Abstract
Women are born with all of their oocytes. The oocyte proteome must be maintained with minimal damage throughout the woman’s reproductive life, and hence for decades. Here we report that oocyte and ovarian proteostasis involves extreme protein longevity. Mouse ovaries had more extremely long-lived proteins than other tissues, including brain. These long-lived proteins had diverse functions, including in mitochondria, the cytoskeleton, chromatin and proteostasis. The stable proteins resided not only in oocytes but also in long-lived ovarian somatic cells. Our data suggest that mammals increase protein longevity and enhance proteostasis by chaperones and cellular antioxidants to maintain the female germline for long periods. Indeed, protein aggregation in oocytes did not increase with age and proteasome activity did not decay. However, increasing protein longevity cannot fully block female germline senescence. Large-scale proteome profiling of ~8,890 proteins revealed a decline in many long-lived proteins of the proteostasis network in the aging ovary, accompanied by massive proteome remodeling, which eventually leads to female fertility decline.
Subject terms: Ageing, Senescence, Systems analysis
Harasimov, Gorry, Welp, Penir, Horokhovskyi et al. analyse proteostasis in mammalian oocytes and ovaries: the maintenance of oocytes involves exceptional protein longevity, and many of the extremely long-lived proteins decline as the ovary ages.
Main
The female ovary is critical for reproduction. It stores the primordial follicles, which contain the oocytes and their associated somatic cells1,2. The primordial follicles are generated in the female fetus and do not appear to be replenished after birth. Females are therefore thought to be born with a finite pool of oocytes, called the ovarian reserve3,4.
The proteome of oocytes must be maintained in a healthy state throughout a woman’s reproductive life to ensure the success of the next generation. Whether germ cells have adapted their proteostasis to maintain and propagate a healthy proteome is unknown5. The identification of such adaptations could reveal the principles involved in resetting the aging clock and inform new therapeutic strategies to delay age-related diseases.
In this study, we analysed proteostasis in mammalian oocytes and ovaries by combining quantitative mass spectrometry (MS), pulse–chase labelling, single-cell RNA-seq and nanoscale secondary ion MS (NanoSIMS). We found that the maintenance of oocytes in the mammalian ovary involves exceptional protein longevity. Many of the extremely long-lived proteins decline as the ovary ages. We propose that extreme protein longevity is required to propagate a healthy germline across generations, yet protein depletion may promote the rapid age-related decline in female fertility.
Results
Oocytes contain many extremely long-lived proteins
To study protein stability in oocytes, we performed a pulse–chase experiment in which female mice were fully labelled with 13C6-lysine (13C6-Lys)6 during their development in utero, transferred to unlabelled (12C6-Lys) foster mothers after birth and fed unlabelled chow after weaning (Fig. 1a, Extended Data Fig. 1a–e). We collected 4,948 fully grown oocytes from 92 eight-week old mice and processed them for bottom-up MS (Fig. 1a, Extended Data Fig. 2a).
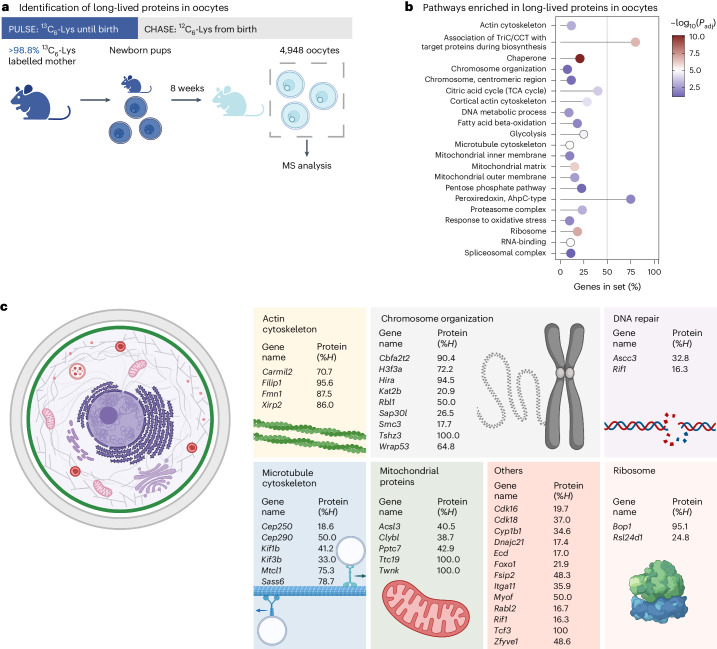
Fig. 1. Oocytes contain a large number of very long-lived proteins.
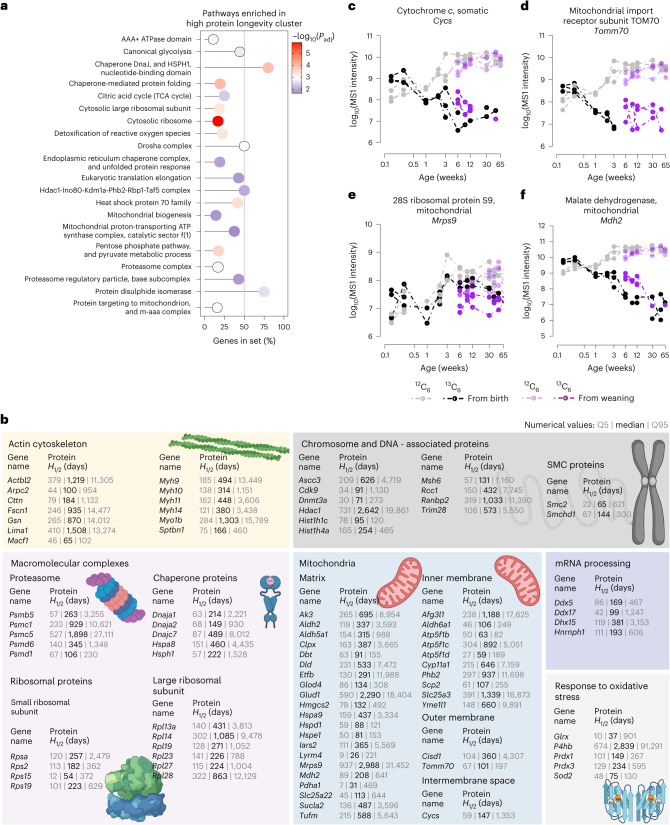
a, Fully labelled 13C6-Lys females were mated and fed 13C6-Lys chow until they gave birth (pulse). Upon birth, the pups were transferred to unlabelled foster mothers (chase) and continued to receive 12C6-Lys chow after weaning. In total, 4,948 oocytes were collected from 92 eight-week-old pubertal female progeny and processed for bottom-up MS. b, Selected pathways enriched with long-lived proteins in oocytes. All 13C6-Lys-labelled proteins detected after 8 weeks were subjected to over-representation analysis. Shown are the percentage of genes of the gene set detected as enriched with their respective adjusted P values for selected pathways. The P values are based on a hypergeometric test and have been adjusted using Benjamini–Hochberg multiple hypothesis testing. For the complete list of enriched pathways with their corresponding exact P values, see Supplementary Table 2. c, Selected biological processes and protein complexes with long-lived proteins. Shown are selected genes with corresponding protein %H values (inferred fraction of 13C6-Lys, mean value over biological replicates). For a complete list of proteins and their corresponding %H values, see Supplementary Table 1.
Extended Data Fig. 1. Generation of fully-13C6-Lys-labelled FVB/N female mice.
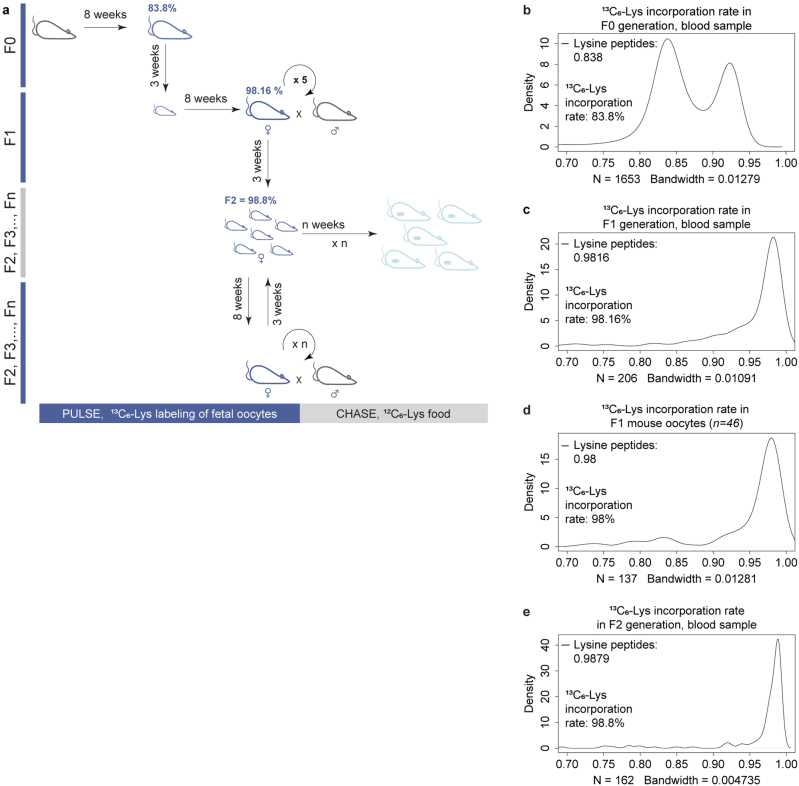
(a) Schematic overview of complete 13C6-Lys labelling of mice. Fully-13C6-Lys-labelled FVB/N female mice were created by successive matings and a diet consisting exclusively of 13C6-Lys feed. F0 females were fed with the 13C6-Lys feed for 8 weeks, after which they were mated with wild-type FVB/N males who were kept on a standard diet. The F0 mother was further fed with the 13C6-Lys feed and the F1 pups born from this mating were fed with the 13C6-Lys feed until weaning. Thereafter, only the F1 offspring was further fed with the 13C6-Lys feed. Upon reaching sexual maturity, the F1 13C6-Lys-fed females were mated with wild-type FVB/N males who were kept on a standard diet. The pups of the F2 generation were the first generation of mice used for experiments. Females of the F1 generation were continuously mated to produce experimental animals. Fully labelled 13C6-Lys breeders were periodically substituted by the pups from the fully labelled offspring, and therefore, the F2 labelling efficiency represents the minimal labelling efficiency, as all subsequent generations had a larger fraction of 13C6-Lys due to longer 13C6-Lys feeding time. The percentage of 13C6-Lys incorporation in blood samples in each generation is indicated in the top left corner of female mouse pictograms. (b-e) Histograms show 13C6-Lys incorporation rates in (b) F0 blood sample for 1,653 lysine-containing peptides, (c) in F1 blood sample for 206 lysine-containing peptides, (d) in F1 oocyte samples for 137 lysine-containing peptides, and (e) in F2 blood sample for 162 lysine-containing peptides. Median 13C6-Lys incorporation rates are indicated. Heavy isotope (13C6) incorporation rates were calculated from available lysine-containing peptides showing heavy (13C6) to light (12C6) lysine ratios. Source numerical data are available in source data.
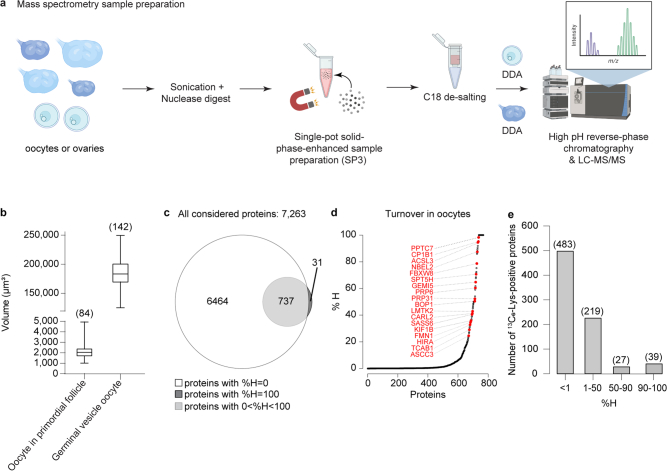
Extended Data Fig. 2. Sample preparation for MS DIA or DDA analysis and protein turnover in oocytes.
(a) Schematic representation of sample preparation for MS DIA or DDA analysis. (b) Box plot showing the volume (µm3) of oocytes in fixed primordial follicles and at germinal vesicle stage from live and fixed samples. Boxplots indicate median, 1st quartile, 3rd quartile, as well as minimum and maximum after outlier removal. Number of oocytes indicated in parenthesis. (c) Venn diagram of 12C6-Lys containing proteins and 13C6-Lys containing proteins detected in mouse oocytes. (d) Rank plot showing inferred remaining fraction of 13C6-Lys labelled proteins (%H) in oocytes for each quantified protein (individual proteins denoted as black circles). Selected examples are highlighted in red. (e) Bar plot showing number of proteins with %H of 0–1, 1–50, 50–90, or 90–100%. Source numerical data are available in source data.
Despite the 8-week chase period and dilution by oocyte growth, we detected many 13C6-Lys-positive proteins (768 out of 7,263 proteins), which must have been present since birth (Extended Data Fig. 2c,d and Supplementary Table 1). Notably, 39 of the proteins were still 90–100% positive for 13C6-Lys, 27 proteins between 50% and <90%, 219 proteins between 1 and <50%, and 483 proteins <1% (Extended Data Fig. 2e). These values represent raw 13C6-Lys over total Lys measurements without correction for oocyte growth. The 768 long-lived proteins were significantly enriched in diverse components (for example, mitochondria, ribosome, spliceosome, proteasome, chromatin, kinetochore and cytoskeleton) and functions (for example, metabolism, chaperones, DNA repair and antioxidants; Fig. 1b,c and Supplementary Table 2).
The 39 proteins that were still >90% positive for 13C6-Lys included many proteins with key cellular functions, such as TWINKLE, a mitochondrial DNA helicase that is essential for mitochondrial DNA replication7; TTC19, which is essential for the assembly and function of complex III of the mitochondrial respiratory chain8; BOP1, a protein that is essential for ribosome assembly9; and HIRA, a histone chaperone (Fig. 1c). We also detected cohesin-related proteins as long-lived, including the cohesin subunits SMC3 and SMC1A, as well as PDS5B (Fig. 1c and Supplementary Table 1), which regulates the association of the cohesin complex with chromatin10–14. The known long-lived cohesin subunit REC8 (refs. 15–17) was not detected by MS, likely because it is present at very low levels in oocytes.
Together, these data establish that oocytes contain many extremely long-lived proteins. Mitochondrial proteins and proteostasis-promoting proteins were prominently enriched in this group, and hence persist with little turnover in oocytes from birth onwards.
Extreme longevity of ovarian proteins
To examine protein longevity in the whole ovary, which contains the oocytes as well as somatic cells, such as granulosa, thecal and stromal cells, we performed two different pulse–chase experiments. First, we repeated our approach above, in which females were labelled with 13C6-Lys during development in utero, and collected 33 ovaries at 11 time points, from 24 hours to 65 weeks of age (Fig. 2a)18. Second, fully labelled 13C6-Lys pups were kept with fully labelled mothers until weaning and then fed 12C6-Lys chow. Ovaries were collected at six time points from 6 weeks to 65 weeks of age (Fig. 2b). The second approach labels all proteins synthesized both in utero and after birth until the end of the first wave of oocyte follicle growth2,4. This dual strategy enabled a comprehensive analysis of the ovary proteome, which changes with age.
