Abstract
IRF6 is a key genetic determinant of syndromic and non-syndromic cleft lip and palate. The ability to interrogate post-embryonic requirements of Irf6 has been hindered, as global Irf6 ablation in the mouse causes neonatal lethality. Prior work analyzing Irf6 in mouse models defined its role in the embryonic surface epithelium and periderm where it is required to regulate cell proliferation and differentiation. Several reports have also described Irf6 gene expression in other cell types, such as muscle, and neuroectoderm. However, analysis of a functional role in non-epithelial cell lineages has been incomplete due to the severity and lethality of the Irf6 knockout model and the paucity of work with a conditional Irf6 allele. Here we describe the generation and characterization of a new Irf6 floxed mouse model and analysis of Irf6 ablation in periderm and neural crest lineages. This work found that loss of Irf6 in periderm recapitulates a mild Irf6 null phenotype, suggesting that Irf6-mediated signaling in periderm plays a crucial role in regulating embryonic development. Further, conditional ablation of Irf6 in neural crest cells resulted in an anterior neural tube defect of variable penetrance. The generation of this conditional Irf6 allele allows for new insights into craniofacial development and new exploration into the post-natal role of Irf6.
Keywords: Irf6, cleft palate, neural tube, neural crest, Van der Woude Syndrome, periderm
Introduction
IRF6 was one of the first genetic determinants of syndromic cleft lip and palate malformation, uncovered from genome-wide association studies of Van der Woude syndrome (VWS) and popliteal pterygium syndrome (PPS) (Kondo et al., 2002). IRF6 gene variants are also major contributors to non-syndromic cleft lip with or without cleft palate (Leslie et al., 2013; Park et al., 2007; Rahimov et al., 2008; Zucchero et al., 2004). Multiple studies using mouse and zebrafish models have shown that Irf6 is expressed in the basal epithelium and periderm during embryonic development with dynamic expression in the oral epithelium during palatogenesis (Carroll et al., 2020; de la Garza et al., 2013; Dougherty et al., 2013; Ferretti et al., 2011; Ingraham et al., 2006; Iwata et al., 2013; Knight et al., 2006; Kousa et al., 2017; Richardson et al., 2006; Xu et al., 2006). Irf6 is necessary for keratinocyte differentiation (Biggs et al., 2012; Ingraham et al., 2006; Restivo et al., 2011; Richardson et al., 2006) and for the development of the periderm (de la Garza et al., 2013; Li et al., 2017; Richardson et al., 2009; Richardson et al., 2014; Sabel et al., 2009). Ablation of Irf6 in mice resulted in severe epithelial adhesions that caused “cocooning” of the embryo and caused adherence of palatal shelves to the tongue in the vertical orientation precluding elevation and fusion of the secondary palate (Ingraham et al., 2006; Richardson et al., 2009; Richardson et al., 2014) and fuse (Iwata et al., 2013).
While most studies have examined the requirement of Irf6 in epithelial differentiation, several studies have described Irf6 function in non-epithelial tissue, in either autonomous or non-cell-autonomous fashion (Goudy et al., 2013; Thompson et al., 2019). Irf6 is expressed in cell types not restricted to surface epithelium during early development, including the craniofacial mesenchyme and neuroectoderm (Carroll et al., 2020; Fakhouri et al., 2017; Goudy et al., 2013; Sabel et al., 2009; Thompson et al., 2019). Further, analysis of murine MCS9.7 enhancer element activity, which replicates endogenous Irf6 expression in most tissues, yielded expression in developing somites, tongue, axial cartilage, and muscle (Fakhouri et al., 2012). We and others have described that mesenchymal-derived craniofacial tissue, such as muscle and cartilage are dysmorphic in the Irf6 null mice (Carroll et al., 2020; Chu et al., 2016; Thompson et al., 2019). However, it remains unclear whether there is a cell-autonomous role of Irf6 in non-epithelial cell types, a non-cell-autonomous role caused by loss of epithelial Irf6, or if these dysmorphologies are associated consequences of the severe epithelial adhesions caused by a dysfunctional epithelium.
In addition to orofacial and epithelial development, Irf6 has been found to have a role in neurulation. Irf6 is expressed in the neuroectoderm of the neural folds and is co-expressed with Tfap2a, a known regulator of neural tube closure (Kousa et al., 2019). Although neural tube defects are not apparent in Irf6 null mice, ablation of other genes in the Irf6 regulatory pathway, i.e. Tfap2A and Grhl3, leads to rostral and caudal neural tube defects (Schorle et al., 1996; Ting et al., 2003; Zhang et al., 1996). Utilizing an Irf6 hypomorph allele and a Krt4:Irf6 transgenic mouse to titrate Irf6 expression levels, Kousa et al. found homeostasis of Irf6 to be required for neurulation (Kousa et al., 2019).
The ability to interrogate non-epithelial and post-natal functions of Irf6 has been impaired by the severe and lethal phenotype of the Irf6 null mouse models. A previously generated Irf6 floxed mouse model has given some insight (Smith et al., 2017). Conditional ablation of Irf6 in oral epithelium via a Pitx2-Cre driver line resulted in tooth development and maturation defects (Chu et al., 2016). Since the previously generated Irf6 floxed allele was reported to show variable recombination efficiency and we remained unsuccessful in acquiring it (Smith et al., 2017), we generated a new conditional Irf6 floxed mouse allele for this work. This Irf6 conditional allele demonstrated complete recombination efficiency with every ubiquitous and tissue-restricted Cre drivers we have tested.
In this study, we describe the generation of a new conditional Irf6 mouse allele and analyze Wnt1-Cre2-mediated disruption of Irf6 in the neural crest cells (NCCs). We also utilized the Krt6ai-Cre driver line to ablate Irf6 function in periderm. These results demonstrate for the first time a cell-autonomous role for Irf6 in the neural crest as well as corroborate the functional role of Irf6 in the periderm during orofacial development.
Materials and Methods
Generation of a new conditional Irf6 mouse allele
All procedures were approved by IACUCs for Massachusetts General Hospital and Harvard University where the initial work was carried out. The Easi-CRISPR protocol was utilized to introduce loxP sites (Miura et al., 2018) flanking exons 3 and 4 of Irf6. As these exons contain the DNA binding region (Kondo et al., 2002), they are predicted to be required for Irf6 transcriptional function and have been previously targeted for conditional ablation of Irf6 (Smith et al., 2017). Guide RNAs (gRNA) were designed within the intronic regions flanking exons 3 and 4 using the CRISPR gRNA design tool from Benchling and were ordered from Synthego. Single-stranded DNA (ssDNA) donor sequences were designed to contain the loxp sequence flanked by homologous arms and were ordered from IDT. Cas9, gRNA and donor ssDNA were injected into mouse zygotes by the Harvard Genome Modification Facility. Resulting viable pups were genotyped by PCR as well as sequenced to ensure the insertion of the loxP sequences within the same DNA strand. A female mouse was identified with the correct genome modifications, was phenotypically normal, and was designated F0. Breeding with a wildtype C57BL/6J mouse generated F1s, which were in-crossed to generate mice homozygous for the floxed Irf6 allele (Irf6fl/fl).
