozaki, Kazuyo Kuwabara, Hiroaki Masuzaki, Tetsuya Matoba, Yuka Matoba, Masaaki Miyata, Toshiharu Ninomiya, Masatsune Ogura, Toshiho Ohtsuki, Yusuke Ohya, Hiroaki Okazaki, Nagako Okuda, Atsuhito Saiki, Kayoko Sato, Shigeyuki Saitoh, Hitoshi Shimano, Daisuke Sugiyama, Hayato Tada, Hirofumi Tomiyama, Kazunori Toyoda, Kazuyo Tsushita, Yukihiko Ueda, Seiji Umemoto, Naoya Yahagi, Kazumasa Yamagishi, Tomoya Yamashita
Advisors
Hiroyuki Daida, Makoto Kinoshita, Shizuya Yamashita, Masayuki Yokode
External Reviewers (Related Societies)
The Japanese Council on Cerebro-Cardiovascular Disease, The Japanese Circulation Society, The Japan Diabetes Society (Yoshihiko Nishio, Iichiro Shimomura), The Japan Endocrine Society (Akiyo Tanabe, Koshi Hashimoto), Japan Epidemiological Association (Katsuyuki Miura), The Japan Geriatrics Society (Sumiko Yoshida), Japanese Society of Health Education and Promotion, The Japan Society of Hepatology (Atsushi Tanaka, Masashi Yoneda), The Japanese Society of Hypertension (Mari Ishida), Japanese Society of Laboratory Medicine (Masato Maekawa, Toshiyuki Yamada), Japan Medical Association (Kazuki Nagai), The Japan Society for Menopause and Women’s Health (Masakazu Terauchi), Japanese Society of Nephrology, The Japanese Society of Clinical Nutrition (Kei Nakajima), Japan Society for the Study of Obesity (Michio Shimabukuro), Japan Pediatric Society (Akira Ohtake), Japanese Society of Clinical Pharmacology and Therapeutics (Shinichiro Ueda), Japanese Society of Physical Fitness and Sports Medicine (Takashi Miyauchi), The Committee on Primary Dyslipidemia under the Research Program on Rare and Intractable Disease of the Ministry of Health, Labour and Welfare of Japan (Mariko Harada-Shiba), The Japan Stroke Society, The Japanese Society on Thrombosis and Hemostasis, The Japan Society of Ultrasonics in Medicine (Toshiko Hirai), Japan Society for Vascular Failure (Toru Miyoshi)
The original version of article appeared in Japanese as “Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022” published by Japan Atherosclerosis Society, Tokyo, in 2022
Table of Contents
Preamble 646
Introduction 647
Conflict of Interest 649
Chapter 1. Clinical Diagnosis of Atherosclerosis 650
1. Morphological Examination Method 650
1.1 Ultrasound Examination 650
1.2 CT 650
1.3 MRI・MRA 651
1.4 Catheterization Examination 651
2. Vascular Function Examination Methods 651
2.1 Ankle Brachial Index (ABI) and Toe Brachial Index (TBI) 651
2.2 Brachialankle Pulse Wave Velocity (baPWV) 651
2.3 Stiffness parameter β, Cardio Ankle Vascular Index (CAVI) 652
2.4 Vascular Endothelial Function 652
3. Risk Prediction for ASCVD by Arterial Wall Assessment and its Problems 652
4. Evaluation of Achilles Tendon Thickness in Familial Hypercholesterolemia 653
Chapter 2. Comprehensive Risk Assessment for ASCVD Prevention 654
1. Risk Factor Assessment 654
1.1 Dyslipidemia 654
(1) Lipid Abnormality 654
(2) Clinical Laboratory Tests for Dyslipidemia 658
1.2 Smoking 660
1.3 Hypertension 662
1.4 Diabetes and Prediabetes 662
1.5 Chronic Kidney Disease (CKD) 667
1.6 Aging, Gender Differences 667
1.7 Family History of CAD 668
1.8 Drinking Alcohol 669
1.9 History of CAD 669
1.10 History of Cerebrovascular Disease (Including TIA) 670
1.11 High Risk Vascular Disease 671
1) Peripheral Arterial Disease: PAD 671
2) Abdominal Aortic Aneurysm: AAA 672
3) Renal Artery Stenosis; RAS 672
1.12 Subclinical Atherosclerosis 673
1.13 MASLD, MASH 676
1.14 Other Risk Factors/Markers To Consider 677
2. Disease Concept and Diagnostic Criteria for Metabolic Syndrome 682
2.1 Importance of Risk Factor Accumulation 682
2.2 Diagnostic Criteria for Metabolic Syndrome 683
2.3 Association of Hyper-LDL Cholesterolemia with Metabolic Syndrome 683
Chapter 3. Comprehensive Risk Management for the Prevention of ASCVD 684
1. Absolute Risk of ASCVD and Lipid Management Targets 684
1.1 Setting Absolute Risk 684
1.2 Approaches to the Management of Dyslipidemia using Absolute Risk 685
1.3 Categorization according to ASCVD Risk 686
1.4 The Concept of Lifetime Risk 688
1.5 Targeted Management of Dyslipidemia from the Perspective of Prevention of ASCVD 688
2. Lifestyle Improvements 690
2.1 Smoking Cessation 690
2.2 Drinking 691
2.3 Management of Obesity and Metabolic Syndrome 692
2.4 Diet Therapy 693
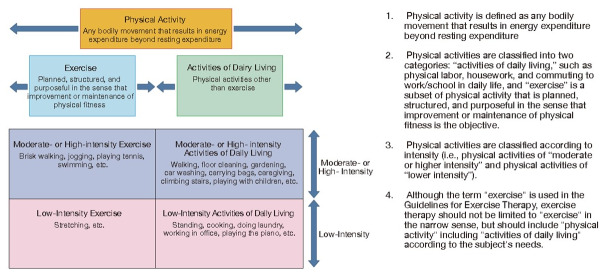
2.5 Exercise Therapy 705
3. Health Counseling Based on Health Behavior Theory 709
3.1 Evidence of Foreign and Domestic Health Counseling on Obesity 709
3.2 Evidence of Overseas and Domestic Health Counseling on Dyslipidemia 710
4. Drug Therapy 711
4.1 Drug Therapy 711
Column: Drug therapy for dyslipidemia with isolated hypo-HDL cholesterolemia 715
4.2 Characteristics and Selection Criteria of Various Drugs 716
4.3 Combination Therapy 720
4.4 Follow-Up of Drug Therapy 721
4.5 Concomitant Use with other Drugs for Prevention of atherosclerosis 722
4.6 Adherence, Treat to Target 724
5. Management of Major High-Risk Pathologies 726
5.1 History of CAD 726
1) Acute Coronary Syndrome 727
2) Familial Hypercholesterolemia 728
3) Diabetes Mellitus 728
4) Atherothrombotic Cerebral Infarction 729
5.2 Diabetes Mellitus 729
1) Risk Factors for ASCVD 729
2) Blood Glucose 729
3) Lipids 730
4) Blood Pressure 730
5) Comprehensive Risk Management 730
5.3 Cerebrovascular Disease 732
1) Frequency of Incidence 732
2) Risk Factors for Incidence 732
3) Lipid-lowering Therapy and Cerebrovascular Disease 733
4) Strategies to prevent cerebrovascular disease 734
5.4 CKD - Chronic Kidney Disease 735
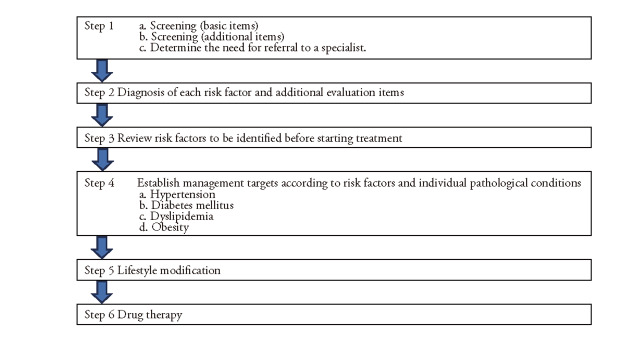
6. Comprehensive Risk Assessment and Management Practices 736
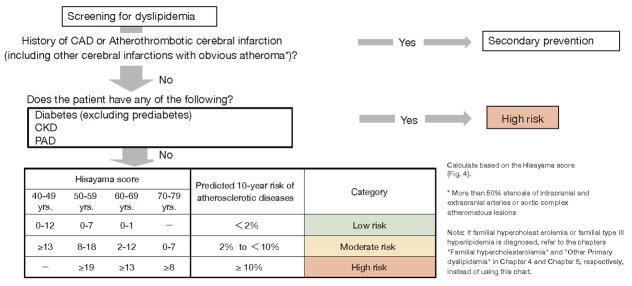
Step 1. Screening for Atherosclerotic Cerebral and Cardiovascular Disease Risk Assessment 736
Step 2. Diagnosis and additional assessment in each risk factor 738
Step 3. Risk Factors to Review Before Initiating Treatment 739
Step 4. Setting Management Targets according to Risk Factors for each Pathological Condition 739
Step 5. Lifestyle Modification 740
Step 6. Drug Therapy 741
Chapter 4. Familial Hypercholesterolemia 742
1. Pathophysiology and Clinical Presentation of FH 744
2. Diagnosis of FH 744
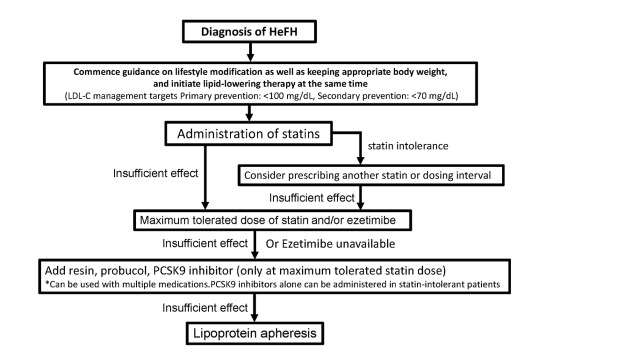
3. Treatment of Adult HeFH (15 years and older) 746
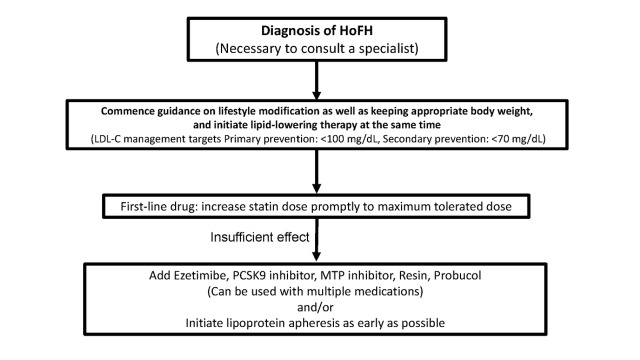
4. Treatment of Adult HoFH (15 years and older) 747
5. Treatment of Pediatric FH (under the Age of 15) 748
6. Pregnancy and delivery of patients with FH 748
Chapter 5. Other Primary Dyslipidemias 750
1. Primary Dyslipidemia and Designated Intractable Diseases 750
2. Familial Combined Hyperlipidemias (FCHL) 750
3. Familial Type III Hyperlipidemia 751
4. Sitosterolemia 753
5. Primary Hypo-HDL Cholesterolemia 753
6. Other 753
Chapter 6. Secondary Dyslipidemia 755
1. Secondary Dyslipidemia 755
2. Diseases and Conditions that Cause 755
2.1 Hypothyroidism 756
2.2 Nephrotic Syndrome 757
2.3 Chronic Kidney Disease: CKD 758
2.4 Primary Biliary Cholangitis and Obstructive Jaundice 758
2.5 Diabetes and Obesity 758
2.6 Cushing’s Syndrome 759
2.7 Pheochromocytoma 759
2.8 Drugs 759
2.9 Heavy Alcohol Consumption 760
Chapter 7. Older People 761
1. Lipid Abnormalities and ASCVD in Older People and their Association with Preventive Effects 761
2. Frailty and Sarcopenia 761
3. ASCVD and Frailty/Sarcopenia 761
Chapter 8. Women 763
1. Current Status of ASCVD in Japanese Women 763
2. Relationship between Risk Factors for Atherosclerosis and ASCVD in Women 763
3. Primary and Secondary Prevention of ASCVD 764
4. Hormone Replacement Therapy (HRT) 766
Chapter 9. Pediatrics 767
1. Early Detection of Dyslipidemia 767
2. Criteria for Lipid Abnormalities in Children 767
3. Primary Dyslipidemia 767
4. Secondary Dyslipidemia 768
5. Maintain Appropriate Weight through Proper Diet and Exercise Habits 768
6. Smoking and Passive Smoking 769
7. Other 769
References 770
Appendix 1. 10‐year risk of incidence of atherosclerotic cardiovascular disease age scored version 841
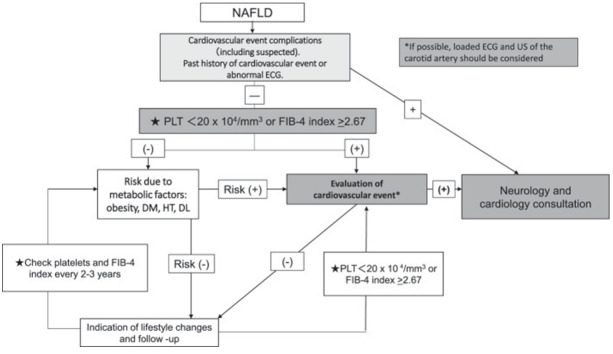
Appendix 2. Flowchart for cardiovascular event screening in NAFLD patients 842
Appendix 3. ‘Where am I?’ chart 843
Appendix 4. Method of Measuring Achilles Tendon Thickness by Ultrasound for FH Screening 844
Appendix 5. Achilles Tendon Radiography for FH Screening 846
Appendix 6. COVID-19 and ASCVD / Thrombosis 850
Preamble
Atherosclerotic cardiovascular diseases (ASCVD), including coronary artery disease (CAD), such as acute myocardial infarction and angina pectoris, and stroke are the major causes of death in Japan, as well as the major cause of the gap between average life expectancy and healthy life expectancy. Even though these are diseases that can be prevented to a certain extent through lifestyle modification and early treatment, ASCVD is a serious problem for the lives and health of the public and has a significant impact on society. Based on this social background, the Cerebrovascular and Cardiovascular Disease Control Act was passed in 2018. And as stipulated by this act, the Cabinet approved the Japanese National Plan for the Promotion of Measures Against Cerebrovascular and Cardiovascular Diseases in 2020, and in this plan the importance of prevention of ASCVD was indicated. Thus, the prevention of the incidence and recurrence of ASCVD is an urgent issue in Japan.
Since the Japan Atherosclerosis Society published its guidelines for the treatment of hyperlipidemia in 1997, it has been revised every five years to incorporate the latest evidence. With the 2012 edition, the risk assessment has been changed from relative risk of CAD to absolute risk of total mortality at 10 years of CAD. For the 2017 edition, items for which evidence is required were subjected to a systematic review (SR) based on clinical questions (CQ), and answers and explanations were included. In this 2022 edition, revisions were made based on recent evidence, including the establishment of cut-off values for triglycerides (TG) at any time (non-fasting) and the adoption of the Hisayama Study score as the absolute risk assessment method. As this guideline changed its name from “medical care” to “prevention” in the 2007 edition, it aims to prevent ASCVD through comprehensive management of the risk of atherosclerosis. It is hoped that ASCVD, which has been on the rise in recent years, will be prevented and an increase in healthy life expectancy will be achieved.
We hope that this guideline will be of use to physicians and medical personnel practicing in the field of ASCVD. It should be noted that this guideline provides information for clinicians to make medical decisions based on past evidence and social and medical situations in Japan and that the final decision on treatment goals and measures for each patient should be made by the physician directly in charge of the patient, depending on the patient’s situation.
Ken-ichi Hirata
President, Japan Atherosclerosis Society
Introduction
In Japan, a super-aged society, deaths from atherosclerotic cardiovascular disease (ASCVD), especially cardiac diseases including coronary artery disease (CAD) such as myocardial infarction and angina pectoris, and cerebrovascular diseases such as cerebral infarction account for about 23% of all deaths and are the leading cause of death comparable to deaths from malignant neoplasms. Therefore, the prevention and treatment of arteriosclerosis, which is the basis for these diseases, will become increasingly important in the future, and the dissemination of prevention and treatment methods based on scientific evidence is an urgent issue.
Since the publication of the Guidelines for the Treatment of Hyperlipidemia in 1997, the Japan Atherosclerosis Society has revised the guidelines every five years to incorporate the large amount of new evidence on treatment and epidemiology published since then. During that time, the name hyperlipidemia was changed to dyslipidemia, total cholesterol changed to LDL cholesterol (LDL-C) among the diagnostic criteria for dyslipidemia, and the risk assessment method for ASCVD was changed from relative risk to absolute risk for CAD. The previous edition (2017) adopted the Suita score, which included data from the statin era, as the absolute risk assessment method.
The main revisions in this 2022 edition are as follows.
1) A cut-off value for non-fasting (including cases where it is unknown whether fasting or not) triglycerides (TG) was established for the first time.
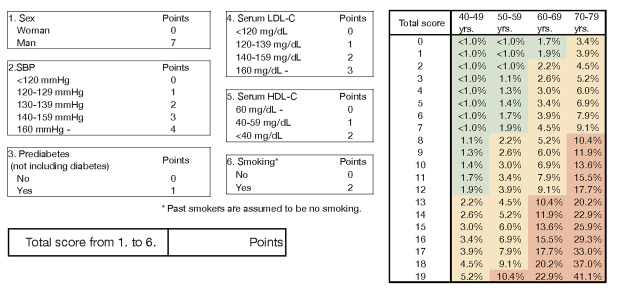
2) The Hisayama score, which used ASCVD combined with CAD and atherothrombotic cerebral infarction (atherothrombotic brain infarction) as endpoints, was used as a method to assess the absolute risk of ASCVD for establishing lipid management targets.
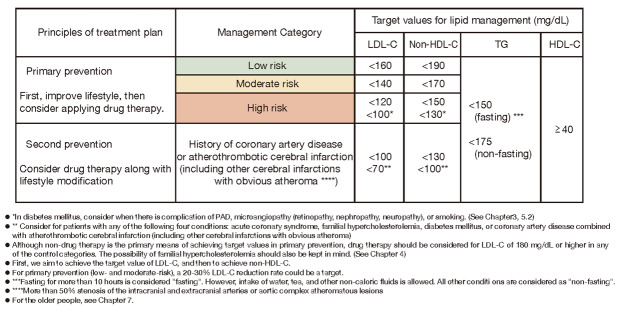
3) In primary prevention, the target for the management of LDL-C in patients with diabetes with peripheral arterial disease, microangiopathy (retinopathy, nephropathy, neuropathy) complications, or smoking is less than 100 mg/dL. Patients with diabetes in the absence of these complications, the target value was set to less than 120 mg/dL as before.
4) In addition to CAD, atherothrombotic cerebral infarction was added as a secondary prevention target and the LDL-C control target was set at less than 100 mg/dL. Furthermore, the target for LDL-C control was established at less than 70 mg/dL for ‘acute coronary syndrome’, ‘familial hypercholesterolemia’, ‘diabetes mellitus’ and ‘complications of CAD and atherothrombotic cerebral infarction’ in secondary prevention.
5) The following items are newly listed based on recent research findings and requests from the clinical field.
a. Clinical laboratory tests for dyslipidemia
b. Subclinical atherosclerosis (current clinical implications of Carotid Intima-media thickness, Pulse wave velocity, Cardio Ankle Vascular Index, etc.)
c. Nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), currently, MASLD/MASH
d. Lifestyle modification for alcohol drinking
e. Health guidance based on health behavior theory
f. Risk management for Chronic kidney disease (CKD)
g. Secondary dyslipidemia
In revising this guideline, the following chapters and sections in particular require the Japan Atherosclerosis Society to present the latest evidence, so a systematic review (SR) was conducted, and a statement was prepared.
• Chapter 2 “Comprehensive Risk Assessment for Prevention of ASCVD”: diagnostic criteria for dyslipidemia, diabetes and prediabetes, subclinical atherosclerosis, NAFLD and NASH (Currently, MASLD/MASH).
• Chapter 3 “Comprehensive Risk Management for Prevention of ASCVD”: absolute risk of ASCVD and lipid management targets, diet, exercise, health guidance based on health behavior theory, drug therapy (including treatment of dyslipidemia in diabetes)
• Chapter 4 “Familial Hypercholesterolemia.”
The procedure proceeded as follows.
1) Establish a Clinical Question (CQ) for the item(s) indicated and designate it as a BQ or FQ using the following definitions.
• BQ (background question): A question about information that provides background information on the topic, such as disease incidence, symptoms, and natural course of disease onset. It is mainly based on epidemiological studies (observational studies) and presents only the level of evidence, without recommendations.
• FQ (foreground question): A question related to decision-making in clinical practice about the choice of medical treatment. Among the foreground questions, clinical issues for which multiple options exist in the current medical process and the balance of benefits and harms is unclear, and for which a recommendation is expected to improve patient outcomes are considered important clinical issues. Basically, PICO (Patient, Intervention, Comparison, Outcome) can be established, and recommendations are made mainly based on randomized controlled trials (RCT). However, in some areas, PECO (Patient, Exposure, Comparison, Outcome) may be used.
2) The level of evidence should be described based on the following criteria. The level of evidence is divided into evidence of treatment interventions and evidence of epidemiological studies.
Classification of the level of evidence for treatment interventions.
| 1+ | High quality RCTs* and their MA/SR |
| 1 | Other RCTs and their MA/SR |
| 2 | Prospective cohort studies, their MA/SR, (predefined) RCT sub analysis |
| 3 | Non-randomized controlled trials, before and after studies, retrospective cohort studies, case-control studies, and their MA/SR and RCT post hoc sub-analysis |
| 4 | Cross-sectional studies, case series |
| Consensus | Consensus of the members of the JAS guideline committee. |
RCT: randomized controlled trial, MA: meta-analysis, SR: systematic review
* A high-quality RCT is defined as (1) large number of subjects, (2) double-blind, independent assessment, (3) high follow-up rate (low dropout rate), and (3) Low protocol deviation (4) Clear random allocation method, etc.
Classification of Evidence Levels for Epidemiological Studies.
| E-1a | Meta-analysis of cohort studies |
| E-1b | Cohort studies |
| E-2 | Case-control studies, cross-sectional studies |
| E-3 | Descriptive studies (case series) |
3) In principle, the text in the FQ is described as “recommend” for Recommendation A, and “suggest” for Recommendation B.
Recommendation Levels.
| A | recommend |
| B | suggest |
4) Those for which a recommendation A/B cannot be determined from the SR, or for which there is no RCT, but which the committee would like to recommend, were designated as “consensus”.
5) The recommendation level is determined by the modified Delphi method. The experts, given the appropriate information on the issues to be considered, will first evaluate them individually (Round 1), and then, after discussion at a meeting using the results of the evaluation as reference material, will evaluate them again individually (Round 2). On the basis of the median value obtained as a result of the second round, a consensus on the recommendation is reached. In the case of polarization, the recommendation will be judged as a disagreement rather than a consensus.
While previous guidelines have focused on the prevention of CAD, this guideline also focuses on atherothrombotic cerebral infarction for the first time, aiming to prevent ASCVD by more comprehensively. This guideline is intended to be used by all physicians, members of the medical team and health administrators who manage the risk of atherosclerosis to prevent the onset and recurrence of ASCVD, including CAD such as myocardial infarction and angina pectoris, and cerebrovascular disorders such as cerebral infarction. However, it should be noted that the diagnosis and treatment of each patient should be decided by the physician in charge of the patient and that this is not a regulation that must be followed.
Conflict of Interest
All members of this guideline committee, as medical and medical specialists in the field of atherosclerotic diseases and related disorders, have prepared to ensure the scientific and medical fairness and validity of the content of the “Guidelines for the Prevention of ASCVD 2022”, to improve the level of care for the diseases covered and to increase the healthy life expectancy and quality of life of the target patients.
All costs associated with the development of this guideline were paid from the annual budget of the Japan Atherosclerosis Society, and no other funding was received.
To ensure fairness and transparency in the selection of the guideline chairperson, a “COI Evaluation Committee for Guideline Committee Members” was established within the Japan Atherosclerosis Society and decided based on the “Guidance on Eligibility Criteria for Participation in the Formulation of Medical Practice Guidelines of the Japan Medical Association”. Furthermore, a wide range of opinions were collected through peer review by related societies and comments from members of the Japan Atherosclerosis Society.
Conflicts of interest of the guideline’s supervisory committee, authors, co-authors and SR members were reviewed based on the “COI Management Guidelines of the Japan Medical Association” for the past three years (January 1, 2016, to December 31, 2018) and the three years after their appointment (January 1, 2019 to December 31, 2021), and the status of conflicts of interest is disclosed on the Japan Atherosclerosis Society website.
https://www.j-athero.org/publications/gl2022_coi_eng.pdf
Conflict of Interest (COI)
Chapter 1.Clinical Diagnosis of Atherosclerosis
From the viewpoint of prevention of ASCVD, it is important to identify the presence and extent of atherosclerotic lesions before clinical symptoms appear, and to manage and treat risk factors with consideration given to prevention of their development or even regression. While invasive diagnostic methods, including angiography, are necessary in the secondary prevention of ASCVD, non-invasive diagnostic methods are the main method to assess arterial stiffness in primary prevention. Here, we will focus on the non-invasive methods currently used to assess atherosclerosis, which are divided into morphological and vascular function testing methods.
1.Morphological Examination Method
1.1 Ultrasound Examination
Ultrasound examinations from the body surface are widely used as a non-invasive imaging method for arteriosclerosis because they are inexpensive, simple, and safe. Ultrasound equipment using a linear 7-10 MHz or higher high frequency probe allows evaluation of peripheral arterial lesions, such as carotid arteries and lower extremity arteries. The use of a convex 3.5 to 6 MHz probe also allows evaluation of the abdominal aorta and renal arteries.
In the carotid artery, the measurement of intima-media thickness (IMT), plaque thickness (localized bulging lesion of 1.1 mm or more), plaque characteristics, and degree of stenosis are recommended as standard evaluation methods for arterial stiffness by the Japan Society of Ultrasonics in Medicine and the Japan Academy of Neurosonology 1) . IMT should be evaluated as age-dependent thickening 2) . IMT is also used as a surrogate indicator for predicting the risk of complications and development of ASCVD (CAD, peripheral arterial disease (PAD), cerebrovascular disease, etc.) 3 - 6) . The standard evaluation method recommends measuring maximum IMT and IMT-C10 (IMT in the distal wall 10 mm proximal to the carotid sinus). The presence of plaque lesions is more important than the IMT index in predicting disease, but in cases without plaque, a high IMT value is the underlying pathology of the appearance of plaque. Referring to the Mannheim consensus, plaques with a maximum thickness greater than 1.5 mm should also be evaluated for characterization 1 , 7) , especially plaques with vulnerability to potential cerebral embolic sources (such as echolucent plaques, ulcerative lesions, mobile lesions, and plaques with large lipid cores). Stenosis should be evaluated if luminal plaque occupancy is greater than 50% on short-axis scanning. In the case of significant stenosis (peak systolic blood flow velocity of 200-230 cm/s or more, corresponding to 70% or more by NASCET method 8) ), carotid endarterectomy and carotid artery stenting should be considered in addition to aggressive medical therapy.
In addition to physical findings such as pulse palpation and blood pressure measurement, diagnostic imaging is especially essential for the diagnosis of PAD in the arteries of the lower extremities. Among these, ultrasound examination increases diagnostic precision when combined with the Ankle Brachial Index (ABI) because of its simplicity, non-invasiveness, and ability to evaluate blood flow 9 , 10) . Basically, plaque characteristics and the degree of stenosis are evaluated, but it is also possible to estimate the site of stenosis by confirming the presence of collateral blood vessels, blood flow wave patterns, and the Transit Time of Vessel Flow (TVF) of the leg 11) .
In the aorta, the main evaluation is for abdominal aortic aneurysms 12) . In particular, the mass diameter (maximum short diameter) and its shape can be useful in determining whether it is eligible for surgical treatment. Ultrasound can also confirm the presence of a mobile component if there is an internal thrombus. Ultrasound is also useful in the diagnosis of atherosclerotic renal artery stenosis in the renal arteries 13 , 14) .
1.2 CT (Computed Tomography)
CT is an examination method that can diagnose atherosclerotic lesions in a short time. By diagnosing the size of the artery, the presence of an aneurysm can be confirmed. It is also excellent for confirming the presence of calcified lesions in head and neck arteries, aorta, and peripheral arteries, since CT values allow some estimation of calcification, fat, and fiber content. Multi-detector row CT (MDCT) is actively used as a non-contrast examination because of its faster imaging speed and superior spatial resolution. It is especially useful to observe the presence of coronary artery calcification by non-contrast MDCT in patients with abnormal glucose metabolism when carotid artery ultrasonography shows IMT thickening or plaque, or when high baPWV and CAVI and low ABI are observed, as described below 15) . In cases of moderate or higher, contrast can be injected through a peripheral vein for a more detailed depiction of the coronary arteries and systemic arteries and is widely used in the evaluation of CAD and PAD. Furthermore, MDCT has high sensitivity and specificity for detecting CAD 16 - 19) , and the presence of organic coronary artery stenosis is almost always ruled out when there is no abnormality with this method. Recently, new methods have been developed to improve the diagnostic performance of CAD. In particular, CT myocardial perfusion and fractional flow reserve CT improve the diagnostic performance of CAD compared to CT angiography 20) .
If chest CT was taken for purposes other than the evaluation of ASCVD, depending on the patient’s profile, it may be desirable to confirm the presence or absence of coronary artery calcification to aid in a further detailed assessment of the patient’s risk.
1.3 MRI, MRA (Magnetic Resonance Imaging, MR Angiography)
MRI is particularly useful for identifying ischemic changes and infarct lesions in the brain. MRA is also excellent in showing stenotic and occlusive lesions not only in the intracranial arteries and carotid arteries, but also in the aorta, renal arteries, and arteries of the lower extremities. Recently, noncontrast MRA examinations are sometimes used in place of angiography. MRI plaque imaging examinations can also be used to evaluate plaque characteristics. Combining MRI/MRA with ultrasound examination improves diagnostic accuracy not only in the evaluation of stenotic or occlusive lesions, but also in the diagnosis of plaque characteristics. There is no clear difference in diagnostic performance between invasive catheter examination and echocardiography or cardiovascular MRI when stable CAD is suspected 21) .
1.4 Catheterization Examination
Catheter-based angiography is an invasive examination and is used as needed when ASCVD is suspected by non-invasive examinations. The stenosis ratio is calculated from the lumen diameter of the stenotic area and the area considered normal, but there are limitations in accurately evaluating the amount of plaque, such as eccentric plaque and compensatory remodeling. On the other hand, intravascular ultrasound (IVUS), optical coherence tomography (OCT), and vascular endoscopy are superior in assessing not only plaque volume but also plaque characteristics. In recent years, aortic endoscopy has been used to observe embolisms caused by plaque disruption. An examination method that measures the ratio of coronary blood flow reserve using a pressure wire has also been implemented as a functional coronary artery stenosis evaluation method.
2.Vascular Function Examination Methods
For examination of vascular function, the Japanese Circulation Society 22) and the Japan Society for Vascular Failure 23) have provided detailed indices for reference.
2.1 Ankle Brachial Index (ABI) and Toe Brachial Index (TBI)
The ABI calculates the ratio of blood pressure at the level of the ankle joint to that at the brachial artery, indicating the presence of a stenotic or occlusive lesion in the main artery central to the ankle joint and the degree of compensation by the collateral blood vessels. Doppler and oscillometric methods are used to measure blood pressure. When measuring with a sphygmomanometer, brachial blood pressure should be measured with a stethoscope and ankle blood pressure should be measured with the Doppler method. Oscillometric methods are used for automatic measurements with automatic sphygmomanometers or specialized devices. Although the correlation between the two is generally good, the accuracy of the oscillometric method decreases with critical limb ischemia. If ABI is less than 0.90, obstructive lesions are suspected 23 , 24) . TBI looks at the ratio of blood pressure at the level of the toes to blood pressure in the brachial artery. When measured with ABI, peripheral ankle joint obstructive lesions can be inferred. The standard value for TBI is 0.7 or higher; if the value is 0.6 or lower, an occlusive lesion of the lower extremity artery is suspected. It should be noted that diabetics and dialysis patients are prone to calcification of the inferior arterial wall, which can result in inaccurate ABI measurements in some cases.
2.2 Brachialankle Pulse Wave Velocity (baPWV)
The arterial pulse wave velocity (PWV) generated by cardiac output reflects the degree of arterial stiffness 25) . It can be easily measured by measuring the pulse wave of an extremity with a dedicated device, but it should be noted that it is an indicator of arterial stiffness and does not necessarily reflect atherosclerosis. The PWV is the speed at which the aortic pulsation (pulse wave) generated by the beating heart propagates to the periphery. PWV is proportional to the stiffness and thickening. There are two types of measurements: cfPWV (carotid-femoral PWV) and baPWV. baPWV is used in actual clinical practice in Japan. It should be noted that baPWV is affected by blood pressure at the time of measurement.
Cardiovascular disease risk factors that increase baPWV include aging 26) , hypertension 27) , diabetes 28) , and pulse rate 26) , which correlate well with the Framingham Risk Score. The normal value of baPWV is less than 1,400 cm/s, and values greater than 1,800 cm/s are judged abnormal 23) . In Japanese data, the addition of baPWV to classical risk factors has been shown to significantly increase the predictive ability of the risk of developing cardiovascular disease 29) , especially in low-risk groups.
2.3 Stiffness Parameter β, Cardio Ankle Vascular Index (CAVI)
The stiffness parameter β is a measure of the degree of stiffness inherent in the local arterial wall. It was devised as an index of arterial elasticity performance that is less sensitive to blood pressure by correcting for blood pressure at the time of measurement 30) . It is calculated from the change in carotid artery caliber and blood pressure as ln (Ps/Pd)/[(Ds-Dd)/Dd] (Ps=systolic blood pressure, Pd=diastolic blood pressure, Ds=carotid end systolic diameter, Dd=carotid end diastolic diameter) and its correlation with carotid artery stiffness has been reported 31 , 32) .
CAVI applies the concept of a stiffness parameter β to arteries with length and is an index of elastic performance of the entire artery from the aortic root to the lower extremity ankle 30) . A characteristic of CAVI is that it is less affected by blood pressure at the time of measurement 32) . CAVI increases with age 33) and in patients with stroke, cardiovascular disease 34) , chronic kidney disease (CKD) and vasculitis, and also increases with hypertension, diabetes, metabolic syndrome, sleep apnea syndrome, smoking, and disaster stress, but it has been reported to improve with treatment for each of these conditions 30) . Prospective cardiovascular event studies have reported that higher levels of CAVI are associated with a higher frequency of cardiovascular events 35 , 36) . The normal value of CAVI is less than 8.0, and a value of 9.0 or higher is considered abnormal 23) .
2.4 Vascular Endothelial Function
Vascular endothelial function is evaluated by measuring changes in forearm blood flow and diameter of the brachial artery in response to endothelium-dependent increases in blood flow caused by drugs such as acetylcholine and reactive hyperemia after 5 minutes of forearm ejection. The most commonly used techniques are flow-mediated dilatation (FMD), which measures changes in brachial artery diameter using ultrasound, and reactive hyperemia peripheral arterial tonometry (RH-PAT), which measures changes in volume pulse wave in the popliteal arterial bed.
FMD is an examination to evaluate the degree of dilation of the brachial artery caused by reactive hyperemia after 5 minutes of inhibition of the forearm and is calculated as follows: FMD (%)=(maximal diameter of the dilated artery - diameter of the resting artery) / diameter of the resting artery x 100. Normal FMD is above 7%, and when endothelial cells are damaged, nitric oxide (NO) production is reduced and FMD is low. Values between 4% and 7% are borderline values, and those less than 4% are considered abnormal 23) . Since FMD declines from the early stages of atherosclerosis 37 , 38) , it is useful in the initial assessment of ASCVD.
RH-PAT uses a dedicated probe to detect the volume pulse wave of the fingertip microvascular bed in each of the left and right fingers. Like FMD, it measures the arterial diastolic function of reactive hyperemia after 5 minutes of forearm inhibition, but unlike FMD, it evaluates the increase over time in the pulse wave of the volume of the finger volume. The normal value of RH-PAT is 2.10 or higher, and when endothelial cells are damaged, RH-PAT is also low; values between 1.67 and 2.10 are borderline and values below 1.67 are considered abnormal 23) .
3.Risk Prediction for ASCVD by Arterial Wall Assessment and its Problems
As mentioned above, carotid IMT/plaque, ABI, baPWV, CAVI, and FMD are considered independent predictors of future development of ASCVD. However, overseas studies have reported that adding carotid IMT measurements does not increase the risk prediction power of the Framingham Risk Score 39) . Although there have been reports on the importance of carotid artery stiffness assessment in Japan 40) , it is not yet clear whether it can contribute to improving the accuracy of existing risk assessment models.
One issue to be aware of when predicting risk is the measurement method and interpretation of the results obtained. For example, carotid IMT and plaque thickness are often measured manually at the time of the actual examination, and it is not always clear whether they are evaluated at the same level at follow-up. On the other hand, vascular function examinations are a standardized measurement method, and the reliability of the obtained values is high, but the values can also vary with body shape, blood pressure level, and arrhythmia, as well as the need to adjust conditions during measurement, such as the interval between meals and room temperature.
More evidence needs to be developed so that abnormal findings in these indices, with the exception of ABI, are reflected in the risk categories of the ASCVD prevention guidelines, leading to stricter management.
4.Evaluation of Achilles Tendon Thickness in Familial Hypercholesterolemia
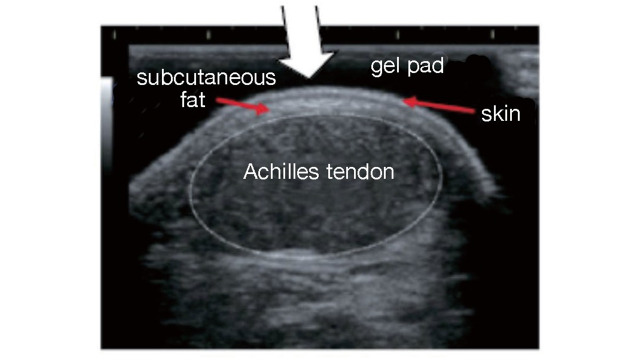
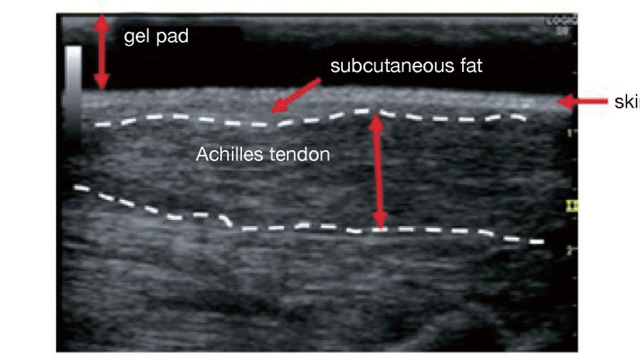
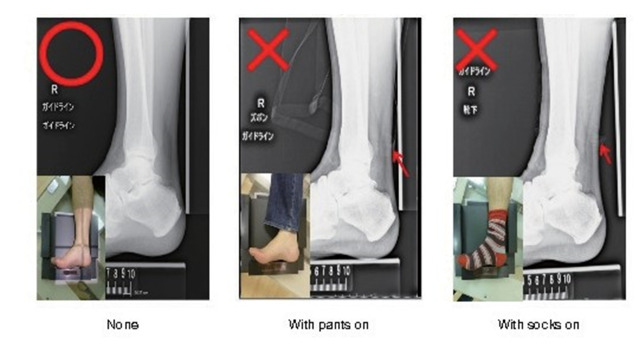
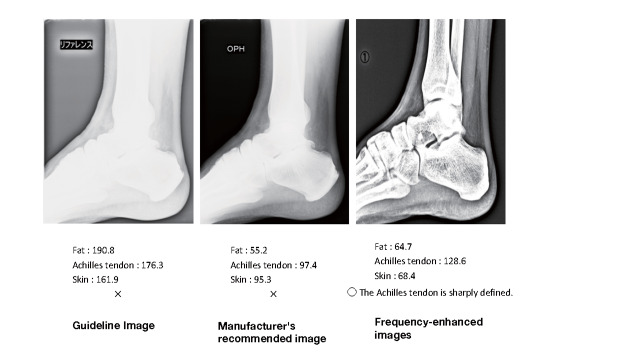
Familial hypercholesterolemia (FH) is at high risk of developing premature CAD, so early diagnosis and treatment are desirable. One of the diagnostic criteria is measuring the thickness of Achilles tendon thickness. The previous diagnostic criteria were 9 mm or more on X-ray (soft X-ray) imaging, but the revised criteria are now 8 mm or more for men and 7.5 mm or more for women.
The boundary between the skin and the Achilles tendon is unclear and difficult to measure when reading X-rays. There is also a discrepancy in that palpation evaluates the transverse width of the Achilles tendon, whereas X-rays evaluate the thickness in the anterior-posterior direction. For this reason, a simple and accurate measurement method was sought in the outpatient examination room. As a result, in 2018, the Japan Atherosclerosis Society and the Japan Society of Ultrasonics in Medicine publicly announced a standard evaluation method for ‘Achilles tendon thickness measurement by ultrasound method’ for screening adult FH 41) . Here, the thickness of the Achilles tendon can be easily measured using a general-purpose ultrasound system with a linear probe of about 7.5 to 24 MHz. Since significant Achilles tendon thickening may be present, different probes are used as needed. The threshold for diagnosing Achilles tendon thickness is a thickening of 6.0 mm or more for men and 5.5 mm or more for women in the anteroposterior diameter. The revised criteria for the diagnosis of FH now include the thickness of the Achilles tendon measured by ultrasound. (See Chapter 4, “Familial Hypercholesterolemia”, Methods of measurement are described at the end of this guideline.)
Chapter 2. Comprehensive Risk Assessment for ASCVD Prevention
1.Risk Factor Assessment
1.1 Dyslipidemia
(1) Lipid Abnormality
BQ1 Does LDL cholesterol predict the incidence and mortality of ASCVD in the Japanese population?
Elevated LDL cholesterol predicts the future incidence and mortality of CAD. LDL cholesterol has been shown to be positively associated with cerebral infarction and negatively associated with hemorrhagic stroke, but there is not enough evidence compared to total cholesterol in the Japanese population. (Level of evidence: E-1b)
As well as many epidemiologic studies in the U.S. and Europe including the Framingham study in the U.S., some cohort studies of Japanese subjects have confirmed that the hazard ratio for the incidence of CAD and death increases with elevated LDL cholesterol (LDL-C) levels 42 - 47) . CIRCS study showed that for every 30 mg/dL increase in LDL-C, the hazard ratio increased 1.3-fold in men and 1.25-fold in women 43) . Thus, it is clear that elevated LDL-C increases the risk of developing CAD even in Japanese. The Suita study showed that the risk of myocardial infarction was positively associated with LDL-C in men, but not in women, but was positively associated with LDL-C in both sexes combined 47) . The probability of developing CAD during one’s lifetime (lifetime risk) was 47.2% for men with LDL-C >160 mg/dL (high LDL-C group) and 13.7% for men with LDL-C <160 mg/dL (low LDL-C group) at age 45, and 44.5% for high LDL-C group and 10.7% for low LDL-C group at age 75. There was a significant difference between the high and low LDL-C groups. Among women, that was 10.2% in the high LDL-C group and 7.1% in the low LDL-C group at age 45, and 7.5% in the high LDL-C group and 6.4% in the low LDL-C group at age 75, which was higher in the high LDL-C group but not significant 48) .
For cerebral infarction, LDL-C was also significantly positively associated with the risk of atherothrombotic cerebral infarction 42) , but conversely, for hemorrhagic stroke (mainly intracerebral hemorrhage), a negative association was reported with a lower hazard ratio in the group with higher LDL-C 46) .
Interventional trials including lifestyle modification for hyper-LDL cholesterolemia have shown to significantly reduce CAD in US and Europe. Large-scale clinical trials have been reported in also Japan 49 - 52) , and it has become clear that treatment of hyper-LDL cholesterolemia reduces CAD in Japanese patients. Meanwhile, lowering LDL-C in these trials has not been associated with an increased risk of intracerebral hemorrhage.
Overlapping risk factors also increase the incidence and mortality of CAD in the Japanese population 53 , 54) . It has been shown that even with the same degree of hypertension, the addition of hyper-LDL cholesterolemia contributes to an increased risk for cardiovascular diseases 55) .
Considering these facts, this guideline sets the screening threshold for Japanese as LDL-C 140 mg/dL or higher, and LDL-C 120 to 139 mg/dL as the borderline range where the influence of overlapping other risk factors should be carefully judged.
BQ2 Does total cholesterol (TC) predict the incidence and mortality of ASCVD in the Japanese population?
Elevated total cholesterol predicts the future incidence and mortality of CAD. Regarding stroke, in common with many studies, total cholesterol predicts stroke incidence and death, with a positive relationship for cerebral infarction and a negative relationship for hemorrhagic stroke. (Level of evidence: E-1a)
As in LDL-C mentioned above, numerous cohort studies in Japan have reported an increase in CAD incidence and mortality with increasing TC 56 - 62) . In NIPPON DATA80 study, from 24 years of follow-up, the hazard ratio for CAD death in the group with TC 220 mg/dL or higher was 1.55 times higher than in the group with TC less than 220 mg/dL, and the population attributable risk factor (PAF) was 18.2% 60) . When this criterion was set at TC 240 mg/dL, the hazard ratio was 1.79 times higher, but the PAF decreased to 11.9%. Although the association between TC and the incidence and mortality of CAD was nearly linear, a statistically significant increase in risk was observed from around TC 220 mg/dL in many studies. The relationship between TC and the risk of CAD mortality was found in both men and women, but the association weakened in those over 65 years 63) .
With regard to stroke, the association between TC and hazard ratio differs depending on cerebral infarction and hemorrhagic stroke (mainly intracerebral hemorrhage). A low TC increased the risk of developing hemorrhagic stroke and intracerebral hemorrhage 63 - 65) , whereas a high TC increased the risk for cerebral infarction as well as CAD 66 , 67) .
EPOCH-JAPAN showed a synergistic effect of blood pressure and TC on CAD mortality 68) . The overlap of systolic blood pressure ≥ 160 mmHg and TC ≥ 220 mg/dL increased the adjusted hazard ratio for CAD death by 4.4-fold compared to the group with blood pressure <120 mmHg and TC <180 mg/dL. On the other hand, death from intracerebral hemorrhage was lower in the group with TC 220 mg/dL or higher, even within normotensive and normotensive ranges of the guideline for the management of hypertension 2019 (JSH 2019). Furthermore, the lifetime risk (LTR) of CAD death at age 35 years in the TC 220 mg/dL or higher group was 7.73% for men and 5.77% for women with degree II or III hypertension, which were 2% higher than those in the TC less than 220 mg/dL group. In the normotensive and normotensive hypertensive groups, the absolute difference in LTR between the TC 220 mg/dL and TC <220 mg/dL groups was 0.25% for men and 0.01% for women. In other words, the increase in LTR of CAD mortality due to high TC was distinct in the hypertensive group 69) .
BQ3 Does non-HDL cholesterol predict the incidence and mortality of ASCVD in the Japanese population?
Elevated non-HDL cholesterol predicts the future incidence and mortality of CAD. On the other hand, there are reports of no association with stroke. (Level of evidence: E-1b)
Non-HDL-C is considered to be a better predictor of ASCVD than LDL-C because it contains all atherosclerosis-inducing lipoproteins, including remnant lipoproteins 70 , 71) . Various epidemiological survey results on the association between non-HDL-C and CAD have been reported in Japan 47 , 59 , 72 - 78) . Non-HDL-C was associated with the development of myocardial infarction as well as LDL-C, and the predictive ability of both was equivalent 47) . On the other hand, the predictive ability of non-HDL-C for myocardial infarction was superior to that of TC 59) . The risk of non-HDL-C for the incidence and mortality of CAD and myocardial infarction has been reported to increase in men, women, or men and women combined from around 140 mg/dL 72 , 75 , 77 , 79) , and all studies showed a clear increase above 170-180 mg/dL. LTR at 45 years for men was significantly higher in the group of 190 mg/dL or higher, 41.5% in the group of non-HDL-C 190 mg/dL or higher and 12.7% in the group of non-HDL-C less than 190 mg/dL, while there was no significant difference in women 48) .
In a report examined the risk of myocardial infarction for non-HDL-C with and without hypertriglyceridemia 71) , a clear increase in risk of myocardial infarction was observed for hypertriglyceridemia (≥ 150 mg/dL) and non-HDL-C ≥ 190 mg/dL. The risk of CAD was significantly higher in the CKD group with a non-HDL-C level of 150 mg/dL or higher compared to those with a non-HDL-C level of less than 150 mg/dL, while the risk of CAD was significantly higher in the non-CKD group with a non-HDL-C level of 190 mg/dL or higher 80) .
It should be noted that LDL-C + 30 mg/dL is a reasonable standard for non-HDL-C in Japanese patients with dyslipidemia as in the U.S. 81 , 82) .
On the other hand, with regard to stroke, while there are reports that the association with any disease type is not clear and others that a positive association with atherothrombotic cerebral infarction has been observed 59 , 75) , there are various reports that the risk of cerebral infarction, especially cardiogenic embolism, is increased when non-HDL-C is low 78) . The JPHC study found a U-shaped association between non-HDL-C and risk of stroke, with an inverse association with intracerebral hemorrhage and a positive association with cortical branch cerebral infarction in men. The lowest risk among women was in the 160-181 mg/dL group for intracerebral hemorrhage and in the 141-159 mg/dL group for embolic infarction 79) .
Based on these results, we concluded that non-HDL-C is a useful indicator that can predict the onset and mortality of CAD, and we set a screening criterion for non-HDL-C of 170 mg/dL or higher in this guideline. In addition, non-HDL-C 150-169 mg/dL was established as a borderline range where the effects of overlapping other risk factzors should be carefully judged.
BQ4 Does HDL cholesterol predict the incidence and mortality of ASCVD in the Japanese population?
Low HDL cholesterol predicts the future incidence and mortality of CAD and cerebral infarction. On the other hand, extremely high HDL cholesterol has been reported to be associated with higher mortality from CAD and cerebral infarction. (Level of evidence: E-1b)
Low levels of HDL-C are associated with the risk of developing CAD and cerebral infarction; conversely, higher levels are associated with a lower risk 45 , 61 , 83 - 87) . In NIPPON DATA90, HDL-C was significantly negatively associated with all-cause mortality and stroke mortality over a 9.6-year observation period 88) . Regional and occupational cohort studies have shown an increased risk of developing CAD at levels below 40 mg/dL 54 , 61 , 85 , 86) , and in J-LIT, a cohort of simvastatin users, the relative risk in the group below 40 mg/dL compared to the group with HDL-C between 40 and 49 mg/dL was 1.3 times higher for primary prevention 89) and 1.6 times higher for secondary prevention 90) . An observational study of the general population in 23 Asian and Oceania regions, including Japan, showed that low HDL-C, especially in Asian regions, is a risk factor for CAD, even if LDL-C and TG are in the normal range and only HDL-C is low 91) . However, a large cohort study limited to Japanese only showed that low HDL-C alone is not a risk factor for CAD or stroke 92 , 93) . Furthermore, a large Japanese cohort study reported a significantly higher risk of death from CAD and cerebral infarction in a group with extremely high HDL-C (>90 mg/dL) compared to a group with HDL-C between 40 and 59 mg/dL. Extremely high HDL-C, >90 mg/dL, was observed in as few as 1.5% of the reported cohort subjects but was more pronounced in those who drank alcohol. Further findings are needed to determine whether hyper-HDL cholesterolemia is a risk factor, taking into account confounders of alcohol consumption 94) .
Considering the above, this guideline set a screening criterion for hypo-HDL cholesterolemia of less than 40 mg/dL. Women generally have higher HDL-C levels than men 54 , 88 , 95) . However, there is currently insufficient evidence regarding the association between gender differences in HDL-C and CAD in men and women 85) , so this guideline uses the same cut-off values as for men.
BQ5 Does triglyceride (TG) predict the incidence and mortality of ASCVD in the Japanese population?
Triglycerides, whether fasting or not fasting, predict the future incidence and mortality of CAD and cerebral infarction. (Level of evidence: E-1b)
High levels of TG have been reported to be associated with the risk of CAD not only in Europe and the United States 96) , but also in Asia and Oceania 97) and Japan 54 , 85 , 98 - 102) . Several of these studies have found an association between TG and CAD even after correcting for HDL-C 96 - 99) . In the United States, the Framingham study defined hypertriglyceridemia as a fasting TG of 150 mg/dL or more (fasting) 103) . Usually, TG has been assessed by fasting blood sampling, but some reports suggest that non-fasting blood sampling is rather more predictive of cardiovascular events 101) . The EAS/EFLM Consensus Statement define hypertriglyceridemia as non-fasting TG of 175 mg/dL or more. Epidemiological studies in Japan have shown that the incidence of CAD increases at fasting TG levels of 150 mg/dL or higher 54 , 104) , myocardial infarction, exertional angina, and sudden death at non-fasting TG levels of 167 mg/dL or higher 98) , and an increased risk of developing ischemic cardiovascular disease from approximately similar TG levels 101) . Furthermore, there are many reports that hypertriglyceridemia is associated with an increased risk of cerebral infarction 54 , 73 , 97 , 101 , 105 , 106) . NIPPON DATA90 showed that the risk of cardiovascular disease mortality was increased when non-fasting TG was 210 mg/dL or higher compared with 150-179 mg/dL, a U-shaped association was found between non-fasting TG and cardiovascular disease mortality, and the risk of cardiovascular death increased with lower non-fasting TG in the group over 65 years of age and with higher non-fasting TG in the group under 65 years of age 107) .
Considering the above, this guideline defines hypertriglyceridemia as fasting TG ≥ 150 mg/dL and non-fasting TG ≥ 175 mg/dL, in consideration of the reports of epidemiological studies in Japan and consistency with the EAS/EFLM Consensus Statement.
Diagnostic Criteria for Dyslipidemia
As shown in the diagnostic criteria for dyslipidemia BQ 1-5, epidemiological studies have shown that the higher the LDL-C, TC, non-HDL-C and TG, and the lower the HDL-C, the higher the incidence of CAD, not only in Europe and the United States, but also in Japan. On the other hand, for cerebral infarction (mainly atherothrombotic cerebral infarction) among strokes, the association is almost the same as for CAD, but for hemorrhagic stroke (mainly intracerebral hemorrhage), the incidence and mortality rates are higher at low levels of LDL-C and TC. The absolute risk (incidence and mortality) of CAD in Japan is currently very low compared to Europe and the United States 108) . However, the management of dyslipidemia is important due to the fact that LDL-C and TC have been increasing in the Japanese population with recent westernization of lifestyles, and TC levels are now equal to or higher than those in the US 109) and reports that the incidence of CAD is beginning to rise in some regions 110 - 112) . Therefore, in this guideline, diagnostic cut-off values for dyslipidemia were established as shown in Table 1 , with emphasis on the prevention of the development of CAD.
Table 1. Dyslipidemia Diagnostic Criteria.
| LDL‐C | ≥ 140 mg/dL | Hyper‐LDL Cholesterolemia |
| 120 ‐ 139 mg/dL | Borderline hyper‐LDLcholesterolemia** | |
| HDL‐C | <40 mg/dL | Hypo‐HDL Cholesterolemia |
| TG | ≥ 150 mg/dL (fasting*) | Hypertriglyceridemia |
| ≥ 175 mg/dL (non‐fasting, any time*) | ||
| Non‐HDL Cholesterol | ≥ 170 mg/dL | Hyper‐non‐HDL cholesterolemia |
| 150 ‐ 169 mg/dL | Borderline hyper‐non‐HDL cholesterolemia** |
*Fasting for more than 10 hours is considered ‘fasting’. However, the consumption of noncaloric fluids such as water and tea is acceptable. If the patient is not confirmed to fast, it is defined as ‘non‐fasting’ or ‘anytime’.
**If screening shows borderline hyper‐LDL cholesterolemia or borderline hyper‐non‐HDL cholesterolemia, investigate whether there are any high‐risk conditions and consider the need for treatment.
‐ LDL‐C is calculated using the Friedewald formula (TC-HDL‐C-TG/5) (only for fasting blood samples), or through a direct method.
‐ If TG is >400 mg/dL or non‐fasting blood is collected, use non‐HDL‐C (=TC-HDL‐C) or LDL‐C direct method. However, when non‐HDL‐ C is used in the screening, the risk should be evaluated with the possibility that the difference from LDL‐C may be less than +30 mg/dL in the absence of hypertriglyceridemia.
‐ The cut‐off value for TG varies depending on whether the blood is collected fasting or non‐fasting.
‐ HDL‐C alone is not a target for drug intervention
The first step in the diagnostic procedure is to measure TC, TG, and HDL-C in the early morning fasting state. LDL-C is calculated using the Friedewald formula (LDL-C=TC-HDL-C-TG/5), but the direct method is also acceptable. The measurement of LDL-C by the direct method was previously pointed out to have accuracy problems 113) . However, the reagents that had been determined to be defective were discontinued, improved, and the standard values were modified, and as a result the performance of the reagents was improved, and the validity of the LDL-C measurement was confirmed within the scope of routine practice 113) . It should be noted, however, that the majority of clinical trials providing evidence for the treatment of hyper-LDL cholesterolemia evaluate LDL-C using the Friedewald formula, and the basis for diagnostic criteria and target treatment values are based on the Friedewald formula. After a meal or when the TG is 400 mg/dL or higher, the non-HDL-C or LDL-C direct method should be used. However, since the direct method is not accurate when TG is 1,000 mg/dL or higher 114) and non-HDLC is not accurate when TG is 600 mg/dL or higher, other methods should be considered for evaluation. For TC, HDL-C, and LDL-C, the direct method uses the same cut-off values even when not fasting, but the TG cut-off values are different for fasting and non-fasting.
(2) Clinical Laboratory Tests for Dyslipidemia
1) Considerations of the Lipid/Lipoprotein Assessment
We recommend that blood samples be drawn for lipid/lipoprotein assessment after fasting for at least 10 hours. Non-fasting blood samples are acceptable for initial screening or assessment of non-fasting TG levels. Since chylomicrons increase in postprandial samples and those with severe hypertriglyceridemia, LDL-C should not be determined by the Friedewald equation in these cases 115) . In addition, alcohol consumption should be avoided on the night before blood collection because it prolongs the duration of TG elevation 116) . Although TC, LDL-C, and HDL-C decrease slightly during the day, these declines average around 5% from the overnight fasting levels 117) . Thus, the timing of blood collection has little effect on these parameters. If TG is less than 1,000 mg/dL, the direct methods for LDL-C and HDL-C are reliable 114) .
Serum lipoprotein concentrations are apparently affected by changes in the circulating plasma volume 117) . To avoid such an effect, blood samples should be drawn in a sitting position after resting for at least 5 minutes 118) . When blood samples are collected in the supine position or from patients receiving vasodilators or a large amount of infusions, lipid levels decrease due to increased circulating plasma volume. In acute myocardial infarction, serum lipids are significantly reduced and remain low for several weeks 118) . Although some reports indicate that lipid levels do not decrease significantly within 24 hours of onset, high-dose heparin administration significantly lowers both TC and TG 119) . Since patients with acute coronary syndromes and percutaneous coronary angioplasty receive heparin, infusions, and vasodilators, serum lipids should be evaluated upon admission 118 , 119) .
2) LDL-C:
LDL-C is usually calculated using the Friedewald formula (TC - HDL-C - TG/5) or measured by the direct method. The former should not be used for postprandial (non-fasting) samples or samples with TG concentrations of 400 mg/dL or higher. There are several reagents for the direct LDL-C method based on different principles. Since the present available reagents have been proven to be unaffected by diet, blood samples can be taken in the non-fasting state 114 , 117) . The measurement of LDL-C by the direct method is reliable even for samples with TG concentrations of 400 mg/dL or higher.
3) HDL-C:
HDL-C is commonly measured by direct methods in clinical practice. Although there are several reagents with different principles for the direct method, any reagent can be used with either fasting or non-fasting samples. In cases whose HDL composition is significantly different from that of normal HDL (HDL-C <20 mg/dL, ≥ 120 mg/dL, cholestatic liver disease, etc.), measured HDL-C values exxxhibit a wide diversity among reagents. To avoid misinterpretation, additional lipid/lipoprotein-related laboratory tests, such as apolipoprotein, should be performed together with HDL-C (mentioned below). When lipoprotein (a) (Lp(a)) is extremely high, some Lp(a) is recovered as HDL-C.
4) TG:
There are two methods for measuring TG. The glycerol blanking method is used in Japan, which eliminates pre-existing free glycerol (FG)xx before TG measurement. On the other hand, the glycerol non-elimination method is used in the U.S. and Europe, which includes pre-existing FG as s part of total glycerides. TG concentrations are affected by food intake. TG increases after meals. Although dyslipidemia, including hypertriglyceridemia, has traditionally been diagnosed with fasting blood samples, elevated postprandial TG levels or postprandial hyperlipidemia is attracting attention as a risk of ASCVD 120) . Fasting TG ≥ 150 mg/dL and non-fasting TG ≥ 175 mg/dL are diagnostic criteria for hypertriglyceridemia. TG measured using the FG blanking method has some merits in detecting postprandial hyperlipidemia 121) .
5) Non-HDL-C:
Non-HDL-C is calculated by subtracting HDL-C from TC. Cholesterol from all atherogenic lipoproteins, that is, LDL (narrowly defined), IDL and remnant lipoprotein, are included. Non-HDL-C shows a good correlation with apolipoprotein B 122) . Since the cholesterol of non-atherogenic lipoproteins, such as normal chylomicrons and normal VLDL, is also included in non-HDL-C, its impact to non-HDL-C cannot be ignored in cases with TG ≥ 600 mg/dL. The reliability of non-HDL-C cannot be endorsed. If the HDL-C direct method is unreliable under the above-mentioned conditions, non-HDL-C is affected by its error.
6) Apolipoprotein (Apoprotein):
Apolipoproteins make up most of the protein constituents of lipoproteins. They act as a ligand for lipoprotein receptors and lipid transporters or activate/inhibit various enzymes. Since apoproteins show little diurnal variation, their postprandial values can be substituted for fasting values 123) . It is useful in patients with marked hyperlipidemia, hypolipidemia, cholestasis, xanthomas, etc. Although it is difficult to distinguish type IIb hyperlipidemia from type III hyperlipidemia by serum lipids, the latter can be diagnosed by the higher apoE/apoCIII ratio 124) .
7) Lipoprotein Fractions:
The main lipoprotein fractions established in a density-gradient or sequential ultracentrifugation are LDL and HDL as cholesterol-rich fractions and chylomicrons, VLDL, and IDL as the TG-rich fractions. After diagnosis of dyslipidemia, patients should be examined for the type of dyslipidemia (type I to V), which is determined by lipoprotein fraction tests with agarose or polyacrylamide gel electrophoresis and anion exchange HPLC (high performance liquid chromatography), as needed. The electrophoresis methods are basically characteristic of a qualitative testing, while the HPLC method is a quantitative assay to measure cholesterol concentrations in each of the five fractions 125 , 126) . The LDL-C value by the Friedewald equation or by the direct method is equivalent to the sum of LDL-C and IDL-C values by the HPLC method.
8) Remnant Lipoproteins:
Remnant lipoproteins are intermediate metabolites generated during the metabolism of chylomicrons and VLDL. Remnant-like lipoprotein cholesterol is measured in the diagnosis and evaluation of dyslipidemia associated with hypertriglyceridemia, including type III hyperlipidemia and familial combined hyperlipidemia. High levels of remnant lipoproteins have been reported to be at an independent risk even when LDL-C is controlled below 100 mg/dL 127) .
In Japan, there are two measurement methods [remnant-like lipoprotein cholesterol by immunosorbent assay (RLP-C) and direct homogenous assay (RemL-C)]. RLP-C reflects chylomicron remnants relatively well while RemL-C tends to have a high correlation with IDL as well although the correlation between the two methods is high 128) .
9) Lipoprotein(a) [Lp(a)]
Lp(a) is a unique LDL-like lipoprotein, and apolipoprotein (a) [apo(a)] is covalently bound to apoB of the LDL particle. Apo(a) consists of repetitive domains, so-called kringles, and carries a number of kringle IV-2 repeats, which are determined by heredity, and thus varies in sizes individually. Circulating Lp(a) concentrations are inversely correlated in most cases with the molecular weights (sizes) of apo(a). The concentration is the combined mass concentration of Lp(a) constituents including proteins and lipids. High levels are a risk factor for ASCVD, especially CAD, but it should be noted that Lp(a) levels can be somewhat elevated due to renal failure or low estrogen, and transiently elevated due to invasive surgical stress or inflammation 129 , 130) . Lp(a) is important as one of the residual risks for ASCVD, and the standardization of Lp(a) measurement is required 131 - 133)
10) Free Fatty Acids and Fatty Acid Fractions:
There are two types of measurements for the determination of fatty acid fractions in clinical practice: 4- and 24-fraction. In the 4-fraction assay, dihomo-γ-linolenic acid (DGLA), arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are measured by gas chromatography. Fatty acid fractions are measured as an adjunct to the diagnosis and evaluation of dyslipidemia and ASCVD. EPA is an n-3 unsaturated fatty acid with anti-inflammatory and antiplatelet effects, while AA is a precursor of lipid mediators with proinflammatory and platelet aggregation effects 134) . The EPA/AA ratio is an indicator of chronic inflammation and is expected to be useful in the risk assessment and clinical follow-up of CAD and stroke 134 - 136) .
11) Lipoprotein Lipase (LPL):
LPL is an enzyme that hydrolyzes TG in lipoproteins and binds to vascular endothelial cells via heparan sulfate proteoglycans 137) . LPL is activated by apo CII and suppressed by apo CIII. Plasma LPL activity or protein after intravenous heparin infusion is measured to diagnose LPL deficiency. Small amounts of LPL protein are also detected in plasma prior to intravenous heparin infusion (preheparin LPL). The low levels of preheparin LPL reflect insulin resistance 138 , 139) .
12) Lecithin Cholesterol Acyltransferase (LCAT), Cholesteryl Ester Transfer Protein (CETP):
LCAT is an enzyme that converts cholesterol from the free form to the ester form. LCAT deficiency results in a decreased cholesteryl ester ratio and marked hypo-HDL cholesterolemia. Acquired causes of marked hypo-HDL cholesterolemia include decreased LCAT synthesis due to severe liver dysfunction and autoantibodies against LCAT 140) . In a study of heterozygous patients carrying LCAT gene mutations using ultrasonography, carotid atherosclerosis was suppressed in familial LCAT deficiency, but accelerated in fish-eye disease, a partial deficiency of LCAT activity 141) . When LCAT activity is markedly reduced, abnormal lipoproteins called lipoprotein-X (Lp-X) increase. Effects of Lp-X on atherosclerosis are controversial 142) .
CETP is a protein that transfers cholesteryl esters from HDL to VLDL and LDL, and its deficiency causes hyper-HDL cholesterolemia. HDL-C can reach 150-200 mg/dL in completely CETP deficient individuals, of which some cases of CAD complications have been reported 143) .
13) Malondialdehyde-LDL (MDA-LDL), Small Dense LDL (sd-LDL)
MDA-LDL is an oxidized LDL formed by oxidative modification of lipids such as phospholipids or apoproteins in LDL under oxidative stress 144 , 145) . Oxidized LDL is presumably involved in a wide range of processes in atherosclerosis, including vascular endothelial cell injury, increased monocyte infiltration into the vessel wall, and foam cell formation 144 , 145) . MDA-LDL is also useful in predicting the prognosis regarding the development of CAD in patients with diabetes with prior CAD and the prognosis regarding restenosis after coronary intervention treatment in patients with diabetes 146) . On the other hand, sd-LDL 147) is a small-sized LDL particle with high density. Its vitamin E concentration is low and as such sd-LDL is susceptible to oxidative modification. In addition, sd-LDL has been reported to be associated with CAD 148 , 149) .
14) Sitosterol and Sterol Fractions:
Sterol fractions such as sitosterol, campesterol, and lathosterol are measured mainly by gas chromatography in Japan 150) . Sitosterolemia is a designated intractable disease with abnormally high levels of sitosterol and is an autosomal recessively inherited disorder of lipid metabolism. Serum sitosterol concentrations in sitosterolemia are elevated above the diagnostic cut-off value of 1 mg/dL (10 µg/mL) and are usually markedly high, ranging from 10 to 65 mg/dL 150 , 151) . In sitosterolemia, functional abnormalities associated with gene mutations in the ABCG5/8 result in impaired excretion of phytosterols, which accumulate in the blood and tissues, leading to xanthomas and premature CAD similar to FH 151) . In addition, sitosterol and campesterol are plant sterols, and their serum concentrations reflect small intestinal absorption of cholesterol, while lathosterol concentrations reflect cholesterol synthesis in the body 152 , 153) .
15) LDL Receptor, PCSK9:
Genetic mutations in the LDLR gene cause FH 154) . Although a genetic test is not essential for the diagnosis of FH heterozygotes, it is valuable when it is difficult to distinguish severe FH heterozygotes from FH homozygotes or when patients are considered to be FH homozygotes. In FH caused by LDLR gene mutations, LDL receptor activity using skin fibroblasts or lymphocytes is markedly reduced to less than 20%. Gain-of-function mutations in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene cause impaired LDL receptor recycling in the liver, resulting in FH. A gene panel testing using a next-generation sequencer is considered for simultaneous analysis of multiple genes responsible for severe hypercholesterolemia. In FH receiving standard lipid-lowering therapy, serum PCSK9 concentration is useful for risk assessment because it correlates with the development of coronary artery lesions and major cardiovascular events 155) . Subjects with PCSK9 gene mutations that reduce LDL-C are at a low risk for CAD 156) .
1.2 Smoking
•Smoking is a risk factor for CAD and stroke, and even smoking one cigarette a day increases the risk.
•Smoking is a risk factor for abdominal aortic aneurysm (AAA) and peripheral arterial disease (PAD).
•Passive smoking is a risk factor for CAD and stroke.
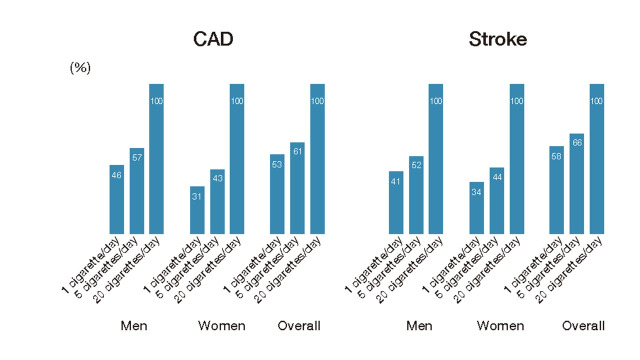
Numerous national and international cohort studies and meta-analyses have reported that smoking is a risk factor for CAD and stroke. The risk of CAD and stroke is higher than that of nonsmokers, and a dose-response relationship exists 157 - 166) . Even if one cigarette is smoked per day, the relative risk of CAD is 1.74 (95% confidence interval: 1.50-2.03) for men and 2.19 (1.84-2.61) for women, and for stroke is 1.30 (1.11-1.53) for men and 1.46 (1.20-1.78) for women, compared to never-smokers in both sexes, an increase in risk of about half that of smoking 20 cigarettes per day 167) ( Fig.1 ) . With respect to the type of tobacco, there are no data showing that even low-tar, low-nicotine cigarettes reduce risk.
Fig.1. Excess relative risk when set at 0% for nonsmoking and 100% for 20 cigarettes/day.
Adapted from Hackshaw A. et al. BMJ, 2018; 360: j5855.
A meta-analysis reported a relative risk of 4.87 (3.9-6.02) for current smokers and 2.10 (1.76-2.50) for ex-smokers for AAA and indicated that the risk for ex-smokers 25 years after quitting smoking is comparable to that of never-smokers 168) . In a meta-analysis of PAD, the odds ratio was 3.08 (2.56-3.69) for smokers and 1.67 (1.53-1.81) for ex-smokers, which was also significantly higher but lower than that of smokers 169) .
On the other hand, a meta-analysis has shown that passive smoking also increases the relative risk of developing CAD to 1.23 (1.16-1.31) 170) , and stroke to 1.25 (1.12-1.38) 171) . Smoking and passive smoking increase the risk of developing type 2 diabetes with relative risks of 1.37 (1.33-1.42) and 1.22 (1.10-1.35), respectively 172) and the risk of developing metabolic syndrome also increases with the number of cigarettes smoked 173) . Meta-analysis has shown that smokers have lower HDL-C and higher LDL-C and TG than non-smokers, and a dose-response relationship has also been observed 174) . Smoking is not only a risk factor for ASCVD on its own, it also contributes to an increased risk of ASCVD, coupled with an increased risk of developing diabetes, dyslipidemia, and metabolic syndrome.
Recently, new types of cigarettes (e.g., heated tobacco products and electronic cigarettes) in forms different from those of conventional cigarettes have become available ( Table 2 ) . In Japan, nicotine is a drug and is therefore regulated by the Pharmaceutical Affairs Law (Law Concerning Quality, Efficacy, and Safety Assurance of Drugs and Medical Devices), and electronic cigarettes containing nicotine are not legally sold in the country. The risk of morbidity and mortality of ASCVD from the new types of cigarettes cannot be determined at this time because they have only been available for a short period of time. However, although heated tobacco products do not contain components produced by combustion, they do contain nicotine and other substances produced by heating tobacco leaves and additives 175) , and a study in humans reported that they cause impairment of vascular endothelial function similar to conventional cigarettes 176) . In addition, various carcinogens have been reported to occur in e-cigarette aerosol, with or without nicotine content 177) , and many cases of electronic cigarettes-associated lung injury (EVALI) have been reported overseas 178) . In the long term, there is a possibility of various health effects including ASCVD and cancer.
Table 2. Classification of New Types of Cigarettes.
| Related Laws | |||
|---|---|---|---|
| 1 |
Heated tobacco products [HTPs] Products that directly heat tobacco leaves (or processed tobacco leaves) and inhale the substance generated, or heat glycerin or other substances and inhale the substance generated by passing it through a tobacco leaf capsule. |
Tobacco Business Law | |
| 2 |
Electronic cigarette [e‐cigarette] Products that attach a cartridge containing a tasting or smelling solution containing nicotine, propylene glycol, glycerin, etc. and inhale the aerosol generated by heating it with a battery. |
||
| a |
Products containing nicotine Sales are prohibited in Japan. However, personal importation is possible via the Internet, etc. |
(Japanese) Pharmaceuticals and Medical Devices Law* | |
| b |
Nicotine‐free product Sold to minors online, in stores, etc. due to lack of regulation |
None | |
*Act on Quality, Efficacy and Safety Assurance of Pharmaceuticals and Medical Devices, etc.
1.3 Hypertension
The higher the blood pressure above normal (systolic blood pressure less than 120 mmHg and diastolic blood pressure less than 80 mmHg), the higher the risk of morbidity and mortality from all cardiovascular diseases, stroke, myocardial infarction, heart failure, atrial fibrillation and chronic kidney disease.
Hypertension is an important risk factor for cerebral and cardiovascular disease like CAD, heart failure, CKD, and other organ damage, and hypertension in middle age also increases the risk of developing vascular dementia in old age 179) . The results of EPOCH-JAPAN, a meta-analysis of 10 cohort studies in Japan (70,000 men and women in total), showed a positive association between blood pressure levels above normal (<120/80 mmHg) and the risk of cerebral cardiovascular disease in all ages above middle age, with the slope steeper the younger the age. The risk of all cerebral and cardiovascular disease mortality tends to increase with blood pressure level even in the older people aged 75-89 years, when deaths during the first 3 years of follow-up were excluded to rule out causal inversion, a significantly increased risk was observed from 130/85 mmHg or higher 180) .
According to EPOCH-JAPAN estimates, 50% of all cerebral and cardiovascular diseases, 52% of stroke deaths, and 59% of CAD deaths were evaluated as deaths attributable to higher than normal blood pressure, with the highest proportion of deaths among degree I hypertensives in all cases 180) . In the J-LIT lipid intervention study, hypertensive patients had a 2.05-fold relative risk of developing CAD in primary prevention subjects compared to non-hypertensive patients: 2.05 times higher in women and 2.15 times higher in men 181) .
Although office blood pressure is often used to measure blood pressure, home blood pressure measurement and ambulatory blood pressure monitoring (ABPM) have also been reported to be more predictive of the occurrence of cardiovascular events than office blood pressure. The Japanese Society of Hypertension’s Guideline for the management of Hypertension 2019 (JSH 2019) clearly states that when there is a diagnostic discrepancy between office blood pressure and home blood pressure, priority should be given to the diagnosis by home blood pressure, including the determination of the antihypertensive effect 179) .
1.4 Diabetes and Prediabetes
BQ6. Are blood glucose and HbA1c associated with the CVD incidence and/or death from CVD in non-diabetic adults?
In adults with prediabetes, blood glucose and HbA1c are associated with increased risk of CVD incidence and/or death from CVD. (Level of evidence: E-1b)
Based on the results of community-based epidemiological studies in Japan, adults with prediabetes have a significantly higher risk of death from cardiovascular disease (CVD) compared to those with normal glucose tolerance. In NIPPON DATA80 with 17.3 years of follow-up, adults with casual blood glucose 140-199 mg/dL have a significantly higher risk of death from CVD compared to those with casual blood glucose less than 94 mg/dL (hazard ratio (HR) 1.46, 95% confidence interval (CI) 1.06-2.01) 182) . In NIPPON DATA90 with 15 years of follow-up, adults with HbA1c 6.0-6.4% have a significantly higher risk of death from CVD compared to those with HbA1c <5.0% (HR 2.18, 95% CI 1.22-3.87) 183) . Furthermore, in the Funagata Study, adults with impaired glucose tolerance based on a 75-g oral glucose tolerance test (OGTT) have a significantly higher risk of death from CVD compared to those with normal glucose tolerance based on a 75-g OGTT (HR 2.22, 95% CI 1.08-4.58) 184) . These results suggested adults with prediabetes such as elevated blood glucose and/or elevated HbA1c levels have a significantly higher risk of death from CVD compared to those with normal glucose tolerance. However, in the J-ECOH study with 7 years of follow-up, which is a recent multicenter epidemiological study among workers, prediabetes which was defined as fasting plasma glucose 100-125 mg/dL and/or HbA1c 5.7-6.4% was not significantly associated with increased risk of death from CVD compared to normal glucose tolerance which was defined as fasting plasma glucose <100 mg/dL and HbA1c <5.7% 185) . When interpreting these results, it is necessary to note that advances in medical technology have made it possible to avoid death from CVD, which has affected the estimates of mortality risk.
The association between indices of glucose metabolism and the incidence of CVD is differed by the criteria for indices of glucose metabolism in a control group. In studies which was set adults with lower levels of glucose and/or HbA1c as a control group, adults with prediabetes have higher risk of CVD incidence. In the Suita study with 11.7 years of follow-up and the J-ECOH Study with 4 years of follow-up, which were examined the associations between fasting plasma glucose and the incidence of CVD and were set adults with fasting plasma glucose <100 mg/dL as controls, adults with fasting plasma glucose 100-125 mg/dL have a significantly higher risk of CVD incidence compared to those with fasting plasma glucose <100 mg/dL (Suita study: HR 1.25, 95% CI 1.00-1.58; J-ECOH study: HR 1.77, 95% CI 1.10-2.86) 186 , 187) . However, in epidemiological studies among workers which were set adults with fasting plasma glucose <110 mg/dL as controls, prediabetes which was defined as fasting plasma glucose 110-125 mg/dL was not significantly associated with increased risk of CVD incidence 188 , 189) . In the Hisayama study with 7 years of follow-up, which was examined the association between HbA1c and the incidence of CVD and were set adults with HbA1c ≤ 5.0% as control, adults with HbA1c 5.5-6.4% have a significantly higher risk of CVD incidence compared to those with HbA1c ≤ 5.0% (HR 2.26, 95% CI 1.29-3.95) 190) . In the JPHC study with 9.4 years of follow-up, adults with HbA1c 6.0-6.4% have a significantly higher risk of CVD incidence compared to those with HbA1c 5.0-5.4% in an analysis excluding those with early events within 3 years of follow-up (HR 1.33, 95% CI 1.01-1.75) 191) . Similarly, in the J-ECOH study with 4 years of follow-up, adults with HbA1c 5.7-6.4% have a significantly higher risk of CVD incidence compared to those with HbA1c <5.7% (HR 1.93, 95% CI 1.21-3.08) 187) . On the other hands, in epidemiological studies which were set adults with HbA1c <6.0% as controls, prediabetes which was defined as HbA1c 6.0-6.4% was not significantly associated with increased risk of CVD incidence 192 , 193) . In a sub-analysis of the MEGA Study, which was a randomized controlled trial examining the effect of pravastatin on the primary prevention of CVD in patients aged 40-70 years with hypercholesterolemia, associations between HbA1c and CVD incidence were evaluated by multivariable Cox proportional hazards model with restricted quadratic spline. As a result, the risk of CVD incidence increased with increasing HbA1c levels, and patients with HbA1c >6.0% have a significantly higher risk of CVD incidence compared to those with HbA1c ≤ 5.5% 192) . Taken together, these findings suggest prediabetes have a significantly higher risk of CVD incidence in non-diabetic adults.
Epidemiological studies examining the association between the indices of glucose metabolism and the incidence of CAD or ischemic stroke or death from CAD or ischemic stroke in non-diabetic adults have not provided consistent results, partly due to the small number of study participants. For example, regarding the association between fasting plasma glucose and the incidence of CAD, in an epidemiological study among male workers with 10 years of follow-up, adults with fasting plasma glucose 110-125 mg/dL have a significantly higher risk of the incidence of CAD compared to those with fasting plasma glucose <100 mg/dL 194) . However, in the Hisayama study with 14 years of follow-up, fasting plasma glucose 110-125 mg/dL was not significantly associated with increased risk of the incidence of CAD compared with fasting plasma glucose <100 mg/dL 195) . Additionally, HbA1c was not significantly associated with increased risk of the incidence of CAD in all previous epidemiological studies 190 - 193) . Regarding the association between HbA1c and the incidence of ischemic stroke, adults with HbA1c 5.5-6.4% have a significantly higher risk of the incidence of ischemic stroke compared to those with HbA1c ≤ 5.0% in the Hisayama study 190) , while HbA1c was not significantly associated with the incidence of ischemic stroke in the JPHC study and the Suita study 191 , 193) . In addition, the level of fasting plasma glucose was not significantly associated with the incidence of ischemic stroke in the Hisayama study 195) . Furthermore, in NIPPON DATA90 with 15 years of follow-up, adults with HbA1c 6.0-6.4% have a significantly higher risk of death from cerebral infarction compared to those with HbA1c <5.0% (HR 5.28, 95% CI 1.66-16.8), while HbA1c was not significantly associated with death from coronary heart disease 183) . The reasons for inconsistent results across epidemiological studies include small incidence rates of events, differences in the definition of incidence among studies, and differences in characteristics of study participants across study settings. Large-scale epidemiological studies should be conducted to resolve these limitations in the future.
Diabetes is a strong risk factor for ASCVD.
Diabetes is an important risk factor for ASCVD 196 , 197) . In the NIPPON DATA80, patients with diabetes showed significantly higher risk of 2.8 times for death from CAD was significantly higher than non-diabetic subjects 182) . The Hisayama study reported that the incidence of CAD was 5.0 / 1,000 person years compared to 1.6 / 1,000 person years in healthy subjects, and the incidence of cerebral infarction was 6.5 / 1,000 person years compared to 1.9 / 1,000 person years in healthy subjects 198) after adjustment for multiple factors including sex and age. In CIRCS, the risk of cerebral infarction incidence was 1.9 times higher in men and 2.2 times higher in women in the diabetes group than non-diabetic subjects 199) . Meta-analyzes have shown that patients with type 2 diabetes have a 1.5- to 3.6-fold increased incidence of CAD or cerebrovascular disease compared to healthy controls 200) . The risk of peripheral arterial disease (PAD) is as much as 3-4 times higher in patients with diabetes 201) , and this risk increases by 26% with every 1% increase in the HbA1c level 202) .
Silent myocardial ischemia often coexists in patients with diabetes, and this may result in delayed diagnosis 203) . Characteristics of coronary artery lesions in patients with diabetes include multivessel disease, highly complicated and diffuse 204 , 205) , and multiple calcified lesions 206) .
Regarding cerebral infarction, the JPHC study has shown that lacunar infarction, atherothrombotic infarction, and thromboembolic infarction occur more common in patients with diabetes 207) . Furthermore, the prognosis of CAD in patients with diabetes is worse 208 - 210) and the recurrence rate of cerebral infarction is higher 211 , 212) than in non-diabetic subjects.
BQ7. Do familial hypercholesterolemia, non-cardiogenic cerebral infarction (cardiogenic cerebral embolism), PAD, microvascular complications, smoking, and persistent poor glycemic control increase the risk of CAD in patients with diabetes without a history of CAD?
Familial hypercholesterolemia, non-cerebral infarction, PAD, microvascular complications, smoking, and persistent poor glycemic control increase the risk of CAD in patients with diabetes without a history of CAD. (Level of evidence: E-1a)
FH
FH with diabetes mellitus has been reported to be another high-risk group for CAD from Canada 213) , China 214) , and Japan 215) , respectively. In a cross-sectional study of 150 FH heterozygotes 40 years or older in Japan, the incidence of CAD in FH with normal glucose tolerance was 43% (46/108 subjects), compared to 59% (16/27 subjects) in FH with impaired glucose tolerance and 87% (13/15 subjects) in FH with diabetes, indicating that CAD increased with the severity of glucose metabolism abnormalities 215) .
Noncardiogenic Cerebral Infarction
The incidence of myocardial infarction in Japan is higher than in the general adult population, ranging from 4.0 to 4.5 per 1,000 person-years 216 , 217) . In patients with diabetes, a history of stroke, especially noncardiogenic cerebral infarction caused by atherosclerotic lesions, is considered a risk for CAD. In a study using an insurance database of 1.17 million patients with type 2 diabetes in six countries, including Japan, a history of stroke was associated with a 1.59, 2.31 and 1.37-fold higher risk of total mortality, cardiovascular death, and incidence of myocardial infarction, respectively 218) . In an analysis of patients with diabetes in the REACH study of patients at high risk for cardiovascular disease conducted in 44 countries including Japan, the cardiovascular mortality rate was 0.7% and the incidence of major cardiovascular events was 2.2% in patients without a history of atherothrombotic disease, while the rates were 2.1% and 6.4%, respectively, in patients with a history of ischemic stroke 219) .
PAD
Patients with peripheral arterial disease (PAD) have a high incidence rate of CAD, but the coexistence of PAD is also a strong risk factor for cardiovascular disease in patients with diabetes 220 - 227) . In a cohort of 474 Swedish men in the general population reaching their 68th birthday followed for 13 years, the incidence of cardiovascular disease was 28.4/1,000 person-years in patients with diabetes with ABI ≥ 0.9 compared to 102.0/1,000 person-years in patients with diabetes with ABI <0.9, indicating a higher incidence of cardiovascular disease in patients with diabetes with suspected PAD 226) . In a Spanish cohort of 262 patients with type 2 diabetes without a diagnosis of PAD followed for 10 years, the incidence of cardiovascular disease was 26.9% in the group with normal ABI (0.91-1.24) compared to 81.9% in the group with abnormal ABI (≤ 0.90) 220) . Furthermore, in a post hoc analysis of ADVANCE in patients with type 2 diabetes at high risk for cardiovascular disease, the total mortality at 10 years and the risk of cardiovascular disease were 1.35- and 1.47-fold higher, respectively, with a history of chronic ulceration of the lower extremity, amputation of the lower extremity due to vascular lesions, and angioplasty or reconstruction of the lower extremity arteries 224) . In a study of 362 patients with PAD and age- and sex-matched non-PAD patients using the Health Insurance Association claims database in Japan, patients with diabetes with PAD had a significantly higher incidence of myocardial infarction, ischemic stroke, coronary artery bypass surgery, peripheral artery reconstruction, coronary artery intervention and leg amputation 222) . In a study conducted in six countries including Japan using an insurance database of 1.17 million patients with type 2 diabetes, the risk of the incidence of total mortality, cardiovascular death, and the incidence of myocardial infarction was 1.72, 2.24, and 2.06 times higher, respectively, for patients with PAD 218) .
Retinopathy
Comorbid retinopathy in patients with diabetes is a risk factor for cardiovascular disease 228 - 239) . In a meta-analysis of 8 studies (7,604 patients) with type 2 diabetes, comorbid diabetic macular edema or proliferative retinopathy was associated with a 2.33 and 1.39-fold higher risk of cardiovascular death or incidence of cardiovascular disease, respectively 240) . A meta-analysis of 20 studies (19,234 patients) with type 1 or type 2 diabetes also found that comorbid diabetic retinopathy was associated with a 2.34-fold higher combined risk of cardiovascular death and cardiovascular disease incidence, particularly in patients with type 1 diabetes, where the risk was 4.10 times higher 241) . In the JDCS, a cohort study of patients with type 2 diabetes in Japan, comorbid diabetic retinopathy was associated with a 1.69-fold higher risk of cardiovascular disease incidence 232) . In an 11.6-year observational study of 233 Japanese patients with diabetes who underwent coronary artery bypass surgery, the risk of death was 4.0 times higher and the risk of revascularization after coronary artery bypass surgery was 3.3 times higher in patients with diabetes who had preoperative diabetic retinopathy comorbidity 235) . In a study of 371 Japanese patients with type 2 diabetes with no history of CAD, the coexistence of proliferative retinopathy was associated with a 6.46-fold higher risk of incidence of CAD 239) . Furthermore, in a cross-sectional study of 1,003 Japanese patients with type 2 diabetes, cardiovascular complications were the lowest in patients without retinopathy, and the frequency of cardiovascular complications increased with the severity of comorbid retinopathy 233) .
Nephropathy
The presence of nephropathy in patients with diabetes is a risk factor for cardiovascular disease 242 - 251) . In NHANES III (the Third National Health and Nutrition Examination Survey), which included 1,430 patients with diabetes in the US, increased albuminuria and low GFR in patients with type 2 diabetes mellitus were independent risk factors for total and cardiovascular death 249) . The annual mortality rate due to cardiovascular disease in the UKPDS of 5,097 newly diagnosed patients with type 2 diabetes in the UK was 0.7% in the normal albuminuria group, 2.0% in the microalbuminuria group, 3.5% in the macroalbuminuria group, and 12.1% in the renal failure group, which increased with progression of nephropathy stage 248) . Furthermore, in a post hoc analysis of the ADVANCE in 10,640 patients with type 2 diabetes at high risk for cardiovascular disease, increased albuminuria and decreased eGFR were associated with an increased risk of cardiovascular disease incidence, with urinary albumin >300 mg/g creatinine and eGFR <60 mL / min / 1.73 m2 was associated with a 3.2-fold higher risk of cardiovascular disease incidence 251) . In a cross-sectional study of 1,493 CKD patients with type 2 diabetes in Japan, the complication rate of cardiovascular disease was 18.6%. By stage of CKD, the rate of cardiovascular disease increased as eGFR decreased: 6.99% for stage 2, 17.78% for stage 3, 52.48% for stage 4, and 55.17% for stage 5 245) . Diabetic nephropathy is typically characterized by the appearance of albuminuria followed by the appearance of apparent proteinuria and then the decline in kidney function. Recently, the term diabetic kidney disease (DKD) has been coined to include atypical diabetes-related kidney disease in which kidney function declines without an increase in proteinuria excretion. A 4-year prospective cohort study of 675 Japanese patients with type 2 diabetes reported a low risk of cardiovascular disease incidence if urinary albumin was normal, even if eGFR was <60 mL/min/1.73 m2 252) .
Neurological Disorder
Diabetic neuropathy includes autonomic neuropathy and sensory/motor neuropathy, both of which are risks of cardiovascular disease. With regard to autonomic neuropathy, studies using cardiovascular autonomic indices have reported a relationship with the risk of cardiovascular disease 253 - 255) . In an ACCORD post hoc analysis of 10,251 patients with type 2 diabetes at high risk of cardiovascular disease, the risk of death from cardiovascular disease was 1.93 to 3.39-fold higher with concomitant cardiac autonomic neuropathy 253) . On the other hand, sensory and motor neuropathy is also a risk of the incidence of cardiovascular disease 256 , 257) . In a study of 13,043 people with type 2 diabetes in the United Kingdom, the risk of cardiovascular disease incidence was 1.33 times higher if there was sensory neuropathy in the foot detected by monofilaments 257) . The risk of cardiovascular disease incidence is high in patients with sensory dullness, foot deformity, skin dryness, and keratinization due to diabetic neuropathy and diabetic foot lesions that occur in association with decreased blood flow due to PAD 258 - 260) . In a meta-analysis of 8 studies (17,830 patients), diabetic patients with diabetic foot lesions had a 1.89, 2.22 and 1.41-fold higher risk of total mortality, fatal myocardial infarction, and cerebral infarction, respectively 258) . In a study of 165,650 patients with diabetes in Italy, the risk of myocardial infarction incidence was 1.84 times higher in men and 1.57 times higher in women with diabetic foot lesions 260) .
Smoking
The fact that smoking is a risk factor for cardiovascular disease has been reported in numerous national and international cohort studies and meta-analyses. Smoking is also a risk factor for cardiovascular disease in patients with diabetes 261 - 265) . In a meta-analysis of 89 studies on smoking in patients with diabetes (1,132,700), the respective risks of cardiovascular death, cardiovascular disease, or incidence of CAD were 1.49, 1.44 and 1.51 times higher for smoking. On the other hand, patients with diabetes who were able to quit smoking had a 1.15, 1.09, and 1.14-fold reduced risk of cardiovascular death, cardiovascular disease, and CAD incidence, respectively 266) . In foreign evidence, smoking was a strong predictor of total mortality and incidence of myocardial infarction in patients with type 2 diabetes in the Swedish National Diabetes Register of 271,174 patients with type 2 diabetes 267) . In a cohort of 59,412 Finnish residents divided into four groups according to diabetes status and smoking status, the risk of CAD mortality in men was 6.15 times higher in diabetics with smoking and 2.62 times higher in diabetics without smoking. The risk of CAD mortality for women was similarly 6.92 and 4.06 times higher, respectively. On the contrary, the risk of CAD incidence in men was 3.27 times higher in diabetics with smoking and 1.56 times higher in diabetics without smoking. The risk of incidence of CAD in women was similarly 4.55 and 2.60 times higher, respectively. In the JDCS, a 7.86-year follow-up study of 1771 patients with type 2 diabetes without a history of cardiovascular disease, the risk of incidence of CAD was 1.41 times that of smoking after adjustment for sex, age, duration of diabetes, BMI, systolic blood pressure, HbA1c, LDL-C, HDL-C, TG and alcohol intake, but the difference was not statistically significant (p=0.12) 268) .
Persistence of Poor Blood Glucose Control
Many epidemiological studies have shown that persistent hyperglycemia is closely related to the risk of cardiovascular disease incidence 269) . In the UKPDS in patients with new-onset type 2 diabetes, a 1% reduction in HbA1c was reported to be associated with a 14% reduction in the incidence of myocardial infarction 270) . In a meta-analysis, a 1% increase in HbA1c increased the incidence of cardiovascular disease by 18%, CAD by 13% and fatal myocardial infarction by 16% 202) . In a study of 14,633 diabetic patients using the Japanese health insurance database, the risk of incidence of CAD increased with poor glycemic control in the groups treated with diet alone, with HbA1c ≤ 7.0%, 7.1-8.0%, and > 8.0%, whereas the risk of incidence of CAD increased in the group treated with insulin or SU drugs, the risk of CAD incidence was higher for both HbA1c ≤ 7.0% and > 8.0% 271) .
1.5 Chronic Kidney Disease (CKD)
CKD is a high-risk condition for ASCVD.
According to the ‘Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018’ of the Japanese Society of Nephrology 13) , CKD is defined as the presence of either of the conditions listed below lasting for more than 3 months.
(1) Findings suggesting kidney damage, i.e., abnormal findings in blood or urinary tests, imaging studies or pathological evaluations. In particular, evidence of proteinuria ≥ 0.15 g/gCr (albuminuria ≥ 30 mg/gCr) is important.
(2) Glomerular filtration rate (GFR) <60 mL/min/1.73 m2
Estimated GFR (eGFR) is used which is calculated by the equation for Japanese patients using serum Cr level, sex, and age. CKD is a high-risk condition not only for kidney failure but also for all-cause and cardiovascular mortality. The risk of these composite outcomes varies greatly depending on the underlying disease (Cause), GFR, and proteinuria (Albuminuria), and these three factors are used to develop the CKD severity classification (CGA Classification of CKD Severity) 13 , 272) .
The risk of cardiovascular mortality among dialysis patients in the United States is 10 to 30 times higher than that of the general population 273) , and a similarly high risk has been shown in Japan 274) . In the Suita Study 275) , the multivariable-adjusted hazard ratios for the incidence of cardiovascular disease (stroke + myocardial infarction) in the eGFR 60-89, 50-59, and <50 mL / min / 1.73 m2 groups compared with the eGFR ≥ 90 mL / min / 1.73 m2 group were 1.21 (0.93-1.58), 1.75 (1.22-2.50), and 2.48 (1.56-3.94), respectively, indicating that the risk of cardiovascular disease incidence is higher in patients with lower eGFR who do not receive dialysis or transplant treatment. There are studies reporting that low eGFR was more significantly associated with myocardial infarction in men and cerebral infarction in women 276 , 277) .
The following information can be used as a basis for positioning CKD as a high-risk condition for ASCVD in Japan. First, according to a cohort analysis of the Case-J study 278) , a multivariable-adjusted Cox analysis of the association of various risk factors for the incidence of cardiovascular disease showed that the hazard ratio of renal impairment (with proteinuria and/or serum Cr ≥ 1.3 mg / dL) was 2.82 (1.18-4.39), which was higher than that of diabetes mellitus, 1.97 (1.26-3.06). In the Suita Study 275) , the risk of cardiovascular disease (stroke + myocardial infarction) in the eGFR 50-59 mL / min / 1.73 m2 group exceeded 10 per 1,000 person-years, equivalent to a 10-year risk of 10%, and in the eGFR <50 mL / min / 1.73 m2 group it was 16 per 1,000 person-years.
The mechanism of increased cardiovascular risk in CKD is explained by the increase in the prevalence and degree of comorbidity of traditional risk factors such as blood pressure, lipids, and glucose metabolism in CKD, as well as the involvement of non-traditional risk factors such as abnormal phosphorus and calcium metabolism in advanced stages of CKD. Furthermore, it is known that the group with lower kidney function has a lower survival rate (higher fatality rate) after the incidence of cardiovascular events 279) , making the prevention of the incidence of cardiovascular disease even more important.
The degree of involvement of each risk factors for ASCVD varies depending upon kidney function. According to a large Canadian cohort study 280) , the association between LDL-C and myocardial infarction was weaker at lower eGFRs, with no significant association observed at eGFRs less than 15 ml / min / 1.73 m2. This result is consistent with the results of randomized controlled trials in hemodialysis patients in which statins did not significantly reduce ASCVD risk 281 , 282) . Thus, these studies suggest the importance of lipid management from the early stages of CKD in addition to appropriate management of hypertension and diabetes.
It is estimated that approximately 13% of adults in Japan have CKD 283) , and screening for CKD is also important for comprehensive risk management of ASCVD 284) .
1.6 Aging, Gender Differences
•Aging is the strongest risk factor for ASCVD, including CAD and cerebrovascular disease.
•The risk of acute myocardial infarction incidence and mortality in women is lower than in men, but myocardial infarction mortality increases after age 70 in women.
The risk of incidence and death from ASCVD, including myocardial infarction, increases with increasing age, and in terms of absolute risk, aging increases the risk of incidence and death from ASCVD more strongly than any other risk factor 285 - 287) .
In addition, women are at a lower risk of incidence and death than men. In a 1999-2001 study in Takashima-cho, Shiga Prefecture, Japan, the age-adjusted incidence rate of acute myocardial infarction (100,000 persons / year) among Japanese women was 35.7, about one-third that of men (100.7) 288) . Furthermore, according to the 2019 Vital Statistics mortality rates by simple cause of death classification (per 100,000 population), the (crude) mortality rate for acute myocardial infarction is 30.1 for men and 21.1 for women. The data showed that the mortality rate for acute myocardial infarction by age was 2.4 for men and 0.4 for women in their 30s, 10.8 for men and 2.0 for women in their 40s, 33.9 for men and 5.2 for women in their 50s, 74.2 for men and 17.6 for women in their 60s, 145.7 for men and 56.8 for women in their 70s, 370.3 for men and 215.9 for women in their 80s, 751.9 for men and 548.2 for women in their 90s, 355.6 for men and 335.0 for women over 100 years of age, indicating that the mortality rate of acute myocardial infarction was lower for women than for men at all ages. However, the risk of ASCVD in older women is not low, as the mortality rate from acute myocardial infarction among women increases beginning in the early 60s and is particularly elevated after the age of 70 289) . The mortality rate from cerebral infarction (per 100,000 population) is 46.8 for men and 49.0 for women, with little difference between the sexes. Age-specific mortality rates (per 100,000 population) were 0.2 for men and 0.2 for women in their 30s; 1.8 for men and 0.8 for women in their 40s; 8.5 for men and 2.3 for women in their 50s; 43.7 for men and 10.5 for women in their 60s; 194.8 for men and 62.3 for women in their 70s; 834.4 for men and 457.7 for women in their 80s;2,367.6 for men and 2,147.4 for women in their 90s, and 1,900.0 for men and 2,188.3 for women over 100 years old. The mortality rate from cerebral infarction in women is lower than in men until the age of 90 years, but the effect of aging is more significant than the effect of gender. Estrogen effects and women’s unique lifestyles (pregnancy, childbirth, childcare, etc.) may contribute to women’s lower risk of ASCVD. In addition, as women’s roles in society increase, we need to be vigilant about the increased risk for women in the future due to lifestyle changes.
1.7 Family History of CAD
Family history of CAD is a risk factor for the incidence of CAD.
In Europe and the United States, a family history of CAD has been reported to be a risk factor for the incidence of this disease since the 1970s 290 - 295) .
A family history of CAD, especially a family history of first-degree relatives (parents, children, brothers, sisters), as well as a family history of premature CAD (age of incidence: <55 years in men and <65 years in women) is a strong risk factor for the development of CAD 296) .
In the Framingham study, when at least one parent had CAD, the age-adjusted odds ratio for the risk of CAD was 2.6 for men and 2.3 for women, and 2.0 for men and 1.7 for women even after all adjustments, including multivariate analysis 293) . In the J-LIT study, a family history of CAD increased the relative risk of CAD incidence about 3-fold in Japan 90) . In the CREDO-Kyoto study, a family history of CAD was also associated with the incidence of major cardiovascular events at younger ages 297) .
Conventional risk factors (high LDL-C, low HDL-C, hypertension, diabetes, and smoking) may be influenced by environmental exposures in the same household, in addition to genetic predisposition. In other words, a family history of CAD may include known genetic and environmental risk factors. However, family history remains a strong risk even after adjusting for all conventional risk factors by multivariate analysis 291 - 293 , 298 - 300) , suggesting the involvement of genetic factors that have not yet been elucidated 295) .
Recent advances in genetic analysis technology have led to the focus on the polygenic risk score (PRS), an algorithm constructed using millions of single nucleotide polymorphisms revealed by genome-wide related analysis to assess genetic risk 301 , 302) . PRS had a limited additive effect on the ACC/AHA risk prediction model for ASCVD (PCE) in some high-risk populations 303) . A family history of premature CAD (age of incidence: <55 years for men and <65 years for women) should be considered particularly high risk.
1.8 Drinking Alcohol
Heavy drinking increases the incidence and death of ASCVD.
Heavy drinking has been shown to be a risk factor for ASCVD in many epidemiological studies and their meta-analyses 304 - 319) . The alcohol consumption that affects the ASCVD are different by diseases. In cerebrovascular diseases, cerebral hemorrhage has the lowest incidence and mortality rate among nondrinkers, with a linear increase in incidence and mortality with increasing alcohol intake, while cerebral infarction has the lowest incidence and mortality rate among light drinkers than non-drinkers 310 - 312) . In cerebral infarction, the relationship is shown as the 'U or J curve' generally, and alcohol intake that inhibits cerebral infarction is often reported to be around 300-400 g/week (approximately 40-50 g/day) 309 - 312) . In addition, with respect to myocardial infarction, the incidence and mortality rate have been shown to be lower in drinkers than in non-drinkers 311 - 316) . Reports have found that even higher alcohol intake (about 400 g/week) has an protective effect on myocardial infarction compared to cerebrovascular disease 316 - 318) . However, the protective association between alcohol intake and myocardial infarction has been shown in domestic and international studies to show a dose-response relationship in the low alcohol intake range, but this relationship decreases with heavy drinking 310 , 317) . Heavy drinking induces atherosclerosis through high TG and insulin resistance. The 'U or J active' mechanism is thought to be an increase in HDL-C due to alcohol consumption 319 , 320) . However, a harmful association has been reported between hyper-HDL cholesterolemia above 90 mg/dL and ASCVD has been reported, especially in drinkers 94 , 321 , 322) . Hyper-HDL cholesterolemia in drinkers should be noted.
It has also been observed that binge drinking, although not habitual, increases the risk of ASCVD 323 - 326) . Binge drinking is defined by US criteria as five drinks (70 g of pure alcohol equivalent) for men and four drinks (56 g of pure alcohol equivalent) for women within two hours 327) , but in Japan it is currently limited to drinking large amounts of alcohol in a short time 328) . Large international case-control studies and meta-analyzes have reported that binge drinking of more than 60 g of pure alcohol (approximately 3 grams of sake per occasion), even with moderate drinking, can increase mortality from ischemic heart disease 323 , 325) .
Furthermore, the relationship between alcohol consumption and all-cause mortality has been reported in a number of cohort studies that show a J-curve relationship 310 , 329 , 330) . However, recent international meta-analyzes have reported that there are no relationships that reduce in mortality or increace mortality or increase in life expectancy among small drinkers 315 , 316) .
Based on these results, considering the increased risk of hemorrhage and health risks in addition to the prevention of ASCVD, it is recommended that consumption be reduced to 25 g or less (guideline amount: up to the equivalent of one sake or one medium beer bottle) in accordance with the existing policy, or as little as possible. It is conceivable that binge drinking and small amounts of alcohol may require more attention in the future.
1.9 History of CAD
Those with a history of CAD are at higher risk for the incidence of coronary events than those without a history of CAD. In particular, those with a history of acute coronary syndromes are at higher risk for the incidence of ASCVD.
It is clear from epidemiological studies and intervention trials in Europe and the United States that people with a history of CAD are at higher risk for the incidence of cardiovascular events than those without a history of CAD 331 - 333) . In Japan, the incidence of coronary artery events in the diet group in the MEGA Study, a statin-based primary prevention trial, was 2.1/1,000 person-years 49) , while in J-LIT study, it was 0.9/1,000 person-years in patients without a history ofCAD 89) compared to 4.5/1,000 person-years in patients with a history of CAD 90) , and in the JELIS study, it was 1.6/1,000 person-years in patients with no history of CAD compared to 6.8/1,000 person-years in patients with CAD 334) . The incidence of coronary events in the JCAD 335) and CREDO-Kyoto Studies 336) , which are registry studies of patients with CAD, is more than 15/1,000 person-years. In particular, the risk of coronary events in acute coronary syndromes is high even with statins, as is the incidence of ASCVD 337 - 342) . The mean LDL-C during the observation period in the statin monotherapy group in HIJ-PROPER in patients with acute coronary syndromes was 84.6 mg/dL, but the incidence of ASCVD for 3.9 years was as high as 128.1/1,000 person-years 341) .