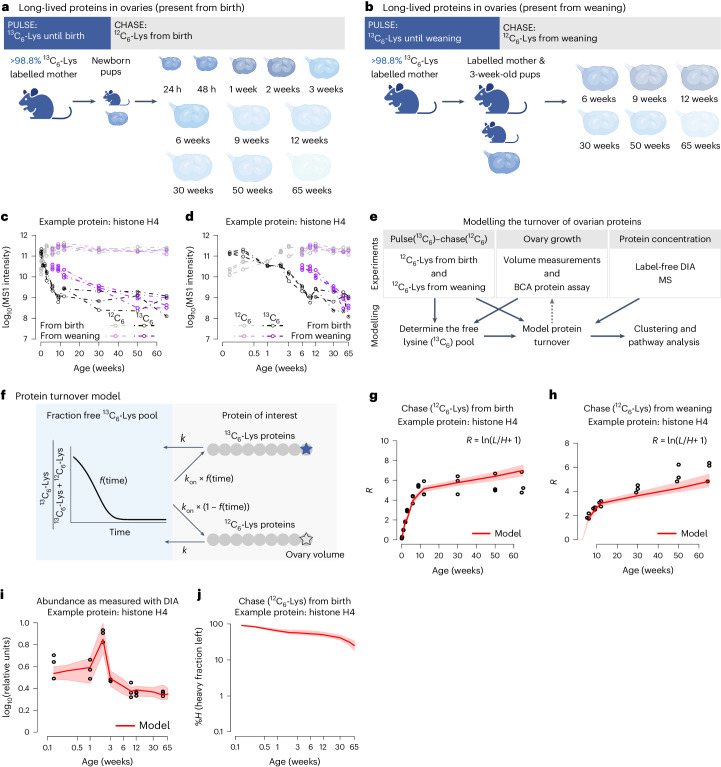
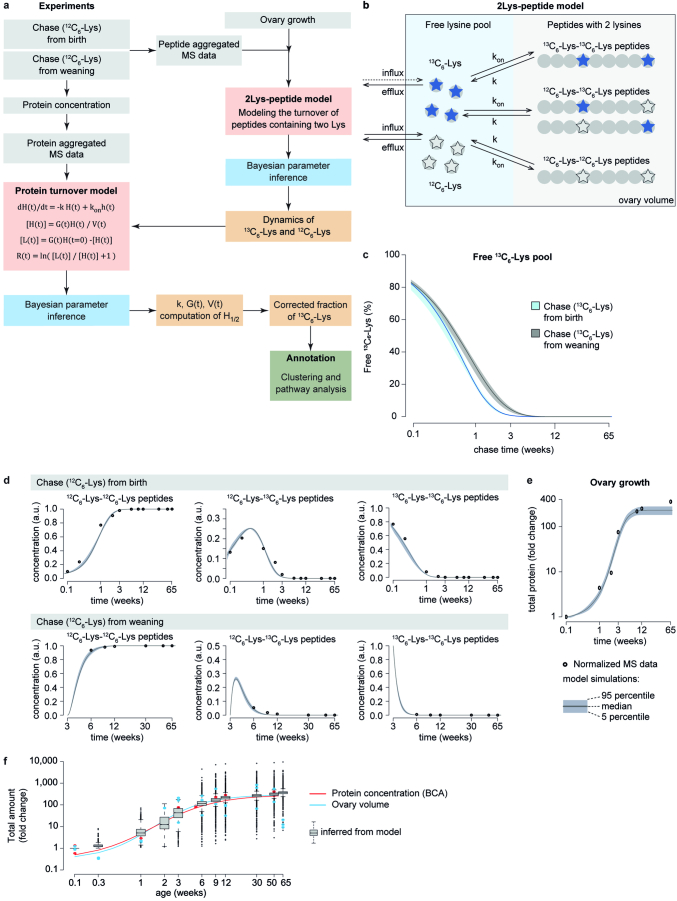
Fig. 2. Modelling protein turnover throughout ovarian development.
a, Fully 13C6-Lys labelled pregnant mice were fed with 13C6-Lys chow until they gave birth (pulse). The pups were subsequently raised on 12C6-Lys (chase). Ovaries were collected from the female progeny at eleven time points (three animals per time point) and processed for DDA MS. b, Progeny from fully 13C6-Lys labelled pregnant mice were fed with 13C6-Lys until weaning. After the weaning period (3 weeks after birth), progeny were fed 12C6-Lys chow. Ovaries were collected from female progeny at six time points (three animals per time point) and processed for DDA MS. c,d, Example data used for modelling protein turnover. Shown are MS1 intensities over time (linear c; log10, d) scale for 13C6-Lys (black and purple) and 12C6-Lys (grey and pink) labelled histone H4, for two pulse lengths (pulse until birth and until weaning). Circles indicate experimental data points for three biological replicates, dashed lines are only for visual aid. e, Schematic of the experimental design and mathematical modelling. Pulse–chase protein concentration data were used to inform the model and estimate protein turnover rates. Dilution factors due to ovary growth were estimated by the model and compared to experimental ovary growth measurements for validation (indicated by a grey dashed arrow). f, Graphical illustration of the employed protein turnover model to estimate H1/2 values in ovaries. g,j, Example (histone H4) of protein turnover model fitting. Experimental data are indicated as black dots, median and confidence ranges of model fit are indicated by the red line and shaded area, respectively. g,h, The ratio (R) between heavy (H) and light (L) labelled protein intensities in the chase (12C6-Lys) from birth (g) and from weaning (h) experiment datasets are shown over time. i, Normalized protein abundance derived from DIA-MS measurements is shown over time for histone H4. j, Proportion of heavy labelled histone H4 compared with all proteins as derived from the estimated protein turnover posterior parameter distribution.
All samples were analysed by MS using data-dependent acquisition (DDA; Fig. 2c,d). In addition, we isolated ovaries from unlabelled mice of eight different ages and analysed the relative abundances of the proteins throughout ovarian development using data-independent acquisition (DIA) MS (Supplementary Data 1).
The turnover rates were determined by mathematical modelling (Fig. 2e,f, Methods and Extended Data Fig. 3a). Briefly, for each protein, we calculated the 12C6-Lys (L) to 13C6-Lys (H) ratio as R = ln(L/H + 1) at the different time points (Fig. 2g,h). These experimentally determined ratios were then compared to ratios obtained from a protein turnover model (Fig. 2f). The protein turnover model describes the abundance of 13C6-Lys-labelled proteins over the chase period, taking into account protein degradation (with rate k) and protein synthesis (with rate kon) due to recycling of free (unincorporated) 13C6-Lys. The rate kon is defined as a function of the free 13C6-Lys pool, such that the higher the remaining free 13C6-Lys, the higher the rate of 13C6-Lys protein synthesis rate due to 13C6-Lys recycling, and, hence, the slower the decay of 13C6-Lys labelled protein.
Extended Data Fig. 3. Modelling protein turnover in the ovary.
(a) Overview of the protein turnover modelling approach described in supplemental materials. (b) Graphical illustration of the 2Lys-peptide model to determine the fraction of free 13C6-Lys during ovarian ageing. (c) Estimated fraction of free 13C6-Lys for the 2Lys-peptide model for the chase (12C6-Lys) from birth and from weaning experiments. Solid lines indicate median; shaded areas indicate 5% and 95% confidence ranges. (d-e) Experimental data and model fits of 2Lys-peptide model (d) and total ovary protein fold change (e) over chase time for the chase (12C6-Lys) from birth and from weaning experiments. Dots indicate experimental data; lines and shaded areas indicate median and confidence ranges of model fits. (f) Change of total protein amount in the ovary over mouse age. Total protein amount was determined from BCA measurements and compared to changes in the volume of the ovary. Boxplots indicate the estimated fold change in total protein amount. Boxplots indicate median, 1st quartile, 3rd quartile, as well as minimum and maximum after outlier removal over 3,078 modelled proteins. Even though the volume of the ovary was not considered in the protein turnover model fitting, the estimated fold changes are in good agreement with experimentally measured total protein amount fold changes. Source numerical data are available in source data.
We determined the free 13C6-Lys and 12C6-Lys pools throughout ovarian development by analysing the concentration of different species of uncleaved peptides containing two lysines19,20 (Extended Data Fig. 3b–e). 2Lys peptides can either be composed of two 13C6-Lys, two 12C6-Lys or a mixture, allowing for the inference of the free 13C6-Lys pool19,20 (Extended Data Fig. 3c–e). As additional parameters, ovarian growth and changes in individual protein concentrations (DIA-MS data) were included in the modelling to account for changes in ovarian protein composition (Extended Data Fig. 3a; Methods). The resulting modelled turnover rates reflect the average behaviour of all proteins in the ovary, and cannot discern whether protein turnover differs depending on interactions or cell types.
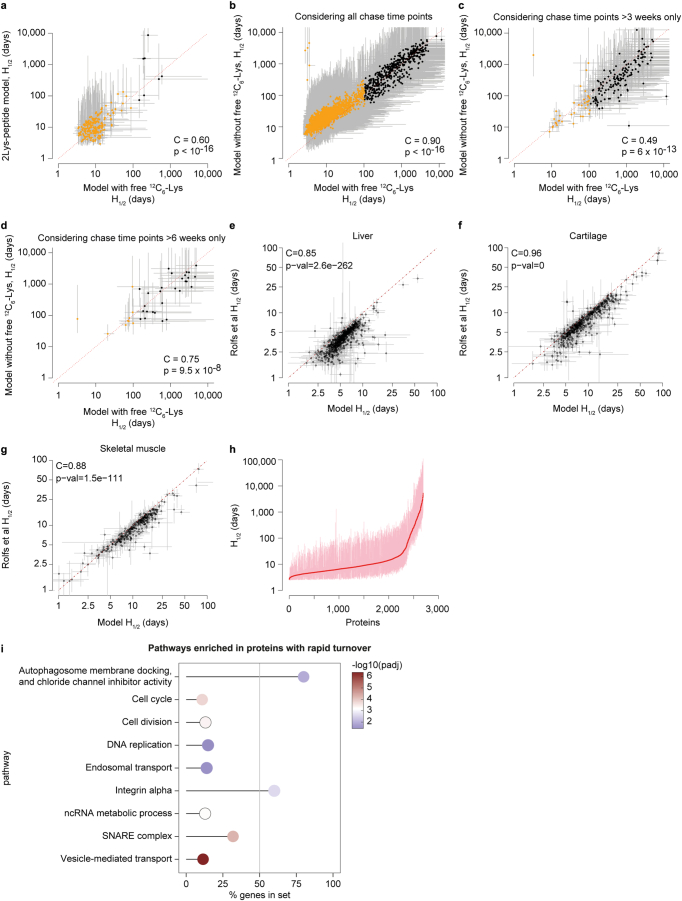
To challenge our approach, we modified our model in various ways and compared the resulting half-lives to the full modelling approach (Extended Data Fig. 4a–d). Specifically, we compared our protein-centric turnover model including re-incorporation of free 13C6-Lys into newly synthesized proteins (Fig. 2f) to (1) a peptide-centric 2Lys-peptide model (Extended Data Fig. 4a) and (2) a protein-centric model not including re-incorporation of free 13C6-Lys into newly synthesized proteins (Fig. 2f with kon = 0) using either all chase time points (Extended Data Fig. 4b), or only chase time points larger than 3 weeks or 6 weeks, respectively (Extended Data Fig. 4c,d). The 2Lys-peptide approach takes advantage of the fact that some very abundant proteins have enough 2Lys peptides resulting from missed tryptic cleavages during sample preparation, so that only peptides with two 13C6-Lys can be taken into consideration for modelling, which are highly unlikely to be generated by reincorporation of 13C6-Lys, and the free 13C6-Lys pool can then be neglected. The half life (H1/2) values calculated in this way were well consistent with our protein-centric turnover model. In addition, we also calculated the H1/2 values from late time points only (larger than 3 weeks or 6 weeks). Growth has substantially slowed down at this stage and the free 13C6-Lys pool is strongly depleted. In further support of our model, modelling of these late time points only (allowing for exclusion of the free 13C6-Lys pool) gave very similar results to our full protein-centric model using all collected data and time points (Extended Data Fig. 4c,d).
Extended Data Fig. 4. Comparison of the H1/2 values estimated from different models.
(a-d) Comparison of estimated H1/2 values resulting from the protein turnover model considering free 13C6-Lys pool with 2Lys-peptide based model (a) and the ‘classical’ protein turnover model not allowing reincorporation of 13C6-Lys into newly synthesized proteins considering all chase time points (b), or only chase time points larger than 3 weeks (c) or 6 weeks (d), respectively. Dots indicate medians, grey lines indicate confidence ranges. All proteins with estimated H1/2 values < 100 days are indicated as orange dots. (e-g) Comparison of previously published H1/2 values (Rolfs et al.20) and H1/2 values calculated for the same published datasets using the protein turnover model developed in this manuscript for ovaries. Shown are comparisons for liver (e), cartilage (f) and skeletal muscles (g). In (a-g) test for association between paired samples using Spearman correlation coefficient (C) was performed with p-values (p) estimated using algorithm AS 89. p < 10−16 indicates approximated p-values. (h) Rank plot showing median (red dots) and confidence ranges (pink lines) of estimated H1/2 values for all modelled proteins in ovaries. (i) Over-representation analysis of proteins with rapid turnover in ovaries. Shown are the percentage of genes detected in the gene set with their respective adjusted p-values for the most prominent pathways. The p-values are based on the hypergeometric test and have been adjusted for multiple hypothesis testing with Benjamini–Hochberg (BH) procedure. For the complete list of enriched pathways and their corresponding exact p-values, see Supplementary Table 4. Source numerical data are available in source data.
Finally, we employed our protein-centric model to pulse–chase data derived from mouse liver, cartilage and skeletal muscle published previously20 to estimate protein half-lives, and to compare them to the original published values (Extended Data Fig. 4e–g). For all three datasets, we obtained good agreement of inferred half-lives with correlation coefficients of 0.85, 0.96 and 0.88 for liver, cartilage and skeletal muscle, respectively, increasing the confidence in our modelling approach.
We summarized all the data in an atlas of 3,078 ovarian proteins (Supplementary Data 2), which shows the raw 12C6-Lys and 13C6-Lys intensities for each of the quantified proteins as determined by MS over time, as well as the modelled data (considering free 13C6-Lys, ovarian growth and changes in protein abundance), H1/2 values (the number of days it takes the 13C6-Lys protein fraction to decrease by half; summarized in Extended Data Fig. 4h) and the changes in the abundance of ovarian proteins throughout development (Fig. 2g–j and Supplementary Table 3).
A subset of 958 13C6-Lys proteins rapidly disappeared from the ovary during the chase (12C6-Lys) in both experiments (Supplementary Table 4). These proteins could not be modelled due to their extremely rapid degradation. This group was enriched in proteins involved in cell cycle regulation, cell division and DNA replication, among others, consistent with the short lifetime of proteins involved in these processes (Extended Data Fig. 4i).