Mouse lines
To validate efficient Cre recombination and to confirm recombination ablates Irf6 function, Irf6fl/fl mice were bred to the Cre deleter lines CMV-Cre (Jackson Labs stock# 006054) and EIIa-Cre (Jackson Labs stock# 003724). The resulting pups (viable and non-viable) were phenotyped and genotyped. Wnt1Cre2 and Sox10Cre were obtained from Jackson Labs (stock# 022501 and 025807, respectively). Krt6ai-Cre came from Vesa Kaartinen. Crect line came from Russ Carstens but originated from Trevor Williams. For timed pregnancies, E0.5 was determined upon observation of a copulatory plug.
Histology and in situ hybridization
Mice were fixed with 4% formaldehyde followed by cryoprotection in 15 and 30% sucrose. Tissues were embedded in OCT and 10 μm sections were made. Hematoxylin and Eosin staining was performed according to a standard protocol (Fischer et al., 2008) and slides were imaged with a Leica DM6 upright microscope and LAS X software.
RNAscope probes for mouse Irf6, Wnt1, and Sox10 were designed and manufactured by Advanced Cell Diagnostics. RNAscope in situ hybridization was performed according to the manufacturer’s protocol (Advanced Cell Diagnostics). Slides were imaged using a confocal laser scanning microscope (Leica SP8) and image processing was performed using ImageJ version 2.0 (2018).
MicroCT analysis and measurements
Scans were performed using a μCT40 benchtop scanner (Scanco Medical AG, Brüttisellen, Switzerland). Scans were acquired with a 15 μm3 isotropic voxel size, 70 kVP peak x-ray tube potential, 114 mA intensity, and 300 ms integration time. Morphometric landmarks were chosen as previously described (Ho et al., 2015) and measurements were made using Avizo software.
Results
Irf6 is expressed in Wnt1+ neuroectoderm and neural crest cell-derived cranial mesenchyme
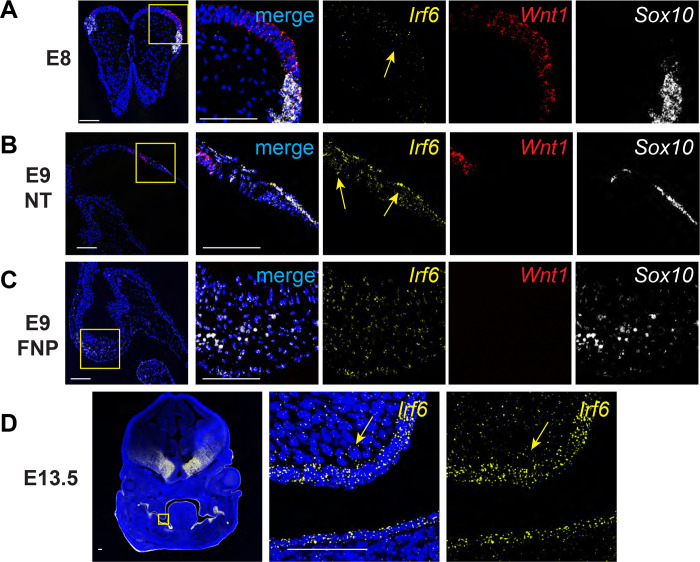
Irf6 null mice exhibit a foreshortened midface as well as malformation of neural crest-derived maxilla (Fakhouri et al., 2017; Richardson et al., 2006; Thompson et al., 2019). Irf6 gene dosage has also been found to impact neural tube closure (Kousa et al., 2019). It was previously reported that Irf6 is expressed in the neuroectoderm and neural folds of mouse embryos (Bertol et al., 2022; Kousa et al., 2019). To examine whether cranial neural crest cells express Irf6, we analyzed Irf6 mRNA expression by RNAscope in situ hybridization in mouse embryos during early craniofacial development. At E8 and E9, we found Irf6 mRNA co-expressed with Wnt1 in the neuroectoderm. Irf6 mRNA expression was also found co-expressed with Sox10, demonstrating Irf6 expression in migratory neural crest cells (Fig 1b). Further, Irf6 mRNA is expressed within the neural crest-derived craniofacial mesenchyme at E9 (Fig 1c) and E13.5 (Fig 1d). Based on these detailed gene expression findings, as well as previously reported expression of Irf6 in the neuroectoderm (Bertol et al., 2022; Kousa et al., 2019), we posited that Irf6 contributes to craniofacial development beyond its established role in the surface epithelium and periderm.
Fig. 1.
Irf6 is expressed with neural crest cell markers Wnt1 and Sox10 in neural folds and neural tube during early embryogenesis. In situ hybridization of Irf6 (yellow), Wnt1 (red), and Sox10 (white) RNA transcripts. A. Coronal section of E8 mouse embryo (dorsal to top) showing the neural fold. In situ hybridization shows RNA expression domains of Irf6, Wnt1, and Sox10, where Irf6 and Wnt1 transcripts are found in the same regions of the neural tube, highlighted by yellow arrow. Box indicates area of higher magnification to the right. B. Sagittal section of E9 mouse embryo (cranial to left). Box indicates a magnified portion of the neural tube. Irf6 is expressed in the neuroectoderm and overlaps with Wnt1 and Sox10 expression (yellow arrows). C. Sagittal section of E9 mouse embryo (cranial to left). Box indicates a magnified portion of frontonasal prominence (FNP). Irf6 is expressed in the FNP mesenchyme, along with the migratory NCC marker Sox10. D. Coronal section of E13.5 embryo (dorsal to top). Box indicates higher magnification of palate shelf epithelium and mesenchyme. Irf6 is highly expressed in the basal epithelium and periderm and the palate mesenchyme (yellow arrow). Blue is dapi. Scale: 100 uM.