1.10 History of Cerebrovascular Disease (Including TIA)
Patients with previous cerebral infarction and transient ischemic attack (TIA) with atherosclerosis are at high risk for recurrent stroke and CAD, and strict lipid management is recommended.
Patients with cerebral infarction or transient ischemia (TIA) have a very high risk of recurrent stroke in the immediate aftermath of incidence. TIAregistry.org, a 5-year follow-up study that registered 4,789 patients with minor cerebral infarction and TIA within 7 days of onset, including patients from Japan, reported that 5.1% had recurrent stroke and 0.4% myocardial infarction in the first year, 9.5% had recurrent stroke, and 1.1% had acute coronary syndrome for 5 years; after the second year, 1.1%/year had recurrent stroke, and 0.2%/year had acute coronary syndrome 343 , 344) . In large clinical trials for cerebral infarction and TIA within 24-48 hours of onset, the CHANCE study 345) in 5,170 patients in China showed a recurrence rate of 10.0% and incidence rate of myocardial infarction of 0.1% within 3 months, the POINT study 346) in 4,881 patients mainly in the US and Europe showed a recurrence rate of 5.6% and incidence rate of myocardial infarction of 0.3% within 3 months, and the SOCRATES study 347) in 13,199 patients showed a recurrence rate of 5.6% and incidence rate of 0.3% within 3 months. In the acute phase of cerebral infarction and TIA, the risk of recurrent stroke greatly exceeds the risk of incidence of myocardial infarction. On the other hand, many clinical trials have been conducted in Japan, mainly in patients with cerebral infarction in the subacute and chronic phase within a week to 6 months. The J-STARS study 348) conducted in Japan on 1,578 patients with noncardiogenic cerebral infarction showed an annual stroke recurrence rate of 2.4% and a myocardial infarction incidence rate of 0.14%. The CSPS2 study 349) on 2,757 patients with noncardiogenic cerebral infarction showed an annual stroke recurrence rate of approximately 3%. The PRASTRO-I trial 350) showed a recurrent stroke rate of 3.9% and incidence of myocardial infarction of 0.3% during a mean follow-up period of 1.8 years, In the CSPS.com study 351) of 1,879 patients with high-risk non-cardiogenic cerebral infarction, the annual recurrent stroke rate was 5.0% and the incidence of acute myocardial infarction was 0.2% during the 1.4-year follow-up period in 947 patients treated with antiplatelet agents alone. In the RESPECT study 352) of 1,263 patients with a history of stroke, the annual recurrent stroke rate was reported to be 2.26% and the incidence of myocardial infarction 0.17% in 633 patients in the usual blood pressure control group. For chronic cerebral infarction, the risk of recurrent stroke is assumed to be 2-3% per year, and the risk of myocardial infarction incidence 0.1-0.3% per year. In the TST study 353) involving 2,860 patients with cerebral infarction or TIA with atherosclerosis, the recurrent stroke rate was 8.0% and the rate of myocardial infarction and emergency coronary revascularization was 1.8% during the 3.5-year follow-up period. It is known from the above that patients with a history of cerebrovascular disease are at high risk of recurrent stroke, especially in the acute phase, but in the chronic phase, they are at high risk of recurrent stroke and CAD. Registries in Japan have reported a 1-year incidence of myocardial infarction of 0.40-0.45% (4.0-4.5 per 1,000 person-years) among people with previous stroke 216 , 217) .
In addition, findings of atherosclerosis in the carotid arteries are an independent risk factor for the incidence of cardiovascular disease 4 , 6) . Cohort studies in Japan have reported that thickened arterial IMT is a significant predictor of cerebral infarction and CAD 354 - 356) , and the USE-IMT study 39) , an international meta-analysis of multiple cohort studies, and the PROG-IMT study 357) also reported that IMT is a risk factor for myocardial infarction and stroke.
1.11 High Risk Vascular Disease
1) Peripheral Arterial Disease: PAD
Peripheral arterial disease of the lower extremities is a high-risk condition that predisposes to a high incidence of CAD and cerebrovascular disease.
Peripheral arterial disease PAD is essentially a term that encompasses all arterial diseases other than the coronary artery and aorta 10) . In Japan, the term ASO (Arteriosclerosis Obliterans) has long been used to describe atherosclerotic disease of the peripheral arteries, and to be consistent with the revised Guideline on the Management of Peripheral Arterial Diseases by the Japanese Circulation Society, we use the term PAD in general and LASO (Lower extremities Arteriosclerosis Obliterans) for diseases based on stenotic or occlusive lesions caused mainly by atherosclerosis of the arteries of the lower extremities in this guideline. The risk of LASO increases with age and with exposure to important cardiovascular risks, including smoking, hypertension, dyslipidemia, and diabetes. As the stenosis/obstruction progresses, symptoms such as coldness of the lower extremities, intermittent claudication, ulceration, and necrosis are observed. In epidemiological studies in Europe and the US, it has been shown that LASO patients are more susceptible to the incidence of other ASCVDs, such as CAD and cerebrovascular disease, and this has also been reported in Japan.
The prevalence of LASO in the general population of middle-aged and older Japanese is estimated to be 1-3%. The prevalence is also increased in populations with risk factors and is estimated to be 3-6% in those 65 years or older, 5-10% in diabetics, 10-20% in patients with CAD or cerebrovascular disease, and 10-20% in patients on hemodialysis for chronic renal failure 358) . In the Hisayama study of the general population, 2,954 people 40 years or older with no cardiovascular disease were followed for an average of 7.1 years, and those with an ABI of 0.9 or less had a 4.13-fold higher risk of incidence of CAD compared to those with a normal ABI 359) . In the CIRCS study of the general population, 939 people aged 60 to 74 years without cardiovascular disease were followed for an average of 9.3 years, and those with an ABI of 0.9 or less had a 2.04-fold higher risk of developing CAD and a 3.39-fold higher risk of developing cerebrovascular disease than those with an ABI of 1.1 or greater 360) . Ohkuma et al. found that the risk of cardiovascular disease was 1.07 times higher in the 1.00-1.09 group, 1.37 times higher in the 0.91-0.99 group, and 1.60 times higher in the 0.90 or lower group compared to those with an ABI of 1.10-1.19 in 720 Japanese subjects with no history of cardiovascular disease who were followed for 7.8 years. Furthermore, an ABI of 1.30 or higher was also 2.42 times higher 361) . The REACH Registry also examined the 1-year incidence of cardiovascular disease in 603 PAD patients out of 5,193 Japanese patients, and found high rates of all-cause mortality (1.25%), cardiovascular death (0.55%), nonfatal myocardial infarction (0.77%) and non-fatal stroke (1.56%) 216) . Shigematsu et al. conducted a prospective observational study of 557 PAD patients and found 6.3% cardiovascular death, 11.3% cardiac disease, 7.0% cerebrovascular disease, and 16.9% lower extremity events over a 3-year period 362) .
As described above, in Japan it has been shown that patients with PAD (LASO) are susceptible to a high incidence of other ASCVD diseases, such as CAD and cerebrovascular disease. Therefore, when a patient with PAD (LASO) is seen, a close examination of the systemic ASCVD is necessary.
As medical therapy in patients with PAD, statins are recommended (Class I, Level A) 10 , 363 - 369) . In particular, the 2017_ESC_Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases refer to target therapeutic values and recommend reducing LDL-C to <70 mg/dL or, or reduce by 50% or more if the reference value is 70-135 mg/dL (Class I, Level C) 10) . In high-risk cases, even lower values can be targeted 366) .
Regarding therapeutic agents other than statins, aggressive LDL-C lowering therapy with small intestinal cholesterol transporter inhibitors and PCSK9 inhibitors has been reported to be useful in preventing the incidence of cerebral and cardiovascular disease in LASO and Polyvasculardisease cases 370 , 371) . Furthermore, a subanalysis of the JELIS study reported that the incidence of cardiovascular disease was significantly reduced when EPA was used in combination with statins, even in LASO patients 372) .
When considering treatment strategies for PAD, it is important to note that the treatment guidelines provided in the guidelines are essentially treatment guidelines for LASO and do not refer comprehensively to other peripheral arterial diseases. Therefore, it is unclear whether the same treatment strategy should be used for non-LASO PAD cases as for LASO cases. In addition, when assessing risk, the indication decision cannot be made solely on the location of the PAD (LASO) lesion or the presence of stenosis or obstruction but must be considered in terms of the incidence risk of all cardiovascular diseases. When judging by LASO alone, the cutoff value for LASO treatment should be symptomatic and low ABI, while in asymptomatic cases, even if the ABI is low, the overall target value for treatment should be considered. In the case of LASO, statins are recommended in addition to general prophylaxis to improve walking distance, especially in intermittent claudication 373) .
2) Abdominal Aortic Aneurysm: AAA
Patients with abdominal aortic aneurysms (AAA) have a high rate of complications of ASCVD.
The abdominal aortic aneurysm (AAA) is believed to be related to atherosclerotic lesions of the coronary arteries due to the presence of atherosclerotic lesions on the wall of the aneurysm and surrounding aorta. A meta-analysis of small-diameter AAAs without indication for surgery reported 3% cardiovascular-related deaths annually, 44.9% overall ischemic heart disease, and 26.8% myocardial infarction 374) . Serious perioperative complications of ASCVD have also been reported to occur at a rate of 2-8% in AAA vascular replacement procedures 375) . Japanese data also include a report of coronary atherosclerosis in 45.7% of preoperative coronary angiograms for subrenal abdominal aortic aneurysms 376) and a report of myocardial ischemia in 37% of myocardial SPECT (single photon emission computed tomography) loaded with ATP (adenosine triphosphate) in patients with abdominal aortic aneurysm patients without a history of CAD 377) . Therefore, the presence of AAA is associated not only with a high rate of coronary atherosclerosis, but also with a certain percentage of atherosclerotic lesions that require treatment. On the other hand, a meta-analysis of data from Europe and the United States found that 8.4% of patients with CAD had AAA, four times as many as those without disease 378) . Thus, AAA and CAD can be considered highly correlated.
Risk factors for AAA are common to those for ASCVD, including aging, smoking, history of CAD and peripheral artery disease, carotid stenosis, hypertension, and hyper-LDL cholesterolemia 375 , 379 , 380) , however, there is a report that diabetes was a suppressor of AAA 375) , so there are some differences. Diastolic blood pressure has been reported as a factor involved in AAA enlargement and rupture in Europe and the United States 381) , but Akai et al. investigated the increase in aneurysm diameter in 374 Japanese AAA patients and reported that hypertension was involved in aneurysm enlargement but was not related to a history of ASCVD or blood cholesterol levels 382) .
However, 5-year survival rate has been reported to improve when statins, antiplatelet agents, and antihypertensive agents are administered to AAA to control risk factors for ASCVD 383) , and since these agents do not directly suppress the increase in AAA aneurysm diameter, it is thought that the effect is due to suppression of cardiovascular complications 384) .
From the above, at this time there are no longitudinal studies, but cross-sectional studies exist, and AAA is not an established risk factor for CAD, but it is a high-risk condition associated with cardiovascular disease 377) . Therefore, it is recommended that smoking cessation, blood pressure control, and exercise promotion, as well as aggressive control of risk factors for atherosclerotic disease, be implemented in AAA patients 384) .
3) Renal Artery Stenosis; RAS
Renal Artery Stenosis (RAS) is a high-risk condition for ASCVD.
Renal artery stenosis (RAS) is an atherosclerotic stenosis in 95% of cases, and RAS has been reported to be found in 20% of Japanese people over 40 years of age at risk of atherosclerosis 385) . RAS is a progressive condition that tends to worsen kidney function but also increases the risk of cardiovascular complications and decreases survival 386 , 387) . Regarding the relationship with CAD, 30% of patients had RAS when the renal artery was examined as a screening during cardiac catheterization 388) and that the survival rate decreases with the degree of stenosis of the RAS 389) , but no prospective study has been reported in Japan. On the release of other hand, the arterial stenosis for RAS has not been shown to have an inhibitory effect on cardiovascular complications or deterioration of kidney function 390 - 393) . However, there have been reports that stenting has an effect on lowering blood pressure and cardiovascular complications in severe cases of RAS 394) , and a prospective study in Japanese subjects has shown that stenting has an antihypertensive effect 395) . It is necessary to examine the effectiveness of stent treatment in a limited number of patients in the future 396) . However, at this time there is not enough evidence that RAS is a direct risk factor for ASCVD, but it is an important high-risk condition for cardiovascular disease with a high rate of CAD lesions.
1.12 Subclinical Atherosclerosis
In this section, we examined scientific evidence for the following question: i.e. whether or not various subclinical atherosclerosis measures improve the predictive ability of prediction models for ASCVD risk (including the Suita Score and others)? The subclinical atherosclerosis measures we examined were: (1) brain magnetic resonance image (MRI), (2) carotid artery ultrasonography (intima-media thickness and plaque), (3) coronary computed tomography (CT), especially coronary artery calcification (CAC), (4) pulse wave velocity (PWV), (5) cardio-ankle vascular index (CAVI) and (6) ankle-brachial index (ABI).
Note that in this section, we evaluated these subclinical atherosclerosis measures with respect to their ability to improve ASCVD risk prediction by adding them to a combination of classical risk factors. We have no intention to defy use of those measures for other (clinical) purposes. Additionally, a subclinical atherosclerosis measure may be useful in motivating classical risk factor management 397 , 398) .
BQ8. Do asymptomatic vascular lesions on brain MRI have a predictive power for the incidence of ASCVD beyond the clustering of classical risk factors (or a prediction model)?
In a general Japanese population, there were no reports evaluating whether asymptomatic vascular lesions in the brain (white matter lesions, lacunar infarction, microbleeds, and vascular stenosis) detected by brain MRI improve the predictability of cardiovascular disease incidence beyond the clustering of classical risk factors. Thus, it is unclear whether asymptomatic vascular lesions on brain MRI improve predictive ability. (Level of evidence: E-1b)
Studies show that brain MRI findings are associated with risk of stroke and cardiovascular incidence independently of classical risk factors in a general Japanese population 399 , 400) . For example, a multicenter study in Japan reported that the severity of asymptomatic periventricular hyperintensity (PVH) and deep subcortical white matter hyperintensity (DSWMH) was a predictor of future symptomatic cerebral infarction 401) , and the findings were generally consistent with those in the United States and Europe. However, we did not find any studies in a general Japanese population that evaluated the improvement of risk prediction beyond a classical risk factor-based prediction model by adding brain MRI abnormalities to the model. Therefore, scientific evidence was insufficient for recommending brain MRI for primary prevention to improve risk prediction beyond the clustering of classical risk factors.
BQ9. Do intima-media thickness or plaque (IMT/plaque) findings by carotid artery ultrasonography have a predictive power for the incidence of ASCVD beyond the clustering of classical risk factors (or a prediction model)?
Very few reports have evaluated whether IMT/plaque by carotid artery ultrasonography improve the prediction of cardiovascular disease incidence beyond the classical risk factors in a general Japanese population. Only one report showed statistically significant improvement in a general Japanese population (Level of evidence: E-1b).
We found two Japanese studies that evaluated the improvement in ASCVD prediction by adding carotid IMT/plaque (assessed with ultrasonography) as a primary prevention. One such study was from the Suita Study, a community-based study following a total of 4,724 Japanese individuals (mean age 59.7 years) for more than 10 years for incidence of cardiovascular disease (stroke=221, CAD events=154). In the study, the addition of Max-CIMT (maximum IMT in the common carotid artery) >1.1 mm or Max-IMT (maximum IMT in all examined carotid arteries) >1.7 mm improved the C statistic compared to the Suita Score alone for the prediction of CVD (i.e. Stroke and CHD) 402) . The net reclassification index (NRI) improved in CVD incidence when max-CIMT >1.1 mm or max-IMT >1.7 mm was added 402) . Another study examined a Japanese population of 783 patients with type 2 diabetes with a mean of 5.46-year follow-up. It revealed that carotid IMT (mean value of the common carotid artery) was a predictor of cardiovascular disease incidence (fatal and non-fatal myocardial infarction, angina, TIA and cerebral infarction. Number of incidences=85) independent of classical risk factors. A slight improvement in ROC was reported from 0.645 to 0.656 when a high carotid IMT (≥ 4th of 5 quintiles) was added to the Framingham Risk Score. However, whether the “improvement” in ROC was statistically significant or not was not presented, thus, unclear 403) .
Expanding to the East Asian region, there were two reports (from Taiwan 404) and China 405) ) showing that carotid artery ultrasonography significantly improved the predictive ability of incident cardiovascular disease beyond the clustering of classical risk factors, while others showed no significant improvement (China 406) ).
Overall, although the evaluation of carotid IMT/plaque by ultrasonography appears promising, the scientific evidence for recommending carotid artery ultrasonography for primary prevention in Japanese individuals to improve risk prediction beyond clustering classical risk factors is not sufficient.
BQ10. Do coronary stenosis and CAC score using coronary CT have predictive ability for the incidence of ASCVD beyond the clustering of classical risk factors (or a prediction model)?
We did not find studies examining whether coronary stenosis or CAC scores assessed with coronary CT have predictive value for incident cardiovascular diseases beyond the clustering of classical risk factors in a general Japanese population free of ASCVD. (Level of evidence: E-1b)
We found no report of coronary CT angiography or CAC scores that may answer this BQ. On the other hand, in guidelines of Europe and the United States, CAC assessed with non-contrast CT has a certain role in the primary prevention of ASCVD. For example, the US AHA/ACC2019 primary prevention guidelines recommend the use of CAC as an adjunct examination in determining risk management goals (primarily statin use) for those aged 40-75 years at an intermediate 10-year risk of ASCVD incidence (7.5% to <20%). Recommendations vary depending on whether the CAC score is 0, 1-99, or 100 or higher (IIa, B-NR) 407) . However, the recommendations are mainly based on studies conducted in western populations, which have a higher incidence of CAD than the Japanese, thus, it would be inappropriate to apply them directly to the Japanese. Furthermore, in Japan, where medical radiation exposure is said to be higher than in Europe or the United States. The problem of increasing radiation exposure to a general population through CT imaging cannot be underestimated. In conclusion, there is little scientific evidence for performing coronary CT for primary prevention in the Japanese population to improve risk prediction beyond the clustering of classical risk factors.
BQ11. Does PWV have predictive ability for the incidence of ASCVD beyond the clustering of classical risk factors (or a prediction model)?
Although baPWV (brachial-ankle pulse wave velocity) is likely to have predictive ability for ASCVD incidence in a general Japanese population beyond the clustering of classical risk factors (or a prediction model), several important unresolved issues make it insufficient as a basis for recommendation at present. (Level of evidence: E-1a)
A pooling study of Japanese cohort data in which baPWV was measured at the individual level assessed whether predictability of ASCVD improve with baPWV added to a prediction model by the criteria of C statistic, NRI, and integrated discrimination improvement (IDI) (J-BAVEL: Japan Brachial-Ankle Pulse Wave Velocity Individual Participants Data Meta-Analysis of Prospective Studies) 29) . The study followed 14,673 people without a history of cardiovascular disease for an average of 6.4 years (death during the observation period=687; incidence of cardiovascular disease=735). According to the results, the addition of baPWV significantly improved the C statistic for cardiovascular disease incidence in the low-risk group (Framingham Risk Score ≤ 5 for men and ≤ 9 for women) or all subjects, while the NRI and IDI were significantly improved in the high- (≥ 9 for men and ≥ 15 for women), intermediate- and low-risk groups. Another study of the Hisayama cohort (N=2,916, mean follow-up 7.1 years; cardiovascular disease incidence=126), one of the cohorts that provided data to J-BAVEL above, reported significant improvements in both the C statistic and the NRI by adding baPWV to its own prediction model. This study proposed 16.6 to 17.6 m/sec as the cutoff point for baPWV 408) .
The J-BAVEL study used the Framingham Risk Score as a predictive model, which was derived from a Caucasian population in the US. Therefore, the applicability of the results to a Japanese population is questionable. Another unresolved issue is that an appropriate cutoff value of baPWV remains uncertain when adding to the predictive model for primary prevention. In another J-BAVEL paper, studying only on hypertensive individuals (N=7566), a cut-off value of 18.3 m/sec was proposed 409) , but whether use of this cut-off value improves predictive ability has not been examined in the paper. These issues deserve further study.
BQ12. Does CAVI have the predictive ability for the incidence of ASCVD beyond the clustering of classical risk factors (or a prediction model)?
There is only one report examining whether CAVI improves the predictive ability for incident cardiovascular disease beyond clustering of classical risk factors in a general Japanese population, and the scientific evidence supporting CAVI for primary prevention in a Japanese population is insufficient. (Level of evidence: E-1b)
Only one study has examined this BQ in a Japanese population. According to the results, in a sample of Japanese obese individuals, CAVI was predictive for ASCVD events (CAD, cerebral infarction/TIA (transient ischemic attack)/atherosclerotic hemorrhage, and arteriosclerosis obliterans) that exceeded the clustering of classical risk factors 410) . However, the prediction model used in this study was derived from the United States (10-year atherosclerotic cardiovascular disease (ASCVD) risk score). The applicability of the score to a Japanese population is unknown. In addition, since the study sample was small (slightly more than 400) with only obese individuals included, the stability of the estimates is not sufficiently high and applicability of the results to a general population is limited. With those considerations, at present, there is insufficient scientific evidence to support the use of CAVI in primary prevention in a Japanese population to improve risk prediction beyond the clustering of classical risk factors.
BQ13. Does ABI have predictive ability for the incidence of ASCVD beyond the clustering of classical risk factors (or a predictive model)?
Only one study has examined whether ABI is predictive beyond the clustering of classical risk factors for the incidence of cardiovascular disease in a sample of Japanese individuals free of cardiovascular disease. The study found no significant improvement in the prediction. The scientific evidence for performing ABI for primary prevention in a Japanese population is therefore lacking. (Level of evidence: E-1a)
According to a collaborative study consists of five cohort data on general populations in Japan (J-BAVEL-ABI; 10,679 individuals, mean follow-up 7.8 years, CVD event=720) 361) , none of the criteria (C statistic, IDI or NRI) showed statistically significant improvement in the prediction with the addition of ABI. Other than the J-BAVEL-ABI, there were few studies on Japanese individuals that could provide answers to this BQ.
Many of the non-Japanese studies conducted on Asian individuals were difficult to obtain the answer to this BQ because the study participants had pre-existing conditions such as CKD 411 , 412) . In a study of patients with type 2 diabetes in China, the improvement in predictive ability by AUC was not statistically significant in participants at low risk of cardiovascular disease 413) . Thus, there is little scientific evidence supporting use of ABI to improve risk prediction beyond the clustering of classical risk factors in primary prevention even in East Asia.
In conclusion, we believe that there is little scientific basis for the use of ABI for the purpose of risk prediction in primary prevention for a Japanese population beyond the clustering of classical risk factors.
1. 13 MASLD, MASH
BQ 14. What dyslipidemia is associated with MASLD/MASH*?
*Originally published as NAFLD/NASH: now described as MASLD(metabolic dysfunction-associated steatotic liver disease)/MASH(metabolic dysfunction-associated steatohepatitis)
•MASLD is associated with hypertriglyceridemia, hyper-LDL cholesterolemia, and hypo-HDL cholesterolemia. (Level of evidence: E-1b)
•sd-LDL and remnant cholesterol are increased in patients with MASLD. (Level of evidence: E-2)
According to the NAFLD / NASH Clinical Practice Guidelines 2020 of the Japanese Society of Gastroenterology and the Japan Society of Hepatology, it had been considered that nonalcoholic fatty liver disease (NAFLD) is often associated with metabolic syndrome, and in which steatotic liver is present in histological or imaging diagnosis and other liver diseases such as alcohol liver disease, viral liver disease, and drug-induced liver disease have been excluded 414) . NAFLD is classified into nonalcoholic fatty liver (NAFL), which rarely progresses, and nonalcoholic steatohepatitis (NASH), which progresses to cirrhosis and hepatocarcinogenesis 414) .
Hamaguchi et al. conducted a prospective observational study of 4401 screening participants and reported that hypo-HDL cholesterolemia and hypertriglyceridemia were associated with the presence or absence of NAFLD at enrollment and with new onset of NAFLD 415) . More than 70% of patients with obesity and nearly 50% of patients with diabetes mellitus have NAFLD as a complication. Among dyslipidemias, hypertriglyceridemia, hyper-LDL cholesterolemia, and hypo-HDL cholesterolemia have the highest complication rates of NAFLD, in that order 416) .
Furthermore, Imajo et al. analyzed lipoprotein subclasses by HPLC in 156 patients with NAFLD (53 patients with NAFLD and 103 patients with NASH) and reported that small, dense LDL (sd-LDL) was significantly increased in patients with NASH 417) . Campanella et al. reported that calculated remnant cholesterol (=TC-HDL-C-LDL-C) was increased in patients with moderate or severe NAFLD 418) .
Thus, in NAFLD/NASH, the association with various abnormalities of lipid metabolism has been reported. While abnormalities in lipid metabolism are a consequence of the pathogenesis of NAFLD/NASH, they also have a causal aspect. Future NAFLD/NASH drug development and intervention trials should be designed to evaluate whether both NAFLD/NASH and serum lipids improve.
BQ15. Is MASLD/MASH a high-risk condition for ASCVD?
Patients with MASLD/MASH have a higher risk of cardiovascular disease incidence and death compared to patients without MASLD. (Level of evidence: E-1a)
According to the NAFLD / NASH Clinical Practice Guidelines 2020 by the Japanese Society of Gastroenterology and the Japan Society of Hepatology, evaluation for potential cerebral and cardiovascular disease is recommended in patients with a platelet count <200,000/mm3 or a liver fibrosis prediction score FIB-4 index ≥ 2.67, even if they have no history of cardiovascular disease 414) .
Among previous overseas studies, a meta-analysis by Musso et al. reported an odds ratio of 2.05 for the incidence of CVD in the NAFLD group versus the control group 414 , 419) .
A subsequent meta-analysis of 34,043 patients (36.3% NAFLD patients) from 16 published studies found that NAFLD patients had an odds ratio of 1.64 (1.26-2.13) for the incidence of CVD/death 420) . Furthermore, an increased odds ratio of 2.58 (1.78-2.13) for the incidence of CVD/death was reported for patients with NAFLD with more severe disease, such as advanced liver fibrosis 420) .
According to the results of a Swedish cohort study of 10,568 patients with liver biopsy followed from 1966 to 2017, 1,199 of 4,338 patients died of CVD, with a hazard ratio of 1.35 (1.26-1.44) for CVD in all NAFLD patients, 1.66 (1.38-2.01) in NASH without liver fibrosis and 1.40 (1.17-1.69) in NASH with liver fibrosis, but increases to 2.11 (1.63-2.73) in cirrhotic NASH 421) .
On the other hand, a meta-analysis of 164,494 participants in 21 cross-sectional studies and 13 cohort studies conducted between 1965 and 2015 of clinical studies reported that NAFLD was associated with the risk of CVD incidence but not CVD mortality 422) .
A study on high-risk coronary plaques by cardiac CT (positive remodeling, CT attenuation <30 HU, napkin ring sign, spotty calcium) in 445 patients (182 patients with NAFLD) found high-risk plaques in 59.3% of patients with NAFLD, compared to only 19.0% of patients without NAFLD 423) . For peripheral arterial stiffness, a meta-analysis of 85,395 patients, including 29,493 patients with NAFLD enrolled in 26 studies, found that NAFLD patients had an odds ratio of 1.74 (1.47-2.06) for carotid intima/plaque, 1.56 (1.24-1.96) for arterial wall stiffness measured by pulse wave velocity, coronary calcification 1.40 (1.22-1.60), and endothelial dysfunction 3.73 (0.99-14.09) 424) .
Although the presence of NAFLD/NASH has been reported to result in a higher rate of cardiovascular complications, no large-scale studies have yet been published that examined the risk of CVD incidence and mortality in Japanese patients with NAFLD. Recently, it has been reported that the prognosis of NAFLD/NASH is most related to the stage of liver fibrosis 425) . It would be highly expected that the mechanisms of increased CVD risk, which cannot be explained by lipid metabolism abnormalities alone, would be elucidated and the development of screening modalities to identify high-CVD risk populations would be developed. Recent drug development for NAFLD/NASH has beem required to ameliorate liver fibrosis. However, it is also worth considering whether drugs developed to target liver fibrosis are also effective in the prevention of CVD associated with NAFLD/NASH. (See Appendix 2 “Flowchart for cardiovascular event screening in NAFLD patients in NAFLD/NASH Clinical Practice Guidelines 2020”).
Note: The European Association for the Study of the Liver (EASL), the American Association for the Study of Liver Diseases (AASLD), and the Latin American Association for the Study of Liver Diseases (ALEH) have announced that the names of fatty liver diseases, such as nonalcoholic fatty liver diseases (NAFLD) and nonalcoholic steatohepatitis (NASH), were changed. The conventional NAFLD and NASH are now diagnosed as metabolic dysfunction associated steatotic liver disease (MASLD) and metabolic dysfunction associated steatohepatitis (MASH) only when some of the criteria for metabolic syndrome are met*. The Japanese Society of Hepatology announced its support for these new disease name changes and classifications on September 29, 2023**. For this reason, Japan Atherosclerosis Society has also decided to follow the change of the notation of NAFLD/NASH to MASLD/MASH in the title of BQ 14 and 15.
*Rinella ME, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023 June 20; DOI: https://doi.org/10.1016/j.jhep.2023.06.003
**https://dx-mice.jp/jsh_cms/files/info/1328/20230929%E3%80%80oshirase62.pdf
1.14 Other Risk Factors/Markers To Consider
•Hyper-Lp (a) lipoproteinemia is a risk factor for ASCVD.
•Measurement of MDA-LDL is useful in predicting the prognosis of the incidence of CAD in diabetic patients with a history of CAD, as well as in non-diabetic patients for prognostic evaluation after coronary intervention therapy.
•Hyper-remnant-lipoproteinemia is a risk factor for ASCVD.
•Postprandial hyperlipidemia is a risk factor for CAD.
•High levels of small dense LDL cholesterol are a risk factor for ASCVD.
•High levels of apoB are a risk factor for ASCVD.
•Ratios of TC / HDL cholesterol, non-HDL cholesterol/HDL cholesterol, the LDL cholesterol, and apoB/AI are markers of ASCVD.
•Elevated levels of fibrinogen and plasminogen activator inhibitor 1 (PAI-1) are markers of ASCVD
Although some of these factors are real risk factors involved in the development of atherosclerosis, others are likely markers of atherosclerosis and should be kept in mind. In addition to the established risk factors mentioned in the previous section, we propose risk factors or markers of ASCVD that should be considered.
1) Lp(a)
Lp(a) is an independent risk factor for CAD and stroke as previously reported in meta-analysis, Mendelian randomization analysis, and genome-wide studies 426 - 436) . The concentration of Lp(a) is higher when the size of apo(a) is smaller because of fewer repeats of Kringle IV-2, and in the case the risk of CVD is also higher 130 , 131 , 427) . Single nucleotide polymorphisms (SNPs) have also been shown to reflect these findings 130 , 131 , 426 - 436) . However, in the enhancement of total mortality risk, a small number of Kringle IV-2 repeats is a significant factor, but the effect of SNPs is not significant 437) . Atherogenic factors of Lp(a) have been proposed, including the promotion of thrombus formation based on the high homology of apo(a) protein with plasminogen 130 , 432 , 438) , its association with oxidized phospholipids 440 , 441) , and the deposition of apo(a) in the arterial wall 441) . Lp(a) is elevated in patients with FH, and Hyper-Lp(a)-lipoproteinemia may further increase the risk of cardiovascular disease in FH 130 , 427 , 442 , 443) .
The contribution of hyper-Lp (a) lipoproteinemia in the primary prevention of ASCVD has been reported mainly from overseas prospective observational studies 428 , 435 , 444 - 449) . Among them, a cohort study of 26,102 patients, which combined data from the United Kingdom and Denmark, showed that the risk due to hyper-Lp(a) lipoproteinemia was attenuated when LDL-C was controlled below 2.5 mmol/L (97 mg/dL) 449) . Regarding the contribution of hyper-Lp (a) lipoproteinemia to the secondary prevention of ASCVD, pos hoc analyses of randomized controlled studies (RCTs) has confirmed that even with adequate control of LDL-C with statins or other treatments, hyper-Lp(a)-lipoproteinemia above 50 mg/dL is associated with increased risk 444 , 450 - 455) In an integrated meta-analysis of high-risk primary and secondary prevention studies using statins, the risk of cardiovascular disease increased linearly at baseline Lp(a) concentrations ≥ 30 mg/dL and at post-therapy Lp(a) values of ≥ 50 mg/dL 455) . In a clinical trial using a PCSK9 inhibitor, which, unlike statins, showed a clear reduction in Lp(a) along with LDL-C, cardiovascular events were reduced by 23% along with a 26.9% reduction in Lp(a), but it needs future studies whether the reduction in Lp(a) leads to prevent cardiovascular events 444 , 453) .
Guidelines such as AHA/ACC set Lp(a) less than 30 mg/dL as no significant ASCVD risk and Lp(a) greater than 50 mg/dL as a risk enhancing factor, and European atherosclerosis guidelines also consider Lp(a) greater than 50 mg / dL as high risk 130 , 456 , 457) . The coexistence of high Lp(a) and a family history of ASCVD or premature ASCVD increased the risk of ASCVD by a hazard ratio of 2.57 and 3.35 times, respectively 458) .
2) MDA-LDL
MDA-LDL is a representative of oxidized LDL, in which LDL is subjected to oxidative stress and phospholipids and other lipids and apoprotein B are oxidatively modified 144, 145) . MDA-LDL is also useful in predicting the prognosis of recurrent CAD and restenosis after PCI in diabetic patients with a history of CAD 146) . In patients with stable angina pectoris on lipid-lowering therapy, the risk of cardiovascular event incidence increased 1.14 times per 10 U/L increase in MDA-LDL after treatment with drug-eluting stents 459) . High MDA-LDL on admission is associated with a worse prognosis in ACS patients undergoing PCI 460) . In an intravascular ultrasound study, high levels of MDA-LDL were significantly associated with plaque instability (intraplaque lipid accumulation and thinning of the fibrous capsule) 461) . In a retrospective study of lipid-lowering therapy-naive patients who underwent coronary CT examination with a diagnosis of stable angina, high MDA-LDL levels were significantly associated with plaque instability 462) . According to the clinical studies in Japan, the percentage of diabetes mellitus among eligible patients is 30-100%, and evidence in non-diabetic patients is accumulating, but the current insurance coverage for the examination of MDA-LDL is limited to diabetic patients.
3) Remnant Lipoprotein
The risk of cardiovascular events in patients with myocardial infarction is higher when the remnant lipoproteins are high and has been found to be an independent risk even when LDL-C is controlled to less than 100 mg/dL 463 , 464) . The utility of measuring remnant lipoproteins has been recognized to assess the risk of recurrence in acute coronary syndrome (ACS) who have undergone coronary intervention and are taking statins, and for assessing ASCVD risk in primary and secondary prevention when type 2 diabetes and CKD are combined 465 , 466) . Hyper-remnant-lipoproteinemia can also explain part of the residual risk of cardiovascular events in patients with CAD controlled at less than 70 mg/dL LDL-C with statins 467) . High levels of remnant lipoprotein appear to contribute to the risk of ASCVD independently of hyper-LDL cholesterolemia to a roughly equivalent extent to LDL-C. A 1 mmol/L (approximately 39 mg/dL) increase in LDL-C and remnant cholesterol levels was associated with a 1.3-fold increase versus a 1.4-fold increase in the risk of myocardial infarction, respectively, in the Copenhagen study, a large observational study, and a 2.1-fold increase in LDL-C versus 1.7-fold in remnant lipoproteins impacted on myocardial infarction risk in the Mendelian randomized study 436 , 468 , 469) . In an observational study of primary prevention in overweight and obese high-risk subjects, high remnant cholesterol levels were a significant risk factor for ASCVD, regardless of high or low LDL-C levels 128) . The evidence on remnant lipoproteins in Japan is based on the evaluation of remnant lipoproteins obtained by an immunosorbent assay or direct measurement, while most of the Western evidence is based on remnant cholesterol calculated by TC-LDL-C (direct method)-HDL-C (direct method) 128 , 470 , 471) . Remnant lipoproteins include not only endogenous apoB-100 as a constituent apolipoprotein but also exogenous apoB-48 in a particle, both of which are useful in the evaluation of abnormal lipid metabolism and atherosclerosis risk 472 - 474) . Note that in evidence from previous studies, measurements of serum apoB-48 concentration measurements have been based primarily on fasting blood samples.
4) Postprandial Hyperlipidemia
Since Zilversmit et al. proposed that postprandial increase in remnant-lipoprotein can induce atherosclerosis, the clinical significance of postprandial hyperlipidemia in terms of ASCVD risk has been established 471 , 475 - 477) . As epidemiological studies in Japan show an increased risk of incidence of CAD due to high levels of non-fasting TG, a 1 mmol/L (88.6 mg/dL) increase in TG increases the relative risk of developing CAD by 1.34 times overall, 1.29 times in men and 1.42 times in women 98) . The risk increases from non-fasting TG 115 mg/dL or higher, and at 167 mg/dL or higher the risk increases more than triples, and it is found similarly even when corrected for HDL-C 98) . In a sub-analysis of MRFIT, non-fasting TG was useful equivalently to or more than fasting TG in managing CAD risk, and the CAD risk was high at non-fasting TG levels of 200 mg/dL or more 478) . Overall postprandial hypertriglyceridemia in a large observational study showed that a non-fasting TG level higher than 175 mg/dL more than doubles the risk of myocardial infarction 475 , 479) . In the United States, persistent hypertriglyceridemia of 175 mg/dL or higher is considered a risk-enhancing factor for ASCVD, and in Europe, a non-fasting serum TG of 175 mg/dL or higher is set as the cutoff value for dyelipidemia 456 , 480) . In Japan, the diagnostic criteria for the clinical diagnosis of dyslipidemia include a fasting TG of 150 mg/dL or more and a non-fasting TG of ≥ 175 mg/dL. In addition, the utility of fasting apoprotein B-48 measurement as a screening marker for postprandial hyperlipidemia is expected 481) . RCTs in Japan have shown that pemafibrate, bezafibrate, ezetimibe, and EPA pharmaceutical formulation are effective in the treatment of postprandial hyperlipidemia 482 - 486) .
5) Small Dense LDL
Among LDL particles, small dense LDL (sdLDL) 487 - 490) , which is small in size and high in density, has been reported to be associated with CAD in numerous sources 149 , 488 - 492) and has also been shown to be associated with PAD and aneurysms 493 , 494) . In addition, sdLDL cholesterol (sdLDL-C) has been shown to be more strongly associated with risk of CAD 495 , 496) , the severity of coronary atherosclerosis 149 , 496) , and the incidence of cardiovascular events in secondary prevention 497) than LDL-C in Japanese population. The following have been proposed as reasons why sdLDL is a strong pro-atherogenic factor: it is easily oxidized 498) and processed by pathways other than the LDL receptor 499) ; it is easily incorporated into the arterial wall 500) and binds readily to the matrix in the arterial wall 501) . An increase in sdLDL is closely associated with hypertriglyceridemia and hypo-HDL cholesterolemia 495 , 502) and is elevated in type 2 diabetes, metabolic syndrome and insulin-resistant state 495 , 503) . In Japan, high levels of sdLDL-C were significantly associated with CVD incidence in the Suita Study of 2,034 subjects, aged 60 years on average, with no history of CVD 504) . Similarly in the Hisayama Study of 3,040 subjects with no history of CVD, high sdLDL-C was a significant marker of the incidence of CHD, with a median sdLDL-C concentration of sdLDL-C of 32.9 mg/dL or greater resulting in twice as many CHD incidences as in the group with less than 32.9 mg/dL and less than 120.1 mg/dL of LDL-C 505) . The clinical cut-off value for the concentration of sdLDL-C in Japanese patients using the ROC curve was 35 mg/dL, which was lower than the cut-off value of 50 mg/dL set in the United States and Europe 506) . In the United States, the association between sdLDL-C and the incidence of CHD has already been verified in the ARIC and the MESA studies, and the clinical assay of sdLDL-C level has been approved by the Food and Drug Administration (FDA) 507 , 508) . Recent results from cohort studies of US women and epidemiological studies in Europe have reported that high sdLDL-C is a risk of ASCVD overall, but particularly significant for myocardial infarction 509 , 510) . In diabetic patients with stable CAD, high sdLDL-C has been reported in Asia to be a significant risk factor for major cardiovascular events (cardiovascular death, non-fatal myocardial infarction, unstable angina that requires hospitalization, emergency revascularization, and stroke) 511) .
6) Apolipoprotein B (apoB)
ApoB (apoB-100) is an apoprotein present in atherosclerosis-inducing lipoprotein particles such as LDL and remnants. As each lipoprotein particle contains one apoB molecule, the value of apoB is proportional to the number of these lipoprotein particles. Longitudinal studies and their meta-analyzes have shown that apoB is a stronger risk factor for cardiovascular events than LDL-C or non-HDL-C and improves the risk assessment of CAD incidence 512 - 514) . A meta-analysis of statin studies has shown that a reduction in apoB is associated with a lower risk of CAD incidence than a reduction in LDL-C and non-HDL-C 515) and that adding apoB to LDL-C and non-HDL-C improves risk prediction 516) . However, only treatments that lower apoB, such as statins and ezetimibe, which increase the LDL receptor, reduce cardiovascular events, and treatment that did not elevate the LDL receptor, such as fibrates, did not have that effect 517) . Meanwhile, Mendelian randomized studies have shown that the LPL pathway, along with the LDL receptor pathway, contributes similarly to apoB concentration and cardiovascular event risk 518) . A Chinese cross-sectional study reported that apoB is associated with CAD, suggesting that apoB is a risk factor for cardiovascular events even in Asians 519) . As described above, apoB is associated with as much or more than LDL-C with respect to the risk of ASCVD, but evidence is insufficient for apoB to replace LDL-C as a therapeutic target 520) . However, since apoB concentrations reflect the number of apoB-containing lipoprotein particles together with low cholesterol concentrations, they are useful in the assessment and clinical practice of residual risk when LDL-C has achieved therapeutic goals 520 , 521) .
7) Ratios of Lipids and Apolipoproteins
Although lipid values such as LDL-C and HDL-C themselves are commonly used as risk factors, rather than these lipid or apolipoprotein values, the ratio of lipids and different lipoprotein cholesterols and the ratio of different apolipoproteins, namely, the TC / HDL-C ratio, non-HDL-C/HDL-C ratio, the LDL-C / HDL-C ratio, TG/HDL-C ratios, HDL-C/apoAI, and apoB/AI ratios have been reported to be risk factors for ASCVD 512 , 522 - 526) . A Chinese study reported that the apoB/AI ratio was more strongly associated with the severity of CAD than the Framingham Risk Score or the TC/HDL-C ratio 527) . The TC/HDL-C ratio, non-HDL-C/HDL-C ratio, LDL-C/HDL-C ratio, and the apoB /AI ratio were related to the severity of CAD in diabetic patients who developed CAD. Although the apoB/AI ratio was particularly involved in the CAD severity, its significance disappeared after correction for confounding factors 528) . In type III hyperlipidemia and dyslipidemia with increased remnant lipoproteins, which is a risk factor for ASCVD, evaluating the non-HDL-C/apoB ratio is useful, and a cut-off value of 6.55 mmol/g (2.53 mg/mg) or higher has been reported 529) . Although some studies in Japan have reported that the TC/HDL-C ratio is significantly associated with coronary artery calcification even after adjusting for confounding factors 530) and that the TC/HDL ratio, rather than TC, HDL-C and non-HDL-C, is a predictor of CAD 531) , the evidence is still insufficient and control goals should be based on absolute values of each lipid level.
8) Inflammatory Marker (CRP, PTX-3, IL-6)
C-reactive protein (CRP) is one of the acute-phase proteins that is used as an inflammatory marker. Chronic inflammation of blood vessels is an important factor in the development of atherosclerosis, and recently it has been reported that high-sensitivity CRP (hs-CRP) may be a risk factor for ASCVD 532 , 533) . In Japan, there are reports that hs-CRP is significantly associated with stroke (especially cerebral infarction and lacunar infarction) 534) . Besides, hs-CRP is associated with the risk of myocardial infarction and incidence of cerebral infarction, of which the association is particularly strong in myocardial infarction 535) . In a meta-analysis of observational studies, CRP was associated with cardiovascular and all-cause mortality 536) . A decrease in CRP with statins has also been reported 537) , and there was an association between baseline CRP and cardiovascular events 538) , however, there was no association between the degree of reduction in CRP with statins and cardiovascular events. In a study of genotypes associated with serum CRP levels and frequency of CAD, CRP levels were not associated with frequency of CAD 539) , and it was also reported that CRP is not a true risk factor but only a biomarker of atherosclerosis in a Mendelian randomized study 436) . Pentraxin (PTX)-3, a member of the same pentraxin family as CRP, is expressed in vascular endothelial cells, smooth muscle cells, and leukocytes, unlike CRP, which is expressed in the liver. The association between CRP and PTX-3 in coronary artery plaques in pathological autopsies showed that the both reflect unstable plaques, but their distribution within the plaque differed between the two, suggesting that they may have different roles. Along with the correlation between changes in FMD and changes in PTX-3 and the lowering effect of statins on PTX-3, therefore, PTX-3 is also expected to be a specific marker reflecting CVD in the future 540 - 542) . Interleukin (IL)-6 is a cytokine secreted by T cells, B cells, macrophages, and other cells. In a meta-analysis of observational studies, IL-6 was an independent risk factor for the incidence of cardiovascular events and cardiovascular death 543 , 544) . An RCT of canakinumab, a fully human anti-IL-1β monoclonal antibody targeting IL-1β, which activates the IL-6 signaling pathway, in patients with a history of myocardial infarction and high sensitivity CRP ≥ 2.0 mg/L, also reported a reduction in recurrent cardiovascular events 545) .
9) Homocysteine
Elevated blood homocysteine (Hcy) levels are a risk factor not only for CAD but also for stroke and PAD 546 - 548) and are an independent predictor of CVD and all-cause mortality 549) . A study involving older individuals, aged ≥ 85 years, who had no history of CVD showed that elevated Hcy levels increased the relative risk of myocardial infarction 550) . Hcy reduction therapy with B vitamins supplementation does not suppress coronary events 551 - 553) , but folic acid supplementation reduces the risk of cerebral infarction and cerebrovascular disease 553) , suggesting that it may be beneficial in the prevention of cerebral infarction 554) . In a meta-analysis of the risk of CAD in young adults, a significant increase in the risk was observed in the group with Hcy concentrations of 15 µmol/L or greater and in Asians with the methylenetetrahydrofolate reductase (MTHFR) gene 677C à T mutation 555) . The background for this finding has been suggested to be the relatively lower intake of folic acid by Asians than that by other races, and this implies a need to conduct further studies of hyperhomocysteinemia as an independent risk factor for CAD in Japan. In a European study using genome-wide association analysis (GWAS), Hcy levels did not contribute to CAD risk in Caucasians 556) . Furthermore, it has been reported that there is no association between genetic mutations that cause elevated Hcy levels and atherosclerosis 557) , and in Mendelian randomized studies, hyperhomocysteinemia is not a risk factor for ASCVD, but only a marker 436) . A meta-analysis examining Hcy concentrations and prognosis in patients with acute coronary syndromes (ACS) found that higher Hcy concentrations were significantly associated with the risk of combined major cardiovascular endpoints and total mortality, but not cardiovascular death 558) . High Hcy levels also increase the risk of restenosis after coronary revascularization, total mortality, and cardiovascular death, but were not significantly associated with restenosis after stenting, except in some studies 559 , 560) . A meta-analysis of Chinese case-control studies examining the association between cerebrovascular disease and Hcy concentrations found that Hcy concentrations were higher in patients with ischemic stroke than in healthy subjects 561) . A meta-analysis on cerebral small vessel disease (20 studies, 8 countries) found that Hcy concentration was significantly associated with cerebral small vessel disease 562) .
10) Blood Coagulation/Fibrinolysis Factors
Fibrinogen has been reported to be an independent risk factor for CVD 563 - 566) . In an integrated analysis of 52 prospective studies, fibrinogen, along with CRP, was a risk factor for new-onset CVD 532) . By contrast, some recent reports studying the association of CRP and fibrinogen with mean IMT and coronary artery calcium score in the Japanese, Japanese American, and Caucasian populations have revealed that no significant association was found in any of the races after adjustment for multivariate model (age, SBP, LDL-C, HDL-C, fasting glucose level, smoking, and alcohol consumption) or for age and BMI 567) . Recent Mendelian randomization model studies have reported a causal relationship between fibrinogen and CAD, but the degree of involvement is small 436 , 565) . The activity of plasminogen activator inhibitor 1 (PAI-1), a fibrinolytic factor secreted by vascular endothelial cells, was elevated in the acute phase of acute myocardial infarction and was lower than in the acute phase but higher than in the control group at the time of discharge about 1-month later 568) . Meta-analyzes have shown that statin treatment decreases plasma PAI-1 569) , and an observational study in middle-aged women reported that PAI-1 was associated with the development of coronary artery calcification 570) . Elevated PAI-1 antigen, but not PAI-1 activity, was also reported to be associated with cardiovascular events 571) .
2.Disease Concept and Diagnostic Criteria for Metabolic Syndrome
Metabolic syndrome is a condition that has a high risk of cardiovascular disease.
Visceral fat accumulation is an established risk of atherosclerosis, and the importance of waist circumference measurement is also a global consensus 572 , 573) . Japan’s dietary habits have clearly changed since the 1970s 574) , and there is concern that cerebrovascular and CAD disorders may increase due to overnutrition and lack of physical activity. Conditions in which risk factors such as impaired glucose tolerance, lipid abnormalities, and elevated blood pressure resulting from a disordered lifestyle are of particular importance. Metabolic syndrome is an ASCVD-prone condition based on visceral fat accumulation and insulin resistance 575 , 576) , and abnormal secretion of bioactive molecules (adipocytokines) is believed to be important in the incidence of the disease.
2.1 Importance of Risk Factor Accumulation
In a survey 577 , 578) by the Ministry of Labor Research Group for Comprehensive Countermeasures against Work-Related Diseases, the results of health examinations of people with CAD incidence were analyzed up to 10 years ago, and it was confirmed that, compared to persons without CAD, those with incidence of CAD had mild but significantly higher body mass index (BMI), blood pressure, fasting blood glucose, and serum lipids, and that this had been persistent for 10 years. The NIPPON DATA80 epidemiological survey also shows that the relative risk of death from CAD and stroke increases with the number of risk factors possessed 577 - 580) ( Fig.2 ) .
Fig.2.Relationship between the number of concurrent risk factors and death due to CAD and stroke.
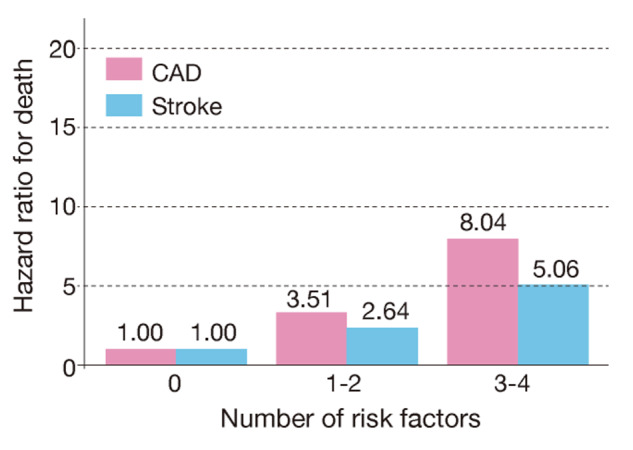
(NIPPON DATA80: 1980-1994) (Nakamura Y et al : Circ J 70: 960-964, 2006)
Therefore, in the incidence of CAD in Japan, a condition with accumulation of risk factors is important, even if the degree of each risk factor is mild. In addition, the odds ratio of having overlapping risk factors in middle-aged and older men in Japan is significantly higher for visceral fat obesity 581) , and to consider obesity as a disease with high risk of health problems that should be treated, the Japan Society for the Study of Obesity proposed the diagnostic criteria for “obesity disease” 582 , 583) .
Obesity is a medical condition that requires weight loss treatment. In Japan, the body mass index (BMI) (weight (kg)/[height (m)]2) is used to determine obesity, and BMI=22, which has the lowest rate of disease complications, is defined as standard weight, while BMI≥ 25 is defined obese 584) . However, obesity is not immediately classified as a disease. Obesity is diagnosed when there are complications of health problems that are caused by or related to obesity and require weight loss, or when there is accumulation of visceral fat that is likely to be accompanied by health problems 585) . The screening criteria for visceral fat accumulation are waist circumference at umbilical height of 85 cm or more for men and 90 cm or more for women 586) , and if visceral fat accumulation is suspected, the area of visceral fat at umbilical height is measured using abdominal CT scan, and visceral tissue of adipose obesity is diagnosed when the area is 100 cm2 or more. Visceral fat accumulation and the clustering of risk factors for arteriosclerosis are common factors in metabolic syndrome, and reduction of visceral fat is expected to improve not only lipid abnormalities, but also hypertension and impaired glucose tolerance 575) .
In fact, approximately half of patients with CAD have visceral fat accumulation 587) , and a cohort study of Japanese Americans has shown that visceral fat accumulation, hypertension, and hyperglycemia are important risk factors for the incidence of CAD 588) .
Overseas epidemiological studies 589 - 592) and meta-analyses 593) , the CIRCS Study 85) , the Tanno and Sobetsu Study 594) , the Hisayama Study 580) and the recently published Japanese integrated prospective cohort study using waist circumference criteria 595) have also shown that accumulation of risk factors, such as metabolic syndrome, increases the risk of atherosclerotic disease. In secondary prevention, cardiovascular events have been reported to occur at a higher rate in the presence of metabolic syndrome, and they are considered an important risk factor for CAD 575) .
2.2 Diagnostic Criteria for Metabolic Syndrome
In 2005, diagnostic criteria for metabolic syndrome, which are based on the accumulation of visceral fat and is complicated by lipid abnormalities, high blood pressure, and hyperglycemia, were established ( Table 3 ) 575) . This diagnostic criterion substitutes visceral fat accumulation for waist circumference and in addition defines the presence of two or more risk factors 575) . The International Diabetes Federation has also published similar diagnostic criteria 576) . The European and American scientific societies later issued a joint declaration proposing that an individual with three of the five risk factors - visceral obesity, hypertriglyceridemia, hypo-HDL cholesterolemia, high BP reading and high glucose level - can be diagnosed as metabolic syndrome 596) , and visceral fat accumulation was not considered necessary condition in the criteria.
Table 3. Diagnostic criteria for metabolic syndrome in Japan.
| Visceral fat accumulation |
(Evaluation Committee on Diagnostic Criteria for Meabolic Syndrome: Internal Medicine, 2005; 94: 794-809, in Japanese) |
|
|---|---|---|
| Waist circumference | Men ≥ 85 cm Women ≥ 90 cm | |
| (The values for both men and women correspond to visceral fat ≥ 100 cm2). | ||
| Two or more of the items mentioned below in addition to the above | ||
|
Hypertriglyceridemia and/or Hypo-HDL cholesterolemia |
≥ 150 mg/dL <40 mg/dL for both men and women |
|
|
Systolic blood pressure and/or Diastolic blood pressure |
130 mmHg ≥ 85 nnHg |
|
| Fasting hyperglycemia | 110 mg/dL | |
The criteria for waist circumference in Japan were determined by the absolute visceral fat area (VFA) of 100 cm2. According to a recent large-scale cross-sectional study conducted in Japan on the visceral fat area and accumulated risk factors, the average number of cardiovascular risk factors (dyslipidemia, high blood pressure, and high blood glucose) was more than 1.0 at 100 cm2 for the visceral fat area in both men and women 597) . The Japanese waist circumference is based on scientific evidence. The rationale is different from the Western standards for waist circumference, which simply use waist circumference corresponding to each country’s definition for obesity. Japan’s diagnostic criteria include the accumulation of visceral fat as a necessary condition, with the goal of reducing risk factors through interventions to reduce visceral fat. Even in the absence of visceral fat accumulation, the accumulation of risk factors is a high risk 595) . Importantly, the intervention methods differ from those with visceral fat accumulation, although it is necessary to keep in mind not only waist circumference but also the accumulation of risk factors without visceral fat accumulation. The mandatory measurement of waist circumference in specified health check-ups and occupational health check-ups that began in FY2008 is an attempt to curb the incidence of diabetes and ASCVD by using the concept of metabolic syndrome.
2.3 Association of Hyper-LDL Cholesterolemia with Metabolic Syndrome
Hyper-LDL cholesterolemia is a major risk factor for atherosclerosis and the significance of its treatment has already been established. Since metabolic syndrome is a high-risk condition for CAD independent of hyper-LDL cholesterolemia, LDL-C is not included in its diagnostic criteria. However, when metabolic syndrome is combined with hyper-LDL cholesterolemia, the risk of CAD is higher, and interventions for both are needed.
Chapter 3.Comprehensive Risk Management for the Prevention of ASCVD
1.Absolute Risk of ASCVD and Lipid Management Targets
BQ16. Are there any evaluation methods to predict the onset and death of ASCVD in Japanese?
There are several evaluation methods for predicting the absolute risk of ASCVD in the Japanese population, and hypertension, diabetes, and smoking are used as predictive indicators of ASCVD. In addition to the above, LDL cholesterol, non-HDL cholesterol, and HDL cholesterol are also used as predictive indices in the evaluation methods for predicting the onset and death of ASCVD including atherothrombotic cerebral infarction. (Evidence level: E-1b)
Clinical guidelines in various countries for the prevention of ASCVD have adopted a lipid management approach based on absolute risk: the probability of developing or deaths from ASCVD. The 2018 U.S. ACC/AHA guideline 456) , a representative example of this approach, pooled five major U.S. cohort studies to create a Pooled Cohort Equation (PCE) that accounts for differences in ASCVD frequency by gender and race and calculated the 10-year absolute ASCVD risk was incorporated into the guideline flow. This PCE can estimate the absolute risk of developing cerebral cardiovascular disease, which includes fatal and non-fatal myocardial infarction and stroke combined, rather than just CAD as the Framingham score does. A similar process is employed in the European SCORE risk chart 598) , which can estimate the absolute risk of death from the same disease.
In Japan, the 2012 edition of the Guidelines for the Prevention of Atherosclerotic Diseases used the NIPPON DATA80 risk chart 286) to predict 10-year CAD mortality, and the 2017 edition used the Suita score 599) to assess absolute risk of developing CAD over 10 years. In developing the 2017 version of the guideline 600) , we conducted the systematic review (SR) with the BQ: “Are there any evaluation methods to predict the onset and death of ASCVD in Japanese?” As a result, nine references were selected, and the Suita score was finally adopted, considering whether the outcome grasps the onset of disease as well as death, or whether LDL-C can be appropriately evaluated as a medical guideline.
Based on this history, in the 2022 edition of the Guidelines for the Prevention of ASCVD, the SR was conducted with the same BQs for references that were published between January 1, 1990, and the end of December 2020. In this SR, outcomes were divided into (1) CAD, (2) cardiovascular disease, and (3) cerebral infarction.
As a result, five new references were selected in addition to those selected in the previous 2017 edition. The summary is shown in Table 4 .
Table 4. Absolute risk prediction tool for ASCVD based on cohort studies in Japan.
| Name of Cohort | Method of risk assessment | Period of risk assessment | Risk factors used for assessment | Outcome |
|---|---|---|---|---|
| Suita Study 287) | Scoring table | 10 years | Age, sex, SBP, DBP, HDL‐C, non‐HDL‐C, LDL‐C (Friedewald formula), diabetes, smoking, urinary protein, electrocardiogram (atrial strain, left ventricular hypertrophy) cerebral hemorrhage) (tool with or without electrocardiogram) | CVD incidence and death (ischemic heart disease, Stroke incl. |
| JALS Study 605) | Scoring table | 5, 10 years | Sex, age, BMI, HDL‐C, blood pressure stage (with or without antihypertensive medication), eGFR, non‐HDL‐C (only in the model of myocardial infarction), diabetes, smoking, presence of atrial fibrillation (a model without atrial fibrillation was also created) | MI incidence. MI+Stroke incidence incl. cerebral hemorrhage, all forms of CVD death |
| Hisayama Study 607) | Scoring table | 10 years | Sex, age, SBP, diabetes, HDL‐C, smoking, exercise | Stroke incl. cerebral hemorrhage, ischemic heart disease, CVD |
| EPOCH‐JAPAN 531) | Scoring table | 10 years | Sex, log‐age, smoking, diabetes, urine protein, log‐ SBP, log‐TC/HDLC, log‐age×log‐SBP, log‐ age× smoking | ischemic heart disease, stroke incl. cerebral hemorrhage, CVD death |
| Hisayama Study 606) | Scoring table | 10 years | Sex, age, SBP, diabetes, HDL‐C, LDL‐C (Friedewald formula), urine protein, smoking, exercise | arteriosclerotic disease (ischemic heart disease, atherothrombotic cerebral infarction) incidence |
1.1 Setting Absolute Risk
The Suita score adopted in the 2017 edition of the Guidelines for the Prevention of Atherosclerotic Diseases was able to estimate the absolute risk of CAD as an outcome. However, the absolute risk was based only on the incidence of CAD, including myocardial infarction, and did not include stroke as shown in the Western risk scores.
Strokes can be broadly classified into subarachnoid hemorrhage, intracerebral hemorrhage, and cerebral infarction. Cerebral infarction is further classified into penetrating artery infarction and cortical artery infarction in epidemiological studies, and the latter into thrombotic and embolic types 601) . Serum TC is negatively associated with cerebral hemorrhage, not associated with penetrating artery infarction, and positively associated with cortical artery infarction 602) . Among cerebral infarctions, atherothrombotic cerebral infarction, whose etiology is atherosclerosis, has been shown in a recent cohort study in Japan to be associated with elevated TC 67) , LDL-C 42) , and non-HDL-C 79) as risk factors. As with CAD, the importance of lipid management has been pointed out in the Japanese Guidelines for the Management of Stroke 401) . In addition, while cerebral hemorrhage and penetrating artery infarction (so-called lacunar infarction) have been the most common types of strokes in Japan, the proportion of cerebral infarction and atherothrombotic cerebral infarction has been increasing in recent years. In the Hisayama study, 22 of 70 (31.3%) men and 20 of 84 (23.8%) women who had cerebral infarction in the third cohort (1988) had atherothrombotic cerebral infarction 603) . In men, the rate increased from 19.4% in the first cohort (1961). On the other hand, in the JPHC study, there was a significant increase in the percentage of cerebral infarction among all strokes in the 2005-2009 cohort, and by stroke type, 61 (26.5%) men and 26 (22.4%) women out of 230 men and 116 women who had cerebral infarction, respectively, had atherothrombotic cerebral infarction 604) . Over the past 10 years, this proportion remained unchanged in men and showed an increasing trend in women. Considering the current situation in Japan, it was considered appropriate to include atherothrombotic cerebral infarction in addition to CAD as ASCVD. The definition of atherothrombotic cerebral infarction in this guideline is broader than the clinical definition of stroke and should be considered as cerebral infarction based on atherosclerosis.
Of the articles newly selected by the SR, only one had death as the outcome 531) , and the others had incidence or death as the endpoint. Regarding lipids, HDL-C was commonly included as a predictive index, but in addition, two articles 287 , 605) used non-HDL-C and two articles 287 , 606) used LDL-C. LDL-C was calculated by the Friedewald formula in all studies. There were differences in the outcome settings among the studies. Some studies used the single event of myocardial infarction, stroke, or death as the outcome, while others used the combined outcome of cardiovascular diseases. Many studies included hemorrhagic stroke and lacunar infarction in stroke 287 , 605 , 607) , but one of the two published scores from the Hisayama study excluded hemorrhagic stroke and only included atherothrombotic cerebral infarction as a combined outcome with CAD 606) .
These results suggest that an absolute risk score for defining lipid management criteria that contribute to the prevention of ASCVD should not be limited to CAD, but should include the incidence of ASCVD, including cerebrovascular disease, as an outcome, and should include LDL-C in conjunction with lipid management. The Suita study and the Hisayama study fall into this category, but the former uses a scoring method based on a combination of non-HDL-C of 170 mg/dL or more/less and LDL-C of 140 mg/dL or more/less, and the LDL-C setting is binary, whereas the Hisayama study 606) categorized as <120 mg/dL (reference), 120-139 mg/dL, 140-159 mg/dL, and ≥ 160 mg/dL, and the reference values were in line with the 2017 control targets. In the Hisayama study, furthermore, the outcome was ASCVD, namely CAD and atherothrombotic cerebral infarction, as mentioned above, which is most consistent with the intended outcome of this guideline. Judging from these factors, the scoring table 606) for the Hisayama study was judged to be the most appropriate.
1.2 Approaches to the Management of Dyslipidemia using Absolute Risk
The U.S. ACC/AHA guidelines 2018 do not set a control target for LDL-C but indicate how much LDL-C should be lowered by statin therapy according to absolute risk 456) . For example, in the case of moderate risk, where the absolute risk of ASCVD is between 7.5% and 20%, a 30% to 49% reduction in LDL-C is recommended. However, the Japanese guideline’s approach to dyslipidemia management using absolute risk was based on the approach presented in the 2017 version of the guideline, which states, “In actual clinical practice in Japan, it is preferable to have a management target from the perspective of patient adherence, and in fact, many practical clinicians use the management target as a guide for treatment. Therefore, the management target value should be maintained as before.”
For setting the grade of absolute risk, the Hisayama study set the probability of developing ASCVD (CAD and atherothrombotic cerebral infarction) within 10 years as less than 2% as low risk, 2% or more, but less than 10% as moderate risk, and more than 10% as high risk 606) , and we adopted those values in this guideline. The 2017 version of the Suita score was the absolute risk for developing CAD, whereas that of the Hisayama study was the absolute risk for ASCVD, which includes CAD and atherothrombotic cerebral infarction. Although absolute risk is affected by differences in the age structure of the study population and the method of ascertaining the outcome, the stratification based on this criterion was almost identical to the stratification in the 2017 version of this guideline.
The U.S. ACC/AHA 2018 defines high risk as an absolute risk of 20% or more for the development of ASCVD, and the ESC/EAS 2016 guidelines, which use death as the outcome, define high risk as 5% to 9% or more and advanced high risk as 10% or more. These criteria are not equally comparable because they are influenced by differences in the incidence and mortality of ASCVD and population-contributed risk ratios in different countries. However, it is unlikely that the situation of ASCVD in Japan has changed significantly since the publication of the 2017 version of this guideline, and we have decided to follow the previous settings.
1.3 Categorization according to ASCVD Risk
Fig.3 shows a flowchart using absolute risk based on the scores of the Hisayama study 606) . To screen for dyslipidemia, first check for a history of CAD, atherothrombotic cerebral infarction or other cerebral infarction with obvious atheroma* (*more than 50% stenosis of intracranial and extracranial arteries or aortic complex atheromatous lesions), and if “yes,” secondary prevention will be applied. In the case of secondary prevention, not only atherothrombotic cerebral infarction but also other types of cerebral infarction were included in the secondary prevention if there was obvious atheroma. In addition, patients with diabetes mellitus, chronic kidney disease (CKD) or peripheral arterial disease (PAD) are classified as high risk. In the absence of these factors, scores from the Hisayama study will be calculated for each age group and assigned to “low risk,” “moderate risk,” or “high risk” groups.
Fig.3.
Flowchart for setting lipid management targets from the viewpoint of ASCVD prevention
In addition, lipid-lowering therapy has been reported to be effective in reducing cardiovascular events in Japanese patients aged 75 years or older (see Chapter 7, “Older people”). However, because the absolute risk increases with age, and there are large individual differences in complications and other factors, the results of the score should not be immediately linked to management targets for primary prevention in patients aged 80 years or older. Therefore, it is not included in the flow chart.
If diagnosed as FH or familial type III hyperlipidemia, you need to follow the appropriate medical policy without using this chart.
Fig.4 shows the prediction model for the incidence of ASCVD based on this guideline, which estimates absolute risk by summing the points for each risk factor as in the 2017 version. The six risk factors are gender (men and women), systolic blood pressure (5 categories), prediabetes (presence or absence), LDL-C (4 categories), HDL-C (3 categories), and smoking (presence or absence). Current smokers (habitual smokers who smoke at least one cigarette daily) are classified as smokers. Past smokers are classified as never smokers. A table mapping the sum of the points to absolute risk is prepared for each age group (10 years). These six items are currently included in the specific health checkups conducted in the community and in the regular health checkups at workplaces and were considered to be highly versatile.
Fig.4.
Prediction model for the onset of ASCVD using the Hisayama score
The Hisayama score used in this guideline differs slightly from the original Hisayama score. In the original score, proteinuria, an indicator of diabetes mellitus and CKD, was selected as a risk factor. In this guideline, however, the presence of diabetes mellitus and CKD is a high-risk condition, so it was excluded from the items that predict absolute risk for setting lipid management targets. However, since prediabetes increases the risk of ASCVD (see Chapter 2, 1.4, “Diabetes and Prediabetes”), one point was added to the score if prediabetes was present. Similarly, the original Hisayama score includes the presence or absence of exercise habits, but it is not used in this guideline because the information may not always be heard in daily practice. In addition, as mentioned above, the score for people aged 80 years or older is not used in this guideline.
The presence of antihypertensive medication was not included in the final model during the development of the Hisayama score. Other medications were not considered as in the Western guidelines. Since it has been reported that the absolute risk of stroke is generally higher in patients taking antihypertensive medication than in those not taking medication at the same blood pressure level 608) , we should consider the possibility that the absolute risk may be underestimated in patients taking antihypertensive medication, even though it was not included in the model. This argument was also made when the 2017 version of the Suita score was adopted and was considered a matter to be considered when assessing absolute risk for medication-takers.
It has long been pointed out that there are differences in the incidence of cerebral and cardiovascular diseases due to regional differences and social factors. Therefore, it is necessary to take such factors into account when assessing absolute risk. It has been reported that there are differences between urban and rural areas 110) , or that NIPPON DATA90 shows an increased risk of cardiovascular disease mortality among workers in small companies compared to workers in large companies and civil servants 609) . Overseas studies have also pointed out the association with socioeconomic factors and have shown that the actual absolute risk is higher than the predicted value in groups with high socioeconomic poverty 610) . The risk score in the 2022 version was created from the data of the Hisayama study and is unlikely to be representative of the national population at absolute risk. However, similar doubts about the representativeness of the underlying data were pointed out in the Suita score used in the 2017 version, and it should be noted that it is necessary to estimate the absolute risk this time based on the regional characteristics of the data. Ideally, the study should be conducted in a stratified randomly selected population of Japanese citizens, as in the 2012 version of NIPPON DATA80, but in that case, it is not possible to conduct an incidence study, and this is an issue for future study.

1.4 The Concept of Lifetime Risk
Absolute risk is naturally strongly influenced by age, and the 10-year probability of developing ASCVD is predicted to be small in the case of mature and middle-aged people. However, it is clear that if people age with risk factors, they will eventually be at a greater risk. Therefore, when considering prevention of ASCVD, it is necessary to present not only the absolute risk for 10 years in the future, but also the relative risk or lifetime risk perspective 611) . In 2018 The ACC/AHA guidelines recommend prioritizing lifetime risk over absolute risk for the age group of 20-39 years and using it as a communication tool between physicians and patients to improve risk factors. Lifetime risk has also been estimated in the EPOCH Japan 612) and Suita studies 48) , which are cohort studies of Japanese subjects. For example, in the Suita study, the lifetime risk of developing CAD at age 45 years with LDL-C of 160 mg/dL or higher was 47.2% in men and 10.2% in women 48) . On the other hand, the 10-year absolute risks for the same age group were 3.7% and 0.0%, respectively. Thus, in people in their 40s and 50s, the 10-year absolute risk and lifetime risk diverged greatly, and it was considered important from the perspective of preventing ASCVD to show the lifetime risk together with the absolute risk. However, at this point, no evidence has been published from Japan to score the prediction of lifetime risk, and this is an issue to be discussed toward the next guideline.
1.5 Targeted Management of Dyslipidemia from the Perspective of Prevention of ASCVD
The target values for the management of dyslipidemia by each category are shown in Table 5 .
Table 5.
Target values for lipid management by risk category
In primary prevention, lifestyle modification should be conducted for 3 to 6 months in principle, and its effects should be evaluated before considering the use of pharmacotherapy. However, if LDL-C of 180 mg/dL or higher persists in any of the control categories, drug therapy may be considered along with lifestyle modification. The LDL-C control targets were determined by the SR of observational and interventional studies: less than 120 mg/dL for the high-risk control group, and less than 160 mg/dL and 140 mg/dL for the low- and moderate-risk control groups, respectively, following the control targets in the 2017 guidelines. These control targets are effort targets. A meta-analysis of randomized controlled trials using statins showed that a 20% to 30% reduction in LDL-C was associated with a 30% reduction in the incidence of CAD 613 , 614) . Therefore, a 20% to 30% reduction in LDL-C may be a target value for low- and moderate-risk management categories. However, in the case of FH (primary prevention) and familial type III hyperlipidemia, other treatment should be considered; since treatment of FH patients is difficult and the risk of future CAD complications is extremely high, referral to a specialist is recommended (see Chapter 4, “Familial hypercholesterolemia” and Chapter 5, “Other primary dyslipidemias”).
In light of the evidence for the effectiveness of interventions for dyslipidemia, the lipid management targets by risk category were developed on the assumption that they would apply to adults younger than 80 years. Whether lipid management is appropriate for individuals younger than 40 years is left to the judgment of the attending physician. The risk chart for the Hisayama study used in this guideline is for persons aged 40 years and older, and absolute risk for persons younger than 40 years cannot be calculated. However, the absolute risk for the 10-year period under 40 years of age is very low, and therefore, motivation should be based on the aforementioned lifetime risk, taking into account the fact that it is calculated higher for younger individuals.
On the other hand, secondary prevention, in which there is already a history of CAD or atherothrombotic infarction (including other cerebral infarctions with obvious atheroma), is considered to require more aggressive treatment and is treated separately from primary prevention. In secondary prevention, LDL-C control targets should be set lower than in primary prevention. Large-scale clinical trials in Europe and the United States have shown that lowering pre-treatment LDL-C, even at average levels, is effective in preventing recurrence of CAD, lowering total mortality, and reducing stroke. Subsequent observational and clinical studies conducted in Japan showed that the lower the LDL-C level up to 100 mg/dL, the lower the frequency of recurrence. Therefore, the target of secondary prevention is to achieve LDL-C of less than 100 mg/dL by pharmacotherapy as well as lifestyle modification, but if it is difficult to achieve LDL-C of less than 100 mg/dL, it is possible to achieve LDL-C reduction of 50% or more. In addition, in secondary prevention, patients with ACS, FH, diabetes mellitus, complications of CAD and atherothrombotic cerebral infarction (other cerebral infarctions with obvious atheroma) as shown in Table 6 are considered to have higher risk and should be considered for more stringent lipid management with a target of LDL-C less than 70 mg/dL and non-HDL-C less than 100 mg/dL. (For details, please refer to “Chapter 3, 5.1 History of CAD (secondary prevention).
Table 6. Patient conditions that require more stringent management in secondary prevention.
|
・Acute coronary syndrome ・Familial hypercholesterolemia ・Diabetes mellitus ・Both coronary artery disease and atherothrombotic cerebral infarction (including other cerebral infarctions with obvious atheroma) |
|---|
Although control targets have been set for each item of dyslipidemia, the first priority should be to achieve the control target for LDL-C, and once that is achieved, if information is obtained, we will consider achieving the control target for non-HDL-C. As in the 2017 guideline, the control target for non-HDL-C was set at 30 mg/ dL to LDL-C as the target value for non-HDL-C control 81 , 82) as per the 2017 guidelines. In addition, if TG is 400 mg/dL or higher and blood is drawn at any time, non-HDL-C should be used as the control target value instead of LDL-C from the beginning. When non-HDL-C is used as a screening test in the general population, it should be noted that the difference between non-HDL-C and LDL-C is smaller than 30 mg/dL and is usually around 20 mg/dL 615 , 616) . On the other hand, as in the previous guideline, it is recommended that TG and HDL-C be managed with the goals of less than 150 mg/dL (fasting) and more than 40 mg/dL for primary and secondary prevention, respectively (less than 175 mg/dL for non-fasting). As for TG, even if the LDL-C control goal is achieved, high non-HDL-C is often associated with hypertriglyceridemia, and its management is important. In addition, there are few effective drugs for low HDL-C, and it has been reported that the risk of CAD is not high when only HDL-C is low and no other lipid abnormalities are present 92) . Therefore, lifestyle modification should be the basic treatment after controlling LDL-C, non-HDL-C, and TG.
2.Lifestyle Improvements
2.1 Smoking Cessation
•Smokers are encouraged to quit smoking for primary and secondary prevention of ASCVD.
•The avoidance of passive smoking is recommended for all individuals for primary and secondary prevention of ASCVD.
•The smoking cessation intervention is the treatment of nicotine dependence, and the use of smoking cessation aids is recommended to increase the success rate of smoking cessation.
Regardless of whether or not a person has a history of ASCVD, smoking cessation reduces the risk of disease progression, morbidity, and mortality, and this effect is independent of age or gender 166) . It is also known that the effects of smoking cessation on ASCVD appear relatively quickly after the start of smoking cessation and that the risk decreases with the length of smoking cessation, including in older people 161 , 617) . Large observational studies have shown that reducing the number of cigarettes smoked does not reduce the risk of cerebral and cardiovascular disease, and that once ex-smokers resume smoking, the risk increases again 618) . Reducing the number of cigarettes smoked or switching to low-nicotine, low-tar cigarettes will not reduce risk. Therefore, cessation of smoking is essential for primary and secondary prevention of ASCVD and should be recommended to all smokers, regardless of age. Passive smoking has also been shown to increase the risk of CAD and stroke 170 , 171) . Meta-analysis has shown that the implementation of passive smoking prevention laws overseas, which prohibit smoking in all indoor areas including restaurants, etc., reduces ACS and strokes 619) . Therefore, it is also important to instruct people to avoid passive smoking.
The first step is to check all patients for smoking history and passive smoking 284) . Encourage smokers to quit smoking and motivate those who do not wish to do so immediately. It is known that when physicians give their patients smoking cessation advice, the cessation rate increases significantly by 1.7 times compared to when they do not 620) . The essence of smoking is nicotine addiction. Therefore, when quitting smoking, as with other drug dependence, there is often the onset of withdrawal symptoms, making it difficult to quit smoking. In addition to counseling, the use of smoking cessation aids (nicotine patch, nicotine gum (OTC drug), varenicline) effective against nicotine dependence increases the success rate of smoking cessation 621 , 622) . In Japan, smoking cessation treatment for 12 weeks is covered by insurance if both the medical facility and the patient meet certain requirements 623) . New types of cigarettes are spreading (see Chapter 2, Table 2 , “Classification of New Types of Cigarettes”). Of these, smoking cessation of heated cigarettes is also covered by smoking cessation insurance treatment. The prescription of an application for smoking cessation that can be downloaded to a smartphone and an exhaled carbon monoxide analyzer as an add-on to varenicline has been shown to increase the success rate of smoking cessation 624) and has been covered by insurance since December 2020. The electronic cigarettes marketed in Japan do not contain nicotine and do not increase the success rate of smoking cessation 625) .
A recent meta-analysis showed a weight gain of 4-5 kg after 1 year of smoking cessation, with most of the gain occurring within the first 3 months of smoking cessation 626) . Although blood glucose and lipid levels may worsen during this period, it has been reported that insulin resistance improves 172) , HDL-C increases 627) , and oxidized LDL complexes decrease 628) , despite weight gain. Although weight gain can prevent initiation of smoking cessation and cause smoking again, two to four years of smoking cessation has been shown to outweigh the disadvantages of weight gain and reduce the risk of cardiovascular disease 629) . Therefore, the long-term benefits of smoking cessation are clear, and it is necessary to educate people about the benefits of smoking cessation, encourage smoking cessation, and support people to continue to do so.
2.2 Drinking
•Avoid heavy drinking to prevent ASCVD.
•Check the drinking status of the drinker.
Heavy drinkers can be instructed to abstain from or reduce alcohol consumption, even with high HDL-C levels. It is important that drinkers reduce their frequency of drinking and alcohol intake more for primary and secondary ASCVD. There is no need to encourage nondrinkers to drink.
It is important to determine the actual drinking status of the drinker, including the amount of alcohol consumed per occasion, whether the drinker has giving liver break days, heavy drinking, and frequency of drinking occasions. To begin with, all patients should be asked to identify their frequency and amount of alcohol consumption. The Alcohol Use Disorders Identification Test (AUDIT) ( Fig.5 ) is a screening test for problem drinkers developed by WHO 630) and used as a tool for early detection and intervention of drinking problems in many countries 631) . In Japan, it was translated more than 20 years ago and has been used in the field of medicine and health guidance. AUDIT consists of a total of 10 questions, and the total score for each item (up to 40 points) can be used to determine the degree of drinking problem. Another feature of AUDIT is the use of the “drink” unit to calculate the amount of alcohol consumed, which is 10 g of pure alcohol equivalent ( Table 7 ) . AUDIT classification points can also be determined according to the characteristics and objectives of group 632) . The “Standard Health Examination and Health Guidance Program (Revised Edition)” used for specific health guidance 633) defines problem drinkers with an AUDIT score of 8 to 14, while the “HAPPY Program”, a typical Japanese method of alcohol reduction guidance developed by the Hizen Psychiatric Center 634) , defines problem drinkers without lifestyle-related diseases with an AUDIT score of 10 to 19 as those who need guidance on reducing alcohol consumption. Those with a score higher than the above are considered to have suspected addiction and are used as a guide for consultation at a medical institution specializing in addiction.
Fig.5.
The Alcohol Use Disorders Identification Test (AUDIT)
Table 7. Equivalences between different alcoholic beverages, amount of alcohol and number of drinks.
| Number of Drinks | Beer Equivalent (ml) | ||
|---|---|---|---|
| Beer | 1 glass | 0.7 | 180 |
| Medium bottle | 2.0 | 500 | |
| Large bottle | 2.5 | 633 | |
| Regular can | 1.4 | 350 | |
| Long can | 2.0 | 500 | |
| Medium beer mug | 1.3 | 320 | |
| Sake (15%) | 180 ml | 2.2 | 540 |
| 30 ml | 0.4 | 90 | |
| Shochu (20%) | 180 ml | 2.9 | 720 |
| Shochu (25%) | 180 ml | 3.6 | 900 |
| Wine (12%) | Wine glass (120 ml) | 1.2 | 288 |
| Half bottle (375 ml) | 3.6 | 900 | |
| Full bottle (750 ml) | 7.2 | 1,800 | |
| Whisky (40%) | Single watered (30 ml with original) Double | 1.0 | 240 |
| watered (60 ml of the original) | 2.0 | 480 | |
| 1 bottle (720 ml) | 23.0 | 5,760 | |
| Umeshu (13%) | 180 ml | 1.9 | 486 |
| 30 ml | 0.3 | 78 |
1 drink = 10g of pure alcohol
Reprinted in translation from National Hospital Organization Kurihama Medical Center HP https://kurihama.hosp.go.jp/hospital/screening/pdf/ drink_img.pdf
2. 3 Management of Obesity and Metabolic Syndrome
For the management of obesity and metabolic syndrome, lifestyle modification is essential for the reduction of body weight and visceral fat.
To achieve and maintain an ideal body weight and optimal waist circumference are important targets for lifestyle modification. Visceral fat accumulation is an independent risk factor for atherosclerosis, and the importance of measuring waist circumference is recognized worldwide 572 , 573) . Obesity and metabolic syndrome promote atherosclerosis directly or indirectly via dyslipidemia, impaired glucose tolerance, hypertension and the dysregulated production of adipocytokines 635 - 638) . Therefore, it is important to achieve lifestyle modification through dietary management and exercise.
Treatment of Obesity and Metabolic Syndrome
The target body weight in the treatment of obese patients should not immediately be set as a BMI of <25. Rapid weight loss resulting from extensive calorie restriction may lead to rapid rebound weight gain 639) . Weight reduction by diet and exercise therapy is expected to provide relatively rapid improvement in moderate abnormalities of plasma lipids, glucose and blood pressure caused by obesity, even if the BMI is within the range of obesity 640) .
Even if medications are needed to treat coexisting diabetes, dyslipidemia, and hypertension, it is necessary that both medical staffs and patients realize the risk reduction through measuring body weight and assessing waist circumference. Recently, the effectiveness of specific health checkups based on the concept of metabolic syndrome has been successively reported 641 , 642) .
Accordingly, it is important to achieve ≥ 3% reduction body weight or waist circumference over 3 - 6 months and to review the patient’s accomplishments over time 583 , 589 , 643) .
2.4 Diet Therapy
[Total Energy]
FQ1. Is limiting total energy intake and maintaining an appropriate body weight effective in preventing ASCVD?
•In obese individuals, weight loss by limiting total energy intake and maintaining an appropriate body weight is recommended because serum lipids improve. (Level of evidence: 1, Recommendation level: A)
•In obese individuals, weight loss by limiting total energy intake and improving metabolic abnormalities, including serum lipid abnormalities, is recommended because it may prevent the incidence of ASCVD. (Level of evidence: Consensus, Level of recommendation: A)
In overweight or obese individuals, there is no direct evidence that reducing total energy intake alone reduces the incidence of ASCVD. However, cohort studies and their meta-analyses have shown that overweight or obese individuals with metabolic abnormalities, such as lipid abnormalities, as well as obese individuals without metabolic abnormalities, are at high risk of ASCVD incidence 644 - 647) . In a Japanese cohort study, those with a BMI of 27.0 kg/m2 or higher had a higher risk of death from CAD than those with a BMI of 23.0-24.9 kg/m2, while those with a BMI of less than 18.5 kg/m2 had a higher risk of death from total stroke and intracerebral hemorrhage than those with a BMI of 23.0-24.9 kg/m2, in men, and women were at higher risk of death from CAD, total stroke, ischemic stroke, and intracerebral hemorrhage 648) . In the UK cohort study mentioned above, the risk of developing CAD was also higher among those with a BMI of less than 18.5 kg/m2 who had one or more metabolic disorders (diabetes, hypertension, lipid abnormalities), and the risk of developing cerebrovascular disease was higher whether they had metabolic disorders or not 644) . In a cohort study (including Asians) of non-smokers without chronic disease, the lowest risk of total mortality was at a BMI of 20.0-25.0 kg/m2 649) .
A meta-analysis of RCTs with weight loss interventions found a significantly reduced risk of total mortality among those assigned to the intervention group 650) , and a meta-analysis of RCTs with physical activity and diet interventions found decreases in blood pressure, TC, LDL-C and TG and increases in HDL-C among those without abnormal glucose metabolism 651) . In a report from Japan, the rate of decrease in LDL-C, TG, blood pressure, items related to blood glucose and uric acid and the rate of increase in HDL-C were significantly greater in obese persons with a weight loss rate of 3% or more after one year of active support for lifestyle guidance through specific health guidance 583 , 652) . Therefore, lifestyle modification, including weight loss, is effective in improving risk factors, including serum lipids, and may reduce the incidence of ASCVD.
Although there is no clear evidence to set total energy intake to improve serum lipids, the target weight is calculated from the following formula, taking into account that the BMI with the lowest total mortality varies with age and has a certain range 653) and the definition of obesity 583) . 18 to 49 years: [height (m)]2 x 18.5 to 24.9 kg/m2, 50 to 64 years: [height (m)] 2 x 20.0 to 24.9 kg/m2, 65 to 74 years: [height (m)] 2 x 21.5 to 24.9 kg/m2, 75 years and older: [height (m)] 2 x 21.5 to 24.9 kg/m2. Optimize total energy intake based on target body weight and daily activity. Total energy intake (kcal/day)=target body weight (kg) x physical activity (25-30 for light exertion, 30-35 for normal exertion, 35- for heavy exertion 583 , 654) . In older people, the decision should be made based on the assessment of current weight, frailty (see Chapter 7, 2. “Frailty and sarcopenia”), decline in (basic) ADL, comorbidities, body composition, reduction in height and feeding and metabolic status 653 , 654) .
[Fat Energy Ratio]
FQ2. For the prevention of ASCVD incidence, is it recommended to maintain an adequate fat energy ratio for the Japanese under an appropriate total energy intake?
•It is recommended to restrict fat-energy ratios with appropriate total energy intake for the purpose of lowering LDL cholesterol. (Level of evidence: 1, Level of recommendation: A)
•It is recommended to modify and restrict lipid intake for obese individuals in addition to weight loss under an appropriate total energy intake, as well as for non-obese individuals, as it improves serum lipids and may reduce the incidence of ASCVD. (Level of evidence: Consensus, Level of recommendation: A)
To date, there is no direct evidence that different fat-energy ratios have an inhibitory effect on the incidence of ASCVD. However, in terms of the energy intake ratio of protein, fat and carbohydrates, a meta-analysis of cohort studies found that a carbohydrate energy ratio of 50-55% had the lowest risk of total mortality, low or high carbohydrate diets increased total mortality risk, and even low carbohydrate diets with high animal fat intake increased total mortality risk, while high vegetable fat intake decreases total mortality risk 655) .
For serum lipids, many comparative studies have been conducted in which the effect of weight loss was examined as the primary endpoint and cardiovascular disease risk items, including blood lipid levels as secondary endpoints in obese subjects with a BMI of 25 kg/m2 or greater, under restriction of total energy intake, with different fat energy ratios [especially low-fat diet intervention and low-carbohydrate diet intervention]. A meta-analysis of RCTs comparing low-carbohydrate and low-fat diets found that low-carbohydrate diets significantly reduced weight loss and TG more than low-fat diets, while significantly increasing LDL-C and HDL-C 656) . A meta-analysis of RCTs comparing low-fat diets (<30%E) with high-fat diets (≥ 30%E) in obese subjects without metabolic abnormalities showed that the low-fat diet significantly decreased TC and LDL-C, increased TG, and decreased HDL-C 657) .
Therefore, limiting the fat energy ratio under adequate total energy intake may reduce LDL-C and prevent ASCVD by improving these risk factors. A meta-analysis summarizing the RCTs of diets that modify or reduce lipid intake did not show a significant effect on the risk of total or cardiovascular mortality, but a 14% reduction in the risk of cardiovascular disease incidence 658) . However, in the same meta-analysis, reducing lipid intake alone did not significantly reduce these risks.
Many sources of animal or vegetable fat are also protein sources. The JPHC study, a cohort study in Japan, examined the association between animal and vegetable protein intake and the risk of cardiovascular disease and found that vegetable protein intake was associated with a lower risk of all-cause, cardiac, and cerebral disease mortality, while animal protein intake was not significantly associated. Using the substitution analysis method, a 34% reduction in the risk of all-cause mortality and a 42% reduction in the risk of cardiovascular disease mortality was found by replacing 3% of total energy from animal meat protein (poultry, fish, and other than processed meat) with vegetable protein, and a 46% reduction in risk of all-cause mortality by replacing processed meat with vegetable protein. Furthermore, replacing 3% of total energy from animal meat protein with fish protein was found to reduce the risk of total mortality by 25% and cardiovascular disease mortality by 33%, and replacing 3% of total energy from processed meat with fish protein reduced the risk of total mortality by 39% 659) .
Furthermore, a meta-analysis of RCTs using a low-fat, high-protein diet with an intervention period of 12 months or more showed no significant differences in body weight, serum lipids, or blood glucose levels, although improvements in fasting blood insulin levels were expected compared to a low-fat, low-protein diet 660) .
Considering the current situation in Japan and its relationship to each state of the disease, it is consistent with the conventionally recommended fat energy ratio of 20-25% and carbohydrates of 50-60% with an appropriate total energy intake. In particular, limiting the fat energy ratio is effective in lowering hyper-LDL cholesterolemia, and a slightly lower carbohydrate energy ratio is recommended within the 50-60% setting in hypertriglyceridemia and hypo-HDL cholesterolemia, taking into account complications such as obesity, diabetes, and hypertension. While considering the sources of protein intake, excessive consumption of meat and processed meats should be avoided, and fish and vegetable fats should be consumed.
*Because many studies in the West consider a low-fat diet to be one with less than 25% or 30% fat, the low-fat diet described here is defined as a dietary pattern with a fat energy ratio of less than 30% of total energy intake. It should be noted that this diet differs from the recommended 20-25% fat energy ratio in Japan and from the fat-restricted diet for hyperchylomicronemia.
[Fatty Acids: Saturated Fatty Acids]
FQ3. For the prevention of ASCVD incidence, is it recommended to reduce saturated fatty acids or replace saturated fatty acid intake with other unsaturated fatty acids (monounsaturated and polyunsaturated fatty acids) with an appropriate total energy intake?
•Reducing saturated fatty acids or replacing saturated fatty acids with polyunsaturated fatty acids with adequate total energy intake is effective in improving serum lipids and is recommended for preventing the incidence of CAD incidence. (Level of evidence: 1+, Level of recommendation: A)
•Under appropriate total energy intake, replacement of saturated fatty acids with monounsaturated fatty acids is recommended to improve serum lipids. (Level of evidence: 1, Level of recommendation: A)
Cohort studies and their meta-analyses have reported both positive and negative findings that saturated fatty acid (SFA) intake is associated with total mortality and cardiovascular disease mortality and incidence 661 - 669) . However, a meta-analysis of RCTs with SFA intake restriction for more than 2 years of intervention did not show a significant reduction in the risk of total mortality and cardiovascular disease mortality, but a 17% reduction in the risk of cardiovascular disease incidence 670) . Replacement of SFA with polyunsaturated fatty acids (PUFA) reduced the risk of cardiovascular disease incidence by 21%, while the effect of replacement with monounsaturated fatty acids (MUFA) was unclear 670) . Other meta-analysis of RCTs have also found a reduction in CAD events by replacing SFA with PUFA 671) . The effect of replacing SFA with protein is unclear 670) .
For meat, the most common source of SFA intake among Japanese, a meta-analysis of cohort studies showed that the total mortality risk increased almost linearly with increasing intake of animal meat and processed meat 672) . The high-intake group of animal and processed meat also showed a non-linear but volume-dependent increase in the risk of CAD, stroke, and heart failure compared to the low-intake group 673) . Although consumption of up to 100 g/day of animal, poultry, and processed meat was not associated with death from ischemic heart disease, stroke death, or all cardiovascular disease death in the Japanese population 674) , in a cohort study of diabetic patients, the risk of cardiovascular disease incidence was approximately three times higher in the group that consumed more than 20 g/day of animal, poultry, and processed meat than in the group that consumed less than 20 g/day 675) .
In an analysis with stroke, the low intake of animal fat and animal protein increased the risk of intracerebral hemorrhage in cohort studies of US women and Japanese 676 , 677) . In Japanese cohort studies and their meta-analyses, SFA intake was negatively associated with the risk of all stroke deaths, intracerebral hemorrhage death or incidence, ischemic stroke death or incidence, and positively associated with the risk of myocardial infarction 678 - 680) .
In terms of the relationship with serum lipids, NIPPON DATA90 showed a positive association between SFA intake and TC and LDL-C 681) . RCTs and meta-analyses of RCTs with restriction of SFA intake found that the intervention reduced TC and LDL-C, but the effects on HDL-C and TG were not significant 670 , 682 - 688) . In the meta-analysis of the RCTs described above, the most significant association with reduced cardiovascular disease was a decrease in TC, which was strongly related to a decrease in SFA intake and more pronounced than the involvement of increased PUFA or MUFA intake 670) (See FQ5 and FQ6 for the effect of replacing SFA with PUFA or MUFA). In the meat-serum lipid relationship, an RCT was conducted in which subjects assigned to the high-SFA or low SFA group were fed three diets of red meat (beef, pork, and lamb), white meat (chicken and other poultry) and non-meat protein for 4 weeks each, and serum lipids were compared during these periods. LDL-C was higher in red meat and white meat, and there was no difference between red and white meat. LDL-C was higher in the high SFA group than in the low SFA group regardless of these foods 689) . Furthermore, in a 12-week RCT in which beef containing the same amount of protein as plant-derived protein was consumed, LDL-C and HDL-C increased significantly in the beef group, with changes in the SFA / fiber ratio contributing significantly to LDL-C and cholesterol/fiber to HDL-C 690) . Dairy products also contain high levels of SFA 691 , 692) , and cohort studies and meta-analyses, as well as RCTs, have shown that dairy intake increases TC or LDL-C 683 , 693 - 698) . On the other hand, the consumption of low-fat milk, fat-free milk, or skimmed milk powder improved serum lipids 699 - 701) . Based on the above, reducing SFA or replacing SFA with PUFA in an appropriate total energy intake is effective in improving serum lipids and can be recommended for the prevention of the incidence of CAD. Replacement of SFA with MUFA can also improve serum lipids. On the other hand, extreme restriction of SFA intake may be associated with the incidence of intracerebral hemorrhage 676 - 680) . Although there is insufficient evidence to establish an appropriate intake of SFA, less than 7% of total energy intake, the conventional recommended intake for patients with dyslipidemia, is considered reasonable considering the current average intake of the Japanese population.
[Fatty Acids: n-3 Polyunsaturated Fatty Acids]
FQ4. For the prevention of ASCVD incidence, is it recommended to increase the intake of n-3 polyunsaturated fatty acids?
•It is recommended to increase fish oil intake among n-3 polyunsaturated fatty acids in order to reduce triglycerides. (Level of evidence: 1 +, Level of recommendation: A)
•It is suggested to increase the intake of fish oil in the diet, since it is expected to reduce the incidence of CAD (Level of evidence: 2, Level of recommendation: B)
RCTs of instructional interventions of fish cooking are scarce, but there is such a report of intervention in patients with secondary prevention of myocardial infarction 702) . Although there was a reduction in all-cause mortality at 2 years in the group that received the instruction compared to the group that did not, there were no significant difference in reinfarction or death from ischemia, and similar results were not seen in later intervention trials in patients with angina pectoris 702 , 703) . Dietary patterns with high fish intake (Japanese diet, Mediterranean diet) are discussed below. Meta-analyses of RCTs of fish oil preparations (eg capsules) that have since been conducted have shown no reduction in total mortality risk with n-3 polyunsaturated fatty acid (n-3PUFA) (fish oil, alpha linolenic acid) intake interventions (both high and low doses) 704 - 706) and controversial results in cardiovascular disease death, cardiovascular disease incidence, and risk of CAD incidence 704 - 708) . Even the significant reduction in risk of CAD incidence shown in several reports was 5-9% 706 , 708) . However, a significant inhibition of the incidence of CAD was observed in the high-risk group with hypertriglyceridemia or hyper-LDL cholesterolemia 709) .
Although the effectiveness of fish intake in the incidence of cardiovascular disease has not been consistent in Western cohort studies 710 - 718) , eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) was found to reduce the risk of total mortality and cardiovascular disease mortality in the United States 668 , 719) , a reduction in the risk of cardiovascular disease mortality in Singapore 720) , and EPA intake was found to reduce the risk of ischemic stroke in Denmark 721) . In the JPHC study, a cohort study of Japanese, the risk of incidence of nonfatal CAD was lower in the group with high fish and n-3PUFA intake 722) . In the JACC study and NIPPON DATA80, the risk of cardiovascular disease mortality was reduced in the group with high fish and n-3PUFA intake 723 , 724) . A meta-analysis of cohort studies found a decreased risk of incidence of CAD and a decreased risk of stroke 725) . Therefore, higher consumption of fish containing EPA and DHA may be expected to reduce the incidence of CAD.
Although we did not find any RCTs that examined the effects of an instructional intervention of fish cooking on serum lipids, a meta-analysis of RCTs in which fish oil was consumed in healthy and dyslipidemic subjects showed a decrease in TG in the fish oil intake group 706 , 726 - 728) and a suppressive effect on the increase in postprandial TG level was obtained in RCTs 729) . Thus, increasing fish oil intake is effective in lowering TG 730) . In this article, we suggest increasing fish oil under an appropriate total energy intake.
The association between alpha-linolenic acid intake and cardiovascular disease has not been consistent in cohort studies, with an increased risk of total mortality 719) , no association with ischemic stroke or peripheral arterial disease 731 , 732) , or a decreased risk of cardiovascular death 720) . Meta-analysis of cohort studies showed a decreased risk of the incidence of composite CAD events and fatal CAD 733) . However, a meta-analysis of RCTs did not show an inhibitory effect on incidence 705 , 706) . RCTs have also not shown an improvement in serum lipids 734) .
[Fatty Acids: n-6 Polyunsaturated Fatty Acids]
FQ5. For the prevention of ASCVD incidence, is it recommended to increase the intake of n-6 polyunsaturated fatty acids?
•To improve serum lipids, it is recommended to increase the intake of n-6 polyunsaturated fatty acids or replace saturated fatty acids with n-6 polyunsaturated fatty acids in an appropriate total energy intake. (Level of evidence: 1, Level of recommendation: A)
•It is suggested to replace saturated fatty acids with n-6 polyunsaturated fatty acids, especially linoleic acid, under an appropriate total energy intake, as it is expected to prevent ASCVD. (Level of evidence: 2, Level of recommendation: B)
Many meta-analyses of RCTs relating the intake of n-6 polyunsaturated fatty acids (n-6PUFA) have already been reported. An analysis of the effect of replacing SFA with PUFA showed a significant 21% reduction in the risk of developing cardiovascular disease 735) and a 10% reduction in the risk of developing CAD events when 5%E SFA was replaced with n-6PUFA 671) . However, in other RCTs, cohort studies, and meta-analyses, it was not associated with a reduction in the incidence of cardiovascular disease 736 - 739) . In a US cohort study of fatty acids, substitution analysis showed that substitution of carbohydrates with linoleic acid decreased the risk of total mortality and cardiovascular disease mortality, while substitution with arachidonic acid increased the risk 668 , 719) , and replacement of 2% SFA with linoleic acid was associated with an 8% reduction in risk of total mortality and a 6% reduction in the risk of cardiovascular mortality 719) . In a meta-analysis of cohort studies, the high linoleic acid intake group had a 15% lower risk of CAD events and a 21% lower risk of CAD death than the low intake group 740) . In summary, replacing SFA with n-6PUFA, especially linoleic acid, may prevent ASCVD, but the effect of increasing the intake of n-6PUFA is not yet clear.
The effect on serum lipids was demonstrated in an RCT in subjects at moderate risk of ASCVD, where the replacement of SFA at an energy intake ratio of 9.6%E with n-6PUFA significantly reduced TC and LDL-C 684) . In another RCT study, corn oil with n-6PUFA 19%E in dyslipidemic subjects decreased TC, LDL-C and TG compared to those receiving butter 687) . A meta-analysis of RCTs also showed a decrease in TC in the high n-6PUFA intake group 739) . In summary, increasing the intake of n-6PUFA without changing energy intake or replacing SFA with n-6PUFA is expected to improve serum lipids.
[Fatty Acids: Monounsaturated Fatty Acids]
FQ6. For the prevention of ASCVD incidence, is it recommended to increase the intake of monounsaturated fatty acids?
•To improve serum lipids, it is recommended to increase the intake of monounsaturated fatty acid or replace saturated fatty acids with monounsaturated fatty acid in an appropriate total energy intake. (Level of evidence: 1, Level of recommendation: A)
•Although the preventive effect of increasing the intake of monounsaturated fatty acids on the incidence of ASCVD is not clear, it is suggested to replace saturated fatty acids with monounsaturated fatty acids from plant foods under an appropriate total energy intake for the prevention of ASCVD. (Level of evidence: 2, Level of recommendation: B)
Monounsaturated fatty acids (MUFAs) are found in many foods, including fats and oils, meat, confectionery, milk, fish, and eggs. In a meta-analysis of RCTs, replacing SFA with MUFA had no effect on the risk of total mortality, incidence of cardiovascular disease, incidence of myocardial infarction, incidence of stroke, or CAD mortality 735) . A meta-analysis using substitution analysis in a cohort study also found no significant association of SFA replacement with MUFA with the risk of developing cardiovascular disease or cardiovascular death 663) , but another meta-analysis found that only increased olive oil intake was negatively associated with the risk of total mortality, cardiovascular death, cardiovascular disease incidence, and stroke 741) . A substitution analysis conducted in a U.S. cohort study found no significant association when carbohydrates were substituted for total MUFA intake, but an increased risk of total mortality when carbohydrates were substituted for MUFA derived from animal foods, and conversely a decreased risk when carbohydrates were substituted for MUFA derived from plant foods, and SFA replacement with MUFA from plant foods was associated with a decreased risk of total mortality and cardiovascular disease mortality 719) . Substitution analysis in another cohort study also found a reduction in the risk of CAD for MUFA from plant foods when substituted for SFA, refined grains, and trans fatty acids, but not for MUFA from animal foods 742) . Therefore, it is desirable to obtain MUFA from plant foods.
In RCTs that examined the effects on serum lipids, a high MUFA diet reduced TC, LDL-C, and HDL-C more than a high SFA diet in patients with dyslipidemia 684 , 743 , 744) . Meta-analysis showed a downward trend in TC and LDL-C, a significant decrease in TG and an increase in HDL-C when carbohydrates were replaced with MUFA, but the effect of lowering LDL-C was weaker than that of replacement with an equivalent amount of PUFA 745) . On the other hand, when MUFA was added (37.8%E) to the AHA STEP1 Diet (30.1%E fat energy ratio), there was no improvement in serum lipids 746) , and no significant differences were observed when comparing high SFA, high MUFA and high PUFA diets in healthy subjects around 38%E fat energy ratio 747) . Furthermore, MUFA intake above 12%E improved body weight, body fat mass, systolic and diastolic blood pressure, but did not have a significant effect on serum lipids compared to below 12%E 748) . In summary, increasing MUFA intake without changing energy intake has the potential to improve serum lipids, but excessive intake may eliminate this effect.
[Fatty Acids: Trans Fatty Acids]
FQ7. For the prevention of ASCVD incidence, is it recommended to limit trans fatty acids?
•To improve serum lipids, it is recommended to replace trans fatty acids with monounsaturated or polyunsaturated fatty acids. (Level of evidence: 1, Level of recommendation: A)
•To prevent CAD, it is recommended to reduce the intake of trans fatty acids. (Level of evidence: 2, recommendation: A)
Trans fatty acids can be naturally occurring (e.g., in beef, lamb, milk, and dairy products) or formed during industrial processing (hydrogenation) and refining (deodorization or high-temperature treatment) of fats and oils. Hydrogenated products include hard margarines, fat spreads, shortenings, and fried foods and confectionery made with these products. It is also found in salad oil, which is a refined vegetable oil. Although there is no consensus on whether naturally occurring trans fatty acids should be treated similarly to industrially produced ones 669 , 749 - 752) , trans fatty acid intake was associated with an increased risk of total and cardiovascular mortality in US cohort studies 668 , 719) . In Japanese, a cross-sectional study found that blood levels of elaidic acid, an industrially derived trans fatty acid, were higher in patients with metabolic syndrome and in young patients with CAD 753) . In Japanese patients with CAD, blood levels of elaidic acid were an independent risk factor for the appearance of unstable plaques 754) . Furthermore, in the Hisayama study, blood levels of elaidic acid were associated with the incidence of total dementia 755) . Therefore, cohorts and their meta-analyses have shown an increased risk of CAD and dementia 661 , 669 , 756 - 759) , but no significant relationship has been found for ischemic stroke 669) .