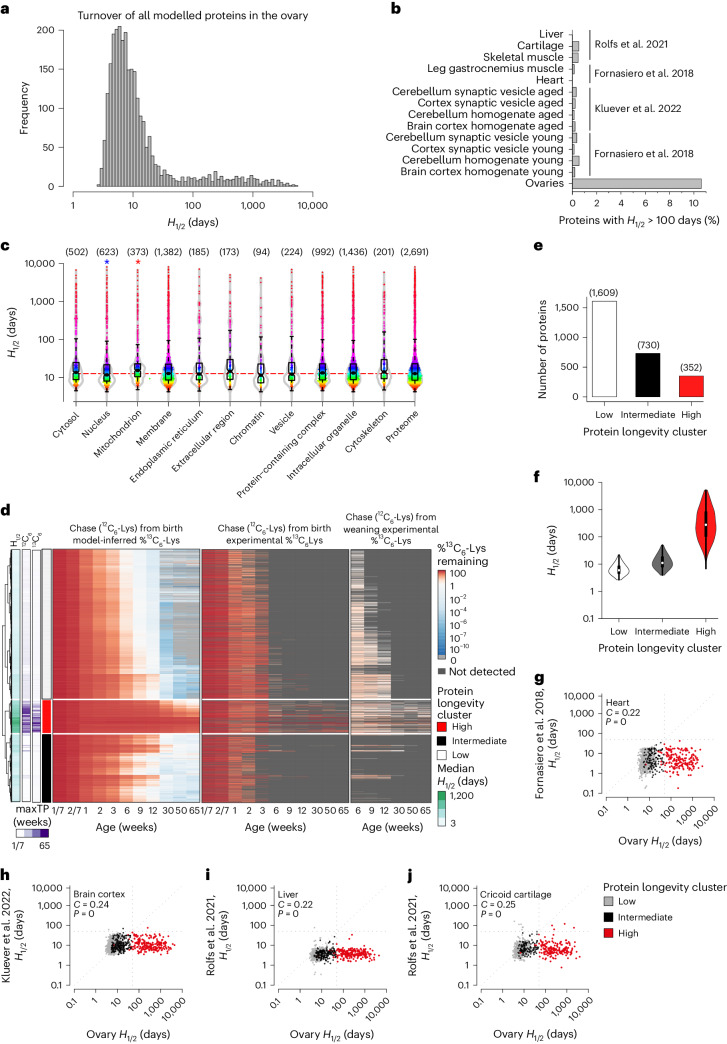
The distribution of H1/2 values in the ovary had a peak at around ~8–10 days, similar to protein turnover in other organs (Fig. 3a and Extended Data Fig. 5a)19–21. However, over 10% of the proteins in the ovary had H1/2 values above 100 days, whereas fewer than 1% of proteins in other organs, including brain, muscle and cartilage, had such high H1/2 values (Fig. 3b and Supplementary Data 3)19–21. Although these extremely long-lived proteins were not restricted to specific cellular locations, mitochondrial proteins had longer H1/2 values, on average, when compared to the total ovary proteome, whereas nuclear proteins had shorter H1/2 values (Fig. 3c). Many mitochondrial proteins (for example, cytochrome c, ATP synthase subunits, malate dehydrogenase and mitochondrial chaperone HSPA9) had H1/2 values that exceeded those reported in brain tissues (Supplementary Data 3).
Fig. 3. Mouse ovaries have a >10-fold higher fraction of extremely long-lived proteins than other post-mitotic tissues.
a, Distribution of the modelled H1/2 values in the ovary. b, Fraction of proteins with H1/2 > 100 days in the mouse ovary samples in this study and various mouse and rat tissues across three studies19–21. c, Distributions of estimated H1/2 values for proteins located in indicated subcellular compartments. Red and blue stars indicate significantly larger and smaller H1/2 distributions compared with the whole modelled proteome, respectively (two-sided Kolmogorov–Smirnov test; P values: nucleus 0.0093; mitochondrion 8.84 × 10–6). Number of proteins in each compartment indicated in parentheses. Boxplots indicate median, first quartile and third quartile, as well as minimum and maximum after outlier removal. d, Cluster analysis of inferred 13C6-Lys levels in the proteins of the aging ovaries. The dendrogram on the left corresponds to the clustering of inferred percentage 13C6-Lys. The leftmost bar shows the medians of inferred proteins half-lives, colouring on log10 scale. The second and third bars indicate the latest time point at which the 13C6-Lys pulse was detected in the data corresponding to chase from birth and weaning. The rightmost bar labels the three identified protein longevity clusters corresponding to proteins with high, intermediate and low amounts of inferred 13C6-Lys content. The leftmost heatmap shows the inferred percentage 13C6-Lys; time points from 6 weeks onwards were used for clustering. Central and rightmost heatmaps show experimental data of the chase 12C6-Lys from birth and weaning, respectively. e, Modelled percentage of 13C6-Lys labelled proteins compared to 12C6-Lys labelled proteins was used to derive three protein longevity clusters (low, intermediate and high inferred 13C6-Lys content). Bar plot showing number of proteins in the low, intermediate and high protein longevity clusters. f, Violin plot showing distributions of inferred half-lives of proteins in the low, intermediate and high protein longevity clusters. Boxplots indicate median, first quartile and third quartile, as well as minimum and maximum after outlier removal. Number of proteins in each cluster as indicated in e. g–i, Dot plots comparing the H1/2 values of different proteins in the ovary (x axis) with organs and tissues (y axis) measured in ref. 19 (g), ref. 21 (h) and ref. 20 (i, liver; j, cricoid cartilage). Test for association between paired samples using Spearman correlation coefficient (C) was performed with P values estimated using algorithm AS 89. Proteins are colour-coded according to the protein longevity cluster they belong to, as in d.
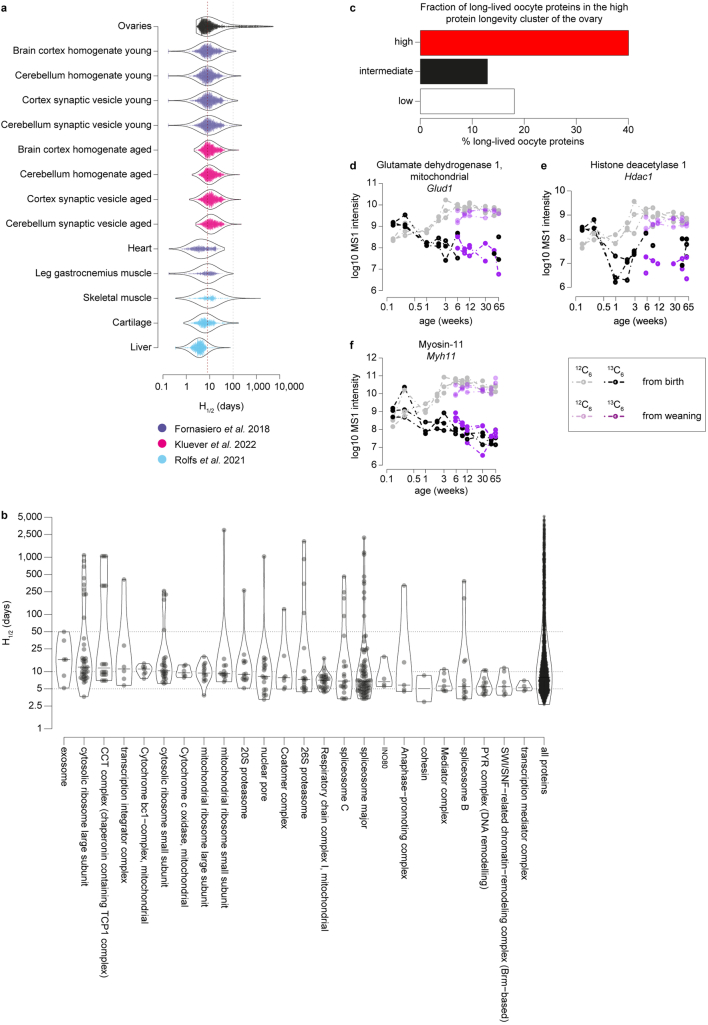
Extended Data Fig. 5. Comparison of the H1/2 values of the different proteins in the ovary with their corresponding H1/2 values in other organs, and examples of long-lived proteins in the ovary.
(a) Distribution of H1/2 determined for various mouse and rat tissues across three studies (Fornasiero et al.19; Kluever et al.21; Rolfs et al.20) compared to the estimated H1/2 distribution in mouse ovary samples in this study. Red line indicates the median H1/2 values in the ovary, while the grey line indicates a H1/2 value of 100. (b) Long-lived members of protein complexes. Shown are protein complexes with long-lived proteins as violin plots. Dots indicate individual proteins. Complexes are sorted by median complex H1/2 values. (c) Fraction of long-lived oocyte proteins in the high protein longevity cluster of the ovary. (d-f) Example MS1 intensities over time in log10 scale for 13C6-Lys- (black and purple) and 12C6-Lys- (grey and pink) labelled peptides for two pulse lengths (pulse with 13C6-Lys until birth and pulse with 13C6-Lys until weaning) for glutamate dehydrogenase 1, mitochondrial (d); histone deacetylase 1 (e); and Myosin-11 (f). Circles indicate experimental data points for three biological replicates, dashed lines are only for visual aid. Source numerical data are available in source data.
Using the CORUM database of protein complexes22, we assessed the distribution of the H1/2 values of a wider range of complexes in the ovary (Extended Data Fig. 5b). The cytoplasmic ribosome, proteasome and spliceosome contained many long-lived proteins with H1/2 values of more than 50 days. Other long-lived complexes included the CCT complex, which is involved in the folding of actin and tubulin, and the transcription integrator complex. Shorter-lived proteins were observed in the MCM complex, the SWI/SNF chromatin remodelling complex and the transcription mediator complex (Extended Data Fig. 5b).
We clustered the proteins according to their longevity (Fig. 3d), based on the modelled 13C6-Lys protein fractions from 6 weeks until 65 weeks of age. This resulted in three distinct protein longevity clusters, corresponding to proteins with high, intermediate and low relative 13C6-Lys levels (Fig. 3d,e and Supplementary Table 3). These clusters comprised 352 extremely long-lived proteins with a mean H1/2 value of 681.0 days, 730 long-lived proteins with a mean H1/2 value of 14.6 days and 1,609 proteins with a mean H1/2 value of 7.0 days (Fig. 3f). Proteins in the high and intermediate protein longevity clusters had significantly higher H1/2 values in the ovary than in other tissues (Fig. 3g–j)19–21.
The 352 extremely long-lived proteins in the ovary contained the long-lived proteins detected in oocytes (Extended Data Fig. 5c) and showed the same over-representation of mitochondria-related and proteostasis-related proteins as mouse oocytes (Fig. 4a,b, Supplementary Table 5 and Supplementary Data 3). Many of these long-lived proteins were 13C6-Lys-positive from birth until the last analysed time point at 65 weeks (Fig. 4c–f, Extended Data Fig. 5d–f and Supplementary Data 2), and have thus persisted in the mouse ovary for 15 months, almost the entire lifespan of FVB/N females.
Fig. 4. The ovary contains long-lived proteins that persist throughout the lifetime of a mouse.
a, Selected pathways enriched in the high protein longevity cluster. Shown are the proportions of genes of the selected gene set detected in the high protein longevity cluster with their respective adjusted P values for selected pathways. The P values are based on the hypergeometric test and have been adjusted with Benjamini–Hochberg multiple hypothesis testing. For the complete list of enriched pathways with their corresponding exact P values, see Supplementary Table 5. b, Selected biological processes and protein complexes with long-lived proteins in ovaries. Shown are gene names with corresponding protein H1/2 values (medians, 5% and 95% quantiles; designated as Q5 and Q95, respectively) in days. For the complete list of proteins and their corresponding H1/2 values, see Supplementary Table 3 and Supplementary Data 2. c–f, MS1 intensities over time in log10 scale for 13C6-Lys (black and purple) and 12C6-Lys (grey and pink) labelled cytochrome c, somatic (c); mitochondrial import receptor subunit TOMM70 (d); 28S ribosomal protein S9, mitochondrial (e); and malate dehydrogenase, mitochondrial (f) for two pulse lengths (pulse with 13C6-Lys until birth and pulse with 13C6-Lys until weaning). Circles indicate experimental data points for three biological replicates, dashed lines are only for visual aid.
To give some specific examples, the mitochondrial proteins cytochrome c (CYC; Fig. 4c), the mitochondrial import receptor subunit TOMM70 (Fig. 4d), the mitochondrial 28S ribosomal protein S9 (MRPS9; Fig. 4e), mitochondrial malate dehydrogenase (MDH2; Fig. 4f) and glutamate dehydrogenase 1 (GLUD1; Extended Data Fig. 5d), as well as the heat shock proteins and chaperones DNAJC7, DNAJA1, DNAJA2, HSPH1, HSPA2, HSPA8, HSPA9 and HYOU1, were extremely long-lived (Fig. 4b and Supplementary Data 3). Notably, DNAJA1 protects cells against apoptosis by negatively regulating the translocation of BAX from the cytosol to mitochondria23,24 (Fig. 4b). Another extremely long-lived protein was SMCHD1, which is a non-canonical member of the structural maintenance of chromosomes (SMC) protein family that plays a key role in epigenetic silencing, and is required for X-inactivation, by mediating XIST spreading (Fig. 4b)25,26. Other proteins involved in epigenetic regulation (including DNMT3A and HDAC1; Fig. 4b, Extended Data Fig. 5e and Supplementary Data 3), as well as proteins involved in nuclear import and export, including RAN, RANBP2, RCC1 and CSE1L (Fig. 4b) and myosins (MYH9, MYH10, MYH11, MYH14 and MYO1B; Extended Data Fig. 5f) were in the high longevity cluster. The extremely long-lived proteins in the ovary thus have essential functions in mitochondria, proteostasis, chromatin maintenance and the cytoskeleton.
Discovery of long-lived somatic cell types in the ovary
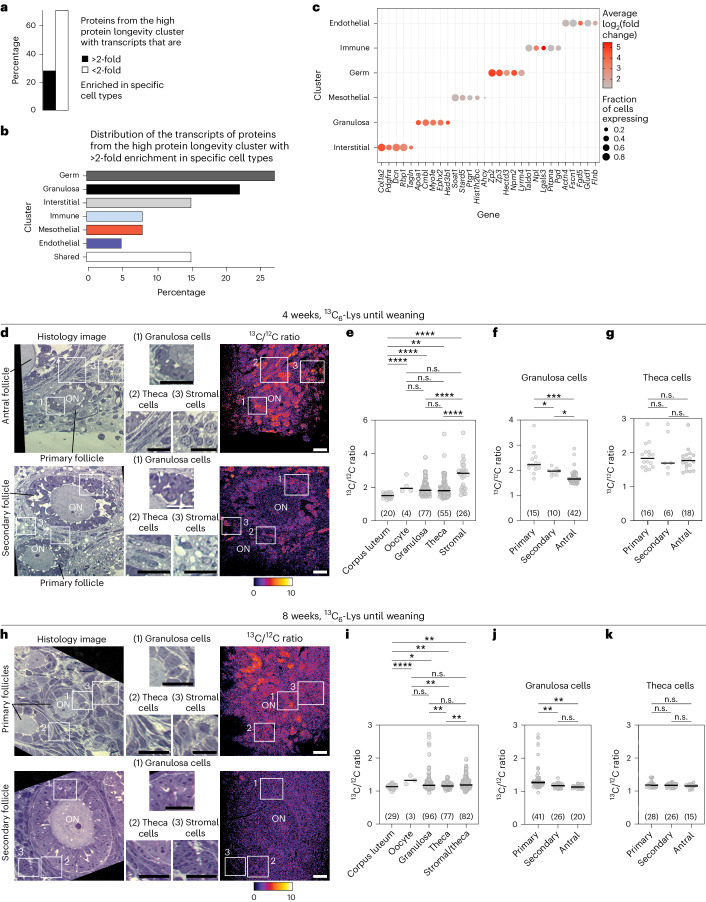
We used single-cell RNA-seq to identify the ovarian cell types that express long-lived proteins (Supplementary Data 4). We examined ovaries from newborn mice (postnatal day 2), as this age refers to the end of the first pulse period, and to the time when the ovarian reserve has been established. Interestingly, some proteins from the high longevity cluster mapped to distinct cell types based on a greater than twofold enrichment of their transcripts (Fig. 5a,b). These cell types included not only oocytes (germ cells), which are generally assumed to reside in the ovary from birth onwards3, but also subsets of somatic cells (Fig. 5b,c). These data suggest that the ovary contains long-lived somatic cells.