Generation and validation of an Irf6 conditional allele
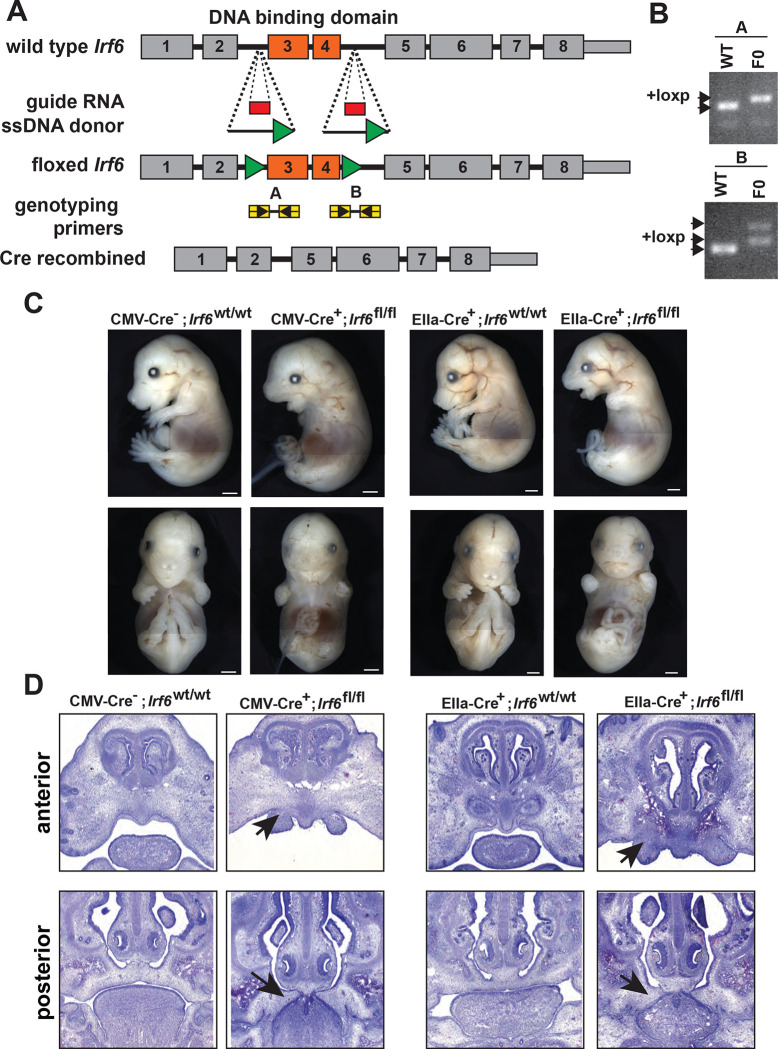
Severe epithelial adhesions and perinatal lethality in the Irf6 complete knockout embryos (Ingraham et al., 2006) and the Irf6 R84C single nucleotide substitution mouse (Richardson et al., 2006) impeded full analysis of Irf6 function. The previously reported Irf6 floxed mouse allele was reported to exhibit inconsistent recombination depending on the Cre driver used, confounding analysis of Irf6 requirement in the multiple tissue types (Smith et al., 2017). Given the complexity of Irf6 gene expression in neuroectoderm and neural crest during early embryogenesis, we generated a new Irf6 floxed allele to better understand Irf6 function. We utilized an CRISPReasi technique (Miura et al., 2018) to insert loxP sequences flanking exon 3 and 4 of the Irf6 gene (Fig 2a). Insertion of the (22bp) loxP sequence was verified by PCR genotyping of potential founders (Fig 2b), followed by Sanger sequencing to confirm loxP insertion without disruption of exonic sequences.
Fig. 2.
Generation and validation of a conditional Irf6 null mouse model. A. Schematic of gene targeting strategy. Introns flanking Irf6 exons 3 and 4 were targeted for CRISPR-Cas9-directed homologous recombination with each donor ssDNA containing loxP sequences (green triangles). Insertion of loxP sites into Irf6 was confirmed by PCR. B. and Sanger sequencing. C. Cre-mediated recombination was validated using the ubiquitous Cre expressing lines CMV-Cre and EIIa-Cre. CMV-Cre+;Irf6fl/fl and EIIa-Cre+; Irf6fl/fl mice phenocopied the Irf6 global KO while Cre−;Irf6fl/fl and Cre+;Irf6wt/wt littermates were normal. D. Hematoxylin and Eosin staining of coronal sections of E15 CMV-Cre or EIIa-Cre knockout embryos and littermate controls. Top row is a relatively anterior section while the bottom row is relatively posterior. CMV-Cre and EIIa-Cre Irf6 KO embryos phenocopy the dysmorphic alveolar bone and the cleft palate with oral adhesions of the total Irf6 knockout mouse (arrows).
To test whether Cre expression resulted in recombination and loss of function, the confirmed founder mouse was bred to two different deleter strains; CMV-Cre and EIIa-Cre. We found that pups that were homozygous for loxP but negative for Cre were phenotypically normal and healthy. Pups that were homozygous for loxP and positive for CMV-Cre or EIIa-Cre recapitulated the epithelial adhesions, limb abnormalities, and cleft palate displayed by Irf6 total knockout mice (Fig 2c,d). Further, we found this phenotype to be completely penetrant. Based on these results, we determined faithful recombination of the Irf6 floxed allele leading to functional Irf6 ablation.
Ablation of Irf6 in the Wnt1 lineage leads to a cranial defect and increased perinatal lethality
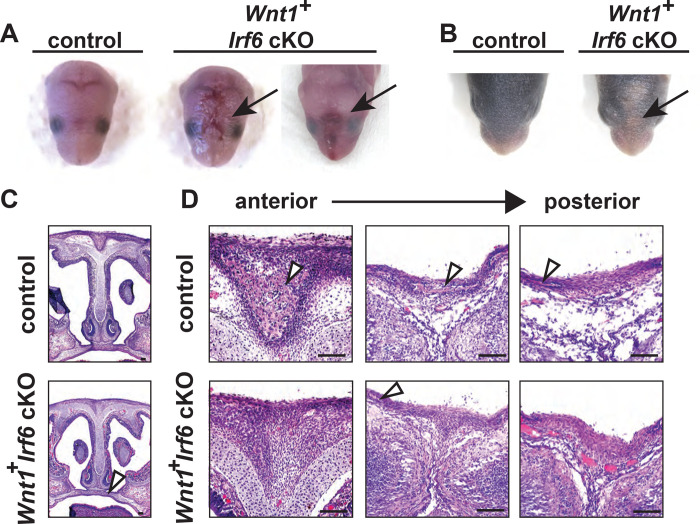
With recombination of the floxed Irf6 allele validated, we tested the effect of Irf6 ablation in the NCC lineage. We utilized the Wnt1-Cre2 and the Sox10-Cre mouse lines to drive the recombination of the floxed genome sequence. Wnt1 is expressed in the neural folds and pre-migratory NCCs (Lewis et al., 2013; Schock et al., 2017) whereas Sox10 is expressed in migratory NCCs (Matsuoka et al., 2005). Analysis of Sox10-Cre Irf6 cKO pups revealed no phenotypic effect of Irf6 ablation in migratory and post-migratory NCCs (data not shown). This finding suggests that although Irf6 mRNA can be found in NCC-derived mesenchymal tissue, its expression is not necessary for craniofacial development. In contrast to Sox10-Cre driven ablation, analysis of Wnt1-Cre2 Irf6 cKO pups revealed a range of phenotype severity with some pups phenotypically normal and viable. We also observed P0 pups that were largely normal but exhibited skin lesions overlying the nasal and frontal bones (Fig 3a). These skin lesions resolved but led to delayed fur growth (Fig 3b). To detect whether Wnt1-Cre2 cKO of Irf6 resulted in differences in pup survival, litter size at P0 was recorded and compared to genotype ratios at 3 weeks of age. Based on total pup numbers and expected ratio (based on parent genotypes), we expected approximately 6 Wnt1-Cre2 Irf6 cKO at weaning. Instead, 1 Irf6 cKO pup was identified at 3 weeks of age, suggesting perinatal lethality between birth and weaning. The numbers of wild-type and heterozygous pups were as expected. We did not find the lethality of the Wnt1-Cre2 Irf6 cKO pups to be due to cleft palate, as histological examination of P0 dead or moribund pups showed palatogenesis to be normal (Fig. 3c).