Trans fatty acids increase LDL-C 749 , 756 , 760 - 762) , increase Lp(a) 760 , 763 , 764) , and decrease HDL-C 749 , 750 , 763) , but there is no consistent view on the variation of TG 761 - 763) . However, a meta-analysis of RCTs in which vegetable oils containing trans fatty acids were replaced with other fats and oils showed a significant decrease in TC, LDL-C, and TG and an increase in HDL-C when MUFA or PUFA was substituted 759) . In a meta-analysis of cohort studies reported in the same article, substitution analysis showed a reduction in the risk of CAD calculated when trans fatty acids were replaced with SFA, MUFA, or PUFA 759) . In contrast, in a crossover intervention study in which stearic acid was replaced by vaccenic acid (which is abundant in naturally occurring products) or elaidic acid by approximately 3% each for 24 days, both increased TC and LDL-C compared to the control group, with vaccenic acid increasing TC, LDL-C and Lp(a) than elaidic acid 765) . The average intake of trans fatty acids by Japanese people is 0.92-0.96 g per person per day, or 0.44-0.47% of the total energy intake 766) , which is below the WHO target (<1% of the total energy intake) 767 , 768) . However, if the diet is unbalanced, such as eating too many fatty sweets, it should be noticed that the intake may be higher than the average. Therefore, it is recommended to reduce the intake of trans fatty acids for the prevention of CAD.
[Cholesterol]
FQ8. For the prevention of ASCVD incidence, is it recommended to limit cholesterol intake?
In patients with hyper-LDL cholesterolemia, restricting cholesterol intake to less than 200 mg / day lower LDL cholesterol and may prevent the development of atherosclerotic disease; therefore, restriction of cholesterol intake is recommended. (Level of evidence: 1, Level of recommendation: A)
In cohort studies in the late 1900s, such as the Framingham Study and the Seven Countries Study, the association between cholesterol intake and the risk of incident CAD or total mortality was not constant 661 , 769 - 771) . However, recently, a pooled analysis of U.S. cohort studies found that increased cholesterol or chicken egg intake was associated with an increased risk of developing cardiovascular disease and total mortality in a dose-dependent manner 772) . A meta-analysis of other major studies also reported a significant dose-dependent association between chicken egg intake and the development of cardiovascular disease 773) .
Regarding serum lipids, the Framingham study found significant positive associations between TC or LDL-C and SFA intake in women, but not with cholesterol intake 774) . However, in an RCT comparing a high cholesterol diet (600 mg/day) with a low cholesterol diet (200 mg/day), the high cholesterol diet significantly increased LDL-C compared to the low cholesterol diet 775) . Under similar conditions, the increase in LDL-C was greater with SFA than with PUFA 776) . In a meta-analysis of combined RCTs and non-RCTs, increased cholesterol intake increased TC, LDL-C, and HDL-C 777) . However, no further significant increase was observed above 900 mg/day 777) . In this report, in a study with cholesterol intake below 200 mg/day as the control group, LDL-C was significantly increased in the group with higher cholesterol intake compared to the control group, while in a study with cholesterol intake higher than 200 mg/day as the control group, the increase in LDL-C in the group with higher cholesterol intake was not significant 777) .
Other RCTs with restriction of cholesterol intake showed a significant decrease in TC 746) with the AHA Step 1 diet [fat energy ratio 30% (30%E), SFA 10%E, cholesterol <250 mg/day], significant reductions in TC, LDL-C and HDL-C in the AHA Step 1 diet with less than 300 mg cholesterol/day 778 , 779) and significant reductions in TC, LDL-C, and HDL-C 780) with Step 2 diet (fat <30%E, SFA <7%E, cholesterol <75 mg/1,000 kcal/day, lifestyle improvement). Another RCT (SFA 8%E, cholesterol <200 mg/day) also showed a decrease in LDL-C 781) . A recent meta-analysis of 55 RCTs also found that increased cholesterol intake increased LDL-C 782) .
The effect of cholesterol intake on serum lipids is complex and varies between individuals (hyper-responder, hypo-responder) 783 , 784) because foods containing cholesterol often also contain SFA, the absorption rate of cholesterol varies greatly between individuals, and cholesterol is synthesized throughout the body, with the liver regulating approximately 70% of serum lipoproteins while synthesis in the liver is only 10%. This has also been observed in intervention studies 775 , 785 - 789) . For example, chicken eggs are a rich in cholesterol, but the relationship between chicken egg intake and serum lipids has not been constant in RCTs of healthy subjects or dyslipidemic patients 786 - 788 , 790 - 799) . However, the meta-analysis shows that TC, LDL-C, and HDL-C increase with egg yolk intake 800) . In a meta-analysis of hyper and hypo-responder groups, chicken egg consumption significantly increased LDL-C in the hyper-responder group, but not in the hypo-responder group 801) . When limited to diabetic patients, cohort studies and their meta-analyses have shown an increased incidence of cardiovascular disease, especially the onset or death of CAD, in groups with high chicken egg intake 802 - 805) . It should be noted that the average daily intake for Japanese people (according to National Health and Nutrition Examination Survey in 2019, more than 20 years old) is 366 mg for men and 317 mg for women, which is higher than the 340 mg for men and 290 mg for women (described in the previous guideline according to National Health and Nutrition Examination Survey in 2015). On the basis of the above, it is recommended that patients with hyper-LDL cholesterolemia should have less than 200 mg/day of cholesterol and less than 7%E of saturated fatty acids to lower LDL-C. Then, the improvement of serum lipids may prevent the development of ASCVD. The Dietary Reference Intakes for Japanese (2020 edition) also states that they should be kept below 200 mg/day from the point of view of preventing the severity of dyslipidemia 653) . It is clear that LDL-C increases with increased cholesterol intake even in those who do not present hyper-LDL cholesterolemia, and although sufficient scientific evidence has not been obtained, it is desirable to keep LDL-C level low from the perspective of preventing ASCVD.
[Dietary Fiber]
FQ9. For the prevention of ASCVD incidence, is it recommended to increase dietary fiber intake?
•To improve serum lipids, it is recommended to increase dietary fiber intake. (Level of evidence: 1+, Level of recommendation: A)
•It is suggested to increase dietary fiber intake to reduce total mortality and prevent cardiovascular disease and stroke. Intake of whole grains, fruits and vegetables are also suggested to reduce total mortality and prevent cardiovascular disease. (Level of evidence: 2, recommendation: B)
Dietary fiber is obtained from foods such as vegetables, grains, seaweed, soybeans, mushrooms, and fruits, and is classified as soluble or insoluble. Its intake prolongs gastric retention time, promotes defecation, inhibits cholesterol absorption, and promotes bile acid synthesis 806 , 807) . Although no RCTs have examined the association of interventions in dietary fiber intake with total mortality or cardiovascular disease, in the JACC study, a Japanese cohort study, soluble, insoluble, and total dietary fiber intake were all negatively associated with the risk of cardiovascular disease mortality in men and women 808) . In the JPHC study, total dietary fiber intake was negatively associated with the risk of cerebral infarction or intracerebral hemorrhage in women 809) . Meta-analysis of cohort studies, including overseas studies, has also shown a reduction in the risk of total mortality 810 - 813) , cardiovascular death 811 - 814) , cardiovascular disease incidence 813 , 815) CAD incidence 813 , 815) , and stroke incidence 813 , 816 - 818) . Therefore, diet fiber intake will prevent total mortality, cardiovascular disease, and stroke.
Regarding the effects on serum lipids, many meta-analyses of RCTs using total and soluble dietary fiber have reported reductions in TC 813 , 819 - 821) , LDL-C 813 , 819 - 823) , and non-HDL-C 822 , 823) , with no effects on HDL-C and TG 819 - 821) . Therefore, dietary fiber intake is effective in improving serum lipids. The most pronounced effects have been observed at 25-29 g/day in preventing the severity of lifestyle-related diseases 653 , 813) . On the basis of the above, a daily intake of 25 g/day or more is generally recommended.
Regarding grains, whole grains have reduced the risk of all-cause mortality 824 - 827) , cardiovascular death 824 - 827) , incidence of CAD 824 , 828) , and incidence of cardiovascular disease 824 , 829) in cohort studies and meta-analyses, including international ones. However, by grain, brown or white rice intake was not significantly associated with the incidence of cardiovascular disease in a pooled analysis of US cohort studies 830) . A Japanese cohort study found no association with cardiovascular disease mortality in rice 831) or with a decrease in cardiovascular disease mortality with higher intake in men 832) . There are no large-scale studies on the effects of brown rice on the incidence of ASCVD, and its effects on serum lipids have not been consistent 833 , 834) . In potatoes, which are high in carbohydrates, potato consumption has not been associated with the incidence of cardiovascular disease risk 835 - 837) .
On the other hand, for serum lipids, the meta-analysis of RCTs showed that TC and LDL-C were significantly lower in whole grains, while HDL-C and TG did not change significantly 838) , and the intake of barley-derived β -glucan was associated with a lower TC, LDL-C and non-HDL-C 839) . Oats and their epidermis have been shown to decrease TC and LDL-C 840 , 841) . Thus, the consumption of barley and oats, which are rich in soluble fiber, improves serum lipids. Buckwheat consumption was also associated with a lower blood glucose, TC, and TG in a meta-analysis of cohort studies 842) . Although the dietary glycemic index and glycemic load affect postprandial blood glucose levels, their effects on total mortality, incidence of cardiovascular disease, and their risk factors are not constant and clear results have not been obtained 843) .
Regarding vegetables and fruits, a meta-analysis of cohort studies, mainly in Europe and the US, found that the consumption of vegetables or fruits, or both together, dose-dependently reduced total mortality, cardiovascular disease mortality and the incidence of CAD, the incidence of stroke, or the risk of developing type 2 diabetes 844 - 850) . These effects are reported to level off or show a J curve at approximately 300 g to 800 g/day or 2-5 servings/day, so be careful not to overdose 844 , 846 , 849 , 850) . Some Japanese cohort studies have also found similar results 851 , 852) and no association 853) . In addition to dietary fiber, the intake of fruits and vegetables can be expected to have an antihypertensive effect due to the high potassium content in these foods. A meta-analysis suggests that potassium intake of approximately 4,500-6,500 mg/day is effective in lowering blood pressure 854) . However, patients with renal dysfunction or those taking antihypertensive medications should consume them appropriately, taking care not to have hyperkalemia.
For serum lipids, neither vegetables nor fruits had an effect on LDL-C throughout the day, although breakfast vegetable intake lowered LDL-C in the EPIC study, a cohort study 855) . In Chinese postmenopausal women, there were fewer patients with high LDL-C in 4 servings/day or more of vegetables 856) . RCT studies using fruits and their components and their meta-analyses have found decreases in TC and LDL-C or increases in HDL-C 857 - 864) and have not been constant with respect to TG 857 - 866) . A meta-analysis of RCTs combining vegetables and fruits showed that TG was reduced by 3 servings/day or more 867) , but lipids did not improve in those with metabolic syndrome 868) , and other conclusions were inconsistent.
In conclusion, the consumption of fruits and vegetables is useful in preventing the incidence of ASCVD, but excessive consumption of fruits should be avoided due to the possibility of elevated TG and uric acid 869) . Furthermore, pickled vegetables should be noted for increased salt intake 870 , 871) . Regarding fruits, it is recommended to consume fresh fruits because canned fruits have been reported to increase total mortality and cardiovascular disease mortality 849 , 872) .
[Processed Foods Containing Fructose]
FQ10. For the prevention of the incidence of ASCVD, is it recommended to reduce the intake of processed foods containing fructose?
Excessive intake of processed foods containing fructose may increase the risk of ASCVD. Since a reduction in the intake of processed foods containing fructose is expected to lower triglycerides, it is recommended to reduce the intake of such foods. (Level of evidence: 2, Level of recommendation: A)
There is concern that high intake of processed foods containing fructose can increase the risk of CAD through excessive energy intake, obesity, elevated TG, exacerbated insulin resistance, and incidence of type 2 diabetes. Meta-analyses of cohort studies, mainly in Europe and the United States, have reported higher risk of total mortality, cardiovascular disease, CAD, stroke, weight gain, hypertension or type 2 diabetes with higher sugar beverage intake, although results are not always consistent among studies 873 - 880) .
In terms of effects on serum lipids, a meta-analysis (including RCTs and non-RCTs) of controlled feeding studies conducted overseas found that fructose had no effect on LDL-C, non-HDL-C HDL-C, or TG when compared to diets in which fructose was replaced with other carbohydrates of equal energy content, while an intervention trial in which fructose was added to a control diet (increasing total energy intake) increased TG 881) . An increase in postprandial TG has also been observed with additional intake 882) . However, analysis of doses including RCTs and non-RCTs did not show a significant increase in fasting TG at less than 100 g/day of fructose and postprandial TG at less than 50 g/day 883) . A meta-analysis of RCTs in which glucose was replaced with isoenergetic fructose did not show a change in maximum postprandial TG and no significant effect on fasting TG 884 , 885) . A meta-analysis of studies with processed products using fructose showed an increase in TG and a decrease in HDL-C, but no significant effects when some reports were excluded due to high heterogeneity 886) .
These results are not consistent with the effects of the consumption of processed foods containing fructose, but their excessive intake may influence ASCVD, and a reduction in TG can be expected by reducing their intake.
[Japanese Dietary Pattern]
FQ11. Is Japanese dietary pattern recommended for the prevention of ASCVD?
A low sodium Japanese diet with a low intake of fatty meat, animal fat (beef fat, lard, butter), and processed meats, combined with soybeans, fish, vegetables, seaweed, mushrooms, fruits, and unrefined grains, is recommended because this diet can improve serum lipids and may prevent ASCVD. (Level of evidence: Consensus, Level of recommendation: A)
The daily meals are prepared from a combination of various foods. Therefore, for their effects on disease incidence and risk factors, it is useful to evaluate the combination of foods consumed (dietary pattern), in addition to individual nutrients 887) .
Epidemiological studies conducted in the 1960s and 1970s, including the Seven Countries Study, drew attention to the extremely low CAD mortality rate in Japan compared to Northern Europe and the United States, and to the characteristic of the Japanese, which are significantly low in meat, fats, oils and dairy products and high in rice, soybeans* and fish 888) . Until the 1960s, the Japanese diet was biased to grains in energy intake, and not only white rice but also barley and low-polished rice were consumed 889) . A domestic cohort study that began in the 1990s showed that cardiovascular disease mortality was lower in dietary patterns with a high contribution of soybeans*, fish, vegetables, seaweed *, mushrooms and fruits 890 - 893) and that the risk of total mortality and CAD mortality was approximately 20% lower in Japanese diet-type dietary patterns with attention to salt reduction 894) . A recent cohort study of 92,969 subjects also showed that those with high rice, miso soup, seaweed*, pickles, green and yellow vegetables, fish, and green tea and low beef and pork intake had a 14% and 11% lower risk of total mortality and cardiovascular mortality, respectively. Among these, seaweed*, pickles, green and yellow vegetables, seafood, and green tea have been shown to be associated with reduced risk 895) . Using a similar approach, another cohort study of 14,764 subjects found a 9% reduction in total mortality risk 896) . However, dietary patterns with a high contribution of meat, butter, and high-fat dairy products had a higher risk of cardiovascular disease mortality 890) . A meta-analysis of several cohort studies conducted in foreign countries reported that consumption of the major food groups comprising the Japanese diet is beneficial for the prevention of ASCVD and that consumption of unrefined cereals reduces the risk of CAD 824) . Thus, if the Japanese dietary pattern is to eat less fatty meat, animal fat (beef fat, lard, butter), and processed meat, and to include soybeans, fish, vegetables, seaweed, mushrooms, fruits, and cereals and unrefined grains, the Japanese dietary pattern improves lipid metabolism 897 - 899) and may be useful in the prevention of ASCVD 900) . The Japan Atherosclerosis Society recommends “Thve Japan Diet” as an example of the Japanese dietary pattern 901) .
The high salt content of the Japanese diet has been an issue and the current salt intake of the Japanese population averages 10.0 g/day 902) . Excess salt intake increases blood pressure and promotes arteriosclerosis, so a goal of less than 6 g/day is recommended for hypertensive patients 903) . (* See “Other nutrients, other dietary patterns and their constituent foods”)
[Diet to Improve Risk Factors]
Dietary modification is the basis for treatment. It is important to assess and adjust the diet based on the Japanese diet with reduced salt according to the condition and lifestyle of the individual patient, and to evaluate the effects of the diet in a timely manner.
•Hyper-LDL Cholesterolemia and Diet
Manage total energy intake appropriately and reduce SFA, cholesterol, and trans fatty acids intake, which raise LDL-C. SFA should be replaced with MUFA or PUFA, SFA should be limited to less than 7% of energy intake, and cholesterol intake should be limited to less than 200 mg per day. Consume fiber actively. Specifically, limit fatty meat and animal fat (beef tallow, lard, butter), processed meat products, dairy, organs, and eggs. In addition, vegetables, including green and yellow vegetables, and soybeans and soy products should be consumed.
•Hypertriglyceridemia and Diet
Consider the total energy intake to maintain or aim for an appropriate body weight. Keep carbohydrate energy ratios slightly lower in the 50-60% setting and limit excessive alcohol intake. Excessive intake of fruits and processed foods containing fructose can increase TG. Increase n-3PUFA intake. Hyperchylomicronemia requires a more stringent lipid restriction. In other words, the fat energy ratio is limited to 15% or less, and medium-chain fatty acids are mainly used 904 , 905) . Combined exercise therapy is effective.
•Hypo-HDL Cholesterolemia and Diet
Consider the total energy intake to maintain or aim for an appropriate weight. Slightly lower carbohydrate to energy ratio and reduce trans fatty acids. Combined exercise therapy is effective.
•Metabolic Syndrome and Diet
Optimize total energy intake according to target body weight and daily activities to reduce visceral fat mass and improve qualitative abnormalities of adipocytes. Avoid rapid weight loss, with the goal of achieving at least a 3% reduction from current weight in 3 to 6 months. Carbohydrate energy should be taken 50-60% of total energy intake, be careful not to lack protein including essential amino acids for avoiding muscle mass reduction and consume more vitamins and minerals. The study leaves room for further consideration regarding carbohydrate intake for weight loss. Combined exercise therapy is effective for improvements in body weight and body fat, serum lipids, and blood pressure are observed.
•Hypertension and Diet 179)
Intensify salt reduction (less than 6 g / day) and consume more fruits and vegetables. Reduce the intake of saturated fatty acids and cholesterol and increase intake of polyunsaturated fatty acids and low-fat dairy products. Maintain appropriate weight and exercise. Limit excessive alcohol consumption, as it increases blood pressure.
•Diabetes and Diet
The total energy intake should be established in accordance with body weight, but the target weight varies according to age and medical condition and should be individualized. The desired BMI ranges from 22 to 25 and should be modified accordingly based on pathology, age, body composition, patient adherence, and changes in metabolic status. The energy intake ratio should be 50-60%E for carbohydrates, 20%E or less for protein, and the remainder should be fats. If fats exceed 25%E, consideration should be given to the composition of fatty acids, such as increasing polyunsaturated fatty acids. The aim is to consume at least 25 g/day of dietary fiber. Consume three meals regularly, chewing them well, and taking the time to eat them.
Other Nutrients, Other Dietary Patterns, and their Constituent Foods
•Vitamins and ASCVD
Adequate intake of vitamins D 906 - 919) , E 920 - 924) , and C 922 - 926) in the normal food intake range and maintaining adequate 25 (OH) vitamin D levels in the blood are desirable to reduce the risk of cardiovascular death or incidence and maintain adequate blood pressure. However, the effects of dietary supplements have been inconsistent, with some finding that they suppress or improve myocardial infarction, arterial stiffness, carotid atherosclerotic lesions, serum lipids and blood pressure 927 - 933) and others without effect 934 - 945) . Rather, an increased risk of stroke has been reported when vitamin D is combined with calcium 946) , heart failure with vitamin E alone 947) , a significantly increased risk of cardiovascular death in postmenopausal patients with diabetes with vitamin C alone 948) , or an increased risk of total mortality in postmenopausal patients with CAD with vitamin E and vitamin C combined intervention 949) , or a significantly increased risk of hemorrhagic stroke with the vitamin E intervention 950 , 951) . Therefore, supplement use is not recommended considering its effectiveness and safety. In addition to the above, care should also be taken to ensure that other supplements are taken appropriately, as even excessive intake of vitamin A or β-carotene, for example, is generally not recommended due to health problems 952) .
•Algae, Soybeans, and Soy Products (Foods Comprising the Japanese Diet)
Seaweed and soybeans are often consumed in the Japanese diet. Japanese dietary pattern that include seaweed have been reported to reduce the risk of total mortality and incidence and death (see “FQ11. Japanese Dietary Patterns”) 890 - 892 , 895 , 896) . Among cohort studies of Japanese subjects, the JPHC study found that the group that consumed seaweed almost daily had a 24% reduced risk of ischemic heart disease incidence in men and a 44% reduction in women compared to the group that consumed little or no seaweed, while finding no association between seaweed intake and risk of stroke 953) . However, the JACC study found a significant reduction in the risk of cardiovascular disease mortality in women in the group that consumed almost daily compared to the group that did not consume at all, but there was no significant reduction in the risk of CAD mortality in either sex 954) . In the CIRCS, the high-take group showed a significant reduction in the risk of total stroke and ischemic stroke in men compared to the low-take group, but not in women, and there was no significant reduction in the risk of incidence of CAD in either sex 955) . Note that seaweeds contain high concentrations of iodine and some have high arsenic content, so care should be taken to avoid excessive intake.
Meta-analyses of cohort studies in Japan and overseas have not yielded consistent results on the association between soy product intake and ASCVD 903 , 956 , 957) . However, Japanese cohort studies have reported a lower risk of stroke with a higher intake of soybeans and soy products 958 , 959) . Meta-analyses and systematic reviews of RCTs on ASCVD risk factors for soy, soy products, soy protein or isoflavones as foods have found a reduction in TC or LDL-C 960 - 962) , while others have not 963 , 964) Totally, consumption of soybeans and soy products may play a role in reducing CAD and stroke.
•Mediterranean Diet
The Lyon diet Heart Study, an RCT that examines secondary prevention of cardiovascular disease in a traditional Mediterranean diet rich in agricultural products such as fruits, vegetables, whole grains, beans, nuts and seafood with lipid-lowering content (with a fat energy ratio of approximately 30% and a MUFA energy ratio of 11-13%), found a reduction in the incidence of heart disease death plus nonfatal myocardial infarction or a composite endpoint of cardiovascular disease 965 , 966) . On the other hand, in an RCT (PREDIMED study) examining the primary prevention effect, only the Mediterranean diet (37% fat energy ratio, 19% MUFA) with nuts added (41% fat energy ratio, 22% MUFA) reduced the risk of stroke incidence compared to a control diet, while olive oil (42% fat energy ratio, 21% MUFA) or the diet added to nuts did not reduce the risk of myocardial infarction incidence 967) . A meta-analysis of RCTs is not clear on the effect of the Mediterranean diet on CAD 968 , 969) . This is presumably due to the diversity in the content of the Mediterranean diet, as well as the excessive lipid characteristics (especially MUFA) as described above. Regarding serum lipids, the Lyon Diet Heart study found no improvement, but another RCT 970) and a cohort study 971) reported a decrease in LDL-C. A study using the results of the 2012 National Health and Nutrition Survey in Japan (about 15,000 people) showed that TC and LDL-C were lower in those with higher Mediterranean diet scores in both men and women, and HDL-C was also lower in women 972) . Therefore, compared to the traditional Mediterranean diet, it has tended to be excessive in lipids (especially MUFA) in recent years and caution should be exercised in menu planning.
•DASH Diet (Dietary Approach to Stop Hypertension Diet)
The DASH diet (rich in vegetables, fruits, whole grains and low-fat dairy products and reduced in red meat, eggs and salt) was found to reduce the risk of cardiovascular disease incidence 973 , 974) and death 975) , and stroke incidence 973) in several cohort studies. Meta-analyses of cross-sectional studies and meta-analyses of RCTs found a reduction in the risk of cardiovascular disease incidence 976 - 978) and death 979 - 982) , as well as stroke incidence 976) and death 979 , 980) . Regarding risk factors, meta-analyses of cohort studies and meta-analyses of RCTs have reported a reduction effect on blood pressure 976 , 977 , 983 , 984) , TC and LDL-C 976 , 977 , 984) . In Japan, a small intervention study of a DASH diet tailored to the Japanese population reported reductions in serum lipids, BMI, systolic and diastolic blood pressure and fasting blood glucose 985 , 986) , but nothing has been done to verify the actual incidence of ASCVD. However, it is helpful in recommending salt reduction and potassium, calcium, and magnesium intake.
•Nuts (Foods that Comprise the Mediterranean and DASH Diets)
Nuts are known as one of the important ingredients that make up the Mediterranean diet, the DASH diet and the vegetarian diet 987) , including almonds, hazelnuts, walnuts, pistachios, cashews, macadamia nuts, and peanuts. Many observational studies have reported a negative association between nut intake and cardiovascular disease risk 988 - 996) . A meta-analysis of cohort studies that observed the association between nuts and cardiovascular disease and CAD reported a 15%, 23%, 18% and 24% reduction in the risk of cardiovascular disease incidence and death, CAD incidence and death, respectively, in those who consumed nuts 997) . On the other hand, the association with stroke is not clear 989 - 991 , 993 , 994 , 997 - 999) . Nut consumption has also been reported to reduce TC, LDL-C, or non-HDL-C 1000 - 1005) . Therefore, the consumption of nuts may be useful in preventing the incidence of ASCVD, but the evidence in Japan is insufficient ( Table 8 ) .
Table 8. Dietary Therapy for Prevention of ASCVD.
| 1. Maintain appropriate weight by avoiding overeating. |
|
•Total energy intake (kcal/day) is generally based on target body weight (kg)* x physical activity (25‐30 for light exertion, 30‐30 for normal 30‐35 exertion, 35‐ for heavy exertion). |
| 2. Do not consume large amounts of meat fat, animal fat, processed meats, or chicken eggs. |
| 3. Increase intake of fish and low‐fat dairy products |
| •Reduce the fat energy ratio to 20‐25%, the saturated fatty acid energy ratio to less than 7%, and cholesterol intake to less than 200 mg/ day |
| •Increase intake of n‐3 polyunsaturated fatty acids |
| •Avoid consumption of trans fatty acids |
| 4. Increase the intake of unrefined grains, vegetables including green and yellow vegetables, seaweed, soy and soy products, and nuts. |
| •Target carbohydrate energy ratio of 50‐60% and fiber intake of at least 25 g/day. |
| 5. Consume fruits with low sugar content appropriately and avoid large amounts of processed foods containing fructose. |
| 6. Limit alcohol to 25 g/day or less and avoid excessive consumption. |
| 7. Salt intake should be less than 6 g/day. |
*18 to 49 years: [height (m)]2 x 18.5 to 24.9 kg/m2, 50 to 64 years: [height (m)] 2 x 20.0 to 24.9 kg/m2, 65 to 74 years: [height (m)]2 x 21.5 to 24.9 kg/m2, and 75 years and older: [height (m)] 2x 21.5 to 24.9 kg/m2 21.5 to 24.9 kg/m2
2.5 Exercise Therapy
FQ12. Is aerobic exercise recommended for adults to improve serum lipids?
In adults, a total of at least 30 minutes of aerobic exercise per day is recommended at least 3 times per week (daily if possible) or at least 150 minutes of moderate intensity aerobic exercise per week, because it improves serum lipids. (Level of evidence: 1, Recommendation level: A)
Systematic reviews and meta-analyses of RCTs have reported that aerobic exercise therapy improves serum lipids 1006 - 1015) . Many reports show that HDL-C increases significantly in the aerobic exercise therapy group (walking, brisk walking, aquatic walking exercise, and supervised or unsupervised training) compared to the nonexercise group 1009 - 1016) , and also significantly decreases TC 1009 , 1011 , 1013 , 1014) , TC/HDL-C 1008 , 1010) , LDL-C 1008 - 1010 , 1014) , TG 1009 , 1011 , 1013) . A meta-analysis of 25 domestic and international RCTs comparing the effects of an exercise therapy group that performed at least 15 minutes of aerobic exercise for at least 8 weeks with a nonexercise group showed that HDL-C increased with exercise therapy, and the degree of increase was positively correlated with the duration of exercise, with HDL-C significantly increased with exercise length more than 120 minutes per week 1012) . A meta-analysis of four RCTs comparing the effects of moderate intensity aerobic exercise (3-5.9 METs, where METs is a unit of activity intensity that indicates the number of times the resting metabolic rate) over a period of 10 weeks to 24 months in Japan with a nonexercised group also showed that exercise increased HDL-C 1016) . A recently reported meta-analysis of 25 RCTs in healthy East Asian subjects also found that aerobic exercise decreased TC and TG and increased HDL-C 1013) . In addition to the above, LDL-C was also shown to be reduced only in studies in which exercise was performed for at least 150 minutes per week 1013) . However, it should be noted that there is variation in subject characteristics such as age, exercise intensity, duration, and serum lipid levels before intervention, as well as significant bias 1017) . Aerobic exercise is highly effective, as RCTs have shown improvement in serum lipids and a correlation between this effect and the volume (duration) of exercise. However, unlike pharmacotherapy, exercise interventions are in principle impossible to double-blind. Furthermore, since cholesterol is not used as an energy source, subjects assigned to exercise therapy can voluntarily improve other lifestyle behaviors (especially diet), which can contribute to the results. It should be noted that the improvement reported with exercise therapy generally tends to be overestimated.
In addition to improving serum lipids, aerobic exercise has been reported in meta-analyses to improve other cardiovascular disease risk factors, including blood pressure 1018) , and the effects are multifaceted. On the other hand, exercise therapy has the potential to cause musculoskeletal disorders 1019) and the risk of sudden death or cardiovascular accidents in people with preexisting or high-risk cardiovascular disease. Exercise is also contraindicated in subjects with extreme high levels of blood pressure or blood glucose, and diabetic patients with severe retinopathy, so it may be necessary to check with the attending physician regarding the appropriateness of exercise therapy. Based on these considerations, the type and volume of exercise should be individually planned.
Tables 9 show the exercise therapy guidelines. Teach the patient to increase physical activity in daily life and incorporate exercise into daily life as appropriate for the individual. Specifically, brisk walking, slow jogging, cycling, dancing, and aquatic exercise are recommended as aerobic exercise. Moderate intensity of exercise (3-5.9 METs; equivalent to walking at normal speed or faster) is most appropriate in terms of effectiveness and safety. Moderate intensity is the degree of modest increase in blood pressure during exercise, which can be performed for long periods of time without strain and without accumulation of lactic acid in the blood. The goal is to perform moderate intensity or higher aerobic exercise (3 METs or higher) for a total of at least 30 minutes per day, at least 3 times per week (daily if possible), or at least 150 minutes per week ( Fig.6 , 7 ) .
Table 9. Guidelines for Exercise Therapy.
| Type | Implement with an emphasis on aerobic exercises such as walking, brisk walking, swimming, aerobic dance, slow jogging, cycling, and bench-srepping | Scale | Perceived |
|---|---|---|---|
| Intensity | Aim for a moderate intensity* or above | 20 | |
| Frequency and duration | Aim to exercise for at least 30 min per day at least 3 days at a week | 19 | |
| Others | Walk or perform other, similar activities frequently and at times other than during exercise therapy and avoid a sedentary lifestyle as much as possible | 18 | Very, very hard |
|
*Moderare intensity means as follows: • An exercise intensity equivalent to walking at normal speed (= walking) • In rerms of METs (a unit that expresses the intensity of exercise as the equivalent number of times the resting metabolism), it is typically 3 METs (walking) but it differs according to individual physical fitness • The perceived exertion during exercise corresponds to 11-13 on the Borg scale, i.e., fairly light to somewhat hard) |
17 | Very hard | |
| 16 | |||
| 15 | Hard | ||
| 14 | |||
| 13 | Somewhat hard | ||
| 12 | |||
| 11 | Fairly light | ||
| 10 | |||
| 9 | Very light | ||
| 8 | |||
| 7 | Very, very light | ||
| 6 | |||
(Borg GA: Med Sci Spans Exerc. 1973; 5: 90-93)
Fig.6. Exercise Guidelines for Health Promotion 2006.
Translated and reprinted from Health Promotion Exercise Guidelines 2003, Ministry of Health, Labour and Welfare, Japan
Fig. 7. Physical Activity Guidelines for Health Promotion 2013.
FQ 13. Is resistance exercise recommended for adults to improve serum lipids?
In adults, resistance exercise improves serum lipids and is suggested. (Level of evidence: 1, Level of recommendation: B)
In resistance exercise RCTs, meta-analysis has reported significant reductions in TC 1015 , 1020 , 1021) , TC/HDL-C ratio 1020) , LDL-C 1015 , 1021) , non-HDL-C 1020) , and TG 1015 , 1020 , 1022) , and significant increases in HDL-C 1015) compared to groups without exercise, but RCTs in Japanese patients are extremely rare. A meta-analysis of RCTs examining the effects of resistance exercise in people with metabolic syndrome found a significant reduction in systolic blood pressure, but no significant differences in serum lipids or fasting blood glucose 1023) . The improvements reported in serum lipids varied markedly in the studies and the effects were relatively small compared to pharmacotherapy. Therefore, the evidence is not sufficient for the effect of resistance exercise. Resistance exercise is recommended in cases where resistance exercise is not contraindicated, as it has been reported not only to improve muscle strength but also to have the potential to bring various benefits to improve quality of life, such as improving blood glucose 1024) in diabetic patients and in combination with aerobic exercise 1025) .
Resistance exercise programs vary widely among studies, and there are few guidelines describing how they should be performed, but training consists of performing approximately several exercises of 1-5 sets (about 3 sets on average) per event, with a rest period of 1-2 minutes, at 50-85% (about 70% on average) of the maximum weight (weight that can be performed once but not twice in a row), with an average of about 12 repetitions per session, 2-3 times per week, continuously 1026) . The ICFSR has issued guidelines for exercise prescriptions for older adults 1027) .
FQ 14. Is exercise therapy recommended in addition to the diet for adults to improve serum lipids?
In adults, a combination of exercise therapy in addition to diet is more likely to improve serum lipids and is suggested. (Level of evidence: 1, Level of recommendation: B)
A meta-analysis of six RCT studies of diet, exercise, and a combination of both versus non-intervention groups has been reported 1028 , 1029) . After 10-104 weeks of intervention, diet and combined diet and exercise significantly reduced TC, LDL-C, and TG 1029) , and combined therapy significantly reduced non-HDL-C 1029) compared to the non-intervention group. The effect of exercise therapy was only seen in the reduction of TG 1028) . The reduction of TC, LDL-C and TG was slightly enhanced by combined diet/exercise therapy compared to diet therapy. Dietary therapy is effective in lowering TC, LDL-C, and TG, but a greater effect can be expected with combined diet and exercise therapy. Exercise therapy is effective in lowering TG, but combined diet and exercise therapy can have a greater effect. However, there are no RCTs on Japanese subjects, and future validation is needed.
BQ 17 Do aerobic exercise and physical activity reduce the incidence of ASCVD in adults?
In adults, aerobic exercise and increased physical activity can be expected to prevent ASCVD and are therefore recommended. (Level of evidence:1)
Numerous meta-analyses and systematic reviews of cohort studies have been reported evaluating the association between different amounts of physical activity, including aerobic exercise (e.g., walking) and activities of daily living, and the incidence of ASCVD and mortality from it have been reported 1030 - 1048) . Significantly less CAD disease 1031 , 1032 , 1035 , 1037 - 1039 , 1043 , 1044) , stroke 1030 , 1031 , 1039 , 1043 , 1044) , cardiovascular disease 1031 , 1033 , 1039 , 1040 , 1043 , 1045) , cardiovascular death 1034 , 1049) and total death 1034 , 1036 , 1040 - 1042 , 1046 - 1048) have been shown in the more physically active group compared to the less active group. Effects were also observed with low amounts 1041 - 1044) and low intensity 1046 - 1048) of physical activity. Cohort studies in Japanese subjects have also reported negative associations between physical activity and cardiovascular disease 1050) , stroke 1050 - 1053) , cardiovascular disease mortality 1054 - 1056) , cardiac disease mortality 1057) , cerebral and cardiovascular disease mortality 1057) , and total mortality 1056 - 1062) . Therefore, a habitual increase in physical activity, including aerobic exercise, is effective in preventing the incidence of ASCVD and improving life expectancy. In 2013, the Ministry of Health, Labour and Welfare (MHLW) established the Physical Activity Standards and Guidelines for Health Promotion (Active Guide) ( Fig.6 , 7 ) . In order to prevent lifestyle-related diseases, the first step is to start with “Plus Ten” (i.e., adding 10 minutes of exercise to one’s current lifestyle), with the ultimate goal of an active lifestyle of at least 60 minutes of moderate-intensity (3-5.9 METs) activity per day and 23 METs·hour/week (product of METs and time) for adults, and at least 40 minutes per day and 10 METs·hour/week for older people 1063) . For older people, the ultimate goal is to lead an active life of at least 40 minutes per day and 10 METs·hour/week 1063) .
BQ 18 Does resistance exercise prevent the incidence of ASCVD in adults?
In adults, resistance exercise has a preventive effect on ASCVD and is suggested. (Level of evidence:2)
A meta-analysis has been reported on the effect of resistance exercise on mortality 1064) . It was a meta-analysis of 1 RCT and 10 cohort studies conducted in North America (370,256 total subjects), with a mean observation period of 8.85 years for the cohort studies. The total mortality risk ratio was significantly lower in the resistance exercise group than in the non-exercise group by 21%. However, there was no dose-response relationship between resistance exercise frequency and reduced mortality. In combination with aerobic exercise, the relative risk of death was significantly lower at 0.60, regardless of the number of resistance exercises performed. Regarding the risk of cardiovascular disease mortality, there was a trend toward a lower risk in the resistance exercise group (risk ratio 0.83 [95% confidence interval 0.67-1.03]), with a significant reduction of 57% when combined with aerobic exercise. Two cohort studies have been reported in the United States examining the association of resistance exercise with CAD or cardiovascular disease 1065 , 1066) . In a cohort study of 44,452 American men, which examined the association with various types of exercise, with 12 years of observation every 2 years, the encouragement of resistance exercise (with weights) was associated with a 23% lower risk of CAD incidence 1065) . In a cohort study of 35,754 American women observed for 10.7 years, the risk ratio of cardiovascular disease incidence for resistance exercise was significantly lower at 0.83 (95% confidence interval 0.72-0.96) 1067) . Compared to aerobic exercise, which has shown results from cohort studies in various populations, the evaluation of the cardiovascular disease prevention benefits of resistance exercise is not as settled. On the other hand, resistance exercise is effective in improving physical fitness and muscle strength and risk factors for ASCVD in older people, whose muscle strength and muscle mass are declining 1067) . Start at 50% of maximum weight and work up to 70-80% intensity, with 6-7 sets of 2-3 sets of 8-10 repetitions per session, 3 days per week 1067) . Although resistance exercise and the combination of aerobic and resistance exercise are expected to prevent ASCVD, there are no reports in Japanese subjects, and the accumulation of evidence is expected in the future.
BQ 19 Does reducing sedentary time prevent the incidence of ASCVD in adults?
In adults, reducing sedentary time has a preventive effect on ASCVD and is suggested. (Level of evidence:2)
Increased sedentary behaviour, defined as “any waking behaviour that by an energy expenditure less than 1.5 METs in the sitting or reclining posture,” has been shown to be associated with worse various health outcomes independent of total physical activity. Several meta-analyses and systematic reviews of cohort studies have been reported that evaluated the relationship between television viewing duration and sedentary time and the incidence and mortality of diabetes and cardiovascular disease 1068 - 1075) . Longer sitting time is associated with a significantly higher incidence of diabetes 1068 , 1069 , 1072 , 1074 , 1075) , cardiovascular disease 1069 , 1071 , 1073 , 1075 , 1076) , CAD, stroke 1074) , cardiovascular death 1069 , 1071 , 1072) , and total mortality 1047 , 1068 - 1072) , with a dose-response relationship observed for the association 1047 , 1068 , 1070 , 1072 , 1073) . In a cohort study of Japanese subjects, the risk of cardiovascular disease mortality was also significantly higher among those who spent more time watching TV 1077) . Multiple meta-analyses have shown that interrupting sedentary behaviour without prolonged continuation improves blood glucose levels and insulin resistance 1078 , 1079) . Therefore, it can be expected that ASCVD can be prevented by reducing the total number of hours of sedentary behaviour, as well as by avoiding prolonged periods of sedentary behaviour and interrupting it frequently.
3. Health Counseling Based on Health Behavior Theory
FQ 15 For the purpose of improving obesity and dyslipidemia, should health counseling based on health behavior theory be recommended over general health counseling?
As health counseling aimed at improving obesity and dyslipidemia, several health counseling based on health behavior theory are recommended because they are more effective than general health counseling in improving lipid levels and promoting the visit to clinic. (Level of evidence: Consensus, Level of recommendation:A)
3. 1 Evidence of Foreign and Domestic Health Counseling on Obesity
Randomized controlled trials (RCT) abroad have shown that lifestyle interventions based on health behavior theory (the idea that shows what kind of factors are existing for increasing the likelihood that a person will engage in healthy behaviors) are effective for improving markers of obesity (weight, BMI or waist circumference), especially in obese individuals. In RCT in the UK, an instruction to improve their exercise and diet based on the Health Action Process Approach 1080) which improves anticipation of behavioral outcomes, perception of risk, and self-efficacy showed a significant weight loss effect after 4 months compared to educational brochures and general health counseling 1081) . In another RCT in the UK, as a weight loss instruction for obese participants (BMI 28 kg/m2 or higher), motivational interview (MI) 1082) significantly increased the amount of walking and significantly decreased weight after 6 months, compared to the information provided by leaflets 1083) . In the US study of a 6-month intervention on weight loss in outpatients with diastolic blood pressure (BP) 90-99 mmHg, Social Learning Theory (SLT) 1084) in combination with cognitive behavioral therapy and self-monitoring was effective for weight loss and maintaining weight loss 4 years after intervention 1085) . Furthermore, in patients with hypertension, dyslipidemia, type 2 diabetes, or obesity, training 3 times a week and small group health counseling for 3 months based on the stage-of-change model 1086) were more effective for a lower mean weight, reductions in waist circumference and diastolic BP compared to the primary care group 1087) . On the other hand, it is reported that there was no significant difference in effectiveness using the behavior change stage theory with and without the staged increase in instructional content 1088) .
In Japan, the specific health checkup including waist circumference measurement is combined with specific health guidance and has been shown to be effective in improving obesity and the risk of cerebral and cardiovascular disease in obese subjects with cardiovascular risk factors. The Amagasaki Visceral Fat Study 1089) is the basis for the diagnostic criteria for metabolic syndrome (MetS) in Japan and is a retrospective case-control study using Amagasaki city employees who underwent a health check-up from 2003 to 2005. In-person health counseling using the modified model of The Health Belief Model (HBM) 1090) and the original health check-up result form, “Where am I chart” (see Appendix 3) for three consecutive years, MetS coverage rate and waist circumference continuously decrease. The MetS ACTION-J study is a retrospective cohort study conducted by the National Database (NDB) using 1,019,688 participants in 2008 who had to receive specific health guidance and were not diabetic or taking medications for diabetes, dyslipidemia or hypertension 642) . Compared to non-users of specific health counseling, users had a significantly higher rate of reductions in waist circumference and BMI of 5% or more and loss of MetS at 3 years.
These studies suggest that the health behavior theory based health counseling is more effective in improving obesity than general health counseling because it improves weight and lipid levels and promotes health care-seeking behavior. In health counseling for obesity reduction, there are some important contents. The feeling that one is expected to do weight loss behavior (Subjective norm) and the feeling that one can do it (Perceived behavioral control, Self-efficacy), are important to arouse the willing to lose weight (Intention). To set the actionable goals (Setting Goal) and to promote observing the behavior of others, learning by imitation, motivation (social learning theory) and encouraging self-determination are suggested to be effective. In promoting instructional interventions based on these behavioral theories, face-to-face, motivational interviewing and group interventions have been reported to be effective.
3. 2 Evidence of Overseas and Domestic Health Counseling on Dyslipidemia
In overseas RCTs have shown the following effects of health counseling on dyslipidemia; health counseling using Self-Determine theory (SDT) 1091) and Motivational Interviewing (MI) for obese and hyper-LDL cholesterolemia patients in the UK significantly reduced TC compared to general counseling 1083) , health counseling using social cognitive theory (self-efficacy theory) 1092) for patients with hyper-LDL cholesterolemia in the US significantly reduced LDL-C compared to general health counseling 1093) , a group health counseling using self-efficacy theory for women with dyslipidemia in the US significantly reduced TC and TG levels compared to the general counceling 1094) . An intervention using the self-efficacy model, MI, and group work for Norwegian workers with hypertension or hyper-LDL cholesterolemia showed a significant decrease in diastolic BP and non-HDL-C levels compared to workers without these methods 1095) . A lifestyle intervention program for weight loss, salt reduction, alcohol reduction, and increased physical activity using social learning theory for patients treated for mild hypertension in the US improves BMI, BP and lipid levels only in population who maintains weight loss for 4 years 1088) .
Furthermore, in a non-RCT, the social learning theory showed a significant reduction in TC level in patients with hyper-LDL cholesterolemia in the US compared to those who didnot use the theoretical model 1096) . A 6-week study of small group-based education and discussion using an empowerment model 1097) for Korean patients with dyslipidemia along with obesity showed significant reductions in BP, blood glucose, and TC 1098) . In a New Zealand-Australian study, a strong 30-day intervention (Complete Health Improvement Program, CHIP) in obese patients showed an improvement in BP and lipid levels along with BMI, this program is effective for improving lipid levels greater in patients with dyslipidemia, increased risk of ASCVD 1099) .
Therefore, health counseling based on several theories of health behavior has been shown to be effective in improving dyslipidemia. There are some studies that show the effectiveness of group intervention methods 1083 , 1094 , 1100) . However, there is insufficient evidence whether the health counseling using behavioral change stages (The stage-of-change model) included in the multi-theoretical integrated model (transtheoretical model) is more effective than general counseling for improving dyslipidemia 1087 , 1088) . Behavioral counseling using cognitive-behavioral therapy 1101) via telephone or Internet did not show differences for improving lipid levels 1102) .
The J-HARP study 1103 , 1104) is a large-scale clinical trial to evaluate the effectiveness of health counseling for people at high risk of cardiovascular disease in Japan. This trial evaluated the interventional effect of a modified health belief model and face-to-face health counseling in terms of the rate of clinical visit compared to general health counseling in 15,710 untreated high-risk individuals. The intervention group whose LDL-C is 180 mg/dL or higher (men) had a significantly higher cumulative proportions of clinical visits than the control group throughout 12 months (multivariate adjusted hazard ratio, 1.65 [95% CI; 1.38-1.97]). The MetS ACTION-J study 642) also showed that, compared to non-users, users of specific health guidance improved their BP and lipid indices (increased HDL-C and decreased TG) as well as obesity markers.
Based on these reports, health counseling based on health behavior theory may improve some lipid levels compared to general health counseling. The work to increase self-efficacy by focusing on cognitive factors, such as learning by imitation and motivation through interrelationships, is effective in health counseling to improve dyslipidemia, and group interventions and motivational interviewing may be effective.
4 Drug Therapy
4.1 Drug Therapy
FQ 16. Can LDL cholesterol-lowering therapy aimed at control targets be recommended for the prevention of ASCVD in Japanese patients?
The usefulness of LDL cholesterol-lowering therapy for the prevention of Atherosclerotic Cardiovascular Disease (ASCVD), including CAD and atherothrombotic cerebral infarction, has been shown in Japanese patients, and we recommend LDL cholesterol management aiming at the control target level. (Level of evidence: Consensus, Level of recommendation: A)
A meta-analysis by the CTT (cholesterol treatment trialists’) Collaboration of large-scale clinical trials conducted overseas using statins showed that the incidence rate of cerebral and cardiovascular disease decreased in proportion to the amount of reduction in LDL-C levels regardless of the individual’s absolute risk, history of CAD and LDL-C levels prior to treatment initiation 331 - 333 , 614) .
In Japan, a 10-year follow-up of the J-LIT study, an observational study of patients taking statins, showed a positive correlation between post-treatment LDL-C levels and the risk of incidence of CAD, regardless of whether the patients had a history of CAD 1105) . In addition, the MEGAStudy 49) , a primary prevention study, has confirmed the efficacy of statin-based LDL-C lowering therapy for patients with hyper-LDL cholesterolemia in preventing cardiovascular events in Japanese patients. Furthermore, in the recent EMPATHY study of diabetes mellitus complicated by retinopathy, a high-risk primary prevention condition, a significant ASCVD prevention benefit was not observed by managing with a lower LDL-C target than conventional one 1106 , 1107) . EWTOPIA75 1108) reported that treatment with ezetimibe 10 mg/day significantly reduced combined cardiovascular events by 34% without an increase in adverse events compared to the dietary guidance group in older patients 75 years or older with hyper-LDL cholesterolemia, suggesting that in primary prevention, LDL-C control is “the lower, the better” in preventing cardiovascular events in high-risk patients.
In Japan, many coronary artery plaque regression studies have been reported using IVUS for secondary prevention of CAD, showing a significant correlation between LDL-C and the rate of change in plaque volume after treatment 1109 - 1113) . On the other hand, a meta-analysis of plaque regression studies conducted overseas reported a significant correlation between the rate of change in plaque volume and the incidence of cardiovascular events 1114) . In recent years, the effect of statin-lowering LDL-C therapy on reducing cardiovascular events has been reported in Japan. REAL-CAD 1115) showed a significant ASCVD suppression of 19% in the high-dose pitavastatin group compared to the low-dose pitavastatin group. The HIJ-PROPER study 341) of ACS did not statistically prove the benefit of active LDL-C lowering therapy with ezetimibe compared to pitavastatin alone, but showed a trend toward the reduction of ASCVD, suggesting that the management of LDL-C in secondary prevention is also “the lower, the better.
In Europe and the United States, where atherothrombotic cerebral infarction is common, a meta-analysis of LDL-C-lowering therapy showed a correlation between the amount of LDL-C reduction and the reduction in the risk of stroke incidence, regardless of whether the patient had previously had a stroke, suggesting that “the lower, the better 331 , 1116 - 1121) .
Epidemiological studies in Japan have not shown a significant correlation between serum cholesterol levels and the incidence of noncardiogenic cerebral infarction 44 , 47 , 59 , 65) , but LDL-C was reported to be a risk factor for atherothrombotic cerebral infarction by type of infarction 42) . In J-STARS, a secondary prevention trial of cerebral infarction, pravastatin-lowering LDL-C therapy was not effective in preventing recurrent stroke or TIA, but it significantly reduced the incidence of atherothrombotic cerebral infarction 348) . Other studies, such as a subanalysis of the MEGA Study 1122) and the secondary endpoint of EMPATHY 1106) , have demonstrated efficacy in the prevention of initial and recurrent cerebral infarction without an increased risk of hemorrhage. These results suggest that LDL-C lowering therapy may be useful for the prevention of ASCVD, including atherothrombotic cerebral infarction, which is a common risk, and may be ‘the lower, the better’. However, a meta-analysis of clinical trials examining the relationship between LDL-C lowering therapy and stroke suggests an increased risk of intracranial hemorrhage in patients receiving aggressive LDL-C lowering therapy for secondary prevention of CAD or high-dose statins 1116 , 1120 , 1121) . Therefore, appropriate LDL-C lowering therapy should be implemented after assessing bleeding risk as well.
FQ 17 Is drug therapy for hypertriglyceridemia recommended for the prevention of ASCVD?
•In high-risk patients with a history of CAD or cerebral infarction, diabetes mellitus, and other conditions in which LDL cholesterol is adequately controlled with statins, concomitant administration of ethyl icosapentate for hypertriglyceridemia is recommended for the prevention of cerebral and cardiovascular disease. (Level of evidence: 1+, recommendation: A)
•In dyslipidemia with hypertriglyceridemia and hypo-HDL cholesterolemia, triglyceride lowering therapy is recommended for the prevention of cerebral and cardiovascular disease with or without statin therapy. (Level of evidence: 1+, Level of recommendation: A)
The REDUCE-IT study, an RCT of 4 g/day of ethyl icosapentate (EPA) versus placebo in hypertriglyceridemia (150-499 mg/dL) in patients 45 ≤ years of age with CAD or 50 ≤ years of age with diabetes and one or more risk factors and appropriately controlled LDL-C <100 mg/dL with oral statin therapy, showed a significant 25% reduction in major vascular events in the EPA-treated group compared to the placebo-treated group 1123) . The study also confirmed the preventive effect against stroke, as well as fatal and nonfatal CAD. A JELIS subanalysis 1124) conducted in Japan reported that the combination of statins and EPA 1.8 g/day in dyslipidemia patients with TG ≥ 150 mg / dL and HDL-C <40 mg/dL showed a significant 53% reduction in coronary events compared to statins alone. In patients with hyper non-HDL cholesterolemia whose LDL-C levels achieved the control target, the combination was also reported to be 38% effective in preventing coronary events. However, it has been suggested that the effect of EPA on the suppression of events of cerebral and cardiovascular disease in both studies may be due to mechanisms other than its effect on TG-lowering. On the other hand, as in the REDUCE-IT study, the STRENGTH study 1125) conducted in high-risk patients with ASCVD or diabetes who had hypertriglyceridemia (180-500 mg/dL) and hypo-HDL cholesterolemia controlled with statins to less than 100 mg/dL of LDL-C level, the combination of high-dose n-3 polyunsaturated fatty acids (EPA and DHA; 4 g/day) could not be proven to prevent cerebral and cardiovascular events, and the study was stopped early.
Although cardiovascular event prevention trials using fibrates and nicotinic acid derivatives have been conducted for some time in dyslipidemic patients with hypertrigceridemia, most of those showing preventive effects against cerebral and cardiovascular events were conducted before statins were made mandatory as part of the study design. FIELD 1126) and ACCORD 1127) , which investigated the effect of fenofibrate on the prevention of ASCVD in patients with type 2 diabetes, did not demonstrate that fenofibrate prevented major cerebral and cardiovascular events. However, a post hoc analysis by FIELD 1128) and a sub-analysis by ACCORD 1127) of patients taking statins showed that fenofibrate significantly reduced cerebral and cardiovascular events in dyslipidemic patients with hypertriglyceridemia and hypo-HDL cholesterolemia. Meta-analyses 1129 , 1130) of clinical trials have shown primary and secondary preventive effects of fibrates on composite cerebral and cardiovascular events compared to placebo-control patients, but the effects are mainly due to inhibition of coronary events, not stroke or in patients taking statins. A meta-analysis of the effect of TG-lowering therapy with fibrates, nicotinic acid derivatives, and n-3 polyunsaturated fatty acids on cerebral and cardiovascular events in dyslipidemic patients with hypertriglyceridemia, or hypertriglyceridemia and hypo-HDL cholesterolemia, with or without statin medication, showed a significant combined cerebral and cardiovascular events prevention effect of 18% in hypertriglyceridemia patients and 29% in hypertriglyceridemia patients and hypo-HDL cholesterolemia patients 1131) . A large meta-analysis of 374,358 patients, combining 24 clinical trials using these TG-lowering agents and 25 clinical trials using statins, found a significant reduction in major vascular events of 16% for a 1 mmol/L reduction in TG and 21% for a 1 mmol/L reduction in non-HDL-C, equivalent to a 20% reduction in LDL-C events 1132) . Additionally, 1 g/day of EPA significantly reduced major vascular events by 7%, while no significant effect was observed for DHA.
FQ 18 Maximal tolerated dose strong statins are recommended as first choice in drug therapy for the secondary prevention of CAD?
In the secondary prevention of CAD, first-line pharmacotherapy with a maximum tolerated dose of strong statin is recommended from the beginning, regardless of the level of LDL cholesterol before starting therapy. Additionally, given the individual’s risk, intensified pharmacotherapy is recommended to achieve LDL cholesterol management goals. (Level of evidence: 1+, Level of recommendation: A)
In Japan, where it is difficult to conduct large-scale clinical trials, it is appropriate to examine the efficacy of LDL-C lowering therapy in ASCVD prevention by referring to the results of coronary plaque regression trials and large-scale overseas clinical trials. A meta-analysis of RCTs conducted overseas confirmed that LDL-C lowering therapy in the early stages of ACS is effective in reducing the incidence of ASCVD in the long term 1133) . In Japan, early and strict LDL-C lowering therapy with statins for ACS has been shown to be effective in inhibiting plaque progression 1109 , 1110 , 1113) and preventing long-term incidence of ASCVD 342 , 1134) by observing coronary artery plaques using IVUS.
A meta-analysis of statin-based RCTs conducted overseas confirmed that aggressive LDL-C lowering therapy using high-intensity statins with LDL-C lowering effect ≥ 50% is significantly more effective than treatment using low- to intermediate-intensity statins with LDL-C lowering effect <50%, regardless of LDL-C before starting therapy, with an additional 15% significant reduction in ASCVD has been demonstrated 331) . Furthermore, a meta-analysis 1135) of RCTs that examined the effect on the prevention of cerebral and cardiovascular events between two groups of different types and doses of statin, statin alone and in combination with the ezetimibe or PCSK9 inhibitor, showed that treatment targeting LDL-C <70 mg/dL is useful when LDL-C at the start of treatment is >155 mg/dL and that high-dose, high-intensity statins are also effective when LDL-C is <100 mg/dL at the start of treatment.
In Japan, the CREDO-Kyoto Registry Cohort-2, a registry-based observational study of patients with CAD, confirmed a significantly lower incidence of cerebral and cardiovascular events in the strong statin treatment group compared to the standard statin treatment group 1136) . In the recent REAL-CAD 1115) secondary prevention study conducted in Japan, a significant reduction in ASCVD was observed in the high-dose (4 mg/day) pitavastatin group compared to the low-dose (1 mg/day) group. The study also confirmed the benefit of aggressive LDL-C lowering therapy with high-dose strong statin regardless of the level of pretreatment LDL-C. Furthermore, EXPLORE-J 342) , a registered observational study of ACS, confirmed that in a population in which potent LDL-C lowering therapy was administered from the beginning, the incidence of ASCVD was suppressed from the beginning and the incidence of ASCVD in the second year was also low. These results suggest that in secondary prevention of CAD, a strong reduction of LDL-C with a maximum tolerated dose of strong statin from early onset may be useful in the prevention of ASCVD.
FQ 19 Is drug therapy targeting LDL cholesterol below 70 mg / dL for the secondary prevention of CAD associated with high-risk conditions recommended?
For the secondary prevention of CAD complicated by acute coronary syndrome, familial hypercholesterolemia, diabetes mellitus, or atherothrombotic cerebral infarction, it is recommended to administer pharmacotherapy with the goal of achieving LDL cholesterol less than 70 mg/dL. (Level of evidence: 1, Level of recommendation: A)
REAL-CAD 1115) , a secondary prevention trial in high-risk patients with stable CAD, 72% of whom had previous ACS and 40% of whom had diabetes mellitus, showed a significant reduction in ASCVD was observed in the pitavastatin high-dose (4 mg/day) group compared to the low-dose (1 mg/day) group, regardless of pretreatment LDL-C levels. The level of LDL-C in the high-dose pitavastatin group in year 3 was 76.6 mg/dL (low-dose group; 91.0 mg/dL), proving that more aggressive management of LDL-C is also useful for the prevention of ASCVD in Japan, in patients with CAD complicated by high-risk conditions. On the other hand, in HIJ-PROPER 341) , a “Treat to Target” study design conducted in patients with ACS, the incidence of ASCVD events was lower in the group treated with the combination of pitavastatin and ezetimibe (mean post-treatment LDL-C: 65.1 mg/dL), targeting LDL-C <70 mg/dL, compared with the group treated with statins alone (mean post-treatment LDL-C: 84.6 mg/dL), but this difference was not statistically significant. The lower than predicted incidence of events in the primary endpoint and the better LDL-C control in the usual care group may have contributed to the smaller difference in on-treatment LDL-C between the groups. However, even in Japanese patients with low absolute risk, LDL-C <70 mg / dL management showed an additive trend towards inhibiting ASCVD without an increase in adverse events, suggesting that LDL-C management in high-risk secondary prevention conditions may be ‘the lower, the better’.
EMPATHY 1106) , a primary prevention trial conducted in diabetic patients with retinopathy, examined the effect of reduction in ASCVD in a group of patients treated with statin monotherapy with active treatment targeting LDL-C less than 70 mg/dL and a group of patients treated with conventional therapy. Although no significant ASCVD preventive effect was observed in the active treatment group, a post hoc analysis limited to patients who achieved the LDL-C control target (107.1 mg/dL in the usual treatment group and 59.7 mg/dL in the active treatment group) confirmed a significant 57% ASCVD preventive effect in the active treatment group 1107) . Diabetes mellitus with retinopathy, a microvascular disorder, is a higher risk condition in primary prevention, but more aggressive LDL-C lowering therapy was found to be useful in preventing the incidence of ASCVD. Aggressive LDL-C lowering therapy targeting LDL-C <70 mg/dL may also be useful in diabetes mellitus complicated by CAD, a high-risk condition for secondary prevention.
According to the results of a meta-analysis of clinical trials conducted mainly overseas, the relationship between post-treatment LDL-C levels and the risk of ASCVD incidence is “the lower, the better” regardless of the dyslipidemia medications 1135 , 1137) . It has also been reported that in patients in secondary prevention of CAD, the benefit of active LDL-C lowering therapy in reducing ASCVD events is greater in patients with concomitant high-risk disease compared to those with low-risk disease 1138 , 1139) . There is no doubt that CAD complicated by FH or atherothrombotic cerebral infarction is a high-risk condition, but no clinical trials have examined the benefit of aggressive LDL-C lowering therapy in these patients. However, in light of the above results, it seems reasonable to implement LDL-C management with a goal of less than 70 mg/dL to prevent the incidence of ASCVD, as is the case with ACS and diabetes. However, in very older patients, patients with ACS without other high-risk complications and without major cardiovascular events for at least 2 years 1140) , and patients with atherothrombotic cerebral infarction with high risk of intracranial bleeding 1116 , 1119 - 1121) , LDL-C control target values and pharmacological therapy should be reviewed periodically.
FQ 20 Is LDL cholesterol lowering therapy with drugs other than statins recommended for the prevention of ASCVD?
The relationship between LDL-C decrement and cerebral and cardiovascular event prevention in non-statin drugs is similar to that of statins, and LDL-C lowering therapy aimed at control targets is recommended in the prevention of ASCVD, regardless of the drug type. (Level of evidence: 1+, Level of recommendation: A)
In a meta-analysis of RCTs 1137) , the effect of dietary therapy and medications that activate LDL receptor expression, such as anion exchange resins and ezetimibe, on the reduction of LDL-C by 1 mmol/L was 25%, which was equivalent to 23% of the effect of statins on the reduction of LDL-C on cerebral and cardiovascular events. In addition, the incidence of cerebral and cardiovascular events was lower with lower post-treatment LDL-C levels, regardless of the presence or absence of CAD complications. A meta-analysis 1132) combining 24 RCTs with fibrates, nicotinic acid derivatives, and n-3 polyunsaturated fatty acids and 25 RCTs with statins also found a significant 20% reduction in major vascular events with a 1 mmol/L reduction in LDL-C, regardless of drug type. Meta-analysis of RCTs of non-statin drug therapy for ischemic stroke also reported a reduction in ischemic stroke risk with LDL-C lowering therapy, regardless of the presence or absence of CAD complications 331 , 1116 - 1121) . These results indicate that LDL-C is “the lower, the better” in preventing the incidence of cerebral and cardiovascular events, and that appropriate management of LDL-C is important regardless of the drug therapy used.
The effect of LDL-C lowering therapy with statins in preventing cerebral and cardiovascular events is well established, and standard pharmacotherapy with statins as the first choice is recommended. However, if the patient is intolerant to statins or statins alone do not provide sufficient LDL-C lowering effect, a change to or combination with other dyslipidemia drugs should be considered. Recent RCTs have reported that aggressive LDL-C lowering therapy with statins plus ezetimibe 341 , 1141 , 1142) or statins plus PCSK9 inhibitors 1143 - 1145) significantly reduces cerebral and cardiovascular events compared to statin therapy alone 1146) . EWTOPIA75 1108) conducted in Japan was a clinical trial to evaluate the primary prevention effect of ezetimibe monotherapy for LDL-C lowering in patients aged 75 years or older with hyper-LDL cholesterolemia, and reported a significant 34% reduction in cerebral and cardiovascular events without any significant adverse events. It is important to understand the characteristics and effects of various drugs, not only statins, and to implement appropriate LDL-C management with safe and effective drugs, taking into consideration statin intolerance, pregnancy and lactation, individual complications, and drug interactions.
Column: Drug therapy for dyslipidemia with isolated hypo-HDL cholesterolemia
Hypo-HDL-C cholesterolemia is an important classical risk factor for CAD, and after the establishment of LDL-C lowering therapy with statins, the residual risk is expected to be the effect of HDL-C elevation on the prevention of cardiovascular events.
Large clinical trials such as AIM-HIGH 1147) and HPS2-THRIVE 1148) conducted with nicotinic acid derivatives in patients treated with standard statin therapy and their meta-analysis 1149 - 1151) have not shown an effect of HDL-C elevation on cerebral and cardiovascular events. In a meta-analysis 1152) of clinical trials using fibrates, a stratified analysis of patients with hypo-HDL cholesterolemia showed a significant suppression effect of cerebral and cardiovascular events regardless of whether they had diabetes or CAD; however, in the limited analysis to patients receiving statins, a trend towards a significant suppression effect of events was observed, but no significant effect was observed. Recently, CETP inhibitors, which exhibit potent HDL-C-raising and moderate LDL-C-lowering effects, have been developed, and large clinical trials 452 , 1153 - 1156) have been conducted in patients with hypo-HDL cholesterolemia and high risk of ASCVD whose LDL-C is adequately controlled with standard statin therapy. Only REVEAL 1153) with anacetrapib demonstrated a significant primary endpoint of coronary event prevention with the addition of anacetrapib compared to statin alone, but other clinical trials 452 , 1154 - 1156) have not demonstrated a benefit of cerebral and cardiovascular event prevention with the addition of a CETP inhibitor. The use of this drug is currently not approved worldwide because it did not demonstrate a benefit in cerebral and cardiovascular disease and was associated with an increase in all-cause mortality in some patients.
At present, when LDL-C is adequately controlled with standard statin therapy, drug therapy primarily aimed at raising HDL-C has not been shown to prevent cerebral and cardiovascular events, and further research results are expected in the future.
4.2 Characteristics and Selection Criteria of Various Drugs
BQ 20. Have indications, efficacy, and safety of drugs for dyslipidemia been established?
The indications, efficacy, and safety of statins, ezetimibe, anion exchange resins, probucol, fibrates, n-3 polyunsaturated fatty acids, and derivatives of nicotinic acid for the treatment of dyslipidemia are well established. Although indications and efficacy of PCSK9 inhibitors have been established, the safety profile for long-term administration has not yet been confirmed. (Level of evidence: 4)
Table 10 shows the classification of dyslipidemia drugs according to their efficacy. In Japan, the effectiveness of these methods has been verified in principle by a double-blind study. It is necessary to understand the characteristics and effects of various drugs and to select safe and effective drugs, taking into account concomitant diseases and drug interactions. The characteristics of various dyslipidemia drugs are described below.
Table 10. Classification of dyslipidemia drugs according to their efficacy.
| Classification | LDL‐C | TG | HDL‐C | non‐ HDL‐C | Main Generic Names |
|---|---|---|---|---|---|
| statins (stratified by LDL‐C lowering effect) | ↓↓ | ↓ | ― ~↑ | ↓↓ | Pravastatin, Simvastatin, Fluvastatin |
| ↓↓↓ | ↓↓↓ | Atorvastatin, Pitavastatin, Rosuvastatin | |||
| small intestinal cholesterol transporter inhibitor | ↓↓ | ↓ | ↑ | ↓↓ | Ezetimibe |
| anion exchange resin | ↓↓ | ↑ | ↑ | ↓↓ | Cholestyramide, Cholestyramine |
| probucol | ↓ | ― | ↓↓ | ↓ | Probucol |
| PCSK9 inhibitor | ↓↓↓↓ | ↓~↓↓ | ― ~↑ | ↓↓↓↓ | Evolocumab |
| MTP inhibitor* | ↓↓↓ | ↓↓↓ | ↓ | ↓↓↓ | Lomitapide |
| fibrate drugs | ↑~↓ | ↓↓↓ | ↑↑ | ↓ | Bezafibrate, Fenofibrate, Clofibrate |
| selective PPARα modulator | ↑~↓ | ↓↓↓ | ↑↑ | ↓ | Pemafibrate |
| nicotinic acid derivative | ↓ | ↓↓ | ↑ | ↓ | Nicomol, Tocopherol Nicotinate |
| n‐3 polyunsaturated fatty acids | ― | ↓ | ― | ― | Ethyl Icosapentate, Ethyl omega‐3 fatty acid |
*indicated for patients with FH homozygotes
↓↓↓↓ : −50% or more, ↓↓↓ : ‐50 to ‐30%, ↓↓ : −30 to ‐20%, ↓ : −20 to ‐10%, ↑ : 10 to 20%, ↑↑ : 20 to 30%, −: −10 to 10%
1) HMG-CoA Reductase Inhibitors (Statins): Pravastatin, Simvastatin, Fluvastatin, Atorvastatin, Pitavastatin, Rosuvastatin
Dyslipidemia with high LDL-C levels is indicated. Since statins have been proven to be effective in FH 1157) , numerous evidences have been presented of their inhibition of atherosclerosis and are currently the mainstay of treatment for dyslipidemia. Statins antagonistically inhibit HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis, thus suppressing cholesterol biosynthesis 1158) and activating SREBP2, which in turn promotes the synthesis of LDL receptors, resulting in a decrease in blood LDL-C 1159) . The effect of lowering LDL-C levels is 20-50%. The decrease in cholesterol biosynthesis in the liver also results in a decrease in TG through suppression of VLDL synthesis and secretion 1160) , but the effect is only 10-20%. Although statins have been suggested to increase the incidence of new-onset diabetes 1161) , evidence of statin-induced suppression of cardiovascular events is thought to outweigh the risk of diabetes incidence 1162) . Side effects include hepatic dysfunction, interstitial pneumonia, myopathy-like symptoms such as increased CK and muscle weakness, and, although extremely rare, rhabdomyolysis characterized by elevated blood and urine myoglobin. This risk is increased in patients with renal dysfunction and with the concomitant use of fibrates, nicotinic acid derivatives, cyclosporine, and erythromycin. Immune-mediated necrotizing myopathy, which is characterized by muscle weakness with predominance of the proximal muscles, marked myalgia and elevated CK, as well as histological evidence of necrosis of muscle fibers without inflammatory cell infiltration and positive anti-HMG-CoA reductase antibody, has been reported in Japan 1163 , 1164) . Because symptoms can persist or progress rapidly after discontinuation of statin therapy, patients should be discontinued immediately if myopathy-like symptoms appear, and their condition should be carefully monitored. Since the nocebo effect (reverse pseudo effect) is involved in muscle symptoms associated with statin use, it is advisable to refer to the guidelines when dealing with patients who have difficulty continuing to take statins 1165) .
There have also been reports of suspected statin-induced teratogenicity in cases of accidental statin use in early pregnancy 1166) , statins should not be used in pregnant or possibly pregnant women, women who wish to become pregnant, and lactating women. Additionally, each drug has its own contraindications, so care should be taken before and during administration. (Simvastatin is contraindicated in preparations containing itraconazole, miconazole, posaconazole, atazanavir, saquinavir mesylate, and cobicistat, in combination with ombitasvir/paritaprevir/ritonavir, atorvastatin in combination with grecaprevir and pivlentavir, and pitavastatin and rosuvastatin are contraindicated with cyclosporine, respectively). Pitavastatin can also be used for pediatric HMF in doses of up to 2 mg for children 10 years of age and older.
2) Small Intestinal Cholesterol Transporter Inhibitor: Ezetimibe
This drug has a blood cholesterol-lowering effect by inhibiting the small intestinal cholesterol transporter (NPC1L1) present in the small intestinal mucosa, thereby inhibiting absorption of dietary and bile-derived cholesterol in the small intestine 1167) . Unlike resins, it is absorbed by the body and about 78% is excreted in the feces after passing through the enterohepatic circulation. Selectively inhibits cholesterol absorption and does not affect the absorption of fat-soluble vitamins, such as vitamins A and D. The usual oral dose (10 mg/day) lowers LDL-C by about 18%, but, like resin, is associated with increased cholesterol synthesis in the liver. Therefore, the combination with a statin is ideal, and the additive effect of the combination can be achieved, with a reduction in LDL-C of approximately 35-50% with 10 mg of ezetimibe plus a regular dose statin 1169 - 1170) . This effect is equivalent to that of a statin alone at a very high dose. A meta-analysis of large clinical trials of ezetimibe and statin combination therapy in high-risk conditions such as patients with FH, ACS, and PAD has confirmed the safety of the combination therapy and its effectiveness in reducing cardiovascular events by lowering LDL-C 1171) . The safety and significant effect of ezetimibe monotherapy on the prevention of cerebral and cardiovascular events was reported in EWTOPIA75 conducted in Japan 1108) . HDL-C increases by 8-9% and TG decreases by 20-30%. The most common side effect is gastrointestinal symptoms, but not significantly different from placebo. As with statins, myopathy-like symptoms such as increased CK and muscle weakness have been reported in rare cases, but concomitant use with statins has not been reported to increase adverse effects. Furthermore, it should be noted that vitamin K absorption from the intestinal tract is reported to be mediated by NPC1L1, and concomitant use of ezetimibe can enhance its effects in patients taking warfarin 1172) .
3) Anion-Exchange Resin (Resin): Cholestimide, Cholestyramine
Dyslipidemia (type IIa) with high LDL-C is indicated. Although statins are the first-line drugs for hyper-LDL cholesterolemia, in patients who cannot tolerate statins due to side effects or other reasons, resins could be a first-line drug. If pharmacotherapy is necessary in women who are pregnant or may become pregnant, statins should be avoided and resins should be used. The most significant aspect of resin administration is its combination therapy with statins. Cholestyramine is the first drug to prove its efficacy in reducing the incidence of CAD through large-scale clinical trials 1173 , 1174) . Resin inhibits cholesterol absorption by adsorbing bile acids in the intestinal tract and promotes the catabolism of cholesterol to bile acids by inhibiting enterohepatic circulation through re-absorption of bile acids. This results in a decrease in the sterol pool in the body and an increase in LDL receptor synthesis in the liver, resulting in a decrease in blood LDL-C 1175) . However, at the same time, it is accompanied by an increase in cholesterol biosynthesis due to the increased activity of HMG-CoA reductase in the liver, so its combination with a statin, an HMG-CoA reductase inhibitor, is very reasonable. On the other hand, bile acids act as ligands for the nuclear receptor FXR and are involved in the regulation of TG metabolism by suppressing SREBP1c expression and increasing LPL activity, so resin treatment results in a decrease in LDL-C, as well as an increase in VLDL synthesis and blood TG due to bile acid adsorption. Side effects are mainly gastrointestinal symptoms such as constipation and abdominal bloating, but no serious side effects have been observed to date as a result of their nonabsorbable nature. Furthermore, since adsorption of drugs such as statins, ezetimibe, digitalis, warfarin, thiazide diuretics, and thyroid preparations has been observed in resins, patient medication instructions should be given to take these drugs at intervals when they are taken concomitantly. In addition, absorption of fat-soluble vitamins (A, D, E, and K) and folic acid may also be inhibited, so supplementation should be considered when taking long-term doses.
4) Probucol
It is indicated for dyslipidemia (type IIa) with high LDL-C. This drug is also characterized by its regressive effect on xanthomas. However, in addition to LDL-C-lowering effects, it also has HDL-C-lowering effects.
The LDL-C-lowering effect of probucol is 15-25%, and the mechanism is thought to be increased LDL catabolism, especially cholesterol excretion into bile. However, suppression of ABCA 1 activity, a membrane protein essential for HDL production, is thought to be a possible mechanism for the decline in HDL-C. Other possible mechanisms include the increased activity of the cholesterol ester transfer protein (CETP) and the HDL receptor, SR-BI. Although the fact that LDL oxidation is an important point in the pathogenesis of atherosclerosis is becoming clear from various aspects, including cell biological facts 1176 , 1177) and immunohistological facts 1178 , 1179) , the drug is incorporated into lipoproteins and has a strong antioxidant effect because of its structure, which consists of two bound antioxidants, BHT, and is fat soluble. Clinically, small RCTs, as well as secondary prevention in heterozygous patients with FH in cohort studies 1180) have reported a reduction in restenosis after percutaneous coronary angioplasty (PTCA) 1181 , 1182) , carotid IMT, and cardiovascular events 1183) . On the other hand, in PQRST, additional administration of probucol to diet and cholestyramine treatment failed to inhibit the development of atherosclerosis in the femoral artery 1184) . The lack of large-scale clinical trials has limited its positioning, such as when statins are not tolerated or used in combination with statins, but recent results suggesting its usefulness have been reported from Japan and other Asian countries. PROSPECTIVE, conducted in Japan, compared the efficacy of standard statin therapy alone with probucol in preventing cerebral and cardiovascular events in patients with hyper-LDL cholesterolemia associated with CAD, found no statistically significant differences, but did find a trend toward lower rates of cerebral and cardiovascular events in the group receiving standard statin therapy plus probucol 1185) . An integrated analysis with IMPACT conducted in Korea and China similarly found a trend for probucol combination therapy to reduce cerebral and cardiovascular events. In particular, a significant suppression effect of cerebral and cardiovascular events was reported in patients whose HDL-C decreased by 6.25 mg/dL or more 1186) . Side effects include QT prolongation and torsade de pointes on the electrocardiogram, in addition to gastrointestinal symptoms, liver problems, and rash.
5) Fibrates: Bezafibrate, Fenofibrate
It is one of the most effective drugs for hypertriglyceridemia. It is particularly effective in type III hyperlipidemia because it also increases the catabolism of remnant lipoproteins. It is also found to increase HDL-C. The main mechanism of action is that fibrates act as ligands for the nuclear receptor PPARα and activate PPARα 1187 , 1188) resulting in: 1) increased β-oxidation of fatty acids and decreased TG production in the liver, 2) increased LPL production, 3) decreased apoC-III production and increased LPL activity to promote TG degradation and VLDL to catabolism to LDL, 4) increased production of apoA-I and A-II, and 5) increased production of ABCA1. As a result, TG decreases and HDL-C increases. With bezafibrate, a TG lowering effect of 30-40%, a TC lowering effect of about 10%, and an HDL-C raising effect of 35-45% are observed. Fenofibrate is characterized by its long half-life and has a uric acid lowering effect in addition to its effect on lipids. The main side effect is a tendency to cause rhabdomyolysis when used in patients with renal dysfunction, especially when used in combination with statins. Bezafibrate is contraindicated in patients with serum creatinine ≥ 2.0 mg/dL and fenofibrate in patients with serum creatinine ≥ 2.5 mg/dL. Fenofibrate is also contraindicated in patients with gallbladder disease due to reports of gallstone formation.
6) Selective PPARα modulator: Pemafibrate
Like fibrates, Pemafibrate is one of the most effective drugs for hypertriglyceridemia. Selective PPARα modulator selectively regulates the transcription of genes involved in TG metabolism, such as apoC-III, apoAV and LPL, and HDL metabolism, such as apoA-I, apoA-II, SR-BI, and ABCA1, among the genes on which PPARα acts, and has been shown to reduce TG by approximately 43%, reduce non-HDL-C by 10-12%, and increase HDL-C by 16-21% 1189) . Additionally, because it has less effect on the liver and kidneys, it is considered safer in combination with statins than fibrates. However, it is contraindicated in patients with gallstones. In addition, concomitant use with cyclosporine and rifampicin is contraindicated, as inhibition of the metabolic pathway may result in increased blood concentrations.
7) n-3 polyunsaturated Fatty Acids: Ethyl Icosapentate, Ethyl Omega-3 Fatty Acid
Dyslipidemia with elevated TG, especially type IIb hypertriglyceridemia and type IV hyperlipidemia, is indicated. EPA and DHA suppress hepatic VLDL synthesis, lower TG, and slightly increase HDL-C. The preventive effect of fish oil and n-3 polyunsaturated fatty acids on cardiovascular events has long been reported in epidemiological studies and in some secondary prevention trials. In the JELIS 1190) study conducted in Japan, EPA was shown to be significantly more effective in preventing major coronary events in the statin plus EPA group than in the statin alone group, confirming the efficacy of EPA itself. The subanalysis showed a significant inhibition of coronary events 1124) in patients with hypertriglyceridemia and hypo-HDL cholesterolemia dyslipidemia without a history of CAD, and a significant prevention of recurrent stroke 1191) in patients with a history of stroke. However, subsequent large-scale clinical trials conducted overseas failed to prove the effect of n-3 polyunsaturated fatty acids in reducing cardiovascular events 707 , 1192 , 1193) . Recently, REDUCEIT reported that the addition of 4 g of high-dose ethyl icosapentate daily significantly reduced cerebral and cardiovascular events in high-risk patients with concomitant cardiovascular disease or diabetes mellitus, taking statins, and with LDL-C less than 100 mg/dL and hypertriglyceridemia 1123) . However, STRENGTH, a study of high-risk patients with cardiovascular disease or diabetes mellitus who took statins and had hypertriglyceridemia and hypo -HDL cholesterolemia, found no effect of an additional 4 g per day of high-dose n-3 polyunsaturated fatty acids in the prevention of cardiovascular events 1125) . At present, EPA is the only n-3 polyunsaturated fatty acid that has been proven to reduce cardiovascular events. It is not clear whether this effect is specific to EPA or depends on the EPA dosage or whether the concomitant use of DHA attenuates the effect of EPA, and more studies are needed. In addition to their effects on lipids, EPA and DHA are also expected to prevent atherosclerosis through their antiplatelet and anti-inflammatory effects. However, an increased risk of new incidence of atrial fibrillation due to high doses of n-3 polyunsaturated fatty acids has been reported, requiring caution 1194) . The main side effects, in addition to gastrointestinal symptoms such as diarrhea, are a tendency to bleeding.
8) Nicotinic Acid Derivatives: Nicomol, Tocopherol Nicotinate
Indications include hyper-LDL cholesteridemia, hypertriglyceridemia, and dyslipidemia with increased remnant-lipoprotein levels. The mechanism of action of this drug is to inhibit lipolysis in peripheral adipose tissue by suppressing the activation of hormone-sensitive lipase, resulting in a decrease in the influx of free fatty acids into the liver, which in turn suppresses lipoprotein synthesis in the liver. In addition, by inhibiting the catabolism of apoprotein A-I, it has an HDL-C raising effect. Nicotinic acid alone (3.0 g/day) has a TG lowering rate of 26% 1196) . Nicotinic acid derivatives also have a lowering effect on Lp(a) 1196 - 1198) . Major side effects include itching, facial flushing due to peripheral vasodilation, and hyperuricemia. It may also exacerbate insulin resistance and should be administered with caution in diabetic patients.
9) PCSK9 Inhibitor (Human Anti-PCSK9 Monoclonal Antibody Drug): Evolocumab
Patients with hyper-LDL cholesterolemia who have FH or are at high risk for the incidence of cardiovascular events, who have had an inadequate response to maximal tolerated statin therapy, or who are not suitable for statin therapy (statin intolerance) are indicated for treatment.
The drug specifically binds to and inhibits the PCSK9 protein (proprotein convertase subtilisin/kexin type 9) that is involved in the degradation of the liver LDL receptor, and thus exhibits effects lowering blood LDL-C by increasing the recycling of LDL receptor 1199) . As noted above, statins, which inhibit cholesterol biosynthesis in the liver and activate SREBP2, increase PCSK9 synthesis as well as LDL receptor synthesis, making their combination with this inhibitor reasonable. The LDL-C lowering effect is the most potent among existing drugs, and in a phase III study in patients at high risk for cardiovascular events, including heterozygous FH, a 70-75% reduction in LDL-C was demonstrated with administration once per two weeks in combination with statin therapy 1200 , 1201) . Another characteristic of this inhibitor is that it lowers Lp(a) by 20-30%, which statins do not have a lowering effect on. TG is reduced by 20-25% and HDL-C is increased by 10-15%. LDL-apheresis can also be combined with a PCSK9 inhibitor, but the anti-PCSK9 antibody is removed during apheresis, so if the combination is used, it should be administered after apheresis.
This drug is a subcutaneous injection and the main side effects are injection site reactions, with other reports of nasopharyngitis and gastroenteritis. To date, there have been no reports of liver or skeletal muscle disorders that are enhanced by concomitant use with statins, and no adverse events have been reported attributable to low levels of LDL-C, but the long-term efficacy and safety of the drug should be carefully monitored.
10) MTP Inhibitor: Lomitapide
Lomitapide is currently the only microsomal triglyceride transfer protein (MTP) inhibitor approved in Europe, the United States, and Japan. MTP inhibition decreases VLDL production and lowers LDL-C and TG. It lowers LDL-C by about 50% even in homozygous FH (HoFH) who do not respond to other drug therapies, but fat accumulation in the liver, abdominal pain, and diarrhea are the major side effects, and future studies are needed to determine its long-term safety. Indication in Japan is limited to patients with HoFH. When HoFH patients were fed a low-fat diet and treated with lomitapide, blood LDL-C and apoB decreased by 50.9% and 55.6%, respectively, after 4 weeks (mean pretreatment LDL-C was 615 mg/dL) 1202) . Significant elevations in AST and ALT and increases in hepatic fat content were observed with lomitapide, but all patients returned to normal 14 weeks after treatment was discontinued. Lomitapide alone or in combination with ezetimibe in 85 hypercholesterolemia patients (mean pretreatment LDL-C 170 mg/dL) in combination with a low-fat diet showed a dose-dependent decrease in LDL-C and apoB 1203) . At the highest dose of lomitapide alone, LDL-C and apoB were reduced by 30% and 24%, respectively, from pretreatment values, while the ezetimibe combination group showed a reduction of 46% and 37%, respectively.
4.3 Combination Therapy
FQ21. Is the addition of cholesterol-lowering non-statin drugs (ezetimibe, anion exchangers, probucol, PCSK9 inhibitors) to statins recommended for the prevention of recurrent ASCVD?
•PCSK9 inhibitors in combination with statins are effective in preventing recurrence of ASCVD in patients with pre-existing ASCVD, and are recommended when LDL cholesterol control targets have not been achieved even with multiple drug therapy. (Level of evidence: 1+, Level of recommendation: A)
•Ezetimibe in combination with statins is effective in preventing recurrent ASCVD in patients with acute coronary syndromes, and is recommended when LDL cholesterol control targets have not been achieved with statins. (Level of evidence: 1, Level of recommendation: A)
The FOURIER study (evolocumab) 1143) and the ODYSSEY OUTCOMES study (alirocumab) 1144) , which investigated the efficacy of PCSK9 inhibitors under statin treatment in patients with a history of ASCVD, showed that the concomitant use of PCSK9 inhibitors significantly reduced the incidence of ASCVD. In both trials, the efficacy of PCSK9 inhibitors was demonstrated in several sub-analysis, including a sub-analysis in diabetic patients and a sub-analysis the endpoint of which was set as all post-treatment events.
The IMPROVE-IT study 1141) was conducted to evaluate the effect of additional administration of ezetimibe in patients whose LDL-C was controlled at 50 to 100 mg/dL with simvastatin after ACS, and demonstrated the efficacy of additional ezetimibe administration to reduce the incidence of ASCVD. Several subanalyses have also shown its efficacy, including that focused on diabetic patients, as well as that examined all event incidences. On the other hands, the HIJ-PROPER study 341) which was conducted in patients with ACS and examined the efficacy of adding ezetimibe to statin (LDL-C target in combination therapy <70 mg/dL, in statin alone 70 to 100 mg/dL) did not disclose the benefit of combination therapy.
Regarding probucol, the PROSPECTIVE study 1185) was conducted in Japan to evaluate the effect of adding probucol to statins in patients with CAD complicated by dyslipidemia. The study showed a trend toward a reduction of the ASCVD event in those who received probucol, but the difference was not significant.
There are no large RCTs that have examined the efficacy of anion exchange resins in combination with statins for the prevention of ASCVD.
FQ22. In patients with hypertriglyceridemia or hypo-HDL cholesterolemia, is the co-administration of fibrates, SPPARMα, nicotinic acid derivatives, or n-3 polyunsaturated fatty acids with statins recommended for the prevention of the incidence of ASCVD?
•The addition of an ethyl icosapentate (EPA) preparation to statin treatment is effective in reducing the incidence of ASCVD in hypertriglyceridemiac cases, and concomitant therapy is recommended. (Level of evidence: 1+, Level of recommendation: A)
•The addition of fibrates to statins is effective in reducing the incidence of ASCVD in patients with hypertriglyceridemia and hypo-HDL cholesterolemia, and combination therapy is suggested. (Level of evidence: 2, Level of recommendation: B)
The REDUCE-IT study 1123) is a study that included diabetic patients with at least one ASCVD risk factor or secondary prevention patients, whose LDL-C levels were cotrolled between 41 and 100 mg/dL with statin treatment and TG levels between 150 mg/dL and 500 mg/dL; it examined the efficacy of adding EPA (at a dose of 4 g/day) to statin treatment and revealed the benefit in reducing ASCVD events with concommitant therapy. A subanalysis of the JELIS study which analyzed primary prevention patients with TG ≥ 151 mg/dL and HDL-C <40 mg/dL showed that the addition of EPA to statins reduced the incidence of coronary events 1124) . On the other hand, the STRENGTH study 1125) which targeted statin-treated patients at high risk of ASCVD with LDL-C <100 mg/dL, TG 180-499 mg / dL and HDL-C <42 mg/dL (men) and <47 mg/dL (women), did not disclose the efficacy of a formulation of EPA / DHA in reducing ASCVD events. The ASCEND 1192) and ORIGIN 1204) studies, which were carried out in patients with diabetes or prediabetes, and the concomitant statin administration rates of which were 75% and 54%, respectively, also did not demonstrate the efficacy of the EPA / DHA formulations.
In the ACCORD-LIPID study conducted in patients with type 2 diabetes (including both primary and secondary prevention), the addition of a fibrate to a statin was not found to reduce ASCVD, but a subanalysis performed in the subjects with TG ≥ 204 mg / dL and HDL-C <34 mg/dL found a reduction in ASCVD 1127) .
The AIM-HIGH study 1205) which targeted ASCVD patients with hypo-HDL cholesterolemia and hypertriglyceridemia, and a subanalysis 1147) of HPS2-THRIVE study (performed in patients with preexisting atherosclerotic diseases) which was conducted in those with the HDL-C <34.8 mg/dL or TG >151 mg/dL, did not show an effectiveness of nicotinic acid derivatives in preventing the incidence of ASCVD when co-administered with statin. However, the control group of AIM-HIGH trial had a higher statin dose and higher ezetimibe administration rate; the HPS2 -THRIVE trial included patients with mean LDL-C levels already as low as 63 mg/dL at study entry, and furthermore, it included only a small number of subjects meeting the subanalysis criteria (HDL-C less than 34.8 mg/dL: 19.1%, TG 151 mg/dL or more: 25.6%).
The PROMINENT trial examined the effect of SPPARMα in combination with statins on ASCVD in diabetic patient with TG 200-499 mg/dL, HDL-C ≤ 40 mg/dL, and LDL-C ≤ 70 mg/dL with statin therapy or LDL-C ≤ 100 mg/dL in statin intolerance. It was terminated midway through the study due to the no significant difference in the primary composite endpoint 1206) .
4.4 Follow-Up of Drug Therapy
BQ21. Is it necessary to regularly perform clinical examinations after the initiation of drug therapy?
After the initiation of drug therapy, regular examinations are recommended to confirm efficacy and safety. Examination items should be selected depending on the drug administered and the patient’s background. (Level of evidence: Consensus)
In addition to monitoring symptoms related to side effects, it is recommended to perform examinations regularly in order to confirm drug effects, perform dose adjustment, find side effects biochemically, and provide lifestyle guidance. The recommended frequencies of examinations are approximately 2 to 3 times during the first 6 months after the initiation of treatment and once every 3 to 6 months thereafter. In addition to lipid examinations, examination items must be selected from liver function tests (AST, ALT, γGT), muscle-related enzyme tests (CK), kidney function tests (BUN, Cre), and blood glucose-related tests (HbA1c, blood glucose level), considering the drugs used and the patient’s background. Some reports 1207) suggest that examinations are sufficient only before drug administration and when symptoms develop, since it is difficult to detect serious complications in a timely manner through regular examinations. However, regular examinations are thought to reduce cardiovascular events by improving adherence and building a positive patient-physician relationship. In addition, because abnormal laboratory values and side effects have been reported to be more likely with combination therapy (statins together with fibrates or nicotinic acid derivatives) 1207 , 1208) than with monotherapy, regular examinations are necessary when combination therapy is used.
If symptoms or abnormal examination values are observed during statin administration, the patient should be treated according to the “Statin Intolerance Clinical Guide 2018” (https://www.jstage.jst.go.jp/article/jat/27/4/27_50948/_pdf/-char/en) 1165) . When elevated or abnormal liver enzymes or muscle enzymes are observed, causes other than statins (e.g., elevated liver enzymes due to fatty liver or elevated muscle enzymes due to exercise) must be ruled out first 1209) . Serious side effects (such as rhabdomyolysis and liver failure) caused by statins are very rare unless they are used together with fibrates and drugs that affect statin metabolism. A meta-analysis of 21 RCTs 1210) or 30 RCTs 1211) showed that the incidence of muscle-related side effects was not significantly different from placebo. Reports from other countries indicate that higher doses, old age, small statue, and being female are at risk of muscle-related side effects 1209 , 1212 - 1216) . In most cases, symptoms appear within six months after the start of statin administration 1217 , 1218) ; post-marketing surveillances in Japan did also show the same results. It should also be noted that although very rare, some cases develop immune-mediated necrotizing myopathy (positive for anti-HMGCR antibody) or idiopathic inflammatory myositis 1219 , 1220) . Although a meta-analysis of large RCTs suggests that statins increase the risk of diabetes (9-13% increase), the frequency is not high (1-2 per 1,000 patients/year) 1161 , 1221 - 1223) and the incidence of diabetes increases mainly in those at high risk of developing diabetes (older people, metabolic syndrome, prediabetes, etc.) 1222 , 1224) . Therefore, it is important to ensure that blood glucose-related examinations are performed regularly in those at high risk for diabetes 1225) .
We need to keep in mind that although most of fibrates-induced creatinine elevations are reversible and mildly elevated, they occasionally become abnormally high. When nicotinic acid derivatives are administered, it is necessary to pay attention to the elevation of blood glucose levels and development of diabetes from metabolic syndrome, but even if they occur, it can be treated with appropriate treatment. As mentioned above, it should be noted that fibrates and nicotinic acid derivatives are prone to liver and muscle disorders when used in combination with statins.
For MTP inhibitor, an agent applicable only to HoFH, diarrhea and liver function abnormalities (fatty liver) occur at a very high rate because of its pharmacological effects. Therefore, monitoring with regular examinations is important, as drug dosages may need to be adjusted or discontinued. In the long term, the progression of liver fibrosis (cirrhosis) should also be kept in mind 1226 - 1231) .
4.5 Concomitant Use with other Drugs for Prevention of atherosclerosis
BQ 22. Does the concomitant use of statins with drugs metabolized by CYPs increase the incidence of adverse effects?
Since there have been many case reports of rhabdomyolysis associated with the concomitant use of fat-soluble statins and drugs metabolized by CYP, careful attention should be paid to the incidence of adverse effects when statins are used concomitantly. (Level of evidence: 3)
Biological foreign substances, such as drugs, are metabolized in the liver by cytochrome P450 proteins (CYPs). Among statins, fat-soluble statins are substrates for CYPs such as CYP3A4 or CYP2C9 and are metabolized and excreted by CYPs. Water-soluble statins, rosuvastatin and pravastatin, are not metabolized by CYPs to any significant extent.
Drugs known to be substrates for CYP include antifungals (fluconazole, itraconazole, etc.), macrolide antibacterials (erythromycin, clarithromycin, etc.), protease inhibitors used in the treatment of HIV, as well as calcium channel blockers, warfarin, nateglinide, glimepiride, and other drugs used in the cardiovascular and metabolic fields. Concomitant use of statins with these drugs can result in adverse events due to elevated blood levels and the potentiation of their effects. Macrolide antibacterial agents, antifungal agents, protease inhibitors, and bergamotine, an ingredient in grapefruit juice, also inhibit CYP, and their concomitant use may increase blood levels of statins. Recently, drugs that induce CYP, such as rifampicin and barbituric acid, are also known and their concomitant use with statins can reduce their efficacy. Statins and the main metabolic and cardiovascular agents metabolized by CYP are listed in Table 11 .
Table 11. Statins and Cardiovascular and Metabolic Drugs Metabolized by CYPs.
| CYP | Statins Metabolized by CYPs | Cardiovascular and Metabolic Drugs Metabolized by CYPs |
|---|---|---|
| CYP3A4 | Atorvastatin | Calcium channel blockers (diltiazem, verapamil, nifedipine, amlodipine, cilnidipine, azelnidipine, benidipine), warfarin, repaglinide* |
| Simvastatin | ||
| CYP2C9 | Fluvastatin | ARBs (losartan, valsartan, candesartan, irbesartan, azilsartan), warfarin, glinides (nateglinide, mitiglinide), glimepiride |
*Repaglinide is mainly metabolized by CYP2C8, but CYP3A4 is also involved in some cases.
Concomitant use of statins with drugs that are substrates of CYP metabolism has been reported to increase the AUC of blood levels of statins 1232) , but no reports examined whether side effects are enhanced in a search conducted since 1990. However, the incidence of rhabdomyolysis due to the concomitant use of statins with drugs that affect CYP metabolism has been reported in a number of cases 1233) . Although this is a relatively small number of overseas studies and cannot be ruled out as a CYP effect, there is a report that when atorvastatin and ezetimibe were combined in patients with anticoagulation for atrial fibrillation, a slight reduction in anticoagulant dose was required in the treatment group, but the dose stabilized after approximately 3 months and did not increase complications such as bleeding 1234) .
In addition to CYPs, dyslipidemia drugs are also affected by transporters such as breast cancer resistance protein, OATP1B1, OATP-C, and P-glycoprotein. Rosuvastatin, a water-soluble statin, is contraindicated in combination with cyclosporine due to increased blood levels, which is noted in the package insert. This is believed to be because cyclosporine inhibits transporters such as breast cancer resistance protein and OATP1B1 in hepatocytes, resulting in decreased uptake of the drug in hepatocytes.
BQ23. Can the use of fixed-dose combination drugs in the treatment of dyslipidemia be recommended for the prevention of serum lipids and the incidence of ASCVD?
The use of fixed-dose combination drugs can be expected to improve adherence by reducing the number of medications taken. Although there are currently no reports that examine the effectiveness of fixed-dose combination drugs in improving adherence and preventing the incidence of ASCVD compared to prescribing each drug individually, its use can be suggested. (Level of evidence: 3)
Many fixed-dose combinations are used for metabolic and cardiovascular diseases to reduce the burden of medication in older people and to treat patients in developing countries who are prone to poor adherence or have difficulty accessing healthcare. In Japan, in addition to a statin and ezetimibe combination drug, a atorvastatin and amlodipine, a calcium channel blocker, combination drug is used for the treatment of dyslipidemia, and a statin and DPP4 inhibitor combination drug is used overseas. The use of combination drugs for antihypertensive and diabetes medications is also widespread, and combination drugs for GLP-1 receptor agonists and injectable insulin are also used in Japan. Combination drugs may improve patient quality of life and improve adherence to medications by reducing financial burden, leading to improved serum lipids and a reduced incidence of cardiovascular disease. Reports from overseas have shown that the use of combination drugs is more effective than separate prescriptions in increasing adherence 1235 , 1236) and especially in maintaining adherence when another drug is added to a patient taking one drug 1237) . In a study comparing a combination drug containing a statin and an antihypertensive drug or aspirin with a placebo, the combination drug was reported to improve serum lipid and blood pressure control 1238) and also reduce the incidence of cardiovascular disease 1239) . However, so far we have not seen reports directly comparing each drug administered separately with a combination drug.
Although the benefits of combination drugs compared to single agents are unknown, the use of combination drugs may be beneficial in terms of adherence, patient convenience, and healthcare economics in Japan’s aging population.
4.6 Adherence, Treat to Target
BQ 24. Is medication adherence related to serum lipid levels and the incidence of ASCVD?
Good adherence to oral statins is associated with an improvement in serum lipids and a reduced incidence of ASCVD. (Level of evidence: 3)
Adherence to medication is an important issue that physicians should be aware of when applying the results of large clinical studies to their patients. Although large clinical studies have demonstrated the efficacy of lipid-lowering therapy with statins and other lipid-lowering therapies in preventing the development of ASCVD, in actual clinical practice, if patients do not actually take the prescribed drugs, the lipid-improving effect will not be achieved, and the cardiovascular disease prevention effect will be reduced. In a retrospective cohort study of statins conducted overseas, the 4- to 5-year mortality rate was 45% lower in the group with better than 90% adherence than in the group with less than 10% adherence 1240) . The JELIS study in Japan also reported a significant reduction in primary endpoints consisting of sudden cardiac death and fatal/non-fatal myocardial infarction in the group of secondary prevention patients who achieved 80% adherence compared to those who did not 1241) .
In clinical studies and trials, adherence is easily maintained with careful guidance and follow-up by physicians and coordinators, but in actual clinical practice, patient adherence is often below 80%. An aspect of the impact of medication adherence is that it is difficult to verify in RCTs or prospective studies. However, retrospective studies using real-world data and interventions to improve adherence have also been reported to improve serum lipids and reduce ASCVD. A systematic review reported in CochraneLibrary showed that the guidance and intervention of physicians and other healthcare professionals improved medication adherence and reduced total cholesterol and LDL-C by 17.15 mg/dL and 19.51 mg / dL, respectively, in less than 6 months, and total cholesterol by 17.57 mg / dL during 6 months in the intervention group compared to the control group 1242) . The study also reported that the achievement of the treatment goal of 100 mg/dL for LDL-C was significantly correlated with adherence to medication, and patients who had achieved the goal also reported significantly higher adherence to medication 1243) . Regarding the incidence of ASCVD, in an overseas registry study, the OR for stroke death increased to 2.04 (95% CI: 1.72-2.43) in the group of hypercholesterolemic patients with low statin adherence (<80%). Among patients with hypercholesterolemia complicated by hypertension, a 1.82 (95% CI: 1.43-2.33) increase was reported in the statin-only non-adherent group and a 7.43 (95% CI: 5.22-10.59) increase in the non-adherent group for statins and antihypertensive drugs 1244) . In a systematic review of 84 real-world studies (including a systematic review-up study of retrospective cohort study systematic review-up study), good adherence was associated with a reduced incidence of cardiovascular events and a favorable prognosis, although no data were collected to allow meta-analysis 1245) .
BQ 25 What factors influence drug adherence?
Factors known to influence medication adherence include age, sex, income, and the presence of cardiovascular disease. Continuous encouragement from health care providers and regular lipid examinations increase adherence. (Level of evidence: 2)
Overseas meta-analyses have found that adherence to statin medications varies by age, sex, income, and whether the patient is receiving treatment for comorbidities. Adherence was lower among women and those with low income, and showed a "U-shaped" distribution in terms of age, with lower adherence among those under age 50 and over age 70. Adherence was higher in secondary prevention patients with a history of cardiovascular disease and lower in primary prevention patients. More frequent lipid examinations and lower payments were also associated with good adherence 1246) . There are also differences between drugs, with anion exchange resins being particularly low, and fibrates, polyunsaturated fatty acid products of n-3, and nicotinic acid products are also known to be low compared to statins 1247) . Discontinuation of medication is known to occur most often within the first year or two of treatment and then declines. Adherence after statin treatment evaluated by PDC (proportion of days converted) was reported to be 79% at 3 months and 50% at 1 year, but 42% at 10 years 1248) ( Table 12 1249) ). In the IMPROVE-IT study, the dropout rate was highest early after inclusion (within 30 days), then decreased and reached a steady state after 1 year 1250) . In clinical research and trials, adherence is good with strict medication management by coordinators, but in real-world clinical practice, it is not as high, and the discontinuation rate is higher.
Table 12. Overseas Reports on Adherence to Dyslipidemia Medications.
| Name of Country | Sample Size | Adherence | Reference |
|---|---|---|---|
| United States | 19,422 |
|
Mann DM, Woodward M, Muntner P, Falzon L, Kronish I: Predictors of nonadherence to statins: a systematic review and meta‐analysis. Ann Pharmacother, 2010; 44: 1410‐ 1421 |
| United Kingdom | 6,262 |
1 year: 66% 5 years: 75% 10 years: 68% |
Wiegand P, McCombs JS, Wang JJ: Factors of hyperlipidemia medication adherence in a nationwide health plan. Am J Manag Care, 2012; 18: 193-199 |
| United States | 4,776 |
6 months: 80%
|
Helin-Salmivaara A, Lavikainen P, Korhonen MJ, Halava H, Junnila SY, Kettunen R, Neuvonen PJ, Martikainen JE, Ruokoniemi P, Saastamoinen LK, Virta L, Huupponen R: Long-term persistence with statin therapy: a nationwide register study in Finland. Clin Ther, 2008; 30 Pt 2: 2228-2240 |
| United States | 34,501 |
3 months: 79% 6 month: 56% 1 years: 50% 10 years: 42% |
Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J: Long- term persistence in use of statin therapy in older patients. JAMA, 2002; 288: 455-461 |
To increase patients’ drug adherence, it is effective to encourage not only physicians but also nurses, pharmacists, and other team medicine personnel to work together. Motivational interviewing 1251) , telephone coaching by pharmacists 1252) , reminder apps 1253) , and financial rewards 1251) were reported to improve medication adherence. Although the mechanism is unknown, it has been suggested that vitamin D decreases statin-induced muscle pain. Vitamin D administration did not change the adherence to statin medication, but was reported to decrease discontinuation of medication 1254) . Regular lipid examinations have also been reported to increase adherence 1246) . Lifestyle management through interviews and personalized medication adjustments by nurses and physicians 1255) , and telephone guidance by pharmacists 1256) not only improved adherence, but also significantly reduced LDL-C in the intervention group. Adherence interventions that have shown a reduction in cardiovascular events have not been reported.
Recognizing the target population with low adherence, such as women, young and old, and primary prevention patients, in addition to providing guidance on lifestyle modification, such as diet and exercise therapy, it is necessary for medical personnel to carefully explain the relationship between dyslipidemia and the incidence of cardiovascular disease, and to make efforts to help them understand the purpose of treatment. It is important to repeatedly explain the need for treatment and to prevent interruptions, especially during the first year or two after the treatment is started.
When prescribing medications, the number of doses should be kept to a minimum, and the timing of dosing, such as before or after meals, should be as easy as possible. Because the use of compound drugs is known to increase adherence compared to separate prescriptions 1235) , the use of compound drugs can be considered in patients who require the administration of multiple drugs. Low self-payment for drugs is also associated with adherence. A US cohort study comparing brand-name and generic statin patients reported not only better adherence but also fewer composite endpoints consisting of hospitalization for ACS and stroke and total mortality in patients prescribed generics 1257) .