Fig. 5. Subsets of granulosa, stromal and theca cells are long-lived in the ovary.
a, Proportion of the proteins from the high protein longevity cluster with greater or less than twofold enrichment of corresponding transcripts in specific cell types in the ovaries of postnatal day 2 mice. b, Distribution of the transcripts of the proteins from the high protein longevity cluster with greater than twofold enrichment in specific cell types of the ovaries of postnatal day 2 mice. c, Dot plot showing the expression patterns of the transcripts of the proteins from the high protein longevity cluster. Size of the dot represents the proportion of the cells expressing the gene, colour denotes log2(fold change) in the one versus all cell types differential gene expression test. d–k, NanoSIMS imaging of ovaries from 4-week-old (d–g) and 8-week-old (h–k) mice that were pulsed with 13C6-Lys until weaning (3 weeks after birth), followed by a chase period with 12C6-Lys. d,h, Histological sections of ovarian tissue (first column on the left). Insets show magnified areas highlighted in the histological sections (middle section of d and h). 13C6/12C6 ratio image for the indicated histological sections and corresponding values are given on the right-hand side of the magnified insets. e,i, Dot plots showing 13C6 /12C6 signal in different ovarian cell types. f,j, Dot plots showing 13C6 /12C6 signal of granulosa cells in the primary, secondary and antral follicles. g,k, Dot plots showing 13C6 /12C6 signal of theca cells in the primary, secondary and antral follicles. Numbers of analysed cells are shown in parentheses. P values were calculated using unpaired two-tailed Student’s t-test. n.s., not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Scale bars, 10 µm.
To test this hypothesis, we used NanoSIMS, which is suitable to assess the local 13C /12C ratio in a tissue27. We collected ovaries from mice that had obtained 13C6-Lys feed until weaning (3 weeks of age), and had subsequently obtained 12C6-Lys feed until 4 (Fig. 5d–g) or 8 weeks of age (Fig. 5h–k). We then measured the ratio of 13C /12C in oocytes, granulosa cells (surround the oocyte inside follicles), theca cells (line the surface of follicles), luteal cells (form the corpus luteum that develops from the follicle upon ovulation) and stromal cells (cells that reside between follicles in the ovary).
Cells of the short-lived corpus luteum had the lowest 13C /12C ratio of all cell types (Fig. 5e,i). Interestingly, the highest 13C /12C ratios were observed for subsets of stromal cells, granulosa cells, theca cells and oocytes (Fig. 5e). Then we examined the 13C /12C ratio for granulosa cells from follicles of different sizes (Fig. 5f,j). The ratios were highest for granulosa cells in small primordial and primary follicles, suggesting that the follicle cells that surround the oocytes during storage are particularly long-lived, and that their long-lived proteins are diluted with newly synthesized proteins as the follicles grow. We cannot currently determine which exact proteins are long-lived in each of these cell types, but this may be possible with future developments in single-cell MS28. We conclude that not only oocytes and their proteins are long-lived in the ovary, but also subsets of somatic cells and their proteins, including granulosa and stromal cells, as well as individual cells in the theca.
Proteostasis is maintained in aged oocytes
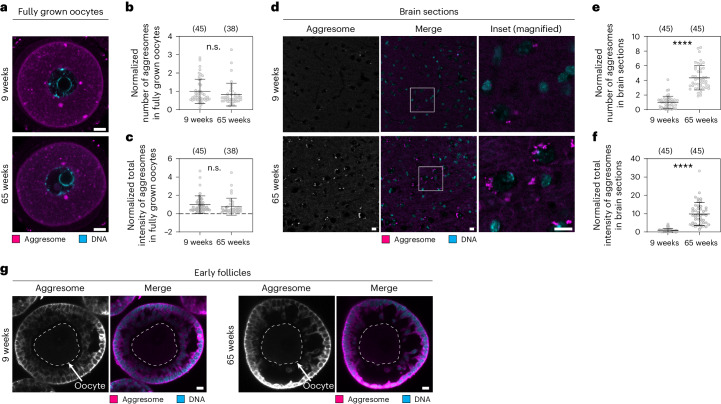
Impaired proteostasis can lead to the accumulation of misfolded proteins, resulting in protein aggregation, which is a major pathological hallmark of various age-related disorders such as Alzheimer’s and Parkinson’s diseases29. To investigate the vulnerability of oocytes to age-related protein aggregation, we stained aggresomes in fully grown oocytes from young (9 weeks) and aged (65 weeks) mice with the ProteoStat dye (Fig. 6a). Strikingly, aggresomes were not increased in aged oocytes compared to young oocytes (Fig. 6a–c). By contrast, we observed increased aggresome staining in brain sections of aged mice compared with young mice, consistent with previous studies30 (Fig. 6d–f). This indicates that protein aggregation does not increase with age in oocytes. In line with this result, aggresomes were also not increased in early follicles from aged mice (Fig. 6g).
Fig. 6. Protein aggregation does not increase in aged oocytes.
a, Representative immunofluorescence images of fully grown, germinal vesicle stage mouse oocytes from 9-week-old and 65-week-old mice stained with the ProteoStat aggresome dye. Magenta, aggresome (ProteoStat); cyan, DNA (Hoechst). b, Dot plot showing number of ProteoStat-positive structures in oocytes as shown in a. c, Dot plot showing total intensity of ProteoStat-positive structures in oocytes as shown in a. d, Representative immunofluorescence images of brain slices from 9-week-old and 65-week-old mice stained with the ProteoStat aggresome dye. Magenta, aggresome (ProteoStat); cyan, DNA (Hoechst). e, Dot plot showing number of ProteoStat-positive structures in brain slices as shown in d. f, Dot plot showing total intensity of ProteoStat-positive structures in brain slices as shown in d. g, Representative immunofluorescence images of early follicles from 9-week-old and 65-week-old mice stained with the ProteoStat aggresome dye. Magenta, aggresome (ProteoStat); cyan, DNA (Hoechst). No obvious aggresome accumulation was detected in either age group. All data from two independent experiments. Number of analysed oocytes and brain areas are in parentheses. Data are shown as mean ± s.d. P values were calculated using unpaired two-tailed Student’s t-test. n.s., not significant; ****P ≤ 0.0001. Scale bars, 10 µm.
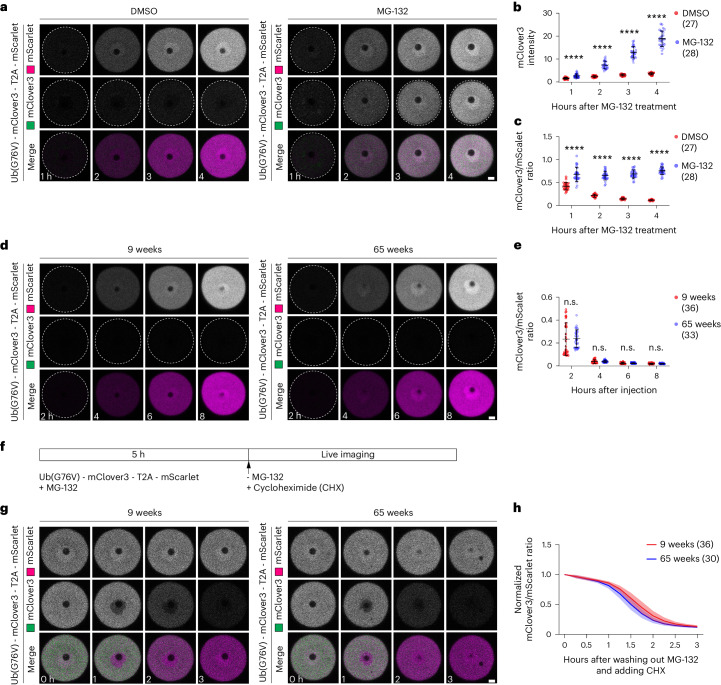
Another hallmark of impaired proteostasis is a decay in proteasomal activity31. We therefore compared proteasomal activity in aged and young oocytes with a reporter assay32. In this assay, a reporter construct consisting of the proteasome substrate Ub(G76V)-mClover3 and mScarlet, separated by a self-cleaving T2A peptide, is expressed. The proteasomal activity can be inferred from the ratio of mClover and mScarlet signal intensity. As expected, proteasome-inhibited oocytes (MG-132) expressing this construct accumulated both mScarlet and Ub(G76V)-mClover3, whereas control oocytes (DMSO) only accumulated mScarlet but not Ub(G76V)-mClover3 due to constant proteasomal degradation (Fig. 7a–c). Ub(G76V)-mClover3 was efficiently degraded in both young (9-week) and aged (65-week) oocytes (Fig. 7d,e), with no significant difference in the mClover3/mScarlet ratio.
Fig. 7. Proteasomal activity does not decay in aged oocytes.
a, Time-lapse images of mouse oocytes from 9-week-old mice expressing Ub(G76V)-mClover3-T2A-mScarlet in the presence of DMSO or 10 μM MG-132. Time is given as hours after DMSO or MG-132 treatment. b, Quantification of the mean fluorescence intensity of mClover3 in oocytes as shown in a. c, Quantification of the fluorescence intensity ratio of mClover3 to mScarlet in oocytes in a. d, Time-lapse images of mouse oocytes from 9-week-old and 65-week-old mice expressing Ub(G76V)-mClover3-T2A-mScarlet. Time is given as hours after injection of the reporter mRNA. e, Quantification of the fluorescence intensity ratio of mClover3 to mScarlet in oocytes in d. f, Schematic diagram of the experiment shown in g. Ub(G76V)-mClover3 and mScarlet were expressed for 5 h in the presence of MG-132, which blocks the degradation of Ub(G76V)-mClover3. MG-132 was then washed out and oocytes were imaged in the presence of the translation inhibitor cycloheximide (CHX), which blocks the synthesis of new proteins. g, Time-lapse images of mouse oocytes from 9-week-old and 65-week-old mouse oocytes expressing Ub(G76V)-mClover3-T2A-mScarlet. Experiment was performed as shown in f. Time is given as hours after MG-132 washout and CHX wash-in. h, Line graph showing normalized fluorescence intensity ratio of mClover3 to mScarlet in oocytes in g. All data from two independent experiments. Number of analysed oocytes in parentheses. Data are shown as mean ± s.d. P values were calculated using unpaired two-tailed Student’s t-test. n.s., not significant; ****P ≤ 0.0001. Scale bars, 10 µm.
To compare the degradation kinetics in both age groups, both young and aged oocytes were allowed to first accumulate Ub(G76V)-mClover3 and mScarlet by MG-132 inhibition, followed by MG-132 washout and live imaging (Fig. 7f). Ub(G76V)-mClover3 was degraded at a similar rate in both young and aged oocytes, with a slightly higher degradation speed in aged oocytes (Fig. 7g,h). Together, these data establish that proteasomal activity does not decay in aged oocytes.
Important long-lived proteins are lost with ovarian aging
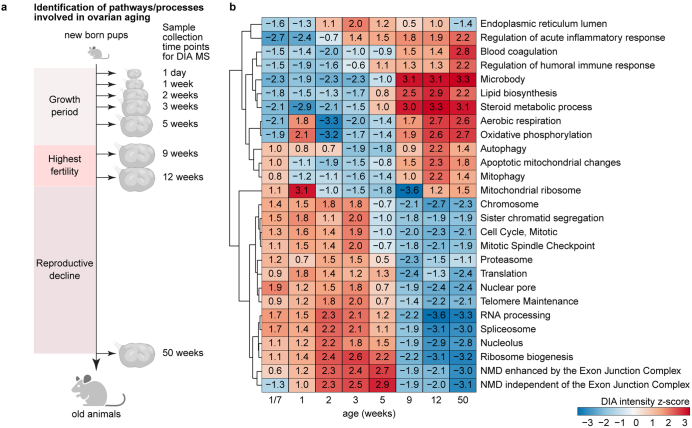
Together, our data establish that proteostasis in oocytes and the ovary involves extreme longevity of a wide range of proteins. Although slow protein turnover can have a positive effect on cellular and organismal longevity33–39, the eventual decline in long-lived proteins can contribute to aging15,40–42. We hence investigated changes in the protein composition of the ovary during ovarian aging (Extended Data Fig. 6a). To this end, we quantified the abundance of 8,890 proteins in ovaries from females at 1 day, and 1, 2, 3, 5, 9, 12 and 50 weeks of age using DIA-MS analysis (Extended Data Fig. 6a, Supplementary Table 6 and Supplementary Data 1).
Extended Data Fig. 6. Changes in enrichment scores of different pathways throughout ovarian development and aging.
(a) Schematic overview of experimental design. Ovaries were collected from female mice at 8 time points (1 day, 1, 2, 3, 5, 9, 12 and 50-week-old mice; three animals per time point analysed). Samples were processed for DIA-MS. (b) Gene set enrichment analysis (GSEA) revealed up- and down-regulated pathways over mouse age. For each time point (1 day, 1, 2, 5, 9, 12 and 50 weeks) normalized DIA-MS data for 8,890 detected proteins were subject to GSEA. Normalized enrichment scores for each pathway and time point are shown as heatmap upon hierarchical clustering (dendrogram shown on the left). Each term is significantly enriched at least in one time point. Source numerical data are available in source data.
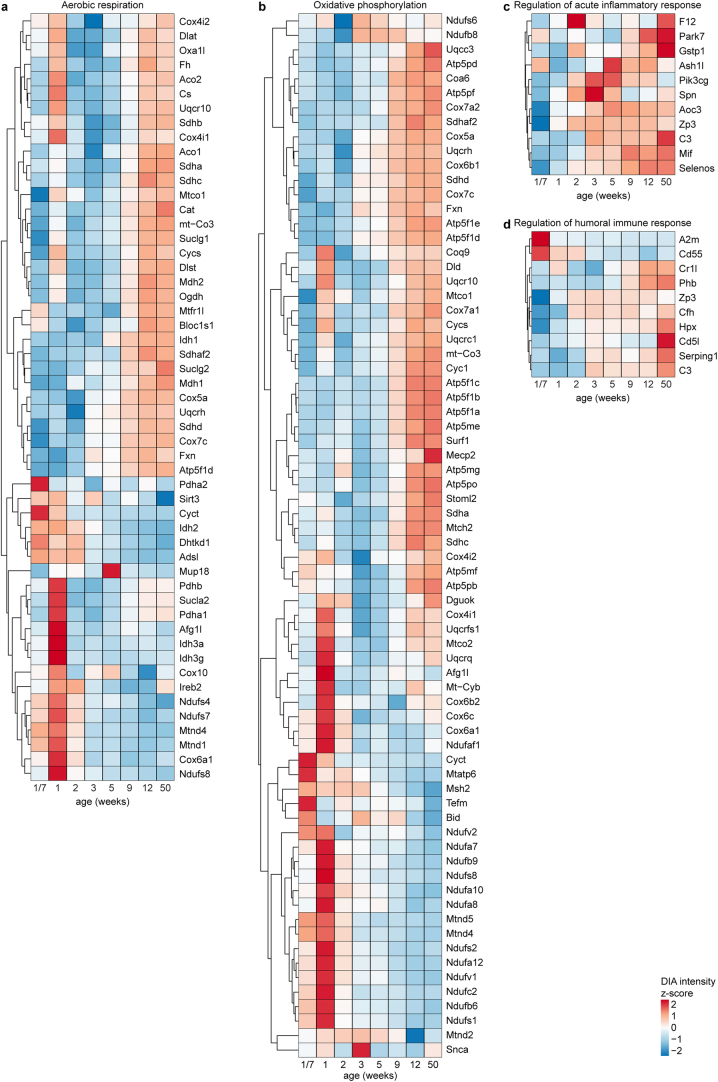
First, we performed a cluster and gene set enrichment analysis (GSEA) on the data (Supplementary Table 7). GSEA revealed a significant upregulation of pathways associated with active cell division and protein biogenesis (including translation, mitotic spindle checkpoint, RNA processing, ribosome biogenesis and meiotic cell cycle) at 3–5 weeks of age, followed by a steep downregulation of these pathways at 9 weeks of age, when FVB/N female mice have reached full fertility (Extended Data Fig. 6a,b). Interestingly, proteins related to aerobic respiration and oxidative phosphorylation (Extended Data Figs. 6b and 7a,b), microbodies, steroid metabolic process, lipid biosynthesis and mitophagy increased around this time. Proteins related to the regulation of acute inflammatory response (Extended Data Figs. 6b and 7c) and the humoral immune response (Extended Data Figs. 6b and 7d) gradually increased in abundance from 3 weeks onwards, reaching a maximum at 50 weeks, which is consistent with the reported increase in inflammation in the ovary with advancing female age43.