Fig. 3.
Wnt1-Cre-dependent Irf6 ablation causes cranial defects. A. Representative images of littermate control and Wnt1-Cre, Irf6 cKO pups at P0. At parturition, Wnt1-Cre+;Irf6fl/fl cKO mice display midline lesions of varying penetrance (arrow). B. Representative images of littermate control and Wnt1-Cre+;Irf6fl/fl cKO pups at P6. As the mouse neonate develops, these frontal lesions resolve but remain evident with deficient or delayed fur growth (arrow). C. Hematoxylin and eosin staining of coronal sections through the palate of E16 Wnt1-Cre+;Irf6fl/fl cKO and littermate control embryos shows normal development (arrow). D. Hematoxylin and eosin staining of coronal sections through the nasal and frontal bones of Wnt1-Cre+;Irf6fl/fl cKO and littermate control. Sections move anterior to posterior from left to right. Bone tissue is indicated with arrows. Wnt1-Cre+;Irf6fl/fl cKO mice have a lack of cranial bone development and suture formation at the midline (bone tissue indicated by arrows). Scale: 100 μM.
To examine whether the underlying calvarial development was affected in the Wnt1-Cre2 Irf6 cKO before parturition, we performed histology on coronal sections taken through the nasal-frontal bone junction of Wnt1-Cre2 Irf6 cKO E16 pups and littermate controls. We found that control mice had bone tissue at the midline, forming a suture between right and left calvaria. In contrast, the Wnt1-Cre2+;Irf6fl/fl cKO mice exhibited a large gap devoid of bone tissue that spanned the midline (Fig 3d). These findings of a midline cranial defect and partial lethality in Wnt1-Cre2 Irf6 cKO mice suggest that Irf6 expression in the pre-migratory NCCs is functionally required for craniofacial development.
Wnt1-Cre2 Irf6 cKO mice exhibited incomplete frontal and parietal bone development
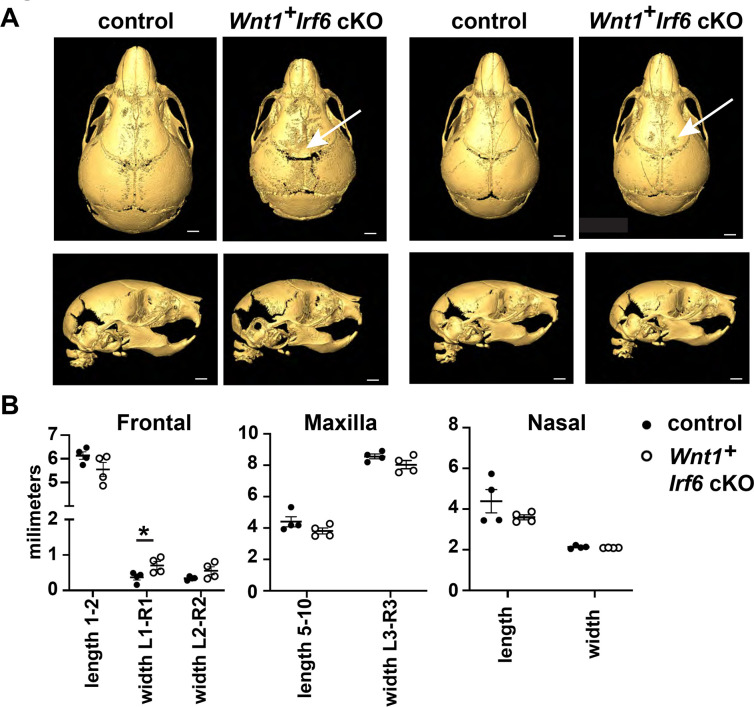
The variable severity of the cranial defect in Wnt1-Cre Irf6 cKO mice spurred us to examine the cranial bone development of the mice more precisely with microCT analysis. Wnt1-Cre cKO pups and sex-matched littermate controls were collected at 10 days of age for microCT scanning. For controls, we analyzed both Wnt1-Cre negative, Irf6fl/fl and Wnt1-Cre positive, Irf6wt/fl pups to account for potential differences caused by the Wnt1-Cre transgene. Wnt1-Cre+;Irf6fl/fl pups exhibited decreased mineralization of the frontal and parietal bones at the midline, although the degree of this defect was variable between individuals (Fig 4a). These observations are similar to previously published microCT analysis of the Irf6 null mouse (Thompson et al., 2019). To quantify potential changes in cranial development in the cKO mice, we performed a series of measurements based on established anatomical landmarks in cKO versus sex-matched littermate Wnt1-Cre negative controls (Fig 4a). Overall, we did not detect significant differences in length or width measurements of the frontal, maxillary, or nasal bones, except that the width across the anterior portion of the frontal bones of the Wnt1-Cre2+;Irf6fl/fl cKO mice was significantly larger (Fig. 4b). Nasal bones of Wnt1-Cre2+;Irf6fl/fl cKO mice tended to be shorter, however, this measurement was variable in the control pups (Fig. 4b). These data demonstrate that Irf6 expressed in NCCs contributes to a midline calvarial bone defect. We hypothesize this to be an indirect effect of loss of Irf6 in the neural folds, as non-NCC derived calvarial tissue, namely parietal bone, is also deficient in the Wnt1-Cre Irf6 cKO mice.
Fig. 4.