BQ 26. Is lipid management by setting a control target (or a goal for cholesterol-lowering rate) effective in preventing ASCVD?
Lipid management of the targeted LDL cholesterol level is an appropriate policy in clinical practice and can be recommended. (Level of evidence: 1)
The efficacy of statin-based LDL-C lowering therapy in preventing atherosclerotic disease has been demonstrated in many clinical studies, including a meta-analysis 331) by the Cholesterol Treatment Trialists published in 2010. Current European and US guidelines also recommend strong lipid-lowering therapy with high-dose statins, PCSK9, and ezetimibe, basically for high-risk primary and secondary prevention cases 456 , 457) . Of these, the European (ESC/EAS) guidelines, like those of Japan, provide a risk-based LDL-C control target value 457) , while the US (AHA / ACC) guidelines do not present LDL-C as a control target value, but rather present the amount of statin to be administered according to risk and the corresponding rate of decrease in LDL-C 456) .
In the US, the 2013 ACC/AHA guidelines established a so-called “fire and forget” policy, in which the amount of statin to be administered is suggested based on risk assessment after defining a group of patients who would benefit the most from statins, and no target LDL-C level is established during treatment is set 1258) . Subsequently, however, the Improve-IT Study 1142) and the ODESSAY OUTCOMES Study 1144) have shown that additional therapy to lower LDL-C with the addition of ezetimibe or a PCSK9 inhibitor to high-dose statins can reduce the incidence of cardiovascular events, especially in high-risk patients after ACS. In light of this, the European and Japanese guidelines have since continued to provide target values for the control. The 2018 guidelines in the United States also indicate the rate of decrease in LDL-C along with the statin dose, and if the target rate of decrease is not reached, the use of drugs other than statins is to be considered.
An overseas study that examined the significance of targeted lipid-lowering therapy (treat to target) in addition to high-dose statin administration showed that a 50% or more LDL-C lipid reduction had an additive event-improving effect, but achieving less than 70 mg/dL was not an independent prognostic factor 1259) . On the other hand, since the rate of reduction in LDL-C depends on baseline LDL-C and the presence or absence of FH, the evaluation based only on this may not be fair 1260) . There have been no RCTs, let alone systematic reviews or meta-analyses, on direct comparisons between setting control targets or not 1261) .
Since 2016, there have been several RCTs examining LDL-C targets, although not comparing treat to target vs. fire and forget. In France, a trial comparing LDL-C <70 mg/dL vs. 90-110 mg/dL in secondary prevention of cerebral infarction showed a significant reduction in cardiovascular events in the group targeting LDL-C 70 mg/dL 353) . Since nearly 30% of the registrants are Korean, the data are also considered to be of significance to the Japanese. In a J-STARS subanalysis of secondary stroke prevention in Japan, the 80-100 mg / dL LDL-C subgroup had the fewest subsequent events 1262) , and further LDL-C lowering therapy did not prove to be justified. In the EMPATHY study, also conducted in Japan, an RCT divided high-risk patients with diabetic retinopathy into two groups, one targeting LDL-C below 70 mg/dL and the other targeting LDL-C between 100 and 120 mg/dL, and failed to show a significant treatment effect, probably because LDL-C in the strong treatment group remained at an average of 76 mg/dL 1106) . Although this study did not use PCSK9 inhibitors, it is evidence that achieving LDL-C less than 70 mg/dL is difficult with high-dose statins alone.
Although there are no studies that directly compare so-called treat-to-target versus fire-and-forget 1261) , meta-analyses 331 , 332) based on studies comparing statins with no statins, as well as high and low statin doses, have shown that lowering LDL-C improves cardiovascular events and life expectancy, and has demonstrated the safety of the treatment 1263) . On the other hand, it has been reported that the achievement rate of LDL-C below 70 mg/dL with high-dose statins alone is only about 60% 1264) , so it is not uncommon for LDL-C reduction to be insufficient in daily clinical practice, and fire and forget with statins alone has its limitations. On the contrary, fire and forget with the addition of a PCSK9 inhibitor or ezetimibe is also not cost-effective or appropriate, as not all high-risk cases require the addition of all drugs to achieve adequate reduction in LDL-C. Considering that elevated LDL-C after initiation of lipid-lowering therapy (the so-called escape phenomenon) is an independent factor in the incidence of subsequent events 1265) and that statin intolerance has also been reported to affect secondary prevention 1266) , treat-to-target is a realistic lipid-lowering therapy in clinical practice.
5. Management of Major High-Risk Pathologies
5.1 History of CAD
In the presence of ACS, familial hypercholesterolemia, diabetes mellitus, or atherothrombotic cerebral infarction, LDL cholesterol should be more strictly controlled, as patients with a history of CAD are at particularly high risk for the incidence of ASCVD.
Patients with a history of CAD should be treated with the goal of LDL-C less than 100 mg/dL. However, coronary atherosclerosis is accelerated by the accumulation of coronary risk factors such as hypertension, dyslipidemia, and diabetes. Among patients with a history of CAD who are already at high risk, those with the four conditions listed in Table 13 are at particularly high risk for the incidence of ASCVD; acute coronary syndrome (ACS), FH, diabetes mellitus, atherothrombotic cerebral infarction, or other cerebral infarction with obvious atherosclerosis (>50% stenosis of the intracranial or extracranial arteries or composite atheromatous lesions). Therefore, strict management with a goal of less than 70 mg/dL LDL-C with high-intensity statins should be used.
Table 13. Conditions requiring particularly strict management in patients with a history of CAD.
| 1. Acute Coronary Syndrome |
| 2. Familial Hypercholesterolemia |
| 3. Diabetes |
| 4. Complicated with atherothrombotic cerebral infarction (including other cerebral infarctions with obvious atheroma) |
1) Acute Coronary Syndrome
Patients who have had a incidence of ACS are at an even higher risk of recurrent cardiovascular events than patients with stable CAD. In the OACIS-LIPID study 338) , which examined the effect of early statin therapy on the prevention of cardiovascular events in patients with acute myocardial infarction in Japan, the incidence of all-cause mortality and non-fatal myocardial infarction was 40/1,000 person-years in patients treated with lipid lowering therapy other than statins, while the statin group also had a significantly higher incidence of cardiovascular events at 30/1,000 person-years. The PACIFIC study 340) , a multicenter registry observational study of patients with ACS, showed a high incidence of fatal and nonfatal myocardial infarction (>35/1,000 person-years) despite statins in approximately 80% of cases. However, it has been reported that LDL-C lowering therapy with high-intensity statins from the early stage of ACS incidence is effective in preventing cardiovascular events 1267) and that LDL-C lowering therapy more severe than usual is effective in preventing cardiovascular events 1268) . A meta-analysis of RCTs in which statins were initiated within 14 days after ACS incidence did not show evidence of prevention of cardiovascular events in the short term of 4 months 1269) , but there was a significant reduction in cardiovascular events during a 2-year observation period 1133) . Regarding the target of LDL-C management, the IMPROVE-IT trial 1141) compared the combination of statin and ezetimibe with statin monotherapy, and the addition of ezetimibe was reported to reduce LDL-C to 53.7 mg/dL and prevent cardiovascular events by an additional 6.4% compared to statin therapy alone. The FOURIER study 1143) , which examined cardiovascular outcomes with evolocumab, a powerful inhibitor of PCSK9 lowering LDL-C, reported that in patients with atherosclerotic cardiovascular disease treated with optimal lipid lowering therapy (equivalent to at least 20 mg of atorvastatin), LDL -C decreased from 92 mg/dL to 30 mg/dL and there was a 15% reduction in cardiovascular events compared to the placebo group. In the ODYSSEY OUTCOME study 1270) conducted in patients post-ACS with LDL-C <70 mg / dL even with a statin maximally tolerated, the PCSK9 inhibitor alirocumab, when used to control LDL-C to 25-50 mg/dL, reduced the risk of cardiovascular events by 15% compared to statin treatment alone. (Alirocumab is currently not used in Japan.) The combination of statins with ezetimibe or PCSK9 inhibitors did not increase adverse events due to marked reduction in LDL-C and significantly reduced cardiovascular events.
In Japan, the efficacy of early LDL-C lowering therapy in ACS has been studied by observation of coronary artery plaque using intravascular ultrasound (IVUS). In the ESTABLISH study 1109) , strict therapy for LDL-C reduction with atorvastatin 20 mg from the onset of ACS resulted in a mean reduction in LDL-C to 70 mg/dL and a 13.1% reduction in plaque volume after 6 months. Changes in plaque volume were also reported to be significantly positively correlated with LDL-C and the rate of reduction in LDL-C after treatment. The study also reported that early strict LDL-C-lowering therapy significantly reduced cardiovascular events after additional case follow-up (average 4.2 years) 1134) . Furthermore, the JAPAN-ACS trial showed that early and strict LDL-C lowering therapy with high-intensity statins for ACS was effective in inhibiting plaque progression, but did not show a significant relationship between the rate of change in LDL-C before and after treatment or post-treatment LDL-C and plaque regression rate 1110) . Although these studies showed results using high intensity statins after the incidence of ACS, the PRECISEIVUS study 1113) reported that the combination of a statin with ezetimibe reduced LDL-C to less than 70 mg/dL, resulting in plaque volume in patients with ACS reported to regress significantly compared to statin monotherapy. On the other hand, the HIJ-PROPER study 341) compared the effect of active treatment with standard-dose statin plus ezetimibe in patients with ACS aiming at LDL-C below 70 mg/dL with that of standard-dose statin alone to control LDL-C at 90-100 mg/dL to prevent cardiovascular events. Ezetimibe combination therapy aimed at lower LDL-C targets showed a trend towards a lower risk of cardiovascular events compared to statin therapy alone, but the difference was not significant. These results suggest that it is desirable to start treatment of patients with ACS with high-intensity statins early in the incidence to achieve an LDL-C of less than 70 mg/dL. Because LDL-C may be temporarily low immediately after the incidence of ACS, early administration of a tolerable high intensity statin is recommended, regardless of preintervention LDL-C levels. Furthermore, although data in Japan are insufficient, large overseas clinical trials have shown the efficacy of high-intensity statins in ACS patients with LDL-C less than 70 mg/dL, and aggressive treatment aimed at even lower levels may be effective.
2) Familial Hypercholesterolemia
The EXPLORE-J study 1271) , a prospective observational study of lipid risk in Japanese patients with ACS, showed that FH was present in 2.7% of patients with ACS, at least 5 times more frequently than in the normal population. The incidence of cardiovascular events has been reported to be related to the sum of lifetime LDL-C (cumulative LDL-C) 1272) , and patients with FH have high levels of LDL-C from birth and reach threshold levels at a young age. Compared to hyper-LDL cholesterolemia without genetic background, the degree of increase in LDL-C is marked and the progression of ASCVD is rapid, so the risk of premature incidence of CAD is extremely high. Therefore, although early diagnosis and early treatment are desirable for FH, it is often not actively treated because it is currently not well recognized and may go unnoticed because lipid-lowering drugs are already being administered.
Observational studies have shown that statin-induced LDL-C reduction therapy for FH reduces the risk of cardiovascular events and delays their incidence 1273 , 1274) . And a 20-year follow-up of statin-treated children with FH was also reported to inhibit the progression of carotid artery thickening and reduce the risk of cardiovascular disease 1275) . Although there is no clear evidence regarding numerical targets because ethical considerations make it difficult to conduct randomized controlled trials in patients with secondary prevention of FH, Since patients with CAD who have FH have a higher risk of recurrence than non-FH patients, it is recommended that LDL-C be promptly lowered and strictly controlled in the prevention of the incidence of CAD for FH. Additional LDL-C lowering effects have been reported with the addition of PCSK9 inhibitors in patients with heterozygous FH treated with statins and ezetimibe 1276 , 1277) , however, the effect of their combination on the reduction of cardiovascular events compared to statin therapy alone is not yet clear.
3) Diabetes Mellitus
In patients with a history of myocardial infarction, the risk of recurrent cardiovascular events has been reported to increase with diabetes mellitus 208 , 1278 - 1280) . The Finnish study 200) followed diabetic patients with a history of myocardial infarction for 7 years and found that reinfarction of myocardial infarction occurred in 45% of diabetic patients, compared to 18.8% of non-diabetic patients. Furthermore, it has been shown that the incidence of myocardial infarction in diabetic patients without CAD is comparable to the incidence of recurrent myocardial infarction in non-diabetic patients with CAD. Epidemiological studies of patients with CAD in Japan have also reported a higher risk of total mortality and cardiovascular events in diabetic patients 209 , 335 , 1281) . An analysis of the J-LIT trial in patients with CAD, a large cohort study of low-dose simvastatin in hyperlipidemic Japanese patients, also showed an approximately 2.5-fold increase in the relative risk of cardiovascular events with concomitant diabetes 90) . According to a meta-analysis 1280) of CholesterolTreatment Trials (CTT) collaborators using 14 randomized controlled trials of statins, a significant reduction in the incidence of major vascular events has been observed with statin-based LDL-C lowering therapy, equally in patients with and without diabetic complications. Furthermore, its event suppressing effect was shown to correlate with the absolute decrease in LDL-C. A subanalysis 1282) of the TNT study in patients with CAD complicated by diabetes reported that high-dose statin treatment significantly reduced cardiovascular and cerebrovascular events by 25% and 31%, respectively, compared to regular-dose statin treatment. Furthermore, the REALCAD study 1115) comparing the effect of high-intensity statins with pitavastatin 1 mg and 4 mg in Japanese patients with stable angina pectoris, including 40% diabetic patients, showed a 19% reduction in cardiovascular events.
A meta-analysis of clinical trials conducted overseas using IVUS reported that diabetes mellitus was an independent risk in patients with coronary plaque development despite treatment with LDL-C below 70 mg/dL 1283) . A significant positive correlation has been found between the development of coronary plaque volume and the incidence of cardiovascular events and post-treatment LDL-C, suggesting that a more stringent LDL-C lowering therapy is important in patients with CAD complicated by diabetes. A subanalysis of the Japan-ACS study 1284) conducted in Japan in ACS also showed that diabetes mellitus was a strong negative risk of plaque regression, and diabetic patients had a markedly lower rate of plaque regression, even though their LDL-C was controlled to the same degree as that of non-diabetic patients. However, significant plaque regression has been reported when LDL-C is controlled below 75 mg/dL 1285) . A subanalysis 1286) of the IMPROVE-IT trial, which compared diabetic and non-diabetic patients, showed that lowering LDL-C more with additional ezetimibe resulted in stronger cardiovascular event prevention in high-risk patients with diabetes.
4) Atherothrombotic Cerebral Infarction
ASCVD, including CAD, cerebrovascular disease, and peripheral arterial disease, all of which have atherosclerosis as a common basis for their incidence, are mutually high-risk conditions for vascular complications. The REACH registry, a registry study of patients with ASCVD (CAD, cerebrovascular disease, and PAD) or overlapped atherosclerotic risk factors, found that approximately 16% of cases were complicated by two or more ASCVDs 1287) . A comparison of the CREDO-Kyoto database in Japan and the Texas Heart Association database in the United States, a registry study of patients who underwent coronary revascularization surgery, showed that the complication rate of cerebrovascular disease was significantly higher in Japan (16.4% vs. 5.0%) and that cerebrovascular disease was a high-risk condition for the development of cardiovascular events in both Japan and the United States 1281) . The results of secondary prevention trials of CAD conducted in Europe and the United States, such as 4S, LIPID, and CARE, have shown that patients with CAD with a history of cerebrovascular disease have a higher risk of recurrent cerebrovascular and cardiovascular events, but that statin-lowering therapy of LDL-C reduces the risk of recurrent cerebrovascular and cardiovascular events 1288 - 1290) .
5.2 Diabetes Mellitus
FQ23. Is comprehensive strict control of blood glucose, lipid, and blood pressure recommended for patients with diabetes from the early stage?
Since patients with diabetes often have multiple risk factors, comprehensive strict management of blood glucose, lipid and blood pressure is recommended from the early stage of the disease. (Level of evidence: 1, Level of recommendation: A)
1) Risk Factors for ASCVD
Risk factors for primary prevention of ASCVD include LDL-C and TG for CAD and systolic blood pressure for stroke in the JDCS patients with type 2 diabetes in Japan 268) . Other Japanese and international studies have identified low HDL-C, high HbA1c, smoking, and high Lp(a) levels as risk factors 1291 , 1292) . The comprehensive management of these risk factors is important for the prevention of ASCVD in patients with diabetes. In patients with metabolic syndrome, interventions focusing on lifestyle modification are especially recommended.
2) Blood Glucose
Meta-analysis has shown that enhanced glycemic control reduces the incidence of ASCVD 1293 , 1294) . In the UKPDS, the study of early stage type 2 diabetes, the intensive glucose lowering for approximately 10 years did not show differences in the incidence of ASCVD compared to the standard care group. However, about 10 years after the end of the intervention, the incidence of acute myocardial infarction and total mortality were significantly reduced in intensive care group 1295) . DCCT/EDIC showed that a significant reduction in total mortality was observed 15 years after the end of intervention in the patients with type 1 diabetes 1296) . Thus, it takes a long period of time to show a beneficial effect of intensive glucose control on the incidence of ASCVD, and good glycemic control from early stage might improve long-term prognosis 1295 , 1297) . On the other hand, strict glycemic control increases the risk of hypoglycemia 1294) . The occurrence of severe hypoglycemia and hypoglycemia-related arrhythmias might be associated with the development of cardiovascular death 1298 , 1299) , thus it requires close attention to hypoglycemia, especially in older people 1300) . Several types of diabetes drugs have been reported to have supressive effects on the development of ASCVD. Multiple trials showed SGLT2 inhibitors 1301 , 1302) and GLP-1 receptor agonists 1303 - 1305) have reduced the incidence of ASCVD, particularly in subjects with high-risk and secondary prevention.
According to the study of Japanese older people, J-EDIT, the risk of stroke incidence was 2.63 times higher in the group with HbA1c of 8.5% or higher compared to the group with HbA1c of 7.0% to 8.4%, while the risk was 2.35 times higher in the group with HbA1c less than 7.0%. Uniformly targeting HbA1c reduction should be avoided in older patients with diabetes, and attention should be paid not only to hyperglycemia but also to the possibility of hypoglycemia underlying excessive glycemic control 1306) .
3) Lipids
Patients with diabetes frequently show hyper-LDL cholesterolemia, hypertriglyceridemia, and hypo-HDL cholesterolemia. In the JDCS , the hazard ratio of CAD increased by 1.49 for each 1 SD increase of LDL-C 268) . In a meta-analysis including CARDS 1307) and other studies, LDL-C lowering therapy by statins significantly reduced the risk of death from cardiovascular disease and cerebral infarction in patients with diabetes, and the effect was comparable to that in patients without diabetes 1280) . In the JDCS, each 1 SD increase in the logarithm of TG lead to a 54% increase in CAD. FIELD, which examined the effect of a fibrate on cardiovascular disease, found no significant difference in the primary CVD outcome, but a 24% reduction in the incidence of nonfatal myocardial infarction was observed 1126) . In UKPDS23, fatal myocardial infarction increased by 19% for patients who has HDL-C less than 38 mg/dL 1291) .
4) Blood Pressure
High blood pressure in patients with diabetes is a risk of ASCVD 1308) . NIPPON DATA80 also shows that higher blood pressure at the beginning of follow-up in patients with diabetes increases the absolute risk of death from ASCVD 286) . In a meta-analysis, the efficacy of antihypertensive therapy was observed in cerebrovascular disease. However, in CAD, the risk reduction was limited, as it was only observed in the group with high blood pressure before starting treatment 1309 , 1310) .
5) Comprehensive Risk Management
The importance of comprehensive and early management of risk factors such as hyperglycemia, hypertension, dyslipidemia, smoking in the prevention of ASCVD in patients with diabetes has been demonstrated in the Steno-2 study 1311 , 1312) . The standard therapy include diet 894) , exercise 1313) and smoking cessation 161) . However, the LOOK AHEAD study in the USA, in which lifestyle interventions were continued for more than 9 years, did not show supression of cardiovascular events despite HbA1c and other factors were improved 1314) . Although there is no clear evidence that lifestyle modification alone reduces the incidence of ASCVD or mortality in patients with diabetes 1315) , the JDCS suggests that combination of lifestyle modification and pharmacotherapy is effective in suppressing ASCVD 1053) . In the J-DOIT3 study, 2,542 Japanese patients with type 2 diabetes mellitus were randomized to conventional therapy or intensive therapy aimed at better control of HbA1c, blood pressure, LDL-C, and obesity. Although there was no statistically significant difference in the primary outcome (myocardial infarction, stroke, revascularization or death), a significant reduction in incidence was observed in the intervention group when predetermined adjustment was performed for factors such as smoking (hazard ratio 0.76, 95% CI 0.59-0.99), demonstrating the importance of comprehensive risk management in Japanese patients 1316) .
FQ24. Is strict LDL cholesterol management recommended in patients with diabetes complicated by PAD, microangiopathy (retinopathy, nephropathy, neuropathy), or in the presence of smoking, along with management of other risk factors?
In patients with diabetes complicated by PAD, microangiopathy (retinopathy, nephropathy, neuropathy), or in the presence of smoking, we suggest targeting LDL-C less than 100 mg/dL along with management of other risk factors. (Level of evidence: 1, Level of recommendation: B)
In primary prevention of ASCVD, patients with diabetes are classified as a high-risk group based on the scoring using the Hisayama study data. In the 2017 edition of this guideline, their treatment goals are less than 120 mg/dL of LDL-C and 150 mg/dL of non-HDL-C. In addition, as indicated in BQ7, the risk of CAD is particularly high in the presence of FH, noncardiogenic cerebral infarction (especially atherothrombotic cerebral infarction), PAD, microangiopathy (retinopathy, nephropathy and neuropathy), or in the presence of smoking. Most randomised controlled trials in patients with diabetes who have these risk factors are performed in Western countries, however in conjunction with Japanese trials, the data suggest that lowering LDL-C to less than 100 mg/dL is appropriate in Japanese patients with primary prevention, as discussed below. The management targets for patients with diabetes with noncardiogenic cerebral infarction or FH are described in Chapter 3, 3.1 and Chapter 4, respectively.
Although the 2019 U.S. guidelines do not set an explicit goal for LDL-C based on cardiovascular risk, moderate or strong statins are recommended for patients with diabetes aged 40-75 years, regardless of lipid levels 1317) . On the other hand, the European ESC/EAS guidelines recommend a control target of LDL-C less than 100 mg/dL for all patients with type 2 diabetes. Furthermore, targeting LDL-C less than 70 mg/dL is recommended for high risk patients who have additional risk factors or more than 10 years’ diabetes duration, and 55 mg/dL for very high risk patients who have organ damages or three or more risk factors 457) . Thus, in Europe and the United States, there is a consensus on strict lipid management for primary prevention in patients with diabetes.
Evidence from Western Country on Primary Prevention in Patients with Diabetes
In the CTT Collaboration, a meta-analysis of 14 RCTs (4S, WOSCOP, CARE, Post-CABG, AFCAPS/TexCAPS, LIPID, GISSI-P, LIPS, HPS, PROSPER, ALLHAT-LLT, ASCOT-LLA, ALERT, CARDS), a sub-analysis of patients with diabetes demonstrated that lowering LDL-C by 39 mg/dL with statins significantly reduced major cardiovascular events by 21%, all-cause mortality by 9%, and cerebrovascular events by 21%, and this effect was not different between patients with diabetes (18, 686 patients) and non-diabetes (71,370 patients). In this study, 63% of patients with diabetes had no history of cardiovascular events, and the event-suppressing effect was observed for both primary and secondary prevention 1280) .
In CARDS, the efficacy of atorvastatin 10 mg/day was studied in patients with type 2 diabetes without a history of CAD who showed LDL-C less than 160 mg/dL and TG less than 600 mg/dL and had one or more risk factors such as retinopathy, albuminuria, current smoking and hypertension. After 4 years, LDL-C decreased to 81.6 mg/dL in the atorvastatin group versus 120.7 mg/dL in the placebo group, with a reduction of 37% in major cardiovascular events and a 27% reduction of all-cause mortality 1307) .
In the Steno-2 extension study, a comprehensive intensified lipid, glucose and blood pressure treatment was compared to a standard therapy in patients with type 2 diabetes with microalbuminuria. LDL-C was 124 mg/dL in the standard group versus 86 mg/dL in the intensified group after a mean intervention period of 7.8 years. After 13.3 years of observation, the intensified therapy group had a reduction of 57%, 59%, 46% in cardiovascular death, cardiovascular events and all-cause mortality, respectively 1312) .
In an analysis of 5,963 patients with diabetes at high risk of cardiovascular events in HPS, simvastatin treatment led to a 22% reduction in the first cardiovascular events. In this study, 49% of patients with diabetes did not have a history of CAD and 18% had vascular disease without CAD. The mean LDL-C after simvastatin treatment was 89.7 mg/dL, a decrease of 39 mg/dL compared to placebo. In patients with diabetes who had no ASCVD at entry, the first cardiovascular events occurred in 9.3% of the statin group, compared to 13.5% in the placebo group. In addition, in high-risk patients with diabetes who had vascular diseases other than CAD (cerebral infarction, PAD, etc.), occurrence of cardiovascular events was significantly less frequent in the statin group compared to the placebo group (25.6%in statin group vs 32.9% placebo group) 1318) .
SPARCL study included 4,731 patients with a history of stroke or TIA and no CAD to assess the effect of high-dose atorvastatin in preventing recurrent strokes and cardiovascular events 1319) . In a post hoc analysis of 794 patients with diabetes and 642 patients with metabolic syndrome, mean LDL-C was reduced to 79.6 mg/dL in the 80 mg atorvastatin group compared to 115.5 mg/dL in the placebo group. The results showed a significant reduction in CAD, cardiovascular events, and any type of revascularization therapy 1320) .
Treat Stroke to Target study examined the incidence of major cardiovascular events in 2,860 patients with TIA or ischemic stroke. The patients were randomized to intensive therapy group targeting LDL-C <70 mg/dL and standard therapy group targeting LDL-C 90-110 mg/dL. In the overall analysis, the intensive therapy group showed a significant 22% cardiovascular event reduction, and sub analysis of 643 patients with diabetes showed significant 40% reduction 353) .
These studies from Europe and United States indicate that suppressing LDL-C to at least less than 100 mg/dL is effective in the primary prevention of patients with diabetes who have risk factors such as retinopathy, nephropathy, cerebrovascular disease, peripheral arterial disease, and in the presence of smoking.
Japanese Evidence on Primary Prevention in Patients with Diabetes
On the other hand, EMPATHY, which compared intensified therapy (target LDL-C <70 mg/dL) with standard therapy (target LDL-C 100-120 mg/dL) in 5,042 Japanese patients with type 2 diabetes with retinopathy and no history of CAD, no significant differences were observed in composite cardiovascular coutcomes after a mean follow-up of 37 months. The mean LDL-C during the study period was 76.5 mg/dL in the intensified therapy group and 104.1 mg/dL in the standard therapy group. However, the relationship between LDL-C reduction and event reduction rate was consistent with the prediction from previous studies. Also, an exploratory analysis showed a 48% reduction in cerebral events in the intensified therapy group 1106) .
In J-DOIT3, which examined the effectiveness of comprehensive intensified therapy for blood glucose, lipids, and blood pressure in Japanese patients with type 2 diabetes with hypertension, the mean LDL-C was 85 mg/dL in the intensified therapy group and 104 mg/dL in the standard therapy group. In the intensified therapy group, the composite primary endpoint of all-cause mortality, coronary events, and cerebrovascular events was significantly reduced by 24% after adjustment. In addition, cerebrovascular events were significantly less frequent by 58% in a post hoc analysis 1316) .
These studies provide a good basis for lipid-lowering therapy with treatment goals less than 100 mg/dL of LDL-C for the primary prevention of Japanese patients with type 2 diabetes at risk of cardiovascular events.
5.3 Cerebrovascular Disease
To prevent recurrent cerebral infarction complicated by atherosclerosis, aim to manage LDL cholesterol to less than 100 mg/dL.
1) Frequency of Incidence
Cerebrovascular disease is classified into three types (cerebral hemorrhage, cerebral infarction, and subarachnoid hemorrhage). According to Stroke Data Bank 2021, the incidence of cerebrovascular disease by type in Japan is reported to be 19.5% for cerebral hemorrhage, 6.5% for subarachnoid hemorrhage and 74.0% for cerebral infarction 1321) . Compared to the incidence of cerebrovascular disease by type in Europe and the United States, hemorrhage is still more common and cerebral infarction is relatively less common 1322) .
Cerebral infarction is further classified into three clinical types (lacunar infarction, atherothrombotic cerebral infarction, and cardiogenic cerebral embolism). In J-MUSIC published in 2000, lacunar infarction was reported in 38.8%, atherothrombotic cerebral infarction in 33.3%, and cardiogenic cerebral embolism in 21.8% 1323) , and in Stroke Data Bank 2021, lacunar infarction was reported in 28.2%, atherothrombotic cerebral infarction in 31.5%, and cardiogenic cerebral embolism in 28.8 1321) . A 2011 Shiga Prefecture survey of the general population reported 155.3 total strokes, 99.8 cerebral infarctions, 39.3 cerebral hemorrhages and 14.3 subarachnoid hemorrhages per 100,000 population, with 25.1 lacunar infarctions, 31.3 atherothrombotic cerebral infarctions, 25.3 cardiogenic cerebral infarctions and 18.1 other types of cerebral infarctions 1325) . and 18.1 other cerebral infarctions 1324) . The frequency of cerebral infarction in Europe and the United States (Caucasians) is reported to be approximately 30% for both lacunar infarction and atherothrombotic cerebral infarction, and approximately 40% for cardiogenic cerebral infarction 1325) . In Japan, atherothrombotic cerebral infarction is believed to account for about 30% of all cerebral infarctions based on atherosclerosis.
2) Risk Factors for Incidence
According to the results of NIPPON DATA80, the factors that influence cerebrovascular disease mortality in the Japanese population are age, systolic blood pressure, smoking, and hyperglycemia, while lipid levels such as TC are not considered risk 58) . Similarly, a summary of 61 observational studies in Europe and the United States (about 900,000 subjects) found no relationship between TC and cerebrovascular disease mortality 1326) . A meta-analysis of 18 cohort studies in Japan and China also showed that blood pressure is the most important risk factor for cerebrovascular disease and that TC involvement is considerably lower than blood pressure 1327) .
When examined individually, the risk factors for hemorrhage are hypertension, heavy drinking, smoking, and antithrombotic drug use, while for subarachnoid hemorrhage, the main risk factors are hypertension, smoking, heavy drinking, and the presence of a cerebral aneurysm. Among cerebral infarctions, atrial fibrillation is the main risk of cardiogenic cerebral infarction 1328 , 1329) .
When considering risk factors limited to non-cardiogenic cerebral infarction, the Stroke Data Bank 2021 reported a frequency of dyslipidemia of 39% in non-cardiogenic cerebral infarction 1321) . However, epidemiological studies in Japan have not shown a significant relationship between serum cholesterol levels (TC, LDL-C and non-HDLC) and incidence 42 , 44 , 46 , 47 , 59 , 65 , 85) . However, in a cohort study of 267,500 subjects in China, TC was significantly positively correlated with the incidence of cerebral infarction incidence 1330) . In Europe and the United States, epidemiological studies such as MRFIT have reported an increased risk of cerebral infarction with increasing TC 1331 - 1333) . A summary of nine cohort studies also reported a significant 15% reduction in cerebral infarction with a lower LDL-C1336) of 1 mmol/L (38.6 mg/dL) lower LDL-C 1334) . A meta-analysis of 21 large clinical trials by the Cholesterol Treatment Trials (CTT) Collaboration similarly found that a reduction of 1 mmol/L (38.6 mg/dL) reduction in LDL-C was associated with a 15% reduction in stroke incidence and a 20% reduction in cerebral infarction incidence 331) . In contrast, there are reports that TC is not a risk of cerebral infarction or has weak involvement 1335 , 1336) .
In the Hisayama study, which examined the risk by type of cerebral infarction, LDL-C was found to be a risk for the incidence of atherothrombotic cerebral infarction after multivariate analysis, but no relationship between LDL-C and the incidence of other types of cerebral infarction was observed 42) . Cholesterol levels are recognized as a risk factor only for atherothrombotic cerebral infarction, and, furthermore, hypertension is considered the primary risk factor for all cerebral infarctions, including atherothrombotic cerebral infarction 1328) .
Hypocholesterolemia has already been reported to be a risk of bleeding in many countries, including Japan 46 , 1337) . A meta-analysis of cohort studies reported a decrease in LDL-C of 1 mmol/L (38. mg/dL) increases cerebral hemorrhage by 19% 1334) , and in Japan, the frequency of hemorrhage is reported to increase when LDL-C is below 80 mg/dL 46) . In China, an increased risk of hemorrhage has been reported at TC levels below 120 mg/dL 1330) . However, as discussed in the following, a meta-analysis of CAD prevention trials has found no increase in hemorrhage with cholesterol-lowering therapy 614) . In the FOURIER study, which examined the efficacy of a PCSK9 inhibitor (evolocumab) in 27,564 patients with a history of atherothrombosis, the treatment group had a decrease in LDL-C to 30 mg/dL over a mean follow-up period of 2.2 years, but did not have an increased risk of hemorrhage 1143) . In many countries, including Japan, it has also been shown that the incidence of cerebral infarction increases with lower HDL-C levels 54 , 84 , 1338 , 1339) .
Many reports have not shown a certain relationship between TG and cerebrovascular disease 1336 , 1340 , 1341) . On the other hand, a positive correlation between TG and the risk of cerebral infarction was observed in China 1330) , and a meta-analysis of epidemiological studies in the Asia-Pacific region reported a 50% increased risk of ischemic stroke in the highest TG group compared to the lowest group 97) when fasting TG was divided into 4 groups.
A cohort study of approximately 14,000 individuals reported an increased frequency of ischemic stroke in both men and women with non-fasting hypertrigceridemia 1342) . The results indicate that a 1 mmol/L (88.5 mg/dL) increase in non-fasting TG results in a 15% increase in ischemic stroke.
3) Lipid-lowering Therapy and Cerebrovascular Disease
Although statins are well established to prevent the incidence of stroke, relatively few studies have been conducted on the prevention of recurrent stroke. Most trials have considered stroke as a secondary endpoint. A meta-analysis of prevention trials conducted in the United States and Europe showed a significant 19% reduction in cerebral infarction after statin-lowering cholesterol therapy. The hemorrhage, on the other hand, did not show a significant change 614) . In the MEGA study conducted in Japan in hypercholesterolemic patients without a history of CAD or stroke, statin treatment showed a trend toward a reduction in stroke with a hazard ratio of 0.66 (men) and 0.63 (women) 49) . In particular, ischemic stroke in men and stroke in women over 55 years of age were significantly reduced 1122 , 1343) . It is not yet clear why statin treatment reduces cerebrovascular disease, although observational studies have not found cholesterol levels to be a risk factor for cerebrovascular disease.
Trials with a primary end point of recurrent stroke in patients with a history of stroke include SPARCL 1319) , J-STARS 348) , and TST 353) . In SPARCL, high-dose statins were administered to patients with a history of stroke or transient ischemic attack (TIA) within 6 months of incidence without CAD, and the rate of recurrent stroke was compared to the placebo group, with a significant reduction in recurrent stroke with a mean reduction in LDL-C of 73 mg/dL in the statin group (-16%, p=0.03), as well as a significant reduction in the incidence of CAD (-35%, p=0.003). Additionally, a post hoc analysis of the breakdown of strokes that occurred as an endpoint showed a significant reduction in cerebral infarction (hazard ratio 0.78), but a significant increase in hemorrhage (hazard ratio 1.66). However, there was no association between increased hemorrhage and LDL-C 1345) . The study showed that statin therapy significantly reduced recurrent stroke in the group whose LDL-C dropped to less than 50% or 70 mg/dL at the beginning of observation 1345) . In patients with carotid artery stenosis, statin treatment significantly prevented recurrent stroke by 33%, but the effect on recurrence was not significant in patients without carotid artery stenosis 1346) . However, in a study by type of disease at the time of enrollment, the effect of statins on preventing recurrent stroke was similar for atherothrombotic cerebral infarction, lacunar infarction, and TIA 1347) . However, it should be noted that the statin doses in this study were significantly higher than the maximum approved doses in Japan. In the J-STARS study of cerebral infarction excluding cardiogenic cerebral infarction in Japanese patients, the incidence of atherothrombotic cerebral infarction was significantly reduced in the pravastatin group, although the primary endpoint of recurrent stroke or TIA was not different between the two groups (hazard ratio 0.33). On the other hand, the incidence of intracranial hemorrhage was comparable to that of the nonstatin group (hazard ratio 1.00) 348) . Post hoc analysis showed that LDL-C 80-100 mg/dL tended to cause the fewest recurrent strokes 1262) . TST 353) , patients with cerebral infarction within the past 3 months or TIA within the past 15 days and atherosclerosis (≥ 50% stenosis in intracranial or extracranial arteries, aortic atheroma, or history of CAD) were randomized to either usual control of LDL-C at 90-110 mg/dL or strict control to lower LDL-C below 70 mg/dL. The primary endpoint of cerebral infarction, myocardial infarction, emergency coronary or carotid revascularization, or cardiovascular death was significantly reduced by 22% 353) . In general, 2,860 patients were entered into the study, with 2,449 cerebral infarctions at enrollment that showed an effective benefit of strict lipid management (hazard ratio 0.67), but the incidence of the primary endpoint was rather higher in the strict lipid management group for 405 transient ischemic events (hazard ratio 2.06). Secondary endpoints of stroke and urgent carotid artery reconstruction tended to decrease by 19% in the strict control group, although not significantly, and the risk of cerebral hemorrhage was similar between the two groups.
A subanalysis of JELIS also showed that statins plus eicosapentaenoic acid (EPA) significantly reduced recurrent stroke by about 20% compared to statins alone 1191) 48). Other reports have also observed a favorable outcome of cerebral infarction that occurs during statin administration 1348) and an increased risk of cerebral infarction with statin discontinuation 1350) .
4) Strategies to prevent cerebrovascular disease
Since hypertension is the most significant risk of cerebrovascular disease, controlling blood pressure is the first priority. Atrial fibrillation is a major risk factor for cardiogenic cerebral embolism, while smoking, heavy alcohol consumption, and the presence of cerebral aneurysms, in addition to hypertension, are major risk factors for subarachnoid hemorrhage. Therefore, it is necessary to treat these risk factors appropriately. The relevant guidelines should be referred to for these management measures 1344) . In Europe and the United States, lipid-lowering therapy is recommended for the prevention of non-cardiogenic cerebral infarction based on the results of meta-analysis of prevention trials and other studies 1326 , 1345) . In Japan, (1) the rate of atherothrombotic cerebral infarction is increasing, (2) the MEGA study has shown that statins are effective in preventing cerebral infarction in patients with dyslipidemia, and (3) strict lipid lowering therapy has been shown to be effective in preventing recurrent cerebral infarction caused by atherosclerosis. Therefore, to prevent cerebral infarction, patients with dyslipidemia should undergo appropriate lipid management along with adequate antihypertensive treatment. For prevention of recurrent non-cardiogenic cerebral infarction (including atherothrombotic cerebral infarction) complicated by atherosclerosis such as more than 50% stenosis in intracranial and extracranial arteries or aortic complex atheroma lesions, LDL-C should be controlled to less than 100 mg/dL in accordance with management criteria for prevention of recurrent CAD.
5.4 CKD - Chronic Kidney Disease
•For the treatment of hypertension in patients with CKD, ACE inhibitor or angiotensin II receptor blocker (ARB) therapy is recommended for renal protection.
•For the treatment of diabetes in patients with CKD, SGLT2 inhibitor therapy is recommended for renal and cardiovascular protection.
•Statin alone or statin in combination with ezetimibe is recommended to control LDL cholesterol and non-HDL cholesterol in patients with CKD.
•Consider the use of n-3 polyunsaturated fatty acids or a selective PPARα modulator which is excreted into bile for the management of hypertriglyceridemia in patients with CKD.
Two points should be considered to reduce the risk of CVD in CKD: (1) prevention or improvement of CKD itself, and (2) proactive intervention for risk factors other than CKD.
As treatment for CKD itself, immunosuppressive therapy has been recommended for glomerulonephritis, antihypertensive therapy for hypertensive nephrosclerosis, and antihypertensive therapy in addition to diabetes treatment for diabetic nephropathy, including ACE inhibitors and angiotensin II receptor blockers (ARBs), which have evidence of effectiveness in reducing albuminuria and improving renal prognosis. Additionally, appropriate intake of energy, protein, and salt have been considered as dietary therapy.
Recently, randomized controlled trials have shown that SGLT2 inhibitor therapy improves renal outcomes in patients with type 2 diabetes (EMPA-REG trial 1350) , CREDENCE trial 1351) ), and in patients with CKD without diabetes (DAPA-CKD trial 1352) ). Furthermore, since SGLT2 inhibitors have been shown to improve cardiovascular outcomes 1301 , 1302 , 1353 , 1354) , they are promising agents which are beneficial both for CKD itself and cardiovascular outcomes. On the other hand, in patients with diabetes who have a decreased eGFR, since the glucose-lowering effect of SGLT2 inhibitors is decreased, appropriate use should be noted in clinical practice according to the instructions in the package insert.
Interventions for risk factors other than CKD, such as medications for hypertension and diabetes, are discussed above. Other than these, weight loss for the metabolic syndrome and management of dyslipidemia are important. It should be noted that although the CVD risk is higher in patients with more advanced stages of CKD, the relative CVD risk reduction achieved by lipid-lowering therapy is smaller 279) . That is, lipid-lowering therapy with statin alone or statin in combination with ezetimibe significantly reduced CVD risk in undialyzed CKD 1355) , but not significantly in CKD patients on dialysis 281 , 282) . In contrast, a post hoc stratified analysis of the RCT results 1356) showed that the relative risk reductions for CAD (by 48%) and stroke (by 73%) in patients with stage G3 CKD (eGFR 30-59 mL/min/1.73 m2) were significant, showing that the relative risk reduction was greater than in the analysis of the entire population. These results suggest that proactive lipid-lowering therapy should be considered for CKD patients from early stages of CKD including stage G3.
Dyslipidemia in CKD patients is characterized by hypertriglyceridemia and hypo-HDL cholesterolemia. Although n-3 polyunsaturated fatty acid preparations can be used to treat hypertriglyceridemia in patients with decreased kidney function, fibrates which are excreted through kidney have been hesitated to use. Pemafibrate, a selective PPARα modulator, has been launched and can be prescribed in clinical practice. Since pemafibrate is excreted mainly into bile, it has been reported that there is no marked increase in blood concentration even in patients with impaired kidney function, including dialysis patients 1357) . Thus, it is expected to be useful in patients with low kidney function. However, since the original package insert stated that it is contraindicated in those with serum Cr of 2.5 mg/dL or higher (Note: the package insert has been revised later and the sentence of cotraindication has been removed), it should be used appropriately.
Although observational studies have shown that hypertriglyceridemia and hypo-HDL cholesterolemia are associated with a higher CVD risk in CKD patients 1358) , there is no solid evidence for a benefit in renal protection or reduction of CVD risk in patients with CKD by pharmacotherapy to improve these conditions. Therefore, when initiating drug therapy for hypertriglyceridemia in CKD patients, it is important to select safe drugs and determine the benefit in each individual case.
6. Comprehensive Risk Assessment and Management Practices
•To prevent atherosclerotic cerebral and cardiovascular disease, management of the major risk factors for ASCVD should be comprehensive from the early stages of the disease.
•Lifestyle modifications such as diet, exercise, and smoking cessation are fundamental and must be continued.
•The introduction or continuation of pharmacotherapy should be done carefully according to individual risk and pathophysiology, and strict treatment is necessary in high-risk cases.
To prevent ASCVD, it is essential to evaluate and manage multiple risk factors such as hypertension, diabetes, dyslipidemia, CKD, and obesity 1359) . Therefore, the 14 societies centered on the Japan Society of Internal Medicine, the Japan Medical Association, and the Japan Medical Association have published the “Comprehensive Management Chart for the Prevention of Cerebral and Cardiovascular Disease” as a comprehensive management guideline 284) . The 2019 edition reflects revisions to each guideline and adds measures and considerations for the prevention of stroke and cardiovascular disease in older people, taking into account the urgent issue of extending healthy life expectancy among the elderly, which is a pressing issue associated with the rapid aging of society.
Comprehensive risk assessment and management based on this comprehensive chart is presented in order from Step 1 to Step 6 ( Fig.8 ) . Although the main target is first-time examinees who need to be evaluated for risk factors for atherosclerosis, patients with a history of ASCVD or already being treated for dyslipidemia, diabetes, or hypertension should also be periodically reevaluated over time for risk and status of treatment according to this section.
Fig.8.
Comprehensive risk management chart for cerebral and cardiovascular disease prevention
Step 1 Screening for Atherosclerotic Cerebral and Cardiovascular Disease Risk Assessment
•Comprehensive screening for major risk factors is important for the comprehensive risk management of the risk of cerebral and cardiovascular disease, which requires not only blood and biochemical tests, but also careful medical history and tests.
•The screening consists of Step 1a, which consists of basic items, Step 1b, which includes additional items, and Step 1c, which describes the criteria for referral to a specialist.
•Blood samples for clinical examinations should be taken in Step 1a, preferably in fasting if possible, and in Step 1b in principle in fasting.
Step 1 consists of Step 1a and Step 1b, which describe the basic and additional screening items, respectively, and Step 1c, which describes the criteria to determine the need for referral to a specialist.
1) Step 1a is the basic screening section, and the following table lists the necessary questions, physical findings, and examinations that must be performed to assess the risk of ASCVD in each patient ( Table 14 ) . During the medical interview, in addition to the standard items of the Specific Health Examination, such as subjective symptoms, complications, medical history, lifestyle (smoking, passive smoking, alcohol), exercise and sleeping habits, home blood pressure, and family history are also recommended. The following basic patient information and physical findings are recommended: age, sex, height, weight, BMI, office blood pressure, pulse rate/minute (normal and irregular), and chest auscultation. Recommended blood examinations include TC, HDL-C, non-HDL-C (TCHDL-C), eGFR (serum creatinine), ALT, γ-GTP, HbA1c, and blood glucose, as well as general urine (qualitative) and ECG (in cases such as atrial fibrillation, refer to a specialist depending on the degree of abnormality).
Table 14. Step 1a Screening (Basic Items).
| Medical interview* |
|
Age, sex, subjective symptoms, family history, complications, medical history, medication history, lifestyle habits (smoking**, passive smoking and alcohol consumption), exercise habits, and sleep and home blood pressure |
| Physical findings |
| Height, body weight, BMI (kg/m2), in‐clinic blood pressure, pulse rate (regular or irregular), and chest auscultation |
| Basic tests (fasting blood preferred) |
| TC, HDL‐C, non‐HDL‐C (TC − HDL‐C), eGFR, ALT, γ‐GT, HbA1c***, blood glucose**, urinalysis (qualitative), and electrocardiography*** |
*Use the standard or additional medical questionnaire of Lifestyle Health Check‐Ups.
**Includes heated cigarettes.
***If only one of HbA1c or blood glucose shows “diabetic type” (HbA1c ≥ 6.5% or fasting blood glucose ≥ 126 mg/dL or non‐fasting blood
glucose ≥ 200 mg/dL), the examination should be repeated on another day.
****The patient can refer to a specialist depending on the degree of abnormality (i.e., atrial fibrillation).
2) Step 1b is an additional screening item that is performed at the same time as Step 1a or when an abnormality is found in Step 1a ( Table 15 ) .
Table 15. Step 1b Screening (Additional Items).
| Physical findings |
| waist circumference, orthostatic blood pressure (after 1–3 min of standing), limb (artery), cervical vascular murmur, and abdominal vascular murmur. |
| Additional tests |
| Blood count, fasting blood glucose, fasting TG, LDL‐C, uric acid, K, chest radiograph, ankle‐ brachial index (ABI), plasma aldosterone concentration/renin activity ratio*, urinary protein/creatinine ratio (random spot urine quantification)** |
*Subjects to be measured: hypokinemia, or under 40 years of age, or blood pressure ≥ 160/100 mmHg. Judgment: If plasma
aldosterone concentration/renin activity ratio (ARR) >200 (CLEIA method) and blood aldosterone concentration (PAC [CLEIA method]) ≥ 60 pg/mL, refer to a specialist, etc.
**Measure when there is an abnormality on the general urine (qualitative) examination.
3) Step 1c describes conditions that may require referral to a specialist based on the screening described above ( Table 16 ) . If only one of HbA1c or blood glucose shows “diabetic type” (HbA1c ≥ 6.5% or fasting blood glucose ≥ 126 mg/dL or non-fasting blood glucose ≥ 200 mg/dL), the examination should be repeated on another day.
Table 16. Step 1c Determine the need for referral to a specialist.
| (1) If the patient is suspected to have a history or is complicated with stroke/transient ischemic attack (TIA), coronary artery disease (CAD), arrhythmia (such as atrial fibrillation), aortic disease, or peripheral arterial disease (PAD) |
| (2) Hypertension |
| Suspected secondary hypertension (early incidence, acute incidence, etc.), pregnancy‐induced hypertension, hypertensive emergency or urgency (untreated diastolic blood pressure ≥ 120 mmHg), treatment‐resistant hypertension (≥ 180/110 mmHg despite treatment or not achieving antihypertensive goal even with concomitant therapy with 3 drugs) |
| (3) Diabetes mellitus |
| Type 1 DM, HbA1c ≥ 8.0%, fasting blood glucose ≥ 200 mg/dL (or non‐fasting blood glucose ≥ 300 mg/dL), acute complications (hyperglycemic emergency), or gestational diabetes |
| (4) Dyslipidemia: |
| LDL‐C ≥ 180 mg/dL, HDL‐C <30 mg/dL, fasting TG ≥ 500 mg/dL, non‐HDL‐C ≥ 210 mg/dL, or suspected primary hyperlipidemia or secondary dyslipidemia |
| (5) Chronic kidney disease (CKD): |
| CKD patients with proteinuria and hematuria |
| eGFR <45 ml/min/1.73 m2 (G3b to 5 ) or proteinuria category A3 (urine albumin/ Cr ratio >300 mg/gCr in diabetes, urine protein/ Cr ratio >0.5 g/Cr otherwise). For patients under 40 years of age or in the A2 category (Urine albumin/Cr ratio 30-299 mg/gCr for diabetes, urine protein/Cr ratio 0.15-0.49 g/Cr for other conditions), referral should be made even if the eGFR is 45-59. |
| (6) Obesity: |
| Severe obesity (BMI ≥ 35). Suspected secondary obesity (symptomatic obesity) |
(1) LDL-C is calculated using the Friedewald formula after TC, HDL-C, and TG are measured simultaneously during fasting. (If TG < 400 mg/dL)
(2) Subjects to be measured: Hypokinesia, or age <40 years, or blood pressure ≥ 160/100 mmHg, if aldosterone/renin activity ratio >200 and the aldosterone concentration >120 mg/dL.
(3) Measure when there is an abnormality in the qualitative analysis of urine.
Step 2. Diagnosis and additional assessment in each risk factor
In Step 2, the diagnosis and additional assessment for each risk factor will be based on the following five items ( Table 17 ). In any of these conditions, carotid echocardiography, echocardiography, vascular echocardiography of the limb, coronary CT, thoracoabdominal CT, MRI, MR angiography, baPWV (pulse wave velocity), and CAVI (cardio-ankle vascular index) are performed as needed 23) .
Table 17. Step 2 Diagnosis and additional assessment of each risk factor.
| 2A Hypertension |
| In‐clinical blood pressure ≥ 140/90 mmHg or home blood pressure ≥ 135/85 mmHg |
| 24‐hour monitoring of blood pressure as needed (to differentiate between nocturnal and workplace hypertension) |
| 2B Diabetes mellitus |
| 2B‐1) When suspicion of diabetes cannot be ruled out |
| HbA1c 5.6‐6.4%, fasting blood glucose 100‐125 mg/dL, non‐fasting blood glucose 140‐199 mg/dL, or a family history of intense diabetes or obesity → 75 gOGTT (except those with obvious diabetic symptoms) |
| 2B‐2) When diagnosed with diabetes |
| If both HbA1c and blood glucose are diabetic type in the same blood sample, or if blood glucose is diabetic type and the patient has typical symptoms (dry mouth, polydipsia, polyuria, weight loss) or definite diabetic retinopathy, or if the diabetic type is reconfirmed by examination on another day (however, at least the initial and second examination, blood glucose must be diabetic type) → Fundus examination, urine albumin/Cr ratio (spot urine determination non‐fasting) |
| 2C Dyslipidemia |
| LDL‐C ≥ 140 mg/dL, HDL‐C <40 mg/dL, fasting TG ≥ 150 mg/dL, or non‐HDL‐C ≥ 170 mg/dL → check for corneal ring / Achilles tendon thickening / skin and tendon xanthoma / rash xanthoma |
| 2D CKD |
| eGFR <60 ml/min/1.73 m2 or proteinuria lasting >3 months |
| 2E Metabolic syndrome |
| Abdominal circumference ≥ 85 cm (men) or ≥ 90 cm (women) and 2 or more of the following: serum lipid abnormalities (HDL‐C <40 mg/dL or fasting TG ≥ 150 mg/dL), high blood pressure (≥ 130/85 mmHg), high blood glucose (fasting blood glucose ≥ 110 mg/dL) |
Step 3 Risk Factors to Review Before Initiating Treatment
Risk factors that should be especially noted at the time of starting treatment are listed. (1) smoking, (2) hypertension, (3) diabetes, (4) dyslipidemia, (5) chronic kidney disease (CKD), (6) obesity (particularly, visceral fat obesity), (7) aging and gender (men or postmenopausal women), (8) family history (history or complications of cerebral and cardiovascular disease or lifestyle-related diseases in your own grandparents, parents, or brothers and sisters related to blood, especially cases occurring at younger ages). It should always be kept in mind that strict management is necessary in cases where multiple risk factors are present. ( Table 18 )
Table 18. Step 3 Risk Factors to Review Before Initiating Treatment.
| (1) Smoking |
| (2) Hypertension |
| (3) Diabetes mellitus (including prediabetes) |
| (4) Dyslipidemia |
| (5) CKD |
| (6) Obesity (especially visceral obesity) |
| (7) Aging and gender (men or postmenopausal women) |
| (8) Family history |
*Always keep in mind that strict management is necessary in cases where multiple risk factors are present.
Step 4 Setting Management Targets according to Risk Factors for each Pathological Condition
In terms of management goals according to risk and individual pathological conditions, dyslipidemia is discussed in Chapter 3, while other risk factors conform to the guidelines of the Japanese Society of Hypertension 179) , the Japanese Diabetes Society 654) , the Japanese Society of Nephrology 13) and other societies ( Table 19 ). However, in older people, management goals should be individualized, taking into account individual circumstances such as activities of daily living (ADL), cognitive function, frailty, and quality of life (QOL).
Table 19. Step 4 Setting Management Targets according to Risk Factors for each Pathological Condition.
| 4A Hypertension: |
| (1) Patients under 75 years of age, patients with cerebrovascular disease (without bilateral carotid stenosis or occlusion of themain cerebral artery occlusion), patients with CKD (positive proteinuria), patients with CAD, diabetes, and taking antithrombotic drugs: <130/80 mmHg (home blood pressure <125/75 mmHg) |
| (2) 75 years of age or older, patients with cerebrovascular disease (with bilateral carotid stenosis or occlusion of the main cerebral artery or not yet evaluated), CKD patients (proteinuria negative): <140/90 mmHg (home blood pressure <135/85 mmHg) |
| 4B Diabetes mellitus: |
| (1) A control indicator of HbA1c <6.0% when the goal is to normalize the blood glucose level |
| (2) A control indicator of HbA1c <7.0% to prevent complications |
| (3) A control indicator of HbA1c <8.0% if intensification of treatment is difficult |
| 4C Dyslipidemia: |
| HDL‐C ≥ 40 mg/dL and TG <150 mg/dL for all risk categories in addition to the following: |
| Low risk: LDL‐C <160 mg/dL (non‐HDL‐C <190 mg/dL) |
| Moderate risk: LDL‐C <140 mg/dL (non‐HDL‐C <170 mg/dL) |
| High risk: LDL‐C <120 mg/dL (non‐HDL‐C <150 mg/dL) |
| 4D Obesity: |
| Improvement of hypertension, diabetes, and dyslipidemia with a reduction of at least 3% in body weight or waist circumference in 3 to 6 months |
*For older people, management targets are established taking into account individual circumstances such as living environment, activities of daily living (ADL), cognitivefunction, and quality of life (QOL), such as living alone or in a nursing home.
Step 5: Lifestyle Modification
Lifestyle improvement is the cornerstone of prevention of ASCVD and the easy initiation of drug therapy should be strictly avoided. During drug therapy, these non-drug therapies should not be neglected, i.e., lifestyle modification guidance should not be neglected. Smoking cessation is the most important cause of ASCVD and should be promoted among all age groups, regardless of gender, for its prevention. ( Table 20 )
Table 20. Step 5 Lifestyle items to be improved.
| Smoking cessation: No smoking is mandatory. Prevent passive smoking. |
| Weight control: |
| Weigh yourself regularly. |
| If BMI <25, maintain an appropriate weight. |
| If BMI ≥ 25, reduce energy intake to less than energy expenditure to lose weight. |
| Dietary management |
| Consume an adequate amount of energy and a good balance of the three macronutrients (protein, fat, and carbohydrates), vitamins, and minerals. |
| Avoid excessive intake of saturated fats and cholesterol. |
| Avoid trans fatty acids. |
| Increase intake of n‐3 polyunsaturated fatty acids. |
| Increase fiber intake. |
| Reduce salt intake, aiming for less than 6 g/day. |
| Physical Activity and Exercise |
| Perform exercise habitually, mainly aerobic exercise of moderate or greater intensity * (aim for a total of at least 30 minutes daily). |
| Remind them to reduce sedentary behavior** and stay active in their daily lives. |
| In addition to aerobic exercise, resistance and flexibility exercises should be performed. |
| Drinking |
| Alcohol consumption should be limited to 25 g*** of ethanol per day or less. Establish a day off from drinking. |
*Moderate or greater means intensity of 3 METs or greater, where METs is a unit of activity intensity that indicates the number of times the resting metabolic rate. **Sedentary behaviors are all arousalbehaviors with an energy expenditure of 1.5 METs or less in the sitting and lying positions. *** Equivalent to approximately 1 cup of sake, 1 medium beer bottle, half cup of shochu, doublewhiskey/brandy, or 2 glasses of wine.
The increased risk of incidence of CAD in non-smokers due to passive smoking is also a serious problem. Adequate energy and nutrient intake and correction of inappropriate eating habits and behaviors are fundamental for patients with risk factors such as dyslipidemia, hypertension, diabetes, and obesity. Eat a low-sodium Japanese diet with a combination of fish, soybeans, vegetables, seaweed, mushrooms, fruits, and unrefined grains, while avoiding animal fats. Avoid heavy alcohol consumption. Exercise has been shown to improve dyslipidemia, lower blood pressure, improve insulin resistance, and lower blood glucose. Aim to perform moderate to vigorous aerobic exercise (intensity of 3 METs or greater) for at least 30 minutes per day, at least 3 times per week (preferably daily). Be sure to check the current physical activity level, intensity, and exercise habits, and if there is no particular exercise habit, instruct the patient to gradually start with light exercise or short-duration exercise. However, in patients with complicated hypertension, exercise therapy is indicated for patients with blood pressure levels below moderate (160-179/100-109 mmHg) and without cardiovascular disease. Exercise therapy should be prohibited or limited in patients with diabetes who have extremely poor metabolic control (fasting blood glucose >25 mg/dL or moderate or high urine ketone positivity) or who have fresh fundus hemorrhage, CAD, or renal failure due to proliferative retinopathy.
In older people, strict dietary restriction and salt reduction may lead to sarcopenia with weight loss, so patients should be instructed to consume at least 1.0-1.2 g/kg standard body weight/day of adequate protein in the absence of severe renal dysfunction. In addition, exercise should be guided by attention to individual motor function and fall risk, and moderate resistance exercise should be performed in addition to aerobic exercise to prevent sarcopenia. Particularly in older people over 75 years of age, dietary guidance should be provided with consideration for the maintenance of dietary intake and quality of life.
Step 6 Drug Therapy
While lifestyle modification must continue and the initiation and continuation of drug therapy must be done with caution according to individual risk and pathophysiology, strict drug therapy is necessary in high-risk cases. Details of drug therapy for hypertension, diabetes, etc. Follow the guidelines for each disease; Special attention should be paid to drug side effects in older people over 75 years of age and in patients with renal dysfunction. In addition, while considering quality of life with regard to the treatment of lifestyle-related diseases for people with end-of-life conditions, discontinuation of drug therapy should be actively considered.
Chapter 4. Familial Hypercholesterolemia
BQ27. What is the prevalence of Familial Hypercholesterolemia in Japan?
In general, it is found in approximately 1 in 300 people in the general population, 1 in 30 people with CAD, and 1 in 15 people with premature CAD or severe hyper-LDL cholesterolemia. (Level of evidence: E-2)
Traditionally, the prevalence of FH was 1 in 500 people, but in recent years, a series of cross-sectional and cohort studies in the United States and Europe have shown a clearly higher prevalence than that. In Japan, Mabuchi et al. examined the frequency of molecular epidemiology in the Hokuriku region and reported a prevalence of 1 in 208 people 1360) .
A systematic review/meta-analysis published in 2017 (not including Japanese) reported a frequency of 1 in 250 people in the general population 1361) . In addition, a subsequent systematic review/meta-analysis (including Japanese) reported a frequency of 1 in 313 1362) and 311 1363) , respectively, in the general population. The meta-analysis that reported a prevalence of 1 in 250 included literature reporting extremely high frequencies due to the so-called founder effect, whereas the latter two reports of 1 in 313 and 311 excluded such studies. As increased frequency due to founder effect is presumed to exist in some regions, it is reasonable to assume that the number of cases is about 1 in 300, despite the report by Mabuchi et al. in Japan. The prevalence of FH is about 1 in 30 for patients with CAD and about 1 in 15 for those with premature CAD or severe hyper-LDL cholesterolemia (defined as >190 mg/dL in the meta-analysis) 1362) . The number of Japanese in the above meta-analysis is small, and it is assumed that differences in frequency may be due to factors such as consanguineous marriages in some regions and demographic bottleneck effects, but there is no evidence of significant differences among races; thus, the results of the above meta-analysis may be applied to Japanese as well.
BQ28. What are the prognosis and main complications of patients with FH?
•CAD: odds ratio 10 - 20 times higher than non-FH (Level of evidence: E-1a*)
•Peripheral arterial disease: odds ratio 5 - 10 times higher than non-FH (Level of evidence: E-1a)
•Stroke: no clear impact (Level of evidence: E-1a)
•Aortic valve stenosis: no epidemiological association was shown, but there have been case reports of FH complicating the disease. (Level of evidence: E-3)
•Abdominal aortic aneurysm: no epidemiological association was shown, but there have been case reports of FH complicating the disease. (Level of evidence: E-3)
*Although there was no meta-analysis of cohort studies, we chose E-1a because of the existence of multiple cohort studies and identical results.
The most important major complication of systemic atherosclerosis in patients with FH is CAD 1364) . In addition, a significantly higher prevalence of peripheral arterial disease and carotid atherosclerosis was reported in FH as compared to non-FH (systematic review/meta-analysis) 1365 , 1366) . On the other hand, regarding stroke, many reports state that its effects remain unclear. With regard to aortic disease and valvular disease (aortic aneurysm, aortic valve stenosis, supravalvular stenosis, etc.), there are case reports showing their association with the disease, although no epidemiological reports have demonstrated such an association.
Although there are no randomized controlled trials (RCTs) or systematic reviews on the prognosis of FH, Mabuchi et al. presented a study comparing the prognosis of FH heterozygotes (HeFH) and FH homozygotes (HoFH) in the pre- and post-statin era in Japan 1367) . This report shows that before the advent of statins, 73% of men and 64% of women with HeFH died of cardiac death, the age of death for HeFH increased from an average of 63 years before statins to 76 years after statins, and for HoFH, the average age of death increased from 28 years before statins to 59 years after statins.
FQ25. Can statins be recommended as the first choice in drug therapy for HeFH?
Strict lipid management with statins as first-line drugs is recommended for the treatment of HeFH. (Level of evidence: 3, Recommendation level: A)
There are 13 randomized double-blind trials examining the LDL-C lowering effect of statins in HeFH 1369 - 1380) , including 9 placebo-controlled trials (2 in adult HeFH 1368 , 1369) , 6 in children to adolescents HeFH 1371 - 1376) , and 1 in adult FH subjects without mention of whether they are HeFH or HoFH 1370) ), and its efficacy and safety have been established in both adult and pediatric cases. Furthermore, a randomized, double-blind, crossover study in HoFH has shown the efficacy of statin 1377) . In Japan, reports have been published showing that statin therapy reduces LDL-C in both adult and pediatric patients 1379 , 1380) , which is consistent with the results of clinical trials in other countries.
A randomized, double-blind, comparative study has been published in adult 1381) and pediatric patients 1376) , respectively, to investigate the efficacy of high intensity statin therapy (80 mg of atorvastatin, twice the approved dose in Japan) compared to standard intensity statin therapy (40 mg simvastatin, twice the approved dose in Japan) in adults and standard intensity statin therapy (20–40 mg pravastatin, no pediatric indication in Japan at the approved dose of 20 mg) compared to placebo in children. Both studies showed inhibition of the development of intima media thickness (IMT). A sub analysis of pediatric cases showed that statin initiation at an earlier age was associated with less IMT thickening 1382) . In addition, an observational study examining 2447 cases from the Netherlands has shown that statin use is associated with a lower incidence of CAD and lower all-cause mortality 1383) . Although scientific evidence for the prevention of atherosclerotic cardiovascular disease by direct comparison is not sufficient, statins appear to be the most recommended drug therapy at this time, given the abundant evidence in non-FH.
Four clinical studies have been published comparing the LDL-C lowering effects of statins and other lipid-lowering drugs in FH (all in adults, all with pravastatin, two in HeFH, and two in FH subjects). In a report comparing pravastatin 40 mg with cholestyramine 4 g (or colestipol 5 g), both groups had significantly lower LDL-C from baseline, but the group of pravastatin had significantly lower LDL-C than the cholestyramine group 1369) . On the other hand, reports comparing 40 mg of pravastatin with 16-24 g of cholestyramine (1.5-2 times the Japanese approved dose) did not show any significant difference between the two groups, although both groups had significantly lower LDL-C from baseline 1368 , 1370 , 1384) . These studies were conducted with pravastatin in the early 1990s, and since the newer generation of statins shows stronger lipid-lowering effects, it is expected to be more effective than cholestyramine. Although there are no direct comparative studies between statins and probucol, small intestinal cholesterol transporter inhibitors (ezetimibe), or proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9), there are reports of trials of ezetimibe and PCSK9 inhibitors on top of statins 1276 , 1277 , 1385 , 1386) .
FQ26. Is lipoprotein apheresis therapy recommended for HoFH and severe HeFH with drug resistance?
For HoFH and severe HeFH with drug resistance, strict control of LDL-C with lipoprotein apheresis therapy is recommended. (Level of evidence: 3, Recommendation level: A)
In the 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk 1387) , although there is no mention of evidence levels, etc., the lipoprotein apheresis has been recommended as a treatment for HoFH.
A systematic review reported in 2016 analyzed a total of 38 articles (8 open-label clinical trials, 11observational studies, 17 reviews/guidelines, and 2 medical technology evaluations) 1388) . Although RCTs were not included, they were noted to have clinical benefits in terms of lowering LDL-C, lowering lipoprotein (a) [Lp (a)], and preventing cardiovascular events. As each country has different rates of diagnosis of FH, availability and access to apheresis treatment, indications, methods, and costs, it will be necessary to evaluate the situation in Japan in the future. However, although this puts physical and social burden on the patient, we believe that lipoprotein apheresis is recommended for patients with FH who do not respond adequately to drug therapy in Japan, where access is relatively easy in terms of transportation and cost.
In 2019, a systematic review examining 76 case reports (209 patients) on HoFH in children was reported 1389) . Although it has not been shown whether lipoprotein apheresis is more or less beneficial than drug therapy alone for cardiovascular outcomes, it has been reported to lower LDL-C and reduce xanthomas with few adverse events, making it generally safe.
FQ27. Is it recommended to start treatment early in pediatric patients with FH?
FH is a high-risk condition for atherosclerotic diseases; thus, early initiation of treatment is recommended, depending on LDL-C levels*. (Level of evidence: Consensus, Level of recommendation: A)
*See Flow chart of pediatric HeFH treatment ( Fig.11 )
Fig. 11.
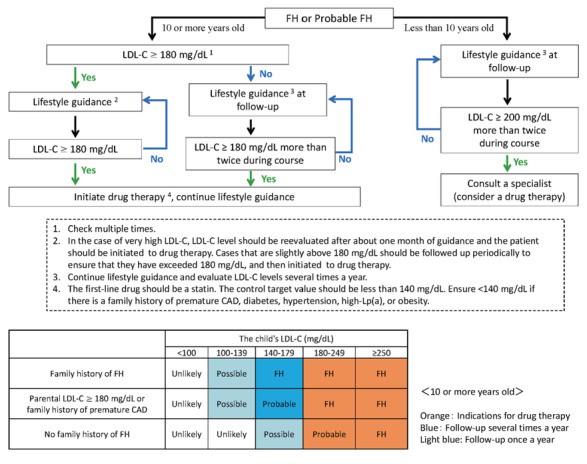
Flowchart of treatment for pediatric FH heterozygotes (under the age of 15)
The US Food and Drug Administration guidelines “approve” pravastatin for pediatric FH starting at age 8 and other statins starting at age 10 1390) . Our policy of “approving” pitavastatin for ages 10 and older, as we stated in Pediatric FH Guide 2017 is in line with the global trend. The 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk 1387) also has a level of evidence/recommendation of Class IIa, Level C, but it states that pediatric FH treatment should begin with statins at 8–10 years of age, with a goal of <135 mg/dL at ages 10 years and older. “Statins for children with familial hypercholesterolemia” which summarized nine studies using statins, found that statin therapy in children with FH successfully and safely reduced LDL-C without affecting liver function, muscle symptoms, muscle damage, and growth. They have also reported that carotid atherosclerosis can be reduced, and some endothelial function improved, although the level of evidence is not high 1391) . Furthermore, to date, RCTs and meta-analyses of RCTs and systematic reviews have shown that the use of statins, resins, and ezetimibe in pediatric patients of 10 years and older is safe and effective in lowering LDL-C levels.
Due to the limited number of RCTs that used statins in children and the short duration of statin treatment, no studies have been able to evaluate the incidence of atherosclerotic cardiovascular disease (ASCVD), cardiovascular death, and long-term safety. However, a 20-year follow-up study showed that starting statins at 13.0±2.9 years (mean LDL-C 237.3 mg/dL) did not cause any mortality related to ASCVD by at least the age of 39 years, although the mean LDL-C level reached 160.7 mg/dL 1275) . Since LDL-C accumulation levels, over time, are believed to be associated with the development of ASCVD, and since FH is a high-risk condition for atherosclerotic disease, early initiation of treatment in children is thus recommended.
1.Pathophysiology and Clinical Presentation of FH
FH is an autosomal hereditary disease characterized by (1) hyper-LDL cholesterolemia (LDL-C), (2) premature CAD, and (3) tendon and skin xanthomas. Except for the rare autosomal recessive hypercholesterolemia (autosomal recessive hypercholesterolemia: ARH), all other cases have a dominant mode of inheritance. In addition to pathogenic genetic mutations in the LDLR, HeFH is known to be caused by pathogenic mutations in apolipoprotein B-100 (APOB) and pathogenic gain-of-function mutations in the proprotein convertase subtilisin/kexin type 9 (PCSK9), both of which play an important role in LDL metabolism.
In addition to early diagnosis and rigorous treatment, family screening (cascade screening) and early intervention for FH can help prevent death at a young age. Diagnosis and treatment of FH in childhood are also important because the progression of atherosclerosis is recognized from childhood.
2.Diagnosis of FH
1) Diagnostic Criteria
The diagnostic criteria for FH in adults (15 years and older) are shown in Table 21 . A family history should be carefully obtained when diagnosing FH. It should be noted that in young patients, thickening of the Achilles tendon and other parts of the tendon is rarely observed. It should be noted that LDL-C levels temporarily decrease when complicated by serious diseases such as acute myocardial infarction. Therefore, when examining a patient with acute myocardial infarction, the Achilles tendon should be palpated, and a family survey should be conducted.
Table 21. Diagnostic criteria for FH in adults (15 years and older).
| 1. Hyper-LDL cholesterolemia (untreated LDL-C >180 mg/dL) |
| 2. Tendon xanthomas (dorsal hand, elbow, knee, etc. or Achilles tendon thickening) or cutaneous nodular xanthomas |
| 3. Family history of FH or premature CAD (first-degree relatives) |
| •Diagnosis is made after excluding other primary and secondary dyslipidemias. |
| •If the patient is already on drug therapy, refer to the lipid level that triggered the therapy. |
| •Achilles tendon thickening is diagnosed by radiography at ≥ 8.0 mm in men and ≥ 7.5 mm in women, or by ultrasound at ≥ 6.0 mm in men and ≥ 5.5 mm in women. |
| •Cutaneous nodular xanthomas do not include xanthelasmas. |
| •Premature CAD is defined as CAD that develops at younger than 55 years of age in men and younger than 65 years of age in women. |
| •FH is diagnosed when two or more items are met. |
| •Even if two or more items are not met, if those whose LDL‐C is 250 mg/dL or higher, or if 2 or 3 are met and LDL‐C is 160 mg/dL or higher, they are classified as probable FH. |
| •Diagnosis of FH is made in the presence of FH pathogenic gene mutations. |
| •If HoFH is suspected, genetic testing is recommended. Genetic testing is also useful for suspected HeFH, which are more difficult to diagnose. |
| •This diagnostic criterion also applies to HoFH. |
| •If FH is diagnosed, it is strongly recommended that family members be examined as well. |
The features of HoFH are serum TC ≥ 600 mg/dL, as well as childhood xanthomas and atherosclerotic diseases, and the parents are HeFH. It may be difficult to distinguish the serious cases of HeFH, so in order to make a definitive diagnosis of HoFH, genetic analysis is required. Since genetic testing for HoFH has been covered by insurance since April 2022 in Japan, it is expected to be used for the definitive diagnosis and selection of treatment options.
The criteria for Achilles tendon thickening have changed. The conventional radiographic cutoff value of 9 mm was published in 1977, and while it had high specificity, it was feared to have low sensitivity. In this present study, based on the analysis of 986 cases, including 485 cases of genetically diagnosed FH in Japan, the cutoff values were reviewed according to the report in 2021 1392) , which set the cutoff value at 7.6 mm for men and 7.0 mm for females and were changed to 8.0 mm or more for males and 7.5 mm or more for females to increase sensitivity while maintaining specificity. The evaluation by ultrasound has also been standardized and adopted in this guideline. However, further considerations of the validity of the changed reference values and the evaluation of sensitivity and specificity will be necessary in the future.
The diagnostic criteria for FH in children (under 15 years of age) are shown in Table 22 1393) . HeFH has few physical symptoms such as xanthomas in childhood, so the diagnosis is based on other two factors: LDL-C and family history. In the new guideline, the cut-off value for LDL-C is 140 mg/dL, which is approximately the 95th percentile value for children. Family history is also handled differently from adult criteria, but a new category of “probable FH” was established to allow for a broader diagnosis. As LDL-C fluctuates in childhood, especially after the onset of puberty, LDL-C should be measured and evaluated multiple times.
Table 22. Diagnostic criteria for pediatric FH (under the age of 15).
| 1. Hyper-LDL cholesterolemia (untreated LDL-C level ≥ 140 mg/dL, confirmed multiple times) |
| 2. Family history of FH (Parents or siblings) |
| 3. Parental LDL-C ≥ 180 mg/dL or family history of premature coronary artery disease (Grandparent or parent) |
| After ruling out other primary and secondary Hyper‐LDL cholesterolemia, |
| ‐ Diagnose FH with items 1 and 2. |
| ‐ Diagnose probable FH with item 1 and 3. If the individual’s LDL‐C is 180 mg/dL or higher, FH is diagnosed. |
| ‐ Even if only criteria 1 is used, a diagnosis of FH above 250 mg/dL and a diagnosis of probable FH above 180 mg/dL should be made. |
| •Differentiate HoFH when LDL‐C is ≥ 250 mg/dL or xanthomas are present. |
| •Diagnose FH if the individual has a pathogenic gene mutation for FH. If a parent, a brother, or a sister is found to have a pathogenic gene mutation for FH, that is considered to be the family history of FH (item 2). |
| •Premature coronary artery disease is defined as coronary artery disease occurring at less than 55 years of age in men and less than 65 years of age in women. |
| •Probable FH cases require further scrutiny and lipid‐lowering therapy. |
2) Evaluation of Achilles Tendon Thickening
2a) Radiography of Achilles Tendon
The diagnosis of thickening is made at 8.0 mm or more in men and 7.5 mm or more in women. (See Appendix 5. “Achilles Tendon Radiography for FH Screening”)
2b) Ultrasonography of Achilles Tendon
The diagnosis of thickening is made at 6.0 mm or more in men and 5.5 mm or more in women. (See Appendix 4. “Method of Measuring Achilles Tendon Thickness by Ultrasound for FH Screening”)
3) Differential Diagnosis
Diseases that cause secondary dyslipidemia (diabetes, hypothyroidism, nephrotic syndrome, obesity, cholestatic liver disease, drug-induced, etc.) and a similar disease, familial combined hyperlipidemia (FCHL) must be differentiated. FCHL is differentiated from FH by LDL-C not being as elevated as FH, absence of tendon xanthomas, presence of small dense LDL, variable lipid phenotype (type IIa, IIb, IV), and presence of dyslipidemia in the family. (See Chapter 5, “Other Primary Dyslipidemia.”)
Primary dyslipidemias with tendon xanthomas include sitosterolemia 1394) and cerebral tendon xanthomatosis1395).
3.Treatment of Adult HeFH (15 years and older)
1) Target Control Levels
As HeFH are at extremely high-risk of developing ASCVD, especially CAD, the risk of developing ASCVD in primary prevention is at least equivalent to that of usual secondary prevention. Therefore, the target control level of LDL-C for patients with HeFH for primary prevention should be less than 100 mg/ dL. It should also be noted that the risk assessment based on the risk chart issued by the Japan Atherosclerosis Society is not applicable in the treatment of FH for primary prevention.
In HeFH patients for secondary prevention, the LDL-C management target level should be less than 70 mg/dL because it can be considered to be at even higher risk.
As it is ethically unacceptable to conduct clinical trials without lipid-lowering therapy in FH, there is no clear evidence for the validity of these numerical targets. The achievement of the management target does not always ensure the absence of future cardiovascular events.
2) Lifestyle Interventions
Lifestyle interventions should be practiced in FH as well and is described in detail in a separate section (Chapter 3, 2 Lifestyle Modification). However, due to the high-risk of ASCVD, screening for ASCVD before administering exercise therapy is essential. Smoking cessation and obesity control are also important.
3) Drug Therapy
Lifestyle interventions alone are usually insufficient to achieve adequate lipid control in patients with HeFH; thus, concomitant pharmacotherapy with statins as first-line agents is recommended. If the standard dose of statin is not sufficiently effective, the dose should be increased to the maximum tolerated dose and ezetimibe should be used in combination with statin. If this is not sufficient, PCSK9 inhibitors, anion exchange resins, and probucol are used ( Fig.9 ) . If the attending physician determines that the patient is a high-risk case, such as a secondary prevention patient or a patient with diabetes, the LDL-C should be promptly reduced. The combination of evolocumab or alirocumab in HeFH patients already treated with statins (and ezetimibe) has demonstrated additional lowering of LDL-C (approximately 60%) and Lp(a) lowering effects 1276 , 1277) and long-term safety has been confirmed up to around 3 years of treatment. Additionally, an average reduction of 35% LDL-C by evolocumab or alirocumab in statin-intolerant FH patients has been noted 1396) . HeFH, excluding statin intolerance, who do not achieve the expected LDL-C lowering effect with PCSK9 inhibitor combination therapy in addition to usual oral therapy, should be referred to a specialist, including genetic testing, as they are most likely to be HoFH. However, it is not yet clear whether these combination therapies are more effective in reducing cardiovascular events in patients with FH than statin therapy alone. In Japan, a retrospective study suggests that the use of probucol delays the recurrence of CAD in HeFH 1180) , but its side effects such as QT prolongation should also be kept in mind.
Fig. 9.
Flowchart of HeFH treatment in adults (15 years and older)
4) Indications for Lipoprotein Apheresis
For HeFH, insurance coverage is allowed in cases where serum LDL-C level exceeds 370 mg/dL in steady state (body weight and serum albumin can be maintained) under diet therapy and does not fall below 170 mg/dL, andif coronary atherosclerosis is evident with xanthoma.
4.Treatment of Adult HoFH (15 years and older)
1) Target Control Levels
In HoFH, it is essential to lower LDL-C as quickly as possible, and aggressive treatment should be implemented. Ideal LDL-C control targets for HoFH are <100 mg/dL for primary prevention and <70 mg/dL for secondary prevention, although this is often difficult to achieve.
2) Lifestyle Interventions
Lifestyle interventions such as diet, exercise, smoking cessation, and obesity control are fundamental to the treatment of HoFH patients. Since HoFH develops atherosclerosis significantly more rapidly than HeFH, before giving guidance in exercise therapy and initiating it, patients should be carefully evaluated for CAD, as well as for valvular disease (particularly AS, supravalvular aortic stenosis) and aortic aneurysms.
3) Drug Therapy
In HoFH, the above lifestyle interventions alone are not sufficient to control the disease, thus, strong LDL-C lowering therapy is required from a young age to prevent the development and progression of CAD ( Fig.10 ) . However, statins, anion exchange resins, and PCSK9 inhibitors all have the primary mechanism of action for increasing the expression (activity) of the LDLR. For the defective type, in which only a small amount of LDLR activity remains, slight efficacy is observed, however, in the negative type, in which LDLR activity is completely absent, no LDL-C lowering effect is observed 1397 , 1398) . In a study examining the LDL-C lowering effect of PCSK9 inhibitors in adult HoFH patients 1385) , although the LDL-C lowering effect of PCSK9 inhibitors (approximately 30%) was confirmed, PCSK9 inhibitor treatment should be discontinued if LDL-C is not reduced at all. However, a retrospective study found that the administration of statin and other drugs was effective in reducing the mortality rates in HoFH 1399) . Furthermore, a microsomal triglyceride transfer protein (MTP) inhibitor, which was developed for patients with HoFH, has been reported to reduce LDL-C by approximately 50% 1202 , 1230) . However, since the frequencies of adverse events such as fatty liver and diarrhea are high with MTP inhibitor, it is essential to strictly control fat and alcohol intake. Probucol reportedly exerts a certain LDL-C lowering effect in HoFH and can cause the regression or disappearance of xanthoma on the skin and Achilles tendon 1400) . However, for LDL-C control, lipoprotein apheresis therapy is still required once every 1–2 weeks in many cases. Liver transplantation is an option for patients who are resistant or intolerant to all of the above treatments, but there are currently very few cases of liver transplantation in Japan 1401 , 1402) .
Fig.10.
Flowchart of HoFH treatment in adults (15 years and older)
4) Lipoprotein Apheresis for HoFH
In patients with HoFH, as it is difficult to sufficiently reduce the LDL-C level using existing drug therapies, continued therapy for lipoprotein apheresis is required from childhood in many cases. The earlier the age at which lipoprotein apheresis therapy is started, the better to control the progression of ASCVD, but it is difficult to implement until the affected child is able to rest during lipoprotein apheresis. The realistic starting time for treatment is around 4-6 years of age, when the child is bedridden and extracorporeal circulation can be performed.
5.Treatment of Pediatric FH (under the Age of 15)
Once FH is diagnosed, lifestyle advice should be provided as early as possible in order to reduce the risk of atherosclerosis, including lowering LDL-C levels. Fig.11 shows a flowchart of pediatric HeFH treatment 1393) . If the level of LDL-C remains above 180 mg/dL despite lifestyle modification, drug therapy should be considered at age 10 or older, regardless of gender. First-line drug therapy is statins, starting with the lowest dose. In Japan, pitavastatin has been indicated for children 10 years of age and older. The target value for the management is an LDL-C level of less than 140 mg/dL. Ensure to maintain below 140 mg/dL in cases with a family history of premature CAD or risk factors such as diabetes. Although it is difficult to achieve the goal in severe cases, try to get as close to the goal with the use of drug combination therapy. Even after the start of drug therapy, lifestyle guidance, including diet, should be provided.
For cases of probable FH ( Table 22 ) , drug therapy should also be considered in cases of persistent hyper-LDL of 180 mg/dL or more 1393) .
6.Pregnancy and delivery of patients with FH
For patients with FH who wish to become pregnant, it is necessary to evaluate the status of atherosclerosis in advance by carotid artery echocardiography, etc. and to provide adequate preconception counseling in preparation for a safe continuation of pregnancy and delivery. The administration of lipid-lowering drugs other than anion exchange resins during pregnancy should be done with caution due to concerns about the risk of incidence of fetal malformations and other problems. According to the National Institute of Health and Clinical Excellence, if pregnancy is discovered while taking a lipid-lowering medication other than an anion exchange resin, the medication should be discontinued immediately, and if a woman wishes to have a baby while taking the medication, she should stop taking the medication for 3 months before trying to conceive. Lipid lowering medications other than anion exchange resin (resin) should also be discontinued during the postpartum lactation period.
It is important to be more deliberate about pregnancy in patients with HoFH. Screening for atherosclerosis by carotid echocardiography, echocardiography, and exercise stress electrocardiography prior to pregnancy to evaluate atherosclerosis status. In HoFH, lipid-lowering medications other than anion exchange resins should be discontinued 3 months before expected pregnancy. Lipoprotein apheresis should be performed during pregnancy due to the high stress on the cardiovascular system in the late pregnancy, especially during the birth. Lipoprotein apheresis therapy can be administered safely during pregnancy.
Chapter 5.Other Primary Dyslipidemias
1.Primary Dyslipidemia and Designated Intractable Diseases
In addition to FH, there are several types of primary dyslipidemias caused by a single gene mutation or a highly heritable component. Their classification based on pathogenesis and genetic abnormalities has been proposed ( Table 23 ) . Of these, HoFH (homozygotes) (designated intractable disease 79) has been recognized since 2009, and lecithin cholesterol acyltransferase deficiency (designated intractable disease 259), sitosterolemia (designated intractable disease 260), Tangier disease (designated intractable disease 261), primary hyperchylomicronemia (designated (designated intractable disease 262), cerebrotendinous xanthomatosis (designated intractable disease 263), abetalipoproteinemia (designated intractable disease 264), and familial hypobetalipoproteinemia (designated intractable disease 336) are each designated as incurable diseases under the national “Law Concerning Medical Care for Patients with Intractable Diseases” (Intractable Disease Law) and are covered by medical expenses subsidies (see https://www.nanbyou.or.jp/). For HoFH (homozygotes) and the six diseases other than cerebrotendinous xanthomatosis, the number of medical beneficiaries is small and there may be many undiagnosed cases, making further disease awareness and education an issue for the future. The outline and diagnostic criteria for these designated intractable diseases were developed by the “Research Group for Primary Hyperlipidemia” (renamed the “Research Group for Primary Dyslipidemia” in FY2021), an Intractable Disease Control Project of the Ministry of Health, Labour and Welfare, and are available on the website of the Japan Intractable Diseases Information Center (https://www.nanbyou.or.jp/). The Japanese-language review can be found on the Research Group’s website (https://nanbyo-lipid.com/).Referral to a specialist is recommended for any of these diseases.
Table 23. Classification of primary dyslipidemia.
| Primary hyperlipidemia | |
| Primary hyperchylomicronemia (Designated intractable disease 262) | •Familial lipoprotein lipase (LPL) deficiency |
| •GPIHBP1 deficiency | |
| •LMF1 deficiency | |
| •Apoprotein A-V deficiency | |
| •Apoprotein C-II deficiency | |
| •Primary hyperlipidemia type V | |
| •Others | |
| Primary hypercholesterolemia | •Familial hypercholesterolemia |
| [LDL receptor abnormalities, PCSK9 abnormalities, | |
| Familial apo B100 abnormalities, | |
| LDLRAP1 abnormalities (autosomal recessive | |
| hypercholesterolemia), other] | |
| - Familial hypercholesterolemia homozygous | |
| (Designated intractable disease 79) | |
| - Familial hypercholesterolemia heterozygous | |
| •Polygenic hypercholesterolemia | |
| •Familial combined hyperlipidemia | |
| Familial type III hyperlipidemia | •Apoprotein E abnormality |
| •Apoprotein E deficiency | |
| Primary hypertriglyceridemia | •Familial type IV hyperlipidemia |
| Primary hyper-HDL cholesterolemia | •CETP deficiency |
| •HL deficiency | |
| •Others | |
| Primary hypolipidemia | |
| Abetalipoproteinemia (MTP abnormality) | •Abetalipoproteinemia (Designated intractable disease 264) |
| Familial hypobetalipoproteinemia (FHBL) | •Familial hypobetalipoproteinemia (FHBL) 1 (apoprotein B abnormalities) (Homozygotes are Designated intractable disease 336) |
| •Familial hypobetalipoproteinemia (FHBL) 2 (ANGPTL3 abnormalities) | |
| •PCSK9 abnormalities | |
| Familial Hypo-HDL cholesterolemia | •Tangier disease(Designated intractable disease 261) |
| •Lecithin cholesterol acyltransferase deficiency (Designated intractable disease 259) | |
| •Apoprotein A-I deficiency | |
| Others | |
| •Sitosterolemia (Designated intractable disease 260) | |
| •Cerebrotendinous Xanthomatosis (Designated Intractable Disease 263) |
Among primary dyslipidemias, FH, sitosterolemia, primary hypo-HDL cholesterolemia, and cerebrotendinous xanthomatosis (non-neurogenic type) have been suggested to be atherosclerosis-inducing 1403 , 1404) . Primary hyperchylomicronemia is a risk for acute pancreatitis and, depending on genetic predisposition, may be atherosclerosis-inducing. In addition, although not designated as an incurable disease, familial combined hyperlipidemia and familial type III hyperlipidemia should be diagnosed and treatment initiated at an early stage because of the increased risk of ASCVD incidence. Primary dyslipidemias that have been suggested to induce ASCVD other than FH are outlined below.
2.Familial Combined Hyperlipidemias (FCHL)
1) Causes
FCHL was proposed as a primary hyperlipidemic disease that is common in patients who survived myocardial infarction 1405) . The disease is a combination of hereditary and acquired factors such as lifestyle to various degrees, presenting with type IIb combined hyperlipidemia. It can also fluctuate to Type IIa and Type IV depending on diet, age, and other factors. First-degree relatives often have type IIa, IIb, and IV patients. It was thought to be a single-gene disease with autosomal dominant inheritance, but a multifactorial basis is now speculated 1406) . In addition to the LPL gene, USF-1 gene, apoprotein B gene, apoprotein C-II gene, apoprotein A-I/C-III/A-IV gene cluster, many related genes have been reported, including the LDL-R gene and PCSK9 gene, and the disease is thought to occur when factors such as overnutrition, obesity and lack of physical activity are present. FCHL is genetically similar to type IV hyperlipidemia 1407) . The frequency is extremely high, about 1% of the general population. In Japan, the frequency is already as high as 0.4% in the general pediatric population 1408) .
2) Clinical Symptoms
Elevation of serum LDL-C is milder than FH, and there are no physical symptoms such as xanthomas. CAD is frequent, although not as frequent as in FH 1409 , 1410) . In Japan, the incidence of myocardial infarction is recognized from the age of 35 years in men and 55 years in women. FCHL is found in 32% of patients under 65 years of age with myocardial infarction, etc 1411) .
3) Examination Findings and Diagnosis
Hyper LDL-C and hypertriglyceridemia are only mild to moderate. Reflecting the above pathology, an increase in apoprotein B and the appearance of small LDL particles (sd-LDL) are observed. Diagnosis is made according to the diagnostic criteria of the Research Group for Primary Hyperlipidemia ( Table 24 ) . Apoprotein B-100/LDL-C ratio >1.0, lipoprotein polyacrylamide disk (PAG) electrophoresis proves sd-LDL.
Table 24. Diagnostic Criteria for Familial Combined Hyperlipidemia (FCHL).
| Items | (1) Type IIb is the standard, but phenotypes IIa and IV can also be taken. |
| (2) Prove the existence of Apoprotein B/LDL cholesterol >1.0 or small dense LDL (LDL particle size <25.5 nm) | |
| (3) Excluding familial hypercholesterolemia and secondary hyperlipidemia such as diabetes mellitus. | |
| (4) Hyperlipidemia of phenotype IIb, IIa, or IV exists in first-degree relatives, and at least one person, including the patient, has phenotype IIb or IIa | |
| Diagnosis | (1) to (4) must all be met for a definite diagnosis, but only (1) to (3) may be used as a simple diagnostic criterion for routine diagnosis. |
(Translated and reprinted from the Ministry of Health and Welfare, Research Group for the Investigation of Primary Hyperlipidemia of Specific Diseases, FY2000 report)
4) Treatment
The treatment of FCHL is essentially similar to that of FH. Lifestyle modification through diet and exercise therapy and correction of obesity are of utmost importance. Their responses to diet and/or drugs are better than those in FH. Statins, fibrates, and ezetimibe are effective. The prognosis is defined by the incidence of CAD and other ASCVD diseases.
3.Familial Type III Hyperlipidemia
1) Causes
Familial Type III Hyperlipidemia is an inherited hyperlipidemia due to an abnormality of apoE, also known as broad beta disease. Because apoE is required for hepatic uptake of remnant lipoproteins, remnant lipoproteins such as IDL, chylomicron remnants, and β-VLDL (cholesterol-rich VLDL that migrates in the β position in electrophoresis) accumulate in the blood 1412 , 1413) . There are three major isoforms of apoE. Besides the most frequent wild-type E3, there are E2 and E4 isoforms. Familial type III hyperlipidemia is caused by a functional abnormality of apoE, mainly apoE2/E2, and in rare cases, genetic mutations such as apoE1, abnormal apoE3, and apoE deficiency have also been reported. ApoE2/E2 alone often does not cause marked lipid abnormalities, which are manifested by complications of other conditions (diabetes, obesity, alcohol consumption, pregnancy, hypothyroidism, etc.) or drugs (estrogens and psychotropic drugs). It is estimated that the frequency of E2/E2 is 0.3-2.0% in Europe and the United States. Less than about 10% of these patients present with type III hyperlipidemia 1414) . E2/E2 is estimated to occur at a frequency of about 0.2% per general population in Japan, but the number of cases diagnosed as familial type III hyperlipidemia is only 0.01-0.02%, and disease awareness is desirable.
2) Clinical Symptoms
Accumulation of remnants in the tissues may result in the appearance of palmar linear xanthomas and cutaneous nodular xanthomas. It is prone to be associated with premature atherosclerotic disease (e.g., CAD, carotid arteriosclerosis, renal arteriosclerosis, PAD) and is also complicated by renal vascular hypertension and intermittent claudication due to PAD. In Europe and the United States, the risk of incidence of CAD increases by 5- to 8-fold 1415) . The frequency of complications of CAD is also high in Japan 1416) .
3) Examination Findings and Diagnosis
Both serum TC and TG are elevated, ranging from slightly above normal to TC 500 mg/dL and TG 2,000 mg/dL in some cases. The diagnostic criteria of the Research Group for Primary Dyslipidemia Study Group ( Table 25 ) are used for diagnosis. The broad β pattern can be demonstrated by polyacrylamide gel electrophoresis in cases where both TC and TG are elevated, and the apoE/TC* ratio exceeds 0.05, etc., which can be screened in routine practice. Other proposed indices include apoE/apoB >0.20 and apoE/apoCIII >1.0 1417) , TC/apoB >2.4 and TG/apoB <8.85 1418) , RLP-C/TG >0.1 1414) , non-HDL-C/apoB >3 1419) , apoB48/TG >0.11 1420) . Compared to those of algorithms using TC and apoB or TC/TG and apoB, non-HDLC/apoB has superior sensitivity and specificity 529) . In lipoprotein analysis by ultracentrifugation or HPLC, LDL-C decreases. In addition, a marked increase in cholesterol in the IDL fraction (1.006 <d <1.019) and a high cholesterol/TG ratio (≥ 0.25) in the VLDL fraction (d <1.006) are confirmed in ultracentrifugation. Abnormalities in apoE isoforms should be confirmed by isoelectric electrophoresis, their Western blotting, or genetic analysis. (*All units are mg/dL except apoB48, which is µg/ml.)
Table 25. Diagnostic Criteria for Familial type III hyperlipidemia.
| Main items | (1) Serum cholesterol and serum triglyceride levels are both elevated |
| (2) Electrophoresis of plasma lipoproteins shows a broad β pattern of continuity from VLDL to LDL | |
| (3) Electrophoresis of apolipoproteins to prove abnormalities of apolipoprotein E (E2/E2, E deficiency, etc.) | |
| Sub items | (1) Xanthoma (especially palmar linear xanthoma) |
| (2) Increased serum apolipoprotein E concentration (apolipoprotein E/total cholesterol ratio >0.05) | |
| (3) VLDL cholesterol/serum TG ratio greater than 0.25 | |
| (4) Decrease in LDL cholesterol | |
| (5) With ASCVD such as arteriosclerosis obliterans and ischemic heart disease | |
| Diagnosis | Definite diagnosis when all three major items are present. |
| Diagnosis as suspected when 2 of the major items and 1 or more of the sub items are present |
(Translated and reprinted from the Ministry of Health and Welfare, Research Group for the Investigation of Primary Hyperlipidemia of Specific Diseases, FY 1986 and 1987 Report)
4) Treatment
Early diagnosis and treatment are important because the disease responds relatively well to lifestyle modifications such as diet like fat restriction and exercise therapy. In cases with complications such as diabetes, obesity, and hypothyroidism, treatment of these complications also improves dyslipidemia. Fibrates are the first-line drugs, but statins and nicotinic acid derivatives are also effective. With early diagnosis and treatment, the prognosis is relatively good. Regular examinations to prevent the incidence of CAD, carotid atherosclerosis, PAD, etc. should be performed, and a visit to a specialist in lipid metabolism is recommended.
4.Sitosterolemia
(Designated Intractable Desease 260: https://www.nanbyou.or.jp/entry/4857)
Sitosterolemia is an autosomal recessive inherited disorder that causes accumulation of sitosterol in the blood or tissues due to decreased excretion of sitosterol, a type of plant sterol found in vegetables and fruits, with clinical manifestations such as skin and tendon xanthomas and premature onset of CAD. Decreased excretion of phytosterols associated with genetic mutations in ATP binding cassettetransporter G5 and G8 (ABCG5/8) is involved in pathogenesis. The clinical presentation is similar to that of FH, and differentiation is important 151) . Ezetimibe and colestimide are effective.
5.Primary Hypo-HDL Cholesterolemia ( Fig.12 )
Fig.12.
Flowchart for Diagnosis of Primary Hypo‐HDL cholesterolemia
1) Lecithin Cholesterol Acyltransferase Deficiency
(Designated Intractable Disease 259: https://www.nanbyou.or.jp/entry/4547)
Lecithin Cholesterol Acyltransferase Deficiency is an autosomal recessively inherited disease, caused by enzyme deficiency or reduced activity of lecithincholesterol acyl transferase (LCAT), an enzyme important for cholesterol esterification, resulting in an increase in serum free cholesterol and lecithin (phosphatidylcholine) that results in a marked decrease in HDL-C and a decrease in serum cholesterol ester ratio (CE/TC). Tissue deposition of altered lipoproteins results in corneal opacity, hemolytic anemia, and renal damage 1421) . It has also been reported that significantly low HDL-C blood levels can lead to ASCVD 1422) .
2) Tangier disease
(Designated Intractable Disease 261: https://www.nanbyou.or.jp/entry/4586)
Tangier disease is an autosomal recessive disorder in which serum HDL-C and apoprotein A-I levels are markedly low due to genetic mutations in ATP binding cassette transporter A1 (ABCA1), which is important for cholesterol withdrawal from cells by apoprotein A-I, characterized by enlarged orange pharyngeal tonsils, hepatosplenomegaly, corneal opacity, peripheral neuropathy, and premature CAD 1422 , 1423) .
3) Apoprotein A-I Deficiency
Apoprotein A-I Deficiency is a condition caused by a deficiency or abnormality of apoprotein A-I, the major constituent apoprotein of HDL. ApoC-III and apoA-IV may be deficient along with apoA-I. Serum HDL-C and apoA-I levels are markedly low. Although there is no orange tonsillar hypertrophy seen in Tangier disease or the decreased cholesterol ester ratio and renal impairment seen in LCAT deficiency, there is a risk of premature CAD complications 1422 , 1424) .
6.Other
Primary hyperchylomicronemia (familial lipoprotein lipase (LPL) deficiency, GPIHBP1 deficiency, LMF1 deficiency, apoprotein C-II deficiency, apoprotein A-V deficiency, etc.) is a marked hyperchylomicronemia resulting in hypertrigceridemia, typically with type I hyperlipidemia but type V hyperlipidemia may also occur. Strict fat restriction (no more than 15-20 g/day) as it often causes acute pancreatitis. Attention should be paid to the possibility that some genetic predispositions (e.g., hypertriglyceridemia associated with apoA-V gene abnormalities) may put the patient at risk for atherosclerosis.
Chapter 6. Secondary Dyslipidemia
For secondary dyslipidemia, the underlying disease should be adequately treated.
1.Secondary Dyslipidemia
Dyslipidemia is classified into primary dyslipidemia, which is based on constitutional or genetic abnormalities, and secondary dyslipidemia, which is caused by various diseases. In other words, secondary dyslipidemia is an abnormality in lipid metabolism caused by other diseases or side effects of drugs. The basis of the treatment of secondary dyslipidemia is to elucidate the cause of the disease or condition and to treat the underlying diseases. Secondary dyslipidemia accounts for 30-40% of all dyslipidemias 1425) . As a general rule, the treatment of secondary dyslipidemia is prioritized over the treatment of the underlying disease. Dyslipidemia can also be cured or improved by changing or discontinuing the causative agent. Caution should be exercised in the treatment of dyslipidemia with statins, etc., which can lead to serious adverse events such as rhabdomyolysis if treatment is easily initiated without the identification of secondary dyslipidemia, such as dyslipidemia due to hypothyroidism.
2.Diseases and Conditions that Cause Secondary Dyslipidemia
Table 26 shows the main conditions that cause secondary dyslipidemia 1426) . Secondary dyslipidemia includes cases of increased cholesterol (e.g., hypothyroidism), increased TG (e.g., alcohol consumption), or increased cholesterol and TG together (nephrotic syndrome) 1426) . The increase or decrease of each lipoprotein is then confirmed in secondary dyslipidemia ( Table 27 ) 1427) .
Table 26. Causes of secondary dyslipidemia.
| Items | Cholesterol | Triglyceride |
|---|---|---|
| 1. Hypothyroidism | ↑ | |
| 2. Nephrotic syndrome | ↑ | ↑ |
| 3. Chronic kidney disease (CKD) | ↑ | |
| 4. Primary biliary cholangitis (PBC) | ↑ | |
| 5. Obstructive jaundice | ↑ | |
| 6. Diabetes | ↑ | ↑ |
| 7. Obesity | ↑ | |
| 8. Cushing’s syndrome | ↑ | ↑ |
| 9. Pheochromocytoma | ↑ | ↑ |
| 10. Drugs | Drug dependent | |
| 11. Alcohol intake | ↑ | |
| 12. Smoking | ↑ | |
Adapted from Secondary dyslipidemia: its treatment and association with atherosclerosis. Glob Health Med 2021; 3: 15‐23.
Table 27. Causes of secondary dyslipidemia in terms of increase or decrease of each lipoprotein.
| LDL | HDL | VLDL | IDL | Chylomicron | LP(a) | ||
|---|---|---|---|---|---|---|---|
| increase | decrease | increase | decrease | increase | increase | increase | increase |
| Hypothyroidism | Severe liver disease | Alcohol consumption | Smoking | Obesity | Multiple myeloma | Autoimmune diseases | CKD |
| Nephrotic syndrome | Malabsorption | Exercise | Type 2 diabetes mellitus | Type 2 diabetes mellitus | Monoclonal gamma globulinemia | Type 2 diabetes mellitus | Nephrotic syndrome |
| Bile stasis | Malnutrition | Exposure to chlorinated hydrocarbons | Obesity | Glycogenic disease | Autoimmune diseases | Inflammation | |
| Acute intermittent porphyria | Gaucher’s disease | Drugs: | Malnutrition | Nephrotic syndrome | Hypothyroidism | Menopause | |
| Anorexia nervosa | Chronic infections | Estrogen | Gaucher's disease | Hepatitis | Spermosuction | ||
| Hepatocellular carcinoma | Hyperthyroidism | Cholesterol ester accumulation | Alcohol consumption | Hypothyroidism | |||
| Drugs: | Drugs: | Drugs: | Renal failure | Acromegaly | |||
| Thiazide diuretics | Niacin addiction | Anabolic steroids | Sepsis | Drugs: | |||
| Cylcosporin | Beta-blockers | Stress | Isotretinoin | ||||
| Carbamazepine | Cushing’s syndrome | ||||||
| Pregnancy | |||||||
| Acromegaly | |||||||
| Lipodystrophy | |||||||
| Drug: | |||||||
| Estrogen | |||||||
| Beta-blocker | |||||||
| Glucocorticoids | |||||||
| Bile acid-binding resins | |||||||
| Retinoic acid | |||||||
HDL, high density lipoprotein; IDL, intermediate density lipoprotein; LDL, low density lipoprotein; Lp(a), lipoprotein(a); VLDL, very low density lipoprotein
New Clinical Internal Medicine, Yoshida H.: Secondary dyslipidemia. Igaku Shoin 2020, Rader DJ, Hobbs HH. 421: Disorders of lipoprotein metabolism. Reprinted with modification from Harrison’s Principles of Internal Medicine, 19e, 2015
2.1 Hypothyroidism
•Hypothyroidism causes secondary dyslipidemia and induces atherosclerosis 1428) . Thyroid hormone replacement improves dyslipidemia and inhibits the development of atherosclerosis.
When considering dyslipidemia due to hypothyroidism, it is necessary to distinguish between overt hypothyroidism, in which thyroid hormone levels are low, and subclinical hypothyroidism, in which thyroid stimulating hormone (TSH) levels are high despite normal thyroid hormone levels. Hypothyroidism should be divided into overt and subclinical hypothyroidism. In overt hypothyroidism, TC, LDL-C, apoB, and Lp(a) increase, and TG shows a normal to mild increase 1429) . In particular, LDL-C has been reported to increase by 30% 1429) . Thyroid hormones are involved in 7α-hydroxylase, the rate-limiting enzyme in the synthesis of bile acids from cholesterol, and in the induction of LDL receptor expression. Therefore, when thyroid hormones are decreased, LDL catabolism and excretion decrease and LDL-C increases 1430) . This condition resembles FH and is important as a differential disease for FH. If dyslipidemia is diagnosed in overt hypothyroidism, thyroid hormone replacement should be administered to normalize thyroid function before administering statins or other drugs for dyslipidemia.