Extended Data Fig. 7. Changes in abundance of different proteins belonging to different gene sets throughout ovarian development and aging.
(a-d) Normalized DIA-MS intensity data showing the abundance changes over time of the proteins in the selected pathways significantly enriched in the gene-set enrichment analysis (GSEA) of the DIA-MS data: aerobic respiration (a), oxidative phosphorylation (b), regulation of acute inflammatory response (c), and regulation of humoral immune response (d). Source numerical data are available in source data.
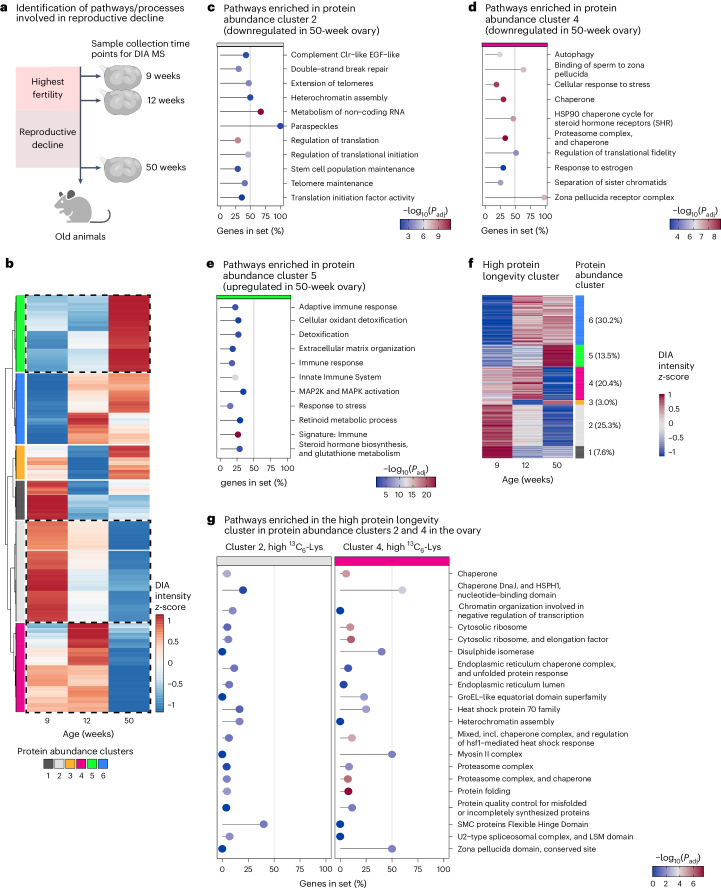
We then focused on the time period from when the females were fully fertile (9 weeks and 12 weeks), to when fertility has markedly declined (50 weeks; Fig. 8a). First, we performed a protein abundance cluster analysis, which revealed six groups of proteins with distinct trends in relative abundance during this period (Fig. 8b and Supplementary Table 8).
Fig. 8. Proteostasis networks are lost with ovarian aging and decreased fertility.
a, Schematic overview of experimental design. Ovaries were collected from female mice at three time points (9-, 12- and 50-week-old mice; three animals per time point analysed). Samples were processed for DIA-MS. b, Cluster analysis of protein abundances during reproductive decline. Normalized DIA-MS data for 9-, 12- and 50-week-old ovaries were subject to clustering to minimize variance within clusters. Dendrogram of resulting clusters is shown on the left. Colours indicate DIA intensities centred to a mean of 0 and s.d. of 1 on per protein basis. Resulting protein abundance clusters are highlighted with different colour keys. (c-e) Pathway over-representation analysis of protein abundance cluster 2 (c), cluster 4 (d) and cluster 5 (e). f, Heatmaps showing protein abundance change for the high protein longevity cluster with assignment to protein abundance clusters. g, Pathway over-representation analysis of high protein longevity cluster proteins in protein abundance clusters 2 and 4 in the ovary. c–e and g show the proportions of genes detected in the gene set with their respective adjusted P values for the most prominent pathways. The P values are based on the hypergeometric test and have been adjusted for multiple hypothesis testing with Benjamini–Hochberg procedure. For the complete list of enriched pathways and their corresponding exact P values, see Supplementary Table 9.
Protein abundance clusters 2 and 4 were of particular interest as they contained proteins that were abundant in 9- and 12-week-old females, but dropped steeply in 50-week-old females, when female fertility declines (Fig. 8c,d). Cluster 2 was significantly enriched in processes with important functions related to ovarian aging or aging in general, including stem cell division proteins, telomere proteins, heterochromatin assembly proteins and DNA double-strand break repair proteins (Fig. 8c and Supplementary Table 9). Cluster 4 was enriched in proteins involved in autophagy (Fig. 8d and Supplementary Table 9), which can promote cellular longevity44, as well as many heat shock proteins and chaperones, which promote cellular longevity by protecting cells against protein misfolding and aggregation38,39. Cluster 4 also contained zona pellucida proteins, which are required for sperm binding, as well as cohesion proteins. Ovarian aging has been attributed to multiple processes that overlap with the enriched gene ontology terms, including a decline in the ability to repair DNA double-strand breaks45–47, a decline of telomere function48,49, alterations in the oocyte’s zona pellucida50,51 and a decline in cohesion function52–55. Changes in the levels of related proteins could contribute to these defects, and also reflect alterations in the cellular composition of the aging ovary, including a decline in follicle numbers.
Protein abundance cluster 5 contained proteins that were steeply upregulated in the ovaries of 50-week-old females (Fig. 8b) and was enriched in inflammatory response proteins, immune proteins, proteins involved in retinoic acid synthesis, detoxification-related proteins, response to stress proteins, oxygen-related proteins, proteins related to mitogen-activated protein kinase (MAPK) signalling, extracellular matrix proteins and hormone biosynthesis proteins (Fig. 8e and Supplementary Table 9). These ontology terms overlap with known alterations in the aging ovary (that is, an increase in inflammation and fibrosis)43,56, and reveal additional candidate pathways that have not yet been linked to ovarian aging, including for instance a potential function for retinoids in the aging ovary.
Importantly, many extremely long-lived proteins also declined in the aging ovary (Fig. 8f; Extended Data Fig. 8). Proteins with protective functions and proteins of the proteostasis network were prominently enriched in this group, including chaperones, heat shock proteins, chaperonins, disulfide isomerases, proteasomal and ribosomal proteins, and other proteins that promote protein folding or protein quality control (Fig. 8g and Supplementary Table 10). Thus, a large number of long-lived proteins with key functions in maintaining proteostasis in cells were significantly decreased in the aged ovary. In addition, long-lived proteins involved in other essential processes, such as heterochromatin organization, were decreased (Fig. 8g). We conclude that ovarian aging is associated with extensive changes in the ovarian proteome and a decrease of important long-lived proteins.
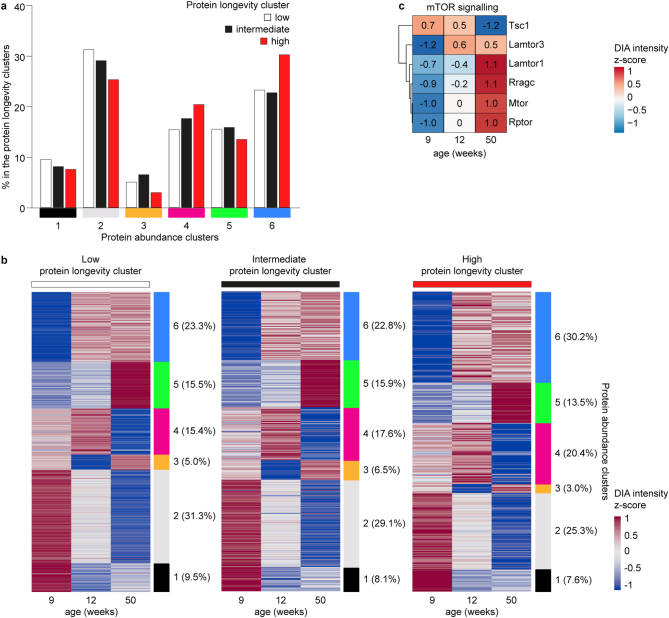
Extended Data Fig. 8. Distribution of ovary proteins in the different protein longevity clusters and protein abundance clusters, and abundance changes over time of proteins in the mTOR signalling pathway.
(a) Percentage of ovary proteins that are detected across the three protein longevity clusters, as well as in one of the six protein abundance clusters derived from the DIA-MS data. (b) Heatmaps showing protein abundance change for low, intermediate, and high protein longevity clusters with assignment to protein abundance clusters. (c) Normalized DIA-MS intensity data showing the abundance changes over time of proteins in the mTOR signalling pathway. Source numerical data are available in source data.
Discussion
Our data establish that many proteins in oocytes and the ovary are unusually stable, with half-lives well above those reported in other cell types and organs, including the liver, heart, cartilage, muscle and the brain19–21,41,57,58. Our data confirm the longevity of various proteins, including histones, components of nuclear pore complexes and mitochondria40,59–62. Unexpectedly, however, the half-lives of many proteins are much higher in the ovary than in other organs, and many additional proteins are uniquely long-lived in the ovary. These include many proteins involved in proteostasis, and in critical cellular machines, such as mitochondria, ribosomes, proteasomes and cytoskeletal assemblies. In addition, proteins in key metabolic pathways, such as glycolysis, the pentose phosphate pathway, the citrate cycle and fatty acid beta oxidation are extremely long-lived.
Oocytes must be stored in the ovary for more than a year in mice, and for decades in humans. Our study shows that germline maintenance involves extraordinary stability of hundreds of proteins, many of which persist throughout the life of the mouse. This extreme protein stability could provide major advantages for safeguarding the germline across generations.
Oocytes and their supporting somatic cells are formed before birth, but only become activated again much later, when puberty resumes63. This likely allows for adaptations in proteostasis that would not be possible in other cell types. A reduction in protein turnover has been shown to promote proteostasis, cellular longevity and high organismal lifespan33–37. Oocytes might similarly use this strategy to preserve a wide range of their components. For example, oocytes provide all of the mitochondria to the developing embryo64,65 and must therefore preserve mitochondria with minimal damage. A wide range of mitochondrial proteins were extremely stable in both oocytes and ovaries, indicating that mitochondrial components persist for exceptionally long periods of time.
High protein turnover is associated with ATP consumption66,67, and therefore requires high mitochondrial activity, which generate reactive oxygen species68, a major driver of cellular damage. Notably, oocyte mitochondria are largely inactive69, and many longevity promoting interventions reduce mitochondrial activity and protein synthesis70,71. Thus, low mitochondrial activity and low protein turnover may be regulatory and functionally coupled in mammalian oocytes. In support of this point, the levels of mTOR signalling-related proteins suggest that mTOR signalling is relatively low in young ovaries and increases with age (Extended Data Fig. 8c). Low mTOR signalling leads to low mitochondrial activity and low protein turnover72–74, and is therefore likely to be a regulatory mechanism that drives high protein longevity and low mitochondrial activity in the young ovary.
The extreme longevity of mitochondrial proteins and core cellular machinery is accompanied by an extreme longevity of several chaperones and heat shock proteins, including the major mitochondrial heat shock protein HSPD1. Proteins that protect against oxidative damage, such as peroxiredoxins, SOD2 and disulfide isomerases, were also extremely long-lived. These proteins likely promote the high longevity, proper folding and function of other proteins in the oocyte and ovary. In addition, the compartmentalization of subsets of proteins into specialized structures, such as the Balbiani bodies75,76 or cytoplasmic lattices77 may help to preserve their stability and function.
Our data suggest that these adaptations in proteostasis help to prevent the accumulation of damage in the germline. Notably, protein aggregation did not increase in aged oocytes and early follicles (Fig. 6a–c,g), and proteasomal activity was not diminished (Fig. 7d–h). This is in stark contrast to the brain, which showed a significant increase in protein aggregation over the same time period (Fig. 6d–f) and exhibits a decay in proteasomal function29–31.
Although reduced protein synthesis has beneficial effects on cellular longevity and proteostasis, the eventual loss of highly stable proteins may contribute to the demise of oocytes and the ovary with age. Previous work has shown that the cohesin subunit REC8 is long-lived in oocytes15–17, and that its age-related decline is a driver of the increase in oocyte aneuploidy with advancing female age52,53. Our data support the hypothesis that other cohesin subunits and regulatory proteins are also very long-lived and show an age-related decline, including SMC3, SMC1A and PDS5B. Many other proteins associated with chromatin organization are also long-lived, including HDAC1, SAP30, HIRA, RIF and DNMT3A, and many of them decrease in abundance in the aging ovary. We also observed a decrease in long-lived proteins required for maintaining protein homeostasis in aged ovaries. Thus, although the mechanism of increasing protein longevity and enhancing proteostasis improves some of the senescent characteristics in oocytes, it cannot fully prevent female germline senescence.
In addition, our analysis suggests candidate proteins and pathways for age-related changes in the ovary that are not yet understood at the molecular level. For example, we identified many apoptosis-promoting proteins that are upregulated in the aging ovary and may be involved in the age-related decline in follicle number. We also identified prominent age-related changes in the abundance of proteins involved in DNA damage repair, protection against oxidative damage, chromatin organization, epigenetic regulation, mitochondria and telomeres, which may contribute to the previously reported age-related changes in oocytes and ovaries45–49,78–87.
Finally, our data show that not only oocytes, but also subsets of ovarian somatic cells are highly enriched in long-lived proteins. These include granulosa cells of early-stage follicles, as well as subsets of thecal and stromal cells. The functions of stromal cells are only poorly understood88, and will be an exciting topic for future studies. Taken together, these findings are consistent with a model in which ovarian aging is driven not only by oocyte aging, but also by the aging of somatic cells in this organ89–92.
Methods
Ethics
The maintenance and handling of all FVB/N and CD1 mice was performed in the MPI-NAT animal facility according to international animal welfare rules (Federation for Laboratory Animal Science Associations guidelines and recommendations). Requirements of formal control of the German national authorities and funding organizations were satisfied, and the study received approval by the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (LAVES).
Mouse oocyte and ovary collection and preparation
FVB/N mice fed with 13C6-Lys feed were kept in static filter cages from Ehret. All other mice were kept in a Blue Line IVC system from Techniplast. All mice were kept in rooms with constant temperature of 21 °C and the humidity of 55%. The light/dark rhythm was 12:12 hours, from 05:00 to 17:00. Health monitoring was carried out in accordance with Federation of European Laboratory Animal Science Associations recommendations with large annual examinations in January and smaller scale in May and September. This study investigates protein turnover and abundance in ovaries and oocytes, and hence, only animals of female sex were analysed.
All 13C6-Lys feed was purchased from Silantes. Fully 13C6-labelled FVB/N female mice were created by successive matings and a diet consisting exclusively of 13C6-Lys feed (Extended Data Fig. 1a). F0 females were fed 13C6-Lys feed for 8 weeks, starting from the week 3 after birth, after which they were mated with wild-type FVB/N males who were kept on a standard diet. The F1 pups born from this mating and the F0 mother were further fed together with 13C6-Lys-feed until weaning. Thereafter, only F1 offspring was further fed 13C6-Lys feed. Upon reaching sexual maturity, F1 13C6-Lys-fed females were mated with wild-type FVB/N males who were kept on a standard diet. The pups of the F2 generation were the first generation of mice used for experiments. Females of F1 generation were mated continuously to produce experimental animals. Fully labelled 13C6-Lys-breeders were periodically substituted by the pups from the fully labelled offspring. The percentage of labelling, as highlighted in Extended Data Fig. 1, was determined by MS analysis of blood samples from F0, F1 and F2 animals as described below (Extended Data Fig. 1b–e). We also analysed percentage of labelling in oocytes of F1 generation (Extended Data Fig. 1d), which was similar to the percentage of labelling observed from blood samples (Extended Data Fig. 1c). The F2 offspring had a 98.7% 13C6-Lys labelled proteome (Extended Data Fig. 1e).