Cranial bone development is impaired in Wnt1-Cre Irf6 cKO mice. A. Representative microCT reconstructions of P10 Wnt1-Cre+;Irf6fl/fl cKO mice and littermate sex-matched controls. Wnt1-Cre+;Irf6fl/fl cKO mice have decreased formation or mineralization of the cranial bones at the midline with variable penetrance (arrows). Scale: 1 mm. B. MicroCT reconstructions were utilized for cranial bone measurements. The space between the left and right frontal bones of Wnt1-Cre+;Irf6fl/fl cKO mice was significantly wider than controls (L1-R1, *p<0.05) and the frontal bones tended to have decreased total length (length 1–2). Maxilla of Wnt1-Cre+;Irf6fl/fl cKO mice tended to be smaller (lower length and width measurements) and the frontal bone of Wnt1-Cre+;Irf6fl/fl cKO mice tended to be shorter, however, these differences were not significantly different. N=4.
Wnt1-Cre dependent Irf6 ablation altered neuroepithelial morphology and Wnt1 expression
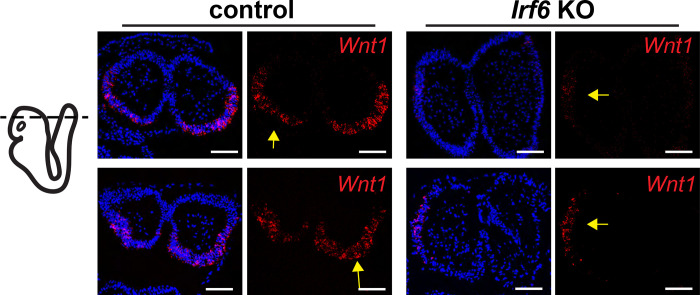
The cranial defect observed in Wnt1-Cre;Irf6 cKO mice involved both the overlying skin (Fig 3a) and the underlying cranial bone (Fig 3d). Further, neural crest-derived frontal bone and non-neural crest-derived parietal bone were affected by Wnt1-Cre;Irf6 cKO (Fig. 4a). Therefore, we reasoned that this phenotype may be the manifestation of a rostral neural tube closure defect. We examined the neural folds of E8 Wnt1-Cre+;Irf6fl/f cKO and littermate control embryos. Transverse sections through the cranial neural folds showed differences in overall morphology, with the cKO embryos tending to be more elongated anterior-posterior as compared to controls (Fig. 5). Further, Wnt1 expression in the Wnt1-Cre+;Irf6fl/fl cKO embryos was laterally displaced relative to the more posterior expression observed in the controls (Fig. 5). These results are consistent with a previous report of dysmorphic neuroectoderm and neural fold morphology in Irf6 null embryos (Bertol et al., 2022).
Fig. 5.
Irf6 ablation in the neuroectoderm and neural crest changes Wnt1 expression domains within the neural folds. A. RNAscope in situ hybridization of transverse sections of Wnt1-Cre+;Irf6fl/fl cKO and littermate control E8 embryos. Rows represent 2 individuals of each genotype. Whereas Wnt1 expression (red) is localized to the caudal-dorsal neural folds in the control embryos, Wnt1 expression in Wnt1-Cre+;Irf6fl/fl cKO embryos is displaced laterally (arrows). Blue is dapi. Scale: 100 μM
Ablation of Irf6 in the periderm causes a milder global disruption phenotype
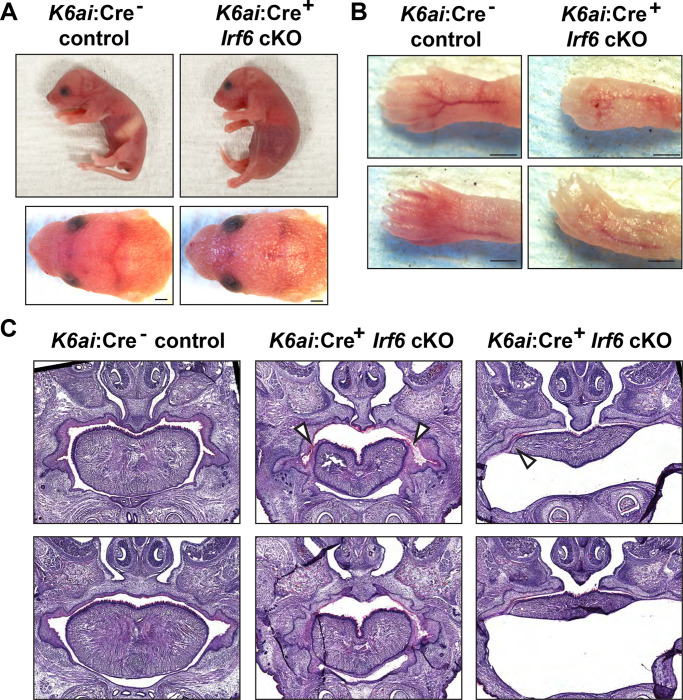
To examine the phenotypic effects of Irf6 ablation in the periderm, we utilized the Krt6ai-Cre driver line where the keratin 6 promoter drives Cre expression predominantly in the oral periderm after E14.5 (Saroya et al., 2023). Krt6ai-Cre+/−;Irf6wt/fl males were bred to Irf6fl/fl females and it was noted that the Krt6ai-Cre+;Irf6fl/fl genotype was not found at 3 weeks of age. As Irf6 global null mice die shortly after birth, we observed neonates at P0 and found that a few lacked a milk spot and appeared to be failing to thrive. Pups were collected and genotyping determined these unhealthy pups to be Krt6ai-Cre+;Irf6fl/fl whereas healthy pups were negative for Cre or were Irf6wt/fl. Closer examination of P0 neonates revealed shiny skin as has been previously noted for KO (Ingraham et al., 2006). Krt6ai-Cre+;Irf6fl/fl pups also exhibited pterygium of the fore and hind limbs consistent with a milder form of the cocooning observed in the global Irf6 null mouse (Ingraham et al., 2006) (Fig. 6A,B). Krt6ai:Cre+;Irf6fl/fl neonates exhibited simple syndactyly digits of the fore and hind limbs (Fig. 6B).
Fig. 6.
Periderm-specific ablation of Irf6 results in a comparable but mild form of the global Irf6 KO phenotype. Krt6ai-Cre+;Irf6fl/fl and littermate control neonates were collected at P1. A. Lateral and caudal representation of neonates comparing control Krt6ai-Cre−;Irf6fl/fl with Krt6ai-Cre+;Irf6fl/fl cKO. B. Krt6ai-Cre−;Irf6fl/fl exhibit normal skin and digits; however Krt6ai-Cre+;Irf6fl/fl reveal abnormal skin and fused digits phenotype. Scale: 500 μM. C. Hematoxylin and Eosin staining of coronal sections through vomeronasal and primary palate of neonates. Krt6ai-Cre−;Irf6fl/fl mice show normal septum and palate. Krt6ai-Cre+;Irf6fl/fl mice reveal abnormal septum and adhesions of the tongue.