In subclinical hypothyroidism, a meta-analysis of 14 observational studies showed an odds ratio of 2.38 (95% CI: 1.53-3.69) for the incidence of CAD after adjustment for other coronary risk factors 1428) . In a meta-analysis looking at the effects of subclinical hypothyroidism on dyslipidemia and carotid intima-media thickening (IMT), subclinical hypothyroidism with TSH ≥10 µU/mL was associated with increased TC, LDL-C, TG and increased IMT 1431) . A meta-analysis that examined the effect of thyroid hormone replacement therapy on dyslipidemia in patients with subclinical hypothyroidism showed that replacement therapy for more than 6 months was associated with a decrease in TC and LDL-C, regardless of TSH levels 1432) . Thyroid hormone replacement therapy for subclinical hypothyroidism has also been shown to decrease IMT 1433 , 1434) . However, at this time, the effect of thyroid hormone replacement therapy on the prevention of cardiovascular events in patients with subclinical hypothyroidism has not been demonstrated 1435) .
Hypothyroidism is a risk factor for statin-induced muscle damage 1436) . There have been reports of cases of overt hypothyroidism in which acute kidney failure due to rhabdomyolysis occurred because a statin was administered prior to thyroid hormone replacement. Hypothyroidism is a causative agent of secondary dyslipidemia that deserves attention.
In older people and patients with thyroid diseases such as chronic thyroiditis, a high intake of iodine from kelp, hijiki, and other sources can cause a Wolf-Chaikoff effect, etc. Therefore, when treating patients with hypothyroidism, which is the cause of secondary dyslipidemia, dietary therapy should also be taken into consideration, including guidance to avoid excessive iodine intake.
2.2 Nephrotic Syndrome
•Nephrotic syndrome is a cause of secondary dyslipidemia and has also been implicated in the incidence of ASCVD.
The mechanisms underlying the incidence of dyslipidemia in nephrotic syndrome include a compensatory increase in hepatic VLDL synthesis and secretion with a subsequent increase in LDL due to protein leakage into the urine, decreased clearance of TG-rich lipoproteins due to decreased activity of lipoprotein lipase (LPL) and hepatic lipase (HL), impaired HDL maturation and other factors 1437 , 1438) . Recently, PCSK9, which is involved in the turnover of LDL receptors, has been implicated in the incidence of dyslipidemia in nephrotic syndrome 1438) . In an observational study comparing patients with nephrotic syndrome with healthy controls, plasma PCSK9 levels were significantly higher in patients with nephrotic syndrome than in healthy controls, and PCSK9 levels showed a significant positive correlation with TC and LDL-C levels 1439) . In a cohort study, unmatched analysis adjusted for hypertension and smoking at diagnosis showed a relative risk of 5.5 (95% CI: 1.6-18.3) of myocardial infarction and 2.8 (95% CI: 0.7-11.3) of coronary death in nephrotic syndrome 1440) .
Dietary intervention studies for nephrotic syndrome have reported that a soy diet produces significant reductions in TC, LDL-C, HDL-C, apo A, apo B, and urinary protein 1441 , 1442) . The n-3 polyunsaturated fatty acids have also been shown to significantly reduce TG, VLDL-C, small dense LDL, remnant-like lipoprotein particle cholesterol (RLP-C) and RLP-TG 1443) . In the dyslipidemia intervention for nephrotic syndrome, RCTs and other studies have shown that statins reduce TC, LDL-C and TG relatively safely, but few studies have demonstrated the effect of statins on renal outcomes 1426 , 1444) . Fibrates have shown significant decreases in TG, TC, LDL-C, and apoB and significant increases in HDL-C with gemfibrozil, but no positive effects on renal outcomes have been reported 1426 , 1444) . The effect of pemafibrate and even ezetimibe on dyslipidemia in nephrotic syndrome is not yet clear. PCSK9 inhibitors are effective in CKD. Its effectiveness in nephrosis is promising, however, further studies are needed 1444 - 1447) . On the other hand, lipoprotein apheresis and prednisone combination therapy in patients with treatment resistant focal glomerulosclerosis/nephrotic syndrome markedly reduced LDL-C and resulted in remission of the nephrotic syndrome in 47.7-71.0% of patients 1426 , 1444) .
A meta-analysis that evaluated cardiovascular events in patients with nephrotic syndrome found no benefit of dyslipidemia medications on all-cause mortality, cardiovascular death, or incidence of nonfatal myocardial infarction 1448) . Considering that most patients with membranous nephropathy presenting with refractory nephrotic syndrome are middle-aged and older people, are prone to arteriovenous thromboembolism, and are treated with prolonged steroid therapy, drugs for dyslipidemia are considered highly necessary 1449) .
2.3 Chronic Kidney Disease: CKD
In CKD, insulin resistance is induced by metabolic acidosis, inflammation, oxidative stress, and uremia 1450) , which may increase VLDL synthesis and increase VLDL, IDL, and RLP-C due to decreased activity of LPL and HL 1451) . CKD is considered to be a highly atherogenic condition 1452) . For a summary of CKD, the level of evidence, its relationship to atherosclerosis, and atherogenic lipoproteins, see the relevant chapters and sections.
2.4 Primary Biliary Cholangitis and Obstructive Jaundice
•Primary biliary cholangitis and obstructive jaundice are causes of secondary dyslipidemia, but their association with the incidence of ASCVD is unknown.
Primary Biliary Cholangitis (PBC)
It is an autoimmune liver disease with positive antimitochondrial antibodies. The secretion of cholesterol and bile acids into bile is impaired, resulting in elevated serum cholesterol. The dyslipidemia seen in this disease is characterized by high LDL-C regardless of the stage of the disease and high HDL-C until the end stage of liver cirrhosis 1453) . A systematic review of the association between PBC and CAD risk found no significant association between PBC and CAD in the general analysis 1453) , although one study showed cardiovascular disease death in 12% of PBC patients 1454) . This suggests that there is a patient population for whom lipid management is necessary for the life prognosis of PBC. Consider treatment for patients with PBC who have other risk factors for atherosclerosis, such as hypertension 1455) .
Obstructive Jaundice
Obstructive jaundice is a condition in which the extrahepatic bile ducts are obstructed by gallstones or tumors, resulting in impaired bile outflow and bile stasis in the liver. Impaired bile excretion into the intestinal tract, impaired fat absorption in the gastrointestinal tract, and increased cholesterol synthesis in the gastrointestinal wall occur. HDL-C also decreases due to impaired synthesis in the liver and impaired synthesis in the intestinal tract due to insufficient bile acid supply. LDL-C is elevated, and HDL-C is low. It is often accompanied by an increase in abnormal lipoprotein, lipoprotein X which is rich in phospholipids and free cholesterol 1456) .
2.5 Diabetes and Obesity
When diabetic ketoacidosis occurs in type 1 diabetes mellitus caused primarily by insulin deficiency, hyperchylomicronemia (CM) with serum TG >1,000 mg/dL may occur, leading to acute pancreatitis. This is due to a marked decrease in LPL activity caused by insulin deficiency. Insulin therapy promptly improves blood glucose and hyperchylomicronemia. Because this is a transient pathological condition, it has not been shown to be related to atherosclerosis.
In type 2 diabetes and obesity, dyslipidemia is induced by insulin resistance 1457) . Increased activity of hormone-sensitive lipase (HSL) in adipose tissue increases free fatty acids in the blood. Increased free fatty acid influx into the liver increases VLDL synthesis. Insulin resistance decreases LPL activity and causes impaired VLDL metabolism, leading to further increases in VLDL. Metabolic disorders in VLDL also cause a decrease in HDL. In type 2 diabetes, there is also an increase in LDL-C due to decreased LDL receptor activity and increased small intestinal cholesterol transporter (Niemann-Pick C1 Like 1). Type 2 diabetes is an important risk factor for atherosclerosis.
For a summary of diabetes and obesity, level of evidence, association with atherosclerosis, and atherogenic lipoproteins, see the relevant chapters and sections.
2.6 Cushing’s Syndrome
•Cushing’s syndrome is a cause of secondary dyslipidemia and has been implicated in the development of atherosclerosis.
Cushing’s syndrome is caused by excessive cortisol secretion and presents with central obesity, impaired glucose tolerance, hypertension, and dyslipidemia. Because cortisol promotes VLDL synthesis in the liver, serum cholesterol and TG are increased in patients with Cushing’s syndrome 1458) . A meta-analysis reported that Cushing’s syndrome is associated with IMT thickening, carotid plaque formation, and vascular endothelial dysfunction 1459) .
2.7 Pheochromocytoma
•Pheochromocytomas cause secondary dyslipidemia.
Tumors of the adrenal medulla and paraganglia, which oversecrete catecholamines such as noradrenaline, resulting in endocrine hypertension and secondary diabetes mellitus. Excess catecholamines activate HSL in adipose tissue, increasing free fatty acids in the blood, and the increased free fatty acids flow into the liver, increasing VLDL synthesis. However, certain observations have not been made in case reports on the dyslipidemic phenotype in pheochromocytoma and the effect of treatment on dyslipidemia 1460 - 1462) .
2.8 Drugs
•The use of diuretics, beta-blockers, steroids, estrogen/progesterone, atypical antipsychotics, HIV medications (protease inhibitors), immunosuppressive drugs, and retinoids can cause secondary dyslipidemia.
Diuretic
Thiazide diuretics are believed to exacerbate insulin resistance and increase VLDL production in the liver, resulting in increased TG, but the mechanism is unclear. No consistent effects on LDL-C or HDL-C have been reported. However, although thiazide diuretics have been reported to affect lipids at high doses as in the past, the doses currently used as fixed-dose combination drugs or even as single agents recommended in the Japanese Society of Hypertension guidelines have little effect on TG, LDL-C, etc. 1463 , 1464) .
Beta-Blocker
Beta-blockers without intrinsic sympathomimetic activity (ISA) or beta1 nonselective beta-blockers have been shown to adversely affect insulin resistance and decrease LPL activity, resulting in increased TG due to increased VLDL.
Steroid
Steroids increase VLDL synthesis and HDL synthesis in the liver. As a result, VLDL, LDL, and HDL increase, presenting as type IV or type IIb hyperlipidemia.
Estrogen/Progesterone
Estrogen increases VLDL synthesis in the liver, suppresses HL activity, and enhances LDL receptor expression 1465 - 1467) . Therefore, estrogen causes a decrease in LDL-C and an increase in HDL-C and TG 1468) . Progesterone antagonizes the effects of estrogen, resulting in an increase in LDL-C and a decrease in TG and HDL-C 1468) . Therefore, their effects on serum lipids vary depending on their ratio. When used as hormone replacement therapy for menopause or as a treatment for prostate cancer, it is known to affect lipid metabolism in a dose-dependent manner. However, dyslipidemia is not usually seen as a problem with low-dose pills for contraceptive purposes.
Immunosuppressant Drugs
Increased serum cholesterol and TG have been observed in more than half of children who underwent liver transplantation and received cyclosporine 1469) . Tacrolimus has less effect on serum lipids than cyclosporine, and TG, LDL-C, and HDL-C decreased when switching from cyclosporine to tacrolimus 1470) . Since patients undergoing transplantation are young, the impact on future cardiovascular events needs to be monitored.
Anti-HIV Drugs
While anti-HIV therapy improves chronic inflammatory conditions and vascular endothelial function by reducing HIV viral load, anti-HIV drugs themselves have been found to increase myocardial infarction as a side effect. The incidence of myocardial infarction in the group that used protease inhibitors for more than 6 years was about 4 times higher than that in the group that did not use protease inhibitors 1471) . It is thought to be due to dyslipidemia such as hyper-LDL cholesterolemia, hypertriglyceridemia, and hypo-HDL cholesterolemia, which are side effects of protease inhibitors 1472 , 1473) . Integrase inhibitors, a new generation of anti-HIV drugs, have little effect on serum lipids 1474) .
Atypical Antipsychotic Agent
Atypical antipsychotics such as olanzapine have been shown to cause obesity, insulin resistance 1475) , hypertriglyceridemia and hypo-HDL cholesterolemia 1476) .
Retinoids
Retinoids are a generic name for vitamin A analogs, used in the treatment of acute promyelocytic leukemia and skin diseases. Hypertriglyceridemia is common, occurring in 17% of patients using retinoids 1477) . Retinoids enhance apoC-III expression via retinoid X receptors, resulting in hypertriglyceridemia 1477 , 1478) . An increase in LDL-C and a decrease in HDL-C were also observed 1477) .
2.9 Heavy Alcohol Consumption
Although moderate alcohol intake has an anti-atherosclerotic effect by increasing HDL and apoprotein A-I 1479) , heavy alcohol consumption is known to exacerbate insulin resistance by increasing inflammatory cytokines 1480) . Hyperlipidemia type IV is caused by increased VLDL synthesis due to worsening insulin resistance and impaired VLDL metabolism. Type V hyperlipidemia with increased CM may also be present. An elevated γ-GT can aid in diagnosis.
Heavy alcohol consumption may induce atherosclerosis via hypertriglyceridemia, insulin resistance, etc. Although the evidence is still insufficient, it has been reported that heavy alcohol drinking is associated with fatality in patients with acute myocardial infarction 1481) and is a risk factor for ischemic stroke 1482) .
Chapter 7.
Older People
•As is the case for nonelderly adults, hyper-LDL cholesterolemia is an important risk factor for CAD among older persons 65-74 years of age.
•Statin therapy can be recommended for the secondary prevention of CAD in older people.
•Statin therapy can be recommended for the primary prevention of CAD and non-cardiogenic cerebral infarction in elderly people 65-74 years of age with hyper-LDL cholesterolemia.
•Lipid-lowering therapy for the primary prevention of CAD and stroke can be suggested in older patients ≥ 75 years of age with hyper-LDL cholesterolemia.
•Frailty is a common complication in older people and a cardiovascular risk. The assessment of frailty can be suggested for comprehensive management in older people.
1.Lipid Abnormalities and ASCVD in Older People and their Association with Preventive Effects
Primary and secondary prevention of ASCVD is extremely important, as it increases with age and adversely affects the quality of life of patients and life prognosis 1483) . Since elderly patients also show an association between TC, LDL-C, and non-HDLC and the incidence of CAD, indicating a secondary prevention effect of statin therapy on CAD and stroke. This is why the JAS guideline 2017 recommended the importance of statin therapy for the secondary prevention of CAD in older people 600) . Regarding primary prevention, the JAS guideline 2017 recommended that statin therapy for hyper-LDL cholesterolemia for the primary prevention of CAD and non-cardiogenic cerebral infarction in elderly persons 65-74 years of age is promising, however, there was no clear evidence regarding the primary preventive effect of lipid-lowering therapy in elderly patients ≥ 75 years of age. Recently, an RCT study was conducted in Japan to address this point. In the EWTOPIA75 trial of 3,796 Japanese elderly patients ≥ 75 years of age with hyper-LDL cholesterolemia, lipid-lowering therapy with ezetimibe prevented 34% of first composite cardiovascular events (sudden death, myocardial infarction, coronary reconstruction, stroke) 1108) . This means that even in hyper-LDL cholesterolemia in elderly patients ≥ 75 years, lipid-lowering treatment is expected to provide primary prevention of CAD and stroke. Note that the EWTOPIA75 subanalysis did not show a clear effective benefit for ages 85 and older, and the above suggestion is for ages 84 and younger.
Recent studies on the relationship between frailty and ASCVD in older people have revealed that frailty is a factor in the incidence of ASCVD, and conversely, ASCVD is also a risk factor for frailty 1484) . Since frailty complications affect the prognosis of ASCVD in older people, it is necessary to properly evaluate and prevent frailty.
2.Frailty and Sarcopenia
As people age, the body’s reserve capacity declines, and when it declines beyond a certain level, it leads to a state of need for nursing care. Frailty is the stage before the need for long-term care, i.e., a fragile state in which a person is able to lead an independent life but is prone to health problems 1485) . Frailty is a condition in which the physiological reserve of the whole body declines in old age due to various factors, which increases vulnerability to stress and makes the person vulnerable to needing care. In addition to physical factors, mental factors such as depression and dementia, as well as social factors such as loneliness and seclusion, can be the cause. The CHS criteria proposed by Fried 1485) and the Japanese version of the CHS criteria (J-CHS criteria), which incorporate questions from the basic checklist 1486) , are used for diagnosis. Both criteria assess five items related to I muscle weakness, II fatigue, III decreased physical activity, IV decreased gait speed, and V weight loss, with a diagnosis of “pre-frailty” when one or two items apply and “frailty” when three or more apply. The physical component of frailty includes sarcopenia, and the criteria of the Asian Working Group for Sarcopenia (AWGS) are used to diagnose it 1487 , 1488) .
3.ASCVD and Frailty/Sarcopenia
In the Women’s Health Initiative Observational Study, a history of CAD was significantly associated with progression to frailty after 3 years in a population of women 65 years and older who were not frail at study entry (odds ratio 1. 47, 95% confidence interval 1.25-1.73) 1489) . The study showed that hypertensive and diabetic populations also have a significantly higher risk of future progression to frailty and that CAD and ischemic heart disease and their risk factors are a series of causes related to frailty progression.
On the other hand, there are studies that show that frailty defines the prognosis for CAD and ischemic heart disease. The Health Aging and Body Composition Study was the first to show that frailty is a risk for the incidence of cardiovascular disease 1490) . In a study of 3,075 subjects aged 70-79 years, the subsequent incidence of cardiovascular disease was significantly increased in the population with a reduced walking speed of more than 362 seconds for 400 m compared to the population without a reduced walking speed of less than 290 seconds for a 400 m (hazard ratio 1.61, 95% confidence interval 1.05-2.45) and a higher 4.9-year total mortality rate of 4.9 years (hazard ratio 3.23, 95% confidence interval 2.11-4.94). Many other similar studies have reported an effect of walking speed on cardiovascular events. The Italian general population cohort study Pro.V.A. examined the effect of pre-frailty on the incidence of cardiovascular disease in the general population aged 65 years and older who were free of frailty, cardiovascular disease, cancer, and dementia at baseline, using new-onset cardiovascular disease (CAD, heart failure, stroke, PAD, and death from cardiovascular disease) as endpoints 1491) . Of 3,099 randomly enrolled Caucasians aged 65 years and older (1,854 women and 1,245 men) from the two regions surrounding the city of Padua, Italy,2 1,567 non-frail older persons, including pre-frail, without cardiovascular complications were followed for 4.4 years. Cardiovascular disease developed in 551 cases (84 cardiovascular deaths, 27 severe angina, 36 acute myocardial infarction, 249 heart failure, 8 stroke, 147 PAD), and there was a significant association between pre-frailty and the incidence of cardiovascular disease. After adjustment for cardiovascular disease risk factors, inflammatory markers, and HbA1c levels, the risk of cardiovascular disease was significantly higher in the 1 and 2 of 5 frailty items group than in the no frailty group (hazard ratio 1.25; 95% confidence interval 1.05-1.64, p=0.03, 1. 79; 1.27-2.52, p=0.001).
CAD, especially myocardial infarction, can lead to heart dysfunction and eventually to heart failure. In the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF), muscle mass was assessed by DXA in 200 patients with chronic heart failure (mean age 66.9±10.4 years), and found that 20% of the patients with chronic heart failure had reduced muscle mass 1492) . It has been pointed out that not only the prognosis of limb but also the prognosis of life is extremely poor in PAD, and one of the reasons for this is that approximately 50% of patients with PAD are complicated by cardiac and cerebrovascular disorders 1493) . The concept of polyvascular disease was introduced to describe a condition in which CAD, cerebrovascular disease, and PAD are combined 1494) . Considering that older people are prone to polyvascular disease and that walking impairment is caused by ischemia in the lower extremities, it is predicted that PAD is associated with frailty and sarcopenia. At present, however, there are few reports on the association between PAD and frailty/sarcopenia. A study reported from Taiwan found that of 1,036 community-dwelling older people aged 65 years or older (539 men and 497 women, mean age 74.2±6.6 years), 143 (13.8%) had frailty and 74 (7.1%) had PAD. Furthermore, the risk of PAD complications was more than three times higher in frail patients 1495) . A retrospective study of cases in which lower limb amputations were performed showed that the readmission rate within 30 days after surgery for 379 eligible patients (mean age 59±15 years) was 22.7%, with the readmission rate increasing with higher frailty scores 1496) . A similar report on the association between prognosis and frailty in PAD includes a study of complicated frailty using the modified frailty index (mFI) in 4,704 patients with PAD (mean age 67.9±11.7 years) who had undergone surgical bypass surgery. In this study, frailty complications were divided into four levels, from mild to severe; 14.6% of the patients had mild frailty complications (1st degree), 55.9% had a second degree, 26.9% had a third degree, and 2.6% had a fourth degree. The higher the mFI, the higher the mortality rate 1497) .
Chapter 8.Women
•Management of risk factors such as hypertension, diabetes, and smoking are important both before and after menopause. In particular, diabetes and smoking are associated with an increased risk of CAD in women compared to men.
•While intensified treatment of hypertension and diabetes will be based on the individual patient, smoking cessation instruction will be given to women of all ages.
•Lifestyle modification is the mainstay of treatment for dyslipidemia in premenopausal women.
•Even before menopause, drug therapy should also be considered for familial hypercholesterolemia and secondary prevention of CAD, and for primary prevention in high-risk patients.
•Lifestyle modification is also a priority for dyslipidemia in postmenopausal women, but drug therapy should be considered for high-risk patients.
1.Current Status of ASCVD in Japanese Women
The ranking of deaths and mortality rates by gender according to the 2018 Vital Statistics shows that for men, malignant neoplasms are the most common cause of death, followed by cerebral and cardiovascular disease. Among women, malignant neoplasms are followed by cardiac disease, senility, and cerebrovascular disease, but the combined number and rate of death from cerebral and cardiovascular disease is higher than that from malignant neoplasm 1498) .
On the other hand, the incidence of myocardial infarction in women is lower than in men 1499 , 1500) . According to epidemiological studies conducted in Japan from 1990 to early 2000, the age-adjusted incidence rate of myocardial infarction in women (100,000 persons/year) is 20-50% of that in men 110 , 288 , 1501 , 1502) . In women, the incidence of myocardial infarction increases after menopause, but the risk is still lower than in men 288) . However, the mortality rate after the incidence of coronary events is reported to be higher in women than in men, not only in Westerners 1503 - 1506) but also in Japanese 1507 , 1508) . As Japanese women are aging, and the incidence of myocardial infarction and mortality increase with advancing age 288) , measures to address these issues will be important in the future.
The age-adjusted incidence of cerebral infarction in Japanese is higher than that of myocardial infarction, although the incidence in women is about 50% to 70% of that in men 110 , 603 , 1501 , 1502 , 1509 , 1510) . The incidence of cerebral infarction in women increases with age, reaching 60% to 90% of that in men 75 years or older, with a smaller gender difference than in myocardial infarction 288 , 1509 , 1510) . On the other hand, a multicenter cross-sectional study of patients with acute-onset cerebral infarction reported that the pathophysiology, including length of hospitalization and conditions at the time of incidence and discharge, was more severe in women than in men 1511) .
Since the incidence of cerebral infarction is higher than that of myocardial infarction in Japanese, the difference in the incidence of cerebral infarction between men and women is smaller than that of myocardial infarction, and the aging of women is increasing, prevention of cerebral infarction and management of heart failure due to ischemic heart disease in women are also important issues for the future.
2.Relationship between Risk Factors for Atherosclerosis and ASCVD in Women
1) Serum Lipids
Age-related changes in serum lipid levels differ significantly between men and women. According to the report of the 2019 National Health and Nutrition Examination Survey, LDL-C and non-HDL-C do not change with time in men, but in women, they are lower than men before age 50, the average age of menopause, but increase after age 50 and become higher than men. The same is true for TG, which remains high for men before 50 years old, but for women it increases after 50 years old and approaches the male value. HDL-C remains high in women but does not change significantly over time in either sex 574) . Thus, in women, one of the major factors causing changes in lipid metabolism at menopause is thought to be related to a decrease in estrogen, and aging and postmenopausal changes in serum lipids, especially LDL-C, are thought to have a significant impact on women’s risk of ASCVD.
Epidemiological studies have been reported that prospectively examined the association of TC or LDL-C with the incidence of CAD in women. JALS-ECC 59) showed a significantly higher risk of multifactor adjusted CAD incidence in the high TC group than in the low TC group. CIRCS also showed a significantly higher multifactor adjusted risk of myocardial infarction incidence of 1.42 for each 30 mg/dL increase in LDL-C 43) .
On the other hand, in EPOCHJAPAN, which examined the relationship with CAD mortality, the risk was significantly higher in the high TC group than in the low TC group among women aged 40-69 years 63) , and also in NIPPONDATA 80, the multifactor-adjusted risk was significantly higher in women in the high TC group 62) . In the Ibaraki Prefectural Health Study, however, no significant association with LDL-C was found 44) 23). These results suggest that cholesterol is a significant risk factor for the incidence of CAD in women and may also increase the risk of death.
The relationship between TC and the risk of incidence of cerebral infarction was examined in the JPHC study 67) and EPOCH-JAPAN 63) , but no significant association was found in women.
Iso et al. reported that high TG levels are a significant risk factor for the incidence of myocardial infarction or ischemic cardiovascular disease in women 98 , 101) . JALS-ECC 59) and CIRCS 72) showed a significant association between the risk of CAD incidence and non-HDL-C in women, but the association with the risk of death was not clear 74) .
These findings suggest that abnormalities in TC, LDL-C, TG, and non-HDL-C are important risk factors for the incidence of CAD in Japanese women.
2) Smoking
In 2019, the habitual smoking rate among men aged 20 years or older was 29.9%, while among women it was only 8.1% 1498) . However, the JPHC Study Cohort 1512) and the Suita Study 162) showed that the incidence of myocardial infarction in smokers was 3 to 8 times higher than in nonsmokers, even among women. The risk of CAD mortality was also significantly higher in women who smoked 1513 , 1514) . A meta-analysis including studies from Japan reported that the effect of smoking on the risk of CAD was greater in women than in men 1515) . The JACSS, a multicenter study of ACS, showed that smoking was associated with an extremely high risk in the presence of smoking in women compared to men, with an odds ratio of 8.2 compared to 4.0 in men 1516) .
Smoking is a significant risk for the incidence of cerebral infarction in women 162) . Passive smoking increases the risk of subarachnoid hemorrhage in Japanese women but has not been associated with cerebral infarction 1517) .
Smoking should be considered an important risk factor for CAD and cerebral infarction in women.
3) Hypertension
Blood pressure increases over time in both men and women 1498) . Although hypertension was not a significant risk factor for CAD in women in an epidemiological study conducted in Japan 1518 , 1519) , a trend toward an increased risk of CAD incidence with increasing blood pressure was observed 1519) . On the other hand, hypertension was reported to be a significant risk factor for cerebral infarction incidence in women 1518 - 1520) . In NIPPON DATA 80, which looked at the association between hypertension and risk of cardiovascular mortality, the increased risk of cardiovascular mortality due to degree II hypertension was seen only in the younger age group of 30-59 years and not in the group over 60 years 1521) .
In conclusion, hypertension is an important risk factor for cerebral infarction in women and should be controlled from a young age.
4) Diabetes
The frequency of diabetes increases over time in both men and women, but the percentage of those with strongly suspected diabetes is higher in men (13.8% in men and 7.7% in women) 1498) . The JPHC Study 207 , 1522 , 1523) , Hisayama Study 195) , and Suita Study 186) reported that the risk of incidence and mortality of CAD and cerebral infarction was significantly higher in patients with diabetes than in the non-diabetic group. NIPPONDATA 80 showed that women who are older and have non-fasting blood glucose of 200 mg/dL or more are at higher risk of CAD 286) . The JACCS reported an increased risk in women, with an odds ratio of 6.12 for the incidence of myocardial infarction in patients with diabetes compared to 2.90 in men 1515) . A meta-analysis of patients with diabetes, including studies from Japan, also reported that women have a 44% 1524) higher risk of CAD and a 27% 1525) higher risk of all strokes than men.
3.Primary and Secondary Prevention of ASCVD
The basis for the prevention of ASCVD is the modification of lifestyle. The U.S. Nurses’ Health Study (NHS) found that the risk of CAD incidence 1525) and sudden cardiac death 1527) decreased with a number of factors, including nonsmoking, increased physical activity, maintenance of proper weight, alcohol restriction, and healthy diet. A combined analysis of the NHS and Health Professionals Follow-up Study reported that the risk of cerebral infarction incidence in women with all five of the above factors was extremely low, at 0.19 in women without any of the above factors 1528) . Furthermore, an NHS study of young women aged 27-44 years also showed that the hazard ratio for CAD in women with all six factors (the above five factors plus reduced TV viewing time) was 0.08, lower than that of the population with none of the above factors 1529) . Maintaining a healthy lifestyle from a young age is the key to ASCVD prevention strategies in women.
The effect of smoking on the risk of CAD is greater in women than in men 1514) . However, the effect decreases when smoking is stopped 162) . Since smoking has a negative effect on pregnancy 1530) and a reduction in the risk of atherosclerosis is observed with smoking cessation regardless of age 161) , it is extremely important to guide women to quit smoking at a young age.
Few large clinical trials have examined the primary prevention of CAD in women with statins. In the MEGA Study conducted in Japan, 68% of the subjects were postmenopausal women under the age of 70. Although the reduction in risk of CAD and cerebral infarction in statin-treated patients was not significant in women 49) , a significant reduction in risk was observed in the age group older than 55 years 1343) when the endpoint was (CAD + cerebral infarction) in the women’s sub analysis. In JUPITER, statin treatment for 3,426 eligible women significantly reduced the risk of unstable angina and revascularization therapy compared to the placebo group, but the effect on the prevention of myocardial infarction and cerebrovascular disease was unclear 1531) . The risk of the primary endpoint including these events was significantly reduced in women aged 65 years and older, but not in those younger than 65 years 1343) . 27 large statin clinical trials and a meta-analysis of CTT in 174,000 patients showed that in patients without previous vascular disease, the reduction in the risk of cardiovascular disease for each reduction of 38.7 mg / dL in LDL-C was significant in men at 0.72, but only showed a trend toward reduction in women at 0.85 333) .
In women, the effect of statins in preventing the first occurrence of ASCVD is less clear than in men, and lifestyle modification is the main focus of treatment. However, drug therapy should be considered in FH, secondary prevention patients, and primary prevention patients considered high risk. There is little evidence of the risk of dyslipidemia for CAD in premenopausal women, and the basic approach is to identify secondary dyslipidemia and manage it through lifestyle modification. Because of the lack of consensus regarding the risk of teratogenicity in fetuses of pregnant women taking statins 1166 , 1532 , 1533) and insufficient studies of milk transfer, statins are contraindicated in pregnant and nursing women.
With regard to secondary prevention, a meta-analysis of 11 studies, including 4S and CARE, reported that statins significantly reduced the risk of cardiovascular events with 0.82 in men and 0.81 in women 1534) . The CTT results also showed that for every 38.7 mg/dL reduction in LDL-C in patients with a history of vascular disease, the reduction in risk of cardiovascular disease was 0.84 in women, which was significant as in men 333) . The J-STARS study was conducted in Japan to investigate the prevention of recurrence with statins in patients with a history of cerebral infarction. The risk of atherothrombotic cerebral infarction was significantly reduced by 67% in a 5-year prospective study, but by gender, women did not show a significant reduction 348) .
In conclusion, women should also be appropriately treated in the secondary prevention of CAD, but the effect of statins on the prevention of recurrent cerebral infarction in women is not clear.
In the treatment of diabetes, strict blood glucose control is effective in the prevention of CAD 1535) , but the effect takes a long time to appear 1295 , 1536) , and the effect is lower than the reduction in the risk of microvascular disease 270) . The risk of hypoglycemia increases, so intensified treatment is necessary considering the patient’s condition is necessary 1535 , 1537) . The impact of diabetes on the incidence of ASCVD is greater in women than in men 1524 , 1525) . The comprehensive management of diabetes in women, including risk factors other than hyperglycemia, is important at an early stage.
Hypertension is an important risk factor for the incidence of cerebral infarction in women and has also been implicated in the association with CAD. The number of hypertensive patients increases with age in both men and women, but after the age of 60, the number of women patients exceeds that of men 1538) . After menopause, LDL-C also increases 1511) and the risk of ASCVD increases, so the management of hypertension in postmenopausal women is important. A meta-analysis of studies on antihypertensive therapy did not report a clear gender difference in the reduction in the risk of cardiovascular disease events 1539) .
There have been no intervention trials for hypertension in premenopausal women. At present, it is appropriate to adequately differentiate secondary hypertension and to proceed with treatment from a young age, focusing on lifestyle modification. The Japanese Society of Hypertension guidelines 179) should be followed for the management of pregnancy-related hypertension and perimenopausal hypertension.
4.Hormone Replacement Therapy (HRT)
Numerous clinical trials have reported the risk of HRT and cardiovascular disease in postmenopausal women for the treatment of menopausal symptoms and the prevention of osteoporosis. HERS using HRT (combined estrogen + medroxyprogesterone acetate) in 2,763 women with CAD showed no reduction in risk of CAD or cerebrovascular disease 1540 , 1541) . The WHI, which examined the effect of HRT (combined estrogen + medroxyprogesterone acetate) in 16,608 healthy postmenopausal women, showed a significantly increased risk of 1.44 1542) for cerebral infarction and 1.24 1543) for CAD. Concurrent conjugated estrogen monotherapy also showed a predominant increase in the risk of incidence of cerebral infarction of 1.55 1544) . However, the increased risk of CAD and cerebrovascular disease with HRT was age-dependent, with no significant increase in either risk among women younger than 60 years, and a rather low trend for CAD 1544 , 1545) .
According to the Hormone Replacement Therapy Guidelines 2017 jointly developed by the Japan Society of Obstetrics and Gynecology and the Japan Society for Menopause and Women’s Health 1546) , HRT is contraindicated in patients with a history of myocardial infarction, atherosclerotic lesions in the coronary arteries and stroke, and new administration of HRT to obese, over 60 years of age, or postmenopausal for more than 10 years is prudent in patients with coronary spasm and microvascular angina, severe hypertriglyceridemia, poorly controlled diabetes, or hypertension. Studies such as WHI and HERS have so far been negative on the effects of HRT on cardiovascular disease risk, however, as the benefits of estrogen in improving lipid metabolism and vascular function have been proven, with transdermal estrogens reported to significantly reduce the risk of myocardial infarction 1547) , future studies are needed on the types, doses, and routes of administration of estrogens and progestins other than those used in WHI and HERS, as well as the age at which HRT should be started. The Japan Society for Menopause and Women’s Health has developed “Management of Primary Prevention of Atherosclerotic Cardiovascular Diseases in Women” specifically for women, in accordance with the JAS Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases. In this guideline, HRT is recommended for women with dyslipidemia and menopausal symptoms, in addition to lifestyle modification 1548) .
Currently, the incidence of CAD in women in Japan is considerably lower than in the United States and Europe 1500) . A reduction in the incidence of cerebrovascular disease has also been observed with the treatment of hypertension 1500) . On the other hand, new concerns about the rise in ASCVD, such as westernization of diets and lack of physical activity, are increasing. Since women live longer than men, early management is especially important given their lifetime risk of ASCVD.
Chapter 9.Pediatrics
•Aggressive early detection of dyslipidemia is important.
•Correctly diagnose primary and secondary dyslipidemia. If necessary, consult with a specialist.
•Familial hypercholesterolemia will be diagnosed using the diagnostic criteria of the “Guidelines for the Diagnosis and Treatment of Pediatric Familial Hypercholesterolemia 2022”, and probable/possible cases should also be followed.
•Follow-up with patients with familial hypercholesterolemia, providing dietary and lifestyle advice, and considering indications for drug therapy. Also, identify new patients within the family.
•Treat the underlying disease adequately for secondary dyslipidemia.
•It is recommended that proper lifestyle habits, including diet, develop from childhood and maintain an appropriate weight.
1.Early Detection of Dyslipidemia
For the prevention of future ASCVD, it is important to take measures and actions from childhood. There is no health examination system that performs blood examinations in childhood, and dyslipidemia has few symptoms in childhood. Therefore, it is necessary to be aware of the aggressive detection of dyslipidemia. If given the opportunity, TC and TG should be examined at least once as early as possible. If any abnormality is found, a close examination is performed. In the case of fasting blood sampling, serum LDL-C is calculated from TC, TG, and HDL-C by the Friedewald method ([LDL-C] =[TC]-[HDL-C]-[TG/5]). In the case of nonfasting blood sampling, the values of LDL-C by the direct method and non-HDL-C ([non-HDL-C]=[TC] - [HDLC]) should be used as a reference.
2.Criteria for Lipid Abnormalities in Children
Cut-off values for dyslipidemia in children are shown in Table 28 . This was set from a national survey of elementary and junior high school students in the 1990s by Okada et al. 1549) . TC, LDL-C and TG are based on 95th percentile values; HDL-C is based on 5th percentile values. The values are represented by one value, although there are some differences depending on the age. The lipid levels in the 2000s survey by Abe et al. 1550) are not much different from those in the 1990s. In addition, a new cut-off value for non-HDL-C was added. 150 mg/dL is approximately the 95th percentile value, and values above this are considered high 1550 , 1551) . Kobayashi et al. 1552) examined postprandial TG and found that its blood level increased within 1 hour and remained almost constant up to 3 hours, with a 95th percentile value of approximately 200 mg/dL.
Table 28. Cut-off values for lipid abnormalities in children (<15 years, fasting).
| Total cholesterol (TC) | ≥ 220 mg/dL |
| LDL cholesterol (LDL-C) | ≥ 140 mg/dL |
| HDL cholesterol (HDL-C) | <40 mg/dL |
| Triglycerides (TG) | ≥ 140 mg/dL |
| non-HDL cholesterol (non-HDL-C) | ≥ 150 mg/dL |
This lipid cut-off value can be used for younger children, but breast-fed infants are more prone to hyperlipidemia, so high values cases should be reexamined after weaning and followed. In addition, since LDL-C decreases during puberty, the adolescent stage should be taken into consideration when treating patients 1553) .
3.Primary Dyslipidemia
In children, primary hypercholesterolemia and primary hyperchylomicronemia are the main problems. Type III hyperlipidemia is considered rare in childhood. (See Chapter 5, “Other Primary Dyslipidemias.”)
1) Primary Hyper-LDL Cholesterolemia
Familial hypercholesterolemia (FH) is a disease that requires appropriate treatments since childhood because of extremely high levels of LDL-C and the rapid progression of ASCVD, caused by genetic abnormalities in the LDL receptor and the related molecules. The frequency is also thought to be higher than previously thought 1393) . In 2022, the diagnostic criteria were revised along with the revised guidelines for pediatric FH 1393) . In order to improve the diagnostic rate, the sensitivity is increased while maintaining specificity. (See Chapter 4, “Familial Hypercholesterolemia.”) Early detection and aggressive treatment from childhood are recommended for FH. If hyper-LDL cholesterolemia of 180 mg/dL or more persists despite lifestyle guidance, drug therapy should be considered at around 10 years of age. Severe cases, such as homozygotes, should be treated in consultation with a specialist. When a child with FH is found, it is also necessary to try to detect new patients in the family (cascade screening) 1393) .
Familial combined hyperlipidemia(FCHL) is a genetic disorder in which both TC and TG are elevated, but there is no evidence for the need for aggressive drug therapy in childhood. A detailed family history should be investigated, and regular follow-up should be done due to fluctuating serum lipid levels.
2) Primary Hypertriglyceridemia
In children, primary hyperchylomicronemia, especially lipoprotein lipase (LPL) deficiency, which may lead to pancreatitis, is important. There have been many reports of genetic mutations in LPL. Apoprotein C-II and apoprotein A-V deficiency, which activate LPL, and autoantibodies are also causes. Homozygotes have extremely high hypertriglyceridemia. Diagnosis and treatment should be consulted with a specialist.
4.Secondary Dyslipidemia
The causes of secondary dyslipidemia are varied. A high frequency is associated with obesity. Furthermore, thyroid hormones should always be examined, as hyper-LDL cholesterolemia may be present in patients with Hashimoto’s disease and other forms of hypothyroidism. Drug-induced dyslipidemia should also be noted. A particularly problematic case, even in children, is diabetes mellitus with hyper-LDL cholesterolemia. Diabetes itself is an important risk factor for atherosclerosis, and blood glucose control alone has little preventive effect on ASCVD (See Chapter 3, 5.2, “Diabetes Mellitus”). The International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines 1554) set lipid management goals of LDL-C <100 mg/dL, HDL-C >35 mg/dL and TG <150 mg/dL. Statins are initiated when LDL-C is 130 mg/dL or higher, even after enhanced blood glucose control and diet and exercise therapy 1554) . In Japan, we also think that LDL-C in particular should be kept below 140 mg/dL 1555) . If hyper-LDL cholesterolemia persists despite continued strict blood glucose control, consult a specialist.
5.Maintain Appropriate Weight through Proper Diet and Exercise Habits
Pathological changes in blood vessels related to atherosclerosis have been reported to occur in childhood 1556 , 1557) . It is important to prevent those changes from occurring and from developing as much as possible. Even in children, obesity, especially excess visceral fat, is accompanied by abnormal adipocytokine secretion, lipid abnormalities, hyperinsulinemia, and hypertension 1551) . In other words, obesity in children, as in adults, acts in the direction of promoting atherosclerosis. It is also known that childhood eating habits tend to be carried over into adulthood and that many cases of childhood obesity lead to adult obesity 1551) . Appropriate weight control from childhood will prevent future ASCVD. Dietary Reference Intakes for Japanese 2020 653) lists the daily energy requirements for each age group according to activity level. For nutritional balance, the target amount of fat-to-energy ratio is 20-30% and that of carbohydrates is 50-65%, which is the same for all ages. In recent years, fat intake has tended to increase due to the westernization of the diet, so fat intake should be moderate. In other words, a balanced intake of fish, soybeans (products), vegetables, fruits, and seaweeds is recommended, with a focus on the traditional Japanese food pattern, without likes and dislikes. Also watch for excessive salt intake. (See Chapter 3, 2.4, “Diet Therapy”).
It is also important to make exercise a habit. Exercise stimulates various cells and tissues and works to prevent atherosclerosis and obesity. For children, it is better to have something that is easy and enjoyable to continue.
For the body size assessment of individual children, BMI itself, as well as the BMI-for-age percentile method used overseas, is not suitable for ages with large height differences 1551 , 1558) . At present, it is better to use Percentage of Overweight (POW, see below), which is compared measured weight with standard weight 1551 , 1558) . In elementary and junior high school students, obesity is assessed as POW of +20% (120% of standard body weight) or more. Waist circumference (abdominal circumference at umbilical height) is used to determine excess visceral fat, as in adults. The standard is 80 cm (75 cm is also used for elementary school students) 1551 , 1558) .
In obesity, LDL-C and TG are likely to be high and HDL-C low, even in children. If the patient has dyslipidemia or other complications, he or she becomes “Obesity disease” and is subject to treatment to reduce POW 1551) . However, the presence of primary dyslipidemia should also always be taken into account, since many cases of childhood obesity are actually normolipidemia.
6.Smoking and Passive Smoking
Smoking is a major independent risk for all ASCVDs, and smoking cessation is known to decrease that risk. Passive smoking has also been reported to increase the risk of CAD and diabetes, so be aware of smoking not only by the patient but also by family members.
7.Other
Primary dyslipidemia is covered by medical expenses subsidized by the government’s measures for chronic specified childhood diseases. The “dyslipidemia” section under “inborn errors of metabolism” is also indicated for diseases other than those listed above. For details, see the website of the “Information Center for Specific Pediatric Chronic Disease, Japan” 1559) .
Childhood Obesity Determination Formula (Percentage of Overweight; POW)
Appendix 1.
Supplemental Fig.1.
10‐year risk of incidence of atherosclerotic cardiovascular disease; age scored version
Appendix 2.
Supplemental Fig.2. Flowchart for cardiovascular event screening in NAFLD* patients .
We have to check for cardiovascular disease (CVD) complications and/or a past history of CVD, and perform an electrocardiogram (ECG). If any abnormality is found, we consult a specialist in cardiology or neurology. In NAFLD with a reduced platelet count or increased FIB-4 index, we should evaluate risk based on cardiovascular examination, such as loaded ECG and/or US of the carotid artery. FIB-4 index Fibrosis-4 index; PLT platelet, DM diabetes mellitus; HT hypertension, DL dyslipidemia; US ultrasonography; ECG electrocardiogram
*NAFLD: Currently MASLD
Tokushige K, et al.: Evidence‐based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020.
J Gastroenterol, 2021; 56: 951‐963
Appendix 3.
Supplemental Fig.3.
‘Where am I?’ chart
Appendix 4. Method of Measuring Achilles Tendon Thickness by Ultrasound for FH Screening
This appendix is excerpted from “Standard Evaluation Method for Measuring Achilles Tendon Thickness by Ultrasound Method for Screening Adult Familial Hypercholesterolemia,” 2018, Japan Atherosclerosis Society and the Japan Society of Ultrasonics in Medicine
Body Position of the Examinee
Performed in bed in (1) kneeling position, (2) seated position, or (3) prone position.
(1) Kneeling · Drooping position: In the “kneeling position” (recommended) on the bed (kneeling position in a backrest chair without casters is also possible: be careful not to fall), the ankle should be bent at about 90 degrees and drooped ( Supplementary Fig.4a ) , with the ankle out from the edge of the bed or chair.
Supplementary Fig.4.
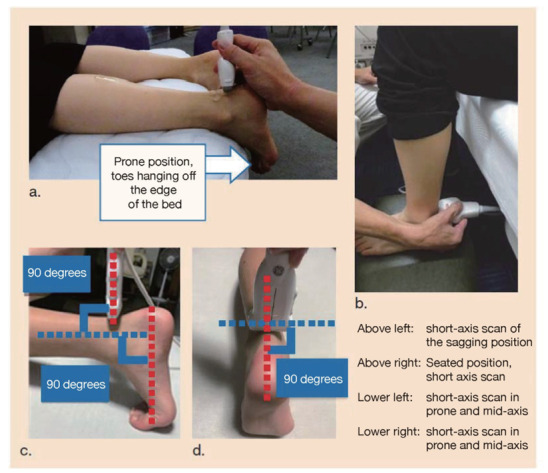
Positions and probes
(2) Seated position: Sit on a chair or bed and observe on a footrest (recommended, Supplementary Fig.1b ).
(3) Prone position: Performed in the prone position on the bed. (i) is recommended.)
i) Sagging position (recommended): In the prone position on the bed, the ankles are placed out from the edge of the bed and allowed to droop with the ankles flexed approximately 90 degrees ( Supplementary Fig.4a ) .
ii) Intermediate position: ankle perpendicular to the ground, ankle flexed approximately 90 degrees ( Supplementary Fig.4c, d ) .
Measurement of Achilles Tendon Thickness
Both short-axis and long-axis images are measured in the “Achilles tendon thickness (ATT) direction” using sufficient echogenic jelly. Gel pad can be used.
Measure at the position where the ATT is thickest. The probe is placed perpendicular to the center line of the foot and the angle between the skin and the probe is approximately 90 degrees ( Supplementary Fig. 4 ).
Tendon thickness is measured in the direction of maximum thickness, not in the anteroposterior direction in the short-axis image ( Supplementary Fig. 5 ).
Supplementary Fig.5. Short-axis cross-sectional image.
Measure the thickness of the tendon in the direction of maximum thickness with the direction of torsion (white arrow) in mind.
The long-axis image is also measured in the same way, taking into account the direction of torsion and depicting the area of maximum thickening to measure tendon thickness ( Supplementary Fig. 6 ).
Supplementary Fig.6. Long-axis cross-sectional image.
Long‐axis cross‐sectional image in the torsion direction from the white arrow site of the short‐axis image
Diagnostic Cut-Off Values for Achilles Tendon Thickening
Suspect Achilles tendon thickening at ≥ 6.0 mm in men and ≥ 5.5 mm in women. If tendon thickening is suspected, the family history and LDL-C should be rechecked, taking care to accurately diagnose FH.
Matters to be considered: Achilles tendon rupture or Achilles tendon area pain, history of rheumatoid arthritis, or sports history. Tendon thickening has also been reported in other conditions such as apoprotein E abnormality and cerebrotendinous xanthomatosis.
In normal subjects, it is about 4 to 5 mm and in FH it is often 4 to 20 mm.
Appendix 5.Achilles Tendon Radiography for FH Screening
Achilles Tendon Evaluation of Familial Hypercholesterolemia Diagnosis Study Group
Representative: Yoshizumi Toru (currently Medical Division, Grom Management Co.)
Participating facilities: Rinku General Medical Center, Keiwakai Osaka Police Hospital, Daini Osaka Police Hospital, Otemae Hospital of the Public Employees Mutual Aid Association, Minoh City Hospital
In Achilles tendon radiography, two matters are important; the first of which is related with image acquisition and imaging techniques, and the second is the image processing and measurement of the obtained images.
(1) Preparation for Radiography
The imaging is performed under conditions in which the subject’s ankle joint is free of any obstructive shadows such as pants or socks ( Supplementary Fig. 7 ).
Supplementary Fig.7.
(2) Radiographic Position
The patient is seated or lying on the side, with the lateral side of the lower leg and the ankle joint attached on the light-receiving surface, so that the lower leg and the plantar surface of the foot forms 90 degrees. Since ankle joint extension, flexion, internal application, and external application affect Achilles tendon thickening measurements, imaging aids and other devices should be used to improve accuracy. The points are summarized below ( Supplementary Fig. 8 ).
Supplementary Fig.8.
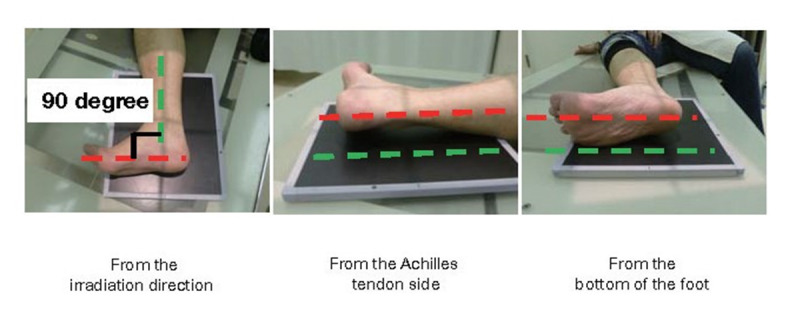
· The patient is placed in a supine position so that the outer margin of the foot of the patient is placed in the light receiving area.
· Positioning so that the lower leg bone is perpendicular to the sole of the foot
· Positioning so that the Achilles tendon (lower leg) and the light-receiving part are as parallel as possible.
· Positioning so that the center of the foot and the light-receiving area are as parallel as possible.
(3) Note
Normally, it is recommended that Achilles tendon radiography is performed with standard method utilizing the foot position as a reference. However, depending on the angle and position of the Achlles tendon at the time of imaging, the limbus of the Achilles tendon may be blurred as shown in the left panel. In this case, the Achilles tendon should be re-imaged using the Achilles tendon as a reference to obtain a clearer image ( Supplementary Fig. 9 ).
Supplementary Fig.9.
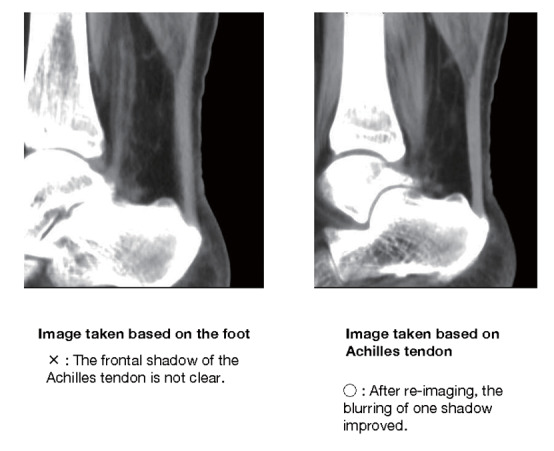
(4) Conditions
50 kV, 5.0 mAs when using digital system (e.g., 100 mA x 0.05 sec, 50 mA x 0.1 sec). Increase or decrease the mAs value as needed.
(5) Distance from X-Ray Tube Focal Point to X-Ray Receiving Surface
The distance should be 120 cm to eliminate the influence of the magnification ratio on the x-ray image. If possible, place a lead scale or similar object (of known size and non-radiopaque) at the same height as the Achilles tendon at the time of imaging to correct for magnification.
(6) X-ray Centerline
Incident to the posterior margin of the endocarp of the tibia perpendicular to the receiving surface.
(7) Image Processing Conditions
For evaluation using a digital radiographic imaging system, processing conditions that enable clear delineation of Achilles tendon, adipose tissue, and skin are recommended ( Supplementary Fig. 10 ).
Supplementary Fig.10.
There are roughly three types of Achilles tendon images. The rightmost image shows the difference between fat and Achilles tendon and Achilles tendon and skin, both of which are suitable for measuring thickening. The numerical difference between each image is large, which makes the image suitable for measuring the Achilles tendon.
(8) Achilles tendon thickening measurement
Currently, Achilles tendon measurement using image reference terminal measurement tools is common. The tool is used to measure the maximum thickened part of the Achilles tendon by the image observer.
If film is used, the image is output at equal magnification and the thickness is measured using calipers or a ruler. Automatic measurement software is currently being commercialized to improve the accuracy of the measurement itself, and its accuracy is currently being verified.
Appendix 6.COVID-19 and ASCVD / Thrombosis
1. Introduction
In February 2020, Coronavirus disease-19 (COVID-19) was brought to Japan’s attention by the Diamond Princess cluster, and the infection quickly spread throughout the country. In Japan, by the end of June 2021, there were approximately 800,000 PCR-positive people and 15,000 deaths. Many academic societies in Japan and abroad have prepared and published recommendations and guidelines on COVID-19 countermeasures in various clinical settings and situations. In this section, we will discuss the effects of COVID-19 on the treatment of atherosclerosis, including risk factor management for atherosclerosis, ASCVD, and thrombosis, as well as the impact of COVID-19 on atherosclerosis as it relates to arteriosclerology.
2. Symptoms of SARS -CoV-2 Virus and COVID-19, etc.
COVID-19 is an infection with SARS coronavirus-2 (SARS -CoV-2), a single-stranded RNA virus.
SARS -CoV-2 is an enveloped virus whose RNA is covered by a lipid bilayer. Spike proteins on its surface bind to angiotensin converting enzyme II (ACE2) on the cell membrane and enter the cell. While SARS, caused by infection with a SARS virus with a close genetic sequence, can cause severe disease if contracted, COVID-19 has very large individual differences, ranging from asymptomatically infected patients to those with severe disease resulting in death. This has made it extremely difficult to control COVID-19 infection socially. Most of the symptoms are not different from those of the common cold: fever, headache, runny nose, sore throat, cough, and phlegm are common. It is noted that COVID-19 rather frequently causes olfactory and taste disturbances in some patients. COVID-19 is often associated with pneumonia, which, like other viral pneumonias, presents with pale shadows on chest radiographs and is multiple. The fever may resolve but worsen again 7-10 days after the incidence. In addition, many cases are characterized by rapid progression of pneumonia when the disease worsens. Some patients develop a severe condition called cytokine storm, in which the lungs become acute respiratory distress syndrome (ARDS)-like and require ventilators or extracorporeal cardioplegia (ECMO). Administration of corticosteroids and anti-interleukin-6 neutralizing antibodies have shown some efficacy in avoiding severe disease1-3).
COVID-19 presents various complications. The most notable complication is thrombosis, but other complications such as myocarditis and takotsubo cardiomyopathy have also been reported4, 5). Furthermore, renal dysfunction6) often occurs in severe cases. It may also leave sequelae such as breathing problems abnormalities of taste and smell, and loss of concentration called ‘brain-fog’ whichh is known as long COVID7, 8).
3. Involvement of ASCVD in the Symptoms and Severity of COVID-19
Although various factors have been identified to contribute to the severity of COVID-19, age was identified as a factor with a large impact9, 10). The most severe cases were among older people, and most of the fatalities were over 70 years of age. The age group for severe disease also tends to decrease in mutant strains and changes in risk factors in mutations should be noted. In the “Guidance for Clinical Practice” provided by the Ministry of Health, Labour and Welfare, COPD, CKD, etc., as well as diabetes, hypertension, dyslipidemia, obesity (BMI over 30), and smoking are listed as risks for COVID-19 serious illness11). These are risk factors for ASCVD, and health care professionals who treat lifestyle-related diseases and ASCVD should strive to prevent infection in their patients and inform them about the risk of serious COVID-19 disease.
4. Involvement of Thrombosis in COVID-19 Severity
One of the characteristics of COVID-19 is its tendency to develop thrombosis.
In addition to typical thrombosis such as deep vein thrombosis, cerebral infarction, and myocardial infarction, microthrombi in the microcirculatory system of COVID-19 complicated pneumonia tissue are also problematic12).
Since the actual status of COVID-19-related thrombosis in Japan was largely unknown, the “Research Group on Blood Coagulation Disorders” of the Ministry of Health, Labour and Welfare’s Research Project for Intractable Diseases, the Japanese Society on Thrombosis and Hemostasis, and the Japan Atherosclerosis Society jointly conducted a survey of hospitals treating COVID-19 nationwide.
The results of the survey were published on December 8, 2020 on the websites of academic societies, and reported in the journal, along with the results of the responses received afterwards13, 14). The following is a summary.
Responses were obtained from 111 hospitals nationwide for 6,202 COVID-19 patients admitted by August 31, 2020. D dimer was measured in 75.0% of the cases, with 9.2% of the cases showing an increase of 3-8 times the cutoff value during hospitalization, and 7.6% of the cases showing an increase of 8 times or more. The thromophilic tendency of COVID-19 is suggested. Symptomatic thrombosis occurred in 108 patients (1.86% of those analyzed), with the following incidence sites (duplicate responses allowed): symptomatic cerebral infarction in 24 patients, myocardial infarction in 7 patients, deep vein thrombosis in 41 patients, pulmonary thromboembolism in 30 patients, and other thrombosis in 22 patients. The incidence of symptomatic cerebral infarction was relatively high, accounting for 22.2% of all thrombosis. Thrombosis occurred in 32 cases (0.59% of mild/moderate cases) and in 52 cases (13.5% of severe cases that required ventilation and even ECMO cases) during ventilator/ECMO use. Anticoagulation was considered desirable in severe cases. Although 67 cases of thrombosis occurred during the exacerbation of symptoms, 26 cases of thrombosis occurred during the recovery period, and thrombosis should be monitored for a while even during the recovery period.
Anticoagulation was administered to 14.6% of patients hospitalized with COVID-19, mostly because of high D-dimer levels or worsening symptoms. Although observational studies have reported that anticoagulant therapy reduced death and serious illness15), there have also been many cases of thrombosis that occurred under the anticoagulant heparin administration. There are also reports that increasing doses of oral selective Xa inhibitors are not effective enough16).
5. COVID-19 Prevention/Vaccine
COVID-19 is transmitted mainly by droplet and aerosol infections, although some contact infections occur. Therefore, it is important to wash hands, wear a mask and avoid the 3 Cs (crowded, close, closed).
SARS -CoV-2 is an enveloped virus, and as with other enveloped viruses, it is effective for disinfecting with ethanol, which has a membrane-disrupting effect, and for washing hands with soap.
As public health measures were taken to control the spread of the disease around the world, a vaccine was developed. In Japan, vaccination has begun, and as of July 4, 2021, more than 31 million people have received at least one dose of the vaccine. As is clear from reports from other countries, the number of new and severely infected cases of COVID-19 were markedly reduced if a large number of the population were vaccinated17). While the vaccines currently approved in Japan all have high efficacy rates, they can cause anaphylactic reactions, fever, malaise, and other symptoms. Although very rare (about 1 in 100,000), myocarditis and pericarditis can occur with mRNA-type vaccines18). Myocarditis and pericarditis are reported to occur 2-3 days after the second vaccination in young men19), and most cases are mild with a hospitalization of about 4 days20). Thrombocytopenic thrombosis syndrome (TTS) has also been reported in young women with a similar frequency with adenoviral-type vaccines21, 22).
Heparin-induced thrombocytopenia and thrombosis (HITT), as well as autoantibodies against PF4 (protein factor 4) have been identified and reported to stimulate platelets, resulting in consumptive thrombocytopenia23). Note that in many cases, anti-PF4 antibodies can only be detected by ELISA, which is not currently used in Japan. In this condition, thrombi are often found in the cerebral venous sinus and visceral veins (such as the splenic vein), which are rarely encountered in daily clinical practice, and are also prone to hemorrhage and other complications during the course of the disease. Along with thrombotic events due to increased thrombogenicity, attention should also be paid to bleeding events due to consumption of factors that contribute to hemostasis. A guide to diagnosis and treatment is published jointly by the Japan Stroke Association and the Japanese Society of Thrombosis and Hemostasis24).
6. Treatment of Acute Atherosclerotic Disease during the COVID-19 Epidemic
Some ASCVDs, such as acute myocardial infarction and cerebral infarction, require urgent attention even during the COVID-19 pandemic. Clinical procedures for COVID-19-infected patients should be determined and addressed ahead of time at each hospital, including the timing of examinations, the flow lines within the hospital (red zone and yellow zone), the method of catheterization and subsequent procedures, and the method of protection and disinfection of inpatient rooms and examination equipment.
7.Refrain from Activities and Outpatient Care during the COVID-19 Pandemic
During the COVID-19 pandemic period, people tend to work from home more and exercise less.
It also increases alcohol consumption and increases complications of obesity, fatty liver, and dyslipidemia. Appropriate outpatient advice should be given to patients at high risk of atherosclerosis and cases of ASCVD.
Furthermore, some chronically ill patients tend to avoid visiting hospitals for fear of infection during transportation and also to avoid infection at medical facilities. In such patients, discontinuation of the medication may also occur. Medical facilities should be informed that infection control measures are adequate and that regular visits to the hospital should not be interrupted.
Furthermore, even in the days of the COVID-19 pandemic, there are some infections that require urgent attention, such as bacterial pneumonia, and patients may be seen for tuberculosis or bronchial asthma. It is important to accept these patients without resistance and not overlook them, even in the COVID-19 pandemic phase.
The results of research on COVID-19 infection, pathogenesis of vaccination, prognosis, prevention, and treatment are continuously being reported. We must always pay attention to the latest medical information.
Appendix References
1)Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva M, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP: Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA, 2020; 324: 1307-1316
2)Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ: Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med, 2021; 384: 693-704
3)Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H: Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A, 2020; 117: 10970-10975
4)Akhmerov A, Marbán E: COVID-19 and the Heart. Circ Res, 2020; 126: 1443-1455
5)Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA: A current review of COVID-19 for the cardiovascular specialist. Am Heart J, 2020; 226: 29-44
6)Ronco C, Reis T, Husain-Syed F: Management of acute kidney injury in patients with COVID-19. Lancet Respir Med, 2020; 8: 738-742
7)Radtke T, Ulyte A, Puhan MA, Kriemler S: Long-term Symptoms After SARS-CoV-2 Infection in Children and Adolescents. JAMA, 2021
8)Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, Lartey S, Onyango TB, Kuwelker K, Sævik M, Bartsch H, Tøndel C, Kittang BR, Cox RJ, Langeland N: Long COVID in a prospective cohort of home-isolated patients. Nat Med, 2021; 27: 1607-1613
9)Terada M, Ohtsu H, Saito S, Hayakawa K, Tsuzuki S, Asai Y, Matsunaga N, Kutsuna S, Sugiura W, Ohmagari N: Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open, 2021; 11: e047007
10)Katzenschlager S, Zimmer AJ, Gottschalk C, Grafeneder J, Schmitz S, Kraker S, Ganslmeier M, Muth A, Seitel A, Maier-Hein L, Benedetti A, Larmann J, Weigand MA, McGrath S, Denkinger CM: Can we predict the severe course of COVID-19 - a systematic review and meta-analysis of indicators of clinical outcome? PLoS One, 2021; 16: e0255154
11)Ministry of Health, Labour and Welfare Medical Practice Guidance Review Committee:A Guide to the Treatment of COVID-19 Infections (5th ed.), https://www.mhlw.go.jp/content/000785119.pdf, 2021 (in Japanese)
12)Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D: Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med, 2020; 383: 120-128
13)Horiuchi H, Morishita E, Urano T, Yokoyama K: COVID-19-Related Thrombosis in Japan: Final Report of a Questionnaire-Based Survey in 2020. J Atheroscler Thromb, 2021; 28: 406-416
14)Horiuchi H, Morishita E, Urano T, Yokoyama K, The Questionnaire-survey Joint Team on The COVID-19-related thrombosis organized by the Research Study Team for Intractable Disease (Blood Coagulation Abnormalities), the Ministry of Health, Labour and Welfaire of Japan, the Japanese Society on Thrombosis and Hemostasis and the Japan Atherosclerosis Society:The final report of the questionnaire-based survey in 2020 on COVID-19 related thrombosis in Japan. Jpn J Thromb Hemost, 2021;32:315-329 (in Japanese)
15)Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, Arvind V, Bose S, Charney AW, Chen MD, Cordon-Cardo C, Dunn AS, Farkouh ME, Glicksberg BS, Kia A, Kohli-Seth R, Levin MA, Timsina P, Zhao S, Fayad ZA, Fuster V: Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol, 2020; 76: 1815-1826
16)Lopes RD, de Barros ESPGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, Barbosa LM, de Aveiro Morata J, Ramacciotti E, de Aquino Martins P, de Oliveira AL, Nunes VS, Ritt LEF, Rocha AT, Tramujas L, Santos SV, Diaz DRA, Viana LS, Melro LMG, de Alcântara Chaud MS, Figueiredo EL, Neuenschwander FC, Dracoulakis MDA, Lima R, de Souza Dantas VC, Fernandes ACS, Gebara OCE, Hernandes ME, Queiroz DAR, Veiga VC, Canesin MF, de Faria LM, Feitosa-Filho GS, Gazzana MB, Liporace IL, de Oliveira Twardowsky A, Maia LN, Machado FR, de Matos Soeiro A, Conceição-Souza GE, Armaganijan L, Guimarães PO, Rosa RG, Azevedo LCP, Alexander JH, Avezum A, Cavalcanti AB, Berwanger O: Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet, 2021; 397: 2253-2263
17)Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ: What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis, 2021; 21: e26-e35
18)Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, Zoabi M, Aisman M, Goldschmid N, Berar Yanay N: Myocarditis following COVID-19 mRNA vaccination. Vaccine, 2021; 39: 3790-3793
19)Bozkurt B, Kamat I, Hotez PJ: Myocarditis with COVID-19 mRNA Vaccines. Circulation, 2021
20)Health IMo: Surveillance of Myocarditis (Inflammation of the Heart Muscle) Cases Between December 2020 and May 2021 (Including). https://www.gov.il/en/departments/news/01062021-03, 2021
21)Thiele T, Ulm L, Holtfreter S, Schönborn L, Kuhn SO, Scheer C, Warkentin TE, Bröker B, Becker K, Aurich K, Selleng K, Hübner NO, Greinacher A: Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood, 2021; 138: 299-303
22)Dias L, Soares-Dos-Reis R, Meira J, Ferrão D, Soares PR, Pastor A, Gama G, Fonseca L, Fagundes V, Carvalho M: Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis, 2021; 30: 105906
23)Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, Holme PA: Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med, 2021; 384: 2124-2130
24)The Japan Stroke Society, The Japanese Society on Thrombosis and Hemostasis: Guide to the diagnosis and treatment of thrombosis with thrombocytopenia after AstraZeneca COVID-19 vaccination, 2nd ed. http://www.jsth.org/wordpress/wp-content/uploads/2021/06/TTS%E6%89%8B%E5%BC%95%E3%81%8Dver.2.3.pdf, 2021 (in Japanese)
References
- 1).Subcommittee for preparing guidelines for ultrasound diagnosis of carotid artery: Standard method for ultrasound evaluation of carotid artery lesions 2017. Jpn J Med Ultrasonics, 2018. https://www.jsum.or.jp/committee/diagnostic/pdf/jsum0515_guideline.pdf; 2018 (in Japanese) [Google Scholar]
- 2).Homma S, Hirose N, Ishida H, Ishii T, Araki G: Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke, 2001; 32: 830-835 [DOI] [PubMed] [Google Scholar]
- 3).Nezu T, Hosomi N, Aoki S, Matsumoto M: Carotid Intima-Media Thickness for Atherosclerosis. J Atheroscler Thromb, 2016; 23: 18-31 [DOI] [PubMed] [Google Scholar]
- 4).O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr.: Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med, 1999; 340: 14-22 [DOI] [PubMed] [Google Scholar]
- 5).del Sol AI, Moons KG, Hollander M, Hofman A, Koudstaal PJ, Grobbee DE, Breteler MM, Witteman JC, Bots ML: Is carotid intima-media thickness useful in cardiovascular disease risk assessment? The Rotterdam Study. Stroke, 2001; 32: 1532-1538 [DOI] [PubMed] [Google Scholar]
- 6).Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M: Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation, 2007; 115: 459-467 [DOI] [PubMed] [Google Scholar]
- 7).Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS: Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis, 2012; 34: 290-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Koga M, Kimura K, Minematsu K, Yamaguchi T: Diagnosis of internal carotid artery stenosis greater than 70% with power Doppler duplex sonography. AJNR Am J Neuroradiol, 2001; 22: 413-417 [PMC free article] [PubMed] [Google Scholar]
- 9).Subcommittee for preparing guidelines for ultrasound diagnosis of peripheral artery: Standard method for ultrasound evaluation of peripheral artery lesions. Jpn J Med Ultrasonics, 2023 submitting (in Japanese) [Google Scholar]
- 10).Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I: 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J, 2018; 39: 763-816 [Google Scholar]
- 11).Mizuta R, Kubota Y, Takeshita S, Akutsu K, Nakamoto F, Tanaka N, Sano M, Okajima T, Tsutsumi Y: Transit Time of Vessel Flow in Below-knee is Useful for Screening of Vessel in Below-knee Lesions. Jpn J Med Ultrasound Technology, 2009; 34: 543-547 [Google Scholar]
- 12).Subcommittee for preparing guidelines for ultrasound diagnosis of aortic lesions: Standard method for ultrasound evaluation of aortic lesions 2020. Jpn J Med Ultrasonics, 2021 https://www.jsum.or.jp/committee/diagnostic/pdf/aorticlesion2020.pdf;2021. (in Japanese) [Google Scholar]
- 13).Japanese Society of Nephrology: Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin Exp Nephrol, 2019; 23: 1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Subcommittee for preparing guidelines on ultrasound diagnosis of renal arteries. Guidelines on methods of standard assessment of ultradound diagnosis of renal arterial lesions. Jpn J Med Ultrasonics, 2015;42:185-200 (in Japanese) [Google Scholar]
- 15).Diagnosis, Prevention, and Treatment of Cardiovascular Diseases in People with Type 2 Diabetesand Pre-Diabetes: A Consensus Statement Jointed from The Japanese Circulation Society and The Japan Diabetes Society. 2020: 11-18 (in Japanese) [DOI] [PubMed] [Google Scholar]
- 16).Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, Hunink MG: Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology, 2007; 244: 419-428 [DOI] [PubMed] [Google Scholar]
- 17).Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J, Ropers D, Schuijf J, Tops LF, Bax JJ: Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J, 2008; 29: 531-556 [DOI] [PubMed] [Google Scholar]
- 18).Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK: Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol, 2008; 52: 1724-1732 [DOI] [PubMed] [Google Scholar]
- 19).Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA: Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med, 2008; 359: 2324-2336 [DOI] [PubMed] [Google Scholar]
- 20).Celeng C, Leiner T, Maurovich-Horvat P, Merkely B, de Jong P, Dankbaar JW, van Es HW, Ghoshhajra BB, Hoffmann U, Takx RAP: Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease: A Meta-Analysis. JACC Cardiovasc Imaging, 2019; 12: 1316-1325 [DOI] [PubMed] [Google Scholar]
- 21).Siontis GC, Mavridis D, Greenwood JP, Coles B, Nikolakopoulou A, Jüni P, Salanti G, Windecker S: Outcomes of non-invasive diagnostic modalities for the detection of coronary artery disease: network meta-analysis of diagnostic randomised controlled trials. BMJ, 2018; 360: k504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).The Japanese Circulation Society. Guidelines for non-invasive vascular function test(JCS 2013). 2013 http://www.j-circ.or.jp/guideline/pdf/JCS2013_yamashina_h.pdf (in Japanese) [Google Scholar]
- 23).Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, Ueda S, Vlachopoulos C, Higashi Y, Inoue T, Node K: Physiological Diagnostic Criteria for Vascular Failure. Hypertension, 2018; 72: 1060-1071 [DOI] [PubMed] [Google Scholar]
- 24).Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME: 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2017; 135: e686-e725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Tomiyama H, Matsumoto C, Shiina K, Yamashina A: Brachial-Ankle PWV: Current Status and Future Directions as a Useful Marker in the Management of Cardiovascular Disease and/or Cardiovascular Risk Factors. J Atheroscler Thromb, 2016; 23: 128-146 [DOI] [PubMed] [Google Scholar]
- 26).Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S: Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis, 2003; 166: 303-309 [DOI] [PubMed] [Google Scholar]
- 27).Yamashina A, Tomiyama H, Arai T, Koji Y, Yambe M, Motobe H, Glunizia Z, Yamamoto Y, Hori S: Nomogram of the relation of brachial-ankle pulse wave velocity with blood pressure. Hypertens Res, 2003; 26: 801-806 [DOI] [PubMed] [Google Scholar]
- 28).Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K: Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: the Tanno and Sobetsu study. Diabetes Care, 2003; 26: 437-440 [DOI] [PubMed] [Google Scholar]
- 29).Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Yamashina A: Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension, 2017; 69: 1045-1052 [DOI] [PubMed] [Google Scholar]
- 30).Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Nagayama D, Ohira M, Endo K, Tatsuno I: The Role of a Novel Arterial Stiffness Parameter, Cardio-Ankle Vascular Index (CAVI), as a Surrogate Marker for Cardiovascular Diseases. J Atheroscler Thromb, 2016; 23: 155-168 [DOI] [PubMed] [Google Scholar]
- 31).Ogawa T, Shimada M, Ishida H, Matsuda N, Fujiu A, Ando Y, Nitta K: Relation of stiffness parameter beta to carotid arteriosclerosis and silent cerebral infarction in patients on chronic hemodialysis. Int Urol Nephrol, 2009; 41: 739-745 [DOI] [PubMed] [Google Scholar]
- 32).Hayashi K, Yamamoto T, Takahara A, Shirai K: Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens, 2015; 33: 1742-1757; discussion 1757 [DOI] [PubMed] [Google Scholar]
- 33).Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M: Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb, 2011; 18: 924-938 [DOI] [PubMed] [Google Scholar]
- 34).Takenaka T, Hoshi H, Kato N, Kobayashi K, Takane H, Shoda J, Suzuki H: Cardio-ankle vascular index to screen cardiovascular diseases in patients with end-stage renal diseases. J Atheroscler Thromb, 2008; 15: 339-344 [DOI] [PubMed] [Google Scholar]
- 35).Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Ohira M, Endo K, Kurosu T, Tomaru T, Shirai K, Tatsuno I: Cardio-Ankle Vascular Index is Independently Associated with Future Cardiovascular Events in Outpatients with Metabolic Disorders. J Atheroscler Thromb, 2016; 23: 596-605 [DOI] [PubMed] [Google Scholar]
- 36).Miyoshi T, Ito H, Shirai K, Horinaka S, Higaki J, Yamamura S, Saiki A, Takahashi M, Masaki M, Okura T, Kotani K, Kubozono T, Yoshioka R, Kihara H, Hasegawa K, Satoh-Asahara N, Orimo H: Predictive Value of the Cardio-Ankle Vascular Index for Cardiovascular Events in Patients at Cardiovascular Risk. J Am Heart Assoc, 2021; 10: e020103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R: Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol, 2002; 39: 257-265 [DOI] [PubMed] [Google Scholar]
- 38).Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE: Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol, 1994; 24: 1468-1474 [DOI] [PubMed] [Google Scholar]
- 39).Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML: Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA, 2012; 308: 796-803 [DOI] [PubMed] [Google Scholar]
- 40).Kadota A, Miura K, Okamura T, Fujiyoshi A, Ohkubo T, Kadowaki T, Takashima N, Hisamatsu T, Nakamura Y, Kasagi F, Maegawa H, Kashiwagi A, Ueshima H: Carotid intima-media thickness and plaque in apparently healthy Japanese individuals with an estimated 10-year absolute risk of CAD death according to the Japan Atherosclerosis Society (JAS) guidelines 2012: the Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA). J Atheroscler Thromb, 2013; 20: 755-766 [DOI] [PubMed] [Google Scholar]
- 41).Subcommittee for preparing guidelines for ultrasound measurement of Achilles tendon thickness. Standard method for ultrasound evaluation of Achilles tendon thickness used for adult familial hypercholesterolemia screening. https://www.jsum.or.jp/committee/diagnostic/pdf/measurement_achilles.pdf, 2018 (in Japanese) [Google Scholar]
- 42).Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y: LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke, 2009; 40: 382-388 [DOI] [PubMed] [Google Scholar]
- 43).Imano H, Noda H, Kitamura A, Sato S, Kiyama M, Sankai T, Ohira T, Nakamura M, Yamagishi K, Ikeda A, Shimamoto T, Iso H: Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Prev Med, 2011; 52: 381-386 [DOI] [PubMed] [Google Scholar]
- 44).Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H: Gender difference of association between LDL cholesterol concentrations and mortality from coronary heart disease amongst Japanese: the Ibaraki Prefectural Health Study. J Intern Med, 2010; 267: 576-587 [DOI] [PubMed] [Google Scholar]
- 45).Yokokawa H, Yasumura S, Tanno K, Ohsawa M, Onoda T, Itai K, Sakata K, Kawamura K, Tanaka F, Yoshida Y, Nakamura M, Terayama Y, Ogawa A, Okayama A: Serum low-density lipoprotein to high-density lipoprotein ratio as a predictor of future acute myocardial infarction among men in a 2.7-year cohort study of a Japanese northern rural population. J Atheroscler Thromb, 2011; 18: 89-98 [DOI] [PubMed] [Google Scholar]
- 46).Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Doi M, Izumi Y, Ohta H: Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation, 2009; 119: 2136-2145 [DOI] [PubMed] [Google Scholar]
- 47).Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, Okayama A: Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis, 2009; 203: 587-592 [DOI] [PubMed] [Google Scholar]
- 48).Sugiyama D, Turin TC, Yeasmin F, Rumana N, Watanabe M, Higashiyama A, Takegami M, Kokubo Y, Okamura T, Miyamoto Y: Hypercholesterolemia and Lifetime Risk of Coronary Heart Disease in the General Japanese Population: Results from the Suita Cohort Study. J Atheroscler Thromb, 2020; 27: 60-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y: Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 50).Pravastatin use and risk of coronary events and cerebral infarction in japanese men with moderate hypercholesterolemia: the Kyushu Lipid Intervention Study. J Atheroscler Thromb, 2000; 7: 110-121 [DOI] [PubMed] [Google Scholar]
- 51).Ito H, Ouchi Y, Ohashi Y, Saito Y, Ishikawa T, Nakamura H, Orimo H: A comparison of low versus standard dose pravastatin therapy for the prevention of cardiovascular events in the elderly: the pravastatin anti-atherosclerosis trial in the elderly (PATE). J Atheroscler Thromb, 2001; 8: 33-44 [DOI] [PubMed] [Google Scholar]
- 52).Chikamori T, Sugimoto K, Hamada T, Kitaoka H, Furuno T, Seo H, Doi Y: Efficacy of cholesterol-lowering treatment in Japanese elderly patients with coronary artery disease and normal cholesterol level using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. J Cardiol, 2000; 35: 95-101 [PubMed] [Google Scholar]
- 53).Saito I, Iso H, Kokubo Y, Inoue M, Tsugane S: Metabolic syndrome and all-cause and cardiovascular disease mortality: Japan Public Health Center-based Prospective (JPHC) Study. Circ J, 2009; 73: 878-884 [DOI] [PubMed] [Google Scholar]
- 54).Noda H, Iso H, Saito I, Konishi M, Inoue M, Tsugane S: The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res, 2009; 32: 289-298 [DOI] [PubMed] [Google Scholar]
- 55).Tsukinoki R, Okamura T, Watanabe M, Kokubo Y, Higashiyama A, Nishimura K, Takegami M, Murakami Y, Okayama A, Miyamoto Y: Blood pressure, low-density lipoprotein cholesterol, and incidences of coronary artery disease and ischemic stroke in Japanese: the Suita study. Am J Hypertens, 2014; 27: 1362-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Kodama K, Sasaki H, Shimizu Y: Trend of coronary heart disease and its relationship to risk factors in a Japanese population: a 26-year follow-up, Hiroshima/Nagasaki study. Jpn Circ J, 1990; 54: 414-421 [DOI] [PubMed] [Google Scholar]
- 57).Konishi M, Iso H, Iida M, Naito Y, Sato S, Komachi Y, Shimamoto T, Doi M, Ito M: Trends for coronary heart disease and its risk factors in Japan: epidemiologic and pathologic studies. Jpn Circ J, 1990; 54: 428-435 [DOI] [PubMed] [Google Scholar]
- 58).Okamura T, Tanaka H, Miyamatsu N, Hayakawa T, Kadowaki T, Kita Y, Nakamura Y, Okayama A, Ueshima H: The relationship between serum total cholesterol and all-cause or cause-specific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis, 2007; 190: 216-223 [DOI] [PubMed] [Google Scholar]
- 59).Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H: Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC. Circ J, 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 60).Wakugami K, Iseki K, Kimura Y, Okumura K, Ikemiya Y, Muratani H, Fukiyama K: Relationship between serum cholesterol and the risk of acute myocardial infarction in a screened cohort in Okinawa, Japan. Jpn Circ J, 1998; 62: 7-14 [DOI] [PubMed] [Google Scholar]
- 61).Kitamura A, Iso H, Naito Y, Iida M, Konishi M, Folsom AR, Sato S, Kiyama M, Nakamura M, Sankai T, et al.: High-density lipoprotein cholesterol and premature coronary heart disease in urban Japanese men. Circulation, 1994; 89: 2533-2539 [DOI] [PubMed] [Google Scholar]
- 62).Sugiyama D, Okamura T, Watanabe M, Higashiyama A, Okuda N, Nakamura Y, Hozawa A, Kita Y, Kadota A, Murakami Y, Miyamatsu N, Ohkubo T, Hayakawa T, Miyamoto Y, Miura K, Okayama A, Ueshima H: Risk of hypercholesterolemia for cardiovascular disease and the population attributable fraction in a 24-year Japanese cohort study. J Atheroscler Thromb, 2015; 22: 95-107 [DOI] [PubMed] [Google Scholar]
- 63).Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H: Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc, 2012; 1: e001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Okumura K, Iseki K, Wakugami K, Kimura Y, Muratani H, Ikemiya Y, Fukiyama K: Low serum cholesterol as a risk factor for hemorrhagic stroke in men: a community-based mass screening in Okinawa, Japan. Jpn Circ J, 1999; 63: 53-58 [DOI] [PubMed] [Google Scholar]
- 65).Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y, Tamakoshi A: Serum total cholesterol levels and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis, 2007; 194: 415-420 [DOI] [PubMed] [Google Scholar]
- 66).Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, Arima H, Tanaka K, Ibayashi S, Fujishima M: Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama study. Stroke, 2000; 31: 2616-2622 [DOI] [PubMed] [Google Scholar]
- 67).Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, Tsugane S: High serum total cholesterol levels is a risk factor of ischemic stroke for general Japanese population: the JPHC study. Atherosclerosis, 2012; 221: 565-569 [DOI] [PubMed] [Google Scholar]
- 68).Satoh M, Ohkubo T, Asayama K, Murakami Y, Sakurai M, Nakagawa H, Iso H, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T: Combined effect of blood pressure and total cholesterol levels on long-term risks of subtypes of cardiovascular death: Evidence for Cardiovascular Prevention from Observational Cohorts in Japan. Hypertension, 2015; 65: 517-524 [DOI] [PubMed] [Google Scholar]
- 69).Satoh M, Ohkubo T, Asayama K, Murakami Y, Sugiyama D, Waki T, Tanaka-Mizuno S, Yamada M, Saitoh S, Sakata K, Irie F, Sairenchi T, Ishikawa S, Kiyama M, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T: A Combination of Blood Pressure and Total Cholesterol Increases the Lifetime Risk of Coronary Heart Disease Mortality: EPOCH-JAPAN. J Atheroscler Thromb, 2021; 28: 6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL: Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med, 2001; 161: 1413-1419 [DOI] [PubMed] [Google Scholar]
- 71).Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB: Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation, 2005; 112: 3375-3383 [DOI] [PubMed] [Google Scholar]
- 72).Kitamura A, Noda H, Nakamura M, Kiyama M, Okada T, Imano H, Ohira T, Sato S, Yamagishi K, Iso H: Association between non-high-density lipoprotein cholesterol levels and the incidence of coronary heart disease among Japanese: the Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb, 2011; 18: 454-463 [DOI] [PubMed] [Google Scholar]
- 73).Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Yoshimasa Y, Okayama A: Triglycerides and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort: the Suita study. Atherosclerosis, 2010; 209: 290-294 [DOI] [PubMed] [Google Scholar]
- 74).Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H: Association between non-high-density lipoprotein cholesterol concentrations and mortality from coronary heart disease among Japanese men and women: the Ibaraki Prefectural Health Study. J Atheroscler Thromb, 2010; 17: 30-36 [DOI] [PubMed] [Google Scholar]
- 75).Imamura T, Doi Y, Ninomiya T, Hata J, Nagata M, Ikeda F, Mukai N, Hirakawa Y, Yoshida D, Fukuhara M, Kitazono T, Kiyohara Y: Non-high-density lipoprotein cholesterol and the development of coronary heart disease and stroke subtypes in a general Japanese population: the Hisayama Study. Atherosclerosis, 2014; 233: 343-348 [DOI] [PubMed] [Google Scholar]
- 76).Takeuchi T, Nemoto K, Takahashi O, Urayama KY, Deshpande GA, Izumo H: Comparison of cardiovascular disease risk associated with 3 lipid measures in Japanese adults. J Clin Lipidol, 2014; 8: 501-509 [DOI] [PubMed] [Google Scholar]
- 77).Tanaka F, Makita S, Onoda T, Tanno K, Ohsawa M, Itai K, Sakata K, Omama S, Yoshida Y, Ogasawara K, Ogawa A, Ishibashi Y, Kuribayashi T, Okayama A, Nakamura M: Predictive value of lipoprotein indices for residual risk of acute myocardial infarction and sudden death in men with low-density lipoprotein cholesterol levels <120 mg/dl. Am J Cardiol, 2013; 112: 1063-1068 [DOI] [PubMed] [Google Scholar]
- 78).Kakehi E, Kotani K, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E: Serum non-high-density lipoprotein cholesterol levels and the incidence of ischemic stroke in a Japanese population: the Jichi Medical School cohort study. Asia Pac J Public Health, 2015; 27: Np535-543 [DOI] [PubMed] [Google Scholar]
- 79).Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S: Non-High-Density Lipoprotein Cholesterol and Risk of Stroke Subtypes and Coronary Heart Disease: The Japan Public Health Center-Based Prospective (JPHC) Study. J Atheroscler Thromb, 2020; 27: 363-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Usui T, Nagata M, Hata J, Mukai N, Hirakawa Y, Yoshida D, Kishimoto H, Kitazono T, Kiyohara Y, Ninomiya T: Serum Non-High-Density Lipoprotein Cholesterol and Risk of Cardiovascular Disease in Community Dwellers with Chronic Kidney Disease: the Hisayama Study. J Atheroscler Thromb, 2017; 24: 706-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Shimano H, Arai H, Harada-Shiba M, Ueshima H, Ohta T, Yamashita S, Gotoda T, Kiyohara Y, Hayashi T, Kobayashi J, Shimamoto K, Bujo H, Ishibashi S, Shirai K, Oikawa S, Saito Y, Yamada N: Proposed guidelines for hypertriglyceridemia in Japan with non-HDL cholesterol as the second target. J Atheroscler Thromb, 2008; 15: 116-121 [DOI] [PubMed] [Google Scholar]
- 82).Sugimoto K, Isobe K, Kawakami Y, Yamada N: The relationship between non-HDL cholesterol and other lipid parameters in Japanese subjects. J Atheroscler Thromb, 2005; 12: 107-110 [DOI] [PubMed] [Google Scholar]
- 83).Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, Cui R, Tanigawa T, Sankai T, Ishikawa Y, Sato S, Hitsumoto S, Iso H: High-density lipoprotein subclasses and risk of stroke and its subtypes in Japanese population: the Circulatory Risk in Communities Study. Stroke, 2013; 44: 327-333 [DOI] [PubMed] [Google Scholar]
- 84).Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Kagamimori S, Nakagawa H: High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: the Oyabe Study. Stroke, 2003; 34: 863-868 [DOI] [PubMed] [Google Scholar]
- 85).Iso H, Sato S, Kitamura A, Imano H, Kiyama M, Yamagishi K, Cui R, Tanigawa T, Shimamoto T: Metabolic syndrome and the risk of ischemic heart disease and stroke among Japanese men and women. Stroke, 2007; 38: 1744-1751 [DOI] [PubMed] [Google Scholar]
- 86).Satoh H, Tomita K, Fujii S, Kishi R, Tsutsui H: Lower high-density lipoprotein cholesterol is a significant and independent risk for coronary artery disease in Japanese men. J Atheroscler Thromb, 2009; 16: 792-798 [DOI] [PubMed] [Google Scholar]
- 87).Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S: Association of high-density lipoprotein cholesterol concentration with different types of stroke and coronary heart disease: The Japan Public Health Center-based prospective (JPHC) study. Atherosclerosis, 2017; 265: 147-154 [DOI] [PubMed] [Google Scholar]
- 88).Okamura T, Hayakawa T, Kadowaki T, Kita Y, Okayama A, Ueshima H: The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis, 2006; 184: 143-150 [DOI] [PubMed] [Google Scholar]
- 89).Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H: Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circ J, 2002; 66: 1087-1095 [DOI] [PubMed] [Google Scholar]
- 90).Mabuchi H, Kita T, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H: Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J, 2002; 66: 1096-1100 [DOI] [PubMed] [Google Scholar]
- 91).Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, Shaw J, Ueshima H, Zimmet P, Jee SH, Patel JV, Caterson I, Perkovic V, Woodward M: Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation, 2011; 124: 2056-2064 [DOI] [PubMed] [Google Scholar]
- 92).Hirata T, Sugiyama D, Nagasawa SY, Murakami Y, Saitoh S, Okayama A, Iso H, Irie F, Sairenchi T, Miyamoto Y, Yamada M, Ishikawa S, Miura K, Ueshima H, Okamura T: A pooled analysis of the association of isolated low levels of high-density lipoprotein cholesterol with cardiovascular mortality in Japan. Eur J Epidemiol, 2017; 32: 547-557 [DOI] [PubMed] [Google Scholar]
- 93).Watanabe J, Kakehi E, Kotani K, Kayaba K, Nakamura Y, Ishikawa S: Isolated low levels of high-density lipoprotein cholesterol and stroke incidence: JMS Cohort Study. J Clin Lab Anal, 2020; 34: e23087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94).Hirata A, Sugiyama D, Watanabe M, Tamakoshi A, Iso H, Kotani K, Kiyama M, Yamada M, Ishikawa S, Murakami Y, Miura K, Ueshima H, Okamura T: Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J Clin Lipidol, 2018; 12: 674-684.e675 [DOI] [PubMed] [Google Scholar]
- 95).Arai H, Yamamoto A, Matsuzawa Y, Saito Y, Yamada N, Oikawa S, Mabuchi H, Teramoto T, Sasaki J, Nakaya N, Itakura H, Ishikawa Y, Ouchi Y, Horibe H, Kita T: Serum lipid survey and its recent trend in the general Japanese population in 2000. J Atheroscler Thromb, 2005; 12: 98-106 [DOI] [PubMed] [Google Scholar]
- 96).Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V: Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation, 2007; 115: 450-458 [DOI] [PubMed] [Google Scholar]
- 97).Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, Woodward M: Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation, 2004; 110: 2678-2686 [DOI] [PubMed] [Google Scholar]
- 98).Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, Shimamoto T, Iida M, Komachi Y: Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol, 2001; 153: 490-499 [DOI] [PubMed] [Google Scholar]
- 99).Satoh H, Nishino T, Tomita K, Tsutsui H: Fasting triglyceride is a significant risk factor for coronary artery disease in middle-aged Japanese men. Circ J, 2006; 70: 227-231 [DOI] [PubMed] [Google Scholar]
- 100).Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Nishimura K, Okayama A, Miyamoto Y: A revised definition of the metabolic syndrome predicts coronary artery disease and ischemic stroke after adjusting for low density lipoprotein cholesterol in a 13-year cohort study of Japanese: the Suita study. Atherosclerosis, 2011; 217: 201-206 [DOI] [PubMed] [Google Scholar]
- 101).Iso H, Imano H, Yamagishi K, Ohira T, Cui R, Noda H, Sato S, Kiyama M, Okada T, Hitsumoto S, Tanigawa T, Kitamura A: Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis, 2014; 237: 361-368 [DOI] [PubMed] [Google Scholar]
- 102).Higashiyama A, Wakabayashi I, Okamura T, Kokubo Y, Watanabe M, Takegami M, Honda-Kohmo K, Okayama A, Miyamoto Y: The Risk of Fasting Triglycerides and its Related Indices for Ischemic Cardiovascular Diseases in Japanese Community Dwellers: the Suita Study. J Atheroscler Thromb, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).Castelli WP: Lipids, risk factors and ischaemic heart disease. Atherosclerosis, 1996; 124 Suppl: S1-9 [DOI] [PubMed] [Google Scholar]
- 104).Higashiyama A, Wakabayashi I, Okamura T, Kokubo Y, Watanabe M, Takegami M, Honda-Kohmo K, Okayama A, Miyamoto Y: The Risk of Fasting Triglycerides and its Related Indices for Ischemic Cardiovascular Diseases in Japanese Community Dwellers: the Suita Study. J Atheroscler Thromb, 2021; 28: 1275-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Labreuche J, Touboul PJ, Amarenco P: Plasma triglyceride levels and risk of stroke and carotid atherosclerosis: a systematic review of the epidemiological studies. Atherosclerosis, 2009; 203: 331-345 [DOI] [PubMed] [Google Scholar]
- 106).Antonios N, Angiolillo DJ, Silliman S: Hypertriglyceridemia and ischemic stroke. Eur Neurol, 2008; 60: 269-278 [DOI] [PubMed] [Google Scholar]
- 107).Hirata A, Okamura T, Hirata T, Sugiyama D, Ohkubo T, Okuda N, Kita Y, Hayakawa T, Kadota A, Kondo K, Miura K, Okayama A, Ueshima H: Relationship Between Non-fasting Triglycerides and Cardiovascular Disease Mortality in a 20-year Follow-up Study of a Japanese General Population: NIPPON DATA90. J Epidemiol, 2022; 32: 303-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Saito I, Folsom AR, Aono H, Ozawa H, Ikebe T, Yamashita T: Comparison of fatal coronary heart disease occurrence based on population surveys in Japan and the USA. Int J Epidemiol, 2000; 29: 837-844 [DOI] [PubMed] [Google Scholar]
- 109).Sekikawa A, Miyamoto Y, Miura K, Nishimura K, Willcox BJ, Masaki KH, Rodriguez B, Tracy RP, Okamura T, Kuller LH: Continuous decline in mortality from coronary heart disease in Japan despite a continuous and marked rise in total cholesterol: Japanese experience after the Seven Countries Study. Int J Epidemiol, 2015; 44: 1614-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Kitamura A, Sato S, Kiyama M, Imano H, Iso H, Okada T, Ohira T, Tanigawa T, Yamagishi K, Nakamura M, Konishi M, Shimamoto T, Iida M, Komachi Y: Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: the Akita-Osaka study. J Am Coll Cardiol, 2008; 52: 71-79 [DOI] [PubMed] [Google Scholar]
- 111).Takii T, Yasuda S, Takahashi J, Ito K, Shiba N, Shirato K, Shimokawa H: Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: report from the MIYAGI-AMI Registry Study. Circ J, 2010; 74: 93-100 [DOI] [PubMed] [Google Scholar]
- 112).Kitamura A, Iso H, Iida M, Naito Y, Sato S, Jacobs DR, Nakamura M, Shimamoto T, Komachi Y: Trends in the incidence of coronary heart disease and stroke and the prevalence of cardiovascular risk factors among Japanese men from 1963 to 1994. Am J Med, 2002; 112: 104-109 [DOI] [PubMed] [Google Scholar]
- 113).Miida T, Nishimura K, Okamura T, Hirayama S, Ohmura H, Yoshida H, Miyashita Y, Ai M, Tanaka A, Sumino H, Murakami M, Inoue I, Kayamori Y, Nakamura M, Nobori T, Miyazawa Y, Teramoto T, Yokoyama S: A multicenter study on the precision and accuracy of homogeneous assays for LDL-cholesterol: comparison with a beta-quantification method using fresh serum obtained from non-diseased and diseased subjects. Atherosclerosis, 2012; 225: 208-215 [DOI] [PubMed] [Google Scholar]
- 114).Miida T, Nishimura K, Hirayama S, Miyamoto Y, Nakamura M, Masuda D, Yamashita S, Ushiyama M, Komori T, Fujita N, Yokoyama S, Teramoto T: Homogeneous Assays for LDL-C and HDL-C are Reliable in Both the Postprandial and Fasting State. J Atheroscler Thromb, 2017; 24: 583-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18: 499-502 [PubMed] [Google Scholar]
- 116).Pownall HJ: Dietary ethanol is associated with reduced lipolysis of intestinally derived lipoproteins. J Lipid Res, 1994; 35: 2105-2113 [PubMed] [Google Scholar]
- 117).Miida T, Nakamura Y, Mezaki T, Hanyu O, Maruyama S, Horikawa Y, Izawa S, Yamada Y, Matsui H, Okada M: LDL-cholesterol and HDL-cholesterol concentrations decrease during the day. Ann Clin Biochem, 2002; 39: 241-249 [DOI] [PubMed] [Google Scholar]
- 118).Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 2002; 106: 3143-3421 [PubMed] [Google Scholar]
- 119).Miida T, Otsuka H, Tsuchiya A, Okada M: Plasma lipoprotein profiles change significantly during cardiac catheterization. Clin Chem, 1998; 44: 517-521 [PubMed] [Google Scholar]
- 120).Nordestgaard BG: A Test in Context: Lipid Profile, Fasting Versus Nonfasting. J Am Coll Cardiol, 2017; 70: 1637-1646 [DOI] [PubMed] [Google Scholar]
- 121).Nakagawa T, Hirayama S, Watanabe T, Yokomura M, Kohno M, Sato T, Bujo H, Sato A, Murata M, Miida T: Triglyceride concentrations should be measured after elimination of free glycerol to exclude interindividual variations due to adiposity and fasting status. Clin Chem Lab Med, 2017; 55: e191-e194 [DOI] [PubMed] [Google Scholar]
- 122).Sniderman A, Williams K, Cobbaert C: ApoB versus non-HDL-C: what to do when they disagree. Curr Atheroscler Rep, 2009; 11: 358-363 [DOI] [PubMed] [Google Scholar]
- 123).Kosuge K, Miida T, Takahashi A, Obayashi K, Ito M, Ito T, Soda S, Ozaki K, Hirayama S, Hanyu O, Aizawa Y, Nakamura Y: Estimating the fasting triglyceride concentration from the postprandial HDL-cholesterol and apolipoprotein CIII concentrations. Atherosclerosis, 2006; 184: 413-419 [DOI] [PubMed] [Google Scholar]
- 124).Todo Y, Kobayashi J, Higashikata T, Kawashiri M, Nohara A, Inazu A, Koizumi J, Mabuchi H: Detailed analysis of serum lipids and lipoproteins from Japanese type III hyperlipoproteinemia with apolipoprotein E2/2 phenotype. Clin Chim Acta, 2004; 348: 35-40 [DOI] [PubMed] [Google Scholar]
- 125).Hirowatari Y, Yoshida H, Kurosawa H, Doumitu KI, Tada N: Measurement of cholesterol of major serum lipoprotein classes by anion-exchange HPLC with perchlorate ion-containing eluent. J Lipid Res, 2003; 44: 1404-1412 [DOI] [PubMed] [Google Scholar]
- 126).Manita D, Hirowatari Y, Yoshida H: A rapid anion-exchange chromatography for measurement of cholesterol concentrations in five lipoprotein classes and estimation of lipoprotein profiles in male volunteers without overt diseases. Ann Clin Biochem, 2015; 52: 638-646 [DOI] [PubMed] [Google Scholar]
- 127).Nordestgaard BG: Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res, 2016; 118: 547-563 [DOI] [PubMed] [Google Scholar]
- 128).Yoshida H, Kurosawa H, Hirowatari Y, Ogura Y, Ikewaki K, Abe I, Saikawa S, Domitsu K, Ito K, Yanai H, Tada N: Characteristic comparison of triglyceride-rich remnant lipoprotein measurement between a new homogenous assay (RemL-C) and a conventional immunoseparation method (RLP-C). Lipids Health Dis, 2008; 7: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129).Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, Moriarty PM, Rader DJ, Remaley AT, Reyes-Soffer G, Santos RD, Thanassoulis G, Witztum JL, Danthi S, Olive M, Liu L: NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J Am Coll Cardiol, 2018; 71: 177-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Tsimikas S: A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol, 2017; 69: 692-711 [DOI] [PubMed] [Google Scholar]
- 131).Marcovina SM, Albers JJ: Lipoprotein (a) measurements for clinical application. J Lipid Res, 2016; 57: 526-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).Yoshida H: Clinical Impact and Significance of Serum Lipoprotein (a) Levels on Cardiovascular Risk in Patients With Coronary Artery Disease. Circ J, 2019; 83: 967-968 [DOI] [PubMed] [Google Scholar]
- 133).Scharnagl H, Stojakovic T, Dieplinger B, Dieplinger H, Erhart G, Kostner GM, Herrmann M, März W, Grammer TB: Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis, 2019; 289: 206-213 [DOI] [PubMed] [Google Scholar]
- 134).Nelson JR, Raskin S: The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad Med, 2019; 131: 268-277 [DOI] [PubMed] [Google Scholar]
- 135).Ninomiya T, Nagata M, Hata J, Hirakawa Y, Ozawa M, Yoshida D, Ohara T, Kishimoto H, Mukai N, Fukuhara M, Kitazono T, Kiyohara Y: Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: the Hisayama Study. Atherosclerosis, 2013; 231: 261-267 [DOI] [PubMed] [Google Scholar]
- 136).Yoshida H, Ito K, Sato R, Kurosawa H, Tomono Y, Hirowatari Y, Shimizu M, Tada N: Clinical relevance of decreased ratios of serum eicosapentaenoic acid/arachidonic acid (AA) and docosahexaenoic acid/AA to impaired arterial stiffness. Int J Cardiol, 2014; 177: 517-519 [DOI] [PubMed] [Google Scholar]
- 137).Wu SA, Kersten S, Qi L: Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol Metab, 2021; 32: 48-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Hanyu O, Miida T, Obayashi K, Ikarashi T, Soda S, Kaneko S, Hirayama S, Suzuki K, Nakamura Y, Yamatani K, Aizawa Y: Lipoprotein lipase (LPL) mass in preheparin serum reflects insulin sensitivity. Atherosclerosis, 2004; 174: 385-390 [DOI] [PubMed] [Google Scholar]
- 139).Hanyu O, Miida T, Kosuge K, Ito T, Soda S, Hirayama S, Wardaningsih E, Fueki Y, Obayashi K, Aizawa Y: Preheparin lipoprotein lipase mass is a practical marker of insulin resistance in ambulatory type 2 diabetic patients treated with oral hypoglycemic agents. Clin Chim Acta, 2007; 384: 118-123 [DOI] [PubMed] [Google Scholar]
- 140).Ishibashi R, Takemoto M, Tsurutani Y, Kuroda M, Ogawa M, Wakabayashi H, Uesugi N, Nagata M, Imai N, Hattori A, Sakamoto K, Kitamoto T, Maezawa Y, Narita I, Hiroi S, Furuta A, Miida T, Yokote K: Immune-mediated acquired lecithin-cholesterol acyltransferase deficiency: A case report and literature review. J Clin Lipidol, 2018; 12: 888-897.e882 [DOI] [PubMed] [Google Scholar]
- 141).Oldoni F, Baldassarre D, Castelnuovo S, Ossoli A, Amato M, van Capelleveen J, Hovingh GK, De Groot E, Bochem A, Simonelli S, Barbieri S, Veglia F, Franceschini G, Kuivenhoven JA, Holleboom AG, Calabresi L: Complete and Partial Lecithin:Cholesterol Acyltransferase Deficiency Is Differentially Associated With Atherosclerosis. Circulation, 2018; 138: 1000-1007 [DOI] [PubMed] [Google Scholar]
- 142).Miida T, Hirayama S: Controversy over the atherogenicity of lipoprotein-X. Curr Opin Endocrinol Diabetes Obes, 2019; 26: 117-123 [DOI] [PubMed] [Google Scholar]
- 143).Nagano M, Nakamura M, Kobayashi N, Kamata J, Hiramori K: Effort angina in a middle-aged woman with abnormally high levels of serum high-density lipoprotein cholesterol: a case of cholesteryl-ester transfer protein deficiency. Circ J, 2005; 69: 609-612 [DOI] [PubMed] [Google Scholar]
- 144).Steinberg D, Witztum JL: Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol, 2010; 30: 2311-2316 [DOI] [PubMed] [Google Scholar]