The offspring of fully labelled females were moved to a wet nurse 12C6-Lys-fed CD1 female within 24 h after birth (Figs. 1a and 2a) or were kept with the 13C6-Lys female breeders until weaning (3 weeks after birth) to provide a longer ‘pulse’ period before the ‘chase’ diet of 12C6-Lys feed (Fig. 2b). All animals were fed ad libitum and had unrestricted access to water.
For MS analyses, oocytes were collected from 8-week-old mice (±24 h). Oocytes were prepared in the homemade M2 medium without bovine serum albumin (BSA, Fisher BioReagents; BP9700100), supplemented with 250 µM dbcAMP (Sigma; D0627) to maintain oocytes in the prophase arrest. Upon collection, oocyte samples were snap-frozen in liquid nitrogen in <5 µl of media and stored at −80 °C. All ovaries collected for MS analysis were prepared in M2 medium without BSA or in phosphate-buffered saline (PBS) prior to snap freezing and storing at −80 °C. Ovaries prepared from mice between the age of 6 and 30 weeks were dissected to remove medulla.
Estimation of the mouse oocyte volume
The diameters of oocytes in primordial follicles were measured from archived paraffin-fixed ovarian slices imaged with the Zeiss LSM800. The diameters of germinal vesicle (GV) oocytes were measured from archived fixed and live confocal images of GV oocytes imaged with the Zeiss LSM800. The volume of the oocytes was then estimated using the formula v = 4/3π × r3, where r is radius.
Estimation of mouse ovarian volume
Ovaries from FVB/N mice were collected at 24 h, 48 h, 1, 2, 3, 6, 9, 12, 30, 50 and 65 weeks after birth. Excess fat and tissue were removed from the organs, before they were placed into a 35 mm imaging dish and measured on a Zeiss LSM800 confocal microscope. A shelf consisting of three glass cover slip pieces was created in order to hold the ovaries in place without impacting the dimensions of the ovaries themselves; the glass cover slip pieces were affixed to the imaging dish using double-sided tape, and placed in a U-shape. Each ovary was placed within the U-shaped shelf and submerged in a drop of homemade M2 medium, before its dimensions were measured using the x, y and z coordinates of the ovary edge as observed using the transmission light on a Zeiss LSM800 microscope. The volume of the ovaries was then estimated by using the formula v = lwh (length × width × height).
Preparation of mouse blood samples for MS measurements
Blood (20 µl), taken from orbital plexus from F0, F1 or F2 13C6-Lys-fed FVB/N males, was supplied with 80 µl urea lysis buffer (6 M urea, 10 mM HEPES-NaOH pH 8.0) following incubation on ice for 20 min and sonication for 10 min with 30 sec on/off cycles at 4 °C using the highest output level (Bioruptor, Diagenode). Lysate was cleared at 13,000g, 10 min, 4 °C. Protein concentrations were determined using Bradford assay (Bio-Rad) and further sample processing was performed on 20 µg protein amount.
Preparation of mouse oocyte samples for MS measurements
For DDA MS measurements, two sets of oocytes (n = 2,475 and n = 2,473) were obtained from 8-week-old mice (12C6-Lys chow after birth until they were 8-weeks old) born from 13C6-Lys-labelled females. The oocytes were suspended in 80 µl SDS lysis buffer (4% [w/v] SDS, 150 mM NaCl, 50 mM HEPES-NaOH pH 7.5, 2 mM DTT, 0.5% [v/v] NP40, 1X Roche complete protease inhibitors-EDTA). Oocytes were lysed for 10 min at 99 °C followed by sonication for 10 min with 30 sec on/off cycles at 20 °C using the highest output level (Bioruptor, Diagenode). Samples were further processed as specified below.
Preparation of mouse ovary samples for MS measurements
For DDA MS measurements, mouse ovaries were collected from female pups pulsed with 13C6-Lys until birth at 24 h, 48 h, 1, 2, 3, 6, 9, 12, 30, 50 and 65 weeks after birth, and for the mice that were pulsed with 13C6-Lys until weaning at 6, 9, 12, 30, 50 and 65 weeks after birth. For protein abundance DIA-MS measurements, mouse ovaries were collected from wild-type FVB/N females at 24 h, 1, 2, 3, 5, 9, 12 and 50 weeks after birth. For all experiments and all time points, three biological replicates were collected, each containing two ovaries from a single animal. Small ovaries (24 h and 48 h time points) were suspended in 40 µl SDS lysis buffer and lysed for 10 min at 99 °C and by sonication for 10 min with 30 sec on/off cycles at 20 °C using the highest output level (Bioruptor, Diagenode). Larger ovaries (1 week onwards) were suspended in 100 µl SDS lysis buffer and ten Zirconia/Silica beads (2.3 mm; BioSpec Products; 11079125Z) were added to each sample. Lysis was performed using a FastPrep-24 benchtop homogenizer (MP Biomedicals) for three 20 sec on/off cycles at 5.5 m s–1 following incubation for 10 min at 99 °C and lysate clearance at 17,000 ×g, 5 min. Protein concentrations were measured for samples derived from 1-week-old and older mice using Bradford (Bio-Rad) or Pierce BCA Protein Assay Kit (ThermoFisher Scientific) according to manufacturer’s instructions and further sample processing was performed starting with 50 or 300 µg protein amount for DIA or DDA ovary analyses, respectively.
Protein digestion and sample cleanup
F0 blood samples were processed by in-gel digestion according to ref. 93. Samples were loaded onto a 4–12% NuPAGE Novex Bis-Tris Minigel (Invitrogen), following Coomassie staining. The protein containing lane was cut into 23 pieces. Proteins were reduced with dithiothreitol (DTT), alkylated with iodoacetamide (IAA) and digested overnight with trypsin (Serva; T6567). Tryptic peptides were extracted from gel pieces, dried in a vacuum concentrator and resuspended in MS buffer for LC-MS/MS measurements. F1 and F2 blood samples were processed in solution. Samples were adjusted to 20 µl using urea lysis buffer followed by reduction of proteins using 5 mM DTT and incubation for 30 min, 25 °C, 650 r.p.m., and alkylation with 20 mM IAA for 30 min, 25 °C, 650 r.p.m., in the dark. Samples were diluted to a final urea concentration of 0.5 M using 50 mM NH4HCO3. Protein digest was performed using trypsin (Promega; V5111) at a 1:20 enzyme-to-protein mass ratio and incubation overnight at 37 °C, 650 r.p.m. Desalting of blood samples was performed as described below.
Oocyte and ovary lysates were diluted to 1% (w/v) final SDS concentration using 100 mM NH4HCO3 (for oocytes) or 50 mM HEPES-NaOH pH 7.5 (for ovaries) and incubated with 250 U (DIA oocyte samples and DDA/DIA ovary samples) or 500 U (DDA oocyte samples) Pierce Universal Nuclease (ThermoFisher Scientific; 88700) and 1 mM MgCl2 for 30 min, 37 °C, 300 r.p.m. Proteins were reduced with 5 mM DTT for 30 min, 37 °C, 300 r.p.m.; alkylated with 10 mM iodoacetamide for 30 min, 25 °C, 300 r.p.m., in the dark; and quenched with 5 mM DTT for 5 min, 25 °C, 300 r.p.m. Proteins were further purified according to the bead-based SP3 preparation method94,95 to remove detergents. Briefly, carboxylate modified magnetic beads (Cytiva; 65152105050350, 45152105050250) were added at a 1:10 protein-to-bead mass ratio and acetonitrile (ACN) was added to 50% (v/v) to induce protein binding to the beads. Washing was performed three to five times with 80% EtOH and once with 100% ACN. For protein digestion, the beads were suspended in 100 mM NH4HCO3 with trypsin and rLys-C (Promega; V5111, V1671) at a 1:20 ratio assuming a protein amount of 25 ng per oocyte and 50 µg total protein amount for 24 h and 48 h old ovaries. The digestion-bead mix was incubated for 16 h at 37 °C, 1,000 r.p.m. and the digested peptides were collected according to the SP3 protocol. The beads were rinsed once with 50 µl 100 mM NH4HCO3 and sonicated for 30 seconds. The supernatant was pooled with the collected peptide mix. From DDA oocyte and ovary samples, unfractionated input samples (3%, ‘input’) were removed before desalting and offline fractionation. Desalting of DIA ovary samples was performed as described below and DIA oocyte samples were dried in a vacuum concentrator and resuspended in MS buffer (2% (v/v) ACN, 0.05% (v/v) trifluoroacetic acid (TFA)) and subjected to LC-MS/MS measurements.
Blood samples, DDA oocyte and ovary samples and DIA ovary samples were desalted using C18 Micro Spin Columns (Harvard Apparatus; 74-4601) according to manufacturer’s instructions. Briefly, samples were adjusted to 0.1% (v/v) formic acid (FA) and loaded onto equilibrated spin columns. Samples were reloaded once and washed three times with 0.1% (v/v) FA. Peptides were eluted using 50% (v/v) ACN, 0.1% (v/v) FA and 80% (v/v) ACN, 0.1% (v/v) FA. Eluants were dried in a vacuum concentrator. Blood samples and DIA ovary samples were resuspended in MS buffer for LC-MS/MS measurements. DDA oocyte and ovary samples were offline fractionated as described below.
Basic reversed phase sample fractionation
Cleaned-up peptides from DDA samples were dissolved in 35 µl 10 mM NH4OH pH 10, 5% (v/v) ACN. Peptides were loaded onto an Xbridge C18 column (Waters; 186003128) using an Agilent 1100 series chromatography system. The column was operated at a flow rate of 60 µl min–1 with a buffer system consisting of 10 mM NH4OH pH 10 (buffer A) and 10 mM NH4OH pH 10, 80% (v/v) ACN (buffer B). The column was equilibrated with 5% B and developed over 64 min using the following gradient: 5% B (0–7 min), 8–30% B (8–42 min), 30–50% B (43–50 min), 90–95% B (51–56 min), 5% B (57–64 min). The first 6 min were collected as one flow-through fraction, followed by 48 × 1 min fractions, which were reduced to 12 fractions by concatenated pooling. For DDA oocyte samples, the last 10 min of each run were collected as one ‘rest’ fraction. All fractions were dissolved in MS buffer for LC-MS/MS measurements.
LC-MS/MS analysis
Blood samples were measured in duplicate on an Orbitrap Fusion Tribrid Mass Spectrometer (ThermoFisher Scientific), coupled to a Dionex Ultimate 3000 RSLCnano system. Analytes were loaded on a Pepmap 300 C18 column (ThermoFisher Scientific) at a flow rate of 10 µl min–1 in 0.1% (v/v) FA (buffer A) and washed for 3 min with buffer A. Samples were separated on an in-house packed C18 column (30 cm; ReproSil-Pur 120 Å, 1.9 µm, C18-AQ; inner diameter, 75 µm) at a flow rate of 300 nl min–1. Sample separation was performed using a buffer system consisting of buffer A and 80% (v/v) ACN, 0.08% (v/v) FA (buffer B). The main column was equilibrated with 5% B, sample was injected and column was washed for 3 min with 5% B. A linear gradient from 10–42% B over 163 min was applied, to separate peptides, followed by 5 min at 90% B and 8 min at 5% B. Peptides were analysed in positive mode using a data-dependent top speed acquisition method with a cycle time of 3 sec. MS1 scans were acquired in an Orbitrap mass analyser with a resolution set to 120,000 FWHM; AGC target was set to standard. MS2 scans were acquired in an Ion Trap with scan rate set to rapid; AGC target was set to 3 × 103 (30%). Precursors selected during MS1 scans (scan range m/z 350–1,500) were fragmented using 34% normalized, higher-energy collision-induced dissociation (HCD) fragmentation. Further MS/MS parameters were set as follows: isolation width, 1.6 m/z; dynamic exclusion, 30 sec; maximum injection times (MS1/MS2), 50 ms/dynamic.
Each DDA oocyte fraction was measured in triplicates on a Q Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer (ThermoFisher Scientific), DDA ovary and DIA oocyte and ovary samples were measured on an Exploris 480 Mass Spectrometer (ThermoFisher Scientific). Mass spectrometers were coupled to Dionex Ultimate 3000 RSLCnano systems. Pre- and main column setup, flow rates and precolumn equilibration and loading were the same as for blood samples. For DDA oocyte and ovary fractions, a linear gradient from 10–42% B over 103 min was applied for separation of peptides on main column. For DDA oocyte input and rest fractions, peptide separation was performed over 163 and 43 min (10–42% B), respectively. DIA oocyte and ovary samples were separated using a linear gradient from 5–14% in 97 min, followed by 14–32% in 100 min and 32–48% in 27 min and a total of 238 min runtime. For all runs, peptide separation was followed by 5 min at 90% B and 8 min at 5% B. For DDA oocyte and ovary analyses, eluting peptides were analysed in positive mode using a data-dependent top 30 acquisition method. MS1 and MS2 resolution were set to 60,000 and 15,000 FWHM, respectively, and AGC targets were 106 and 105. Further MS/MS parameters were set as follows: scan range, m/z 350–1,600; HCD collision energy, 30%; isolation width, 1.4 m/z; dynamic exclusion, 20 sec; maximum injection times (MS1/MS2), 50 ms/54 ms. For DIA analyses, data were acquired using positive mode. MS1 scans were performed using the following settings: resolution, 12,000 FWHM; AGC target, 3 × 106; scan range, 350–1,600 m/z; maximum injection time, 20 ms. Following each MS1 scan, MS2 scans were acquired using tMS2 option in Thermo Xcalibur Instrument Setup software, in 70 defined, variable m/z windows (Supplementary Table 11). Further MS2 parameters were set as follows: resolution, 30,000 FWHM; AGC target, 106; maximum injection time, 55 ms; HCD collision energy, 30%. For all DDA and DIA measurements, the lock mass option (m/z 445.120025) was used for internal calibration.
Peptide database search
Database searches for DDA data were performed using MaxQuant software (version 1.5.2.8 for blood samples and 1.6.0.1 for oocyte and ovary samples)96,97. For blood samples, a reviewed (Swiss-Prot) Mus musculus (strain C57BL/6J) reference proteome database, including canonical protein sequences, was downloaded from UniProt Knowledgebase (date of download: 6 January 2017; 10,090 proteins); for oocyte and ovary samples, a similar database was used that included canonical and isoform protein sequences (date of download: 10 September 2021; 17,077 proteins). For long-lived protein analysis, data were searched with the following settings: enzyme, trypsin/P; multiplicity, 2; heavy labels, Lys6; fixed modifications, carbamidomethyl (C); variable modifications (included in protein quantification), oxidation (M), acetyl (protein N− term) for blood sample data, and oxidation (M), acetyl (protein N− term), deamidation (N), methyl (KR), for other data; match between runs, enabled. For mixed peptide analysis, settings were the same except for multiplicity was set to 1 and Lys6 (13C6-K) was set as variable modification. MaxQuant results for F0, F1 and F2 13C6-Lys-fed FVB/N male blood samples were further processed using R studio98 to determine median peptide 13C6-Lys incorporation rates for lysine-containing peptides showing H/L ratios. DIA data were analysed with Spectronaut version 15.7.220308.50606 (ref. 99). A spectral library was generated from all DDA oocyte and ovary data, which were analysed using Pulsar search engine platform and the following settings: enzyme, trypsin/P; minimum peptide length, 7; maximum peptide length, 52; fixed modifications, carbamidomethyl (C); variable modifications oxidation (M), acetyl (protein N-term). BGS Factory Settings were used for identification and quantification of proteins from DIA data except for maximum. Top N precursors used for quantity calculation was set to 5. A minimum of two unique peptides per isotope was required for protein quantification.