The lack of a milk spot in the Krt6ai-Cre+;Irf6fl/fl neonates suggested impaired feeding and possible palate defects and oral adhesions as occur with global Irf6 ablation. Histological examination revealed that Krt6ai-Cre+;Irf6fl/fl mice present with lateral adhesions of the tongue to the oral cavity and a cleft of the secondary palate of variable penetrance (Fig. 6C). In some Krt6ai-Cre+;Irf6fl/fl individuals we found sublingual fluid accumulation that we presume to be caused by the oral adhesions. No differences were observed in the lip or primary palate.
To compare Krt6ai periderm-specific Irf6 ablation findings to pan-epithelial ablation, we utilized the Crect driver line. The Crect mouse has been previously utilized to conditionally ablate gene expression in the ectoderm, including the oral and cranial epithelium (Reid et al., 2011; Schock et al., 2017). Crect+;Irf6fl/fl embryos were examined at approximately E17 and were found to recapitulate the Irf6 knockout phenotype with abnormal skin, foreshortened limbs, and deficient development of the maxilla and mandible (Fig. S1). Histology of these mice showed adhesion of the tongue to the palate, similar to Irf6 global null mice (Fig. S1). This finding suggests that Crect expression largely overlaps with the expression of endogenous Irf6 gene expression, leading to complete Irf6 ablation in the Crect+;Irf6fl/fl cKO mouse.
Discussion
Mutations in IRF6 underlie VWS and PPS, which are characterized by varying degrees of cleft lip, cleft palate, lip pits, skin folds, syndactyly, and oral adhesions (REF). Irf6 null and the Irf6R84C mutant mouse models recapitulate aspects of these syndromes with severe oral adhesions, surface epithelium adhesions, and dysfunctional keratinocytes which cause neonatal lethality (Ingraham et al., 2006; Kondo et al., 2002). IRF6 is also associated with non-syndromic cleft lip and palate (Leslie et al., 2016), and yet the severe adhesions of the tongue within the oral cavity in the Irf6 null and Irf6R84C mutant mouse models complicate a direct comparison to the human condition. This study generated a new Irf6 conditional knockout mouse model and demonstrated reliable recombination of the conditional allele when tested with various Cre driver lines. This new conditional Irf6 allele facilitated the investigation of tissue-specific roles of Irf6.
IRF6, TFAP2A, and GRHL3 share a genetic regulatory pathway and ablation of each of these genes in mice causes similar cleft, skin, and limb defects (Ingraham et al., 2006; Kousa et al., 2019; Richardson et al., 2006; Schorle et al., 1996; Siewert et al., 2023; Smith et al., 2017; Ting et al., 2003; Zhang et al., 1996). As such, it is intriguing that Tfap2a and Grhl3 are associated with neural tube defects, whereas defects are not observed in the Irf6 ablated mice (Schorle et al., 1996; Ting et al., 2003; Zhang et al., 1996). To investigate this phenomenon Kousa et al., developed an Irf6 loss-of-function and gain-of-function allelic series in mice and found rostral neural tube defects associated with Irf6 overexpression and caudal defects associated with Irf6 loss of function (Kousa et al., 2019). We hypothesized that the severe epithelial adhesions resulting from periderm dysfunction in the Irf6 null mouse may mask neural tube defects and we therefore generated a conditional KO where Irf6 would be ablated in Wnt1 expressing neuroectoderm and neural crest cells, including those in the neural folds. We found a rostromedial defect in these mice of varying severity that affected the skin and calvarial bone. Further, we found changes to neural fold morphology and Wnt1 expression patterns in these embryos. Together, these data corroborate a role for Irf6 in the patterning and morphogenesis of the rostral neural tube in mice. Differences in phenotype and severity between our results and Kousa et al. may be attributed to spatial and temporal differences in the respective overexpression and knockout drivers that were utilized (Krt14 versus Wnt1). Further, additional neural tube phenotypes may become apparent in the Wnt1-Cre Irf6 cKO upon combinatorial genetic disruption of Tfap2a or Grhl3.
Irf6 is widely expressed in the pan-epithelium and its specific role in various epithelial populations (i.e. basal epithelium versus periderm) and those contributions to the mutant phenotype have had limited direct investigation. Kousa et al. previously investigated the role of Irf6 in the basal epithelium by utilizing the Krt14 promoter to express Irf6 in the basal epithelium on an Irf6 global null background. It was found that Irf6 expression in the basal epithelium partially rescued some aspects of the Irf6 null phenotype, namely the skin adhesions of the axial and appendicular skeleton but did not rescue the cleft palate (Kousa et al., 2017). Utilizing our Irf6 floxed mouse and the Krt6ai-Cre driver, we found that ablation of Irf6 in the periderm largely phenocopied the Krt14:Irf6tg rescue. Limb defects were similar in that the limbs were not adhered to the body yet syndactyly of the digits were observed. Whereas Kousa et al. reported oral adhesions slightly less severe than the global KO and cleft palate, the periderm-specific Irf6 KO mice had relatively mild oral adhesion and cleft of the palate was incompletely penetrant. Therefore, our data coincide with previous findings, and differences in phenotype and severity are likely due to differences in cell specificity and timing of expression.
Irf6 has a key role in the regulation of epithelial proliferation and differentiation (Bailey et al., 2008; Biggs et al., 2012; Girousi et al., 2021; Oberbeck et al., 2019). As such, IRF6 is implicated in epidermal wound healing and children with VWS have an increased risk of wound complications following surgical repair of orofacial clefts (Hixon et al., 2017; Jones et al., 2010; Rhea et al., 2020). Further, loss of Irf6 expression is associated with epidermal malignancy (Botti et al., 2011; Darido et al., 2016; Parisi et al., 2022; Yan et al., 2023). Investigation into these roles of Irf6 have, until now, depended on human patient-derived cells, genetically manipulated cell lines, and gene association studies. The availability of this Irf6 conditional mouse allele will allow post-natal ablation of Irf6 and facilitate mechanistic studies of epithelial biology in a mouse model.
This study successfully generated and validated a conditional Irf6 mouse allele. This mouse model will serve as an invaluable tool for advancing our comprehension of Irf6’s multifaceted functions and for developing targeted interventions for conditions like orofacial clefts, wound healing complications, and various cancers.
Supplementary Material
Acknowledgments
CRISPR design consultation, zygote microinjection, and embryo implantation were performed by The Genome Modification Facility and Harvard University.
MicroCT scanning was performed by the Center for Musculoskeletal Research Imaging and Biomechanical Testing Core (NIH P30 AR070542).
Funding sources
This work was supported by R01DE027983 to ECL, research support from Children’s Hospital of Philadelphia, and research grants from the Shriners Hospitals for Children.