- 145).Yoshida H, Kisugi R: Mechanisms of LDL oxidation. Clin Chim Acta, 2010; 411: 1875-1882 [DOI] [PubMed] [Google Scholar]
- 146).Kotani K, Tashiro J, Yamazaki K, Nakamura Y, Miyazaki A, Bujo H, Saito Y, Kanno T, Maekawa M: Investigation of MDA-LDL (malondialdehyde-modified low-density lipoprotein) as a prognostic marker for coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta, 2015; 450: 145-150 [DOI] [PubMed] [Google Scholar]
- 147).Krauss RM: Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol, 2010; 21: 305-311 [DOI] [PubMed] [Google Scholar]
- 148).Hirano T: Pathophysiology of Diabetic Dyslipidemia. J Atheroscler Thromb, 2018; 25: 771-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149).Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, Iso Y, Suzuki H, Katagiri T: Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis, 2006; 189: 206-214 [DOI] [PubMed] [Google Scholar]
- 150).Yoshida H, Tada H, Ito K, Kishimoto Y, Yanai H, Okamura T, Ikewaki K, Inagaki K, Shoji T, Bujo H, Miida T, Yoshida M, Kuzuya M, Yamashita S: Reference Intervals of Serum Non-Cholesterol Sterols by Gender in Healthy Japanese Individuals. J Atheroscler Thromb, 2020; 27: 409-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151).Tada H, Nohara A, Inazu A, Sakuma N, Mabuchi H, Kawashiri MA: Sitosterolemia, Hypercholesterolemia, and Coronary Artery Disease. J Atheroscler Thromb, 2018; 25: 783-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152).Miettinen TA, Tilvis RS, Kesäniemi YA: Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol, 1990; 131: 20-31 [DOI] [PubMed] [Google Scholar]
- 153).Simonen P, Gylling H, Miettinen TA: The validity of serum squalene and non-cholesterol sterols as surrogate markers of cholesterol synthesis and absorption in type 2 diabetes. Atherosclerosis, 2008; 197: 883-888 [DOI] [PubMed] [Google Scholar]
- 154).Harada-Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T, Ogura M, Dobashi K, Nohara A, Bujo H, Miyauchi K, Yamashita S, Yokote K: Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 751-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155).Cao YX, Jin JL, Sun D, Liu HH, Guo YL, Wu NQ, Xu RX, Zhu CG, Dong Q, Sun J, Li JJ: Circulating PCSK9 and cardiovascular events in FH patients with standard lipid-lowering therapy. J Transl Med, 2019; 17: 367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156).Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH: Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med, 2006; 354: 1264-1272 [DOI] [PubMed] [Google Scholar]
- 157).Kono S, Ikeda M, Tokudome S, Nishizumi M, Kuratsune M: Smoking and mortalities from cancer, coronary heart disease and stroke in male Japanese physicians. J Cancer Res Clin Oncol, 1985; 110: 161-164 [DOI] [PubMed] [Google Scholar]
- 158).Irie F ST, Iso H, Shimamoto T.: Prediction of mortality from findings of annual health checkups utility for health care programs. Nihon Koshu Eisei Zasshi, 2001; 48: 95-108 [PubMed] [Google Scholar]
- 159).Yamagishi K, Iso H, Kitamura A, Sankai T, Tanigawa T, Naito Y, Sato S, Imano H, Ohira T, Shimamoto T: Smoking raises the risk of total and ischemic strokes in hypertensive men. Hypertens Res, 2003; 26: 209-217 [DOI] [PubMed] [Google Scholar]
- 160).Ueshima H, Choudhury SR, Okayama A, Hayakawa T, Kita Y, Kadowaki T, Okamura T, Minowa M, Iimura O: Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke, 2004; 35: 1836-1841 [DOI] [PubMed] [Google Scholar]
- 161).Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Wada Y, Kondo T, Inaba Y, Tamakoshi A: Smoking cessation and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Am J Epidemiol, 2005; 161: 170-179 [DOI] [PubMed] [Google Scholar]
- 162).Higashiyama A, Okamura T, Ono Y, Watanabe M, Kokubo Y, Okayama A: Risk of smoking and metabolic syndrome for incidence of cardiovascular disease--comparison of relative contribution in urban Japanese population: the Suita study. Circ J, 2009; 73: 2258-2263 [DOI] [PubMed] [Google Scholar]
- 163).Hata J, Doi Y, Ninomiya T, Fukuhara M, Ikeda F, Mukai N, Hirakawa Y, Kitazono T, Kiyohara Y: Combined effects of smoking and hypercholesterolemia on the risk of stroke and coronary heart disease in Japanese: the Hisayama study. Cerebrovasc Dis, 2011; 31: 477-484 [DOI] [PubMed] [Google Scholar]
- 164).Kondo T, Osugi S, Shimokata K, Honjo H, Morita Y, Maeda K, Yamashita K, Muramatsu T, Shintani S, Matsushita K, Murohara T: Smoking and smoking cessation in relation to all-cause mortality and cardiovascular events in 25,464 healthy male Japanese workers. Circ J, 2011; 75: 2885-2892 [DOI] [PubMed] [Google Scholar]
- 165).Eshak ES, Iso H, Yamagishi K, Kokubo Y, Saito I, Yatsuya H, Sawada N, Inoue M, Tsugane S: Modification of the excess risk of coronary heart disease due to smoking by seafood/fish intake. Am J Epidemiol, 2014; 179: 1173-1181 [DOI] [PubMed] [Google Scholar]
- 166).National Center for Chronic Disease P, Health Promotion Office on S, Health: Reports of the Surgeon General.In: Reports of the Surgeon General, Centers for Disease Control and Prevention (US), Atlanta (GA), 2014 [Google Scholar]
- 167).Hackshaw A, Morris JK, Boniface S, Tang JL, Milenković D: Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ, 2018; 360: j5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168).Aune D, Schlesinger S, Norat T, Riboli E: Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci Rep, 2018; 8: 14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169).Lu L, Mackay DF, Pell JP: Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart, 2014; 100: 414-423 [DOI] [PubMed] [Google Scholar]
- 170).Lv X, Sun J, Bi Y, Xu M, Lu J, Zhao L, Xu Y: Risk of all-cause mortality and cardiovascular disease associated with secondhand smoke exposure: a systematic review and meta-analysis. Int J Cardiol, 2015; 199: 106-115 [DOI] [PubMed] [Google Scholar]
- 171).Oono IP, Mackay DF, Pell JP: Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf), 2011; 33: 496-502 [DOI] [PubMed] [Google Scholar]
- 172).Pan A, Wang Y, Talaei M, Hu FB, Wu T: Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol, 2015; 3: 958-967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173).Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M: Association between cigarette smoking, metabolic syndrome, and carotid arteriosclerosis in Japanese individuals. Atherosclerosis, 2005; 181: 381-388 [DOI] [PubMed] [Google Scholar]
- 174).Craig WY, Palomaki GE, Haddow JE: Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ, 1989; 298: 784-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175).Simonavicius E, McNeill A, Shahab L, Brose LS: Heat-not-burn tobacco products: a systematic literature review. Tob Control, 2019; 28: 582-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176).Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, De Falco E, Chimenti I, Cammisotto V, Valenti V, Coluzzi F, Cavarretta E, Carrizzo A, Prati F, Carnevale R, Frati G: Acute Effects of Heat-Not-Burn, Electronic Vaping, and Traditional Tobacco Combustion Cigarettes: The Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking ( SUR - VAPES ) 2 Randomized Trial. J Am Heart Assoc, 2019; 8: e010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177).Uchiyama S, Senoo Y, Hayashida H, Inaba Y, Nakagome H, Kunugita N: Determination of Chemical Compounds Generated from Second-generation E-cigarettes Using a Sorbent Cartridge Followed by a Two-step Elution Method. Anal Sci, 2016; 32: 549-555 [DOI] [PubMed] [Google Scholar]
- 178).Kiernan E, Click ES, Melstrom P, Evans ME, Layer MR, Weissman DN, Reagan-Steiner S, Wiltz JL, Hocevar S, Goodman AB, Twentyman E: A Brief Overview of the National Outbreak of e-Cigarette, or Vaping, Product Use-Associated Lung Injury and the Primary Causes. Chest, 2021; 159: 426-431 [DOI] [PubMed] [Google Scholar]
- 179).Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res, 2019; 42: 1235-1481 [DOI] [PubMed] [Google Scholar]
- 180).Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, Ueshima H: Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res, 2012; 35: 947-953 [DOI] [PubMed] [Google Scholar]
- 181).Sasaki J, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Shimamoto K, Kono S, Itakura H: Gender difference in coronary events in relation to risk factors in Japanese hypercholesterolemic patients treated with low-dose simvastatin. Circ J, 2006; 70: 810-814 [DOI] [PubMed] [Google Scholar]
- 182).Kadowaki S, Okamura T, Hozawa A, Kadowaki T, Kadota A, Murakami Y, Nakamura K, Saitoh S, Nakamura Y, Hayakawa T, Kita Y, Okayama A, Ueshima H: Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia, 2008; 51: 575-582 [DOI] [PubMed] [Google Scholar]
- 183).Sakurai M, Saitoh S, Miura K, Nakagawa H, Ohnishi H, Akasaka H, Kadota A, Kita Y, Hayakawa T, Ohkubo T, Okayama A, Okamura T, Ueshima H: HbA1c and the risks for all-cause and cardiovascular mortality in the general Japanese population: NIPPON DATA90. Diabetes Care, 2013; 36: 3759-3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184).Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A: Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care, 1999; 22: 920-924 [DOI] [PubMed] [Google Scholar]
- 185).Islam Z, Akter S, Inoue Y, Hu H, Kuwahara K, Nakagawa T, Honda T, Yamamoto S, Okazaki H, Miyamoto T, Ogasawara T, Sasaki N, Uehara A, Yamamoto M, Kochi T, Eguchi M, Shirasaka T, Shimizu M, Nagahama S, Hori A, Imai T, Nishihara A, Tomita K, Sone T, Konishi M, Kabe I, Mizoue T, Dohi S: Prediabetes, Diabetes, and the Risk of All-Cause and Cause-Specific Mortality in a Japanese Working Population: Japan Epidemiology Collaboration on Occupational Health Study. Diabetes Care, 2021; 44: 757-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186).Kokubo Y, Okamura T, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Furukawa Y, Kamide K, Kawanishi K, Okayama A, Yoshimasa Y: The combined impact of blood pressure category and glucose abnormality on the incidence of cardiovascular diseases in a Japanese urban cohort: the Suita Study. Hypertens Res, 2010; 33: 1238-1243 [DOI] [PubMed] [Google Scholar]
- 187).Hu H, Mizoue T, Sasaki N, Ogasawara T, Tomita K, Nagahama S, Hori A, Nishihara A, Imai T, Yamamoto M, Eguchi M, Kochi T, Miyamoto T, Honda T, Nakagawa T, Yamamoto S, Okazaki H, Uehara A, Shimizu M, Murakami T, Kuwahara K, Nanri A, Konishi M, Kabe I, Dohi S: Prediabetes and cardiovascular disease risk: A nested case-control study. Atherosclerosis, 2018; 278: 1-6 [DOI] [PubMed] [Google Scholar]
- 188).Hirokawa W, Nakamura K, Sakurai M, Morikawa Y, Miura K, Ishizaki M, Yoshita K, Kido T, Naruse Y, Nakagawa H: Mild metabolic abnormalities, abdominal obesity and the risk of cardiovascular diseases in middle-aged Japanese men. J Atheroscler Thromb, 2010; 17: 934-943 [DOI] [PubMed] [Google Scholar]
- 189).Nakanishi N, Takatorige T, Fukuda H, Shirai K, Li W, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K: Components of the metabolic syndrome as predictors of cardiovascular disease and type 2 diabetes in middle-aged Japanese men. Diabetes Res Clin Pract, 2004; 64: 59-70 [DOI] [PubMed] [Google Scholar]
- 190).Ikeda F, Doi Y, Ninomiya T, Hirakawa Y, Mukai N, Hata J, Shikata K, Yoshida D, Matsumoto T, Kitazono T, Kiyohara Y: Haemoglobin A1c even within non-diabetic level is a predictor of cardiovascular disease in a general Japanese population: the Hisayama Study. Cardiovasc Diabetol, 2013; 12: 164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191).Goto A, Noda M, Matsushita Y, Goto M, Kato M, Isogawa A, Takahashi Y, Kurotani K, Oba S, Nanri A, Mizoue T, Yamagishi K, Yatsuya H, Saito I, Kokubo Y, Sawada N, Inoue M, Iso H, Kadowaki T, Tsugane S: Hemoglobin a1c levels and the risk of cardiovascular disease in people without known diabetes: a population-based cohort study in Japan. Medicine (Baltimore), 2015; 94: e785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192).Nishimura R, Nakagami T, Sone H, Ohashi Y, Tajima N: Relationship between hemoglobin A1c and cardiovascular disease in mild-to-moderate hypercholesterolemic Japanese individuals: subanalysis of a large-scale randomized controlled trial. Cardiovasc Diabetol, 2011; 10: 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193).Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Okayama A, Okamura T: New diagnosis criteria for diabetes with hemoglobin A1c and risks of macro-vascular complications in an urban Japanese cohort: the Suita study. Diabetes Res Clin Pract, 2010; 88: e20-23 [DOI] [PubMed] [Google Scholar]
- 194).Satoh H, Nishino T, Tomita K, Saijo Y, Kishi R, Tsutsui H: Risk factors and the incidence of coronary artery disease in young middle-aged Japanese men: results from a 10-year cohort study. Intern Med, 2006; 45: 235-239 [DOI] [PubMed] [Google Scholar]
- 195).Doi Y, Ninomiya T, Hata J, Fukuhara M, Yonemoto K, Iwase M, Iida M, Kiyohara Y: Impact of glucose tolerance status on development of ischemic stroke and coronary heart disease in a general Japanese population: the Hisayama study. Stroke, 2010; 41: 203-209 [DOI] [PubMed] [Google Scholar]
- 196).Kannel WB, McGee DL: Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care, 1979; 2: 120-126 [DOI] [PubMed] [Google Scholar]
- 197).Vaccaro O, Stamler J, Neaton JD: Sixteen-year coronary mortality in black and white men with diabetes screened for the Multiple Risk Factor Intervention Trial (MRFIT). Int J Epidemiol, 1998; 27: 636-641 [DOI] [PubMed] [Google Scholar]
- 198).Fujishima M, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Yoshitake T: Diabetes and cardiovascular disease in a prospective population survey in Japan: The Hisayama Study. Diabetes, 1996; 45 Suppl 3: S14-16 [DOI] [PubMed] [Google Scholar]
- 199).Iso H, Imano H, Kitamura A, Sato S, Naito Y, Tanigawa T, Ohira T, Yamagishi K, Iida M, Shimamoto T: Type 2 diabetes and risk of non-embolic ischaemic stroke in Japanese men and women. Diabetologia, 2004; 47: 2137-2144 [DOI] [PubMed] [Google Scholar]
- 200).Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med, 1998; 339: 229-234 [DOI] [PubMed] [Google Scholar]
- 201).Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG: Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg, 2007; 45 Suppl S: S5-67 [DOI] [PubMed] [Google Scholar]
- 202).Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH: Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med, 2004; 141: 421-431 [DOI] [PubMed] [Google Scholar]
- 203).Nesto RW, Phillips RT, Kett KG, Hill T, Perper E, Young E, Leland OS, Jr.: Angina and exertional myocardial ischemia in diabetic and nondiabetic patients: assessment by exercise thallium scintigraphy. Ann Intern Med, 1988; 108: 170-175 [DOI] [PubMed] [Google Scholar]
- 204).Goraya TY, Leibson CL, Palumbo PJ, Weston SA, Killian JM, Pfeifer EA, Jacobsen SJ, Frye RL, Roger VL: Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol, 2002; 40: 946-953 [DOI] [PubMed] [Google Scholar]
- 205).Kataoka Y, Yasuda S, Morii I, Otsuka Y, Kawamura A, Miyazaki S: Quantitative coronary angiographic studies of patients with angina pectoris and impaired glucose tolerance. Diabetes Care, 2005; 28: 2217-2222 [DOI] [PubMed] [Google Scholar]
- 206).Hoff JA, Quinn L, Sevrukov A, Lipton RB, Daviglus M, Garside DB, Ajmere NK, Gandhi S, Kondos GT: The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol, 2003; 41: 1008-1012 [DOI] [PubMed] [Google Scholar]
- 207).Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, Tsugane S: Diabetes mellitus and risk of stroke and its subtypes among Japanese: the Japan public health center study. Stroke, 2011; 42: 2611-2614 [DOI] [PubMed] [Google Scholar]
- 208).Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM: Diabetes and mortality following acute coronary syndromes. JAMA, 2007; 298: 765-775 [DOI] [PubMed] [Google Scholar]
- 209).Takara A, Ogawa H, Endoh Y, Mori F, Yamaguchi J, Takagi A, Koyanagi R, Shiga T, Kasanuki H, Hagiwara N: Long-term prognosis of diabetic patients with acute myocardial infarction in the era of acute revascularization. Cardiovasc Diabetol, 2010; 9: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210).Kuramitsu S, Yokoi H, Domei T, Nomura A, Watanabe H, Yamaji K, Soga Y, Arita T, Kondo K, Shirai S, Ando K, Sakai K, Iwabuchi M, Nosaka H, Nobuyoshi M: Impact of post-challenge hyperglycemia on clinical outcomes in Japanese patients with stable angina undergoing percutaneous coronary intervention. Cardiovasc Diabetol, 2013; 12: 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211).Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD: Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke, 2003; 34: 1457-1463 [DOI] [PubMed] [Google Scholar]
- 212).Shinohara Y, Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, Itoh E, Matsuda T, Sawada T, Yamaguchi T, Nishimaru K, Ohashi Y: Antiplatelet cilostazol is beneficial in diabetic and/or hypertensive ischemic stroke patients. Subgroup analysis of the cilostazol stroke prevention study. Cerebrovasc Dis, 2008; 26: 63-70 [DOI] [PubMed] [Google Scholar]
- 213).Paquette M, Bernard S, Ruel I, Blank DW, Genest J, Baass A: Diabetes is associated with an increased risk of cardiovascular disease in patients with familial hypercholesterolemia. J Clin Lipidol, 2019; 13: 123-128 [DOI] [PubMed] [Google Scholar]
- 214).Sun D, Cao YX, You XD, Zhou BY, Li S, Guo YL, Zhang Y, Wu NQ, Zhu CG, Gao Y, Dong QT, Liu G, Dong Q, Li JJ: Clinical and genetic characteristics of familial hypercholesterolemia patients with type 2 diabetes. J Endocrinol Invest, 2019; 42: 591-598 [DOI] [PubMed] [Google Scholar]
- 215).Yanagi K, Yamashita S, Kihara S, Nakamura T, Nozaki S, Nagai Y, Funahashi T, Kameda-Takemura K, Ueyama Y, Jiao S, Kubo M, Tokunaga K, Matsuzawa Y: Characteristics of coronary artery disease and lipoprotein abnormalities in patients with heterozygous familial hypercholesterolemia associated with diabetes mellitus or impaired glucose tolerance. Atherosclerosis, 1997; 132: 43-51 [DOI] [PubMed] [Google Scholar]
- 216).Uchiyama S, Goto S, Matsumoto M, Nagai R, Origasa H, Yamazaki T, Shigematsu H, Shimada K, Yamada N, Bhatt DL, Steg PG, Ikeda Y: Cardiovascular event rates in patients with cerebrovascular disease and atherothrombosis at other vascular locations: results from 1-year outcomes in the Japanese REACH Registry. J Neurol Sci, 2009; 287: 45-51 [DOI] [PubMed] [Google Scholar]
- 217).Goto S, Ikeda Y, Shimada K, Uchiyama S, Origasa H, Kobayashi H: One-year cardiovascular event rates in Japanese outpatients with myocardial infarction, stroke, and atrial fibrillation. -Results From the Japan Thrombosis Registry for Atrial Fibrillation, Coronary, or Cerebrovascular Events (J-TRACE). Circ J, 2011; 75: 2598-2604 [DOI] [PubMed] [Google Scholar]
- 218).Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, Banerjee A, Thuresson M, Okami S, Garal-Pantaler E, Overbeek J, Mamza JB, Zhang R, Yajima T, Komuro I, Kadowaki T: Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab, 2020; 22: 1607-1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219).Krempf M, Parhofer KG, Steg PG, Bhatt DL, Ohman EM, Röther J, Goto S, Pasquet B, Wilson PW: Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the REduction of Atherothrombosis for Continued Health [REACH] Registry). Am J Cardiol, 2010; 105: 667-671 [DOI] [PubMed] [Google Scholar]
- 220).Bundó M, Muñoz L, Pérez C, Montero JJ, Montellà N, Torán P, Pera G: Asymptomatic peripheral arterial disease in type 2 diabetes patients: a 10-year follow-up study of the utility of the ankle brachial index as a prognostic marker of cardiovascular disease. Ann Vasc Surg, 2010; 24: 985-993 [DOI] [PubMed] [Google Scholar]
- 221).Chang LH, Lin HD, Kwok CF, Won JG, Chen HS, Chu CH, Hwu CM, Kuo CS, Jap TS, Shih KC, Lin LY: The combination of the ankle brachial index and brachial ankle pulse wave velocity exhibits a superior association with outcomes in diabetic patients. Intern Med, 2014; 53: 2425-2431 [DOI] [PubMed] [Google Scholar]
- 222).Hosaka A, Miyata T, Onishi Y, Liao L, Zhang Q: Clinical and economic burden in patients with diagnosis of peripheral arterial disease in a claims database in Japan. Clin Ther, 2014; 36: 1223-1230, 1230.e1221-1224 [DOI] [PubMed] [Google Scholar]
- 223).Leibson CL, Ransom JE, Olson W, Zimmerman BR, O’Fallon W M, Palumbo PJ: Peripheral arterial disease, diabetes, and mortality. Diabetes Care, 2004; 27: 2843-2849 [DOI] [PubMed] [Google Scholar]
- 224).Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Colagiuri S, Hamet P, Harrap S, Poulter N, Matthews DR, Marre M, Chalmers J: Presentations of major peripheral arterial disease and risk of major outcomes in patients with type 2 diabetes: results from the ADVANCE-ON study. Cardiovasc Diabetol, 2016; 15: 129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225).Norman PE, Davis WA, Bruce DG, Davis TM: Peripheral arterial disease and risk of cardiac death in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care, 2006; 29: 575-580 [DOI] [PubMed] [Google Scholar]
- 226).Ogren M, Hedblad B, Engström G, Janzon L: Prevalence and prognostic significance of asymptomatic peripheral arterial disease in 68-year-old men with diabetes. Results from the population study ‘Men born in 1914’ from Malmö, Sweden. Eur J Vasc Endovasc Surg, 2005; 29: 182-189 [DOI] [PubMed] [Google Scholar]
- 227).Saely CH, Schindewolf M, Zanolin D, Heinzle CF, Vonbank A, Silbernagel G, Leiherer A, Drexel H, Baumgartner I: Single and combined effects of peripheral artery disease and of type 2 diabetes mellitus on the risk of cardiovascular events: A prospective cohort study. Atherosclerosis, 2018; 279: 32-37 [DOI] [PubMed] [Google Scholar]
- 228).Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY: Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care, 2007; 30: 1742-1746 [DOI] [PubMed] [Google Scholar]
- 229).Cusick M, Meleth AD, Agrón E, Fisher MR, Reed GF, Knatterud GL, Barton FB, Davis MD, Ferris FL, 3rd, Chew EY: Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care, 2005; 28: 617-625 [DOI] [PubMed] [Google Scholar]
- 230).Gerstein HC, Ambrosius WT, Danis R, Ismail-Beigi F, Cushman W, Calles J, Banerji M, Schubart U, Chew EY: Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care, 2013; 36: 1266-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231).Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care, 2007; 30: 292-299 [DOI] [PubMed] [Google Scholar]
- 232).Kawasaki R, Tanaka S, Tanaka S, Abe S, Sone H, Yokote K, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N, Yamashita H: Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology, 2013; 120: 574-582 [DOI] [PubMed] [Google Scholar]
- 233).Kawasaki S, Misawa H, Tamura Y, Kondo Y, Satoh S, Hasegawa O, Kato S, Terauchi Y: Relationship between coronary artery disease and retinopathy in patients with type 2 diabetes mellitus. Intern Med, 2013; 52: 2483-2487 [DOI] [PubMed] [Google Scholar]
- 234).Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälà K, Laakso M: Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care, 1996; 19: 1445-1448 [DOI] [PubMed] [Google Scholar]
- 235).Ono T, Kobayashi J, Sasako Y, Bando K, Tagusari O, Niwaya K, Imanaka H, Nakatani T, Kitamura S: The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol, 2002; 40: 428-436 [DOI] [PubMed] [Google Scholar]
- 236).Rajala U, Pajunpää H, Koskela P, Keinänen-Kiukaanniemi S: High cardiovascular disease mortality in subjects with visual impairment caused by diabetic retinopathy. Diabetes Care, 2000; 23: 957-961 [DOI] [PubMed] [Google Scholar]
- 237).Seferovic JP, Bentley-Lewis R, Claggett B, Diaz R, Gerstein HC, Køber LV, Lawson FC, Lewis EF, Maggioni AP, McMurray JJV, Probstfield JL, Riddle MC, Solomon SD, Tardif JC, Pfeffer MA: Retinopathy, Neuropathy, and Subsequent Cardiovascular Events in Patients with Type 2 Diabetes and Acute Coronary Syndrome in the ELIXA: The Importance of Disease Duration. J Diabetes Res, 2018; 2018: 1631263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238).Targher G, Bertolini L, Zenari L, Lippi G, Pichiri I, Zoppini G, Muggeo M, Arcaro G: Diabetic retinopathy is associated with an increased incidence of cardiovascular events in Type 2 diabetic patients. Diabet Med, 2008; 25: 45-50 [DOI] [PubMed] [Google Scholar]
- 239).Yamada T, Itoi T, Kiuchi Y, Nemoto M, Yamashita S: Proliferative diabetic retinopathy is a predictor of coronary artery disease in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract, 2012; 96: e4-6 [DOI] [PubMed] [Google Scholar]
- 240).Xie J, Ikram MK, Cotch MF, Klein B, Varma R, Shaw JE, Klein R, Mitchell P, Lamoureux EL, Wong TY: Association of Diabetic Macular Edema and Proliferative Diabetic Retinopathy With Cardiovascular Disease: A Systematic Review and Meta-analysis. JAMA Ophthalmol, 2017; 135: 586-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241).Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M: Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab, 2019; 21: 467-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242).Kim JJ, Hwang BH, Choi IJ, Choo EH, Lim S, Koh YS, Lee JM, Kim PJ, Seung KB, Lee SH, Cho JH, Jung JI, Chang K: A prospective two-center study on the associations between microalbuminuria, coronary atherosclerosis and long-term clinical outcome in asymptomatic patients with type 2 diabetes mellitus: evaluation by coronary CT angiography. Int J Cardiovasc Imaging, 2015; 31: 193-203 [DOI] [PubMed] [Google Scholar]
- 243).Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Johnson J, Hu G: Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int, 2014; 85: 1192-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244).Hsieh MC, Hsiao JY, Tien KJ, Chang SJ, Hsu SC, Liang HT, Chen HC, Lin SR, Tu ST: Chronic kidney disease as a risk factor for coronary artery disease in Chinese with type 2 diabetes. Am J Nephrol, 2008; 28: 317-323 [DOI] [PubMed] [Google Scholar]
- 245).Tanaka K, Hara S, Kushiyama A, Ubara Y, Yoshida Y, Mizuiri S, Aikawa A, Kawatzu S: Risk of macrovascular disease stratified by stage of chronic kidney disease in type 2 diabetic patients: critical level of the estimated glomerular filtration rate and the significance of hyperuricemia. Clin Exp Nephrol, 2011; 15: 391-397 [DOI] [PubMed] [Google Scholar]
- 246).Bo S, Ciccone G, Rosato R, Gancia R, Grassi G, Merletti F, Pagano GF: Renal damage in patients with Type 2 diabetes: a strong predictor of mortality. Diabet Med, 2005; 22: 258-265 [DOI] [PubMed] [Google Scholar]
- 247).Asakawa H, Tokunaga K, Kawakami F: Comparison of risk factors of macrovascular complications. Peripheral vascular disease, cerebral vascular disease, and coronary heart disease in Japanese type 2 diabetes mellitus patients. J Diabetes Complications, 2000; 14: 307-313 [DOI] [PubMed] [Google Scholar]
- 248).Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int, 2003; 63: 225-232 [DOI] [PubMed] [Google Scholar]
- 249).Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH: Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol, 2013; 24: 302-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250).de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation, 2004; 110: 921-927 [DOI] [PubMed] [Google Scholar]
- 251).Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J: Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol, 2009; 20: 1813-1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252).Ito H, Antoku S, Izutsu T, Kusano E, Matsumoto S, Yamasaki T, Mori T, Togane M: The prognosis of subjects showing a reduced estimated glomerular filtration rate without albuminuria in Japanese patients with type 2 diabetes: a cohort study for diabetic kidney disease. Clin Exp Nephrol, 2020; 24: 1033-1043 [DOI] [PubMed] [Google Scholar]
- 253).Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R: Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care, 2010; 33: 1578-1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254).Yun JS, Park YM, Cha SA, Ahn YB, Ko SH: Progression of cardiovascular autonomic neuropathy and cardiovascular disease in type 2 diabetes. Cardiovasc Diabetol, 2018; 17: 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255).Lee KH, Jang HJ, Kim YH, Lee EJ, Choe YS, Choi Y, Lee MG, Lee SH, Kim BT: Prognostic value of cardiac autonomic neuropathy independent and incremental to perfusion defects in patients with diabetes and suspected coronary artery disease. Am J Cardiol, 2003; 92: 1458-1461 [DOI] [PubMed] [Google Scholar]
- 256).Baltzis D, Roustit M, Grammatikopoulou MG, Katsaboukas D, Athanasiou V, Iakovou I, Veves A, Manes C, Trakatelli MC: Diabetic Peripheral Neuropathy as a Predictor of Asymptomatic Myocardial Ischemia in Type 2 Diabetes Mellitus: A Cross-Sectional Study. Adv Ther, 2016; 33: 1840-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257).Brownrigg JR, de Lusignan S, McGovern A, Hughes C, Thompson MM, Ray KK, Hinchliffe RJ: Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart, 2014; 100: 1837-1843 [DOI] [PubMed] [Google Scholar]
- 258).Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, Hinchliffe RJ: The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia, 2012; 55: 2906-2912 [DOI] [PubMed] [Google Scholar]
- 259).Pinto A, Tuttolomondo A, Di Raimondo D, Fernandez P, La Placa S, Di Gati M, Licata G: Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metabolism, 2008; 57: 676-682 [DOI] [PubMed] [Google Scholar]
- 260).Seghieri G, Policardo L, Gualdani E, Anichini R, Francesconi P: Gender difference in the risk for cardiovascular events or mortality of patients with diabetic foot syndrome. Acta Diabetol, 2019; 56: 561-567 [DOI] [PubMed] [Google Scholar]
- 261).Hamada S, Gulliford MC: Multiple risk factor control, mortality and cardiovascular events in type 2 diabetes and chronic kidney disease: a population-based cohort study. BMJ Open, 2018; 8: e019950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262).Blomster JI, Woodward M, Zoungas S, Hillis GS, Harrap S, Neal B, Poulter N, Mancia G, Chalmers J, Huxley R: The harms of smoking and benefits of smoking cessation in women compared with men with type 2 diabetes: an observational analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron modified release Controlled Evaluation) trial. BMJ Open, 2016; 6: e009668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263).Stevens RJ, Kothari V, Adler AI, Stratton IM: The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond), 2001; 101: 671-679 [PubMed] [Google Scholar]
- 264).Rosengren A, Welin L, Tsipogianni A, Wilhelmsen L: Impact of cardiovascular risk factors on coronary heart disease and mortality among middle aged diabetic men: a general population study. BMJ, 1989; 299: 1127-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265).Hadaegh F, Derakhshan A, Mozaffary A, Hasheminia M, Khalili D, Azizi F: Twelve-Year Cardiovascular and Mortality Risk in Relation to Smoking Habits in Type 2 Diabetic and Non-Diabetic Men: Tehran Lipid and Glucose Study. PLoS One, 2016; 11: e0149780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266).Pan A, Wang Y, Talaei M, Hu FB: Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation, 2015; 132: 1795-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267).Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S: Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med, 2018; 379: 633-644 [DOI] [PubMed] [Google Scholar]
- 268).Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N: Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab, 2011; 96: 3448-3456 [DOI] [PubMed] [Google Scholar]
- 269).Roussel R, Steg PG, Mohammedi K, Marre M, Potier L: Prevention of cardiovascular disease through reduction of glycaemic exposure in type 2 diabetes: A perspective on glucose-lowering interventions. Diabetes Obes Metab, 2018; 20: 238-244 [DOI] [PubMed] [Google Scholar]
- 270).Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ, 2000; 321: 405-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271).Harada M, Fujihara K, Osawa T, Yamamoto M, Kaneko M, Ishizawa M, Matsubayashi Y, Yamada T, Yamanaka N, Seida H, Kodama S, Ogawa W, Sone H: Association of treatment-achieved HbA(1c) with incidence of coronary artery disease and severe eye disease in diabetes patients. Diabetes Metab, 2020; 46: 331-334 [DOI] [PubMed] [Google Scholar]
- 272).Subcommittee for Renal Health Examination, Committee on Renal Disease Control, Japanese Society of Nephrology: Health guidance for the examinees of kidney medical checkup and suggestions to the referral criteria to medical institutions, Jpn J Nephrol 2017;59:38-42 (in Japanese) [Google Scholar]
- 273).Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension, 2003; 42: 1050-1065 [DOI] [PubMed] [Google Scholar]
- 274).Shoji T, Abe T, Matsuo H, Egusa G, Yamasaki Y, Kashihara N, Shirai K, Kashiwagi A: Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb, 2012; 19: 299-315 [DOI] [PubMed] [Google Scholar]
- 275).Kokubo Y, Nakamura S, Okamura T, Yoshimasa Y, Makino H, Watanabe M, Higashiyama A, Kamide K, Kawanishi K, Okayama A, Kawano Y: Relationship between blood pressure category and incidence of stroke and myocardial infarction in an urban Japanese population with and without chronic kidney disease: the Suita Study. Stroke, 2009; 40: 2674-2679 [DOI] [PubMed] [Google Scholar]
- 276).Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, Wakugawa Y, Hata J, Oishi Y, Shikata K, Yonemoto K, Hirakata H, Iida M: Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int, 2005; 68: 228-236 [DOI] [PubMed] [Google Scholar]
- 277).Ninomiya T, Kiyohara Y, Tokuda Y, Doi Y, Arima H, Harada A, Ohashi Y, Ueshima H: Impact of kidney disease and blood pressure on the development of cardiovascular disease: an overview from the Japan Arteriosclerosis Longitudinal Study. Circulation, 2008; 118: 2694-2701 [DOI] [PubMed] [Google Scholar]
- 278).Yasuno S, Ueshima K, Oba K, Fujimoto A, Ogihara T, Saruta T, Nakao K: Clinical significance of left ventricular hypertrophy and changes in left ventricular mass in high-risk hypertensive patients: a subanalysis of the Candesartan Antihypertensive Survival Evaluation in Japan trial. J Hypertens, 2009; 27: 1705-1712 [DOI] [PubMed] [Google Scholar]
- 279).Wanner C, Amann K, Shoji T: The heart and vascular system in dialysis. Lancet, 2016; 388: 276-284 [DOI] [PubMed] [Google Scholar]
- 280).Tonelli M, Muntner P, Lloyd A, Manns B, Klarenbach S, Pannu N, James M, Hemmelgarn B: Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol, 2013; 24: 979-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281).Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med, 2005; 353: 238-248 [DOI] [PubMed] [Google Scholar]
- 282).Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med, 2009; 360: 1395-1407 [DOI] [PubMed] [Google Scholar]
- 283).Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S: Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol, 2009; 13: 621-630 [DOI] [PubMed] [Google Scholar]
- 284).The Japanese Counsil on Cerebro-Cardiovascular Disease: Comprehensive risk management chart for cerebro-cardiovascular disease 2019. Nihon Naika Gakkai Zasshi, 2019; 108: 1024-1074 (in Japanese) [DOI] [PubMed] [Google Scholar]
- 285).Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847 [DOI] [PubMed] [Google Scholar]
- 286).Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative population. Circ J, 2006; 70: 1249-1255 [DOI] [PubMed] [Google Scholar]
- 287).Nakai M, Watanabe M, Kokubo Y, Nishimura K, Higashiyama A, Takegami M, Nakao YM, Okamura T, Miyamoto Y: Development of a Cardiovascular Disease Risk Prediction Model Using the Suita Study, a Population-Based Prospective Cohort Study in Japan. J Atheroscler Thromb, 2020; 27: 1160-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288).Rumana N, Kita Y, Turin TC, Murakami Y, Sugihara H, Morita Y, Tomioka N, Okayama A, Nakamura Y, Abbott RD, Ueshima H: Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI Registry, 1990-2001. Am J Epidemiol, 2008; 167: 1358-1364 [DOI] [PubMed] [Google Scholar]
- 289).Ministry of Health LaW: Vital Statistics in Japan, 2020 [Google Scholar]
- 290).Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ: Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study II. Am J Epidemiol, 1998; 147: 1133-1139 [DOI] [PubMed] [Google Scholar]
- 291).Li R, Bensen JT, Hutchinson RG, Province MA, Hertz-Picciotto I, Sprafka JM, Tyroler HA: Family risk score of coronary heart disease (CHD) as a predictor of CHD: the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI family heart study. Genet Epidemiol, 2000; 18: 236-250 [DOI] [PubMed] [Google Scholar]
- 292).Williams RR, Hunt SC, Heiss G, Province MA, Bensen JT, Higgins M, Chamberlain RM, Ware J, Hopkins PN: Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study). Am J Cardiol, 2001; 87: 129-135 [DOI] [PubMed] [Google Scholar]
- 293).Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr., Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ: Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA, 2004; 291: 2204-2211 [DOI] [PubMed] [Google Scholar]
- 294).Myers RH, Kiely DK, Cupples LA, Kannel WB: Parental history is an independent risk factor for coronary artery disease: the Framingham Study. Am Heart J, 1990; 120: 963-969 [DOI] [PubMed] [Google Scholar]
- 295).Watkins H, Farrall M: Genetic susceptibility to coronary artery disease: from promise to progress. Nat Rev Genet, 2006; 7: 163-173 [DOI] [PubMed] [Google Scholar]
- 296).Nasir K, Budoff MJ, Wong ND, Scheuner M, Herrington D, Arnett DK, Szklo M, Greenland P, Blumenthal RS: Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA). Circulation, 2007; 116: 619-626 [DOI] [PubMed] [Google Scholar]
- 297).Furukawa Y, Ehara N, Taniguchi R, Haruna Y, Ozasa N, Saito N, Doi T, Hoshino K, Tamura T, Shizuta S, Abe M, Toma M, Morimoto T, Teramukai S, Fukushima M, Kita T, Kimura T: Coronary risk factor profile and prognostic factors for young Japanese patients undergoing coronary revascularization. Circ J, 2009; 73: 1459-1465 [DOI] [PubMed] [Google Scholar]
- 298).Wahrenberg A, Kuja-Halkola R, Magnusson PKE, Häbel H, Warnqvist A, Hambraeus K, Jernberg T, Svensson P: Cardiovascular Family History Increases the Risk of Disease Recurrence After a First Myocardial Infarction. J Am Heart Assoc, 2021; 10: e022264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299).Timmerman N, de Kleijn DPV, de Borst GJ, den Ruijter HM, Asselbergs FW, Pasterkamp G, Haitjema S, van der Laan SW: Family history and polygenic risk of cardiovascular disease: Independent factors associated with secondary cardiovascular events in patients undergoing carotid endarterectomy. Atherosclerosis, 2020; 307: 121-129 [DOI] [PubMed] [Google Scholar]
- 300).Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM: Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA, 2012; 308: 788-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301).Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S: Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet, 2018; 50: 1219-1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302).Koyama S, Ito K, Terao C, Akiyama M, Horikoshi M, Momozawa Y, Matsunaga H, Ieki H, Ozaki K, Onouchi Y, Takahashi A, Nomura S, Morita H, Akazawa H, Kim C, Seo JS, Higasa K, Iwasaki M, Yamaji T, Sawada N, Tsugane S, Koyama T, Ikezaki H, Takashima N, Tanaka K, Arisawa K, Kuriki K, Naito M, Wakai K, Suna S, Sakata Y, Sato H, Hori M, Sakata Y, Matsuda K, Murakami Y, Aburatani H, Kubo M, Matsuda F, Kamatani Y, Komuro I: Population-specific and trans-ancestry genome-wide analyses identify distinct and shared genetic risk loci for coronary artery disease. Nat Genet, 2020; 52: 1169-1177 [DOI] [PubMed] [Google Scholar]
- 303).Elliott J, Bodinier B, Bond TA, Chadeau-Hyam M, Evangelou E, Moons KGM, Dehghan A, Muller DC, Elliott P, Tzoulaki I: Predictive Accuracy of a Polygenic Risk Score-Enhanced Prediction Model vs a Clinical Risk Score for Coronary Artery Disease. JAMA, 2020; 323: 636-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304).Roerecke M, Rehm J: Chronic heavy drinking and ischaemic heart disease: a systematic review and meta-analysis. Open Heart, 2014; 1: e000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305).Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, Rehm J: Alcohol consumption and the risk of morbidity and mortality for different stroke types--a systematic review and meta-analysis. BMC Public Health, 2010; 10: 258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306).Higashiyama A, Wakabayashi I, Ono Y, Watanabe M, Kokubo Y, Okayama A, Miyamoto Y, Okamura T: Association with serum gamma-glutamyltransferase levels and alcohol consumption on stroke and coronary artery disease: the Suita study. Stroke, 2011; 42: 1764-1767 [DOI] [PubMed] [Google Scholar]
- 307).Camargo CA, Jr.: Moderate alcohol consumption and stroke. The epidemiologic evidence. Stroke, 1989; 20: 1611-1626 [DOI] [PubMed] [Google Scholar]
- 308).Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J: Alcohol consumption and risk of stroke: a meta-analysis. JAMA, 2003; 289: 579-588 [DOI] [PubMed] [Google Scholar]
- 309).Ikehara S, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Wada Y, Inaba Y, Tamakoshi A: Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: the Japan collaborative cohort study. Stroke, 2008; 39: 2936-2942 [DOI] [PubMed] [Google Scholar]
- 310).Iso H, Baba S, Mannami T, Sasaki S, Okada K, Konishi M, Tsugane S: Alcohol consumption and risk of stroke among middle-aged men: the JPHC Study Cohort I. Stroke, 2004; 35: 1124-1129 [DOI] [PubMed] [Google Scholar]
- 311).Kawano Y: Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res, 2010; 33: 181-191 [DOI] [PubMed] [Google Scholar]
- 312).Higashiyama A, Okamura T, Watanabe M, Kokubo Y, Wakabayashi I, Okayama A, Miyamoto Y: Alcohol consumption and cardiovascular disease incidence in men with and without hypertension: the Suita study. Hypertens Res, 2013; 36: 58-64 [DOI] [PubMed] [Google Scholar]
- 313).Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA: Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ, 2011; 342: d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314).Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, Hemingway H: Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ, 2017; 356: j909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315).GBD2016Alcohol-Collaborators.: Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 2018; 392: 1015-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316).Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG, 2nd, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després JP, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J: Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet, 2018; 391: 1513-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317).Kitamura A, Iso H, Sankai T, Naito Y, Sato S, Kiyama M, Okamura T, Nakagawa Y, Iida M, Shimamoto T, Komachi Y: Alcohol intake and premature coronary heart disease in urban Japanese men. Am J Epidemiol, 1998; 147: 59-65 [DOI] [PubMed] [Google Scholar]
- 318).Saito E, Inoue M, Sawada N, Charvat H, Shimazu T, Yamaji T, Iwasaki M, Sasazuki S, Mizoue T, Iso H, Tsugane S: Impact of Alcohol Intake and Drinking Patterns on Mortality From All Causes and Major Causes of Death in a Japanese Population. J Epidemiol, 2018; 28: 140-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319).Langer RD, Criqui MH, Reed DM: Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation, 1992; 85: 910-915 [DOI] [PubMed] [Google Scholar]
- 320).Mukamal KJ, Jensen MK, Grønbaek M, Stampfer MJ, Manson JE, Pischon T, Rimm EB: Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation, 2005; 112: 1406-1413 [DOI] [PubMed] [Google Scholar]
- 321).Hirata A, Okamura T, Sugiyama D, Kuwabara K, Kadota A, Fujiyoshi A, Miura K, Okuda N, Ohkubo T, Okayama A, Ueshima H: The Relationship between Very High Levels of Serum High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in a 20-Year Follow-Up Study of Japanese General Population. J Atheroscler Thromb, 2016; 23: 800-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322).Madsen CM, Varbo A, Nordestgaard BG: Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J, 2017; 38: 2478-2486 [DOI] [PubMed] [Google Scholar]
- 323).Roerecke M, Rehm J: Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Epidemiol, 2010; 171: 633-644 [DOI] [PubMed] [Google Scholar]
- 324).O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S: Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet, 2016; 388: 761-775 [DOI] [PubMed] [Google Scholar]
- 325).Mostofsky E, Chahal HS, Mukamal KJ, Rimm EB, Mittleman MA: Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation, 2016; 133: 979-987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326).Leong DP, Smyth A, Teo KK, McKee M, Rangarajan S, Pais P, Liu L, Anand SS, Yusuf S: Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation, 2014; 130: 390-398 [DOI] [PubMed] [Google Scholar]
- 327).National Institute of Alcohol Abuse and Alcoholism: NIAAA council approves definition of binge drinking., 2004;3:3 [Google Scholar]
- 328).Higuchi S: Promotion of Terms and Research, Alcohol and Health Impairment Countermeasures Conference Materials, Ministry of Health, Labour and Welfare, 2015 (in Japanese) [Google Scholar]
- 329).Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G: Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med, 2006; 166: 2437-2445 [DOI] [PubMed] [Google Scholar]
- 330).Tsugane S, Fahey MT, Sasaki S, Baba S: Alcohol consumption and all-cause and cancer mortality among middle-aged Japanese men: seven-year follow-up of the JPHC study Cohort I. Japan Public Health Center. Am J Epidemiol, 1999; 150: 1201-1207 [DOI] [PubMed] [Google Scholar]
- 331).Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R: Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332).Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C: The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet, 2012; 380: 581-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333).Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A: Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 334).Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y: Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J, 2009; 73: 1283-1290 [DOI] [PubMed] [Google Scholar]
- 335).Current status of the background of patients with coronary artery disease in Japan. Circ J, 2006; 70: 1256-1262 [DOI] [PubMed] [Google Scholar]
- 336).Furukawa Y, Taniguchi R, Ehara N, Ozasa N, Haruna Y, Saito N, Doi T, Hoshino K, Shizuta S, Morimoto T, Imai Y, Teramukai S, Fukushima M, Kita T, Kimura T: Better survival with statin administration after revascularization therapy in Japanese patients with coronary artery disease: perspectives from the CREDO-Kyoto registry. Circ J, 2008; 72: 1937-1945 [DOI] [PubMed] [Google Scholar]
- 337).Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, Sakaino N, Kitagawa A: Effects of early statin treatment on symptomatic heart failure and ischemic events after acute myocardial infarction in Japanese. Am J Cardiol, 2006; 97: 1165-1171 [DOI] [PubMed] [Google Scholar]
- 338).Sato H, Kinjo K, Ito H, Hirayama A, Nanto S, Fukunami M, Nishino M, Lim YJ, Kijima Y, Koretsune Y, Nakatani D, Mizuno H, Shimizu M, Hori M: Effect of early use of low-dose pravastatin on major adverse cardiac events in patients with acute myocardial infarction: the OACIS-LIPID Study. Circ J, 2008; 72: 17-22 [DOI] [PubMed] [Google Scholar]
- 339).Miura T, Izawa A, Motoki H, Miyashita Y, Kashima Y, Ebisawa S, Tomita T, Koyama J, Ikeda U: Clinical Impact of Rapid Reduction of Low-Density Lipoprotein Cholesterol Level on Long-Term Outcome of Acute Myocardial Infarction in the Statin Era: Subanalysis of the ALPS-AMI Study. PLoS One, 2015; 10: e0127835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340).Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T: Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J, 2013; 77: 934-943 [DOI] [PubMed] [Google Scholar]
- 341).Hagiwara N, Kawada-Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, Saito K, Uchida T, Matsui K, Ogawa H: Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J, 2017; 38: 2264-2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342).Nakamura M, Ako J, Arai H, Hirayama A, Nohara A, Murakami Y, Ozaki A, Harada-Shiba M: Lipid Management and 2-Year Clinical Outcomes in Japanese Patients with Acute Coronary Syndrome: EXPLORE-J. J Atheroscler Thromb, 2021; 28: 1307-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343).Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina C, Rothwell PM, Sissani L, Školoudík D, Steg PG, Touboul PJ, Uchiyama S, Vicaut É, Wong LK: One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med, 2016; 374: 1533-1542 [DOI] [PubMed] [Google Scholar]
- 344).Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, Anticoli S, Audebert H, Bornstein NM, Caplan LR, Correia M, Donnan GA, Ferro JM, Gongora-Rivera F, Heide W, Hennerici MG, Kelly PJ, Král M, Lin HF, Molina C, Park JM, Purroy F, Rothwell PM, Segura T, Školoudík D, Steg PG, Touboul PJ, Uchiyama S, Vicaut É, Wang Y, Wong LKS: Five-Year Risk of Stroke after TIA or Minor Ischemic Stroke. N Engl J Med, 2018; 378: 2182-2190 [DOI] [PubMed] [Google Scholar]
- 345).Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC: Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med, 2013; 369: 11-19 [DOI] [PubMed] [Google Scholar]
- 346).Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY: Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med, 2018; 379: 215-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347).Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, Wang Y, Wong KS: Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N Engl J Med, 2016; 375: 35-43 [DOI] [PubMed] [Google Scholar]
- 348).Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, Takagi M, Ibayashi S, Nakamura M, Origasa H, Fukushima M, Mori E, Minematsu K, Uchiyama S, Shinohara Y, Yamaguchi T, Matsumoto M: The Japan Statin Treatment Against Recurrent Stroke (J-STARS): A Multicenter, Randomized, Open-label, Parallel-group Study. EBioMedicine, 2015; 2: 1071-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349).Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, Kitagawa Y, Kusuoka H, Nishimaru K, Tsushima M, Koretsune Y, Sawada T, Hamada C: Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol, 2010; 9: 959-968 [DOI] [PubMed] [Google Scholar]
- 350).Ogawa A, Toyoda K, Kitagawa K, Kitazono T, Nagao T, Yamagami H, Uchiyama S, Tanahashi N, Matsumoto M, Minematsu K, Nagata I, Nishikawa M, Nanto S, Abe K, Ikeda Y: Comparison of prasugrel and clopidogrel in patients with non-cardioembolic ischaemic stroke: a phase 3, randomised, non-inferiority trial (PRASTRO-I). Lancet Neurol, 2019; 18: 238-247 [DOI] [PubMed] [Google Scholar]
- 351).Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, Sakai N, Okada Y, Tanaka K, Origasa H, Naritomi H, Houkin K, Yamaguchi K, Isobe M, Minematsu K: Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol, 2019; 18: 539-548 [DOI] [PubMed] [Google Scholar]
- 352).Kitagawa K, Yamamoto Y, Arima H, Maeda T, Sunami N, Kanzawa T, Eguchi K, Kamiyama K, Minematsu K, Ueda S, Rakugi H, Ohya Y, Kohro T, Yonemoto K, Okada Y, Higaki J, Tanahashi N, Kimura G, Umemura S, Matsumoto M, Shimamoto K, Ito S, Saruta T, Shimada K: Effect of Standard vs Intensive Blood Pressure Control on the Risk of Recurrent Stroke: A Randomized Clinical Trial and Meta-analysis. JAMA Neurol, 2019; 76: 1309-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353).Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha JK, Ducrocq G, Giroud M, Guidoux C, Hobeanu C, Kim YJ, Lapergue B, Lavallée PC, Lee BC, Lee KB, Leys D, Mahagne MH, Meseguer E, Nighoghossian N, Pico F, Samson Y, Sibon I, Steg PG, Sung SM, Touboul PJ, Touzé E, Varenne O, Vicaut É, Yelles N, Bruckert E: A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N Engl J Med, 2020; 382: 9 [Google Scholar]
- 354).Kitagawa K, Hougaku H, Yamagami H, Hashimoto H, Itoh T, Shimizu Y, Takahashi D, Murata S, Seike Y, Kondo K, Hoshi T, Furukado S, Abe Y, Yagita Y, Sakaguchi M, Tagaya M, Etani H, Fukunaga R, Nagai Y, Matsumoto M, Hori M: Carotid intima-media thickness and risk of cardiovascular events in high-risk patients. Results of the Osaka Follow-Up Study for Carotid Atherosclerosis 2 (OSACA2 Study). Cerebrovasc Dis, 2007; 24: 35-42 [DOI] [PubMed] [Google Scholar]
- 355).Irie Y, Katakami N, Kaneto H, Kasami R, Sumitsuji S, Yamasaki K, Tachibana K, Kuroda T, Sakamoto K, Umayahara Y, Ueda Y, Kosugi K, Shimomura I: Maximum carotid intima-media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis, 2012; 221: 438-444 [DOI] [PubMed] [Google Scholar]
- 356).Hirano M, Nakamura T, Kitta Y, Takishima I, Deyama J, Kobayashi T, Fujioka D, Saito Y, Watanabe K, Watanabe Y, Kawabata K, Obata JE, Kugiyama K: Short-term progression of maximum intima-media thickness of carotid plaque is associated with future coronary events in patients with coronary artery disease. Atherosclerosis, 2011; 215: 507-512 [DOI] [PubMed] [Google Scholar]
- 357).Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG: Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet, 2012; 379: 2053-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358).JCS Joint research group: Guidelines for the management of peripheral arterial occlusive diseases (JCS 2015) https://plaza.umin.ac.jp/~jscvs/wordpress/wp-content/uploads/2020/06/JCS2015_miyata_h.pdf (in Japanese) [Google Scholar]
- 359).Kojima I, Ninomiya T, Hata J, Fukuhara M, Hirakawa Y, Mukai N, Yoshida D, Kitazono T, Kiyohara Y: A low ankle brachial index is associated with an increased risk of cardiovascular disease: the Hisayama study. J Atheroscler Thromb, 2014; 21: 966-973 [DOI] [PubMed] [Google Scholar]
- 360).Cui R, Yamagishi K, Imano H, Ohira T, Tanigawa T, Hitsumoto S, Kiyama M, Okada T, Kitamura A, Iso H: Relationship between the ankle-brachial index and the risk of coronary heart disease and stroke: the circulatory risk in communities study. J Atheroscler Thromb, 2014; 21: 1283-1289 [DOI] [PubMed] [Google Scholar]
- 361).Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Aboyans V, Yamashina A: Ankle-brachial index measured by oscillometry is predictive for cardiovascular disease and premature death in the Japanese population: An individual participant data meta-analysis. Atherosclerosis, 2018; 275: 141-148 [DOI] [PubMed] [Google Scholar]
- 362).Shigematsu H, Nishibe T, Obitsu Y, Matsuzaki K, Ishida A, Miyata T, Shindo S, Hida K, Ohta T, Ando M, Kawasaki T, Yasugi T, Matsumoto T: Three-year cardiovascular events and disease progress in patients with peripheral arterial disease: results from the Japan Medication Therapy for Peripheral Arterial Disease (J-METHOD). Int Angiol, 2010; 29: 2-13 [PubMed] [Google Scholar]
- 363).Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME: 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2017; 135: e726-e779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364).Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC: Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev, 2007; 2007: Cd000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365).Antoniou GA, Fisher RK, Georgiadis GS, Antoniou SA, Torella F: Statin therapy in lower limb peripheral arterial disease: Systematic review and meta-analysis. Vascul Pharmacol, 2014; 63: 79-87 [DOI] [PubMed] [Google Scholar]
- 366).Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg, 2007; 45: 645-654; discussion 653-644 [DOI] [PubMed] [Google Scholar]
- 367).Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC, Jr., Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, Banerjee S, Creager MA, Bhatt DL: Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J, 2014; 35: 2864-2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368).Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, Sala J, Grau M, Martí R, Ponjoan A, Alves-Cabratosa L, Blanch J, Bolíbar B: Statins for Prevention of Cardiovascular Events in a Low-Risk Population With Low Ankle Brachial Index. J Am Coll Cardiol, 2016; 67: 630-640 [DOI] [PubMed] [Google Scholar]
- 369).Vogel TR, Dombrovskiy VY, Galiñanes EL, Kruse RL: Preoperative statins and limb salvage after lower extremity revascularization in the Medicare population. Circ Cardiovasc Interv, 2013; 6: 694-700 [DOI] [PubMed] [Google Scholar]
- 370).Bonaca MP, Gutierrez JA, Cannon C, Giugliano R, Blazing M, Park JG, White J, Tershakovec A, Braunwald E: Polyvascular disease, type 2 diabetes, and long-term vascular risk: a secondary analysis of the IMPROVE-IT trial. Lancet Diabetes Endocrinol, 2018; 6: 934-943 [DOI] [PubMed] [Google Scholar]
- 371).Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR, Sabatine MS: Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation, 2018; 137: 338-350 [DOI] [PubMed] [Google Scholar]
- 372).Ishikawa Y, Yokoyama M, Saito Y, Matsuzaki M, Origasa H, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y: Preventive effects of eicosapentaenoic acid on coronary artery disease in patients with peripheral artery disease. Circ J, 2010; 74: 1451-1457 [DOI] [PubMed] [Google Scholar]
- 373).Mohler ER, 3rd, Hiatt WR, Creager MA: Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation, 2003; 108: 1481-1486 [DOI] [PubMed] [Google Scholar]
- 374).Bath MF, Gokani VJ, Sidloff DA, Jones LR, Choke E, Sayers RD, Bown MJ: Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg, 2015; 102: 866-872 [DOI] [PubMed] [Google Scholar]
- 375).Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW: The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg, 2018; 67: 2-77.e72 [DOI] [PubMed] [Google Scholar]
- 376).Kioka Y, Tanabe A, Kotani Y, Yamada N, Nakahama M, Ueda T, Seitou T, Maruyama M: Review of coronary artery disease in patients with infrarenal abdominal aortic aneurysm. Circ J, 2002; 66: 1110-1112 [DOI] [PubMed] [Google Scholar]
- 377).Hirose K, Chikamori T, Hida S, Tanaka H, Igarashi Y, Watanabe Y, Koizumi N, Kawaguchi S, Obitsu Y, Shigematsu H, Yamashina A: Prevalence of coronary heart disease in patients with aortic aneurysm and/or peripheral artery disease. Am J Cardiol, 2009; 103: 1215-1220 [DOI] [PubMed] [Google Scholar]
- 378).Elkalioubie A, Haulon S, Duhamel A, Rosa M, Rauch A, Staels B, Susen S, Van Belle E, Dupont A: Meta-Analysis of Abdominal Aortic Aneurysm in Patients With Coronary Artery Disease. Am J Cardiol, 2015; 116: 1451-1456 [DOI] [PubMed] [Google Scholar]
- 379).Hobbs SD, Claridge MW, Quick CR, Day NE, Bradbury AW, Wilmink AB: LDL cholesterol is associated with small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg, 2003; 26: 618-622 [DOI] [PubMed] [Google Scholar]
- 380).Törnwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK: Life-style factors and risk for abdominal aortic aneurysm in a cohort of Finnish male smokers. Epidemiology, 2001; 12: 94-100 [DOI] [PubMed] [Google Scholar]
- 381).Bhak RH, Wininger M, Johnson GR, Lederle FA, Messina LM, Ballard DJ, Wilson SE: Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg, 2015; 150: 44-50 [DOI] [PubMed] [Google Scholar]
- 382).Akai A, Watanabe Y, Hoshina K, Obitsu Y, Deguchi J, Sato O, Shigematsu K, Miyata T: Family history of aortic aneurysm is an independent risk factor for more rapid growth of small abdominal aortic aneurysms in Japan. J Vasc Surg, 2015; 61: 287-290 [DOI] [PubMed] [Google Scholar]
- 383).Bahia SS, Vidal-Diez A, Seshasai SR, Shpitser I, Brownrigg JR, Patterson BO, Ray KK, Holt PJ, Thompson MM, Karthikesalingam A: Cardiovascular risk prevention and all-cause mortality in primary care patients with an abdominal aortic aneurysm. Br J Surg, 2016; 103: 1626-1633 [DOI] [PubMed] [Google Scholar]
- 384).Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z, Esvs Guidelines C, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F, Document R, Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein HH, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco JB, Verhagen H: Editor’s Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg, 2019; 57: 8-93 [DOI] [PubMed] [Google Scholar]
- 385).Tanemoto M, Saitoh H, Satoh F, Satoh H, Abe T, Ito S: Predictors of undiagnosed renal artery stenosis among Japanese patients with risk factors of atherosclerosis. Hypertens Res, 2005; 28: 237-242 [DOI] [PubMed] [Google Scholar]
- 386).Tollefson DF, Ernst CB: Natural history of atherosclerotic renal artery stenosis associated with aortic disease. J Vasc Surg, 1991; 14: 327-331 [PubMed] [Google Scholar]
- 387).Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE, Jr.: A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens, 1996; 9: 1055-1061 [DOI] [PubMed] [Google Scholar]
- 388).White CJ, Jaff MR, Haskal ZJ, Jones DJ, Olin JW, Rocha-Singh KJ, Rosenfield KA, Rundback JH, Linas SL: Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation, 2006; 114: 1892-1895 [DOI] [PubMed] [Google Scholar]
- 389).Conlon PJ, Athirakul K, Kovalik E, Schwab SJ, Crowley J, Stack R, McCants CB, Jr., Mark DB, Bashore TM, Albers F: Survival in renal vascular disease. J Am Soc Nephrol, 1998; 9: 252-256 [DOI] [PubMed] [Google Scholar]
- 390).Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr., Dworkin LD: Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med, 2014; 370: 13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391).Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J: Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med, 2009; 361: 1953-1962 [DOI] [PubMed] [Google Scholar]
- 392).Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ: Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med, 2009; 150: 840-848, w150-841 [DOI] [PubMed] [Google Scholar]
- 393).Riaz IB, Husnain M, Riaz H, Asawaeer M, Bilal J, Pandit A, Shetty R, Lee KS: Meta-analysis of revascularization versus medical therapy for atherosclerotic renal artery stenosis. Am J Cardiol, 2014; 114: 1116-1123 [DOI] [PubMed] [Google Scholar]
- 394).Raman G, Adam GP, Halladay CW, Langberg VN, Azodo IA, Balk EM: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis: An Updated Systematic Review. Ann Intern Med, 2016; 165: 635-649 [DOI] [PubMed] [Google Scholar]
- 395).Fujihara M, Yokoi Y, Abe T, Soga Y, Yamashita T, Miyashita Y, Nakamura M, Yokoi H, Ito S: Clinical outcome of renal artery stenting for hypertension and chronic kidney disease up to 12 months in the J-RAS Study – prospective, single-arm, multicenter clinical study. Circ J, 2015; 79: 351-359 [DOI] [PubMed] [Google Scholar]
- 396).Prince M, Tafur JD, White CJ: When and How Should We Revascularize Patients With Atherosclerotic Renal Artery Stenosis? JACC Cardiovasc Interv, 2019; 12: 505-517 [DOI] [PubMed] [Google Scholar]
- 397).Bovet P, Perret F, Cornuz J, Quilindo J, Paccaud F: Improved smoking cessation in smokers given ultrasound photographs of their own atherosclerotic plaques. Prev Med, 2002; 34: 215-220 [DOI] [PubMed] [Google Scholar]
- 398).Taylor AJ, Bindeman J, Feuerstein I, Le T, Bauer K, Byrd C, Wu H, O’Malley PG: Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol, 2008; 51: 1337-1341 [DOI] [PubMed] [Google Scholar]
- 399).Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, Oguro H, Takahashi K: Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis, 2006; 15: 57-63 [DOI] [PubMed] [Google Scholar]
- 400).Matsui R, Nakagawa T, Takayoshi H, Onoda K, Oguro H, Nagai A, Yamaguchi S: A Prospective Study of Asymptomatic Intracranial Atherosclerotic Stenosis in Neurologically Normal Volunteers in a Japanese Cohort. Front Neurol, 2016; 7: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401).The Japan Stroke Society,: Japanese Guidelines for the Management of Stroke 2015 [addendum 2019], 2019 (in Japanese) [Google Scholar]
- 402).Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Nakamura F, Miyamoto Y: Impact of Intima-Media Thickness Progression in the Common Carotid Arteries on the Risk of Incident Cardiovascular Disease in the Suita Study. J Am Heart Assoc, 2018; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403).Yoshida M, Mita T, Yamamoto R, Shimizu T, Ikeda F, Ohmura C, Kanazawa A, Hirose T, Kawamori R, Watada H: Combination of the Framingham risk score and carotid intima-media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care, 2012; 35: 178-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404).Yang CW, Guo YC, Li CI, Liu CS, Lin CH, Liu CH, Wang MC, Yang SY, Li TC, Lin CC: Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. Int J Environ Res Public Health, 2020; 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405).Xie W, Wu Y, Wang W, Zhao D, Liang L, Wang M, Yang Y, Sun J, Shi P, Huo Y: A longitudinal study of carotid plaque and risk of ischemic cardiovascular disease in the Chinese population. J Am Soc Echocardiogr, 2011; 24: 729-737 [DOI] [PubMed] [Google Scholar]
- 406).Chien KL, Su TC, Jeng JS, Hsu HC, Chang WT, Chen MF, Lee YT, Hu FB: Carotid artery intima-media thickness, carotid plaque and coronary heart disease and stroke in Chinese. PLoS One, 2008; 3: e3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407).Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Jr., Virani SS, Williams KA, Sr., Yeboah J, Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 140: e596-e646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408).Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, Kitazono T, Kiyohara Y: Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens, 2013; 31: 477-483; discussion 483 [DOI] [PubMed] [Google Scholar]
- 409).Ohkuma T, Tomiyama H, Ninomiya T, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Yamashina A: Proposed Cutoff Value of Brachial-Ankle Pulse Wave Velocity for the Management of Hypertension. Circ J, 2017; 81: 1540-1542 [DOI] [PubMed] [Google Scholar]
- 410).Satoh-Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, Oishi M, Shimatsu A: Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis, 2015; 242: 461-468 [DOI] [PubMed] [Google Scholar]
- 411).Woo J, Leung J: Does measurement of ankle-brachial index contribute to prediction of adverse health outcomes in older Chinese people? Intern Med J, 2013; 43: 1017-1023 [DOI] [PubMed] [Google Scholar]
- 412).Wang Y, Guo X, Li J, Hu D, Zhao D, Ma H, Mou Q, Liu J, Xu Y: Predictive value of ankle-brachial index to all-cause mortality and cardiovascular mortality in Chinese patients with chronic kidney disease. Vasa, 2012; 41: 205-213 [DOI] [PubMed] [Google Scholar]
- 413).Wang Y, Mou Q, Zhao D, Xu Y, Hu D, Ma H, Liu J, Guo X, Li J: Predictive value of ankle-brachial index and blood glucose on the outcomes of six-year all-cause mortality and cardiovascular mortality in a Chinese population of type 2 diabetes patients. Int Angiol, 2012; 31: 586-594 [PubMed] [Google Scholar]
- 414).The Japanese Society of Gastroenterology, Hepatology TJSo: NAFLD/NASH Clinical Practice Guidelines 2020, Nankodo, 2020 [Google Scholar]
- 415).Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K: The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med, 2005; 143: 722-728 [DOI] [PubMed] [Google Scholar]
- 416).Nakahara T, Hyogo H, Yoneda M, Sumida Y, Eguchi Y, Fujii H, Ono M, Kawaguchi T, Imajo K, Aikata H, Tanaka S, Kanemasa K, Fujimoto K, Anzai K, Saibara T, Sata M, Nakajima A, Itoh Y, Chayama K, Okanoue T: Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol, 2014; 49: 1477-1484 [DOI] [PubMed] [Google Scholar]
- 417).Imajo K, Hyogo H, Yoneda M, Honda Y, Kessoku T, Tomeno W, Ogawa Y, Taguri M, Mawatari H, Nozaki Y, Fujita K, Kirikoshi H, Saito S, Sumida Y, Ono M, Wada K, Nakajima A, Eguchi Y: LDL-migration index (LDL-MI), an indicator of small dense low-density lipoprotein (sdLDL), is higher in non-alcoholic steatohepatitis than in non-alcoholic fatty liver: a multicenter cross-sectional study. PLoS One, 2014; 9: e115403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418).Campanella A, Iacovazzi PA, Misciagna G, Bonfiglio C, Mirizzi A, Franco I, Bianco A, Sorino P, Caruso MG, Cisternino AM, Buongiorno C, Liuzzi R, Osella AR: The Effect of Three Mediterranean Diets on Remnant Cholesterol and Non-Alcoholic Fatty Liver Disease: A Secondary Analysis. Nutrients, 2020; 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419).Musso G, Gambino R, Cassader M, Pagano G: Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med, 2011; 43: 617-649 [DOI] [PubMed] [Google Scholar]
- 420).Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C: Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol, 2016; 65: 589-600 [DOI] [PubMed] [Google Scholar]
- 421).Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF: Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut, 2021; 70: 1375-1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422).Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z: Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep, 2016; 6: 33386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423).Puchner SB, Lu MT, Mayrhofer T, Liu T, Pursnani A, Ghoshhajra BB, Truong QA, Wiviott SD, Fleg JL, Hoffmann U, Ferencik M: High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology, 2015; 274: 693-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424).Zhou YY, Zhou XD, Wu SJ, Fan DH, Van Poucke S, Chen YP, Fu SW, Zheng MH: Nonalcoholic fatty liver disease contributes to subclinical atherosclerosis: A systematic review and meta-analysis. Hepatol Commun, 2018; 2: 376-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425).Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F: Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology, 2015; 149: 389-397.e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426).McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, Scanu AM, Lawn RM: cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature, 1987; 330: 132-137 [DOI] [PubMed] [Google Scholar]
- 427).Kronenberg F, Utermann G: Lipoprotein(a): resurrected by genetics. J Intern Med, 2013; 273: 6-30 [DOI] [PubMed] [Google Scholar]
- 428).Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG: Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA, 2009; 301: 2331-2339 [DOI] [PubMed] [Google Scholar]
- 429).Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI: Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention Trial. JAMA, 1994; 271: 999-1003 [DOI] [PubMed] [Google Scholar]
- 430).Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GD, Danesh J, Gudnason V: Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med, 2008; 168: 598-608 [DOI] [PubMed] [Google Scholar]
- 431).Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J: Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA, 2009; 302: 412-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432).Marcovina SM, Koschinsky ML: Evaluation of lipoprotein(a) as a prothrombotic factor: progress from bench to bedside. Curr Opin Lipidol, 2003; 14: 361-366 [DOI] [PubMed] [Google Scholar]
- 433).Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M: Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med, 2009; 361: 2518-2528 [DOI] [PubMed] [Google Scholar]
- 434).Kyriakou T, Seedorf U, Goel A, Hopewell JC, Clarke R, Watkins H, Farrall M: A common LPA null allele associates with lower lipoprotein(a) levels and coronary artery disease risk. Arterioscler Thromb Vasc Biol, 2014; 34: 2095-2099 [DOI] [PubMed] [Google Scholar]
- 435).Saleheen D, Haycock PC, Zhao W, Rasheed A, Taleb A, Imran A, Abbas S, Majeed F, Akhtar S, Qamar N, Zaman KS, Yaqoob Z, Saghir T, Rizvi SNH, Memon A, Mallick NH, Ishaq M, Rasheed SZ, Memon FU, Mahmood K, Ahmed N, Frossard P, Tsimikas S, Witztum JL, Marcovina S, Sandhu M, Rader DJ, Danesh J: Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol, 2017; 5: 524-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436).Jansen H, Samani NJ, Schunkert H: Mendelian randomization studies in coronary artery disease. Eur Heart J, 2014; 35: 1917-1924 [DOI] [PubMed] [Google Scholar]
- 437).Langsted A, Kamstrup PR, Nordestgaard BG: High lipoprotein(a) and high risk of mortality. Eur Heart J, 2019; 40: 2760-2770 [DOI] [PubMed] [Google Scholar]
- 438).Boffa MB, Koschinsky ML: Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res, 2016; 57: 745-757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439).Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB: Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med, 2005; 353: 46-57 [DOI] [PubMed] [Google Scholar]
- 440).Tsimikas S, Witztum JL: The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol, 2008; 19: 369-377 [DOI] [PubMed] [Google Scholar]
- 441).Nielsen LB: Atherogenecity of lipoprotein(a) and oxidized low density lipoprotein: insight from in vivo studies of arterial wall influx, degradation and efflux. Atherosclerosis, 1999; 143: 229-243 [DOI] [PubMed] [Google Scholar]
- 442).Pavanello C, Pirazzi C, Bjorkman K, Sandstedt J, Tarlarini C, Mosca L, Romeo S, Calabresi L, Mancina RM: Individuals with familial hypercholesterolemia and cardiovascular events have higher circulating Lp(a) levels. J Clin Lipidol, 2019; 13: 778-787.e776 [DOI] [PubMed] [Google Scholar]
- 443).Alonso R, Andres E, Mata N, Fuentes-Jiménez F, Badimón L, López-Miranda J, Padró T, Muñiz O, Díaz-Díaz JL, Mauri M, Ordovás JM, Mata P: Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol, 2014; 63: 1982-1989 [DOI] [PubMed] [Google Scholar]
- 444).Shah NP, Pajidipati NJ, McGarrah RW, Navar AM, Vemulapalli S, Blazing MA, Shah SH, Hernandez AF, Patel MR: Lipoprotein (a): An Update on a Marker of Residual Risk and Associated Clinical Manifestations. Am J Cardiol, 2020; 126: 94-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445).Cook NR, Mora S, Ridker PM: Lipoprotein(a) and Cardiovascular Risk Prediction Among Women. J Am Coll Cardiol, 2018; 72: 287-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446).Kouvari M, Panagiotakos DB, Chrysohoou C, Georgousopoulou EN, Yannakoulia M, Tousoulis D, Pitsavos C: Lipoprotein (a) and 10-year Cardiovascular Disease Incidence in Apparently Healthy Individuals: A Sex-based Sensitivity Analysis from ATTICA Cohort Study. Angiology, 2019; 70: 819-829 [DOI] [PubMed] [Google Scholar]
- 447).Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, Ndumele C, Shahar E, Heiss G, Boerwinkle E, Konety S, Hoogeveen RC, Ballantyne CM: The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities study. Atherosclerosis, 2017; 262: 131-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448).Aronis KN, Zhao D, Hoogeveen RC, Alonso A, Ballantyne CM, Guallar E, Jones SR, Martin SS, Nazarian S, Steffen BT, Virani SS, Michos ED: Associations of Lipoprotein(a) Levels With Incident Atrial Fibrillation and Ischemic Stroke: The ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc, 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449).Verbeek R, Hoogeveen RM, Langsted A, Stiekema LCA, Verweij SL, Hovingh GK, Wareham NJ, Khaw KT, Boekholdt SM, Nordestgaard BG, Stroes ESG: Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J, 2018; 39: 2589-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450).Albers JJ, Slee A, O’Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Jr., Xu P, Marcovina SM: Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol, 2013; 62: 1575-1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451).Nestel PJ, Barnes EH, Tonkin AM, Simes J, Fournier M, White HD, Colquhoun DM, Blankenberg S, Sullivan DR: Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol, 2013; 33: 2902-2908 [DOI] [PubMed] [Google Scholar]
- 452).Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE: Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med, 2017; 376: 1933-1942 [DOI] [PubMed] [Google Scholar]
- 453).O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, Im K, Lira Pineda A, Wasserman SM, Češka R, Ezhov MV, Jukema JW, Jensen HK, Tokgözoğlu SL, Mach F, Huber K, Sever PS, Keech AC, Pedersen TR, Sabatine MS: Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation, 2019; 139: 1483-1492 [DOI] [PubMed] [Google Scholar]
- 454).Gaudet D, Watts GF, Robinson JG, Minini P, Sasiela WJ, Edelberg J, Louie MJ, Raal FJ: Effect of Alirocumab on Lipoprotein(a) Over ≥1.5 Years (from the Phase 3 ODYSSEY Program). Am J Cardiol, 2017; 119: 40-46 [DOI] [PubMed] [Google Scholar]
- 455).Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, Drechsler C, Wanner C, Mora S, Lesogor A, Tsimikas S: Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet, 2018; 392: 1311-1320 [DOI] [PubMed] [Google Scholar]
- 456).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS, Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 139: e1082-e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457).Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J, 2020; 41: 111-188 [DOI] [PubMed] [Google Scholar]
- 458).Mehta A, Virani SS, Ayers CR, Sun W, Hoogeveen RC, Rohatgi A, Berry JD, Joshi PH, Ballantyne CM, Khera A: Lipoprotein(a) and Family History Predict Cardiovascular Disease Risk. J Am Coll Cardiol, 2020; 76: 781-793 [DOI] [PubMed] [Google Scholar]
- 459).Ito T, Fujita H, Tani T, Ohte N: Malondialdehyde-modified low-density lipoprotein is a predictor of cardiac events in patients with stable angina on lipid-lowering therapy after percutaneous coronary intervention using drug-eluting stent. Atherosclerosis, 2015; 239: 311-317 [DOI] [PubMed] [Google Scholar]
- 460).Amioka N, Miyoshi T, Otsuka H, Yamada D, Takaishi A, Ueeda M, Hirohata S, Ito H: Serum malondialdehyde-modified low-density lipoprotein levels on admission predict prognosis in patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Cardiol, 2019; 74: 258-266 [DOI] [PubMed] [Google Scholar]
- 461).Ikenaga H, Kurisu S, Kono S, Sumimoto Y, Watanabe N, Shimonaga T, Higaki T, Iwasaki T, Mitsuba N, Ishibashi K, Dohi Y, Fukuda Y, Kihara Y: Impact of Malondialdehyde-Modified Low-Density Lipoprotein on Tissue Characteristics in Patients With Stable Coronary Artery Disease - Integrated Backscatter-Intravascular Ultrasound Study. Circ J, 2016; 80: 2173-2182 [DOI] [PubMed] [Google Scholar]
- 462).Ito T, Ichihashi T, Fujita H, Sugiura T, Ohte N: Impact of malondialdehyde-modified low-density lipoprotein on coronary plaque vulnerability in patients not receiving lipid-lowering therapy: a whole coronary analysis with multislice-computed tomography. Heart Vessels, 2018; 33: 351-357 [DOI] [PubMed] [Google Scholar]
- 463).Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, Tsunoda R, Sakamoto T, Nakano T, Nakajima K, Ogawa H, Sugiyama S, Yoshimura M, Yasue H: Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation, 1999; 99: 2858-2860 [DOI] [PubMed] [Google Scholar]
- 464).Nakamura T, Obata JE, Hirano M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Watanabe K, Watanabe Y, Mishina H, Kugiyama K: Predictive value of remnant lipoprotein for cardiovascular events in patients with coronary artery disease after achievement of LDL-cholesterol goals. Atherosclerosis, 2011; 218: 163-167 [DOI] [PubMed] [Google Scholar]
- 465).Nguyen SV, Nakamura T, Kugiyama K: High remnant lipoprotein predicts recurrent cardiovascular events on statin treatment after acute coronary syndrome. Circ J, 2014; 78: 2492-2500 [DOI] [PubMed] [Google Scholar]
- 466).Nguyen SV, Nakamura T, Uematsu M, Fujioka D, Watanabe K, Watanabe Y, Obata JE, Nakamura K, Kugiyama K: Remnant lipoproteinemia predicts cardiovascular events in patients with type 2 diabetes and chronic kidney disease. J Cardiol, 2017; 69: 529-535 [DOI] [PubMed] [Google Scholar]
- 467).Fujihara Y, Nakamura T, Horikoshi T, Obata JE, Fujioka D, Watanabe Y, Watanabe K, Kugiyama K: Remnant Lipoproteins Are Residual Risk Factor for Future Cardiovascular Events in Patients With Stable Coronary Artery Disease and On-Statin Low-Density Lipoprotein Cholesterol Levels <70 mg/dL. Circ J, 2019; 83: 1302-1308 [DOI] [PubMed] [Google Scholar]
- 468).Salinas CAA, Chapman MJ: Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol, 2020; 31: 132-139 [DOI] [PubMed] [Google Scholar]
- 469).Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, Nordestgaard BG, Watts GF, Bruckert E, Fazio S, Ference BA, Graham I, Horton JD, Landmesser U, Laufs U, Masana L, Pasterkamp G, Raal FJ, Ray KK, Schunkert H, Taskinen MR, van de Sluis B, Wiklund O, Tokgozoglu L, Catapano AL, Ginsberg HN: Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J, 2020; 41: 2313-2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470).McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ: Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis, 2001; 154: 229-236 [DOI] [PubMed] [Google Scholar]
- 471).Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG: Increased Remnant Cholesterol Explains Part of Residual Risk of All-Cause Mortality in 5414 Patients with Ischemic Heart Disease. Clin Chem, 2016; 62: 593-604 [DOI] [PubMed] [Google Scholar]
- 472).Ginsberg HN, Packard CJ, Chapman MJ, Borén J, Aguilar-Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, Lewis GF, Lichtenstein AH, Moulin P, Nordestgaard BG, Remaley AT, Staels B, Stroes ESG, Taskinen MR, Tokgözoğlu LS, Tybjaerg-Hansen A, Stock JK, Catapano AL: Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J, 2021; 42: 4791-4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473).Masuda D, Sugimoto T, Tsujii K, Inagaki M, Nakatani K, Yuasa-Kawase M, Tsubakio-Yamamoto K, Ohama T, Nishida M, Ishigami M, Kawamoto T, Matsuyama A, Sakai N, Komuro I, Yamashita S: Correlation of fasting serum apolipoprotein B-48 with coronary artery disease prevalence. Eur J Clin Invest, 2012; 42: 992-999 [DOI] [PubMed] [Google Scholar]
- 474).Tian J, Chen H, Liu P, Wang C, Chen Y: Fasting apolipoprotein B48 is associated with large artery atherosclerotic stroke: a case-control study. Sci Rep, 2019; 9: 3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475).Nordestgaard BG, Varbo A: Triglycerides and cardiovascular disease. Lancet, 2014; 384: 626-635 [DOI] [PubMed] [Google Scholar]
- 476).Zilversmit DB: Atherogenesis: a postprandial phenomenon. Circulation, 1979; 60: 473-485 [DOI] [PubMed] [Google Scholar]
- 477).Havel RJ: Remnant lipoproteins as therapeutic targets. Curr Opin Lipidol, 2000; 11: 615-620 [DOI] [PubMed] [Google Scholar]
- 478).Eberly LE, Stamler J, Neaton JD: Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med, 2003; 163: 1077-1083 [DOI] [PubMed] [Google Scholar]
- 479).Driver SL, Martin SS, Gluckman TJ, Clary JM, Blumenthal RS, Stone NJ: Fasting or Nonfasting Lipid Measurements: It Depends on the Question. J Am Coll Cardiol, 2016; 67: 1227-1234 [DOI] [PubMed] [Google Scholar]
- 480).Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Borén J, Chapman MJ, Cobbaert C, Descamps OS, von Eckardstein A, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M: Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J, 2016; 37: 1944-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481).Masuda D, Sakai N, Sugimoto T, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Yuasa-Kawase M, Tsubakio-Yamamoto K, Ohama T, Nakagawa-Toyama Y, Nishida M, Ishigami M, Masuda Y, Matsuyama A, Komuro I, Yamashita S: Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb, 2011; 18: 1062-1070 [DOI] [PubMed] [Google Scholar]
- 482).Yamashita S, Arai H, Yokote K, Araki E, Suganami H, Ishibashi S: Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol, 2018; 12: 1267-1279.e1264 [DOI] [PubMed] [Google Scholar]
- 483).Ohno Y, Miyoshi T, Noda Y, Oe H, Toh N, Nakamura K, Kohno K, Morita H, Ito H: Bezafibrate improves postprandial hypertriglyceridemia and associated endothelial dysfunction in patients with metabolic syndrome: a randomized crossover study. Cardiovasc Diabetol, 2014; 13: 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484).Kurozumi A, Okada Y, Mori H, Kobayashi T, Masuda D, Yamashita S, Tanaka Y: Detrimental effects of high-fat diet loading on vascular endothelial function and therapeutic efficacy of ezetimibe and statins in patients with type 2 diabetes. Endocr J, 2016; 63: 431-440 [DOI] [PubMed] [Google Scholar]
- 485).Nakamura A, Sato K, Kanazawa M, Kondo M, Endo H, Takahashi T, Nozaki E: Impact of decreased insulin resistance by ezetimibe on postprandial lipid profiles and endothelial functions in obese, non-diabetic-metabolic syndrome patients with coronary artery disease. Heart Vessels, 2019; 34: 916-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486).Sawada T, Tsubata H, Hashimoto N, Takabe M, Miyata T, Aoki K, Yamashita S, Oishi S, Osue T, Yokoi K, Tsukishiro Y, Onishi T, Shimane A, Taniguchi Y, Yasaka Y, Ohara T, Kawai H, Yokoyama M: Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol, 2016; 15: 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487).Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM: Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA, 1988; 260: 1917-1921 [PubMed] [Google Scholar]
- 488).Krauss R: Low-density lipoprotein subclasses and risk of coronary artery disease. Curr Opin Lipidol, 1991; 2: 248-242 [Google Scholar]
- 489).St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard PM, Després JP, Lamarche B: Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol, 2005; 25: 553-559 [DOI] [PubMed] [Google Scholar]
- 490).Arsenault BJ, Lemieux I, Després JP, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM: Cholesterol levels in small LDL particles predict the risk of coronary heart disease in the EPIC-Norfolk prospective population study. Eur Heart J, 2007; 28: 2770-2777 [DOI] [PubMed] [Google Scholar]
- 491).El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM: Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol, 2007; 49: 547-553 [DOI] [PubMed] [Google Scholar]
- 492).Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, Sharp DS: Low-density lipoprotein particle size, triglycerides, and high-density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men. Am J Cardiol, 2000; 86: 412-416 [DOI] [PubMed] [Google Scholar]
- 493).Rizzo M, Pernice V, Frasheri A, Berneis K: Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis, 2008; 197: 237-241 [DOI] [PubMed] [Google Scholar]
- 494).Rizzo M, Krayenbühl PA, Pernice V, Frasheri A, Battista Rini G, Berneis K: LDL size and subclasses in patients with abdominal aortic aneurysm. Int J Cardiol, 2009; 134: 406-408 [DOI] [PubMed] [Google Scholar]
- 495).Hirano T, Ito Y, Koba S, Toyoda M, Ikejiri A, Saegusa H, Yamazaki J, Yoshino G: Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol, 2004; 24: 558-563 [DOI] [PubMed] [Google Scholar]
- 496).Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, Kobayashi Y, Katagiri T: Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb, 2008; 15: 250-260 [DOI] [PubMed] [Google Scholar]
- 497).Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T, Kobayashi Y: Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb, 2014; 21: 755-767 [DOI] [PubMed] [Google Scholar]
- 498).de Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, Stalenhoef AF: Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb, 1991; 11: 298-306 [DOI] [PubMed] [Google Scholar]
- 499).Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ: Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res, 1998; 39: 1263-1273 [PubMed] [Google Scholar]
- 500).Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM: Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med, 2009; 150: 474-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501).Berneis KK, Krauss RM: Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res, 2002; 43: 1363-1379 [DOI] [PubMed] [Google Scholar]
- 502).Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM: Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest, 1993; 92: 141-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503).Austin MA, Edwards KL: Small, dense low density lipoproteins, the insulin resistance syndrome and noninsulin-dependent diabetes. Curr Opin Lipidol, 1996; 7: 167-171 [DOI] [PubMed] [Google Scholar]
- 504).Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y: Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb, 2013; 20: 195-203 [DOI] [PubMed] [Google Scholar]
- 505).Higashioka M, Sakata S, Honda T, Hata J, Yoshida D, Hirakawa Y, Shibata M, Goto K, Kitazono T, Osawa H, Ninomiya T: Small Dense Low-Density Lipoprotein Cholesterol and the Risk of Coronary Heart Disease in a Japanese Community. J Atheroscler Thromb, 2020; 27: 669-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506).Higashioka M, Sakata S, Honda T, Hata J, Shibata M, Yoshida D, Goto K, Kitazono T, Osawa H, Ninomiya T: The Association of Small Dense Low-Density Lipoprotein Cholesterol and Coronary Heart Disease in Subjects at High Cardiovascular Risk. J Atheroscler Thromb, 2021; 28: 79-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507).Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM: Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508).Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT: New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol, 2014; 34: 196-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509).Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD: Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J Am Coll Cardiol, 2020; 75: 2122-2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510).Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S: Small Dense Low-Density Lipoprotein Cholesterol Predicts Atherosclerotic Cardiovascular Disease in the Copenhagen General Population Study. J Am Coll Cardiol, 2020; 75: 2873-2875 [DOI] [PubMed] [Google Scholar]
- 511).Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, Zhang Y, Wu NQ, Zhu CG, Xu RX, Gao Y, Li XL, Cui CJ, Liu G, Sun J, Dong Q, Guo YL, Li JJ: Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: a prospective, observational cohort study. Cardiovasc Diabetol, 2020; 19: 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512).Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE: Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA, 2005; 294: 326-333 [DOI] [PubMed] [Google Scholar]
- 513).Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD: A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes, 2011; 4: 337-345 [DOI] [PubMed] [Google Scholar]
- 514).Pencina MJ, D’Agostino RB, Zdrojewski T, Williams K, Thanassoulis G, Furberg CD, Peterson ED, Vasan RS, Sniderman AD: Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol, 2015; 22: 1321-1327 [DOI] [PubMed] [Google Scholar]
- 515).Thanassoulis G, Williams K, Ye K, Brook R, Couture P, Lawler PR, de Graaf J, Furberg CD, Sniderman A: Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: a meta-analysis of randomized trials. J Am Heart Assoc, 2014; 3: e000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516).Robinson JG, Wang S, Jacobson TA: Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol, 2012; 110: 1468-1476 [DOI] [PubMed] [Google Scholar]
- 517).Khan SU, Khan MU, Valavoor S, Khan MS, Okunrintemi V, Mamas MA, Leucker TM, Blaha MJ, Michos ED: Association of lowering apolipoprotein B with cardiovascular outcomes across various lipid-lowering therapies: Systematic review and meta-analysis of trials. Eur J Prev Cardiol, 2020; 27: 1255-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518).Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Nicholls SJ, Bhatt DL, Sabatine MS, Catapano AL: Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants With Risk of Coronary Heart Disease. JAMA, 2019; 321: 364-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519).Ling Y, Jiang J, Wu B, Gao X: Serum triglyceride, high-density lipoprotein cholesterol, apolipoprotein B, and coronary heart disease in a Chinese population undergoing coronary angiography. J Clin Lipidol, 2017; 11: 646-656 [DOI] [PubMed] [Google Scholar]
- 520).Nordestgaard BG, Langlois MR, Langsted A, Chapman MJ, Aakre KM, Baum H, Borén J, Bruckert E, Catapano A, Cobbaert C, Collinson P, Descamps OS, Duff CJ, von Eckardstein A, Hammerer-Lercher A, Kamstrup PR, Kolovou G, Kronenberg F, Mora S, Pulkki K, Remaley AT, Rifai N, Ros E, Stankovic S, Stavljenic-Rukavina A, Sypniewska G, Watts GF, Wiklund O, Laitinen P: Quantifying atherogenic lipoproteins for lipid-lowering strategies: Consensus-based recommendations from EAS and EFLM. Atherosclerosis, 2020; 294: 46-61 [DOI] [PubMed] [Google Scholar]
- 521).Welsh C, Celis-Morales CA, Brown R, Mackay DF, Lewsey J, Mark PB, Gray SR, Ferguson LD, Anderson JJ, Lyall DM, Cleland JG, Jhund PS, Gill JMR, Pell JP, Sattar N, Welsh P: Comparison of Conventional Lipoprotein Tests and Apolipoproteins in the Prediction of Cardiovascular Disease. Circulation, 2019; 140: 542-552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522).McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S: Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet, 2008; 372: 224-233 [DOI] [PubMed] [Google Scholar]
- 523).Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, Deedwania P, Olsson AG, Boekholdt SM, Demicco DA, Szarek M, LaRosa JC, Pedersen TR, Grundy SM: Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation, 2008; 117: 3002-3009 [DOI] [PubMed] [Google Scholar]
- 524).Ray KK, Cannon CP, Cairns R, Morrow DA, Ridker PM, Braunwald E: Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: results from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol, 2009; 29: 424-430 [Google Scholar]
- 525).Sung KC, Ryu S, Wild SH, Byrne CD: An increased high-density lipoprotein cholesterol/apolipoprotein A-I ratio is associated with increased cardiovascular and all-cause mortality. Heart, 2015; 101: 553-558 [DOI] [PubMed] [Google Scholar]
- 526).Chang TI, Streja E, Soohoo M, Kim TW, Rhee CM, Kovesdy CP, Kashyap ML, Vaziri ND, Kalantar-Zadeh K, Moradi H: Association of Serum Triglyceride to HDL Cholesterol Ratio with All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients. Clin J Am Soc Nephrol, 2017; 12: 591-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527).Tian M, Li R, Shan Z, Wang DW, Jiang J, Cui G: Comparison of Apolipoprotein B/A1 ratio, Framingham risk score and TC/HDL-c for predicting clinical outcomes in patients undergoing percutaneous coronary intervention. Lipids Health Dis, 2019; 18: 202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528).Hong LF, Yan XN, Fan Y, Wu Q, Luo SH, Yang B, Li JJ: Is the ratio of apoB/apoA-1 the best predictor for the severity of coronary artery lesions in Chinese diabetics with stable angina pectoris? An assessment based on Gensini scores. J Geriatr Cardiol, 2015; 12: 402-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529).Boot CS, Middling E, Allen J, Neely RDG: Evaluation of the Non-HDL Cholesterol to Apolipoprotein B Ratio as a Screening Test for Dysbetalipoproteinemia. Clin Chem, 2019; 65: 313-320 [DOI] [PubMed] [Google Scholar]
- 530).Hisamatsu T, Fujiyoshi A, Miura K, Ohkubo T, Kadota A, Kadowaki S, Kadowaki T, Yamamoto T, Miyagawa N, Zaid M, Torii S, Takashima N, Murakami Y, Okamura T, Horie M, Ueshima H: Lipoprotein particle profiles compared with standard lipids in association with coronary artery calcification in the general Japanese population. Atherosclerosis, 2014; 236: 237-243 [DOI] [PubMed] [Google Scholar]
- 531).Li Y, Yatsuya H, Tanaka S, Iso H, Okayama A, Tsuji I, Sakata K, Miyamoto Y, Ueshima H, Miura K, Murakami Y, Okamura T: Estimation of 10-Year Risk of Death from Coronary Heart Disease, Stroke, and Cardiovascular Disease in a Pooled Analysis of Japanese Cohorts: EPOCH-JAPAN. J Atheroscler Thromb, 2021; 28: 816-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532).Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D’Agostino RB, Sr., Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engström G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jørgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J: C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med, 2012; 367: 1310-1320 [Google Scholar]
- 533).Kawase Ishihara K, Kokubo Y, Yokota C, Hida E, Miyata T, Toyoda K, Matsumoto M, Minematsu K, Miyamoto Y: Effect of Plasma Fibrinogen, High-Sensitive C-Reactive Protein, and Cigarette Smoking on Carotid Atherosclerosis: The Suita Study. J Stroke Cerebrovasc Dis, 2015; 24: 2385-2389 [DOI] [PubMed] [Google Scholar]
- 534).Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, Cui R, Tanigawa T, Sankai T, Ishikawa Y, Sato S, Iso H: C-reactive protein levels and risk of stroke and its subtype in Japanese: The Circulatory Risk in Communities Study (CIRCS). Atherosclerosis, 2011; 217: 187-193 [DOI] [PubMed] [Google Scholar]
- 535).Iso H, Noda H, Ikeda A, Yamagishi K, Inoue M, Iwasaki M, Tsugane S: The impact of C-reactive protein on risk of stroke, stroke subtypes, and ischemic heart disease in middle-aged Japanese: the Japan public health center-based study. J Atheroscler Thromb, 2012; 19: 756-766 [PubMed] [Google Scholar]
- 536).Tian R, Tian M, Wang L, Qian H, Zhang S, Pang H, Liu Z, Fang L, Shen Z: C-reactive protein for predicting cardiovascular and all-cause mortality in type 2 diabetic patients: A meta-analysis. Cytokine, 2019; 117: 59-64 [DOI] [PubMed] [Google Scholar]
- 537).Tabrizi R, Tamtaji OR, Mirhosseini N, Lankarani KB, Akbari M, Dadgostar E, Borhani-Haghighi A, Peymani P, Ahmadizar F, Asemi Z: The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res, 2019; 141: 85-103 [DOI] [PubMed] [Google Scholar]
- 538).Zhang XL, Lan RF, Zhang XW, Xu W, Wang L, Kang LN, Xu B: Association Between Baseline, Achieved, and Reduction of CRP and Cardiovascular Outcomes After LDL Cholesterol Lowering with Statins or Ezetimibe: A Systematic Review and Meta-Analysis. J Am Heart Assoc, 2019; 8: e012428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539).Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey-Smith G, Nordestgaard BG, Saleheen D, Samani NJ, Sandhu M, Anand S, Pepys MB, Smeeth L, Whittaker J, Casas JP, Thompson SG, Hingorani AD, Danesh J: Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ, 2011; 342: d548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540).Matsuura Y, Hatakeyama K, Imamura T, Tsuruda T, Shibata Y, Kodama T, Kitamura K, Asada Y: Different distribution of pentraxin 3 and C-reactive protein in coronary atherosclerotic plaques. J Atheroscler Thromb, 2012; 19: 837-845 [DOI] [PubMed] [Google Scholar]
- 541).Iwata A, Miura S, Tanaka T, Ike A, Sugihara M, Nishikawa H, Kawamura A, Saku K: Plasma pentraxin-3 levels are associated with coronary plaque vulnerability and are decreased by statin. Coron Artery Dis, 2012; 23: 315-321 [DOI] [PubMed] [Google Scholar]
- 542).Yasunaga T, Ikeda S, Koga S, Nakata T, Yoshida T, Masuda N, Kohno S, Maemura K: Plasma pentraxin 3 is a more potent predictor of endothelial dysfunction than high-sensitive C-reactive protein. Int Heart J, 2014; 55: 160-164 [DOI] [PubMed] [Google Scholar]
- 543).Li H, Liu W, Xie J: Circulating interleukin-6 levels and cardiovascular and all-cause mortality in the elderly population: A meta-analysis. Arch Gerontol Geriatr, 2017; 73: 257-262 [DOI] [PubMed] [Google Scholar]
- 544).Zhang B, Li XL, Zhao CR, Pan CL, Zhang Z: Interleukin-6 as a Predictor of the Risk of Cardiovascular Disease: A Meta-Analysis of Prospective Epidemiological Studies. Immunol Invest, 2018; 47: 689-699 [DOI] [PubMed] [Google Scholar]
- 545).Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ: Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet, 2017; 390: 1833-1842 [DOI] [PubMed] [Google Scholar]
- 546).Gerhard GT, Duell PB: Homocysteine and atherosclerosis. Curr Opin Lipidol, 1999; 10: 417-428 [DOI] [PubMed] [Google Scholar]
- 547).Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA, 2002; 288: 2015-2022 [DOI] [PubMed] [Google Scholar]
- 548).Verhoef P, Stampfer MJ: Prospective studies of homocysteine and cardiovascular disease. Nutr Rev, 1995; 53: 283-288 [DOI] [PubMed] [Google Scholar]
- 549).Peng HY, Man CF, Xu J, Fan Y: Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: a meta-analysis of prospective studies. J Zhejiang Univ Sci B, 2015; 16: 78-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550).de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, Gussekloo J: Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ, 2009; 338: a3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551).Lewis SJ, Ebrahim S, Davey Smith G: Meta-analysis of MTHFR 677C->T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ, 2005; 331: 1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552).Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M: Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev, 2017; 8: Cd006612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553).Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB: Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc, 2016; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554).Zeng R, Xu CH, Xu YN, Wang YL, Wang M: The effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk: a meta-analysis. Public Health Nutr, 2015; 18: 1514-1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555).Hou X, Chen X, Shi J: Genetic polymorphism of MTHFR C677T and premature coronary artery disease susceptibility: A meta-analysis. Gene, 2015; 565: 39-44 [DOI] [PubMed] [Google Scholar]
- 556).van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, Cotlarciuc I, Yuan X, Mälarstig A, Bandinelli S, Bis JC, Blom H, Brown MJ, Chen C, Chen YD, Clarke RJ, Dehghan A, Erdmann J, Ferrucci L, Hamsten A, Hofman A, Hunter DJ, Goel A, Johnson AD, Kathiresan S, Kampman E, Kiel DP, Kiemeney LA, Chambers JC, Kraft P, Lindemans J, McKnight B, Nelson CP, O’Donnell CJ, Psaty BM, Ridker PM, Rivadeneira F, Rose LM, Seedorf U, Siscovick DS, Schunkert H, Selhub J, Ueland PM, Vollenweider P, Waeber G, Waterworth DM, Watkins H, Witteman JC, den Heijer M, Jacques P, Uitterlinden AG, Kooner JS, Rader DJ, Reilly MP, Mooser V, Chasman DI, Samani NJ, Ahmadi KR: Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr, 2013; 98: 668-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557).Fuster V: Lewis A. Conner Memorial Lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation, 1994; 90: 2126-2146 [DOI] [PubMed] [Google Scholar]
- 558).Zhu M, Mao M, Lou X: Elevated homocysteine level and prognosis in patients with acute coronary syndrome: a meta-analysis. Biomarkers, 2019; 24: 309-316 [DOI] [PubMed] [Google Scholar]
- 559).Zhang Z, Xiao S, Yang C, Ye R, Hu X, Chen X: Association of Elevated Plasma Homocysteine Level with Restenosis and Clinical Outcomes After Percutaneous Coronary Interventions: a Systemic Review and Meta-analysis. Cardiovasc Drugs Ther, 2019; 33: 353-361 [DOI] [PubMed] [Google Scholar]
- 560).Yeh JK, Chen CC, Hsieh MJ, Tsai ML, Yang CH, Chen DY, Chang SH, Wang CY, Lee CH, Hsieh IC: Impact of Homocysteine Level on Long-term Cardiovascular Outcomes in Patients after Coronary Artery Stenting. J Atheroscler Thromb, 2017; 24: 696-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561).Zhang T, Jiang Y, Zhang S, Tie T, Cheng Y, Su X, Man Z, Hou J, Sun L, Tian M, Zhang Y, Li J, Ma Y: The association between homocysteine and ischemic stroke subtypes in Chinese: A meta-analysis. Medicine (Baltimore), 2020; 99: e19467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562).Piao X, Wu G, Yang P, Shen J, De A, Wu J, Qu Q: Association between Homocysteine and Cerebral Small Vessel Disease: A Meta-Analysis. J Stroke Cerebrovasc Dis, 2018; 27: 2423-2430 [DOI] [PubMed] [Google Scholar]
- 563).Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D’Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Piñango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssönen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Després JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A: Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA, 2005; 294: 1799-1809 [DOI] [PubMed] [Google Scholar]
- 564).Kunutsor SK, Kurl S, Zaccardi F, Laukkanen JA: Baseline and long-term fibrinogen levels and risk of sudden cardiac death: A new prospective study and meta-analysis. Atherosclerosis, 2016; 245: 171-180 [DOI] [PubMed] [Google Scholar]
- 565).Ward-Caviness CK, de Vries PS, Wiggins KL, Huffman JE, Yanek LR, Bielak LF, Giulianini F, Guo X, Kleber ME, Kacprowski T, Groß S, Petersman A, Davey Smith G, Hartwig FP, Bowden J, Hemani G, Müller-Nuraysid M, Strauch K, Koenig W, Waldenberger M, Meitinger T, Pankratz N, Boerwinkle E, Tang W, Fu YP, Johnson AD, Song C, de Maat MPM, Uitterlinden AG, Franco OH, Brody JA, McKnight B, Chen YI, Psaty BM, Mathias RA, Becker DM, Peyser PA, Smith JA, Bielinski SJ, Ridker PM, Taylor KD, Yao J, Tracy R, Delgado G, Trompet S, Sattar N, Jukema JW, Becker LC, Kardia SLR, Rotter JI, März W, Dörr M, Chasman DI, Dehghan A, O’Donnell CJ, Smith NL, Peters A, Morrison AC: Mendelian randomization evaluation of causal effects of fibrinogen on incident coronary heart disease. PLoS One, 2019; 14: e0216222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566).Tabakcı MM, Gerin F, Sunbul M, Toprak C, Durmuş H, Demir S, Arslantaş U, Cerşit S, Batgerel U, Kargın R: Relation of Plasma Fibrinogen Level With the Presence, Severity, and Complexity of Coronary Artery Disease. Clin Appl Thromb Hemost, 2017; 23: 638-644 [DOI] [PubMed] [Google Scholar]
- 567).Nagasawa SY, Ohkubo T, Masaki K, Barinas-Mitchell E, Miura K, Seto T, El-Saed A, Kadowaki T, Willcox BJ, Edmundowicz D, Kadota A, Evans RW, Kadowaki S, Fujiyoshi A, Hisamatsu T, Bertolet MH, Okamura T, Nakamura Y, Kuller LH, Ueshima H, Sekikawa A: Associations between Inflammatory Markers and Subclinical Atherosclerosis in Middle-aged White, Japanese-American and Japanese Men: The ERA-JUMP Study. J Atheroscler Thromb, 2015; 22: 590-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568).Sakamoto T, Yasue H, Ogawa H, Misumi I, Masuda T: Association of patency of the infarct-related coronary artery with plasma levels of plasminogen activator inhibitor activity in acute myocardial infarction. Am J Cardiol, 1992; 70: 271-276 [DOI] [PubMed] [Google Scholar]
- 569).Sahebkar A, Catena C, Ray KK, Vallejo-Vaz AJ, Reiner Ž, Sechi LA, Colussi G: Impact of statin therapy on plasma levels of plasminogen activator inhibitor-1. A systematic review and meta-analysis of randomised controlled trials. Thromb Haemost, 2016; 116: 162-171 [DOI] [PubMed] [Google Scholar]
- 570).Wang NC, Matthews KA, Barinas-Mitchell EJ, Chang CC, El Khoudary SR: Inflammatory/Hemostatic Biomarkers and Coronary Artery Calcium Progression in Women at Midlife (from the Study of Women’s Health Across the Nation, Heart Study). Am J Cardiol, 2016; 118: 311-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571).Jung RG, Motazedian P, Ramirez FD, Simard T, Di Santo P, Visintini S, Faraz MA, Labinaz A, Jung Y, Hibbert B: Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thromb J, 2018; 16: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572).Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin B, Zambon A, Barter P, Fruchart JC, Eckel RH: Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol, 2019; 7: 715-725 [DOI] [PubMed] [Google Scholar]
- 573).Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, Griffin BA, Zambon A, Barter P, Fruchart JC, Eckel RH, Matsuzawa Y, Després JP: Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol, 2020; 16: 177-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574).Ministry of Health, Labour and Welfare: National health and nutrition survey in Japan, 2019., 2020 (in Japanese) [Google Scholar]
- 575).Committee to Evaluate Diagnostic Standards for Metabolic Syndrome: Definition and the diagnostic standard for metabolic syndrome. J Jpn Soc Intern Med, 2005; 94: 794-809 (in Japanese) [PubMed] [Google Scholar]
- 576).Alberti KG, Zimmet P, Shaw J: The metabolic syndrome--a new worldwide definition. Lancet, 2005; 366: 1059-1062 [DOI] [PubMed] [Google Scholar]
- 577).Nakamura T, Tsubono Y, Kameda-Takemura K, Funahashi T, Yamashita S, Hisamichi S, Kita T, Yamamura T, Matsuzawa Y: Magnitude of sustained multiple risk factors for ischemic heart disease in Japanese employees: a case-control study. Jpn Circ J, 2001; 65: 11-17 [DOI] [PubMed] [Google Scholar]
- 578).The 1994 Report of the Group of the Research for the Association between Host Origin and Atherosclerotic Diseases under the Preventive Measure for Work-related Diseases of the Japanese Labor Ministry Significance of host factors contributing to the development of atherosclerosis, 1994 (in Japanese) [Google Scholar]
- 579).Nakamura Y, Yamamoto T, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Saitoh S, Okayama A, Ueshima H: Combined cardiovascular risk factors and outcome: NIPPON DATA80, 1980-1994. Circ J, 2006; 70: 960-964 [DOI] [PubMed] [Google Scholar]
- 580).Okubo K, Kiyohara Y.: Frequency of the metabolic syndrome in the general inhabitant. Rinsho to Kenkyu, 2004; 81: 1736-1740 [Google Scholar]
- 581).Matsuzawa Y.: Multicenter follow-up study of insulin resistance and the lifestyle basis in patients at high risk of diabetes mellitus-establishment of significance of abdominal obesity for Intervention. Health Science Research Project, Ministry of Health, Labour and Welfare,2001, in Japanese [Google Scholar]
- 582).Matsuzawa Y, Inoue S, Ikeda Y, Sakata T, Saitou Y,Satoh H, Shirai A, Oono J, Miyazaki S, Tokunaga M,Fukagawa H, Yamanouchi K, Nakamura M.: New evaluation of obesity and diagnostic criteria of obesity. J Jpn Soc Study of Obesity, 2000; 6: 18-28, in Japanese [Google Scholar]
- 583).Japan Society for the Study of Obesity: Guidelines for the management of obesity disease 2016. 2016 (in Japanese) [Google Scholar]
- 584).Saito Y, Shirai K, Nakamura T: Diagnostic criteria for obesity. J Jpn Soc Study of Obesity, 2011; 17: 1-78 (in Japanese) [Google Scholar]
- 585).The Examination Committee of Criteria for Obesity Disease in Japan (2002) Japan Society for the Study of Obesity:New criteria for ‘obesity disease’ in Japan. Circ J, 2002; 66: 987-992 [DOI] [PubMed] [Google Scholar]
- 586).Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH: Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation, 2004; 110: 2952-2967 [DOI] [PubMed] [Google Scholar]
- 587).Nakamura T, Tokunaga K, Shimomura I, Nishida M, Yoshida S, Kotani K, Islam AH, Keno Y, Kobatake T, Nagai Y, et al.: Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis, 1994; 107: 239-246 [DOI] [PubMed] [Google Scholar]
- 588).Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW: Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care, 1999; 22: 1808-1812 [DOI] [PubMed] [Google Scholar]
- 589).Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR: Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation, 2004; 110: 1245-1250 [DOI] [PubMed] [Google Scholar]