Single-cell RNA sequencing
Collection and dissociation of postnatal ovaries
The postnatal ovary samples were collected in 1X PBS. For the generation of each single-cell sequencing library, 6 ovaries were collected from 3 different pups from a single pregnant female mouse. The ovaries were dissociated into single cells using 1 mg ml–1 Collagenase Type IV (Gibco; 17104019) at 37 °C for 30 min with pipetting at regular intervals, followed by incubation with Accumax (PAN-Biotech; P10-21200) at 25 °C for 5 min with continuous shaking at 500 r.p.m. The sample was triturated for 1 min using wide orifice low retention tips (Mettler Toledo). The reaction was stopped with 0.04% fetal bovine serum, and the cell suspension was passed through 35 µm (Corning; 352235) and 40 µm (Merck; 136800040) cell strainers respectively. Finally, the sample was centrifuged at 400g for 5 min at 4 °C, the supernatant was aspirated and the pellet was resuspended in 1X PBS with 0.04% BSA.
Generation of single-cell RNA libraries and sequencing
The ovarian single-cell suspensions were loaded onto the 10X Genomics Chromium Single Cell system using Chromium Single Cell 3ʹ Reagent Kits v3 as per manufacturer’s instructions. Each reaction well was loaded with approximately 12,000 cells to achieve a recovery estimate of about 7,000 cells per library. Single cells were then partitioned into Gel Bead-In Emulsions (GEMs) in the Chromium controller followed by generation and amplification of cDNA molecules with unique 10X barcodes. The single-cell RNA-seq libraries were subjected to pair-end sequencing on Illumina HiSeq 4000 (Sequencing Core Facility, MPI-MG, Berlin, Germany).
Protein turnover analysis in oocytes and ovaries, and scRNAseq analysis of mouse ovaries
All related information about the oocyte and ovarian proteome analysis, and the single-cell RNA sequencing of mouse ovaries are described in detail in the Supplementary Note 1.
Aggresome staining
Aggresomes were stained using the ProteoStat Aggresome Detection Kit (Enzo Life Sciences; ENZ-51035-K100) according to the manufacturer’s instructions with some modifications. Early-stage follicles or full-grown oocytes were isolated from 9-week-old or 65-week-old mice and fixed with 2% methanol-free formaldehyde in 1×Assay Buffer for 1 h at room temperature. Fixed oocytes/follicles were washed and permeabilized with 0.5% Triton X-100 in 1×Assay Buffer containing 3 mM EDTA (pH 8.0) with shaking on ice for 1 h. After a brief wash with 1×Assay Buffer containing 0.1% BSA, oocytes/follicles were incubated in 1×Assay Buffer containing 0.1% BSA, 1:2,000 diluted ProteoStat aggresome dye, and 20 µg ml–1 Hoechst 33342 for 1 h with shaking at room temperature. The samples were then extensively washed with 1×Assay Buffer containing 0.1% BSA and scanned using a Zeiss LSM880. The ProteoStat aggresome dye was excited with a 561 nm laser line and detected at 579 to 623 nm. Brains isolated from three 9-week-old and three 65-week-old mice were fixed with 4% paraformaldehyde in PBS for at least 5 h on ice and then washed thoroughly with PBS. The fixed samples were placed in 25% sucrose in PBS and gently shaken overnight in a cold room. They were then embedded in OCT Compound (Tissue-Tek; 4583) and sectioned at 14 µm. The brain slices were washed briefly with PBS and treated with the same permeabilization solution as described above for 30 min at room temperature. They were then washed again with PBS and stained with 1:2,000 diluted ProteoStat aggresome dye in 1×Assay Buffer for 30 min at room temperature. After extensive washing with PBS, Vectashield Antifade Mounting Medium with DAPI (VECTOR; H-1200) was added, and the slides were sealed for imaging. Fifteen areas of the cerebral cortex were randomly selected and scanned in each brain.
Proteasome activity assay
To construct the proteasome activity reporter pGEMHE-Ub(G76V)-mClover3-T2A-mScarlet, Ub(G76V) was amplified from Ub(G76V)-EGFP (a gift from Nico Dantuma, Addgene plasmid 11941)32 and assembled into pGEMHE together with three mClover3 sequences and T2A-mScarlet. pGEMHE-Ub(G76V)-mClover3-T2A-mScarlet was linearized with PacI and then transcribed in vitro using the HiScribe T7 ARCA mRNA Kit (NEB; E2065S). The transcribed mRNA was further purified using the RNeasy Mini Kit (Qiagen; 74104). To test whether Ub(G76V)-mClover3 is efficiently degraded in oocytes, 4 pl of 0.3 µM Ub(G76V)-mClover3-T2A-mScarlet mRNA was injected into oocytes collected from either 9-week-old or 65-week-old mice, and then imaged in the presence or absence of 10 µM MG-132. After injection, two proteins, Ub(G76V)-mClover3 and mScarlet, were generated because of the presence of the self-cleaving T2A peptide. Ub(G76V)-mClover3 is a proteasome substrate, while mScarlet serves as a control. To follow the degradation kinetics of Ub(G76V)-mClover3, 4 pl of 0.3 µM Ub(G76V)-mClover3-T2A-mScarlet mRNA was injected into oocytes collected from either 9-week-old or 65-week-old mice and expressed for 5 h in the presence of 10 µM MG-132. MG-132 can prevent the degradation of Ub(G76V)-mClover3, allowing it to accumulate in oocytes. MG-132 was then washed out and the oocytes were imaged in medium containing 100 µg ml–1 of the translation inhibitor cycloheximide and using a Zeiss LSM880.
Secondary ion mass spectrometry
Sample preparation and embedding for SIMS imaging
Ovaries were collected at 4 and 8 weeks from mice kept with the fully 13C6-Lys-labelled FVB/N mothers until weaning (3 weeks after birth) and cut carefully into three or four slices (approximately 100–200 µm in thickness, depending on size of ovary), on PBS-soaked filter paper under a stereoscope. Slices were transferred to 2% (w/v) EM-grade glutaraldehyde for fixation (RT, 2 h). Slices were washed in PBS three times, before quenching in 100 mM ammonium chloride for 30 min at RT. Slices were washed in PBS three more times before embedding in LR White resin (medium grade, London Resin Company), according to published protocols100. In brief, the samples were dehydrated using a series of ethanol dilutions (30%, 50%, 70% in ddH2O), and were then incubated in a 1:1 mixture of LR White and 70% ethanol, before being placed in pure LR White resin, and being finally placed in resin containing LR White accelerator (London Resin Company). A final polymerization was performed at 60 °C. The samples were then processed to 200 nm thin sections, employing an ultramicrotome (type EM UC6, Leica Microsystems), and the sections were mounted on silicon wafers (Siegert Wafer GmbH). Consecutive sections were mounted on glass slides and were stained with a dilution of toluidine blue (1% in H2O containing 2% sodium borate), before histological imaging.
SIMS imaging and image processing
Imaging was performed using a NanoSIMS 50L instrument (Cameca, France). The negative ion mode was employed, using a 8 kV Cs+ primary ion source. The selected imaging area was pre-implanted before imaging for several minutes, with an ion current of approximately 600 pA, to achieve a proper steady-state of ionization. The entrance and aperture slits were optimized for separating isobaric mass peaks optimally, concentrating on imaging 12C14N− and 13C14N−. Imaging was performed using a primary ion current of 2.5 pA, with an empirically adjusted dwell time. The images of individual samples were taken simultaneously, with identical dwell time, to enable precise isotope ratio calculations. Histology images of the samples were acquired using an Axio Imager M2 upright microscope (Zeiss). These images were overlapped onto the SIMS images, using Adobe Photoshop (2020). 13C/12C ratios were then determined in regions of interest (ROIs) identified by an experienced user in the histology images, using a self-written routine in MATLAB (the Mathworks) as described previously100.
Statistics and reproducibility
Sample size
In the single-cell RNA sequencing and proteomics datasets, no groups of conditions were compared to each other, and therefore, no sample-size calculation was performed.
For the oocyte protein aggregation and proteosomal activity experiments, no statistical methods were used to predetermine sample size. Retrospectively, achieved sample sizes were determined to be adequate based on the magnitude and consistency of measurable differences between groups. Most importantly, sample size per experiment was dictated by the number of oocytes that could be processed for microinjection and live imaging within a reasonable time by the researcher without affecting oocyte quality.
Data exclusion
In the oocyte protein turnover experiments (DDA MS), median over technical replicates were only computed if the DDA intensity of 13C6-Lys labelled protein was detected in at least two out of four technical replicates, otherwise the data point is omitted (set as n.a.). The mean and s.d. of F (fractions of 13C6-Lys labelled proteins) were calculated if F was detected in at least one of the two biological replicates.
A protein was defined as ‘heavy only’ if it was detected as 13C6-Lys labelled protein in at least two out of four technical replicates in at least one biological replicate and there is no signal for 12C6-Lys labelled protein in any of the four technical replicates of both biological replicates detected. Accordingly, a protein was defined as ‘light only’ if it was detected as 12C6-Lys labelled protein in at least two out of four technical replicates in at least one biological replicate and if there is no 13C6-Lys labelled protein in any of the four technical replicates of both biological replicates detected.
No data points were excluded in the oocyte protein aggregation and proteosomal activity experiments.
Replication
In the oocyte protein turnover experiments (DDA MS), two sets of oocytes (n = 2,475, n = 2,473) were obtained from a total of 8-week old mice (12C6-Lys chow after birth until they were 8-weeks old) born from 13C6-Lys labelled females. Oocytes from multiple animals were pooled for a single replicate to reach the minimum input required for the MS processing. Each DDA oocyte fraction was measured in quadruplicate.
In the ovary protein turnover experiments (DDA MS) with 13C6-Lys pulse until birth and 12C6-Lys chase from birth, ovaries were collected from the female progeny at 11 time points (24 hours, 48 hours, 1, 2, 3, 6, 9, 12, 30, 50 and 65 weeks). For each time point, three biological replicates were collected, each containing two ovaries from a single animal.
In the ovary protein turnover experiments (DDA MS) with 13C6-Lys pulse until birth and 12C6-Lys chase from weaning, ovaries were collected from the female progeny at six time points (6, 9, 12, 30, 50 and 65 weeks). For each time point, three biological replicates were collected, each containing two ovaries from a single animal.
In the protein abundance experiments (DIA-MS), ovaries were collected from unlabelled FVB/N female mice at eight time points (24 hours, 1, 2, 3, 5, 9, 12, and 50 weeks). For each time point, three biological replicates were collected, each containing two ovaries from a single animal.
In the single-cell RNA sequencing experiments, each library was generated from six ovaries collected from three different pups in a single pregnant CD1 female mouse.
All data in the oocyte protein aggregation and proteosomal activity experiments are from at least two independent experiments or multiple biological replicates. All attempts at replication were successful.
Randomization
The single-cell RNA sequencing and proteomics datasets did not have different treatments or conditions that could be randomized. For the oocyte protein aggregation and proteosomal activity experiments, mouse oocytes were collected from multiple ovaries or animals and then pooled. They were then randomly assigned to different experimental groups.
Blinding
The single-cell RNA sequencing and proteomics datasets did not have different groups of conditions, and therefore, these experiments did not require blinding.
For the oocyte protein aggregation and proteosomal activity experiments, the investigators were not blinded to allocation during experiments and outcome assessment, as each experiment was performed by one researcher alone. Thus, blinding during group allocation was not possible to ensure samples received the right treatment or manipulation during experiment. Blinding was also not possible during data analysis as it was performed by the same researcher that conducted the experiment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41556-024-01442-7.
Supplementary information
Supplementary information.
Supplementary Tables 1–13.
Plots of raw abundance values of proteins in unlabelled ovaries at eight different ages (1 day; 1, 2, 3, 5, 9, 12 and 50 weeks) measured using DIA mass spectrometry.
An atlas of the 2,691 ovarian proteins. The atlas shows the proteins’ raw and log-scaled 13C6-Lys and 12C6-Lys MS1 intensities determined by DDA mass spectrometry over time, the modelled data, H1/2 values (describing the number of days over which the 13C6-Lys protein fraction decreased by half, considering ovarian growth, changes in protein concentration and the free heavy lysine pool), the ratio of 12C6-Lys (L) and 13C6-Lys (H), as well as the changes in their abundance in the ovary throughout development.
Heatmaps of half-lives of different proteins belonging to different protein complexes and enriched GO terms in the ovary and organs from other studies19–21.
Characterization of the localization of the transcripts encoding the proteins from the high protein longevity in the ovary using single-cell RNA-sequencing of postnatal day 2 ovaries.
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Acknowledgements
We thank the staff from the Animal Facility and Proteomics Facility at the Max Planck Institute for Multidisciplinary Sciences for technical assistance and support; M. Daniel, S. Schlott and L. Wartosch for their assistance with maintenance and handling of fully labelled 13C6-Lys mice; S. Schlott for preparing mouse brain and ovary slices, and oocyte isolation; M. Eggert Martínez for help in establishing the proteasome activity assay; Life Science Editors for critical comments on the manuscript; and all the members of Urlaub, Liepe and Schuh labs for helpful discussions. The research leading to these results received financial support from the Max Planck Society, a Deutsche Forschungsgemeinschaft (DFG) Leibniz Prize to M.S. (SCHU 3047/1-1), a European Research Council (ERC) Starting Grant (ERC-StG 945528 IMAP) to J.L. and the DFG SFB1565 (project number 469281184) to H.U.; S.M.P. and Y.H. were supported by the International Max Planck Research School for Genome Science, part of the Göttingen Graduate Center for Neurosciences, Biophysics and Molecular Biosciences. Some graphics in Figs. 1a,c, 2a,b, 4b and 8a, and Extended Data Figs. 2a and 6a were created with Biorender.com.
Extended data
Author contributions
M.S. conceived the study. K.H., L.M.W., A.L.T.-T., H.U., J.L. and M.S. designed the screen to identify long-lived proteins in mouse oocytes and ovaries. R.L.G., K.H., L.M.W., Y.H., H.U., J.L. and M.S. designed experiments and analyses to determine relative protein abundances throughout the lifetime of a mouse. K.H. and V.S. created fully labelled 13C6-Lys mice. K.H. performed and collected all samples for oocyte and ovary MS analyses with help from A.-S.F.; K.H., L.M.W., A.S., A.-S.F. and H.U. optimized protocols for processing mouse oocytes and ovaries for MS. A.S. processed oocyte samples for MS analysis. L.M.W. and M.R. processed ovary samples for MS analysis. L.M.W. performed the initial processing of all MS data. J.L. developed methods to identify long-lived proteins in mouse oocytes with input from L.M.W., H.U., R.L.G. and M.S.; J.L. conducted all modelling related to the determination of ovarian protein turnover and changes in ovarian protein abundance, with input from Y.H., R.L.G., K.H., L.M.W., S.M.P., H.U. and M.S.; Y.H. and J.L. performed over-representation analysis, gene set enrichment analysis and clustering analysis with input from S.M.P., R.L.G., K.H., L.M.W., H.U. and M.S.; R.L.G., K.H., K.G., S.O.R. and M.S. designed NanoSIMS experiments. R.L.G. and K.G. processed samples for NanoSIMS imaging. K.G. performed NanoSIMS experiments. R.L.G. analysed NanoSIMS data with input from S.O.R. and M.S.; S.H. and M.S. designed the single-cell RNA sequencing experiment. S.H. established protocols for sample preparation of mouse postnatal ovaries for single-cell RNA sequencing. S.H. and D.S. processed mouse postnatal ovaries for single-cell RNA sequencing. S.M.P. analysed the single-cell RNA sequencing data. S.C. and K.T. designed the aggresome staining and proteasomal activity measurement experiments. K.T. designed and constructed the proteasomal activity reporter and performed assay characterization. S.C. performed all aggresome staining and proteasomal activity experiments and analysed the data. M.S. prepared the first draft of the main text. Subsequent editing and modifications to the text were done by all authors. The Supplementary Information was written by K.H., R.L.G., S.M.P., D.S., L.M.W., Y.H., K.G., S.O.R. and J.L. with input from all authors. Figure legends were written by K.H., S.M.P., Y.H. and J.L. with input from all authors. K.H., S.M.P., R.L.G., J.L. and M.S. prepared the figures with input from all the authors. Funding for this study was secured by J.L., H.U. and M.S.; H.U., J.L. and M.S. supervised the study.