References
- Bailey C.M., Abbott D.E., Margaryan N.V., Khalkhali-Ellis Z., and Hendrix M.J. (2008). Interferon regulatory factor 6 promotes cell cycle arrest and is regulated by the proteasome in a cell cycle-dependent manner. Molecular and cellular biology 28, 2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertol J.W., Johnston S., Ahmed R., Xie V.K., Hubka K.M., Cruz L., Nitschke L., Stetsiv M., Goering J.P., Nistor P., et al. (2022). TWIST1 interacts with beta/delta-catenins during neural tube development and regulates fate transition in cranial neural crest cells. Development 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs L.C., Rhea L., Schutte B.C., and Dunnwald M. (2012). Interferon regulatory factor 6 is necessary, but not sufficient, for keratinocyte differentiation. The Journal of investigative dermatology 132, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti E., Spallone G., Moretti F., Marinari B., Pinetti V., Galanti S., De Meo P.D., De Nicola F., Ganci F., Castrignano T., et al. (2011). Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proceedings of the National Academy of Sciences of the United States of America 108, 13710–13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S.H., Macias Trevino C., Li E.B., Kawasaki K., Myers N., Hallett S.A., Alhazmi N., Cotney J., Carstens R.P., and Liao E.C. (2020). An Irf6-Esrp1/2 regulatory axis controls midface morphogenesis in vertebrates. Development 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E.Y., Tamasas B., Fong H., Foster B.L., LaCourse M.R., Tran A.B., Martin J.F., Schutte B.C., Somerman M.J., and Cox T.C. (2016). Full Spectrum of Postnatal Tooth Phenotypes in a Novel Irf6 Cleft Lip Model. Journal of dental research 95, 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darido C., Georgy S.R., and Jane S.M. (2016). The role of barrier genes in epidermal malignancy. Oncogene 35, 5705–5712. [DOI] [PubMed] [Google Scholar]
- de la Garza G., Schleiffarth J.R., Dunnwald M., Mankad A., Weirather J.L., Bonde G., Butcher S., Mansour T.A., Kousa Y.A., Fukazawa C.F., et al. (2013). Interferon regulatory factor 6 promotes differentiation of the periderm by activating expression of Grainyhead-like 3. The Journal of investigative dermatology 133, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty M., Kamel G., Grimaldi M., Gfrerer L., Shubinets V., Ethier R., Hickey G., Cornell R.A., and Liao E.C. (2013). Distinct requirements for wnt9a and irf6 in extension and integration mechanisms during zebrafish palate morphogenesis. Development 140, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri W.D., Metwalli K., Naji A., Bakhiet S., Quispe-Salcedo A., Nitschke L., Kousa Y.A., and Schutte B.C. (2017). Intercellular Genetic Interaction Between Irf6 and Twist1 during Craniofacial Development. Scientific reports 7, 7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri W.D., Rhea L., Du T., Sweezer E., Morrison H., Fitzpatrick D., Yang B., Dunnwald M., and Schutte B.C. (2012). MCS9.7 enhancer activity is highly, but not completely, associated with expression of Irf6 and p63. Developmental dynamics : an official publication of the American Association of Anatomists 241, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti E., Li B., Zewdu R., Wells V., Hebert J.M., Karner C., Anderson M.J., Williams T., Dixon J., Dixon M.J., et al. (2011). A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Developmental cell 21, 627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.H., Jacobson K.A., Rose J., and Zeller R. (2008). Hematoxylin and eosin staining of tissue and cell sections. CSH protocols 2008, pdb prot4986. [DOI] [PubMed] [Google Scholar]
- Girousi E., Muerner L., Parisi L., Rihs S., von Gunten S., Katsaros C., and Degen M. (2021). Lack of IRF6 Disrupts Human Epithelial Homeostasis by Altering Colony Morphology, Migration Pattern, and Differentiation Potential of Keratinocytes. Frontiers in cell and developmental biology 9, 718066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudy S., Angel P., Jacobs B., Hill C., Mainini V., Smith A.L., Kousa Y.A., Caprioli R., Prince L.S., Baldwin S., et al. (2013). Cell-autonomous and non-cell-autonomous roles for IRF6 during development of the tongue. PloS one 8, e56270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixon K., Rhea L., Standley J., Canady F.J., Canady J.W., and Dunnwald M. (2017). Interferon Regulatory Factor 6 Controls Proliferation of Keratinocytes From Children With Van der Woude Syndrome. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association 54, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.V., Iwata J., Ho H.A., Grimes W.C., Park S., Sanchez-Lara P.A., and Chai Y. (2015). Integration of comprehensive 3D microCT and signaling analysis reveals differential regulatory mechanisms of craniofacial bone development. Developmental biology 400, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham C.R., Kinoshita A., Kondo S., Yang B., Sajan S., Trout K.J., Malik M.I., Dunnwald M., Goudy S.L., Lovett M., et al. (2006). Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nature genetics 38, 1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J., Suzuki A., Pelikan R.C., Ho T.V., Sanchez-Lara P.A., Urata M., Dixon M.J., and Chai Y. (2013). Smad4-Irf6 genetic interaction and TGFbeta-mediated IRF6 signaling cascade are crucial for palatal fusion in mice. Development 140, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.L., Canady J.W., Brookes J.T., Wehby G.L., L’Heureux J., Schutte B.C., Murray J.C., and Dunnwald M. (2010). Wound complications after cleft repair in children with Van der Woude syndrome. The Journal of craniofacial surgery 21, 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A.S., Schutte B.C., Jiang R., and Dixon M.J. (2006). Developmental expression analysis of the mouse and chick orthologues of IRF6: the gene mutated in Van der Woude syndrome. Developmental dynamics : an official publication of the American Association of Anatomists 235, 1441–1447. [DOI] [PubMed] [Google Scholar]
- Kondo S., Schutte B.C., Richardson R.J., Bjork B.C., Knight A.S., Watanabe Y., Howard E., de Lima R.L., Daack-Hirsch S., Sander A., et al. (2002). Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nature genetics 32, 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousa Y.A., Roushangar R., Patel N., Walter A., Marangoni P., Krumlauf R., Klein O.D., and Schutte B.C. (2017). IRF6 and SPRY4 Signaling Interact in Periderm Development. Journal of dental research 96, 1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousa Y.A., Zhu H., Fakhouri W.D., Lei Y., Kinoshita A., Roushangar R.R., Patel N.K., Agopian A.J., Yang W., Leslie E.J., et al. (2019). The TFAP2A-IRF6-GRHL3 genetic pathway is conserved in neurulation. Human molecular genetics 28, 1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie E.J., Koboldt D.C., Kang C.J., Ma L., Hecht J.T., Wehby G.L., Christensen K., Czeizel A.E., Deleyiannis F.W., Fulton R.S., et al. (2016). IRF6 mutation screening in non-syndromic orofacial clefting: analysis of 1521 families. Clinical genetics 90, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie E.J., Mancuso J.L., Schutte B.C., Cooper M.E., Durda K.M., L’Heureux J., Zucchero T.M., Marazita M.L., and Murray J.C. (2013). Search for genetic modifiers of IRF6 and genotype-phenotype correlations in Van der Woude and popliteal pterygium syndromes. American journal of medical genetics Part A 161A, 2535–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A.E., Vasudevan H.N., O’Neill A.K., Soriano P., and Bush J.O. (2013). The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Developmental biology 379, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E.B., Truong D., Hallett S.A., Mukherjee K., Schutte B.C., and Liao E.C. (2017). Rapid functional analysis of computationally complex rare human IRF6 gene variants using a novel zebrafish model. PLoS genetics 13, e1007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Ahlberg P.E., Kessaris N., Iannarelli P., Dennehy U., Richardson W.D., McMahon A.P., and Koentges G. (2005). Neural crest origins of the neck and shoulder. Nature 436, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H., Quadros R.M., Gurumurthy C.B., and Ohtsuka M. (2018). Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nature protocols 13, 195–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbeck N., Pham V.C., Webster J.D., Reja R., Huang C.S., Zhang Y., Roose-Girma M., Warming S., Li Q., Birnberg A., et al. (2019). The RIPK4-IRF6 signalling axis safeguards epidermal differentiation and barrier function. Nature 574, 249–253. [DOI] [PubMed] [Google Scholar]
- Parisi L., Mockenhaupt C., Rihs S., Mansour F., Katsaros C., and Degen M. (2022). Consistent downregulation of the cleft lip/palate-associated genes IRF6 and GRHL3 in carcinomas. Frontiers in oncology 12, 1023072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.W., McIntosh I., Hetmanski J.B., Jabs E.W., Vander Kolk C.A., Wu-Chou Y.H., Chen P.K., Chong S.S., Yeow V., Jee S.H., et al. (2007). Association between IRF6 and nonsyndromic cleft lip with or without cleft palate in four populations. Genetics in medicine : official journal of the American College of Medical Genetics 9, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimov F., Marazita M.L., Visel A., Cooper M.E., Hitchler M.J., Rubini M., Domann F.E., Govil M., Christensen K., Bille C., et al. (2008). Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nature genetics 40, 1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B.S., Yang H., Melvin V.S., Taketo M.M., and Williams T. (2011). Ectodermal Wnt/beta-catenin signaling shapes the mouse face. Developmental biology 349, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo G., Nguyen B.C., Dziunycz P., Ristorcelli E., Ryan R.J., Ozuysal O.Y., Di Piazza M., Radtke F., Dixon M.J., Hofbauer G.F., et al. (2011). IRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytes. The EMBO journal 30, 4571–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea L., Canady F.J., Le M., Reeb T., Canady J.W., Kacmarynski D.S.F., Avvari R., Biggs L.C., and Dunnwald M. (2020). Interferon regulatory factor 6 is required for proper wound healing in vivo. Developmental dynamics : an official publication of the American Association of Anatomists 249, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R.J., Dixon J., Jiang R., and Dixon M.J. (2009). Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Human molecular genetics 18, 2632–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R.J., Dixon J., Malhotra S., Hardman M.J., Knowles L., Boot-Handford R.P., Shore P., Whitmarsh A., and Dixon M.J. (2006). Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nature genetics 38, 1329–1334. [DOI] [PubMed] [Google Scholar]
- Richardson R.J., Hammond N.L., Coulombe P.A., Saloranta C., Nousiainen H.O., Salonen R., Berry A., Hanley N., Headon D., Karikoski R., et al. (2014). Periderm prevents pathological epithelial adhesions during embryogenesis. The Journal of clinical investigation 124, 3891–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel J.L., d’Alencon C., O’Brien E.K., Van Otterloo E., Lutz K., Cuykendall T.N., Schutte B.C., Houston D.W., and Cornell R.A. (2009). Maternal Interferon Regulatory Factor 6 is required for the differentiation of primary superficial epithelia in Danio and Xenopus embryos. Developmental biology 325, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroya G., Hu J., Hu M., Panaretos C., Mann J., Kim S., Bush J.O., and Kaartinen V. (2023). Periderm Fate during Palatogenesis: TGF-beta and Periderm Dedifferentiation. Journal of dental research 102, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock E.N., Struve J.N., Chang C.F., Williams T.J., Snedeker J., Attia A.C., Stottmann R.W., and Brugmann S.A. (2017). A tissue-specific role for intraflagellar transport genes during craniofacial development. PloS one 12, e0174206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorle H., Meier P., Buchert M., Jaenisch R., and Mitchell P.J. (1996). Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 381, 235–238. [DOI] [PubMed] [Google Scholar]
- Siewert A., Reiz B., Krug C., Heggemann J., Mangold E., Dickten H., and Ludwig K.U. (2023). Analysis of candidate genes for cleft lip +/− cleft palate using murine single-cell expression data. Frontiers in cell and developmental biology 11, 1091666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.L., Kousa Y.A., Kinoshita A., Fodor K., Yang B., and Schutte B.C. (2017). Generation and characterization of a conditional allele of Interferon Regulatory Factor 6. Genesis 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Mendoza F., Tan E., Bertol J.W., Gaggar A.S., Jun G., Biguetti C., and Fakhouri W.D. (2019). A cleft lip and palate gene, Irf6, is involved in osteoblast differentiation of craniofacial bone. Developmental dynamics : an official publication of the American Association of Anatomists 248, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting S.B., Wilanowski T., Auden A., Hall M., Voss A.K., Thomas T., Parekh V., Cunningham J.M., and Jane S.M. (2003). Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nat Med 9, 1513–1519. [DOI] [PubMed] [Google Scholar]
- Xu X., Han J., Ito Y., Bringas P. Jr., Urata M.M., and Chai Y. (2006). Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Developmental biology 297, 238–248. [DOI] [PubMed] [Google Scholar]
- Yan Y., Gauthier M.A., Malik A., Fotiadou I., Ostrovski M., Dervovic D., Ghadban L., Tsai R., Gish G., Loganathan S.K., et al. (2023). The NOTCH-RIPK4-IRF6-ELOVL4 Axis Suppresses Squamous Cell Carcinoma. Cancers (Basel) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Hagopian-Donaldson S., Serbedzija G., Elsemore J., Plehn-Dujowich D., McMahon A.P., Flavell R.A., and Williams T. (1996). Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381, 238–241. [DOI] [PubMed] [Google Scholar]
- Zucchero T.M., Cooper M.E., Maher B.S., Daack-Hirsch S., Nepomuceno B., Ribeiro L., Caprau D., Christensen K., Suzuki Y., Machida J., et al. (2004). Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. The New England journal of medicine 351, 769–780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.