- 590).Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP: National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation, 2004; 110: 1251-1257 [DOI] [PubMed] [Google Scholar]
- 591).Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L: Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care, 2001; 24: 683-689 [DOI] [PubMed] [Google Scholar]
- 592).Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA, 2002; 288: 2709-2716 [DOI] [PubMed] [Google Scholar]
- 593).Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ: The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol, 2010; 56: 1113-1132 [DOI] [PubMed] [Google Scholar]
- 594).Takeuchi H, Saitoh S, Takagi S, Ohnishi H, Ohhata J, Isobe T, Shimamoto K: Metabolic syndrome and cardiac disease in Japanese men: applicability of the concept of metabolic syndrome defined by the National Cholesterol Education Program-Adult Treatment Panel III to Japanese men--the Tanno and Sobetsu Study. Hypertens Res, 2005; 28: 203-208 [DOI] [PubMed] [Google Scholar]
- 595).Iso H, Cui R, Takamoto I, Kiyama M, Saito I, Okamura T, Miyamoto Y, Higashiyama A, Kiyohara Y, Ninomiya T, Yamada M, Nakagawa H, Sakurai M, Shimabukuro M, Higa M, Shimamoto K, Saito S, Daimon M, Kayama T, Noda M, Ito S, Yokote K, Ito C, Nakao K, Yamauchi T, Kadowaki T: Risk Classification for Metabolic Syndrome and the Incidence of Cardiovascular Disease in Japan With Low Prevalence of Obesity: A Pooled Analysis of 10 Prospective Cohort Studies. J Am Heart Assoc, 2021: e020760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596).Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr.: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 2009; 120: 1640-1645 [DOI] [PubMed] [Google Scholar]
- 597).Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, Suzuki S, Takaya N, Nakagawa T, Fukui T, Fukuda H, Watanabe N, Yoshizumi T, Nakamura T, Matsuzawa Y, Yamakado M, Shimomura I: Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med, 2012; 44: 82-92 [DOI] [PubMed] [Google Scholar]
- 598).Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT: 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J, 2016; 37: 2999-3058 [Google Scholar]
- 599).Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y: Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the framingham risk score: the suita study. J Atheroscler Thromb, 2014; 21: 784-798 [DOI] [PubMed] [Google Scholar]
- 600).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601).Sankai T, Miyagaki T, Iso H, Shimamoto T, Iida M, Tanigaki M, Naito Y, Sato S, Kiyama M, Kitamura A, et al.: [A population-based study of the proportion by type of stroke determined by computed tomography scan]. Nihon Koshu Eisei Zasshi, 1991; 38: 901-909 [PubMed] [Google Scholar]
- 602).Konishi M, Iso H, Komachi Y, Iida M, Shimamoto T, Jacobs DR, Jr., Terao A, Baba S, Sankai T, Ito M: Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita Pathology Study. Stroke, 1993; 24: 954-964 [DOI] [PubMed] [Google Scholar]
- 603).Kubo M, Hata J, Doi Y, Tanizaki Y, Iida M, Kiyohara Y: Secular trends in the incidence of and risk factors for ischemic stroke and its subtypes in Japanese population. Circulation, 2008; 118: 2672-2678 [DOI] [PubMed] [Google Scholar]
- 604).Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, Sawada N, Tsugane S: Trends in the proportions of stroke subtypes and coronary heart disease in the Japanese men and women from 1995 to 2009. Atherosclerosis, 2016; 248: 219-223 [DOI] [PubMed] [Google Scholar]
- 605).Harada A, Ueshima H, Kinoshita Y, Miura K, Ohkubo T, Asayama K, Ohashi Y: Absolute risk score for stroke, myocardial infarction, and all cardiovascular disease: Japan Arteriosclerosis Longitudinal Study. Hypertens Res, 2019; 42: 567-579 [DOI] [PubMed] [Google Scholar]
- 606).Honda T, Chen S, Hata J, Yoshida D, Hirakawa Y, Furuta Y, Shibata M, Sakata S, Kitazono T, Ninomiya T: Development and Validation of a Risk Prediction Model for Atherosclerotic Cardiovascular Disease in Japanese Adults: The Hisayama Study. J Atheroscler Thromb, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607).Honda T, Yoshida D, Hata J, Hirakawa Y, Ishida Y, Shibata M, Sakata S, Kitazono T, Ninomiya T: Development and validation of modified risk prediction models for cardiovascular disease and its subtypes: The Hisayama Study. Atherosclerosis, 2018; 279: 38-44 [DOI] [PubMed] [Google Scholar]
- 608).Yatsuya H, Iso H, Yamagishi K, Kokubo Y, Saito I, Suzuki K, Sawada N, Inoue M, Tsugane S: Development of a point-based prediction model for the incidence of total stroke: Japan public health center study. Stroke, 2013; 44: 1295-1302 [DOI] [PubMed] [Google Scholar]
- 609).Okuda N, Kadota A, Nishi N, Miura K, Ohkubo T, Miyagawa N, Satoh A, Kita Y, Hayakawa T, Takashima N, Fujiyoshi A, Okayama A, Okamura T, Ueshima H: Association of Work Situation With Cardiovascular Disease Mortality Risk Among Working-Age Japanese Men - A 20-Year Follow-up of NIPPON DATA90. Circ J, 2019; 83: 1506-1513 [DOI] [PubMed] [Google Scholar]
- 610).Dalton JE, Perzynski AT, Zidar DA, Rothberg MB, Coulton CJ, Milinovich AT, Einstadter D, Karichu JK, Dawson NV: Accuracy of Cardiovascular Risk Prediction Varies by Neighborhood Socioeconomic Position: A Retrospective Cohort Study. Ann Intern Med, 2017; 167: 456-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611).Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM: Lifetime risks of cardiovascular disease. N Engl J Med, 2012; 366: 321-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612).Satoh M, Ohkubo T, Asayama K, Murakami Y, Sugiyama D, Yamada M, Saitoh S, Sakata K, Irie F, Sairenchi T, Ishikawa S, Kiyama M, Ohnishi H, Miura K, Imai Y, Ueshima H, Okamura T: Lifetime Risk of Stroke and Coronary Heart Disease Deaths According to Blood Pressure Level: EPOCH-JAPAN (Evidence for Cardiovascular Prevention From Observational Cohorts in Japan). Hypertension, 2019; 73: 52-59 [DOI] [PubMed] [Google Scholar]
- 613).Fager G, Wiklund O: Cholesterol reduction and clinical benefit. Are there limits to our expectations? Arterioscler Thromb Vasc Biol, 1997; 17: 3527-3533 [DOI] [PubMed] [Google Scholar]
- 614).Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 615).Saiki Y, Otsuka T, Kato K, Kawada T: A Proposal for the Optimal Management Target for Serum Non-High-Density Lipoprotein Cholesterol Level in Low-Risk Japanese Workers. J Atheroscler Thromb, 2016; 23: 422-430 [DOI] [PubMed] [Google Scholar]
- 616).Kuwabara K, Harada S, Sugiyama D, Kurihara A, Kubota Y, Higashiyama A, Hirata T, Nishida Y, Kawasaki M, Takebayashi T, Okamura T: Relationship between Non-High-Density Lipoprotein Cholesterol and Low-Density Lipoprotein Cholesterol in the General Population. J Atheroscler Thromb, 2016; 23: 477-490 [DOI] [PubMed] [Google Scholar]
- 617).Mons U, Müezzinler A, Gellert C, Schöttker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O’Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H: Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ, 2015; 350: h1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618).Jeong SM, Jeon KH, Shin DW, Han K, Kim D, Park SH, Cho MH, Lee CM, Nam KW, Lee SP: Smoking cessation, but not reduction, reduces cardiovascular disease incidence. Eur Heart J, 2021; 42: 4141-4153 [DOI] [PubMed] [Google Scholar]
- 619).Tan CE, Glantz SA: Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation, 2012; 126: 2177-2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620).Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T: Physician advice for smoking cessation. Cochrane Database Syst Rev, 2013; 2013: Cd000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621).Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T: Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev, 2018; 5: Cd000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622).Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T: Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev, 2016; 2016: Cd006103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623).The Japanese Circulation Society, The Japan Lung Cancer Society, The Japanese Respiratory Society: Standard Procedures for Smoking Cessation Treatment, 8th ed., http://j-circ.or.jp/kinen/anti_smoke_std/pdf/anti_smoke_std_rev8_.pdf, 2021 (in Japanese) [Google Scholar]
- 624).Masaki K, Tateno H, Nomura A, Muto T, Suzuki S, Satake K, Hida E, Fukunaga K: A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. NPJ Digit Med, 2020; 3: 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625).Hirano T, Tabuchi T, Nakahara R, Kunugita N, Mochizuki-Kobayashi Y: Electronic Cigarette Use and Smoking Abstinence in Japan: A Cross-Sectional Study of Quitting Methods. Int J Environ Res Public Health, 2017; 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626).Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P: Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ, 2012; 345: e4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627).Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH: Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J, 2011; 161: 145-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628).Komiyama M, Shimada S, Wada H, Yamakage H, Satoh-Asahara N, Shimatsu A, Akao M, Morimoto T, Takahashi Y, Hasegawa K: Time-dependent Changes of Atherosclerotic LDL Complexes after Smoking Cessation. J Atheroscler Thromb, 2016; 23: 1270-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629).Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, Willett WC, Manson JE, Hu FB, Sun Q: Smoking Cessation, Weight Change, Type 2 Diabetes, and Mortality. N Engl J Med, 2018; 379: 623-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630).Reinert DF, Allen JP: The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res, 2002; 26: 272-279 [PubMed] [Google Scholar]
- 631).Álvarez-Bueno C, Rodríguez-Martín B, García-Ortiz L, Gómez-Marcos M, Martínez-Vizcaíno V: Effectiveness of brief interventions in primary health care settings to decrease alcohol consumption by adult non-dependent drinkers: a systematic review of systematic reviews. Prev Med, 2015; 76 Suppl: S33-38 [DOI] [PubMed] [Google Scholar]
- 632).Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Jr., Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB: Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA, 2018; 320: 1899-1909 [DOI] [PubMed] [Google Scholar]
- 633).Maesato H: Measures to Alcohol Problem in Japan, Revised Manual for Alcohol Health Guidance, 2016 [Google Scholar]
- 634).Yuzuriha T: Early Intervention Programs for Alcohol-Related Problems, HAPPY. Journal of clinical and experimental medicine, 2007; 9: 728-732 [Google Scholar]
- 635).Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjöström L: Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed), 1984; 289: 1257-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636).Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G: Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed), 1984; 288: 1401-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637).Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE: Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes, 2004; 53: 2087-2094 [DOI] [PubMed] [Google Scholar]
- 638).Matsuzawa Y: The metabolic syndrome and adipocytokines. FEBS Lett, 2006; 580: 2917-2921 [DOI] [PubMed] [Google Scholar]
- 639).Anderson JW, Konz EC, Frederich RC, Wood CL: Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr, 2001; 74: 579-584 [DOI] [PubMed] [Google Scholar]
- 640).Muramoto A, Matsushita M, Kato A, Yamamoto N, Koike G, Nakamura M, Numata T, Tamakoshi A, Tsushita K: Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract, 2014; 8: e466-475 [DOI] [PubMed] [Google Scholar]
- 641).Tsushita K, A SH, Miura K, Ito Y, Fukuda T, Kitamura A, Tatara K: Rationale and Descriptive Analysis of Specific Health Guidance: the Nationwide Lifestyle Intervention Program Targeting Metabolic Syndrome in Japan. J Atheroscler Thromb, 2018; 25: 308-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642).Nakao YM, Miyamoto Y, Ueshima K, Nakao K, Nakai M, Nishimura K, Yasuno S, Hosoda K, Ogawa Y, Itoh H, Ogawa H, Kangawa K, Nakao K: Effectiveness of nationwide screening and lifestyle intervention for abdominal obesity and cardiometabolic risks in Japan: The metabolic syndrome and comprehensive lifestyle intervention study on nationwide database in Japan (MetS ACTION-J study). PLoS One, 2018; 13: e0190862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643).Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr., Tomaselli GF: 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 2014; 129: S102-138 [Google Scholar]
- 644).Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K: Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J Am Coll Cardiol, 2017; 70: 1429-1437 [DOI] [PubMed] [Google Scholar]
- 645).Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der AD, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado-Yohar SM, Engström G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina-Portillo E, Moreno-Iribas C, Norberg M, Overvad K, Panico S, Quirós JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjønneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS: Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J, 2018; 39: 397-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646).Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB: Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol, 2018; 6: 714-724 [DOI] [PubMed] [Google Scholar]
- 647).Kramer CK, Zinman B, Retnakaran R: Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med, 2013; 159: 758-769 [DOI] [PubMed] [Google Scholar]
- 648).Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Wada Y, Inaba Y, Tamakoshi A: Body mass index and mortality from cardiovascular disease among Japanese men and women: the JACC study. Stroke, 2005; 36: 1377-1382 [DOI] [PubMed] [Google Scholar]
- 649).Global B.M.I. Mortality Collaboration, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson Ch L, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O’Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Z, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB: Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet, 2016; 388: 776-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650).Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, Sharma P, Fraser C, MacLennan G: Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ, 2017; 359: j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651).Zhang X, Devlin HM, Smith B, Imperatore G, Thomas W, Lobelo F, Ali MK, Norris K, Gruss S, Bardenheier B, Cho P, Garcia de Quevedo I, Mudaliar U, Jones CD, Durthaler JM, Saaddine J, Geiss LS, Gregg EW: Effect of lifestyle interventions on cardiovascular risk factors among adults without impaired glucose tolerance or diabetes: A systematic review and meta-analysis. PLoS One, 2017; 12: e0176436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652).Tsushita K: Health Labour Sciences Research: Research on the Effects of Lifestyle-related Disease Prevention Activities and Disease Management on Health Indicators and the Effects of Medical Cost Optimization, 2011 (in Japanese) [Google Scholar]
- 653).Ministry of Labour and Welfare: Dietary Reference Intakes for Japanese 2020 in Japanese https://www.mhlw.go.jp/content/10904750/000586553.pdf (in Japanese) [Google Scholar]
- 654).Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K, Yoshioka N: Japanese Clinical Practice Guideline for Diabetes 2019. J Diabetes Investig, 2020; 11: 1020-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655).Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, Rimm EB, Willett WC, Solomon SD: Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health, 2018; 3: e419-e428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656).Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K: Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr, 2016; 115: 466-479 [DOI] [PubMed] [Google Scholar]
- 657).Lu M, Wan Y, Yang B, Huggins CE, Li D: Effects of low-fat compared with high-fat diet on cardiometabolic indicators in people with overweight and obesity without overt metabolic disturbance: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr, 2018; 119: 96-108 [DOI] [PubMed] [Google Scholar]
- 658).Hooper L, Summerbell CD, Higgins JP, Thompson RL, Clements G, Capps N, Davey S, Riemersma RA, Ebrahim S: Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev, 2001: Cd002137 [DOI] [PubMed] [Google Scholar]
- 659).Budhathoki S, Sawada N, Iwasaki M, Yamaji T, Goto A, Kotemori A, Ishihara J, Takachi R, Charvat H, Mizoue T, Iso H, Tsugane S, Japan Public Health Center-based Prospective Study G: Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality in a Japanese Cohort. JAMA Intern Med, 2019; 179: 1509-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660).Schwingshackl L, Hoffmann G: Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J, 2013; 12: 48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661).Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A, et al.: Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med, 1995; 24: 308-315 [DOI] [PubMed] [Google Scholar]
- 662).Tanasescu M, Cho E, Manson JE, Hu FB: Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am J Clin Nutr, 2004; 79: 999-1005 [DOI] [PubMed] [Google Scholar]
- 663).Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A: Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr, 2009; 89: 1425-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664).Guasch-Ferré M, Babio N, Martínez-González MA, Corella D, Ros E, Martín-Peláez S, Estruch R, Arós F, Gómez-Gracia E, Fiol M, Santos-Lozano JM, Serra-Majem L, Bulló M, Toledo E, Barragán R, Fitó M, Gea A, Salas-Salvadó J: Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr, 2015; 102: 1563-1573 [DOI] [PubMed] [Google Scholar]
- 665).Blekkenhorst LC, Prince RL, Hodgson JM, Lim WH, Zhu K, Devine A, Thompson PL, Lewis JR: Dietary saturated fat intake and atherosclerotic vascular disease mortality in elderly women: a prospective cohort study. Am J Clin Nutr, 2015; 101: 1263-1268 [DOI] [PubMed] [Google Scholar]
- 666).Puaschitz NG, Strand E, Norekvål TM, Dierkes J, Dahl L, Svingen GF, Assmus J, Schartum-Hansen H, Øyen J, Pedersen EK, Drevon CA, Tell GS, Nygård O: Dietary intake of saturated fat is not associated with risk of coronary events or mortality in patients with established coronary artery disease. J Nutr, 2015; 145: 299-305 [DOI] [PubMed] [Google Scholar]
- 667).Siri-Tarino PW, Sun Q, Hu FB, Krauss RM: Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr, 2010; 91: 535-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668).Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, Willett WC, Hu FB: Association of Specific Dietary Fats With Total and Cause-Specific Mortality. JAMA Intern Med, 2016; 176: 1134-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669).de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, Anand SS: Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ, 2015; 351: h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670).Hooper L, Martin N, Abdelhamid A, Davey Smith G: Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev, 2015 [DOI] [PubMed] [Google Scholar]
- 671).Mozaffarian D, Micha R, Wallace S: Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med, 2010; 7: e1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672).Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H: Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr, 2017; 105: 1462-1473 [DOI] [PubMed] [Google Scholar]
- 673).Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger S, Schwingshackl L: Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr, 2019; 59: 1071-1090 [DOI] [PubMed] [Google Scholar]
- 674).Nagao M, Iso H, Yamagishi K, Date C, Tamakoshi A: Meat consumption in relation to mortality from cardiovascular disease among Japanese men and women. Eur J Clin Nutr, 2012; 66: 687-693 [DOI] [PubMed] [Google Scholar]
- 675).Horikawa C, Kamada C, Tanaka S, Tanaka S, Araki A, Ito H, Matsunaga S, Fujihara K, Yoshimura Y, Ohashi Y, Akanuma Y, Sone H: Meat intake and incidence of cardiovascular disease in Japanese patients with type 2 diabetes: analysis of the Japan Diabetes Complications Study (JDCS). Eur J Nutr, 2019; 58: 281-290 [DOI] [PubMed] [Google Scholar]
- 676).Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu F, Hennekens CH, Colditz GA, Speizer FE, Willett WC: Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation, 2001; 103: 856-863 [DOI] [PubMed] [Google Scholar]
- 677).Iso H, Sato S, Kitamura A, Naito Y, Shimamoto T, Komachi Y: Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol, 2003; 157: 32-39 [DOI] [PubMed] [Google Scholar]
- 678).Yamagishi K, Iso H, Yatsuya H, Tanabe N, Date C, Kikuchi S, Yamamoto A, Inaba Y, Tamakoshi A: Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am J Clin Nutr, 2010; 92: 759-765 [DOI] [PubMed] [Google Scholar]
- 679).Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Tsugane S: Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC Study. Eur Heart J, 2013; 34: 1225-1232 [DOI] [PubMed] [Google Scholar]
- 680).Muto M, Ezaki O: High Dietary Saturated Fat is Associated with a Low Risk of Intracerebral Hemorrhage and Ischemic Stroke in Japanese but not in Non-Japanese: A Review and Meta-Analysis of Prospective Cohort Studies. J Atheroscler Thromb, 2018; 25: 375-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681).Nakamura Y, Okuda N, Turin TC, Fujiyoshi A, Okamura T, Hayakawa T, Yoshita K, Miura K, Ueshima H: Fatty acids intakes and serum lipid profiles: NIPPON DATA90 and the national nutrition monitoring. J Epidemiol, 2010; 20 Suppl 3: S544-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682).Fattore E, Bosetti C, Brighenti F, Agostoni C, Fattore G: Palm oil and blood lipid-related markers of cardiovascular disease: a systematic review and meta-analysis of dietary intervention trials. Am J Clin Nutr, 2014; 99: 1331-1350 [DOI] [PubMed] [Google Scholar]
- 683).Engel S, Tholstrup T: Butter increased total and LDL cholesterol compared with olive oil but resulted in higher HDL cholesterol compared with a habitual diet. Am J Clin Nutr, 2015; 102: 309-315 [DOI] [PubMed] [Google Scholar]
- 684).Vafeiadou K, Weech M, Altowaijri H, Todd S, Yaqoob P, Jackson KG, Lovegrove JA: Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr, 2015; 102: 40-48 [DOI] [PubMed] [Google Scholar]
- 685).Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed R, Stewart K, Stewart P, Phillips K, Anderson N: Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1. Arterioscler Thromb Vasc Biol, 1998; 18: 441-449 [DOI] [PubMed] [Google Scholar]
- 686).Barr SL, Ramakrishnan R, Johnson C, Holleran S, Dell RB, Ginsberg HN: Reducing total dietary fat without reducing saturated fatty acids does not significantly lower total plasma cholesterol concentrations in normal males. Am J Clin Nutr, 1992; 55: 675-681 [DOI] [PubMed] [Google Scholar]
- 687).Wardlaw GM, Snook JT: Effect of diets high in butter, corn oil, or high-oleic acid sunflower oil on serum lipids and apolipoproteins in men. Am J Clin Nutr, 1990; 51: 815-821 [DOI] [PubMed] [Google Scholar]
- 688).Temme EH, Mensink RP, Hornstra G: Comparison of the effects of diets enriched in lauric, palmitic, or oleic acids on serum lipids and lipoproteins in healthy women and men. Am J Clin Nutr, 1996; 63: 897-903 [DOI] [PubMed] [Google Scholar]
- 689).Bergeron N, Chiu S, Williams PT, S MK, Krauss RM: Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. Am J Clin Nutr, 2019; 110: 24-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690).Haub MD, Wells AM, Campbell WW: Beef and soy-based food supplements differentially affect serum lipoprotein-lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive-train. Metabolism, 2005; 54: 769-774 [DOI] [PubMed] [Google Scholar]
- 691).Jensen RG: The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci, 2002; 85: 295-350 [DOI] [PubMed] [Google Scholar]
- 692).Willett WC, Ludwig DS: Milk and Health. N Engl J Med, 2020; 382: 644-654 [DOI] [PubMed] [Google Scholar]
- 693).Huang LY, Wahlqvist ML, Huang YC, Lee MS: Optimal dairy intake is predicated on total, cardiovascular, and stroke mortalities in a Taiwanese cohort. J Am Coll Nutr, 2014; 33: 426-436 [DOI] [PubMed] [Google Scholar]
- 694).Yu E, Hu FB: Dairy Products, Dairy Fatty Acids, and the Prevention of Cardiometabolic Disease: a Review of Recent Evidence. Curr Atheroscler Rep, 2018; 20: 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695).Roy SJ, Lapierre SS, Baker BD, Delfausse LA, Machin DR, Tanaka H: High dietary intake of whole milk and full-fat dairy products does not exert hypotensive effects in adults with elevated blood pressure. Nutr Res, 2019; 64: 72-81 [DOI] [PubMed] [Google Scholar]
- 696).Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA: Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: a randomized trial. J Am Coll Nutr, 2007; 26: 669-677 [DOI] [PubMed] [Google Scholar]
- 697).Khaw KT, Sharp SJ, Finikarides L, Afzal I, Lentjes M, Luben R, Forouhi NG: Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open, 2018; 8: e020167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698).Chisholm A, Mann J, Sutherland W, Duncan A, Skeaff M, Frampton C: Effect on lipoprotein profile of replacing butter with margarine in a low fat diet: randomised crossover study with hypercholesterolaemic subjects. BMJ, 1996; 312: 931-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699).Hendrie GA, Golley RK: Changing from regular-fat to low-fat dairy foods reduces saturated fat intake but not energy intake in 4-13-y-old children. Am J Clin Nutr, 2011; 93: 1117-1127 [DOI] [PubMed] [Google Scholar]
- 700).Hidaka H, Takiwaki M, Yamashita M, Kawasaki K, Sugano M, Honda T: Consumption of nonfat milk results in a less atherogenic lipoprotein profile: a pilot study. Ann Nutr Metab, 2012; 61: 111-116 [DOI] [PubMed] [Google Scholar]
- 701).Villalpando S, Lara Zamudio Y, Shamah-Levy T, Mundo-Rosas V, Manzano AC, Lamadrid-Figueroa H: Substitution of whole cows’ milk with defatted milk for 4 months reduced serum total cholesterol, HDL-cholesterol and total apoB in a sample of Mexican school-age children (6-16 years of age). Br J Nutr, 2015; 114: 788-795 [DOI] [PubMed] [Google Scholar]
- 702).Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM: Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet, 1989; 2: 757-761 [DOI] [PubMed] [Google Scholar]
- 703).Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC: Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr, 2003; 57: 193-200 [DOI] [PubMed] [Google Scholar]
- 704).Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B: Omega 3 Fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes, 2012; 5: 808-818 [DOI] [PubMed] [Google Scholar]
- 705).Balk EM, Adams GP, Langberg V, Halladay C, Chung M, Lin L, Robertson S, Yip A, Steele D, Smith BT, Lau J, Lichtenstein AH, Trikalinos TA: Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systematic Review. Evid Rep Technol Assess (Full Rep), 2016: 1-1252 [DOI] [PubMed] [Google Scholar]
- 706).Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, AlAbdulghafoor FK, Summerbell CD, Worthington HV, Song F, Hooper L: Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2018; 11: Cd003177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707).Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R: Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol, 2018; 3: 225-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708).Hu Y, Hu FB, Manson JE: Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J Am Heart Assoc, 2019; 8: e013543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709).Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC: A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin Proc, 2017; 92: 15-29 [DOI] [PubMed] [Google Scholar]
- 710).Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE: Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA, 2002; 287: 1815-1821 [DOI] [PubMed] [Google Scholar]
- 711).Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC: Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA, 2001; 285: 304-312 [DOI] [PubMed] [Google Scholar]
- 712).Strøm M, Halldorsson TI, Mortensen EL, Torp-Pedersen C, Olsen SF: Fish, n-3 fatty acids, and cardiovascular diseases in women of reproductive age: a prospective study in a large national cohort. Hypertension, 2012; 59: 36-43 [DOI] [PubMed] [Google Scholar]
- 713).de Goede J, Geleijnse JM, Boer JM, Kromhout D, Verschuren WM: Marine (n-3) fatty acids, fish consumption, and the 10-year risk of fatal and nonfatal coronary heart disease in a large population of Dutch adults with low fish intake. J Nutr, 2010; 140: 1023-1028 [DOI] [PubMed] [Google Scholar]
- 714).Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC: Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med, 1995; 332: 977-982 [DOI] [PubMed] [Google Scholar]
- 715).Manger MS, Strand E, Ebbing M, Seifert R, Refsum H, Nordrehaug JE, Nilsen DW, Drevon CA, Tell GS, Bleie O, Vollset SE, Pedersen ER, Nygård O: Dietary intake of n-3 long-chain polyunsaturated fatty acids and coronary events in Norwegian patients with coronary artery disease. Am J Clin Nutr, 2010; 92: 244-251 [DOI] [PubMed] [Google Scholar]
- 716).Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D: Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: the Zutphen study. Eur Heart J, 2008; 29: 2024-2030 [DOI] [PubMed] [Google Scholar]
- 717).Lentjes MAH, Keogh RH, Welch AA, Mulligan AA, Luben RN, Wareham NJ, Khaw KT: Longitudinal associations between marine omega-3 supplement users and coronary heart disease in a UK population-based cohort. BMJ Open, 2017; 7: e017471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718).Saber H, Yakoob MY, Shi P, Longstreth WT, Jr., Lemaitre RN, Siscovick D, Rexrode KM, Willett WC, Mozaffarian D: Omega-3 Fatty Acids and Incident Ischemic Stroke and Its Atherothrombotic and Cardioembolic Subtypes in 3 US Cohorts. Stroke, 2017; 48: 2678-2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719).Zhuang P, Zhang Y, He W, Chen X, Chen J, He L, Mao L, Wu F, Jiao J: Dietary Fats in Relation to Total and Cause-Specific Mortality in a Prospective Cohort of 521 120 Individuals With 16 Years of Follow-Up. Circ Res, 2019; 124: 757-768 [DOI] [PubMed] [Google Scholar]
- 720).Koh AS, Pan A, Wang R, Odegaard AO, Pereira MA, Yuan JM, Koh WP: The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol, 2015; 22: 364-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721).Venø SK, Bork CS, Jakobsen MU, Lundbye-Christensen S, McLennan PL, Bach FW, Overvad K, Schmidt EB: Marine n-3 Polyunsaturated Fatty Acids and the Risk of Ischemic Stroke. Stroke, 2019; 50: 274-282 [DOI] [PubMed] [Google Scholar]
- 722).Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S: Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC) Study Cohort I. Circulation, 2006; 113: 195-202 [DOI] [PubMed] [Google Scholar]
- 723).Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A: Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol, 2008; 52: 988-996 [DOI] [PubMed] [Google Scholar]
- 724).Miyagawa N, Miura K, Okuda N, Kadowaki T, Takashima N, Nagasawa SY, Nakamura Y, Matsumura Y, Hozawa A, Fujiyoshi A, Hisamatsu T, Yoshita K, Sekikawa A, Ohkubo T, Abbott RD, Okamura T, Okayama A, Ueshima H: Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: a 24-year follow-up of NIPPON DATA80. Atherosclerosis, 2014; 232: 384-389 [DOI] [PubMed] [Google Scholar]
- 725).Cheng P, Huang W, Bai S, Wu Y, Yu J, Zhu X, Qi Z, Shao W, Xie P: BMI Affects the Relationship between Long Chain N-3 Polyunsaturated Fatty Acid Intake and Stroke Risk: a Meta-Analysis. Sci Rep, 2015; 5: 14161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726).Leslie MA, Cohen DJ, Liddle DM, Robinson LE, Ma DW: A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis, 2015; 14: 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727).Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A: Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol, 2009; 136: 4-16 [DOI] [PubMed] [Google Scholar]
- 728).Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J: Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis, 2006; 189: 19-30 [DOI] [PubMed] [Google Scholar]
- 729).Agren JJ, Hänninen O, Julkunen A, Fogelholm L, Vidgren H, Schwab U, Pynnönen O, Uusitupa M: Fish diet, fish oil and docosahexaenoic acid rich oil lower fasting and postprandial plasma lipid levels. Eur J Clin Nutr, 1996; 50: 765-771 [PubMed] [Google Scholar]
- 730).Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK: Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation, 2019; 140: e673-e691 [DOI] [PubMed] [Google Scholar]
- 731).Bork CS, Venø SK, Lundbye-Christensen S, Jakobsen MU, Tjønneland A, Schmidt EB, Overvad K: Dietary Intake of α-Linolenic Acid Is Not Appreciably Associated with Risk of Ischemic Stroke among Middle-Aged Danish Men and Women. J Nutr, 2018; 148: 952-958 [DOI] [PubMed] [Google Scholar]
- 732).Bork CS, Lasota AN, Lundbye-Christensen S, Jakobsen MU, Tjønneland A, Calder PC, Schmidt EB, Overvad K: Intake of α-linolenic acid is not consistently associated with a lower risk of peripheral artery disease: results from a Danish cohort study. Br J Nutr, 2019; 122: 86-92 [DOI] [PubMed] [Google Scholar]
- 733).Wei J, Hou R, Xi Y, Kowalski A, Wang T, Yu Z, Hu Y, Chandrasekar EK, Sun H, Ali MK: The association and dose-response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br J Nutr, 2018; 119: 83-89 [DOI] [PubMed] [Google Scholar]
- 734).Zhou Q, Zhang Z, Wang P, Zhang B, Chen C, Zhang C, Su Y: EPA+DHA, but not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mol Nutr Food Res, 2019; 63: e1801157 [DOI] [PubMed] [Google Scholar]
- 735).Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS: Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev, 2020; 5: Cd011737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736).de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Jr., Mozaffarian D: Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2013; 2: e000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737).Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E: Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med, 2014; 160: 398-406 [DOI] [PubMed] [Google Scholar]
- 738).Hamley S: The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: a meta-analysis of randomised controlled trials. Nutr J, 2017; 16: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739).Hooper L, Al-Khudairy L, Abdelhamid AS, Rees K, Brainard JS, Brown TJ, Ajabnoor SM, O’Brien AT, Winstanley LE, Donaldson DH, Song F, Deane KH: Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2018; 11: Cd011094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740).Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB: Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation, 2014; 130: 1568-1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741).Schwingshackl L, Hoffmann G: Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis, 2014; 13: 154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742).Zong G, Li Y, Sampson L, Dougherty LW, Willett WC, Wanders AJ, Alssema M, Zock PL, Hu FB, Sun Q: Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr, 2018; 107: 445-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743).Foley M, Ball M, Chisholm A, Duncan A, Spears G, Mann J: Should mono- or poly-unsaturated fats replace saturated fat in the diet? Eur J Clin Nutr, 1992; 46: 429-436 [PubMed] [Google Scholar]
- 744).Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, Pearson TA, Dennis BH, Roheim PS, Ramakrishnan R, Reed R, Stewart K, Phillips KM: Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr, 2007; 86: 1611-1620 [DOI] [PubMed] [Google Scholar]
- 745).Mensink RP, Katan MB: Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb, 1992; 12: 911-919 [DOI] [PubMed] [Google Scholar]
- 746).Ginsberg HN, Barr SL, Gilbert A, Karmally W, Deckelbaum R, Kaplan K, Ramakrishnan R, Holleran S, Dell RB: Reduction of plasma cholesterol levels in normal men on an American Heart Association Step 1 diet or a Step 1 diet with added monounsaturated fat. N Engl J Med, 1990; 322: 574-579 [DOI] [PubMed] [Google Scholar]
- 747).Thijssen MA, Mensink RP: Small differences in the effects of stearic acid, oleic acid, and linoleic acid on the serum lipoprotein profile of humans. Am J Clin Nutr, 2005; 82: 510-516 [DOI] [PubMed] [Google Scholar]
- 748).Schwingshackl L, Strasser B, Hoffmann G: Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab, 2011; 59: 176-186 [DOI] [PubMed] [Google Scholar]
- 749).de Roos B, Wanders AJ, Wood S, Horgan G, Rucklige G, Reid M, Siebelink E, Brouwer IA: A high intake of industrial or ruminant trans fatty acids does not affect the plasma proteome in healthy men. Proteomics, 2011; 11: 3928-3934 [DOI] [PubMed] [Google Scholar]
- 750).Wanders AJ, Brouwer IA, Siebelink E, Katan MB: Effect of a high intake of conjugated linoleic acid on lipoprotein levels in healthy human subjects. PLoS One, 2010; 5: e9000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751).Lacroix E, Charest A, Cyr A, Baril-Gravel L, Lebeuf Y, Paquin P, Chouinard PY, Couture P, Lamarche B: Randomized controlled study of the effect of a butter naturally enriched in trans fatty acids on blood lipids in healthy women. Am J Clin Nutr, 2012; 95: 318-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752).Gayet-Boyer C, Tenenhaus-Aziza F, Prunet C, Marmonier C, Malpuech-Brugère C, Lamarche B, Chardigny JM: Is there a linear relationship between the dose of ruminant trans-fatty acids and cardiovascular risk markers in healthy subjects: results from a systematic review and meta-regression of randomised clinical trials. Br J Nutr, 2014; 112: 1914-1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753).Mori K, Ishida T, Yasuda T, Hasokawa M, Monguchi T, Sasaki M, Kondo K, Nakajima H, Shinohara M, Shinke T, Irino Y, Toh R, Nishimura K, Hirata K: Serum Trans-Fatty Acid Concentration Is Elevated in Young Patients With Coronary Artery Disease in Japan. Circ J, 2015; 79: 2017-2025 [DOI] [PubMed] [Google Scholar]
- 754).Nagasawa Y, Shinke T, Toh R, Ishida T, Otake H, Takaya T, Sugiyama D, Toba T, Kuroda M, Takahashi H, Terashita D, Tahara N, Shinkura Y, Uzu K, Kashiwagi D, Kuroda K, Nagano Y, Yamamoto H, Yanaka K, Tsukiyama Y, Hirata KI: The impact of serum trans fatty acids concentration on plaque vulnerability in patients with coronary artery disease: Assessment via optical coherence tomography. Atherosclerosis, 2017; 265: 312-317 [DOI] [PubMed] [Google Scholar]
- 755).Honda T, Ohara T, Shinohara M, Hata J, Toh R, Yoshida D, Shibata M, Ishida T, Hirakawa Y, Irino Y, Sakata S, Uchida K, Kitazono T, Kanba S, Hirata KI, Ninomiya T: Serum elaidic acid concentration and risk of dementia: The Hisayama Study. Neurology, 2019; 93: e2053-e2064 [DOI] [PubMed] [Google Scholar]
- 756).Mozaffarian D, Aro A, Willett WC: Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr, 2009; 63 Suppl 2: S5-21 [DOI] [PubMed] [Google Scholar]
- 757).Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J: Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol, 1997; 145: 876-887 [DOI] [PubMed] [Google Scholar]
- 758).Oomen CM, Ocké MC, Feskens EJ, van Erp-Baart MA, Kok FJ, Kromhout D: Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet, 2001; 357: 746-751 [DOI] [PubMed] [Google Scholar]
- 759).Mozaffarian D, Clarke R: Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr, 2009; 63 Suppl 2: S22-33 [DOI] [PubMed] [Google Scholar]
- 760).Nestel P, Noakes M, Belling B, McArthur R, Clifton P, Janus E, Abbey M: Plasma lipoprotein lipid and Lp[a] changes with substitution of elaidic acid for oleic acid in the diet. J Lipid Res, 1992; 33: 1029-1036 [PubMed] [Google Scholar]
- 761).Vega-López S, Ausman LM, Jalbert SM, Erkkilä AT, Lichtenstein AH: Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr, 2006; 84: 54-62 [DOI] [PubMed] [Google Scholar]
- 762).Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA: trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr, 2013; 97: 854-861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763).Aro A, Jauhiainen M, Partanen R, Salminen I, Mutanen M: Stearic acid, trans fatty acids, and dairy fat: effects on serum and lipoprotein lipids, apolipoproteins, lipoprotein(a), and lipid transfer proteins in healthy subjects. Am J Clin Nutr, 1997; 65: 1419-1426 [DOI] [PubMed] [Google Scholar]
- 764).Mensink RP, Zock PL, Katan MB, Hornstra G: Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J Lipid Res, 1992; 33: 1493-1501 [PubMed] [Google Scholar]
- 765).Gebauer SK, Destaillats F, Dionisi F, Krauss RM, Baer DJ: Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: a double-blind, randomized controlled trial. Am J Clin Nutr, 2015; 102: 1339-1346 [DOI] [PubMed] [Google Scholar]
- 766).Ministry of Agriculture, Forestry and Fisheries: Information on trans fatty acids, https://www.maff.go.jp/j/syouan/seisaku/trans_fat/ 2022, (in Japanese) [Google Scholar]
- 767).Joint-WHO/FAO-ExpertConsultation: Diet, Nutrition and the Prevention of Chonic Diseases, http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf;jsessionid=3C16B0B5645DCBA1E6146E7F4C1761F0?sequence=1, 2003 [Google Scholar]
- 768).Uauy R, Aro A, Clarke R, Ghafoorunissa., L’Abbe M, Mozaffarian D, Skeaff M, Stender S, Tacke M: WHO Scientific Update on trans fatty acids: summary and conclusions. Eur J Clin Nutr, 2009; 63: S68–S75 [Google Scholar]
- 769).McGee D, Reed D, Stemmerman G, Rhoads G, Yano K, Feinleib M: The relationship of dietary fat and cholesterol to mortality in 10 years: the Honolulu Heart Program. Int J Epidemiol, 1985; 14: 97-105 [DOI] [PubMed] [Google Scholar]
- 770).Posner BM, Cobb JL, Belanger AJ, Cupples LA, D’Agostino RB, Stokes J, 3rd: Dietary lipid predictors of coronary heart disease in men. The Framingham Study. Arch Intern Med, 1991; 151: 1181-1187 [PubMed] [Google Scholar]
- 771).Xu J, Eilat-Adar S, Loria C, Goldbourt U, Howard BV, Fabsitz RR, Zephier EM, Mattil C, Lee ET: Dietary fat intake and risk of coronary heart disease: the Strong Heart Study. Am J Clin Nutr, 2006; 84: 894-902 [DOI] [PubMed] [Google Scholar]
- 772).Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM, Allen NB: Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality. JAMA, 2019; 321: 1081-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773).Li Y, Zhou C, Zhou X, Li L: Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis, 2013; 229: 524-530 [DOI] [PubMed] [Google Scholar]
- 774).Millen BE, Franz MM, Quatromoni PA, Gagnon DR, Sonnenberg LM, Ordovas JM, Wilson PW, Schaefer EJ, Cupples LA: Diet and plasma lipids in women. I. Macronutrients and plasma total and low-density lipoprotein cholesterol in women: the Framingham nutrition studies. J Clin Epidemiol, 1996; 49: 657-663 [DOI] [PubMed] [Google Scholar]
- 775).Johnson C, Greenland P: Effects of exercise, dietary cholesterol, and dietary fat on blood lipids. Arch Intern Med, 1990; 150: 137-141 [PubMed] [Google Scholar]
- 776).Fielding CJ, Havel RJ, Todd KM, Yeo KE, Schloetter MC, Weinberg V, Frost PH: Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young men. J Clin Invest, 1995; 95: 611-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777).Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ: Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr, 2015; 102: 276-294 [DOI] [PubMed] [Google Scholar]
- 778).Nicklas BJ, Katzel LI, Bunyard LB, Dennis KE, Goldberg AP: Effects of an American Heart Association diet and weight loss on lipoprotein lipids in obese, postmenopausal women. Am J Clin Nutr, 1997; 66: 853-859 [DOI] [PubMed] [Google Scholar]
- 779).Dengel JL, Katzel LI, Goldberg AP: Effect of an American Heart Association diet, with or without weight loss, on lipids in obese middle-aged and older men. Am J Clin Nutr, 1995; 62: 715-721 [DOI] [PubMed] [Google Scholar]
- 780).Lichtenstein AH, Ausman LM, Jalbert SM, Vilella-Bach M, Jauhiainen M, McGladdery S, Erkkilä AT, Ehnholm C, Frohlich J, Schaefer EJ: Efficacy of a Therapeutic Lifestyle Change/Step 2 diet in moderately hypercholesterolemic middle-aged and elderly female and male subjects. J Lipid Res, 2002; 43: 264-273 [PubMed] [Google Scholar]
- 781).Rivellese AA, Auletta P, Marotta G, Saldalamacchia G, Giacco A, Mastrilli V, Vaccaro O, Riccardi G: Long term metabolic effects of two dietary methods of treating hyperlipidaemia. BMJ, 1994; 308: 227-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782).Vincent MJ, Allen B, Palacios OM, Haber LT, Maki KC: Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am J Clin Nutr, 2019; 109: 7-16 [DOI] [PubMed] [Google Scholar]
- 783).Katan MB, Beynen AC, de Vries JH, Nobels A: Existence of consistent hypo- and hyperresponders to dietary cholesterol in man. Am J Epidemiol, 1986; 123: 221-234 [DOI] [PubMed] [Google Scholar]
- 784).Djoussé L, Gaziano JM: Dietary cholesterol and coronary artery disease: a systematic review. Curr Atheroscler Rep, 2009; 11: 418-422 [DOI] [PubMed] [Google Scholar]
- 785).Clifton PM, Kestin M, Abbey M, Drysdale M, Nestel PJ: Relationship between sensitivity to dietary fat and dietary cholesterol. Arteriosclerosis, 1990; 10: 394-401 [DOI] [PubMed] [Google Scholar]
- 786).Ginsberg HN, Karmally W, Siddiqui M, Holleran S, Tall AR, Rumsey SC, Deckelbaum RJ, Blaner WS, Ramakrishnan R: A dose-response study of the effects of dietary cholesterol on fasting and postprandial lipid and lipoprotein metabolism in healthy young men. Arterioscler Thromb, 1994; 14: 576-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787).Chakrabarty G, Manjunatha S, Bijlani RL, Ray RB, Mahapatra SC, Mehta N, Lakshmy R, Vashisht S, Manchanda SC: The effect of ingestion of egg on the serum lipid profile of healthy young Indians. Indian J Physiol Pharmacol, 2004; 48: 286-292 [PubMed] [Google Scholar]
- 788).Herron KL, Vega-Lopez S, Conde K, Ramjiganesh T, Shachter NS, Fernandez ML: Men classified as hypo- or hyperresponders to dietary cholesterol feeding exhibit differences in lipoprotein metabolism. J Nutr, 2003; 133: 1036-1042 [DOI] [PubMed] [Google Scholar]
- 789).Flaim E, Ferreri LF, Thye FW, Hill JE, Ritchey SJ: Plasma lipid and lipoprotein cholesterol concentrations in adult males consuming normal and high cholesterol diets under controlled conditions. Am J Clin Nutr, 1981; 34: 1103-1108 [DOI] [PubMed] [Google Scholar]
- 790).Sacks FM, Salazar J, Miller L, Foster JM, Sutherland M, Samonds KW, Albers JJ, Kass EH: Ingestion of egg raises plasma low density lipoproteins in free-living subjects. Lancet, 1984; 1: 647-649 [DOI] [PubMed] [Google Scholar]
- 791).Roberts SL, McMurry MP, Connor WE: Does egg feeding (i.e., dietary cholesterol) affect plasma cholesterol levels in humans? The results of a double-blind study. Am J Clin Nutr, 1981; 34: 2092-2099 [DOI] [PubMed] [Google Scholar]
- 792).Knopp RH, Retzlaff BM, Walden CE, Dowdy AA, Tsunehara CH, Austin MA, Nguyen T: A double-blind, randomized, controlled trial of the effects of two eggs per day in moderately hypercholesterolemic and combined hyperlipidemic subjects taught the NCEP step I diet. J Am Coll Nutr, 1997; 16: 551-561 [PubMed] [Google Scholar]
- 793).Severins N, Mensink RP, Plat J: Effects of lutein-enriched egg yolk in buttermilk or skimmed milk on serum lipids & lipoproteins of mildly hypercholesterolemic subjects. Nutr Metab Cardiovasc Dis, 2015; 25: 210-217 [DOI] [PubMed] [Google Scholar]
- 794).Baumgartner S, Kelly ER, van der Made S, Berendschot TT, Husche C, Lütjohann D, Plat J: The influence of consuming an egg or an egg-yolk buttermilk drink for 12 wk on serum lipids, inflammation, and liver function markers in human volunteers. Nutrition, 2013; 29: 1237-1244 [DOI] [PubMed] [Google Scholar]
- 795).Flynn MA, Nolph GB, Flynn TC, Kahrs R, Krause G: Effect of dietary egg on human serum cholesterol and triglycerides. Am J Clin Nutr, 1979; 32: 1051-1057 [DOI] [PubMed] [Google Scholar]
- 796).Fuller NR, Caterson ID, Sainsbury A, Denyer G, Fong M, Gerofi J, Baqleh K, Williams KH, Lau NS, Markovic TP: The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: the Diabetes and Egg (DIABEGG) study-a 3-mo randomized controlled trial. Am J Clin Nutr, 2015; 101: 705-713 [DOI] [PubMed] [Google Scholar]
- 797).Katz DL, Gnanaraj J, Treu JA, Ma Y, Kavak Y, Njike VY: Effects of egg ingestion on endothelial function in adults with coronary artery disease: a randomized, controlled, crossover trial. Am Heart J, 2015; 169: 162-169 [DOI] [PubMed] [Google Scholar]
- 798).Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML: Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism, 2013; 62: 400-410 [DOI] [PubMed] [Google Scholar]
- 799).Pearce KL, Clifton PM, Noakes M: Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br J Nutr, 2011; 105: 584-592 [DOI] [PubMed] [Google Scholar]
- 800).Weggemans RM, Zock PL, Katan MB: Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a meta-analysis. Am J Clin Nutr, 2001; 73: 885-891 [DOI] [PubMed] [Google Scholar]
- 801).Rouhani MH, Rashidi-Pourfard N, Salehi-Abargouei A, Karimi M, Haghighatdoost F: Effects of Egg Consumption on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Am Coll Nutr, 2018; 37: 99-110 [DOI] [PubMed] [Google Scholar]
- 802).Hu FB, Stampfer MJ, Rimm EB, Manson JE, Ascherio A, Colditz GA, Rosner BA, Spiegelman D, Speizer FE, Sacks FM, Hennekens CH, Willett WC: A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA, 1999; 281: 1387-1394 [DOI] [PubMed] [Google Scholar]
- 803).Qureshi AI, Suri FK, Ahmed S, Nasar A, Divani AA, Kirmani JF: Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med Sci Monit, 2007; 13: Cr1-8 [PubMed] [Google Scholar]
- 804).Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, Newman AB, Visser M, Kritchevsky SB: Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC Study. Nutr Metab Cardiovasc Dis, 2011; 21: 430-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805).Shin JY, Xun P, Nakamura Y, He K: Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr, 2013; 98: 146-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806).Soliman GA: Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients, 2019; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807).Wang Y, Harding SV, Thandapilly SJ, Tosh SM, Jones PJH, Ames NP: Barley β-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br J Nutr, 2017; 118: 822-829 [DOI] [PubMed] [Google Scholar]
- 808).Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Wakai K, Tamakoshi A: Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr, 2010; 140: 1445-1453 [DOI] [PubMed] [Google Scholar]
- 809).Kokubo Y, Iso H, Saito I, Yamagishi K, Ishihara J, Inoue M, Tsugane S: Dietary fiber intake and risk of cardiovascular disease in the Japanese population: the Japan Public Health Center-based study cohort. Eur J Clin Nutr, 2011; 65: 1233-1241 [DOI] [PubMed] [Google Scholar]
- 810).Yang Y, Zhao LG, Wu QJ, Ma X, Xiang YB: Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J Epidemiol, 2015; 181: 83-91 [DOI] [PubMed] [Google Scholar]
- 811).Liu L, Wang S, Liu J: Fiber consumption and all-cause, cardiovascular, and cancer mortalities: a systematic review and meta-analysis of cohort studies. Mol Nutr Food Res, 2015; 59: 139-146 [DOI] [PubMed] [Google Scholar]
- 812).Hajishafiee M, Saneei P, Benisi-Kohansal S, Esmaillzadeh A: Cereal fibre intake and risk of mortality from all causes, CVD, cancer and inflammatory diseases: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr, 2016; 116: 343-352 [DOI] [PubMed] [Google Scholar]
- 813).Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L: Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet, 2019; 393: 434-445 [DOI] [PubMed] [Google Scholar]
- 814).Kim Y, Je Y: Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch Cardiovasc Dis, 2016; 109: 39-54 [DOI] [PubMed] [Google Scholar]
- 815).Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ: Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ, 2013; 347: f6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816).Chen GC, Lv DB, Pang Z, Dong JY, Liu QF: Dietary fiber intake and stroke risk: a meta-analysis of prospective cohort studies. Eur J Clin Nutr, 2013; 67: 96-100 [DOI] [PubMed] [Google Scholar]
- 817).Zhang Z, Xu G, Liu D, Zhu W, Fan X, Liu X: Dietary fiber consumption and risk of stroke. Eur J Epidemiol, 2013; 28: 119-130 [DOI] [PubMed] [Google Scholar]
- 818).Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ: Dietary fiber intake and risk of first stroke: a systematic review and meta-analysis. Stroke, 2013; 44: 1360-1368 [DOI] [PubMed] [Google Scholar]
- 819).Hartley L, May MD, Loveman E, Colquitt JL, Rees K: Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2016; 2016: Cd011472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820).Whitehead A, Beck EJ, Tosh S, Wolever TM: Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am J Clin Nutr, 2014; 100: 1413-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821).Brown L, Rosner B, Willett WW, Sacks FM: Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr, 1999; 69: 30-42 [DOI] [PubMed] [Google Scholar]
- 822).Ho HV, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, Jenkins AL, Vuksan V: The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: a systematic review and meta-analysis of randomised-controlled trials. Br J Nutr, 2016; 116: 1369-1382 [DOI] [PubMed] [Google Scholar]
- 823).Ho HVT, Jovanovski E, Zurbau A, Blanco Mejia S, Sievenpiper JL, Au-Yeung F, Jenkins AL, Duvnjak L, Leiter L, Vuksan V: A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr, 2017; 105: 1239-1247 [DOI] [PubMed] [Google Scholar]
- 824).Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T: Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ, 2016; 353: i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825).Zong G, Gao A, Hu FB, Sun Q: Whole Grain Intake and Mortality From All Causes, Cardiovascular Disease, and Cancer: A Meta-Analysis of Prospective Cohort Studies. Circulation, 2016; 133: 2370-2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826).Zhang B, Zhao Q, Guo W, Bao W, Wang X: Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: a systematic review and dose-response meta-analysis from prospective cohort studies. Eur J Clin Nutr, 2018; 72: 57-65 [DOI] [PubMed] [Google Scholar]
- 827).Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q: Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med, 2015; 175: 373-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828).Tang G, Wang D, Long J, Yang F, Si L: Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol, 2015; 115: 625-629 [DOI] [PubMed] [Google Scholar]
- 829).Mellen PB, Walsh TF, Herrington DM: Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis, 2008; 18: 283-290 [DOI] [PubMed] [Google Scholar]
- 830).Muraki I, Wu H, Imamura F, Laden F, Rimm EB, Hu FB, Willett WC, Sun Q: Rice consumption and risk of cardiovascular disease: results from a pooled analysis of 3 U.S. cohorts. Am J Clin Nutr, 2015; 101: 164-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831).Eshak ES, Iso H, Yamagishi K, Kokubo Y, Saito I, Yatsuya H, Sawada N, Inoue M, Tsugane S: Rice consumption is not associated with risk of cardiovascular disease morbidity or mortality in Japanese men and women: a large population-based, prospective cohort study. Am J Clin Nutr, 2014; 100: 199-207 [DOI] [PubMed] [Google Scholar]
- 832).Eshak ES, Iso H, Date C, Yamagishi K, Kikuchi S, Watanabe Y, Wada Y, Tamakoshi A: Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr, 2011; 141: 595-602 [DOI] [PubMed] [Google Scholar]
- 833).Shimabukuro M, Higa M, Kinjo R, Yamakawa K, Tanaka H, Kozuka C, Yabiku K, Taira S, Sata M, Masuzaki H: Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr, 2014; 111: 310-320 [DOI] [PubMed] [Google Scholar]
- 834).Kondo K, Morino K, Nishio Y, Ishikado A, Arima H, Nakao K, Nakagawa F, Nikami F, Sekine O, Nemoto KI, Suwa M, Matsumoto M, Miura K, Makino T, Ugi S, Maegawa H: Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS One, 2017; 12: e0179869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835).Larsson SC, Wolk A: Potato consumption and risk of cardiovascular disease: 2 prospective cohort studies. Am J Clin Nutr, 2016; 104: 1245-1252 [DOI] [PubMed] [Google Scholar]
- 836).Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A: Mediterranean diet and CHD: the Greek European Prospective Investigation into Cancer and Nutrition cohort. Br J Nutr, 2012; 108: 699-709 [DOI] [PubMed] [Google Scholar]
- 837).Schwingshackl L, Schwedhelm C, Hoffmann G, Boeing H: Potatoes and risk of chronic disease: a systematic review and dose-response meta-analysis. Eur J Nutr, 2019; 58: 2243-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838).Hollænder PL, Ross AB, Kristensen M: Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr, 2015; 102: 556-572 [DOI] [PubMed] [Google Scholar]
- 839).Ho HV, Sievenpiper JL, Zurbau A, Blanco Mejia S, Jovanovski E, Au-Yeung F, Jenkins AL, Vuksan V: A systematic review and meta-analysis of randomized controlled trials of the effect of barley β-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reduction(i-iv). Eur J Clin Nutr, 2016; 70: 1239-1245 [DOI] [PubMed] [Google Scholar]
- 840).Hui S, Liu K, Lang H, Liu Y, Wang X, Zhu X, Doucette S, Yi L, Mi M: Comparative effects of different whole grains and brans on blood lipid: a network meta-analysis. Eur J Nutr, 2019; 58: 2779-2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841).Ripsin CM, Keenan JM, Jacobs DR, Jr., Elmer PJ, Welch RR, Van Horn L, Liu K, Turnbull WH, Thye FW, Kestin M, et al.: Oat products and lipid lowering. A meta-analysis. JAMA, 1992; 267: 3317-3325 [PubMed] [Google Scholar]
- 842).Li L, Lietz G, Seal C: Buckwheat and CVD Risk Markers: A Systematic Review and Meta-Analysis. Nutrients, 2018; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843).Vega-López S, Venn BJ, Slavin JL: Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients, 2018; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844).Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB: Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ, 2014; 349: g4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845).Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S: Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol, 2017; 46: 1029-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846).Gan Y, Tong X, Li L, Cao S, Yin X, Gao C, Herath C, Li W, Jin Z, Chen Y, Lu Z: Consumption of fruit and vegetable and risk of coronary heart disease: a meta-analysis of prospective cohort studies. Int J Cardiol, 2015; 183: 129-137 [DOI] [PubMed] [Google Scholar]
- 847).Dauchet L, Amouyel P, Dallongeville J: Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology, 2005; 65: 1193-1197 [DOI] [PubMed] [Google Scholar]
- 848).Hu D, Huang J, Wang Y, Zhang D, Qu Y: Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke, 2014; 45: 1613-1619 [DOI] [PubMed] [Google Scholar]
- 849).Yip CSC, Chan W, Fielding R: The Associations of Fruit and Vegetable Intakes with Burden of Diseases: A Systematic Review of Meta-Analyses. J Acad Nutr Diet, 2019; 119: 464-481 [DOI] [PubMed] [Google Scholar]
- 850).Li M, Fan Y, Zhang X, Hou W, Tang Z: Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open, 2014; 4: e005497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851).Okuda N, Miura K, Okayama A, Okamura T, Abbott RD, Nishi N, Fujiyoshi A, Kita Y, Nakamura Y, Miyagawa N, Hayakawa T, Ohkubo T, Kiyohara Y, Ueshima H: Fruit and vegetable intake and mortality from cardiovascular disease in Japan: a 24-year follow-up of the NIPPON DATA80 Study. Eur J Clin Nutr, 2015; 69: 482-488 [DOI] [PubMed] [Google Scholar]
- 852).Sauvaget C, Nagano J, Allen N, Kodama K: Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke, 2003; 34: 2355-2360 [DOI] [PubMed] [Google Scholar]
- 853).Yoshizaki T, Ishihara J, Kotemori A, Yamamoto J, Kokubo Y, Saito I, Yatsuya H, Yamagishi K, Sawada N, Iwasaki M, Iso H, Tsugane S: Association of Vegetable, Fruit, and Okinawan Vegetable Consumption With Incident Stroke and Coronary Heart Disease. J Epidemiol, 2020; 30: 37-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854).Filippini T, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, Moreira P, Malavolti M, Orsini N, Whelton PK, Vinceti M: Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc, 2020; 9: e015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855).Schwedhelm C, Schwingshackl L, Agogo GO, Sonestedt E, Boeing H, Knüppel S: Associations of food groups and cardiometabolic and inflammatory biomarkers: does the meal matter? Br J Nutr, 2019; 122: 707-716 [DOI] [PubMed] [Google Scholar]
- 856).Chung GKK, Yu RHY, Ho SSY, Woo J, Ho SC: Associations of consuming specific fruit and vegetable subgroups with LDL-C status in early postmenopausal Chinese women. Menopause, 2018; 25: 436-443 [DOI] [PubMed] [Google Scholar]
- 857).Hadi A, Askarpour M, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E: Effects of strawberry supplementation on cardiovascular risk factors: a comprehensive systematic review and meta-analysis of randomized controlled trials. Food Funct, 2019; 10: 6987-6998 [DOI] [PubMed] [Google Scholar]
- 858).Curtis PJ, van der Velpen V, Berends L, Jennings A, Feelisch M, Umpleby AM, Evans M, Fernandez BO, Meiss MS, Minnion M, Potter J, Minihane AM, Kay CD, Rimm EB, Cassidy A: Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am J Clin Nutr, 2019; 109: 1535-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859).Luís Â, Domingues F, Pereira L: Association between berries intake and cardiovascular diseases risk factors: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct, 2018; 9: 740-757 [DOI] [PubMed] [Google Scholar]
- 860).Huang H, Chen G, Liao D, Zhu Y, Xue X: Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci Rep, 2016; 6: 23625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861).Ghaedi E, Moradi S, Aslani Z, Kord-Varkaneh H, Miraghajani M, Mohammadi H: Effects of grape products on blood lipids: a systematic review and dose-response meta-analysis of randomized controlled trials. Food Funct, 2019; 10: 6399-6416 [DOI] [PubMed] [Google Scholar]
- 862).Tenore GC, Caruso D, Buonomo G, D'Urso E, D'Avino M, Campiglia P, Marinelli L, Novellino E: Annurca (Malus pumila Miller cv. Annurca) apple as a functional food for the contribution to a healthy balance of plasma cholesterol levels: results of a randomized clinical trial. J Sci Food Agric, 2017; 97: 2107-2115 [DOI] [PubMed] [Google Scholar]
- 863).Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, Leontowicz M, Tashma Z, Katrich E, Feng S, Trakhtenberg S: Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J Agric Food Chem, 2006; 54: 1887-1892 [DOI] [PubMed] [Google Scholar]
- 864).Gorinstein S, Caspi A, Libman I, Katrich E, Lerner HT, Trakhtenberg S: Preventive effects of diets supplemented with sweetie fruits in hypercholesterolemic patients suffering from coronary artery disease. Prev Med, 2004; 38: 841-847 [DOI] [PubMed] [Google Scholar]
- 865).Moazzen H, Alizadeh M: Effects of Pomegranate Juice on Cardiovascular Risk Factors in Patients with Metabolic Syndrome: a Double-Blinded, Randomized Crossover Controlled Trial. Plant Foods Hum Nutr, 2017; 72: 126-133 [DOI] [PubMed] [Google Scholar]
- 866).Gammon CS, Kruger R, Conlon CA, von Hurst PR, Jones B, Stonehouse W: Inflammatory status modulates plasma lipid and inflammatory marker responses to kiwifruit consumption in hypercholesterolaemic men. Nutr Metab Cardiovasc Dis, 2014; 24: 91-99 [DOI] [PubMed] [Google Scholar]
- 867).Toh DWK, Koh ES, Kim JE: Incorporating healthy dietary changes in addition to an increase in fruit and vegetable intake further improves the status of cardiovascular disease risk factors: A systematic review, meta-regression, and meta-analysis of randomized controlled trials. Nutr Rev, 2020; 78: 532-545 [DOI] [PubMed] [Google Scholar]
- 868).Shin JY, Kim JY, Kang HT, Han KH, Shim JY: Effect of fruits and vegetables on metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Int J Food Sci Nutr, 2015; 66: 416-425 [DOI] [PubMed] [Google Scholar]
- 869).Committee for the Revision of Guidelines of the Japanese Society of Gout and Uric & nucleic Acids: Guideline for the management of hyperuricemia and gout. Ver3. 2019, Shindan to Chiryo sha, 2019 (in Japanese) [Google Scholar]
- 870).Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, Ueshima H, Kesteloot H, Miura K, Curb JD, Yoshita K, Elliott P, Yamamoto ME, Stamler J: Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc, 2010; 110: 736-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871).Miura K, Okuda N, Turin TC, Takashima N, Nakagawa H, Nakamura K, Yoshita K, Okayama A, Ueshima H: Dietary salt intake and blood pressure in a representative Japanese population: baseline analyses of NIPPON DATA80. J Epidemiol, 2010; 20 Suppl 3: S524-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872).Kwok CS, Gulati M, Michos ED, Potts J, Wu P, Watson L, Loke YK, Mallen C, Mamas MA: Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prev Cardiol, 2019; 26: 1415-1429 [DOI] [PubMed] [Google Scholar]
- 873).Khan TA, Tayyiba M, Agarwal A, Mejia SB, de Souza RJ, Wolever TMS, Leiter LA, Kendall CWC, Jenkins DJA, Sievenpiper JL: Relation of Total Sugars, Sucrose, Fructose, and Added Sugars With the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin Proc, 2019; 94: 2399-2414 [DOI] [PubMed] [Google Scholar]
- 874).Micha R, Shulkin ML, Peñalvo JL, Khatibzadeh S, Singh GM, Rao M, Fahimi S, Powles J, Mozaffarian D: Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One, 2017; 12: e0175149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 875).Auerbach BJ, Dibey S, Vallila-Buchman P, Kratz M, Krieger J: Review of 100% Fruit Juice and Chronic Health Conditions: Implications for Sugar-Sweetened Beverage Policy. Adv Nutr, 2018; 9: 78-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876).Collin LJ, Judd S, Safford M, Vaccarino V, Welsh JA: Association of Sugary Beverage Consumption With Mortality Risk in US Adults: A Secondary Analysis of Data From the REGARDS Study. JAMA Netw Open, 2019; 2: e193121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877).de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB: Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation, 2012; 125: 1735-1741, s1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878).Xi B, Huang Y, Reilly KH, Li S, Zheng R, Barrio-Lopez MT, Martinez-Gonzalez MA, Zhou D: Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr, 2015; 113: 709-717 [DOI] [PubMed] [Google Scholar]
- 879).Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG: Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br J Sports Med, 2016; 50: 496-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880).Eshak ES, Iso H, Kokubo Y, Saito I, Yamagishi K, Inoue M, Tsugane S: Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre-based study cohort I. Am J Clin Nutr, 2012; 96: 1390-1397 [DOI] [PubMed] [Google Scholar]
- 881).Chiavaroli L, de Souza RJ, Ha V, Cozma AI, Mirrahimi A, Wang DD, Yu M, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ, Sievenpiper JL: Effect of Fructose on Established Lipid Targets: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. J Am Heart Assoc, 2015; 4: e001700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882).David Wang D, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ: Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis, 2014; 232: 125-133 [DOI] [PubMed] [Google Scholar]
- 883).Livesey G, Taylor R: Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr, 2008; 88: 1419-1437 [DOI] [PubMed] [Google Scholar]
- 884).Evans RA, Frese M, Romero J, Cunningham JH, Mills KE: Fructose replacement of glucose or sucrose in food or beverages lowers postprandial glucose and insulin without raising triglycerides: a systematic review and meta-analysis. Am J Clin Nutr, 2017; 106: 506-518 [DOI] [PubMed] [Google Scholar]
- 885).Evans RA, Frese M, Romero J, Cunningham JH, Mills KE: Chronic fructose substitution for glucose or sucrose in food or beverages has little effect on fasting blood glucose, insulin, or triglycerides: a systematic review and meta-analysis. Am J Clin Nutr, 2017; 106: 519-529 [DOI] [PubMed] [Google Scholar]
- 886).Kelishadi R, Mansourian M, Heidari-Beni M: Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition, 2014; 30: 503-510 [DOI] [PubMed] [Google Scholar]
- 887).Mozaffarian D, Appel LJ, Van Horn L: Components of a cardioprotective diet: new insights. Circulation, 2011; 123: 2870-2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888).Kromhout D, Keys A, Aravanis C, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, et al.: Food consumption patterns in the 1960s in seven countries. Am J Clin Nutr, 1989; 49: 889-894 [DOI] [PubMed] [Google Scholar]
- 889).National Institute of Health and Nutrition: Current Status of National Nutrition, https://www.nibiohn.go.jp/eiken/chosa/kokumin_eiyou/ (in Japanese) [Google Scholar]
- 890).Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, Nishino Y, Tsubono Y, Tsuji I: Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol, 2007; 36: 600-609 [DOI] [PubMed] [Google Scholar]
- 891).Maruyama K, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A: Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC Study. Nutr Metab Cardiovasc Dis, 2013; 23: 519-527 [DOI] [PubMed] [Google Scholar]
- 892).Okada E, Nakamura K, Ukawa S, Wakai K, Date C, Iso H, Tamakoshi A: The Japanese food score and risk of all-cause, CVD and cancer mortality: the Japan Collaborative Cohort Study. Br J Nutr, 2018; 120: 464-471 [DOI] [PubMed] [Google Scholar]
- 893).Nanri A, Mizoue T, Shimazu T, Ishihara J, Takachi R, Noda M, Iso H, Sasazuki S, Sawada N, Tsugane S: Dietary patterns and all-cause, cancer, and cardiovascular disease mortality in Japanese men and women: The Japan public health center-based prospective study. PLoS One, 2017; 12: e0174848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894).Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Abbott RD, Okayama A: A Japanese diet and 19-year mortality: national integrated project for prospective observation of non-communicable diseases and its trends in the aged, 1980. Br J Nutr, 2009; 101: 1696-1705 [DOI] [PubMed] [Google Scholar]
- 895).Matsuyama S, Sawada N, Tomata Y, Zhang S, Goto A, Yamaji T, Iwasaki M, Inoue M, Tsuji I, Tsugane S: Association between adherence to the Japanese diet and all-cause and cause-specific mortality: the Japan Public Health Center-based Prospective Study. Eur J Nutr, 2021; 60: 1327-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896).Abe S, Zhang S, Tomata Y, Tsuduki T, Sugawara Y, Tsuji I: Japanese diet and survival time: The Ohsaki Cohort 1994 study. Clin Nutr, 2020; 39: 298-303 [DOI] [PubMed] [Google Scholar]
- 897).Maruyama C, Nakano R, Shima M, Mae A, Shijo Y, Nakamura E, Okabe Y, Park S, Kameyama N, Hirai S, Nakanishi M, Uchida K, Nishiyama H: Effects of a Japan Diet Intake Program on Metabolic Parameters in Middle-Aged Men. J Atheroscler Thromb, 2017; 24: 393-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898).Shijo Y, Maruyama C, Nakamura E, Nakano R, Shima M, Mae A, Okabe Y, Park S, Kameyama N, Hirai S: Japan Diet Intake Changes Serum Phospholipid Fatty Acid Compositions in Middle-Aged Men: A Pilot Study. J Atheroscler Thromb, 2019; 26: 3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899).Maruyama C, Shijo Y, Kameyama N, Umezawa A, Sato A, Nishitani A, Ayaori M, Ikewaki K, Waki M, Teramoto T: Effects of Nutrition Education Program for the Japan Diet on Serum LDL-Cholesterol Concentration in Patients with Dyslipidemia: A Randomized Controlled Trial. J Atheroscler Thromb, 2021; 28: 1035-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900).Tada N, Maruyama C, Koba S, Tanaka H, Birou S, Teramoto T, Sasaki J: Japanese dietary lifestyle and cardiovascular disease. J Atheroscler Thromb, 2011; 18: 723-734 [DOI] [PubMed] [Google Scholar]
- 901).Maruyama C, Kameyama N, Doi E, Nagai N, Okuda N, Fujioka Y, Masuda D, Morino K, Yoshida H, Waki M, Hirai S: The Japan Diet - Diet for the Prevention of ASCVD, https://www.j-athero.org/jp/wp-content/uploads/en/publications/pdf/TheJapanDiet_DigestEdition.pdf, 2020 [Google Scholar]
- 902).Ministry of Health, Labour and Welfare: the National Health and Nutrition Survey. https://www.mhlw.go.jp/content/000711006.pdf, 2020 in Japanese [Google Scholar]
- 903).Namazi N, Saneei P, Larijani B, Esmaillzadeh A: Soy product consumption and the risk of all-cause, cardiovascular and cancer mortality: a systematic review and meta-analysis of cohort studies. Food Funct, 2018; 9: 2576-2588 [DOI] [PubMed] [Google Scholar]
- 904).Shirai K, Kobayashi J, Inadera H, Ohkubo Y, Mori S, Saito Y, Yoshida S: Type I hyperlipoproteinemia caused by lipoprotein lipase defect in lipid-interface recognition was relieved by administration of medium-chain triglyceride. Metabolism, 1992; 41: 1161-1164 [DOI] [PubMed] [Google Scholar]
- 905).Rouis M, Dugi KA, Previato L, Patterson AP, Brunzell JD, Brewer HB, Santamarina-Fojo S: Therapeutic response to medium-chain triglycerides and omega-3 fatty acids in a patient with the familial chylomicronemia syndrome. Arterioscler Thromb Vasc Biol, 1997; 17: 1400-1406 [DOI] [PubMed] [Google Scholar]
- 906).Sheerah HA, Eshak ES, Cui R, Imano H, Iso H, Tamakoshi A: Relationship Between Dietary Vitamin D and Deaths From Stroke and Coronary Heart Disease: The Japan Collaborative Cohort Study. Stroke, 2018; 49: 454-457 [DOI] [PubMed] [Google Scholar]
- 907).Shi H, Chen H, Zhang Y, Li J, Fu K, Xue W, Teng W, Tian L: 25-Hydroxyvitamin D level, vitamin D intake, and risk of stroke: A dose-response meta-analysis. Clin Nutr, 2020; 39: 2025-2034 [DOI] [PubMed] [Google Scholar]
- 908).Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH: Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas, 2010; 65: 225-236 [DOI] [PubMed] [Google Scholar]
- 909).Grandi NC, Breitling LP, Brenner H: Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med, 2010; 51: 228-233 [DOI] [PubMed] [Google Scholar]
- 910).Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD: Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes, 2012; 5: 819-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911).Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM: 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke, 2012; 43: 1470-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912).Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, Gu H, Wang Y, Wang Y, Liu G: Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr, 2017; 105: 810-819 [DOI] [PubMed] [Google Scholar]
- 913).Gholami F, Moradi G, Zareei B, Rasouli MA, Nikkhoo B, Roshani D, Ghaderi E: The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord, 2019; 19: 248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914).Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH: Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ, 2014; 348: g1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915).Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG: 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol, 2012; 32: 2794-2802 [DOI] [PubMed] [Google Scholar]
- 916).Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH: Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol, 2012; 27: 581-591 [DOI] [PubMed] [Google Scholar]
- 917).Zhou R, Wang M, Huang H, Li W, Hu Y, Wu T: Lower Vitamin D Status Is Associated with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Nutrients, 2018; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918).Kunutsor SK, Apekey TA, Steur M: Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol, 2013; 28: 205-221 [DOI] [PubMed] [Google Scholar]
- 919).Qi D, Nie XL, Wu S, Cai J: Vitamin D and hypertension: Prospective study and meta-analysis. PLoS One, 2017; 12: e0174298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920).Cheng P, Wang L, Ning S, Liu Z, Lin H, Chen S, Zhu J: Vitamin E intake and risk of stroke: a meta-analysis. Br J Nutr, 2018; 120: 1181-1188 [DOI] [PubMed] [Google Scholar]
- 921).Nagao M, Moriyama Y, Yamagishi K, Iso H, Tamakoshi A: Relation of serum α- and γ-tocopherol levels to cardiovascular disease-related mortality among Japanese men and women. J Epidemiol, 2012; 22: 402-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922).Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T: Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr, 2018; 108: 1069-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923).Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S: Dietary and circulating vitamin C, vitamin E, β-carotene and risk of total cardiovascular mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr, 2019; 22: 1872-1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924).Chen GC, Lu DB, Pang Z, Liu QF: Vitamin C intake, circulating vitamin C and risk of stroke: a meta-analysis of prospective studies. J Am Heart Assoc, 2013; 2: e000329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925).Kubota Y, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A: Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: the Japan Collaborative Cohort Study (JACC) study. Stroke, 2011; 42: 1665-1672 [DOI] [PubMed] [Google Scholar]
- 926).Knekt P, Ritz J, Pereira MA, O'Reilly EJ, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Rimm EB, Ascherio A: Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr, 2004; 80: 1508-1520 [DOI] [PubMed] [Google Scholar]
- 927).Mirhosseini N, Rainsbury J, Kimball SM: Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front Cardiovasc Med, 2018; 5: 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928).Dibaba DT: Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev, 2019; 77: 890-902 [DOI] [PubMed] [Google Scholar]
- 929).Loffredo L, Perri L, Di Castelnuovo A, Iacoviello L, De Gaetano G, Violi F: Supplementation with vitamin E alone is associated with reduced myocardial infarction: a meta-analysis. Nutr Metab Cardiovasc Dis, 2015; 25: 354-363 [DOI] [PubMed] [Google Scholar]
- 930).Ashor AW, Siervo M, Lara J, Oggioni C, Mathers JC: Antioxidant vitamin supplementation reduces arterial stiffness in adults: a systematic review and meta-analysis of randomized controlled trials. J Nutr, 2014; 144: 1594-1602 [DOI] [PubMed] [Google Scholar]
- 931).Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, Tuomainen TP, Valkonen VP, Ristonmaa U, Lakka HM, Vanharanta M, Salonen JT, Poulsen HE: Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation, 2003; 107: 947-953 [DOI] [PubMed] [Google Scholar]
- 932).Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S: Vitamin E As a Potential Interventional Treatment for Metabolic Syndrome: Evidence from Animal and Human Studies. Front Pharmacol, 2017; 8: 444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933).Wong SK, Kamisah Y, Mohamed N, Muhammad N, Masbah N, Fahami NAM, Mohamed IN, Shuid AN, Saad QM, Abdullah A, Mohamad NV, Ibrahim NI, Pang KL, Chow YY, Thong BKS, Subramaniam S, Chan CY, Ima-Nirwana S, Chin AK: Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients, 2020; 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934).Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM: Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med, 2010; 152: 307-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935).Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, Yelangi A, Sundus S, Bachuwa G, Alkotob ML, Manson JE: Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol, 2019; 4: 765-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936).Swart KM, Lips P, Brouwer IA, Jorde R, Heymans MW, Grimnes G, Grübler MR, Gaksch M, Tomaschitz A, Pilz S, Eiriksdottir G, Gudnason V, Wamberg L, Rejnmark L, Sempos CT, Durazo-Arvizu RA, Dowling KG, Hull G, Škrabáková Z, Kiely M, Cashman KD, van Schoor NM: Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: an individual participant data meta-analysis of randomized controlled trials. Am J Clin Nutr, 2018; 107: 1043-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937).Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP: Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ, 2014; 348: g2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938).Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM: Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab, 2011; 96: 1931-1942 [DOI] [PubMed] [Google Scholar]
- 939).Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M: Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr, 2014; 100: 746-755 [DOI] [PubMed] [Google Scholar]
- 940).Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ: Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet, 2003; 361: 2017-2023 [DOI] [PubMed] [Google Scholar]
- 941).Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH: Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med, 2004; 164: 1552-1556 [DOI] [PubMed] [Google Scholar]
- 942).Ashor AW, Brown R, Keenan PD, Willis ND, Siervo M, Mathers JC: Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. Nutr Res, 2019; 61: 1-12 [DOI] [PubMed] [Google Scholar]
- 943).Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, Paquette M, Patel D, Mitchell S, Kavanagh M, Tsirakis T, Bachiri L, Maran A, Umatheva N, McKay T, Trinidad G, Bernstein D, Chowdhury A, Correa-Betanzo J, Del Principe G, Hajizadeh A, Jayaraman R, Jenkins A, Jenkins W, Kalaichandran R, Kirupaharan G, Manisekaran P, Qutta T, Shahid R, Silver A, Villegas C, White J, Kendall CWC, Pichika SC, Sievenpiper JL: Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J Am Coll Cardiol, 2018; 71: 2570-2584 [DOI] [PubMed] [Google Scholar]
- 944).Bleys J, Miller ER, 3rd, Pastor-Barriuso R, Appel LJ, Guallar E: Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr, 2006; 84: 880-887; quiz 954-885 [DOI] [PubMed] [Google Scholar]
- 945).Ye Y, Li J, Yuan Z: Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One, 2013; 8: e56803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946).Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, Okunrintemi V, Riaz IB, Khan MS, Kaluski E, Murad MH, Blaha MJ, Guallar E, Michos ED: Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes: An Umbrella Review and Evidence Map. Ann Intern Med, 2019; 171: 190-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947).Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR: Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA, 2005; 293: 1338-1347 [DOI] [PubMed] [Google Scholar]
- 948).Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR, Jr.: Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr, 2004; 80: 1194-1200 [DOI] [PubMed] [Google Scholar]
- 949).Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higginson L, Bittner V, Steffes M, Gordon DJ, Proschan M, Younes N, Verter JI: Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA, 2002; 288: 2432-2440 [DOI] [PubMed] [Google Scholar]
- 950).Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T: Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ, 2010; 341: c5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 951).Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM: Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA, 2008; 300: 2123-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952).Food Safety Commission of Japan, Cabinet Office: Effects of excessive vitamin A intake, https://www.fsc.go.jp/topics/factsheet-vitamin-a.pdf (in Japanese) [Google Scholar]
- 953).Murai U, Yamagishi K, Sata M, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Sawada N, Iso H, Tsugane S: Seaweed intake and risk of cardiovascular disease: the Japan Public Health Center-based Prospective (JPHC) Study. Am J Clin Nutr, 2019; 110: 1449-1455 [DOI] [PubMed] [Google Scholar]
- 954).Kishida R, Yamagishi K, Muraki I, Sata M, Tamakoshi A, Iso H: Frequency of Seaweed Intake and Its Association with Cardiovascular Disease Mortality: The JACC Study. J Atheroscler Thromb, 2020; 27: 1340-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955).Chichibu H, Yamagishi K, Kishida R, Maruyama K, Hayama-Terada M, Shimizu Y, Muraki I, Umesawa M, Cui R, Imano H, Ohira T, Tanigawa T, Sankai T, Okada T, Kitamura A, Kiyama M, Iso H: Seaweed Intake and Risk of Cardiovascular Disease: The Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb, 2021; 28: 1298-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956).Yan Z, Zhang X, Li C, Jiao S, Dong W: Association between consumption of soy and risk of cardiovascular disease: A meta-analysis of observational studies. Eur J Prev Cardiol, 2017; 24: 735-747 [DOI] [PubMed] [Google Scholar]
- 957).Lou D, Li Y, Yan G, Bu J, Wang H: Soy Consumption with Risk of Coronary Heart Disease and Stroke: A Meta-Analysis of Observational Studies. Neuroepidemiology, 2016; 46: 242-252 [DOI] [PubMed] [Google Scholar]
- 958).Nguyen HN, Miyagawa N, Miura K, Okuda N, Yoshita K, Arai Y, Nakagawa H, Sakata K, Ojima T, Kadota A, Takashima N, Fujiyoshi A, Ohkubo T, Abbott RD, Okamura T, Okayama A, Ueshima H: Dietary tofu intake and long-term risk of death from stroke in a general population. Clin Nutr, 2018; 37: 182-188 [DOI] [PubMed] [Google Scholar]
- 959).Nagata C, Wada K, Tamura T, Konishi K, Goto Y, Koda S, Kawachi T, Tsuji M, Nakamura K: Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: the Takayama study. Am J Clin Nutr, 2017; 105: 426-431 [DOI] [PubMed] [Google Scholar]
- 960).Anderson JW, Bush HM: Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr, 2011; 30: 79-91 [DOI] [PubMed] [Google Scholar]
- 961).Blanco Mejia S, Messina M, Li SS, Viguiliouk E, Chiavaroli L, Khan TA, Srichaikul K, Mirrahimi A, Sievenpiper JL, Kris-Etherton P, Jenkins DJA: A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J Nutr, 2019; 149: 968-981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962).Tokede OA, Onabanjo TA, Yansane A, Gaziano JM, Djoussé L: Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br J Nutr, 2015; 114: 831-843 [DOI] [PubMed] [Google Scholar]
- 963).Eslami O, Shidfar F: Soy milk: A functional beverage with hypocholesterolemic effects? A systematic review of randomized controlled trials. Complement Ther Med, 2019; 42: 82-88 [DOI] [PubMed] [Google Scholar]
- 964).Weggemans RM, Trautwein EA: Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr, 2003; 57: 940-946 [DOI] [PubMed] [Google Scholar]
- 965).de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J: Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet, 1994; 343: 1454-1459 [DOI] [PubMed] [Google Scholar]
- 966).de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N: Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation, 1999; 99: 779-785 [DOI] [PubMed] [Google Scholar]
- 967).Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA: Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med, 2018; 378: e34 [Google Scholar]
- 968).Liyanage T, Ninomiya T, Wang A, Neal B, Jun M, Wong MG, Jardine M, Hillis GS, Perkovic V: Effects of the Mediterranean Diet on Cardiovascular Outcomes-A Systematic Review and Meta-Analysis. PLoS One, 2016; 11: e0159252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969).Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A, Das A, Hartley L, Stranges S: Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2019; 3: Cd009825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970).Panizza CE, Lim U, Yonemori KM, Cassel KD, Wilkens LR, Harvie MN, Maskarinec G, Delp EJ, Lampe JW, Shepherd JA, Le Marchand L, Boushey CJ: Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients, 2019; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971).Yang J, Farioli A, Korre M, Kales SN: Modified Mediterranean diet score and cardiovascular risk in a North American working population. PLoS One, 2014; 9: e87539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 972).Murakami K, Livingstone MBE, Sasaki S: Diet quality scores in relation to metabolic risk factors in Japanese adults: a cross-sectional analysis from the 2012 National Health and Nutrition Survey, Japan. Eur J Nutr, 2019; 58: 2037-2050 [DOI] [PubMed] [Google Scholar]
- 973).Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB: Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med, 2008; 168: 713-720 [DOI] [PubMed] [Google Scholar]
- 974).Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB: Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation, 2015; 132: 2212-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975).Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF: Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr, 2014; 144: 881-889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976).Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, Salas-Salvadó J, Kendall CW, Sievenpiper JL: DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients, 2019; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977).Kahleova H, Salas-Salvadó J, Rahelić D, Kendall CW, Rembert E, Sievenpiper JL: Dietary Patterns and Cardiometabolic Outcomes in Diabetes: A Summary of Systematic Reviews and Meta-Analyses. Nutrients, 2019; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978).Yang ZQ, Yang Z, Duan ML: Dietary approach to stop hypertension diet and risk of coronary artery disease: a meta-analysis of prospective cohort studies. Int J Food Sci Nutr, 2019; 70: 668-674 [DOI] [PubMed] [Google Scholar]
- 979).Soltani S, Arablou T, Jayedi A, Salehi-Abargouei A: Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutr J, 2020; 19: 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980).Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L: Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition, 2013; 29: 611-618 [DOI] [PubMed] [Google Scholar]
- 981).Schwingshackl L, Bogensberger B, Hoffmann G: Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J Acad Nutr Diet, 2018; 118: 74-100.e111 [DOI] [PubMed] [Google Scholar]
- 982).Schwingshackl L, Hoffmann G: Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet, 2015; 115: 780-800.e785 [DOI] [PubMed] [Google Scholar]
- 983).Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, Chrysochoou CA, Nihoyannopoulos PI, Tousoulis DM: Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr, 2020; 11: 1150-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984).Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC: Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr, 2015; 113: 1-15 [DOI] [PubMed] [Google Scholar]
- 985).Kawamura A, Kajiya K, Kishi H, Inagaki J, Mitarai M, Oda H, Umemoto S, Kobayashi S: Effects of the DASH-JUMP dietary intervention in Japanese participants with high-normal blood pressure and stage 1 hypertension: an open-label single-arm trial. Hypertens Res, 2016; 39: 777-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986).Umemoto S, Onaka U, Kawano R, Kawamura A, Motoi S, Honda N, Kanazashi H, Mitarai M: Effects of a Japanese Cuisine-Based Antihypertensive Diet and Fish Oil on Blood Pressure and Its Variability in Participants with Untreated Normal High Blood Pressure or Stage I Hypertension: A Feasibility Randomized Controlled Study. J Atheroscler Thromb, 2022; 29: 152-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987).Ros E: Health benefits of nut consumption. Nutrients, 2010; 2: 652-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988).Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T: Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med, 2016; 14: 207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989).Aune D: Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv Nutr, 2019; 10: S404-s421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990).Brown RC, Gray AR, Tey SL, Chisholm A, Burley V, Greenwood DC, Cade J: Associations between Nut Consumption and Health Vary between Omnivores, Vegetarians, and Vegans. Nutrients, 2017; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991).Chen GC, Zhang R, Martínez-González MA, Zhang ZL, Bonaccio M, van Dam RM, Qin LQ: Nut consumption in relation to all-cause and cause-specific mortality: a meta-analysis 18 prospective studies. Food Funct, 2017; 8: 3893-3905 [DOI] [PubMed] [Google Scholar]
- 992).Grosso G, Yang J, Marventano S, Micek A, Galvano F, Kales SN: Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr, 2015; 101: 783-793 [DOI] [PubMed] [Google Scholar]
- 993).Mayhew AJ, de Souza RJ, Meyre D, Anand SS, Mente A: A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr, 2016; 115: 212-225 [DOI] [PubMed] [Google Scholar]
- 994).Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu M, Hu FB, Liu L: Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr, 2014; 100: 256-269 [DOI] [PubMed] [Google Scholar]
- 995).Weng YQ, Yao J, Guo ML, Qin QJ, Li P: Association between nut consumption and coronary heart disease: a meta-analysis. Coron Artery Dis, 2016; 27: 227-232 [DOI] [PubMed] [Google Scholar]
- 996).Ma L, Wang F, Guo W, Yang H, Liu Y, Zhang W: Nut consumption and the risk of coronary artery disease: a dose-response meta-analysis of 13 prospective studies. Thromb Res, 2014; 134: 790-794 [DOI] [PubMed] [Google Scholar]
- 997).Becerra-Tomás N, Paz-Graniel I, C WCK, Kahleova H, Rahelić D, Sievenpiper JL, Salas-Salvadó J: Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Nutr Rev, 2019; 77: 691-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998).Zhang Z, Xu G, Wei Y, Zhu W, Liu X: Nut consumption and risk of stroke. Eur J Epidemiol, 2015; 30: 189-196 [DOI] [PubMed] [Google Scholar]
- 999).Shao C, Tang H, Zhao W, He J: Nut intake and stroke risk: A dose-response meta-analysis of prospective cohort studies. Sci Rep, 2016; 6: 30394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000).Sabaté J, Oda K, Ros E: Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med, 2010; 170: 821-827 [DOI] [PubMed] [Google Scholar]
- 1001).Tey SL, Brown RC, Chisholm AW, Delahunty CM, Gray AR, Williams SM: Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr, 2011; 65: 117-124 [DOI] [PubMed] [Google Scholar]
- 1002).Tey SL, Delahunty C, Gray A, Chisholm A, Brown RC: Effects of regular consumption of different forms of almonds and hazelnuts on acceptance and blood lipids. Eur J Nutr, 2015; 54: 483-487 [DOI] [PubMed] [Google Scholar]
- 1003).Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, Koletzko B, Stark RG, Nagel JM, Mantzoros CS, Parhofer KG: Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism, 2014; 63: 382-391 [DOI] [PubMed] [Google Scholar]
- 1004).Banel DK, Hu FB: Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr, 2009; 90: 56-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005).Altamimi M, Zidan S, Badrasawi M: Effect of Tree Nuts Consumption on Serum Lipid Profile in Hyperlipidemic Individuals: A Systematic Review. Nutr Metab Insights, 2020; 13: 1178638820926521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006).Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD: Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med, 2001; 31: 1033-1062 [DOI] [PubMed] [Google Scholar]
- 1007).Leon AS, Sanchez OA: Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc, 2001; 33: S502-515; discussion S528-509 [DOI] [PubMed] [Google Scholar]
- 1008).Kelley GA, Kelley KS, Tran ZV: Walking, lipids, and lipoproteins: a meta-analysis of randomized controlled trials. Prev Med, 2004; 38: 651-661 [DOI] [PubMed] [Google Scholar]
- 1009).Kelley GA, Kelley KS, Tran ZV: Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Womens Health (Larchmt), 2004; 13: 1148-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010).Kelley GA, Kelley KS, Tran ZV: Exercise, lipids, and lipoproteins in older adults: a meta-analysis. Prev Cardiol, 2005; 8: 206-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011).Kelley GA, Kelley KS: Aerobic exercise and lipids and lipoproteins in men: a meta-analysis of randomized controlled trials. J Mens Health Gend, 2006; 3: 61-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012).Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H: Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med, 2007; 167: 999-1008 [DOI] [PubMed] [Google Scholar]
- 1013).Igarashi Y, Akazawa N, Maeda S: Effects of Aerobic Exercise Alone on Lipids in Healthy East Asians: A Systematic Review and Meta-Analysis. J Atheroscler Thromb, 2019; 26: 488-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014).Igarashi Y, Nogami Y: Response of Lipids and Lipoproteins to Regular Aquatic Endurance Exercise: A Meta-Analysis of Randomized Controlled Trials. J Atheroscler Thromb, 2019; 26: 14-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015).Costa RR, Buttelli ACK, Vieira AF, Coconcelli L, Magalhães RL, Delevatti RS, Kruel LFM: Effect of Strength Training on Lipid and Inflammatory Outcomes: Systematic Review With Meta-Analysis and Meta-Regression. J Phys Act Health, 2019; 16: 477-491 [DOI] [PubMed] [Google Scholar]
- 1016).Koba S, Tanaka H, Maruyama C, Tada N, Birou S, Teramoto T, Sasaki J: Physical activity in the Japan population: association with blood lipid levels and effects in reducing cardiovascular and all-cause mortality. J Atheroscler Thromb, 2011; 18: 833-845 [DOI] [PubMed] [Google Scholar]
- 1017).Palazón-Bru A, Hernández-Lozano D, Gil-Guillén VF: Which Physical Exercise Interventions Increase HDL-Cholesterol Levels? A Systematic Review of Meta-analyses of Randomized Controlled Trials. Sports Med, 2021; 51: 243-253 [DOI] [PubMed] [Google Scholar]
- 1018).Igarashi Y, Akazawa N, Maeda S: Regular aerobic exercise and blood pressure in East Asians: A meta-analysis of randomized controlled trials. Clin Exp Hypertens, 2018; 40: 378-389 [DOI] [PubMed] [Google Scholar]
- 1019).Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN: Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Med Sci Sports Exerc, 2002; 34: 838-844 [DOI] [PubMed] [Google Scholar]
- 1020).Kelley GA, Kelley KS: Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med, 2009; 48: 9-19 [DOI] [PubMed] [Google Scholar]
- 1021).Yang Z, Scott CA, Mao C, Tang J, Farmer AJ: Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med, 2014; 44: 487-499 [DOI] [PubMed] [Google Scholar]
- 1022).Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L: Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension, 2011; 58: 950-958 [DOI] [PubMed] [Google Scholar]
- 1023).Lemes Í R, Ferreira PH, Linares SN, Machado AF, Pastre CM, Netto JJ: Resistance training reduces systolic blood pressure in metabolic syndrome: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med, 2016; 50: 1438-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024).Liu Y, Ye W, Chen Q, Zhang Y, Kuo CH, Korivi M: Resistance Exercise Intensity is Correlated with Attenuation of HbA1c and Insulin in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health, 2019; 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025).Hayashino Y, Jackson JL, Fukumori N, Nakamura F, Fukuhara S: Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract, 2012; 98: 349-360 [DOI] [PubMed] [Google Scholar]
- 1026).Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ: Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation, 2007; 116: 572-584 [DOI] [PubMed] [Google Scholar]
- 1027).Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, Aubertin-Leheudre M, Bernabei R, Cadore EL, Cesari M, Chen LK, de Souto Barreto P, Duque G, Ferrucci L, Fielding RA, García-Hermoso A, Gutiérrez-Robledo LM, Harridge SDR, Kirk B, Kritchevsky S, Landi F, Lazarus N, Martin FC, Marzetti E, Pahor M, Ramírez-Vélez R, Rodriguez-Mañas L, Rolland Y, Ruiz JG, Theou O, Villareal DT, Waters DL, Won Won C, Woo J, Vellas B, Fiatarone Singh M: International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging, 2021; 25: 824-853 [DOI] [PubMed] [Google Scholar]
- 1028).Kelley GA, Kelley KS, Roberts S, Haskell W: Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clin Nutr, 2012; 31: 156-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029).Kelley GA, Kelley KS: Effects of Diet, Aerobic Exercise, or Both on Non-HDL-C in Adults: A Meta-Analysis of Randomized Controlled Trials. Cholesterol, 2012; 2012: 840935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030).Lee CD, Folsom AR, Blair SN: Physical activity and stroke risk: a meta-analysis. Stroke, 2003; 34: 2475-2481 [DOI] [PubMed] [Google Scholar]
- 1031).Oguma Y, Shinoda-Tagawa T: Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med, 2004; 26: 407-418 [DOI] [PubMed] [Google Scholar]
- 1032).Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF: Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil, 2008; 15: 247-257 [DOI] [PubMed] [Google Scholar]
- 1033).Hamer M, Chida Y: Active commuting and cardiovascular risk: a meta-analytic review. Prev Med, 2008; 46: 9-13 [DOI] [PubMed] [Google Scholar]
- 1034).Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN: Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil, 2008; 15: 239-246 [DOI] [PubMed] [Google Scholar]
- 1035).Zheng H, Orsini N, Amin J, Wolk A, Nguyen VT, Ehrlich F: Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol, 2009; 24: 181-192 [DOI] [PubMed] [Google Scholar]
- 1036).Löllgen H, Böckenhoff A, Knapp G: Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. Int J Sports Med, 2009; 30: 213-224 [DOI] [PubMed] [Google Scholar]
- 1037).Diep L, Kwagyan J, Kurantsin-Mills J, Weir R, Jayam-Trouth A: Association of physical activity level and stroke outcomes in men and women: a meta-analysis. J Womens Health (Larchmt), 2010; 19: 1815-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038).Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM: Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation, 2011; 124: 789-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1039).Li J, Siegrist J: Physical activity and risk of cardiovascular disease--a meta-analysis of prospective cohort studies. Int J Environ Res Public Health, 2012; 9: 391-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040).Kodama S, Tanaka S, Heianza Y, Fujihara K, Horikawa C, Shimano H, Saito K, Yamada N, Ohashi Y, Sone H: Association between physical activity and risk of all-cause mortality and cardiovascular disease in patients with diabetes: a meta-analysis. Diabetes Care, 2013; 36: 471-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041).Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Matthews CE: Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med, 2015; 175: 959-967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042).Hupin D, Roche F, Gremeaux V, Chatard JC, Oriol M, Gaspoz JM, Barthélémy JC, Edouard P: Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med, 2015; 49: 1262-1267 [DOI] [PubMed] [Google Scholar]
- 1043).Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, Kaur A, Friedemann Smith C, Wilkins E, Rayner M, Roberts N, Scarborough P: Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J Am Heart Assoc, 2016; 5: e002495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044).Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJ, Forouzanfar MH: Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ, 2016; 354: i3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045).Nordengen S, Andersen LB, Solbraa AK, Riiser A: Cycling is associated with a lower incidence of cardiovascular diseases and death: Part 1 - systematic review of cohort studies with meta-analysis. Br J Sports Med, 2019; 53: 870-878 [DOI] [PubMed] [Google Scholar]
- 1046).Ramakrishnan R, He JR, Ponsonby AL, Woodward M, Rahimi K, Blair SN, Dwyer T: Objectively measured physical activity and all cause mortality: A systematic review and meta-analysis. Prev Med, 2021; 143: 106356 [DOI] [PubMed] [Google Scholar]
- 1047).Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn IM, Hagströmer M, Edwardson C, Yates T, Shiroma E, Anderssen SA, Lee IM: Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ, 2019; 366: l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048).Amagasa S, Machida M, Fukushima N, Kikuchi H, Takamiya T, Odagiri Y, Inoue S: Is objectively measured light-intensity physical activity associated with health outcomes after adjustment for moderate-to-vigorous physical activity in adults? A systematic review. Int J Behav Nutr Phys Act, 2018; 15: 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1049).Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W: Associations of leisure-time physical activity with cardiovascular mortality: A systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol, 2018; 25: 1864-1872 [DOI] [PubMed] [Google Scholar]
- 1050).Kubota Y, Iso H, Yamagishi K, Sawada N, Tsugane S: Daily Total Physical Activity and Incident Cardiovascular Disease in Japanese Men and Women: Japan Public Health Center-Based Prospective Study. Circulation, 2017; 135: 1471-1473 [DOI] [PubMed] [Google Scholar]
- 1051).Nakayama T, Date C, Yokoyama T, Yoshiike N, Yamaguchi M, Tanaka H: A 15.5-year follow-up study of stroke in a Japanese provincial city. The Shibata Study. Stroke, 1997; 28: 45-52 [DOI] [PubMed] [Google Scholar]
- 1052).Sone H, Tanaka S, Tanaka S, Suzuki S, Seino H, Hanyu O, Sato A, Toyonaga T, Okita K, Ishibashi S, Kodama S, Akanuma Y, Yamada N: Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with type 2 diabetes: analysis from the Japan Diabetes Complications Study (JDCS). Diabetologia, 2013; 56: 1021-1030 [DOI] [PubMed] [Google Scholar]
- 1053).Kubota Y, Iso H, Yamagishi K, Sawada N, Tsugane S: Daily Total Physical Activity and Incident Stroke: The Japan Public Health Center-Based Prospective Study. Stroke, 2017; 48: 1730-1736 [DOI] [PubMed] [Google Scholar]
- 1054).Noda H, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A: Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol, 2005; 46: 1761-1767 [DOI] [PubMed] [Google Scholar]
- 1055).Shibata Y, Hayasaka S, Yamada T, Goto Y, Ojima T, Ishikawa S, Kayaba K, Gotoh T, Nakamura Y: Physical activity and cardiovascular disease in Japan: the Jichi Medical School Cohort Study. J Epidemiol, 2010; 20: 225-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056).Ueshima K, Ishikawa-Takata K, Yorifuji T, Suzuki E, Kashima S, Takao S, Sugiyama M, Ohta T, Doi H: Physical activity and mortality risk in the Japanese elderly: a cohort study. Am J Prev Med, 2010; 38: 410-418 [DOI] [PubMed] [Google Scholar]
- 1057).Inoue M, Iso H, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S: Daily total physical activity level and premature death in men and women: results from a large-scale population-based cohort study in Japan (JPHC study). Ann Epidemiol, 2008; 18: 522-530 [DOI] [PubMed] [Google Scholar]
- 1058).Fujita K, Takahashi H, Miura C, Ohkubo T, Sato Y, Ugajin T, Kurashima K, Tsubono Y, Tsuji I, Fukao A, Hisamichi S: Walking and mortality in Japan: the Miyagi Cohort Study. J Epidemiol, 2004; 14 Suppl 1: S26-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059).Seki N: [Relationships between walking hours, sleeping hours, meaningfulness of life (ikigai) and mortality in the elderly: prospective cohort study]. Nihon Eiseigaku Zasshi, 2001; 56: 535-540 [DOI] [PubMed] [Google Scholar]
- 1060).Hayasaka S, Shibata Y, Ishikawa S, Kayaba K, Gotoh T, Noda T, Murata C, Yamada T, Goto Y, Nakamura Y, Ojima T: Physical activity and all-cause mortality in Japan: the Jichi Medical School (JMS) Cohort Study. J Epidemiol, 2009; 19: 24-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1061).Kikuchi H, Inoue S, Lee IM, Odagiri Y, Sawada N, Inoue M, Tsugane S: Impact of Moderate-Intensity and Vigorous-Intensity Physical Activity on Mortality. Med Sci Sports Exerc, 2018; 50: 715-721 [DOI] [PubMed] [Google Scholar]
- 1062).Yamamoto N, Miyazaki H, Shimada M, Nakagawa N, Sawada SS, Nishimuta M, Kimura Y, Kawakami R, Nagayama H, Asai H, Lee IM, Blair SN, Yoshitake Y: Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health, 2018; 18: 540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1063).Kamagata K, Shimomitsu T, Suzuki S, Suzuki T, Sudo M, Tanaka K, Tabata I, Toyama Y, Naitoh Y, Fukunaga T, Fujikawa M, Michinaga M, Miyachi M: Report of the Study Group on Revision of Exercise Standards and Guidelines. https://www.mhlw.go.jp/content/000306883.pdf, 2013 (in Japanese) [Google Scholar]
- 1064).Saeidifard F, Medina-Inojosa JR, West CP, Olson TP, Somers VK, Bonikowske AR, Prokop LJ, Vinciguerra M, Lopez-Jimenez F: The association of resistance training with mortality: A systematic review and meta-analysis. Eur J Prev Cardiol, 2019; 26: 1647-1665 [DOI] [PubMed] [Google Scholar]
- 1065).Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB: Exercise type and intensity in relation to coronary heart disease in men. JAMA, 2002; 288: 1994-2000 [DOI] [PubMed] [Google Scholar]
- 1066).Shiroma EJ, Cook NR, Manson JE, Moorthy MV, Buring JE, Rimm EB, Lee IM: Strength Training and the Risk of Type 2 Diabetes and Cardiovascular Disease. Med Sci Sports Exerc, 2017; 49: 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067).Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, Aubertin-Leheudre M, Bernabei R, Cadore EL, Cesari M, Chen LK, De Souto Barreto P, Duque G, Ferrucci L, Fielding RA, García-Hermoso A, Gutiérrez-Robledo LM, Harridge SDR, Kirk B, Kritchevsky S, Landi F, Lazarus N, Martin FC, Marzetti E, Pahor M, Ramírez-Vélez R, Rodriguez-Mañas L, Rolland Y, Ruiz JG, Theou O, Villareal DT, Waters DL, Won CW, Woo J, Vellas B, Singh MF: International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging, 2021; 25: 824-853 [DOI] [PubMed] [Google Scholar]
- 1068).Grøntved A, Hu FB: Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA, 2011; 305: 2448-2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069).Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA: Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med, 2015; 162: 123-132 [DOI] [PubMed] [Google Scholar]
- 1070).Zhao R, Bu W, Chen Y, Chen X: The Dose-Response Associations of Sedentary Time with Chronic Diseases and the Risk for All-Cause Mortality Affected by Different Health Status: A Systematic Review and Meta-Analysis. J Nutr Health Aging, 2020; 24: 63-70 [DOI] [PubMed] [Google Scholar]
- 1071).Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ: Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia, 2012; 55: 2895-2905 [DOI] [PubMed] [Google Scholar]