Peer review
Peer review information
Nature Cell Biology thanks Thorsten Hoppe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Max Planck Society.
Data availability
The scRNAseq datasets are deposited in the NCBI Gene Expression Omnibus database101 under accession number GSE237012. The proteomics data are deposited to the ProteomeXchange Consortium via the UCSD MassIVE repository with the dataset identifier MSV000092528 and PXD044113, for MassIVE and ProteomeXchange, respectively102. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
R scripts for data processing and visualization of proteomics and single-cell RNA sequencing data are available on figshare (10.6084/m9.figshare.25604232.v1).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Katarina Harasimov, Rebecca L. Gorry, Luisa M. Welp, Sarah Mae Penir, Yehor Horokhovskyi.
Contributor Information
Henning Urlaub, Email: henning.urlaub@mpinat.mpg.de.
Juliane Liepe, Email: juliane.liepe@mpinat.mpg.de.
Melina Schuh, Email: melina.schuh@mpinat.mpg.de.
Extended data
is available for this paper at 10.1038/s41556-024-01442-7.
Supplementary information
The online version contains supplementary material available at 10.1038/s41556-024-01442-7.
References
- 1.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 2.Hirshfield AN. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/S0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 3.Wallace WHB, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles*. Endocr. Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 5.Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019;20:421–435. doi: 10.1038/s41580-019-0101-y. [DOI] [PubMed] [Google Scholar]
- 6.Krüger M, et al. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134:353–364. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Milenkovic D, et al. TWINKLE is an essential mitochondrial helicase required for synthesis of nascent D-loop strands and complete mtDNA replication. Hum. Mol. Genet. 2013;22:1983–1993. doi: 10.1093/hmg/ddt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghezzi D, et al. Mutations in TTC19 cause mitochondrial complex III deficiency and neurological impairment in humans and flies. Nat. Genet. 2011;43:259–U109. doi: 10.1038/ng.761. [DOI] [PubMed] [Google Scholar]
- 9.Borah N, Nukala KM, Vadlapudi V, Gubbala SP, Amanchy R. BOP1-a key player of ribosomal biogenesis. Curr. Sci. India. 2019;117:422–433. doi: 10.18520/cs/v117/i3/422-433. [DOI] [Google Scholar]
- 10.Gause M, Misulovin Z, Bilyeu A, Dorsett D. Dosage-sensitive regulation of cohesin chromosome binding and dynamics by Nipped-B, Pds5, and Wapl. Mol. Cell. Biol. 2010;30:4940–4940. doi: 10.1128/MCB.00642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiyama T, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 2010;143:737–749. doi: 10.1016/j.cell.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Sutani T, Kawaguchi T, Kanno R, Itoh T, Shirahige K. Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr. Biol. 2009;19:492–497. doi: 10.1016/j.cub.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Coutinho LE, Pati D. PDS5A and PDS5B in cohesin function and human disease. Int. J. Mol. Sci. 2021;22:5868. doi: 10.3390/ijms22115868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang N, Pati D. Sororin is a master regulator of sister chromatid cohesion and separation. Cell Cycle. 2012;11:2073–2083. doi: 10.4161/cc.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana-Konwalski K, et al. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–2516. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhardt S, et al. Chromosome cohesion established by Rec8-cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice. Curr. Biol. 2016;26:678–685. doi: 10.1016/j.cub.2015.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr. Biol. 2010;20:1529–1533. doi: 10.1016/j.cub.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically-induced animal model of menopause. Brain Res. 2011;1379:176–187. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fornasiero EF, et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 2018;9:4230. doi: 10.1038/s41467-018-06519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolfs Z, et al. An atlas of protein turnover rates in mouse tissues. Nat. Commun. 2021;12:6778. doi: 10.1038/s41467-021-26842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluever V, et al. Protein lifetimes in aged brains reveal a proteostatic adaptation linking physiological aging to neurodegeneration. Sci. Adv. 2022;8:eabn4437. doi: 10.1126/sciadv.abn4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giurgiu M, et al. CORUM: the comprehensive resource of mammalian protein complexes-2019. Nucleic Acids Res. 2019;47:D559–D563. doi: 10.1093/nar/gky973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanazawa M, Terada K, Kato S, Mori M. HSDJ, a human homolog of DnaJ, is farnesylated and is involved in protein import into mitochondria. J. Biochem. 1997;121:890–895. doi: 10.1093/oxfordjournals.jbchem.a021670. [DOI] [PubMed] [Google Scholar]
- 24.Gotoh T, Terada K, Oyadomari S, Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- 25.Wang CY, Jégu T, Chu HP, Oh HJ, Lee JT. SMCHD1 merges chromosome compartments and assists formation of super-structures on the inactive X. Cell. 2018;174:406–421.e425. doi: 10.1016/j.cell.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blewitt ME, et al. SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat. Genet. 2008;40:663–669. doi: 10.1038/ng.142. [DOI] [PubMed] [Google Scholar]
- 27.Steinhauser ML, et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–519. doi: 10.1038/nature10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad R, Budnik B. A review of the current state of single-cell proteomics and future perspective. Anal. Bioanal. Chem. 2023;415:6889–6899. doi: 10.1007/s00216-023-04759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med. 2008;14:451–464. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuanalo-Contreras K, et al. Extensive accumulation of misfolded protein aggregates during natural aging and senescence. Front. Aging Neurosci. 2022;14:1090109. doi: 10.3389/fnagi.2022.1090109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meller A, Shalgi R. The aging proteostasis decline: from nematode to human. Exp. Cell. Res. 2021;399:112474. doi: 10.1016/j.yexcr.2021.112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dantuma NP, Lindsten K, Glas R, Jellne M, Masucci MG. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat. Biotechnol. 2000;18:538–543. doi: 10.1038/75406. [DOI] [PubMed] [Google Scholar]
- 33.Rayon T, et al. Species-specific pace of development is associated with differences in protein stability. Science. 2020;369:eaba7667. doi: 10.1126/science.aba7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basisty N, Meyer JG, Schilling B. Protein turnover in aging and longevity. Proteomics. 2018;18:1700108. doi: 10.1002/pmic.201700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swovick K, et al. Interspecies differences in proteome turnover kinetics are correlated with life spans and energetic demands. Mol. Cell. Proteom. 2021;20:100041. doi: 10.1074/mcp.RA120.002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson ACS, et al. Reduced in vivo hepatic proteome replacement rates but not cell proliferation rates predict maximum lifespan extension in mice. Aging Cell. 2016;15:118–127. doi: 10.1111/acel.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher M, et al. Proteome-wide changes in protein turnover rates in C. elegans models of longevity and age-related disease. Cell Rep. 2016;16:3041–3051. doi: 10.1016/j.celrep.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Söti C, Csermely P. Molecular chaperones and the aging process. Biogerontology. 2000;1:225–233. doi: 10.1023/A:1010082129022. [DOI] [PubMed] [Google Scholar]
- 39.Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging – a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savas JN, Toyama BH, Xu T, Yates JR, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335:942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toyama BH, Hetzer MW. Protein homeostasis: live long, won’t prosper. Nat. Rev. Mol. Cell Biol. 2013;14:55–61. doi: 10.1038/nrm3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navarro-Pando JM, et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci. Adv. 2021;7:eabc7409. doi: 10.1126/sciadv.abc7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018;19:579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update. 2020;26:43–57. doi: 10.1093/humupd/dmz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol. Reprod. 2015;93:67–68. doi: 10.1095/biolreprod.115.132290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Titus S, et al. Impairment of BRCA1-related DNA double strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013;5:172ra121. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamagata Y, et al. Changes in telomerase activity in experimentally induced atretic follicles of immature rats. Endocr. J. 2002;49:589–595. doi: 10.1507/endocrj.49.589. [DOI] [PubMed] [Google Scholar]
- 49.Yamada-Fukunaga T, et al. Age-associated telomere shortening in mouse oocytes. Reprod. Biol. Endocrinol. 2013;11:108. doi: 10.1186/1477-7827-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogués C, Ponsà M, Vidal F, Boada M, Egozcue J. Effects of aging on the zona pellucida surface of mouse oocytes. J. In Vitro Fert. Embryo Transf. 1988;5:225–229. doi: 10.1007/BF01131126. [DOI] [PubMed] [Google Scholar]
- 51.Longo FJ. Changes in the zonae pellucidae and plasmalemmae of aging mouse eggs. Biol. Reprod. 1981;25:399–411. doi: 10.1095/biolreprod25.2.399. [DOI] [PubMed] [Google Scholar]
- 52.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 2010;20:1522–1528. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lister LM, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 54.Duncan FE, et al. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging cell. 2012;11:1121–1124. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zielinska AP, Holubcova Z, Blayney M, Elder K, Schuh M. Sister kinetochore splitting and precocious disintegration of bivalents could explain the maternal age effect. eLife. 2015;4:e11389. doi: 10.7554/eLife.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briley SM, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152:245–260. doi: 10.1530/REP-16-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyama BH, et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toyama BH, et al. Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. J. Cell Biol. 2019;218:433–444. doi: 10.1083/jcb.201809123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol. 2004;6:1114–1121. doi: 10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- 60.Commerford SL, Carsten AL, Cronkite EP. Histone turnover within nonproliferating cells. Proc. Natl Acad. Sci. USA. 1982;79:1163–1165. doi: 10.1073/pnas.79.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duerre JA, Lee CT. In vivo methylation and turnover of rat brain histones. J. Neurochem. 1974;23:541–547. doi: 10.1111/j.1471-4159.1974.tb06057.x. [DOI] [PubMed] [Google Scholar]
- 62.Krishna S, et al. Identification of long-lived proteins in the mitochondria reveals increased stability of the electron transport chain. Dev. Cell. 2021;56:2952–2965.e2959. doi: 10.1016/j.devcel.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 64.Al Rawi S, et al. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–1147. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- 65.Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- 66.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian-cells. Biochem. J. 1995;312:163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li GW, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez-Nuevo A, et al. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature. 2022;607:756–761. doi: 10.1038/s41586-022-04979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu, J. K. Antiaging agents: safe interventions to slow aging and healthy life span extension. Nat. Prod. Bioprospect.12, 18 (2022). [DOI] [PMC free article] [PubMed]
- 71.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 73.Zhao J, Zhai B, Gygi SP, Goldberg AL. MTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl Acad. Sci. USA. 2015;112:15790–15797. doi: 10.1073/pnas.1521919112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morita M, et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14:473–480. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boke E, et al. Amyloid-like self-assembly of a cellular compartment. Cell. 2016;166:637–650. doi: 10.1016/j.cell.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl Acad. Sci. USA. 2007;104:187–192. doi: 10.1073/pnas.0609923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jentoft IMA, et al. Mammalian oocytes store proteins for the early embryo on cytoplasmic lattices. Cell. 2023;186:5308–5327.e5325. doi: 10.1016/j.cell.2023.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Govindaraj V, Krishnagiri H, Chakraborty P, Vasudevan M, Rao AJ. Age-related changes in gene expression patterns of immature and aged rat primordial follicles. Syst. Biol. Reprod. Med. 2017;63:37–48. doi: 10.1080/19396368.2016.1267820. [DOI] [PubMed] [Google Scholar]
- 79.Yue MX, et al. Abnormal DNA methylation in oocytes could be associated with a decrease in reproductive potential in old mice. J. Assist. Reprod. Genet. 2012;29:643–650. doi: 10.1007/s10815-012-9780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall KL, Wang J, Ji T, Rivera RM. The effects of biological aging on global DNA methylation, histone modification, and epigenetic modifiers in the mouse germinal vesicle stage oocyte. Anim. Reprod. 2018;15:1253–1267. doi: 10.21451/1984-3143-AR2018-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manosalva I, González A. Aging changes the chromatin configuration and histone methylation of mouse oocytes at germinal vesicle stage. Theriogenology. 2010;74:1539–1547. doi: 10.1016/j.theriogenology.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 82.Van Den Berg IM, et al. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum. Reprod. 2011;26:1181–1190. doi: 10.1093/humrep/der030. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, et al. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021;236:7966–7983. doi: 10.1002/jcp.30468. [DOI] [PubMed] [Google Scholar]
- 84.May-Panloup P, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update. 2016;22:725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 85.Xi X, et al. Dynamic changes of DNA methylation and transcriptome expression in porcine ovaries during aging. Biomed. Res. Int. 2019;2019:8732023. doi: 10.1155/2019/8732023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uysal F, Ozturk S. The loss of global DNA methylation due to decreased DNMT expression in the postnatal mouse ovaries may associate with infertility emerging during ovarian aging. Histochemistry Cell Biol. 2020;154:301–314. doi: 10.1007/s00418-020-01890-w. [DOI] [PubMed] [Google Scholar]
- 87.Jiang ZX, et al. The m6A mRNA demethylase FTO in granulosa cells retards FOS-dependent ovarian aging. Cell Death Dis. 2021;12:744. doi: 10.1038/s41419-021-04016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kinnear HM, et al. The ovarian stroma as a new frontier. Reproduction. 2020;160:R25–R39. doi: 10.1530/REP-19-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwata H. Age-associated changes in granulosa cells and follicular fluid in cows. J. Reprod. Dev. 2017;63:339–345. doi: 10.1262/jrd.2017-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tatone C, Amicarelli F. The aging ovary—the poor granulosa cells. Fertil. Steril. 2013;99:12–17. doi: 10.1016/j.fertnstert.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 91.Zhang D, et al. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J. Assist. Reprod. Genet. 2015;32:1069–1078. doi: 10.1007/s10815-015-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180:585–600.e519. doi: 10.1016/j.cell.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Shevchenko A, Tomas H, Havliš J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 94.Moggridge S, Sorensen PH, Morin GB, Hughes CS. Extending the compatibility of the SP3 paramagnetic bead processing approach for proteomics. J. Proteome Res. 2018;17:1730–1740. doi: 10.1021/acs.jproteome.7b00913. [DOI] [PubMed] [Google Scholar]
- 95.Hughes CS, et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 96.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 97.Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 98.R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2021).
- 99.Bruderer R, et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol. Cell. Proteom. 2015;14:1400–1410. doi: 10.1074/mcp.M114.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jahne S, et al. Presynaptic activity and protein turnover are correlated at the single-synapse level. Cell Rep. 2021;34:108841. doi: 10.1016/j.celrep.2021.108841. [DOI] [PubMed] [Google Scholar]
- 101.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perez-Riverol Y, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary Tables 1–13.
Plots of raw abundance values of proteins in unlabelled ovaries at eight different ages (1 day; 1, 2, 3, 5, 9, 12 and 50 weeks) measured using DIA mass spectrometry.
An atlas of the 2,691 ovarian proteins. The atlas shows the proteins’ raw and log-scaled 13C6-Lys and 12C6-Lys MS1 intensities determined by DDA mass spectrometry over time, the modelled data, H1/2 values (describing the number of days over which the 13C6-Lys protein fraction decreased by half, considering ovarian growth, changes in protein concentration and the free heavy lysine pool), the ratio of 12C6-Lys (L) and 13C6-Lys (H), as well as the changes in their abundance in the ovary throughout development.
Heatmaps of half-lives of different proteins belonging to different protein complexes and enriched GO terms in the ovary and organs from other studies19–21.
Characterization of the localization of the transcripts encoding the proteins from the high protein longevity in the ovary using single-cell RNA-sequencing of postnatal day 2 ovaries.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Data Availability Statement
The scRNAseq datasets are deposited in the NCBI Gene Expression Omnibus database101 under accession number GSE237012. The proteomics data are deposited to the ProteomeXchange Consortium via the UCSD MassIVE repository with the dataset identifier MSV000092528 and PXD044113, for MassIVE and ProteomeXchange, respectively102. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
R scripts for data processing and visualization of proteomics and single-cell RNA sequencing data are available on figshare (10.6084/m9.figshare.25604232.v1).