- 1072).Patterson R, McNamara E, Tainio M, de Sá TH, Smith AD, Sharp SJ, Edwards P, Woodcock J, Brage S, Wijndaele K: Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol, 2018; 33: 811-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073).Pandey A, Salahuddin U, Garg S, Ayers C, Kulinski J, Anand V, Mayo H, Kumbhani DJ, de Lemos J, Berry JD: Continuous Dose-Response Association Between Sedentary Time and Risk for Cardiovascular Disease: A Meta-analysis. JAMA Cardiol, 2016; 1: 575-583 [DOI] [PubMed] [Google Scholar]
- 1074).Kivimäki M, Singh-Manoux A, Pentti J, Sabia S, Nyberg ST, Alfredsson L, Goldberg M, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Nordin M, Oksanen T, Strandberg T, Suominen SB, Theorell T, Vahtera J, Väänänen A, Virtanen M, Westerholm P, Westerlund H, Zins M, Seshadri S, Batty GD, Sipilä PN, Shipley MJ, Lindbohm JV, Ferrie JE, Jokela M: Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ, 2019; 365: l1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1075).Bailey DP, Hewson DJ, Champion RB, Sayegh SM: Sitting Time and Risk of Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. Am J Prev Med, 2019; 57: 408-416 [DOI] [PubMed] [Google Scholar]
- 1076).Takagi H, Hari Y, Nakashima K, Kuno T, Ando T: Meta-analysis of the Relation of Television-Viewing Time and Cardiovascular Disease. Am J Cardiol, 2019; 124: 1674-1683 [DOI] [PubMed] [Google Scholar]
- 1077).Ikehara S, Iso H, Wada Y, Tanabe N, Watanabe Y, Kikuchi S, Tamakoshi A: Television viewing time and mortality from stroke and coronary artery disease among Japanese men and women -- the Japan Collaborative Cohort Study. Circ J, 2015; 79: 2389-2395 [DOI] [PubMed] [Google Scholar]
- 1078).Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ: Effects of Interrupting Prolonged Sitting with Physical Activity Breaks on Blood Glucose, Insulin and Triacylglycerol Measures: A Systematic Review and Meta-analysis. Sports Med, 2020; 50: 295-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1079).Chastin SF, Egerton T, Leask C, Stamatakis E: Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity (Silver Spring), 2015; 23: 1800-1810 [DOI] [PubMed] [Google Scholar]
- 1080).Schwarzer R: Health action process approach (HAPA) as a theoretical framework to understand behavior change. Actualidades en Psicología, 2016; 30: 119-130 [Google Scholar]
- 1081).Greaves C, Gillison F, Stathi A, Bennett P, Reddy P, Dunbar J, Perry R, Messom D, Chandler R, Francis M, Davis M, Green C, Evans P, Taylor G: Waste the waist: a pilot randomised controlled trial of a primary care based intervention to support lifestyle change in people with high cardiovascular risk. Int J Behav Nutr Phys Act, 2015; 12: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082).Hettema J, Steele J, Miller WR: Motivational interviewing. Annu Rev Clin Psychol, 2005; 1: 91-111 [DOI] [PubMed] [Google Scholar]
- 1083).Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS: Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12-month post-intervention follow-up. Int J Behav Nutr Phys Act, 2013; 10: 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084).Albert B: Social learning theory Englewood Cliffs, N.J. : Prentice Hall, 1977 [Google Scholar]
- 1085).Elmer PJ, Grimm R, Jr., Laing B, Grandits G, Svendsen K, Van Heel N, Betz E, Raines J, Link M, Stamler J, et al.: Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS). Prev Med, 1995; 24: 378-388 [DOI] [PubMed] [Google Scholar]
- 1086).Greene GW, Rossi SR, Reed GR, Willey C, Prochaska JO: Stages of change for reducing dietary fat to 30% of energy or less. J Am Diet Assoc, 1994; 94: 1105-1110; quiz 1111-1102 [DOI] [PubMed] [Google Scholar]
- 1087).Eriksson KM, Westborg CJ, Eliasson MC: A randomized trial of lifestyle intervention in primary healthcare for the modification of cardiovascular risk factors. Scand J Public Health, 2006; 34: 453-461 [DOI] [PubMed] [Google Scholar]
- 1088).Nasser R, Cook SL, Dorsch KD, Haennel RG: Comparison of two nutrition education approaches to reduce dietary fat intake and serum lipids reveals registered dietitians are effective at disseminating information regardless of the educational approach. J Am Diet Assoc, 2006; 106: 850-859 [DOI] [PubMed] [Google Scholar]
- 1089).Ryo M, Nakamura T, Funahashi T, Noguchi M, Kishida K, Okauchi Y, Nishizawa H, Ogawa T, Kojima S, Ohira T, Okita K, Iwahashi H, Imagawa A, Matsuzawa Y, Shimomura I: Health education "Hokenshido" program reduced metabolic syndrome in the Amagasaki visceral fat study. Three-year follow-up study of 3,174 Japanese employees. Intern Med, 2011; 50: 1643-1648 [DOI] [PubMed] [Google Scholar]
- 1090).Rosenstock IM, Strecher VJ, Becker MH: Social learning theory and the Health Belief Model. Health Educ Q, 1988; 15: 175-183 [DOI] [PubMed] [Google Scholar]
- 1091).Deci E, Ryan R: Intrinsic Motivation and Self-Determination in Human Behavior (Perspectives in Social Psychology), Springer, 1985 [Google Scholar]
- 1092).Bandura A, Adams NE: Analysis of self-efficacy theory of behavioral change. Cognit Ther Res, 1977; 1: 287-310 [Google Scholar]
- 1093).Burke LE, Dunbar-Jacob J, Orchard TJ, Sereika SM: Improving adherence to a cholesterol-lowering diet: a behavioral intervention study. Patient Educ Couns, 2005; 57: 134-142 [DOI] [PubMed] [Google Scholar]
- 1094).Flannery K, Resnick B, Galik E, Lipscomb J, McPhaul K, Shaughnessy M: The Worksite Heart Health Improvement Project (WHHIP): feasibility and efficacy. Public Health Nurs, 2012; 29: 455-466 [DOI] [PubMed] [Google Scholar]
- 1095).Pedersen C, Halvari H, Williams G: Worksite intervention effects on motivation, physical activity, and health: A cluster randomized controlled trial,. Psychol Sport Exerc, 2018; 35: 171-180 [Google Scholar]
- 1096).Murray DM, Kurth C, Mullis R, Jeffery RW: Cholesterol reduction through low-intensity interventions: results from the Minnesota Heart Health Program. Prev Med, 1990; 19: 181-189 [DOI] [PubMed] [Google Scholar]
- 1097).Perkins DD, Zimmerman MA: Empowerment theory, research, and application. Am J Community Psychol, 1995; 23: 569-579 [DOI] [PubMed] [Google Scholar]
- 1098).Ok Ham K, Jeong Kim B: Evaluation of a cardiovascular health promotion programme offered to low-income women in Korea. J Clin Nurs, 2011; 20: 1245-1254 [DOI] [PubMed] [Google Scholar]
- 1099).Morton DP, Rankin P, Morey P, Kent L, Hurlow T, Chang E, Diehl H: The effectiveness of the Complete Health Improvement Program (CHIP) in Australasia for reducing selected chronic disease risk factors: a feasibility study. N Z Med J, 2013; 126: 43-54 [PubMed] [Google Scholar]
- 1100).Salinardi TC, Batra P, Roberts SB, Urban LE, Robinson LM, Pittas AG, Lichtenstein AH, Deckersbach T, Saltzman E, Das SK: Lifestyle intervention reduces body weight and improves cardiometabolic risk factors in worksites. Am J Clin Nutr, 2013; 97: 667-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1101).Kendall P, Hollon S: Cognitive-behavioral interventions: Overview and current status, Cognitive-behavioral interventions: theory, research, and procedures., Academic Press, 1979 [Google Scholar]
- 1102).Dekkers JC, van Wier MF, Ariëns GA, Hendriksen IJ, Pronk NP, Smid T, van Mechelen W: Comparative effectiveness of lifestyle interventions on cardiovascular risk factors among a Dutch overweight working population: a randomized controlled trial. BMC Public Health, 2011; 11: 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1103).Noguchi M, Kojima S, Sairenchi T, Kinuta M, Yamakawa M, Nishizawa H, Takahara M, Imano H, Kitamura A, Yoshida T, Shintani A, Saito I, Yokoyama T, Shimomura I, Iso H: Japan Trial in High-Risk Individuals to Enhance Their Referral to Physicians (J-HARP)-A Nurse-Led, Community-Based Prevention Program of Lifestyle-Related Disease. J Epidemiol, 2020; 30: 194-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1104).Iso H, Noguchi M, Yokoyama T, Yoshida T, Saito I, Shintani A, Sairenchi T, Nishizawa H, Imano H, Kitamura A, Shimomura I, for J-HARP Research Group. Effect of a Community-Based Program to Accelerate Referral to Physicians for Individuals at High-Risk of Lifestyle-Related Diseases: A Cluster Randomized Trial, J Atheroscler Thromb. 2023; 30: 1389-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105).Itakura H, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K: Relationship between coronary events and serum cholesterol during 10 years of low-dose simvastatin therapy: long-term efficacy and safety in Japanese patients with hypercholesterolemia in the Japan Lipid Intervention Trial (J-LIT) Extension 10 Study, a prospective large-scale observational cohort study. Circ J, 2008; 72: 1218-1224 [DOI] [PubMed] [Google Scholar]
- 1106).Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y, Fujita H, Higaki J, Hirata KI, Ishibashi S, Isshiki T, Ito S, Kashiwagi A, Kato S, Kitagawa K, Kitakaze M, Kitazono T, Kurabayashi M, Miyauchi K, Murakami T, Murohara T, Node K, Ogawa S, Saito Y, Seino Y, Shigeeda T, Shindo S, Sugawara M, Sugiyama S, Terauchi Y, Tsutsui H, Ueshima K, Utsunomiya K, Yamagishi M, Yamazaki T, Yo S, Yokote K, Yoshida K, Yoshimura M, Yoshimura N, Nakao K, Nagai R: Intensive Treat-to-Target Statin Therapy in High-Risk Japanese Patients With Hypercholesterolemia and Diabetic Retinopathy: Report of a Randomized Study. Diabetes Care, 2018; 41: 1275-1284 [DOI] [PubMed] [Google Scholar]
- 1107).Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y, Fujita H, Higaki J, Hirata KI, Ishibashi S, Isshiki T, Ito S, Kashiwagi A, Kato S, Kitagawa K, Kitakaze M, Kitazono T, Kurabayashi M, Miyauchi K, Murakami T, Murohara T, Node K, Ogawa S, Saito Y, Seino Y, Shigeeda T, Shindo S, Sugawara M, Sugiyama S, Terauchi Y, Tsutsui H, Ueshima K, Utsunomiya K, Yamagishi M, Yamazaki T, Yo S, Yokote K, Yoshida K, Yoshimura M, Yoshimura N, Nakao K, Nagai R: Achieving LDL cholesterol target levels <1.81 mmol/L may provide extra cardiovascular protection in patients at high risk: Exploratory analysis of the Standard Versus Intensive Statin Therapy for Patients with Hypercholesterolaemia and Diabetic Retinopathy study. Diabetes Obes Metab, 2019; 21: 791-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1108).Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, Urabe T, Uchida Y, Hayashi M, Yokota N, Nishida H, Otonari T, Arai T, Sakuma I, Sakabe K, Yamamoto M, Kobayashi T, Oikawa S, Yamashita S, Rakugi H, Imai T, Tanaka S, Ohashi Y, Kuwabara M, Ito H: Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial. Circulation, 2019; 140: 992-1003 [DOI] [PubMed] [Google Scholar]
- 1109).Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H: Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation, 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 1110).Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M: Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302 [DOI] [PubMed] [Google Scholar]
- 1111).Takayama T, Hiro T, Yamagishi M, Daida H, Hirayama A, Saito S, Yamaguchi T, Matsuzaki M: Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ J, 2009; 73: 2110-2117 [DOI] [PubMed] [Google Scholar]
- 1112).Nakajima N, Miyauchi K, Yokoyama T, Manabu O, Tadashi M, Hiroshi T, Akihisa N, Ken Y, Sinya O, Takeshi K, Satoru S, Hiroyuki D: Effect of combination of ezetimibe and a statin on coronary plaque regression in patients with acute coronary syndrome: ZEUS trial (eZEtimibe Ultrasound Study),. IJC Metabolic & Endocrine, 2014; 3: 8-13 [Google Scholar]
- 1113).Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T, Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K, Oshima S, Kaikita K, Hokimoto S, Ogawa H: Impact of Dual Lipid-Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: The Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol, 2015; 66: 495-507 [DOI] [PubMed] [Google Scholar]
- 1114).Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, Uno K, Tuzcu EM, Nissen SE: Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol, 2010; 55: 2399-2407 [DOI] [PubMed] [Google Scholar]
- 1115).Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Miyauchi K, Yamazaki T, Ito H, Otsuji Y, Kimura K, Takahashi J, Hirayama A, Yokoi H, Kitagawa K, Urabe T, Okada Y, Terayama Y, Toyoda K, Nagao T, Matsumoto M, Ohashi Y, Kaneko T, Fujita R, Ohtsu H, Ogawa H, Daida H, Shimokawa H, Saito Y, Kimura T, Inoue T, Matsuzaki M, Nagai R: High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial. Circulation, 2018; 137: 1997-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1116).Amarenco P, Labreuche J: Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol, 2009; 8: 453-463 [DOI] [PubMed] [Google Scholar]
- 1117).McKinney JS, Kostis WJ: Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke, 2012; 43: 2149-2156 [DOI] [PubMed] [Google Scholar]
- 1118).Salvatore T, Morganti R, Marchioli R, De Caterina R: Cholesterol Lowering and Stroke: No Longer Room for Pleiotropic Effects of Statins - Confirmation from PCSK9 Inhibitor Studies. Am J Med, 2020; 133: 95-99.e96 [DOI] [PubMed] [Google Scholar]
- 1119).Pandit AK, Kumar P, Kumar A, Chakravarty K, Misra S, Prasad K: High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand, 2016; 134: 22-28 [DOI] [PubMed] [Google Scholar]
- 1120).Judge C, Ruttledge S, Costello M, Murphy R, Loughlin E, Alvarez-Iglesias A, Ferguson J, Gorey S, Nolan A, Canavan M, O'Halloran M, O'Donnell MJ: Lipid Lowering Therapy, Low-Density Lipoprotein Level and Risk of Intracerebral Hemorrhage - A Meta-Analysis. J Stroke Cerebrovasc Dis, 2019; 28: 1703-1709 [DOI] [PubMed] [Google Scholar]
- 1121).Cheng Y, Qiao L, Jiang Z, Dong X, Feng H, Gui Q, Lu Y, Liang Y: Significant reduction in the LDL cholesterol increases the risk of intracerebral hemorrhage: a systematic review and meta-analysis of 33 randomized controlled trials. Am J Transl Res, 2020; 12: 463-477 [PMC free article] [PubMed] [Google Scholar]
- 1122).Uchiyama S, Nakaya N, Mizuno K, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Nakamura H: Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: analysis of data from the MEGA Study, a large randomized controlled trial. J Neurol Sci, 2009; 284: 72-76 [DOI] [PubMed] [Google Scholar]
- 1123).Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM: Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med, 2019; 380: 11-22 [DOI] [PubMed] [Google Scholar]
- 1124).Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis, 2008; 200: 135-140 [DOI] [PubMed] [Google Scholar]
- 1125).Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K, Nissen SE: Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA, 2020; 324: 2268-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126).Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M: Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet, 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 1127).Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr., Cushman WC, Simons-Morton DG, Byington RP: Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128).Scott R, O'Brien R, Fulcher G, Pardy C, D'Emden M, Tse D, Taskinen MR, Ehnholm C, Keech A: Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care, 2009; 32: 493-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1129).Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-González I, Briel M: Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev, 2016; 11: Cd009753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130).Wang D, Liu B, Tao W, Hao Z, Liu M: Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev, 2015; 2015: Cd009580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131).Maki KC, Guyton JR, Orringer CE, Hamilton-Craig I, Alexander DD, Davidson MH: Triglyceride-lowering therapies reduce cardiovascular disease event risk in subjects with hypertriglyceridemia. J Clin Lipidol, 2016; 10: 905-914 [DOI] [PubMed] [Google Scholar]
- 1132).Marston NA, Giugliano RP, Im K, Silverman MG, O'Donoghue ML, Wiviott SD, Ference BA, Sabatine MS: Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation, 2019; 140: 1308-1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1133).Hulten E, Jackson JL, Douglas K, George S, Villines TC: The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med, 2006; 166: 1814-1821 [DOI] [PubMed] [Google Scholar]
- 1134).Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, Kojima T, Yokoyama K, Kurata T, Daida H: Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (Extended-ESTABLISH trial): a follow-up study. Atherosclerosis, 2010; 210: 497-502 [DOI] [PubMed] [Google Scholar]
- 1135).Soran H, Kwok S, Adam S, Ho JH, Durrington PN: Evidence for more intensive cholesterol lowering. Curr Opin Lipidol, 2017; 28: 291-299 [DOI] [PubMed] [Google Scholar]
- 1136).Natsuaki M, Furukawa Y, Morimoto T, Nakagawa Y, Ono K, Kaburagi S, Inada T, Mitsuoka H, Taniguchi R, Nakano A, Kita T, Sakata R, Kimura T: Intensity of statin therapy, achieved low-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after coronary revascularization. Perspectives from the CREDO-Kyoto registry cohort-2. Circ J, 2012; 76: 1369-1379 [DOI] [PubMed] [Google Scholar]
- 1137).Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS: Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA, 2016; 316: 1289-1297 [DOI] [PubMed] [Google Scholar]
- 1138).Puymirat E, Bonaca M, Fumery M, Tea V, Aissaoui N, Lemesles G, Bonello L, Ducrocq G, Cayla G, Ferrières J, Schiele F, Simon T, Danchin N: Atherothrombotic risk stratification after acute myocardial infarction: The Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention in the light of the French Registry of Acute ST Elevation or non-ST Elevation Myocardial Infarction registries. Clin Cardiol, 2019; 42: 227-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139).Bohula EA, Morrow DA, Giugliano RP, Blazing MA, He P, Park JG, Murphy SA, White JA, Kesaniemi YA, Pedersen TR, Brady AJ, Mitchel Y, Cannon CP, Braunwald E: Atherothrombotic Risk Stratification and Ezetimibe for Secondary Prevention. J Am Coll Cardiol, 2017; 69: 911-921 [DOI] [PubMed] [Google Scholar]
- 1140).Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, Kuder JF, Murphy SA, Wiviott SD, Kurtz CE, Honarpour N, Keech AC, Sever PS, Pedersen TR: Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: Analysis From FOURIER. Circulation, 2018; 138: 756-766 [DOI] [PubMed] [Google Scholar]
- 1141).Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM: Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 1142).Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X: Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst Rev, 2018; 11: Cd012502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143).Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR: Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 1144).Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM: Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med, 2018; 379: 2097-2107 [DOI] [PubMed] [Google Scholar]
- 1145).Schmidt AF, Carter JL, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP: PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2020; 10: Cd011748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146).Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Räber L, Mach F, Windecker S: Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J, 2018; 39: 1172-1180 [DOI] [PubMed] [Google Scholar]
- 1147).Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J: Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med, 2014; 371: 203-212 [DOI] [PubMed] [Google Scholar]
- 1148).HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J, 2013; 34: 1279-1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149).Schandelmaier S, Briel M, Saccilotto R, Olu KK, Arpagaus A, Hemkens LG, Nordmann AJ: Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev, 2017; 6: Cd009744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150).D'Andrea E, Hey SP, Ramirez CL, Kesselheim AS: Assessment of the Role of Niacin in Managing Cardiovascular Disease Outcomes: A Systematic Review and Meta-analysis. JAMA Netw Open, 2019; 2: e192224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1151).Keene D, Price C, Shun-Shin MJ, Francis DP: Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ, 2014; 349: g4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1152).Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B: Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysis. Atherosclerosis, 2011; 217: 492-498 [DOI] [PubMed] [Google Scholar]
- 1153).Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ: Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med, 2017; 377: 1217-1227 [DOI] [PubMed] [Google Scholar]
- 1154).Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B: Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 1155).Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, Zafarino J, Mitchel Y, Barter P: Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med, 2010; 363: 2406-2415 [DOI] [PubMed] [Google Scholar]
- 1156).Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS: Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 1157).Mabuchi H, Haba T, Tatami R, Miyamoto S, Sakai Y, Wakasugi T, Watanabe A, Koizumi J, Takeda R: Effect of an inhibitor of 3-hydroxy-3-methyglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10-levels in patients with familial hypercholesterolemia. N Engl J Med, 1981; 305: 478-482 [DOI] [PubMed] [Google Scholar]
- 1158).Endo A: The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res, 1992; 33: 1569-1582 [PubMed] [Google Scholar]
- 1159).Bilheimer DW, Grundy SM, Brown MS, Goldstein JL: Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc Natl Acad Sci U S A, 1983; 80: 4124-4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1160).Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, Keilson LM, Brown WV, Miller VT, Shurzinske LJ, Black DM: Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA, 1996; 275: 128-133 [PubMed] [Google Scholar]
- 1161).Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK: Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA, 2011; 305: 2556-2564 [DOI] [PubMed] [Google Scholar]
- 1162).Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL, 2nd, Goldstein LB, Chin C, Tannock LR, Miller M, Raghuveer G, Duell PB, Brinton EA, Pollak A, Braun LT, Welty FK: Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol, 2019; 39: e38-e81 [DOI] [PubMed] [Google Scholar]
- 1163).Phamaceuticals and Medical Devices Agency: Revision of "Precautions" for preparations containing HMG-CoA reductase inhibitors. https://www.pmda.go.jp/files/000214541.pdf, 2016 (in Japanese) [Google Scholar]
- 1164).Mammen AL: Statin-Associated Autoimmune Myopathy. N Engl J Med, 2016; 374: 664-669 [DOI] [PubMed] [Google Scholar]
- 1165).Kajinami K, Tsukamoto K, Koba S, Inoue I, Yamakawa M, Suzuki S, Hamano T, Saito H, Saito Y, Masuda S, Nakayama T, Okamura T, Yamashita S, Kagawa T, Kaneyama J, Kuriyama A, Tanaka R, Hirata A: Statin Intolerance Clinical Guide 2018. J Atheroscler Thromb, 2020; 27: 375-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166).Edison RJ, Muenke M: Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med, 2004; 350: 1579-1582 [DOI] [PubMed] [Google Scholar]
- 1167).Gagné C, Gaudet D, Bruckert E: Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation, 2002; 105: 2469-2475 [DOI] [PubMed] [Google Scholar]
- 1168).Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT: Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med, 1990; 323: 1289-1298 [DOI] [PubMed] [Google Scholar]
- 1169).Mabuchi H, Kobayashi J, Kajinami K: Effect of MCI-196 in combination with pravastatin on hypercholesterolemia (I) - a study in patients with familial hypercholesterolemia (FH), Rinsho Iyaku Inc., 1996 (in Japanese) [Google Scholar]
- 1170).Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP: Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation, 2003; 107: 2409-2415 [DOI] [PubMed] [Google Scholar]
- 1171).Thomopoulos C, Skalis G, Michalopoulou H, Tsioufis C, Makris T: Effect of Low-Density Lipoprotein Cholesterol Lowering by Ezetimibe/Simvastatin on Outcome Incidence: Overview, Meta-Analyses, and Meta-Regression Analyses of Randomized Trials. Clin Cardiol, 2015; 38: 763-769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172).Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, Yamamoto H, Suzuki H: NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci Transl Med, 2015; 7: 275ra223 [DOI] [PubMed] [Google Scholar]
- 1173).The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA, 1984; 251: 351-364 [DOI] [PubMed] [Google Scholar]
- 1174).The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA, 1984; 251: 365-374 [PubMed] [Google Scholar]
- 1175).Rudling MJ, Reihnér E, Einarsson K, Ewerth S, Angelin B: Low density lipoprotein receptor-binding activity in human tissues: quantitative importance of hepatic receptors and evidence for regulation of their expression in vivo. Proc Natl Acad Sci U S A, 1990; 87: 3469-3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1176).Kodama T, Reddy P, Kishimoto C, Krieger M: Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci U S A, 1988; 85: 9238-9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177).Kita T, Ishii K, Yokode M, Kume N, Nagano Y, Arai H, Kawai C: The role of oxidized low density lipoprotein in the pathogenesis of atherosclerosis. Eur Heart J, 1990; 11 Suppl E: 122-127 [DOI] [PubMed] [Google Scholar]
- 1178).Itabe H, Takeshima E, Iwasaki H, Kimura J, Yoshida Y, Imanaka T, Takano T: A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J Biol Chem, 1994; 269: 15274-15279 [PubMed] [Google Scholar]
- 1179).Itabe H, Yamamoto H, Suzuki M, Kawai Y, Nakagawa Y, Suzuki A, Imanaka T, Takano T: Oxidized phosphatidylcholines that modify proteins. Analysis by monoclonal antibody against oxidized low density lipoprotein. J Biol Chem, 1996; 271: 33208-33217 [DOI] [PubMed] [Google Scholar]
- 1180).Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y: Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 1181).Tardif JC, Cöté G, Lespérance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P: Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med, 1997; 337: 365-372 [DOI] [PubMed] [Google Scholar]
- 1182).Yokoi H, Daida H, Kuwabara Y, Nishikawa H, Takatsu F, Tomihara H, Nakata Y, Kutsumi Y, Ohshima S, Nishiyama S, Seki A, Kato K, Nishimura S, Kanoh T, Yamaguchi H: Effectiveness of an antioxidant in preventing restenosis after percutaneous transluminal coronary angioplasty: the Probucol Angioplasty Restenosis Trial. J Am Coll Cardiol, 1997; 30: 855-862 [DOI] [PubMed] [Google Scholar]
- 1183).Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, Hayashi J: Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol, 2002; 39: 610-616 [DOI] [PubMed] [Google Scholar]
- 1184).Walldius G, Erikson U, Olsson AG, Bergstrand L, Hådell K, Johansson J, Kaijser L, Lassvik C, Mölgaard J, Nilsson S, et al.: The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol, 1994; 74: 875-883 [DOI] [PubMed] [Google Scholar]
- 1185).Yamashita S, Arai H, Bujo H, Masuda D, Ohama T, Ishibashi T, Yanagi K, Doi Y, Nakagawa S, Yamashiro K, Tanabe K, Kita T, Matsuzaki M, Saito Y, Fukushima M, Matsuzawa Y: Probucol Trial for Secondary Prevention of Atherosclerotic Events in Patients with Coronary Heart Disease (PROSPECTIVE). J Atheroscler Thromb, 2021; 28: 103-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1186).Arai H, Bujo H, Masuda D, Ishibashi T, Nakagawa S, Tanabe K, Kagimura T, Kang HJ, Kim MH, Sung J, Kim SH, Kim CH, Park JE, Ge J, Oh BH, Kita T, Saito Y, Fukushima M, Matsuzawa Y, Yamashita S: Integrated Analysis of Two Probucol Trials for the Secondary Prevention of Atherosclerotic Cardiovascular Events: PROSPECTIVE and IMPACT. J Atheroscler Thromb, 2022; 29: 850-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1187).Schoonjans K, Staels B, Auwerx J: Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res, 1996; 37: 907-925 [PubMed] [Google Scholar]
- 1188).Fruchart JC, Brewer HB, Jr., Leitersdorf E: Consensus for the use of fibrates in the treatment of dyslipoproteinemia and coronary heart disease. Fibrate Consensus Group. Am J Cardiol, 1998; 81: 912-917 [DOI] [PubMed] [Google Scholar]
- 1189).Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H, Fruchart JC, Kodama T: Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: A randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis, 2016; 249: 36-43 [DOI] [PubMed] [Google Scholar]
- 1190).Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 1191).Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke, 2008; 39: 2052-2058 [DOI] [PubMed] [Google Scholar]
- 1192).Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J: Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med, 2018; 379: 1540-1550 [DOI] [PubMed] [Google Scholar]
- 1193).Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE: Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N Engl J Med, 2019; 380: 23-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1194).Lombardi M, Chiabrando JG, Vescovo GM, Bressi E, Del Buono MG, Carbone S, Koenig RA, Van Tassell BW, Abbate A, Biondi-Zoccai G, Dixon DL: Impact of Different Doses of Omega-3 Fatty Acids on Cardiovascular Outcomes: a Pairwise and Network Meta-analysis. Curr Atheroscler Rep, 2020; 22: 45 [DOI] [PubMed] [Google Scholar]
- 1195).Alderman JD, Pasternak RC, Sacks FM, Smith HS, Monrad ES, Grossman W: Effect of a modified, well-tolerated niacin regimen on serum total cholesterol, high density lipoprotein cholesterol and the cholesterol to high density lipoprotein ratio. Am J Cardiol, 1989; 64: 725-729 [DOI] [PubMed] [Google Scholar]
- 1196).Teramoto T, Yamada N, Shimano H, Oka Y, Itakura H, Saito Y, Morisaki N, Shirai K, Ishikawa T, Tada N, Ito H, Yamanouchi T, Matsushima T, Kawakami M, Murase T, Okubo M, Totsuka Y, Kikuchi M: Dose-dependent effect of niceritrol on plasma lipoprotein-a. Scand J Clin Lab Invest, 1996; 56: 359-365 [DOI] [PubMed] [Google Scholar]
- 1197).Carlson LA, Hamsten A, Asplund A: Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med, 1989; 226: 271-276 [DOI] [PubMed] [Google Scholar]
- 1198).Matsunaga A, Handa K, Mori T, Moriyama K, Hidaka K, Yuki M, Sasaki J, Arakawa K: Effects of niceritrol on levels of serum lipids, lipoprotein(a), and fibrinogen in patients with primary hypercholesterolemia. Atherosclerosis, 1992; 94: 241-248 [DOI] [PubMed] [Google Scholar]
- 1199).Ni YG, Condra JH, Orsatti L, Shen X, Di Marco S, Pandit S, Bottomley MJ, Ruggeri L, Cummings RT, Cubbon RM, Santoro JC, Ehrhardt A, Lewis D, Fisher TS, Ha S, Njimoluh L, Wood DD, Hammond HA, Wisniewski D, Volpari C, Noto A, Lo Surdo P, Hubbard B, Carfí A, Sitlani A: A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J Biol Chem, 2010; 285: 12882-12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200).Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A: A Phase 3 Study of Evolocumab (AMG 145) in Statin-Treated Japanese Patients at High Cardiovascular Risk. Am J Cardiol, 2016; 117: 40-47 [DOI] [PubMed] [Google Scholar]
- 1201).Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, Uno K, Baccara-Dinet MT, Nohara A: Efficacy and Safety of Alirocumab in Japanese Patients With Heterozygous Familial Hypercholesterolemia or at High Cardiovascular Risk With Hypercholesterolemia Not Adequately Controlled With Statins - ODYSSEY JAPAN Randomized Controlled Trial. Circ J, 2016; 80: 1980-1987 [DOI] [PubMed] [Google Scholar]
- 1202).Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ: Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med, 2007; 356: 148-156 [DOI] [PubMed] [Google Scholar]
- 1203).Samaha FF, McKenney J, Bloedon LT, Sasiela WJ, Rader DJ: Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med, 2008; 5: 497-505 [DOI] [PubMed] [Google Scholar]
- 1204).Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S: n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med, 2012; 367: 309-318 [DOI] [PubMed] [Google Scholar]
- 1205).Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 1206).Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, Campbell SE, Oshima R, Amarenco P, Blom DJ, Brinton EA, Eckel RH, Elam MB, Felicio JS, Ginsberg HN, Goudev A, Ishibashi S, Joseph J, Kodama T, Koenig W, Leiter LA, Lorenzatti AJ, Mankovsky B, Marx N, Nordestgaard BG, Pall D, Ray KK, Santos RD, Soran H, Susekov A, Tendera M, Yokote K, Paynter NP, Buring JE, Libby P, Ridker PM, for the PROMINENT Investigator: Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N Engl J Med, 2022; 387: 1923-1934 [DOI] [PubMed] [Google Scholar]
- 1207).Wiklund O, Pirazzi C, Romeo S: Monitoring of lipids, enzymes, and creatine kinase in patients on lipid-lowering drug therapy. Curr Cardiol Rep, 2013; 15: 397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1208).Hedrington MS, Davis SN: Peroxisome proliferator-activated receptor alpha-mediated drug toxicity in the liver. Expert Opin Drug Metab Toxicol, 2018; 14: 671-677 [DOI] [PubMed] [Google Scholar]
- 1209).Law M, Rudnicka AR: Statin safety: a systematic review. Am J Cardiol, 2006; 97: 52c-60c [DOI] [PubMed] [Google Scholar]
- 1210).Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, Krumholz HM: Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation, 2006; 114: 2788-2797 [DOI] [PubMed] [Google Scholar]
- 1211).Thompson PD, Clarkson P, Karas RH: Statin-associated myopathy. JAMA, 2003; 289: 1681-1690 [DOI] [PubMed] [Google Scholar]
- 1212).Bays H: Statin safety: an overview and assessment of the data--2005. Am J Cardiol, 2006; 97: 6c-26c [DOI] [PubMed] [Google Scholar]
- 1213).Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R: SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med, 2008; 359: 789-799 [DOI] [PubMed] [Google Scholar]
- 1214).Joy TR, Hegele RA: Narrative review: statin-related myopathy. Ann Intern Med, 2009; 150: 858-868 [DOI] [PubMed] [Google Scholar]
- 1215).Katz DH, Intwala SS, Stone NJ: Addressing statin adverse effects in the clinic: the 5 Ms. J Cardiovasc Pharmacol Ther, 2014; 19: 533-542 [DOI] [PubMed] [Google Scholar]
- 1216).Yu S, Jin J, Chen Z, Luo X: High-intensity statin therapy yields better outcomes in acute coronary syndrome patients: a meta-analysis involving 26,497 patients. Lipids Health Dis, 2020; 19: 194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1217).Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA: Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med, 2005; 118: 618-624 [DOI] [PubMed] [Google Scholar]
- 1218).Jacobson TA: Toward "pain-free" statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc, 2008; 83: 687-700 [DOI] [PubMed] [Google Scholar]
- 1219).Bitzur R, Cohen H, Kamari Y, Harats D: Intolerance to statins: mechanisms and management. Diabetes Care, 2013; 36 Suppl 2: S325-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1220).Azemawah V, Movahed MR, Centuori P, Penaflor R, Riel PL, Situ S, Shadmehr M, Hashemzadeh M: State of the Art Comprehensive Review of Individual Statins, Their Differences, Pharmacology, and Clinical Implications. Cardiovasc Drugs Ther, 2019; 33: 625-639 [DOI] [PubMed] [Google Scholar]
- 1221).Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM: Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care, 2009; 32: 1924-1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1222).Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I: Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet, 2010; 375: 735-742 [DOI] [PubMed] [Google Scholar]
- 1223).Casula M, Mozzanica F, Scotti L, Tragni E, Pirillo A, Corrao G, Catapano AL: Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis, 2017; 27: 396-406 [DOI] [PubMed] [Google Scholar]
- 1224).Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ: Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet, 2012; 380: 565-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1225).Cybulska B, Kłosiewicz-Latoszek L: How do we know that statins are diabetogenic, and why? Is it an important issue in the clinical practice? Kardiol Pol, 2018; 76: 1217-1223 [DOI] [PubMed] [Google Scholar]
- 1226).Davis KA, Miyares MA: Lomitapide: A novel agent for the treatment of homozygous familial hypercholesterolemia. Am J Health Syst Pharm, 2014; 71: 1001-1008 [DOI] [PubMed] [Google Scholar]
- 1227).Sacks FM, Stanesa M, Hegele RA: Severe hypertriglyceridemia with pancreatitis: thirteen years' treatment with lomitapide. JAMA Intern Med, 2014; 174: 443-447 [DOI] [PubMed] [Google Scholar]
- 1228).France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, Ramaswami U, Seed M, Neely D, Cramb R, Shoulders C, Barbir M, Pottle A, Eatough R, Martin S, Bayly G, Simpson B, Halcox J, Edwards R, Main L, Payne J, Soran H: HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis, 2016; 255: 128-139 [DOI] [PubMed] [Google Scholar]
- 1229).Blom DJ, Raal FJ, Santos RD, Marais AD: Lomitapide and Mipomersen-Inhibiting Microsomal Triglyceride Transfer Protein (MTP) and apoB100 Synthesis. Curr Atheroscler Rep, 2019; 21: 48 [DOI] [PubMed] [Google Scholar]
- 1230).Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, Chang Q, Foulds P: Efficacy and Safety of Lomitapide in Japanese Patients with Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb, 2017; 24: 402-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1231).Nohara A, Otsubo Y, Yanagi K, Yoshida M, Ikewaki K, Harada-Shiba M, Jurecka A: Safety and Efficacy of Lomitapide in Japanese Patients with Homozygous Familial Hypercholesterolemia (HoFH): Results from the AEGR-733-301 Long-Term Extension Study. J Atheroscler Thromb, 2019; 26: 368-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232).Hirota T, Ieiri I: Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol, 2015; 11: 1435-1447 [DOI] [PubMed] [Google Scholar]
- 1233).Omar MA, Wilson JP, Cox TS: Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother, 2001; 35: 1096-1107 [DOI] [PubMed] [Google Scholar]
- 1234).Enajat M, Teerenstra S, van Kuilenburg JT, van Sorge-Greve AH, Albers-Akkers MT, Verheugt FW, Pop GA: Safety of the combination of intensive cholesterol-lowering therapy with oral anticoagulation medication in elderly patients with atrial fibrillation: a randomized, double-blind, placebo-controlled study. Drugs Aging, 2009; 26: 585-593 [DOI] [PubMed] [Google Scholar]
- 1235).Patel BV, Leslie RS, Thiebaud P, Nichol MB, Tang SS, Solomon H, Honda D, Foody JM: Adherence with single-pill amlodipine/atorvastatin vs a two-pill regimen. Vasc Health Risk Manag, 2008; 4: 673-681 [PMC free article] [PubMed] [Google Scholar]
- 1236).Weisser B, Predel HG, Gillessen A, Hacke C, Vor dem Esche J, Rippin G, Noetel A, Randerath O: Single Pill Regimen Leads to Better Adherence and Clinical Outcome in Daily Practice in Patients Suffering from Hypertension and/or Dyslipidemia: Results of a Meta-Analysis. High Blood Press Cardiovasc Prev, 2020; 27: 157-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1237).Hussein MA, Chapman RH, Benner JS, Tang SS, Solomon HA, Joyce A, Foody JM: Does a single-pill antihypertensive/lipid-lowering regimen improve adherence in US managed care enrolees? A non-randomized, observational, retrospective study. Am J Cardiovasc Drugs, 2010; 10: 193-202 [DOI] [PubMed] [Google Scholar]
- 1238).Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, Yunis C: The use of a single-pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo-controlled, multicenter study. J Clin Hypertens (Greenwich), 2009; 11: 22-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1239).Yusuf S, Joseph P, Dans A, Gao P, Teo K, Xavier D, López-Jaramillo P, Yusoff K, Santoso A, Gamra H, Talukder S, Christou C, Girish P, Yeates K, Xavier F, Dagenais G, Rocha C, McCready T, Tyrwhitt J, Bosch J, Pais P: Polypill with or without Aspirin in Persons without Cardiovascular Disease. N Engl J Med, 2021; 384: 216-228 [Google Scholar]
- 1240).Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD: Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med, 2009; 169: 260-268 [DOI] [PubMed] [Google Scholar]
- 1241).Origasa H, Yokoyama M, Matsuzaki M, Saito Y, Matsuzawa Y: Clinical importance of adherence to treatment with eicosapentaenoic acid by patients with hypercholesterolemia. Circ J, 2010; 74: 510-517 [DOI] [PubMed] [Google Scholar]
- 1242).van Driel ML, Morledge MD, Ulep R, Shaffer JP, Davies P, Deichmann R: Interventions to improve adherence to lipid-lowering medication. Cochrane Database Syst Rev, 2016; 12: Cd004371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1243).Parris ES, Lawrence DB, Mohn LA, Long LB: Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care, 2005; 28: 595-599 [DOI] [PubMed] [Google Scholar]
- 1244).Herttua K, Martikainen P, Batty GD, Kivimäki M: Poor Adherence to Statin and Antihypertensive Therapies as Risk Factors for Fatal Stroke. J Am Coll Cardiol, 2016; 67: 1507-1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1245).Deshpande S, Quek RG, Forbes CA, de Kock S, Kleijnen J, Gandra SR, Simpson RJ, Jr.: A systematic review to assess adherence and persistence with statins. Curr Med Res Opin, 2017; 33: 769-778 [DOI] [PubMed] [Google Scholar]
- 1246).Mann DM, Woodward M, Muntner P, Falzon L, Kronish I: Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother, 2010; 44: 1410-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1247).Wiegand P, McCombs JS, Wang JJ: Factors of hyperlipidemia medication adherence in a nationwide health plan. Am J Manag Care, 2012; 18: 193-199 [PubMed] [Google Scholar]
- 1248).Helin-Salmivaara A, Lavikainen P, Korhonen MJ, Halava H, Junnila SY, Kettunen R, Neuvonen PJ, Martikainen JE, Ruokoniemi P, Saastamoinen LK, Virta L, Huupponen R: Long-term persistence with statin therapy: a nationwide register study in Finland. Clin Ther, 2008; 30 Pt 2: 2228-2240 [DOI] [PubMed] [Google Scholar]
- 1249).Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J: Long-term persistence in use of statin therapy in elderly patients. JAMA, 2002; 288: 455-461 [DOI] [PubMed] [Google Scholar]
- 1250).Navar AM, Roe MT, White JA, Cannon CP, Lokhnygina Y, Newby LK, Giugliano RP, Tershakovec AM, Braunwald E, Califf RM, Blazing MA: Medication Discontinuation in the IMPROVE-IT Trial. Circ Cardiovasc Qual Outcomes, 2019; 12: e005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1251).Barker-Collo S, Krishnamurthi R, Witt E, Feigin V, Jones A, McPherson K, Starkey N, Parag V, Jiang Y, Barber PA, Rush E, Bennett D, Aroll B: Improving Adherence to Secondary Stroke Prevention Strategies Through Motivational Interviewing: Randomized Controlled Trial. Stroke, 2015; 46: 3451-3458 [DOI] [PubMed] [Google Scholar]
- 1252).Lyons I, Barber N, Raynor DK, Wei L: The Medicines Advice Service Evaluation (MASE): a randomised controlled trial of a pharmacist-led telephone based intervention designed to improve medication adherence. BMJ Qual Saf, 2016; 25: 759-769 [DOI] [PubMed] [Google Scholar]
- 1253).Santo K, Singleton A, Rogers K, Thiagalingam A, Chalmers J, Chow CK, Redfern J: Medication reminder applications to improve adherence in coronary heart disease: a randomised clinical trial. Heart, 2019; 105: 323-329 [DOI] [PubMed] [Google Scholar]
- 1254).Wu Z, Camargo CA, Jr., Khaw KT, Waayer D, Lawes CMM, Toop L, Scragg R: Effects of vitamin D supplementation on adherence to and persistence with long-term statin therapy: Secondary analysis from the randomized, double-blind, placebo-controlled ViDA study. Atherosclerosis, 2018; 273: 59-66 [DOI] [PubMed] [Google Scholar]
- 1255).Daniel H, Christian W, Robin H, Lars S, Thomas M: Statin treatment after acute coronary syndrome: Adherence and reasons for non-adherence in a randomized controlled intervention trial. Sci Rep, 2019; 9: 12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1256).Oñatibia-Astibia A, Malet-Larrea A, Larrañaga B, Gastelurrutia M, Calvo B, Ramírez D, Cantero I, Garay Á, Goyenechea E: Tailored interventions by community pharmacists and general practitioners improve adherence to statins in a Spanish randomized controlled trial. Health Serv Res, 2019; 54: 658-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1257).Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, Brennan TA, Avorn J, Shrank WH: Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med, 2014; 161: 400-407 [DOI] [PubMed] [Google Scholar]
- 1258).Smith SC, Jr., Grundy SM: 2013 ACC/AHA guideline recommends fixed-dose strategies instead of targeted goals to lower blood cholesterol. J Am Coll Cardiol, 2014; 64: 601-612 [DOI] [PubMed] [Google Scholar]
- 1259).Bangalore S, Fayyad R, Kastelein JJ, Laskey R, Amarenco P, DeMicco DA, Waters DD: 2013 Cholesterol Guidelines Revisited: Percent LDL Cholesterol Reduction or Attained LDL Cholesterol Level or Both for Prognosis? Am J Med, 2016; 129: 384-391 [DOI] [PubMed] [Google Scholar]
- 1260).Bacquer D, Smedt D, Reiner Ž, Tokgözoğlu L, Clays E, Kotseva K, Rydén L, Wood D, Backer G: Percentage low-density lipoprotein-cholesterol response to a given statin dose is not fixed across the pre-treatment range: Real world evidence from clinical practice: Data from the ESC-EORP EUROASPIRE V Study. Eur J Prev Cardiol, 2020; 27: 1630-1636 [DOI] [PubMed] [Google Scholar]
- 1261).Hayward RA, Krumholz HM: Three reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes, 2012; 5: 2-5 [DOI] [PubMed] [Google Scholar]
- 1262).Hosomi N, Kitagawa K, Nagai Y, Nakagawa Y, Aoki S, Nezu T, Kagimura T, Maruyama H, Origasa H, Minematsu K, Uchiyama S, Matsumoto M: Desirable Low-Density Lipoprotein Cholesterol Levels for Preventing Stroke Recurrence: A Post Hoc Analysis of the J-STARS Study (Japan Statin Treatment Against Recurrent Stroke). Stroke, 2018; 49: 865-871 [DOI] [PubMed] [Google Scholar]
- 1263).Wang N, Fulcher J, Abeysuriya N, Park L, Kumar S, Di Tanna GL, Wilcox I, Keech A, Rodgers A, Lal S: Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol, 2020; 8: 36-49 [DOI] [PubMed] [Google Scholar]
- 1264).Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Jr., Ridker PM, Grundy SM, Kastelein JJ: Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol, 2014; 64: 485-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265).Ota T, Ishii H, Suzuki S, Shibata Y, Tatami Y, Harata S, Shimbo Y, Takayama Y, Tanaka A, Kawamura Y, Osugi N, Maeda K, Kondo T, Murohara T: Impact of the statin escape phenomenon on long-term clinical outcomes in patients with acute myocardial infarction: Subgroup analysis of the Nagoya Acute Myocardial Infarction Study (NAMIS). Atherosclerosis, 2015; 242: 155-160 [DOI] [PubMed] [Google Scholar]
- 1266).Tanaka S, Ikari Y, Ijichi T, Nakazawa G: Treat-to-target lipid control is effective but highlighted poor prognosis without indication of statin following percutaneous coronary intervention. Cardiovasc Interv Ther, 2017; 32: 358-364 [DOI] [PubMed] [Google Scholar]
- 1267).Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T: Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA, 2001; 285: 1711-1718 [DOI] [PubMed] [Google Scholar]
- 1268).Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM: Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med, 2004; 350: 1495-1504 [DOI] [PubMed] [Google Scholar]
- 1269).Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, Kayikçioglu M, Arntz HR, den Hartog FR, Veeger NJ, Colivicchi F, Dupuis J, Okazaki S, Wright RS, Bucher HC, Nordmann AJ: Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA, 2006; 295: 2046-2056 [DOI] [PubMed] [Google Scholar]
- 1270).Steg PG, Szarek M, Bhatt DL, Bittner VA, Brégeault MF, Dalby AJ, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Ostadal P, Parkhomenko A, Pordy R, Roe MT, Tricoci P, Vogel R, White HD, Zeiher AM, Schwartz GG: Effect of Alirocumab on Mortality After Acute Coronary Syndromes. Circulation, 2019; 140: 103-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271).Harada-Shiba M, Ako J, Arai H, Hirayama A, Murakami Y, Nohara A, Ozaki A, Uno K, Nakamura M: Prevalence of familial hypercholesterolemia in patients with acute coronary syndrome in Japan: Results of the EXPLORE-J study. Atherosclerosis, 2018; 277: 362-368 [DOI] [PubMed] [Google Scholar]
- 1272).Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Borén J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjærg-Hansen A: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1273).Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE: Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J, 2008; 29: 2625-2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1274).Harada-Shiba M, Sugisawa T, Makino H, Abe M, Tsushima M, Yoshimasa Y, Yamashita T, Miyamoto Y, Yamamoto A, Tomoike H, Yokoyama S: Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2010; 17: 667-674 [DOI] [PubMed] [Google Scholar]
- 1275).Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA: 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N Engl J Med, 2019; 381: 1547-1556 [DOI] [PubMed] [Google Scholar]
- 1276).Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara-Dinet MT, Gipe DA, Geiger MJ, Farnier M: ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J, 2015; 36: 2996-3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1277).Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D: PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet, 2015; 385: 331-340 [DOI] [PubMed] [Google Scholar]
- 1278).Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, Schweiger C, Tavazzi L, Tognoni G, Valagussa F, Marchioli R: Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol, 2005; 46: 277-283 [DOI] [PubMed] [Google Scholar]
- 1279).Costa J, Borges M, David C, Vaz Carneiro A: Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ, 2006; 332: 1115-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1280).Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C: Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet, 2008; 371: 117-125 [DOI] [PubMed] [Google Scholar]
- 1281).Kohsaka S, Kimura T, Goto M, Lee VV, Elayda M, Furukawa Y, Fukushima M, Komeda M, Sakata R, Willerson JT, Wilson JM, Kita T: Difference in patient profiles and outcomes in Japanese versus American patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the Texas Heart Institute Research Database). Am J Cardiol, 2010; 105: 1698-1704 [DOI] [PubMed] [Google Scholar]
- 1282).Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, Waters D: Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care, 2006; 29: 1220-1226 [DOI] [PubMed] [Google Scholar]
- 1283).Bayturan O, Kapadia S, Nicholls SJ, Tuzcu EM, Shao M, Uno K, Shreevatsa A, Lavoie AJ, Wolski K, Schoenhagen P, Nissen SE: Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol, 2010; 55: 2736-2742 [DOI] [PubMed] [Google Scholar]
- 1284).Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M: Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome--serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS Trial). Circ J, 2010; 74: 1165-1174 [DOI] [PubMed] [Google Scholar]
- 1285).Arai H, Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M: More intensive lipid lowering is associated with regression of coronary atherosclerosis in diabetic patients with acute coronary syndrome--sub-analysis of JAPAN-ACS study. J Atheroscler Thromb, 2010; 17: 1096-1107 [DOI] [PubMed] [Google Scholar]
- 1286).Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalán R, Špinar J, Park JG, White JA, Bohula EA, Braunwald E: Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients With Versus Without Diabetes Mellitus: Results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation, 2018; 137: 1571-1582 [DOI] [PubMed] [Google Scholar]
- 1287).Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW: International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA, 2006; 295: 180-189 [DOI] [PubMed] [Google Scholar]
- 1288).Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet, 1994; 344: 1383-1389 [PubMed] [Google Scholar]
- 1289).Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med, 1998; 339: 1349-1357 [DOI] [PubMed] [Google Scholar]
- 1290).Plehn JF, Davis BR, Sacks FM, Rouleau JL, Pfeffer MA, Bernstein V, Cuddy TE, Moyé LA, Piller LB, Rutherford J, Simpson LM, Braunwald E: Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. The Care Investigators. Circulation, 1999; 99: 216-223 [DOI] [PubMed] [Google Scholar]
- 1291).Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR: Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ, 1998; 316: 823-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1292).Murakami K, Ishibashi S, Yoshida Y, Yamada N, Akanuma Y: Lipoprotein(a) as a coronary risk factor in Japanese patients with Type II (non-insulin-dependent) diabetes mellitus. Relation with apolipoprotein(a) phenotypes. Diabetologia, 1998; 41: 1397-1398 [DOI] [PubMed] [Google Scholar]
- 1293).Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N: Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet, 2009; 373: 1765-1772 [DOI] [PubMed] [Google Scholar]
- 1294).Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C: Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ, 2011; 343: d4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1295).Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med, 2008; 359: 1577-1589 [DOI] [PubMed] [Google Scholar]
- 1296).Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM: Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA, 2015; 313: 45-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1297).Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ: Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care, 2003; 26: 1374-1379 [DOI] [PubMed] [Google Scholar]
- 1298).Goto A, Goto M, Terauchi Y, Yamaguchi N, Noda M: Association Between Severe Hypoglycemia and Cardiovascular Disease Risk in Japanese Patients With Type 2 Diabetes. J Am Heart Assoc, 2016; 5: e002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1299).Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, Bigger T, Cushman W, Goff D, Soliman EZ, Thomas A, Papademetriou V: Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol, 2014; 114: 1217-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1300).Miller ME, Williamson JD, Gerstein HC, Byington RP, Cushman WC, Ginsberg HN, Ambrosius WT, Lovato L, Applegate WB: Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care, 2014; 37: 634-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1301).Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE: Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med, 2015; 373: 2117-2128 [DOI] [PubMed] [Google Scholar]
- 1302).Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR: Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med, 2017; 377: 644-657 [DOI] [PubMed] [Google Scholar]
- 1303).Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB: Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med, 2016; 375: 311-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1304).Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T: Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med, 2016; 375: 1834-1844 [DOI] [PubMed] [Google Scholar]
- 1305).Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Botros FT, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T: Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet, 2019; 394: 131-138 [Google Scholar]
- 1306).Araki A, Iimuro S, Sakurai T, Umegaki H, Iijima K, Nakano H, Oba K, Yokono K, Sone H, Yamada N, Ako J, Kozaki K, Miura H, Kashiwagi A, Kikkawa R, Yoshimura Y, Nakano T, Ohashi Y, Ito H: Non-high-density lipoprotein cholesterol: an important predictor of stroke and diabetes-related mortality in Japanese elderly diabetic patients. Geriatr Gerontol Int, 2012; 12 Suppl 1: 18-28 [DOI] [PubMed] [Google Scholar]
- 1307).Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH: Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet, 2004; 364: 685-696 [DOI] [PubMed] [Google Scholar]
- 1308).Kengne AP, Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Gu DF, Suh I, Woodward M: Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region. J Hypertens, 2007; 25: 1205-1213 [DOI] [PubMed] [Google Scholar]
- 1309).Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A: Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA, 2015; 313: 603-615 [DOI] [PubMed] [Google Scholar]
- 1310).McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, Tonelli M, Leiter LA, Klarenbach SW, Manns BJ: Intensive and Standard Blood Pressure Targets in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis. Arch Intern Med, 2012; 172: 1296-1303 [DOI] [PubMed] [Google Scholar]
- 1311).Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med, 2003; 348: 383-393 [DOI] [PubMed] [Google Scholar]
- 1312).Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med, 2008; 358: 580-591 [DOI] [PubMed] [Google Scholar]
- 1313).Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS: Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med, 2002; 347: 716-725 [DOI] [PubMed] [Google Scholar]
- 1314).Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ: Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med, 2013; 369: 145-154 [Google Scholar]
- 1315).Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C: Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med, 2013; 159: 543-551 [DOI] [PubMed] [Google Scholar]
- 1316).Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, Haraguchi M, Morita A, Ohashi K, Hara K, Morise A, Izumi K, Ishizuka N, Ohashi Y, Noda M, Kadowaki T: Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol, 2017; 5: 951-964 [DOI] [PubMed] [Google Scholar]
- 1317).Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Jr., Virani SS, Williams KA, Sr., Yeboah J, Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 140: e563-e595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1318).Collins R, Armitage J, Parish S, Sleigh P, Peto R: MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet, 2003; 361: 2005-2016 [DOI] [PubMed] [Google Scholar]
- 1319).Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA: High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med, 2006; 355: 549-559 [DOI] [PubMed] [Google Scholar]
- 1320).Callahan A, Amarenco P, Goldstein LB, Sillesen H, Messig M, Samsa GP, Altafullah I, Ledbetter LY, MacLeod MJ, Scott R, Hennerici M, Zivin JA, Welch KM: Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch Neurol, 2011; 68: 1245-1251 [DOI] [PubMed] [Google Scholar]
- 1321).National Cerebral and Cardiovascular Center, Stroke Data Bank Editorial Committee: Stroke Treatment Data Bank,2021, Nakayama Shoten Co,.Ltd,2021 (in Japanese) [Google Scholar]
- 1322).Tanaka H, Iso H, Yokoyama T, et al.: Cerebrovasculardisease.In: Cerebrovasculardisease, pp1193-1226, OxfordPress, 2001 [Google Scholar]
- 1323).Kimura K, Kazui S, Minematsu K, Yamaguchi T: Analysis of 16,922 patients with acute ischemic stroke and transient ischemic attack in Japan. A hospital-based prospective registration study. Cerebrovasc Dis, 2004; 18: 47-56 [DOI] [PubMed] [Google Scholar]
- 1324).Takashima N, Arima H, Kita Y, Fujii T, Miyamatsu N, Komori M, Sugimoto Y, Nagata S, Miura K, Nozaki K: Incidence, Management and Short-Term Outcome of Stroke in a General Population of 1.4 Million Japanese - Shiga Stroke Registry. Circ J, 2017; 81: 1636-1646 [DOI] [PubMed] [Google Scholar]
- 1325).Petty GW, Brown RD, Jr., Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO: Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke, 1999; 30: 2513-2516 [DOI] [PubMed] [Google Scholar]
- 1326).Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R: Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet, 2007; 370: 1829-1839 [DOI] [PubMed] [Google Scholar]
- 1327).Blood pressure, cholesterol, and stroke in eastern Asia. Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Lancet, 1998; 352: 1801-1807 [PubMed] [Google Scholar]
- 1328).Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J: Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke, 2004; 35: 1552-1556 [DOI] [PubMed] [Google Scholar]
- 1329).Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU: Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke, 2001; 32: 2735-2740 [DOI] [PubMed] [Google Scholar]
- 1330).Gu X, Li Y, Chen S, Yang X, Liu F, Li Y, Li J, Cao J, Liu X, Chen J, Shen C, Yu L, Huang J, Lam TH, Fang X, He Y, Zhang X, Lu X, Wu S, Gu D: Association of Lipids With Ischemic and Hemorrhagic Stroke: A Prospective Cohort Study Among 267 500 Chinese. Stroke, 2019; 50: 3376-3384 [DOI] [PubMed] [Google Scholar]
- 1331).Iso H, Jacobs DR, Jr., Wentworth D, Neaton JD, Cohen JD: Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med, 1989; 320: 904-910 [DOI] [PubMed] [Google Scholar]
- 1332).Leppälä JM, Virtamo J, Fogelholm R, Albanes D, Heinonen OP: Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke, 1999; 30: 2535-2540 [DOI] [PubMed] [Google Scholar]
- 1333).Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, MacMahon S, Woodward M: Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol, 2003; 32: 563-572 [DOI] [PubMed] [Google Scholar]
- 1334).Law MR, Wald NJ, Rudnicka AR: Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ, 2003; 326: 1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335).Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE: Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health, 2002; 56 Suppl 1: i19-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1336).Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR: Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke, 2003; 34: 623-631 [DOI] [PubMed] [Google Scholar]
- 1337).Sturgeon JD, Folsom AR, Longstreth WT, Jr., Shahar E, Rosamond WD, Cushman M: Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke, 2007; 38: 2718-2725 [DOI] [PubMed] [Google Scholar]
- 1338).Lindenstrøm E, Boysen G, Nyboe J: Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: the Copenhagen City Heart Study. BMJ, 1994; 309: 11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1339).Wannamethee SG, Shaper AG, Ebrahim S: HDL-Cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke, 2000; 31: 1882-1888 [DOI] [PubMed] [Google Scholar]
- 1340).Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, Gaziano JM: Cholesterol and the risk of ischemic stroke. Stroke, 2003; 34: 2930-2934 [DOI] [PubMed] [Google Scholar]
- 1341).Håheim LL, Holme I, Hjermann I, Leren P: Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke, 1993; 24: 1484-1489 [DOI] [PubMed] [Google Scholar]
- 1342).Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG: Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA, 2008; 300: 2142-2152 [DOI] [PubMed] [Google Scholar]
- 1343).Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Uchiyama S, Nakamura H: Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA study). Circulation, 2008; 117: 494-502 [DOI] [PubMed] [Google Scholar]
- 1344).Goldstein LB, Amarenco P, Szarek M, Callahan A, 3rd, Hennerici M, Sillesen H, Zivin JA, Welch KM: Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology, 2008; 70: 2364-2370 [DOI] [PubMed] [Google Scholar]
- 1345).Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, 3rd, Hennerici M, Simunovic L, Zivin JA, Welch KM: Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke, 2007; 38: 3198-3204 [DOI] [PubMed] [Google Scholar]
- 1346).Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM: Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke, 2008; 39: 3297-3302 [DOI] [PubMed] [Google Scholar]
- 1347).Amarenco P, Benavente O, Goldstein LB, Callahan A, 3rd, Sillesen H, Hennerici MG, Gilbert S, Rudolph AE, Simunovic L, Zivin JA, Welch KM: Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke, 2009; 40: 1405-1409 [DOI] [PubMed] [Google Scholar]
- 1348).Martí-Fàbregas J, Gomis M, Arboix A, Aleu A, Pagonabarraga J, Belvís R, Cocho D, Roquer J, Rodríguez A, García MD, Molina-Porcel L, Díaz-Manera J, Martí-Vilalta JL: Favorable outcome of ischemic stroke in patients pretreated with statins. Stroke, 2004; 35: 1117-1121 [DOI] [PubMed] [Google Scholar]
- 1349).Colivicchi F, Bassi A, Santini M, Caltagirone C: Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke, 2007; 38: 2652-2657 [DOI] [PubMed] [Google Scholar]
- 1350).Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B: Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med, 2016; 375: 323-334 [DOI] [PubMed] [Google Scholar]
- 1351).Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW: Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med, 2019; 380: 2295-2306 [Google Scholar]
- 1352).Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC: Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med, 2020; 383: 1436-1446 [Google Scholar]
- 1353).McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM: Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med, 2019; 381: 1995-2008 [Google Scholar]
- 1354).Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS: Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med, 2019; 380: 347-357 [Google Scholar]
- 1355).Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R: The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet, 2011; 377: 2181-2192 [Google Scholar]
- 1356).Nakamura H, Mizuno K, Ohashi Y, Yoshida T, Hirao K, Uchida Y: Pravastatin and cardiovascular risk in moderate chronic kidney disease. Atherosclerosis, 2009; 206: 512-517 [DOI] [PubMed] [Google Scholar]
- 1357).Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S, Of The KSGOB: Long-Term Efficacy and Safety of Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated Receptor-α Modulator (SPPARMα), in Dyslipidemic Patients with Renal Impairment. Int J Mol Sci, 2019; 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1358).Lamprea-Montealegre JA, Staplin N, Herrington WG, Haynes R, Emberson J, Baigent C, de Boer IH: Apolipoprotein B, Triglyceride-Rich Lipoproteins, and Risk of Cardiovascular Events in Persons with CKD. Clin J Am Soc Nephrol, 2020; 15: 47-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1359).Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL: 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis, 2016; 253: 281-344 [DOI] [PubMed] [Google Scholar]
- 1360).Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J: Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]
- 1361).Akioyamen LE, Genest J, Shan SD, Reel RL, Albaum JM, Chu A, Tu JV: Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open, 2017; 7: e016461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1362).Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG: Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J Am Coll Cardiol, 2020; 75: 2553-2566 [DOI] [PubMed] [Google Scholar]
- 1363).Hu P, Dharmayat KI, Stevens CAT, Sharabiani MTA, Jones RS, Watts GF, Genest J, Ray KK, Vallejo-Vaz AJ: Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation, 2020; 141: 1742-1759 [DOI] [PubMed] [Google Scholar]
- 1364).Austin MA, Hutter CM, Zimmern RL, Humphries SE: Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol, 2004; 160: 421-429 [DOI] [PubMed] [Google Scholar]
- 1365).Hutter CM, Austin MA, Humphries SE: Familial hypercholesterolemia, peripheral arterial disease, and stroke: a HuGE minireview. Am J Epidemiol, 2004; 160: 430-435 [DOI] [PubMed] [Google Scholar]
- 1366).Akioyamen LE, Tu JV, Genest J, Ko DT, Coutin AJS, Shan SD, Chu A: Risk of Ischemic Stroke and Peripheral Arterial Disease in Heterozygous Familial Hypercholesterolemia: A Meta-Analysis. Angiology, 2019; 70: 726-736 [DOI] [PubMed] [Google Scholar]
- 1367).Mabuchi H: Half a Century Tales of Familial Hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1368).Betteridge DJ, Bhatnager D, Bing RF, Durrington PN, Evans GR, Flax H, Jay RH, Lewis-Barned N, Mann J, Matthews DR, et al.: Treatment of familial hypercholesterolaemia. United Kingdom lipid clinics study of pravastatin and cholestyramine. BMJ, 1992; 304: 1335-1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1369).Hoogerbrugge N, Mol MJ, Van Dormaal JJ, Rustemeijer C, Muls E, Stalenhoef AF, Birkenhäger JC: The efficacy and safety of pravastatin, compared to and in combination with bile acid binding resins, in familial hypercholesterolaemia. J Intern Med, 1990; 228: 261-266 [DOI] [PubMed] [Google Scholar]
- 1370).Wiklund O, Angelin B, Fager G, Eriksson M, Olofsson SO, Berglund L, Lindén T, Sjöberg A, Bondjers G: Treatment of familial hypercholesterolaemia: a controlled trial of the effects of pravastatin or cholestyramine therapy on lipoprotein and apolipoprotein levels. J Intern Med, 1990; 228: 241-247 [DOI] [PubMed] [Google Scholar]
- 1371).Avis HJ, Hutten BA, Gagné C, Langslet G, McCrindle BW, Wiegman A, Hsia J, Kastelein JJ, Stein EA: Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J Am Coll Cardiol, 2010; 55: 1121-1126 [DOI] [PubMed] [Google Scholar]
- 1372).Clauss SB, Holmes KW, Hopkins P, Stein E, Cho M, Tate A, Johnson-Levonas AO, Kwiterovich PO: Efficacy and safety of lovastatin therapy in adolescent girls with heterozygous familial hypercholesterolemia. Pediatrics, 2005; 116: 682-688 [DOI] [PubMed] [Google Scholar]
- 1373).de Jongh S, Ose L, Szamosi T, Gagné C, Lambert M, Scott R, Perron P, Dobbelaere D, Saborio M, Tuohy MB, Stepanavage M, Sapre A, Gumbiner B, Mercuri M, van Trotsenburg AS, Bakker HD, Kastelein JJ: Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation, 2002; 106: 2231-2237 [DOI] [PubMed] [Google Scholar]
- 1374).Knipscheer HC, Boelen CC, Kastelein JJ, van Diermen DE, Groenemeijer BE, van den Ende A, Büller HR, Bakker HD: Short-term efficacy and safety of pravastatin in 72 children with familial hypercholesterolemia. Pediatr Res, 1996; 39: 867-871 [DOI] [PubMed] [Google Scholar]
- 1375).Stein EA, Illingworth DR, Kwiterovich PO, Jr., Liacouras CA, Siimes MA, Jacobson MS, Brewster TG, Hopkins P, Davidson M, Graham K, Arensman F, Knopp RH, DuJovne C, Williams CL, Isaacsohn JL, Jacobsen CA, Laskarzewski PM, Ames S, Gormley GJ: Efficacy and safety of lovastatin in adolescent males with heterozygous familial hypercholesterolemia: a randomized controlled trial. JAMA, 1999; 281: 137-144 [DOI] [PubMed] [Google Scholar]
- 1376).Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Büller HR, Sijbrands EJ, Kastelein JJ: Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA, 2004; 292: 331-337 [DOI] [PubMed] [Google Scholar]
- 1377).Stein EA, Dann EJ, Wiegman A, Skovby F, Gaudet D, Sokal E, Charng MJ, Mohamed M, Luirink I, Raichlen JS, Sundén M, Carlsson SC, Raal FJ, Kastelein JJP: Efficacy of Rosuvastatin in Children With Homozygous Familial Hypercholesterolemia and Association With Underlying Genetic Mutations. J Am Coll Cardiol, 2017; 70: 1162-1170 [DOI] [PubMed] [Google Scholar]
- 1378).Lambert M, Lupien PJ, Gagné C, Lévy E, Blaichman S, Langlois S, Hayden M, Rose V, Clarke JT, Wolfe BM, Clarson C, Parsons H, Stephure DK, Potvin D, Lambert J: Treatment of familial hypercholesterolemia in children and adolescents: effect of lovastatin. Canadian Lovastatin in Children Study Group. Pediatrics, 1996; 97: 619-628 [PubMed] [Google Scholar]
- 1379).Harada-Shiba M, Arisaka O, Ohtake A, Okada T, Suganami H: Efficacy and Safety of Pitavastatin in Japanese Male Children with Familial Hypercholesterolemia. J Atheroscler Thromb, 2016; 23: 48-55 [DOI] [PubMed] [Google Scholar]
- 1380).Nozue T, Michishita I, Ito Y, Hirano T: Effects of statin on small dense low-density lipoprotein cholesterol and remnant-like particle cholesterol in heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2008; 15: 146-153 [DOI] [PubMed] [Google Scholar]
- 1381).Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF: Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet, 2001; 357: 577-581 [DOI] [PubMed] [Google Scholar]
- 1382).Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg AS, van der Graaf A, de Groot E, Wijburg FA, Kastelein JJ, Hutten BA: Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation, 2007; 116: 664-668 [DOI] [PubMed] [Google Scholar]
- 1383).Besseling J, Hovingh GK, Huijgen R, Kastelein JJP, Hutten BA: Statins in Familial Hypercholesterolemia: Consequences for Coronary Artery Disease and All-Cause Mortality. J Am Coll Cardiol, 2016; 68: 252-260 [DOI] [PubMed] [Google Scholar]
- 1384).Jay RH, Sturley RH, Stirling C, McGarrigle HH, Katz M, Reckless JP, Betteridge DJ: Effects of pravastatin and cholestyramine on gonadal and adrenal steroid production in familial hypercholesterolaemia. Br J Clin Pharmacol, 1991; 32: 417-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1385).Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA: Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet, 2015; 385: 341-350 [DOI] [PubMed] [Google Scholar]
- 1386).Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E: Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med, 2008; 358: 1431-1443 [DOI] [PubMed] [Google Scholar]
- 1387).Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis 2019; 290: 140-205 [DOI] [PubMed] [Google Scholar]
- 1388).Wang A, Richhariya A, Gandra SR, Calimlim B, Kim L, Quek RG, Nordyke RJ, Toth PP: Systematic Review of Low-Density Lipoprotein Cholesterol Apheresis for the Treatment of Familial Hypercholesterolemia. J Am Heart Assoc, 2016; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1389).Luirink IK, Determeijer J, Hutten BA, Wiegman A, Bruckert E, Schmitt CP, Groothoff JW: Efficacy and safety of lipoprotein apheresis in children with homozygous familial hypercholesterolemia: A systematic review. J Clin Lipidol, 2019; 13: 31-39 [DOI] [PubMed] [Google Scholar]
- 1390).Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128 Suppl 5: S213-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1391).Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Tonstad S, Wiegman A, Drogari E, Ramaswami U: Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev, 2019; 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1392).Tada H, Hori M, Matsuki K, Ogura M, Nohara A, Kawashiri MA, Harada-Shiba M: Achilles Tendon Thickness Assessed by X-ray Predicting a Pathogenic Mutation in Familial Hypercholesterolemia Gene. J Atheroscler Thromb, 2022; 29: 816-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1393).Harada-Shiba M, Ohtake A, Sugiyama D, Tada H, Dobashi K, Matsuki K, Minamino T, Yamashita S, Yamamoto Y: Guidelines for the Diagnosis and Treatment of Pediatric Familial Hypercholesterolemia 2022. J Atheroscler Thromb, 2023; 30: 531-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1394).Tada H, Nomura A, Ogura M, Ikewaki K, Ishigaki Y, Inagaki K, Tsukamoto K, Dobashi K, Nakamura K, Hori M, Matsuki K, Yamashita S, Yokoyama S, Kawashiri MA, Harada-Shiba M: Diagnosis and Management of Sitosterolemia 2021. J Atheroscler Thromb, 2021; 28: 791-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1395).Koyama S, Sekijima Y, Ogura M, Hori M, Matsuki K, Miida T, Harada-Shiba M: Cerebrotendinous Xanthomatosis: Molecular Pathogenesis, Clinical Spectrum, Diagnosis, and Disease-Modifying Treatments. J Atheroscler Thromb, 2021; 28: 905-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1396).Qian LJ, Gao Y, Zhang YM, Chu M, Yao J, Xu D: Therapeutic efficacy and safety of PCSK9-monoclonal antibodies on familial hypercholesterolemia and statin-intolerant patients: A meta-analysis of 15 randomized controlled trials. Sci Rep, 2017; 7: 238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397).Uauy R, Vega GL, Grundy SM, Bilheimer DM: Lovastatin therapy in receptor-negative homozygous familial hypercholesterolemia: lack of effect on low-density lipoprotein concentrations or turnover. J Pediatr, 1988; 113: 387-392 [DOI] [PubMed] [Google Scholar]
- 1398).Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ: Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation, 2013; 128: 2113-2120 [DOI] [PubMed] [Google Scholar]
- 1399).Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, Marais AD: Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation, 2011; 124: 2202-2207 [DOI] [PubMed] [Google Scholar]
- 1400).Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B: Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29h-35h [DOI] [PubMed] [Google Scholar]
- 1401).Shirahata Y, Ohkohchi N, Kawagishi N, Syouji M, Tsukamoto S, Sekiguchi S, Koyamada N, Oikawa S, Satomi S: Living-donor liver transplantation for homozygous familial hypercholesterolemia from a donor with heterozygous hypercholesterolemia. Transpl Int, 2003; 16: 276-279 [DOI] [PubMed] [Google Scholar]
- 1402).Kawagishi N, Satoh K, Akamatsu Y, Sekiguchi S, Ishigaki Y, Oikawa S, Satomi S: Long-term outcome after living donor liver transplantation for two cases of homozygous familial hypercholesterolemia from a heterozygous donor. J Atheroscler Thromb, 2007; 14: 94-98 [DOI] [PubMed] [Google Scholar]
- 1403).Bujo H, Takahashi K, Saito Y, Maruyama T, Yamashita S, Matsuzawa Y, Ishibashi S, Shionoiri F, Yamada N, Kita T: Clinical features of familial hypercholesterolemia in Japan in a database from 1996-1998 by the research committee of the ministry of health, labour and welfare of Japan. J Atheroscler Thromb, 2004; 11: 146-151 [DOI] [PubMed] [Google Scholar]
- 1404).Maruyama T, Yamashita S, Matsuzawa Y, Bujo H, Takahashi K, Saito Y, Ishibashi S, Ohashi K, Shionoiri F, Gotoda T, Yamada N, Kita T: Mutations in Japanese subjects with primary hyperlipidemia--results from the Research Committee of the Ministry of Health and Welfare of Japan since 1996. J Atheroscler Thromb, 2004; 11: 131-145 [DOI] [PubMed] [Google Scholar]
- 1405).Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG: Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest, 1973; 52: 1544-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1406).Brahm AJ, Hegele RA: Combined hyperlipidemia: familial but not (usually) monogenic. Curr Opin Lipidol, 2016; 27: 131-140 [DOI] [PubMed] [Google Scholar]
- 1407).Gill PK, Dron JS, Berberich AJ, Wang J, McIntyre AD, Cao H, Hegele RA: Combined hyperlipidemia is genetically similar to isolated hypertriglyceridemia. J Clin Lipidol, 2021; 15: 79-87 [DOI] [PubMed] [Google Scholar]
- 1408).Iwata F, Okada T, Kuromori Y, Hara M, Harada K: Screening for familial combined hyperlipidemia in children using lipid phenotypes. J Atheroscler Thromb, 2003; 10: 299-303 [DOI] [PubMed] [Google Scholar]
- 1409).Austin MA, McKnight B, Edwards KL, Bradley CM, McNeely MJ, Psaty BM, Brunzell JD, Motulsky AG: Cardiovascular disease mortality in familial forms of hypertriglyceridemia: A 20-year prospective study. Circulation, 2000; 101: 2777-2782 [DOI] [PubMed] [Google Scholar]
- 1410).Pitsavos C, Skoumas I, Masoura C, Aznaouridis K, Papadimitriou L, Chrysohoou C, Giotsas N, Toutouza M, Stefanadis C: Prevalence and determinants of coronary artery disease in males and females with familial combined hyperlipidaemia. Atherosclerosis, 2008; 199: 402-407 [DOI] [PubMed] [Google Scholar]
- 1411).Mabuchi H, Koizumi J: Serum lipids and coronary atherosclerosis in familial combined hyperlipidemia diagnosed definitively by family survey, Ministry of Health and Welfare Primary Hyperlipidemia Research Group for Specific Diseases, 1998. (in Japanese) [Google Scholar]
- 1412).Mahley RW, Huang Y, Rall SC, Jr.: Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res, 1999; 40: 1933-1949 [PubMed] [Google Scholar]
- 1413).Hopkins PN, Brinton EA, Nanjee MN: Hyperlipoproteinemia type 3: the forgotten phenotype. Curr Atheroscler Rep, 2014; 16: 440 [DOI] [PubMed] [Google Scholar]
- 1414).LaRosa JC, Chambless LE, Criqui MH, Frantz ID, Glueck CJ, Heiss G, Morrison JA: Patterns of dyslipoproteinemia in selected North American populations. The Lipid Research Clinics Program Prevalence Study. Circulation, 1986; 73: I12-29 [PubMed] [Google Scholar]
- 1415).Hopkins PN, Nanjee MN, Wu LL, McGinty MG, Brinton EA, Hunt SC, Anderson JL: Altered composition of triglyceride-rich lipoproteins and coronary artery disease in a large case-control study. Atherosclerosis, 2009; 207: 559-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1416).Eto M, Saito M, Nakata H, Iwashima Y, Watanabe K, Ikoda A, Kaku K: Type III hyperlipoproteinema with apolipoprotein E2/2 genotype in Japan. Clin Genet, 2002; 61: 416-422 [DOI] [PubMed] [Google Scholar]
- 1417).Yamamura T: Familial type III hyperlipidemia. Nihon Naika Gakkai Zasshi, 1992 (in Japanese) [Google Scholar]
- 1418).Sniderman A, Tremblay A, Bergeron J, Gagné C, Couture P: Diagnosis of type III hyperlipoproteinemia from plasma total cholesterol, triglyceride, and apolipoprotein B. J Clin Lipidol, 2007; 1: 256-263 [DOI] [PubMed] [Google Scholar]
- 1419).Murase T, Okubo M, Takeuchi I: Non-HDL-cholesterol/apolipoprotein B ratio: a useful distinguishing feature in the screening for type III hyperlipoproteinemia. J Clin Lipidol, 2010; 4: 99-104 [DOI] [PubMed] [Google Scholar]
- 1420).Yuasa-Kawase M, Masuda D, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Tsubakio-Yamamoto K, Ohama T, Toyama-Nakagawa Y, Nishida M, Ishigami M, Saito M, Eto M, Matsuyama A, Komuro I, Yamashita S: Apolipoprotein B-48 to triglyceride ratio is a novel and useful marker for detection of type III hyperlipidemia after antihyperlipidemic intervention. J Atheroscler Thromb, 2012; 19: 862-871 [DOI] [PubMed] [Google Scholar]
- 1421).Ossoli A, Simonelli S, Vitali C, Franceschini G, Calabresi L: Role of LCAT in Atherosclerosis. J Atheroscler Thromb, 2016; 23: 119-127 [DOI] [PubMed] [Google Scholar]
- 1422).Tietjen I, Hovingh GK, Singaraja R, Radomski C, McEwen J, Chan E, Mattice M, Legendre A, Kastelein JJ, Hayden MR: Increased risk of coronary artery disease in Caucasians with extremely low HDL cholesterol due to mutations in ABCA1, APOA1, and LCAT. Biochim Biophys Acta, 2012; 1821: 416-424 [DOI] [PubMed] [Google Scholar]
- 1423).Muratsu J, Koseki M, Masuda D, Yasuga Y, Tomoyama S, Ataka K, Yagi Y, Nakagawa A, Hamada H, Fujita S, Hattori H, Ohama T, Nishida M, Hiraoka H, Matsuzawa Y, Yamashita S: Accelerated Atherogenicity in Tangier Disease. J Atheroscler Thromb, 2018; 25: 1076-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1424).Zanoni P, von Eckardstein A: Inborn errors of apolipoprotein A-I metabolism: implications for disease, research and development. Curr Opin Lipidol, 2020; 31: 62-70 [DOI] [PubMed] [Google Scholar]
- 1425).Vodnala D, Rubenfire M, Brook RD: Secondary causes of dyslipidemia. Am J Cardiol, 2012; 110: 823-825 [DOI] [PubMed] [Google Scholar]
- 1426).Yanai H, Yoshida H: Secondary dyslipidemia: its treatments and association with atherosclerosis. Glob Health Med, 2021; 3: 15-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1427).Rader D, Hobbs H: Disorders of lipoprotein metabolism, 2015 [Google Scholar]
- 1428).Rodondi N, Aujesky D, Vittinghoff E, Cornuz J, Bauer DC: Subclinical hypothyroidism and the risk of coronary heart disease: a meta-analysis. Am J Med, 2006; 119: 541-551 [DOI] [PubMed] [Google Scholar]
- 1429).Pearce EN: Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep, 2004; 6: 451-456 [DOI] [PubMed] [Google Scholar]
- 1430).Duntas LH, Brenta G: Thyroid hormones: a potential ally to LDL-cholesterol-lowering agents. Hormones (Athens), 2016; 15: 500-510 [DOI] [PubMed] [Google Scholar]
- 1431).Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH: Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis, 2013; 227: 18-25 [DOI] [PubMed] [Google Scholar]
- 1432).Li X, Wang Y, Guan Q, Zhao J, Gao L: The lipid-lowering effect of levothyroxine in patients with subclinical hypothyroidism: A systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf), 2017; 87: 1-9 [DOI] [PubMed] [Google Scholar]
- 1433).Aziz M, Kandimalla Y, Machavarapu A, Saxena A, Das S, Younus A, Nguyen M, Malik R, Anugula D, Latif MA, Humayun C, Khan IM, Adus A, Rasool A, Veledar E, Nasir K: Effect of Thyroxin Treatment on Carotid Intima-Media Thickness (CIMT) Reduction in Patients with Subclinical Hypothyroidism (SCH): a Meta-Analysis of Clinical Trials. J Atheroscler Thromb, 2017; 24: 643-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434).Zhao T, Chen B, Zhou Y, Wang X, Zhang Y, Wang H, Shan Z: Effect of levothyroxine on the progression of carotid intima-media thickness in subclinical hypothyroidism patients: a meta-analysis. BMJ Open, 2017; 7: e016053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1435).Andersen MN, Olsen AS, Madsen JC, Kristensen SL, Faber J, Torp-Pedersen C, Gislason GH, Selmer C: Long-Term Outcome in Levothyroxine Treated Patients With Subclinical Hypothyroidism and Concomitant Heart Disease. J Clin Endocrinol Metab, 2016; 101: 4170-4177 [DOI] [PubMed] [Google Scholar]
- 1436).Ramkumar S, Raghunath A, Raghunath S: Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol Sin, 2016; 32: 631-639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1437).Wheeler DC, Bernard DB: Lipid abnormalities in the nephrotic syndrome: causes, consequences, and treatment. Am J Kidney Dis, 1994; 23: 331-346 [DOI] [PubMed] [Google Scholar]
- 1438).Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE: Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol, 2018; 14: 57-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1439).Shen H, Feng S, Lu Y, Jiang L, Yang T, Wang Z: Correlation between plasma proprotein convertase subtilisin/kexin type 9 and blood lipids in patients with newly diagnosed primary nephrotic syndrome. Ren Fail, 2020; 42: 405-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1440).Ordoñez JD, Hiatt RA, Killebrew EJ, Fireman BH: The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int, 1993; 44: 638-642 [DOI] [PubMed] [Google Scholar]
- 1441).D'Amico G, Gentile MG, Manna G, Fellin G, Ciceri R, Cofano F, Petrini C, Lavarda F, Perolini S, Porrini M: Effect of vegetarian soy diet on hyperlipidaemia in nephrotic syndrome. Lancet, 1992; 339: 1131-1134 [DOI] [PubMed] [Google Scholar]
- 1442).Gentile MG, Fellin G, Cofano F, Delle Fave A, Manna G, Ciceri R, Petrini C, Lavarda F, Pozzi F, D'Amico G: Treatment of proteinuric patients with a vegetarian soy diet and fish oil. Clin Nephrol, 1993; 40: 315-320 [PubMed] [Google Scholar]
- 1443).Bell S, Cooney J, Packard CJ, Caslake MJ, Deighan CJ: The effect of omega-3 fatty acids on the atherogenic lipoprotein phenotype in patients with nephrotic range proteinuria. Clin Nephrol, 2012; 77: 445-453 [DOI] [PubMed] [Google Scholar]
- 1444).Yanai H: Hyperlipidemia due to Nephrotic Syndrome: Its Effects and Effects of Interventions on Atherogenesis, Cardiovascular and Renal Outcomes. J Endocrinol Metab, 2020; 10: 63-73 [Google Scholar]
- 1445).Zheng-Lin B, Ortiz A: Lipid Management in Chronic Kidney Disease: Systematic Review of PCSK9 Targeting. Drugs, 2018; 78: 215-229 [DOI] [PubMed] [Google Scholar]
- 1446).Sjuls S, Jensen U, Littmann K, Bruchfeld A, Brinck J: Effective cholesterol lowering after myocardial infarction in patients with nephrotic syndrome may require a multi-pharmacological approach: a case report. Eur Heart J Case Rep, 2021; 5: ytab151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1447).Jatem E, Lima J, Montoro B, Torres-Bondia F, Segarra A: Efficacy and Safety of PCSK9 Inhibitors in Hypercholesterolemia Associated With Refractory Nephrotic Syndrome. Kidney Int Rep, 2021; 6: 101-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1448).Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L: Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev, 2013: Cd005425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1449).Alawami M, Wimalasena S, Ghashi R, Alnasrallah B: Acute arterial cardiovascular events risk in patients with primary membranous nephropathy. Intern Med J, 2019; 49: 855-858 [DOI] [PubMed] [Google Scholar]
- 1450).Xu H, Carrero JJ: Insulin resistance in chronic kidney disease. Nephrology (Carlton), 2017; 22 Suppl 4: 31-34 [DOI] [PubMed] [Google Scholar]
- 1451).Eto M, Saito M, Okada M, Kume Y, Kawasaki F, Matsuda M, Yoneda M, Matsuki M, Takigami S, Kaku K: Apolipoprotein E genetic polymorphism, remnant lipoproteins, and nephropathy in type 2 diabetic patients. Am J Kidney Dis, 2002; 40: 243-251 [DOI] [PubMed] [Google Scholar]
- 1452).Rosenblit PD: Extreme Atherosclerotic Cardiovascular Disease (ASCVD) Risk Recognition. Curr Diab Rep, 2019; 19: 61 [DOI] [PubMed] [Google Scholar]
- 1453).Sorokin A, Brown JL, Thompson PD: Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis, 2007; 194: 293-299 [DOI] [PubMed] [Google Scholar]
- 1454).Van Dam GM, Gips CH: Primary biliary cirrhosis in The Netherlands. An analysis of associated diseases, cardiovascular risk, and malignancies on the basis of mortality figures. Scand J Gastroenterol, 1997; 32: 77-83 [DOI] [PubMed] [Google Scholar]
- 1455).Wang C, Zhao P, Liu W: Risk of incident coronary artery disease in patients with primary biliary cirrhosis. Int J Clin Exp Med, 2014; 7: 2921-2924 [PMC free article] [PubMed] [Google Scholar]
- 1456).Miller JP: Dyslipoproteinaemia of liver disease. Baillieres Clin Endocrinol Metab, 1990; 4: 807-832 [DOI] [PubMed] [Google Scholar]
- 1457).Yanai H, Hirowatari Y, Yoshida H: Diabetic dyslipidemia: evaluation and mechanism. Glob Health Med, 2019; 1: 30-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1458).Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A: Complications of Cushing's syndrome: state of the art. Lancet Diabetes Endocrinol, 2016; 4: 611-629 [DOI] [PubMed] [Google Scholar]
- 1459).Lupoli R, Ambrosino P, Tortora A, Barba L, Lupoli GA, Di Minno MN: Markers of atherosclerosis in patients with Cushing's syndrome: a meta-analysis of literature studies. Ann Med, 2017; 49: 206-216 [DOI] [PubMed] [Google Scholar]
- 1460).Rofougaran R, Mooraki A, Bastani B: Insulin-requiring diabetes mellitus, hyperlipidemia, and anginal chest pains as prominent features of pheochromocytoma. Am J Nephrol, 1997; 17: 474-476 [DOI] [PubMed] [Google Scholar]
- 1461).Winocour PH, Masud T, Clark F, Cooper BG, Laker MF, Alberti KG: Lipid and lipoprotein metabolism in familial combined hyperlipidaemia during treatment of sporadic phaeochromocytoma: a case study. Postgrad Med J, 1992; 68: 371-375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462).Yamamoto M, Hosokawa T, Suehiro T, Numata S, Yamano T, Ono F: [A case of pheochromocytoma with hyper-HDL-cholesterolemia]. Nihon Naika Gakkai Zasshi, 1991; 80: 1678-1679 [PubMed] [Google Scholar]
- 1463).Ueda S, Morimoto T, Ando S, Takishita S, Kawano Y, Shimamoto K, Ogihara T, Saruta T: A randomised controlled trial for the evaluation of risk for type 2 diabetes in hypertensive patients receiving thiazide diuretics: Diuretics In the Management of Essential hypertension (DIME) study. BMJ Open, 2014; 4: e004576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1464).Carlsen JE, Køber L, Torp-Pedersen C, Johansen P: Relation between dose of bendrofluazide, antihypertensive effect, and adverse biochemical effects. BMJ, 1990; 300: 975-978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1465).Basdevant A: Steroids and lipid metabolism: mechanism of action. Int J Fertil, 1992; 37 Suppl 2: 93-97 [PubMed] [Google Scholar]
- 1466).Lobo RA: Cardiovascular implications of estrogen replacement therapy. Obstet Gynecol, 1990; 75: 18S-25S; discussion 31S-35S [DOI] [PubMed] [Google Scholar]
- 1467).Arca M, Vega GL, Grundy SM: Hypercholesterolemia in postmenopausal women. Metabolic defects and response to low-dose lovastatin. JAMA, 1994; 271: 453-459 [DOI] [PubMed] [Google Scholar]
- 1468).Donahoo WT, Kosmiski LA, Eckel RH: Drugs causing dyslipoproteinemia. Endocrinol Metab Clin North Am, 1998; 27: 677-697 [DOI] [PubMed] [Google Scholar]
- 1469).McDiarmid SV, Gornbein JA, Fortunat M, Saikali D, Vargas JH, Busuttil RW, Ament ME: Serum lipid abnormalities in pediatric liver transplant patients. Transplantation, 1992; 53: 109-115 [DOI] [PubMed] [Google Scholar]
- 1470).Seymen P, Yildiz M, Türkmen MF, Titiz MI, Seymen HO: Effects of cyclosporine-tacrolimus switching in posttransplantation hyperlipidemia on high-density lipoprotein 2/3, lipoprotein a1/b, and other lipid parameters. Transplant Proc, 2009; 41: 4181-4183 [DOI] [PubMed] [Google Scholar]
- 1471).Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD: Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med, 2007; 356: 1723-1735 [DOI] [PubMed] [Google Scholar]
- 1472).Ergin HE, Inga EE, Maung TZ, Javed M, Khan S: HIV, Antiretroviral Therapy and Metabolic Alterations: A Review. Cureus, 2020; 12: e8059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1473).Lagathu C, Béréziat V, Gorwood J, Fellahi S, Bastard JP, Vigouroux C, Boccara F, Capeau J: Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin Drug Saf, 2019; 18: 829-840 [DOI] [PubMed] [Google Scholar]
- 1474).Maggi P, Di Biagio A, Rusconi S, Cicalini S, D'Abbraccio M, d'Ettorre G, Martinelli C, Nunnari G, Sighinolfi L, Spagnuolo V, Squillace N: Cardiovascular risk and dyslipidemia among persons living with HIV: a review. BMC Infect Dis, 2017; 17: 551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1475).Gonçalves P, Araújo JR, Martel F: Antipsychotics-induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur Neuropsychopharmacol, 2015; 25: 1-16 [DOI] [PubMed] [Google Scholar]
- 1476).Kang SH, Lee JI: Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatry Investig, 2015; 12: 242-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1477).Bershad S, Rubinstein A, Paterniti JR, Le NA, Poliak SC, Heller B, Ginsberg HN, Fleischmajer R, Brown WV: Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med, 1985; 313: 981-985 [DOI] [PubMed] [Google Scholar]
- 1478).Shenoy C, Shenoy MM, Rao GK: Dyslipidemia in Dermatological Disorders. N Am J Med Sci, 2015; 7: 421-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1479).Imhof A, Koenig W: Alcohol inflammation and coronary heart disease. Addict Biol, 2003; 8: 271-277 [DOI] [PubMed] [Google Scholar]
- 1480).González-Reimers E, Santolaria-Fernández F, Martín-González MC, Fernández-Rodríguez CM, Quintero-Platt G: Alcoholism: a systemic proinflammatory condition. World J Gastroenterol, 2014; 20: 14660-14671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1481).Quintana HK, Janszky I, Kanar A, Gigante B, Druid H, Ahlbom A, de Faire U, Hallqvist J, Leander K: Comorbidities in relation to fatality of first myocardial infarction. Cardiovasc Pathol, 2018; 32: 32-37 [DOI] [PubMed] [Google Scholar]
- 1482).Allen CL, Bayraktutan U: Risk factors for ischaemic stroke. Int J Stroke, 2008; 3: 105-116 [DOI] [PubMed] [Google Scholar]
- 1483).Arai H, Mortaki K, Rane P, Quinn C, Zhao Z, Qian Y: Estimating Years of Life Lost Due to Cardiovascular Disease in Japan. Circ J, 2019; 83: 1006-1010 [DOI] [PubMed] [Google Scholar]
- 1484).Uchikado Y, Ikeda Y, Ohishi M: Current Understanding of the Role of Frailty in Cardiovascular Disease. Circ J, 2020; 84: 1903-1908 [DOI] [PubMed] [Google Scholar]
- 1485).Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA: Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 2001; 56: M146-156 [DOI] [PubMed] [Google Scholar]
- 1486).Satake S, Arai H: The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr Gerontol Int, 2020; 20: 992-993 [DOI] [PubMed] [Google Scholar]
- 1487).Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H: Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc, 2020; 21: 300-307.e302 [DOI] [PubMed] [Google Scholar]
- 1488).Akishita M, Kozaki K, Iijima K, Tanaka T, Shibasaki K, Ogawa S, Arai H: Chapter 1 Definitions and diagnosis of sarcopenia. Geriatr Gerontol Int, 2018; 18 Suppl 1: 7-12 [DOI] [PubMed] [Google Scholar]
- 1489).Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB: Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc, 2005; 53: 1321-1330 [DOI] [PubMed] [Google Scholar]
- 1490).Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB: Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA, 2006; 295: 2018-2026 [DOI] [PubMed] [Google Scholar]
- 1491).Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E: Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol, 2015; 65: 976-983 [DOI] [PubMed] [Google Scholar]
- 1492).Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S: Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J, 2013; 34: 512-519 [DOI] [PubMed] [Google Scholar]
- 1493).Cacoub PP, Abola MT, Baumgartner I, Bhatt DL, Creager MA, Liau CS, Goto S, Röther J, Steg PG, Hirsch AT: Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis, 2009; 204: e86-92 [DOI] [PubMed] [Google Scholar]
- 1494).Setacci C, de Donato G, Setacci F, Chisci E: Diabetic patients: epidemiology and global impact. J Cardiovasc Surg (Torino), 2009; 50: 263-273 [PubMed] [Google Scholar]
- 1495).Lin CH, Chou CY, Liu CS, Huang CY, Li TC, Lin CC: Association between frailty and subclinical peripheral vascular disease in a community-dwelling geriatric population: Taichung Community Health Study for Elders. Geriatr Gerontol Int, 2015; 15: 261-267 [DOI] [PubMed] [Google Scholar]
- 1496).Fang ZB, Hu FY, Arya S, Gillespie TW, Rajani RR: Preoperative frailty is predictive of complications after major lower extremity amputation. J Vasc Surg, 2017; 65: 804-811 [DOI] [PubMed] [Google Scholar]
- 1497).Ali TZ, Lehman EB, Aziz F: Modified Frailty Index Can Be Used to Predict Adverse Outcomes and Mortality after Lower Extremity Bypass Surgery. Ann Vasc Surg, 2018; 46: 168-177 [DOI] [PubMed] [Google Scholar]
- 1498).Ministry of Health, Labour and Welfare: Vital Statistics in Japan, 2018, https: //www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai18/dl/gaikyou30-190626.pdf, 2019 (in Japanese) [Google Scholar]
- 1499).Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A: Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation, 1994; 90: 583-612 [DOI] [PubMed] [Google Scholar]
- 1500).Ueshima H: Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb, 2007; 14: 278-286 [DOI] [PubMed] [Google Scholar]
- 1501).Fukiyama K, Kimura Y, Wakugami K, Muratani H: Incidence and long-term prognosis of initial stroke and acute myocardial infarction in Okinawa, Japan. Hypertens Res, 2000; 23: 127-135 [DOI] [PubMed] [Google Scholar]
- 1502).Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Arima H, Tanaka K, Nakamura H, Okubo K, Iida M: Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke, 2003; 34: 2349-2354 [DOI] [PubMed] [Google Scholar]
- 1503).Kudenchuk PJ, Maynard C, Martin JS, Wirkus M, Weaver WD: Comparison of presentation, treatment, and outcome of acute myocardial infarction in men versus women (the Myocardial Infarction Triage and Intervention Registry). Am J Cardiol, 1996; 78: 9-14 [DOI] [PubMed] [Google Scholar]
- 1504).Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M: Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Arch Intern Med, 1998; 158: 981-988 [DOI] [PubMed] [Google Scholar]
- 1505).Vakili BA, Kaplan RC, Brown DL: Sex-based differences in early mortality of patients undergoing primary angioplasty for first acute myocardial infarction. Circulation, 2001; 104: 3034-3038 [DOI] [PubMed] [Google Scholar]
- 1506).Marso SP, Gowda M, O'Keefe JH, Coen MM, McCallister BD, Giorgi LV, Huber KC, Laster SB, Johnson WL, Rutherford BD: Improving in-hospital mortality in the setting of an increasing risk profile among patients undergoing catheter-based reperfusion for an acute myocardial infarction without cardiogenic shock. J Invasive Cardiol, 2003; 15: 711-716 [PubMed] [Google Scholar]
- 1507).Kimura Y, Takishita S, Muratani H, Kinjo K, Shinzato Y, Muratani A, Fukiyama K: Demographic study of first-ever stroke and acute myocardial infarction in Okinawa, Japan. Intern Med, 1998; 37: 736-745 [DOI] [PubMed] [Google Scholar]
- 1508).Kosuge M, Kimura K, Kojima S, Sakamoto T, Ishihara M, Asada Y, Tei C, Miyazaki S, Sonoda M, Tsuchihashi K, Yamagishi M, Ikeda Y, Shirai M, Hiraoka H, Inoue T, Saito F, Ogawa H: Sex differences in early mortality of patients undergoing primary stenting for acute myocardial infarction. Circ J, 2006; 70: 217-221 [DOI] [PubMed] [Google Scholar]
- 1509).Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y, Hirose K, Okayama A, Miura K, Ueshima H: Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988-2004. Stroke, 2010; 41: 1871-1876 [DOI] [PubMed] [Google Scholar]
- 1510).Kita Y, Turin TC, Ichikawa M, Sugihara H, Morita Y, Tomioka N, Rumana N, Okayama A, Nakamura Y, Abbott RD, Ueshima H: Trend of stroke incidence in a Japanese population: Takashima stroke registry, 1990-2001. Int J Stroke, 2009; 4: 241-249 [DOI] [PubMed] [Google Scholar]
- 1511).Maeda K, Toyoda K, Minematsu K, Kobayashi S: Effects of sex difference on clinical features of acute ischemic stroke in Japan. J Stroke Cerebrovasc Dis, 2013; 22: 1070-1075 [DOI] [PubMed] [Google Scholar]
- 1512).Baba S, Iso H, Mannami T, Sasaki S, Okada K, Konishi M: Cigarette smoking and risk of coronary heart disease incidence among middle-aged Japanese men and women: the JPHC Study Cohort I. Eur J Cardiovasc Prev Rehabil, 2006; 13: 207-213 [DOI] [PubMed] [Google Scholar]
- 1513).Honjo K, Iso H, Tsugane S, Tamakoshi A, Satoh H, Tajima K, Suzuki T, Sobue T: The effects of smoking and smoking cessation on mortality from cardiovascular disease among Japanese: pooled analysis of three large-scale cohort studies in Japan. Tob Control, 2010; 19: 50-57 [DOI] [PubMed] [Google Scholar]
- 1514).Nakamura K, Nakagawa H, Sakurai M, Murakami Y, Irie F, Fujiyoshi A, Okamura T, Miura K, Ueshima H: Influence of smoking combined with another risk factor on the risk of mortality from coronary heart disease and stroke: pooled analysis of 10 Japanese cohort studies. Cerebrovasc Dis, 2012; 33: 480-491 [DOI] [PubMed] [Google Scholar]
- 1515).Huxley RR, Woodward M: Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet, 2011; 378: 1297-1305 [DOI] [PubMed] [Google Scholar]
- 1516).Kawano H, Soejima H, Kojima S, Kitagawa A, Ogawa H: Sex differences of risk factors for acute myocardial infarction in Japanese patients. Circ J, 2006; 70: 513-517 [DOI] [PubMed] [Google Scholar]
- 1517).Nishino Y, Tsuji I, Tanaka H, Nakayama T, Nakatsuka H, Ito H, Suzuki T, Katanoda K, Sobue T, Tominaga S: Stroke mortality associated with environmental tobacco smoke among never-smoking Japanese women: a prospective cohort study. Prev Med, 2014; 67: 41-45 [DOI] [PubMed] [Google Scholar]
- 1518).Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, Takahashi A, Nishinaga M, Soejima H, Ueshima H: Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: a meta-analysis of 16 cohort studies. Circulation, 2009; 119: 1892-1898 [DOI] [PubMed] [Google Scholar]
- 1519).Ikeda A, Iso H, Yamagishi K, Inoue M, Tsugane S: Blood pressure and the risk of stroke, cardiovascular disease, and all-cause mortality among Japanese: the JPHC Study. Am J Hypertens, 2009; 22: 273-280 [DOI] [PubMed] [Google Scholar]
- 1520).Arima H, Tanizaki Y, Yonemoto K, Doi Y, Ninomiya T, Hata J, Fukuhara M, Matsumura K, Iida M, Kiyohara Y: Impact of blood pressure levels on different types of stroke: the Hisayama study. J Hypertens, 2009; 27: 2437-2443 [DOI] [PubMed] [Google Scholar]
- 1521).Takashima N, Ohkubo T, Miura K, Okamura T, Murakami Y, Fujiyoshi A, Nagasawa SY, Kadota A, Kita Y, Miyagawa N, Hisamatsu T, Hayakawa T, Okayama A, Ueshima H: Long-term risk of BP values above normal for cardiovascular mortality: a 24-year observation of Japanese aged 30 to 92 years. J Hypertens, 2012; 30: 2299-2306 [DOI] [PubMed] [Google Scholar]
- 1522).Saito I, Kokubo Y, Yamagishi K, Iso H, Inoue M, Tsugane S: Diabetes and the risk of coronary heart disease in the general Japanese population: the Japan Public Health Center-based prospective (JPHC) study. Atherosclerosis, 2011; 216: 187-191 [DOI] [PubMed] [Google Scholar]
- 1523).Kato M, Noda M, Mizoue T, Goto A, Takahashi Y, Matsushita Y, Nanri A, Iso H, Inoue M, Sawada N, Tsugane S: Diagnosed diabetes and premature death among middle-aged Japanese: results from a large-scale population-based cohort study in Japan (JPHC study). BMJ Open, 2015; 5: e007736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1524).Peters SA, Huxley RR, Woodward M: Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia, 2014; 57: 1542-1551 [DOI] [PubMed] [Google Scholar]
- 1525).Peters SA, Huxley RR, Woodward M: Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet, 2014; 383: 1973-1980 [DOI] [PubMed] [Google Scholar]
- 1526).Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC: Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med, 2000; 343: 16-22 [DOI] [PubMed] [Google Scholar]
- 1527).Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM: Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA, 2011; 306: 62-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1528).Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB: Primary prevention of stroke by healthy lifestyle. Circulation, 2008; 118: 947-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1529).Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB: Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol, 2015; 65: 43-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1530).Hackshaw A, Rodeck C, Boniface S: Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update, 2011; 17: 589-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1531).Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM: Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation, 2010; 121: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1532).Edison RJ, Muenke M: Gestational exposure to lovastatin followed by cardiac malformation misclassified as holoprosencephaly. N Engl J Med, 2005; 352: 2759 [DOI] [PubMed] [Google Scholar]
- 1533).Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM, Desai RJ, Allen-Coleman C, Mogun H, Avorn J, Huybrechts KF: Statins and congenital malformations: cohort study. BMJ, 2015; 350: h1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1534).Gutierrez J, Ramirez G, Rundek T, Sacco RL: Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med, 2012; 172: 909-919 [DOI] [PubMed] [Google Scholar]
- 1535).Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J: Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ, 2011; 343: d6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1536).Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med, 2005; 353: 2643-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1537).Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J, Inzucchi SE, Kosiborod M, Nelson RG, Patel MJ, Pignone M, Quinn L, Schauer PR, Selvin E, Vafiadis DK: Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care, 2015; 38: 1777-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1538).Miura K, Nagai M, Ohkubo T: Epidemiology of hypertension in Japan: where are we now? Circ J, 2013; 77: 2226-2231 [DOI] [PubMed] [Google Scholar]
- 1539).Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, Perkovic V, Li N, MacMahon S: Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J, 2008; 29: 2669-2680 [DOI] [PubMed] [Google Scholar]
- 1540).Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E: Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA, 1998; 280: 605-613 [DOI] [PubMed] [Google Scholar]
- 1541).Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N: Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA, 2002; 288: 49-57 [DOI] [PubMed] [Google Scholar]
- 1542).Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ: Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA, 2003; 289: 2673-2684 [DOI] [PubMed] [Google Scholar]
- 1543).Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M: Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med, 2003; 349: 523-534 [DOI] [PubMed] [Google Scholar]
- 1544).Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J: Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation, 2006; 113: 2425-2434 [DOI] [PubMed] [Google Scholar]
- 1545).Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML: Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA, 2007; 297: 1465-1477 [DOI] [PubMed] [Google Scholar]
- 1546).The Japan Society of Obstetrics and Gynecology and the Japan Society for Menopause and Women's Health: Hormone Replacement Therapy Guidelines 2017. Japan Society of Obstetrics and Gynecology, 2017 (in Japanese) [Google Scholar]
- 1547).Løkkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard Ø: Hormone therapy and risk of myocardial infarction: a national register study. Eur Heart J, 2008; 29: 2660-2668 [DOI] [PubMed] [Google Scholar]
- 1548).Committee for the Preparation of the Management for Primary Prevention of Atherosclerotic Cardiovascular Diseases in Women 2018:Management for Primary Prevention of Atherosclerotic Cardiovascular Diseases in Women 2018, The Japan Society for Menopause and Women's Health, Shindan to Chiryo sha Inc., 2018 (in Japanese) [Google Scholar]
- 1549).Okada T, Murata M, Yamauchi K, Harada K: New criteria of normal serum lipid levels in Japanese children: the nationwide study. Pediatr Int, 2002; 44: 596-601 [DOI] [PubMed] [Google Scholar]
- 1550).Abe Y, Okada T, Sugiura R, Yamauchi K, Murata M: Reference Ranges for the Non-High-Density Lipoprotein Cholesterol Levels in Japanese Children and Adolescents. J Atheroscler Thromb, 2015; 22: 669-675 [DOI] [PubMed] [Google Scholar]
- 1551).Japan Society for the Study of Obesity: Guidelines for the management of obesity disease in children and adolescents 2017, Life Science Publishing, 2017 [Google Scholar]
- 1552).Kobayashi Y, Sugihara S, Tanaka Y, Ishihara H, Ohno K, Fujita H, Takizawa N, Tsuchihashi M: A consideration on criteria for blood collection after a meal in medical examinations of life-style related diseases in children. The Journal of the Japan Peiatric Society, 2011; 115: 1255-1264 (in Japanese) [Google Scholar]
- 1553).Dobashi K: Changes in Serum Cholesterol in Childhood and its Tracking to Adulthood. J Atheroscler Thromb, 2022; 29: 5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1554).Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, Maahs D: ISPAD Clinical Practice Consensus Guidelines 2014. Type 2 diabetes in the child and adolescent. Pediatr Diabetes, 2014; 15 Suppl 20: 26-46 [DOI] [PubMed] [Google Scholar]
- 1555).Japan Diabetes Society and Japanese Society for Pediatric Endocrinology: Consensus Guidelines for Diabetes Mellitus in Children and Adolescents, Nankodo, 2015 (in Japanese) [Google Scholar]
- 1556).Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA, 1990; 264: 3018-3024 [DOI] [PubMed] [Google Scholar]
- 1557).Newman WP, 3rd, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, Williamson GD, Webber LS, Berenson GS: Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med, 1986; 314: 138-144 [DOI] [PubMed] [Google Scholar]
- 1558).Dobashi K: Evaluation of Obesity in School-Age Children. J Atheroscler Thromb, 2016; 23: 32-38 [DOI] [PubMed] [Google Scholar]
- 1559).Information Center for Specific Pediatric Chronic Diseases, Japan https: //www.shouman.jp/disease/details/08_12_130/ (in Japanese) [Google Scholar]